
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 146
1,3-Dichloropropene, 1,2-Dichloropropane
and Mixtures
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
1,3-Dichloropropene, 1,2-dichloropropane and mixtures.
(Environmental health criteria: 146)
1. Environmental exposure 2. Hydrocarbons, Chlorinated - adverse
effects 3. Hydrocarbons, Chlorinated - poisoning 4. Hydrocarbons,
Chlorinated - toxicity 5. Occupational exposure I.Series
ISBN 92 4 157146 2 (NLM Classification QV 633)
ISSN 0250-8634
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland,
Which will be glad to provide the latest information on any changes
made to the text, plans for new editions, and reprints and
translations already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the impression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of every country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,3-DICHLOROPROPENE,
1,2-DICHLOROPROPANE, AND MIXTURES
PART A. 1,3-DICHLOROPROPENE
PART B. 1,2-DICHLOROPROPANE
PART C. MIXTURES OF DICHLOROPROPENES AND DICHLOROPROPANE
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES, Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH
CRITERIA FOR 1,3-DICHLOROPROPENE,
1,2-DICHLOROPROPANE, AND MIXTURES
Members
Dr V. Benes, Department of Toxicology and Reference Laboratory,
Institute of Hygiene & Epidemiology, Prague, Czechoslovakia
Dr R. Drew, Key Centre for Toxicology, Department of Applied
Biology, Royal Melbourne Institute for Technology, Melbourne,
Victoria, Australia (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr J.I. Kundiev, Research Institute of Labour Hygiene & Occupational
Diseases, Kiev, Ukraine (Vice-Chairman)
Dr K. Mitsumori, Division of Pathology, Biological Safety Research
Center, National Institute of Hygienic Sciences, Tokyo, Japan
Dr Richard F. Shore, Ecotoxicology and Pollution Section, Institute
of Terrestrial Ecology, Monks Wood Experimental Station, Abbots
Ripton, Huntingdon, United Kingdom
Dr G.J. van Esch, Oranje, Bilthoven, Netherlands (Rapporteur)
Dr E.A.H. van Heemstra-Lequin, Laren, Netherlands (Joint
Rapporteur)
Dr S. Wong, Bureau of Chemical Hazards, Environmental Health
Directorate, Department of National Health and Welfare,
Tunney's Pasture, Ottawa, Ontario, Canada
Observers
Dr D.E. Owen, Shell Internationale Petroleum Maatschappij BV, The
Hague, Netherlands
Members from the Host Institution
Dr W.H. Gross, Fraunhofer Institute of Toxicology & Aerosol
Research, Hanover, Germany
Dr J.R. Kielhorn, Fraunhofer Institute of Toxicology & Aerosol
Research, Hanover, Germany
Dr C.M. Melber, Fraunhofer Institute of Toxicology & Aerosol
Research, Hanover, Germany
Secretariat
Dr R.F. Hertel, Fraunhofer Institute of Toxicology & Aerosol
Research, Hanover, Germany
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mme C. Partensky, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer (IARC), Lyon,
France
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
* * *
Note: The proprietary information contained in this monograph
cannot replace documentation for registration purposes, because the
latter has to be closely linked to the source, the manufacturing
route, and the purity/impurities of the substance to be registered.
The data should be used in accordance with paragraphs 82-84 and
recommendations paragraph 90 of the Second FAO Government
Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR
1,3-DICHLOROPROPENE, 1,2-DICHLOROPROPANE, AND MIXTURES
The meeting of the WHO Task Group on Environmental Health
Criteria for 1,3-dichloropropene, 1,2-dichloropropane, and mixtures,
which was held at the Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany, from 16 to 20 September 1990, was
sponsored by the German Ministry of the Environment. Dr R.F. Hertel
welcomed the participants on behalf of the host institute. Dr K.W.
Jager, IPCS, welcomed the participants on behalf of Dr M. Mercier,
Director of the IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Group reviewed and revised the draft criteria
monograph and made an evaluation of the risks for human health and
the environment from exposure to 1,3-dichloropropene, 1,2-
dichloropropane, and mixtures of dichloropropenes and
dichloropropane.
Dr E.A.H. van Heemstra-Lequin and Dr G.J. van Esch of the
Netherlands cooperated in the preparation of the first draft of the
EHC monograph. Dr van Esch prepared the second draft, incorporating
the comments received following circulation of the first draft to
the IPCS contact points for Environmental Health Criteria
monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monographs, and Mrs M.O. Head of Oxford
for the editing.
The fact that Shell and Dow Chemical made their proprietary
toxicological information on their products available to the IPCS
and the Task Group is gratefully acknowledged. This allowed the Task
Group to make their evaluation on a more complete data base.
The efforts of all who helped in the preparation and
finalization of the publications are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
monograph was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
PART A
ENVIRONMENTAL HEALTH CRITERIA FOR 1,3-DICHLOROPROPENE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,3-DICHLOROPROPENE
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
2.4.1 Sampling
2.4.2 Determination of residues in crops and soil
2.4.3 Determination of residues in water
2.4.4 Determination of residues in air
2.4.5 Determination of residues in food
2.4.6 Determination of 3-chloroallyl alcohol
2.4.7 Determination of mercapturic acids in urine
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.2 Use
3.2.3 Sources of pollution
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Water
4.1.3 Soil
4.1.3.1 Hydrolysis
4.1.3.2 Volatilization
4.1.3.3 Uptake in crops
4.1.3.4 Movement in soil
4.1.3.5 Loss under field conditions
4.1.3.6 Results of supervised field trials
4.2 Bioconcentration
4.3 Abiotic degradation
4.3.1 Photodegradation
4.4 Biodegradation and biotransformation
4.4.1 Miscellaneous
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Air
5.2 Water
5.3 Crops
5.4 Occupational exposure
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.1.1 Rat
6.1.1.2 Mouse
6.1.2 Inhalation
6.1.2.1 Rat
6.2 Influence on tissue levels of glutathione
6.2.1 Oral
6.2.2 Inhalation
6.3 Biotransformation
6.3.1 Rat
6.3.2 Humans
6.4 Reaction with macromolecules
6.4.1 Mouse
6.4.2 Rat
6.5 Appraisal
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Acute toxicity
7.1.1 Microorganisms
7.1.2 Algae
7.1.3 Invertebrates
7.1.4 Honey bees
7.1.5 Fish
7.1.6 Birds
7.2 Short-term/long-term toxicity
7.2.1 Invertebrates
7.2.2 Fish
7.2.3 Field studies
7.2.4 Phytotoxicity
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.2 Short-term exposures
8.2.1 Oral
8.2.2 Inhalation
8.2.2.1 Mouse
8.2.2.2 Rat
8.2.2.3 Other animal species
8.3 Skin and eye irritation, sensitization
8.3.1 Skin irritation
8.3.2 Eye irritation
8.3.2.1 In vitro studies
8.3.3 Sensitization
8.4 Long-term exposure
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Inhalation (rat)
8.5.1.2 Intraperitoneal (mouse)
8.5.2 Teratogenicity
8.5.2.1 Inhalation (rat)
8.5.2.2 Inhalation (rabbit)
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies
8.6.1.1 Microorganisms
8.6.1.2 Effects of glutathione on bacterial
mutagenesis
8.6.1.3 Mammalian cells
8.6.1.4 DNA damage
8.6.1.5 Chromosomal effects
8.6.2 In vivo studies
8.6.3 Appraisal
8.7 Carcinogenicity
8.7.1 Oral
8.7.1.1 Mouse
8.7.1.2 Rat
8.7.2 Inhalation
8.7.2.1 Mouse
8.7.2.2 Rat
8.7.3 Appraisal
8.7.4 Dermal and subcutaneous (mouse)
8.8 Factors modifying toxicity, toxicity of metabolites, mode
of action
8.8.1 Toxicity of metabolites, cis- and trans-
1,3-dichloropropene oxide
8.8.1.1 Mutagenicity
8.8.1.2 Carcinogenicity
8.8.2 Role of oxidation
8.8.3 Role of glutathione
8.8.4 Effect on liver enzyme activity
9. EFFECTS ON HUMANS
9.1 General population
9.1.1 Acute toxicity - poisoning incidents
9.1.2 Controlled human studies
9.2 Occupational exposure
9.2.1 General
9.2.2 Acute toxicity - poisoning incidents
9.2.3 Effects of short- and long-term exposure
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
"1,3-Dichloropropene" was introduced in 1956 as part of a
mixture, containing 1,3-dichloropropenes, 1,2-dichloropropane, and
other halogenated hydrocarbons, and has been widely used in
agriculture as a pre-plant soil fumigant for the control of
nematodes in vegetables, potatoes, and tobacco. Application is
primarily by soil injection. The commercial formulation of 1,3-
dichloropropene is a mixture of cis- and trans-isomers (in
approximately equal proportions), which is a colourless to amber
liquid with a penetrating, irritating, chloroform-like odour. The
vapour pressure is 3.7 kPa at 20 °C. The technical product has a
purity of 92% and may contain a number of impurities, such as 1,2-
dichloropropane. The log P octanol/water partition coefficient is
1.98.
In air, decomposition of 1,3-dichloropropene is mainly by
reaction with free radicals and ozone. The half-lives of the cis-
and trans-isomers in the reaction with free radicals are 12 and
7 h, respectively, and in the reaction with ozone, 52 and 12 days,
respectively. Direct photo-transformation seems to be insignificant,
but may be enhanced in the presence of atmospheric particles.
In water, 1,3-dichloropropene is likely to disappear rapidly,
because of its relatively low water solubility and high volatility;
half-lives of less than 5 h have been reported.
The distribution of 1,3-dichloropropene in soil compartments is
dependent on the vapour pressure, diffusion coefficient,
temperature, and moisture content of the soil. The persistence of
1,3-dichloropropene in soil is influenced by volatilization,
chemical and biological transformation, photochemical
transformation, and organism uptake. Volatilization and diffusion in
the vapour phase are the most significant mechanisms for
environmental dispersion and dilution.
Transformation of 1,3-dichloropropene is initially by
hydrolysis to 3-chloroallyl alcohol and then by microbial
transformation to 3-chloroacrolein and 3-chloroacrylic acid. In a
laboratory study, the half-lives for the hydrolysis of the cis-
and trans-isomers of 1,3-dichloropropene at 15 °C and 29 °C were
11.0 and 2.0 days, respectively, for the cis-isomer and 13.0 and
2.0 days for the trans-isomer. In soil with a pH of 7 and a
temperature of 25 °C, the half-life for hydrolysis for both isomers
was 4.6 days. Because of its relatively rapid disappearance from
soil, residues are unlikely to accumulate when the fumigant is
applied at the recommended rate and frequency.
1,3-Dichloropropene is potentially mobile in soil, especially
in open-textured, sandy soil with a low moisture content. Downward
movement is enhanced by deep cultivation of soils with low porosity.
1,3-Dichloropropene has been detected in "upper groundwater" (up to
2 m below the surface), but not in deep groundwater, which is more
likely to be used for drinking-water.
1,3-Dichloropropene can be taken up by crops. However,
significant residues are unlikely to occur in edible crops, because
these are not normally planted until most of the fumigant has
dissipated.
Bioaccumulation of 1,3-dichloropropene is unlikely, because of
its relatively high water solubility (> 1 g/kg), low log P octanol
water partition coefficient, and rapid elimination from mammals and
other organisms.
1.1.2 Kinetics and metabolism
1,3-Dichloropropene administered orally to rodents is rapidly
eliminated. The major route of elimination is in the urine where 81%
of the cis-isomer and 56% of the trans-isomer are eliminated
within 24 h of dosing. The half-life of elimination in the urine is
5-6 h. Faecal elimination is minor. Expired carbon dioxide accounts
for 4 and 24% of the elimination of the cis- and trans-isomers
of 1,3-dichloropropene, respectively. Tissue concentrations after
oral administration are low; the highest residual concentrations are
found in the stomach wall, followed by lower amounts in the kidneys,
liver, and bladder.
Unchanged 1,3-dichloropropene is not found in the urine. The
cis- and trans-isomers are substrates for hepatic glutathione-
S-alkyl transferase, forming mercapturic acids, which are excreted
in the urine. The trans-isomer is conjugated 4-5 times more slowly
than the cis-isomer. The principal urinary metabolite in rats and
mice is N-acetyl- S-(3-chloroprop-2-enyl)L-cysteine; this
compound can be used for biological monitoring in humans. A second,
minor metabolic pathway has been identified for the cis-isomer
that involves mono-oxygenation to cis-1,3-dichloropropene oxide,
which can also be conjugated with glutathione. The high proportion
of the trans-isomer that occurs in expired air results from an
alternative metabolic pathway to conjugation that has a higher
specificity for the trans- than for the cis-isomer.
Inhalation exposure of rats to 1,3-dichloropropene did not lead
to increases in blood concentrations proportional with dose. At a
dose of 408.6 mg/m3 (90 ppm), respiratory frequency and
respiratory minute volume were decreased and saturation of
metabolism occurred at 1362 mg/m3 (300 ppm). Cis- and trans-
isomers were rapidly eliminated from the blood, the half-life of
elimination being 3-6 min at concentrations below 1362 mg/m3 but
considerably longer (33-43 min) at higher concentrations.
1.1.3 Effects on organisms in the environment
The EC50 values for growth (96 h) for the freshwater alga,
Selenastrum capricornutum, and the estuarine diatom, Skeletoneria
costatum, are 4.95 mg/litre and 1 mg/litre, respectively. The
acute toxicity (96-h LC50) of 1,3-dichloropropene for fish is of
the order of 1-7.9 mg/litre. In an embryo-larval test on Fathead
minnow, the maximum no-effect level was 0.24 mg/litre. These data
and the fact that 1,3-dichloropropene is unlikely to persist in
water, indicate that the hazard for fish lies in acute toxic
effects, with little potential for additional effects resulting from
long-term exposure.
1,3-dichloropropene at dose levels of 30-60 mg/kg can reduce
the abundance of fungi and the rate of microbial enzyme activity,
but the effect is not usually long lasting (< 7 days) and does not
occur in all soil types. In some studies, there was a significant
increase in microbial numbers following application.
1,3-Dichloropropene is phytotoxic, however, its toxicity for
Honey bees is low. Using a dusting technique, the 48-h LD50 was
6.6 µg/bee. Birds are relatively non-sensitive to 1,3-
dichloropropene. LC50s (8-day) of > 10 g/kg were reported for
Mallard duck and Bobwhite quail.
1.1.4 Effects on experimental animals and in vitro test systems
The acute oral toxicity of 1,3-dichloropropene in animals is
moderate to high. The LD50 values reported in rats ranged between
127 and 713 mg/kg body weight. The oral LD50 values in rats for
the cis- and trans-isomers were 85 and 94 mg/kg body weight,
respectively.
Acute dermal exposure is moderately toxic. Dermal LD50s of
423 mg/kg body weight and 504 mg/kg body weight have been reported
for the rat and the rabbit, respectively. The LD50 values for the
cis- and trans-isomers were 1090 and 1575 mg/kg body weight,
respectively.
Inhalation exposure (4 h) of rats indicated LC50s of 3310
mg/m3 (729 ppm) for 1,3-dichloropropene; 3042-3514 mg/m3 for the
cis-isomer, and 4880-5403 mg/m3 for the trans-isomer.
Acute intoxication showed central nervous and respiratory
system involvement.
Severe reactions were seen in rabbit skin and eye irritation
tests, but recovery occurred in 14-21 days. The results of skin
sensitization tests on guinea-pigs were positive.
Several short-term inhalation toxicity studies have been
conducted on mice, rats, guinea-pigs, rabbits, and dogs. In mice,
the nasal mucosa and urinary bladder were the target organs.
Degeneration of the olfactory epithelium and hyperplasia of the
respiratory epithelium were observed. Moderate hyperplasia of the
transitional epithelium in the urinary bladder was found. A no-
observed-effect level (NOEL) of 136 mg/m3 (30 ppm) in mice can be
estimated.
Similar degenerative changes of the olfactory epithelium and
hyperplasia have been demonstrated in rats. The reported NOEL value
for 1,3-dichloropropene from a well-designed study was 45.4 mg/m3;
a NOEL of 136 mg/m3 has been reported for the cis-isomer.
A 90-day oral study on rats indicated a NOEL of 3 mg/kg body
weight. The only observed effect at the next higher dose level of 10
mg/kg body weight was an increase in relative kidney weight in the
male.
In a 2-generation, 2-litter, inhalation study on rats, doses of
up to 408.6 mg/m3 (90 ppm) did not show adverse effects on the
reproduction parameters examined. However, the highest dose level of
408.6 mg/m3 induced maternal toxicity, as evidenced by decreased
growth and histopathological changes in the nasal mucosa. A NOEL of
136.2 mg/m3 (30 ppm) was established for maternal toxicity.
Inhalation teratogenicity studies on rats and rabbits did not
indicate teratogenic potential for 1,3-dichloropropene at exposure
levels up to 1362 mg/m3, but embryotoxicity (reduction in litter
size and increase in resorption rates) was seen in the rat. Maternal
toxicity was observed in both rats and rabbits at dose levels of
544.8 mg/m3 (120 ppm) or more.
In most of the studies, cis- and trans-1,3-dichloropropene
and mixtures were mutagenic in bacteria with, and without, metabolic
activation. Pure 1,3-dichloropropene and pure cis-1,3-
dichloropropene were found to be negative in bacteria. Glutathione
was shown to prevent the mutagenic activity of 1,3-dichloropropene
in bacteria. Cis-1,3-dichloropropene was negative in a gene
mutation assay with V79 Chinese hamster cells as well as in the
Chinese hamster ovary HPRT test.
Cis- and trans-1,3-dichloropropene induced unscheduled DNA
synthesis in HeLa S3 cells. In rat hepatocytes, 1,3-
dichloropropene did not elicit significant DNA repair. 1,3-
Dichloropropene was positive in the Bacillus subtilis strain H17
microsome rec-assay with metabolic activation.
In Chinese hamster ovary cells, cis- and trans-1,3-
dichloropropene induced chromosome damage in the presence of
metabolic activation but, in another study, 1,3-dichloropropene was
positive without metabolic activation. Cis-1,3-dichloropropene did
not induce chromosomal damage in rat liver cells, but induced sister
chromatid exchange in Chinese hamster ovary cells with, and without,
metabolic activation and in Chinese hamster V79 cells without
activation.
1,3-Dichloropropene was negative in a bone marrow micronucleus
test on mice and in a sex-linked, recessive lethal assay on
Drosophila melanogaster.
Carcinogenicity studies were carried out on mice and rats.
Technical 1,3-dichloropropene (containing 1% epichlorhydrin) was
administered by gavage for 2 years. In mice, a significant increase
in epithelial hyperplasia and transitional cell carcinomas in the
urinary bladder, an increase in lung tumours, a slight increase in
tumours of the liver, and an increase in epithelial hyperplasia and
squamous cell papillomas or carcinomas in the forestomach were
found. In rats, increases in the incidence of neoplastic nodules in
the liver and of squamous cell papillomas or carcinomas of the
forestomach were observed.
The carcinogenicity in mice and rats of 1,3-dichloropropene
(without epichlorohydrin) was investigated in 2-year inhalation
studies. In mice, increased incidences of hyperplasia of the urinary
bladder, the forestomach, and the nasal mucosa were observed. There
was an increase in the incidence of benign lung tumours. Some toxic
changes in the olfactory mucosa of the nasal cavity were also seen
in rats, but no increase in tumour incidence.
Epichlorohydrin was shown to produce forestomach tumours in a
gavage study and nasal cavity tumours in an inhalation study on
rats; a carcinogenic effect on the urinary bladder cannot be
excluded for 1,3-dichloropropene administered orally to mice.
Mode of Action
Given that the major metabolic route of elimination of 1,3-
dichloropropene is via conjugation with glutathione, it is to be
expected that situations that affect tissue glutathione (non-protein
sulfhydryl) concentrations may modify the effects of the compound.
1,3-Dichloropropene itself depletes the glutathione content of a
variety of tissues, especially those that are the initial points of
entry into the body, i.e., predominantly the forestomach and liver
following gavage administration, and the nasal tissue after
inhalation exposure. Decreases in nasal epithelium and forestomach
glutathione occurred in mice after inhalation of 1,3-dichloropropene
concentrations exceeding 22.7 mg/m3 (5 ppm) and 113.5 mg/m3 (25
ppm), respectively.
The toxicity of 1,3-dichloropropene in animals occurs at
exposures that deplete glutathione and prior reduction of tissue
glutathione exacerbates it. Long-term inhalation of concentrations
higher than 90.8 mg/m3 (20 ppm) results in degeneration and
hyperplasia of nasal and stomach epithelia in mice, and long-term
inhalation at 272.4 mg/m3 (60 ppm) causes degeneration of nasal
tissue in rats.
The protective role of glutathione has been further highlighted
by studies demonstrating that covalent binding of 14C-1,3-
dichloropropene to mouse forestomach increased as the non-protein
sulfhydryl content decreased. Similarly, in in vitro test systems,
the genotoxicity of 1,3-dichloropropene and its minor oxidative
(cytochrome P-450) metabolite (1,3-dichloropropene oxide) was
markedly ameliorated by glutathione.
1.1.5 Effects on human beings
The exposure of the general population through air, water, or
food is unlikely.
Studies have shown that occupational exposures are generally
below 4.54 mg/m3 (1 ppm), but higher levels have also been
reported (up to 18.3 mg/m3 during filling or nozzle changing).
Occupational exposure is likely to be through inhalation and via the
skin. Irritation of the eyes and the upper respiratory mucosa
appears promptly after exposure. Inhalation of air containing
concentrations of > 6810 mg/m3 (> 1500 ppm) resulted in serious
signs and symptoms of poisoning; lower exposures resulted in
depression of the central nervous system and irritation of the
respiratory system. Dermal exposure caused severe skin irritation.
Some liver and kidney function changes were reported in a group
of 1,3-dichloroprepene applicators at the end of the application
season. However, the cause-effect relationship has been contested.
Some poisoning incidents have occurred in which persons were
hospitalized with signs and symptoms of irritation of the mucous
membrane, chest discomfort, headache, nausea, vomiting, dizziness,
and, occasionally, loss of consciousness and decreased libido. Three
cases of haematological malignancies have been attributed to an
earlier accidental overexposure to 1,3-dichloropropene, but the
cause-effect relationship remains uncertain.
The fertility status of workers employed in the production of
chlorinated three-carbon compounds was compared with a control
group. There was no indication of an association between decreased
fertility and exposure.
1.2 Conclusions
General population: In view of the low or non-existent exposure to
1,3-dichloropropene, the risk to the general population is
negligible.
Occupational exposure: Filling operations and field applications
may lead to operator exposure exceeding the maximum allowable
concentration, when appropriate safety precautions have not been
taken.
Environment: Provided that 1,3-dichloropropene is used at the
recommended rate, it is unlikely to attain levels of environmental
significance and is unlikely to have adverse effects on populations
of terrestrial and aquatic organisms.
1.3 Recommendations
* Filling operations and field applications of 1,3-
dichloropropene should only be conducted taking appropriate
safety precautions, in order to ensure that exposure levels do
not exceed the maximum allowable concentrations of 1,3-
dichloropropene.
* Studies should be conducted to investigate the metabolic fate
of trans-1,3-dichloropropene in mammals and the potential
role that oxidative metabolites of this isomer may have in
mediating 1,3-dichloropropene toxicity.
* Glutathione transferase mediates the protective effect of
glutathione against the toxicity of 1,3-dichloropropene. It is
recommended that studies should be carried out to compare the
relative enzyme kinetics of human glutathione S-transferase
from various tissues with enzyme activity from comparable
animal tissues.
* The available data on the protective role of glutathione should
be consolidated and published in the open literature.
* Part of the genotoxicity of dichloropropene is mediated by
oxidative metabolism. It is recommended that studies be
undertaken to identify the responsible cytochrome P-450
isoenzyme and compare its activity with human P-450 isoenzymes.
* The confounding role of epichlorohydrin in oral gavage
carcinogenicity studies should be clarified.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Primary constituents
Chemical structure
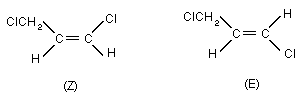 Chemical formula C3H4Cl2
Relative molecular mass 110.98
Chemical name 1,3-dichloropropene; (IUPAC);
dichloro-1,3-propene; (F-ISO);
1,3-dichloro-1-propene; (CA).
Common synonyms gamma-chloroallylchloride,
1,3-dichloropropylene
Trade name TELONE II(R), D-D 92
CAS registry number 542-75-6 ( cis- and trans-isomers)
cis-isomer: 10061-01-5
trans-isomer: 10061-02-6
RTECS registry number UC8310000
EINECS number 208-826-5
The commercial product is a mixture of cis- and trans-
isomers and is more than 92% pure. In the past, 1% epichlorohydrin
was added as a stabilizer, but nowadays an epoxidized vegetable oil
is used.
Other names are: Dedisol C, Nematox II, D-D 95, Telone 2000
(Hayes, 1982; Worthing & Hance, 1991).
2.2 Physical and chemical properties
Freezing point - 85 °Ca ( cis-isomer)
Boiling point 103.8-105.2 °C ( cis-isomer)a
111.0-112.0 °C ( trans-isomer)b
108.0 °C (1,3-dichloropropene)
Vapour pressure at 25 °C 4850 Pa ( cis-isomer)a
3560 Pa ( trans-isomer)b
3.7 kPa (20 °C) (1,3-dichloropropene)
Relative density (D 23/4) 1.221 kg/litre ( cis-isomer)a
(D 20/4) 1.214 kg/litre ( trans-isomer)b
Water solubility 2.45 ( cis-isomer)a
(at 20 °C, in g/litre) 2.49 ( trans-isomer)b
2.0 (1,3-dichloropropene)
Flash point 28.5 °C ( cis-isomer)a
28.0 °C ( trans-isomer)b
25.0 °C (1,3-dichloropropene)
Self-ignition 555 °C ( cis-isomer)a
534 °C ( trans-isomer)b
Log P octanol/water 1.82 at 20 °C ( cis-isomer)a
partition coefficient 2.22 at 25 °C ( trans-isomer)b
1.4-2.0 (1,3-dichloropropene)
K(OM/Vc 14 ( cis-isomer)
15 ( trans-isomer)
K (OM/V)c 14 ( cis-isomer)
15 ( trans-isomer)
a purity 98.1%;
b purity 96.7%;
c K(OM/V) = µg adsorbed per g of organic matter (soil)
µg dissolved per ml water phase
From: Leistra (1970), Krijgsheld & van der Gen (1986), Bennett &
Ridge (1989), Schuurman (1989), van Hooidonk (1989), O'Connor
(1990a).
Neither the cis- nor the trans-isomer produces gas in
contact with water, and they are not highly flammable in contact
with diatomite.
1.3-Dichloropropene is a colourless to amber coloured liquid
with a penetrating, irritating, chloroform-like odour. The technical
product is > 92% pure. The physical properties of a cis/trans
mixture depend on the ratio of the isomers (Yang, 1986).
Saturated atmosphere: 167 980 mg/m3 (37 000 ppm) at 25 °C.
Explosive limit: 195 220 mg/m3 (43 000 ppm) (80 °C). Miscible with
acetone, benzene, carbon tetrachloride, heptane, and methanol
(Sittig, 1980; Hayes, 1982; Worthing & Hance, 1991).
Van Hooidonk (1989) and O'Connor (1990a) described methods to
determine the water- and/or fat solubility of cis- and trans-
1,3-dichloropropene using gas chromatography and ECD and/or FID
detection.
Details on ultraviolet/visible, infrared, and nuclear magnetic
resonance spectra are given by O'Connor (1990a).
2.3 Conversion factors
1 ppm (91.2% 1,3-dichloropropene) = 4.54 mg/m3 at 25 °C at 1
atm (Krijgsheld & Van der Gen, 1986; Breslin et al., 1987).
2.4 Analytical methods
Methods have been developed for the determination of 1,3-
dichloropropene ( cis- and trans-isomers) and of 1,2-
dichloropropane in air, soil, water, and crops, and the degradation
product 3-chloroallyl alcohol ( cis- and trans-isomers) in soil
and crops (see Tables 1 and 2). Current methods are based on gas
chromatography (GC).
2.4.1 Sampling
In the case of crops and soil, the need for special care in the
handling of samples and extracts has been stressed, because of the
high volatility of 1,3-dichloropropene.
To minimize loss of residue by volatilization, soil samples
should be deep frozen as soon as possible after sampling, and
shipped to the laboratory for analysis in sealed containers with a
minimum of delay (Rexilius & Schmidt, 1982). The period of storage
of deep frozen samples in the laboratory should also be kept as
short as possible (Wallace, 1979). At -20 °C, Hermann & Matsuyama
(1982) found a slow decline in the contents of all components of
"MIX D/D", indicating a maximum acceptable storage period of 2
months. No loss occurred in 4 months at a temperature of -80 °C.
Table 1. Methods of analysis for 1,3-dichloropropene and 1,2-dichloropropane in food and biological media
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantitation determination
Crops, steam distillation absorption chromatography gas chromatography -a 0.01 mg/kg Rexilius & Schmidt
Soil and diethyl ether on acidic alumina with ECD and FID (1,3-dichloropropene) (1982); Shell (1985);
extraction 0.1 mg/kg Wallace (1974)
(1,2-dichloropropane)
trapped in - gas chromatography -a - Shell (1980)
ethyl acetate with ECD
Water steam distillation absorption chromatography gas chromatography -a 0.001 mg/kg Shell (1985)
and diethyl ether on acidic alumina with ECD (1,2-dichloropropane)
extraction
Air - absorption on Tenax GC, gas chromatography -a -a Leiber & Berk (1984)
desorption with isooctane with ECD
Air - absorption on charcoal, gas chromatography 90-100% 0.005 mg/m3 Van Sittert et al.
desorption with with FID (1977); Sherren &
carbon disulfide Woodbridge (1987a,b)
Air - absorption on charcoal, gas chromatography 85% 23 ngb Albrecht et al.
desorption with with ECD (1986)
methanol/benzene
Blood hexane - gas chromatography 90% cis and trans Kastl & Hermann
with 63Ni-ECD or 1,3-dichloropropene, (1983)
GS-MS (SIM) 5.3-5.9 ng/litre
a Data on recovery and/or limit of determination not given.
b Given as mass/tube.
Table 2. Methods of analysis for 3-chloroallyl alcohol in food and biological media
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantitation determination
Crop, diethyl ether derivatization with 3,5- gas chromatography - crops: 0.05 mg/kg Rexilius & Schmidt
Soil dinitrobenzoyl chloride with ECD soil: 0.02 mg/kg (1982)
and pyridine, absorption Wallace (1974)
chromatography on acidic
alumina
Crop, steam-distillation, esterification with capillary gas- - - Shell (1978)
Soil, hexane extraction trifluoroacetic chromatography - -
Water with diethyl ether anhydride with ECD - water: 0.002 mg/kg Shell (1985)
Crop samples should be deep frozen as soon as possible after
sampling, and water samples should be chilled or deep frozen; both
should be shipped and stored under the same precautions as soil
(Wallace, 1976b; Rexilius & Schmidt, 1982).
2.4.2 Determination of residues in crops and soil
A combined method for the determination and confirmation of 1,3-
dichloropropene, 1,2-dichloropropane, and chloroallyl alcohol (3-
CAA) in crops and soil has been developed (Wallace, 1974; Shell,
1976). After steam distillation and extraction and clean up, the
determination of residues is carried out using gas chromatography
(electron capture (ECD) and flame ionization (FID)). The chloroallyl
alcohol is derivatized, followed by a clean up and determination
using ECD. Confirmation of the identity of residues is carried out
by combined gas chromatography-mass spectrometry (GC-MS).
With this method, the lower limit of determination in most crop
and soil samples is 0.01 mg/kg for 1,3-dichloropropene and 0.1 mg/kg
for 1,2-dichloropropane. For 3-chloroallyl alcohol, the lower limit
of determination is 0.05 mg/kg for crops and 0.02 mg/kg for soil
(Wallace, 1974; Rexilius & Schmidt, 1982; Shell, 1985).
Alternative methods are described by Shell (1980) in which 1,3-
dichloropropene and 1,2-dichloropropane are trapped in ethyl acetate
and directly determined, without clean up by capillary GC with ECD.
The 3-chloroallyl alcohol residues are steam-distilled without acid
or alkali and "free residues" are washed with hexane, and extracted
into diethyl ether. The alcohol residues are then esterified by
trifluoroacetic anhydride and determined with capillary GC with ECD
(Shell, 1978).
Shell (1984) described a method based on the previously
mentioned techniques of extraction and preparation of extracts;
however, in both crops and soil, residues are determined by
capillary GC with a Hall electrolytic conductivity detector (HECD).
In addition, residues of 3-chloroallyl alcohol are determined
without derivatization. The lower limit of determination is 0.01
mg/kg.
2.4.3 Determination of residues in water
The methods described in section 2.4.2 can be adapted for the
determination of residues of 1,3-dichloropropene, 1,2-
dichloropropane, and 3-chloroallyl alcohol in water (Wallace, 1974).
The alternative methods mentioned under section 2.4.2 also include
procedures for water analysis (Wallace, 1974; Shell, 1978) (see
Table 1).
A laboratory analytical method (US EPA method 524.2), developed
to monitor drinking-water, involves a standard inert (helium) gas
purge extraction, isolation on a solid-phase trap (gas
chromatography with a fused silica capillary column (FSCC) coated
with a film of cyanopropylphenyl-dimethylpolysiloxane polymer),
thermal desorption, and gas chromatography and identification and
measurement with a low-cost, bench-top ion trap detector (ITD),
which functions as a mass spectrometer. At a concentration of 0.2
µg/litre, the total mean measurement accuracy was 99% for trans-
1,3-dichloropropene ( cis-isomer not measured) and 103% for 1,2-
dichloropropane (Eichelberger et al., 1990).
Telliard (1990) described broad-range methods for the
determination of pollutants in waste water. US EPA method 1624 is
used to determine purgeable organic compounds by calibrated isotope
dilution or internal standard GC-MS and by reverse search of a GS-MS
run for the analytes. The first technique can be used to determine
1,2-dichloropropane and the second, 1,3-dichloropropene.
2.4.4 Determination of residues in air
Methods based on the use of solid absorbent traps or direct gas
sampling procedures in conjunction with GC analysis have been
described for the determination of 1,3-dichloropropenes and 1,2-
dichloropropane in air.
Leiber & Berk (1984) used Tenax-GC as an absorbent to monitor
concentrations of chlorinated aliphatic hydrocarbons in workspace
air. Isooctane, containing 1,3,5-tribromobenzene as internal
standard, was used for the desorption of the hydrocarbons.
Recoveries of 1,3-dichloropropenes were in the range of 1.8-18
mg/m3. A similar method was used by Van Sittert et al. (1977) and
Albrecht et al. (1986), but, in this case, the trapping medium was
activated charcoal. It appears that charcoal had a better trapping
capacity than Tenax-GC (Brown & Purnell, 1979) for 1,3-
dichloropropenes. Trapped vapours were desorbed using carbon
disulfide (recovery 90-100%) (van Sittert et al., 1977; HSE, 1990)
or 1% v/v methanol-benzene mixture (mean recovery 85%) (Albrecht et
al., 1986). Van Sittert et al. (1977) could determine 0.05 mg/m3
of the cis- and trans-isomers of 1,3-dichloropropene in air.
All authors warned that care should be taken in the handling of
trapped samples.
Parker et al. (1982) used charcoal filters to determine 1,3-
dichloropropene and 1,2-dichloropropane levels in air.
Others have used more direct gas sampling procedures. Air from
the head space above soil and water in sealed containers has been
sampled and directly determined by GC with ECD or FID. Gas samples
were trapped by injecting the air into an organic solvent, such as
xylene or hexane, before GC analysis (Williams, 1968; Leistra, 1970;
Abdalla, 1974; Abdalla et al., 1974; McKenry & Thomason, 1974; van
Dijk, 1980).
2.4.5 Determination of residues in food
Reinert et al. (1983) described a dynamic heated headspace
analysis of organic compounds including 1,2-dichloropropane in fish
and shellfish tissue samples. The method included solvent (carbon
disulfide) desorption of activated carbon adsorbent and
determination with capillary column gas chromatography with a flame
ionization detector. Recoveries were rather low (approximately 40-
70%). Hiatt (1983) described a vacuum distillation apparatus and a
procedure developed for the analysis of fish tissue. The volatile
compounds were distilled from the sample and characterized by gas
chromatography/mass spectrometry using fused silica capillary column
(FSCC).
A method was described by Daft (1989) to determine fumigants and
related chemicals in fatty and non-fatty foods. The method started
with liquid extraction with isooctane, when necessary with co-
extraction with a mixture of acetone/NaCl in 25% phosphoric acid and
isooctane. The isooctane extracts were analysed using gas
chromatography. Excess fat was removed by micro-Florisil columns.
The determination was done by ECD and HECD (Hall electroconductivity
detection). Overall mean recovery was 73% from fatty foods and 78%
from non-fatty foods; the recovery from both sample types after
further Florisil chromatography was 55%.
2.4.6 Determination of 3-chloroallyl alcohol
In Table 2, analytical methods are described to determine 3-
chloroallyl alcohol in food and biological media.
2.4.7 Determination of mercapturic acids in urine
In Table 3, methods are described to determine metabolites of
1,3-dichloropropene in urine.
Van Welie et al. (1989) used an analytical method to determine
N-acetyl- S-( cis- and trans)-3-chloroprop-2-enyl-L-cysteine
( cis- and trans-DCP-MA) in urine, based on capillary gas
chromatography with sulfur-selective detection. An internal standard
N-acetyl- S-(benzyl)-L-cysteine and hydrochloric acid (resulting
in a pH 1-2) were added to urine samples. The samples were extracted
with ethyl acetate and the latter evaporated; the residues were
methylated and determined using gas chromatography-flame photometric
detection (GC-FPD). GC-MS was used for identification. The limits of
determination were 0.107 mg/litre for cis-DCP-MA and 0.115
mg/litre for trans-DCP-MA.
Table 3. Methods of analysis for metabolites of 1,3-dichloropropene in urine
Sample Extraction Clean-up Detection and Recovery Limit of Reference
derivatization derivatization quantitation determination
N-acetyl-S[cis-chloroprop-2-enyl]cysteine
Urine ether derivatization with gas chromatography with - - Osterloh et
(human) diazomethane etherate electron impact ionization al. (1984)
silicone membrane
separator, mass spectrometry
Urine ethyl acetate derivatization with gas chromatography with cis-isomer and for cis- and van Welie
(human) diazomethane etherate fused silica WCOT columns, trans-isomer trans-isomer range et al. (1989)
sulfur-selective detection 105% 107-115 ng/ml
Urine ethyl acetate derivatization with gas chromatography with cis-isomer for the different Onkenhout et
(rat) diazomethane nitrogen selective detection 66-83% methods and for al. (1986)
or negative chemical trans-isomer cis- and trans-isomer
ionization/mass spectrometry 56-85% range 20-550 ng/ml
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
As far as is known, 1,3-dichloropropene does not occur
naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
1,3-Dichloropropene is produced by the high-temperature
chlorination of propylene or from 1,3-dichloro-2-propanol by
dehydration with POCl3 or with P2O5 in benzene.
1,3-Dichloropropene is a by-product in the synthesis of allyl
chloride; 1,2-dichloropropane and to a lesser extent, 2,3-
dichloropropene are also formed. In some commercial products,
marketed for soil fumigation (mix D/D, Telone), 1,3-dichloropropene
is the major and active ingredient (50-80% of total), but 1,2-
dichloropropane (20-40%) and 2,3-dichloropropene (5-6.5%) are also
present (Krijgsheld & Van der Gen, 1986).
Before 1978, about 25 000 tonnes of 1,3-dichloropropene were
produced annually in the USA (Flessel et al., 1978). In Italy, 2187
tonnes were produced in 1972 (De Lorenzo et al., 1977). Over 1285
tonnes of 1,3-dichloropropene-containing pesticides were used in
California in 1971 (Yang, 1986), while in the period 1970-77, the
amount applied was approximately 1.8-2.7 million kg. In 1981, over
7.2 million kg of 1,2-dichloropropane- and 1,3-dichloropropene-
containing fumigants were used in California (California State Water
Resources Control Board, 1983).
The estimated production in Europe in 1979 was 6-7
kilotonnes/year.
1,2-Dichloropropane, present as an impurity in the fumigant,
does not add to the desired biological effects, but may, on the
contrary, have unwanted ecotoxicological consequences. Therefore,
there has been a more recent development to stop the use of the
"impure" fumigant and to move to a purer preparation of 1,3-
dichloropropene (> 90%) (Krijgsheld & Van der Gen, 1986).
3.2.2 Use
1,3-Dichloropropene, the main ingredient of Telone II, was
introduced in 1956 as a commercial preplant soil fumigant for the
control of nematodes in crops, such as vegetables, potatoes, and
tobacco. It is applied from a tractor-drawn, high pressure injection
system into the soil. The soil is treated prior to the planting of
crops (De Lorenzo et al., 1977; Hayes, 1982; Maddy et al., 1982).
1,3-Dichloropropene is effective against soil nematodes
including root-knot, meadow, sting and dagger, spiral and sugar beet
nematodes. The rates of application are determined according to the
crop to be grown and the soil conditions, but generally lie within
the range of 75-200 kg/ha (occasional maximum of 700 kg/ha)
(Krijgsheld & van der Gen, 1986; Shell, IPM, 1990).
3.2.3 Sources of pollution
1,3-Dichloropropene is used extensively as a soil fumigant for
the treatment of agricultural land. After application, part of the
chemical will evaporate and escape from the soil. Although
significant biodegradation and abiotic decomposition will occur in
the soil, there is a limited risk of leaching down to groundwater
level (see section 4.1.3). The 1,3-dichloropropene that is used for
fumigation is contaminated with 1,2-dichloropropane and 2,3-
dichloropropene. At application rates of "MIX D/D" ranging from 200
to 400 kg/ha, this may mean an input of 40-160 kg of 1,2-
dichloropropane and 10-25 kg of 2,3-dichloropropene per hectare of
land (Krijgsheld & van der Gen, 1986). The potential for groundwater
contamination has been reduced by reducing the 1,2-dichloropropane
content of the products used in agriculture.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As with other fumigants, the performance of 1,3-dichloropropene
as a nematocide is dependent on a number of important factors
influencing the movement of soil fumigants, e.g., the chemical and
adsorptive characteristics of the toxicant (vapour pressure,
solubility, diffusion coefficient, the distribution of the fumigant
through air, water, and solid phases of the soil) and physical
factors, such as temperature, moisture, organic matter, soil
texture, and soil profile variability (Munnecke & van Gundy, 1979;
NTP, 1985; Yang, 1986).
Dichloropropenes can enter the aquatic environment as discharges
from industrial effluents, through run off from agricultural land,
and from municipal effluents.
The stability and mobility of 1,3-dichloropropene and 1,2-
dichloropropane in air, soil, and groundwater are influenced by
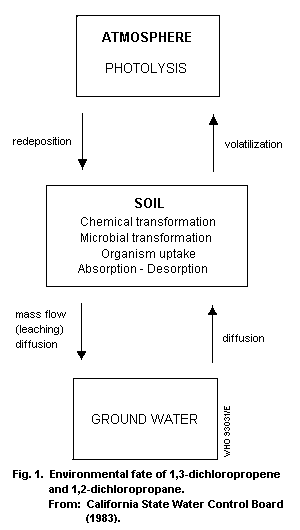
several processes, as shown in Fig. 1.
4.1 Transport and distribution between media
(See also section 4.1 of 1,2-dichloropropane and subsections).
4.1.1 Air
Tuazon et al. (1984) calculated that, at a daytime OH-radical
concentration of 2 x 106/cm3 (8 x 10-8 ppm) in the
troposphere, the half-lives of cis- and trans-1,3-
dichloropropene would be 12 and 7 h, respectively. The half-life for
1,2-dichloropropane is > 313 days for a 24-h average OH-radical
concentration of 1 x 106/cm3. For the reaction with ozone at a
background level in the troposphere of 80 µg/m3 (0.04 ppm), the
half-lives of cis- and trans-1,3 dichloropropene, were
calculated to be 52 and 12 days. Direct phototransformation seems to
be insignificant compared with the two other reactions, but may be
enhanced in the presence of atmospheric particulates.
4.1.2 Water
Since the chloropropenes have a relatively low water solubility
and high volatility, they will have a tendency to disappear rapidly
from an aqueous medium. The half-life of evaporation of a chemical
from a certain body of water will increase with the depth of the
water and continuous evaporation will become increasingly dependent
on sufficient agitation in the water. Evaporation can be expected to
contribute significantly to the disappearance from the aquatic
environment (Krijgsheld & van der Gen, 1986).
Chemical formula C3H4Cl2
Relative molecular mass 110.98
Chemical name 1,3-dichloropropene; (IUPAC);
dichloro-1,3-propene; (F-ISO);
1,3-dichloro-1-propene; (CA).
Common synonyms gamma-chloroallylchloride,
1,3-dichloropropylene
Trade name TELONE II(R), D-D 92
CAS registry number 542-75-6 ( cis- and trans-isomers)
cis-isomer: 10061-01-5
trans-isomer: 10061-02-6
RTECS registry number UC8310000
EINECS number 208-826-5
The commercial product is a mixture of cis- and trans-
isomers and is more than 92% pure. In the past, 1% epichlorohydrin
was added as a stabilizer, but nowadays an epoxidized vegetable oil
is used.
Other names are: Dedisol C, Nematox II, D-D 95, Telone 2000
(Hayes, 1982; Worthing & Hance, 1991).
2.2 Physical and chemical properties
Freezing point - 85 °Ca ( cis-isomer)
Boiling point 103.8-105.2 °C ( cis-isomer)a
111.0-112.0 °C ( trans-isomer)b
108.0 °C (1,3-dichloropropene)
Vapour pressure at 25 °C 4850 Pa ( cis-isomer)a
3560 Pa ( trans-isomer)b
3.7 kPa (20 °C) (1,3-dichloropropene)
Relative density (D 23/4) 1.221 kg/litre ( cis-isomer)a
(D 20/4) 1.214 kg/litre ( trans-isomer)b
Water solubility 2.45 ( cis-isomer)a
(at 20 °C, in g/litre) 2.49 ( trans-isomer)b
2.0 (1,3-dichloropropene)
Flash point 28.5 °C ( cis-isomer)a
28.0 °C ( trans-isomer)b
25.0 °C (1,3-dichloropropene)
Self-ignition 555 °C ( cis-isomer)a
534 °C ( trans-isomer)b
Log P octanol/water 1.82 at 20 °C ( cis-isomer)a
partition coefficient 2.22 at 25 °C ( trans-isomer)b
1.4-2.0 (1,3-dichloropropene)
K(OM/Vc 14 ( cis-isomer)
15 ( trans-isomer)
K (OM/V)c 14 ( cis-isomer)
15 ( trans-isomer)
a purity 98.1%;
b purity 96.7%;
c K(OM/V) = µg adsorbed per g of organic matter (soil)
µg dissolved per ml water phase
From: Leistra (1970), Krijgsheld & van der Gen (1986), Bennett &
Ridge (1989), Schuurman (1989), van Hooidonk (1989), O'Connor
(1990a).
Neither the cis- nor the trans-isomer produces gas in
contact with water, and they are not highly flammable in contact
with diatomite.
1.3-Dichloropropene is a colourless to amber coloured liquid
with a penetrating, irritating, chloroform-like odour. The technical
product is > 92% pure. The physical properties of a cis/trans
mixture depend on the ratio of the isomers (Yang, 1986).
Saturated atmosphere: 167 980 mg/m3 (37 000 ppm) at 25 °C.
Explosive limit: 195 220 mg/m3 (43 000 ppm) (80 °C). Miscible with
acetone, benzene, carbon tetrachloride, heptane, and methanol
(Sittig, 1980; Hayes, 1982; Worthing & Hance, 1991).
Van Hooidonk (1989) and O'Connor (1990a) described methods to
determine the water- and/or fat solubility of cis- and trans-
1,3-dichloropropene using gas chromatography and ECD and/or FID
detection.
Details on ultraviolet/visible, infrared, and nuclear magnetic
resonance spectra are given by O'Connor (1990a).
2.3 Conversion factors
1 ppm (91.2% 1,3-dichloropropene) = 4.54 mg/m3 at 25 °C at 1
atm (Krijgsheld & Van der Gen, 1986; Breslin et al., 1987).
2.4 Analytical methods
Methods have been developed for the determination of 1,3-
dichloropropene ( cis- and trans-isomers) and of 1,2-
dichloropropane in air, soil, water, and crops, and the degradation
product 3-chloroallyl alcohol ( cis- and trans-isomers) in soil
and crops (see Tables 1 and 2). Current methods are based on gas
chromatography (GC).
2.4.1 Sampling
In the case of crops and soil, the need for special care in the
handling of samples and extracts has been stressed, because of the
high volatility of 1,3-dichloropropene.
To minimize loss of residue by volatilization, soil samples
should be deep frozen as soon as possible after sampling, and
shipped to the laboratory for analysis in sealed containers with a
minimum of delay (Rexilius & Schmidt, 1982). The period of storage
of deep frozen samples in the laboratory should also be kept as
short as possible (Wallace, 1979). At -20 °C, Hermann & Matsuyama
(1982) found a slow decline in the contents of all components of
"MIX D/D", indicating a maximum acceptable storage period of 2
months. No loss occurred in 4 months at a temperature of -80 °C.
Table 1. Methods of analysis for 1,3-dichloropropene and 1,2-dichloropropane in food and biological media
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantitation determination
Crops, steam distillation absorption chromatography gas chromatography -a 0.01 mg/kg Rexilius & Schmidt
Soil and diethyl ether on acidic alumina with ECD and FID (1,3-dichloropropene) (1982); Shell (1985);
extraction 0.1 mg/kg Wallace (1974)
(1,2-dichloropropane)
trapped in - gas chromatography -a - Shell (1980)
ethyl acetate with ECD
Water steam distillation absorption chromatography gas chromatography -a 0.001 mg/kg Shell (1985)
and diethyl ether on acidic alumina with ECD (1,2-dichloropropane)
extraction
Air - absorption on Tenax GC, gas chromatography -a -a Leiber & Berk (1984)
desorption with isooctane with ECD
Air - absorption on charcoal, gas chromatography 90-100% 0.005 mg/m3 Van Sittert et al.
desorption with with FID (1977); Sherren &
carbon disulfide Woodbridge (1987a,b)
Air - absorption on charcoal, gas chromatography 85% 23 ngb Albrecht et al.
desorption with with ECD (1986)
methanol/benzene
Blood hexane - gas chromatography 90% cis and trans Kastl & Hermann
with 63Ni-ECD or 1,3-dichloropropene, (1983)
GS-MS (SIM) 5.3-5.9 ng/litre
a Data on recovery and/or limit of determination not given.
b Given as mass/tube.
Table 2. Methods of analysis for 3-chloroallyl alcohol in food and biological media
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantitation determination
Crop, diethyl ether derivatization with 3,5- gas chromatography - crops: 0.05 mg/kg Rexilius & Schmidt
Soil dinitrobenzoyl chloride with ECD soil: 0.02 mg/kg (1982)
and pyridine, absorption Wallace (1974)
chromatography on acidic
alumina
Crop, steam-distillation, esterification with capillary gas- - - Shell (1978)
Soil, hexane extraction trifluoroacetic chromatography - -
Water with diethyl ether anhydride with ECD - water: 0.002 mg/kg Shell (1985)
Crop samples should be deep frozen as soon as possible after
sampling, and water samples should be chilled or deep frozen; both
should be shipped and stored under the same precautions as soil
(Wallace, 1976b; Rexilius & Schmidt, 1982).
2.4.2 Determination of residues in crops and soil
A combined method for the determination and confirmation of 1,3-
dichloropropene, 1,2-dichloropropane, and chloroallyl alcohol (3-
CAA) in crops and soil has been developed (Wallace, 1974; Shell,
1976). After steam distillation and extraction and clean up, the
determination of residues is carried out using gas chromatography
(electron capture (ECD) and flame ionization (FID)). The chloroallyl
alcohol is derivatized, followed by a clean up and determination
using ECD. Confirmation of the identity of residues is carried out
by combined gas chromatography-mass spectrometry (GC-MS).
With this method, the lower limit of determination in most crop
and soil samples is 0.01 mg/kg for 1,3-dichloropropene and 0.1 mg/kg
for 1,2-dichloropropane. For 3-chloroallyl alcohol, the lower limit
of determination is 0.05 mg/kg for crops and 0.02 mg/kg for soil
(Wallace, 1974; Rexilius & Schmidt, 1982; Shell, 1985).
Alternative methods are described by Shell (1980) in which 1,3-
dichloropropene and 1,2-dichloropropane are trapped in ethyl acetate
and directly determined, without clean up by capillary GC with ECD.
The 3-chloroallyl alcohol residues are steam-distilled without acid
or alkali and "free residues" are washed with hexane, and extracted
into diethyl ether. The alcohol residues are then esterified by
trifluoroacetic anhydride and determined with capillary GC with ECD
(Shell, 1978).
Shell (1984) described a method based on the previously
mentioned techniques of extraction and preparation of extracts;
however, in both crops and soil, residues are determined by
capillary GC with a Hall electrolytic conductivity detector (HECD).
In addition, residues of 3-chloroallyl alcohol are determined
without derivatization. The lower limit of determination is 0.01
mg/kg.
2.4.3 Determination of residues in water
The methods described in section 2.4.2 can be adapted for the
determination of residues of 1,3-dichloropropene, 1,2-
dichloropropane, and 3-chloroallyl alcohol in water (Wallace, 1974).
The alternative methods mentioned under section 2.4.2 also include
procedures for water analysis (Wallace, 1974; Shell, 1978) (see
Table 1).
A laboratory analytical method (US EPA method 524.2), developed
to monitor drinking-water, involves a standard inert (helium) gas
purge extraction, isolation on a solid-phase trap (gas
chromatography with a fused silica capillary column (FSCC) coated
with a film of cyanopropylphenyl-dimethylpolysiloxane polymer),
thermal desorption, and gas chromatography and identification and
measurement with a low-cost, bench-top ion trap detector (ITD),
which functions as a mass spectrometer. At a concentration of 0.2
µg/litre, the total mean measurement accuracy was 99% for trans-
1,3-dichloropropene ( cis-isomer not measured) and 103% for 1,2-
dichloropropane (Eichelberger et al., 1990).
Telliard (1990) described broad-range methods for the
determination of pollutants in waste water. US EPA method 1624 is
used to determine purgeable organic compounds by calibrated isotope
dilution or internal standard GC-MS and by reverse search of a GS-MS
run for the analytes. The first technique can be used to determine
1,2-dichloropropane and the second, 1,3-dichloropropene.
2.4.4 Determination of residues in air
Methods based on the use of solid absorbent traps or direct gas
sampling procedures in conjunction with GC analysis have been
described for the determination of 1,3-dichloropropenes and 1,2-
dichloropropane in air.
Leiber & Berk (1984) used Tenax-GC as an absorbent to monitor
concentrations of chlorinated aliphatic hydrocarbons in workspace
air. Isooctane, containing 1,3,5-tribromobenzene as internal
standard, was used for the desorption of the hydrocarbons.
Recoveries of 1,3-dichloropropenes were in the range of 1.8-18
mg/m3. A similar method was used by Van Sittert et al. (1977) and
Albrecht et al. (1986), but, in this case, the trapping medium was
activated charcoal. It appears that charcoal had a better trapping
capacity than Tenax-GC (Brown & Purnell, 1979) for 1,3-
dichloropropenes. Trapped vapours were desorbed using carbon
disulfide (recovery 90-100%) (van Sittert et al., 1977; HSE, 1990)
or 1% v/v methanol-benzene mixture (mean recovery 85%) (Albrecht et
al., 1986). Van Sittert et al. (1977) could determine 0.05 mg/m3
of the cis- and trans-isomers of 1,3-dichloropropene in air.
All authors warned that care should be taken in the handling of
trapped samples.
Parker et al. (1982) used charcoal filters to determine 1,3-
dichloropropene and 1,2-dichloropropane levels in air.
Others have used more direct gas sampling procedures. Air from
the head space above soil and water in sealed containers has been
sampled and directly determined by GC with ECD or FID. Gas samples
were trapped by injecting the air into an organic solvent, such as
xylene or hexane, before GC analysis (Williams, 1968; Leistra, 1970;
Abdalla, 1974; Abdalla et al., 1974; McKenry & Thomason, 1974; van
Dijk, 1980).
2.4.5 Determination of residues in food
Reinert et al. (1983) described a dynamic heated headspace
analysis of organic compounds including 1,2-dichloropropane in fish
and shellfish tissue samples. The method included solvent (carbon
disulfide) desorption of activated carbon adsorbent and
determination with capillary column gas chromatography with a flame
ionization detector. Recoveries were rather low (approximately 40-
70%). Hiatt (1983) described a vacuum distillation apparatus and a
procedure developed for the analysis of fish tissue. The volatile
compounds were distilled from the sample and characterized by gas
chromatography/mass spectrometry using fused silica capillary column
(FSCC).
A method was described by Daft (1989) to determine fumigants and
related chemicals in fatty and non-fatty foods. The method started
with liquid extraction with isooctane, when necessary with co-
extraction with a mixture of acetone/NaCl in 25% phosphoric acid and
isooctane. The isooctane extracts were analysed using gas
chromatography. Excess fat was removed by micro-Florisil columns.
The determination was done by ECD and HECD (Hall electroconductivity
detection). Overall mean recovery was 73% from fatty foods and 78%
from non-fatty foods; the recovery from both sample types after
further Florisil chromatography was 55%.
2.4.6 Determination of 3-chloroallyl alcohol
In Table 2, analytical methods are described to determine 3-
chloroallyl alcohol in food and biological media.
2.4.7 Determination of mercapturic acids in urine
In Table 3, methods are described to determine metabolites of
1,3-dichloropropene in urine.
Van Welie et al. (1989) used an analytical method to determine
N-acetyl- S-( cis- and trans)-3-chloroprop-2-enyl-L-cysteine
( cis- and trans-DCP-MA) in urine, based on capillary gas
chromatography with sulfur-selective detection. An internal standard
N-acetyl- S-(benzyl)-L-cysteine and hydrochloric acid (resulting
in a pH 1-2) were added to urine samples. The samples were extracted
with ethyl acetate and the latter evaporated; the residues were
methylated and determined using gas chromatography-flame photometric
detection (GC-FPD). GC-MS was used for identification. The limits of
determination were 0.107 mg/litre for cis-DCP-MA and 0.115
mg/litre for trans-DCP-MA.
Table 3. Methods of analysis for metabolites of 1,3-dichloropropene in urine
Sample Extraction Clean-up Detection and Recovery Limit of Reference
derivatization derivatization quantitation determination
N-acetyl-S[cis-chloroprop-2-enyl]cysteine
Urine ether derivatization with gas chromatography with - - Osterloh et
(human) diazomethane etherate electron impact ionization al. (1984)
silicone membrane
separator, mass spectrometry
Urine ethyl acetate derivatization with gas chromatography with cis-isomer and for cis- and van Welie
(human) diazomethane etherate fused silica WCOT columns, trans-isomer trans-isomer range et al. (1989)
sulfur-selective detection 105% 107-115 ng/ml
Urine ethyl acetate derivatization with gas chromatography with cis-isomer for the different Onkenhout et
(rat) diazomethane nitrogen selective detection 66-83% methods and for al. (1986)
or negative chemical trans-isomer cis- and trans-isomer
ionization/mass spectrometry 56-85% range 20-550 ng/ml
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
As far as is known, 1,3-dichloropropene does not occur
naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
1,3-Dichloropropene is produced by the high-temperature
chlorination of propylene or from 1,3-dichloro-2-propanol by
dehydration with POCl3 or with P2O5 in benzene.
1,3-Dichloropropene is a by-product in the synthesis of allyl
chloride; 1,2-dichloropropane and to a lesser extent, 2,3-
dichloropropene are also formed. In some commercial products,
marketed for soil fumigation (mix D/D, Telone), 1,3-dichloropropene
is the major and active ingredient (50-80% of total), but 1,2-
dichloropropane (20-40%) and 2,3-dichloropropene (5-6.5%) are also
present (Krijgsheld & Van der Gen, 1986).
Before 1978, about 25 000 tonnes of 1,3-dichloropropene were
produced annually in the USA (Flessel et al., 1978). In Italy, 2187
tonnes were produced in 1972 (De Lorenzo et al., 1977). Over 1285
tonnes of 1,3-dichloropropene-containing pesticides were used in
California in 1971 (Yang, 1986), while in the period 1970-77, the
amount applied was approximately 1.8-2.7 million kg. In 1981, over
7.2 million kg of 1,2-dichloropropane- and 1,3-dichloropropene-
containing fumigants were used in California (California State Water
Resources Control Board, 1983).
The estimated production in Europe in 1979 was 6-7
kilotonnes/year.
1,2-Dichloropropane, present as an impurity in the fumigant,
does not add to the desired biological effects, but may, on the
contrary, have unwanted ecotoxicological consequences. Therefore,
there has been a more recent development to stop the use of the
"impure" fumigant and to move to a purer preparation of 1,3-
dichloropropene (> 90%) (Krijgsheld & Van der Gen, 1986).
3.2.2 Use
1,3-Dichloropropene, the main ingredient of Telone II, was
introduced in 1956 as a commercial preplant soil fumigant for the
control of nematodes in crops, such as vegetables, potatoes, and
tobacco. It is applied from a tractor-drawn, high pressure injection
system into the soil. The soil is treated prior to the planting of
crops (De Lorenzo et al., 1977; Hayes, 1982; Maddy et al., 1982).
1,3-Dichloropropene is effective against soil nematodes
including root-knot, meadow, sting and dagger, spiral and sugar beet
nematodes. The rates of application are determined according to the
crop to be grown and the soil conditions, but generally lie within
the range of 75-200 kg/ha (occasional maximum of 700 kg/ha)
(Krijgsheld & van der Gen, 1986; Shell, IPM, 1990).
3.2.3 Sources of pollution
1,3-Dichloropropene is used extensively as a soil fumigant for
the treatment of agricultural land. After application, part of the
chemical will evaporate and escape from the soil. Although
significant biodegradation and abiotic decomposition will occur in
the soil, there is a limited risk of leaching down to groundwater
level (see section 4.1.3). The 1,3-dichloropropene that is used for
fumigation is contaminated with 1,2-dichloropropane and 2,3-
dichloropropene. At application rates of "MIX D/D" ranging from 200
to 400 kg/ha, this may mean an input of 40-160 kg of 1,2-
dichloropropane and 10-25 kg of 2,3-dichloropropene per hectare of
land (Krijgsheld & van der Gen, 1986). The potential for groundwater
contamination has been reduced by reducing the 1,2-dichloropropane
content of the products used in agriculture.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As with other fumigants, the performance of 1,3-dichloropropene
as a nematocide is dependent on a number of important factors
influencing the movement of soil fumigants, e.g., the chemical and
adsorptive characteristics of the toxicant (vapour pressure,
solubility, diffusion coefficient, the distribution of the fumigant
through air, water, and solid phases of the soil) and physical
factors, such as temperature, moisture, organic matter, soil
texture, and soil profile variability (Munnecke & van Gundy, 1979;
NTP, 1985; Yang, 1986).
Dichloropropenes can enter the aquatic environment as discharges
from industrial effluents, through run off from agricultural land,
and from municipal effluents.
The stability and mobility of 1,3-dichloropropene and 1,2-
dichloropropane in air, soil, and groundwater are influenced by
several processes, as shown in Fig. 1.
4.1 Transport and distribution between media
(See also section 4.1 of 1,2-dichloropropane and subsections).
4.1.1 Air
Tuazon et al. (1984) calculated that, at a daytime OH-radical
concentration of 2 x 106/cm3 (8 x 10-8 ppm) in the
troposphere, the half-lives of cis- and trans-1,3-
dichloropropene would be 12 and 7 h, respectively. The half-life for
1,2-dichloropropane is > 313 days for a 24-h average OH-radical
concentration of 1 x 106/cm3. For the reaction with ozone at a
background level in the troposphere of 80 µg/m3 (0.04 ppm), the
half-lives of cis- and trans-1,3 dichloropropene, were
calculated to be 52 and 12 days. Direct phototransformation seems to
be insignificant compared with the two other reactions, but may be
enhanced in the presence of atmospheric particulates.
4.1.2 Water
Since the chloropropenes have a relatively low water solubility
and high volatility, they will have a tendency to disappear rapidly
from an aqueous medium. The half-life of evaporation of a chemical
from a certain body of water will increase with the depth of the
water and continuous evaporation will become increasingly dependent
on sufficient agitation in the water. Evaporation can be expected to
contribute significantly to the disappearance from the aquatic
environment (Krijgsheld & van der Gen, 1986).
 Dilling et al. (1975) determined the rate of evaporation of 1,3-
dichloropropene ( cis- and trans-) from water at the 1 mg/litre
level under ambient conditions. The time required for the compound
to be reduced by 50% was 31 min and by 90%, 98 min.
Yon et al. (1991) determined that the half-life of evaporation
of 1,3-dichloropropene ( cis- and trans-isomers) from water was
less than 5 h.
4.1.3 Soil
The persistence of 1,3-dichloropropene depends on chemical
degradation, volatilization, microbial transformation, photochemical
transformation, type of soil, water content of soil, and uptake into
organisms. Thomason & McKenry (1974) studied the quantitative as
well as the qualitative aspects of the movement and fate of 1,3-
dichloropropene under various conditions in different types of soil.
Since 1,3-dichloropropene is used as soil fumigant, some
information is available on the distribution of the compound in
soil. The adsorption of 1,3-DCP on soil was found to be proportional
to the organic matter content of the soil. The K(om/v)sa for cis-
and trans-1,3-dichloropropene were estimated to be 14 and 15,
respectively, independent of ambient temperature (Leistra, 1970).
Similar soil/water distribution coefficients (23 and 26), based on
organic carbon content, were reported by Kenaga (1980).
McKenry & Thomason (1974) demonstrated that high soil moisture
was a major limiting factor in the total diffusion when soil
moisture in the field approached field capacity. In contrast,
Munnecke & van Gundy (1979) stated that soil moisture was a very
important factor in that gaseous compounds are most effective in
killing organisms when they are in a moist environment.
Environmental transformation of 1,3-dichloropropene results from
microbial action, with the exception of the initial hydrolysis of
cis- and trans-1,3-dichloropropene to 3-chloroallyl alcohol
(Castro & Belser, 1966; Belser & Castro, 1971). The pathway for the
transformation of 1,3-dichloropropene is given in Fig. 2.
a See section 2.2.
4.1.3.1 Hydrolysis
Cis- and trans-1,3-dichloropropene can be hydrolysed in soil
to 3-chloroallyl alcohol (see Fig. 2). Hydrolysis rates for 1,3-
dichloropropenes range from 1 to 3.4% per day, depending on
temperature and moisture content. Hydrolysis rates also vary with
soil type (particle size) because of differences in chemical
diffusion rate and sorption capacity (California State Water
Resources Control Board, 1983).
Using [14C]-radiolabelled 1.3-dichloropropene in sterile
buffered water at pH 5, 7, or 9 and temperatures of 10, 20, or 30
°C, McCall (1987) found that the rate of hydrolysis was independent
of pH at each temperature, and that the half-lives at temperatures
of 30, 20, and 10 °C were 3.1, 11.3, and 51 days, respectively. One
hydrolysis product, formed during the course of the study, was
identified as 3-chloroallyl alcohol. The alcohol appeared to be
stable to further hydrolytic conversion and was formed in the same
cis-:trans-ratio as the initial 1,3-dichloropropene.
The hydrolysis of cis-1,3-dichloropropene (98.1%) was studied
by O'Connor (1990b). The degradation reactions at all pH values were
shown to follow pseudo first-order behaviour in the EEC-test,
independent of the concentration. The degradation rate constants and
environmental half-lives for cis-1,3-dichloropropene at 25 °C at
pH 4, pH 7, and pH 9 (extrapolated by measuring degradation at
temperatures of 50, 60, and 70 °C, using Arrhenius relationships)
were 100 h, 54.5 h, and 38 h, respectively. (Remark: although the
rate of hydrolysis of cis-1,3-dichloropropene did show some slight
pH dependence, the author stated that this was probably within
experimental error). It is hypothesized that the degradation
proceeds via a resonance stabilized carbonium ion intermediate,
resulting in the formation of a mixture of 3-chloroallyl alcohol and
propenal (see Fig. 3).
Connors et al. (1990) studied the hydrolysis of 1,3-
dichloropropene into 3-chloroallyl alcohol, under laboratory
conditions. A 1.0 µg/litre cis- and trans-1,3-dichloropropene
solution was prepared in a pH 5.5 or pH 7.0 buffer. The half-lives
for the cis- and trans-isomers at 15 and 29 °C (pH 5.5) were
11.0, 2.0 and 13.0, 2.0 days, respectively. At pH 7.0 and 25 °C, the
value was 4.6 days for both isomers.
Dilling et al. (1975) determined the rate of evaporation of 1,3-
dichloropropene ( cis- and trans-) from water at the 1 mg/litre
level under ambient conditions. The time required for the compound
to be reduced by 50% was 31 min and by 90%, 98 min.
Yon et al. (1991) determined that the half-life of evaporation
of 1,3-dichloropropene ( cis- and trans-isomers) from water was
less than 5 h.
4.1.3 Soil
The persistence of 1,3-dichloropropene depends on chemical
degradation, volatilization, microbial transformation, photochemical
transformation, type of soil, water content of soil, and uptake into
organisms. Thomason & McKenry (1974) studied the quantitative as
well as the qualitative aspects of the movement and fate of 1,3-
dichloropropene under various conditions in different types of soil.
Since 1,3-dichloropropene is used as soil fumigant, some
information is available on the distribution of the compound in
soil. The adsorption of 1,3-DCP on soil was found to be proportional
to the organic matter content of the soil. The K(om/v)sa for cis-
and trans-1,3-dichloropropene were estimated to be 14 and 15,
respectively, independent of ambient temperature (Leistra, 1970).
Similar soil/water distribution coefficients (23 and 26), based on
organic carbon content, were reported by Kenaga (1980).
McKenry & Thomason (1974) demonstrated that high soil moisture
was a major limiting factor in the total diffusion when soil
moisture in the field approached field capacity. In contrast,
Munnecke & van Gundy (1979) stated that soil moisture was a very
important factor in that gaseous compounds are most effective in
killing organisms when they are in a moist environment.
Environmental transformation of 1,3-dichloropropene results from
microbial action, with the exception of the initial hydrolysis of
cis- and trans-1,3-dichloropropene to 3-chloroallyl alcohol
(Castro & Belser, 1966; Belser & Castro, 1971). The pathway for the
transformation of 1,3-dichloropropene is given in Fig. 2.
a See section 2.2.
4.1.3.1 Hydrolysis
Cis- and trans-1,3-dichloropropene can be hydrolysed in soil
to 3-chloroallyl alcohol (see Fig. 2). Hydrolysis rates for 1,3-
dichloropropenes range from 1 to 3.4% per day, depending on
temperature and moisture content. Hydrolysis rates also vary with
soil type (particle size) because of differences in chemical
diffusion rate and sorption capacity (California State Water
Resources Control Board, 1983).
Using [14C]-radiolabelled 1.3-dichloropropene in sterile
buffered water at pH 5, 7, or 9 and temperatures of 10, 20, or 30
°C, McCall (1987) found that the rate of hydrolysis was independent
of pH at each temperature, and that the half-lives at temperatures
of 30, 20, and 10 °C were 3.1, 11.3, and 51 days, respectively. One
hydrolysis product, formed during the course of the study, was
identified as 3-chloroallyl alcohol. The alcohol appeared to be
stable to further hydrolytic conversion and was formed in the same
cis-:trans-ratio as the initial 1,3-dichloropropene.
The hydrolysis of cis-1,3-dichloropropene (98.1%) was studied
by O'Connor (1990b). The degradation reactions at all pH values were
shown to follow pseudo first-order behaviour in the EEC-test,
independent of the concentration. The degradation rate constants and
environmental half-lives for cis-1,3-dichloropropene at 25 °C at
pH 4, pH 7, and pH 9 (extrapolated by measuring degradation at
temperatures of 50, 60, and 70 °C, using Arrhenius relationships)
were 100 h, 54.5 h, and 38 h, respectively. (Remark: although the
rate of hydrolysis of cis-1,3-dichloropropene did show some slight
pH dependence, the author stated that this was probably within
experimental error). It is hypothesized that the degradation
proceeds via a resonance stabilized carbonium ion intermediate,
resulting in the formation of a mixture of 3-chloroallyl alcohol and
propenal (see Fig. 3).
Connors et al. (1990) studied the hydrolysis of 1,3-
dichloropropene into 3-chloroallyl alcohol, under laboratory
conditions. A 1.0 µg/litre cis- and trans-1,3-dichloropropene
solution was prepared in a pH 5.5 or pH 7.0 buffer. The half-lives
for the cis- and trans-isomers at 15 and 29 °C (pH 5.5) were
11.0, 2.0 and 13.0, 2.0 days, respectively. At pH 7.0 and 25 °C, the
value was 4.6 days for both isomers.
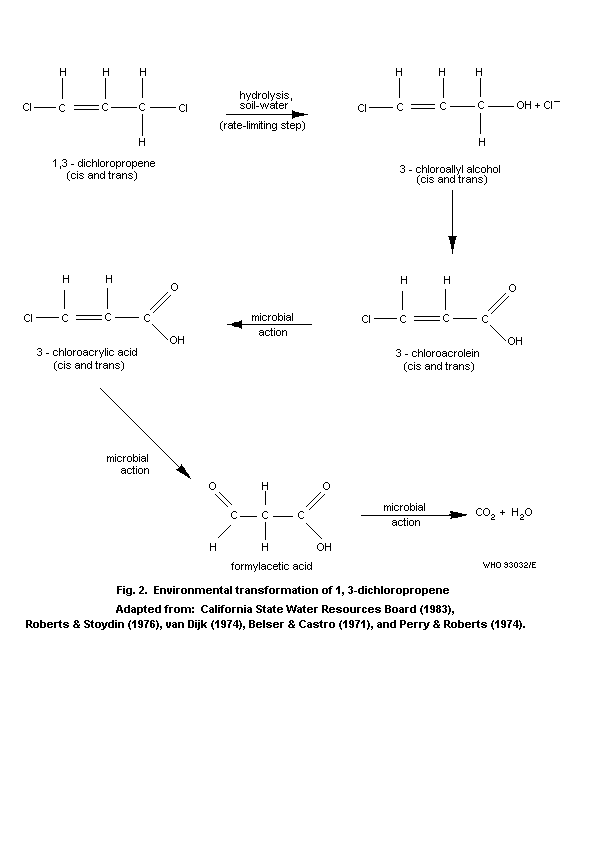
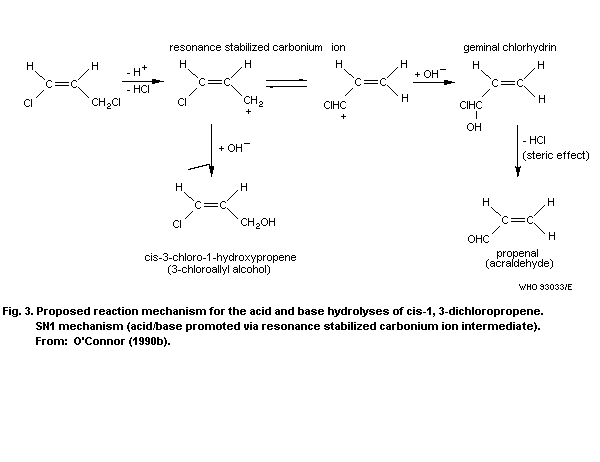 Determination of the rate of hydrolysis of 1,3-dichloropropene
at 25 °C in 50% aqueous ethanol indicated a half-time of 4 days for
both the cis- and trans-isomers and appeared independent of the
concentration in the range of 10-1000 mg/litre. Only small
differences were observed in disappearance rates at pH levels of 5.5
and 7.5. The effect of temperature was clearly demonstrated: at 29
°C, the half-life for cis-1,3-dichloropropene was 1.5-2.0 days,
while, at 2 °C, the half-life was estimated to be 91-100 days
(Krijgsheld & Van der Gen, 1986).
The rates of transformation of the cis- and trans-isomers in
soil layers of 0.1-0.2 m and 0.4-0.5 m in a bulb field in the
Netherlands were determined in the laboratory. The initial contents
of added 1,3-dichloropropene were approximately 12 and 62 mg/kg.
Incubation took place at 15 °C. The half transformation time was
about 4 days for both isomers. After 2 weeks, only small amounts
(1%) of the initial amount were left. The transformation was slower
in soil with the higher initial content (62 mg/kg) than in soil with
12 mg/kg. The half-life was approximately 19 days for both isomers.
Only small amounts were left after one month (Van der Pas & Leistra,
1987).
The behaviour of technical grade 1,3-dichloropropene in the soil
from 4 fields (soil containing 13.2-24.6% of organic matter) was
studied in the laboratory. The transformation rates of cis- and
trans-1,3-dichloropropene were measured in soil samples taken from
the ploughed layer of the fields. Pure 1,3-dichloropropene was added
at 35 µlitre/kg moist soil. The transformation in soil from one of
the fields could be approximated with first- order kinetics during
the whole incubation period of 21 days. The half-lives of the cis-
and trans-isomers at 10 °C were 17 and 20 days, respectively. In
soil from the 3 other fields, transformation of 1,3-dichloropropene
with approximate first-order kinetics in the initial period of 7-14
days was followed by a period of accelerated transformation. The
concentration dropped below the limit of determination (0.1 mg/kg
dry soil), 14-21 days after the start of the incubation. Presumably,
soil microorganisms adapted their enzymes, resulting in an increased
rate of transformation (Van den Berg & Leistra, 1989).
In 6 loamy soils, transformation was gradual and pseudo first-
order for 3-6 days, and then, very rapid. There was no difference
between the transformation of the cis- and trans-isomers of 1,3-
dichloropropene in these soils. When the initial content in dry soil
was 62-80 mg/kg, less than 0.2% remained after a week (temperature
15 °C). The greatly accelerated transformation that occurred after a
short time lag suggests that the soils contained microorganisms that
could transform 1,3-dichloropropene effectively (Smelt et al.,
1989).
Rapid transformation was found in 6 loamy soils from fields
fumigated once or twice previously, as well as from fields never
treated; after 7 days, less than 0.2% of the applied dose (3.7, 18,
or 92 mg 1,3-dichloropropene/kg) remained. The incubation
temperature was 15 °C. However, with an initial content of 470
mg/kg, the transformation was suppressed with a half-life of 33
days. In another loamy soil, which showed no accelerated
transformation pattern, the pseudo half-lives increased from 4.3 to
36 days, when initial content of 1,3-dichloropropene was raised from
3.7 to 470 mg/kg (Smelt et al., 1989).
4.1.3.2 Volatilization
Volatilization and diffusion in the vapour phase are the most
significant mechanisms for the environmental dispersal and dilution
of 1,3-dichloropropene and 1,2-dichloropropane. Volatilization rates
from soil surfaces depend on water solubility and vapour density as
well as on soil properties, such as temperature and moisture
content, the depth of application, and surface wind velocity.
Estimates of volatilization of cis-1,3-dichloropropene from soil
have ranged from 20 to 75%.
D-D 92 was applied to sandy clay loam soil in a polyethylene
tunnel and the air in the tunnel was monitored continuously for 1,3-
dichloropropene for 4 weeks. The temperature in the tunnel was 18-29
°C. D-D 92 was injected by hand at a dose rate of 225 kg/ha, at a
depth of 15 cm. About 45% of the applied D-D 92 was volatilized as
1,3-dichloropropene in the first week, increasing to 54% after 4
weeks. No more than 5% was found as 1,3-dichloropropene or 3-
chloroallyl alcohol in the soil at the end of the 4-week period
(Sherren & Woodbridge, 1987c).
4.1.3.3 Uptake in crops
Residues in edible crops arising from the use of "MIX D/D" or
1,3-dichloropropene have only been detected in small amounts (<
0.02 mg/kg). The most obvious reason for this is the fact that crops
are not normally planted until most of the product has been
eliminated. Under certain conditions, where low concentrations of
1,3-dichloropropene persist for long periods of time, plants will
absorb measurable quantities. Uptake has been shown to occur in
potato tubers in sandy loam soil treated with 14C-1,2-
dichloropropane and 14C-1,3-dichloropropene 6 months prior to
planting (application rate 290 litre/ha). The total radioactivity
(expressed as 1,3-dichloropropene equivalents) in the tubers was 7
µg/kg (Roberts & Stoydin, 1976).
Tomatoes, bush beans, and carrots absorbed 14C-1,3-
dichloropropene from vermiculite culture solution and sand. During
24 h, the compound was absorbed and translocated through the plants.
3-Chloroallyl alcohol was also readily absorbed, but to a lesser
extent than dichloropropene. Comparison of the metabolism of 1,3-
dichloropropene and 3-chloroallyl alcohol showed rapid reversion to
the general carbon pool, the half-lives for 1,3-dichloropropene and
3-chloroallyl alcohol being 1.5 and 4.4 h, respectively (Berry et
al., 1980).
4.1.3.4 Movement in soil
Vapour diffusion is usually the most important mode of downward
movement for "MIX D/D". McKenry & Thomason (1974) injected either
Telone or "MIX D/D" into a series of soils at 11 different sites in
California. The moisture levels, temperatures, cultivation, and soil
profiles at the sites varied. The movement was studied during 13 and
69 days. The application rates ranged from 600 up to 2300 kg/ha. It
was concluded that:
* There was a substantial and downward movement of all the
components.
* Downward movement was greatest in open-textured soils that were
sufficiently moist but not saturated; the fumigant was
detectable at a depth of a few metres.
* Downward movement was encouraged by deep cultivation in soils
with horizons of low porosity.
In the United Kingdom, however, Wallace (1979) found only traces
of fumigant in the 40-60 cm layer, after an injection at a depth of
18 cm. Wallace (1976a) had found comparable results in soil in
Germany. In the European studies, the diffusion was slower, because
the applications were made in late autumn; soils were wetter,
colder, and heavier in texture. Thus, results from studies carried
out under different agronomic and climatic conditions are not
necessarily comparable.
The vertical and horizontal movements of 1,3-dichloropropene
were studied in a tree-nursery region in the north of the Federal
Republic of Germany. Sounding pipes were used to collect water
samples down to a depth of 4 m using the percussion-boring method.
Further borings were set to a depth of 3 m on days 10-91 after
application of a formulation containing cis- and trans-1,3-
dichloropropene, methylisothiocyanate and 1,2-dichloropropane at 50
ml/m2. Soil cores were analysed. 1,3-Dichloropropene showed a
rather high mobility in the soil, as it could be detected at a depth
of 4 m in all soil layers on the fourth day of application. In
samples of the near-surface groundwater, collected 140 days after
application, a concentration of 1.36 µg 1,3-dichloropropene per
litre was found. Ten to 25 m from the treated area, 1,3-
dichloropropene was also found in groundwater after 59 and 140 days
(Rexilius & Schmidt, 1982).
4.1.3.5 Loss under field conditions
Williams (1968) studied the loss of 1,3-dichloropropene under
field conditions in sandy loam and peat soils in Canada. The
application rates were approximately 1000 and 2000 litre "Mix
D/D"/ha, respectively. Eight months later, samples were collected
and residues determined (Table 4).
In studies in the Federal Republic of Germany, Netherlands, and
the United Kingdom, only very low residues (1%) of the amount
originally applied remained after 3 months in the soil (Wallace,
1976a,b; Wallace, 1979).
A comparative trial was carried out in the United Kingdom in
which "MIX D/D" and 1,3-dichloropropene were injected, at a depth of
15 cm, in clay loam at concentrations of 410 and 240 litre/ha,
respectively (Table 5, see also section 4.3.2 of "MIX D/D"). Samples
of soil were taken at depths of 0-20 cm, 20-40 cm, and 40-60 cm, at
6 intervals up to 9´ months after application. As part of normal
recommended agricultural practice, the soil was ploughed 5 weeks
after treatment. Soil samples were analysed for residues of cis-
and trans-1,3-dichloropropene, 1,2-dichloropropane, and cis- and
trans-3-chloroallyl alcohol. There was no significant difference
between the residues of the 1,3-dichloropropene or the 3-chloroallyl
alcohol resulting from the 2 treatments. As expected, no 1,2-
dichloropropane residues were detected in soil samples treated with
1,3-dichloropropene. Residues of the cis- and trans-1,3-
dichloropropenes and cis- and trans-3-chloroallyl alcohols were
detected in all samples up to 9´ months after treatment and down to
the 20-40 cm soil layer. Before the soil was ploughed, the
concentrations of these substances showed little change, and they
were present in all 3 layers, but, after ploughing, the
concentrations decreased gradually (Wallace, 1979).
Table 4. Recovery of cis- and trans-1,3-dichloropropene from
sandy loam or peat soils, 8 months after application of 1000 or 2000
litre "MIX D/D"/ha, respectively
Soil Depth in cm Residue in mg/kg soil
cis-1,3- trans-1,3-
dichloropropene dichloropropene
Peat 0-10 1.4 3.2
10-20 1.8 4.8
Sandy loam 0-10 - -
10-20 0.3 0.4
From: Williams (1968)
Table 5. Residues from the plot treated with 1,3-dichloropropene at 240 litre/haa
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 2.02 2.54 < 0.1 1.01 1.01
20-40 5.98 7.32 0.2 3.16 3.34
40-60 0.14 0.15 < 0.1 1.57b 1.88b
10 0-20 6.29 7.66 0.1 1.23 1.23
20-40 1.79 2.10 < 0.1 1.09 1.14
40-60 0.52 0.55 < 0.1 3.01b 3.24b
23 0-20 6.10 6.10 0.2 2.39 2.39
20-40 3.26 3.20 0.2 1.32 1.32
40-60 0.09 0.08 < 0.1 0.04 0.04
34 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.95 1.10 < 0.1 0.45 0.45
20-40 0.97 0.90 < 0.1 0.62 0.62
40-60 0.06 0.04 < 0.1 < 0.02 < 0.02
67 0-20 0.28 0.36 < 0.1 0.70 0.70
20-40 0.04 0.05 < 0.1 0.32 0.26
40-60 0.11 0.09 < 0.1 0.05 0.04
Table 5 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.08 0.06 < 0.1 0.20 0.20
9´ months 20-40 0.02c 0.02c < 0.1 0.04 0.03
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
Pre-treatment 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a From: Wallace (1979).
Note: All residues are on a dry weight basis.
b Anomalous results.
c Results confirmed by GC/MS.
1,3-Dichloropropene (D-D 95 and Telone II, containing > 92%),
at concentrations of 240, 280, and 290 litre/ha, was injected into
the soil of 3 bulb fields in the Netherlands in the summer. Nine
points were sampled per field and the samples were taken at various
times down to a depth of 3 m. Within a month, the concentrations
decreased to less than 0.2 mg/kg and continued to decline gradually
with time (Van der Pas & Leistra, 1987).
In 2 fields in the Netherlands (soil containing 15.7-24.6% of
organic matter), the spread of the fumigant (application rate 150
litre/ha) through the soil was measured. Only low fumigant
concentrations (about 0.1-0.4 mg/kg) were measured at a depth of 0.3
m. Around the depth of injection (0.15-0.2 m), the ratio of cis-
and trans-isomers changed with time in favour of the trans-
isomer. Cumulative emissions into the air over a period of 3 weeks
were calculated to range from 10 to 20% of the dosage of the cis-
isomer, and 4 to 15% of the trans-isomer (Van den Berg & Leistra,
1989).
4.1.3.6 Results of supervised field trials
A field study was undertaken in France in 1988, in which D-D 92
was applied to the soil prior to planting vines, and the air in the
vicinity of the treated area was monitored for 1,3-dichloropropene.
D-D 92 was applied at approximately 600 kg/ha at a depth of 30-40
cm. The air levels were monitored for 10 days. No samples contained
1,2-dichloropropane at levels above the limit of determination of
0.02 mg/m3. The highest 1,3-dichloropropene concentration found
during the first 24 h (perimeter of the field) was 2.1 mg/m3 and
this declined to 0.02-0.04 mg/m3 after 10 days. Air concentrations
also decreased with increasing distance, downwind (Sherren, 1990).
4.2 Bioconcentration
No data are available on bioconcentration.
4.3 Abiotic degradation
4.3.1 Photodegradation
Li (1979) obtained results comparable with those of Tuazon et
al. (1984) working with ozone, by irradiation of vapour of cis-
and trans-1,3-dichloropropene with a GE-RS sunlamp (see section
4.1.1). The main reaction product was 3-chloropropionyl chloride
with smaller quantities of 3-chloropropionic acid, CO2, and
phosgene. In this process, the initial reaction was epoxidation of
the double bond. There is evidence of the importance of a surface
reaction in the atmosphere, adsorption on to particulate matter
seems to be necessary for an appreciable direct phototransformation
to occur. Vapour phase photolysis of 1,3-dichloropropene was not
detected after prolonged simulated sunlight irradiation in a
reaction chamber. Photolysis occurred on the photoreactor surface
walls suggesting surface-catalysing reactions. The reaction products
suggest that 12-13% was totally degraded to CO2 after 5 days of
irradiation. Over 20% was transformed to phosgene.
No data on the photolytic decomposition of the chloropropenes
in water are available. Nevertheless, UVR of these chemicals in
methanol, in a frozen state, or as inclusion in adamantine matrices,
may cause the production of allyl radicals, by cleavage of the
allylic C-Cl bond (Krijgsheld & van der Gen, 1986).
4.4 Biodegradation and biotransformation
Several studies have been performed on the persistence of 1,3-
DCP in soil, after application as a fumigant. Biodegradation by soil
microorganisms does occur, depending on soil type, temperature, and
moisture content. The rate of disappearance ranges from a half-life
of 3 days to one of 37 days, without any consistent correlation with
organic matter content of the soil, or with pH. In sterile soils,
the effect of temperature was minimal (Van Dijk, 1974; Tabak et al.,
1981; California State Water Resources Control Board, 1983). In
general, the rates of disappearance of the cis- and trans-
isomers are similar and tend to increase with moisture content and
temperature, conditions that may increase, not only biodegradation,
but also loss by volatilization or chemical hydrolysis. Although
between 15 and 80% decomposition of field applications of 1,3-
dichloropropene has been shown, the large amount that can be
absorbed (80-90%) can result in soil residues existing months after
application is completed (Van Dijk, 1974; Roberts & Stoydin, 1976;
Sittig, 1980; Krijgsheld & van der Gen, 1986).
In biodegradability studies using a synthetic medium that
contained 5 mg of yeast extract/litre and was inoculated with waste
water, loss of 1,3-dichloropropene was determined after 7 days of
incubation. Significant degradation was observed at 5 and 10 mg of
1,3-dichloropropene/litre and gradual adaptation was shown in
subcultures. The original culture degraded about 50% of the 1,3-
dichloropropene in 7 days, while the third subculture was able to
degrade approximately 85% at both substrate concentrations, in the
same period of time (Tabak et al., 1981).
Battersby (1990a) determined the "ready biodegradability" of
trans-1,3-dichloropropene (95.4% trans- and 0.3% cis-isomer)
using the closed bottle procedure. The substance was not degraded in
this system with a negligible proportion of the theoretical oxygen
demand being consumed during the 28-day incubation period.
The EEC-activated sludge respiration inhibition test was used
to determine the effect of a cis- (51.2-52.2%) + trans- (43.9-
44.1%) mixture of 1,3-dichloropropene containing 0.33% of 1,2-
dichloropropane on the respiration rate of activated sludge. The
EC50 for this mixture was 188 mg/litre (Battersby, 1990b).
The EEC-activated sludge respiration inhibition test was also
used to determine the effect of cis-1,3-dichloropropene (94.5-
97.5% cis-, 1.5% trans-isomer and 0.25% 1,2-dichloropropane) on
the respiration rate of activated sludge. The EC50 for the cis-
1,3-dichloropropene was 279 mg/litre (Battersby, 1990c).
Biodehalogenation by soil organisms has been demonstrated for
1,3-dichloropropene. The fumigant appeared to be chemically
hydrolysed to 3-chloroallyl alcohol and then converted to 3-
chloroacrylic acid. The chlorine is removed and the intermediate
products are converted to carbon dioxide and water. The rate of
disappearance of 1,3-dichloropropene at 15-20 °C was 2-3.5% per day
in sandy soil and up to 25% per day in clay soils. The chloroallyl
alcohol disappeared at rates of 20-60% per day at 15 °C (Van Dijk,
1974). Leistra et al. (1991) incubated 1,3-dichloropropene and its
transformation product 3-chloroallyl alcohol in water-saturated
subsoil material at 10 °C. The times for 50% and 95% transformation
ranged from 15 to 47 days and from 27 to 79 days, respectively, for
1,3-dichloropropene. The corresponding 50% and 95% transformation
times for 3 chloroallyl alcohol were 0.8-4.2 and 4.0-6.5 days,
respectively.
Chemical hydrolysis is the first step in the transformation of
1,3-dichloropropene. Further transformation is thought to result
from microbial action; 3-chloroacrolein and 3-chloroacrylic acid
have been isolated from the metabolism of 3-chloroallyl alcohol by
Pseudomonas species (see Fig. 4) (Belser & Castro, 1971; Roberts &
Stoydin, 1976).
Soil culture studies using media enriched with 1,3-
dichloropropenes, 1,2-dichloropropane, and "Mix D/D" at
concentrations of up to 100 mg/kg, produced abundant growth of all
microorganisms tested, indicating the use of the fumigants as carbon
sources. Several of these organisms (Rhizobium leguminosarum,
Bacillus subtilis, Arthrobacter globiformis, and Pseudomonas
fluorescens) produced greater amounts of amino acids (Altman &
Lawlor, 1966; Altman, 1969). The cis- and trans-isomers of 1,3-
dichloropropene have undergone biodehalogenation by a Pseudomonas
sp. isolated from the soil. Cis- and trans-1,3-dichloropropene
can be chemically hydrolysed in moist soils to the corresponding 3-
chloroallyl alcohols, which can be metabolized to carbon dioxide and
water by Pseudomonas sp. (Fig. 4).
Determination of the rate of hydrolysis of 1,3-dichloropropene
at 25 °C in 50% aqueous ethanol indicated a half-time of 4 days for
both the cis- and trans-isomers and appeared independent of the
concentration in the range of 10-1000 mg/litre. Only small
differences were observed in disappearance rates at pH levels of 5.5
and 7.5. The effect of temperature was clearly demonstrated: at 29
°C, the half-life for cis-1,3-dichloropropene was 1.5-2.0 days,
while, at 2 °C, the half-life was estimated to be 91-100 days
(Krijgsheld & Van der Gen, 1986).
The rates of transformation of the cis- and trans-isomers in
soil layers of 0.1-0.2 m and 0.4-0.5 m in a bulb field in the
Netherlands were determined in the laboratory. The initial contents
of added 1,3-dichloropropene were approximately 12 and 62 mg/kg.
Incubation took place at 15 °C. The half transformation time was
about 4 days for both isomers. After 2 weeks, only small amounts
(1%) of the initial amount were left. The transformation was slower
in soil with the higher initial content (62 mg/kg) than in soil with
12 mg/kg. The half-life was approximately 19 days for both isomers.
Only small amounts were left after one month (Van der Pas & Leistra,
1987).
The behaviour of technical grade 1,3-dichloropropene in the soil
from 4 fields (soil containing 13.2-24.6% of organic matter) was
studied in the laboratory. The transformation rates of cis- and
trans-1,3-dichloropropene were measured in soil samples taken from
the ploughed layer of the fields. Pure 1,3-dichloropropene was added
at 35 µlitre/kg moist soil. The transformation in soil from one of
the fields could be approximated with first- order kinetics during
the whole incubation period of 21 days. The half-lives of the cis-
and trans-isomers at 10 °C were 17 and 20 days, respectively. In
soil from the 3 other fields, transformation of 1,3-dichloropropene
with approximate first-order kinetics in the initial period of 7-14
days was followed by a period of accelerated transformation. The
concentration dropped below the limit of determination (0.1 mg/kg
dry soil), 14-21 days after the start of the incubation. Presumably,
soil microorganisms adapted their enzymes, resulting in an increased
rate of transformation (Van den Berg & Leistra, 1989).
In 6 loamy soils, transformation was gradual and pseudo first-
order for 3-6 days, and then, very rapid. There was no difference
between the transformation of the cis- and trans-isomers of 1,3-
dichloropropene in these soils. When the initial content in dry soil
was 62-80 mg/kg, less than 0.2% remained after a week (temperature
15 °C). The greatly accelerated transformation that occurred after a
short time lag suggests that the soils contained microorganisms that
could transform 1,3-dichloropropene effectively (Smelt et al.,
1989).
Rapid transformation was found in 6 loamy soils from fields
fumigated once or twice previously, as well as from fields never
treated; after 7 days, less than 0.2% of the applied dose (3.7, 18,
or 92 mg 1,3-dichloropropene/kg) remained. The incubation
temperature was 15 °C. However, with an initial content of 470
mg/kg, the transformation was suppressed with a half-life of 33
days. In another loamy soil, which showed no accelerated
transformation pattern, the pseudo half-lives increased from 4.3 to
36 days, when initial content of 1,3-dichloropropene was raised from
3.7 to 470 mg/kg (Smelt et al., 1989).
4.1.3.2 Volatilization
Volatilization and diffusion in the vapour phase are the most
significant mechanisms for the environmental dispersal and dilution
of 1,3-dichloropropene and 1,2-dichloropropane. Volatilization rates
from soil surfaces depend on water solubility and vapour density as
well as on soil properties, such as temperature and moisture
content, the depth of application, and surface wind velocity.
Estimates of volatilization of cis-1,3-dichloropropene from soil
have ranged from 20 to 75%.
D-D 92 was applied to sandy clay loam soil in a polyethylene
tunnel and the air in the tunnel was monitored continuously for 1,3-
dichloropropene for 4 weeks. The temperature in the tunnel was 18-29
°C. D-D 92 was injected by hand at a dose rate of 225 kg/ha, at a
depth of 15 cm. About 45% of the applied D-D 92 was volatilized as
1,3-dichloropropene in the first week, increasing to 54% after 4
weeks. No more than 5% was found as 1,3-dichloropropene or 3-
chloroallyl alcohol in the soil at the end of the 4-week period
(Sherren & Woodbridge, 1987c).
4.1.3.3 Uptake in crops
Residues in edible crops arising from the use of "MIX D/D" or
1,3-dichloropropene have only been detected in small amounts (<
0.02 mg/kg). The most obvious reason for this is the fact that crops
are not normally planted until most of the product has been
eliminated. Under certain conditions, where low concentrations of
1,3-dichloropropene persist for long periods of time, plants will
absorb measurable quantities. Uptake has been shown to occur in
potato tubers in sandy loam soil treated with 14C-1,2-
dichloropropane and 14C-1,3-dichloropropene 6 months prior to
planting (application rate 290 litre/ha). The total radioactivity
(expressed as 1,3-dichloropropene equivalents) in the tubers was 7
µg/kg (Roberts & Stoydin, 1976).
Tomatoes, bush beans, and carrots absorbed 14C-1,3-
dichloropropene from vermiculite culture solution and sand. During
24 h, the compound was absorbed and translocated through the plants.
3-Chloroallyl alcohol was also readily absorbed, but to a lesser
extent than dichloropropene. Comparison of the metabolism of 1,3-
dichloropropene and 3-chloroallyl alcohol showed rapid reversion to
the general carbon pool, the half-lives for 1,3-dichloropropene and
3-chloroallyl alcohol being 1.5 and 4.4 h, respectively (Berry et
al., 1980).
4.1.3.4 Movement in soil
Vapour diffusion is usually the most important mode of downward
movement for "MIX D/D". McKenry & Thomason (1974) injected either
Telone or "MIX D/D" into a series of soils at 11 different sites in
California. The moisture levels, temperatures, cultivation, and soil
profiles at the sites varied. The movement was studied during 13 and
69 days. The application rates ranged from 600 up to 2300 kg/ha. It
was concluded that:
* There was a substantial and downward movement of all the
components.
* Downward movement was greatest in open-textured soils that were
sufficiently moist but not saturated; the fumigant was
detectable at a depth of a few metres.
* Downward movement was encouraged by deep cultivation in soils
with horizons of low porosity.
In the United Kingdom, however, Wallace (1979) found only traces
of fumigant in the 40-60 cm layer, after an injection at a depth of
18 cm. Wallace (1976a) had found comparable results in soil in
Germany. In the European studies, the diffusion was slower, because
the applications were made in late autumn; soils were wetter,
colder, and heavier in texture. Thus, results from studies carried
out under different agronomic and climatic conditions are not
necessarily comparable.
The vertical and horizontal movements of 1,3-dichloropropene
were studied in a tree-nursery region in the north of the Federal
Republic of Germany. Sounding pipes were used to collect water
samples down to a depth of 4 m using the percussion-boring method.
Further borings were set to a depth of 3 m on days 10-91 after
application of a formulation containing cis- and trans-1,3-
dichloropropene, methylisothiocyanate and 1,2-dichloropropane at 50
ml/m2. Soil cores were analysed. 1,3-Dichloropropene showed a
rather high mobility in the soil, as it could be detected at a depth
of 4 m in all soil layers on the fourth day of application. In
samples of the near-surface groundwater, collected 140 days after
application, a concentration of 1.36 µg 1,3-dichloropropene per
litre was found. Ten to 25 m from the treated area, 1,3-
dichloropropene was also found in groundwater after 59 and 140 days
(Rexilius & Schmidt, 1982).
4.1.3.5 Loss under field conditions
Williams (1968) studied the loss of 1,3-dichloropropene under
field conditions in sandy loam and peat soils in Canada. The
application rates were approximately 1000 and 2000 litre "Mix
D/D"/ha, respectively. Eight months later, samples were collected
and residues determined (Table 4).
In studies in the Federal Republic of Germany, Netherlands, and
the United Kingdom, only very low residues (1%) of the amount
originally applied remained after 3 months in the soil (Wallace,
1976a,b; Wallace, 1979).
A comparative trial was carried out in the United Kingdom in
which "MIX D/D" and 1,3-dichloropropene were injected, at a depth of
15 cm, in clay loam at concentrations of 410 and 240 litre/ha,
respectively (Table 5, see also section 4.3.2 of "MIX D/D"). Samples
of soil were taken at depths of 0-20 cm, 20-40 cm, and 40-60 cm, at
6 intervals up to 9´ months after application. As part of normal
recommended agricultural practice, the soil was ploughed 5 weeks
after treatment. Soil samples were analysed for residues of cis-
and trans-1,3-dichloropropene, 1,2-dichloropropane, and cis- and
trans-3-chloroallyl alcohol. There was no significant difference
between the residues of the 1,3-dichloropropene or the 3-chloroallyl
alcohol resulting from the 2 treatments. As expected, no 1,2-
dichloropropane residues were detected in soil samples treated with
1,3-dichloropropene. Residues of the cis- and trans-1,3-
dichloropropenes and cis- and trans-3-chloroallyl alcohols were
detected in all samples up to 9´ months after treatment and down to
the 20-40 cm soil layer. Before the soil was ploughed, the
concentrations of these substances showed little change, and they
were present in all 3 layers, but, after ploughing, the
concentrations decreased gradually (Wallace, 1979).
Table 4. Recovery of cis- and trans-1,3-dichloropropene from
sandy loam or peat soils, 8 months after application of 1000 or 2000
litre "MIX D/D"/ha, respectively
Soil Depth in cm Residue in mg/kg soil
cis-1,3- trans-1,3-
dichloropropene dichloropropene
Peat 0-10 1.4 3.2
10-20 1.8 4.8
Sandy loam 0-10 - -
10-20 0.3 0.4
From: Williams (1968)
Table 5. Residues from the plot treated with 1,3-dichloropropene at 240 litre/haa
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 2.02 2.54 < 0.1 1.01 1.01
20-40 5.98 7.32 0.2 3.16 3.34
40-60 0.14 0.15 < 0.1 1.57b 1.88b
10 0-20 6.29 7.66 0.1 1.23 1.23
20-40 1.79 2.10 < 0.1 1.09 1.14
40-60 0.52 0.55 < 0.1 3.01b 3.24b
23 0-20 6.10 6.10 0.2 2.39 2.39
20-40 3.26 3.20 0.2 1.32 1.32
40-60 0.09 0.08 < 0.1 0.04 0.04
34 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.95 1.10 < 0.1 0.45 0.45
20-40 0.97 0.90 < 0.1 0.62 0.62
40-60 0.06 0.04 < 0.1 < 0.02 < 0.02
67 0-20 0.28 0.36 < 0.1 0.70 0.70
20-40 0.04 0.05 < 0.1 0.32 0.26
40-60 0.11 0.09 < 0.1 0.05 0.04
Table 5 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.08 0.06 < 0.1 0.20 0.20
9´ months 20-40 0.02c 0.02c < 0.1 0.04 0.03
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
Pre-treatment 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a From: Wallace (1979).
Note: All residues are on a dry weight basis.
b Anomalous results.
c Results confirmed by GC/MS.
1,3-Dichloropropene (D-D 95 and Telone II, containing > 92%),
at concentrations of 240, 280, and 290 litre/ha, was injected into
the soil of 3 bulb fields in the Netherlands in the summer. Nine
points were sampled per field and the samples were taken at various
times down to a depth of 3 m. Within a month, the concentrations
decreased to less than 0.2 mg/kg and continued to decline gradually
with time (Van der Pas & Leistra, 1987).
In 2 fields in the Netherlands (soil containing 15.7-24.6% of
organic matter), the spread of the fumigant (application rate 150
litre/ha) through the soil was measured. Only low fumigant
concentrations (about 0.1-0.4 mg/kg) were measured at a depth of 0.3
m. Around the depth of injection (0.15-0.2 m), the ratio of cis-
and trans-isomers changed with time in favour of the trans-
isomer. Cumulative emissions into the air over a period of 3 weeks
were calculated to range from 10 to 20% of the dosage of the cis-
isomer, and 4 to 15% of the trans-isomer (Van den Berg & Leistra,
1989).
4.1.3.6 Results of supervised field trials
A field study was undertaken in France in 1988, in which D-D 92
was applied to the soil prior to planting vines, and the air in the
vicinity of the treated area was monitored for 1,3-dichloropropene.
D-D 92 was applied at approximately 600 kg/ha at a depth of 30-40
cm. The air levels were monitored for 10 days. No samples contained
1,2-dichloropropane at levels above the limit of determination of
0.02 mg/m3. The highest 1,3-dichloropropene concentration found
during the first 24 h (perimeter of the field) was 2.1 mg/m3 and
this declined to 0.02-0.04 mg/m3 after 10 days. Air concentrations
also decreased with increasing distance, downwind (Sherren, 1990).
4.2 Bioconcentration
No data are available on bioconcentration.
4.3 Abiotic degradation
4.3.1 Photodegradation
Li (1979) obtained results comparable with those of Tuazon et
al. (1984) working with ozone, by irradiation of vapour of cis-
and trans-1,3-dichloropropene with a GE-RS sunlamp (see section
4.1.1). The main reaction product was 3-chloropropionyl chloride
with smaller quantities of 3-chloropropionic acid, CO2, and
phosgene. In this process, the initial reaction was epoxidation of
the double bond. There is evidence of the importance of a surface
reaction in the atmosphere, adsorption on to particulate matter
seems to be necessary for an appreciable direct phototransformation
to occur. Vapour phase photolysis of 1,3-dichloropropene was not
detected after prolonged simulated sunlight irradiation in a
reaction chamber. Photolysis occurred on the photoreactor surface
walls suggesting surface-catalysing reactions. The reaction products
suggest that 12-13% was totally degraded to CO2 after 5 days of
irradiation. Over 20% was transformed to phosgene.
No data on the photolytic decomposition of the chloropropenes
in water are available. Nevertheless, UVR of these chemicals in
methanol, in a frozen state, or as inclusion in adamantine matrices,
may cause the production of allyl radicals, by cleavage of the
allylic C-Cl bond (Krijgsheld & van der Gen, 1986).
4.4 Biodegradation and biotransformation
Several studies have been performed on the persistence of 1,3-
DCP in soil, after application as a fumigant. Biodegradation by soil
microorganisms does occur, depending on soil type, temperature, and
moisture content. The rate of disappearance ranges from a half-life
of 3 days to one of 37 days, without any consistent correlation with
organic matter content of the soil, or with pH. In sterile soils,
the effect of temperature was minimal (Van Dijk, 1974; Tabak et al.,
1981; California State Water Resources Control Board, 1983). In
general, the rates of disappearance of the cis- and trans-
isomers are similar and tend to increase with moisture content and
temperature, conditions that may increase, not only biodegradation,
but also loss by volatilization or chemical hydrolysis. Although
between 15 and 80% decomposition of field applications of 1,3-
dichloropropene has been shown, the large amount that can be
absorbed (80-90%) can result in soil residues existing months after
application is completed (Van Dijk, 1974; Roberts & Stoydin, 1976;
Sittig, 1980; Krijgsheld & van der Gen, 1986).
In biodegradability studies using a synthetic medium that
contained 5 mg of yeast extract/litre and was inoculated with waste
water, loss of 1,3-dichloropropene was determined after 7 days of
incubation. Significant degradation was observed at 5 and 10 mg of
1,3-dichloropropene/litre and gradual adaptation was shown in
subcultures. The original culture degraded about 50% of the 1,3-
dichloropropene in 7 days, while the third subculture was able to
degrade approximately 85% at both substrate concentrations, in the
same period of time (Tabak et al., 1981).
Battersby (1990a) determined the "ready biodegradability" of
trans-1,3-dichloropropene (95.4% trans- and 0.3% cis-isomer)
using the closed bottle procedure. The substance was not degraded in
this system with a negligible proportion of the theoretical oxygen
demand being consumed during the 28-day incubation period.
The EEC-activated sludge respiration inhibition test was used
to determine the effect of a cis- (51.2-52.2%) + trans- (43.9-
44.1%) mixture of 1,3-dichloropropene containing 0.33% of 1,2-
dichloropropane on the respiration rate of activated sludge. The
EC50 for this mixture was 188 mg/litre (Battersby, 1990b).
The EEC-activated sludge respiration inhibition test was also
used to determine the effect of cis-1,3-dichloropropene (94.5-
97.5% cis-, 1.5% trans-isomer and 0.25% 1,2-dichloropropane) on
the respiration rate of activated sludge. The EC50 for the cis-
1,3-dichloropropene was 279 mg/litre (Battersby, 1990c).
Biodehalogenation by soil organisms has been demonstrated for
1,3-dichloropropene. The fumigant appeared to be chemically
hydrolysed to 3-chloroallyl alcohol and then converted to 3-
chloroacrylic acid. The chlorine is removed and the intermediate
products are converted to carbon dioxide and water. The rate of
disappearance of 1,3-dichloropropene at 15-20 °C was 2-3.5% per day
in sandy soil and up to 25% per day in clay soils. The chloroallyl
alcohol disappeared at rates of 20-60% per day at 15 °C (Van Dijk,
1974). Leistra et al. (1991) incubated 1,3-dichloropropene and its
transformation product 3-chloroallyl alcohol in water-saturated
subsoil material at 10 °C. The times for 50% and 95% transformation
ranged from 15 to 47 days and from 27 to 79 days, respectively, for
1,3-dichloropropene. The corresponding 50% and 95% transformation
times for 3 chloroallyl alcohol were 0.8-4.2 and 4.0-6.5 days,
respectively.
Chemical hydrolysis is the first step in the transformation of
1,3-dichloropropene. Further transformation is thought to result
from microbial action; 3-chloroacrolein and 3-chloroacrylic acid
have been isolated from the metabolism of 3-chloroallyl alcohol by
Pseudomonas species (see Fig. 4) (Belser & Castro, 1971; Roberts &
Stoydin, 1976).
Soil culture studies using media enriched with 1,3-
dichloropropenes, 1,2-dichloropropane, and "Mix D/D" at
concentrations of up to 100 mg/kg, produced abundant growth of all
microorganisms tested, indicating the use of the fumigants as carbon
sources. Several of these organisms (Rhizobium leguminosarum,
Bacillus subtilis, Arthrobacter globiformis, and Pseudomonas
fluorescens) produced greater amounts of amino acids (Altman &
Lawlor, 1966; Altman, 1969). The cis- and trans-isomers of 1,3-
dichloropropene have undergone biodehalogenation by a Pseudomonas
sp. isolated from the soil. Cis- and trans-1,3-dichloropropene
can be chemically hydrolysed in moist soils to the corresponding 3-
chloroallyl alcohols, which can be metabolized to carbon dioxide and
water by Pseudomonas sp. (Fig. 4).
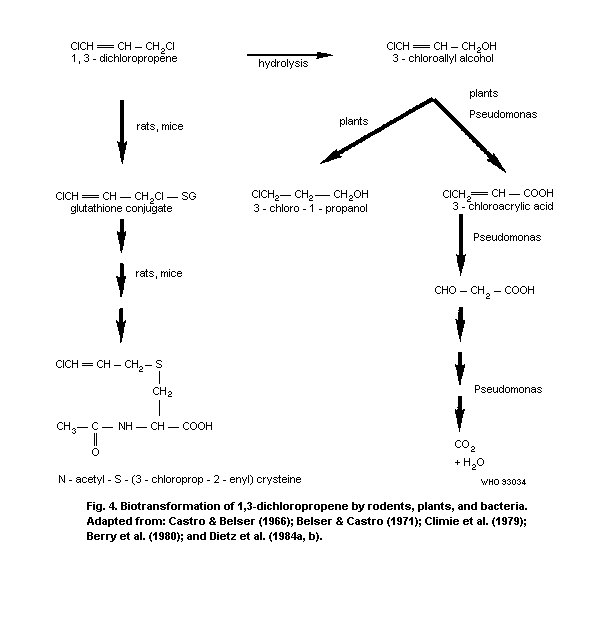 The degradation of Telone II (92% 1,3-dichloropropene cis-
and trans-isomers; 2% 1,2-dichloropropane and 5% mixture of
propenes and hexenes, and 1% epichlorohydrin) in soil was studied
using 14C-1,3-dichloropropene in Fuquay loamy sand samples
collected from a field in Florida. The samples were collected
before, and one, and two weeks, and 2 years following application at
a rate of 15 kg/ha, at depths of 0-36 cm or 36-65 cm. After 28 days
incubation of 14C-1,3-dichloropropene in the soil, it was degraded
into 14CO2 (44%), water-soluble metabolites (probably 3-
chloroallyl alcohol), bound residues, and possibly some microbial
mass. Little or no difference was observed in the degradation of
14C-1,3-dichloropropene in soil samples collected one week prior
to the field application of Telone II, or two weeks and two years
after application. A mixed bacteria culture isolated from the soil
in the presence of a carbon source, completely degraded 14C-1,3-
dichloropropene into 14CO2, water-soluble products and microbial
mass (Ou, 1989).
4.4.1 Miscellaneous
Laboratory experiments were conducted to determine the effects
of 1,3-dichloropropene on the activity of invertase in a sandy soil.
The rates of application were 30 and 60 mg/kg. No inhibition was
found. The same dose levels were used to test the influence of the
compound on amylase in sandy soil. After 3 days, stimulation of the
formation of glucose from the added starch was seen, especially at
the lowest dose level. Microbial respiration was also tested in
sandy loam. The treatment did not significantly decrease oxygen
consumption (Tu, 1988).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Air
Telone II, at a rate of 293 litre/ha, was applied, at a depth
of 0.45 m, simultaneously with pineapple crown planting. Each row of
pineapple was covered with black polyethylene film at the time of
planting. Air samples were taken inside the cover and at ground
level, 10, 20, 22, 27, and 30 days after fumigation. The
concentration inside the cover remained steady, at least until day
9; thereafter, a decrease was noticed and, after 22 days, the
substance was no longer detectable. At ground level, the
concentration fell gradually and was non-detectable after 30 days
(Albrecht & Chenchin, 1985).
A small-scale, field study was undertaken in 1986, when air
concentrations of 1,3-dichloropropene were measured in the vicinity
of ground treated with D-D 92 (93.8%), by hand injector at a dose
rate of 330 kg/ha. The air was monitored for 2 weeks. The
concentration of 1,3-dichloropropene varied between 0.004 and 0.88
mg/m3 during the first week. Levels of 1,3-dichloropropene in the
second week were below the limit of determination (0.002 mg/m3)
(Sherren & Woodbridge, 1987a).
5.2 Water
An investigation of 1,3-dichloropropene in well-water in
California was carried out by Maddy et al. (1982). Fifty-four wells
were selected in locations of high nematocide use. No samples showed
levels above the limit of determination of 0.1 µg/litre. In a
survey, 72 water samples from wells in California were analysed for
1,3-dichloropropene, but no samples contained levels above 1
µg/litre (limit of determination) (Peoples et al., 1980).
Connors et al. (1990) analysed potable water samples collected
in 8 homes in 3 communities in Connecticut and did not find 1,3-
dichloropropene (< 0.1 µg/litre).
Dowty et al. (1975) conducted a survey on drinking-water in New
Orleans, they found 1,3-dichloropropene, but did not give actual
levels or frequency of occurrence.
No 1,3-dichloropropene (limit of determination 1 µg/litre) was
found in 30 Canadian potable water facilities (Otson et al., 1982).
Apparently, chlorination of organic materials in water may lead
to traces of 1,3-dichloropropene (< 1 µg/litre). Therefore, this
process may be responsible for the observed presence of the
substance in tap water (Otson et al., 1982; Krijgsheld & Van der
Gen, 1986).
1,3-Dichloropropene has been identified in the waste water from
a textile plant. A level of 2 µg cis-isomer/litre was measured in
the influent of the waste water treatment plant, while higher
concentrations of cis-1,3-dichloropropene (e.g., 6 µg/litre) were
found in the effluent, together with the trans-isomer (0.9-4
µg/litre). Similarly, no 1,3-dichloropropene was detected in the
influent of a municipal waste treatment plant, but, after "super-
chlorination", a mean concentration of approximately 10 µg/litre
could be detected in the liquid sludge (Krijgsheld & Van der Gen,
1986).
Hallberg (1989) reported studies on the presence of pesticides
in groundwater in different States of the USA. 1,3-Dichloropropene
was found only in Oregon, but no concentration(s) were reported.
Van Beek et al. (1988) examined 33 groundwater wells up to a
depth of 50 m in the northern Netherlands for the presence of 1,3-
dichloropropene. In this area, "MIX D/D" had been used on a large
scale as a nematocide in potato growing since 1967. 1,2-
Dichloropropane was present in the groundwater, but no 1,3-
dichloropropene (> 0.1 µg/litre) was found in 45 samples from these
wells.
Samples of upper groundwater (from 1-2 m below the water level)
below 4 sandy soils were analysed in the Netherlands, for 2.5 years
in 8 sampling rounds. 1,3-Dichloropropene was detected in the
groundwater in 6/34 samples at concentrations in the range of
< 0.1-80 µg/litre. These observations were made below fields with
potato, maize, and bulb flower crops, all on low-humic to moderately
humic sandy soils (Loch & Verdam, 1989).
Lagas et al. (1989) analysed groundwater (up to 6 m depth) in 5
areas (4 of which are described by Loch & Verdam, 1989), and found
1,3-dichloropropene levels above the limit of detection (0.1
µg/litre) in 2 out of 22 samples (range: < 0.1-0.2 µg/litre) taken
from underneath potato crops and in 1 out of 8 samples (< 0.1-2.5
µg/litre) from below maize and bulb crops.
On 5 sites in a polder in the Netherlands, samples of surface
water were taken monthly in 1987-88 and analysed. The area is
situated next to the dunes (where groundwater is being pumped up for
the preparation of drinking-water), and is extensively used for
bulb-culture. The maximum concentration found for 1,3-
dichloropropenes ( cis- and trans-) was 2.5 µg/litre (Greve et
al., 1989).
In the Netherlands and the Federal Republic of Germany, 1,3-
dichloropropene was found in areas with extensive agriculture and
horticulture. 1,3-Dichloropropene was found in the upper groundwater
(depth 1-5 m) and the average levels ranged from 0.6 to 2530
µg/litre (maximum level 8620 µg/litre). In bores for irrigation (11-
24 m depth), an average of 0.23 (< 0.02-0.89) µg/litre was found
(Leistra & Boesten, 1989).
Ahlsdorf et al. (1989) determined the presence of 1,3-
dichloropropene in the upper groundwater of an area used for potato
growing, which was treated with this nematocide (about 140 kg/ha) in
1984. Very low levels of 1,3-dichloropropene (1-4 µg/litre) were
found in soil with a high organic matter content, but concentrations
of up to 8620 µg/litre were found in the groundwater of a clay
podsol soil containing a high sand content, after one month.
1,3-Dichloropropene was detected in irrigation wells that were
close to a piece of land that was treated with the chemical (10-25 m
distance) in Schleswig Holstein (Germany). In the well water,
concentrations of 1,3-dichloropropene varied between 0.06 and 0.89
µg/litre (Rexilius & Schmidt, 1982).
5.3 Crops
Residues in edible crop commodities, arising from the use of
1,3-dichloropropene or "MIX D/D", are reported to be generally below
the limit of detection. The obvious reason for this, is the fact
that crops are not normally planted until most of the product
applied has dissipated. Another reason is that any 1,3-
dichloropropene or "MIX D/D" taken up by the plant, would have to
survive the whole crop cycle to be detected in the harvest
commodity.
Supervised trials with "MIX D/D", with 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
5.4 Occupational exposure
Albrecht (1987) carried out a survey to assess the exposure of
72 workers on a Hawaiian pineapple farm (attendants, crown
unloaders, (truck) drivers, irrigation workers, supervisors, mulch
coverers, and planters). Exposures were predominantly below 4.54
mg/m3 (1 ppm). The concentrations in these workers ranged between
0.032 and 4.626 mg/m3 (0.007-1.019 ppm).
Brouwer et al. (1991a) studied the inhalation of cis- and
trans-1,3-dichloropropene in 12 commercial applicators in the
Netherlands. The time-weighted average (TWA) concentrations of 1,3-
dichloropropene ranged from 1.9 to 18.9 mg/m3. Short-term exposure
levels during tank-loading and repair ranged up to 30 mg/m3. No
correlation was observed between exposure and total area injected
with 1,3-dichloropropene. Emission of 1,3-dichloropropene vapour
from the soil or from spilled liquid dripping from the nozzles on to
the soil may contribute to exposure.
An employee air-monitoring study to determine the amount of
Telone II to which personnel would be exposed, removing soil core
samples in the immediate area of the drilling, was carried out. The
concentration in the air was between 0.0982 and 1.79 mg/m3 on the
first day, and between 0.202 and 3.056 mg/m3 on second day. The
time-weighted averages from personal monitoring on days one and two
were 0.65 and 0.90 mg/m3, respectively. The time-weighted averages
from air monitoring on days one and two were 0.39 and 0.59 mg/m3
(Fong & Maykoski, 1985).
A study on a single operator during a one-day application was
carried out in the Federal Republic of Germany in 1986. Short-term
inhalation exposures to 1,3-dichloropropene were observed during the
filling operation (5.6-16.3 mg/m3) and during nozzle changing
(18.3 mg/m3). The overall exposure during 11 h exceeded the
recommended TWA value (Eadsforth et al., 1987).
An air monitoring study on exposure to 1,3-dichloropropene
during the application of "Mix D/D" (not less than 50%) and D-D 92
(not less than 92%) was carried out at different locations near
Nimes in France in 1988. The 8-h time-weighted average (TWA) air
concentrations of total 1,3-dichloropropene for the applicator on
the 2 days of application were 11.3 and 13.2 mg/m3, respectively,
and for the tractor driver on the second day, 14.4 mg/m3.
Relatively high, short-term inhalation exposures of the applicator
were measured during filling operations; the concentrations varied
between 6.4 and 83.5 mg/m3. These short-term exposures were found
to contribute significantly to the overall time-weighted average
exposures over the working period (Rocchi & van Sittert, 1989).
Albrecht & Chenchin (1985) found measurable concentrations of
1,3-dichloropropene in the range of 2.4-18.5 mg/m3 during a 8-h
shift in 8 out of 15 workers, planting pineapple crowns by hand,
simultaneously with 1,3-dichloropropene (Telone II) treatment of the
soil at 293 litre/ha.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.1.1 Rat
Groups of 6 adult male and 6 female Carworth Farm E rats
received, by stomach tube, 2.5-2.7 mg cis-1,3-dichloro-[2-
14C]propene or trans-1,3-dichloro-[2-14C]propene in 0.5 ml
arachis oil per rat, and excretion was followed. After 4 days, the
animals were killed and the radioactivity measured in skin and
carcasses. The excretion of radioactivity was very rapid, 80-90% was
eliminated in the faeces, urine, and expired air in the first 24 h.
The urine was the major route of elimination, i.e., 80.7 and 56.5%
(average of males and females) of the dose for cis- and trans-
1,3-dichloropropene, respectively. Only 2.6 and 2.2% of the 2
isomers, respectively, were eliminated in the faeces in 4 days,
while 3.9 and 23.5%, respectively, were eliminated as 14CO2 in 4
days in the expired air. Levels of the other volatile compounds in
air were only 1-3% of the dose. Up to 1% of the dose in the skin and
carcass was found. The difference in the amount of labelled CO2 in
expired air and urine indicated a difference in the kinetics of the
2 isomers (Hutson et al., 1971).
Groups of 8 adult Fischer 344 rats/sex were given non
radiolabelled 1,3-dichloropropene at 5 mg/kg body weight, in corn
oil, by gavage, for 14 consecutive days, prior to a single dose of 5
mg 14C-1,3-dichloropropene/kg body weight (actual 4.5 mg)
(uniformly labelled) (96.3%; 53.3% cis- and 43.0% trans-),
administered to 5 out of the 8 rats on day 15. The remaining 3
rats/sex were sacrificed. The distribution of radioactivity found in
the tissues (4-6%) of repeatedly dosed rats, 48 h after dosing, was
similar to that of single dosed animals. There was no sex difference
in the distribution of the radioactivity. In addition to the
repeatedly dosed rats, 2 rats of each sex, which had not been
previously dosed, received a single gavage dose of 5 mg 14C-1,3-
dichloropropene/kg body weight. The urine was the major route of
elimination of the radioactivity derived from 14C-1,3-
dichloropropene, which ranged from 60 to 65% of the administered
dose in 48 h in the rats with repeated doses and a single dose.
Elimination of 1,3-dichloropropene as 14CO2 was approximately
(average) 26% of the administrated radioactivity with about 4-5% of
the dose eliminated in the faeces, for all groups (Waechter & Kastl,
1988).
In another study, the fate of 14C- cis- and 14C- trans-
1,3-dichloropropene (97%; 62% cis and 38% trans) was determined
after a single oral dose of 1 or 50 mg/kg body weight to male
Fischer 344 rats (3 animals per dose level). Urine, faeces, expired
air, tissues, and remaining carcasses were analysed after 48 h.
Urine was the major route of excretion, 51-61% of the administered
dose being excreted over 48 h. In the carcass, 6% of the dose was
found at the end of 48 h. On the basis of interval excretion data,
half-lives for urinary excretion ranged from 5 to 6 h. Faeces and
expired CO2 accounted for roughly 18% and 6%, respectively. The
tissue concentrations of 14C activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, skin,
and fat (Dietz et al., 1984a,b, 1985).
6.1.1.2 Mouse
The fate of 14C- cis- and 14C- trans-1,3-dichloropropene
(97%; 62% cis and 38% trans) was studied after oral dosing of
male B6C3F1 mice with 1 or 100 mg/kg body weight (3
animals/dose level). Urine, faeces, expired air, tissues, and
remaining carcasses were analysed after 48 h. Urine was the major
route of excretion, with 63 and 79%, respectively, of the
administered doses (1 and 100 mg/kg body weight) being excreted over
48 h. Half-lives for urinary excretion ranged from 5 to 6 h. Faeces
and expired CO2 accounted for 15 and 14% of the 14C-
radioactivity, respectively. In the carcass, 2% was found. The
tissue concentrations of 14C-activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, fat,
and skin (Dietz et al., 1984a,b, 1985).
6.1.2 Inhalation
6.1.2.1 Rat
Stott & Kastl (1985, 1986) studied the pharmacokinetics of the
uptake of vapours of technical grade 1,3-dichloropropene (49.3%
cis- and 42.8% trans-isomer) and the disappearance of cis- and
trans-1,3-dichloropropene from the blood in groups of 3-6 male
Fischer 344 rats exposed to actual concentrations of 136, 409, 1362,
and 4086 mg/m3 for 3 h.
The uptake of 1,3-dichloropropene did not increase
proportionately with increasing exposure concentration due to an
exposure level-related decrease in the respiration rate and
respiration min/volume of rats exposed to > 409 mg 1,3-
dichloropropene/m3 and the saturation of metabolism of 1,3-
dichloropropene in rats exposed to > 1362 mg/m3. Absorption of
inhaled 1,3-dichloropropene occurred via the lungs, primarily in the
lower respiratory tract (approximately 50% of total inhaled), with a
small amount via the nasal mucosa (11-16%).
Following exposure to < 1362 mg/m3, both isomers were
rapidly eliminated from the blood, with a half-life of 3-6 min.
There was no interaction in the kinetics of both isomers. In
addition, data obtained on rats exposed to 1362 mg/m3 revealed
that this rapid elimination phase was followed by a slower
elimination phase having a half-life of 33-43 min. These data
demonstrated that a combination of saturable metabolism and
chemically-induced changes in respiration control 1,3-
dichloropropene uptake and body-burden in rats. However, only
decreases in respiration appear to influence vapour uptake.
Fisher & Kilgore (1988a) studied the excretion of the
mercapturic acid of cis-dichloropropene in Sprague-Dawley rats. In
a nose-only exposure system, groups of 3 rats were exposed for 1 h
to Telone II (94%) at average concentrations of 0, 181.6, 485.8,
1289.4, 1806.9, or 3582.1 mg/m3. Urine samples (24 h) were
collected and analysed for the mercapturic derivative of cis-
dichloropropene. At the lower exposure levels (< 1289.4 mg/m3),
urinary excretion of the mercapturic acid derivative increased with
exposure level. With exposure to 1806.9 or 3582.1 mg/m3, no
further increase was found, suggesting saturation of the metabolic
process.
6.2 Influence on tissue levels of glutathione
6.2.1 Oral
Oral administration of 1,3-dichloropropene to rats or mice
resulted in significant, dose-related reductions in the levels of
non-protein sulfhydryls (NPS) (indicator of tissue glutathione
concentration) in the forestomach and to a lesser extent in the
glandular stomach and liver (Dietz et al., 1984b, 1985, see also
section 6.4).
6.2.2 Inhalation
Shortly after inhalation exposure of rats to cis-1,3-
dichloropropene, kidney and liver NPS contents were reduced in a
dose-related manner, but returned to control values 18 h after
exposure. Lung NPS levels were not affected (Stott & Kastl, 1986,
see section 6.1.2.1; Nitschke & Lomax, 1990, see section 8.2.2.2).
Male Sprague-Dawley rats (200-250 g) were exposed through
inhalation to 1,3-dichloropropene (Telone II, 94%) concentrations of
0, 9.1, 22.7, 150, 1384.7, 3504.9, 4335.7, or 7790.6 mg/m3 to
assess the relationship between 1,3-dichloropropene exposure
concentration and tissue levels of reduced glutathione (GSH).
Animals were exposed for 1 h in a dynamic, nose-only system. GSH
contents were measured in the heart, kidneys, liver, lung, nasal
mucosa, and testes, 2 h after 1,3-dichloropropene exposure. A
decrease in nasal GSH, first seen at 22.7 mg/m3, followed an
exposure concentration-dependent curve. Exposure to concentrations
above 150 mg/m3 reduced the level of liver GSH. Lung GSH remained
relatively constant at 75% of control concentrations up to 4335.7
mg/m3. Significantly decreased GSH levels were observed in the
heart, liver, lung, and testes at 7790.6 mg/m3. Kidney GSH content
was not significantly decreased. Unchanged 1,3-dichloropropene was
not present in the blood of animals 2 h after exposure to 4335.7
mg/m3 or less. Serum lactic dehydrogenase activity was affected
only at 7790.6 mg/m3. Lung weight, measured 2 and 6 h after
exposure, did not differ from controls for any exposure level
(Fisher & Kilgore, 1988b).
Four male Sprague-Dawley rats (200-250 g) were exposed to
Telone II (94%) for 1 h, in a dynamic, nose-only exposure system.
The actual 1,3-dichloropropene concentration was 354.1 ± 49.9, 703.7
± 408.6, and 1834.2 ± 113.5 mg/m3 (relative concentrations of
cis- and trans-isomers were approximately 62 and 38%,
respectively). The GSH conjugation of 1,3-dichloropropene (GSCP) in
the blood of rats following exposure showed that there was no
significant difference between the regression line expressed as
either monophasic or biphasic decay at any exposure concentration.
Moreover, no differences were found in the regression lines between
the exposure concentrations. The elimination half-time of GSCP was
approximately 17 h following exposure to 354.1, 703.7, or 1834.2
mg/m3, and, thus, was not dose-dependent. This fits a one-
compartment model (Fischer & Kilgore, 1989).
6.3 Biotransformation
6.3.1 Rat
In urine from rats and mice treated orally with 14C-
dichloropropene, no unchanged parent compound, but 2 major and 2
minor metabolites were found. The predominant metabolite was N-
acetyl- S-(3-chloroprop-2-enyl) cysteine with its sulfoxide or
sulfone. These data indicate that conjugation with glutathione is a
major route of 1,3-dichloropropene metabolism in the rat (Dietz et
al., 1984a,b, 1985) (see Fig. 4 and section 6.1.1).
Although the spontaneous reaction of cis-1,3-dichloropropene
with glutathione is slow in the rat, the rapid urinary excretion is
due to hepatic glutathione transferase, which catalyses its
conjugation with glutathione. The transferase is present in the rat
liver cytosol fraction and little microsomally mediated metabolism
occurs. The cis-isomer is a better substrate than the trans-
isomer for glutathione transferase. The conjugation then follows a
classic mercapturic acid pathway (Boyland & Chasseaud, 1969). The
conjugated product N-acetyl- S-(3-chloroprop-2-enyl) cysteine and
its sulfoxide are excreted in the urine of rats and mice (Climie et
al., 1979; Dietz et al., 1984b; van Sittert, 1984, 1989).
It has been shown that a minor metabolic pathway of the cis-
1,3-dichloropropene is mono-oxygenase catalysed oxygenation, leading
to the possible formation of the metabolite cis-1,3-
dichloropropene-oxide (II in Fig. 5) (Van Sittert, 1989).
Rats administered 25-450 µg cis- and trans-1,3-
dichloropropene/kg body weight, intraperitoneally, showed excretion
of N-acetyl- S-( cis- and trans-3-chloroprop-2-enyl)-L-
cysteine for 55% (cis-) and 45% (trans-) of the dose within 24 h
(Onkenhout et al., 1986).
In the study of Waechter & Kastl (1988) (see section 6.1.1.1),
in which rats were administered daily doses of 5 mg of non-labelled
1,3-dichloropropene/kg body weight followed by a single dose of 5 mg
14-C (uniformly) labelled 1,3-dichloropropene, or a single dose of
5 mg/kg body weight, the major urinary metabolites were the
mercapturic acid of 1,3-dichloropropene (1,3-D-MA) and its
sulfoxide. The repeatedly dosed rats excreted slightly higher
percentages of the dose as mercapturic acids than the single dosed
rats (28.5% vs 22.7% for males and 25.5% vs 14.3% for females). The
isomeric ratio of the 2,3-D-MA was approximately 80% cis- and 20%
trans- for all groups.
6.3.2 Humans
Van Welie et al. (1989, 1991) determined the relationship
between respiratory occupational exposure to cis- and trans-1,3-
dichloropropene and urinary excretion of 2 mercapturic acid
metabolites, N-acetyl- S-( cis- and trans-)-3-chloroprop-2-
enyl)-L-cysteine ( cis- and trans-DCP-MA) by 12, 1,3-
dichloropropene applicators in the Netherlands. Urinary excretion of
these mercapturic acids followed first-order elimination kinetics.
Urinary half-lives of elimination were 5.0 ± 1.2 h for the cis-
mercapturic acid and 4.7 ± 1.3 h for the transform. These values
were not statistically significantly different. A clear correlation
was observed between the 8-h time-weighted average (TWA) exposure to
cis- and trans-1,3-dichloropropene and complete cumulative
urinary excretion of cis- and trans-DCP-MA. The cis-DCP-MA
yielded 3 times more mercapturic acid (45%) than the trans- form
(14%), probably because of differences in kinetics. It was concluded
that the uptake of cis- and trans-1,3-dichloropropene, their
metabolism to the corresponding mercapturic acids, and urinary
excretion was a rapid process.
The degradation of Telone II (92% 1,3-dichloropropene cis-
and trans-isomers; 2% 1,2-dichloropropane and 5% mixture of
propenes and hexenes, and 1% epichlorohydrin) in soil was studied
using 14C-1,3-dichloropropene in Fuquay loamy sand samples
collected from a field in Florida. The samples were collected
before, and one, and two weeks, and 2 years following application at
a rate of 15 kg/ha, at depths of 0-36 cm or 36-65 cm. After 28 days
incubation of 14C-1,3-dichloropropene in the soil, it was degraded
into 14CO2 (44%), water-soluble metabolites (probably 3-
chloroallyl alcohol), bound residues, and possibly some microbial
mass. Little or no difference was observed in the degradation of
14C-1,3-dichloropropene in soil samples collected one week prior
to the field application of Telone II, or two weeks and two years
after application. A mixed bacteria culture isolated from the soil
in the presence of a carbon source, completely degraded 14C-1,3-
dichloropropene into 14CO2, water-soluble products and microbial
mass (Ou, 1989).
4.4.1 Miscellaneous
Laboratory experiments were conducted to determine the effects
of 1,3-dichloropropene on the activity of invertase in a sandy soil.
The rates of application were 30 and 60 mg/kg. No inhibition was
found. The same dose levels were used to test the influence of the
compound on amylase in sandy soil. After 3 days, stimulation of the
formation of glucose from the added starch was seen, especially at
the lowest dose level. Microbial respiration was also tested in
sandy loam. The treatment did not significantly decrease oxygen
consumption (Tu, 1988).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Air
Telone II, at a rate of 293 litre/ha, was applied, at a depth
of 0.45 m, simultaneously with pineapple crown planting. Each row of
pineapple was covered with black polyethylene film at the time of
planting. Air samples were taken inside the cover and at ground
level, 10, 20, 22, 27, and 30 days after fumigation. The
concentration inside the cover remained steady, at least until day
9; thereafter, a decrease was noticed and, after 22 days, the
substance was no longer detectable. At ground level, the
concentration fell gradually and was non-detectable after 30 days
(Albrecht & Chenchin, 1985).
A small-scale, field study was undertaken in 1986, when air
concentrations of 1,3-dichloropropene were measured in the vicinity
of ground treated with D-D 92 (93.8%), by hand injector at a dose
rate of 330 kg/ha. The air was monitored for 2 weeks. The
concentration of 1,3-dichloropropene varied between 0.004 and 0.88
mg/m3 during the first week. Levels of 1,3-dichloropropene in the
second week were below the limit of determination (0.002 mg/m3)
(Sherren & Woodbridge, 1987a).
5.2 Water
An investigation of 1,3-dichloropropene in well-water in
California was carried out by Maddy et al. (1982). Fifty-four wells
were selected in locations of high nematocide use. No samples showed
levels above the limit of determination of 0.1 µg/litre. In a
survey, 72 water samples from wells in California were analysed for
1,3-dichloropropene, but no samples contained levels above 1
µg/litre (limit of determination) (Peoples et al., 1980).
Connors et al. (1990) analysed potable water samples collected
in 8 homes in 3 communities in Connecticut and did not find 1,3-
dichloropropene (< 0.1 µg/litre).
Dowty et al. (1975) conducted a survey on drinking-water in New
Orleans, they found 1,3-dichloropropene, but did not give actual
levels or frequency of occurrence.
No 1,3-dichloropropene (limit of determination 1 µg/litre) was
found in 30 Canadian potable water facilities (Otson et al., 1982).
Apparently, chlorination of organic materials in water may lead
to traces of 1,3-dichloropropene (< 1 µg/litre). Therefore, this
process may be responsible for the observed presence of the
substance in tap water (Otson et al., 1982; Krijgsheld & Van der
Gen, 1986).
1,3-Dichloropropene has been identified in the waste water from
a textile plant. A level of 2 µg cis-isomer/litre was measured in
the influent of the waste water treatment plant, while higher
concentrations of cis-1,3-dichloropropene (e.g., 6 µg/litre) were
found in the effluent, together with the trans-isomer (0.9-4
µg/litre). Similarly, no 1,3-dichloropropene was detected in the
influent of a municipal waste treatment plant, but, after "super-
chlorination", a mean concentration of approximately 10 µg/litre
could be detected in the liquid sludge (Krijgsheld & Van der Gen,
1986).
Hallberg (1989) reported studies on the presence of pesticides
in groundwater in different States of the USA. 1,3-Dichloropropene
was found only in Oregon, but no concentration(s) were reported.
Van Beek et al. (1988) examined 33 groundwater wells up to a
depth of 50 m in the northern Netherlands for the presence of 1,3-
dichloropropene. In this area, "MIX D/D" had been used on a large
scale as a nematocide in potato growing since 1967. 1,2-
Dichloropropane was present in the groundwater, but no 1,3-
dichloropropene (> 0.1 µg/litre) was found in 45 samples from these
wells.
Samples of upper groundwater (from 1-2 m below the water level)
below 4 sandy soils were analysed in the Netherlands, for 2.5 years
in 8 sampling rounds. 1,3-Dichloropropene was detected in the
groundwater in 6/34 samples at concentrations in the range of
< 0.1-80 µg/litre. These observations were made below fields with
potato, maize, and bulb flower crops, all on low-humic to moderately
humic sandy soils (Loch & Verdam, 1989).
Lagas et al. (1989) analysed groundwater (up to 6 m depth) in 5
areas (4 of which are described by Loch & Verdam, 1989), and found
1,3-dichloropropene levels above the limit of detection (0.1
µg/litre) in 2 out of 22 samples (range: < 0.1-0.2 µg/litre) taken
from underneath potato crops and in 1 out of 8 samples (< 0.1-2.5
µg/litre) from below maize and bulb crops.
On 5 sites in a polder in the Netherlands, samples of surface
water were taken monthly in 1987-88 and analysed. The area is
situated next to the dunes (where groundwater is being pumped up for
the preparation of drinking-water), and is extensively used for
bulb-culture. The maximum concentration found for 1,3-
dichloropropenes ( cis- and trans-) was 2.5 µg/litre (Greve et
al., 1989).
In the Netherlands and the Federal Republic of Germany, 1,3-
dichloropropene was found in areas with extensive agriculture and
horticulture. 1,3-Dichloropropene was found in the upper groundwater
(depth 1-5 m) and the average levels ranged from 0.6 to 2530
µg/litre (maximum level 8620 µg/litre). In bores for irrigation (11-
24 m depth), an average of 0.23 (< 0.02-0.89) µg/litre was found
(Leistra & Boesten, 1989).
Ahlsdorf et al. (1989) determined the presence of 1,3-
dichloropropene in the upper groundwater of an area used for potato
growing, which was treated with this nematocide (about 140 kg/ha) in
1984. Very low levels of 1,3-dichloropropene (1-4 µg/litre) were
found in soil with a high organic matter content, but concentrations
of up to 8620 µg/litre were found in the groundwater of a clay
podsol soil containing a high sand content, after one month.
1,3-Dichloropropene was detected in irrigation wells that were
close to a piece of land that was treated with the chemical (10-25 m
distance) in Schleswig Holstein (Germany). In the well water,
concentrations of 1,3-dichloropropene varied between 0.06 and 0.89
µg/litre (Rexilius & Schmidt, 1982).
5.3 Crops
Residues in edible crop commodities, arising from the use of
1,3-dichloropropene or "MIX D/D", are reported to be generally below
the limit of detection. The obvious reason for this, is the fact
that crops are not normally planted until most of the product
applied has dissipated. Another reason is that any 1,3-
dichloropropene or "MIX D/D" taken up by the plant, would have to
survive the whole crop cycle to be detected in the harvest
commodity.
Supervised trials with "MIX D/D", with 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
5.4 Occupational exposure
Albrecht (1987) carried out a survey to assess the exposure of
72 workers on a Hawaiian pineapple farm (attendants, crown
unloaders, (truck) drivers, irrigation workers, supervisors, mulch
coverers, and planters). Exposures were predominantly below 4.54
mg/m3 (1 ppm). The concentrations in these workers ranged between
0.032 and 4.626 mg/m3 (0.007-1.019 ppm).
Brouwer et al. (1991a) studied the inhalation of cis- and
trans-1,3-dichloropropene in 12 commercial applicators in the
Netherlands. The time-weighted average (TWA) concentrations of 1,3-
dichloropropene ranged from 1.9 to 18.9 mg/m3. Short-term exposure
levels during tank-loading and repair ranged up to 30 mg/m3. No
correlation was observed between exposure and total area injected
with 1,3-dichloropropene. Emission of 1,3-dichloropropene vapour
from the soil or from spilled liquid dripping from the nozzles on to
the soil may contribute to exposure.
An employee air-monitoring study to determine the amount of
Telone II to which personnel would be exposed, removing soil core
samples in the immediate area of the drilling, was carried out. The
concentration in the air was between 0.0982 and 1.79 mg/m3 on the
first day, and between 0.202 and 3.056 mg/m3 on second day. The
time-weighted averages from personal monitoring on days one and two
were 0.65 and 0.90 mg/m3, respectively. The time-weighted averages
from air monitoring on days one and two were 0.39 and 0.59 mg/m3
(Fong & Maykoski, 1985).
A study on a single operator during a one-day application was
carried out in the Federal Republic of Germany in 1986. Short-term
inhalation exposures to 1,3-dichloropropene were observed during the
filling operation (5.6-16.3 mg/m3) and during nozzle changing
(18.3 mg/m3). The overall exposure during 11 h exceeded the
recommended TWA value (Eadsforth et al., 1987).
An air monitoring study on exposure to 1,3-dichloropropene
during the application of "Mix D/D" (not less than 50%) and D-D 92
(not less than 92%) was carried out at different locations near
Nimes in France in 1988. The 8-h time-weighted average (TWA) air
concentrations of total 1,3-dichloropropene for the applicator on
the 2 days of application were 11.3 and 13.2 mg/m3, respectively,
and for the tractor driver on the second day, 14.4 mg/m3.
Relatively high, short-term inhalation exposures of the applicator
were measured during filling operations; the concentrations varied
between 6.4 and 83.5 mg/m3. These short-term exposures were found
to contribute significantly to the overall time-weighted average
exposures over the working period (Rocchi & van Sittert, 1989).
Albrecht & Chenchin (1985) found measurable concentrations of
1,3-dichloropropene in the range of 2.4-18.5 mg/m3 during a 8-h
shift in 8 out of 15 workers, planting pineapple crowns by hand,
simultaneously with 1,3-dichloropropene (Telone II) treatment of the
soil at 293 litre/ha.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.1.1 Rat
Groups of 6 adult male and 6 female Carworth Farm E rats
received, by stomach tube, 2.5-2.7 mg cis-1,3-dichloro-[2-
14C]propene or trans-1,3-dichloro-[2-14C]propene in 0.5 ml
arachis oil per rat, and excretion was followed. After 4 days, the
animals were killed and the radioactivity measured in skin and
carcasses. The excretion of radioactivity was very rapid, 80-90% was
eliminated in the faeces, urine, and expired air in the first 24 h.
The urine was the major route of elimination, i.e., 80.7 and 56.5%
(average of males and females) of the dose for cis- and trans-
1,3-dichloropropene, respectively. Only 2.6 and 2.2% of the 2
isomers, respectively, were eliminated in the faeces in 4 days,
while 3.9 and 23.5%, respectively, were eliminated as 14CO2 in 4
days in the expired air. Levels of the other volatile compounds in
air were only 1-3% of the dose. Up to 1% of the dose in the skin and
carcass was found. The difference in the amount of labelled CO2 in
expired air and urine indicated a difference in the kinetics of the
2 isomers (Hutson et al., 1971).
Groups of 8 adult Fischer 344 rats/sex were given non
radiolabelled 1,3-dichloropropene at 5 mg/kg body weight, in corn
oil, by gavage, for 14 consecutive days, prior to a single dose of 5
mg 14C-1,3-dichloropropene/kg body weight (actual 4.5 mg)
(uniformly labelled) (96.3%; 53.3% cis- and 43.0% trans-),
administered to 5 out of the 8 rats on day 15. The remaining 3
rats/sex were sacrificed. The distribution of radioactivity found in
the tissues (4-6%) of repeatedly dosed rats, 48 h after dosing, was
similar to that of single dosed animals. There was no sex difference
in the distribution of the radioactivity. In addition to the
repeatedly dosed rats, 2 rats of each sex, which had not been
previously dosed, received a single gavage dose of 5 mg 14C-1,3-
dichloropropene/kg body weight. The urine was the major route of
elimination of the radioactivity derived from 14C-1,3-
dichloropropene, which ranged from 60 to 65% of the administered
dose in 48 h in the rats with repeated doses and a single dose.
Elimination of 1,3-dichloropropene as 14CO2 was approximately
(average) 26% of the administrated radioactivity with about 4-5% of
the dose eliminated in the faeces, for all groups (Waechter & Kastl,
1988).
In another study, the fate of 14C- cis- and 14C- trans-
1,3-dichloropropene (97%; 62% cis and 38% trans) was determined
after a single oral dose of 1 or 50 mg/kg body weight to male
Fischer 344 rats (3 animals per dose level). Urine, faeces, expired
air, tissues, and remaining carcasses were analysed after 48 h.
Urine was the major route of excretion, 51-61% of the administered
dose being excreted over 48 h. In the carcass, 6% of the dose was
found at the end of 48 h. On the basis of interval excretion data,
half-lives for urinary excretion ranged from 5 to 6 h. Faeces and
expired CO2 accounted for roughly 18% and 6%, respectively. The
tissue concentrations of 14C activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, skin,
and fat (Dietz et al., 1984a,b, 1985).
6.1.1.2 Mouse
The fate of 14C- cis- and 14C- trans-1,3-dichloropropene
(97%; 62% cis and 38% trans) was studied after oral dosing of
male B6C3F1 mice with 1 or 100 mg/kg body weight (3
animals/dose level). Urine, faeces, expired air, tissues, and
remaining carcasses were analysed after 48 h. Urine was the major
route of excretion, with 63 and 79%, respectively, of the
administered doses (1 and 100 mg/kg body weight) being excreted over
48 h. Half-lives for urinary excretion ranged from 5 to 6 h. Faeces
and expired CO2 accounted for 15 and 14% of the 14C-
radioactivity, respectively. In the carcass, 2% was found. The
tissue concentrations of 14C-activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, fat,
and skin (Dietz et al., 1984a,b, 1985).
6.1.2 Inhalation
6.1.2.1 Rat
Stott & Kastl (1985, 1986) studied the pharmacokinetics of the
uptake of vapours of technical grade 1,3-dichloropropene (49.3%
cis- and 42.8% trans-isomer) and the disappearance of cis- and
trans-1,3-dichloropropene from the blood in groups of 3-6 male
Fischer 344 rats exposed to actual concentrations of 136, 409, 1362,
and 4086 mg/m3 for 3 h.
The uptake of 1,3-dichloropropene did not increase
proportionately with increasing exposure concentration due to an
exposure level-related decrease in the respiration rate and
respiration min/volume of rats exposed to > 409 mg 1,3-
dichloropropene/m3 and the saturation of metabolism of 1,3-
dichloropropene in rats exposed to > 1362 mg/m3. Absorption of
inhaled 1,3-dichloropropene occurred via the lungs, primarily in the
lower respiratory tract (approximately 50% of total inhaled), with a
small amount via the nasal mucosa (11-16%).
Following exposure to < 1362 mg/m3, both isomers were
rapidly eliminated from the blood, with a half-life of 3-6 min.
There was no interaction in the kinetics of both isomers. In
addition, data obtained on rats exposed to 1362 mg/m3 revealed
that this rapid elimination phase was followed by a slower
elimination phase having a half-life of 33-43 min. These data
demonstrated that a combination of saturable metabolism and
chemically-induced changes in respiration control 1,3-
dichloropropene uptake and body-burden in rats. However, only
decreases in respiration appear to influence vapour uptake.
Fisher & Kilgore (1988a) studied the excretion of the
mercapturic acid of cis-dichloropropene in Sprague-Dawley rats. In
a nose-only exposure system, groups of 3 rats were exposed for 1 h
to Telone II (94%) at average concentrations of 0, 181.6, 485.8,
1289.4, 1806.9, or 3582.1 mg/m3. Urine samples (24 h) were
collected and analysed for the mercapturic derivative of cis-
dichloropropene. At the lower exposure levels (< 1289.4 mg/m3),
urinary excretion of the mercapturic acid derivative increased with
exposure level. With exposure to 1806.9 or 3582.1 mg/m3, no
further increase was found, suggesting saturation of the metabolic
process.
6.2 Influence on tissue levels of glutathione
6.2.1 Oral
Oral administration of 1,3-dichloropropene to rats or mice
resulted in significant, dose-related reductions in the levels of
non-protein sulfhydryls (NPS) (indicator of tissue glutathione
concentration) in the forestomach and to a lesser extent in the
glandular stomach and liver (Dietz et al., 1984b, 1985, see also
section 6.4).
6.2.2 Inhalation
Shortly after inhalation exposure of rats to cis-1,3-
dichloropropene, kidney and liver NPS contents were reduced in a
dose-related manner, but returned to control values 18 h after
exposure. Lung NPS levels were not affected (Stott & Kastl, 1986,
see section 6.1.2.1; Nitschke & Lomax, 1990, see section 8.2.2.2).
Male Sprague-Dawley rats (200-250 g) were exposed through
inhalation to 1,3-dichloropropene (Telone II, 94%) concentrations of
0, 9.1, 22.7, 150, 1384.7, 3504.9, 4335.7, or 7790.6 mg/m3 to
assess the relationship between 1,3-dichloropropene exposure
concentration and tissue levels of reduced glutathione (GSH).
Animals were exposed for 1 h in a dynamic, nose-only system. GSH
contents were measured in the heart, kidneys, liver, lung, nasal
mucosa, and testes, 2 h after 1,3-dichloropropene exposure. A
decrease in nasal GSH, first seen at 22.7 mg/m3, followed an
exposure concentration-dependent curve. Exposure to concentrations
above 150 mg/m3 reduced the level of liver GSH. Lung GSH remained
relatively constant at 75% of control concentrations up to 4335.7
mg/m3. Significantly decreased GSH levels were observed in the
heart, liver, lung, and testes at 7790.6 mg/m3. Kidney GSH content
was not significantly decreased. Unchanged 1,3-dichloropropene was
not present in the blood of animals 2 h after exposure to 4335.7
mg/m3 or less. Serum lactic dehydrogenase activity was affected
only at 7790.6 mg/m3. Lung weight, measured 2 and 6 h after
exposure, did not differ from controls for any exposure level
(Fisher & Kilgore, 1988b).
Four male Sprague-Dawley rats (200-250 g) were exposed to
Telone II (94%) for 1 h, in a dynamic, nose-only exposure system.
The actual 1,3-dichloropropene concentration was 354.1 ± 49.9, 703.7
± 408.6, and 1834.2 ± 113.5 mg/m3 (relative concentrations of
cis- and trans-isomers were approximately 62 and 38%,
respectively). The GSH conjugation of 1,3-dichloropropene (GSCP) in
the blood of rats following exposure showed that there was no
significant difference between the regression line expressed as
either monophasic or biphasic decay at any exposure concentration.
Moreover, no differences were found in the regression lines between
the exposure concentrations. The elimination half-time of GSCP was
approximately 17 h following exposure to 354.1, 703.7, or 1834.2
mg/m3, and, thus, was not dose-dependent. This fits a one-
compartment model (Fischer & Kilgore, 1989).
6.3 Biotransformation
6.3.1 Rat
In urine from rats and mice treated orally with 14C-
dichloropropene, no unchanged parent compound, but 2 major and 2
minor metabolites were found. The predominant metabolite was N-
acetyl- S-(3-chloroprop-2-enyl) cysteine with its sulfoxide or
sulfone. These data indicate that conjugation with glutathione is a
major route of 1,3-dichloropropene metabolism in the rat (Dietz et
al., 1984a,b, 1985) (see Fig. 4 and section 6.1.1).
Although the spontaneous reaction of cis-1,3-dichloropropene
with glutathione is slow in the rat, the rapid urinary excretion is
due to hepatic glutathione transferase, which catalyses its
conjugation with glutathione. The transferase is present in the rat
liver cytosol fraction and little microsomally mediated metabolism
occurs. The cis-isomer is a better substrate than the trans-
isomer for glutathione transferase. The conjugation then follows a
classic mercapturic acid pathway (Boyland & Chasseaud, 1969). The
conjugated product N-acetyl- S-(3-chloroprop-2-enyl) cysteine and
its sulfoxide are excreted in the urine of rats and mice (Climie et
al., 1979; Dietz et al., 1984b; van Sittert, 1984, 1989).
It has been shown that a minor metabolic pathway of the cis-
1,3-dichloropropene is mono-oxygenase catalysed oxygenation, leading
to the possible formation of the metabolite cis-1,3-
dichloropropene-oxide (II in Fig. 5) (Van Sittert, 1989).
Rats administered 25-450 µg cis- and trans-1,3-
dichloropropene/kg body weight, intraperitoneally, showed excretion
of N-acetyl- S-( cis- and trans-3-chloroprop-2-enyl)-L-
cysteine for 55% (cis-) and 45% (trans-) of the dose within 24 h
(Onkenhout et al., 1986).
In the study of Waechter & Kastl (1988) (see section 6.1.1.1),
in which rats were administered daily doses of 5 mg of non-labelled
1,3-dichloropropene/kg body weight followed by a single dose of 5 mg
14-C (uniformly) labelled 1,3-dichloropropene, or a single dose of
5 mg/kg body weight, the major urinary metabolites were the
mercapturic acid of 1,3-dichloropropene (1,3-D-MA) and its
sulfoxide. The repeatedly dosed rats excreted slightly higher
percentages of the dose as mercapturic acids than the single dosed
rats (28.5% vs 22.7% for males and 25.5% vs 14.3% for females). The
isomeric ratio of the 2,3-D-MA was approximately 80% cis- and 20%
trans- for all groups.
6.3.2 Humans
Van Welie et al. (1989, 1991) determined the relationship
between respiratory occupational exposure to cis- and trans-1,3-
dichloropropene and urinary excretion of 2 mercapturic acid
metabolites, N-acetyl- S-( cis- and trans-)-3-chloroprop-2-
enyl)-L-cysteine ( cis- and trans-DCP-MA) by 12, 1,3-
dichloropropene applicators in the Netherlands. Urinary excretion of
these mercapturic acids followed first-order elimination kinetics.
