
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 173
Tris(2,3-dibromopropyl) phosphate and
Bis(2,3-dibromopropyl) phosphate.
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. G.J. van Esch,
Bilthoven, Netherlands
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Tris(2,3-dibromopropyl) phosphate and Bis(2,3-dibromopropyl)
phosphate.
(Environmental health criteria ; 173)
1.Phosphoric acid esters 2.Environmental exposure
3.Flame retardants I.Series
ISBN 92 4 157173 X (NLM Classification: QP 981.P49)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TRIS(2,3-DIBROMOPROPYL)
PHOSPHATE AND BIS(2,3-DIBROMOPROPYL) PHOSPHATE
INTRODUCTION
TRIS(2,3-DIBROMOPROPYL) PHOSPHATE
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Production and use
1.1.2. Physical and chemical properties
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals
and humans
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory
and field
1.2. Conclusions
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
2.3.1. General
2.3.2. Urine
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Sources of human and environmental exposure
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Transformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.3. Interaction with other physical, chemical, or
biological factors
4.4. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Fish
5.2. General population exposure
5.2.1. Subpopulation at special risk
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Elimination
6.2.1. Different routes (rat and rabbit)
6.2.2. Dermal exposure (rat and rabbit)
6.2.2.1 TBPP
6.2.2.2 TBPP-treated fibres
6.2.3. Dermal exposure (human)
6.3. Distribution
6.3.1. Rat
6.3.1.1 Oral
6.3.1.2 Intravenous
6.3.2. Dermal (rabbit)
6.4. Metabolic transformation
6.4.1. In vivo studies
6.4.1.1 Oral (rat)
6.4.2. In vitro studies
6.5. Covalent binding to macromolecules
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.2.1. Oral exposure (rat)
7.2.1.1 TBPP
7.2.1.2 TBPP-treated fibres
7.2.2. Oral exposure (dog)
7.2.2.1 TBPP
7.2.2.2 TBPP-treated fibres
7.2.3. Dermal exposure
7.2.3.1 Rabbit
7.2.3.2 Dog
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.4.1. Skin irritation
7.4.2. Eye irritation
7.4.3. Sensitization
7.5. Reproductive toxicity, embryotoxicity, and
teratogenicity
7.5.1. Reproductive toxicity
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.6.1. DNA damage
7.6.1.1 In vivo
7.6.1.2 In vitro
7.6.2. Mutation assay with Salmonella
typhimurium strains
7.6.3. Mutations by urine of rats treated with TBPP65
7.6.4. Other mutation assays
7.6.5. Chromosomal effects
7.6.6. Cell transformation
7.6.7. Miscellaneous tests
7.6.8. Mechanisms of TBPP genotoxicity
7.7. Carcinogenicity
7.7.1. Oral
7.7.1.1 Mouse
7.7.1.2 Rat
7.7.2. Dermal
7.7.2.1 Mouse
7.8. Special studies
7.8.1. Kidneys
7.9. Factors modifying toxicity; toxicity of metabolites
7.9.1. Toxicity of metabolites
7.9.2. Mutagenicity of metabolites
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory studies
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.2.1 Invertebrates
9.1.2.2 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
BIS(2,3-DIBROMOPROPYL) PHOSPHATE AND SALTS
A1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
A2.1 Identity
A2.2 Physical and chemical properties
A2.3 Analytical methods
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
A3.2.2 Uses
A3.3 Contamination of the environment
A3.4 Environmental transport, distribution,
transformation, and exposure levels
A4. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
A4.1 Absorption, distribution, elimination,
and biotransformation
A5. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A5.1 Single exposure
A5.2 Short-term exposure
A5.3 Long-term exposure
A5.3.1 Mutagenicity and related end-points
A5.3.2 Carcinogenicity
A5.4 Special studies
A5.4.1 Kidneys
A5.5 Effects on humans and other organisms
in the laboratory and field
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone
No. 9799111).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
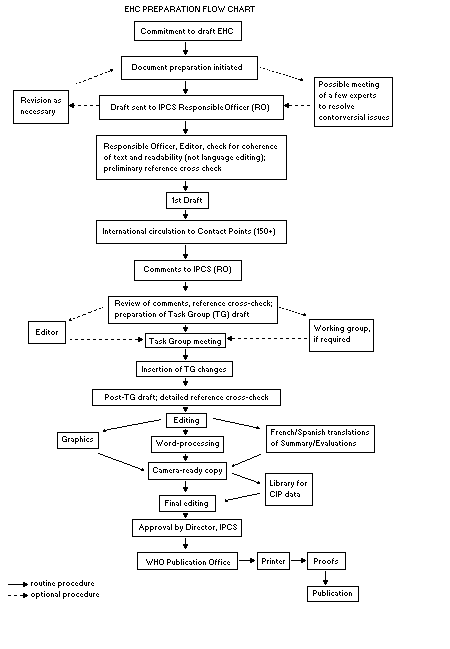
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
 All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND
BIS(2,3-DIBROMOPROPYL)PHOSPHATE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
United Kingdom
Dr D. Osborn, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Soderlund, National Institute of Public Health, Oslo,
Norway (Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research,
Stockholm University, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and
Aerosol Research, Hannover, Germany
Dr R.D. Kimbrough, Institute for Evaluating Health Risks,
Washington DC, USA (Vice-chairman)
Dr Wai-On Phoon, Department of Occupational Health,
University of Sydney, Sydney, Australia (Chairman)
Dr R. Benson, Drinking Water Branch, US EPA, Denver, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo,
Japan (Rapporteur)
Observers
Dr M.L. Hardy, Toxicology Advisor, Albemarle Corporation,
Baton Rouge, USA
Dr D.L. McAllister, Director, Quality, Environment, Health
and Safety, and Research Support, Great Lakes Chemical
Corporation, West Lafayette, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND BIS(2,3-DIBROMOPROPYL)
PHOSPHATE
A WHO Task Group on Environmental Health Criteria for tris- and
bis(2,3-dibromopropyl) phosphate met at BIBRA Toxicology
International, Carshalton, United Kingdom, from 6 to 11 June 1994.
Dr K.W. Jager, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the
draft and made an evaluation of the risks for human health and the
environment from exposure to tris- and bis(2,3-dibromopropyl)
phosphate.
The first draft was prepared by Dr G.J. van Esch, the
Netherlands, who also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS Contact Points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs M.O. Head of Oxford for
the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
INTRODUCTION
The IPCS is preparing several EHC monographs on Flame Retardants,
which will provide additional information relevant to TBPP.
There will be one monograph, "Flame Retardants - A General
Introduction" (in preparation), giving a general introduction to the
use, the mode of action, and the potential risks of flame retardants,
and listing the substances used as flame retardants with a general
indication of the data available on them.
Flame retardants in wide use are discussed in separate
monographs, e.g., EHC 162: Brominated Diphenyl Ethers (IPCS, 1994a)
and EHC 172: Tetrabromobisphenol-A (IPCS, 1995).
Some flame retardants considered hazardous for humans and the
environment have been reviewed in separate monographs including EHC
152: Polybrominated Biphenyls (IPCS, 1994b), and EHC 173: Tris- and
Bis(2,3-dibromopropyl) phosphate (this monograph).
Because of the possibility of the formation of halogenated
dibenzodioxins and dibenzofurans under certain circumstances, such as
pyrolysis, the following monographs have been developed: EHC 88:
Polychlorinated Dibenzodioxins and Dibenzofurans (IPCS, 1989) and
Polybrominated Dibenzodioxins and Dibenzofurans (in preparation).
The reader should consult these monographs for further
information.
Tris(2,3-dibromopropyl) phosphate was an important commercial
flame retardant ("TRIS"), especially for children's sleepwear. In
1977, the US Consumer Product Safety Commission banned children's
clothing treated with tris(2,3-dibromopropyl) phosphate. Since then,
in several other countries, the use of this compound as a flame
retardant has been severely restricted in consumer products and
prohibited in textiles.
Because tris(2,3-dibromopropyl) phosphate can also be used for
other applications, the information available on physical and chemical
properties, behaviour in the environment, occurrence in the
environment and humans, kinetics and metabolism, toxicity for
laboratory animals and in the field, and the exposure of the general
population and workers, is summarized in this Environmental Health
Criteria monograph. General properties and uses of brominated flame
retardants are given in "Flame Retardants - A General Introduction"
(in preparation).
ABBREVIATIONS
BA 2-bromoacrolein
BBPP bis(2,3-dibromopropyl) phosphate
DBCP 1,2-dibromo-3-chloropropane
DBP 2,3-dibromopropanol
DMBA dimethylbenzanthracene
mono-BPP mono(2,3-dibromopropyl) phosphate
TBPP tris(2,3-dibromopropyl) phosphate
TPA tetradecanoyl phorbolacetate
TRIS-(2,3-DIBROMOPROPYL) PHOSPHATE
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Production and use
Tris(2,3-dibromopropyl) phosphate (TBPP) was first produced in
about 1950; commercial production was reported in 1959. Production of
TBPP, in the USA, in 1975, was estimated to be between 4100 and
5400 tonnes. As far as is known, TBPP is not produced or used
currently in the world as a flame retardant in textiles, but may be
added to polymers used for other purposes. TBPP was an important
flame retardant for cellulose and tri-acetate and polyester fabrics,
especially in children's sleepwear, but has been banned for these
applications in several countries in Europe, the USA (1977), and Japan
(1978).
TBPP exists both in, and on, the fabric. When it is in the
fabric, it is not extractable with solvents and, therefore, probably
not available for dermal absorption. However, when it is on the fibre
surface, it can be extracted during laundering, and by acetic acid,
other solvents, and saliva, and is available for dermal absorption. In
this case, substantial losses of surface TBPP from fabrics during use
and/or laundering of the finished products, will occur, and will
contaminate the environment. Furthermore, release of TBPP into the
environment has been reported from textile-finishing plants and the
ultimate disposal of solid wastes, containing TBPP.
1.1.2 Physical and chemical properties
TBPP is available in at least two grades. The high-purity grade
is a clear, pale-yellow, viscous liquid, with up to 1.5% volatiles.
The low-purity grade may contain up to 10% volatiles.
TBPP (purity > 97%), has a boiling point of 390°C, a melting
point of 5.5°C, and a vapour pressure of 1.9 × 10-4mmHg at 25°C.
The solubility of TBPP in water is low (8 mg/litre).
When heated to decomposition, above 260-300°C, TBPP emits
compounds containing bromine and phosphorus. The n-octanol/water
partition coefficient (log Pow) is 3.02.
Analytical methods to determine TBPP and its metabolites in
biological samples and other matrices are available.
1.1.3 Environmental transport, distribution, and transformation
The limited information available suggests that TBPP is
relatively persistent in the environment. Oxidation and
photodegradation are not likely to be significant fate processes.
However, hydrolysis involving the bromine atoms on the propyl group
may occur, especially under basic conditions. Volatilization from
water may occur, but no actual data are available. Although
biodegradation of TBPP (half-life 19.7 h) in activated sewage is
reported to occur, it is not thought to be an important process in
natural soils and waters. In sterilized sludge, almost no breakdown
takes place. Bis(2,3-dibromopropyl) phosphate (BBPP) was found as a
major breakdown product. Because TBPP is virtually insoluble in
water, adsorption on particulate matter and sediment may be an
important process.
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water
solubility, TBPP is expected to leach only slowly to groundwater. TBPP
will tend to accumulate in rubbish dumps and other disposal sites,
which may result in biological accumulation. A bioaccumulation study
with fathead minnow showed a bioconcentration factor of 2.7, which is
low, while the n-octanol/water partition coefficient (Log Pow) was
3.02. Because of its low vapour pressure, TBPP is expected to be
mostly sorbed on particulate matter in air. Thermal oxidative
degradation of TBPP at 370°C showed that hydrogen bromide and
C3-brominated compounds, such as bromopropenes, dibromopropenes, and
diand tribromopropanes, are formed.
1.1.4 Environmental levels and human exposure
Data on environmental levels and human exposure are limited.
Studies carried out in Japan in 1975 showed that 20 samples of water,
soil, and fish did not contain TBPP. TBPP was identified, but not
quantified, in air particulates in the surroundings of an industry.
Children wearing TBPP-treated sleepwear were the group of the
general population particularly exposed to this flame retardant. The
estimated intake via the skin of children wearing TBPPtreated
sleepwear in the USA was calculated to be 9 µg/kg body weight per day.
The Consumer Product Safety Commission of the USA calculated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight or more.
1.1.5 Kinetics and metabolism in laboratory animals and humans
TBPP is absorbed readily from the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. In rats, TBPP or its
metabolites are eliminated within 5 days. Approximately 50% is
eliminated via the urine, 10% via the faeces, and 10-20% is exhaled as
CO2.
One day after oral administration of labelled TBPP to rats,
radioactivity was found in the blood, liver, kidneys, muscles, fat,
and skin, in a range of 0.2-6.6%. The half-life of clearance of
radioactivity from these organs was approximately 2-4 days. After 8
h, only bis(2,3-dibromopropyl) (BBPP) phosphate was still present in
substantial concentrations in most tissues.
After oral administration of TBPP to rats, six metabolites
were identified in the urine and bile. The main metabolite in
the urine, faeces, bile, and tissues was BBPP. The metabolite
2,3-dibromopropanol (DBP) was also identified in rats and in children
wearing TBPP-treated clothing.
Liver microsomes metabolize TBPP in the presence of NADPH and
oxygen. The main metabolites are BBPP and 2,3-dibromopropanol (DBP).
It has been shown that BBPP is formed by oxidation at the C3 and,
possibly, also at the C2 position of TBPP. In addition to BBPP,
2-bromoacrolein, 2-bromoacrylic acid, and propyl-hydroxylated
compounds and metabolites conjugated with glutathione have been found.
S-(2,3-dihydroxypropyl) glutathione was identified in the bile
of rats, and, it was suggested that TBPP and/or BBPP are conjugated
directly with glutathione by glutathione S-transferase with the
formation of episulfonium ion metabolites.
TBPP has been shown to be activated to form products that bind
covalently to proteins and DNA in vivo and in vitro. After
intraperitoneal injections of tritiated-TBPP, male mice, hamsters, and
guinea-pigs, which are less sensitive to TBPP-induced nephrotoxicity
than rats, showed similar levels of covalent binding to proteins in
the liver and kidneys. In the male rat, which is highly susceptible to
TBPP-induced nephrotoxicity, much higher amounts of radiolabel were
bound to kidney proteins than to liver proteins.
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to genotoxic intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from the rodents.
The binding of labelled TBPP and analogues in rats at a
nephrotoxic dose showed that the covalent protein binding was highest
in the kidneys followed by the liver and testes. The results of
comparative in vitro and in vivo studies on renal DNA damage
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C2 or C3 of TBPP. BBPP is transported to the kidneys, where
it is metabolized to reactive intermediates that cause DNA damage and
bind to kidney proteins. The activation occurring in the kidney
appears not to involve P450 but seems to be mediated by GSH-dependent
metabolism. In vitro studies with labelled TBPP and analogues
showed that oxidation of TBPP incorporates one atom of oxygen from
water. This implies that oxidation at C2 of the propyl moiety yields
a reactive alphabromoketone that can alkylate protein directly or
hydrolyse to BBPP and a reactive bromo-alpha-hydroxyketone.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute and short-term oral, and the acute dermal, toxicities
of TBPP are low. The oral LD50 for the rat > 2 g/kg and the dermal
LD50 for the rabbit > 8 g/kg body weight. Extensive kidney damage
(necrosis of renal proximal tubular cells) was noted in male rats
following a single ip injection of 100 mg TBPP/kg body weight.
Four-week, and 90-day, oral toxicity tests with TBPP (by gavage
or in the diet) in rats showed a dose-related increase in the
incidence and severity of chronic nephritis at dose levels of 25 mg/kg
body weight or more.
In rabbits, daily dermal applications of 2.2 g TBPP/kg body
weight or more, for 4 weeks, resulted in degenerative changes in the
liver and kidneys. All rabbits died within four weeks. No deaths
occurred in another study with dose levels of up to 250 mg/kg body
weight.
In a 90-day test on rabbits, weekly application of 2.27 g/kg body
weight to the skin resulted in kidney changes, testicular atrophy, and
aspermatogenesis.
No skin or eye irritation was observed in rabbits with dose
levels of 1.1 g or 0.22 g TBPP and no skin sensitization was observed
in guinea-pigs.
Two teratogenicity studies were carried out on rats. In one
study with dose levels of up to 125 mg/kg body weight, no
teratogenicity was observed. In another study with a dose level of
200 mg/kg body weight, a significant increase in skeletal variations
in the fetuses was observed, and, with 50 and 100 mg/kg body weight, a
significantly lower viability index was found. The authors concluded
that the observed effect resulted from maternal toxicity.
Extensive DNA damage was found in various organs of rats
administered TBPP. In vitro, TBPP has been shown to induce DNA
strand breaks in human KB cells. It induced unscheduled DNA synthesis
in rat liver hepatocytes, but not in human foreskin epithelial cells.
TBPP was mutagenic in several studies on Salmonella typhimurium,
especially in base-pair substituting strains with, and without,
metabolic activation.
Forward gene mutation assays using Chinese hamster V79 cells,
with, and without, metabolic activation were negative. However, a
positive effect in the presence of liver microsomes of rats pretreated
with phenobarbital was obtained. A weak positive effect was obtained
with mouse lymphoma cells (L5178YTK locus).
TBPP increased the number of sister chromatid exchanges (SCEs) in
Chinese hamster V 79 cells, but no chromosomal aberrations were
induced in Chinese hamster cells, mouse bone marrow cells, or in
cultured human lymphoid cells. SCEs but no chromosomal aberrations
were found with diploid human fibroblastic cells (line HE 2144)
without metabolic activation. However, in an in vitro chromosome
aberration test with the Chinese hamster cell line (CHL), TBPP was
positive.
A positive result was obtained with TBPP in a micronucleus test
on Chinese hamster bone marrow cells. Another micronucleus study with
mice showed a weak positive effect.
Studies with Drosophila melanogaster showed that TBPP increased
sex-linked recessive lethals in male germ cells and in adult males,
reciprocal translocations were induced. TBPP showed a strong positive
response in the w/w+ eye mosaic assay.
Several studies have been directed towards the elucidation of the
mechanisms involved in TBPP-induced mutagenicity and/or genotoxicity.
Bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. TBPP is activated by cytochrome P450 in a
reaction depending on NADPH and oxygen. Microsomes prepared from
livers of animals treated with phenobarbital or PCBs give increased
mutagenicity. The mono-and bis(2,3-dibromopropyl) phosphates are less
mutagenic than TBPP. In vitro studies have shown that oxidation at
C3 of the TBPP molecule yields the potent direct acting mutagen
2bromoacrolein that also binds to DNA.
Species differences in the bioactivation of TBPP to metabolites
mutagenic to Salmonella typhimurium TA 100 have been reported. Liver
microsomes from mice were more effective than those from guinea-pigs,
hamsters, and rats.
Three studies in which C3H/10T1/2 cells were used to study cell
transformation were carried out. In one study, a positive effect was
noted, but, in the other two studies, the results were negative.
TBPP was tested on mice and rats by oral administration and on
female mice by skin application in long-term studies. In mice,
following oral administration, TBPP produced tumours of the
fore-stomach and lung in the animals of both sexes, benign and
malignant liver tumours in females, and benign and malignant tumours
of the kidneys in males. In rats, TBPP produced benign and malignant
tumours of the kidneys in males and benign kidney tumours in females.
After skin application to female mice, TBPP produced tumours of the
skin, lung, fore-stomach, and oral cavity. From these studies, it can
be concluded that TBPP has carcinogenic potential in mice and rats.
When the TBPP metabolite BBPP was administered to rats orally, it
caused tumours in both sexes in the digestive system. The tumours
found included papillomas and adenocarcinomas of the tongue,
oesophagus, and forestomach, adenocarcinomas of the intestine, and
hepatocellular adenomas and carcinomas.
Another metabolite of TBPP, DBP, was tested on rats and mice by
dermal application. In male rats, there was an increased incidence of
neoplasms in skin, nose, oral mucosa, oesophagus, forestomach, small
and large intestine, Zymbal's gland, liver, kidney, tunica vaginalis,
and spleen. In female rats, there was an increased incidence of
neoplasms of the skin, nose, oral mucosa, oesophagus, forestomach,
small and large intestine, Zymbal's gland, liver, kidney, clitoral
gland, and mammary gland. In male mice, there was an increased
incidence of neoplasms in the skin, forestomach, liver, and lung, and
in female mice, there was an increased incidence of neoplasms of the
skin and the forestomach.
1.1.7 Effects on humans
Limited data are available regarding the effects of TBPP on
humans.
TBPP has been tested for skin sensitization potential in a few
studies on humans. The results of these studies indicate that TBPP
has a low sensitization potential and no skin irritation was reported.
However, persons who showed a positive sensitization response to pure
TBPP also reacted when exposed to fabrics
treated with TBPP.
1.1.8 Effects on other organisms in the laboratory and field
There are very few data on the effects of TBPP on other
organisms. All 6 goldfish (Carassius auratus), exposed to 1 mg
TBPP/litre, died within 5 days.
The EC50 for growth inhibition in oat seed was 1000 mg/kg soil.
This concentration caused a 100% inhibition of growth in turnip seed
(Brassica rapa sp.).
1.2 Conclusions
TBPP has been used as a flame retardant in fabrics, particularly
in children's sleepwear, but there is inadequate information on its
use in other applications. Exposure of the general population was
primarily through contact with fabrics treated with TBPP.
There is little information on the exposure of, and hazards to,
workers from the commercial production of TBPP and its use in a
variety of products.
Because of the paucity of data, no firm conclusions can be drawn
as to the exposure levels and hazards of TBPP for organisms in the
environment, other than humans.
Animal studies have shown that TBPP can be absorbed from the
gastrointestinal tract and, to a lesser extent, from the skin. TBPP
can also be absorbed through the skin of humans. In the rat, TBPP
appears to be extensively metabolized in the liver to BBPP, which is
the major metabolite detected in the urine and, to a lesser extent, to
DBP. In addition, other brominated metabolites of TBPP have been
found in small amounts. DBP has also been detected in humans wearing
TBPP-treated fabrics. The main route of elimination is the urine and
very little is excreted as the parent compound. The main metabolic
pathway seems to be through metabolism by cytochrome P450 and
glutathione S-transferases.
From the available data, it can be concluded that TBPP has a low
acute toxicity for experimental animals. Repeated dose studies with
relatively high doses of TBPP have revealed kidney and liver damage in
rats and also testicular toxicity in rabbits. TBPP has elicited a
clear genotoxic effect in several test systems, both in vitro and
in vivo. Carcinogenic effects were found in rats and mice. The
metabolites BBPP and DBP have also been shown to produce carcinogenic
effects in experimental animals. No irritation effects were found in
animals and a low sensitization potential in humans was noted.
In 1977, the US Consumer Product Safety Commission banned
children's clothing treated with TBPP, because of concerns that the
chemical might be a human carcinogen, and, because of the possibility
of significant human exposure through contact with treated fabrics.
Since then, the use of this substance as a flame retardant in consumer
products has been severely restricted in several other countries and
it has been prohibited in textiles.
1.3 Recommendations
Because of its toxic effects, TBPP should no longer be used
commercially.
If uses are identified for which there are no less hazardous
alternatives to TBPP, studies to demonstrate the absence of exposure
of, and hazards for, humans and the environment should be conducted.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Chemical formula C9H15Br6O4P
Chemical structure
BrCH2-CHBr-CH2O
\
BrCH2-CHBr-CH2O - P = O
/
BrCH2-CHBr-CH2O
Relative molecular mass 697.7
Synonyms tris(2,3-dibromopropyl) phosphate;
tris(2,3-dibromopropyl) phosphoric
acid ester; phosphoric acid, tris(2,3-
dibromo-propyl) ester;
tris(dibromopropyl) phosphate
CAS registry number 126-72-7
CAS chemical name 2,3-dibromo-1-propanol-phosphate (3:1)
RTECS registry number UB0350000
Trade names T 23 P; TP-69; DBP-TP; Apex
(emulsion) 462-5; Hamcogard FR;
Fyrol 59; Tanotard PN-2; Cav Gard
FR 1811 and FR 1812; Pyrosan 497;
Firemaster LV-T23P and T23P-LV;
Firemaster 200; Glotard PE-2; PE 10;
Anfram 3PB; Bromkal P 67-6HP; ES
685; Firemaster T23 and T23P;
Flacavon R; Flamex T23P; Flammex
AP; Zetofex ZN; Fyrol HB-32; NCI-
CO3270; Phoscon PE60; Phoscon UF-
S; RCRA waste number U 235;
USAF-DO-41 (LeBlanc, 1976; IARC,
1979; Ulsamer et al., 1980; IRPTC,
1987). FR 2406; Berkflam T23 P;
Flammex LVT 23P; 3PBR; TDBP;
TDBPP; TRIS; TRIS-BP; Zetifex ZN;
(Andersen, 1977).
2.1.1 Technical product
Commercial TBPP contains up to 0.2% of the following impurities:
2,3-dibromopropanol, 1,2,3-tribromopropane, and 1,2-dibromo-
3-chloropropane (DBCP) (Blum & Ames, 1977; Van Duuren et al., 1978;
Ulsamer et al., 1980).
2.2 Physical and chemical properties
Two grades of TBPP were available in the USA. The highpurity
grade had the following typical properties: a clear, pale-yellow,
viscous liquid; relative density at 25°C, 2.20-2.26; refractive index
at 25°C, 1.576-1.577; viscosity at 25°C, 3900-4200 centistokes; acid
number (mg KOH/g), 0.05 max; volatiles, 1.5% max; bromine content,
68.7%, and phosphorus content, 4,0%. Typical properties for a lower
grade are as follows: density at 25°C, 2.2-2.3; viscosity at 25°C,
1400-1700 centistokes; acid number (mg KOH/g), 0.05 max; and
volatiles, 10% max. (US EPA, 1976; IARC, 1979).
Osterberg et al. (1977) reported a viscosity of 9200 cP (25°C)
for TBPP of a purity of 99.76%. Firemaster LVT 23P has a viscosity of
9200 cP (Kerst, 1974).
Specific gravity 2.27 (2.2-2.3) g/ml at 25°C
(density) (Kerst, 1974)
Boiling point: 390°C (Dybing et al., 1989)
Melting point: 5.5°C (Dybing et al., 1989)
Vapour pressure: 1.9 × 10-4 mmHg at 25°C
1.2 × 10-3 mmHg at 45°C
4.8 × 10-3 mmHg at 65°C
(Kerst, 1974)
Solubility: Virtually insoluble in water
(6.3 mg/litre at 20°C) and hexane;
miscible in organic solvents, such
as carbon tetrachloride, acetone,
chloroform, methylene chloride,
dimethyl formamide, methanol,
xylene, benzene, toluene, and ethyl
acetate (Kerst, 1974)
Stability:
Heat stability: Major decomposition begins at
about 260-300°C; when heated to
decomposition, TBPP emits toxic
fumes of Br- and POx (Sax, 1984)
Light stability: Stable in sunlight
Hydrolytic stability: Hydrolysed by acids and bases
(IRPTC, 1987)
n-Octanol/water partition
coefficient (log Pow): 3.02 (IARC, 1979)
2.3 Analytical methods
2.3.1 General
TBPP is determined using a gas chromatograph equipped with a
flame photometric detector with possible cleaning processes. Direct
mass spectrometry, GC-MS, and HPLC are also used for the analysis of
biological samples containing TBPP and its metabolites (Cope, 1973;
Lynn et al., 1980, 1982; Pearson et al., 1993a).
Recovery and limits of determination vary, depending on sampling
procedures and matrices. GC analysis shows that TBPP can be
determined at the 10 ng level by using a column packed with a high
liquid loaded support. In an indirect analytical method, TBPP is
determined by spectrophotometry, by complexing phosphor with
molybdenum blue after hydrolysis of the TBPP by hydrobromic acid
(Nakamura, 1980; Gutenmann & Lisk, 1975).
Gardner (1979) described a densitometric method using thin-layer
chromatography. TBPP was chromatographed on silicagel thin-layer
plates, using ethyl acetate hexane (30:70) as a developing solvent.
TBPP was visualized by spraying the chromatograms with 1% aqueous
silver nitrate followed by exposure to UVR for 40 min. The spots were
quantified by densitometry at 600 nm. The lower level of sensitivity
was 50 ng; calibration plots were linear from 50 to 800 ng. The
recovery of TBPP from sewage sludge samples fortified at the 1.0 ppm
level was 97%.
Techniques for the qualitative detection of TBPP in textiles have
been described, including thin-layer chromatography, HPLC, and NMR
(Iliano et al., 1982).
2.3.2 Urine
In mammalian species, organophosphates undergo enzymatic or
chemical hydrolysis to form the corresponding acids and alcohols. The
alcohols are often excreted in the urine as soluble conjugates. Since
the hydrolysis of TBPP yields 2,3-dibromopropanol (DBP), an analytical
method has been developed to determine free, and conjugated, DBP.
Extraction of urine by diethylether/hydrochloric acid, followed by
methylation with diazomethane gives the methylether of DBP.
Determination is by electron affinity gas chromatography. The limits
of determination in rat and human urine were 0.4 and 0.2 mg/litre,
respectively (St. John et al., 1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
TBPP is not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
It is estimated that TBPP was first produced in 1950, when it was
prepared by the addition of bromine to a solution of triallyl
phosphate in benzene. However, it is synthesized in the USA by a
two-step process in which bromine is added to allyl alcohol to give
2,3-dibromopropanol (DBP). This is then reacted with phosphorus
oxychloride, in the presence of a Lewis acid such as, aluminum
chloride or stannium chloride as a catalyst (Overbeek & Nametz, 1962).
The commercial production of TBPP was reported in 1959 and US
production in 1975 has been estimated to have been between 4100-5400
tonnes (US EPA, 1976). Prior to 1977, 4500 tonnes of TBPP were
produced annually in the USA by 6 manufacturers. There was no
evidence of production of TBPP in the USA in 1986.
Production of TBPP in Japan in 1976 and 1977 is estimated to have
been 100 and 300 tonnes per year, respectively, made by one
manufacturer. No TBPP is produced in Japan at present.
It has not been possible to assess whether TBPP is currently
produced. However, no reports are available that describe any
production of TBPP.
3.2.2 Uses
TBPP has been used as a flame retardant for cellulose and
triacetate and polyester fabrics, which are widely used in children's
sleepwear. It has also been used as a flame retardant in other
materials, such as urethane foam and acrylic carpets and sheets,
polyvinyl- and phenolic resins, polystyrene foam, paints, lacquers,
paper coatings and styrene-butadiene rubber, latexes, and cured
unsaturated polyesters products. Rigid foams containing TBPP were
used in insulation, furniture, automobile interior parts, and water
flotation devices. About 65% of the 4500 tonnes of TBPP that were
produced annually in the USA by 6 manufacturers was applied to fabrics
used for children's clothing. TBPP was added to these children's
garments to an extent of 5-10% by weight (US EPA, 1976; Kirk-Othmer,
19781984).
TBPP was applied to cellulose acetate and triacetate by addition
to the melt prior to spinning. The process involved the thermal
diffusion of TBPP by driving it into the fibre under pressure dying.
For materials such as, polyesters, nylons, and acrylics, the TBPP was
either "padded on" at 5-10% by weight with heat fixation to the woven
or knitted material or applied via emulsion from conventional batch
dying equipment (Prival, 1975).
Fire-retarded polyurethane required about 0.5% phosphor and 4-7%
bromine; being equivalent to about 10% TBPP by weight in the product
(US EPA, 1976).
By actions taken on 8 April and 1 June 1977, on the basis of the
genotoxic and possible carcinogenic effects of TBPP, the US Consumer
Product Safety Commission banned children's clothing treated with
TBPP, the chemical itself when used or intended to be used in
children's clothing, and fabric, yarn, or fibre containing it, when
intended for use in such clothing (US Consumer Product Safety
Commission, 1977a,b; US Consumer Product Safety Commission, 1977a,b).
In March 1978, The Consumer Product Safety Commission listed 22
products that contained TBPP and were available to USA consumers.
These included children's clothing, industrial uniforms, draperies,
tent fabric, automobile headliners, epoxy resins for the electronics
industry, Christmas decorations, and polyester thread (IARC, 1979).
In Japan, the use of TBPP as a fire-retardant in textile products
was banned in 1981, because the chemical might be a human carcinogen
and genotoxicant.
As from December 1987, TBPP could not be used in the EC in
textile articles such as, garments, under-garments, and linen intended
to come into contact with the skin (EEC, 1976, 1979).
Several other countries including Finland, New Zealand, and
Sweden have also banned, or severely restricted, the use of TBPP in
textiles and textile articles (UN, 1991).
3.2.3 Sources of human and environmental exposure
Potential sources of human exposure and environmental
contamination include: the manufacturing of the flame retardant, its
application to materials, leaching out of the flame retardant during
use and/or washing, and ultimate disposal of the material.
Studies indicated substantial losses of surface TBPP from fabrics
after laundering, but TBPP was not completely removed after repeated
laundering. For example, acetate fabrics (65-600 mg TBPP/kg) showed
up to 85% reduction in surface concentration after one laundering,
and, polyester fabrics (260-37 500 mg TBPP/kg), from 21 to 82%
reduction after one laundering. A significant portion, approximately
10% of the total production reached the environment from
textile-finishing plants and laundries. Most of the rest will find
its way into solid wastes (US EPA, 1976).
Surface TBPP can be extracted from treated fabric by saliva (up
to 3%) as well as by water, acetic acid, sodium bicarbonate, and salt
(Ulsamer et al., 1980).
Gutenmann & Lisk (1975) heated polyester flannel material,
treated with TBPP, in distilled water at 60°C for 20 min, simulating
a laundering operation. It was calculated from the extraction rate
that laundering of flame-retarded sheets could result in a
concentration of 6 mg/litre in combined washing and rinsing water.
This release was maintained during several subsequent launderings.
The presence of detergents may increase the extraction rate.
TBPP exists both in, and on, the fabric. In the fabric fibres,
it is not extractable with a benzene/hexane mixture and, therefore,
is probably not available for dermal absorption. However, when it is
on the fibre surface, it is extractable and is available for dermal
absorption (Morrow et al., 1976; Ulsamer et al., 1980).
While most of the TBPP is within the fabric in both polyester and
acetate, polyester contains considerably more surface TBPP as a result
of differences in methods of addition. Concentrations of surface
bromine in polyester fabric ranged from 2000 to 37 500 mg/kg with the
actual TBPP content ranging from 20 to 90% of the bromine value. The
non-TBPP organic bromides have not yet been identified (Ulsamer et
al., 1980).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water solubility
of the technical chemical (8.0 mg/litre), TBPP is expected to leach
only slowly into groundwater. The water solubility of pure TBPP may
be lower than the solubility of the technical grade chemical and so
the extent of leaching of the pure chemical may be even lower than the
Koc above suggests (Kenaga, 1980; Lyman, 1982; Verschueren, 1983; US
EPA, 1985).
Although hydrolysis of the phosphate ester is not expected to be
significant, hydrolysis involving the bromine atoms on the propyl
groups may occur, especially under basic conditions. Direct
photolysis is not expected to be a major process, since TBPP should
not absorb light of wavelengths found in sunlight (> 290 nm) (Mabey &
Mill, 1978).
No data on volatilization from water or soil are available. Using
measured water solubility (8.0 mg/litre) and vapour pressure of 1.9 ×
10-4 mmHg, volatilization half-life values were estimated. The
half-life values for TBPP volatilization from streams, rivers, and
lakes were 3.64, 4.66, and 392 days, respectively, assuming current
velocities of 3, 1, and 0.01 m/second, respectively. The river and
stream depths were assumed to be 1 m, while the lake was assumed to be
50 m deep (Verschueren, 1983).
4.2 Transformation
4.2.1 Biodegradation
The biodegradability of TBPP was determined following a
shake-flask test. TBPP was incubated with a microbial inoculum of raw
sewage. Samples of the test solutions were taken at 0, 5, 10, and 15
days for final analysis using neutron activation to determine the
bromine content of the liquid. Assuming the increased bromide content
of the inoculated samples relative to the blank samples is due to
biodegradation, and the solubility of TBPP is 1.6 mg/litre, an amount
of TBPP equal to 2.4 times the dissolved TBPP was degraded in 5 days
(Kerst, 1974).
Activated return sludge (at 21°C), used within 1 h of
collection, diluted with a basal medium, with an added 2 mg
14C-labelled TBPP/kg, showed that 6% of the added radio-activity was
evolved as 14CO2. A major metabolite bis(2,3-dibromopropyl)
phosphate (BBPP) was identified, but neither dibromopropanol (DBP) nor
dibromopropionic acid was detected. The half-life of TBPP was 19.7 h
(by least squares regression analysis). In a sterilized sludge
control study, 93% of the added TBPP was found and metabolites were
not identified (Alvarez et al., 1982).
A biodegradation study on TBPP (100 mg/litre) was carried out
under sewage treatment condition with sludge (30 mg/litre). The
degree of biodegradation, as measured by BOD, was 1.8% of TBPP after a
2-week incubation period (Chemicals Inspection & Testing Institute,
1992).
4.2.2 Abiotic degradation
No data available.
4.2.3 Bioaccumulation
Tissue residue analysis of rats fed TBPP for a period of 28 days
at levels of 100 or 1000 mg/kg diet has shown dose-related residue
levels (measured as total bromine) in the muscle, liver, and body fat,
of the treated animals (see section 7.2.1.).
Groups of 30 adult fathead minnow (Pimephales promelas) (six
months old), were exposed to 47.7 µg TBPP/litre for 2-32 days in a
flow-through system. The temperature of the water was 25°C, pH 7.49,
dissolved oxygen > 5 mg/litre, and hardness
45.5 mg/litre. The bioconcentration factor determined was 2.7 (Veith
et al., 1979).
Bioconcentration of TBPP (0.1 mg/litre, 0.03 mg/litre) from water
to carp was estimated to be between < 0.7 to 1.9, and < 2.2 to 4.3,
respectively, after 6 weeks of exposure (Chemicals Inspection &
Testing Institute, 1992).
4.3 Interaction with other physical, chemical, or biological factors
The thermal oxidative degradation at 370°C of TBPP produced
hydrogen bromide and the C3-brominated species - bromopropenes,
dibromopropenes, dibromopropanes and tribromopropanes, accounting for
87% of the volatiles. The detection of chlorinated species can only
be explained by the presence of chlorinated impurities in the original
ester. The residue (ether soluble aliquot) was composed mainly of
1,2,3-tribromopropane, whereas the aqueous layer contained the
phosphoric acid produced. The gas chromatographic analyses of the
volatiles showed a number of isomeric dibromopropenes. It was
established that 1,3-dibromopropene was the major dibromopropene
formed (Paciorek et al., 1978).
4.4 Ultimate fate following use
It is to be expected that TBPP would be released into the
environment in wastewater after laundering articles coated with TBPP
flame retardant.
With regard to disposal, it must be assumed that clothes and
other products containing TBPP ultimately end up in landfills, which
may result in some biological accumulation. Incineration should be
carried out at high temperature with scrubbers or the equivalent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
TBPP was identified, but not quantified, in Arkansas air
particulates (DeCarlo, 1979).
5.1.2 Water
In 1975, 20 water samples were collected at different places in
Japan and analysed for the presence of TBPP. None of the samples
contained the compound (limit of determination 1 µg/litre)
(Environment Agency Japan, 1978, 1987).
5.1.3 Soil
In 1975, 20 sediment samples were collected at different places
in Japan and analysed for the presence of TBPP. None of the samples
contained TBPP (limit of determination 0.4-10 mg/kg) (Environment
Agency Japan, 1978, 1987).
TBPP was identified, but not quantified, in Arkansas soil
(DeCarlo, 1979).
5.1.4 Fish
In 1975, 20 fish samples, collected at different places in Japan,
were analysed for the presence of TBPP. None of the samples contained
TBPP (limit of determination 1 mg/kg) (Environment Agency Japan, 1978,
1987).
5.2 General population exposure
5.2.1 Subpopulation at special risk
Tests for the extraction of TBPP from fabrics by water at various
pH values and by a simulated saliva solution failed to reveal any TBPP
in the extracts, but sodium bromide and hydrobromic acid were detected
(limits of determination not mentioned) (Prival, 1975). However,
surface TBPP can be extracted from treated fabric by saliva (up to 3%)
as well as by water, acetic acid, sodium bicarbonate, and salt
(Ulsamer et al., 1980).
In the USA, the estimated intake via the skin of children,
wearing sleepwear treated with the compound, was estimated to be
9 µg/kg body weight (Blum et al., 1978).
The Consumer Product Safety Commission of the USA stated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight and there are
indications that this may be even higher (IRPTC, 1987).
5.3 Occupational exposure
There are no data on levels of exposure to TBPP during
manufacture or further processing.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
TBPP is absorbed readily by the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. Studies on children
revealed that TBPP is dermally absorbed from TBPP-treated sleepwear
(Kerst, 1974; Blum et al., 1978; Ulsamer et al., 1978, 1980).
Following the dermal application of 14C-TBPP to the clipped
backs of New Zealand White rabbits (2-3 kg), 3.5-3.8% of the 0.9 ml/kg
dose and 15.2% of the 0.05 ml/kg dose were absorbed over 96 h.
Osborne Mendel rats (200-250 g) absorbed approximately 1/6 as much
14C-TBPP at each dose, when TBPP was applied to an equivalent area
of skin/kg. The dermal uptake of 14C-TBPP by rats and rabbits
showed that the primary elimination was via the kidneys (Ulsamer et
al., 1980).
6.2 Elimination
6.2.1 Different routes (rat and rabbit)
Four male Sprague-Dawley rats (290-310 g) were administered
14C-TBPP (98%) intravenously. The animals were housed in metabolism
cages for 5 days. Urine, faeces, and air samples were collected for
5 days, and bile for 1 day. In 5 days, 58% of the administered
radioactivity was found in the urine; 9% in the faeces and 19% in the
air as CO2. In 24 h, bile contained 34% of the radioactivity while
9% was found in the bodies of the rats. In three additional rats,
it was found that biliary excretion and enterohepatic recirculation
was a major route in the disposition of TBPP. Bis(2,3-dibromopropyl)
phosphate (BBPP) was detected in the urine of male rats (290-310 g)
dosed iv with 25 mg 14C-TBPP (98%)/animal (in Emulphor) in
amounts of 7.8% of the dose during 5 days following administration.
BBPP was identified in the urine, faeces, bile, and tissues.
2,3-Dibromopropanol (DBP) was found in tissues and DBP and a few other
metabolites were found in urine, but TBPP was not detected (Lynn et
al., 1980, 1982).
An adult male Sprague-Dawley rat (150-200 g) was administered
(iv or orally) 1.39 mg 14C(propyl)-TBPP (99%)/kg body weight. One
day after iv administration, 17% of the administered radioactivity was
found in the urine, 7.4% in the faeces and 20% in the air (as CO2).
One day after oral administration of TBPP, the concentrations were 24%
in the urine and 11.5% in the faeces, but no radioactivity was
detected in the air. Mainly metabolites were excreted in the urine
and bile (Nomeir & Matthews, 1983).
Small amounts of DBP and conjugates appeared in urine, when the
rat was allowed to chew on TBPP-finished polyester fabric (St. John et
al., 1976).
Radiolabel from 14C-TBPP, applied to the skin, was excreted
primarily in the urine (70% for rabbits and 50% for rats) with lesser
amounts appearing in the faeces and 12 and 18% exhaled as CO2,
respectively. TBPP itself did not appear in the urine, but a number
of metabolites including DBP were found (section 6.4) (Ulsamer et al.,
1980).
6.2.2 Dermal exposure (rat and rabbit)
6.2.2.1 TBPP
One hundred mg of TBPP was spread over the surface of a gauze pad
(one square inch) bandage and pressed tightly against the shaved skin
of a rat. Urine was assayed for free and conjugated (released by acid
hydrolysis) DBP. By day 7, the total concentrations of free and
conjugated DBP in the urine were 17.61 and 23.58 mg/litre,
respectively (St. John et al., 1976).
6.2.2.2 TBPP-treated fibres
TBPP has been shown to penetrate rabbit skin from 14C-TBPP
labelled polyester cloth containing 15 000 mg TBPP/kg of surface (4.3%
of the radioactivity in 96 h) (Ulsamer et al., 1980).
A shaved rat wore a garment made of 100% polyester flannel
(4 × 6 inches), treated with TBPP, for 9 days. No DBP could be
detected in the urine (limit of determination 0.4 mg/litre) (St. John
et al., 1976).
6.2.3 Dermal exposure (human)
The skin of a 7-year-old child was exposed on days 1, 2, and
8-12, by wearing repeatedly washed sleepwear that may have been
TBPP-treated. On days 3-7, she wore new TBPP-treated pyjamas. Urine
samples were collected daily from the child. In the urine, a maximum
concentration of DBP of 29 µg/litre was found 2 days after wearing the
new treated pyjamas. DBP at a concentration of 0.4 µg/litre was
present in the urine, prior to wearing the new treated pyjamas. DBP
was still excreted 5 days after the child stopped wearing the new
TBPP-treated pyjamas. Urine samples were collected from 10 other
children and one adult. All samples were analysed for DBP; it was not
found in the urine of one child and one adult (who had never used
washed TBPP-treated sleepwear). Seven children had levels of about
0.5 µg DBP/litre in the urine and one child had a level of 5 µg/litre.
Approximately 180 µg/day (9 µg/kg body weight) was absorbed through
the skin of children wearing pyjamas treated with TBPP (Blum et al.,
(1978).
No DBP could be detected in the urine of an adult or in the urine
of a 5-year-old boy who wore 100% polyester knit pyjamas, treated with
TBPP, for 7 nights. Morning urine samples were collected daily
throughout this period and up to 8 days thereafter (limit of
determination 0.2 mg/litre) (St. John et al., 1976).
6.3 Distribution
6.3.1 Rat
6.3.1.1 Oral
Male adult Sprague-Dawley rats (150-200 g) were administered
1.39 mg 14C(propyl)-TBPP (99%) orally. The percentages of the total
dose of radioactivity, found after one day, in the blood, liver,
kidneys, lung, muscles, fat, and skin, were 6.6, 3.4, 0.7, 0.2, 5.5,
1.3, and 3.4%; 24 and 11.5% of the total dose were found in the urine
and faeces, respectively. The terminal clearance of TBPP-derived
radioactivity from most of the tissues was described by a single
component exponential decay with a half-life of 2.5 days. The
half-life of TBPP in the liver and kidneys was 3.8 days (Nomeir &
Matthews, 1983).
Dose-related bromine concentrations were detected by neutron
activation analysis in the muscles, liver, and fat of male rats fed
TBPP for 28 days. The levels decreased to control levels during the
six-week withdrawal period (Kerst, 1974).
6.3.1.2 Intravenous
Eight male Sprague-Dawley rats (290-310 g) were administered
14C-TBPP (98%) by the iv route and the distribution was studied.
All tissues contained TBPP-derived radioactivity. The concentrations
of TBPP-derived radioactivity declined rapidly in most tissues, but
the concentration of radioactivity in kidneys was 11 times the average
body concentration, five days after dosing. No TBPP was detected,
though bis(2,3-dibromopropyl) phosphate (BBPP) was still present in
substantial concentrations. By day five, only small quantities of
this metabolite were detected. The concentration of TBPP increased
in the fat during the first 5-30 min, but, after 8 h, TBPP was no
longer detectable. In contrast to the rapid disappearance of TBPP,
the half-life of BBPP was relatively long in most tissues. BBPP
represented a major portion of the radioactivity in several tissues
including the lung, muscles, fat, and blood. In blood, it accounted
for 90% of the radioactivity at 30 min and 8 h. By 5 min, 75% of the
radioactivity in plasma was BBPP. The initial plasma half-life of
this metabolite was 6 h. For 5 days it was 36 h. TBPP was not
detectable in plasma after 1 h (Lynn et al., 1982).
6.3.2 Dermal (rabbit)
Substantially more TBPP-derived radiolabel was detected in the
kidneys and liver than in other organs of New Zealand rabbits,
dermally treated with polyester fabrics containing 14C-TBPP (Ulsamer
et al., 1978).
6.4 Metabolic transformation
6.4.1 In vivo studies
6.4.1.1 Oral (rat)
TBPP was readily metabolized in rats. The main metabolite found
in the urine, faeces, bile, and tissues of rats was BBPP.
2,3-Dibromopropanol (DBP) was also identified in tissues and urine.
Only small amounts of unchanged TBPP were found in the excreta (Lynn
et al., 1982; Nomeir & Matthews, 1983).
Male adult rats (150-200 g) were administered 1.39 mg
14C(propyl)-TBPP (99%) orally (by intubation), and the urine and
bile were analysed for metabolites. Six metabolites were identified
in urine and bile, respectively:
- 2,3-dibromopropanol; 1.0 and 1.1%;
- bis(2,3-dibromopropyl) phosphate; 2.8 and 25.8%;
- 2-bromo-2-propenyl 2,3-dibromopropyl phosphate; 4.8 and 13.8%;
- bis(2-bromo-2-propenyl) phosphate; 10.3 and 5.2%;
- 2,3-dibromopropyl phosphate; 4.1 and 2.6%;
- 2-bromo-2-propenyl phosphate; 9.5 and 2.4%
and TBPP was found in concentrations of 0.8 and 2.0%, respectively.
These data are expressed as a percentage of total radioactivity
excreted in the urine in 24 h, and, bile in 3 h. The total quantity
of metabolites eliminated in the urine and bile were, in these
periods, 33.3 and 52.9% of the radioactivity administered,
respectively (Nomeir & Matthews, 1983).
The formation of BBPP has been studied using selectively
deuterated analogues of TBPP. Plasma concentrations of BBPP in rats
dosed with either C2-D1- or C3-D2-TBPP were substantially lower than
levels obtained with TBPP up to 4-6 h after administration. This
indicates that oxidative metabolism of TBPP to form BBPP is important
in vivo. Furthermore, in addition to oxidation at C3, BBPP
formation may result from oxidation at C2. This latter reaction may
be of particular importance with phenobarbital-pretreated microsomes
(Pearson et al., 1993a; Dybing et al., 1989).
In addition to these TBPP metabolites, 2-bromoacrolein,
2-bromoacrylic acid, bis(2,3-dibromopropyl)-3-hydroxypropyl phosphate,
S-(2,3-dihydroxypropyl) glutathione, S-(3hydroxypropyl)
glutathione and S-(2-carboxyethyl) glutathione have been detected
in vitro and/or in vivo (Marsden & Casida, 1982; Nelson et al.,
1984).
2-Bromoacrylic acid has been detected in the urine of rats
administered TBPP. It was suggested that 2-bromoacrylic acid is an
oxidation product of 2-bromoacrolein and that 2-bromoacrolein is
formed spontaneously from DBP generated via initial cytochrome
P450-mediated oxidation of TBPP (Marsden & Casida, 1982; Soderlund et
al., 1984).
Recent data indicate that the formation of 2-bromoacrolein occurs
mainly from oxidative dehalogenation at the C3 position (Pearson et
al., 1993a).
Although glutathione acts as a detoxifying agent for reactive
TBPP metabolites (Soderlund et al., 1984), conjugation could also
result in the formation of reactive episulfonium ion intermediates
(Pearson et al., 1993b). Van Beerendonk (1994) noted that there is
S-(2,3-dihydroxypropyl) glutathione in the bile of Sprague-Dawley
rats. They suggested that TBPP and/or BBPP are conjugated directly
with glutathione by glutathione S-transferases, with subsequent
formation of episulfonium ions.
6.4.2 In vitro studies
TBPP is readily metabolized by microsomal and cytosolic rat liver
fractions. Liver microsomes metabolized TBPP in the presence of NADPH
and oxygen, as evidenced by the release of bromine and the formation
of BBPP (Kerst, 1974; Nomeir & Matthews, 1983).
The role of debromination in the formation of reactive
metabolites was demonstrated in a series of TBPP analogues (Soderlund
et al., 1984). The rate of NADPH-dependent metabolism was increased
5-10 times with microsomes from phenobarbital-pretreated rats compared
with control microsomes and was reduced in the presence of cytochrome
P450 inhibitors, indicating that cytochrome P450 is responsible for
microsomal TBPP biotransformation (Soderlund et al., 1979, 1981, 1984;
Nomeir & Matthews, 1983).
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to reactive intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from rodents (Soderlund et al.,
1982a).
In addition, a 1.5 to 2-fold increase in the rate of TBPP
metabolism occurred when phenobarbital-pretreated microsomes were
fortified with GSH, indicating that microsomal GSH- S-tranferases are
able to conjugate TBPP with GSH. Dialysed rat liver cytosolic
fractions, supplemented with GSH, metabolized TBPP at rates that were
3 times higher than those observed with control microsomes and NADPH
(Nomeir & Matthews, 1983; Soderlund et al., 1981, 1984). Thus, in
animals, GSH-dependent metabolism may be an important route in the in
vivo biotransformation of TBPP to more water-soluble products.
Soderlund et al. (1984) detected the in vitro formation of
2-bromoacrolein, by a reaction catalysed by cytochrome P450, in a
process liberating bromide ions with subsequent formation of BBPP
using rat liver microsomes (Soderlund et al., 1984). Mass spectral
analysis of 2-bromoacrolein, formed from selectively deuterated
analogues of TBPP, revealed that the primary mechanism for the
formation of 2-bromoacrolein involves the initial oxidative
dehalogenation at C-3 followed by a betaelimination reaction (Nelson
et al., 1984).
