
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 191
Acrylic Acid
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared at the National Institute of Health Sciences,
Tokyo, Japan, and the Institute of Terrestrial Ecology, Monk's Wood,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Acrylic Acid
(Environmental health criteria ; 191)
1.Acrylates - adverse affects 2.Acrylates - toxicity
3.Environmental exposure 4.Occupational exposure
I.Series
ISBN 92 4 157191 8 (NLM Classification: QV 50)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
PREAMBLE
ABBREVIATIONS
1. SUMMARY AND RECOMMENDATIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.2.1. Physical properties
2.2.2. Chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. In air
2.4.2. In industrial effluents
2.4.3. In polyacrylate materials
2.4.4. In biological samples
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.1.1 Manufacturing process
3.2.1.2 Impurities
3.2.1.3 Other sources
3.2.1.4 Production data
3.2.2. Experimental production of acrylic
acid by bacterial isolates
3.2.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Transformation
4.2.1. Abiotic degradation
4.2.2. Biodegradation
4.2.2.1 Aerobic biodegradation
4.2.2.2 Anaerobic biodegradation
4.2.3. Bioaccumulation and biomagnification
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure during manufacture,
formulation or use
6. KINETICS AND METABOLISM
6.1. Human studies
6.2. Studies on experimental animals
6.2.1. Absorption, distribution and excretion
6.2.1.1 Oral exposure
6.2.1.2 Inhalation exposure
6.2.1.3 Dermal exposure
6.2.1.4 Intravenous administration
6.2.2. Metabolism
6.2.2.1 In vitro investigations
6.2.2.2 In vivo investigations
6.2.2.3 Metabolic pathways
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Irritation and sensitization
7.2.1. Eye irritation
7.2.2. Skin irritation and sensitization
7.2.2.1 Skin irritation
7.2.2.2 Skin sensitization
7.2.3. Upper respiratory tract irritation
7.3. Short-term exposure
7.3.1. Oral
7.3.2. Inhalation
7.4. Long-term exposure
7.5. Reproduction, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Oral
7.5.2.2 Inhalation
7.5.2.3 Intraperitoneal
7.6. Mutagenicity and related end-points
7.6.1. In vitro and insect studies
7.6.2. In vivo mammalian studies
7.7. Carcinogenicity
7.8. Other studies
7.9. Factors modifying toxicity
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Acute toxicity
8.1.1.1 Poisoning accidents
8.2. Occupational exposure
8.2.1. Poisoning accidents
8.2.2. Effects of short- and long-term exposure
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure of the general population
10.1.2. Occupational exposure
10.1.3. Toxic effects
10.1.3.1 Carcinogenic and mutagenic effects
10.1.3.2 Non-cancer effects
10.1.4. Risk evaluation
10.1.4.1 Inhalation exposure
10.1.4.2 Oral exposure
10.2. Evaluation of effects on the environment
10.2.1. Exposure
10.2.2. Effects
10.2.3. Risk evaluation
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1. Conclusions
11.2. Recommendations for protection of human health
12. FUTURE RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
14. REFERENCES
RESUME ET RECOMMANDATIONS
RESUMEN Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in thecriteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
* * *
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for this
publication.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined
below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
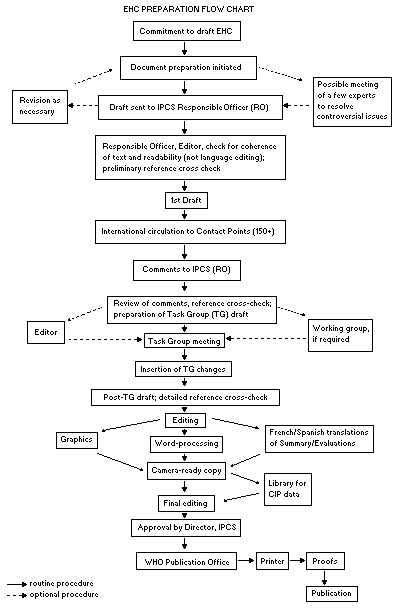 The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
Members
Dr B.I. Ghanayem, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr D. Guth, Office of Research and Development, National Centre
for Environmental Assessment, Research Triangle Park North
Carolina, USA
Mr L. Heiskanen, Environmental Health and Safety Unit,
Department of Health and Family Services, Canberra, Australia
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, United Kingdom ( Co-rapporteur)
Dr P. Lundberg, Department of Toxicology and Chemistry,
National Institute for Working Life, Sweden ( Chairman)
Dr K. Rydzynski, The Nofer Institute of Occupational Medicine,
Lodz, Poland ( Co-rapporteur)
Dr R.O. Shillaker, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries & Food, United Kingdom
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Observers
Dr M. Wooder, Rohm and Haas Uk, Ltd., Croydon, Surrey, United
Kingdom (representing the American Industrial Health Council)
Dr A. Lombard, Service Hygiène Industrielle Toxicologique, ELF-
ATOCHEM, Paris, France (representing the Centre for Ecotoxicology
and Toxicology of Chemicals)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
A WHO Task Group on Environmental Health Criteria for Acrylic
Acid met at the Institute of Terrestrial Ecology, Monks Wood,
Huntington, United Kingdom, from 16 to 19 April 1996. Dr S. Dobson
opened the meeting and welcomed the participants on behalf of the
Institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to acrylic acid.
Dr K. Rydzynski, the Nofer Institute of Occupational Medicine,
Poland, prepared the first draft of this monograph. Dr R.O.
Shillaker, Pesticides Safety Directorate, Ministry of Agriculture,
Fisheries and Food, United Kingdom, contributed to the preparation of
the first draft. The second draft was prepared by Dr K. Rydzynski
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria.
Dr D. Guth, National Centre for Environmental Protection, USA,
contributed to the preparation of the final text of the evaluation.
The meeting was chaired by Dr P. Lundberg, National Institute for
Working Life, Sweden.
Dr B.H. Chen and Dr P.G. Jenkins, IPCS Central Unit, were
responsible for the overall scientific content and technical editing,
respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial
Hygienists
CHO Chinese hamster ovary
EC50 median effective concentration
FID flame ionization detector
GC gas chromatography
GSH reduced glutathione
GV guidance value
HPLC high performance liquid chromatography
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect concentration
NMR nuclear magnetic resonance
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
TCA tricarboxylic acid cycle
TI tolerable intake
TT toxicity threshold
UDS unscheduled DNA synthesis
1. SUMMARY AND RECOMMENDATIONS
Acrylic acid is a colourless liquid, with an irritating acrid
odour, at room temperature and pressure. The odour threshold of
acrylic acid is low (0.20-3.14 mg/m3). It is miscible with water and
most organic solvents.
Acrylic acid is commercially available in two grades; technical
grade and glacial grade. Acrylic acid polymerizes easily when exposed
to heat, light or metals, and a polymerization inhibitor is therefore
added to commercial products.
The worldwide production of acrylic acid in 1994 was estimated to
be approximately 2 million tonnes. It is used primarily as a starting
material in the production of acrylic esters as a monomer for
polyacrylic acid and salts and as a co-monomer with acrylamide for
polymers used as flocculants, with ethylene for ion-exchange resin
polymers, with methyl ester for polymers, and with itatonic acid for
other co-polymers.
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
are 14 ppm in air and 1 ppm in other media.
Acrylic acid has been reported to occur naturally in marine algae
and has been found in the rumen fluid of sheep.
Being miscible with water, acrylic acid would not be expected to
adsorb significantly to soil or sediment. Under soil conditions,
chemicals with low Henry's Law constants are essentially non-volatile.
However, the vapour pressure of acrylic acid suggests that it
volatilizes from surface and dry soil.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
Acrylic acid may be formed by hydrolysis of acrylamide monomer
from industrial waste in soil, especially under aerobic conditions.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. Acrylic acid is rapidly oxidized in water and can
therefore potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
No quantitative data on levels of acrylic acid in ambient air,
drinking-water or soil are available. However, acrylic acid occurs in
wastewater effluent from its production via the oxidation of
propylene.
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as water-based paints. People living in the vicinity of plants
producing acrylic acid or its esters or polymers may be exposed to
acrylic acid in the ambient air. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters.
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxy propionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route and moderate acute toxicity by the inhalation or
dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats, the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg body weight per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). A lowest-observed-adverse-effect level (LOAEL)
of 15 mg/m3 (5 ppm) by the inhalation route was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in
in vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay gave negative results. No firm conclusions can be
drawn from an in vivo DNA binding study or from a dominant lethal
assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization) for
Daphnia magna are 54 mg/litre (24 h) and 95 mg/litre (48 h). Acrylic
acid is more toxic to daphnids than is the alkaline salt. Acute
toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre (72-h
LC50) for the golden orfe. The 96-h NOEC for acrylic acid toxicity to
rainbow trout was found to be 6.3 mg/litre, based on a lack of
sublethal/behavioural responses.
Because of its low octanol-water partition coefficient, acrylic
acid is unlikely to bioconcentrate in aquatic organisms. There have
been no reports of biomagnification in food chains.
No data are available concerning the effects of acrylic acid on
terrestrial organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: acrylic acid
CAS name: 2-propenoic acid
CAS registry number: 79-10-7
EEC No: 607-061-00-8
DOT UN: 22-18-29
RTECS Number: AS 4375000
Synonyms: acroleic acid (Sax & Lewis, 1989)
2-propenoic acid (Sax, 1984)
vinylformic acid (Sittig, 1985)
propene acid (Sax, 1984)
ethylenecarboxylic acid
(Verschueren, 1983)
UN 2218
propenoic acid (Weast et al., 1989)
ethene carboxylic acid (IUPAC name)
Chemical formula: C3H4O2
Chemical structure:
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
Members
Dr B.I. Ghanayem, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr D. Guth, Office of Research and Development, National Centre
for Environmental Assessment, Research Triangle Park North
Carolina, USA
Mr L. Heiskanen, Environmental Health and Safety Unit,
Department of Health and Family Services, Canberra, Australia
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, United Kingdom ( Co-rapporteur)
Dr P. Lundberg, Department of Toxicology and Chemistry,
National Institute for Working Life, Sweden ( Chairman)
Dr K. Rydzynski, The Nofer Institute of Occupational Medicine,
Lodz, Poland ( Co-rapporteur)
Dr R.O. Shillaker, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries & Food, United Kingdom
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Observers
Dr M. Wooder, Rohm and Haas Uk, Ltd., Croydon, Surrey, United
Kingdom (representing the American Industrial Health Council)
Dr A. Lombard, Service Hygiène Industrielle Toxicologique, ELF-
ATOCHEM, Paris, France (representing the Centre for Ecotoxicology
and Toxicology of Chemicals)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
A WHO Task Group on Environmental Health Criteria for Acrylic
Acid met at the Institute of Terrestrial Ecology, Monks Wood,
Huntington, United Kingdom, from 16 to 19 April 1996. Dr S. Dobson
opened the meeting and welcomed the participants on behalf of the
Institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to acrylic acid.
Dr K. Rydzynski, the Nofer Institute of Occupational Medicine,
Poland, prepared the first draft of this monograph. Dr R.O.
Shillaker, Pesticides Safety Directorate, Ministry of Agriculture,
Fisheries and Food, United Kingdom, contributed to the preparation of
the first draft. The second draft was prepared by Dr K. Rydzynski
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria.
Dr D. Guth, National Centre for Environmental Protection, USA,
contributed to the preparation of the final text of the evaluation.
The meeting was chaired by Dr P. Lundberg, National Institute for
Working Life, Sweden.
Dr B.H. Chen and Dr P.G. Jenkins, IPCS Central Unit, were
responsible for the overall scientific content and technical editing,
respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial
Hygienists
CHO Chinese hamster ovary
EC50 median effective concentration
FID flame ionization detector
GC gas chromatography
GSH reduced glutathione
GV guidance value
HPLC high performance liquid chromatography
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect concentration
NMR nuclear magnetic resonance
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
TCA tricarboxylic acid cycle
TI tolerable intake
TT toxicity threshold
UDS unscheduled DNA synthesis
1. SUMMARY AND RECOMMENDATIONS
Acrylic acid is a colourless liquid, with an irritating acrid
odour, at room temperature and pressure. The odour threshold of
acrylic acid is low (0.20-3.14 mg/m3). It is miscible with water and
most organic solvents.
Acrylic acid is commercially available in two grades; technical
grade and glacial grade. Acrylic acid polymerizes easily when exposed
to heat, light or metals, and a polymerization inhibitor is therefore
added to commercial products.
The worldwide production of acrylic acid in 1994 was estimated to
be approximately 2 million tonnes. It is used primarily as a starting
material in the production of acrylic esters as a monomer for
polyacrylic acid and salts and as a co-monomer with acrylamide for
polymers used as flocculants, with ethylene for ion-exchange resin
polymers, with methyl ester for polymers, and with itatonic acid for
other co-polymers.
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
are 14 ppm in air and 1 ppm in other media.
Acrylic acid has been reported to occur naturally in marine algae
and has been found in the rumen fluid of sheep.
Being miscible with water, acrylic acid would not be expected to
adsorb significantly to soil or sediment. Under soil conditions,
chemicals with low Henry's Law constants are essentially non-volatile.
However, the vapour pressure of acrylic acid suggests that it
volatilizes from surface and dry soil.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
Acrylic acid may be formed by hydrolysis of acrylamide monomer
from industrial waste in soil, especially under aerobic conditions.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. Acrylic acid is rapidly oxidized in water and can
therefore potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
No quantitative data on levels of acrylic acid in ambient air,
drinking-water or soil are available. However, acrylic acid occurs in
wastewater effluent from its production via the oxidation of
propylene.
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as water-based paints. People living in the vicinity of plants
producing acrylic acid or its esters or polymers may be exposed to
acrylic acid in the ambient air. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters.
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxy propionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route and moderate acute toxicity by the inhalation or
dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats, the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg body weight per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). A lowest-observed-adverse-effect level (LOAEL)
of 15 mg/m3 (5 ppm) by the inhalation route was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in
in vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay gave negative results. No firm conclusions can be
drawn from an in vivo DNA binding study or from a dominant lethal
assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization) for
Daphnia magna are 54 mg/litre (24 h) and 95 mg/litre (48 h). Acrylic
acid is more toxic to daphnids than is the alkaline salt. Acute
toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre (72-h
LC50) for the golden orfe. The 96-h NOEC for acrylic acid toxicity to
rainbow trout was found to be 6.3 mg/litre, based on a lack of
sublethal/behavioural responses.
Because of its low octanol-water partition coefficient, acrylic
acid is unlikely to bioconcentrate in aquatic organisms. There have
been no reports of biomagnification in food chains.
No data are available concerning the effects of acrylic acid on
terrestrial organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: acrylic acid
CAS name: 2-propenoic acid
CAS registry number: 79-10-7
EEC No: 607-061-00-8
DOT UN: 22-18-29
RTECS Number: AS 4375000
Synonyms: acroleic acid (Sax & Lewis, 1989)
2-propenoic acid (Sax, 1984)
vinylformic acid (Sittig, 1985)
propene acid (Sax, 1984)
ethylenecarboxylic acid
(Verschueren, 1983)
UN 2218
propenoic acid (Weast et al., 1989)
ethene carboxylic acid (IUPAC name)
Chemical formula: C3H4O2
Chemical structure:
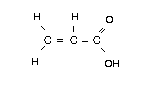 Relative molecular mass: 72.06
2.1.2 Technical product
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins (IARC, 1979; CHRIS, 1989). Acrylic acid polymerizes easily when
exposed to heat, light or metals, and so a polymerization inhibitor is
added to commercial acrylic acid to prevent the strong exothermic
polymerization (NLM, 1989). The inhibitors that are usually used in
acrylic acid preparations are the monomethyl ether of hydroquinone
(methoxyphenol) at 200 ± 20 ppm, phenothiazine at 0.1% and
hydroquinone at 0.1%. Methylene blue at 0.5 to 1.0% and N,N'-
diphenyl- p-phenylenediamine at 0.05% can also be used (IARC, 1979;
CHRIS, 1989; OHM/TADS, 1989; BASF, 1993).
2.2 Physical and chemical properties
2.2.1 Physical properties
Acrylic acid is a colourless liquid at room temperature and
pressure (IARC, 1979; Windholz, 1983; CHRIS, 1985). It has an
irritating acrid odour and it is totally miscible with water and most
organic solvents. Some of the most important physical properties of
acrylic acid are summarized in Table 1.
Table 1. Physical and chemical properties of acrylic acid
Property Value References
Odour threshold concentration (mg/m3) 0.20-3.14 Fazzalari, 1978; Amoore & Hautala, 1983;
Ruth, 1986; Grudzinski, 1988; HSDB, 1989
Melting point (°C at 1 atm) 12.3-14.0 CHRIS, 1989; Weast et al., 1989
Boiling point (°C at 1 atm) 141.3-141.6 CHRIS, 1989; Weast et al., 1989
Flash point (°C)
open cup
closed cup 54.0-68.3 IARC, 1979; Kirk-Othmer, 1984; Sax & Lewis 1989;
46-48.5 Elf Atochem, 1992; BASF, 1994a
Autoignition temperature (°C) 390-446 IARC, 1979; HSDB, 1989; BASF, 1992; Elf Atochem, 1992
Flammable limits (%)
lower 28 HSDB, 1989
upper
Burning rate (mm/min) 1.6 CHRIS, 1989
Specific gravity (g/ml at 20°C) 1.0497-1.0511 IARC, 1979; CHRIS, 1989; Weast et al., 1989
Relative vapour density (air =1 at 20°C) 2.5 HSDB, 1989
Viscosity (mPa.s at 20°C) 1.22-1.30 BASF, 1992; Elf Atochem, 1992
Saturated concentration in air
(g/m3 at 20°C) 22.8 Verschueren, 1983
Volatility (mmHg at 20°C) 3.1; 7.76 Riddick et al., 1986
Vapour pressure (mmHg)
at 39°C 10 OHM/TADS, 1989
at 75°C 60
Table 1. (contd)
Property Value References
Henry's law constant (atm.m3/mol) 3.2 × 10-7 Singh et al., 1984
Surface tension (dyne/cm) 28.1 at 30°C Dean, 1987
Heat of fusion (cal/g) 30.03-37.03 CHRIS, 1989; Weast et al., 1989
Heat of polymerization (cal/g) -257 CHRIS, 1989
Heat of combustion (cal/g) -327 at 25°C Weast et al., 1989
Heat of vaporization (cal/g) 10.955 Weast et al., 1989
Activated carbon absorbability (g/g) 0.129 Verschueren, 1983
Partition coefficient (log Kow at 20-25°C) 0.161-0.46 Korenman & Lunicheva, 1972; GEMS, 1983; Hansch & Leo, 1987;
BASF, 1988
Dissociation constant (pKa at 25°C) 4.25 Weast et al., 1989
Critical temperature (°C) 342 CHRIS, 1985
Critical pressure (atm) 57 CHRIS, 1985
Solubility: in water and most organic completely Dean, 1987; Sax & Lewis, 1989; Weast et al., 1989
solvents (alcohol, chloroform, benzene) miscible
Refractive index (nD20-25) 1.4224-1.4185 Kirk-Othmer, 1984
Maximum absorption (nm, in methanol) 252 Weast et al., 1989
2.2.2 Chemical properties
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according to
supplier's recommendations. Heating can cause vigorous polymerization
in some circumstances. Acrylic acid reacts readily with free radicals
and electrophilic or nucleophilic agents (Kirk-Othmer, 1984). It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of an inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C (CHRIS, 1989). The mechanism of auto-
accelerating polymerization of acrylic acid in hexane-methanol
solution, which can become explosive, has been studied by Bretherick
(1985).
Acrylic acid rapidly decomposes in the atmosphere by
photochemical attack on the double bond (NLM, 1989; OHM/TADS, 1989).
Acrylic acid is corrosive to many metals but not to stainless
steel or aluminium (Kirk-Othmer, 1984; AAR, 1987).
2.3 Conversion factors
In air:
1 ppm = 3.0 mg/m3 (NLM, 1989)
1 mg/m3 = 0.33 ppm (NLM, 1989)
2.4 Analytical methods
2.4.1 In air
A summary of methods for the detection of acrylic acid in air is
given in Table 2.
Table 2. Methods for the analysis of acrylic acid in air
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples absorbed GC on a glass column FID 33 mg/ml acetone The method is Vincent &
on silica gel treated packed with 1% FFAP (lower) to significantly Guient, 1982
with p-methoxyphenol on Chromosorb T 2084 mg/ml acetone affected by high
followed by desorption (upper); this is humidity. Samples
with acetone (94% equivalent to can be stored for
recovery) concentrations ranging up to 11 days at
from 0.5 ppm to 30 ppm room temperature or
(1.5-90 mg/m3) of under refrigeration
acrylic acid in a without affecting
48-litre sample volume recovery. Recommended
as useful for
determining acrylic
acid in the
occupational
environment
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples Reverse phase UV detector 1 g per sample; The sensitivity of OSHA, 1981
collected by HPLC 210 nm assuming 24 litre the analytical
drawing a known sample volume, this method permits
volume of air column: 25 cm × is equivalent to sampling times
through two 4.6 mm i.d. 0.042 mg/m3 as short as
XAD-8 sampling stainless steel (0.014 ppm) 15 min. Under
tubes connected column packed conditions of
in series, with Zorbax 8 m this procedure,
followed by ODS-bound, spherical the possibility
desorption with silica particles of interference
methanol/water from acetaldehyde,
(1:1) mobile phase: acetic acid,
96:4 (V/V) acrylamide,
water/acetonitrile acrolein,
containing 0.1% by acrylonitrile,
volume phosphoric methacrylic
acid; flow rate: acid is excluded.
1 ml/min; injection Method recommended
volume: 25 litre and fully validated
retention by OSHA for acrylic
time:6 min acid determinations
in workplace air
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air is pumped HPLC equipped Conductivity 1 mg/m3 air The method is Simon et
through a florisil with Aminex HPX detector (10 litre rapid, easy and al., 1989
tube at a rate of OFH organic acid sample volume) appears suitable for
1 litre/min. The analysis column the determination
sorbent is mixed (300 mm × 7.8 mm). of acrylic acid
with water (5 ml) Eluent, 2.5 × 10-4 when present in
and 1N H2SO4 (10 µl) M benzoic acid industrial emissions
prior to injection is pumped at containing other
to the chromatographic 0.8 ml/min aliphatic acids
system
a FID = Flame ionisation detector
Air samples are collected on silica gel treated with
p-methoxyhydroquinone used as an inhibitor of polymerization
(Vincent & Guient 1982) or on XAD-8 sampling tubes (OSHA, 1981). XAD-8
sampling tubes contain solid sorbent, i.e. acrylic ester polymer, of
16-50 mesh (OSHA, 1981).
After separation with gas chromatographic (GC) technique or
reverse phase high performance liquid chromatography (HPLC), flame
ionization detection (Vincent & Guient 1982) or UV detection at 210 nm
(OSHA, 1981) are utilized, respectively. The latter method was
modified and recommended by OSHA as a fully validated method for the
determination of acrylic acid in workplace air (OSHA, 1981). This
method, when coupled with an ion suppression technique, proved
successful for the retention and separation of acrylic acid.
A retention time of approximately 6 min is obtained with a Dupont
Zorbax ODS 8-µm silica packed column and a water/acetonitrile (96:4)
mobile phase containing 0.1% (by volume) phosphoric acid. The
phosphoric acid serves to suppress the ionization of acrylic acid
resulting in the retention of the undissociated form of the molecule.
Under these conditions acrylic acid is separated from potential
interfering substances: methacrylic acid, acrylamide, acrolein,
acrylonitrile and acetic acid. Propanoic acid, a saturated precursor
of acrylic acid, can be resolved from acrylic acid in a 13-min
analysis at 1 ml/min flow rate using a 0.1% aqueous phosphoric acid
mobile phase. Detection of acrylic acid at 210 nm is approximately 100
times more sensitive than that of propanoic acid, owing to the
unsaturated nature of acrylic acid. This method permits the detection
of acrylic acid in the presence of very high levels of propanoic acid.
A third method utilizes high-performance ion-exclusion
chromatography with conductimetric detection (Simon et al., 1989). The
use of 2.5 × 10-4 M benzoic acid as the mobile phase in this method
allows the separation of acrylic acid from propionic acid and other
aliphatic acids.
2.4.2 In industrial effluents
A gas chromatographic method has been developed for the analysis
of acrylic acid and some other related pollutants present in small
quantities in the effluent from a methyl acrylate plant in India
(Singh & Thomas, 1985). In this method, effluent samples were injected
directly to the GC system without prior extraction or concentration. A
Porapak Q (4 feet × 1/8 inch I.D.) column and a FID were utilized in
this method. The experimental parameters for the analysis are: column
temperature, 165°C; injector and detector temperature, 250°C; carrier
gas, N2 at 50 ml/min; hydrogen pressure, 1.3 kg/cm2; air pressure,
2.2 kg/cm2 and injection volume, 1-10 µl. The method was found to be
sensitive for detecting acrylic acid at concentrations as low as
1 ppm.
2.4.3 In polyacrylate materials
A differential pulse polarographic method was used for the
determination of residual acrylic acid in sodium polyacrylate
polymeric systems (Husain et al., 1991). The method has the advantage
of analysing acrylic acid in trace quantities directly without
resorting to separation techniques. Sample solutions of the tested
polymers were extracted with N,N-dimethylformamide several times and
the extraction mixture was made up to 25 ml, with the solvent
tert-butyl ammonium iodide (0.02 M) in N,N-dimethylformamide
serving as the supporting electrolyte. The polarographic measurements
were performed with a Metrohm E-506 Polarecord equipped with a three-
electrode system (a dropping mercury electrode (DME), Ag/AgCl
(saturated KCl) reference electrode, and an auxiliary platinum
electrode). Using this method, free acrylic acid in polymers at levels
of 10-100 ppm can be measured with a precision of ± 3%.
2.4.4 In biological samples
Methods for the analysis of acrylic acid in aqueous samples and
tissues extracts in metabolic studies have been reported (Mao et al.,
1994; Mitchell & Petersen, 1988; Black et al., 1995). In these
methods, high-performance ion-exclusion chromatography and/or reverse
phase HPLC with radiometric, refractive index, photo diode-array and
UV detectors were used for the separation and quantification of
acrylic acid.