Urinary half-lives of elimination were 5.0 ± 1.2 h for the cis-
mercapturic acid and 4.7 ± 1.3 h for the transform. These values
were not statistically significantly different. A clear correlation
was observed between the 8-h time-weighted average (TWA) exposure to
cis- and trans-1,3-dichloropropene and complete cumulative
urinary excretion of cis- and trans-DCP-MA. The cis-DCP-MA
yielded 3 times more mercapturic acid (45%) than the trans- form
(14%), probably because of differences in kinetics. It was concluded
that the uptake of cis- and trans-1,3-dichloropropene, their
metabolism to the corresponding mercapturic acids, and urinary
excretion was a rapid process.
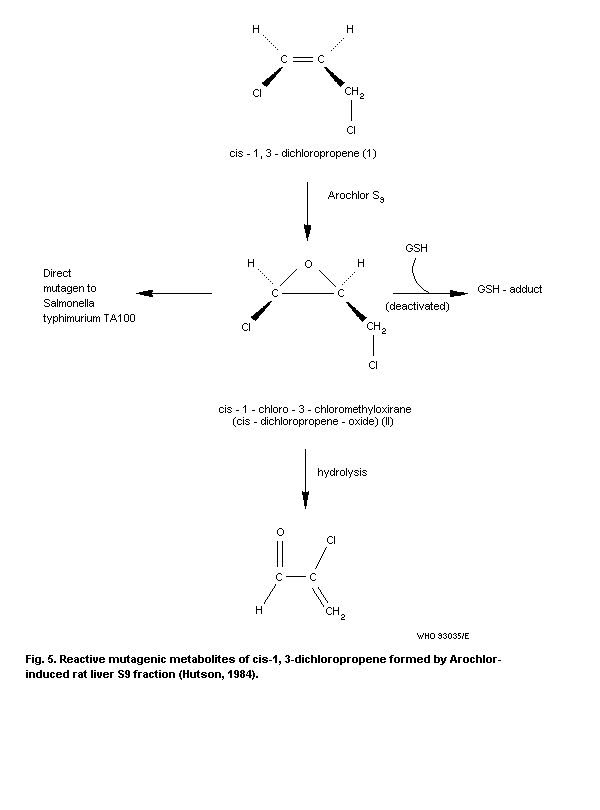 In California, applicators of 1,3-dichloropropene were also
studied for personal air exposure and urinary excretion of
mercapturic acid metabolites. The amount excreted was correlated
with the product of the duration of exposure x TWA. The highest
urinary metabolite concentration occurred during the application
period, indicating rapid excretion. Skin absorption of vapour was
not a significant route of exposure (Osterloh et al., 1984, 1989,
see also section 9.2.1).
Air and biological monitoring of 6 operators exposed to "Mix
DD" soil fumigant during filling operations in the Netherlands was
carried out in 1985-86. There was rapid metabolism and elimination:
the half-lives of mercapturic acid excretion were 4-5 h, with a
return to background levels after 24 h. It was calculated that,
under linear, non-saturation conditions, approximately 23% of the
inhalation dose of the cis-isomer and 10% of the trans-isomer
are excreted in the urine as mercapturic acids (Eadsforth, 1987).
6.4 Reaction with macromolecules
6.4.1 Mouse
The non-protein sulfhydryl (NPS) content, e.g., GSH, and
covalent binding to macromolecules were determined in the tissues of
male B6C3F1 mice. Single oral doses of 0, 1, 5, 25, 50, or 100
mg 1,3-dichloropropene 97% ( cis- 62% : trans-isomer 38%)/kg body
weight were given for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-
dichloropropene/kg body weight for binding studies. Non-glandular
forestomach, glandular stomach, liver, kidneys, and bladder were
analysed, 2 h after dosing. Although NPS depletion and dose-related
increases in macromolecular binding were noted in several tissues of
rats, these effects were more pronounced in the non-glandular
stomach than in any other tissue (including glandular stomach,
liver, kidneys, and bladder). The no-observed-effect level (NOEL)
for NPS depletion in rat non-glandular stomach was 1 mg/kg body
weight. NPS levels in non-glandular forestomach were significantly
decreased at doses of 25 mg or higher and, in the liver, at 100
mg/kg body weight. Binding in the non-glandular forestomach was
greatest at dose levels that caused the most depletion of tissue
NPS. Limited binding occurred in the liver, kidneys, and bladder
(Dietz et al., 1984b, 1985).
6.4.2 Rat
Groups of 3-9 male Fischer 344 rats (200-260 g) were
administered 50 mg cis-1,3-dichloropropene (94.1% cis- and 2.5%
trans-) or 50 mg trans-1,3-dichloropropene (97.3% trans- and
0.8 cis-)/kg body weight, by gavage. The rats were sacrificed at
various intervals after dosing, to determine the tissue non-protein
sulfhydryls (NPS) in the liver, kidneys, forestomach, glandular
stomach, and bladder. Blood samples were also taken to determine the
presence of unchanged 1,3-dichloropropene. Cis-1,3-dichloropropene
was only detected in the blood (6.58 µg/litre) 15 min after dosing,
the blood levels of trans-1,3-dichloropropene were 11.72 and 8.38
µg/litre, respectively, 15 and 45 min after dosing. A statistically
significant decrease in the non-protein sulfhydryl contents of the
liver, kidneys, forestomach, and glandular stomach was found. This
depletion reached a maximum, approximately 2-h after dosing. No
depletion was noted in the bladder. It is not possible to
distinguish the effects of cis- and trans-1,3-dichloropropene on
NPS, as the results for the individual isomers were not reported.
The results indicated that orally administered 1,3-dichloropropene
produces a rapid and significant depletion of tissue non-protein
sulfhydryls in the rat (Dietz et al., 1982).
The non-protein sulfhydryl (NPS) contents and covalent binding
to macromolecules were determined in the tissues of male Fischer 344
rats. Single, oral doses of 0, 1, 5, 25, 50, or 100 mg 1,3-
dichloropropene 97% ( cis-62% and trans-isomer 38%) were given
for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-dichloropropene/kg
body weight for binding studies. NPS levels in non- glandular
forestomach were significantly decreased with doses of 25 mg/kg body
weight or more. Binding in the non-glandular forestomach was
greatest at dose levels that caused a significant depletion of
tissue NPS. Limited binding was noted in the liver, kidneys, and
bladder (Dietz et al., 1984b, 1985).
6.5 Appraisal
Mice, rats and humans metabolize 1,3-dichloropropene
predominantly by conjugation with reduced glutathione (GSH). The
glutathione conjugate is further metabolized to the corresponding
mercapturic acid, which is then excreted in the urine. Consistent
with the function of GSH as a detoxication mechanism, the
genotoxicity of 1,3-dichloropropene was decreased in Salmonella
typhimurium when the concentration of GSH was increased to a
mammalian physiological concentration (see sections 8.6.1.2, 8.9.2).
The levels of GSH (measured as non-protein sulfhydryl content)
were decreased at locations consistent with the route of exposure,
i.e., predominantly in the forestomach and to a lesser extent in the
glandular stomach and liver, following oral administration, and in
the nasal tissue after inhalation exposure. For oral exposure, the
extent of covalent binding of 1,3-dichloropropene to macromolecules
was correlated with the decrease in non-protein sulfhydryl content.
It is anticipated that toxic effects of 1,3-dichloropropene will
occur at doses that deplete tissue sulfhydryls and will be
manifested in the organs described above (forestomach, liver, and
nasal tissue).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The United States Environmental Protection Agency published a
Health and Environmental Effects Profile for 1,3-Dichloropropene in
1985 (US EPA, 1985).
7.1 Acute toxicity
7.1.1 Microorganisms
The major effect of 1,3-dichloropropene on soil microorganisms
is on nitrogen transformation. The oxidation of ammonium from soil
organic nitrogen is reduced by 1,3-dichloropropene; soil ammonium
levels were significantly greater and soil nitrate levels were
significantly lower in soils treated with 1,3-dichloropropene
compared with untreated controls (Tu, 1973; Elliot et al., 1974,
1977).
In a series of studies, the effects of 1,3-dichloropropene at
30 or 60 mg/kg soil were evaluated, in parallel, under laboratory
conditions. In general, neither dose affected soil microorganisms or
function appreciably. The effects of 1,3-dichloropropene on soil
microorganisms and enzyme activity were not consistent in 3 soil
types (sandy-, clay-, and organic soils). The numbers of fungi, 2
days after fumigation, were significantly reduced in clay and sandy
soils, but not in organic soil, but recovered after 7 days. 1,3-
Dichloropropene either increased or decreased the number of non-
symbiotic nitrogen fixers and enzyme activity in different soils
(Tu, 1978, 1979, 1981a,b).
Moje et al. (1957) studied the individual components of "MIX
D/D" in old citrus soil and found that the toxicity was mainly due
to its 1,3-dichloropropene content, in particular that of the cis-
isomer (see also section 7.1.1 of "MIX D/D"). Results were as
tabulated on the next page.
1,3-Dichloropropene was converted by the methanotrophic
bacterium Methylosinus trichosporium OB3b, grown in aerobic
continuous cultures. The substance was added at a concentration of
0.2 mmol/litre. After 24 h, 85% of the substance added was degraded
(Oldenhuis et al., 1989).
In field experiments with continuous potato cropping, it was
found that sustained annual applications of 1,3-dichloropropene led
to insufficient control of Globodera rostochiensis. In the
laboratory, 1,3-dichloropropene rapidly disappeared from these
soils. This did not occur when the soil was sterilized. A bacterium
was isolated from these soils, which was found to decompose 1,3-
dichloropropene using it as a carbon and energy source. The
bacterium was Pseudomonas sp. Repeated application of 1,3-
dichloropropene led to accelerated degradation of the substance
(Lebbink et al., 1989).
Reduction in
Compound Fungi Bacteria and
actinomycetes
Cis-1,3-dichloropropene 85-95% reduction 85-100% reduction
at 25 mg/kg soil at 250 mg/kg soil
Trans-1,3-dichloropropene 100% reduction 100% reduction at
at 250 mg/kg soil 1000 mg/kg soil
7.1.2 Algae
The 96-h EC50 value for growth, based on the concentration of
chlorophyll a and also on cell numbers of the freshwater green algae
Selenastrum capricornutum in a static system, was 4.95 mg/litre
for 1,3-dichloropropene. The estuarine diatome, Skeletonema
costatum, showed a 96-h EC50 value for growth, based on the
concentration of chlorophyll a in culture, of 1 mg/litre (Leblanc,
1984). The EC50 calculated from cell numbers was 1.04 mg/litre (US
EPA, 1980). The compound is moderately toxic for marine algae.
The toxicity of cis-, trans- and a mixture of cis- and
trans-1,3-dichloropropenes for Selenastrum capricornutum was
determined in a sealed 72-h growth inhibition test. The 72-h EC50
values (percentage reduction in area under the growth curve),
expressed in terms of the mean measured concentration in the test
media, were; cis-isomer 2.8 mg/litre; trans-isomer 11 mg/litre;
and the mixture 8.2 mg/litre. The EC50 (percentage reduction in
mean specific growth rate), (24-48 h) and EC50 (24-72 h) values
determined by analysis of average specific growth rates were: cis-
isomer 4.6 and 3.1 mg/litre; trans-isomer 6.6 and 7.5 mg/litre;
and, for the mixture, 11 and 3.6 mg/litre, respectively (Girling,
1989a,b,c).
Rapid evaporation and sensitivity to hydrolysis are features of
the chloropropenes that may interfere with proper determination of
toxic concentrations of these chemicals for aquatic species, using
the standard techniques. Significant loss of the test substance may
have occurred during experiments carried out at temperatures above
20 °C and under "static" conditions, i.e., lasting for several days
without refreshing the medium. Determination of the actual
concentrations has seldom been carried out, and it is suspected that
several of the data reported for the chloropropenes present an
underestimation of the toxic potential of these chemicals for
aquatic organisms. On the other hand, the products of hydrolysis of
the chloropropenes, for instance, chloroallyl alcohols are
themselves toxic (Krijgsheld & Van der Gen, 1986).
7.1.3 Invertebrates
The acute toxicity of 1,3-dichloropropene for non-target
aquatic crustacea is summarized in Tables 6 and 7.
Table 6. Acute toxicity of 1,3-dichloropropene for non-target aquatic crustacea
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (mg/litre) (mg/litre)
Water flea 21 0.090a - Mayer &
(Daphnia magna) Ellersieck (1986)
Mysid shrimp - 0.79 US EPA (1980);
(Mysidopsis bahia) Leblanc (1984)
a 48-h EC50 6 mg/litre (US EPA, 1980; Leblanc, 1980) in a static system.
Leblanc (1980) calculated a no discernable effect concentration
of 0.41 mg/litre for 1,3-dichloropropene in Daphnia magna, under
static conditions. However, the result was based on nominal
concentrations and was higher than the 48-h LC50 given by Mayer &
Ellersieck (1986).
7.1.4 Honey bees
1,3-Dichloropropene has been tested on worker Honey bees (Apis
mellifera) using a dusting technique. The 48-h LD50 was 6.6
µg/bee and 1,3-dichloropropene was rated as "relatively non-toxic"
for Honey bees (Atkins et al., 1973).
Table 7. Acute toxicity of cis- and/or trans-1,3-dichloropropenes in Daphnia magna
Substance Age System Temperature 48-h EC50 Reference
(°C) (mg/litre)
Cis-1,3 DCP 24 h statica 18-22 1.4 Girling (1989a)
(96%)
Trans-1,3 DCP 24 h staticb 18-22 3.1 Girling (1989c)
(95.4% trans +
0.3% cis)
Cis- + trans- 24 h staticc 18-23 3.1 Girling (1989b)
mixture
(51-52% cis +
44% trans)
a pH 7.6-8.4; total hardness 176 mg/litre; dissolved oxygen 8.8-9.8 mg/litre.
b pH 7.8-8.1; total hardness 170 mg/litre; dissolved oxygen 8.6-9.2 mg/litre.
c pH 7.9-8.3; total hardness 179 mg/litre; dissolved oxygen 8.6-9.9 mg/litre.
7.1.5 Fish
The data summarized in Tables 8 and 9, suggest that 1,3-
dichloropropene is moderately toxic for fish.
Heitmuller et al. (1981) exposed sheepshead minnows to 1,3-
dichloropropene under static conditions. They calculated a no-
observed-effect concentration of 1.2 mg/litre, but this was based on
nominal concentrations.
7.1.6 Birds
Worthing & Hance (1991) reported an LC50 (8-day) for Mallard
duck and Bobwhite quail of > 10 000 mg/kg diet.
7.2 Short-term/long-term toxicity
7.2.1 Invertebrates
No data are available.
Table 8. Acute toxicity of 1,3-dichloropropene for fish
Species Type of test Size (g, mm) Temperature 96-h LC50 References
(°C) (mg/litre)
Fathead minnow static 0.9 g 18 4.1 Mayer & Ellersieck (1986)
(Pimephales promelas) (3.39-4.97)
Largemouth bass static 1.0 g 18 3.65a Mayer & Ellersieck (1986)
(Micropterus salmoides) (3.52-3.78)
Walleye static 1.3 g 18 1.08 Mayer & Ellersieck (1986)
(Stizostedion vitreum) (0.99-1.18)
Golden orfe - 2.8 g 20 9b Reiff (1978); Krijgsheld & Van
(Idus idus melanotus) (8-11) der Gen (1986)
Sheepshead minnow static 8-15 mm 25-31 1.8c,d US EPA (1980); Heitmuller
(Cyprinodon variegatus) (0.7-4.5) et al. (1981); Leblanc (1984)
Rainbow trout - - - 3.9 Worthing & Hance (1991)
(Salmo gairdneri)
Bluegill static 0.32-1.2 g 21-23 6.1c,d Buccafusco et al. (1981)
(Lepomis macrochirus) (5.1-6.8) US EPA (1980); Leblanc (1984)
Guppy semi-static - 22 0.5e Krijgsheld & Van der Gen (1986)
(Poecilia reticulata)
Goldfish static 1.0 g 18 < 7.5 Mayer & Ellersieck (1986)
(Carasius auratus)
Table 8 (continued)
a Tested in hard water, 272 mg CaCO3/litre.
b Tested in dechlorinated water, 260 mg CaCO3/litre.
c Tested in well water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration 9.7 mg/litre reduced
at the beginning to 0.3 mg/litre after 96-h exposure.
d Nominal concentrations.
e 14-day test.
Table 9. Acute toxicity of cis- and/or trans-1,3-dichloropropenes
in rainbow trout (Salmo gairdneri)
Substance Mean size System Temperature 96-h LC50 Reference
(cm, g) (°C) (mg/litre)
Cis-1,3 DCP 4.7 cm semi- 13-17 1.6 Girling (1989a)
(96%) (1.1 g) statica
Trans-1,3 DCP 4.0 cm semi- 15-17 4.5 Girling (1989c)
(95.4% trans + (0.67 g) staticb
0.3% cis)
Cis- + trans- 4.2 cm semi- 13-17 2.0 Girling (1989b)
mixture (0.68 g) staticc
(51-52% cis +
44% trans)
a pH 7-7.8; total hardness, 226-258 mg/litre as CaCO3: dissolved oxygen, 6.4-10.2 mg/litre.
b pH 7.2-7.8; total hardness, 234-264 mg/litre as CaCO3: dissolved oxygen, 8.2-9.9 mg/litre.
c pH 7.5-8.3; total hardness, 251-284 mg/litre as CaCO3: dissolved oxygen, 8.1-10.2 mg/litre.
7.2.2 Fish
Embryo-larval tests have been conducted on Fathead minnows
(Pimephales promelas) exposed to 1,3-dichloropropene. The maximum
no-effect concentration was 0.24 mg/litre (no details are available)
(US EPA, 1980).
7.2.3 Field studies
See section 7.3.2 of "Mixtures of dichloropropenes and
dichloropropane" for effects of "MIX D/D" on worms.
7.2.4 Phytotoxicity
1,3-Dichloropropene is highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The United States Environmental Protection Agency, published a
Health and Environmental Effects Profile for 1,3-dichloropropene in
1985 (US EPA, 1985).
In two other documents of the US EPA, health risk assessment
information is given, on the basis of a comprehensive review of the
toxicity data (US EPA, 1990, 1991).
8.1 Single exposures
8.1.1 Oral
The acute oral LD50s for mice and rats are summarized in
Table 10.
The following signs of toxicity were observed after oral
administration: hunched posture, lethargy, pilo-erection, decreased
respiratory rate, ptosis, diarrhoea, diuresis, ataxia, tip-toe gait,
red/brown staining around the snout, tremors, emaciation, and pallor
of the extremities. Haemorrhages and congestion were found in the
lungs and gastrointestinal tract. The livers showed patchy areas of
pallor (Jones & Collier, 1986a; Jeffrey et al., 1987; Gardner,
1989a,b,c).
8.1.2 Inhalation
The acute inhalation LC50s for 1,3-dichloropropene are
summarized in Table 11.
During the exposure and observation periods, the following
symptoms were observed: partial closing of the eyes, pilo-erection
salivation, lacrimation, lethargy, diarrhoea, reduction in
respiratory rate, irregular respiratory movements (lung congestion
was observed in dead animals) and hunched posture, brown staining of
fur and fur loss, and reddening of ears, tail, and feet.
Pathological signs were cardiopulmonary failure, acute tubular
necrosis in the kidneys, and local effects on the respiratory tract.
It was suggested that the irritating properties of the vapour
might serve as a warning of its presence in air. Signs of
intoxication included hypoactivity, anorexia, and chromodacryorrhoea
(Coombs & Carter, 1976b).
Table 10. Acute oral toxicity (LD50) of 1,3-dichloropropene
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse (CD 1) undiluted 215 Coombs & Carter
(1976b)
Mouse 92% (in corn oil) 640 (582-704)a Toyoshima et al.
(JCL:ICR) 640 (547-749)b (1978a)
Rat 94.5-97.5% cis-, 1.5% 85 Jeffrey et al.
(Fischer 344) trans-, 0.25% (1987); Gardner
1,2-dichloropropane, (1989b)
undiluted
Rat 96.7%, trans-, 94 Gardner (1989c)
(Fischer 344) undiluted 117a
78b
Rat (CD) undiluted 127 (112-141) Coombs & Carter
(1976b)
Rat (not 92% (in corn oil) 470b Torkelson & Oyen
specified) 713a (1977)
Rat (Sprague- 97.2% 130 (110-170)a Jones & Collier
Dawley) between 110 and 250b (1986a)
Rat D-D 95, undiluted 57 Gardner (1989a)
(Fischer 344) (cis + trans 1 : 1)
Rat 92% (in corn oil) 560 (452-695)a Toyoshima et al.
(Wistar) 510 (480-726)b (1978b)
Table 10 (contd)
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Rat 97.5% 300a Jeffrey et al. (1987)
(Fischer 344) 224b
a Male.
b Female.
Table 11. The acute inhalation toxicity LC50 for 1,3-dichloropropene (4-h exposure)
Species Concentration LC50 (mg/m3) Reference
of substance
Rat 51% cis-, 43.4% 3309.7 Blair (1977)
(Wistar) trans-isomer, 1%
epichlorohydrin
Rat Telone II 2.70-3.07c Cracknell et al.
(Wistar) (98.4%) (1987)
Rat 95.6% cis- and 3041.8a Nitschke et
(Fischer 344) 1.5% trans-isomer 3377.8b al. (1990)
Rat Telone II (97.5%; > 3881.7-< 4698.9a Streeter et al.
(Fischer 344) 52.6% cis- and 4014.2b (1987)
44.9% trans-isomer)
Rat 95.4% trans- and 4880.5b Collins (1989)
(Crb:CD(SD)Br) 0.3% cis-isomer 5402.6a
a male.
b female.
c mg/litre.
A 2-h exposure to 1,3-dichloropropene at 4540 mg/m3 was
lethal to rats, whereas guinea-pigs died following a single 7-h
exposure to 1816 mg/m3 (Torkelson & Oyen, 1977).
One female and 3 male Rhesus monkeys (4-5 kg) were exposed to
Telone II at 0, 113.5, 227, 454, 908, or 2724 mg/m3 for a single
behavioural session of 1 h, with a 1-week recovery period after
exposure. Data were obtained on all monkeys for each of the 5
atmospheric concentrations. At concentrations ranging up to 908
mg/m3, there were no significant indications of toxicity or
alterations in behaviour (the monkeys were trained to perform on a
dual component FRFR (Fixed Ratio followed by a Fixed Ratio) chained
schedule, with light stimulation). Only slight eye irritation was
observed in all monkeys at concentrations exceeding 454 mg/m3
(Rosenblum & Talley, 1979).
8.1.3 Dermal
The acute dermal and subcutaneous LD50s are summarized in
Table 12.
After dermal application, the signs of intoxication were:
diarrhoea, lethargy, hunched posture, decreased respiratory rate,
with lacrimation, salivation, ataxia or abasia, loss of righting
reflex, diarrhoea, diuresis, and red/brown staining around the eyes,
snout or mouth. The lungs and gastrointestinal tract showed
haemorrhages and irritation. Signs of skin irritation manifested by
oedema, eschar formation, or subcutaneous haemorrhage were apparent
(Jones & Collier, 1986b).
One 24-h application of undiluted 1,3-dichloropropene to
intact, occluded New Zealand white rabbit skin caused extremely
severe eschar, resulting in black necrotic tissue. The skin had
hardened and was cracking after 7 days. The animals used for dermal
LD50 estimation with 1,3-dichloropropene showed lethargy and
hypothermia at dose levels above 300 mg/kg body weight (Coombs &
Carter, 1976b).
8.2 Short-term exposures
8.2.1 Oral
Albino rats derived from the Wistar strain, were used in a 90-
day test. Groups of 10 male and 10 female rats received doses (by
gavage) of 0, 1, 3, 10, or 30 mg 1,3-dichloropropene (40% cis- and
28% trans-isomer)/kg body weight on 6 days/week. General
Table 12. Acute dermal and subcutaneous LD50s for 1,3-dichloropropene
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(JCL:ICR) (1978a)
Rat dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(Wistar) (1978b)
Rat (CD) dermal undiluted 0.423 Coombs & Carter
(0.336-0.555) (1976b)
Rat (Sprague- dermal 97.2% 1.0 (0.8-1.3)a Jones & Collier
Dawley) between 1.3 and 2.0b (1986b)
Rat dermal 96.7%, trans-isomer 1.575 Gardner (1989c)
(Fischer 344) undiluted
Rat dermal 94.5-97.5% 1.09 Gardner (1989b)
(Fischer 344) cis-, 1.5%
trans-, 0.25%
1,2-dichloropropane
undiluted
Rabbit (not dermal 92% 0.504 Torkelson & Oyen
specified) undiluted (0.22-1.15) (1977)
Mouse subcutaneous 92% (in corn oil) 0.33 (0.29-0.376)a Toyoshima et
(JCL:ICR) 0.345 (0.30-0.40)b al. (1978a)
Table 12. (contd)
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Rat subcutaneous 92% (in corn oil) 0.40 (0.345-0.464)a Toyoshima et
(Wistar) 0.366 (0.305-0.439)b al. (1978b)
a Male.
b Female.
condition, behaviour, and survival were not affected at any dose
level. No distinct differences in haematological indices, serum
enzyme activities, or urinalysis were observed. The relative kidney
weights were significantly increased in the 30-mg group in both
sexes and also in the males receiving 10 mg/kg. The relative liver
weights of females at 30 mg/kg were significantly increased. Gross
and microscopic examination did not reveal any abnormalities in the
main organs. The 3 mg/kg body weight dose was without effect (Til et
al., 1973).
8.2.2 Inhalation
8.2.2.1 Mouse
CD-1 Albino mice, 10/sex per group were exposed to production
grade Telone II (whole-body exposure) at 0, 45.4, 136.2, or 408.6
mg/m3 for 6 h/day, 5 days/week, for 90 days. No significant
differences in lesions were found between the control and treated
groups upon gross and histological examination, except that there
were compound-related effects on the nasal turbinates. These effects
were decreased height of the nasal epithelial cells resulting from a
loss of cytoplasm, and disorganization of the nuclei. Necrotic cells
were only observed among the females exposed to 408.6 mg/m3. No
alterations were found in the mice exposed to 136.2 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 10 male and 10 female B6C3F1 mice were exposed
(whole body) by inhalation to technical grade 1,3-dichloropropene
90.9% ( cis 48.6% and trans 42.3%) containing 2.4% 1,2-
dichloropropane, 5.5% mixed isomers of chlorohexane, chlorohexene,
and trichloropropene, and epichlorohydrin 1.2% (as stabilizer). The
actual exposures were to levels of 0, 45.4, 136, 409, or 681 mg/m3
for 6 h/day, 5 days/week for 13 weeks. Extensive haematological,
clinical-chemical, and urinalysis studies, and histopathological
examination of organs and tissues were performed.
A treatment-related depression in body weight was seen at doses
of 409 and 681 mg/m3. In animals exposed to 681 mg/m3, transient
brown discoloration of the fur with a strong mercaptan odour in the
coats and urine was found. The poor growth rate was reflected in a
decrease or increase in the relative weights of a number of organs,
but without histological alterations. An exposure-related decrease
in BUN levels was observed in male mice exposed to 409 and 681
mg/m3. Furthermore, alanine transaminase levels were increased in
mice exposed to 681 mg/m3. No other changes were found. The
primary target tissues of inhaled 1,3-dichloropropenes were the
nasal mucosa and urinary bladder. The changes in the nasal mucosa
including slight degeneration of the olfactory epithelium and slight
hyperplasia of respiratory epithelium in animals exposed to 409 and
681 mg/m3 were dose-related. The animals also had small focal
areas of nasal metaplasia, a condition in which the damaged sensory
olfactory epithelium is replaced by ciliated respiratory epithelium
(only in animals exposed to 681 mg/m3).
The urinary bladders of female mice exposed to 409 and 681
mg/m3 (7/10 and 6/10, respectively) had areas of moderate
hyperplasia of the transitional epithelium (7-10 layers thick in
contrast with 2-3 layers in control mice). Submucosal aggregates of
lymphoid cells in the bladder, not associated with hyperplasia and
not treatment-related, were found in female mice exposed to
concentrations of 136 mg/m3 or more. No treatment-related effects
were found in mice exposed to 45.4 mg/m3 (Stott et al., 1984,
1988).
8.2.2.2 Rat
Fischer 344 rats, 10/sex per group, were exposed to 0, 45.4,
136, or 409 mg/m3 of production grade Telone II, for 6 h/day, 5
days/week for 90 days. No significant differences in lesions were
found between the control and treated groups on gross or
histological examination, except that compound-related effects on
the nasal turbinates were found. These effects included decreased
height of the nasal epithelial cells resulting from a loss of
cytoplasm, and a disorganization of the nuclei. Necrotic cells were
observed in all of the male and female rats exposed to 409 mg/m3
and in 6/10 of the female rats exposed to 136 mg/m3. No
alterations were found in the animals with 45.4 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 5 male rats were exposed to atmospheres containing
concentrations of 1,3 cis-/trans-dichloropropene (92%) of 0 or
13.6 mg/m3 for 0.5, 1, 2, or 4 h/day, 5 days/week for 6 months.
The only effect in all the exposed groups was a slight, apparently
reversible change, seen microscopically in the kidneys of male rats
(Torkelson & Oyen, 1977).
Groups of 24 male and 24 female rats were exposed repeatedly to
air containing 0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7
h/day, 5 days/week for 6 months. Changes attributable to 1,3-
dichloropropene were limited to cloudy swellings in the renal
tubular epithelium of male rats in the 13.6 mg/m3 group. Female
rats in this group showed an increased liver to body weight ratio,
though no histopathological changes were observed. A recovery group
of male and female rats was maintained for 3 months following the 6-
month exposure to 4.54 or 13.6 mg/m3. No changes were observed in
the renal epithelium following this recovery period (Torkelson &
Oyen, 1977).
In a well-designed study (Stott et al., 1984, 1988), groups of
10 male and 10 female Fischer 344 rats were exposed through
inhalation to technical grade 1,3-dichloropropene 90.9% (for
composition see section 8.2.2.1) in actual concentrations of 0,
45.4, 136, 409, or 681 mg/m3, for 6 h/day, 5 days/week for 13
weeks. Haematological, clinical-chemical studies, urinalysis, and
histopathological studies of organs and tissues were carried out.
In the rats exposed to 681 mg/m3, transient brown
discoloration of the fur with a strong mercaptan odour in the coats
and urine was found. The body weights of rats exposed to 409 or 681
mg/m3 were decreased in an exposure-related manner. The relative
weights of the testes were increased and thymus weights decreased.
Rats exposed to 409 and 681 mg/m3 had slightly lower levels of
serum proteins. No other treatment-related changes in clinical-
chemical or haematological parameters were found.
The primary target tissues of inhaled 1,3-dichloropropenes were
the nasal mucosa in male and female rats and the uteri in females.
The effects consisted of dose-related degenerative effects on the
nasal olfactory epithelium or mild hyperplasia of the respiratory
epithelium or both, in all animals exposed to 409 and 681 mg/m3,
and 2 of the 10 male animals in the 136 mg/m3 group.
The uteri of 7 out of 10 female rats exposed to 681 mg/m3
were not developed as completely as those of control animals,
suggesting hypoplasia of the uterine tissues. The only other change
noted histologically was the atrophic appearance of the mesenteric
adipose tissue in the rats exposed to 681 mg/m3. No treatment-
related effects were observed in rats of either sex exposed to 45.4
mg 1,3-dichloropropenes/m3 (Stott et al., 1984, 1988).
In a study to investigate the effects of 1,3-dichloropropene on
tissue sulfhydryl levels, groups of 15 male and 15 female Fischer
344 rats were exposed to vapours of cis-1,3-dichloropropene (94.3-
95.6% cis-, 1.5% trans-1,3-dichloropropene and 0.2% 1,2-
dichloropropane) at 0, 45.4, 272.4, or 681.0 mg/m3 for 6 h/day, 5
days/week for 9 exposures. Whole-body exposures occurred under
dynamic air-flow conditions. On the day after the last exposure, 5
males and 5 females/dose level were necropsied. Major organs were
weighed and selected tissues were evaluated histopathologically.
Groups of 5 animals/sex per dose level were used to determine the
non-protein sulfhydryl (NPS) contents of the liver, kidneys, and
lung, 1 and 18 h following the last exposure.
Rats exposed to 681 mg/m3 lost weight, but not those exposed
to 272.4 mg/m3. There were concentration-related decreases in
liver, kidney, and lung NPS levels in male rats, when measured after
1 h. After 18 h, the NPS levels were higher than those of the
controls and there were no associated gross or histopathological
changes in these organs. The NPS measurements for females were
unremarkable. Histopathological examination revealed changes of
moderate severity in the respiratory and olfactory mucosa in the
nasal cavity of males and females exposed to 681 mg/m3. There were
no changes in the animals exposed to 272.4 mg/m3 (Nitschke &
Lomax, 1990).
Groups of 10 male and 10 female Fischer 344 rats (6 weeks old)
were exposed to cis-1,3-dichloropropene at 0, 45.4, 136, or 409
mg/m3 for 6 h/day, 5 days/week for 13 weeks. The test material was
reported to consist of 94.3% (95.6%) cis-1,3-dichloropropene, 1.5%
trans-1,3-dichloropropene and 0.2% 1,2- dichloropropane. Whole-
body exposures occurred under dynamic conditions. No exposure-
related effects were noted in haematology or clinical chemistry. At
409 mg/m3, body weights of male rats were significantly decreased
compared with controls throughout the 13-week exposure period; body
weights of female rats were significantly decreased during the first
6 weeks, but were comparable with those of the controls at the end
of the study. As a result of the decreased body weight in male rats,
the relative liver, kidney, lung, and testes weights were
significantly elevated in comparison with control values. The
relative liver weight in females was also increased. These organ
weight changes were not accompanied by gross or histopathological
changes. Exposure-related changes only occurred in the nasal
cavities of the rats of both sexes exposed to 409 mg/m3 and
consisted of multifocal bilateral degeneration of olfactory
epithelium and slight bilateral multifocal hyperplasia of
respiratory epithelium. No effects were noted in rats exposed to
45.4 or 136 mg/m3. The NOEL was considered to be 136 mg cis-1,3-
dichloropropene/m3 (Nitschke et al., 1991).
8.2.2.3 Other animal species
Groups of 12 male and 12 female guinea-pigs, 3 male and 3
female rabbits and 2 dogs were exposed repeatedly to air containing
0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7 h/day, 5
days/week for 6 months. No changes resulting from 1,3-
dichloropropene exposure were observed in guinea-pigs, rabbits, or
dogs (Torkelson & Oyen, 1977).
8.3 Skin and eye irritation, sensitization
8.3.1 Skin irritation
1,3-Dichloropropene is extremely irritating to the skin
(Worthing & Hance, 1991).
Telone II (52.63% cis- and 44.91% trans-1,3,-
dichloropropene), 0.5 ml, was applied for 4 h to the back (clipped
free of fur) of 2 male and 4 female New Zealand White rabbits. After
4 h, the wrapping and gauze patch and any residual test substance
were removed. Dermal irritation characterized as slight to moderate
erythema and moderate to severe oedema was observed at the site of
application immediately following the 4 h exposure period.
Subsequent observations revealed slight to moderate erythema,
oedema, and exfoliation. These changes were still present in some of
the animals after 14 days (Jeffrey, 1987a).
In a 4-h rabbit skin irritancy test, undiluted cis-1,3-
dichloropropene (94.5-97.5% cis-, 1.5% trans-1,3-dichloropropene
and 0.25% 1,2-dichloropropane) caused well defined erythema and
moderate oedema shortly after removal of the semi-occlusive
dressings in New Zealand White rabbits (3-5 months of age). After
removal of the dressings, the skin was washed after 4 h. Resolution
of the irritancy reaction was first apparent on the day after
treatment and was complete within 14 days (Gardner, 1989b).
In another 4-h rabbit skin irritancy test, 0.5 ml of undiluted
trans-1,3-dichloropropene (96.7%) caused irritation not exceeding
well-defined erythema and slight oedema. New Zealand White rabbits
(3-5 months of age) were used. After the 4-h exposure, the dressings
were removed and the skin washed. Resolution of the irritation was
first apparent 72 h after treatment and was complete by day 21
(Gardner, 1989c).
8.3.2 Eye irritation
1,3-Dichloropropene is a severe eye irritant (Worthing & Hance,
1991).
Aliquots of 0.1 ml Telone II (52.63% cis- and 44.91% trans-
1,3-dichloropropene) were instilled into the conjunctival sac of one
eye of 4 male and 2 female New Zealand White rabbits. The eyes of
all rabbits remained unwashed. Slight to marked redness and slight
to moderate chemosis were found after the treatment. The treated
eyes had a slight to marked amount of discharge as well as reddening
of the iris. In one animal, opacity was found which resolved as did
all the other changes within 14 days following treatment (Jeffrey,
1987b).
In the rabbit eye irritancy test, 0.1 ml trans-1,3-
dichloropropene (96.7%) caused moderate to severe conjunctival
irritation and minor irritation changes of the cornea (opacities),
chemosis, and iridial responses within 24 h following instillation
into the eye. Resolution of the irritant effects of trans-1,3-
dichloropropene was advanced 7 days after treatment and complete one
week later. There was an initial pain response (Gardner, 1989c).
Ten male and 10 female Sprague-Dawley rats (Spartan substrain)
were exposed to an aerosol (mean size 2.96 µm; 99% of particles were
6 µm or less in diameter) in a glass chamber for 1 h at a nominal
concentration of 5.2 mg/litre of air. Five male and 5 female animals
were maintained under ambient conditions as controls. Slight
transitory eye irritation was observed during the exposure (Yakel &
Kociba, 1977).
8.3.2.1 In vitro studies
The corneal thickness of isolated eye preparations subjected to
application of cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) increased
by more than 20% within 3 h. The isolated rabbit eye test described
by Price & Andrews (1985) was used. Corneal uptake of fluorescein
was demonstrated at the conclusion of the test. The results
indicated that application of cis-1,3-dichloropropene to the eye
in vivo would cause significant tissue damage (Gardner, 1989b).
8.3.3 Sensitization
1,3-Dichloropropene was applied in corn oil in a 5% v/v
concentration 3 times topically to the skin of guinea-pigs followed
by a 1% concentration as a challenge following the method of Buehler
(1965). A positive reaction was obtained in 5 out of 20 guinea-pigs
of the "P" strain. The reaction was considered to be mild to
moderate skin sensitization (Coombs & Carter 1976b).
Ten male, Hartley albion guinea-pigs received 3 dermal
applications on the back of 0.4 ml of 0.1% (v/v) Telone II (52.63%
cis- and 44.91% trans-1,3-dichloropropene) in mineral oil;
another group received only the vehicle during the induction phase
of the study. The dermal sensitization potential was tested using
the modified Buehler method. A positive control group received 2
applications of 10% epoxy resin and a third application of 5% epoxy
resin. All groups were challenged dermally 2 weeks after the last
induction application. The control group did not show any signs of
sensitization, while 5 out of the 10 animals of the positive control
group revealed slight erythema. Nine out of 10 guinea-pigs
challenged with 0.1% Telone II revealed slight to moderate erythema.
Telone II was considered a potential skin sensitizer at the
concentrations tested (Jeffrey, 1987c).
In the guinea-pig maximization test of Magnusson & Kligman, all
20 test animals showed positive responses, 24 and 48 h after removal
of the challenge patches. Guinea-pigs of the Dunkin-Hartley strain
(5-9 weeks of age) were used in this study. In a study by Gardner
(1989b), 0.1% cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) was
injected intradermally; topical induction was carried out using a 5%
solution in corn oil, and the topical challenge using 2.5% in corn
oil.
In the guinea-pig maximization test of Magnussen & Kligman, 16
out of 20 test animals showed positive responses 24 and/or 48 h
after removal of the challenge patches. Guinea-pigs of the Dunkin-
Hartley strain (age 5-9 weeks) were used. Intradermal injection,
carried out at concentrations of 0.05% trans-1,3-dichloropropene
(96.7%) or more resulted in red areas with defined edges. The
concentration selected (10%) for topical induction did give slight
irritation, but the concentration for the topical challenge of 5%
was without effect. The solvent was corn oil (Gardner, 1989c).
8.4 Long-term exposure
See section 8.7 (Carcinogenicity).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Inhalation (rat)
Groups of 30 male and 30 female Fischer 344 rats (6 weeks of
age) were exposed to Telone II (1,3-dichloropropene containing 2%
epoxidized soybean oil) at 0, 45.4, 136, or 409 mg/m3 (first 7
days of study: 0, 22.7, 90.8, or 272.4 mg/m3) for 6 h/day, 5
days/week during the premating period and for 7 days/week during
breeding, gestation, and lactation, for 2 generations. The Telone II
used had a purity of 92% and the remainder of the test material
comprised chlorinated and unchlorinated alkanes and alkenes as well
as approximately 2.0% epoxidized soybean oil. Following 10 weeks of
exposure, the adult rats (F0) were mated twice to produce the
F1a and F1b litters. After weaning, 30 pups/sex per exposure
from the F1b litters were selected and, after 12 weeks, used to
produce the F2a and F2b litters.
All litters were examined on the day of parturition, the
following indices of fertility being recorded: gestation length,
litter size, pup survival indices, number of live pups on days 1 up
to 28 postpartum, sex and weight of litters, lactation and
individual body weights, and any visible physical abnormalities.
Gross necropsy was carried out on adult rats F0 and F1 and
weanling F1b and F2b rats and histopathological studies on adult
rats. Inhalation of up to 409 mg/m3 for 2 generations did not
adversely affect reproduction or neonatal growth or survival.
Exposure to 409 mg/m3 resulted, however, in parental toxicity
(F0, F1), as indicated by decreases in body weight and
histopathological effects in the nasal mucosa (slight, focal
hyperplasia of the respiratory mucosal epithelium and/or focal
degenerative changes in the olfactory epithelium). No adverse
effects were observed in the parents in the 136 mg/m3 group. The
reproductive no-effect level was 409 mg/m3 (Breslin et al., 1987,
1989).
8.5.1.2 Intraperitoneal (mouse)
1,3-Dichloropropene (Telone II) in corn oil was injected
intraperitoneally in B6C3F1 male mice (4 per group) at dose
levels ranging from 10 mg up to 600 mg/kg body weight, daily for 5
days, to study sperm morphology, epididymal sperm counts, and testes
weights. Testicular toxicity was assessed at day 35. No animals
survived at dose levels of 150 mg/kg body weight or more. No effects
on the testes were found at dose levels up to 75 mg/kg body weight
(Osterloh et al., 1983).
8.5.2 Teratogenicity
8.5.2.1 Inhalation (rat)
In a range-finding study, groups of 7 or 8 female Fischer 344
rats were exposed to Telone II (92.1%; 47.7% cis- and 42.4%
trans-1,3-dichloropropene, impurities, and 1.8% epichlorohydrin)
at 0, 230, 680, or 1360 mg/m3 for 6 h/day on days 6-15 of
gestation.
Consumption of food and drinking-water was decreased during the
period of exposure in rats exposed to 680 or 1360 mg/m3 and the
rats showed a significant decrease in body weight gain. Rats were
sacrificed on day 16 and examined. During exposure, nasal exudate
and red crusty material around the eyes were observed. In the group
exposed to 1360 mg/m3, a significant decrease in litter size and
significant increase in resorption were seen, while in the groups
exposed to 230 and 680 mg/m3, the same changes were observed, but
were not statistically significant (Kloes et al., 1983).
Groups of 30 Fischer 344 rats were exposed, via inhalation, to
1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-isomer) at
0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-15 of
gestation. Food consumption and maternal body weight gain were
depressed in all treated groups in a dose-related manner. A decrease
in water consumption was found in rats at the highest dose-level. No
consistent or dose-related effects on reproductive performance were
found. The number of implantations, resorptions, litter size, fetal
weights, and fetal lengths were comparable with the controls. A
slight, but statistically significant, dose-related increase in the
incidence of delayed ossification of the vertebral centra was found
in rats, but considered of little toxicological significance in the
light of the maternal toxicity observed. The authors stated that
there was no evidence of a teratogenic or embryotoxic response at
exposure levels up to and including 545 mg/m3 (John et al., 1983;
Hanley et al., 1987).
8.5.2.2 Inhalation (rabbit)
In a range-finding study, groups of 7 New Zealand White rabbits
were exposed to Telone II (92.1%, 47.7% cis- and 42.4% trans-
1,3-dichloropropene, impurities and 1.8% epichlorohydrin) at 0, 230,
680 or 1360 mg/m3 for 6 h/day on days 6-18 of gestation. The
rabbits were sacrificed on day 19 and examined. Six out of 7 rabbits
exposed to 1360 mg/m3 showed signs of toxicity, such as rear limb
ataxia, decreased or absence of righting reflex, and flaccid hind
limb muscles; these animals died or were sacrificed. Histologically,
no effects were detected in the brains of these animals. Maternal
body weight was statistically significantly decreased during
exposure in the 680 mg/m3 group, but weights in the 230 mg/m3
group were comparable with those of the controls. There were no
differences in the reproductive parameters between the groups
exposed to 230 and 680 mg/m3 and the controls. No teratological
effects were found (Kloes et al., 1983).
Groups of 25-31 inseminated New Zealand white rabbits were
exposed to 1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-
isomer) at 0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-18
of gestation. Decreased weight gain was observed among rabbits at
272 and 545 mg/m3, but no pronounced maternal toxicity was
observed. No evidence of teratogenic or embryotoxic responses was
observed (John et al., 1983; Hanley et al., 1987).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies
8.6.1.1 Microorganisms
Cis- and trans-1,3-dichloropropene or a mixture were tested
for their mutagenic activity in Salmonella typhimurium and in
Saccharomyces cerevisiae, with and without metabolic activation.
Most of the studies showed a positive effect, especially with
Salmonella typhimurium TA100 and TA1535, with, and without,
metabolic activation. With TA98 and TA1537, positive and negative
effects were found. Saccharomyces cerevisiae was positive. The
results are summarized in Table 13. Samples of highly purified 1,3-
dichloropropene or cis-1,3-dichloropropene, however, were not
mutagenic to Salmonella typhimurium TA100, indicating that trace
impurities, such as 1,3-dichloropropene oxide, were the cause of the
activity (Talcott & King, 1984; Watson et al., 1987, see section
8.8.2).
There was a difference in mutagenicity (expressed as rev/µmol)
between the cis and trans isomers of 1,3-dichloropropene in
Salmonella typhimurium TA100, with, and without, metabolic
activation. The cis-isomer induced a greater number of revertants,
with, and without, S9 mix, than the trans-isomer and also showed
stronger alkylating properties in the NBP-test. Furthermore, a
longer preincubation time invariably led to higher mutagenic
activity, and an increase in protein added to the activating system
increased the efficiency of the metabolic activation (Neudecker et
al., 1980, Neudecker & Henschler, 1986).
Salmonella typhimurium TA100 was used to test for the
mutagenicity of cis- and trans-3-chloroallyl alcohol (99%),
with, and without, metabolic activation. No mutagenicity was
observed without S9 mix, over a range of 0.01-1000 µg/plate.
However, a positive effect was obtained with S9 mix and cis-3-
chloroallyl alcohol (in high concentration) (Connors et al., 1990).
Von der Hude et al. (1988) used the SOS chromotest with
Escherichia coli PQ 37, to examine 1,3-dichloropropene (in DMSO)
at concentrations of 0, 1.0, 3.3 or 10.0 mmol/litre, without S9. In
this strain, the structural gene for beta-galactosidase lacZ is
placed under the control of the SOS-gene sfiA. The expression of
this gene, induced by DNA-damage, is measured indirectly by
determination of the beta-galactosidase activity. The test was
positive with concentrations of 1.0 mmol/litre or more.
8.6.1.2 Effects of glutathione on bacterial mutagenesis
The addition of glutathione (GSH), at physiological
concentrations, to in vitro bacterial test systems of Salmonella
typhimurium TA100 has shown a clear protective effect, i.e., a
virtual elimination of the mutagenic response of 1,3-dichloropropene
(Brooks et al., 1978; Climie et al., 1979; Creedy & Hutson, 1982;
Wright & Creedy, 1982; Creedy, 1983; Creedy et al., 1984; Brooks &
Wiggins, 1990). This protective effect occurs in either the presence
or the absence of S9 for both the (cis)- and (trans)-isomers.
Protection, in the presence of S9, is consistent with the operation
of glutathione transferase enzyme occurring in the added S9
fraction. This enzyme is also present in mammalian cells, and as the
metabolic studies have shown, it plays a key role in the rapid
detoxification of cis-1,3-dichloropropene in mammalian tissue
(Climie et al., 1979; Brooks & Wiggins, 1991).
Even in the absence of S9, this protection by GSH still exists
against the mutagenic action of 1,3-dichloropropene. Thus, it is
likely that an additional mechanism for protection exists that
probably reflects a reaction between the mutagenic component(s) and
GSH. This additional mechanism may also play an important role in
the detoxification of 1,3-dichloropropene via a spontaneous
nucleophilic substitution reaction between the chloromethyl carbon
of 1,3-dichloropropene and the sulfur atom of glutathione.
Table 13. Mutagenicity tests with 1,3-dichloropropenes (1,3-DCP) on microorganisms
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
Salmonella typhimurium
Cis-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 top agar S9 mix/none + Neudecker et al. (1977)
(99.9%) µg/ml Kier et al. (1986)
Cis-1,3-DCP TA98, TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
(> 99%)
TA1538 20-2000 µg plate S9 mix/none - Brooks et al. (1978)
Cis-1,3-DCP TA100, TA1535 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5%, + TA98, TA1537, TA1538 78-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1990)
1,2-DCP 0.25%)
Cis-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 µg/ml top agar S9 mix/none + Neudecker et al. (1977)
(97.5%) Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535 39-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1989a)
(98% + cis-
1,3-DCP 0.3%) TA98, TA1537, TA1538 39-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1989a)
1,3-DCP (51.3% TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
cis, 43.7%
trans, 0.6% TA98, TA1538 20-2000 µg plate S9 mix/none ± Brooks et al. (1978)
epichlorohydrin)
Table 13 (contd)
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
1,3-DCP *** TA100, TA1535 3-333 µg/ml plate S9 mix/none + Haworth et al. (1983)
TA98, TA1537 3-333 µg/ml plate S9 mix/none - Haworth et al. (1983)
1,3-DCP *** TA100, TA1535 1000 µg/ml * S9 mix/none + Priston et al. (1983)
TA98, TA1537 1000 µg/ml * S9 mix/none - Priston et al. (1983)
1,3-DCP *** TA100 0.1-10 µmol plate S9 mix/none + Stolzenberg & Hine (1980)
1,3-DCP *** TA98 100 µg ** none + Vithayathil et al. (1983)
Saccharomyces cerevisiae
1,3-DCP *** JD1 1000 or 5000 liquid S9 mix/none + Priston et al. (1983)
µg/ml
culture
Escherichia coli
Cis-1,3-DCP WP2 UVRA pKM 101 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5% +
1,2-DCP 0.25%)
Trans-1,3-DCP WP2 UVRA pKM 101 39-1250 µg/ml * none - Brooks & Wiggins (1989a)
(98% + cis-1,3- S9 mix +
DCP 0.3%)
* Assays were performed by the pre-incubation method in sealed containers.
** Salmonella/microsome multiple indicator test.
*** Details of composition not given.
These 2 mechanisms with GSH afford complete protection against
the mutagenic activity of the trans-isomer in bacterial test
systems, in the presence and absence of S9 (Hutson & Stoydin, 1977;
Creedy & Hutson, 1982; Wright & Creedy, 1982).
In the presence of Aroclor-induced rat liver S-9 fraction and a
rat liver microsomal mono-oxygenase system, cis-1,3-
dichloropropene (I) is apparently metabolized to a mutagenic
metabolite, cis-1-chloro-3-chloromethyloxirane (II), which is not
as effectively deactivated by glutathione as the parent compound.
Cis-dichloropropeneoxide was shown not to be stable in aqueous
medium, 2-chloroacroleine (III) was the product of hydrolysis,
another direct-acting mutagen for Salmonella typhimurium TA100
(Hutson, 1984) (see Fig. 5). Glutathione transferase systems afford
efficient protection against this bioactivated product of 1,3-
dichloropropene. The protective action of glutathione-linked systems
against the mutagenicity observed in S. typhimurium has also been
shown to occur in vivo.
8.6.1.3 Mammalian cells
In a gene mutation assay, V79 Chinese hamster cells were
exposed to cis-1,3-dichloropropene (99.9%) in DMSO at dose levels
of 2.5 to 20 µg/ml. No indication for an increased mutation
frequency at the HGPRT locus was found in these cells (Meyer, 1980).
Telone II (48.9% cis- and 43.2% trans-1,3-dichloropropene)
was tested in the Chinese hamster ovary cell/HGPRT mutagenicity
assay, with the cell line designated as CHO-K1-BH4, with, and
without, metabolic activation. Three tests were carried out without
activation. The first test with 50, 100, 150, 200, and 250
mmol/litre, showed an increase in mutation frequency at the 200 and
250 mmol/litre dose levels. However, the biological significance is
doubtful because of the extreme toxicity at these high dose levels.
In a repeat of this study using the same concentrations, no increase
in mutation frequency was found. The results of a third study using
concentrations of 50, 100, 125, 150, and 200 mmol/litre were also
negative. The fourth test was with activation, with dose levels of
50, 100, 125, 150, and 200 mmol/litre. The relative survival ranged
from 98% at 50 mmol/litre to 14% at 200 mmol/litre. No increase in
mutation frequency was observed. The results indicated that Telone
II was not mutagenic in the CHO/HGPRT assay, with, or without,
metabolic activation (Mendrala, 1986).
8.6.1.4 DNA damage
Cis- and trans-1,3-dichloropropene were tested for their
ability to induce unscheduled DNA synthesis (UDS) in Hela S3
cells. The lowest dose, 10-4 mol/litre was the dose at which UDS
occurred, the dose-response curves being rather shallow (Schiffmann
et al., 1983).
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in the rat hepatocyte unscheduled DNA synthesis assay
at concentrations from 1 x 10-6 up to 3 x 10-3 mol/litre. In an
initial and a repeat assay, toxic effects on the hepatocyte cultures
(indicated as detachment of the cells and/or a granular appearance)
occurred at 1 x 10-5 mol/litre and at 1 x 10-4 mol/litre or
more, respectively. In both tests, Telone II failed to elicit
significant DNA repair in the primary cultures of rat hepatocytes,
over the wide range of concentrations tested, suggesting an apparent
lack of genetic activity (Mendrala, 1985).
The liquid Bacillus subtilis strain H17 (arg-, trp-, recE+)
microsome rec-assay was used to evaluate the DNA-damaging effect of
1,3-dichloropropene with, and without, S9 mix. The concentrations of
1,3-dichloropropene used with, and without, S9 mix were for
CR50Rec+/CR50Rec-, 7.62 x 10 / 2.49 x 10 and 4.79 x 102/1.01 x
103 mg/litre. With S9 mix, there was a strong DNA-damaging effect,
but, without S9 mix, a reverse effect was observed (Matsui et al.,
1989).
8.6.1.5 Chromosomal effects
Rat liver (RL1) cells were exposed to culture medium
containing cis-1,3-dichloropropene (99.9%) in DMSO, at
concentrations of 2.5, 5.0, or 10 µg/ml. No indication was found
that the compound induced chromosome damage in the liver cells
(Meyer, 1980).
The clastogenic potential of trans-1,3-dichloropropene (95.4%
trans-isomer and 0.3% cis-isomer) was assessed from assays
designed to monitor chromosome damage in CHO cells. Cultures were
grown in incubated medium containing the test compound, for either 3
h in the presence of S9-mix (concentration 0, 0.5, 2.5 or 5.0 µg/ml)
or 24 h in the absence of S9 mix (concentration 0, 3, 15 or 30
µg/ml). Methyl methane sulfonate and cyclophosphamide were used as
positive controls. Metaphase cells were used for the analysis of
chromosome aberrations after 8, 12, and 24 h, in the case of
cultures with S9 mix, and, after 24 h, in the case of the cultures
without S9 mix. At the 24-h sample time, it was concluded that
trans-1,3-dichloropropene induced chromosome damage (gaps, breaks,
and exchange figures) in the presence of S9 mix, but no effect was
seen in its absence (Brooks & Wiggins, 1989b).
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce chromosomal aberrations in cultured CHO cells.
In the first study, without S9, 49.1 µg/ml produced a strong
positive response, i.e., 16% of cells with aberrations (large number
of gaps). No metaphase cells were seen at the 98.0 µg/ml dose level.
In a repeat study, these results could not be confirmed with 50, 75,
and 100 µg/ml. All 3 doses produced 50% decreases in cell
confluency. When the compound was tested with S9 mix, no chromosomal
aberrations were found at 50 µg/ml, the highest dose at which
metaphase cells could be found.
Brooks & Wiggins (1991) assessed the mutagenic activity of
cis-1,3-dichloropropene (94.5-97.5% cis-isomer, 1.5% trans-
isomer, 0.25% 1,2-dichloropropane), in Chinese hamster ovary cells.
The cultures were grown in medium containing the test substance for
either 3 h in the presence of S9 mix or 24 h in the absence of S9
mix. Metaphase cells were prepared for analysis for chromosome
aberrations, 24 h following initiation of exposure with, and
without, S9 mix. From the data obtained at the 24-h sample time, it
was concluded that cis-1,3-dichloropropene at 1, 5, or 10 µg/ml
induced chromosome damage (gaps, breaks, and exchange figures) in
the presence of S9 mix. No increase in metaphase chromosome damage
was found with doses of up to 10 µg/ml in the absence of S9 mix.
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce Sister Chromatid Exchanges (SCEs), in cultured
Chinese hamster ovary cells. The test was carried out with, and
without, rat liver S9 fraction. DMSO was used as the solvent. 1,3-
Dichloropropene induced SCEs without S9 at a dose level of 29.9
µg/ml. A positive response was also found with S9.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
(SCE) test in vitro in Chinese hamster V79 cells without S9 mix,
to evaluate the effect of 1,3-dichloropropene (80%). The
concentrations tested were 0 (DMSO), and 0.1 up to 0.8 mmol/litre.
The 0.8 mmol/litre dose was toxic to the cells. A dose-related
increase in the number of SCEs was found. Negative results were
obtained with S9, at dose levels of 0.1-3.3 mmol/litre.
8.6.2 In vivo studies
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in a mouse bone marrow micronucleus test to detect
chromosomal aberrations and spindle malfunction. The substance
(dissolved in corn oil) was administered to CD-1 (ICR) BR mice by
single oral gavage at dose levels of 0 (corn oil), 38, 115, or 380
mg/kg body weight. Groups of animals were sacrificed at intervals of
24 and 48 h. A positive control group received cyclophosphamide at a
dose of 120 mg/kg. There were no significant increases in the
frequencies of micronucleated polychromatic erythrocytes in the
Telone II groups compared with the controls. Positive results were
obtained with the positive control group. Telone II was considered
negative in the mouse bone marrow micronucleus test (Gollapudi et
al., 1985).
Valencia et al. (1985) tested 1,3-dichloropropene (95.5%) for
mutagenicity in Drosophila melanogaster. The compound was tested
for the induction of sex-linked recessive lethals (SLRLs) by feeding
the substance in a 5% aqueous sucrose solution. The dose level fed
was 0 or 5750 mg/litre. The mortality rate was 33% and sterility
10%. 1,3-Dichloropropene induced an increased number of SLRLs, but
no reciprocal translocations were induced.
The results of both dominant lethal and host-mediated assays
carried out with "MIX D/D" were negative (see "Mixtures of
dichloropropenes and dichloropropane", section 8.6.2).
8.6.3 Appraisal
1,3-Dichloropropene ( cis- and trans-isomer) showed
mutagenic activity in Salmonella typhimurium, especially in
strains TA100 and TA1535 with, and without, metabolic activation.
There is a difference in mutagenic potential between the cis- and
trans-isomers in TA100 with, and without, activation. The cis-
isomer induces a greater number of revertants than the trans-
isomer.
1,3-Dichloropropene does not possess a genotoxic potential in a
variety of non-bacterial studies. Gene mutation has not been
detected in in vitro assays using the eukaryotic cell lines of
either V79 or CHO (HGRPT locus) (Meyer, 1980; Mendrala, 1986).
Chromosomal damage has not been observed in vitro with rat liver
cell cultures or in an in vivo mouse micronucleus study using
single oral doses of up to 380 mg/kg (Gollapudi et al., 1985).
Interaction with DNA was not observed, even at cytotoxic doses, in
an in vitro rat liver unscheduled DNA synthesis study (Mendrala,
1985). However, Schiffmann et al. (1983) found liver unscheduled DNA
synthesis in Hela S3 cells.
8.7 Carcinogenicity
8.7.1 Oral
8.7.1.1 Mouse
A carcinogenicity study was carried out on groups of 50 male
and 50 female (4-6 weeks old) B6C3F1 mice at dose levels of 0,
50, or 100 mg (stabilized technical grade 1,3-dichloropropene)/kg
body weight, administered in corn oil (5 ml/kg body weight), by
gavage, 3 times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, nine other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). The initial mean body weights of
the treated mice were lower (6-22%) than those of the controls.
These differences were caused by failure to fully randomize the
distribution of the animals. No clinical signs were observed.
However, the survival of vehicle control male mice was significantly
lower than that in either dose group. Thirty-nine control male mice
died with myocarditis, 25, between weeks 48 and 51. A slight
increase in mortality was found in the female mice at a dose level
of 100 mg/kg body weight.
The reduced survival of the control male mice did not allow for
an adequate evaluation of carcinogenicity in males in this study.
But, in both sexes, there was evidence for a 1,3-dichloropropene-
related increase in transitional cell carcinomas of the urinary
bladder (without the presence of calculi), liver tumours, squamous
cell papillomas and/or carcinomas of the forestomach, and of
alveolar/bronchiolar adenomas and carcinomas of the lung, at 50 or
100 mg/kg body weight. In the female mice, an increase in basal cell
or epithelial cell hyperplasia in the forestomach was also found
(Table 14). It was stated that the presence of 1% epichlorohydrin, a
direct acting mutagen and carcinogen (especially for the
forestomach), may have influenced the development of forestomach
lesions (Haseman et al., 1984; NTP, 1985; Yang, 1986; Yang et al.,
1986).
8.7.1.2 Rat
A long-term carcinogenicity study was carried out on groups of
52 F344/N rats of each sex (aged 6 weeks), at dose levels of 0, 25,
or 50 mg stabilized technical grade 1,3-dichloropropene/kg body
weight, administered in corn oil (5 ml/kg body weight), by gavage, 3
times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, 9 other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). Additional groups of 25 rats of
each sex were assigned to each dose group. At 9, 16, 21, 24, and 27
months of dosing, 5 rats/sex per group were killed and organs and
tissues studied microscopically. Haematological and clinical-
chemical studies were carried out on groups of 20 rats of each sex,
11-13 times in the first 70 weeks of the study. The mean body weight
of high-dose male rats was about 5% lower than those of the control
and low-dose rats. No differences in body weight were observed in
female animals. Mortality was comparable with the controls. The
primary organs affected were the forestomach and liver. The
incidences of non-neoplastic and neoplastic lesions are summarized
in Table 15.
Table 14. Occurrence of microscopic lesions in mice in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Urinary bladder
Epithelial hyperplasia b 2/50 (4%) 9/50 (18%) 15/50 (30%) 18/50 (36%) 19/48 (40%)
Transitional cell carcinoma b 0/50 (0%) 0/50 (0%) 8/50 (16%) 2/50 (4%) 21/48 (44%)
Lung
Alveolar/bronchiolar
adenoma or carcinoma 1/50c 2/50 (4%) 13/50 (26%) 4/50 (8%) 12/50 (24%) 8/50 (16%)*
Forestomach
Epithelial hyperplasia b 1/50 (2%) 0/50 (0%) 1/50 (2%) 4/50 (8%) 21/50 (42%)*
Squamous cell papilloma b 0/50 (0%) 2/50 (4%) 1/50 (2%) 3/50 (6%)d 4/50 (8%)*
or carcinoma
Liver
Hepatocellular adenoma 5/50b 1/50 (2%) 7/50 (14%) 8/50 (16%)* 13/50 (26%) 3/50 (6%)
or carcinoma
Kidneys
Hydronephrosis b 0/50 (0%) 0/50 (0%) 2/50 (4%) 0/50 (0%) 14/50 (28%)
a From: NTP (1985).
b Too many animals died during the study, but no epithelial hyperplasia or neoplasia were observed in these animals.
c As b but, a lung adenoma was found in one animal.
d Only squamous cell papilloma.
* P = < 0.05.
Table 15. Occurrence of microscopic lesions in rats in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Forestomach
Epithelial hyperplasia 2/52 (4%) 1/52 (2%) 5/52 (10%) 0/52 (0%) 13/52 (25%)c 16/52 (31%)c
Squamous cell papilloma 1/52 (2%) 0/52 (0%) 1/52 (2%) 2/52 (4%)b 13/52 (25%)c 3/52 (6%)b
or carcinoma
Liver
Neoplastic nodule 1/52 (2%) 6/52 (12%) 6/52 (12%)c 6/52 (12%)c 7/52 (13%)c 10/52 (19%)
or carcinoma
a From: NTP (1985).
b Only squamous cell papilloma.
c P = ¾ 0.05
Under the conditions of the study, 1,3-dichloropropene induced
an increased incidence of squamous cell papillomas and carcinomas of
the forestomach. In addition, a dose-related trend was observed in
the incidence of neoplastic nodules in the livers of male rats. It
was stated that the presence of 1% epichlorohydrin, a direct acting
mutagen and carcinogen (especially for the forestomach), may have
influenced the development of forestomach lesions (Haseman et al.,
1984; NTP, 1985; Yang, 1986; Yang et al., 1986).
In the ancillary study, dose-related lesions were observed in
the forestomach and liver. The development of the forestomach basal-
cell hyperplasia and squamous-cell papilloma followed a time-
dependent trend in high-dose males and females. Basal-cell
hyperplasia was seen 9-16 months after dosing started. The neoplasms
of the forestomach and liver were not seen until 24 months after
dosing began.
8.7.2 Inhalation
8.7.2.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (6-7 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days per
week for up to 24 months. In addition, 2 ancillary groups, each with
10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The mice were exposed to 1,3-
dichloropropane vapour at 0, 22.7, 90.8, or 272 mg/m3. The
composition of the technical-grade 1,3-dichloropropene was 1,3-
dichloropropene 92.1% ( cis-49.5% and trans 42.6%); 1,2-
dichloropropane 0.7%; and 5.2% mixtures of hexanes and hexadienes.
Epoxidized soybean oil (approximately 2%) was added as stabilizing
agent. Besides body weights, clinical-chemical parameters in the
blood and urine and haematological parameters were determined for
all animals terminated at 6 and 12 months and for 20 animals/sex per
group at 24 months. At 6, 12, and 24 months, animals were sacrificed
and the weight of 5 organs determined; a large number of organs and
tissues were examined histopathologically.
No significant differences in survival rates were observed
between the groups. The body weights of male mice exposed to 272 mg
1,3-dichloropropene/m3 were statistically significantly depressed
in comparison with the controls. Examination of haematological and
clinical-chemical parameters and urinalysis did not indicate any
toxicity resulting from exposure to 1,3-dichloropropene for 6, 12,
or 24 months.
The mean relative liver weight of male animals exposed to 272
mg/m3 showed a statistically significant decrease. Gross patho-
logical examination of mice revealed morphological alterations
involving the urinary bladder and lung, which were attributed to
exposure to 1,3-dichloropropene. The bladder mucosal surface in
females exposed to 272 mg/m3 for 12 months and 90.8 and 272
mg/m3 for 24 months had a roughened appearance. In addition, a
statistically significantly increased number of the females exposed
to 90.8 and 272 mg/m3 showed inflammation and epithelial
hyperplasia of the bladder mucosa, after 24 months. An increased
number of male animals exposed to 272 mg/m3 also showed
inflammation.
Female mice exposed to 90.8 or 272 mg/m3 and males exposed to
272 mg/m3 showed hypertrophy and hyperplasia of the nasal
epithelium and degeneration of the olfactory epithelium. Additional
microscopic changes, considered exposure-related, were hyperplasia
and hyperkeratosis in the forestomach of 8/50 male mice following 24
months exposure to 272 mg/m3. A statistically significant increase
in the incidence of a benign tumour, bronchio-alveolar adenoma, was
observed in male mice exposed to 272 mg 1,3-dichloropropene
vapour/m3 for 24 months [22/50 (44%) vs 9/50 (18%) in controls].
No statistically significant increase in tumour incidence was found
in the groups exposed to 1,3-dichloropropene at 22.7 and 90.8
mg/m3 (Table 16). The incidence of lung tumours in the males
exposed to 272 mg/m3 was somewhat higher (7-32%) than the range of
historical control values for this type of tumour in male
B6C3F1 mice in 7 previous, long-term studies. The NOAEL in
this study on mice for hypertrophy/hyperplasia of the nasal
epithelium was 22.7 mg/m3 (Yano et al., 1985; Stott et al., 1987;
Lomax et al., 1989).
8.7.2.2 Rat
Groups of 50 male and 50 female Fischer 344 rats (7-9 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days/week
for up to 24 months. In addition, 2 ancillary groups, each
comprising 10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The animals were exposed to 0,
22.7, 90.8, or 272 mg/m3. The chemical composition of the test
material was 92.1% 1,3-dichloropropene ( cis-49.5% and trans-
42.6%), 0.7% 1,2-dichloropropane, and mixtures of hexanes and
hexadiens. Epoxidized soybean oil (approximately 2%) was present as
stabilizer. Besides body weights of the animals, clinical-chemical
parameters in the blood and urine and haematological parameters were
determined for all animals terminated at 6 and 12 months and for 20
animals/sex from each exposure group at 24 months.
Table 16. Incidence of various types of lesions observed in a mouse inhalation study with 1,3-dichloropropenea
Males (mg/m3) Females (mg/m3)
0 22.7 90.8 272 0 22.7 90.8 272
Urinary bladder
Hyperplasia mucosa 4/48 (9%) 7/48 (15%) 11/48 (23%) 37/47 (79%)b 1/47 (2%) 4/46 (9%) 21/48 (44%)b 44/45 (98%)b
(simple or nodular)
Lungs
Bronchio-alveolar 9/50 (18%) 6/50 (12%) 13/50 (26%) 22/50 (44%)b 4/50 (8%) 3/50 (6%) 5/50 (6%) 3/50 (6%)
adenoma
Nasal tissues
Degeneration of 1/50 (2%) 0/50 1/50 (2%) 48/50 (96%)b 0/50 0/50 1/50 (2%) 45/50 (90%)b
olfactory epithelium
Hyperplasia and 5/50 (10%) 1/50 (2%) 4/50 (8%) 48/50 (96%)b 4/50 (8%) 4/50 (8%) 28/50 (56%) 49/50 (98%)b
hypertrophy of
respiratory epithelium
Stomach
Squamous papilloma 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50(6%)
a From: Lomax et al. (1989).
b Statistical difference from control mean identified by using Yate's kappa2 pairwise test, alpha = 0.05.
At 6, 12, or 24 months, animals were sacrificed and the weight
of 5 organs determined; a large number of organs and tissues were
examined histopathologically. No significant influence on survival
was observed. Mean body weights of both male and female rats exposed
to 272 mg/m3 were statistically significantly decreased compared
with mean control values. Examination of haematological and
clinical-chemical parameters, urinalysis, and organ weights did not
indicate any toxicity resulting from exposure to 1,3-dichloropropene
for 6, 12, or 24 months.
Gross pathological examination did not indicate any exposure-
related effects after 6, 12, or 24 months. Exposure-related
histological effects occurred in nasal tissues of rats exposed to
272 mg/m3 for 24 months (Table 17), but not for 6 or 12 months.
The microscopic changes were located in the olfactory mucosa, which
covers the upper portions of the nasal cavity, nasal septum, and
turbinates. The changes were characterized by unilateral or
bilateral decreased thickness of olfactory epithelium and fibrosis
of the submucosal tissues underlying eroded olfactory epithelium. At
the lower dose levels, females did not show histopathological
changes in the nasal tissue, while one male exposed to 22.7 mg/m3
and one exposed to 90.8 mg/m3 showed decreased thickness of the
olfactory epithelium. No effects were seen in the controls. No
statistically significant increase in tumour incidence was found in
exposed rats compared with controls (Lomax et al., 1987, 1989).
8.7.3 Appraisal
Exposure to 1,3-dichloropropene, through inhalation, for up to
24 months did not have any demonstrable effects on survival or
spontaneous tumour development in male and female Fischer 344 rats.
In mice, an increased incidence of bronchio-alveolar adenomas was
found in the lungs of male mice, exposed to 272 mg/m3, but not at
the lower dose level (90.8 mg/m3). The oral gavage study with 1,3-
dichloropropene demonstrated an increased incidence of forestomach
neoplasms in rats of both sexes at dose levels of 50 mg/kg body
weight, administered 3 times/week for 24 months. Male rats treated
with 25 or 50 mg/kg also had an increased incidence of neoplastic
nodules in the liver.
In both the oral gavage and inhalation mouse bioassays with
1,3-dichloropropene, a tumorigenic response was noted in tissues
with which 1,3-dichloropropene had direct contact, i.e., the stomach
and the lung. However, in the gavage study, tumours were also
induced at sites distant from that at the primary "portal-of-entry"
(lung and urinary bladder). This was not the case in the inhalation
study, despite the fact that the dose of 1,3-dichloropropene
received on a mg/kg body weight per day basis by mice exposed for 5
days/week through inhalation was approximately 2-3 times higher than
the dose levels administered orally 3 times/week in the NTP (1985).
Table 17. Microscopic changes in the nasal tissues of rats exposed to
Telone II at 272 mg/m3a
Microscopic change Vehicle control Overall incidence
(male and female) Male Female
Decreased thickness of 0/100 20/50b (40%) 15/50b (31%)
olfactory epithelium
Erosion of olfactory 0/100 15/50b (30%) 6/50 (12%)
epithelium
Submucosal fibrosis 0/100 6/50b (12%) 2/50 (4%)
a From: Lomax et al. (1989).
b Statistical difference identified from control mean of
Yate's kappa2 pairwise test, alpha = 0.05.
The degeneration and subsequent hyperplasia of nasal and forestomach
epithelium occurred only at concentrations that are known to deplete
glutathione levels in these tissues. Tumorigenic effects occur at
doses higher than those causing glutathione depletion and tissue
damage.
The same mouse strain and similar test materials relative to
1,3-dichloropropene were used in both bioassays, but there was a
difference in the stabilizing agents in the test materials. The 1,3-
dichloropropene used in the NTP study was stabilized with 1%
epichlorohydrin, a carcinogen. It has been suggested that
epichlorohydrin may have played a role in the tumorigenic response
obtained in the gavage study because of the bolus nature of its
administration. However, it is not known whether the increased
incidence of tumours in the urinary bladder, lungs, forestomach, and
liver in the mouse gavage study are attributable to the treatment
with 1,3-dichloropropene or the effect of epichlorohydrin, since
carcinogenicity studies of epichlorohydrin in mice have not been
performed yet. In the inhalation study carried out by Lomax et al.
(1989), the 1,3-dichloropropene was stabilized with the relatively
nontoxic epoxidized soybean oil. The role of the stabilizing
additive epichlorohydrin in generating the different tumours seen in
the oral gavage studies on 1,3-dichloropropene is still uncertain.
This question has to be further investigated before a more definite
conclusion about the carcinogenic potential of 1,3-dichloropropene
can be drawn.
8.7.4 Dermal and subcutaneous (mouse)
Groups of 30 female Ha:ICR Swiss strain mice (6-8 weeks old)
were treated with 0.2 ml acetone containing 41 or 122 mg purified
cis-1,3 dichloropropene, applied to shaven skin 3 times weekly,
for approximately 18 months. Control mice received acetone. In the
group treated with 41 mg, no papillomas were found, but at the 122
mg dose, 3 animals showed papillomas, and 2, carcinomas. No tumours
were found at distant sites. Cis-1,3-dichloropropene, applied once
on the skin at a dose of 122 mg/mouse, was followed after 14 days by
the application of 5 µg phorbol myristate acetate in 0.2 ml acetone,
3 times weekly until the end of the study. No skin tumour-initiating
activity was observed (van Duuren et al., 1979).
A group of 30 female Ha:ICR Swiss mice were given weekly
subcutaneous injections in the left flank of 0.05 ml trioctanoin
containing 3 mg purified cis-1,3-dichloropropene per injection.
The study lasted 538 days. Control animals received only the
vehicle. Six out of 30 mice showed local fibrosarcomas, whereas
vehicle control animals did not. A positive control of 0.3 mg beta-
propiolactone produced local sarcomas in 24 out of 30 mice during a
378-day period (van Duuren et al., 1979).
The relevance of the subcutaneous route for the assessment of
carcinogenic properties remains questionable, especially when
injections of an irritant material are made. It is probable that the
persistent and physical properties rather than the chemical
characteristics of cis-1,3-dichloropropene are responsible for
production of local sarcomas.
8.8 Factors modifying toxicity, toxicity of metabolites, mode of
action
8.8.1 Toxicity of the metabolites, cis- and trans-1,3-
dichloropropene oxide
There is some evidence that a small proportion of cis-1,3-
dichloropropene is metabolized to cis-1,3-dichloropropene oxide
(Fig. 5; Hutson, 1984).
8.8.1.1 Mutagenicity
Cis- and trans-1,3-dichloropropene oxide were tested for
mutagenicity, in the absence of metabolic activation, in Salmonella
typhimurium TA1535 and Escherichia coli WP2 uvr A, and for
preferential inhibition of growth of DNA-repair-polymerase-deficient
E. coli. Both oxides were potent mutagens and DNA modifiers. In
Salmonella typhimurium TA1535, treated with 0.025 µmol/ml and in
E. coli WP2 uvr A treated with 0.05 µmol/ml of bacterial
suspension, a significant increase in revertant colonies was found.
A gene mutation test with E. coli (pol A1-/pol A1+) in the
absence of metabolic activation, already showed an effect with
0.0005 µmol/ml (Kline et al., 1982).
In the absence of an S9-fraction, cis-dichloropropene oxide
was strongly mutagenic towards S. typhimurium TA100. The
mutagenicity reached a maximum at 25 µg/plate. Above 300 µg/plate,
marked cytotoxicity was observed. Glutathione (5 mmol/litre) caused
a significant inhibitory effect on the mutagenicity and cytotoxicity
of this epoxide, but did not offer complete protection. Inclusion of
glutathione (5 mmol/litre) together with S9-fraction afforded
complete protection over the range of concentrations of cis-
dichloropropene oxide up to 100 µg/plate (Hutson 1984; Watson et
al., 1986a).
Cis- and trans-1,3-dichloropropene oxide were tested in a
quantitative Syrian hamster embryo cell model. Both compounds at
dose levels of 0.005, 0.01, or 0.02 mmol/litre ( cis-isomer) and
0.01, 0.025, or 0.05 mmol/litre ( trans-isomer) induced
morphological transformation of the Syrian hamster embryo cells
(DiPaolo & Doniger, 1982).
8.8.1.2 Carcinogenicity
Female ICR/Ha Swiss mice (30 per group) were treated 3 times
weekly, with cis-1,3-dichloropropene oxide or trans-1,3-
dichloropropene oxide (containing 10-15% of m-dichlorobenzene) on
the skin. The dose level was 10 mg in 0.1 ml of acetone. The
controls received only acetone. The median survival time was
comparable with that of the controls (over 500 days). With cis-
dichloropropene oxide, 16/30 mice had local papillomas and 10/30
squamous cell carcinomas of the skin; with trans-dichloropropene
oxide, this was 20/30 and 17/30, respectively. No tumours were found
in the control animals (van Duuren et al., 1983).
A study was also carried out on the same strain of mice using
subcutaneous injections. Thirty female mice received 500 µg cis-
dichloropropene oxide or trans-dichloropropene oxide in 0.05 ml
tricaprylin once weekly. The median survival time was comparable to
that of controls. With cis-dichloropropene oxide, 4 animals had a
local (fibro)sarcoma and one carcinoma, and, with trans-
dichloropropene oxide, 5 animals had fibrosarcomas. No tumours were
found in the vehicle controls (van Duuren et al., 1983).
8.8.2 Role of oxidation
When purified cis-1,3-dichloropropene was heated for a few
hours in an oxygen atmosphere, in either the light or dark, the non-
mutagenic cis-1,3-dichloropropene became strongly mutagenic.
Heating under nitrogen was negative. Storage of cis-1,3-
dichloropropene at room temperature, in the presence of oxygen, for
two months, made it mutagenic (Watson et al., 1987). Talcott & King
(1984) demonstrated that purified samples of 1,3-dichloropropene
were not mutagenic to Salmonella typhimurium TA100. Four
preparations of 1,3-dichloropropene were separated into different
fractions and analysed for mutagenic activity. The fraction
containing polar metabolites was found to be mutagenic. Its
composition was too complex to characterize completely, but 2
mutagens, epichlorohydrin and 1,3-dichloro-2-propanol, were
identified.
Watson et al. (1986 a,b; 1987) confirmed that the direct
mutagenicity, inducing base-pair mutations, previously observed in
Salmonella typhimurium TA100 treated with cis-1,3-
dichloropropene, was caused by trace impurities. These impurities
resulted from the autooxidation of cis- and trans-1,3-
dichloropropene and were identified as cis- and trans-
dichloropropene oxides. The dichloropropene oxides made a
significant contribution towards the intrinsic mutagenicity, when
tested in S. typhimurium TA100 (see section 8.8.1.1).
The proposed formation of cis- and trans-dichloropropene
oxides is shown in Fig. 6.
In California, applicators of 1,3-dichloropropene were also
studied for personal air exposure and urinary excretion of
mercapturic acid metabolites. The amount excreted was correlated
with the product of the duration of exposure x TWA. The highest
urinary metabolite concentration occurred during the application
period, indicating rapid excretion. Skin absorption of vapour was
not a significant route of exposure (Osterloh et al., 1984, 1989,
see also section 9.2.1).
Air and biological monitoring of 6 operators exposed to "Mix
DD" soil fumigant during filling operations in the Netherlands was
carried out in 1985-86. There was rapid metabolism and elimination:
the half-lives of mercapturic acid excretion were 4-5 h, with a
return to background levels after 24 h. It was calculated that,
under linear, non-saturation conditions, approximately 23% of the
inhalation dose of the cis-isomer and 10% of the trans-isomer
are excreted in the urine as mercapturic acids (Eadsforth, 1987).
6.4 Reaction with macromolecules
6.4.1 Mouse
The non-protein sulfhydryl (NPS) content, e.g., GSH, and
covalent binding to macromolecules were determined in the tissues of
male B6C3F1 mice. Single oral doses of 0, 1, 5, 25, 50, or 100
mg 1,3-dichloropropene 97% ( cis- 62% : trans-isomer 38%)/kg body
weight were given for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-
dichloropropene/kg body weight for binding studies. Non-glandular
forestomach, glandular stomach, liver, kidneys, and bladder were
analysed, 2 h after dosing. Although NPS depletion and dose-related
increases in macromolecular binding were noted in several tissues of
rats, these effects were more pronounced in the non-glandular
stomach than in any other tissue (including glandular stomach,
liver, kidneys, and bladder). The no-observed-effect level (NOEL)
for NPS depletion in rat non-glandular stomach was 1 mg/kg body
weight. NPS levels in non-glandular forestomach were significantly
decreased at doses of 25 mg or higher and, in the liver, at 100
mg/kg body weight. Binding in the non-glandular forestomach was
greatest at dose levels that caused the most depletion of tissue
NPS. Limited binding occurred in the liver, kidneys, and bladder
(Dietz et al., 1984b, 1985).
6.4.2 Rat
Groups of 3-9 male Fischer 344 rats (200-260 g) were
administered 50 mg cis-1,3-dichloropropene (94.1% cis- and 2.5%
trans-) or 50 mg trans-1,3-dichloropropene (97.3% trans- and
0.8 cis-)/kg body weight, by gavage. The rats were sacrificed at
various intervals after dosing, to determine the tissue non-protein
sulfhydryls (NPS) in the liver, kidneys, forestomach, glandular
stomach, and bladder. Blood samples were also taken to determine the
presence of unchanged 1,3-dichloropropene. Cis-1,3-dichloropropene
was only detected in the blood (6.58 µg/litre) 15 min after dosing,
the blood levels of trans-1,3-dichloropropene were 11.72 and 8.38
µg/litre, respectively, 15 and 45 min after dosing. A statistically
significant decrease in the non-protein sulfhydryl contents of the
liver, kidneys, forestomach, and glandular stomach was found. This
depletion reached a maximum, approximately 2-h after dosing. No
depletion was noted in the bladder. It is not possible to
distinguish the effects of cis- and trans-1,3-dichloropropene on
NPS, as the results for the individual isomers were not reported.
The results indicated that orally administered 1,3-dichloropropene
produces a rapid and significant depletion of tissue non-protein
sulfhydryls in the rat (Dietz et al., 1982).
The non-protein sulfhydryl (NPS) contents and covalent binding
to macromolecules were determined in the tissues of male Fischer 344
rats. Single, oral doses of 0, 1, 5, 25, 50, or 100 mg 1,3-
dichloropropene 97% ( cis-62% and trans-isomer 38%) were given
for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-dichloropropene/kg
body weight for binding studies. NPS levels in non- glandular
forestomach were significantly decreased with doses of 25 mg/kg body
weight or more. Binding in the non-glandular forestomach was
greatest at dose levels that caused a significant depletion of
tissue NPS. Limited binding was noted in the liver, kidneys, and
bladder (Dietz et al., 1984b, 1985).
6.5 Appraisal
Mice, rats and humans metabolize 1,3-dichloropropene
predominantly by conjugation with reduced glutathione (GSH). The
glutathione conjugate is further metabolized to the corresponding
mercapturic acid, which is then excreted in the urine. Consistent
with the function of GSH as a detoxication mechanism, the
genotoxicity of 1,3-dichloropropene was decreased in Salmonella
typhimurium when the concentration of GSH was increased to a
mammalian physiological concentration (see sections 8.6.1.2, 8.9.2).
The levels of GSH (measured as non-protein sulfhydryl content)
were decreased at locations consistent with the route of exposure,
i.e., predominantly in the forestomach and to a lesser extent in the
glandular stomach and liver, following oral administration, and in
the nasal tissue after inhalation exposure. For oral exposure, the
extent of covalent binding of 1,3-dichloropropene to macromolecules
was correlated with the decrease in non-protein sulfhydryl content.
It is anticipated that toxic effects of 1,3-dichloropropene will
occur at doses that deplete tissue sulfhydryls and will be
manifested in the organs described above (forestomach, liver, and
nasal tissue).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The United States Environmental Protection Agency published a
Health and Environmental Effects Profile for 1,3-Dichloropropene in
1985 (US EPA, 1985).
7.1 Acute toxicity
7.1.1 Microorganisms
The major effect of 1,3-dichloropropene on soil microorganisms
is on nitrogen transformation. The oxidation of ammonium from soil
organic nitrogen is reduced by 1,3-dichloropropene; soil ammonium
levels were significantly greater and soil nitrate levels were
significantly lower in soils treated with 1,3-dichloropropene
compared with untreated controls (Tu, 1973; Elliot et al., 1974,
1977).
In a series of studies, the effects of 1,3-dichloropropene at
30 or 60 mg/kg soil were evaluated, in parallel, under laboratory
conditions. In general, neither dose affected soil microorganisms or
function appreciably. The effects of 1,3-dichloropropene on soil
microorganisms and enzyme activity were not consistent in 3 soil
types (sandy-, clay-, and organic soils). The numbers of fungi, 2
days after fumigation, were significantly reduced in clay and sandy
soils, but not in organic soil, but recovered after 7 days. 1,3-
Dichloropropene either increased or decreased the number of non-
symbiotic nitrogen fixers and enzyme activity in different soils
(Tu, 1978, 1979, 1981a,b).
Moje et al. (1957) studied the individual components of "MIX
D/D" in old citrus soil and found that the toxicity was mainly due
to its 1,3-dichloropropene content, in particular that of the cis-
isomer (see also section 7.1.1 of "MIX D/D"). Results were as
tabulated on the next page.
1,3-Dichloropropene was converted by the methanotrophic
bacterium Methylosinus trichosporium OB3b, grown in aerobic
continuous cultures. The substance was added at a concentration of
0.2 mmol/litre. After 24 h, 85% of the substance added was degraded
(Oldenhuis et al., 1989).
In field experiments with continuous potato cropping, it was
found that sustained annual applications of 1,3-dichloropropene led
to insufficient control of Globodera rostochiensis. In the
laboratory, 1,3-dichloropropene rapidly disappeared from these
soils. This did not occur when the soil was sterilized. A bacterium
was isolated from these soils, which was found to decompose 1,3-
dichloropropene using it as a carbon and energy source. The
bacterium was Pseudomonas sp. Repeated application of 1,3-
dichloropropene led to accelerated degradation of the substance
(Lebbink et al., 1989).
Reduction in
Compound Fungi Bacteria and
actinomycetes
Cis-1,3-dichloropropene 85-95% reduction 85-100% reduction
at 25 mg/kg soil at 250 mg/kg soil
Trans-1,3-dichloropropene 100% reduction 100% reduction at
at 250 mg/kg soil 1000 mg/kg soil
7.1.2 Algae
The 96-h EC50 value for growth, based on the concentration of
chlorophyll a and also on cell numbers of the freshwater green algae
Selenastrum capricornutum in a static system, was 4.95 mg/litre
for 1,3-dichloropropene. The estuarine diatome, Skeletonema
costatum, showed a 96-h EC50 value for growth, based on the
concentration of chlorophyll a in culture, of 1 mg/litre (Leblanc,
1984). The EC50 calculated from cell numbers was 1.04 mg/litre (US
EPA, 1980). The compound is moderately toxic for marine algae.
The toxicity of cis-, trans- and a mixture of cis- and
trans-1,3-dichloropropenes for Selenastrum capricornutum was
determined in a sealed 72-h growth inhibition test. The 72-h EC50
values (percentage reduction in area under the growth curve),
expressed in terms of the mean measured concentration in the test
media, were; cis-isomer 2.8 mg/litre; trans-isomer 11 mg/litre;
and the mixture 8.2 mg/litre. The EC50 (percentage reduction in
mean specific growth rate), (24-48 h) and EC50 (24-72 h) values
determined by analysis of average specific growth rates were: cis-
isomer 4.6 and 3.1 mg/litre; trans-isomer 6.6 and 7.5 mg/litre;
and, for the mixture, 11 and 3.6 mg/litre, respectively (Girling,
1989a,b,c).
Rapid evaporation and sensitivity to hydrolysis are features of
the chloropropenes that may interfere with proper determination of
toxic concentrations of these chemicals for aquatic species, using
the standard techniques. Significant loss of the test substance may
have occurred during experiments carried out at temperatures above
20 °C and under "static" conditions, i.e., lasting for several days
without refreshing the medium. Determination of the actual
concentrations has seldom been carried out, and it is suspected that
several of the data reported for the chloropropenes present an
underestimation of the toxic potential of these chemicals for
aquatic organisms. On the other hand, the products of hydrolysis of
the chloropropenes, for instance, chloroallyl alcohols are
themselves toxic (Krijgsheld & Van der Gen, 1986).
7.1.3 Invertebrates
The acute toxicity of 1,3-dichloropropene for non-target
aquatic crustacea is summarized in Tables 6 and 7.
Table 6. Acute toxicity of 1,3-dichloropropene for non-target aquatic crustacea
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (mg/litre) (mg/litre)
Water flea 21 0.090a - Mayer &
(Daphnia magna) Ellersieck (1986)
Mysid shrimp - 0.79 US EPA (1980);
(Mysidopsis bahia) Leblanc (1984)
a 48-h EC50 6 mg/litre (US EPA, 1980; Leblanc, 1980) in a static system.
Leblanc (1980) calculated a no discernable effect concentration
of 0.41 mg/litre for 1,3-dichloropropene in Daphnia magna, under
static conditions. However, the result was based on nominal
concentrations and was higher than the 48-h LC50 given by Mayer &
Ellersieck (1986).
7.1.4 Honey bees
1,3-Dichloropropene has been tested on worker Honey bees (Apis
mellifera) using a dusting technique. The 48-h LD50 was 6.6
µg/bee and 1,3-dichloropropene was rated as "relatively non-toxic"
for Honey bees (Atkins et al., 1973).
Table 7. Acute toxicity of cis- and/or trans-1,3-dichloropropenes in Daphnia magna
Substance Age System Temperature 48-h EC50 Reference
(°C) (mg/litre)
Cis-1,3 DCP 24 h statica 18-22 1.4 Girling (1989a)
(96%)
Trans-1,3 DCP 24 h staticb 18-22 3.1 Girling (1989c)
(95.4% trans +
0.3% cis)
Cis- + trans- 24 h staticc 18-23 3.1 Girling (1989b)
mixture
(51-52% cis +
44% trans)
a pH 7.6-8.4; total hardness 176 mg/litre; dissolved oxygen 8.8-9.8 mg/litre.
b pH 7.8-8.1; total hardness 170 mg/litre; dissolved oxygen 8.6-9.2 mg/litre.
c pH 7.9-8.3; total hardness 179 mg/litre; dissolved oxygen 8.6-9.9 mg/litre.
7.1.5 Fish
The data summarized in Tables 8 and 9, suggest that 1,3-
dichloropropene is moderately toxic for fish.
Heitmuller et al. (1981) exposed sheepshead minnows to 1,3-
dichloropropene under static conditions. They calculated a no-
observed-effect concentration of 1.2 mg/litre, but this was based on
nominal concentrations.
7.1.6 Birds
Worthing & Hance (1991) reported an LC50 (8-day) for Mallard
duck and Bobwhite quail of > 10 000 mg/kg diet.
7.2 Short-term/long-term toxicity
7.2.1 Invertebrates
No data are available.
Table 8. Acute toxicity of 1,3-dichloropropene for fish
Species Type of test Size (g, mm) Temperature 96-h LC50 References
(°C) (mg/litre)
Fathead minnow static 0.9 g 18 4.1 Mayer & Ellersieck (1986)
(Pimephales promelas) (3.39-4.97)
Largemouth bass static 1.0 g 18 3.65a Mayer & Ellersieck (1986)
(Micropterus salmoides) (3.52-3.78)
Walleye static 1.3 g 18 1.08 Mayer & Ellersieck (1986)
(Stizostedion vitreum) (0.99-1.18)
Golden orfe - 2.8 g 20 9b Reiff (1978); Krijgsheld & Van
(Idus idus melanotus) (8-11) der Gen (1986)
Sheepshead minnow static 8-15 mm 25-31 1.8c,d US EPA (1980); Heitmuller
(Cyprinodon variegatus) (0.7-4.5) et al. (1981); Leblanc (1984)
Rainbow trout - - - 3.9 Worthing & Hance (1991)
(Salmo gairdneri)
Bluegill static 0.32-1.2 g 21-23 6.1c,d Buccafusco et al. (1981)
(Lepomis macrochirus) (5.1-6.8) US EPA (1980); Leblanc (1984)
Guppy semi-static - 22 0.5e Krijgsheld & Van der Gen (1986)
(Poecilia reticulata)
Goldfish static 1.0 g 18 < 7.5 Mayer & Ellersieck (1986)
(Carasius auratus)
Table 8 (continued)
a Tested in hard water, 272 mg CaCO3/litre.
b Tested in dechlorinated water, 260 mg CaCO3/litre.
c Tested in well water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration 9.7 mg/litre reduced
at the beginning to 0.3 mg/litre after 96-h exposure.
d Nominal concentrations.
e 14-day test.
Table 9. Acute toxicity of cis- and/or trans-1,3-dichloropropenes
in rainbow trout (Salmo gairdneri)
Substance Mean size System Temperature 96-h LC50 Reference
(cm, g) (°C) (mg/litre)
Cis-1,3 DCP 4.7 cm semi- 13-17 1.6 Girling (1989a)
(96%) (1.1 g) statica
Trans-1,3 DCP 4.0 cm semi- 15-17 4.5 Girling (1989c)
(95.4% trans + (0.67 g) staticb
0.3% cis)
Cis- + trans- 4.2 cm semi- 13-17 2.0 Girling (1989b)
mixture (0.68 g) staticc
(51-52% cis +
44% trans)
a pH 7-7.8; total hardness, 226-258 mg/litre as CaCO3: dissolved oxygen, 6.4-10.2 mg/litre.
b pH 7.2-7.8; total hardness, 234-264 mg/litre as CaCO3: dissolved oxygen, 8.2-9.9 mg/litre.
c pH 7.5-8.3; total hardness, 251-284 mg/litre as CaCO3: dissolved oxygen, 8.1-10.2 mg/litre.
7.2.2 Fish
Embryo-larval tests have been conducted on Fathead minnows
(Pimephales promelas) exposed to 1,3-dichloropropene. The maximum
no-effect concentration was 0.24 mg/litre (no details are available)
(US EPA, 1980).
7.2.3 Field studies
See section 7.3.2 of "Mixtures of dichloropropenes and
dichloropropane" for effects of "MIX D/D" on worms.
7.2.4 Phytotoxicity
1,3-Dichloropropene is highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The United States Environmental Protection Agency, published a
Health and Environmental Effects Profile for 1,3-dichloropropene in
1985 (US EPA, 1985).
In two other documents of the US EPA, health risk assessment
information is given, on the basis of a comprehensive review of the
toxicity data (US EPA, 1990, 1991).
8.1 Single exposures
8.1.1 Oral
The acute oral LD50s for mice and rats are summarized in
Table 10.
The following signs of toxicity were observed after oral
administration: hunched posture, lethargy, pilo-erection, decreased
respiratory rate, ptosis, diarrhoea, diuresis, ataxia, tip-toe gait,
red/brown staining around the snout, tremors, emaciation, and pallor
of the extremities. Haemorrhages and congestion were found in the
lungs and gastrointestinal tract. The livers showed patchy areas of
pallor (Jones & Collier, 1986a; Jeffrey et al., 1987; Gardner,
1989a,b,c).
8.1.2 Inhalation
The acute inhalation LC50s for 1,3-dichloropropene are
summarized in Table 11.
During the exposure and observation periods, the following
symptoms were observed: partial closing of the eyes, pilo-erection
salivation, lacrimation, lethargy, diarrhoea, reduction in
respiratory rate, irregular respiratory movements (lung congestion
was observed in dead animals) and hunched posture, brown staining of
fur and fur loss, and reddening of ears, tail, and feet.
Pathological signs were cardiopulmonary failure, acute tubular
necrosis in the kidneys, and local effects on the respiratory tract.
It was suggested that the irritating properties of the vapour
might serve as a warning of its presence in air. Signs of
intoxication included hypoactivity, anorexia, and chromodacryorrhoea
(Coombs & Carter, 1976b).
Table 10. Acute oral toxicity (LD50) of 1,3-dichloropropene
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse (CD 1) undiluted 215 Coombs & Carter
(1976b)
Mouse 92% (in corn oil) 640 (582-704)a Toyoshima et al.
(JCL:ICR) 640 (547-749)b (1978a)
Rat 94.5-97.5% cis-, 1.5% 85 Jeffrey et al.
(Fischer 344) trans-, 0.25% (1987); Gardner
1,2-dichloropropane, (1989b)
undiluted
Rat 96.7%, trans-, 94 Gardner (1989c)
(Fischer 344) undiluted 117a
78b
Rat (CD) undiluted 127 (112-141) Coombs & Carter
(1976b)
Rat (not 92% (in corn oil) 470b Torkelson & Oyen
specified) 713a (1977)
Rat (Sprague- 97.2% 130 (110-170)a Jones & Collier
Dawley) between 110 and 250b (1986a)
Rat D-D 95, undiluted 57 Gardner (1989a)
(Fischer 344) (cis + trans 1 : 1)
Rat 92% (in corn oil) 560 (452-695)a Toyoshima et al.
(Wistar) 510 (480-726)b (1978b)
Table 10 (contd)
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Rat 97.5% 300a Jeffrey et al. (1987)
(Fischer 344) 224b
a Male.
b Female.
Table 11. The acute inhalation toxicity LC50 for 1,3-dichloropropene (4-h exposure)
Species Concentration LC50 (mg/m3) Reference
of substance
Rat 51% cis-, 43.4% 3309.7 Blair (1977)
(Wistar) trans-isomer, 1%
epichlorohydrin
Rat Telone II 2.70-3.07c Cracknell et al.
(Wistar) (98.4%) (1987)
Rat 95.6% cis- and 3041.8a Nitschke et
(Fischer 344) 1.5% trans-isomer 3377.8b al. (1990)
Rat Telone II (97.5%; > 3881.7-< 4698.9a Streeter et al.
(Fischer 344) 52.6% cis- and 4014.2b (1987)
44.9% trans-isomer)
Rat 95.4% trans- and 4880.5b Collins (1989)
(Crb:CD(SD)Br) 0.3% cis-isomer 5402.6a
a male.
b female.
c mg/litre.
A 2-h exposure to 1,3-dichloropropene at 4540 mg/m3 was
lethal to rats, whereas guinea-pigs died following a single 7-h
exposure to 1816 mg/m3 (Torkelson & Oyen, 1977).
One female and 3 male Rhesus monkeys (4-5 kg) were exposed to
Telone II at 0, 113.5, 227, 454, 908, or 2724 mg/m3 for a single
behavioural session of 1 h, with a 1-week recovery period after
exposure. Data were obtained on all monkeys for each of the 5
atmospheric concentrations. At concentrations ranging up to 908
mg/m3, there were no significant indications of toxicity or
alterations in behaviour (the monkeys were trained to perform on a
dual component FRFR (Fixed Ratio followed by a Fixed Ratio) chained
schedule, with light stimulation). Only slight eye irritation was
observed in all monkeys at concentrations exceeding 454 mg/m3
(Rosenblum & Talley, 1979).
8.1.3 Dermal
The acute dermal and subcutaneous LD50s are summarized in
Table 12.
After dermal application, the signs of intoxication were:
diarrhoea, lethargy, hunched posture, decreased respiratory rate,
with lacrimation, salivation, ataxia or abasia, loss of righting
reflex, diarrhoea, diuresis, and red/brown staining around the eyes,
snout or mouth. The lungs and gastrointestinal tract showed
haemorrhages and irritation. Signs of skin irritation manifested by
oedema, eschar formation, or subcutaneous haemorrhage were apparent
(Jones & Collier, 1986b).
One 24-h application of undiluted 1,3-dichloropropene to
intact, occluded New Zealand white rabbit skin caused extremely
severe eschar, resulting in black necrotic tissue. The skin had
hardened and was cracking after 7 days. The animals used for dermal
LD50 estimation with 1,3-dichloropropene showed lethargy and
hypothermia at dose levels above 300 mg/kg body weight (Coombs &
Carter, 1976b).
8.2 Short-term exposures
8.2.1 Oral
Albino rats derived from the Wistar strain, were used in a 90-
day test. Groups of 10 male and 10 female rats received doses (by
gavage) of 0, 1, 3, 10, or 30 mg 1,3-dichloropropene (40% cis- and
28% trans-isomer)/kg body weight on 6 days/week. General
Table 12. Acute dermal and subcutaneous LD50s for 1,3-dichloropropene
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(JCL:ICR) (1978a)
Rat dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(Wistar) (1978b)
Rat (CD) dermal undiluted 0.423 Coombs & Carter
(0.336-0.555) (1976b)
Rat (Sprague- dermal 97.2% 1.0 (0.8-1.3)a Jones & Collier
Dawley) between 1.3 and 2.0b (1986b)
Rat dermal 96.7%, trans-isomer 1.575 Gardner (1989c)
(Fischer 344) undiluted
Rat dermal 94.5-97.5% 1.09 Gardner (1989b)
(Fischer 344) cis-, 1.5%
trans-, 0.25%
1,2-dichloropropane
undiluted
Rabbit (not dermal 92% 0.504 Torkelson & Oyen
specified) undiluted (0.22-1.15) (1977)
Mouse subcutaneous 92% (in corn oil) 0.33 (0.29-0.376)a Toyoshima et
(JCL:ICR) 0.345 (0.30-0.40)b al. (1978a)
Table 12. (contd)
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Rat subcutaneous 92% (in corn oil) 0.40 (0.345-0.464)a Toyoshima et
(Wistar) 0.366 (0.305-0.439)b al. (1978b)
a Male.
b Female.
condition, behaviour, and survival were not affected at any dose
level. No distinct differences in haematological indices, serum
enzyme activities, or urinalysis were observed. The relative kidney
weights were significantly increased in the 30-mg group in both
sexes and also in the males receiving 10 mg/kg. The relative liver
weights of females at 30 mg/kg were significantly increased. Gross
and microscopic examination did not reveal any abnormalities in the
main organs. The 3 mg/kg body weight dose was without effect (Til et
al., 1973).
8.2.2 Inhalation
8.2.2.1 Mouse
CD-1 Albino mice, 10/sex per group were exposed to production
grade Telone II (whole-body exposure) at 0, 45.4, 136.2, or 408.6
mg/m3 for 6 h/day, 5 days/week, for 90 days. No significant
differences in lesions were found between the control and treated
groups upon gross and histological examination, except that there
were compound-related effects on the nasal turbinates. These effects
were decreased height of the nasal epithelial cells resulting from a
loss of cytoplasm, and disorganization of the nuclei. Necrotic cells
were only observed among the females exposed to 408.6 mg/m3. No
alterations were found in the mice exposed to 136.2 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 10 male and 10 female B6C3F1 mice were exposed
(whole body) by inhalation to technical grade 1,3-dichloropropene
90.9% ( cis 48.6% and trans 42.3%) containing 2.4% 1,2-
dichloropropane, 5.5% mixed isomers of chlorohexane, chlorohexene,
and trichloropropene, and epichlorohydrin 1.2% (as stabilizer). The
actual exposures were to levels of 0, 45.4, 136, 409, or 681 mg/m3
for 6 h/day, 5 days/week for 13 weeks. Extensive haematological,
clinical-chemical, and urinalysis studies, and histopathological
examination of organs and tissues were performed.
A treatment-related depression in body weight was seen at doses
of 409 and 681 mg/m3. In animals exposed to 681 mg/m3, transient
brown discoloration of the fur with a strong mercaptan odour in the
coats and urine was found. The poor growth rate was reflected in a
decrease or increase in the relative weights of a number of organs,
but without histological alterations. An exposure-related decrease
in BUN levels was observed in male mice exposed to 409 and 681
mg/m3. Furthermore, alanine transaminase levels were increased in
mice exposed to 681 mg/m3. No other changes were found. The
primary target tissues of inhaled 1,3-dichloropropenes were the
nasal mucosa and urinary bladder. The changes in the nasal mucosa
including slight degeneration of the olfactory epithelium and slight
hyperplasia of respiratory epithelium in animals exposed to 409 and
681 mg/m3 were dose-related. The animals also had small focal
areas of nasal metaplasia, a condition in which the damaged sensory
olfactory epithelium is replaced by ciliated respiratory epithelium
(only in animals exposed to 681 mg/m3).
The urinary bladders of female mice exposed to 409 and 681
mg/m3 (7/10 and 6/10, respectively) had areas of moderate
hyperplasia of the transitional epithelium (7-10 layers thick in
contrast with 2-3 layers in control mice). Submucosal aggregates of
lymphoid cells in the bladder, not associated with hyperplasia and
not treatment-related, were found in female mice exposed to
concentrations of 136 mg/m3 or more. No treatment-related effects
were found in mice exposed to 45.4 mg/m3 (Stott et al., 1984,
1988).
8.2.2.2 Rat
Fischer 344 rats, 10/sex per group, were exposed to 0, 45.4,
136, or 409 mg/m3 of production grade Telone II, for 6 h/day, 5
days/week for 90 days. No significant differences in lesions were
found between the control and treated groups on gross or
histological examination, except that compound-related effects on
the nasal turbinates were found. These effects included decreased
height of the nasal epithelial cells resulting from a loss of
cytoplasm, and a disorganization of the nuclei. Necrotic cells were
observed in all of the male and female rats exposed to 409 mg/m3
and in 6/10 of the female rats exposed to 136 mg/m3. No
alterations were found in the animals with 45.4 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 5 male rats were exposed to atmospheres containing
concentrations of 1,3 cis-/trans-dichloropropene (92%) of 0 or
13.6 mg/m3 for 0.5, 1, 2, or 4 h/day, 5 days/week for 6 months.
The only effect in all the exposed groups was a slight, apparently
reversible change, seen microscopically in the kidneys of male rats
(Torkelson & Oyen, 1977).
Groups of 24 male and 24 female rats were exposed repeatedly to
air containing 0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7
h/day, 5 days/week for 6 months. Changes attributable to 1,3-
dichloropropene were limited to cloudy swellings in the renal
tubular epithelium of male rats in the 13.6 mg/m3 group. Female
rats in this group showed an increased liver to body weight ratio,
though no histopathological changes were observed. A recovery group
of male and female rats was maintained for 3 months following the 6-
month exposure to 4.54 or 13.6 mg/m3. No changes were observed in
the renal epithelium following this recovery period (Torkelson &
Oyen, 1977).
In a well-designed study (Stott et al., 1984, 1988), groups of
10 male and 10 female Fischer 344 rats were exposed through
inhalation to technical grade 1,3-dichloropropene 90.9% (for
composition see section 8.2.2.1) in actual concentrations of 0,
45.4, 136, 409, or 681 mg/m3, for 6 h/day, 5 days/week for 13
weeks. Haematological, clinical-chemical studies, urinalysis, and
histopathological studies of organs and tissues were carried out.
In the rats exposed to 681 mg/m3, transient brown
discoloration of the fur with a strong mercaptan odour in the coats
and urine was found. The body weights of rats exposed to 409 or 681
mg/m3 were decreased in an exposure-related manner. The relative
weights of the testes were increased and thymus weights decreased.
Rats exposed to 409 and 681 mg/m3 had slightly lower levels of
serum proteins. No other treatment-related changes in clinical-
chemical or haematological parameters were found.
The primary target tissues of inhaled 1,3-dichloropropenes were
the nasal mucosa in male and female rats and the uteri in females.
The effects consisted of dose-related degenerative effects on the
nasal olfactory epithelium or mild hyperplasia of the respiratory
epithelium or both, in all animals exposed to 409 and 681 mg/m3,
and 2 of the 10 male animals in the 136 mg/m3 group.
The uteri of 7 out of 10 female rats exposed to 681 mg/m3
were not developed as completely as those of control animals,
suggesting hypoplasia of the uterine tissues. The only other change
noted histologically was the atrophic appearance of the mesenteric
adipose tissue in the rats exposed to 681 mg/m3. No treatment-
related effects were observed in rats of either sex exposed to 45.4
mg 1,3-dichloropropenes/m3 (Stott et al., 1984, 1988).
In a study to investigate the effects of 1,3-dichloropropene on
tissue sulfhydryl levels, groups of 15 male and 15 female Fischer
344 rats were exposed to vapours of cis-1,3-dichloropropene (94.3-
95.6% cis-, 1.5% trans-1,3-dichloropropene and 0.2% 1,2-
dichloropropane) at 0, 45.4, 272.4, or 681.0 mg/m3 for 6 h/day, 5
days/week for 9 exposures. Whole-body exposures occurred under
dynamic air-flow conditions. On the day after the last exposure, 5
males and 5 females/dose level were necropsied. Major organs were
weighed and selected tissues were evaluated histopathologically.
Groups of 5 animals/sex per dose level were used to determine the
non-protein sulfhydryl (NPS) contents of the liver, kidneys, and
lung, 1 and 18 h following the last exposure.
Rats exposed to 681 mg/m3 lost weight, but not those exposed
to 272.4 mg/m3. There were concentration-related decreases in
liver, kidney, and lung NPS levels in male rats, when measured after
1 h. After 18 h, the NPS levels were higher than those of the
controls and there were no associated gross or histopathological
changes in these organs. The NPS measurements for females were
unremarkable. Histopathological examination revealed changes of
moderate severity in the respiratory and olfactory mucosa in the
nasal cavity of males and females exposed to 681 mg/m3. There were
no changes in the animals exposed to 272.4 mg/m3 (Nitschke &
Lomax, 1990).
Groups of 10 male and 10 female Fischer 344 rats (6 weeks old)
were exposed to cis-1,3-dichloropropene at 0, 45.4, 136, or 409
mg/m3 for 6 h/day, 5 days/week for 13 weeks. The test material was
reported to consist of 94.3% (95.6%) cis-1,3-dichloropropene, 1.5%
trans-1,3-dichloropropene and 0.2% 1,2- dichloropropane. Whole-
body exposures occurred under dynamic conditions. No exposure-
related effects were noted in haematology or clinical chemistry. At
409 mg/m3, body weights of male rats were significantly decreased
compared with controls throughout the 13-week exposure period; body
weights of female rats were significantly decreased during the first
6 weeks, but were comparable with those of the controls at the end
of the study. As a result of the decreased body weight in male rats,
the relative liver, kidney, lung, and testes weights were
significantly elevated in comparison with control values. The
relative liver weight in females was also increased. These organ
weight changes were not accompanied by gross or histopathological
changes. Exposure-related changes only occurred in the nasal
cavities of the rats of both sexes exposed to 409 mg/m3 and
consisted of multifocal bilateral degeneration of olfactory
epithelium and slight bilateral multifocal hyperplasia of
respiratory epithelium. No effects were noted in rats exposed to
45.4 or 136 mg/m3. The NOEL was considered to be 136 mg cis-1,3-
dichloropropene/m3 (Nitschke et al., 1991).
8.2.2.3 Other animal species
Groups of 12 male and 12 female guinea-pigs, 3 male and 3
female rabbits and 2 dogs were exposed repeatedly to air containing
0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7 h/day, 5
days/week for 6 months. No changes resulting from 1,3-
dichloropropene exposure were observed in guinea-pigs, rabbits, or
dogs (Torkelson & Oyen, 1977).
8.3 Skin and eye irritation, sensitization
8.3.1 Skin irritation
1,3-Dichloropropene is extremely irritating to the skin
(Worthing & Hance, 1991).
Telone II (52.63% cis- and 44.91% trans-1,3,-
dichloropropene), 0.5 ml, was applied for 4 h to the back (clipped
free of fur) of 2 male and 4 female New Zealand White rabbits. After
4 h, the wrapping and gauze patch and any residual test substance
were removed. Dermal irritation characterized as slight to moderate
erythema and moderate to severe oedema was observed at the site of
application immediately following the 4 h exposure period.
Subsequent observations revealed slight to moderate erythema,
oedema, and exfoliation. These changes were still present in some of
the animals after 14 days (Jeffrey, 1987a).
In a 4-h rabbit skin irritancy test, undiluted cis-1,3-
dichloropropene (94.5-97.5% cis-, 1.5% trans-1,3-dichloropropene
and 0.25% 1,2-dichloropropane) caused well defined erythema and
moderate oedema shortly after removal of the semi-occlusive
dressings in New Zealand White rabbits (3-5 months of age). After
removal of the dressings, the skin was washed after 4 h. Resolution
of the irritancy reaction was first apparent on the day after
treatment and was complete within 14 days (Gardner, 1989b).
In another 4-h rabbit skin irritancy test, 0.5 ml of undiluted
trans-1,3-dichloropropene (96.7%) caused irritation not exceeding
well-defined erythema and slight oedema. New Zealand White rabbits
(3-5 months of age) were used. After the 4-h exposure, the dressings
were removed and the skin washed. Resolution of the irritation was
first apparent 72 h after treatment and was complete by day 21
(Gardner, 1989c).
8.3.2 Eye irritation
1,3-Dichloropropene is a severe eye irritant (Worthing & Hance,
1991).
Aliquots of 0.1 ml Telone II (52.63% cis- and 44.91% trans-
1,3-dichloropropene) were instilled into the conjunctival sac of one
eye of 4 male and 2 female New Zealand White rabbits. The eyes of
all rabbits remained unwashed. Slight to marked redness and slight
to moderate chemosis were found after the treatment. The treated
eyes had a slight to marked amount of discharge as well as reddening
of the iris. In one animal, opacity was found which resolved as did
all the other changes within 14 days following treatment (Jeffrey,
1987b).
In the rabbit eye irritancy test, 0.1 ml trans-1,3-
dichloropropene (96.7%) caused moderate to severe conjunctival
irritation and minor irritation changes of the cornea (opacities),
chemosis, and iridial responses within 24 h following instillation
into the eye. Resolution of the irritant effects of trans-1,3-
dichloropropene was advanced 7 days after treatment and complete one
week later. There was an initial pain response (Gardner, 1989c).
Ten male and 10 female Sprague-Dawley rats (Spartan substrain)
were exposed to an aerosol (mean size 2.96 µm; 99% of particles were
6 µm or less in diameter) in a glass chamber for 1 h at a nominal
concentration of 5.2 mg/litre of air. Five male and 5 female animals
were maintained under ambient conditions as controls. Slight
transitory eye irritation was observed during the exposure (Yakel &
Kociba, 1977).
8.3.2.1 In vitro studies
The corneal thickness of isolated eye preparations subjected to
application of cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) increased
by more than 20% within 3 h. The isolated rabbit eye test described
by Price & Andrews (1985) was used. Corneal uptake of fluorescein
was demonstrated at the conclusion of the test. The results
indicated that application of cis-1,3-dichloropropene to the eye
in vivo would cause significant tissue damage (Gardner, 1989b).
8.3.3 Sensitization
1,3-Dichloropropene was applied in corn oil in a 5% v/v
concentration 3 times topically to the skin of guinea-pigs followed
by a 1% concentration as a challenge following the method of Buehler
(1965). A positive reaction was obtained in 5 out of 20 guinea-pigs
of the "P" strain. The reaction was considered to be mild to
moderate skin sensitization (Coombs & Carter 1976b).
Ten male, Hartley albion guinea-pigs received 3 dermal
applications on the back of 0.4 ml of 0.1% (v/v) Telone II (52.63%
cis- and 44.91% trans-1,3-dichloropropene) in mineral oil;
another group received only the vehicle during the induction phase
of the study. The dermal sensitization potential was tested using
the modified Buehler method. A positive control group received 2
applications of 10% epoxy resin and a third application of 5% epoxy
resin. All groups were challenged dermally 2 weeks after the last
induction application. The control group did not show any signs of
sensitization, while 5 out of the 10 animals of the positive control
group revealed slight erythema. Nine out of 10 guinea-pigs
challenged with 0.1% Telone II revealed slight to moderate erythema.
Telone II was considered a potential skin sensitizer at the
concentrations tested (Jeffrey, 1987c).
In the guinea-pig maximization test of Magnusson & Kligman, all
20 test animals showed positive responses, 24 and 48 h after removal
of the challenge patches. Guinea-pigs of the Dunkin-Hartley strain
(5-9 weeks of age) were used in this study. In a study by Gardner
(1989b), 0.1% cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) was
injected intradermally; topical induction was carried out using a 5%
solution in corn oil, and the topical challenge using 2.5% in corn
oil.
In the guinea-pig maximization test of Magnussen & Kligman, 16
out of 20 test animals showed positive responses 24 and/or 48 h
after removal of the challenge patches. Guinea-pigs of the Dunkin-
Hartley strain (age 5-9 weeks) were used. Intradermal injection,
carried out at concentrations of 0.05% trans-1,3-dichloropropene
(96.7%) or more resulted in red areas with defined edges. The
concentration selected (10%) for topical induction did give slight
irritation, but the concentration for the topical challenge of 5%
was without effect. The solvent was corn oil (Gardner, 1989c).
8.4 Long-term exposure
See section 8.7 (Carcinogenicity).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Inhalation (rat)
Groups of 30 male and 30 female Fischer 344 rats (6 weeks of
age) were exposed to Telone II (1,3-dichloropropene containing 2%
epoxidized soybean oil) at 0, 45.4, 136, or 409 mg/m3 (first 7
days of study: 0, 22.7, 90.8, or 272.4 mg/m3) for 6 h/day, 5
days/week during the premating period and for 7 days/week during
breeding, gestation, and lactation, for 2 generations. The Telone II
used had a purity of 92% and the remainder of the test material
comprised chlorinated and unchlorinated alkanes and alkenes as well
as approximately 2.0% epoxidized soybean oil. Following 10 weeks of
exposure, the adult rats (F0) were mated twice to produce the
F1a and F1b litters. After weaning, 30 pups/sex per exposure
from the F1b litters were selected and, after 12 weeks, used to
produce the F2a and F2b litters.
All litters were examined on the day of parturition, the
following indices of fertility being recorded: gestation length,
litter size, pup survival indices, number of live pups on days 1 up
to 28 postpartum, sex and weight of litters, lactation and
individual body weights, and any visible physical abnormalities.
Gross necropsy was carried out on adult rats F0 and F1 and
weanling F1b and F2b rats and histopathological studies on adult
rats. Inhalation of up to 409 mg/m3 for 2 generations did not
adversely affect reproduction or neonatal growth or survival.
Exposure to 409 mg/m3 resulted, however, in parental toxicity
(F0, F1), as indicated by decreases in body weight and
histopathological effects in the nasal mucosa (slight, focal
hyperplasia of the respiratory mucosal epithelium and/or focal
degenerative changes in the olfactory epithelium). No adverse
effects were observed in the parents in the 136 mg/m3 group. The
reproductive no-effect level was 409 mg/m3 (Breslin et al., 1987,
1989).
8.5.1.2 Intraperitoneal (mouse)
1,3-Dichloropropene (Telone II) in corn oil was injected
intraperitoneally in B6C3F1 male mice (4 per group) at dose
levels ranging from 10 mg up to 600 mg/kg body weight, daily for 5
days, to study sperm morphology, epididymal sperm counts, and testes
weights. Testicular toxicity was assessed at day 35. No animals
survived at dose levels of 150 mg/kg body weight or more. No effects
on the testes were found at dose levels up to 75 mg/kg body weight
(Osterloh et al., 1983).
8.5.2 Teratogenicity
8.5.2.1 Inhalation (rat)
In a range-finding study, groups of 7 or 8 female Fischer 344
rats were exposed to Telone II (92.1%; 47.7% cis- and 42.4%
trans-1,3-dichloropropene, impurities, and 1.8% epichlorohydrin)
at 0, 230, 680, or 1360 mg/m3 for 6 h/day on days 6-15 of
gestation.
Consumption of food and drinking-water was decreased during the
period of exposure in rats exposed to 680 or 1360 mg/m3 and the
rats showed a significant decrease in body weight gain. Rats were
sacrificed on day 16 and examined. During exposure, nasal exudate
and red crusty material around the eyes were observed. In the group
exposed to 1360 mg/m3, a significant decrease in litter size and
significant increase in resorption were seen, while in the groups
exposed to 230 and 680 mg/m3, the same changes were observed, but
were not statistically significant (Kloes et al., 1983).
Groups of 30 Fischer 344 rats were exposed, via inhalation, to
1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-isomer) at
0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-15 of
gestation. Food consumption and maternal body weight gain were
depressed in all treated groups in a dose-related manner. A decrease
in water consumption was found in rats at the highest dose-level. No
consistent or dose-related effects on reproductive performance were
found. The number of implantations, resorptions, litter size, fetal
weights, and fetal lengths were comparable with the controls. A
slight, but statistically significant, dose-related increase in the
incidence of delayed ossification of the vertebral centra was found
in rats, but considered of little toxicological significance in the
light of the maternal toxicity observed. The authors stated that
there was no evidence of a teratogenic or embryotoxic response at
exposure levels up to and including 545 mg/m3 (John et al., 1983;
Hanley et al., 1987).
8.5.2.2 Inhalation (rabbit)
In a range-finding study, groups of 7 New Zealand White rabbits
were exposed to Telone II (92.1%, 47.7% cis- and 42.4% trans-
1,3-dichloropropene, impurities and 1.8% epichlorohydrin) at 0, 230,
680 or 1360 mg/m3 for 6 h/day on days 6-18 of gestation. The
rabbits were sacrificed on day 19 and examined. Six out of 7 rabbits
exposed to 1360 mg/m3 showed signs of toxicity, such as rear limb
ataxia, decreased or absence of righting reflex, and flaccid hind
limb muscles; these animals died or were sacrificed. Histologically,
no effects were detected in the brains of these animals. Maternal
body weight was statistically significantly decreased during
exposure in the 680 mg/m3 group, but weights in the 230 mg/m3
group were comparable with those of the controls. There were no
differences in the reproductive parameters between the groups
exposed to 230 and 680 mg/m3 and the controls. No teratological
effects were found (Kloes et al., 1983).
Groups of 25-31 inseminated New Zealand white rabbits were
exposed to 1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-
isomer) at 0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-18
of gestation. Decreased weight gain was observed among rabbits at
272 and 545 mg/m3, but no pronounced maternal toxicity was
observed. No evidence of teratogenic or embryotoxic responses was
observed (John et al., 1983; Hanley et al., 1987).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies
8.6.1.1 Microorganisms
Cis- and trans-1,3-dichloropropene or a mixture were tested
for their mutagenic activity in Salmonella typhimurium and in
Saccharomyces cerevisiae, with and without metabolic activation.
Most of the studies showed a positive effect, especially with
Salmonella typhimurium TA100 and TA1535, with, and without,
metabolic activation. With TA98 and TA1537, positive and negative
effects were found. Saccharomyces cerevisiae was positive. The
results are summarized in Table 13. Samples of highly purified 1,3-
dichloropropene or cis-1,3-dichloropropene, however, were not
mutagenic to Salmonella typhimurium TA100, indicating that trace
impurities, such as 1,3-dichloropropene oxide, were the cause of the
activity (Talcott & King, 1984; Watson et al., 1987, see section
8.8.2).
There was a difference in mutagenicity (expressed as rev/µmol)
between the cis and trans isomers of 1,3-dichloropropene in
Salmonella typhimurium TA100, with, and without, metabolic
activation. The cis-isomer induced a greater number of revertants,
with, and without, S9 mix, than the trans-isomer and also showed
stronger alkylating properties in the NBP-test. Furthermore, a
longer preincubation time invariably led to higher mutagenic
activity, and an increase in protein added to the activating system
increased the efficiency of the metabolic activation (Neudecker et
al., 1980, Neudecker & Henschler, 1986).
Salmonella typhimurium TA100 was used to test for the
mutagenicity of cis- and trans-3-chloroallyl alcohol (99%),
with, and without, metabolic activation. No mutagenicity was
observed without S9 mix, over a range of 0.01-1000 µg/plate.
However, a positive effect was obtained with S9 mix and cis-3-
chloroallyl alcohol (in high concentration) (Connors et al., 1990).
Von der Hude et al. (1988) used the SOS chromotest with
Escherichia coli PQ 37, to examine 1,3-dichloropropene (in DMSO)
at concentrations of 0, 1.0, 3.3 or 10.0 mmol/litre, without S9. In
this strain, the structural gene for beta-galactosidase lacZ is
placed under the control of the SOS-gene sfiA. The expression of
this gene, induced by DNA-damage, is measured indirectly by
determination of the beta-galactosidase activity. The test was
positive with concentrations of 1.0 mmol/litre or more.
8.6.1.2 Effects of glutathione on bacterial mutagenesis
The addition of glutathione (GSH), at physiological
concentrations, to in vitro bacterial test systems of Salmonella
typhimurium TA100 has shown a clear protective effect, i.e., a
virtual elimination of the mutagenic response of 1,3-dichloropropene
(Brooks et al., 1978; Climie et al., 1979; Creedy & Hutson, 1982;
Wright & Creedy, 1982; Creedy, 1983; Creedy et al., 1984; Brooks &
Wiggins, 1990). This protective effect occurs in either the presence
or the absence of S9 for both the (cis)- and (trans)-isomers.
Protection, in the presence of S9, is consistent with the operation
of glutathione transferase enzyme occurring in the added S9
fraction. This enzyme is also present in mammalian cells, and as the
metabolic studies have shown, it plays a key role in the rapid
detoxification of cis-1,3-dichloropropene in mammalian tissue
(Climie et al., 1979; Brooks & Wiggins, 1991).
Even in the absence of S9, this protection by GSH still exists
against the mutagenic action of 1,3-dichloropropene. Thus, it is
likely that an additional mechanism for protection exists that
probably reflects a reaction between the mutagenic component(s) and
GSH. This additional mechanism may also play an important role in
the detoxification of 1,3-dichloropropene via a spontaneous
nucleophilic substitution reaction between the chloromethyl carbon
of 1,3-dichloropropene and the sulfur atom of glutathione.
Table 13. Mutagenicity tests with 1,3-dichloropropenes (1,3-DCP) on microorganisms
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
Salmonella typhimurium
Cis-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 top agar S9 mix/none + Neudecker et al. (1977)
(99.9%) µg/ml Kier et al. (1986)
Cis-1,3-DCP TA98, TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
(> 99%)
TA1538 20-2000 µg plate S9 mix/none - Brooks et al. (1978)
Cis-1,3-DCP TA100, TA1535 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5%, + TA98, TA1537, TA1538 78-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1990)
1,2-DCP 0.25%)
Cis-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 µg/ml top agar S9 mix/none + Neudecker et al. (1977)
(97.5%) Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535 39-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1989a)
(98% + cis-
1,3-DCP 0.3%) TA98, TA1537, TA1538 39-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1989a)
1,3-DCP (51.3% TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
cis, 43.7%
trans, 0.6% TA98, TA1538 20-2000 µg plate S9 mix/none ± Brooks et al. (1978)
epichlorohydrin)
Table 13 (contd)
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
1,3-DCP *** TA100, TA1535 3-333 µg/ml plate S9 mix/none + Haworth et al. (1983)
TA98, TA1537 3-333 µg/ml plate S9 mix/none - Haworth et al. (1983)
1,3-DCP *** TA100, TA1535 1000 µg/ml * S9 mix/none + Priston et al. (1983)
TA98, TA1537 1000 µg/ml * S9 mix/none - Priston et al. (1983)
1,3-DCP *** TA100 0.1-10 µmol plate S9 mix/none + Stolzenberg & Hine (1980)
1,3-DCP *** TA98 100 µg ** none + Vithayathil et al. (1983)
Saccharomyces cerevisiae
1,3-DCP *** JD1 1000 or 5000 liquid S9 mix/none + Priston et al. (1983)
µg/ml
culture
Escherichia coli
Cis-1,3-DCP WP2 UVRA pKM 101 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5% +
1,2-DCP 0.25%)
Trans-1,3-DCP WP2 UVRA pKM 101 39-1250 µg/ml * none - Brooks & Wiggins (1989a)
(98% + cis-1,3- S9 mix +
DCP 0.3%)
* Assays were performed by the pre-incubation method in sealed containers.
** Salmonella/microsome multiple indicator test.
*** Details of composition not given.
These 2 mechanisms with GSH afford complete protection against
the mutagenic activity of the trans-isomer in bacterial test
systems, in the presence and absence of S9 (Hutson & Stoydin, 1977;
Creedy & Hutson, 1982; Wright & Creedy, 1982).
In the presence of Aroclor-induced rat liver S-9 fraction and a
rat liver microsomal mono-oxygenase system, cis-1,3-
dichloropropene (I) is apparently metabolized to a mutagenic
metabolite, cis-1-chloro-3-chloromethyloxirane (II), which is not
as effectively deactivated by glutathione as the parent compound.
Cis-dichloropropeneoxide was shown not to be stable in aqueous
medium, 2-chloroacroleine (III) was the product of hydrolysis,
another direct-acting mutagen for Salmonella typhimurium TA100
(Hutson, 1984) (see Fig. 5). Glutathione transferase systems afford
efficient protection against this bioactivated product of 1,3-
dichloropropene. The protective action of glutathione-linked systems
against the mutagenicity observed in S. typhimurium has also been
shown to occur in vivo.
8.6.1.3 Mammalian cells
In a gene mutation assay, V79 Chinese hamster cells were
exposed to cis-1,3-dichloropropene (99.9%) in DMSO at dose levels
of 2.5 to 20 µg/ml. No indication for an increased mutation
frequency at the HGPRT locus was found in these cells (Meyer, 1980).
Telone II (48.9% cis- and 43.2% trans-1,3-dichloropropene)
was tested in the Chinese hamster ovary cell/HGPRT mutagenicity
assay, with the cell line designated as CHO-K1-BH4, with, and
without, metabolic activation. Three tests were carried out without
activation. The first test with 50, 100, 150, 200, and 250
mmol/litre, showed an increase in mutation frequency at the 200 and
250 mmol/litre dose levels. However, the biological significance is
doubtful because of the extreme toxicity at these high dose levels.
In a repeat of this study using the same concentrations, no increase
in mutation frequency was found. The results of a third study using
concentrations of 50, 100, 125, 150, and 200 mmol/litre were also
negative. The fourth test was with activation, with dose levels of
50, 100, 125, 150, and 200 mmol/litre. The relative survival ranged
from 98% at 50 mmol/litre to 14% at 200 mmol/litre. No increase in
mutation frequency was observed. The results indicated that Telone
II was not mutagenic in the CHO/HGPRT assay, with, or without,
metabolic activation (Mendrala, 1986).
8.6.1.4 DNA damage
Cis- and trans-1,3-dichloropropene were tested for their
ability to induce unscheduled DNA synthesis (UDS) in Hela S3
cells. The lowest dose, 10-4 mol/litre was the dose at which UDS
occurred, the dose-response curves being rather shallow (Schiffmann
et al., 1983).
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in the rat hepatocyte unscheduled DNA synthesis assay
at concentrations from 1 x 10-6 up to 3 x 10-3 mol/litre. In an
initial and a repeat assay, toxic effects on the hepatocyte cultures
(indicated as detachment of the cells and/or a granular appearance)
occurred at 1 x 10-5 mol/litre and at 1 x 10-4 mol/litre or
more, respectively. In both tests, Telone II failed to elicit
significant DNA repair in the primary cultures of rat hepatocytes,
over the wide range of concentrations tested, suggesting an apparent
lack of genetic activity (Mendrala, 1985).
The liquid Bacillus subtilis strain H17 (arg-, trp-, recE+)
microsome rec-assay was used to evaluate the DNA-damaging effect of
1,3-dichloropropene with, and without, S9 mix. The concentrations of
1,3-dichloropropene used with, and without, S9 mix were for
CR50Rec+/CR50Rec-, 7.62 x 10 / 2.49 x 10 and 4.79 x 102/1.01 x
103 mg/litre. With S9 mix, there was a strong DNA-damaging effect,
but, without S9 mix, a reverse effect was observed (Matsui et al.,
1989).
8.6.1.5 Chromosomal effects
Rat liver (RL1) cells were exposed to culture medium
containing cis-1,3-dichloropropene (99.9%) in DMSO, at
concentrations of 2.5, 5.0, or 10 µg/ml. No indication was found
that the compound induced chromosome damage in the liver cells
(Meyer, 1980).
The clastogenic potential of trans-1,3-dichloropropene (95.4%
trans-isomer and 0.3% cis-isomer) was assessed from assays
designed to monitor chromosome damage in CHO cells. Cultures were
grown in incubated medium containing the test compound, for either 3
h in the presence of S9-mix (concentration 0, 0.5, 2.5 or 5.0 µg/ml)
or 24 h in the absence of S9 mix (concentration 0, 3, 15 or 30
µg/ml). Methyl methane sulfonate and cyclophosphamide were used as
positive controls. Metaphase cells were used for the analysis of
chromosome aberrations after 8, 12, and 24 h, in the case of
cultures with S9 mix, and, after 24 h, in the case of the cultures
without S9 mix. At the 24-h sample time, it was concluded that
trans-1,3-dichloropropene induced chromosome damage (gaps, breaks,
and exchange figures) in the presence of S9 mix, but no effect was
seen in its absence (Brooks & Wiggins, 1989b).
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce chromosomal aberrations in cultured CHO cells.
In the first study, without S9, 49.1 µg/ml produced a strong
positive response, i.e., 16% of cells with aberrations (large number
of gaps). No metaphase cells were seen at the 98.0 µg/ml dose level.
In a repeat study, these results could not be confirmed with 50, 75,
and 100 µg/ml. All 3 doses produced 50% decreases in cell
confluency. When the compound was tested with S9 mix, no chromosomal
aberrations were found at 50 µg/ml, the highest dose at which
metaphase cells could be found.
Brooks & Wiggins (1991) assessed the mutagenic activity of
cis-1,3-dichloropropene (94.5-97.5% cis-isomer, 1.5% trans-
isomer, 0.25% 1,2-dichloropropane), in Chinese hamster ovary cells.
The cultures were grown in medium containing the test substance for
either 3 h in the presence of S9 mix or 24 h in the absence of S9
mix. Metaphase cells were prepared for analysis for chromosome
aberrations, 24 h following initiation of exposure with, and
without, S9 mix. From the data obtained at the 24-h sample time, it
was concluded that cis-1,3-dichloropropene at 1, 5, or 10 µg/ml
induced chromosome damage (gaps, breaks, and exchange figures) in
the presence of S9 mix. No increase in metaphase chromosome damage
was found with doses of up to 10 µg/ml in the absence of S9 mix.
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce Sister Chromatid Exchanges (SCEs), in cultured
Chinese hamster ovary cells. The test was carried out with, and
without, rat liver S9 fraction. DMSO was used as the solvent. 1,3-
Dichloropropene induced SCEs without S9 at a dose level of 29.9
µg/ml. A positive response was also found with S9.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
(SCE) test in vitro in Chinese hamster V79 cells without S9 mix,
to evaluate the effect of 1,3-dichloropropene (80%). The
concentrations tested were 0 (DMSO), and 0.1 up to 0.8 mmol/litre.
The 0.8 mmol/litre dose was toxic to the cells. A dose-related
increase in the number of SCEs was found. Negative results were
obtained with S9, at dose levels of 0.1-3.3 mmol/litre.
8.6.2 In vivo studies
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in a mouse bone marrow micronucleus test to detect
chromosomal aberrations and spindle malfunction. The substance
(dissolved in corn oil) was administered to CD-1 (ICR) BR mice by
single oral gavage at dose levels of 0 (corn oil), 38, 115, or 380
mg/kg body weight. Groups of animals were sacrificed at intervals of
24 and 48 h. A positive control group received cyclophosphamide at a
dose of 120 mg/kg. There were no significant increases in the
frequencies of micronucleated polychromatic erythrocytes in the
Telone II groups compared with the controls. Positive results were
obtained with the positive control group. Telone II was considered
negative in the mouse bone marrow micronucleus test (Gollapudi et
al., 1985).
Valencia et al. (1985) tested 1,3-dichloropropene (95.5%) for
mutagenicity in Drosophila melanogaster. The compound was tested
for the induction of sex-linked recessive lethals (SLRLs) by feeding
the substance in a 5% aqueous sucrose solution. The dose level fed
was 0 or 5750 mg/litre. The mortality rate was 33% and sterility
10%. 1,3-Dichloropropene induced an increased number of SLRLs, but
no reciprocal translocations were induced.
The results of both dominant lethal and host-mediated assays
carried out with "MIX D/D" were negative (see "Mixtures of
dichloropropenes and dichloropropane", section 8.6.2).
8.6.3 Appraisal
1,3-Dichloropropene ( cis- and trans-isomer) showed
mutagenic activity in Salmonella typhimurium, especially in
strains TA100 and TA1535 with, and without, metabolic activation.
There is a difference in mutagenic potential between the cis- and
trans-isomers in TA100 with, and without, activation. The cis-
isomer induces a greater number of revertants than the trans-
isomer.
1,3-Dichloropropene does not possess a genotoxic potential in a
variety of non-bacterial studies. Gene mutation has not been
detected in in vitro assays using the eukaryotic cell lines of
either V79 or CHO (HGRPT locus) (Meyer, 1980; Mendrala, 1986).
Chromosomal damage has not been observed in vitro with rat liver
cell cultures or in an in vivo mouse micronucleus study using
single oral doses of up to 380 mg/kg (Gollapudi et al., 1985).
Interaction with DNA was not observed, even at cytotoxic doses, in
an in vitro rat liver unscheduled DNA synthesis study (Mendrala,
1985). However, Schiffmann et al. (1983) found liver unscheduled DNA
synthesis in Hela S3 cells.
8.7 Carcinogenicity
8.7.1 Oral
8.7.1.1 Mouse
A carcinogenicity study was carried out on groups of 50 male
and 50 female (4-6 weeks old) B6C3F1 mice at dose levels of 0,
50, or 100 mg (stabilized technical grade 1,3-dichloropropene)/kg
body weight, administered in corn oil (5 ml/kg body weight), by
gavage, 3 times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, nine other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). The initial mean body weights of
the treated mice were lower (6-22%) than those of the controls.
These differences were caused by failure to fully randomize the
distribution of the animals. No clinical signs were observed.
However, the survival of vehicle control male mice was significantly
lower than that in either dose group. Thirty-nine control male mice
died with myocarditis, 25, between weeks 48 and 51. A slight
increase in mortality was found in the female mice at a dose level
of 100 mg/kg body weight.
The reduced survival of the control male mice did not allow for
an adequate evaluation of carcinogenicity in males in this study.
But, in both sexes, there was evidence for a 1,3-dichloropropene-
related increase in transitional cell carcinomas of the urinary
bladder (without the presence of calculi), liver tumours, squamous
cell papillomas and/or carcinomas of the forestomach, and of
alveolar/bronchiolar adenomas and carcinomas of the lung, at 50 or
100 mg/kg body weight. In the female mice, an increase in basal cell
or epithelial cell hyperplasia in the forestomach was also found
(Table 14). It was stated that the presence of 1% epichlorohydrin, a
direct acting mutagen and carcinogen (especially for the
forestomach), may have influenced the development of forestomach
lesions (Haseman et al., 1984; NTP, 1985; Yang, 1986; Yang et al.,
1986).
8.7.1.2 Rat
A long-term carcinogenicity study was carried out on groups of
52 F344/N rats of each sex (aged 6 weeks), at dose levels of 0, 25,
or 50 mg stabilized technical grade 1,3-dichloropropene/kg body
weight, administered in corn oil (5 ml/kg body weight), by gavage, 3
times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, 9 other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). Additional groups of 25 rats of
each sex were assigned to each dose group. At 9, 16, 21, 24, and 27
months of dosing, 5 rats/sex per group were killed and organs and
tissues studied microscopically. Haematological and clinical-
chemical studies were carried out on groups of 20 rats of each sex,
11-13 times in the first 70 weeks of the study. The mean body weight
of high-dose male rats was about 5% lower than those of the control
and low-dose rats. No differences in body weight were observed in
female animals. Mortality was comparable with the controls. The
primary organs affected were the forestomach and liver. The
incidences of non-neoplastic and neoplastic lesions are summarized
in Table 15.
Table 14. Occurrence of microscopic lesions in mice in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Urinary bladder
Epithelial hyperplasia b 2/50 (4%) 9/50 (18%) 15/50 (30%) 18/50 (36%) 19/48 (40%)
Transitional cell carcinoma b 0/50 (0%) 0/50 (0%) 8/50 (16%) 2/50 (4%) 21/48 (44%)
Lung
Alveolar/bronchiolar
adenoma or carcinoma 1/50c 2/50 (4%) 13/50 (26%) 4/50 (8%) 12/50 (24%) 8/50 (16%)*
Forestomach
Epithelial hyperplasia b 1/50 (2%) 0/50 (0%) 1/50 (2%) 4/50 (8%) 21/50 (42%)*
Squamous cell papilloma b 0/50 (0%) 2/50 (4%) 1/50 (2%) 3/50 (6%)d 4/50 (8%)*
or carcinoma
Liver
Hepatocellular adenoma 5/50b 1/50 (2%) 7/50 (14%) 8/50 (16%)* 13/50 (26%) 3/50 (6%)
or carcinoma
Kidneys
Hydronephrosis b 0/50 (0%) 0/50 (0%) 2/50 (4%) 0/50 (0%) 14/50 (28%)
a From: NTP (1985).
b Too many animals died during the study, but no epithelial hyperplasia or neoplasia were observed in these animals.
c As b but, a lung adenoma was found in one animal.
d Only squamous cell papilloma.
* P = < 0.05.
Table 15. Occurrence of microscopic lesions in rats in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Forestomach
Epithelial hyperplasia 2/52 (4%) 1/52 (2%) 5/52 (10%) 0/52 (0%) 13/52 (25%)c 16/52 (31%)c
Squamous cell papilloma 1/52 (2%) 0/52 (0%) 1/52 (2%) 2/52 (4%)b 13/52 (25%)c 3/52 (6%)b
or carcinoma
Liver
Neoplastic nodule 1/52 (2%) 6/52 (12%) 6/52 (12%)c 6/52 (12%)c 7/52 (13%)c 10/52 (19%)
or carcinoma
a From: NTP (1985).
b Only squamous cell papilloma.
c P = ¾ 0.05
Under the conditions of the study, 1,3-dichloropropene induced
an increased incidence of squamous cell papillomas and carcinomas of
the forestomach. In addition, a dose-related trend was observed in
the incidence of neoplastic nodules in the livers of male rats. It
was stated that the presence of 1% epichlorohydrin, a direct acting
mutagen and carcinogen (especially for the forestomach), may have
influenced the development of forestomach lesions (Haseman et al.,
1984; NTP, 1985; Yang, 1986; Yang et al., 1986).
In the ancillary study, dose-related lesions were observed in
the forestomach and liver. The development of the forestomach basal-
cell hyperplasia and squamous-cell papilloma followed a time-
dependent trend in high-dose males and females. Basal-cell
hyperplasia was seen 9-16 months after dosing started. The neoplasms
of the forestomach and liver were not seen until 24 months after
dosing began.
8.7.2 Inhalation
8.7.2.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (6-7 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days per
week for up to 24 months. In addition, 2 ancillary groups, each with
10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The mice were exposed to 1,3-
dichloropropane vapour at 0, 22.7, 90.8, or 272 mg/m3. The
composition of the technical-grade 1,3-dichloropropene was 1,3-
dichloropropene 92.1% ( cis-49.5% and trans 42.6%); 1,2-
dichloropropane 0.7%; and 5.2% mixtures of hexanes and hexadienes.
Epoxidized soybean oil (approximately 2%) was added as stabilizing
agent. Besides body weights, clinical-chemical parameters in the
blood and urine and haematological parameters were determined for
all animals terminated at 6 and 12 months and for 20 animals/sex per
group at 24 months. At 6, 12, and 24 months, animals were sacrificed
and the weight of 5 organs determined; a large number of organs and
tissues were examined histopathologically.
No significant differences in survival rates were observed
between the groups. The body weights of male mice exposed to 272 mg
1,3-dichloropropene/m3 were statistically significantly depressed
in comparison with the controls. Examination of haematological and
clinical-chemical parameters and urinalysis did not indicate any
toxicity resulting from exposure to 1,3-dichloropropene for 6, 12,
or 24 months.
The mean relative liver weight of male animals exposed to 272
mg/m3 showed a statistically significant decrease. Gross patho-
logical examination of mice revealed morphological alterations
involving the urinary bladder and lung, which were attributed to
exposure to 1,3-dichloropropene. The bladder mucosal surface in
females exposed to 272 mg/m3 for 12 months and 90.8 and 272
mg/m3 for 24 months had a roughened appearance. In addition, a
statistically significantly increased number of the females exposed
to 90.8 and 272 mg/m3 showed inflammation and epithelial
hyperplasia of the bladder mucosa, after 24 months. An increased
number of male animals exposed to 272 mg/m3 also showed
inflammation.
Female mice exposed to 90.8 or 272 mg/m3 and males exposed to
272 mg/m3 showed hypertrophy and hyperplasia of the nasal
epithelium and degeneration of the olfactory epithelium. Additional
microscopic changes, considered exposure-related, were hyperplasia
and hyperkeratosis in the forestomach of 8/50 male mice following 24
months exposure to 272 mg/m3. A statistically significant increase
in the incidence of a benign tumour, bronchio-alveolar adenoma, was
observed in male mice exposed to 272 mg 1,3-dichloropropene
vapour/m3 for 24 months [22/50 (44%) vs 9/50 (18%) in controls].
No statistically significant increase in tumour incidence was found
in the groups exposed to 1,3-dichloropropene at 22.7 and 90.8
mg/m3 (Table 16). The incidence of lung tumours in the males
exposed to 272 mg/m3 was somewhat higher (7-32%) than the range of
historical control values for this type of tumour in male
B6C3F1 mice in 7 previous, long-term studies. The NOAEL in
this study on mice for hypertrophy/hyperplasia of the nasal
epithelium was 22.7 mg/m3 (Yano et al., 1985; Stott et al., 1987;
Lomax et al., 1989).
8.7.2.2 Rat
Groups of 50 male and 50 female Fischer 344 rats (7-9 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days/week
for up to 24 months. In addition, 2 ancillary groups, each
comprising 10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The animals were exposed to 0,
22.7, 90.8, or 272 mg/m3. The chemical composition of the test
material was 92.1% 1,3-dichloropropene ( cis-49.5% and trans-
42.6%), 0.7% 1,2-dichloropropane, and mixtures of hexanes and
hexadiens. Epoxidized soybean oil (approximately 2%) was present as
stabilizer. Besides body weights of the animals, clinical-chemical
parameters in the blood and urine and haematological parameters were
determined for all animals terminated at 6 and 12 months and for 20
animals/sex from each exposure group at 24 months.
Table 16. Incidence of various types of lesions observed in a mouse inhalation study with 1,3-dichloropropenea
Males (mg/m3) Females (mg/m3)
0 22.7 90.8 272 0 22.7 90.8 272
Urinary bladder
Hyperplasia mucosa 4/48 (9%) 7/48 (15%) 11/48 (23%) 37/47 (79%)b 1/47 (2%) 4/46 (9%) 21/48 (44%)b 44/45 (98%)b
(simple or nodular)
Lungs
Bronchio-alveolar 9/50 (18%) 6/50 (12%) 13/50 (26%) 22/50 (44%)b 4/50 (8%) 3/50 (6%) 5/50 (6%) 3/50 (6%)
adenoma
Nasal tissues
Degeneration of 1/50 (2%) 0/50 1/50 (2%) 48/50 (96%)b 0/50 0/50 1/50 (2%) 45/50 (90%)b
olfactory epithelium
Hyperplasia and 5/50 (10%) 1/50 (2%) 4/50 (8%) 48/50 (96%)b 4/50 (8%) 4/50 (8%) 28/50 (56%) 49/50 (98%)b
hypertrophy of
respiratory epithelium
Stomach
Squamous papilloma 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50(6%)
a From: Lomax et al. (1989).
b Statistical difference from control mean identified by using Yate's kappa2 pairwise test, alpha = 0.05.
At 6, 12, or 24 months, animals were sacrificed and the weight
of 5 organs determined; a large number of organs and tissues were
examined histopathologically. No significant influence on survival
was observed. Mean body weights of both male and female rats exposed
to 272 mg/m3 were statistically significantly decreased compared
with mean control values. Examination of haematological and
clinical-chemical parameters, urinalysis, and organ weights did not
indicate any toxicity resulting from exposure to 1,3-dichloropropene
for 6, 12, or 24 months.
Gross pathological examination did not indicate any exposure-
related effects after 6, 12, or 24 months. Exposure-related
histological effects occurred in nasal tissues of rats exposed to
272 mg/m3 for 24 months (Table 17), but not for 6 or 12 months.
The microscopic changes were located in the olfactory mucosa, which
covers the upper portions of the nasal cavity, nasal septum, and
turbinates. The changes were characterized by unilateral or
bilateral decreased thickness of olfactory epithelium and fibrosis
of the submucosal tissues underlying eroded olfactory epithelium. At
the lower dose levels, females did not show histopathological
changes in the nasal tissue, while one male exposed to 22.7 mg/m3
and one exposed to 90.8 mg/m3 showed decreased thickness of the
olfactory epithelium. No effects were seen in the controls. No
statistically significant increase in tumour incidence was found in
exposed rats compared with controls (Lomax et al., 1987, 1989).
8.7.3 Appraisal
Exposure to 1,3-dichloropropene, through inhalation, for up to
24 months did not have any demonstrable effects on survival or
spontaneous tumour development in male and female Fischer 344 rats.
In mice, an increased incidence of bronchio-alveolar adenomas was
found in the lungs of male mice, exposed to 272 mg/m3, but not at
the lower dose level (90.8 mg/m3). The oral gavage study with 1,3-
dichloropropene demonstrated an increased incidence of forestomach
neoplasms in rats of both sexes at dose levels of 50 mg/kg body
weight, administered 3 times/week for 24 months. Male rats treated
with 25 or 50 mg/kg also had an increased incidence of neoplastic
nodules in the liver.
In both the oral gavage and inhalation mouse bioassays with
1,3-dichloropropene, a tumorigenic response was noted in tissues
with which 1,3-dichloropropene had direct contact, i.e., the stomach
and the lung. However, in the gavage study, tumours were also
induced at sites distant from that at the primary "portal-of-entry"
(lung and urinary bladder). This was not the case in the inhalation
study, despite the fact that the dose of 1,3-dichloropropene
received on a mg/kg body weight per day basis by mice exposed for 5
days/week through inhalation was approximately 2-3 times higher than
the dose levels administered orally 3 times/week in the NTP (1985).
Table 17. Microscopic changes in the nasal tissues of rats exposed to
Telone II at 272 mg/m3a
Microscopic change Vehicle control Overall incidence
(male and female) Male Female
Decreased thickness of 0/100 20/50b (40%) 15/50b (31%)
olfactory epithelium
Erosion of olfactory 0/100 15/50b (30%) 6/50 (12%)
epithelium
Submucosal fibrosis 0/100 6/50b (12%) 2/50 (4%)
a From: Lomax et al. (1989).
b Statistical difference identified from control mean of
Yate's kappa2 pairwise test, alpha = 0.05.
The degeneration and subsequent hyperplasia of nasal and forestomach
epithelium occurred only at concentrations that are known to deplete
glutathione levels in these tissues. Tumorigenic effects occur at
doses higher than those causing glutathione depletion and tissue
damage.
The same mouse strain and similar test materials relative to
1,3-dichloropropene were used in both bioassays, but there was a
difference in the stabilizing agents in the test materials. The 1,3-
dichloropropene used in the NTP study was stabilized with 1%
epichlorohydrin, a carcinogen. It has been suggested that
epichlorohydrin may have played a role in the tumorigenic response
obtained in the gavage study because of the bolus nature of its
administration. However, it is not known whether the increased
incidence of tumours in the urinary bladder, lungs, forestomach, and
liver in the mouse gavage study are attributable to the treatment
with 1,3-dichloropropene or the effect of epichlorohydrin, since
carcinogenicity studies of epichlorohydrin in mice have not been
performed yet. In the inhalation study carried out by Lomax et al.
(1989), the 1,3-dichloropropene was stabilized with the relatively
nontoxic epoxidized soybean oil. The role of the stabilizing
additive epichlorohydrin in generating the different tumours seen in
the oral gavage studies on 1,3-dichloropropene is still uncertain.
This question has to be further investigated before a more definite
conclusion about the carcinogenic potential of 1,3-dichloropropene
can be drawn.
8.7.4 Dermal and subcutaneous (mouse)
Groups of 30 female Ha:ICR Swiss strain mice (6-8 weeks old)
were treated with 0.2 ml acetone containing 41 or 122 mg purified
cis-1,3 dichloropropene, applied to shaven skin 3 times weekly,
for approximately 18 months. Control mice received acetone. In the
group treated with 41 mg, no papillomas were found, but at the 122
mg dose, 3 animals showed papillomas, and 2, carcinomas. No tumours
were found at distant sites. Cis-1,3-dichloropropene, applied once
on the skin at a dose of 122 mg/mouse, was followed after 14 days by
the application of 5 µg phorbol myristate acetate in 0.2 ml acetone,
3 times weekly until the end of the study. No skin tumour-initiating
activity was observed (van Duuren et al., 1979).
A group of 30 female Ha:ICR Swiss mice were given weekly
subcutaneous injections in the left flank of 0.05 ml trioctanoin
containing 3 mg purified cis-1,3-dichloropropene per injection.
The study lasted 538 days. Control animals received only the
vehicle. Six out of 30 mice showed local fibrosarcomas, whereas
vehicle control animals did not. A positive control of 0.3 mg beta-
propiolactone produced local sarcomas in 24 out of 30 mice during a
378-day period (van Duuren et al., 1979).
The relevance of the subcutaneous route for the assessment of
carcinogenic properties remains questionable, especially when
injections of an irritant material are made. It is probable that the
persistent and physical properties rather than the chemical
characteristics of cis-1,3-dichloropropene are responsible for
production of local sarcomas.
8.8 Factors modifying toxicity, toxicity of metabolites, mode of
action
8.8.1 Toxicity of the metabolites, cis- and trans-1,3-
dichloropropene oxide
There is some evidence that a small proportion of cis-1,3-
dichloropropene is metabolized to cis-1,3-dichloropropene oxide
(Fig. 5; Hutson, 1984).
8.8.1.1 Mutagenicity
Cis- and trans-1,3-dichloropropene oxide were tested for
mutagenicity, in the absence of metabolic activation, in Salmonella
typhimurium TA1535 and Escherichia coli WP2 uvr A, and for
preferential inhibition of growth of DNA-repair-polymerase-deficient
E. coli. Both oxides were potent mutagens and DNA modifiers. In
Salmonella typhimurium TA1535, treated with 0.025 µmol/ml and in
E. coli WP2 uvr A treated with 0.05 µmol/ml of bacterial
suspension, a significant increase in revertant colonies was found.
A gene mutation test with E. coli (pol A1-/pol A1+) in the
absence of metabolic activation, already showed an effect with
0.0005 µmol/ml (Kline et al., 1982).
In the absence of an S9-fraction, cis-dichloropropene oxide
was strongly mutagenic towards S. typhimurium TA100. The
mutagenicity reached a maximum at 25 µg/plate. Above 300 µg/plate,
marked cytotoxicity was observed. Glutathione (5 mmol/litre) caused
a significant inhibitory effect on the mutagenicity and cytotoxicity
of this epoxide, but did not offer complete protection. Inclusion of
glutathione (5 mmol/litre) together with S9-fraction afforded
complete protection over the range of concentrations of cis-
dichloropropene oxide up to 100 µg/plate (Hutson 1984; Watson et
al., 1986a).
Cis- and trans-1,3-dichloropropene oxide were tested in a
quantitative Syrian hamster embryo cell model. Both compounds at
dose levels of 0.005, 0.01, or 0.02 mmol/litre ( cis-isomer) and
0.01, 0.025, or 0.05 mmol/litre ( trans-isomer) induced
morphological transformation of the Syrian hamster embryo cells
(DiPaolo & Doniger, 1982).
8.8.1.2 Carcinogenicity
Female ICR/Ha Swiss mice (30 per group) were treated 3 times
weekly, with cis-1,3-dichloropropene oxide or trans-1,3-
dichloropropene oxide (containing 10-15% of m-dichlorobenzene) on
the skin. The dose level was 10 mg in 0.1 ml of acetone. The
controls received only acetone. The median survival time was
comparable with that of the controls (over 500 days). With cis-
dichloropropene oxide, 16/30 mice had local papillomas and 10/30
squamous cell carcinomas of the skin; with trans-dichloropropene
oxide, this was 20/30 and 17/30, respectively. No tumours were found
in the control animals (van Duuren et al., 1983).
A study was also carried out on the same strain of mice using
subcutaneous injections. Thirty female mice received 500 µg cis-
dichloropropene oxide or trans-dichloropropene oxide in 0.05 ml
tricaprylin once weekly. The median survival time was comparable to
that of controls. With cis-dichloropropene oxide, 4 animals had a
local (fibro)sarcoma and one carcinoma, and, with trans-
dichloropropene oxide, 5 animals had fibrosarcomas. No tumours were
found in the vehicle controls (van Duuren et al., 1983).