In vitro studies were carried out with deuterated analogues of
TBPP, or, analogues labelled at specific positions with carbon-14,
phosphorus-32, or oxygen-18, or dual-labelled with both deuterium and
tritium. These were used as metabolic probes to study the chemical
and metabolic events in the bioactivation of TBPP to chemically
reactive metabolites in the liver microsomal preparations of male
Sprague-Dawley rats. Studies with deuterated analogues of TBPP
implicated oxidation at C-2 of the propyl moiety as a major pathway
that leads to protein binding, which is enhanced by phenobarbital
pretreatment of rats. Investigations with 18O-TBPP and H218O
showed that the BBPP that is formed from the oxidation of TBPP
incorporates one atom of oxygen from water. These results imply that
oxidation at C-2 yields a reactive alpha-bromoketone that can alkylate
proteins directly, or, hydrolyse to BBPP and a reactive alpha-
bromoalpha'-hydroxyketone that alkylates microsomal proteins (Pearson
et al., 1993a). These studies also showed that TBPP is oxidized at
C-3, yielding the direct acting mutagen 2-bromoacrolein as the major
metabolite that binds to DNA. This is consistent with earlier studies
that indicate that 2-bromoacrolein is the major reactive metabolite
formed in in vitro microsomal incubations (Nelson et al., 1984;
Dybing et al., 1989).
6.5 Covalent binding to macromolecules
TBPP has been shown to be activated to products that bind
covalently to proteins (total macromolecules) and DNA in vitro and
in vivo (Soderlund et al., 1981, 1984; Pearson et al., 1993a,b).
The covalent binding of radiolabel TBPP to macromolecules was
dependent on microsomes and NADPH, and was reduced by carbon monoxide,
inhibitors of P450, and glutathione (Soderlund et al., 1981). The
extent of TBPP covalent binding in vivo was five times higher in the
kidneys than in the liver, whereas the rate of in vitro covalent
binding was much higher with liver microsomes than with kidney
microsomes. The low levels of TBPP binding in the liver in vivo may
be the result of an extensive detoxification of TBPP to non-reactive
metabolites or to low tissue concentrations of the proximate
metabolite(s).
Male NMRI and female B6C3F1 mice (20-25 g), male F344 rats
(200-250 g), and guinea-pigs (80-100 g) were injected ip once with
250 mg 3H-TBPP/kg body weight in DMSO. The animals were killed 9 h
after injection. All species showed similar levels of covalent
binding to proteins in the liver and kidneys except for the rat which
had much higher amounts of radiolabel bound to kidney proteins
(Soderlund et al., 1982a).
The binding of TBPP and analogues has also been studied in
vivo. Analogues of TBPP either labelled at specific positions with
carbon-14, and phosphorus-32 or dual-labelled with both deuterium and
tritium were administered to male Wistar rats at a nephrotoxic dose of
360 µmol/kg body weight. The covalent binding of TBPP metabolites to
rat hepatic, renal, and testicular proteins was determined after 9 and
24 h. The covalent protein binding was 5 times higher in the kidneys
than in the liver and approximately 25 times higher than that in the
testes. The results of comparative studies on renal DNA damage
induced by TBPP and BBPP labelled with deuterium at C-2 or C-3
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C-2 or C-3 of TBPP. BBPP is then transported to the
kidneys, where it is subsequently metabolized to reactive
intermediates that cause DNA damage and bind to kidney proteins in a
process, independent of cytochrome P450, involving activation by
conjugation with glutathione (Pearson et al., 1993b).
Van Beerendonk et al. (1992) studied the formation of thymidine
adducts and the cross-linking potential of 2-bromoacrolein (BA), a
reactive metabolite of TBPP. In this study, [3-3H]BA was reacted
with single-stranded (ss) DNA or double-stranded (ds) DNA and
subsequently incubated with methoxylamine to covert the reaction
product to an unstable BA:thymidine adduct. Because the unstable
BA:thymidine adduct may have the potential to form cross-links, the
reaction with various nucleophiles in vitro was studied. A reaction
occurred between the adduct and cystein, but not with lysine or
desoxynucleosides. Reaction of BA with ssDNA in the presence of
[3H]glutathione also resulted in the binding of radiolabelled GSH to
DNA. The results indicated that the reactive aldehyde group of the
adduct can react with thiol groups in proteins to form protein-DNA
cross-links. When the possibility that tris- and bis-(2,3-
dibromopropyl) phosphates form such cross-links was examined in vivo
in Drosophila, it was found that TBPP was a cross-linking agent,
whereas BBPP was not.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The oral LD50 for TBPP was calculated to be 5.24 g/kg body
weight, when administered as a suspension in propyleneglycol to male
albino Spartan rats with a weight of 202-250 g. The observation
period was 14 days (Kerst, 1974). In another study, TBPP dissolved in
propyleneglycol or in ethanol was given to Osborn-Mendel rats. Oral
LD50s of 1.88 and 3.12 g/kg body weight, respectively, were obtained
(Ulsamer et al., 1980).
A dermal toxicity study showed an acute LD50 for rabbits of
17.6 g/kg body weight (Ulsamer et al., 1980). In another study, TBPP
was applied once to the back of four groups of two male and two female
New Zealand white rabbits (2.56-2.96 kg) in concentrations of 1, 2, 4,
or 8 g/kg body weight. The application area was wrapped with a gauze
bandage and occluded; after 24 h, the bandages were removed and the
skin washed with water. The observation time was 14 days. An LD50
of > 8 g/kg body weight was found (Kerst, 1974).
A dose of 2 g TBPP was applied to the intact and abraded skin of
10 albino rabbits. No deaths were observed during a 14-day
observation period (Moldovan, 1972).
7.2 Short-term exposure
7.2.1 Oral exposure (rat)
7.2.1.1 TBPP
Groups of male rats received daily doses of 250 mg TBPP/kg in
either propyleneglycol or saline, by gavage, and were sacrificed after
1, 2, 4, 6, 8, or 10 days. Liver and testes were unaffected by any
treatment, but nephrotic changes were observed to commence on day 2
and to become progressively more severe with time. In addition to the
tubular lesions, the glomeruli were adversely affected, an observation
not seen in the 13-week study (Osterberg et al., 1979).
In a pilot study, groups of 10 non-pregnant rats were
administered TBPP for 10 days at dose levels of 100, 150, 500, or
1000 mg/kg body weight per day. Mortality rates were 0, 0, 70, and
100%, respectively (Seabaugh et al., 1981).
Male weanling rats were fed TBPP at concentrations of 100 or
1000 mg/kg diet for 28 days. The animals were then sacrificed
immediately or after 2 or 6 additional weeks of recovery. The results
showed a decrease in food efficiency (approximately 10% at the
highest dose), decreased body weight gain (approximately 20% at the
highest dose), and decreased organ to body weight ratios for heart,
liver, spleen, kidney, and gonads (approximately 20% for each organ at
the highest dose). Haematology, blood chemistry, urinalysis, and
histopathology did not differ from the control values. In the
recovery period, the body weight gain became normal. The authors
suggested that the effect might be because of the palatability of the
substance. Tissue residues (measured as bromine) increased 40-50
times in the first 4 weeks of treatment in the fat, liver, and
muscles. By the end of the 6-week withdrawal period, the residues
were at control levels (Kerst, 1974).
Groups of rats were gavaged with TBPP in corn oil at 10, 50, or
100 mg/kg per day for 4 weeks. One half of each group was sacrificed
at 4 weeks and the remainder at 6 weeks. While no adverse responses
were observed, elevated bromine levels in blood were reported (Brieger
et al., 1968).
A 90-day study was carried out on rats administered TBPP in
propylene glycol, daily (by gavage), at 25, 100, or 250 mg/kg body
weight. The control groups received either the vehicle, normal
saline, or no treatment. Weight gain for males was 34-50% less and,
for females, 40% less in the test groups and vehicle group compared
with the control values. Liver/body weight ratios were lower for both
sexes in the low TBPP group, but higher in females in the highest dose
group, compared with those in the control group. Kidney/body weight
ratios were 18% lower than in controls. Testes/body weight ratios in
the TBPP groups were 25% lower. There was an increased incidence and
severity of chronic nephritis associated with regenerative epithelium,
hypertrophy, and dysplasia of renal tubular epithelial cells in all
TBPP-treated rats. The complex of changes was more severe with higher
dose, and among males (Osterberg et al., 1978).
7.2.1.2 TBPP-treated fibres
The results of a 2-week study on rats fed 15% shredded
TBPP-treated acetate fibres in their food (3 times/week) showed no
changes in blood-bromine levels and no adverse effects (Ulsamer et
al., 1980).
7.2.2 Oral exposure (dog)
7.2.2.1 TBPP
In a study on dogs, doses of 50 or 100 mg TBPP/kg body weight
were given in the diet for four weeks. A decrease in body weight was
noted in the treated dogs as well as increased blood-bromine levels.
Cholinesterase activity was reported to be unaffected (Brieger et al.,
1968). No further details were available for this study.
7.2.2.2 TBPP-treated fibres
In a 2-week study on dogs fed 15% shredded TBPP-treated acetate
fibres in their food (3 times/week), no changes in blood-bromine
levels or adverse effects were seen. Two additional, 3-week studies
on dogs using TBPP-treated shredded rayon and acetate fibres added to
foods did not show any detectable changes in health or in
blood-bromine levels (Brieger et al., 1968).
7.2.3 Dermal exposure
7.2.3.1 Rabbit
Short-term dermal studies have been performed using groups of
clipped rabbits dosed with 2.2, 4.4, or 8.8 g TBPP/kg body weight,
daily, for 4 weeks. A dose-related increase in bromine was found in
the blood and urine. All rabbits died within 4 weeks. Significant
degenerative changes in the kidneys and the liver were found. Slight
decreases in cholinesterase activity were recorded (Brieger et al.,
1968). In another study in which the animals were administered dose
levels of 50 and 250 mg/kg body weight, bromide levels in the blood
and urine were increased, but no deaths occurred (Ulsamer et al.,
1980).
A 13-week study was carried out on 12 young (3 months old) New
Zealand white rabbits, 6 with intact, and 6 with abraded, dorsal skin.
They were treated with a weekly application of 2.27 g TBPP (99.76%)/kg
body weight for 13 weeks. In a third group, 6 rabbits were initially
clipped and maintained untreated as controls. The TBPP-treated sites
were not occluded with a patch, but the animals were fitted with a
collar. Besides a statistically significant increase in relative
liver weights in the rabbits with intact and abraded skin (53% and
59%, respectively), a significant decrease in testes weight (54% and
40%, respectively) was observed. Microscopically, chronic
interstitial nephritis (in 6/8 males) with tubule involvement and
bizarre nuclei as well as testicular atrophy and aspermatogenesis
(spermatogonia were present in seminiferous tubules, and also
secondary spermatocytes, but no spermatozoa) were observed in 7/8
males of the test groups. Female rabbits did not exhibit any adverse
responses. No histopathological changes were seen in the liver
(Osterberg et al., 1977, 1978).
In a study in which TBPP-treated rayon cloth was applied to the
clipped skin of rabbits for 4 weeks, no significant effects were found
(bromine levels were not increased) in treated animals (Ulsamer et
al., 1980). No further details were available for this study.
7.2.3.2 Dog
When TBPP-treated rayon cloth was applied to the clipped skin of
dogs for 4 weeks, no significant effects (no increased bromine levels)
were found in the treated animals (Brieger et al., 1968). No further
details were available for this study.
7.3 Long-term exposure
Apart from carcinogenicity studies, no long-term toxicity studies
are available (see section 7.7).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
TBPP (1.1 g) was applied to the abraded or intact skin of six
albino rabbits. The animals were fitted with collars for 24 h. After
this period, the coverings were removed and the test material washed
off. The extent of erythema and oedema was determined after 24 and
72 h. No signs of irritation were observed (Kerst, 1974).
7.4.2 Eye irritation
Administration of 0.22 g TBPP to the eyes of 6 adult rabbits did
not cause noticeable irritation or damage to the cornea, iris, or
palpebral conjunctiva during a 72-h observation period (Kerst, 1974;
US EPA, 1976).
7.4.3 Sensitization
TBPP was tested for skin sensitization in groups of 5-10
guinea-pigs using a modified Landsteiner method and the footpad
technique. No sensitization was noted in either test (no details
given) (Morrow et al., 1976).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
7.5.1 Reproductive system
Groups of 6 adult male Sprague-Dawley rats (56-60 days of age)
were used in a study to investigate the effects of TBPP on the
reproductive system. Six rats were injected with 0.1 ml
propyleneglycol intraperitoneally, three times/week, and, six rats
were untreated controls. Nine groups of 6 rats were given (ip
injection), three times/week, 0.4, 0.9, 1.8, 3.5, 7.1, 14.2, 28.4,
56.8, or 113.5 mg TBPP in propyleneglycol for a period of 72 days. The
four highest dose levels of TBPP did not dissolve completely and were
injected as an emulsion. The rats were treated for a minimum of 72
days (6 cycles of the germinal epithelium) before being killed. The
three highest dose levels (28.4-113.5 mg/injection) caused significant
dose-related declines in the weights of the testes and prostate,
epididymides, and seminal vesicles. Sperm production of testes and
sperm storage in the epididymides were reduced, and the percentage of
the motile sperm and the motility index were decreased. Histological
examination of the testes revealed that the seminiferous tubules were
affected. The affected tubules contained very few germinal cells and
the macrophages in the interstitium of the affected testes appeared to
be phagocytically active. The Leydig cells were normal. TBPP did
not have any significant effects on the serum concentration of
testosterone or on the in vitro testicular capacity for testosterone
secretion (Cochran & Wiedow, 1986).
The effects on the testes were also reported in a 13-week study
on New Zealand white rabbits, treated with weekly dermal applications
of 2.27 g TBPP on the intact or abraded skin. Decreased testes
weights and, microscopically, testicular atrophy and aspermatogenesis
were found in male rabbits (Osterberg et al., 1977).
B6C3F1 mice (15 weeks old) were administered (ip) TBPP in corn
oil at dose levels of 0, approximately 200, 400, 600, 800, and
1000 mg/kg body weight daily, for 5 days. The mice were killed 35
days after the fifth treatment. Their epididymides were removed and
abnormal sperm heads determined. The frequency of abnormal sperm
heads in TBPP-treated mice was significantly greater than in controls,
predominantly at dose levels of 800 mg/kg body weight or more
(Salamone & Katz, 1981).
7.5.2 Teratogenicity
In a pilot study on groups of ten pregnant Sprague-Dawley rats,
orally intubated with 0, 250, or 1000 mg TBPP/kg body weight on days
6-15 of gestation, an increase in maternal mortality was observed.
The mortality rates were 0, 10, and 100% respectively. The rats given
1000 mg/kg died on days 9-11 of gestation (Seabaugh et al., 1981).
Sexually mature, timed-pregnant Sprague-Dawley rats, 30 animals
per group, were intubated on days 6-15 of gestation with TBPP (99.7%
TBPP, 0.14% 1,2,3-tribromopropane, and 0.17% 2,3-dibromopropanol) in
undiluted propyleneglycol at levels of 0, 5, 25, or 125 mg/kg body
weight per day. Maternal body weight gain was decreased at the
highest dose level. No effects of treatment were apparent on the
number of corpora lutea, implantations, or early or late deaths.
Furthermore, the percentage of females with resorptions, the number of
viable fetuses, the percentage of resorptions, and the percentage of
pre-implantation losses, did not show compound-related changes. Fetal
body weight and crown-rump length were not affected. Some fetal soft
tissue and skeletal variations found were not dose-related or
statistically significant. It was concluded that TBPP was not
teratogenic in this study (Seabaugh et al., 1981).
Female Wistar rats were exposed orally to 25, 50, 100, or 200 mg
TBPP in olive oil/kg body weight on days 7-15 of gestation. A
significant increase in skeletal variation was found in the fetuses at
200 mg/kg. A significantly lower viability index was observed in the
50 and 100 mg/kg groups. The authors concluded that TBPP did not
produce teratogenic effects in rats. A dose of 200 mg/kg elicited
maternal toxicity (Kawashima et al., 1983).
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
7.6.1.1 In vivo
When male Wistar rats (250-320 g) were given a single ip
injection of 350 µmol TBPP/kg (250 mg/kg) body weight and assayed for
DNA damage 2 h later, single strand breaks/alkali labile sites were
found in the DNA from nuclei isolated from several organs. DNA damage
was detected using an automated alkaline elution system. Extensive
DNA damage was detected in the liver, kidneys, and small intestines.
In addition, substantial DNA damage was found in the brain and lungs;
less DNA damage was detected in the testes, spleen, and large
intestines (Holme et al., 1983; Soderlund et al., 1992). DNA damage
was clearly detectable in the kidneys 20 min after a single ip dose of
36 µmol TBPP/kg (25 mg/kg) body weight (Pearson et al., 1993b).
7.6.1.2 In vitro
Monolayer cultures of human (KB) cells were grown with
[3H]-thymidine for 30 h, and without, for another 17 h. The cells
were then exposed to TBPP (2 µl/ml of growth medium devoid of serum)
for 4.5 h and processed for analysis of the DNA on alkaline-sucrose
gradients. They were re-incubated for various intervals to permit DNA
repair. TBPP was shown to have induced DNA repair, which indicated a
specific action on human cellular DNA. TBPP was found to damage human
DNA in vitro and to cause unscheduled DNA synthesis in human cells
in tissue culture (Gutter & Rosenkranz, 1977; Blum & Ames, 1977).
A semiquantitative, in vitro method for measuring unscheduled
DNA synthesis (UDS) was developed by Lake et al. (1978). Normal
foreskin epithelial cells from a cryopreserved skin pool were grown
from explants and replanted in replicate culture wells. Cultures were
then treated for 3 days in an arginine-deficient medium and further
inhibited in S-phase DNA-synthesis by a 2-h (10 mmol/litre)
hydroxyurea treatment. 3H-Thymidine and TBPP were added
simultaneously and the UDS, accumulated over a 24-h incubation period,
was determined by direct scintillation counting of acid-precipitable
whole-cell radioactivity. TBPP did not induce an UDS response in this
assay, with input dose ranges of 10-99 and 100-400 µg/ml.
UDS was detected in rat liver hepatocytes, grown as monolayer
cultures, exposed to 0.01-0.1 mmol TBPP/litre for 18-19 h in the
presence of [3H]-thymidine and hydroxyurea. UDS was determined by
scintillation counting (Holme et al., 1983; Holme & Soderlund, 1984;
Gordon et al., 1985; Soderlund et al., 1985).
In in vitro test systems, DNA damage was detected in isolated
rat hepatocytes exposed to concentrations as low as 5 µmol TBPP/litre,
while a 10-fold higher concentration was necessary to induce DNA
damage in testicular cells (Soderlund et al., 1992). No DNA damage
was found in cultured Reuber rat hepatoma cells, without the addition
of an exogenous metabolism system (Gordon et al., 1985).
7.6.2 Mutation assay with Salmonella typhimurium strains
Species differences in the bioactivation of TBPP to metabolites,
mutagenic to Salmonella typhimurium TA 100, have been reported.
Liver microsomes from mice (NMRI strain) were more effective in
activating TBPP to mutagenic intermediates than those from
guinea-pigs, hamsters, and rats. Phenobarbitalinduced liver
microsomes from NMRI mice were especially effective (Soderlund et al.,
1982a).
TBPP was activated to mutagens in the Salmonella/microsome
test. S9-fractions from rats pretreated with phenobarbital increased
the mutagenicity of 0.05 mmol TBPP/litre in TA 100 strain compared
with liver microsomes from untreated rats (Holme et al., 1983).
It was demonstrated that the metabolic activation is dependent on
the presence of NADPH and oxygen, which indicates that TBPP is
metabolized by cytochrome P450 enzymes to mutagenic products. In
studies conducted in an anaerobic atmosphere or in the presence of
GSH, the mutagenicity of TBPP was significantly decreased (Soderlund
et al., 1979, 1984).
TBPP (97%) in DMSO was tested in concentrations of 0.0110 µlitre
on Salmonella typhimurium TA 100, TA 1535, TA 1537, and TA 1538,
using the plate assay, in the absence, and presence, of a metabolic
activation system from rat liver. A mutagenic effect was found with
TA 100 and TA 1535 with, and without, metabolic activation. TA 1537
and TA 1538 gave negative results (Blum & Ames, 1977; Brusick et al.,
1978; Prival et al., 1977).
TBPP was tested on Salmonella typhimurium tester strains
TA 1535 and TA 1538 in the absence, and presence, of metabolic
activation derived from Aroclor-induced rat liver. Dose levels of 0,
0.1, and 1.0 µlitre/plate were used. Weak mutagenic activity was
observed in TA 1535 without activation, but a strong effect was seen
with microsomal activation. TA 1538 gave negative results (Carr &
Rosenkranz, 1978).
MacGregor et al. (1980) confirmed the mutagenicity of TBPP in the
Salmonella typhimurium strains TA 100, TA 98, and TA 1535, with dose
levels ranging from 10 to 1000 µg/plate, with metabolic activation.
Without activation, no mutagenicity was found. A negative result was
obtained in strain TA 1537 with, and without, activation.
Nakamura et al. (1979) tested TBPP on Salmonella typhimurium
strains TA 100 and TA 1535 with, and without, metabolic activation at
dose levels of 0.3-100 µmol/plate. A positive effect was seen in both
strains, without and with S9 mix. McCann & Ames (1977) found a
mutagenic effect in Salmonella typhimurium TA 100 with dose levels
up to 100 µg/plate, in the presence of liver S9 fraction of rats
treated with Aroclor.
TBPP at dose levels of 0, 112, 224, 2240, 4480, and
11 200 µg/plate was tested on Salmonella typhimurium strain TA100
with, and without, metabolic activation by Aroclor 1254-induced rat
liver S9 fraction. With the S9 fraction, all dose levels showed a
mutagenic effect. Without the S9 fraction, TBPP showed direct-acting
properties only at dose levels of 2240 µg/plate or more (Salamone &
Katz, 1981).
In an interlaboratory study, TBPP and 62 other chemicals were
tested for mutagenic activity. TBPP was tested on the Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, and on
Escherichia coli WP2uvrA. The dose levels were between 0.3 and
10 000 µg/plate. TBPP was tested without metabolic activation and
with liver S9 fractions from uninduced and Aroclor 1254-induced F344
rats, B6C3F1 mice, and Syrian hamsters. TBPP tested positive in all
four laboratories involved in this study (Dunkel et al., 1985).
Results obtained by Prival et al. (1977) indicated that TBPP
induces mutations of the base-pair substitution type in Salmonella
typhimurium TA100. Although, at higher concentrations
(> 1 µl/plate), TBPP behaves as a direct acting mutagen not requiring
metabolic activation, at a much lower concentration (0.01 µl/plate) it
demonstrates significant genetic activity only with metabolic
activation.
Brusick and coworkers demonstrated that amounts of 50 µg/plate or
more were clearly mutagenic for Salmonella typhimurium TA 100
(Brusick et al., 1980). When tested for bacterial mutagenicity in
Salmonella typhimurium TA 100, a 4-fold interindividual variation in
the capability to activate TBPP was noted with human liver microsomes
prepared from 5 liver donors (Soderlund et al., 1982a).
The CASE structure-activity method was applied to a Gene-Tox
derived Salmonella mutagenicity data base. Strains TA 97, TA 98, TA
100, TA 1535, TA 1537, and TA 1538 with, or without, exogenous
metabolic activation, were used. TBPP was found to be positive
(Klopman et al., 1990).
7.6.3 Mutations by urine of rats treated with TBPP
The urine was collected of rats exposed to TBPP directly by
either the oral or dermal route, or from treated fabric. Salmonella
typhimurium TA 1535 was used as indicator organism. TBPP was
dermally applied at doses of 5, 50, 500, or 5000 mg/kg body weight or
given orally at 5, 50, or 500 mg/kg body weight. In the oral study,
only 500 mg/kg produced a positive response. In the dermal studies, a
dose of 500 mg/kg produced a weak positive response, while 5000 mg/kg
produced a definitive positive response. When fabrics with surface
TBPP levels of 3000, 28 000, and 67 000 mg/kg product were applied
dermally, no mutagenic responses were detected in the urine of the
rats over the 5-day period (data were lacking on whether or not
metabolic activation was used) (Brusick et al., 1978; Ulsamer et al.,
1980).
TBPP at 500 mg/kg body weight in corn oil was applied dermally to
CD-1 mice. Urine was collected over approximately 16 h and the
bacterial mutagenicity of 0.3 ml urine samples was assayed in
Salmonella typhimurium TA1535, TA1537, and TA100. A positive
response was found only with TA100 (Brusick et al., 1982).
7.6.4 Other mutation assays
TBPP was tested in the forward mutation assay with mouse-lymphoma
cells (L5178YTK locus). While the results at lower doses were
inconclusive, a 2 to 3-fold increase in mutations was consistently
produced at 5 mg/litre (Brusick et al., 1978; Ulsamer et al., 1980).
TBPP has been reported to induce increased mutation frequencies
(6-TG resistance) in V79 Chinese hamster cells incubated with
0.02 mmol TBPP/litre in the presence of liver microsomes of rats
pretreated with phenobarbital as an exogenous metabolism system (Holme
et al., 1983; Soderlund et al., 1985). However, in a similar study,
concentrations of TBPP up to 150 µg/ml did not increase the frequency
of 6-TG resistance, both with, and without, an exogenous metabolism
system (Sala et al., 1982).
7.6.5 Chromosomal effects
Using Chinese hamster V79 cells, TBPP severely inhibited the
colony-forming activity and significantly increased sisterchromatid
exchanges, but no significant increase in chromosome aberrations was
found (Furukawa et al., 1978). Interestingly, chromosomal aberrations
were not significantly increased in Chinese hamster cells, in mouse
bone-marrow cells, or in cultured human lymphoid cells. The lack of a
TBPP effect on rat bone-marrow chromosomes was also observed after
rats received 25, 250, or 2500 mg TBPP/kg body weight, by gavage,
after either a single dose, or, 5 daily doses/week for 13 weeks
(Osterberg, 1977; Nakanishi & Schneider, 1979).
TBPP was tested for the induction of chromosome aberrations, and
sister chromatid exchanges in the diploid human fibroblastic cell line
HE 2144 (from a 10-week-old male embryo) without metabolic activation
(Sasaki et al., 1980). The dose levels used were 0.349, 0.070, and
0.035 mg/ml. Sister chromatid exchanges were induced with 0.070 mg
TBPP/ml in the human HE 2144 cell line. No chromosomal aberrations
were found.
In a comparative study, Brusick and coworkers found that TBPP
gave a positive response in tests for sister chromatid exchanges and
chromosomal aberrations in the mouse lymphoma L5178Y cell line at
concentrations of 0.005 and 0.01 µlitre/ml, respectively (Brusick et
al., 1980).
TBPP was tested in an in-vitro test for sister chromatid
exchanges in Chinese hamster V79 cells with, and without, S9 fraction
of livers of Wistar rats administered (ip) methylcholanthrene.
Acetone was used as solvent. The dose levels 17.2, 35, 100, and
200 µg/ml were tested only without S9 fraction, while levels of 24.5
and 50 µg/ml were tested with, and without, metabolic activation. A
significant increase in sister chromatid exchanges was found at dose
levels of more than 35 µg/ml (Sala et al., 1982).
Two male and two female Chinese hamsters per group were used in a
micronucleus test. The dose levels were 200, 400, and 800 mg TBPP/kg
body weight administered by ip injection. The solvent was DMSO.
Bone-marrow samples were obtained after 24 h. Two thousand
polychromatic erythrocytes/animal were analyzed for the presence of
micronuclei. Levels of 400 and 800 mg/kg body weight showed a
positive effect (Sala et al., 1982).
Salamone & Katz (1981) studied the clastogenic effect of TBPP in
a bone marrow micronucleus test. B6C3F1 mice (15 weeks old) were
given two ip treatments of TBPP in corn oil. Dose levels of 0, 204,
408, 612, 816, 1020, 1275, and 1530 mg/kg body weight were tested. In
this test, TBPP showed a weak clastogenic effect.
An in vitro chromosome aberration test was carried out with
TBPP, using a Chinese hamster CHL cell line of lung fibroblast origin.
CHL cells cultured in plates were exposed to different dose levels of
TBPP including the 50% growth inhibition dose. The number of
polyploid cells and cells with structural aberrations, such as
chromatid-type gaps, breaks, exchanges, and rings, were scored. A
microsome fraction (S9-mix) from the liver of Wistar rats, pretreated
with the PCB; KC-400, was used. TBPP was positive in this test. A
dose level of 0.25 mg/ml showed chromosomal aberrations in 20% of the
metaphases (Ishidate et al., 1981).
Vogel & Nivard (1993) studied the effects of TBPP in the
(white/white+) (w/w+) eye mosaic assay, and an in vivo, short-term
test measuring genetic damage in the somatic cells of Drosophila
melanogaster, after treatment of the larvae. The genetic principle
of this system is the loss of heterozygosity for the wild-type
reporter gene w+, an event predominantly resulting from homologous,
interchromosomal, mitotic recombination between the two X-chromosomes
of female genotypes. The w/w+ eye mosaic test detects a broad
spectrum of DNA modifications. Between 12 and 15 pairs of flies were
permitted to lay eggs for three days on food supplemented with 0.25,
0.5, or 1.0 mmol TBPP/litre (dissolved in 3% ethanol). TBPP gave a
strong positive response in the w/w+ bioassay.
7.6.6 Cell transformation
TBPP was tested for its ability to induce malignant
transformation in vitro using mouse BALB/3T3 cells. The results of
this test showed that TBPP can transform mammalian cells in vitro,
perhaps indicating a potential for the induction of carcinogenic
responses (Brusick et al., 1978; Ulsamer et al., 1980).
C3H/10T1/2 cells were treated with TBPP, with or without S9 mix
from the liver of Wistar rats administered methylchloanthrene
intraperitoneally. Some cell samples were additionally treated
several times with tetradecanoyl phorbolacetate (TPA) (0.1 µg/ml).
The TBPP concentrations tested were 40 µg/ml (with and without S9
fraction) and 80 µg/ml (without S9 fraction). A very low frequency of
transformed type 3 foci was obtained and the authors considered the
results of this study to be negative (Sala et al., 1982). Dunkel et
al. (1988) also found a negative result for TBPP in the C3H/10T1/2
cell transformation assay. The dose levels tested were between 0.16
and 20 µg/ml.
7.6.7 Miscellaneous tests
TBPP induced a significant increase in sex-linked recessive
lethal mutations in male germ-cell stages of Drosophila melanogaster
at a dose of 1000 mg/kg. The spermatids were the most sensitive
(Valencia, 1978).
Adult Canto-S male Drosophila flies aged 7 days were fed for
48 h on a 1% solution of glucose containing 1000 or 10 000 mg
TBPP/litre. The TBPP-exposed males were mated immediately after
treatment to brown ebony virgin females. The results showed that TBPP
caused reciprocal translocations in Drosophila. There was no
difference between the translocation recoveries at the two dose levels
(Berkowitz, 1978).
7.6.8 Mechanisms of TBPP genotoxicity
Following the initial mutagenicity reports, several studies have
been directed towards the elucidation of the mechanisms involved in
TBPP-induced genotoxicity. Such studies include investigations into
the enzyme systems involved in the bioactivation of TBPP to genotoxic
metabolites, delineating structural requirements for genotoxicity, and
the characterization and identification of the genotoxicity of
possible TBPP metabolites. Some of these investigations have recently
been reviewed (Dybing et al., 1989).
The bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. Several studies have shown that TBPP is
activated to metabolites, mutagenic to Salmonella typhimurium TA100,
by cytochrome P450 in a reaction depending on NADPH and oxygen
(Soderlund et al., 1979; Dybing et al., 1989). The mutagenicity of
TBPP was increased in the presence of microsomes prepared from livers
of rodents pretreated with phenobarbital or PCBs, but not from those
pretreated with 3-methylcholanthrene or beta-naphthoflavone (Soderlund
et al., 1979; 1982a).
The metabolism of TBPP by soluble enzymes (e.g., glutathione
S-transferase) appears to be of minor importance in the
bioactivation of TBPP to mutagenic species. The available data
indicate that reductive metabolism and episulfonium ion formation are
not major activation pathways in TBPP-induced mutagenicity (Dybing et
al., 1989). However, it has recently been suggested that human
glutathione transferases may further metabolize P-450-generated TBPP
intermediates to more potent mutagenic species (Simula et al., 1993).
Interestingly, singlet oxygen, obtained from the illumination of
riboflavin, may also activate TBPP to mutagenic metabolites (McCoy et
al., 1980).
Studies investigating the bacterial mutagenicity of known and
postulated metabolites of TBPP have given little information regarding
the mechanisms of its bioactivation. With the exception of
2-bromoacrolein (BA) and DBP, other postulated or identified TBPP
metabolites were less mutagenic than TBPP when tested with, or
without, an exogenous metabolism system (Prival et al., 1977;
Soderlund et al., 1979; Zeiger et al., 1982; Holme et al., 1983;
Gordon et al., 1985). Such metabolites include the two major TBPP
metabolites 2,3-dibromopropanol (DBP) and bis(2,3-dibromopropyl)
phosphate (BBPP) as well as mono(2,3-dibromopropyl) phosphate (mono-
BP) (McCann & Ames, 1977; Prival et al., 1977; Soderlund et al.,
1982b; Holme et al., 1983). These metabolites were also less
mutagenic than TBPP in V79 Chinese hamster lung cells with microsomal
activation (Holme et al., 1983).
Eight coded samples of different lots of TBPP from different
manufacturers were tested in TA 1535. All were positive (Prival,
1975).
Several commercial TBPP samples and a few known contaminants
(1,2,3-tribromopropane, DBP, and 1,2-dibromo-3-chloropropane) were
tested in amounts of 0.01 up to 10 µl/plate on Salmonella strains TA
100, TA 1535, and TA 1538 with, and without, Aroclor 1254-activated,
or non-activated, rat liver S9 fractions. The results indicated that
the highest levels of mutagenicity obtained with commercial TBPP were
probably not due to the presence of the contaminants (Prival et al.,
1977).
The bacterial mutagenicity of compounds structurally related to
TBPP, including monobrominated and chlorinated analogues, unsaturated
and saturated methyl esters, and halogenated propanols, indicate the
following structural effects on mutagenic activity: (a) a decrease in
the number of alkyl chains decreases mutagenicity; (b) a decrease in
the number of halogens in the alkyl chain decreases mutagenicity; (c)
compounds with vicinal halogens are more mutagenic than 1,3-dihalo-
isopropyl analogues; (d) brominated compounds are more mutagenic than
the corresponding chlorinated ones; and (e) salts of mono- and
diphosphate esters are more mutagenic than the free base (Carr &
Rosenkranz, 1978; Nakamura et al., 1979, 1983; Zeiger et al., 1982;
Holme et al., 1983).
Mutagenicity testing of TBPP metabolites has yielded little
information on the mechanisms of activation of TBPP to mutagenic
metabolites, since none of the metabolites were more mutagenic than
the parent compound. The first evidence for the possible formation of
a potent bacterial mutagen from TBPP came when 2-bromoacrylic acid was
found in the urine of rats, administered large doses of TBPP (Marsden
& Casida, 1982). These authors proposed that TBPP is initially
oxidized at the C-1 position to yield 2,3-dibromopropanal, which
then spontaneously dehydrobrominates to give BA. BA and 2,3-
dibromopropanal, which are potent, direct-acting, bacterial mutagens
in Salmonella typhimurium TA100, caused DNA damage in Reuber rat
hepatoma cells, and transformation of Syrian hamster embryo cells
(Rosen et al., 1980; Gordon et al., 1985).
In 1984, BA was detected in incubations of TBPP with rat liver
microsomes, using GC/MS. In these experiments, substitution of
deuterium atoms for hydrogen at the C-3 position decreased the
mutagenicity of TBPP by approximately 80%, whereas only a small
deuterium isotope effect was noted at C-2 and C-1. These data
indicate that an initial oxidation at the terminal carbon atom is the
key step in the formation of mutagenic metabolites from TBPP (Nelson
et al., 1984; Soderlund et al., 1984). Subsequent experiments with
variously deuterated TBPP analogues revealed that, according to the
number of deuteriums retained in the BA formed, oxidation at C-3 is
the major pathway for BA formation (Nelson et al., 1984). The results
of the studies with deuterated TBPP analogues were later substantiated
with selectively methylated analogues. These findings demonstrated
that the initial oxidation of TBPP at C-3 was followed by spontaneous
dehydrohalogenation and dehydrophosphorylation, with the subsequent
formation of BA and BBPP (Omichinski et al., 1987).
Although BA is the most potent TBPP metabolite with regard to
bacterial mutagenicity in vitro, additional TBPP metabolites appear
to be involved in TBPP-induced DNA damage in mammalian cells. The
formation of these metabolites has not yet been elucidated, but is
likely to involve conjugation with glutathione (Soderlund et al.,
1992).
All the known metabolites, including the two major metabolites,
DBP and BBPP, are considerably less mutagenic than the parent
compound, when tested directly or in the presence of an activation
system (Blum et al., 1978; Soderlund et al., 1979, 1982b; Zeiger et
al., 1982). However, BA is a more potent mutagen than TBPP (Rosen et
al., 1980; Nelson et al., 1984; Gordon et al., 1985).
7.7 Carcinogenicity
7.7.1 Oral
7.7.1.1 Mouse
Groups of 50 male, and 50 female, B6C3F1 hybrid mice, 6 weeks
old, were fed technical TBPP (containing no detectable 1,2-dibromo-3-
chloropropane) at concentrations of 500 or 1000 mg/kg diet in the diet
for 103 weeks followed by a 1-week observation period. The
experimental design of the study is shown in Table 1. Of the males,
44/55 matched controls, 38/50 low-dose mice and 43/50 high-dose mice
survived until the end of the study; of the females 44/55 controls,
37/50 low-dose mice, and 38/50 high-dose mice survived. TBPP
increased the incidence of squamous-cell carcinomas and papillomas of
the fore-stomach and of adenomas and carcinomas of the lungs in both
male and female treated animals compared with the controls. There was
also an increased incidence of renal tubular cell adenomas and
adenocarcinomas in treated male mice and liver cell adenomas and
carcinomas in treated female mice. Neoplastic lesions associated with
the administration of TBPP are summarized in Table 1. The incidence
of "preneoplastic" kidney changes, dysplasia, and hyperplasia, were:
controls (males and females) 0/109, low-dose females 20/50, low-dose
males 46/50, high-dose females 40/46 and high-dose males 49/50 (US
NCI, 1978; Reznik et al., 1979; IARC, 1979).
7.7.1.2 Rat
Groups of 55 male, and 55 female Fischer 344 rats, 6 weeks old,
were fed diets containing concentrations of 50 or 100 mg technical
TBPP/kg for 103 weeks, followed by a 1- or 2-week observation period.
The experimental design of the study is shown in Table 1. Of the
males 39/55 control, 35/55 low-dose, and 40/55 high-dose rats survived
until the end of the study; of the females 36/55 control, 44/55
low-dose, and 36/55 high-dose rats survived. The compound increased
the incidences of both renal tubular cell adenomas in rats of both
sexes and tubular cell adenocarcinomas in high-dose males.
Neoplastic lesions associated with the administration of TBPP are
summarized in Table 1. The incidence of "preneoplastic" kidney
changes, dysplasia, and hyperplasia, were; controls (males and
females) 0/105, low-dose females 25/54, low-dose males 53/54,
high-dose females 46/54, and high-dose males 39/54 (US NCI, 1978;
IARC, 1979; Reznik et al., 1979).
Table 1. Tumour incidences in mice and rats fed tris(2,3-dibromopropyl) phosphate (TBPP)
Species Sex Number Concentration Duration Number of tumour-bearing animals/number of animals examined
of animals (mg/kg (weeks)
treated diet) Forestomach** Lung** Kidneys* Liver**
(squamous-cell (adenomas or (tubular-cell (adenomas or
carcinomas or carcinomas) adenomas or carcinomas)
papillomas) adenocarcinomas)
Mouse male 55 0 105 0/51 12/54 0/54 28/54
male 50 500 103 10/47a 18/44c 6/50 31/49
mate 50 1000 103 13/48b 25/50d 17/49e 23/49
female 55 0 105 2/53 4/55 0/55 11/54
female 50 500 103 14/48b 9/50 3/50 23/50f
female 50 1000 103 22/44b 17/50d 3/46 35/49f
Rat male 55 0 107 - 0/54 0/53 0/54
male 55 50 103 - 3/55 30/54e 1/55
male 55 100 103 - 0/55 30/54a 4/54
female 55 0 107 - - 0/52 -
female 55 50 103 - - 4/54 -
female 55 100 103 - - 13/54a -
From: * Reznik et al. (1979); ** US-NCI (1978).
Fisher analysis of treated group versus control:
a Squamous-cell papillomas; P < 0.01; b Squamous-cell carcinomas and papillomas; P < 0.01; c Alveolar/bronchiolar
adenomas and carcinomas; P < 0.05; d Alveolar/bronchiolar adenomas and carcinomas: P < 0.01: e Tubular-cell adenomas
and adenocarcinomas: P < 0.01; f Hepatocellular adenomas and carcinomas; P < 0.01.
Male F344 rats, 4 weeks old, were administered (by gavage) 0
(untreated), and 0 (vegetable oil), or 100 mg TBPP in vegetable oil/kg
body weight per day. Treatment was given for 5 days per week and
continued for 4 or 52 weeks. Selected rats of the treated and control
groups were killed at various time intervals. Control and treated
animals (2-9 animals) were killed after 1, 5, 10, 20, 50, 75, and 260
treatments. After 4 weeks (20 treatments), the TBPP group was divided
into two subgroups; TBPP administration was continued in one subgroup
of 15 rats and, in the second subgroup, 15 other rats (treated for
four weeks) received only vegetable oil for the remainder of the
study. The histomorphology and ultrastructure of the kidneys were
studied. Twenty-four hours after the first TBPP treatment, the
epithelial cells at the corticomedullary junction developed increased
nucleus/cytoplasm ratios, cytomegaly, and nuclear vacuolization and
pleomorphism. These changes increased in severity to a toxic tubular
nephrosis as treatment continued, and extended to the peripheral
cortex by 52 weeks. After 52 weeks of TBPP treatment, small tubular
papillary hyperplasia had developed in three animals and an
adenocarcinoma was observed in one of the five animals killed at the
end of the study. Electron microscopy showed loss of microvilli and
polarity in the epithelium of the proximal convoluted tubules. At the
ultrastructural level, the cytoplasm of the neoplastic cells was
poorly differentiated and, in many areas, the surfaces of the cells
were covered by microvilli. In the animals in which treatment was
discontinued after 4 weeks, a gradual, but incomplete, restoration of
the tubular epithelia to nearly normal morphology was observed.
Nuclear changes persisted after cytoplasmic abnormalities had
disappeared. At 52 weeks, three out of five surviving rats,
administered TBPP throughout the study, were found to have polyploid
adenomas of the descending colon (Reznik et al., 1981).
Cunningham et al. (1992, 1993) examined the mechanisms whereby
chemicals produce mutagenicity in short-term in vitro assays, yet
fail to produce carcinogenicity in long-term rodent bioassays.
Previous studies indicated that mutagenic carcinogens increased the
amount of cell turnover in the target organ, but that mutagenic
noncarcinogens failed to do so. An association of cell-proliferation,
as determined by labelling with bromodeoxyuridine, and tumour
development was investigated in groups of five male Fischer 344 rats
(150 g). Administration of TBPP in the diet at dose levels of 0, 50,
or 100 mg/kg, for 14 days, resulted in increased incidences of cell
proliferation in the kidneys. A further association of cell
proliferation with tumour development in the kidneys was suggested by
their similar location in the kidneys, i.e., the renal outer medulla.
7.7.2 Dermal
7.7.2.1 Mouse
Female ICR/Ha Swiss mice (29 or 30 per group) (6-8 weeks old)
were treated with 10 or 30 mg TBPP (97%) in 0.2 ml acetone, 3 times
weekly, on the shaved skin, for 496 and 474 days, respectively. Two
control groups were used; 30 mice received the acetone and 249 mice
were untreated. Besides a significant increase in skin tumours
(papillomas and/or carcinomas), a substantial number of tumours were
also found at distant sites, such as squamous cell carcinomas of the
tongue and in the gingival area; papillomas and carcinomas were
observed in the forestomach (Table 2) (Van Duuren et al., 1978).
TBPP was tested in an in vivo, short-term skin test for
sebaceous gland suppression and the induction of epidermal
hyperplasia. Groups of 25 Swiss mice (aged 45 days) received dorsal
applications of TBPP in acetone on days 1, 3, and 5. The dose levels
applied on the skin were 49.5, 82.5, and 115.5 mg (total dose applied
in three applications in acetone). TBPP did not suppress the
sebaceous gland and did not induce hyperplasia (Sala et al., 1982).
Groups of 28-34 female (60 days old) Swiss mice were given a
single application of dimethylbenzanthracene (DMBA) (50 µg) or TBPP
(110 µg) in acetone, on the dorsal skin. In the tumour promotion
studies, the mice received, for 78 weeks, twice weekly applications to
the same area of the dorsal skin of an acetone solution of
tetradecanoyl phorbolacetate (TPA) (1 µg) or TBPP (33 mg), started one
week after the initiation with DMBA or TBPP. In order to test TBPP
for its ability to act as a complete carcinogen, a second series of
mice received the same twice weekly applications as the promoted mice,
but without any initiation treatment. The total TPA dose applied, was
156 µg/mouse and that of TBPP, 5.1 g/mouse. TBPP did not have any
effect as a complete carcinogen on the skin, with a total dose of
5.1 g/mouse. The same dose did not have a promoting activity after
DMBA initiation. However, TBPP initiated a significant number of skin
tumours, when TPA was used as promoter. Furthermore, the number of
lung adenomas increased significantly (Table 3) (Sala et al., 1982).
Table 2. Tumour incidences in female Swiss mice after dermal application of
tris(2,3-dibromopropyl) phosphate (TBPP)a
Number of Dose Number of mice with tumours/number necropsiedb
animals (mg/animal)
treated Forestomach Lung Skin Oral cavity
29 0 1/29 7/29 0/29 0/29
29 10 10/29 26/29 2/29 2/29
30 30 20/30 28/30 5/30 4/30
a From: Van Duuren et al. (1978).
b Increases in incidences of tumours of the forestomach, lung, skin, and oral
cavity in treated animals were statistically significant compared with those
in controls ( P < 0.05).
7.8 Special studies
7.8.1 Kidneys
Osterberg et al. (1978) found an increased incidence and severity
of chronic nephritis in a 90-day gavage study on rats, administered
TBPP at dose levels of 25, 100, or 250 mg/kg body weight in
propyleneglycol. The renal changes were associated with regenerative
epithelium, hypertrophy, and dysplasia of renal tubular epithelial
cells and were found at all three dose levels (section 7.2.1.1).
TBPP caused proximal tubular damage and acute renal failure in
rats, with elevation of serum creatinine and urea, and depression of
organic anion and cation transport (Soderlund et al., 1980; Elliott et
al., 1982; Lynn et al., 1982). It has been demonstrated that TBPP
caused urinary excretion of renal cytoplasmic enzymes associated with
the initial damage (characterized by increased membrane permeability),
followed by the excretion of cell organelle-linked enzymes with
necrosis of the renal tubular epithelium (Nomiyama et al., 1974;
Emanuelli et al., 1979; Fukuoka et al., 1987, 1988a,b). TBPP also
produced impairment of the tubular reabsorption capacity for metabolic
fuels such as, lactate, glucose, and citrate, maintained across the
brush border membrane by the sodium co-transport system (Kurokawa et
al., 1985; Pitts, 1987).
Male Wistar rats (56 rats in test group and 15 as controls; 7
weeks of age) were given a single oral dose in olive oil of 286.8 µmol
TBPP (98%)/kg, to study TBPP nephrotoxicity. TBPP caused tubular
necrosis. The animals received a single dose and some were killed
daily for 10 days. The following effects were observed: on day 1,
pyknosis of the renal tubular epithelial cells, necrosis on day 2,
regeneration from day 3 and large nuclei formation from day 4.
13C-NMR spectra were applied to clarify changes of the renal
low-molecular weight components in the kidneys injured by TBPP; sialic
acid and inositol were found to be the desired marker components. The
lesions produced by TBPP were characterized by changes in the renal
components and enzyme activities. Increases in the sialic acid
content of the kidneys were observed on day 1, suggesting destruction
of the epithelial cell membrane. On day 5, regeneration accompanied
by an increase in inositol contents was found. Renal activity of the
cytoplasmic enzyme, alanine aminopeptidase, was increased on days 2,
5, 6, and 7 (Fukuoka et al., 1988a).
There are large species differences in TBPP nephrotoxicity, since
neither hamsters, guinea-pigs, nor mice developed acute kidney damage
at doses of 500-1000 mg/kg body weight (Soderlund et al., 1982a).
Table 3. Development of tumours in female Swiss mice in initiation-promotion studies carried out with TBPPa
Treatment Number of mice with tumours
Group Initiation Promotion or Number Skin Lung Number Other tumours
repeated treatment of mice tumours adenomas Type
1 - TBPPb 33 0 14 3 2: lymphosarcoma,
hepatoma
2 TBPP TPAc 34 26d 7 0
3 DMBA TBPPa 33 3 11 2 mammary tumour,
perianal carcinoma
7 - TPAc 28 12 5 0
a From: Sala et al. (1982).
b TBPP: Total dose, 5.1 g/animal (170 g/kg body weight).
c TPA: Total dose, 156 µg/animal.
d Two squamous cell carcinomas in each group.
BBPP was clearly more nephrotoxic for Wistar and Sprague-Dawley
rats than TBPP, whereas mono(2,3-dibrompropyl) phosphate (mono-BPP)
was less nephrotoxic (Elliott et al., 1982; Soderlund et al., 1982b).
However, female Sprague-Dawley rats appeared to be resistant to BBPP
nephrotoxicity (Elliott et al., 1982), paralleling the carcinogenicity
of TBPP in female animals.
The nephrotoxicity of TBPP has been compared to that of BBPP,
using equimolar doses. Both chemicals caused reversible acute renal
failure, accompanied by tubular necrosis. Polyuria, high urinary
glucose, lactate, and enzyme levels, and increased serum creatinine
levels were observed. It was suggested, therefore, that BBPP or a
metabolite of this compound, mediated the nephrotoxicity associated
with TBPP (Takada et al., 1991a).
The finding that BBPP was a major urinary metabolite of TBPP and
that this compound was at least as nephrotoxic as TBPP, indicates that
it is a proximate nephrotoxic metabolite of TBPP (Lynn et al., 1980;
Soderlund et al., 1982b).
Generally, attempts to modulate TBPP nephrotoxicity by various
pretreatments have not been very successful. Pretreatment of Wistar
rats with the cytochrome P-450 inducers, phenobarbital and
polychlorinated biphenyls, known to increase the metabolism of TBPP,
had no effect on its nephrotoxicity. However, pretreatment with
cobaltous chloride resulted in a moderate reduction in TBPP
nephrotoxicity. Interestingly, depletion of glutathione in vivo
with diethyl maleate did not alter TBPP nephrotoxicity (Soderlund et
al., 1980).
The indication that the oxidative metabolism of TBPP or BBPP
plays only a minor role in its nephrotoxicity, is confirmed by the
findings that none of the selectively deuterated analogues (see below)
significantly altered morphological evidence of nephrotoxicity
compared with the protio compounds. It appears that C-H bond cleavage
was not the rate-limiting step in the overall process leading to
nephrotoxicity (Soderlund et al., 1988). However, recent studies have
revealed that deuterium substitution at C-2 and C-3 of TBPP results in
a considerable decrease in the plasma levels of BBPP at earlier time
points compared with those after protio TBPP. The time-integrated
plasma concentrations of the resulting deuterated BBPP analogues at
later time points are not significantly different from that of BBPP,
indicating that the target organ exposures to BBPP and deuterated BBPP
analogues are similar (Pearson et al., 1993b). This may explain the
lack of deuterium isotope effect on nephrotoxicity. Thus, a role of
oxidative metabolism cannot be completely ruled out.
Activation of nephrotoxic alkyl halides with glutathione by the
beta-lyase pathway is documented. However, known inhibitors of the
beta-lyase pathway (e.g., AT-125 and aminooxyacetic acid) did not
affect the extent of nephrotoxicity in rats caused by a single ip dose
of TBPP (Soderlund et al., 1988).
Because of its acidic nature, BBPP may be the species transported
into the renal tubular cells. Probenecid, an inhibitor of the organic
anion transport system in the kidneys, reduces the nephrotoxicity of
BBPP (Soderlund et al., 1988). The nature of the toxic metabolites
formed from BBPP has not been identified. One possible candidate is
an episulfonium ion generated by the conjugation of BBPP with
glutathione.
At present, the mechanisms involved in TBPP organ toxicity are
not known with certainty. DNA damage, as measured by alkaline
elution, was detected after 20 min in the kidneys of Wistar rats given
a single ip dose of 25 mg/kg body weight. Thus, DNA appears to be an
early target in TBPP nephrotoxicity, leading to cell death (Pearson et
al., 1993b).
7.9 Factors modifying toxicity; toxicity of metabolites
7.9.1 Toxicity of metabolites
2,3-Dibromo-1-propanol (DBP), a metabolite of TBPP, was tested in
2-year dermal carcinogenicity studies on F344/N rats and B6C3F1
mice. The doses used were 0, 188, or 375 mg/kg body weight in rats
and 0, 88, or 177 mg/kg body weight in mice. Under the conditions of
these studies, there was clear evidence of carcinogenic activity in
both sexes of both species in a variety of organs (US NTP, 1992). In
male rats, there was an increased incidence of neoplasms of the skin,
nose, oral mucosa, oesophagus, forestomach, small and large intestine,
Zymbal's gland, liver, kidney, tunica vaginalis, and spleen. In
female rats, there was an increased incidence of neoplasms of the
skin, nose, oral mucosa, oesophagus, forestomach, small and large
intestine, Zymbal's gland, liver, kidney, clitoral gland, and mammary
gland. In male mice, there was an increased incidence of neoplasms of
the skin, forestomach, liver, and lung. In female mice, there was an
increased incidence of neoplasms of the skin and forestomach.