In another study, residues of acrylic acid in an anaerobic
degradation mixture were quantified using a gas chromatographic
technique with a flame ionization detector (FID) (Stewart et al.,
1995). The column was an 80/120 Carbopak B-DA/4% Carbowax 20 M. The
column and FID temperatures were 175 and 200°C, respectively. The
carrier gas was helium at a flow rate of 24 ml/min. The detection
limit was 1 mg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acrylic acid has been reported to occur naturally in the
following species of marine algae: 9 species of Chlorophyceae, 10
species of Rhodophyceae and 11 species of Phaeophyceae (Sieburth,
1960, Glombitza, 1970a,b, 1979). It is also produced in
Phaeodactylum tricornutum, Phaeocystis spp. and Polysiphonia
lanosa (Brown et al., 1977), as a result of hydrolysis of dimethyl-ß-
propiothetin (Verschueren, 1983).
Acrylic acid has been identified as an antibacterial substance in
oysters (Brown et al., 1977), scallops, ( Patinopecten yessoensis)
(Kodama & Ogata, 1983), and the digestive tract of penguins (Sieburth,
1960; Herwig 1978). It is thought to originate from the phytoplankton
Protogonyaulax (Kodama & Ogata, 1983), Phaeocystis spp (Sieburth,
1960) and Phaeodactylum tricornutum (Brown et al., 1977) on which
the molluscs and penguins fed. It has also been shown that under
natural conditions acrylic acid is generated by certain species of
algae and acts as a microbiocide (Glombitza, 1970a,b, 1979; Heyser &
Glombitza, 1972). It has also been identified as the agent responsible
for the antimicrobial activity of the marine algae Gracilaria
corticata and Ulva lactuca (Bandara et al., 1988).
Acrylic acid has been found in the rumen fluid of sheep as a
result of bacterial fermentation of carbohydrates (Noble & Czerkawski,
1973), where it is converted by rumen microorganisms to propionic acid
(Whanger & Matrone, 1967). It can also be produced from lactic acid by
the anaerobic rumen bacterium Megasphaera elsdenii in the presence
of 3-butynoic acid (Sanseverino et al., 1989).
Acrylic acid has been found in agricultural rum obtained by
fermentation of sugarcane juice by the action of Micrococci spp
(Ganou-Parfait et al., 1988).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
The first commercial process for the manufacture of acrylic acid
and its esters involved hydrolysis of ethylene cyanohydrin in sulfuric
acid. This route is no longer commercially significant (Kirk-Othmer
1984).
Most commercial acrylic acid is now produced via a process in
which propylene is vapour-oxidized to acrolein, which is in turn
oxidized at 300°C with molybdenum-vanadium catalyst to acrylic acid
(NLM, 1989). Other methods of production are as follows:
* a modification of the Reppe process by the reaction of acetylene,
carbon monoxide and alcohol with a nickel catalyst;
* by hydrolysis of acrylonitrile;
* condensation of ethylene oxide with hydrocyanic acid followed by
reaction with sulfuric acid at 160°C;
* a process in which formaldehyde undergoes a type of aldol
reaction with a large molar excess of acetic acid in the vapour
phase in a catalyst tube containing calcium Decalso (Kirk-
Othmer, 1984);
* a heterolytic dehydration pathway of lactic acid in supercritical
water (Mok et al., 1989).
3.2.1.2 Impurities
Commercial acrylic acid is available in two grades: technical and
glacial. Glacial grade is 98-99.5% acrylic acid (NLM, 1989). This may
contain, as impurities, water up to 0.3% w/w and acrylic acid dimer up
to 0.1% w/w (BASF, 1992; Elf Atochem, 1992).
3.2.1.3 Other sources
Acrylic acid has also been detected in trace amounts in
commercial propionic acid (Kostanyan et al., 1969).
3.2.1.4 Production data
Available data on the production of acrylic acid are shown in
Table 3.
Table 3. Production data
Country Year Production of Reference
acrylic acid
(in kilotonnes)
China 1994 105 CEFICA (1995)a
European Community 1975 155 IARC (1979)
1994 665 CEFIC (1995)a
Japan 1976 70 IARC (1979)
1994 420 CEFIC (1995)a
Korea 1994 60 CEFIC (1995)a
Taiwan 1994 50 CEFIC (1995)a
USA 1993 332 US ITC (1983)
1985 361 US ITC (1985)
1986 348 US ITC (1986)
1987 499 US ITC (1987)
1988 480 US ITC (1988)
1991 554 NLM (1991)
1994 685 CEFIC (1995)a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union Chemicals risk
assessment document.
The worldwide production of acrylic acid was approximately
1.13 million tonnes in 1991 (Chemical Marketing Reporter, 1992).
Worldwide capacity for acrylic acid production was reported to be
2 million tonnes in 1994 (CEFIC, 1995).
3.2.2 Experimental production of acrylic acid by bacterial isolates
The following bacterial species have been utilized in
experimental systems to produce acrylic acid:
* from acrylonitrile: (1) by the action of epsilon-caprolactum-
induced Rhodococcus rhodochrous J1 (Nagasawa et al., 1990);
with a periodic substrate feeding system the highest accumulation
(390 g/litre) was obtained; (2) by Arthrobacter sp. isolated
from petrochemical industry waste (Narayanasamy et al., 1990).
* from acrylamide by the action of Pseudomonas sp. and
Xanthomonas maltophilia isolated from herbicide-contaminated
soils (Nawaz et al., 1993, 1994); batch culture of these bacteria
completely degraded 62.8 mM acrylamide to acrylic acid and
ammonia in 24 and 48 h, respectively.
3.2.3 Uses
Acrylic acid is used primarily: as a starting chemical for ethyl
acrylate, n-butyl acrylate, methyl acrylate, 2-ethylhexyl acrylate;
as a monomer for polyacrylic acid and salts, cross-linked high (and
low) molecular weight polymers; as a co-monomer with acrylamide for
polymers used as flocculants; with ethylene for ion-exchange resin
polymers; with methyl ester for polymers; and with methylene succinic
acid (itaconic acid) for other co-polymers (SRI, 1981; NLM, 1989).
In 1987, 25% of the acrylic acid produced in the USA was used for
surface coatings; 20% for polyacrylic acid and salts, including super-
absorbent polymers, detergents, water treatment and dispersants; 13%
for textiles and non-wovens; and 9% for adhesives and sealants
(Kavaler, 1987).
Until 1979, in the European Union countries more than 80% of
acrylic acid was used for the production of polyacrylates and in Japan
90% was used in the production of acrylic esters (IARC, 1979). In
1988, European use of acrylic acid was 69% for esters, 10% for
detergents, 8.5% for flocculants and dispersants and 6.5% for super-
absorbers (CEFIC, 1995).a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Other uses are in the production of copolymers for dental
adhesives (Bowen, 1979), in the production of hydrogels used for
contact lenses (Kirk-Othmer, 1984), in surface coating formulations
(Kirk-Othmer, 1984), and in latex applications to increase stability
in order to prevent premature coagulation (Kirk-Othmer, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Acrylic acid is miscible with water (Riddick et al., 1986) and
therefore would not be expected to adsorb significantly to soil or
sediment (Lyman et al., 1982). The Henry's Law constant for acrylic
acid is reported to be 3.2 × 10-7 atm m3/mol (Singh et al., 1984).
Under soil conditions, chemicals with such low Henry's Law constants
are essentially non-volatile (Lyman et al., 1982). However, the vapour
pressure of acrylic acid suggests that it volatilizes from surface and
dry soil (Howard, 1991).
The adsorption and desorption of acrylic acid were examined in
five different soils: an aquatic sandy loam sediment, a loamy sand, a
clay loam and two loams. The average Koc for the adsorption of
acrylic acid to soil was 43, and ranged from 23 to 63. The Koc values
for the desorption data were widely scattered with values ranging from
18 to 837. This indicates that the degree of adsorption is not
correlated to the organic carbon content (OC), which ranged from 0.46%
for the loamy sand to 4.58% for one of the loams. The results of this
study indicate a high mobility of acrylic acid through soil (Archer &
Horvath, 1991).
Using the fugacity model of Mackay & Peterson (1981) the
theoretical distribution of acrylic acid has been estimated. About 97%
of acrylic acid released to the environment should be associated with
the aquatic environment (the water phase), approximately 1.6% in air,
1% in sediment and < 1% in soils, suspended solids and biota
(Staples, 1993).
Since the atmospheric lifetime of acrylic acid is less than one
month (Atkinson, 1987), there is no potential for long-range transport
of this compound.
4.2 Transformation
4.2.1 Abiotic degradation
The UV absorption band of acrylic acid extends to about 320 nm
(Weast & Astle, 1985). Vapour phase acrylic acid reacts with
photochemically produced hydroxyl radicals primarily by addition to
the double bond and with atmospheric ozone, resulting in an estimated
overall half-life of 6.6 h to 6.5 days (Atkinson & Carter, 1984).
Based upon the estimated rate constant for vapour phase reactions and
assuming hydroxyl radical concentrations of 5 × 105 radicals per cm3
and an ozone concentrations of 7 × 1011 molecules per cm3 (Atkinson
and Carter, 1984; Atkinson, 1987), a half-life of 2.5-23.8 h was
estimated by Howard et al. (1991).
Acrylic acid was found to be stable to hydrolysis at pH values
between 3.7 and 11 (Shah, 1990).
4.2.2 Biodegradation
4.2.2.1 Aerobic biodegradation
When added to water, acrylic acid is rapidly oxidized, and
wastewater containing the compound can deplete reservoirs of oxygen
(Ekhina & Ampleeva, 1977).
Several biodegradability studies show that acrylic acid will
readily biodegrade (Lyman et al., 1982; Keystone Environmental
Resources, 1989a; Douglas & Bell, 1992). The BOD5 (biological
oxygen demand, 5 days) for glacial acrylic acid, using acclimated,
fresh dilution water and raw sewage from a local treatment plant as
the inoculum, was determined to be 0.315 g of oxygen consumed per gram
of product. The COD (chemical oxygen demand) under the same conditions
was 1.48 g/g (Keystone Environmental Resources, 1989)a; therefore,
the BOD5/COD ratio was 0.21. A BOD5/COD ratio of 0.26 was also
reported by Lyman et al. (1982). Biodegradation of acrylic acid in a
14-day BOD test was up to 68% (CITI, 1992). Acrylic acid at a
concentration of 3 mg/litre attained 81% biodegradation within 28 days
in a closed-bottle test based on the consumption of oxygen (Douglas &
Bell, 1992). The pass level of 60% was reached within 10 days of
exceeding the 10% level, and so acrylic acid is considered to be
"readily biodegradable" according to EC classification criteria (EEC,
1988).
The metabolism of 14C-acrylic acid in sandy loam soil has been
studied under aerobic conditions for up to 28 days after treatment at
a rate of 100 mg/kg. Acrylic acid was rapidly metabolized; after 3
days no acrylic acid was detected in soil extracts. Carbon dioxide
evolution accounted for 72.9% of applied radioactivity by day 3 and a
total of 81.1% over the 28-day study period. The half-life for acrylic
acid under these conditions was estimated to be less than 1 day
(Hawkins et al., 1992).
Acrylic acid formed from hydrolysis of acrylamide added to soil
was totally degraded within 15 days of its formation (Nishikawa et
al., 1979). In a 42-day screening study using a sewage seed inoculum,
71% of acrylic acid was mineralized under aerobic conditions. After
previous acclimatization, 81% of acrylic acid degraded to carbon
dioxide in 22 days (Pahren & Bloodgood 1961; Chou et al., 1978).
a Report sent by J.M. Flaherty to J. McLanghlin, Rohm and Haas
Spring House (work order numbers M8903002 and M8902005).
A collection of strains utilizing acrylonitrile, acrylamide and
acrylic acid as sole carbon and/or nitrogen source was isolated from
environmental samples. Strains with maximum decomposing activity were
identified as Pseudomonas pseudoalcaligenes 6p; P.alkaligenes 5g
and Brevibacterium spp. 13 PA (Moiseeva et al., 1991).
An aerobic gram-negative bacterium ( Pseudomonas sp.) isolated
from tropical garden soil was found to be able to degrade a high
concentration of acrylamide (4 mg/litre) to acrylic acid and ammonia,
which were utilized as sole carbon and nitrogen sources, respectively,
for growth (Shanker et al., 1990).
A strain of Byssochlamys sp. produced ß-hydroxypropionic acid
(ß-HPA) when grown on media containing high concentrations of acrylic
acid. The maximal production of ß-HPA was 4.8% when the initial
culture medium contained 7% acrylic acid and 2% glucose and the
initial culture pH was adjusted to 7.0 (Takamizawa et al., 1993).
Acrylic acid has been reported to be significantly degraded
(> 30%) in the MITI test, a biodegradability screening test of the
Japanese Ministry of International Trade and Industry (Sasaki, 1978).
Acrylic acid was completely degraded in a standard Zahn-Wellens test
and the authors concluded that it is biodegradable (BASF, 1993).
Acrylic acid has been found to be degraded by a strain of
Alcaligenes denitrificans isolated from a landfill soil. The
bacterium degraded acrylic acid through the intermediate formation of
L-(+)-lactic and acetic acids, which were further metabolized
(Andreoni et al., 1990).
4.2.2.2 Anaerobic biodegradation
Speece (1983) reported that acrylic acid can undergo ultimate
anaerobic biodegradation. In an anaerobic screening study utilizing
10% sludge from a secondary digester as an inoculum, acrylic acid was
judged to be degradable, with over 75% of theoretical methane being
produced within 8 weeks of incubation (Shelton & Tiedje, 1984).
In another study, acrylic acid was toxic to unacclimated
anaerobic acetate-enriched cultures and was poorly utilized (21%) in a
completely mixed anaerobic reactor with a 20-day hydraulic retention
time after a 90-day acclimatization period (Chou et al., 1978). A
possible explanation for the conflicting results of anaerobic
degradation is the observation that acetate cultures have to exhaust
the acetic acid as carbon and energy source before they can utilize a
cross-fed compound (Chou et al., 1978).
The biodegradability of acrylic acid using methanogenic acetate
enrichment culture was studied by Stewart et al. (1995). Acrylic acid
was degraded with almost no effect on methanogens with spikes up to
100 mg/litre. However, concentrations of 500, 1000 and 1500 mg/litre
were found to inhibit the methanogens for several days before
recovery. Acrylic acid was eventually degraded to less than 1 mg/litre
(> 99% of initial concentration) in all cases by the end of the
study (55 days).
4.2.3 Bioaccumulation and biomagnification
From the low value for log Kow, ranging from 0.161 to 0.46
(Hansch & Leo, 1987; BASF, 1988), one would expect the
bioconcentration of acrylic acid in organisms to be negligible Bysshe
(1990) using a regression equation calculated theoretical
bioconcentration factors ranging from 0.78 to 1.3. Veith et al. (1979)
estimated the bioconcentration factor to be in the range of 1.6 to
2.4.
There have been no reports of biomagnification of acrylic acid in
the food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
No quantitative data are available for environmental levels of
acrylic acid in ambient air, water or soil. Acrylic acid has been
found to occur naturally in some marine algae (Sieburth, 1960; Brown
et al., 1977) and some molluscs (Kodama & Ogata, 1983). The acrylic
acid content of Phaeocystis spp. can be 7.4% of dry weight
(Sieburth, 1960). Other marine algae have been found to contain
acrylic acid: Chlorophyceae, 0.124-16.5 mg/g dry weight;
Rhodophyceae, 0-0.131 mg/kg dry weight; and Phaeophyceae 0-0.02 mg/g
dry weight (Glombitza, 1970a, 1979).
5.2 General population exposure
No data are available for general population exposure. However,
consumers may be exposed to unreacted acrylic acid in the following
household goods: polishes, paints and coatings, adhesives, rug
backing, plastics, textiles and paper finishes (USEPA, 1981).
Information on the typical content of unreacted acrylic acid in these
kinds of products is unavailable.
Populations living in the vicinity of plants producing acrylic
acid or manufacturing its esters or polymers may be exposed to acrylic
acid in the ambient air. The concentrations of emitted vapours of
acrylic acid in the plume from such plants were found to vary from 22
to 183 mg/m3 (Grudzinski, 1988). However, there are no data on
concentrations of acrylic acid in the ambient air of populated areas.
Acrylic acid occurs in wastewater effluents from its production
by the oxidation of propylene at concentrations not exceeding
0.5 mg/litre (Wise & Fahrentholdt, 1981). After treatment of
wastewater from a production facility in Europe, acrylic acid levels
were below the limit of detection (0.1 mg/litre) (CEFIC, 1995)a.
However, effluent from a methyl acrylate plant in India was found to
contain 2500 mg/litre as acrylic acid (Singh & Thomas, 1985).
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Since there is evidence that acrylic acid esters are hydrolysed
to acrylic acid in laboratory animals (Ghanayen et al., 1987) and in
human tissues in vitro (Wiegand, 1990), a potential source of
internal exposure to acrylic acid may result from metabolism of
absorbed acrylic acid esters (Frederick et al., 1994; Sanders et al.,
1988).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure is the most important means of human
exposure to acrylic acid. Inhalation and contact with skin are
important routes of exposure.
The National Institute of Occupational Safety and Health (NIOSH)
conducted two observational nationwide surveys, a decade apart, to
determine the extent of exposure of workers to a variety of substances
in their work environment. The National Occupational Hazard Survey
(NOHS) was conducted during 1972-1974 using a stratified probability
sample of 4636 businesses in 67 metropolitan areas throughout the USA
employing nearly 900 000 workers (NIOSH 1974, 1977). According to the
NOHS, an estimated 28 600 workers were potentially exposed to acrylic
acid, approximately 10% of whom were exposed to acrylic acid and 90%
to trade-name products. However, this estimation excluded the exposure
of the general population to trade-name chemicals possibly containing
acrylic acid. Acrylic acid was seen in 16 major industry groups and in
41 occupational groups in the NOHS.
During 1981-1983 NIOSH conducted the National Occupational
Exposure Survey (NOES), using a stratified probability sample of 4490
businesses in 98 different geographic locations of the USA employing
nearly 1.8 million workers (NIOSH, 1990). According to the NOES, an
estimated 96 500 workers were potentially exposed to acrylic acid,
approximately 10% of whom were exposed to acrylic acid and 90% to
trade-name products. Acrylic acid was seen in 25 major industry groups
and in 67 occupational groups (NIOSH, 1990).
One study conducted at a large manufacturing facility of the Rohm
and Haas Company in the USA, where several chemicals including acrylic
acid and a variety of acrylates and methacrylates were used, indicated
that ethyl acrylate and acrylic acid levels varied from 0.01 to
56 ppm. Most areas of the plant had levels (as 8-h time-weighted
averages) well below the hygiene standards recommended at that time by
the OSHA and the ACGIH of 10 ppm for acrylic acid and 5 ppm for ethyl
acrylate (ACGIH, 1988, OSHA, 1989). Many of the available industrial
hygiene data were specific for high, short-term exposure tasks
(5-40 min samples) when chemicals were pumped into containers for
shipping or when lines were open for new connections, or to obtain
samples. They revealed levels at the high end of the above-mentioned
range (Schwartz et al., 1989).
Exposures of workers to acrylic acid for short periods of time of
less than 15 min and for full shift expressed as time-weighted average
(TWA) concentration have been compiled from four producing companies.
Operators had a mean short-term exposure limit (STEL) value of
8.4 mg/m3 (range < 0.3 to 189 mg/m3); loading/unloading operations
a mean of 3.9 mg/m3 (range 1.2 to 12 mg/m3); and those engaged in
quality assurance a mean of 0.3 mg/m3 (range of less than 0.3 to
0.6 mg/m3). Concerning the 8-h TWA, the operators showed levels of
0.48 mg/m3 (range of 0.03 to 3 mg/m3) and loading/unloading
operations a mean of 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3)
(Casciery & Clary, 1993).
No such data are available from other countries.
6. KINETICS AND METABOLISM
6.1 Human studies
Apart from in vitro skin absorption studies, no data are
available on kinetics, metabolism or elimination or acrylic acid in
humans.
The absorption of 14C-acrylic acid (site of label unspecified)
dissolved in acetone, water or phosphate buffer (pH 6.5) was tested
using samples of excised (postmortem) human and mouse skin in vitro
(Corrigan & Scott, 1988). Acrylic acid concentrations of 0.01, 0.1,
1.0 and 4.0% were applied at 100 µl/cm2 under occlusive conditions.
Samples were taken from the receptor fluid up to 32 h. Rates of
absorption decreased in the order of magnitude as follows: acetone >
water > phosphate buffer. Independent of the vehicle, the absorption
rate increased as a function of acrylic acid concentration.
Permeability coefficients, which ideally are concentration-independent
expressions of absorption rate, for human skin ranged from 0.37 to
0.72 × 10-3 cm/h for water and from 0.47 to 1.81 × 10-4 cm/h for
phosphate buffer. Permeability coefficients were not calculated for
acetone because of evaporation of this volatile vehicle during the
course of the experiments (Corrigan & Scott, 1988).
A briefly reported in vitro percutaneous penetration study
using excised human cadaver skin indicated that 14C-acrylic acid
absorption can vary significantly as a function of pH and delivery
vehicle. In vitro flux, estimated after a 1 mg dose was applied,
varied by 600 times within the treatments studied and decreased in the
order: acetone (600 µg/cm2 per h) > phosphate buffer pH 6.0
(23 µg/cm2 per h) > ethylene glycol (15 µg/cm2 per h) > phosphate
buffer pH 7.4 (1 µg/cm2 per h) (D'Souza and Francis, 1988).
6.2 Studies on experimental animals
6.2.1. Absorption, distribution and excretion
6.2.1.1 Oral exposure
After oral gavage administration of an aqueous solution of
(1-11C)-acrylic acid (26 µg/kg body weight) to female Sprague-Dawley
rats, it was rapidly absorbed and expired mainly as 11CO2 within 1 h
post-administration. The uptake appeared biphasic. The short alpha-
phase had an apparent first-order absorption constant (Ka) of 19% of
the available dose per minute (biological half-time = 3.6 min) and the
Ka of the ß-phase was 30% (biological half-time = 23 min). Relative
retention of radiolabel (dpm per g tissue versus dpm per g body
weight) after 65 min was above unity in liver (2.6), adipose tissue
(1.9), small intestine (1.5), kidneys (1.2) and spleen (1.0).
Approximately 6% of the radiolabel was excreted in the urine within
65 min (Kutzman et al., 1982).
In another study, single gavage doses of 4, 40 or 400 mg/kg body
weight of (2,3-14C)-acrylic acid in 0.5% aqueous methylcellulose
solution were administered to male Sprague-Dawley rats. Approximately
35, 55 and 60%, respectively, of the administrated dose were
eliminated, mostly as 14CO2, within 8 h. By 24 h 50-65% of the dosed
radioactivity was eliminated and the excretion of radioactivity had
virtually ceased. After 72 h, 44-65% of the administrated
radioactivity had been eliminated as 14CO2; 2.9-4.3% in urine, 2.4-
3.6% in the faeces and 18.9-24.6% remained in the tissues examined
(liver, stomach, muscle, blood, plasma, adipose tissue). The residual
radioactivity was highest in the adipose tissue (9-15%), followed by
muscle (6.5-7.5%) and liver (1.7-2.2%) (De Bethizy et al., 1987).
The disposition of (1-14C)-acrylic acid was also determined in
male Sprague-Dawley rats following oral administration by gavage in
water at 400 mg/kg body weight. Excretion of acrylic acid-derived
radioactivity was determined by collection of urine, faeces and
expired air for 72 h following administration. The predominant route
of excretion was in the expired air with approximately 80% of the
radioactivity exhaled as 14CO2 within 24 h and 83.2% after 72 h.
Elimination of radioactivity as exhaled volatile organic compounds was
negligible (less than 0.5% of the radiolabel). Within 24 h of dose
administration, excretion of radioactivity accounted for 5.0% in the
urine and 8.8% of the radiolabel in faeces. Tissue concentrations of
radioactivity after 72 h were generally low: 0.4% of the total dose in
the liver, 0.39% in muscle and 0.18% in skin (Winter & Sipes, 1993).
A comparative bioavailability and disposition study in male
Fischer-344 rats and male C3H mice after a single administration of
(1-14C)-acrylic acid (40 or 150 mg/kg body weight in water) by gavage
has been conducted. This study confirmed that acrylic acid is rapidly
absorbed and metabolized. In rats and mice about 80-90% of the dose
was exhaled as 14CO2 within 24 h (Black et al., 1995). In rats,
excretion of radiolabel in urine and faeces within 72 h accounted for
< 5% and < 1% of the dose, respectively. Elimination of
radioactivity in rats as exhaled organic volatile compounds was less
than 0.5% of the radiolabel. Similar patterns were observed in male
mice (Black et al., 1995).
6.2.1.2 Inhalation exposure
A tissue distribution study has been conducted in 39 female
Sprague-Dawley rats nose-exposed to (1-11C)-acrylic acid vapour for
1 min (concentration not indicated). Radioactivity was widely
distributed; 90 seconds after exposure 18.3% of the delivered dose
remained in the rats. Approximately 28.0% of this radioactivity was
associated with the snout and 42.9% of the radioactivity was found in
the head; this was considered to be solubilized in the mucous of the
turbinates and the nasopharynx. After 65 min, the activity in the
snout was reduced to 8.1% and approximately 60% of the label was
expired as 11CO2. The elimination of labelled CO2 appeared to be
biphasic, with a half-time of approximately 30.6 min during the
alpha-phase. The amount of radioactivity retained in liver and fat
increased markedly between 1.5 and 65 min post-exposure (Kutzman et
al., 1982).
6.2.1.3 Dermal exposure
In one in vitro experiment, the dermal penetration capacity of
(1-14C)-acrylic acid was tested using excised mouse skin. Skin slices
were treated in a diffusion chamber with 0.01, 0.1, 1 and 4% (w/v)
100 µl/cm2 of acrylic acid dissolved in acetone, water or phosphate
buffer pH 6.5. The results were comparable with the study performed on
excised human skin (section 6.1). Permeability coefficients for mouse
skin were 0.96-1.73 × 10-3 cm/h for water and 1.91-3.1 × 10-4 cm/h
for phosphate buffer. The permeability coefficients and steady-state
absorption rate data indicate that mouse skin is approximately three
times more permeable than human skin to acrylic acid (Corrigan &
Scott, 1988). This difference may not be biologically significant.
In a briefly reported study, male Sprague-Dawley rats were
administered dermally 5 mg 14C-acrylic acid per kg body weight
(D'Souza & Francis, 1988). Phosphate buffer of pH 6 or 7.4 or acetone
was used as a formulating agent. In each case the formulation was
applied to the shaved back of the rats and covered with a glass
chamber. The rate of appearance of 14CO2 measured at 0.5, 1, 2, 4,
8, 16 and 24 h after application was used as a measure of the
absorption rate of acrylic acid. The absorption rate was dependent on
the vehicle and decreased in the following order, acetone > phosphate
buffer of pH 6 > phosphate buffer of pH 7.4. Cumulative absorption
after 24 h was 22% from acetone, approximately 19% from phosphate
buffer of pH 6, and 9% from phosphate buffer of pH 7.4. The results of
the in vivo investigations were comparable to those of the in
vitro studies obtained by the same authors (D'Souza & Francis, 1988).