8.8.2 Role of oxidation
When purified cis-1,3-dichloropropene was heated for a few
hours in an oxygen atmosphere, in either the light or dark, the non-
mutagenic cis-1,3-dichloropropene became strongly mutagenic.
Heating under nitrogen was negative. Storage of cis-1,3-
dichloropropene at room temperature, in the presence of oxygen, for
two months, made it mutagenic (Watson et al., 1987). Talcott & King
(1984) demonstrated that purified samples of 1,3-dichloropropene
were not mutagenic to Salmonella typhimurium TA100. Four
preparations of 1,3-dichloropropene were separated into different
fractions and analysed for mutagenic activity. The fraction
containing polar metabolites was found to be mutagenic. Its
composition was too complex to characterize completely, but 2
mutagens, epichlorohydrin and 1,3-dichloro-2-propanol, were
identified.
Watson et al. (1986 a,b; 1987) confirmed that the direct
mutagenicity, inducing base-pair mutations, previously observed in
Salmonella typhimurium TA100 treated with cis-1,3-
dichloropropene, was caused by trace impurities. These impurities
resulted from the autooxidation of cis- and trans-1,3-
dichloropropene and were identified as cis- and trans-
dichloropropene oxides. The dichloropropene oxides made a
significant contribution towards the intrinsic mutagenicity, when
tested in S. typhimurium TA100 (see section 8.8.1.1).
The proposed formation of cis- and trans-dichloropropene
oxides is shown in Fig. 6.
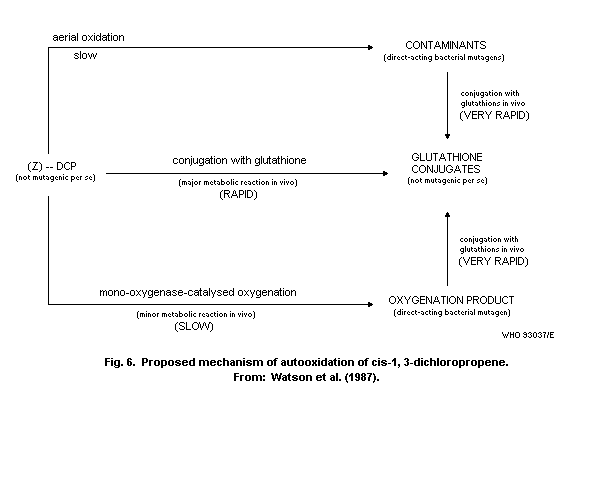 Autooxidation of cis-1,3-dichloropropene occurs after radical
initiation (5) to give the alkyl peroxy radical (6), which reacts
with a second molecule of cis-1,3-dichloropropene to give the free
radical intermediate (7). Free rotation can occur in this molecule,
prior to expulsion of the alkoxy radical (8), with concomitant
formation of both cis- and trans-dichloropropene oxides (3) and
(4). The alkoxy radical (8) may further abstract a proton or
chlorine from cis-1,3-dichloropropene, thus, continuing the chain
reaction. Autooxidation reactions often proceed via several pathways
and in the case of cis-dichloropropene there are minor products,
such as a 2-hydroxyperoxy intermediate, and unstable 1,2-dioxetanes
leading to 1,3-dichloro-2-propanol and aldehydes, respectively.
Trans-dichloropropene was considerably more resistant to
autooxidations than cis-dichloropropene (Watson et al., 1987).
8.8.3 Role of glutathione
Glutathione (GSH) at physiological concentrations in in vitro
bacterial test systems of Salmonella typhimurium TA100 has been
shown to have a protective effect, i.e., a virtual elimination of
the mutagenic response to 1,3-dichloropropene (Hutson & Stoydin,
1977; DeLorenzo et al., 1977; Brooks et al., 1978; Climie et al.,
1979; Wright & Creedy, 1982; Creedy & Hutson, 1982).
This protective effect occurs in either the absence or the
presence of S9 for both the cis- and trans-isomer. In the
absence of a rat liver fraction, the chemical reaction of cis-1,3-
dichloropropene with glutathione is slow, and, in the presence of
the rat liver fraction, the reaction is rapid due to enzyme
catalysis. The trans-isomer (in the presence of the cis
compound) was degraded 4-5 times more slowly than the cis-isomer
(Hutson & Stoydin, 1977; Climie et al., 1979).
This protective effect in the presence of S9 is consistent with
the operation of a glutathione transferase enzyme occurring in the
added S9 fraction. This enzyme, which conjugates cis-1,3-
dichloropropene with glutathione, is also present in mammalian cells
and the metabolic studies have shown that it plays a key role in the
rapid detoxification of cis-1,3-dichloropropene in mammalian
tissue (Climie et al., 1979; Brooks & Wiggins, 1991).
There is some evidence that the mutagenicity of these
preparations is due to contaminants and that the protective action
of glutathione is due to spontaneous conjugation reactions between
these contaminants and glutathione. Pure cis-dichloropropene did
undergo metabolic activation catalysed by microsomal mono-oxygenase
system from the rat liver. Thus, a small, but significant, dose-
dependent increase in mutation was observed when cis-
dichloropropene was tested in S. typhimurium TA100, in the
presence of S9-liver fraction. When this S9 fraction was replaced by
washed microsomes, which remove the glutathione activity, the
mutagenic effect of cis-1,3-dichloropropene was increased.
Replacement of the glutathione S-alkyl transferase(s) in the
microsomal fraction from an S100 fraction, restored the glutathione-
conjugating activity and afforded complete protection against cis-
1,3-dichloropropene. Cis-dichloropropene undergoes rapid
conjugation with glutathione in the presence of the mentioned
transferase(s), which limits the availability of cis-
dichloropropene to undergo mono-oxygenase-catalysed bioactivation.
These results also provide some evidence that these glutathione-
linked conjugation systems also afford efficient protection against
the mutagenic hazard posed by the bioactivation products of cis-
dichloropropene. Thus, the microbial mutagenicity of cis-
dichloropropene oxide was significantly reduced by glutathione (5
mmol/litre) and this protective action was strongly enhanced in the
presence of glutathione S-alkyl transferases from the S100 (Fig.
7).
Autooxidation of cis-1,3-dichloropropene occurs after radical
initiation (5) to give the alkyl peroxy radical (6), which reacts
with a second molecule of cis-1,3-dichloropropene to give the free
radical intermediate (7). Free rotation can occur in this molecule,
prior to expulsion of the alkoxy radical (8), with concomitant
formation of both cis- and trans-dichloropropene oxides (3) and
(4). The alkoxy radical (8) may further abstract a proton or
chlorine from cis-1,3-dichloropropene, thus, continuing the chain
reaction. Autooxidation reactions often proceed via several pathways
and in the case of cis-dichloropropene there are minor products,
such as a 2-hydroxyperoxy intermediate, and unstable 1,2-dioxetanes
leading to 1,3-dichloro-2-propanol and aldehydes, respectively.
Trans-dichloropropene was considerably more resistant to
autooxidations than cis-dichloropropene (Watson et al., 1987).
8.8.3 Role of glutathione
Glutathione (GSH) at physiological concentrations in in vitro
bacterial test systems of Salmonella typhimurium TA100 has been
shown to have a protective effect, i.e., a virtual elimination of
the mutagenic response to 1,3-dichloropropene (Hutson & Stoydin,
1977; DeLorenzo et al., 1977; Brooks et al., 1978; Climie et al.,
1979; Wright & Creedy, 1982; Creedy & Hutson, 1982).
This protective effect occurs in either the absence or the
presence of S9 for both the cis- and trans-isomer. In the
absence of a rat liver fraction, the chemical reaction of cis-1,3-
dichloropropene with glutathione is slow, and, in the presence of
the rat liver fraction, the reaction is rapid due to enzyme
catalysis. The trans-isomer (in the presence of the cis
compound) was degraded 4-5 times more slowly than the cis-isomer
(Hutson & Stoydin, 1977; Climie et al., 1979).
This protective effect in the presence of S9 is consistent with
the operation of a glutathione transferase enzyme occurring in the
added S9 fraction. This enzyme, which conjugates cis-1,3-
dichloropropene with glutathione, is also present in mammalian cells
and the metabolic studies have shown that it plays a key role in the
rapid detoxification of cis-1,3-dichloropropene in mammalian
tissue (Climie et al., 1979; Brooks & Wiggins, 1991).
There is some evidence that the mutagenicity of these
preparations is due to contaminants and that the protective action
of glutathione is due to spontaneous conjugation reactions between
these contaminants and glutathione. Pure cis-dichloropropene did
undergo metabolic activation catalysed by microsomal mono-oxygenase
system from the rat liver. Thus, a small, but significant, dose-
dependent increase in mutation was observed when cis-
dichloropropene was tested in S. typhimurium TA100, in the
presence of S9-liver fraction. When this S9 fraction was replaced by
washed microsomes, which remove the glutathione activity, the
mutagenic effect of cis-1,3-dichloropropene was increased.
Replacement of the glutathione S-alkyl transferase(s) in the
microsomal fraction from an S100 fraction, restored the glutathione-
conjugating activity and afforded complete protection against cis-
1,3-dichloropropene. Cis-dichloropropene undergoes rapid
conjugation with glutathione in the presence of the mentioned
transferase(s), which limits the availability of cis-
dichloropropene to undergo mono-oxygenase-catalysed bioactivation.
These results also provide some evidence that these glutathione-
linked conjugation systems also afford efficient protection against
the mutagenic hazard posed by the bioactivation products of cis-
dichloropropene. Thus, the microbial mutagenicity of cis-
dichloropropene oxide was significantly reduced by glutathione (5
mmol/litre) and this protective action was strongly enhanced in the
presence of glutathione S-alkyl transferases from the S100 (Fig.
7).
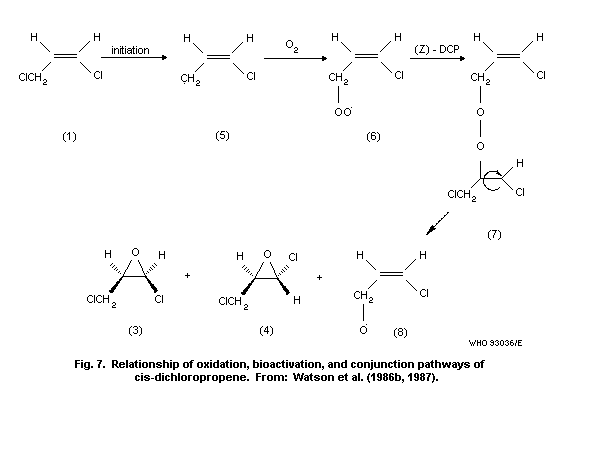 It was concluded that the degree to which the genotoxic
potential of cis-dichloropropene or its autooxidation products is
expressed in vivo is likely to be lower than that found by
microbial mutation assays. Cis-dichloropropene is efficiently
detoxified in mammals by the operation of a glutathione-dependent
S-alkyl transferase (Watson et al., 1987).
8.8.4 Effect on liver enzyme activity
Miyaoka et al. (1990) studied the mechanism of 1,3-
dichloropropene-induced hepatotoxicity in male mice of the ICR
strain (6 weeks old). 1,3-Dichloropropene (300 mg/kg body weight),
administered by gavage in corn oil, increased plasma GOT and GPT
activities significantly, and centrilobular swelling occurred in the
liver, 15 h after treatment. No such effect was found with 100 mg/kg
body weight. Pretreatment of piperonylbutoxide (PIB, a cytochrome
P450 inhibitor), at 200 mg/kg body weight i.p., significantly
suppressed the elevation of plasma GOT and GPT activities caused by
300 mg 1,3-dichloropropene/kg body weight, but increased the 1,3-
dichloropropene concentration in the liver. The PIB pretreatment
decreased the cytochrome P450 contents in liver microsomes, but
prevented further reduction of cytochrome P450 after 1,3-
dichloropropene treatment.
With pretreatment with L-buthionine-S,R sulfoximine (a GSH
depleting agent) at 1600 mg/kg body weight, plasma GOT activities
increased significantly in animals receiving 100 mg 1,3-
dichloropropene, whereas liver GSH contents and GST activity
decreased. Cysteine administration, 2 h after 1,3-dichloropropene
treatment, did not decrease the cytochrome P450 content, though it
prevented the elevation of GOT and GPT activities and increased
hepatic GSH concentration. The results suggest that 1,3-
dichloropropene is biotransformed via cytochrome P450, and that the
metabolites induce liver damage. GSH plays an important role in the
detoxification of 1,3-dichloropropene (Miyaoka et al., 1990).
9. EFFECTS ON HUMANS
9.1 General population
9.1.1 Acute toxicity - poisoning incidents
In a truck accident in California in 1975, about 4500 litres of
1,3-dichloropropene (92%) was slowly spilled on to the highway. An
estimated 80 persons were exposed to the vapour. Forty-six persons
were examined at hospitals. The following symptoms were found in a
small number of persons (4-6), headache, vomiting and nausea,
dizziness, irritation of mucous membranes, and chest discomfort.
Three persons lost consciousness at the scene of the accident. In 11
out of 41 persons, slightly elevated SGOT and/or SGPT values were
found. Twenty-eight patients were interviewed 1 or 2 weeks later.
The most common symptoms were: headache (12), abdominal discomfort
(6), chest discomfort (5), and malaise (5). Twenty-one patients were
interviewed after 2 years; 10 patients complained of severe or
unusual headache, 10 of chest pain or discomfort, and 13 of
"personality changes" (fatigue, irritability, difficulty in
concentrating, or decreased libido). The frequency of these long-
persisting symptoms was not associated with the intensity of the
exposure (Flessel et al., 1978).
Markovitz & Crosby (1984) reported 9 cases of acute poisoning
following accidental over-exposure to 1,3-dichloropropene. The
chemical spilled as the driver jack-knifed the container. Two of
these cases died 6 years later, due to diffuse histocytic lymphoma.
Authors have reported another case of myelo-monocytic leukaemia
where the patient had been accidentally over-exposed to 1,3-
dichloropropene (see section 9.2.2).
9.1.2 Controlled human studies
A smell detection test with 10 human volunteers was carried
out. A level of 13.6 mg 1,3-dichloropropene/m3 air was detected by
7 out of 10 volunteers. Some reported that the sense of smell
diminished after a few minutes. Even a level of 4.54 mg/m3 was
detected by these 7 persons, but it was noticeably fainter than 13.6
mg/m3 (Torkelson & Oyen, 1977).
In a study designed to determine the odour threshold of Telone
II among 22 individuals, the lowest concentration at which odour was
detected was 20 ± 14 mg/m3. This level is slightly above the US
threshold limit value time-weighted average of 4.54 mg/m3, but
below the short-term exposure limit of 45.4 mg/m3 (Rick & McCarty,
1988).
9.2 Occupational exposure
9.2.1 General
The most likely routes of human exposure to 1,3-dichloropropene
are through inhalation and the skin. Irritation of the eyes and
upper respiratory mucosa, accompanied by lacrimation, appear
promptly after exposure to vapours (Gosselin et al., 1976).
Inhalation by humans of air containing concentrations greater
than 6810 mg/m3 produces headaches, mucous membrane irritation,
dizziness, nausea, vomiting, gasping, coughing, substernal pain, and
respiratory distress (Gosselin et al., 1976; Flessel et al., 1978).
Lower concentrations produce central nervous system depression and
moderate irritation of the respiratory system.
A 44-year-old male process operator at a pesticide plant had
acute bullous dermatitis on both feet in 1988. Approximately one
year later, an identical dermatitis developed. In both periods, he
contaminated his shoes with a 1,3-dichloropropene formulation (D-D-
95). In a patch test with 1,3-dichloropropene, even a concentration
of 0.005% produced a positive reaction. Twenty volunteers did not
react in this patch test at a concentration of 0.05% (Bousema et
al., 1991).
Maddy et al. (1990) summarized the pesticides that caused
occupational illness/injury, reported by physicians in California
during 1987. 1,3-Dichloropropene caused one case of systemic illness
in that period. In the year 1986, 3 cases were mentioned, one with
systemic effects, one with skin effects, and one with eye injury
(Edmiston & Maddy, 1987).
Fifteen applicators of 1,3-dichloropropene were studied for
personal air exposure, urinary excretion of the metabolite, and
excretion of the renal tubular enzyme N-acetyl glucosaminidase
(NAG). Each was studied for four, 6-8 h consecutive intervals
following base-line determinations. The duration of exposure ranged
from 120 to 697 min and the personal air concentrations ranged from
0.3 to 9.4 mg/m3. The 24-h urinary excretion of the metabolite
(average 2.6 mg, range: 0.5-9.2 mg) correlated well with the 1,3-
dichloropropene air exposure product (minutes exposed x mg/m3).
The mean excretion of NAG for all intervals was 2.6 mU/mg of
creatinine (range: 1.0-7.7 mU/mg of creatinine); in 24 h, the mean
was 4940 mU with a range of 278-8956 mU. Four of the 15 workers had
an NAG activity of > 4 mU/mg creatinine in any of their urine
collected after the base line. Nine workers showed increases in NAG
excretion of more than 25% compared with the base line. The authors
concluded that the elevated excretion of NAG indicated a possible
subclinical nephrotoxic effect in the workers, though no complaints
or cases of renal injury were reported (Osterloh et al., 1989).
Stott et al. (1990) commented that the slight increase in NAG in the
urine might be a result of the stimulation of exocytosis or an
increase (induction) in the NAG activity in the kidneys, rather than
an indication of nephrotoxicity. Taken together with the known
metabolism and toxicity of 1,3-dichloropropene in laboratory
animals, the findings by Osterloh et al. (1989) do not suggest any
untoward effects in workers exposed occupationally to low levels of
1,3-dichloropropene.
Fourteen workers applying 1,3-dichloropropene were monitored at
the start of the season, in July, and at the end of the season, in
October, for liver function. The following parameters were measured;
alanine aminotransferase, aspartate aminotransferase, alkaline
phosphatase, lactic dehydrogenase, gamma-glutamyltrans-peptidase,
and total bilirubin. Total bilirubin was significantly decreased at
the end of the season. In combination with an increase in serum
gamma-glutamyltranspeptidase activity this indicates moderate
hepatic enzyme induction. The renal function was also studied by
measuring creatinine and beta-2-microglobulin in serum and beta-2-
microglobulin, albumin, alanine-aminopeptidase, beta-galactosidase,
and retinol-binding protein in urine. The glomerular function
parameters (increased albumin in urine and decreased creatinine in
serum) changed significantly during the season. The tubular function
(retinol-binding protein) also increased. On the basis of these
data, a subclinical nephrotoxic effect cannot be excluded. Effects
on the glutathione conjugation capacity were studied by measuring
erythrocyte glutathione- S-transferase activity and blood
glutathione concentration. Both parameters were significantly
decreased (Brouwer et al., 1991b). The cause-effect relationship
with 1,3-dichloropropene exposure has been questioned (Van Sittert
et al., 1991).
9.2.2 Acute toxicity - poisoning incidents
A farmer in good health developed pain in the right ear, nasal
mucosa, and pharynx after applying 1,3-dichloropropene to his fields
from his tractor for 30 days. Hospital examination showed a red and
painful external ear, hyperaemia, and superficial ulcerations of the
nasal mucosa, and inflammation of the pharynx. The hose containing
the 1,3-dichloropropene had a small leak, which had sprayed the
chemical near the right side of his face. Over the following year,
the man developed myelo-monocytic leukaemia. The man died of
pneumonia 5 weeks after entering the hospital (Markovitz & Crosby,
1984; NTP, 1985; Yang, 1986). The Task Group considers that the
cause-effect relationship in this case is doubtful.
9.2.3 Effects of short- and long-term exposure
The fertility status of 63 males employed in the production of
chlorinated three-carbon compounds were investigated in comparison
with 63 non-exposed persons (at least 5 years without exposure).
Data from reproductive medical history, hormone determination, and
semen analysis were used. There were no indications for an
association between lowered fertility by the standard fertility
parameters, and exposure to allyl chloride, epichlorohydrin, and
1,3-dichloropropene in the quantities occurring in the working
environment. A possible source of bias in this study stems from the
relatively low (64%) volunteer rate from the exposed group and the
lack of an estimate of the individual variation (Venable et al.,
1980).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,3-Dichloropropene (technical grade) was considered by working
groups of the International Agency for Research on Cancer (IARC) in
1986 (IARC, 1986) and 1987 (IARC,1987). In the updating of 1987, it
was evaluated as follows. "There is sufficient evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in
experimental animals. There is inadequate evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in humans.
The agent is possibly carcinogenic to humans (Group 2B)".
Based on the results from 2-year gavage studies on rats and
mice, using the linearized multistage model, the drinking-water
concentration for an excess life-time cancer risk of 104, 105,
or 106 is estimated to be 20, 2.0, or 0.2 µg/litre, respectively
(WHO/EURO, 1990).
PART B
ENVIRONMENTAL HEALTH CRITERIA
FOR
1,2-DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Soil
4.1.2.1 Volatilization
4.1.2.2 Uptake in crops
4.1.2.3 Movement in soil
4.2 Biotransformation
4.3 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water and soil
5.1.3 Crops
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.2 Inhalation
6.1.3 Intraperitoneal
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
7.1.2 Invertebrates
7.1.3 Fish
7.1.3.1 Acute toxicity
7.1.3.2 Short-term/long-term toxicity
7.2 Terrestrial organisms
7.2.1 Earthworms
7.2.2 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.2 Inhalation
8.2.2.1 Mouse
8.2.2.2 Rat
8.2.2.3 Rabbit
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
8.3.2.2 Oral (rabbit)
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
8.4.1.2 Mammalian cells
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
8.4.2.2 Dominant lethal test
8.4.2.3 Miscellaneous
8.5 Carcinogenicity
8.5.1 Oral (mouse)
8.5.2 Oral (rat)
8.6 Factors modifying toxicity
8.7 Special studies
8.7.1 Liver
8.7.2 Kidneys
8.7.3 Central nervous system
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
9.2 Occupational exposure
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1,2-Dichloropropane is a liquid with a boiling point of 96.8 °C
and a vapour pressure of 42 mmHg at 20 °C. The substance is soluble
in water, ethanol, and ethyl ether. When heated, it emits highly
toxic fumes of phosgene. The log P octanol/water partition
coefficient is 2.28.
This substance is used in furniture finish, dry cleaning fluid,
and paint remover, gum processing, metal degreasing, oil processing,
and as a rubber- and wax-making agent, and a chemical intermediate
in the production of tetrachloroethylene and carbon tetrachloride.
It is a component of the "MIX D/D", used as a pre-plant fumigant.
Concentrations of 1,2-dichloropropane in city air have been
measured at 1.2 µg/m3 (mean value), 0.021-0.040 µg/m3, and
0.0065-1.4 µg/m3 in Philadelphia, Portland, and Japan,
respectively. Decomposition in the atmosphere is slow; on the basis
of reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days. Phototransformation is likely to be
the dominant process for the decomposition. Adsorption on to
particulate matter is necessary for appreciable phototransformation.
Volatilization is likely to be the major route of loss from water.
In soil, the main routes of loss are volatilization and
diffusion. 1,2-dichloropropane is persistent in soil. More than 98%
of the 1,2-dichloropropane applied to loam soil was recovered 12-20
weeks after treatment.
Leaching of 1,2-dichloropropane occurs from soil and can
contaminate upper and lower groundwater in areas where "MIX D/D" has
been used as a soil fumigant. In well water and groundwater in the
USA, concentrations of up to 440 µg/litre and 51 µg/litre,
respectively, have been found. In the Netherlands, concentrations of
up to 160 µg/litre have been measured in well water and 1,2-
dichloropropane has been found to a depth of 13 m.
1,2-Dichloropropane can be taken up by edible crops, but the
residues detected have been low (< 0.01 mg/kg) and are unlikely to
be biologically significant.
Bioaccumulation of 1,2-dichloropropane is unlikely, because of
its high water solubility (2.7 g/kg) and low log P octanol/water
partition coefficient.
1.1.2 Kinetics and metabolism
1,2-Dichloropropane administered orally to rats is rapidly
eliminated (80-90% within 24 h). There are no major differences in
kinetics or elimination between males and females. Urine is the
major route of elimination, up to half an oral dose being eliminated
by this route within 24 h. Less than 10% is eliminated via the
faeces. Approximately one-third is eliminated through expired air,
both as carbon dioxide and as a mixture of volatile materials.
Tissue concentrations are low, the highest concentration being found
in the liver. Rapid elimination also occurs following the inhalation
exposure of rats; 55-65% of a dose is eliminated in the urine and
16-23% in expired air. The half-life of elimination from the blood
is 24-30 min.
Unchanged 1,2-dichloropropane is not found in urine. Three
major urinary metabolites have been identified. These metabolites
result from oxidative and conjugation pathways, which yield the
mercapturates, N-acetyl- S-(2-hydroxypropyl)-L-cysteine, N-
acetyl- S-(2-oxypropyl)-L-cysteine, and N-acetyl- S-(1-carboxy-
ethyl)-L-cysteine. 1,2-Dichloropropane can also be oxidized to
lactate with resultant carbon dioxide or acetyl co-enzyme A
production.
Oral administration of 1,2-dichloropropane (2 ml/kg) to rats
significantly depleted tissue glutathione contents. There was a
correlation between tissue glutathione loss and expression of
toxicity in the liver, kidneys, and red blood cells. Prior depletion
of intracellular glutathione exacerbated 1,2-dichloropropane
toxicity, whereas pretreatment with precursors for glutathione
synthesis ameliorated the toxicity. These results demonstrate the
protective effect of glutathione against 1,2-dichloropropane
toxicity.
1.1.3 Effects on organisms in the environment
EC50 for freshwater algae have not been calculated because of
difficulties with volatilization of the chemical from the test
solution. The acute toxicity of 1,2-dichloropropane for aquatic
invertebrates and fish is low to moderate; 48-h LC50 values for
invertebrates range between 52 and > 100 mg/litre and 96-h LC50
values for fish lie between 61 and 320 mg/litre. A short-term
toxicity test on Fathead minnows demonstrated a maximum no-effect
level of 82 mg/litre. A 32-day test on early life stage toxicity in
the same species demonstrated that larval growth and survival were
the most sensitive parameters. The estimated maximum acceptable
toxicant concentration (MATC) was between 6 and 11 mg/litre. Growth
inhibition was noted in Sheepshead minnows after 33 days at a 1,2-
dichloropropane concentration of 164 mg/litre.
1,2-Dichloropropane is phytotoxic.
Contact tests on 4 species of earthworm showed an LC50 of 44-
84 µg/cm2 (mean values) of filter paper. In artificial soil, the
LC50 values were 3880-5300 mg/kg soil (dry weight).
1.1.4 Effects on experimental animals and in vitro test
systems
The acute oral toxicity of 1,2-dichloropropane in experimental
animals is low. The oral LD50 for the rat is 1.9 g/kg body weight,
and the dermal LD50 in rabbits is 8.75 ml/kg body weight.
Short-term, oral toxicity studies of 1,2-dichloropropane in
mice and rats showed growth inhibition, clinical toxic signs
associated with central nervous system depression, and/or increased
mortality at dose levels of 250 mg/kg body weight per day or higher.
In rats given 250 mg/kg per day for 10 days, there were changes in
serum enzymes indicative of slight hepatotoxicity with a NOEL of 100
mg/kg per day.
In a 13-week mouse inhalation study (highest dose 681 mg/m3),
no adverse effects were observed. In a similar study on rats exposed
to 68.1, 227, or 681 mg/m3, a decrease in body weight and minimal
damage to nasal tissues occurred in the 2 highest dose groups.
In a 2-generation reproduction study, rats exposed to 1,2-
dichloropropane in drinking-water at 0.024, 0.1, 0.24% (equivalent
to 33.6, 140 and 336 mg/kg body weight per day) resulted in lower
maternal body weight gain and decreased water consumption at the mid
and high dose levels. Neonatal body weights were lower at the high
dose level. The NOAELs established for maternal and reproductive
toxicity were 33.6 and 140 mg/kg body weight per day, respectively.
Studies did not indicate any teratogenic activity of 1,2-
dichloropropane at oral dose levels up to 125 mg/kg body weight in
the rat and 150 mg/kg body weight in the rabbit. However, at these
dose levels, 1,2-dichloropropane was maternally toxic and fetotoxic,
as evidenced by central nervous system associated clinical signs,
decreased maternal body weight gain, and delayed ossification of
bones in the fetuses. The NOELs are 30 and 50 mg/kg body weight per
day for the rat and rabbit, respectively.
1,2-Dichloropropane was mutagenic in bacteria in most studies
with, and without, metabolic activation, but very high dose levels
were used of up to 10 mg/plate. In Chinese hamster ovary cells, 1,2-
dichloropropane caused chromosomal aberrations and sister chromatid
exchange; in Chinese hamster V79 cells, it increased the sister
chromatid exchange. In an in vitro system with human lymphocytes,
the tritiated thymidine uptake and cell viability in cultures grown
with, and without, rat liver metabolizing system, were similar to
those in control cultures. The results of a sex-linked recessive
lethal test in Drosophila melanogaster were negative. A dominant
lethal test in rats, dosed for 14 weeks via drinking-water
containing 1,2-dichloropropane, followed by 2 weeks of mating, was
negative.
In a carcinogenicity study on mice administered 125 or 250 mg
1,2-dichloropropane/kg body weight by gavage, a dose-related
increase in the incidence of liver adenomas was observed. The
incidence of liver adenomas in treated groups was higher than that
in the concurrent control group, but was within the historical
control range.
In rats administered dose levels of 125 and 250 mg/kg body
weight (females) and 62 and 125 mg/kg body weight (males), by
gavage, for 5 days per week over 113 weeks, a slight increase in the
incidence of mammary gland adenocarcinomas exceeding the historical
range was observed in high-dose females.
1.1.5 Effects on human beings
Exposure of the general population to 1,2-dichloropropane via
air and water is unlikely, except in areas where there is extensive
use of 1,2-dichloropropane and "MIX D/D" in agriculture. Residues of
1,2-dichloropropane in edible crops are generally below the limit of
detection. In view of these low exposures to 1,2-dichloropropane,
the risk to the general population is negligible.
Several cases of acute poisoning have been reported due to
accidental or intentional (suicide) over-exposure to 1,2-
dichloropropane. Effects have been mainly on the central nervous
system, liver, and kidneys. Haemolytic anaemia and disseminated
intravascular coagulation have also been reported. In one case,
delirium progressed to irreversible shock, cardiac failure, and
death.
Occupational exposures can be via both skin and inhalation.
Several cases of dermatitis and skin sensitization have been
reported in workers using solvent mixtures containing 1,2-
dichloropropane.
1.2 Conclusions
* General population: There is low or non-existent exposure of
the general population to 1,2-dichloropropane from air and
food. However, in certain areas, exposure may occur when
groundwater is contaminated.
* Occupational exposure: With good work practices, hygienic
measures, and safety precautions, the use of 1,2-
dichloropropane is unlikely to present a risk for those
occupationally exposed to it.
* Environment: 1,2-Dichloropropane is unlikely to attain levels
of environmental significance when used at the recommended
rate. It is unlikely to have adverse effects on populations of
terrestrial and aquatic organisms.
1.3 Recommendations
* Studies should be conducted to assess acute inhalation
toxicity, eye and skin irritancy, and skin sensitization
potential.
* Appropriate safety precautions should be taken, when handling
1,2-dichloropropane, in order to avoid exposures exceeding the
maximum allowable concentration.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
Primary constituent
Chemical structure Cl
'
ClCH2CHCH3
Chemical formula C3H6Cl2
Relative molecular mass 112.99
Chemical name 1,2-dichloropropane,
dichloro-1,2-propane
Common synonyms propylene dichloride
CAS registry number 78-87-5
RTECS registry number TX9625000
EINECS number 201-152-2
2.2 Physical and chemical properties
1,2-Dichloropropane is a liquid with a boiling point of 96.8
°C. The vapour pressure is 42 mmHg at 20 °C (27.9 kPa at 19.6 °C).
Its solubility is 2.7 g/kg water at 20 °C. It is soluble in ethanol
and diethyl ether. The density is 1.1595 g/ml at 20 °C, and 1.437
g/ml at 25 °C. It is flammable; the flash point is 21 °C (Cleveland
open cup). When heated to decomposition, 1,2-dichloropropane emits
highly toxic fumes of phosgene.
The log P octanol/water partition coefficient is 2.28 (NTP,
1983).
2.3 Conversion factors
1 ppm = 4.66 mg/m3
1 mg/m3 = 0.214 ppm
2.4 Analytical methods
The analytical methods used are the same as those used for 1,3-
dichloropropene.
Boyd et al. (1981) developed a method to collect vapours of
1,2-dichloropropane from air with solid sorbents (petroleum
charcoal) in tandem with a personal sampling pump; desorption of the
sorbed compound in acetone/cyclohexane and analysis of the extracts
by gas chromatography. A Carbowax or Chromosorb column was used. The
Hall electrolytic conductivity detector (in the halogen mode)
offered better sensitivity than the electron-capture detector and
the flame ionization detector.
An analytical method is described and issued in 1985 by NIOSH,
method 1013, for the determination of 1,2-dichloropropane in air.
The working range is 0.25-600 mg/m3. The limit of determination
0.1 µg/sample (NIOSH, 1985).
In method MDHS 28, a description is given for the determination
of the time-weighted-average concentrations of chlorinated
hydrocarbon solvent vapours in workplace atmospheres. The method is
suitable for sampling over periods in the range of 10 min to 8 h.
The method can also be used for the determination of personal
exposure. The method is suitable for the measurement of airborne
vapours containing concentrations in the range of approximately 1-
1000 mg/m3 (about 0.2-200 ppm v/v) for samples of 10 litres of
air. Charcoal is used as adsorbent and 15% (v/v) acetone in
cyclohexane is recommended as a desorption solvent for 1,2-
dichloropropane; determination takes place with a gas chromatograph
fitted with a flame ionization detector (HSE, 1990).
See section 2.4 of 1,3-dichloropropene.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
1,2-Dichloropropane is not known to occur naturally.
3.2 Man-made sources
1,2-Dichloropropane is produced by the chlorination of
propylene.
The production of 1,2-dichloropropane in the USA in 1974 was 66
million kg: in 1976, it was 32.2 million, and in 1980, 35 million kg
(Fishbein, 1979).
1,3-Dichloropropene, contaminated with 1,2-dichloropropane and
2,3-dichloropropene, used for fumigation in the Netherlands at
application rates ranging from 200 to 400 kg/ha, would mean an input
of 40-160 kg of 1,2-dichloropropane and 10-25 kg of 2,3-
dichloropropenes/ha (Krijgsheld & van der Gen, 1986). 1,2-
Dichloropropane is more persistent in the environment than 1,3-
dichloropropene and has a greater potential for contaminating
groundwater, because of its slow chemical degradation. By reducing
the 1,2-dichloropropane content of the products used in agriculture,
the contamination of groundwater will decrease.
3.3 Uses
1,2-Dichloropropane is a solvent for fats and oils. It has also
been used as an insecticide fumigant on grain and soil and to
control peach tree borers. Other uses are in gum processing, oil
processing, and organic chemical synthesis, in rubber making, wax
making, and the making of scouring compounds (Fishbein, 1979;
Sittig, 1980; Baruffini et al., 1989). It is used in furniture
finishing, dry cleaning fluid, paint remover, and metal degreasing,
and is a chemical intermediate for the production of
tetrachloroethylene and carbon tetrachloride (Fishbein, 1979).
It is a component of "MIX D/D" (Sittig, 1980; Worthing & Hance,
1991).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Phototransformation is likely to be the dominant process for
the decomposition of 1,2-dichloropropane, but vapour phase
photolysis was not detected after prolonged simulated sunlight
irradiation in a reaction chamber (California State Water Resources
Control Board, 1983).
Adsorption on to particulate matter seems to be necessary for
appreciable direct phototransformation. The decomposition is rather
slow in the atmosphere. It was calculated that, on the basis of
reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days for a 24-h average OH-radical
concentration of 1 x 106 cm3 (Tuazon et al., 1984).
See also section 4.1.1 of 1,3-dichloropropene.
4.1.2 Soil
The persistence of 1,3-dichloropropene and 1,2-dichloropropane
depends on chemical transformation, volatilization, microbial
transformation, photochemical transformation, and uptake into
organisms (see section 4.1.3.5 and Table 5 on 1,3-dichloropropene
and section 4.3.2 and Table 23 on "MIX D/D"). The persistence and
degradation depend on the type of soil and the temperature (Sittig,
1980).
Little or no chemical degradation of 1,2-dichloropropane has
been observed in laboratory and field studies. More than 98% of 1,2-
dichloropropane applied to a sandy loam soil and medium loam soil
was recovered 12-20 weeks after treatment (Roberts & Stoydin, 1976).
4.1.2.1 Volatilization
See section 4.1.3.2 of 1,3-dichloropropene.
4.1.2.2 Uptake in crops
See section 4.1.3.3 of 1,3-dichloropropene.
4.1.2.3 Movement in soil
See section 4.1.3.4 of 1,3-dichloropropene.
4.2 Biotransformation
In a well-run, waste-water treatment plant, Bi-Chem mutant
bacteria can remove chlorinated aliphatic hydrocarbons, such as 1,2-
dichloropropane (Straley et al., 1982).
Oldenhuis et al. (1989) studied the conversion of 1,2-
dichloropropane by the methanotrophic bacterium Methylosinus
trichosporium OB3b, grown in continuous cultures. 1,2-
Dichloropropane was added at a concentration of 0.2 mmol/litre.
After 24 h, complete degradation was found. 2,3-Dichloro-1-propanol
was identified as a degradation product.
4.3 Bioconcentration
No data on bioconcentration are available.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The City of Philadelphia made an emission inventory of 99
pollutants. In the periods 11 December 1983 to 31 March 1984 and 6
February 1984 to 31 March 1984, the mean concentration of 1,2-
dichloropropane observed in the air was 1.2 µg/m3 (Haemisegger et
al., 1985; Sullivan et al., 1985).
In Portland, Oregon, the atmospheric gas-phase concentration of
1,2-dichloropropane during rain events in the period February-April
1984, ranged between 21 and 40 ng/m3 (Ligocki et al., 1985).
In Japan, ambient air levels of 1,2-dichloropropane were
monitored in 13 cities in 1989. The substance was detected in 11 out
of 36 samples at levels ranging from 0.0065 to 1.4 µg/m3 (Japan
Environment Agency, 1991, letter from Sawamura, Ministry of Health
and Welfare, Tokyo, Japan, to the IPCS).
5.1.2 Water and soil
It was reported from the USA that 1.4% of 945 wells contained a
median level of 0.9 µg 1,2-dichloropropane/litre. In Maryland, 13
out of 36 wells contained 1,2-dichloropropane at levels ranging from
1 to 440 µg/litre. In California, 3 wells out of 64 were positive
for 1,2-dichloropropane, in another study, 12 out of 95 wells were
positive with levels ranging from 0.4 to 16 µg/litre. In the North
Coast area, 18 out of 24 wells were positive, with 4 with levels
above 10 µg/litre (California State Water Resources Control Board,
1983).
Hallberg (1989) also reported on the presence of 1,2-
dichloropropane in groundwater in the USA; 1,2-dichloropropane was
found in 7 States, with a maximum concentration of 51 µg/litre in
Massachusetts. Connors et al. (1990) found levels of 0.7-19.0 µg
1,2-dichloropropane/litre in potable water samples collected in 8
homes in 3 communities in Connecticut.
In 1984, micropollutants were analysed in well water in the
northern part of the Netherlands in an area in which "MIX D/D" had
been used for at least 20 years. 1,2-Dichloropropane was present in
14 out of 26 wells at levels ranging from 0.1 up to 9.2 µg/litre
water (Hoogsteen, 1986).
Van Beek et al. (1988) studied the presence of 1,2-
dichloropropane in well water in Noordbargeres in the potato growing
area in the Northern Netherlands. Thirty-three wells were monitored
and levels higher than 0.1 µg/litre were found in 17/45 samples. The
highest concentration observed was 160 µg/litre. The 1,2-
dichloropropane was found in well water up to a depth of 13 m. The
average concentration of 1,2-dichloropropane (16 samples) found in
Puttenveld in the northern Netherlands in a potato growing area, in
the period 1985-86, was 1.1 µg/litre (range < 0.05-21.0 µg/litre)
(Beugelink, 1987).
In 1987-88, samples of surface water up to 1 m depth were taken
monthly at 5 sites in a polder in the Netherlands and analysed. The
area is situated next to the dunes (where groundwater is being
pumped up for the preparation of drinking-water) and is extensively
used for bulb culture. The maximum concentration of 1,2-
dichloropropane found was 16.2 µg/litre (Greve et al., 1989).
Lagas et al. (1989) analysed groundwater in 5 areas where
potato, maize, and bulb crops were grown in the Netherlands. 1,2-
Dichloropropane was found in 15 out of 22 samples of water collected
to a depth of 6 m below potato crops in concentrations of < 0.1-200
µg/litre and in 6 out of 8 samples below maize and bulb crops in
concentrations of < 1-14 µg/litre.
Cotruvo (1985) reported the occurrence of 1,2-dichloropropane
in 6 out of 466 samples of groundwater (source not specified) in the
USA. The median and maximum concentrations were 0.9 µg/litre and 21
µg/litre, respectively.
In Japan, 1,2-dichloropropane levels in water and bottom
sediment were monitored at 26 points (7 mouth of river, 4 lake, 8
port and 7 bay areas) in 1989. The substance was detected in 20 out
of 78 water samples with levels ranging from 0.00001 to 0.14
µg/litre and in 9 out of 78 sediment samples with levels ranging
from 0.16 to 10.0 µg/kg (Japan Environment Agency, 1991, letter from
Sawamura, Ministry of Health and Welfare, Tokyo, Japan, to the
IPCS).
5.1.3 Crops
Residues of 1,2-dichloropropane in edible crop commodities,
arising from the use of 1,3-dichloropropene or "MIX D/D", are
generally below the limit of detection. The obvious reason for this,
is that crops are not normally planted until most of the product
applied has been dissipated. Another reason is that any 1,2-
dichloropropane taken up by the plant, would have to survive the
whole crop cycle to be detected in the harvested commodity.
Supervised trials with "MIX D/D", in 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
Adult male and female Carworth Farm E rats received 1,2-
dichloro[1-14C]propane in 0.5 ml arachis oil, by stomach tube, and
excretion was followed. After 4 days, the animals were killed and
the radioactivity measured in the skin and carcass. The 24-h
excretion of radioactivity was very rapid, 80-90% was eliminated in
the faeces, urine, and expired air. The urine, the major route of
excretion, contained 50.2% (average of male and female animals) of
the administered dose. In expired air, 19.3% was found as labelled
CO2 and 23.1% as other volatile radioactivity. Only 4.4% was
detected in the faeces in first 24 h. The skin and carcass contained
1.5 and 3.7% of the dose, respectively, on day 4 (Hutson et al.,
1971).
In Sprague-Dawley rats, dosed orally with 20 mg 1,2-
dichloropropane/kg body weight per day for 4 consecutive days,
unchanged 1,2-dichloropropane was found in expired air, but not in
the urine (Jones & Gibson, 1980).
14C-1,2-Dichloropropane (99.9%) was administered orally to
groups of 4 Fischer 344 rats/sex in a single dose of 1 or 100 mg/kg
body weight, followed by 1 mg/kg per day non-radiolabelled compound
for 7 days, and a single 1 mg dose of 14C-1,2-dichloropropane/kg
body weight on day 8. 14C-1,2-Dichloropropane was rapidly
absorbed, metabolized, and excreted in both sexes. In all treated
groups, the principal routes of elimination were via the urine (37-
52%), expired air (31-36%) and faeces (5.5-7.9%); a total of 80-90%
was eliminated in the first 24 h. The tissues and carcass contained
7.1-10.6% of the dose. In general, the radioactivity was well
distributed among the 13 organs and tissues analysed, 48 h after
treatment. The liver contained the highest 14C-activity in all
groups; from 0.229 to 0.416% of the dose/g wet weight. Peak
concentrations were found in the blood 4 h after treatment. The
quantities of volatile organic compounds found ranged from 0.14 to
1.13% in the 1 mg/kg group and from 10 to 16% in the 100 mg/kg
group; approximately 82% of the exhaled volatile organic compounds
were in the form of 1,2-dichloropropane. Multiple exposure resulted
in a statistically significant reduction in the amount of
radioactivity eliminated in the urine, while the metabolism of 1,2-
dichloropropane to CO2 was enhanced. At 100 mg/kg, there was a
significant reduction of CO2 formation and enhancement of 14C
elimination as volatile organic compounds (14.7-33.4%) compared with
a single dose. No parent 1,2-dichloropropane was found in the urine,
but 3 mercapturic acid metabolites of 1,2-dichloropropane were
identified (Timchalk et al., 1989).
6.1.2 Inhalation
Groups of 4 Fischer 344 rats/sex were exposed to 14C-1,2-
dichloropropane vapour for a 6-h period in head-only inhalation
chambers at target concentrations of 23.3, 233, or 466 mg/m3. The
14C-1,2-dichloropropane was rapidly absorbed, metabolized, and
excreted. The urine contained between 55 and 65% of the dose, and
expired air contained 16-23% of 14CO2. With increasing dose, a
greater percentage of the recovered dose was eliminated as expired
organic volatile compounds, i.e., 1.7, 2.1-3.4, and 6.3-6.7%,
respectively. At 466 mg/m3, this increase was statistically
significant. The faeces contained 6.3-9.7% of the dose and the
tissues and carcass accounted for 5.8-10.0%. No sex difference was
noted. Radioactivity was well distributed among all 13 organs when
the tissues were analysed after 48 h. The liver and kidneys had the
highest concentration ranging from 0.154 to 0.292% and from 0.098 to
0.252% of the dose/g wet weight, respectively. Peak blood
concentrations of 0.06, 0.92, and 3.87 µg/g blood for the 3 dose
levels, respectively, were found at 4 h. Half-lives for elimination
from blood were 24 and 30 min for females and males, respectively.
No parent 1.2-dichloropropane was found in the urine, but 3
mercapturic acid metabolites were identified (section 6.2) (Timchalk
et al., 1989).
6.1.3 Intraperitoneal
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight in 0.5 ml corn oil for 5 days (once daily) or for 4 weeks
(five days/week). Urinary mercapturic acid excretion was monitored.
A significant increase in mercapturic acid excretion was observed at
all dose levels, with no further increase during the treatment: at
lower doses, a return to baseline values occurred within 48 h of the
end of the treatment. Mercapturic acid excretion at the end of weeks
2, 3, and 4 was significantly lower than that observed at the end of
the first week (Trevisan et al., 1989).
6.2 Metabolic transformation
1,2-Dichloropropane is metabolized to form a variety of
metabolic products. Dichloropropane oxidation yielded the
mercapturic acid, N-acetyl- S-(2-hydroxypropyl)cysteine (Jones &
Gibson, 1980). Three mercapturic acid metabolites were identified in
the urine of Fischer 344 rats (110-140 g) administered 1,2-
dichloropropane orally (100 mg/kg body weight) or by inhalation (466
mg/m3 per 6 h). These compounds are N-acetyl- S-(2-
hydroxypropyl)-L-cysteine, N-acetyl- S-(2-oxopropyl)-L-cysteine
and N-acetyl- S-(1-carboxyethyl)-L-cysteine. Fischer 344 rats
were given a single oral dose of deuterium (D6)-labelled
dichloropropane (105 mg/kg body weight) in a mechanistic study
conducted to determine whether the conjugated metabolites are
generated through a sulfonium ion intermediate. The results suggest
that dichloropropane undergoes oxidation either prior to, or
subsequent to, glutathione conjugation. There was no evidence to
support the existence of a sulfonium intermediate in the formation
of the 2-hydroxypropyl-mercapturic acid metabolite of
dichloropropane (Fig. 8). Instead, this metabolite is thought to
arise via the direct oxidation of 1,2-dichloropropane, either prior
to, or following, conjugation with glutathione (Fig. 8) (Timchalk et
al., 1989; Bartels & Timchalk, 1990).
It was concluded that the degree to which the genotoxic
potential of cis-dichloropropene or its autooxidation products is
expressed in vivo is likely to be lower than that found by
microbial mutation assays. Cis-dichloropropene is efficiently
detoxified in mammals by the operation of a glutathione-dependent
S-alkyl transferase (Watson et al., 1987).
8.8.4 Effect on liver enzyme activity
Miyaoka et al. (1990) studied the mechanism of 1,3-
dichloropropene-induced hepatotoxicity in male mice of the ICR
strain (6 weeks old). 1,3-Dichloropropene (300 mg/kg body weight),
administered by gavage in corn oil, increased plasma GOT and GPT
activities significantly, and centrilobular swelling occurred in the
liver, 15 h after treatment. No such effect was found with 100 mg/kg
body weight. Pretreatment of piperonylbutoxide (PIB, a cytochrome
P450 inhibitor), at 200 mg/kg body weight i.p., significantly
suppressed the elevation of plasma GOT and GPT activities caused by
300 mg 1,3-dichloropropene/kg body weight, but increased the 1,3-
dichloropropene concentration in the liver. The PIB pretreatment
decreased the cytochrome P450 contents in liver microsomes, but
prevented further reduction of cytochrome P450 after 1,3-
dichloropropene treatment.
With pretreatment with L-buthionine-S,R sulfoximine (a GSH
depleting agent) at 1600 mg/kg body weight, plasma GOT activities
increased significantly in animals receiving 100 mg 1,3-
dichloropropene, whereas liver GSH contents and GST activity
decreased. Cysteine administration, 2 h after 1,3-dichloropropene
treatment, did not decrease the cytochrome P450 content, though it
prevented the elevation of GOT and GPT activities and increased
hepatic GSH concentration. The results suggest that 1,3-
dichloropropene is biotransformed via cytochrome P450, and that the
metabolites induce liver damage. GSH plays an important role in the
detoxification of 1,3-dichloropropene (Miyaoka et al., 1990).
9. EFFECTS ON HUMANS
9.1 General population
9.1.1 Acute toxicity - poisoning incidents
In a truck accident in California in 1975, about 4500 litres of
1,3-dichloropropene (92%) was slowly spilled on to the highway. An
estimated 80 persons were exposed to the vapour. Forty-six persons
were examined at hospitals. The following symptoms were found in a
small number of persons (4-6), headache, vomiting and nausea,
dizziness, irritation of mucous membranes, and chest discomfort.
Three persons lost consciousness at the scene of the accident. In 11
out of 41 persons, slightly elevated SGOT and/or SGPT values were
found. Twenty-eight patients were interviewed 1 or 2 weeks later.
The most common symptoms were: headache (12), abdominal discomfort
(6), chest discomfort (5), and malaise (5). Twenty-one patients were
interviewed after 2 years; 10 patients complained of severe or
unusual headache, 10 of chest pain or discomfort, and 13 of
"personality changes" (fatigue, irritability, difficulty in
concentrating, or decreased libido). The frequency of these long-
persisting symptoms was not associated with the intensity of the
exposure (Flessel et al., 1978).
Markovitz & Crosby (1984) reported 9 cases of acute poisoning
following accidental over-exposure to 1,3-dichloropropene. The
chemical spilled as the driver jack-knifed the container. Two of
these cases died 6 years later, due to diffuse histocytic lymphoma.
Authors have reported another case of myelo-monocytic leukaemia
where the patient had been accidentally over-exposed to 1,3-
dichloropropene (see section 9.2.2).
9.1.2 Controlled human studies
A smell detection test with 10 human volunteers was carried
out. A level of 13.6 mg 1,3-dichloropropene/m3 air was detected by
7 out of 10 volunteers. Some reported that the sense of smell
diminished after a few minutes. Even a level of 4.54 mg/m3 was
detected by these 7 persons, but it was noticeably fainter than 13.6
mg/m3 (Torkelson & Oyen, 1977).
In a study designed to determine the odour threshold of Telone
II among 22 individuals, the lowest concentration at which odour was
detected was 20 ± 14 mg/m3. This level is slightly above the US
threshold limit value time-weighted average of 4.54 mg/m3, but
below the short-term exposure limit of 45.4 mg/m3 (Rick & McCarty,
1988).
9.2 Occupational exposure
9.2.1 General
The most likely routes of human exposure to 1,3-dichloropropene
are through inhalation and the skin. Irritation of the eyes and
upper respiratory mucosa, accompanied by lacrimation, appear
promptly after exposure to vapours (Gosselin et al., 1976).
Inhalation by humans of air containing concentrations greater
than 6810 mg/m3 produces headaches, mucous membrane irritation,
dizziness, nausea, vomiting, gasping, coughing, substernal pain, and
respiratory distress (Gosselin et al., 1976; Flessel et al., 1978).
Lower concentrations produce central nervous system depression and
moderate irritation of the respiratory system.
A 44-year-old male process operator at a pesticide plant had
acute bullous dermatitis on both feet in 1988. Approximately one
year later, an identical dermatitis developed. In both periods, he
contaminated his shoes with a 1,3-dichloropropene formulation (D-D-
95). In a patch test with 1,3-dichloropropene, even a concentration
of 0.005% produced a positive reaction. Twenty volunteers did not
react in this patch test at a concentration of 0.05% (Bousema et
al., 1991).
Maddy et al. (1990) summarized the pesticides that caused
occupational illness/injury, reported by physicians in California
during 1987. 1,3-Dichloropropene caused one case of systemic illness
in that period. In the year 1986, 3 cases were mentioned, one with
systemic effects, one with skin effects, and one with eye injury
(Edmiston & Maddy, 1987).
Fifteen applicators of 1,3-dichloropropene were studied for
personal air exposure, urinary excretion of the metabolite, and
excretion of the renal tubular enzyme N-acetyl glucosaminidase
(NAG). Each was studied for four, 6-8 h consecutive intervals
following base-line determinations. The duration of exposure ranged
from 120 to 697 min and the personal air concentrations ranged from
0.3 to 9.4 mg/m3. The 24-h urinary excretion of the metabolite
(average 2.6 mg, range: 0.5-9.2 mg) correlated well with the 1,3-
dichloropropene air exposure product (minutes exposed x mg/m3).
The mean excretion of NAG for all intervals was 2.6 mU/mg of
creatinine (range: 1.0-7.7 mU/mg of creatinine); in 24 h, the mean
was 4940 mU with a range of 278-8956 mU. Four of the 15 workers had
an NAG activity of > 4 mU/mg creatinine in any of their urine
collected after the base line. Nine workers showed increases in NAG
excretion of more than 25% compared with the base line. The authors
concluded that the elevated excretion of NAG indicated a possible
subclinical nephrotoxic effect in the workers, though no complaints
or cases of renal injury were reported (Osterloh et al., 1989).
Stott et al. (1990) commented that the slight increase in NAG in the
urine might be a result of the stimulation of exocytosis or an
increase (induction) in the NAG activity in the kidneys, rather than
an indication of nephrotoxicity. Taken together with the known
metabolism and toxicity of 1,3-dichloropropene in laboratory
animals, the findings by Osterloh et al. (1989) do not suggest any
untoward effects in workers exposed occupationally to low levels of
1,3-dichloropropene.
Fourteen workers applying 1,3-dichloropropene were monitored at
the start of the season, in July, and at the end of the season, in
October, for liver function. The following parameters were measured;
alanine aminotransferase, aspartate aminotransferase, alkaline
phosphatase, lactic dehydrogenase, gamma-glutamyltrans-peptidase,
and total bilirubin. Total bilirubin was significantly decreased at
the end of the season. In combination with an increase in serum
gamma-glutamyltranspeptidase activity this indicates moderate
hepatic enzyme induction. The renal function was also studied by
measuring creatinine and beta-2-microglobulin in serum and beta-2-
microglobulin, albumin, alanine-aminopeptidase, beta-galactosidase,
and retinol-binding protein in urine. The glomerular function
parameters (increased albumin in urine and decreased creatinine in
serum) changed significantly during the season. The tubular function
(retinol-binding protein) also increased. On the basis of these
data, a subclinical nephrotoxic effect cannot be excluded. Effects
on the glutathione conjugation capacity were studied by measuring
erythrocyte glutathione- S-transferase activity and blood
glutathione concentration. Both parameters were significantly
decreased (Brouwer et al., 1991b). The cause-effect relationship
with 1,3-dichloropropene exposure has been questioned (Van Sittert
et al., 1991).
9.2.2 Acute toxicity - poisoning incidents
A farmer in good health developed pain in the right ear, nasal
mucosa, and pharynx after applying 1,3-dichloropropene to his fields
from his tractor for 30 days. Hospital examination showed a red and
painful external ear, hyperaemia, and superficial ulcerations of the
nasal mucosa, and inflammation of the pharynx. The hose containing
the 1,3-dichloropropene had a small leak, which had sprayed the
chemical near the right side of his face. Over the following year,
the man developed myelo-monocytic leukaemia. The man died of
pneumonia 5 weeks after entering the hospital (Markovitz & Crosby,
1984; NTP, 1985; Yang, 1986). The Task Group considers that the
cause-effect relationship in this case is doubtful.
9.2.3 Effects of short- and long-term exposure
The fertility status of 63 males employed in the production of
chlorinated three-carbon compounds were investigated in comparison
with 63 non-exposed persons (at least 5 years without exposure).
Data from reproductive medical history, hormone determination, and
semen analysis were used. There were no indications for an
association between lowered fertility by the standard fertility
parameters, and exposure to allyl chloride, epichlorohydrin, and
1,3-dichloropropene in the quantities occurring in the working
environment. A possible source of bias in this study stems from the
relatively low (64%) volunteer rate from the exposed group and the
lack of an estimate of the individual variation (Venable et al.,
1980).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,3-Dichloropropene (technical grade) was considered by working
groups of the International Agency for Research on Cancer (IARC) in
1986 (IARC, 1986) and 1987 (IARC,1987). In the updating of 1987, it
was evaluated as follows. "There is sufficient evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in
experimental animals. There is inadequate evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in humans.
The agent is possibly carcinogenic to humans (Group 2B)".
Based on the results from 2-year gavage studies on rats and
mice, using the linearized multistage model, the drinking-water
concentration for an excess life-time cancer risk of 104, 105,
or 106 is estimated to be 20, 2.0, or 0.2 µg/litre, respectively
(WHO/EURO, 1990).
PART B
ENVIRONMENTAL HEALTH CRITERIA
FOR
1,2-DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Soil
4.1.2.1 Volatilization
4.1.2.2 Uptake in crops
4.1.2.3 Movement in soil
4.2 Biotransformation
4.3 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water and soil
5.1.3 Crops
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.2 Inhalation
6.1.3 Intraperitoneal
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
7.1.2 Invertebrates
7.1.3 Fish
7.1.3.1 Acute toxicity
7.1.3.2 Short-term/long-term toxicity
7.2 Terrestrial organisms
7.2.1 Earthworms
7.2.2 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.2 Inhalation
8.2.2.1 Mouse
8.2.2.2 Rat
8.2.2.3 Rabbit
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
8.3.2.2 Oral (rabbit)
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
8.4.1.2 Mammalian cells
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
8.4.2.2 Dominant lethal test
8.4.2.3 Miscellaneous
8.5 Carcinogenicity
8.5.1 Oral (mouse)
8.5.2 Oral (rat)
8.6 Factors modifying toxicity
8.7 Special studies
8.7.1 Liver
8.7.2 Kidneys
8.7.3 Central nervous system
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
9.2 Occupational exposure
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1,2-Dichloropropane is a liquid with a boiling point of 96.8 °C
and a vapour pressure of 42 mmHg at 20 °C. The substance is soluble
in water, ethanol, and ethyl ether. When heated, it emits highly
toxic fumes of phosgene. The log P octanol/water partition
coefficient is 2.28.
This substance is used in furniture finish, dry cleaning fluid,
and paint remover, gum processing, metal degreasing, oil processing,
and as a rubber- and wax-making agent, and a chemical intermediate
in the production of tetrachloroethylene and carbon tetrachloride.
It is a component of the "MIX D/D", used as a pre-plant fumigant.
Concentrations of 1,2-dichloropropane in city air have been
measured at 1.2 µg/m3 (mean value), 0.021-0.040 µg/m3, and
0.0065-1.4 µg/m3 in Philadelphia, Portland, and Japan,
respectively. Decomposition in the atmosphere is slow; on the basis
of reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days. Phototransformation is likely to be
the dominant process for the decomposition. Adsorption on to
particulate matter is necessary for appreciable phototransformation.
Volatilization is likely to be the major route of loss from water.
In soil, the main routes of loss are volatilization and
diffusion. 1,2-dichloropropane is persistent in soil. More than 98%
of the 1,2-dichloropropane applied to loam soil was recovered 12-20
weeks after treatment.
Leaching of 1,2-dichloropropane occurs from soil and can
contaminate upper and lower groundwater in areas where "MIX D/D" has
been used as a soil fumigant. In well water and groundwater in the
USA, concentrations of up to 440 µg/litre and 51 µg/litre,
respectively, have been found. In the Netherlands, concentrations of
up to 160 µg/litre have been measured in well water and 1,2-
dichloropropane has been found to a depth of 13 m.
1,2-Dichloropropane can be taken up by edible crops, but the
residues detected have been low (< 0.01 mg/kg) and are unlikely to
be biologically significant.
Bioaccumulation of 1,2-dichloropropane is unlikely, because of
its high water solubility (2.7 g/kg) and low log P octanol/water
partition coefficient.
1.1.2 Kinetics and metabolism
1,2-Dichloropropane administered orally to rats is rapidly
eliminated (80-90% within 24 h). There are no major differences in
kinetics or elimination between males and females. Urine is the
major route of elimination, up to half an oral dose being eliminated
by this route within 24 h. Less than 10% is eliminated via the
faeces. Approximately one-third is eliminated through expired air,
both as carbon dioxide and as a mixture of volatile materials.
Tissue concentrations are low, the highest concentration being found
in the liver. Rapid elimination also occurs following the inhalation
exposure of rats; 55-65% of a dose is eliminated in the urine and
16-23% in expired air. The half-life of elimination from the blood
is 24-30 min.
Unchanged 1,2-dichloropropane is not found in urine. Three
major urinary metabolites have been identified. These metabolites
result from oxidative and conjugation pathways, which yield the
mercapturates, N-acetyl- S-(2-hydroxypropyl)-L-cysteine, N-
acetyl- S-(2-oxypropyl)-L-cysteine, and N-acetyl- S-(1-carboxy-
ethyl)-L-cysteine. 1,2-Dichloropropane can also be oxidized to
lactate with resultant carbon dioxide or acetyl co-enzyme A
production.
Oral administration of 1,2-dichloropropane (2 ml/kg) to rats
significantly depleted tissue glutathione contents. There was a
correlation between tissue glutathione loss and expression of
toxicity in the liver, kidneys, and red blood cells. Prior depletion
of intracellular glutathione exacerbated 1,2-dichloropropane
toxicity, whereas pretreatment with precursors for glutathione
synthesis ameliorated the toxicity. These results demonstrate the
protective effect of glutathione against 1,2-dichloropropane
toxicity.
1.1.3 Effects on organisms in the environment
EC50 for freshwater algae have not been calculated because of
difficulties with volatilization of the chemical from the test
solution. The acute toxicity of 1,2-dichloropropane for aquatic
invertebrates and fish is low to moderate; 48-h LC50 values for
invertebrates range between 52 and > 100 mg/litre and 96-h LC50
values for fish lie between 61 and 320 mg/litre. A short-term
toxicity test on Fathead minnows demonstrated a maximum no-effect
level of 82 mg/litre. A 32-day test on early life stage toxicity in
the same species demonstrated that larval growth and survival were
the most sensitive parameters. The estimated maximum acceptable
toxicant concentration (MATC) was between 6 and 11 mg/litre. Growth
inhibition was noted in Sheepshead minnows after 33 days at a 1,2-
dichloropropane concentration of 164 mg/litre.
1,2-Dichloropropane is phytotoxic.
Contact tests on 4 species of earthworm showed an LC50 of 44-
84 µg/cm2 (mean values) of filter paper. In artificial soil, the
LC50 values were 3880-5300 mg/kg soil (dry weight).
1.1.4 Effects on experimental animals and in vitro test
systems
The acute oral toxicity of 1,2-dichloropropane in experimental
animals is low. The oral LD50 for the rat is 1.9 g/kg body weight,
and the dermal LD50 in rabbits is 8.75 ml/kg body weight.
Short-term, oral toxicity studies of 1,2-dichloropropane in
mice and rats showed growth inhibition, clinical toxic signs
associated with central nervous system depression, and/or increased
mortality at dose levels of 250 mg/kg body weight per day or higher.
In rats given 250 mg/kg per day for 10 days, there were changes in
serum enzymes indicative of slight hepatotoxicity with a NOEL of 100
mg/kg per day.
In a 13-week mouse inhalation study (highest dose 681 mg/m3),
no adverse effects were observed. In a similar study on rats exposed
to 68.1, 227, or 681 mg/m3, a decrease in body weight and minimal
damage to nasal tissues occurred in the 2 highest dose groups.
In a 2-generation reproduction study, rats exposed to 1,2-
dichloropropane in drinking-water at 0.024, 0.1, 0.24% (equivalent
to 33.6, 140 and 336 mg/kg body weight per day) resulted in lower
maternal body weight gain and decreased water consumption at the mid
and high dose levels. Neonatal body weights were lower at the high
dose level. The NOAELs established for maternal and reproductive
toxicity were 33.6 and 140 mg/kg body weight per day, respectively.
Studies did not indicate any teratogenic activity of 1,2-
dichloropropane at oral dose levels up to 125 mg/kg body weight in
the rat and 150 mg/kg body weight in the rabbit. However, at these
dose levels, 1,2-dichloropropane was maternally toxic and fetotoxic,
as evidenced by central nervous system associated clinical signs,
decreased maternal body weight gain, and delayed ossification of
bones in the fetuses. The NOELs are 30 and 50 mg/kg body weight per
day for the rat and rabbit, respectively.
1,2-Dichloropropane was mutagenic in bacteria in most studies
with, and without, metabolic activation, but very high dose levels
were used of up to 10 mg/plate. In Chinese hamster ovary cells, 1,2-
dichloropropane caused chromosomal aberrations and sister chromatid
exchange; in Chinese hamster V79 cells, it increased the sister
chromatid exchange. In an in vitro system with human lymphocytes,
the tritiated thymidine uptake and cell viability in cultures grown
with, and without, rat liver metabolizing system, were similar to
those in control cultures. The results of a sex-linked recessive
lethal test in Drosophila melanogaster were negative. A dominant
lethal test in rats, dosed for 14 weeks via drinking-water
containing 1,2-dichloropropane, followed by 2 weeks of mating, was
negative.
In a carcinogenicity study on mice administered 125 or 250 mg
1,2-dichloropropane/kg body weight by gavage, a dose-related
increase in the incidence of liver adenomas was observed. The
incidence of liver adenomas in treated groups was higher than that
in the concurrent control group, but was within the historical
control range.
In rats administered dose levels of 125 and 250 mg/kg body
weight (females) and 62 and 125 mg/kg body weight (males), by
gavage, for 5 days per week over 113 weeks, a slight increase in the
incidence of mammary gland adenocarcinomas exceeding the historical
range was observed in high-dose females.
1.1.5 Effects on human beings
Exposure of the general population to 1,2-dichloropropane via
air and water is unlikely, except in areas where there is extensive
use of 1,2-dichloropropane and "MIX D/D" in agriculture. Residues of
1,2-dichloropropane in edible crops are generally below the limit of
detection. In view of these low exposures to 1,2-dichloropropane,
the risk to the general population is negligible.
Several cases of acute poisoning have been reported due to
accidental or intentional (suicide) over-exposure to 1,2-
dichloropropane. Effects have been mainly on the central nervous
system, liver, and kidneys. Haemolytic anaemia and disseminated
intravascular coagulation have also been reported. In one case,
delirium progressed to irreversible shock, cardiac failure, and
death.
Occupational exposures can be via both skin and inhalation.
Several cases of dermatitis and skin sensitization have been
reported in workers using solvent mixtures containing 1,2-
dichloropropane.
1.2 Conclusions
* General population: There is low or non-existent exposure of
the general population to 1,2-dichloropropane from air and
food. However, in certain areas, exposure may occur when
groundwater is contaminated.
* Occupational exposure: With good work practices, hygienic
measures, and safety precautions, the use of 1,2-
dichloropropane is unlikely to present a risk for those
occupationally exposed to it.
* Environment: 1,2-Dichloropropane is unlikely to attain levels
of environmental significance when used at the recommended
rate. It is unlikely to have adverse effects on populations of
terrestrial and aquatic organisms.
1.3 Recommendations
* Studies should be conducted to assess acute inhalation
toxicity, eye and skin irritancy, and skin sensitization
potential.
* Appropriate safety precautions should be taken, when handling
1,2-dichloropropane, in order to avoid exposures exceeding the
maximum allowable concentration.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
Primary constituent
Chemical structure Cl
'
ClCH2CHCH3
Chemical formula C3H6Cl2
Relative molecular mass 112.99
Chemical name 1,2-dichloropropane,
dichloro-1,2-propane
Common synonyms propylene dichloride
CAS registry number 78-87-5
RTECS registry number TX9625000
EINECS number 201-152-2
2.2 Physical and chemical properties
1,2-Dichloropropane is a liquid with a boiling point of 96.8
°C. The vapour pressure is 42 mmHg at 20 °C (27.9 kPa at 19.6 °C).
Its solubility is 2.7 g/kg water at 20 °C. It is soluble in ethanol
and diethyl ether. The density is 1.1595 g/ml at 20 °C, and 1.437
g/ml at 25 °C. It is flammable; the flash point is 21 °C (Cleveland
open cup). When heated to decomposition, 1,2-dichloropropane emits
highly toxic fumes of phosgene.
The log P octanol/water partition coefficient is 2.28 (NTP,
1983).
2.3 Conversion factors
1 ppm = 4.66 mg/m3
1 mg/m3 = 0.214 ppm
2.4 Analytical methods
The analytical methods used are the same as those used for 1,3-
dichloropropene.
Boyd et al. (1981) developed a method to collect vapours of
1,2-dichloropropane from air with solid sorbents (petroleum
charcoal) in tandem with a personal sampling pump; desorption of the
sorbed compound in acetone/cyclohexane and analysis of the extracts
by gas chromatography. A Carbowax or Chromosorb column was used. The
Hall electrolytic conductivity detector (in the halogen mode)
offered better sensitivity than the electron-capture detector and
the flame ionization detector.
An analytical method is described and issued in 1985 by NIOSH,
method 1013, for the determination of 1,2-dichloropropane in air.
The working range is 0.25-600 mg/m3. The limit of determination
0.1 µg/sample (NIOSH, 1985).
In method MDHS 28, a description is given for the determination
of the time-weighted-average concentrations of chlorinated
hydrocarbon solvent vapours in workplace atmospheres. The method is
suitable for sampling over periods in the range of 10 min to 8 h.
The method can also be used for the determination of personal
exposure. The method is suitable for the measurement of airborne
vapours containing concentrations in the range of approximately 1-
1000 mg/m3 (about 0.2-200 ppm v/v) for samples of 10 litres of
air. Charcoal is used as adsorbent and 15% (v/v) acetone in
cyclohexane is recommended as a desorption solvent for 1,2-
dichloropropane; determination takes place with a gas chromatograph
fitted with a flame ionization detector (HSE, 1990).
See section 2.4 of 1,3-dichloropropene.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
1,2-Dichloropropane is not known to occur naturally.
3.2 Man-made sources
1,2-Dichloropropane is produced by the chlorination of
propylene.
The production of 1,2-dichloropropane in the USA in 1974 was 66
million kg: in 1976, it was 32.2 million, and in 1980, 35 million kg
(Fishbein, 1979).
1,3-Dichloropropene, contaminated with 1,2-dichloropropane and
2,3-dichloropropene, used for fumigation in the Netherlands at
application rates ranging from 200 to 400 kg/ha, would mean an input
of 40-160 kg of 1,2-dichloropropane and 10-25 kg of 2,3-
dichloropropenes/ha (Krijgsheld & van der Gen, 1986). 1,2-
Dichloropropane is more persistent in the environment than 1,3-
dichloropropene and has a greater potential for contaminating
groundwater, because of its slow chemical degradation. By reducing
the 1,2-dichloropropane content of the products used in agriculture,
the contamination of groundwater will decrease.
3.3 Uses
1,2-Dichloropropane is a solvent for fats and oils. It has also
been used as an insecticide fumigant on grain and soil and to
control peach tree borers. Other uses are in gum processing, oil
processing, and organic chemical synthesis, in rubber making, wax
making, and the making of scouring compounds (Fishbein, 1979;
Sittig, 1980; Baruffini et al., 1989). It is used in furniture
finishing, dry cleaning fluid, paint remover, and metal degreasing,
and is a chemical intermediate for the production of
tetrachloroethylene and carbon tetrachloride (Fishbein, 1979).
It is a component of "MIX D/D" (Sittig, 1980; Worthing & Hance,
1991).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Phototransformation is likely to be the dominant process for
the decomposition of 1,2-dichloropropane, but vapour phase
photolysis was not detected after prolonged simulated sunlight
irradiation in a reaction chamber (California State Water Resources
Control Board, 1983).
Adsorption on to particulate matter seems to be necessary for
appreciable direct phototransformation. The decomposition is rather
slow in the atmosphere. It was calculated that, on the basis of
reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days for a 24-h average OH-radical
concentration of 1 x 106 cm3 (Tuazon et al., 1984).
See also section 4.1.1 of 1,3-dichloropropene.
4.1.2 Soil
The persistence of 1,3-dichloropropene and 1,2-dichloropropane
depends on chemical transformation, volatilization, microbial
transformation, photochemical transformation, and uptake into
organisms (see section 4.1.3.5 and Table 5 on 1,3-dichloropropene
and section 4.3.2 and Table 23 on "MIX D/D"). The persistence and
degradation depend on the type of soil and the temperature (Sittig,
1980).
Little or no chemical degradation of 1,2-dichloropropane has
been observed in laboratory and field studies. More than 98% of 1,2-
dichloropropane applied to a sandy loam soil and medium loam soil
was recovered 12-20 weeks after treatment (Roberts & Stoydin, 1976).
4.1.2.1 Volatilization
See section 4.1.3.2 of 1,3-dichloropropene.
4.1.2.2 Uptake in crops
See section 4.1.3.3 of 1,3-dichloropropene.
4.1.2.3 Movement in soil
See section 4.1.3.4 of 1,3-dichloropropene.
4.2 Biotransformation
In a well-run, waste-water treatment plant, Bi-Chem mutant
bacteria can remove chlorinated aliphatic hydrocarbons, such as 1,2-
dichloropropane (Straley et al., 1982).
Oldenhuis et al. (1989) studied the conversion of 1,2-
dichloropropane by the methanotrophic bacterium Methylosinus
trichosporium OB3b, grown in continuous cultures. 1,2-
Dichloropropane was added at a concentration of 0.2 mmol/litre.
After 24 h, complete degradation was found. 2,3-Dichloro-1-propanol
was identified as a degradation product.
4.3 Bioconcentration
No data on bioconcentration are available.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The City of Philadelphia made an emission inventory of 99
pollutants. In the periods 11 December 1983 to 31 March 1984 and 6
February 1984 to 31 March 1984, the mean concentration of 1,2-
dichloropropane observed in the air was 1.2 µg/m3 (Haemisegger et
al., 1985; Sullivan et al., 1985).
In Portland, Oregon, the atmospheric gas-phase concentration of
1,2-dichloropropane during rain events in the period February-April
1984, ranged between 21 and 40 ng/m3 (Ligocki et al., 1985).
In Japan, ambient air levels of 1,2-dichloropropane were
monitored in 13 cities in 1989. The substance was detected in 11 out
of 36 samples at levels ranging from 0.0065 to 1.4 µg/m3 (Japan
Environment Agency, 1991, letter from Sawamura, Ministry of Health
and Welfare, Tokyo, Japan, to the IPCS).
5.1.2 Water and soil
It was reported from the USA that 1.4% of 945 wells contained a
median level of 0.9 µg 1,2-dichloropropane/litre. In Maryland, 13
out of 36 wells contained 1,2-dichloropropane at levels ranging from
1 to 440 µg/litre. In California, 3 wells out of 64 were positive
for 1,2-dichloropropane, in another study, 12 out of 95 wells were
positive with levels ranging from 0.4 to 16 µg/litre. In the North
Coast area, 18 out of 24 wells were positive, with 4 with levels
above 10 µg/litre (California State Water Resources Control Board,
1983).
Hallberg (1989) also reported on the presence of 1,2-
dichloropropane in groundwater in the USA; 1,2-dichloropropane was
found in 7 States, with a maximum concentration of 51 µg/litre in
Massachusetts. Connors et al. (1990) found levels of 0.7-19.0 µg
1,2-dichloropropane/litre in potable water samples collected in 8
homes in 3 communities in Connecticut.
In 1984, micropollutants were analysed in well water in the
northern part of the Netherlands in an area in which "MIX D/D" had
been used for at least 20 years. 1,2-Dichloropropane was present in
14 out of 26 wells at levels ranging from 0.1 up to 9.2 µg/litre
water (Hoogsteen, 1986).
Van Beek et al. (1988) studied the presence of 1,2-
dichloropropane in well water in Noordbargeres in the potato growing
area in the Northern Netherlands. Thirty-three wells were monitored
and levels higher than 0.1 µg/litre were found in 17/45 samples. The
highest concentration observed was 160 µg/litre. The 1,2-
dichloropropane was found in well water up to a depth of 13 m. The
average concentration of 1,2-dichloropropane (16 samples) found in
Puttenveld in the northern Netherlands in a potato growing area, in
the period 1985-86, was 1.1 µg/litre (range < 0.05-21.0 µg/litre)
(Beugelink, 1987).
In 1987-88, samples of surface water up to 1 m depth were taken
monthly at 5 sites in a polder in the Netherlands and analysed. The
area is situated next to the dunes (where groundwater is being
pumped up for the preparation of drinking-water) and is extensively
used for bulb culture. The maximum concentration of 1,2-
dichloropropane found was 16.2 µg/litre (Greve et al., 1989).
Lagas et al. (1989) analysed groundwater in 5 areas where
potato, maize, and bulb crops were grown in the Netherlands. 1,2-
Dichloropropane was found in 15 out of 22 samples of water collected
to a depth of 6 m below potato crops in concentrations of < 0.1-200
µg/litre and in 6 out of 8 samples below maize and bulb crops in
concentrations of < 1-14 µg/litre.
Cotruvo (1985) reported the occurrence of 1,2-dichloropropane
in 6 out of 466 samples of groundwater (source not specified) in the
USA. The median and maximum concentrations were 0.9 µg/litre and 21
µg/litre, respectively.
In Japan, 1,2-dichloropropane levels in water and bottom
sediment were monitored at 26 points (7 mouth of river, 4 lake, 8
port and 7 bay areas) in 1989. The substance was detected in 20 out
of 78 water samples with levels ranging from 0.00001 to 0.14
µg/litre and in 9 out of 78 sediment samples with levels ranging
from 0.16 to 10.0 µg/kg (Japan Environment Agency, 1991, letter from
Sawamura, Ministry of Health and Welfare, Tokyo, Japan, to the
IPCS).
5.1.3 Crops
Residues of 1,2-dichloropropane in edible crop commodities,
arising from the use of 1,3-dichloropropene or "MIX D/D", are
generally below the limit of detection. The obvious reason for this,
is that crops are not normally planted until most of the product
applied has been dissipated. Another reason is that any 1,2-
dichloropropane taken up by the plant, would have to survive the
whole crop cycle to be detected in the harvested commodity.
Supervised trials with "MIX D/D", in 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
Adult male and female Carworth Farm E rats received 1,2-
dichloro[1-14C]propane in 0.5 ml arachis oil, by stomach tube, and
excretion was followed. After 4 days, the animals were killed and
the radioactivity measured in the skin and carcass. The 24-h
excretion of radioactivity was very rapid, 80-90% was eliminated in
the faeces, urine, and expired air. The urine, the major route of
excretion, contained 50.2% (average of male and female animals) of
the administered dose. In expired air, 19.3% was found as labelled
CO2 and 23.1% as other volatile radioactivity. Only 4.4% was
detected in the faeces in first 24 h. The skin and carcass contained
1.5 and 3.7% of the dose, respectively, on day 4 (Hutson et al.,
1971).
In Sprague-Dawley rats, dosed orally with 20 mg 1,2-
dichloropropane/kg body weight per day for 4 consecutive days,
unchanged 1,2-dichloropropane was found in expired air, but not in
the urine (Jones & Gibson, 1980).
14C-1,2-Dichloropropane (99.9%) was administered orally to
groups of 4 Fischer 344 rats/sex in a single dose of 1 or 100 mg/kg
body weight, followed by 1 mg/kg per day non-radiolabelled compound
for 7 days, and a single 1 mg dose of 14C-1,2-dichloropropane/kg
body weight on day 8. 14C-1,2-Dichloropropane was rapidly
absorbed, metabolized, and excreted in both sexes. In all treated
groups, the principal routes of elimination were via the urine (37-
52%), expired air (31-36%) and faeces (5.5-7.9%); a total of 80-90%
was eliminated in the first 24 h. The tissues and carcass contained
7.1-10.6% of the dose. In general, the radioactivity was well
distributed among the 13 organs and tissues analysed, 48 h after
treatment. The liver contained the highest 14C-activity in all
groups; from 0.229 to 0.416% of the dose/g wet weight. Peak
concentrations were found in the blood 4 h after treatment. The
quantities of volatile organic compounds found ranged from 0.14 to
1.13% in the 1 mg/kg group and from 10 to 16% in the 100 mg/kg
group; approximately 82% of the exhaled volatile organic compounds
were in the form of 1,2-dichloropropane. Multiple exposure resulted
in a statistically significant reduction in the amount of
radioactivity eliminated in the urine, while the metabolism of 1,2-
dichloropropane to CO2 was enhanced. At 100 mg/kg, there was a
significant reduction of CO2 formation and enhancement of 14C
elimination as volatile organic compounds (14.7-33.4%) compared with
a single dose. No parent 1,2-dichloropropane was found in the urine,
but 3 mercapturic acid metabolites of 1,2-dichloropropane were
identified (Timchalk et al., 1989).
6.1.2 Inhalation
Groups of 4 Fischer 344 rats/sex were exposed to 14C-1,2-
dichloropropane vapour for a 6-h period in head-only inhalation
chambers at target concentrations of 23.3, 233, or 466 mg/m3. The
14C-1,2-dichloropropane was rapidly absorbed, metabolized, and
excreted. The urine contained between 55 and 65% of the dose, and
expired air contained 16-23% of 14CO2. With increasing dose, a
greater percentage of the recovered dose was eliminated as expired
organic volatile compounds, i.e., 1.7, 2.1-3.4, and 6.3-6.7%,
respectively. At 466 mg/m3, this increase was statistically
significant. The faeces contained 6.3-9.7% of the dose and the
tissues and carcass accounted for 5.8-10.0%. No sex difference was
noted. Radioactivity was well distributed among all 13 organs when
the tissues were analysed after 48 h. The liver and kidneys had the
highest concentration ranging from 0.154 to 0.292% and from 0.098 to
0.252% of the dose/g wet weight, respectively. Peak blood
concentrations of 0.06, 0.92, and 3.87 µg/g blood for the 3 dose
levels, respectively, were found at 4 h. Half-lives for elimination
from blood were 24 and 30 min for females and males, respectively.
No parent 1.2-dichloropropane was found in the urine, but 3
mercapturic acid metabolites were identified (section 6.2) (Timchalk
et al., 1989).
6.1.3 Intraperitoneal
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight in 0.5 ml corn oil for 5 days (once daily) or for 4 weeks
(five days/week). Urinary mercapturic acid excretion was monitored.
A significant increase in mercapturic acid excretion was observed at
all dose levels, with no further increase during the treatment: at
lower doses, a return to baseline values occurred within 48 h of the
end of the treatment. Mercapturic acid excretion at the end of weeks
2, 3, and 4 was significantly lower than that observed at the end of
the first week (Trevisan et al., 1989).
6.2 Metabolic transformation
1,2-Dichloropropane is metabolized to form a variety of
metabolic products. Dichloropropane oxidation yielded the
mercapturic acid, N-acetyl- S-(2-hydroxypropyl)cysteine (Jones &
Gibson, 1980). Three mercapturic acid metabolites were identified in
the urine of Fischer 344 rats (110-140 g) administered 1,2-
dichloropropane orally (100 mg/kg body weight) or by inhalation (466
mg/m3 per 6 h). These compounds are N-acetyl- S-(2-
hydroxypropyl)-L-cysteine, N-acetyl- S-(2-oxopropyl)-L-cysteine
and N-acetyl- S-(1-carboxyethyl)-L-cysteine. Fischer 344 rats
were given a single oral dose of deuterium (D6)-labelled
dichloropropane (105 mg/kg body weight) in a mechanistic study
conducted to determine whether the conjugated metabolites are
generated through a sulfonium ion intermediate. The results suggest
that dichloropropane undergoes oxidation either prior to, or
subsequent to, glutathione conjugation. There was no evidence to
support the existence of a sulfonium intermediate in the formation
of the 2-hydroxypropyl-mercapturic acid metabolite of
dichloropropane (Fig. 8). Instead, this metabolite is thought to
arise via the direct oxidation of 1,2-dichloropropane, either prior
to, or following, conjugation with glutathione (Fig. 8) (Timchalk et
al., 1989; Bartels & Timchalk, 1990).
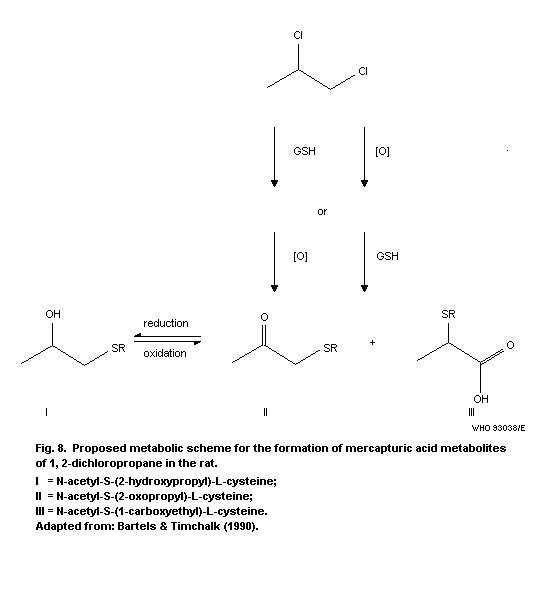 Imberti et al. (1990) investigated the effects of a single dose
of 1,2-dichloropropane of 2 ml/kg body weight (by gavage) on the
intracellular glutathione (GSH) content of the liver, kidneys, and
blood of male Wistar rats (180-250 g). 1,2-Dichloropropane,
administered orally, caused a significant depletion of GSH within 24
h of treatment, followed by a slow recovery, approaching normal
levels after 96 h. The GSH depletion was associated with a marked
increase in serum GOT, GPT, 5'-nucleotidase, gamma-glutamyl
transpeptidase, alkaline phosphatase, urea, and creatinine, and a
significant degree of haemolysis. The administration of L-
buthionine-S,R sulfoximine (BSO) (0.5 g/kg body weight, i.p.), 4 h
before 1,2-dichloropropane treatment, resulted in a significant
increase in overall mortality. The administration of a GSH
precursor, N-acetylcysteine (NAC), i.p., at 250 mg/kg body weight,
2 and 16 h after 1,2-dichloropropane treatment, prevented the loss
of cellular GSH and reduced the extent of injury in the target
tissues. There was a correlation between the depletion of liver GSH
and the increases in GOT, GPT, and 5'-nucleotidase, between the
depletion of GSH in the kidneys and the increase in serum urea and
creatinine, and the depletion of GSH in blood and the occurrence of
haemolysis.
Both 1-chloro-2-hydroxypropane (II) and 1,2-epoxypropane (III)
are proposed as intermediates in the metabolism to the mercapturic
acid. 1,2-Epoxypropane can also be metabolized to propanediol (IV),
which is further metabolized to pyruvate and enters the
tricarboxylic acid cycle; carbon dioxide is released and expired.
Epoxypropane may also be conjugated with glutathione (VI) and
excreted in the urine. Jones & Gibson (1980) further proposed that
the 1-chloro-2-hydroxypropane (II) may be metabolized to beta-
chlorolactaldehyde (VII) and beta-chlorolactate (VIII) (Fig. 9).
Imberti et al. (1990) investigated the effects of a single dose
of 1,2-dichloropropane of 2 ml/kg body weight (by gavage) on the
intracellular glutathione (GSH) content of the liver, kidneys, and
blood of male Wistar rats (180-250 g). 1,2-Dichloropropane,
administered orally, caused a significant depletion of GSH within 24
h of treatment, followed by a slow recovery, approaching normal
levels after 96 h. The GSH depletion was associated with a marked
increase in serum GOT, GPT, 5'-nucleotidase, gamma-glutamyl
transpeptidase, alkaline phosphatase, urea, and creatinine, and a
significant degree of haemolysis. The administration of L-
buthionine-S,R sulfoximine (BSO) (0.5 g/kg body weight, i.p.), 4 h
before 1,2-dichloropropane treatment, resulted in a significant
increase in overall mortality. The administration of a GSH
precursor, N-acetylcysteine (NAC), i.p., at 250 mg/kg body weight,
2 and 16 h after 1,2-dichloropropane treatment, prevented the loss
of cellular GSH and reduced the extent of injury in the target
tissues. There was a correlation between the depletion of liver GSH
and the increases in GOT, GPT, and 5'-nucleotidase, between the
depletion of GSH in the kidneys and the increase in serum urea and
creatinine, and the depletion of GSH in blood and the occurrence of
haemolysis.
Both 1-chloro-2-hydroxypropane (II) and 1,2-epoxypropane (III)
are proposed as intermediates in the metabolism to the mercapturic
acid. 1,2-Epoxypropane can also be metabolized to propanediol (IV),
which is further metabolized to pyruvate and enters the
tricarboxylic acid cycle; carbon dioxide is released and expired.
Epoxypropane may also be conjugated with glutathione (VI) and
excreted in the urine. Jones & Gibson (1980) further proposed that
the 1-chloro-2-hydroxypropane (II) may be metabolized to beta-
chlorolactaldehyde (VII) and beta-chlorolactate (VIII) (Fig. 9).
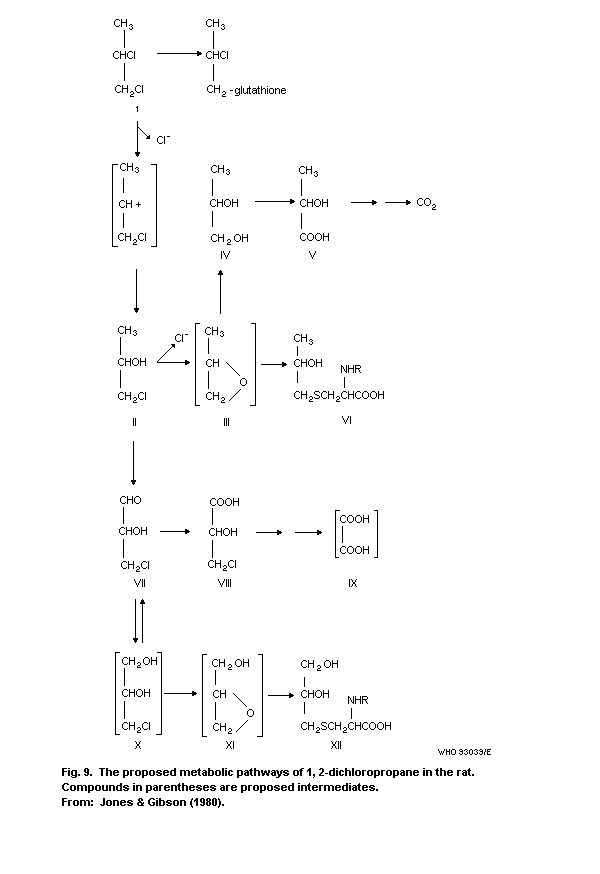 7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
The EC50 for CO2 uptake by marine algae (Phaeodactylum
tricornutum) was 50 mg/litre. 1,2-Dichloropropane was not found to
exhibit any algistatic or algicidal effects in an acute toxicity
study on Selenastrum capricornutum, though the problems of losses
of 1,2-dichloropropane from the test flasks, made it impossible to
calculate EC values (Dynamic Corporation, 1991).
A general trend of decreasing algal population growth with
increasing nominal concentrations of 1,2-dichloropropane was
observed in an acute toxicity test on Skeletonema costatum.
Because the concentrations measured for each nominal value were
variable, it was not appropriate to determine EC values. A NOEL of
18 mg/litre was determined, though it was not possible to
distinguish algistatic from algicidal effects (Dynamic Corporation,
1991).
7.1.2 Invertebrates
The acute toxicity of 1,2-dichloropropane for non-target
invertebrates is summarized in Table 18; 1,2-dichloropropane has a
48-h LC50 of 50-100 mg/litre for these aquatic organisms. No
discernible effects on Daphnia magna were found at concentrations
of less than 22 mg/litre (Leblanc, 1980).
Table 18. Acute toxicity of 1,2-dichloropropane in non-target
aquatic insects and crustacea
Species Temperature 48-h LC50 References
(°C) mg/litre
Barnacle nauplii - 53 Pearson & McConnell
(Elminius moderatus) (1975)
Brown shrimp - > 100 Portmann & Wilson
(Crangon crangon) (1971)
7.1.3 Fish
7.1.3.1 Acute toxicity
The 96-h LC50 of 1,2-dichloropropane for freshwater and
marine fish is 61-320 mg/litre (Table 19).
7.1.3.2 Short-term/long-term toxicity
Two embryo-larval tests have been conducted on Fathead minnow
(Pimephales promelas) exposed to 1,2-dichloropropane. The maximum
no-effect levels were 8.1 and 60 mg/litre, respectively (US EPA,
1980).
In the marine environment, growth inhibition was noted in the
Sheepshead minnow (Cyprinodon variegatus) after 33 days exposure
to 164 mg 1,2-dichloropropane/litre (US EPA, 1980).
A 32-day test to study early life stage toxicity in Fathead
minnows (Pimephales promelas) demonstrated that larval growth and
survival (28-day-old fish) were the most sensitive indicators of
toxic effects. Embryo hatch and larval deformities at hatch were the
least sensitive indicators of toxicity. The 1,2-dichloropropane
(98%) was tested at dose levels of 0, 6, 11, 25, 51, and 110
mg/litre (temperature of the water 25 °C, hardness 45 mg/litre as
CaCO3, pH 7.4). The estimated maximum acceptable toxicant
concentration (MATC) was between 6 and 11 mg/litre (Benoit et al.,
1982).
7.2 Terrestrial organisms
7.2.1 Earthworms
Neuhauser et al. (1985a,b; 1986) studied the toxicity of 1,2-
dichloropropane for 4 species of earthworm (Allolobophora
tuberculata, Eisenia foetida, Eudrilus eugeniae and Perionyx
excavatus) using EEC earthworm artificial soil and a contact
testing procedure (Edwards, 1983). The LC50 values and 95%
confidence limits in the contact test were 84(65-110); 64(59-70);
44(38-51); and 63(56-72) µg/cm2 of filter paper for the 4 species
of earthworms, respectively. In the artificial soil test, the LC50
values were 4272, 4240, 5300, and 3880 mg/kg dry weight artificial
soil, respectively.
7.2.2 Plants
1,2-Dichloropropane is highly phytotoxic.
Table 19. Acute toxicity of 1,2-dichloropropane in fish
Species Size or age Temperature 96-h LC50 References
(°C) mg/litre
Guppy 2-3 months 21-23 3.01 µmol/litref Könemann (1981)
(Poecilia reticulata)
Bluegill sunfish 33-75 mm 23a 320 Dawson et al. (1977)
(Lepomis macrochirus) 0.32-1.2 g 21-23 280b,c Buccafusco et al. (1981)
(220-340)
Fathead minnow 30-35 days 25d,e 140d Wallbridge (1983)
(Pimephales promelas) (131-150)
Tidewater silversides 40-100 mm 20a 240 Dawson et al. (1977)
(Menida beryllina
Dab 15-20 cm - 61 Pearson & McConnell (1975)
(Pleuronectes limanda)
a Hardness 55 mg/litre (as CaCO3), pH 7.6-7.9.
b Tested in well-water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration at the beginning
9.7 to 0.3 mg/litre after 96-h exposure.
c Static system (nominal concentrations).
d Flow-through system.
e Hardness 42-45 mg/litre (as CaCO3) pH 6.7-7.6.
f 7-day LC50 (static system, renewal).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
The acute oral LD50 for the rat has been reported to be 1.9
(1.7-2.1) g/kg body weight and the dermal LD50 after a single skin
exposure of rabbits was 8.75 (8.3-9.2) ml/kg body weight (Smyth et
al., 1969; California State Water Resources Control Board, 1983).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
Groups of 5 male and 5 female B6C3F1 mice were
administered (by gavage) 0, 125, 250, 500, 1000, or 2000 mg 1,2-
dichloropropane (99.4%) per kg body weight, in corn oil. The doses
were given for 14 consecutive days (range-finding study) followed by
1 day of observation. All male mice in the 1000 and 2000 mg/kg
groups and female mice in the 2000 mg/kg group died. Also 3 out of 5
males receiving 500 mg/kg and 4 out of 5 females receiving 1000
mg/kg died. No growth inhibition was seen in the surviving animals
(NTP, 1986).
Groups of 10 male and 10 female B6C3F1 mice were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks, at doses of 0, 30, 60, 125, 250, or 500
mg/kg body weight. Mortality was not significantly increased, and
mean body weight changes were not dose-related. No compound-related
histopathological changes were found at the highest dose level (NTP,
1986).
8.2.1.2 Rat
Groups of 5 male and 5 female F344/N rats were administered
1,2-dichloropropane (99.4%) at 0, 125, 250, 500, 1000, and 2000
mg/kg body weight, in corn oil, by gavage, for 14 consecutive days
(range-finding study) followed by 1 day of observation. All rats
receiving the highest dose level died. Growth inhibition was seen in
the surviving animals in the 1000 mg/kg group. At necropsy, renal
medullae were red in the 2000 mg/kg group, not in lower dose levels
(NTP, 1986).
Groups of 10 male and 10 female F344/N rats were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks at doses of 0, 60, 125, 250, 500, or 1000
mg/kg body weight. All animals administered 1000 mg/kg and half of
the males administered 500 mg/kg died. Growth inhibition was seen in
the 500 mg/kg group. Centrilobular congestion of the liver occurred
in the 1000 mg/kg group and 2 females of this group showed hepatic
changes and centrilobular necrosis. Lower dose levels did not
produce effects (NTP, 1986).
A 2-week study of 1,2-dichloropropane (99.9%) was conducted
using groups of 10 Fischer 344 rats/sex to select doses for a
subsequent 13-week study. Dose levels were 0 (corn oil), 300, or 500
mg/kg body weight per day (by gavage) for 14 days. Data were
obtained on body weight, clinical effects, body temperature,
functional observational battery, motor activity, haematology,
liver, kidney, and spleen weights, gross pathology and histological
examination of the liver and kidneys. In both groups, transient
clinical effects (tearing, blinking, and lethargy) were seen, and
body weights were significantly decreased. The body temperatures of
treated animals in both groups, recorded 1 h after dosing on day 13,
were decreased by 0.3-0.5 °C. No effects on motor activity and
haematology were noted. Liver and kidney weights were increased and
spleen weight decreased. Histopathological changes (prominent
nucleoli of hepatocytes in the centrilobular region, degeneration
and necrosis of the liver cells) were found in the livers of animals
in both the treated groups (Gorzinski & Johnson, 1989).
In an oral toxicity study, adult male Sprague-Dawley rats were
administered 0, 100, 250, 500, or 1000 mg 1,2-dichloropropane/kg
body weight, in corn oil (by gavage), once a day, for up to 10
consecutive days. Some rats were killed at each dose level 24 h
after 1, 5, and 10 daily doses. After dosing, changes in body weight
and clinical toxic signs were assessed. In addition, measurements of
clinical chemistry parameters, urinary levels of glucose, alkaline
and acid phosphatase and N-acetyl glycosaminidase activities, and
the histopathology of major organs, liver enzyme levels, as well as
levels of non-protein sulfhydryls in the liver and kidneys were
determined. Definite central nervous system depression and body
weight loss, and increased renal nonprotein sulfhydryl (NPS) levels
were seen at 250 mg/kg. Liver damage (both morphological and enzyme
changes) was observed only at the 500 and 1000 mg/kg dose levels. In
this study, "resistance" to 1,2-dichloropropane hepatotoxicity
developed over the 10 consecutive days of exposure, as reflected by
progressively lower serum enzyme levels and by decreases in the
severity and incidence of toxic hepatitis and periportal
vacuolization. Nucleolar enlargement in hepatocytes, however, was
observed at all dose levels at both 5 and 10 days. There were a
number of manifestations of haemolytic anaemia in various tissues,
but no evidence of nephrotoxicity. On the basis of the parameters
examined, a NOEL of 100 mg/kg body weight can be established
(Bruckner et al., 1989).
In a similar oral study, male Sprague-Dawley adult rats were
treated with 0, 100, 250, 500, or 750 mg 1,2-dichloropropane/kg body
weight, in corn oil, by gavage, 5 times weekly for up to 13 weeks.
Over one-half of the animals in the 750 mg/kg group died within 10
days. Histopathological changes included mild hepatitis and splenic
haemosiderosis, adrenal medullary vacuolization and cortical
lipidosis, testicular degeneration and a reduction in sperm, and an
increased number of degenerated spermatogonia in the epididymis.
Similar testicular and epididymal degenerative changes were observed
in some animals in the 500 mg/kg group after 13 weeks of dosing.
While no deaths occurred in the 100 or 250 mg/kg groups, more than
50% in the 500 mg/kg group had died by 13 weeks. A dose-dependent
decrease in body weight gain was observed. 1,2-Dichloropropane
exhibited very limited hepatotoxic potential and no apparent
nephrotoxic potential in this 13-week study (Bruckner et al., 1989).
8.2.2 Inhalation
8.2.2.1 Mouse
B6C3F1 mice (aged 6-8 weeks) were exposed to target
concentrations of 1,2-dichloropropane (99.9%) of 0, 69.9, 233, or
699 mg/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. There were no
effects on haematology, gross pathology, and histopathology at
concentrations as high as 699 mg/m3, in this study (Nitschke et
al., 1988).
8.2.2.2 Rat
Fischer 344 rats (aged 6-8 weeks) were exposed to target
concentrations of 0, 69.9, 233, or 699 mg 1,2-dichloropropane
(99.9%)/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. The body weights
of rats exposed to 233 and 699 mg/m3 were significantly decreased.
Minimal effects were observed microscopically in the nasal tissues
of rats exposed to 233 and 699 mg/m3. A few rats exposed to 69.9
mg/m3 also had slight thickening of a small portion of the
respiratory nasal mucosa. No treatment-related effects were noted in
haematology, clinical chemistry, or urinalysis, even at 699 mg/m3
(Nitschke et al., 1988). A NOEL of 74.5 mg/m3 can be established.
8.2.2.3 Rabbit
New Zealand white rabbits (age 6-7 months) were exposed to 1,2-
dichloropropane at 0, 699, 2330, or 4660 mg/m3 for 6 h/day, 5
days/week, for 13 weeks. The mean actual concentrations were 0, 694,
2204, or 4436 mg/m3. Minimal effects on nasal tissues were present
in male rabbits only at the 4660 mg/m3 level. The primary effects,
decreased red blood cell parameters (red cell blood count,
haemoglobin, and packed cell volume), were observed in male rabbits
exposed to 699 (minimal effects) - 4660 mg/m3, and in female
rabbits exposed to 2330 and 4660 mg/m3 (Nitschke et al., 1988). No
NOEL has been established.
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
Groups of 30 male and 30 female Sprague-Dawley rats each were
provided with drinking-water containing 1,2-dichloropropane (99.9%)
at concentrations of 0, 0.24, 1, or 2.4 g/litre (w/v) (equivalent to
0, 33.6, 140, or 336 mg/kg body weight) over 2 generations. A
concentration of 2.4 g/litre represented the maximum practicable
attainable concentration, based on solubility. Adult rats were
evaluated for body weights, water and feed consumption, reproductive
performance, and gross pathological and histological changes. The
litters were evaluated for size, neonatal growth, and survival.
Decreases in water consumption reflective of rejection because of
unpalatability, were observed at all levels tested in both sexes in
the F0 and F1 generations. These decreases in water consumption
resulted in significantly lower body weights in both generations
administered 2.4 g/litre. These differences in water consumption and
body weights were also evident among the females during gestation
and/or lactation. The 0.24 g/litre dose level had a minor effect on
water consumption and body weights, but no adverse effects on the
animals. No treatment-related gross pathological changes were noted
in any dose group and the histological changes were limited to
increased hepatocellular granularity in both sexes in both
generations at all dose levels.
There were no histological changes in the reproductive tracts
of either sex in either generation. Reproductive function, measured
by fertility and litter size, was unaffected. The decreases in water
consumption among females at 2.4 g 1,2-dichloropropane/litre
resulted in significantly lower neonatal body weights and slightly
increased neonatal mortality in their litters. These neonatal
effects were considered secondary to the substantial decreases in
maternal water consumption, rather than a direct effect of the
substance. There were no neonatal effects at the 2 lower
concentrations. The NOAEL for adults is 0.24 g/litre, and the
reproductive NOAEL is 1 g/litre (equivalent to 140 mg/kg body
weight) (Kirk et al., 1990).
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
Groups of 30 Sprague-Dawley rats were administered 1,2-
dichloropropane (99.9%) in corn oil, by gavage, on gestation days 6-
15 at dose levels of 0, 10, 30, or 125 mg/kg body weight per day.
Parameters evaluated included maternal body weight and weight gain,
feed and water consumption, fetal weights, and fetal morphology. The
highest dose level produced transient decreases in respiration,
movement, muscle tone and extensor thrust reflex, and increases in
salivation and lacrimation, within 1 h of dosing on day 6 of
gestation. A decrease in the frequency of these clinical symptoms
occurred on the second day. In rats, 1,2-dichloropropane produced
transient central nervous system depression and decreased maternal
body weight gain and feed consumption at 125 mg/kg body weight.
Fetal effects in rats were limited to a significant increase in the
incidence of delayed ossification of the bones of the skull in the
125 mg/kg group, secondary to maternal effects. A level of 30 mg/kg
was considered the NOEL for maternal and fetal effects (Kirk et al.,
1989; Hanley et al., 1990).
8.3.2.2 Oral (rabbit)
Groups of 18 New Zealand white rabbits were administered 1,2-
dichloropropane (99.9%) at dose levels of 0, 15, 50, or 150 mg/kg
body weight per day on gestation days 7-19. On day 28 of gestation,
Caesarean section was performed and the fetuses evaluated.
Parameters evaluated included maternal body weight and weight gain,
haematology, fetal body weights, and fetal morphology. In maternal
rabbits, a dose of 150 mg/kg per day produced anorexia, significant
decreases in weight gain, and anaemia, with microscopic examination
revealing slight to moderate anisocytosis, poikilocytosis, and/or
polychromasia, indicative of a regenerative anaemia. The only fetal
effect in rabbits was a significant increase in the incidence of
delayed ossification of the bones of the skull at the highest dose
level, secondary to maternal effects. No effects were observed in
the 15 and 50 mg/kg dose groups. The NOEL for both maternal and
fetal effects was 50 mg/kg body weight (Hanley et al., 1989a).
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
1,2-Dichloropropane was tested for its mutagenic activity in
Salmonella typhimurium, Saccharomyces cerevisiae with, and without
metabolic activation, and in Aspergillus nidulans and
Streptomyces coelicolor. Results in most studies on S.
typhimurium TA100 and TA1535 were positive, but negative results
were obtained with TA98, TA1537, and TA1538, S. cerevisiae, and
S. coelicolor. In Aspergillus nidulans, a forward mutation to 8-
azaguanine resistance and methionine suppression was found with S9
activation.
The results are summarized in Table 20.
Table 20. Mutagenicity tests with 1,2-dichloropropane on microorganisms
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Salmonella typhimurium
TA100, TA1535 1,2-DCP 10-50 mg plate S9 mix/none + De Lorenzo et al.
TA1978 S9 mix/none - (1977)
TA100 1,2-DCP (65% 62.5-8000 suspension S9 mix + Priston et al.
TA1535 1,2-DCP + 25% µg/mld S9 mix/none + (1983)
1,3-DCPropene)
TA98, TA1537 S9 mix/none -
TA100 1,2-DCP 1, 10, plate S9 mix/none - Stolzenberg & Hine
100 µmola (1980)
TA100, TA1535 1,2-DCP 1-10 µlitre plate S9 mix/none + Carere & Morpurgo
(1981)
TA98, TA1537, TA1538 S9 mix/none -
TA100, TA1535 1,2-DCP 0.33-10 mg plate S9 mix/none + Haworth et al. (1983)
(rat/hamster)
TA98, TA1537 S9 mix/none -
(rat/hamster)
TA100, TA1535, TA1537 1,2-DCP (99%) 33-2000 µg plate S9 mix - NTP (1983)
S9 none (+)
TA98 S9 mix/none -
(rat/hamster)
Saccharomyces cerevisiae
JD1 1,2-DCP (65% 62.5-8000 - S9 mix - Priston et al.
1,2-DCP + 25% µg/mld S9 none (+) (1983)
1,3-DC propene)
Table 20 (contd)
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Streptomyces coelicolarb 1,2-DCP 2-100 µlitre plate none - Carere & Morpurgo
A3 (1981)
Aspergillus nidulansc 100-400 µlitre plate none + Carere & Morpurgo
(1981)
Aspergillus nidulans 1,2-DCP (99%) 154 mmol/litre - Crebelli et al.
(1984)
a The highest dose level, 100 µmol/plate gave complete inhibition of bacterial growth.
b Resistance to streptomycin.
c Resistance to 8-azaguanine.
d Concentrations of 1000 µg/ml and higher were cytotoxic.
Crebelli et al. (1984) studied the induction of somatic
segregation in Aspergillus nidulans. Induction of haploidization,
mitotic non-disjunction, and mitotic crossing-over was studied in
heterozygous colonies exposed to 1,2-dichloropropane, 99%. No
significant rise in frequency of any kind of segregated sectors was
found. The concentration that was used was 154 mmol/litre.
8.4.1.2 Mammalian cells
In cytogenetic studies using Chinese hamster ovary cells, 1,2-
dichloropropane (99.4%) caused both chromosome aberrations and
sister chromatid exchanges. Dose levels tested were 0.46-1.50 and
0.113-1.13 mg/ml, respectively (Galloway et al., 1987).
Priston et al. (1983) also studied the ability of 1,2-
dichloropropane (containing 65% 1,2-dichloropropane and 25% 1,3-
dichloropropane) to induce chromosome damage using rat liver (RL4)
cells, in concentrations of 5-20 µg/ml. An indication for a small
increase in the frequency of chromatid gaps, chromatid and
chromosome aberrations, was noticed, but only in the presence of
cytotoxic effects. The Task Group considered this study inadequate.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
test in vitro in Chinese hamster V79 cells to evaluate the effect
of 1,2-dichloropropane (99%). The concentrations tested were 0
(DMSO), 1.0, 3.3, and 10.0 mmol/litre without S9 mix. A dose-related
increase in SCEs was observed. With S9 mix and the same
concentrations, the SCEs frequency was increased but was less than
without S9 mix.
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
Woodruff et al. (1985) tested 1,2-dichloropropane at 0 and 4200
mg/litre by injection in germ cells and 0 and 33 552 mg/m3 by
inhalation in Drosophila melanogaster, using the sex-linked
recessive lethal mutation for their mutagenicity. The results by
injection and inhalation were negative.
8.4.2.2 Dominant lethal test
Groups of 30 male Sprague-Dawley rats were given 1,2-
dichloropropane (99.9%) in the drinking-water at concentrations of
0, 0.24, 1, or 2.4 g/litre, continuously, for a period of
approximately 14 weeks, as part of a combined reproduction/ dominant
lethal study (section 8.3.1). Exposed males (F0) were used for a
dominant lethal test following completion of the breeding for the
F1 litters of the reproduction study. They were mated with pairs
of naive, untreated adult females for 2 successive periods of 1 week
each. A separate, positive control group of 30 male rats was
administered a single oral dose of 100 mg cyclophosphamide/kg body
weight, 48 h prior to mating with untreated females. The uterine
contents of the females were evaluated for evidence of dominant
lethal effects as manifested by an increase in the resorption rate.
Among 1,2-dichloropropane-treated males, concentration-related
decreases in water consumption were noted at all levels and
decreased body weights were noted in males given 1 or 2.4 g/litre in
the water. Mating performance was unaffected in these animals.
Resorption rates among these groups revealed that the weekly values
for the females mated to treated males ranged from 2.2 to 8.1%, well
within the historical control range. Resorption rates among the
concurrent controls were low, ranging from 3.5 to 5.4%. The
significant increases from the concurrent control values, identified
during the first week of breeding in the resorption rates in the
groups given 0.24 or 2.4 g/litre, were considered to be within the
normal variation. Cyclophosphamide resulted in a 10-fold increase in
the resorption rate. 1,2-Dichloropropane was not mutagenic in a
dominant lethal assay in male Sprague-Dawley rats exposed
continuously to concentrations of up to 2.4 g/litre in the drinking-
water (Hanley et al., 1989b).
8.4.2.3 Miscellaneous
Perocco et al. (1983) studied the tritiated thymidine uptake
and cell viability in human lymphocyte cultures grown with, or
without, rat liver metabolizing system. Both the [3H]TdR uptake
and the percentage of viable cells showed values very similar to
control values. The 1,2-dichloropropane concentrations used ranged
from 10-2 to 10-4 mmol/litre.
8.5 Carcinogenicity
8.5.1 Oral (mouse)
Groups of 7 to 9-week-old hybrid B6C3F1 mice (50 males
and 50 females) were administered 0, 125, or 250 mg 1,2-
dichloropropane (99.4%)/kg body weight, in corn oil, by gavage, 5
days/week for 113 weeks. No influence on growth was observed. The
survival of the female animals at the highest dose level was
significantly decreased. The incidence of non-neoplastic lesions
showed lesions of the spleen (haemosiderosis and haematopoiesis were
increased) in female mice at the highest dose level.
The incidences of neoplastic lesions are summarized in Table
21.
Table 21. The incidence of neoplastic lesions in a mouse study with 1,2-dichloropropanea
Sex Control 125 mg/kg 250 mg/kg
Adenoma (liver) male 7/35 (20%) 9/33 (27%) 15/35 (43%)
Carcinoma (liver) male 8/35 (23%) 10/33 (30%) 9/35 (26%)
Adenoma and carcinoma (liver) male 15/35 (43%) 18/33 (55%) 24/35 (69%)b
Adenoma (liver) female 1/35 (3%) 5/29 (17%) 5/26 (19%)b
Carcinoma (liver) female 1/35 (3%) 2/29 (7%) 2/26 (8%)
Adenoma and carcinoma (liver) female 2/35 (6%) 7/29 (24%)b 7/26 (27%)b
Follicular cell adenoma and female 1/34 (3%) 0/27 (0%) 5/24 (21%)b
carcinoma (thyroid)
Alveolar/bronchiolar adenoma female 5/35 (14%) 0/29 (0%) 1/26 (4%)
or carcinoma (lung)
Squamous cell male 0/50 (0%) 1/48 (2%) 3/49 (6%)
papillomas (forestomach) female 0/50 (0%) 2/50 (4%) 2/50 (4%)c
a From: NTP (1986).
b P = < 0.05 - > 0.001.
c One high-dose female mouse had a squamous cell carcinoma.
On the basis of the results of this study, the NTP concluded
that 1,2-dichloropropane induces an increased incidence of liver
tumours in male and female B6C3F1 mice (Haseman et al., 1984;
NTP, 1986). However, the Task Group noted that the incidences of
liver adenomas and carcinomas in the treated groups were within the
historical ranges of this species (Haseman et al., 1985). The
increased incidence of thyroid tumours is equivocal.
8.5.2 Oral (rat)
Groups of 50 male and 50 female F344/N rats (aged 7-9 weeks)
were administered, by gavage, 0, 125 or 250 (female) and 0, 62, or
125 mg (male) 1,2-dichloropropane (99.4%)/kg body weight, in corn
oil, 5 days/week for 103 weeks. The highest dose level showed growth
depression in both sexes. Survival of female rats at the high dose
level was significantly lower. The incidence of non-neoplastic
lesions was not significantly different from that in the controls,
except for an increased incidence of foci of clear cell change and
of necrosis in the liver in high-dose female rats.
The incidences of neoplastic lesions are summarized in Table
22. Apart from these tumours, squamous cell papillomas of the
forestomach were found in 1 control male and 1 female rat. In high-
dose females, there was an increased incidence of mammary gland
adenocarcinomas, but not of mammary gland adenomas. Apart from these
tumours, squamous cell papillomas of the fore-stomach were found in
two high-dose females (not significantly increased compared to
controls). There were no effects on tumour incidences in male rats
(Haseman et al., 1984; NTP, 1986).
8.6 Factors modifying toxicity
Weanling Wistar rats, fed for several weeks on low protein
choline-deficient diets, were more susceptible to the effects of
inhalation of 4660 mg 1,2-dichloropropane/m3 than rats on a
control diet. This increased susceptibility could be corrected by
dietary supplements of 1-methionine or 1-cysteine plus choline
chloride (Heppel et al., 1946).
8.7 Special studies
8.7.1 Liver
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight, in corn oil, for 5 days (once daily) or for 4 weeks (five
days/week), or a single dose of 0, 50, 100, or 250 mg/kg body
weight, to investigate the biochemical and histological liver
changes. Reduced glutathione (GSH), glutathione- S-transferase,
cytochrome P450, and protein contents were measured. A dose-
Table 22. The incidence of neoplastic lesions in a rat study with 1,2-dichloropropanea
Sex Control 62 mg/kg male 125 mg/kg male
125 mg/kg female 250 mg/kg female
Fibroadenoma female 14/37 (38%) 20/43 (47%) 5/16 (31%)
(mammary gland)
Adenocarcinoma female 1/37 (3%) 2/43 (5%) 4/16 (25%)b
(mammary gland)
Endometrial stromal female 8/37 (22%) 14/42 (33%) 6/16 (38%)b
polyps (uterine
tumours)
Liver tumours male 3/50 (6%) 3/50 (6%) 2/50 (4%)
female 0/50 (0%) 0/50 (0%) 0/50 (0%)
Islet cell adenoma male 4/38 (11%) 1/42 (2%) 5/41 (12%)
and carcinoma
(pancreas)
a From: NTP (1986).
b P = < 0.05.
dependent decrease in liver-reduced glutathione was observed after a
single injection and a dose-dependent increase after 4 weeks. The
liver biochemical pattern after 4 weeks, characterized by a decrease
of cytochrome P450 and by an increase in reduced glutathione and
glutathione- S-transferase activity, suggests a hyperplastic
evolution of the liver cells, probably a repair mechanism induced by
the early depletion of glutathione. Histologically, the alterations
confirm the regenerative nature (atypical mitosis and hyperplastic
nodules) of the changes (Trevisan et al., 1989).
The hepatotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose-levels of 0, 50, 100, 250, or 500 mg/kg body weight per day,
in corn oil, for 4 weeks. Biochemical changes in the liver were
demonstrated. Significant findings included reduction of aminopyrine
demethylase activity at 100 mg/kg or more, increased levels of
reduced glutathione and glutathione- S-transferase activity at 50
mg/kg or more, and decreased cytochrome P450 activity at 500 mg/kg.
The activity of aniline hydroxylase was not affected. Duplicate
groups of rats treated with 1,2-dichloropropane, but allowed a
period of 4 weeks of recovery before being subjected to examination,
showed that the induced biochemical changes in the liver were
completely reversible (Trevisan et al., 1991).
1,2-Dichloropropane toxicity is actually preferentially
mediated by GSH depletion. This is suggested by the fact that GSH
loss is correlated with an increase in the biochemical indices of
liver and renal injury, and with the extent of haemolysis.
Pretreatment of 1,2-dichloropropane-intoxicated rats with
buthionine-sulfoximine (BSO) (depleting GSH) markedly increased the
overall mortality. Furthermore, the administration of the GSH
precursor N-acetyl-cysteine (NAC), preventing GSH depletion,
reduced the damage in target tissues, as demonstrated by a smaller
increase in some biochemical indices of cell injury and a smaller
degree of haemolysis. A possible explanation for these findings is
that, when the GSH level falls below a certain threshold value,
irrespective of the causative agent, a series of common reactions is
triggered, inducing peroxidation of membrane lipids, disturbances of
Ca2 homeostasis, and DNA damage, which quickly lead to
irreversible liver injury. Furthermore, it is also possible that
electrophilic metabolites of 1,2-dichloropropane, formed in the
absence of GSH, could directly attack a variety of cell
macromolecules the function of which is essentially to ensure the
physiological survival of the hepatocytes (Mitchell et al., 1973;
Bellomo & Orrenius, 1985; Casini et al., 1987; Orrenius et al.,
1989).
Male Wistar rats were exposed (by gavage) to a single oral dose
of 55 mg 1,2-dichloropropane/kg body weight in propylene glycol. The
animals were killed 1-6 days after exposure. Glutathione (GSH),
lipid peroxidation and protein of liver homogenate were measured.
Reduction of hepatic GSH and total protein and enhanced hepatic
lipid peroxidation still persisted 6 days after 1,2-dichloropropane
administration (Di Nucci et al., 1988).
Groups of rats were treated orally, by gavage, with single
doses of 1,2-dichloropropane ranging from 55 to 400 mg/kg body
weight. 1,2-Dichloropropane was no longer detectable in the blood 24
h after dosing at any dose level (Di Nucci et al., 1988).
8.7.2 Kidneys
Renal failure caused by 1,2-dichloropropane has been reported
by several authors who found an increase in serum creatinine and
urea in intoxicated animals and humans and fatty degeneration or
acute tubular damage in the kidney parenchyma (Heppel et al., 1946,
Ponticelli et al., 1968; Pozzi et al., 1985; Imberti et al., 1987).
Imberti et al. (1990) suggested that, on the basis of the
demonstration of 1,2-dichloropropane-induced depletion of kidney
GSH, it may be postulated that the biochemical mechanisms involved
in liver toxicity may also apply to the kidneys (Brezis et al.,
1983). In addition, since most of the glutathione and cysteine
conjugates of 1,2-dichloropropane are recovered in the urine, a
direct toxicity of the conjugates to the kidneys seems to play an
important role, as previously demonstrated for other conjugates
(Stevens et al., 1988).
It has been demonstrated that GSH plays an essential role in
the maintenance of the physiological structure of the erythrocyte,
preventing the formation of inter-protein or intra-protein
disulfides within the membrane skeleton. Depletion of erythrocyte
glutathione causes an increased fragility of this cell and
subsequent haemolysis. The involvement of such a mechanism in 1,2-
dichloropropane-induced haemolysis in intoxicated animals is
supported by the demonstration of a concomitant loss in erythrocyte
GSH and a correlation between these 2 phenomena (Imberti et al.,
1990).
Nephrotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose levels of 0, 50, 100, 250, or 500 mg/kg body weight per day
for 4 weeks. Biochemical and histopathological alterations of the
kidneys were demonstrated. Kidney pathology involved a decrease in
the activity of angiotensin-converting enzyme in the proximal tubule
brush border and fraying of microvilli with epithelial coagulative
necrosis. Duplicate groups of rats treated similarly with 1,2-
dichloropropane, but allowed a period of 4 weeks of recovery before
being subjected to examination, showed that the treatment-induced
biochemical changes in the kidneys were completely reversible
(Trevisan et al., 1988, 1991).
8.7.3 Central nervous system
In the study of Gorzinski & Johnson (1989), in which Fischer
344 rats were treated with 0, 300, or 500 mg 1,2-dichloropropane per
kg body weight, by gavage, for 14 days, motor activity and a
functional observational battery (unusual responses in behaviour,
presence of convulsions, tremors, etc., and sensory function) were
evaluated (section 8.2.1.2). No effects were seen on these
parameters.
Specific tests in groups of 15 Fischer 344 rats/sex given, by
gavage, 0 (corn oil), 20, 65, or 200 mg 1,2-dichloropropane
(99.9%)/kg body weight, 5 days/week for 13 weeks, included monthly
evaluation of a functional observation battery, hind limb grip
strength, and motor activity; a comprehensive neuropathology study
was carried out at the end of the study. Clinical observations
included measurement of body weight gain and body temperature. Body
weights were decreased for male rats given 200 mg/kg body weight.
The body temperature was within normal limits, except in the females
given 200 mg/kg, which showed a slightly lower temperature. No
functional effects were noted. No gross or histopathological effects
on either the central or peripheral nervous system were observed
(Johnson & Gorzinski, 1988).
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
Ingestion of cleaning solvent (50 ml) containing 1,2-
dichloropropane (other components not known) by a man produced coma
followed by delirium, irreversible shock, cardiac failure, and
death. Histopathologically, centri- and medio-lobular hepatic
necrosis was found (Larcan et al., 1977).
Two cases of disseminated intravascular coagulation syndrome
(DIC) have been described in association with acute intoxication by
1,2-dichloropropane. Effects on the central nervous system, liver,
and kidney functions were also observed, but no details were given
(Perbellini et al., 1985).
Pozzi et al. (1985), from Italy, reported clinical observations
on 3 other hospitalized persons with 1,2-dichloropropane poisoning.
Two out of the 3 persons ingested the substance, while the third
person was exposed through inhalation. The clinical symptoms were
similar, despite different routes of exposure. All 3 suffered from
acute renal and hepatic damage. Kidney biopsy, carried out on 1
person, showed acute tubular necrosis. Haemolytic anaemia and
disseminated intravascular coagulation were noted in all 3 patients,
1 of whom died on the seventh day. The other patients recovered
after treatment.
Toxic hepatitis with portal hypertension has been described in
a 49-year-old man, who ingested 1,2-dichloropropane in an attempted
suicide (Thorel et al., 1986).
9.2 Occupational exposure
Baruffini et al. (1989) studied 10 cases of 1,2-dichloropropane
dermatitis in the period 1985-88. Patients were painters or metal-
workers in the engineering industry and they all had known contact
with mixtures of solvents containing 10-40% 1,2-dichloropropane. On
examination, the workers had itchy erythematous, oedematous,
vesicular lesions on the fingers and dorsa of the hands. Two
subjects also had scaling and fissuring of the palms. Cessation of
exposure produced quick resolution of the dermatitis in all
subjects. Patch tests were carried out. As controls, 120 subjects
were similarly tested with 1,2-dichloropropane. All patients showed
a positive response to concentrations of 2% 1,2-dichloropropane or
more (allergic contact dermatitis). The results of the tests on the
control subjects were negative.
Grzywa & Rudzki (1981) described cases of dermatitis in 2 women
(47 and 55 years old), in a group of 60 persons, who had been
exposed to Silform aerosols for 6 and 4 years, respectively. Silform
aerosols contained 7.4, 11.0, or 12.7% 1,2-dichloropropane;
methylsilicone oils, and Freon. The patch tests that were carried
out were positive for 1,2-dichloropropane, but negative for
methylsilicone oils.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloropropane was considered by working groups of the
International Agency for Research on Cancer (IARC) in 1986 (IARC,
1986) and in 1987 (IARC, 1987). In the updating of 1987, it was
evaluated as follows: "There is limited evidence for the
carcinogenicity of 1,2-dichloropropane in experimental animals.
There are no data in humans. The agent is not classifiable as to its
carcinogenicity to humans (Group 3)".
WHO (in preparation) has proposed a provisional guideline value
for drinking-water of 20 µg 1,2-dichloropropane/litre.
PART C
ENVIRONMENTAL HEALTH CRITERIA
FOR
MIXTURES OF DICHLOROPROPENES
AND DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MIXTURES OF DICHLOROPROPENES AND
DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on humans
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
4.2 Water
4.3 Soil
4.3.1 Microbial transformation
4.3.2 Loss under field conditions
4.3.3 Soil function
4.4 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Groundwater
5.2 Occupational exposure
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Terrestrial microorganisms
7.1.1.1 Effects on nitrification
7.1.1.2 Recovery studies with microorganisms
7.2 Aquatic organisms
7.2.1 Invertebrates
7.2.2 Fish
7.3 Terrestrial organisms
7.3.1 Birds
7.3.2 Soil fauna
7.3.3 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.2 Inhalation
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
8.3.2 Sensitization
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
8.5.1.2 Inhalation
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
8.6.2 In vivo studies
9. EFFECTS ON HUMANS
9.1 General population exposure
9.2 Occupational exposure
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
The technical mixture of dichloropropenes and dichloropropane
(abbreviated in this text to "MIX D/D") is a clear amber liquid with
a pungent odour; it has a vapour pressure of 35 mmHg at 20 °C, and
is soluble in halogenated solvents, esters, and ketones.
"MIX D/D" typically contains not less than 50% 1,3-
dichloropropene (ratio of cis- and trans-isomers approximately
1:1), the other main constituents being 1,2-dichloropropane and
related compounds. It was widely used as a soil nematocide before
planting.
The environmental transport, distribution, and fate of the
major constituents of "MIX D/D" in air, water, and soil is described
in the sections 4 of the parts of this EHC monograph that deal with
1,3-dichloropropene and 1,2-dichloropropane.
There is a significant potential for 1,2-dichloropropane
derived from "MIX D/D" to leach from the soil and contaminate well
water and groundwater. In an irrigation bore (68 m deep) in Western
Europe, mean 1,2-dichloropropane concentrations at different depths
ranged between 0.8 and 8.5 µg/litre, and the maximum concentration
recorded was 165 µg/litre.
Significant uptake of the constituents of "MIX D/D" by crops is
unlikely (see other parts of this EHC monograph). Bioaccumulation of
the constituents of "MIX D/D" is also unlikely because of their low
log P octanol/water partition coefficient and their relatively high
water solubility.
1.1.2 Kinetics and metabolism
No metabolic studies have been carried out on "MIX D/D". The
two major components, 1,3-dichloropropene and 1,2-dichloropropane,
are rapidly eliminated, primarily in the urine and, to a lesser
extent, via expired air. The components of "MIX D/D" are metabolized
by oxidative and conjunction pathways. The major urinary metabolites
are mercapturic acids.
1.1.3 Effects on organisms in the environment
"MIX D/D" is moderately toxic for fish; 96-h LC50 values
range between 1 and 6 mg/litre. The 1,3-dichloropropene is largely
responsible for the toxicity of "MIX D/D".
When used at recommended application rates, the main effects of
"MIX D/D" are a transient (< 7 days) reduction in soil fungi and
inhibition of the oxidation of ammonium ions to nitrate. "MIX D/D"
is toxic for nitrifying bacteria. Soon after "MIX D/D" disappears
from the soil, recolonization by bacteria takes place. In field
trials, "MIX D/D" (applied at 600 litre/ha) killed soil
invertebrates. Recolonization times ranged between 6 and 24 months.
"MIX D/D" is highly phytotoxic.
1.1.4 Effects on experimental animals and in vitro test
systems
The acute toxicity of "MIX D/D" for laboratory animals is
moderate to high. The oral LD50 values in rats and mice range from
132 to 300 mg/kg body weight. The dermal LD50 values for rats and
rabbits are 779 and 2100 mg/kg body weight, respectively. The
inhalation LC50 (4 h) for rats is approximately 4540 mg/m3.
Acute exposure results in clinical signs associated with central
nervous system depression. "MIX D/D" is a severe eye and skin
irritant and it is a moderate dermal sensitizer.
The results of the available short-term toxicity studies in
rats and dogs are inadequate to assess properly the toxicity
potential of "MIX D/D", because the relatively low doses tested do
not demonstrate any biologically significant effects. Several short-
term inhalation (whole-body) studies have been conducted in rats.
"MIX D/D" at levels up to 145 mg/m3 does not cause any toxic
effects. At levels of 1362 mg/m3 or higher, toxic effects
associated with central nervous system depression are evident. An
exposure to 443 mg/m3 for 10 weeks leads to reduced body weight
gain and increased absolute kidney weight.
An oral teratology study in rats was inadequate for assessment
of the teratological potential of "MIX D/D".
In an inhalation rat study to investigate male and female
fertility, no effects were found at dose levels up to 443 mg/m3
for 10 weeks. Complete evaluation of reproductive effects of "MIX
D/D" was not possible owing to inadequate protocol designs.
"MIX D/D" is mutagenic in Salmonella typhimurium strains
TA100 and TA1535, as well as in Escherichia coli WP2 HCR, without
metabolic activation. There was no such effect in Salmonella
strains TA98, TA1537, and TA1538.
In a long-term study on rats fed diets containing up to 120 mg
"MIX D/D" per kg (equivalent to 6 mg/kg body weight) for 2 years, no
toxic or carcinogenic effects were seen.
1.1.5 Effects on humans
"MIX D/D" is no longer widely used, and, thus, exposure of the
general population via air, water, and food is unlikely.
The exposure of workers filling drums and of field applicators
was generally below 4.5 mg 1,3-dichloropropene/m3 when recommended
procedures were used; in other situations, levels up to 36.32
mg/m3 have been measured.
One case of acute fatal poisoning has been reported following
accidental ingestion of "MIX D/D".
Several cases of contact dermatitis and skin sensitization due
to "MIX D/D" exposure have been reported.
1.2 Conclusions
- General population. As "MIX D/D" is no longer widely used,
the exposure of the general population to 1,3-dichloropropene
via air, water, and food is negligible, but, in certain areas,
exposure to 1,2-dichloropropane may occur when groundwater is
contaminated.
- Occupational exposure. Filling operations and field
applications of "MIX D/D" can lead to exposure of operators to
1,3-dichloropropene that exceed maximum allowable
concentrations, especially under warm climatic conditions.
- Environment. "MIX D/D" is unlikely to reach biologically
significant levels in either the terrestrial or the aquatic
environment when used at the recommended rate. Lasting adverse
effects on organisms in the environment are unlikely to occur.
1.3 Recommendations
- "MIX D/D" should not be used as a soil fumigant because of
potential leaching to groundwater.
- Monitoring of residues in surface water and groundwater should
be carried out in areas where "MIX D/D" has been used.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
The technical mixture of dichloropropenes and dichloropropane
(abbreviated to "MIX D/D") contains not less than 50% of the cis-
and trans-isomers (ratio approximately 1:1) of 1,3-dichloropropene
and the other main constituent was 1,2-dichloropropane. Yang (1986)
described a commercial "MIX D/D" containing 25% cis-
dichloropropene, 27% of trans-dichloropropene, 29% 1,2-
dichloropropane, and 19% other related chlorinated hydrocarbons. It
may also contain 1% epichlorohydrin as a stabilizer.
CAS registry number: 8003-19-8.
For the physical and chemical properties of the main
constituents, see the sections 2.2 of the parts of this monograph
dealing with 1,3-dichloropropene and 1,2-dichloropropane.
Major trade names are D-D mixture, Nemafene, Nemax, Vidden-D.
Other formulations on the market are Ditrapex and Vortex
(mixtures of 1,2-dichloropropane, 1,3-dichloropropene, and
methylisothiocyanate), Ditrapex CP (the same mixture as Ditrapex,
but also containing chloropicrin).
2.2 Physical and chemical properties
Technical "MIX D/D" is a clear amber liquid with a pungent
odour. It has a vapour pressure of 4.6 kPa (20 °C) (35 mmHg at 20
°C), flash distils over the range of 59-115 °C, and has a relative
density of 1.17-1.22 g/cm3 at 20 °C; its flash point is 17.5 °C.
Its solubility at room temperature is approximately 2 g/kg in water,
but it is soluble at room temperature in hydrocarbon and halogenated
solvents, esters, and ketones. The mixture is stable up to 500 °C
(therefore stabilizers are not needed) but reacts with dilute
organic bases, concentrated acids, halogens, and some metal salts.
It is corrosive to some metals (e.g., aluminium, magnesium, and
their alloys, and may remove lacquer from lacquer-lined containers.
It is not corrosive to mild steel.
2.3 Analytical methods
The same methods can be used as for 1,3-dichloropropene.
See section 2.4 of the part of this monograph that deals with
1,3-dichloropropene.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
"MIX D/D" does not occur naturally.
3.2 Man-made sources
"MIX D/D" is manufactured by high temperature chlorination of
propene.
For sources of pollution, see section 3.2.3 of the part of this
monograph that deals with 1,3-dichloropropene and section 3.2 of
1,2-dichloropropane.
3.3 Uses
"MIX D/D" is a preplant nematocide, effective against soil
nematodes including root knot, meadow, sting and dagger, spiral, and
sugar beet nematodes. It is usually applied by injection into the
soil or through tractor-drawn hollow tines, to a depth of 15-20 cm
at a rate of 150-400 litre/ha (occasionally to a maximum of 1000
litre/ha), depending on soil type and the following crop. The soil
surface is sealed by rolling. "MIX D/D" volatilizes and diffuses as
a vapour and, thus, its effectiveness depends on how readily this
can occur. Because the components of "MIX D/D" are highly
phytotoxic, it is essential that, after an application of 220
litre/ha or more, a period of not less than 14 days should elapse
before planting or sowing (Shell, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.2 Water
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.3 Soil
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.3.1 Microbial transformation
It has been demonstrated under in vitro and in vivo
conditions that indigenous soil microflora can utilize C3
chlorinated hydrocarbons. Four species, Bacillus subtilis,
Arthrobacter globiformis, Pseudomonas fluorescens, and Rhizobium
leguminosarum, were successfully grown on media including "MIX
D/D" (Altman & Lawlor, 1966; Altman, 1969).
Toxicity tests were carried out with Rhizobium phaseoli and
Azotobacter beinjerinckii, both nitrogen-fixing bacteria, in
cultures using unsterilized Hanford sandy loam. "MIX D/D" at
concentrations ranging from 10 to 100 mg/kg and 100 to 1000 mg/kg
caused growth inhibition in Azotobacter beinjerinckii and
Rhizobium phaseoli, respectively (Rader & Love, 1977a).
4.3.2 Loss under field conditions
A trial has been carried out in the United Kingdom in which
"MIX D/D" was applied at 410 litre/ha (see section 4.1.3.5 of 1,3-
dichloropropene). Samples of soil were taken at 3 depths: 0-20 cm,
20-40 cm, and 40-60 cm, at 6 intervals up to 9´ months after
application. The results are summarized in Table 23. Residues of
1,3-dichloropropenes, 1,2-dichloropropane, and 3-chloroallyl
alcohols were present in all 3 soil layers, especially before
ploughing. They showed little change in the period before ploughing,
but, thereafter, the concentrations decreased gradually (Wallace,
1979).
4.3.3 Soil function
"MIX D/D" soil fumigant, 337 and 3370 kg active ingredient per
ha, was used to evaluate the effect on nodulation of pinto bean
plants in soil and on the growth of root nodule bacteria in culture.
Unsterilized Hanford sandy loam was used. After 4 weeks' growing
time, low and high doses of "MIX D/D" resulted in a reduction in
Table 23. Residues from the plot treated with "Mix D/D" at 410 litre/ha
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl
application (cm) alcohols (days)
cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 6.82 6.05 8.7 2.02 1.98
20-40 6.83 7.08 9.8 1.36 1.35
40-60 0.85 0.90 1.4 0.24 0.24
11 0-20 3.50 3.50 5.4 0.63 0.53
20-40 4.84 5.05 6.5 1.86 2.11
40-60 0.35 0.35 0.9 0.18 0.17
24 0-20 5.73 5.55 9.4 1.0 1.0
20-40 6.07 6.21 12.5 2.0 2.0
40-60 0.70 0.63 2.1 0.29 0.29
33 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.73 0.86 0.77 0.58 0.44
20-40 1.54 1.79 1.90 0.41 0.37
40-60 0.30 0.30 1.00 0.14 0.15
73 0-20 0.26 0.25 0.2 0.16 0.11
20-40 0.64 0.62 1.5 0.36 0.28
40-60 0.51 0.44 2.9 0.22 0.19
Table 23 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl
application (cm) alcohols (days)
cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.06 0.05 0.1 0.16 0.08
9´ months 20-40 0.03a 0.02a 0.2 0.07 0.03
40-60 < 0.01 < 0.01 0.2 < 0.02 < 0.02
Pre- 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
treatment 20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a Results confirmed by GC/MS.
From: Wallace (1979).
Note: All residues are on a dry weight basis.
root nodules of approximately 70 and 80%, respectively; the
percentages of germination were about 90 and 50%, respectively
(Rader & Love, 1977a).
Unsterilized Oakdale loamy sand soil (81.6% sand, 11.2% silt
and 1.06% organic carbon), treated with "MIX D/D" soil fumigant at
337 and 1348 kg active ingredient/ha, in 1978, showed no decrease in
soil phosphatase activity after 0, 4, and 8 weeks at an incubation
temperature of 27 °C (Rader, 1979c).
The effects were studied of "MIX D/D" soil fumigant (337 and
3370 kg/ha) on proteolytic enzyme activity in the treated soil. The
soil proteases were assayed by the release of tyrosine from sodium
caseinate in the treated soil, after incubation periods of 0, 4, 8,
and 12 weeks, at 27 °C. No inhibitory effects were found (Rader,
1979b).
"MIX D/D", at a high dose of 100-1000 mg/kg, caused a temporary
loss of cellulytic activity in Trichoderma viride (Rader & Love,
1977b).
Laboratory studies were conducted to determine the effects of
"MIX D/D" on the activities of invertase in a sandy soil. The rates
of application were 150 and 300 mg/kg. No inhibition was found. The
same dose levels were used to study the influence of "MIX D/D" on
amylase activity in this soil. After 3 days, stimulation of glucose
formation from the added starch was observed, especially at the
lowest dose level. Microbial respiration was also studied. The
treatments did not significantly decrease oxygen consumption (Tu,
1988).
4.4 Bioconcentration
No data on bioconcentration are available.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Analyses have been made to determine whether residues of the
active 1,3-dichloropropene isomers could be found in the edible
parts of a number of crops, including potatoes, carrots, onions,
cucurbits, rice, and sugar beet. At recommended rates and pre-
planting intervals, residues of these propenes ( cis- and trans-
isomers) have not been detected (limit of determination 0.02 mg/kg)
(see section 2.4 of 1,3-dichloropropene).
5.1 Groundwater
1,2-Dichloropropane levels of 0.8-8.5 µg/litre, with a maximum
of 165 µg/litre, were reported in groundwater in the Netherlands
from bores for irrigation at depths up to 68 m. These levels
resulted from previous applications of "MIX D/D" (Leistra & Boesten,
1989) (see section 5.1.2 of 1,2-dichloropropane).
5.2 Occupational exposure
Field studies were carried out in several locations in the
Netherlands and in France to monitor personal exposure and
environmental concentrations during application of "MIX D/D". In
most of the 11 locations in the Netherlands, the time-weighted
average of the exposure of the operators, as measured with personal
sampler pumps during application, was of the order of 4.54 mg total
cis- and trans-1,3-dichloropropene per m3. When filling
operations are carried out in the recommended way, personal
exposures can be limited to a maximum of 4.54 mg/m3. Levels of the
unsaturated components of "MIX D/D" in the air 1 m above the soil
surface shortly after application were below 0.454 mg/m3.
In most of the 9 locations in France, the time-weighted average
exposure levels of the operators, as measured with personal sampler
pumps, was of the order of 4.54-9.08 mg total cis- and trans-
1,3-dichloropropene per m3. Peak exposures of up to 36.32 mg/m3
were measured when the recommended safety precautions were
insufficiently observed. Levels of the unsaturated components of
"MIX D/D" in the air 1 m above the soil surface during application
ranged from 1 to 6.4 mg/m3 (van Sittert et al., 1977; van Sittert,
1978).
6. KINETICS AND METABOLISM
See section 6 in the parts of this monograph that deal with
both 1,3-dichloropropene and 1,2-dichloropropane.
No information is available on the kinetics and metabolism of
other compounds, impurities, and stabilizers that may be present in
"MIX D/D".
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
(See also relevant sections of 1,3-dichloropropene and 1,2-
dichloropropane.)
7.1.1 Terrestrial microorganisms
The effects of "MIX D/D" on microorganisms and on soil function
have been widely studied in Europe (Pochon et al., 1951; Bakker,
1968; Sommer, 1970; Kämpfe, 1973; Lebbink & Kolenbrander, 1974), in
Canada (Wensley, 1953; Elliot et al., 1972, 1974, 1977; Tu, 1972,
1973, 1978, 1979, 1981a,b), in the USA (Thornton, 1951; Moje et al.,
1957), and elsewhere (Dommergues, 1959; Mehta et al., 1963; Dubey et
al., 1975; Ross & McNeilly, 1975). Main interest has been in the
microorganisms involved in the nitrogen balance of the soil, as this
bears strongly upon the yields of crops grown in treated soil.
During the search for effective soil fumigants, the toxicity of
"MIX D/D" for soil microorganisms has been investigated on 2 soil
types, a sandy loam and a soil with high organic matter. At a dosage
of 1200 mg/kg soil, the following reductions in soil microorganisms
were found after 96 h: bacteria 90%, actinomycetes 99%, and fungi
98%. The toxicity of the major components of "MIX D/D" for these
same major groups of microorganisms has been studied in soil samples
from old citrus plantations under laboratory conditions, and it was
found that the toxicity of 1,2-dichloropropane for fungi and
bacteria and actinomycetes was low to moderate (Moje et al., 1957).
The studies of Wensley (1953) and of Moje et al. (1957) are
reasonably consistent and indicate that bacteria are more tolerant
to "MIX D/D" than fungi, and, again, that the toxicity of "MIX D/D"
is related mainly to its 1,3-dichloropropene content, in particular
the cis-isomer.
Rader et al. (1978) studied the correlation between the numbers
of soil microorganisms and the O2/CO2 exchange in treated soil.
Unsterilized Hanford sandy loam (57.6% sand, 26.6% silt, 15.8% clay,
and 0.7% organic carbon) was used and the soil had a moisture
content of 6%. "MIX D/D" soil fumigant (dosages 336 and 2240 kg
active ingredient/ha) caused a decrease in the populations of
actinomycetes, bacteria, and fungi, and also reduced the oxygen
utilization by soil microorganisms.
7.1.1.1 Effects on nitrification
Kämpfe (1973) studied the inhibition by "MIX D/D" of
nitrification in a black earth soil. Nitrogen (180 mg/kg, NH3 and
NH3-water) and "MIX D/D" (80 mg/kg) were applied at different
temperatures. The time of incubation was 30-120 days. At
temperatures between 5 °C and 10 °C, nitrification of NH3 was
severely inhibited for 90 days. After 120 days of incubation at 10
°C, 70% of the nitrogen applied was retrieved as ammoniumion.
The inhibition of ammonium nitrogen oxidation has led to the
build-up of ammonium nitrogen in treated soil under both laboratory
(Sommer, 1970) and field conditions (Thornton, 1951; Mehta et al.,
1963; Elliot et al., 1974; Ross & McNeilly, 1975).
"MIX D/D" was extremely toxic to nitrifying bacteria in silty
clay loam or loam soils. Doses of 200, 2000, and 20 000 mg "MIX
D/D"/kg soil were tested. Inhibition of NH4+-oxidizing bacteria
was found, but not of Nitrobacter spp. Nitrogen mineralization was
progressively depressed with increasing levels of "MIX D/D". At 200
mg "MIX D/D"/kg soil, nitrate formation from ammonium nitrogen was
very markedly reduced, while mineralization of nitrogen was only
slightly reduced at 20 000 mg/kg soil (Dubey et al., 1975). These
results are in agreement with the results of Bromley & Cook (1981).
Bromley & Cook (1981) also studied the influence of "MIX D/D"
on the nitrification processes in soil. Transient inhibition of
nitrification (< 20 days) occurred in sandy clay treated with 200
or 1000 mg "MIX D/D"/kg. Considerable (80% reduction compared with
controls) and total inhibition occurred in soil treated with 2000
and 10 000 mg "MIX D/D"/kg, respectively. There was no difference
between the inhibitory effects of "MIX D/D" and those of a purified
dichloropropene isomer mixture, indicating that the dichloropropenes
were the active inhibitors in "MIX D/D". During inhibition of
nitrification by "MIX D/D", ammonium accumulated but no significant
nitrite accumulation was observed. This indicates that
ammonification was unaffected and that inhibition of nitrification
resulted from the specific inhibition of Nitrosomonas spp.
Overall, it was concluded that "MIX D/D" was not very active as a
nitrification inhibitor, compared with commercially available
inhibitors.
Unsterilized Handford sandy loam was fumigated with "MIX D/D"
soil fumigant. The soil was moistened to 75% moisture content, and
was composed as follows: 0.7% organic carbon, 57.5% sand, 26.6%
silt, and 15.8% clay. The activity of the nitrifying bacteria
(oxidation of nitrite-nitrogen to nitrate-nitrogen) was monitored.
After the treated soil had been incubated for 1-4 months at 27 °C,
nitrite could not be detected. "MIX D/D" soil fumigant did reduce
the oxidation of the ammonia to nitrite by Nitrosomonas spp.,
especially at a concentration four times normal field use. There was
no effect on Nitrobacter spp. (Rader, 1979a).
"MIX D/D" application kills a considerable part of the biomass.
Lysis of the killed biomass provides the surviving microflora with a
new and, to some extent, readily available substrate. The
mineralization of this substrate, together with a small priming
effect, gives an extra contribution to the inorganic nitrogen
content of the soil (so-called "flush"). This contribution depends
on the amount of biomass, which is related to the type of soil. The
gain in nitrogen is approximately 5-10 kg N/ha. The nitrogen gain in
spring, after autumn fumigation, can be attributed to a reduction in
loss of nitrogen by diminished leaching and diminished
denitrification of nitrate, and depends on the rate of
mineralization, time of recovery of nitrification, weather
conditions during the winter, and soil type (Lebbink & Kolenbrander,
1974).
In field trials, the influence of "MIX D/D" on nitrification
was studied in loamy sand and black earth soils. Nitrogen
application was 100 kg/ha; and the application of "MIX D/D" was
between 37.1 and 46.6 kg/ha. When "MIX D/D" had been applied to the
loamy sand, 61.5% of the September-applied nitrogen was retrieved in
the top 20 cm in March of the following year. This percentage went
up to 72 and 100%, respectively, when fertilizer was applied in
October or November. On loamy sand, the yield obtained from the
following crop was significantly increased. On black earth, "MIX
D/D" application resulted in only a slight increase in crop yield
(Kämpfe, 1973).
7.1.1.2 Recovery studies with microorganisms
"MIX D/D" was added to soil at 2 rates, approximately 10 and
100 times the normal recommended treatment rates. A number of
microbial assessments were made (see below).
Parameter % reduction over control at:
3000 litre/ha 30 000 litre/ha
Evolution of carbon dioxide 10 100
Density of total bacteria nda 98
Density of proteolytic bacteria 44 99
Density of cellulolytic bacteria 30 100
Mineralization of nitrogen 9 44
Mineralization of asparagin 0 27
a Not determined.
From: Dommergues (1959).
Soon after the "MIX D/D" disappears from the soil,
recolonization by bacteria takes place and the number of the
different types of bacteria may be higher than before the "MIX D/D"
treatment; this may lead to the production of higher levels of
nitrogen/ha, perhaps because of a belated recovery of bacteriophages
(Bakker, 1968).
With "MIX D/D" (120 and 600 mg/kg), Tu (1972) found a decrease
in bacterial and fungal populations in a loamy sand, but recovery to
the same levels as the controls was rapid. In a series of studies by
Tu (1978, 1979, 1981a,b), "MIX D/D" at 150 and 300 mg/kg soil, and
1,3-dichloropropene at 30 and 60 mg/kg, were evaluated in parallel
under laboratory conditions. In general, neither had much effect on
either the numbers of soil microorganisms or their activity.
Acetylene reduction, the population of non-symbiotic nitrogen-fixing
organisms, and the viability of indigenous microorganisms were not
affected. There was some stimulation of microbial numbers in some
experiments. Soil enzyme activity was either not affected or only
very slightly affected.
Overall, the main effect of "MIX D/D" on soil microbial
function, at normal usage levels, is to reduce the rate of turnover
of ammonium. After autumn treatment, this is an advantage, as
ammonium leaches from the soil less readily than nitrate and so is
available in the spring for crop growth (Elliot et al., 1974). This
effect, together with increased chlorine content, contributes to the
yield increases beyond those expected from pest control alone, that
are often noted following "MIX D/D" treatment (Goffart & Heiling,
1958; Ennik et al., 1964; Bakker, 1968).
7.2 Aquatic organisms
7.2.1 Invertebrates
Varanka (1979) studied the toxicity of "MIX D/D" (50% 1,3-
dichloropropene + 1,2-dichloropropane) for the larvae (glochidia)
of freshwater mussels ( Anodonta cygnea L.). The decrease of
tryptamine-induced adductor muscle activity was used as an indicator
of the effect of the pesticide. The presence of toxicants reduces
the ability of the larval adductor muscle to contract when
stimulated by tryptamine. In each experiment, 200-300 larvae
originating from 4-5 adult mussels were used. The concentration of
"MIX D/D" causing 50% reduction in adductor muscle activity, over a
30-min exposure, was 18 mg/litre (20 ppm v/v).
7.2.2 Fish
"MIX D/D" is moderately toxic for fish (Table 24). The toxicity
is largely due to the 1,3-dichloropropene component, 1,2-
dichloropropane being about two orders of magnitude less toxic than
"MIX D/D" (see section 7.1.3 of 1,2-dichloropropane).
Harlequin fish (Rasbora heteromorpha) were exposed in water
containing 10 mg "MIX D/D"/litre. After 2 h, no deaths were found.
The fish were then transferred to clean water and 3 days later there
were still no deaths. In another study, 5 fish were exposed
continuously in water containing 5 mg "MIX D/D"/litre. Three fish
had died by 45 h, but no further deaths had occurred by 96 h (Reiff,
1975).
Application of "MIX D/D" (1000 litre/ha) to a vineyard in
France caused contamination of a natural spring, which in turn led
to contamination of fish-breeding basins and a pond. "MIX D/D" was
detected in the spring water 20 days after the death of trout and
carp. The concentrations in the spring and pond water ranged from
0.4 to 2.4 mg/litre. Within 3 months, the concentration decreased to
less than 0.1 mg/litre (Elgar et al., 1965).
7.3 Terrestrial organisms
7.3.1 Birds
No data were available.
7.3.2 Soil fauna
In one study in the United Kingdom, 99% of soil arthropods were
killed following "MIX D/D" application. It took more than 2 years
for the population to recover completely, although the chemical
itself disappeared in about 4 weeks. Collembola populations
started to build up again after 6 months and exceeded 50% of the
initial population after 10 months. Populations were not monitored
beyond 10 months (Edwards & Lofty, 1969).
Studies on a light sandy soil in Belgium showed somewhat faster
recolonization rates. In an unreplicated experiment, 10-11 months
after the last of 5 successive yearly treatments of 600 litre "MIX
D/D"/ha, it was found that populations of phytophagous nematodes,
but not of saprophagous nematodes, were depressed. Earthworms,
enchytraeid worms, mites, and Collembola were present, mainly in
Table 24. Acute toxicity of "Mix D/D" to fish
Species Size Temperature 96-h LC50 References
(°C) (mg/litre)
Rainbow trout 1.1 g 12 5.5a Mayer & Ellersieck
(Oncorhynchus mykiss) (3.6-8.4) (1986)
1.1 g 12 1.97b
(1.2-3.2)
Cutthroat trout 1.0 g 12 1-10 Mayer & Ellersieck
(Salmo clarki) (1986)
Walleye 1.3 g 18 0.98b Mayer & Ellersieck
(Stizostedion vitreum) (1986)
Largemouth bass 0.9 g 18 3.4b Mayer & Ellersieck
(Micropterus salmoides) (1986)
Bluegill sunfish 1.4 g 18 3.9b Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Channel catfish 1.1 g 18 4.4b Mayer & Ellersieck
(Ictalurus punctatus) (1986)
Harlequin fish 2-3 cm 20-22c 4-5 Reiff (1975)
(Rasbora heteromorpha)
Carp - - 6d Reiff (1975)
(Cyprimus carpio)
a Water hardness, 44 mg CaCO3/litre.
b Water hardness, 272 mg CaCO3/litre.
c Hard, chlorine-free water (260 mg CaCO3/litre).
d At 24 h.
the top 5 cm of the soil. Populations of these groups were not
significantly depressed relative to the untreated plot. Populations
of earthworms and mites were significantly increased (van den Brande
& Heungens, 1969).
In a replicated study, the influence of four, successive,
yearly applications of "MIX D/D" (600 litre/ha) on the soil fauna
was studied in a sandy soil in which begonias were grown. Again,
recolonization was found between 6 and 12 months after treatment. Of
the groups studied 12 months after the last treatment, there was
little or no effect on the populations of Enchytraeidae, Gamasina,
Onychiuridae, Lumbricidae, and Acaridae. In the remaining 5 groups
(of mites and Collembola), the populations were also similar to
those in untreated plots (Heungens & van Daele, 1974).
7.3.3 Plants
The components of "MIX D/D" are highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
The acute oral toxicity of "MIX D/D" (containing 51.5% of 1,3-
dichloropropene) was moderate to high when it was administered to
mice, rats, and dogs (Table 25). Signs of intoxication in rats and
mice were hyperexcitability, followed by incoordination, depression,
dyspnoea, and chromodacryorrhoea. Most of the surviving animals had
recovered within 24 h of dosing (Hine et al., 1953; Coombs & Carter,
1976a).
8.1.2 Inhalation
Long-Evans rats were exposed for 4 h in atmospheres containing
2043-81 720 mg "MIX D/D"/m3. The animals were observed for 10
days. The LC50 was approximately 4540 mg/m3. The rats that died
showed severe oedema of the lung, with haemorrhages. Congestion,
cloudy swelling, and fatty degeneration of the liver were also
observed (Hine et al., 1952, 1953).
8.1.3 Dermal
The acute toxicity of "MIX D/D" is low when it is applied in a
single percutaneous dose to rats. Signs of intoxication included
lethargy and chromodacryorrhoea that disappeared in survivors within
4 days after dosing (Hine et al., 1953; Coombs & Carter, 1976a).
Nineteen adult rabbits were treated with 1.2-4.8 g "MIX D/D"/kg
body weight, on the skin, for 24 h. Inhalation of the vapour was not
possible. A mucous nasal discharge was noted in one rabbit treated
with 3 g "MIX D/D"/kg body weight. The skin showed extremely severe
eschar with intense oedema, resulting in black necrotic tissue.