BBPP, DBP, as well as TBPP, are acute nephrotoxins, BBPP being
the most potent (Lynn et al., 1982). In three groups of rats, the
24-h urine volume was measured after ip injection of equimolar amounts
(in 1 ml Emulphor) of TBPP (50 mg), BBPP (36 mg), or DBP (39 mg). The
single dose of TBPP caused a 5-fold increase in urine volume after two
days. After one week, urine volume had returned to normal. In
contrast, the single dose of BBPP resulted in a 7 to 8-fold increase
in urine volume from days 2-5. Urine volume had not returned to
normal after 10 days. The single dose of DBP produced a 2 to 3-fold
increase in urine volume, which rapidly returned to normal.
In another study, rats received a single ip injection of TBPP,
BBPP, or mono-BPP (in dimethyl-sulfoxide, 0.25 ml per 100 g) of 0, 10,
25, 50, 100, or 200 mg/kg body weight; the animals were killed 48 h
later (except the high dose group which was killed 40 h later). A
significant increase in kidney/body weight ratio occurred with all
three compounds at the 200 mg/kg dose. Enlarged kidneys were pale,
oedematous, and showed a prominent necrotic band in the inner part of
the cortex. Histologically, tubular kidney necrosis was demonstrated
in rats receiving 100 mg TBPP or mono-BP/kg or more and, in animals
receiving 50 mg BBPP/kg or more. Plasma creatinine was significantly
elevated at doses from 10 mg BBPP/kg, 25 mg mono-BP/kg, and 50 mg
TBPP/kg upwards. Plasma urea was significantly elevated after doses
of 100 mg mono-BP/kg or more and 200 mg BBPP/kg. Plasma GDT was also
significantly increased at the highest doses of BBPP and mono-BP
(Soderlund et al., 1982b).
Comparable results were reported by Fukuoka et al. (1988b).
Rats received a single oral dose of 0, 71.7, 143.4, or 286.8 µmol
BBPP/kg and were evaluated for seven days after the dose. In
addition to polyuria, changes in the excretion of lactate, uric acid,
and glucose, and changes in the activities of urinary enzymes
(alkaline phosphatase, aspartate aminotransferase, and gamma-
glutamyltransferase) at various times after dosing, histopatho-logical
changes occurred in the kidney. The histopathological changes
included pyknosis, necrosis, and desquamation.
7.9.2 Mutagenicity of metabolites
The following TBPP metabolites have been identified in in vivo
and in vitro test systems: BBPP, mono(2,3-dibromopropyl) phosphate
(mono-BPP), 2-bromoacrolein (BA), 2,3-dibromo-1propanol (DBP),
2,3-dibromopropyl-2-bromopropen-2-yl, bis(2-bromopropen-2-yl)
phosphate and 3,3-dibromopropyl phosphate (Lynn et al., 1980, 1982;
Zeiger et al., 1982; Nelson et al., 1984). Several of these TBPP
metabolites have been tested for their mutagenic potential.
All the identified TBPP metabolites were mutagenic in Salmonella
typhimurium TA 100 (Prival et al., 1977; Zeiger et al., 1982; Holme
et al 1983; Nakamura et al., 1983; Gordon et al., 1985). DBP was
mutagenic in Salmonella typhimurium TA 100 and TA 1535, but not in
TA 1538 (Carr & Rosenkranz, 1978). However, only BA was more
mutagenic than the parent compound (Gordon et al., 1985). Unscheduled
DNA synthesis was induced in monolayer cultures of rat hepatocytes by
DBP and, to a lesser extent, by BBPP and mono(2,3-dibromopropyl)
phosphate (mono-BPP). A similar relative response of DBP, BBPP, and
mono-BPP was found with respect to mutagenicity in V79 Chinese hamster
lung cells with liver microsomal activation. The concentration of
BBPP that was tested was 0.05 mmol/litre (Holme et al., 1983).
BBPP was less potent than TBPP in causing DNA damage in both the
liver and testicular cells. DNA damage, as measured by alkaline
elution, was found in isolated rat liver cells exposed to 100
micromolar BBPP and to a lesser extent in testicular cells. DNA damage
caused by BBPP phosphate could be decreased by diethyl maleate-
pretreatment in testicular cells, but not in the liver cells
(Soderlund et al., 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Fifty-two out of 61 (22 males and 39 females) human volunteers
completed a repeated insult patch study in which TBPP (1.1 g) was
applied 10 times over a 24-day period, followed by a single challenge
patch 14 to 21 days later. Fifty persons showed no reaction. After
the 6th or 7th application 2 showed itching (or pruritis), and
urticaria. The study was stopped for a month in these 2 subjects, and
then restarted. No adverse effects were seen the second time. The
conclusion of the author was that TBPP did not produce primary skin
irritation, skin fatigue, or skin sensitization (Kerst, 1974; US EPA,
1976).
A maximization test was carried out and 8 out of 24 human
volunteers, exposed to a 100% TBPP solution, showed a sensitization
reaction; while a 20% solution in a petroleum ether sensitized 2 out
of 25 subjects. The TBPP was considered to be a mild skin sensitizer
in humans. Subjects who had been sensitized by the 20% solution also
showed reactions when tested with TBPP-treated fabrics. The response
varied with the type of fabric substrate. The degree of sensitization
appeared to depend upon the availability of the agent at the surface
of the fibre. This is different for different types of fibres and the
methods in which the flame retardant is applied. Washing the fibre
reduced surface concentrations (Morrow et al., 1976).
Andersen (1977) reported the incidence of sensitization to TBPP
in human subjects from seven European countries, patch-tested with
TBPP (5% in petrolatum), and found two positives among 1103 patients.
Since TBPP-treated fabrics can be expected to contact the skin
for long exposure periods, patches from both rayon and acetate
fabrics, previously treated with TBPP were applied to humans, but skin
reactions were not elicited (Brieger et al., 1968).
8.2 Occupational exposure
No case reports or epidemiological studies are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory studies
9.1.1 Microorganisms
Nitrifying return activated sludge diluted with fresh settled
sewage, filtered, aerated and containing Nitromonas and Nitrobacter
was used to test nitrification. No inhibition was found with 300 mg
TBPP/litre and no depression of the BOD was noticed at 170 mg/litre
(Wood et al., 1981).
9.1.2 Aquatic organisms
9.1.2.1 Invertebrates
A 57% inhibition of southern armyworm (Spodoptera eridania)
microsomal p-chloro- N-methylanaline N-demethylase was measured
at 1 mg TBPP/ml, in an in vitro incubation mixture (Eldefrawi et
al., 1977).
9.1.2.2 Vertebrates
Six goldfish (Carassius auratus) were exposed to 1 mg TBPP
(dissolved in acetone) per litre water. All died within 5 days. The
fish appeared to swim in a disoriented manner prior to death. The
fish showed necrosis of the kidneys (Gutenmann & Lisk, 1975).
TBPP (1 mmol/litre) inhibited by 19% the acetylcholinesterase
(AChE) activity in the electric organ of the electric ray (Torpedo
ocellata). The binding of acetylcholine to its electric organ
receptor was not inhibited (Eldefrawi et al. 1977).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Seed of oat (Avena sativa) was added to loamy sand soil (1.5%
organic carbon) and exposed to 1, 10, 100 or 1000 mg TBPP/kg soil for
14 days. The temperature of the soil was 20°C, the pH 6.0 and 16 h
light/8 h dark cycle was used. The study was performed according to a
modified OECD terrestrial plant-growth test. The EC50 for growth
inhibition was at 1000 mg/kg soil (Pestemer, 1988). In a comparable
study, turnip seed (Brassica rapa sp.) was tested under the same
conditions as the oat seed. With 1000 mg TBPP/kg soil, 100%
inhibition of growth was obtained (Pestemer, 1988).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC concluded in 1979 that there is sufficient evidence that
TBPP is carcinogenic in mice and rats. In the absence of adequate
data on humans, it is reasonable, for practical purposes, to regard
TBPP as if it presented a carcinogenic risk to humans (IARC, 1979).
In 1987, colon tumours were reported in a short-term study on male
rats. The overall evaluation made by IARC (1987) was that TBPP is
probably carcinogenic to humans (Group 2A) (IARC, 1987).
BIS(2,3-DIBROMOPROPYL) PHOSPHATE AND SALTS
A1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
The data base on bis(2,3-dibromopropyl) phosphate (BBPP) and its
salts is inadequate for an evaluation, and to support its commercial
use.
From the available data, there is some indication that the
substance may be mutagenic and carcinogenic.
The substance cannot be evaluated unless additional data become
available on physical and chemical properties, production and use,
environmental transport, distribution, and transformation,
environmental levels and human exposure, kinetics and metabolism in
animals and humans, effects on laboratory mammals, humans, and in
vitro test systems, and effects on other organisms in the laboratory
and field. More mutagenicity data on at least two end-points are also
needed.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Chemical formula C6H11Br4O4P
Chemical structure
(BrCH2-BrCH-CH2O)2P - OH
'
(BrCH2-BrCH-CH2O)2P - O - P = O
H '
OH
Relative molecular 497.8
mass
CAS Chemical name 2,3-dibromo-1-propanol-hydrogen
phosphate
Common name bis(2,3-dibromopropyl) phosphate;
Synonyms bis(2,3-dibromopropyl)hydrogen
phosphate; bis(2,3-dibromopropyl)
phosphoric acid
CAS registry number 5412-25-9
The ammonium, magnesium, potassium, and sodium salts have also
been proposed.
A2.2 Physical and chemical properties
No data are available on this subject.
A2.3 Analytical methods
See section 2.3, TBPP.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
BBPP and its salts are not known to occur as natural products.
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
Bis(2,3-dibromopropyl)ammonium phosphate has been prepared by a
reaction of bis(2,3-dibromopropyl) phosphate with NH4OH (Mischutin,
1972).
A3.2.2 Uses
In the 1960s and 1970s, BBPP and its magnesium and ammonium salts
were proposed for use as fire-proofing agents for textiles and
plastics.
No evidence was found that BBPP or its salts are currently used
for commercial applications.
A3.3 Contamination of the environment
BBPP has been identified as a major biodegradation product of
TBPP, in a laboratory activated sludge system (Alvarez et al., 1982).
A3.4 Environmental transport, distribution, transformation,
and exposure levels
No data are available.
A4. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
A4.1 Absorption, distribution, elimination, and biotransformation
BBPP has been identified as a metabolite of TBPP (Lynn et al.,
1982). Following intravenous administration of [14C]-tris(2,3-
dibromopropyl) phosphate to male Sprague-Dawley rats, approximately
half of the label appeared in the urine within 120 h and 7.8% of the
recovered urinary label was BBPP. BBPP also constituted 21.5% of the
biliary label (33.9% of administered dose in 24 h). The tissue
distribution of BBPP was studied at different intervals (5 and 30 min,
8, 24, and 120 h, and 5 days) after administration of the tris
compound. BBPP was identified in nearly all organs within 5 min of
administration. Tissue levels declined after 5 or 30 min at all sites
except the large intestines and carcass. Five min after
administration, 75% of the plasma label was BBPP. The plasma
concentration of BBPP increased between 0 and 1 h and declined
biphasically thereafter, with an initial plasma half-life of 6 h,
declining to approximately 36 h, by one to five days.
A5. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A5.1 Single exposure
Adult male Wistar rats, 5 per group, were administered a single
ip injection of 0, 10, 25, 50, 100, or 200 mg BBPP/kg body weight in
DMSO (2.5 ml/kg). All rats were killed 40-48 h after dosing. One rat
of the highest dose group died. Kidneys and body weight ratios were
increased in 200 mg/kg rats. The kidneys were pale and oedematous
with necrosis of the inner cortex. Microscopically, tubular cell
necrosis was observed in rats with 50 mg/kg or more. However, plasma
creatinine was significantly elevated at doses of 10 mg/kg body weight
or more and plasma urea and plasma-GOT were elevated at 200 mg/kg body
weight (Soderlund et al., 1982b).
Elliot et al. (1982) carried out a comparable study with only
one dose level of 120 mg/kg body weight, administered to male
Sprague-Dawley rats intraperitoneally. Rats were killed after 48 h.
Serum creatinine was elevated, and renal cortical slices showed
decreased uptake of para-aminohippuric acid and N-[14C]-
methylnicotinamide. Microscopically, tubular cell necrosis of the
loops of Henle was found.
When the Mg salt of BBPP was administered by oral intubation to
Wistar rats, eyelid closure, crouching, shivering, and staggering gait
were observed. LD50 values in male and female rats were 283 and
261 mg/kg, respectively (Takada et al., 1991b).
A5.2 Short-term exposure
The Mg salt of BBPP was given to Wistar rats (5/sex per group) in
the diet at levels of 0, 30, 100, 300, or 1000 mg/kg for 45 days.
There was no difference between treated and control animals in body
weight and food consumption. At 1000 mg/kg, a significant increase in
liver and kidney weights was observed in the males. Desquamation,
swelling and large nuclei formation of the tubular epithelium, and
tubular dilation of kidney were observed. It was concluded that
BBPP-Mg has apparent renal toxicity (Takada et al., 1991b).
A5.3 Long-term exposure
No data were available on the following subjects:
* Skin and eye irritation; sensitization
* Reproductive toxicity, embryotoxicity, and teratogenicity
A5.3.1 Mutagenicity and related end-points
2,3-Dibromo-1-propanol (DBP) was mutagenic in Salmonella
typhimurium TA 100 and TA 1535, but not in TA 1538 (Carr &
Rosenkranz, 1978).
Magnesium BBPP at doses of 3-100 µmol/plate was more mutagenic
to Salmonella typhimurium TA 1535 and TA 100 in the presence of
metabolic activation by Kanechlor 500-induced rat-liver S9 than in its
absence; it was weakly mutagenic to TA 98 with S9, but showed no
mutagenic activity in TA 1537 and TA 1538 (Nakamura et al., 1979).
The ammonium salt of BBPP was more mutagenic than the magnesium salt,
which itself was more mutagenic than the free acid (Nakamura et al.,
1983).
BBPP purified as a urinary metabolite from rats treated with TBPP
was mutagenic to Salmonella typhimurium TA1535 and TA100 in the
presence of metabolic activation (Aroclor 1254-induced rat liver S9)
at doses ranging from 0.05 to 1.0 µmol (Lynn et al., 1982).
Mutagenic activity in Salmonella typhimurium TA100 was detected
when BBPP at concentrations of 50 and 100 µmol/litre was incubated for
30 min with hepatic microsomal fractions from untreated rats or rats
pretreated with phenobarbital (Soderlund et al., 1982b).
A5.3.2 Carcinogenicity
Four groups of 40 Wistar rats (5 weeks old) of each sex per dose
level were fed diets containing 0, 80, 400, or 2000 mg/kg of the
magnesium salt of bis(2,3-dibromopropyl) phosphate (BBPP), for 24
months. Food consumption and body weight gain were measured
immediately prior to the beginning of the study, and then weekly for 6
weeks, biweekly for 6 months, and monthly, thereafter. Blood samples
of 8-13 rats/sex per dose were taken at 12, 18, and 24 months. Body
weight gain was reduced significantly at a level of 2000 mg/kg. Male
and female rats receiving 2000 mg/kg and female rats receiving
400 mg/kg showed a significant increase in absolute and relative liver
and kidney weights. A high incidence of tumours was observed in both
sexes. In the digestive system, papillomas and adenocarcinomas were
found in the tongue, oesophagus, and forestomach, and adenocarcinomas
of the intestines. In the liver, hepatocellular adenomas (neoplastic
nodules) and carcinomas were found (Table 4). Pre-terminal
mortalities were associated with an increased incidence of forestomach
papillomas in both sexes, adenocarcinomas of the small intestines in
male rats, and hepatocellular carcinomas in females. Non-neoplastic
lesions were mainly found in the kidneys of the rats of the 2000 mg/kg
group and, in a few instances, also in the 400 mg/kg group. The
changes were epithelial swelling and desquamation, large bizarre
nuclei, pyknosis, and basement membrane thickening. Serum
biochemistry was performed using commercially available assay kits for
the diagnosis of liver and kidney function and of disorders of the
digestive system. Eighteen parameters were studied. Significant
increases or decreases in the parameters were mainly observed in the
2000 mg/kg group with a few in the 400 mg/kg group. Statistically
significant decreases were seen in the serum, in total protein,
albumin, and cholinesterase; and significant increases were seen in
blood urea nitrogen, total cholesterol, alkaline phosphatase,
gamma-glutamyl transferase, magnesium, GOT, and GPT (Takada et al.,
1991a).
A5.4 Special studies
A5.4.1 Kidneys
Many nephrotoxic agents exert their effects primarily on the
cells of the proximal tubules. Isolated tubular cells were used to
study the uptake of alpha-methylglucose as indicator of effects on the
functional integrity of the cells. BBPP, which is acutely nephrotoxic
in vivo, inhibited the uptake of alpha-methylglucose at low
concentrations (Boogaard et al., 1989).
See also TBPP section 7.8.1 and 7.9.1 on the nephrotoxicity of
BBPP.
A5.5 Effects on humans and other organisms in the laboratory
and field
No data are available.
Table 4. Neoplastic lesions and tumour incidence in Wistar rats fed diets containing
BBP magnesium salta
Organ Sex Dose level Papillomas Squamous cell
carcinomas
Tongue male 400 1/40 1/40
female 400 1/40 1/40
2000 5/40 0/40
Oesophagus male 400 6/40 1/40
2000 2/40 0/40
female 2000 6/40b 0/40
Forestomach male 400 8/40b 1/40
2000 17/40c 2/40
female 400 4/40 2/40
2000 20/40c 4/40
Adenoma Adenocarcinoma
Small intestines male 400 0/40 2/40
2000 2/40 14/40c
female 2000 0/40 9/40c
Table 4 (contd).
Organ Sex Dose level Neoplastic Hepatic cell
nodules carcinomas
Liver male 0 7/40 1/40
80 3/40 2/40
400 7/40 2140
2000 2/40 2/40
female 0 1/40 0/40
80 2/40 1/40
400 5/40 7/40b
2000 5/40 24/40c
Adenoma Carcinoma
Kidneys male 400 5/40 1/40
2000 0/40 0/40
female 2000 1/40 1/40
a From: Takada et al, (1991a).
No tumours were found in the groups not mentioned in the table.
The figures given in this table are the total numbers of the tumours
found at the end of the study. The 2000 mg/kg treated rats did not survive
until the end of the 18th or/and 24th month.
b P < 0.05.
c p < 0.01 compared with the controls.
REFERENCES
Alvarez GH, Page SW, & Ku Y (1982) Biodegradation of 14C-tris(2,3-
dibromopropyl) phosphate in a laboratory activated sludge system. Bull
Environ Contam Toxicol, 28: 85-90.
Andersen KE (1977) Sensitivity to a flame retardant, tris(2,3-
dibromopropyl) phosphate (Firemaster LVT 23P). Contact Dermatitis, 3:
297-300.
Berkowitz S (1978) The induction of II-III translocations by
tris(2,3-dibromopropyl) phosphate in Drosophila. Mutat Res, 57:
385-387.
Blum A & Ames BN (1977) Flame-retardant additives as possible cancer
hazards. Science, 195(4273): 17-23.
Blum A, Gold MD, Ames BN, Kenyon C, Jones FR, Hett EA, Dougherty RC,
Horning EC, Dzidic I, Carroll DI, Stillwell RN, & Thenol JP (1978)
Children absorb Tris-BP flame retardant from sleepwear: Urine contains
the mutagenic metabolite, 2,3-dibromopropanol. Science, 201:
1020-1023.
Boogaard PJ, Mulder GJ, & Nagelkerke JF (1989) Isolated proximal
tubular cells from rat kidney as an in vitro model for studies on
nephrotoxicity. II. Alpha-methylglucose uptake as a sensitive
parameter for mechanistic studies of acute toxicity by xenobiotics.
Toxicol Appl Pharmacol, 101(1): 144-157.
Brieger H, Gabriel K, & Rieders F (1968) Toxicology and safe
utilization of flame retardants. Presented at the American Industrial
Hygiene Conference, St. Louis, 15 May 1968.
Brusick D, Matheson D, & Bakshi K (1978) Evaluation of tris(2,3-
dibromopropyl) phosphate for mutagenic and transforming activity in a
battery of test systems. Proceedings of the Second International
Conference on Environmental Mutagens, Edinburgh, 11-15 July 1977.
Mutat Res., 53(2): 31-160.
Brusick D Matheson D, Jagannath DR, Goode S, Lebowitz H, Reed M, Roy
G, & Benson S (1980) A comparison of the genotoxic properties of
tris(2,3-dibromopropyl) phosphate and tris(1,3-dichloro-2-propyl)
phosphate in a battery of short-term bioassays. J Environ Pathol
Toxicol 3: 207-226.
Brusick DJ, Jagannath DR, & Matheson D (1982) The activity of
2,(2',4'-diaminophenoxy) ethanol in 3 genetic toxicity bioassays.
Mutat Res, 102: 361-372.
Carr HS & Rosenkranz HS (1978) Mutagenicity of derivatives of the
flame retardant tris(2,3-dibromopropyl) phosphate: halogenated
propanols. Mutat Res, 57: 381-384.
Chemicals Inspection & Testing Institute (1992) Biodegradation and
bioaccumulation data of existing chemicals based on the CSCL. Tokyo,
Japan Chemical Industry, Ecology-Toxicology Information Centre,
pp 2-110.
Cochran RC & Wiedow MA (1986) The effects of tris(2,3-dibromopropyl)
phosphate on the reproductive system of male rats. J Am Coll Toxicol,
5(2): 153-160.
Cope JF (1973) Identification of flame retardant textile finishes by
pyrolysis-gas chromatography. Anal Chem, 45: 562-564.
Cunningham ML, Elwell MR, & Matthews HB (1992) Site-specific
proliferation in renal tubular cells by the renal tubular carcinogen
tris(2,3-dibromopropyl) phosphate (TRIS). Toxicologist, 12(1): 266,
(Abstract No. 1013).
Cunningham ML, Elwell MR, & Matthews HB (1993) Site-specific cell
proliferation in renal tubular cells by the renal tubular carcinogen
tris(2,3-dibromopropyl) phosphate. Environ Health Perspect, 101(Suppl
5): 253-257.
DeCarlo VJ (1979) Studies on brominated chemicals in the environment.
Ann NY Acad Sci, 320: 678-681.
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli. Environ Mutagen,
7(Suppl 5): 1-248.
Dunkel VC, Schechtman LM, Tu AS, Sivak A, Lubet RA, & Cameron TP
(1988) Interlaboratory evaluation of the C3H/10T1/2 cell
transformation assay. Environ Mol Mutagen, 12: 21-31.
Dybing E, Omichinski JG, Soderlund EJ, Brunborg G, Lag M, Holme JA, &
Nelson SD (1989) Mutagenicity and organ damage of 1,2-dibromo-3-
chloropropane (DBCP) and tris(2,3-dibromopropyl) phosphate (tris-BP):
Role of metabolic activation. In: Hodgson E, Philpot RM, & Bend JR ed.
Reviews in biochemical toxicology. Amsterdam, Oxford, New York,
Elsevier Science Publishers, vol 10, pp 139-186.
EEC (1976) Council directive 76/769/EEC. Off J Eur Communities.
EEC (1979) Council directive 79/663/EEC. Off J Eur Communities.
Eldefrawi AT, Mansour NA, Brattsten LB, Ahrens VD, & Lisk DJ (1977)
Further toxicologic studies with commercial and candidate flame
retardant chemicals. Part II. Bull Environ Contam Toxicol, 17(6):
720-726.
Elliott WC, Lynn RK, Houghton DC, Kennish JM, & Bennett WM (1982)
Nephrotoxicity of the flame retardant tris(2,3-dibromopropyl)
phosphate and its metabolites. Toxicol Appl Pharmacol, 62, 179-182.
Emanuelli G, Calcamuggi G, Cestonaro G, Gatti G, Anfossi G, & Cotto A
(1979) Urinary enzyme activities in human and experimental renal
damage. Excerpta Med Int Congr Ser, 502: 780-781.
Environment Agency Japan (1978) Environmental monitoring of
chemicals. Environmental survey report of 1977 F.Y. Tokyo, Department
of Environmental Health, Office of Health Studies.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Department of Environmental Health, Office of
Health Studies.
Fukuoka M, Takabashi T, Tanaka A, Yamaha T, Naito K, Nakaji Y,
Kobayashi K, & Tobe M (1987) Nephrotoxic effect of tris(2,3-
dibromopropyl) phosphate on the urinary metabolites: assessment from
13C-NMR spectra of urines and biochemical and histopathological
examinations. J Appl Toxicol, 7(1): 23-34.
Fukuoka M, Tanaka A, Yamaha T, Naito K, Takada K, Kobayashi K, & Tobe
M (1988a) Tris(2,3-dibromopropyl) phosphate nephrotoxicity in the rat:
Histological and biochemical changes in renal components by 13C-NMR-
spectra. J Appl Toxicol, 8(6): 411-416.
Fukuoka M, Takahashi T, Naito K, & Takada K (1988b) Comparative
studies on nephrotoxic effects of tris(2,3-dibromopropyl) phosphate
and bis(2,3-dibromopropyl) phosphate on rat urinary metabolites. J
Appl Toxicol, 8: 43-52.
Furukawa M, Sirianni SR, Tan JC, & Huang CC (1978) Sister chromatid
exchanges and growth inhibition induced by the flame retardant
tris(2,3-dibromopropyl) phosphate in Chinese hamster cells: Brief
communication. J Natl Cancer Inst, 60: 1179-1181.
Gardner AM (1979) Quantitative determination of tris(2,3-
dibromopropyl) phosphate by densitometry of thin layer chromatograms.
J Assoc Off Anal Chem, 62(6): 1315-1318.
Gordon WP, Soderlund EJ, Holme JA, Nelson SD, Iyer L, Rivedal E, &
Dybing E (1985) The genotoxicity of 2-bromoacrolein and 2,3-
dibromopropanal. Carcinogenesis, 6: 705-709.
Gutenmann WH & Lisk DJ (1975) Flame retardant release from fabrics
during laundering and their toxicity to fish. Bull Environ Contam
Toxicol, 14(1): 61-64.
Gutter B & Rosenkranz HS (1977) The flame retardant tris(2,3-
dibromopropyl) phosphate: Alteration of human cellular DNA. Mutat Res,
56: 89-90.
Holme JA, Soderlund EJ, Hongslo JK, Nelson SD, & Dybing E (1983)
Comparative genotoxicity studies of the flame retardant tris(2,3-
dibromopropyl) phosphate and possible metabolites. Mutat Res, 124:
213-224.
Holme JA & Soderlund EJ (1984) Unscheduled DNA synthesis of rat
hepatocytes in monolayer culture. Mutat Res, 126: 205-214.
IARC (1979) Tris(2,3-dibromopropyl) phosphate. In: Some halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
575-588 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 20).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, pp 369-370 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Iliano B, Grimee R, & Storms M (1982) Identification du tris-(2,3-
dibromopropyl) phosphate, produit ignifuge pour textiles. Anal Lett,
15(9): 797-809.
IPCS (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo- para-dioxins and dibenzofurans. Geneva, World Health
Organization, 409 pp.
IPCS (1994a) Environmental Health Criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, 347 pp.
IPCS (1994b) Environmental Health Criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, 577 pp.
IPCS (1995) Environmental Health Criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization.
IRPTC (1987) Data profile on flame retardants. Geneva, International
Register on Potentially Toxic Chemicals, United Nations Environment
Programme. Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monogr Cancer Res,
27: 95-108.
Kawashima K, Tanaka S, Nakamura S, Nagao S, Endo T, Onada K, Takanaka
A, & Omori Y (1983) Effect of phosphoric tri-esters flame retardants
on the prenatal and postnatal developments of the rats. J Toxicol Sci,
8: 339.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kerst AF (1974) Toxicology of tris(2,3-dibromopropyl) phosphate. J
Fire Flammabil Fire Retard Chem, 1: 205-217.
Kirk-Othmer (1978-1984) Encyclopedia of chemical technology, 3rd ed.
Chichester, Brisbane, Toronto, John Wiley and Sons, vol 10,
pp 486-490.
Klopman G, Frierson MR, & Rosenkranz HS (1990) The structural basis of
the mutagenicity of chemicals in Salmonella typhimurium: The
Gene-Tox data base. Mutat Res, 228: 1-50.
Kurokawa K, Nagami GT, & Yamaguchi DT (1985) Transport and substrate
metabolism of the kidney. In: Kinne RKH ed. Renal biochemistry, cells,
membranes, molecules. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 175-223.
Lake RS, Kropko ML, Pezzutti MR, Shoemaker RH, & Igel HJ (1978)
Chemical induction of unscheduled DNA synthesis in human skin
epithelial cell cultures. Cancer Res, 38: 2091-2098.
LeBlanc RB (1976) What's available for flame retardant textiles. Text
ind, February: 28-31, 35, 37, 39, 41, 43 and 88.
Lyman WJ (1982) Handbook of chemical property estimation methods.
Environmental behaviour of organic compounds. New York, McGraw-Hill,
pp 4-9.
Lynn RK, Wong K, Dickinson RG, Gerber N, & Kennish JM (1980) Diester
metabolites of the flame retardant chemicals tris(1,3-dichloro-2-
propyl) phosphate and tris(2,3-dibromopropyl) phosphate in the rat:
Identification and quantification. Res Commun Chem Pathol Pharmacol,
28: 351-360.
Lynn RK, Garvie-Gould C, Wong K, & Kennish JM (1982) Metabolism,
distribution and excretion of the flame retardant tris(2,3-
dibromopropyl) phosphate (Tris-BP) in rats: Identification of
mutagenic and nephrotoxic metabolites. Toxicol Appl Pharmacol, 63:
105-119.
Mabey W & Mill T (1978) Critical review of hydrolysis of organic
compounds in water under environmental conditions. J Phys Chem Ref
Data, 7: 383-415.
McCann J & Ames BN (1977) The Salmonella/microsome mutagenicity
test: Predictive value for animal carcinogenicity. In: Hiatt HH,
Watson JD, & Winsten JA ed. Origins of human cancer. Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 1431-1450.
McCoy E, Hyman J, & Rosenkranz HS (1980) A new mutagenic and genotoxic
response of the flame retardant tris(2,3-dibromopropyl) phosphate.
Mutat Res, 77: 209-214.
MacGregor JT Diamond MJ, Mazzeno LW Jr, & Friedman M (1980)
Mutagenicity tests of fabric-finishing agents in Salmonella
typhimurium: Fiber-reactive wool dyes and cotton flame retardants.
Environ Mutagen, 2: 405-418.
Marsden PJ & Casida JE (1982) 2-Halocrylic acids as indicators of
mutagenic 2-haloacrolein intermediates in mammalian metabolism of
selected promutagens and carcinogens. J Agric Food Chem, 30: 627-631.
Morrow RW, Hornberger CS, Kligman AM, & Maibach HI (1976) Tris(2,3-
dibromo-propyl) phosphate: Human contact sensitization. Am Ind Hyg
Assoc J, 37: 192-197.
Nakamura A (1980) Quantitative gas chromatographic determination of
tris(2,3-dibromopropyl) phosphate in the 10 ng range by using a 0.8 mm
i.d. column packed with a high liquid loaded support. J Chromatogr,
196: 133-141.
Nakamura A, Tateno N, Kojima S, Kaniwa M-A, & Kawamura T (1979) The
mutagenicity of halogenated alkanols and their phosphoric acid esters
for Salmonella typhimurium. Mutat Res, 66: 373-380.
Nakamura A, Tateno N, Iwata T, Kojima S, Kaniwa M-A, & Kawamura T
(1983) Mutagenicity of bis- and mono-(2,3-dibromopropyl) phosphate,
and their salts used as flame retardants, in the Salmonella/
microsome system. Mutat Res, 117: 1-8.
Nakanishi Y & Schneider EL (1979) In vivo sister-chromatid
exchange: A sensitive measure of DNA damage. Mutat Res, 60: 329-337.
Nelson SD, Omichinski JG, Iyer L, Gordon WP, Soderlund EJ, & Dybing E
(1984) Activation mechanism of tris(2,3-dibromopropyl) phosphate to
the potent mutagen, 2-bromoacrolein. Biochem Biophys Res Commun,
121: 213-219.
Nomeir AA & Matthews HB (1983) Metabolism and disposition of the
flame retardant tris(2,3-dibromopropyl) phosphate in the rat. Toxicol
Appl Pharmacol, 67: 357-369.
Nomiyama K, Yamamoto A, & Sato C (1974) Assay of urinary enzymes in
toxic nephropathy. Toxicol Appl Pharmacol, 27: 484-490.
NTP (1992) NTP Technical report on the toxicology and carcinogenesis
studies of 2,3-dibromo-1-propanol (CAS No. 96-13-9) in F344/N rats and
B6C3F1 mice (dermal studies). Research Triangle Park, North Carolina,
National Toxicology Program (NTP Technical Report Series No. 400; NIH
Publication No. 92-2855).
Omichinski JG, Soderlund EJ, Bausano JA, Dybing E, & Nelson SD (1987)
Synthesis and mutagenicity of selectively methylated analogues of
tris(2,3-dibromopropyl) phosphate and 1,2-dibromo-3-chloropropane.
Mutagenesis, 2(4): 287-292.
Osterberg RE (1977) Children's wearing apparel containing TRIS;
interpretation as banned hazardous substance. Fed Reg, 42:
18850-18854.
Osterberg RE, Bierbower GW, & Hehir RM (1977) Renal and testicular
damage following dermal application of the flame retardant tris(2,3-
dibromopropyl) phosphate. J Toxicol Environ Health, 3: 979-987.
Osterberg RE, Bierbower GW, Ulsamer AG, Porter WK Jr, & Jones S (1978)
The com-parative toxicity of the flame retardant tris(2,3-
dibromopropyl) phosphate and the meta-bolite 2,3-dibromopropanol in
the rat. Toxicol Appl Pharmacol, 45: 254 (Abstract No. 82).
Overbeek DE & Nametz RC (1962) Method for the preparation of
tris(2,3-dibromopropyl) phosphate. US Patent 3,046,297, 25 July, to
Michigan Chemical Corporation.
Paciorek KJL, Kratzer RH, Kaufman J, Nakahara JH, Christos T, &
Hartstein AM (1978) Thermal oxidative degradation studies of
phosphate esters. Am Ind Hyg Assoc J, 39: 633-639.
Pearson PG, Omichinski JG, McClanahan RH, Soderlund EJ, Dybing E, &
Nelson SD (1993a) Metabolic activation of tris(2,3-dibromopropyl)
phosphate to reactive intermediates. I. Covalent binding and reactive
metabolite formation in vitro. Toxicol Appl Pharmacol, 118: 186-195.
Pearson PG, Omichinski JG, Holme JA, McClanahan RH, Brunborg G,
Soderlund EJ, Dybing E, & Nelson SD (1993b) Metabolic activation of
tris(2,3-dibromopropyl) phosphate to reactive intermediates. II.
Covalent binding, reactive metabolite formation, and differential
metabolite-specific DNA damage in vivo. Toxicol Appl Pharmacol,
118: 196-204.
Pestemer W (1988) [Special reports of the Jülich Nuclear Research
Centre]. Anl Jülich, 11: 93-109 (in German).
Pitts RF (1987) Production of CO2 by the intact functioning kidney
of the dog. Med Clin North Am, 59: 507-518.
Prival MJ (1975) Information available to date relevant to the
mutagenicity of tris(2,3-dibromopropyl) phosphate. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
Prival MJ, McCoy EC, Gutter B, & Rosenkranz HS (1977) Tris(2,3-
dibromopropyl) phosphate: Mutagenicity of a widely used flame
retardant. Science, 195(4273): 76-78.
Reznik G, Ward JM, Hardisty JF, & Russfield A (1979) Renal
carcinogenic and nephrotoxic effects of the flame retardant tris(2,3-
dibromopropyl) phosphate in F344 rats and (C57Bl/6N × C3H/HeN)F1 mice.
J Natl Cancer Inst, 63(1): 205-212.
Reznik G, Reznik-Schüller HM, Rice JM, & Hague BF Jr (1981)
Pathogenesis of toxic and neoplastic renal lesions induced by the
flame retardant tris(2,3-dibromopropyl) phosphate in F344 rats, and
development of colonic adenomas after prolonged oral administration.
Lab Invest, 44(1): 74-83.
Rosen JD, Segall Y, & Casida JE (1980) Mutagenic potency of
haloacroleins and related compounds. Mutat Res, 78: 113-120.
St. John LE Jr, Eldefrawi ME, & Lisk DJ (1976) Studies of possible
absorption of a flame retardant from treated fabrics worn by rats and
humans. Bull Environ Contam Toxicol, 15(2): 192-197.
Sala M, Gu ZG, Moens G, & Chouroulinkov I (1982) In vivo and in vitro
biological effects of the flame retardants tris(2,3-dibromopropyl)
phosphate and tris(2-chloroethyl) orthophosphate. Eur J Cancer Clin
Oncol, 18(12): 1337-1344.
Salamone MF & Katz M (1981) Mutagenicity of tris(2,3-dibromopropyl)
phosphate in mammalian gonad and bone marrow tissue. J Natl Cancer
Inst, 4: 691-695.
Sasaki M, Sugimura K, Yoshida MA, & Abe S (1980) Cytogenetic effects
of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo, II(20): 574-584.
Seabaugh VM, Collins TFX, Hoheisel CA, Bierbower GW, & McLaughlin J
(1981) Rat teratology study of orally administered tris(2,3-
dibromopropyl) phosphate. Food Cosmet Toxicol, 19: 67-72.
Simula TP, Glancey MJ, Soderlund EJ, Dybing E, & Wolf CR (1993)
Increased mutagenicity of 1,2-dibromo-3 chloropropane and tris(2,3,-
dibromopropyl) phosphate in Salmonella TA100 expressing human
glutathione S-transferases. Carcinogenesis, 14(11): 2303-2307.
Soderlund EJ, Nelson SD, & Dybing E (1979) Mutagenic activation of
tris(2,3-dibromopropyl) phosphate: The role of microsomal oxidative
metabolism. Acta Pharmacol. Toxicol., 45: 112-121.
Soderlund E, Dybing E, & Nelson SD (1980) Nephrotoxicity and
hepatotoxicity of tris(2,3-dibromopropyl) phosphate in the rat.
Toxicol Appl Pharmacol, 56: 171-181.
Soderlund EJ, Nelson SD, & Dybing E (1981) In vitro and in vivo
covalent binding of the kidney toxicant and carcinogen tris(2,3-
dibromopropyl) phosphate. Toxicology, 21: 291-304.
Soderlund EJ, Nelson SD, von Bahr C, & Dybing E (1982a) Species
differences in kidney toxicity and metabolic activation of tris(2,3-
dibromopropyl) phosphate. Fundam Appl Toxicol, 2: 187-194.
Soderlund E, Nelson SD, & Dybing E (1982b) Mutagenicity and
nephrotoxicity of two tris(2,3-dibromopropyl) phosphate analogs:
Bis(2,3-dibromopropyl) phosphate and 2,3-dibromopropyl phosphate. Acta
Pharmacol Toxicol, 51: 76-80.
Soderlund EJ, Gordon WP, Nelson SD, Omichinski JG, & Dybing E (1984)
Metabolism in vitro of tris(2,3-dibromopropyl) phosphate: Oxidative
debromination and bis(2,3-dibromopropyl) phosphate formation as
correlates to mutagenicity and covalent protein binding. Biochem
Pharmacol, 33: 4017-4023.
Soderlund EJ, Dybing E, Holme JA, Hongslo JK, Rivedal E, Sanner T,
Nelson SD (1985) Comparative genotoxicity and nephrotoxicity studies
of the two halogenated flame retardants tris(1,3-dichloro-2-propyl)
phosphate and tris(2,3-dibromopropyl) phosphate. Acta Pharmacol
Toxicol, 56: 20-29.
Soderlund EJ, Omichinski JG, Dahl JE, Nelson SD, & Dybing E (1988)
Nephrotoxicity of selectively deuterated and methylated analogues of
Tris-BP and Bis-BP in the rat. Pharmacol Toxicol, 62: 142-149.
Soderlund EJ, Brunberg G, Dybing E, Trygg B, Nelson SD, & Holme JA
(1992) Organ-specific DNA damage of tris(2,3-dibromopropyl) phosphate
and its diester metabolite in the rat. Chem-Biol Interact, 82:
195-207.
Takada K, Naito K, Kobayashi K, Tobe M, Kurokawa Y, & Fukuoka M
(1991a) Carcinogenic effect of bis(2,3-dibromopropyl) phosphate in
Wistar rats. J Appl Toxicol, 11(5): 323-331.
Takada K, Naito K, Aida Y, Momma J, Yoshimoto H, Nakaji Y, Kurokawa Y,
& Tobe M (1991b) Acute and subacute toxicity studies of bis(2,3-
dibromopropyl) phosphate magnesium in rats. Bull Natl Inst Health Sci
Jpn, 109: 25-31.
Ulsamer AG, Porter WK, & Osterberg RE (1978) Percutaneous absorption
of radiolabelled TRIS from flame retarded fabric. J Environ Pathol
Toxicol, 1: 543-549.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
UN (1991) Consolidated list of products whose consumption and/or sale
have been banned, withdrawn, severely restricted or not approved by
Governments, 4th ed. New York, United Nations.
US Consumer Product Safety Commission (1977a) Children's wearing
apparel containing Tris; interpretation as banned hazardous substance.
Fed Reg, 42: 18850-18854.
US Consumer Product Safety Commission (1977b) Children's wearing
apparel containing tris; tris and fabric, yarn or fiber containing
tris; withdrawal of interpretations as banned hazardous substances.
Fed Reg, 43: 61593-61594, 61621-61622.
US EPA (1976) Tris(2,3-dibromopropyl) phosphate (TBPP). Washington,
DC, US Environmental Protection Agency, pp 53-55 (EPA 560/4-76-004).
US EPA (1985) GEMS - Graphical exposure modelling system. Fate of
atmospheric pollutants (FAP) data base. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
US NCI (1978) Bioassay of tris(2,3-dibromopropyl) phosphate for
possible carcinogenicity. Washington, DC, National Cancer Institute
(Technical Report Series No. 76; NIH Publication No. 78-1326).
Valencia R (1978) Drosophila mutagenicity tests of saccharin, Tris,
PtCl4 and other compounds. (Abstract Cb-11). In: 9th Annual Meeting
of the American Environmental Mutagen Society, San Francisco. New
York, Alan R. Liss, Inc.
Van Beerendonk GJM (1994) Genotoxicity of the flame retardant
tris(2,3-dibromopropyl) phosphate: aspects of bioactivation and DNA
adduct formation. Leiden, The Netherlands, State University of Leiden,
82 pp.
Van Beerendonk GJM, Nivard MJM, Vogel EW, Nelson SD, & Meerman JHN
(1992) Formation of thymidine adducts and cross-linking potential of
2-bromoacrolein, a reactive metabolite of tris(2,3-dibromopropyl)
phosphate. Mutagenesis, 7(1): 19-24.
Van Duuren BL, Loewengart G, Seidman I, Smith AC, & Melchionne S
(1978) Mouse skin carcinogenicity tests of the flame retardants
tris(2,3-dibromopropyl) phosphate, tetrakis(hydroxymethyl)
phosphoniumchloride and polyvinylbromide. Cancer Res, 38: 3236-3240.
Veith GD, DeFoe D, & Bergstedt BV (1979) Measuring and estimating the
bioconcentration factor of chemicals in fish. J Fish Res Board Can,
36(9): 1040-1048.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company, 1173 pp.
Vogel EW & Nivard MJM (1993) Performance of 181 chemicals in a
Drosophila assay predominantly monitoring interchromosomal mitotic
recombination. Mutagenesis, 8(1): 57-81.
Wood LB, Hurley BJE, & Matthews PJ (1981) Some observations on the
biochemistry and inhibition of nitrification. Water Res, 15: 543-551.
Zeiger E, Pagano DA, & Nomeir AA (1982) Structure-activity studies on
the mutagenicity of tris(2,3-dibromopropyl) phosphate (Tris-BP) and
its metabolites in Salmonella. Environ Mutagen, 4: 271-277.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Tris-2,3-dibromopropyle
1.1 Résumé et évaluation
1.1.1 Production et usage
La production de phosphate de tris-2,3-dibromopropyle (TBPP) a
commencé pour la première fois vers 1950 et sa production commerciale
est documentée à partir de 1959. Aux Etats-Unis, en 1975, la
production de TBPP se situait, selon les estimations, entre 4100 et
5400 tonnes. Autant qu'on sache, le TBPP n'est ni produit ni utilisé
actuellement dans le monde comme retardateur de flamme dans les
textiles, mais il peut être incorporé à des polymères utilisés à
d'autres fins. Le TBPP a constitué un important retardateur de
flamme, que l'on ajoutait aux tissus de cellulose, de triacétate et de
polyester, en particulier pour les vêtements de nuit destinés aux
enfants, mais il a été depuis interdit pour cet usage dans plusieurs
pays d'Europe, aux Etats-Unis d'Amérique (1977) ainsi qu'au Japon
(1978).
Le TBPP peut se trouver à l'intérieur ou à la surface du tissu.
Lorsqu'il se trouve à l'intérieur, on ne peut pas l'extraire par
solvants et il est donc probable qu'il ne peut pas non plus être
absorbé par voie percutanée. Toutefois, lorsqu'il se trouve à la
surface de la fibre, il peut être extrait lors de la lessive ainsi
qu'au moyen d'acide acétique ou d'autres solvants ou encore par la
salive, et peut être alors absorbé par voie percutanée. Dans ce cas,
il peut y avoir en cours d'utilisation ou pendant la lessive des
produits finis une perte importante du TBPP qui se trouve à leur
surface, d'où contamination de l'environnement. En outre, on a
signalé la décharge de TBPP dans l'environnement par des ateliers de
finissage et le rejet final de déchets solides.
1.1.2 Propriétés physiques et chimiques
Le TBPP existe en au moins deux qualités. Le produit de haute
qualité est un liquide visqueux clair, de couleur jaune pâle, qui
contient jusqu'à 1,5% de matière volatile. Le produit de basse
qualité peut contenir jusqu'à 10% de matière volatile.
Le TBPP (degré de pureté > à 97%) a un point d'ébullition égal à
390°C, un point de fusion de 5,5°C et une tension de vapeur de
1,9 × 10-4 mmHg à 25°C. Il est faiblement soluble dans l'eau
(8 mg/litre).
Lorsqu'on le chauffe jusqu'à décomposition, c'est-à-dire au-dessus
de 260-300°C, le TBPP libère des composés contenant du brome et du
phosphore. Son coefficient de partage entre le n-octanol et l'eau
(log Pow) est égal à 3,02.
Il existe des méthodes d'analyse permettant le dosage du TBPP et
de ses métabolites dans des échantillons biologiques ou d'autres
matrices.
1.1.3 Transport, distribution et transformation dans l'environnement
Le peu de données dont on dispose incitent à penser que le TBPP
est relativement persistant dans l'environnement. Il ne semble pas
que l'oxydation et la photodécomposition jouent un rôle important dans
sa destinée. Cependant, il peut y avoir une hydrolyse impliquant les
atomes de brome du groupement propyle, en particulier en milieu
basique. Il peut s'évaporer de l'eau mais on ne dispose pas
véritablement de données sur ce point. Bien qu'on ait signalé la
possibilité d'une biodécomposition du TBPP (demi-vie 19,7 h.) dans les
boues activées, on ne pense pas que cela constitue un processus
important dans les sols et les eaux naturels. Dans la boue
stérilisée, il n'y a pratiquement pas de décomposition. On a constaté
que l'un des principaux produits de décomposition du TBPP était le
phosphate de bis-2,3-dibromo-propyle. Le TBPP étant pratiquement
insoluble dans l'eau, il est possible que l'adsorption sur les
matières particulaires et sur les sédiments joue un rôle important.
La valeur estimative du log de Koc (3,29) indique qu'il y a une
forte adsorption au sol. Sur la base de cette valeur de Koc et de
la faible solubilité dans l'eau du TBPP, on pense que ce composé n'est
que lentement lessivé vers les eaux souterraines. Le TBPP va avoir
tendance à s'accumuler dans les décharges publiques et autres lieux de
ce genre, avec pour conséquence la possibilité d'une bioaccumulation.
D'ailleurs, une étude portant sur un cyprin, Pimephales promelas, a
permis de mettre en évidence un facteur de bioconcentration de 2,7, ce
qui est faible, alors que le coefficient de partage n-octanol/eau
(log de Pow) est de 3,02. En raison de sa faible tension de vapeur,
on peut penser que le TBPP sera essentiellement sorbé sur les
particules en suspension dans l'air. La décomposition thermique en
milieu oxydant du TBPP à la température de 370°C entraîne la formation
de bromure d'hydrogène et de composés bromés en C3 tels que des
bromopropènes, des dibromopropènes ainsi que des di-et-tribromopropanes.
1.1.4 Concentrations dans l'environnement et exposition humaine
On ne dispose que de données limitées sur les concentrations dans
l'environnement et l'exposition humaine. Lors d'études effectuées au
Japon en 1975, on a analysé 20 échantillons d'eau, de sol et de
poissons sans y trouver de TBPP. En revanche, on a mis en évidence,
sans le doser, du TBPP dans des particules en suspension dans l'air
prélevé aux alentours d'une installation industrielle.
Ce sont les enfants portant des vêtements de nuit traités par du
TBPP qui, lorsque ce produit était autorisé, constituaient le groupe
de population le plus particulièrement exposé à ce retardateur de
flamme. On estime qu'à l'époque, la dose absorbée à travers la peau
par ces enfants aux Etats-Unis d'Amérique était de l'ordre de 9 µg/kg
de poids corporel et par jour. La Consumer Product Safety Commission
des Etats-Unis a calculé que, sur une période de six ans, un enfant
portant de tels vêtements pouvait absorber une dose totale d'au moins
2 à 77 mg de TBPP/kg de poids corporel.
1.1.5 Cinétique et métabolisme chez les animaux de laboratoire
et l'homme
Le TBPP est rapidement absorbé au niveau des voies digestives et
à un rythme plus modéré par la voie percutanée chez les rats et les
lapins. Chez le rat, le TBPP ou ses métabolites sont éliminés dans
les 5 jours. L'élimination se produit à hauteur d'environ 50% dans
les urines, 10% dans les matières fécales, 10-20% de son carbone étant
rejetés sous forme de CO2 dans l'air expiré.
Un jour après avoir administré par voie orale du TBPP radiomarqué
à des rats, la radioactivité s'est retrouvée dans les limites de
0,2-6,6% au niveau du sang, du foie, des reins, des muscles, des
tissus adipeux et de la peau. Le temps de demi-élimination de la
radioactivité de tous ces organes était d'environ 2-4 jours. Au bout
de 8 h., il ne restait encore, en concentrations notables, que du
phosphate de bis-2,3-dibromopropyle dans la plupart des tissus.
Après administration orale de TBPP à des rats, on a mis en
évidence six métabolites dans les urines et la bile. Le principal
métabolite urinaire, fécal, biliaire et tissulaire était le phosphate
de bis-2,3-dibromopropyle. Un autre métabolite, le 2,3-dibromo-
propanol, a été également mis en évidence chez des rats et des enfants
qui portaient des vêtements traités par du TBPP.
Les microsomes hépatiques métabolisent le TBPP en présence de
NADPH et d'oxygène. Les principaux métabolites obtenus sont le
phosphate de bis-2,3-dibromopropyle et le 2,3-dibromopropanol. On a
montré qu'il se formait du phosphate de bis-2,3-dibromopropyle par
oxydation au niveau du C3 et peut-être également en position C2 du
TBPP. Outre le phosphate bis-2,3-dibromopropyle, on retrouve de la
2-bromoacroléine, de l'acide 2-bromoacrylique ainsi que des composés
propylliques hydroxylés et des métabolites conjugués avec du
glutathion.
Du S-(2,3-dihydroxypropyl)glutathion ayant été mis en évidence
dans la bile de rats, on a avancé l'hypothèse que le TBPP et/ou le
phosphate de bis-2,3-dibromopropyle subissent une conjugaison directe
par le glutathion en présence de glutathion S-transférase avec
formation, comme métabolites, d'ions épisulfonium.
Par activation, le TBPP forme des produits qui se fixent par
liaisons covalentes aux protéines et à l'ADN in vivo et in vitro.
Après injection intrapéritonéale de TBPP tritié à des souris, à des
hamsters et à des cobayes mâles, qui sont moins sensibles à la
néphrotoxicité induite par cette substance que les rats, on a observé
un degré analogue de liaison covalente aux protéines dans le foie et
les reins. Chez le rat mâle, qui est beaucoup plus sensible à la
néphrotoxicité induite par ce produit, on a constaté qu'il y avait
beaucoup plus de composé radiomarqué fixé aux protéines rénales qu'aux
protéines hépatiques.
Mis en présence de microsomes hépatiques provenant de souris, de
cobayes, de hamsters et de sujets humains, le TBPP est dans tous les
cas métabolisé en intermédiaires génotoxiques. Toutefois, les
métabolites réactifs du TBPP se forment beaucoup plus lentement en
présence de microsomes hépatiques d'origine humaine qu'en présence de
microsomes prélevés sur rongeurs.