The disposition of (1-14C)-acrylic acid was determined in male
Sprague-Dawley rats after topical application of 100 µl of a 4% (v/v)
solution of acrylic acid in acetone to an area of 8.4 cm2 of the skin
(501 µg/cm2) using a skin-mounted, charcoal-containing trap covered
with fixed aluminium discs to ensure complete recovery of the label.
Excretion of acrylic-acid-derived radioactivity was determined by
collection of urine, faeces and expired air for 72 h following
administration of acrylic acid. Approximately 73% of the radioactivity
volatilized from the skin and was trapped in the charcoal sorbent.
After 72 h, 6% of radioactivity was detected at the site of
application in the skin or on the skin surface. Approximately 75% of
the absorbed dose, representing about 16% of the applied dose, was
exhaled as 14CO2 within 12 h. Excretion of radioactivity in the
urine accounted for approximately 9% of the applied radioactivity
(approximately 4% of the absorbed dose), the faeces containing only
negligible amounts of radioactivity. After 72 h, less than 0.4% of the
applied dose was retained in tissues other than skin (Winter & Sipes,
1993).
In another study, 1% (v/v) acetone solutions of 14C-acrylic acid
at doses of 10 or 40 mg/kg were applied to the clipped skin of the
shoulder region of male F-344 rats or male C3H/HeN Crl BR mice. A non-
occlusive "frame" device was cemented to the skin surface of animals
to allow for free evaporation of acrylic acid, which was trapped using
on-line volatile organic traps. Since this technique was inefficient,
activated-charcoal-impregnated filter paper sheets were placed
occlusively on the treated skin surface of a second high dose group of
animals to provide for absorption of evaporating acrylic acid (Black
et al., 1995). In rats, the reported 72-h recovery was low and ranged
from 50 to 60% of the applied dose. Evaporation accounted for most of
the applied acrylic acid, but approximately 26 and 19% of the applied
high and low doses were absorbed in rats within 72 h, respectively.
The major route of elimination of absorbed acrylic acid was via
exhalation of 14CO2 and accounted for 69.5 and 77% of the absorbed
low and high doses, respectively. Minimal faecal elimination of
absorbed acrylic-acid-derived radioactivity was reported (< 1%), and
tissues and carcasses contained approximately 2-3% of the absorbed
chemical at 72 h.
In mice, the 72-h recovery ranged from 61.5 to 84.0% of the
applied acrylic acid dose. As in the rat experiments, while most of
the applied acrylic acid was lost to evaporation, absorption accounted
for 11-12% of the applied dose. Exhalation of 14CO2 accounted for
83.5 and 77.7% of the absorbed high and low doses, respectively.
Elimination via other routes was negligible, and less than 1% of the
absorbed dose remained in the tissues and carcasses at 72 h (Black et
al., 1995).
6.2.1.4 Intravenous administration
Single i.v. doses of (1-14C)-labelled acrylic acid (10 mg/kg
body weight in phosphate-buffered saline) were given to male F-344
rats and male C3H/HeNCrlBR mice into the tail veins. In rats 63% of
the 14C-dose was eliminated as 14CO2 after 4 h and 68% after 72 h,
while almost no 14C was recovered as exhaled organic volatiles.
Tissue samples (liver, kidney and fat) and plasma contained 1.9% at 1
h, 0.4% at 8 h, and 0.2% at 72 h of the recovered dose. Overall the
recovery was 72.8 ± 10.8%. In mice, 51% of the radioactivity was
exhaled as 14CO2 over the 72-h collection period, the majority
exhaled in the first 4 h. The volatile radioactive fractions were
about 0.6% of the total dose. Overall, 55.7 ± 6.6% of this intravenous
10 mg/kg dose was recovered in mice (Frantz & Beskitt, 1993).
6.2.2 Metabolism
6.2.2.1 In vitro investigations
Oxidation of (2,3-14C)-acrylic acid was studied by incubating
acrylic acid with hepatic microsomal preparations obtained from male
Sprague-Dawley rats. No metabolites were detected by HPLC and acrylic
acid was recovered unchanged from the incubation mixture (De Bethizy
et al., 1987).
Results of the in vitro metabolism of (1-14C)-acrylic acid
incubated with freshly isolated hepatocytes and liver homogenates of
male F-344 rats or mitochondria isolated from liver homogenates of
male F-344 rats indicate that acrylic acid is rapidly metabolized to
14CO2. Addition of equimolar amounts of propionic acid, 3-hydroxy-
propionic acid or 3-mercaptopropionic acid caused a significant
inhibition of the oxidation of acrylic acid by isolated mitochondria.
A single major metabolite co-eluting with 3-hydroxypropionic acid was
found by HPLC analysis in the mitochondrial incubation mixtures. The
authors suggested that acrylic acid is metabolized in vitro by
mammalian enzymes to CO2 via 3-hydroxypropionate by the non-vitamin-
B12 - dependent pathway for propionate metabolism (Finch & Frederick,
1992).
The oxidation rate of acrylic acid in 13 different tissues
(liver, kidney, forestomach, glandular stomach, small and large
intestine, spleen, brain, heart, lung, skeletal muscle, fat and skin)
of male and female C3H/HeNCrlBR mice was measured by incubating tissue
slices with (1-14C)-acrylic acid and collecting 14CO2. All the
tissues studied oxidized acrylic acid to a certain extent, but
activity in kidney, followed by liver, was much higher than in other
tissues. Oxidation of acrylic acid followed pseudo-Michaelis-Menten
kinetics in the liver, kidney and skin, with a Km for all these
tissues of approximately 0.67 mM. Marked differences were observed in
the Vmax values, 2890 ± 436 nmol/h per g for kidney, 616 ± 62 nmol/h
per g for liver and 47.9 ± 5.8 nmol/h per g for skin. Half-lives in
these tissues were 0.13, 0.867 and 10.2 h, respectively. Lung,
glandular stomach, heart, spleen, fat and large intestine preparations
oxidized acrylic acid at rates from 10 to 40% of the rate determined
in the liver; in the remaining tissues reaction rates were less than
10% of those in the liver. Rates of metabolism in tissues from male
and female mice were similar.3-Hydroxypropionic acid was the only
metabolite detected by HPLC analysis following incubation of tissues
with (1-14C)-acrylic acid. To determine if CO2 was formed from the
C1 carbon, and if acetyl-CoA was derived from carbons 2 and 3 of
acrylic acid, the authors incubated (2,3-14C)-acrylic acid and
(1-14C)-acetate with liver and kidney slices and measured the rate of
14CO2 formation. It was concluded that CO2 originated from C1, but
that acetyl-CoA was derived from carbons 2 and 3 of acrylic acid. Both
substrates were oxidized well by the tissues, thus providing for the
complete metabolism of acrylic acid to CO2. The results demonstrate
that the rate of acrylic acid metabolism varies significantly among
mouse tissues and suggested that the kidneys and liver are major sites
of acrylic acid metabolism (Black et al., 1993).
6.2.2.2 In vivo investigations
After oral administration of (2,3-14C)-acrylic acid (4, 40 or
400 mg/kg body weight in 0.5% methylcellulose) to male Sprague-Dawley
rats, the major portion of the radioactivity (up to 65%) was exhaled
as 14CO2 within 24 h. In urine four metabolites were identified by
HPLC analysis. One of the two major metabolites eluted very near to
the solvent front and did not co-elute with acetic acid pyruvic acid
or lactic acid. The second metabolite co-eluted with 3-hydroxypro-
pionic acid. Traces of two other unidentified residues were also
detected. Radioactivity could not be detected at the retention times
corresponding to that of 2,3-epoxypropionic acid, glyceric acid or
N-acetyl- S-(2-carboxy-2-hydroxyethyl)-cysteine, suggesting that
acrylic acid is not epoxidized to 2,3-epoxypropionic acid in vivo.
It was suggested that acrylic acid was metabolized by the non-vitamin-
B12-dependent pathway for propionic acid metabolism, with degradation
to CO2 being the main route of elimination. Residual radioactivity in
tissues may be due to incorporation of 14C from acrylic acid into
acetyl-CoA (De Bethizy et al., 1987).
Using HPLC and NMR analysis, 3-hydroxypropionic acid, N-acetyl-
S-2-(2-carboxyethyl)-cysteine and N-acetyl- S-(2-carboxyethyl)-
cysteine-S-oxide were identified as urinary metabolites after oral
administration of (2,3-14C)-acrylic acid (400 mg/kg body weight in
water by gavage) to male Sprague-Dawley rats. According to the
authors, the detection of mercapturates may be a consequence of the
high dose used in this study (Winter et al., 1992).
HPLC analysis for acrylic acid and its metabolites in rats
revealed that a metabolite that coeluted with 3-hydroxypropionic acid
was found in the urine, plasma and liver of rats that had received
acrylic acid by gavage. Furthermore, a material that co-eluted with
authentic acrylic acid was detected in the urine and liver, but not in
the plasma, of these rats. Acrylic acid, but not 3-hydroxypropionic
acid, was also detected in the urine of rats after cutaneous
application (Black et al., 1995). In mice, 3-hydroxypropionic acid was
identified in the liver after gavage administration of acrylic acid.
No acrylic acid was detected in the liver of these animals (Black et
al., 1995).
6.2.2.3 Metabolic pathways
Acrylic acid is rapidly metabolized to CO2, a major metabolite
formed via acrylyl-CoA by the non-vitamin-B12-dependent pathway of
mammalian propionate catabolism (Finch & Frederick, 1992; Winter et
al., 1992; Black et al., 1993; Winter & Sipes, 1993). This pathway
occurs in the mitochondrion (Finch & Frederick, 1992) and consist of
reactions analogous to fatty acid ß-oxidation (Schultz, 1991).
ß-oxidation is the major route of propionate catabolism in many
invertebrates and plants (Wegner et al., 1968; Halarnkar & Blomquist,
1989); however the primary pathway of propionate catabolism in mammals
is that involving the vitamin-B12-dependent enzyme, methyl-malonyl-
CoA mutase (Black et al., 1993). A small amount of 3-hydroxypropionic
acid was identified as the major urinary metabolite of acrylic acid
(De Bethizy et al., 1987; Winter et al., 1992). There is no evidence
to suggest that epoxide intermediates are formed during the metabolism
of acrylic acid (De Bethizy et al., 1987). N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl) cysteine-
S-oxide were identified in the urine of rats that had received
400 mg/kg (2,3,-14C)-acrylic acid by gavage (Winter et al., 1992),
suggesting a direct reaction between acrylic acid and reduced
glutathione.
The major route of metabolism for acrylic acid esters has been
shown to involve the rapid cleavage of the ester bond by carboxyl
esterases (see Fig. 1) (Ghanayem et al., 1987; Sanders et al., 1988;
Frederik et al., 1994). Thus exposure to acrylic acid esters may
constitute a significant internal exposure to acrylic acid. A
secondary metabolic pathway involves conjugation of the acrylic acid
ester with glutathione to yield acetyl- S-(2-carboxyethyl) cysteine
alkylesters. (Ghanayem et al., 1987; Sanders et al., 1988). This
intermediate may be further metabolized to N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl)-cysteine-
S-oxide. However, it is currently uncertain what proportion of N-
acetyl- S-(2-carboxyethyl) cysteine, or its oxide, formed from the
metabolism of the acrylic acid esters originates from the reaction of
the intact ester with glutathione and what proportion originates from
the conjugation of the released acrylic acid with glutathione (see Fig
1).
On the basis of available information, proposed metabolic
pathways for acrylic acid are summarized in Fig. 1. The proposed
scheme also includes relationships between metabolism of acrylic acid
and its esters (e.g., ethyl acrylate) and metabolism of propionate via
the major vitamin-B12-dependent pathway.
Relative molecular mass: 72.06
2.1.2 Technical product
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins (IARC, 1979; CHRIS, 1989). Acrylic acid polymerizes easily when
exposed to heat, light or metals, and so a polymerization inhibitor is
added to commercial acrylic acid to prevent the strong exothermic
polymerization (NLM, 1989). The inhibitors that are usually used in
acrylic acid preparations are the monomethyl ether of hydroquinone
(methoxyphenol) at 200 ± 20 ppm, phenothiazine at 0.1% and
hydroquinone at 0.1%. Methylene blue at 0.5 to 1.0% and N,N'-
diphenyl- p-phenylenediamine at 0.05% can also be used (IARC, 1979;
CHRIS, 1989; OHM/TADS, 1989; BASF, 1993).
2.2 Physical and chemical properties
2.2.1 Physical properties
Acrylic acid is a colourless liquid at room temperature and
pressure (IARC, 1979; Windholz, 1983; CHRIS, 1985). It has an
irritating acrid odour and it is totally miscible with water and most
organic solvents. Some of the most important physical properties of
acrylic acid are summarized in Table 1.
Table 1. Physical and chemical properties of acrylic acid
Property Value References
Odour threshold concentration (mg/m3) 0.20-3.14 Fazzalari, 1978; Amoore & Hautala, 1983;
Ruth, 1986; Grudzinski, 1988; HSDB, 1989
Melting point (°C at 1 atm) 12.3-14.0 CHRIS, 1989; Weast et al., 1989
Boiling point (°C at 1 atm) 141.3-141.6 CHRIS, 1989; Weast et al., 1989
Flash point (°C)
open cup
closed cup 54.0-68.3 IARC, 1979; Kirk-Othmer, 1984; Sax & Lewis 1989;
46-48.5 Elf Atochem, 1992; BASF, 1994a
Autoignition temperature (°C) 390-446 IARC, 1979; HSDB, 1989; BASF, 1992; Elf Atochem, 1992
Flammable limits (%)
lower 28 HSDB, 1989
upper
Burning rate (mm/min) 1.6 CHRIS, 1989
Specific gravity (g/ml at 20°C) 1.0497-1.0511 IARC, 1979; CHRIS, 1989; Weast et al., 1989
Relative vapour density (air =1 at 20°C) 2.5 HSDB, 1989
Viscosity (mPa.s at 20°C) 1.22-1.30 BASF, 1992; Elf Atochem, 1992
Saturated concentration in air
(g/m3 at 20°C) 22.8 Verschueren, 1983
Volatility (mmHg at 20°C) 3.1; 7.76 Riddick et al., 1986
Vapour pressure (mmHg)
at 39°C 10 OHM/TADS, 1989
at 75°C 60
Table 1. (contd)
Property Value References
Henry's law constant (atm.m3/mol) 3.2 × 10-7 Singh et al., 1984
Surface tension (dyne/cm) 28.1 at 30°C Dean, 1987
Heat of fusion (cal/g) 30.03-37.03 CHRIS, 1989; Weast et al., 1989
Heat of polymerization (cal/g) -257 CHRIS, 1989
Heat of combustion (cal/g) -327 at 25°C Weast et al., 1989
Heat of vaporization (cal/g) 10.955 Weast et al., 1989
Activated carbon absorbability (g/g) 0.129 Verschueren, 1983
Partition coefficient (log Kow at 20-25°C) 0.161-0.46 Korenman & Lunicheva, 1972; GEMS, 1983; Hansch & Leo, 1987;
BASF, 1988
Dissociation constant (pKa at 25°C) 4.25 Weast et al., 1989
Critical temperature (°C) 342 CHRIS, 1985
Critical pressure (atm) 57 CHRIS, 1985
Solubility: in water and most organic completely Dean, 1987; Sax & Lewis, 1989; Weast et al., 1989
solvents (alcohol, chloroform, benzene) miscible
Refractive index (nD20-25) 1.4224-1.4185 Kirk-Othmer, 1984
Maximum absorption (nm, in methanol) 252 Weast et al., 1989
2.2.2 Chemical properties
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according to
supplier's recommendations. Heating can cause vigorous polymerization
in some circumstances. Acrylic acid reacts readily with free radicals
and electrophilic or nucleophilic agents (Kirk-Othmer, 1984). It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of an inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C (CHRIS, 1989). The mechanism of auto-
accelerating polymerization of acrylic acid in hexane-methanol
solution, which can become explosive, has been studied by Bretherick
(1985).
Acrylic acid rapidly decomposes in the atmosphere by
photochemical attack on the double bond (NLM, 1989; OHM/TADS, 1989).
Acrylic acid is corrosive to many metals but not to stainless
steel or aluminium (Kirk-Othmer, 1984; AAR, 1987).
2.3 Conversion factors
In air:
1 ppm = 3.0 mg/m3 (NLM, 1989)
1 mg/m3 = 0.33 ppm (NLM, 1989)
2.4 Analytical methods
2.4.1 In air
A summary of methods for the detection of acrylic acid in air is
given in Table 2.
Table 2. Methods for the analysis of acrylic acid in air
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples absorbed GC on a glass column FID 33 mg/ml acetone The method is Vincent &
on silica gel treated packed with 1% FFAP (lower) to significantly Guient, 1982
with p-methoxyphenol on Chromosorb T 2084 mg/ml acetone affected by high
followed by desorption (upper); this is humidity. Samples
with acetone (94% equivalent to can be stored for
recovery) concentrations ranging up to 11 days at
from 0.5 ppm to 30 ppm room temperature or
(1.5-90 mg/m3) of under refrigeration
acrylic acid in a without affecting
48-litre sample volume recovery. Recommended
as useful for
determining acrylic
acid in the
occupational
environment
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples Reverse phase UV detector 1 g per sample; The sensitivity of OSHA, 1981
collected by HPLC 210 nm assuming 24 litre the analytical
drawing a known sample volume, this method permits
volume of air column: 25 cm × is equivalent to sampling times
through two 4.6 mm i.d. 0.042 mg/m3 as short as
XAD-8 sampling stainless steel (0.014 ppm) 15 min. Under
tubes connected column packed conditions of
in series, with Zorbax 8 m this procedure,
followed by ODS-bound, spherical the possibility
desorption with silica particles of interference
methanol/water from acetaldehyde,
(1:1) mobile phase: acetic acid,
96:4 (V/V) acrylamide,
water/acetonitrile acrolein,
containing 0.1% by acrylonitrile,
volume phosphoric methacrylic
acid; flow rate: acid is excluded.
1 ml/min; injection Method recommended
volume: 25 litre and fully validated
retention by OSHA for acrylic
time:6 min acid determinations
in workplace air
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air is pumped HPLC equipped Conductivity 1 mg/m3 air The method is Simon et
through a florisil with Aminex HPX detector (10 litre rapid, easy and al., 1989
tube at a rate of OFH organic acid sample volume) appears suitable for
1 litre/min. The analysis column the determination
sorbent is mixed (300 mm × 7.8 mm). of acrylic acid
with water (5 ml) Eluent, 2.5 × 10-4 when present in
and 1N H2SO4 (10 µl) M benzoic acid industrial emissions
prior to injection is pumped at containing other
to the chromatographic 0.8 ml/min aliphatic acids
system
a FID = Flame ionisation detector
Air samples are collected on silica gel treated with
p-methoxyhydroquinone used as an inhibitor of polymerization
(Vincent & Guient 1982) or on XAD-8 sampling tubes (OSHA, 1981). XAD-8
sampling tubes contain solid sorbent, i.e. acrylic ester polymer, of
16-50 mesh (OSHA, 1981).
After separation with gas chromatographic (GC) technique or
reverse phase high performance liquid chromatography (HPLC), flame
ionization detection (Vincent & Guient 1982) or UV detection at 210 nm
(OSHA, 1981) are utilized, respectively. The latter method was
modified and recommended by OSHA as a fully validated method for the
determination of acrylic acid in workplace air (OSHA, 1981). This
method, when coupled with an ion suppression technique, proved
successful for the retention and separation of acrylic acid.
A retention time of approximately 6 min is obtained with a Dupont
Zorbax ODS 8-µm silica packed column and a water/acetonitrile (96:4)
mobile phase containing 0.1% (by volume) phosphoric acid. The
phosphoric acid serves to suppress the ionization of acrylic acid
resulting in the retention of the undissociated form of the molecule.
Under these conditions acrylic acid is separated from potential
interfering substances: methacrylic acid, acrylamide, acrolein,
acrylonitrile and acetic acid. Propanoic acid, a saturated precursor
of acrylic acid, can be resolved from acrylic acid in a 13-min
analysis at 1 ml/min flow rate using a 0.1% aqueous phosphoric acid
mobile phase. Detection of acrylic acid at 210 nm is approximately 100
times more sensitive than that of propanoic acid, owing to the
unsaturated nature of acrylic acid. This method permits the detection
of acrylic acid in the presence of very high levels of propanoic acid.
A third method utilizes high-performance ion-exclusion
chromatography with conductimetric detection (Simon et al., 1989). The
use of 2.5 × 10-4 M benzoic acid as the mobile phase in this method
allows the separation of acrylic acid from propionic acid and other
aliphatic acids.
2.4.2 In industrial effluents
A gas chromatographic method has been developed for the analysis
of acrylic acid and some other related pollutants present in small
quantities in the effluent from a methyl acrylate plant in India
(Singh & Thomas, 1985). In this method, effluent samples were injected
directly to the GC system without prior extraction or concentration. A
Porapak Q (4 feet × 1/8 inch I.D.) column and a FID were utilized in
this method. The experimental parameters for the analysis are: column
temperature, 165°C; injector and detector temperature, 250°C; carrier
gas, N2 at 50 ml/min; hydrogen pressure, 1.3 kg/cm2; air pressure,
2.2 kg/cm2 and injection volume, 1-10 µl. The method was found to be
sensitive for detecting acrylic acid at concentrations as low as
1 ppm.
2.4.3 In polyacrylate materials
A differential pulse polarographic method was used for the
determination of residual acrylic acid in sodium polyacrylate
polymeric systems (Husain et al., 1991). The method has the advantage
of analysing acrylic acid in trace quantities directly without
resorting to separation techniques. Sample solutions of the tested
polymers were extracted with N,N-dimethylformamide several times and
the extraction mixture was made up to 25 ml, with the solvent
tert-butyl ammonium iodide (0.02 M) in N,N-dimethylformamide
serving as the supporting electrolyte. The polarographic measurements
were performed with a Metrohm E-506 Polarecord equipped with a three-
electrode system (a dropping mercury electrode (DME), Ag/AgCl
(saturated KCl) reference electrode, and an auxiliary platinum
electrode). Using this method, free acrylic acid in polymers at levels
of 10-100 ppm can be measured with a precision of ± 3%.
2.4.4 In biological samples
Methods for the analysis of acrylic acid in aqueous samples and
tissues extracts in metabolic studies have been reported (Mao et al.,
1994; Mitchell & Petersen, 1988; Black et al., 1995). In these
methods, high-performance ion-exclusion chromatography and/or reverse
phase HPLC with radiometric, refractive index, photo diode-array and
UV detectors were used for the separation and quantification of
acrylic acid.
In another study, residues of acrylic acid in an anaerobic
degradation mixture were quantified using a gas chromatographic
technique with a flame ionization detector (FID) (Stewart et al.,
1995). The column was an 80/120 Carbopak B-DA/4% Carbowax 20 M. The
column and FID temperatures were 175 and 200°C, respectively. The
carrier gas was helium at a flow rate of 24 ml/min. The detection
limit was 1 mg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acrylic acid has been reported to occur naturally in the
following species of marine algae: 9 species of Chlorophyceae, 10
species of Rhodophyceae and 11 species of Phaeophyceae (Sieburth,
1960, Glombitza, 1970a,b, 1979). It is also produced in
Phaeodactylum tricornutum, Phaeocystis spp. and Polysiphonia
lanosa (Brown et al., 1977), as a result of hydrolysis of dimethyl-ß-
propiothetin (Verschueren, 1983).
Acrylic acid has been identified as an antibacterial substance in
oysters (Brown et al., 1977), scallops, ( Patinopecten yessoensis)
(Kodama & Ogata, 1983), and the digestive tract of penguins (Sieburth,
1960; Herwig 1978). It is thought to originate from the phytoplankton
Protogonyaulax (Kodama & Ogata, 1983), Phaeocystis spp (Sieburth,
1960) and Phaeodactylum tricornutum (Brown et al., 1977) on which
the molluscs and penguins fed. It has also been shown that under
natural conditions acrylic acid is generated by certain species of
algae and acts as a microbiocide (Glombitza, 1970a,b, 1979; Heyser &
Glombitza, 1972). It has also been identified as the agent responsible
for the antimicrobial activity of the marine algae Gracilaria
corticata and Ulva lactuca (Bandara et al., 1988).
Acrylic acid has been found in the rumen fluid of sheep as a
result of bacterial fermentation of carbohydrates (Noble & Czerkawski,
1973), where it is converted by rumen microorganisms to propionic acid
(Whanger & Matrone, 1967). It can also be produced from lactic acid by
the anaerobic rumen bacterium Megasphaera elsdenii in the presence
of 3-butynoic acid (Sanseverino et al., 1989).
Acrylic acid has been found in agricultural rum obtained by
fermentation of sugarcane juice by the action of Micrococci spp
(Ganou-Parfait et al., 1988).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
The first commercial process for the manufacture of acrylic acid
and its esters involved hydrolysis of ethylene cyanohydrin in sulfuric
acid. This route is no longer commercially significant (Kirk-Othmer
1984).
Most commercial acrylic acid is now produced via a process in
which propylene is vapour-oxidized to acrolein, which is in turn
oxidized at 300°C with molybdenum-vanadium catalyst to acrylic acid
(NLM, 1989). Other methods of production are as follows:
* a modification of the Reppe process by the reaction of acetylene,
carbon monoxide and alcohol with a nickel catalyst;
* by hydrolysis of acrylonitrile;
* condensation of ethylene oxide with hydrocyanic acid followed by
reaction with sulfuric acid at 160°C;
* a process in which formaldehyde undergoes a type of aldol
reaction with a large molar excess of acetic acid in the vapour
phase in a catalyst tube containing calcium Decalso (Kirk-
Othmer, 1984);
* a heterolytic dehydration pathway of lactic acid in supercritical
water (Mok et al., 1989).
3.2.1.2 Impurities
Commercial acrylic acid is available in two grades: technical and
glacial. Glacial grade is 98-99.5% acrylic acid (NLM, 1989). This may
contain, as impurities, water up to 0.3% w/w and acrylic acid dimer up
to 0.1% w/w (BASF, 1992; Elf Atochem, 1992).
3.2.1.3 Other sources
Acrylic acid has also been detected in trace amounts in
commercial propionic acid (Kostanyan et al., 1969).
3.2.1.4 Production data
Available data on the production of acrylic acid are shown in
Table 3.