Seven of the 10 animals receiving the three higher dose levels died
in 8-48 h. The LD50 was 2.1 g/kg body weight (see Table 25) (Hine
et al., 1952, 1953).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
Carworth Farm E-rats (12 of each sex per group) were dosed
orally, by gavage, with emulsions of "MIX D/D" in corn oil at 0,
0.0125, 0.025, 0.125, or 3.125 mg "MIX D/D"/kg body weight per day,
for 3 months. The "MIX D/D" contained 55% 1,3-dichloropropenes, 26%
1,2-dichloropropane, and 0.7% epichlorohydrin. There were no effects
on general health, behaviour, growth rate, food intake, or blood
Table 25. Acute toxicity of "Mix D/D"
Species Route Vehicle Sex LD50 (mg/kg References
body weight)a
Mouse oral propylene glycol male 300 Hine et al. (1952, 1953)
(Swiss)
Mouse (CD-1) oral undiluted 314 (276-365) Coombs & Carter (1976a)
Mouse (CF-1) oral 3% D-D in DMSO 234 (208-262) Carter (1975)
Rat oral suspension in 140 Hine et al. (1952, 1953)
(Long-Evans) propylene glycol
Rat (CD) oral undiluted male 227 (180-540) Coombs & Carter (1976a)
female 132 (108-156)
Dog oral undiluted > 230b Carter (1975)
Rat (CD) dermal undiluted 779 (630-1103) Coombs & Carter (1976a)
Rabbit dermal undiluted male 2100 (1540-2660) Hine et al. (1952, 1953)
a With 95% confidence limits.
b Screening value from test with only 2 dogs/dose. Apart from vomiting in dogs, no signs of
intoxication were observed.
chemistry throughout the experiment. No changes in organ weights or
pathological lesions attributable to "MIX D/D" were detected at any
dose level.
When rats were dosed with 3.125 mg/kg body weight, there were
slight reductions in the haemoglobin concentration and erythrocyte
count in the males, and at 0.125 mg/kg body weight there were
reductions in the erythrocyte and total leukocyte counts in the
females, but since these reductions were not dose-related, they were
of doubtful toxicological significance. With a dose of 0.025 mg/kg
body weight there were no effects attributable to the "MIX D/D"
(Walker, 1968a). (Remark: The Task Group noted that the dose levels
used were too low for a proper assessment of the toxicity).
8.2.1.2 Dog
Beagle dogs (3 of each sex per dose group, with 5 of each sex
as controls) were orally dosed by capsule with 0, 0.0125, 0.025, or
3.125 mg "MIX D/D"/kg body weight per day, for 3 months. The
composition of the "MIX D/D" was 55% 1,3-dichloropropenes, 26% 1,2-
dichloropropane, and 0.7% epichlorohydrin. There were no effects on
general health, behaviour, growth rate, haematology, or clinical
chemistry at any dose level. No pathological lesions attributable to
"MIX D/D" were detected, even at the highest dose level (Walker,
1968b). (Remark: The Task Group noted that the dose levels used were
too low for a proper assessment of the toxicity).
Groups of 4 pure-bred Beagle dogs and 4 bitches were dosed with
0, 0.25, 0.75, 2.50, or 7.50 mg/kg body weight "MIX D/D" (containing
28.2% cis-, 29.0% trans-1,3-dichloropropene, and 34.0% 1,2-
dichloropropane, without epichlorohydrin), suspended in olive oil in
gelatin capsules, 7 days/week, for 2 years. Haematological and blood
chemistry studies and urinalyses were conducted on each animal just
prior to the inception of the study and after 3, 6, 9, 12, 18, and
24 months of testing. Ten organs were weighed and histopathological
examination was carried out of 28 organs and tissues.
The overall body weights of the bitches were lower in the 7.5
mg/kg group compared to untreated bitches. The mean corpuscular
volume and mean corpuscular haemoglobin level were lower in the
bitches of the 7.5 mg/kg group, while their erythrocyte count was
raised above that of untreated bitches. No other effects were found.
At the dose level of 2.5 mg/kg, no differences were found in the
body weight, food intake, behaviour, mortality, haematology,
clinical chemistry, urinalysis, organ weight, or gross or
histopathological appearance between treated and control animals
(Industr. Biotest Lab., 1977b). An audit of the study data concluded
that no deviations from the protocol were observed which influenced
the overall interpretation. Repeated oral dosing of dogs with 7.5 mg
"MIX D/D"/kg body weight per day, for 2 years, did not result in any
significant biological effects (Schweizer & Parker, 1980; Parker,
1980).
8.2.2 Inhalation
Male or female Long-Evans rats (6 per group) were exposed,
whole body, in concentrations of 0, 340.5, 1362, or 2724 mg "MIX
D/D"/m3, 1 h/day, 5 days a week, for 2 weeks, or until the animals
died. Rats exposed to the two highest dose levels showed evidence of
weight loss, central nervous system depression, and moderate
irritation. At 2724 mg/m3, 3 animals died. There were no deaths in
the other groups and no gross or microscopic lesions were observed.
No effects were seen in the 340.5 mg/m3 group (Hine et al., 1952).
A further group of six rats was exposed to 1362 mg "MIX D/D"/m3, 1
h/day, for 3 days. They were killed to find out whether there were
any lesions immediately after exposure. No gross or microscopic
lesions were found (Hine et al., 1952).
Groups of 28 male and 28 female Fischer 344 albino rats and CD-
1 albino mice were exposed to atmospheres containing 0, 22.7, 68.1,
or 227 mg "MIX D/D"/m3, for 6 h/day, 5 days/week for 6 or 12
weeks. The actual mean concentrations were 0, 21.16, 65.38, and
243.8 mg/m3. No unusual signs of toxicity were observed in rats or
mice during the study. Body weights and mortality were similar in
all groups at both 6 and 12 weeks. There were no treatment-related
changes in haematology, clinical chemistry, or urinalysis apart from
the detection of small to moderate amounts of occult blood in the
urine of female mice exposed to 22.7, 68.1, or 227 mg "MIX D/D"/m3
for 6 weeks. No treatment-related changes were observed in absolute
organ weights of rats or mice exposed to "MIX D/D" atmospheres up
to, and including, 227 mg/m3, for 6 or 12 weeks. Small changes
attributable to "MIX D/D" exposure were observed in male rats
exposed to 227 mg/m3 (increased liver/body weight ratio) at 6 and
12 weeks and in female rats exposed to 227 mg/m3 (increased
kidney/body weight ratio) at 12 weeks. No treatment-related
histopathological changes were observed in either rats or mice,
other than slight to moderate diffuse hepatocytic enlargement in
male mice (12/21) after 12 weeks exposure to 227 mg "MIX D/D"/m3.
No significant changes related to toxicity were found in the body
weight, behaviour, haematology, clinical chemistry, or gross or
histological appearance of rats or mice exposed to atmospheres
containing up to, and including, 227 mg "MIX D/D"/m3, for 6 or 12
weeks. No adverse effects were seen at the top dose level (Hazleton
Laboratories America Inc., 1979; Parker et al., 1982).
Groups of 30 male and 24 female Wistar SPF albino rats were
exposed to actual concentrations of 0, 64, 145, or 443 mg "MIX
D/D"/m3, 6 h/day 5 days per week, for 10 weeks. The "MIX D/D"
contained 28.1% cis-1,3-dichloropropene, 25.6% trans-1,3-
dichloropropene, and 25.6% 1,2-dichloropropane, without
epichlorohydrin. A subgroup was used for the reproduction study (see
also section 8.5.1.2). There were no compound-related changes in the
urinalysis, haematology, clinical chemistry, or in the gross or
histological appearance of the reproductive tract in male and female
rats. Males and females exposed to 443 mg "MIX D/D"/m3 showed a
reduced weight gain compared to controls, indicative of a mild toxic
response at this top dose. Absolute kidney weights in females were
higher in the 443 mg/m3 group compared to controls, but apart from
a slight increase in amorphous protein casts in the proximal
convoluted tubular lumina, no histopathological effects were
observed. This increase in kidney weight was probably related to the
efficient renal excretion of "MIX D/D". A no-observed-effect level
(NOEL) of 145 mg/m3 was considered (Clark, 1980).
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
Undiluted "MIX D/D" was applied to the skin of 12 rabbits in
single doses of 0.5 ml. The liquid was allowed to be in contact with
the skin for 24 h. The mean score was 7 on the scale (up to 8) of
the method of Draize et al. (1944): rating it as a severe irritant.
Signs were severe eschar, intense oedema, and black necrotic tissue
(Hine et al., 1952, 1953; Coombs & Carter, 1976a).
Six young New Zealand albino rabbits were used in a primary
skin-irritation test. 0.5 ml of undiluted "MIX D/D" soil fumigant
was applied on the skin for 4 h. The mean scores for erythema and
oedema after 4, 24, and 72 h were averaged. The primary irritation
score was 8, rating it as corrosive. The signs were erythema,
oedema, escharosis, and necrosis (Industr. Biotest Lab., 1972).
Undiluted "MIX D/D" was instilled into the eyes of 10 rabbits
in doses of 0.005 or 0.02 ml. After 18-24 h, the eyes were examined
and the reactions scored. The eye injury was scored as grade 5
(severe irritation) (Hine et al., 1952, 1953).
8.3.2 Sensitization
In a test with 20 "P" strain guinea-pigs, a 5% w/v
concentration of "MIX D/D" in corn oil was used and three topical
induction applications were followed by a 1% w/v concentration for
the topical challenge (method of Buehler, 1965). In 13 out of the 20
guinea-pigs, a positive reaction was obtained. "MIX D/D" has a
moderate skin sensitizing potential (Coombs & Carter, 1976a).
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
Groups of 50 male and 50 female albino rats (age 5 weeks) were
fed diets containing nominal concentrations of 0, 10, 30, 100, or
300 mg "MIX D/D"/kg diet for 2 years. Blood samples were collected
by suborbital sinus puncture at 3, 6, 12, 18, and 24 months. The
"MIX D/D" contained 28.2% cis-, 29.0% trans-1,3-dichloropropene
and 34% 1,2-dichloropropane, without epichloro- hydrin.
There were no significant differences throughout the 2-year
period between the body weights, food intakes, behaviour, or
mortalities of male and female rats fed diets containing "MIX D/D"
and those of control animals. There were no consistent compound-
related changes in the haematological parameters.
Statistically significant increases were noted, at 3, 6, and 24
months, in the fasting serum glucose values of rats receiving 300
mg/kg diet and in the serum alkaline phosphatase levels at 12 months
compared with controls. These effects were not considered to be
biologically significant, because of the size of the response and
the lack of a consistent response throughout the study. No other
changes were observed in the haematology and clinical chemistry of
rats exposed to "MIX D/D" for 2 years.
Urinalyses were performed at 3, 6, 12, 18, and 24 months, and
the only statistically significant difference recorded was a lowered
specific gravity of urine at 3 months in males receiving 300 mg/kg
diet compared with controls. This effect was not correlated with any
other changes, and the effect did not occur consistently throughout
the study; it was therefore considered to be of doubtful biological
significance. No changes attributable to feeding diets containing
"MIX D/D" were observed in the organ weights and histopathology at 1
and 2 years (Industr. Biotest Lab., 1978). An audit performed on the
study data revealed no major factors that would affect the
conclusions of this study. The nominal concentrations of "MIX D/D"
specified in the protocol could not be considered representative of
the actual concentrations fed to animals. Subsequent work on the
volatility of "MIX D/D" in the diet revealed that the average
dietary concentrations present at the time of feeding were 40% of
the nominal values.
In conclusion, no compound-related effects were observed in
rats fed diets containing an average concentration of up to and
including 120 mg/kg diet (= nominal concentration of 300 mg/kg diet)
"MIX D/D" for two years (Jud et al., 1980a).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
Charles River CD-strain albino rats (10 males and 20 females)
were fed diets containing 0, 10, 30, or 100 mg "MIX D/D" ( cis-1,3-
dichloropropene 28.2%, trans-1,3-dichloropropene 29%, 1,2-
dichloropropane 34%, without epichlorohydrin) per kg diet in a 3-
generation, 2-litter, reproduction study. No statistically
significant differences were observed in parental body weights, food
consumption, behaviour, or mortalities throughout each 10-week pre-
breeding period. Gross pathology, histopathology, and organ weights
were similar in all groups. The feeding of diets containing "MIX
D/D" did not affect the reproductive performance (mating, fecundity,
fertility indices, and parturition incidence) or dam body weights
during gestation or post-partum and post-weaning periods. There were
no signs of external abnormalities in newborn pups. There were
significantly more pups alive at day 1 of lactation in the F3a
litters of the 30 mg/kg group, compared with controls. No
differences that were directly related to treatment were noted for
survival over a 21-day period, number of pups delivered, or
stillbirths. No gross pathological or histopathological differences
were observed between treatment and control offspring. Significantly
higher mean body weights were recorded at weaning in the F2b males
of the 30 mg/kg group and in males and females of the 100 mg/kg
group. Increases were also recorded at weaning in the F3b
generation of 10 mg/kg males and 100 mg/kg females. These increases
in mean body weight did not show any consistent relationship with
dose or sex and were not considered attributable to "MIX D/D"
exposure (Industr. Biotest Lab., 1977a).
An audit of the study data demonstrated that compliance with
the protocol was satisfactory and that agreement of the results with
more recent data was sufficient to support the conclusions of this
study. Subsequent work on the volatility of "MIX D/D" in diet
revealed that average concentrations present at the time of feeding
were 40% of the nominal values. Thus "MIX D/D" did not produce any
effects on the reproductive performance or growth of offspring of
rats fed diets containing up to and including 40 mg "MIX D/D"/kg
diet (= nominal concentration of 100 mg/kg diet) for 3 generations
(40 mg/kg diet, the highest dose level tested is equivalent with 2
mg/kg body weight) (Jud et al., 1980b).
8.5.1.2 Inhalation
In one study (Linnett et al., 1988), groups of 20 male SPF
Wistar-derived rats (15 weeks old) and 24 virgin female rats (10
weeks old) were exposed by inhalation to nominal concentrations of
0, 45.4, 136, or 408 mg "MIX D/D"/m3 (actual concentrations of 0,
64, 145, or 443 mg/m3 for 6 h/day, 5 days/week, for 10 weeks. The
composition of the "MIX D/D" was 28.1% (w/w) cis-1,3-
dichloropropene, 25.6% trans-1,3-dichloropropene, and 25.6% 1,2-
dichloropropane and a number of other chlorinated components in
percentages up to 5%, but it contained no epichlorohydrin.
Treated males of proven fertility were paired with untreated
virgin females at intervals during and after exposure. Groups of 15
treated females were paired with untreated males immediately after
the 10-week period. Various aspects of reproduction performance and
general toxicity were assessed. Mortality and haematological,
clinical-chemical, and urinary parameters were comparable with the
controls. Exposure to "MIX D/D" did not produce any adverse effects
on the libido, fertility, or morphology of the reproductive tracts
of rats of either sex. No treatment-related dominant lethal effects
were observed in male rats. Mean body weights of male and female
rats at 408 mg/m3 were significantly decreased. The mean liver and
kidney weights were significantly increased in the animals of both
sexes exposed to the highest dose level. Histological examination of
the organs did not reveal treatment-related changes. This study
demonstrates that male and female rats exposed to atmospheres
containing up to 443 mg/m3 "MIX D/D" vapour for 10 weeks did not
suffer any impairment of reproductive performance.
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
Charles River, albino, female rats received gastric intubations
of 0, 30, or 100 mg "MIX D/D"/kg body weight (containing 28% cis-
1,3-dichloropropene, 29% trans-1,3-dichloropropene, and 34% 1,2-
dichloropropane, without epichlorohydrin) dissolved in corn oil,
during days 6 through 15 of pregnancy. The control group was dosed
with corn oil. A significant decrease in body weight of the dams
receiving 100 mg/kg, compared with the controls, was observed at day
15 of gestation. While no statistically significant reproductive
effects occurred, there appears to have been a trend for an increase
in the percentage of females with one or more resorption sites. The
lack of statistical significance is probably due to the small
numbers of animals involved (3 of 17, 6 of 19, and 5 of 15, for the
0, 30, and 100 mg/kg groups, respectively). The maternal toxicity
observed in the highest-dose group would account for the apparent
increase in resorption sites.
There was a dose-related increase in the incidence of
supernumerary ribs (2.8% for controls, 7.6% for the 30 mg/kg group,
and 32.3% for the 100 mg/kg per group) and the same was found for
the occurrence of non-ossified sternum sections. The incidence of
supernumerary ribs over approximately 50 rat teratology studies, in
this laboratory, has been within the range 0-26%.
A significant decrease in mean body weight of fetuses from
females dosed with 100 mg "MIX D/D"/kg was observed. Maternal
toxicity was observed in the 100 mg/kg group, but no malformations,
other than supernumerary ribs and non-ossified sternebrae, were
observed in this group (Industr. Biotest Lab., 1975).
An audit of the study data demonstrated that there were no
major factors to alter the conclusions of this study (O'Sullivan et
al., 1980).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
DeLorenzo et al. (1977) tested "MIX D/D" (40% 1,3-
dichloropropene and 40% 1,2-dichloropropane) on Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1978, with
and without activation. Dose levels of 0, 0.5, 5, 15, and 25
mg/plate were tested. Mutagenic effects were seen with TA100,
TA1535, and TA1978, but not with TA98 and TA1537.
Shirasu et al. (1981) studied the mutagenicity of "MIX D/D" in
reverse mutation tests using Salmonella typhimurium strain TA100,
and Moriya et al. (1983) used 5 strains of Salmonella typhimurium,
TA98, TA100, TA1535, TA1537, and TA1538, and Escherichia coli
strain WP2 hcr, with and without metabolic activation. "MIX D/D"
was tested in dose levels up to 5000 µg/plate. The mutagenic potency
in Salmonella typhimurium TA100 was 0.0087 revertants/nmole. "MIX
D/D" was a direct-acting mutagen in TA100, TA1535, and E. coli WP2
hcr, but was not mutagenic in TA1537, TA1538, or TA98.
8.6.2 In vivo studies
Dominant lethality was tested in rats exposed to an atmosphere
containing "MIX D/D" vapour for 10 weeks (Linnett et al., 1988, see
section 8.5.1.2). No effects on fertility or on implantations were
observed even at 443 mg/m3, the highest concentration tested. This
result demonstrates the lack of any significant effects on germ-cell
production in rats.
Further evidence of rapid detoxification was found in the
negative results of a host-mediated assay using S. typhimurium,
strain G46, in mice dosed orally with either 60 or 200 mg "MIX
D/D"/kg over a 24-h period (Shirasu et al., 1981).
9. EFFECTS ON HUMANS
9.1 General population exposure
A case of acute poisoning occurred a few hours after the
accidental ingestion of "MIX D/D". The victim experienced abdominal
pain and vomiting. He became semicomatose and exhibited muscle
twitching and died (Gosselin et al., 1976; NTP, 1985).
9.2 Occupational exposure
In the period from 1966 to 1971, a total of 13 cases of
untoward skin reactions to pesticides were reported in the northern
part of the Netherlands. Seven were due to contact with "MIX D/D"
caused by inadvertent dripping into the shoes of the applicators
during spraying operations. In all cases, acute vesicular dermatitis
occurred. Three other cases of dermatitis were described, one being
of a contact allergic nature, as confirmed by patch testing, and
were diagnosed as orthoergic contact reactions (Nater & Gooskens,
1976).
Nater & Gooskens (1976) and Van Joost & De Jong (1988)
described dermatitis in a farmer who had sprayed "MIX D/D" and in a
process operator, caused by direct contact. The persons had had
previous contact with this substance. Both patients reacted
positively in a patch test, with 1% and 0.02% "MIX D/D",
respectively.
REFERENCES
Abdalla NA (1974) Distribution, persistence and nematocidal activity
of monobromomethane and a pesticide containing 1,3-dichloropropene
in soil. Diss Abstr Int, B35: 296.
Abdalla NA, Raski DJ, Lear B, & Schmitt RV (1974) Movement,
persistence and nematocidal activity of a pesticide containing 1,3-
dichloropropene in soils treated for nematode control in replant
vineyards. Plant Dis Reptr, 58: 562-566.
Ahlsdorf B, Stock R, Muller-Wegener U, & Milde G (1989) [The
behaviour of selected pesticides in ground water close to the
surface in heterogenous loose sediments.] Schriftenr Ver Wasser-
Boden- Lufthyg, 79: 375-385 (in German).
Albrecht WN (1987) Occupational exposure to 1,3-dichloropropene
(Telone(R) II) in Hawaiian pineapple culture. Arch Environ Health,
42(5): 286-291.
Albrecht WN & Chenchin K (1985) Dissipation of 1,2-dibromo-3-
chloropropane (DBCP), cis-1,3-dichloropropene (1,3-DCP) and
dichloropropenes from soil to atmosphere. Bull Environ Contam
Toxicol, 34: 824-831.
Albrecht WN, Hagadone MR, & Chenchin K (1986) Charcoal air sampling
tube storage stability and desorption efficiencies of 1,2-dibromo-3-
chloropropane (DBCP) and 1,3-dichloropropene (DCP). Bull Environ
Contam Toxicol, 36: 629-634.
Altman J (1969) Effect of chlorinated C3 hydrocarbons on amino
acid production by indigenous soil bacteria. Phytopathology, 59:
762-766.
Altman J & Lawlor S (1966) The effect of some chlorinated
hydrocarbons on certain soil bacteria. J Appl Bacteriol, 29: 260-
265.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside,
California, University of California, Agricultural Extension (Report
No. M16 5M Rev. 9/73).
Bakker Y (1968) [Influence of soil decontamination with DD on
nitrogen manuring.] Landbouwvoorlichting, 25(2): 87-88 (in Dutch).
Bartels MJ & Timchalk C (1990) 1,2-Dichloropropane: Investigation of
the mechanism of mercapturic acid formation in the rat. Xenobiotica,
20(10): 1035-1042.
Baruffini A, Cirla AM, Pisati G, Ratti R, & Zedda S (1989) Allergic
contact dermatitis from 1,2-dichloropropane. Contact Dermatitis, 20:
379-380.
Battersby NS (1990a) Trans-1,3-dichloropropene: An assessment of
ready bio-degradability. Internal Report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 89.179, submitted to WHO by
Shell).
Battersby NS (1990b) An assessment of the inhibitory effect of cis-
+ trans-mixture 1,3-dichloropropene on the respiration rate of
activated sludge. Internal Report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 90.007, submitted to WHO by
Shell).
Battersby NS (1990c) An assessment of the inhibitory effect of cis-
1,3-dichloropropene on the respiration rate of activated sludge.
Internal Report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 90.005, submitted to WHO by Shell).
Bellomo G & Orrenius S (1985) Altered thiol and calcium homeostasis
in oxidative hepatocellular injury. Hepatology, 5: 876-882.
Belser NO & Castro CE (1971) Biodehalogenation - the metabolism of
the nematocides cis- and trans-3-chloroallyl alcohol by a
bacterium isolated from soil. J Agric Food Chem, 19: 23-26.
Bennett D & Ridge MA (1989) Trans-1,3-dichloropropene:
Determination of the N-octanol/water partition coefficient using a
reverse-phase HPLC method. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 89.133, submitted to WHO by
Shell).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow,
Pimephales promelas, early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berry DL, Campbell WF, Street JC, & Salunkhe DK (1980) Uptake and
metabolism of 1,3-dichloropropene in plants. J Food Saf, 2(4): 247-
255.
Beugelink GP (1987) [Chemicals used in soil decontamination heralds
of a serious contamination.] H2O, 20(21): 522-526 (in Dutch).
Blair D (1977) Toxicity of soil fumigants: acute inhalation toxicity
of 1,3-dichloropropene. Sittingbourne, Shell Research Ltd,
(Unpublished proprietary report TLTR 0002.77, submitted to WHO by
Shell).
Bousema MT, Wiemer GR, & Van Joost TH (1991) A classic case of
sensitization to DD-95. Contact Dermatitis, 24: 132-133.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds.
Washington, DC, American Chemical Society, pp 49-64 (ACS Symposium
Series No. 149).
Boyland E & Chasseaud LF (1969) The role of glutathione and
glutathione-S-transferases in mercapturic acid biosynthesis. Adv
Enzymol, 32: 173-219.
Breslin WJ, Kirk HD, Streeter CM, Quast JF, & Szabo JR (1987) Telone
II soil fumigant: Two generation inhalation reproduction study in
Fischer 344 rats. (Study No. M003993-015). Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Breslin WJ, Kirk HD, Streeter CM, Quast JF, & Szabo JR (1989) 1,3-
Dichloropropene: Two generation inhalation reproduction study in
Fischer 344 rats. Fundam Appl Toxicol, 12: 129-143.
Brezis M, Rosen S, Silva P, & Epstein FH (1983) Selective
glutathione depletion on function and structure of the isolated
perfused rat kidney. Kidney Int, 24: 178-184.
Bromley S & Cook KA (1981) Evaluation of the soil fumigant, DD, as a
nitrification inhibitor. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report SBGR 81.295, submitted to WHO by
Shell).
Brooks TM, Dean BJ, & Wright AS (1978) Toxicity studies with
dichloropropenes; Mutation studies with 1,3-D and cis-1,3-
dichloropropene and the influence of glutathione on the mutagenicity
of cis-1,3 dichloropropene in Salmonella typhimurium.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
TLGR 0081.78, submitted to WHO by Shell).
Brooks TM & Wiggins DE (1989a) Bacterial mutagenicity studies with
trans-1,3-dichloropropene. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 88.247, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1989b) Genotoxicity studies with trans-
1,3-dichloropropene: In vitro chromosome studies with trans-1,3-
dichloropropene. Internal report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 89.086, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1990) Bacterial mutagenicity studies with
cis-1,3-dichloropropene. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 89.205, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1991) Genotoxicity studies with cis-1,3-
dichloropropene: In vitro chromosome studies. Internal report.
London, Shell Research Ltd (Unpublished proprietary report SBGR
90.049, submitted to WHO by Shell).
Brouwer DH, Brouwer EJ, De Vreede JAF, Van Welie RTH, Vermeulen NPE,
& Van Hemmen JJ (1991a) Inhalation exposure to 1,3-dichloropropene
in the Dutch flower-bulb culture. Part. I. Environmental monitoring.
Arch Environ Contam Toxicol, 20: 1-5.
Brouwer EJ, Evelo CTA, Verplanke AJW, Van Welie RTH, & De Wolff FA
(1991b) Biological effect monitoring of occupational exposure to
1,3-dichloropropene: Effects on liver and renal function and on
glutathione conjugation. Br J Ind Med, 48: 167-172.
Brown RH & Purnell CJ (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. The performance of
a Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Bruckner JV, Mackenzie WF, Ramanathan R, Muralidhara S, Kim HJ, &
Dallas CE (1989) Oral toxicity of 1,2-dichloropropane: Acute, short-
term and long-term studies in rats. Fundam Appl Toxicol, 12: 713-
730.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull
Environ Contam Toxicol, 26: 446-452.
Buehler EV (1965) Delayed contact hypersensitivity in the guinea-
pig. Arch Dermatol, 91: 171-177.
California State Water Resources Control Board (1983) 1,2-
Dichloropropene (1,2-D) and 1,3-dichloropropene (1,3-D). California
State Water Resources Control Board, Toxic Substances Control
Programme (Unpublished special project draft report).
Carere A & Morpurgo G (1981) Comparison of the mutagenic activity of
pesticides in vitro in various short-term assays. In: Kappas A ed.
Progress in environmental mutagenesis and carcinogenesis. Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp 87-104 (Progress
in Mutation Research, Volume 2).
Carter B (1975) Toxicity of soil fumigants: acute oral toxicity of
D-D to mice and dogs. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0034.75, submitted to WHO by Shell).
Casini AF, Maellaro E, Pompella A, Ferrali M, & Comporti M (1987)
Lipid peroxidation, protein thiols and calcium homeostasis in
bromobenzene-induced liver damage. Biochem Pharmacol, 36: 3689-3695.
Castro CE & Belser NO (1966) Hydrolysis of cis- and trans-1,3-
dichloropropene in wet soil. J Agric Food Chem, 14(1): 69-70.
Clark DG (1980) D-D: A 10-week inhalation study of mating behaviour,
fertility and toxicity in male and female rats. Sittingbourne, Shell
Research Ltd (Unpublished proprietary report TLGR 80.023, submitted
to WHO by Shell).
Climie IJG, Hutson DH, Morrison BJ, & Stoydin G (1979) Glutathione
conjugation in the detoxication of (Z)-1-3-dichloropropene (a
component of the nematocide D-D) in the rat. Xenobiotica, 9(3): 149-
156.
Coate WB & Voelker RW (1979b) Final report: 90-day inhalation
toxicity study in rats and mice. Telone II. Vienna, Virginia,
Hazleton Laboratories America Inc. (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Coate WB & Voelker RW (1979a) Addendum to final report: 90-day
inhalation toxicity study in rats and mice. Telone II. Vienna,
Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Collins CJ (1989) Trans-1,3-dichloropropene: Acute inhalation
toxicity study - LC50 rats (4-hour exposure). Harrogate, United
Kingdom, Hazleton Laboratories (Unpublished proprietary report
submitted to WHO by Shell).
Connors TF, Stuart JD, & Cope JB (1990) Chromatographic and
mutagenic analyses of 1,2-dichloropropane and 1,3-dichloropropylene
and their degradation products. Bull Environ Contam Toxicol, 44:
288-293.
Coombs AD & Carter BI (1976a) The toxicity of soil fumigants: Acute
toxicity, skin irritation and skin sensitizing potential of current
product D-D. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLTR 0021.76, submitted to WHO by Shell).
Coombs AD & Carter BI (1976b) The toxicity of soil fumigants: Acute
toxicity, skin irritation and skin sensitizing potential of 1,3-
dichloropropene (95% w/w a.m.). Sittingbourne, Shell Research Ltd
(Unpublished proprietary report TLTR 0023.76, submitted to WHO by
Shell).
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cracknell S, Jackson GC, & Hardy CJ (1987) Telone II (1,3-
dichloropropene): acute inhalation study in rats. 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre Ltd (Prepared
for Dow Chemical Europe, Horgen, Switzerland) (Unpublished
proprietary report submitted to WHO by Dow Chemical Company).
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Creedy CL (1983) The modulation of the bacterial mutagenicity of the
(Z)- and (E)-isomers of 1,3-dichloropropene by glutathione and
subcellular fractions from rat liver. Sittingbourne, Shell Research
Ltd (Unpublished proprietary report SBGR 83.380, submitted to WHO by
Shell).
Creedy CL & Hutson DH (1982) The bacterial mutagenicity of (E)-1,3-
dichloropropene and its modulation by glutathione. Sittingbourne,
Shell Research Ltd (Unpublished proprietary report SBGR 82.335,
submitted to WHO by Shell).
Creedy CL, Brooks TM, Dean BJ, Hutson DH, & Wright AS (1984) The
protective action of glutathione on the microbial mutagenicity of
the Z- and E-isomers of 1,3-dichloropropene. Chem-Biol Interact,
50(1): 39-48.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and non-fatty foods. J Agric Food Chem, 37: 560-564.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and salt water fishes.
J Hazard Mater, 1: 303-318.
De Lorenzo F, Degl'Innocenti S, Ruocco A, Silengo L, & Cortese R
(1977) Mutagenicity of pesticides containing 1,3-dichloropropene.
Cancer Res, 37: 1915-1917.
Dietz FK, Dittenber DA, & Kastl PE (1982) 1,3-Dichloropropene:
Effects on tissue non-protein sulfhydryl content and blood
concentration time profile, probe study. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Dietz FK, Hermann EA, & Ramsey JC (1984a) The pharmacokinetics of
14C-1,3-dichloropropene in rats and mice following oral
administration. (Presented as poster No. 585 at 23rd Annual Society
of Toxicology Conference, Atlanta, Georgia, 15 March 1984).
Toxicologist, 4: 147 (Abstract 585).
Dietz FK, Dittenber DA, Kirk HD, & Ramsey JC (1984b) Non-protein
sulfhydryl content and macro-molecular binding in rats and mice
following oral administration of 1,3-dichloropropene. (Presented as
poster No. 586 at 23rd Annual Society of Toxicology Conference,
Atlanta, Georgia, 15 March 1984). Toxicologist, 4(1): 147 (Abstract
586).
Dietz FK, Hermann EA, Kastl PE, Dittenber DA, & Ramsey JC (1985)
1,3-Dichloropropene: Pharmacokinetics, effect on tissue non-protein
sulfhydryls, and macromolecular binding in Fischer 344 rats and
B6C3F1 mice following oral administration. Freeport, Texas,
Dow Chemical Company USA (Unpublished proprietary report HET: K-
6409-11, submitted to WHO by Dow Chemical).
Dilling WL, Tefertiller NB, & Kallos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1.1.1-
trichloroethane, trichloroethylene, tetrachloroethylene and other
chlorinated compounds in dilute aqueous solutions. Environ Sci
Technol, 9(9): 833-838.
Di Nucci A, Imbriani M, Ghittori S, Gregotti C, Baldi C, Locatelli
C, Manzo L, & Capodaglio E (1988) 1,2-Dichloropropane-induced liver
toxicity. Clinical data and preliminary studies in rats. The target
organ and the toxic process. Arch Toxicol, 12(Suppl): 370-374.
Dipaolo JA & Doniger J (1982) Neoplastic transformation of Syrian
hamster cells by putative epoxide metabolites of commercially
utilized chloroalkenes. J Natl Cancer Inst, 69(2): 531-534.
Dommergues Y (1959) Influence des nématocides sur l'activité
biologique du sol. Fruits (Paris), 14(4): 177-181.
Dowty B, Carlisle D, & Laseter JL (1975) Halogenated hydrocarbons in
New Orleans drinking water and blood plasma. Science, 187: 75-77.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Dubey HD, Riera A, & Rodriguez RL (1975) Effects of the nematocides
Nemagon and DD on mineralization, nitrification, soil microbial
population, and soil fertility status of two tropical soils. J Agric
Univ Puerto Rico, 59(1): 43-50.
Dynamic Corporation (1991) Herd profile: 1,2-Dichloropropane. CAS
No. 78-87-5 (Draft). Washington, DC, US Environmental Protection
Agency, Health and Environmental Review Division, 12 pp.
Eadsforth CV (1987) Biological monitoring of operators for exposure
to D-D during filling operations in CFD loods 2, SNR/C Pernis during
1985-86. Internal report. Rotterdam, Shell Research Ltd, Pernis
Biomedical Laboratory (Unpublished proprietary report submitted to
WHO by Shell).
Eadsforth CV, Rocchi PSJ, & Tuinman CP (1987) A monitoring study of
the exposure to 1,3-dichloropropene during a single application in
Germany of Shell D-D 92 nematocide. Internal report. The Hague,
Shell Research Ltd, Safety and Environmental Division (Unpublished
proprietary report submitted to WHO by Shell).
Edmiston S & Maddy KT (1987) Summary of illness and injuries
reported in California by physicians in 1986 as potentially related
to pesticides. Vet Hum Toxicol, 29(5): 391-397.
Edwards CA & Lofty JR (1969) The influence of agricultural practice
on soil micro-arthropod populations. Syst Assoc Publ, 8: 237-247.
Edwards CA (1983) Development of a standardized laboratory method
for assessing the toxicity of chemical substances to earthworms.
Brussels, Commission of the European Community (Report EUR 8714 EN).
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with USEPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 28: 460-
467.
Elgar KE, Hughes DG, & Reiff B (1965) Residues of DD in samples of
water from France (Technical Memorandum 55/65). Sittingbourne,
Woodstock Agricultural Research Centre, Technical Service Laboratory
(Unpublished proprietary data submitted to WHO by Shell).
Elliot JM, Marks CF, & Tu CM (1972) Effects of nematicides on
Pratylenchus penetrans, soil microflora, and flue-cured tobacco.
Can J Plant Sci, 52(1): 1-11.
Elliot JM, Marks CF, & Tu CM (1974) Effects of the nematicides DD
and Mocap on soil nitrogen, soil microflora, populations of
Pratylenchus penetrans, and flue-cured tobacco. Can J Plant Sci,
54: 801-809.
Elliot JM, Marks CF, & Tu CM (1977) Effects of certain nematicides
on soil nitrogen, soil nitrifiers, and populations of Pratylenchus
penetrans in flue-cured tobacco. Can J Plant Sci, 57: 143-154.
Ennik GC, Kort J, & Luesink B (1964) The influence of soil
disinfection with DD, certain components of DD and some other
compounds with nematocidal activity on the growth of white clover.
Neth J Plant Pathol, 70: 117-135.
Fishbein L (1979) Potential halogenated industrial carcinogenic and
mutagenic chemicals. Sci Total Environ, 11: 223-257.
Fisher GD & Kilgore WW (1988a) Mercapturic acid excretion by rats
following inhalation exposure to 1,3-dichloropropene. Fundam Appl
Toxicol, 11: 300-307.
Fisher GD & Kilgore WW (1988b) Tissue levels of glutathione
following acute inhalation of 1,3-dichloropropene. J Toxicol Environ
Health, 2: 171-182.
Fisher GD & Kilgore WW (1989) Pharmacokinetics of S-[3-chloroprop-2-
enyl]-glutathione in rats following acute inhalation exposure to
1,3-dichloropropene. Xenobiotica, 19(3): 269-278.
Flessel P, Goldsmith JR, Kahn E, & Wesolowski JJ (1978) Acute and
possible long-term effects of 1,3-dichloropropene-California. Morb
Mortal Wkly Rep, 271: 50, 55.
Fong HR & Maykoski R (1985) Air exposure monitoring of EHAP
personnel during a Telone II soil translocation study in Fresno
County. Sacramento, California, California Department of Food and
Agriculture, Division of Pest Management, Environmental Protection
and Worker Safety, Worker Health and Safety Unit, 6 pp (Unpublished
report HS-1299).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BB, Resnick
MA, Anderson B, & Zieger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluation of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gardner JR (1989a) DD-95 ( cis- + trans-mixture 1,3-
dichloropropene): Acute oral toxicity. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 89.005,
submitted to WHO by Shell).
Gardner JR (1989b) Cis-1,3-dichloropropene: Acute oral and dermal
toxicity, skin and eye irritancy and skin sensitisation potential.
Internal report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 89.007, submitted to WHO by Shell).
Gardner JR (1989c) Trans-1,3-dichloropropene: Acute oral and
dermal toxicity, skin and eye irritancy and skin sensitising
potential. Internal report. London, Shell Research Ltd (Unpublished
proprietary report SBGR 88.286, submitted to WHO by Shell).
Girling AE (1989a) Cis-1,3-dichloropropene: Acute toxicity to
Salmo gairdneri, Daphnia magna and Selenastrum capricornutum.
London, Shell Research Ltd (Unpublished proprietary report SBGR
89.118, submitted to WHO by Shell).
Girling AE (1989b) Cis + trans-1,3-dichloropropene: Acute
toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. London, Shell Research Ltd (Unpublished proprietary
report SBGR 89.159, submitted to WHO by Shell).
Girling AE (1989c) Trans-1,3-dichloropropene: Acute toxicity to
Salmo gairdneri, Daphnia magna and Selenastrum capricornutum.
London, Shell Research Ltd (Unpublished proprietary report SBGR
89.160, submitted to WHO by Shell).
Goffart H & Heiling A (1958) [Side effects of nematode control with
Shell D-D and related agents.] Nematologica, 3: 213-228 (in German
with English summary).
Gollapudi BB, Bruce RJ, & Hinze CA (1985) Evaluation of Telone II
soil fumigant in the mouse bone marrow micronucleus test. Freeport,
Texas, Dow Chemical Company USA (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Gorzinski SJ & Johnson KA (1989) Neurotoxicologic examination of
Fischer 344 rats exposed to 1,2-dichloropropane (DCP) via gavage for
2 weeks. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Gosselin R, Hodge H, Smith R, & Gleason M (1976) Clinical toxicology
of commercial products, 4th ed. Baltimore, Maryland, Williams and
Wilkins Co., pp 119-121.
Greve PA, Klapwijk SP, & Linders JBHJ (1989) [Pesticides in surface
water in the bulb-growing area near Langeveld.] Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection (Unpublished report No. 638812001) (in Dutch).
Grzywa Z & Rudzki E (1981) Dermatitis from dichloropropene. Contact
Dermatitis, 7: 151-152.
Haemisegger ER, Jones AD, & Reinhardt FL (1985) EPA's experience
with assessment of site-specific environmental problems: A review of
IEMD's geographic study of Philadelphia. (Prepared for presentations
at the 78th Annual Meeting of the Air Pollution Control Association,
Detroit, Michigan, 16-21 June 1985). Washington, DC, US
Environmental Protection Agency, pp 1-20 (Report 85-63-6).
Hallberg GR (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hanley TR Jr, John-Greene JA, Young JT, Calhoun LL, & Rao KS (1987)
Evaluation of the effects of inhalation exposure to 1,3-
dichloropropene on fetal development in rats and rabbits. Fundam
Appl Toxicol, 8: 562-570.
Hanley TR Jr, Berdasco NM, Battjes JE, & Johnson KA (1989a)
Propylene dichloride: Oral teratology study in New Zealand white
rabbits. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Hanley TR Jr, Kirk HD, Bond DM, Firchau HM, & Johnson KA (1989b)
Propylene dichloride: Dominant lethal study in Sprague-Dawley rats.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Hanley TR Jr, Kirk HD, Berdasco NM, & Johnson KA (1990) Evaluation
of the developmental toxicity of propylene dichloride in rats and
rabbits. Teratology, 41(5): 562 (Abstract P54).
Haseman JK, Crawford DD, Huff JE, Boorman GA, & McConnell EE (1984)
Results from 86 two-year carcinogenicity studies conducted by the
national toxicology program. J Toxicol Environ Health, 14: 621-639.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen, 1(Suppl): 3-142.
Hayes WJ Jr (1982) Pesticides studied in man. Baltimore, Maryland,
Williams and Wilkins Co., pp 162, 163.
Hazleton Laboratories America, Inc. (1979) Subacute inhalation
toxicity study of D-D soil fumigant in CD-1 mice and albino Fischer
344 rats. Vienna, Virginia, Hazleton Laboratories America Inc.
(Prepared for Shell Oil Company, Houston, Texas) (Unpublished
proprietary report No. 776-132, submitted to WHO by Shell).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of
54 industrial chemicals to sheephead minnows (Cyprinodon
variegatus). Bull Environ Contam Toxicol, 27: 596-604.
Heppel LA, Highman B, & Porterfield VT (1946) Toxicology of 1,2-
dichloropropane (propylene dichloride). II Influence of dietary
factors on the toxicity of dichloropropane. J Pharmacol Exp Ther,
87: 11-17.
Hermann BW & Matsuyama H (1982) Freezer storage stability of D-D
soil fumigant: Shell Development Company's monthly research summary
(July 1982). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Heungens A & Van Daele E (1974) Evolution of the soil fauna in
Begonia culture by repeated applications of dichloropropane-
dichloropropene. Agric Environ, 1: 251-258.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hine CH, Anderson HH, Kodama JK, Morse M, & McDaniel HC (1952)
Studies on the toxicity of CBP-55 and D-D. San Francisco, University
of California, School of Medicine, Division of Pharmacology and
Experimental Therapeutics (Prepared for Shell Development Company,
California) (Unpublished proprietary report No. U.C. 184, submitted
to WHO by Shell).
Hine CH, Anderson HH, Moon HD, Kodama JK, Morse M, & Jacobsen NW
(1953) Toxicology and safe handling of CBP-55 (Technical 1-Chloro-3-
bromopropene-1). Arch Ind Hyg Occup Med, 7: 118-136.
Hoogsteen KJ (1986) [Micro pollution in 3 water-winning areas.]
H2O, 19(3): 48-52 (in Dutch).
HSE (1990) Chlorinated hydrocarbon solvent vapours in air. Methods
for the determination of hazardous substances (MDHS 28). London,
Health and Safety Executive, Occupational Medicine and Hygiene
Laboratory, pp. 1-7.
Hutson DH (1984) The role of oxidation in the bacterial mutagenicity
of (Z)-1,3-dichloropropene. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report SBGR 83.049, submitted to WHO by
Shell).
Hutson DH & Stoydin G (1977) The reaction of D-D components with
glutathione. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0041-77, submitted to WHO by Shell).
Hutson DH, Moss JA, & Pickering BA (1971) The excretion and
retention of components of the soil fumigant D-D, and their
metabolites in the rat. Food Cosmet Toxicol, 9(5): 677-680.
IARC (1986) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer, pp 113-130, 131-
147 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, Volumes 1 to 42. Lyon, International Agency for
Research on Cancer, pp 195-196 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Imberti R, Calabrese SR, Emilio G, Marchi L, & Giuffrida L (1987)
[Acute solvent poisoning: aliphatic chlorinated hydrocarbons.] Min
Anest, 53: 399-403 (in Italian).
Imberti R, Mapelli A, Colombo P, Richelmi P, Berte F, & Bellomo G
(1990) 1,2-Dichloropropane (DCP) toxicity is correlated with DCP-
induced glutathione (GSH) depletion and is modulated by factors
affecting intracellular GSH. Arch Toxicol, 64: 459-465.
Industrial Biotest Laboratories Inc. (1972) Primary skin irritation
tests with three samples in albino rabbits. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Unpublished proprietary report
No. IBT A.2484, submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1975) Teratogenic study with
D-D in albino rats. Northbrook, Illinois, Industrial Biotest
Laboratories Inc. (Prepared for Shell Kagaku Kabushiki Kaisha)
(Unpublished proprietary report No. IBT 623-06212, submitted to WHO
by Shell).
Industrial Biotest Laboratories Inc. (1977a) Three generation
reproduction study with D-D in albino rats. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Prepared for Shell Kagaku
Kabushiki Kaisha) (Unpublished proprietary report No. IBT 621-06002,
submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1977b) Two year chronic and
toxicity study with D-D compound in beagle dogs. Northbrook,
Illinois, Industrial Biotest Laboratories Inc. (Prepared for Shell
Kagaku Kabushiki Kaisha) (Unpublished proprietary report No. IBT
651-06000, submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1978) Two year chronic and
toxicity study with D-D in albino rats. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Prepared for Shell Kagaku
Kabushiki Kaisha) (Unpublished proprietary report No. IBT 621-06001,
submitted to WHO by Shell).
Jeffrey MM (1987a) Telone II soil fumigant: Primary dermal
irritation study in New Zealand White rabbits. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report No. M-003993-
017B, submitted to WHO by Dow Chemical).
Jeffrey MM (1987b) Telone II soil fumigant: Primary eye irritation
study in New Zealand White rabbits. Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report No. M-003993-017C, submitted
to WHO by Dow Chemical).
Jeffrey MM (1987c) Telone II soil fumigant: Dermal sensitization
potential in the Hartley albino guinea-pig. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report No. DR-003993-017E,
submitted to WHO by Dow Chemical).
Jeffrey MM, Battjer JE, & Lomax LG (1987) Telone II soil fumigant.
Acute oral toxicity study in Fischer 344 rats. Internal report.
Midland, Michigan, Dow Chemical Company (Unpublished proprietary
report submitted to WHO by Dow Chemical).
John JA, Kloes PM, Calhoun LL, & Young JT (1983) Technical grade
1,3-dichloropropene: Inhalation teratology study in Fischer 344 rats
and New Zealand White rabbits. Internal report. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Johnson KA & Gorzinski SJ (1988) Neurotoxicologic examination of
rats exposed to 1,2-dichloropropane (DCP) via gavage for 13 weeks.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Jones AR & Gibson J (1980) 1,2-dichloropropane: metabolism and fate
in the rat. Xenobiotica, 10(11): 835-846.
Jones JR & Collier TA (1986a) Telone II: OECD 401 Acute oral
toxicity test in the rat (Project No. 44/83). Horgen, Switzerland,
Dow Chemical Europe S.A. (Unpublished proprietary report submitted
to WHO by Dow Chemical).
Jones JR & Collier TA (1986b) Telone II: OECD 402 Acute dermal
toxicity test in the rat (Project No. 44/84). Horgen, Switzerland,
Dow Chemical Europe S.A. (Unpublished proprietary report submitted
to WHO by Dow Chemical).
Jud VA, Kincke VL, O'Sullivan PK, Schweizer AD, & Parker CM (1980a)
Audit of Industrial Bio-Test Laboratories Study No. 621-06001: Two
year chronic oral toxicity study with D-D in albino rats (Regulatory
Information Record No. WRC RIR-5). Houston, Texas, Shell Development
Company (Unpublished proprietary report submitted to WHO by Shell).
Jud VA, Kincke VL, Schweizer AD, & Lu CC (1980b) Audit of Industrial
Bio-Test Laboratories Study No. 621-06002: Three generation
reproduction study with D-D in albino rats (Regulatory Information
Record No. WRC RIR-7). Houston, Texas, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Kämpfe K (1973) [Results of the combined application of anhydrous
ammonia and a mixture of dichloropropane and dichloropropene (DD) as
a nitrification inhibitor in autumn.] Arch Acker-Pflanzenbau
Bodenkd, 17(10): 827-835 (in German).
Kastl PE & Hermann EA (1983) Determination of cis- and trans-
1,3-dichloropropene in whole rat blood by gas chromatography and gas
chromatography-chemical ionization mass spectrometry with selected
ion monitoring. J Chromatogr, 265: 277-283.
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
Environ Saf, 4: 26-38.
Kier LE, Brusick DJ, Auletta AE, Von Halle ES, Brown MM, Simmon VF,
Dunkel V, McCann J, Mortelmans K, Prival M, Rao TK, & Ray V (1986)
The Salmonella typhimurium/mammalian microsomal assay. A report of
the US Environmental Protection Agency gene-Tox program. Mutat Res,
16: 69-240.
Kirk HD, Hanley TR Jr, Johnson KA, & Dietz FK (1989) Propylene
dichloride: Oral teratology study in Sprague-Dawley rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Kirk HD, Hanley TR Jr, Bond DM, Firchau HM, Peck CN, Stebbins KE, &
Johnson KA (1990) Propylene dichloride: Two-generation reproduction
study in Sprague-Dawley rats. Internal report. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Kline SA, McCoy EC, Rosenkranz HS, & Van Duuren BL (1982)
Mutagenicity of chloroalkene epoxides in bacterial systems. Mutat
Res, 101: 115-125.
Kloes PM, Calhoun LL, Young JT, & John JA (1983) Telone II:
Inhalation teratology probe study in Fischer 344 rats and New
Zealand White rabbits. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Toxicology, 19: 209-221.
Krijgsheld KR & Van der Gen A (1986) Assessment of the impact of the
emission of certain organochlorine compounds on the aquatic
environment. Part II: Allylchloride, 1,3- and 2,3-dichloropropene.
Chemosphere, 15(7): 861-880.
Lagas P, Verdam B, & Loch JPG (1989) Threat to groundwater quality
by pesticides in the Netherlands. In: Groundwater management:
Quantity and quality. Proceedings of the Benidorm Symposium, October
1989, pp 171-180 (IAHS Publication No. 188).
Larcan A, Lambert H, Laprevote MC, & Gustin B (1977) Acute poisoning
induced by dichloropropane. Acta Pharmacol Toxicol, 41: 330.
Lebbink G & Kolenbrander GJ (1974) Quantitative effect of fumigation
with 1,3-dichloropropene mixtures and with metham sodium on the soil
nitrogen status. Agric Environ, 1: 283-292.
Lebbink G, Proper B, & Nipshagen A (1989) Accelerated degradation of
1,3-dichloropropene. Acta Hortic, 255: 361-371.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc GA (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Leiber MA & Berk HC (1984) Development and validation of an air
monitoring method for 1,3-dichloropropene, trans-1,2,3-
trichloropropene cis-1,2,3-trichloropropene 1,1,2,3-
tetrachloropropene, 2,3,3-trichloro-2-propen-1-ol and 1,1,2,2,3-
pentachloropropane. Anal Chem, 56: 2134-2137.
Leistra M (1970) Distribution of 1,3-dichloropropene over the phases
in soil. J Agric Food Chem, 18(6): 1124-1126.
Leistra M & Boesten JJTI (1989) Pesticide contamination of
groundwater in Western Europe. Agric Ecosyst Environ, 26: 369-389.
Leistra M, Groen AE, Crum SJH, & Van der Pas LJT (1991)
Transformation rate of 1,3-dichloropropene and 3-chloroallyl alcohol
in topsoil and subsoil material of flower-bulb fields. Pestic Sci,
31: 197-207.
Li M (1979) A systems approach to controlling pesticides in the San
Joaquin Valley, Ecosystem studies of the National Science
Foundation, University of California, Davis.
Ligocki MP, Leuenberger C, & Pankow JG (1985) Trace organic
compounds in rain - II. Gas scavenging of neutral organic compounds.
Atmos Environ, 19(10): 1609-1617.
Linnett SL, Clark DG, Blair D, & Cassidy SL (1988) Effects of
subchronic inhalation of D-D (1,3-dichloropropene/1,2-
dichloropropane) on reproduction in male and female rats. Fundam
Appl Toxicol, 10: 214-223.
Loch JPG & Verdam B (1989) Pesticide residues in groundwater in the
Netherlands: State of observations and future directions of
research. Schriftenr Ver Wasser- Boden- Lufthyg, 79: 349-363.
Lomax LG, Calhoun LL, Stott WT, & Franson LE (1987) Telone II soil
fumigant: 2-year inhalation chronic toxicity-oncogenicity study in
rats (Study No. M 003993-009R). Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report submitted to WHO by Dow
Chemical).
Lomax LG, Stott WT, Johnson KA, Calhoun LL, Yano BL, & Quast JF
(1989) The chronic toxicity and oncogenicity of inhaled technical-
grade 1,3-dichloropropene in rats and mice. Fundam Appl Toxicol, 12:
418-431.
Loveday KS, Lugo MH, Resnick MA, Anderson BE, & Zeiger E (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro: II. Results with 20 chemicals.
Environ Mol Mutagen, 13: 60-94.
McCall PJ (1987) Hydrolysis of 1,3-dichloropropene in dilute aqueous
solution. Pestic Sci, 19: 235-242.
McKenry MV & Thomason IJ (1974) Dosage values obtained following
pre-plant fumigation for perennials. I 1,3-dichloropropene
nematicides in eleven field situations. Pestic Sci, 7: 521-534.
Maddy KT, Fong HR, Lowe JA, Conrad DW, & Fredrickson AS (1982) A
study of well water in selected Californian communities for residues
of 1,3-dichloropropene, chloroallyl alcohol and 49 organophosphate
or chlorinated hydrocarbon pesticides. Bull Environ Contam Toxicol,
29: 354-359.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. Rev Environ
Contam Toxicol, 114: 58-99.
Markovitz A & Crosby WH (1984) Chemical carcinogenesis: A soil
fumigant, 1,3-dichloropropene as possible cause of hematologic
malignancies. Arch Intern Med, 144: 1409-1411.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus
subtilis/microsome REC-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci Technol, 21: 875-887.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 506-553 (Resource Publication No.
160).
Mehta BV, Patel GJ, & Dangarwala RT (1963) Effects of fumigation and
other measures for nematode control on the production of nitrate in
Goradu soil. J Indian Soc Soil Sci, 11: 361-371.
Mendrala AL (1985) Evaluation of Telone II in the rat hepatocyte
unscheduled DNA synthesis assay. Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report submitted to WHO by Dow
Chemical).
Mendrala AL (1986) The evaluation of Telone II soil fumigant in the
Chinese hamster ovary cell/hypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Meyer AL (1980) Mutagenicity studies with cis-1,3-dichloropropene
in cultured mammalian cells. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report TLGR 80.022, submitted to WHO by
Shell).
Mitchell JR, Jollow DJ, Potter WZ, Gilette JR, & Brodie BB (1973)
Acetaminophen induced hepatic damage. IV. Protective role of
glutathione. J Pharmacol Exp Ther, 1877: 211-217.
Miyaoka T, Yamashita E, Hasegawa T, Akiyama M, Tsuda S, & Shirasu Y
(1990) Mechanism of 1,3-dichloropropene-induced hepatotoxicity in
mice. J Pestic Sci, 15: 419-425.
Moje W, Martin JP, & Baines RC (1957) Structural effects of some
organic compounds on soil organisms and citrus seedlings grown in an
old citrus soil. J Agric Food Chem, 5(10): 32-36.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol,
17: 405-429.
Nater JP & Gooskens VHJ (1976) Occupational dermatosis due to a soil
fumigant. Contact Dermatitis, 2: 227-229.
Neudecker T & Henschler D (1986) Mutagenicity of chloro-olefins in
the Salmonella/mammalian microsome test. III. Metabolic activation
of the allylic chloropropenes allylchloride, 1,3-dichloropropene,
2,3-dichloro-1-propene, 1,2,3-trichloropropene, 1,1,2,3-tetrachloro-
2-propene and hexachloropropene by S9 mix via two different
metabolic pathways. Mutat Res, 170: 1-9.
Neudecker T, Stefani A, & Henschler D (1977) In vitro mutagenicity
of the soil nematicide 1,3-dichloropropene. Experientia (Basel), 33:
1084-1085.
Neudecker T, Lutz D, Eder E, & Henschler D (1980) Structure-activity
relationship in halogen and alkyl substituted allyl and allylic
compounds: Correlation of alkylating and mutagenic properties.
Biochem Pharmacol, 29: 2611-2617.
Neuhauser EF, Loehr RC, & Malecki MR (1985a) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in
soil. In: Petros JK Jr, Lacy WJ, & Conway W ed. Hazardous and
industrial solid waste: 4th Symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM Special
Technical Publication No. 886).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985b)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Neuhauser EF, Durkin PR, Malecki MR, & Anatra M (1986) Comparative
toxicity of ten organic chemicals to four earthworm species. Comp
Biochem Physiol, 83C(1): 197-200.
NIOSH (1985) 1,2-Dichloropropane: Analytical method 1013. In: Eller
PM ed. NIOSH Manual of analytical methods. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 1, pp 1-
4.
Nitschke KD & Lomax LG (1990) Cis-1,3-dichloropropene: 2-Week
vapor inhalation toxicity study in Fischer 344 rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Nitschke KD, Johnson KA, Wackerle DL, Phillips JE, & Dittenber DA
(1988) Propylene dichloride: 13-Week inhalation toxicity study with
rats, mice and rabbits. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Nitschke KD, Crissman JW, & Schuetz DJ (1990) Cis-1,3-
dichloropropene: Acute inhalation study with Fischer 344 rats.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Nitschke KD, Lomax LG, & Sanderson TG (1991) Cis-1,3-
dichloropropene: 13-Week vapor inhalation toxicity study in Fischer
344 rats. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
NTP (1983) Carcinogenesis bioassay of 1,2-dichloropropane (propylene
dichloride) in F344/N rats and B6C3F1 mice (gavage study).
Research Triangle Park, North Carolina, National Toxicology Program
(NTP Technical Report No. 82-092; NIH publication No. 82-2519).
NTP (1985) Toxicology and carcinogenesis studies of Telone II, in
F344/N rats and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NTP
Technical Report No. 269; NIH-publication No. 85-2525).
NTP (1986) Toxicology and carcinogenesis studies of 1,2-
dichloropropane (propylene dichloride) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP Technical Report No. 263;
NIH Publication No. 86-2519).
O'Connor J (1990a) Cis-1,3-dichloropropene: Determination of
physico-chemical properties. Eye, United Kingdom, Life Science
Research Ltd (Unpublished proprietary report No. 90/SLK005/0461,
submitted to WHO by Shell).
O'Connor J (1990b) Cis-1,3-dichloropropene: Determination of
hydrolysis as a function of pH. Eye, United Kingdom, Life Science
Research Ltd (Unpublished proprietary report No. 90/SLK006/0479,
submitted to WHO by Shell).
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation
of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium
OB3b expressing soluble methane monooxygenase. Appl Environ
Microbiol, 55(11): 2819-2826.
Onkenhout W, Mulder PPJ, Boogaard PJ, Buys W, & Vermeulen NPE (1986)
Identification and quantitative determination of mercapturic acids
formed from Z- and E-1,3-dichloropropene by the rat, using
gaschromatography with three different detection techniques. Arch
Toxicol, 59: 235-241.
Orrenius S, Thor H, McConkey D, Nicotera P, & Bellomo G (1989)
Mechanism of cell toxicity: the thiol/calcium hypothesis. In:
Proceedings of the VII International Symposium on Microsomes and
Drug Oxidation. London, Taylor & Francis, pp 329-337.
Osterloh J, Letz G, Pond S, & Becker C (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse-sperm
morphology assay. Mutat Res, 116: 407-415.
Osterloh JD, Cohen BS, Popendorf W, & Pond SM (1984) Urinary
excretion of the N-acetyl cysteine conjugate of cis-1,3-
dichloropropene by exposed individuals. Arch Environ Health, 39(4):
271-275.
Osterloh JD, Wang R, Schneider F, & Maddy K (1989) Biological
monitoring of dichloropropene: Air concentrations, urinary
metabolite, and renal enzyme excretion. Arch Environ Health, 44(4):
207-213.
O'Sullivan PK, Schweizer AK, & Lu CC (1980) Audit of Industrial Bio-
Test Laboratories Study No. 623-06212: Teratogenic study with D-D in
albino rats (Regulatory Information Record No. WRC RIR-6). Houston,
Texas, Shell Development Company (Unpublished proprietary report
submitted to WHO by Shell).
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic
compounds in water and thirty Canadian potable water treatment
facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Ou L-T (1989) Degradation of Telone II in contaminated and
noncontaminated soils. J Environ Sci Health, B24(6): 661-574.
Parker CM (1980) Histopathologic evaluation of tissues and organs
from beagle dogs in a two-year chronic oral toxicity study fed D-D
compound (Regulatory Information Record No. WRC RIR-3). Houston,
Texas, Shell Development Company (Unpublished proprietary report
submitted to WHO by Shell).
Parker CM, Coate WB, & Voelker RW (1982) Subchronic inhalation
toxicity of 1,3-dichloropropene/1,2-dichloropropane (D-D) in mice
and rats. J Toxicol Environ Health, 9: 899-910.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc R Soc Lond, B189: 305-
332.
Peoples SA, Maddy KT, Cusick W, Jackson T, Cooper C, & Frederickson
AS (1980) A study of samples of well water collected from selected
areas in California to determine the presence of DBCP and certain
other pesticide residues. Bull Environ Contam Toxicol, 24: 611-618.
Perbellini L, Zedda A, Schiavon R, & Franchi GL (1985) [Two cases of
disseminated intravascular coagulation syndrome (DIC) caused by
exposure to 1,2-dichloropropane (commercial trichloroethylene).] Med
Lav, 76(5): 412-417 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol Lett, 16: 69-75.
Perry J & Roberts TR (1974) The degradation and leaching of Z and E-
3-chloroacrylic acids in soils. Sittingbourne, Shell Research Ltd.
(Unpublished proprietary report WKGR 0098.74, submitted to WHO by
Shell).
Pochon J, Lajudie J, & Coppier O (1951) Remarques sur les recherches
relatives à l'action de certaines substances antiparasitaires sur la
microflore du sol. Ann Inst Pasteur, 80: 517-519.
Ponticelli C, Imbasciati E, Redaelli B, & Salvadeo A (1968) [Acute
hepatorenal insufficiency due to trielene.] Lav Um, 20: 205-212 (in
Italian).
Portmann JE & Wilson KW (1971) The toxicity of 140 substances to the
Brown shrimp and other marine animals. London, Ministry of
Agriculture, Fisheries and Food (Shellfish Information Leaflet No.
22).
Pozzi C, Marai P, Ponti R, Dell'Oro C, Sala C, Zedda S, & Locatelli
F (1985) Toxicity in man due to stain removers containing 1,2-
dichloropropane. Br J Ind Med, 42: 770-772.
Price JB & Andrews IJ (1985) The in vitro assessment of eye
irritancy using enucleated eyes. Food Chem Toxicol, 23(2): 313-315.
Priston RAJ, Brooks TM, Hodson-Walker G, & Wiggins DE (1983)
Genotoxicity studies with 1,2-dichloropropane. Sittingbourne, Shell
Research Ltd (Unpublished proprietary report SBGR 83.083, submitted
to WHO by Shell).
Rader WE & Love JW (1977a) Impact of commercial Shell products on
microorganisms in the soil. Part. I. Effect on modulation on pinto
beans and on the nitrogen fixation bacteria (Technical Information
Record No. TIR-22-112-77, Part I). Modesto, California, Shell
Development Company (Unpublished proprietary report submitted to WHO
by Shell).
Rader WE & Love JW (1977b) Impact of commercial Shell pesticides on
microorganisms in the soil. Part. II. Effect on decomposition of
cellulose in the soil (Technical Information Record No. TIR-22-112-
77, Part II). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE, Love JW, & Chai EY (1978) Impact of commercial Shell
pesticides on microorganisms in the soil. Part III. Effect of
compounds on the populations of microorganisms and the O2/CO2
exchange in treated soil (Technical Information Record No. TIR-22-
112-77, Part III). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE (1979a) Impact of commercial Shell pesticides on
microorganisms in the soil. Part IV. The effect of the compounds on
soil nitrification (Technical Information Record No. TIR-22-112-77,
Part IV). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE (1979b) The effects of Shell pesticides on protein
decomposition (Technical Information Record No. TIR-51-108-79).
Modesto, California, Shell Development Company (Unpublished
proprietary report submitted to WHO by Shell).
Rader WE (1979c) The effect of Shell pesticides on soil phosphatase
activity (Technical Information Record No. TIR-51-110-79). Modesto,
California, Shell Development Company (Unpublished proprietary
report submitted to WHO by Shell).
Rexilius L & Schmidt H (1982) Investigations into the migratory
behaviour of 1,3-dichloropropene and methylisothiocyanate in the
soil of the nursery areas. Nachrichtenbl Dtsch Pflanzenschutzd,
34(11): 161-165.
Reiff B (1975) Soil fumigant D-D: its toxicity to fish.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
TLGR 0035.75, submitted to WHO by Shell).
Reiff B (1978) The acute toxicity of 1,3-dichloropropene to the
Golden orfe (Idus idus melanotus). Sittingbourne, Shell Research
Ltd (Unpublished proprietary report TLGR 0057.78, submitted to WHO
by Shell).
Reinert KH, Hunter JV, & Sabatine T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue
samples. J Agric Food Chem, 31: 1057-1060.
Rick DL & McCarty LP (1988) The determination of the odor threshold
of Telone(R) II soil fumigant by a new method. Appl Ind Hyg, 3(11):
299-302.
Roberts TR & Stoydin G (1976) The degradation of (Z)-and (E)-1,3-
dichloropropenes and 1,2-dichloropropane in soil. Pestic Sci, 7:
325-335.
Rocchi PSJ & Van Sittert NJ (1989) A monitoring study of the
exposure to 1,3-dichloropropene during application in France of
Shell D-D and Shell D-D 92 nematocide. Internal report. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Unpublished
proprietary report submitted to WHO by Shell).
Rosenblum I & Talley W (1979) Evaluation of instrumental behavioural
performance of Rhesus monkeys after acute exposure to Telone II.
Albany, New York, Institute of Comparative and Human Toxicology
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Ross DJ & McNeilly BA (1975) Influence of four nematicides on soil
nitrogen mineralisation and nitrogen uptake by white clover in a
yellow-grey earth. NZ J Agric Res, 18: 155-162.
Schiffmann D, Eder E, Neudecker T, & Henschler D (1983) Induction of
unscheduled DNA synthesis in Hela cells by allylic compounds. Cancer
Lett, 20(3): 263-269.
Schuurman P (1989) Physico-chemical properties of trans-1,3-
dichloropropene. Part II. Rijswijk, The Netherlands, Organization
for Applied Scientific Research (Unpublished proprietary report No.
PML 1989-C35, submitted to WHO by Shell).
Schweizer AK & Parker CM (1980) Audit of Industrial Bio-test
Laboratories Study No. 651-06000: Two-year chronic oral toxicity
study with D-D compound in beagle dogs (Regulatory Information
Record No. WRC RIR-4). Houston, Texas, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Shell (1976) Determination of residues of 1,2-dichloropropane, 1,3-
dichloropropene (Z and E isomers), and 3-chloro-allyl alcohol (Z and
E isomers) in crops and soil. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report No. WAMS 222-1, submitted to WHO by
Shell).
Shell (1978) Residue determination of the E-and Z-isomers of 3-
chloroallylalcohol (CAA) in crops and soil. Modesto, California,
Shell Development Company (Unpublished proprietary report No. MMS-R-
481-1, submitted to WHO by Shell).
Shell (1980) Residue determination of (E) and (Z)-isomers of 3-
chloroallylalcohol in agricultural commodities and water.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
No. MMS-R-506-1, submitted to WHO by Shell).
Shell (1984) Residue determination of 1,2-dichloropropane and the Z-
and E-isomers of 1,3-dichloropropene in agricultural commodities,
soil and water. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report No. MMS-R-505-3, submitted to WHO by Shell).
Shell (1985) D-D/D-D92. Review of the occurrence and fate of
residues in crops and the environment. London, Shell International
Chemical Company Ltd, Regulatory Affairs, Crop Protection Division
(Unpublished proprietary report submitted to WHO by Shell).
Shell IPM (1990) Review of environmental toxicology D-D92 (1,3-
dichloropropene). The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Review Series HSE 90.001) (Unpublished
proprietary report submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987a) "D-D 92" residue and
environmental fate studies - Small scale field studies. Internal
report. London, Shell Research Ltd (Unpublished proprietary report
SBGR 87.170, submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987b) "D-D 92" residue and
environmental fate studies - Development of air monitoring methods.
Internal report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 87.141, submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987c) "D-D 92" residue and
environmental fate studies - Volatilization of "D-D 92" following
treatment of soil in an outdoor enclosure. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 87.157,
submitted to WHO by Shell).
Sherren AJ (1990) D-D 92 residue and environmental fate studies.
Field study - France - Air monitoring. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 89.127,
submitted to WHO by Shell).
Shirasu Y, Moriya M, Tezuka H, Teramoto S, Ohta T, & Inoue T (1981)
Mutagenicity screening studies on pesticides. In: Environmental
mutagens and carcinogens: Proceedings of the Third International
Conference on Environmental Mutagens, Tokyo, Mishima and Kyoto, 21-
27 September 1981, pp 331-335.
Sittig M (1980) Priority toxic pollutants. Health impacts and
allowable limits. Park Ridge, New Jersey, Noyes Data Corporation, pp
192-196.
Smelt JH, Teunissen W, Crum SJH, & Leistra M (1989) Accelerated
transformation of 1,3-dichloropropene in loamy soils. Neth J Agric
Sci, 37: 173-183.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani VC, Striegel JA, & Nycum
JS (1969) Range-finding toxicity data: List VII. Am Ind Hyg Assoc J,
30(5): 470-476.
Sommer K (1970) [Influence of different pesticides on nitrification
and nitrogen transformation in soils.] Landwirtsch Forsch, 25: 22-30
(in German with English summary).
Stevens JL, Ayoubi N, & Robbins JD (1988) The role of mitochondrial
matrix enzymes in the metabolism and toxicity of cysteine
conjugates. J Biol Chem, 263: 3395-3401.
Stolzenberg SJ & Hine CH (1980) Mutagenicity of 2- and 3-carbon
halogenated compounds in the Salmonella/mammalian microsome test.
Environ Mutagen, 2(1): 59-66.
Stott WT & Kastl PE (1985) Inhalation pharmacokinetics of technical
grade 1,3-dichloropropene in rats. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Stott WT & Kastl PE (1986) Inhalation pharmacokinetics of technical
grade 1,3-dichloropropene in rats. Toxicol Appl Pharmacol, 85(3):
332-341.
Stott WT, Young JT, Calhoun LL, & Battjes JE (1984) Telone II, soil
fumigant: 13-week inhalation study in rats and mice. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Stott WT, Johnson KA, Calhoun LL, Weiss SK, & Franson LE (1987)
Telone II soil fumigant: 2-year inhalation chronic toxicity-
oncogenicity study in mice (Study No. M-003993-009). Midland,
Michigan Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Stott WT, Young JT, Calhoun LL, & Battjes JE (1988) Subchronic
toxicity of inhaled technical grade 1,3-dichloropropene in rats and
mice. Fundam Appl Toxicol, 11: 207-220.
Stott WT, Waechter JM, & Quast JT (1990) Letter to the editor. Arch
Environ Health, 45: 250-253.
Straley JP, Christiansen JA, & Kopecky AL (1982) Improved biological
degradation chlorinated hydrocarbons using mutant bacteria. Proc Int
Water Conf Eng Soc West, 43: 523-531.
Streeter CM, Battjes JE, & Lomax LG (1987) Telone II soil fumigant.
An acute vapor inhalation study in Fischer 344 rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Sullivan DA, Jones AD, & Williams JG (1985) Results of the US
Environmental Protection Agency's air toxics analysis in
Philadelphia (Prepared for presentation at the 78th Annual Meeting
of the Air Pollution Control Association, Detroit, Michigan, 16-21
June 1985). Washington, DC, US Environmental Protection Agency, pp
1-15 (Report 85-17.5).
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503.
Talcott RE & King J (1984) Mutagenic impurities in 1,3-
dichloropropene preparations. J Natl Cancer Inst, 72(5): 1113-1116.
Telliard WA (1990) Broad-range methods for determination of
pollutants in wastewater. J Chromatogr Sci, 28: 453-459.
Thomason IJ & McKenry MV (1974) Part I. Movement and fate as
affected by various conditions in several soils. Hilgardia, 42: 392-
420.
Thorel JM, Bercoff E, Massari Ph, Droy JM, Chassagne Ph, Proust B,
Hemet J, & Bourreille J (1986) Toxicité du 1,2-dichloropropane: à
propos d'un cas avec hypertension portale. J Toxicol Clin Exp, 6(4):
247-252.
Thornton GD (1951) Some effects of D-D, EDB and chloropicrin on
microbiological action in several Florida soils. Proc Soil Sci Soc
Florida, 1951(11): 68-71.
Til HP, Spanjers MTh, Feron VJ, & Renzel PJG (1973) Sub-chronic (90-
day) toxicity study with Telone in albino rats (Final report).
Zeist, The Netherlands, Central Institute for Nutrition and Food
Research (Unpublished proprietary report No. R-4002, submitted to
WHO by Dow Chemical).
Timchalk C, Bartels MJ, Dryzga MD, & Smith FA (1989) Propylene
dichloride: Pharmacokinetics and metabolism in Fischer 344 rats
following oral and inhalation exposure. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Torkelson TR & Oyen F (1977) The toxicity of 1,3-dichloropropene as
determined by repeated exposure of laboratory animals. Am Ind Hyg
Assoc J, 38: 217-223.
Toyoshima S, Sato R, & Sato S (1978a) The acute toxicity test of
Telone II in mice (Unpublished proprietary report submitted to WHO
by Dow Chemical).
Toyoshima S, Sato R, & Sato S (1978b) The acute toxicity test of
Telone II in rats. (Unpublished proprietary report submitted to WHO
by Dow Chemical).
Trevisan A, Rizzi E, Bungaro A, Pozzobon L, Gioffre F, Scapinello A,
Valeri A, & Chiesura P (1988) Proximal tubule brush border
angiotensin converting enzyme behaviour and nephrotoxicity due to
1,2-dichloropropane. The target organ and the toxic process. Arch
Toxicol, 12(Suppl): 190-192.
Trevisan A, Rizzi E, Scapinello A, Gioffre F, & Chiesura P (1989)
Liver toxicity due to 1,2-dichloropropane in the rat. Arch Toxicol,
63: 445-449.
Trevisan A, Troso O, & Maso S (1991) Recovery of biochemical changes
induced by 1,2-dichloropropane in rat liver and kidney. Hum Exp
Toxicol, 10: 241-244.
Tu CM (1972) Effects of four nematocides on activities of
microorganisms in soil. Appl Microbiol, 23(2): 398-401.
Tu CM (1973) Effects of Mocap. N-Serve, Telone, and Vorlex at two
temperatures on populations and activities of microorganisms in
soil. Can J Plant Sci, 53: 401-405.
Tu CM (1978) Effect of pesticides on acetylene reduction and
microorganisms in a sandy loam. Soil Biol Biochem, 10: 451-456.
Tu CM (1979) Influence of pesticides on acetylene reduction and
growth of microorganisms in an organic soil. J Environ Sci Health,
B14(6): 617-624.
Tu CM (1981a) Effects of pesticides on activities of enzymes and
microorganisms in a clay soil. J Environ Sci Health, B16(2): 179-
191.
Tu CM (1981b) Effects of some pesticides on enzyme activities in an
organic soil. Bull Environ Contam Toxicol, 27: 109-114.
Tu CM (1988) Effects of selected pesticides on activities of
invertase, amylase and microbial respiration in sandy soil.
Chemosphere, 17(1): 159-163.
Tuazon EC, Atkinson R, Winer AM, & Pitts JN Jr (1984) A study of the
atmospheric reactions of 1,3-dichloropropene and other selected
organochlorine compounds. Arch Environ Contam Toxicol, 13: 691-700.
US EPA (1980) Ambient water quality criteria for dichloropropane and
dichloropropene. Washington, DC, US Environmental Protection Agency,
Office of Water Regulations and Standards Criteria (Report No.
440/5-80-043, PB 81-117541).
US EPA (1985) Health and environmental effects profile for 1,3-
dichloropropene. US Cincinnati, Ohio, Environmental Protection
Agency, Environmental Criteria and Assessment Office (EPA/600/X-85-
399).
US EPA (1990) Inhalation reference concentration for 1,3-
dichloropropene (542-75-6). Fairfax, Virginia, Clement International
Corporation (Prepared for the US Environmental Protection Agency,
Washington).
US EPA (1991) 1,3-Dichloropropene. CAS No. 542-75-6. Washington, DC,
US Environmental Protection Agency (Unpublished report).
Valencia R, Mason JM, Woodruff RC, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 325-348.
Van Beek CGEM, Janssen HMJ, & Puijker LM (1988) [Pesticides in
groundwater.] H2O, 21(4): 80-85 (in Dutch).
Van Den Berg F & Leistra M (1989) Behaviour of the fumigant 1,3-
dichloropropene in soil and its emission into the air. Internal
report. Wageningen, The Netherlands, The Winand Staring Centre for
Integrated Land, Soil and Water Research.
Van Den Brande J & Heungens A (1969) Influence of repeated
applications of nematicides on the soil fauna in Begonia culture.
Neth J Plant Pathol, 75: 40-44.
Van Der Pas LJT & Leistra M (1987) Movement and transformation of
1,3-dichloropropene in the soil of flower-bulb fields. Arch Environ
Contam Toxicol, 16: 417-422.
Van Dijk H (1974) Degradation of 1,3-dichloropropenes in the soil.
Agro-ecosystems, 1: 193-204.
Van Dijk H (1980) Dissipation rates in soil of 1,2-dichloropropane
and 1,3- and 2,3-dichloropropenes. Pestic Sci, 11: 625-632.
Van Duuren BL, Goldschmidt BM, Loevengart G, Smith AC, Melchionne S,
Seidman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63(6): 1433-
1439.
Van Duuren BL, Kline SA, Melchionne S, & Seidman J (1983) Chemical
structure and carcinogenicity relationships of some chloroalkene
oxides and their parent olefins. Cancer Res, 43: 159-162.
Van Hooidonk C (1989) Physico-chemical properties of trans-1,3-
dichloropropene. Rijswijk, The Netherlands, Organization for Applied
Scientific Research (Unpublished proprietary report PML 1989-C118,
submitted to WHO by Shell).
Van Joost Th & De Jong G (1988) Sensitization to DD soil fumigant
during manufacture. Contact Dermatitis, 18(5): 307-308.
Van Sittert NJ (1978) D-D monitoring studies on field workers in
France. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Unpublished proprietary report TOX 78-001, submitted to WHO by
Shell).
Van Sittert NJ (1984) Biomonitoring of chemicals and their
metabolites. In: Berlin A, Draper M, Hemminki K, & Vainio H ed.
Monitoring human exposure to carcinogenic and mutagenic agents.
Lyon, International Agency for Research on Cancer, pp 153-172 (IARC
Scientific Publications No. 59).
Van Sittert NJ (1989) Individual exposure monitoring from plasma or
urinary metabolite determination. Arch Toxicol 13(Suppl): 91-100.
Van Sittert NJ, Van der Harst J, & Verhoeven PF (1977) D-D
monitoring studies on field workers in Holland. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Unpublished proprietary
report TOX 77-001, submitted to WHO by Shell).
Van Welie RTH, Van Duyn P, & Vermeulen NPE (1989) Determination of
two mercapturic acid metabolites of 1,3-dichloropropene in human
urine with gas chromatography and sulphur-selective detection. J
Chromatogr, 496: 463-471.
Van Welie RTH, Van Duyn P, Brouwer DH, Van Hemmen JJ, Brouwer EJ, &
Vermeulen NPE (1991) Inhalation exposure to 1,3-dichloropropene in
the Dutch flower-bulb culture. Part.II. Biological monitoring by
measurement of urinary excretion of two mercapturic acid
metabolites. Arch Environ Contam Toxicol, 20: 6-12.
Varanka I (1979) Effect of some pesticides on the rhythmic adductor
muscle activity of fresh-water mussel larvae. Symp Biol Hung, 19:
177-196.
Venable JR, McClimans CD, Flake RE, & Dimick DB (1980) A fertility
study of male employees engaged in the manufacture of glycerine. J
Occup Med, 22(2): 87-91.
Vithayathil AJ, McClure C, & Myers JW (1983) Salmonella/microsome
multiple indicator mutagenicity test. Mutat Res, 121:(1) 33-37.
Von Der Hude W, Scheutwinkel M, Gramlich U, Fiszler B, & Basler A
(1987) Genotoxicity of three-carbon compounds evaluated in the SCE
test in vitro. Environ Mutagen, 9: 401-410.
Von Der Hude W, Behm C, Gurtler R, & Basler A (1988) Evaluation of
the SOS chromotest. Mutat Res, 203: 81-94.
Waechter JM Jr & Kastl PE (1988) 1,3-Dichloropropene:
Pharmacokinetics and metabolism in Fischer 344 rats following
repeated oral administration. Internal report. Midland, Michigan,
Dow Chemical Company, (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Wallbridge CT, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol, 12:
661-666.
Walker AIT (1968a) The toxicity of D-D soil fumigant (1,2-
dichloropropane: 1,3-dichloropropene). 13-week oral toxicity
experiment in rats. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0018.68, submitted to WHO by Shell).
Walker AIT (1968b) The toxicity of D-D soil fumigant (1,2-
dichloropropane: 1,3-dichloropropene). 13-week oral toxicity
experiment in dogs. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0019.68, submitted to WHO by Shell).
Wallace BG (1974) Development of methods for the determination of
residues of free and bound 3-chloroallylalcohol in soil and crops.
London, Shell Research Ltd (Unpublished proprietary report WKGR
0067.74, submitted to WHO by Shell).
Wallace BG (1976a) Residues of the major components of D-D and
primary metabolites in soil from Germany. London, Shell Research Ltd
(Unpublished proprietary report WKGR 0051.76, submitted to WHO by
Shell).
Wallace BG (1976b) Residues of the major components of D-D and
primary metabolites in soil and crops from Holland. London, Shell
Research Ltd (Unpublished proprietary report WKGR 0052.76, submitted
to WHO by Shell).
Wallace BG (1979) Residues of D-D and 1,3-D in soil from the UK.
London, Shell Research Ltd (Unpublished proprietary report BLGR
79.093, submitted to WHO by Shell).
Watson WP, Lang KL, Brooks TM, Huckle KR, & Wright AS (1986a)
Genotoxicity studies with (Z)-1,3-dichloropropene: Mutagenic
contaminants. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report SBGR 85.246, submitted to WHO by Shell).
Watson WP, Lang KL, Brooks TM, & Wright AS (1986b) Genotoxicity
studies with (Z)-1,3-dichloropropene: Bioactivation by mammalian
mono-oxygenases and the protective role of glutathione.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
SBGR 86.056, submitted to WHO by Shell).
Watson WP, Brooks TM, Huckle KR, Hutson DH, Lang KL, Smith RJ, &
Wright AS (1987) Microbial mutagenicity studies with (Z)-1,3-
dichloropropene. Chem-Biol Interact, 61: 17-30.
Wensley RN (1953) Microbiological studies of the action of some
selected soil fumigants. Can J Bot, 31: 277-308.
WHO (in preparation) Guidelines for drinking-water quality, 2nd ed.
Geneva, World Health Organization.
Williams IH (1968) Recovery of cis- and trans-dichloropropene
residues from 2 types of soil and their detection and determination
by electron capture gas chromatography. J Econ Entomol, 61(5): 1432-
1435.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 677-702.
Worthing CR & Hance RJ (1991) The pesticide manual: A world
compendium, 9th ed. Croydon, The British Crop Protection Council, pp
254-255.
Wright AS & Creedy CL (1982) The protective action of glutathione on
the microbial mutagenicity of (Z)-1,3-dichloropropene.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
SBGR 81.317, submitted to WHO by Shell).
Yakel HO & Kociba RJ (1977) Acute inhalation toxicity of M-3993
(Telone II) in rats. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Yang RSH (1986) 1,3-Dichloropropene. Residue Rev, 97: 19-35.
Yang RSH, Huff JE, Boorman GA, Haseman JK, Kornreich M, & Stookey JL
(1986) Chronic toxicology and carcinogenesis studies of Telone II by
gavage in Fischer 344 rats and B6C3F1 mice. J Toxicol Environ
Health, 18: 377-392.
Yano BL, Calhoun LL, Stott W, Johnson KA, & Schuetz DJ (1985) Telone
II soil fumigant: 2 year inhalation chronic toxicity-oncogenicity
study in mice. Interim report: 6- and 12-month exposures. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Yon DA, Morrison GA, & McGibbon, A.S. (1991) The dissipation of 1,3-
dichloropropene in ditch bottom sediment and associated aerobic
ditch water. Pestic Sci, 32: 147-159.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1,3-DICHLOROPROPENE
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le "1,3-dichloropropène" a été introduit en agriculture en
1956, mélangé à des 1,3-dichloropropènes, du 1,2-dichloropropane et
d'autres hydrocarbures halogénés. On l'utilise depuis largement
comme fumigant du sol avant plantation pour lutter contre les
nématodes qui parasitent les légumes, les pommes de terre et le
tabac. L'application s'effectue essentiellement par injection dans
le sol. La formulation du commerce consiste en un mélange d'isomères
cis et trans (en proportions approximativement égales), et se
présente sous la forme d'un liquide incolore à ambré dont l'odeur
pénétrante et irritante rappelle celle du chloroforme. Sa tension de
vapeur est de 3.7 kPa à 20 °C. Le produit technique a une pureté de
92% et peut contenir certaines impuretés, comme le 1,2-
dichloropropane. Le coefficient de partage octanol/eau (log Kow)
est égal à 1,98.
Dans l'air, la décomposition du 1,3-dichloropropène s'effectue
principalement par réaction avec des radicaux libres et l'ozone.
Dans le cas de la réaction avec les radicaux libres, la demi-vie des
isomères cis- et trans- est respectivement égale à 12 et 7
heures et dans le cas de la réaction avec l'ozone, de 52 et 12
jours. Il semble que la phototransformation directe soit négligeable
mais elle pourrait être favorisée par la présence de particules en
suspension dans l'atmosphère.
Dans l'eau, il est probable que le 1,3-dichloropropène
disparaît rapidement du fait de sa solubilité relativement faible et
de sa forte volatilité; on a fait état d'une demi-vie de moins de 5
heures.
La distribution du 1,3-dichloropropène dans les différents
compartiments du sol dépend de la tension de vapeur, du coefficient
de diffusion, de la température et de la teneur en eau. La
persistance du 1,3-dichloropropène dans le sol dépend de sa
volatilisation, des transformations chimiques, photo-chimiques ou
biologiques qu'il subit et de sa fixation par les êtres vivants. La
volatilisation et la diffusion en phase gazeuse sont les mécanismes
les plus importants de sa dispersion et de la dilution dans le
milieu.
Dans l'environnement, la transformation du 1,3-dichloropropène
commence par une hydrolyse en alcool 3-chloro-allylique puis, sous
l'action des microorganismes, en 3-chloro-acroléine et en acide 3-
chloro-acrylique. Une étude de laboratoire a montré que le temps de
demi-hydrolyse des isomères cis- et trans- du 1,3-
dichloropropène à 15 °C et à 29 °C était respectivement égal, pour
l'isomère cis, à 11 et 2 jours et pour l'isomère trans, à 13 et
2 jours. Dans le sol, à un pH de 7, on a observé un temps de demi-
hydrolyse à 25 °C de 4,6 jours pour les deux isomères. Du fait que
le composé disparaît relativement vite du sol, il est peu probable
que des résidus s'y accumulent après application du fumigant à la
dose et selon la périodicité recommandées.
Le 1,3-dichloropropène est potentiellement mobile dans le sol,
en particulier dans les sols sableux à texture lâche dont la teneur
en eau est faible. Son cheminement en profondeur est favorisé par
les cultures profondes dans des sols de faible porosité. On a décelé
du 1,3-dichloropropène dans les nappes souterraines peu profondes
(jusqu'à 2 m en-dessous de la surface) mais non dans les eaux
profondes, c'est-à-dire celles qui ont le plus de chances d'être
utilisées pour la consommation humaine.
Le 1,3-dichloropropène peut-être fixé par les plantes
cultivées. Toutefois, il est peu probable qu'il donne lieu à des
résidus importants dans les cultures vivrières car celles-ci sont en
principe plantées lorsque la majeure partie du fumigant s'est
dissipée.
La bioaccumulation du 1,3-dichloropropène est peu probable car
il possède une solubilité dans l'eau relativement forte (> 1 g/kg),
un coefficient de partage octanol/eau faible (log Kow) et il est
rapidement éliminé chez les mammifères et autres organismes.
1.2 Cinétique et métabolisme
Après administration par voie orale à des rongeurs, le 1,3-
dichloropropène est rapidement éliminé. La principale voie
d'élimination est la voie urinaire, avec 81% de l'isomère cis et
56% de l'isomère de trans excrétés dans les 24 heures suivant
l'administration. La demi-vie d'élimination dans l'urine est de 5 à
6 heures. L'élimination dans les matières fécales est minime. Le
1,3-dichloropropène est éliminé à hauteur de 4% (isomère cis) et
de 24% (isomère trans) dans le dioxyde de carbone expiré. Après
l'administration, les concentrations tissulaires sont faibles; les
concentrations résiduelles les plus élevées se retrouvent dans la
paroi gastrique, puis, à des valeurs plus faibles, dans les reins,
le foie et la vessie.
On ne retrouve pas de 1,3-dichloropropène non modifié dans les
urines. Les isomères cis et trans tiennent lieu de substrats à
la glutathion- S-alkyltransférase hépatique qui les transforme en
acides mercapturiques, excrétés ensuite dans les urines. Le
principal métabolite urinaire chez le rat et la souris est la N-
acétyl- S-(3-chloroprop-2-ényl)L-cystéine; ce composé peut être
utilisé pour la surveillance biologique chez l'homme. On a observé
une deuxième voie métabolique d'importance secondaire dans le cas de
l'isomère cis; il s'agit d'une mono-oxygénation en cis-1,3-
dichloropropène oxyde, composé qui peut ensuite être conjugué avec
le glutathion. La forte proportion d'isomère trans présente dans
l'air expiré résulte d'une autre voie métabolique conduisant à la
conjugaison, voie qui est plus spécifique de l'isomère trans que
de l'isomère cis.
Exposés par voie respiratoire à du 1,3-dichloropropène, des
rats n'ont pas présenté une augmentation du taux sanguin
proportionnelle à la dose. A la dose de 408,6 mg/m3 (90 ppm), la
fréquence respiratoire et le volume expiratoire-minute étaient
réduits et l'on notait une saturation du métabolisme à 1362 mg/m3
(300 ppm). Les isomères cis et trans ont été rapidement éliminés
du courant sanguin, avec une demi-vie d'élimination de 3 à 6 minutes
pour des concentrations inférieures à 1362 mg/m3, mais beaucoup
plus longue (33 à 43 minutes) à plus forte concentration.
1.3 Effets sur les êtres vivant dans leur milieu naturel
Les valeurs de la CE50 relatives à la croissance (à 96 h)
chez une algue d'eau douce, Selenastrum capricornutum, et chez une
diatomée estuarielle, Skeletoneria costatum, sont respectivement
égales à 4,95 mg/litre et 1 mg/litre. Pour les poissons, la toxicité
aiguë du 1,3-dichloropropène (CL50 à 96 h), est de l'ordre de 1 à
7,9 mg/litre. Un test effectué sur les stades embryo-larvaires d'un
vairon, Pimephales promelas, ont donné une dose maximale sans
effet observable de 0,24 mg/litre. Ces données, jointes au fait que
le 1,3-dichloropropène ne persiste vraisemblablement pas dans l'eau,
indiquent que le danger pour les poissons réside dans les effets
toxiques aigus de ce composé, mais qu'il y a peu de risques d'effets
supplémentaires résultant d'une exposition à long terme.
Aux doses de 30 à 60 mg/kg, le 1,3-dichloropropène peut réduire
l'abondance des champignons et l'activité des enzymes microbiennes,
mais cet effet n'est généralement pas de longue durée (< 7 jours)
et ne se produit pas dans tous les types de sol. Certaines études
ont fait ressortir un accroissement sensible du nombre de
microorganismes après application du fumigant.
Le 1,3-dichloropropène est phytotoxique mais en revanche, il
est peu toxique pour les abeilles. On a constaté, en épandant du
1,3-dichloropropène par poudrage, que la DL50 à 48 h était de 6,6
µg/abeille. Les oiseaux sont relativement insensibles au 1,3-
dichloropropène. La CL50 (8 jours) est inférieure à 10 g/kg de
nourriture pour le col-vert et les cailles du genre Colinis.
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale du 1,3-dichloropropène est
modérée à forte chez les animaux d'expérience. En ce qui concerne le
rat, on a fait état, pour la DL50, de valeurs se situant entre 127
et 713 mg/kg de poids corporel. Par voie orale, ces valeurs étaient
respectivement de 85 et 94 mg/kg de poids corporel pour les isomères
cis et trans.
En cas d'exposition cutanée, le composé est modérément toxique.
Chez le rat et le lapin, on a obtenu pour la DL50 des valeurs
respectivement égales à 423 et 504 mg/kg de poids corporel. Ces
valeurs étaient respectivement de 1090 mg pour l'isomère cis et de
1575 mg/kg de poids corporel pour l'isomère trans.
Chez des rats exposés pendant 4 h par la voie respiratoire à du
1,3-dichloropropène, on a obtenu une CL50 de 3310 mg/m3 (729
ppm); les valeurs allaient de 3042 mg/m3 à 3514 mg/m3 pour
l'isomère cis et de 4880 mg/m3 à 5403 mg/m3 pour l'isomère
trans.
En cas d'intoxication aiguë, on observe une atteinte nerveuse
centrale et une atteinte respiratoire.
Des réactions graves ont été observées chez le lapin lors
d'épreuves d'irritation cutanée et oculaire mais les animaux ont
récupéré en l'espace de 14 à 21 jours. Les épreuves de
sensibilisation cutanée chez le cobaye se sont révélées positives.
Plusieurs études de toxicité respiratoire à court terme ont été
effectuées sur des souris, des rats, des cobayes, des lapins et des
chiens. Chez la souris, c'est les muqueuses nasales et la vessie qui
étaient les organes cibles. On a observé une dégénérescence de
l'épithélium olfactif et une hyperplasie de l'épithélium
respiratoire. Au niveau de la vessie, on a observé une hyperplasie
modérée de l'épithélium de transition. On a pu fixer à 136 mg/kg,
soit 30 ppm, la dose sans effet observable chez la souris.
Chez le rat, on a observé également une hyperplasie ainsi que
des modifications dégénératives analogues au niveau de l'épithélium
olfactif. Une étude bien conçue a permis d'obtenir une valeur de
4,35 mg/m3 pour la dose sans effet observable; dans le cas précis
de l'isomère cis, cette dose était égale à 136 mg/m3.
Une étude de 90 jours consistant à administrer le composé par
voie orale à des rats a permis de fixer à 3 mg/kg de poids corporel
la dose sans effet observable. Le seul effet observé à la dose
immédiatement supérieure (10 mg/kg de poids corporel) consistait en
une augmentation du poids relatif des reins chez les mâles.
Une étude de reproduction portant sur deux générations et deux
portées de rats, à des doses allant jusqu'à 408,6 mg/m3 (90 ppm),
n'a pas fait ressortir d'effets indésirables sur les paramètres
examinés. Toutefois, la dose la plus forte (408,6 mg/m3) était
toxique pour les mères, comme l'ont montré les deux anomalies
observées: réduction de la croissance et modification
histopathologique au niveau de la muqueuse nasale. Les résultats de
cette étude ont permis de fixer à 136,2 mg/m3 (30 ppm) la dose
maximale sans effet toxique observable chez les mères.
Des études de tératogénicité au cours desquelles du 1,3-
dichloropropène a été administré à des rats et à des lapins par la
voie respiratoire, n'ont pas permis de relever d'indices de
tératogénicité jusqu'à la dose de 1362 mg/m3, mais on a observé
une embryotoxicité chez le rat (réduction de la taille des portées
et augmentation du taux de résorption). Le composé s'est révélé
toxique pour les mères, tant chez les rattes que chez les lapines,
aux doses supérieures ou égales à 544,8 mg/m3 (120 ppm).
Dans la plupart des études, les isomères cis et trans du
1,3-dichloropropène, ainsi que les mélanges, se sont révélés
mutagènes chez les bactéries, sans ou avec activation métabolique. A
l'état pur, le 1,3-dichloropropène et le cis-1,3-dichloropropène
étaient négatifs à cet égard chez les bactéries. On a montré que le
glutathion bloquait l'activité mutagène du 1,3-dichloropropène chez
les bactéries. Lors d'une épreuve de mutation génique sur des
cellules V79 de hamster chinois ainsi que dans une épreuve HGPRT sur
des cellules ovariennes du même animal, le cis-1,3-dichloropropène
a donné des résultats négatifs.
Le cis- et le trans-1,3-dichloropropène provoquent une
synthèse non programmée de l'ADN dans les cellules HeLa S3. Dans
des hépatocytes de rat, le 1,3-dichloropropène n'a pas entraîné de
réparation importante de l'ADN. L'épreuve rec sur des microsomes de
la souche H17 de Bacillus subtilis a donné un résultat positif
avec le 1,3-dichloropropène en présence d'activation métabolique.
Dans des cellules ovariennes de hamster chinois, le cis- et
le trans-1,3-dichloropropène ont entraîné des lésions
chromosomiques en présence d'activation métabolique; toutefois dans
une autre étude, le 1,3-dichloropropène a produit les mêmes effets
sans activation métabolique. Le cis-1,3-dichloropropène n'a pas
provoqué de lésions chromosomiques dans des cellules de foie de rat,
mais il a entraîné des échanges entre chromatides soeurs dans des
cellules ovariennes de hamster chinois, en présence ou en l'absence
d'activation métabolique, ainsi que dans des cellules V79 du même
animal, cette fois sans activation métabolique.
Une épreuve de recherche des micronoyaux dans la moelle osseuse
de souris a donné des résultats négatifs avec le 1,3-
dichloropropène. Les résultats ont été également négatifs dans le
cas d'une épreuve de mutation létale récessive liée au sexe sur
Drosophila melanogaster.
Des études de cancérogénicité ont été effectuées sur des souris
et des rats. On leur a administré par gavage pendant deux ans du
1,3-dichloropropène technique contenant 1% d'épichlorhydrine. Chez
les souris, on a noté un accroissement sensible des hyperplasies
épithéliales ainsi que des carcinomes de type transitionnel au
niveau de la vessie, un accroissement des tumeurs pulmonaires, une
légère augmentation des tumeurs hépatiques et, au niveau de la
portion cardiaque de l'estomac, une augmentation de l'hyperplasie
épithéliale ainsi que des papillomes ou des carcinomes spino-
cellulaires. Chez le rat, on a observé une augmentation de
l'incidence des nodules néoplasiques au niveau du foie ainsi que des
papillomes ou des carcinomes spino-cellulaires dans la portion
cardiaque de l'estomac.
Une étude par inhalation de deux ans a permis d'étudier la
cancérogénicité du 1,3-dichloropropène (sans épichlorhydrine) sur
des rats et des souris. Chez les souris, on a observé une incidence
accrue des hyperplasies, au niveau de la vessie, de la portion
cardiaque de l'estomac et des muqueuses nasales. Il y avait
également augmentation dans l'incidence des tumeurs pulmonaires
bénignes. Un certain nombre de modifications d'origine toxique ont
été constatées dans la muqueuse olfactive nasale chez le rat, mais
sans accroissement de l'incidence tumorale.
On a montré que l'épichlorhydrine provoquait des tumeurs de la
partie proximale de l'estomac lors d'une étude où cette substance
était administrée par gavage ainsi que des tumeurs des fosses
nasales lors d'une étude par inhalation sur des rats; les résultats
de l'étude au cours de laquelle du 1,3-dichloropropène a été
administré par voie orale à des souris ne permettent pas d'exclure
un effet cancérogène sur la vessie.
Mode d'action
Etant donné que la principale voie métabolique d'élimination du
1,3-dichloropropène consiste dans une conjugaison avec le
glutathion, on peut penser que toute situation affectant la
concentration en glutathion tissulaire (groupements sulfhydriles non
protéiques) est susceptible de modifier les effets du composé. Le
1,3-dichloropropène lui-même provoque une déplétion en glutathion
dans les divers tissus, en particulier ceux qui constituent la porte
d'entrée dans l'organisme, c'est-à-dire essentiellement la portion
cardiaque de l'estomac et le foie dans le cas où l'administration se
fait par gavage et les tissus des fosses nasales dans le cas des
études par inhalation. On a observé chez la souris une diminution du
glutathion, au niveau de l'épithélium nasal aux doses supérieurs
22,7 mg/m3 (5 ppm) et au niveau de la portion cardiaque de
l'estomac aux doses supérieurs à 113,5 mg/m3 (25 ppm).
La toxicité du 1,3-dichloropropène pour les animaux
d'expérience se manifeste lorsque l'exposition entraîne une
déplétion en glutathion et une réduction préalable de la teneur en
glutathion tissulaire exacerbe cet effet toxique. L'inhalation
pendant une longue période de concentrations supérieures à 90,8
mg/m3 (20 ppm) entraîne une dégénérescence et une hyperplasie de
l'épithélium nasal et stomacal chez la souris; chez le rat et dans
les mêmes conditions, la dose de 272,4 mg/m3 (60 ppm) a également
provoqué une dégénérescence du tissu des fosses nasales.
Le rôle protecteur du glutathion a également été mis en lumière
par des études chez la souris qui ont montré que lorsque la teneur
en groupements sulfhydriles non protéiques diminuait, il y avait
augmentation du taux de liaison covalente du dichloropropène
radiomarqué (au carbone 14) aux cellules de la portion cardiaque de
l'estomac. De même, on a observé, dans des systèmes d'épreuves in
vitro, que la présence de glutathion réduisait sensiblement la
génotoxicité du 1,3-dichloropropène et d'un des ses métabolites
mineurs d'oxydation (cytochrome P-450), à savoir le 1,3-
dichloropropène-oxyde.
1.5 Effets sur l'homme
Il est improbable qu'il y ait exposition de la population
générale par l'intermédiaire de l'air, de l'eau ou des aliments.
On a montré que l'exposition professionnelle se situe en
général en-dessous de 4,54 mg/m3 (1 ppm), mais on a également fait
état de concentrations plus élevées (jusqu'à 18,3 mg/m3 lors
d'opérations de remplissage ou de changement de buses). L'exposition
professionnelle se produit vraisemblablement par la voie
respiratoire ou par la voie cutanée. Très peu de temps après
l'exposition, il y a irritation des yeux et des muqueuses des voies
respiratoires supérieures. On a observé de graves symptômes
d'intoxication après inhalation d'air contenant des concentrations
supérieures à 6810 mg/m3 (> 1500 ppm); à plus faible
concentration, il y avait dépression du système nerveux central et
irritation des voies respiratoires. L'exposition de la peau entraîne
également de graves irritations à ce niveau.
Chez un groupe de personnes chargées d'épandre du 1,3-
dichloropropène, on a observé en fin de saison un certain nombre
d'anomalies de la fonction hépatique et rénale. Toutefois,
l'existence d'une relation de cause à effet reste controversée.
Il y a eu des cas d'intoxication qui ont entraîné
l'hospitalisation des intéressés avec des symptômes d'irritation des
muqueuses, une sensation de gêne thoracique, des maux de tête, des
nausées, des vomissements, des vertiges et parfois une perte de
conscience et une diminution de la libido. En outre, trois cas
d'affections hématologiques malignes ont été attribués à une
surexposition accidentelle antérieure au 1,3-dichloropropène, mais
là encore, l'existence d'une relation de cause à effet reste
incertaine.
En comparant à un groupe témoin la fécondité d'employés
travaillant à la production d'hydrocarbures chlorés à trois atomes
de carbone, on n'a pas mis en évidence d'association entre
l'exposition et une réduction éventuelle de la fécondité.
2. Conclusions
Population générale: Du fait que l'exposition au 1,3-dichloropropène
est faible voire inexistante, le risque pour la population générale
est négligeable.
Exposition professionnelle: Lors d'opérations de remplissage et lors
des épandages, il peut y avoir exposition des opérateurs à des
concentrations dépassant la limite maximale autorisée, si des
mesures de sécurité appropriées ne sont pas prises.
Environnement: Dans la mesure où le 1,3-dichloropropène est utilisé
à la dose recommandée, il est vraisemblable qu'il ne s'accumulera
pas dans l'environnement à des concentrations susceptibles de poser
un problème écologique et il est improbable qu'il puisse avoir des
effets nocifs sur les organismes terrestres et aquatiques.
3. Recommandations
* Les opérations de remplissage et l'épandage du 1,3-
dichloropropène doivent obligatoirement s'accompagner des
mesures de sécurité appropriées afin de faire en sorte que
l'exposition ne dépasse pas les concentrations maximales
autorisées.
* Il faudrait étudier la destinée métabolique du trans-1,3-
dichloropropène chez les mammifères ainsi que le rôle que
pourraient avoir les métabolites d'oxydation de cet isomère
dans la toxicité du composé.
* L'effet protecteur du glutathion vis-à-vis du 1,3-
dichloropropène est dû à l'action de la glutathion-transférase.
Il est donc recommandé de procéder à des études afin de
comparer la cinétique de l'action enzymatique de la glutathion-
S-transférase humaine provenant des divers tissus à
l'activité de l'enzyme d'origine animale provenant des tissus
correspondants.
* Il conviendrait de rassembler et de publier les données dont on
dispose sur le rôle protecteur du glutathion.
* La génotoxicité du dichloropropène est due pour une part à son
métabolisme oxydatif. Il est recommandé d'entreprendre des
études pour identifier l'isoenzyme responsable et la comparer à
l'activité des isoenzymes du cytochrome P-450 humain.
* Dans les études de cancérogénicité où l'on procède par gavage
des animaux, il conviendrait d'élucider le rôle de
l'épichlorhydrine en tant que facteur de confusion éventuel.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1,2-DICHLOROPROPANE
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le 1,2-dichloropropane est un liquide dont le point
d'ébullition est de 96,8 °C et la tension de vapeur de 42 mmHg à 20
°C. Il est soluble dans l'eau, l'éthanol et l'éther éthylique. Par
chauffage, il émet des vapeurs de phosgène hautement toxiques. Son
coefficient de partage entre l'octanol et l'eau (log Kow) est égal
à 2,28.
Ce produit entre dans la composition des vernis pour meubles,
des liquides de nettoyage à sec, des décapants pour peintures, des
produits pour le dégraissage des surfaces métalliques, le traitement
des huiles; il sert à la fabrication de caoutchoucs et de cires,
ainsi que comme intermédiaire dans la production du
tétrachloréthylène et du tétrachlorure de carbone. Il entre
également dans la composition du mélange appelé D/D que l'on utilise
comme fumigant avant la plantation.
Les mesures de la concentration du 1,2-dichloropropane dans
l'air urbain ont donné des valeurs respectives de 1,2 µg/m3
(valeur moyenne), 0,021-0,040 µg/m3 et 0,0065-1,4 µg/m3
respectivement à Philadelphie, Portland et au Japon. La
décomposition dans l'atmosphère est lente; sur la base de la
réaction avec les radicaux hydroxyles, on a évalué le temps de demi-
décomposition du 1,2-dichloropropane à plus de 313 jours. Il est
probable que la décomposition est essentiellement de nature
photochimique. Pour que cette décomposition photochimique soit
appréciable, il est nécessaire que le composé soit adsorbé sur des
particules. C'est, semble-t-il, principalement par volatilisation
que le 1,2-dichloropropane s'élimine de l'eau.
Dans le sol, les principales voies d'élimination sont la
volatilisation et la diffusion. Le 1,2-dichloropropane persiste dans
le sol. Plus de 98% du 1,2-dichloropropane appliqué sur du terreau
ont été récupérés 12 à 20 semaines après ce traitement.
Il peut y avoir lessivage du 1,2-dichloropropane présent dans
le sol et contamination des eaux souterraines à faible ou grande
profondeur dans les secteurs traités avec des fumigants du type "MIX
D/D". Aux Etats-Unis d'Amérique, on a trouvé des concentrations dans
l'eau de puits et les eaux souterraines allant respectivement
jusqu'à 440 µg/litre et 51 µg/litre. Aux Pays-Bas, des
concentrations atteignant 160 µg/litre ont été observées dans l'eau
de puits et on a retrouvé du 1,2-dichloropropane jusqu'à une
profondeur de 13 mètres.
Les plantes vivrières peuvent fixer le 1,2-dichloropropane mais
les résidus qu'on y a décelés sont faibles (< 0,01 mg/kg) et
probablement sans conséquence biologique.
La bioaccumulation du 1,2-dichloropropane est improbable en
raison de sa forte solubilité dans l'eau (2,7 g/kg) et de la faible
valeur de son coefficient de partage entre l'octanol et l'eau (log
Kow).
1.2 Cinétique et métabolisme
Administré par voie orale à des rats, le 1,2-dichloropropane
est rapidement éliminé (80 à 90% en 24 h). Il n'y a pas de
différences majeures dans la cinétique ou dans l'élimination entre
les mâles et les femelles. La principale voie d'élimination est la
voie urinaire, et jusqu'à la moitié de la dose orale initiale est
éliminée dans les 24 h. La proportion éliminée par la voie fécale
est inférieure à 10%. Le 1,2-dichloropropane est éliminé à hauteur
de 33% dans l'air expiré, à la fois sous forme de dioxyde de carbone
et d'un mélange de produits volatils. Les concentrations tissulaires
sont faibles, la plus élevée étant observée au niveau du foie. Après
exposition de rats par la voie respiratoire on note une élimination
rapide du 1,2-dichloropropane; 55 à 65% de la dose initiale sont
éliminés dans les urines et 16 à 23% dans l'air expiré. La demi-vie
d'élimination à partir du sang est de 24 à 30 minutes.
On ne retrouve pas le 1,2-dichloropropane initial dans les
urines. On y a identifié trois métabolites principaux. Ces
métabolites résultent de l'oxydation et de la conjugaison du composé
et aboutissent à la formation de mercapturates, de N-acétyl- S-
(2-hydroxypropyl)-L-cystéine, de N-acétyl- S-(2-oxypropyl)-L-
cystéine et de N-acétyl- S-(1-carboxyéthyl)-L-cystéine. Le 1,2-
dichloropropane peut également subir une oxydation en lactate avec
production de dioxyde de carbone ou d'acétyl-coenzyme A.
Après administration de 1,2-dichloropropane par voie orale à
des rats à raison de 2 mg/kg, on a constaté une forte diminution du
glutathion tissulaire. Il y avait également corrélation entre la
diminution du glutathion tissulaire et les manifestations toxiques
au niveau du foie, des reins et des hématies. Une réduction
préalable du glutathion intracellulaire a provoqué une exacerbation
de la toxicité du 1,2-dichloropropane, alors qu'un traitement
préalable par des précurseurs de la synthèse du glutathion réduisait
cette toxicité. Ces résultats montrent que le glutathion a un effet
protecteur vis-à-vis des propriétés toxiques du 1,2-dichloropropane.
1.3 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas déterminé la CE50 pour les algues d'eau douce car
la volatilisation du composé à partir de la solution d'épreuve rend
cette détermination difficile. La toxicité aiguë du 1,2-
dichloropropane pour les invertébrés aquatiques et les poissons est
faible à modérée; pour les invertébrés, les valeurs de la CL50 à
48 h varient de 52 à > 100 mg/litre, tandis que la CL50 à 96 h pour
les poissons se situe entre 61 et 320 mg/litre. Une épreuve de
toxicité à court terme effectuée sur des vairons du genre
Pimephales promelas a montré que la dose maximale sans effet était
de 82 mg/litre. Lors d'une épreuve de 32 jours sur les larves de ce
vairon, on a constaté que les paramètres biologiques les plus
sensibles à la toxicité du 1,2-dichloropropane étaient la croissance
des larves et leur survie. On estime que la concentration maximale
acceptable de substance toxique est de 6 à 11 mg/litre. Chez des
poissons du genre Pimelometopon on a constaté une inhibition de la
croissance après exposition de 33 jours à une concentration de 164
mg/litre.
Le 1,2-dichloropropane est phytotoxique.
Des épreuves par contact effectuées sur quatre espèces de
lombrics ont montré que la CL50 se situait entre 44 et 84 µg/cm2
(valeur moyenne) de papier filtre. Sur sol artificiel, les valeurs
de la CL50 oscillaient entre 3880 et 5300 mg/kg de sol (en poids
sec).
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Chez les animaux d'expérience, la toxicité aiguë par voie orale
de ce composé est faible. Ainsi, la DL50 par voie orale est de 1,9
g/kg de poids corporel pour le rat et la DL50 cutanée est de 8,75
mg/kg de poids corporel chez le lapin.
Des études de toxicité de courte durée comportant
l'administration de 1,2-dichloropropane par voie orale à des souris
et à des rats ont montré qu'à des doses quotidiennes égales ou
supérieures à 250 mg/kg de poids corporel, il y avait inhibition de
la croissance, apparition de signes cliniques d'intoxication
correspondant à une dépression du système nerveux central et
accroissement de la mortalité. A la dose quotidienne de 250 mg/kg
pendant dix jours, on a noté chez des rats une modification des
enzymes sériques trahissant une légère hépatotoxicité, la dose sans
effet observable étant de 100 mg/kg par jour.
Lors d'une étude par inhalation de 13 semaines effectuée sur
des souris (à la dose maximale de 681 mg/m3), on n'a pas observé
d'effets nocifs. Lors d'une étude analogue sur des rats exposés à
des doses de 68,1, 227 et 681 mg/m3, on a observé une réduction du
poids corporel et des lésions mineures du tissu des fosses nasales
dans les groupes soumis aux deux plus fortes doses.
Lors d'une étude de reproduction portant sur deux générations
de rats, on a donné aux animaux une eau de boisson contenant des
concentrations de 1,2-dichloropropane respectivement égales à 0,024,
0,1, 0,24% (soit l'équivalent de 33,6, 140 et 336 mg/kg de poids
corporel par jour); il en est résulté une réduction du gain de poids
maternel et une diminution de la consommation d'eau, à la dose
médiane et à la plus forte dose. Chez les animaux nouveaunés, le
poids corporel était réduit à la dose la plus forte. La dose sans
effet nocif observable s'établissait respectivement à 33,6 et 140
mg/kg de poids corporel par jour pour les effets toxiques sur la
mère et sur la fonction de reproduction.
Les études ne mettent en évidence aucune activité tératogène du
1,2-dichloropropane à des doses orales allant jusqu'à 125 mg/kg de
poids corporel chez le rat et 150 mg/kg de poids corporel chez le
lapin. Toutefois à ces doses, on a observé une toxicité du produit
pour les mères et pour les foetus, à en juger d'après certains
signes cliniques témoignant d'une atteinte du système nerveux
central, la réduction du gain de poids maternel et, chez les foetus,
un retard d'ossification. La dose sans effet observable est égale à
30 mg/kg de poids corporel par jour chez le rat et à 50 mg/kg de
poids corporel par jour chez le lapin.
La plupart des études ont mis en évidence une mutagénicité du
1,2-dichloropropane chez les bactéries avec ou sans activation
métabolique, mais il est vrai qu'on avait utilisé des doses
extrêmement élevées (jusqu'à 10 mg/boîte). Le 1,2-dichloropropane
provoque des aberrations chromosomiques et des échanges entre
chromatides soeurs dans les cellules ovariennes de hamster chinois;
il y a également accroissement des échanges entre chromatides soeurs
dans des cellules V79 de hamster chinois en présence de 1,2-
dichloropropane. Des lymphocytes humains ont été cultivés en
présence ou en l'absence d'un système métabolisant de foie de rat;
on a constaté que ces cellules fixaient la thymidine tritiée de la
même manière que les cultures témoins et qu'elles présentaient la
même viabilité. Une épreuve de mutation létale récessive liée au
sexe effectuée sur Drosophila melanogaster a donné des résultats
négatifs. Une épreuve de létalité dominante chez des rats soumis
pendant 14 semaines à des doses de 1,2-dichloropropane mêlé à leur
eau de boisson, puis accouplés au cours des deux semaines suivantes,
a donné des résultats également négatifs.
Lors d'une étude de cancérogénicité effectuée sur des souris,
on a administré aux animaux par gavage, 125 ou 250 mg de 1,2-
dichloropropane par kg de poids corporel; on a observé une
augmentation, liée à la dose, de l'incidence des adénomes
hépatiques. L'incidence des adénomes était plus élevée dans les
groupes traités que dans le groupe témoin mais elle se situait
malgré tout dans les limites normales pour les témoins historiques.
Chez des rats soumis à des doses de 125 et de 250 mg/kg de
poids corporel (femelles) ou 62 et 125 mg/kg de poids corporel
(mâles) par gavage, cinq jours par semaines pendant 113 semaines, on
a noté une légère augmentation dans l'incidence des adénocarcinomes
mammaires chez les femelles soumises à la dose la plus forte,
augmentation qui était supérieure aux limites normales pour les
témoins historique.
1.5 Effets sur l'homme
Il est improbable que la population générale soit exposée au
1,2-dichloropropane par l'intermédiaire de l'air et de l'eau, sauf
dans les zones où l'on utilise largement le 1,2-dichloropropoane ou
le "D/D MIX" à des fins agricoles. Les résidus de 1,2-
dichloropropane présents dans les plantes vivrières sont
généralement inférieurs à la limite de détection. L'exposition étant
faible, on peut considérer que le risque est négligeable pour la
population générale.
On a signalé plusieurs cas d'intoxication aiguë accidentelle ou
intentionnelle (suicide) dus à une surexposition au 1,2-
dichloropropane. Les effets en étaient essentiellement observables
au niveau du système nerveux central, du foie et des reins. On a
également fait état d'une anémie hémolytique et d'une coagulation
intravasculaire disséminée. Dans un cas, le malade se trouvait dans
un état de délire qui a évolué vers un état de choc irréversible et
une insuffisance cardiaque fatale.
Il peut y avoir exposition professionnelle par voie cutanée ou
respiratoire. On a fait état de plusieurs cas de dermatite ou de
sensibilisation cutanée chez des travailleurs qui utilisaient des
solvants contenant du 1,2-dichloropropane.
2. Conclusions
* Population générale: L'exposition de la population générale au
1,2-dichloropropane à partir de l'air ou de la nourriture est
faible, voire inexistante. Toutefois dans certains secteurs, il
peut y avoir exposition en cas de contamination des eaux
souterraines.
* Exposition professionnelle: Moyennant de bonnes méthodes de
travail et des précautions d'hygiène et de sécurité, il est peu
probable que l'utilisation du 1,2-dichloropropane comporte un
risque pour les personnes qui y sont exposées de par leur
profession.
* Environnement: Il est improbable que le 1,2-dichloropropane
s'accumule dans l'environnement à des concentrations
écologiquement nocives lorsqu'on l'utilise à la dose
recommandée. Il est également improbable qu'il produise des
effets nocifs sur les populations d'organismes terrestres ou
aquatiques.
3. Recommandations
* Il faudrait évaluer la toxicité aiguë par voie respiratoire
ainsi que le pouvoir irritant pour les yeux et la peau et le
pouvoir sensibilisant cutané de ce composé.
* Lorsqu'on manipule du 1,2-dichloropropane il faut prendre des
mesures de sécurité appropriées afin d'éviter toute exposition
supérieure à la concentration maximale admissible.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
"MIX D/D"
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le mélange technique de dichloropropènes et de dichloropropane
(désignés dans la suite du texte par l'abréviation "MIX D/D") est un
liquide limpide de couleur ambrée doté d'une odeur piquante; sa
tension de vapeur est de 35 mmHg à 20 °C et il est soluble dans les
solvants halogénés, les esters et les cétones.
Le MIX D/D présente une composition caractéristique, à savoir:
au moins 50% de 1,3-dichloropropènes (proportion des isomères cis-
et trans-, environ 1/1), les autres constituants principaux étant
le 1,2-dichloropropane et les composés voisins. On l'a beaucoup
utilisé comme nématocide en application sur le sol avant la
plantation.
Le transport, la distribution et la destinée des principaux
constituants du MIX D/D dans l'air, l'eau et le sol sont décrits à
la section 4 des chapitres consacrées au 1,3-dichloropropène et au
1,2-dichloropropane.
Le 1,2-dichloropropane provenant du MIX D/D présent dans le sol
a une certaine tendance à contaminer l'eau des puits et les eaux
souterraines en général, par suite d'un phénomène de lessivage. Lors
d'une opération de forage à des fins d'irrigation en Europe
occidentale (68 m de profondeur) on a constaté que les
concentrations moyennes de 1,2-dichloropropane à différentes
profondeurs variaient entre 0,8 et 8,5 µg/litre, la concentration la
plus élevée étant de 165 µg/litre.
Il est peu probable que les cultures fixent en proportion
importante les constituants du MIX D/D (voir les autres chapitres de
la présente monographie). Il est également peu probable que ces
constituants subissent une bioaccumulation du fait que leur
coefficient de partage entre l'octanol et l'eau (log Kow) est
faible et que leur solubilité dans l'eau est relativement forte.
1.2 Cinétique et métabolisme
On n'a pas procédé à des études métaboliques sur le MIX D/D.
Les deux principaux constituants, le 1,3-dichloropropène et le 1,2-
dichloropropane sont rapidement éliminés, essentiellement dans
l'urine et en moindre proportion, dans l'air expiré. Les
constituants du MIX D/D sont métabolisés selon un processus qui
comporte une oxydation et une conjugaison. Les principaux
métabolites urinaires sont des acides mercapturiques.
1.3 Effets sur les organismes vivants dans leur milieu naturel
Le MIX D/D est modérément toxique pour les poissons; les
valeurs de la CL50 à 96 h varient de 1 à 6 mg/litre. La toxicité
du MIX D/D est largement imputable au 1,3-dichloropropène.
Lorsqu'on utilise ce mélange aux doses recommandées, ses effets
principaux consistent dans une diminution passagère (moins de sept
jours) des populations de champignons terricoles et une inhibition
de l'oxydation de ions ammonium en nitrates. Le MIX D/D est toxique
pour les bactéries nitrifiantes. Peu après la disparition du MIX
D/D, le sol est recolonisé par les bactéries. Lors d'essais en
situation réelle, on a constaté que le MIX D/D, appliqué à raison de
600 litres/hectare, provoquait la destruction des invertébrés
terricoles. Il faut de 6 à 24 mois pour que la recolonisation
s'effectue.
Le MIX D/D est extrêmement phytotoxique.
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le MIX D/D présente une toxicité aiguë modérée à forte pour les
animaux de laboratoire. Chez le rat et la souris, la DL50 par voie
orale varie de 132 à 300 mg/kg de poids corporel. En ce qui concerne
la DL50 par voie cutanée, les valeurs pour le rat et le lapin sont
respectivement de 779 et de 2100 mg/kg de poids corporel. Chez le
rat, la CL50 à 4 h par inhalation est approximativement égale à
4540 mg/m3. En cas d'exposition, les signes cliniques observés
sont ceux d'une dépression du système nerveux central. Le MIX D/D
est fortement irritant pour les yeux et la peau et il est doté d'un
pouvoir sensibilisateur cutané modéré.
Les résultats des études toxicologiques à court terme
effectuées sur des rats et des chiens sont insuffisants pour qu'on
puisse déterminer convenablement la toxicité du MIX D/D, car aux
doses relativement faibles que l'on a étudiées, on n'observe pas
d'effets biologiquement significatifs. Plusieurs études d'inhalation
de courte durée (corps entier) ont été effectuées sur des rats. A
des doses allant jusqu'à 145 mg/m3, le MIX D/D n'a pas produit
d'effets toxiques. A partir de 1362 mg/m3, les effets toxiques
étaient évidents et correspondaient à une dépression du système
nerveux central. L'exposition des animaux à la dose de 443 mg/m3
pendant 10 semaines a entraîné une réduction du gain de poids et une
augmentation du poids des reins.
Une étude tératologique comportant l'administration de MIX D/D
par voie orale n'a pas permis, en raison de ses insuffisances,
d'évaluer le pouvoir tératogène de ce composé chez le rat.
Lors d'une étude d'inhalation chez le rat destinée à étudier
les effets du MIX D/D sur la fécondité des mâles et des femelles, on
n'a pas observé d'effets à des doses allant jusqu' 443 mg/m3 sur
une durée de dix semaines. Il n'a pas été possible de procéder à une
évaluation complète des effets du MIX D/D sur la reproduction, en
raison du caractère incomplet du protocole de ces études.
Le MIX D/D s'est révélé mutagène pour les souches TA100 et
TA1535 de Salmonella typhimurium ainsi que pour la souche WP2 HCR
d'Escherichia coli, sans activation métabolique. Cet effet n'a pas
été observé sur les souches TA98, TA1537 et TA1538 de salmonelles.
Lors d'une étude à long terme, au cours de laquelle on avait
administré à des rats un régime alimentaire contenant jusqu'à 120
mg/kg (soit 6 mg/kg de poids corporel) pendant deux ans, on n'a pas
observé d'effets toxiques ou cancérogènes.
1.5 Effets sur l'homme
Le MIX D/D n'est plus guère utilisé et par conséquent il est
peu probable que la population générale puisse être exposée par
l'intermédiaire de l'air, de l'eau et des aliments. L'exposition des
personnels qui remplissent les fûts ou qui sont chargés de
l'épandage du produit s'est en général située en-dessous de 4,5 mg
de 1,3-dichloropropène/m3 lorsque l'on respectait la marche à
suivre recommandée; dans d'autres cas, on a mesuré des
concentrations allant jusqu'à 36,32 mg/m3.
On a cité un cas d'intoxication aiguë mortelle par suite de
l'ingestion accidentelle de MIX D/D.
2. Conclusions
* Population générale. Etant donné que l'on l'utilise plus guère
le MIX D/D, l'exposition de la population générale au 1,3-
dichloropropène par l'intermédiaire de l'air, de l'eau et des
aliments est négligeable, mais dans certaines zones, il peut y
avoir exposition au 1,2-dichloropropane en cas de contamination
des eaux souterraines.
* Exposition professionnelle. Lors du remplissage des fûts et de
l'épandage du MIX D/D, il peut y avoir exposition des
personnels concernés au 1,3-dichloropropène, à des
concentrations qui dépassent les valeurs maximales admissibles,
en particulier sous les climats chauds.
* Environnement. Il est peu probable que le MIX D/D atteigne dans
l'environnement des concentrations biologiquement nocives pour
la faune et la flore terrestre ou aquatique, lorsqu'on utilise
la dose recommandée. Il est également improbable qu'il puisse
exercer des effets nocifs durables sur les organismes vivants
dans leur milieu naturel.
3. Recommandations
* Le MIX D/D ne doit pas être utilisé comme fumigant pour traiter
le sol du fait qu'il risque de passer dans les eaux
souterraines par lessivage.
* Dans les zones où l'on a utilisé du MIX D/D, il faut surveiller
les eaux de surface et les eaux souterraines à la recherche de
résidus éventuels.
RESUMEN Y EVALUACION, CONCLUSIONES, Y RECOMENDACIONES
1,3-DICLOROPROPENO
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
El "1,3-dicloropropeno" se introdujo en 1956 como parte de una
mezcla, que contenía 1,3-dicloropropeno, 1,2-dicloropropano y otros
hidrocarburos halogenados, y se ha utilizado ampliamente en la
agricultura como fumigante del suelo que se destina a nuevas
plantaciones para combatir los nematodos de las hortalizas, las
papas y el tabaco. Se aplica fundamentalmente mediante inyección en
el suelo. La formulación comercial del 1,3-dicloropropeno es una
mezcla de isómeros cis y trans (aproximadamente en proporciones
iguales), que forman un líquido entre incoloro y ámbar, con un olor
penetrante e irritante, parecido al del cloroformo. La presión del
vapor es de 3,7 kPa a 20 °C. El producto técnico tiene una pureza
del 92% y puede contener varias impurezas, como 1,2-dicloropropano.
El log P del coeficiente de reparto octanol/agua es de 1,98.
En el aire, la descomposición del 1,3-dicloropropeno tiene
lugar sobre todo por reacción con radicales libres y con el ozono.
La semivida de los isómeros cis y trans en la reacción con
radicales libres es de 12 y 7 horas respectivamente, y en la
reacción con el ozono de 52 y 12 días respectivamente. La
fototransformación directa parece ser insignificante, pero puede
aumentar en presencia de partículas atmosféricas.
En el agua, el 1,3-dicloropropeno tiende a desaparecer con
rapidez, debido a su solubilidad relativamente baja en ella y su
elevada volatilidad; se han notificado semividas de menos de 5 h.
La distribución del 1,3-dicloropropeno en los compartimentos
del suelo depende de la presión del vapor, el coeficiente de
difusión, la temperatura y el contenido de humedad del mismo. En la
persistencia del 1,3-dicloropropeno en el suelo influyen la
volatilización, la transformación química y biológica, la
transformación fotoquímica y la absorción por los organismos. Los
mecanismos más importantes de dispersión y dilución en el medio
ambiente son la volatilización y la difusión en la fase de vapor.
La transformación del 1,3-dicloropropeno se produce
inicialmente por hidrólisis a 3-cloroalilalcohol y luego por
transformación microbiana a 3-cloroacroleína y ácido 3-
cloroacrílico. En un estudio de laboratorio, la semivida de la
hidrólisis de los isómeros cis y trans del 1,3-dicloropropeno a
15 °C y 29 °C fue de 11,0 y 2,0 días respectivamente para el isómero
cis y de 13,0 y 2,0 días para el isómero trans. En el suelo, con
un pH 7 y una temperatura de 25 °C, la semivida de la hidrólisis fue
de 4,6 días para ambos isómeros. Debido a su desaparición
relativamente rápida del suelo, no es probable que se acumulen
residuos cuando se aplica el fumigante con la dosis y la frecuencia
recomendadas.
El 1,3-dicloropropeno puede desplazarse en el suelo, sobre todo
si es arenoso, de textura gruesa y con un contenido bajo de humedad.
El desplazamiento descendente se ve favorecido por el cultivo
profundo de los suelos con escasa porosidad. Se ha detectado 1,3-
dicloropropeno en "aguas subterráneas altas" (hasta 2 m por debajo
de la superficie), pero no en las profundas, que son las que suelen
utilizarse para beber.
Los cultivos pueden absorber 1,3-dicloropropeno. Sin embargo,
no es probable que aparezca una cantidad apreciable de residuos en
las plantas cultivadas comestibles, que se suelen sembrar cuando ya
ha desaparecido la mayor parte del fumigante.
Es poco probable la bioacumulación de 1,3-dicloropropeno,
debido a su solubilidad relativamente alta en agua (< 1 g/kg), al
bajo log P del coeficiente de reparto octanol/agua y a la
eliminación rápida en mamíferos y otros organismos.
1.2 Cinética y metabolismo
El 1,3-dicloropropeno se elimina rápidamente tras su
administración oral a roedores. La principal vía de eliminación es
la orina, donde a ella el 81% de los isómeros cis y el 56% de los
trans se eliminan en las 24 horas siguientes a la dosificación. La
semivida de la eliminación en la orina es de 5 a 6 horas. La
eliminación fecal es escasa. El anhídrido carbónico representa el 4
y el 24%, respectivamente, de los isómeros cis y trans del 1,3-
dicloropropeno eliminados. Las concentraciones en los tejidos tras
la administración oral son bajas; los niveles residuales más
elevados se encuentran en la pared estomacal, seguidos por
cantidades más bajas en los riñones y la vejiga.
No se ha detectado en la orina la presencia de 1,3-
dicloropropeno inalterado. La glutatión- S-alquiltransferasa actúa
sobre los isómeros cis y trans formando ácidos mercaptúricos,
que se excretan por la orina. El isómero trans se conjuga de 4 a 5
veces más lentamente que el cis. El principal metabolito urinario
en ratas y ratones es la N-acetil- S-(3-cloroprop-2-enil)L-
cisteína; este metabolito se puede utilizar para la vigilancia
biológica en el ser humano. Para el isómero cis se ha identificado
una segunda ruta metabólica de menor importancia, en la que se
produce una monooxigenación a óxido de cis-1,3-dicloropropeno, que
se puede conjugar también con el glutatión. La elevada proporción
del isómero trans que se encuentra en el aire expirado procede de
una ruta metabólica distinta de la conjugación, que tiene una
especificidad más elevada para el isómero trans que para el cis.
La exposición de ratas al 1,3-dicloropropeno por inhalación no
produce un aumento de la concentración en sangre proporcional a la
dosis. A una dosis de 408,6 mg/m3 (90 ppm), disminuyeron la
frecuencia respiratoria y el volumen respiratorio por minuto, y la
saturación del metabolismo se produjo a 1362 mg/m3 (300 ppm). Los
isómeros cis y trans se eliminaron rápidamente de la sangre,
siendo la semivida de la eliminación de 3 a 6 minutos para
concentraciones inferiores a 1362 mg/m3, pero considerablemente
más larga (33-43 min.) a concentraciones más elevadas.
1.3 Efectos en los seres vivos del medio ambiente
Los valores de la CE50 para el crecimiento (96 h) del alga de
agua dulce Selenastrum capricornutum y la diatomea de los
estuarios Skeletoneria costatum son 4,95 mg/litro y 1 mg/litro
respectivamente. La toxicidad aguda (CL50 a las 96 h) del 1,3-
dicloropropeno para los peces es del orden de 1 a 7,9 mg/litro. En
una prueba en embriones-larvas de Pimephales promelas, el nivel
máximo sin efectos fue de 0,24 mg/litro. Estos datos, junto con el
hecho de que no es probable que el 1,3-dicloropropeno persista en el
agua, indican que el peligro para los peces lo constituyen los
efectos tóxicos agudos, con escasas posibilidades de efectos
adicionales debidos a la exposición durante un tiempo prolongado.
En dosis de 30 a 60 mg/kg, el 1,3-dicloropropeno puede reducir
la concentración de hongos y la tasa de actividad enzimática
microbiana, pero el efecto no suele ser duradero (< 7 días) y no se
produce en todos los tipos de suelos. En algunos estudios, aumentó
significativamente el número de microorganismos tras la aplicación.
El 1,3-dicloropropeno es fitotóxico, pero su toxicidad para las
abejas es escasa. Utilizando una técnica de espolvoreo, la DL50 a
las 48 horas fue de 6,6 µg/abeja. Las aves tienen una sensibilidad
relativamente baja al 1,3-dicloropropeno. Para el pato real (Anas
platyrhynchos) y la codorniz (Colinus virginianus) se ha
informado de CL50 (8 días) de > 10 g/kg de la dieta.
1.4 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda por vía oral del 1,3-dicloropropeno en
animales es de moderada a alta. Se han notificado valores de la
DL50 en ratas que oscilan entre 127 y 713 mg/kg de peso corporal.
Los valores de la DL50 por vía oral en ratas para los isómeros
cis y trans fueron de 85 y 94 mg/kg de peso corporal,
respectivamente.
La exposición aguda cutánea es moderadamente tóxica. En ratas y
conejos se ha reportado de una DL50 de 423 mg/kg y 504 mg/kg de
peso corporal, respectivamente. Los valores de la DL50 para los
isómeros cis y trans fueron 1090 y 1575 mg/kg de peso corporal,
respectivamente.
La exposición por inhalación (4 h) en ratas dio como resultado
valores de la DL50 de 3310 mg/m3 (729 ppm) para el 1,3-
dicloropropeno; 3042 mg/m3 -3514 mg/m3 para el isómero cis y
4880 mg/m3 - 5403 mg/m3 para el trans.
La intoxicación aguda afectó el sistema nervioso central y el
aparato respiratorio.
En pruebas cutáneas y de irritación ocular con conejos se
observaron reacciones graves, pero la recuperación se produjo en un
período de 14-21 días. Los resultados de las pruebas de
sensibilización en cobayos fueron positivos.
Se han realizado varios estudios de toxicidad por inhalación
durante un tiempo breve en ratones, ratas, cobayos, conejos y
perros. En los ratones los órganos afectados fueron la mucosa nasal
y la vejiga urinaria. Se observó degeneración del epitelio olfatorio
e hiperplasia del epitelio respiratorio. Se detectó hiperplasia del
epitelio de transición de la vejiga urinaria. En ratones se puede
estimar que el nivel sin efectos observados (NOEL) es de 136 mg/m3
(30 ppm).
En ratas también se han detectado cambios degenerativos
similares en el epitelio olfatorio, así como hiperplasia. En un
estudio bien diseñado se encontró un valor del NOEL para el 1,3-
dicloropropeno de 45,4 mg/m3, siendo el valor del NOEL para el
isómero cis de 136 mg/m3.
En un estudio de administración por vía oral durante 90 días a
ratas, el NOEL fue de 3 mg/kg de peso corporal. El único efecto
observado con la dosis inmediatamente superior, de 10 mg/kg de peso
corporal, fue un aumento relativo del peso de los riñones en los
machos.
En un estudio de inhalación sobre la reproducción de dos
camadas en dos generaciones de ratas, las dosis de hasta 408,6
mg/m3 (90 ppm) no produjeron efectos adversos sobre los parámetros
de la reproducción examinados. Sin embargo, la dosis más alta, de
408,6 mg/m3, indujo toxicidad materna, que se puso de manifiesto
por la disminución del crecimiento y por cambios histopatológicos de
la mucosa nasal. Se estableció un NOEL para la toxicidad materna de
136,2 mg/m3 (30 ppm).
En estudios de teratogenicidad por inhalación en ratas y
conejos, el 1,3-dicloropropeno no mostró potencial teratogénico a
niveles de exposición de hasta 1362 mg/m3, pero se observó
embriotoxicidad (reducción del tamaño de la camada y aumento del
índice de reabsorciones) en ratas. Con dosis de 544,8 mg/m3 (120
ppm) o superiores se advirtió toxicidad materna tanto en ratas como
en conejos.
En la mayor parte de los estudios, el 1,3-dicloropropeno cis
y trans y la mezcla de ambos fueron mutagénicos en bacterias, con
y sin activación metabólica. Se encontró que el 1,3-dicloropropeno
puro y el cis-1,3-dicloropropeno puro carecían de efecto sobre las
bacterias. Se demostró que el glutatión impedía la actividad
mutagénica del 1,3-dicloropropeno en bacterias. En un ensayo de
mutación genética con células de hámster chino V79, así como en la
prueba del locus HPRT de ovario de hámster chino, el cis-1,3-
dicloropropeno dio un resultado negativo.
El 1,3-dicloropropeno cis y trans indujo una síntesis no
programada de ADN en células S3 HeLa. En hepatocitos de rata, el
1,3-dicloropropeno no produjo una reparación significativa del ADN.
En el ensayo del locus rec con microsomas de la cepa H17 de
Bacillus subtilis con activación metabólica, el 1,3-dicloropropeno
dio resultado positivo.
En células de ovario de hámster chino, el 1,3-dicloropropeno
cis y trans indujo daños cromosómicos en condiciones de
activación metabólica, pero en otro estudio también dio resultado
positivo sin que hubiera activación. El isómero cis no indujo
lesiones cromosómicas en células hepáticas de rata, pero sí un
intercambio de cromátidas hermanas en células de ovario de hámster
chino con activación metabólica y sin ella, y en células de hámster
chino V79 sin activación.
En una prueba con micronúcleos de médula ósea en ratones, y en
otra de letalidad recesiva ligada al sexo en Drosophila
melanogaster, el 1,3-dicloropropeno fue negativo.
Se realizaron estudios de carcinogenicidad en ratones y ratas.
Se administró 1,3-dicloropropeno de calidad técnica (con un 1% de
epiclorhidrina) mediante sonda durante dos años. En los ratones se
observó un aumento significativo de la hiperplasia epitelial y los
carcinomas celulares transitorios en la vejiga urinaria, una
incidencia mayor de tumores pulmonares, un ligero aumento de tumores
hepáticos y una mayor proporción de hiperplasia epitelial y
papilomas o carcinomas de las células escamosas de la parte cardíaca
del estómago.
En estudios de inhalación de dos años se investigó la
carcinogenicidad del 1,3-dicloropropeno (sin epiclorhidrina) en
ratones y ratas. En ratones se detectó una mayor incidencia de
hiperplasia en la vejiga urinaria, la parte cardíaca del estómago y
la mucosa nasal. Aumentó la incidencia de los tumores pulmonares
benignos. También se observaron en ratas algunos cambios tóxicos en
la mucosa olfativa de la cavidad nasal, pero sin aumento de la
incidencia de tumores.
En un estudio de administración con sonda se puso de manifiesto
que la epiclorhidrina producía tumores en la parte cardíaca del
estómago, y en otro estudio de inhalación en ratas aparecieron
tumores en la cavidad nasal; en el caso de la administración de 1,3-
dicloropropeno por vía oral a ratones no se puede excluir un efecto
carcinogénico sobre la vejiga urinaria.
Mecanismo de acción
Dado que la principal ruta metabólica de eliminación del 1,3-
dicloropropeno es mediante la conjugación con el glutatión, cabe
esperar que las condiciones que alteran la concentración de
glutatión (sulfhidrilo no proteico) en los tejidos puedan modificar
los efectos del compuesto. El mismo 1,3-dicloropropeno agota el
contenido de glutatión de diversos tejidos, especialmente los
situados en puntos de entrada en el organismo, es decir, sobre todo
la parte cardíaca del estómago y el hígado tras la administración
con sonda y el tejido nasal después de la exposición por inhalación.
Tras la inhalación de concentraciones de 1,3-dicloropropeno
superiores a 22,7 mg/m3 (5 ppm) y 113,5 mg/m3 (25 ppm) se
produjo, respectivamente, una disminución de los niveles de
glutatión en el epitelio nasal y en la parte cardíaca del estómago
en ratones.
La toxicidad del 1,3-dicloropropeno en animales se produce con
niveles de exposiciones que agotan el glutatión de los tejidos y la
disminución previa de la concentración de éste la agrava. La
inhalación durante un tiempo prolongado de concentraciones
superiores a 90,8 mg/m3 (20 ppm) da lugar en ratones a
degeneración e hiperplasia del epitelio nasal y gástrico, mientras
que en ratas la inhalación durante un tiempo prolongado de una
concentración de 272,4 mg/m3 (60 ppm) produce degeneración del
tejido nasal.
La función protectora del glutatión se ha puesto de relieve
ulteriormente en estudios que han demostrado que la unión mediante
enlaces covalentes del 14C-1,3-dicloropropeno a la parte cardíaca
del estómago de ratón aumentaba a medida que disminuía el contenido
de sulfhidrilo no proteico. De igual forma, en sistemas de prueba
in vitro el glutatión mejoró notablemente la genotoxicidad del
1,3-dicloropropeno y del óxido 1,3-dicloropropeno, su metabolito
oxidativo secundario (citocromo P-450).
1.5 Efectos en el ser humano
No es probable la exposición de la población general a través
del aire, el agua o los alimentos.
En los estudios realizados se ha puesto de manifiesto que la
exposición profesional está en general por debajo de 4,54 mg/m3 (1
ppm), pero también se han notificado niveles más elevados (hasta
18,3 mg/m3 durante el llenado o el cambio de la boquilla). Es
probable que la exposición profesional se produzca por inhalación y
por vía cutánea. Tras la exposición aparece inmediatamente
irritación de los ojos y de la parte superior de la mucosa
respiratoria. La inhalación de aire con concentraciones de > 6810
mg/m3 (> 1500 ppm) produjo signos y síntomas de intoxicación
grave; las exposiciones más bajas dieron lugar a una depresión del
sistema nervioso central y a la irritación del aparato respiratorio.
La exposición cutánea produjo una irritación grave de la piel.
Se notificó que un grupo de aplicadores de 1,3-dicloropropeno
tuvieron algunos cambios en las funciones renal y hepática al final
de la temporada de aplicación. Sin embargo, se ha rebatido la
relación causa-efecto.
Se han producido algunos casos de intoxicación con
hospitalización de los afectados, que presentaban signos y síntomas
de irritación de la membrana mucosa, malestar torácico, dolor de
cabeza, náuseas, vómitos, mareos y, en ocasiones, pérdida del
conocimiento y disminución de la libido. Se han atribuido tres casos
de enfermedades malignas sanguíneas a una sobreexposición accidental
anterior al 1,3-dicloropropeno, pero la relación causa-efecto sigue
siendo dudosa.
Se comparó el estado de fecundidad de un grupo de personas que
trabajaban en la producción de compuestos clorados de tres carbonos
con otro testigo. No se demostró la existencia de una asociación
entre la disminución de la fecundidad y la exposición.
2. Conclusiones
Población general: A la vista del grado de exposición bajo o nulo
al 1,3-dicloropropeno, el riesgo para la población general es
insignificante.
Exposición profesional: Cuando no se adoptan las precauciones
adecuadas de seguridad, las actividades de llenado y aplicación en
el campo pueden dar lugar a una exposición del operador a
concentraciones que superan el máximo permisible.
Medio ambiente: Siempre que se utilice el 1,3-dicloropropeno en la
proporción recomendada, no es probable que se alcancen niveles
importantes para el medio ambiente, y tampoco es probable que
produzca efectos secundarios sobre poblaciones de seres vivos
terrestres y acuáticos.
3. Recomendaciones
* Las actividades de llenado y la aplicación en el campo del 1,3-
dicloropropeno sólo deben realizarse tomando las precauciones
de seguridad adecuadas, a fin de tener la garantía de que los
niveles de exposición no exceden las concentraciones máximas
permisibles de este producto.
* Se deben realizar estudios a fin de investigar el destino
metabólico del isómero trans del 1,3-dicloropropeno en
mamíferos y la posible función que los metabolitos oxidativos
de este isómero pueden tener como intermediarios en la
toxicidad del 1,3-dicloropropeno.
* La glutatión transferasa interviene en el efecto protector del
glutatión frente a la toxicidad del 1,3-dicloropropeno. Se
recomienda la realización de estudios que permitan comparar la
cinética enzimática relativa de la glutatión S-transferasa
humana de diversos tejidos con la actividad enzimática de
tejidos animales comparables.
* Se deben agrupar y publicar en revistas con una difusión amplia
los datos disponibles acerca de la función protectora del
glutatión.
* Parte de la genotoxicidad del dicloropropeno se debe al
metabolismo oxidativo. Se recomienda la realización de estudios
para identificar la isoenzima del citocromo P-450 que lleva a
cabo esta acción y comparar su actividad con la de las
isoenzimas del citocromo P-450 humano.
* Hay que aclarar la confusa función de la epiclorhidrina en los
estudios de carcinogenicidad por vía oral con sonda.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1,2-DICLOROPROPANO
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
El 1,2-dicloropropano es un líquido con un punto de ebullición
de 96,8 °C y una presión del vapor de 42 mm de Hg a 20 °C. Es
soluble en agua, etanol y éter etílico. Al calentarlo desprende
vapores de fosgeno enormemente tóxicos. El log P del coeficiente de
reparto octanol/agua es de 2,28.
Es una sustancia que se usa en el acabado de muebles, líquidos
de limpieza en seco, decapantes para pinturas, tratamiento de la
cola, desengrasado de metales, tratamiento del petróleo y como
ingrediente en la fabricación de caucho y de cera y como producto
químico intermedio en la fabricación de tetracloroetileno y
tetracloruro de carbono. Forma parte de la mezcla D/D, utilizada
como fumigante antes de la siembra.
Se han determinado las concentraciones de 1,2-dicloropropano en
el aire de las ciudades, con 1,2 µg/m3 (valor medio), 0,021-0,040
µg/m3 y 0,0065-1,4 µg/m3 en Filadelfia, Portland y Japón,
respectivamente. Su descomposición en la atmósfera es lenta; en
función de su reacción con los radicales oxhidrilos, la semivida del
1,2-dicloropropano fue de > 313 días. Probablemente el proceso
predominante en su descomposición es la fototrans-formación. Para
que ésta sea apreciable es necesario que haya adsorción sobre
material particulado. Es probable que la volatilización sea la
principal vía de escape del agua.
En el suelo, los principales mecanismos de eliminación son la
volatilización y la difusión. El 1,2-dicloropropano es persistente
en el suelo. Más del 98% del aplicado a suelos de marga se recuperó
a las 12-20 semanas del tratamiento.
En zonas en las que se ha utilizado la "mezcla D/D" para
fumigar el suelo, el 1,2-dicloropropano puede contaminar por
lixiviación las aguas subterráneas altas y profundas. En los Estados
Unidos se han encontrado en el agua de pozo y la subterránea
concentraciones de hasta 440 µg/litro y 51 µg/litro,
respectivamente. En los Países Bajos se han medido concentraciones
de hasta 160 µg/litro en agua de pozo, y se ha encontrado 1,2-
dicloropropano a una profundidad de 13 metros.
El 1,2-dicloropropano se puede ingerir con los cultivos
comestibles, pero los residuos detectados eran bajos (< 0,01 mg/kg)
y no parece que puedan tener significación biológica.
No es probable la bioacumulación de 1,2-dicloropropano, debido
a su elevada solubilidad en agua (2,7 g/kg) y al bajo log P del
coeficiente de reparto octanol/agua.
1.2 Cinética y metabolismo
El 1,2-dicloropropano administrado a ratas por vía oral se
elimina rápidamente (80-90% en 24 horas). No existen grandes
diferencias entre machos y hembras en cuanto a la cinética o la
eliminación. La principal vía de eliminación es la orina,
excretandose en 24 horas hasta la mitad de una dosis oral. Por las
heces se elimina menos del 10%. Un tercio se expulsa en el aire
expirado, en forma de anhídrido carbónico y como mezcla de productos
volátiles. Las concentraciones en los tejidos son bajas,
detectándose la más alta en el hígado. Tras la exposición de ratas
por inhalación, también se produce una eliminación rápida; en la
orina se expulsa el 55-65% de la dosis, y el 16-23% en el aire
expirado. La semivida en la sangre es de 24-30 minutos.
No se ha encontrado en la orina 1,2-dicloropropano inalterado.
Se han identificado tres metabolitos urinarios principales. Estos
proceden de las vías oxidativa y de conjugación, que dan lugar a los
mercapturatos, N-acetil- S-(2-hidroxipropil)-L-cisteína, N-
acetil- S-(2-oxipropil)-L-cisteína y N-acetil- S-(1-
carboxietil)-L-cisteína. El 1,2-dicloropropano también se puede
oxidar a lactato y dar anhídrido carbónico o acetil-CoA como
producto final.
La administración de 1,2-dicloropropano por vía oral a ratas (2
ml/kg) produjo una notable reducción de la concentración de
glutatión en los tejidos. Había una correlación entre la pérdida de
glutatión y las características de la toxicidad en el hígado, los
riñones y los eritrocitos. La disminución previa del glutatión
intracelular agravaba la toxicidad del 1,2-dicloropropano, mientras
que un tratamiento anterior con precursores de la síntesis del
glutatión mejoraba la toxicidad. Estos resultados demuestran el
efecto protector del glutatión frente a la toxicidad del 1,2-
dicloropropano.
1.3 Efectos en los seres vivos del medio ambiente
No se ha calculado la CE50 para las algas de agua dulce por
las dificultades que plantea la volatilización del producto de la
solución de prueba. La toxicidad aguda del 1,2-dicloropropano para
los invertebrados acuáticos y los peces es de baja a moderada; los
valores de la CL50 a las 48 h para los invertebrados oscila entre
52 y > 100 mg/litro, y para los peces a las 96 h varía entre 61 y
320 mg/litro. En las pruebas de toxicidad durante un período corto
con Pimephales promelas se calculó un nivel máximo sin efectos
observados de 82 mg/litro. En una prueba de toxicidad de 32 días en
las primeras fases de vida de la misma especie se puso de manifiesto
que el crecimiento y la supervivencia de las larvas eran los
parámetros más sensibles. La máxima concentración tóxica aceptable
(MCTA) se estimó entre 6 y 11 mg/litro. A los 33 días de exposición
a concentraciones de 164 mg/litro de 1,2-dicloropropano se advirtió
inhibición del crecimiento en Cyprinodon variegatus.
El 1,2-dicloropropano es fitotóxico.
En las pruebas de contacto con cuatro especies de lombrices de
tierra se obtuvo una CL50 de 44-84 µg/cm2 (valores medios) de
papel de filtro. Los valores de la CL50 en suelo artificial fueron
de 3880-5300 mg/kg de suelo (peso seco).
1.4 Efectos en los animales de experimentación y en
sistemas de prueba in vitro
La toxicidad aguda por vía oral del 1,2-dicloropropano en
animales de experimentación es baja. La DL50 por vía oral para la
rata es de 1,9 g/kg de peso corporal, y por vía cutánea en conejos
de 8,75 ml/kg de peso corporal.
En los estudios de toxicidad oral durante un período corto en
ratones y ratas se puso de manifiesto una inhibición del
crecimiento, signos clínicos de toxicidad asociados con una
depresión del sistema nervioso central y/o un aumento de la
mortalidad con dosis de 250 mg/kg de peso corporal al día o
superiores. En ratas con una dosis diaria de 250 mg/kg de peso
corporal durante 10 días se observaron cambios en las enzimas del
suero que indicaban una ligera hepatotoxicidad, con un NOEL de 100
mg/kg al día.
En un estudio de inhalación durante 13 semanas en ratones
(dosis máxima de 681 mg/m3) no se observaron efectos adversos. En
un estudio similar en el que se expusieron ratas a 68,1, 227 ó 681
mg/m3, se produjo una disminución del peso corporal y ligeras
lesiones en el tejido nasal en los grupos con las dos dosis más
elevadas.
En un estudio de reproducción en dos generaciones, la
exposición de ratas a proporciones de 1,2-dicloropropano en el agua
de bebida del 0,024, el 0,1 y el 0,24% (equivalentes a 33,6, 140 y
336 mg/kg de peso corporal al día) dio lugar a un menor aumento del
peso corporal en la madre y a un consumo reducido de agua con las
dosis media y alta. El peso corporal de los recién nacidos fue menor
con las dosis más altas. Se estableció un NOAEL para la toxicidad en
las madres y en la reproducción de 33,6 y 140 mg/kg de peso corporal
al día, respectivamente.
En los estudios realizados no se observó actividad teratogénica
alguna del 1,2-dicloropropano con dosis orales de hasta 125 mg/kg de
peso corporal en la rata y 150 mg/kg de peso corporal en el conejo.
Sin embargo, a estas dosis el 1,2-dicloropropano era tóxico para las
madres y los fetos, como pusieron de manifiesto los signos clínicos
asociados al sistema nervioso central, el menor aumento del peso
corporal de las madres y el retraso de la osificación en los fetos.
Los NOEL son de 30 y 50 mg/kg de peso corporal al día para la rata y
el conejo, respectivamente.
En la mayor parte de los estudios con bacterias, con activación
metabólica o sin ella, el 1,2-dicloropropano mostró efectos
mutagénicos, pero se utilizaron dosis muy elevadas, de hasta 10
mg/placa. En células de ovario de hámster chino se produjeron
aberraciones cromosómicas e intercambio de cromátidas hermanas. En
células V79 de hámster chino aumentó el intercambio de cromátidas.
En un sistema in vitro con linfocitos humanos, la absorción de
timidina tritiada y la viabilidad de las células cultivadas con un
sistema metabolizante hepático de la rata y sin él fueron análogas a
las de los cultivos testigo. Los resultados de una prueba de
letalidad recesiva ligada al sexo en Drosophila melanogaster
fueron negativos. En una prueba de letalidad dominante en ratas, en
la que se administró el 1,2-dicloropropano con el agua de bebida
durante 14 semanas, seguidas de dos semanas de apareamiento, se
obtuvieron resultados negativos.
En un estudio de carcinogenicidad en ratones se administraron
125 ó 250 mg de 1,2-dicloropropano/kg de peso corporal mediante
sonda, observándose un aumento relacionado con la dosis en la
incidencia de adenomas hepáticos. Esta fue mayor en los grupos
tratados que en el grupo testigo, pero se mantuvo dentro de los
valores habituales de los testigos.
La administración por sonda en ratas, a concentraciones de 125
y 250 mg/kg de peso corporal (hembras) y 62 y 125 mg/kg de peso
corporal (machos), cinco días a la semana durante 113 semanas,
produjo en las hembras con la dosificación más alta un ligero
aumento de la incidencia de adenocarcinomas de las glándulas
mamarias, por encima de los valores habituales.
1.5 Efectos en el ser humano
No es probable la exposición de la población general al 1,2-
dicloropropano a través del aire y el agua, excepto en zonas con un
uso abundante de 1,2-dicloropropano y de mezcla D/D en la
agricultura. Los residuos de 1,2-dicloropropano en los cultivos
comestibles se suelen mantener por debajo del límite de detección.
En vista de estos bajos niveles de exposición, el riesgo para la
población general es insignificante.
Se han notificado varios casos de intoxicación aguda por 1,2-
dicloropropano, debidos a una exposición excesiva accidental o
intencionada (suicidio). Los efectos se han concentrado
principalmente en el sistema nervioso central, el hígado y los
riñones. También se ha descrito la aparición de anemia hemolítica y
coagulación intravascular diseminada. En un caso, el delirio
evolucionó hacia un shock irreversible, insuficiencia cardíaca y la
muerte.
Se puede producir exposición profesional a través de la piel o
por inhalación. Se ha informado de varios casos de dermatitis y de
sensibilización cutánea en trabajadores que utilizaban mezclas de
disolventes con 1,2-dicloropropano.
2. Conclusiones
* Población general: La exposición de la población general al
1,2-dicloropropano a partir del aire o los alimentos es baja o
nula. Sin embargo, se puede producir en determinadas zonas una
exposición debida a la contaminación de las aguas subterráneas.
* Exposición profesional: Con unas buenas prácticas de trabajo,
medidas higiénicas y precauciones de seguridad, no es probable
que el 1,2-dicloropropano represente un riesgo para las
personas profesionalmente expuestas a él.
* Medio ambiente: Utilizado en las dosis recomendadas, no es
probable que el 1,2-dicloropropano alcance niveles
significativos en el medio ambiente. Tampoco es probable que
tenga efectos adversos sobre las poblaciones de seres vivos
terrestres y acuáticos.
3. Recomendaciones
* Se deben realizar estudios a fin de evaluar la toxicidad aguda
por inhalación, la irritación ocular y cutánea y la posible
sensibilización de la piel.
* Se han de adoptar las precauciones de seguridad apropiadas
cuando se maneje 1,2-dicloropropano, a fin de evitar
exposiciones que superen la concentración máxima permisible.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
"MEZCLA D/D"
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
La mezcla técnica de dicloropropenos y dicloropropano
(abreviada en el presente texto como "mezcla D/D") es un líquido de
color ámbar claro y olor acre; la presión del vapor es de 35 mm de
Hg a 20 °C, y es soluble en disolventes halogenados, ésteres y
cetonas.