Après avoir administré à des rats une dose néphrotoxique de TBPP
et de ses analogues radiomarqués, on a constaté que le degré de
liaison covalente aux protéines décroissait dans l'ordre suivant:
reins, foie et testicules. D'après les résultats d'études in vitro
et in vivo au cours desquelles on a comparé les lésions produites au
niveau de l'ADN rénal, on est incité à penser qu'il y a formation de
phosphate de bis-2,3-dibromopropyle au niveau du foie par oxydation,
catalysée par le P450, du TBPP en position C2 ou C3. Ce phosphate de
bis-2,3-dibromopropyle est ensuite transporté vers les reins où il est
métabolisé en intermédiaire réactif qui endommage l'ADN et se fixe aux
protéines rénales. L'activation qui se produit au niveau du rein ne
semble pas impliquer le P450 mais s'effectuer plutôt par
l'intermédiaire d'un métabolisme dépendant du GSH. Des études in
vitro avec du TBPP et certains de ses analogues radiomarqués ont
montré que l'oxydation du TBPP comportait l'incorporation d'un atome
d'oxygène provenant de l'eau. Cela implique que l'oxydation en
position C2 du reste propyle donne naissance à une alpha-bromocétone
réactive qui est capable d'alkyler directement les protéines ou de
s'hydrolyser pour donner du phosphate de bis-2,3-dibromopropyle et une
bromo-alpha-hydroxycétone réactive.
1.1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La toxicité du TBPP est faible, qu'il s'agisse de la toxicité
aiguë par voie orale à court terme ou de la toxicité aiguë par voie
percutanée. Pour le rat, la DL50 par voie orale est > 2 g/kg et
pour le lapin, la DL50 par voie cutanée dépasse 8 g/kg de poids
corporel. On a observé une atteinte rénale très étendue (nécrose
cellulaire au niveau des tubules proximaux) chez des rats mâles à qui
l'on avait injecté par voie intrapéritonéale une seule dose de 100 mg
de TBPP par kg de poids corporel.
Chez des rats soumis respectivement pendant quatre semaines ou
90 jours à des épreuves de toxicité orale au cours desquelles du TBPP
leur avait été administré par gavage ou mêlé à leur nourriture, on a
observé une augmentation de l'incidence et de la gravité des néphrites
chroniques aux doses supérieures ou égales à 25 mg/kg de poids
corporel.
Chez des lapins, l'application cutanée quotidienne de TBPP à des
doses supérieures ou égales à 2,2 g/kg de poids corporel pendant 4
semaines a entraîné une dégénérescence au niveau du foie et des reins.
Tous les lapins sont morts dans les quatre semaines. En revanche,
aucun animal n'est mort lors d'une autre étude avec des doses allant
jusqu'à 250 mg/kg de poids corporel.
Des lapins qui avaient subi chaque semaine pendant 90 jours une
application cutanée de 2,27 g de TBPP/kg de poids corporel ont
présenté des anomalies rénales, une atrophie testiculaire et une
aspermatogénèse.
Aux doses de 1,1 ou 0,22g de TBPP, on n'a observé aucune
irritation cutanée ou oculaire chez les lapins et il n'y a pas eu non
plus de sensibilisation cutanée chez des cobayes.
Deux études de tératogénicité ont été effectuées sur des rats.
L'une d'entre elles comportait des doses allant jusqu'à 125 mg/kg de
poids corporel et n'a pas permis de mettre en évidence d'effet
tératogène. Lors d'une autre étude où la dose administrée était de
200 mg/kg de poids corporel, on a observé une augmentation
significative des variations affectant le squelette chez les foetus
et, aux doses de 50 et 100 mg/kg de poids corporel, une diminution
sensible de l'indice de viabilité. Les auteurs en ont conclu que
l'effet observé était dû à l'action toxique du composé sur les
femelles gestantes.
Chez des rats auxquels on avait administré du TBPP, on a constaté
des lésions étendues de l'ADN dans divers organes. In vitro, on a
constaté que le TBPP produisait la rupture des brins d'ADN dans des
cellules KB d'origine humaine. Le TBPP a également induit une
synthèse non programmée de l'ADN dans des hépatocytes de foie de rat,
ce phénomène n'étant toutefois pas constaté dans des cellules
épithéliales de prépuce humain.
Plusieurs études ont révélé que le TBPP provoquait des mutations,
notamment par substitution des paires de base, chez des souches de
Salmonella typhimurium, avec ou sans activation métabolique.
L'étude des mutations géniques directes sur cellules de hamsters
chinois V79 avec ou sans activation métabolique, a donné des résultats
négatifs. Toutefois, en présence de microsomes hépatiques de rats
préalablement traités par du phénobarbital, on a observé un effet
positif. Un effet faiblement positif a également été observé avec des
cellules lymphomateuses de souris (locus L5178YTK).
Dans des cellules V79 de hamsters chinois, on a constaté que le
TBPP augmentait le nombre d'échanges entre chromatides soeurs. En
revanche il n'y avait pas d'augmentation du nombre des aberrations
chromosomiques, ni dans les cellules de hamsters chinois, ni dans les
cellules de moelle osseuse murine, ni dans les cellules lymphoïdes
humaines en culture. Dans des cellules fibroblastiques humaines
diploïdes (lignée HE 2144), on a observé des échanges entre
chromatides soeurs mais pas d'aberrations chromosomiques, l'épreuve
étant effectuée sans activation métabolique. Toutefois la recherche
in vitro d'aberrations chromo-somiques dans des lignées cellulaires
de hamsters chinois a donné un résultat positif.
Un résultat positif a également été obtenu lors de la recherche
de micronoyaux dans des cellules de moelle osseuse provenant de
hamsters chinois. Une autre épreuve de ce genre, portant cette fois
sur des souris, a permis d'observer un effet faiblement positif.
Les études effectuées sur Drosophila melanogaster ont montré
que le TBPP augmentait les mutations récessives létales liées au sexe
dans les gamètes mâles ainsi que chez les mâles adultes et il y avait
induction de translocations réciproques. Dans l'épreuve de l'oeil en
mosaïque w/w+, le TBPP a suscité une réaction fortement positive.
Un certain nombre d'études ont été menées pour tenter d'élucider
les mécanismes qui sont à la base de la mutagénicité et/ou de la
génotoxicité induites par le TBPP. Ainsi la mutagénicité du TBPP pour
les bactéries s'effectue par l'intermédiaire du système des
monooxygénases microsomiques. Par ailleurs, lors d'une réaction qui
est sous la dépendance du NADPH et de l'oxygène, il y a activation du
TBPP par le cytochrome P450. Des microsomes préparés à partir de
foies d'animaux traités par du phénobarbital ou des PCBs entraînent un
accroissement de la mutagénicité. Le phosphate de mono- et de
bis-2,3-dibromopropyle sont moins mutagènes que le TBPP. Des études
in vitro ont montré que l'oxydation de la molécule de TBPP au niveau
du C3 donnait naissance à un puissant mutagène à action directe, la
2-bromoacroléine qui se lie également à l'ADN.
On a mis en évidence des différences interspécifiques dans la
bioactivation du TBPP en métabolites mutagènes pour la souche TA 100
de Salmonella typhimurium. A cet égard, les microsomes hépatiques
de souris étaient plus efficaces que ceux de cobayes, de hamsters et
de rats.
Trois études de transformation cellulaire ont été menées à l'aide
de cellules C3H/10T1/2. Dans l'une d'entre elles, on a obtenu un
effet positif, mais les deux autres ont donné des résultats négatifs.
Lors d'études à long terme, on a administré par voie orale du
TBPP à des souris et à des rats et on en a appliqué sur la peau de
souris femelles. Chez les souris, on a constaté, après administration
par voie orale, qu'il se formait chez les deux sexes des tumeurs au
niveau de la portion cardiaque de l'estomac et des poumons, ainsi que
des tumeurs bénignes ou malignes au niveau du foie chez les femelles
et au niveau des reins chez les mâles. Chez les rats, des tumeurs
bénignes ou malignes se sont formées au niveau des reins chez les
mâles, les tumeurs rénales étant bénignes chez les femelles.
L'application de TBPP sur la peau de souris femelles a entraîné
l'apparition de tumeurs de la peau, des poumons, de la portion
cardiaque de l'estomac et de la cavité buccale. On peut conclure de
ces études que le TBPP est doté de pouvoir cancérogène chez la souris
et le rat.
Après administration d'un métabolite du TBPP, le phosphate de
bis-2,3-dibromopropyle, par voie orale à des rats, on a constaté
l'apparition de tumeurs digestives chez les deux sexes. Il s'agissait
de papillomes et d'adénocarcinomes de la langue, de l'oesophage et de
la portion cardiaque de l'estomac, ainsi que d'adénocarcinomes de
l'intestin avec en outre des adénomes et des carcinomes
hépatocellulaires.
On a également procédé à l'application cutanée d'un autre
métabolite du TBPP, le 2,3-dibromo-1-propanol, à des souris et à des
rats. Chez les rats mâles, on a constaté un accroissement de
l'incidence des tumeurs malignes de la peau, du nez, de la muqueuse
buccale, de l'oesophage, de la portion cardiaque de l'estomac, de
l'intestin grêle et du gros intestin, de la glande de Zymbal, du foie,
du rein, de la vaginale, et de la rate. Chez les rats femelles, on
constatait une incidence accrue de tumeurs malignes affectant la peau,
le nez, la muqueuse buccale, l'oesophage, la portion cardiaque de
l'estomac, l'intestin grêle et le gros intestin, la glande de Zymbal,
le foie, le rein, la glande clitoridienne, et les glandes mammaires.
Chez les souris mâles, il y avait également une incidence plus élevée
des tumeurs malignes au niveau de la peau, de la portion cardiaque de
l'estomac, du foie et des poumons, tandis que chez les femelles
l'accroissement des tumeurs malignes se manifestait au niveau de la
peau et de la portion cardiaque de l'estomac.
1.1.7 Effets sur l'homme
On ne dispose que de données limitées concernant les effets du
TBPP sur l'homme.
Quelques études ont été consacrées à la recherche chez l'homme
d'un effet sensibilisateur que le TBPP pourrait avoir sur la peau.
Les résultats obtenus montrent que ce produit n'a qu'un faible pouvoir
sensibilisateur et aucune irritation cutanée n'a été observée.
Toutefois les personnes qui avaient présenté une réaction positive au
TBPP pur ont également réagi lorsqu'on les a mises en contact avec des
tissus qui en contenaient.
1.1.8 Effets sur les autres êtres vivants au laboratoire et
dans leur milieu naturel
On ne possède que très peu de données concernant les effets du
TBPP sur les autres êtres vivants. Par exemple, des poissons rouges
(Caraccius auratus) qui avaient été exposés à du TBPP à raison de
1 mg/litre sont morts tous les 6 en l'espace de 5 jours.
La CE50 relative à l'inhibition de la croissance de la semence
d'avoine se situait à 1000 mg/kg de terre. Cette concentration a
provoqué l'inhibition totale de la croissance des semences de navet
( Brassica rapa sp.).
1.2 Conclusions
Le TBPP a été utilisé naguère comme retardateur de flamme pour en
imprégner les tissus, en particulier destinés à la confection de
vêtements de nuit pour enfants, mais on est guère renseigné sur ses
autres applications. C'est essentiellement par contact avec des
tissus traités par ce composé que la population générale a pu être
contaminée.
On n'a guère de renseignements non plus sur l'exposition des
ouvriers employés à la production commerciale du TBPP ainsi qu'à son
utilisation pour la fabrication de divers produits, ni d'ailleurs sur
les dangers qu'il représente.
En raison de la rareté des données, il n'est pas possible de
parvenir à des conclusions définitives quant aux niveaux d'exposition
ou aux dangers que le TBPP fait courir aux êtres vivants dans leur
milieu naturel, l'homme mis à part.
Les études sur l'animal ont montré que le TBPP pouvait être
absorbé au niveau des voies digestives et, dans une moindre
proportion, par la voie percutanée. Il peut également être résorbé
par cette dernière voie chez l'homme. Chez le rat, il se révèle être
très largement métabolisé dans le foie en phosphate de bis-2,3-
dibromopropyle, qui constitue le principal métabolite mis en évidence
dans les urines, et, dans une moindre proportion, en 2,3-
dibromopropanol. En outre, on a retrouvé de petites quantités
d'autres métabolites bromés du TBPP. La présence de
2,3-dibromopropanol a été également observée chez des personnes qui
portaient des tissus traités par le TBPP. La principale voie
d'élimination est la voie urinaire, le composé étant excrété en très
faible proportion sous sa forme initiale. Quant à la principale voie
métabolique, elle semble faire intervenir le cytochrome P450 et les
glutathion- S-transférases.
D'après les données dont on dispose, on peut conclure que le TBPP
ne présente qu'une faible toxicité aiguë chez l'animal de laboratoire.
Des études au cours desquelles on a administré de manière répétée de
fortes doses de TBPP, on permis de mettre en évidence des lésions
rénales et hépatiques chez le rat ainsi qu'une atteinte testiculaire
chez le lapin. Le composé a également un indéniable effet génotoxique
dans plusieurs systèmes d'épreuve, tant in vitro qu' in vivo. Des
effets cancérogènes ont également été relevés chez le rat et la
souris. Deux de ses métabolites, le phosphate de bis-2,3-
dibromopropyle et le 2,3-dibromopropanol, produisent également des
effets cancérogènes chez l'animal de laboratoire. Il n'est pas
irritant chez l'animal et son pouvoir sensibilisateur chez l'homme est
faible.
En 1977, la Consumer Product Safety Commission des Etats-Unis
d'Amérique (Commission pour protection du consommateur) a interdit
l'utilisation de vêtements d'enfants traités par le phosphate de
tris-2,3-dibromoproyl, par crainte que ce composé ne soit cancérogène
pour l'homme et en raison du risque non négligeable encouru par les
personnes portant des vêtements confectionnés à l'aide de tissus
imprégnés. Depuis lors, l'utilisation de ce composé comme retardateur
de flamme dans les produits destinés à la consommation courante est
très sévèrement réglementée dans un certain nombre d'autres pays et
son utilisation dans les textiles est interdite.
1.3 Recommandations
En raison de ses effets toxiques, le TBPP ne doit plus être
utilisé dans le commerce.
Au cas où, pour certains usages, il n'existerait pas de
substituts moins dangereux au TBPP, il faudrait entreprendre des
études pour s'assurer de l'absence d'exposition et de danger pour
l'homme et l'environnement.
2. Bis-2,3-dibromopropyle
La base de données relatives au phosphate de
bis-2,3-dibromopropyle et à ses sels est insuffisante pour en
permettre l'évaluation ou en justifier l'usage commercial.
D'après les données disponibles on peut penser que ce composé
pourrait être mutagène et cancérogène.
Il ne sera pas possible d'évaluer ce produit tant qu'on ne
disposera pas de données complémentaires sur ses propriétés physiques
et chimiques, sa production et son usage, son transport, sa
distribution, sa transformation et sa concentration dans
l'environnement ainsi que l'exposition humaine auxquels il donne lieu,
sa cinétique et son métabolisme chez l'animal et l'homme, ses effets
sur les animaux de laboratoire, l'homme et les systèmes d'épreuve in
vitro ainsi que son action sur les autres êtres vivants au
laboratoire et dans leur milieu naturel. Il est également nécessaire
d'obtenir davantage de données concernant son pouvoir mutagène sur au
moins deux points d'aboutissement.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. El fosfato de tris(2,3-dibromopropilo)
1.1 Resumen y evaluación
1.1.1 Producción y utilización
El fosfato de tris(2,3-dibromopropilo) (FTBP) se produjo por
primera vez hacia 1950. Se sabe que en 1959 hubo producción con fines
comerciales. En 1975 la producción de FTBP en los Estados Unidos de
América se estimó entre 4100 y 5400 toneladas. Que se sepa, el FTBP
no se produce ni utiliza corrientemente en la actualidad a nivel
mundial como retardador de ignición en productos textiles, pero puede
utilizarse en polímeros empleados para otros fines. El FTBP era un
importante retardador de ignición de la celulosa y de tejidos de
triacetato y de poliéster, especialmente en ropa de dormir para
niños, pero este empleo se ha prohibido en varios países de Europa,
los Estados Unidos de América (1977) y el Japón (1978).
El FTBP se utiliza tanto dentro del tejido como sobre el mismo.
Si se encuentra dentro del tejido, no puede extraerse con disolventes
y, por consiguiente, probablemente no esté disponible para su
absorción cutánea. Sin embargo, si se halla en la superficie de la
fibra, puede extraerse durante el lavado o por acción del ácido
acético, de otros disolventes y de la saliva y está disponible para la
absorción cutánea. En este caso habrá pérdida sustancial de FTBP
superficial del tejido durante la utilización y/o el lavado de los
productos acabados, y se contaminará el medio ambiente. Además, se
sabe que hay emisión de FTBP al medio ambiente en las operaciones de
acabado de textiles y en la evacuación final de desechos sólidos que
contienen FTBP.
1.1.2 Propiedades físicas y químicas
Puede obtenerse FTBP de dos calidades por lo menos. El producto
de alto grado de pureza es un líquido transparente, amarillo pálido y
viscoso, que tiene hasta un 1,5% de componentes volátiles. La calidad
de baja pureza puede contener hasta un 10% de componentes volátiles.
El punto de ebullición del FTBP (pureza > 97%) es de 390°C, su
punto de fusión de 5,5°C y su presión de vapor de 1,9 × 10-4 mmHg a
25°C. La solubilidad del FTBP en el agua es baja (8 mg/litro).
Cuando se calienta hasta su descomposición, a una temperatura
superior a 260-300°C el FTBP emite compuestos que contienen bromo y
fósforo. El coeficiente de reparto n-octanol/agua (log Pow) es de
3,02.
Se dispone de métodos analíticos para determinar la presencia de
FTBP y sus metabolitos en muestras biológicas y otras matrices.
1.1.3 Transporte, distribución y transformación en el medio ambiente
La limitada información disponible sugiere que el FTBP es
relativamente persistente en el medio ambiente. La oxidación y la
fotodegradación probablemente no tengan un efecto significativo en su
destino. Sin embargo, puede haber hidrólisis de los átomos de bromo
del grupo propílico, especialmente en condiciones básicas. Puede
producirse volatilización a partir del agua, pero no se dispone de
datos efectivos. Aunque se han notificado casos de biodegradación del
FTBP (semivida 19,7 h) en aguas residuales activadas, no se considera
que ésta constituya un proceso importante en suelos y aguas naturales.
En fangos esterilizados casi no se produce descomposición. Se
encontró fosfato de bis(2,3-dibromopropilo) como principal producto de
la descomposición. Como el FTBP es prácticamente insoluble en agua,
la adsorción en partículas en sedimento puede ser un proceso
importante.
Un log Koc estimado (3,29) sugiere una fuerte adsorción en el
suelo. Sobre la base de este valor de Koc y de la baja solubilidad
medida en agua, sólo se prevé una lixiviación lenta del FTBP a las
aguas subterráneas. El FTBP tenderá a acumularse en basureros y otros
vertederos de desechos, lo que tal vez dé lugar a la acumulación
biológica. Un estudio sobre bioacumulación en Pimephales
promelas mostró un factor de bioconcentración de 2,7, que
es bajo, mientras que el coeficiente de reparto n-octanol/agua (Log
Pow) es de 3,02. Debido a su baja presión de vapor, se prevé que el
FTBP sea principalmente objeto de sorción en las partículas en
suspensión en el aire. La degradación oxidativa térmica del FTBP a
370 oC mostró que se forman bromuro de hidrógeno y compuestos
C3-bromados, tales como bromopropenos, dibromopropenos, y
dibromopropanos y tribromopropanos.
1.1.4 Niveles ambientales y exposición humana
Los datos sobre los niveles ambientales y la exposición humana
son limitados. Estudios realizados en el Japón en 1975 mostraron que
20 muestras de agua, suelo y peces no contenían FTBP. Se identificó
la presencia de FTBP en partículas en suspensión en el aire en los
alrededores de una planta industrial, pero no se cuantificaron.
Los niños que llevaban ropa de dormir tratada con FTBP fueron el
grupo de la población general particularmente expuesto a este
retardador de ignición. La absorción estimada a través de la piel de
los niños que llevaban ropa de dormir tratada con FTBP en los Estados
Unidos de América se calculó en 9 µg/kg de peso corporal por día. La
Comisión de Seguridad de los Productos de Consumo de los Estados
Unidos de América calculó que, en un periodo de seis años, un niño que
lleve ropa tratada con FTBP podría absorber en total 2-77 mg de
FTBP/kg de peso corporal o más.
1.1.5 Cinética y metabolismo en animales de laboratorio y en
seres humanos
El FTBP se absorbe rápidamente a través del tracto gastro-
intestinal y a una velocidad moderada a través de la piel en la rata y
el conejo. En la rata, el FTBP o sus metabolitos se eliminan en cinco
días. Aproximadamente el 50% se elimina por la orina, el 10% por las
heces y el 10-20% se exhala en forma de CO2.
Un día después de la administración oral de FTBP marcado a ratas,
se encontró radiactividad en la sangre, el hígado, los riñones, los
músculos, la grasa y la piel con valores comprendidos entre el 0,2% y
el 6,6%. El periodo de semieliminación de la radiactividad de dichos
órganos fue de aproximadamente 2-4 días. Después de ocho horas,
solamente el fosfato de bis(2,3-dibromopropilo) seguía presente en
concentraciones sustanciales en la mayor parte de los tejidos.
Después de la administración oral de FTBP a ratas, se
identificaron seis metabolitos en la orina y en la bilis. El
principal metabolito en la orina, las heces, la bilis y los tejidos
fue el fosfato de bis(2,3-dibromopropilo). También se identificó el
metabolito 2,3-dibromopropanol en ratas y en niños que llevaban ropa
tratada con FTBP.
Los microsomas del hígado metabolizan el FTBP en presencia de
NADPH y oxígeno. Los principales metabolitos son el fosfato de
bis(2,3-dibromopropilo) y el 2,3-dibromopropanol. Se ha mostrado que
el fosfato de bis(2,3-dibromopropilo) se forma por oxidación en la
posición C3 y posiblemente también en la posición C2 del FTBP.
Además del fosfato de bis(2,3-dibromopropilo), se han encontrado
2-bromoacroleína, ácido 2-bromoacrílico, y compuestos propil-
hidroxilados y metabolitos conjugados con glutatión.
Se identificó la presencia de S-(2,3-dihidroxipropil)glutatión en
la bilis de ratas y se sugirió que el FTBP y/o el fosfato de bis(2,3-
dibromopropilo) son conjugados directamente con el glutatión por la
glutatión S-transferasa, formándose iones episulfonio como
metabolitos.
Se ha mostrado que el FTBP se activa para formar productos que se
enlazan de forma covalente con las proteínas y el ADN in vivo e in
vitro. Después de inyecciones intraperitoneales de FTBP tritiado,
el ratón, el hámster y el cobayo machos, que son menos sensibles a la
nefrotoxicidad inducida por el FTBP que la rata, mostraron niveles
semejantes de enlace covalente con las proteínas en el hígado y los
riñones. En la rata macho, muy susceptible a la nefrotoxicidad
inducida por el FTBP, los átomos marcados se habían fijado a las
proteínas de riñon en cantidad mucho mayor que las proteínas del
hígado.
Los microsomas del hígado de ratón, cobayo, hámster y humano
metabolizaron todos ellos el FTBP formando productos intermedios
genotóxicos. Sin embargo, la tasa de formación de metabolitos
reactivos del FTBP por acción de los microsomas del hígado humano fue
menor a la de los microsomas del hígado de roedores.
El enlace del FTBP marcado y análogos en ratas a las que se había
administrado una dosis nefrotóxica mostró que el número de enlaces
covalentes a las proteínas era máximo en los riñones; les seguían el
hígado y los testículos. Los resultados de estudios comparativos in
vitro e in vivo sobre lesiones del ADN renal parecen indicar que
el fosfato de bis(2,3-dibromopropilo) se forma en el hígado por
oxidación mediada por el P450 en las posiciones C2 o C3 del FTBP. El
fosfato de bis(2,3 dibromopropilo) se transporta a los riñones, donde
se metaboliza formando productos intermedios reactivos que lesionan el
ADN y se enlazan con las proteínas del riñón. La activación en el
riñón no parece realizarse con intervención del P450 sino por medio
del metabolismo depen-diente del glutatión. Estudios in vitro con
FTBP marcado y productos análogos mostraron que, en la oxidación, al
FTBP se incorpora un átomo de oxígeno del agua. Ello significa que la
oxidación en la posición C2 del grupo propílico produce una alpha-
bromocetona reactiva que puede alkilizar la proteína directamente o
hidrolizarla produciendo fosfato de bis(2,3-dibromopropilo) y una
bromo-alpha-hidroxicetona reactiva.
1.1.6 Efectos en mamíferos de laboratorio y en sistemas de
prueba in vitro
La toxicidad oral aguda y de corto plazo y la toxicidad cutánea
aguda del FTBP son bajas. La DL50 oral para la rata es > 2 g/kg y
la DL50 dérmica para el conejo es > 8 g/kg de peso corporal en
ambos casos. Se observaron lesiones renales extensas (necrosis de las
células de los tubos proximales) en ratas macho después de una sola
inyección intraperitoneal de 100 mg de FTBP/kg de peso corporal.
Estudios de cuatro semanas y de 90 días sobre toxicidad oral del
FTBP (administrado por sonda o en la alimentación) a ratas mostraron
un aumento relacionado con la dosis en la incidencia de nefritis
crónica y su gravedad a niveles de dosis de 25 mg/kg de peso corporal
o más.
En conejos, aplicaciones cutáneas cotidianas de 2,2 g de FTBP/kg
de peso corporal o más durante cuatro semanas ocasionaron cambios
degenerativos en el hígado y los riñones. Todos los conejos murieron
en cuatro semanas. No se registraron muertes en otro estudio con
niveles de dosis de hasta 250 mg/kg de peso corporal.
En una prueba de 90 días en conejos, aplicaciones cutáneas
semanales de 2,27 g/kg de peso corporal dieron lugar a cambios
renales, atrofia testicular y aspermatogénesis.
No se observó irritación cutánea ni ocular en conejos a niveles
de dosis de 1,1 g ó 0,22 g de FTBP y tampoco se observó
sensibilización cutánea en cobayos.
Se realizaron dos estudios sobre teratogenicidad en ratas. En un
estudio, a niveles de dosis de hasta 125 mg/kg de peso corporal no se
observó teratogenicidad. En otro estudio, a un nivel de dosis de
200 mg/kg de peso corporal se observó un aumento significativo en las
variaciones esqueléticas de los fetos, y con 50 y 100 mg/kg de peso
corporal se obtuvo un índice de viabilidad significativamente más
bajo. Los autores llegaron a la conclusión de que el efecto observado
se debía a toxicidad materna.
Se encontraron lesiones extensas del ADN en diversos órganos de
ratas a las que se había administrado FTBP. In vitro, se ha
mostrado que el FTBP induce ruptura de las hebras de ADN en las
células KB humanas. Indujo síntesis imprevista del ADN en hepatocitos
de ratas, pero no en células epiteliales del prepucio en el hombre.
El FTBP resultó mutagénico en varios estudios realizados en
Salmonella typhimurium, especialmente en cepas sensibles a la
sustitución de pares de bases, con y sin activación metabólica.
Las valoraciones de mutación génica anterógrada efectuadas en
células V79 de hámster de China, con y sin activación metabólica,
dieron resultados negativos. Sin embargo, se obtuvo un efecto
positivo en presencia de microsomas del hígado de ratas tratadas
previamente con fenobarbital. Se obtuvo un efecto positivo débil con
células de linfoma de ratón (locus L5178YTK).
El FTBP aumentó el número de intercambios de cromátides hermanas
en células V79 de hámster de China, pero no indujo aberraciones
cromosómicas en células de hámster de China, células de médula ósea de
ratón y células linfoides humanas de cultivo. Se encontraron
intercambios de cromátides hermanas, pero no aberraciones
cromosómicas, en fibroblastos humanos diploides (línea HE 2144) sin
activación metabólica. Sin embargo, en una prueba in vitro sobre
aberración cromosómica con la línea celular de hámster de China, el
FTBP dio resultados positivos.
Se obtuvo un resultado positivo con FTBP en una prueba de
formación de micronúcleos en células de médula ósea de hámster de
China. Otro estudio en ratones sobre formación de micronúcleos mostró
un efecto positivo débil.
Estudios con Drosophila melanogaster mostraron que el FTBP
hacía aumentar el número de efectos recesivos letales ligados al sexo
en células germinales masculinas y en machos adultos y se inducían
traslocaciones recíprocas. El FTBP mostró una respuesta fuertemente
positiva en la valoración de mosaicismo ocular w/w+.
Se han realizado varios estudios para dilucidar los mecanismos de
la mutagenicidad y/o la genotoxicidad inducidas por el FTBP. La
mutagenicidad bacteriana ocasionada por el FTBP está mediada por el
sistema de la monooxigenasa microsómica. El citocromo P450 activa el
FTBP en una reacción que depende del NADPH y del oxígeno. Los
microsomas preparados a partir del hígado de animales tratados con
fenobarbital o con bifenilos policlorados acusaron un aumento de la
mutagenicidad. Los fosfatos de mono(2,3-dibromopropilo) y
bis(2,3-dibromopropilo) son menos mutagénicos que el FTBP. Estudios
in vitro han mostrado que la oxidación en la posición C3 de la
molécula de FTBP produce el potente mutágeno 2-bromoacroleína de
acción directa, que también se enlaza con el ADN.
Se han notificado diferencias entre especies en la bioactivación
del FTBP con transformación en metabolitos mutagénicos para cepas
TA 100 de Salmonella typhimurium. Los microsomas del hígado de
ratones fueron más eficaces que los de cobayos, hámsters y ratas.
Se realizaron tres estudios sobre transformación celular en los
que se utilizaron células C3H/10T1/2. En un estudio se observó un
efecto positivo, pero en los otros dos los resultados fueron
negativos.
Se probó el FTBP en ratones y ratas por administración oral y en
ratones hembra por aplicación cutánea en estudios a largo plazo. En
los ratones, el FTBP administrado por vía oral produjo tumores de
preestómago y pulmón en los animales de ambos sexos, tumores hepáticos
benignos y malignos en las hembras y tumores renales benignos y
malignos en los machos. En ratas, el FTBP produjo tumores renales
benignos y malignos en los machos y tumores renales benignos en las
hembras. Después de la aplicación cutánea a ratones hembra, el FTBP
produjo tumores en la piel, el pulmón, el preestómago y la cavidad
bucal. De esos estudios puede concluirse que el FTBP tiene un
potencial carcinogénico en ratones y ratas.
El fosfato de bis(2,3-dibromilpropilo), un metabolito del FTBP
administrado por vía oral a ratas produjo tumores del sistema
digestivo en ambos sexos. Entre los tumores encontrados había
papilomas y adenocarcinomas de lengua, esófago y preestómago,
adenocarcinomas de intestino, y adenomas y carcinomas
hepato-celulares.
Otro metabolito del FTBP, el 2,3-dibromo-1-propanol, se ensayó en
ratas y ratones por aplicación cutánea. En ratas macho se observó
mayor incidencia de neoplasias de piel, nariz, mucosa bucal, esófago,
preestómago, intestino delgado y grueso, glándula de Zymbal, hígado,
riñón, túnica vaginal y bazo. En ratas hembra se registró mayor
incidencia de neoplasias de piel, nariz, mucosa bucal, esófago,
preestómago, intestino delgado y grueso, glándula de Zymbal, hígado,
riñón, glándula clitorídea y mama. En ratones macho se observó un
aumento de la incidencia de neoplasias de piel, preestómago, hígado y
pulmón, y en ratones hembra, un aumento de la incidencia de neoplasias
de piel y preestómago.
1.1.7 Efectos en el ser humano
Se dispone de datos limitados sobre los efectos del FTBP en el
ser humano.
Se ha ensayado el FTBP para determinar su potencial de
sensibilización cutánea en unos pocos estudios en seres humanos. Los
resultados de éstos indican que el FTBP tiene un bajo potencial de
sensibilización y no ha habido irritación cutánea. Sin embargo, las
personas que mostraron una respuesta positiva de sensibilización al
FTBP puro también reaccionaron cuando se expusieron a tejidos tratados
con FTBP.
1.1.8 Efectos en otros organismos en laboratorio y en el medio
natural
Hay muy pocos datos sobre los efectos del FTBP en otros
organismos. Seis carpas doradas (Carassius auratus) expuestas a
1 mg de FTBP/litro murieron todas a los cinco días.
La CE50 de inhibición del crecimiento en semillas de avena fue
de 1000 mg/kg de suelo. Esta concentración causó una inhibición del
100% del crecimiento en semillas de nabo ( Brassica rapa sp.).
1.2 Conclusiones
El FTBP se ha utilizado como retardador de ignición en tejidos,
en particular en ropa de dormir para niños, pero hay información
insuficiente sobre su utilización para otros fines. La exposición de
la población general se ha efectuado principalmente por contacto con
tejidos tratados con FTBP.
Hay poca información sobre la exposición de los trabajadores y
los riesgos que para éstos entrañan la producción comercial de FTBP y
su utilización en diversos productos.
Debido a la escasez de datos, no pueden sacarse conclusiones
firmes respecto de los niveles de exposición y los riesgos del FTBP
para organismos en el medio ambiente distintos del ser humano.
Estudios en animales han mostrado que el FTBP puede absorberse a
través del tracto gastrointestinal y, en menor medida, de la piel. El
FTBP también puede absorberse a través de la piel en el ser humano.
En la rata, el FTBP parece metabolizarse extensamente en el hígado
convirtiéndose en fosfato de bis(2,3-dibromopropilo), que es el
principal metabolito detectado en la orina, y, en menor medida, en
2,3-dibromopropanol. Además, se han encontrado pequeñas cantidades de
otros metabolitos bromados del FTBP. También se ha detectado
2,3-dibromopropanol en seres humanos que llevaron tejidos tratados con
FTBP. La principal vía de eliminación es la orina y una cantidad muy
pequeña se excreta en la forma del compuesto originario. La principal
vía metabólica parece ser la del metabolismo de las S-transferasas
del citocromo P450 y del glutatión.
Sobre la base de los datos disponibles se puede concluir que el
FTBP tiene una baja toxicidad aguda para animales de experimentación.
Estudios sobre la administración repetida de dosis relativamente
elevadas de FTBP han revelado lesiones renales y hepáticas en ratas y
también toxicidad testicular en conejos. El FTBP ha producido un
claro efecto genotóxico en varios sistemas de prueba, tanto in vitro
como in vivo. Se observaron efectos carcinogénicos en ratas y
ratones. Se ha observado que los metabolitos fosfato de
bis(2,3-dibromopropilo) y 2,3-dibromopropanol también tienen efectos
carcinogénicos en animales de experimentación. No se registraron
efectos irritativos en animales y se observó un bajo potencial de
sensibilización en seres humanos.
En 1977, la Comisión de Seguridad de los Productos de Consumo de
los Estados Unidos de América prohibió las prendas de vestir para
niños tratadas con fosfato de tris(2,3-dibromo-propilo), debido a la
preocupación de que esta sustancia química pudiera ser carcinogénica
para el ser humano y a la posibilidad de una exposición humana
significativa por contacto con los tejidos tratados. Desde entonces,
la utilización de esta sustancia como retardador de ignición en
productos de consumo se ha restringido rigurosamente en varios otros
países y se ha prohibido en los productos textiles.
1.3 Recomendaciones
Debido a sus efectos tóxicos, el FTBP no se debería utilizar ya
comercialmente.
Si se identifican aplicaciones para las cuales no hay
alternativas menos peligrosas que el FTBP, deberían realizarse
estudios para demostrar la ausencia de exposición humana y ambiental y
de riesgos para el ser humano y para el medio ambiente.
2. El fosfato de bis(2,3-dibromopropilo)
La base de datos sobre el fosfato de bis(2,3-dibromopropilo) y
sus sales es insuficiente para hacer una evaluación y para respaldar
su utilización comercial.
Sobre la base de los datos disponibles, hay algunos indicios de
que esta sustancia puede ser mutagénica y carcinogénica.
Esta sustancia no puede evaluarse a menos que llegue a disponerse
de datos adicionales sobre sus propiedades físicas y químicas; su
producción y utilización; su transporte, distribución y transformación
en el medio ambiente; los niveles ambientales y la exposición humana;
su cinética y metabolismo en animales y en seres humanos; sus efectos
en mamíferos de laboratorio, en seres humanos y en sistemas de prueba
in vitro; y sus efectos en otros organismos en el laboratorio y en
el medio natural. También se necesitan más datos sobre mutagenicidad
en relación con dos variables de evaluación por lo menos.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND
BIS(2,3-DIBROMOPROPYL)PHOSPHATE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
United Kingdom
Dr D. Osborn, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Soderlund, National Institute of Public Health, Oslo,
Norway (Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research,
Stockholm University, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and
Aerosol Research, Hannover, Germany
Dr R.D. Kimbrough, Institute for Evaluating Health Risks,
Washington DC, USA (Vice-chairman)
Dr Wai-On Phoon, Department of Occupational Health,
University of Sydney, Sydney, Australia (Chairman)
Dr R. Benson, Drinking Water Branch, US EPA, Denver, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo,
Japan (Rapporteur)
Observers
Dr M.L. Hardy, Toxicology Advisor, Albemarle Corporation,
Baton Rouge, USA
Dr D.L. McAllister, Director, Quality, Environment, Health
and Safety, and Research Support, Great Lakes Chemical
Corporation, West Lafayette, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND BIS(2,3-DIBROMOPROPYL)
PHOSPHATE
A WHO Task Group on Environmental Health Criteria for tris- and
bis(2,3-dibromopropyl) phosphate met at BIBRA Toxicology
International, Carshalton, United Kingdom, from 6 to 11 June 1994.
Dr K.W. Jager, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the
draft and made an evaluation of the risks for human health and the
environment from exposure to tris- and bis(2,3-dibromopropyl)
phosphate.
The first draft was prepared by Dr G.J. van Esch, the
Netherlands, who also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS Contact Points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs M.O. Head of Oxford for
the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
INTRODUCTION
The IPCS is preparing several EHC monographs on Flame Retardants,
which will provide additional information relevant to TBPP.
There will be one monograph, "Flame Retardants - A General
Introduction" (in preparation), giving a general introduction to the
use, the mode of action, and the potential risks of flame retardants,
and listing the substances used as flame retardants with a general
indication of the data available on them.
Flame retardants in wide use are discussed in separate
monographs, e.g., EHC 162: Brominated Diphenyl Ethers (IPCS, 1994a)
and EHC 172: Tetrabromobisphenol-A (IPCS, 1995).
Some flame retardants considered hazardous for humans and the
environment have been reviewed in separate monographs including EHC
152: Polybrominated Biphenyls (IPCS, 1994b), and EHC 173: Tris- and
Bis(2,3-dibromopropyl) phosphate (this monograph).
Because of the possibility of the formation of halogenated
dibenzodioxins and dibenzofurans under certain circumstances, such as
pyrolysis, the following monographs have been developed: EHC 88:
Polychlorinated Dibenzodioxins and Dibenzofurans (IPCS, 1989) and
Polybrominated Dibenzodioxins and Dibenzofurans (in preparation).
The reader should consult these monographs for further
information.
Tris(2,3-dibromopropyl) phosphate was an important commercial
flame retardant ("TRIS"), especially for children's sleepwear. In
1977, the US Consumer Product Safety Commission banned children's
clothing treated with tris(2,3-dibromopropyl) phosphate. Since then,
in several other countries, the use of this compound as a flame
retardant has been severely restricted in consumer products and
prohibited in textiles.
Because tris(2,3-dibromopropyl) phosphate can also be used for
other applications, the information available on physical and chemical
properties, behaviour in the environment, occurrence in the
environment and humans, kinetics and metabolism, toxicity for
laboratory animals and in the field, and the exposure of the general
population and workers, is summarized in this Environmental Health
Criteria monograph. General properties and uses of brominated flame
retardants are given in "Flame Retardants - A General Introduction"
(in preparation).
ABBREVIATIONS
BA 2-bromoacrolein
BBPP bis(2,3-dibromopropyl) phosphate
DBCP 1,2-dibromo-3-chloropropane
DBP 2,3-dibromopropanol
DMBA dimethylbenzanthracene
mono-BPP mono(2,3-dibromopropyl) phosphate
TBPP tris(2,3-dibromopropyl) phosphate
TPA tetradecanoyl phorbolacetate
TRIS-(2,3-DIBROMOPROPYL) PHOSPHATE
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Production and use
Tris(2,3-dibromopropyl) phosphate (TBPP) was first produced in
about 1950; commercial production was reported in 1959. Production of
TBPP, in the USA, in 1975, was estimated to be between 4100 and
5400 tonnes. As far as is known, TBPP is not produced or used
currently in the world as a flame retardant in textiles, but may be
added to polymers used for other purposes. TBPP was an important
flame retardant for cellulose and tri-acetate and polyester fabrics,
especially in children's sleepwear, but has been banned for these
applications in several countries in Europe, the USA (1977), and Japan
(1978).
TBPP exists both in, and on, the fabric. When it is in the
fabric, it is not extractable with solvents and, therefore, probably
not available for dermal absorption. However, when it is on the fibre
surface, it can be extracted during laundering, and by acetic acid,
other solvents, and saliva, and is available for dermal absorption. In
this case, substantial losses of surface TBPP from fabrics during use
and/or laundering of the finished products, will occur, and will
contaminate the environment. Furthermore, release of TBPP into the
environment has been reported from textile-finishing plants and the
ultimate disposal of solid wastes, containing TBPP.
1.1.2 Physical and chemical properties
TBPP is available in at least two grades. The high-purity grade
is a clear, pale-yellow, viscous liquid, with up to 1.5% volatiles.
The low-purity grade may contain up to 10% volatiles.
TBPP (purity > 97%), has a boiling point of 390°C, a melting
point of 5.5°C, and a vapour pressure of 1.9 × 10-4mmHg at 25°C.
The solubility of TBPP in water is low (8 mg/litre).
When heated to decomposition, above 260-300°C, TBPP emits
compounds containing bromine and phosphorus. The n-octanol/water
partition coefficient (log Pow) is 3.02.
Analytical methods to determine TBPP and its metabolites in
biological samples and other matrices are available.
1.1.3 Environmental transport, distribution, and transformation
The limited information available suggests that TBPP is
relatively persistent in the environment. Oxidation and
photodegradation are not likely to be significant fate processes.
However, hydrolysis involving the bromine atoms on the propyl group
may occur, especially under basic conditions. Volatilization from
water may occur, but no actual data are available. Although
biodegradation of TBPP (half-life 19.7 h) in activated sewage is
reported to occur, it is not thought to be an important process in
natural soils and waters. In sterilized sludge, almost no breakdown
takes place. Bis(2,3-dibromopropyl) phosphate (BBPP) was found as a
major breakdown product. Because TBPP is virtually insoluble in
water, adsorption on particulate matter and sediment may be an
important process.
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water
solubility, TBPP is expected to leach only slowly to groundwater. TBPP
will tend to accumulate in rubbish dumps and other disposal sites,
which may result in biological accumulation. A bioaccumulation study
with fathead minnow showed a bioconcentration factor of 2.7, which is
low, while the n-octanol/water partition coefficient (Log Pow) was
3.02. Because of its low vapour pressure, TBPP is expected to be
mostly sorbed on particulate matter in air. Thermal oxidative
degradation of TBPP at 370°C showed that hydrogen bromide and
C3-brominated compounds, such as bromopropenes, dibromopropenes, and
diand tribromopropanes, are formed.
1.1.4 Environmental levels and human exposure
Data on environmental levels and human exposure are limited.
Studies carried out in Japan in 1975 showed that 20 samples of water,
soil, and fish did not contain TBPP. TBPP was identified, but not
quantified, in air particulates in the surroundings of an industry.
Children wearing TBPP-treated sleepwear were the group of the
general population particularly exposed to this flame retardant. The
estimated intake via the skin of children wearing TBPPtreated
sleepwear in the USA was calculated to be 9 µg/kg body weight per day.
The Consumer Product Safety Commission of the USA calculated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight or more.
1.1.5 Kinetics and metabolism in laboratory animals and humans
TBPP is absorbed readily from the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. In rats, TBPP or its
metabolites are eliminated within 5 days. Approximately 50% is
eliminated via the urine, 10% via the faeces, and 10-20% is exhaled as
CO2.
One day after oral administration of labelled TBPP to rats,
radioactivity was found in the blood, liver, kidneys, muscles, fat,
and skin, in a range of 0.2-6.6%. The half-life of clearance of
radioactivity from these organs was approximately 2-4 days. After 8
h, only bis(2,3-dibromopropyl) (BBPP) phosphate was still present in
substantial concentrations in most tissues.
After oral administration of TBPP to rats, six metabolites
were identified in the urine and bile. The main metabolite in
the urine, faeces, bile, and tissues was BBPP. The metabolite
2,3-dibromopropanol (DBP) was also identified in rats and in children
wearing TBPP-treated clothing.
Liver microsomes metabolize TBPP in the presence of NADPH and
oxygen. The main metabolites are BBPP and 2,3-dibromopropanol (DBP).
It has been shown that BBPP is formed by oxidation at the C3 and,
possibly, also at the C2 position of TBPP. In addition to BBPP,
2-bromoacrolein, 2-bromoacrylic acid, and propyl-hydroxylated
compounds and metabolites conjugated with glutathione have been found.
S-(2,3-dihydroxypropyl) glutathione was identified in the bile
of rats, and, it was suggested that TBPP and/or BBPP are conjugated
directly with glutathione by glutathione S-transferase with the
formation of episulfonium ion metabolites.
TBPP has been shown to be activated to form products that bind
covalently to proteins and DNA in vivo and in vitro. After
intraperitoneal injections of tritiated-TBPP, male mice, hamsters, and
guinea-pigs, which are less sensitive to TBPP-induced nephrotoxicity
than rats, showed similar levels of covalent binding to proteins in
the liver and kidneys. In the male rat, which is highly susceptible to
TBPP-induced nephrotoxicity, much higher amounts of radiolabel were
bound to kidney proteins than to liver proteins.
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to genotoxic intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from the rodents.
The binding of labelled TBPP and analogues in rats at a
nephrotoxic dose showed that the covalent protein binding was highest
in the kidneys followed by the liver and testes. The results of
comparative in vitro and in vivo studies on renal DNA damage
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C2 or C3 of TBPP. BBPP is transported to the kidneys, where
it is metabolized to reactive intermediates that cause DNA damage and
bind to kidney proteins. The activation occurring in the kidney
appears not to involve P450 but seems to be mediated by GSH-dependent
metabolism. In vitro studies with labelled TBPP and analogues
showed that oxidation of TBPP incorporates one atom of oxygen from
water. This implies that oxidation at C2 of the propyl moiety yields
a reactive alphabromoketone that can alkylate protein directly or
hydrolyse to BBPP and a reactive bromo-alpha-hydroxyketone.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute and short-term oral, and the acute dermal, toxicities
of TBPP are low. The oral LD50 for the rat > 2 g/kg and the dermal
LD50 for the rabbit > 8 g/kg body weight. Extensive kidney damage
(necrosis of renal proximal tubular cells) was noted in male rats
following a single ip injection of 100 mg TBPP/kg body weight.
Four-week, and 90-day, oral toxicity tests with TBPP (by gavage
or in the diet) in rats showed a dose-related increase in the
incidence and severity of chronic nephritis at dose levels of 25 mg/kg
body weight or more.
In rabbits, daily dermal applications of 2.2 g TBPP/kg body
weight or more, for 4 weeks, resulted in degenerative changes in the
liver and kidneys. All rabbits died within four weeks. No deaths
occurred in another study with dose levels of up to 250 mg/kg body
weight.
In a 90-day test on rabbits, weekly application of 2.27 g/kg body
weight to the skin resulted in kidney changes, testicular atrophy, and
aspermatogenesis.
No skin or eye irritation was observed in rabbits with dose
levels of 1.1 g or 0.22 g TBPP and no skin sensitization was observed
in guinea-pigs.
Two teratogenicity studies were carried out on rats. In one
study with dose levels of up to 125 mg/kg body weight, no
teratogenicity was observed. In another study with a dose level of
200 mg/kg body weight, a significant increase in skeletal variations
in the fetuses was observed, and, with 50 and 100 mg/kg body weight, a
significantly lower viability index was found. The authors concluded
that the observed effect resulted from maternal toxicity.
Extensive DNA damage was found in various organs of rats
administered TBPP. In vitro, TBPP has been shown to induce DNA
strand breaks in human KB cells. It induced unscheduled DNA synthesis
in rat liver hepatocytes, but not in human foreskin epithelial cells.
TBPP was mutagenic in several studies on Salmonella typhimurium,
especially in base-pair substituting strains with, and without,
metabolic activation.
Forward gene mutation assays using Chinese hamster V79 cells,
with, and without, metabolic activation were negative. However, a
positive effect in the presence of liver microsomes of rats pretreated
with phenobarbital was obtained. A weak positive effect was obtained
with mouse lymphoma cells (L5178YTK locus).
TBPP increased the number of sister chromatid exchanges (SCEs) in
Chinese hamster V 79 cells, but no chromosomal aberrations were
induced in Chinese hamster cells, mouse bone marrow cells, or in
cultured human lymphoid cells. SCEs but no chromosomal aberrations
were found with diploid human fibroblastic cells (line HE 2144)
without metabolic activation. However, in an in vitro chromosome
aberration test with the Chinese hamster cell line (CHL), TBPP was
positive.
A positive result was obtained with TBPP in a micronucleus test
on Chinese hamster bone marrow cells. Another micronucleus study with
mice showed a weak positive effect.
Studies with Drosophila melanogaster showed that TBPP increased
sex-linked recessive lethals in male germ cells and in adult males,
reciprocal translocations were induced. TBPP showed a strong positive
response in the w/w+ eye mosaic assay.
Several studies have been directed towards the elucidation of the
mechanisms involved in TBPP-induced mutagenicity and/or genotoxicity.
Bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. TBPP is activated by cytochrome P450 in a
reaction depending on NADPH and oxygen. Microsomes prepared from
livers of animals treated with phenobarbital or PCBs give increased
mutagenicity. The mono-and bis(2,3-dibromopropyl) phosphates are less
mutagenic than TBPP. In vitro studies have shown that oxidation at
C3 of the TBPP molecule yields the potent direct acting mutagen
2bromoacrolein that also binds to DNA.
Species differences in the bioactivation of TBPP to metabolites
mutagenic to Salmonella typhimurium TA 100 have been reported. Liver
microsomes from mice were more effective than those from guinea-pigs,
hamsters, and rats.
Three studies in which C3H/10T1/2 cells were used to study cell
transformation were carried out. In one study, a positive effect was
noted, but, in the other two studies, the results were negative.
TBPP was tested on mice and rats by oral administration and on
female mice by skin application in long-term studies. In mice,
following oral administration, TBPP produced tumours of the
fore-stomach and lung in the animals of both sexes, benign and
malignant liver tumours in females, and benign and malignant tumours
of the kidneys in males. In rats, TBPP produced benign and malignant
tumours of the kidneys in males and benign kidney tumours in females.
After skin application to female mice, TBPP produced tumours of the
skin, lung, fore-stomach, and oral cavity. From these studies, it can
be concluded that TBPP has carcinogenic potential in mice and rats.
When the TBPP metabolite BBPP was administered to rats orally, it
caused tumours in both sexes in the digestive system. The tumours
found included papillomas and adenocarcinomas of the tongue,
oesophagus, and forestomach, adenocarcinomas of the intestine, and
hepatocellular adenomas and carcinomas.
Another metabolite of TBPP, DBP, was tested on rats and mice by
dermal application. In male rats, there was an increased incidence of
neoplasms in skin, nose, oral mucosa, oesophagus, forestomach, small
and large intestine, Zymbal's gland, liver, kidney, tunica vaginalis,
and spleen. In female rats, there was an increased incidence of
neoplasms of the skin, nose, oral mucosa, oesophagus, forestomach,
small and large intestine, Zymbal's gland, liver, kidney, clitoral
gland, and mammary gland. In male mice, there was an increased
incidence of neoplasms in the skin, forestomach, liver, and lung, and
in female mice, there was an increased incidence of neoplasms of the
skin and the forestomach.
1.1.7 Effects on humans
Limited data are available regarding the effects of TBPP on
humans.
TBPP has been tested for skin sensitization potential in a few
studies on humans. The results of these studies indicate that TBPP
has a low sensitization potential and no skin irritation was reported.
However, persons who showed a positive sensitization response to pure
TBPP also reacted when exposed to fabrics
treated with TBPP.
1.1.8 Effects on other organisms in the laboratory and field
There are very few data on the effects of TBPP on other
organisms. All 6 goldfish (Carassius auratus), exposed to 1 mg
TBPP/litre, died within 5 days.
The EC50 for growth inhibition in oat seed was 1000 mg/kg soil.
This concentration caused a 100% inhibition of growth in turnip seed
(Brassica rapa sp.).
1.2 Conclusions
TBPP has been used as a flame retardant in fabrics, particularly
in children's sleepwear, but there is inadequate information on its
use in other applications. Exposure of the general population was
primarily through contact with fabrics treated with TBPP.
There is little information on the exposure of, and hazards to,
workers from the commercial production of TBPP and its use in a
variety of products.
Because of the paucity of data, no firm conclusions can be drawn
as to the exposure levels and hazards of TBPP for organisms in the
environment, other than humans.