Table 3. Production data
Country Year Production of Reference
acrylic acid
(in kilotonnes)
China 1994 105 CEFICA (1995)a
European Community 1975 155 IARC (1979)
1994 665 CEFIC (1995)a
Japan 1976 70 IARC (1979)
1994 420 CEFIC (1995)a
Korea 1994 60 CEFIC (1995)a
Taiwan 1994 50 CEFIC (1995)a
USA 1993 332 US ITC (1983)
1985 361 US ITC (1985)
1986 348 US ITC (1986)
1987 499 US ITC (1987)
1988 480 US ITC (1988)
1991 554 NLM (1991)
1994 685 CEFIC (1995)a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union Chemicals risk
assessment document.
The worldwide production of acrylic acid was approximately
1.13 million tonnes in 1991 (Chemical Marketing Reporter, 1992).
Worldwide capacity for acrylic acid production was reported to be
2 million tonnes in 1994 (CEFIC, 1995).
3.2.2 Experimental production of acrylic acid by bacterial isolates
The following bacterial species have been utilized in
experimental systems to produce acrylic acid:
* from acrylonitrile: (1) by the action of epsilon-caprolactum-
induced Rhodococcus rhodochrous J1 (Nagasawa et al., 1990);
with a periodic substrate feeding system the highest accumulation
(390 g/litre) was obtained; (2) by Arthrobacter sp. isolated
from petrochemical industry waste (Narayanasamy et al., 1990).
* from acrylamide by the action of Pseudomonas sp. and
Xanthomonas maltophilia isolated from herbicide-contaminated
soils (Nawaz et al., 1993, 1994); batch culture of these bacteria
completely degraded 62.8 mM acrylamide to acrylic acid and
ammonia in 24 and 48 h, respectively.
3.2.3 Uses
Acrylic acid is used primarily: as a starting chemical for ethyl
acrylate, n-butyl acrylate, methyl acrylate, 2-ethylhexyl acrylate;
as a monomer for polyacrylic acid and salts, cross-linked high (and
low) molecular weight polymers; as a co-monomer with acrylamide for
polymers used as flocculants; with ethylene for ion-exchange resin
polymers; with methyl ester for polymers; and with methylene succinic
acid (itaconic acid) for other co-polymers (SRI, 1981; NLM, 1989).
In 1987, 25% of the acrylic acid produced in the USA was used for
surface coatings; 20% for polyacrylic acid and salts, including super-
absorbent polymers, detergents, water treatment and dispersants; 13%
for textiles and non-wovens; and 9% for adhesives and sealants
(Kavaler, 1987).
Until 1979, in the European Union countries more than 80% of
acrylic acid was used for the production of polyacrylates and in Japan
90% was used in the production of acrylic esters (IARC, 1979). In
1988, European use of acrylic acid was 69% for esters, 10% for
detergents, 8.5% for flocculants and dispersants and 6.5% for super-
absorbers (CEFIC, 1995).a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Other uses are in the production of copolymers for dental
adhesives (Bowen, 1979), in the production of hydrogels used for
contact lenses (Kirk-Othmer, 1984), in surface coating formulations
(Kirk-Othmer, 1984), and in latex applications to increase stability
in order to prevent premature coagulation (Kirk-Othmer, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Acrylic acid is miscible with water (Riddick et al., 1986) and
therefore would not be expected to adsorb significantly to soil or
sediment (Lyman et al., 1982). The Henry's Law constant for acrylic
acid is reported to be 3.2 × 10-7 atm m3/mol (Singh et al., 1984).
Under soil conditions, chemicals with such low Henry's Law constants
are essentially non-volatile (Lyman et al., 1982). However, the vapour
pressure of acrylic acid suggests that it volatilizes from surface and
dry soil (Howard, 1991).
The adsorption and desorption of acrylic acid were examined in
five different soils: an aquatic sandy loam sediment, a loamy sand, a
clay loam and two loams. The average Koc for the adsorption of
acrylic acid to soil was 43, and ranged from 23 to 63. The Koc values
for the desorption data were widely scattered with values ranging from
18 to 837. This indicates that the degree of adsorption is not
correlated to the organic carbon content (OC), which ranged from 0.46%
for the loamy sand to 4.58% for one of the loams. The results of this
study indicate a high mobility of acrylic acid through soil (Archer &
Horvath, 1991).
Using the fugacity model of Mackay & Peterson (1981) the
theoretical distribution of acrylic acid has been estimated. About 97%
of acrylic acid released to the environment should be associated with
the aquatic environment (the water phase), approximately 1.6% in air,
1% in sediment and < 1% in soils, suspended solids and biota
(Staples, 1993).
Since the atmospheric lifetime of acrylic acid is less than one
month (Atkinson, 1987), there is no potential for long-range transport
of this compound.
4.2 Transformation
4.2.1 Abiotic degradation
The UV absorption band of acrylic acid extends to about 320 nm
(Weast & Astle, 1985). Vapour phase acrylic acid reacts with
photochemically produced hydroxyl radicals primarily by addition to
the double bond and with atmospheric ozone, resulting in an estimated
overall half-life of 6.6 h to 6.5 days (Atkinson & Carter, 1984).
Based upon the estimated rate constant for vapour phase reactions and
assuming hydroxyl radical concentrations of 5 × 105 radicals per cm3
and an ozone concentrations of 7 × 1011 molecules per cm3 (Atkinson
and Carter, 1984; Atkinson, 1987), a half-life of 2.5-23.8 h was
estimated by Howard et al. (1991).
Acrylic acid was found to be stable to hydrolysis at pH values
between 3.7 and 11 (Shah, 1990).
4.2.2 Biodegradation
4.2.2.1 Aerobic biodegradation
When added to water, acrylic acid is rapidly oxidized, and
wastewater containing the compound can deplete reservoirs of oxygen
(Ekhina & Ampleeva, 1977).
Several biodegradability studies show that acrylic acid will
readily biodegrade (Lyman et al., 1982; Keystone Environmental
Resources, 1989a; Douglas & Bell, 1992). The BOD5 (biological
oxygen demand, 5 days) for glacial acrylic acid, using acclimated,
fresh dilution water and raw sewage from a local treatment plant as
the inoculum, was determined to be 0.315 g of oxygen consumed per gram
of product. The COD (chemical oxygen demand) under the same conditions
was 1.48 g/g (Keystone Environmental Resources, 1989)a; therefore,
the BOD5/COD ratio was 0.21. A BOD5/COD ratio of 0.26 was also
reported by Lyman et al. (1982). Biodegradation of acrylic acid in a
14-day BOD test was up to 68% (CITI, 1992). Acrylic acid at a
concentration of 3 mg/litre attained 81% biodegradation within 28 days
in a closed-bottle test based on the consumption of oxygen (Douglas &
Bell, 1992). The pass level of 60% was reached within 10 days of
exceeding the 10% level, and so acrylic acid is considered to be
"readily biodegradable" according to EC classification criteria (EEC,
1988).
The metabolism of 14C-acrylic acid in sandy loam soil has been
studied under aerobic conditions for up to 28 days after treatment at
a rate of 100 mg/kg. Acrylic acid was rapidly metabolized; after 3
days no acrylic acid was detected in soil extracts. Carbon dioxide
evolution accounted for 72.9% of applied radioactivity by day 3 and a
total of 81.1% over the 28-day study period. The half-life for acrylic
acid under these conditions was estimated to be less than 1 day
(Hawkins et al., 1992).
Acrylic acid formed from hydrolysis of acrylamide added to soil
was totally degraded within 15 days of its formation (Nishikawa et
al., 1979). In a 42-day screening study using a sewage seed inoculum,
71% of acrylic acid was mineralized under aerobic conditions. After
previous acclimatization, 81% of acrylic acid degraded to carbon
dioxide in 22 days (Pahren & Bloodgood 1961; Chou et al., 1978).
a Report sent by J.M. Flaherty to J. McLanghlin, Rohm and Haas
Spring House (work order numbers M8903002 and M8902005).
A collection of strains utilizing acrylonitrile, acrylamide and
acrylic acid as sole carbon and/or nitrogen source was isolated from
environmental samples. Strains with maximum decomposing activity were
identified as Pseudomonas pseudoalcaligenes 6p; P.alkaligenes 5g
and Brevibacterium spp. 13 PA (Moiseeva et al., 1991).
An aerobic gram-negative bacterium ( Pseudomonas sp.) isolated
from tropical garden soil was found to be able to degrade a high
concentration of acrylamide (4 mg/litre) to acrylic acid and ammonia,
which were utilized as sole carbon and nitrogen sources, respectively,
for growth (Shanker et al., 1990).
A strain of Byssochlamys sp. produced ß-hydroxypropionic acid
(ß-HPA) when grown on media containing high concentrations of acrylic
acid. The maximal production of ß-HPA was 4.8% when the initial
culture medium contained 7% acrylic acid and 2% glucose and the
initial culture pH was adjusted to 7.0 (Takamizawa et al., 1993).
Acrylic acid has been reported to be significantly degraded
(> 30%) in the MITI test, a biodegradability screening test of the
Japanese Ministry of International Trade and Industry (Sasaki, 1978).
Acrylic acid was completely degraded in a standard Zahn-Wellens test
and the authors concluded that it is biodegradable (BASF, 1993).
Acrylic acid has been found to be degraded by a strain of
Alcaligenes denitrificans isolated from a landfill soil. The
bacterium degraded acrylic acid through the intermediate formation of
L-(+)-lactic and acetic acids, which were further metabolized
(Andreoni et al., 1990).
4.2.2.2 Anaerobic biodegradation
Speece (1983) reported that acrylic acid can undergo ultimate
anaerobic biodegradation. In an anaerobic screening study utilizing
10% sludge from a secondary digester as an inoculum, acrylic acid was
judged to be degradable, with over 75% of theoretical methane being
produced within 8 weeks of incubation (Shelton & Tiedje, 1984).
In another study, acrylic acid was toxic to unacclimated
anaerobic acetate-enriched cultures and was poorly utilized (21%) in a
completely mixed anaerobic reactor with a 20-day hydraulic retention
time after a 90-day acclimatization period (Chou et al., 1978). A
possible explanation for the conflicting results of anaerobic
degradation is the observation that acetate cultures have to exhaust
the acetic acid as carbon and energy source before they can utilize a
cross-fed compound (Chou et al., 1978).
The biodegradability of acrylic acid using methanogenic acetate
enrichment culture was studied by Stewart et al. (1995). Acrylic acid
was degraded with almost no effect on methanogens with spikes up to
100 mg/litre. However, concentrations of 500, 1000 and 1500 mg/litre
were found to inhibit the methanogens for several days before
recovery. Acrylic acid was eventually degraded to less than 1 mg/litre
(> 99% of initial concentration) in all cases by the end of the
study (55 days).
4.2.3 Bioaccumulation and biomagnification
From the low value for log Kow, ranging from 0.161 to 0.46
(Hansch & Leo, 1987; BASF, 1988), one would expect the
bioconcentration of acrylic acid in organisms to be negligible Bysshe
(1990) using a regression equation calculated theoretical
bioconcentration factors ranging from 0.78 to 1.3. Veith et al. (1979)
estimated the bioconcentration factor to be in the range of 1.6 to
2.4.
There have been no reports of biomagnification of acrylic acid in
the food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
No quantitative data are available for environmental levels of
acrylic acid in ambient air, water or soil. Acrylic acid has been
found to occur naturally in some marine algae (Sieburth, 1960; Brown
et al., 1977) and some molluscs (Kodama & Ogata, 1983). The acrylic
acid content of Phaeocystis spp. can be 7.4% of dry weight
(Sieburth, 1960). Other marine algae have been found to contain
acrylic acid: Chlorophyceae, 0.124-16.5 mg/g dry weight;
Rhodophyceae, 0-0.131 mg/kg dry weight; and Phaeophyceae 0-0.02 mg/g
dry weight (Glombitza, 1970a, 1979).
5.2 General population exposure
No data are available for general population exposure. However,
consumers may be exposed to unreacted acrylic acid in the following
household goods: polishes, paints and coatings, adhesives, rug
backing, plastics, textiles and paper finishes (USEPA, 1981).
Information on the typical content of unreacted acrylic acid in these
kinds of products is unavailable.
Populations living in the vicinity of plants producing acrylic
acid or manufacturing its esters or polymers may be exposed to acrylic
acid in the ambient air. The concentrations of emitted vapours of
acrylic acid in the plume from such plants were found to vary from 22
to 183 mg/m3 (Grudzinski, 1988). However, there are no data on
concentrations of acrylic acid in the ambient air of populated areas.
Acrylic acid occurs in wastewater effluents from its production
by the oxidation of propylene at concentrations not exceeding
0.5 mg/litre (Wise & Fahrentholdt, 1981). After treatment of
wastewater from a production facility in Europe, acrylic acid levels
were below the limit of detection (0.1 mg/litre) (CEFIC, 1995)a.
However, effluent from a methyl acrylate plant in India was found to
contain 2500 mg/litre as acrylic acid (Singh & Thomas, 1985).
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Since there is evidence that acrylic acid esters are hydrolysed
to acrylic acid in laboratory animals (Ghanayen et al., 1987) and in
human tissues in vitro (Wiegand, 1990), a potential source of
internal exposure to acrylic acid may result from metabolism of
absorbed acrylic acid esters (Frederick et al., 1994; Sanders et al.,
1988).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure is the most important means of human
exposure to acrylic acid. Inhalation and contact with skin are
important routes of exposure.
The National Institute of Occupational Safety and Health (NIOSH)
conducted two observational nationwide surveys, a decade apart, to
determine the extent of exposure of workers to a variety of substances
in their work environment. The National Occupational Hazard Survey
(NOHS) was conducted during 1972-1974 using a stratified probability
sample of 4636 businesses in 67 metropolitan areas throughout the USA
employing nearly 900 000 workers (NIOSH 1974, 1977). According to the
NOHS, an estimated 28 600 workers were potentially exposed to acrylic
acid, approximately 10% of whom were exposed to acrylic acid and 90%
to trade-name products. However, this estimation excluded the exposure
of the general population to trade-name chemicals possibly containing
acrylic acid. Acrylic acid was seen in 16 major industry groups and in
41 occupational groups in the NOHS.
During 1981-1983 NIOSH conducted the National Occupational
Exposure Survey (NOES), using a stratified probability sample of 4490
businesses in 98 different geographic locations of the USA employing
nearly 1.8 million workers (NIOSH, 1990). According to the NOES, an
estimated 96 500 workers were potentially exposed to acrylic acid,
approximately 10% of whom were exposed to acrylic acid and 90% to
trade-name products. Acrylic acid was seen in 25 major industry groups
and in 67 occupational groups (NIOSH, 1990).
One study conducted at a large manufacturing facility of the Rohm
and Haas Company in the USA, where several chemicals including acrylic
acid and a variety of acrylates and methacrylates were used, indicated
that ethyl acrylate and acrylic acid levels varied from 0.01 to
56 ppm. Most areas of the plant had levels (as 8-h time-weighted
averages) well below the hygiene standards recommended at that time by
the OSHA and the ACGIH of 10 ppm for acrylic acid and 5 ppm for ethyl
acrylate (ACGIH, 1988, OSHA, 1989). Many of the available industrial
hygiene data were specific for high, short-term exposure tasks
(5-40 min samples) when chemicals were pumped into containers for
shipping or when lines were open for new connections, or to obtain
samples. They revealed levels at the high end of the above-mentioned
range (Schwartz et al., 1989).
Exposures of workers to acrylic acid for short periods of time of
less than 15 min and for full shift expressed as time-weighted average
(TWA) concentration have been compiled from four producing companies.
Operators had a mean short-term exposure limit (STEL) value of
8.4 mg/m3 (range < 0.3 to 189 mg/m3); loading/unloading operations
a mean of 3.9 mg/m3 (range 1.2 to 12 mg/m3); and those engaged in
quality assurance a mean of 0.3 mg/m3 (range of less than 0.3 to
0.6 mg/m3). Concerning the 8-h TWA, the operators showed levels of
0.48 mg/m3 (range of 0.03 to 3 mg/m3) and loading/unloading
operations a mean of 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3)
(Casciery & Clary, 1993).
No such data are available from other countries.
6. KINETICS AND METABOLISM
6.1 Human studies
Apart from in vitro skin absorption studies, no data are
available on kinetics, metabolism or elimination or acrylic acid in
humans.
The absorption of 14C-acrylic acid (site of label unspecified)
dissolved in acetone, water or phosphate buffer (pH 6.5) was tested
using samples of excised (postmortem) human and mouse skin in vitro
(Corrigan & Scott, 1988). Acrylic acid concentrations of 0.01, 0.1,
1.0 and 4.0% were applied at 100 µl/cm2 under occlusive conditions.
Samples were taken from the receptor fluid up to 32 h. Rates of
absorption decreased in the order of magnitude as follows: acetone >
water > phosphate buffer. Independent of the vehicle, the absorption
rate increased as a function of acrylic acid concentration.
Permeability coefficients, which ideally are concentration-independent
expressions of absorption rate, for human skin ranged from 0.37 to
0.72 × 10-3 cm/h for water and from 0.47 to 1.81 × 10-4 cm/h for
phosphate buffer. Permeability coefficients were not calculated for
acetone because of evaporation of this volatile vehicle during the
course of the experiments (Corrigan & Scott, 1988).
A briefly reported in vitro percutaneous penetration study
using excised human cadaver skin indicated that 14C-acrylic acid
absorption can vary significantly as a function of pH and delivery
vehicle. In vitro flux, estimated after a 1 mg dose was applied,
varied by 600 times within the treatments studied and decreased in the
order: acetone (600 µg/cm2 per h) > phosphate buffer pH 6.0
(23 µg/cm2 per h) > ethylene glycol (15 µg/cm2 per h) > phosphate
buffer pH 7.4 (1 µg/cm2 per h) (D'Souza and Francis, 1988).
6.2 Studies on experimental animals
6.2.1. Absorption, distribution and excretion
6.2.1.1 Oral exposure
After oral gavage administration of an aqueous solution of
(1-11C)-acrylic acid (26 µg/kg body weight) to female Sprague-Dawley
rats, it was rapidly absorbed and expired mainly as 11CO2 within 1 h
post-administration. The uptake appeared biphasic. The short alpha-
phase had an apparent first-order absorption constant (Ka) of 19% of
the available dose per minute (biological half-time = 3.6 min) and the
Ka of the ß-phase was 30% (biological half-time = 23 min). Relative
retention of radiolabel (dpm per g tissue versus dpm per g body
weight) after 65 min was above unity in liver (2.6), adipose tissue
(1.9), small intestine (1.5), kidneys (1.2) and spleen (1.0).
Approximately 6% of the radiolabel was excreted in the urine within
65 min (Kutzman et al., 1982).
In another study, single gavage doses of 4, 40 or 400 mg/kg body
weight of (2,3-14C)-acrylic acid in 0.5% aqueous methylcellulose
solution were administered to male Sprague-Dawley rats. Approximately
35, 55 and 60%, respectively, of the administrated dose were
eliminated, mostly as 14CO2, within 8 h. By 24 h 50-65% of the dosed
radioactivity was eliminated and the excretion of radioactivity had
virtually ceased. After 72 h, 44-65% of the administrated
radioactivity had been eliminated as 14CO2; 2.9-4.3% in urine, 2.4-
3.6% in the faeces and 18.9-24.6% remained in the tissues examined
(liver, stomach, muscle, blood, plasma, adipose tissue). The residual
radioactivity was highest in the adipose tissue (9-15%), followed by
muscle (6.5-7.5%) and liver (1.7-2.2%) (De Bethizy et al., 1987).
The disposition of (1-14C)-acrylic acid was also determined in
male Sprague-Dawley rats following oral administration by gavage in
water at 400 mg/kg body weight. Excretion of acrylic acid-derived
radioactivity was determined by collection of urine, faeces and
expired air for 72 h following administration. The predominant route
of excretion was in the expired air with approximately 80% of the
radioactivity exhaled as 14CO2 within 24 h and 83.2% after 72 h.
Elimination of radioactivity as exhaled volatile organic compounds was
negligible (less than 0.5% of the radiolabel). Within 24 h of dose
administration, excretion of radioactivity accounted for 5.0% in the
urine and 8.8% of the radiolabel in faeces. Tissue concentrations of
radioactivity after 72 h were generally low: 0.4% of the total dose in
the liver, 0.39% in muscle and 0.18% in skin (Winter & Sipes, 1993).
A comparative bioavailability and disposition study in male
Fischer-344 rats and male C3H mice after a single administration of
(1-14C)-acrylic acid (40 or 150 mg/kg body weight in water) by gavage
has been conducted. This study confirmed that acrylic acid is rapidly
absorbed and metabolized. In rats and mice about 80-90% of the dose
was exhaled as 14CO2 within 24 h (Black et al., 1995). In rats,
excretion of radiolabel in urine and faeces within 72 h accounted for
< 5% and < 1% of the dose, respectively. Elimination of
radioactivity in rats as exhaled organic volatile compounds was less
than 0.5% of the radiolabel. Similar patterns were observed in male
mice (Black et al., 1995).
6.2.1.2 Inhalation exposure
A tissue distribution study has been conducted in 39 female
Sprague-Dawley rats nose-exposed to (1-11C)-acrylic acid vapour for
1 min (concentration not indicated). Radioactivity was widely
distributed; 90 seconds after exposure 18.3% of the delivered dose
remained in the rats. Approximately 28.0% of this radioactivity was
associated with the snout and 42.9% of the radioactivity was found in
the head; this was considered to be solubilized in the mucous of the
turbinates and the nasopharynx. After 65 min, the activity in the
snout was reduced to 8.1% and approximately 60% of the label was
expired as 11CO2. The elimination of labelled CO2 appeared to be
biphasic, with a half-time of approximately 30.6 min during the
alpha-phase. The amount of radioactivity retained in liver and fat
increased markedly between 1.5 and 65 min post-exposure (Kutzman et
al., 1982).
6.2.1.3 Dermal exposure
In one in vitro experiment, the dermal penetration capacity of
(1-14C)-acrylic acid was tested using excised mouse skin. Skin slices
were treated in a diffusion chamber with 0.01, 0.1, 1 and 4% (w/v)
100 µl/cm2 of acrylic acid dissolved in acetone, water or phosphate
buffer pH 6.5. The results were comparable with the study performed on
excised human skin (section 6.1). Permeability coefficients for mouse
skin were 0.96-1.73 × 10-3 cm/h for water and 1.91-3.1 × 10-4 cm/h
for phosphate buffer. The permeability coefficients and steady-state
absorption rate data indicate that mouse skin is approximately three
times more permeable than human skin to acrylic acid (Corrigan &
Scott, 1988). This difference may not be biologically significant.
In a briefly reported study, male Sprague-Dawley rats were
administered dermally 5 mg 14C-acrylic acid per kg body weight
(D'Souza & Francis, 1988). Phosphate buffer of pH 6 or 7.4 or acetone
was used as a formulating agent. In each case the formulation was
applied to the shaved back of the rats and covered with a glass
chamber. The rate of appearance of 14CO2 measured at 0.5, 1, 2, 4,
8, 16 and 24 h after application was used as a measure of the
absorption rate of acrylic acid. The absorption rate was dependent on
the vehicle and decreased in the following order, acetone > phosphate
buffer of pH 6 > phosphate buffer of pH 7.4. Cumulative absorption
after 24 h was 22% from acetone, approximately 19% from phosphate
buffer of pH 6, and 9% from phosphate buffer of pH 7.4. The results of
the in vivo investigations were comparable to those of the in
vitro studies obtained by the same authors (D'Souza & Francis, 1988).
The disposition of (1-14C)-acrylic acid was determined in male
Sprague-Dawley rats after topical application of 100 µl of a 4% (v/v)
solution of acrylic acid in acetone to an area of 8.4 cm2 of the skin
(501 µg/cm2) using a skin-mounted, charcoal-containing trap covered
with fixed aluminium discs to ensure complete recovery of the label.
Excretion of acrylic-acid-derived radioactivity was determined by
collection of urine, faeces and expired air for 72 h following
administration of acrylic acid. Approximately 73% of the radioactivity
volatilized from the skin and was trapped in the charcoal sorbent.
After 72 h, 6% of radioactivity was detected at the site of
application in the skin or on the skin surface. Approximately 75% of
the absorbed dose, representing about 16% of the applied dose, was
exhaled as 14CO2 within 12 h. Excretion of radioactivity in the
urine accounted for approximately 9% of the applied radioactivity
(approximately 4% of the absorbed dose), the faeces containing only
negligible amounts of radioactivity. After 72 h, less than 0.4% of the
applied dose was retained in tissues other than skin (Winter & Sipes,
1993).
In another study, 1% (v/v) acetone solutions of 14C-acrylic acid
at doses of 10 or 40 mg/kg were applied to the clipped skin of the
shoulder region of male F-344 rats or male C3H/HeN Crl BR mice. A non-
occlusive "frame" device was cemented to the skin surface of animals
to allow for free evaporation of acrylic acid, which was trapped using
on-line volatile organic traps. Since this technique was inefficient,
activated-charcoal-impregnated filter paper sheets were placed
occlusively on the treated skin surface of a second high dose group of
animals to provide for absorption of evaporating acrylic acid (Black
et al., 1995). In rats, the reported 72-h recovery was low and ranged
from 50 to 60% of the applied dose. Evaporation accounted for most of
the applied acrylic acid, but approximately 26 and 19% of the applied
high and low doses were absorbed in rats within 72 h, respectively.
The major route of elimination of absorbed acrylic acid was via
exhalation of 14CO2 and accounted for 69.5 and 77% of the absorbed
low and high doses, respectively. Minimal faecal elimination of
absorbed acrylic-acid-derived radioactivity was reported (< 1%), and
tissues and carcasses contained approximately 2-3% of the absorbed
chemical at 72 h.
In mice, the 72-h recovery ranged from 61.5 to 84.0% of the
applied acrylic acid dose. As in the rat experiments, while most of
the applied acrylic acid was lost to evaporation, absorption accounted
for 11-12% of the applied dose. Exhalation of 14CO2 accounted for
83.5 and 77.7% of the absorbed high and low doses, respectively.
Elimination via other routes was negligible, and less than 1% of the
absorbed dose remained in the tissues and carcasses at 72 h (Black et
al., 1995).
6.2.1.4 Intravenous administration
Single i.v. doses of (1-14C)-labelled acrylic acid (10 mg/kg
body weight in phosphate-buffered saline) were given to male F-344
rats and male C3H/HeNCrlBR mice into the tail veins. In rats 63% of
the 14C-dose was eliminated as 14CO2 after 4 h and 68% after 72 h,
while almost no 14C was recovered as exhaled organic volatiles.