La "mezcla D/D" suele contener no menos del 50% de 1,3-
dicloropropeno (con isómeros cis y trans en una proporción
aproximada de 1:1), y los demás ingredientes principales son el 1,2-
dicloropropano y compuestos afines. Esta mezcla se utiliza mucho
como nematocida del suelo antes de la siembra.
El transporte, la distribución y el destino en el medio
ambiente de los componentes principales de la "mezcla D/D" en el
aire, el agua y el suelo se describen en sección 4 de los apartados
de la presente monografía de EHC que tratan del 1,3-dicloropropeno y
el 1,2-dicloropropano.
El 1,2-dicloropropano procedente de la "mezcla D/D" tiene
considerables posibilidades de escapar del suelo por lixiviación y
contaminar las aguas de los pozos y las subterráneas. En un pozo de
riego (68 m de profundidad) de Europa occidental se registraron unas
concentraciones medias de 1,2-dicloropropano a diferentes
profundidades que oscilaban entre 0,8 y 8,5 µg/litro, con una
concentración máxima de 165 µg/litro.
No es probable que los cultivos absorban cantidades importantes
de los componentes de la "mezcla D/D" (véase en otros apartados de
la presente monografía). Tampoco es probable la bioacumulación de
los componentes de la mezcla debido a su bajo log P del coeficiente
de reparto octanol/agua y a su solubilidad relativamente grande en
agua.
1.2 Cinética y metabolismo
No se han realizado estudios metabólicos con la "mezcla D/D".
Los dos componentes principales, el 1,3-dicloropropeno y el 1,2-
dicloropropano, se eliminan con rapidez, principalmente por la orina
y, en menor cantidad, por el aire expirado. Los componentes de la
"mezcla D/D" se metabolizan por oxidación y conjugación. Los
principales metabolitos urinarios son los ácidos mercaptúricos.
1.3 Efectos en los seres vivos del medio ambiente
La "mezcla D/D" es moderadamente tóxica para los peces; los
valores de la CL50 a las 96 h oscilan entre 1 y 6 mg/litro. La
toxicidad de la mezcla se debe fundamentalmente al 1,3-
dicloropropeno.
Cuando se utilizan las dosis de aplicación recomendadas, los
principales efectos de la "mezcla D/D" son la reducción transitoria
(< 7 días) de los hongos del suelo y la inhibición de la oxidación
de los iones amonio a nitrato. La mezcla es tóxica para las
bacterias nitrificantes. Inmediatamente después de desaparecer del
suelo, las bacterias comienzan a colonizar la zona de nuevo. En
ensayos de campo, la "mezcla D/D" (aplicada a 600 litros/ha) eliminó
los invertebrados del suelo. La recolonización requirió entre 6 y 24
meses.
La "mezcla D/D" tiene una elevada fitotoxicidad.
1.4 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la "mezcla D/D" para los animales de
laboratorio es de moderada a alta. Los valores de la DL50 por vía
oral en ratas y ratones oscilan entre 132 y 300 mg/kg de peso
corporal. Los valores de la DL50 por vía cutánea en ratas y
conejos son de 779 y 2100 mg/kg de peso corporal, respectivamente.
La CL50 (4 h) por vía respiratoria para ratas es aproximadamente
de 4540 mg/m3. La exposición aguda produjo signos clínicos
asociados a depresión del sistema nervioso central. La "mezcla D/D"
tiene un fuerte efecto irritante en los ojos y la piel y una
moderada capacidad de sensibilización cutánea.
Los resultados de los estudios disponibles de toxicidad durante
un período breve en ratas y perros son insuficientes para evaluar de
manera correcta la posible toxicidad de la mezcla, porque las dosis
relativamente bajas ensayadas no demuestran ningún efecto
biológicamente significativo. Se han realizado varios estudios de
exposición por inhalación (todo el cuerpo) durante un período corto
en ratas. Las concentraciones de hasta 145 mg/m3 de la "mezcla
D/D" no tienen ningún efecto tóxico. Con niveles de 1362 mg/m3 o
superiores se detectan claramente efectos tóxicos asociados con
depresión del sistema nervioso central. Una exposición a 443 mg/m3
durante 10 semanas da lugar a una disminución del aumento del peso
corporal y a un mayor peso absoluto de los riñones.
Un estudio teratológico en ratas por vía oral de la "mezcla
D/D" fue inadecuado para evaluar su posible acción en este sentido.
En un estudio de inhalación en ratas con dosis de hasta 443
mg/m3 durante 10 semanas, para investigar la fecundidad de machos
y hembras, no se observó ningún efecto. Debido a un diseño
inadecuado del procedimiento, no fue posible evaluar completamente
los efectos de la "mezcla D/D" sobre la reproducción.
La mezcla tiene efectos mutagénicos en las cepas TA100 y TA1535
de Salmonella typhimurium, así como en la WP2 HCR de Escherichia
coli, sin activación metabólica. Sin embargo, no se produjeron
tales efectos en las cepas TA98, TA1537 y TA1538 de Salmonella.
En un estudio prolongado en ratas alimentadas con dietas que
contenían hasta 120 mg de la "mezcla D/D" por kg (equivalentes a 6
mg/kg de peso corporal) durante dos años no se detectaron efectos
tóxicos ni carcinógenos.
1.5 Efectos en el ser humano
Ya no se utiliza la "mezcla D/D" tanto como antes, por lo que
es improbable la exposición de la población general a través del
aire, el agua y los alimentos.
Cuando se utilizaban los procedimientos recomendados, la
exposición de los trabajadores que llenaban los bidones y de los
aplicadores en el campo fue en general inferior a 4,5 mg de 1,3-
dicloropropeno/m3. En otras condiciones se han medido
concentraciones de hasta 36,32 mg/m3.
Se ha informado de un caso de intoxicación aguda con desenlace
fatal tras la ingestión accidental de la mezcla.
Se han notificado varios casos de dermatitis por contacto y de
sensibilización cutánea debidas a la exposición accidental a la
"mezcla D/D".
2. Conclusiones
* Población general: Dado que la "mezcla D/D" no tiene ya un uso
tan generalizado, la exposición de la población al 1,3-
dicloropropeno a través del aire, el agua y los alimentos es
insignificante, pero, en ciertas zonas, cuando se contaminan
las aguas subterráneas, se puede producir una exposición al
1,2-dicloropropano.
* Exposición profesional: Durante las actividades de llenado y de
aplicación de la "mezcla D/D" en los campos se puede producir
una exposición de los manipuladores a concentraciones de 1,3-
dicloropropeno superiores a la máxima permisible, especialmente
en condiciones climáticas cálidas.
* Medio ambiente: No es probable que la "mezcla D/D" alcance
niveles biológicamente significativos en el medio terrestre o
acuático, siempre que se utilice en las dosis recomendadas.
Tampoco es probable que se produzcan efectos adversos duraderos
en los organismos vivos del medio ambiente.
3. Recomendaciones
* No se debe utilizar la "mezcla D/D" para fumigar el suelo,
debido a su capacidad de lixiviar y alcanzar las aguas
subterráneas.
* En las zonas en las que se use la mezcla, se han de vigilar los
residuos en las aguas superficiales y subterráneas.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
The EC50 for CO2 uptake by marine algae (Phaeodactylum
tricornutum) was 50 mg/litre. 1,2-Dichloropropane was not found to
exhibit any algistatic or algicidal effects in an acute toxicity
study on Selenastrum capricornutum, though the problems of losses
of 1,2-dichloropropane from the test flasks, made it impossible to
calculate EC values (Dynamic Corporation, 1991).
A general trend of decreasing algal population growth with
increasing nominal concentrations of 1,2-dichloropropane was
observed in an acute toxicity test on Skeletonema costatum.
Because the concentrations measured for each nominal value were
variable, it was not appropriate to determine EC values. A NOEL of
18 mg/litre was determined, though it was not possible to
distinguish algistatic from algicidal effects (Dynamic Corporation,
1991).
7.1.2 Invertebrates
The acute toxicity of 1,2-dichloropropane for non-target
invertebrates is summarized in Table 18; 1,2-dichloropropane has a
48-h LC50 of 50-100 mg/litre for these aquatic organisms. No
discernible effects on Daphnia magna were found at concentrations
of less than 22 mg/litre (Leblanc, 1980).
Table 18. Acute toxicity of 1,2-dichloropropane in non-target
aquatic insects and crustacea
Species Temperature 48-h LC50 References
(°C) mg/litre
Barnacle nauplii - 53 Pearson & McConnell
(Elminius moderatus) (1975)
Brown shrimp - > 100 Portmann & Wilson
(Crangon crangon) (1971)
7.1.3 Fish
7.1.3.1 Acute toxicity
The 96-h LC50 of 1,2-dichloropropane for freshwater and
marine fish is 61-320 mg/litre (Table 19).
7.1.3.2 Short-term/long-term toxicity
Two embryo-larval tests have been conducted on Fathead minnow
(Pimephales promelas) exposed to 1,2-dichloropropane. The maximum
no-effect levels were 8.1 and 60 mg/litre, respectively (US EPA,
1980).
In the marine environment, growth inhibition was noted in the
Sheepshead minnow (Cyprinodon variegatus) after 33 days exposure
to 164 mg 1,2-dichloropropane/litre (US EPA, 1980).
A 32-day test to study early life stage toxicity in Fathead
minnows (Pimephales promelas) demonstrated that larval growth and
survival (28-day-old fish) were the most sensitive indicators of
toxic effects. Embryo hatch and larval deformities at hatch were the
least sensitive indicators of toxicity. The 1,2-dichloropropane
(98%) was tested at dose levels of 0, 6, 11, 25, 51, and 110
mg/litre (temperature of the water 25 °C, hardness 45 mg/litre as
CaCO3, pH 7.4). The estimated maximum acceptable toxicant
concentration (MATC) was between 6 and 11 mg/litre (Benoit et al.,
1982).
7.2 Terrestrial organisms
7.2.1 Earthworms
Neuhauser et al. (1985a,b; 1986) studied the toxicity of 1,2-
dichloropropane for 4 species of earthworm (Allolobophora
tuberculata, Eisenia foetida, Eudrilus eugeniae and Perionyx
excavatus) using EEC earthworm artificial soil and a contact
testing procedure (Edwards, 1983). The LC50 values and 95%
confidence limits in the contact test were 84(65-110); 64(59-70);
44(38-51); and 63(56-72) µg/cm2 of filter paper for the 4 species
of earthworms, respectively. In the artificial soil test, the LC50
values were 4272, 4240, 5300, and 3880 mg/kg dry weight artificial
soil, respectively.
7.2.2 Plants
1,2-Dichloropropane is highly phytotoxic.
Table 19. Acute toxicity of 1,2-dichloropropane in fish
Species Size or age Temperature 96-h LC50 References
(°C) mg/litre
Guppy 2-3 months 21-23 3.01 µmol/litref Könemann (1981)
(Poecilia reticulata)
Bluegill sunfish 33-75 mm 23a 320 Dawson et al. (1977)
(Lepomis macrochirus) 0.32-1.2 g 21-23 280b,c Buccafusco et al. (1981)
(220-340)
Fathead minnow 30-35 days 25d,e 140d Wallbridge (1983)
(Pimephales promelas) (131-150)
Tidewater silversides 40-100 mm 20a 240 Dawson et al. (1977)
(Menida beryllina
Dab 15-20 cm - 61 Pearson & McConnell (1975)
(Pleuronectes limanda)
a Hardness 55 mg/litre (as CaCO3), pH 7.6-7.9.
b Tested in well-water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration at the beginning
9.7 to 0.3 mg/litre after 96-h exposure.
c Static system (nominal concentrations).
d Flow-through system.
e Hardness 42-45 mg/litre (as CaCO3) pH 6.7-7.6.
f 7-day LC50 (static system, renewal).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
The acute oral LD50 for the rat has been reported to be 1.9
(1.7-2.1) g/kg body weight and the dermal LD50 after a single skin
exposure of rabbits was 8.75 (8.3-9.2) ml/kg body weight (Smyth et
al., 1969; California State Water Resources Control Board, 1983).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
Groups of 5 male and 5 female B6C3F1 mice were
administered (by gavage) 0, 125, 250, 500, 1000, or 2000 mg 1,2-
dichloropropane (99.4%) per kg body weight, in corn oil. The doses
were given for 14 consecutive days (range-finding study) followed by
1 day of observation. All male mice in the 1000 and 2000 mg/kg
groups and female mice in the 2000 mg/kg group died. Also 3 out of 5
males receiving 500 mg/kg and 4 out of 5 females receiving 1000
mg/kg died. No growth inhibition was seen in the surviving animals
(NTP, 1986).
Groups of 10 male and 10 female B6C3F1 mice were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks, at doses of 0, 30, 60, 125, 250, or 500
mg/kg body weight. Mortality was not significantly increased, and
mean body weight changes were not dose-related. No compound-related
histopathological changes were found at the highest dose level (NTP,
1986).
8.2.1.2 Rat
Groups of 5 male and 5 female F344/N rats were administered
1,2-dichloropropane (99.4%) at 0, 125, 250, 500, 1000, and 2000
mg/kg body weight, in corn oil, by gavage, for 14 consecutive days
(range-finding study) followed by 1 day of observation. All rats
receiving the highest dose level died. Growth inhibition was seen in
the surviving animals in the 1000 mg/kg group. At necropsy, renal
medullae were red in the 2000 mg/kg group, not in lower dose levels
(NTP, 1986).
Groups of 10 male and 10 female F344/N rats were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks at doses of 0, 60, 125, 250, 500, or 1000
mg/kg body weight. All animals administered 1000 mg/kg and half of
the males administered 500 mg/kg died. Growth inhibition was seen in
the 500 mg/kg group. Centrilobular congestion of the liver occurred
in the 1000 mg/kg group and 2 females of this group showed hepatic
changes and centrilobular necrosis. Lower dose levels did not
produce effects (NTP, 1986).
A 2-week study of 1,2-dichloropropane (99.9%) was conducted
using groups of 10 Fischer 344 rats/sex to select doses for a
subsequent 13-week study. Dose levels were 0 (corn oil), 300, or 500
mg/kg body weight per day (by gavage) for 14 days. Data were
obtained on body weight, clinical effects, body temperature,
functional observational battery, motor activity, haematology,
liver, kidney, and spleen weights, gross pathology and histological
examination of the liver and kidneys. In both groups, transient
clinical effects (tearing, blinking, and lethargy) were seen, and
body weights were significantly decreased. The body temperatures of
treated animals in both groups, recorded 1 h after dosing on day 13,
were decreased by 0.3-0.5 °C. No effects on motor activity and
haematology were noted. Liver and kidney weights were increased and
spleen weight decreased. Histopathological changes (prominent
nucleoli of hepatocytes in the centrilobular region, degeneration
and necrosis of the liver cells) were found in the livers of animals
in both the treated groups (Gorzinski & Johnson, 1989).
In an oral toxicity study, adult male Sprague-Dawley rats were
administered 0, 100, 250, 500, or 1000 mg 1,2-dichloropropane/kg
body weight, in corn oil (by gavage), once a day, for up to 10
consecutive days. Some rats were killed at each dose level 24 h
after 1, 5, and 10 daily doses. After dosing, changes in body weight
and clinical toxic signs were assessed. In addition, measurements of
clinical chemistry parameters, urinary levels of glucose, alkaline
and acid phosphatase and N-acetyl glycosaminidase activities, and
the histopathology of major organs, liver enzyme levels, as well as
levels of non-protein sulfhydryls in the liver and kidneys were
determined. Definite central nervous system depression and body
weight loss, and increased renal nonprotein sulfhydryl (NPS) levels
were seen at 250 mg/kg. Liver damage (both morphological and enzyme
changes) was observed only at the 500 and 1000 mg/kg dose levels. In
this study, "resistance" to 1,2-dichloropropane hepatotoxicity
developed over the 10 consecutive days of exposure, as reflected by
progressively lower serum enzyme levels and by decreases in the
severity and incidence of toxic hepatitis and periportal
vacuolization. Nucleolar enlargement in hepatocytes, however, was
observed at all dose levels at both 5 and 10 days. There were a
number of manifestations of haemolytic anaemia in various tissues,
but no evidence of nephrotoxicity. On the basis of the parameters
examined, a NOEL of 100 mg/kg body weight can be established
(Bruckner et al., 1989).
In a similar oral study, male Sprague-Dawley adult rats were
treated with 0, 100, 250, 500, or 750 mg 1,2-dichloropropane/kg body
weight, in corn oil, by gavage, 5 times weekly for up to 13 weeks.
Over one-half of the animals in the 750 mg/kg group died within 10
days. Histopathological changes included mild hepatitis and splenic
haemosiderosis, adrenal medullary vacuolization and cortical
lipidosis, testicular degeneration and a reduction in sperm, and an
increased number of degenerated spermatogonia in the epididymis.
Similar testicular and epididymal degenerative changes were observed
in some animals in the 500 mg/kg group after 13 weeks of dosing.
While no deaths occurred in the 100 or 250 mg/kg groups, more than
50% in the 500 mg/kg group had died by 13 weeks. A dose-dependent
decrease in body weight gain was observed. 1,2-Dichloropropane
exhibited very limited hepatotoxic potential and no apparent
nephrotoxic potential in this 13-week study (Bruckner et al., 1989).
8.2.2 Inhalation
8.2.2.1 Mouse
B6C3F1 mice (aged 6-8 weeks) were exposed to target
concentrations of 1,2-dichloropropane (99.9%) of 0, 69.9, 233, or
699 mg/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. There were no
effects on haematology, gross pathology, and histopathology at
concentrations as high as 699 mg/m3, in this study (Nitschke et
al., 1988).
8.2.2.2 Rat
Fischer 344 rats (aged 6-8 weeks) were exposed to target
concentrations of 0, 69.9, 233, or 699 mg 1,2-dichloropropane
(99.9%)/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. The body weights
of rats exposed to 233 and 699 mg/m3 were significantly decreased.
Minimal effects were observed microscopically in the nasal tissues
of rats exposed to 233 and 699 mg/m3. A few rats exposed to 69.9
mg/m3 also had slight thickening of a small portion of the
respiratory nasal mucosa. No treatment-related effects were noted in
haematology, clinical chemistry, or urinalysis, even at 699 mg/m3
(Nitschke et al., 1988). A NOEL of 74.5 mg/m3 can be established.
8.2.2.3 Rabbit
New Zealand white rabbits (age 6-7 months) were exposed to 1,2-
dichloropropane at 0, 699, 2330, or 4660 mg/m3 for 6 h/day, 5
days/week, for 13 weeks. The mean actual concentrations were 0, 694,
2204, or 4436 mg/m3. Minimal effects on nasal tissues were present
in male rabbits only at the 4660 mg/m3 level. The primary effects,
decreased red blood cell parameters (red cell blood count,
haemoglobin, and packed cell volume), were observed in male rabbits
exposed to 699 (minimal effects) - 4660 mg/m3, and in female
rabbits exposed to 2330 and 4660 mg/m3 (Nitschke et al., 1988). No
NOEL has been established.
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
Groups of 30 male and 30 female Sprague-Dawley rats each were
provided with drinking-water containing 1,2-dichloropropane (99.9%)
at concentrations of 0, 0.24, 1, or 2.4 g/litre (w/v) (equivalent to
0, 33.6, 140, or 336 mg/kg body weight) over 2 generations. A
concentration of 2.4 g/litre represented the maximum practicable
attainable concentration, based on solubility. Adult rats were
evaluated for body weights, water and feed consumption, reproductive
performance, and gross pathological and histological changes. The
litters were evaluated for size, neonatal growth, and survival.
Decreases in water consumption reflective of rejection because of
unpalatability, were observed at all levels tested in both sexes in
the F0 and F1 generations. These decreases in water consumption
resulted in significantly lower body weights in both generations
administered 2.4 g/litre. These differences in water consumption and
body weights were also evident among the females during gestation
and/or lactation. The 0.24 g/litre dose level had a minor effect on
water consumption and body weights, but no adverse effects on the
animals. No treatment-related gross pathological changes were noted
in any dose group and the histological changes were limited to
increased hepatocellular granularity in both sexes in both
generations at all dose levels.
There were no histological changes in the reproductive tracts
of either sex in either generation. Reproductive function, measured
by fertility and litter size, was unaffected. The decreases in water
consumption among females at 2.4 g 1,2-dichloropropane/litre
resulted in significantly lower neonatal body weights and slightly
increased neonatal mortality in their litters. These neonatal
effects were considered secondary to the substantial decreases in
maternal water consumption, rather than a direct effect of the
substance. There were no neonatal effects at the 2 lower
concentrations. The NOAEL for adults is 0.24 g/litre, and the
reproductive NOAEL is 1 g/litre (equivalent to 140 mg/kg body
weight) (Kirk et al., 1990).
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
Groups of 30 Sprague-Dawley rats were administered 1,2-
dichloropropane (99.9%) in corn oil, by gavage, on gestation days 6-
15 at dose levels of 0, 10, 30, or 125 mg/kg body weight per day.
Parameters evaluated included maternal body weight and weight gain,
feed and water consumption, fetal weights, and fetal morphology. The
highest dose level produced transient decreases in respiration,
movement, muscle tone and extensor thrust reflex, and increases in
salivation and lacrimation, within 1 h of dosing on day 6 of
gestation. A decrease in the frequency of these clinical symptoms
occurred on the second day. In rats, 1,2-dichloropropane produced
transient central nervous system depression and decreased maternal
body weight gain and feed consumption at 125 mg/kg body weight.
Fetal effects in rats were limited to a significant increase in the
incidence of delayed ossification of the bones of the skull in the
125 mg/kg group, secondary to maternal effects. A level of 30 mg/kg
was considered the NOEL for maternal and fetal effects (Kirk et al.,
1989; Hanley et al., 1990).
8.3.2.2 Oral (rabbit)
Groups of 18 New Zealand white rabbits were administered 1,2-
dichloropropane (99.9%) at dose levels of 0, 15, 50, or 150 mg/kg
body weight per day on gestation days 7-19. On day 28 of gestation,
Caesarean section was performed and the fetuses evaluated.
Parameters evaluated included maternal body weight and weight gain,
haematology, fetal body weights, and fetal morphology. In maternal
rabbits, a dose of 150 mg/kg per day produced anorexia, significant
decreases in weight gain, and anaemia, with microscopic examination
revealing slight to moderate anisocytosis, poikilocytosis, and/or
polychromasia, indicative of a regenerative anaemia. The only fetal
effect in rabbits was a significant increase in the incidence of
delayed ossification of the bones of the skull at the highest dose
level, secondary to maternal effects. No effects were observed in
the 15 and 50 mg/kg dose groups. The NOEL for both maternal and
fetal effects was 50 mg/kg body weight (Hanley et al., 1989a).
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
1,2-Dichloropropane was tested for its mutagenic activity in
Salmonella typhimurium, Saccharomyces cerevisiae with, and without
metabolic activation, and in Aspergillus nidulans and
Streptomyces coelicolor. Results in most studies on S.
typhimurium TA100 and TA1535 were positive, but negative results
were obtained with TA98, TA1537, and TA1538, S. cerevisiae, and
S. coelicolor. In Aspergillus nidulans, a forward mutation to 8-
azaguanine resistance and methionine suppression was found with S9
activation.
The results are summarized in Table 20.
Table 20. Mutagenicity tests with 1,2-dichloropropane on microorganisms
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Salmonella typhimurium
TA100, TA1535 1,2-DCP 10-50 mg plate S9 mix/none + De Lorenzo et al.
TA1978 S9 mix/none - (1977)
TA100 1,2-DCP (65% 62.5-8000 suspension S9 mix + Priston et al.
TA1535 1,2-DCP + 25% µg/mld S9 mix/none + (1983)
1,3-DCPropene)
TA98, TA1537 S9 mix/none -
TA100 1,2-DCP 1, 10, plate S9 mix/none - Stolzenberg & Hine
100 µmola (1980)
TA100, TA1535 1,2-DCP 1-10 µlitre plate S9 mix/none + Carere & Morpurgo
(1981)
TA98, TA1537, TA1538 S9 mix/none -
TA100, TA1535 1,2-DCP 0.33-10 mg plate S9 mix/none + Haworth et al. (1983)
(rat/hamster)
TA98, TA1537 S9 mix/none -
(rat/hamster)
TA100, TA1535, TA1537 1,2-DCP (99%) 33-2000 µg plate S9 mix - NTP (1983)
S9 none (+)
TA98 S9 mix/none -
(rat/hamster)
Saccharomyces cerevisiae
JD1 1,2-DCP (65% 62.5-8000 - S9 mix - Priston et al.
1,2-DCP + 25% µg/mld S9 none (+) (1983)
1,3-DC propene)
Table 20 (contd)
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Streptomyces coelicolarb 1,2-DCP 2-100 µlitre plate none - Carere & Morpurgo
A3 (1981)
Aspergillus nidulansc 100-400 µlitre plate none + Carere & Morpurgo
(1981)
Aspergillus nidulans 1,2-DCP (99%) 154 mmol/litre - Crebelli et al.
(1984)
a The highest dose level, 100 µmol/plate gave complete inhibition of bacterial growth.
b Resistance to streptomycin.
c Resistance to 8-azaguanine.
d Concentrations of 1000 µg/ml and higher were cytotoxic.
Crebelli et al. (1984) studied the induction of somatic
segregation in Aspergillus nidulans. Induction of haploidization,
mitotic non-disjunction, and mitotic crossing-over was studied in
heterozygous colonies exposed to 1,2-dichloropropane, 99%. No
significant rise in frequency of any kind of segregated sectors was
found. The concentration that was used was 154 mmol/litre.
8.4.1.2 Mammalian cells
In cytogenetic studies using Chinese hamster ovary cells, 1,2-
dichloropropane (99.4%) caused both chromosome aberrations and
sister chromatid exchanges. Dose levels tested were 0.46-1.50 and
0.113-1.13 mg/ml, respectively (Galloway et al., 1987).
Priston et al. (1983) also studied the ability of 1,2-
dichloropropane (containing 65% 1,2-dichloropropane and 25% 1,3-
dichloropropane) to induce chromosome damage using rat liver (RL4)
cells, in concentrations of 5-20 µg/ml. An indication for a small
increase in the frequency of chromatid gaps, chromatid and
chromosome aberrations, was noticed, but only in the presence of
cytotoxic effects. The Task Group considered this study inadequate.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
test in vitro in Chinese hamster V79 cells to evaluate the effect
of 1,2-dichloropropane (99%). The concentrations tested were 0
(DMSO), 1.0, 3.3, and 10.0 mmol/litre without S9 mix. A dose-related
increase in SCEs was observed. With S9 mix and the same
concentrations, the SCEs frequency was increased but was less than
without S9 mix.
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
Woodruff et al. (1985) tested 1,2-dichloropropane at 0 and 4200
mg/litre by injection in germ cells and 0 and 33 552 mg/m3 by
inhalation in Drosophila melanogaster, using the sex-linked
recessive lethal mutation for their mutagenicity. The results by
injection and inhalation were negative.
8.4.2.2 Dominant lethal test
Groups of 30 male Sprague-Dawley rats were given 1,2-
dichloropropane (99.9%) in the drinking-water at concentrations of
0, 0.24, 1, or 2.4 g/litre, continuously, for a period of
approximately 14 weeks, as part of a combined reproduction/ dominant
lethal study (section 8.3.1). Exposed males (F0) were used for a
dominant lethal test following completion of the breeding for the
F1 litters of the reproduction study. They were mated with pairs
of naive, untreated adult females for 2 successive periods of 1 week
each. A separate, positive control group of 30 male rats was
administered a single oral dose of 100 mg cyclophosphamide/kg body
weight, 48 h prior to mating with untreated females. The uterine
contents of the females were evaluated for evidence of dominant
lethal effects as manifested by an increase in the resorption rate.
Among 1,2-dichloropropane-treated males, concentration-related
decreases in water consumption were noted at all levels and
decreased body weights were noted in males given 1 or 2.4 g/litre in
the water. Mating performance was unaffected in these animals.
Resorption rates among these groups revealed that the weekly values
for the females mated to treated males ranged from 2.2 to 8.1%, well
within the historical control range. Resorption rates among the
concurrent controls were low, ranging from 3.5 to 5.4%. The
significant increases from the concurrent control values, identified
during the first week of breeding in the resorption rates in the
groups given 0.24 or 2.4 g/litre, were considered to be within the
normal variation. Cyclophosphamide resulted in a 10-fold increase in
the resorption rate. 1,2-Dichloropropane was not mutagenic in a
dominant lethal assay in male Sprague-Dawley rats exposed
continuously to concentrations of up to 2.4 g/litre in the drinking-
water (Hanley et al., 1989b).
8.4.2.3 Miscellaneous
Perocco et al. (1983) studied the tritiated thymidine uptake
and cell viability in human lymphocyte cultures grown with, or
without, rat liver metabolizing system. Both the [3H]TdR uptake
and the percentage of viable cells showed values very similar to
control values. The 1,2-dichloropropane concentrations used ranged
from 10-2 to 10-4 mmol/litre.
8.5 Carcinogenicity
8.5.1 Oral (mouse)
Groups of 7 to 9-week-old hybrid B6C3F1 mice (50 males
and 50 females) were administered 0, 125, or 250 mg 1,2-
dichloropropane (99.4%)/kg body weight, in corn oil, by gavage, 5
days/week for 113 weeks. No influence on growth was observed. The
survival of the female animals at the highest dose level was
significantly decreased. The incidence of non-neoplastic lesions
showed lesions of the spleen (haemosiderosis and haematopoiesis were
increased) in female mice at the highest dose level.
The incidences of neoplastic lesions are summarized in Table
21.
Table 21. The incidence of neoplastic lesions in a mouse study with 1,2-dichloropropanea
Sex Control 125 mg/kg 250 mg/kg
Adenoma (liver) male 7/35 (20%) 9/33 (27%) 15/35 (43%)
Carcinoma (liver) male 8/35 (23%) 10/33 (30%) 9/35 (26%)
Adenoma and carcinoma (liver) male 15/35 (43%) 18/33 (55%) 24/35 (69%)b
Adenoma (liver) female 1/35 (3%) 5/29 (17%) 5/26 (19%)b
Carcinoma (liver) female 1/35 (3%) 2/29 (7%) 2/26 (8%)
Adenoma and carcinoma (liver) female 2/35 (6%) 7/29 (24%)b 7/26 (27%)b
Follicular cell adenoma and female 1/34 (3%) 0/27 (0%) 5/24 (21%)b
carcinoma (thyroid)
Alveolar/bronchiolar adenoma female 5/35 (14%) 0/29 (0%) 1/26 (4%)
or carcinoma (lung)
Squamous cell male 0/50 (0%) 1/48 (2%) 3/49 (6%)
papillomas (forestomach) female 0/50 (0%) 2/50 (4%) 2/50 (4%)c
a From: NTP (1986).
b P = < 0.05 - > 0.001.
c One high-dose female mouse had a squamous cell carcinoma.
On the basis of the results of this study, the NTP concluded
that 1,2-dichloropropane induces an increased incidence of liver
tumours in male and female B6C3F1 mice (Haseman et al., 1984;
NTP, 1986). However, the Task Group noted that the incidences of
liver adenomas and carcinomas in the treated groups were within the
historical ranges of this species (Haseman et al., 1985). The
increased incidence of thyroid tumours is equivocal.
8.5.2 Oral (rat)
Groups of 50 male and 50 female F344/N rats (aged 7-9 weeks)
were administered, by gavage, 0, 125 or 250 (female) and 0, 62, or
125 mg (male) 1,2-dichloropropane (99.4%)/kg body weight, in corn
oil, 5 days/week for 103 weeks. The highest dose level showed growth
depression in both sexes. Survival of female rats at the high dose
level was significantly lower. The incidence of non-neoplastic
lesions was not significantly different from that in the controls,
except for an increased incidence of foci of clear cell change and
of necrosis in the liver in high-dose female rats.
The incidences of neoplastic lesions are summarized in Table
22. Apart from these tumours, squamous cell papillomas of the
forestomach were found in 1 control male and 1 female rat. In high-
dose females, there was an increased incidence of mammary gland
adenocarcinomas, but not of mammary gland adenomas. Apart from these
tumours, squamous cell papillomas of the fore-stomach were found in
two high-dose females (not significantly increased compared to
controls). There were no effects on tumour incidences in male rats
(Haseman et al., 1984; NTP, 1986).
8.6 Factors modifying toxicity
Weanling Wistar rats, fed for several weeks on low protein
choline-deficient diets, were more susceptible to the effects of
inhalation of 4660 mg 1,2-dichloropropane/m3 than rats on a
control diet. This increased susceptibility could be corrected by
dietary supplements of 1-methionine or 1-cysteine plus choline
chloride (Heppel et al., 1946).
8.7 Special studies
8.7.1 Liver
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight, in corn oil, for 5 days (once daily) or for 4 weeks (five
days/week), or a single dose of 0, 50, 100, or 250 mg/kg body
weight, to investigate the biochemical and histological liver
changes. Reduced glutathione (GSH), glutathione- S-transferase,
cytochrome P450, and protein contents were measured. A dose-
Table 22. The incidence of neoplastic lesions in a rat study with 1,2-dichloropropanea
Sex Control 62 mg/kg male 125 mg/kg male
125 mg/kg female 250 mg/kg female
Fibroadenoma female 14/37 (38%) 20/43 (47%) 5/16 (31%)
(mammary gland)
Adenocarcinoma female 1/37 (3%) 2/43 (5%) 4/16 (25%)b
(mammary gland)
Endometrial stromal female 8/37 (22%) 14/42 (33%) 6/16 (38%)b
polyps (uterine
tumours)
Liver tumours male 3/50 (6%) 3/50 (6%) 2/50 (4%)
female 0/50 (0%) 0/50 (0%) 0/50 (0%)
Islet cell adenoma male 4/38 (11%) 1/42 (2%) 5/41 (12%)
and carcinoma
(pancreas)
a From: NTP (1986).
b P = < 0.05.
dependent decrease in liver-reduced glutathione was observed after a
single injection and a dose-dependent increase after 4 weeks. The
liver biochemical pattern after 4 weeks, characterized by a decrease
of cytochrome P450 and by an increase in reduced glutathione and
glutathione- S-transferase activity, suggests a hyperplastic
evolution of the liver cells, probably a repair mechanism induced by
the early depletion of glutathione. Histologically, the alterations
confirm the regenerative nature (atypical mitosis and hyperplastic
nodules) of the changes (Trevisan et al., 1989).
The hepatotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose-levels of 0, 50, 100, 250, or 500 mg/kg body weight per day,
in corn oil, for 4 weeks. Biochemical changes in the liver were
demonstrated. Significant findings included reduction of aminopyrine
demethylase activity at 100 mg/kg or more, increased levels of
reduced glutathione and glutathione- S-transferase activity at 50
mg/kg or more, and decreased cytochrome P450 activity at 500 mg/kg.
The activity of aniline hydroxylase was not affected. Duplicate
groups of rats treated with 1,2-dichloropropane, but allowed a
period of 4 weeks of recovery before being subjected to examination,
showed that the induced biochemical changes in the liver were
completely reversible (Trevisan et al., 1991).
1,2-Dichloropropane toxicity is actually preferentially
mediated by GSH depletion. This is suggested by the fact that GSH
loss is correlated with an increase in the biochemical indices of
liver and renal injury, and with the extent of haemolysis.
Pretreatment of 1,2-dichloropropane-intoxicated rats with
buthionine-sulfoximine (BSO) (depleting GSH) markedly increased the
overall mortality. Furthermore, the administration of the GSH
precursor N-acetyl-cysteine (NAC), preventing GSH depletion,
reduced the damage in target tissues, as demonstrated by a smaller
increase in some biochemical indices of cell injury and a smaller
degree of haemolysis. A possible explanation for these findings is
that, when the GSH level falls below a certain threshold value,
irrespective of the causative agent, a series of common reactions is
triggered, inducing peroxidation of membrane lipids, disturbances of
Ca2 homeostasis, and DNA damage, which quickly lead to
irreversible liver injury. Furthermore, it is also possible that
electrophilic metabolites of 1,2-dichloropropane, formed in the
absence of GSH, could directly attack a variety of cell
macromolecules the function of which is essentially to ensure the
physiological survival of the hepatocytes (Mitchell et al., 1973;
Bellomo & Orrenius, 1985; Casini et al., 1987; Orrenius et al.,
1989).
Male Wistar rats were exposed (by gavage) to a single oral dose
of 55 mg 1,2-dichloropropane/kg body weight in propylene glycol. The
animals were killed 1-6 days after exposure. Glutathione (GSH),
lipid peroxidation and protein of liver homogenate were measured.
Reduction of hepatic GSH and total protein and enhanced hepatic
lipid peroxidation still persisted 6 days after 1,2-dichloropropane
administration (Di Nucci et al., 1988).
Groups of rats were treated orally, by gavage, with single
doses of 1,2-dichloropropane ranging from 55 to 400 mg/kg body
weight. 1,2-Dichloropropane was no longer detectable in the blood 24
h after dosing at any dose level (Di Nucci et al., 1988).
8.7.2 Kidneys
Renal failure caused by 1,2-dichloropropane has been reported
by several authors who found an increase in serum creatinine and
urea in intoxicated animals and humans and fatty degeneration or
acute tubular damage in the kidney parenchyma (Heppel et al., 1946,
Ponticelli et al., 1968; Pozzi et al., 1985; Imberti et al., 1987).
Imberti et al. (1990) suggested that, on the basis of the
demonstration of 1,2-dichloropropane-induced depletion of kidney
GSH, it may be postulated that the biochemical mechanisms involved
in liver toxicity may also apply to the kidneys (Brezis et al.,
1983). In addition, since most of the glutathione and cysteine
conjugates of 1,2-dichloropropane are recovered in the urine, a
direct toxicity of the conjugates to the kidneys seems to play an
important role, as previously demonstrated for other conjugates
(Stevens et al., 1988).
It has been demonstrated that GSH plays an essential role in
the maintenance of the physiological structure of the erythrocyte,
preventing the formation of inter-protein or intra-protein
disulfides within the membrane skeleton. Depletion of erythrocyte
glutathione causes an increased fragility of this cell and
subsequent haemolysis. The involvement of such a mechanism in 1,2-
dichloropropane-induced haemolysis in intoxicated animals is
supported by the demonstration of a concomitant loss in erythrocyte
GSH and a correlation between these 2 phenomena (Imberti et al.,
1990).
Nephrotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose levels of 0, 50, 100, 250, or 500 mg/kg body weight per day
for 4 weeks. Biochemical and histopathological alterations of the
kidneys were demonstrated. Kidney pathology involved a decrease in
the activity of angiotensin-converting enzyme in the proximal tubule
brush border and fraying of microvilli with epithelial coagulative
necrosis. Duplicate groups of rats treated similarly with 1,2-
dichloropropane, but allowed a period of 4 weeks of recovery before
being subjected to examination, showed that the treatment-induced
biochemical changes in the kidneys were completely reversible
(Trevisan et al., 1988, 1991).
8.7.3 Central nervous system
In the study of Gorzinski & Johnson (1989), in which Fischer
344 rats were treated with 0, 300, or 500 mg 1,2-dichloropropane per
kg body weight, by gavage, for 14 days, motor activity and a
functional observational battery (unusual responses in behaviour,
presence of convulsions, tremors, etc., and sensory function) were
evaluated (section 8.2.1.2). No effects were seen on these
parameters.
Specific tests in groups of 15 Fischer 344 rats/sex given, by
gavage, 0 (corn oil), 20, 65, or 200 mg 1,2-dichloropropane
(99.9%)/kg body weight, 5 days/week for 13 weeks, included monthly
evaluation of a functional observation battery, hind limb grip
strength, and motor activity; a comprehensive neuropathology study
was carried out at the end of the study. Clinical observations
included measurement of body weight gain and body temperature. Body
weights were decreased for male rats given 200 mg/kg body weight.
The body temperature was within normal limits, except in the females
given 200 mg/kg, which showed a slightly lower temperature. No
functional effects were noted. No gross or histopathological effects
on either the central or peripheral nervous system were observed
(Johnson & Gorzinski, 1988).
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
Ingestion of cleaning solvent (50 ml) containing 1,2-
dichloropropane (other components not known) by a man produced coma
followed by delirium, irreversible shock, cardiac failure, and
death. Histopathologically, centri- and medio-lobular hepatic
necrosis was found (Larcan et al., 1977).
Two cases of disseminated intravascular coagulation syndrome
(DIC) have been described in association with acute intoxication by
1,2-dichloropropane. Effects on the central nervous system, liver,
and kidney functions were also observed, but no details were given
(Perbellini et al., 1985).
Pozzi et al. (1985), from Italy, reported clinical observations
on 3 other hospitalized persons with 1,2-dichloropropane poisoning.
Two out of the 3 persons ingested the substance, while the third
person was exposed through inhalation. The clinical symptoms were
similar, despite different routes of exposure. All 3 suffered from
acute renal and hepatic damage. Kidney biopsy, carried out on 1
person, showed acute tubular necrosis. Haemolytic anaemia and
disseminated intravascular coagulation were noted in all 3 patients,
1 of whom died on the seventh day. The other patients recovered
after treatment.
Toxic hepatitis with portal hypertension has been described in
a 49-year-old man, who ingested 1,2-dichloropropane in an attempted
suicide (Thorel et al., 1986).
9.2 Occupational exposure
Baruffini et al. (1989) studied 10 cases of 1,2-dichloropropane
dermatitis in the period 1985-88. Patients were painters or metal-
workers in the engineering industry and they all had known contact
with mixtures of solvents containing 10-40% 1,2-dichloropropane. On
examination, the workers had itchy erythematous, oedematous,
vesicular lesions on the fingers and dorsa of the hands. Two
subjects also had scaling and fissuring of the palms. Cessation of
exposure produced quick resolution of the dermatitis in all
subjects. Patch tests were carried out. As controls, 120 subjects
were similarly tested with 1,2-dichloropropane. All patients showed
a positive response to concentrations of 2% 1,2-dichloropropane or
more (allergic contact dermatitis). The results of the tests on the
control subjects were negative.
Grzywa & Rudzki (1981) described cases of dermatitis in 2 women
(47 and 55 years old), in a group of 60 persons, who had been
exposed to Silform aerosols for 6 and 4 years, respectively. Silform
aerosols contained 7.4, 11.0, or 12.7% 1,2-dichloropropane;
methylsilicone oils, and Freon. The patch tests that were carried
out were positive for 1,2-dichloropropane, but negative for
methylsilicone oils.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloropropane was considered by working groups of the
International Agency for Research on Cancer (IARC) in 1986 (IARC,
1986) and in 1987 (IARC, 1987). In the updating of 1987, it was
evaluated as follows: "There is limited evidence for the
carcinogenicity of 1,2-dichloropropane in experimental animals.
There are no data in humans. The agent is not classifiable as to its
carcinogenicity to humans (Group 3)".
WHO (in preparation) has proposed a provisional guideline value
for drinking-water of 20 µg 1,2-dichloropropane/litre.
PART C
ENVIRONMENTAL HEALTH CRITERIA
FOR
MIXTURES OF DICHLOROPROPENES
AND DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MIXTURES OF DICHLOROPROPENES AND
DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on humans
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
4.2 Water
4.3 Soil
4.3.1 Microbial transformation
4.3.2 Loss under field conditions
4.3.3 Soil function
4.4 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Groundwater
5.2 Occupational exposure
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Terrestrial microorganisms
7.1.1.1 Effects on nitrification
7.1.1.2 Recovery studies with microorganisms
7.2 Aquatic organisms
7.2.1 Invertebrates
7.2.2 Fish
7.3 Terrestrial organisms
7.3.1 Birds
7.3.2 Soil fauna
7.3.3 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.2 Inhalation
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
8.3.2 Sensitization
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
8.5.1.2 Inhalation
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
8.6.2 In vivo studies
9. EFFECTS ON HUMANS
9.1 General population exposure
9.2 Occupational exposure
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
The technical mixture of dichloropropenes and dichloropropane
(abbreviated in this text to "MIX D/D") is a clear amber liquid with
a pungent odour; it has a vapour pressure of 35 mmHg at 20 °C, and
is soluble in halogenated solvents, esters, and ketones.
"MIX D/D" typically contains not less than 50% 1,3-
dichloropropene (ratio of cis- and trans-isomers approximately
1:1), the other main constituents being 1,2-dichloropropane and
related compounds. It was widely used as a soil nematocide before
planting.
The environmental transport, distribution, and fate of the
major constituents of "MIX D/D" in air, water, and soil is described
in the sections 4 of the parts of this EHC monograph that deal with
1,3-dichloropropene and 1,2-dichloropropane.
There is a significant potential for 1,2-dichloropropane
derived from "MIX D/D" to leach from the soil and contaminate well
water and groundwater. In an irrigation bore (68 m deep) in Western
Europe, mean 1,2-dichloropropane concentrations at different depths
ranged between 0.8 and 8.5 µg/litre, and the maximum concentration
recorded was 165 µg/litre.
Significant uptake of the constituents of "MIX D/D" by crops is
unlikely (see other parts of this EHC monograph). Bioaccumulation of
the constituents of "MIX D/D" is also unlikely because of their low
log P octanol/water partition coefficient and their relatively high
water solubility.
1.1.2 Kinetics and metabolism
No metabolic studies have been carried out on "MIX D/D". The
two major components, 1,3-dichloropropene and 1,2-dichloropropane,
are rapidly eliminated, primarily in the urine and, to a lesser
extent, via expired air. The components of "MIX D/D" are metabolized
by oxidative and conjunction pathways. The major urinary metabolites
are mercapturic acids.
1.1.3 Effects on organisms in the environment
"MIX D/D" is moderately toxic for fish; 96-h LC50 values
range between 1 and 6 mg/litre. The 1,3-dichloropropene is largely
responsible for the toxicity of "MIX D/D".
When used at recommended application rates, the main effects of
"MIX D/D" are a transient (< 7 days) reduction in soil fungi and
inhibition of the oxidation of ammonium ions to nitrate. "MIX D/D"
is toxic for nitrifying bacteria. Soon after "MIX D/D" disappears
from the soil, recolonization by bacteria takes place. In field
trials, "MIX D/D" (applied at 600 litre/ha) killed soil
invertebrates. Recolonization times ranged between 6 and 24 months.
"MIX D/D" is highly phytotoxic.
1.1.4 Effects on experimental animals and in vitro test
systems
The acute toxicity of "MIX D/D" for laboratory animals is
moderate to high. The oral LD50 values in rats and mice range from
132 to 300 mg/kg body weight. The dermal LD50 values for rats and
rabbits are 779 and 2100 mg/kg body weight, respectively. The
inhalation LC50 (4 h) for rats is approximately 4540 mg/m3.
Acute exposure results in clinical signs associated with central
nervous system depression. "MIX D/D" is a severe eye and skin
irritant and it is a moderate dermal sensitizer.
The results of the available short-term toxicity studies in
rats and dogs are inadequate to assess properly the toxicity
potential of "MIX D/D", because the relatively low doses tested do
not demonstrate any biologically significant effects. Several short-
term inhalation (whole-body) studies have been conducted in rats.
"MIX D/D" at levels up to 145 mg/m3 does not cause any toxic
effects. At levels of 1362 mg/m3 or higher, toxic effects
associated with central nervous system depression are evident. An
exposure to 443 mg/m3 for 10 weeks leads to reduced body weight
gain and increased absolute kidney weight.
An oral teratology study in rats was inadequate for assessment
of the teratological potential of "MIX D/D".
In an inhalation rat study to investigate male and female
fertility, no effects were found at dose levels up to 443 mg/m3
for 10 weeks. Complete evaluation of reproductive effects of "MIX
D/D" was not possible owing to inadequate protocol designs.
"MIX D/D" is mutagenic in Salmonella typhimurium strains
TA100 and TA1535, as well as in Escherichia coli WP2 HCR, without
metabolic activation. There was no such effect in Salmonella
strains TA98, TA1537, and TA1538.
In a long-term study on rats fed diets containing up to 120 mg
"MIX D/D" per kg (equivalent to 6 mg/kg body weight) for 2 years, no
toxic or carcinogenic effects were seen.
1.1.5 Effects on humans
"MIX D/D" is no longer widely used, and, thus, exposure of the
general population via air, water, and food is unlikely.
The exposure of workers filling drums and of field applicators
was generally below 4.5 mg 1,3-dichloropropene/m3 when recommended
procedures were used; in other situations, levels up to 36.32
mg/m3 have been measured.
One case of acute fatal poisoning has been reported following
accidental ingestion of "MIX D/D".
Several cases of contact dermatitis and skin sensitization due
to "MIX D/D" exposure have been reported.
1.2 Conclusions
- General population. As "MIX D/D" is no longer widely used,
the exposure of the general population to 1,3-dichloropropene
via air, water, and food is negligible, but, in certain areas,
exposure to 1,2-dichloropropane may occur when groundwater is
contaminated.
- Occupational exposure. Filling operations and field
applications of "MIX D/D" can lead to exposure of operators to
1,3-dichloropropene that exceed maximum allowable
concentrations, especially under warm climatic conditions.
- Environment. "MIX D/D" is unlikely to reach biologically
significant levels in either the terrestrial or the aquatic
environment when used at the recommended rate. Lasting adverse
effects on organisms in the environment are unlikely to occur.
1.3 Recommendations
- "MIX D/D" should not be used as a soil fumigant because of
potential leaching to groundwater.
- Monitoring of residues in surface water and groundwater should
be carried out in areas where "MIX D/D" has been used.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
The technical mixture of dichloropropenes and dichloropropane
(abbreviated to "MIX D/D") contains not less than 50% of the cis-
and trans-isomers (ratio approximately 1:1) of 1,3-dichloropropene
and the other main constituent was 1,2-dichloropropane. Yang (1986)
described a commercial "MIX D/D" containing 25% cis-
dichloropropene, 27% of trans-dichloropropene, 29% 1,2-
dichloropropane, and 19% other related chlorinated hydrocarbons. It
may also contain 1% epichlorohydrin as a stabilizer.
CAS registry number: 8003-19-8.
For the physical and chemical properties of the main
constituents, see the sections 2.2 of the parts of this monograph
dealing with 1,3-dichloropropene and 1,2-dichloropropane.
Major trade names are D-D mixture, Nemafene, Nemax, Vidden-D.
Other formulations on the market are Ditrapex and Vortex
(mixtures of 1,2-dichloropropane, 1,3-dichloropropene, and
methylisothiocyanate), Ditrapex CP (the same mixture as Ditrapex,
but also containing chloropicrin).
2.2 Physical and chemical properties
Technical "MIX D/D" is a clear amber liquid with a pungent
odour. It has a vapour pressure of 4.6 kPa (20 °C) (35 mmHg at 20
°C), flash distils over the range of 59-115 °C, and has a relative
density of 1.17-1.22 g/cm3 at 20 °C; its flash point is 17.5 °C.
Its solubility at room temperature is approximately 2 g/kg in water,
but it is soluble at room temperature in hydrocarbon and halogenated
solvents, esters, and ketones. The mixture is stable up to 500 °C
(therefore stabilizers are not needed) but reacts with dilute
organic bases, concentrated acids, halogens, and some metal salts.
It is corrosive to some metals (e.g., aluminium, magnesium, and
their alloys, and may remove lacquer from lacquer-lined containers.
It is not corrosive to mild steel.
2.3 Analytical methods
The same methods can be used as for 1,3-dichloropropene.
See section 2.4 of the part of this monograph that deals with
1,3-dichloropropene.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
"MIX D/D" does not occur naturally.
3.2 Man-made sources
"MIX D/D" is manufactured by high temperature chlorination of
propene.
For sources of pollution, see section 3.2.3 of the part of this
monograph that deals with 1,3-dichloropropene and section 3.2 of
1,2-dichloropropane.
3.3 Uses
"MIX D/D" is a preplant nematocide, effective against soil
nematodes including root knot, meadow, sting and dagger, spiral, and
sugar beet nematodes. It is usually applied by injection into the
soil or through tractor-drawn hollow tines, to a depth of 15-20 cm
at a rate of 150-400 litre/ha (occasionally to a maximum of 1000
litre/ha), depending on soil type and the following crop. The soil
surface is sealed by rolling. "MIX D/D" volatilizes and diffuses as
a vapour and, thus, its effectiveness depends on how readily this
can occur. Because the components of "MIX D/D" are highly
phytotoxic, it is essential that, after an application of 220
litre/ha or more, a period of not less than 14 days should elapse
before planting or sowing (Shell, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.2 Water
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.3 Soil
(See section 4 of 1,3-dichloropropene and 1,2-dichloropropane).
4.3.1 Microbial transformation
It has been demonstrated under in vitro and in vivo
conditions that indigenous soil microflora can utilize C3
chlorinated hydrocarbons. Four species, Bacillus subtilis,
Arthrobacter globiformis, Pseudomonas fluorescens, and Rhizobium
leguminosarum, were successfully grown on media including "MIX
D/D" (Altman & Lawlor, 1966; Altman, 1969).
Toxicity tests were carried out with Rhizobium phaseoli and
Azotobacter beinjerinckii, both nitrogen-fixing bacteria, in
cultures using unsterilized Hanford sandy loam. "MIX D/D" at
concentrations ranging from 10 to 100 mg/kg and 100 to 1000 mg/kg
caused growth inhibition in Azotobacter beinjerinckii and
Rhizobium phaseoli, respectively (Rader & Love, 1977a).
4.3.2 Loss under field conditions
A trial has been carried out in the United Kingdom in which
"MIX D/D" was applied at 410 litre/ha (see section 4.1.3.5 of 1,3-
dichloropropene). Samples of soil were taken at 3 depths: 0-20 cm,
20-40 cm, and 40-60 cm, at 6 intervals up to 9´ months after
application. The results are summarized in Table 23. Residues of
1,3-dichloropropenes, 1,2-dichloropropane, and 3-chloroallyl
alcohols were present in all 3 soil layers, especially before
ploughing. They showed little change in the period before ploughing,
but, thereafter, the concentrations decreased gradually (Wallace,
1979).
4.3.3 Soil function
"MIX D/D" soil fumigant, 337 and 3370 kg active ingredient per
ha, was used to evaluate the effect on nodulation of pinto bean
plants in soil and on the growth of root nodule bacteria in culture.
Unsterilized Hanford sandy loam was used. After 4 weeks' growing
time, low and high doses of "MIX D/D" resulted in a reduction in
Table 23. Residues from the plot treated with "Mix D/D" at 410 litre/ha
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl
application (cm) alcohols (days)
cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 6.82 6.05 8.7 2.02 1.98
20-40 6.83 7.08 9.8 1.36 1.35
40-60 0.85 0.90 1.4 0.24 0.24
11 0-20 3.50 3.50 5.4 0.63 0.53
20-40 4.84 5.05 6.5 1.86 2.11
40-60 0.35 0.35 0.9 0.18 0.17
24 0-20 5.73 5.55 9.4 1.0 1.0
20-40 6.07 6.21 12.5 2.0 2.0
40-60 0.70 0.63 2.1 0.29 0.29
33 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.73 0.86 0.77 0.58 0.44
20-40 1.54 1.79 1.90 0.41 0.37
40-60 0.30 0.30 1.00 0.14 0.15
73 0-20 0.26 0.25 0.2 0.16 0.11
20-40 0.64 0.62 1.5 0.36 0.28
40-60 0.51 0.44 2.9 0.22 0.19
Table 23 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl
application (cm) alcohols (days)
cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.06 0.05 0.1 0.16 0.08
9´ months 20-40 0.03a 0.02a 0.2 0.07 0.03
40-60 < 0.01 < 0.01 0.2 < 0.02 < 0.02
Pre- 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
treatment 20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a Results confirmed by GC/MS.
From: Wallace (1979).
Note: All residues are on a dry weight basis.
root nodules of approximately 70 and 80%, respectively; the
percentages of germination were about 90 and 50%, respectively
(Rader & Love, 1977a).
Unsterilized Oakdale loamy sand soil (81.6% sand, 11.2% silt
and 1.06% organic carbon), treated with "MIX D/D" soil fumigant at
337 and 1348 kg active ingredient/ha, in 1978, showed no decrease in
soil phosphatase activity after 0, 4, and 8 weeks at an incubation
temperature of 27 °C (Rader, 1979c).
The effects were studied of "MIX D/D" soil fumigant (337 and
3370 kg/ha) on proteolytic enzyme activity in the treated soil. The
soil proteases were assayed by the release of tyrosine from sodium
caseinate in the treated soil, after incubation periods of 0, 4, 8,
and 12 weeks, at 27 °C. No inhibitory effects were found (Rader,
1979b).
"MIX D/D", at a high dose of 100-1000 mg/kg, caused a temporary
loss of cellulytic activity in Trichoderma viride (Rader & Love,
1977b).
Laboratory studies were conducted to determine the effects of
"MIX D/D" on the activities of invertase in a sandy soil. The rates
of application were 150 and 300 mg/kg. No inhibition was found. The
same dose levels were used to study the influence of "MIX D/D" on
amylase activity in this soil. After 3 days, stimulation of glucose
formation from the added starch was observed, especially at the
lowest dose level. Microbial respiration was also studied. The
treatments did not significantly decrease oxygen consumption (Tu,
1988).
4.4 Bioconcentration
No data on bioconcentration are available.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Analyses have been made to determine whether residues of the
active 1,3-dichloropropene isomers could be found in the edible
parts of a number of crops, including potatoes, carrots, onions,
cucurbits, rice, and sugar beet. At recommended rates and pre-
planting intervals, residues of these propenes ( cis- and trans-
isomers) have not been detected (limit of determination 0.02 mg/kg)
(see section 2.4 of 1,3-dichloropropene).
5.1 Groundwater
1,2-Dichloropropane levels of 0.8-8.5 µg/litre, with a maximum
of 165 µg/litre, were reported in groundwater in the Netherlands
from bores for irrigation at depths up to 68 m. These levels
resulted from previous applications of "MIX D/D" (Leistra & Boesten,
1989) (see section 5.1.2 of 1,2-dichloropropane).
5.2 Occupational exposure
Field studies were carried out in several locations in the
Netherlands and in France to monitor personal exposure and
environmental concentrations during application of "MIX D/D". In
most of the 11 locations in the Netherlands, the time-weighted
average of the exposure of the operators, as measured with personal
sampler pumps during application, was of the order of 4.54 mg total
cis- and trans-1,3-dichloropropene per m3. When filling
operations are carried out in the recommended way, personal
exposures can be limited to a maximum of 4.54 mg/m3. Levels of the
unsaturated components of "MIX D/D" in the air 1 m above the soil
surface shortly after application were below 0.454 mg/m3.
In most of the 9 locations in France, the time-weighted average
exposure levels of the operators, as measured with personal sampler
pumps, was of the order of 4.54-9.08 mg total cis- and trans-
1,3-dichloropropene per m3. Peak exposures of up to 36.32 mg/m3
were measured when the recommended safety precautions were
insufficiently observed. Levels of the unsaturated components of
"MIX D/D" in the air 1 m above the soil surface during application
ranged from 1 to 6.4 mg/m3 (van Sittert et al., 1977; van Sittert,
1978).
6. KINETICS AND METABOLISM
See section 6 in the parts of this monograph that deal with
both 1,3-dichloropropene and 1,2-dichloropropane.
No information is available on the kinetics and metabolism of
other compounds, impurities, and stabilizers that may be present in
"MIX D/D".
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
(See also relevant sections of 1,3-dichloropropene and 1,2-
dichloropropane.)
7.1.1 Terrestrial microorganisms
The effects of "MIX D/D" on microorganisms and on soil function
have been widely studied in Europe (Pochon et al., 1951; Bakker,
1968; Sommer, 1970; Kämpfe, 1973; Lebbink & Kolenbrander, 1974), in
Canada (Wensley, 1953; Elliot et al., 1972, 1974, 1977; Tu, 1972,
1973, 1978, 1979, 1981a,b), in the USA (Thornton, 1951; Moje et al.,
1957), and elsewhere (Dommergues, 1959; Mehta et al., 1963; Dubey et
al., 1975; Ross & McNeilly, 1975). Main interest has been in the
microorganisms involved in the nitrogen balance of the soil, as this
bears strongly upon the yields of crops grown in treated soil.
During the search for effective soil fumigants, the toxicity of
"MIX D/D" for soil microorganisms has been investigated on 2 soil
types, a sandy loam and a soil with high organic matter. At a dosage
of 1200 mg/kg soil, the following reductions in soil microorganisms
were found after 96 h: bacteria 90%, actinomycetes 99%, and fungi
98%. The toxicity of the major components of "MIX D/D" for these
same major groups of microorganisms has been studied in soil samples
from old citrus plantations under laboratory conditions, and it was
found that the toxicity of 1,2-dichloropropane for fungi and
bacteria and actinomycetes was low to moderate (Moje et al., 1957).
The studies of Wensley (1953) and of Moje et al. (1957) are
reasonably consistent and indicate that bacteria are more tolerant
to "MIX D/D" than fungi, and, again, that the toxicity of "MIX D/D"
is related mainly to its 1,3-dichloropropene content, in particular
the cis-isomer.
Rader et al. (1978) studied the correlation between the numbers
of soil microorganisms and the O2/CO2 exchange in treated soil.
Unsterilized Hanford sandy loam (57.6% sand, 26.6% silt, 15.8% clay,
and 0.7% organic carbon) was used and the soil had a moisture
content of 6%. "MIX D/D" soil fumigant (dosages 336 and 2240 kg
active ingredient/ha) caused a decrease in the populations of
actinomycetes, bacteria, and fungi, and also reduced the oxygen
utilization by soil microorganisms.
7.1.1.1 Effects on nitrification
Kämpfe (1973) studied the inhibition by "MIX D/D" of
nitrification in a black earth soil. Nitrogen (180 mg/kg, NH3 and
NH3-water) and "MIX D/D" (80 mg/kg) were applied at different
temperatures. The time of incubation was 30-120 days. At
temperatures between 5 °C and 10 °C, nitrification of NH3 was
severely inhibited for 90 days. After 120 days of incubation at 10
°C, 70% of the nitrogen applied was retrieved as ammoniumion.
The inhibition of ammonium nitrogen oxidation has led to the
build-up of ammonium nitrogen in treated soil under both laboratory
(Sommer, 1970) and field conditions (Thornton, 1951; Mehta et al.,
1963; Elliot et al., 1974; Ross & McNeilly, 1975).
"MIX D/D" was extremely toxic to nitrifying bacteria in silty
clay loam or loam soils. Doses of 200, 2000, and 20 000 mg "MIX
D/D"/kg soil were tested. Inhibition of NH4+-oxidizing bacteria
was found, but not of Nitrobacter spp. Nitrogen mineralization was
progressively depressed with increasing levels of "MIX D/D". At 200
mg "MIX D/D"/kg soil, nitrate formation from ammonium nitrogen was
very markedly reduced, while mineralization of nitrogen was only
slightly reduced at 20 000 mg/kg soil (Dubey et al., 1975). These
results are in agreement with the results of Bromley & Cook (1981).
Bromley & Cook (1981) also studied the influence of "MIX D/D"
on the nitrification processes in soil. Transient inhibition of
nitrification (< 20 days) occurred in sandy clay treated with 200
or 1000 mg "MIX D/D"/kg. Considerable (80% reduction compared with
controls) and total inhibition occurred in soil treated with 2000
and 10 000 mg "MIX D/D"/kg, respectively. There was no difference
between the inhibitory effects of "MIX D/D" and those of a purified
dichloropropene isomer mixture, indicating that the dichloropropenes
were the active inhibitors in "MIX D/D". During inhibition of
nitrification by "MIX D/D", ammonium accumulated but no significant
nitrite accumulation was observed. This indicates that
ammonification was unaffected and that inhibition of nitrification
resulted from the specific inhibition of Nitrosomonas spp.
Overall, it was concluded that "MIX D/D" was not very active as a
nitrification inhibitor, compared with commercially available
inhibitors.
Unsterilized Handford sandy loam was fumigated with "MIX D/D"
soil fumigant. The soil was moistened to 75% moisture content, and
was composed as follows: 0.7% organic carbon, 57.5% sand, 26.6%
silt, and 15.8% clay. The activity of the nitrifying bacteria
(oxidation of nitrite-nitrogen to nitrate-nitrogen) was monitored.
After the treated soil had been incubated for 1-4 months at 27 °C,
nitrite could not be detected. "MIX D/D" soil fumigant did reduce
the oxidation of the ammonia to nitrite by Nitrosomonas spp.,
especially at a concentration four times normal field use. There was
no effect on Nitrobacter spp. (Rader, 1979a).
"MIX D/D" application kills a considerable part of the biomass.
Lysis of the killed biomass provides the surviving microflora with a
new and, to some extent, readily available substrate. The
mineralization of this substrate, together with a small priming
effect, gives an extra contribution to the inorganic nitrogen
content of the soil (so-called "flush"). This contribution depends
on the amount of biomass, which is related to the type of soil. The
gain in nitrogen is approximately 5-10 kg N/ha. The nitrogen gain in
spring, after autumn fumigation, can be attributed to a reduction in
loss of nitrogen by diminished leaching and diminished
denitrification of nitrate, and depends on the rate of
mineralization, time of recovery of nitrification, weather
conditions during the winter, and soil type (Lebbink & Kolenbrander,
1974).
In field trials, the influence of "MIX D/D" on nitrification
was studied in loamy sand and black earth soils. Nitrogen
application was 100 kg/ha; and the application of "MIX D/D" was
between 37.1 and 46.6 kg/ha. When "MIX D/D" had been applied to the
loamy sand, 61.5% of the September-applied nitrogen was retrieved in
the top 20 cm in March of the following year. This percentage went
up to 72 and 100%, respectively, when fertilizer was applied in
October or November. On loamy sand, the yield obtained from the
following crop was significantly increased. On black earth, "MIX
D/D" application resulted in only a slight increase in crop yield
(Kämpfe, 1973).
7.1.1.2 Recovery studies with microorganisms
"MIX D/D" was added to soil at 2 rates, approximately 10 and
100 times the normal recommended treatment rates. A number of
microbial assessments were made (see below).
Parameter % reduction over control at:
3000 litre/ha 30 000 litre/ha
Evolution of carbon dioxide 10 100
Density of total bacteria nda 98
Density of proteolytic bacteria 44 99
Density of cellulolytic bacteria 30 100
Mineralization of nitrogen 9 44
Mineralization of asparagin 0 27
a Not determined.
From: Dommergues (1959).
Soon after the "MIX D/D" disappears from the soil,
recolonization by bacteria takes place and the number of the
different types of bacteria may be higher than before the "MIX D/D"
treatment; this may lead to the production of higher levels of
nitrogen/ha, perhaps because of a belated recovery of bacteriophages
(Bakker, 1968).
With "MIX D/D" (120 and 600 mg/kg), Tu (1972) found a decrease
in bacterial and fungal populations in a loamy sand, but recovery to
the same levels as the controls was rapid. In a series of studies by
Tu (1978, 1979, 1981a,b), "MIX D/D" at 150 and 300 mg/kg soil, and
1,3-dichloropropene at 30 and 60 mg/kg, were evaluated in parallel
under laboratory conditions. In general, neither had much effect on
either the numbers of soil microorganisms or their activity.
Acetylene reduction, the population of non-symbiotic nitrogen-fixing
organisms, and the viability of indigenous microorganisms were not
affected. There was some stimulation of microbial numbers in some
experiments. Soil enzyme activity was either not affected or only
very slightly affected.
Overall, the main effect of "MIX D/D" on soil microbial
function, at normal usage levels, is to reduce the rate of turnover
of ammonium. After autumn treatment, this is an advantage, as
ammonium leaches from the soil less readily than nitrate and so is
available in the spring for crop growth (Elliot et al., 1974). This
effect, together with increased chlorine content, contributes to the
yield increases beyond those expected from pest control alone, that
are often noted following "MIX D/D" treatment (Goffart & Heiling,
1958; Ennik et al., 1964; Bakker, 1968).
7.2 Aquatic organisms
7.2.1 Invertebrates
Varanka (1979) studied the toxicity of "MIX D/D" (50% 1,3-
dichloropropene + 1,2-dichloropropane) for the larvae (glochidia)
of freshwater mussels ( Anodonta cygnea L.). The decrease of
tryptamine-induced adductor muscle activity was used as an indicator
of the effect of the pesticide. The presence of toxicants reduces
the ability of the larval adductor muscle to contract when
stimulated by tryptamine. In each experiment, 200-300 larvae
originating from 4-5 adult mussels were used. The concentration of
"MIX D/D" causing 50% reduction in adductor muscle activity, over a
30-min exposure, was 18 mg/litre (20 ppm v/v).
7.2.2 Fish
"MIX D/D" is moderately toxic for fish (Table 24). The toxicity
is largely due to the 1,3-dichloropropene component, 1,2-
dichloropropane being about two orders of magnitude less toxic than
"MIX D/D" (see section 7.1.3 of 1,2-dichloropropane).
Harlequin fish (Rasbora heteromorpha) were exposed in water
containing 10 mg "MIX D/D"/litre. After 2 h, no deaths were found.
The fish were then transferred to clean water and 3 days later there
were still no deaths. In another study, 5 fish were exposed
continuously in water containing 5 mg "MIX D/D"/litre. Three fish
had died by 45 h, but no further deaths had occurred by 96 h (Reiff,
1975).
Application of "MIX D/D" (1000 litre/ha) to a vineyard in
France caused contamination of a natural spring, which in turn led
to contamination of fish-breeding basins and a pond. "MIX D/D" was
detected in the spring water 20 days after the death of trout and
carp. The concentrations in the spring and pond water ranged from
0.4 to 2.4 mg/litre. Within 3 months, the concentration decreased to
less than 0.1 mg/litre (Elgar et al., 1965).
7.3 Terrestrial organisms
7.3.1 Birds
No data were available.
7.3.2 Soil fauna
In one study in the United Kingdom, 99% of soil arthropods were
killed following "MIX D/D" application. It took more than 2 years
for the population to recover completely, although the chemical
itself disappeared in about 4 weeks. Collembola populations
started to build up again after 6 months and exceeded 50% of the
initial population after 10 months. Populations were not monitored
beyond 10 months (Edwards & Lofty, 1969).
Studies on a light sandy soil in Belgium showed somewhat faster
recolonization rates. In an unreplicated experiment, 10-11 months
after the last of 5 successive yearly treatments of 600 litre "MIX
D/D"/ha, it was found that populations of phytophagous nematodes,
but not of saprophagous nematodes, were depressed. Earthworms,
enchytraeid worms, mites, and Collembola were present, mainly in
Table 24. Acute toxicity of "Mix D/D" to fish
Species Size Temperature 96-h LC50 References
(°C) (mg/litre)
Rainbow trout 1.1 g 12 5.5a Mayer & Ellersieck
(Oncorhynchus mykiss) (3.6-8.4) (1986)
1.1 g 12 1.97b
(1.2-3.2)
Cutthroat trout 1.0 g 12 1-10 Mayer & Ellersieck
(Salmo clarki) (1986)
Walleye 1.3 g 18 0.98b Mayer & Ellersieck
(Stizostedion vitreum) (1986)
Largemouth bass 0.9 g 18 3.4b Mayer & Ellersieck
(Micropterus salmoides) (1986)
Bluegill sunfish 1.4 g 18 3.9b Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Channel catfish 1.1 g 18 4.4b Mayer & Ellersieck
(Ictalurus punctatus) (1986)
Harlequin fish 2-3 cm 20-22c 4-5 Reiff (1975)
(Rasbora heteromorpha)
Carp - - 6d Reiff (1975)
(Cyprimus carpio)
a Water hardness, 44 mg CaCO3/litre.
b Water hardness, 272 mg CaCO3/litre.
c Hard, chlorine-free water (260 mg CaCO3/litre).
d At 24 h.
the top 5 cm of the soil. Populations of these groups were not
significantly depressed relative to the untreated plot. Populations
of earthworms and mites were significantly increased (van den Brande
& Heungens, 1969).
In a replicated study, the influence of four, successive,
yearly applications of "MIX D/D" (600 litre/ha) on the soil fauna
was studied in a sandy soil in which begonias were grown. Again,
recolonization was found between 6 and 12 months after treatment. Of
the groups studied 12 months after the last treatment, there was
little or no effect on the populations of Enchytraeidae, Gamasina,
Onychiuridae, Lumbricidae, and Acaridae. In the remaining 5 groups
(of mites and Collembola), the populations were also similar to
those in untreated plots (Heungens & van Daele, 1974).
7.3.3 Plants
The components of "MIX D/D" are highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
The acute oral toxicity of "MIX D/D" (containing 51.5% of 1,3-
dichloropropene) was moderate to high when it was administered to
mice, rats, and dogs (Table 25). Signs of intoxication in rats and
mice were hyperexcitability, followed by incoordination, depression,
dyspnoea, and chromodacryorrhoea. Most of the surviving animals had
recovered within 24 h of dosing (Hine et al., 1953; Coombs & Carter,
1976a).
8.1.2 Inhalation
Long-Evans rats were exposed for 4 h in atmospheres containing
2043-81 720 mg "MIX D/D"/m3. The animals were observed for 10
days. The LC50 was approximately 4540 mg/m3. The rats that died
showed severe oedema of the lung, with haemorrhages. Congestion,
cloudy swelling, and fatty degeneration of the liver were also
observed (Hine et al., 1952, 1953).
8.1.3 Dermal
The acute toxicity of "MIX D/D" is low when it is applied in a
single percutaneous dose to rats. Signs of intoxication included
lethargy and chromodacryorrhoea that disappeared in survivors within
4 days after dosing (Hine et al., 1953; Coombs & Carter, 1976a).
Nineteen adult rabbits were treated with 1.2-4.8 g "MIX D/D"/kg
body weight, on the skin, for 24 h. Inhalation of the vapour was not
possible. A mucous nasal discharge was noted in one rabbit treated
with 3 g "MIX D/D"/kg body weight. The skin showed extremely severe
eschar with intense oedema, resulting in black necrotic tissue.
Seven of the 10 animals receiving the three higher dose levels died
in 8-48 h. The LD50 was 2.1 g/kg body weight (see Table 25) (Hine
et al., 1952, 1953).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
Carworth Farm E-rats (12 of each sex per group) were dosed
orally, by gavage, with emulsions of "MIX D/D" in corn oil at 0,
0.0125, 0.025, 0.125, or 3.125 mg "MIX D/D"/kg body weight per day,
for 3 months. The "MIX D/D" contained 55% 1,3-dichloropropenes, 26%
1,2-dichloropropane, and 0.7% epichlorohydrin. There were no effects
on general health, behaviour, growth rate, food intake, or blood
Table 25. Acute toxicity of "Mix D/D"
Species Route Vehicle Sex LD50 (mg/kg References
body weight)a
Mouse oral propylene glycol male 300 Hine et al. (1952, 1953)
(Swiss)
Mouse (CD-1) oral undiluted 314 (276-365) Coombs & Carter (1976a)
Mouse (CF-1) oral 3% D-D in DMSO 234 (208-262) Carter (1975)
Rat oral suspension in 140 Hine et al. (1952, 1953)
(Long-Evans) propylene glycol
Rat (CD) oral undiluted male 227 (180-540) Coombs & Carter (1976a)
female 132 (108-156)
Dog oral undiluted > 230b Carter (1975)
Rat (CD) dermal undiluted 779 (630-1103) Coombs & Carter (1976a)
Rabbit dermal undiluted male 2100 (1540-2660) Hine et al. (1952, 1953)
a With 95% confidence limits.
b Screening value from test with only 2 dogs/dose. Apart from vomiting in dogs, no signs of
intoxication were observed.
chemistry throughout the experiment. No changes in organ weights or
pathological lesions attributable to "MIX D/D" were detected at any
dose level.
When rats were dosed with 3.125 mg/kg body weight, there were
slight reductions in the haemoglobin concentration and erythrocyte
count in the males, and at 0.125 mg/kg body weight there were
reductions in the erythrocyte and total leukocyte counts in the
females, but since these reductions were not dose-related, they were
of doubtful toxicological significance. With a dose of 0.025 mg/kg
body weight there were no effects attributable to the "MIX D/D"
(Walker, 1968a). (Remark: The Task Group noted that the dose levels
used were too low for a proper assessment of the toxicity).
8.2.1.2 Dog
Beagle dogs (3 of each sex per dose group, with 5 of each sex
as controls) were orally dosed by capsule with 0, 0.0125, 0.025, or
3.125 mg "MIX D/D"/kg body weight per day, for 3 months. The
composition of the "MIX D/D" was 55% 1,3-dichloropropenes, 26% 1,2-
dichloropropane, and 0.7% epichlorohydrin. There were no effects on
general health, behaviour, growth rate, haematology, or clinical
chemistry at any dose level. No pathological lesions attributable to
"MIX D/D" were detected, even at the highest dose level (Walker,
1968b). (Remark: The Task Group noted that the dose levels used were
too low for a proper assessment of the toxicity).
Groups of 4 pure-bred Beagle dogs and 4 bitches were dosed with
0, 0.25, 0.75, 2.50, or 7.50 mg/kg body weight "MIX D/D" (containing
28.2% cis-, 29.0% trans-1,3-dichloropropene, and 34.0% 1,2-
dichloropropane, without epichlorohydrin), suspended in olive oil in
gelatin capsules, 7 days/week, for 2 years. Haematological and blood
chemistry studies and urinalyses were conducted on each animal just
prior to the inception of the study and after 3, 6, 9, 12, 18, and
24 months of testing. Ten organs were weighed and histopathological
examination was carried out of 28 organs and tissues.
The overall body weights of the bitches were lower in the 7.5
mg/kg group compared to untreated bitches. The mean corpuscular
volume and mean corpuscular haemoglobin level were lower in the
bitches of the 7.5 mg/kg group, while their erythrocyte count was
raised above that of untreated bitches. No other effects were found.
At the dose level of 2.5 mg/kg, no differences were found in the
body weight, food intake, behaviour, mortality, haematology,
clinical chemistry, urinalysis, organ weight, or gross or
histopathological appearance between treated and control animals
(Industr. Biotest Lab., 1977b). An audit of the study data concluded
that no deviations from the protocol were observed which influenced
the overall interpretation. Repeated oral dosing of dogs with 7.5 mg
"MIX D/D"/kg body weight per day, for 2 years, did not result in any
significant biological effects (Schweizer & Parker, 1980; Parker,
1980).
8.2.2 Inhalation
Male or female Long-Evans rats (6 per group) were exposed,
whole body, in concentrations of 0, 340.5, 1362, or 2724 mg "MIX
D/D"/m3, 1 h/day, 5 days a week, for 2 weeks, or until the animals
died. Rats exposed to the two highest dose levels showed evidence of
weight loss, central nervous system depression, and moderate
irritation. At 2724 mg/m3, 3 animals died. There were no deaths in
the other groups and no gross or microscopic lesions were observed.
No effects were seen in the 340.5 mg/m3 group (Hine et al., 1952).
A further group of six rats was exposed to 1362 mg "MIX D/D"/m3, 1
h/day, for 3 days. They were killed to find out whether there were
any lesions immediately after exposure. No gross or microscopic
lesions were found (Hine et al., 1952).
Groups of 28 male and 28 female Fischer 344 albino rats and CD-
1 albino mice were exposed to atmospheres containing 0, 22.7, 68.1,
or 227 mg "MIX D/D"/m3, for 6 h/day, 5 days/week for 6 or 12
weeks. The actual mean concentrations were 0, 21.16, 65.38, and
243.8 mg/m3. No unusual signs of toxicity were observed in rats or
mice during the study. Body weights and mortality were similar in
all groups at both 6 and 12 weeks. There were no treatment-related
changes in haematology, clinical chemistry, or urinalysis apart from
the detection of small to moderate amounts of occult blood in the
urine of female mice exposed to 22.7, 68.1, or 227 mg "MIX D/D"/m3
for 6 weeks. No treatment-related changes were observed in absolute
organ weights of rats or mice exposed to "MIX D/D" atmospheres up
to, and including, 227 mg/m3, for 6 or 12 weeks. Small changes
attributable to "MIX D/D" exposure were observed in male rats
exposed to 227 mg/m3 (increased liver/body weight ratio) at 6 and
12 weeks and in female rats exposed to 227 mg/m3 (increased
kidney/body weight ratio) at 12 weeks. No treatment-related
histopathological changes were observed in either rats or mice,
other than slight to moderate diffuse hepatocytic enlargement in
male mice (12/21) after 12 weeks exposure to 227 mg "MIX D/D"/m3.
No significant changes related to toxicity were found in the body
weight, behaviour, haematology, clinical chemistry, or gross or
histological appearance of rats or mice exposed to atmospheres
containing up to, and including, 227 mg "MIX D/D"/m3, for 6 or 12
weeks. No adverse effects were seen at the top dose level (Hazleton
Laboratories America Inc., 1979; Parker et al., 1982).
Groups of 30 male and 24 female Wistar SPF albino rats were
exposed to actual concentrations of 0, 64, 145, or 443 mg "MIX
D/D"/m3, 6 h/day 5 days per week, for 10 weeks. The "MIX D/D"
contained 28.1% cis-1,3-dichloropropene, 25.6% trans-1,3-
dichloropropene, and 25.6% 1,2-dichloropropane, without
epichlorohydrin. A subgroup was used for the reproduction study (see
also section 8.5.1.2). There were no compound-related changes in the
urinalysis, haematology, clinical chemistry, or in the gross or
histological appearance of the reproductive tract in male and female
rats. Males and females exposed to 443 mg "MIX D/D"/m3 showed a
reduced weight gain compared to controls, indicative of a mild toxic
response at this top dose. Absolute kidney weights in females were
higher in the 443 mg/m3 group compared to controls, but apart from
a slight increase in amorphous protein casts in the proximal
convoluted tubular lumina, no histopathological effects were
observed. This increase in kidney weight was probably related to the
efficient renal excretion of "MIX D/D". A no-observed-effect level
(NOEL) of 145 mg/m3 was considered (Clark, 1980).
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
Undiluted "MIX D/D" was applied to the skin of 12 rabbits in
single doses of 0.5 ml. The liquid was allowed to be in contact with
the skin for 24 h. The mean score was 7 on the scale (up to 8) of
the method of Draize et al. (1944): rating it as a severe irritant.
Signs were severe eschar, intense oedema, and black necrotic tissue
(Hine et al., 1952, 1953; Coombs & Carter, 1976a).
Six young New Zealand albino rabbits were used in a primary
skin-irritation test. 0.5 ml of undiluted "MIX D/D" soil fumigant
was applied on the skin for 4 h. The mean scores for erythema and
oedema after 4, 24, and 72 h were averaged. The primary irritation
score was 8, rating it as corrosive. The signs were erythema,
oedema, escharosis, and necrosis (Industr. Biotest Lab., 1972).
Undiluted "MIX D/D" was instilled into the eyes of 10 rabbits
in doses of 0.005 or 0.02 ml. After 18-24 h, the eyes were examined
and the reactions scored. The eye injury was scored as grade 5
(severe irritation) (Hine et al., 1952, 1953).
8.3.2 Sensitization
In a test with 20 "P" strain guinea-pigs, a 5% w/v
concentration of "MIX D/D" in corn oil was used and three topical
induction applications were followed by a 1% w/v concentration for
the topical challenge (method of Buehler, 1965). In 13 out of the 20
guinea-pigs, a positive reaction was obtained. "MIX D/D" has a
moderate skin sensitizing potential (Coombs & Carter, 1976a).
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
Groups of 50 male and 50 female albino rats (age 5 weeks) were
fed diets containing nominal concentrations of 0, 10, 30, 100, or
300 mg "MIX D/D"/kg diet for 2 years. Blood samples were collected
by suborbital sinus puncture at 3, 6, 12, 18, and 24 months. The
"MIX D/D" contained 28.2% cis-, 29.0% trans-1,3-dichloropropene
and 34% 1,2-dichloropropane, without epichloro- hydrin.
There were no significant differences throughout the 2-year
period between the body weights, food intakes, behaviour, or
mortalities of male and female rats fed diets containing "MIX D/D"
and those of control animals. There were no consistent compound-
related changes in the haematological parameters.
Statistically significant increases were noted, at 3, 6, and 24
months, in the fasting serum glucose values of rats receiving 300
mg/kg diet and in the serum alkaline phosphatase levels at 12 months
compared with controls. These effects were not considered to be
biologically significant, because of the size of the response and
the lack of a consistent response throughout the study. No other
changes were observed in the haematology and clinical chemistry of
rats exposed to "MIX D/D" for 2 years.
Urinalyses were performed at 3, 6, 12, 18, and 24 months, and
the only statistically significant difference recorded was a lowered
specific gravity of urine at 3 months in males receiving 300 mg/kg
diet compared with controls. This effect was not correlated with any
other changes, and the effect did not occur consistently throughout
the study; it was therefore considered to be of doubtful biological
significance. No changes attributable to feeding diets containing
"MIX D/D" were observed in the organ weights and histopathology at 1
and 2 years (Industr. Biotest Lab., 1978). An audit performed on the
study data revealed no major factors that would affect the
conclusions of this study. The nominal concentrations of "MIX D/D"
specified in the protocol could not be considered representative of
the actual concentrations fed to animals. Subsequent work on the
volatility of "MIX D/D" in the diet revealed that the average
dietary concentrations present at the time of feeding were 40% of
the nominal values.
In conclusion, no compound-related effects were observed in
rats fed diets containing an average concentration of up to and
including 120 mg/kg diet (= nominal concentration of 300 mg/kg diet)
"MIX D/D" for two years (Jud et al., 1980a).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
Charles River CD-strain albino rats (10 males and 20 females)
were fed diets containing 0, 10, 30, or 100 mg "MIX D/D" ( cis-1,3-
dichloropropene 28.2%, trans-1,3-dichloropropene 29%, 1,2-
dichloropropane 34%, without epichlorohydrin) per kg diet in a 3-
generation, 2-litter, reproduction study. No statistically
significant differences were observed in parental body weights, food
consumption, behaviour, or mortalities throughout each 10-week pre-
breeding period. Gross pathology, histopathology, and organ weights
were similar in all groups. The feeding of diets containing "MIX
D/D" did not affect the reproductive performance (mating, fecundity,
fertility indices, and parturition incidence) or dam body weights
during gestation or post-partum and post-weaning periods. There were
no signs of external abnormalities in newborn pups. There were
significantly more pups alive at day 1 of lactation in the F3a
litters of the 30 mg/kg group, compared with controls. No
differences that were directly related to treatment were noted for
survival over a 21-day period, number of pups delivered, or
stillbirths. No gross pathological or histopathological differences
were observed between treatment and control offspring. Significantly
higher mean body weights were recorded at weaning in the F2b males
of the 30 mg/kg group and in males and females of the 100 mg/kg
group. Increases were also recorded at weaning in the F3b
generation of 10 mg/kg males and 100 mg/kg females. These increases
in mean body weight did not show any consistent relationship with
dose or sex and were not considered attributable to "MIX D/D"
exposure (Industr. Biotest Lab., 1977a).
An audit of the study data demonstrated that compliance with
the protocol was satisfactory and that agreement of the results with
more recent data was sufficient to support the conclusions of this
study. Subsequent work on the volatility of "MIX D/D" in diet
revealed that average concentrations present at the time of feeding
were 40% of the nominal values. Thus "MIX D/D" did not produce any
effects on the reproductive performance or growth of offspring of
rats fed diets containing up to and including 40 mg "MIX D/D"/kg
diet (= nominal concentration of 100 mg/kg diet) for 3 generations
(40 mg/kg diet, the highest dose level tested is equivalent with 2
mg/kg body weight) (Jud et al., 1980b).
8.5.1.2 Inhalation
In one study (Linnett et al., 1988), groups of 20 male SPF
Wistar-derived rats (15 weeks old) and 24 virgin female rats (10
weeks old) were exposed by inhalation to nominal concentrations of
0, 45.4, 136, or 408 mg "MIX D/D"/m3 (actual concentrations of 0,
64, 145, or 443 mg/m3 for 6 h/day, 5 days/week, for 10 weeks. The
composition of the "MIX D/D" was 28.1% (w/w) cis-1,3-
dichloropropene, 25.6% trans-1,3-dichloropropene, and 25.6% 1,2-
dichloropropane and a number of other chlorinated components in
percentages up to 5%, but it contained no epichlorohydrin.
Treated males of proven fertility were paired with untreated
virgin females at intervals during and after exposure. Groups of 15
treated females were paired with untreated males immediately after
the 10-week period. Various aspects of reproduction performance and
general toxicity were assessed. Mortality and haematological,
clinical-chemical, and urinary parameters were comparable with the
controls. Exposure to "MIX D/D" did not produce any adverse effects
on the libido, fertility, or morphology of the reproductive tracts
of rats of either sex. No treatment-related dominant lethal effects
were observed in male rats. Mean body weights of male and female
rats at 408 mg/m3 were significantly decreased. The mean liver and
kidney weights were significantly increased in the animals of both
sexes exposed to the highest dose level. Histological examination of
the organs did not reveal treatment-related changes. This study
demonstrates that male and female rats exposed to atmospheres
containing up to 443 mg/m3 "MIX D/D" vapour for 10 weeks did not
suffer any impairment of reproductive performance.
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
Charles River, albino, female rats received gastric intubations
of 0, 30, or 100 mg "MIX D/D"/kg body weight (containing 28% cis-
1,3-dichloropropene, 29% trans-1,3-dichloropropene, and 34% 1,2-
dichloropropane, without epichlorohydrin) dissolved in corn oil,
during days 6 through 15 of pregnancy. The control group was dosed
with corn oil. A significant decrease in body weight of the dams
receiving 100 mg/kg, compared with the controls, was observed at day
15 of gestation. While no statistically significant reproductive
effects occurred, there appears to have been a trend for an increase
in the percentage of females with one or more resorption sites. The
lack of statistical significance is probably due to the small
numbers of animals involved (3 of 17, 6 of 19, and 5 of 15, for the
0, 30, and 100 mg/kg groups, respectively). The maternal toxicity
observed in the highest-dose group would account for the apparent
increase in resorption sites.
There was a dose-related increase in the incidence of
supernumerary ribs (2.8% for controls, 7.6% for the 30 mg/kg group,
and 32.3% for the 100 mg/kg per group) and the same was found for
the occurrence of non-ossified sternum sections. The incidence of
supernumerary ribs over approximately 50 rat teratology studies, in
this laboratory, has been within the range 0-26%.
A significant decrease in mean body weight of fetuses from
females dosed with 100 mg "MIX D/D"/kg was observed. Maternal
toxicity was observed in the 100 mg/kg group, but no malformations,
other than supernumerary ribs and non-ossified sternebrae, were
observed in this group (Industr. Biotest Lab., 1975).
An audit of the study data demonstrated that there were no
major factors to alter the conclusions of this study (O'Sullivan et
al., 1980).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
DeLorenzo et al. (1977) tested "MIX D/D" (40% 1,3-
dichloropropene and 40% 1,2-dichloropropane) on Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1978, with
and without activation. Dose levels of 0, 0.5, 5, 15, and 25
mg/plate were tested. Mutagenic effects were seen with TA100,
TA1535, and TA1978, but not with TA98 and TA1537.
Shirasu et al. (1981) studied the mutagenicity of "MIX D/D" in
reverse mutation tests using Salmonella typhimurium strain TA100,
and Moriya et al. (1983) used 5 strains of Salmonella typhimurium,
TA98, TA100, TA1535, TA1537, and TA1538, and Escherichia coli
strain WP2 hcr, with and without metabolic activation. "MIX D/D"
was tested in dose levels up to 5000 µg/plate. The mutagenic potency
in Salmonella typhimurium TA100 was 0.0087 revertants/nmole. "MIX
D/D" was a direct-acting mutagen in TA100, TA1535, and E. coli WP2
hcr, but was not mutagenic in TA1537, TA1538, or TA98.
8.6.2 In vivo studies
Dominant lethality was tested in rats exposed to an atmosphere
containing "MIX D/D" vapour for 10 weeks (Linnett et al., 1988, see
section 8.5.1.2). No effects on fertility or on implantations were
observed even at 443 mg/m3, the highest concentration tested. This
result demonstrates the lack of any significant effects on germ-cell
production in rats.
Further evidence of rapid detoxification was found in the
negative results of a host-mediated assay using S. typhimurium,
strain G46, in mice dosed orally with either 60 or 200 mg "MIX
D/D"/kg over a 24-h period (Shirasu et al., 1981).
9. EFFECTS ON HUMANS
9.1 General population exposure
A case of acute poisoning occurred a few hours after the
accidental ingestion of "MIX D/D". The victim experienced abdominal
pain and vomiting. He became semicomatose and exhibited muscle
twitching and died (Gosselin et al., 1976; NTP, 1985).
9.2 Occupational exposure
In the period from 1966 to 1971, a total of 13 cases of
untoward skin reactions to pesticides were reported in the northern
part of the Netherlands. Seven were due to contact with "MIX D/D"
caused by inadvertent dripping into the shoes of the applicators
during spraying operations. In all cases, acute vesicular dermatitis
occurred. Three other cases of dermatitis were described, one being
of a contact allergic nature, as confirmed by patch testing, and
were diagnosed as orthoergic contact reactions (Nater & Gooskens,
1976).
Nater & Gooskens (1976) and Van Joost & De Jong (1988)
described dermatitis in a farmer who had sprayed "MIX D/D" and in a
process operator, caused by direct contact. The persons had had
previous contact with this substance. Both patients reacted
positively in a patch test, with 1% and 0.02% "MIX D/D",
respectively.
REFERENCES
Abdalla NA (1974) Distribution, persistence and nematocidal activity
of monobromomethane and a pesticide containing 1,3-dichloropropene
in soil. Diss Abstr Int, B35: 296.
Abdalla NA, Raski DJ, Lear B, & Schmitt RV (1974) Movement,
persistence and nematocidal activity of a pesticide containing 1,3-
dichloropropene in soils treated for nematode control in replant
vineyards. Plant Dis Reptr, 58: 562-566.
Ahlsdorf B, Stock R, Muller-Wegener U, & Milde G (1989) [The
behaviour of selected pesticides in ground water close to the
surface in heterogenous loose sediments.] Schriftenr Ver Wasser-
Boden- Lufthyg, 79: 375-385 (in German).
Albrecht WN (1987) Occupational exposure to 1,3-dichloropropene
(Telone(R) II) in Hawaiian pineapple culture. Arch Environ Health,
42(5): 286-291.
Albrecht WN & Chenchin K (1985) Dissipation of 1,2-dibromo-3-
chloropropane (DBCP), cis-1,3-dichloropropene (1,3-DCP) and
dichloropropenes from soil to atmosphere. Bull Environ Contam
Toxicol, 34: 824-831.
Albrecht WN, Hagadone MR, & Chenchin K (1986) Charcoal air sampling
tube storage stability and desorption efficiencies of 1,2-dibromo-3-
chloropropane (DBCP) and 1,3-dichloropropene (DCP). Bull Environ
Contam Toxicol, 36: 629-634.
Altman J (1969) Effect of chlorinated C3 hydrocarbons on amino
acid production by indigenous soil bacteria. Phytopathology, 59:
762-766.
Altman J & Lawlor S (1966) The effect of some chlorinated
hydrocarbons on certain soil bacteria. J Appl Bacteriol, 29: 260-
265.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside,
California, University of California, Agricultural Extension (Report
No. M16 5M Rev. 9/73).
Bakker Y (1968) [Influence of soil decontamination with DD on
nitrogen manuring.] Landbouwvoorlichting, 25(2): 87-88 (in Dutch).
Bartels MJ & Timchalk C (1990) 1,2-Dichloropropane: Investigation of
the mechanism of mercapturic acid formation in the rat. Xenobiotica,
20(10): 1035-1042.
Baruffini A, Cirla AM, Pisati G, Ratti R, & Zedda S (1989) Allergic
contact dermatitis from 1,2-dichloropropane. Contact Dermatitis, 20:
379-380.
Battersby NS (1990a) Trans-1,3-dichloropropene: An assessment of
ready bio-degradability. Internal Report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 89.179, submitted to WHO by
Shell).
Battersby NS (1990b) An assessment of the inhibitory effect of cis-
+ trans-mixture 1,3-dichloropropene on the respiration rate of
activated sludge. Internal Report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 90.007, submitted to WHO by
Shell).
Battersby NS (1990c) An assessment of the inhibitory effect of cis-
1,3-dichloropropene on the respiration rate of activated sludge.
Internal Report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 90.005, submitted to WHO by Shell).
Bellomo G & Orrenius S (1985) Altered thiol and calcium homeostasis
in oxidative hepatocellular injury. Hepatology, 5: 876-882.
Belser NO & Castro CE (1971) Biodehalogenation - the metabolism of
the nematocides cis- and trans-3-chloroallyl alcohol by a
bacterium isolated from soil. J Agric Food Chem, 19: 23-26.
Bennett D & Ridge MA (1989) Trans-1,3-dichloropropene:
Determination of the N-octanol/water partition coefficient using a
reverse-phase HPLC method. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 89.133, submitted to WHO by
Shell).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow,
Pimephales promelas, early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berry DL, Campbell WF, Street JC, & Salunkhe DK (1980) Uptake and
metabolism of 1,3-dichloropropene in plants. J Food Saf, 2(4): 247-
255.
Beugelink GP (1987) [Chemicals used in soil decontamination heralds
of a serious contamination.] H2O, 20(21): 522-526 (in Dutch).
Blair D (1977) Toxicity of soil fumigants: acute inhalation toxicity
of 1,3-dichloropropene. Sittingbourne, Shell Research Ltd,
(Unpublished proprietary report TLTR 0002.77, submitted to WHO by
Shell).
Bousema MT, Wiemer GR, & Van Joost TH (1991) A classic case of
sensitization to DD-95. Contact Dermatitis, 24: 132-133.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds.
Washington, DC, American Chemical Society, pp 49-64 (ACS Symposium
Series No. 149).
Boyland E & Chasseaud LF (1969) The role of glutathione and
glutathione-S-transferases in mercapturic acid biosynthesis. Adv
Enzymol, 32: 173-219.
Breslin WJ, Kirk HD, Streeter CM, Quast JF, & Szabo JR (1987) Telone
II soil fumigant: Two generation inhalation reproduction study in
Fischer 344 rats. (Study No. M003993-015). Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Breslin WJ, Kirk HD, Streeter CM, Quast JF, & Szabo JR (1989) 1,3-
Dichloropropene: Two generation inhalation reproduction study in
Fischer 344 rats. Fundam Appl Toxicol, 12: 129-143.
Brezis M, Rosen S, Silva P, & Epstein FH (1983) Selective
glutathione depletion on function and structure of the isolated
perfused rat kidney. Kidney Int, 24: 178-184.
Bromley S & Cook KA (1981) Evaluation of the soil fumigant, DD, as a
nitrification inhibitor. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report SBGR 81.295, submitted to WHO by
Shell).
Brooks TM, Dean BJ, & Wright AS (1978) Toxicity studies with
dichloropropenes; Mutation studies with 1,3-D and cis-1,3-
dichloropropene and the influence of glutathione on the mutagenicity
of cis-1,3 dichloropropene in Salmonella typhimurium.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
TLGR 0081.78, submitted to WHO by Shell).
Brooks TM & Wiggins DE (1989a) Bacterial mutagenicity studies with
trans-1,3-dichloropropene. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 88.247, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1989b) Genotoxicity studies with trans-
1,3-dichloropropene: In vitro chromosome studies with trans-1,3-
dichloropropene. Internal report. London, Shell Research Ltd
(Unpublished proprietary report SBGR 89.086, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1990) Bacterial mutagenicity studies with
cis-1,3-dichloropropene. Internal report. London, Shell Research
Ltd (Unpublished proprietary report SBGR 89.205, submitted to WHO by
Shell).
Brooks TM & Wiggins DE (1991) Genotoxicity studies with cis-1,3-
dichloropropene: In vitro chromosome studies. Internal report.
London, Shell Research Ltd (Unpublished proprietary report SBGR
90.049, submitted to WHO by Shell).
Brouwer DH, Brouwer EJ, De Vreede JAF, Van Welie RTH, Vermeulen NPE,
& Van Hemmen JJ (1991a) Inhalation exposure to 1,3-dichloropropene
in the Dutch flower-bulb culture. Part. I. Environmental monitoring.
Arch Environ Contam Toxicol, 20: 1-5.
Brouwer EJ, Evelo CTA, Verplanke AJW, Van Welie RTH, & De Wolff FA
(1991b) Biological effect monitoring of occupational exposure to
1,3-dichloropropene: Effects on liver and renal function and on
glutathione conjugation. Br J Ind Med, 48: 167-172.
Brown RH & Purnell CJ (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. The performance of
a Tenax-GC adsorbent tube. J Chromatogr, 178: 79-90.
Bruckner JV, Mackenzie WF, Ramanathan R, Muralidhara S, Kim HJ, &
Dallas CE (1989) Oral toxicity of 1,2-dichloropropane: Acute, short-
term and long-term studies in rats. Fundam Appl Toxicol, 12: 713-
730.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull
Environ Contam Toxicol, 26: 446-452.
Buehler EV (1965) Delayed contact hypersensitivity in the guinea-
pig. Arch Dermatol, 91: 171-177.
California State Water Resources Control Board (1983) 1,2-
Dichloropropene (1,2-D) and 1,3-dichloropropene (1,3-D). California
State Water Resources Control Board, Toxic Substances Control
Programme (Unpublished special project draft report).
Carere A & Morpurgo G (1981) Comparison of the mutagenic activity of
pesticides in vitro in various short-term assays. In: Kappas A ed.
Progress in environmental mutagenesis and carcinogenesis. Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp 87-104 (Progress
in Mutation Research, Volume 2).
Carter B (1975) Toxicity of soil fumigants: acute oral toxicity of
D-D to mice and dogs. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0034.75, submitted to WHO by Shell).
Casini AF, Maellaro E, Pompella A, Ferrali M, & Comporti M (1987)
Lipid peroxidation, protein thiols and calcium homeostasis in
bromobenzene-induced liver damage. Biochem Pharmacol, 36: 3689-3695.
Castro CE & Belser NO (1966) Hydrolysis of cis- and trans-1,3-
dichloropropene in wet soil. J Agric Food Chem, 14(1): 69-70.
Clark DG (1980) D-D: A 10-week inhalation study of mating behaviour,
fertility and toxicity in male and female rats. Sittingbourne, Shell
Research Ltd (Unpublished proprietary report TLGR 80.023, submitted
to WHO by Shell).
Climie IJG, Hutson DH, Morrison BJ, & Stoydin G (1979) Glutathione
conjugation in the detoxication of (Z)-1-3-dichloropropene (a
component of the nematocide D-D) in the rat. Xenobiotica, 9(3): 149-
156.
Coate WB & Voelker RW (1979b) Final report: 90-day inhalation
toxicity study in rats and mice. Telone II. Vienna, Virginia,
Hazleton Laboratories America Inc. (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Coate WB & Voelker RW (1979a) Addendum to final report: 90-day
inhalation toxicity study in rats and mice. Telone II. Vienna,
Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Collins CJ (1989) Trans-1,3-dichloropropene: Acute inhalation
toxicity study - LC50 rats (4-hour exposure). Harrogate, United
Kingdom, Hazleton Laboratories (Unpublished proprietary report
submitted to WHO by Shell).
Connors TF, Stuart JD, & Cope JB (1990) Chromatographic and
mutagenic analyses of 1,2-dichloropropane and 1,3-dichloropropylene
and their degradation products. Bull Environ Contam Toxicol, 44:
288-293.
Coombs AD & Carter BI (1976a) The toxicity of soil fumigants: Acute
toxicity, skin irritation and skin sensitizing potential of current
product D-D. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLTR 0021.76, submitted to WHO by Shell).
Coombs AD & Carter BI (1976b) The toxicity of soil fumigants: Acute
toxicity, skin irritation and skin sensitizing potential of 1,3-
dichloropropene (95% w/w a.m.). Sittingbourne, Shell Research Ltd
(Unpublished proprietary report TLTR 0023.76, submitted to WHO by
Shell).
Cotruvo JA (1985) Organic micropollutants in drinking water: An
overview. Sci Total Environ, 47: 7-26.
Cracknell S, Jackson GC, & Hardy CJ (1987) Telone II (1,3-
dichloropropene): acute inhalation study in rats. 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre Ltd (Prepared
for Dow Chemical Europe, Horgen, Switzerland) (Unpublished
proprietary report submitted to WHO by Dow Chemical Company).
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Creedy CL (1983) The modulation of the bacterial mutagenicity of the
(Z)- and (E)-isomers of 1,3-dichloropropene by glutathione and
subcellular fractions from rat liver. Sittingbourne, Shell Research
Ltd (Unpublished proprietary report SBGR 83.380, submitted to WHO by
Shell).
Creedy CL & Hutson DH (1982) The bacterial mutagenicity of (E)-1,3-
dichloropropene and its modulation by glutathione. Sittingbourne,
Shell Research Ltd (Unpublished proprietary report SBGR 82.335,
submitted to WHO by Shell).
Creedy CL, Brooks TM, Dean BJ, Hutson DH, & Wright AS (1984) The
protective action of glutathione on the microbial mutagenicity of
the Z- and E-isomers of 1,3-dichloropropene. Chem-Biol Interact,
50(1): 39-48.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and non-fatty foods. J Agric Food Chem, 37: 560-564.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1977) The acute
toxicity of 47 industrial chemicals to fresh and salt water fishes.
J Hazard Mater, 1: 303-318.
De Lorenzo F, Degl'Innocenti S, Ruocco A, Silengo L, & Cortese R
(1977) Mutagenicity of pesticides containing 1,3-dichloropropene.
Cancer Res, 37: 1915-1917.
Dietz FK, Dittenber DA, & Kastl PE (1982) 1,3-Dichloropropene:
Effects on tissue non-protein sulfhydryl content and blood
concentration time profile, probe study. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Dietz FK, Hermann EA, & Ramsey JC (1984a) The pharmacokinetics of
14C-1,3-dichloropropene in rats and mice following oral
administration. (Presented as poster No. 585 at 23rd Annual Society
of Toxicology Conference, Atlanta, Georgia, 15 March 1984).
Toxicologist, 4: 147 (Abstract 585).
Dietz FK, Dittenber DA, Kirk HD, & Ramsey JC (1984b) Non-protein
sulfhydryl content and macro-molecular binding in rats and mice
following oral administration of 1,3-dichloropropene. (Presented as
poster No. 586 at 23rd Annual Society of Toxicology Conference,
Atlanta, Georgia, 15 March 1984). Toxicologist, 4(1): 147 (Abstract
586).
Dietz FK, Hermann EA, Kastl PE, Dittenber DA, & Ramsey JC (1985)
1,3-Dichloropropene: Pharmacokinetics, effect on tissue non-protein
sulfhydryls, and macromolecular binding in Fischer 344 rats and
B6C3F1 mice following oral administration. Freeport, Texas,
Dow Chemical Company USA (Unpublished proprietary report HET: K-
6409-11, submitted to WHO by Dow Chemical).
Dilling WL, Tefertiller NB, & Kallos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1.1.1-
trichloroethane, trichloroethylene, tetrachloroethylene and other
chlorinated compounds in dilute aqueous solutions. Environ Sci
Technol, 9(9): 833-838.
Di Nucci A, Imbriani M, Ghittori S, Gregotti C, Baldi C, Locatelli
C, Manzo L, & Capodaglio E (1988) 1,2-Dichloropropane-induced liver
toxicity. Clinical data and preliminary studies in rats. The target
organ and the toxic process. Arch Toxicol, 12(Suppl): 370-374.
Dipaolo JA & Doniger J (1982) Neoplastic transformation of Syrian
hamster cells by putative epoxide metabolites of commercially
utilized chloroalkenes. J Natl Cancer Inst, 69(2): 531-534.
Dommergues Y (1959) Influence des nématocides sur l'activité
biologique du sol. Fruits (Paris), 14(4): 177-181.
Dowty B, Carlisle D, & Laseter JL (1975) Halogenated hydrocarbons in
New Orleans drinking water and blood plasma. Science, 187: 75-77.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Dubey HD, Riera A, & Rodriguez RL (1975) Effects of the nematocides
Nemagon and DD on mineralization, nitrification, soil microbial
population, and soil fertility status of two tropical soils. J Agric
Univ Puerto Rico, 59(1): 43-50.
Dynamic Corporation (1991) Herd profile: 1,2-Dichloropropane. CAS
No. 78-87-5 (Draft). Washington, DC, US Environmental Protection
Agency, Health and Environmental Review Division, 12 pp.
Eadsforth CV (1987) Biological monitoring of operators for exposure
to D-D during filling operations in CFD loods 2, SNR/C Pernis during
1985-86. Internal report. Rotterdam, Shell Research Ltd, Pernis
Biomedical Laboratory (Unpublished proprietary report submitted to
WHO by Shell).
Eadsforth CV, Rocchi PSJ, & Tuinman CP (1987) A monitoring study of
the exposure to 1,3-dichloropropene during a single application in
Germany of Shell D-D 92 nematocide. Internal report. The Hague,
Shell Research Ltd, Safety and Environmental Division (Unpublished
proprietary report submitted to WHO by Shell).
Edmiston S & Maddy KT (1987) Summary of illness and injuries
reported in California by physicians in 1986 as potentially related
to pesticides. Vet Hum Toxicol, 29(5): 391-397.
Edwards CA & Lofty JR (1969) The influence of agricultural practice
on soil micro-arthropod populations. Syst Assoc Publ, 8: 237-247.
Edwards CA (1983) Development of a standardized laboratory method
for assessing the toxicity of chemical substances to earthworms.
Brussels, Commission of the European Community (Report EUR 8714 EN).
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with USEPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 28: 460-
467.
Elgar KE, Hughes DG, & Reiff B (1965) Residues of DD in samples of
water from France (Technical Memorandum 55/65). Sittingbourne,
Woodstock Agricultural Research Centre, Technical Service Laboratory
(Unpublished proprietary data submitted to WHO by Shell).
Elliot JM, Marks CF, & Tu CM (1972) Effects of nematicides on
Pratylenchus penetrans, soil microflora, and flue-cured tobacco.
Can J Plant Sci, 52(1): 1-11.
Elliot JM, Marks CF, & Tu CM (1974) Effects of the nematicides DD
and Mocap on soil nitrogen, soil microflora, populations of
Pratylenchus penetrans, and flue-cured tobacco. Can J Plant Sci,
54: 801-809.
Elliot JM, Marks CF, & Tu CM (1977) Effects of certain nematicides
on soil nitrogen, soil nitrifiers, and populations of Pratylenchus
penetrans in flue-cured tobacco. Can J Plant Sci, 57: 143-154.
Ennik GC, Kort J, & Luesink B (1964) The influence of soil
disinfection with DD, certain components of DD and some other
compounds with nematocidal activity on the growth of white clover.
Neth J Plant Pathol, 70: 117-135.
Fishbein L (1979) Potential halogenated industrial carcinogenic and
mutagenic chemicals. Sci Total Environ, 11: 223-257.
Fisher GD & Kilgore WW (1988a) Mercapturic acid excretion by rats
following inhalation exposure to 1,3-dichloropropene. Fundam Appl
Toxicol, 11: 300-307.
Fisher GD & Kilgore WW (1988b) Tissue levels of glutathione
following acute inhalation of 1,3-dichloropropene. J Toxicol Environ
Health, 2: 171-182.
Fisher GD & Kilgore WW (1989) Pharmacokinetics of S-[3-chloroprop-2-
enyl]-glutathione in rats following acute inhalation exposure to
1,3-dichloropropene. Xenobiotica, 19(3): 269-278.
Flessel P, Goldsmith JR, Kahn E, & Wesolowski JJ (1978) Acute and
possible long-term effects of 1,3-dichloropropene-California. Morb
Mortal Wkly Rep, 271: 50, 55.
Fong HR & Maykoski R (1985) Air exposure monitoring of EHAP
personnel during a Telone II soil translocation study in Fresno
County. Sacramento, California, California Department of Food and
Agriculture, Division of Pest Management, Environmental Protection
and Worker Safety, Worker Health and Safety Unit, 6 pp (Unpublished
report HS-1299).
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BB, Resnick
MA, Anderson B, & Zieger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluation of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gardner JR (1989a) DD-95 ( cis- + trans-mixture 1,3-
dichloropropene): Acute oral toxicity. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 89.005,
submitted to WHO by Shell).
Gardner JR (1989b) Cis-1,3-dichloropropene: Acute oral and dermal
toxicity, skin and eye irritancy and skin sensitisation potential.
Internal report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 89.007, submitted to WHO by Shell).
Gardner JR (1989c) Trans-1,3-dichloropropene: Acute oral and
dermal toxicity, skin and eye irritancy and skin sensitising
potential. Internal report. London, Shell Research Ltd (Unpublished
proprietary report SBGR 88.286, submitted to WHO by Shell).
Girling AE (1989a) Cis-1,3-dichloropropene: Acute toxicity to
Salmo gairdneri, Daphnia magna and Selenastrum capricornutum.
London, Shell Research Ltd (Unpublished proprietary report SBGR
89.118, submitted to WHO by Shell).
Girling AE (1989b) Cis + trans-1,3-dichloropropene: Acute
toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. London, Shell Research Ltd (Unpublished proprietary
report SBGR 89.159, submitted to WHO by Shell).
Girling AE (1989c) Trans-1,3-dichloropropene: Acute toxicity to
Salmo gairdneri, Daphnia magna and Selenastrum capricornutum.
London, Shell Research Ltd (Unpublished proprietary report SBGR
89.160, submitted to WHO by Shell).
Goffart H & Heiling A (1958) [Side effects of nematode control with
Shell D-D and related agents.] Nematologica, 3: 213-228 (in German
with English summary).
Gollapudi BB, Bruce RJ, & Hinze CA (1985) Evaluation of Telone II
soil fumigant in the mouse bone marrow micronucleus test. Freeport,
Texas, Dow Chemical Company USA (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Gorzinski SJ & Johnson KA (1989) Neurotoxicologic examination of
Fischer 344 rats exposed to 1,2-dichloropropane (DCP) via gavage for
2 weeks. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Gosselin R, Hodge H, Smith R, & Gleason M (1976) Clinical toxicology
of commercial products, 4th ed. Baltimore, Maryland, Williams and
Wilkins Co., pp 119-121.
Greve PA, Klapwijk SP, & Linders JBHJ (1989) [Pesticides in surface
water in the bulb-growing area near Langeveld.] Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Protection (Unpublished report No. 638812001) (in Dutch).
Grzywa Z & Rudzki E (1981) Dermatitis from dichloropropene. Contact
Dermatitis, 7: 151-152.
Haemisegger ER, Jones AD, & Reinhardt FL (1985) EPA's experience
with assessment of site-specific environmental problems: A review of
IEMD's geographic study of Philadelphia. (Prepared for presentations
at the 78th Annual Meeting of the Air Pollution Control Association,
Detroit, Michigan, 16-21 June 1985). Washington, DC, US
Environmental Protection Agency, pp 1-20 (Report 85-63-6).
Hallberg GR (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hanley TR Jr, John-Greene JA, Young JT, Calhoun LL, & Rao KS (1987)
Evaluation of the effects of inhalation exposure to 1,3-
dichloropropene on fetal development in rats and rabbits. Fundam
Appl Toxicol, 8: 562-570.
Hanley TR Jr, Berdasco NM, Battjes JE, & Johnson KA (1989a)
Propylene dichloride: Oral teratology study in New Zealand white
rabbits. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Hanley TR Jr, Kirk HD, Bond DM, Firchau HM, & Johnson KA (1989b)
Propylene dichloride: Dominant lethal study in Sprague-Dawley rats.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Hanley TR Jr, Kirk HD, Berdasco NM, & Johnson KA (1990) Evaluation
of the developmental toxicity of propylene dichloride in rats and
rabbits. Teratology, 41(5): 562 (Abstract P54).
Haseman JK, Crawford DD, Huff JE, Boorman GA, & McConnell EE (1984)
Results from 86 two-year carcinogenicity studies conducted by the
national toxicology program. J Toxicol Environ Health, 14: 621-639.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen, 1(Suppl): 3-142.
Hayes WJ Jr (1982) Pesticides studied in man. Baltimore, Maryland,
Williams and Wilkins Co., pp 162, 163.
Hazleton Laboratories America, Inc. (1979) Subacute inhalation
toxicity study of D-D soil fumigant in CD-1 mice and albino Fischer
344 rats. Vienna, Virginia, Hazleton Laboratories America Inc.
(Prepared for Shell Oil Company, Houston, Texas) (Unpublished
proprietary report No. 776-132, submitted to WHO by Shell).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of
54 industrial chemicals to sheephead minnows (Cyprinodon
variegatus). Bull Environ Contam Toxicol, 27: 596-604.
Heppel LA, Highman B, & Porterfield VT (1946) Toxicology of 1,2-
dichloropropane (propylene dichloride). II Influence of dietary
factors on the toxicity of dichloropropane. J Pharmacol Exp Ther,
87: 11-17.
Hermann BW & Matsuyama H (1982) Freezer storage stability of D-D
soil fumigant: Shell Development Company's monthly research summary
(July 1982). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Heungens A & Van Daele E (1974) Evolution of the soil fauna in
Begonia culture by repeated applications of dichloropropane-
dichloropropene. Agric Environ, 1: 251-258.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hine CH, Anderson HH, Kodama JK, Morse M, & McDaniel HC (1952)
Studies on the toxicity of CBP-55 and D-D. San Francisco, University
of California, School of Medicine, Division of Pharmacology and
Experimental Therapeutics (Prepared for Shell Development Company,
California) (Unpublished proprietary report No. U.C. 184, submitted
to WHO by Shell).
Hine CH, Anderson HH, Moon HD, Kodama JK, Morse M, & Jacobsen NW
(1953) Toxicology and safe handling of CBP-55 (Technical 1-Chloro-3-
bromopropene-1). Arch Ind Hyg Occup Med, 7: 118-136.
Hoogsteen KJ (1986) [Micro pollution in 3 water-winning areas.]
H2O, 19(3): 48-52 (in Dutch).
HSE (1990) Chlorinated hydrocarbon solvent vapours in air. Methods
for the determination of hazardous substances (MDHS 28). London,
Health and Safety Executive, Occupational Medicine and Hygiene
Laboratory, pp. 1-7.
Hutson DH (1984) The role of oxidation in the bacterial mutagenicity
of (Z)-1,3-dichloropropene. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report SBGR 83.049, submitted to WHO by
Shell).
Hutson DH & Stoydin G (1977) The reaction of D-D components with
glutathione. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0041-77, submitted to WHO by Shell).
Hutson DH, Moss JA, & Pickering BA (1971) The excretion and
retention of components of the soil fumigant D-D, and their
metabolites in the rat. Food Cosmet Toxicol, 9(5): 677-680.
IARC (1986) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer, pp 113-130, 131-
147 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, Volumes 1 to 42. Lyon, International Agency for
Research on Cancer, pp 195-196 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Imberti R, Calabrese SR, Emilio G, Marchi L, & Giuffrida L (1987)
[Acute solvent poisoning: aliphatic chlorinated hydrocarbons.] Min
Anest, 53: 399-403 (in Italian).
Imberti R, Mapelli A, Colombo P, Richelmi P, Berte F, & Bellomo G
(1990) 1,2-Dichloropropane (DCP) toxicity is correlated with DCP-
induced glutathione (GSH) depletion and is modulated by factors
affecting intracellular GSH. Arch Toxicol, 64: 459-465.
Industrial Biotest Laboratories Inc. (1972) Primary skin irritation
tests with three samples in albino rabbits. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Unpublished proprietary report
No. IBT A.2484, submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1975) Teratogenic study with
D-D in albino rats. Northbrook, Illinois, Industrial Biotest
Laboratories Inc. (Prepared for Shell Kagaku Kabushiki Kaisha)
(Unpublished proprietary report No. IBT 623-06212, submitted to WHO
by Shell).
Industrial Biotest Laboratories Inc. (1977a) Three generation
reproduction study with D-D in albino rats. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Prepared for Shell Kagaku
Kabushiki Kaisha) (Unpublished proprietary report No. IBT 621-06002,
submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1977b) Two year chronic and
toxicity study with D-D compound in beagle dogs. Northbrook,
Illinois, Industrial Biotest Laboratories Inc. (Prepared for Shell
Kagaku Kabushiki Kaisha) (Unpublished proprietary report No. IBT
651-06000, submitted to WHO by Shell).
Industrial Biotest Laboratories Inc. (1978) Two year chronic and
toxicity study with D-D in albino rats. Northbrook, Illinois,
Industrial Biotest Laboratories Inc. (Prepared for Shell Kagaku
Kabushiki Kaisha) (Unpublished proprietary report No. IBT 621-06001,
submitted to WHO by Shell).
Jeffrey MM (1987a) Telone II soil fumigant: Primary dermal
irritation study in New Zealand White rabbits. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report No. M-003993-
017B, submitted to WHO by Dow Chemical).
Jeffrey MM (1987b) Telone II soil fumigant: Primary eye irritation
study in New Zealand White rabbits. Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report No. M-003993-017C, submitted
to WHO by Dow Chemical).
Jeffrey MM (1987c) Telone II soil fumigant: Dermal sensitization
potential in the Hartley albino guinea-pig. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report No. DR-003993-017E,
submitted to WHO by Dow Chemical).
Jeffrey MM, Battjer JE, & Lomax LG (1987) Telone II soil fumigant.
Acute oral toxicity study in Fischer 344 rats. Internal report.
Midland, Michigan, Dow Chemical Company (Unpublished proprietary
report submitted to WHO by Dow Chemical).
John JA, Kloes PM, Calhoun LL, & Young JT (1983) Technical grade
1,3-dichloropropene: Inhalation teratology study in Fischer 344 rats
and New Zealand White rabbits. Internal report. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Johnson KA & Gorzinski SJ (1988) Neurotoxicologic examination of
rats exposed to 1,2-dichloropropane (DCP) via gavage for 13 weeks.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Jones AR & Gibson J (1980) 1,2-dichloropropane: metabolism and fate
in the rat. Xenobiotica, 10(11): 835-846.
Jones JR & Collier TA (1986a) Telone II: OECD 401 Acute oral
toxicity test in the rat (Project No. 44/83). Horgen, Switzerland,
Dow Chemical Europe S.A. (Unpublished proprietary report submitted
to WHO by Dow Chemical).
Jones JR & Collier TA (1986b) Telone II: OECD 402 Acute dermal
toxicity test in the rat (Project No. 44/84). Horgen, Switzerland,
Dow Chemical Europe S.A. (Unpublished proprietary report submitted
to WHO by Dow Chemical).
Jud VA, Kincke VL, O'Sullivan PK, Schweizer AD, & Parker CM (1980a)
Audit of Industrial Bio-Test Laboratories Study No. 621-06001: Two
year chronic oral toxicity study with D-D in albino rats (Regulatory
Information Record No. WRC RIR-5). Houston, Texas, Shell Development
Company (Unpublished proprietary report submitted to WHO by Shell).
Jud VA, Kincke VL, Schweizer AD, & Lu CC (1980b) Audit of Industrial
Bio-Test Laboratories Study No. 621-06002: Three generation
reproduction study with D-D in albino rats (Regulatory Information
Record No. WRC RIR-7). Houston, Texas, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Kämpfe K (1973) [Results of the combined application of anhydrous
ammonia and a mixture of dichloropropane and dichloropropene (DD) as
a nitrification inhibitor in autumn.] Arch Acker-Pflanzenbau
Bodenkd, 17(10): 827-835 (in German).
Kastl PE & Hermann EA (1983) Determination of cis- and trans-
1,3-dichloropropene in whole rat blood by gas chromatography and gas
chromatography-chemical ionization mass spectrometry with selected
ion monitoring. J Chromatogr, 265: 277-283.
Kenaga EE (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals. Ecotoxicol
Environ Saf, 4: 26-38.
Kier LE, Brusick DJ, Auletta AE, Von Halle ES, Brown MM, Simmon VF,
Dunkel V, McCann J, Mortelmans K, Prival M, Rao TK, & Ray V (1986)
The Salmonella typhimurium/mammalian microsomal assay. A report of
the US Environmental Protection Agency gene-Tox program. Mutat Res,
16: 69-240.
Kirk HD, Hanley TR Jr, Johnson KA, & Dietz FK (1989) Propylene
dichloride: Oral teratology study in Sprague-Dawley rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Kirk HD, Hanley TR Jr, Bond DM, Firchau HM, Peck CN, Stebbins KE, &
Johnson KA (1990) Propylene dichloride: Two-generation reproduction
study in Sprague-Dawley rats. Internal report. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Kline SA, McCoy EC, Rosenkranz HS, & Van Duuren BL (1982)
Mutagenicity of chloroalkene epoxides in bacterial systems. Mutat
Res, 101: 115-125.
Kloes PM, Calhoun LL, Young JT, & John JA (1983) Telone II:
Inhalation teratology probe study in Fischer 344 rats and New
Zealand White rabbits. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Toxicology, 19: 209-221.
Krijgsheld KR & Van der Gen A (1986) Assessment of the impact of the
emission of certain organochlorine compounds on the aquatic
environment. Part II: Allylchloride, 1,3- and 2,3-dichloropropene.
Chemosphere, 15(7): 861-880.
Lagas P, Verdam B, & Loch JPG (1989) Threat to groundwater quality
by pesticides in the Netherlands. In: Groundwater management:
Quantity and quality. Proceedings of the Benidorm Symposium, October
1989, pp 171-180 (IAHS Publication No. 188).
Larcan A, Lambert H, Laprevote MC, & Gustin B (1977) Acute poisoning
induced by dichloropropane. Acta Pharmacol Toxicol, 41: 330.
Lebbink G & Kolenbrander GJ (1974) Quantitative effect of fumigation
with 1,3-dichloropropene mixtures and with metham sodium on the soil
nitrogen status. Agric Environ, 1: 283-292.
Lebbink G, Proper B, & Nipshagen A (1989) Accelerated degradation of
1,3-dichloropropene. Acta Hortic, 255: 361-371.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc GA (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Leiber MA & Berk HC (1984) Development and validation of an air
monitoring method for 1,3-dichloropropene, trans-1,2,3-
trichloropropene cis-1,2,3-trichloropropene 1,1,2,3-
tetrachloropropene, 2,3,3-trichloro-2-propen-1-ol and 1,1,2,2,3-
pentachloropropane. Anal Chem, 56: 2134-2137.
Leistra M (1970) Distribution of 1,3-dichloropropene over the phases
in soil. J Agric Food Chem, 18(6): 1124-1126.
Leistra M & Boesten JJTI (1989) Pesticide contamination of
groundwater in Western Europe. Agric Ecosyst Environ, 26: 369-389.
Leistra M, Groen AE, Crum SJH, & Van der Pas LJT (1991)
Transformation rate of 1,3-dichloropropene and 3-chloroallyl alcohol
in topsoil and subsoil material of flower-bulb fields. Pestic Sci,
31: 197-207.
Li M (1979) A systems approach to controlling pesticides in the San
Joaquin Valley, Ecosystem studies of the National Science
Foundation, University of California, Davis.
Ligocki MP, Leuenberger C, & Pankow JG (1985) Trace organic
compounds in rain - II. Gas scavenging of neutral organic compounds.
Atmos Environ, 19(10): 1609-1617.
Linnett SL, Clark DG, Blair D, & Cassidy SL (1988) Effects of
subchronic inhalation of D-D (1,3-dichloropropene/1,2-
dichloropropane) on reproduction in male and female rats. Fundam
Appl Toxicol, 10: 214-223.
Loch JPG & Verdam B (1989) Pesticide residues in groundwater in the
Netherlands: State of observations and future directions of
research. Schriftenr Ver Wasser- Boden- Lufthyg, 79: 349-363.
Lomax LG, Calhoun LL, Stott WT, & Franson LE (1987) Telone II soil
fumigant: 2-year inhalation chronic toxicity-oncogenicity study in
rats (Study No. M 003993-009R). Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report submitted to WHO by Dow
Chemical).
Lomax LG, Stott WT, Johnson KA, Calhoun LL, Yano BL, & Quast JF
(1989) The chronic toxicity and oncogenicity of inhaled technical-
grade 1,3-dichloropropene in rats and mice. Fundam Appl Toxicol, 12:
418-431.
Loveday KS, Lugo MH, Resnick MA, Anderson BE, & Zeiger E (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro: II. Results with 20 chemicals.
Environ Mol Mutagen, 13: 60-94.
McCall PJ (1987) Hydrolysis of 1,3-dichloropropene in dilute aqueous
solution. Pestic Sci, 19: 235-242.
McKenry MV & Thomason IJ (1974) Dosage values obtained following
pre-plant fumigation for perennials. I 1,3-dichloropropene
nematicides in eleven field situations. Pestic Sci, 7: 521-534.
Maddy KT, Fong HR, Lowe JA, Conrad DW, & Fredrickson AS (1982) A
study of well water in selected Californian communities for residues
of 1,3-dichloropropene, chloroallyl alcohol and 49 organophosphate
or chlorinated hydrocarbon pesticides. Bull Environ Contam Toxicol,
29: 354-359.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. Rev Environ
Contam Toxicol, 114: 58-99.
Markovitz A & Crosby WH (1984) Chemical carcinogenesis: A soil
fumigant, 1,3-dichloropropene as possible cause of hematologic
malignancies. Arch Intern Med, 144: 1409-1411.
Matsui S, Yamamoto R, & Yamada H (1989) The Bacillus
subtilis/microsome REC-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci Technol, 21: 875-887.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 506-553 (Resource Publication No.
160).
Mehta BV, Patel GJ, & Dangarwala RT (1963) Effects of fumigation and
other measures for nematode control on the production of nitrate in
Goradu soil. J Indian Soc Soil Sci, 11: 361-371.
Mendrala AL (1985) Evaluation of Telone II in the rat hepatocyte
unscheduled DNA synthesis assay. Midland, Michigan, Dow Chemical
Company (Unpublished proprietary report submitted to WHO by Dow
Chemical).
Mendrala AL (1986) The evaluation of Telone II soil fumigant in the
Chinese hamster ovary cell/hypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Midland, Michigan,
Dow Chemical Company (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Meyer AL (1980) Mutagenicity studies with cis-1,3-dichloropropene
in cultured mammalian cells. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report TLGR 80.022, submitted to WHO by
Shell).
Mitchell JR, Jollow DJ, Potter WZ, Gilette JR, & Brodie BB (1973)
Acetaminophen induced hepatic damage. IV. Protective role of
glutathione. J Pharmacol Exp Ther, 1877: 211-217.
Miyaoka T, Yamashita E, Hasegawa T, Akiyama M, Tsuda S, & Shirasu Y
(1990) Mechanism of 1,3-dichloropropene-induced hepatotoxicity in
mice. J Pestic Sci, 15: 419-425.
Moje W, Martin JP, & Baines RC (1957) Structural effects of some
organic compounds on soil organisms and citrus seedlings grown in an
old citrus soil. J Agric Food Chem, 5(10): 32-36.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol,
17: 405-429.
Nater JP & Gooskens VHJ (1976) Occupational dermatosis due to a soil
fumigant. Contact Dermatitis, 2: 227-229.
Neudecker T & Henschler D (1986) Mutagenicity of chloro-olefins in
the Salmonella/mammalian microsome test. III. Metabolic activation
of the allylic chloropropenes allylchloride, 1,3-dichloropropene,
2,3-dichloro-1-propene, 1,2,3-trichloropropene, 1,1,2,3-tetrachloro-
2-propene and hexachloropropene by S9 mix via two different
metabolic pathways. Mutat Res, 170: 1-9.
Neudecker T, Stefani A, & Henschler D (1977) In vitro mutagenicity
of the soil nematicide 1,3-dichloropropene. Experientia (Basel), 33:
1084-1085.
Neudecker T, Lutz D, Eder E, & Henschler D (1980) Structure-activity
relationship in halogen and alkyl substituted allyl and allylic
compounds: Correlation of alkylating and mutagenic properties.
Biochem Pharmacol, 29: 2611-2617.
Neuhauser EF, Loehr RC, & Malecki MR (1985a) Contact and artificial
soil tests using earthworms to evaluate the impact of wastes in
soil. In: Petros JK Jr, Lacy WJ, & Conway W ed. Hazardous and
industrial solid waste: 4th Symposium. Philadelphia, Pennsylvania,
American Society for Testing and Materials, pp 192-203 (ASTM Special
Technical Publication No. 886).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985b)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Neuhauser EF, Durkin PR, Malecki MR, & Anatra M (1986) Comparative
toxicity of ten organic chemicals to four earthworm species. Comp
Biochem Physiol, 83C(1): 197-200.
NIOSH (1985) 1,2-Dichloropropane: Analytical method 1013. In: Eller
PM ed. NIOSH Manual of analytical methods. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, vol 1, pp 1-
4.
Nitschke KD & Lomax LG (1990) Cis-1,3-dichloropropene: 2-Week
vapor inhalation toxicity study in Fischer 344 rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Nitschke KD, Johnson KA, Wackerle DL, Phillips JE, & Dittenber DA
(1988) Propylene dichloride: 13-Week inhalation toxicity study with
rats, mice and rabbits. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Nitschke KD, Crissman JW, & Schuetz DJ (1990) Cis-1,3-
dichloropropene: Acute inhalation study with Fischer 344 rats.
Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Nitschke KD, Lomax LG, & Sanderson TG (1991) Cis-1,3-
dichloropropene: 13-Week vapor inhalation toxicity study in Fischer
344 rats. Internal report. Midland, Michigan, Dow Chemical Company
(Unpublished proprietary report submitted to WHO by Dow Chemical).
NTP (1983) Carcinogenesis bioassay of 1,2-dichloropropane (propylene
dichloride) in F344/N rats and B6C3F1 mice (gavage study).
Research Triangle Park, North Carolina, National Toxicology Program
(NTP Technical Report No. 82-092; NIH publication No. 82-2519).
NTP (1985) Toxicology and carcinogenesis studies of Telone II, in
F344/N rats and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NTP
Technical Report No. 269; NIH-publication No. 85-2525).
NTP (1986) Toxicology and carcinogenesis studies of 1,2-
dichloropropane (propylene dichloride) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP Technical Report No. 263;
NIH Publication No. 86-2519).
O'Connor J (1990a) Cis-1,3-dichloropropene: Determination of
physico-chemical properties. Eye, United Kingdom, Life Science
Research Ltd (Unpublished proprietary report No. 90/SLK005/0461,
submitted to WHO by Shell).
O'Connor J (1990b) Cis-1,3-dichloropropene: Determination of
hydrolysis as a function of pH. Eye, United Kingdom, Life Science
Research Ltd (Unpublished proprietary report No. 90/SLK006/0479,
submitted to WHO by Shell).
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation
of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium
OB3b expressing soluble methane monooxygenase. Appl Environ
Microbiol, 55(11): 2819-2826.
Onkenhout W, Mulder PPJ, Boogaard PJ, Buys W, & Vermeulen NPE (1986)
Identification and quantitative determination of mercapturic acids
formed from Z- and E-1,3-dichloropropene by the rat, using
gaschromatography with three different detection techniques. Arch
Toxicol, 59: 235-241.
Orrenius S, Thor H, McConkey D, Nicotera P, & Bellomo G (1989)
Mechanism of cell toxicity: the thiol/calcium hypothesis. In:
Proceedings of the VII International Symposium on Microsomes and
Drug Oxidation. London, Taylor & Francis, pp 329-337.
Osterloh J, Letz G, Pond S, & Becker C (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse-sperm
morphology assay. Mutat Res, 116: 407-415.
Osterloh JD, Cohen BS, Popendorf W, & Pond SM (1984) Urinary
excretion of the N-acetyl cysteine conjugate of cis-1,3-
dichloropropene by exposed individuals. Arch Environ Health, 39(4):
271-275.
Osterloh JD, Wang R, Schneider F, & Maddy K (1989) Biological
monitoring of dichloropropene: Air concentrations, urinary
metabolite, and renal enzyme excretion. Arch Environ Health, 44(4):
207-213.
O'Sullivan PK, Schweizer AK, & Lu CC (1980) Audit of Industrial Bio-
Test Laboratories Study No. 623-06212: Teratogenic study with D-D in
albino rats (Regulatory Information Record No. WRC RIR-6). Houston,
Texas, Shell Development Company (Unpublished proprietary report
submitted to WHO by Shell).
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic
compounds in water and thirty Canadian potable water treatment
facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Ou L-T (1989) Degradation of Telone II in contaminated and
noncontaminated soils. J Environ Sci Health, B24(6): 661-574.
Parker CM (1980) Histopathologic evaluation of tissues and organs
from beagle dogs in a two-year chronic oral toxicity study fed D-D
compound (Regulatory Information Record No. WRC RIR-3). Houston,
Texas, Shell Development Company (Unpublished proprietary report
submitted to WHO by Shell).
Parker CM, Coate WB, & Voelker RW (1982) Subchronic inhalation
toxicity of 1,3-dichloropropene/1,2-dichloropropane (D-D) in mice
and rats. J Toxicol Environ Health, 9: 899-910.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc R Soc Lond, B189: 305-
332.
Peoples SA, Maddy KT, Cusick W, Jackson T, Cooper C, & Frederickson
AS (1980) A study of samples of well water collected from selected
areas in California to determine the presence of DBCP and certain
other pesticide residues. Bull Environ Contam Toxicol, 24: 611-618.
Perbellini L, Zedda A, Schiavon R, & Franchi GL (1985) [Two cases of
disseminated intravascular coagulation syndrome (DIC) caused by
exposure to 1,2-dichloropropane (commercial trichloroethylene).] Med
Lav, 76(5): 412-417 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol Lett, 16: 69-75.
Perry J & Roberts TR (1974) The degradation and leaching of Z and E-
3-chloroacrylic acids in soils. Sittingbourne, Shell Research Ltd.
(Unpublished proprietary report WKGR 0098.74, submitted to WHO by
Shell).
Pochon J, Lajudie J, & Coppier O (1951) Remarques sur les recherches
relatives à l'action de certaines substances antiparasitaires sur la
microflore du sol. Ann Inst Pasteur, 80: 517-519.
Ponticelli C, Imbasciati E, Redaelli B, & Salvadeo A (1968) [Acute
hepatorenal insufficiency due to trielene.] Lav Um, 20: 205-212 (in
Italian).
Portmann JE & Wilson KW (1971) The toxicity of 140 substances to the
Brown shrimp and other marine animals. London, Ministry of
Agriculture, Fisheries and Food (Shellfish Information Leaflet No.
22).
Pozzi C, Marai P, Ponti R, Dell'Oro C, Sala C, Zedda S, & Locatelli
F (1985) Toxicity in man due to stain removers containing 1,2-
dichloropropane. Br J Ind Med, 42: 770-772.
Price JB & Andrews IJ (1985) The in vitro assessment of eye
irritancy using enucleated eyes. Food Chem Toxicol, 23(2): 313-315.
Priston RAJ, Brooks TM, Hodson-Walker G, & Wiggins DE (1983)
Genotoxicity studies with 1,2-dichloropropane. Sittingbourne, Shell
Research Ltd (Unpublished proprietary report SBGR 83.083, submitted
to WHO by Shell).
Rader WE & Love JW (1977a) Impact of commercial Shell products on
microorganisms in the soil. Part. I. Effect on modulation on pinto
beans and on the nitrogen fixation bacteria (Technical Information
Record No. TIR-22-112-77, Part I). Modesto, California, Shell
Development Company (Unpublished proprietary report submitted to WHO
by Shell).
Rader WE & Love JW (1977b) Impact of commercial Shell pesticides on
microorganisms in the soil. Part. II. Effect on decomposition of
cellulose in the soil (Technical Information Record No. TIR-22-112-
77, Part II). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE, Love JW, & Chai EY (1978) Impact of commercial Shell
pesticides on microorganisms in the soil. Part III. Effect of
compounds on the populations of microorganisms and the O2/CO2
exchange in treated soil (Technical Information Record No. TIR-22-
112-77, Part III). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE (1979a) Impact of commercial Shell pesticides on
microorganisms in the soil. Part IV. The effect of the compounds on
soil nitrification (Technical Information Record No. TIR-22-112-77,
Part IV). Modesto, California, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Rader WE (1979b) The effects of Shell pesticides on protein
decomposition (Technical Information Record No. TIR-51-108-79).
Modesto, California, Shell Development Company (Unpublished
proprietary report submitted to WHO by Shell).
Rader WE (1979c) The effect of Shell pesticides on soil phosphatase
activity (Technical Information Record No. TIR-51-110-79). Modesto,
California, Shell Development Company (Unpublished proprietary
report submitted to WHO by Shell).
Rexilius L & Schmidt H (1982) Investigations into the migratory
behaviour of 1,3-dichloropropene and methylisothiocyanate in the
soil of the nursery areas. Nachrichtenbl Dtsch Pflanzenschutzd,
34(11): 161-165.
Reiff B (1975) Soil fumigant D-D: its toxicity to fish.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
TLGR 0035.75, submitted to WHO by Shell).
Reiff B (1978) The acute toxicity of 1,3-dichloropropene to the
Golden orfe (Idus idus melanotus). Sittingbourne, Shell Research
Ltd (Unpublished proprietary report TLGR 0057.78, submitted to WHO
by Shell).
Reinert KH, Hunter JV, & Sabatine T (1983) Dynamic heated headspace
analysis of volatile organic compounds present in fish tissue
samples. J Agric Food Chem, 31: 1057-1060.
Rick DL & McCarty LP (1988) The determination of the odor threshold
of Telone(R) II soil fumigant by a new method. Appl Ind Hyg, 3(11):
299-302.
Roberts TR & Stoydin G (1976) The degradation of (Z)-and (E)-1,3-
dichloropropenes and 1,2-dichloropropane in soil. Pestic Sci, 7:
325-335.
Rocchi PSJ & Van Sittert NJ (1989) A monitoring study of the
exposure to 1,3-dichloropropene during application in France of
Shell D-D and Shell D-D 92 nematocide. Internal report. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Unpublished
proprietary report submitted to WHO by Shell).
Rosenblum I & Talley W (1979) Evaluation of instrumental behavioural
performance of Rhesus monkeys after acute exposure to Telone II.
Albany, New York, Institute of Comparative and Human Toxicology
(Unpublished proprietary report submitted to WHO by Dow Chemical).
Ross DJ & McNeilly BA (1975) Influence of four nematicides on soil
nitrogen mineralisation and nitrogen uptake by white clover in a
yellow-grey earth. NZ J Agric Res, 18: 155-162.
Schiffmann D, Eder E, Neudecker T, & Henschler D (1983) Induction of
unscheduled DNA synthesis in Hela cells by allylic compounds. Cancer
Lett, 20(3): 263-269.
Schuurman P (1989) Physico-chemical properties of trans-1,3-
dichloropropene. Part II. Rijswijk, The Netherlands, Organization
for Applied Scientific Research (Unpublished proprietary report No.
PML 1989-C35, submitted to WHO by Shell).
Schweizer AK & Parker CM (1980) Audit of Industrial Bio-test
Laboratories Study No. 651-06000: Two-year chronic oral toxicity
study with D-D compound in beagle dogs (Regulatory Information
Record No. WRC RIR-4). Houston, Texas, Shell Development Company
(Unpublished proprietary report submitted to WHO by Shell).
Shell (1976) Determination of residues of 1,2-dichloropropane, 1,3-
dichloropropene (Z and E isomers), and 3-chloro-allyl alcohol (Z and
E isomers) in crops and soil. Sittingbourne, Shell Research Ltd
(Unpublished proprietary report No. WAMS 222-1, submitted to WHO by
Shell).
Shell (1978) Residue determination of the E-and Z-isomers of 3-
chloroallylalcohol (CAA) in crops and soil. Modesto, California,
Shell Development Company (Unpublished proprietary report No. MMS-R-
481-1, submitted to WHO by Shell).
Shell (1980) Residue determination of (E) and (Z)-isomers of 3-
chloroallylalcohol in agricultural commodities and water.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
No. MMS-R-506-1, submitted to WHO by Shell).
Shell (1984) Residue determination of 1,2-dichloropropane and the Z-
and E-isomers of 1,3-dichloropropene in agricultural commodities,
soil and water. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report No. MMS-R-505-3, submitted to WHO by Shell).
Shell (1985) D-D/D-D92. Review of the occurrence and fate of
residues in crops and the environment. London, Shell International
Chemical Company Ltd, Regulatory Affairs, Crop Protection Division
(Unpublished proprietary report submitted to WHO by Shell).
Shell IPM (1990) Review of environmental toxicology D-D92 (1,3-
dichloropropene). The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Review Series HSE 90.001) (Unpublished
proprietary report submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987a) "D-D 92" residue and
environmental fate studies - Small scale field studies. Internal
report. London, Shell Research Ltd (Unpublished proprietary report
SBGR 87.170, submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987b) "D-D 92" residue and
environmental fate studies - Development of air monitoring methods.
Internal report. London, Shell Research Ltd (Unpublished proprietary
report SBGR 87.141, submitted to WHO by Shell).
Sherren AJ & Woodbridge AP (1987c) "D-D 92" residue and
environmental fate studies - Volatilization of "D-D 92" following
treatment of soil in an outdoor enclosure. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 87.157,
submitted to WHO by Shell).
Sherren AJ (1990) D-D 92 residue and environmental fate studies.
Field study - France - Air monitoring. Internal report. London,
Shell Research Ltd (Unpublished proprietary report SBGR 89.127,
submitted to WHO by Shell).
Shirasu Y, Moriya M, Tezuka H, Teramoto S, Ohta T, & Inoue T (1981)
Mutagenicity screening studies on pesticides. In: Environmental
mutagens and carcinogens: Proceedings of the Third International
Conference on Environmental Mutagens, Tokyo, Mishima and Kyoto, 21-
27 September 1981, pp 331-335.
Sittig M (1980) Priority toxic pollutants. Health impacts and
allowable limits. Park Ridge, New Jersey, Noyes Data Corporation, pp
192-196.
Smelt JH, Teunissen W, Crum SJH, & Leistra M (1989) Accelerated
transformation of 1,3-dichloropropene in loamy soils. Neth J Agric
Sci, 37: 173-183.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani VC, Striegel JA, & Nycum
JS (1969) Range-finding toxicity data: List VII. Am Ind Hyg Assoc J,
30(5): 470-476.
Sommer K (1970) [Influence of different pesticides on nitrification
and nitrogen transformation in soils.] Landwirtsch Forsch, 25: 22-30
(in German with English summary).
Stevens JL, Ayoubi N, & Robbins JD (1988) The role of mitochondrial
matrix enzymes in the metabolism and toxicity of cysteine
conjugates. J Biol Chem, 263: 3395-3401.
Stolzenberg SJ & Hine CH (1980) Mutagenicity of 2- and 3-carbon
halogenated compounds in the Salmonella/mammalian microsome test.
Environ Mutagen, 2(1): 59-66.
Stott WT & Kastl PE (1985) Inhalation pharmacokinetics of technical
grade 1,3-dichloropropene in rats. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Stott WT & Kastl PE (1986) Inhalation pharmacokinetics of technical
grade 1,3-dichloropropene in rats. Toxicol Appl Pharmacol, 85(3):
332-341.
Stott WT, Young JT, Calhoun LL, & Battjes JE (1984) Telone II, soil
fumigant: 13-week inhalation study in rats and mice. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Stott WT, Johnson KA, Calhoun LL, Weiss SK, & Franson LE (1987)
Telone II soil fumigant: 2-year inhalation chronic toxicity-
oncogenicity study in mice (Study No. M-003993-009). Midland,
Michigan Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Stott WT, Young JT, Calhoun LL, & Battjes JE (1988) Subchronic
toxicity of inhaled technical grade 1,3-dichloropropene in rats and
mice. Fundam Appl Toxicol, 11: 207-220.
Stott WT, Waechter JM, & Quast JT (1990) Letter to the editor. Arch
Environ Health, 45: 250-253.
Straley JP, Christiansen JA, & Kopecky AL (1982) Improved biological
degradation chlorinated hydrocarbons using mutant bacteria. Proc Int
Water Conf Eng Soc West, 43: 523-531.
Streeter CM, Battjes JE, & Lomax LG (1987) Telone II soil fumigant.
An acute vapor inhalation study in Fischer 344 rats. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Sullivan DA, Jones AD, & Williams JG (1985) Results of the US
Environmental Protection Agency's air toxics analysis in
Philadelphia (Prepared for presentation at the 78th Annual Meeting
of the Air Pollution Control Association, Detroit, Michigan, 16-21
June 1985). Washington, DC, US Environmental Protection Agency, pp
1-15 (Report 85-17.5).
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503.
Talcott RE & King J (1984) Mutagenic impurities in 1,3-
dichloropropene preparations. J Natl Cancer Inst, 72(5): 1113-1116.
Telliard WA (1990) Broad-range methods for determination of
pollutants in wastewater. J Chromatogr Sci, 28: 453-459.
Thomason IJ & McKenry MV (1974) Part I. Movement and fate as
affected by various conditions in several soils. Hilgardia, 42: 392-
420.
Thorel JM, Bercoff E, Massari Ph, Droy JM, Chassagne Ph, Proust B,
Hemet J, & Bourreille J (1986) Toxicité du 1,2-dichloropropane: à
propos d'un cas avec hypertension portale. J Toxicol Clin Exp, 6(4):
247-252.
Thornton GD (1951) Some effects of D-D, EDB and chloropicrin on
microbiological action in several Florida soils. Proc Soil Sci Soc
Florida, 1951(11): 68-71.
Til HP, Spanjers MTh, Feron VJ, & Renzel PJG (1973) Sub-chronic (90-
day) toxicity study with Telone in albino rats (Final report).
Zeist, The Netherlands, Central Institute for Nutrition and Food
Research (Unpublished proprietary report No. R-4002, submitted to
WHO by Dow Chemical).
Timchalk C, Bartels MJ, Dryzga MD, & Smith FA (1989) Propylene
dichloride: Pharmacokinetics and metabolism in Fischer 344 rats
following oral and inhalation exposure. Internal report. Midland,
Michigan, Dow Chemical Company (Unpublished proprietary report
submitted to WHO by Dow Chemical).
Torkelson TR & Oyen F (1977) The toxicity of 1,3-dichloropropene as
determined by repeated exposure of laboratory animals. Am Ind Hyg
Assoc J, 38: 217-223.
Toyoshima S, Sato R, & Sato S (1978a) The acute toxicity test of
Telone II in mice (Unpublished proprietary report submitted to WHO
by Dow Chemical).
Toyoshima S, Sato R, & Sato S (1978b) The acute toxicity test of
Telone II in rats. (Unpublished proprietary report submitted to WHO
by Dow Chemical).
Trevisan A, Rizzi E, Bungaro A, Pozzobon L, Gioffre F, Scapinello A,
Valeri A, & Chiesura P (1988) Proximal tubule brush border
angiotensin converting enzyme behaviour and nephrotoxicity due to
1,2-dichloropropane. The target organ and the toxic process. Arch
Toxicol, 12(Suppl): 190-192.
Trevisan A, Rizzi E, Scapinello A, Gioffre F, & Chiesura P (1989)
Liver toxicity due to 1,2-dichloropropane in the rat. Arch Toxicol,
63: 445-449.
Trevisan A, Troso O, & Maso S (1991) Recovery of biochemical changes
induced by 1,2-dichloropropane in rat liver and kidney. Hum Exp
Toxicol, 10: 241-244.
Tu CM (1972) Effects of four nematocides on activities of
microorganisms in soil. Appl Microbiol, 23(2): 398-401.
Tu CM (1973) Effects of Mocap. N-Serve, Telone, and Vorlex at two
temperatures on populations and activities of microorganisms in
soil. Can J Plant Sci, 53: 401-405.
Tu CM (1978) Effect of pesticides on acetylene reduction and
microorganisms in a sandy loam. Soil Biol Biochem, 10: 451-456.
Tu CM (1979) Influence of pesticides on acetylene reduction and
growth of microorganisms in an organic soil. J Environ Sci Health,
B14(6): 617-624.
Tu CM (1981a) Effects of pesticides on activities of enzymes and
microorganisms in a clay soil. J Environ Sci Health, B16(2): 179-
191.
Tu CM (1981b) Effects of some pesticides on enzyme activities in an
organic soil. Bull Environ Contam Toxicol, 27: 109-114.
Tu CM (1988) Effects of selected pesticides on activities of
invertase, amylase and microbial respiration in sandy soil.
Chemosphere, 17(1): 159-163.
Tuazon EC, Atkinson R, Winer AM, & Pitts JN Jr (1984) A study of the
atmospheric reactions of 1,3-dichloropropene and other selected
organochlorine compounds. Arch Environ Contam Toxicol, 13: 691-700.
US EPA (1980) Ambient water quality criteria for dichloropropane and
dichloropropene. Washington, DC, US Environmental Protection Agency,
Office of Water Regulations and Standards Criteria (Report No.
440/5-80-043, PB 81-117541).
US EPA (1985) Health and environmental effects profile for 1,3-
dichloropropene. US Cincinnati, Ohio, Environmental Protection
Agency, Environmental Criteria and Assessment Office (EPA/600/X-85-
399).
US EPA (1990) Inhalation reference concentration for 1,3-
dichloropropene (542-75-6). Fairfax, Virginia, Clement International
Corporation (Prepared for the US Environmental Protection Agency,
Washington).
US EPA (1991) 1,3-Dichloropropene. CAS No. 542-75-6. Washington, DC,
US Environmental Protection Agency (Unpublished report).
Valencia R, Mason JM, Woodruff RC, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 325-348.
Van Beek CGEM, Janssen HMJ, & Puijker LM (1988) [Pesticides in
groundwater.] H2O, 21(4): 80-85 (in Dutch).
Van Den Berg F & Leistra M (1989) Behaviour of the fumigant 1,3-
dichloropropene in soil and its emission into the air. Internal
report. Wageningen, The Netherlands, The Winand Staring Centre for
Integrated Land, Soil and Water Research.
Van Den Brande J & Heungens A (1969) Influence of repeated
applications of nematicides on the soil fauna in Begonia culture.
Neth J Plant Pathol, 75: 40-44.
Van Der Pas LJT & Leistra M (1987) Movement and transformation of
1,3-dichloropropene in the soil of flower-bulb fields. Arch Environ
Contam Toxicol, 16: 417-422.
Van Dijk H (1974) Degradation of 1,3-dichloropropenes in the soil.
Agro-ecosystems, 1: 193-204.
Van Dijk H (1980) Dissipation rates in soil of 1,2-dichloropropane
and 1,3- and 2,3-dichloropropenes. Pestic Sci, 11: 625-632.
Van Duuren BL, Goldschmidt BM, Loevengart G, Smith AC, Melchionne S,
Seidman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63(6): 1433-
1439.
Van Duuren BL, Kline SA, Melchionne S, & Seidman J (1983) Chemical
structure and carcinogenicity relationships of some chloroalkene
oxides and their parent olefins. Cancer Res, 43: 159-162.
Van Hooidonk C (1989) Physico-chemical properties of trans-1,3-
dichloropropene. Rijswijk, The Netherlands, Organization for Applied
Scientific Research (Unpublished proprietary report PML 1989-C118,
submitted to WHO by Shell).
Van Joost Th & De Jong G (1988) Sensitization to DD soil fumigant
during manufacture. Contact Dermatitis, 18(5): 307-308.
Van Sittert NJ (1978) D-D monitoring studies on field workers in
France. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Unpublished proprietary report TOX 78-001, submitted to WHO by
Shell).
Van Sittert NJ (1984) Biomonitoring of chemicals and their
metabolites. In: Berlin A, Draper M, Hemminki K, & Vainio H ed.
Monitoring human exposure to carcinogenic and mutagenic agents.
Lyon, International Agency for Research on Cancer, pp 153-172 (IARC
Scientific Publications No. 59).
Van Sittert NJ (1989) Individual exposure monitoring from plasma or
urinary metabolite determination. Arch Toxicol 13(Suppl): 91-100.
Van Sittert NJ, Van der Harst J, & Verhoeven PF (1977) D-D
monitoring studies on field workers in Holland. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Unpublished proprietary
report TOX 77-001, submitted to WHO by Shell).
Van Welie RTH, Van Duyn P, & Vermeulen NPE (1989) Determination of
two mercapturic acid metabolites of 1,3-dichloropropene in human
urine with gas chromatography and sulphur-selective detection. J
Chromatogr, 496: 463-471.
Van Welie RTH, Van Duyn P, Brouwer DH, Van Hemmen JJ, Brouwer EJ, &
Vermeulen NPE (1991) Inhalation exposure to 1,3-dichloropropene in
the Dutch flower-bulb culture. Part.II. Biological monitoring by
measurement of urinary excretion of two mercapturic acid
metabolites. Arch Environ Contam Toxicol, 20: 6-12.
Varanka I (1979) Effect of some pesticides on the rhythmic adductor
muscle activity of fresh-water mussel larvae. Symp Biol Hung, 19:
177-196.
Venable JR, McClimans CD, Flake RE, & Dimick DB (1980) A fertility
study of male employees engaged in the manufacture of glycerine. J
Occup Med, 22(2): 87-91.
Vithayathil AJ, McClure C, & Myers JW (1983) Salmonella/microsome
multiple indicator mutagenicity test. Mutat Res, 121:(1) 33-37.
Von Der Hude W, Scheutwinkel M, Gramlich U, Fiszler B, & Basler A
(1987) Genotoxicity of three-carbon compounds evaluated in the SCE
test in vitro. Environ Mutagen, 9: 401-410.
Von Der Hude W, Behm C, Gurtler R, & Basler A (1988) Evaluation of
the SOS chromotest. Mutat Res, 203: 81-94.
Waechter JM Jr & Kastl PE (1988) 1,3-Dichloropropene:
Pharmacokinetics and metabolism in Fischer 344 rats following
repeated oral administration. Internal report. Midland, Michigan,
Dow Chemical Company, (Unpublished proprietary report submitted to
WHO by Dow Chemical).
Wallbridge CT, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol, 12:
661-666.
Walker AIT (1968a) The toxicity of D-D soil fumigant (1,2-
dichloropropane: 1,3-dichloropropene). 13-week oral toxicity
experiment in rats. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0018.68, submitted to WHO by Shell).
Walker AIT (1968b) The toxicity of D-D soil fumigant (1,2-
dichloropropane: 1,3-dichloropropene). 13-week oral toxicity
experiment in dogs. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report TLGR 0019.68, submitted to WHO by Shell).
Wallace BG (1974) Development of methods for the determination of
residues of free and bound 3-chloroallylalcohol in soil and crops.
London, Shell Research Ltd (Unpublished proprietary report WKGR
0067.74, submitted to WHO by Shell).
Wallace BG (1976a) Residues of the major components of D-D and
primary metabolites in soil from Germany. London, Shell Research Ltd
(Unpublished proprietary report WKGR 0051.76, submitted to WHO by
Shell).
Wallace BG (1976b) Residues of the major components of D-D and
primary metabolites in soil and crops from Holland. London, Shell
Research Ltd (Unpublished proprietary report WKGR 0052.76, submitted
to WHO by Shell).
Wallace BG (1979) Residues of D-D and 1,3-D in soil from the UK.
London, Shell Research Ltd (Unpublished proprietary report BLGR
79.093, submitted to WHO by Shell).
Watson WP, Lang KL, Brooks TM, Huckle KR, & Wright AS (1986a)
Genotoxicity studies with (Z)-1,3-dichloropropene: Mutagenic
contaminants. Sittingbourne, Shell Research Ltd (Unpublished
proprietary report SBGR 85.246, submitted to WHO by Shell).
Watson WP, Lang KL, Brooks TM, & Wright AS (1986b) Genotoxicity
studies with (Z)-1,3-dichloropropene: Bioactivation by mammalian
mono-oxygenases and the protective role of glutathione.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
SBGR 86.056, submitted to WHO by Shell).