Animal studies have shown that TBPP can be absorbed from the
gastrointestinal tract and, to a lesser extent, from the skin. TBPP
can also be absorbed through the skin of humans. In the rat, TBPP
appears to be extensively metabolized in the liver to BBPP, which is
the major metabolite detected in the urine and, to a lesser extent, to
DBP. In addition, other brominated metabolites of TBPP have been
found in small amounts. DBP has also been detected in humans wearing
TBPP-treated fabrics. The main route of elimination is the urine and
very little is excreted as the parent compound. The main metabolic
pathway seems to be through metabolism by cytochrome P450 and
glutathione S-transferases.
From the available data, it can be concluded that TBPP has a low
acute toxicity for experimental animals. Repeated dose studies with
relatively high doses of TBPP have revealed kidney and liver damage in
rats and also testicular toxicity in rabbits. TBPP has elicited a
clear genotoxic effect in several test systems, both in vitro and
in vivo. Carcinogenic effects were found in rats and mice. The
metabolites BBPP and DBP have also been shown to produce carcinogenic
effects in experimental animals. No irritation effects were found in
animals and a low sensitization potential in humans was noted.
In 1977, the US Consumer Product Safety Commission banned
children's clothing treated with TBPP, because of concerns that the
chemical might be a human carcinogen, and, because of the possibility
of significant human exposure through contact with treated fabrics.
Since then, the use of this substance as a flame retardant in consumer
products has been severely restricted in several other countries and
it has been prohibited in textiles.
1.3 Recommendations
Because of its toxic effects, TBPP should no longer be used
commercially.
If uses are identified for which there are no less hazardous
alternatives to TBPP, studies to demonstrate the absence of exposure
of, and hazards for, humans and the environment should be conducted.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Chemical formula C9H15Br6O4P
Chemical structure
BrCH2-CHBr-CH2O
\
BrCH2-CHBr-CH2O - P = O
/
BrCH2-CHBr-CH2O
Relative molecular mass 697.7
Synonyms tris(2,3-dibromopropyl) phosphate;
tris(2,3-dibromopropyl) phosphoric
acid ester; phosphoric acid, tris(2,3-
dibromo-propyl) ester;
tris(dibromopropyl) phosphate
CAS registry number 126-72-7
CAS chemical name 2,3-dibromo-1-propanol-phosphate (3:1)
RTECS registry number UB0350000
Trade names T 23 P; TP-69; DBP-TP; Apex
(emulsion) 462-5; Hamcogard FR;
Fyrol 59; Tanotard PN-2; Cav Gard
FR 1811 and FR 1812; Pyrosan 497;
Firemaster LV-T23P and T23P-LV;
Firemaster 200; Glotard PE-2; PE 10;
Anfram 3PB; Bromkal P 67-6HP; ES
685; Firemaster T23 and T23P;
Flacavon R; Flamex T23P; Flammex
AP; Zetofex ZN; Fyrol HB-32; NCI-
CO3270; Phoscon PE60; Phoscon UF-
S; RCRA waste number U 235;
USAF-DO-41 (LeBlanc, 1976; IARC,
1979; Ulsamer et al., 1980; IRPTC,
1987). FR 2406; Berkflam T23 P;
Flammex LVT 23P; 3PBR; TDBP;
TDBPP; TRIS; TRIS-BP; Zetifex ZN;
(Andersen, 1977).
2.1.1 Technical product
Commercial TBPP contains up to 0.2% of the following impurities:
2,3-dibromopropanol, 1,2,3-tribromopropane, and 1,2-dibromo-
3-chloropropane (DBCP) (Blum & Ames, 1977; Van Duuren et al., 1978;
Ulsamer et al., 1980).
2.2 Physical and chemical properties
Two grades of TBPP were available in the USA. The highpurity
grade had the following typical properties: a clear, pale-yellow,
viscous liquid; relative density at 25°C, 2.20-2.26; refractive index
at 25°C, 1.576-1.577; viscosity at 25°C, 3900-4200 centistokes; acid
number (mg KOH/g), 0.05 max; volatiles, 1.5% max; bromine content,
68.7%, and phosphorus content, 4,0%. Typical properties for a lower
grade are as follows: density at 25°C, 2.2-2.3; viscosity at 25°C,
1400-1700 centistokes; acid number (mg KOH/g), 0.05 max; and
volatiles, 10% max. (US EPA, 1976; IARC, 1979).
Osterberg et al. (1977) reported a viscosity of 9200 cP (25°C)
for TBPP of a purity of 99.76%. Firemaster LVT 23P has a viscosity of
9200 cP (Kerst, 1974).
Specific gravity 2.27 (2.2-2.3) g/ml at 25°C
(density) (Kerst, 1974)
Boiling point: 390°C (Dybing et al., 1989)
Melting point: 5.5°C (Dybing et al., 1989)
Vapour pressure: 1.9 × 10-4 mmHg at 25°C
1.2 × 10-3 mmHg at 45°C
4.8 × 10-3 mmHg at 65°C
(Kerst, 1974)
Solubility: Virtually insoluble in water
(6.3 mg/litre at 20°C) and hexane;
miscible in organic solvents, such
as carbon tetrachloride, acetone,
chloroform, methylene chloride,
dimethyl formamide, methanol,
xylene, benzene, toluene, and ethyl
acetate (Kerst, 1974)
Stability:
Heat stability: Major decomposition begins at
about 260-300°C; when heated to
decomposition, TBPP emits toxic
fumes of Br- and POx (Sax, 1984)
Light stability: Stable in sunlight
Hydrolytic stability: Hydrolysed by acids and bases
(IRPTC, 1987)
n-Octanol/water partition
coefficient (log Pow): 3.02 (IARC, 1979)
2.3 Analytical methods
2.3.1 General
TBPP is determined using a gas chromatograph equipped with a
flame photometric detector with possible cleaning processes. Direct
mass spectrometry, GC-MS, and HPLC are also used for the analysis of
biological samples containing TBPP and its metabolites (Cope, 1973;
Lynn et al., 1980, 1982; Pearson et al., 1993a).
Recovery and limits of determination vary, depending on sampling
procedures and matrices. GC analysis shows that TBPP can be
determined at the 10 ng level by using a column packed with a high
liquid loaded support. In an indirect analytical method, TBPP is
determined by spectrophotometry, by complexing phosphor with
molybdenum blue after hydrolysis of the TBPP by hydrobromic acid
(Nakamura, 1980; Gutenmann & Lisk, 1975).
Gardner (1979) described a densitometric method using thin-layer
chromatography. TBPP was chromatographed on silicagel thin-layer
plates, using ethyl acetate hexane (30:70) as a developing solvent.
TBPP was visualized by spraying the chromatograms with 1% aqueous
silver nitrate followed by exposure to UVR for 40 min. The spots were
quantified by densitometry at 600 nm. The lower level of sensitivity
was 50 ng; calibration plots were linear from 50 to 800 ng. The
recovery of TBPP from sewage sludge samples fortified at the 1.0 ppm
level was 97%.
Techniques for the qualitative detection of TBPP in textiles have
been described, including thin-layer chromatography, HPLC, and NMR
(Iliano et al., 1982).
2.3.2 Urine
In mammalian species, organophosphates undergo enzymatic or
chemical hydrolysis to form the corresponding acids and alcohols. The
alcohols are often excreted in the urine as soluble conjugates. Since
the hydrolysis of TBPP yields 2,3-dibromopropanol (DBP), an analytical
method has been developed to determine free, and conjugated, DBP.
Extraction of urine by diethylether/hydrochloric acid, followed by
methylation with diazomethane gives the methylether of DBP.
Determination is by electron affinity gas chromatography. The limits
of determination in rat and human urine were 0.4 and 0.2 mg/litre,
respectively (St. John et al., 1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
TBPP is not known to occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
It is estimated that TBPP was first produced in 1950, when it was
prepared by the addition of bromine to a solution of triallyl
phosphate in benzene. However, it is synthesized in the USA by a
two-step process in which bromine is added to allyl alcohol to give
2,3-dibromopropanol (DBP). This is then reacted with phosphorus
oxychloride, in the presence of a Lewis acid such as, aluminum
chloride or stannium chloride as a catalyst (Overbeek & Nametz, 1962).
The commercial production of TBPP was reported in 1959 and US
production in 1975 has been estimated to have been between 4100-5400
tonnes (US EPA, 1976). Prior to 1977, 4500 tonnes of TBPP were
produced annually in the USA by 6 manufacturers. There was no
evidence of production of TBPP in the USA in 1986.
Production of TBPP in Japan in 1976 and 1977 is estimated to have
been 100 and 300 tonnes per year, respectively, made by one
manufacturer. No TBPP is produced in Japan at present.
It has not been possible to assess whether TBPP is currently
produced. However, no reports are available that describe any
production of TBPP.
3.2.2 Uses
TBPP has been used as a flame retardant for cellulose and
triacetate and polyester fabrics, which are widely used in children's
sleepwear. It has also been used as a flame retardant in other
materials, such as urethane foam and acrylic carpets and sheets,
polyvinyl- and phenolic resins, polystyrene foam, paints, lacquers,
paper coatings and styrene-butadiene rubber, latexes, and cured
unsaturated polyesters products. Rigid foams containing TBPP were
used in insulation, furniture, automobile interior parts, and water
flotation devices. About 65% of the 4500 tonnes of TBPP that were
produced annually in the USA by 6 manufacturers was applied to fabrics
used for children's clothing. TBPP was added to these children's
garments to an extent of 5-10% by weight (US EPA, 1976; Kirk-Othmer,
19781984).
TBPP was applied to cellulose acetate and triacetate by addition
to the melt prior to spinning. The process involved the thermal
diffusion of TBPP by driving it into the fibre under pressure dying.
For materials such as, polyesters, nylons, and acrylics, the TBPP was
either "padded on" at 5-10% by weight with heat fixation to the woven
or knitted material or applied via emulsion from conventional batch
dying equipment (Prival, 1975).
Fire-retarded polyurethane required about 0.5% phosphor and 4-7%
bromine; being equivalent to about 10% TBPP by weight in the product
(US EPA, 1976).
By actions taken on 8 April and 1 June 1977, on the basis of the
genotoxic and possible carcinogenic effects of TBPP, the US Consumer
Product Safety Commission banned children's clothing treated with
TBPP, the chemical itself when used or intended to be used in
children's clothing, and fabric, yarn, or fibre containing it, when
intended for use in such clothing (US Consumer Product Safety
Commission, 1977a,b; US Consumer Product Safety Commission, 1977a,b).
In March 1978, The Consumer Product Safety Commission listed 22
products that contained TBPP and were available to USA consumers.
These included children's clothing, industrial uniforms, draperies,
tent fabric, automobile headliners, epoxy resins for the electronics
industry, Christmas decorations, and polyester thread (IARC, 1979).
In Japan, the use of TBPP as a fire-retardant in textile products
was banned in 1981, because the chemical might be a human carcinogen
and genotoxicant.
As from December 1987, TBPP could not be used in the EC in
textile articles such as, garments, under-garments, and linen intended
to come into contact with the skin (EEC, 1976, 1979).
Several other countries including Finland, New Zealand, and
Sweden have also banned, or severely restricted, the use of TBPP in
textiles and textile articles (UN, 1991).
3.2.3 Sources of human and environmental exposure
Potential sources of human exposure and environmental
contamination include: the manufacturing of the flame retardant, its
application to materials, leaching out of the flame retardant during
use and/or washing, and ultimate disposal of the material.
Studies indicated substantial losses of surface TBPP from fabrics
after laundering, but TBPP was not completely removed after repeated
laundering. For example, acetate fabrics (65-600 mg TBPP/kg) showed
up to 85% reduction in surface concentration after one laundering,
and, polyester fabrics (260-37 500 mg TBPP/kg), from 21 to 82%
reduction after one laundering. A significant portion, approximately
10% of the total production reached the environment from
textile-finishing plants and laundries. Most of the rest will find
its way into solid wastes (US EPA, 1976).
Surface TBPP can be extracted from treated fabric by saliva (up
to 3%) as well as by water, acetic acid, sodium bicarbonate, and salt
(Ulsamer et al., 1980).
Gutenmann & Lisk (1975) heated polyester flannel material,
treated with TBPP, in distilled water at 60°C for 20 min, simulating
a laundering operation. It was calculated from the extraction rate
that laundering of flame-retarded sheets could result in a
concentration of 6 mg/litre in combined washing and rinsing water.
This release was maintained during several subsequent launderings.
The presence of detergents may increase the extraction rate.
TBPP exists both in, and on, the fabric. In the fabric fibres,
it is not extractable with a benzene/hexane mixture and, therefore,
is probably not available for dermal absorption. However, when it is
on the fibre surface, it is extractable and is available for dermal
absorption (Morrow et al., 1976; Ulsamer et al., 1980).
While most of the TBPP is within the fabric in both polyester and
acetate, polyester contains considerably more surface TBPP as a result
of differences in methods of addition. Concentrations of surface
bromine in polyester fabric ranged from 2000 to 37 500 mg/kg with the
actual TBPP content ranging from 20 to 90% of the bromine value. The
non-TBPP organic bromides have not yet been identified (Ulsamer et
al., 1980).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water solubility
of the technical chemical (8.0 mg/litre), TBPP is expected to leach
only slowly into groundwater. The water solubility of pure TBPP may
be lower than the solubility of the technical grade chemical and so
the extent of leaching of the pure chemical may be even lower than the
Koc above suggests (Kenaga, 1980; Lyman, 1982; Verschueren, 1983; US
EPA, 1985).
Although hydrolysis of the phosphate ester is not expected to be
significant, hydrolysis involving the bromine atoms on the propyl
groups may occur, especially under basic conditions. Direct
photolysis is not expected to be a major process, since TBPP should
not absorb light of wavelengths found in sunlight (> 290 nm) (Mabey &
Mill, 1978).
No data on volatilization from water or soil are available. Using
measured water solubility (8.0 mg/litre) and vapour pressure of 1.9 ×
10-4 mmHg, volatilization half-life values were estimated. The
half-life values for TBPP volatilization from streams, rivers, and
lakes were 3.64, 4.66, and 392 days, respectively, assuming current
velocities of 3, 1, and 0.01 m/second, respectively. The river and
stream depths were assumed to be 1 m, while the lake was assumed to be
50 m deep (Verschueren, 1983).
4.2 Transformation
4.2.1 Biodegradation
The biodegradability of TBPP was determined following a
shake-flask test. TBPP was incubated with a microbial inoculum of raw
sewage. Samples of the test solutions were taken at 0, 5, 10, and 15
days for final analysis using neutron activation to determine the
bromine content of the liquid. Assuming the increased bromide content
of the inoculated samples relative to the blank samples is due to
biodegradation, and the solubility of TBPP is 1.6 mg/litre, an amount
of TBPP equal to 2.4 times the dissolved TBPP was degraded in 5 days
(Kerst, 1974).
Activated return sludge (at 21°C), used within 1 h of
collection, diluted with a basal medium, with an added 2 mg
14C-labelled TBPP/kg, showed that 6% of the added radio-activity was
evolved as 14CO2. A major metabolite bis(2,3-dibromopropyl)
phosphate (BBPP) was identified, but neither dibromopropanol (DBP) nor
dibromopropionic acid was detected. The half-life of TBPP was 19.7 h
(by least squares regression analysis). In a sterilized sludge
control study, 93% of the added TBPP was found and metabolites were
not identified (Alvarez et al., 1982).
A biodegradation study on TBPP (100 mg/litre) was carried out
under sewage treatment condition with sludge (30 mg/litre). The
degree of biodegradation, as measured by BOD, was 1.8% of TBPP after a
2-week incubation period (Chemicals Inspection & Testing Institute,
1992).
4.2.2 Abiotic degradation
No data available.
4.2.3 Bioaccumulation
Tissue residue analysis of rats fed TBPP for a period of 28 days
at levels of 100 or 1000 mg/kg diet has shown dose-related residue
levels (measured as total bromine) in the muscle, liver, and body fat,
of the treated animals (see section 7.2.1.).
Groups of 30 adult fathead minnow (Pimephales promelas) (six
months old), were exposed to 47.7 µg TBPP/litre for 2-32 days in a
flow-through system. The temperature of the water was 25°C, pH 7.49,
dissolved oxygen > 5 mg/litre, and hardness
45.5 mg/litre. The bioconcentration factor determined was 2.7 (Veith
et al., 1979).
Bioconcentration of TBPP (0.1 mg/litre, 0.03 mg/litre) from water
to carp was estimated to be between < 0.7 to 1.9, and < 2.2 to 4.3,
respectively, after 6 weeks of exposure (Chemicals Inspection &
Testing Institute, 1992).
4.3 Interaction with other physical, chemical, or biological factors
The thermal oxidative degradation at 370°C of TBPP produced
hydrogen bromide and the C3-brominated species - bromopropenes,
dibromopropenes, dibromopropanes and tribromopropanes, accounting for
87% of the volatiles. The detection of chlorinated species can only
be explained by the presence of chlorinated impurities in the original
ester. The residue (ether soluble aliquot) was composed mainly of
1,2,3-tribromopropane, whereas the aqueous layer contained the
phosphoric acid produced. The gas chromatographic analyses of the
volatiles showed a number of isomeric dibromopropenes. It was
established that 1,3-dibromopropene was the major dibromopropene
formed (Paciorek et al., 1978).
4.4 Ultimate fate following use
It is to be expected that TBPP would be released into the
environment in wastewater after laundering articles coated with TBPP
flame retardant.
With regard to disposal, it must be assumed that clothes and
other products containing TBPP ultimately end up in landfills, which
may result in some biological accumulation. Incineration should be
carried out at high temperature with scrubbers or the equivalent.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
TBPP was identified, but not quantified, in Arkansas air
particulates (DeCarlo, 1979).
5.1.2 Water
In 1975, 20 water samples were collected at different places in
Japan and analysed for the presence of TBPP. None of the samples
contained the compound (limit of determination 1 µg/litre)
(Environment Agency Japan, 1978, 1987).
5.1.3 Soil
In 1975, 20 sediment samples were collected at different places
in Japan and analysed for the presence of TBPP. None of the samples
contained TBPP (limit of determination 0.4-10 mg/kg) (Environment
Agency Japan, 1978, 1987).
TBPP was identified, but not quantified, in Arkansas soil
(DeCarlo, 1979).
5.1.4 Fish
In 1975, 20 fish samples, collected at different places in Japan,
were analysed for the presence of TBPP. None of the samples contained
TBPP (limit of determination 1 mg/kg) (Environment Agency Japan, 1978,
1987).
5.2 General population exposure
5.2.1 Subpopulation at special risk
Tests for the extraction of TBPP from fabrics by water at various
pH values and by a simulated saliva solution failed to reveal any TBPP
in the extracts, but sodium bromide and hydrobromic acid were detected
(limits of determination not mentioned) (Prival, 1975). However,
surface TBPP can be extracted from treated fabric by saliva (up to 3%)
as well as by water, acetic acid, sodium bicarbonate, and salt
(Ulsamer et al., 1980).
In the USA, the estimated intake via the skin of children,
wearing sleepwear treated with the compound, was estimated to be
9 µg/kg body weight (Blum et al., 1978).
The Consumer Product Safety Commission of the USA stated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight and there are
indications that this may be even higher (IRPTC, 1987).
5.3 Occupational exposure
There are no data on levels of exposure to TBPP during
manufacture or further processing.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
TBPP is absorbed readily by the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. Studies on children
revealed that TBPP is dermally absorbed from TBPP-treated sleepwear
(Kerst, 1974; Blum et al., 1978; Ulsamer et al., 1978, 1980).
Following the dermal application of 14C-TBPP to the clipped
backs of New Zealand White rabbits (2-3 kg), 3.5-3.8% of the 0.9 ml/kg
dose and 15.2% of the 0.05 ml/kg dose were absorbed over 96 h.
Osborne Mendel rats (200-250 g) absorbed approximately 1/6 as much
14C-TBPP at each dose, when TBPP was applied to an equivalent area
of skin/kg. The dermal uptake of 14C-TBPP by rats and rabbits
showed that the primary elimination was via the kidneys (Ulsamer et
al., 1980).
6.2 Elimination
6.2.1 Different routes (rat and rabbit)
Four male Sprague-Dawley rats (290-310 g) were administered
14C-TBPP (98%) intravenously. The animals were housed in metabolism
cages for 5 days. Urine, faeces, and air samples were collected for
5 days, and bile for 1 day. In 5 days, 58% of the administered
radioactivity was found in the urine; 9% in the faeces and 19% in the
air as CO2. In 24 h, bile contained 34% of the radioactivity while
9% was found in the bodies of the rats. In three additional rats,
it was found that biliary excretion and enterohepatic recirculation
was a major route in the disposition of TBPP. Bis(2,3-dibromopropyl)
phosphate (BBPP) was detected in the urine of male rats (290-310 g)
dosed iv with 25 mg 14C-TBPP (98%)/animal (in Emulphor) in
amounts of 7.8% of the dose during 5 days following administration.
BBPP was identified in the urine, faeces, bile, and tissues.
2,3-Dibromopropanol (DBP) was found in tissues and DBP and a few other
metabolites were found in urine, but TBPP was not detected (Lynn et
al., 1980, 1982).
An adult male Sprague-Dawley rat (150-200 g) was administered
(iv or orally) 1.39 mg 14C(propyl)-TBPP (99%)/kg body weight. One
day after iv administration, 17% of the administered radioactivity was
found in the urine, 7.4% in the faeces and 20% in the air (as CO2).
One day after oral administration of TBPP, the concentrations were 24%
in the urine and 11.5% in the faeces, but no radioactivity was
detected in the air. Mainly metabolites were excreted in the urine
and bile (Nomeir & Matthews, 1983).
Small amounts of DBP and conjugates appeared in urine, when the
rat was allowed to chew on TBPP-finished polyester fabric (St. John et
al., 1976).
Radiolabel from 14C-TBPP, applied to the skin, was excreted
primarily in the urine (70% for rabbits and 50% for rats) with lesser
amounts appearing in the faeces and 12 and 18% exhaled as CO2,
respectively. TBPP itself did not appear in the urine, but a number
of metabolites including DBP were found (section 6.4) (Ulsamer et al.,
1980).
6.2.2 Dermal exposure (rat and rabbit)
6.2.2.1 TBPP
One hundred mg of TBPP was spread over the surface of a gauze pad
(one square inch) bandage and pressed tightly against the shaved skin
of a rat. Urine was assayed for free and conjugated (released by acid
hydrolysis) DBP. By day 7, the total concentrations of free and
conjugated DBP in the urine were 17.61 and 23.58 mg/litre,
respectively (St. John et al., 1976).
6.2.2.2 TBPP-treated fibres
TBPP has been shown to penetrate rabbit skin from 14C-TBPP
labelled polyester cloth containing 15 000 mg TBPP/kg of surface (4.3%
of the radioactivity in 96 h) (Ulsamer et al., 1980).
A shaved rat wore a garment made of 100% polyester flannel
(4 × 6 inches), treated with TBPP, for 9 days. No DBP could be
detected in the urine (limit of determination 0.4 mg/litre) (St. John
et al., 1976).
6.2.3 Dermal exposure (human)
The skin of a 7-year-old child was exposed on days 1, 2, and
8-12, by wearing repeatedly washed sleepwear that may have been
TBPP-treated. On days 3-7, she wore new TBPP-treated pyjamas. Urine
samples were collected daily from the child. In the urine, a maximum
concentration of DBP of 29 µg/litre was found 2 days after wearing the
new treated pyjamas. DBP at a concentration of 0.4 µg/litre was
present in the urine, prior to wearing the new treated pyjamas. DBP
was still excreted 5 days after the child stopped wearing the new
TBPP-treated pyjamas. Urine samples were collected from 10 other
children and one adult. All samples were analysed for DBP; it was not
found in the urine of one child and one adult (who had never used
washed TBPP-treated sleepwear). Seven children had levels of about
0.5 µg DBP/litre in the urine and one child had a level of 5 µg/litre.
Approximately 180 µg/day (9 µg/kg body weight) was absorbed through
the skin of children wearing pyjamas treated with TBPP (Blum et al.,
(1978).
No DBP could be detected in the urine of an adult or in the urine
of a 5-year-old boy who wore 100% polyester knit pyjamas, treated with
TBPP, for 7 nights. Morning urine samples were collected daily
throughout this period and up to 8 days thereafter (limit of
determination 0.2 mg/litre) (St. John et al., 1976).
6.3 Distribution
6.3.1 Rat
6.3.1.1 Oral
Male adult Sprague-Dawley rats (150-200 g) were administered
1.39 mg 14C(propyl)-TBPP (99%) orally. The percentages of the total
dose of radioactivity, found after one day, in the blood, liver,
kidneys, lung, muscles, fat, and skin, were 6.6, 3.4, 0.7, 0.2, 5.5,
1.3, and 3.4%; 24 and 11.5% of the total dose were found in the urine
and faeces, respectively. The terminal clearance of TBPP-derived
radioactivity from most of the tissues was described by a single
component exponential decay with a half-life of 2.5 days. The
half-life of TBPP in the liver and kidneys was 3.8 days (Nomeir &
Matthews, 1983).
Dose-related bromine concentrations were detected by neutron
activation analysis in the muscles, liver, and fat of male rats fed
TBPP for 28 days. The levels decreased to control levels during the
six-week withdrawal period (Kerst, 1974).
6.3.1.2 Intravenous
Eight male Sprague-Dawley rats (290-310 g) were administered
14C-TBPP (98%) by the iv route and the distribution was studied.
All tissues contained TBPP-derived radioactivity. The concentrations
of TBPP-derived radioactivity declined rapidly in most tissues, but
the concentration of radioactivity in kidneys was 11 times the average
body concentration, five days after dosing. No TBPP was detected,
though bis(2,3-dibromopropyl) phosphate (BBPP) was still present in
substantial concentrations. By day five, only small quantities of
this metabolite were detected. The concentration of TBPP increased
in the fat during the first 5-30 min, but, after 8 h, TBPP was no
longer detectable. In contrast to the rapid disappearance of TBPP,
the half-life of BBPP was relatively long in most tissues. BBPP
represented a major portion of the radioactivity in several tissues
including the lung, muscles, fat, and blood. In blood, it accounted
for 90% of the radioactivity at 30 min and 8 h. By 5 min, 75% of the
radioactivity in plasma was BBPP. The initial plasma half-life of
this metabolite was 6 h. For 5 days it was 36 h. TBPP was not
detectable in plasma after 1 h (Lynn et al., 1982).
6.3.2 Dermal (rabbit)
Substantially more TBPP-derived radiolabel was detected in the
kidneys and liver than in other organs of New Zealand rabbits,
dermally treated with polyester fabrics containing 14C-TBPP (Ulsamer
et al., 1978).
6.4 Metabolic transformation
6.4.1 In vivo studies
6.4.1.1 Oral (rat)
TBPP was readily metabolized in rats. The main metabolite found
in the urine, faeces, bile, and tissues of rats was BBPP.
2,3-Dibromopropanol (DBP) was also identified in tissues and urine.
Only small amounts of unchanged TBPP were found in the excreta (Lynn
et al., 1982; Nomeir & Matthews, 1983).
Male adult rats (150-200 g) were administered 1.39 mg
14C(propyl)-TBPP (99%) orally (by intubation), and the urine and
bile were analysed for metabolites. Six metabolites were identified
in urine and bile, respectively:
- 2,3-dibromopropanol; 1.0 and 1.1%;
- bis(2,3-dibromopropyl) phosphate; 2.8 and 25.8%;
- 2-bromo-2-propenyl 2,3-dibromopropyl phosphate; 4.8 and 13.8%;
- bis(2-bromo-2-propenyl) phosphate; 10.3 and 5.2%;
- 2,3-dibromopropyl phosphate; 4.1 and 2.6%;
- 2-bromo-2-propenyl phosphate; 9.5 and 2.4%
and TBPP was found in concentrations of 0.8 and 2.0%, respectively.
These data are expressed as a percentage of total radioactivity
excreted in the urine in 24 h, and, bile in 3 h. The total quantity
of metabolites eliminated in the urine and bile were, in these
periods, 33.3 and 52.9% of the radioactivity administered,
respectively (Nomeir & Matthews, 1983).
The formation of BBPP has been studied using selectively
deuterated analogues of TBPP. Plasma concentrations of BBPP in rats
dosed with either C2-D1- or C3-D2-TBPP were substantially lower than
levels obtained with TBPP up to 4-6 h after administration. This
indicates that oxidative metabolism of TBPP to form BBPP is important
in vivo. Furthermore, in addition to oxidation at C3, BBPP
formation may result from oxidation at C2. This latter reaction may
be of particular importance with phenobarbital-pretreated microsomes
(Pearson et al., 1993a; Dybing et al., 1989).
In addition to these TBPP metabolites, 2-bromoacrolein,
2-bromoacrylic acid, bis(2,3-dibromopropyl)-3-hydroxypropyl phosphate,
S-(2,3-dihydroxypropyl) glutathione, S-(3hydroxypropyl)
glutathione and S-(2-carboxyethyl) glutathione have been detected
in vitro and/or in vivo (Marsden & Casida, 1982; Nelson et al.,
1984).
2-Bromoacrylic acid has been detected in the urine of rats
administered TBPP. It was suggested that 2-bromoacrylic acid is an
oxidation product of 2-bromoacrolein and that 2-bromoacrolein is
formed spontaneously from DBP generated via initial cytochrome
P450-mediated oxidation of TBPP (Marsden & Casida, 1982; Soderlund et
al., 1984).
Recent data indicate that the formation of 2-bromoacrolein occurs
mainly from oxidative dehalogenation at the C3 position (Pearson et
al., 1993a).
Although glutathione acts as a detoxifying agent for reactive
TBPP metabolites (Soderlund et al., 1984), conjugation could also
result in the formation of reactive episulfonium ion intermediates
(Pearson et al., 1993b). Van Beerendonk (1994) noted that there is
S-(2,3-dihydroxypropyl) glutathione in the bile of Sprague-Dawley
rats. They suggested that TBPP and/or BBPP are conjugated directly
with glutathione by glutathione S-transferases, with subsequent
formation of episulfonium ions.
6.4.2 In vitro studies
TBPP is readily metabolized by microsomal and cytosolic rat liver
fractions. Liver microsomes metabolized TBPP in the presence of NADPH
and oxygen, as evidenced by the release of bromine and the formation
of BBPP (Kerst, 1974; Nomeir & Matthews, 1983).
The role of debromination in the formation of reactive
metabolites was demonstrated in a series of TBPP analogues (Soderlund
et al., 1984). The rate of NADPH-dependent metabolism was increased
5-10 times with microsomes from phenobarbital-pretreated rats compared
with control microsomes and was reduced in the presence of cytochrome
P450 inhibitors, indicating that cytochrome P450 is responsible for
microsomal TBPP biotransformation (Soderlund et al., 1979, 1981, 1984;
Nomeir & Matthews, 1983).
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to reactive intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from rodents (Soderlund et al.,
1982a).
In addition, a 1.5 to 2-fold increase in the rate of TBPP
metabolism occurred when phenobarbital-pretreated microsomes were
fortified with GSH, indicating that microsomal GSH- S-tranferases are
able to conjugate TBPP with GSH. Dialysed rat liver cytosolic
fractions, supplemented with GSH, metabolized TBPP at rates that were
3 times higher than those observed with control microsomes and NADPH
(Nomeir & Matthews, 1983; Soderlund et al., 1981, 1984). Thus, in
animals, GSH-dependent metabolism may be an important route in the in
vivo biotransformation of TBPP to more water-soluble products.
Soderlund et al. (1984) detected the in vitro formation of
2-bromoacrolein, by a reaction catalysed by cytochrome P450, in a
process liberating bromide ions with subsequent formation of BBPP
using rat liver microsomes (Soderlund et al., 1984). Mass spectral
analysis of 2-bromoacrolein, formed from selectively deuterated
analogues of TBPP, revealed that the primary mechanism for the
formation of 2-bromoacrolein involves the initial oxidative
dehalogenation at C-3 followed by a betaelimination reaction (Nelson
et al., 1984).
In vitro studies were carried out with deuterated analogues of
TBPP, or, analogues labelled at specific positions with carbon-14,
phosphorus-32, or oxygen-18, or dual-labelled with both deuterium and
tritium. These were used as metabolic probes to study the chemical
and metabolic events in the bioactivation of TBPP to chemically
reactive metabolites in the liver microsomal preparations of male
Sprague-Dawley rats. Studies with deuterated analogues of TBPP
implicated oxidation at C-2 of the propyl moiety as a major pathway
that leads to protein binding, which is enhanced by phenobarbital
pretreatment of rats. Investigations with 18O-TBPP and H218O
showed that the BBPP that is formed from the oxidation of TBPP
incorporates one atom of oxygen from water. These results imply that
oxidation at C-2 yields a reactive alpha-bromoketone that can alkylate
proteins directly, or, hydrolyse to BBPP and a reactive alpha-
bromoalpha'-hydroxyketone that alkylates microsomal proteins (Pearson
et al., 1993a). These studies also showed that TBPP is oxidized at
C-3, yielding the direct acting mutagen 2-bromoacrolein as the major
metabolite that binds to DNA. This is consistent with earlier studies
that indicate that 2-bromoacrolein is the major reactive metabolite
formed in in vitro microsomal incubations (Nelson et al., 1984;
Dybing et al., 1989).
6.5 Covalent binding to macromolecules
TBPP has been shown to be activated to products that bind
covalently to proteins (total macromolecules) and DNA in vitro and
in vivo (Soderlund et al., 1981, 1984; Pearson et al., 1993a,b).
The covalent binding of radiolabel TBPP to macromolecules was
dependent on microsomes and NADPH, and was reduced by carbon monoxide,
inhibitors of P450, and glutathione (Soderlund et al., 1981). The
extent of TBPP covalent binding in vivo was five times higher in the
kidneys than in the liver, whereas the rate of in vitro covalent
binding was much higher with liver microsomes than with kidney
microsomes. The low levels of TBPP binding in the liver in vivo may
be the result of an extensive detoxification of TBPP to non-reactive
metabolites or to low tissue concentrations of the proximate
metabolite(s).
Male NMRI and female B6C3F1 mice (20-25 g), male F344 rats
(200-250 g), and guinea-pigs (80-100 g) were injected ip once with
250 mg 3H-TBPP/kg body weight in DMSO. The animals were killed 9 h
after injection. All species showed similar levels of covalent
binding to proteins in the liver and kidneys except for the rat which
had much higher amounts of radiolabel bound to kidney proteins
(Soderlund et al., 1982a).
The binding of TBPP and analogues has also been studied in
vivo. Analogues of TBPP either labelled at specific positions with
carbon-14, and phosphorus-32 or dual-labelled with both deuterium and
tritium were administered to male Wistar rats at a nephrotoxic dose of
360 µmol/kg body weight. The covalent binding of TBPP metabolites to
rat hepatic, renal, and testicular proteins was determined after 9 and
24 h. The covalent protein binding was 5 times higher in the kidneys
than in the liver and approximately 25 times higher than that in the
testes. The results of comparative studies on renal DNA damage
induced by TBPP and BBPP labelled with deuterium at C-2 or C-3
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C-2 or C-3 of TBPP. BBPP is then transported to the
kidneys, where it is subsequently metabolized to reactive
intermediates that cause DNA damage and bind to kidney proteins in a
process, independent of cytochrome P450, involving activation by
conjugation with glutathione (Pearson et al., 1993b).
Van Beerendonk et al. (1992) studied the formation of thymidine
adducts and the cross-linking potential of 2-bromoacrolein (BA), a
reactive metabolite of TBPP. In this study, [3-3H]BA was reacted
with single-stranded (ss) DNA or double-stranded (ds) DNA and
subsequently incubated with methoxylamine to covert the reaction
product to an unstable BA:thymidine adduct. Because the unstable
BA:thymidine adduct may have the potential to form cross-links, the
reaction with various nucleophiles in vitro was studied. A reaction
occurred between the adduct and cystein, but not with lysine or
desoxynucleosides. Reaction of BA with ssDNA in the presence of
[3H]glutathione also resulted in the binding of radiolabelled GSH to
DNA. The results indicated that the reactive aldehyde group of the
adduct can react with thiol groups in proteins to form protein-DNA
cross-links. When the possibility that tris- and bis-(2,3-
dibromopropyl) phosphates form such cross-links was examined in vivo
in Drosophila, it was found that TBPP was a cross-linking agent,
whereas BBPP was not.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The oral LD50 for TBPP was calculated to be 5.24 g/kg body
weight, when administered as a suspension in propyleneglycol to male
albino Spartan rats with a weight of 202-250 g. The observation
period was 14 days (Kerst, 1974). In another study, TBPP dissolved in
propyleneglycol or in ethanol was given to Osborn-Mendel rats. Oral
LD50s of 1.88 and 3.12 g/kg body weight, respectively, were obtained
(Ulsamer et al., 1980).
A dermal toxicity study showed an acute LD50 for rabbits of
17.6 g/kg body weight (Ulsamer et al., 1980). In another study, TBPP
was applied once to the back of four groups of two male and two female
New Zealand white rabbits (2.56-2.96 kg) in concentrations of 1, 2, 4,
or 8 g/kg body weight. The application area was wrapped with a gauze
bandage and occluded; after 24 h, the bandages were removed and the
skin washed with water. The observation time was 14 days. An LD50
of > 8 g/kg body weight was found (Kerst, 1974).
A dose of 2 g TBPP was applied to the intact and abraded skin of
10 albino rabbits. No deaths were observed during a 14-day
observation period (Moldovan, 1972).
7.2 Short-term exposure
7.2.1 Oral exposure (rat)
7.2.1.1 TBPP
Groups of male rats received daily doses of 250 mg TBPP/kg in
either propyleneglycol or saline, by gavage, and were sacrificed after
1, 2, 4, 6, 8, or 10 days. Liver and testes were unaffected by any
treatment, but nephrotic changes were observed to commence on day 2
and to become progressively more severe with time. In addition to the
tubular lesions, the glomeruli were adversely affected, an observation
not seen in the 13-week study (Osterberg et al., 1979).
In a pilot study, groups of 10 non-pregnant rats were
administered TBPP for 10 days at dose levels of 100, 150, 500, or
1000 mg/kg body weight per day. Mortality rates were 0, 0, 70, and
100%, respectively (Seabaugh et al., 1981).
Male weanling rats were fed TBPP at concentrations of 100 or
1000 mg/kg diet for 28 days. The animals were then sacrificed
immediately or after 2 or 6 additional weeks of recovery. The results
showed a decrease in food efficiency (approximately 10% at the
highest dose), decreased body weight gain (approximately 20% at the
highest dose), and decreased organ to body weight ratios for heart,
liver, spleen, kidney, and gonads (approximately 20% for each organ at
the highest dose). Haematology, blood chemistry, urinalysis, and
histopathology did not differ from the control values. In the
recovery period, the body weight gain became normal. The authors
suggested that the effect might be because of the palatability of the
substance. Tissue residues (measured as bromine) increased 40-50
times in the first 4 weeks of treatment in the fat, liver, and
muscles. By the end of the 6-week withdrawal period, the residues
were at control levels (Kerst, 1974).
Groups of rats were gavaged with TBPP in corn oil at 10, 50, or
100 mg/kg per day for 4 weeks. One half of each group was sacrificed
at 4 weeks and the remainder at 6 weeks. While no adverse responses
were observed, elevated bromine levels in blood were reported (Brieger
et al., 1968).
A 90-day study was carried out on rats administered TBPP in
propylene glycol, daily (by gavage), at 25, 100, or 250 mg/kg body
weight. The control groups received either the vehicle, normal
saline, or no treatment. Weight gain for males was 34-50% less and,
for females, 40% less in the test groups and vehicle group compared
with the control values. Liver/body weight ratios were lower for both
sexes in the low TBPP group, but higher in females in the highest dose
group, compared with those in the control group. Kidney/body weight
ratios were 18% lower than in controls. Testes/body weight ratios in
the TBPP groups were 25% lower. There was an increased incidence and
severity of chronic nephritis associated with regenerative epithelium,
hypertrophy, and dysplasia of renal tubular epithelial cells in all
TBPP-treated rats. The complex of changes was more severe with higher
dose, and among males (Osterberg et al., 1978).
7.2.1.2 TBPP-treated fibres
The results of a 2-week study on rats fed 15% shredded
TBPP-treated acetate fibres in their food (3 times/week) showed no
changes in blood-bromine levels and no adverse effects (Ulsamer et
al., 1980).
7.2.2 Oral exposure (dog)
7.2.2.1 TBPP
In a study on dogs, doses of 50 or 100 mg TBPP/kg body weight
were given in the diet for four weeks. A decrease in body weight was
noted in the treated dogs as well as increased blood-bromine levels.
Cholinesterase activity was reported to be unaffected (Brieger et al.,
1968). No further details were available for this study.
7.2.2.2 TBPP-treated fibres
In a 2-week study on dogs fed 15% shredded TBPP-treated acetate
fibres in their food (3 times/week), no changes in blood-bromine
levels or adverse effects were seen. Two additional, 3-week studies
on dogs using TBPP-treated shredded rayon and acetate fibres added to
foods did not show any detectable changes in health or in
blood-bromine levels (Brieger et al., 1968).
7.2.3 Dermal exposure
7.2.3.1 Rabbit
Short-term dermal studies have been performed using groups of
clipped rabbits dosed with 2.2, 4.4, or 8.8 g TBPP/kg body weight,
daily, for 4 weeks. A dose-related increase in bromine was found in
the blood and urine. All rabbits died within 4 weeks. Significant
degenerative changes in the kidneys and the liver were found. Slight
decreases in cholinesterase activity were recorded (Brieger et al.,
1968). In another study in which the animals were administered dose
levels of 50 and 250 mg/kg body weight, bromide levels in the blood
and urine were increased, but no deaths occurred (Ulsamer et al.,
1980).
A 13-week study was carried out on 12 young (3 months old) New
Zealand white rabbits, 6 with intact, and 6 with abraded, dorsal skin.
They were treated with a weekly application of 2.27 g TBPP (99.76%)/kg
body weight for 13 weeks. In a third group, 6 rabbits were initially
clipped and maintained untreated as controls. The TBPP-treated sites
were not occluded with a patch, but the animals were fitted with a
collar. Besides a statistically significant increase in relative
liver weights in the rabbits with intact and abraded skin (53% and
59%, respectively), a significant decrease in testes weight (54% and
40%, respectively) was observed. Microscopically, chronic
interstitial nephritis (in 6/8 males) with tubule involvement and
bizarre nuclei as well as testicular atrophy and aspermatogenesis
(spermatogonia were present in seminiferous tubules, and also
secondary spermatocytes, but no spermatozoa) were observed in 7/8
males of the test groups. Female rabbits did not exhibit any adverse
responses. No histopathological changes were seen in the liver
(Osterberg et al., 1977, 1978).
In a study in which TBPP-treated rayon cloth was applied to the
clipped skin of rabbits for 4 weeks, no significant effects were found
(bromine levels were not increased) in treated animals (Ulsamer et
al., 1980). No further details were available for this study.
7.2.3.2 Dog
When TBPP-treated rayon cloth was applied to the clipped skin of
dogs for 4 weeks, no significant effects (no increased bromine levels)
were found in the treated animals (Brieger et al., 1968). No further
details were available for this study.
7.3 Long-term exposure
Apart from carcinogenicity studies, no long-term toxicity studies
are available (see section 7.7).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
TBPP (1.1 g) was applied to the abraded or intact skin of six
albino rabbits. The animals were fitted with collars for 24 h. After
this period, the coverings were removed and the test material washed
off. The extent of erythema and oedema was determined after 24 and
72 h. No signs of irritation were observed (Kerst, 1974).
7.4.2 Eye irritation
Administration of 0.22 g TBPP to the eyes of 6 adult rabbits did
not cause noticeable irritation or damage to the cornea, iris, or
palpebral conjunctiva during a 72-h observation period (Kerst, 1974;
US EPA, 1976).
7.4.3 Sensitization
TBPP was tested for skin sensitization in groups of 5-10
guinea-pigs using a modified Landsteiner method and the footpad
technique. No sensitization was noted in either test (no details
given) (Morrow et al., 1976).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
7.5.1 Reproductive system
Groups of 6 adult male Sprague-Dawley rats (56-60 days of age)
were used in a study to investigate the effects of TBPP on the
reproductive system. Six rats were injected with 0.1 ml
propyleneglycol intraperitoneally, three times/week, and, six rats
were untreated controls. Nine groups of 6 rats were given (ip
injection), three times/week, 0.4, 0.9, 1.8, 3.5, 7.1, 14.2, 28.4,
56.8, or 113.5 mg TBPP in propyleneglycol for a period of 72 days. The
four highest dose levels of TBPP did not dissolve completely and were
injected as an emulsion. The rats were treated for a minimum of 72
days (6 cycles of the germinal epithelium) before being killed. The
three highest dose levels (28.4-113.5 mg/injection) caused significant
dose-related declines in the weights of the testes and prostate,
epididymides, and seminal vesicles. Sperm production of testes and
sperm storage in the epididymides were reduced, and the percentage of
the motile sperm and the motility index were decreased. Histological
examination of the testes revealed that the seminiferous tubules were
affected. The affected tubules contained very few germinal cells and
the macrophages in the interstitium of the affected testes appeared to
be phagocytically active. The Leydig cells were normal. TBPP did
not have any significant effects on the serum concentration of
testosterone or on the in vitro testicular capacity for testosterone
secretion (Cochran & Wiedow, 1986).
The effects on the testes were also reported in a 13-week study
on New Zealand white rabbits, treated with weekly dermal applications
of 2.27 g TBPP on the intact or abraded skin. Decreased testes
weights and, microscopically, testicular atrophy and aspermatogenesis
were found in male rabbits (Osterberg et al., 1977).
B6C3F1 mice (15 weeks old) were administered (ip) TBPP in corn
oil at dose levels of 0, approximately 200, 400, 600, 800, and
1000 mg/kg body weight daily, for 5 days. The mice were killed 35
days after the fifth treatment. Their epididymides were removed and
abnormal sperm heads determined. The frequency of abnormal sperm
heads in TBPP-treated mice was significantly greater than in controls,
predominantly at dose levels of 800 mg/kg body weight or more
(Salamone & Katz, 1981).
7.5.2 Teratogenicity
In a pilot study on groups of ten pregnant Sprague-Dawley rats,
orally intubated with 0, 250, or 1000 mg TBPP/kg body weight on days
6-15 of gestation, an increase in maternal mortality was observed.
The mortality rates were 0, 10, and 100% respectively. The rats given
1000 mg/kg died on days 9-11 of gestation (Seabaugh et al., 1981).
Sexually mature, timed-pregnant Sprague-Dawley rats, 30 animals
per group, were intubated on days 6-15 of gestation with TBPP (99.7%
TBPP, 0.14% 1,2,3-tribromopropane, and 0.17% 2,3-dibromopropanol) in
undiluted propyleneglycol at levels of 0, 5, 25, or 125 mg/kg body
weight per day. Maternal body weight gain was decreased at the
highest dose level. No effects of treatment were apparent on the
number of corpora lutea, implantations, or early or late deaths.
Furthermore, the percentage of females with resorptions, the number of
viable fetuses, the percentage of resorptions, and the percentage of
pre-implantation losses, did not show compound-related changes. Fetal
body weight and crown-rump length were not affected. Some fetal soft
tissue and skeletal variations found were not dose-related or
statistically significant. It was concluded that TBPP was not
teratogenic in this study (Seabaugh et al., 1981).
Female Wistar rats were exposed orally to 25, 50, 100, or 200 mg
TBPP in olive oil/kg body weight on days 7-15 of gestation. A
significant increase in skeletal variation was found in the fetuses at
200 mg/kg. A significantly lower viability index was observed in the
50 and 100 mg/kg groups. The authors concluded that TBPP did not
produce teratogenic effects in rats. A dose of 200 mg/kg elicited
maternal toxicity (Kawashima et al., 1983).
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
7.6.1.1 In vivo
When male Wistar rats (250-320 g) were given a single ip
injection of 350 µmol TBPP/kg (250 mg/kg) body weight and assayed for
DNA damage 2 h later, single strand breaks/alkali labile sites were
found in the DNA from nuclei isolated from several organs. DNA damage
was detected using an automated alkaline elution system. Extensive
DNA damage was detected in the liver, kidneys, and small intestines.
In addition, substantial DNA damage was found in the brain and lungs;
less DNA damage was detected in the testes, spleen, and large
intestines (Holme et al., 1983; Soderlund et al., 1992). DNA damage
was clearly detectable in the kidneys 20 min after a single ip dose of
36 µmol TBPP/kg (25 mg/kg) body weight (Pearson et al., 1993b).
7.6.1.2 In vitro
Monolayer cultures of human (KB) cells were grown with
[3H]-thymidine for 30 h, and without, for another 17 h. The cells
were then exposed to TBPP (2 µl/ml of growth medium devoid of serum)
for 4.5 h and processed for analysis of the DNA on alkaline-sucrose
gradients. They were re-incubated for various intervals to permit DNA
repair. TBPP was shown to have induced DNA repair, which indicated a
specific action on human cellular DNA. TBPP was found to damage human
DNA in vitro and to cause unscheduled DNA synthesis in human cells
in tissue culture (Gutter & Rosenkranz, 1977; Blum & Ames, 1977).
A semiquantitative, in vitro method for measuring unscheduled
DNA synthesis (UDS) was developed by Lake et al. (1978). Normal
foreskin epithelial cells from a cryopreserved skin pool were grown
from explants and replanted in replicate culture wells. Cultures were
then treated for 3 days in an arginine-deficient medium and further
inhibited in S-phase DNA-synthesis by a 2-h (10 mmol/litre)
hydroxyurea treatment. 3H-Thymidine and TBPP were added
simultaneously and the UDS, accumulated over a 24-h incubation period,
was determined by direct scintillation counting of acid-precipitable
whole-cell radioactivity. TBPP did not induce an UDS response in this
assay, with input dose ranges of 10-99 and 100-400 µg/ml.
UDS was detected in rat liver hepatocytes, grown as monolayer
cultures, exposed to 0.01-0.1 mmol TBPP/litre for 18-19 h in the
presence of [3H]-thymidine and hydroxyurea. UDS was determined by
scintillation counting (Holme et al., 1983; Holme & Soderlund, 1984;
Gordon et al., 1985; Soderlund et al., 1985).
In in vitro test systems, DNA damage was detected in isolated
rat hepatocytes exposed to concentrations as low as 5 µmol TBPP/litre,
while a 10-fold higher concentration was necessary to induce DNA
damage in testicular cells (Soderlund et al., 1992). No DNA damage
was found in cultured Reuber rat hepatoma cells, without the addition
of an exogenous metabolism system (Gordon et al., 1985).
7.6.2 Mutation assay with Salmonella typhimurium strains
Species differences in the bioactivation of TBPP to metabolites,
mutagenic to Salmonella typhimurium TA 100, have been reported.
Liver microsomes from mice (NMRI strain) were more effective in
activating TBPP to mutagenic intermediates than those from
guinea-pigs, hamsters, and rats. Phenobarbitalinduced liver
microsomes from NMRI mice were especially effective (Soderlund et al.,
1982a).
TBPP was activated to mutagens in the Salmonella/microsome
test. S9-fractions from rats pretreated with phenobarbital increased
the mutagenicity of 0.05 mmol TBPP/litre in TA 100 strain compared
with liver microsomes from untreated rats (Holme et al., 1983).
It was demonstrated that the metabolic activation is dependent on
the presence of NADPH and oxygen, which indicates that TBPP is
metabolized by cytochrome P450 enzymes to mutagenic products. In
studies conducted in an anaerobic atmosphere or in the presence of
GSH, the mutagenicity of TBPP was significantly decreased (Soderlund
et al., 1979, 1984).
TBPP (97%) in DMSO was tested in concentrations of 0.0110 µlitre
on Salmonella typhimurium TA 100, TA 1535, TA 1537, and TA 1538,
using the plate assay, in the absence, and presence, of a metabolic
activation system from rat liver. A mutagenic effect was found with
TA 100 and TA 1535 with, and without, metabolic activation. TA 1537
and TA 1538 gave negative results (Blum & Ames, 1977; Brusick et al.,
1978; Prival et al., 1977).
TBPP was tested on Salmonella typhimurium tester strains
TA 1535 and TA 1538 in the absence, and presence, of metabolic
activation derived from Aroclor-induced rat liver. Dose levels of 0,
0.1, and 1.0 µlitre/plate were used. Weak mutagenic activity was
observed in TA 1535 without activation, but a strong effect was seen
with microsomal activation. TA 1538 gave negative results (Carr &
Rosenkranz, 1978).
MacGregor et al. (1980) confirmed the mutagenicity of TBPP in the
Salmonella typhimurium strains TA 100, TA 98, and TA 1535, with dose
levels ranging from 10 to 1000 µg/plate, with metabolic activation.
Without activation, no mutagenicity was found. A negative result was
obtained in strain TA 1537 with, and without, activation.
Nakamura et al. (1979) tested TBPP on Salmonella typhimurium
strains TA 100 and TA 1535 with, and without, metabolic activation at
dose levels of 0.3-100 µmol/plate. A positive effect was seen in both
strains, without and with S9 mix. McCann & Ames (1977) found a
mutagenic effect in Salmonella typhimurium TA 100 with dose levels
up to 100 µg/plate, in the presence of liver S9 fraction of rats
treated with Aroclor.
TBPP at dose levels of 0, 112, 224, 2240, 4480, and
11 200 µg/plate was tested on Salmonella typhimurium strain TA100
with, and without, metabolic activation by Aroclor 1254-induced rat
liver S9 fraction. With the S9 fraction, all dose levels showed a
mutagenic effect. Without the S9 fraction, TBPP showed direct-acting
properties only at dose levels of 2240 µg/plate or more (Salamone &
Katz, 1981).