Tissue samples (liver, kidney and fat) and plasma contained 1.9% at 1
h, 0.4% at 8 h, and 0.2% at 72 h of the recovered dose. Overall the
recovery was 72.8 ± 10.8%. In mice, 51% of the radioactivity was
exhaled as 14CO2 over the 72-h collection period, the majority
exhaled in the first 4 h. The volatile radioactive fractions were
about 0.6% of the total dose. Overall, 55.7 ± 6.6% of this intravenous
10 mg/kg dose was recovered in mice (Frantz & Beskitt, 1993).
6.2.2 Metabolism
6.2.2.1 In vitro investigations
Oxidation of (2,3-14C)-acrylic acid was studied by incubating
acrylic acid with hepatic microsomal preparations obtained from male
Sprague-Dawley rats. No metabolites were detected by HPLC and acrylic
acid was recovered unchanged from the incubation mixture (De Bethizy
et al., 1987).
Results of the in vitro metabolism of (1-14C)-acrylic acid
incubated with freshly isolated hepatocytes and liver homogenates of
male F-344 rats or mitochondria isolated from liver homogenates of
male F-344 rats indicate that acrylic acid is rapidly metabolized to
14CO2. Addition of equimolar amounts of propionic acid, 3-hydroxy-
propionic acid or 3-mercaptopropionic acid caused a significant
inhibition of the oxidation of acrylic acid by isolated mitochondria.
A single major metabolite co-eluting with 3-hydroxypropionic acid was
found by HPLC analysis in the mitochondrial incubation mixtures. The
authors suggested that acrylic acid is metabolized in vitro by
mammalian enzymes to CO2 via 3-hydroxypropionate by the non-vitamin-
B12 - dependent pathway for propionate metabolism (Finch & Frederick,
1992).
The oxidation rate of acrylic acid in 13 different tissues
(liver, kidney, forestomach, glandular stomach, small and large
intestine, spleen, brain, heart, lung, skeletal muscle, fat and skin)
of male and female C3H/HeNCrlBR mice was measured by incubating tissue
slices with (1-14C)-acrylic acid and collecting 14CO2. All the
tissues studied oxidized acrylic acid to a certain extent, but
activity in kidney, followed by liver, was much higher than in other
tissues. Oxidation of acrylic acid followed pseudo-Michaelis-Menten
kinetics in the liver, kidney and skin, with a Km for all these
tissues of approximately 0.67 mM. Marked differences were observed in
the Vmax values, 2890 ± 436 nmol/h per g for kidney, 616 ± 62 nmol/h
per g for liver and 47.9 ± 5.8 nmol/h per g for skin. Half-lives in
these tissues were 0.13, 0.867 and 10.2 h, respectively. Lung,
glandular stomach, heart, spleen, fat and large intestine preparations
oxidized acrylic acid at rates from 10 to 40% of the rate determined
in the liver; in the remaining tissues reaction rates were less than
10% of those in the liver. Rates of metabolism in tissues from male
and female mice were similar.3-Hydroxypropionic acid was the only
metabolite detected by HPLC analysis following incubation of tissues
with (1-14C)-acrylic acid. To determine if CO2 was formed from the
C1 carbon, and if acetyl-CoA was derived from carbons 2 and 3 of
acrylic acid, the authors incubated (2,3-14C)-acrylic acid and
(1-14C)-acetate with liver and kidney slices and measured the rate of
14CO2 formation. It was concluded that CO2 originated from C1, but
that acetyl-CoA was derived from carbons 2 and 3 of acrylic acid. Both
substrates were oxidized well by the tissues, thus providing for the
complete metabolism of acrylic acid to CO2. The results demonstrate
that the rate of acrylic acid metabolism varies significantly among
mouse tissues and suggested that the kidneys and liver are major sites
of acrylic acid metabolism (Black et al., 1993).
6.2.2.2 In vivo investigations
After oral administration of (2,3-14C)-acrylic acid (4, 40 or
400 mg/kg body weight in 0.5% methylcellulose) to male Sprague-Dawley
rats, the major portion of the radioactivity (up to 65%) was exhaled
as 14CO2 within 24 h. In urine four metabolites were identified by
HPLC analysis. One of the two major metabolites eluted very near to
the solvent front and did not co-elute with acetic acid pyruvic acid
or lactic acid. The second metabolite co-eluted with 3-hydroxypro-
pionic acid. Traces of two other unidentified residues were also
detected. Radioactivity could not be detected at the retention times
corresponding to that of 2,3-epoxypropionic acid, glyceric acid or
N-acetyl- S-(2-carboxy-2-hydroxyethyl)-cysteine, suggesting that
acrylic acid is not epoxidized to 2,3-epoxypropionic acid in vivo.
It was suggested that acrylic acid was metabolized by the non-vitamin-
B12-dependent pathway for propionic acid metabolism, with degradation
to CO2 being the main route of elimination. Residual radioactivity in
tissues may be due to incorporation of 14C from acrylic acid into
acetyl-CoA (De Bethizy et al., 1987).
Using HPLC and NMR analysis, 3-hydroxypropionic acid, N-acetyl-
S-2-(2-carboxyethyl)-cysteine and N-acetyl- S-(2-carboxyethyl)-
cysteine-S-oxide were identified as urinary metabolites after oral
administration of (2,3-14C)-acrylic acid (400 mg/kg body weight in
water by gavage) to male Sprague-Dawley rats. According to the
authors, the detection of mercapturates may be a consequence of the
high dose used in this study (Winter et al., 1992).
HPLC analysis for acrylic acid and its metabolites in rats
revealed that a metabolite that coeluted with 3-hydroxypropionic acid
was found in the urine, plasma and liver of rats that had received
acrylic acid by gavage. Furthermore, a material that co-eluted with
authentic acrylic acid was detected in the urine and liver, but not in
the plasma, of these rats. Acrylic acid, but not 3-hydroxypropionic
acid, was also detected in the urine of rats after cutaneous
application (Black et al., 1995). In mice, 3-hydroxypropionic acid was
identified in the liver after gavage administration of acrylic acid.
No acrylic acid was detected in the liver of these animals (Black et
al., 1995).
6.2.2.3 Metabolic pathways
Acrylic acid is rapidly metabolized to CO2, a major metabolite
formed via acrylyl-CoA by the non-vitamin-B12-dependent pathway of
mammalian propionate catabolism (Finch & Frederick, 1992; Winter et
al., 1992; Black et al., 1993; Winter & Sipes, 1993). This pathway
occurs in the mitochondrion (Finch & Frederick, 1992) and consist of
reactions analogous to fatty acid ß-oxidation (Schultz, 1991).
ß-oxidation is the major route of propionate catabolism in many
invertebrates and plants (Wegner et al., 1968; Halarnkar & Blomquist,
1989); however the primary pathway of propionate catabolism in mammals
is that involving the vitamin-B12-dependent enzyme, methyl-malonyl-
CoA mutase (Black et al., 1993). A small amount of 3-hydroxypropionic
acid was identified as the major urinary metabolite of acrylic acid
(De Bethizy et al., 1987; Winter et al., 1992). There is no evidence
to suggest that epoxide intermediates are formed during the metabolism
of acrylic acid (De Bethizy et al., 1987). N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl) cysteine-
S-oxide were identified in the urine of rats that had received
400 mg/kg (2,3,-14C)-acrylic acid by gavage (Winter et al., 1992),
suggesting a direct reaction between acrylic acid and reduced
glutathione.
The major route of metabolism for acrylic acid esters has been
shown to involve the rapid cleavage of the ester bond by carboxyl
esterases (see Fig. 1) (Ghanayem et al., 1987; Sanders et al., 1988;
Frederik et al., 1994). Thus exposure to acrylic acid esters may
constitute a significant internal exposure to acrylic acid. A
secondary metabolic pathway involves conjugation of the acrylic acid
ester with glutathione to yield acetyl- S-(2-carboxyethyl) cysteine
alkylesters. (Ghanayem et al., 1987; Sanders et al., 1988). This
intermediate may be further metabolized to N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl)-cysteine-
S-oxide. However, it is currently uncertain what proportion of N-
acetyl- S-(2-carboxyethyl) cysteine, or its oxide, formed from the
metabolism of the acrylic acid esters originates from the reaction of
the intact ester with glutathione and what proportion originates from
the conjugation of the released acrylic acid with glutathione (see Fig
1).
On the basis of available information, proposed metabolic
pathways for acrylic acid are summarized in Fig. 1. The proposed
scheme also includes relationships between metabolism of acrylic acid
and its esters (e.g., ethyl acrylate) and metabolism of propionate via
the major vitamin-B12-dependent pathway.
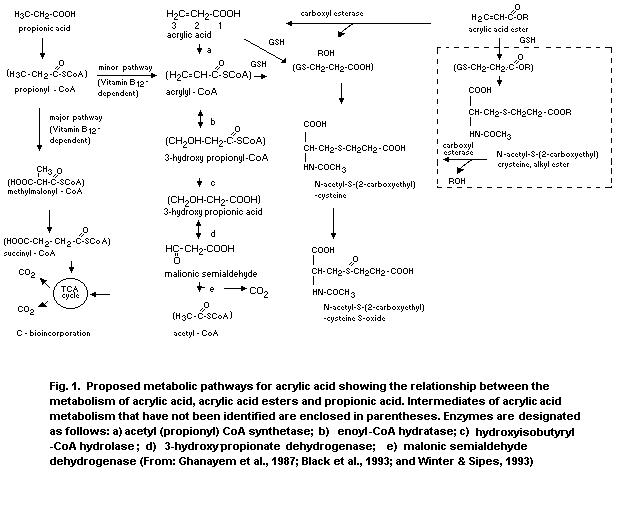 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of acrylic acid is difficult to ascertain,
owing to the wide range of LD50 values reported (Table 4). However
most data indicate that the substance is of low to moderate toxicity
by the oral and inhalation routes and of moderate toxicity by the
dermal route. It has been proposed that the wide variation in oral
LD50 values may be due to the different forms in which acrylic acid
has been applied, i.e. undiluted, in aqueous solution at various
concentrations or in neutralized solution (BG Chemie, 1991).
Stomach lesions, necrosis and haemorrhage have been reported
following oral dosing of rats with acrylic acid (Ghanayem et al.,
1985; DeBethizy et al., 1987). The lowest dose at which lesions were
seen was 144 mg/kg.
CrL:CDBR rats were exposed (whole body) to aerosol (mean mass
median diameter of 2.3 µm ± 2.3) concentrations of acrylic acid
ranging from 8775 to 14 145 mg/m3 (2925 to 4715 ppm) for 30 min, 8139
to 12 624 mg/m3 (2713 to 4208 ppm) for 60 min, and 3669 to
10 239 mg/m3 (1223 to 3413 ppm) for 120 min. Additionally, rats were
exposed (whole body) to acrylic acid vapour concentrations ranging from
2784 to 6426 mg/m3 (928 to 2142 ppm) for 60 min. Exposure to acrylic
acid produced treatment-related signs of nasal mucosa, upper airway and
lower airway irritation, ocular irritation, corneal opacities and
dermal toxicity in all experimental groups. Deaths, as a function of
both aerosol concentration and exposure duration, were seen in the
30-, 60- and 120-min aerosol exposures. No deaths resulted from the
vapour exposures. Following the 14-day observation period, necropsies
revealed treatment-related alterations of the lungs, eyes and skin
consistent with that of an irritant. However, comparison of LC values
suggested no difference in toxicity between the aerosol and vapour
(Hagan & Emmons, 1991).
Table 4. The acute toxicity (LD50 and LC50) of acrylic acid for experimental animals
Species Route Parameter Dose Reference
Mouse oral LD50 830 mg/kg Klimkina et al., 1969
Mouse oral LD50 1200 mg/kg Zeller, 1958
Rat oral LD50 193 mg/kg IARC, 1979
Rat oral LD50 340 mg/kg Carpenter et al., 1974
Rat oral LD50 1350 mg/kg Majka et al., 1974
Rat oral LD50 1500 mg/kg Zeller, 1958
Rat oral LD50 2520 mg/kg Fassett, 1963
Rat oral LD50 2100-3200 mg/kg Miller, 1964
Rat oral LD50 2500 mg/kg Verschueren, 1983
Mouse subcutaneous LD50 1590 mg/kg Sittig, 1985
Rabbit percutaneous LD50 295 mg/kg Carpenter et al., 1974
Rabbit percutaneous LD50 640 mg/kg Gelbke & Hofman, 1979
Rabbit percutaneous LD50 750 mg/kg IARC, 1979
Rabbit percutaneous LD50 950 mg/kg Fassett, 1963
Mouse inhalation LC50 (2 h) 5300 mg/m3 RTECS, 1989
Rat inhalation LC50 (30 min) 26 000 mg/m3 Hagan & Emmons, 1991
LC50 (60 min) 11 100 mg/m3
LC50 (120 min) 7500 mg/m3
Table 4. (contd.)
Species Route Parameter Dose Reference
Rat inhalation LC50 (4 h) 3600 mg/m3 Majka et al., 1974
Rat inhalation LC50 (4 h) > 5100 mg/m3 Klimisch & Zeller, 1980
Mouse intraperitoneal LD50 17 mg/kg Lawrence et al., 1972
Mouse intraperitoneal LD50 128 mg/kg RTECS, 1989
Mouse intraperitoneal LD50 140 mg/kg Zeller, 1958
Rat intraperitoneal LD50 24 mg/kg Majka et al., 1974
Rat intraperitoneal LD50 24 mg/kg Singh et al., 1972
In a single inhalation study in rats, no deaths occurred when
six animals were exposed to acrylic acid at a concentration of
12 000 mg/m3 (4000 ppm) for 4 h and observed over 14 days (Union
Carbide Corp., 1977).
A single 4-h exposure of six rats to 6000 mg/m3 (2000 ppm) of
acrylic acid caused no death (Carpenter et al., 1974).
One 5-h exposure to an atmosphere saturated with acrylic acid
(6000 ppm or 17 700 mg/m3) given to four rats (2 male, 2 female)
produced nose and eye irritation, respiratory difficulty and
unresponsiveness in all rats. One rat died. Histopathological
examination showed lung haemorrhage and degenerative changes in the
liver and kidney tubules of all rats (Gage, 1970), but these were
possibly secondary changes in dying animals.
Rats exposed for 1 h to acrylic acid concentrations of 300, 900
or 1500 mg/m3 (100, 300 or 500 ppm) exhibited exposure-dependent
decreases in both respiratory frequency and minute volume (Silver et
al., 1981).
In a sensory irritation study, the single exposure to acrylic
acid vapour estimated for a 50% reduction of the respiratory rate
(RD50) was 1539 mg/m3 (513 ppm) in F344/N rats and 2055 mg/m3
(685 ppm) in B6C3F1 mice. During exposure to 225 mg/m3 (75 ppm) of
acrylic acid vapour for 6 h, a 20-30% decrease in minute volume was
observed in both species (Buckley et al., 1984).
7.2 Irritation and sensitization
7.2.1 Eye irritation
Application of acrylic acid in different concentrations (glacial,
10%, 3% and 1%) to rabbit eyes revealed that it is corrosive in high
concentrations, i.e. glacial and 10%; 1% and 3% solution caused eye
irritancy (Majka et al., 1974). There are also other reports of
undiluted acrylic acid causing eye irritation and corneal damage
(Carpenter et al. 1974; BG Chemie, 1991).
7.2.2 Skin irritation and sensitization
7.2.2.1 Skin irritation
Undiluted acrylic acid is corrosive to rabbit skin (Carpenter et
al., 1974; Majka et al., 1974; BG Chemie, 1991). A study with rabbits
reported that a one-minute exposure to a 50% or 20% aqueous solution
caused, respectively, erythema and oedema or slight erythema (BG
Chemie, 1991). Another study reported a 10% solution to be corrosive
when applied to rabbit skin and that a 0.6-5% solution caused
irritation of various severity (Majka et al., 1974).
The irritant effects of repeated dermal exposure have also been
investigated. A 5% acrylic acid solution in acetone caused skin
irritation in the mouse after daily non-occlusive application for 14
days (DePass et al., 1984). No irritation was seen with a 1% solution.
In another study, groups of three strains of mice received dermal
applications of 0.1 ml acrylic acid in acetone 3 times a week for 13
weeks at concentrations of 0, 1 or 4% (Tegeris et al., 1987, 1988). At
4%, there were signs of significant skin irritation (desquamation,
fissures and eschar), with proliferative, degenerative and
inflammatory changes being detected histologically in the epidermis
and dermis, from weeks 1 to 2. At 1%, minimal proliferative changes,
detected histologically, were the only effects seen. No differences
were found between the response of the three strains of mice.
7.2.2.2 Skin sensitization
Acrylic acid has been tested for contact sensitivity in guinea-
pigs. In one study, a 20% aqueous solution of pure unstabilized
acrylic acid was applied to the skin once a day until definite skin
irritation was seen. When challenged topically 11 days later with a 2%
solution, there was no evidence of skin sensitization up to 24 h post-
challenge (BG Chemie, 1991).
In another study, the highest non-irritating concentration of
acrylic acid (not specified) was applied topically four times in 10
days. At the time of the third application, Freund's adjuvant was
injected intradermally. When challenged two weeks later, none of the
10 guinea-pigs showed evidence of skin sensitization (Rao et al.,
1981).
Three out of six guinea-pigs exposed to acrylic acid, said to be
99% pure, showed a skin sensitization response in a Polak test (Parker
& Turk, 1983). Induction was by dermal injections of a total of 1 mg
acrylic acid, together with adjuvant, followed by topical challenge
with 5% acrylic acid. However, the impurities and inhibitors of the
acrylic acid used were not mentioned in the report.
Acrylic acid was found to be an extreme sensitizer by the guinea
pig maximization test and a weak sensitizer by the Landsteiner Draize
test. The compound used for testing was considered pure, but no
analytical data were provided (Magnusson & Kligman, 1969).
Acrylic acid gave a clearly positive result in the Freunds
Complete Adjuvant test in guinea-pigs (Waegemaekers & van der Walle,
1984). Induction was by three intradermal injections of 1.2% followed
by topical application of 2.2 or 7.2%. The positive response was
believed to be due to the historical impurity, alpha,ß-
diacryloxypropionic acid. This impurity was identified in acrylic acid
from just one of three suppliers. Limited testing of acrylic acid from
the other two suppliers gave negative skin sensitization results. It
should be noted that the impurity is not present in acrylic acid
resulting from current production methods involving distillation.
Commercial acrylic acid also contains a small amount of
polymerization inhibitors, usually hydroquinone monomethyl ether
(methoxyphenol). This is a known skin sensitizer in guinea-pigs (van
der Walle et al., 1982). Other inhibitors used with acrylic acid have
also been reported to have skin-sensitizing properties, namely pheno-
thiazine (Costellati et al., 1990) and diphenyl- p-phenylenediamine
(Magnusson et al., 1968; Kalimo et al., 1989). However, it is unclear
whether the small amount of one of these inhibitors present
(0.02-0.1%) could contribute to the skin-sensitizing properties of
commercial acrylic acid.
7.2.3 Upper respiratory tract irritation
Olfactory cell proliferation, as measured by tritiated thymidine
incorporation, was investigated in male F-344 rats and B6C3F1 mice
exposed to 224 mg/m3 (75 ppm) acrylic acid 6 h daily for 5 days. A
17-fold increase in cell proliferation occurred in mice and a 4-fold
increase in rats (Swenberg et al, 1986). Further information on upper
respiratory tract irritation is given in sections 7.1 and 7.3.2.
7.3 Short-term exposure
Results of key studies on the short-term repeated exposure
effects of acrylic acid are presented in Table 5.
Table 5. Key studies on the noncarcinogenic effects of repeated exposures to acrylic acid
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Fisher-344 oral, 250 mg/kg bw/day 83 mg/kg bw/day Decreased body weight, De Pass et al.,
drinking-water, 0, 83, reduced water and food 1983
250, 750 mg/kg body consumption, changes in
weight/day for 3 months organ weights
Rat, Wistar, gavage, 0, 150 mg/kg bw/day 50% mortality in both Hellwig et al.,
150, 375 mg/kg bw/day, treatment groups, dose- 1993
5 times/week for 3 dependent irritation in the
months forestomach and
glandular stomach,
purulent rhinitis, tubular
nephroses
Rat, Wistar, oral,
drinking-water, 0, 9, 61,
140, 331 mg/kg body
weight/day for 3 months 331 mg/kg bw/day 140 mg/kg bw/day Reduced water and food Hellwig et al.,
consumption in males 1993
Rat, Wistar, oral, 140 mg/kg bw/day 61 mg/kg bw/day Reduced water and food Hellwig et al.,
drinking-water, 0, 9, 61, consumption in males 1993
140, 331 mg/kg body
weight/day for 12 months
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Wistar, oral, 78 mg/kg bw/day No treatment-related Hellwig et al.,
drinking-water, 0, 8, 27, toxic effects including 1993
78 mg/kg body tumorogenicity
weight/day for 26
(males) or 28 (females)
months
Rat, inhalation, 80, 300 300 ppm 80 ppm Nose irritation, lethargy Gage, 1970
ppm, 6 h/day 5 (900 mg/m3) (240 mg/m3) reduced body weight gain
days/week,
20 exposures
Rat, Fisher-344 225 ppm (675 75 ppm Decrease of adipose Miller et al.,
inhalation, 0, 25, 75, mg/m3) (225 mg/m3) tissue in females, lesions 1979
225 ppm, 6 h/day, of nasal mucosa
5 days/week for 2 weeks
Rat, Fisher 344 75 ppm 25 ppm Lesions of nasal olfactory Miller et al.,
inhalation, 0, 5, 25, (225 mg/m3) (75 mg/m3) epithelium 1981
75 ppm, 6 h/day,
5 days/week for
13 weeks
Rat, F-344, and mouse 75 ppm Olfactory cell proliferation Swenberg et al.,
B6C3F1 inhalation, (225 mg/m3) 17-fold in mice, 4-fold in 1986
75 ppm, 6 h/day, 5 days rats
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Mouse, B6C3F1 25 ppm Decrease in body weight Miller et al.,
inhalation, 0, 25, 74, (75 mg/m3) gain, lesions in nasal 1979
223 ppm, 6 h/day mucosa
5 days/week for 2 weeks
Mouse, B6C3F inhalation 5 ppm 5 ppm Atrophy, disorganization, Lomax et al.,
0, 5, 25 ppm for 6 or 22 22 h/day 6 h/day necrosis of the olfactory 1994
h/day and 25 ppm for epithelium of nasal
4.4 h/day for 2 weeks, cavity. Recovery after 6
6 weeks recovery period weeks except for mice
exposed to 25 ppm for
22 h/day where
metaplasia was seen
Mouse, B6C3F1 5 ppm Slight focal lesions of Miller et al.,
inhalation, 0, 5, 25, (15 mg/m3) nasal olfactory 1981
75 ppm, 6 h/day 5 epithelium
days/week for 13 weeks
a LOEL = lowest-observed-effect level; NOEL = no-observed-effect level
7.3.1 Oral
Acrylic acid was administered via oral gavage to ten rats for 20
days with doses increasing by 50% every fourth day (range:
135 mg/kg to 684 mg/kg). Reduction in body weight gain and minor
histopathological changes in the stomach were found at higher doses
(Majka et al., 1974).
In a 3-month study (Hellwig et al., 1993), groups of 10 male and
10 female Wistar rats were gavaged, 5 times per week, with acrylic
acid at doses of 150 or 375 mg/kg body weight. A control group of 10
males and 10 females was gavaged with water. A high mortality rate was
observed in experimental groups; 50% of both males and females in the
low-dose group and 60% (males) and 90% (females) in the high-dose
group died. Cyanosis, dyspnoea and irritation ulceration of
forestomach and glandular stomach, purulent rhinitis and lung
emphysema and alveolar hyperaemia were the main findings reported.
Necrotizing tubular nephroses were seen in the animals that died
during the study. The symptoms and histopathological findings were
substantially the same in both groups, but they were less pronounced
and observed in a smaller number of animals given acrylic acid at
150 mg/kg body weight.
Acrylic acid was given to Wistar rats in drinking-water for
3 months as part of a 12-month study (Hellwig et al., 1993). Further
details are given in section 7.4.
In a subchronic study acrylic acid was incorporated into the
drinking-water of rats (15/sex/group) for 3 months, resulting in doses
of 0,83, 250 and 750 mg/kg per day. At the high and intermediate dose
levels, reduction in body weight gain and changes in organ weights
were observed. These effects coincided with a dose-related reduction
in food and water consumption. At the 83 mg/kg dose, the only effect
was a slight reduction in water consumption. No significant treatment-
related histological effects were seen at any dose level (DePass et
al., 1983).
7.3.2 Inhalation
In a short-term inhalation study (Gage, 1970) no adverse effects
were observed in eight rats (four males and four females) exposed to
240 mg/m3 (80 ppm) acrylic acid vapour, 6 h/day, 5 days/week for 20
exposures. Eight rats (four males and four females) exposed at
900 mg/m3 (300 ppm) showed signs of nasal irritation, lethargy and
reduced body weight gain. Histological and haematological examinations
were normal. Higher doses for shorter periods of time, i.e. 4500 mg/m3
(1500 ppm) for 4 × 6 h, resulted in nasal discharge, lethargy,
retarded weight gain and kidney congestion.
In a 2-week subacute inhalation study by Miller et al. (1979a,
1981a), F-344 rats and B6C3F1 mice (5/sex/group) were exposed to
actual concentrations of 0, 75, 225 or 675 mg/m3 (0, 25, 75 or
225 ppm) acrylic acid vapour for 6 h/day, 5 days/week for 2 weeks.
Significant decreases in body weight gain were seen in exposed groups
at 675 mg/m3. Decreased body weight gain in male mice at 75 and
225 mg/m3 was not considered to be exposure-related because of the low
initial weight and unusually large weight gain in the controls. A
reduction of adipose tissue was observed in female rats at 675 mg/m3.
Rats had lesions of the nasal mucosa at 675 mg/m3. In mice lesions of
the nasal mucosa were observed in 2/5 males and 4/5 females at 75 mg/m3
and in all mice exposed to 225 or 675 mg/m3. The lesions were
described as slight focal degeneration in the olfactory epithelium. No
effects on lung or trachea were observed in rats or mice.
Fifteen F-344 rats/sex/group and 15 B6C3F1 mice/sex/group were
exposed to actual concentrations measured by infrared analysis of 0,
15, 75 or 225 mg/m3 (0, 5, 25 or 75 ppm) acrylic acid (Miller et al.,
1979a, 1981a). The exposure was 6 h/day for 5 days/week for 13 weeks.