Watson WP, Brooks TM, Huckle KR, Hutson DH, Lang KL, Smith RJ, &
Wright AS (1987) Microbial mutagenicity studies with (Z)-1,3-
dichloropropene. Chem-Biol Interact, 61: 17-30.
Wensley RN (1953) Microbiological studies of the action of some
selected soil fumigants. Can J Bot, 31: 277-308.
WHO (in preparation) Guidelines for drinking-water quality, 2nd ed.
Geneva, World Health Organization.
Williams IH (1968) Recovery of cis- and trans-dichloropropene
residues from 2 types of soil and their detection and determination
by electron capture gas chromatography. J Econ Entomol, 61(5): 1432-
1435.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ
Mutagen, 7: 677-702.
Worthing CR & Hance RJ (1991) The pesticide manual: A world
compendium, 9th ed. Croydon, The British Crop Protection Council, pp
254-255.
Wright AS & Creedy CL (1982) The protective action of glutathione on
the microbial mutagenicity of (Z)-1,3-dichloropropene.
Sittingbourne, Shell Research Ltd (Unpublished proprietary report
SBGR 81.317, submitted to WHO by Shell).
Yakel HO & Kociba RJ (1977) Acute inhalation toxicity of M-3993
(Telone II) in rats. Internal report. Midland, Michigan, Dow
Chemical Company (Unpublished proprietary report submitted to WHO by
Dow Chemical).
Yang RSH (1986) 1,3-Dichloropropene. Residue Rev, 97: 19-35.
Yang RSH, Huff JE, Boorman GA, Haseman JK, Kornreich M, & Stookey JL
(1986) Chronic toxicology and carcinogenesis studies of Telone II by
gavage in Fischer 344 rats and B6C3F1 mice. J Toxicol Environ
Health, 18: 377-392.
Yano BL, Calhoun LL, Stott W, Johnson KA, & Schuetz DJ (1985) Telone
II soil fumigant: 2 year inhalation chronic toxicity-oncogenicity
study in mice. Interim report: 6- and 12-month exposures. Internal
report. Midland, Michigan, Dow Chemical Company (Unpublished
proprietary report submitted to WHO by Dow Chemical).
Yon DA, Morrison GA, & McGibbon, A.S. (1991) The dissipation of 1,3-
dichloropropene in ditch bottom sediment and associated aerobic
ditch water. Pestic Sci, 32: 147-159.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1,3-DICHLOROPROPENE
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le "1,3-dichloropropène" a été introduit en agriculture en
1956, mélangé à des 1,3-dichloropropènes, du 1,2-dichloropropane et
d'autres hydrocarbures halogénés. On l'utilise depuis largement
comme fumigant du sol avant plantation pour lutter contre les
nématodes qui parasitent les légumes, les pommes de terre et le
tabac. L'application s'effectue essentiellement par injection dans
le sol. La formulation du commerce consiste en un mélange d'isomères
cis et trans (en proportions approximativement égales), et se
présente sous la forme d'un liquide incolore à ambré dont l'odeur
pénétrante et irritante rappelle celle du chloroforme. Sa tension de
vapeur est de 3.7 kPa à 20 °C. Le produit technique a une pureté de
92% et peut contenir certaines impuretés, comme le 1,2-
dichloropropane. Le coefficient de partage octanol/eau (log Kow)
est égal à 1,98.
Dans l'air, la décomposition du 1,3-dichloropropène s'effectue
principalement par réaction avec des radicaux libres et l'ozone.
Dans le cas de la réaction avec les radicaux libres, la demi-vie des
isomères cis- et trans- est respectivement égale à 12 et 7
heures et dans le cas de la réaction avec l'ozone, de 52 et 12
jours. Il semble que la phototransformation directe soit négligeable
mais elle pourrait être favorisée par la présence de particules en
suspension dans l'atmosphère.
Dans l'eau, il est probable que le 1,3-dichloropropène
disparaît rapidement du fait de sa solubilité relativement faible et
de sa forte volatilité; on a fait état d'une demi-vie de moins de 5
heures.
La distribution du 1,3-dichloropropène dans les différents
compartiments du sol dépend de la tension de vapeur, du coefficient
de diffusion, de la température et de la teneur en eau. La
persistance du 1,3-dichloropropène dans le sol dépend de sa
volatilisation, des transformations chimiques, photo-chimiques ou
biologiques qu'il subit et de sa fixation par les êtres vivants. La
volatilisation et la diffusion en phase gazeuse sont les mécanismes
les plus importants de sa dispersion et de la dilution dans le
milieu.
Dans l'environnement, la transformation du 1,3-dichloropropène
commence par une hydrolyse en alcool 3-chloro-allylique puis, sous
l'action des microorganismes, en 3-chloro-acroléine et en acide 3-
chloro-acrylique. Une étude de laboratoire a montré que le temps de
demi-hydrolyse des isomères cis- et trans- du 1,3-
dichloropropène à 15 °C et à 29 °C était respectivement égal, pour
l'isomère cis, à 11 et 2 jours et pour l'isomère trans, à 13 et
2 jours. Dans le sol, à un pH de 7, on a observé un temps de demi-
hydrolyse à 25 °C de 4,6 jours pour les deux isomères. Du fait que
le composé disparaît relativement vite du sol, il est peu probable
que des résidus s'y accumulent après application du fumigant à la
dose et selon la périodicité recommandées.
Le 1,3-dichloropropène est potentiellement mobile dans le sol,
en particulier dans les sols sableux à texture lâche dont la teneur
en eau est faible. Son cheminement en profondeur est favorisé par
les cultures profondes dans des sols de faible porosité. On a décelé
du 1,3-dichloropropène dans les nappes souterraines peu profondes
(jusqu'à 2 m en-dessous de la surface) mais non dans les eaux
profondes, c'est-à-dire celles qui ont le plus de chances d'être
utilisées pour la consommation humaine.
Le 1,3-dichloropropène peut-être fixé par les plantes
cultivées. Toutefois, il est peu probable qu'il donne lieu à des
résidus importants dans les cultures vivrières car celles-ci sont en
principe plantées lorsque la majeure partie du fumigant s'est
dissipée.
La bioaccumulation du 1,3-dichloropropène est peu probable car
il possède une solubilité dans l'eau relativement forte (> 1 g/kg),
un coefficient de partage octanol/eau faible (log Kow) et il est
rapidement éliminé chez les mammifères et autres organismes.
1.2 Cinétique et métabolisme
Après administration par voie orale à des rongeurs, le 1,3-
dichloropropène est rapidement éliminé. La principale voie
d'élimination est la voie urinaire, avec 81% de l'isomère cis et
56% de l'isomère de trans excrétés dans les 24 heures suivant
l'administration. La demi-vie d'élimination dans l'urine est de 5 à
6 heures. L'élimination dans les matières fécales est minime. Le
1,3-dichloropropène est éliminé à hauteur de 4% (isomère cis) et
de 24% (isomère trans) dans le dioxyde de carbone expiré. Après
l'administration, les concentrations tissulaires sont faibles; les
concentrations résiduelles les plus élevées se retrouvent dans la
paroi gastrique, puis, à des valeurs plus faibles, dans les reins,
le foie et la vessie.
On ne retrouve pas de 1,3-dichloropropène non modifié dans les
urines. Les isomères cis et trans tiennent lieu de substrats à
la glutathion- S-alkyltransférase hépatique qui les transforme en
acides mercapturiques, excrétés ensuite dans les urines. Le
principal métabolite urinaire chez le rat et la souris est la N-
acétyl- S-(3-chloroprop-2-ényl)L-cystéine; ce composé peut être
utilisé pour la surveillance biologique chez l'homme. On a observé
une deuxième voie métabolique d'importance secondaire dans le cas de
l'isomère cis; il s'agit d'une mono-oxygénation en cis-1,3-
dichloropropène oxyde, composé qui peut ensuite être conjugué avec
le glutathion. La forte proportion d'isomère trans présente dans
l'air expiré résulte d'une autre voie métabolique conduisant à la
conjugaison, voie qui est plus spécifique de l'isomère trans que
de l'isomère cis.
Exposés par voie respiratoire à du 1,3-dichloropropène, des
rats n'ont pas présenté une augmentation du taux sanguin
proportionnelle à la dose. A la dose de 408,6 mg/m3 (90 ppm), la
fréquence respiratoire et le volume expiratoire-minute étaient
réduits et l'on notait une saturation du métabolisme à 1362 mg/m3
(300 ppm). Les isomères cis et trans ont été rapidement éliminés
du courant sanguin, avec une demi-vie d'élimination de 3 à 6 minutes
pour des concentrations inférieures à 1362 mg/m3, mais beaucoup
plus longue (33 à 43 minutes) à plus forte concentration.
1.3 Effets sur les êtres vivant dans leur milieu naturel
Les valeurs de la CE50 relatives à la croissance (à 96 h)
chez une algue d'eau douce, Selenastrum capricornutum, et chez une
diatomée estuarielle, Skeletoneria costatum, sont respectivement
égales à 4,95 mg/litre et 1 mg/litre. Pour les poissons, la toxicité
aiguë du 1,3-dichloropropène (CL50 à 96 h), est de l'ordre de 1 à
7,9 mg/litre. Un test effectué sur les stades embryo-larvaires d'un
vairon, Pimephales promelas, ont donné une dose maximale sans
effet observable de 0,24 mg/litre. Ces données, jointes au fait que
le 1,3-dichloropropène ne persiste vraisemblablement pas dans l'eau,
indiquent que le danger pour les poissons réside dans les effets
toxiques aigus de ce composé, mais qu'il y a peu de risques d'effets
supplémentaires résultant d'une exposition à long terme.
Aux doses de 30 à 60 mg/kg, le 1,3-dichloropropène peut réduire
l'abondance des champignons et l'activité des enzymes microbiennes,
mais cet effet n'est généralement pas de longue durée (< 7 jours)
et ne se produit pas dans tous les types de sol. Certaines études
ont fait ressortir un accroissement sensible du nombre de
microorganismes après application du fumigant.
Le 1,3-dichloropropène est phytotoxique mais en revanche, il
est peu toxique pour les abeilles. On a constaté, en épandant du
1,3-dichloropropène par poudrage, que la DL50 à 48 h était de 6,6
µg/abeille. Les oiseaux sont relativement insensibles au 1,3-
dichloropropène. La CL50 (8 jours) est inférieure à 10 g/kg de
nourriture pour le col-vert et les cailles du genre Colinis.
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale du 1,3-dichloropropène est
modérée à forte chez les animaux d'expérience. En ce qui concerne le
rat, on a fait état, pour la DL50, de valeurs se situant entre 127
et 713 mg/kg de poids corporel. Par voie orale, ces valeurs étaient
respectivement de 85 et 94 mg/kg de poids corporel pour les isomères
cis et trans.
En cas d'exposition cutanée, le composé est modérément toxique.
Chez le rat et le lapin, on a obtenu pour la DL50 des valeurs
respectivement égales à 423 et 504 mg/kg de poids corporel. Ces
valeurs étaient respectivement de 1090 mg pour l'isomère cis et de
1575 mg/kg de poids corporel pour l'isomère trans.
Chez des rats exposés pendant 4 h par la voie respiratoire à du
1,3-dichloropropène, on a obtenu une CL50 de 3310 mg/m3 (729
ppm); les valeurs allaient de 3042 mg/m3 à 3514 mg/m3 pour
l'isomère cis et de 4880 mg/m3 à 5403 mg/m3 pour l'isomère
trans.
En cas d'intoxication aiguë, on observe une atteinte nerveuse
centrale et une atteinte respiratoire.
Des réactions graves ont été observées chez le lapin lors
d'épreuves d'irritation cutanée et oculaire mais les animaux ont
récupéré en l'espace de 14 à 21 jours. Les épreuves de
sensibilisation cutanée chez le cobaye se sont révélées positives.
Plusieurs études de toxicité respiratoire à court terme ont été
effectuées sur des souris, des rats, des cobayes, des lapins et des
chiens. Chez la souris, c'est les muqueuses nasales et la vessie qui
étaient les organes cibles. On a observé une dégénérescence de
l'épithélium olfactif et une hyperplasie de l'épithélium
respiratoire. Au niveau de la vessie, on a observé une hyperplasie
modérée de l'épithélium de transition. On a pu fixer à 136 mg/kg,
soit 30 ppm, la dose sans effet observable chez la souris.
Chez le rat, on a observé également une hyperplasie ainsi que
des modifications dégénératives analogues au niveau de l'épithélium
olfactif. Une étude bien conçue a permis d'obtenir une valeur de
4,35 mg/m3 pour la dose sans effet observable; dans le cas précis
de l'isomère cis, cette dose était égale à 136 mg/m3.
Une étude de 90 jours consistant à administrer le composé par
voie orale à des rats a permis de fixer à 3 mg/kg de poids corporel
la dose sans effet observable. Le seul effet observé à la dose
immédiatement supérieure (10 mg/kg de poids corporel) consistait en
une augmentation du poids relatif des reins chez les mâles.
Une étude de reproduction portant sur deux générations et deux
portées de rats, à des doses allant jusqu'à 408,6 mg/m3 (90 ppm),
n'a pas fait ressortir d'effets indésirables sur les paramètres
examinés. Toutefois, la dose la plus forte (408,6 mg/m3) était
toxique pour les mères, comme l'ont montré les deux anomalies
observées: réduction de la croissance et modification
histopathologique au niveau de la muqueuse nasale. Les résultats de
cette étude ont permis de fixer à 136,2 mg/m3 (30 ppm) la dose
maximale sans effet toxique observable chez les mères.
Des études de tératogénicité au cours desquelles du 1,3-
dichloropropène a été administré à des rats et à des lapins par la
voie respiratoire, n'ont pas permis de relever d'indices de
tératogénicité jusqu'à la dose de 1362 mg/m3, mais on a observé
une embryotoxicité chez le rat (réduction de la taille des portées
et augmentation du taux de résorption). Le composé s'est révélé
toxique pour les mères, tant chez les rattes que chez les lapines,
aux doses supérieures ou égales à 544,8 mg/m3 (120 ppm).
Dans la plupart des études, les isomères cis et trans du
1,3-dichloropropène, ainsi que les mélanges, se sont révélés
mutagènes chez les bactéries, sans ou avec activation métabolique. A
l'état pur, le 1,3-dichloropropène et le cis-1,3-dichloropropène
étaient négatifs à cet égard chez les bactéries. On a montré que le
glutathion bloquait l'activité mutagène du 1,3-dichloropropène chez
les bactéries. Lors d'une épreuve de mutation génique sur des
cellules V79 de hamster chinois ainsi que dans une épreuve HGPRT sur
des cellules ovariennes du même animal, le cis-1,3-dichloropropène
a donné des résultats négatifs.
Le cis- et le trans-1,3-dichloropropène provoquent une
synthèse non programmée de l'ADN dans les cellules HeLa S3. Dans
des hépatocytes de rat, le 1,3-dichloropropène n'a pas entraîné de
réparation importante de l'ADN. L'épreuve rec sur des microsomes de
la souche H17 de Bacillus subtilis a donné un résultat positif
avec le 1,3-dichloropropène en présence d'activation métabolique.
Dans des cellules ovariennes de hamster chinois, le cis- et
le trans-1,3-dichloropropène ont entraîné des lésions
chromosomiques en présence d'activation métabolique; toutefois dans
une autre étude, le 1,3-dichloropropène a produit les mêmes effets
sans activation métabolique. Le cis-1,3-dichloropropène n'a pas
provoqué de lésions chromosomiques dans des cellules de foie de rat,
mais il a entraîné des échanges entre chromatides soeurs dans des
cellules ovariennes de hamster chinois, en présence ou en l'absence
d'activation métabolique, ainsi que dans des cellules V79 du même
animal, cette fois sans activation métabolique.
Une épreuve de recherche des micronoyaux dans la moelle osseuse
de souris a donné des résultats négatifs avec le 1,3-
dichloropropène. Les résultats ont été également négatifs dans le
cas d'une épreuve de mutation létale récessive liée au sexe sur
Drosophila melanogaster.
Des études de cancérogénicité ont été effectuées sur des souris
et des rats. On leur a administré par gavage pendant deux ans du
1,3-dichloropropène technique contenant 1% d'épichlorhydrine. Chez
les souris, on a noté un accroissement sensible des hyperplasies
épithéliales ainsi que des carcinomes de type transitionnel au
niveau de la vessie, un accroissement des tumeurs pulmonaires, une
légère augmentation des tumeurs hépatiques et, au niveau de la
portion cardiaque de l'estomac, une augmentation de l'hyperplasie
épithéliale ainsi que des papillomes ou des carcinomes spino-
cellulaires. Chez le rat, on a observé une augmentation de
l'incidence des nodules néoplasiques au niveau du foie ainsi que des
papillomes ou des carcinomes spino-cellulaires dans la portion
cardiaque de l'estomac.
Une étude par inhalation de deux ans a permis d'étudier la
cancérogénicité du 1,3-dichloropropène (sans épichlorhydrine) sur
des rats et des souris. Chez les souris, on a observé une incidence
accrue des hyperplasies, au niveau de la vessie, de la portion
cardiaque de l'estomac et des muqueuses nasales. Il y avait
également augmentation dans l'incidence des tumeurs pulmonaires
bénignes. Un certain nombre de modifications d'origine toxique ont
été constatées dans la muqueuse olfactive nasale chez le rat, mais
sans accroissement de l'incidence tumorale.
On a montré que l'épichlorhydrine provoquait des tumeurs de la
partie proximale de l'estomac lors d'une étude où cette substance
était administrée par gavage ainsi que des tumeurs des fosses
nasales lors d'une étude par inhalation sur des rats; les résultats
de l'étude au cours de laquelle du 1,3-dichloropropène a été
administré par voie orale à des souris ne permettent pas d'exclure
un effet cancérogène sur la vessie.
Mode d'action
Etant donné que la principale voie métabolique d'élimination du
1,3-dichloropropène consiste dans une conjugaison avec le
glutathion, on peut penser que toute situation affectant la
concentration en glutathion tissulaire (groupements sulfhydriles non
protéiques) est susceptible de modifier les effets du composé. Le
1,3-dichloropropène lui-même provoque une déplétion en glutathion
dans les divers tissus, en particulier ceux qui constituent la porte
d'entrée dans l'organisme, c'est-à-dire essentiellement la portion
cardiaque de l'estomac et le foie dans le cas où l'administration se
fait par gavage et les tissus des fosses nasales dans le cas des
études par inhalation. On a observé chez la souris une diminution du
glutathion, au niveau de l'épithélium nasal aux doses supérieurs
22,7 mg/m3 (5 ppm) et au niveau de la portion cardiaque de
l'estomac aux doses supérieurs à 113,5 mg/m3 (25 ppm).
La toxicité du 1,3-dichloropropène pour les animaux
d'expérience se manifeste lorsque l'exposition entraîne une
déplétion en glutathion et une réduction préalable de la teneur en
glutathion tissulaire exacerbe cet effet toxique. L'inhalation
pendant une longue période de concentrations supérieures à 90,8
mg/m3 (20 ppm) entraîne une dégénérescence et une hyperplasie de
l'épithélium nasal et stomacal chez la souris; chez le rat et dans
les mêmes conditions, la dose de 272,4 mg/m3 (60 ppm) a également
provoqué une dégénérescence du tissu des fosses nasales.
Le rôle protecteur du glutathion a également été mis en lumière
par des études chez la souris qui ont montré que lorsque la teneur
en groupements sulfhydriles non protéiques diminuait, il y avait
augmentation du taux de liaison covalente du dichloropropène
radiomarqué (au carbone 14) aux cellules de la portion cardiaque de
l'estomac. De même, on a observé, dans des systèmes d'épreuves in
vitro, que la présence de glutathion réduisait sensiblement la
génotoxicité du 1,3-dichloropropène et d'un des ses métabolites
mineurs d'oxydation (cytochrome P-450), à savoir le 1,3-
dichloropropène-oxyde.
1.5 Effets sur l'homme
Il est improbable qu'il y ait exposition de la population
générale par l'intermédiaire de l'air, de l'eau ou des aliments.
On a montré que l'exposition professionnelle se situe en
général en-dessous de 4,54 mg/m3 (1 ppm), mais on a également fait
état de concentrations plus élevées (jusqu'à 18,3 mg/m3 lors
d'opérations de remplissage ou de changement de buses). L'exposition
professionnelle se produit vraisemblablement par la voie
respiratoire ou par la voie cutanée. Très peu de temps après
l'exposition, il y a irritation des yeux et des muqueuses des voies
respiratoires supérieures. On a observé de graves symptômes
d'intoxication après inhalation d'air contenant des concentrations
supérieures à 6810 mg/m3 (> 1500 ppm); à plus faible
concentration, il y avait dépression du système nerveux central et
irritation des voies respiratoires. L'exposition de la peau entraîne
également de graves irritations à ce niveau.
Chez un groupe de personnes chargées d'épandre du 1,3-
dichloropropène, on a observé en fin de saison un certain nombre
d'anomalies de la fonction hépatique et rénale. Toutefois,
l'existence d'une relation de cause à effet reste controversée.
Il y a eu des cas d'intoxication qui ont entraîné
l'hospitalisation des intéressés avec des symptômes d'irritation des
muqueuses, une sensation de gêne thoracique, des maux de tête, des
nausées, des vomissements, des vertiges et parfois une perte de
conscience et une diminution de la libido. En outre, trois cas
d'affections hématologiques malignes ont été attribués à une
surexposition accidentelle antérieure au 1,3-dichloropropène, mais
là encore, l'existence d'une relation de cause à effet reste
incertaine.
En comparant à un groupe témoin la fécondité d'employés
travaillant à la production d'hydrocarbures chlorés à trois atomes
de carbone, on n'a pas mis en évidence d'association entre
l'exposition et une réduction éventuelle de la fécondité.
2. Conclusions
Population générale: Du fait que l'exposition au 1,3-dichloropropène
est faible voire inexistante, le risque pour la population générale
est négligeable.
Exposition professionnelle: Lors d'opérations de remplissage et lors
des épandages, il peut y avoir exposition des opérateurs à des
concentrations dépassant la limite maximale autorisée, si des
mesures de sécurité appropriées ne sont pas prises.
Environnement: Dans la mesure où le 1,3-dichloropropène est utilisé
à la dose recommandée, il est vraisemblable qu'il ne s'accumulera
pas dans l'environnement à des concentrations susceptibles de poser
un problème écologique et il est improbable qu'il puisse avoir des
effets nocifs sur les organismes terrestres et aquatiques.
3. Recommandations
* Les opérations de remplissage et l'épandage du 1,3-
dichloropropène doivent obligatoirement s'accompagner des
mesures de sécurité appropriées afin de faire en sorte que
l'exposition ne dépasse pas les concentrations maximales
autorisées.
* Il faudrait étudier la destinée métabolique du trans-1,3-
dichloropropène chez les mammifères ainsi que le rôle que
pourraient avoir les métabolites d'oxydation de cet isomère
dans la toxicité du composé.
* L'effet protecteur du glutathion vis-à-vis du 1,3-
dichloropropène est dû à l'action de la glutathion-transférase.
Il est donc recommandé de procéder à des études afin de
comparer la cinétique de l'action enzymatique de la glutathion-
S-transférase humaine provenant des divers tissus à
l'activité de l'enzyme d'origine animale provenant des tissus
correspondants.
* Il conviendrait de rassembler et de publier les données dont on
dispose sur le rôle protecteur du glutathion.
* La génotoxicité du dichloropropène est due pour une part à son
métabolisme oxydatif. Il est recommandé d'entreprendre des
études pour identifier l'isoenzyme responsable et la comparer à
l'activité des isoenzymes du cytochrome P-450 humain.
* Dans les études de cancérogénicité où l'on procède par gavage
des animaux, il conviendrait d'élucider le rôle de
l'épichlorhydrine en tant que facteur de confusion éventuel.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1,2-DICHLOROPROPANE
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le 1,2-dichloropropane est un liquide dont le point
d'ébullition est de 96,8 °C et la tension de vapeur de 42 mmHg à 20
°C. Il est soluble dans l'eau, l'éthanol et l'éther éthylique. Par
chauffage, il émet des vapeurs de phosgène hautement toxiques. Son
coefficient de partage entre l'octanol et l'eau (log Kow) est égal
à 2,28.
Ce produit entre dans la composition des vernis pour meubles,
des liquides de nettoyage à sec, des décapants pour peintures, des
produits pour le dégraissage des surfaces métalliques, le traitement
des huiles; il sert à la fabrication de caoutchoucs et de cires,
ainsi que comme intermédiaire dans la production du
tétrachloréthylène et du tétrachlorure de carbone. Il entre
également dans la composition du mélange appelé D/D que l'on utilise
comme fumigant avant la plantation.
Les mesures de la concentration du 1,2-dichloropropane dans
l'air urbain ont donné des valeurs respectives de 1,2 µg/m3
(valeur moyenne), 0,021-0,040 µg/m3 et 0,0065-1,4 µg/m3
respectivement à Philadelphie, Portland et au Japon. La
décomposition dans l'atmosphère est lente; sur la base de la
réaction avec les radicaux hydroxyles, on a évalué le temps de demi-
décomposition du 1,2-dichloropropane à plus de 313 jours. Il est
probable que la décomposition est essentiellement de nature
photochimique. Pour que cette décomposition photochimique soit
appréciable, il est nécessaire que le composé soit adsorbé sur des
particules. C'est, semble-t-il, principalement par volatilisation
que le 1,2-dichloropropane s'élimine de l'eau.
Dans le sol, les principales voies d'élimination sont la
volatilisation et la diffusion. Le 1,2-dichloropropane persiste dans
le sol. Plus de 98% du 1,2-dichloropropane appliqué sur du terreau
ont été récupérés 12 à 20 semaines après ce traitement.
Il peut y avoir lessivage du 1,2-dichloropropane présent dans
le sol et contamination des eaux souterraines à faible ou grande
profondeur dans les secteurs traités avec des fumigants du type "MIX
D/D". Aux Etats-Unis d'Amérique, on a trouvé des concentrations dans
l'eau de puits et les eaux souterraines allant respectivement
jusqu'à 440 µg/litre et 51 µg/litre. Aux Pays-Bas, des
concentrations atteignant 160 µg/litre ont été observées dans l'eau
de puits et on a retrouvé du 1,2-dichloropropane jusqu'à une
profondeur de 13 mètres.
Les plantes vivrières peuvent fixer le 1,2-dichloropropane mais
les résidus qu'on y a décelés sont faibles (< 0,01 mg/kg) et
probablement sans conséquence biologique.
La bioaccumulation du 1,2-dichloropropane est improbable en
raison de sa forte solubilité dans l'eau (2,7 g/kg) et de la faible
valeur de son coefficient de partage entre l'octanol et l'eau (log
Kow).
1.2 Cinétique et métabolisme
Administré par voie orale à des rats, le 1,2-dichloropropane
est rapidement éliminé (80 à 90% en 24 h). Il n'y a pas de
différences majeures dans la cinétique ou dans l'élimination entre
les mâles et les femelles. La principale voie d'élimination est la
voie urinaire, et jusqu'à la moitié de la dose orale initiale est
éliminée dans les 24 h. La proportion éliminée par la voie fécale
est inférieure à 10%. Le 1,2-dichloropropane est éliminé à hauteur
de 33% dans l'air expiré, à la fois sous forme de dioxyde de carbone
et d'un mélange de produits volatils. Les concentrations tissulaires
sont faibles, la plus élevée étant observée au niveau du foie. Après
exposition de rats par la voie respiratoire on note une élimination
rapide du 1,2-dichloropropane; 55 à 65% de la dose initiale sont
éliminés dans les urines et 16 à 23% dans l'air expiré. La demi-vie
d'élimination à partir du sang est de 24 à 30 minutes.
On ne retrouve pas le 1,2-dichloropropane initial dans les
urines. On y a identifié trois métabolites principaux. Ces
métabolites résultent de l'oxydation et de la conjugaison du composé
et aboutissent à la formation de mercapturates, de N-acétyl- S-
(2-hydroxypropyl)-L-cystéine, de N-acétyl- S-(2-oxypropyl)-L-
cystéine et de N-acétyl- S-(1-carboxyéthyl)-L-cystéine. Le 1,2-
dichloropropane peut également subir une oxydation en lactate avec
production de dioxyde de carbone ou d'acétyl-coenzyme A.
Après administration de 1,2-dichloropropane par voie orale à
des rats à raison de 2 mg/kg, on a constaté une forte diminution du
glutathion tissulaire. Il y avait également corrélation entre la
diminution du glutathion tissulaire et les manifestations toxiques
au niveau du foie, des reins et des hématies. Une réduction
préalable du glutathion intracellulaire a provoqué une exacerbation
de la toxicité du 1,2-dichloropropane, alors qu'un traitement
préalable par des précurseurs de la synthèse du glutathion réduisait
cette toxicité. Ces résultats montrent que le glutathion a un effet
protecteur vis-à-vis des propriétés toxiques du 1,2-dichloropropane.
1.3 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas déterminé la CE50 pour les algues d'eau douce car
la volatilisation du composé à partir de la solution d'épreuve rend
cette détermination difficile. La toxicité aiguë du 1,2-
dichloropropane pour les invertébrés aquatiques et les poissons est
faible à modérée; pour les invertébrés, les valeurs de la CL50 à
48 h varient de 52 à > 100 mg/litre, tandis que la CL50 à 96 h pour
les poissons se situe entre 61 et 320 mg/litre. Une épreuve de
toxicité à court terme effectuée sur des vairons du genre
Pimephales promelas a montré que la dose maximale sans effet était
de 82 mg/litre. Lors d'une épreuve de 32 jours sur les larves de ce
vairon, on a constaté que les paramètres biologiques les plus
sensibles à la toxicité du 1,2-dichloropropane étaient la croissance
des larves et leur survie. On estime que la concentration maximale
acceptable de substance toxique est de 6 à 11 mg/litre. Chez des
poissons du genre Pimelometopon on a constaté une inhibition de la
croissance après exposition de 33 jours à une concentration de 164
mg/litre.
Le 1,2-dichloropropane est phytotoxique.
Des épreuves par contact effectuées sur quatre espèces de
lombrics ont montré que la CL50 se situait entre 44 et 84 µg/cm2
(valeur moyenne) de papier filtre. Sur sol artificiel, les valeurs
de la CL50 oscillaient entre 3880 et 5300 mg/kg de sol (en poids
sec).
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Chez les animaux d'expérience, la toxicité aiguë par voie orale
de ce composé est faible. Ainsi, la DL50 par voie orale est de 1,9
g/kg de poids corporel pour le rat et la DL50 cutanée est de 8,75
mg/kg de poids corporel chez le lapin.
Des études de toxicité de courte durée comportant
l'administration de 1,2-dichloropropane par voie orale à des souris
et à des rats ont montré qu'à des doses quotidiennes égales ou
supérieures à 250 mg/kg de poids corporel, il y avait inhibition de
la croissance, apparition de signes cliniques d'intoxication
correspondant à une dépression du système nerveux central et
accroissement de la mortalité. A la dose quotidienne de 250 mg/kg
pendant dix jours, on a noté chez des rats une modification des
enzymes sériques trahissant une légère hépatotoxicité, la dose sans
effet observable étant de 100 mg/kg par jour.
Lors d'une étude par inhalation de 13 semaines effectuée sur
des souris (à la dose maximale de 681 mg/m3), on n'a pas observé
d'effets nocifs. Lors d'une étude analogue sur des rats exposés à
des doses de 68,1, 227 et 681 mg/m3, on a observé une réduction du
poids corporel et des lésions mineures du tissu des fosses nasales
dans les groupes soumis aux deux plus fortes doses.
Lors d'une étude de reproduction portant sur deux générations
de rats, on a donné aux animaux une eau de boisson contenant des
concentrations de 1,2-dichloropropane respectivement égales à 0,024,
0,1, 0,24% (soit l'équivalent de 33,6, 140 et 336 mg/kg de poids
corporel par jour); il en est résulté une réduction du gain de poids
maternel et une diminution de la consommation d'eau, à la dose
médiane et à la plus forte dose. Chez les animaux nouveaunés, le
poids corporel était réduit à la dose la plus forte. La dose sans
effet nocif observable s'établissait respectivement à 33,6 et 140
mg/kg de poids corporel par jour pour les effets toxiques sur la
mère et sur la fonction de reproduction.
Les études ne mettent en évidence aucune activité tératogène du
1,2-dichloropropane à des doses orales allant jusqu'à 125 mg/kg de
poids corporel chez le rat et 150 mg/kg de poids corporel chez le
lapin. Toutefois à ces doses, on a observé une toxicité du produit
pour les mères et pour les foetus, à en juger d'après certains
signes cliniques témoignant d'une atteinte du système nerveux
central, la réduction du gain de poids maternel et, chez les foetus,
un retard d'ossification. La dose sans effet observable est égale à
30 mg/kg de poids corporel par jour chez le rat et à 50 mg/kg de
poids corporel par jour chez le lapin.
La plupart des études ont mis en évidence une mutagénicité du
1,2-dichloropropane chez les bactéries avec ou sans activation
métabolique, mais il est vrai qu'on avait utilisé des doses
extrêmement élevées (jusqu'à 10 mg/boîte). Le 1,2-dichloropropane
provoque des aberrations chromosomiques et des échanges entre
chromatides soeurs dans les cellules ovariennes de hamster chinois;
il y a également accroissement des échanges entre chromatides soeurs
dans des cellules V79 de hamster chinois en présence de 1,2-
dichloropropane. Des lymphocytes humains ont été cultivés en
présence ou en l'absence d'un système métabolisant de foie de rat;
on a constaté que ces cellules fixaient la thymidine tritiée de la
même manière que les cultures témoins et qu'elles présentaient la
même viabilité. Une épreuve de mutation létale récessive liée au
sexe effectuée sur Drosophila melanogaster a donné des résultats
négatifs. Une épreuve de létalité dominante chez des rats soumis
pendant 14 semaines à des doses de 1,2-dichloropropane mêlé à leur
eau de boisson, puis accouplés au cours des deux semaines suivantes,
a donné des résultats également négatifs.
Lors d'une étude de cancérogénicité effectuée sur des souris,
on a administré aux animaux par gavage, 125 ou 250 mg de 1,2-
dichloropropane par kg de poids corporel; on a observé une
augmentation, liée à la dose, de l'incidence des adénomes
hépatiques. L'incidence des adénomes était plus élevée dans les
groupes traités que dans le groupe témoin mais elle se situait
malgré tout dans les limites normales pour les témoins historiques.
Chez des rats soumis à des doses de 125 et de 250 mg/kg de
poids corporel (femelles) ou 62 et 125 mg/kg de poids corporel
(mâles) par gavage, cinq jours par semaines pendant 113 semaines, on
a noté une légère augmentation dans l'incidence des adénocarcinomes
mammaires chez les femelles soumises à la dose la plus forte,
augmentation qui était supérieure aux limites normales pour les
témoins historique.
1.5 Effets sur l'homme
Il est improbable que la population générale soit exposée au
1,2-dichloropropane par l'intermédiaire de l'air et de l'eau, sauf
dans les zones où l'on utilise largement le 1,2-dichloropropoane ou
le "D/D MIX" à des fins agricoles. Les résidus de 1,2-
dichloropropane présents dans les plantes vivrières sont
généralement inférieurs à la limite de détection. L'exposition étant
faible, on peut considérer que le risque est négligeable pour la
population générale.
On a signalé plusieurs cas d'intoxication aiguë accidentelle ou
intentionnelle (suicide) dus à une surexposition au 1,2-
dichloropropane. Les effets en étaient essentiellement observables
au niveau du système nerveux central, du foie et des reins. On a
également fait état d'une anémie hémolytique et d'une coagulation
intravasculaire disséminée. Dans un cas, le malade se trouvait dans
un état de délire qui a évolué vers un état de choc irréversible et
une insuffisance cardiaque fatale.
Il peut y avoir exposition professionnelle par voie cutanée ou
respiratoire. On a fait état de plusieurs cas de dermatite ou de
sensibilisation cutanée chez des travailleurs qui utilisaient des
solvants contenant du 1,2-dichloropropane.
2. Conclusions
* Population générale: L'exposition de la population générale au
1,2-dichloropropane à partir de l'air ou de la nourriture est
faible, voire inexistante. Toutefois dans certains secteurs, il
peut y avoir exposition en cas de contamination des eaux
souterraines.
* Exposition professionnelle: Moyennant de bonnes méthodes de
travail et des précautions d'hygiène et de sécurité, il est peu
probable que l'utilisation du 1,2-dichloropropane comporte un
risque pour les personnes qui y sont exposées de par leur
profession.
* Environnement: Il est improbable que le 1,2-dichloropropane
s'accumule dans l'environnement à des concentrations
écologiquement nocives lorsqu'on l'utilise à la dose
recommandée. Il est également improbable qu'il produise des
effets nocifs sur les populations d'organismes terrestres ou
aquatiques.
3. Recommandations
* Il faudrait évaluer la toxicité aiguë par voie respiratoire
ainsi que le pouvoir irritant pour les yeux et la peau et le
pouvoir sensibilisant cutané de ce composé.
* Lorsqu'on manipule du 1,2-dichloropropane il faut prendre des
mesures de sécurité appropriées afin d'éviter toute exposition
supérieure à la concentration maximale admissible.
RESUME ET EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
"MIX D/D"
1. Résumé et évaluation
1.1 Usage, destinée et concentrations dans l'environnement
Le mélange technique de dichloropropènes et de dichloropropane
(désignés dans la suite du texte par l'abréviation "MIX D/D") est un
liquide limpide de couleur ambrée doté d'une odeur piquante; sa
tension de vapeur est de 35 mmHg à 20 °C et il est soluble dans les
solvants halogénés, les esters et les cétones.
Le MIX D/D présente une composition caractéristique, à savoir:
au moins 50% de 1,3-dichloropropènes (proportion des isomères cis-
et trans-, environ 1/1), les autres constituants principaux étant
le 1,2-dichloropropane et les composés voisins. On l'a beaucoup
utilisé comme nématocide en application sur le sol avant la
plantation.
Le transport, la distribution et la destinée des principaux
constituants du MIX D/D dans l'air, l'eau et le sol sont décrits à
la section 4 des chapitres consacrées au 1,3-dichloropropène et au
1,2-dichloropropane.
Le 1,2-dichloropropane provenant du MIX D/D présent dans le sol
a une certaine tendance à contaminer l'eau des puits et les eaux
souterraines en général, par suite d'un phénomène de lessivage. Lors
d'une opération de forage à des fins d'irrigation en Europe
occidentale (68 m de profondeur) on a constaté que les
concentrations moyennes de 1,2-dichloropropane à différentes
profondeurs variaient entre 0,8 et 8,5 µg/litre, la concentration la
plus élevée étant de 165 µg/litre.
Il est peu probable que les cultures fixent en proportion
importante les constituants du MIX D/D (voir les autres chapitres de
la présente monographie). Il est également peu probable que ces
constituants subissent une bioaccumulation du fait que leur
coefficient de partage entre l'octanol et l'eau (log Kow) est
faible et que leur solubilité dans l'eau est relativement forte.
1.2 Cinétique et métabolisme
On n'a pas procédé à des études métaboliques sur le MIX D/D.
Les deux principaux constituants, le 1,3-dichloropropène et le 1,2-
dichloropropane sont rapidement éliminés, essentiellement dans
l'urine et en moindre proportion, dans l'air expiré. Les
constituants du MIX D/D sont métabolisés selon un processus qui
comporte une oxydation et une conjugaison. Les principaux
métabolites urinaires sont des acides mercapturiques.
1.3 Effets sur les organismes vivants dans leur milieu naturel
Le MIX D/D est modérément toxique pour les poissons; les
valeurs de la CL50 à 96 h varient de 1 à 6 mg/litre. La toxicité
du MIX D/D est largement imputable au 1,3-dichloropropène.
Lorsqu'on utilise ce mélange aux doses recommandées, ses effets
principaux consistent dans une diminution passagère (moins de sept
jours) des populations de champignons terricoles et une inhibition
de l'oxydation de ions ammonium en nitrates. Le MIX D/D est toxique
pour les bactéries nitrifiantes. Peu après la disparition du MIX
D/D, le sol est recolonisé par les bactéries. Lors d'essais en
situation réelle, on a constaté que le MIX D/D, appliqué à raison de
600 litres/hectare, provoquait la destruction des invertébrés
terricoles. Il faut de 6 à 24 mois pour que la recolonisation
s'effectue.
Le MIX D/D est extrêmement phytotoxique.
1.4 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le MIX D/D présente une toxicité aiguë modérée à forte pour les
animaux de laboratoire. Chez le rat et la souris, la DL50 par voie
orale varie de 132 à 300 mg/kg de poids corporel. En ce qui concerne
la DL50 par voie cutanée, les valeurs pour le rat et le lapin sont
respectivement de 779 et de 2100 mg/kg de poids corporel. Chez le
rat, la CL50 à 4 h par inhalation est approximativement égale à
4540 mg/m3. En cas d'exposition, les signes cliniques observés
sont ceux d'une dépression du système nerveux central. Le MIX D/D
est fortement irritant pour les yeux et la peau et il est doté d'un
pouvoir sensibilisateur cutané modéré.
Les résultats des études toxicologiques à court terme
effectuées sur des rats et des chiens sont insuffisants pour qu'on
puisse déterminer convenablement la toxicité du MIX D/D, car aux
doses relativement faibles que l'on a étudiées, on n'observe pas
d'effets biologiquement significatifs. Plusieurs études d'inhalation
de courte durée (corps entier) ont été effectuées sur des rats. A
des doses allant jusqu'à 145 mg/m3, le MIX D/D n'a pas produit
d'effets toxiques. A partir de 1362 mg/m3, les effets toxiques
étaient évidents et correspondaient à une dépression du système
nerveux central. L'exposition des animaux à la dose de 443 mg/m3
pendant 10 semaines a entraîné une réduction du gain de poids et une
augmentation du poids des reins.
Une étude tératologique comportant l'administration de MIX D/D
par voie orale n'a pas permis, en raison de ses insuffisances,
d'évaluer le pouvoir tératogène de ce composé chez le rat.
Lors d'une étude d'inhalation chez le rat destinée à étudier
les effets du MIX D/D sur la fécondité des mâles et des femelles, on
n'a pas observé d'effets à des doses allant jusqu' 443 mg/m3 sur
une durée de dix semaines. Il n'a pas été possible de procéder à une
évaluation complète des effets du MIX D/D sur la reproduction, en
raison du caractère incomplet du protocole de ces études.
Le MIX D/D s'est révélé mutagène pour les souches TA100 et
TA1535 de Salmonella typhimurium ainsi que pour la souche WP2 HCR
d'Escherichia coli, sans activation métabolique. Cet effet n'a pas
été observé sur les souches TA98, TA1537 et TA1538 de salmonelles.
Lors d'une étude à long terme, au cours de laquelle on avait
administré à des rats un régime alimentaire contenant jusqu'à 120
mg/kg (soit 6 mg/kg de poids corporel) pendant deux ans, on n'a pas
observé d'effets toxiques ou cancérogènes.
1.5 Effets sur l'homme
Le MIX D/D n'est plus guère utilisé et par conséquent il est
peu probable que la population générale puisse être exposée par
l'intermédiaire de l'air, de l'eau et des aliments. L'exposition des
personnels qui remplissent les fûts ou qui sont chargés de
l'épandage du produit s'est en général située en-dessous de 4,5 mg
de 1,3-dichloropropène/m3 lorsque l'on respectait la marche à
suivre recommandée; dans d'autres cas, on a mesuré des
concentrations allant jusqu'à 36,32 mg/m3.
On a cité un cas d'intoxication aiguë mortelle par suite de
l'ingestion accidentelle de MIX D/D.
2. Conclusions
* Population générale. Etant donné que l'on l'utilise plus guère
le MIX D/D, l'exposition de la population générale au 1,3-
dichloropropène par l'intermédiaire de l'air, de l'eau et des
aliments est négligeable, mais dans certaines zones, il peut y
avoir exposition au 1,2-dichloropropane en cas de contamination
des eaux souterraines.
* Exposition professionnelle. Lors du remplissage des fûts et de
l'épandage du MIX D/D, il peut y avoir exposition des
personnels concernés au 1,3-dichloropropène, à des
concentrations qui dépassent les valeurs maximales admissibles,
en particulier sous les climats chauds.
* Environnement. Il est peu probable que le MIX D/D atteigne dans
l'environnement des concentrations biologiquement nocives pour
la faune et la flore terrestre ou aquatique, lorsqu'on utilise
la dose recommandée. Il est également improbable qu'il puisse
exercer des effets nocifs durables sur les organismes vivants
dans leur milieu naturel.
3. Recommandations
* Le MIX D/D ne doit pas être utilisé comme fumigant pour traiter
le sol du fait qu'il risque de passer dans les eaux
souterraines par lessivage.
* Dans les zones où l'on a utilisé du MIX D/D, il faut surveiller
les eaux de surface et les eaux souterraines à la recherche de
résidus éventuels.
RESUMEN Y EVALUACION, CONCLUSIONES, Y RECOMENDACIONES
1,3-DICLOROPROPENO
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
El "1,3-dicloropropeno" se introdujo en 1956 como parte de una
mezcla, que contenía 1,3-dicloropropeno, 1,2-dicloropropano y otros
hidrocarburos halogenados, y se ha utilizado ampliamente en la
agricultura como fumigante del suelo que se destina a nuevas
plantaciones para combatir los nematodos de las hortalizas, las
papas y el tabaco. Se aplica fundamentalmente mediante inyección en
el suelo. La formulación comercial del 1,3-dicloropropeno es una
mezcla de isómeros cis y trans (aproximadamente en proporciones
iguales), que forman un líquido entre incoloro y ámbar, con un olor
penetrante e irritante, parecido al del cloroformo. La presión del
vapor es de 3,7 kPa a 20 °C. El producto técnico tiene una pureza
del 92% y puede contener varias impurezas, como 1,2-dicloropropano.
El log P del coeficiente de reparto octanol/agua es de 1,98.
En el aire, la descomposición del 1,3-dicloropropeno tiene
lugar sobre todo por reacción con radicales libres y con el ozono.
La semivida de los isómeros cis y trans en la reacción con
radicales libres es de 12 y 7 horas respectivamente, y en la
reacción con el ozono de 52 y 12 días respectivamente. La
fototransformación directa parece ser insignificante, pero puede
aumentar en presencia de partículas atmosféricas.
En el agua, el 1,3-dicloropropeno tiende a desaparecer con
rapidez, debido a su solubilidad relativamente baja en ella y su
elevada volatilidad; se han notificado semividas de menos de 5 h.
La distribución del 1,3-dicloropropeno en los compartimentos
del suelo depende de la presión del vapor, el coeficiente de
difusión, la temperatura y el contenido de humedad del mismo. En la
persistencia del 1,3-dicloropropeno en el suelo influyen la
volatilización, la transformación química y biológica, la
transformación fotoquímica y la absorción por los organismos. Los
mecanismos más importantes de dispersión y dilución en el medio
ambiente son la volatilización y la difusión en la fase de vapor.
La transformación del 1,3-dicloropropeno se produce
inicialmente por hidrólisis a 3-cloroalilalcohol y luego por
transformación microbiana a 3-cloroacroleína y ácido 3-
cloroacrílico. En un estudio de laboratorio, la semivida de la
hidrólisis de los isómeros cis y trans del 1,3-dicloropropeno a
15 °C y 29 °C fue de 11,0 y 2,0 días respectivamente para el isómero
cis y de 13,0 y 2,0 días para el isómero trans. En el suelo, con
un pH 7 y una temperatura de 25 °C, la semivida de la hidrólisis fue
de 4,6 días para ambos isómeros. Debido a su desaparición
relativamente rápida del suelo, no es probable que se acumulen
residuos cuando se aplica el fumigante con la dosis y la frecuencia
recomendadas.
El 1,3-dicloropropeno puede desplazarse en el suelo, sobre todo
si es arenoso, de textura gruesa y con un contenido bajo de humedad.
El desplazamiento descendente se ve favorecido por el cultivo
profundo de los suelos con escasa porosidad. Se ha detectado 1,3-
dicloropropeno en "aguas subterráneas altas" (hasta 2 m por debajo
de la superficie), pero no en las profundas, que son las que suelen
utilizarse para beber.
Los cultivos pueden absorber 1,3-dicloropropeno. Sin embargo,
no es probable que aparezca una cantidad apreciable de residuos en
las plantas cultivadas comestibles, que se suelen sembrar cuando ya
ha desaparecido la mayor parte del fumigante.
Es poco probable la bioacumulación de 1,3-dicloropropeno,
debido a su solubilidad relativamente alta en agua (< 1 g/kg), al
bajo log P del coeficiente de reparto octanol/agua y a la
eliminación rápida en mamíferos y otros organismos.
1.2 Cinética y metabolismo
El 1,3-dicloropropeno se elimina rápidamente tras su
administración oral a roedores. La principal vía de eliminación es
la orina, donde a ella el 81% de los isómeros cis y el 56% de los
trans se eliminan en las 24 horas siguientes a la dosificación. La
semivida de la eliminación en la orina es de 5 a 6 horas. La
eliminación fecal es escasa. El anhídrido carbónico representa el 4
y el 24%, respectivamente, de los isómeros cis y trans del 1,3-
dicloropropeno eliminados. Las concentraciones en los tejidos tras
la administración oral son bajas; los niveles residuales más
elevados se encuentran en la pared estomacal, seguidos por
cantidades más bajas en los riñones y la vejiga.
No se ha detectado en la orina la presencia de 1,3-
dicloropropeno inalterado. La glutatión- S-alquiltransferasa actúa
sobre los isómeros cis y trans formando ácidos mercaptúricos,
que se excretan por la orina. El isómero trans se conjuga de 4 a 5
veces más lentamente que el cis. El principal metabolito urinario
en ratas y ratones es la N-acetil- S-(3-cloroprop-2-enil)L-
cisteína; este metabolito se puede utilizar para la vigilancia
biológica en el ser humano. Para el isómero cis se ha identificado
una segunda ruta metabólica de menor importancia, en la que se
produce una monooxigenación a óxido de cis-1,3-dicloropropeno, que
se puede conjugar también con el glutatión. La elevada proporción
del isómero trans que se encuentra en el aire expirado procede de
una ruta metabólica distinta de la conjugación, que tiene una
especificidad más elevada para el isómero trans que para el cis.
La exposición de ratas al 1,3-dicloropropeno por inhalación no
produce un aumento de la concentración en sangre proporcional a la
dosis. A una dosis de 408,6 mg/m3 (90 ppm), disminuyeron la
frecuencia respiratoria y el volumen respiratorio por minuto, y la
saturación del metabolismo se produjo a 1362 mg/m3 (300 ppm). Los
isómeros cis y trans se eliminaron rápidamente de la sangre,
siendo la semivida de la eliminación de 3 a 6 minutos para
concentraciones inferiores a 1362 mg/m3, pero considerablemente
más larga (33-43 min.) a concentraciones más elevadas.
1.3 Efectos en los seres vivos del medio ambiente
Los valores de la CE50 para el crecimiento (96 h) del alga de
agua dulce Selenastrum capricornutum y la diatomea de los
estuarios Skeletoneria costatum son 4,95 mg/litro y 1 mg/litro
respectivamente. La toxicidad aguda (CL50 a las 96 h) del 1,3-
dicloropropeno para los peces es del orden de 1 a 7,9 mg/litro. En
una prueba en embriones-larvas de Pimephales promelas, el nivel
máximo sin efectos fue de 0,24 mg/litro. Estos datos, junto con el
hecho de que no es probable que el 1,3-dicloropropeno persista en el
agua, indican que el peligro para los peces lo constituyen los
efectos tóxicos agudos, con escasas posibilidades de efectos
adicionales debidos a la exposición durante un tiempo prolongado.
En dosis de 30 a 60 mg/kg, el 1,3-dicloropropeno puede reducir
la concentración de hongos y la tasa de actividad enzimática
microbiana, pero el efecto no suele ser duradero (< 7 días) y no se
produce en todos los tipos de suelos. En algunos estudios, aumentó
significativamente el número de microorganismos tras la aplicación.
El 1,3-dicloropropeno es fitotóxico, pero su toxicidad para las
abejas es escasa. Utilizando una técnica de espolvoreo, la DL50 a
las 48 horas fue de 6,6 µg/abeja. Las aves tienen una sensibilidad
relativamente baja al 1,3-dicloropropeno. Para el pato real (Anas
platyrhynchos) y la codorniz (Colinus virginianus) se ha
informado de CL50 (8 días) de > 10 g/kg de la dieta.
1.4 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda por vía oral del 1,3-dicloropropeno en
animales es de moderada a alta. Se han notificado valores de la
DL50 en ratas que oscilan entre 127 y 713 mg/kg de peso corporal.
Los valores de la DL50 por vía oral en ratas para los isómeros
cis y trans fueron de 85 y 94 mg/kg de peso corporal,
respectivamente.
La exposición aguda cutánea es moderadamente tóxica. En ratas y
conejos se ha reportado de una DL50 de 423 mg/kg y 504 mg/kg de
peso corporal, respectivamente. Los valores de la DL50 para los
isómeros cis y trans fueron 1090 y 1575 mg/kg de peso corporal,
respectivamente.
La exposición por inhalación (4 h) en ratas dio como resultado
valores de la DL50 de 3310 mg/m3 (729 ppm) para el 1,3-
dicloropropeno; 3042 mg/m3 -3514 mg/m3 para el isómero cis y
4880 mg/m3 - 5403 mg/m3 para el trans.
La intoxicación aguda afectó el sistema nervioso central y el
aparato respiratorio.
En pruebas cutáneas y de irritación ocular con conejos se
observaron reacciones graves, pero la recuperación se produjo en un
período de 14-21 días. Los resultados de las pruebas de
sensibilización en cobayos fueron positivos.
Se han realizado varios estudios de toxicidad por inhalación
durante un tiempo breve en ratones, ratas, cobayos, conejos y
perros. En los ratones los órganos afectados fueron la mucosa nasal
y la vejiga urinaria. Se observó degeneración del epitelio olfatorio
e hiperplasia del epitelio respiratorio. Se detectó hiperplasia del
epitelio de transición de la vejiga urinaria. En ratones se puede
estimar que el nivel sin efectos observados (NOEL) es de 136 mg/m3
(30 ppm).
En ratas también se han detectado cambios degenerativos
similares en el epitelio olfatorio, así como hiperplasia. En un
estudio bien diseñado se encontró un valor del NOEL para el 1,3-
dicloropropeno de 45,4 mg/m3, siendo el valor del NOEL para el
isómero cis de 136 mg/m3.
En un estudio de administración por vía oral durante 90 días a
ratas, el NOEL fue de 3 mg/kg de peso corporal. El único efecto
observado con la dosis inmediatamente superior, de 10 mg/kg de peso
corporal, fue un aumento relativo del peso de los riñones en los
machos.
En un estudio de inhalación sobre la reproducción de dos
camadas en dos generaciones de ratas, las dosis de hasta 408,6
mg/m3 (90 ppm) no produjeron efectos adversos sobre los parámetros
de la reproducción examinados. Sin embargo, la dosis más alta, de
408,6 mg/m3, indujo toxicidad materna, que se puso de manifiesto
por la disminución del crecimiento y por cambios histopatológicos de
la mucosa nasal. Se estableció un NOEL para la toxicidad materna de
136,2 mg/m3 (30 ppm).
En estudios de teratogenicidad por inhalación en ratas y
conejos, el 1,3-dicloropropeno no mostró potencial teratogénico a
niveles de exposición de hasta 1362 mg/m3, pero se observó
embriotoxicidad (reducción del tamaño de la camada y aumento del
índice de reabsorciones) en ratas. Con dosis de 544,8 mg/m3 (120
ppm) o superiores se advirtió toxicidad materna tanto en ratas como
en conejos.
En la mayor parte de los estudios, el 1,3-dicloropropeno cis
y trans y la mezcla de ambos fueron mutagénicos en bacterias, con
y sin activación metabólica. Se encontró que el 1,3-dicloropropeno
puro y el cis-1,3-dicloropropeno puro carecían de efecto sobre las
bacterias. Se demostró que el glutatión impedía la actividad
mutagénica del 1,3-dicloropropeno en bacterias. En un ensayo de
mutación genética con células de hámster chino V79, así como en la
prueba del locus HPRT de ovario de hámster chino, el cis-1,3-
dicloropropeno dio un resultado negativo.
El 1,3-dicloropropeno cis y trans indujo una síntesis no
programada de ADN en células S3 HeLa. En hepatocitos de rata, el
1,3-dicloropropeno no produjo una reparación significativa del ADN.
En el ensayo del locus rec con microsomas de la cepa H17 de
Bacillus subtilis con activación metabólica, el 1,3-dicloropropeno
dio resultado positivo.
En células de ovario de hámster chino, el 1,3-dicloropropeno
cis y trans indujo daños cromosómicos en condiciones de
activación metabólica, pero en otro estudio también dio resultado
positivo sin que hubiera activación. El isómero cis no indujo
lesiones cromosómicas en células hepáticas de rata, pero sí un
intercambio de cromátidas hermanas en células de ovario de hámster
chino con activación metabólica y sin ella, y en células de hámster
chino V79 sin activación.
En una prueba con micronúcleos de médula ósea en ratones, y en
otra de letalidad recesiva ligada al sexo en Drosophila
melanogaster, el 1,3-dicloropropeno fue negativo.
Se realizaron estudios de carcinogenicidad en ratones y ratas.
Se administró 1,3-dicloropropeno de calidad técnica (con un 1% de
epiclorhidrina) mediante sonda durante dos años. En los ratones se
observó un aumento significativo de la hiperplasia epitelial y los
carcinomas celulares transitorios en la vejiga urinaria, una
incidencia mayor de tumores pulmonares, un ligero aumento de tumores
hepáticos y una mayor proporción de hiperplasia epitelial y
papilomas o carcinomas de las células escamosas de la parte cardíaca
del estómago.
En estudios de inhalación de dos años se investigó la
carcinogenicidad del 1,3-dicloropropeno (sin epiclorhidrina) en
ratones y ratas. En ratones se detectó una mayor incidencia de
hiperplasia en la vejiga urinaria, la parte cardíaca del estómago y
la mucosa nasal. Aumentó la incidencia de los tumores pulmonares
benignos. También se observaron en ratas algunos cambios tóxicos en
la mucosa olfativa de la cavidad nasal, pero sin aumento de la
incidencia de tumores.
En un estudio de administración con sonda se puso de manifiesto
que la epiclorhidrina producía tumores en la parte cardíaca del
estómago, y en otro estudio de inhalación en ratas aparecieron
tumores en la cavidad nasal; en el caso de la administración de 1,3-
dicloropropeno por vía oral a ratones no se puede excluir un efecto
carcinogénico sobre la vejiga urinaria.
Mecanismo de acción
Dado que la principal ruta metabólica de eliminación del 1,3-
dicloropropeno es mediante la conjugación con el glutatión, cabe
esperar que las condiciones que alteran la concentración de
glutatión (sulfhidrilo no proteico) en los tejidos puedan modificar
los efectos del compuesto. El mismo 1,3-dicloropropeno agota el
contenido de glutatión de diversos tejidos, especialmente los
situados en puntos de entrada en el organismo, es decir, sobre todo
la parte cardíaca del estómago y el hígado tras la administración
con sonda y el tejido nasal después de la exposición por inhalación.
Tras la inhalación de concentraciones de 1,3-dicloropropeno
superiores a 22,7 mg/m3 (5 ppm) y 113,5 mg/m3 (25 ppm) se
produjo, respectivamente, una disminución de los niveles de
glutatión en el epitelio nasal y en la parte cardíaca del estómago
en ratones.
La toxicidad del 1,3-dicloropropeno en animales se produce con
niveles de exposiciones que agotan el glutatión de los tejidos y la
disminución previa de la concentración de éste la agrava. La
inhalación durante un tiempo prolongado de concentraciones
superiores a 90,8 mg/m3 (20 ppm) da lugar en ratones a
degeneración e hiperplasia del epitelio nasal y gástrico, mientras
que en ratas la inhalación durante un tiempo prolongado de una
concentración de 272,4 mg/m3 (60 ppm) produce degeneración del
tejido nasal.
La función protectora del glutatión se ha puesto de relieve
ulteriormente en estudios que han demostrado que la unión mediante
enlaces covalentes del 14C-1,3-dicloropropeno a la parte cardíaca
del estómago de ratón aumentaba a medida que disminuía el contenido
de sulfhidrilo no proteico. De igual forma, en sistemas de prueba
in vitro el glutatión mejoró notablemente la genotoxicidad del
1,3-dicloropropeno y del óxido 1,3-dicloropropeno, su metabolito
oxidativo secundario (citocromo P-450).
1.5 Efectos en el ser humano
No es probable la exposición de la población general a través
del aire, el agua o los alimentos.
En los estudios realizados se ha puesto de manifiesto que la
exposición profesional está en general por debajo de 4,54 mg/m3 (1
ppm), pero también se han notificado niveles más elevados (hasta
18,3 mg/m3 durante el llenado o el cambio de la boquilla). Es
probable que la exposición profesional se produzca por inhalación y
por vía cutánea. Tras la exposición aparece inmediatamente
irritación de los ojos y de la parte superior de la mucosa
respiratoria. La inhalación de aire con concentraciones de > 6810
mg/m3 (> 1500 ppm) produjo signos y síntomas de intoxicación
grave; las exposiciones más bajas dieron lugar a una depresión del
sistema nervioso central y a la irritación del aparato respiratorio.
La exposición cutánea produjo una irritación grave de la piel.
Se notificó que un grupo de aplicadores de 1,3-dicloropropeno
tuvieron algunos cambios en las funciones renal y hepática al final
de la temporada de aplicación. Sin embargo, se ha rebatido la
relación causa-efecto.
Se han producido algunos casos de intoxicación con
hospitalización de los afectados, que presentaban signos y síntomas
de irritación de la membrana mucosa, malestar torácico, dolor de
cabeza, náuseas, vómitos, mareos y, en ocasiones, pérdida del
conocimiento y disminución de la libido. Se han atribuido tres casos
de enfermedades malignas sanguíneas a una sobreexposición accidental
anterior al 1,3-dicloropropeno, pero la relación causa-efecto sigue
siendo dudosa.
Se comparó el estado de fecundidad de un grupo de personas que
trabajaban en la producción de compuestos clorados de tres carbonos
con otro testigo. No se demostró la existencia de una asociación
entre la disminución de la fecundidad y la exposición.
2. Conclusiones
Población general: A la vista del grado de exposición bajo o nulo
al 1,3-dicloropropeno, el riesgo para la población general es
insignificante.
Exposición profesional: Cuando no se adoptan las precauciones
adecuadas de seguridad, las actividades de llenado y aplicación en
el campo pueden dar lugar a una exposición del operador a
concentraciones que superan el máximo permisible.
Medio ambiente: Siempre que se utilice el 1,3-dicloropropeno en la
proporción recomendada, no es probable que se alcancen niveles
importantes para el medio ambiente, y tampoco es probable que
produzca efectos secundarios sobre poblaciones de seres vivos
terrestres y acuáticos.
3. Recomendaciones
* Las actividades de llenado y la aplicación en el campo del 1,3-
dicloropropeno sólo deben realizarse tomando las precauciones
de seguridad adecuadas, a fin de tener la garantía de que los
niveles de exposición no exceden las concentraciones máximas
permisibles de este producto.
* Se deben realizar estudios a fin de investigar el destino
metabólico del isómero trans del 1,3-dicloropropeno en
mamíferos y la posible función que los metabolitos oxidativos
de este isómero pueden tener como intermediarios en la
toxicidad del 1,3-dicloropropeno.
* La glutatión transferasa interviene en el efecto protector del
glutatión frente a la toxicidad del 1,3-dicloropropeno. Se
recomienda la realización de estudios que permitan comparar la
cinética enzimática relativa de la glutatión S-transferasa
humana de diversos tejidos con la actividad enzimática de
tejidos animales comparables.
* Se deben agrupar y publicar en revistas con una difusión amplia
los datos disponibles acerca de la función protectora del
glutatión.
* Parte de la genotoxicidad del dicloropropeno se debe al
metabolismo oxidativo. Se recomienda la realización de estudios
para identificar la isoenzima del citocromo P-450 que lleva a
cabo esta acción y comparar su actividad con la de las
isoenzimas del citocromo P-450 humano.
* Hay que aclarar la confusa función de la epiclorhidrina en los
estudios de carcinogenicidad por vía oral con sonda.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1,2-DICLOROPROPANO
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
El 1,2-dicloropropano es un líquido con un punto de ebullición
de 96,8 °C y una presión del vapor de 42 mm de Hg a 20 °C. Es
soluble en agua, etanol y éter etílico. Al calentarlo desprende
vapores de fosgeno enormemente tóxicos. El log P del coeficiente de
reparto octanol/agua es de 2,28.
Es una sustancia que se usa en el acabado de muebles, líquidos
de limpieza en seco, decapantes para pinturas, tratamiento de la
cola, desengrasado de metales, tratamiento del petróleo y como
ingrediente en la fabricación de caucho y de cera y como producto
químico intermedio en la fabricación de tetracloroetileno y
tetracloruro de carbono. Forma parte de la mezcla D/D, utilizada
como fumigante antes de la siembra.
Se han determinado las concentraciones de 1,2-dicloropropano en
el aire de las ciudades, con 1,2 µg/m3 (valor medio), 0,021-0,040
µg/m3 y 0,0065-1,4 µg/m3 en Filadelfia, Portland y Japón,
respectivamente. Su descomposición en la atmósfera es lenta; en
función de su reacción con los radicales oxhidrilos, la semivida del
1,2-dicloropropano fue de > 313 días. Probablemente el proceso
predominante en su descomposición es la fototrans-formación. Para
que ésta sea apreciable es necesario que haya adsorción sobre
material particulado. Es probable que la volatilización sea la
principal vía de escape del agua.
En el suelo, los principales mecanismos de eliminación son la
volatilización y la difusión. El 1,2-dicloropropano es persistente
en el suelo. Más del 98% del aplicado a suelos de marga se recuperó
a las 12-20 semanas del tratamiento.
En zonas en las que se ha utilizado la "mezcla D/D" para
fumigar el suelo, el 1,2-dicloropropano puede contaminar por
lixiviación las aguas subterráneas altas y profundas. En los Estados
Unidos se han encontrado en el agua de pozo y la subterránea
concentraciones de hasta 440 µg/litro y 51 µg/litro,
respectivamente. En los Países Bajos se han medido concentraciones
de hasta 160 µg/litro en agua de pozo, y se ha encontrado 1,2-
dicloropropano a una profundidad de 13 metros.
El 1,2-dicloropropano se puede ingerir con los cultivos
comestibles, pero los residuos detectados eran bajos (< 0,01 mg/kg)
y no parece que puedan tener significación biológica.
No es probable la bioacumulación de 1,2-dicloropropano, debido
a su elevada solubilidad en agua (2,7 g/kg) y al bajo log P del
coeficiente de reparto octanol/agua.
1.2 Cinética y metabolismo
El 1,2-dicloropropano administrado a ratas por vía oral se
elimina rápidamente (80-90% en 24 horas). No existen grandes
diferencias entre machos y hembras en cuanto a la cinética o la
eliminación. La principal vía de eliminación es la orina,
excretandose en 24 horas hasta la mitad de una dosis oral. Por las
heces se elimina menos del 10%. Un tercio se expulsa en el aire
expirado, en forma de anhídrido carbónico y como mezcla de productos
volátiles. Las concentraciones en los tejidos son bajas,
detectándose la más alta en el hígado. Tras la exposición de ratas
por inhalación, también se produce una eliminación rápida; en la
orina se expulsa el 55-65% de la dosis, y el 16-23% en el aire
expirado. La semivida en la sangre es de 24-30 minutos.
No se ha encontrado en la orina 1,2-dicloropropano inalterado.
Se han identificado tres metabolitos urinarios principales. Estos
proceden de las vías oxidativa y de conjugación, que dan lugar a los
mercapturatos, N-acetil- S-(2-hidroxipropil)-L-cisteína, N-
acetil- S-(2-oxipropil)-L-cisteína y N-acetil- S-(1-
carboxietil)-L-cisteína. El 1,2-dicloropropano también se puede
oxidar a lactato y dar anhídrido carbónico o acetil-CoA como
producto final.
La administración de 1,2-dicloropropano por vía oral a ratas (2
ml/kg) produjo una notable reducción de la concentración de
glutatión en los tejidos. Había una correlación entre la pérdida de
glutatión y las características de la toxicidad en el hígado, los
riñones y los eritrocitos. La disminución previa del glutatión
intracelular agravaba la toxicidad del 1,2-dicloropropano, mientras
que un tratamiento anterior con precursores de la síntesis del
glutatión mejoraba la toxicidad. Estos resultados demuestran el
efecto protector del glutatión frente a la toxicidad del 1,2-
dicloropropano.
1.3 Efectos en los seres vivos del medio ambiente
No se ha calculado la CE50 para las algas de agua dulce por
las dificultades que plantea la volatilización del producto de la
solución de prueba. La toxicidad aguda del 1,2-dicloropropano para
los invertebrados acuáticos y los peces es de baja a moderada; los
valores de la CL50 a las 48 h para los invertebrados oscila entre
52 y > 100 mg/litro, y para los peces a las 96 h varía entre 61 y
320 mg/litro. En las pruebas de toxicidad durante un período corto
con Pimephales promelas se calculó un nivel máximo sin efectos
observados de 82 mg/litro. En una prueba de toxicidad de 32 días en
las primeras fases de vida de la misma especie se puso de manifiesto
que el crecimiento y la supervivencia de las larvas eran los
parámetros más sensibles. La máxima concentración tóxica aceptable
(MCTA) se estimó entre 6 y 11 mg/litro. A los 33 días de exposición
a concentraciones de 164 mg/litro de 1,2-dicloropropano se advirtió
inhibición del crecimiento en Cyprinodon variegatus.
El 1,2-dicloropropano es fitotóxico.
En las pruebas de contacto con cuatro especies de lombrices de
tierra se obtuvo una CL50 de 44-84 µg/cm2 (valores medios) de
papel de filtro. Los valores de la CL50 en suelo artificial fueron
de 3880-5300 mg/kg de suelo (peso seco).
1.4 Efectos en los animales de experimentación y en
sistemas de prueba in vitro
La toxicidad aguda por vía oral del 1,2-dicloropropano en
animales de experimentación es baja. La DL50 por vía oral para la
rata es de 1,9 g/kg de peso corporal, y por vía cutánea en conejos
de 8,75 ml/kg de peso corporal.
En los estudios de toxicidad oral durante un período corto en
ratones y ratas se puso de manifiesto una inhibición del
crecimiento, signos clínicos de toxicidad asociados con una
depresión del sistema nervioso central y/o un aumento de la
mortalidad con dosis de 250 mg/kg de peso corporal al día o
superiores. En ratas con una dosis diaria de 250 mg/kg de peso
corporal durante 10 días se observaron cambios en las enzimas del
suero que indicaban una ligera hepatotoxicidad, con un NOEL de 100
mg/kg al día.
En un estudio de inhalación durante 13 semanas en ratones
(dosis máxima de 681 mg/m3) no se observaron efectos adversos. En
un estudio similar en el que se expusieron ratas a 68,1, 227 ó 681
mg/m3, se produjo una disminución del peso corporal y ligeras
lesiones en el tejido nasal en los grupos con las dos dosis más
elevadas.
En un estudio de reproducción en dos generaciones, la
exposición de ratas a proporciones de 1,2-dicloropropano en el agua
de bebida del 0,024, el 0,1 y el 0,24% (equivalentes a 33,6, 140 y
336 mg/kg de peso corporal al día) dio lugar a un menor aumento del
peso corporal en la madre y a un consumo reducido de agua con las
dosis media y alta. El peso corporal de los recién nacidos fue menor
con las dosis más altas. Se estableció un NOAEL para la toxicidad en
las madres y en la reproducción de 33,6 y 140 mg/kg de peso corporal
al día, respectivamente.
En los estudios realizados no se observó actividad teratogénica
alguna del 1,2-dicloropropano con dosis orales de hasta 125 mg/kg de
peso corporal en la rata y 150 mg/kg de peso corporal en el conejo.
Sin embargo, a estas dosis el 1,2-dicloropropano era tóxico para las
madres y los fetos, como pusieron de manifiesto los signos clínicos
asociados al sistema nervioso central, el menor aumento del peso
corporal de las madres y el retraso de la osificación en los fetos.
Los NOEL son de 30 y 50 mg/kg de peso corporal al día para la rata y
el conejo, respectivamente.
En la mayor parte de los estudios con bacterias, con activación
metabólica o sin ella, el 1,2-dicloropropano mostró efectos
mutagénicos, pero se utilizaron dosis muy elevadas, de hasta 10
mg/placa. En células de ovario de hámster chino se produjeron
aberraciones cromosómicas e intercambio de cromátidas hermanas. En
células V79 de hámster chino aumentó el intercambio de cromátidas.
En un sistema in vitro con linfocitos humanos, la absorción de
timidina tritiada y la viabilidad de las células cultivadas con un
sistema metabolizante hepático de la rata y sin él fueron análogas a
las de los cultivos testigo. Los resultados de una prueba de
letalidad recesiva ligada al sexo en Drosophila melanogaster
fueron negativos. En una prueba de letalidad dominante en ratas, en
la que se administró el 1,2-dicloropropano con el agua de bebida
durante 14 semanas, seguidas de dos semanas de apareamiento, se
obtuvieron resultados negativos.
En un estudio de carcinogenicidad en ratones se administraron
125 ó 250 mg de 1,2-dicloropropano/kg de peso corporal mediante
sonda, observándose un aumento relacionado con la dosis en la
incidencia de adenomas hepáticos. Esta fue mayor en los grupos
tratados que en el grupo testigo, pero se mantuvo dentro de los
valores habituales de los testigos.
La administración por sonda en ratas, a concentraciones de 125
y 250 mg/kg de peso corporal (hembras) y 62 y 125 mg/kg de peso
corporal (machos), cinco días a la semana durante 113 semanas,
produjo en las hembras con la dosificación más alta un ligero
aumento de la incidencia de adenocarcinomas de las glándulas
mamarias, por encima de los valores habituales.
1.5 Efectos en el ser humano
No es probable la exposición de la población general al 1,2-
dicloropropano a través del aire y el agua, excepto en zonas con un
uso abundante de 1,2-dicloropropano y de mezcla D/D en la
agricultura. Los residuos de 1,2-dicloropropano en los cultivos
comestibles se suelen mantener por debajo del límite de detección.
En vista de estos bajos niveles de exposición, el riesgo para la
población general es insignificante.
Se han notificado varios casos de intoxicación aguda por 1,2-
dicloropropano, debidos a una exposición excesiva accidental o
intencionada (suicidio). Los efectos se han concentrado
principalmente en el sistema nervioso central, el hígado y los
riñones. También se ha descrito la aparición de anemia hemolítica y
coagulación intravascular diseminada. En un caso, el delirio
evolucionó hacia un shock irreversible, insuficiencia cardíaca y la
muerte.
Se puede producir exposición profesional a través de la piel o
por inhalación. Se ha informado de varios casos de dermatitis y de
sensibilización cutánea en trabajadores que utilizaban mezclas de
disolventes con 1,2-dicloropropano.
2. Conclusiones
* Población general: La exposición de la población general al
1,2-dicloropropano a partir del aire o los alimentos es baja o
nula. Sin embargo, se puede producir en determinadas zonas una
exposición debida a la contaminación de las aguas subterráneas.
* Exposición profesional: Con unas buenas prácticas de trabajo,
medidas higiénicas y precauciones de seguridad, no es probable
que el 1,2-dicloropropano represente un riesgo para las
personas profesionalmente expuestas a él.
* Medio ambiente: Utilizado en las dosis recomendadas, no es
probable que el 1,2-dicloropropano alcance niveles
significativos en el medio ambiente. Tampoco es probable que
tenga efectos adversos sobre las poblaciones de seres vivos
terrestres y acuáticos.
3. Recomendaciones
* Se deben realizar estudios a fin de evaluar la toxicidad aguda
por inhalación, la irritación ocular y cutánea y la posible
sensibilización de la piel.
* Se han de adoptar las precauciones de seguridad apropiadas
cuando se maneje 1,2-dicloropropano, a fin de evitar
exposiciones que superen la concentración máxima permisible.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
"MEZCLA D/D"
1. Resumen y evaluación
1.1 Uso, destino y niveles en el medio ambiente
La mezcla técnica de dicloropropenos y dicloropropano
(abreviada en el presente texto como "mezcla D/D") es un líquido de
color ámbar claro y olor acre; la presión del vapor es de 35 mm de
Hg a 20 °C, y es soluble en disolventes halogenados, ésteres y
cetonas.
La "mezcla D/D" suele contener no menos del 50% de 1,3-
dicloropropeno (con isómeros cis y trans en una proporción
aproximada de 1:1), y los demás ingredientes principales son el 1,2-
dicloropropano y compuestos afines. Esta mezcla se utiliza mucho
como nematocida del suelo antes de la siembra.
El transporte, la distribución y el destino en el medio
ambiente de los componentes principales de la "mezcla D/D" en el
aire, el agua y el suelo se describen en sección 4 de los apartados
de la presente monografía de EHC que tratan del 1,3-dicloropropeno y
el 1,2-dicloropropano.
El 1,2-dicloropropano procedente de la "mezcla D/D" tiene
considerables posibilidades de escapar del suelo por lixiviación y
contaminar las aguas de los pozos y las subterráneas. En un pozo de
riego (68 m de profundidad) de Europa occidental se registraron unas
concentraciones medias de 1,2-dicloropropano a diferentes
profundidades que oscilaban entre 0,8 y 8,5 µg/litro, con una
concentración máxima de 165 µg/litro.
No es probable que los cultivos absorban cantidades importantes
de los componentes de la "mezcla D/D" (véase en otros apartados de
la presente monografía). Tampoco es probable la bioacumulación de
los componentes de la mezcla debido a su bajo log P del coeficiente
de reparto octanol/agua y a su solubilidad relativamente grande en
agua.
1.2 Cinética y metabolismo
No se han realizado estudios metabólicos con la "mezcla D/D".
Los dos componentes principales, el 1,3-dicloropropeno y el 1,2-
dicloropropano, se eliminan con rapidez, principalmente por la orina
y, en menor cantidad, por el aire expirado. Los componentes de la
"mezcla D/D" se metabolizan por oxidación y conjugación. Los
principales metabolitos urinarios son los ácidos mercaptúricos.
1.3 Efectos en los seres vivos del medio ambiente
La "mezcla D/D" es moderadamente tóxica para los peces; los
valores de la CL50 a las 96 h oscilan entre 1 y 6 mg/litro. La
toxicidad de la mezcla se debe fundamentalmente al 1,3-
dicloropropeno.
Cuando se utilizan las dosis de aplicación recomendadas, los
principales efectos de la "mezcla D/D" son la reducción transitoria
(< 7 días) de los hongos del suelo y la inhibición de la oxidación
de los iones amonio a nitrato. La mezcla es tóxica para las
bacterias nitrificantes. Inmediatamente después de desaparecer del
suelo, las bacterias comienzan a colonizar la zona de nuevo. En
ensayos de campo, la "mezcla D/D" (aplicada a 600 litros/ha) eliminó
los invertebrados del suelo. La recolonización requirió entre 6 y 24
meses.
La "mezcla D/D" tiene una elevada fitotoxicidad.
1.4 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la "mezcla D/D" para los animales de
laboratorio es de moderada a alta. Los valores de la DL50 por vía
oral en ratas y ratones oscilan entre 132 y 300 mg/kg de peso
corporal. Los valores de la DL50 por vía cutánea en ratas y
conejos son de 779 y 2100 mg/kg de peso corporal, respectivamente.
La CL50 (4 h) por vía respiratoria para ratas es aproximadamente
de 4540 mg/m3. La exposición aguda produjo signos clínicos
asociados a depresión del sistema nervioso central. La "mezcla D/D"
tiene un fuerte efecto irritante en los ojos y la piel y una
moderada capacidad de sensibilización cutánea.
Los resultados de los estudios disponibles de toxicidad durante
un período breve en ratas y perros son insuficientes para evaluar de
manera correcta la posible toxicidad de la mezcla, porque las dosis
relativamente bajas ensayadas no demuestran ningún efecto
biológicamente significativo. Se han realizado varios estudios de
exposición por inhalación (todo el cuerpo) durante un período corto
en ratas. Las concentraciones de hasta 145 mg/m3 de la "mezcla
D/D" no tienen ningún efecto tóxico. Con niveles de 1362 mg/m3 o
superiores se detectan claramente efectos tóxicos asociados con
depresión del sistema nervioso central. Una exposición a 443 mg/m3
durante 10 semanas da lugar a una disminución del aumento del peso
corporal y a un mayor peso absoluto de los riñones.
Un estudio teratológico en ratas por vía oral de la "mezcla
D/D" fue inadecuado para evaluar su posible acción en este sentido.
En un estudio de inhalación en ratas con dosis de hasta 443
mg/m3 durante 10 semanas, para investigar la fecundidad de machos
y hembras, no se observó ningún efecto. Debido a un diseño
inadecuado del procedimiento, no fue posible evaluar completamente
los efectos de la "mezcla D/D" sobre la reproducción.
La mezcla tiene efectos mutagénicos en las cepas TA100 y TA1535
de Salmonella typhimurium, así como en la WP2 HCR de Escherichia
coli, sin activación metabólica. Sin embargo, no se produjeron
tales efectos en las cepas TA98, TA1537 y TA1538 de Salmonella.
En un estudio prolongado en ratas alimentadas con dietas que
contenían hasta 120 mg de la "mezcla D/D" por kg (equivalentes a 6
mg/kg de peso corporal) durante dos años no se detectaron efectos
tóxicos ni carcinógenos.
1.5 Efectos en el ser humano
Ya no se utiliza la "mezcla D/D" tanto como antes, por lo que
es improbable la exposición de la población general a través del
aire, el agua y los alimentos.
Cuando se utilizaban los procedimientos recomendados, la
exposición de los trabajadores que llenaban los bidones y de los
aplicadores en el campo fue en general inferior a 4,5 mg de 1,3-
dicloropropeno/m3. En otras condiciones se han medido
concentraciones de hasta 36,32 mg/m3.
Se ha informado de un caso de intoxicación aguda con desenlace
fatal tras la ingestión accidental de la mezcla.
Se han notificado varios casos de dermatitis por contacto y de
sensibilización cutánea debidas a la exposición accidental a la
"mezcla D/D".
2. Conclusiones
* Población general: Dado que la "mezcla D/D" no tiene ya un uso
tan generalizado, la exposición de la población al 1,3-
dicloropropeno a través del aire, el agua y los alimentos es
insignificante, pero, en ciertas zonas, cuando se contaminan
las aguas subterráneas, se puede producir una exposición al
1,2-dicloropropano.
* Exposición profesional: Durante las actividades de llenado y de
aplicación de la "mezcla D/D" en los campos se puede producir
una exposición de los manipuladores a concentraciones de 1,3-
dicloropropeno superiores a la máxima permisible, especialmente
en condiciones climáticas cálidas.
* Medio ambiente: No es probable que la "mezcla D/D" alcance
niveles biológicamente significativos en el medio terrestre o
acuático, siempre que se utilice en las dosis recomendadas.
Tampoco es probable que se produzcan efectos adversos duraderos
en los organismos vivos del medio ambiente.
3. Recomendaciones
* No se debe utilizar la "mezcla D/D" para fumigar el suelo,
debido a su capacidad de lixiviar y alcanzar las aguas
subterráneas.
* En las zonas en las que se use la mezcla, se han de vigilar los
residuos en las aguas superficiales y subterráneas.
 Chemical formula C3H4Cl2
Relative molecular mass 110.98
Chemical name 1,3-dichloropropene; (IUPAC);
dichloro-1,3-propene; (F-ISO);
1,3-dichloro-1-propene; (CA).
Common synonyms gamma-chloroallylchloride,
1,3-dichloropropylene
Trade name TELONE II(R), D-D 92
CAS registry number
Chemical formula C3H4Cl2
Relative molecular mass 110.98
Chemical name 1,3-dichloropropene; (IUPAC);
dichloro-1,3-propene; (F-ISO);
1,3-dichloro-1-propene; (CA).
Common synonyms gamma-chloroallylchloride,
1,3-dichloropropylene
Trade name TELONE II(R), D-D 92
CAS registry number  Dilling et al. (1975) determined the rate of evaporation of 1,3-
dichloropropene ( cis- and trans-) from water at the 1 mg/litre
level under ambient conditions. The time required for the compound
to be reduced by 50% was 31 min and by 90%, 98 min.
Yon et al. (1991) determined that the half-life of evaporation
of 1,3-dichloropropene ( cis- and trans-isomers) from water was
less than 5 h.
4.1.3 Soil
The persistence of 1,3-dichloropropene depends on chemical
degradation, volatilization, microbial transformation, photochemical
transformation, type of soil, water content of soil, and uptake into
organisms. Thomason & McKenry (1974) studied the quantitative as
well as the qualitative aspects of the movement and fate of 1,3-
dichloropropene under various conditions in different types of soil.
Since 1,3-dichloropropene is used as soil fumigant, some
information is available on the distribution of the compound in
soil. The adsorption of 1,3-DCP on soil was found to be proportional
to the organic matter content of the soil. The K(om/v)sa for cis-
and trans-1,3-dichloropropene were estimated to be 14 and 15,
respectively, independent of ambient temperature (Leistra, 1970).
Similar soil/water distribution coefficients (23 and 26), based on
organic carbon content, were reported by Kenaga (1980).
McKenry & Thomason (1974) demonstrated that high soil moisture
was a major limiting factor in the total diffusion when soil
moisture in the field approached field capacity. In contrast,
Munnecke & van Gundy (1979) stated that soil moisture was a very
important factor in that gaseous compounds are most effective in
killing organisms when they are in a moist environment.
Environmental transformation of 1,3-dichloropropene results from
microbial action, with the exception of the initial hydrolysis of
cis- and trans-1,3-dichloropropene to 3-chloroallyl alcohol
(Castro & Belser, 1966; Belser & Castro, 1971). The pathway for the
transformation of 1,3-dichloropropene is given in Fig. 2.
a See section 2.2.
4.1.3.1 Hydrolysis
Cis- and trans-1,3-dichloropropene can be hydrolysed in soil
to 3-chloroallyl alcohol (see Fig. 2). Hydrolysis rates for 1,3-
dichloropropenes range from 1 to 3.4% per day, depending on
temperature and moisture content. Hydrolysis rates also vary with
soil type (particle size) because of differences in chemical
diffusion rate and sorption capacity (California State Water
Resources Control Board, 1983).
Using [14C]-radiolabelled 1.3-dichloropropene in sterile
buffered water at pH 5, 7, or 9 and temperatures of 10, 20, or 30
°C, McCall (1987) found that the rate of hydrolysis was independent
of pH at each temperature, and that the half-lives at temperatures
of 30, 20, and 10 °C were 3.1, 11.3, and 51 days, respectively. One
hydrolysis product, formed during the course of the study, was
identified as 3-chloroallyl alcohol. The alcohol appeared to be
stable to further hydrolytic conversion and was formed in the same
cis-:trans-ratio as the initial 1,3-dichloropropene.
The hydrolysis of cis-1,3-dichloropropene (98.1%) was studied
by O'Connor (1990b). The degradation reactions at all pH values were
shown to follow pseudo first-order behaviour in the EEC-test,
independent of the concentration. The degradation rate constants and
environmental half-lives for cis-1,3-dichloropropene at 25 °C at
pH 4, pH 7, and pH 9 (extrapolated by measuring degradation at
temperatures of 50, 60, and 70 °C, using Arrhenius relationships)
were 100 h, 54.5 h, and 38 h, respectively. (Remark: although the
rate of hydrolysis of cis-1,3-dichloropropene did show some slight
pH dependence, the author stated that this was probably within
experimental error). It is hypothesized that the degradation
proceeds via a resonance stabilized carbonium ion intermediate,
resulting in the formation of a mixture of 3-chloroallyl alcohol and
propenal (see Fig. 3).
Connors et al. (1990) studied the hydrolysis of 1,3-
dichloropropene into 3-chloroallyl alcohol, under laboratory
conditions. A 1.0 µg/litre cis- and trans-1,3-dichloropropene
solution was prepared in a pH 5.5 or pH 7.0 buffer. The half-lives
for the cis- and trans-isomers at 15 and 29 °C (pH 5.5) were
11.0, 2.0 and 13.0, 2.0 days, respectively. At pH 7.0 and 25 °C, the
value was 4.6 days for both isomers.
Dilling et al. (1975) determined the rate of evaporation of 1,3-
dichloropropene ( cis- and trans-) from water at the 1 mg/litre
level under ambient conditions. The time required for the compound
to be reduced by 50% was 31 min and by 90%, 98 min.
Yon et al. (1991) determined that the half-life of evaporation
of 1,3-dichloropropene ( cis- and trans-isomers) from water was
less than 5 h.
4.1.3 Soil
The persistence of 1,3-dichloropropene depends on chemical
degradation, volatilization, microbial transformation, photochemical
transformation, type of soil, water content of soil, and uptake into
organisms. Thomason & McKenry (1974) studied the quantitative as
well as the qualitative aspects of the movement and fate of 1,3-
dichloropropene under various conditions in different types of soil.
Since 1,3-dichloropropene is used as soil fumigant, some
information is available on the distribution of the compound in
soil. The adsorption of 1,3-DCP on soil was found to be proportional
to the organic matter content of the soil. The K(om/v)sa for cis-
and trans-1,3-dichloropropene were estimated to be 14 and 15,
respectively, independent of ambient temperature (Leistra, 1970).
Similar soil/water distribution coefficients (23 and 26), based on
organic carbon content, were reported by Kenaga (1980).
McKenry & Thomason (1974) demonstrated that high soil moisture
was a major limiting factor in the total diffusion when soil
moisture in the field approached field capacity. In contrast,
Munnecke & van Gundy (1979) stated that soil moisture was a very
important factor in that gaseous compounds are most effective in
killing organisms when they are in a moist environment.
Environmental transformation of 1,3-dichloropropene results from
microbial action, with the exception of the initial hydrolysis of
cis- and trans-1,3-dichloropropene to 3-chloroallyl alcohol
(Castro & Belser, 1966; Belser & Castro, 1971). The pathway for the
transformation of 1,3-dichloropropene is given in Fig. 2.
a See section 2.2.
4.1.3.1 Hydrolysis
Cis- and trans-1,3-dichloropropene can be hydrolysed in soil
to 3-chloroallyl alcohol (see Fig. 2). Hydrolysis rates for 1,3-
dichloropropenes range from 1 to 3.4% per day, depending on
temperature and moisture content. Hydrolysis rates also vary with
soil type (particle size) because of differences in chemical
diffusion rate and sorption capacity (California State Water
Resources Control Board, 1983).
Using [14C]-radiolabelled 1.3-dichloropropene in sterile
buffered water at pH 5, 7, or 9 and temperatures of 10, 20, or 30
°C, McCall (1987) found that the rate of hydrolysis was independent
of pH at each temperature, and that the half-lives at temperatures
of 30, 20, and 10 °C were 3.1, 11.3, and 51 days, respectively. One
hydrolysis product, formed during the course of the study, was
identified as 3-chloroallyl alcohol. The alcohol appeared to be
stable to further hydrolytic conversion and was formed in the same
cis-:trans-ratio as the initial 1,3-dichloropropene.
The hydrolysis of cis-1,3-dichloropropene (98.1%) was studied
by O'Connor (1990b). The degradation reactions at all pH values were
shown to follow pseudo first-order behaviour in the EEC-test,
independent of the concentration. The degradation rate constants and
environmental half-lives for cis-1,3-dichloropropene at 25 °C at
pH 4, pH 7, and pH 9 (extrapolated by measuring degradation at
temperatures of 50, 60, and 70 °C, using Arrhenius relationships)
were 100 h, 54.5 h, and 38 h, respectively. (Remark: although the
rate of hydrolysis of cis-1,3-dichloropropene did show some slight
pH dependence, the author stated that this was probably within
experimental error). It is hypothesized that the degradation
proceeds via a resonance stabilized carbonium ion intermediate,
resulting in the formation of a mixture of 3-chloroallyl alcohol and
propenal (see Fig. 3).
Connors et al. (1990) studied the hydrolysis of 1,3-
dichloropropene into 3-chloroallyl alcohol, under laboratory
conditions. A 1.0 µg/litre cis- and trans-1,3-dichloropropene
solution was prepared in a pH 5.5 or pH 7.0 buffer. The half-lives
for the cis- and trans-isomers at 15 and 29 °C (pH 5.5) were
11.0, 2.0 and 13.0, 2.0 days, respectively. At pH 7.0 and 25 °C, the
value was 4.6 days for both isomers.

 Determination of the rate of hydrolysis of 1,3-dichloropropene
at 25 °C in 50% aqueous ethanol indicated a half-time of 4 days for
both the cis- and trans-isomers and appeared independent of the
concentration in the range of 10-1000 mg/litre. Only small
differences were observed in disappearance rates at pH levels of 5.5
and 7.5. The effect of temperature was clearly demonstrated: at 29
°C, the half-life for cis-1,3-dichloropropene was 1.5-2.0 days,
while, at 2 °C, the half-life was estimated to be 91-100 days
(Krijgsheld & Van der Gen, 1986).
The rates of transformation of the cis- and trans-isomers in
soil layers of 0.1-0.2 m and 0.4-0.5 m in a bulb field in the
Netherlands were determined in the laboratory. The initial contents
of added 1,3-dichloropropene were approximately 12 and 62 mg/kg.
Incubation took place at 15 °C. The half transformation time was
about 4 days for both isomers. After 2 weeks, only small amounts
(1%) of the initial amount were left. The transformation was slower
in soil with the higher initial content (62 mg/kg) than in soil with
12 mg/kg. The half-life was approximately 19 days for both isomers.
Only small amounts were left after one month (Van der Pas & Leistra,
1987).
The behaviour of technical grade 1,3-dichloropropene in the soil
from 4 fields (soil containing 13.2-24.6% of organic matter) was
studied in the laboratory. The transformation rates of cis- and
trans-1,3-dichloropropene were measured in soil samples taken from
the ploughed layer of the fields. Pure 1,3-dichloropropene was added
at 35 µlitre/kg moist soil. The transformation in soil from one of
the fields could be approximated with first- order kinetics during
the whole incubation period of 21 days. The half-lives of the cis-
and trans-isomers at 10 °C were 17 and 20 days, respectively. In
soil from the 3 other fields, transformation of 1,3-dichloropropene
with approximate first-order kinetics in the initial period of 7-14
days was followed by a period of accelerated transformation. The
concentration dropped below the limit of determination (0.1 mg/kg
dry soil), 14-21 days after the start of the incubation. Presumably,
soil microorganisms adapted their enzymes, resulting in an increased
rate of transformation (Van den Berg & Leistra, 1989).
In 6 loamy soils, transformation was gradual and pseudo first-
order for 3-6 days, and then, very rapid. There was no difference
between the transformation of the cis- and trans-isomers of 1,3-
dichloropropene in these soils. When the initial content in dry soil
was 62-80 mg/kg, less than 0.2% remained after a week (temperature
15 °C). The greatly accelerated transformation that occurred after a
short time lag suggests that the soils contained microorganisms that
could transform 1,3-dichloropropene effectively (Smelt et al.,
1989).
Rapid transformation was found in 6 loamy soils from fields
fumigated once or twice previously, as well as from fields never
treated; after 7 days, less than 0.2% of the applied dose (3.7, 18,
or 92 mg 1,3-dichloropropene/kg) remained. The incubation
temperature was 15 °C. However, with an initial content of 470
mg/kg, the transformation was suppressed with a half-life of 33
days. In another loamy soil, which showed no accelerated
transformation pattern, the pseudo half-lives increased from 4.3 to
36 days, when initial content of 1,3-dichloropropene was raised from
3.7 to 470 mg/kg (Smelt et al., 1989).
4.1.3.2 Volatilization
Volatilization and diffusion in the vapour phase are the most
significant mechanisms for the environmental dispersal and dilution
of 1,3-dichloropropene and 1,2-dichloropropane. Volatilization rates
from soil surfaces depend on water solubility and vapour density as
well as on soil properties, such as temperature and moisture
content, the depth of application, and surface wind velocity.
Estimates of volatilization of cis-1,3-dichloropropene from soil
have ranged from 20 to 75%.
D-D 92 was applied to sandy clay loam soil in a polyethylene
tunnel and the air in the tunnel was monitored continuously for 1,3-
dichloropropene for 4 weeks. The temperature in the tunnel was 18-29
°C. D-D 92 was injected by hand at a dose rate of 225 kg/ha, at a
depth of 15 cm. About 45% of the applied D-D 92 was volatilized as
1,3-dichloropropene in the first week, increasing to 54% after 4
weeks. No more than 5% was found as 1,3-dichloropropene or 3-
chloroallyl alcohol in the soil at the end of the 4-week period
(Sherren & Woodbridge, 1987c).
4.1.3.3 Uptake in crops
Residues in edible crops arising from the use of "MIX D/D" or
1,3-dichloropropene have only been detected in small amounts (<
0.02 mg/kg). The most obvious reason for this is the fact that crops
are not normally planted until most of the product has been
eliminated. Under certain conditions, where low concentrations of
1,3-dichloropropene persist for long periods of time, plants will
absorb measurable quantities. Uptake has been shown to occur in
potato tubers in sandy loam soil treated with 14C-1,2-
dichloropropane and 14C-1,3-dichloropropene 6 months prior to
planting (application rate 290 litre/ha). The total radioactivity
(expressed as 1,3-dichloropropene equivalents) in the tubers was 7
µg/kg (Roberts & Stoydin, 1976).
Tomatoes, bush beans, and carrots absorbed 14C-1,3-
dichloropropene from vermiculite culture solution and sand. During
24 h, the compound was absorbed and translocated through the plants.
3-Chloroallyl alcohol was also readily absorbed, but to a lesser
extent than dichloropropene. Comparison of the metabolism of 1,3-
dichloropropene and 3-chloroallyl alcohol showed rapid reversion to
the general carbon pool, the half-lives for 1,3-dichloropropene and
3-chloroallyl alcohol being 1.5 and 4.4 h, respectively (Berry et
al., 1980).
4.1.3.4 Movement in soil
Vapour diffusion is usually the most important mode of downward
movement for "MIX D/D". McKenry & Thomason (1974) injected either
Telone or "MIX D/D" into a series of soils at 11 different sites in
California. The moisture levels, temperatures, cultivation, and soil
profiles at the sites varied. The movement was studied during 13 and
69 days. The application rates ranged from 600 up to 2300 kg/ha. It
was concluded that:
* There was a substantial and downward movement of all the
components.
* Downward movement was greatest in open-textured soils that were
sufficiently moist but not saturated; the fumigant was
detectable at a depth of a few metres.
* Downward movement was encouraged by deep cultivation in soils
with horizons of low porosity.
In the United Kingdom, however, Wallace (1979) found only traces
of fumigant in the 40-60 cm layer, after an injection at a depth of
18 cm. Wallace (1976a) had found comparable results in soil in
Germany. In the European studies, the diffusion was slower, because
the applications were made in late autumn; soils were wetter,
colder, and heavier in texture. Thus, results from studies carried
out under different agronomic and climatic conditions are not
necessarily comparable.
The vertical and horizontal movements of 1,3-dichloropropene
were studied in a tree-nursery region in the north of the Federal
Republic of Germany. Sounding pipes were used to collect water
samples down to a depth of 4 m using the percussion-boring method.
Further borings were set to a depth of 3 m on days 10-91 after
application of a formulation containing cis- and trans-1,3-
dichloropropene, methylisothiocyanate and 1,2-dichloropropane at 50
ml/m2. Soil cores were analysed. 1,3-Dichloropropene showed a
rather high mobility in the soil, as it could be detected at a depth
of 4 m in all soil layers on the fourth day of application. In
samples of the near-surface groundwater, collected 140 days after
application, a concentration of 1.36 µg 1,3-dichloropropene per
litre was found. Ten to 25 m from the treated area, 1,3-
dichloropropene was also found in groundwater after 59 and 140 days
(Rexilius & Schmidt, 1982).
4.1.3.5 Loss under field conditions
Williams (1968) studied the loss of 1,3-dichloropropene under
field conditions in sandy loam and peat soils in Canada. The
application rates were approximately 1000 and 2000 litre "Mix
D/D"/ha, respectively. Eight months later, samples were collected
and residues determined (Table 4).
In studies in the Federal Republic of Germany, Netherlands, and
the United Kingdom, only very low residues (1%) of the amount
originally applied remained after 3 months in the soil (Wallace,
1976a,b; Wallace, 1979).
A comparative trial was carried out in the United Kingdom in
which "MIX D/D" and 1,3-dichloropropene were injected, at a depth of
15 cm, in clay loam at concentrations of 410 and 240 litre/ha,
respectively (Table 5, see also section 4.3.2 of "MIX D/D"). Samples
of soil were taken at depths of 0-20 cm, 20-40 cm, and 40-60 cm, at
6 intervals up to 9´ months after application. As part of normal
recommended agricultural practice, the soil was ploughed 5 weeks
after treatment. Soil samples were analysed for residues of cis-
and trans-1,3-dichloropropene, 1,2-dichloropropane, and cis- and
trans-3-chloroallyl alcohol. There was no significant difference
between the residues of the 1,3-dichloropropene or the 3-chloroallyl
alcohol resulting from the 2 treatments. As expected, no 1,2-
dichloropropane residues were detected in soil samples treated with
1,3-dichloropropene. Residues of the cis- and trans-1,3-
dichloropropenes and cis- and trans-3-chloroallyl alcohols were
detected in all samples up to 9´ months after treatment and down to
the 20-40 cm soil layer. Before the soil was ploughed, the
concentrations of these substances showed little change, and they
were present in all 3 layers, but, after ploughing, the
concentrations decreased gradually (Wallace, 1979).
Table 4. Recovery of cis- and trans-1,3-dichloropropene from
sandy loam or peat soils, 8 months after application of 1000 or 2000
litre "MIX D/D"/ha, respectively
Soil Depth in cm Residue in mg/kg soil
cis-1,3- trans-1,3-
dichloropropene dichloropropene
Peat 0-10 1.4 3.2
10-20 1.8 4.8
Sandy loam 0-10 - -
10-20 0.3 0.4
From: Williams (1968)
Table 5. Residues from the plot treated with 1,3-dichloropropene at 240 litre/haa
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 2.02 2.54 < 0.1 1.01 1.01
20-40 5.98 7.32 0.2 3.16 3.34
40-60 0.14 0.15 < 0.1 1.57b 1.88b
10 0-20 6.29 7.66 0.1 1.23 1.23
20-40 1.79 2.10 < 0.1 1.09 1.14
40-60 0.52 0.55 < 0.1 3.01b 3.24b
23 0-20 6.10 6.10 0.2 2.39 2.39
20-40 3.26 3.20 0.2 1.32 1.32
40-60 0.09 0.08 < 0.1 0.04 0.04
34 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.95 1.10 < 0.1 0.45 0.45
20-40 0.97 0.90 < 0.1 0.62 0.62
40-60 0.06 0.04 < 0.1 < 0.02 < 0.02
67 0-20 0.28 0.36 < 0.1 0.70 0.70
20-40 0.04 0.05 < 0.1 0.32 0.26
40-60 0.11 0.09 < 0.1 0.05 0.04
Table 5 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.08 0.06 < 0.1 0.20 0.20
9´ months 20-40 0.02c 0.02c < 0.1 0.04 0.03
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
Pre-treatment 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a From: Wallace (1979).
Note: All residues are on a dry weight basis.
b Anomalous results.
c Results confirmed by GC/MS.
1,3-Dichloropropene (D-D 95 and Telone II, containing > 92%),
at concentrations of 240, 280, and 290 litre/ha, was injected into
the soil of 3 bulb fields in the Netherlands in the summer. Nine
points were sampled per field and the samples were taken at various
times down to a depth of 3 m. Within a month, the concentrations
decreased to less than 0.2 mg/kg and continued to decline gradually
with time (Van der Pas & Leistra, 1987).
In 2 fields in the Netherlands (soil containing 15.7-24.6% of
organic matter), the spread of the fumigant (application rate 150
litre/ha) through the soil was measured. Only low fumigant
concentrations (about 0.1-0.4 mg/kg) were measured at a depth of 0.3
m. Around the depth of injection (0.15-0.2 m), the ratio of cis-
and trans-isomers changed with time in favour of the trans-
isomer. Cumulative emissions into the air over a period of 3 weeks
were calculated to range from 10 to 20% of the dosage of the cis-
isomer, and 4 to 15% of the trans-isomer (Van den Berg & Leistra,
1989).
4.1.3.6 Results of supervised field trials
A field study was undertaken in France in 1988, in which D-D 92
was applied to the soil prior to planting vines, and the air in the
vicinity of the treated area was monitored for 1,3-dichloropropene.
D-D 92 was applied at approximately 600 kg/ha at a depth of 30-40
cm. The air levels were monitored for 10 days. No samples contained
1,2-dichloropropane at levels above the limit of determination of
0.02 mg/m3. The highest 1,3-dichloropropene concentration found
during the first 24 h (perimeter of the field) was 2.1 mg/m3 and
this declined to 0.02-0.04 mg/m3 after 10 days. Air concentrations
also decreased with increasing distance, downwind (Sherren, 1990).
4.2 Bioconcentration
No data are available on bioconcentration.
4.3 Abiotic degradation
4.3.1 Photodegradation
Li (1979) obtained results comparable with those of Tuazon et
al. (1984) working with ozone, by irradiation of vapour of cis-
and trans-1,3-dichloropropene with a GE-RS sunlamp (see section
4.1.1). The main reaction product was 3-chloropropionyl chloride
with smaller quantities of 3-chloropropionic acid, CO2, and
phosgene. In this process, the initial reaction was epoxidation of
the double bond. There is evidence of the importance of a surface
reaction in the atmosphere, adsorption on to particulate matter
seems to be necessary for an appreciable direct phototransformation
to occur. Vapour phase photolysis of 1,3-dichloropropene was not
detected after prolonged simulated sunlight irradiation in a
reaction chamber. Photolysis occurred on the photoreactor surface
walls suggesting surface-catalysing reactions. The reaction products
suggest that 12-13% was totally degraded to CO2 after 5 days of
irradiation. Over 20% was transformed to phosgene.
No data on the photolytic decomposition of the chloropropenes
in water are available. Nevertheless, UVR of these chemicals in
methanol, in a frozen state, or as inclusion in adamantine matrices,
may cause the production of allyl radicals, by cleavage of the
allylic C-Cl bond (Krijgsheld & van der Gen, 1986).
4.4 Biodegradation and biotransformation
Several studies have been performed on the persistence of 1,3-
DCP in soil, after application as a fumigant. Biodegradation by soil
microorganisms does occur, depending on soil type, temperature, and
moisture content. The rate of disappearance ranges from a half-life
of 3 days to one of 37 days, without any consistent correlation with
organic matter content of the soil, or with pH. In sterile soils,
the effect of temperature was minimal (Van Dijk, 1974; Tabak et al.,
1981; California State Water Resources Control Board, 1983). In
general, the rates of disappearance of the cis- and trans-
isomers are similar and tend to increase with moisture content and
temperature, conditions that may increase, not only biodegradation,
but also loss by volatilization or chemical hydrolysis. Although
between 15 and 80% decomposition of field applications of 1,3-
dichloropropene has been shown, the large amount that can be
absorbed (80-90%) can result in soil residues existing months after
application is completed (Van Dijk, 1974; Roberts & Stoydin, 1976;
Sittig, 1980; Krijgsheld & van der Gen, 1986).
In biodegradability studies using a synthetic medium that
contained 5 mg of yeast extract/litre and was inoculated with waste
water, loss of 1,3-dichloropropene was determined after 7 days of
incubation. Significant degradation was observed at 5 and 10 mg of
1,3-dichloropropene/litre and gradual adaptation was shown in
subcultures. The original culture degraded about 50% of the 1,3-
dichloropropene in 7 days, while the third subculture was able to
degrade approximately 85% at both substrate concentrations, in the
same period of time (Tabak et al., 1981).
Battersby (1990a) determined the "ready biodegradability" of
trans-1,3-dichloropropene (95.4% trans- and 0.3% cis-isomer)
using the closed bottle procedure. The substance was not degraded in
this system with a negligible proportion of the theoretical oxygen
demand being consumed during the 28-day incubation period.
The EEC-activated sludge respiration inhibition test was used
to determine the effect of a cis- (51.2-52.2%) + trans- (43.9-
44.1%) mixture of 1,3-dichloropropene containing 0.33% of 1,2-
dichloropropane on the respiration rate of activated sludge. The
EC50 for this mixture was 188 mg/litre (Battersby, 1990b).
The EEC-activated sludge respiration inhibition test was also
used to determine the effect of cis-1,3-dichloropropene (94.5-
97.5% cis-, 1.5% trans-isomer and 0.25% 1,2-dichloropropane) on
the respiration rate of activated sludge. The EC50 for the cis-
1,3-dichloropropene was 279 mg/litre (Battersby, 1990c).
Biodehalogenation by soil organisms has been demonstrated for
1,3-dichloropropene. The fumigant appeared to be chemically
hydrolysed to 3-chloroallyl alcohol and then converted to 3-
chloroacrylic acid. The chlorine is removed and the intermediate
products are converted to carbon dioxide and water. The rate of
disappearance of 1,3-dichloropropene at 15-20 °C was 2-3.5% per day
in sandy soil and up to 25% per day in clay soils. The chloroallyl
alcohol disappeared at rates of 20-60% per day at 15 °C (Van Dijk,
1974). Leistra et al. (1991) incubated 1,3-dichloropropene and its
transformation product 3-chloroallyl alcohol in water-saturated
subsoil material at 10 °C. The times for 50% and 95% transformation
ranged from 15 to 47 days and from 27 to 79 days, respectively, for
1,3-dichloropropene. The corresponding 50% and 95% transformation
times for 3 chloroallyl alcohol were 0.8-4.2 and 4.0-6.5 days,
respectively.
Chemical hydrolysis is the first step in the transformation of
1,3-dichloropropene. Further transformation is thought to result
from microbial action; 3-chloroacrolein and 3-chloroacrylic acid
have been isolated from the metabolism of 3-chloroallyl alcohol by
Pseudomonas species (see Fig. 4) (Belser & Castro, 1971; Roberts &
Stoydin, 1976).
Soil culture studies using media enriched with 1,3-
dichloropropenes, 1,2-dichloropropane, and "Mix D/D" at
concentrations of up to 100 mg/kg, produced abundant growth of all
microorganisms tested, indicating the use of the fumigants as carbon
sources. Several of these organisms (Rhizobium leguminosarum,
Bacillus subtilis, Arthrobacter globiformis, and Pseudomonas
fluorescens) produced greater amounts of amino acids (Altman &
Lawlor, 1966; Altman, 1969). The cis- and trans-isomers of 1,3-
dichloropropene have undergone biodehalogenation by a Pseudomonas
sp. isolated from the soil. Cis- and trans-1,3-dichloropropene
can be chemically hydrolysed in moist soils to the corresponding 3-
chloroallyl alcohols, which can be metabolized to carbon dioxide and
water by Pseudomonas sp. (Fig. 4).
Determination of the rate of hydrolysis of 1,3-dichloropropene
at 25 °C in 50% aqueous ethanol indicated a half-time of 4 days for
both the cis- and trans-isomers and appeared independent of the
concentration in the range of 10-1000 mg/litre. Only small
differences were observed in disappearance rates at pH levels of 5.5
and 7.5. The effect of temperature was clearly demonstrated: at 29
°C, the half-life for cis-1,3-dichloropropene was 1.5-2.0 days,
while, at 2 °C, the half-life was estimated to be 91-100 days
(Krijgsheld & Van der Gen, 1986).
The rates of transformation of the cis- and trans-isomers in
soil layers of 0.1-0.2 m and 0.4-0.5 m in a bulb field in the
Netherlands were determined in the laboratory. The initial contents
of added 1,3-dichloropropene were approximately 12 and 62 mg/kg.
Incubation took place at 15 °C. The half transformation time was
about 4 days for both isomers. After 2 weeks, only small amounts
(1%) of the initial amount were left. The transformation was slower
in soil with the higher initial content (62 mg/kg) than in soil with
12 mg/kg. The half-life was approximately 19 days for both isomers.
Only small amounts were left after one month (Van der Pas & Leistra,
1987).
The behaviour of technical grade 1,3-dichloropropene in the soil
from 4 fields (soil containing 13.2-24.6% of organic matter) was
studied in the laboratory. The transformation rates of cis- and
trans-1,3-dichloropropene were measured in soil samples taken from
the ploughed layer of the fields. Pure 1,3-dichloropropene was added
at 35 µlitre/kg moist soil. The transformation in soil from one of
the fields could be approximated with first- order kinetics during
the whole incubation period of 21 days. The half-lives of the cis-
and trans-isomers at 10 °C were 17 and 20 days, respectively. In
soil from the 3 other fields, transformation of 1,3-dichloropropene
with approximate first-order kinetics in the initial period of 7-14
days was followed by a period of accelerated transformation. The
concentration dropped below the limit of determination (0.1 mg/kg
dry soil), 14-21 days after the start of the incubation. Presumably,
soil microorganisms adapted their enzymes, resulting in an increased
rate of transformation (Van den Berg & Leistra, 1989).
In 6 loamy soils, transformation was gradual and pseudo first-
order for 3-6 days, and then, very rapid. There was no difference
between the transformation of the cis- and trans-isomers of 1,3-
dichloropropene in these soils. When the initial content in dry soil
was 62-80 mg/kg, less than 0.2% remained after a week (temperature
15 °C). The greatly accelerated transformation that occurred after a
short time lag suggests that the soils contained microorganisms that
could transform 1,3-dichloropropene effectively (Smelt et al.,
1989).
Rapid transformation was found in 6 loamy soils from fields
fumigated once or twice previously, as well as from fields never
treated; after 7 days, less than 0.2% of the applied dose (3.7, 18,
or 92 mg 1,3-dichloropropene/kg) remained. The incubation
temperature was 15 °C. However, with an initial content of 470
mg/kg, the transformation was suppressed with a half-life of 33
days. In another loamy soil, which showed no accelerated
transformation pattern, the pseudo half-lives increased from 4.3 to
36 days, when initial content of 1,3-dichloropropene was raised from
3.7 to 470 mg/kg (Smelt et al., 1989).
4.1.3.2 Volatilization
Volatilization and diffusion in the vapour phase are the most
significant mechanisms for the environmental dispersal and dilution
of 1,3-dichloropropene and 1,2-dichloropropane. Volatilization rates
from soil surfaces depend on water solubility and vapour density as
well as on soil properties, such as temperature and moisture
content, the depth of application, and surface wind velocity.
Estimates of volatilization of cis-1,3-dichloropropene from soil
have ranged from 20 to 75%.
D-D 92 was applied to sandy clay loam soil in a polyethylene
tunnel and the air in the tunnel was monitored continuously for 1,3-
dichloropropene for 4 weeks. The temperature in the tunnel was 18-29
°C. D-D 92 was injected by hand at a dose rate of 225 kg/ha, at a
depth of 15 cm. About 45% of the applied D-D 92 was volatilized as
1,3-dichloropropene in the first week, increasing to 54% after 4
weeks. No more than 5% was found as 1,3-dichloropropene or 3-
chloroallyl alcohol in the soil at the end of the 4-week period
(Sherren & Woodbridge, 1987c).
4.1.3.3 Uptake in crops
Residues in edible crops arising from the use of "MIX D/D" or
1,3-dichloropropene have only been detected in small amounts (<
0.02 mg/kg). The most obvious reason for this is the fact that crops
are not normally planted until most of the product has been
eliminated. Under certain conditions, where low concentrations of
1,3-dichloropropene persist for long periods of time, plants will
absorb measurable quantities. Uptake has been shown to occur in
potato tubers in sandy loam soil treated with 14C-1,2-
dichloropropane and 14C-1,3-dichloropropene 6 months prior to
planting (application rate 290 litre/ha). The total radioactivity
(expressed as 1,3-dichloropropene equivalents) in the tubers was 7
µg/kg (Roberts & Stoydin, 1976).
Tomatoes, bush beans, and carrots absorbed 14C-1,3-
dichloropropene from vermiculite culture solution and sand. During
24 h, the compound was absorbed and translocated through the plants.
3-Chloroallyl alcohol was also readily absorbed, but to a lesser
extent than dichloropropene. Comparison of the metabolism of 1,3-
dichloropropene and 3-chloroallyl alcohol showed rapid reversion to
the general carbon pool, the half-lives for 1,3-dichloropropene and
3-chloroallyl alcohol being 1.5 and 4.4 h, respectively (Berry et
al., 1980).
4.1.3.4 Movement in soil
Vapour diffusion is usually the most important mode of downward
movement for "MIX D/D". McKenry & Thomason (1974) injected either
Telone or "MIX D/D" into a series of soils at 11 different sites in
California. The moisture levels, temperatures, cultivation, and soil
profiles at the sites varied. The movement was studied during 13 and
69 days. The application rates ranged from 600 up to 2300 kg/ha. It
was concluded that:
* There was a substantial and downward movement of all the
components.
* Downward movement was greatest in open-textured soils that were
sufficiently moist but not saturated; the fumigant was
detectable at a depth of a few metres.
* Downward movement was encouraged by deep cultivation in soils
with horizons of low porosity.
In the United Kingdom, however, Wallace (1979) found only traces
of fumigant in the 40-60 cm layer, after an injection at a depth of
18 cm. Wallace (1976a) had found comparable results in soil in
Germany. In the European studies, the diffusion was slower, because
the applications were made in late autumn; soils were wetter,
colder, and heavier in texture. Thus, results from studies carried
out under different agronomic and climatic conditions are not
necessarily comparable.
The vertical and horizontal movements of 1,3-dichloropropene
were studied in a tree-nursery region in the north of the Federal
Republic of Germany. Sounding pipes were used to collect water
samples down to a depth of 4 m using the percussion-boring method.
Further borings were set to a depth of 3 m on days 10-91 after
application of a formulation containing cis- and trans-1,3-
dichloropropene, methylisothiocyanate and 1,2-dichloropropane at 50
ml/m2. Soil cores were analysed. 1,3-Dichloropropene showed a
rather high mobility in the soil, as it could be detected at a depth
of 4 m in all soil layers on the fourth day of application. In
samples of the near-surface groundwater, collected 140 days after
application, a concentration of 1.36 µg 1,3-dichloropropene per
litre was found. Ten to 25 m from the treated area, 1,3-
dichloropropene was also found in groundwater after 59 and 140 days
(Rexilius & Schmidt, 1982).
4.1.3.5 Loss under field conditions
Williams (1968) studied the loss of 1,3-dichloropropene under
field conditions in sandy loam and peat soils in Canada. The
application rates were approximately 1000 and 2000 litre "Mix
D/D"/ha, respectively. Eight months later, samples were collected
and residues determined (Table 4).
In studies in the Federal Republic of Germany, Netherlands, and
the United Kingdom, only very low residues (1%) of the amount
originally applied remained after 3 months in the soil (Wallace,
1976a,b; Wallace, 1979).
A comparative trial was carried out in the United Kingdom in
which "MIX D/D" and 1,3-dichloropropene were injected, at a depth of
15 cm, in clay loam at concentrations of 410 and 240 litre/ha,
respectively (Table 5, see also section 4.3.2 of "MIX D/D"). Samples
of soil were taken at depths of 0-20 cm, 20-40 cm, and 40-60 cm, at
6 intervals up to 9´ months after application. As part of normal
recommended agricultural practice, the soil was ploughed 5 weeks
after treatment. Soil samples were analysed for residues of cis-
and trans-1,3-dichloropropene, 1,2-dichloropropane, and cis- and
trans-3-chloroallyl alcohol. There was no significant difference
between the residues of the 1,3-dichloropropene or the 3-chloroallyl
alcohol resulting from the 2 treatments. As expected, no 1,2-
dichloropropane residues were detected in soil samples treated with
1,3-dichloropropene. Residues of the cis- and trans-1,3-
dichloropropenes and cis- and trans-3-chloroallyl alcohols were
detected in all samples up to 9´ months after treatment and down to
the 20-40 cm soil layer. Before the soil was ploughed, the
concentrations of these substances showed little change, and they
were present in all 3 layers, but, after ploughing, the
concentrations decreased gradually (Wallace, 1979).
Table 4. Recovery of cis- and trans-1,3-dichloropropene from
sandy loam or peat soils, 8 months after application of 1000 or 2000
litre "MIX D/D"/ha, respectively
Soil Depth in cm Residue in mg/kg soil
cis-1,3- trans-1,3-
dichloropropene dichloropropene
Peat 0-10 1.4 3.2
10-20 1.8 4.8
Sandy loam 0-10 - -
10-20 0.3 0.4
From: Williams (1968)
Table 5. Residues from the plot treated with 1,3-dichloropropene at 240 litre/haa
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
3 0-20 2.02 2.54 < 0.1 1.01 1.01
20-40 5.98 7.32 0.2 3.16 3.34
40-60 0.14 0.15 < 0.1 1.57b 1.88b
10 0-20 6.29 7.66 0.1 1.23 1.23
20-40 1.79 2.10 < 0.1 1.09 1.14
40-60 0.52 0.55 < 0.1 3.01b 3.24b
23 0-20 6.10 6.10 0.2 2.39 2.39
20-40 3.26 3.20 0.2 1.32 1.32
40-60 0.09 0.08 < 0.1 0.04 0.04
34 NORMAL CULTIVATION (ploughing of the soil)
40 0-20 0.95 1.10 < 0.1 0.45 0.45
20-40 0.97 0.90 < 0.1 0.62 0.62
40-60 0.06 0.04 < 0.1 < 0.02 < 0.02
67 0-20 0.28 0.36 < 0.1 0.70 0.70
20-40 0.04 0.05 < 0.1 0.32 0.26
40-60 0.11 0.09 < 0.1 0.05 0.04
Table 5 (contd)
Concentration in soil (mg/kg)
Interval since Soil depth 1,3-dichloropropenes 1,2-dichloropropane 3-chloroallyl alcohol
application (cm)
(days) cis-isomer trans-isomer cis-isomer trans-isomer
At harvest 0-20 0.08 0.06 < 0.1 0.20 0.20
9´ months 20-40 0.02c 0.02c < 0.1 0.04 0.03
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
Pre-treatment 0-20 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
20-40 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
40-60 < 0.01 < 0.01 < 0.1 < 0.02 < 0.02
a From: Wallace (1979).
Note: All residues are on a dry weight basis.
b Anomalous results.
c Results confirmed by GC/MS.
1,3-Dichloropropene (D-D 95 and Telone II, containing > 92%),
at concentrations of 240, 280, and 290 litre/ha, was injected into
the soil of 3 bulb fields in the Netherlands in the summer. Nine
points were sampled per field and the samples were taken at various
times down to a depth of 3 m. Within a month, the concentrations
decreased to less than 0.2 mg/kg and continued to decline gradually
with time (Van der Pas & Leistra, 1987).
In 2 fields in the Netherlands (soil containing 15.7-24.6% of
organic matter), the spread of the fumigant (application rate 150
litre/ha) through the soil was measured. Only low fumigant
concentrations (about 0.1-0.4 mg/kg) were measured at a depth of 0.3
m. Around the depth of injection (0.15-0.2 m), the ratio of cis-
and trans-isomers changed with time in favour of the trans-
isomer. Cumulative emissions into the air over a period of 3 weeks
were calculated to range from 10 to 20% of the dosage of the cis-
isomer, and 4 to 15% of the trans-isomer (Van den Berg & Leistra,
1989).
4.1.3.6 Results of supervised field trials
A field study was undertaken in France in 1988, in which D-D 92
was applied to the soil prior to planting vines, and the air in the
vicinity of the treated area was monitored for 1,3-dichloropropene.
D-D 92 was applied at approximately 600 kg/ha at a depth of 30-40
cm. The air levels were monitored for 10 days. No samples contained
1,2-dichloropropane at levels above the limit of determination of
0.02 mg/m3. The highest 1,3-dichloropropene concentration found
during the first 24 h (perimeter of the field) was 2.1 mg/m3 and
this declined to 0.02-0.04 mg/m3 after 10 days. Air concentrations
also decreased with increasing distance, downwind (Sherren, 1990).
4.2 Bioconcentration
No data are available on bioconcentration.
4.3 Abiotic degradation
4.3.1 Photodegradation
Li (1979) obtained results comparable with those of Tuazon et
al. (1984) working with ozone, by irradiation of vapour of cis-
and trans-1,3-dichloropropene with a GE-RS sunlamp (see section
4.1.1). The main reaction product was 3-chloropropionyl chloride
with smaller quantities of 3-chloropropionic acid, CO2, and
phosgene. In this process, the initial reaction was epoxidation of
the double bond. There is evidence of the importance of a surface
reaction in the atmosphere, adsorption on to particulate matter
seems to be necessary for an appreciable direct phototransformation
to occur. Vapour phase photolysis of 1,3-dichloropropene was not
detected after prolonged simulated sunlight irradiation in a
reaction chamber. Photolysis occurred on the photoreactor surface
walls suggesting surface-catalysing reactions. The reaction products
suggest that 12-13% was totally degraded to CO2 after 5 days of
irradiation. Over 20% was transformed to phosgene.
No data on the photolytic decomposition of the chloropropenes
in water are available. Nevertheless, UVR of these chemicals in
methanol, in a frozen state, or as inclusion in adamantine matrices,
may cause the production of allyl radicals, by cleavage of the
allylic C-Cl bond (Krijgsheld & van der Gen, 1986).
4.4 Biodegradation and biotransformation
Several studies have been performed on the persistence of 1,3-
DCP in soil, after application as a fumigant. Biodegradation by soil
microorganisms does occur, depending on soil type, temperature, and
moisture content. The rate of disappearance ranges from a half-life
of 3 days to one of 37 days, without any consistent correlation with
organic matter content of the soil, or with pH. In sterile soils,
the effect of temperature was minimal (Van Dijk, 1974; Tabak et al.,
1981; California State Water Resources Control Board, 1983). In
general, the rates of disappearance of the cis- and trans-
isomers are similar and tend to increase with moisture content and
temperature, conditions that may increase, not only biodegradation,
but also loss by volatilization or chemical hydrolysis. Although
between 15 and 80% decomposition of field applications of 1,3-
dichloropropene has been shown, the large amount that can be
absorbed (80-90%) can result in soil residues existing months after
application is completed (Van Dijk, 1974; Roberts & Stoydin, 1976;
Sittig, 1980; Krijgsheld & van der Gen, 1986).
In biodegradability studies using a synthetic medium that
contained 5 mg of yeast extract/litre and was inoculated with waste
water, loss of 1,3-dichloropropene was determined after 7 days of
incubation. Significant degradation was observed at 5 and 10 mg of
1,3-dichloropropene/litre and gradual adaptation was shown in
subcultures. The original culture degraded about 50% of the 1,3-
dichloropropene in 7 days, while the third subculture was able to
degrade approximately 85% at both substrate concentrations, in the
same period of time (Tabak et al., 1981).
Battersby (1990a) determined the "ready biodegradability" of
trans-1,3-dichloropropene (95.4% trans- and 0.3% cis-isomer)
using the closed bottle procedure. The substance was not degraded in
this system with a negligible proportion of the theoretical oxygen
demand being consumed during the 28-day incubation period.
The EEC-activated sludge respiration inhibition test was used
to determine the effect of a cis- (51.2-52.2%) + trans- (43.9-
44.1%) mixture of 1,3-dichloropropene containing 0.33% of 1,2-
dichloropropane on the respiration rate of activated sludge. The
EC50 for this mixture was 188 mg/litre (Battersby, 1990b).
The EEC-activated sludge respiration inhibition test was also
used to determine the effect of cis-1,3-dichloropropene (94.5-
97.5% cis-, 1.5% trans-isomer and 0.25% 1,2-dichloropropane) on
the respiration rate of activated sludge. The EC50 for the cis-
1,3-dichloropropene was 279 mg/litre (Battersby, 1990c).
Biodehalogenation by soil organisms has been demonstrated for
1,3-dichloropropene. The fumigant appeared to be chemically
hydrolysed to 3-chloroallyl alcohol and then converted to 3-
chloroacrylic acid. The chlorine is removed and the intermediate
products are converted to carbon dioxide and water. The rate of
disappearance of 1,3-dichloropropene at 15-20 °C was 2-3.5% per day
in sandy soil and up to 25% per day in clay soils. The chloroallyl
alcohol disappeared at rates of 20-60% per day at 15 °C (Van Dijk,
1974). Leistra et al. (1991) incubated 1,3-dichloropropene and its
transformation product 3-chloroallyl alcohol in water-saturated
subsoil material at 10 °C. The times for 50% and 95% transformation
ranged from 15 to 47 days and from 27 to 79 days, respectively, for
1,3-dichloropropene. The corresponding 50% and 95% transformation
times for 3 chloroallyl alcohol were 0.8-4.2 and 4.0-6.5 days,
respectively.
Chemical hydrolysis is the first step in the transformation of
1,3-dichloropropene. Further transformation is thought to result
from microbial action; 3-chloroacrolein and 3-chloroacrylic acid
have been isolated from the metabolism of 3-chloroallyl alcohol by
Pseudomonas species (see Fig. 4) (Belser & Castro, 1971; Roberts &
Stoydin, 1976).
Soil culture studies using media enriched with 1,3-
dichloropropenes, 1,2-dichloropropane, and "Mix D/D" at
concentrations of up to 100 mg/kg, produced abundant growth of all
microorganisms tested, indicating the use of the fumigants as carbon
sources. Several of these organisms (Rhizobium leguminosarum,
Bacillus subtilis, Arthrobacter globiformis, and Pseudomonas
fluorescens) produced greater amounts of amino acids (Altman &
Lawlor, 1966; Altman, 1969). The cis- and trans-isomers of 1,3-
dichloropropene have undergone biodehalogenation by a Pseudomonas
sp. isolated from the soil. Cis- and trans-1,3-dichloropropene
can be chemically hydrolysed in moist soils to the corresponding 3-
chloroallyl alcohols, which can be metabolized to carbon dioxide and
water by Pseudomonas sp. (Fig. 4).
 The degradation of Telone II (92% 1,3-dichloropropene cis-
and trans-isomers; 2% 1,2-dichloropropane and 5% mixture of
propenes and hexenes, and 1% epichlorohydrin) in soil was studied
using 14C-1,3-dichloropropene in Fuquay loamy sand samples
collected from a field in Florida. The samples were collected
before, and one, and two weeks, and 2 years following application at
a rate of 15 kg/ha, at depths of 0-36 cm or 36-65 cm. After 28 days
incubation of 14C-1,3-dichloropropene in the soil, it was degraded
into 14CO2 (44%), water-soluble metabolites (probably 3-
chloroallyl alcohol), bound residues, and possibly some microbial
mass. Little or no difference was observed in the degradation of
14C-1,3-dichloropropene in soil samples collected one week prior
to the field application of Telone II, or two weeks and two years
after application. A mixed bacteria culture isolated from the soil
in the presence of a carbon source, completely degraded 14C-1,3-
dichloropropene into 14CO2, water-soluble products and microbial
mass (Ou, 1989).
4.4.1 Miscellaneous
Laboratory experiments were conducted to determine the effects
of 1,3-dichloropropene on the activity of invertase in a sandy soil.
The rates of application were 30 and 60 mg/kg. No inhibition was
found. The same dose levels were used to test the influence of the
compound on amylase in sandy soil. After 3 days, stimulation of the
formation of glucose from the added starch was seen, especially at
the lowest dose level. Microbial respiration was also tested in
sandy loam. The treatment did not significantly decrease oxygen
consumption (Tu, 1988).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Air
Telone II, at a rate of 293 litre/ha, was applied, at a depth
of 0.45 m, simultaneously with pineapple crown planting. Each row of
pineapple was covered with black polyethylene film at the time of
planting. Air samples were taken inside the cover and at ground
level, 10, 20, 22, 27, and 30 days after fumigation. The
concentration inside the cover remained steady, at least until day
9; thereafter, a decrease was noticed and, after 22 days, the
substance was no longer detectable. At ground level, the
concentration fell gradually and was non-detectable after 30 days
(Albrecht & Chenchin, 1985).
A small-scale, field study was undertaken in 1986, when air
concentrations of 1,3-dichloropropene were measured in the vicinity
of ground treated with D-D 92 (93.8%), by hand injector at a dose
rate of 330 kg/ha. The air was monitored for 2 weeks. The
concentration of 1,3-dichloropropene varied between 0.004 and 0.88
mg/m3 during the first week. Levels of 1,3-dichloropropene in the
second week were below the limit of determination (0.002 mg/m3)
(Sherren & Woodbridge, 1987a).
5.2 Water
An investigation of 1,3-dichloropropene in well-water in
California was carried out by Maddy et al. (1982). Fifty-four wells
were selected in locations of high nematocide use. No samples showed
levels above the limit of determination of 0.1 µg/litre. In a
survey, 72 water samples from wells in California were analysed for
1,3-dichloropropene, but no samples contained levels above 1
µg/litre (limit of determination) (Peoples et al., 1980).
Connors et al. (1990) analysed potable water samples collected
in 8 homes in 3 communities in Connecticut and did not find 1,3-
dichloropropene (< 0.1 µg/litre).
Dowty et al. (1975) conducted a survey on drinking-water in New
Orleans, they found 1,3-dichloropropene, but did not give actual
levels or frequency of occurrence.
No 1,3-dichloropropene (limit of determination 1 µg/litre) was
found in 30 Canadian potable water facilities (Otson et al., 1982).
Apparently, chlorination of organic materials in water may lead
to traces of 1,3-dichloropropene (< 1 µg/litre). Therefore, this
process may be responsible for the observed presence of the
substance in tap water (Otson et al., 1982; Krijgsheld & Van der
Gen, 1986).
1,3-Dichloropropene has been identified in the waste water from
a textile plant. A level of 2 µg cis-isomer/litre was measured in
the influent of the waste water treatment plant, while higher
concentrations of cis-1,3-dichloropropene (e.g., 6 µg/litre) were
found in the effluent, together with the trans-isomer (0.9-4
µg/litre). Similarly, no 1,3-dichloropropene was detected in the
influent of a municipal waste treatment plant, but, after "super-
chlorination", a mean concentration of approximately 10 µg/litre
could be detected in the liquid sludge (Krijgsheld & Van der Gen,
1986).
Hallberg (1989) reported studies on the presence of pesticides
in groundwater in different States of the USA. 1,3-Dichloropropene
was found only in Oregon, but no concentration(s) were reported.
Van Beek et al. (1988) examined 33 groundwater wells up to a
depth of 50 m in the northern Netherlands for the presence of 1,3-
dichloropropene. In this area, "MIX D/D" had been used on a large
scale as a nematocide in potato growing since 1967. 1,2-
Dichloropropane was present in the groundwater, but no 1,3-
dichloropropene (> 0.1 µg/litre) was found in 45 samples from these
wells.
Samples of upper groundwater (from 1-2 m below the water level)
below 4 sandy soils were analysed in the Netherlands, for 2.5 years
in 8 sampling rounds. 1,3-Dichloropropene was detected in the
groundwater in 6/34 samples at concentrations in the range of
< 0.1-80 µg/litre. These observations were made below fields with
potato, maize, and bulb flower crops, all on low-humic to moderately
humic sandy soils (Loch & Verdam, 1989).
Lagas et al. (1989) analysed groundwater (up to 6 m depth) in 5
areas (4 of which are described by Loch & Verdam, 1989), and found
1,3-dichloropropene levels above the limit of detection (0.1
µg/litre) in 2 out of 22 samples (range: < 0.1-0.2 µg/litre) taken
from underneath potato crops and in 1 out of 8 samples (< 0.1-2.5
µg/litre) from below maize and bulb crops.
On 5 sites in a polder in the Netherlands, samples of surface
water were taken monthly in 1987-88 and analysed. The area is
situated next to the dunes (where groundwater is being pumped up for
the preparation of drinking-water), and is extensively used for
bulb-culture. The maximum concentration found for 1,3-
dichloropropenes ( cis- and trans-) was 2.5 µg/litre (Greve et
al., 1989).
In the Netherlands and the Federal Republic of Germany, 1,3-
dichloropropene was found in areas with extensive agriculture and
horticulture. 1,3-Dichloropropene was found in the upper groundwater
(depth 1-5 m) and the average levels ranged from 0.6 to 2530
µg/litre (maximum level 8620 µg/litre). In bores for irrigation (11-
24 m depth), an average of 0.23 (< 0.02-0.89) µg/litre was found
(Leistra & Boesten, 1989).
Ahlsdorf et al. (1989) determined the presence of 1,3-
dichloropropene in the upper groundwater of an area used for potato
growing, which was treated with this nematocide (about 140 kg/ha) in
1984. Very low levels of 1,3-dichloropropene (1-4 µg/litre) were
found in soil with a high organic matter content, but concentrations
of up to 8620 µg/litre were found in the groundwater of a clay
podsol soil containing a high sand content, after one month.
1,3-Dichloropropene was detected in irrigation wells that were
close to a piece of land that was treated with the chemical (10-25 m
distance) in Schleswig Holstein (Germany). In the well water,
concentrations of 1,3-dichloropropene varied between 0.06 and 0.89
µg/litre (Rexilius & Schmidt, 1982).
5.3 Crops
Residues in edible crop commodities, arising from the use of
1,3-dichloropropene or "MIX D/D", are reported to be generally below
the limit of detection. The obvious reason for this, is the fact
that crops are not normally planted until most of the product
applied has dissipated. Another reason is that any 1,3-
dichloropropene or "MIX D/D" taken up by the plant, would have to
survive the whole crop cycle to be detected in the harvest
commodity.
Supervised trials with "MIX D/D", with 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
5.4 Occupational exposure
Albrecht (1987) carried out a survey to assess the exposure of
72 workers on a Hawaiian pineapple farm (attendants, crown
unloaders, (truck) drivers, irrigation workers, supervisors, mulch
coverers, and planters). Exposures were predominantly below 4.54
mg/m3 (1 ppm). The concentrations in these workers ranged between
0.032 and 4.626 mg/m3 (0.007-1.019 ppm).
Brouwer et al. (1991a) studied the inhalation of cis- and
trans-1,3-dichloropropene in 12 commercial applicators in the
Netherlands. The time-weighted average (TWA) concentrations of 1,3-
dichloropropene ranged from 1.9 to 18.9 mg/m3. Short-term exposure
levels during tank-loading and repair ranged up to 30 mg/m3. No
correlation was observed between exposure and total area injected
with 1,3-dichloropropene. Emission of 1,3-dichloropropene vapour
from the soil or from spilled liquid dripping from the nozzles on to
the soil may contribute to exposure.
An employee air-monitoring study to determine the amount of
Telone II to which personnel would be exposed, removing soil core
samples in the immediate area of the drilling, was carried out. The
concentration in the air was between 0.0982 and 1.79 mg/m3 on the
first day, and between 0.202 and 3.056 mg/m3 on second day. The
time-weighted averages from personal monitoring on days one and two
were 0.65 and 0.90 mg/m3, respectively. The time-weighted averages
from air monitoring on days one and two were 0.39 and 0.59 mg/m3
(Fong & Maykoski, 1985).
A study on a single operator during a one-day application was
carried out in the Federal Republic of Germany in 1986. Short-term
inhalation exposures to 1,3-dichloropropene were observed during the
filling operation (5.6-16.3 mg/m3) and during nozzle changing
(18.3 mg/m3). The overall exposure during 11 h exceeded the
recommended TWA value (Eadsforth et al., 1987).
An air monitoring study on exposure to 1,3-dichloropropene
during the application of "Mix D/D" (not less than 50%) and D-D 92
(not less than 92%) was carried out at different locations near
Nimes in France in 1988. The 8-h time-weighted average (TWA) air
concentrations of total 1,3-dichloropropene for the applicator on
the 2 days of application were 11.3 and 13.2 mg/m3, respectively,
and for the tractor driver on the second day, 14.4 mg/m3.
Relatively high, short-term inhalation exposures of the applicator
were measured during filling operations; the concentrations varied
between 6.4 and 83.5 mg/m3. These short-term exposures were found
to contribute significantly to the overall time-weighted average
exposures over the working period (Rocchi & van Sittert, 1989).
Albrecht & Chenchin (1985) found measurable concentrations of
1,3-dichloropropene in the range of 2.4-18.5 mg/m3 during a 8-h
shift in 8 out of 15 workers, planting pineapple crowns by hand,
simultaneously with 1,3-dichloropropene (Telone II) treatment of the
soil at 293 litre/ha.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.1.1 Rat
Groups of 6 adult male and 6 female Carworth Farm E rats
received, by stomach tube, 2.5-2.7 mg cis-1,3-dichloro-[2-
14C]propene or trans-1,3-dichloro-[2-14C]propene in 0.5 ml
arachis oil per rat, and excretion was followed. After 4 days, the
animals were killed and the radioactivity measured in skin and
carcasses. The excretion of radioactivity was very rapid, 80-90% was
eliminated in the faeces, urine, and expired air in the first 24 h.
The urine was the major route of elimination, i.e., 80.7 and 56.5%
(average of males and females) of the dose for cis- and trans-
1,3-dichloropropene, respectively. Only 2.6 and 2.2% of the 2
isomers, respectively, were eliminated in the faeces in 4 days,
while 3.9 and 23.5%, respectively, were eliminated as 14CO2 in 4
days in the expired air. Levels of the other volatile compounds in
air were only 1-3% of the dose. Up to 1% of the dose in the skin and
carcass was found. The difference in the amount of labelled CO2 in
expired air and urine indicated a difference in the kinetics of the
2 isomers (Hutson et al., 1971).
Groups of 8 adult Fischer 344 rats/sex were given non
radiolabelled 1,3-dichloropropene at 5 mg/kg body weight, in corn
oil, by gavage, for 14 consecutive days, prior to a single dose of 5
mg 14C-1,3-dichloropropene/kg body weight (actual 4.5 mg)
(uniformly labelled) (96.3%; 53.3% cis- and 43.0% trans-),
administered to 5 out of the 8 rats on day 15. The remaining 3
rats/sex were sacrificed. The distribution of radioactivity found in
the tissues (4-6%) of repeatedly dosed rats, 48 h after dosing, was
similar to that of single dosed animals. There was no sex difference
in the distribution of the radioactivity. In addition to the
repeatedly dosed rats, 2 rats of each sex, which had not been
previously dosed, received a single gavage dose of 5 mg 14C-1,3-
dichloropropene/kg body weight. The urine was the major route of
elimination of the radioactivity derived from 14C-1,3-
dichloropropene, which ranged from 60 to 65% of the administered
dose in 48 h in the rats with repeated doses and a single dose.
Elimination of 1,3-dichloropropene as 14CO2 was approximately
(average) 26% of the administrated radioactivity with about 4-5% of
the dose eliminated in the faeces, for all groups (Waechter & Kastl,
1988).
In another study, the fate of 14C- cis- and 14C- trans-
1,3-dichloropropene (97%; 62% cis and 38% trans) was determined
after a single oral dose of 1 or 50 mg/kg body weight to male
Fischer 344 rats (3 animals per dose level). Urine, faeces, expired
air, tissues, and remaining carcasses were analysed after 48 h.
Urine was the major route of excretion, 51-61% of the administered
dose being excreted over 48 h. In the carcass, 6% of the dose was
found at the end of 48 h. On the basis of interval excretion data,
half-lives for urinary excretion ranged from 5 to 6 h. Faeces and
expired CO2 accounted for roughly 18% and 6%, respectively. The
tissue concentrations of 14C activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, skin,
and fat (Dietz et al., 1984a,b, 1985).
6.1.1.2 Mouse
The fate of 14C- cis- and 14C- trans-1,3-dichloropropene
(97%; 62% cis and 38% trans) was studied after oral dosing of
male B6C3F1 mice with 1 or 100 mg/kg body weight (3
animals/dose level). Urine, faeces, expired air, tissues, and
remaining carcasses were analysed after 48 h. Urine was the major
route of excretion, with 63 and 79%, respectively, of the
administered doses (1 and 100 mg/kg body weight) being excreted over
48 h. Half-lives for urinary excretion ranged from 5 to 6 h. Faeces
and expired CO2 accounted for 15 and 14% of the 14C-
radioactivity, respectively. In the carcass, 2% was found. The
tissue concentrations of 14C-activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, fat,
and skin (Dietz et al., 1984a,b, 1985).
6.1.2 Inhalation
6.1.2.1 Rat
Stott & Kastl (1985, 1986) studied the pharmacokinetics of the
uptake of vapours of technical grade 1,3-dichloropropene (49.3%
cis- and 42.8% trans-isomer) and the disappearance of cis- and
trans-1,3-dichloropropene from the blood in groups of 3-6 male
Fischer 344 rats exposed to actual concentrations of 136, 409, 1362,
and 4086 mg/m3 for 3 h.
The uptake of 1,3-dichloropropene did not increase
proportionately with increasing exposure concentration due to an
exposure level-related decrease in the respiration rate and
respiration min/volume of rats exposed to > 409 mg 1,3-
dichloropropene/m3 and the saturation of metabolism of 1,3-
dichloropropene in rats exposed to > 1362 mg/m3. Absorption of
inhaled 1,3-dichloropropene occurred via the lungs, primarily in the
lower respiratory tract (approximately 50% of total inhaled), with a
small amount via the nasal mucosa (11-16%).
Following exposure to < 1362 mg/m3, both isomers were
rapidly eliminated from the blood, with a half-life of 3-6 min.
There was no interaction in the kinetics of both isomers. In
addition, data obtained on rats exposed to 1362 mg/m3 revealed
that this rapid elimination phase was followed by a slower
elimination phase having a half-life of 33-43 min. These data
demonstrated that a combination of saturable metabolism and
chemically-induced changes in respiration control 1,3-
dichloropropene uptake and body-burden in rats. However, only
decreases in respiration appear to influence vapour uptake.
Fisher & Kilgore (1988a) studied the excretion of the
mercapturic acid of cis-dichloropropene in Sprague-Dawley rats. In
a nose-only exposure system, groups of 3 rats were exposed for 1 h
to Telone II (94%) at average concentrations of 0, 181.6, 485.8,
1289.4, 1806.9, or 3582.1 mg/m3. Urine samples (24 h) were
collected and analysed for the mercapturic derivative of cis-
dichloropropene. At the lower exposure levels (< 1289.4 mg/m3),
urinary excretion of the mercapturic acid derivative increased with
exposure level. With exposure to 1806.9 or 3582.1 mg/m3, no
further increase was found, suggesting saturation of the metabolic
process.
6.2 Influence on tissue levels of glutathione
6.2.1 Oral
Oral administration of 1,3-dichloropropene to rats or mice
resulted in significant, dose-related reductions in the levels of
non-protein sulfhydryls (NPS) (indicator of tissue glutathione
concentration) in the forestomach and to a lesser extent in the
glandular stomach and liver (Dietz et al., 1984b, 1985, see also
section 6.4).
6.2.2 Inhalation
Shortly after inhalation exposure of rats to cis-1,3-
dichloropropene, kidney and liver NPS contents were reduced in a
dose-related manner, but returned to control values 18 h after
exposure. Lung NPS levels were not affected (Stott & Kastl, 1986,
see section 6.1.2.1; Nitschke & Lomax, 1990, see section 8.2.2.2).
Male Sprague-Dawley rats (200-250 g) were exposed through
inhalation to 1,3-dichloropropene (Telone II, 94%) concentrations of
0, 9.1, 22.7, 150, 1384.7, 3504.9, 4335.7, or 7790.6 mg/m3 to
assess the relationship between 1,3-dichloropropene exposure
concentration and tissue levels of reduced glutathione (GSH).
Animals were exposed for 1 h in a dynamic, nose-only system. GSH
contents were measured in the heart, kidneys, liver, lung, nasal
mucosa, and testes, 2 h after 1,3-dichloropropene exposure. A
decrease in nasal GSH, first seen at 22.7 mg/m3, followed an
exposure concentration-dependent curve. Exposure to concentrations
above 150 mg/m3 reduced the level of liver GSH. Lung GSH remained
relatively constant at 75% of control concentrations up to 4335.7
mg/m3. Significantly decreased GSH levels were observed in the
heart, liver, lung, and testes at 7790.6 mg/m3. Kidney GSH content
was not significantly decreased. Unchanged 1,3-dichloropropene was
not present in the blood of animals 2 h after exposure to 4335.7
mg/m3 or less. Serum lactic dehydrogenase activity was affected
only at 7790.6 mg/m3. Lung weight, measured 2 and 6 h after
exposure, did not differ from controls for any exposure level
(Fisher & Kilgore, 1988b).
Four male Sprague-Dawley rats (200-250 g) were exposed to
Telone II (94%) for 1 h, in a dynamic, nose-only exposure system.
The actual 1,3-dichloropropene concentration was 354.1 ± 49.9, 703.7
± 408.6, and 1834.2 ± 113.5 mg/m3 (relative concentrations of
cis- and trans-isomers were approximately 62 and 38%,
respectively). The GSH conjugation of 1,3-dichloropropene (GSCP) in
the blood of rats following exposure showed that there was no
significant difference between the regression line expressed as
either monophasic or biphasic decay at any exposure concentration.
Moreover, no differences were found in the regression lines between
the exposure concentrations. The elimination half-time of GSCP was
approximately 17 h following exposure to 354.1, 703.7, or 1834.2
mg/m3, and, thus, was not dose-dependent. This fits a one-
compartment model (Fischer & Kilgore, 1989).
6.3 Biotransformation
6.3.1 Rat
In urine from rats and mice treated orally with 14C-
dichloropropene, no unchanged parent compound, but 2 major and 2
minor metabolites were found. The predominant metabolite was N-
acetyl- S-(3-chloroprop-2-enyl) cysteine with its sulfoxide or
sulfone. These data indicate that conjugation with glutathione is a
major route of 1,3-dichloropropene metabolism in the rat (Dietz et
al., 1984a,b, 1985) (see Fig. 4 and section 6.1.1).
Although the spontaneous reaction of cis-1,3-dichloropropene
with glutathione is slow in the rat, the rapid urinary excretion is
due to hepatic glutathione transferase, which catalyses its
conjugation with glutathione. The transferase is present in the rat
liver cytosol fraction and little microsomally mediated metabolism
occurs. The cis-isomer is a better substrate than the trans-
isomer for glutathione transferase. The conjugation then follows a
classic mercapturic acid pathway (Boyland & Chasseaud, 1969). The
conjugated product N-acetyl- S-(3-chloroprop-2-enyl) cysteine and
its sulfoxide are excreted in the urine of rats and mice (Climie et
al., 1979; Dietz et al., 1984b; van Sittert, 1984, 1989).
It has been shown that a minor metabolic pathway of the cis-
1,3-dichloropropene is mono-oxygenase catalysed oxygenation, leading
to the possible formation of the metabolite cis-1,3-
dichloropropene-oxide (II in Fig. 5) (Van Sittert, 1989).
Rats administered 25-450 µg cis- and trans-1,3-
dichloropropene/kg body weight, intraperitoneally, showed excretion
of N-acetyl- S-( cis- and trans-3-chloroprop-2-enyl)-L-
cysteine for 55% (cis-) and 45% (trans-) of the dose within 24 h
(Onkenhout et al., 1986).
In the study of Waechter & Kastl (1988) (see section 6.1.1.1),
in which rats were administered daily doses of 5 mg of non-labelled
1,3-dichloropropene/kg body weight followed by a single dose of 5 mg
14-C (uniformly) labelled 1,3-dichloropropene, or a single dose of
5 mg/kg body weight, the major urinary metabolites were the
mercapturic acid of 1,3-dichloropropene (1,3-D-MA) and its
sulfoxide. The repeatedly dosed rats excreted slightly higher
percentages of the dose as mercapturic acids than the single dosed
rats (28.5% vs 22.7% for males and 25.5% vs 14.3% for females). The
isomeric ratio of the 2,3-D-MA was approximately 80% cis- and 20%
trans- for all groups.
6.3.2 Humans
Van Welie et al. (1989, 1991) determined the relationship
between respiratory occupational exposure to cis- and trans-1,3-
dichloropropene and urinary excretion of 2 mercapturic acid
metabolites, N-acetyl- S-( cis- and trans-)-3-chloroprop-2-
enyl)-L-cysteine ( cis- and trans-DCP-MA) by 12, 1,3-
dichloropropene applicators in the Netherlands. Urinary excretion of
these mercapturic acids followed first-order elimination kinetics.
Urinary half-lives of elimination were 5.0 ± 1.2 h for the cis-
mercapturic acid and 4.7 ± 1.3 h for the transform. These values
were not statistically significantly different. A clear correlation
was observed between the 8-h time-weighted average (TWA) exposure to
cis- and trans-1,3-dichloropropene and complete cumulative
urinary excretion of cis- and trans-DCP-MA. The cis-DCP-MA
yielded 3 times more mercapturic acid (45%) than the trans- form
(14%), probably because of differences in kinetics. It was concluded
that the uptake of cis- and trans-1,3-dichloropropene, their
metabolism to the corresponding mercapturic acids, and urinary
excretion was a rapid process.
The degradation of Telone II (92% 1,3-dichloropropene cis-
and trans-isomers; 2% 1,2-dichloropropane and 5% mixture of
propenes and hexenes, and 1% epichlorohydrin) in soil was studied
using 14C-1,3-dichloropropene in Fuquay loamy sand samples
collected from a field in Florida. The samples were collected
before, and one, and two weeks, and 2 years following application at
a rate of 15 kg/ha, at depths of 0-36 cm or 36-65 cm. After 28 days
incubation of 14C-1,3-dichloropropene in the soil, it was degraded
into 14CO2 (44%), water-soluble metabolites (probably 3-
chloroallyl alcohol), bound residues, and possibly some microbial
mass. Little or no difference was observed in the degradation of
14C-1,3-dichloropropene in soil samples collected one week prior
to the field application of Telone II, or two weeks and two years
after application. A mixed bacteria culture isolated from the soil
in the presence of a carbon source, completely degraded 14C-1,3-
dichloropropene into 14CO2, water-soluble products and microbial
mass (Ou, 1989).
4.4.1 Miscellaneous
Laboratory experiments were conducted to determine the effects
of 1,3-dichloropropene on the activity of invertase in a sandy soil.
The rates of application were 30 and 60 mg/kg. No inhibition was
found. The same dose levels were used to test the influence of the
compound on amylase in sandy soil. After 3 days, stimulation of the
formation of glucose from the added starch was seen, especially at
the lowest dose level. Microbial respiration was also tested in
sandy loam. The treatment did not significantly decrease oxygen
consumption (Tu, 1988).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Air
Telone II, at a rate of 293 litre/ha, was applied, at a depth
of 0.45 m, simultaneously with pineapple crown planting. Each row of
pineapple was covered with black polyethylene film at the time of
planting. Air samples were taken inside the cover and at ground
level, 10, 20, 22, 27, and 30 days after fumigation. The
concentration inside the cover remained steady, at least until day
9; thereafter, a decrease was noticed and, after 22 days, the
substance was no longer detectable. At ground level, the
concentration fell gradually and was non-detectable after 30 days
(Albrecht & Chenchin, 1985).
A small-scale, field study was undertaken in 1986, when air
concentrations of 1,3-dichloropropene were measured in the vicinity
of ground treated with D-D 92 (93.8%), by hand injector at a dose
rate of 330 kg/ha. The air was monitored for 2 weeks. The
concentration of 1,3-dichloropropene varied between 0.004 and 0.88
mg/m3 during the first week. Levels of 1,3-dichloropropene in the
second week were below the limit of determination (0.002 mg/m3)
(Sherren & Woodbridge, 1987a).
5.2 Water
An investigation of 1,3-dichloropropene in well-water in
California was carried out by Maddy et al. (1982). Fifty-four wells
were selected in locations of high nematocide use. No samples showed
levels above the limit of determination of 0.1 µg/litre. In a
survey, 72 water samples from wells in California were analysed for
1,3-dichloropropene, but no samples contained levels above 1
µg/litre (limit of determination) (Peoples et al., 1980).
Connors et al. (1990) analysed potable water samples collected
in 8 homes in 3 communities in Connecticut and did not find 1,3-
dichloropropene (< 0.1 µg/litre).
Dowty et al. (1975) conducted a survey on drinking-water in New
Orleans, they found 1,3-dichloropropene, but did not give actual
levels or frequency of occurrence.
No 1,3-dichloropropene (limit of determination 1 µg/litre) was
found in 30 Canadian potable water facilities (Otson et al., 1982).
Apparently, chlorination of organic materials in water may lead
to traces of 1,3-dichloropropene (< 1 µg/litre). Therefore, this
process may be responsible for the observed presence of the
substance in tap water (Otson et al., 1982; Krijgsheld & Van der
Gen, 1986).
1,3-Dichloropropene has been identified in the waste water from
a textile plant. A level of 2 µg cis-isomer/litre was measured in
the influent of the waste water treatment plant, while higher
concentrations of cis-1,3-dichloropropene (e.g., 6 µg/litre) were
found in the effluent, together with the trans-isomer (0.9-4
µg/litre). Similarly, no 1,3-dichloropropene was detected in the
influent of a municipal waste treatment plant, but, after "super-
chlorination", a mean concentration of approximately 10 µg/litre
could be detected in the liquid sludge (Krijgsheld & Van der Gen,
1986).
Hallberg (1989) reported studies on the presence of pesticides
in groundwater in different States of the USA. 1,3-Dichloropropene
was found only in Oregon, but no concentration(s) were reported.
Van Beek et al. (1988) examined 33 groundwater wells up to a
depth of 50 m in the northern Netherlands for the presence of 1,3-
dichloropropene. In this area, "MIX D/D" had been used on a large
scale as a nematocide in potato growing since 1967. 1,2-
Dichloropropane was present in the groundwater, but no 1,3-
dichloropropene (> 0.1 µg/litre) was found in 45 samples from these
wells.
Samples of upper groundwater (from 1-2 m below the water level)
below 4 sandy soils were analysed in the Netherlands, for 2.5 years
in 8 sampling rounds. 1,3-Dichloropropene was detected in the
groundwater in 6/34 samples at concentrations in the range of
< 0.1-80 µg/litre. These observations were made below fields with
potato, maize, and bulb flower crops, all on low-humic to moderately
humic sandy soils (Loch & Verdam, 1989).
Lagas et al. (1989) analysed groundwater (up to 6 m depth) in 5
areas (4 of which are described by Loch & Verdam, 1989), and found
1,3-dichloropropene levels above the limit of detection (0.1
µg/litre) in 2 out of 22 samples (range: < 0.1-0.2 µg/litre) taken
from underneath potato crops and in 1 out of 8 samples (< 0.1-2.5
µg/litre) from below maize and bulb crops.
On 5 sites in a polder in the Netherlands, samples of surface
water were taken monthly in 1987-88 and analysed. The area is
situated next to the dunes (where groundwater is being pumped up for
the preparation of drinking-water), and is extensively used for
bulb-culture. The maximum concentration found for 1,3-
dichloropropenes ( cis- and trans-) was 2.5 µg/litre (Greve et
al., 1989).