In an interlaboratory study, TBPP and 62 other chemicals were
tested for mutagenic activity. TBPP was tested on the Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, and on
Escherichia coli WP2uvrA. The dose levels were between 0.3 and
10 000 µg/plate. TBPP was tested without metabolic activation and
with liver S9 fractions from uninduced and Aroclor 1254-induced F344
rats, B6C3F1 mice, and Syrian hamsters. TBPP tested positive in all
four laboratories involved in this study (Dunkel et al., 1985).
Results obtained by Prival et al. (1977) indicated that TBPP
induces mutations of the base-pair substitution type in Salmonella
typhimurium TA100. Although, at higher concentrations
(> 1 µl/plate), TBPP behaves as a direct acting mutagen not requiring
metabolic activation, at a much lower concentration (0.01 µl/plate) it
demonstrates significant genetic activity only with metabolic
activation.
Brusick and coworkers demonstrated that amounts of 50 µg/plate or
more were clearly mutagenic for Salmonella typhimurium TA 100
(Brusick et al., 1980). When tested for bacterial mutagenicity in
Salmonella typhimurium TA 100, a 4-fold interindividual variation in
the capability to activate TBPP was noted with human liver microsomes
prepared from 5 liver donors (Soderlund et al., 1982a).
The CASE structure-activity method was applied to a Gene-Tox
derived Salmonella mutagenicity data base. Strains TA 97, TA 98, TA
100, TA 1535, TA 1537, and TA 1538 with, or without, exogenous
metabolic activation, were used. TBPP was found to be positive
(Klopman et al., 1990).
7.6.3 Mutations by urine of rats treated with TBPP
The urine was collected of rats exposed to TBPP directly by
either the oral or dermal route, or from treated fabric. Salmonella
typhimurium TA 1535 was used as indicator organism. TBPP was
dermally applied at doses of 5, 50, 500, or 5000 mg/kg body weight or
given orally at 5, 50, or 500 mg/kg body weight. In the oral study,
only 500 mg/kg produced a positive response. In the dermal studies, a
dose of 500 mg/kg produced a weak positive response, while 5000 mg/kg
produced a definitive positive response. When fabrics with surface
TBPP levels of 3000, 28 000, and 67 000 mg/kg product were applied
dermally, no mutagenic responses were detected in the urine of the
rats over the 5-day period (data were lacking on whether or not
metabolic activation was used) (Brusick et al., 1978; Ulsamer et al.,
1980).
TBPP at 500 mg/kg body weight in corn oil was applied dermally to
CD-1 mice. Urine was collected over approximately 16 h and the
bacterial mutagenicity of 0.3 ml urine samples was assayed in
Salmonella typhimurium TA1535, TA1537, and TA100. A positive
response was found only with TA100 (Brusick et al., 1982).
7.6.4 Other mutation assays
TBPP was tested in the forward mutation assay with mouse-lymphoma
cells (L5178YTK locus). While the results at lower doses were
inconclusive, a 2 to 3-fold increase in mutations was consistently
produced at 5 mg/litre (Brusick et al., 1978; Ulsamer et al., 1980).
TBPP has been reported to induce increased mutation frequencies
(6-TG resistance) in V79 Chinese hamster cells incubated with
0.02 mmol TBPP/litre in the presence of liver microsomes of rats
pretreated with phenobarbital as an exogenous metabolism system (Holme
et al., 1983; Soderlund et al., 1985). However, in a similar study,
concentrations of TBPP up to 150 µg/ml did not increase the frequency
of 6-TG resistance, both with, and without, an exogenous metabolism
system (Sala et al., 1982).
7.6.5 Chromosomal effects
Using Chinese hamster V79 cells, TBPP severely inhibited the
colony-forming activity and significantly increased sisterchromatid
exchanges, but no significant increase in chromosome aberrations was
found (Furukawa et al., 1978). Interestingly, chromosomal aberrations
were not significantly increased in Chinese hamster cells, in mouse
bone-marrow cells, or in cultured human lymphoid cells. The lack of a
TBPP effect on rat bone-marrow chromosomes was also observed after
rats received 25, 250, or 2500 mg TBPP/kg body weight, by gavage,
after either a single dose, or, 5 daily doses/week for 13 weeks
(Osterberg, 1977; Nakanishi & Schneider, 1979).
TBPP was tested for the induction of chromosome aberrations, and
sister chromatid exchanges in the diploid human fibroblastic cell line
HE 2144 (from a 10-week-old male embryo) without metabolic activation
(Sasaki et al., 1980). The dose levels used were 0.349, 0.070, and
0.035 mg/ml. Sister chromatid exchanges were induced with 0.070 mg
TBPP/ml in the human HE 2144 cell line. No chromosomal aberrations
were found.
In a comparative study, Brusick and coworkers found that TBPP
gave a positive response in tests for sister chromatid exchanges and
chromosomal aberrations in the mouse lymphoma L5178Y cell line at
concentrations of 0.005 and 0.01 µlitre/ml, respectively (Brusick et
al., 1980).
TBPP was tested in an in-vitro test for sister chromatid
exchanges in Chinese hamster V79 cells with, and without, S9 fraction
of livers of Wistar rats administered (ip) methylcholanthrene.
Acetone was used as solvent. The dose levels 17.2, 35, 100, and
200 µg/ml were tested only without S9 fraction, while levels of 24.5
and 50 µg/ml were tested with, and without, metabolic activation. A
significant increase in sister chromatid exchanges was found at dose
levels of more than 35 µg/ml (Sala et al., 1982).
Two male and two female Chinese hamsters per group were used in a
micronucleus test. The dose levels were 200, 400, and 800 mg TBPP/kg
body weight administered by ip injection. The solvent was DMSO.
Bone-marrow samples were obtained after 24 h. Two thousand
polychromatic erythrocytes/animal were analyzed for the presence of
micronuclei. Levels of 400 and 800 mg/kg body weight showed a
positive effect (Sala et al., 1982).
Salamone & Katz (1981) studied the clastogenic effect of TBPP in
a bone marrow micronucleus test. B6C3F1 mice (15 weeks old) were
given two ip treatments of TBPP in corn oil. Dose levels of 0, 204,
408, 612, 816, 1020, 1275, and 1530 mg/kg body weight were tested. In
this test, TBPP showed a weak clastogenic effect.
An in vitro chromosome aberration test was carried out with
TBPP, using a Chinese hamster CHL cell line of lung fibroblast origin.
CHL cells cultured in plates were exposed to different dose levels of
TBPP including the 50% growth inhibition dose. The number of
polyploid cells and cells with structural aberrations, such as
chromatid-type gaps, breaks, exchanges, and rings, were scored. A
microsome fraction (S9-mix) from the liver of Wistar rats, pretreated
with the PCB; KC-400, was used. TBPP was positive in this test. A
dose level of 0.25 mg/ml showed chromosomal aberrations in 20% of the
metaphases (Ishidate et al., 1981).
Vogel & Nivard (1993) studied the effects of TBPP in the
(white/white+) (w/w+) eye mosaic assay, and an in vivo, short-term
test measuring genetic damage in the somatic cells of Drosophila
melanogaster, after treatment of the larvae. The genetic principle
of this system is the loss of heterozygosity for the wild-type
reporter gene w+, an event predominantly resulting from homologous,
interchromosomal, mitotic recombination between the two X-chromosomes
of female genotypes. The w/w+ eye mosaic test detects a broad
spectrum of DNA modifications. Between 12 and 15 pairs of flies were
permitted to lay eggs for three days on food supplemented with 0.25,
0.5, or 1.0 mmol TBPP/litre (dissolved in 3% ethanol). TBPP gave a
strong positive response in the w/w+ bioassay.
7.6.6 Cell transformation
TBPP was tested for its ability to induce malignant
transformation in vitro using mouse BALB/3T3 cells. The results of
this test showed that TBPP can transform mammalian cells in vitro,
perhaps indicating a potential for the induction of carcinogenic
responses (Brusick et al., 1978; Ulsamer et al., 1980).
C3H/10T1/2 cells were treated with TBPP, with or without S9 mix
from the liver of Wistar rats administered methylchloanthrene
intraperitoneally. Some cell samples were additionally treated
several times with tetradecanoyl phorbolacetate (TPA) (0.1 µg/ml).
The TBPP concentrations tested were 40 µg/ml (with and without S9
fraction) and 80 µg/ml (without S9 fraction). A very low frequency of
transformed type 3 foci was obtained and the authors considered the
results of this study to be negative (Sala et al., 1982). Dunkel et
al. (1988) also found a negative result for TBPP in the C3H/10T1/2
cell transformation assay. The dose levels tested were between 0.16
and 20 µg/ml.
7.6.7 Miscellaneous tests
TBPP induced a significant increase in sex-linked recessive
lethal mutations in male germ-cell stages of Drosophila melanogaster
at a dose of 1000 mg/kg. The spermatids were the most sensitive
(Valencia, 1978).
Adult Canto-S male Drosophila flies aged 7 days were fed for
48 h on a 1% solution of glucose containing 1000 or 10 000 mg
TBPP/litre. The TBPP-exposed males were mated immediately after
treatment to brown ebony virgin females. The results showed that TBPP
caused reciprocal translocations in Drosophila. There was no
difference between the translocation recoveries at the two dose levels
(Berkowitz, 1978).
7.6.8 Mechanisms of TBPP genotoxicity
Following the initial mutagenicity reports, several studies have
been directed towards the elucidation of the mechanisms involved in
TBPP-induced genotoxicity. Such studies include investigations into
the enzyme systems involved in the bioactivation of TBPP to genotoxic
metabolites, delineating structural requirements for genotoxicity, and
the characterization and identification of the genotoxicity of
possible TBPP metabolites. Some of these investigations have recently
been reviewed (Dybing et al., 1989).
The bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. Several studies have shown that TBPP is
activated to metabolites, mutagenic to Salmonella typhimurium TA100,
by cytochrome P450 in a reaction depending on NADPH and oxygen
(Soderlund et al., 1979; Dybing et al., 1989). The mutagenicity of
TBPP was increased in the presence of microsomes prepared from livers
of rodents pretreated with phenobarbital or PCBs, but not from those
pretreated with 3-methylcholanthrene or beta-naphthoflavone (Soderlund
et al., 1979; 1982a).
The metabolism of TBPP by soluble enzymes (e.g., glutathione
S-transferase) appears to be of minor importance in the
bioactivation of TBPP to mutagenic species. The available data
indicate that reductive metabolism and episulfonium ion formation are
not major activation pathways in TBPP-induced mutagenicity (Dybing et
al., 1989). However, it has recently been suggested that human
glutathione transferases may further metabolize P-450-generated TBPP
intermediates to more potent mutagenic species (Simula et al., 1993).
Interestingly, singlet oxygen, obtained from the illumination of
riboflavin, may also activate TBPP to mutagenic metabolites (McCoy et
al., 1980).
Studies investigating the bacterial mutagenicity of known and
postulated metabolites of TBPP have given little information regarding
the mechanisms of its bioactivation. With the exception of
2-bromoacrolein (BA) and DBP, other postulated or identified TBPP
metabolites were less mutagenic than TBPP when tested with, or
without, an exogenous metabolism system (Prival et al., 1977;
Soderlund et al., 1979; Zeiger et al., 1982; Holme et al., 1983;
Gordon et al., 1985). Such metabolites include the two major TBPP
metabolites 2,3-dibromopropanol (DBP) and bis(2,3-dibromopropyl)
phosphate (BBPP) as well as mono(2,3-dibromopropyl) phosphate (mono-
BP) (McCann & Ames, 1977; Prival et al., 1977; Soderlund et al.,
1982b; Holme et al., 1983). These metabolites were also less
mutagenic than TBPP in V79 Chinese hamster lung cells with microsomal
activation (Holme et al., 1983).
Eight coded samples of different lots of TBPP from different
manufacturers were tested in TA 1535. All were positive (Prival,
1975).
Several commercial TBPP samples and a few known contaminants
(1,2,3-tribromopropane, DBP, and 1,2-dibromo-3-chloropropane) were
tested in amounts of 0.01 up to 10 µl/plate on Salmonella strains TA
100, TA 1535, and TA 1538 with, and without, Aroclor 1254-activated,
or non-activated, rat liver S9 fractions. The results indicated that
the highest levels of mutagenicity obtained with commercial TBPP were
probably not due to the presence of the contaminants (Prival et al.,
1977).
The bacterial mutagenicity of compounds structurally related to
TBPP, including monobrominated and chlorinated analogues, unsaturated
and saturated methyl esters, and halogenated propanols, indicate the
following structural effects on mutagenic activity: (a) a decrease in
the number of alkyl chains decreases mutagenicity; (b) a decrease in
the number of halogens in the alkyl chain decreases mutagenicity; (c)
compounds with vicinal halogens are more mutagenic than 1,3-dihalo-
isopropyl analogues; (d) brominated compounds are more mutagenic than
the corresponding chlorinated ones; and (e) salts of mono- and
diphosphate esters are more mutagenic than the free base (Carr &
Rosenkranz, 1978; Nakamura et al., 1979, 1983; Zeiger et al., 1982;
Holme et al., 1983).
Mutagenicity testing of TBPP metabolites has yielded little
information on the mechanisms of activation of TBPP to mutagenic
metabolites, since none of the metabolites were more mutagenic than
the parent compound. The first evidence for the possible formation of
a potent bacterial mutagen from TBPP came when 2-bromoacrylic acid was
found in the urine of rats, administered large doses of TBPP (Marsden
& Casida, 1982). These authors proposed that TBPP is initially
oxidized at the C-1 position to yield 2,3-dibromopropanal, which
then spontaneously dehydrobrominates to give BA. BA and 2,3-
dibromopropanal, which are potent, direct-acting, bacterial mutagens
in Salmonella typhimurium TA100, caused DNA damage in Reuber rat
hepatoma cells, and transformation of Syrian hamster embryo cells
(Rosen et al., 1980; Gordon et al., 1985).
In 1984, BA was detected in incubations of TBPP with rat liver
microsomes, using GC/MS. In these experiments, substitution of
deuterium atoms for hydrogen at the C-3 position decreased the
mutagenicity of TBPP by approximately 80%, whereas only a small
deuterium isotope effect was noted at C-2 and C-1. These data
indicate that an initial oxidation at the terminal carbon atom is the
key step in the formation of mutagenic metabolites from TBPP (Nelson
et al., 1984; Soderlund et al., 1984). Subsequent experiments with
variously deuterated TBPP analogues revealed that, according to the
number of deuteriums retained in the BA formed, oxidation at C-3 is
the major pathway for BA formation (Nelson et al., 1984). The results
of the studies with deuterated TBPP analogues were later substantiated
with selectively methylated analogues. These findings demonstrated
that the initial oxidation of TBPP at C-3 was followed by spontaneous
dehydrohalogenation and dehydrophosphorylation, with the subsequent
formation of BA and BBPP (Omichinski et al., 1987).
Although BA is the most potent TBPP metabolite with regard to
bacterial mutagenicity in vitro, additional TBPP metabolites appear
to be involved in TBPP-induced DNA damage in mammalian cells. The
formation of these metabolites has not yet been elucidated, but is
likely to involve conjugation with glutathione (Soderlund et al.,
1992).
All the known metabolites, including the two major metabolites,
DBP and BBPP, are considerably less mutagenic than the parent
compound, when tested directly or in the presence of an activation
system (Blum et al., 1978; Soderlund et al., 1979, 1982b; Zeiger et
al., 1982). However, BA is a more potent mutagen than TBPP (Rosen et
al., 1980; Nelson et al., 1984; Gordon et al., 1985).
7.7 Carcinogenicity
7.7.1 Oral
7.7.1.1 Mouse
Groups of 50 male, and 50 female, B6C3F1 hybrid mice, 6 weeks
old, were fed technical TBPP (containing no detectable 1,2-dibromo-3-
chloropropane) at concentrations of 500 or 1000 mg/kg diet in the diet
for 103 weeks followed by a 1-week observation period. The
experimental design of the study is shown in Table 1. Of the males,
44/55 matched controls, 38/50 low-dose mice and 43/50 high-dose mice
survived until the end of the study; of the females 44/55 controls,
37/50 low-dose mice, and 38/50 high-dose mice survived. TBPP
increased the incidence of squamous-cell carcinomas and papillomas of
the fore-stomach and of adenomas and carcinomas of the lungs in both
male and female treated animals compared with the controls. There was
also an increased incidence of renal tubular cell adenomas and
adenocarcinomas in treated male mice and liver cell adenomas and
carcinomas in treated female mice. Neoplastic lesions associated with
the administration of TBPP are summarized in Table 1. The incidence
of "preneoplastic" kidney changes, dysplasia, and hyperplasia, were:
controls (males and females) 0/109, low-dose females 20/50, low-dose
males 46/50, high-dose females 40/46 and high-dose males 49/50 (US
NCI, 1978; Reznik et al., 1979; IARC, 1979).
7.7.1.2 Rat
Groups of 55 male, and 55 female Fischer 344 rats, 6 weeks old,
were fed diets containing concentrations of 50 or 100 mg technical
TBPP/kg for 103 weeks, followed by a 1- or 2-week observation period.
The experimental design of the study is shown in Table 1. Of the
males 39/55 control, 35/55 low-dose, and 40/55 high-dose rats survived
until the end of the study; of the females 36/55 control, 44/55
low-dose, and 36/55 high-dose rats survived. The compound increased
the incidences of both renal tubular cell adenomas in rats of both
sexes and tubular cell adenocarcinomas in high-dose males.
Neoplastic lesions associated with the administration of TBPP are
summarized in Table 1. The incidence of "preneoplastic" kidney
changes, dysplasia, and hyperplasia, were; controls (males and
females) 0/105, low-dose females 25/54, low-dose males 53/54,
high-dose females 46/54, and high-dose males 39/54 (US NCI, 1978;
IARC, 1979; Reznik et al., 1979).
Table 1. Tumour incidences in mice and rats fed tris(2,3-dibromopropyl) phosphate (TBPP)
Species Sex Number Concentration Duration Number of tumour-bearing animals/number of animals examined
of animals (mg/kg (weeks)
treated diet) Forestomach** Lung** Kidneys* Liver**
(squamous-cell (adenomas or (tubular-cell (adenomas or
carcinomas or carcinomas) adenomas or carcinomas)
papillomas) adenocarcinomas)
Mouse male 55 0 105 0/51 12/54 0/54 28/54
male 50 500 103 10/47a 18/44c 6/50 31/49
mate 50 1000 103 13/48b 25/50d 17/49e 23/49
female 55 0 105 2/53 4/55 0/55 11/54
female 50 500 103 14/48b 9/50 3/50 23/50f
female 50 1000 103 22/44b 17/50d 3/46 35/49f
Rat male 55 0 107 - 0/54 0/53 0/54
male 55 50 103 - 3/55 30/54e 1/55
male 55 100 103 - 0/55 30/54a 4/54
female 55 0 107 - - 0/52 -
female 55 50 103 - - 4/54 -
female 55 100 103 - - 13/54a -
From: * Reznik et al. (1979); ** US-NCI (1978).
Fisher analysis of treated group versus control:
a Squamous-cell papillomas; P < 0.01; b Squamous-cell carcinomas and papillomas; P < 0.01; c Alveolar/bronchiolar
adenomas and carcinomas; P < 0.05; d Alveolar/bronchiolar adenomas and carcinomas: P < 0.01: e Tubular-cell adenomas
and adenocarcinomas: P < 0.01; f Hepatocellular adenomas and carcinomas; P < 0.01.
Male F344 rats, 4 weeks old, were administered (by gavage) 0
(untreated), and 0 (vegetable oil), or 100 mg TBPP in vegetable oil/kg
body weight per day. Treatment was given for 5 days per week and
continued for 4 or 52 weeks. Selected rats of the treated and control
groups were killed at various time intervals. Control and treated
animals (2-9 animals) were killed after 1, 5, 10, 20, 50, 75, and 260
treatments. After 4 weeks (20 treatments), the TBPP group was divided
into two subgroups; TBPP administration was continued in one subgroup
of 15 rats and, in the second subgroup, 15 other rats (treated for
four weeks) received only vegetable oil for the remainder of the
study. The histomorphology and ultrastructure of the kidneys were
studied. Twenty-four hours after the first TBPP treatment, the
epithelial cells at the corticomedullary junction developed increased
nucleus/cytoplasm ratios, cytomegaly, and nuclear vacuolization and
pleomorphism. These changes increased in severity to a toxic tubular
nephrosis as treatment continued, and extended to the peripheral
cortex by 52 weeks. After 52 weeks of TBPP treatment, small tubular
papillary hyperplasia had developed in three animals and an
adenocarcinoma was observed in one of the five animals killed at the
end of the study. Electron microscopy showed loss of microvilli and
polarity in the epithelium of the proximal convoluted tubules. At the
ultrastructural level, the cytoplasm of the neoplastic cells was
poorly differentiated and, in many areas, the surfaces of the cells
were covered by microvilli. In the animals in which treatment was
discontinued after 4 weeks, a gradual, but incomplete, restoration of
the tubular epithelia to nearly normal morphology was observed.
Nuclear changes persisted after cytoplasmic abnormalities had
disappeared. At 52 weeks, three out of five surviving rats,
administered TBPP throughout the study, were found to have polyploid
adenomas of the descending colon (Reznik et al., 1981).
Cunningham et al. (1992, 1993) examined the mechanisms whereby
chemicals produce mutagenicity in short-term in vitro assays, yet
fail to produce carcinogenicity in long-term rodent bioassays.
Previous studies indicated that mutagenic carcinogens increased the
amount of cell turnover in the target organ, but that mutagenic
noncarcinogens failed to do so. An association of cell-proliferation,
as determined by labelling with bromodeoxyuridine, and tumour
development was investigated in groups of five male Fischer 344 rats
(150 g). Administration of TBPP in the diet at dose levels of 0, 50,
or 100 mg/kg, for 14 days, resulted in increased incidences of cell
proliferation in the kidneys. A further association of cell
proliferation with tumour development in the kidneys was suggested by
their similar location in the kidneys, i.e., the renal outer medulla.
7.7.2 Dermal
7.7.2.1 Mouse
Female ICR/Ha Swiss mice (29 or 30 per group) (6-8 weeks old)
were treated with 10 or 30 mg TBPP (97%) in 0.2 ml acetone, 3 times
weekly, on the shaved skin, for 496 and 474 days, respectively. Two
control groups were used; 30 mice received the acetone and 249 mice
were untreated. Besides a significant increase in skin tumours
(papillomas and/or carcinomas), a substantial number of tumours were
also found at distant sites, such as squamous cell carcinomas of the
tongue and in the gingival area; papillomas and carcinomas were
observed in the forestomach (Table 2) (Van Duuren et al., 1978).
TBPP was tested in an in vivo, short-term skin test for
sebaceous gland suppression and the induction of epidermal
hyperplasia. Groups of 25 Swiss mice (aged 45 days) received dorsal
applications of TBPP in acetone on days 1, 3, and 5. The dose levels
applied on the skin were 49.5, 82.5, and 115.5 mg (total dose applied
in three applications in acetone). TBPP did not suppress the
sebaceous gland and did not induce hyperplasia (Sala et al., 1982).
Groups of 28-34 female (60 days old) Swiss mice were given a
single application of dimethylbenzanthracene (DMBA) (50 µg) or TBPP
(110 µg) in acetone, on the dorsal skin. In the tumour promotion
studies, the mice received, for 78 weeks, twice weekly applications to
the same area of the dorsal skin of an acetone solution of
tetradecanoyl phorbolacetate (TPA) (1 µg) or TBPP (33 mg), started one
week after the initiation with DMBA or TBPP. In order to test TBPP
for its ability to act as a complete carcinogen, a second series of
mice received the same twice weekly applications as the promoted mice,
but without any initiation treatment. The total TPA dose applied, was
156 µg/mouse and that of TBPP, 5.1 g/mouse. TBPP did not have any
effect as a complete carcinogen on the skin, with a total dose of
5.1 g/mouse. The same dose did not have a promoting activity after
DMBA initiation. However, TBPP initiated a significant number of skin
tumours, when TPA was used as promoter. Furthermore, the number of
lung adenomas increased significantly (Table 3) (Sala et al., 1982).
Table 2. Tumour incidences in female Swiss mice after dermal application of
tris(2,3-dibromopropyl) phosphate (TBPP)a
Number of Dose Number of mice with tumours/number necropsiedb
animals (mg/animal)
treated Forestomach Lung Skin Oral cavity
29 0 1/29 7/29 0/29 0/29
29 10 10/29 26/29 2/29 2/29
30 30 20/30 28/30 5/30 4/30
a From: Van Duuren et al. (1978).
b Increases in incidences of tumours of the forestomach, lung, skin, and oral
cavity in treated animals were statistically significant compared with those
in controls ( P < 0.05).
7.8 Special studies
7.8.1 Kidneys
Osterberg et al. (1978) found an increased incidence and severity
of chronic nephritis in a 90-day gavage study on rats, administered
TBPP at dose levels of 25, 100, or 250 mg/kg body weight in
propyleneglycol. The renal changes were associated with regenerative
epithelium, hypertrophy, and dysplasia of renal tubular epithelial
cells and were found at all three dose levels (section 7.2.1.1).
TBPP caused proximal tubular damage and acute renal failure in
rats, with elevation of serum creatinine and urea, and depression of
organic anion and cation transport (Soderlund et al., 1980; Elliott et
al., 1982; Lynn et al., 1982). It has been demonstrated that TBPP
caused urinary excretion of renal cytoplasmic enzymes associated with
the initial damage (characterized by increased membrane permeability),
followed by the excretion of cell organelle-linked enzymes with
necrosis of the renal tubular epithelium (Nomiyama et al., 1974;
Emanuelli et al., 1979; Fukuoka et al., 1987, 1988a,b). TBPP also
produced impairment of the tubular reabsorption capacity for metabolic
fuels such as, lactate, glucose, and citrate, maintained across the
brush border membrane by the sodium co-transport system (Kurokawa et
al., 1985; Pitts, 1987).
Male Wistar rats (56 rats in test group and 15 as controls; 7
weeks of age) were given a single oral dose in olive oil of 286.8 µmol
TBPP (98%)/kg, to study TBPP nephrotoxicity. TBPP caused tubular
necrosis. The animals received a single dose and some were killed
daily for 10 days. The following effects were observed: on day 1,
pyknosis of the renal tubular epithelial cells, necrosis on day 2,
regeneration from day 3 and large nuclei formation from day 4.
13C-NMR spectra were applied to clarify changes of the renal
low-molecular weight components in the kidneys injured by TBPP; sialic
acid and inositol were found to be the desired marker components. The
lesions produced by TBPP were characterized by changes in the renal
components and enzyme activities. Increases in the sialic acid
content of the kidneys were observed on day 1, suggesting destruction
of the epithelial cell membrane. On day 5, regeneration accompanied
by an increase in inositol contents was found. Renal activity of the
cytoplasmic enzyme, alanine aminopeptidase, was increased on days 2,
5, 6, and 7 (Fukuoka et al., 1988a).
There are large species differences in TBPP nephrotoxicity, since
neither hamsters, guinea-pigs, nor mice developed acute kidney damage
at doses of 500-1000 mg/kg body weight (Soderlund et al., 1982a).
Table 3. Development of tumours in female Swiss mice in initiation-promotion studies carried out with TBPPa
Treatment Number of mice with tumours
Group Initiation Promotion or Number Skin Lung Number Other tumours
repeated treatment of mice tumours adenomas Type
1 - TBPPb 33 0 14 3 2: lymphosarcoma,
hepatoma
2 TBPP TPAc 34 26d 7 0
3 DMBA TBPPa 33 3 11 2 mammary tumour,
perianal carcinoma
7 - TPAc 28 12 5 0
a From: Sala et al. (1982).
b TBPP: Total dose, 5.1 g/animal (170 g/kg body weight).
c TPA: Total dose, 156 µg/animal.
d Two squamous cell carcinomas in each group.
BBPP was clearly more nephrotoxic for Wistar and Sprague-Dawley
rats than TBPP, whereas mono(2,3-dibrompropyl) phosphate (mono-BPP)
was less nephrotoxic (Elliott et al., 1982; Soderlund et al., 1982b).
However, female Sprague-Dawley rats appeared to be resistant to BBPP
nephrotoxicity (Elliott et al., 1982), paralleling the carcinogenicity
of TBPP in female animals.
The nephrotoxicity of TBPP has been compared to that of BBPP,
using equimolar doses. Both chemicals caused reversible acute renal
failure, accompanied by tubular necrosis. Polyuria, high urinary
glucose, lactate, and enzyme levels, and increased serum creatinine
levels were observed. It was suggested, therefore, that BBPP or a
metabolite of this compound, mediated the nephrotoxicity associated
with TBPP (Takada et al., 1991a).
The finding that BBPP was a major urinary metabolite of TBPP and
that this compound was at least as nephrotoxic as TBPP, indicates that
it is a proximate nephrotoxic metabolite of TBPP (Lynn et al., 1980;
Soderlund et al., 1982b).
Generally, attempts to modulate TBPP nephrotoxicity by various
pretreatments have not been very successful. Pretreatment of Wistar
rats with the cytochrome P-450 inducers, phenobarbital and
polychlorinated biphenyls, known to increase the metabolism of TBPP,
had no effect on its nephrotoxicity. However, pretreatment with
cobaltous chloride resulted in a moderate reduction in TBPP
nephrotoxicity. Interestingly, depletion of glutathione in vivo
with diethyl maleate did not alter TBPP nephrotoxicity (Soderlund et
al., 1980).
The indication that the oxidative metabolism of TBPP or BBPP
plays only a minor role in its nephrotoxicity, is confirmed by the
findings that none of the selectively deuterated analogues (see below)
significantly altered morphological evidence of nephrotoxicity
compared with the protio compounds. It appears that C-H bond cleavage
was not the rate-limiting step in the overall process leading to
nephrotoxicity (Soderlund et al., 1988). However, recent studies have
revealed that deuterium substitution at C-2 and C-3 of TBPP results in
a considerable decrease in the plasma levels of BBPP at earlier time
points compared with those after protio TBPP. The time-integrated
plasma concentrations of the resulting deuterated BBPP analogues at
later time points are not significantly different from that of BBPP,
indicating that the target organ exposures to BBPP and deuterated BBPP
analogues are similar (Pearson et al., 1993b). This may explain the
lack of deuterium isotope effect on nephrotoxicity. Thus, a role of
oxidative metabolism cannot be completely ruled out.
Activation of nephrotoxic alkyl halides with glutathione by the
beta-lyase pathway is documented. However, known inhibitors of the
beta-lyase pathway (e.g., AT-125 and aminooxyacetic acid) did not
affect the extent of nephrotoxicity in rats caused by a single ip dose
of TBPP (Soderlund et al., 1988).
Because of its acidic nature, BBPP may be the species transported
into the renal tubular cells. Probenecid, an inhibitor of the organic
anion transport system in the kidneys, reduces the nephrotoxicity of
BBPP (Soderlund et al., 1988). The nature of the toxic metabolites
formed from BBPP has not been identified. One possible candidate is
an episulfonium ion generated by the conjugation of BBPP with
glutathione.
At present, the mechanisms involved in TBPP organ toxicity are
not known with certainty. DNA damage, as measured by alkaline
elution, was detected after 20 min in the kidneys of Wistar rats given
a single ip dose of 25 mg/kg body weight. Thus, DNA appears to be an
early target in TBPP nephrotoxicity, leading to cell death (Pearson et
al., 1993b).
7.9 Factors modifying toxicity; toxicity of metabolites
7.9.1 Toxicity of metabolites
2,3-Dibromo-1-propanol (DBP), a metabolite of TBPP, was tested in
2-year dermal carcinogenicity studies on F344/N rats and B6C3F1
mice. The doses used were 0, 188, or 375 mg/kg body weight in rats
and 0, 88, or 177 mg/kg body weight in mice. Under the conditions of
these studies, there was clear evidence of carcinogenic activity in
both sexes of both species in a variety of organs (US NTP, 1992). In
male rats, there was an increased incidence of neoplasms of the skin,
nose, oral mucosa, oesophagus, forestomach, small and large intestine,
Zymbal's gland, liver, kidney, tunica vaginalis, and spleen. In
female rats, there was an increased incidence of neoplasms of the
skin, nose, oral mucosa, oesophagus, forestomach, small and large
intestine, Zymbal's gland, liver, kidney, clitoral gland, and mammary
gland. In male mice, there was an increased incidence of neoplasms of
the skin, forestomach, liver, and lung. In female mice, there was an
increased incidence of neoplasms of the skin and forestomach.
BBPP, DBP, as well as TBPP, are acute nephrotoxins, BBPP being
the most potent (Lynn et al., 1982). In three groups of rats, the
24-h urine volume was measured after ip injection of equimolar amounts
(in 1 ml Emulphor) of TBPP (50 mg), BBPP (36 mg), or DBP (39 mg). The
single dose of TBPP caused a 5-fold increase in urine volume after two
days. After one week, urine volume had returned to normal. In
contrast, the single dose of BBPP resulted in a 7 to 8-fold increase
in urine volume from days 2-5. Urine volume had not returned to
normal after 10 days. The single dose of DBP produced a 2 to 3-fold
increase in urine volume, which rapidly returned to normal.
In another study, rats received a single ip injection of TBPP,
BBPP, or mono-BPP (in dimethyl-sulfoxide, 0.25 ml per 100 g) of 0, 10,
25, 50, 100, or 200 mg/kg body weight; the animals were killed 48 h
later (except the high dose group which was killed 40 h later). A
significant increase in kidney/body weight ratio occurred with all
three compounds at the 200 mg/kg dose. Enlarged kidneys were pale,
oedematous, and showed a prominent necrotic band in the inner part of
the cortex. Histologically, tubular kidney necrosis was demonstrated
in rats receiving 100 mg TBPP or mono-BP/kg or more and, in animals
receiving 50 mg BBPP/kg or more. Plasma creatinine was significantly
elevated at doses from 10 mg BBPP/kg, 25 mg mono-BP/kg, and 50 mg
TBPP/kg upwards. Plasma urea was significantly elevated after doses
of 100 mg mono-BP/kg or more and 200 mg BBPP/kg. Plasma GDT was also
significantly increased at the highest doses of BBPP and mono-BP
(Soderlund et al., 1982b).
Comparable results were reported by Fukuoka et al. (1988b).
Rats received a single oral dose of 0, 71.7, 143.4, or 286.8 µmol
BBPP/kg and were evaluated for seven days after the dose. In
addition to polyuria, changes in the excretion of lactate, uric acid,
and glucose, and changes in the activities of urinary enzymes
(alkaline phosphatase, aspartate aminotransferase, and gamma-
glutamyltransferase) at various times after dosing, histopatho-logical
changes occurred in the kidney. The histopathological changes
included pyknosis, necrosis, and desquamation.
7.9.2 Mutagenicity of metabolites
The following TBPP metabolites have been identified in in vivo
and in vitro test systems: BBPP, mono(2,3-dibromopropyl) phosphate
(mono-BPP), 2-bromoacrolein (BA), 2,3-dibromo-1propanol (DBP),
2,3-dibromopropyl-2-bromopropen-2-yl, bis(2-bromopropen-2-yl)
phosphate and 3,3-dibromopropyl phosphate (Lynn et al., 1980, 1982;
Zeiger et al., 1982; Nelson et al., 1984). Several of these TBPP
metabolites have been tested for their mutagenic potential.
All the identified TBPP metabolites were mutagenic in Salmonella
typhimurium TA 100 (Prival et al., 1977; Zeiger et al., 1982; Holme
et al 1983; Nakamura et al., 1983; Gordon et al., 1985). DBP was
mutagenic in Salmonella typhimurium TA 100 and TA 1535, but not in
TA 1538 (Carr & Rosenkranz, 1978). However, only BA was more
mutagenic than the parent compound (Gordon et al., 1985). Unscheduled
DNA synthesis was induced in monolayer cultures of rat hepatocytes by
DBP and, to a lesser extent, by BBPP and mono(2,3-dibromopropyl)
phosphate (mono-BPP). A similar relative response of DBP, BBPP, and
mono-BPP was found with respect to mutagenicity in V79 Chinese hamster
lung cells with liver microsomal activation. The concentration of
BBPP that was tested was 0.05 mmol/litre (Holme et al., 1983).
BBPP was less potent than TBPP in causing DNA damage in both the
liver and testicular cells. DNA damage, as measured by alkaline
elution, was found in isolated rat liver cells exposed to 100
micromolar BBPP and to a lesser extent in testicular cells. DNA damage
caused by BBPP phosphate could be decreased by diethyl maleate-
pretreatment in testicular cells, but not in the liver cells
(Soderlund et al., 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Fifty-two out of 61 (22 males and 39 females) human volunteers
completed a repeated insult patch study in which TBPP (1.1 g) was
applied 10 times over a 24-day period, followed by a single challenge
patch 14 to 21 days later. Fifty persons showed no reaction. After
the 6th or 7th application 2 showed itching (or pruritis), and
urticaria. The study was stopped for a month in these 2 subjects, and
then restarted. No adverse effects were seen the second time. The
conclusion of the author was that TBPP did not produce primary skin
irritation, skin fatigue, or skin sensitization (Kerst, 1974; US EPA,
1976).
A maximization test was carried out and 8 out of 24 human
volunteers, exposed to a 100% TBPP solution, showed a sensitization
reaction; while a 20% solution in a petroleum ether sensitized 2 out
of 25 subjects. The TBPP was considered to be a mild skin sensitizer
in humans. Subjects who had been sensitized by the 20% solution also
showed reactions when tested with TBPP-treated fabrics. The response
varied with the type of fabric substrate. The degree of sensitization
appeared to depend upon the availability of the agent at the surface
of the fibre. This is different for different types of fibres and the
methods in which the flame retardant is applied. Washing the fibre
reduced surface concentrations (Morrow et al., 1976).
Andersen (1977) reported the incidence of sensitization to TBPP
in human subjects from seven European countries, patch-tested with
TBPP (5% in petrolatum), and found two positives among 1103 patients.
Since TBPP-treated fabrics can be expected to contact the skin
for long exposure periods, patches from both rayon and acetate
fabrics, previously treated with TBPP were applied to humans, but skin
reactions were not elicited (Brieger et al., 1968).
8.2 Occupational exposure
No case reports or epidemiological studies are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory studies
9.1.1 Microorganisms
Nitrifying return activated sludge diluted with fresh settled
sewage, filtered, aerated and containing Nitromonas and Nitrobacter
was used to test nitrification. No inhibition was found with 300 mg
TBPP/litre and no depression of the BOD was noticed at 170 mg/litre
(Wood et al., 1981).
9.1.2 Aquatic organisms
9.1.2.1 Invertebrates
A 57% inhibition of southern armyworm (Spodoptera eridania)
microsomal p-chloro- N-methylanaline N-demethylase was measured
at 1 mg TBPP/ml, in an in vitro incubation mixture (Eldefrawi et
al., 1977).
9.1.2.2 Vertebrates
Six goldfish (Carassius auratus) were exposed to 1 mg TBPP
(dissolved in acetone) per litre water. All died within 5 days. The
fish appeared to swim in a disoriented manner prior to death. The
fish showed necrosis of the kidneys (Gutenmann & Lisk, 1975).
TBPP (1 mmol/litre) inhibited by 19% the acetylcholinesterase
(AChE) activity in the electric organ of the electric ray (Torpedo
ocellata). The binding of acetylcholine to its electric organ
receptor was not inhibited (Eldefrawi et al. 1977).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Seed of oat (Avena sativa) was added to loamy sand soil (1.5%
organic carbon) and exposed to 1, 10, 100 or 1000 mg TBPP/kg soil for
14 days. The temperature of the soil was 20°C, the pH 6.0 and 16 h
light/8 h dark cycle was used. The study was performed according to a
modified OECD terrestrial plant-growth test. The EC50 for growth
inhibition was at 1000 mg/kg soil (Pestemer, 1988). In a comparable
study, turnip seed (Brassica rapa sp.) was tested under the same
conditions as the oat seed. With 1000 mg TBPP/kg soil, 100%
inhibition of growth was obtained (Pestemer, 1988).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC concluded in 1979 that there is sufficient evidence that
TBPP is carcinogenic in mice and rats. In the absence of adequate
data on humans, it is reasonable, for practical purposes, to regard
TBPP as if it presented a carcinogenic risk to humans (IARC, 1979).
In 1987, colon tumours were reported in a short-term study on male
rats. The overall evaluation made by IARC (1987) was that TBPP is
probably carcinogenic to humans (Group 2A) (IARC, 1987).
BIS(2,3-DIBROMOPROPYL) PHOSPHATE AND SALTS
A1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
The data base on bis(2,3-dibromopropyl) phosphate (BBPP) and its
salts is inadequate for an evaluation, and to support its commercial
use.
From the available data, there is some indication that the
substance may be mutagenic and carcinogenic.
The substance cannot be evaluated unless additional data become
available on physical and chemical properties, production and use,
environmental transport, distribution, and transformation,
environmental levels and human exposure, kinetics and metabolism in
animals and humans, effects on laboratory mammals, humans, and in
vitro test systems, and effects on other organisms in the laboratory
and field. More mutagenicity data on at least two end-points are also
needed.
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Chemical formula C6H11Br4O4P
Chemical structure
(BrCH2-BrCH-CH2O)2P - OH
'
(BrCH2-BrCH-CH2O)2P - O - P = O
H '
OH
Relative molecular 497.8
mass
CAS Chemical name 2,3-dibromo-1-propanol-hydrogen
phosphate
Common name bis(2,3-dibromopropyl) phosphate;
Synonyms bis(2,3-dibromopropyl)hydrogen
phosphate; bis(2,3-dibromopropyl)
phosphoric acid
CAS registry number 5412-25-9
The ammonium, magnesium, potassium, and sodium salts have also
been proposed.
A2.2 Physical and chemical properties
No data are available on this subject.
A2.3 Analytical methods
See section 2.3, TBPP.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
BBPP and its salts are not known to occur as natural products.
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
Bis(2,3-dibromopropyl)ammonium phosphate has been prepared by a
reaction of bis(2,3-dibromopropyl) phosphate with NH4OH (Mischutin,
1972).
A3.2.2 Uses
In the 1960s and 1970s, BBPP and its magnesium and ammonium salts
were proposed for use as fire-proofing agents for textiles and
plastics.
No evidence was found that BBPP or its salts are currently used
for commercial applications.
A3.3 Contamination of the environment
BBPP has been identified as a major biodegradation product of
TBPP, in a laboratory activated sludge system (Alvarez et al., 1982).
A3.4 Environmental transport, distribution, transformation,
and exposure levels
No data are available.
A4. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
A4.1 Absorption, distribution, elimination, and biotransformation
BBPP has been identified as a metabolite of TBPP (Lynn et al.,
1982). Following intravenous administration of [14C]-tris(2,3-
dibromopropyl) phosphate to male Sprague-Dawley rats, approximately
half of the label appeared in the urine within 120 h and 7.8% of the
recovered urinary label was BBPP. BBPP also constituted 21.5% of the
biliary label (33.9% of administered dose in 24 h). The tissue
distribution of BBPP was studied at different intervals (5 and 30 min,
8, 24, and 120 h, and 5 days) after administration of the tris
compound. BBPP was identified in nearly all organs within 5 min of
administration. Tissue levels declined after 5 or 30 min at all sites
except the large intestines and carcass. Five min after
administration, 75% of the plasma label was BBPP. The plasma
concentration of BBPP increased between 0 and 1 h and declined
biphasically thereafter, with an initial plasma half-life of 6 h,
declining to approximately 36 h, by one to five days.
A5. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A5.1 Single exposure
Adult male Wistar rats, 5 per group, were administered a single
ip injection of 0, 10, 25, 50, 100, or 200 mg BBPP/kg body weight in
DMSO (2.5 ml/kg). All rats were killed 40-48 h after dosing. One rat
of the highest dose group died. Kidneys and body weight ratios were
increased in 200 mg/kg rats. The kidneys were pale and oedematous
with necrosis of the inner cortex. Microscopically, tubular cell
necrosis was observed in rats with 50 mg/kg or more. However, plasma
creatinine was significantly elevated at doses of 10 mg/kg body weight
or more and plasma urea and plasma-GOT were elevated at 200 mg/kg body
weight (Soderlund et al., 1982b).
Elliot et al. (1982) carried out a comparable study with only
one dose level of 120 mg/kg body weight, administered to male
Sprague-Dawley rats intraperitoneally. Rats were killed after 48 h.
Serum creatinine was elevated, and renal cortical slices showed
decreased uptake of para-aminohippuric acid and N-[14C]-
methylnicotinamide. Microscopically, tubular cell necrosis of the
loops of Henle was found.
When the Mg salt of BBPP was administered by oral intubation to
Wistar rats, eyelid closure, crouching, shivering, and staggering gait
were observed. LD50 values in male and female rats were 283 and
261 mg/kg, respectively (Takada et al., 1991b).
A5.2 Short-term exposure
The Mg salt of BBPP was given to Wistar rats (5/sex per group) in
the diet at levels of 0, 30, 100, 300, or 1000 mg/kg for 45 days.
There was no difference between treated and control animals in body
weight and food consumption. At 1000 mg/kg, a significant increase in
liver and kidney weights was observed in the males. Desquamation,
swelling and large nuclei formation of the tubular epithelium, and
tubular dilation of kidney were observed. It was concluded that
BBPP-Mg has apparent renal toxicity (Takada et al., 1991b).
A5.3 Long-term exposure
No data were available on the following subjects:
* Skin and eye irritation; sensitization
* Reproductive toxicity, embryotoxicity, and teratogenicity
A5.3.1 Mutagenicity and related end-points
2,3-Dibromo-1-propanol (DBP) was mutagenic in Salmonella
typhimurium TA 100 and TA 1535, but not in TA 1538 (Carr &
Rosenkranz, 1978).
Magnesium BBPP at doses of 3-100 µmol/plate was more mutagenic
to Salmonella typhimurium TA 1535 and TA 100 in the presence of
metabolic activation by Kanechlor 500-induced rat-liver S9 than in its
absence; it was weakly mutagenic to TA 98 with S9, but showed no
mutagenic activity in TA 1537 and TA 1538 (Nakamura et al., 1979).
The ammonium salt of BBPP was more mutagenic than the magnesium salt,
which itself was more mutagenic than the free acid (Nakamura et al.,
1983).
BBPP purified as a urinary metabolite from rats treated with TBPP
was mutagenic to Salmonella typhimurium TA1535 and TA100 in the
presence of metabolic activation (Aroclor 1254-induced rat liver S9)
at doses ranging from 0.05 to 1.0 µmol (Lynn et al., 1982).
Mutagenic activity in Salmonella typhimurium TA100 was detected
when BBPP at concentrations of 50 and 100 µmol/litre was incubated for
30 min with hepatic microsomal fractions from untreated rats or rats
pretreated with phenobarbital (Soderlund et al., 1982b).
A5.3.2 Carcinogenicity
Four groups of 40 Wistar rats (5 weeks old) of each sex per dose
level were fed diets containing 0, 80, 400, or 2000 mg/kg of the
magnesium salt of bis(2,3-dibromopropyl) phosphate (BBPP), for 24
months. Food consumption and body weight gain were measured
immediately prior to the beginning of the study, and then weekly for 6
weeks, biweekly for 6 months, and monthly, thereafter. Blood samples
of 8-13 rats/sex per dose were taken at 12, 18, and 24 months. Body
weight gain was reduced significantly at a level of 2000 mg/kg. Male
and female rats receiving 2000 mg/kg and female rats receiving
400 mg/kg showed a significant increase in absolute and relative liver
and kidney weights. A high incidence of tumours was observed in both
sexes. In the digestive system, papillomas and adenocarcinomas were
found in the tongue, oesophagus, and forestomach, and adenocarcinomas
of the intestines. In the liver, hepatocellular adenomas (neoplastic
nodules) and carcinomas were found (Table 4). Pre-terminal
mortalities were associated with an increased incidence of forestomach
papillomas in both sexes, adenocarcinomas of the small intestines in
male rats, and hepatocellular carcinomas in females. Non-neoplastic
lesions were mainly found in the kidneys of the rats of the 2000 mg/kg
group and, in a few instances, also in the 400 mg/kg group. The
changes were epithelial swelling and desquamation, large bizarre
nuclei, pyknosis, and basement membrane thickening. Serum
biochemistry was performed using commercially available assay kits for
the diagnosis of liver and kidney function and of disorders of the
digestive system. Eighteen parameters were studied. Significant
increases or decreases in the parameters were mainly observed in the
2000 mg/kg group with a few in the 400 mg/kg group. Statistically
significant decreases were seen in the serum, in total protein,
albumin, and cholinesterase; and significant increases were seen in
blood urea nitrogen, total cholesterol, alkaline phosphatase,
gamma-glutamyl transferase, magnesium, GOT, and GPT (Takada et al.,
1991a).
A5.4 Special studies
A5.4.1 Kidneys
Many nephrotoxic agents exert their effects primarily on the
cells of the proximal tubules. Isolated tubular cells were used to
study the uptake of alpha-methylglucose as indicator of effects on the
functional integrity of the cells. BBPP, which is acutely nephrotoxic
in vivo, inhibited the uptake of alpha-methylglucose at low
concentrations (Boogaard et al., 1989).
See also TBPP section 7.8.1 and 7.9.1 on the nephrotoxicity of
BBPP.
A5.5 Effects on humans and other organisms in the laboratory
and field
No data are available.
Table 4. Neoplastic lesions and tumour incidence in Wistar rats fed diets containing
BBP magnesium salta
Organ Sex Dose level Papillomas Squamous cell
carcinomas
Tongue male 400 1/40 1/40
female 400 1/40 1/40
2000 5/40 0/40
Oesophagus male 400 6/40 1/40
2000 2/40 0/40
female 2000 6/40b 0/40
Forestomach male 400 8/40b 1/40
2000 17/40c 2/40
female 400 4/40 2/40
2000 20/40c 4/40
Adenoma Adenocarcinoma
Small intestines male 400 0/40 2/40
2000 2/40 14/40c
female 2000 0/40 9/40c
Table 4 (contd).
Organ Sex Dose level Neoplastic Hepatic cell
nodules carcinomas
Liver male 0 7/40 1/40
80 3/40 2/40
400 7/40 2140
2000 2/40 2/40
female 0 1/40 0/40
80 2/40 1/40
400 5/40 7/40b
2000 5/40 24/40c
Adenoma Carcinoma
Kidneys male 400 5/40 1/40
2000 0/40 0/40
female 2000 1/40 1/40
a From: Takada et al, (1991a).
No tumours were found in the groups not mentioned in the table.
The figures given in this table are the total numbers of the tumours
found at the end of the study. The 2000 mg/kg treated rats did not survive
until the end of the 18th or/and 24th month.
b P < 0.05.
c p < 0.01 compared with the controls.
REFERENCES
Alvarez GH, Page SW, & Ku Y (1982) Biodegradation of 14C-tris(2,3-
dibromopropyl) phosphate in a laboratory activated sludge system. Bull
Environ Contam Toxicol, 28: 85-90.
Andersen KE (1977) Sensitivity to a flame retardant, tris(2,3-
dibromopropyl) phosphate (Firemaster LVT 23P). Contact Dermatitis, 3:
297-300.
Berkowitz S (1978) The induction of II-III translocations by
tris(2,3-dibromopropyl) phosphate in Drosophila. Mutat Res, 57:
385-387.
Blum A & Ames BN (1977) Flame-retardant additives as possible cancer
hazards. Science, 195(4273): 17-23.
Blum A, Gold MD, Ames BN, Kenyon C, Jones FR, Hett EA, Dougherty RC,
Horning EC, Dzidic I, Carroll DI, Stillwell RN, & Thenol JP (1978)
Children absorb Tris-BP flame retardant from sleepwear: Urine contains
the mutagenic metabolite, 2,3-dibromopropanol. Science, 201:
1020-1023.
Boogaard PJ, Mulder GJ, & Nagelkerke JF (1989) Isolated proximal
tubular cells from rat kidney as an in vitro model for studies on
nephrotoxicity. II. Alpha-methylglucose uptake as a sensitive
parameter for mechanistic studies of acute toxicity by xenobiotics.
Toxicol Appl Pharmacol, 101(1): 144-157.
Brieger H, Gabriel K, & Rieders F (1968) Toxicology and safe
utilization of flame retardants. Presented at the American Industrial
Hygiene Conference, St. Louis, 15 May 1968.
Brusick D, Matheson D, & Bakshi K (1978) Evaluation of tris(2,3-
dibromopropyl) phosphate for mutagenic and transforming activity in a
battery of test systems. Proceedings of the Second International
Conference on Environmental Mutagens, Edinburgh, 11-15 July 1977.
Mutat Res., 53(2): 31-160.
Brusick D Matheson D, Jagannath DR, Goode S, Lebowitz H, Reed M, Roy
G, & Benson S (1980) A comparison of the genotoxic properties of
tris(2,3-dibromopropyl) phosphate and tris(1,3-dichloro-2-propyl)
phosphate in a battery of short-term bioassays. J Environ Pathol
Toxicol 3: 207-226.
Brusick DJ, Jagannath DR, & Matheson D (1982) The activity of
2,(2',4'-diaminophenoxy) ethanol in 3 genetic toxicity bioassays.
Mutat Res, 102: 361-372.
Carr HS & Rosenkranz HS (1978) Mutagenicity of derivatives of the
flame retardant tris(2,3-dibromopropyl) phosphate: halogenated
propanols. Mutat Res, 57: 381-384.
Chemicals Inspection & Testing Institute (1992) Biodegradation and
bioaccumulation data of existing chemicals based on the CSCL. Tokyo,
Japan Chemical Industry, Ecology-Toxicology Information Centre,
pp 2-110.