Animals were observed twice per day. There were no treatment-related
deaths in rats or mice during the study period. Mean body weight gains
of female mice in the 75 and 225 mg/m3 exposure groups after 12 weeks
of exposure were significantly lower than controls. There were no
significant differences in organ weights, clinical chemistry
parameters, urinalysis parameters or gross pathology that could
clearly be related to exposure. Slight focal degeneration of the nasal
olfactory epithelium was observed in rats at 225 mg/m3 but no effects
were seen at 15 or 75 mg/m3. In mice, there was a clear exposure-
related increase in focal degeneration of the olfactory nasal
epithelium at > 25 mg/m3. Lesions of the olfactory epithelium were
detected histopathologically in all male and female mice in the
225 mg/m3 exposure group, in all males and 9/10 females in the
75 mg/m3 group and in 1/10 males and 4/10 females in the
15 mg/m3 group. The lesions were described as very slight at 15 mg/m3,
slight at 75 mg/m3 and slight to moderate at 225 mg/m3.
In a whole-body inhalation study, groups of 10 B6C3F1 female
mice were exposed to 0, 15 or 75 mg/m3 (0, 5 or 25 ppm) acrylic acid
vapour for 6 or 22 h/day for 2 weeks. An additional group of mice was
exposed to 75 mg/m3 for 4.4 h/day for 2 weeks. No histopathological
lesions in the nasal cavity were detected in mice exposed to 0 or 15
mg/m3 for 6 h/day. Histopathological lesions were observed in the
olfactory epithelium of the dorsal meatus of the nasal cavity in all
other groups. These lesions included atrophy, disorganization,
necrosis, desquamation of the epithelium and nasal cell hypertrophy.
Lesions were more severe after the 22 h exposure at 75 mg/m3. After a
6-week recovery period, the olfactory epithelium was normal except for
mice exposed to 75 mg/m3 for 22 h/day where metaplasia was seen
(replacement of olfactory epithelium with respiratory-like epithelium)
(Lomax et al., 1994).
Animals exposed to 720 mg/m3 (240 ppm) for 4 h/day, 6 days/week,
for 5 weeks exhibited decreased body weight gain, increased urinary
secretion of phenol red, reduced urine-concentrating ability, nasal
discharge and increased reticulocyte count. Histopathological
examination revealed lesions of the gastric mucosa after repeated
doses of acrylic acid. Inflammation of the upper respiratory tract was
also seen (Majka et al., 1974).
7.4 Long-term exposure
Results of the studies of long-term repeated exposure effects to
acrylic acid are presented in Table 5.
In a 12-month study (Hellwig et al., 1993), Wistar rats
(20/group/sex) were given drinking-water containing 120, 800, 2000 or
5000 mg/litre (equivalent to doses of 9, 61, 140, 331 mg/kg body
weight/day, respectively). Satellite groups (10 rats/group/sex) were
treated concurrently for 3 months. Both in satellite groups and
12-months exposure groups, a reduction in drinking-water and/or feed
consumption and retarded body weight gain were observed in males at
140 and 331 mg/kg per day (2000 and 5000 mg/litre), probably due to
the unpalatability of acrylic acid at these concentrations.
Differences between groups in the results of clinico-chemical,
haematological and urinalytical examinations as well as in gross
pathological and histopathological findings were not treatment-
related. The no-observed-effect level (NOEL) was 61 mg/kg body weight
(800 mg/litre).
Acrylic acid was given to Wistar rats in drinking-water for 26
(males) and 28 (females) months in a chronic/carcinogenicity study
(Hellwig et al., 1993). Further details are given in section 7.7.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
Results of key studies on the reproductive system are shown in
Table 6.
Table 6. Reproductive effects of acrylic acid exposure in female rats and mice and developmental effects in their offspring
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Reproduction Rat Fischer 250 mg/kg 83 mg/kg 750 mg/kg 250 mg/kg Decreased body Decrease in
and development 344; oral, bw/day bw/day bw/day bw/day weight and food body weight gain
(dePass et al., drinking- consumption of male and
1983) water; 0, 83, female pups,
250, 750 mg/kg decrease in
bw/day for 3 liver, kidney,
months before heart and spleen
mating and weight in male
through-out pups and liver
gestation and weight in female
lactation pups
Two-generation Rat Wistar; 240 mg/kg 53 mg/kg 240 mg/kg 53 mg/kg Fertility not Retarded growth
reproductive oral, drinking- bw/day bw/day bw/day bw/day affected. Reduced of F1 and F2 pups
toxicity water, 0, 500, food and water
(BASF, 1994a) 2500, 5000 ppm consumption,
(53, 240, reduced body
460 mg/kg/day) weights, gross
and
histopathological
changes in stomach
Developmental Rat Sprague- - - 2.4 mg/kg No data Increased
toxicity Dawley, bw resorptions
(Singh et al., intraperitoneal and number
1972) 0, 2.4, 4, 8, of gross and
8.0 mg/kg bw skeletal
i.p. on days 5, abnormalities
10 and 15 of
gestation
Table 6. (contd.)
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Developmental Rat Sprague- 360 mg/m3 - - 1080 Decreased None
toxicity Dawley, (120 ppm) mg/m3 body weight,
(Klimisch & inhalation, (360 ppm) food consumption
Hellwig, 0, 40, 120, uterus-to-body
1991) 360 ppm for weight; eye and
10 days during nose irritation
gestation
(days 6
through
15)
Developmental Rabbit New 180 90 mg/m3 - -- Squamous -
toxicity Zealand white, mg/m3 (30 ppm) metaplasia
(Chun et al., inhalation, 0, (60 ppm) erosions,
1993) 30, 60, 125, ulcerations in
250 ppm (0, olfactory
90, 180, 375, epithelium
750 mg/m3)
during
gestation
(days 10-23)
Developmental Rabbit New 225 mg/m3 75 mg/m3 - 675 Perinasal, None
toxicity Zealand white, (75 ppm) (25 ppm) mg/m3 perioral
(Neeper- inhalation (225 ppm) wetness,
Bradley & 0, 25, 75, nasal
Kubena, 1993) 225 ppm congestion,
(0, 75, 225, blepharospasm
675 mg/m3)
during
gestation
(days 6-18)
A one-generation study was conducted in which 10 male and 20
female rats received acrylic acid orally, in drinking-water, at doses
of 83, 250 or 750 mg/kg body weight per day for 3 months, after which
the animals were mated; this formed part of the subchronic study
reported in section 7.3.1. (DePass et al., 1983). Exposure was
continued throughout gestation and lactation.
Maternal toxicity at the two highest doses included decreased
body weight gain and decreased food consumption. An apparent decrease
in the fertility of females, as well as a reduction in gestation
index, number of live pups/litter and percentage of pups weaned at the
highest dose, was observed, but this difference was not statistically
significant when compared with the control group. Unusually low
fertility in the control group makes interpretation difficult.
In a two-generation reproductive toxicity study (BASF, 1994a),
Wistar rats were exposed to acrylic acid in drinking-water at levels
of 500, 2500 or 5000 mg/litre. Acrylic acid did not impair
reproductive function in either of the parental generations exposed to
any dose level. Clear signs of toxicity, including reduced food and
water consumption, reduced body weight and/or body weight gain, and
gross and histopathological changes in the stomach, were observed in
both parental generations exposed to 5000 mg/litre. At the
2500 mg/litre level, signs of toxicity were present in the second
parental generation animals. No adverse effects were observed in either
parental generation exposed to 500 mg/litre. Signs of developmental
toxicity, including retarded growth of F1 and F2 pups and some
delays in physical development of F2 pups only, were observed at
5000 mg/litre but less so at 2500 mg/litre. It is difficult to evaluate
the role of acrylic acid in the decreased pup weights, because of the
combined effects of reduced water consumption and the poor
palatability of water containing acrylic acid. The greatest effects on
body weight occurred during the period of early life around weaning.
Developmental toxicity was not observed at 500 mg/litre. Thus,
500 mg/litre (equivalent to 53 mg/kg per day) was a NOEL for both
generations of offspring and also a NOAEL for general toxicity in the
second parental generation. The NOEL for general toxicity in the first
parental generation was 2500 mg/litre (equivalent to 240 mg/kg per
day).
In a study by Vojtisek et al. (1991) cows were given 16 kg/day
clover grass silage, treated with 3 litres acrylic acid/tonne, from
day 57.6 ± 21.1 before parturition, for 107 days. Control groups of
eight cows were given the same amount of silage, but treated with 4
litres formic acid/tonne from day 55.5 ± 21.9 before parturition.
Changes in clinical chemistry and haematological parameters observed
in the course of the experiment (on days 0, 39, 65 and 107) were of no
toxicological importance. No differences in colostrum density, body
weight, clinico-chemical or haematological examinations of live calves
were seen.
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Oral
In the experiment of DePass et al., (1983) (see also section
7.5.1) there was a statistically significant decrease in body weight
of the male and female pups at the highest dose (750 mg/kg per day),
which was maternally toxic. The male pups also exhibited significant
decreases in absolute and relative liver weights and in absolute
kidney and heart weights at 750 mg/kg per day. The female pups showed
a significant decrease in absolute and relative spleen weight and in
absolute liver weight at the highest dose. There was an increase in
relative brain weight in both sexes at this dose.
7.5.2.2 Inhalation
Groups of 30 pregnant Sprague-Dawley rats were exposed to nominal
concentrations of 0, 120, 360 or 1080 mg/m3 (0, 40, 120, or 360 ppm)
acrylic acid vapour for 6 h/day for 10 days during gestation (day 6 to
day 15 of gestation) (Klimisch & Hellwig, 1991). There was clear
evidence of maternal toxicity at 1080 mg/m3 consisting of eye and
nose irritation, as well as reduced body weight gain and food
consumption. The latter two effects were also seen at 360 mg/m3 and
there was an indication of minimal maternal toxicity at 40 ppm
(reduced body weight gain). There were no effects on preimplantation
loss, the number of live fetuses and resorption, fetal size or on the
appearance of the soft tissues and skeleton of the fetuses. This study
identified a NOAEL for developmental effects of 1080 mg/m3 and a
LOAEL for maternal toxicity of 1080 mg/m3.
An inhalation developmental study has also been reported in
rabbits (Neeper-Bradley & Kubena, 1993). In the range-finding study
(Chun et al., 1993), groups of eight pregnant New Zealand white
rabbits were exposed to 0, 90, 180, 375 or 750 mg/m3 (0, 30, 60, 125
or 250 ppm) acrylic acid vapour for 6 h/day on days 10-23 of
gestation. Three animals per group were necropsied on day 23 of
gestation, and the remaining animals were examined on day 29.
Exposure-related maternal toxicity at 375 and 750 mg/m3 was observed,
including signs of nasal irritation and reduced body weight. Final
body weight was reduced to a lesser degree in animals exposed to 90
and 180 mg/m3. Histopathological examination of a single section of
the nose showed adverse effects in the olfactory epithelium. The
lesions included squamous metaplasia, epithelial erosion and
ulceration of the epithelium, and increased in severity with
increasing exposure concentration. The effect first appeared in the
90 mg/m3 group at day 23 and in the 180 mg/m3 group at day 29.
In the main developmental study (Neeper-Bradley & Kubena, 1993), groups
of 16 pregnant rabbits were exposed to 0, 75, 225 or 675 mg/m3 (0, 25,
75 or 225 ppm) acrylic acid vapour for 6 h/day on gestation days 6-18.
Maternal toxicity was evident in groups exposed to 225 or 675 mg/m3,
but not to 75 mg/m3 (NOEL). Signs of nasal irritation, including
perinasal wetness and nasal congestion, were observed. Significant
decrements in food consumption and body weight gain were observed
occasionally during exposure, but the body weights at the end of the
exposure were not significantly affected. Histopathological
examination of maternal tissues was not performed. No exposure-
related adverse effects were observed in the number of corpora lutea
and total, viable or nonviable implantations; preimplantation loss;
fetal length or weight; or on morphological abnormalities (skeletal
or soft tissue). This study identified a NOEL for developmental
effects of 675 mg/m3.
7.5.2.3 Intraperitoneal
When acrylic acid was injected intraperitoneally into pregnant
female Sprague Dawley rats at doses of 0, 2.4, 4.8, or 8.0 mg/kg on
days 5, 10 and 15 of gestation, the chemical was both embryotoxic and
teratogenic (Singh et al., 1972). The number of resorptions and the
number of gross and skeletal abnormalities increased with increasing
acrylic acid concentration, and the NOEL was identified at the low
dose (i.e. 2.4 mg/kg). Fetotoxicity (decreased number of live fetuses
and mean fetal weight) was observed at a dose of 2.4 mg/kg. This study
is difficult to interpret as the control groups treated with distilled
water, normal saline and cotton-seed oil also showed gross and
skeleton abnormalities. There was also no information about maternal
toxicity.
7.6 Mutagenicity and related end-points
7.6.1 In vitro and insect studies
Acrylic acid was found to be without mutagenic activity in five
test strains of Salmonella typhimurium with and without activation
by rat and hamster liver microsomal preparations using both plate and
liquid suspension assays (Lijinsky & Andrews, 1980). Although negative
responses were reported in all strains, no cytotoxicity was observed
at the concentrations tested (up to 1000 µg/plate).
Acrylic acid was also evaluated for mutagenicity in the
Salmonella/microsome preincubation assay using the standard protocol
approved by the National Toxicology Program. Acrylic acid was tested
at doses of 0, 10, 33, 100, 333, 1000 and 3333 µg/plate in four
Salmonella typhimurium strains (TA98, TA100, TA1535 and TA1537) in
the presence and absence of Aroclor-1254-induced rat or hamster liver
S9. Acrylic acid was negative in these tests and the highest non-toxic
dose level tested in any Salmonella test strain was 1000 µg/plate. The
3333 µg/plate dose level was toxic and caused a complete clearing of
the background lawn (Zeiger et al., 1987).
In another study (Cameron et al., 1991) acrylic acid was also
found to be negative in the Salmonella assay, both in the presence and
the absence of both Aroclor-1254-induced rat or hamster liver S9 mix.
Three mammalian gene mutation studies have been reported. No
increase in mutation frequency was seen in a CHO/HPRT gene mutation
assay with or without Aroclor 1254-induced rat liver S9 (McCarthy et
al., 1992). The upper dose levels in this single experiment resulted
in a reasonable amount of toxicity (survival reduced by up to 65-76%).
In two mouse lymphoma L5178Y TK+/- studies positive results have been
obtained. A concentration-related increase in mutant frequency was
observed with and without rat liver S9 in association with an
acceptable level of toxicity (Cameron et al., 1991). The authors did
not state if there was any adjustment of pH. In the other study,
conducted without exogenous metabolic activation, there were
concentration-related increases in mutant frequency in two separate
experiments in the presence of marked but not excessive toxicity.
Small colonies predominated, which suggested a clastogenic effect;
this was confirmed by chromosome analysis. Acrylic acid was tested at
concentrations of 300, 450 and 500 µg/ml in this study but the authors
did not state if there was any adjustment of pH (Moore et al., 1988).
A single experiment was conducted with CHO cells exposed to
acrylic acid solutions adjusted to pH 7 at concentrations that reduced
cloning efficiency by up to 58-65% (McCarthy et al., 1992). A
concentration-related increase in the percentage of cells with
chromosome aberrations, primarily chromatid breaks and exchanges, was
observed in the presence and absence of rat liver S9. A positive
result has also been briefly reported for this in vitro chromosome
aberration assay using CHL cells in the absence of exogenous metabolic
activation (Ishidate, 1988).
The effect of acrylic acid on unscheduled DNA synthesis (UDS) in
rat hepatocytes has been investigated in one unreplicated assay
(McCarthy et al., 1992). There was no increase in UDS at
concentrations up to those closely approaching a very toxic level.
Similar dose levels of acrylic acid in a single UDS experiment with
SHE cells gave a negative result (Wiegand et al., 1989).
Negative results have also been obtained in a micronucleus test
and a transformation test, both with SHE cells, as well as in the
Drosophila sex-linked recessive lethal assay (Wiegand et al., 1989;
McCarthy et al., 1992).
In a study by Segal et al. (1987), it was reported that acrylic
acid forms 2-carboxyethyl adducts with adenine, guanine and thymine
following in vitro reaction with calf thymus DNA. These adducts are
identical to those formed with the same bases following in vitro
reaction of carcinogen ß-propionolactone. The relevance of the results
of this study is questionable because appropriate control treatments
were not conducted and the 2-carboxyethyl adducts were formed after
long treatment period.
In contrast to the results above, Frederick & Reynolds (1989)
found that incubation of the negatively charged acrylate anion with
two representative nucleophiles, methylamine and imidazole, did not
result in the formation of adducts of the acrylate ion to the
nucleophile.
It is suggested that binding of acrylic acid to cellular
nucleophiles might be due to small amounts of the unionized acid in
the equilibrium between acrylate anion and acrylic acid at cellular
pH. However, this event is considered to be insignificant in vivo,
based upon the rapid metabolism and excretion of acrylic acid
(Frederick & Reynolds, 1989).
7.6.2 In vivo mammalian studies
Preliminary results indicated no DNA adducts in the stomach and
liver of rats after oral dosing (Sagelsdorf et al., 1988). Although
DNA adducts were found in the skin of mice after dermal application,
the investigators concluded that further work was needed to confirm
the significance of these findings. No firm conclusions can therefore
be drawn from these preliminary results.
No increase in the incidence of chromosomal aberrations was
observed in the bone marrow of rats following acute or repeated
exposure to acrylic acid (McCarthy et al., 1992). Rats either received
a single gavage dose of up to 1000 mg/kg acrylic acid or were exposed
to up to 5000 mg/litre in the drinking-water for 5 days. There was no
effect on the mitotic index of the bone marrow but there was evidence
of systemic toxicity.
Negative results were reported in a dominant lethal assay with
mice following acute or repeated exposure to acrylic acid (McCarthy et
al., 1992). Male CD-1 mice received either a single gavage dose of up
to 324 mg/kg or daily gavage doses of up to 162 mg/kg for 5 days. The
fact that the dose levels were selected not to exceed the LD1 for
acute dosing and 0.5 LD1 for repeat dosing limits the value of this
study.
7.7 Carcinogenicity
In a carcinogenicity study (Hellwig et al., 1993), Wistar rats
(50/group/sex) were given acrylic acid in the drinking-water at
concentrations of 0, 120, 400 or 1200 mg/litre (0, 8, 27, or
78 mg/kg body weight per day, respectively) over 26 (males) or 28
(females) months. The highest concentration was selected because of
evidence of palatability problems at 2000 and 5000 mg/litre in an
earlier chronic drinking-water study. At 1200 mg/litre there was some
evidence for slightly reduced water consumption. In comparison with
the controls, mortality was not increased by administering acrylic
acid. No clear toxic effects were revealed in the groups. The few
statistically significant differences in some haematological
parameters between groups were considered to be of an incidental
nature. The extensive histopathological examination revealed no clear
treatment-related non-neoplastic tissue changes. The incidence and
organ distribution of the tumours found in the groups treated with
acrylic acid for 26/28 months did not differ from those of the
controls. In this study the NOEL was 78 mg/kg body weight per day.
It has been reported that when 25 µl of a 1% solution
(i.e. 0.25 mg) of acrylic acid was applied topically in acetone
on the dorsal skin three times a week for their lifetime to 40
male C3H/HeJ mice, no malignancies were observed at the site of
application (De Pass et al., 1984).
When 1 mg (14 µmol) of acrylic acid was applied topically in
acetone 3 times a week to 30 female ICR/HA (currently designated
Hsd:(ICR)Br) mice for 1.5 years, squamous cell carcinomas were
observed in two of the mice (Cote et al., 1986). No conclusion can be
drawn from this study, because only an abstract was issued, it was not
subsequently published in full and an independent review of this study
(Sivak, 1987) uncovered many inconsistencies and hence questioned the
validity of the findings.
Acrylic acid was assayed for carcinogenic activity in female
Hsd:(ICR)bR mice by subcutaneous injection of 20 µmol (approx 1.4 mg)
in 0.05 µl trioctanoin, once a week for 52 weeks. The mice were then
observed for an additional 93 days (total 450 days) when the survivors
were killed. Two mice with sarcomas at the site of application were
observed out of 30 mice (Segal et al., 1987). This would be an
expected finding as an irritant solution was repeatedly given by
subcutaneous injection. Injections for this length of time frequently
result in sarcomas, no matter what the compound, because of repeated
insult.
7.8 Other studies
In contrast to some of its esters, acrylic acid did not
significantly decrease non-protein sulfhydryl content (NPSH) in the
liver, blood or forestomach after oral dosing of rats with up to
400 mg/kg body weight (as 8% acrylic acid solution in 0.5% methyl-
cellulose solution). However, a significant depletion of NPSH was
observed in the glandular stomach at doses above 4 mg/kg (as 0.8%
acrylic acid solution) (Miller et al., 1981b; De Bethizy et al., 1987).
Acrylic acid was found to be ineffective in inducing GSH
depletion, lipid peroxidation and haemolysis (Ferrali et al., 1989).
It was reported that methylethyl- n-butyl and 2-ethyl-hexyl
acrylates, when inhaled by male Wistar rats, induced hyperglycaemia
but that acrylic acid was without effect (Vodicka et al., 1990).
Rats received neutralized acrylic acid (50 mg/kg) intra-
peritoneally for 8 consecutive days and were observed for central and
peripheral nervous system effects. They showed a 25 % decrease in body
weight gain but no signs of neurotoxicity (Kohriyama et al., 1994).
In an in vitro study, acrylic acid was more potent than
acrylamide as an inhibitor of creatinine kenase (CK) activity in rat
brain homogenates. However, when the two chemicals were given
intraperitoneally 50 mg/kg per day for 8 days to rats, only acrylamide
inhibited CK activity in the brain. The 14C level in the brain, 24 h
after the injection of 14C-labelled chemicals, was more than seven
times greater with acrylamide then with acrylic acid (Kohriyama et
al., 1994).
Single intraperitoneal injections of up to 2.2 mg/kg body weight
of acrylic acid to Wistar rats had no effect on the hepatic activity
of ornithine decarboxylase, which was measured for tumour-promoting
activity (van de Zande et al., 1986).
Laparotomies were carried out on pregnant Sprague-Dawley rats on
day 13 of pregnancy under anaesthesia, and half of the developing
fetuses were injected with 10, 100 or 1000 µg acrylic acid per fetus.
One fetus injected with 100 µg acrylic acid showed slight
hydrocephalus and microcephalia. A dose of 1000 µg resulted in 78%
resorption (Slott & Hales, 1985).
It was reported that acrylic acid interferes with incorporation
of thymidine into DNA and uracil into RNA in Staphylococcus aureus
and Escherichia coli (Glombitza & Heyser, 1971).
7.9 Factors modifying toxicity
No studies on factors modifying the toxicity of acrylic acid have
been reported.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Acute toxicity
8.1.1.1 Poisoning accidents
There have been no reports on poisoning accidents in the general
population or on the short- or long-term effects of acrylic acid on
the general population.
8.2 Occupational exposure
8.2.1 Poisoning accidents
There have been no reports on poisoning accidents due to
occupational exposure.
8.2.2 Effects of short- and long-term exposure
Fowler (1990) described the case of a male chemical worker who
developed acute generalized urticaria after working with acrylic acid
and acrylate compounds. Immediate hypersensitivity testing showed a
severe local reaction to 2% acrylic acid (which was apparently not
irritant), but no reaction to other acrylate compounds. The acrylic
acid used for testing was thought to be pure but it was not analysed
for the presence of alpha,ß-diacryloxypropionic acid. Re-exposure of
the worker in the workplace to acrylic acid resulted in generalized
urticaria.
No cross-sensitization to 0.1% acrylic acid was observed in six
patients who had exhibited hypersensitivity to acrylate-based sealants
(Conde-Salazar et al., 1988).
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
9.1 Microorganisms
The toxic threshold (EC3) level for inhibition of growth of the
bacterium, Pseudomonas putida was 41 mg/litre after exposure for
16 h to a neutralized solution of acrylic acid (Bringmann & Kühn,
1980). Stewart et al. (1995) studied the anaerobic degradation of
acrylic acid in 55-day tests in glucose-acetate enrichment culture. At
100 mg/litre of acrylic acid there was minimal effect on acetate
utilization by methanogens; concentrations of 500, 1000 and
1500 mg/litre inhibited acetate utilization for a period of time
before recovery.
In a study of the effect of acrylic acid on the soil carbon
cycle, it was shown that acrylic acid in soil (sandy loam soil), at
levels of up to 100 mg/kg soil, had no effect on the respiration of
the soil microflora. However, a concentration of 1000 mg/kg acrylic
acid completely suppressed respiration (Hossack et al., 1992).
9.2 Aquatic organisms
The toxicity of acrylic acid to aquatic organisms is summarized
in Table 7. Algae appear to be the most sensitive group with EC50
values, based on growth, ranging from 0.04 to 63 mg/litre; and a NOEC
for the most sensitive species of 0.008 mg/litre. Other acute toxicity
studies range from 27 mg/litre (96-h LC50) for the rainbow trout to
315 mg/litre (72-h LC50) for the golden orfe. A 96-h NOEC for glacial
acrylic acid toxicity to rainbow trout was found to be 6.3 mg/litre
based on a lack of sublethal/behavioural responses, e.g., quiescence,
fish on bottom of test vessel, loss of equilibrium and erratic
swimming, at this concentration (Bowman, 1990). Neutralized acrylic
acid was found to be less toxic to daphnids than the non-neutralized
solution (Bringmann & Kühn, 1982).
9.3 Terrestrial organisms
No data on terrestrial organisms have been reported.
Table 7. Toxicity of acrylic acid to aquatic organisms
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Blue-green CMIT Exposure duration 8 days; TT (EC3) 0.15 the toxic threshold Bringmann &
alga temp. 27°C; neutralized with extinction Kühn, 1978
Microcystis pollutant solution; static values > 3% lower
aeruginosa closed flasks shaken once than for controls
a day; the
concentration of the
algae suspension measured
turbidimetrically at 578 nm
Green alga CMIT Exposure duration 7 days; TT (EC3) 18 as above Bringmann &
Scenedesmus temp. 27°C; neutralized Kühn, 1980
quadricauda pollutant solution; static
closed flasks shaken once a
day; the concentration of
the algal suspension measured
turbidi-metrically at 578 nm
Green alga CMIT 96-h static algal assay; EC50 0.17 50% algal Forbis, 1989
Selenastrum acrylic acid exposure growth
capricornutum concentrations ranged from inhibition
(Printz) 0.15 to 1.9 mg/litre
Green alga algal Exposure duration EC50 0.04 50% algal BASF, 1994b
Scenedesmus inhibition 72 h biomass
subspicatus test inhibition
EC50 0.13 50% algal
growth rate
LOEC 0.016 inhibition
NOEC 0.008
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Green alga algal Exposure duration EC50 1.53 50% algal SNF, 1995
Chlorella inhibition 72 h; nominal acrylic biomass
vulgaris test (OECD acid exposure inhibition
No. 201) concentrations
ranged from 0.2 to EC50 63.0 50% algal
4.7 mg/litre for growth rate
biomass test and were inhibition
50 and 100 mg/litre
for growth rate
inhibition test
Protozoan CMIT Exposure duration TT (EC5) 20 the toxic Bringmann &
Entosiphon 72 h; temp. 25°C; threshold with Kühn, 1982
sulcatum pollutant solution extinction
(Stein) adjusted to pH 6.9; values > 5%
static closed flasks; lower than for
the number of controls
protozoans was
determined by
means of cell counter
Water flea Daphnia Exposure duration EC50 765 after 24 h Bringmann &
Daphnia immobilization 24 h; temp. 20°C; 50% of exposed Kühn, 1982
magna test pH 7.8-8.2; organisms were
(Straus) performed in 50 ml immobilized in
beakers covered with neutralized test.
filter paper;
10 organisms/
20 ml test solution;
9 h illumination/
15 h dark
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
EC100 5000 100% immobilized
after 24 h in
neutralized test
Acrylic acid in
non-neutralized
test
EC50 54 in non-neutralized
test
EC100 91
Water flea Daphnia Dynamic test. Acrylic acid EC50 95 after 48 h 50% of Burgess, 1990
Daphnia magna immobilization exposure concentrations exposed organisms
test ranged from 7.9-110 were immobilized
mg/litre.