In the Netherlands and the Federal Republic of Germany, 1,3-
dichloropropene was found in areas with extensive agriculture and
horticulture. 1,3-Dichloropropene was found in the upper groundwater
(depth 1-5 m) and the average levels ranged from 0.6 to 2530
µg/litre (maximum level 8620 µg/litre). In bores for irrigation (11-
24 m depth), an average of 0.23 (< 0.02-0.89) µg/litre was found
(Leistra & Boesten, 1989).
Ahlsdorf et al. (1989) determined the presence of 1,3-
dichloropropene in the upper groundwater of an area used for potato
growing, which was treated with this nematocide (about 140 kg/ha) in
1984. Very low levels of 1,3-dichloropropene (1-4 µg/litre) were
found in soil with a high organic matter content, but concentrations
of up to 8620 µg/litre were found in the groundwater of a clay
podsol soil containing a high sand content, after one month.
1,3-Dichloropropene was detected in irrigation wells that were
close to a piece of land that was treated with the chemical (10-25 m
distance) in Schleswig Holstein (Germany). In the well water,
concentrations of 1,3-dichloropropene varied between 0.06 and 0.89
µg/litre (Rexilius & Schmidt, 1982).
5.3 Crops
Residues in edible crop commodities, arising from the use of
1,3-dichloropropene or "MIX D/D", are reported to be generally below
the limit of detection. The obvious reason for this, is the fact
that crops are not normally planted until most of the product
applied has dissipated. Another reason is that any 1,3-
dichloropropene or "MIX D/D" taken up by the plant, would have to
survive the whole crop cycle to be detected in the harvest
commodity.
Supervised trials with "MIX D/D", with 23 crops in 8 countries
showed that residues in edible crop commodities were below the
limits of determination (< 0.01 mg/kg), for 1,3-dichloropropene,
1,2-dichloropropane, and 3-chloroallyl alcohol.
5.4 Occupational exposure
Albrecht (1987) carried out a survey to assess the exposure of
72 workers on a Hawaiian pineapple farm (attendants, crown
unloaders, (truck) drivers, irrigation workers, supervisors, mulch
coverers, and planters). Exposures were predominantly below 4.54
mg/m3 (1 ppm). The concentrations in these workers ranged between
0.032 and 4.626 mg/m3 (0.007-1.019 ppm).
Brouwer et al. (1991a) studied the inhalation of cis- and
trans-1,3-dichloropropene in 12 commercial applicators in the
Netherlands. The time-weighted average (TWA) concentrations of 1,3-
dichloropropene ranged from 1.9 to 18.9 mg/m3. Short-term exposure
levels during tank-loading and repair ranged up to 30 mg/m3. No
correlation was observed between exposure and total area injected
with 1,3-dichloropropene. Emission of 1,3-dichloropropene vapour
from the soil or from spilled liquid dripping from the nozzles on to
the soil may contribute to exposure.
An employee air-monitoring study to determine the amount of
Telone II to which personnel would be exposed, removing soil core
samples in the immediate area of the drilling, was carried out. The
concentration in the air was between 0.0982 and 1.79 mg/m3 on the
first day, and between 0.202 and 3.056 mg/m3 on second day. The
time-weighted averages from personal monitoring on days one and two
were 0.65 and 0.90 mg/m3, respectively. The time-weighted averages
from air monitoring on days one and two were 0.39 and 0.59 mg/m3
(Fong & Maykoski, 1985).
A study on a single operator during a one-day application was
carried out in the Federal Republic of Germany in 1986. Short-term
inhalation exposures to 1,3-dichloropropene were observed during the
filling operation (5.6-16.3 mg/m3) and during nozzle changing
(18.3 mg/m3). The overall exposure during 11 h exceeded the
recommended TWA value (Eadsforth et al., 1987).
An air monitoring study on exposure to 1,3-dichloropropene
during the application of "Mix D/D" (not less than 50%) and D-D 92
(not less than 92%) was carried out at different locations near
Nimes in France in 1988. The 8-h time-weighted average (TWA) air
concentrations of total 1,3-dichloropropene for the applicator on
the 2 days of application were 11.3 and 13.2 mg/m3, respectively,
and for the tractor driver on the second day, 14.4 mg/m3.
Relatively high, short-term inhalation exposures of the applicator
were measured during filling operations; the concentrations varied
between 6.4 and 83.5 mg/m3. These short-term exposures were found
to contribute significantly to the overall time-weighted average
exposures over the working period (Rocchi & van Sittert, 1989).
Albrecht & Chenchin (1985) found measurable concentrations of
1,3-dichloropropene in the range of 2.4-18.5 mg/m3 during a 8-h
shift in 8 out of 15 workers, planting pineapple crowns by hand,
simultaneously with 1,3-dichloropropene (Telone II) treatment of the
soil at 293 litre/ha.
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.1.1 Rat
Groups of 6 adult male and 6 female Carworth Farm E rats
received, by stomach tube, 2.5-2.7 mg cis-1,3-dichloro-[2-
14C]propene or trans-1,3-dichloro-[2-14C]propene in 0.5 ml
arachis oil per rat, and excretion was followed. After 4 days, the
animals were killed and the radioactivity measured in skin and
carcasses. The excretion of radioactivity was very rapid, 80-90% was
eliminated in the faeces, urine, and expired air in the first 24 h.
The urine was the major route of elimination, i.e., 80.7 and 56.5%
(average of males and females) of the dose for cis- and trans-
1,3-dichloropropene, respectively. Only 2.6 and 2.2% of the 2
isomers, respectively, were eliminated in the faeces in 4 days,
while 3.9 and 23.5%, respectively, were eliminated as 14CO2 in 4
days in the expired air. Levels of the other volatile compounds in
air were only 1-3% of the dose. Up to 1% of the dose in the skin and
carcass was found. The difference in the amount of labelled CO2 in
expired air and urine indicated a difference in the kinetics of the
2 isomers (Hutson et al., 1971).
Groups of 8 adult Fischer 344 rats/sex were given non
radiolabelled 1,3-dichloropropene at 5 mg/kg body weight, in corn
oil, by gavage, for 14 consecutive days, prior to a single dose of 5
mg 14C-1,3-dichloropropene/kg body weight (actual 4.5 mg)
(uniformly labelled) (96.3%; 53.3% cis- and 43.0% trans-),
administered to 5 out of the 8 rats on day 15. The remaining 3
rats/sex were sacrificed. The distribution of radioactivity found in
the tissues (4-6%) of repeatedly dosed rats, 48 h after dosing, was
similar to that of single dosed animals. There was no sex difference
in the distribution of the radioactivity. In addition to the
repeatedly dosed rats, 2 rats of each sex, which had not been
previously dosed, received a single gavage dose of 5 mg 14C-1,3-
dichloropropene/kg body weight. The urine was the major route of
elimination of the radioactivity derived from 14C-1,3-
dichloropropene, which ranged from 60 to 65% of the administered
dose in 48 h in the rats with repeated doses and a single dose.
Elimination of 1,3-dichloropropene as 14CO2 was approximately
(average) 26% of the administrated radioactivity with about 4-5% of
the dose eliminated in the faeces, for all groups (Waechter & Kastl,
1988).
In another study, the fate of 14C- cis- and 14C- trans-
1,3-dichloropropene (97%; 62% cis and 38% trans) was determined
after a single oral dose of 1 or 50 mg/kg body weight to male
Fischer 344 rats (3 animals per dose level). Urine, faeces, expired
air, tissues, and remaining carcasses were analysed after 48 h.
Urine was the major route of excretion, 51-61% of the administered
dose being excreted over 48 h. In the carcass, 6% of the dose was
found at the end of 48 h. On the basis of interval excretion data,
half-lives for urinary excretion ranged from 5 to 6 h. Faeces and
expired CO2 accounted for roughly 18% and 6%, respectively. The
tissue concentrations of 14C activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, skin,
and fat (Dietz et al., 1984a,b, 1985).
6.1.1.2 Mouse
The fate of 14C- cis- and 14C- trans-1,3-dichloropropene
(97%; 62% cis and 38% trans) was studied after oral dosing of
male B6C3F1 mice with 1 or 100 mg/kg body weight (3
animals/dose level). Urine, faeces, expired air, tissues, and
remaining carcasses were analysed after 48 h. Urine was the major
route of excretion, with 63 and 79%, respectively, of the
administered doses (1 and 100 mg/kg body weight) being excreted over
48 h. Half-lives for urinary excretion ranged from 5 to 6 h. Faeces
and expired CO2 accounted for 15 and 14% of the 14C-
radioactivity, respectively. In the carcass, 2% was found. The
tissue concentrations of 14C-activity were highest in the stomach
wall, followed in decreasing order by kidneys, liver, bladder, fat,
and skin (Dietz et al., 1984a,b, 1985).
6.1.2 Inhalation
6.1.2.1 Rat
Stott & Kastl (1985, 1986) studied the pharmacokinetics of the
uptake of vapours of technical grade 1,3-dichloropropene (49.3%
cis- and 42.8% trans-isomer) and the disappearance of cis- and
trans-1,3-dichloropropene from the blood in groups of 3-6 male
Fischer 344 rats exposed to actual concentrations of 136, 409, 1362,
and 4086 mg/m3 for 3 h.
The uptake of 1,3-dichloropropene did not increase
proportionately with increasing exposure concentration due to an
exposure level-related decrease in the respiration rate and
respiration min/volume of rats exposed to > 409 mg 1,3-
dichloropropene/m3 and the saturation of metabolism of 1,3-
dichloropropene in rats exposed to > 1362 mg/m3. Absorption of
inhaled 1,3-dichloropropene occurred via the lungs, primarily in the
lower respiratory tract (approximately 50% of total inhaled), with a
small amount via the nasal mucosa (11-16%).
Following exposure to < 1362 mg/m3, both isomers were
rapidly eliminated from the blood, with a half-life of 3-6 min.
There was no interaction in the kinetics of both isomers. In
addition, data obtained on rats exposed to 1362 mg/m3 revealed
that this rapid elimination phase was followed by a slower
elimination phase having a half-life of 33-43 min. These data
demonstrated that a combination of saturable metabolism and
chemically-induced changes in respiration control 1,3-
dichloropropene uptake and body-burden in rats. However, only
decreases in respiration appear to influence vapour uptake.
Fisher & Kilgore (1988a) studied the excretion of the
mercapturic acid of cis-dichloropropene in Sprague-Dawley rats. In
a nose-only exposure system, groups of 3 rats were exposed for 1 h
to Telone II (94%) at average concentrations of 0, 181.6, 485.8,
1289.4, 1806.9, or 3582.1 mg/m3. Urine samples (24 h) were
collected and analysed for the mercapturic derivative of cis-
dichloropropene. At the lower exposure levels (< 1289.4 mg/m3),
urinary excretion of the mercapturic acid derivative increased with
exposure level. With exposure to 1806.9 or 3582.1 mg/m3, no
further increase was found, suggesting saturation of the metabolic
process.
6.2 Influence on tissue levels of glutathione
6.2.1 Oral
Oral administration of 1,3-dichloropropene to rats or mice
resulted in significant, dose-related reductions in the levels of
non-protein sulfhydryls (NPS) (indicator of tissue glutathione
concentration) in the forestomach and to a lesser extent in the
glandular stomach and liver (Dietz et al., 1984b, 1985, see also
section 6.4).
6.2.2 Inhalation
Shortly after inhalation exposure of rats to cis-1,3-
dichloropropene, kidney and liver NPS contents were reduced in a
dose-related manner, but returned to control values 18 h after
exposure. Lung NPS levels were not affected (Stott & Kastl, 1986,
see section 6.1.2.1; Nitschke & Lomax, 1990, see section 8.2.2.2).
Male Sprague-Dawley rats (200-250 g) were exposed through
inhalation to 1,3-dichloropropene (Telone II, 94%) concentrations of
0, 9.1, 22.7, 150, 1384.7, 3504.9, 4335.7, or 7790.6 mg/m3 to
assess the relationship between 1,3-dichloropropene exposure
concentration and tissue levels of reduced glutathione (GSH).
Animals were exposed for 1 h in a dynamic, nose-only system. GSH
contents were measured in the heart, kidneys, liver, lung, nasal
mucosa, and testes, 2 h after 1,3-dichloropropene exposure. A
decrease in nasal GSH, first seen at 22.7 mg/m3, followed an
exposure concentration-dependent curve. Exposure to concentrations
above 150 mg/m3 reduced the level of liver GSH. Lung GSH remained
relatively constant at 75% of control concentrations up to 4335.7
mg/m3. Significantly decreased GSH levels were observed in the
heart, liver, lung, and testes at 7790.6 mg/m3. Kidney GSH content
was not significantly decreased. Unchanged 1,3-dichloropropene was
not present in the blood of animals 2 h after exposure to 4335.7
mg/m3 or less. Serum lactic dehydrogenase activity was affected
only at 7790.6 mg/m3. Lung weight, measured 2 and 6 h after
exposure, did not differ from controls for any exposure level
(Fisher & Kilgore, 1988b).
Four male Sprague-Dawley rats (200-250 g) were exposed to
Telone II (94%) for 1 h, in a dynamic, nose-only exposure system.
The actual 1,3-dichloropropene concentration was 354.1 ± 49.9, 703.7
± 408.6, and 1834.2 ± 113.5 mg/m3 (relative concentrations of
cis- and trans-isomers were approximately 62 and 38%,
respectively). The GSH conjugation of 1,3-dichloropropene (GSCP) in
the blood of rats following exposure showed that there was no
significant difference between the regression line expressed as
either monophasic or biphasic decay at any exposure concentration.
Moreover, no differences were found in the regression lines between
the exposure concentrations. The elimination half-time of GSCP was
approximately 17 h following exposure to 354.1, 703.7, or 1834.2
mg/m3, and, thus, was not dose-dependent. This fits a one-
compartment model (Fischer & Kilgore, 1989).
6.3 Biotransformation
6.3.1 Rat
In urine from rats and mice treated orally with 14C-
dichloropropene, no unchanged parent compound, but 2 major and 2
minor metabolites were found. The predominant metabolite was N-
acetyl- S-(3-chloroprop-2-enyl) cysteine with its sulfoxide or
sulfone. These data indicate that conjugation with glutathione is a
major route of 1,3-dichloropropene metabolism in the rat (Dietz et
al., 1984a,b, 1985) (see Fig. 4 and section 6.1.1).
Although the spontaneous reaction of cis-1,3-dichloropropene
with glutathione is slow in the rat, the rapid urinary excretion is
due to hepatic glutathione transferase, which catalyses its
conjugation with glutathione. The transferase is present in the rat
liver cytosol fraction and little microsomally mediated metabolism
occurs. The cis-isomer is a better substrate than the trans-
isomer for glutathione transferase. The conjugation then follows a
classic mercapturic acid pathway (Boyland & Chasseaud, 1969). The
conjugated product N-acetyl- S-(3-chloroprop-2-enyl) cysteine and
its sulfoxide are excreted in the urine of rats and mice (Climie et
al., 1979; Dietz et al., 1984b; van Sittert, 1984, 1989).
It has been shown that a minor metabolic pathway of the cis-
1,3-dichloropropene is mono-oxygenase catalysed oxygenation, leading
to the possible formation of the metabolite cis-1,3-
dichloropropene-oxide (II in Fig. 5) (Van Sittert, 1989).
Rats administered 25-450 µg cis- and trans-1,3-
dichloropropene/kg body weight, intraperitoneally, showed excretion
of N-acetyl- S-( cis- and trans-3-chloroprop-2-enyl)-L-
cysteine for 55% (cis-) and 45% (trans-) of the dose within 24 h
(Onkenhout et al., 1986).
In the study of Waechter & Kastl (1988) (see section 6.1.1.1),
in which rats were administered daily doses of 5 mg of non-labelled
1,3-dichloropropene/kg body weight followed by a single dose of 5 mg
14-C (uniformly) labelled 1,3-dichloropropene, or a single dose of
5 mg/kg body weight, the major urinary metabolites were the
mercapturic acid of 1,3-dichloropropene (1,3-D-MA) and its
sulfoxide. The repeatedly dosed rats excreted slightly higher
percentages of the dose as mercapturic acids than the single dosed
rats (28.5% vs 22.7% for males and 25.5% vs 14.3% for females). The
isomeric ratio of the 2,3-D-MA was approximately 80% cis- and 20%
trans- for all groups.
6.3.2 Humans
Van Welie et al. (1989, 1991) determined the relationship
between respiratory occupational exposure to cis- and trans-1,3-
dichloropropene and urinary excretion of 2 mercapturic acid
metabolites, N-acetyl- S-( cis- and trans-)-3-chloroprop-2-
enyl)-L-cysteine ( cis- and trans-DCP-MA) by 12, 1,3-
dichloropropene applicators in the Netherlands. Urinary excretion of
these mercapturic acids followed first-order elimination kinetics.
Urinary half-lives of elimination were 5.0 ± 1.2 h for the cis-
mercapturic acid and 4.7 ± 1.3 h for the transform. These values
were not statistically significantly different. A clear correlation
was observed between the 8-h time-weighted average (TWA) exposure to
cis- and trans-1,3-dichloropropene and complete cumulative
urinary excretion of cis- and trans-DCP-MA. The cis-DCP-MA
yielded 3 times more mercapturic acid (45%) than the trans- form
(14%), probably because of differences in kinetics. It was concluded
that the uptake of cis- and trans-1,3-dichloropropene, their
metabolism to the corresponding mercapturic acids, and urinary
excretion was a rapid process.
 In California, applicators of 1,3-dichloropropene were also
studied for personal air exposure and urinary excretion of
mercapturic acid metabolites. The amount excreted was correlated
with the product of the duration of exposure x TWA. The highest
urinary metabolite concentration occurred during the application
period, indicating rapid excretion. Skin absorption of vapour was
not a significant route of exposure (Osterloh et al., 1984, 1989,
see also section 9.2.1).
Air and biological monitoring of 6 operators exposed to "Mix
DD" soil fumigant during filling operations in the Netherlands was
carried out in 1985-86. There was rapid metabolism and elimination:
the half-lives of mercapturic acid excretion were 4-5 h, with a
return to background levels after 24 h. It was calculated that,
under linear, non-saturation conditions, approximately 23% of the
inhalation dose of the cis-isomer and 10% of the trans-isomer
are excreted in the urine as mercapturic acids (Eadsforth, 1987).
6.4 Reaction with macromolecules
6.4.1 Mouse
The non-protein sulfhydryl (NPS) content, e.g., GSH, and
covalent binding to macromolecules were determined in the tissues of
male B6C3F1 mice. Single oral doses of 0, 1, 5, 25, 50, or 100
mg 1,3-dichloropropene 97% ( cis- 62% : trans-isomer 38%)/kg body
weight were given for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-
dichloropropene/kg body weight for binding studies. Non-glandular
forestomach, glandular stomach, liver, kidneys, and bladder were
analysed, 2 h after dosing. Although NPS depletion and dose-related
increases in macromolecular binding were noted in several tissues of
rats, these effects were more pronounced in the non-glandular
stomach than in any other tissue (including glandular stomach,
liver, kidneys, and bladder). The no-observed-effect level (NOEL)
for NPS depletion in rat non-glandular stomach was 1 mg/kg body
weight. NPS levels in non-glandular forestomach were significantly
decreased at doses of 25 mg or higher and, in the liver, at 100
mg/kg body weight. Binding in the non-glandular forestomach was
greatest at dose levels that caused the most depletion of tissue
NPS. Limited binding occurred in the liver, kidneys, and bladder
(Dietz et al., 1984b, 1985).
6.4.2 Rat
Groups of 3-9 male Fischer 344 rats (200-260 g) were
administered 50 mg cis-1,3-dichloropropene (94.1% cis- and 2.5%
trans-) or 50 mg trans-1,3-dichloropropene (97.3% trans- and
0.8 cis-)/kg body weight, by gavage. The rats were sacrificed at
various intervals after dosing, to determine the tissue non-protein
sulfhydryls (NPS) in the liver, kidneys, forestomach, glandular
stomach, and bladder. Blood samples were also taken to determine the
presence of unchanged 1,3-dichloropropene. Cis-1,3-dichloropropene
was only detected in the blood (6.58 µg/litre) 15 min after dosing,
the blood levels of trans-1,3-dichloropropene were 11.72 and 8.38
µg/litre, respectively, 15 and 45 min after dosing. A statistically
significant decrease in the non-protein sulfhydryl contents of the
liver, kidneys, forestomach, and glandular stomach was found. This
depletion reached a maximum, approximately 2-h after dosing. No
depletion was noted in the bladder. It is not possible to
distinguish the effects of cis- and trans-1,3-dichloropropene on
NPS, as the results for the individual isomers were not reported.
The results indicated that orally administered 1,3-dichloropropene
produces a rapid and significant depletion of tissue non-protein
sulfhydryls in the rat (Dietz et al., 1982).
The non-protein sulfhydryl (NPS) contents and covalent binding
to macromolecules were determined in the tissues of male Fischer 344
rats. Single, oral doses of 0, 1, 5, 25, 50, or 100 mg 1,3-
dichloropropene 97% ( cis-62% and trans-isomer 38%) were given
for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-dichloropropene/kg
body weight for binding studies. NPS levels in non- glandular
forestomach were significantly decreased with doses of 25 mg/kg body
weight or more. Binding in the non-glandular forestomach was
greatest at dose levels that caused a significant depletion of
tissue NPS. Limited binding was noted in the liver, kidneys, and
bladder (Dietz et al., 1984b, 1985).
6.5 Appraisal
Mice, rats and humans metabolize 1,3-dichloropropene
predominantly by conjugation with reduced glutathione (GSH). The
glutathione conjugate is further metabolized to the corresponding
mercapturic acid, which is then excreted in the urine. Consistent
with the function of GSH as a detoxication mechanism, the
genotoxicity of 1,3-dichloropropene was decreased in Salmonella
typhimurium when the concentration of GSH was increased to a
mammalian physiological concentration (see sections 8.6.1.2, 8.9.2).
The levels of GSH (measured as non-protein sulfhydryl content)
were decreased at locations consistent with the route of exposure,
i.e., predominantly in the forestomach and to a lesser extent in the
glandular stomach and liver, following oral administration, and in
the nasal tissue after inhalation exposure. For oral exposure, the
extent of covalent binding of 1,3-dichloropropene to macromolecules
was correlated with the decrease in non-protein sulfhydryl content.
It is anticipated that toxic effects of 1,3-dichloropropene will
occur at doses that deplete tissue sulfhydryls and will be
manifested in the organs described above (forestomach, liver, and
nasal tissue).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The United States Environmental Protection Agency published a
Health and Environmental Effects Profile for 1,3-Dichloropropene in
1985 (US EPA, 1985).
7.1 Acute toxicity
7.1.1 Microorganisms
The major effect of 1,3-dichloropropene on soil microorganisms
is on nitrogen transformation. The oxidation of ammonium from soil
organic nitrogen is reduced by 1,3-dichloropropene; soil ammonium
levels were significantly greater and soil nitrate levels were
significantly lower in soils treated with 1,3-dichloropropene
compared with untreated controls (Tu, 1973; Elliot et al., 1974,
1977).
In a series of studies, the effects of 1,3-dichloropropene at
30 or 60 mg/kg soil were evaluated, in parallel, under laboratory
conditions. In general, neither dose affected soil microorganisms or
function appreciably. The effects of 1,3-dichloropropene on soil
microorganisms and enzyme activity were not consistent in 3 soil
types (sandy-, clay-, and organic soils). The numbers of fungi, 2
days after fumigation, were significantly reduced in clay and sandy
soils, but not in organic soil, but recovered after 7 days. 1,3-
Dichloropropene either increased or decreased the number of non-
symbiotic nitrogen fixers and enzyme activity in different soils
(Tu, 1978, 1979, 1981a,b).
Moje et al. (1957) studied the individual components of "MIX
D/D" in old citrus soil and found that the toxicity was mainly due
to its 1,3-dichloropropene content, in particular that of the cis-
isomer (see also section 7.1.1 of "MIX D/D"). Results were as
tabulated on the next page.
1,3-Dichloropropene was converted by the methanotrophic
bacterium Methylosinus trichosporium OB3b, grown in aerobic
continuous cultures. The substance was added at a concentration of
0.2 mmol/litre. After 24 h, 85% of the substance added was degraded
(Oldenhuis et al., 1989).
In field experiments with continuous potato cropping, it was
found that sustained annual applications of 1,3-dichloropropene led
to insufficient control of Globodera rostochiensis. In the
laboratory, 1,3-dichloropropene rapidly disappeared from these
soils. This did not occur when the soil was sterilized. A bacterium
was isolated from these soils, which was found to decompose 1,3-
dichloropropene using it as a carbon and energy source. The
bacterium was Pseudomonas sp. Repeated application of 1,3-
dichloropropene led to accelerated degradation of the substance
(Lebbink et al., 1989).
Reduction in
Compound Fungi Bacteria and
actinomycetes
Cis-1,3-dichloropropene 85-95% reduction 85-100% reduction
at 25 mg/kg soil at 250 mg/kg soil
Trans-1,3-dichloropropene 100% reduction 100% reduction at
at 250 mg/kg soil 1000 mg/kg soil
7.1.2 Algae
The 96-h EC50 value for growth, based on the concentration of
chlorophyll a and also on cell numbers of the freshwater green algae
Selenastrum capricornutum in a static system, was 4.95 mg/litre
for 1,3-dichloropropene. The estuarine diatome, Skeletonema
costatum, showed a 96-h EC50 value for growth, based on the
concentration of chlorophyll a in culture, of 1 mg/litre (Leblanc,
1984). The EC50 calculated from cell numbers was 1.04 mg/litre (US
EPA, 1980). The compound is moderately toxic for marine algae.
The toxicity of cis-, trans- and a mixture of cis- and
trans-1,3-dichloropropenes for Selenastrum capricornutum was
determined in a sealed 72-h growth inhibition test. The 72-h EC50
values (percentage reduction in area under the growth curve),
expressed in terms of the mean measured concentration in the test
media, were; cis-isomer 2.8 mg/litre; trans-isomer 11 mg/litre;
and the mixture 8.2 mg/litre. The EC50 (percentage reduction in
mean specific growth rate), (24-48 h) and EC50 (24-72 h) values
determined by analysis of average specific growth rates were: cis-
isomer 4.6 and 3.1 mg/litre; trans-isomer 6.6 and 7.5 mg/litre;
and, for the mixture, 11 and 3.6 mg/litre, respectively (Girling,
1989a,b,c).
Rapid evaporation and sensitivity to hydrolysis are features of
the chloropropenes that may interfere with proper determination of
toxic concentrations of these chemicals for aquatic species, using
the standard techniques. Significant loss of the test substance may
have occurred during experiments carried out at temperatures above
20 °C and under "static" conditions, i.e., lasting for several days
without refreshing the medium. Determination of the actual
concentrations has seldom been carried out, and it is suspected that
several of the data reported for the chloropropenes present an
underestimation of the toxic potential of these chemicals for
aquatic organisms. On the other hand, the products of hydrolysis of
the chloropropenes, for instance, chloroallyl alcohols are
themselves toxic (Krijgsheld & Van der Gen, 1986).
7.1.3 Invertebrates
The acute toxicity of 1,3-dichloropropene for non-target
aquatic crustacea is summarized in Tables 6 and 7.
Table 6. Acute toxicity of 1,3-dichloropropene for non-target aquatic crustacea
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (mg/litre) (mg/litre)
Water flea 21 0.090a - Mayer &
(Daphnia magna) Ellersieck (1986)
Mysid shrimp - 0.79 US EPA (1980);
(Mysidopsis bahia) Leblanc (1984)
a 48-h EC50 6 mg/litre (US EPA, 1980; Leblanc, 1980) in a static system.
Leblanc (1980) calculated a no discernable effect concentration
of 0.41 mg/litre for 1,3-dichloropropene in Daphnia magna, under
static conditions. However, the result was based on nominal
concentrations and was higher than the 48-h LC50 given by Mayer &
Ellersieck (1986).
7.1.4 Honey bees
1,3-Dichloropropene has been tested on worker Honey bees (Apis
mellifera) using a dusting technique. The 48-h LD50 was 6.6
µg/bee and 1,3-dichloropropene was rated as "relatively non-toxic"
for Honey bees (Atkins et al., 1973).
Table 7. Acute toxicity of cis- and/or trans-1,3-dichloropropenes in Daphnia magna
Substance Age System Temperature 48-h EC50 Reference
(°C) (mg/litre)
Cis-1,3 DCP 24 h statica 18-22 1.4 Girling (1989a)
(96%)
Trans-1,3 DCP 24 h staticb 18-22 3.1 Girling (1989c)
(95.4% trans +
0.3% cis)
Cis- + trans- 24 h staticc 18-23 3.1 Girling (1989b)
mixture
(51-52% cis +
44% trans)
a pH 7.6-8.4; total hardness 176 mg/litre; dissolved oxygen 8.8-9.8 mg/litre.
b pH 7.8-8.1; total hardness 170 mg/litre; dissolved oxygen 8.6-9.2 mg/litre.
c pH 7.9-8.3; total hardness 179 mg/litre; dissolved oxygen 8.6-9.9 mg/litre.
7.1.5 Fish
The data summarized in Tables 8 and 9, suggest that 1,3-
dichloropropene is moderately toxic for fish.
Heitmuller et al. (1981) exposed sheepshead minnows to 1,3-
dichloropropene under static conditions. They calculated a no-
observed-effect concentration of 1.2 mg/litre, but this was based on
nominal concentrations.
7.1.6 Birds
Worthing & Hance (1991) reported an LC50 (8-day) for Mallard
duck and Bobwhite quail of > 10 000 mg/kg diet.
7.2 Short-term/long-term toxicity
7.2.1 Invertebrates
No data are available.
Table 8. Acute toxicity of 1,3-dichloropropene for fish
Species Type of test Size (g, mm) Temperature 96-h LC50 References
(°C) (mg/litre)
Fathead minnow static 0.9 g 18 4.1 Mayer & Ellersieck (1986)
(Pimephales promelas) (3.39-4.97)
Largemouth bass static 1.0 g 18 3.65a Mayer & Ellersieck (1986)
(Micropterus salmoides) (3.52-3.78)
Walleye static 1.3 g 18 1.08 Mayer & Ellersieck (1986)
(Stizostedion vitreum) (0.99-1.18)
Golden orfe - 2.8 g 20 9b Reiff (1978); Krijgsheld & Van
(Idus idus melanotus) (8-11) der Gen (1986)
Sheepshead minnow static 8-15 mm 25-31 1.8c,d US EPA (1980); Heitmuller
(Cyprinodon variegatus) (0.7-4.5) et al. (1981); Leblanc (1984)
Rainbow trout - - - 3.9 Worthing & Hance (1991)
(Salmo gairdneri)
Bluegill static 0.32-1.2 g 21-23 6.1c,d Buccafusco et al. (1981)
(Lepomis macrochirus) (5.1-6.8) US EPA (1980); Leblanc (1984)
Guppy semi-static - 22 0.5e Krijgsheld & Van der Gen (1986)
(Poecilia reticulata)
Goldfish static 1.0 g 18 < 7.5 Mayer & Ellersieck (1986)
(Carasius auratus)
Table 8 (continued)
a Tested in hard water, 272 mg CaCO3/litre.
b Tested in dechlorinated water, 260 mg CaCO3/litre.
c Tested in well water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration 9.7 mg/litre reduced
at the beginning to 0.3 mg/litre after 96-h exposure.
d Nominal concentrations.
e 14-day test.
Table 9. Acute toxicity of cis- and/or trans-1,3-dichloropropenes
in rainbow trout (Salmo gairdneri)
Substance Mean size System Temperature 96-h LC50 Reference
(cm, g) (°C) (mg/litre)
Cis-1,3 DCP 4.7 cm semi- 13-17 1.6 Girling (1989a)
(96%) (1.1 g) statica
Trans-1,3 DCP 4.0 cm semi- 15-17 4.5 Girling (1989c)
(95.4% trans + (0.67 g) staticb
0.3% cis)
Cis- + trans- 4.2 cm semi- 13-17 2.0 Girling (1989b)
mixture (0.68 g) staticc
(51-52% cis +
44% trans)
a pH 7-7.8; total hardness, 226-258 mg/litre as CaCO3: dissolved oxygen, 6.4-10.2 mg/litre.
b pH 7.2-7.8; total hardness, 234-264 mg/litre as CaCO3: dissolved oxygen, 8.2-9.9 mg/litre.
c pH 7.5-8.3; total hardness, 251-284 mg/litre as CaCO3: dissolved oxygen, 8.1-10.2 mg/litre.
7.2.2 Fish
Embryo-larval tests have been conducted on Fathead minnows
(Pimephales promelas) exposed to 1,3-dichloropropene. The maximum
no-effect concentration was 0.24 mg/litre (no details are available)
(US EPA, 1980).
7.2.3 Field studies
See section 7.3.2 of "Mixtures of dichloropropenes and
dichloropropane" for effects of "MIX D/D" on worms.
7.2.4 Phytotoxicity
1,3-Dichloropropene is highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The United States Environmental Protection Agency, published a
Health and Environmental Effects Profile for 1,3-dichloropropene in
1985 (US EPA, 1985).
In two other documents of the US EPA, health risk assessment
information is given, on the basis of a comprehensive review of the
toxicity data (US EPA, 1990, 1991).
8.1 Single exposures
8.1.1 Oral
The acute oral LD50s for mice and rats are summarized in
Table 10.
The following signs of toxicity were observed after oral
administration: hunched posture, lethargy, pilo-erection, decreased
respiratory rate, ptosis, diarrhoea, diuresis, ataxia, tip-toe gait,
red/brown staining around the snout, tremors, emaciation, and pallor
of the extremities. Haemorrhages and congestion were found in the
lungs and gastrointestinal tract. The livers showed patchy areas of
pallor (Jones & Collier, 1986a; Jeffrey et al., 1987; Gardner,
1989a,b,c).
8.1.2 Inhalation
The acute inhalation LC50s for 1,3-dichloropropene are
summarized in Table 11.
During the exposure and observation periods, the following
symptoms were observed: partial closing of the eyes, pilo-erection
salivation, lacrimation, lethargy, diarrhoea, reduction in
respiratory rate, irregular respiratory movements (lung congestion
was observed in dead animals) and hunched posture, brown staining of
fur and fur loss, and reddening of ears, tail, and feet.
Pathological signs were cardiopulmonary failure, acute tubular
necrosis in the kidneys, and local effects on the respiratory tract.
It was suggested that the irritating properties of the vapour
might serve as a warning of its presence in air. Signs of
intoxication included hypoactivity, anorexia, and chromodacryorrhoea
(Coombs & Carter, 1976b).
Table 10. Acute oral toxicity (LD50) of 1,3-dichloropropene
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse (CD 1) undiluted 215 Coombs & Carter
(1976b)
Mouse 92% (in corn oil) 640 (582-704)a Toyoshima et al.
(JCL:ICR) 640 (547-749)b (1978a)
Rat 94.5-97.5% cis-, 1.5% 85 Jeffrey et al.
(Fischer 344) trans-, 0.25% (1987); Gardner
1,2-dichloropropane, (1989b)
undiluted
Rat 96.7%, trans-, 94 Gardner (1989c)
(Fischer 344) undiluted 117a
78b
Rat (CD) undiluted 127 (112-141) Coombs & Carter
(1976b)
Rat (not 92% (in corn oil) 470b Torkelson & Oyen
specified) 713a (1977)
Rat (Sprague- 97.2% 130 (110-170)a Jones & Collier
Dawley) between 110 and 250b (1986a)
Rat D-D 95, undiluted 57 Gardner (1989a)
(Fischer 344) (cis + trans 1 : 1)
Rat 92% (in corn oil) 560 (452-695)a Toyoshima et al.
(Wistar) 510 (480-726)b (1978b)
Table 10 (contd)
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Rat 97.5% 300a Jeffrey et al. (1987)
(Fischer 344) 224b
a Male.
b Female.
Table 11. The acute inhalation toxicity LC50 for 1,3-dichloropropene (4-h exposure)
Species Concentration LC50 (mg/m3) Reference
of substance
Rat 51% cis-, 43.4% 3309.7 Blair (1977)
(Wistar) trans-isomer, 1%
epichlorohydrin
Rat Telone II 2.70-3.07c Cracknell et al.
(Wistar) (98.4%) (1987)
Rat 95.6% cis- and 3041.8a Nitschke et
(Fischer 344) 1.5% trans-isomer 3377.8b al. (1990)
Rat Telone II (97.5%; > 3881.7-< 4698.9a Streeter et al.
(Fischer 344) 52.6% cis- and 4014.2b (1987)
44.9% trans-isomer)
Rat 95.4% trans- and 4880.5b Collins (1989)
(Crb:CD(SD)Br) 0.3% cis-isomer 5402.6a
a male.
b female.
c mg/litre.
A 2-h exposure to 1,3-dichloropropene at 4540 mg/m3 was
lethal to rats, whereas guinea-pigs died following a single 7-h
exposure to 1816 mg/m3 (Torkelson & Oyen, 1977).
One female and 3 male Rhesus monkeys (4-5 kg) were exposed to
Telone II at 0, 113.5, 227, 454, 908, or 2724 mg/m3 for a single
behavioural session of 1 h, with a 1-week recovery period after
exposure. Data were obtained on all monkeys for each of the 5
atmospheric concentrations. At concentrations ranging up to 908
mg/m3, there were no significant indications of toxicity or
alterations in behaviour (the monkeys were trained to perform on a
dual component FRFR (Fixed Ratio followed by a Fixed Ratio) chained
schedule, with light stimulation). Only slight eye irritation was
observed in all monkeys at concentrations exceeding 454 mg/m3
(Rosenblum & Talley, 1979).
8.1.3 Dermal
The acute dermal and subcutaneous LD50s are summarized in
Table 12.
After dermal application, the signs of intoxication were:
diarrhoea, lethargy, hunched posture, decreased respiratory rate,
with lacrimation, salivation, ataxia or abasia, loss of righting
reflex, diarrhoea, diuresis, and red/brown staining around the eyes,
snout or mouth. The lungs and gastrointestinal tract showed
haemorrhages and irritation. Signs of skin irritation manifested by
oedema, eschar formation, or subcutaneous haemorrhage were apparent
(Jones & Collier, 1986b).
One 24-h application of undiluted 1,3-dichloropropene to
intact, occluded New Zealand white rabbit skin caused extremely
severe eschar, resulting in black necrotic tissue. The skin had
hardened and was cracking after 7 days. The animals used for dermal
LD50 estimation with 1,3-dichloropropene showed lethargy and
hypothermia at dose levels above 300 mg/kg body weight (Coombs &
Carter, 1976b).
8.2 Short-term exposures
8.2.1 Oral
Albino rats derived from the Wistar strain, were used in a 90-
day test. Groups of 10 male and 10 female rats received doses (by
gavage) of 0, 1, 3, 10, or 30 mg 1,3-dichloropropene (40% cis- and
28% trans-isomer)/kg body weight on 6 days/week. General
Table 12. Acute dermal and subcutaneous LD50s for 1,3-dichloropropene
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(JCL:ICR) (1978a)
Rat dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(Wistar) (1978b)
Rat (CD) dermal undiluted 0.423 Coombs & Carter
(0.336-0.555) (1976b)
Rat (Sprague- dermal 97.2% 1.0 (0.8-1.3)a Jones & Collier
Dawley) between 1.3 and 2.0b (1986b)
Rat dermal 96.7%, trans-isomer 1.575 Gardner (1989c)
(Fischer 344) undiluted
Rat dermal 94.5-97.5% 1.09 Gardner (1989b)
(Fischer 344) cis-, 1.5%
trans-, 0.25%
1,2-dichloropropane
undiluted
Rabbit (not dermal 92% 0.504 Torkelson & Oyen
specified) undiluted (0.22-1.15) (1977)
Mouse subcutaneous 92% (in corn oil) 0.33 (0.29-0.376)a Toyoshima et
(JCL:ICR) 0.345 (0.30-0.40)b al. (1978a)
Table 12. (contd)
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Rat subcutaneous 92% (in corn oil) 0.40 (0.345-0.464)a Toyoshima et
(Wistar) 0.366 (0.305-0.439)b al. (1978b)
a Male.
b Female.
condition, behaviour, and survival were not affected at any dose
level. No distinct differences in haematological indices, serum
enzyme activities, or urinalysis were observed. The relative kidney
weights were significantly increased in the 30-mg group in both
sexes and also in the males receiving 10 mg/kg. The relative liver
weights of females at 30 mg/kg were significantly increased. Gross
and microscopic examination did not reveal any abnormalities in the
main organs. The 3 mg/kg body weight dose was without effect (Til et
al., 1973).
8.2.2 Inhalation
8.2.2.1 Mouse
CD-1 Albino mice, 10/sex per group were exposed to production
grade Telone II (whole-body exposure) at 0, 45.4, 136.2, or 408.6
mg/m3 for 6 h/day, 5 days/week, for 90 days. No significant
differences in lesions were found between the control and treated
groups upon gross and histological examination, except that there
were compound-related effects on the nasal turbinates. These effects
were decreased height of the nasal epithelial cells resulting from a
loss of cytoplasm, and disorganization of the nuclei. Necrotic cells
were only observed among the females exposed to 408.6 mg/m3. No
alterations were found in the mice exposed to 136.2 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 10 male and 10 female B6C3F1 mice were exposed
(whole body) by inhalation to technical grade 1,3-dichloropropene
90.9% ( cis 48.6% and trans 42.3%) containing 2.4% 1,2-
dichloropropane, 5.5% mixed isomers of chlorohexane, chlorohexene,
and trichloropropene, and epichlorohydrin 1.2% (as stabilizer). The
actual exposures were to levels of 0, 45.4, 136, 409, or 681 mg/m3
for 6 h/day, 5 days/week for 13 weeks. Extensive haematological,
clinical-chemical, and urinalysis studies, and histopathological
examination of organs and tissues were performed.
A treatment-related depression in body weight was seen at doses
of 409 and 681 mg/m3. In animals exposed to 681 mg/m3, transient
brown discoloration of the fur with a strong mercaptan odour in the
coats and urine was found. The poor growth rate was reflected in a
decrease or increase in the relative weights of a number of organs,
but without histological alterations. An exposure-related decrease
in BUN levels was observed in male mice exposed to 409 and 681
mg/m3. Furthermore, alanine transaminase levels were increased in
mice exposed to 681 mg/m3. No other changes were found. The
primary target tissues of inhaled 1,3-dichloropropenes were the
nasal mucosa and urinary bladder. The changes in the nasal mucosa
including slight degeneration of the olfactory epithelium and slight
hyperplasia of respiratory epithelium in animals exposed to 409 and
681 mg/m3 were dose-related. The animals also had small focal
areas of nasal metaplasia, a condition in which the damaged sensory
olfactory epithelium is replaced by ciliated respiratory epithelium
(only in animals exposed to 681 mg/m3).
The urinary bladders of female mice exposed to 409 and 681
mg/m3 (7/10 and 6/10, respectively) had areas of moderate
hyperplasia of the transitional epithelium (7-10 layers thick in
contrast with 2-3 layers in control mice). Submucosal aggregates of
lymphoid cells in the bladder, not associated with hyperplasia and
not treatment-related, were found in female mice exposed to
concentrations of 136 mg/m3 or more. No treatment-related effects
were found in mice exposed to 45.4 mg/m3 (Stott et al., 1984,
1988).
8.2.2.2 Rat
Fischer 344 rats, 10/sex per group, were exposed to 0, 45.4,
136, or 409 mg/m3 of production grade Telone II, for 6 h/day, 5
days/week for 90 days. No significant differences in lesions were
found between the control and treated groups on gross or
histological examination, except that compound-related effects on
the nasal turbinates were found. These effects included decreased
height of the nasal epithelial cells resulting from a loss of
cytoplasm, and a disorganization of the nuclei. Necrotic cells were
observed in all of the male and female rats exposed to 409 mg/m3
and in 6/10 of the female rats exposed to 136 mg/m3. No
alterations were found in the animals with 45.4 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 5 male rats were exposed to atmospheres containing
concentrations of 1,3 cis-/trans-dichloropropene (92%) of 0 or
13.6 mg/m3 for 0.5, 1, 2, or 4 h/day, 5 days/week for 6 months.
The only effect in all the exposed groups was a slight, apparently
reversible change, seen microscopically in the kidneys of male rats
(Torkelson & Oyen, 1977).
Groups of 24 male and 24 female rats were exposed repeatedly to
air containing 0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7
h/day, 5 days/week for 6 months. Changes attributable to 1,3-
dichloropropene were limited to cloudy swellings in the renal
tubular epithelium of male rats in the 13.6 mg/m3 group. Female
rats in this group showed an increased liver to body weight ratio,
though no histopathological changes were observed. A recovery group
of male and female rats was maintained for 3 months following the 6-
month exposure to 4.54 or 13.6 mg/m3. No changes were observed in
the renal epithelium following this recovery period (Torkelson &
Oyen, 1977).
In a well-designed study (Stott et al., 1984, 1988), groups of
10 male and 10 female Fischer 344 rats were exposed through
inhalation to technical grade 1,3-dichloropropene 90.9% (for
composition see section 8.2.2.1) in actual concentrations of 0,
45.4, 136, 409, or 681 mg/m3, for 6 h/day, 5 days/week for 13
weeks. Haematological, clinical-chemical studies, urinalysis, and
histopathological studies of organs and tissues were carried out.
In the rats exposed to 681 mg/m3, transient brown
discoloration of the fur with a strong mercaptan odour in the coats
and urine was found. The body weights of rats exposed to 409 or 681
mg/m3 were decreased in an exposure-related manner. The relative
weights of the testes were increased and thymus weights decreased.
Rats exposed to 409 and 681 mg/m3 had slightly lower levels of
serum proteins. No other treatment-related changes in clinical-
chemical or haematological parameters were found.
The primary target tissues of inhaled 1,3-dichloropropenes were
the nasal mucosa in male and female rats and the uteri in females.
The effects consisted of dose-related degenerative effects on the
nasal olfactory epithelium or mild hyperplasia of the respiratory
epithelium or both, in all animals exposed to 409 and 681 mg/m3,
and 2 of the 10 male animals in the 136 mg/m3 group.
The uteri of 7 out of 10 female rats exposed to 681 mg/m3
were not developed as completely as those of control animals,
suggesting hypoplasia of the uterine tissues. The only other change
noted histologically was the atrophic appearance of the mesenteric
adipose tissue in the rats exposed to 681 mg/m3. No treatment-
related effects were observed in rats of either sex exposed to 45.4
mg 1,3-dichloropropenes/m3 (Stott et al., 1984, 1988).
In a study to investigate the effects of 1,3-dichloropropene on
tissue sulfhydryl levels, groups of 15 male and 15 female Fischer
344 rats were exposed to vapours of cis-1,3-dichloropropene (94.3-
95.6% cis-, 1.5% trans-1,3-dichloropropene and 0.2% 1,2-
dichloropropane) at 0, 45.4, 272.4, or 681.0 mg/m3 for 6 h/day, 5
days/week for 9 exposures. Whole-body exposures occurred under
dynamic air-flow conditions. On the day after the last exposure, 5
males and 5 females/dose level were necropsied. Major organs were
weighed and selected tissues were evaluated histopathologically.
Groups of 5 animals/sex per dose level were used to determine the
non-protein sulfhydryl (NPS) contents of the liver, kidneys, and
lung, 1 and 18 h following the last exposure.
Rats exposed to 681 mg/m3 lost weight, but not those exposed
to 272.4 mg/m3. There were concentration-related decreases in
liver, kidney, and lung NPS levels in male rats, when measured after
1 h. After 18 h, the NPS levels were higher than those of the
controls and there were no associated gross or histopathological
changes in these organs. The NPS measurements for females were
unremarkable. Histopathological examination revealed changes of
moderate severity in the respiratory and olfactory mucosa in the
nasal cavity of males and females exposed to 681 mg/m3. There were
no changes in the animals exposed to 272.4 mg/m3 (Nitschke &
Lomax, 1990).
Groups of 10 male and 10 female Fischer 344 rats (6 weeks old)
were exposed to cis-1,3-dichloropropene at 0, 45.4, 136, or 409
mg/m3 for 6 h/day, 5 days/week for 13 weeks. The test material was
reported to consist of 94.3% (95.6%) cis-1,3-dichloropropene, 1.5%
trans-1,3-dichloropropene and 0.2% 1,2- dichloropropane. Whole-
body exposures occurred under dynamic conditions. No exposure-
related effects were noted in haematology or clinical chemistry. At
409 mg/m3, body weights of male rats were significantly decreased
compared with controls throughout the 13-week exposure period; body
weights of female rats were significantly decreased during the first
6 weeks, but were comparable with those of the controls at the end
of the study. As a result of the decreased body weight in male rats,
the relative liver, kidney, lung, and testes weights were
significantly elevated in comparison with control values. The
relative liver weight in females was also increased. These organ
weight changes were not accompanied by gross or histopathological
changes. Exposure-related changes only occurred in the nasal
cavities of the rats of both sexes exposed to 409 mg/m3 and
consisted of multifocal bilateral degeneration of olfactory
epithelium and slight bilateral multifocal hyperplasia of
respiratory epithelium. No effects were noted in rats exposed to
45.4 or 136 mg/m3. The NOEL was considered to be 136 mg cis-1,3-
dichloropropene/m3 (Nitschke et al., 1991).
8.2.2.3 Other animal species
Groups of 12 male and 12 female guinea-pigs, 3 male and 3
female rabbits and 2 dogs were exposed repeatedly to air containing
0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7 h/day, 5
days/week for 6 months. No changes resulting from 1,3-
dichloropropene exposure were observed in guinea-pigs, rabbits, or
dogs (Torkelson & Oyen, 1977).
8.3 Skin and eye irritation, sensitization
8.3.1 Skin irritation
1,3-Dichloropropene is extremely irritating to the skin
(Worthing & Hance, 1991).
Telone II (52.63% cis- and 44.91% trans-1,3,-
dichloropropene), 0.5 ml, was applied for 4 h to the back (clipped
free of fur) of 2 male and 4 female New Zealand White rabbits. After
4 h, the wrapping and gauze patch and any residual test substance
were removed. Dermal irritation characterized as slight to moderate
erythema and moderate to severe oedema was observed at the site of
application immediately following the 4 h exposure period.
Subsequent observations revealed slight to moderate erythema,
oedema, and exfoliation. These changes were still present in some of
the animals after 14 days (Jeffrey, 1987a).
In a 4-h rabbit skin irritancy test, undiluted cis-1,3-
dichloropropene (94.5-97.5% cis-, 1.5% trans-1,3-dichloropropene
and 0.25% 1,2-dichloropropane) caused well defined erythema and
moderate oedema shortly after removal of the semi-occlusive
dressings in New Zealand White rabbits (3-5 months of age). After
removal of the dressings, the skin was washed after 4 h. Resolution
of the irritancy reaction was first apparent on the day after
treatment and was complete within 14 days (Gardner, 1989b).
In another 4-h rabbit skin irritancy test, 0.5 ml of undiluted
trans-1,3-dichloropropene (96.7%) caused irritation not exceeding
well-defined erythema and slight oedema. New Zealand White rabbits
(3-5 months of age) were used. After the 4-h exposure, the dressings
were removed and the skin washed. Resolution of the irritation was
first apparent 72 h after treatment and was complete by day 21
(Gardner, 1989c).
8.3.2 Eye irritation
1,3-Dichloropropene is a severe eye irritant (Worthing & Hance,
1991).
Aliquots of 0.1 ml Telone II (52.63% cis- and 44.91% trans-
1,3-dichloropropene) were instilled into the conjunctival sac of one
eye of 4 male and 2 female New Zealand White rabbits. The eyes of
all rabbits remained unwashed. Slight to marked redness and slight
to moderate chemosis were found after the treatment. The treated
eyes had a slight to marked amount of discharge as well as reddening
of the iris. In one animal, opacity was found which resolved as did
all the other changes within 14 days following treatment (Jeffrey,
1987b).
In the rabbit eye irritancy test, 0.1 ml trans-1,3-
dichloropropene (96.7%) caused moderate to severe conjunctival
irritation and minor irritation changes of the cornea (opacities),
chemosis, and iridial responses within 24 h following instillation
into the eye. Resolution of the irritant effects of trans-1,3-
dichloropropene was advanced 7 days after treatment and complete one
week later. There was an initial pain response (Gardner, 1989c).
Ten male and 10 female Sprague-Dawley rats (Spartan substrain)
were exposed to an aerosol (mean size 2.96 µm; 99% of particles were
6 µm or less in diameter) in a glass chamber for 1 h at a nominal
concentration of 5.2 mg/litre of air. Five male and 5 female animals
were maintained under ambient conditions as controls. Slight
transitory eye irritation was observed during the exposure (Yakel &
Kociba, 1977).
8.3.2.1 In vitro studies
The corneal thickness of isolated eye preparations subjected to
application of cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) increased
by more than 20% within 3 h. The isolated rabbit eye test described
by Price & Andrews (1985) was used. Corneal uptake of fluorescein
was demonstrated at the conclusion of the test. The results
indicated that application of cis-1,3-dichloropropene to the eye
in vivo would cause significant tissue damage (Gardner, 1989b).
8.3.3 Sensitization
1,3-Dichloropropene was applied in corn oil in a 5% v/v
concentration 3 times topically to the skin of guinea-pigs followed
by a 1% concentration as a challenge following the method of Buehler
(1965). A positive reaction was obtained in 5 out of 20 guinea-pigs
of the "P" strain. The reaction was considered to be mild to
moderate skin sensitization (Coombs & Carter 1976b).
Ten male, Hartley albion guinea-pigs received 3 dermal
applications on the back of 0.4 ml of 0.1% (v/v) Telone II (52.63%
cis- and 44.91% trans-1,3-dichloropropene) in mineral oil;
another group received only the vehicle during the induction phase
of the study. The dermal sensitization potential was tested using
the modified Buehler method. A positive control group received 2
applications of 10% epoxy resin and a third application of 5% epoxy
resin. All groups were challenged dermally 2 weeks after the last
induction application. The control group did not show any signs of
sensitization, while 5 out of the 10 animals of the positive control
group revealed slight erythema. Nine out of 10 guinea-pigs
challenged with 0.1% Telone II revealed slight to moderate erythema.
Telone II was considered a potential skin sensitizer at the
concentrations tested (Jeffrey, 1987c).
In the guinea-pig maximization test of Magnusson & Kligman, all
20 test animals showed positive responses, 24 and 48 h after removal
of the challenge patches. Guinea-pigs of the Dunkin-Hartley strain
(5-9 weeks of age) were used in this study. In a study by Gardner
(1989b), 0.1% cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) was
injected intradermally; topical induction was carried out using a 5%
solution in corn oil, and the topical challenge using 2.5% in corn
oil.
In the guinea-pig maximization test of Magnussen & Kligman, 16
out of 20 test animals showed positive responses 24 and/or 48 h
after removal of the challenge patches. Guinea-pigs of the Dunkin-
Hartley strain (age 5-9 weeks) were used. Intradermal injection,
carried out at concentrations of 0.05% trans-1,3-dichloropropene
(96.7%) or more resulted in red areas with defined edges. The
concentration selected (10%) for topical induction did give slight
irritation, but the concentration for the topical challenge of 5%
was without effect. The solvent was corn oil (Gardner, 1989c).
8.4 Long-term exposure
See section 8.7 (Carcinogenicity).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Inhalation (rat)
Groups of 30 male and 30 female Fischer 344 rats (6 weeks of
age) were exposed to Telone II (1,3-dichloropropene containing 2%
epoxidized soybean oil) at 0, 45.4, 136, or 409 mg/m3 (first 7
days of study: 0, 22.7, 90.8, or 272.4 mg/m3) for 6 h/day, 5
days/week during the premating period and for 7 days/week during
breeding, gestation, and lactation, for 2 generations. The Telone II
used had a purity of 92% and the remainder of the test material
comprised chlorinated and unchlorinated alkanes and alkenes as well
as approximately 2.0% epoxidized soybean oil. Following 10 weeks of
exposure, the adult rats (F0) were mated twice to produce the
F1a and F1b litters. After weaning, 30 pups/sex per exposure
from the F1b litters were selected and, after 12 weeks, used to
produce the F2a and F2b litters.
All litters were examined on the day of parturition, the
following indices of fertility being recorded: gestation length,
litter size, pup survival indices, number of live pups on days 1 up
to 28 postpartum, sex and weight of litters, lactation and
individual body weights, and any visible physical abnormalities.
Gross necropsy was carried out on adult rats F0 and F1 and
weanling F1b and F2b rats and histopathological studies on adult
rats. Inhalation of up to 409 mg/m3 for 2 generations did not
adversely affect reproduction or neonatal growth or survival.
Exposure to 409 mg/m3 resulted, however, in parental toxicity
(F0, F1), as indicated by decreases in body weight and
histopathological effects in the nasal mucosa (slight, focal
hyperplasia of the respiratory mucosal epithelium and/or focal
degenerative changes in the olfactory epithelium). No adverse
effects were observed in the parents in the 136 mg/m3 group. The
reproductive no-effect level was 409 mg/m3 (Breslin et al., 1987,
1989).
8.5.1.2 Intraperitoneal (mouse)
1,3-Dichloropropene (Telone II) in corn oil was injected
intraperitoneally in B6C3F1 male mice (4 per group) at dose
levels ranging from 10 mg up to 600 mg/kg body weight, daily for 5
days, to study sperm morphology, epididymal sperm counts, and testes
weights. Testicular toxicity was assessed at day 35. No animals
survived at dose levels of 150 mg/kg body weight or more. No effects
on the testes were found at dose levels up to 75 mg/kg body weight
(Osterloh et al., 1983).
8.5.2 Teratogenicity
8.5.2.1 Inhalation (rat)
In a range-finding study, groups of 7 or 8 female Fischer 344
rats were exposed to Telone II (92.1%; 47.7% cis- and 42.4%
trans-1,3-dichloropropene, impurities, and 1.8% epichlorohydrin)
at 0, 230, 680, or 1360 mg/m3 for 6 h/day on days 6-15 of
gestation.
Consumption of food and drinking-water was decreased during the
period of exposure in rats exposed to 680 or 1360 mg/m3 and the
rats showed a significant decrease in body weight gain. Rats were
sacrificed on day 16 and examined. During exposure, nasal exudate
and red crusty material around the eyes were observed. In the group
exposed to 1360 mg/m3, a significant decrease in litter size and
significant increase in resorption were seen, while in the groups
exposed to 230 and 680 mg/m3, the same changes were observed, but
were not statistically significant (Kloes et al., 1983).
Groups of 30 Fischer 344 rats were exposed, via inhalation, to
1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-isomer) at
0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-15 of
gestation. Food consumption and maternal body weight gain were
depressed in all treated groups in a dose-related manner. A decrease
in water consumption was found in rats at the highest dose-level. No
consistent or dose-related effects on reproductive performance were
found. The number of implantations, resorptions, litter size, fetal
weights, and fetal lengths were comparable with the controls. A
slight, but statistically significant, dose-related increase in the
incidence of delayed ossification of the vertebral centra was found
in rats, but considered of little toxicological significance in the
light of the maternal toxicity observed. The authors stated that
there was no evidence of a teratogenic or embryotoxic response at
exposure levels up to and including 545 mg/m3 (John et al., 1983;
Hanley et al., 1987).
8.5.2.2 Inhalation (rabbit)
In a range-finding study, groups of 7 New Zealand White rabbits
were exposed to Telone II (92.1%, 47.7% cis- and 42.4% trans-
1,3-dichloropropene, impurities and 1.8% epichlorohydrin) at 0, 230,
680 or 1360 mg/m3 for 6 h/day on days 6-18 of gestation. The
rabbits were sacrificed on day 19 and examined. Six out of 7 rabbits
exposed to 1360 mg/m3 showed signs of toxicity, such as rear limb
ataxia, decreased or absence of righting reflex, and flaccid hind
limb muscles; these animals died or were sacrificed. Histologically,
no effects were detected in the brains of these animals. Maternal
body weight was statistically significantly decreased during
exposure in the 680 mg/m3 group, but weights in the 230 mg/m3
group were comparable with those of the controls. There were no
differences in the reproductive parameters between the groups
exposed to 230 and 680 mg/m3 and the controls. No teratological
effects were found (Kloes et al., 1983).
Groups of 25-31 inseminated New Zealand white rabbits were
exposed to 1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-
isomer) at 0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-18
of gestation. Decreased weight gain was observed among rabbits at
272 and 545 mg/m3, but no pronounced maternal toxicity was
observed. No evidence of teratogenic or embryotoxic responses was
observed (John et al., 1983; Hanley et al., 1987).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies
8.6.1.1 Microorganisms
Cis- and trans-1,3-dichloropropene or a mixture were tested
for their mutagenic activity in Salmonella typhimurium and in
Saccharomyces cerevisiae, with and without metabolic activation.
Most of the studies showed a positive effect, especially with
Salmonella typhimurium TA100 and TA1535, with, and without,
metabolic activation. With TA98 and TA1537, positive and negative
effects were found. Saccharomyces cerevisiae was positive. The
results are summarized in Table 13. Samples of highly purified 1,3-
dichloropropene or cis-1,3-dichloropropene, however, were not
mutagenic to Salmonella typhimurium TA100, indicating that trace
impurities, such as 1,3-dichloropropene oxide, were the cause of the
activity (Talcott & King, 1984; Watson et al., 1987, see section
8.8.2).
There was a difference in mutagenicity (expressed as rev/µmol)
between the cis and trans isomers of 1,3-dichloropropene in
Salmonella typhimurium TA100, with, and without, metabolic
activation. The cis-isomer induced a greater number of revertants,
with, and without, S9 mix, than the trans-isomer and also showed
stronger alkylating properties in the NBP-test. Furthermore, a
longer preincubation time invariably led to higher mutagenic
activity, and an increase in protein added to the activating system
increased the efficiency of the metabolic activation (Neudecker et
al., 1980, Neudecker & Henschler, 1986).
Salmonella typhimurium TA100 was used to test for the
mutagenicity of cis- and trans-3-chloroallyl alcohol (99%),
with, and without, metabolic activation. No mutagenicity was
observed without S9 mix, over a range of 0.01-1000 µg/plate.
However, a positive effect was obtained with S9 mix and cis-3-
chloroallyl alcohol (in high concentration) (Connors et al., 1990).
Von der Hude et al. (1988) used the SOS chromotest with
Escherichia coli PQ 37, to examine 1,3-dichloropropene (in DMSO)
at concentrations of 0, 1.0, 3.3 or 10.0 mmol/litre, without S9. In
this strain, the structural gene for beta-galactosidase lacZ is
placed under the control of the SOS-gene sfiA. The expression of
this gene, induced by DNA-damage, is measured indirectly by
determination of the beta-galactosidase activity. The test was
positive with concentrations of 1.0 mmol/litre or more.
8.6.1.2 Effects of glutathione on bacterial mutagenesis
The addition of glutathione (GSH), at physiological
concentrations, to in vitro bacterial test systems of Salmonella
typhimurium TA100 has shown a clear protective effect, i.e., a
virtual elimination of the mutagenic response of 1,3-dichloropropene
(Brooks et al., 1978; Climie et al., 1979; Creedy & Hutson, 1982;
Wright & Creedy, 1982; Creedy, 1983; Creedy et al., 1984; Brooks &
Wiggins, 1990). This protective effect occurs in either the presence
or the absence of S9 for both the (cis)- and (trans)-isomers.
Protection, in the presence of S9, is consistent with the operation
of glutathione transferase enzyme occurring in the added S9
fraction. This enzyme is also present in mammalian cells, and as the
metabolic studies have shown, it plays a key role in the rapid
detoxification of cis-1,3-dichloropropene in mammalian tissue
(Climie et al., 1979; Brooks & Wiggins, 1991).
Even in the absence of S9, this protection by GSH still exists
against the mutagenic action of 1,3-dichloropropene. Thus, it is
likely that an additional mechanism for protection exists that
probably reflects a reaction between the mutagenic component(s) and
GSH. This additional mechanism may also play an important role in
the detoxification of 1,3-dichloropropene via a spontaneous
nucleophilic substitution reaction between the chloromethyl carbon
of 1,3-dichloropropene and the sulfur atom of glutathione.
Table 13. Mutagenicity tests with 1,3-dichloropropenes (1,3-DCP) on microorganisms
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
Salmonella typhimurium
Cis-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 top agar S9 mix/none + Neudecker et al. (1977)
(99.9%) µg/ml Kier et al. (1986)
Cis-1,3-DCP TA98, TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
(> 99%)
TA1538 20-2000 µg plate S9 mix/none - Brooks et al. (1978)
Cis-1,3-DCP TA100, TA1535 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5%, + TA98, TA1537, TA1538 78-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1990)
1,2-DCP 0.25%)
Cis-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 µg/ml top agar S9 mix/none + Neudecker et al. (1977)
(97.5%) Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535 39-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1989a)
(98% + cis-
1,3-DCP 0.3%) TA98, TA1537, TA1538 39-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1989a)
1,3-DCP (51.3% TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
cis, 43.7%
trans, 0.6% TA98, TA1538 20-2000 µg plate S9 mix/none ± Brooks et al. (1978)
epichlorohydrin)
Table 13 (contd)
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
1,3-DCP *** TA100, TA1535 3-333 µg/ml plate S9 mix/none + Haworth et al. (1983)
TA98, TA1537 3-333 µg/ml plate S9 mix/none - Haworth et al. (1983)
1,3-DCP *** TA100, TA1535 1000 µg/ml * S9 mix/none + Priston et al. (1983)
TA98, TA1537 1000 µg/ml * S9 mix/none - Priston et al. (1983)
1,3-DCP *** TA100 0.1-10 µmol plate S9 mix/none + Stolzenberg & Hine (1980)
1,3-DCP *** TA98 100 µg ** none + Vithayathil et al. (1983)
Saccharomyces cerevisiae
1,3-DCP *** JD1 1000 or 5000 liquid S9 mix/none + Priston et al. (1983)
µg/ml
culture
Escherichia coli
Cis-1,3-DCP WP2 UVRA pKM 101 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5% +
1,2-DCP 0.25%)
Trans-1,3-DCP WP2 UVRA pKM 101 39-1250 µg/ml * none - Brooks & Wiggins (1989a)
(98% + cis-1,3- S9 mix +
DCP 0.3%)
* Assays were performed by the pre-incubation method in sealed containers.
** Salmonella/microsome multiple indicator test.
*** Details of composition not given.
These 2 mechanisms with GSH afford complete protection against
the mutagenic activity of the trans-isomer in bacterial test
systems, in the presence and absence of S9 (Hutson & Stoydin, 1977;
Creedy & Hutson, 1982; Wright & Creedy, 1982).
In the presence of Aroclor-induced rat liver S-9 fraction and a
rat liver microsomal mono-oxygenase system, cis-1,3-
dichloropropene (I) is apparently metabolized to a mutagenic
metabolite, cis-1-chloro-3-chloromethyloxirane (II), which is not
as effectively deactivated by glutathione as the parent compound.
Cis-dichloropropeneoxide was shown not to be stable in aqueous
medium, 2-chloroacroleine (III) was the product of hydrolysis,
another direct-acting mutagen for Salmonella typhimurium TA100
(Hutson, 1984) (see Fig. 5). Glutathione transferase systems afford
efficient protection against this bioactivated product of 1,3-
dichloropropene. The protective action of glutathione-linked systems
against the mutagenicity observed in S. typhimurium has also been
shown to occur in vivo.
8.6.1.3 Mammalian cells
In a gene mutation assay, V79 Chinese hamster cells were
exposed to cis-1,3-dichloropropene (99.9%) in DMSO at dose levels
of 2.5 to 20 µg/ml. No indication for an increased mutation
frequency at the HGPRT locus was found in these cells (Meyer, 1980).
Telone II (48.9% cis- and 43.2% trans-1,3-dichloropropene)
was tested in the Chinese hamster ovary cell/HGPRT mutagenicity
assay, with the cell line designated as CHO-K1-BH4, with, and
without, metabolic activation. Three tests were carried out without
activation. The first test with 50, 100, 150, 200, and 250
mmol/litre, showed an increase in mutation frequency at the 200 and
250 mmol/litre dose levels. However, the biological significance is
doubtful because of the extreme toxicity at these high dose levels.
In a repeat of this study using the same concentrations, no increase
in mutation frequency was found. The results of a third study using
concentrations of 50, 100, 125, 150, and 200 mmol/litre were also
negative. The fourth test was with activation, with dose levels of
50, 100, 125, 150, and 200 mmol/litre. The relative survival ranged
from 98% at 50 mmol/litre to 14% at 200 mmol/litre. No increase in
mutation frequency was observed. The results indicated that Telone
II was not mutagenic in the CHO/HGPRT assay, with, or without,
metabolic activation (Mendrala, 1986).
8.6.1.4 DNA damage
Cis- and trans-1,3-dichloropropene were tested for their
ability to induce unscheduled DNA synthesis (UDS) in Hela S3
cells. The lowest dose, 10-4 mol/litre was the dose at which UDS
occurred, the dose-response curves being rather shallow (Schiffmann
et al., 1983).
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in the rat hepatocyte unscheduled DNA synthesis assay
at concentrations from 1 x 10-6 up to 3 x 10-3 mol/litre. In an
initial and a repeat assay, toxic effects on the hepatocyte cultures
(indicated as detachment of the cells and/or a granular appearance)
occurred at 1 x 10-5 mol/litre and at 1 x 10-4 mol/litre or
more, respectively. In both tests, Telone II failed to elicit
significant DNA repair in the primary cultures of rat hepatocytes,
over the wide range of concentrations tested, suggesting an apparent
lack of genetic activity (Mendrala, 1985).
The liquid Bacillus subtilis strain H17 (arg-, trp-, recE+)
microsome rec-assay was used to evaluate the DNA-damaging effect of
1,3-dichloropropene with, and without, S9 mix. The concentrations of
1,3-dichloropropene used with, and without, S9 mix were for
CR50Rec+/CR50Rec-, 7.62 x 10 / 2.49 x 10 and 4.79 x 102/1.01 x
103 mg/litre. With S9 mix, there was a strong DNA-damaging effect,
but, without S9 mix, a reverse effect was observed (Matsui et al.,
1989).
8.6.1.5 Chromosomal effects
Rat liver (RL1) cells were exposed to culture medium
containing cis-1,3-dichloropropene (99.9%) in DMSO, at
concentrations of 2.5, 5.0, or 10 µg/ml. No indication was found
that the compound induced chromosome damage in the liver cells
(Meyer, 1980).
The clastogenic potential of trans-1,3-dichloropropene (95.4%
trans-isomer and 0.3% cis-isomer) was assessed from assays
designed to monitor chromosome damage in CHO cells. Cultures were
grown in incubated medium containing the test compound, for either 3
h in the presence of S9-mix (concentration 0, 0.5, 2.5 or 5.0 µg/ml)
or 24 h in the absence of S9 mix (concentration 0, 3, 15 or 30
µg/ml). Methyl methane sulfonate and cyclophosphamide were used as
positive controls. Metaphase cells were used for the analysis of
chromosome aberrations after 8, 12, and 24 h, in the case of
cultures with S9 mix, and, after 24 h, in the case of the cultures
without S9 mix. At the 24-h sample time, it was concluded that
trans-1,3-dichloropropene induced chromosome damage (gaps, breaks,
and exchange figures) in the presence of S9 mix, but no effect was
seen in its absence (Brooks & Wiggins, 1989b).
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce chromosomal aberrations in cultured CHO cells.
In the first study, without S9, 49.1 µg/ml produced a strong
positive response, i.e., 16% of cells with aberrations (large number
of gaps). No metaphase cells were seen at the 98.0 µg/ml dose level.
In a repeat study, these results could not be confirmed with 50, 75,
and 100 µg/ml. All 3 doses produced 50% decreases in cell
confluency. When the compound was tested with S9 mix, no chromosomal
aberrations were found at 50 µg/ml, the highest dose at which
metaphase cells could be found.
Brooks & Wiggins (1991) assessed the mutagenic activity of
cis-1,3-dichloropropene (94.5-97.5% cis-isomer, 1.5% trans-
isomer, 0.25% 1,2-dichloropropane), in Chinese hamster ovary cells.
The cultures were grown in medium containing the test substance for
either 3 h in the presence of S9 mix or 24 h in the absence of S9
mix. Metaphase cells were prepared for analysis for chromosome
aberrations, 24 h following initiation of exposure with, and
without, S9 mix. From the data obtained at the 24-h sample time, it
was concluded that cis-1,3-dichloropropene at 1, 5, or 10 µg/ml
induced chromosome damage (gaps, breaks, and exchange figures) in
the presence of S9 mix. No increase in metaphase chromosome damage
was found with doses of up to 10 µg/ml in the absence of S9 mix.
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce Sister Chromatid Exchanges (SCEs), in cultured
Chinese hamster ovary cells. The test was carried out with, and
without, rat liver S9 fraction. DMSO was used as the solvent. 1,3-
Dichloropropene induced SCEs without S9 at a dose level of 29.9
µg/ml. A positive response was also found with S9.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
(SCE) test in vitro in Chinese hamster V79 cells without S9 mix,
to evaluate the effect of 1,3-dichloropropene (80%). The
concentrations tested were 0 (DMSO), and 0.1 up to 0.8 mmol/litre.
The 0.8 mmol/litre dose was toxic to the cells. A dose-related
increase in the number of SCEs was found. Negative results were
obtained with S9, at dose levels of 0.1-3.3 mmol/litre.
8.6.2 In vivo studies
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in a mouse bone marrow micronucleus test to detect
chromosomal aberrations and spindle malfunction. The substance
(dissolved in corn oil) was administered to CD-1 (ICR) BR mice by
single oral gavage at dose levels of 0 (corn oil), 38, 115, or 380
mg/kg body weight. Groups of animals were sacrificed at intervals of
24 and 48 h. A positive control group received cyclophosphamide at a
dose of 120 mg/kg. There were no significant increases in the
frequencies of micronucleated polychromatic erythrocytes in the
Telone II groups compared with the controls. Positive results were
obtained with the positive control group. Telone II was considered
negative in the mouse bone marrow micronucleus test (Gollapudi et
al., 1985).
Valencia et al. (1985) tested 1,3-dichloropropene (95.5%) for
mutagenicity in Drosophila melanogaster. The compound was tested
for the induction of sex-linked recessive lethals (SLRLs) by feeding
the substance in a 5% aqueous sucrose solution. The dose level fed
was 0 or 5750 mg/litre. The mortality rate was 33% and sterility
10%. 1,3-Dichloropropene induced an increased number of SLRLs, but
no reciprocal translocations were induced.
The results of both dominant lethal and host-mediated assays
carried out with "MIX D/D" were negative (see "Mixtures of
dichloropropenes and dichloropropane", section 8.6.2).
8.6.3 Appraisal
1,3-Dichloropropene ( cis- and trans-isomer) showed
mutagenic activity in Salmonella typhimurium, especially in
strains TA100 and TA1535 with, and without, metabolic activation.
There is a difference in mutagenic potential between the cis- and
trans-isomers in TA100 with, and without, activation. The cis-
isomer induces a greater number of revertants than the trans-
isomer.
1,3-Dichloropropene does not possess a genotoxic potential in a
variety of non-bacterial studies. Gene mutation has not been
detected in in vitro assays using the eukaryotic cell lines of
either V79 or CHO (HGRPT locus) (Meyer, 1980; Mendrala, 1986).
Chromosomal damage has not been observed in vitro with rat liver
cell cultures or in an in vivo mouse micronucleus study using
single oral doses of up to 380 mg/kg (Gollapudi et al., 1985).
Interaction with DNA was not observed, even at cytotoxic doses, in
an in vitro rat liver unscheduled DNA synthesis study (Mendrala,
1985). However, Schiffmann et al. (1983) found liver unscheduled DNA
synthesis in Hela S3 cells.
8.7 Carcinogenicity
8.7.1 Oral
8.7.1.1 Mouse
A carcinogenicity study was carried out on groups of 50 male
and 50 female (4-6 weeks old) B6C3F1 mice at dose levels of 0,
50, or 100 mg (stabilized technical grade 1,3-dichloropropene)/kg
body weight, administered in corn oil (5 ml/kg body weight), by
gavage, 3 times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, nine other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). The initial mean body weights of
the treated mice were lower (6-22%) than those of the controls.
These differences were caused by failure to fully randomize the
distribution of the animals. No clinical signs were observed.
However, the survival of vehicle control male mice was significantly
lower than that in either dose group. Thirty-nine control male mice
died with myocarditis, 25, between weeks 48 and 51. A slight
increase in mortality was found in the female mice at a dose level
of 100 mg/kg body weight.
The reduced survival of the control male mice did not allow for
an adequate evaluation of carcinogenicity in males in this study.
But, in both sexes, there was evidence for a 1,3-dichloropropene-
related increase in transitional cell carcinomas of the urinary
bladder (without the presence of calculi), liver tumours, squamous
cell papillomas and/or carcinomas of the forestomach, and of
alveolar/bronchiolar adenomas and carcinomas of the lung, at 50 or
100 mg/kg body weight. In the female mice, an increase in basal cell
or epithelial cell hyperplasia in the forestomach was also found
(Table 14). It was stated that the presence of 1% epichlorohydrin, a
direct acting mutagen and carcinogen (especially for the
forestomach), may have influenced the development of forestomach
lesions (Haseman et al., 1984; NTP, 1985; Yang, 1986; Yang et al.,
1986).
8.7.1.2 Rat
A long-term carcinogenicity study was carried out on groups of
52 F344/N rats of each sex (aged 6 weeks), at dose levels of 0, 25,
or 50 mg stabilized technical grade 1,3-dichloropropene/kg body
weight, administered in corn oil (5 ml/kg body weight), by gavage, 3
times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, 9 other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). Additional groups of 25 rats of
each sex were assigned to each dose group. At 9, 16, 21, 24, and 27
months of dosing, 5 rats/sex per group were killed and organs and
tissues studied microscopically. Haematological and clinical-
chemical studies were carried out on groups of 20 rats of each sex,
11-13 times in the first 70 weeks of the study. The mean body weight
of high-dose male rats was about 5% lower than those of the control
and low-dose rats. No differences in body weight were observed in
female animals. Mortality was comparable with the controls. The
primary organs affected were the forestomach and liver. The
incidences of non-neoplastic and neoplastic lesions are summarized
in Table 15.
Table 14. Occurrence of microscopic lesions in mice in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Urinary bladder
Epithelial hyperplasia b 2/50 (4%) 9/50 (18%) 15/50 (30%) 18/50 (36%) 19/48 (40%)
Transitional cell carcinoma b 0/50 (0%) 0/50 (0%) 8/50 (16%) 2/50 (4%) 21/48 (44%)
Lung
Alveolar/bronchiolar
adenoma or carcinoma 1/50c 2/50 (4%) 13/50 (26%) 4/50 (8%) 12/50 (24%) 8/50 (16%)*
Forestomach
Epithelial hyperplasia b 1/50 (2%) 0/50 (0%) 1/50 (2%) 4/50 (8%) 21/50 (42%)*
Squamous cell papilloma b 0/50 (0%) 2/50 (4%) 1/50 (2%) 3/50 (6%)d 4/50 (8%)*
or carcinoma
Liver
Hepatocellular adenoma 5/50b 1/50 (2%) 7/50 (14%) 8/50 (16%)* 13/50 (26%) 3/50 (6%)
or carcinoma
Kidneys
Hydronephrosis b 0/50 (0%) 0/50 (0%) 2/50 (4%) 0/50 (0%) 14/50 (28%)
a From: NTP (1985).
b Too many animals died during the study, but no epithelial hyperplasia or neoplasia were observed in these animals.
c As b but, a lung adenoma was found in one animal.
d Only squamous cell papilloma.
* P = < 0.05.
Table 15. Occurrence of microscopic lesions in rats in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Forestomach
Epithelial hyperplasia 2/52 (4%) 1/52 (2%) 5/52 (10%) 0/52 (0%) 13/52 (25%)c 16/52 (31%)c
Squamous cell papilloma 1/52 (2%) 0/52 (0%) 1/52 (2%) 2/52 (4%)b 13/52 (25%)c 3/52 (6%)b
or carcinoma
Liver
Neoplastic nodule 1/52 (2%) 6/52 (12%) 6/52 (12%)c 6/52 (12%)c 7/52 (13%)c 10/52 (19%)
or carcinoma
a From: NTP (1985).
b Only squamous cell papilloma.
c P = ¾ 0.05
Under the conditions of the study, 1,3-dichloropropene induced
an increased incidence of squamous cell papillomas and carcinomas of
the forestomach. In addition, a dose-related trend was observed in
the incidence of neoplastic nodules in the livers of male rats. It
was stated that the presence of 1% epichlorohydrin, a direct acting
mutagen and carcinogen (especially for the forestomach), may have
influenced the development of forestomach lesions (Haseman et al.,
1984; NTP, 1985; Yang, 1986; Yang et al., 1986).
In the ancillary study, dose-related lesions were observed in
the forestomach and liver. The development of the forestomach basal-
cell hyperplasia and squamous-cell papilloma followed a time-
dependent trend in high-dose males and females. Basal-cell
hyperplasia was seen 9-16 months after dosing started. The neoplasms
of the forestomach and liver were not seen until 24 months after
dosing began.
8.7.2 Inhalation
8.7.2.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (6-7 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days per
week for up to 24 months. In addition, 2 ancillary groups, each with
10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The mice were exposed to 1,3-
dichloropropane vapour at 0, 22.7, 90.8, or 272 mg/m3. The
composition of the technical-grade 1,3-dichloropropene was 1,3-
dichloropropene 92.1% ( cis-49.5% and trans 42.6%); 1,2-
dichloropropane 0.7%; and 5.2% mixtures of hexanes and hexadienes.
Epoxidized soybean oil (approximately 2%) was added as stabilizing
agent. Besides body weights, clinical-chemical parameters in the
blood and urine and haematological parameters were determined for
all animals terminated at 6 and 12 months and for 20 animals/sex per
group at 24 months. At 6, 12, and 24 months, animals were sacrificed
and the weight of 5 organs determined; a large number of organs and
tissues were examined histopathologically.
No significant differences in survival rates were observed
between the groups. The body weights of male mice exposed to 272 mg
1,3-dichloropropene/m3 were statistically significantly depressed
in comparison with the controls. Examination of haematological and
clinical-chemical parameters and urinalysis did not indicate any
toxicity resulting from exposure to 1,3-dichloropropene for 6, 12,
or 24 months.
The mean relative liver weight of male animals exposed to 272
mg/m3 showed a statistically significant decrease. Gross patho-
logical examination of mice revealed morphological alterations
involving the urinary bladder and lung, which were attributed to
exposure to 1,3-dichloropropene. The bladder mucosal surface in
females exposed to 272 mg/m3 for 12 months and 90.8 and 272
mg/m3 for 24 months had a roughened appearance. In addition, a
statistically significantly increased number of the females exposed
to 90.8 and 272 mg/m3 showed inflammation and epithelial
hyperplasia of the bladder mucosa, after 24 months. An increased
number of male animals exposed to 272 mg/m3 also showed
inflammation.
Female mice exposed to 90.8 or 272 mg/m3 and males exposed to
272 mg/m3 showed hypertrophy and hyperplasia of the nasal
epithelium and degeneration of the olfactory epithelium. Additional
microscopic changes, considered exposure-related, were hyperplasia
and hyperkeratosis in the forestomach of 8/50 male mice following 24
months exposure to 272 mg/m3. A statistically significant increase
in the incidence of a benign tumour, bronchio-alveolar adenoma, was
observed in male mice exposed to 272 mg 1,3-dichloropropene
vapour/m3 for 24 months [22/50 (44%) vs 9/50 (18%) in controls].
No statistically significant increase in tumour incidence was found
in the groups exposed to 1,3-dichloropropene at 22.7 and 90.8
mg/m3 (Table 16). The incidence of lung tumours in the males
exposed to 272 mg/m3 was somewhat higher (7-32%) than the range of
historical control values for this type of tumour in male
B6C3F1 mice in 7 previous, long-term studies. The NOAEL in
this study on mice for hypertrophy/hyperplasia of the nasal
epithelium was 22.7 mg/m3 (Yano et al., 1985; Stott et al., 1987;
Lomax et al., 1989).
8.7.2.2 Rat
Groups of 50 male and 50 female Fischer 344 rats (7-9 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days/week
for up to 24 months. In addition, 2 ancillary groups, each
comprising 10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The animals were exposed to 0,
22.7, 90.8, or 272 mg/m3. The chemical composition of the test
material was 92.1% 1,3-dichloropropene ( cis-49.5% and trans-
42.6%), 0.7% 1,2-dichloropropane, and mixtures of hexanes and
hexadiens. Epoxidized soybean oil (approximately 2%) was present as
stabilizer. Besides body weights of the animals, clinical-chemical
parameters in the blood and urine and haematological parameters were
determined for all animals terminated at 6 and 12 months and for 20
animals/sex from each exposure group at 24 months.
Table 16. Incidence of various types of lesions observed in a mouse inhalation study with 1,3-dichloropropenea
Males (mg/m3) Females (mg/m3)
0 22.7 90.8 272 0 22.7 90.8 272
Urinary bladder
Hyperplasia mucosa 4/48 (9%) 7/48 (15%) 11/48 (23%) 37/47 (79%)b 1/47 (2%) 4/46 (9%) 21/48 (44%)b 44/45 (98%)b
(simple or nodular)
Lungs
Bronchio-alveolar 9/50 (18%) 6/50 (12%) 13/50 (26%) 22/50 (44%)b 4/50 (8%) 3/50 (6%) 5/50 (6%) 3/50 (6%)
adenoma
Nasal tissues
Degeneration of 1/50 (2%) 0/50 1/50 (2%) 48/50 (96%)b 0/50 0/50 1/50 (2%) 45/50 (90%)b
olfactory epithelium
Hyperplasia and 5/50 (10%) 1/50 (2%) 4/50 (8%) 48/50 (96%)b 4/50 (8%) 4/50 (8%) 28/50 (56%) 49/50 (98%)b
hypertrophy of
respiratory epithelium
Stomach
Squamous papilloma 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50(6%)
a From: Lomax et al. (1989).
b Statistical difference from control mean identified by using Yate's kappa2 pairwise test, alpha = 0.05.
At 6, 12, or 24 months, animals were sacrificed and the weight
of 5 organs determined; a large number of organs and tissues were
examined histopathologically. No significant influence on survival
was observed. Mean body weights of both male and female rats exposed
to 272 mg/m3 were statistically significantly decreased compared
with mean control values. Examination of haematological and
clinical-chemical parameters, urinalysis, and organ weights did not
indicate any toxicity resulting from exposure to 1,3-dichloropropene
for 6, 12, or 24 months.
Gross pathological examination did not indicate any exposure-
related effects after 6, 12, or 24 months. Exposure-related
histological effects occurred in nasal tissues of rats exposed to
272 mg/m3 for 24 months (Table 17), but not for 6 or 12 months.
The microscopic changes were located in the olfactory mucosa, which
covers the upper portions of the nasal cavity, nasal septum, and
turbinates. The changes were characterized by unilateral or
bilateral decreased thickness of olfactory epithelium and fibrosis
of the submucosal tissues underlying eroded olfactory epithelium. At
the lower dose levels, females did not show histopathological
changes in the nasal tissue, while one male exposed to 22.7 mg/m3
and one exposed to 90.8 mg/m3 showed decreased thickness of the
olfactory epithelium. No effects were seen in the controls. No
statistically significant increase in tumour incidence was found in
exposed rats compared with controls (Lomax et al., 1987, 1989).
8.7.3 Appraisal
Exposure to 1,3-dichloropropene, through inhalation, for up to
24 months did not have any demonstrable effects on survival or
spontaneous tumour development in male and female Fischer 344 rats.
In mice, an increased incidence of bronchio-alveolar adenomas was
found in the lungs of male mice, exposed to 272 mg/m3, but not at
the lower dose level (90.8 mg/m3). The oral gavage study with 1,3-
dichloropropene demonstrated an increased incidence of forestomach
neoplasms in rats of both sexes at dose levels of 50 mg/kg body
weight, administered 3 times/week for 24 months. Male rats treated
with 25 or 50 mg/kg also had an increased incidence of neoplastic
nodules in the liver.
In both the oral gavage and inhalation mouse bioassays with
1,3-dichloropropene, a tumorigenic response was noted in tissues
with which 1,3-dichloropropene had direct contact, i.e., the stomach
and the lung. However, in the gavage study, tumours were also
induced at sites distant from that at the primary "portal-of-entry"
(lung and urinary bladder). This was not the case in the inhalation
study, despite the fact that the dose of 1,3-dichloropropene
received on a mg/kg body weight per day basis by mice exposed for 5
days/week through inhalation was approximately 2-3 times higher than
the dose levels administered orally 3 times/week in the NTP (1985).
Table 17. Microscopic changes in the nasal tissues of rats exposed to
Telone II at 272 mg/m3a
Microscopic change Vehicle control Overall incidence
(male and female) Male Female
Decreased thickness of 0/100 20/50b (40%) 15/50b (31%)
olfactory epithelium
Erosion of olfactory 0/100 15/50b (30%) 6/50 (12%)
epithelium
Submucosal fibrosis 0/100 6/50b (12%) 2/50 (4%)
a From: Lomax et al. (1989).
b Statistical difference identified from control mean of
Yate's kappa2 pairwise test, alpha = 0.05.
The degeneration and subsequent hyperplasia of nasal and forestomach
epithelium occurred only at concentrations that are known to deplete
glutathione levels in these tissues. Tumorigenic effects occur at
doses higher than those causing glutathione depletion and tissue
damage.
The same mouse strain and similar test materials relative to
1,3-dichloropropene were used in both bioassays, but there was a
difference in the stabilizing agents in the test materials. The 1,3-
dichloropropene used in the NTP study was stabilized with 1%
epichlorohydrin, a carcinogen. It has been suggested that
epichlorohydrin may have played a role in the tumorigenic response
obtained in the gavage study because of the bolus nature of its
administration. However, it is not known whether the increased
incidence of tumours in the urinary bladder, lungs, forestomach, and
liver in the mouse gavage study are attributable to the treatment
with 1,3-dichloropropene or the effect of epichlorohydrin, since
carcinogenicity studies of epichlorohydrin in mice have not been
performed yet. In the inhalation study carried out by Lomax et al.
(1989), the 1,3-dichloropropene was stabilized with the relatively
nontoxic epoxidized soybean oil. The role of the stabilizing
additive epichlorohydrin in generating the different tumours seen in
the oral gavage studies on 1,3-dichloropropene is still uncertain.
This question has to be further investigated before a more definite
conclusion about the carcinogenic potential of 1,3-dichloropropene
can be drawn.
8.7.4 Dermal and subcutaneous (mouse)
Groups of 30 female Ha:ICR Swiss strain mice (6-8 weeks old)
were treated with 0.2 ml acetone containing 41 or 122 mg purified
cis-1,3 dichloropropene, applied to shaven skin 3 times weekly,
for approximately 18 months. Control mice received acetone. In the
group treated with 41 mg, no papillomas were found, but at the 122
mg dose, 3 animals showed papillomas, and 2, carcinomas. No tumours
were found at distant sites. Cis-1,3-dichloropropene, applied once
on the skin at a dose of 122 mg/mouse, was followed after 14 days by
the application of 5 µg phorbol myristate acetate in 0.2 ml acetone,
3 times weekly until the end of the study. No skin tumour-initiating
activity was observed (van Duuren et al., 1979).
A group of 30 female Ha:ICR Swiss mice were given weekly
subcutaneous injections in the left flank of 0.05 ml trioctanoin
containing 3 mg purified cis-1,3-dichloropropene per injection.
The study lasted 538 days. Control animals received only the
vehicle. Six out of 30 mice showed local fibrosarcomas, whereas
vehicle control animals did not. A positive control of 0.3 mg beta-
propiolactone produced local sarcomas in 24 out of 30 mice during a
378-day period (van Duuren et al., 1979).
The relevance of the subcutaneous route for the assessment of
carcinogenic properties remains questionable, especially when
injections of an irritant material are made. It is probable that the
persistent and physical properties rather than the chemical
characteristics of cis-1,3-dichloropropene are responsible for
production of local sarcomas.
8.8 Factors modifying toxicity, toxicity of metabolites, mode of
action
8.8.1 Toxicity of the metabolites, cis- and trans-1,3-
dichloropropene oxide
There is some evidence that a small proportion of cis-1,3-
dichloropropene is metabolized to cis-1,3-dichloropropene oxide
(Fig. 5; Hutson, 1984).
8.8.1.1 Mutagenicity
Cis- and trans-1,3-dichloropropene oxide were tested for
mutagenicity, in the absence of metabolic activation, in Salmonella
typhimurium TA1535 and Escherichia coli WP2 uvr A, and for
preferential inhibition of growth of DNA-repair-polymerase-deficient
E. coli. Both oxides were potent mutagens and DNA modifiers. In
Salmonella typhimurium TA1535, treated with 0.025 µmol/ml and in
E. coli WP2 uvr A treated with 0.05 µmol/ml of bacterial
suspension, a significant increase in revertant colonies was found.
A gene mutation test with E. coli (pol A1-/pol A1+) in the
absence of metabolic activation, already showed an effect with
0.0005 µmol/ml (Kline et al., 1982).
In the absence of an S9-fraction, cis-dichloropropene oxide
was strongly mutagenic towards S. typhimurium TA100. The
mutagenicity reached a maximum at 25 µg/plate. Above 300 µg/plate,
marked cytotoxicity was observed. Glutathione (5 mmol/litre) caused
a significant inhibitory effect on the mutagenicity and cytotoxicity
of this epoxide, but did not offer complete protection. Inclusion of
glutathione (5 mmol/litre) together with S9-fraction afforded
complete protection over the range of concentrations of cis-
dichloropropene oxide up to 100 µg/plate (Hutson 1984; Watson et
al., 1986a).
Cis- and trans-1,3-dichloropropene oxide were tested in a
quantitative Syrian hamster embryo cell model. Both compounds at
dose levels of 0.005, 0.01, or 0.02 mmol/litre ( cis-isomer) and
0.01, 0.025, or 0.05 mmol/litre ( trans-isomer) induced
morphological transformation of the Syrian hamster embryo cells
(DiPaolo & Doniger, 1982).
8.8.1.2 Carcinogenicity
Female ICR/Ha Swiss mice (30 per group) were treated 3 times
weekly, with cis-1,3-dichloropropene oxide or trans-1,3-
dichloropropene oxide (containing 10-15% of m-dichlorobenzene) on
the skin. The dose level was 10 mg in 0.1 ml of acetone. The
controls received only acetone. The median survival time was
comparable with that of the controls (over 500 days). With cis-
dichloropropene oxide, 16/30 mice had local papillomas and 10/30
squamous cell carcinomas of the skin; with trans-dichloropropene
oxide, this was 20/30 and 17/30, respectively. No tumours were found
in the control animals (van Duuren et al., 1983).
A study was also carried out on the same strain of mice using
subcutaneous injections. Thirty female mice received 500 µg cis-
dichloropropene oxide or trans-dichloropropene oxide in 0.05 ml
tricaprylin once weekly. The median survival time was comparable to
that of controls. With cis-dichloropropene oxide, 4 animals had a
local (fibro)sarcoma and one carcinoma, and, with trans-
dichloropropene oxide, 5 animals had fibrosarcomas. No tumours were
found in the vehicle controls (van Duuren et al., 1983).
8.8.2 Role of oxidation
When purified cis-1,3-dichloropropene was heated for a few
hours in an oxygen atmosphere, in either the light or dark, the non-
mutagenic cis-1,3-dichloropropene became strongly mutagenic.
Heating under nitrogen was negative. Storage of cis-1,3-
dichloropropene at room temperature, in the presence of oxygen, for
two months, made it mutagenic (Watson et al., 1987). Talcott & King
(1984) demonstrated that purified samples of 1,3-dichloropropene
were not mutagenic to Salmonella typhimurium TA100. Four
preparations of 1,3-dichloropropene were separated into different
fractions and analysed for mutagenic activity. The fraction
containing polar metabolites was found to be mutagenic. Its
composition was too complex to characterize completely, but 2
mutagens, epichlorohydrin and 1,3-dichloro-2-propanol, were
identified.
Watson et al. (1986 a,b; 1987) confirmed that the direct
mutagenicity, inducing base-pair mutations, previously observed in
Salmonella typhimurium TA100 treated with cis-1,3-
dichloropropene, was caused by trace impurities. These impurities
resulted from the autooxidation of cis- and trans-1,3-
dichloropropene and were identified as cis- and trans-
dichloropropene oxides. The dichloropropene oxides made a
significant contribution towards the intrinsic mutagenicity, when
tested in S. typhimurium TA100 (see section 8.8.1.1).
The proposed formation of cis- and trans-dichloropropene
oxides is shown in Fig. 6.
In California, applicators of 1,3-dichloropropene were also
studied for personal air exposure and urinary excretion of
mercapturic acid metabolites. The amount excreted was correlated
with the product of the duration of exposure x TWA. The highest
urinary metabolite concentration occurred during the application
period, indicating rapid excretion. Skin absorption of vapour was
not a significant route of exposure (Osterloh et al., 1984, 1989,
see also section 9.2.1).
Air and biological monitoring of 6 operators exposed to "Mix
DD" soil fumigant during filling operations in the Netherlands was
carried out in 1985-86. There was rapid metabolism and elimination:
the half-lives of mercapturic acid excretion were 4-5 h, with a
return to background levels after 24 h. It was calculated that,
under linear, non-saturation conditions, approximately 23% of the
inhalation dose of the cis-isomer and 10% of the trans-isomer
are excreted in the urine as mercapturic acids (Eadsforth, 1987).
6.4 Reaction with macromolecules
6.4.1 Mouse
The non-protein sulfhydryl (NPS) content, e.g., GSH, and
covalent binding to macromolecules were determined in the tissues of
male B6C3F1 mice. Single oral doses of 0, 1, 5, 25, 50, or 100
mg 1,3-dichloropropene 97% ( cis- 62% : trans-isomer 38%)/kg body
weight were given for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-
dichloropropene/kg body weight for binding studies. Non-glandular
forestomach, glandular stomach, liver, kidneys, and bladder were
analysed, 2 h after dosing. Although NPS depletion and dose-related
increases in macromolecular binding were noted in several tissues of
rats, these effects were more pronounced in the non-glandular
stomach than in any other tissue (including glandular stomach,
liver, kidneys, and bladder). The no-observed-effect level (NOEL)
for NPS depletion in rat non-glandular stomach was 1 mg/kg body
weight. NPS levels in non-glandular forestomach were significantly
decreased at doses of 25 mg or higher and, in the liver, at 100
mg/kg body weight. Binding in the non-glandular forestomach was
greatest at dose levels that caused the most depletion of tissue
NPS. Limited binding occurred in the liver, kidneys, and bladder
(Dietz et al., 1984b, 1985).
6.4.2 Rat
Groups of 3-9 male Fischer 344 rats (200-260 g) were
administered 50 mg cis-1,3-dichloropropene (94.1% cis- and 2.5%
trans-) or 50 mg trans-1,3-dichloropropene (97.3% trans- and
0.8 cis-)/kg body weight, by gavage. The rats were sacrificed at
various intervals after dosing, to determine the tissue non-protein
sulfhydryls (NPS) in the liver, kidneys, forestomach, glandular
stomach, and bladder. Blood samples were also taken to determine the
presence of unchanged 1,3-dichloropropene. Cis-1,3-dichloropropene
was only detected in the blood (6.58 µg/litre) 15 min after dosing,
the blood levels of trans-1,3-dichloropropene were 11.72 and 8.38
µg/litre, respectively, 15 and 45 min after dosing. A statistically
significant decrease in the non-protein sulfhydryl contents of the
liver, kidneys, forestomach, and glandular stomach was found. This
depletion reached a maximum, approximately 2-h after dosing. No
depletion was noted in the bladder. It is not possible to
distinguish the effects of cis- and trans-1,3-dichloropropene on
NPS, as the results for the individual isomers were not reported.
The results indicated that orally administered 1,3-dichloropropene
produces a rapid and significant depletion of tissue non-protein
sulfhydryls in the rat (Dietz et al., 1982).
The non-protein sulfhydryl (NPS) contents and covalent binding
to macromolecules were determined in the tissues of male Fischer 344
rats. Single, oral doses of 0, 1, 5, 25, 50, or 100 mg 1,3-
dichloropropene 97% ( cis-62% and trans-isomer 38%) were given
for NPS studies and 0, 1, 50, or 100 mg 14C-1,3-dichloropropene/kg
body weight for binding studies. NPS levels in non- glandular
forestomach were significantly decreased with doses of 25 mg/kg body
weight or more. Binding in the non-glandular forestomach was
greatest at dose levels that caused a significant depletion of
tissue NPS. Limited binding was noted in the liver, kidneys, and
bladder (Dietz et al., 1984b, 1985).
6.5 Appraisal
Mice, rats and humans metabolize 1,3-dichloropropene
predominantly by conjugation with reduced glutathione (GSH). The
glutathione conjugate is further metabolized to the corresponding
mercapturic acid, which is then excreted in the urine. Consistent
with the function of GSH as a detoxication mechanism, the
genotoxicity of 1,3-dichloropropene was decreased in Salmonella
typhimurium when the concentration of GSH was increased to a
mammalian physiological concentration (see sections 8.6.1.2, 8.9.2).
The levels of GSH (measured as non-protein sulfhydryl content)
were decreased at locations consistent with the route of exposure,
i.e., predominantly in the forestomach and to a lesser extent in the
glandular stomach and liver, following oral administration, and in
the nasal tissue after inhalation exposure. For oral exposure, the
extent of covalent binding of 1,3-dichloropropene to macromolecules
was correlated with the decrease in non-protein sulfhydryl content.
It is anticipated that toxic effects of 1,3-dichloropropene will
occur at doses that deplete tissue sulfhydryls and will be
manifested in the organs described above (forestomach, liver, and
nasal tissue).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The United States Environmental Protection Agency published a
Health and Environmental Effects Profile for 1,3-Dichloropropene in
1985 (US EPA, 1985).
7.1 Acute toxicity
7.1.1 Microorganisms
The major effect of 1,3-dichloropropene on soil microorganisms
is on nitrogen transformation. The oxidation of ammonium from soil
organic nitrogen is reduced by 1,3-dichloropropene; soil ammonium
levels were significantly greater and soil nitrate levels were
significantly lower in soils treated with 1,3-dichloropropene
compared with untreated controls (Tu, 1973; Elliot et al., 1974,
1977).
In a series of studies, the effects of 1,3-dichloropropene at
30 or 60 mg/kg soil were evaluated, in parallel, under laboratory
conditions. In general, neither dose affected soil microorganisms or
function appreciably. The effects of 1,3-dichloropropene on soil
microorganisms and enzyme activity were not consistent in 3 soil
types (sandy-, clay-, and organic soils). The numbers of fungi, 2
days after fumigation, were significantly reduced in clay and sandy
soils, but not in organic soil, but recovered after 7 days. 1,3-
Dichloropropene either increased or decreased the number of non-
symbiotic nitrogen fixers and enzyme activity in different soils
(Tu, 1978, 1979, 1981a,b).
Moje et al. (1957) studied the individual components of "MIX
D/D" in old citrus soil and found that the toxicity was mainly due
to its 1,3-dichloropropene content, in particular that of the cis-
isomer (see also section 7.1.1 of "MIX D/D"). Results were as
tabulated on the next page.
1,3-Dichloropropene was converted by the methanotrophic
bacterium Methylosinus trichosporium OB3b, grown in aerobic
continuous cultures. The substance was added at a concentration of
0.2 mmol/litre. After 24 h, 85% of the substance added was degraded
(Oldenhuis et al., 1989).
In field experiments with continuous potato cropping, it was
found that sustained annual applications of 1,3-dichloropropene led
to insufficient control of Globodera rostochiensis. In the
laboratory, 1,3-dichloropropene rapidly disappeared from these
soils. This did not occur when the soil was sterilized. A bacterium
was isolated from these soils, which was found to decompose 1,3-
dichloropropene using it as a carbon and energy source. The
bacterium was Pseudomonas sp. Repeated application of 1,3-
dichloropropene led to accelerated degradation of the substance
(Lebbink et al., 1989).
Reduction in
Compound Fungi Bacteria and
actinomycetes
Cis-1,3-dichloropropene 85-95% reduction 85-100% reduction
at 25 mg/kg soil at 250 mg/kg soil
Trans-1,3-dichloropropene 100% reduction 100% reduction at
at 250 mg/kg soil 1000 mg/kg soil
7.1.2 Algae
The 96-h EC50 value for growth, based on the concentration of
chlorophyll a and also on cell numbers of the freshwater green algae
Selenastrum capricornutum in a static system, was 4.95 mg/litre
for 1,3-dichloropropene. The estuarine diatome, Skeletonema
costatum, showed a 96-h EC50 value for growth, based on the
concentration of chlorophyll a in culture, of 1 mg/litre (Leblanc,
1984). The EC50 calculated from cell numbers was 1.04 mg/litre (US
EPA, 1980). The compound is moderately toxic for marine algae.
The toxicity of cis-, trans- and a mixture of cis- and
trans-1,3-dichloropropenes for Selenastrum capricornutum was
determined in a sealed 72-h growth inhibition test. The 72-h EC50
values (percentage reduction in area under the growth curve),
expressed in terms of the mean measured concentration in the test
media, were; cis-isomer 2.8 mg/litre; trans-isomer 11 mg/litre;
and the mixture 8.2 mg/litre. The EC50 (percentage reduction in
mean specific growth rate), (24-48 h) and EC50 (24-72 h) values
determined by analysis of average specific growth rates were: cis-
isomer 4.6 and 3.1 mg/litre; trans-isomer 6.6 and 7.5 mg/litre;
and, for the mixture, 11 and 3.6 mg/litre, respectively (Girling,
1989a,b,c).
Rapid evaporation and sensitivity to hydrolysis are features of
the chloropropenes that may interfere with proper determination of
toxic concentrations of these chemicals for aquatic species, using
the standard techniques. Significant loss of the test substance may
have occurred during experiments carried out at temperatures above
20 °C and under "static" conditions, i.e., lasting for several days
without refreshing the medium. Determination of the actual
concentrations has seldom been carried out, and it is suspected that
several of the data reported for the chloropropenes present an
underestimation of the toxic potential of these chemicals for
aquatic organisms. On the other hand, the products of hydrolysis of
the chloropropenes, for instance, chloroallyl alcohols are
themselves toxic (Krijgsheld & Van der Gen, 1986).
7.1.3 Invertebrates
The acute toxicity of 1,3-dichloropropene for non-target
aquatic crustacea is summarized in Tables 6 and 7.
Table 6. Acute toxicity of 1,3-dichloropropene for non-target aquatic crustacea
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (mg/litre) (mg/litre)
Water flea 21 0.090a - Mayer &
(Daphnia magna) Ellersieck (1986)
Mysid shrimp - 0.79 US EPA (1980);
(Mysidopsis bahia) Leblanc (1984)
a 48-h EC50 6 mg/litre (US EPA, 1980; Leblanc, 1980) in a static system.
Leblanc (1980) calculated a no discernable effect concentration
of 0.41 mg/litre for 1,3-dichloropropene in Daphnia magna, under
static conditions. However, the result was based on nominal
concentrations and was higher than the 48-h LC50 given by Mayer &
Ellersieck (1986).
7.1.4 Honey bees
1,3-Dichloropropene has been tested on worker Honey bees (Apis
mellifera) using a dusting technique. The 48-h LD50 was 6.6
µg/bee and 1,3-dichloropropene was rated as "relatively non-toxic"
for Honey bees (Atkins et al., 1973).
Table 7. Acute toxicity of cis- and/or trans-1,3-dichloropropenes in Daphnia magna
Substance Age System Temperature 48-h EC50 Reference
(°C) (mg/litre)
Cis-1,3 DCP 24 h statica 18-22 1.4 Girling (1989a)
(96%)
Trans-1,3 DCP 24 h staticb 18-22 3.1 Girling (1989c)
(95.4% trans +
0.3% cis)
Cis- + trans- 24 h staticc 18-23 3.1 Girling (1989b)
mixture
(51-52% cis +
44% trans)
a pH 7.6-8.4; total hardness 176 mg/litre; dissolved oxygen 8.8-9.8 mg/litre.
b pH 7.8-8.1; total hardness 170 mg/litre; dissolved oxygen 8.6-9.2 mg/litre.
c pH 7.9-8.3; total hardness 179 mg/litre; dissolved oxygen 8.6-9.9 mg/litre.
7.1.5 Fish
The data summarized in Tables 8 and 9, suggest that 1,3-
dichloropropene is moderately toxic for fish.
Heitmuller et al. (1981) exposed sheepshead minnows to 1,3-
dichloropropene under static conditions. They calculated a no-
observed-effect concentration of 1.2 mg/litre, but this was based on
nominal concentrations.
7.1.6 Birds
Worthing & Hance (1991) reported an LC50 (8-day) for Mallard
duck and Bobwhite quail of > 10 000 mg/kg diet.
7.2 Short-term/long-term toxicity
7.2.1 Invertebrates
No data are available.
Table 8. Acute toxicity of 1,3-dichloropropene for fish
Species Type of test Size (g, mm) Temperature 96-h LC50 References
(°C) (mg/litre)
Fathead minnow static 0.9 g 18 4.1 Mayer & Ellersieck (1986)
(Pimephales promelas) (3.39-4.97)
Largemouth bass static 1.0 g 18 3.65a Mayer & Ellersieck (1986)
(Micropterus salmoides) (3.52-3.78)
Walleye static 1.3 g 18 1.08 Mayer & Ellersieck (1986)
(Stizostedion vitreum) (0.99-1.18)
Golden orfe - 2.8 g 20 9b Reiff (1978); Krijgsheld & Van
(Idus idus melanotus) (8-11) der Gen (1986)
Sheepshead minnow static 8-15 mm 25-31 1.8c,d US EPA (1980); Heitmuller
(Cyprinodon variegatus) (0.7-4.5) et al. (1981); Leblanc (1984)
Rainbow trout - - - 3.9 Worthing & Hance (1991)
(Salmo gairdneri)
Bluegill static 0.32-1.2 g 21-23 6.1c,d Buccafusco et al. (1981)
(Lepomis macrochirus) (5.1-6.8) US EPA (1980); Leblanc (1984)
Guppy semi-static - 22 0.5e Krijgsheld & Van der Gen (1986)
(Poecilia reticulata)
Goldfish static 1.0 g 18 < 7.5 Mayer & Ellersieck (1986)
(Carasius auratus)
Table 8 (continued)
a Tested in hard water, 272 mg CaCO3/litre.
b Tested in dechlorinated water, 260 mg CaCO3/litre.
c Tested in well water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration 9.7 mg/litre reduced
at the beginning to 0.3 mg/litre after 96-h exposure.
d Nominal concentrations.
e 14-day test.
Table 9. Acute toxicity of cis- and/or trans-1,3-dichloropropenes
in rainbow trout (Salmo gairdneri)
Substance Mean size System Temperature 96-h LC50 Reference
(cm, g) (°C) (mg/litre)
Cis-1,3 DCP 4.7 cm semi- 13-17 1.6 Girling (1989a)
(96%) (1.1 g) statica
Trans-1,3 DCP 4.0 cm semi- 15-17 4.5 Girling (1989c)
(95.4% trans + (0.67 g) staticb
0.3% cis)
Cis- + trans- 4.2 cm semi- 13-17 2.0 Girling (1989b)
mixture (0.68 g) staticc
(51-52% cis +
44% trans)
a pH 7-7.8; total hardness, 226-258 mg/litre as CaCO3: dissolved oxygen, 6.4-10.2 mg/litre.
b pH 7.2-7.8; total hardness, 234-264 mg/litre as CaCO3: dissolved oxygen, 8.2-9.9 mg/litre.
c pH 7.5-8.3; total hardness, 251-284 mg/litre as CaCO3: dissolved oxygen, 8.1-10.2 mg/litre.
7.2.2 Fish
Embryo-larval tests have been conducted on Fathead minnows
(Pimephales promelas) exposed to 1,3-dichloropropene. The maximum
no-effect concentration was 0.24 mg/litre (no details are available)
(US EPA, 1980).
7.2.3 Field studies
See section 7.3.2 of "Mixtures of dichloropropenes and
dichloropropane" for effects of "MIX D/D" on worms.
7.2.4 Phytotoxicity
1,3-Dichloropropene is highly phytotoxic.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The United States Environmental Protection Agency, published a
Health and Environmental Effects Profile for 1,3-dichloropropene in
1985 (US EPA, 1985).
In two other documents of the US EPA, health risk assessment
information is given, on the basis of a comprehensive review of the
toxicity data (US EPA, 1990, 1991).
8.1 Single exposures
8.1.1 Oral
The acute oral LD50s for mice and rats are summarized in
Table 10.
The following signs of toxicity were observed after oral
administration: hunched posture, lethargy, pilo-erection, decreased
respiratory rate, ptosis, diarrhoea, diuresis, ataxia, tip-toe gait,
red/brown staining around the snout, tremors, emaciation, and pallor
of the extremities. Haemorrhages and congestion were found in the
lungs and gastrointestinal tract. The livers showed patchy areas of
pallor (Jones & Collier, 1986a; Jeffrey et al., 1987; Gardner,
1989a,b,c).
8.1.2 Inhalation
The acute inhalation LC50s for 1,3-dichloropropene are
summarized in Table 11.
During the exposure and observation periods, the following
symptoms were observed: partial closing of the eyes, pilo-erection
salivation, lacrimation, lethargy, diarrhoea, reduction in
respiratory rate, irregular respiratory movements (lung congestion
was observed in dead animals) and hunched posture, brown staining of
fur and fur loss, and reddening of ears, tail, and feet.
Pathological signs were cardiopulmonary failure, acute tubular
necrosis in the kidneys, and local effects on the respiratory tract.
It was suggested that the irritating properties of the vapour
might serve as a warning of its presence in air. Signs of
intoxication included hypoactivity, anorexia, and chromodacryorrhoea
(Coombs & Carter, 1976b).
Table 10. Acute oral toxicity (LD50) of 1,3-dichloropropene
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse (CD 1) undiluted 215 Coombs & Carter
(1976b)
Mouse 92% (in corn oil) 640 (582-704)a Toyoshima et al.
(JCL:ICR) 640 (547-749)b (1978a)
Rat 94.5-97.5% cis-, 1.5% 85 Jeffrey et al.
(Fischer 344) trans-, 0.25% (1987); Gardner
1,2-dichloropropane, (1989b)
undiluted
Rat 96.7%, trans-, 94 Gardner (1989c)
(Fischer 344) undiluted 117a
78b
Rat (CD) undiluted 127 (112-141) Coombs & Carter
(1976b)
Rat (not 92% (in corn oil) 470b Torkelson & Oyen
specified) 713a (1977)
Rat (Sprague- 97.2% 130 (110-170)a Jones & Collier
Dawley) between 110 and 250b (1986a)
Rat D-D 95, undiluted 57 Gardner (1989a)
(Fischer 344) (cis + trans 1 : 1)
Rat 92% (in corn oil) 560 (452-695)a Toyoshima et al.
(Wistar) 510 (480-726)b (1978b)
Table 10 (contd)
Species Concentration LD50 (mg/kg body Reference
of substance weight, with 95%
confidence limits)
Rat 97.5% 300a Jeffrey et al. (1987)
(Fischer 344) 224b
a Male.
b Female.
Table 11. The acute inhalation toxicity LC50 for 1,3-dichloropropene (4-h exposure)
Species Concentration LC50 (mg/m3) Reference
of substance
Rat 51% cis-, 43.4% 3309.7 Blair (1977)
(Wistar) trans-isomer, 1%
epichlorohydrin
Rat Telone II 2.70-3.07c Cracknell et al.
(Wistar) (98.4%) (1987)
Rat 95.6% cis- and 3041.8a Nitschke et
(Fischer 344) 1.5% trans-isomer 3377.8b al. (1990)
Rat Telone II (97.5%; > 3881.7-< 4698.9a Streeter et al.
(Fischer 344) 52.6% cis- and 4014.2b (1987)
44.9% trans-isomer)
Rat 95.4% trans- and 4880.5b Collins (1989)
(Crb:CD(SD)Br) 0.3% cis-isomer 5402.6a
a male.
b female.
c mg/litre.
A 2-h exposure to 1,3-dichloropropene at 4540 mg/m3 was
lethal to rats, whereas guinea-pigs died following a single 7-h
exposure to 1816 mg/m3 (Torkelson & Oyen, 1977).
One female and 3 male Rhesus monkeys (4-5 kg) were exposed to
Telone II at 0, 113.5, 227, 454, 908, or 2724 mg/m3 for a single
behavioural session of 1 h, with a 1-week recovery period after
exposure. Data were obtained on all monkeys for each of the 5
atmospheric concentrations. At concentrations ranging up to 908
mg/m3, there were no significant indications of toxicity or
alterations in behaviour (the monkeys were trained to perform on a
dual component FRFR (Fixed Ratio followed by a Fixed Ratio) chained
schedule, with light stimulation). Only slight eye irritation was
observed in all monkeys at concentrations exceeding 454 mg/m3
(Rosenblum & Talley, 1979).
8.1.3 Dermal
The acute dermal and subcutaneous LD50s are summarized in
Table 12.
After dermal application, the signs of intoxication were:
diarrhoea, lethargy, hunched posture, decreased respiratory rate,
with lacrimation, salivation, ataxia or abasia, loss of righting
reflex, diarrhoea, diuresis, and red/brown staining around the eyes,
snout or mouth. The lungs and gastrointestinal tract showed
haemorrhages and irritation. Signs of skin irritation manifested by
oedema, eschar formation, or subcutaneous haemorrhage were apparent
(Jones & Collier, 1986b).
One 24-h application of undiluted 1,3-dichloropropene to
intact, occluded New Zealand white rabbit skin caused extremely
severe eschar, resulting in black necrotic tissue. The skin had
hardened and was cracking after 7 days. The animals used for dermal
LD50 estimation with 1,3-dichloropropene showed lethargy and
hypothermia at dose levels above 300 mg/kg body weight (Coombs &
Carter, 1976b).
8.2 Short-term exposures
8.2.1 Oral
Albino rats derived from the Wistar strain, were used in a 90-
day test. Groups of 10 male and 10 female rats received doses (by
gavage) of 0, 1, 3, 10, or 30 mg 1,3-dichloropropene (40% cis- and
28% trans-isomer)/kg body weight on 6 days/week. General
Table 12. Acute dermal and subcutaneous LD50s for 1,3-dichloropropene
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Mouse dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(JCL:ICR) (1978a)
Rat dermal 92% (in corn oil) > 1.211 Toyoshima et al.
(Wistar) (1978b)
Rat (CD) dermal undiluted 0.423 Coombs & Carter
(0.336-0.555) (1976b)
Rat (Sprague- dermal 97.2% 1.0 (0.8-1.3)a Jones & Collier
Dawley) between 1.3 and 2.0b (1986b)
Rat dermal 96.7%, trans-isomer 1.575 Gardner (1989c)
(Fischer 344) undiluted
Rat dermal 94.5-97.5% 1.09 Gardner (1989b)
(Fischer 344) cis-, 1.5%
trans-, 0.25%
1,2-dichloropropane
undiluted
Rabbit (not dermal 92% 0.504 Torkelson & Oyen
specified) undiluted (0.22-1.15) (1977)
Mouse subcutaneous 92% (in corn oil) 0.33 (0.29-0.376)a Toyoshima et
(JCL:ICR) 0.345 (0.30-0.40)b al. (1978a)
Table 12. (contd)
Species Route Concentration LD50 (g/kg body Reference
of substance weight, with 95%
confidence limits)
Rat subcutaneous 92% (in corn oil) 0.40 (0.345-0.464)a Toyoshima et
(Wistar) 0.366 (0.305-0.439)b al. (1978b)
a Male.
b Female.
condition, behaviour, and survival were not affected at any dose
level. No distinct differences in haematological indices, serum
enzyme activities, or urinalysis were observed. The relative kidney
weights were significantly increased in the 30-mg group in both
sexes and also in the males receiving 10 mg/kg. The relative liver
weights of females at 30 mg/kg were significantly increased. Gross
and microscopic examination did not reveal any abnormalities in the
main organs. The 3 mg/kg body weight dose was without effect (Til et
al., 1973).
8.2.2 Inhalation
8.2.2.1 Mouse
CD-1 Albino mice, 10/sex per group were exposed to production
grade Telone II (whole-body exposure) at 0, 45.4, 136.2, or 408.6
mg/m3 for 6 h/day, 5 days/week, for 90 days. No significant
differences in lesions were found between the control and treated
groups upon gross and histological examination, except that there
were compound-related effects on the nasal turbinates. These effects
were decreased height of the nasal epithelial cells resulting from a
loss of cytoplasm, and disorganization of the nuclei. Necrotic cells
were only observed among the females exposed to 408.6 mg/m3. No
alterations were found in the mice exposed to 136.2 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 10 male and 10 female B6C3F1 mice were exposed
(whole body) by inhalation to technical grade 1,3-dichloropropene
90.9% ( cis 48.6% and trans 42.3%) containing 2.4% 1,2-
dichloropropane, 5.5% mixed isomers of chlorohexane, chlorohexene,
and trichloropropene, and epichlorohydrin 1.2% (as stabilizer). The
actual exposures were to levels of 0, 45.4, 136, 409, or 681 mg/m3
for 6 h/day, 5 days/week for 13 weeks. Extensive haematological,
clinical-chemical, and urinalysis studies, and histopathological
examination of organs and tissues were performed.
A treatment-related depression in body weight was seen at doses
of 409 and 681 mg/m3. In animals exposed to 681 mg/m3, transient
brown discoloration of the fur with a strong mercaptan odour in the
coats and urine was found. The poor growth rate was reflected in a
decrease or increase in the relative weights of a number of organs,
but without histological alterations. An exposure-related decrease
in BUN levels was observed in male mice exposed to 409 and 681
mg/m3. Furthermore, alanine transaminase levels were increased in
mice exposed to 681 mg/m3. No other changes were found. The
primary target tissues of inhaled 1,3-dichloropropenes were the
nasal mucosa and urinary bladder. The changes in the nasal mucosa
including slight degeneration of the olfactory epithelium and slight
hyperplasia of respiratory epithelium in animals exposed to 409 and
681 mg/m3 were dose-related. The animals also had small focal
areas of nasal metaplasia, a condition in which the damaged sensory
olfactory epithelium is replaced by ciliated respiratory epithelium
(only in animals exposed to 681 mg/m3).
The urinary bladders of female mice exposed to 409 and 681
mg/m3 (7/10 and 6/10, respectively) had areas of moderate
hyperplasia of the transitional epithelium (7-10 layers thick in
contrast with 2-3 layers in control mice). Submucosal aggregates of
lymphoid cells in the bladder, not associated with hyperplasia and
not treatment-related, were found in female mice exposed to
concentrations of 136 mg/m3 or more. No treatment-related effects
were found in mice exposed to 45.4 mg/m3 (Stott et al., 1984,
1988).
8.2.2.2 Rat
Fischer 344 rats, 10/sex per group, were exposed to 0, 45.4,
136, or 409 mg/m3 of production grade Telone II, for 6 h/day, 5
days/week for 90 days. No significant differences in lesions were
found between the control and treated groups on gross or
histological examination, except that compound-related effects on
the nasal turbinates were found. These effects included decreased
height of the nasal epithelial cells resulting from a loss of
cytoplasm, and a disorganization of the nuclei. Necrotic cells were
observed in all of the male and female rats exposed to 409 mg/m3
and in 6/10 of the female rats exposed to 136 mg/m3. No
alterations were found in the animals with 45.4 mg/m3 (Coate &
Voelker, 1979a,b).
Groups of 5 male rats were exposed to atmospheres containing
concentrations of 1,3 cis-/trans-dichloropropene (92%) of 0 or
13.6 mg/m3 for 0.5, 1, 2, or 4 h/day, 5 days/week for 6 months.
The only effect in all the exposed groups was a slight, apparently
reversible change, seen microscopically in the kidneys of male rats
(Torkelson & Oyen, 1977).
Groups of 24 male and 24 female rats were exposed repeatedly to
air containing 0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7
h/day, 5 days/week for 6 months. Changes attributable to 1,3-
dichloropropene were limited to cloudy swellings in the renal
tubular epithelium of male rats in the 13.6 mg/m3 group. Female
rats in this group showed an increased liver to body weight ratio,
though no histopathological changes were observed. A recovery group
of male and female rats was maintained for 3 months following the 6-
month exposure to 4.54 or 13.6 mg/m3. No changes were observed in
the renal epithelium following this recovery period (Torkelson &
Oyen, 1977).
In a well-designed study (Stott et al., 1984, 1988), groups of
10 male and 10 female Fischer 344 rats were exposed through
inhalation to technical grade 1,3-dichloropropene 90.9% (for
composition see section 8.2.2.1) in actual concentrations of 0,
45.4, 136, 409, or 681 mg/m3, for 6 h/day, 5 days/week for 13
weeks. Haematological, clinical-chemical studies, urinalysis, and
histopathological studies of organs and tissues were carried out.
In the rats exposed to 681 mg/m3, transient brown
discoloration of the fur with a strong mercaptan odour in the coats
and urine was found. The body weights of rats exposed to 409 or 681
mg/m3 were decreased in an exposure-related manner. The relative
weights of the testes were increased and thymus weights decreased.
Rats exposed to 409 and 681 mg/m3 had slightly lower levels of
serum proteins. No other treatment-related changes in clinical-
chemical or haematological parameters were found.
The primary target tissues of inhaled 1,3-dichloropropenes were
the nasal mucosa in male and female rats and the uteri in females.
The effects consisted of dose-related degenerative effects on the
nasal olfactory epithelium or mild hyperplasia of the respiratory
epithelium or both, in all animals exposed to 409 and 681 mg/m3,
and 2 of the 10 male animals in the 136 mg/m3 group.
The uteri of 7 out of 10 female rats exposed to 681 mg/m3
were not developed as completely as those of control animals,
suggesting hypoplasia of the uterine tissues. The only other change
noted histologically was the atrophic appearance of the mesenteric
adipose tissue in the rats exposed to 681 mg/m3. No treatment-
related effects were observed in rats of either sex exposed to 45.4
mg 1,3-dichloropropenes/m3 (Stott et al., 1984, 1988).
In a study to investigate the effects of 1,3-dichloropropene on
tissue sulfhydryl levels, groups of 15 male and 15 female Fischer
344 rats were exposed to vapours of cis-1,3-dichloropropene (94.3-
95.6% cis-, 1.5% trans-1,3-dichloropropene and 0.2% 1,2-
dichloropropane) at 0, 45.4, 272.4, or 681.0 mg/m3 for 6 h/day, 5
days/week for 9 exposures. Whole-body exposures occurred under
dynamic air-flow conditions. On the day after the last exposure, 5
males and 5 females/dose level were necropsied. Major organs were
weighed and selected tissues were evaluated histopathologically.
Groups of 5 animals/sex per dose level were used to determine the
non-protein sulfhydryl (NPS) contents of the liver, kidneys, and
lung, 1 and 18 h following the last exposure.
Rats exposed to 681 mg/m3 lost weight, but not those exposed
to 272.4 mg/m3. There were concentration-related decreases in
liver, kidney, and lung NPS levels in male rats, when measured after
1 h. After 18 h, the NPS levels were higher than those of the
controls and there were no associated gross or histopathological
changes in these organs. The NPS measurements for females were
unremarkable. Histopathological examination revealed changes of
moderate severity in the respiratory and olfactory mucosa in the
nasal cavity of males and females exposed to 681 mg/m3. There were
no changes in the animals exposed to 272.4 mg/m3 (Nitschke &
Lomax, 1990).
Groups of 10 male and 10 female Fischer 344 rats (6 weeks old)
were exposed to cis-1,3-dichloropropene at 0, 45.4, 136, or 409
mg/m3 for 6 h/day, 5 days/week for 13 weeks. The test material was
reported to consist of 94.3% (95.6%) cis-1,3-dichloropropene, 1.5%
trans-1,3-dichloropropene and 0.2% 1,2- dichloropropane. Whole-
body exposures occurred under dynamic conditions. No exposure-
related effects were noted in haematology or clinical chemistry. At
409 mg/m3, body weights of male rats were significantly decreased
compared with controls throughout the 13-week exposure period; body
weights of female rats were significantly decreased during the first
6 weeks, but were comparable with those of the controls at the end
of the study. As a result of the decreased body weight in male rats,
the relative liver, kidney, lung, and testes weights were
significantly elevated in comparison with control values. The
relative liver weight in females was also increased. These organ
weight changes were not accompanied by gross or histopathological
changes. Exposure-related changes only occurred in the nasal
cavities of the rats of both sexes exposed to 409 mg/m3 and
consisted of multifocal bilateral degeneration of olfactory
epithelium and slight bilateral multifocal hyperplasia of
respiratory epithelium. No effects were noted in rats exposed to
45.4 or 136 mg/m3. The NOEL was considered to be 136 mg cis-1,3-
dichloropropene/m3 (Nitschke et al., 1991).
8.2.2.3 Other animal species
Groups of 12 male and 12 female guinea-pigs, 3 male and 3
female rabbits and 2 dogs were exposed repeatedly to air containing
0, 4.54, or 13.6 mg 1,3-dichloropropene/m3 for 7 h/day, 5
days/week for 6 months. No changes resulting from 1,3-
dichloropropene exposure were observed in guinea-pigs, rabbits, or
dogs (Torkelson & Oyen, 1977).
8.3 Skin and eye irritation, sensitization
8.3.1 Skin irritation
1,3-Dichloropropene is extremely irritating to the skin
(Worthing & Hance, 1991).
Telone II (52.63% cis- and 44.91% trans-1,3,-
dichloropropene), 0.5 ml, was applied for 4 h to the back (clipped
free of fur) of 2 male and 4 female New Zealand White rabbits. After
4 h, the wrapping and gauze patch and any residual test substance
were removed. Dermal irritation characterized as slight to moderate
erythema and moderate to severe oedema was observed at the site of
application immediately following the 4 h exposure period.
Subsequent observations revealed slight to moderate erythema,
oedema, and exfoliation. These changes were still present in some of
the animals after 14 days (Jeffrey, 1987a).
In a 4-h rabbit skin irritancy test, undiluted cis-1,3-
dichloropropene (94.5-97.5% cis-, 1.5% trans-1,3-dichloropropene
and 0.25% 1,2-dichloropropane) caused well defined erythema and
moderate oedema shortly after removal of the semi-occlusive
dressings in New Zealand White rabbits (3-5 months of age). After
removal of the dressings, the skin was washed after 4 h. Resolution
of the irritancy reaction was first apparent on the day after
treatment and was complete within 14 days (Gardner, 1989b).
In another 4-h rabbit skin irritancy test, 0.5 ml of undiluted
trans-1,3-dichloropropene (96.7%) caused irritation not exceeding
well-defined erythema and slight oedema. New Zealand White rabbits
(3-5 months of age) were used. After the 4-h exposure, the dressings
were removed and the skin washed. Resolution of the irritation was
first apparent 72 h after treatment and was complete by day 21
(Gardner, 1989c).
8.3.2 Eye irritation
1,3-Dichloropropene is a severe eye irritant (Worthing & Hance,
1991).
Aliquots of 0.1 ml Telone II (52.63% cis- and 44.91% trans-
1,3-dichloropropene) were instilled into the conjunctival sac of one
eye of 4 male and 2 female New Zealand White rabbits. The eyes of
all rabbits remained unwashed. Slight to marked redness and slight
to moderate chemosis were found after the treatment. The treated
eyes had a slight to marked amount of discharge as well as reddening
of the iris. In one animal, opacity was found which resolved as did
all the other changes within 14 days following treatment (Jeffrey,
1987b).
In the rabbit eye irritancy test, 0.1 ml trans-1,3-
dichloropropene (96.7%) caused moderate to severe conjunctival
irritation and minor irritation changes of the cornea (opacities),
chemosis, and iridial responses within 24 h following instillation
into the eye. Resolution of the irritant effects of trans-1,3-
dichloropropene was advanced 7 days after treatment and complete one
week later. There was an initial pain response (Gardner, 1989c).
Ten male and 10 female Sprague-Dawley rats (Spartan substrain)
were exposed to an aerosol (mean size 2.96 µm; 99% of particles were
6 µm or less in diameter) in a glass chamber for 1 h at a nominal
concentration of 5.2 mg/litre of air. Five male and 5 female animals
were maintained under ambient conditions as controls. Slight
transitory eye irritation was observed during the exposure (Yakel &
Kociba, 1977).
8.3.2.1 In vitro studies
The corneal thickness of isolated eye preparations subjected to
application of cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) increased
by more than 20% within 3 h. The isolated rabbit eye test described
by Price & Andrews (1985) was used. Corneal uptake of fluorescein
was demonstrated at the conclusion of the test. The results
indicated that application of cis-1,3-dichloropropene to the eye
in vivo would cause significant tissue damage (Gardner, 1989b).
8.3.3 Sensitization
1,3-Dichloropropene was applied in corn oil in a 5% v/v
concentration 3 times topically to the skin of guinea-pigs followed
by a 1% concentration as a challenge following the method of Buehler
(1965). A positive reaction was obtained in 5 out of 20 guinea-pigs
of the "P" strain. The reaction was considered to be mild to
moderate skin sensitization (Coombs & Carter 1976b).
Ten male, Hartley albion guinea-pigs received 3 dermal
applications on the back of 0.4 ml of 0.1% (v/v) Telone II (52.63%
cis- and 44.91% trans-1,3-dichloropropene) in mineral oil;
another group received only the vehicle during the induction phase
of the study. The dermal sensitization potential was tested using
the modified Buehler method. A positive control group received 2
applications of 10% epoxy resin and a third application of 5% epoxy
resin. All groups were challenged dermally 2 weeks after the last
induction application. The control group did not show any signs of
sensitization, while 5 out of the 10 animals of the positive control
group revealed slight erythema. Nine out of 10 guinea-pigs
challenged with 0.1% Telone II revealed slight to moderate erythema.
Telone II was considered a potential skin sensitizer at the
concentrations tested (Jeffrey, 1987c).
In the guinea-pig maximization test of Magnusson & Kligman, all
20 test animals showed positive responses, 24 and 48 h after removal
of the challenge patches. Guinea-pigs of the Dunkin-Hartley strain
(5-9 weeks of age) were used in this study. In a study by Gardner
(1989b), 0.1% cis-1,3-dichloropropene (94.5-97.5% cis-, 1.5%
trans-1,3-dichloropropene and 0.25% 1,2-dichloropropane) was
injected intradermally; topical induction was carried out using a 5%
solution in corn oil, and the topical challenge using 2.5% in corn
oil.
In the guinea-pig maximization test of Magnussen & Kligman, 16
out of 20 test animals showed positive responses 24 and/or 48 h
after removal of the challenge patches. Guinea-pigs of the Dunkin-
Hartley strain (age 5-9 weeks) were used. Intradermal injection,
carried out at concentrations of 0.05% trans-1,3-dichloropropene
(96.7%) or more resulted in red areas with defined edges. The
concentration selected (10%) for topical induction did give slight
irritation, but the concentration for the topical challenge of 5%
was without effect. The solvent was corn oil (Gardner, 1989c).
8.4 Long-term exposure
See section 8.7 (Carcinogenicity).
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Inhalation (rat)
Groups of 30 male and 30 female Fischer 344 rats (6 weeks of
age) were exposed to Telone II (1,3-dichloropropene containing 2%
epoxidized soybean oil) at 0, 45.4, 136, or 409 mg/m3 (first 7
days of study: 0, 22.7, 90.8, or 272.4 mg/m3) for 6 h/day, 5
days/week during the premating period and for 7 days/week during
breeding, gestation, and lactation, for 2 generations. The Telone II
used had a purity of 92% and the remainder of the test material
comprised chlorinated and unchlorinated alkanes and alkenes as well
as approximately 2.0% epoxidized soybean oil. Following 10 weeks of
exposure, the adult rats (F0) were mated twice to produce the
F1a and F1b litters. After weaning, 30 pups/sex per exposure
from the F1b litters were selected and, after 12 weeks, used to
produce the F2a and F2b litters.
All litters were examined on the day of parturition, the
following indices of fertility being recorded: gestation length,
litter size, pup survival indices, number of live pups on days 1 up
to 28 postpartum, sex and weight of litters, lactation and
individual body weights, and any visible physical abnormalities.
Gross necropsy was carried out on adult rats F0 and F1 and
weanling F1b and F2b rats and histopathological studies on adult
rats. Inhalation of up to 409 mg/m3 for 2 generations did not
adversely affect reproduction or neonatal growth or survival.
Exposure to 409 mg/m3 resulted, however, in parental toxicity
(F0, F1), as indicated by decreases in body weight and
histopathological effects in the nasal mucosa (slight, focal
hyperplasia of the respiratory mucosal epithelium and/or focal
degenerative changes in the olfactory epithelium). No adverse
effects were observed in the parents in the 136 mg/m3 group. The
reproductive no-effect level was 409 mg/m3 (Breslin et al., 1987,
1989).
8.5.1.2 Intraperitoneal (mouse)
1,3-Dichloropropene (Telone II) in corn oil was injected
intraperitoneally in B6C3F1 male mice (4 per group) at dose
levels ranging from 10 mg up to 600 mg/kg body weight, daily for 5
days, to study sperm morphology, epididymal sperm counts, and testes
weights. Testicular toxicity was assessed at day 35. No animals
survived at dose levels of 150 mg/kg body weight or more. No effects
on the testes were found at dose levels up to 75 mg/kg body weight
(Osterloh et al., 1983).
8.5.2 Teratogenicity
8.5.2.1 Inhalation (rat)
In a range-finding study, groups of 7 or 8 female Fischer 344
rats were exposed to Telone II (92.1%; 47.7% cis- and 42.4%
trans-1,3-dichloropropene, impurities, and 1.8% epichlorohydrin)
at 0, 230, 680, or 1360 mg/m3 for 6 h/day on days 6-15 of
gestation.
Consumption of food and drinking-water was decreased during the
period of exposure in rats exposed to 680 or 1360 mg/m3 and the
rats showed a significant decrease in body weight gain. Rats were
sacrificed on day 16 and examined. During exposure, nasal exudate
and red crusty material around the eyes were observed. In the group
exposed to 1360 mg/m3, a significant decrease in litter size and
significant increase in resorption were seen, while in the groups
exposed to 230 and 680 mg/m3, the same changes were observed, but
were not statistically significant (Kloes et al., 1983).
Groups of 30 Fischer 344 rats were exposed, via inhalation, to
1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-isomer) at
0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-15 of
gestation. Food consumption and maternal body weight gain were
depressed in all treated groups in a dose-related manner. A decrease
in water consumption was found in rats at the highest dose-level. No
consistent or dose-related effects on reproductive performance were
found. The number of implantations, resorptions, litter size, fetal
weights, and fetal lengths were comparable with the controls. A
slight, but statistically significant, dose-related increase in the
incidence of delayed ossification of the vertebral centra was found
in rats, but considered of little toxicological significance in the
light of the maternal toxicity observed. The authors stated that
there was no evidence of a teratogenic or embryotoxic response at
exposure levels up to and including 545 mg/m3 (John et al., 1983;
Hanley et al., 1987).
8.5.2.2 Inhalation (rabbit)
In a range-finding study, groups of 7 New Zealand White rabbits
were exposed to Telone II (92.1%, 47.7% cis- and 42.4% trans-
1,3-dichloropropene, impurities and 1.8% epichlorohydrin) at 0, 230,
680 or 1360 mg/m3 for 6 h/day on days 6-18 of gestation. The
rabbits were sacrificed on day 19 and examined. Six out of 7 rabbits
exposed to 1360 mg/m3 showed signs of toxicity, such as rear limb
ataxia, decreased or absence of righting reflex, and flaccid hind
limb muscles; these animals died or were sacrificed. Histologically,
no effects were detected in the brains of these animals. Maternal
body weight was statistically significantly decreased during
exposure in the 680 mg/m3 group, but weights in the 230 mg/m3
group were comparable with those of the controls. There were no
differences in the reproductive parameters between the groups
exposed to 230 and 680 mg/m3 and the controls. No teratological
effects were found (Kloes et al., 1983).
Groups of 25-31 inseminated New Zealand white rabbits were
exposed to 1,3-dichloropropene 90.1% (47.7% cis- and 42.4% trans-
isomer) at 0, 91, 272, or 545 mg/m3 air for 6 h/day on days 6-18
of gestation. Decreased weight gain was observed among rabbits at
272 and 545 mg/m3, but no pronounced maternal toxicity was
observed. No evidence of teratogenic or embryotoxic responses was
observed (John et al., 1983; Hanley et al., 1987).
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies
8.6.1.1 Microorganisms
Cis- and trans-1,3-dichloropropene or a mixture were tested
for their mutagenic activity in Salmonella typhimurium and in
Saccharomyces cerevisiae, with and without metabolic activation.
Most of the studies showed a positive effect, especially with
Salmonella typhimurium TA100 and TA1535, with, and without,
metabolic activation. With TA98 and TA1537, positive and negative
effects were found. Saccharomyces cerevisiae was positive. The
results are summarized in Table 13. Samples of highly purified 1,3-
dichloropropene or cis-1,3-dichloropropene, however, were not
mutagenic to Salmonella typhimurium TA100, indicating that trace
impurities, such as 1,3-dichloropropene oxide, were the cause of the
activity (Talcott & King, 1984; Watson et al., 1987, see section
8.8.2).
There was a difference in mutagenicity (expressed as rev/µmol)
between the cis and trans isomers of 1,3-dichloropropene in
Salmonella typhimurium TA100, with, and without, metabolic
activation. The cis-isomer induced a greater number of revertants,
with, and without, S9 mix, than the trans-isomer and also showed
stronger alkylating properties in the NBP-test. Furthermore, a
longer preincubation time invariably led to higher mutagenic
activity, and an increase in protein added to the activating system
increased the efficiency of the metabolic activation (Neudecker et
al., 1980, Neudecker & Henschler, 1986).
Salmonella typhimurium TA100 was used to test for the
mutagenicity of cis- and trans-3-chloroallyl alcohol (99%),
with, and without, metabolic activation. No mutagenicity was
observed without S9 mix, over a range of 0.01-1000 µg/plate.
However, a positive effect was obtained with S9 mix and cis-3-
chloroallyl alcohol (in high concentration) (Connors et al., 1990).
Von der Hude et al. (1988) used the SOS chromotest with
Escherichia coli PQ 37, to examine 1,3-dichloropropene (in DMSO)
at concentrations of 0, 1.0, 3.3 or 10.0 mmol/litre, without S9. In
this strain, the structural gene for beta-galactosidase lacZ is
placed under the control of the SOS-gene sfiA. The expression of
this gene, induced by DNA-damage, is measured indirectly by
determination of the beta-galactosidase activity. The test was
positive with concentrations of 1.0 mmol/litre or more.
8.6.1.2 Effects of glutathione on bacterial mutagenesis
The addition of glutathione (GSH), at physiological
concentrations, to in vitro bacterial test systems of Salmonella
typhimurium TA100 has shown a clear protective effect, i.e., a
virtual elimination of the mutagenic response of 1,3-dichloropropene
(Brooks et al., 1978; Climie et al., 1979; Creedy & Hutson, 1982;
Wright & Creedy, 1982; Creedy, 1983; Creedy et al., 1984; Brooks &
Wiggins, 1990). This protective effect occurs in either the presence
or the absence of S9 for both the (cis)- and (trans)-isomers.
Protection, in the presence of S9, is consistent with the operation
of glutathione transferase enzyme occurring in the added S9
fraction. This enzyme is also present in mammalian cells, and as the
metabolic studies have shown, it plays a key role in the rapid
detoxification of cis-1,3-dichloropropene in mammalian tissue
(Climie et al., 1979; Brooks & Wiggins, 1991).
Even in the absence of S9, this protection by GSH still exists
against the mutagenic action of 1,3-dichloropropene. Thus, it is
likely that an additional mechanism for protection exists that
probably reflects a reaction between the mutagenic component(s) and
GSH. This additional mechanism may also play an important role in
the detoxification of 1,3-dichloropropene via a spontaneous
nucleophilic substitution reaction between the chloromethyl carbon
of 1,3-dichloropropene and the sulfur atom of glutathione.
Table 13. Mutagenicity tests with 1,3-dichloropropenes (1,3-DCP) on microorganisms
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
Salmonella typhimurium
Cis-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 top agar S9 mix/none + Neudecker et al. (1977)
(99.9%) µg/ml Kier et al. (1986)
Cis-1,3-DCP TA98, TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
(> 99%)
TA1538 20-2000 µg plate S9 mix/none - Brooks et al. (1978)
Cis-1,3-DCP TA100, TA1535 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5%, + TA98, TA1537, TA1538 78-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1990)
1,2-DCP 0.25%)
Cis-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA1535, TA1537, TA1538 0.1-1.0 µg/ml top agar S9 mix/none + Neudecker et al. (1977)
(97.5%) Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535, TA1978 20-100 µg plate S9 mix/none + DeLorenzo et al. (1977)
Kier et al. (1986)
Trans-1,3-DCP TA100, TA1535 39-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1989a)
(98% + cis-
1,3-DCP 0.3%) TA98, TA1537, TA1538 39-1250 µg/ml * S9 mix/none - Brooks & Wiggins (1989a)
1,3-DCP (51.3% TA100, TA1535 20-2000 µg plate S9 mix/none + Brooks et al. (1978)
cis, 43.7%
trans, 0.6% TA98, TA1538 20-2000 µg plate S9 mix/none ± Brooks et al. (1978)
epichlorohydrin)
Table 13 (contd)
Substance Organism/strain Dose Type of Metabolic Result Reference
test activation
1,3-DCP *** TA100, TA1535 3-333 µg/ml plate S9 mix/none + Haworth et al. (1983)
TA98, TA1537 3-333 µg/ml plate S9 mix/none - Haworth et al. (1983)
1,3-DCP *** TA100, TA1535 1000 µg/ml * S9 mix/none + Priston et al. (1983)
TA98, TA1537 1000 µg/ml * S9 mix/none - Priston et al. (1983)
1,3-DCP *** TA100 0.1-10 µmol plate S9 mix/none + Stolzenberg & Hine (1980)
1,3-DCP *** TA98 100 µg ** none + Vithayathil et al. (1983)
Saccharomyces cerevisiae
1,3-DCP *** JD1 1000 or 5000 liquid S9 mix/none + Priston et al. (1983)
µg/ml
culture
Escherichia coli
Cis-1,3-DCP WP2 UVRA pKM 101 78-1250 µg/ml * S9 mix/none + Brooks & Wiggins (1990)
(96.3% + trans-
1,3-DCP 1.5% +
1,2-DCP 0.25%)
Trans-1,3-DCP WP2 UVRA pKM 101 39-1250 µg/ml * none - Brooks & Wiggins (1989a)
(98% + cis-1,3- S9 mix +
DCP 0.3%)
* Assays were performed by the pre-incubation method in sealed containers.
** Salmonella/microsome multiple indicator test.
*** Details of composition not given.
These 2 mechanisms with GSH afford complete protection against
the mutagenic activity of the trans-isomer in bacterial test
systems, in the presence and absence of S9 (Hutson & Stoydin, 1977;
Creedy & Hutson, 1982; Wright & Creedy, 1982).
In the presence of Aroclor-induced rat liver S-9 fraction and a
rat liver microsomal mono-oxygenase system, cis-1,3-
dichloropropene (I) is apparently metabolized to a mutagenic
metabolite, cis-1-chloro-3-chloromethyloxirane (II), which is not
as effectively deactivated by glutathione as the parent compound.
Cis-dichloropropeneoxide was shown not to be stable in aqueous
medium, 2-chloroacroleine (III) was the product of hydrolysis,
another direct-acting mutagen for Salmonella typhimurium TA100
(Hutson, 1984) (see Fig. 5). Glutathione transferase systems afford
efficient protection against this bioactivated product of 1,3-
dichloropropene. The protective action of glutathione-linked systems
against the mutagenicity observed in S. typhimurium has also been
shown to occur in vivo.
8.6.1.3 Mammalian cells
In a gene mutation assay, V79 Chinese hamster cells were
exposed to cis-1,3-dichloropropene (99.9%) in DMSO at dose levels
of 2.5 to 20 µg/ml. No indication for an increased mutation
frequency at the HGPRT locus was found in these cells (Meyer, 1980).
Telone II (48.9% cis- and 43.2% trans-1,3-dichloropropene)
was tested in the Chinese hamster ovary cell/HGPRT mutagenicity
assay, with the cell line designated as CHO-K1-BH4, with, and
without, metabolic activation. Three tests were carried out without
activation. The first test with 50, 100, 150, 200, and 250
mmol/litre, showed an increase in mutation frequency at the 200 and
250 mmol/litre dose levels. However, the biological significance is
doubtful because of the extreme toxicity at these high dose levels.
In a repeat of this study using the same concentrations, no increase
in mutation frequency was found. The results of a third study using
concentrations of 50, 100, 125, 150, and 200 mmol/litre were also
negative. The fourth test was with activation, with dose levels of
50, 100, 125, 150, and 200 mmol/litre. The relative survival ranged
from 98% at 50 mmol/litre to 14% at 200 mmol/litre. No increase in
mutation frequency was observed. The results indicated that Telone
II was not mutagenic in the CHO/HGPRT assay, with, or without,
metabolic activation (Mendrala, 1986).
8.6.1.4 DNA damage
Cis- and trans-1,3-dichloropropene were tested for their
ability to induce unscheduled DNA synthesis (UDS) in Hela S3
cells. The lowest dose, 10-4 mol/litre was the dose at which UDS
occurred, the dose-response curves being rather shallow (Schiffmann
et al., 1983).
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in the rat hepatocyte unscheduled DNA synthesis assay
at concentrations from 1 x 10-6 up to 3 x 10-3 mol/litre. In an
initial and a repeat assay, toxic effects on the hepatocyte cultures
(indicated as detachment of the cells and/or a granular appearance)
occurred at 1 x 10-5 mol/litre and at 1 x 10-4 mol/litre or
more, respectively. In both tests, Telone II failed to elicit
significant DNA repair in the primary cultures of rat hepatocytes,
over the wide range of concentrations tested, suggesting an apparent
lack of genetic activity (Mendrala, 1985).
The liquid Bacillus subtilis strain H17 (arg-, trp-, recE+)
microsome rec-assay was used to evaluate the DNA-damaging effect of
1,3-dichloropropene with, and without, S9 mix. The concentrations of
1,3-dichloropropene used with, and without, S9 mix were for
CR50Rec+/CR50Rec-, 7.62 x 10 / 2.49 x 10 and 4.79 x 102/1.01 x
103 mg/litre. With S9 mix, there was a strong DNA-damaging effect,
but, without S9 mix, a reverse effect was observed (Matsui et al.,
1989).
8.6.1.5 Chromosomal effects
Rat liver (RL1) cells were exposed to culture medium
containing cis-1,3-dichloropropene (99.9%) in DMSO, at
concentrations of 2.5, 5.0, or 10 µg/ml. No indication was found
that the compound induced chromosome damage in the liver cells
(Meyer, 1980).
The clastogenic potential of trans-1,3-dichloropropene (95.4%
trans-isomer and 0.3% cis-isomer) was assessed from assays
designed to monitor chromosome damage in CHO cells. Cultures were
grown in incubated medium containing the test compound, for either 3
h in the presence of S9-mix (concentration 0, 0.5, 2.5 or 5.0 µg/ml)
or 24 h in the absence of S9 mix (concentration 0, 3, 15 or 30
µg/ml). Methyl methane sulfonate and cyclophosphamide were used as
positive controls. Metaphase cells were used for the analysis of
chromosome aberrations after 8, 12, and 24 h, in the case of
cultures with S9 mix, and, after 24 h, in the case of the cultures
without S9 mix. At the 24-h sample time, it was concluded that
trans-1,3-dichloropropene induced chromosome damage (gaps, breaks,
and exchange figures) in the presence of S9 mix, but no effect was
seen in its absence (Brooks & Wiggins, 1989b).
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce chromosomal aberrations in cultured CHO cells.
In the first study, without S9, 49.1 µg/ml produced a strong
positive response, i.e., 16% of cells with aberrations (large number
of gaps). No metaphase cells were seen at the 98.0 µg/ml dose level.
In a repeat study, these results could not be confirmed with 50, 75,
and 100 µg/ml. All 3 doses produced 50% decreases in cell
confluency. When the compound was tested with S9 mix, no chromosomal
aberrations were found at 50 µg/ml, the highest dose at which
metaphase cells could be found.
Brooks & Wiggins (1991) assessed the mutagenic activity of
cis-1,3-dichloropropene (94.5-97.5% cis-isomer, 1.5% trans-
isomer, 0.25% 1,2-dichloropropane), in Chinese hamster ovary cells.
The cultures were grown in medium containing the test substance for
either 3 h in the presence of S9 mix or 24 h in the absence of S9
mix. Metaphase cells were prepared for analysis for chromosome
aberrations, 24 h following initiation of exposure with, and
without, S9 mix. From the data obtained at the 24-h sample time, it
was concluded that cis-1,3-dichloropropene at 1, 5, or 10 µg/ml
induced chromosome damage (gaps, breaks, and exchange figures) in
the presence of S9 mix. No increase in metaphase chromosome damage
was found with doses of up to 10 µg/ml in the absence of S9 mix.
Loveday et al. (1989) tested 1,3-dichloropropene (97.1%) for
its ability to induce Sister Chromatid Exchanges (SCEs), in cultured
Chinese hamster ovary cells. The test was carried out with, and
without, rat liver S9 fraction. DMSO was used as the solvent. 1,3-
Dichloropropene induced SCEs without S9 at a dose level of 29.9
µg/ml. A positive response was also found with S9.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
(SCE) test in vitro in Chinese hamster V79 cells without S9 mix,
to evaluate the effect of 1,3-dichloropropene (80%). The
concentrations tested were 0 (DMSO), and 0.1 up to 0.8 mmol/litre.
The 0.8 mmol/litre dose was toxic to the cells. A dose-related
increase in the number of SCEs was found. Negative results were
obtained with S9, at dose levels of 0.1-3.3 mmol/litre.
8.6.2 In vivo studies
Telone II (49.5% cis- and 42.6% trans-1,3-dichloropropene)
was evaluated in a mouse bone marrow micronucleus test to detect
chromosomal aberrations and spindle malfunction. The substance
(dissolved in corn oil) was administered to CD-1 (ICR) BR mice by
single oral gavage at dose levels of 0 (corn oil), 38, 115, or 380
mg/kg body weight. Groups of animals were sacrificed at intervals of
24 and 48 h. A positive control group received cyclophosphamide at a
dose of 120 mg/kg. There were no significant increases in the
frequencies of micronucleated polychromatic erythrocytes in the
Telone II groups compared with the controls. Positive results were
obtained with the positive control group. Telone II was considered
negative in the mouse bone marrow micronucleus test (Gollapudi et
al., 1985).
Valencia et al. (1985) tested 1,3-dichloropropene (95.5%) for
mutagenicity in Drosophila melanogaster. The compound was tested
for the induction of sex-linked recessive lethals (SLRLs) by feeding
the substance in a 5% aqueous sucrose solution. The dose level fed
was 0 or 5750 mg/litre. The mortality rate was 33% and sterility
10%. 1,3-Dichloropropene induced an increased number of SLRLs, but
no reciprocal translocations were induced.
The results of both dominant lethal and host-mediated assays
carried out with "MIX D/D" were negative (see "Mixtures of
dichloropropenes and dichloropropane", section 8.6.2).
8.6.3 Appraisal
1,3-Dichloropropene ( cis- and trans-isomer) showed
mutagenic activity in Salmonella typhimurium, especially in
strains TA100 and TA1535 with, and without, metabolic activation.
There is a difference in mutagenic potential between the cis- and
trans-isomers in TA100 with, and without, activation. The cis-
isomer induces a greater number of revertants than the trans-
isomer.
1,3-Dichloropropene does not possess a genotoxic potential in a
variety of non-bacterial studies. Gene mutation has not been
detected in in vitro assays using the eukaryotic cell lines of
either V79 or CHO (HGRPT locus) (Meyer, 1980; Mendrala, 1986).
Chromosomal damage has not been observed in vitro with rat liver
cell cultures or in an in vivo mouse micronucleus study using
single oral doses of up to 380 mg/kg (Gollapudi et al., 1985).
Interaction with DNA was not observed, even at cytotoxic doses, in
an in vitro rat liver unscheduled DNA synthesis study (Mendrala,
1985). However, Schiffmann et al. (1983) found liver unscheduled DNA
synthesis in Hela S3 cells.
8.7 Carcinogenicity
8.7.1 Oral
8.7.1.1 Mouse
A carcinogenicity study was carried out on groups of 50 male
and 50 female (4-6 weeks old) B6C3F1 mice at dose levels of 0,
50, or 100 mg (stabilized technical grade 1,3-dichloropropene)/kg
body weight, administered in corn oil (5 ml/kg body weight), by
gavage, 3 times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, nine other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). The initial mean body weights of
the treated mice were lower (6-22%) than those of the controls.
These differences were caused by failure to fully randomize the
distribution of the animals. No clinical signs were observed.
However, the survival of vehicle control male mice was significantly
lower than that in either dose group. Thirty-nine control male mice
died with myocarditis, 25, between weeks 48 and 51. A slight
increase in mortality was found in the female mice at a dose level
of 100 mg/kg body weight.
The reduced survival of the control male mice did not allow for
an adequate evaluation of carcinogenicity in males in this study.
But, in both sexes, there was evidence for a 1,3-dichloropropene-
related increase in transitional cell carcinomas of the urinary
bladder (without the presence of calculi), liver tumours, squamous
cell papillomas and/or carcinomas of the forestomach, and of
alveolar/bronchiolar adenomas and carcinomas of the lung, at 50 or
100 mg/kg body weight. In the female mice, an increase in basal cell
or epithelial cell hyperplasia in the forestomach was also found
(Table 14). It was stated that the presence of 1% epichlorohydrin, a
direct acting mutagen and carcinogen (especially for the
forestomach), may have influenced the development of forestomach
lesions (Haseman et al., 1984; NTP, 1985; Yang, 1986; Yang et al.,
1986).
8.7.1.2 Rat
A long-term carcinogenicity study was carried out on groups of
52 F344/N rats of each sex (aged 6 weeks), at dose levels of 0, 25,
or 50 mg stabilized technical grade 1,3-dichloropropene/kg body
weight, administered in corn oil (5 ml/kg body weight), by gavage, 3
times per week for 104 weeks.
The technical product contained 41.6% cis- and 45.9% trans-
isomer of 1,3-dichloropropene, 2.5% 1,2-dichloropropane, 1.5%
trichloropropene isomer, 9 other impurities, (7.5%) and
epichlorohydrin 1% (as stabilizer). Additional groups of 25 rats of
each sex were assigned to each dose group. At 9, 16, 21, 24, and 27
months of dosing, 5 rats/sex per group were killed and organs and
tissues studied microscopically. Haematological and clinical-
chemical studies were carried out on groups of 20 rats of each sex,
11-13 times in the first 70 weeks of the study. The mean body weight
of high-dose male rats was about 5% lower than those of the control
and low-dose rats. No differences in body weight were observed in
female animals. Mortality was comparable with the controls. The
primary organs affected were the forestomach and liver. The
incidences of non-neoplastic and neoplastic lesions are summarized
in Table 15.