Cochran RC & Wiedow MA (1986) The effects of tris(2,3-dibromopropyl)
phosphate on the reproductive system of male rats. J Am Coll Toxicol,
5(2): 153-160.
Cope JF (1973) Identification of flame retardant textile finishes by
pyrolysis-gas chromatography. Anal Chem, 45: 562-564.
Cunningham ML, Elwell MR, & Matthews HB (1992) Site-specific
proliferation in renal tubular cells by the renal tubular carcinogen
tris(2,3-dibromopropyl) phosphate (TRIS). Toxicologist, 12(1): 266,
(Abstract No. 1013).
Cunningham ML, Elwell MR, & Matthews HB (1993) Site-specific cell
proliferation in renal tubular cells by the renal tubular carcinogen
tris(2,3-dibromopropyl) phosphate. Environ Health Perspect, 101(Suppl
5): 253-257.
DeCarlo VJ (1979) Studies on brominated chemicals in the environment.
Ann NY Acad Sci, 320: 678-681.
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli. Environ Mutagen,
7(Suppl 5): 1-248.
Dunkel VC, Schechtman LM, Tu AS, Sivak A, Lubet RA, & Cameron TP
(1988) Interlaboratory evaluation of the C3H/10T1/2 cell
transformation assay. Environ Mol Mutagen, 12: 21-31.
Dybing E, Omichinski JG, Soderlund EJ, Brunborg G, Lag M, Holme JA, &
Nelson SD (1989) Mutagenicity and organ damage of 1,2-dibromo-3-
chloropropane (DBCP) and tris(2,3-dibromopropyl) phosphate (tris-BP):
Role of metabolic activation. In: Hodgson E, Philpot RM, & Bend JR ed.
Reviews in biochemical toxicology. Amsterdam, Oxford, New York,
Elsevier Science Publishers, vol 10, pp 139-186.
EEC (1976) Council directive 76/769/EEC. Off J Eur Communities.
EEC (1979) Council directive 79/663/EEC. Off J Eur Communities.
Eldefrawi AT, Mansour NA, Brattsten LB, Ahrens VD, & Lisk DJ (1977)
Further toxicologic studies with commercial and candidate flame
retardant chemicals. Part II. Bull Environ Contam Toxicol, 17(6):
720-726.
Elliott WC, Lynn RK, Houghton DC, Kennish JM, & Bennett WM (1982)
Nephrotoxicity of the flame retardant tris(2,3-dibromopropyl)
phosphate and its metabolites. Toxicol Appl Pharmacol, 62, 179-182.
Emanuelli G, Calcamuggi G, Cestonaro G, Gatti G, Anfossi G, & Cotto A
(1979) Urinary enzyme activities in human and experimental renal
damage. Excerpta Med Int Congr Ser, 502: 780-781.
Environment Agency Japan (1978) Environmental monitoring of
chemicals. Environmental survey report of 1977 F.Y. Tokyo, Department
of Environmental Health, Office of Health Studies.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Department of Environmental Health, Office of
Health Studies.
Fukuoka M, Takabashi T, Tanaka A, Yamaha T, Naito K, Nakaji Y,
Kobayashi K, & Tobe M (1987) Nephrotoxic effect of tris(2,3-
dibromopropyl) phosphate on the urinary metabolites: assessment from
13C-NMR spectra of urines and biochemical and histopathological
examinations. J Appl Toxicol, 7(1): 23-34.
Fukuoka M, Tanaka A, Yamaha T, Naito K, Takada K, Kobayashi K, & Tobe
M (1988a) Tris(2,3-dibromopropyl) phosphate nephrotoxicity in the rat:
Histological and biochemical changes in renal components by 13C-NMR-
spectra. J Appl Toxicol, 8(6): 411-416.
Fukuoka M, Takahashi T, Naito K, & Takada K (1988b) Comparative
studies on nephrotoxic effects of tris(2,3-dibromopropyl) phosphate
and bis(2,3-dibromopropyl) phosphate on rat urinary metabolites. J
Appl Toxicol, 8: 43-52.
Furukawa M, Sirianni SR, Tan JC, & Huang CC (1978) Sister chromatid
exchanges and growth inhibition induced by the flame retardant
tris(2,3-dibromopropyl) phosphate in Chinese hamster cells: Brief
communication. J Natl Cancer Inst, 60: 1179-1181.
Gardner AM (1979) Quantitative determination of tris(2,3-
dibromopropyl) phosphate by densitometry of thin layer chromatograms.
J Assoc Off Anal Chem, 62(6): 1315-1318.
Gordon WP, Soderlund EJ, Holme JA, Nelson SD, Iyer L, Rivedal E, &
Dybing E (1985) The genotoxicity of 2-bromoacrolein and 2,3-
dibromopropanal. Carcinogenesis, 6: 705-709.
Gutenmann WH & Lisk DJ (1975) Flame retardant release from fabrics
during laundering and their toxicity to fish. Bull Environ Contam
Toxicol, 14(1): 61-64.
Gutter B & Rosenkranz HS (1977) The flame retardant tris(2,3-
dibromopropyl) phosphate: Alteration of human cellular DNA. Mutat Res,
56: 89-90.
Holme JA, Soderlund EJ, Hongslo JK, Nelson SD, & Dybing E (1983)
Comparative genotoxicity studies of the flame retardant tris(2,3-
dibromopropyl) phosphate and possible metabolites. Mutat Res, 124:
213-224.
Holme JA & Soderlund EJ (1984) Unscheduled DNA synthesis of rat
hepatocytes in monolayer culture. Mutat Res, 126: 205-214.
IARC (1979) Tris(2,3-dibromopropyl) phosphate. In: Some halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
575-588 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 20).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, pp 369-370 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Iliano B, Grimee R, & Storms M (1982) Identification du tris-(2,3-
dibromopropyl) phosphate, produit ignifuge pour textiles. Anal Lett,
15(9): 797-809.
IPCS (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo- para-dioxins and dibenzofurans. Geneva, World Health
Organization, 409 pp.
IPCS (1994a) Environmental Health Criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, 347 pp.
IPCS (1994b) Environmental Health Criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, 577 pp.
IPCS (1995) Environmental Health Criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization.
IRPTC (1987) Data profile on flame retardants. Geneva, International
Register on Potentially Toxic Chemicals, United Nations Environment
Programme. Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monogr Cancer Res,
27: 95-108.
Kawashima K, Tanaka S, Nakamura S, Nagao S, Endo T, Onada K, Takanaka
A, & Omori Y (1983) Effect of phosphoric tri-esters flame retardants
on the prenatal and postnatal developments of the rats. J Toxicol Sci,
8: 339.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kerst AF (1974) Toxicology of tris(2,3-dibromopropyl) phosphate. J
Fire Flammabil Fire Retard Chem, 1: 205-217.
Kirk-Othmer (1978-1984) Encyclopedia of chemical technology, 3rd ed.
Chichester, Brisbane, Toronto, John Wiley and Sons, vol 10,
pp 486-490.
Klopman G, Frierson MR, & Rosenkranz HS (1990) The structural basis of
the mutagenicity of chemicals in Salmonella typhimurium: The
Gene-Tox data base. Mutat Res, 228: 1-50.
Kurokawa K, Nagami GT, & Yamaguchi DT (1985) Transport and substrate
metabolism of the kidney. In: Kinne RKH ed. Renal biochemistry, cells,
membranes, molecules. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 175-223.
Lake RS, Kropko ML, Pezzutti MR, Shoemaker RH, & Igel HJ (1978)
Chemical induction of unscheduled DNA synthesis in human skin
epithelial cell cultures. Cancer Res, 38: 2091-2098.
LeBlanc RB (1976) What's available for flame retardant textiles. Text
ind, February: 28-31, 35, 37, 39, 41, 43 and 88.
Lyman WJ (1982) Handbook of chemical property estimation methods.
Environmental behaviour of organic compounds. New York, McGraw-Hill,
pp 4-9.
Lynn RK, Wong K, Dickinson RG, Gerber N, & Kennish JM (1980) Diester
metabolites of the flame retardant chemicals tris(1,3-dichloro-2-
propyl) phosphate and tris(2,3-dibromopropyl) phosphate in the rat:
Identification and quantification. Res Commun Chem Pathol Pharmacol,
28: 351-360.
Lynn RK, Garvie-Gould C, Wong K, & Kennish JM (1982) Metabolism,
distribution and excretion of the flame retardant tris(2,3-
dibromopropyl) phosphate (Tris-BP) in rats: Identification of
mutagenic and nephrotoxic metabolites. Toxicol Appl Pharmacol, 63:
105-119.
Mabey W & Mill T (1978) Critical review of hydrolysis of organic
compounds in water under environmental conditions. J Phys Chem Ref
Data, 7: 383-415.
McCann J & Ames BN (1977) The Salmonella/microsome mutagenicity
test: Predictive value for animal carcinogenicity. In: Hiatt HH,
Watson JD, & Winsten JA ed. Origins of human cancer. Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 1431-1450.
McCoy E, Hyman J, & Rosenkranz HS (1980) A new mutagenic and genotoxic
response of the flame retardant tris(2,3-dibromopropyl) phosphate.
Mutat Res, 77: 209-214.
MacGregor JT Diamond MJ, Mazzeno LW Jr, & Friedman M (1980)
Mutagenicity tests of fabric-finishing agents in Salmonella
typhimurium: Fiber-reactive wool dyes and cotton flame retardants.
Environ Mutagen, 2: 405-418.
Marsden PJ & Casida JE (1982) 2-Halocrylic acids as indicators of
mutagenic 2-haloacrolein intermediates in mammalian metabolism of
selected promutagens and carcinogens. J Agric Food Chem, 30: 627-631.
Morrow RW, Hornberger CS, Kligman AM, & Maibach HI (1976) Tris(2,3-
dibromo-propyl) phosphate: Human contact sensitization. Am Ind Hyg
Assoc J, 37: 192-197.
Nakamura A (1980) Quantitative gas chromatographic determination of
tris(2,3-dibromopropyl) phosphate in the 10 ng range by using a 0.8 mm
i.d. column packed with a high liquid loaded support. J Chromatogr,
196: 133-141.
Nakamura A, Tateno N, Kojima S, Kaniwa M-A, & Kawamura T (1979) The
mutagenicity of halogenated alkanols and their phosphoric acid esters
for Salmonella typhimurium. Mutat Res, 66: 373-380.
Nakamura A, Tateno N, Iwata T, Kojima S, Kaniwa M-A, & Kawamura T
(1983) Mutagenicity of bis- and mono-(2,3-dibromopropyl) phosphate,
and their salts used as flame retardants, in the Salmonella/
microsome system. Mutat Res, 117: 1-8.
Nakanishi Y & Schneider EL (1979) In vivo sister-chromatid
exchange: A sensitive measure of DNA damage. Mutat Res, 60: 329-337.
Nelson SD, Omichinski JG, Iyer L, Gordon WP, Soderlund EJ, & Dybing E
(1984) Activation mechanism of tris(2,3-dibromopropyl) phosphate to
the potent mutagen, 2-bromoacrolein. Biochem Biophys Res Commun,
121: 213-219.
Nomeir AA & Matthews HB (1983) Metabolism and disposition of the
flame retardant tris(2,3-dibromopropyl) phosphate in the rat. Toxicol
Appl Pharmacol, 67: 357-369.
Nomiyama K, Yamamoto A, & Sato C (1974) Assay of urinary enzymes in
toxic nephropathy. Toxicol Appl Pharmacol, 27: 484-490.
NTP (1992) NTP Technical report on the toxicology and carcinogenesis
studies of 2,3-dibromo-1-propanol (CAS No. 96-13-9) in F344/N rats and
B6C3F1 mice (dermal studies). Research Triangle Park, North Carolina,
National Toxicology Program (NTP Technical Report Series No. 400; NIH
Publication No. 92-2855).
Omichinski JG, Soderlund EJ, Bausano JA, Dybing E, & Nelson SD (1987)
Synthesis and mutagenicity of selectively methylated analogues of
tris(2,3-dibromopropyl) phosphate and 1,2-dibromo-3-chloropropane.
Mutagenesis, 2(4): 287-292.
Osterberg RE (1977) Children's wearing apparel containing TRIS;
interpretation as banned hazardous substance. Fed Reg, 42:
18850-18854.
Osterberg RE, Bierbower GW, & Hehir RM (1977) Renal and testicular
damage following dermal application of the flame retardant tris(2,3-
dibromopropyl) phosphate. J Toxicol Environ Health, 3: 979-987.
Osterberg RE, Bierbower GW, Ulsamer AG, Porter WK Jr, & Jones S (1978)
The com-parative toxicity of the flame retardant tris(2,3-
dibromopropyl) phosphate and the meta-bolite 2,3-dibromopropanol in
the rat. Toxicol Appl Pharmacol, 45: 254 (Abstract No. 82).
Overbeek DE & Nametz RC (1962) Method for the preparation of
tris(2,3-dibromopropyl) phosphate. US Patent 3,046,297, 25 July, to
Michigan Chemical Corporation.
Paciorek KJL, Kratzer RH, Kaufman J, Nakahara JH, Christos T, &
Hartstein AM (1978) Thermal oxidative degradation studies of
phosphate esters. Am Ind Hyg Assoc J, 39: 633-639.
Pearson PG, Omichinski JG, McClanahan RH, Soderlund EJ, Dybing E, &
Nelson SD (1993a) Metabolic activation of tris(2,3-dibromopropyl)
phosphate to reactive intermediates. I. Covalent binding and reactive
metabolite formation in vitro. Toxicol Appl Pharmacol, 118: 186-195.
Pearson PG, Omichinski JG, Holme JA, McClanahan RH, Brunborg G,
Soderlund EJ, Dybing E, & Nelson SD (1993b) Metabolic activation of
tris(2,3-dibromopropyl) phosphate to reactive intermediates. II.
Covalent binding, reactive metabolite formation, and differential
metabolite-specific DNA damage in vivo. Toxicol Appl Pharmacol,
118: 196-204.
Pestemer W (1988) [Special reports of the Jülich Nuclear Research
Centre]. Anl Jülich, 11: 93-109 (in German).
Pitts RF (1987) Production of CO2 by the intact functioning kidney
of the dog. Med Clin North Am, 59: 507-518.
Prival MJ (1975) Information available to date relevant to the
mutagenicity of tris(2,3-dibromopropyl) phosphate. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
Prival MJ, McCoy EC, Gutter B, & Rosenkranz HS (1977) Tris(2,3-
dibromopropyl) phosphate: Mutagenicity of a widely used flame
retardant. Science, 195(4273): 76-78.
Reznik G, Ward JM, Hardisty JF, & Russfield A (1979) Renal
carcinogenic and nephrotoxic effects of the flame retardant tris(2,3-
dibromopropyl) phosphate in F344 rats and (C57Bl/6N × C3H/HeN)F1 mice.
J Natl Cancer Inst, 63(1): 205-212.
Reznik G, Reznik-Schüller HM, Rice JM, & Hague BF Jr (1981)
Pathogenesis of toxic and neoplastic renal lesions induced by the
flame retardant tris(2,3-dibromopropyl) phosphate in F344 rats, and
development of colonic adenomas after prolonged oral administration.
Lab Invest, 44(1): 74-83.
Rosen JD, Segall Y, & Casida JE (1980) Mutagenic potency of
haloacroleins and related compounds. Mutat Res, 78: 113-120.
St. John LE Jr, Eldefrawi ME, & Lisk DJ (1976) Studies of possible
absorption of a flame retardant from treated fabrics worn by rats and
humans. Bull Environ Contam Toxicol, 15(2): 192-197.
Sala M, Gu ZG, Moens G, & Chouroulinkov I (1982) In vivo and in vitro
biological effects of the flame retardants tris(2,3-dibromopropyl)
phosphate and tris(2-chloroethyl) orthophosphate. Eur J Cancer Clin
Oncol, 18(12): 1337-1344.
Salamone MF & Katz M (1981) Mutagenicity of tris(2,3-dibromopropyl)
phosphate in mammalian gonad and bone marrow tissue. J Natl Cancer
Inst, 4: 691-695.
Sasaki M, Sugimura K, Yoshida MA, & Abe S (1980) Cytogenetic effects
of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo, II(20): 574-584.
Seabaugh VM, Collins TFX, Hoheisel CA, Bierbower GW, & McLaughlin J
(1981) Rat teratology study of orally administered tris(2,3-
dibromopropyl) phosphate. Food Cosmet Toxicol, 19: 67-72.
Simula TP, Glancey MJ, Soderlund EJ, Dybing E, & Wolf CR (1993)
Increased mutagenicity of 1,2-dibromo-3 chloropropane and tris(2,3,-
dibromopropyl) phosphate in Salmonella TA100 expressing human
glutathione S-transferases. Carcinogenesis, 14(11): 2303-2307.
Soderlund EJ, Nelson SD, & Dybing E (1979) Mutagenic activation of
tris(2,3-dibromopropyl) phosphate: The role of microsomal oxidative
metabolism. Acta Pharmacol. Toxicol., 45: 112-121.
Soderlund E, Dybing E, & Nelson SD (1980) Nephrotoxicity and
hepatotoxicity of tris(2,3-dibromopropyl) phosphate in the rat.
Toxicol Appl Pharmacol, 56: 171-181.
Soderlund EJ, Nelson SD, & Dybing E (1981) In vitro and in vivo
covalent binding of the kidney toxicant and carcinogen tris(2,3-
dibromopropyl) phosphate. Toxicology, 21: 291-304.
Soderlund EJ, Nelson SD, von Bahr C, & Dybing E (1982a) Species
differences in kidney toxicity and metabolic activation of tris(2,3-
dibromopropyl) phosphate. Fundam Appl Toxicol, 2: 187-194.
Soderlund E, Nelson SD, & Dybing E (1982b) Mutagenicity and
nephrotoxicity of two tris(2,3-dibromopropyl) phosphate analogs:
Bis(2,3-dibromopropyl) phosphate and 2,3-dibromopropyl phosphate. Acta
Pharmacol Toxicol, 51: 76-80.
Soderlund EJ, Gordon WP, Nelson SD, Omichinski JG, & Dybing E (1984)
Metabolism in vitro of tris(2,3-dibromopropyl) phosphate: Oxidative
debromination and bis(2,3-dibromopropyl) phosphate formation as
correlates to mutagenicity and covalent protein binding. Biochem
Pharmacol, 33: 4017-4023.
Soderlund EJ, Dybing E, Holme JA, Hongslo JK, Rivedal E, Sanner T,
Nelson SD (1985) Comparative genotoxicity and nephrotoxicity studies
of the two halogenated flame retardants tris(1,3-dichloro-2-propyl)
phosphate and tris(2,3-dibromopropyl) phosphate. Acta Pharmacol
Toxicol, 56: 20-29.
Soderlund EJ, Omichinski JG, Dahl JE, Nelson SD, & Dybing E (1988)
Nephrotoxicity of selectively deuterated and methylated analogues of
Tris-BP and Bis-BP in the rat. Pharmacol Toxicol, 62: 142-149.
Soderlund EJ, Brunberg G, Dybing E, Trygg B, Nelson SD, & Holme JA
(1992) Organ-specific DNA damage of tris(2,3-dibromopropyl) phosphate
and its diester metabolite in the rat. Chem-Biol Interact, 82:
195-207.
Takada K, Naito K, Kobayashi K, Tobe M, Kurokawa Y, & Fukuoka M
(1991a) Carcinogenic effect of bis(2,3-dibromopropyl) phosphate in
Wistar rats. J Appl Toxicol, 11(5): 323-331.
Takada K, Naito K, Aida Y, Momma J, Yoshimoto H, Nakaji Y, Kurokawa Y,
& Tobe M (1991b) Acute and subacute toxicity studies of bis(2,3-
dibromopropyl) phosphate magnesium in rats. Bull Natl Inst Health Sci
Jpn, 109: 25-31.
Ulsamer AG, Porter WK, & Osterberg RE (1978) Percutaneous absorption
of radiolabelled TRIS from flame retarded fabric. J Environ Pathol
Toxicol, 1: 543-549.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
UN (1991) Consolidated list of products whose consumption and/or sale
have been banned, withdrawn, severely restricted or not approved by
Governments, 4th ed. New York, United Nations.
US Consumer Product Safety Commission (1977a) Children's wearing
apparel containing Tris; interpretation as banned hazardous substance.
Fed Reg, 42: 18850-18854.
US Consumer Product Safety Commission (1977b) Children's wearing
apparel containing tris; tris and fabric, yarn or fiber containing
tris; withdrawal of interpretations as banned hazardous substances.
Fed Reg, 43: 61593-61594, 61621-61622.
US EPA (1976) Tris(2,3-dibromopropyl) phosphate (TBPP). Washington,
DC, US Environmental Protection Agency, pp 53-55 (EPA 560/4-76-004).
US EPA (1985) GEMS - Graphical exposure modelling system. Fate of
atmospheric pollutants (FAP) data base. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
US NCI (1978) Bioassay of tris(2,3-dibromopropyl) phosphate for
possible carcinogenicity. Washington, DC, National Cancer Institute
(Technical Report Series No. 76; NIH Publication No. 78-1326).
Valencia R (1978) Drosophila mutagenicity tests of saccharin, Tris,
PtCl4 and other compounds. (Abstract Cb-11). In: 9th Annual Meeting
of the American Environmental Mutagen Society, San Francisco. New
York, Alan R. Liss, Inc.
Van Beerendonk GJM (1994) Genotoxicity of the flame retardant
tris(2,3-dibromopropyl) phosphate: aspects of bioactivation and DNA
adduct formation. Leiden, The Netherlands, State University of Leiden,
82 pp.
Van Beerendonk GJM, Nivard MJM, Vogel EW, Nelson SD, & Meerman JHN
(1992) Formation of thymidine adducts and cross-linking potential of
2-bromoacrolein, a reactive metabolite of tris(2,3-dibromopropyl)
phosphate. Mutagenesis, 7(1): 19-24.
Van Duuren BL, Loewengart G, Seidman I, Smith AC, & Melchionne S
(1978) Mouse skin carcinogenicity tests of the flame retardants
tris(2,3-dibromopropyl) phosphate, tetrakis(hydroxymethyl)
phosphoniumchloride and polyvinylbromide. Cancer Res, 38: 3236-3240.
Veith GD, DeFoe D, & Bergstedt BV (1979) Measuring and estimating the
bioconcentration factor of chemicals in fish. J Fish Res Board Can,
36(9): 1040-1048.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company, 1173 pp.
Vogel EW & Nivard MJM (1993) Performance of 181 chemicals in a
Drosophila assay predominantly monitoring interchromosomal mitotic
recombination. Mutagenesis, 8(1): 57-81.
Wood LB, Hurley BJE, & Matthews PJ (1981) Some observations on the
biochemistry and inhibition of nitrification. Water Res, 15: 543-551.
Zeiger E, Pagano DA, & Nomeir AA (1982) Structure-activity studies on
the mutagenicity of tris(2,3-dibromopropyl) phosphate (Tris-BP) and
its metabolites in Salmonella. Environ Mutagen, 4: 271-277.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Tris-2,3-dibromopropyle
1.1 Résumé et évaluation
1.1.1 Production et usage
La production de phosphate de tris-2,3-dibromopropyle (TBPP) a
commencé pour la première fois vers 1950 et sa production commerciale
est documentée à partir de 1959. Aux Etats-Unis, en 1975, la
production de TBPP se situait, selon les estimations, entre 4100 et
5400 tonnes. Autant qu'on sache, le TBPP n'est ni produit ni utilisé
actuellement dans le monde comme retardateur de flamme dans les
textiles, mais il peut être incorporé à des polymères utilisés à
d'autres fins. Le TBPP a constitué un important retardateur de
flamme, que l'on ajoutait aux tissus de cellulose, de triacétate et de
polyester, en particulier pour les vêtements de nuit destinés aux
enfants, mais il a été depuis interdit pour cet usage dans plusieurs
pays d'Europe, aux Etats-Unis d'Amérique (1977) ainsi qu'au Japon
(1978).
Le TBPP peut se trouver à l'intérieur ou à la surface du tissu.
Lorsqu'il se trouve à l'intérieur, on ne peut pas l'extraire par
solvants et il est donc probable qu'il ne peut pas non plus être
absorbé par voie percutanée. Toutefois, lorsqu'il se trouve à la
surface de la fibre, il peut être extrait lors de la lessive ainsi
qu'au moyen d'acide acétique ou d'autres solvants ou encore par la
salive, et peut être alors absorbé par voie percutanée. Dans ce cas,
il peut y avoir en cours d'utilisation ou pendant la lessive des
produits finis une perte importante du TBPP qui se trouve à leur
surface, d'où contamination de l'environnement. En outre, on a
signalé la décharge de TBPP dans l'environnement par des ateliers de
finissage et le rejet final de déchets solides.
1.1.2 Propriétés physiques et chimiques
Le TBPP existe en au moins deux qualités. Le produit de haute
qualité est un liquide visqueux clair, de couleur jaune pâle, qui
contient jusqu'à 1,5% de matière volatile. Le produit de basse
qualité peut contenir jusqu'à 10% de matière volatile.
Le TBPP (degré de pureté > à 97%) a un point d'ébullition égal à
390°C, un point de fusion de 5,5°C et une tension de vapeur de
1,9 × 10-4 mmHg à 25°C. Il est faiblement soluble dans l'eau
(8 mg/litre).
Lorsqu'on le chauffe jusqu'à décomposition, c'est-à-dire au-dessus
de 260-300°C, le TBPP libère des composés contenant du brome et du
phosphore. Son coefficient de partage entre le n-octanol et l'eau
(log Pow) est égal à 3,02.
Il existe des méthodes d'analyse permettant le dosage du TBPP et
de ses métabolites dans des échantillons biologiques ou d'autres
matrices.
1.1.3 Transport, distribution et transformation dans l'environnement
Le peu de données dont on dispose incitent à penser que le TBPP
est relativement persistant dans l'environnement. Il ne semble pas
que l'oxydation et la photodécomposition jouent un rôle important dans
sa destinée. Cependant, il peut y avoir une hydrolyse impliquant les
atomes de brome du groupement propyle, en particulier en milieu
basique. Il peut s'évaporer de l'eau mais on ne dispose pas
véritablement de données sur ce point. Bien qu'on ait signalé la
possibilité d'une biodécomposition du TBPP (demi-vie 19,7 h.) dans les
boues activées, on ne pense pas que cela constitue un processus
important dans les sols et les eaux naturels. Dans la boue
stérilisée, il n'y a pratiquement pas de décomposition. On a constaté
que l'un des principaux produits de décomposition du TBPP était le
phosphate de bis-2,3-dibromo-propyle. Le TBPP étant pratiquement
insoluble dans l'eau, il est possible que l'adsorption sur les
matières particulaires et sur les sédiments joue un rôle important.
La valeur estimative du log de Koc (3,29) indique qu'il y a une
forte adsorption au sol. Sur la base de cette valeur de Koc et de
la faible solubilité dans l'eau du TBPP, on pense que ce composé n'est
que lentement lessivé vers les eaux souterraines. Le TBPP va avoir
tendance à s'accumuler dans les décharges publiques et autres lieux de
ce genre, avec pour conséquence la possibilité d'une bioaccumulation.
D'ailleurs, une étude portant sur un cyprin, Pimephales promelas, a
permis de mettre en évidence un facteur de bioconcentration de 2,7, ce
qui est faible, alors que le coefficient de partage n-octanol/eau
(log de Pow) est de 3,02. En raison de sa faible tension de vapeur,
on peut penser que le TBPP sera essentiellement sorbé sur les
particules en suspension dans l'air. La décomposition thermique en
milieu oxydant du TBPP à la température de 370°C entraîne la formation
de bromure d'hydrogène et de composés bromés en C3 tels que des
bromopropènes, des dibromopropènes ainsi que des di-et-tribromopropanes.
1.1.4 Concentrations dans l'environnement et exposition humaine
On ne dispose que de données limitées sur les concentrations dans
l'environnement et l'exposition humaine. Lors d'études effectuées au
Japon en 1975, on a analysé 20 échantillons d'eau, de sol et de
poissons sans y trouver de TBPP. En revanche, on a mis en évidence,
sans le doser, du TBPP dans des particules en suspension dans l'air
prélevé aux alentours d'une installation industrielle.
Ce sont les enfants portant des vêtements de nuit traités par du
TBPP qui, lorsque ce produit était autorisé, constituaient le groupe
de population le plus particulièrement exposé à ce retardateur de
flamme. On estime qu'à l'époque, la dose absorbée à travers la peau
par ces enfants aux Etats-Unis d'Amérique était de l'ordre de 9 µg/kg
de poids corporel et par jour. La Consumer Product Safety Commission
des Etats-Unis a calculé que, sur une période de six ans, un enfant
portant de tels vêtements pouvait absorber une dose totale d'au moins
2 à 77 mg de TBPP/kg de poids corporel.
1.1.5 Cinétique et métabolisme chez les animaux de laboratoire
et l'homme
Le TBPP est rapidement absorbé au niveau des voies digestives et
à un rythme plus modéré par la voie percutanée chez les rats et les
lapins. Chez le rat, le TBPP ou ses métabolites sont éliminés dans
les 5 jours. L'élimination se produit à hauteur d'environ 50% dans
les urines, 10% dans les matières fécales, 10-20% de son carbone étant
rejetés sous forme de CO2 dans l'air expiré.
Un jour après avoir administré par voie orale du TBPP radiomarqué
à des rats, la radioactivité s'est retrouvée dans les limites de
0,2-6,6% au niveau du sang, du foie, des reins, des muscles, des
tissus adipeux et de la peau. Le temps de demi-élimination de la
radioactivité de tous ces organes était d'environ 2-4 jours. Au bout
de 8 h., il ne restait encore, en concentrations notables, que du
phosphate de bis-2,3-dibromopropyle dans la plupart des tissus.
Après administration orale de TBPP à des rats, on a mis en
évidence six métabolites dans les urines et la bile. Le principal
métabolite urinaire, fécal, biliaire et tissulaire était le phosphate
de bis-2,3-dibromopropyle. Un autre métabolite, le 2,3-dibromo-
propanol, a été également mis en évidence chez des rats et des enfants
qui portaient des vêtements traités par du TBPP.
Les microsomes hépatiques métabolisent le TBPP en présence de
NADPH et d'oxygène. Les principaux métabolites obtenus sont le
phosphate de bis-2,3-dibromopropyle et le 2,3-dibromopropanol. On a
montré qu'il se formait du phosphate de bis-2,3-dibromopropyle par
oxydation au niveau du C3 et peut-être également en position C2 du
TBPP. Outre le phosphate bis-2,3-dibromopropyle, on retrouve de la
2-bromoacroléine, de l'acide 2-bromoacrylique ainsi que des composés
propylliques hydroxylés et des métabolites conjugués avec du
glutathion.
Du S-(2,3-dihydroxypropyl)glutathion ayant été mis en évidence
dans la bile de rats, on a avancé l'hypothèse que le TBPP et/ou le
phosphate de bis-2,3-dibromopropyle subissent une conjugaison directe
par le glutathion en présence de glutathion S-transférase avec
formation, comme métabolites, d'ions épisulfonium.
Par activation, le TBPP forme des produits qui se fixent par
liaisons covalentes aux protéines et à l'ADN in vivo et in vitro.
Après injection intrapéritonéale de TBPP tritié à des souris, à des
hamsters et à des cobayes mâles, qui sont moins sensibles à la
néphrotoxicité induite par cette substance que les rats, on a observé
un degré analogue de liaison covalente aux protéines dans le foie et
les reins. Chez le rat mâle, qui est beaucoup plus sensible à la
néphrotoxicité induite par ce produit, on a constaté qu'il y avait
beaucoup plus de composé radiomarqué fixé aux protéines rénales qu'aux
protéines hépatiques.
Mis en présence de microsomes hépatiques provenant de souris, de
cobayes, de hamsters et de sujets humains, le TBPP est dans tous les
cas métabolisé en intermédiaires génotoxiques. Toutefois, les
métabolites réactifs du TBPP se forment beaucoup plus lentement en
présence de microsomes hépatiques d'origine humaine qu'en présence de
microsomes prélevés sur rongeurs.
Après avoir administré à des rats une dose néphrotoxique de TBPP
et de ses analogues radiomarqués, on a constaté que le degré de
liaison covalente aux protéines décroissait dans l'ordre suivant:
reins, foie et testicules. D'après les résultats d'études in vitro
et in vivo au cours desquelles on a comparé les lésions produites au
niveau de l'ADN rénal, on est incité à penser qu'il y a formation de
phosphate de bis-2,3-dibromopropyle au niveau du foie par oxydation,
catalysée par le P450, du TBPP en position C2 ou C3. Ce phosphate de
bis-2,3-dibromopropyle est ensuite transporté vers les reins où il est
métabolisé en intermédiaire réactif qui endommage l'ADN et se fixe aux
protéines rénales. L'activation qui se produit au niveau du rein ne
semble pas impliquer le P450 mais s'effectuer plutôt par
l'intermédiaire d'un métabolisme dépendant du GSH. Des études in
vitro avec du TBPP et certains de ses analogues radiomarqués ont
montré que l'oxydation du TBPP comportait l'incorporation d'un atome
d'oxygène provenant de l'eau. Cela implique que l'oxydation en
position C2 du reste propyle donne naissance à une alpha-bromocétone
réactive qui est capable d'alkyler directement les protéines ou de
s'hydrolyser pour donner du phosphate de bis-2,3-dibromopropyle et une
bromo-alpha-hydroxycétone réactive.
1.1.6 Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La toxicité du TBPP est faible, qu'il s'agisse de la toxicité
aiguë par voie orale à court terme ou de la toxicité aiguë par voie
percutanée. Pour le rat, la DL50 par voie orale est > 2 g/kg et
pour le lapin, la DL50 par voie cutanée dépasse 8 g/kg de poids
corporel. On a observé une atteinte rénale très étendue (nécrose
cellulaire au niveau des tubules proximaux) chez des rats mâles à qui
l'on avait injecté par voie intrapéritonéale une seule dose de 100 mg
de TBPP par kg de poids corporel.
Chez des rats soumis respectivement pendant quatre semaines ou
90 jours à des épreuves de toxicité orale au cours desquelles du TBPP
leur avait été administré par gavage ou mêlé à leur nourriture, on a
observé une augmentation de l'incidence et de la gravité des néphrites
chroniques aux doses supérieures ou égales à 25 mg/kg de poids
corporel.
Chez des lapins, l'application cutanée quotidienne de TBPP à des
doses supérieures ou égales à 2,2 g/kg de poids corporel pendant 4
semaines a entraîné une dégénérescence au niveau du foie et des reins.
Tous les lapins sont morts dans les quatre semaines. En revanche,
aucun animal n'est mort lors d'une autre étude avec des doses allant
jusqu'à 250 mg/kg de poids corporel.
Des lapins qui avaient subi chaque semaine pendant 90 jours une
application cutanée de 2,27 g de TBPP/kg de poids corporel ont
présenté des anomalies rénales, une atrophie testiculaire et une
aspermatogénèse.
Aux doses de 1,1 ou 0,22g de TBPP, on n'a observé aucune
irritation cutanée ou oculaire chez les lapins et il n'y a pas eu non
plus de sensibilisation cutanée chez des cobayes.
Deux études de tératogénicité ont été effectuées sur des rats.
L'une d'entre elles comportait des doses allant jusqu'à 125 mg/kg de
poids corporel et n'a pas permis de mettre en évidence d'effet
tératogène. Lors d'une autre étude où la dose administrée était de
200 mg/kg de poids corporel, on a observé une augmentation
significative des variations affectant le squelette chez les foetus
et, aux doses de 50 et 100 mg/kg de poids corporel, une diminution
sensible de l'indice de viabilité. Les auteurs en ont conclu que
l'effet observé était dû à l'action toxique du composé sur les
femelles gestantes.
Chez des rats auxquels on avait administré du TBPP, on a constaté
des lésions étendues de l'ADN dans divers organes. In vitro, on a
constaté que le TBPP produisait la rupture des brins d'ADN dans des
cellules KB d'origine humaine. Le TBPP a également induit une
synthèse non programmée de l'ADN dans des hépatocytes de foie de rat,
ce phénomène n'étant toutefois pas constaté dans des cellules
épithéliales de prépuce humain.
Plusieurs études ont révélé que le TBPP provoquait des mutations,
notamment par substitution des paires de base, chez des souches de
Salmonella typhimurium, avec ou sans activation métabolique.
L'étude des mutations géniques directes sur cellules de hamsters
chinois V79 avec ou sans activation métabolique, a donné des résultats
négatifs. Toutefois, en présence de microsomes hépatiques de rats
préalablement traités par du phénobarbital, on a observé un effet
positif. Un effet faiblement positif a également été observé avec des
cellules lymphomateuses de souris (locus L5178YTK).
Dans des cellules V79 de hamsters chinois, on a constaté que le
TBPP augmentait le nombre d'échanges entre chromatides soeurs. En
revanche il n'y avait pas d'augmentation du nombre des aberrations
chromosomiques, ni dans les cellules de hamsters chinois, ni dans les
cellules de moelle osseuse murine, ni dans les cellules lymphoïdes
humaines en culture. Dans des cellules fibroblastiques humaines
diploïdes (lignée HE 2144), on a observé des échanges entre
chromatides soeurs mais pas d'aberrations chromosomiques, l'épreuve
étant effectuée sans activation métabolique. Toutefois la recherche
in vitro d'aberrations chromo-somiques dans des lignées cellulaires
de hamsters chinois a donné un résultat positif.
Un résultat positif a également été obtenu lors de la recherche
de micronoyaux dans des cellules de moelle osseuse provenant de
hamsters chinois. Une autre épreuve de ce genre, portant cette fois
sur des souris, a permis d'observer un effet faiblement positif.
Les études effectuées sur Drosophila melanogaster ont montré
que le TBPP augmentait les mutations récessives létales liées au sexe
dans les gamètes mâles ainsi que chez les mâles adultes et il y avait
induction de translocations réciproques. Dans l'épreuve de l'oeil en
mosaïque w/w+, le TBPP a suscité une réaction fortement positive.
Un certain nombre d'études ont été menées pour tenter d'élucider
les mécanismes qui sont à la base de la mutagénicité et/ou de la
génotoxicité induites par le TBPP. Ainsi la mutagénicité du TBPP pour
les bactéries s'effectue par l'intermédiaire du système des
monooxygénases microsomiques. Par ailleurs, lors d'une réaction qui
est sous la dépendance du NADPH et de l'oxygène, il y a activation du
TBPP par le cytochrome P450. Des microsomes préparés à partir de
foies d'animaux traités par du phénobarbital ou des PCBs entraînent un
accroissement de la mutagénicité. Le phosphate de mono- et de
bis-2,3-dibromopropyle sont moins mutagènes que le TBPP. Des études
in vitro ont montré que l'oxydation de la molécule de TBPP au niveau
du C3 donnait naissance à un puissant mutagène à action directe, la
2-bromoacroléine qui se lie également à l'ADN.
On a mis en évidence des différences interspécifiques dans la
bioactivation du TBPP en métabolites mutagènes pour la souche TA 100
de Salmonella typhimurium. A cet égard, les microsomes hépatiques
de souris étaient plus efficaces que ceux de cobayes, de hamsters et
de rats.
Trois études de transformation cellulaire ont été menées à l'aide
de cellules C3H/10T1/2. Dans l'une d'entre elles, on a obtenu un
effet positif, mais les deux autres ont donné des résultats négatifs.
Lors d'études à long terme, on a administré par voie orale du
TBPP à des souris et à des rats et on en a appliqué sur la peau de
souris femelles. Chez les souris, on a constaté, après administration
par voie orale, qu'il se formait chez les deux sexes des tumeurs au
niveau de la portion cardiaque de l'estomac et des poumons, ainsi que
des tumeurs bénignes ou malignes au niveau du foie chez les femelles
et au niveau des reins chez les mâles. Chez les rats, des tumeurs
bénignes ou malignes se sont formées au niveau des reins chez les
mâles, les tumeurs rénales étant bénignes chez les femelles.
L'application de TBPP sur la peau de souris femelles a entraîné
l'apparition de tumeurs de la peau, des poumons, de la portion
cardiaque de l'estomac et de la cavité buccale. On peut conclure de
ces études que le TBPP est doté de pouvoir cancérogène chez la souris
et le rat.
Après administration d'un métabolite du TBPP, le phosphate de
bis-2,3-dibromopropyle, par voie orale à des rats, on a constaté
l'apparition de tumeurs digestives chez les deux sexes. Il s'agissait
de papillomes et d'adénocarcinomes de la langue, de l'oesophage et de
la portion cardiaque de l'estomac, ainsi que d'adénocarcinomes de
l'intestin avec en outre des adénomes et des carcinomes
hépatocellulaires.
On a également procédé à l'application cutanée d'un autre
métabolite du TBPP, le 2,3-dibromo-1-propanol, à des souris et à des
rats. Chez les rats mâles, on a constaté un accroissement de
l'incidence des tumeurs malignes de la peau, du nez, de la muqueuse
buccale, de l'oesophage, de la portion cardiaque de l'estomac, de
l'intestin grêle et du gros intestin, de la glande de Zymbal, du foie,
du rein, de la vaginale, et de la rate. Chez les rats femelles, on
constatait une incidence accrue de tumeurs malignes affectant la peau,
le nez, la muqueuse buccale, l'oesophage, la portion cardiaque de
l'estomac, l'intestin grêle et le gros intestin, la glande de Zymbal,
le foie, le rein, la glande clitoridienne, et les glandes mammaires.
Chez les souris mâles, il y avait également une incidence plus élevée
des tumeurs malignes au niveau de la peau, de la portion cardiaque de
l'estomac, du foie et des poumons, tandis que chez les femelles
l'accroissement des tumeurs malignes se manifestait au niveau de la
peau et de la portion cardiaque de l'estomac.
1.1.7 Effets sur l'homme
On ne dispose que de données limitées concernant les effets du
TBPP sur l'homme.
Quelques études ont été consacrées à la recherche chez l'homme
d'un effet sensibilisateur que le TBPP pourrait avoir sur la peau.
Les résultats obtenus montrent que ce produit n'a qu'un faible pouvoir
sensibilisateur et aucune irritation cutanée n'a été observée.
Toutefois les personnes qui avaient présenté une réaction positive au
TBPP pur ont également réagi lorsqu'on les a mises en contact avec des
tissus qui en contenaient.
1.1.8 Effets sur les autres êtres vivants au laboratoire et
dans leur milieu naturel
On ne possède que très peu de données concernant les effets du
TBPP sur les autres êtres vivants. Par exemple, des poissons rouges
(Caraccius auratus) qui avaient été exposés à du TBPP à raison de
1 mg/litre sont morts tous les 6 en l'espace de 5 jours.
La CE50 relative à l'inhibition de la croissance de la semence
d'avoine se situait à 1000 mg/kg de terre. Cette concentration a
provoqué l'inhibition totale de la croissance des semences de navet
( Brassica rapa sp.).
1.2 Conclusions
Le TBPP a été utilisé naguère comme retardateur de flamme pour en
imprégner les tissus, en particulier destinés à la confection de
vêtements de nuit pour enfants, mais on est guère renseigné sur ses
autres applications. C'est essentiellement par contact avec des
tissus traités par ce composé que la population générale a pu être
contaminée.
On n'a guère de renseignements non plus sur l'exposition des
ouvriers employés à la production commerciale du TBPP ainsi qu'à son
utilisation pour la fabrication de divers produits, ni d'ailleurs sur
les dangers qu'il représente.
En raison de la rareté des données, il n'est pas possible de
parvenir à des conclusions définitives quant aux niveaux d'exposition
ou aux dangers que le TBPP fait courir aux êtres vivants dans leur
milieu naturel, l'homme mis à part.
Les études sur l'animal ont montré que le TBPP pouvait être
absorbé au niveau des voies digestives et, dans une moindre
proportion, par la voie percutanée. Il peut également être résorbé
par cette dernière voie chez l'homme. Chez le rat, il se révèle être
très largement métabolisé dans le foie en phosphate de bis-2,3-
dibromopropyle, qui constitue le principal métabolite mis en évidence
dans les urines, et, dans une moindre proportion, en 2,3-
dibromopropanol. En outre, on a retrouvé de petites quantités
d'autres métabolites bromés du TBPP. La présence de
2,3-dibromopropanol a été également observée chez des personnes qui
portaient des tissus traités par le TBPP. La principale voie
d'élimination est la voie urinaire, le composé étant excrété en très
faible proportion sous sa forme initiale. Quant à la principale voie
métabolique, elle semble faire intervenir le cytochrome P450 et les
glutathion- S-transférases.
D'après les données dont on dispose, on peut conclure que le TBPP
ne présente qu'une faible toxicité aiguë chez l'animal de laboratoire.
Des études au cours desquelles on a administré de manière répétée de
fortes doses de TBPP, on permis de mettre en évidence des lésions
rénales et hépatiques chez le rat ainsi qu'une atteinte testiculaire
chez le lapin. Le composé a également un indéniable effet génotoxique
dans plusieurs systèmes d'épreuve, tant in vitro qu' in vivo. Des
effets cancérogènes ont également été relevés chez le rat et la
souris. Deux de ses métabolites, le phosphate de bis-2,3-
dibromopropyle et le 2,3-dibromopropanol, produisent également des
effets cancérogènes chez l'animal de laboratoire. Il n'est pas
irritant chez l'animal et son pouvoir sensibilisateur chez l'homme est
faible.
En 1977, la Consumer Product Safety Commission des Etats-Unis
d'Amérique (Commission pour protection du consommateur) a interdit
l'utilisation de vêtements d'enfants traités par le phosphate de
tris-2,3-dibromoproyl, par crainte que ce composé ne soit cancérogène
pour l'homme et en raison du risque non négligeable encouru par les
personnes portant des vêtements confectionnés à l'aide de tissus
imprégnés. Depuis lors, l'utilisation de ce composé comme retardateur
de flamme dans les produits destinés à la consommation courante est
très sévèrement réglementée dans un certain nombre d'autres pays et
son utilisation dans les textiles est interdite.
1.3 Recommandations
En raison de ses effets toxiques, le TBPP ne doit plus être
utilisé dans le commerce.
Au cas où, pour certains usages, il n'existerait pas de
substituts moins dangereux au TBPP, il faudrait entreprendre des
études pour s'assurer de l'absence d'exposition et de danger pour
l'homme et l'environnement.
2. Bis-2,3-dibromopropyle
La base de données relatives au phosphate de
bis-2,3-dibromopropyle et à ses sels est insuffisante pour en
permettre l'évaluation ou en justifier l'usage commercial.
D'après les données disponibles on peut penser que ce composé
pourrait être mutagène et cancérogène.
Il ne sera pas possible d'évaluer ce produit tant qu'on ne
disposera pas de données complémentaires sur ses propriétés physiques
et chimiques, sa production et son usage, son transport, sa
distribution, sa transformation et sa concentration dans
l'environnement ainsi que l'exposition humaine auxquels il donne lieu,
sa cinétique et son métabolisme chez l'animal et l'homme, ses effets
sur les animaux de laboratoire, l'homme et les systèmes d'épreuve in
vitro ainsi que son action sur les autres êtres vivants au
laboratoire et dans leur milieu naturel. Il est également nécessaire
d'obtenir davantage de données concernant son pouvoir mutagène sur au
moins deux points d'aboutissement.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. El fosfato de tris(2,3-dibromopropilo)
1.1 Resumen y evaluación
1.1.1 Producción y utilización
El fosfato de tris(2,3-dibromopropilo) (FTBP) se produjo por
primera vez hacia 1950. Se sabe que en 1959 hubo producción con fines
comerciales. En 1975 la producción de FTBP en los Estados Unidos de
América se estimó entre 4100 y 5400 toneladas. Que se sepa, el FTBP
no se produce ni utiliza corrientemente en la actualidad a nivel
mundial como retardador de ignición en productos textiles, pero puede
utilizarse en polímeros empleados para otros fines. El FTBP era un
importante retardador de ignición de la celulosa y de tejidos de
triacetato y de poliéster, especialmente en ropa de dormir para
niños, pero este empleo se ha prohibido en varios países de Europa,
los Estados Unidos de América (1977) y el Japón (1978).
El FTBP se utiliza tanto dentro del tejido como sobre el mismo.
Si se encuentra dentro del tejido, no puede extraerse con disolventes
y, por consiguiente, probablemente no esté disponible para su
absorción cutánea. Sin embargo, si se halla en la superficie de la
fibra, puede extraerse durante el lavado o por acción del ácido
acético, de otros disolventes y de la saliva y está disponible para la
absorción cutánea. En este caso habrá pérdida sustancial de FTBP
superficial del tejido durante la utilización y/o el lavado de los
productos acabados, y se contaminará el medio ambiente. Además, se
sabe que hay emisión de FTBP al medio ambiente en las operaciones de
acabado de textiles y en la evacuación final de desechos sólidos que
contienen FTBP.
1.1.2 Propiedades físicas y químicas
Puede obtenerse FTBP de dos calidades por lo menos. El producto
de alto grado de pureza es un líquido transparente, amarillo pálido y
viscoso, que tiene hasta un 1,5% de componentes volátiles. La calidad
de baja pureza puede contener hasta un 10% de componentes volátiles.
El punto de ebullición del FTBP (pureza > 97%) es de 390°C, su
punto de fusión de 5,5°C y su presión de vapor de 1,9 × 10-4 mmHg a
25°C. La solubilidad del FTBP en el agua es baja (8 mg/litro).
Cuando se calienta hasta su descomposición, a una temperatura
superior a 260-300°C el FTBP emite compuestos que contienen bromo y
fósforo. El coeficiente de reparto n-octanol/agua (log Pow) es de
3,02.
Se dispone de métodos analíticos para determinar la presencia de
FTBP y sus metabolitos en muestras biológicas y otras matrices.
1.1.3 Transporte, distribución y transformación en el medio ambiente
La limitada información disponible sugiere que el FTBP es
relativamente persistente en el medio ambiente. La oxidación y la
fotodegradación probablemente no tengan un efecto significativo en su
destino. Sin embargo, puede haber hidrólisis de los átomos de bromo
del grupo propílico, especialmente en condiciones básicas. Puede
producirse volatilización a partir del agua, pero no se dispone de
datos efectivos. Aunque se han notificado casos de biodegradación del
FTBP (semivida 19,7 h) en aguas residuales activadas, no se considera
que ésta constituya un proceso importante en suelos y aguas naturales.
En fangos esterilizados casi no se produce descomposición. Se
encontró fosfato de bis(2,3-dibromopropilo) como principal producto de
la descomposición. Como el FTBP es prácticamente insoluble en agua,
la adsorción en partículas en sedimento puede ser un proceso
importante.
Un log Koc estimado (3,29) sugiere una fuerte adsorción en el
suelo. Sobre la base de este valor de Koc y de la baja solubilidad
medida en agua, sólo se prevé una lixiviación lenta del FTBP a las
aguas subterráneas. El FTBP tenderá a acumularse en basureros y otros
vertederos de desechos, lo que tal vez dé lugar a la acumulación
biológica. Un estudio sobre bioacumulación en Pimephales
promelas mostró un factor de bioconcentración de 2,7, que
es bajo, mientras que el coeficiente de reparto n-octanol/agua (Log
Pow) es de 3,02. Debido a su baja presión de vapor, se prevé que el
FTBP sea principalmente objeto de sorción en las partículas en
suspensión en el aire. La degradación oxidativa térmica del FTBP a
370 oC mostró que se forman bromuro de hidrógeno y compuestos
C3-bromados, tales como bromopropenos, dibromopropenos, y
dibromopropanos y tribromopropanos.
1.1.4 Niveles ambientales y exposición humana
Los datos sobre los niveles ambientales y la exposición humana
son limitados. Estudios realizados en el Japón en 1975 mostraron que
20 muestras de agua, suelo y peces no contenían FTBP. Se identificó
la presencia de FTBP en partículas en suspensión en el aire en los
alrededores de una planta industrial, pero no se cuantificaron.
Los niños que llevaban ropa de dormir tratada con FTBP fueron el
grupo de la población general particularmente expuesto a este
retardador de ignición. La absorción estimada a través de la piel de
los niños que llevaban ropa de dormir tratada con FTBP en los Estados
Unidos de América se calculó en 9 µg/kg de peso corporal por día. La
Comisión de Seguridad de los Productos de Consumo de los Estados
Unidos de América calculó que, en un periodo de seis años, un niño que
lleve ropa tratada con FTBP podría absorber en total 2-77 mg de
FTBP/kg de peso corporal o más.
1.1.5 Cinética y metabolismo en animales de laboratorio y en
seres humanos
El FTBP se absorbe rápidamente a través del tracto gastro-
intestinal y a una velocidad moderada a través de la piel en la rata y
el conejo. En la rata, el FTBP o sus metabolitos se eliminan en cinco
días. Aproximadamente el 50% se elimina por la orina, el 10% por las
heces y el 10-20% se exhala en forma de CO2.
Un día después de la administración oral de FTBP marcado a ratas,
se encontró radiactividad en la sangre, el hígado, los riñones, los
músculos, la grasa y la piel con valores comprendidos entre el 0,2% y
el 6,6%. El periodo de semieliminación de la radiactividad de dichos
órganos fue de aproximadamente 2-4 días. Después de ocho horas,
solamente el fosfato de bis(2,3-dibromopropilo) seguía presente en
concentraciones sustanciales en la mayor parte de los tejidos.
Después de la administración oral de FTBP a ratas, se
identificaron seis metabolitos en la orina y en la bilis. El
principal metabolito en la orina, las heces, la bilis y los tejidos
fue el fosfato de bis(2,3-dibromopropilo). También se identificó el
metabolito 2,3-dibromopropanol en ratas y en niños que llevaban ropa
tratada con FTBP.