Exposure duration 48 h; NOEC 23 based on mortality
temp. 19-20°C; pH 6.7-7.7;
dissolved O2 7.9-8.0 mg/litre
Golden orfe L15-Fish test Exposure duration LC0 210 no lethality Juhnke &
Leuciscus idus German 48 h; temp. 19-21°C; pH 7-8; Luedemann,
melanotus standard dissolved O2 1978
method for the > 5 mg/litre; 10-fish LC50 315 death of 50%
contaminants per 10 litre test of exposed
of water, solution; continuous fish
waste water aeration
and sludge
LC100 420 death of all
exposed fish
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Rainbow trout A flow-through Exposure duration 96 h; LC50 27 death of 50% Bowman,
Oncorhynchus toxicity test measured glacial acrylic of exposed 1990
mykiss acid exposure NOEC 6.3 fish
concentrations ranged lack of
from 6.3 to 90 mg/litre; sublethal/
20 fish per group behavioural
responses
Common carp Exposure duration LC100 100 Nishiuchi,
Cyprinus 24 h 1975
carpio
a CMIT = Cell multiplication inhibition test
b TT = Toxicity threshold
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure of the general population
Very limited data are available on general population exposure.
Exposure of the general population to acrylic acid may occur by the
dermal route via contact with unreacted acrylic acid in household
goods such as water-based paints or detergents. Exposure is also
possible from inhalation due to evaporation of acrylic acid from
paint.
Exposure of the general population through drinking-water and via
ambient air might be assumed. Exposure through drinking-water is
possible from surface or groundwater contamination while inhalation
exposure could occur in the vicinity of industrial emissions. However,
there is no data on measured ambient air concentrations in populated
areas and no data are available on acrylic acid concentrations in
drinking-water. It was reported that acrylic acid might occur in
wastewater effluent from industrial facilities at concentrations not
exceeding 0.5 mg/litre. However, effluent levels of 2500 mg/litre were
reported at a methyl acrylate production facility in India. After
treatment acrylic acid concentration was below the limit of detention
(0.1 mg/litre) in wastewater from a production facility in Europe.
Food is an unlikely source of exposure to commercially derived
acrylic acid because it is not known to be used in applications that
involve direct contact with food.
10.1.2 Occupational exposure
The most important means of human exposure to acrylic acid is
occupational exposure via inhalation and skin contact. On the basis of
statistical estimates derived from a NIOSH survey conducted in
1972-1974, 28 600 workers were potentially exposed to acrylic acid in
the USA. More recently, NIOSH estimated that 96 500 workers were
potentially exposed in the USA.
One study conducted at a factory, where a number of chemicals
including acrylic acid and a variety of acrylates and methacrylates
were used, indicated that levels in air of acrylic acid and ethyl
acrylate varied from 0.03 to 168 mg/m3 (0.01 to 56 ppm). However, the
8-h TWA levels of most areas of the plant were well below the hygienic
standard of 30 mg/m3 (10 ppm) for acrylic acid recommended by OSHA
and ACGIH at the time of the study.
Exposures of workers to acrylic acid have been compiled from four
producing companies. Operators had a mean 8 h-exposure value of
0.48 mg/m3 (range of 0.03 to 3 mg/m3); during loading/unloading
the mean 8 h-exposure was 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3).
10.1.3 Toxic effects
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, moderate acute toxicity by the inhalation route and
moderate acute toxicity by the dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. Although a 1% solution has
been reported to irritate rabbit skin and eyes, no externally visible
irritation was seen in one mouse study following repeated dermal
application of 1%. Acrylic acid is also a strong irritant to the
respiratory tract.
Both positive and negative results have been obtained in skin
sensitization tests with acrylic acid, but it appears that the
positive findings may have been due to the impurity alpha,ß-
diacryloxypropionic acid.
10.1.3.1 Carcinogenic and mutagenic effects
Both positive and negative results have been obtained in
in vitro genotoxicity tests. Notably, acrylic acid was negative in
the Ames test and in vitro UDS assay, positive in a chromosome
aberration assay (with pH adjustment) and in the in vitro lymphoma
cell assay. Chromosome damage was also demonstrated in one lymphoma
assay. An in vivo bone marrow chromosome aberration assay was
negative. No firm conclusions can be drawn from an in vivo
DNA-binding study or from a dominant lethal assay. There are no
satisfactory in vivo genotoxicity data for sites of initial contact,
e.g., nasal epithelium or skin.
A single chronic cancer bioassay of acrylic acid, administered in
drinking-water, has been reported. Male and female Wistar rats were
given drinking-water containing 120, 400 or 1200 mg/litre (estimated
doses were 8, 27 and 78 mg/kg body weight per day), after preliminary
3- and 12-month studies showed reduced water consumption and body
weight gain in groups of rats receiving drinking-water containing 2000
or 5000 mg/litre. No systemic effects, including weight change or
histopathology, and no increase in tumour incidence resulted from
acrylic acid exposure. Although the lack of any toxic effects in this
study suggests that the maximum tolerated dose was not achieved, it
appears that the high dose in this study was approaching a maximum
tolerance dose (MTD), based on the mild body weight effects noted at
140 mg/kg per day in a 12-month study and at 240 mg/kg per day in a
two-generation reproductive study. Apart from not reaching the MTD,
the study used adequate number of animals (50), was well documented
and reported complete histopathology.
There has been no cancer bioassay conducted in a second species
by the oral route, nor any inhalation cancer bioassay. Available data
are inadequate to evaluate the carcinogenic potential of acrylic acid
via the dermal route.
There have been several chronic bioassays conducted on related
compounds that are relevant to evaluation of the potential
carcinogenicity of acrylic acid. Chronic inhalation studies have been
reported for methyl acrylate, butyl acrylate and ethyl acrylate. All
of these studies exposed rats to 0, 5, 25 or 75 ppm of the acrylates
for 2 years, which was followed by complete necropsy and histopa-
thological evaluation. The acrylate esters are rapidly metabolized to
acrylic acid and alcohols by nasal, blood and other tissue carboxyl-
esterases. In all of these studies a concentration-dependent increase
in nasal olfactory degeneration was observed, and the response was
very similar to the response observed in a subchronic inhalation study
of acrylic acid. There were no treatment-related increases in tumour
incidence in these studies. Although there may have been quantitative
differences in the tissue doses of acrylic acid, the acrylate ester
studies provide supportive evidence that carcinogenic effects do not
occur in tissues exposed chronically to acrylic acid.
Two cancer bioassays via oral exposure have been conducted with
ethyl acrylate, a gavage study in F-344 rats and B6C3F1 mice (NTP,
1986) and a drinking-water study in Wistar rats. The highest dose was
similar in the two studies and produced lesions of the forestomach.
However, the gavage study resulted in a dose-dependent increase in
tumour incidence in both sexes of rats and mice while the drinking-
water study did not. The gavage study suggests a potential for
carcinogenic effects for ethyl acrylate, and, by extension, for the
other acrylate esters and acrylic acid. The greater potency for this
effect in a gavage study may relate to the contact site toxicity of
these compounds. Gavage may not always be an appropriate exposure
method for direct irritants because of the extremely high local doses
that are achieved, compared to the local doses in a drinking-water
study.
A similar result can be seen in 3-month gavage and drinking-water
studies. The drinking-water study caused mildly reduced body weight
gain compared to controls, while the gavage study, at very similar
doses, resulted in lethality in most of the dosed animals. These
results suggest that the gavage route of exposure should play little
role in the risk assessment of acrylic acid because human exposures
are not likely to result in repeated high-concentration doses.
Current practice requires strong evidence to conclude that a
chemical is not a carcinogenic hazard, usually including adequate
chronic bioassays in two species. A second species chronic bioassay is
not available for acrylic acid, and the acrylate ester inhalation and
drinking-water bioassays described above were carried out on rats. The
existing acrylic acid bioassay is adequate and demonstrated no cancer
effects. No epidemiological evidence regarding potential
carcinogenicity of acrylic acid is available. Based on the available
data, one cannot definitively conclude that acrylic acid does not pose
a carcinogenic hazard to humans, although substantial relevant data do
not suggest a cancer hazard.
10.1.3.2 Non-cancer effects
a) Route-specific effects
Both oral and inhalation exposures show effects at the site of
contact. Inhalation exposures resulted in nasal effects in studies of
2 weeks to 3 months in duration and at various exposure
concentrations. High concentration drinking-water exposure to acrylic
acid resulted in forestomach lesions. No other specific organ
pathology has been consistently demonstrated in either oral or
inhalation studies. Metabolic studies show rapid metabolism and
clearance of acrylic acid that is similar for several routes of
exposure, and there is no evidence of bioaccumulation of this
chemical. The available evidence supports the conclusion that systemic
toxicity is not likely to occur, and that the effects of oral and
inhalation exposure can be treated as independent events with no
additivity of doses received by the different routes of exposure. For
this reason, guidance values will be derived separately for oral and
inhalation routes.
b) Developmental and reproductive effects
The toxicity data base for acrylic acid includes inhalation
developmental studies in rats and rabbits and oral reproductive
studies in rats. No specific reproductive or developmental effects
were observed in any of these studies. The only effects observed
included nasal effects in the inhalation studies and stomach lesions
and reduced body weight gain in the drinking-water studies. Based on
these results and the evidence for specific site-of-contact effects
described above, the potential hazards for developmental and
reproductive effects have been adequately studied and are not critical
to the human health risk assessment.
c) Oral gavage studies
As described above (section 10.1.3.1) the 3-month gavage and
drinking-water studies showed important differences in toxicity,
depending on the method of dosing. The drinking-water study revealed
reduced body weight gain compared to controls, while the gavage study,
at very similar daily doses, showed lethality in most of the dosed
animals. Therefore, the gavage route of exposure should be of little
importance in the risk assessment of acrylic acid because human
exposures are not likely to result in repeated high-concentration oral
doses.
d) Critical oral studies
Potential critical studies include a 3-month drinking-water study
in rats, 3-, 12- and 26- to 28-month drinking-water studies in rats,
and a two-generation reproductive study in rats. Table 8 summarizes
the effects seen in these studies and the determination of which
effects are considered adverse.
Table 8. Summary of adverse effect levels in drinking-water studies on ratsa
LOAEL NOAEL Effectb Reference
750 250 Body weight; 81 and 84% of controls in males and females, DePass et al., 1983
respectively, at 750; 95% of C in females at 250
none 331 Body weight: Hellwig et al., 1993
males: 93% of C at 331; (3-month study)
females: no effect
331 140 Body weight: Hellwig et al., 1993
males: 91% of C at 331, 94% of C at 140; (12-month study)
females; no effect
none 78 no effects Hellwig et al., 1993
(26- to 28-month studies)
460 240 460: stomach irritation, body weight: 91% of C in F0 males, BASF, 1993
65% of C in F1 pups, 85% of C in F1 (both sexes), 68% of C
in F2 pups
240: body weight: 89% of C in F1 pups, 88% of C in F2 pups
a concentrations are given in mg/kg body weight per day
b C = control group; F0 = first parental generation; F1 = second parental generation
A consistent finding throughout the drinking-water studies was
decreased water consumption, probably due to taste aversion and
sometimes accompanied by decreased food consumption. Reductions in
body weight appear to parallel decreased water consumption and were
worse in the pups in the reproductive study. The water consumption
effect makes the body weight changes difficult to interpret as a
direct toxic effect of acrylic acid, but the possibility that the
effect was at least partly a direct result of acrylic-acid-induced
irritation cannot be ruled out. The body weight effects are therefore
considered to be adverse when the magnitude of the effect approaches a
10% reduction. A somewhat larger change in the pup weight would be
required to be considered adverse, because the largest weight
decrements were seen at the end of active nursing and the beginning of
weaning; they may be more severely affected by the reduced water
consumption because of the greater maternal need for water intake
during this time and the unknown effects of taste aversion on weaning
behaviour. Based on these considerations and the presence of stomach
histopathology at 460 mg/kg body weight per day, the NOAEL in the two-
generation reproduction study was 240 mg/kg body weight per day. The
NOAEL based on body weight changes in the 12-month studies was
140 mg/kg body weight per day.
e) Critical inhalation studies
In a subchronic study, which is the only study that is
potentially a critical study for inhalation, lesions in the nasal
epithelium were seen in rats at 225 mg/m3 (75 ppm) and in mice at
15 mg/m3 (5 ppm). The lesion in mice was described as very slight at
15 mg/m3 (5 ppm), slight at 75 mg/m3 (25 ppm), and slight to
moderate at 225 mg/m3 (75 ppm). Despite the mild nature of the
response at 15 mg/m3 (5 ppm), there was a clear increase in incidence
and severity of this lesion with increasing exposure concentration, so
it must be considered adverse.
f) Progression of lesions
There is suggestive evidence from both inhalation and oral routes
of exposure that the effects caused by acrylic acid are largely
determined by the exposure concentration and are relatively less
affected by the duration of exposure in repeated exposure studies.
In the oral studies, even if different LOAEL and NOAEL values
could be defined by the 3-month and 12-month oral drinking studies,
the effects on weight are similar for the two exposure durations. In
inhalation studies in mice, the severity of the lesions in animals
exposed for 6 h/day to 75 mg/m3 (25 ppm) was very similar in the
2-week and the 90-day studies, although limited description of the
lesions in the 2-week study makes this conclusion tenuous. Additional
2-week studies included groups exposed to 15 and 75 mg/m3 (5 and
25 ppm) for 6 h/day. The 75 mg/m3 (25 ppm) group showed effects similar
to those seen in the subchronic study, suggesting limited progression
over this time-frame. The 15 mg/m3 (5 ppm) group exposed for 6 h/day
showed no effect after 2 weeks, so slight progression does occur at
low concentrations, given that lesions were observed in the 90-day
study. The 2-week study also included recovery groups, which showed
that the lesions were completely reversed in animals exposed to
15 mg/m3 (5 ppm) for 22 h and 75 mg/m3 (25 ppm) for 6 h, but
not in animals exposed to 75 mg/m3 (25 ppm) for 22 h/day.
Comparison of the subchronic and chronic inhalation studies of
the acrylate esters also supports the conclusion that there is limited
progression of nasal lesions. Some progression of lesions does occur,
but much less than would be predicted by an assumption of a constant
concentration × time relationship.
10.1.4 Risk evaluation
10.1.4.1 Inhalation exposure
The LOAEL of 15 mg/m3 (5 ppm) from the subchronic study is used
as the basis for the guidance value. The appropriate uncertainty
factors (UF) include 5 for inter-individual differences and 10 as a
composite UF for interspecies, LOAEL to NOAEL and subchronic to
chronic extrapolations. Reduced uncertainty for these aspects of the
guidance value (GV) for inhalation results from the information
discussed previously in this monograph. The inter-individual
uncertainty is reduced because of the direct-acting nature of the
toxicity, which does not involve metabolism. The uncertainty in
extrapolating from a LOAEL to a NOAEL is reduced because the lesion at
the LOAEL is very mild and reversible. The uncertainty in
extrapolating from a subchronic to a chronic exposure is reduced
because of the evidence discussed above showing limited progression.
The interspecies uncertainty is reduced because of the direct-acting
nature of the toxicity of acrylic acid and because the mouse is
apparently very sensitive. The toxicity of acrylic acid is clearly
limited to the site of deposition and is not dependent on metabolic
activation. Since the deposition is controlled by physical
interactions, there is little reason to expect significantly greater
deposition in humans.
Based on these considerations, the GVair for inhalation exposure
of the general population may be calculated as follows:
GVair = 15mg/m3 × 6 × 5 × 1 = 54 µg/m3
24 5 50
where:
15 mg/m3 = LOAEL for mice
6/24 and 5/7 = duration of exposure in h/day and weeks/day,
respectively
50 = total uncertainty factor
10.1.4.2 Oral exposure
For oral exposure the GV can be calculated based on NOAEL values
in the 12-month oral study (140 mg/kg body weight per day), the two-
generation reproductive study (240 mg/kg body weight per day) or the
NOAEL in the chronic study (78 mg/kg body weight day). The chronic
study is used to derive the GV because a lifetime exposure study is
preferred to a short-duration study. The chronic study found no effect
at the highest dose tested. The NOAEL is supported by the finding of
adverse effects on body weight at 331 mg/kg body weight per day in the
12-month study and body weight effects and stomach pathology in the
two-generation reproductive study at 460 mg/kg body weight per day.
With these supporting studies, the NOAEL from the chronic study can be
used with confidence. A total uncertainty factor of 25 is based on a
factor of 5 for inter-individual variability, and a factor of 5 for
interspecies variability and for the lack of an adequate oral study in
a second species. Reduced uncertainty factors are used for
interspecies and inter-individual differences because of the direct-
acting nature of the toxicity, as described in section 10.1.4.1. The
need for a second species is suggested by the apparent differences in
sensitivity between rats and mice in the subchronic inhalation study.
Based on these considerations, the GVoral for drinking-water
exposure may be calculated as follows:
Tolerance intake = 78 = 3.1 mg/kg
25
From the above calculation a guidance value for oral intake of
acrylic acid may be derived according to the following formula:
GVoral = 3.1 × 64 ‰ 2 = 99 mg/litre
where:
3.1 mg/kg body weight per day = tolerable intake (TI)
64 = average weight (kg) of human body
25 = uncertainty factor
2 = average intake of drinking water (litres/day)
However, the Task Group expressed significant concern regarding
the guidance value for oral intake calculated above, because of the
difference in toxicity between drinking-water and gavage exposures.
Exposure levels only 50-fold above the GV calculated above were lethal
to 50% of the animals in the 90-day gavage study, and comparison with
effects in the 90-day drinking-water study revealed a very significant
difference between the two dosing methods. High doses taken over a
short time period have much more severe effects.
The concern is reduced by the fact that the local effects in the
stomach might be determined by both concentration of delivered dose
and total dose, so the same total dose delivered in more dilute
solutions results in lesser effects, as shown for ethyl acrylate. The
concentration in drinking-water needed to deliver the TI dose is
probably far less than that required to cause direct effects, and is
not a public health concern if small amounts are ingested over the
course of the day, approximating to continuous exposure. The
possibility remains that a large part of the TI could be delivered
over a short time via drinking-water.
Because of potential differences in individual drinking
behaviour, the Task Group decided that a risk of effects still exists
at the TI calculated above. For this reason an additional uncertainty
factor of 10 was applied to derive the GV for drinking-water.
Thus, the final guidance value for oral intake of acrylic acid
is:
GVoral = 99 mg/litre = 9.9 mg/litre
10
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Acrylic acid has been found to occur naturally in some species of
marine alga. No quantitative data are available regarding
environmental levels in ambient air or soil. It was reported that
acrylic acid might occur in wastewater effluent from industrial
facilities at concentrations not exceeding 0.5 mg/litre. Effluent
levels of (2500 mg/litre) have been reported at a methyl acrylate
production facility in India. After treatment acrylic acid was below
the limit of detection (0.1 mg/litre) in wastewater from a production
facility in Europe.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Acrylic acid is
eliminated from the environment by both abiotic and biotic
degradation. Photolysis reactions are possible processes, but aerobic
degradation constitutes the major route of breakdown. Microorganisms
are capable of degrading acrylic acid under aerobic and anaerobic
conditions.
In soil, acrylic acid biodegrades very rapidly, the half-life
being less than one day. There is no potential for long-range
atmospheric transport of acrylic acid since it has an atmospheric
lifetime of less than one month.
Bioaccumulation of acrylic acid in organisms, based on the low
octanol-water partition coefficient, is likely to be negligible. There
has been no report of biomagnification of acrylic acid in food chains.
10.2.2 Effects
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values based on growth ranging from 0.04 to 63 mg/litre and
a NOEC for the most sensitive species of 0.008 mg/litre. Acrylic acid
has low to moderate acute toxicity for invertebrates and fish with
LC50 values ranging from 27 to 315 mg/litre. The 96-h NOEC was
6.3 mg/litre for rainbow trout, based on a lack of sublethal/
behavioural responses.
No data are available concerning the effects of acrylic acid on
terrestrial organisms in the environment.
10.2.3 Risk evaluation
If released into the environment acrylic acid will partition to
water where it will be readily degraded. Therefore, it is unlikely to
pose a problem in the general environment. In the case of spills,
acrylic acid is likely to cause localized mortality to aquatic
organisms both from direct toxicity and oxygen depletion. There is
likely to be a problem near to outfalls from plants discharging
acrylic acid if there is inadequate sewage treatment. The toxicity of
acrylic acid to bacteria and soil microorganisms is low. No data are
available concerning the effects of acrylic acid on terrestrial
organisms in the environment; however, data from laboratory mammals
suggest a low risk.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure for the general
population. However, no obvious adverse effects on the general
population have been identified.
Acrylic acid poses minimal risk for the general environment
except in the case of uncontrolled discharge.
11.2 Recommendations for protection of human health
It is recommended that exposure of the general population to
acrylic acid in the ambient air and drinking-water does not exceed the
guidance values given in this monograph.
12. FUTURE RESEARCH
a) Monitoring of acrylic acid concentrations in ambient air, water,
soil and effluent should be carried out. Research should be
undertaken to lower the analytical detection limit in water.
b) Further in vivo studies on the genotoxic potential of acrylic
acid at the initial site of contact are recommended.
c) The carcinogenicity of acrylic acid should be assessed in a well-
controlled two-species bioassay (with both sexes) using proper
exposure levels via inhalation or dermal exposure.
d) Better understanding of the relationship between persistent
tissue damage and tumour formation would aid the risk assessment
for an irritant chemical such as acrylic acid.
e) Additional research on the development of models describing the
disposition and kinetics of acrylic acid is needed. Such models
should focus on species differences in order to allow more
confident extrapolation from animals to humans. In addition,
models allowing comparison of acrylic acid dose for various
acrylate esters would be useful to allow complete use of
available data.
f) Since many workers are exposed to acrylic acid, epidemiological
studies are needed, which should concentrate on the following
end-points:
i) rhinitis, laryngitis and olfactory function;
ii) respiratory tract disease;
iii) skin disease.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer (IARC, 1979, 1987). Data
on the carcinogenicity of the compound for humans were considered
inadequate. There was inadequate evidence for carcinogenicity in
animals. Therefore, IARC considered acrylic acid not classifiable as
to its carcinogenicity to humans.
REFERENCES
AAR (1987) Emergency handling of hazardous materials in surface
transportation. Washington, DC, Association of America Railroads,
Hazardous Systems (BOE), 11 pp.
ACGIH (1988) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1988-1989.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
ACGIH (1990) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1990-1991.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists, p 9.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Andreoni V, Bernasconi S, Sorlini C, & Villa M (1990) Microbial
degradation of acrylic acid. Ann Microbiol, 40: 279-287.
Archer G & Horvath MK (1991) Adsorption and desorption of acrylic acid
to soils. Painesville, Ohio, Ricerca Inc., Department of Environmental
Sciences (Document No. 3193-88-0214-EF-001).
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reactions of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
Atkinson R & Carter WPL (1984) Kinetics and mechanisms of the gas-
phase reactions of ozone with organic compounds under atmospheric
conditions. Chem Rev, 84: 437-470.
Bandara BMR, Gunatilaka AAL, Kumar NS, & Wimalasiri WR (1988)
Antimicrobial activity of some marine algae of Sri Lanka. J Natl Sci
Counc (Sri Lanka), 16(2): 209-221.
BASF (1958) [Report on biological tests for crude and pure acrylic
acid.] Ludwigshafen, Germany, BASF AG, Toxicology Department
(Unpublished report No. VII/365-366) (in German).
BASF (1988) [Determination of the distribution coefficient log Pow of
acrylic acid in 1-octanol/water at room temperature (25°C).]
Ludwigshafen, Germany, BASF AG, Analytical Laboratory, pp 1-4 (in
German).
BASF (1992) Acrylic acid glacial P. Technical information monomers.
Ludwigshafen, Germany, BASF AG.
BASF (1993) [Examination of the biological degradability or the
elimination potential, respectively, of acrylic acid (pure P)
according to the experimental design of Zahn-Wellens (DUU/OM Project
No. 92/2641/10/1).] Ludwigshafen, Germany, BASF AG (in German).
BASF (1994a) Continuous administration in the drinking-water over 2
generations (1 liter in the first and 1 liter in the second
generation) (Project No. 71RO114/92011). Ludwigshafen, Germany, BASF
AG.
BASF (1994b) [Determination of the inhibitory effect of acrylic acid
(pure) on the cell replication of the green algae Scenedesmus
supsicatus.] Ludwingshafen, Germany, BASF AG (Unpublished
experiment No. 94/0840/60/1) (in German).
BG Chemie (1991) Acrylic acid. In: Toxicological evaluations, Volume
2. Berlin, Springer-Verlag, pp 39-73.
Black KA (1993) 14C-Acrylic acid. Comparative bioavailability study
in male mice and rats -analysis of tissues. Spring House,
Pennsylvania, Rohm and Haas Company, Toxicology Department (Report
No. 93R-200).
Black KA, Finch L, & Frederick CB (1993) Metabolism of acrylic acid to
carbon dioxide in mouse tissues. Fundam Appl Toxicol, 21: 97-104.
Black KA, Beskitt JL, Tallant MJ, & Frantz SW (1994) Disposition of
acrylic acid (AA) in C3H mice after the oral, cutaneous and
intravenous routes (Rohm and Haas Company, Spring House, PA and Bushy
Run Research Center, Export, PA). Toxicologist, 14(1): 430 (Abstract
1705).