Table 14. Occurrence of microscopic lesions in mice in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Urinary bladder
Epithelial hyperplasia b 2/50 (4%) 9/50 (18%) 15/50 (30%) 18/50 (36%) 19/48 (40%)
Transitional cell carcinoma b 0/50 (0%) 0/50 (0%) 8/50 (16%) 2/50 (4%) 21/48 (44%)
Lung
Alveolar/bronchiolar
adenoma or carcinoma 1/50c 2/50 (4%) 13/50 (26%) 4/50 (8%) 12/50 (24%) 8/50 (16%)*
Forestomach
Epithelial hyperplasia b 1/50 (2%) 0/50 (0%) 1/50 (2%) 4/50 (8%) 21/50 (42%)*
Squamous cell papilloma b 0/50 (0%) 2/50 (4%) 1/50 (2%) 3/50 (6%)d 4/50 (8%)*
or carcinoma
Liver
Hepatocellular adenoma 5/50b 1/50 (2%) 7/50 (14%) 8/50 (16%)* 13/50 (26%) 3/50 (6%)
or carcinoma
Kidneys
Hydronephrosis b 0/50 (0%) 0/50 (0%) 2/50 (4%) 0/50 (0%) 14/50 (28%)
a From: NTP (1985).
b Too many animals died during the study, but no epithelial hyperplasia or neoplasia were observed in these animals.
c As b but, a lung adenoma was found in one animal.
d Only squamous cell papilloma.
* P = < 0.05.
Table 15. Occurrence of microscopic lesions in rats in a 2-year gavage study of 1,3-dichloropropenea
Lesions Vehicle 50 mg/kg 100 mg/kg
control body weight body weight
male female male female male female
Forestomach
Epithelial hyperplasia 2/52 (4%) 1/52 (2%) 5/52 (10%) 0/52 (0%) 13/52 (25%)c 16/52 (31%)c
Squamous cell papilloma 1/52 (2%) 0/52 (0%) 1/52 (2%) 2/52 (4%)b 13/52 (25%)c 3/52 (6%)b
or carcinoma
Liver
Neoplastic nodule 1/52 (2%) 6/52 (12%) 6/52 (12%)c 6/52 (12%)c 7/52 (13%)c 10/52 (19%)
or carcinoma
a From: NTP (1985).
b Only squamous cell papilloma.
c P = ¾ 0.05
Under the conditions of the study, 1,3-dichloropropene induced
an increased incidence of squamous cell papillomas and carcinomas of
the forestomach. In addition, a dose-related trend was observed in
the incidence of neoplastic nodules in the livers of male rats. It
was stated that the presence of 1% epichlorohydrin, a direct acting
mutagen and carcinogen (especially for the forestomach), may have
influenced the development of forestomach lesions (Haseman et al.,
1984; NTP, 1985; Yang, 1986; Yang et al., 1986).
In the ancillary study, dose-related lesions were observed in
the forestomach and liver. The development of the forestomach basal-
cell hyperplasia and squamous-cell papilloma followed a time-
dependent trend in high-dose males and females. Basal-cell
hyperplasia was seen 9-16 months after dosing started. The neoplasms
of the forestomach and liver were not seen until 24 months after
dosing began.
8.7.2 Inhalation
8.7.2.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (6-7 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days per
week for up to 24 months. In addition, 2 ancillary groups, each with
10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The mice were exposed to 1,3-
dichloropropane vapour at 0, 22.7, 90.8, or 272 mg/m3. The
composition of the technical-grade 1,3-dichloropropene was 1,3-
dichloropropene 92.1% ( cis-49.5% and trans 42.6%); 1,2-
dichloropropane 0.7%; and 5.2% mixtures of hexanes and hexadienes.
Epoxidized soybean oil (approximately 2%) was added as stabilizing
agent. Besides body weights, clinical-chemical parameters in the
blood and urine and haematological parameters were determined for
all animals terminated at 6 and 12 months and for 20 animals/sex per
group at 24 months. At 6, 12, and 24 months, animals were sacrificed
and the weight of 5 organs determined; a large number of organs and
tissues were examined histopathologically.
No significant differences in survival rates were observed
between the groups. The body weights of male mice exposed to 272 mg
1,3-dichloropropene/m3 were statistically significantly depressed
in comparison with the controls. Examination of haematological and
clinical-chemical parameters and urinalysis did not indicate any
toxicity resulting from exposure to 1,3-dichloropropene for 6, 12,
or 24 months.
The mean relative liver weight of male animals exposed to 272
mg/m3 showed a statistically significant decrease. Gross patho-
logical examination of mice revealed morphological alterations
involving the urinary bladder and lung, which were attributed to
exposure to 1,3-dichloropropene. The bladder mucosal surface in
females exposed to 272 mg/m3 for 12 months and 90.8 and 272
mg/m3 for 24 months had a roughened appearance. In addition, a
statistically significantly increased number of the females exposed
to 90.8 and 272 mg/m3 showed inflammation and epithelial
hyperplasia of the bladder mucosa, after 24 months. An increased
number of male animals exposed to 272 mg/m3 also showed
inflammation.
Female mice exposed to 90.8 or 272 mg/m3 and males exposed to
272 mg/m3 showed hypertrophy and hyperplasia of the nasal
epithelium and degeneration of the olfactory epithelium. Additional
microscopic changes, considered exposure-related, were hyperplasia
and hyperkeratosis in the forestomach of 8/50 male mice following 24
months exposure to 272 mg/m3. A statistically significant increase
in the incidence of a benign tumour, bronchio-alveolar adenoma, was
observed in male mice exposed to 272 mg 1,3-dichloropropene
vapour/m3 for 24 months [22/50 (44%) vs 9/50 (18%) in controls].
No statistically significant increase in tumour incidence was found
in the groups exposed to 1,3-dichloropropene at 22.7 and 90.8
mg/m3 (Table 16). The incidence of lung tumours in the males
exposed to 272 mg/m3 was somewhat higher (7-32%) than the range of
historical control values for this type of tumour in male
B6C3F1 mice in 7 previous, long-term studies. The NOAEL in
this study on mice for hypertrophy/hyperplasia of the nasal
epithelium was 22.7 mg/m3 (Yano et al., 1985; Stott et al., 1987;
Lomax et al., 1989).
8.7.2.2 Rat
Groups of 50 male and 50 female Fischer 344 rats (7-9 weeks of
age) were exposed to 1,3-dichloropropene for 6 h/day, 5 days/week
for up to 24 months. In addition, 2 ancillary groups, each
comprising 10 animals/sex per exposure level, were exposed to 1,3-
dichloropropene for 6 or 12 months. The animals were exposed to 0,
22.7, 90.8, or 272 mg/m3. The chemical composition of the test
material was 92.1% 1,3-dichloropropene ( cis-49.5% and trans-
42.6%), 0.7% 1,2-dichloropropane, and mixtures of hexanes and
hexadiens. Epoxidized soybean oil (approximately 2%) was present as
stabilizer. Besides body weights of the animals, clinical-chemical
parameters in the blood and urine and haematological parameters were
determined for all animals terminated at 6 and 12 months and for 20
animals/sex from each exposure group at 24 months.
Table 16. Incidence of various types of lesions observed in a mouse inhalation study with 1,3-dichloropropenea
Males (mg/m3) Females (mg/m3)
0 22.7 90.8 272 0 22.7 90.8 272
Urinary bladder
Hyperplasia mucosa 4/48 (9%) 7/48 (15%) 11/48 (23%) 37/47 (79%)b 1/47 (2%) 4/46 (9%) 21/48 (44%)b 44/45 (98%)b
(simple or nodular)
Lungs
Bronchio-alveolar 9/50 (18%) 6/50 (12%) 13/50 (26%) 22/50 (44%)b 4/50 (8%) 3/50 (6%) 5/50 (6%) 3/50 (6%)
adenoma
Nasal tissues
Degeneration of 1/50 (2%) 0/50 1/50 (2%) 48/50 (96%)b 0/50 0/50 1/50 (2%) 45/50 (90%)b
olfactory epithelium
Hyperplasia and 5/50 (10%) 1/50 (2%) 4/50 (8%) 48/50 (96%)b 4/50 (8%) 4/50 (8%) 28/50 (56%) 49/50 (98%)b
hypertrophy of
respiratory epithelium
Stomach
Squamous papilloma 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50 (6%) 2/50 (4%) 0/50 (0%) 3/50(6%)
a From: Lomax et al. (1989).
b Statistical difference from control mean identified by using Yate's kappa2 pairwise test, alpha = 0.05.
At 6, 12, or 24 months, animals were sacrificed and the weight
of 5 organs determined; a large number of organs and tissues were
examined histopathologically. No significant influence on survival
was observed. Mean body weights of both male and female rats exposed
to 272 mg/m3 were statistically significantly decreased compared
with mean control values. Examination of haematological and
clinical-chemical parameters, urinalysis, and organ weights did not
indicate any toxicity resulting from exposure to 1,3-dichloropropene
for 6, 12, or 24 months.
Gross pathological examination did not indicate any exposure-
related effects after 6, 12, or 24 months. Exposure-related
histological effects occurred in nasal tissues of rats exposed to
272 mg/m3 for 24 months (Table 17), but not for 6 or 12 months.
The microscopic changes were located in the olfactory mucosa, which
covers the upper portions of the nasal cavity, nasal septum, and
turbinates. The changes were characterized by unilateral or
bilateral decreased thickness of olfactory epithelium and fibrosis
of the submucosal tissues underlying eroded olfactory epithelium. At
the lower dose levels, females did not show histopathological
changes in the nasal tissue, while one male exposed to 22.7 mg/m3
and one exposed to 90.8 mg/m3 showed decreased thickness of the
olfactory epithelium. No effects were seen in the controls. No
statistically significant increase in tumour incidence was found in
exposed rats compared with controls (Lomax et al., 1987, 1989).
8.7.3 Appraisal
Exposure to 1,3-dichloropropene, through inhalation, for up to
24 months did not have any demonstrable effects on survival or
spontaneous tumour development in male and female Fischer 344 rats.
In mice, an increased incidence of bronchio-alveolar adenomas was
found in the lungs of male mice, exposed to 272 mg/m3, but not at
the lower dose level (90.8 mg/m3). The oral gavage study with 1,3-
dichloropropene demonstrated an increased incidence of forestomach
neoplasms in rats of both sexes at dose levels of 50 mg/kg body
weight, administered 3 times/week for 24 months. Male rats treated
with 25 or 50 mg/kg also had an increased incidence of neoplastic
nodules in the liver.
In both the oral gavage and inhalation mouse bioassays with
1,3-dichloropropene, a tumorigenic response was noted in tissues
with which 1,3-dichloropropene had direct contact, i.e., the stomach
and the lung. However, in the gavage study, tumours were also
induced at sites distant from that at the primary "portal-of-entry"
(lung and urinary bladder). This was not the case in the inhalation
study, despite the fact that the dose of 1,3-dichloropropene
received on a mg/kg body weight per day basis by mice exposed for 5
days/week through inhalation was approximately 2-3 times higher than
the dose levels administered orally 3 times/week in the NTP (1985).
Table 17. Microscopic changes in the nasal tissues of rats exposed to
Telone II at 272 mg/m3a
Microscopic change Vehicle control Overall incidence
(male and female) Male Female
Decreased thickness of 0/100 20/50b (40%) 15/50b (31%)
olfactory epithelium
Erosion of olfactory 0/100 15/50b (30%) 6/50 (12%)
epithelium
Submucosal fibrosis 0/100 6/50b (12%) 2/50 (4%)
a From: Lomax et al. (1989).
b Statistical difference identified from control mean of
Yate's kappa2 pairwise test, alpha = 0.05.
The degeneration and subsequent hyperplasia of nasal and forestomach
epithelium occurred only at concentrations that are known to deplete
glutathione levels in these tissues. Tumorigenic effects occur at
doses higher than those causing glutathione depletion and tissue
damage.
The same mouse strain and similar test materials relative to
1,3-dichloropropene were used in both bioassays, but there was a
difference in the stabilizing agents in the test materials. The 1,3-
dichloropropene used in the NTP study was stabilized with 1%
epichlorohydrin, a carcinogen. It has been suggested that
epichlorohydrin may have played a role in the tumorigenic response
obtained in the gavage study because of the bolus nature of its
administration. However, it is not known whether the increased
incidence of tumours in the urinary bladder, lungs, forestomach, and
liver in the mouse gavage study are attributable to the treatment
with 1,3-dichloropropene or the effect of epichlorohydrin, since
carcinogenicity studies of epichlorohydrin in mice have not been
performed yet. In the inhalation study carried out by Lomax et al.
(1989), the 1,3-dichloropropene was stabilized with the relatively
nontoxic epoxidized soybean oil. The role of the stabilizing
additive epichlorohydrin in generating the different tumours seen in
the oral gavage studies on 1,3-dichloropropene is still uncertain.
This question has to be further investigated before a more definite
conclusion about the carcinogenic potential of 1,3-dichloropropene
can be drawn.
8.7.4 Dermal and subcutaneous (mouse)
Groups of 30 female Ha:ICR Swiss strain mice (6-8 weeks old)
were treated with 0.2 ml acetone containing 41 or 122 mg purified
cis-1,3 dichloropropene, applied to shaven skin 3 times weekly,
for approximately 18 months. Control mice received acetone. In the
group treated with 41 mg, no papillomas were found, but at the 122
mg dose, 3 animals showed papillomas, and 2, carcinomas. No tumours
were found at distant sites. Cis-1,3-dichloropropene, applied once
on the skin at a dose of 122 mg/mouse, was followed after 14 days by
the application of 5 µg phorbol myristate acetate in 0.2 ml acetone,
3 times weekly until the end of the study. No skin tumour-initiating
activity was observed (van Duuren et al., 1979).
A group of 30 female Ha:ICR Swiss mice were given weekly
subcutaneous injections in the left flank of 0.05 ml trioctanoin
containing 3 mg purified cis-1,3-dichloropropene per injection.
The study lasted 538 days. Control animals received only the
vehicle. Six out of 30 mice showed local fibrosarcomas, whereas
vehicle control animals did not. A positive control of 0.3 mg beta-
propiolactone produced local sarcomas in 24 out of 30 mice during a
378-day period (van Duuren et al., 1979).
The relevance of the subcutaneous route for the assessment of
carcinogenic properties remains questionable, especially when
injections of an irritant material are made. It is probable that the
persistent and physical properties rather than the chemical
characteristics of cis-1,3-dichloropropene are responsible for
production of local sarcomas.
8.8 Factors modifying toxicity, toxicity of metabolites, mode of
action
8.8.1 Toxicity of the metabolites, cis- and trans-1,3-
dichloropropene oxide
There is some evidence that a small proportion of cis-1,3-
dichloropropene is metabolized to cis-1,3-dichloropropene oxide
(Fig. 5; Hutson, 1984).
8.8.1.1 Mutagenicity
Cis- and trans-1,3-dichloropropene oxide were tested for
mutagenicity, in the absence of metabolic activation, in Salmonella
typhimurium TA1535 and Escherichia coli WP2 uvr A, and for
preferential inhibition of growth of DNA-repair-polymerase-deficient
E. coli. Both oxides were potent mutagens and DNA modifiers. In
Salmonella typhimurium TA1535, treated with 0.025 µmol/ml and in
E. coli WP2 uvr A treated with 0.05 µmol/ml of bacterial
suspension, a significant increase in revertant colonies was found.
A gene mutation test with E. coli (pol A1-/pol A1+) in the
absence of metabolic activation, already showed an effect with
0.0005 µmol/ml (Kline et al., 1982).
In the absence of an S9-fraction, cis-dichloropropene oxide
was strongly mutagenic towards S. typhimurium TA100. The
mutagenicity reached a maximum at 25 µg/plate. Above 300 µg/plate,
marked cytotoxicity was observed. Glutathione (5 mmol/litre) caused
a significant inhibitory effect on the mutagenicity and cytotoxicity
of this epoxide, but did not offer complete protection. Inclusion of
glutathione (5 mmol/litre) together with S9-fraction afforded
complete protection over the range of concentrations of cis-
dichloropropene oxide up to 100 µg/plate (Hutson 1984; Watson et
al., 1986a).
Cis- and trans-1,3-dichloropropene oxide were tested in a
quantitative Syrian hamster embryo cell model. Both compounds at
dose levels of 0.005, 0.01, or 0.02 mmol/litre ( cis-isomer) and
0.01, 0.025, or 0.05 mmol/litre ( trans-isomer) induced
morphological transformation of the Syrian hamster embryo cells
(DiPaolo & Doniger, 1982).
8.8.1.2 Carcinogenicity
Female ICR/Ha Swiss mice (30 per group) were treated 3 times
weekly, with cis-1,3-dichloropropene oxide or trans-1,3-
dichloropropene oxide (containing 10-15% of m-dichlorobenzene) on
the skin. The dose level was 10 mg in 0.1 ml of acetone. The
controls received only acetone. The median survival time was
comparable with that of the controls (over 500 days). With cis-
dichloropropene oxide, 16/30 mice had local papillomas and 10/30
squamous cell carcinomas of the skin; with trans-dichloropropene
oxide, this was 20/30 and 17/30, respectively. No tumours were found
in the control animals (van Duuren et al., 1983).
A study was also carried out on the same strain of mice using
subcutaneous injections. Thirty female mice received 500 µg cis-
dichloropropene oxide or trans-dichloropropene oxide in 0.05 ml
tricaprylin once weekly. The median survival time was comparable to
that of controls. With cis-dichloropropene oxide, 4 animals had a
local (fibro)sarcoma and one carcinoma, and, with trans-
dichloropropene oxide, 5 animals had fibrosarcomas. No tumours were
found in the vehicle controls (van Duuren et al., 1983).
8.8.2 Role of oxidation
When purified cis-1,3-dichloropropene was heated for a few
hours in an oxygen atmosphere, in either the light or dark, the non-
mutagenic cis-1,3-dichloropropene became strongly mutagenic.
Heating under nitrogen was negative. Storage of cis-1,3-
dichloropropene at room temperature, in the presence of oxygen, for
two months, made it mutagenic (Watson et al., 1987). Talcott & King
(1984) demonstrated that purified samples of 1,3-dichloropropene
were not mutagenic to Salmonella typhimurium TA100. Four
preparations of 1,3-dichloropropene were separated into different
fractions and analysed for mutagenic activity. The fraction
containing polar metabolites was found to be mutagenic. Its
composition was too complex to characterize completely, but 2
mutagens, epichlorohydrin and 1,3-dichloro-2-propanol, were
identified.
Watson et al. (1986 a,b; 1987) confirmed that the direct
mutagenicity, inducing base-pair mutations, previously observed in
Salmonella typhimurium TA100 treated with cis-1,3-
dichloropropene, was caused by trace impurities. These impurities
resulted from the autooxidation of cis- and trans-1,3-
dichloropropene and were identified as cis- and trans-
dichloropropene oxides. The dichloropropene oxides made a
significant contribution towards the intrinsic mutagenicity, when
tested in S. typhimurium TA100 (see section 8.8.1.1).
The proposed formation of cis- and trans-dichloropropene
oxides is shown in Fig. 6.
 Autooxidation of cis-1,3-dichloropropene occurs after radical
initiation (5) to give the alkyl peroxy radical (6), which reacts
with a second molecule of cis-1,3-dichloropropene to give the free
radical intermediate (7). Free rotation can occur in this molecule,
prior to expulsion of the alkoxy radical (8), with concomitant
formation of both cis- and trans-dichloropropene oxides (3) and
(4). The alkoxy radical (8) may further abstract a proton or
chlorine from cis-1,3-dichloropropene, thus, continuing the chain
reaction. Autooxidation reactions often proceed via several pathways
and in the case of cis-dichloropropene there are minor products,
such as a 2-hydroxyperoxy intermediate, and unstable 1,2-dioxetanes
leading to 1,3-dichloro-2-propanol and aldehydes, respectively.
Trans-dichloropropene was considerably more resistant to
autooxidations than cis-dichloropropene (Watson et al., 1987).
8.8.3 Role of glutathione
Glutathione (GSH) at physiological concentrations in in vitro
bacterial test systems of Salmonella typhimurium TA100 has been
shown to have a protective effect, i.e., a virtual elimination of
the mutagenic response to 1,3-dichloropropene (Hutson & Stoydin,
1977; DeLorenzo et al., 1977; Brooks et al., 1978; Climie et al.,
1979; Wright & Creedy, 1982; Creedy & Hutson, 1982).
This protective effect occurs in either the absence or the
presence of S9 for both the cis- and trans-isomer. In the
absence of a rat liver fraction, the chemical reaction of cis-1,3-
dichloropropene with glutathione is slow, and, in the presence of
the rat liver fraction, the reaction is rapid due to enzyme
catalysis. The trans-isomer (in the presence of the cis
compound) was degraded 4-5 times more slowly than the cis-isomer
(Hutson & Stoydin, 1977; Climie et al., 1979).
This protective effect in the presence of S9 is consistent with
the operation of a glutathione transferase enzyme occurring in the
added S9 fraction. This enzyme, which conjugates cis-1,3-
dichloropropene with glutathione, is also present in mammalian cells
and the metabolic studies have shown that it plays a key role in the
rapid detoxification of cis-1,3-dichloropropene in mammalian
tissue (Climie et al., 1979; Brooks & Wiggins, 1991).
There is some evidence that the mutagenicity of these
preparations is due to contaminants and that the protective action
of glutathione is due to spontaneous conjugation reactions between
these contaminants and glutathione. Pure cis-dichloropropene did
undergo metabolic activation catalysed by microsomal mono-oxygenase
system from the rat liver. Thus, a small, but significant, dose-
dependent increase in mutation was observed when cis-
dichloropropene was tested in S. typhimurium TA100, in the
presence of S9-liver fraction. When this S9 fraction was replaced by
washed microsomes, which remove the glutathione activity, the
mutagenic effect of cis-1,3-dichloropropene was increased.
Replacement of the glutathione S-alkyl transferase(s) in the
microsomal fraction from an S100 fraction, restored the glutathione-
conjugating activity and afforded complete protection against cis-
1,3-dichloropropene. Cis-dichloropropene undergoes rapid
conjugation with glutathione in the presence of the mentioned
transferase(s), which limits the availability of cis-
dichloropropene to undergo mono-oxygenase-catalysed bioactivation.
These results also provide some evidence that these glutathione-
linked conjugation systems also afford efficient protection against
the mutagenic hazard posed by the bioactivation products of cis-
dichloropropene. Thus, the microbial mutagenicity of cis-
dichloropropene oxide was significantly reduced by glutathione (5
mmol/litre) and this protective action was strongly enhanced in the
presence of glutathione S-alkyl transferases from the S100 (Fig.
7).
Autooxidation of cis-1,3-dichloropropene occurs after radical
initiation (5) to give the alkyl peroxy radical (6), which reacts
with a second molecule of cis-1,3-dichloropropene to give the free
radical intermediate (7). Free rotation can occur in this molecule,
prior to expulsion of the alkoxy radical (8), with concomitant
formation of both cis- and trans-dichloropropene oxides (3) and
(4). The alkoxy radical (8) may further abstract a proton or
chlorine from cis-1,3-dichloropropene, thus, continuing the chain
reaction. Autooxidation reactions often proceed via several pathways
and in the case of cis-dichloropropene there are minor products,
such as a 2-hydroxyperoxy intermediate, and unstable 1,2-dioxetanes
leading to 1,3-dichloro-2-propanol and aldehydes, respectively.
Trans-dichloropropene was considerably more resistant to
autooxidations than cis-dichloropropene (Watson et al., 1987).
8.8.3 Role of glutathione
Glutathione (GSH) at physiological concentrations in in vitro
bacterial test systems of Salmonella typhimurium TA100 has been
shown to have a protective effect, i.e., a virtual elimination of
the mutagenic response to 1,3-dichloropropene (Hutson & Stoydin,
1977; DeLorenzo et al., 1977; Brooks et al., 1978; Climie et al.,
1979; Wright & Creedy, 1982; Creedy & Hutson, 1982).
This protective effect occurs in either the absence or the
presence of S9 for both the cis- and trans-isomer. In the
absence of a rat liver fraction, the chemical reaction of cis-1,3-
dichloropropene with glutathione is slow, and, in the presence of
the rat liver fraction, the reaction is rapid due to enzyme
catalysis. The trans-isomer (in the presence of the cis
compound) was degraded 4-5 times more slowly than the cis-isomer
(Hutson & Stoydin, 1977; Climie et al., 1979).
This protective effect in the presence of S9 is consistent with
the operation of a glutathione transferase enzyme occurring in the
added S9 fraction. This enzyme, which conjugates cis-1,3-
dichloropropene with glutathione, is also present in mammalian cells
and the metabolic studies have shown that it plays a key role in the
rapid detoxification of cis-1,3-dichloropropene in mammalian
tissue (Climie et al., 1979; Brooks & Wiggins, 1991).
There is some evidence that the mutagenicity of these
preparations is due to contaminants and that the protective action
of glutathione is due to spontaneous conjugation reactions between
these contaminants and glutathione. Pure cis-dichloropropene did
undergo metabolic activation catalysed by microsomal mono-oxygenase
system from the rat liver. Thus, a small, but significant, dose-
dependent increase in mutation was observed when cis-
dichloropropene was tested in S. typhimurium TA100, in the
presence of S9-liver fraction. When this S9 fraction was replaced by
washed microsomes, which remove the glutathione activity, the
mutagenic effect of cis-1,3-dichloropropene was increased.
Replacement of the glutathione S-alkyl transferase(s) in the
microsomal fraction from an S100 fraction, restored the glutathione-
conjugating activity and afforded complete protection against cis-
1,3-dichloropropene. Cis-dichloropropene undergoes rapid
conjugation with glutathione in the presence of the mentioned
transferase(s), which limits the availability of cis-
dichloropropene to undergo mono-oxygenase-catalysed bioactivation.
These results also provide some evidence that these glutathione-
linked conjugation systems also afford efficient protection against
the mutagenic hazard posed by the bioactivation products of cis-
dichloropropene. Thus, the microbial mutagenicity of cis-
dichloropropene oxide was significantly reduced by glutathione (5
mmol/litre) and this protective action was strongly enhanced in the
presence of glutathione S-alkyl transferases from the S100 (Fig.
7).
 It was concluded that the degree to which the genotoxic
potential of cis-dichloropropene or its autooxidation products is
expressed in vivo is likely to be lower than that found by
microbial mutation assays. Cis-dichloropropene is efficiently
detoxified in mammals by the operation of a glutathione-dependent
S-alkyl transferase (Watson et al., 1987).
8.8.4 Effect on liver enzyme activity
Miyaoka et al. (1990) studied the mechanism of 1,3-
dichloropropene-induced hepatotoxicity in male mice of the ICR
strain (6 weeks old). 1,3-Dichloropropene (300 mg/kg body weight),
administered by gavage in corn oil, increased plasma GOT and GPT
activities significantly, and centrilobular swelling occurred in the
liver, 15 h after treatment. No such effect was found with 100 mg/kg
body weight. Pretreatment of piperonylbutoxide (PIB, a cytochrome
P450 inhibitor), at 200 mg/kg body weight i.p., significantly
suppressed the elevation of plasma GOT and GPT activities caused by
300 mg 1,3-dichloropropene/kg body weight, but increased the 1,3-
dichloropropene concentration in the liver. The PIB pretreatment
decreased the cytochrome P450 contents in liver microsomes, but
prevented further reduction of cytochrome P450 after 1,3-
dichloropropene treatment.
With pretreatment with L-buthionine-S,R sulfoximine (a GSH
depleting agent) at 1600 mg/kg body weight, plasma GOT activities
increased significantly in animals receiving 100 mg 1,3-
dichloropropene, whereas liver GSH contents and GST activity
decreased. Cysteine administration, 2 h after 1,3-dichloropropene
treatment, did not decrease the cytochrome P450 content, though it
prevented the elevation of GOT and GPT activities and increased
hepatic GSH concentration. The results suggest that 1,3-
dichloropropene is biotransformed via cytochrome P450, and that the
metabolites induce liver damage. GSH plays an important role in the
detoxification of 1,3-dichloropropene (Miyaoka et al., 1990).
9. EFFECTS ON HUMANS
9.1 General population
9.1.1 Acute toxicity - poisoning incidents
In a truck accident in California in 1975, about 4500 litres of
1,3-dichloropropene (92%) was slowly spilled on to the highway. An
estimated 80 persons were exposed to the vapour. Forty-six persons
were examined at hospitals. The following symptoms were found in a
small number of persons (4-6), headache, vomiting and nausea,
dizziness, irritation of mucous membranes, and chest discomfort.
Three persons lost consciousness at the scene of the accident. In 11
out of 41 persons, slightly elevated SGOT and/or SGPT values were
found. Twenty-eight patients were interviewed 1 or 2 weeks later.
The most common symptoms were: headache (12), abdominal discomfort
(6), chest discomfort (5), and malaise (5). Twenty-one patients were
interviewed after 2 years; 10 patients complained of severe or
unusual headache, 10 of chest pain or discomfort, and 13 of
"personality changes" (fatigue, irritability, difficulty in
concentrating, or decreased libido). The frequency of these long-
persisting symptoms was not associated with the intensity of the
exposure (Flessel et al., 1978).
Markovitz & Crosby (1984) reported 9 cases of acute poisoning
following accidental over-exposure to 1,3-dichloropropene. The
chemical spilled as the driver jack-knifed the container. Two of
these cases died 6 years later, due to diffuse histocytic lymphoma.
Authors have reported another case of myelo-monocytic leukaemia
where the patient had been accidentally over-exposed to 1,3-
dichloropropene (see section 9.2.2).
9.1.2 Controlled human studies
A smell detection test with 10 human volunteers was carried
out. A level of 13.6 mg 1,3-dichloropropene/m3 air was detected by
7 out of 10 volunteers. Some reported that the sense of smell
diminished after a few minutes. Even a level of 4.54 mg/m3 was
detected by these 7 persons, but it was noticeably fainter than 13.6
mg/m3 (Torkelson & Oyen, 1977).
In a study designed to determine the odour threshold of Telone
II among 22 individuals, the lowest concentration at which odour was
detected was 20 ± 14 mg/m3. This level is slightly above the US
threshold limit value time-weighted average of 4.54 mg/m3, but
below the short-term exposure limit of 45.4 mg/m3 (Rick & McCarty,
1988).
9.2 Occupational exposure
9.2.1 General
The most likely routes of human exposure to 1,3-dichloropropene
are through inhalation and the skin. Irritation of the eyes and
upper respiratory mucosa, accompanied by lacrimation, appear
promptly after exposure to vapours (Gosselin et al., 1976).
Inhalation by humans of air containing concentrations greater
than 6810 mg/m3 produces headaches, mucous membrane irritation,
dizziness, nausea, vomiting, gasping, coughing, substernal pain, and
respiratory distress (Gosselin et al., 1976; Flessel et al., 1978).
Lower concentrations produce central nervous system depression and
moderate irritation of the respiratory system.
A 44-year-old male process operator at a pesticide plant had
acute bullous dermatitis on both feet in 1988. Approximately one
year later, an identical dermatitis developed. In both periods, he
contaminated his shoes with a 1,3-dichloropropene formulation (D-D-
95). In a patch test with 1,3-dichloropropene, even a concentration
of 0.005% produced a positive reaction. Twenty volunteers did not
react in this patch test at a concentration of 0.05% (Bousema et
al., 1991).
Maddy et al. (1990) summarized the pesticides that caused
occupational illness/injury, reported by physicians in California
during 1987. 1,3-Dichloropropene caused one case of systemic illness
in that period. In the year 1986, 3 cases were mentioned, one with
systemic effects, one with skin effects, and one with eye injury
(Edmiston & Maddy, 1987).
Fifteen applicators of 1,3-dichloropropene were studied for
personal air exposure, urinary excretion of the metabolite, and
excretion of the renal tubular enzyme N-acetyl glucosaminidase
(NAG). Each was studied for four, 6-8 h consecutive intervals
following base-line determinations. The duration of exposure ranged
from 120 to 697 min and the personal air concentrations ranged from
0.3 to 9.4 mg/m3. The 24-h urinary excretion of the metabolite
(average 2.6 mg, range: 0.5-9.2 mg) correlated well with the 1,3-
dichloropropene air exposure product (minutes exposed x mg/m3).
The mean excretion of NAG for all intervals was 2.6 mU/mg of
creatinine (range: 1.0-7.7 mU/mg of creatinine); in 24 h, the mean
was 4940 mU with a range of 278-8956 mU. Four of the 15 workers had
an NAG activity of > 4 mU/mg creatinine in any of their urine
collected after the base line. Nine workers showed increases in NAG
excretion of more than 25% compared with the base line. The authors
concluded that the elevated excretion of NAG indicated a possible
subclinical nephrotoxic effect in the workers, though no complaints
or cases of renal injury were reported (Osterloh et al., 1989).
Stott et al. (1990) commented that the slight increase in NAG in the
urine might be a result of the stimulation of exocytosis or an
increase (induction) in the NAG activity in the kidneys, rather than
an indication of nephrotoxicity. Taken together with the known
metabolism and toxicity of 1,3-dichloropropene in laboratory
animals, the findings by Osterloh et al. (1989) do not suggest any
untoward effects in workers exposed occupationally to low levels of
1,3-dichloropropene.
Fourteen workers applying 1,3-dichloropropene were monitored at
the start of the season, in July, and at the end of the season, in
October, for liver function. The following parameters were measured;
alanine aminotransferase, aspartate aminotransferase, alkaline
phosphatase, lactic dehydrogenase, gamma-glutamyltrans-peptidase,
and total bilirubin. Total bilirubin was significantly decreased at
the end of the season. In combination with an increase in serum
gamma-glutamyltranspeptidase activity this indicates moderate
hepatic enzyme induction. The renal function was also studied by
measuring creatinine and beta-2-microglobulin in serum and beta-2-
microglobulin, albumin, alanine-aminopeptidase, beta-galactosidase,
and retinol-binding protein in urine. The glomerular function
parameters (increased albumin in urine and decreased creatinine in
serum) changed significantly during the season. The tubular function
(retinol-binding protein) also increased. On the basis of these
data, a subclinical nephrotoxic effect cannot be excluded. Effects
on the glutathione conjugation capacity were studied by measuring
erythrocyte glutathione- S-transferase activity and blood
glutathione concentration. Both parameters were significantly
decreased (Brouwer et al., 1991b). The cause-effect relationship
with 1,3-dichloropropene exposure has been questioned (Van Sittert
et al., 1991).
9.2.2 Acute toxicity - poisoning incidents
A farmer in good health developed pain in the right ear, nasal
mucosa, and pharynx after applying 1,3-dichloropropene to his fields
from his tractor for 30 days. Hospital examination showed a red and
painful external ear, hyperaemia, and superficial ulcerations of the
nasal mucosa, and inflammation of the pharynx. The hose containing
the 1,3-dichloropropene had a small leak, which had sprayed the
chemical near the right side of his face. Over the following year,
the man developed myelo-monocytic leukaemia. The man died of
pneumonia 5 weeks after entering the hospital (Markovitz & Crosby,
1984; NTP, 1985; Yang, 1986). The Task Group considers that the
cause-effect relationship in this case is doubtful.
9.2.3 Effects of short- and long-term exposure
The fertility status of 63 males employed in the production of
chlorinated three-carbon compounds were investigated in comparison
with 63 non-exposed persons (at least 5 years without exposure).
Data from reproductive medical history, hormone determination, and
semen analysis were used. There were no indications for an
association between lowered fertility by the standard fertility
parameters, and exposure to allyl chloride, epichlorohydrin, and
1,3-dichloropropene in the quantities occurring in the working
environment. A possible source of bias in this study stems from the
relatively low (64%) volunteer rate from the exposed group and the
lack of an estimate of the individual variation (Venable et al.,
1980).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,3-Dichloropropene (technical grade) was considered by working
groups of the International Agency for Research on Cancer (IARC) in
1986 (IARC, 1986) and 1987 (IARC,1987). In the updating of 1987, it
was evaluated as follows. "There is sufficient evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in
experimental animals. There is inadequate evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in humans.
The agent is possibly carcinogenic to humans (Group 2B)".
Based on the results from 2-year gavage studies on rats and
mice, using the linearized multistage model, the drinking-water
concentration for an excess life-time cancer risk of 104, 105,
or 106 is estimated to be 20, 2.0, or 0.2 µg/litre, respectively
(WHO/EURO, 1990).
PART B
ENVIRONMENTAL HEALTH CRITERIA
FOR
1,2-DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Soil
4.1.2.1 Volatilization
4.1.2.2 Uptake in crops
4.1.2.3 Movement in soil
4.2 Biotransformation
4.3 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water and soil
5.1.3 Crops
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.2 Inhalation
6.1.3 Intraperitoneal
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
7.1.2 Invertebrates
7.1.3 Fish
7.1.3.1 Acute toxicity
7.1.3.2 Short-term/long-term toxicity
7.2 Terrestrial organisms
7.2.1 Earthworms
7.2.2 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.2 Inhalation
8.2.2.1 Mouse
8.2.2.2 Rat
8.2.2.3 Rabbit
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
8.3.2.2 Oral (rabbit)
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
8.4.1.2 Mammalian cells
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
8.4.2.2 Dominant lethal test
8.4.2.3 Miscellaneous
8.5 Carcinogenicity
8.5.1 Oral (mouse)
8.5.2 Oral (rat)
8.6 Factors modifying toxicity
8.7 Special studies
8.7.1 Liver
8.7.2 Kidneys
8.7.3 Central nervous system
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
9.2 Occupational exposure
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1,2-Dichloropropane is a liquid with a boiling point of 96.8 °C
and a vapour pressure of 42 mmHg at 20 °C. The substance is soluble
in water, ethanol, and ethyl ether. When heated, it emits highly
toxic fumes of phosgene. The log P octanol/water partition
coefficient is 2.28.
This substance is used in furniture finish, dry cleaning fluid,
and paint remover, gum processing, metal degreasing, oil processing,
and as a rubber- and wax-making agent, and a chemical intermediate
in the production of tetrachloroethylene and carbon tetrachloride.
It is a component of the "MIX D/D", used as a pre-plant fumigant.
Concentrations of 1,2-dichloropropane in city air have been
measured at 1.2 µg/m3 (mean value), 0.021-0.040 µg/m3, and
0.0065-1.4 µg/m3 in Philadelphia, Portland, and Japan,
respectively. Decomposition in the atmosphere is slow; on the basis
of reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days. Phototransformation is likely to be
the dominant process for the decomposition. Adsorption on to
particulate matter is necessary for appreciable phototransformation.
Volatilization is likely to be the major route of loss from water.
In soil, the main routes of loss are volatilization and
diffusion. 1,2-dichloropropane is persistent in soil. More than 98%
of the 1,2-dichloropropane applied to loam soil was recovered 12-20
weeks after treatment.
Leaching of 1,2-dichloropropane occurs from soil and can
contaminate upper and lower groundwater in areas where "MIX D/D" has
been used as a soil fumigant. In well water and groundwater in the
USA, concentrations of up to 440 µg/litre and 51 µg/litre,
respectively, have been found. In the Netherlands, concentrations of
up to 160 µg/litre have been measured in well water and 1,2-
dichloropropane has been found to a depth of 13 m.
1,2-Dichloropropane can be taken up by edible crops, but the
residues detected have been low (< 0.01 mg/kg) and are unlikely to
be biologically significant.
Bioaccumulation of 1,2-dichloropropane is unlikely, because of
its high water solubility (2.7 g/kg) and low log P octanol/water
partition coefficient.
1.1.2 Kinetics and metabolism
1,2-Dichloropropane administered orally to rats is rapidly
eliminated (80-90% within 24 h). There are no major differences in
kinetics or elimination between males and females. Urine is the
major route of elimination, up to half an oral dose being eliminated
by this route within 24 h. Less than 10% is eliminated via the
faeces. Approximately one-third is eliminated through expired air,
both as carbon dioxide and as a mixture of volatile materials.
Tissue concentrations are low, the highest concentration being found
in the liver. Rapid elimination also occurs following the inhalation
exposure of rats; 55-65% of a dose is eliminated in the urine and
16-23% in expired air. The half-life of elimination from the blood
is 24-30 min.
Unchanged 1,2-dichloropropane is not found in urine. Three
major urinary metabolites have been identified. These metabolites
result from oxidative and conjugation pathways, which yield the
mercapturates, N-acetyl- S-(2-hydroxypropyl)-L-cysteine, N-
acetyl- S-(2-oxypropyl)-L-cysteine, and N-acetyl- S-(1-carboxy-
ethyl)-L-cysteine. 1,2-Dichloropropane can also be oxidized to
lactate with resultant carbon dioxide or acetyl co-enzyme A
production.
Oral administration of 1,2-dichloropropane (2 ml/kg) to rats
significantly depleted tissue glutathione contents. There was a
correlation between tissue glutathione loss and expression of
toxicity in the liver, kidneys, and red blood cells. Prior depletion
of intracellular glutathione exacerbated 1,2-dichloropropane
toxicity, whereas pretreatment with precursors for glutathione
synthesis ameliorated the toxicity. These results demonstrate the
protective effect of glutathione against 1,2-dichloropropane
toxicity.
1.1.3 Effects on organisms in the environment
EC50 for freshwater algae have not been calculated because of
difficulties with volatilization of the chemical from the test
solution. The acute toxicity of 1,2-dichloropropane for aquatic
invertebrates and fish is low to moderate; 48-h LC50 values for
invertebrates range between 52 and > 100 mg/litre and 96-h LC50
values for fish lie between 61 and 320 mg/litre. A short-term
toxicity test on Fathead minnows demonstrated a maximum no-effect
level of 82 mg/litre. A 32-day test on early life stage toxicity in
the same species demonstrated that larval growth and survival were
the most sensitive parameters. The estimated maximum acceptable
toxicant concentration (MATC) was between 6 and 11 mg/litre. Growth
inhibition was noted in Sheepshead minnows after 33 days at a 1,2-
dichloropropane concentration of 164 mg/litre.
1,2-Dichloropropane is phytotoxic.
Contact tests on 4 species of earthworm showed an LC50 of 44-
84 µg/cm2 (mean values) of filter paper. In artificial soil, the
LC50 values were 3880-5300 mg/kg soil (dry weight).
1.1.4 Effects on experimental animals and in vitro test
systems
The acute oral toxicity of 1,2-dichloropropane in experimental
animals is low. The oral LD50 for the rat is 1.9 g/kg body weight,
and the dermal LD50 in rabbits is 8.75 ml/kg body weight.
Short-term, oral toxicity studies of 1,2-dichloropropane in
mice and rats showed growth inhibition, clinical toxic signs
associated with central nervous system depression, and/or increased
mortality at dose levels of 250 mg/kg body weight per day or higher.
In rats given 250 mg/kg per day for 10 days, there were changes in
serum enzymes indicative of slight hepatotoxicity with a NOEL of 100
mg/kg per day.
In a 13-week mouse inhalation study (highest dose 681 mg/m3),
no adverse effects were observed. In a similar study on rats exposed
to 68.1, 227, or 681 mg/m3, a decrease in body weight and minimal
damage to nasal tissues occurred in the 2 highest dose groups.
In a 2-generation reproduction study, rats exposed to 1,2-
dichloropropane in drinking-water at 0.024, 0.1, 0.24% (equivalent
to 33.6, 140 and 336 mg/kg body weight per day) resulted in lower
maternal body weight gain and decreased water consumption at the mid
and high dose levels. Neonatal body weights were lower at the high
dose level. The NOAELs established for maternal and reproductive
toxicity were 33.6 and 140 mg/kg body weight per day, respectively.
Studies did not indicate any teratogenic activity of 1,2-
dichloropropane at oral dose levels up to 125 mg/kg body weight in
the rat and 150 mg/kg body weight in the rabbit. However, at these
dose levels, 1,2-dichloropropane was maternally toxic and fetotoxic,
as evidenced by central nervous system associated clinical signs,
decreased maternal body weight gain, and delayed ossification of
bones in the fetuses. The NOELs are 30 and 50 mg/kg body weight per
day for the rat and rabbit, respectively.
1,2-Dichloropropane was mutagenic in bacteria in most studies
with, and without, metabolic activation, but very high dose levels
were used of up to 10 mg/plate. In Chinese hamster ovary cells, 1,2-
dichloropropane caused chromosomal aberrations and sister chromatid
exchange; in Chinese hamster V79 cells, it increased the sister
chromatid exchange. In an in vitro system with human lymphocytes,
the tritiated thymidine uptake and cell viability in cultures grown
with, and without, rat liver metabolizing system, were similar to
those in control cultures. The results of a sex-linked recessive
lethal test in Drosophila melanogaster were negative. A dominant
lethal test in rats, dosed for 14 weeks via drinking-water
containing 1,2-dichloropropane, followed by 2 weeks of mating, was
negative.
In a carcinogenicity study on mice administered 125 or 250 mg
1,2-dichloropropane/kg body weight by gavage, a dose-related
increase in the incidence of liver adenomas was observed. The
incidence of liver adenomas in treated groups was higher than that
in the concurrent control group, but was within the historical
control range.
In rats administered dose levels of 125 and 250 mg/kg body
weight (females) and 62 and 125 mg/kg body weight (males), by
gavage, for 5 days per week over 113 weeks, a slight increase in the
incidence of mammary gland adenocarcinomas exceeding the historical
range was observed in high-dose females.
1.1.5 Effects on human beings
Exposure of the general population to 1,2-dichloropropane via
air and water is unlikely, except in areas where there is extensive
use of 1,2-dichloropropane and "MIX D/D" in agriculture. Residues of
1,2-dichloropropane in edible crops are generally below the limit of
detection. In view of these low exposures to 1,2-dichloropropane,
the risk to the general population is negligible.
Several cases of acute poisoning have been reported due to
accidental or intentional (suicide) over-exposure to 1,2-
dichloropropane. Effects have been mainly on the central nervous
system, liver, and kidneys. Haemolytic anaemia and disseminated
intravascular coagulation have also been reported. In one case,
delirium progressed to irreversible shock, cardiac failure, and
death.
Occupational exposures can be via both skin and inhalation.
Several cases of dermatitis and skin sensitization have been
reported in workers using solvent mixtures containing 1,2-
dichloropropane.
1.2 Conclusions
* General population: There is low or non-existent exposure of
the general population to 1,2-dichloropropane from air and
food. However, in certain areas, exposure may occur when
groundwater is contaminated.
* Occupational exposure: With good work practices, hygienic
measures, and safety precautions, the use of 1,2-
dichloropropane is unlikely to present a risk for those
occupationally exposed to it.
* Environment: 1,2-Dichloropropane is unlikely to attain levels
of environmental significance when used at the recommended
rate. It is unlikely to have adverse effects on populations of
terrestrial and aquatic organisms.
1.3 Recommendations
* Studies should be conducted to assess acute inhalation
toxicity, eye and skin irritancy, and skin sensitization
potential.
* Appropriate safety precautions should be taken, when handling
1,2-dichloropropane, in order to avoid exposures exceeding the
maximum allowable concentration.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
Primary constituent
Chemical structure Cl
'
ClCH2CHCH3
Chemical formula C3H6Cl2
Relative molecular mass 112.99
Chemical name 1,2-dichloropropane,
dichloro-1,2-propane
Common synonyms propylene dichloride
CAS registry number
It was concluded that the degree to which the genotoxic
potential of cis-dichloropropene or its autooxidation products is
expressed in vivo is likely to be lower than that found by
microbial mutation assays. Cis-dichloropropene is efficiently
detoxified in mammals by the operation of a glutathione-dependent
S-alkyl transferase (Watson et al., 1987).
8.8.4 Effect on liver enzyme activity
Miyaoka et al. (1990) studied the mechanism of 1,3-
dichloropropene-induced hepatotoxicity in male mice of the ICR
strain (6 weeks old). 1,3-Dichloropropene (300 mg/kg body weight),
administered by gavage in corn oil, increased plasma GOT and GPT
activities significantly, and centrilobular swelling occurred in the
liver, 15 h after treatment. No such effect was found with 100 mg/kg
body weight. Pretreatment of piperonylbutoxide (PIB, a cytochrome
P450 inhibitor), at 200 mg/kg body weight i.p., significantly
suppressed the elevation of plasma GOT and GPT activities caused by
300 mg 1,3-dichloropropene/kg body weight, but increased the 1,3-
dichloropropene concentration in the liver. The PIB pretreatment
decreased the cytochrome P450 contents in liver microsomes, but
prevented further reduction of cytochrome P450 after 1,3-
dichloropropene treatment.
With pretreatment with L-buthionine-S,R sulfoximine (a GSH
depleting agent) at 1600 mg/kg body weight, plasma GOT activities
increased significantly in animals receiving 100 mg 1,3-
dichloropropene, whereas liver GSH contents and GST activity
decreased. Cysteine administration, 2 h after 1,3-dichloropropene
treatment, did not decrease the cytochrome P450 content, though it
prevented the elevation of GOT and GPT activities and increased
hepatic GSH concentration. The results suggest that 1,3-
dichloropropene is biotransformed via cytochrome P450, and that the
metabolites induce liver damage. GSH plays an important role in the
detoxification of 1,3-dichloropropene (Miyaoka et al., 1990).
9. EFFECTS ON HUMANS
9.1 General population
9.1.1 Acute toxicity - poisoning incidents
In a truck accident in California in 1975, about 4500 litres of
1,3-dichloropropene (92%) was slowly spilled on to the highway. An
estimated 80 persons were exposed to the vapour. Forty-six persons
were examined at hospitals. The following symptoms were found in a
small number of persons (4-6), headache, vomiting and nausea,
dizziness, irritation of mucous membranes, and chest discomfort.
Three persons lost consciousness at the scene of the accident. In 11
out of 41 persons, slightly elevated SGOT and/or SGPT values were
found. Twenty-eight patients were interviewed 1 or 2 weeks later.
The most common symptoms were: headache (12), abdominal discomfort
(6), chest discomfort (5), and malaise (5). Twenty-one patients were
interviewed after 2 years; 10 patients complained of severe or
unusual headache, 10 of chest pain or discomfort, and 13 of
"personality changes" (fatigue, irritability, difficulty in
concentrating, or decreased libido). The frequency of these long-
persisting symptoms was not associated with the intensity of the
exposure (Flessel et al., 1978).
Markovitz & Crosby (1984) reported 9 cases of acute poisoning
following accidental over-exposure to 1,3-dichloropropene. The
chemical spilled as the driver jack-knifed the container. Two of
these cases died 6 years later, due to diffuse histocytic lymphoma.
Authors have reported another case of myelo-monocytic leukaemia
where the patient had been accidentally over-exposed to 1,3-
dichloropropene (see section 9.2.2).
9.1.2 Controlled human studies
A smell detection test with 10 human volunteers was carried
out. A level of 13.6 mg 1,3-dichloropropene/m3 air was detected by
7 out of 10 volunteers. Some reported that the sense of smell
diminished after a few minutes. Even a level of 4.54 mg/m3 was
detected by these 7 persons, but it was noticeably fainter than 13.6
mg/m3 (Torkelson & Oyen, 1977).
In a study designed to determine the odour threshold of Telone
II among 22 individuals, the lowest concentration at which odour was
detected was 20 ± 14 mg/m3. This level is slightly above the US
threshold limit value time-weighted average of 4.54 mg/m3, but
below the short-term exposure limit of 45.4 mg/m3 (Rick & McCarty,
1988).
9.2 Occupational exposure
9.2.1 General
The most likely routes of human exposure to 1,3-dichloropropene
are through inhalation and the skin. Irritation of the eyes and
upper respiratory mucosa, accompanied by lacrimation, appear
promptly after exposure to vapours (Gosselin et al., 1976).
Inhalation by humans of air containing concentrations greater
than 6810 mg/m3 produces headaches, mucous membrane irritation,
dizziness, nausea, vomiting, gasping, coughing, substernal pain, and
respiratory distress (Gosselin et al., 1976; Flessel et al., 1978).
Lower concentrations produce central nervous system depression and
moderate irritation of the respiratory system.
A 44-year-old male process operator at a pesticide plant had
acute bullous dermatitis on both feet in 1988. Approximately one
year later, an identical dermatitis developed. In both periods, he
contaminated his shoes with a 1,3-dichloropropene formulation (D-D-
95). In a patch test with 1,3-dichloropropene, even a concentration
of 0.005% produced a positive reaction. Twenty volunteers did not
react in this patch test at a concentration of 0.05% (Bousema et
al., 1991).
Maddy et al. (1990) summarized the pesticides that caused
occupational illness/injury, reported by physicians in California
during 1987. 1,3-Dichloropropene caused one case of systemic illness
in that period. In the year 1986, 3 cases were mentioned, one with
systemic effects, one with skin effects, and one with eye injury
(Edmiston & Maddy, 1987).
Fifteen applicators of 1,3-dichloropropene were studied for
personal air exposure, urinary excretion of the metabolite, and
excretion of the renal tubular enzyme N-acetyl glucosaminidase
(NAG). Each was studied for four, 6-8 h consecutive intervals
following base-line determinations. The duration of exposure ranged
from 120 to 697 min and the personal air concentrations ranged from
0.3 to 9.4 mg/m3. The 24-h urinary excretion of the metabolite
(average 2.6 mg, range: 0.5-9.2 mg) correlated well with the 1,3-
dichloropropene air exposure product (minutes exposed x mg/m3).
The mean excretion of NAG for all intervals was 2.6 mU/mg of
creatinine (range: 1.0-7.7 mU/mg of creatinine); in 24 h, the mean
was 4940 mU with a range of 278-8956 mU. Four of the 15 workers had
an NAG activity of > 4 mU/mg creatinine in any of their urine
collected after the base line. Nine workers showed increases in NAG
excretion of more than 25% compared with the base line. The authors
concluded that the elevated excretion of NAG indicated a possible
subclinical nephrotoxic effect in the workers, though no complaints
or cases of renal injury were reported (Osterloh et al., 1989).
Stott et al. (1990) commented that the slight increase in NAG in the
urine might be a result of the stimulation of exocytosis or an
increase (induction) in the NAG activity in the kidneys, rather than
an indication of nephrotoxicity. Taken together with the known
metabolism and toxicity of 1,3-dichloropropene in laboratory
animals, the findings by Osterloh et al. (1989) do not suggest any
untoward effects in workers exposed occupationally to low levels of
1,3-dichloropropene.
Fourteen workers applying 1,3-dichloropropene were monitored at
the start of the season, in July, and at the end of the season, in
October, for liver function. The following parameters were measured;
alanine aminotransferase, aspartate aminotransferase, alkaline
phosphatase, lactic dehydrogenase, gamma-glutamyltrans-peptidase,
and total bilirubin. Total bilirubin was significantly decreased at
the end of the season. In combination with an increase in serum
gamma-glutamyltranspeptidase activity this indicates moderate
hepatic enzyme induction. The renal function was also studied by
measuring creatinine and beta-2-microglobulin in serum and beta-2-
microglobulin, albumin, alanine-aminopeptidase, beta-galactosidase,
and retinol-binding protein in urine. The glomerular function
parameters (increased albumin in urine and decreased creatinine in
serum) changed significantly during the season. The tubular function
(retinol-binding protein) also increased. On the basis of these
data, a subclinical nephrotoxic effect cannot be excluded. Effects
on the glutathione conjugation capacity were studied by measuring
erythrocyte glutathione- S-transferase activity and blood
glutathione concentration. Both parameters were significantly
decreased (Brouwer et al., 1991b). The cause-effect relationship
with 1,3-dichloropropene exposure has been questioned (Van Sittert
et al., 1991).
9.2.2 Acute toxicity - poisoning incidents
A farmer in good health developed pain in the right ear, nasal
mucosa, and pharynx after applying 1,3-dichloropropene to his fields
from his tractor for 30 days. Hospital examination showed a red and
painful external ear, hyperaemia, and superficial ulcerations of the
nasal mucosa, and inflammation of the pharynx. The hose containing
the 1,3-dichloropropene had a small leak, which had sprayed the
chemical near the right side of his face. Over the following year,
the man developed myelo-monocytic leukaemia. The man died of
pneumonia 5 weeks after entering the hospital (Markovitz & Crosby,
1984; NTP, 1985; Yang, 1986). The Task Group considers that the
cause-effect relationship in this case is doubtful.
9.2.3 Effects of short- and long-term exposure
The fertility status of 63 males employed in the production of
chlorinated three-carbon compounds were investigated in comparison
with 63 non-exposed persons (at least 5 years without exposure).
Data from reproductive medical history, hormone determination, and
semen analysis were used. There were no indications for an
association between lowered fertility by the standard fertility
parameters, and exposure to allyl chloride, epichlorohydrin, and
1,3-dichloropropene in the quantities occurring in the working
environment. A possible source of bias in this study stems from the
relatively low (64%) volunteer rate from the exposed group and the
lack of an estimate of the individual variation (Venable et al.,
1980).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,3-Dichloropropene (technical grade) was considered by working
groups of the International Agency for Research on Cancer (IARC) in
1986 (IARC, 1986) and 1987 (IARC,1987). In the updating of 1987, it
was evaluated as follows. "There is sufficient evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in
experimental animals. There is inadequate evidence for the
carcinogenicity of 1,3-dichloropropene (technical-grade) in humans.
The agent is possibly carcinogenic to humans (Group 2B)".
Based on the results from 2-year gavage studies on rats and
mice, using the linearized multistage model, the drinking-water
concentration for an excess life-time cancer risk of 104, 105,
or 106 is estimated to be 20, 2.0, or 0.2 µg/litre, respectively
(WHO/EURO, 1990).
PART B
ENVIRONMENTAL HEALTH CRITERIA
FOR
1,2-DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Soil
4.1.2.1 Volatilization
4.1.2.2 Uptake in crops
4.1.2.3 Movement in soil
4.2 Biotransformation
4.3 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water and soil
5.1.3 Crops
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Oral
6.1.2 Inhalation
6.1.3 Intraperitoneal
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
7.1.2 Invertebrates
7.1.3 Fish
7.1.3.1 Acute toxicity
7.1.3.2 Short-term/long-term toxicity
7.2 Terrestrial organisms
7.2.1 Earthworms
7.2.2 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.2 Inhalation
8.2.2.1 Mouse
8.2.2.2 Rat
8.2.2.3 Rabbit
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
8.3.2.2 Oral (rabbit)
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
8.4.1.2 Mammalian cells
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
8.4.2.2 Dominant lethal test
8.4.2.3 Miscellaneous
8.5 Carcinogenicity
8.5.1 Oral (mouse)
8.5.2 Oral (rat)
8.6 Factors modifying toxicity
8.7 Special studies
8.7.1 Liver
8.7.2 Kidneys
8.7.3 Central nervous system
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
9.2 Occupational exposure
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1,2-Dichloropropane is a liquid with a boiling point of 96.8 °C
and a vapour pressure of 42 mmHg at 20 °C. The substance is soluble
in water, ethanol, and ethyl ether. When heated, it emits highly
toxic fumes of phosgene. The log P octanol/water partition
coefficient is 2.28.
This substance is used in furniture finish, dry cleaning fluid,
and paint remover, gum processing, metal degreasing, oil processing,
and as a rubber- and wax-making agent, and a chemical intermediate
in the production of tetrachloroethylene and carbon tetrachloride.
It is a component of the "MIX D/D", used as a pre-plant fumigant.
Concentrations of 1,2-dichloropropane in city air have been
measured at 1.2 µg/m3 (mean value), 0.021-0.040 µg/m3, and
0.0065-1.4 µg/m3 in Philadelphia, Portland, and Japan,
respectively. Decomposition in the atmosphere is slow; on the basis
of reaction with hydroxyl radicals, the half-life of 1,2-
dichloropropane was > 313 days. Phototransformation is likely to be
the dominant process for the decomposition. Adsorption on to
particulate matter is necessary for appreciable phototransformation.
Volatilization is likely to be the major route of loss from water.
In soil, the main routes of loss are volatilization and
diffusion. 1,2-dichloropropane is persistent in soil. More than 98%
of the 1,2-dichloropropane applied to loam soil was recovered 12-20
weeks after treatment.
Leaching of 1,2-dichloropropane occurs from soil and can
contaminate upper and lower groundwater in areas where "MIX D/D" has
been used as a soil fumigant. In well water and groundwater in the
USA, concentrations of up to 440 µg/litre and 51 µg/litre,
respectively, have been found. In the Netherlands, concentrations of
up to 160 µg/litre have been measured in well water and 1,2-
dichloropropane has been found to a depth of 13 m.
1,2-Dichloropropane can be taken up by edible crops, but the
residues detected have been low (< 0.01 mg/kg) and are unlikely to
be biologically significant.
Bioaccumulation of 1,2-dichloropropane is unlikely, because of
its high water solubility (2.7 g/kg) and low log P octanol/water
partition coefficient.
1.1.2 Kinetics and metabolism
1,2-Dichloropropane administered orally to rats is rapidly
eliminated (80-90% within 24 h). There are no major differences in
kinetics or elimination between males and females. Urine is the
major route of elimination, up to half an oral dose being eliminated
by this route within 24 h. Less than 10% is eliminated via the
faeces. Approximately one-third is eliminated through expired air,
both as carbon dioxide and as a mixture of volatile materials.
Tissue concentrations are low, the highest concentration being found
in the liver. Rapid elimination also occurs following the inhalation
exposure of rats; 55-65% of a dose is eliminated in the urine and
16-23% in expired air. The half-life of elimination from the blood
is 24-30 min.
Unchanged 1,2-dichloropropane is not found in urine. Three
major urinary metabolites have been identified. These metabolites
result from oxidative and conjugation pathways, which yield the
mercapturates, N-acetyl- S-(2-hydroxypropyl)-L-cysteine, N-
acetyl- S-(2-oxypropyl)-L-cysteine, and N-acetyl- S-(1-carboxy-
ethyl)-L-cysteine. 1,2-Dichloropropane can also be oxidized to
lactate with resultant carbon dioxide or acetyl co-enzyme A
production.
Oral administration of 1,2-dichloropropane (2 ml/kg) to rats
significantly depleted tissue glutathione contents. There was a
correlation between tissue glutathione loss and expression of
toxicity in the liver, kidneys, and red blood cells. Prior depletion
of intracellular glutathione exacerbated 1,2-dichloropropane
toxicity, whereas pretreatment with precursors for glutathione
synthesis ameliorated the toxicity. These results demonstrate the
protective effect of glutathione against 1,2-dichloropropane
toxicity.
1.1.3 Effects on organisms in the environment
EC50 for freshwater algae have not been calculated because of
difficulties with volatilization of the chemical from the test
solution. The acute toxicity of 1,2-dichloropropane for aquatic
invertebrates and fish is low to moderate; 48-h LC50 values for
invertebrates range between 52 and > 100 mg/litre and 96-h LC50
values for fish lie between 61 and 320 mg/litre. A short-term
toxicity test on Fathead minnows demonstrated a maximum no-effect
level of 82 mg/litre. A 32-day test on early life stage toxicity in
the same species demonstrated that larval growth and survival were
the most sensitive parameters. The estimated maximum acceptable
toxicant concentration (MATC) was between 6 and 11 mg/litre. Growth
inhibition was noted in Sheepshead minnows after 33 days at a 1,2-
dichloropropane concentration of 164 mg/litre.
1,2-Dichloropropane is phytotoxic.
Contact tests on 4 species of earthworm showed an LC50 of 44-
84 µg/cm2 (mean values) of filter paper. In artificial soil, the
LC50 values were 3880-5300 mg/kg soil (dry weight).
1.1.4 Effects on experimental animals and in vitro test
systems
The acute oral toxicity of 1,2-dichloropropane in experimental
animals is low. The oral LD50 for the rat is 1.9 g/kg body weight,
and the dermal LD50 in rabbits is 8.75 ml/kg body weight.
Short-term, oral toxicity studies of 1,2-dichloropropane in
mice and rats showed growth inhibition, clinical toxic signs
associated with central nervous system depression, and/or increased
mortality at dose levels of 250 mg/kg body weight per day or higher.
In rats given 250 mg/kg per day for 10 days, there were changes in
serum enzymes indicative of slight hepatotoxicity with a NOEL of 100
mg/kg per day.
In a 13-week mouse inhalation study (highest dose 681 mg/m3),
no adverse effects were observed. In a similar study on rats exposed
to 68.1, 227, or 681 mg/m3, a decrease in body weight and minimal
damage to nasal tissues occurred in the 2 highest dose groups.
In a 2-generation reproduction study, rats exposed to 1,2-
dichloropropane in drinking-water at 0.024, 0.1, 0.24% (equivalent
to 33.6, 140 and 336 mg/kg body weight per day) resulted in lower
maternal body weight gain and decreased water consumption at the mid
and high dose levels. Neonatal body weights were lower at the high
dose level. The NOAELs established for maternal and reproductive
toxicity were 33.6 and 140 mg/kg body weight per day, respectively.
Studies did not indicate any teratogenic activity of 1,2-
dichloropropane at oral dose levels up to 125 mg/kg body weight in
the rat and 150 mg/kg body weight in the rabbit. However, at these
dose levels, 1,2-dichloropropane was maternally toxic and fetotoxic,
as evidenced by central nervous system associated clinical signs,
decreased maternal body weight gain, and delayed ossification of
bones in the fetuses. The NOELs are 30 and 50 mg/kg body weight per
day for the rat and rabbit, respectively.
1,2-Dichloropropane was mutagenic in bacteria in most studies
with, and without, metabolic activation, but very high dose levels
were used of up to 10 mg/plate. In Chinese hamster ovary cells, 1,2-
dichloropropane caused chromosomal aberrations and sister chromatid
exchange; in Chinese hamster V79 cells, it increased the sister
chromatid exchange. In an in vitro system with human lymphocytes,
the tritiated thymidine uptake and cell viability in cultures grown
with, and without, rat liver metabolizing system, were similar to
those in control cultures. The results of a sex-linked recessive
lethal test in Drosophila melanogaster were negative. A dominant
lethal test in rats, dosed for 14 weeks via drinking-water
containing 1,2-dichloropropane, followed by 2 weeks of mating, was
negative.
In a carcinogenicity study on mice administered 125 or 250 mg
1,2-dichloropropane/kg body weight by gavage, a dose-related
increase in the incidence of liver adenomas was observed. The
incidence of liver adenomas in treated groups was higher than that
in the concurrent control group, but was within the historical
control range.
In rats administered dose levels of 125 and 250 mg/kg body
weight (females) and 62 and 125 mg/kg body weight (males), by
gavage, for 5 days per week over 113 weeks, a slight increase in the
incidence of mammary gland adenocarcinomas exceeding the historical
range was observed in high-dose females.
1.1.5 Effects on human beings
Exposure of the general population to 1,2-dichloropropane via
air and water is unlikely, except in areas where there is extensive
use of 1,2-dichloropropane and "MIX D/D" in agriculture. Residues of
1,2-dichloropropane in edible crops are generally below the limit of
detection. In view of these low exposures to 1,2-dichloropropane,
the risk to the general population is negligible.
Several cases of acute poisoning have been reported due to
accidental or intentional (suicide) over-exposure to 1,2-
dichloropropane. Effects have been mainly on the central nervous
system, liver, and kidneys. Haemolytic anaemia and disseminated
intravascular coagulation have also been reported. In one case,
delirium progressed to irreversible shock, cardiac failure, and
death.
Occupational exposures can be via both skin and inhalation.
Several cases of dermatitis and skin sensitization have been
reported in workers using solvent mixtures containing 1,2-
dichloropropane.
1.2 Conclusions
* General population: There is low or non-existent exposure of
the general population to 1,2-dichloropropane from air and
food. However, in certain areas, exposure may occur when
groundwater is contaminated.
* Occupational exposure: With good work practices, hygienic
measures, and safety precautions, the use of 1,2-
dichloropropane is unlikely to present a risk for those
occupationally exposed to it.
* Environment: 1,2-Dichloropropane is unlikely to attain levels
of environmental significance when used at the recommended
rate. It is unlikely to have adverse effects on populations of
terrestrial and aquatic organisms.
1.3 Recommendations
* Studies should be conducted to assess acute inhalation
toxicity, eye and skin irritancy, and skin sensitization
potential.
* Appropriate safety precautions should be taken, when handling
1,2-dichloropropane, in order to avoid exposures exceeding the
maximum allowable concentration.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
Primary constituent
Chemical structure Cl
'
ClCH2CHCH3
Chemical formula C3H6Cl2
Relative molecular mass 112.99
Chemical name 1,2-dichloropropane,
dichloro-1,2-propane
Common synonyms propylene dichloride
CAS registry number  Imberti et al. (1990) investigated the effects of a single dose
of 1,2-dichloropropane of 2 ml/kg body weight (by gavage) on the
intracellular glutathione (GSH) content of the liver, kidneys, and
blood of male Wistar rats (180-250 g). 1,2-Dichloropropane,
administered orally, caused a significant depletion of GSH within 24
h of treatment, followed by a slow recovery, approaching normal
levels after 96 h. The GSH depletion was associated with a marked
increase in serum GOT, GPT, 5'-nucleotidase, gamma-glutamyl
transpeptidase, alkaline phosphatase, urea, and creatinine, and a
significant degree of haemolysis. The administration of L-
buthionine-S,R sulfoximine (BSO) (0.5 g/kg body weight, i.p.), 4 h
before 1,2-dichloropropane treatment, resulted in a significant
increase in overall mortality. The administration of a GSH
precursor, N-acetylcysteine (NAC), i.p., at 250 mg/kg body weight,
2 and 16 h after 1,2-dichloropropane treatment, prevented the loss
of cellular GSH and reduced the extent of injury in the target
tissues. There was a correlation between the depletion of liver GSH
and the increases in GOT, GPT, and 5'-nucleotidase, between the
depletion of GSH in the kidneys and the increase in serum urea and
creatinine, and the depletion of GSH in blood and the occurrence of
haemolysis.
Both 1-chloro-2-hydroxypropane (II) and 1,2-epoxypropane (III)
are proposed as intermediates in the metabolism to the mercapturic
acid. 1,2-Epoxypropane can also be metabolized to propanediol (IV),
which is further metabolized to pyruvate and enters the
tricarboxylic acid cycle; carbon dioxide is released and expired.
Epoxypropane may also be conjugated with glutathione (VI) and
excreted in the urine. Jones & Gibson (1980) further proposed that
the 1-chloro-2-hydroxypropane (II) may be metabolized to beta-
chlorolactaldehyde (VII) and beta-chlorolactate (VIII) (Fig. 9).
Imberti et al. (1990) investigated the effects of a single dose
of 1,2-dichloropropane of 2 ml/kg body weight (by gavage) on the
intracellular glutathione (GSH) content of the liver, kidneys, and
blood of male Wistar rats (180-250 g). 1,2-Dichloropropane,
administered orally, caused a significant depletion of GSH within 24
h of treatment, followed by a slow recovery, approaching normal
levels after 96 h. The GSH depletion was associated with a marked
increase in serum GOT, GPT, 5'-nucleotidase, gamma-glutamyl
transpeptidase, alkaline phosphatase, urea, and creatinine, and a
significant degree of haemolysis. The administration of L-
buthionine-S,R sulfoximine (BSO) (0.5 g/kg body weight, i.p.), 4 h
before 1,2-dichloropropane treatment, resulted in a significant
increase in overall mortality. The administration of a GSH
precursor, N-acetylcysteine (NAC), i.p., at 250 mg/kg body weight,
2 and 16 h after 1,2-dichloropropane treatment, prevented the loss
of cellular GSH and reduced the extent of injury in the target
tissues. There was a correlation between the depletion of liver GSH
and the increases in GOT, GPT, and 5'-nucleotidase, between the
depletion of GSH in the kidneys and the increase in serum urea and
creatinine, and the depletion of GSH in blood and the occurrence of
haemolysis.
Both 1-chloro-2-hydroxypropane (II) and 1,2-epoxypropane (III)
are proposed as intermediates in the metabolism to the mercapturic
acid. 1,2-Epoxypropane can also be metabolized to propanediol (IV),
which is further metabolized to pyruvate and enters the
tricarboxylic acid cycle; carbon dioxide is released and expired.
Epoxypropane may also be conjugated with glutathione (VI) and
excreted in the urine. Jones & Gibson (1980) further proposed that
the 1-chloro-2-hydroxypropane (II) may be metabolized to beta-
chlorolactaldehyde (VII) and beta-chlorolactate (VIII) (Fig. 9).
 7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
The EC50 for CO2 uptake by marine algae (Phaeodactylum
tricornutum) was 50 mg/litre. 1,2-Dichloropropane was not found to
exhibit any algistatic or algicidal effects in an acute toxicity
study on Selenastrum capricornutum, though the problems of losses
of 1,2-dichloropropane from the test flasks, made it impossible to
calculate EC values (Dynamic Corporation, 1991).
A general trend of decreasing algal population growth with
increasing nominal concentrations of 1,2-dichloropropane was
observed in an acute toxicity test on Skeletonema costatum.
Because the concentrations measured for each nominal value were
variable, it was not appropriate to determine EC values. A NOEL of
18 mg/litre was determined, though it was not possible to
distinguish algistatic from algicidal effects (Dynamic Corporation,
1991).
7.1.2 Invertebrates
The acute toxicity of 1,2-dichloropropane for non-target
invertebrates is summarized in Table 18; 1,2-dichloropropane has a
48-h LC50 of 50-100 mg/litre for these aquatic organisms. No
discernible effects on Daphnia magna were found at concentrations
of less than 22 mg/litre (Leblanc, 1980).
Table 18. Acute toxicity of 1,2-dichloropropane in non-target
aquatic insects and crustacea
Species Temperature 48-h LC50 References
(°C) mg/litre
Barnacle nauplii - 53 Pearson & McConnell
(Elminius moderatus) (1975)
Brown shrimp - > 100 Portmann & Wilson
(Crangon crangon) (1971)
7.1.3 Fish
7.1.3.1 Acute toxicity
The 96-h LC50 of 1,2-dichloropropane for freshwater and
marine fish is 61-320 mg/litre (Table 19).
7.1.3.2 Short-term/long-term toxicity
Two embryo-larval tests have been conducted on Fathead minnow
(Pimephales promelas) exposed to 1,2-dichloropropane. The maximum
no-effect levels were 8.1 and 60 mg/litre, respectively (US EPA,
1980).
In the marine environment, growth inhibition was noted in the
Sheepshead minnow (Cyprinodon variegatus) after 33 days exposure
to 164 mg 1,2-dichloropropane/litre (US EPA, 1980).
A 32-day test to study early life stage toxicity in Fathead
minnows (Pimephales promelas) demonstrated that larval growth and
survival (28-day-old fish) were the most sensitive indicators of
toxic effects. Embryo hatch and larval deformities at hatch were the
least sensitive indicators of toxicity. The 1,2-dichloropropane
(98%) was tested at dose levels of 0, 6, 11, 25, 51, and 110
mg/litre (temperature of the water 25 °C, hardness 45 mg/litre as
CaCO3, pH 7.4). The estimated maximum acceptable toxicant
concentration (MATC) was between 6 and 11 mg/litre (Benoit et al.,
1982).
7.2 Terrestrial organisms
7.2.1 Earthworms
Neuhauser et al. (1985a,b; 1986) studied the toxicity of 1,2-
dichloropropane for 4 species of earthworm (Allolobophora
tuberculata, Eisenia foetida, Eudrilus eugeniae and Perionyx
excavatus) using EEC earthworm artificial soil and a contact
testing procedure (Edwards, 1983). The LC50 values and 95%
confidence limits in the contact test were 84(65-110); 64(59-70);
44(38-51); and 63(56-72) µg/cm2 of filter paper for the 4 species
of earthworms, respectively. In the artificial soil test, the LC50
values were 4272, 4240, 5300, and 3880 mg/kg dry weight artificial
soil, respectively.
7.2.2 Plants
1,2-Dichloropropane is highly phytotoxic.
Table 19. Acute toxicity of 1,2-dichloropropane in fish
Species Size or age Temperature 96-h LC50 References
(°C) mg/litre
Guppy 2-3 months 21-23 3.01 µmol/litref Könemann (1981)
(Poecilia reticulata)
Bluegill sunfish 33-75 mm 23a 320 Dawson et al. (1977)
(Lepomis macrochirus) 0.32-1.2 g 21-23 280b,c Buccafusco et al. (1981)
(220-340)
Fathead minnow 30-35 days 25d,e 140d Wallbridge (1983)
(Pimephales promelas) (131-150)
Tidewater silversides 40-100 mm 20a 240 Dawson et al. (1977)
(Menida beryllina
Dab 15-20 cm - 61 Pearson & McConnell (1975)
(Pleuronectes limanda)
a Hardness 55 mg/litre (as CaCO3), pH 7.6-7.9.
b Tested in well-water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration at the beginning
9.7 to 0.3 mg/litre after 96-h exposure.
c Static system (nominal concentrations).
d Flow-through system.
e Hardness 42-45 mg/litre (as CaCO3) pH 6.7-7.6.
f 7-day LC50 (static system, renewal).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
The acute oral LD50 for the rat has been reported to be 1.9
(1.7-2.1) g/kg body weight and the dermal LD50 after a single skin
exposure of rabbits was 8.75 (8.3-9.2) ml/kg body weight (Smyth et
al., 1969; California State Water Resources Control Board, 1983).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
Groups of 5 male and 5 female B6C3F1 mice were
administered (by gavage) 0, 125, 250, 500, 1000, or 2000 mg 1,2-
dichloropropane (99.4%) per kg body weight, in corn oil. The doses
were given for 14 consecutive days (range-finding study) followed by
1 day of observation. All male mice in the 1000 and 2000 mg/kg
groups and female mice in the 2000 mg/kg group died. Also 3 out of 5
males receiving 500 mg/kg and 4 out of 5 females receiving 1000
mg/kg died. No growth inhibition was seen in the surviving animals
(NTP, 1986).
Groups of 10 male and 10 female B6C3F1 mice were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks, at doses of 0, 30, 60, 125, 250, or 500
mg/kg body weight. Mortality was not significantly increased, and
mean body weight changes were not dose-related. No compound-related
histopathological changes were found at the highest dose level (NTP,
1986).
8.2.1.2 Rat
Groups of 5 male and 5 female F344/N rats were administered
1,2-dichloropropane (99.4%) at 0, 125, 250, 500, 1000, and 2000
mg/kg body weight, in corn oil, by gavage, for 14 consecutive days
(range-finding study) followed by 1 day of observation. All rats
receiving the highest dose level died. Growth inhibition was seen in
the surviving animals in the 1000 mg/kg group. At necropsy, renal
medullae were red in the 2000 mg/kg group, not in lower dose levels
(NTP, 1986).
Groups of 10 male and 10 female F344/N rats were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks at doses of 0, 60, 125, 250, 500, or 1000
mg/kg body weight. All animals administered 1000 mg/kg and half of
the males administered 500 mg/kg died. Growth inhibition was seen in
the 500 mg/kg group. Centrilobular congestion of the liver occurred
in the 1000 mg/kg group and 2 females of this group showed hepatic
changes and centrilobular necrosis. Lower dose levels did not
produce effects (NTP, 1986).
A 2-week study of 1,2-dichloropropane (99.9%) was conducted
using groups of 10 Fischer 344 rats/sex to select doses for a
subsequent 13-week study. Dose levels were 0 (corn oil), 300, or 500
mg/kg body weight per day (by gavage) for 14 days. Data were
obtained on body weight, clinical effects, body temperature,
functional observational battery, motor activity, haematology,
liver, kidney, and spleen weights, gross pathology and histological
examination of the liver and kidneys. In both groups, transient
clinical effects (tearing, blinking, and lethargy) were seen, and
body weights were significantly decreased. The body temperatures of
treated animals in both groups, recorded 1 h after dosing on day 13,
were decreased by 0.3-0.5 °C. No effects on motor activity and
haematology were noted. Liver and kidney weights were increased and
spleen weight decreased. Histopathological changes (prominent
nucleoli of hepatocytes in the centrilobular region, degeneration
and necrosis of the liver cells) were found in the livers of animals
in both the treated groups (Gorzinski & Johnson, 1989).
In an oral toxicity study, adult male Sprague-Dawley rats were
administered 0, 100, 250, 500, or 1000 mg 1,2-dichloropropane/kg
body weight, in corn oil (by gavage), once a day, for up to 10
consecutive days. Some rats were killed at each dose level 24 h
after 1, 5, and 10 daily doses. After dosing, changes in body weight
and clinical toxic signs were assessed. In addition, measurements of
clinical chemistry parameters, urinary levels of glucose, alkaline
and acid phosphatase and N-acetyl glycosaminidase activities, and
the histopathology of major organs, liver enzyme levels, as well as
levels of non-protein sulfhydryls in the liver and kidneys were
determined. Definite central nervous system depression and body
weight loss, and increased renal nonprotein sulfhydryl (NPS) levels
were seen at 250 mg/kg. Liver damage (both morphological and enzyme
changes) was observed only at the 500 and 1000 mg/kg dose levels. In
this study, "resistance" to 1,2-dichloropropane hepatotoxicity
developed over the 10 consecutive days of exposure, as reflected by
progressively lower serum enzyme levels and by decreases in the
severity and incidence of toxic hepatitis and periportal
vacuolization. Nucleolar enlargement in hepatocytes, however, was
observed at all dose levels at both 5 and 10 days. There were a
number of manifestations of haemolytic anaemia in various tissues,
but no evidence of nephrotoxicity. On the basis of the parameters
examined, a NOEL of 100 mg/kg body weight can be established
(Bruckner et al., 1989).
In a similar oral study, male Sprague-Dawley adult rats were
treated with 0, 100, 250, 500, or 750 mg 1,2-dichloropropane/kg body
weight, in corn oil, by gavage, 5 times weekly for up to 13 weeks.
Over one-half of the animals in the 750 mg/kg group died within 10
days. Histopathological changes included mild hepatitis and splenic
haemosiderosis, adrenal medullary vacuolization and cortical
lipidosis, testicular degeneration and a reduction in sperm, and an
increased number of degenerated spermatogonia in the epididymis.
Similar testicular and epididymal degenerative changes were observed
in some animals in the 500 mg/kg group after 13 weeks of dosing.
While no deaths occurred in the 100 or 250 mg/kg groups, more than
50% in the 500 mg/kg group had died by 13 weeks. A dose-dependent
decrease in body weight gain was observed. 1,2-Dichloropropane
exhibited very limited hepatotoxic potential and no apparent
nephrotoxic potential in this 13-week study (Bruckner et al., 1989).
8.2.2 Inhalation
8.2.2.1 Mouse
B6C3F1 mice (aged 6-8 weeks) were exposed to target
concentrations of 1,2-dichloropropane (99.9%) of 0, 69.9, 233, or
699 mg/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. There were no
effects on haematology, gross pathology, and histopathology at
concentrations as high as 699 mg/m3, in this study (Nitschke et
al., 1988).
8.2.2.2 Rat
Fischer 344 rats (aged 6-8 weeks) were exposed to target
concentrations of 0, 69.9, 233, or 699 mg 1,2-dichloropropane
(99.9%)/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. The body weights
of rats exposed to 233 and 699 mg/m3 were significantly decreased.
Minimal effects were observed microscopically in the nasal tissues
of rats exposed to 233 and 699 mg/m3. A few rats exposed to 69.9
mg/m3 also had slight thickening of a small portion of the
respiratory nasal mucosa. No treatment-related effects were noted in
haematology, clinical chemistry, or urinalysis, even at 699 mg/m3
(Nitschke et al., 1988). A NOEL of 74.5 mg/m3 can be established.
8.2.2.3 Rabbit
New Zealand white rabbits (age 6-7 months) were exposed to 1,2-
dichloropropane at 0, 699, 2330, or 4660 mg/m3 for 6 h/day, 5
days/week, for 13 weeks. The mean actual concentrations were 0, 694,
2204, or 4436 mg/m3. Minimal effects on nasal tissues were present
in male rabbits only at the 4660 mg/m3 level. The primary effects,
decreased red blood cell parameters (red cell blood count,
haemoglobin, and packed cell volume), were observed in male rabbits
exposed to 699 (minimal effects) - 4660 mg/m3, and in female
rabbits exposed to 2330 and 4660 mg/m3 (Nitschke et al., 1988). No
NOEL has been established.
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
Groups of 30 male and 30 female Sprague-Dawley rats each were
provided with drinking-water containing 1,2-dichloropropane (99.9%)
at concentrations of 0, 0.24, 1, or 2.4 g/litre (w/v) (equivalent to
0, 33.6, 140, or 336 mg/kg body weight) over 2 generations. A
concentration of 2.4 g/litre represented the maximum practicable
attainable concentration, based on solubility. Adult rats were
evaluated for body weights, water and feed consumption, reproductive
performance, and gross pathological and histological changes. The
litters were evaluated for size, neonatal growth, and survival.
Decreases in water consumption reflective of rejection because of
unpalatability, were observed at all levels tested in both sexes in
the F0 and F1 generations. These decreases in water consumption
resulted in significantly lower body weights in both generations
administered 2.4 g/litre. These differences in water consumption and
body weights were also evident among the females during gestation
and/or lactation. The 0.24 g/litre dose level had a minor effect on
water consumption and body weights, but no adverse effects on the
animals. No treatment-related gross pathological changes were noted
in any dose group and the histological changes were limited to
increased hepatocellular granularity in both sexes in both
generations at all dose levels.
There were no histological changes in the reproductive tracts
of either sex in either generation. Reproductive function, measured
by fertility and litter size, was unaffected. The decreases in water
consumption among females at 2.4 g 1,2-dichloropropane/litre
resulted in significantly lower neonatal body weights and slightly
increased neonatal mortality in their litters. These neonatal
effects were considered secondary to the substantial decreases in
maternal water consumption, rather than a direct effect of the
substance. There were no neonatal effects at the 2 lower
concentrations. The NOAEL for adults is 0.24 g/litre, and the
reproductive NOAEL is 1 g/litre (equivalent to 140 mg/kg body
weight) (Kirk et al., 1990).
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
Groups of 30 Sprague-Dawley rats were administered 1,2-
dichloropropane (99.9%) in corn oil, by gavage, on gestation days 6-
15 at dose levels of 0, 10, 30, or 125 mg/kg body weight per day.
Parameters evaluated included maternal body weight and weight gain,
feed and water consumption, fetal weights, and fetal morphology. The
highest dose level produced transient decreases in respiration,
movement, muscle tone and extensor thrust reflex, and increases in
salivation and lacrimation, within 1 h of dosing on day 6 of
gestation. A decrease in the frequency of these clinical symptoms
occurred on the second day. In rats, 1,2-dichloropropane produced
transient central nervous system depression and decreased maternal
body weight gain and feed consumption at 125 mg/kg body weight.
Fetal effects in rats were limited to a significant increase in the
incidence of delayed ossification of the bones of the skull in the
125 mg/kg group, secondary to maternal effects. A level of 30 mg/kg
was considered the NOEL for maternal and fetal effects (Kirk et al.,
1989; Hanley et al., 1990).
8.3.2.2 Oral (rabbit)
Groups of 18 New Zealand white rabbits were administered 1,2-
dichloropropane (99.9%) at dose levels of 0, 15, 50, or 150 mg/kg
body weight per day on gestation days 7-19. On day 28 of gestation,
Caesarean section was performed and the fetuses evaluated.
Parameters evaluated included maternal body weight and weight gain,
haematology, fetal body weights, and fetal morphology. In maternal
rabbits, a dose of 150 mg/kg per day produced anorexia, significant
decreases in weight gain, and anaemia, with microscopic examination
revealing slight to moderate anisocytosis, poikilocytosis, and/or
polychromasia, indicative of a regenerative anaemia. The only fetal
effect in rabbits was a significant increase in the incidence of
delayed ossification of the bones of the skull at the highest dose
level, secondary to maternal effects. No effects were observed in
the 15 and 50 mg/kg dose groups. The NOEL for both maternal and
fetal effects was 50 mg/kg body weight (Hanley et al., 1989a).
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
1,2-Dichloropropane was tested for its mutagenic activity in
Salmonella typhimurium, Saccharomyces cerevisiae with, and without
metabolic activation, and in Aspergillus nidulans and
Streptomyces coelicolor. Results in most studies on S.
typhimurium TA100 and TA1535 were positive, but negative results
were obtained with TA98, TA1537, and TA1538, S. cerevisiae, and
S. coelicolor. In Aspergillus nidulans, a forward mutation to 8-
azaguanine resistance and methionine suppression was found with S9
activation.
The results are summarized in Table 20.
Table 20. Mutagenicity tests with 1,2-dichloropropane on microorganisms
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Salmonella typhimurium
TA100, TA1535 1,2-DCP 10-50 mg plate S9 mix/none + De Lorenzo et al.
TA1978 S9 mix/none - (1977)
TA100 1,2-DCP (65% 62.5-8000 suspension S9 mix + Priston et al.
TA1535 1,2-DCP + 25% µg/mld S9 mix/none + (1983)
1,3-DCPropene)
TA98, TA1537 S9 mix/none -
TA100 1,2-DCP 1, 10, plate S9 mix/none - Stolzenberg & Hine
100 µmola (1980)
TA100, TA1535 1,2-DCP 1-10 µlitre plate S9 mix/none + Carere & Morpurgo
(1981)
TA98, TA1537, TA1538 S9 mix/none -
TA100, TA1535 1,2-DCP 0.33-10 mg plate S9 mix/none + Haworth et al. (1983)
(rat/hamster)
TA98, TA1537 S9 mix/none -
(rat/hamster)
TA100, TA1535, TA1537 1,2-DCP (99%) 33-2000 µg plate S9 mix - NTP (1983)
S9 none (+)
TA98 S9 mix/none -
(rat/hamster)
Saccharomyces cerevisiae
JD1 1,2-DCP (65% 62.5-8000 - S9 mix - Priston et al.
1,2-DCP + 25% µg/mld S9 none (+) (1983)
1,3-DC propene)
Table 20 (contd)
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Streptomyces coelicolarb 1,2-DCP 2-100 µlitre plate none - Carere & Morpurgo
A3 (1981)
Aspergillus nidulansc 100-400 µlitre plate none + Carere & Morpurgo
(1981)
Aspergillus nidulans 1,2-DCP (99%) 154 mmol/litre - Crebelli et al.
(1984)
a The highest dose level, 100 µmol/plate gave complete inhibition of bacterial growth.
b Resistance to streptomycin.
c Resistance to 8-azaguanine.
d Concentrations of 1000 µg/ml and higher were cytotoxic.
Crebelli et al. (1984) studied the induction of somatic
segregation in Aspergillus nidulans. Induction of haploidization,
mitotic non-disjunction, and mitotic crossing-over was studied in
heterozygous colonies exposed to 1,2-dichloropropane, 99%. No
significant rise in frequency of any kind of segregated sectors was
found. The concentration that was used was 154 mmol/litre.
8.4.1.2 Mammalian cells
In cytogenetic studies using Chinese hamster ovary cells, 1,2-
dichloropropane (99.4%) caused both chromosome aberrations and
sister chromatid exchanges. Dose levels tested were 0.46-1.50 and
0.113-1.13 mg/ml, respectively (Galloway et al., 1987).
Priston et al. (1983) also studied the ability of 1,2-
dichloropropane (containing 65% 1,2-dichloropropane and 25% 1,3-
dichloropropane) to induce chromosome damage using rat liver (RL4)
cells, in concentrations of 5-20 µg/ml. An indication for a small
increase in the frequency of chromatid gaps, chromatid and
chromosome aberrations, was noticed, but only in the presence of
cytotoxic effects. The Task Group considered this study inadequate.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
test in vitro in Chinese hamster V79 cells to evaluate the effect
of 1,2-dichloropropane (99%). The concentrations tested were 0
(DMSO), 1.0, 3.3, and 10.0 mmol/litre without S9 mix. A dose-related
increase in SCEs was observed. With S9 mix and the same
concentrations, the SCEs frequency was increased but was less than
without S9 mix.
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
Woodruff et al. (1985) tested 1,2-dichloropropane at 0 and 4200
mg/litre by injection in germ cells and 0 and 33 552 mg/m3 by
inhalation in Drosophila melanogaster, using the sex-linked
recessive lethal mutation for their mutagenicity. The results by
injection and inhalation were negative.
8.4.2.2 Dominant lethal test
Groups of 30 male Sprague-Dawley rats were given 1,2-
dichloropropane (99.9%) in the drinking-water at concentrations of
0, 0.24, 1, or 2.4 g/litre, continuously, for a period of
approximately 14 weeks, as part of a combined reproduction/ dominant
lethal study (section 8.3.1). Exposed males (F0) were used for a
dominant lethal test following completion of the breeding for the
F1 litters of the reproduction study. They were mated with pairs
of naive, untreated adult females for 2 successive periods of 1 week
each. A separate, positive control group of 30 male rats was
administered a single oral dose of 100 mg cyclophosphamide/kg body
weight, 48 h prior to mating with untreated females. The uterine
contents of the females were evaluated for evidence of dominant
lethal effects as manifested by an increase in the resorption rate.
Among 1,2-dichloropropane-treated males, concentration-related
decreases in water consumption were noted at all levels and
decreased body weights were noted in males given 1 or 2.4 g/litre in
the water. Mating performance was unaffected in these animals.
Resorption rates among these groups revealed that the weekly values
for the females mated to treated males ranged from 2.2 to 8.1%, well
within the historical control range. Resorption rates among the
concurrent controls were low, ranging from 3.5 to 5.4%. The
significant increases from the concurrent control values, identified
during the first week of breeding in the resorption rates in the
groups given 0.24 or 2.4 g/litre, were considered to be within the
normal variation. Cyclophosphamide resulted in a 10-fold increase in
the resorption rate. 1,2-Dichloropropane was not mutagenic in a
dominant lethal assay in male Sprague-Dawley rats exposed
continuously to concentrations of up to 2.4 g/litre in the drinking-
water (Hanley et al., 1989b).
8.4.2.3 Miscellaneous
Perocco et al. (1983) studied the tritiated thymidine uptake
and cell viability in human lymphocyte cultures grown with, or
without, rat liver metabolizing system. Both the [3H]TdR uptake
and the percentage of viable cells showed values very similar to
control values. The 1,2-dichloropropane concentrations used ranged
from 10-2 to 10-4 mmol/litre.
8.5 Carcinogenicity
8.5.1 Oral (mouse)
Groups of 7 to 9-week-old hybrid B6C3F1 mice (50 males
and 50 females) were administered 0, 125, or 250 mg 1,2-
dichloropropane (99.4%)/kg body weight, in corn oil, by gavage, 5
days/week for 113 weeks. No influence on growth was observed. The
survival of the female animals at the highest dose level was
significantly decreased. The incidence of non-neoplastic lesions
showed lesions of the spleen (haemosiderosis and haematopoiesis were
increased) in female mice at the highest dose level.
The incidences of neoplastic lesions are summarized in Table
21.
Table 21. The incidence of neoplastic lesions in a mouse study with 1,2-dichloropropanea
Sex Control 125 mg/kg 250 mg/kg
Adenoma (liver) male 7/35 (20%) 9/33 (27%) 15/35 (43%)
Carcinoma (liver) male 8/35 (23%) 10/33 (30%) 9/35 (26%)
Adenoma and carcinoma (liver) male 15/35 (43%) 18/33 (55%) 24/35 (69%)b
Adenoma (liver) female 1/35 (3%) 5/29 (17%) 5/26 (19%)b
Carcinoma (liver) female 1/35 (3%) 2/29 (7%) 2/26 (8%)
Adenoma and carcinoma (liver) female 2/35 (6%) 7/29 (24%)b 7/26 (27%)b
Follicular cell adenoma and female 1/34 (3%) 0/27 (0%) 5/24 (21%)b
carcinoma (thyroid)
Alveolar/bronchiolar adenoma female 5/35 (14%) 0/29 (0%) 1/26 (4%)
or carcinoma (lung)
Squamous cell male 0/50 (0%) 1/48 (2%) 3/49 (6%)
papillomas (forestomach) female 0/50 (0%) 2/50 (4%) 2/50 (4%)c
a From: NTP (1986).
b P = < 0.05 - > 0.001.
c One high-dose female mouse had a squamous cell carcinoma.
On the basis of the results of this study, the NTP concluded
that 1,2-dichloropropane induces an increased incidence of liver
tumours in male and female B6C3F1 mice (Haseman et al., 1984;
NTP, 1986). However, the Task Group noted that the incidences of
liver adenomas and carcinomas in the treated groups were within the
historical ranges of this species (Haseman et al., 1985). The
increased incidence of thyroid tumours is equivocal.
8.5.2 Oral (rat)
Groups of 50 male and 50 female F344/N rats (aged 7-9 weeks)
were administered, by gavage, 0, 125 or 250 (female) and 0, 62, or
125 mg (male) 1,2-dichloropropane (99.4%)/kg body weight, in corn
oil, 5 days/week for 103 weeks. The highest dose level showed growth
depression in both sexes. Survival of female rats at the high dose
level was significantly lower. The incidence of non-neoplastic
lesions was not significantly different from that in the controls,
except for an increased incidence of foci of clear cell change and
of necrosis in the liver in high-dose female rats.
The incidences of neoplastic lesions are summarized in Table
22. Apart from these tumours, squamous cell papillomas of the
forestomach were found in 1 control male and 1 female rat. In high-
dose females, there was an increased incidence of mammary gland
adenocarcinomas, but not of mammary gland adenomas. Apart from these
tumours, squamous cell papillomas of the fore-stomach were found in
two high-dose females (not significantly increased compared to
controls). There were no effects on tumour incidences in male rats
(Haseman et al., 1984; NTP, 1986).
8.6 Factors modifying toxicity
Weanling Wistar rats, fed for several weeks on low protein
choline-deficient diets, were more susceptible to the effects of
inhalation of 4660 mg 1,2-dichloropropane/m3 than rats on a
control diet. This increased susceptibility could be corrected by
dietary supplements of 1-methionine or 1-cysteine plus choline
chloride (Heppel et al., 1946).
8.7 Special studies
8.7.1 Liver
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight, in corn oil, for 5 days (once daily) or for 4 weeks (five
days/week), or a single dose of 0, 50, 100, or 250 mg/kg body
weight, to investigate the biochemical and histological liver
changes. Reduced glutathione (GSH), glutathione- S-transferase,
cytochrome P450, and protein contents were measured. A dose-
Table 22. The incidence of neoplastic lesions in a rat study with 1,2-dichloropropanea
Sex Control 62 mg/kg male 125 mg/kg male
125 mg/kg female 250 mg/kg female
Fibroadenoma female 14/37 (38%) 20/43 (47%) 5/16 (31%)
(mammary gland)
Adenocarcinoma female 1/37 (3%) 2/43 (5%) 4/16 (25%)b
(mammary gland)
Endometrial stromal female 8/37 (22%) 14/42 (33%) 6/16 (38%)b
polyps (uterine
tumours)
Liver tumours male 3/50 (6%) 3/50 (6%) 2/50 (4%)
female 0/50 (0%) 0/50 (0%) 0/50 (0%)
Islet cell adenoma male 4/38 (11%) 1/42 (2%) 5/41 (12%)
and carcinoma
(pancreas)
a From: NTP (1986).
b P = < 0.05.
dependent decrease in liver-reduced glutathione was observed after a
single injection and a dose-dependent increase after 4 weeks. The
liver biochemical pattern after 4 weeks, characterized by a decrease
of cytochrome P450 and by an increase in reduced glutathione and
glutathione- S-transferase activity, suggests a hyperplastic
evolution of the liver cells, probably a repair mechanism induced by
the early depletion of glutathione. Histologically, the alterations
confirm the regenerative nature (atypical mitosis and hyperplastic
nodules) of the changes (Trevisan et al., 1989).
The hepatotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose-levels of 0, 50, 100, 250, or 500 mg/kg body weight per day,
in corn oil, for 4 weeks. Biochemical changes in the liver were
demonstrated. Significant findings included reduction of aminopyrine
demethylase activity at 100 mg/kg or more, increased levels of
reduced glutathione and glutathione- S-transferase activity at 50
mg/kg or more, and decreased cytochrome P450 activity at 500 mg/kg.
The activity of aniline hydroxylase was not affected. Duplicate
groups of rats treated with 1,2-dichloropropane, but allowed a
period of 4 weeks of recovery before being subjected to examination,
showed that the induced biochemical changes in the liver were
completely reversible (Trevisan et al., 1991).
1,2-Dichloropropane toxicity is actually preferentially
mediated by GSH depletion. This is suggested by the fact that GSH
loss is correlated with an increase in the biochemical indices of
liver and renal injury, and with the extent of haemolysis.
Pretreatment of 1,2-dichloropropane-intoxicated rats with
buthionine-sulfoximine (BSO) (depleting GSH) markedly increased the
overall mortality. Furthermore, the administration of the GSH
precursor N-acetyl-cysteine (NAC), preventing GSH depletion,
reduced the damage in target tissues, as demonstrated by a smaller
increase in some biochemical indices of cell injury and a smaller
degree of haemolysis. A possible explanation for these findings is
that, when the GSH level falls below a certain threshold value,
irrespective of the causative agent, a series of common reactions is
triggered, inducing peroxidation of membrane lipids, disturbances of
Ca2 homeostasis, and DNA damage, which quickly lead to
irreversible liver injury. Furthermore, it is also possible that
electrophilic metabolites of 1,2-dichloropropane, formed in the
absence of GSH, could directly attack a variety of cell
macromolecules the function of which is essentially to ensure the
physiological survival of the hepatocytes (Mitchell et al., 1973;
Bellomo & Orrenius, 1985; Casini et al., 1987; Orrenius et al.,
1989).
Male Wistar rats were exposed (by gavage) to a single oral dose
of 55 mg 1,2-dichloropropane/kg body weight in propylene glycol. The
animals were killed 1-6 days after exposure. Glutathione (GSH),
lipid peroxidation and protein of liver homogenate were measured.
Reduction of hepatic GSH and total protein and enhanced hepatic
lipid peroxidation still persisted 6 days after 1,2-dichloropropane
administration (Di Nucci et al., 1988).
Groups of rats were treated orally, by gavage, with single
doses of 1,2-dichloropropane ranging from 55 to 400 mg/kg body
weight. 1,2-Dichloropropane was no longer detectable in the blood 24
h after dosing at any dose level (Di Nucci et al., 1988).
8.7.2 Kidneys
Renal failure caused by 1,2-dichloropropane has been reported
by several authors who found an increase in serum creatinine and
urea in intoxicated animals and humans and fatty degeneration or
acute tubular damage in the kidney parenchyma (Heppel et al., 1946,
Ponticelli et al., 1968; Pozzi et al., 1985; Imberti et al., 1987).
Imberti et al. (1990) suggested that, on the basis of the
demonstration of 1,2-dichloropropane-induced depletion of kidney
GSH, it may be postulated that the biochemical mechanisms involved
in liver toxicity may also apply to the kidneys (Brezis et al.,
1983). In addition, since most of the glutathione and cysteine
conjugates of 1,2-dichloropropane are recovered in the urine, a
direct toxicity of the conjugates to the kidneys seems to play an
important role, as previously demonstrated for other conjugates
(Stevens et al., 1988).
It has been demonstrated that GSH plays an essential role in
the maintenance of the physiological structure of the erythrocyte,
preventing the formation of inter-protein or intra-protein
disulfides within the membrane skeleton. Depletion of erythrocyte
glutathione causes an increased fragility of this cell and
subsequent haemolysis. The involvement of such a mechanism in 1,2-
dichloropropane-induced haemolysis in intoxicated animals is
supported by the demonstration of a concomitant loss in erythrocyte
GSH and a correlation between these 2 phenomena (Imberti et al.,
1990).
Nephrotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose levels of 0, 50, 100, 250, or 500 mg/kg body weight per day
for 4 weeks. Biochemical and histopathological alterations of the
kidneys were demonstrated. Kidney pathology involved a decrease in
the activity of angiotensin-converting enzyme in the proximal tubule
brush border and fraying of microvilli with epithelial coagulative
necrosis. Duplicate groups of rats treated similarly with 1,2-
dichloropropane, but allowed a period of 4 weeks of recovery before
being subjected to examination, showed that the treatment-induced
biochemical changes in the kidneys were completely reversible
(Trevisan et al., 1988, 1991).
8.7.3 Central nervous system
In the study of Gorzinski & Johnson (1989), in which Fischer
344 rats were treated with 0, 300, or 500 mg 1,2-dichloropropane per
kg body weight, by gavage, for 14 days, motor activity and a
functional observational battery (unusual responses in behaviour,
presence of convulsions, tremors, etc., and sensory function) were
evaluated (section 8.2.1.2). No effects were seen on these
parameters.
Specific tests in groups of 15 Fischer 344 rats/sex given, by
gavage, 0 (corn oil), 20, 65, or 200 mg 1,2-dichloropropane
(99.9%)/kg body weight, 5 days/week for 13 weeks, included monthly
evaluation of a functional observation battery, hind limb grip
strength, and motor activity; a comprehensive neuropathology study
was carried out at the end of the study. Clinical observations
included measurement of body weight gain and body temperature. Body
weights were decreased for male rats given 200 mg/kg body weight.
The body temperature was within normal limits, except in the females
given 200 mg/kg, which showed a slightly lower temperature. No
functional effects were noted. No gross or histopathological effects
on either the central or peripheral nervous system were observed
(Johnson & Gorzinski, 1988).
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
Ingestion of cleaning solvent (50 ml) containing 1,2-
dichloropropane (other components not known) by a man produced coma
followed by delirium, irreversible shock, cardiac failure, and
death. Histopathologically, centri- and medio-lobular hepatic
necrosis was found (Larcan et al., 1977).
Two cases of disseminated intravascular coagulation syndrome
(DIC) have been described in association with acute intoxication by
1,2-dichloropropane. Effects on the central nervous system, liver,
and kidney functions were also observed, but no details were given
(Perbellini et al., 1985).
Pozzi et al. (1985), from Italy, reported clinical observations
on 3 other hospitalized persons with 1,2-dichloropropane poisoning.
Two out of the 3 persons ingested the substance, while the third
person was exposed through inhalation. The clinical symptoms were
similar, despite different routes of exposure. All 3 suffered from
acute renal and hepatic damage. Kidney biopsy, carried out on 1
person, showed acute tubular necrosis. Haemolytic anaemia and
disseminated intravascular coagulation were noted in all 3 patients,
1 of whom died on the seventh day. The other patients recovered
after treatment.
Toxic hepatitis with portal hypertension has been described in
a 49-year-old man, who ingested 1,2-dichloropropane in an attempted
suicide (Thorel et al., 1986).
9.2 Occupational exposure
Baruffini et al. (1989) studied 10 cases of 1,2-dichloropropane
dermatitis in the period 1985-88. Patients were painters or metal-
workers in the engineering industry and they all had known contact
with mixtures of solvents containing 10-40% 1,2-dichloropropane. On
examination, the workers had itchy erythematous, oedematous,
vesicular lesions on the fingers and dorsa of the hands. Two
subjects also had scaling and fissuring of the palms. Cessation of
exposure produced quick resolution of the dermatitis in all
subjects. Patch tests were carried out. As controls, 120 subjects
were similarly tested with 1,2-dichloropropane. All patients showed
a positive response to concentrations of 2% 1,2-dichloropropane or
more (allergic contact dermatitis). The results of the tests on the
control subjects were negative.
Grzywa & Rudzki (1981) described cases of dermatitis in 2 women
(47 and 55 years old), in a group of 60 persons, who had been
exposed to Silform aerosols for 6 and 4 years, respectively. Silform
aerosols contained 7.4, 11.0, or 12.7% 1,2-dichloropropane;
methylsilicone oils, and Freon. The patch tests that were carried
out were positive for 1,2-dichloropropane, but negative for
methylsilicone oils.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloropropane was considered by working groups of the
International Agency for Research on Cancer (IARC) in 1986 (IARC,
1986) and in 1987 (IARC, 1987). In the updating of 1987, it was
evaluated as follows: "There is limited evidence for the
carcinogenicity of 1,2-dichloropropane in experimental animals.
There are no data in humans. The agent is not classifiable as to its
carcinogenicity to humans (Group 3)".
WHO (in preparation) has proposed a provisional guideline value
for drinking-water of 20 µg 1,2-dichloropropane/litre.
PART C
ENVIRONMENTAL HEALTH CRITERIA
FOR
MIXTURES OF DICHLOROPROPENES
AND DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MIXTURES OF DICHLOROPROPENES AND
DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on humans
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
4.2 Water
4.3 Soil
4.3.1 Microbial transformation
4.3.2 Loss under field conditions
4.3.3 Soil function
4.4 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Groundwater
5.2 Occupational exposure
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Terrestrial microorganisms
7.1.1.1 Effects on nitrification
7.1.1.2 Recovery studies with microorganisms
7.2 Aquatic organisms
7.2.1 Invertebrates
7.2.2 Fish
7.3 Terrestrial organisms
7.3.1 Birds
7.3.2 Soil fauna
7.3.3 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.2 Inhalation
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
8.3.2 Sensitization
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
8.5.1.2 Inhalation
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
8.6.2 In vivo studies
9. EFFECTS ON HUMANS
9.1 General population exposure
9.2 Occupational exposure
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
The technical mixture of dichloropropenes and dichloropropane
(abbreviated in this text to "MIX D/D") is a clear amber liquid with
a pungent odour; it has a vapour pressure of 35 mmHg at 20 °C, and
is soluble in halogenated solvents, esters, and ketones.
"MIX D/D" typically contains not less than 50% 1,3-
dichloropropene (ratio of cis- and trans-isomers approximately
1:1), the other main constituents being 1,2-dichloropropane and
related compounds. It was widely used as a soil nematocide before
planting.
The environmental transport, distribution, and fate of the
major constituents of "MIX D/D" in air, water, and soil is described
in the sections 4 of the parts of this EHC monograph that deal with
1,3-dichloropropene and 1,2-dichloropropane.
There is a significant potential for 1,2-dichloropropane
derived from "MIX D/D" to leach from the soil and contaminate well
water and groundwater. In an irrigation bore (68 m deep) in Western
Europe, mean 1,2-dichloropropane concentrations at different depths
ranged between 0.8 and 8.5 µg/litre, and the maximum concentration
recorded was 165 µg/litre.
Significant uptake of the constituents of "MIX D/D" by crops is
unlikely (see other parts of this EHC monograph). Bioaccumulation of
the constituents of "MIX D/D" is also unlikely because of their low
log P octanol/water partition coefficient and their relatively high
water solubility.
1.1.2 Kinetics and metabolism
No metabolic studies have been carried out on "MIX D/D". The
two major components, 1,3-dichloropropene and 1,2-dichloropropane,
are rapidly eliminated, primarily in the urine and, to a lesser
extent, via expired air. The components of "MIX D/D" are metabolized
by oxidative and conjunction pathways. The major urinary metabolites
are mercapturic acids.
1.1.3 Effects on organisms in the environment
"MIX D/D" is moderately toxic for fish; 96-h LC50 values
range between 1 and 6 mg/litre. The 1,3-dichloropropene is largely
responsible for the toxicity of "MIX D/D".
When used at recommended application rates, the main effects of
"MIX D/D" are a transient (< 7 days) reduction in soil fungi and
inhibition of the oxidation of ammonium ions to nitrate. "MIX D/D"
is toxic for nitrifying bacteria. Soon after "MIX D/D" disappears
from the soil, recolonization by bacteria takes place. In field
trials, "MIX D/D" (applied at 600 litre/ha) killed soil
invertebrates. Recolonization times ranged between 6 and 24 months.
"MIX D/D" is highly phytotoxic.
1.1.4 Effects on experimental animals and in vitro test
systems
The acute toxicity of "MIX D/D" for laboratory animals is
moderate to high. The oral LD50 values in rats and mice range from
132 to 300 mg/kg body weight. The dermal LD50 values for rats and
rabbits are 779 and 2100 mg/kg body weight, respectively. The
inhalation LC50 (4 h) for rats is approximately 4540 mg/m3.
Acute exposure results in clinical signs associated with central
nervous system depression. "MIX D/D" is a severe eye and skin
irritant and it is a moderate dermal sensitizer.
The results of the available short-term toxicity studies in
rats and dogs are inadequate to assess properly the toxicity
potential of "MIX D/D", because the relatively low doses tested do
not demonstrate any biologically significant effects. Several short-
term inhalation (whole-body) studies have been conducted in rats.
"MIX D/D" at levels up to 145 mg/m3 does not cause any toxic
effects. At levels of 1362 mg/m3 or higher, toxic effects
associated with central nervous system depression are evident. An
exposure to 443 mg/m3 for 10 weeks leads to reduced body weight
gain and increased absolute kidney weight.
An oral teratology study in rats was inadequate for assessment
of the teratological potential of "MIX D/D".
In an inhalation rat study to investigate male and female
fertility, no effects were found at dose levels up to 443 mg/m3
for 10 weeks. Complete evaluation of reproductive effects of "MIX
D/D" was not possible owing to inadequate protocol designs.
"MIX D/D" is mutagenic in Salmonella typhimurium strains
TA100 and TA1535, as well as in Escherichia coli WP2 HCR, without
metabolic activation. There was no such effect in Salmonella
strains TA98, TA1537, and TA1538.
In a long-term study on rats fed diets containing up to 120 mg
"MIX D/D" per kg (equivalent to 6 mg/kg body weight) for 2 years, no
toxic or carcinogenic effects were seen.
1.1.5 Effects on humans
"MIX D/D" is no longer widely used, and, thus, exposure of the
general population via air, water, and food is unlikely.
The exposure of workers filling drums and of field applicators
was generally below 4.5 mg 1,3-dichloropropene/m3 when recommended
procedures were used; in other situations, levels up to 36.32
mg/m3 have been measured.
One case of acute fatal poisoning has been reported following
accidental ingestion of "MIX D/D".
Several cases of contact dermatitis and skin sensitization due
to "MIX D/D" exposure have been reported.
1.2 Conclusions
- General population. As "MIX D/D" is no longer widely used,
the exposure of the general population to 1,3-dichloropropene
via air, water, and food is negligible, but, in certain areas,
exposure to 1,2-dichloropropane may occur when groundwater is
contaminated.
- Occupational exposure. Filling operations and field
applications of "MIX D/D" can lead to exposure of operators to
1,3-dichloropropene that exceed maximum allowable
concentrations, especially under warm climatic conditions.
- Environment. "MIX D/D" is unlikely to reach biologically
significant levels in either the terrestrial or the aquatic
environment when used at the recommended rate. Lasting adverse
effects on organisms in the environment are unlikely to occur.
1.3 Recommendations
- "MIX D/D" should not be used as a soil fumigant because of
potential leaching to groundwater.
- Monitoring of residues in surface water and groundwater should
be carried out in areas where "MIX D/D" has been used.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
The technical mixture of dichloropropenes and dichloropropane
(abbreviated to "MIX D/D") contains not less than 50% of the cis-
and trans-isomers (ratio approximately 1:1) of 1,3-dichloropropene
and the other main constituent was 1,2-dichloropropane. Yang (1986)
described a commercial "MIX D/D" containing 25% cis-
dichloropropene, 27% of trans-dichloropropene, 29% 1,2-
dichloropropane, and 19% other related chlorinated hydrocarbons. It
may also contain 1% epichlorohydrin as a stabilizer.
CAS registry number:
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Algae
The EC50 for CO2 uptake by marine algae (Phaeodactylum
tricornutum) was 50 mg/litre. 1,2-Dichloropropane was not found to
exhibit any algistatic or algicidal effects in an acute toxicity
study on Selenastrum capricornutum, though the problems of losses
of 1,2-dichloropropane from the test flasks, made it impossible to
calculate EC values (Dynamic Corporation, 1991).
A general trend of decreasing algal population growth with
increasing nominal concentrations of 1,2-dichloropropane was
observed in an acute toxicity test on Skeletonema costatum.
Because the concentrations measured for each nominal value were
variable, it was not appropriate to determine EC values. A NOEL of
18 mg/litre was determined, though it was not possible to
distinguish algistatic from algicidal effects (Dynamic Corporation,
1991).
7.1.2 Invertebrates
The acute toxicity of 1,2-dichloropropane for non-target
invertebrates is summarized in Table 18; 1,2-dichloropropane has a
48-h LC50 of 50-100 mg/litre for these aquatic organisms. No
discernible effects on Daphnia magna were found at concentrations
of less than 22 mg/litre (Leblanc, 1980).
Table 18. Acute toxicity of 1,2-dichloropropane in non-target
aquatic insects and crustacea
Species Temperature 48-h LC50 References
(°C) mg/litre
Barnacle nauplii - 53 Pearson & McConnell
(Elminius moderatus) (1975)
Brown shrimp - > 100 Portmann & Wilson
(Crangon crangon) (1971)
7.1.3 Fish
7.1.3.1 Acute toxicity
The 96-h LC50 of 1,2-dichloropropane for freshwater and
marine fish is 61-320 mg/litre (Table 19).
7.1.3.2 Short-term/long-term toxicity
Two embryo-larval tests have been conducted on Fathead minnow
(Pimephales promelas) exposed to 1,2-dichloropropane. The maximum
no-effect levels were 8.1 and 60 mg/litre, respectively (US EPA,
1980).
In the marine environment, growth inhibition was noted in the
Sheepshead minnow (Cyprinodon variegatus) after 33 days exposure
to 164 mg 1,2-dichloropropane/litre (US EPA, 1980).
A 32-day test to study early life stage toxicity in Fathead
minnows (Pimephales promelas) demonstrated that larval growth and
survival (28-day-old fish) were the most sensitive indicators of
toxic effects. Embryo hatch and larval deformities at hatch were the
least sensitive indicators of toxicity. The 1,2-dichloropropane
(98%) was tested at dose levels of 0, 6, 11, 25, 51, and 110
mg/litre (temperature of the water 25 °C, hardness 45 mg/litre as
CaCO3, pH 7.4). The estimated maximum acceptable toxicant
concentration (MATC) was between 6 and 11 mg/litre (Benoit et al.,
1982).
7.2 Terrestrial organisms
7.2.1 Earthworms
Neuhauser et al. (1985a,b; 1986) studied the toxicity of 1,2-
dichloropropane for 4 species of earthworm (Allolobophora
tuberculata, Eisenia foetida, Eudrilus eugeniae and Perionyx
excavatus) using EEC earthworm artificial soil and a contact
testing procedure (Edwards, 1983). The LC50 values and 95%
confidence limits in the contact test were 84(65-110); 64(59-70);
44(38-51); and 63(56-72) µg/cm2 of filter paper for the 4 species
of earthworms, respectively. In the artificial soil test, the LC50
values were 4272, 4240, 5300, and 3880 mg/kg dry weight artificial
soil, respectively.
7.2.2 Plants
1,2-Dichloropropane is highly phytotoxic.
Table 19. Acute toxicity of 1,2-dichloropropane in fish
Species Size or age Temperature 96-h LC50 References
(°C) mg/litre
Guppy 2-3 months 21-23 3.01 µmol/litref Könemann (1981)
(Poecilia reticulata)
Bluegill sunfish 33-75 mm 23a 320 Dawson et al. (1977)
(Lepomis macrochirus) 0.32-1.2 g 21-23 280b,c Buccafusco et al. (1981)
(220-340)
Fathead minnow 30-35 days 25d,e 140d Wallbridge (1983)
(Pimephales promelas) (131-150)
Tidewater silversides 40-100 mm 20a 240 Dawson et al. (1977)
(Menida beryllina
Dab 15-20 cm - 61 Pearson & McConnell (1975)
(Pleuronectes limanda)
a Hardness 55 mg/litre (as CaCO3), pH 7.6-7.9.
b Tested in well-water, 32-48 mg/litre CaCO3, pH 6.5-7.9, oxygen concentration at the beginning
9.7 to 0.3 mg/litre after 96-h exposure.
c Static system (nominal concentrations).
d Flow-through system.
e Hardness 42-45 mg/litre (as CaCO3) pH 6.7-7.6.
f 7-day LC50 (static system, renewal).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
The acute oral LD50 for the rat has been reported to be 1.9
(1.7-2.1) g/kg body weight and the dermal LD50 after a single skin
exposure of rabbits was 8.75 (8.3-9.2) ml/kg body weight (Smyth et
al., 1969; California State Water Resources Control Board, 1983).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Mouse
Groups of 5 male and 5 female B6C3F1 mice were
administered (by gavage) 0, 125, 250, 500, 1000, or 2000 mg 1,2-
dichloropropane (99.4%) per kg body weight, in corn oil. The doses
were given for 14 consecutive days (range-finding study) followed by
1 day of observation. All male mice in the 1000 and 2000 mg/kg
groups and female mice in the 2000 mg/kg group died. Also 3 out of 5
males receiving 500 mg/kg and 4 out of 5 females receiving 1000
mg/kg died. No growth inhibition was seen in the surviving animals
(NTP, 1986).
Groups of 10 male and 10 female B6C3F1 mice were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks, at doses of 0, 30, 60, 125, 250, or 500
mg/kg body weight. Mortality was not significantly increased, and
mean body weight changes were not dose-related. No compound-related
histopathological changes were found at the highest dose level (NTP,
1986).
8.2.1.2 Rat
Groups of 5 male and 5 female F344/N rats were administered
1,2-dichloropropane (99.4%) at 0, 125, 250, 500, 1000, and 2000
mg/kg body weight, in corn oil, by gavage, for 14 consecutive days
(range-finding study) followed by 1 day of observation. All rats
receiving the highest dose level died. Growth inhibition was seen in
the surviving animals in the 1000 mg/kg group. At necropsy, renal
medullae were red in the 2000 mg/kg group, not in lower dose levels
(NTP, 1986).
Groups of 10 male and 10 female F344/N rats were then
administered 1,2-dichloropropane (99.4%) in corn oil, by gavage, 5
days per week for 13 weeks at doses of 0, 60, 125, 250, 500, or 1000
mg/kg body weight. All animals administered 1000 mg/kg and half of
the males administered 500 mg/kg died. Growth inhibition was seen in
the 500 mg/kg group. Centrilobular congestion of the liver occurred
in the 1000 mg/kg group and 2 females of this group showed hepatic
changes and centrilobular necrosis. Lower dose levels did not
produce effects (NTP, 1986).
A 2-week study of 1,2-dichloropropane (99.9%) was conducted
using groups of 10 Fischer 344 rats/sex to select doses for a
subsequent 13-week study. Dose levels were 0 (corn oil), 300, or 500
mg/kg body weight per day (by gavage) for 14 days. Data were
obtained on body weight, clinical effects, body temperature,
functional observational battery, motor activity, haematology,
liver, kidney, and spleen weights, gross pathology and histological
examination of the liver and kidneys. In both groups, transient
clinical effects (tearing, blinking, and lethargy) were seen, and
body weights were significantly decreased. The body temperatures of
treated animals in both groups, recorded 1 h after dosing on day 13,
were decreased by 0.3-0.5 °C. No effects on motor activity and
haematology were noted. Liver and kidney weights were increased and
spleen weight decreased. Histopathological changes (prominent
nucleoli of hepatocytes in the centrilobular region, degeneration
and necrosis of the liver cells) were found in the livers of animals
in both the treated groups (Gorzinski & Johnson, 1989).
In an oral toxicity study, adult male Sprague-Dawley rats were
administered 0, 100, 250, 500, or 1000 mg 1,2-dichloropropane/kg
body weight, in corn oil (by gavage), once a day, for up to 10
consecutive days. Some rats were killed at each dose level 24 h
after 1, 5, and 10 daily doses. After dosing, changes in body weight
and clinical toxic signs were assessed. In addition, measurements of
clinical chemistry parameters, urinary levels of glucose, alkaline
and acid phosphatase and N-acetyl glycosaminidase activities, and
the histopathology of major organs, liver enzyme levels, as well as
levels of non-protein sulfhydryls in the liver and kidneys were
determined. Definite central nervous system depression and body
weight loss, and increased renal nonprotein sulfhydryl (NPS) levels
were seen at 250 mg/kg. Liver damage (both morphological and enzyme
changes) was observed only at the 500 and 1000 mg/kg dose levels. In
this study, "resistance" to 1,2-dichloropropane hepatotoxicity
developed over the 10 consecutive days of exposure, as reflected by
progressively lower serum enzyme levels and by decreases in the
severity and incidence of toxic hepatitis and periportal
vacuolization. Nucleolar enlargement in hepatocytes, however, was
observed at all dose levels at both 5 and 10 days. There were a
number of manifestations of haemolytic anaemia in various tissues,
but no evidence of nephrotoxicity. On the basis of the parameters
examined, a NOEL of 100 mg/kg body weight can be established
(Bruckner et al., 1989).
In a similar oral study, male Sprague-Dawley adult rats were
treated with 0, 100, 250, 500, or 750 mg 1,2-dichloropropane/kg body
weight, in corn oil, by gavage, 5 times weekly for up to 13 weeks.
Over one-half of the animals in the 750 mg/kg group died within 10
days. Histopathological changes included mild hepatitis and splenic
haemosiderosis, adrenal medullary vacuolization and cortical
lipidosis, testicular degeneration and a reduction in sperm, and an
increased number of degenerated spermatogonia in the epididymis.
Similar testicular and epididymal degenerative changes were observed
in some animals in the 500 mg/kg group after 13 weeks of dosing.
While no deaths occurred in the 100 or 250 mg/kg groups, more than
50% in the 500 mg/kg group had died by 13 weeks. A dose-dependent
decrease in body weight gain was observed. 1,2-Dichloropropane
exhibited very limited hepatotoxic potential and no apparent
nephrotoxic potential in this 13-week study (Bruckner et al., 1989).
8.2.2 Inhalation
8.2.2.1 Mouse
B6C3F1 mice (aged 6-8 weeks) were exposed to target
concentrations of 1,2-dichloropropane (99.9%) of 0, 69.9, 233, or
699 mg/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. There were no
effects on haematology, gross pathology, and histopathology at
concentrations as high as 699 mg/m3, in this study (Nitschke et
al., 1988).
8.2.2.2 Rat
Fischer 344 rats (aged 6-8 weeks) were exposed to target
concentrations of 0, 69.9, 233, or 699 mg 1,2-dichloropropane
(99.9%)/m3 for 6 h/day, 5 days/week, for 13 weeks. The mean actual
concentrations were 0, 74.5, 233, or 694 mg/m3. The body weights
of rats exposed to 233 and 699 mg/m3 were significantly decreased.
Minimal effects were observed microscopically in the nasal tissues
of rats exposed to 233 and 699 mg/m3. A few rats exposed to 69.9
mg/m3 also had slight thickening of a small portion of the
respiratory nasal mucosa. No treatment-related effects were noted in
haematology, clinical chemistry, or urinalysis, even at 699 mg/m3
(Nitschke et al., 1988). A NOEL of 74.5 mg/m3 can be established.
8.2.2.3 Rabbit
New Zealand white rabbits (age 6-7 months) were exposed to 1,2-
dichloropropane at 0, 699, 2330, or 4660 mg/m3 for 6 h/day, 5
days/week, for 13 weeks. The mean actual concentrations were 0, 694,
2204, or 4436 mg/m3. Minimal effects on nasal tissues were present
in male rabbits only at the 4660 mg/m3 level. The primary effects,
decreased red blood cell parameters (red cell blood count,
haemoglobin, and packed cell volume), were observed in male rabbits
exposed to 699 (minimal effects) - 4660 mg/m3, and in female
rabbits exposed to 2330 and 4660 mg/m3 (Nitschke et al., 1988). No
NOEL has been established.
8.3 Reproduction, embryotoxicity, and teratogenicity
8.3.1 Reproduction
Groups of 30 male and 30 female Sprague-Dawley rats each were
provided with drinking-water containing 1,2-dichloropropane (99.9%)
at concentrations of 0, 0.24, 1, or 2.4 g/litre (w/v) (equivalent to
0, 33.6, 140, or 336 mg/kg body weight) over 2 generations. A
concentration of 2.4 g/litre represented the maximum practicable
attainable concentration, based on solubility. Adult rats were
evaluated for body weights, water and feed consumption, reproductive
performance, and gross pathological and histological changes. The
litters were evaluated for size, neonatal growth, and survival.
Decreases in water consumption reflective of rejection because of
unpalatability, were observed at all levels tested in both sexes in
the F0 and F1 generations. These decreases in water consumption
resulted in significantly lower body weights in both generations
administered 2.4 g/litre. These differences in water consumption and
body weights were also evident among the females during gestation
and/or lactation. The 0.24 g/litre dose level had a minor effect on
water consumption and body weights, but no adverse effects on the
animals. No treatment-related gross pathological changes were noted
in any dose group and the histological changes were limited to
increased hepatocellular granularity in both sexes in both
generations at all dose levels.
There were no histological changes in the reproductive tracts
of either sex in either generation. Reproductive function, measured
by fertility and litter size, was unaffected. The decreases in water
consumption among females at 2.4 g 1,2-dichloropropane/litre
resulted in significantly lower neonatal body weights and slightly
increased neonatal mortality in their litters. These neonatal
effects were considered secondary to the substantial decreases in
maternal water consumption, rather than a direct effect of the
substance. There were no neonatal effects at the 2 lower
concentrations. The NOAEL for adults is 0.24 g/litre, and the
reproductive NOAEL is 1 g/litre (equivalent to 140 mg/kg body
weight) (Kirk et al., 1990).
8.3.2 Teratogenicity
8.3.2.1 Oral (rat)
Groups of 30 Sprague-Dawley rats were administered 1,2-
dichloropropane (99.9%) in corn oil, by gavage, on gestation days 6-
15 at dose levels of 0, 10, 30, or 125 mg/kg body weight per day.
Parameters evaluated included maternal body weight and weight gain,
feed and water consumption, fetal weights, and fetal morphology. The
highest dose level produced transient decreases in respiration,
movement, muscle tone and extensor thrust reflex, and increases in
salivation and lacrimation, within 1 h of dosing on day 6 of
gestation. A decrease in the frequency of these clinical symptoms
occurred on the second day. In rats, 1,2-dichloropropane produced
transient central nervous system depression and decreased maternal
body weight gain and feed consumption at 125 mg/kg body weight.
Fetal effects in rats were limited to a significant increase in the
incidence of delayed ossification of the bones of the skull in the
125 mg/kg group, secondary to maternal effects. A level of 30 mg/kg
was considered the NOEL for maternal and fetal effects (Kirk et al.,
1989; Hanley et al., 1990).
8.3.2.2 Oral (rabbit)
Groups of 18 New Zealand white rabbits were administered 1,2-
dichloropropane (99.9%) at dose levels of 0, 15, 50, or 150 mg/kg
body weight per day on gestation days 7-19. On day 28 of gestation,
Caesarean section was performed and the fetuses evaluated.
Parameters evaluated included maternal body weight and weight gain,
haematology, fetal body weights, and fetal morphology. In maternal
rabbits, a dose of 150 mg/kg per day produced anorexia, significant
decreases in weight gain, and anaemia, with microscopic examination
revealing slight to moderate anisocytosis, poikilocytosis, and/or
polychromasia, indicative of a regenerative anaemia. The only fetal
effect in rabbits was a significant increase in the incidence of
delayed ossification of the bones of the skull at the highest dose
level, secondary to maternal effects. No effects were observed in
the 15 and 50 mg/kg dose groups. The NOEL for both maternal and
fetal effects was 50 mg/kg body weight (Hanley et al., 1989a).
8.4 Mutagenicity and related end-points
8.4.1 In vitro studies
8.4.1.1 Microorganisms
1,2-Dichloropropane was tested for its mutagenic activity in
Salmonella typhimurium, Saccharomyces cerevisiae with, and without
metabolic activation, and in Aspergillus nidulans and
Streptomyces coelicolor. Results in most studies on S.
typhimurium TA100 and TA1535 were positive, but negative results
were obtained with TA98, TA1537, and TA1538, S. cerevisiae, and
S. coelicolor. In Aspergillus nidulans, a forward mutation to 8-
azaguanine resistance and methionine suppression was found with S9
activation.
The results are summarized in Table 20.
Table 20. Mutagenicity tests with 1,2-dichloropropane on microorganisms
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Salmonella typhimurium
TA100, TA1535 1,2-DCP 10-50 mg plate S9 mix/none + De Lorenzo et al.
TA1978 S9 mix/none - (1977)
TA100 1,2-DCP (65% 62.5-8000 suspension S9 mix + Priston et al.
TA1535 1,2-DCP + 25% µg/mld S9 mix/none + (1983)
1,3-DCPropene)
TA98, TA1537 S9 mix/none -
TA100 1,2-DCP 1, 10, plate S9 mix/none - Stolzenberg & Hine
100 µmola (1980)
TA100, TA1535 1,2-DCP 1-10 µlitre plate S9 mix/none + Carere & Morpurgo
(1981)
TA98, TA1537, TA1538 S9 mix/none -
TA100, TA1535 1,2-DCP 0.33-10 mg plate S9 mix/none + Haworth et al. (1983)
(rat/hamster)
TA98, TA1537 S9 mix/none -
(rat/hamster)
TA100, TA1535, TA1537 1,2-DCP (99%) 33-2000 µg plate S9 mix - NTP (1983)
S9 none (+)
TA98 S9 mix/none -
(rat/hamster)
Saccharomyces cerevisiae
JD1 1,2-DCP (65% 62.5-8000 - S9 mix - Priston et al.
1,2-DCP + 25% µg/mld S9 none (+) (1983)
1,3-DC propene)
Table 20 (contd)
Organism/strain Substance Dose Type of test Metabolic activation Result Reference
Streptomyces coelicolarb 1,2-DCP 2-100 µlitre plate none - Carere & Morpurgo
A3 (1981)
Aspergillus nidulansc 100-400 µlitre plate none + Carere & Morpurgo
(1981)
Aspergillus nidulans 1,2-DCP (99%) 154 mmol/litre - Crebelli et al.
(1984)
a The highest dose level, 100 µmol/plate gave complete inhibition of bacterial growth.
b Resistance to streptomycin.
c Resistance to 8-azaguanine.
d Concentrations of 1000 µg/ml and higher were cytotoxic.
Crebelli et al. (1984) studied the induction of somatic
segregation in Aspergillus nidulans. Induction of haploidization,
mitotic non-disjunction, and mitotic crossing-over was studied in
heterozygous colonies exposed to 1,2-dichloropropane, 99%. No
significant rise in frequency of any kind of segregated sectors was
found. The concentration that was used was 154 mmol/litre.
8.4.1.2 Mammalian cells
In cytogenetic studies using Chinese hamster ovary cells, 1,2-
dichloropropane (99.4%) caused both chromosome aberrations and
sister chromatid exchanges. Dose levels tested were 0.46-1.50 and
0.113-1.13 mg/ml, respectively (Galloway et al., 1987).
Priston et al. (1983) also studied the ability of 1,2-
dichloropropane (containing 65% 1,2-dichloropropane and 25% 1,3-
dichloropropane) to induce chromosome damage using rat liver (RL4)
cells, in concentrations of 5-20 µg/ml. An indication for a small
increase in the frequency of chromatid gaps, chromatid and
chromosome aberrations, was noticed, but only in the presence of
cytotoxic effects. The Task Group considered this study inadequate.
Von der Hude et al. (1987) used the Sister Chromatid Exchange
test in vitro in Chinese hamster V79 cells to evaluate the effect
of 1,2-dichloropropane (99%). The concentrations tested were 0
(DMSO), 1.0, 3.3, and 10.0 mmol/litre without S9 mix. A dose-related
increase in SCEs was observed. With S9 mix and the same
concentrations, the SCEs frequency was increased but was less than
without S9 mix.
8.4.2 In vivo studies
8.4.2.1 Drosophila melanogaster
Woodruff et al. (1985) tested 1,2-dichloropropane at 0 and 4200
mg/litre by injection in germ cells and 0 and 33 552 mg/m3 by
inhalation in Drosophila melanogaster, using the sex-linked
recessive lethal mutation for their mutagenicity. The results by
injection and inhalation were negative.
8.4.2.2 Dominant lethal test
Groups of 30 male Sprague-Dawley rats were given 1,2-
dichloropropane (99.9%) in the drinking-water at concentrations of
0, 0.24, 1, or 2.4 g/litre, continuously, for a period of
approximately 14 weeks, as part of a combined reproduction/ dominant
lethal study (section 8.3.1). Exposed males (F0) were used for a
dominant lethal test following completion of the breeding for the
F1 litters of the reproduction study. They were mated with pairs
of naive, untreated adult females for 2 successive periods of 1 week
each. A separate, positive control group of 30 male rats was
administered a single oral dose of 100 mg cyclophosphamide/kg body
weight, 48 h prior to mating with untreated females. The uterine
contents of the females were evaluated for evidence of dominant
lethal effects as manifested by an increase in the resorption rate.
Among 1,2-dichloropropane-treated males, concentration-related
decreases in water consumption were noted at all levels and
decreased body weights were noted in males given 1 or 2.4 g/litre in
the water. Mating performance was unaffected in these animals.
Resorption rates among these groups revealed that the weekly values
for the females mated to treated males ranged from 2.2 to 8.1%, well
within the historical control range. Resorption rates among the
concurrent controls were low, ranging from 3.5 to 5.4%. The
significant increases from the concurrent control values, identified
during the first week of breeding in the resorption rates in the
groups given 0.24 or 2.4 g/litre, were considered to be within the
normal variation. Cyclophosphamide resulted in a 10-fold increase in
the resorption rate. 1,2-Dichloropropane was not mutagenic in a
dominant lethal assay in male Sprague-Dawley rats exposed
continuously to concentrations of up to 2.4 g/litre in the drinking-
water (Hanley et al., 1989b).
8.4.2.3 Miscellaneous
Perocco et al. (1983) studied the tritiated thymidine uptake
and cell viability in human lymphocyte cultures grown with, or
without, rat liver metabolizing system. Both the [3H]TdR uptake
and the percentage of viable cells showed values very similar to
control values. The 1,2-dichloropropane concentrations used ranged
from 10-2 to 10-4 mmol/litre.
8.5 Carcinogenicity
8.5.1 Oral (mouse)
Groups of 7 to 9-week-old hybrid B6C3F1 mice (50 males
and 50 females) were administered 0, 125, or 250 mg 1,2-
dichloropropane (99.4%)/kg body weight, in corn oil, by gavage, 5
days/week for 113 weeks. No influence on growth was observed. The
survival of the female animals at the highest dose level was
significantly decreased. The incidence of non-neoplastic lesions
showed lesions of the spleen (haemosiderosis and haematopoiesis were
increased) in female mice at the highest dose level.
The incidences of neoplastic lesions are summarized in Table
21.
Table 21. The incidence of neoplastic lesions in a mouse study with 1,2-dichloropropanea
Sex Control 125 mg/kg 250 mg/kg
Adenoma (liver) male 7/35 (20%) 9/33 (27%) 15/35 (43%)
Carcinoma (liver) male 8/35 (23%) 10/33 (30%) 9/35 (26%)
Adenoma and carcinoma (liver) male 15/35 (43%) 18/33 (55%) 24/35 (69%)b
Adenoma (liver) female 1/35 (3%) 5/29 (17%) 5/26 (19%)b
Carcinoma (liver) female 1/35 (3%) 2/29 (7%) 2/26 (8%)
Adenoma and carcinoma (liver) female 2/35 (6%) 7/29 (24%)b 7/26 (27%)b
Follicular cell adenoma and female 1/34 (3%) 0/27 (0%) 5/24 (21%)b
carcinoma (thyroid)
Alveolar/bronchiolar adenoma female 5/35 (14%) 0/29 (0%) 1/26 (4%)
or carcinoma (lung)
Squamous cell male 0/50 (0%) 1/48 (2%) 3/49 (6%)
papillomas (forestomach) female 0/50 (0%) 2/50 (4%) 2/50 (4%)c
a From: NTP (1986).
b P = < 0.05 - > 0.001.
c One high-dose female mouse had a squamous cell carcinoma.
On the basis of the results of this study, the NTP concluded
that 1,2-dichloropropane induces an increased incidence of liver
tumours in male and female B6C3F1 mice (Haseman et al., 1984;
NTP, 1986). However, the Task Group noted that the incidences of
liver adenomas and carcinomas in the treated groups were within the
historical ranges of this species (Haseman et al., 1985). The
increased incidence of thyroid tumours is equivocal.
8.5.2 Oral (rat)
Groups of 50 male and 50 female F344/N rats (aged 7-9 weeks)
were administered, by gavage, 0, 125 or 250 (female) and 0, 62, or
125 mg (male) 1,2-dichloropropane (99.4%)/kg body weight, in corn
oil, 5 days/week for 103 weeks. The highest dose level showed growth
depression in both sexes. Survival of female rats at the high dose
level was significantly lower. The incidence of non-neoplastic
lesions was not significantly different from that in the controls,
except for an increased incidence of foci of clear cell change and
of necrosis in the liver in high-dose female rats.
The incidences of neoplastic lesions are summarized in Table
22. Apart from these tumours, squamous cell papillomas of the
forestomach were found in 1 control male and 1 female rat. In high-
dose females, there was an increased incidence of mammary gland
adenocarcinomas, but not of mammary gland adenomas. Apart from these
tumours, squamous cell papillomas of the fore-stomach were found in
two high-dose females (not significantly increased compared to
controls). There were no effects on tumour incidences in male rats
(Haseman et al., 1984; NTP, 1986).
8.6 Factors modifying toxicity
Weanling Wistar rats, fed for several weeks on low protein
choline-deficient diets, were more susceptible to the effects of
inhalation of 4660 mg 1,2-dichloropropane/m3 than rats on a
control diet. This increased susceptibility could be corrected by
dietary supplements of 1-methionine or 1-cysteine plus choline
chloride (Heppel et al., 1946).
8.7 Special studies
8.7.1 Liver
Groups of 5 male Wistar rats (200 g) were administered, i.p.,
0, 10, 25, 50, 100, 250, or 500 mg 1,2-dichloropropane (97%)/kg body
weight, in corn oil, for 5 days (once daily) or for 4 weeks (five
days/week), or a single dose of 0, 50, 100, or 250 mg/kg body
weight, to investigate the biochemical and histological liver
changes. Reduced glutathione (GSH), glutathione- S-transferase,
cytochrome P450, and protein contents were measured. A dose-
Table 22. The incidence of neoplastic lesions in a rat study with 1,2-dichloropropanea
Sex Control 62 mg/kg male 125 mg/kg male
125 mg/kg female 250 mg/kg female
Fibroadenoma female 14/37 (38%) 20/43 (47%) 5/16 (31%)
(mammary gland)
Adenocarcinoma female 1/37 (3%) 2/43 (5%) 4/16 (25%)b
(mammary gland)
Endometrial stromal female 8/37 (22%) 14/42 (33%) 6/16 (38%)b
polyps (uterine
tumours)
Liver tumours male 3/50 (6%) 3/50 (6%) 2/50 (4%)
female 0/50 (0%) 0/50 (0%) 0/50 (0%)
Islet cell adenoma male 4/38 (11%) 1/42 (2%) 5/41 (12%)
and carcinoma
(pancreas)
a From: NTP (1986).
b P = < 0.05.
dependent decrease in liver-reduced glutathione was observed after a
single injection and a dose-dependent increase after 4 weeks. The
liver biochemical pattern after 4 weeks, characterized by a decrease
of cytochrome P450 and by an increase in reduced glutathione and
glutathione- S-transferase activity, suggests a hyperplastic
evolution of the liver cells, probably a repair mechanism induced by
the early depletion of glutathione. Histologically, the alterations
confirm the regenerative nature (atypical mitosis and hyperplastic
nodules) of the changes (Trevisan et al., 1989).
The hepatotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose-levels of 0, 50, 100, 250, or 500 mg/kg body weight per day,
in corn oil, for 4 weeks. Biochemical changes in the liver were
demonstrated. Significant findings included reduction of aminopyrine
demethylase activity at 100 mg/kg or more, increased levels of
reduced glutathione and glutathione- S-transferase activity at 50
mg/kg or more, and decreased cytochrome P450 activity at 500 mg/kg.
The activity of aniline hydroxylase was not affected. Duplicate
groups of rats treated with 1,2-dichloropropane, but allowed a
period of 4 weeks of recovery before being subjected to examination,
showed that the induced biochemical changes in the liver were
completely reversible (Trevisan et al., 1991).
1,2-Dichloropropane toxicity is actually preferentially
mediated by GSH depletion. This is suggested by the fact that GSH
loss is correlated with an increase in the biochemical indices of
liver and renal injury, and with the extent of haemolysis.
Pretreatment of 1,2-dichloropropane-intoxicated rats with
buthionine-sulfoximine (BSO) (depleting GSH) markedly increased the
overall mortality. Furthermore, the administration of the GSH
precursor N-acetyl-cysteine (NAC), preventing GSH depletion,
reduced the damage in target tissues, as demonstrated by a smaller
increase in some biochemical indices of cell injury and a smaller
degree of haemolysis. A possible explanation for these findings is
that, when the GSH level falls below a certain threshold value,
irrespective of the causative agent, a series of common reactions is
triggered, inducing peroxidation of membrane lipids, disturbances of
Ca2 homeostasis, and DNA damage, which quickly lead to
irreversible liver injury. Furthermore, it is also possible that
electrophilic metabolites of 1,2-dichloropropane, formed in the
absence of GSH, could directly attack a variety of cell
macromolecules the function of which is essentially to ensure the
physiological survival of the hepatocytes (Mitchell et al., 1973;
Bellomo & Orrenius, 1985; Casini et al., 1987; Orrenius et al.,
1989).
Male Wistar rats were exposed (by gavage) to a single oral dose
of 55 mg 1,2-dichloropropane/kg body weight in propylene glycol. The
animals were killed 1-6 days after exposure. Glutathione (GSH),
lipid peroxidation and protein of liver homogenate were measured.
Reduction of hepatic GSH and total protein and enhanced hepatic
lipid peroxidation still persisted 6 days after 1,2-dichloropropane
administration (Di Nucci et al., 1988).
Groups of rats were treated orally, by gavage, with single
doses of 1,2-dichloropropane ranging from 55 to 400 mg/kg body
weight. 1,2-Dichloropropane was no longer detectable in the blood 24
h after dosing at any dose level (Di Nucci et al., 1988).
8.7.2 Kidneys
Renal failure caused by 1,2-dichloropropane has been reported
by several authors who found an increase in serum creatinine and
urea in intoxicated animals and humans and fatty degeneration or
acute tubular damage in the kidney parenchyma (Heppel et al., 1946,
Ponticelli et al., 1968; Pozzi et al., 1985; Imberti et al., 1987).
Imberti et al. (1990) suggested that, on the basis of the
demonstration of 1,2-dichloropropane-induced depletion of kidney
GSH, it may be postulated that the biochemical mechanisms involved
in liver toxicity may also apply to the kidneys (Brezis et al.,
1983). In addition, since most of the glutathione and cysteine
conjugates of 1,2-dichloropropane are recovered in the urine, a
direct toxicity of the conjugates to the kidneys seems to play an
important role, as previously demonstrated for other conjugates
(Stevens et al., 1988).
It has been demonstrated that GSH plays an essential role in
the maintenance of the physiological structure of the erythrocyte,
preventing the formation of inter-protein or intra-protein
disulfides within the membrane skeleton. Depletion of erythrocyte
glutathione causes an increased fragility of this cell and
subsequent haemolysis. The involvement of such a mechanism in 1,2-
dichloropropane-induced haemolysis in intoxicated animals is
supported by the demonstration of a concomitant loss in erythrocyte
GSH and a correlation between these 2 phenomena (Imberti et al.,
1990).
Nephrotoxicity of 1,2-dichloropropane (97%) in adult male
Wistar rats (5 per group) was studied following daily i.p. injection
at dose levels of 0, 50, 100, 250, or 500 mg/kg body weight per day
for 4 weeks. Biochemical and histopathological alterations of the
kidneys were demonstrated. Kidney pathology involved a decrease in
the activity of angiotensin-converting enzyme in the proximal tubule
brush border and fraying of microvilli with epithelial coagulative
necrosis. Duplicate groups of rats treated similarly with 1,2-
dichloropropane, but allowed a period of 4 weeks of recovery before
being subjected to examination, showed that the treatment-induced
biochemical changes in the kidneys were completely reversible
(Trevisan et al., 1988, 1991).
8.7.3 Central nervous system
In the study of Gorzinski & Johnson (1989), in which Fischer
344 rats were treated with 0, 300, or 500 mg 1,2-dichloropropane per
kg body weight, by gavage, for 14 days, motor activity and a
functional observational battery (unusual responses in behaviour,
presence of convulsions, tremors, etc., and sensory function) were
evaluated (section 8.2.1.2). No effects were seen on these
parameters.
Specific tests in groups of 15 Fischer 344 rats/sex given, by
gavage, 0 (corn oil), 20, 65, or 200 mg 1,2-dichloropropane
(99.9%)/kg body weight, 5 days/week for 13 weeks, included monthly
evaluation of a functional observation battery, hind limb grip
strength, and motor activity; a comprehensive neuropathology study
was carried out at the end of the study. Clinical observations
included measurement of body weight gain and body temperature. Body
weights were decreased for male rats given 200 mg/kg body weight.
The body temperature was within normal limits, except in the females
given 200 mg/kg, which showed a slightly lower temperature. No
functional effects were noted. No gross or histopathological effects
on either the central or peripheral nervous system were observed
(Johnson & Gorzinski, 1988).
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute toxicity - poisoning incidents
Ingestion of cleaning solvent (50 ml) containing 1,2-
dichloropropane (other components not known) by a man produced coma
followed by delirium, irreversible shock, cardiac failure, and
death. Histopathologically, centri- and medio-lobular hepatic
necrosis was found (Larcan et al., 1977).
Two cases of disseminated intravascular coagulation syndrome
(DIC) have been described in association with acute intoxication by
1,2-dichloropropane. Effects on the central nervous system, liver,
and kidney functions were also observed, but no details were given
(Perbellini et al., 1985).
Pozzi et al. (1985), from Italy, reported clinical observations
on 3 other hospitalized persons with 1,2-dichloropropane poisoning.
Two out of the 3 persons ingested the substance, while the third
person was exposed through inhalation. The clinical symptoms were
similar, despite different routes of exposure. All 3 suffered from
acute renal and hepatic damage. Kidney biopsy, carried out on 1
person, showed acute tubular necrosis. Haemolytic anaemia and
disseminated intravascular coagulation were noted in all 3 patients,
1 of whom died on the seventh day. The other patients recovered
after treatment.
Toxic hepatitis with portal hypertension has been described in
a 49-year-old man, who ingested 1,2-dichloropropane in an attempted
suicide (Thorel et al., 1986).
9.2 Occupational exposure
Baruffini et al. (1989) studied 10 cases of 1,2-dichloropropane
dermatitis in the period 1985-88. Patients were painters or metal-
workers in the engineering industry and they all had known contact
with mixtures of solvents containing 10-40% 1,2-dichloropropane. On
examination, the workers had itchy erythematous, oedematous,
vesicular lesions on the fingers and dorsa of the hands. Two
subjects also had scaling and fissuring of the palms. Cessation of
exposure produced quick resolution of the dermatitis in all
subjects. Patch tests were carried out. As controls, 120 subjects
were similarly tested with 1,2-dichloropropane. All patients showed
a positive response to concentrations of 2% 1,2-dichloropropane or
more (allergic contact dermatitis). The results of the tests on the
control subjects were negative.
Grzywa & Rudzki (1981) described cases of dermatitis in 2 women
(47 and 55 years old), in a group of 60 persons, who had been
exposed to Silform aerosols for 6 and 4 years, respectively. Silform
aerosols contained 7.4, 11.0, or 12.7% 1,2-dichloropropane;
methylsilicone oils, and Freon. The patch tests that were carried
out were positive for 1,2-dichloropropane, but negative for
methylsilicone oils.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloropropane was considered by working groups of the
International Agency for Research on Cancer (IARC) in 1986 (IARC,
1986) and in 1987 (IARC, 1987). In the updating of 1987, it was
evaluated as follows: "There is limited evidence for the
carcinogenicity of 1,2-dichloropropane in experimental animals.
There are no data in humans. The agent is not classifiable as to its
carcinogenicity to humans (Group 3)".
WHO (in preparation) has proposed a provisional guideline value
for drinking-water of 20 µg 1,2-dichloropropane/litre.
PART C
ENVIRONMENTAL HEALTH CRITERIA
FOR
MIXTURES OF DICHLOROPROPENES
AND DICHLOROPROPANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MIXTURES OF DICHLOROPROPENES AND
DICHLOROPROPANE
1. SUMMARY AND EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
1.1.2 Kinetics and metabolism
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
test systems
1.1.5 Effects on humans
1.2 Conclusions
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.3 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Air
4.2 Water
4.3 Soil
4.3.1 Microbial transformation
4.3.2 Loss under field conditions
4.3.3 Soil function
4.4 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Groundwater
5.2 Occupational exposure
6. KINETICS AND METABOLISM
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Terrestrial microorganisms
7.1.1.1 Effects on nitrification
7.1.1.2 Recovery studies with microorganisms
7.2 Aquatic organisms
7.2.1 Invertebrates
7.2.2 Fish
7.3 Terrestrial organisms
7.3.1 Birds
7.3.2 Soil fauna
7.3.3 Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.2 Inhalation
8.3 Skin and eye irritation; sensitization
8.3.1 Skin and eye irritation
8.3.2 Sensitization
8.4 Long-term exposures/carcinogenicity
8.4.1 Oral
8.4.1.1 Rat
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction
8.5.1.1 Oral
8.5.1.2 Inhalation
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
8.6 Mutagenicity and related end-points
8.6.1 In vitro studies (microorganisms)
8.6.2 In vivo studies
9. EFFECTS ON HUMANS
9.1 General population exposure
9.2 Occupational exposure
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Use, environmental fate, and environmental levels
The technical mixture of dichloropropenes and dichloropropane
(abbreviated in this text to "MIX D/D") is a clear amber liquid with
a pungent odour; it has a vapour pressure of 35 mmHg at 20 °C, and
is soluble in halogenated solvents, esters, and ketones.
"MIX D/D" typically contains not less than 50% 1,3-
dichloropropene (ratio of cis- and trans-isomers approximately
1:1), the other main constituents being 1,2-dichloropropane and
related compounds. It was widely used as a soil nematocide before
planting.
The environmental transport, distribution, and fate of the
major constituents of "MIX D/D" in air, water, and soil is described
in the sections 4 of the parts of this EHC monograph that deal with
1,3-dichloropropene and 1,2-dichloropropane.
There is a significant potential for 1,2-dichloropropane
derived from "MIX D/D" to leach from the soil and contaminate well
water and groundwater. In an irrigation bore (68 m deep) in Western
Europe, mean 1,2-dichloropropane concentrations at different depths
ranged between 0.8 and 8.5 µg/litre, and the maximum concentration
recorded was 165 µg/litre.
Significant uptake of the constituents of "MIX D/D" by crops is
unlikely (see other parts of this EHC monograph). Bioaccumulation of
the constituents of "MIX D/D" is also unlikely because of their low
log P octanol/water partition coefficient and their relatively high
water solubility.
1.1.2 Kinetics and metabolism
No metabolic studies have been carried out on "MIX D/D". The
two major components, 1,3-dichloropropene and 1,2-dichloropropane,
are rapidly eliminated, primarily in the urine and, to a lesser
extent, via expired air. The components of "MIX D/D" are metabolized
by oxidative and conjunction pathways. The major urinary metabolites
are mercapturic acids.
1.1.3 Effects on organisms in the environment
"MIX D/D" is moderately toxic for fish; 96-h LC50 values
range between 1 and 6 mg/litre. The 1,3-dichloropropene is largely
responsible for the toxicity of "MIX D/D".
When used at recommended application rates, the main effects of
"MIX D/D" are a transient (< 7 days) reduction in soil fungi and
inhibition of the oxidation of ammonium ions to nitrate. "MIX D/D"
is toxic for nitrifying bacteria. Soon after "MIX D/D" disappears
from the soil, recolonization by bacteria takes place. In field
trials, "MIX D/D" (applied at 600 litre/ha) killed soil
invertebrates. Recolonization times ranged between 6 and 24 months.
"MIX D/D" is highly phytotoxic.
1.1.4 Effects on experimental animals and in vitro test
systems
The acute toxicity of "MIX D/D" for laboratory animals is
moderate to high. The oral LD50 values in rats and mice range from
132 to 300 mg/kg body weight. The dermal LD50 values for rats and
rabbits are 779 and 2100 mg/kg body weight, respectively. The
inhalation LC50 (4 h) for rats is approximately 4540 mg/m3.
Acute exposure results in clinical signs associated with central
nervous system depression. "MIX D/D" is a severe eye and skin
irritant and it is a moderate dermal sensitizer.
The results of the available short-term toxicity studies in
rats and dogs are inadequate to assess properly the toxicity
potential of "MIX D/D", because the relatively low doses tested do
not demonstrate any biologically significant effects. Several short-
term inhalation (whole-body) studies have been conducted in rats.
"MIX D/D" at levels up to 145 mg/m3 does not cause any toxic
effects. At levels of 1362 mg/m3 or higher, toxic effects
associated with central nervous system depression are evident. An
exposure to 443 mg/m3 for 10 weeks leads to reduced body weight
gain and increased absolute kidney weight.
An oral teratology study in rats was inadequate for assessment
of the teratological potential of "MIX D/D".
In an inhalation rat study to investigate male and female
fertility, no effects were found at dose levels up to 443 mg/m3
for 10 weeks. Complete evaluation of reproductive effects of "MIX
D/D" was not possible owing to inadequate protocol designs.
"MIX D/D" is mutagenic in Salmonella typhimurium strains
TA100 and TA1535, as well as in Escherichia coli WP2 HCR, without
metabolic activation. There was no such effect in Salmonella
strains TA98, TA1537, and TA1538.
In a long-term study on rats fed diets containing up to 120 mg
"MIX D/D" per kg (equivalent to 6 mg/kg body weight) for 2 years, no
toxic or carcinogenic effects were seen.
1.1.5 Effects on humans
"MIX D/D" is no longer widely used, and, thus, exposure of the
general population via air, water, and food is unlikely.
The exposure of workers filling drums and of field applicators
was generally below 4.5 mg 1,3-dichloropropene/m3 when recommended
procedures were used; in other situations, levels up to 36.32
mg/m3 have been measured.
One case of acute fatal poisoning has been reported following
accidental ingestion of "MIX D/D".
Several cases of contact dermatitis and skin sensitization due
to "MIX D/D" exposure have been reported.
1.2 Conclusions
- General population. As "MIX D/D" is no longer widely used,
the exposure of the general population to 1,3-dichloropropene
via air, water, and food is negligible, but, in certain areas,
exposure to 1,2-dichloropropane may occur when groundwater is
contaminated.
- Occupational exposure. Filling operations and field
applications of "MIX D/D" can lead to exposure of operators to
1,3-dichloropropene that exceed maximum allowable
concentrations, especially under warm climatic conditions.
- Environment. "MIX D/D" is unlikely to reach biologically
significant levels in either the terrestrial or the aquatic
environment when used at the recommended rate. Lasting adverse
effects on organisms in the environment are unlikely to occur.
1.3 Recommendations
- "MIX D/D" should not be used as a soil fumigant because of
potential leaching to groundwater.
- Monitoring of residues in surface water and groundwater should
be carried out in areas where "MIX D/D" has been used.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
The technical mixture of dichloropropenes and dichloropropane
(abbreviated to "MIX D/D") contains not less than 50% of the cis-
and trans-isomers (ratio approximately 1:1) of 1,3-dichloropropene
and the other main constituent was 1,2-dichloropropane. Yang (1986)
described a commercial "MIX D/D" containing 25% cis-
dichloropropene, 27% of trans-dichloropropene, 29% 1,2-
dichloropropane, and 19% other related chlorinated hydrocarbons. It
may also contain 1% epichlorohydrin as a stabilizer.
CAS registry number: 