Los microsomas del hígado metabolizan el FTBP en presencia de
NADPH y oxígeno. Los principales metabolitos son el fosfato de
bis(2,3-dibromopropilo) y el 2,3-dibromopropanol. Se ha mostrado que
el fosfato de bis(2,3-dibromopropilo) se forma por oxidación en la
posición C3 y posiblemente también en la posición C2 del FTBP.
Además del fosfato de bis(2,3-dibromopropilo), se han encontrado
2-bromoacroleína, ácido 2-bromoacrílico, y compuestos propil-
hidroxilados y metabolitos conjugados con glutatión.
Se identificó la presencia de S-(2,3-dihidroxipropil)glutatión en
la bilis de ratas y se sugirió que el FTBP y/o el fosfato de bis(2,3-
dibromopropilo) son conjugados directamente con el glutatión por la
glutatión S-transferasa, formándose iones episulfonio como
metabolitos.
Se ha mostrado que el FTBP se activa para formar productos que se
enlazan de forma covalente con las proteínas y el ADN in vivo e in
vitro. Después de inyecciones intraperitoneales de FTBP tritiado,
el ratón, el hámster y el cobayo machos, que son menos sensibles a la
nefrotoxicidad inducida por el FTBP que la rata, mostraron niveles
semejantes de enlace covalente con las proteínas en el hígado y los
riñones. En la rata macho, muy susceptible a la nefrotoxicidad
inducida por el FTBP, los átomos marcados se habían fijado a las
proteínas de riñon en cantidad mucho mayor que las proteínas del
hígado.
Los microsomas del hígado de ratón, cobayo, hámster y humano
metabolizaron todos ellos el FTBP formando productos intermedios
genotóxicos. Sin embargo, la tasa de formación de metabolitos
reactivos del FTBP por acción de los microsomas del hígado humano fue
menor a la de los microsomas del hígado de roedores.
El enlace del FTBP marcado y análogos en ratas a las que se había
administrado una dosis nefrotóxica mostró que el número de enlaces
covalentes a las proteínas era máximo en los riñones; les seguían el
hígado y los testículos. Los resultados de estudios comparativos in
vitro e in vivo sobre lesiones del ADN renal parecen indicar que
el fosfato de bis(2,3-dibromopropilo) se forma en el hígado por
oxidación mediada por el P450 en las posiciones C2 o C3 del FTBP. El
fosfato de bis(2,3 dibromopropilo) se transporta a los riñones, donde
se metaboliza formando productos intermedios reactivos que lesionan el
ADN y se enlazan con las proteínas del riñón. La activación en el
riñón no parece realizarse con intervención del P450 sino por medio
del metabolismo depen-diente del glutatión. Estudios in vitro con
FTBP marcado y productos análogos mostraron que, en la oxidación, al
FTBP se incorpora un átomo de oxígeno del agua. Ello significa que la
oxidación en la posición C2 del grupo propílico produce una alpha-
bromocetona reactiva que puede alkilizar la proteína directamente o
hidrolizarla produciendo fosfato de bis(2,3-dibromopropilo) y una
bromo-alpha-hidroxicetona reactiva.
1.1.6 Efectos en mamíferos de laboratorio y en sistemas de
prueba in vitro
La toxicidad oral aguda y de corto plazo y la toxicidad cutánea
aguda del FTBP son bajas. La DL50 oral para la rata es > 2 g/kg y
la DL50 dérmica para el conejo es > 8 g/kg de peso corporal en
ambos casos. Se observaron lesiones renales extensas (necrosis de las
células de los tubos proximales) en ratas macho después de una sola
inyección intraperitoneal de 100 mg de FTBP/kg de peso corporal.
Estudios de cuatro semanas y de 90 días sobre toxicidad oral del
FTBP (administrado por sonda o en la alimentación) a ratas mostraron
un aumento relacionado con la dosis en la incidencia de nefritis
crónica y su gravedad a niveles de dosis de 25 mg/kg de peso corporal
o más.
En conejos, aplicaciones cutáneas cotidianas de 2,2 g de FTBP/kg
de peso corporal o más durante cuatro semanas ocasionaron cambios
degenerativos en el hígado y los riñones. Todos los conejos murieron
en cuatro semanas. No se registraron muertes en otro estudio con
niveles de dosis de hasta 250 mg/kg de peso corporal.
En una prueba de 90 días en conejos, aplicaciones cutáneas
semanales de 2,27 g/kg de peso corporal dieron lugar a cambios
renales, atrofia testicular y aspermatogénesis.
No se observó irritación cutánea ni ocular en conejos a niveles
de dosis de 1,1 g ó 0,22 g de FTBP y tampoco se observó
sensibilización cutánea en cobayos.
Se realizaron dos estudios sobre teratogenicidad en ratas. En un
estudio, a niveles de dosis de hasta 125 mg/kg de peso corporal no se
observó teratogenicidad. En otro estudio, a un nivel de dosis de
200 mg/kg de peso corporal se observó un aumento significativo en las
variaciones esqueléticas de los fetos, y con 50 y 100 mg/kg de peso
corporal se obtuvo un índice de viabilidad significativamente más
bajo. Los autores llegaron a la conclusión de que el efecto observado
se debía a toxicidad materna.
Se encontraron lesiones extensas del ADN en diversos órganos de
ratas a las que se había administrado FTBP. In vitro, se ha
mostrado que el FTBP induce ruptura de las hebras de ADN en las
células KB humanas. Indujo síntesis imprevista del ADN en hepatocitos
de ratas, pero no en células epiteliales del prepucio en el hombre.
El FTBP resultó mutagénico en varios estudios realizados en
Salmonella typhimurium, especialmente en cepas sensibles a la
sustitución de pares de bases, con y sin activación metabólica.
Las valoraciones de mutación génica anterógrada efectuadas en
células V79 de hámster de China, con y sin activación metabólica,
dieron resultados negativos. Sin embargo, se obtuvo un efecto
positivo en presencia de microsomas del hígado de ratas tratadas
previamente con fenobarbital. Se obtuvo un efecto positivo débil con
células de linfoma de ratón (locus L5178YTK).
El FTBP aumentó el número de intercambios de cromátides hermanas
en células V79 de hámster de China, pero no indujo aberraciones
cromosómicas en células de hámster de China, células de médula ósea de
ratón y células linfoides humanas de cultivo. Se encontraron
intercambios de cromátides hermanas, pero no aberraciones
cromosómicas, en fibroblastos humanos diploides (línea HE 2144) sin
activación metabólica. Sin embargo, en una prueba in vitro sobre
aberración cromosómica con la línea celular de hámster de China, el
FTBP dio resultados positivos.
Se obtuvo un resultado positivo con FTBP en una prueba de
formación de micronúcleos en células de médula ósea de hámster de
China. Otro estudio en ratones sobre formación de micronúcleos mostró
un efecto positivo débil.
Estudios con Drosophila melanogaster mostraron que el FTBP
hacía aumentar el número de efectos recesivos letales ligados al sexo
en células germinales masculinas y en machos adultos y se inducían
traslocaciones recíprocas. El FTBP mostró una respuesta fuertemente
positiva en la valoración de mosaicismo ocular w/w+.
Se han realizado varios estudios para dilucidar los mecanismos de
la mutagenicidad y/o la genotoxicidad inducidas por el FTBP. La
mutagenicidad bacteriana ocasionada por el FTBP está mediada por el
sistema de la monooxigenasa microsómica. El citocromo P450 activa el
FTBP en una reacción que depende del NADPH y del oxígeno. Los
microsomas preparados a partir del hígado de animales tratados con
fenobarbital o con bifenilos policlorados acusaron un aumento de la
mutagenicidad. Los fosfatos de mono(2,3-dibromopropilo) y
bis(2,3-dibromopropilo) son menos mutagénicos que el FTBP. Estudios
in vitro han mostrado que la oxidación en la posición C3 de la
molécula de FTBP produce el potente mutágeno 2-bromoacroleína de
acción directa, que también se enlaza con el ADN.
Se han notificado diferencias entre especies en la bioactivación
del FTBP con transformación en metabolitos mutagénicos para cepas
TA 100 de Salmonella typhimurium. Los microsomas del hígado de
ratones fueron más eficaces que los de cobayos, hámsters y ratas.
Se realizaron tres estudios sobre transformación celular en los
que se utilizaron células C3H/10T1/2. En un estudio se observó un
efecto positivo, pero en los otros dos los resultados fueron
negativos.
Se probó el FTBP en ratones y ratas por administración oral y en
ratones hembra por aplicación cutánea en estudios a largo plazo. En
los ratones, el FTBP administrado por vía oral produjo tumores de
preestómago y pulmón en los animales de ambos sexos, tumores hepáticos
benignos y malignos en las hembras y tumores renales benignos y
malignos en los machos. En ratas, el FTBP produjo tumores renales
benignos y malignos en los machos y tumores renales benignos en las
hembras. Después de la aplicación cutánea a ratones hembra, el FTBP
produjo tumores en la piel, el pulmón, el preestómago y la cavidad
bucal. De esos estudios puede concluirse que el FTBP tiene un
potencial carcinogénico en ratones y ratas.
El fosfato de bis(2,3-dibromilpropilo), un metabolito del FTBP
administrado por vía oral a ratas produjo tumores del sistema
digestivo en ambos sexos. Entre los tumores encontrados había
papilomas y adenocarcinomas de lengua, esófago y preestómago,
adenocarcinomas de intestino, y adenomas y carcinomas
hepato-celulares.
Otro metabolito del FTBP, el 2,3-dibromo-1-propanol, se ensayó en
ratas y ratones por aplicación cutánea. En ratas macho se observó
mayor incidencia de neoplasias de piel, nariz, mucosa bucal, esófago,
preestómago, intestino delgado y grueso, glándula de Zymbal, hígado,
riñón, túnica vaginal y bazo. En ratas hembra se registró mayor
incidencia de neoplasias de piel, nariz, mucosa bucal, esófago,
preestómago, intestino delgado y grueso, glándula de Zymbal, hígado,
riñón, glándula clitorídea y mama. En ratones macho se observó un
aumento de la incidencia de neoplasias de piel, preestómago, hígado y
pulmón, y en ratones hembra, un aumento de la incidencia de neoplasias
de piel y preestómago.
1.1.7 Efectos en el ser humano
Se dispone de datos limitados sobre los efectos del FTBP en el
ser humano.
Se ha ensayado el FTBP para determinar su potencial de
sensibilización cutánea en unos pocos estudios en seres humanos. Los
resultados de éstos indican que el FTBP tiene un bajo potencial de
sensibilización y no ha habido irritación cutánea. Sin embargo, las
personas que mostraron una respuesta positiva de sensibilización al
FTBP puro también reaccionaron cuando se expusieron a tejidos tratados
con FTBP.
1.1.8 Efectos en otros organismos en laboratorio y en el medio
natural
Hay muy pocos datos sobre los efectos del FTBP en otros
organismos. Seis carpas doradas (Carassius auratus) expuestas a
1 mg de FTBP/litro murieron todas a los cinco días.
La CE50 de inhibición del crecimiento en semillas de avena fue
de 1000 mg/kg de suelo. Esta concentración causó una inhibición del
100% del crecimiento en semillas de nabo ( Brassica rapa sp.).
1.2 Conclusiones
El FTBP se ha utilizado como retardador de ignición en tejidos,
en particular en ropa de dormir para niños, pero hay información
insuficiente sobre su utilización para otros fines. La exposición de
la población general se ha efectuado principalmente por contacto con
tejidos tratados con FTBP.
Hay poca información sobre la exposición de los trabajadores y
los riesgos que para éstos entrañan la producción comercial de FTBP y
su utilización en diversos productos.
Debido a la escasez de datos, no pueden sacarse conclusiones
firmes respecto de los niveles de exposición y los riesgos del FTBP
para organismos en el medio ambiente distintos del ser humano.
Estudios en animales han mostrado que el FTBP puede absorberse a
través del tracto gastrointestinal y, en menor medida, de la piel. El
FTBP también puede absorberse a través de la piel en el ser humano.
En la rata, el FTBP parece metabolizarse extensamente en el hígado
convirtiéndose en fosfato de bis(2,3-dibromopropilo), que es el
principal metabolito detectado en la orina, y, en menor medida, en
2,3-dibromopropanol. Además, se han encontrado pequeñas cantidades de
otros metabolitos bromados del FTBP. También se ha detectado
2,3-dibromopropanol en seres humanos que llevaron tejidos tratados con
FTBP. La principal vía de eliminación es la orina y una cantidad muy
pequeña se excreta en la forma del compuesto originario. La principal
vía metabólica parece ser la del metabolismo de las S-transferasas
del citocromo P450 y del glutatión.
Sobre la base de los datos disponibles se puede concluir que el
FTBP tiene una baja toxicidad aguda para animales de experimentación.
Estudios sobre la administración repetida de dosis relativamente
elevadas de FTBP han revelado lesiones renales y hepáticas en ratas y
también toxicidad testicular en conejos. El FTBP ha producido un
claro efecto genotóxico en varios sistemas de prueba, tanto in vitro
como in vivo. Se observaron efectos carcinogénicos en ratas y
ratones. Se ha observado que los metabolitos fosfato de
bis(2,3-dibromopropilo) y 2,3-dibromopropanol también tienen efectos
carcinogénicos en animales de experimentación. No se registraron
efectos irritativos en animales y se observó un bajo potencial de
sensibilización en seres humanos.
En 1977, la Comisión de Seguridad de los Productos de Consumo de
los Estados Unidos de América prohibió las prendas de vestir para
niños tratadas con fosfato de tris(2,3-dibromo-propilo), debido a la
preocupación de que esta sustancia química pudiera ser carcinogénica
para el ser humano y a la posibilidad de una exposición humana
significativa por contacto con los tejidos tratados. Desde entonces,
la utilización de esta sustancia como retardador de ignición en
productos de consumo se ha restringido rigurosamente en varios otros
países y se ha prohibido en los productos textiles.
1.3 Recomendaciones
Debido a sus efectos tóxicos, el FTBP no se debería utilizar ya
comercialmente.
Si se identifican aplicaciones para las cuales no hay
alternativas menos peligrosas que el FTBP, deberían realizarse
estudios para demostrar la ausencia de exposición humana y ambiental y
de riesgos para el ser humano y para el medio ambiente.
2. El fosfato de bis(2,3-dibromopropilo)
La base de datos sobre el fosfato de bis(2,3-dibromopropilo) y
sus sales es insuficiente para hacer una evaluación y para respaldar
su utilización comercial.
Sobre la base de los datos disponibles, hay algunos indicios de
que esta sustancia puede ser mutagénica y carcinogénica.
Esta sustancia no puede evaluarse a menos que llegue a disponerse
de datos adicionales sobre sus propiedades físicas y químicas; su
producción y utilización; su transporte, distribución y transformación
en el medio ambiente; los niveles ambientales y la exposición humana;
su cinética y metabolismo en animales y en seres humanos; sus efectos
en mamíferos de laboratorio, en seres humanos y en sistemas de prueba
in vitro; y sus efectos en otros organismos en el laboratorio y en
el medio natural. También se necesitan más datos sobre mutagenicidad
en relación con dos variables de evaluación por lo menos.
 All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND
BIS(2,3-DIBROMOPROPYL)PHOSPHATE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
United Kingdom
Dr D. Osborn, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Soderlund, National Institute of Public Health, Oslo,
Norway (Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research,
Stockholm University, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and
Aerosol Research, Hannover, Germany
Dr R.D. Kimbrough, Institute for Evaluating Health Risks,
Washington DC, USA (Vice-chairman)
Dr Wai-On Phoon, Department of Occupational Health,
University of Sydney, Sydney, Australia (Chairman)
Dr R. Benson, Drinking Water Branch, US EPA, Denver, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo,
Japan (Rapporteur)
Observers
Dr M.L. Hardy, Toxicology Advisor, Albemarle Corporation,
Baton Rouge, USA
Dr D.L. McAllister, Director, Quality, Environment, Health
and Safety, and Research Support, Great Lakes Chemical
Corporation, West Lafayette, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND BIS(2,3-DIBROMOPROPYL)
PHOSPHATE
A WHO Task Group on Environmental Health Criteria for tris- and
bis(2,3-dibromopropyl) phosphate met at BIBRA Toxicology
International, Carshalton, United Kingdom, from 6 to 11 June 1994.
Dr K.W. Jager, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the
draft and made an evaluation of the risks for human health and the
environment from exposure to tris- and bis(2,3-dibromopropyl)
phosphate.
The first draft was prepared by Dr G.J. van Esch, the
Netherlands, who also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS Contact Points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs M.O. Head of Oxford for
the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
INTRODUCTION
The IPCS is preparing several EHC monographs on Flame Retardants,
which will provide additional information relevant to TBPP.
There will be one monograph, "Flame Retardants - A General
Introduction" (in preparation), giving a general introduction to the
use, the mode of action, and the potential risks of flame retardants,
and listing the substances used as flame retardants with a general
indication of the data available on them.
Flame retardants in wide use are discussed in separate
monographs, e.g., EHC 162: Brominated Diphenyl Ethers (IPCS, 1994a)
and EHC 172: Tetrabromobisphenol-A (IPCS, 1995).
Some flame retardants considered hazardous for humans and the
environment have been reviewed in separate monographs including EHC
152: Polybrominated Biphenyls (IPCS, 1994b), and EHC 173: Tris- and
Bis(2,3-dibromopropyl) phosphate (this monograph).
Because of the possibility of the formation of halogenated
dibenzodioxins and dibenzofurans under certain circumstances, such as
pyrolysis, the following monographs have been developed: EHC 88:
Polychlorinated Dibenzodioxins and Dibenzofurans (IPCS, 1989) and
Polybrominated Dibenzodioxins and Dibenzofurans (in preparation).
The reader should consult these monographs for further
information.
Tris(2,3-dibromopropyl) phosphate was an important commercial
flame retardant ("TRIS"), especially for children's sleepwear. In
1977, the US Consumer Product Safety Commission banned children's
clothing treated with tris(2,3-dibromopropyl) phosphate. Since then,
in several other countries, the use of this compound as a flame
retardant has been severely restricted in consumer products and
prohibited in textiles.
Because tris(2,3-dibromopropyl) phosphate can also be used for
other applications, the information available on physical and chemical
properties, behaviour in the environment, occurrence in the
environment and humans, kinetics and metabolism, toxicity for
laboratory animals and in the field, and the exposure of the general
population and workers, is summarized in this Environmental Health
Criteria monograph. General properties and uses of brominated flame
retardants are given in "Flame Retardants - A General Introduction"
(in preparation).
ABBREVIATIONS
BA 2-bromoacrolein
BBPP bis(2,3-dibromopropyl) phosphate
DBCP 1,2-dibromo-3-chloropropane
DBP 2,3-dibromopropanol
DMBA dimethylbenzanthracene
mono-BPP mono(2,3-dibromopropyl) phosphate
TBPP tris(2,3-dibromopropyl) phosphate
TPA tetradecanoyl phorbolacetate
TRIS-(2,3-DIBROMOPROPYL) PHOSPHATE
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Production and use
Tris(2,3-dibromopropyl) phosphate (TBPP) was first produced in
about 1950; commercial production was reported in 1959. Production of
TBPP, in the USA, in 1975, was estimated to be between 4100 and
5400 tonnes. As far as is known, TBPP is not produced or used
currently in the world as a flame retardant in textiles, but may be
added to polymers used for other purposes. TBPP was an important
flame retardant for cellulose and tri-acetate and polyester fabrics,
especially in children's sleepwear, but has been banned for these
applications in several countries in Europe, the USA (1977), and Japan
(1978).
TBPP exists both in, and on, the fabric. When it is in the
fabric, it is not extractable with solvents and, therefore, probably
not available for dermal absorption. However, when it is on the fibre
surface, it can be extracted during laundering, and by acetic acid,
other solvents, and saliva, and is available for dermal absorption. In
this case, substantial losses of surface TBPP from fabrics during use
and/or laundering of the finished products, will occur, and will
contaminate the environment. Furthermore, release of TBPP into the
environment has been reported from textile-finishing plants and the
ultimate disposal of solid wastes, containing TBPP.
1.1.2 Physical and chemical properties
TBPP is available in at least two grades. The high-purity grade
is a clear, pale-yellow, viscous liquid, with up to 1.5% volatiles.
The low-purity grade may contain up to 10% volatiles.
TBPP (purity > 97%), has a boiling point of 390°C, a melting
point of 5.5°C, and a vapour pressure of 1.9 × 10-4mmHg at 25°C.
The solubility of TBPP in water is low (8 mg/litre).
When heated to decomposition, above 260-300°C, TBPP emits
compounds containing bromine and phosphorus. The n-octanol/water
partition coefficient (log Pow) is 3.02.
Analytical methods to determine TBPP and its metabolites in
biological samples and other matrices are available.
1.1.3 Environmental transport, distribution, and transformation
The limited information available suggests that TBPP is
relatively persistent in the environment. Oxidation and
photodegradation are not likely to be significant fate processes.
However, hydrolysis involving the bromine atoms on the propyl group
may occur, especially under basic conditions. Volatilization from
water may occur, but no actual data are available. Although
biodegradation of TBPP (half-life 19.7 h) in activated sewage is
reported to occur, it is not thought to be an important process in
natural soils and waters. In sterilized sludge, almost no breakdown
takes place. Bis(2,3-dibromopropyl) phosphate (BBPP) was found as a
major breakdown product. Because TBPP is virtually insoluble in
water, adsorption on particulate matter and sediment may be an
important process.
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water
solubility, TBPP is expected to leach only slowly to groundwater. TBPP
will tend to accumulate in rubbish dumps and other disposal sites,
which may result in biological accumulation. A bioaccumulation study
with fathead minnow showed a bioconcentration factor of 2.7, which is
low, while the n-octanol/water partition coefficient (Log Pow) was
3.02. Because of its low vapour pressure, TBPP is expected to be
mostly sorbed on particulate matter in air. Thermal oxidative
degradation of TBPP at 370°C showed that hydrogen bromide and
C3-brominated compounds, such as bromopropenes, dibromopropenes, and
diand tribromopropanes, are formed.
1.1.4 Environmental levels and human exposure
Data on environmental levels and human exposure are limited.
Studies carried out in Japan in 1975 showed that 20 samples of water,
soil, and fish did not contain TBPP. TBPP was identified, but not
quantified, in air particulates in the surroundings of an industry.
Children wearing TBPP-treated sleepwear were the group of the
general population particularly exposed to this flame retardant. The
estimated intake via the skin of children wearing TBPPtreated
sleepwear in the USA was calculated to be 9 µg/kg body weight per day.
The Consumer Product Safety Commission of the USA calculated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight or more.
1.1.5 Kinetics and metabolism in laboratory animals and humans
TBPP is absorbed readily from the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. In rats, TBPP or its
metabolites are eliminated within 5 days. Approximately 50% is
eliminated via the urine, 10% via the faeces, and 10-20% is exhaled as
CO2.
One day after oral administration of labelled TBPP to rats,
radioactivity was found in the blood, liver, kidneys, muscles, fat,
and skin, in a range of 0.2-6.6%. The half-life of clearance of
radioactivity from these organs was approximately 2-4 days. After 8
h, only bis(2,3-dibromopropyl) (BBPP) phosphate was still present in
substantial concentrations in most tissues.
After oral administration of TBPP to rats, six metabolites
were identified in the urine and bile. The main metabolite in
the urine, faeces, bile, and tissues was BBPP. The metabolite
2,3-dibromopropanol (DBP) was also identified in rats and in children
wearing TBPP-treated clothing.
Liver microsomes metabolize TBPP in the presence of NADPH and
oxygen. The main metabolites are BBPP and 2,3-dibromopropanol (DBP).
It has been shown that BBPP is formed by oxidation at the C3 and,
possibly, also at the C2 position of TBPP. In addition to BBPP,
2-bromoacrolein, 2-bromoacrylic acid, and propyl-hydroxylated
compounds and metabolites conjugated with glutathione have been found.
S-(2,3-dihydroxypropyl) glutathione was identified in the bile
of rats, and, it was suggested that TBPP and/or BBPP are conjugated
directly with glutathione by glutathione S-transferase with the
formation of episulfonium ion metabolites.
TBPP has been shown to be activated to form products that bind
covalently to proteins and DNA in vivo and in vitro. After
intraperitoneal injections of tritiated-TBPP, male mice, hamsters, and
guinea-pigs, which are less sensitive to TBPP-induced nephrotoxicity
than rats, showed similar levels of covalent binding to proteins in
the liver and kidneys. In the male rat, which is highly susceptible to
TBPP-induced nephrotoxicity, much higher amounts of radiolabel were
bound to kidney proteins than to liver proteins.
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to genotoxic intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from the rodents.
The binding of labelled TBPP and analogues in rats at a
nephrotoxic dose showed that the covalent protein binding was highest
in the kidneys followed by the liver and testes. The results of
comparative in vitro and in vivo studies on renal DNA damage
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C2 or C3 of TBPP. BBPP is transported to the kidneys, where
it is metabolized to reactive intermediates that cause DNA damage and
bind to kidney proteins. The activation occurring in the kidney
appears not to involve P450 but seems to be mediated by GSH-dependent
metabolism. In vitro studies with labelled TBPP and analogues
showed that oxidation of TBPP incorporates one atom of oxygen from
water. This implies that oxidation at C2 of the propyl moiety yields
a reactive alphabromoketone that can alkylate protein directly or
hydrolyse to BBPP and a reactive bromo-alpha-hydroxyketone.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute and short-term oral, and the acute dermal, toxicities
of TBPP are low. The oral LD50 for the rat > 2 g/kg and the dermal
LD50 for the rabbit > 8 g/kg body weight. Extensive kidney damage
(necrosis of renal proximal tubular cells) was noted in male rats
following a single ip injection of 100 mg TBPP/kg body weight.
Four-week, and 90-day, oral toxicity tests with TBPP (by gavage
or in the diet) in rats showed a dose-related increase in the
incidence and severity of chronic nephritis at dose levels of 25 mg/kg
body weight or more.
In rabbits, daily dermal applications of 2.2 g TBPP/kg body
weight or more, for 4 weeks, resulted in degenerative changes in the
liver and kidneys. All rabbits died within four weeks. No deaths
occurred in another study with dose levels of up to 250 mg/kg body
weight.
In a 90-day test on rabbits, weekly application of 2.27 g/kg body
weight to the skin resulted in kidney changes, testicular atrophy, and
aspermatogenesis.
No skin or eye irritation was observed in rabbits with dose
levels of 1.1 g or 0.22 g TBPP and no skin sensitization was observed
in guinea-pigs.
Two teratogenicity studies were carried out on rats. In one
study with dose levels of up to 125 mg/kg body weight, no
teratogenicity was observed. In another study with a dose level of
200 mg/kg body weight, a significant increase in skeletal variations
in the fetuses was observed, and, with 50 and 100 mg/kg body weight, a
significantly lower viability index was found. The authors concluded
that the observed effect resulted from maternal toxicity.
Extensive DNA damage was found in various organs of rats
administered TBPP. In vitro, TBPP has been shown to induce DNA
strand breaks in human KB cells. It induced unscheduled DNA synthesis
in rat liver hepatocytes, but not in human foreskin epithelial cells.
TBPP was mutagenic in several studies on Salmonella typhimurium,
especially in base-pair substituting strains with, and without,
metabolic activation.
Forward gene mutation assays using Chinese hamster V79 cells,
with, and without, metabolic activation were negative. However, a
positive effect in the presence of liver microsomes of rats pretreated
with phenobarbital was obtained. A weak positive effect was obtained
with mouse lymphoma cells (L5178YTK locus).
TBPP increased the number of sister chromatid exchanges (SCEs) in
Chinese hamster V 79 cells, but no chromosomal aberrations were
induced in Chinese hamster cells, mouse bone marrow cells, or in
cultured human lymphoid cells. SCEs but no chromosomal aberrations
were found with diploid human fibroblastic cells (line HE 2144)
without metabolic activation. However, in an in vitro chromosome
aberration test with the Chinese hamster cell line (CHL), TBPP was
positive.
A positive result was obtained with TBPP in a micronucleus test
on Chinese hamster bone marrow cells. Another micronucleus study with
mice showed a weak positive effect.
Studies with Drosophila melanogaster showed that TBPP increased
sex-linked recessive lethals in male germ cells and in adult males,
reciprocal translocations were induced. TBPP showed a strong positive
response in the w/w+ eye mosaic assay.
Several studies have been directed towards the elucidation of the
mechanisms involved in TBPP-induced mutagenicity and/or genotoxicity.
Bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. TBPP is activated by cytochrome P450 in a
reaction depending on NADPH and oxygen. Microsomes prepared from
livers of animals treated with phenobarbital or PCBs give increased
mutagenicity. The mono-and bis(2,3-dibromopropyl) phosphates are less
mutagenic than TBPP. In vitro studies have shown that oxidation at
C3 of the TBPP molecule yields the potent direct acting mutagen
2bromoacrolein that also binds to DNA.
Species differences in the bioactivation of TBPP to metabolites
mutagenic to Salmonella typhimurium TA 100 have been reported. Liver
microsomes from mice were more effective than those from guinea-pigs,
hamsters, and rats.
Three studies in which C3H/10T1/2 cells were used to study cell
transformation were carried out. In one study, a positive effect was
noted, but, in the other two studies, the results were negative.
TBPP was tested on mice and rats by oral administration and on
female mice by skin application in long-term studies. In mice,
following oral administration, TBPP produced tumours of the
fore-stomach and lung in the animals of both sexes, benign and
malignant liver tumours in females, and benign and malignant tumours
of the kidneys in males. In rats, TBPP produced benign and malignant
tumours of the kidneys in males and benign kidney tumours in females.
After skin application to female mice, TBPP produced tumours of the
skin, lung, fore-stomach, and oral cavity. From these studies, it can
be concluded that TBPP has carcinogenic potential in mice and rats.
When the TBPP metabolite BBPP was administered to rats orally, it
caused tumours in both sexes in the digestive system. The tumours
found included papillomas and adenocarcinomas of the tongue,
oesophagus, and forestomach, adenocarcinomas of the intestine, and
hepatocellular adenomas and carcinomas.
Another metabolite of TBPP, DBP, was tested on rats and mice by
dermal application. In male rats, there was an increased incidence of
neoplasms in skin, nose, oral mucosa, oesophagus, forestomach, small
and large intestine, Zymbal's gland, liver, kidney, tunica vaginalis,
and spleen. In female rats, there was an increased incidence of
neoplasms of the skin, nose, oral mucosa, oesophagus, forestomach,
small and large intestine, Zymbal's gland, liver, kidney, clitoral
gland, and mammary gland. In male mice, there was an increased
incidence of neoplasms in the skin, forestomach, liver, and lung, and
in female mice, there was an increased incidence of neoplasms of the
skin and the forestomach.
1.1.7 Effects on humans
Limited data are available regarding the effects of TBPP on
humans.
TBPP has been tested for skin sensitization potential in a few
studies on humans. The results of these studies indicate that TBPP
has a low sensitization potential and no skin irritation was reported.
However, persons who showed a positive sensitization response to pure
TBPP also reacted when exposed to fabrics
treated with TBPP.
1.1.8 Effects on other organisms in the laboratory and field
There are very few data on the effects of TBPP on other
organisms. All 6 goldfish (Carassius auratus), exposed to 1 mg
TBPP/litre, died within 5 days.
The EC50 for growth inhibition in oat seed was 1000 mg/kg soil.
This concentration caused a 100% inhibition of growth in turnip seed
(Brassica rapa sp.).
1.2 Conclusions
TBPP has been used as a flame retardant in fabrics, particularly
in children's sleepwear, but there is inadequate information on its
use in other applications. Exposure of the general population was
primarily through contact with fabrics treated with TBPP.
There is little information on the exposure of, and hazards to,
workers from the commercial production of TBPP and its use in a
variety of products.
Because of the paucity of data, no firm conclusions can be drawn
as to the exposure levels and hazards of TBPP for organisms in the
environment, other than humans.
Animal studies have shown that TBPP can be absorbed from the
gastrointestinal tract and, to a lesser extent, from the skin. TBPP
can also be absorbed through the skin of humans. In the rat, TBPP
appears to be extensively metabolized in the liver to BBPP, which is
the major metabolite detected in the urine and, to a lesser extent, to
DBP. In addition, other brominated metabolites of TBPP have been
found in small amounts. DBP has also been detected in humans wearing
TBPP-treated fabrics. The main route of elimination is the urine and
very little is excreted as the parent compound. The main metabolic
pathway seems to be through metabolism by cytochrome P450 and
glutathione S-transferases.
From the available data, it can be concluded that TBPP has a low
acute toxicity for experimental animals. Repeated dose studies with
relatively high doses of TBPP have revealed kidney and liver damage in
rats and also testicular toxicity in rabbits. TBPP has elicited a
clear genotoxic effect in several test systems, both in vitro and
in vivo. Carcinogenic effects were found in rats and mice. The
metabolites BBPP and DBP have also been shown to produce carcinogenic
effects in experimental animals. No irritation effects were found in
animals and a low sensitization potential in humans was noted.
In 1977, the US Consumer Product Safety Commission banned
children's clothing treated with TBPP, because of concerns that the
chemical might be a human carcinogen, and, because of the possibility
of significant human exposure through contact with treated fabrics.
Since then, the use of this substance as a flame retardant in consumer
products has been severely restricted in several other countries and
it has been prohibited in textiles.
1.3 Recommendations
Because of its toxic effects, TBPP should no longer be used
commercially.
If uses are identified for which there are no less hazardous
alternatives to TBPP, studies to demonstrate the absence of exposure
of, and hazards for, humans and the environment should be conducted.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Chemical formula C9H15Br6O4P
Chemical structure
BrCH2-CHBr-CH2O
\
BrCH2-CHBr-CH2O - P = O
/
BrCH2-CHBr-CH2O
Relative molecular mass 697.7
Synonyms tris(2,3-dibromopropyl) phosphate;
tris(2,3-dibromopropyl) phosphoric
acid ester; phosphoric acid, tris(2,3-
dibromo-propyl) ester;
tris(dibromopropyl) phosphate
CAS registry number
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND
BIS(2,3-DIBROMOPROPYL)PHOSPHATE
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
United Kingdom
Dr D. Osborn, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Soderlund, National Institute of Public Health, Oslo,
Norway (Rapporteur)
Dr B. Jansson, Institute of Applied Environmental Research,
Stockholm University, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and
Aerosol Research, Hannover, Germany
Dr R.D. Kimbrough, Institute for Evaluating Health Risks,
Washington DC, USA (Vice-chairman)
Dr Wai-On Phoon, Department of Occupational Health,
University of Sydney, Sydney, Australia (Chairman)
Dr R. Benson, Drinking Water Branch, US EPA, Denver, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo,
Japan (Rapporteur)
Observers
Dr M.L. Hardy, Toxicology Advisor, Albemarle Corporation,
Baton Rouge, USA
Dr D.L. McAllister, Director, Quality, Environment, Health
and Safety, and Research Support, Great Lakes Chemical
Corporation, West Lafayette, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR TRIS- AND BIS(2,3-DIBROMOPROPYL)
PHOSPHATE
A WHO Task Group on Environmental Health Criteria for tris- and
bis(2,3-dibromopropyl) phosphate met at BIBRA Toxicology
International, Carshalton, United Kingdom, from 6 to 11 June 1994.
Dr K.W. Jager, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the
draft and made an evaluation of the risks for human health and the
environment from exposure to tris- and bis(2,3-dibromopropyl)
phosphate.
The first draft was prepared by Dr G.J. van Esch, the
Netherlands, who also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS Contact Points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs M.O. Head of Oxford for
the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
INTRODUCTION
The IPCS is preparing several EHC monographs on Flame Retardants,
which will provide additional information relevant to TBPP.
There will be one monograph, "Flame Retardants - A General
Introduction" (in preparation), giving a general introduction to the
use, the mode of action, and the potential risks of flame retardants,
and listing the substances used as flame retardants with a general
indication of the data available on them.
Flame retardants in wide use are discussed in separate
monographs, e.g., EHC 162: Brominated Diphenyl Ethers (IPCS, 1994a)
and EHC 172: Tetrabromobisphenol-A (IPCS, 1995).
Some flame retardants considered hazardous for humans and the
environment have been reviewed in separate monographs including EHC
152: Polybrominated Biphenyls (IPCS, 1994b), and EHC 173: Tris- and
Bis(2,3-dibromopropyl) phosphate (this monograph).
Because of the possibility of the formation of halogenated
dibenzodioxins and dibenzofurans under certain circumstances, such as
pyrolysis, the following monographs have been developed: EHC 88:
Polychlorinated Dibenzodioxins and Dibenzofurans (IPCS, 1989) and
Polybrominated Dibenzodioxins and Dibenzofurans (in preparation).
The reader should consult these monographs for further
information.
Tris(2,3-dibromopropyl) phosphate was an important commercial
flame retardant ("TRIS"), especially for children's sleepwear. In
1977, the US Consumer Product Safety Commission banned children's
clothing treated with tris(2,3-dibromopropyl) phosphate. Since then,
in several other countries, the use of this compound as a flame
retardant has been severely restricted in consumer products and
prohibited in textiles.
Because tris(2,3-dibromopropyl) phosphate can also be used for
other applications, the information available on physical and chemical
properties, behaviour in the environment, occurrence in the
environment and humans, kinetics and metabolism, toxicity for
laboratory animals and in the field, and the exposure of the general
population and workers, is summarized in this Environmental Health
Criteria monograph. General properties and uses of brominated flame
retardants are given in "Flame Retardants - A General Introduction"
(in preparation).
ABBREVIATIONS
BA 2-bromoacrolein
BBPP bis(2,3-dibromopropyl) phosphate
DBCP 1,2-dibromo-3-chloropropane
DBP 2,3-dibromopropanol
DMBA dimethylbenzanthracene
mono-BPP mono(2,3-dibromopropyl) phosphate
TBPP tris(2,3-dibromopropyl) phosphate
TPA tetradecanoyl phorbolacetate
TRIS-(2,3-DIBROMOPROPYL) PHOSPHATE
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Production and use
Tris(2,3-dibromopropyl) phosphate (TBPP) was first produced in
about 1950; commercial production was reported in 1959. Production of
TBPP, in the USA, in 1975, was estimated to be between 4100 and
5400 tonnes. As far as is known, TBPP is not produced or used
currently in the world as a flame retardant in textiles, but may be
added to polymers used for other purposes. TBPP was an important
flame retardant for cellulose and tri-acetate and polyester fabrics,
especially in children's sleepwear, but has been banned for these
applications in several countries in Europe, the USA (1977), and Japan
(1978).
TBPP exists both in, and on, the fabric. When it is in the
fabric, it is not extractable with solvents and, therefore, probably
not available for dermal absorption. However, when it is on the fibre
surface, it can be extracted during laundering, and by acetic acid,
other solvents, and saliva, and is available for dermal absorption. In
this case, substantial losses of surface TBPP from fabrics during use
and/or laundering of the finished products, will occur, and will
contaminate the environment. Furthermore, release of TBPP into the
environment has been reported from textile-finishing plants and the
ultimate disposal of solid wastes, containing TBPP.
1.1.2 Physical and chemical properties
TBPP is available in at least two grades. The high-purity grade
is a clear, pale-yellow, viscous liquid, with up to 1.5% volatiles.
The low-purity grade may contain up to 10% volatiles.
TBPP (purity > 97%), has a boiling point of 390°C, a melting
point of 5.5°C, and a vapour pressure of 1.9 × 10-4mmHg at 25°C.
The solubility of TBPP in water is low (8 mg/litre).
When heated to decomposition, above 260-300°C, TBPP emits
compounds containing bromine and phosphorus. The n-octanol/water
partition coefficient (log Pow) is 3.02.
Analytical methods to determine TBPP and its metabolites in
biological samples and other matrices are available.
1.1.3 Environmental transport, distribution, and transformation
The limited information available suggests that TBPP is
relatively persistent in the environment. Oxidation and
photodegradation are not likely to be significant fate processes.
However, hydrolysis involving the bromine atoms on the propyl group
may occur, especially under basic conditions. Volatilization from
water may occur, but no actual data are available. Although
biodegradation of TBPP (half-life 19.7 h) in activated sewage is
reported to occur, it is not thought to be an important process in
natural soils and waters. In sterilized sludge, almost no breakdown
takes place. Bis(2,3-dibromopropyl) phosphate (BBPP) was found as a
major breakdown product. Because TBPP is virtually insoluble in
water, adsorption on particulate matter and sediment may be an
important process.
An estimated log Koc (3.29) suggests strong adsorption on soil.
On the basis of this Koc value and the low measured water
solubility, TBPP is expected to leach only slowly to groundwater. TBPP
will tend to accumulate in rubbish dumps and other disposal sites,
which may result in biological accumulation. A bioaccumulation study
with fathead minnow showed a bioconcentration factor of 2.7, which is
low, while the n-octanol/water partition coefficient (Log Pow) was
3.02. Because of its low vapour pressure, TBPP is expected to be
mostly sorbed on particulate matter in air. Thermal oxidative
degradation of TBPP at 370°C showed that hydrogen bromide and
C3-brominated compounds, such as bromopropenes, dibromopropenes, and
diand tribromopropanes, are formed.
1.1.4 Environmental levels and human exposure
Data on environmental levels and human exposure are limited.
Studies carried out in Japan in 1975 showed that 20 samples of water,
soil, and fish did not contain TBPP. TBPP was identified, but not
quantified, in air particulates in the surroundings of an industry.
Children wearing TBPP-treated sleepwear were the group of the
general population particularly exposed to this flame retardant. The
estimated intake via the skin of children wearing TBPPtreated
sleepwear in the USA was calculated to be 9 µg/kg body weight per day.
The Consumer Product Safety Commission of the USA calculated that,
over a 6-year period, a child wearing TBPP-treated clothing could
absorb a total of 2-77 mg TBPP/kg body weight or more.
1.1.5 Kinetics and metabolism in laboratory animals and humans
TBPP is absorbed readily from the gastrointestinal tract and at a
moderate rate via the skin in rats and rabbits. In rats, TBPP or its
metabolites are eliminated within 5 days. Approximately 50% is
eliminated via the urine, 10% via the faeces, and 10-20% is exhaled as
CO2.
One day after oral administration of labelled TBPP to rats,
radioactivity was found in the blood, liver, kidneys, muscles, fat,
and skin, in a range of 0.2-6.6%. The half-life of clearance of
radioactivity from these organs was approximately 2-4 days. After 8
h, only bis(2,3-dibromopropyl) (BBPP) phosphate was still present in
substantial concentrations in most tissues.
After oral administration of TBPP to rats, six metabolites
were identified in the urine and bile. The main metabolite in
the urine, faeces, bile, and tissues was BBPP. The metabolite
2,3-dibromopropanol (DBP) was also identified in rats and in children
wearing TBPP-treated clothing.
Liver microsomes metabolize TBPP in the presence of NADPH and
oxygen. The main metabolites are BBPP and 2,3-dibromopropanol (DBP).
It has been shown that BBPP is formed by oxidation at the C3 and,
possibly, also at the C2 position of TBPP. In addition to BBPP,
2-bromoacrolein, 2-bromoacrylic acid, and propyl-hydroxylated
compounds and metabolites conjugated with glutathione have been found.
S-(2,3-dihydroxypropyl) glutathione was identified in the bile
of rats, and, it was suggested that TBPP and/or BBPP are conjugated
directly with glutathione by glutathione S-transferase with the
formation of episulfonium ion metabolites.
TBPP has been shown to be activated to form products that bind
covalently to proteins and DNA in vivo and in vitro. After
intraperitoneal injections of tritiated-TBPP, male mice, hamsters, and
guinea-pigs, which are less sensitive to TBPP-induced nephrotoxicity
than rats, showed similar levels of covalent binding to proteins in
the liver and kidneys. In the male rat, which is highly susceptible to
TBPP-induced nephrotoxicity, much higher amounts of radiolabel were
bound to kidney proteins than to liver proteins.
Liver microsomes from mice, guinea-pigs, hamsters, and humans all
metabolized TBPP to genotoxic intermediates. However, the rate of
formation of reactive TBPP metabolites with human liver microsomes was
lower than with liver microsomes from the rodents.
The binding of labelled TBPP and analogues in rats at a
nephrotoxic dose showed that the covalent protein binding was highest
in the kidneys followed by the liver and testes. The results of
comparative in vitro and in vivo studies on renal DNA damage
suggested that BBPP is formed in the liver by P450-mediated oxidation
at either C2 or C3 of TBPP. BBPP is transported to the kidneys, where
it is metabolized to reactive intermediates that cause DNA damage and
bind to kidney proteins. The activation occurring in the kidney
appears not to involve P450 but seems to be mediated by GSH-dependent
metabolism. In vitro studies with labelled TBPP and analogues
showed that oxidation of TBPP incorporates one atom of oxygen from
water. This implies that oxidation at C2 of the propyl moiety yields
a reactive alphabromoketone that can alkylate protein directly or
hydrolyse to BBPP and a reactive bromo-alpha-hydroxyketone.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute and short-term oral, and the acute dermal, toxicities
of TBPP are low. The oral LD50 for the rat > 2 g/kg and the dermal
LD50 for the rabbit > 8 g/kg body weight. Extensive kidney damage
(necrosis of renal proximal tubular cells) was noted in male rats
following a single ip injection of 100 mg TBPP/kg body weight.
Four-week, and 90-day, oral toxicity tests with TBPP (by gavage
or in the diet) in rats showed a dose-related increase in the
incidence and severity of chronic nephritis at dose levels of 25 mg/kg
body weight or more.
In rabbits, daily dermal applications of 2.2 g TBPP/kg body
weight or more, for 4 weeks, resulted in degenerative changes in the
liver and kidneys. All rabbits died within four weeks. No deaths
occurred in another study with dose levels of up to 250 mg/kg body
weight.
In a 90-day test on rabbits, weekly application of 2.27 g/kg body
weight to the skin resulted in kidney changes, testicular atrophy, and
aspermatogenesis.
No skin or eye irritation was observed in rabbits with dose
levels of 1.1 g or 0.22 g TBPP and no skin sensitization was observed
in guinea-pigs.
Two teratogenicity studies were carried out on rats. In one
study with dose levels of up to 125 mg/kg body weight, no
teratogenicity was observed. In another study with a dose level of
200 mg/kg body weight, a significant increase in skeletal variations
in the fetuses was observed, and, with 50 and 100 mg/kg body weight, a
significantly lower viability index was found. The authors concluded
that the observed effect resulted from maternal toxicity.
Extensive DNA damage was found in various organs of rats
administered TBPP. In vitro, TBPP has been shown to induce DNA
strand breaks in human KB cells. It induced unscheduled DNA synthesis
in rat liver hepatocytes, but not in human foreskin epithelial cells.
TBPP was mutagenic in several studies on Salmonella typhimurium,
especially in base-pair substituting strains with, and without,
metabolic activation.
Forward gene mutation assays using Chinese hamster V79 cells,
with, and without, metabolic activation were negative. However, a
positive effect in the presence of liver microsomes of rats pretreated
with phenobarbital was obtained. A weak positive effect was obtained
with mouse lymphoma cells (L5178YTK locus).
TBPP increased the number of sister chromatid exchanges (SCEs) in
Chinese hamster V 79 cells, but no chromosomal aberrations were
induced in Chinese hamster cells, mouse bone marrow cells, or in
cultured human lymphoid cells. SCEs but no chromosomal aberrations
were found with diploid human fibroblastic cells (line HE 2144)
without metabolic activation. However, in an in vitro chromosome
aberration test with the Chinese hamster cell line (CHL), TBPP was
positive.
A positive result was obtained with TBPP in a micronucleus test
on Chinese hamster bone marrow cells. Another micronucleus study with
mice showed a weak positive effect.
Studies with Drosophila melanogaster showed that TBPP increased
sex-linked recessive lethals in male germ cells and in adult males,
reciprocal translocations were induced. TBPP showed a strong positive
response in the w/w+ eye mosaic assay.
Several studies have been directed towards the elucidation of the
mechanisms involved in TBPP-induced mutagenicity and/or genotoxicity.
Bacterial mutagenicity of TBPP is mediated by the microsomal
monooxygenase system. TBPP is activated by cytochrome P450 in a
reaction depending on NADPH and oxygen. Microsomes prepared from
livers of animals treated with phenobarbital or PCBs give increased
mutagenicity. The mono-and bis(2,3-dibromopropyl) phosphates are less
mutagenic than TBPP. In vitro studies have shown that oxidation at
C3 of the TBPP molecule yields the potent direct acting mutagen
2bromoacrolein that also binds to DNA.
Species differences in the bioactivation of TBPP to metabolites
mutagenic to Salmonella typhimurium TA 100 have been reported. Liver
microsomes from mice were more effective than those from guinea-pigs,
hamsters, and rats.
Three studies in which C3H/10T1/2 cells were used to study cell
transformation were carried out. In one study, a positive effect was
noted, but, in the other two studies, the results were negative.
TBPP was tested on mice and rats by oral administration and on
female mice by skin application in long-term studies. In mice,
following oral administration, TBPP produced tumours of the
fore-stomach and lung in the animals of both sexes, benign and
malignant liver tumours in females, and benign and malignant tumours
of the kidneys in males. In rats, TBPP produced benign and malignant
tumours of the kidneys in males and benign kidney tumours in females.
After skin application to female mice, TBPP produced tumours of the
skin, lung, fore-stomach, and oral cavity. From these studies, it can
be concluded that TBPP has carcinogenic potential in mice and rats.
When the TBPP metabolite BBPP was administered to rats orally, it
caused tumours in both sexes in the digestive system. The tumours
found included papillomas and adenocarcinomas of the tongue,
oesophagus, and forestomach, adenocarcinomas of the intestine, and
hepatocellular adenomas and carcinomas.
Another metabolite of TBPP, DBP, was tested on rats and mice by
dermal application. In male rats, there was an increased incidence of
neoplasms in skin, nose, oral mucosa, oesophagus, forestomach, small
and large intestine, Zymbal's gland, liver, kidney, tunica vaginalis,
and spleen. In female rats, there was an increased incidence of
neoplasms of the skin, nose, oral mucosa, oesophagus, forestomach,
small and large intestine, Zymbal's gland, liver, kidney, clitoral
gland, and mammary gland. In male mice, there was an increased
incidence of neoplasms in the skin, forestomach, liver, and lung, and
in female mice, there was an increased incidence of neoplasms of the
skin and the forestomach.
1.1.7 Effects on humans
Limited data are available regarding the effects of TBPP on
humans.
TBPP has been tested for skin sensitization potential in a few
studies on humans. The results of these studies indicate that TBPP
has a low sensitization potential and no skin irritation was reported.
However, persons who showed a positive sensitization response to pure
TBPP also reacted when exposed to fabrics
treated with TBPP.
1.1.8 Effects on other organisms in the laboratory and field
There are very few data on the effects of TBPP on other
organisms. All 6 goldfish (Carassius auratus), exposed to 1 mg
TBPP/litre, died within 5 days.
The EC50 for growth inhibition in oat seed was 1000 mg/kg soil.
This concentration caused a 100% inhibition of growth in turnip seed
(Brassica rapa sp.).
1.2 Conclusions
TBPP has been used as a flame retardant in fabrics, particularly
in children's sleepwear, but there is inadequate information on its
use in other applications. Exposure of the general population was
primarily through contact with fabrics treated with TBPP.
There is little information on the exposure of, and hazards to,
workers from the commercial production of TBPP and its use in a
variety of products.
Because of the paucity of data, no firm conclusions can be drawn
as to the exposure levels and hazards of TBPP for organisms in the
environment, other than humans.
Animal studies have shown that TBPP can be absorbed from the
gastrointestinal tract and, to a lesser extent, from the skin. TBPP
can also be absorbed through the skin of humans. In the rat, TBPP
appears to be extensively metabolized in the liver to BBPP, which is
the major metabolite detected in the urine and, to a lesser extent, to
DBP. In addition, other brominated metabolites of TBPP have been
found in small amounts. DBP has also been detected in humans wearing
TBPP-treated fabrics. The main route of elimination is the urine and
very little is excreted as the parent compound. The main metabolic
pathway seems to be through metabolism by cytochrome P450 and
glutathione S-transferases.
From the available data, it can be concluded that TBPP has a low
acute toxicity for experimental animals. Repeated dose studies with
relatively high doses of TBPP have revealed kidney and liver damage in
rats and also testicular toxicity in rabbits. TBPP has elicited a
clear genotoxic effect in several test systems, both in vitro and
in vivo. Carcinogenic effects were found in rats and mice. The
metabolites BBPP and DBP have also been shown to produce carcinogenic
effects in experimental animals. No irritation effects were found in
animals and a low sensitization potential in humans was noted.
In 1977, the US Consumer Product Safety Commission banned
children's clothing treated with TBPP, because of concerns that the
chemical might be a human carcinogen, and, because of the possibility
of significant human exposure through contact with treated fabrics.
Since then, the use of this substance as a flame retardant in consumer
products has been severely restricted in several other countries and
it has been prohibited in textiles.
1.3 Recommendations
Because of its toxic effects, TBPP should no longer be used
commercially.
If uses are identified for which there are no less hazardous
alternatives to TBPP, studies to demonstrate the absence of exposure
of, and hazards for, humans and the environment should be conducted.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Chemical formula C9H15Br6O4P
Chemical structure
BrCH2-CHBr-CH2O
\
BrCH2-CHBr-CH2O - P = O
/
BrCH2-CHBr-CH2O
Relative molecular mass 697.7
Synonyms tris(2,3-dibromopropyl) phosphate;
tris(2,3-dibromopropyl) phosphoric
acid ester; phosphoric acid, tris(2,3-
dibromo-propyl) ester;
tris(dibromopropyl) phosphate
CAS registry number 