Black KA, Beskitt JL, Finch L, Tallant MJ, Udinsky JR, & Frantz ST
(1995) Disposition and metabolism of acrylic acid in C3H mice and
Fischer 344 rats after oral or cutaneous administration. J Toxicol
Environ Health, 45: 291-311.
Borzelleca JF, Larson PS, Hennigar GR, Huf EG, Crawford EM, & Smith RB
(1964) Ethyl acrylate and methyl methacrylate: Studies on the chronic
oral toxicity of monomeric ethyl acrylate and methyl methacrylate.
Toxicol Appl Pharmacol, 6: 29-36.
Bowen RL (1979) Adhesive bonding of various materials to hard tooth
tissues. XVIII: Synthesis of a polyfunctional surface-active
comonomer. J Dent Res, 58: 1101-1107.
Bowman JE (1990) Acute flow-through toxicity of glacial acrylic acid
to rainbow trout ( Salmo gairdneri). Columbia, Missouri, Analytical
Bio-Chemistry Laboratories Inc., Aquatic Toxicology Division (Report
No. 37343).
Bretherick L (1985) Handbook of reactive chemical hazards, 3rd ed.
Boston, Massachusetts, Butterworth, 351 pp.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: model organisms Microcytis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Z Wasser-Abwasser-Forsch, 15: 1-6 (in
German).
Brown RK, McMeekin TA, & Balis C (1977) Effect of some unicellular
algae on Escherichia coli populations in sea water and oysters. J
Appl Bacteriol, 43: 129-136.
Buckley LA, James RA, & Barrow CS (1984) Differences in nasal cavity
toxicity between rats and mice exposed to acrylic vapor. Toxicologist,
4(1): 1 (Abstract only).
Burgess D (1990) Acute flow-through toxicity of acrylic acid to
Daphnia magna. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37344).
Bysshe SE (1990) Bioconcentration factor in aquatic organisms. In:
Lyman WJ, Reehle WF, & Rosenblatt DN ed. Handbook of chemical property
estimation methods for environmental behaviour of organic compounds.
Washington, DC, American Chemical Society, vol 15, pp 1-30.
Cameron TP, Rogers-Back AM, Lawlor TE, Harbel JW, Seifried HE, &
Dunkel VC (1991) Genotoxicity of multifunctional acrylates in the
Salmonella/mammalian-microsome assay and mouse lymphoma TK+/- assay.
Environ Mol Mutagen, 17: 264-271.
Carpenter CP, Weil CS, & Smyth HF Jr (1974) Range-finding toxicity
data: List VIII. Toxicol Appl Pharmacol, 28: 313-319.
Casciery T & Clary J (1993) Acrylic acid health effects overview. In:
Taylor TB, Murphy SR, & Hunt EK ed. Health effect assessments of the
basic acrylates. Boca Raton, Florida, CRC Press, p 13.
Chemical Marketing Reporter (1992) Acrylic grows at rapid pace, but so
does the world's capacity. Chem Mark Report, July 20.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation
of petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CHRIS (1985) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado).
CHRIS (1989) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado.
Chun JS, Kubena MF, & Neeper-Bradley TL (1993) Developmental toxicity
dose range-finding study of inhaled acrylic acid vapor in New Zealand
white rabbits. Export, Pennsylvania, Union Carbide Corporation (Bushy
Run Research Center Report No. 92N1007).
CITI (Japanese Chemicals Inspection and Testing Institute) ed. (1992)
Acrylic acid. In: Biodegradation and bioaccumulation: Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology and Information Center, pp 2-83.
Conde-Salazar L, Guimaraens D, & Romero LV (1988) Occupational
allergic contact dermatitis from anaerobic acrylic reactants. Contact
Dermatitis, 18: 129-132.
Corrigan MA & Scott R (1988) Acrylic acid: In vitro absorption
through human and mouse skin. Macclesfield, United Kingdom, Imperial
Chemical Industries PLC, Central Toxicology Laboratory (Report No.
CTL/P/2047).
Costellati L, Guglielmin AM, Vistoli O, Carboni GP, & Rambaldi R
(1990) [Allergic contact dermatitis - evaluation of patch tests in
animal fodder plant workers.] Med Lav, 81: 296-300 (in Italian).
Cote IL, Hochwalt A, Seidman I, Budzilovich G, Solomon JJ, & Segal A
(1986) Acrylic acid: Skin carcinogenesis in ICR/HA mice. Toxicologist,
6: 235.
Dean JA (1987) Handbook of organic chemistry. New York, McGraw-Hill
Book Co., pp 1-76.
De Bethizy ID, Udinsky JR, & Scribner HE (1987) The disposition and
metabolism of acrylic acid and ethyl acrylate in male Sprague-Dawley
rats. Fundam Appl Toxicol, 8(4) 549-561.
DePass LR, Woodside MD, Garman RH, & Weil CS (1983) Subchronic and
reproductive toxicology studies on acrylic acid in the drinking water
of the rat. Drug Chem Toxicol, 6(1): 1-20.
DePass LR, Fowler EH, Meckley DR, & Weil CS (1984) Dermal oncogenicity
bioassays of acrylic acid, ethyl acrylate and butyl acrylate. J
Toxicol Environ Health, 14: 115-120.
Douglas MT & Bell G (1992) Assessment of ready biodegradability of
acrylic acid (Closed bottle test). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 1/91329).
D'Souza RW & Francis WR (1988) Vehicle and pH effects on the dermal
penetration of acrylic acid: in vitro- in vivo correlation.
Toxicologist, 8: 831.
EEC (1988) Methods for the determination of ecotoxicity,
biodegradation. Activated sewage sludge respiration inhibition test.
In: Council Directive of 18 November 1987 adapting to technical
progress for the ninth time Directive 67/548/EEC on the approximation
of the laws, regulations and administrative provisions relating to the
classification, packaging and labelling of dangerous substances
(88/302/EEC). Off J Eur Comm, L133: 118-122.
Ekhina RS & Ampleeva GP (1977) [Combined effect of six acrylates on
the sanitary status of a reservoir.] Vodemov, 1977: 157-164 (in
Russian).
Elf Atochem (1992) Norsocryl AA, acrylic acid specifications. Paris,
Elf Atochem.
Fassett DW (1963) Organic acids, anhydrides, lactones, acid halides
and amides, thioacids. In: Patty FA ed. Industrial hygiene and
toxicology, 2nd ed. New York, Wiley-Interscience, vol 2, p 1794.
Fazzalari FA ed. (1978) Compilation of odor and taste threshold values
data. Philadelphia, Pennsylvania, American Society for Testing and
Materials, p 141.
Ferrali M, Ciccoli L, & Comporti M (1989) Allyl alcohol-induced
hemolysis and its relation to iron release and lipid peroxidation.
Biochem Pharmacol, 38(1): 1819-1826.
Finch L & Frederick CB (1992) Rate and route of oxidation of acrylic
acid to carbon dioxide in rat liver. Fundam Appl Toxicol, 19:
498-504.
Forbis AD (1989) Acute toxicity of acrylic acid to Selenastrum
capricornutum Printz. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc., Aquatic Toxicology Division (Report No. 37345).
Fowler JH Jr (1990) Immediate contact hypersensitivity to acrylic
acid. Dermatol Clin, 8: 193-195.
Frantz SW & Beskitt JL (1993) 14C-Acrylic acid: comparative
bioavailability study in male mice and rats. Export, Pennsylvania,
Bushy Run Research Center (Report No. 92 N 1005).
Frantz SW, Beskitt JL, Tallant MJ, & Black KA (1994) Disposition of
acrylic acid (AA) in Fischer rats after the oral, cutaneous and
intravenous routes. Toxicologist, 14(1): 430 (Abstract 1704).
Frederick CB & Reynolds CH (1989) Modeling the reactivity of acrylic
acid and acrylate anion with biological nucleophiles. Toxicol Lett,
47(3): 241-247.
Frederick CB, Udinsky JR, & Finch L (1994) The regional hydrolysis of
ethyl acrylate to acrylic acid in the rat nasal cavity. Toxicol Lett,
70: 49-56.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Ganou-Parfait B, Fahrasmane L, Celestine-Myrtil DA, Parfait A, & Galzy
P (1988) Micrococcus in rum technology in the French West Indies.
Microbiol Aliments Nutr, 6(3): 273-277.
Gelbke HP & Hofmann HTh (1979) [Report on testing for acute inhalation
risk of acrylic acid (pure) in Sprague-Dawley rats.] Ludwigshafen,
Germany, BASF AG, Department of Toxicology (in German).
Ghanayem BI, Maronpot RR, & Matthews HB (1985) Ethyl acrylate-induced
gastric toxicity: II. Structure-toxicity relationships and mechanism.
Toxicol Appl Pharmacol, 80: 336-344.
Ghanayem BI, Burka LT, & Matthews HB (1987) Ethyl acrylate
distribution, macromolecular binding, excretion and metabolism in male
Fischer 344 rats. Fundam Appl Toxicol, 9: 389-397.
Glombitza KW (1970a) [Antimicrobial contents of algae: 2. Presence of
acrylic acid in different sea algae.] Planta Med, 18(3): 210-221 (in
German).
Glombitza KW (1970b) [Antimicrobiol contents in algae: 3. Quantitative
determination of acrylic acid in sea algae.] Planta Med, 18(4):
231-234 (in German).
Glombitza KW (1979) Antibiotics from algae. In: Hoppe HA, Levring T, &
Tanaka J ed. Marine algae in pharmaceutical science. Berlin, New York,
Walter de Gruyter, pp 303-307.
Glombitza KW & Heyser R (1971) [Antimicrobial constituent compounds in
algae - Part 4: Effect of acrylic acid on respiration and synthesis of
macromolecular in Staphylococcus aureus and Escherichia coli.]
Helgoal Wiss Meeresunters, 22(3/4): 442-453 (in German).
Grudzinski UJ (1988) [Substantiation of single maximum permissible
levels of acrylic acid in the air of populated regions.] Gig I Sanit,
9: 64-65 (in Russian).
Hagan JV & Emmons HF (1991) Acrylic acid: Acute inhalation study in
rats (Protocol No. 87P-240). Spring House, Pennsylvania, Rohm and Haas
Company, Toxicology Department (Report No 87R-106).
Halarnkar PJ & Blomquist GJ (1989) Comparative aspects of propionate
metabolism. Comp Biochem Physiol, 92B: 227-231.
Hansch C & Leo A (1987) The log P database. Claremont, California,
Pomona College, p 391.
Hawkins DR, Kirkpatrick D, Aikens PJ, & Saxton JE (1992) The
metabolism of acrylic acid in soil under aerobic conditions.
Huntingdon, United Kingdom, Huntingdon Research Centre (HRC
Confidential Report No. 93A/920625).
Hellwig J, Deckerdt K, & Freisberg KO (1993) Subchronic and chronic
studies of the effects of oral administration acrylic acid to rats.
Food Chem Toxicol, 31: 1-18.
Herwig N (1978) Nutritious antibiotics. Mar Aquarist, 8(5): 48-50.
Heyser R & Glombitza KW (1972) Effect of acrylic acid on glycolysis
and citric acid cycle in bacteria: 5. Antimicrobiol components in
algae. Arch Pharm, 305(11): 863-870.
Hossack DJN, Thomas FJ, & Ruckman SM (1992) Acrylic acid: Effects on
soil carbon cycle (respiration). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 2/920228).
Howard PH, Boethlings RS, Jarris WF, Meylan WM, & Midalenko EM (1991)
Handbook on environmental degradation rates. Chelsea, Michigan, Lewis
Publishers.
Husain S, Sastry GSR, Prasad PR, & Sarma VR (1991) Differential pulse
polarographic determination of residual acrylic acid in sodium
polyacrylate. Electroanalysis, 3: 71-72.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein. Lyon, International Agency for Research on Cancer, pp 47-71
(IARC Monograph on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 19).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 56 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1992) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M (1988) Data book of chromosomal aberration test in vitro.
Amsterdam, Oxford, New York, Elsevier Science Publishers.
Juhnke I & Luedemann D (1978) [Results of testing of 200 chemical
compounds for acute fish toxicity using the Golden Orfe test.]
Z. Wasser Abwasser Forschung, 11(5): 61-164 (in German).
Kalimo K, Jolanki R, Estlander T, & Kanerva L (1989) Contact allergy
to antioxidants in industrial greases. Contact Dermatitis, 20:
151-152.
Kavaler AR (1987) Chemical profile on acrylic acid. Chem Mark Report,
232(23): 62.
Kirk-Othmer (1978-1984) Kirk-Othmer encyclopedia of chemical
technology, Volumes 1-26, 3rd ed. New York, John Wiley and Sons.
Klimisch HJ & Hellwig J (1991) The prenatal inhalation toxicity of
acrylic acid in rats. Fundam Appl Toxicol, 16(4): 656-666.
Klimisch HJ & Zeller H (1980) [Acute inhalation toxicity in the rat.]
Ludwigshafen/Rhine, Germany, BASF AG, Department of Toxicology (in
German).
Klimkina NV, Bolding ZN, & Sergeev AN (1969) [Experimental basis for
maximum permissible content of acrylic acid in reservoir waters.] Prom
Zag Vodoemov, 9: 171-185 (in Russian, with English abstract).
Kodama M & Ogata T (1983) Acrylic acid, as an antibacterial substance
in scallop. Bull Jpn Soc Sci Fish, 49(7): 1103-1107.
Kohriyama K, Matsuoka M, & Igisu H (1994) Effects of acrylamide and
acrylic acid on creatinine kinase activity in the rat brain. Arch
Toxicol, 68(1): 67-70.
Korenman IM & Lunicheva EV (1972) Distribution of acrylic acid between
organic solvents and water. J Appl Chem (USSR), 45: 1101-1105.
Kostanyan GG, Dadayan AA, & Safuryan GE (1969) [Polarographic
determination of acrylic acid in commercial propionic acid.] Arm Khem,
22: 1044 (in Russian with English abstract).
Kutzman RS, Meyer GI, & Wolf AP (1982) The biodistribution and
metabolic fate of (11C) acrylic acid in the rat after acute inhalation
exposure or stomach intubation. J Toxicol Environ Health, 10(6):
969-980.
Lawrence WH, Bass GE, Purcell WP, & Autian J (1972) Use of
mathematical models in the study of structure-toxicity relationships
of dental compounds: I. Esters of acrylic acid and methacrylic acids.
J Dent Res, 51: 526.
Lijinsky W & Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratog Carcinog Mutagen, 1: 259-267.
Lomax LG, Brown OW, & Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic acid
for either 6 or 22 h per day. Abstract presented at a Meeting on Nasal
Toxicity and Dosimetry of Inhaled Xenobiotics: Implications for human
health, Durham, North Carolina, 20-22 September 1993. Inhal Toxicol,
6: 445-449.
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods: Environmental behavior of organic
compounds. New York, McGraw-Hill Book Co., pp 9-65.
McCarthy KL, Thomas WC, Aardema MJ, Seymour JL, Putman DL, Yang LL,
Curren RD, & Valencia R (1992) Genetic toxicology of acrylic acid.
Food Chem Toxicol, 30: 505-515.
Mackay D & Peterson S (1981) Calculating fugacity. Environ Sci
Technol, 15: 1006-1014.
Magnusson B & Kligman AM (1969) The identification of contact
allergens by animal assay: The Guinea pig maximization test. J Invest
Dermatol, 52: 268-276.
Magnusson B, Blohm S, Fregert S, Hjorth N, Hovding G, Pirila V, & Skog
E (1968) Routine patch testing: IV. Supplementary series of test
substances for Scandinavian countries. Acta Dermato-Venereol, 48:
110-114.
Majka J, Knobloch K, & Stetkiewicz J (1974) [Evaluation of acute and
subacute toxicity of acrylic acid.] Med Prac, 25: 427-35 (in
Polish).
Mao J, Doane R, & Kovacs F Jr (1994) Separation of acrolein and its
possible metabolites using different modes of high performance liquid
chromatography. J Liq Chromatogr, 17: 1811-1819.
Miller ML (1964) Acrylic acid polymers. In: Bikales NM ed.
Encyclopedia of polymer science and technology, plastics, resins,
rubbers, fibers. New York, Interscience Publishers, vol 1, pp 197-226.
Miller RR, Ayres JA, & Jersey GC (1979a) Acrylic acid 90-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1024).
Miller RR, Ayres JA, & Jersey GC (1979b) Acrylic acid 10-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1015).
Miller RR, Ayres JA, Jersey GC, & McKenna MJ (1981a) Inhalation
toxicity of acrylic acid. Fundam Appl Toxicol, 1(3): 271-277.
Miller RR, Ayres JA, Rampy LW, & McKenna MJ (1981b) The metabolism of
acrylate esters in rat tissue homogenates. Fundam Appl Toxicol,
1(6): 410-414.
Miller RR, Young JT, Kociba RJ, Keyes DG, Bodner KM, Calhoun LL, &
Ayers JA (1985) Chronic toxicity and oncogenicity bioassay of inhaled
ethyl acrylate in Fischer 344 rats and B6C31 mice. Drug Chem Toxicol,
8: 1-42.
Mitchell DY & Petersen DR (1988) Inhibition of rat liver aldehyde
dehydrogenases by acrolein. Drug Metab Dispos, 16: 37-42.
Moiseeva TN, Kozulin SV, Kulikova LK, & Voronin SP (1991) Screening of
strains decomposing acrylic acid and its derivatives. Soviet-
Biotechnology, 1991(6): 119-125.
Mok WSL (1989) Formation of acrylic acid from lactic acid in
supercritical water. J Org Chem, 54(19): 4596-4602.
Moore MM, Amtower A, Doerr CL, Brock KH, & Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environ Mol Mutagen, 11: 49-63.
Nagasawa T, Nakamura T, & Yamada H (1990) Production of acrylic acid
and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase.
Appl Microbiol Biotechnol, 34(3): 322-324.
Narayanasamy K, Shukla S, & Parekh LJ (1990) Utilization of
acrylonitrile by bacteria isolated from petrochemical waste waters.
Indian J Exp Biol, 28(10): 968-971.
Nawaz MS, Franklin W, & Cerniglia CE (1993) Degradation of acrylamide
by immobilized cells of Xanthomonas maltophilia. Can J Microbiol,
39(2): 207-212.
Nawaz MS, Franklin W, & Cerniglia CE (1994) Degradation of aliphatic
amide mixture by immobilized and nonimmobilized cells of Pseudomonas
sp. Environ Sci Technol, 28(6): 1106-1109.
Neeper-Bradley TL & Kubena MF (1993) Developmental toxicity evaluation
of inhaled acrylic acid vapor in New Zealand white rabbits. Export,
Pennsylvania, Union Carbide Corporation (Bushy Run Research Center
Report No. 92N1008).
NIOSH (1974) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 74-127).
NIOSH (1977) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 77-213).
NIOSH (1989) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (CD-ROM version by Canadian Centre for Occupational Safety and
Health, Hamilton, Ontario).
NIOSH (1990) National occupational exposure survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard, Evaluations, and Field Studies (Unpublished
provisional data as of January 7, 1990).
Nishikawa H, Hosomura H, Sonoda H, & Inagaki M (1979) [Behaviour of
acrylamide in a soil-vegetation system.] Kenkyu Hokoku-Gifu-Ken Kogyo
Gijutsu Senta, 11: 31-34 (in Japanese).
Nishiuchi Y (1975) Toxicity of formulated pesticides to some
freshwater organisms. Suisan Zoshoku, 23: 132.
NLM (1989) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
NLM (1991) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
Noble RC & Czerkawski JW (1973) A gas-chromatographic method for the
determination of low concentrations of acrylic acid in mixtures of C2
to C5 fatty acids in biological materials. Analyst, 98: 122-125.
NTP (1986) Carcinogenesis studies of ethyl acrylates (CAS No.
140-88-5) in F344/N and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program, 224 pp
(NTP Technical Report Series No. 259; NIH Publication No. 87-2515).
OHM/TADS (1989) Oil and hazardous material - Technical assistance
Washington, DC, US Environmental Protection Agency (CD-ROM version by
Micromedex Inc., Denver, Colorado).
OSHA (1981) OSHA analytical methods manual. Part 1 - Organic
substances: Method 28. Washington, DC, US Department of Labor,
Occupational Safety and Health Administration.
OSHA (1989) Department of Labor, Occupational Safety and Health
Administration - 29 CFR, Part 1910: Air Contaminants. Final Rule. Fed
Reg, 54(12): 2332-2983.
Pahren HR & Bloodgood DE (1961) Biological oxidation of several vinyl
compounds. J Water Pollut Control Fed, 33(3): 233-238.
Parker D & Turk JL (1983) Contact sensitivity to acrylate compounds in
guinea pigs. Contact Dermatitis, 9(1): 55-60.
Rao KS, Betso JE, & Olson KJ (1981) A collection of guinea pig
sensitisation test results -grouped by chemical class. Drug Chem
Toxicol, 4: 331-351.
Reininghaus W, Koestner A, & Klimisch HJ (1991) Chronic toxicity and
oncogenicity of inhaled methyl acrylate and n-butyl acrylate in
Sprague-Dawley rats. Food Chem Toxicol, 29(5): 329-339.
Riddick JA, Bunger WB, & Sakano T (1986) Organic solvents physical
properties and methods of purification. In: Techniques of chemistry,
4th ed. New York, Wiley-Interscience, vol 2, p 1325.
Ruth JH (1986) Odour thresholds and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A/143.
Sagelsdorf P, Lutz WK, & Schlatter C (1988) Investigation of the
potential for covalent binding of acrylic acid in vivo after oral
and dermal administration, preliminary results. Schwerzenbach bei
Zurich, Switzerland, Toxicology Institute of the University of
Zurich's Swiss Federal Institute of Technology (Report No.TOXETHZ
1044K).
Sanders JM, Burka LT, & Matthews HB (1988) Metabolism and disposition
of n-butyl acrylate in male Fischer rats. Drug Metab Dispos,
16(3): 429-434.
Sanseverino J, Montenecourt BS, & Sands JA (1989) Detection of acrylic
acid formation in Megasphaera elsdenii in the presence of 3-butynoic
acid. Appl Microbiol Biotechnol, 30(3): 239-242.
Sasaki S (1978) In: Hutizinger O ed. The scientific aspects of the
chemical substance control law in Japan in aquatic pollutants
transformation and biological effects. Oxford, New York, Pergamon
Press, pp 283-298.
Sax NI (1984) Dangerous properties of industrial chemicals, 6th ed.
New York, Van Nostrand Reinhold Co., p 129.
Sax NI & Lewis R (1989) Dangerous properties of industrial chemicals,
7th ed. New York, Van Nostrand Reinhold Co., pp 71-72.
Schultz H (1991) Beta-oxidation of fatty acid. Biochem Biophys,
1081: 109-120.
Schwartz BS, Doty RL, Moroe C, Frye R, & Barker S (1989) Olfactory
function in chemical workers exposed to acrylate and methacrylate
vapors. Am J Public Health, 79(5): 613-618.
Segal A, Fedyk J, Meclchionne S, & Seidman I (1987) The isolation and
characterization of 2-carboxyethyl adducts following in vitro
reaction of acrylic acid with calf thymus DNA and bioassay of acrylic
acid in female Hsd:(ICR)Br mice. Chem-Biol Interact, 61: 189-197.
Shah JF (1990) A hydrolysis study of 14C-acrylic acid. Painesville,
Ohio, Ricerca Inc.
Shanker R, Ramakrishna C, & Seth PK (1990) Microbial degradation of
acrylamide monomer. Arch Microbiol, 154(2): 192-198.
Shelton DR & Tiedje JM (1984) General method for determining anaerobic
biodegradation potential. Appl Environ Microbiol, 47: 850-857.
Sieburth JMN (1960) Acrylic acid, an "antibiotic" principle in
Phaeocystis blooms in Antarctic waters. Science, 132: 676-677.
Silver EH, Leith DE, & Murphy SD (1981) Potentiation by triorthotolyl
phosphate of acrylate ester-induced alterations in respiration.
Toxicology, 22(3): 193-203.
Simon P, Brand F, & Lemacon C (1989) Florisil sorbent sampling and ion
chromatographic determination of airborn aliphatic carboxylic acids. J
Chromatogr, 479: 445-451.
Singh M & Thomas M (1985) Analysis of effluent from a methyl acrylate
plant by gas chromatography. Indian J Environ Health, 27: 361-364.
Singh AR, Lawrence WH, & Autian J (1972) Embryonic-fetal toxicity and
teratogenic effects of a group of methacrylate esters in rats. J Dent
Res, 51(6): 1632-1638.
Singh HB, Jaber HM, & Davenport HE (1984) Reactivity/volatility
classification of selected organic chemicals: Existing data. Menlo
Park, California, SRI International, p 190 (EPA-600/3-84-082).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Data Corporation, p
43.
Sivak A (1987) Evaluation of acrylic acid mouse skin tumour bioassay
and DNA adduct study performed at New York University Medical Center.
New York, New York University (Report No. ADL/558.46 to the Celanese
Corporation).
Slott VL & Hales BF (1985) Teratogenicity and embryolethality of
acrolein and structurally related compounds in rats. Teratology,
32(1): 65-72.
SNF (1995) Inhibition test (72 h) in freshwater unicellular algae.
Licata-Messana L, SEPC (Société d'Ecotoxicologie et de Physico-
Chimie). Saint Etienne, France, Société Nouvelle Floerger.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17: A416-A427.
SRI International (1981) Production and use profile for CHIP of
acrylic acid. Menlo Park, California, SRI International (US EPA
Technical Directive 5.16).
Staples RE (1993) Evaluation of the environmental fate and
ecotoxicological parameters for several acrylic monomers. Washington,
DC, Assessment Technologies BAMM.
Stewart JM, Bhattacharya SK, Madura RL, Mason SH, & Schonberg JC
(1995) Anaerobic treatability of selected organic toxicants in
petrochemical wastes. Water Res, 29: 2730-2738.
Swenberg JA, Gross EA, & Randall HW (1986) Localization and
quantitation of cell proliferation following exposure to nasal
irritants. In: Barrow CS ed. Toxicology of nasal passages. Washington,
DC, Hemisphere Publishing Corporation, pp 291-300.
Takamizawa K, Horitsu H, Ichikawa T, Tawai K, & Suzuki T (1993) Beta-
hydroxypropionic acid production by Byssochlamys sp grown on acrylic
acid. Appl Microbiol Biotechnol, 40(2/3): 196-200.
Tegeris AS, Balmer MF, Morton MF, Buckley IR, & Garner FM (1987)
13-week mouse comparative skin irritation study with acrylic acid.
Washington, DC, Basic Acrylate Monomer Manufacturers Association
(Unpublished report).
Tegeris AS, Balmer MF, Garner FM, Thomas WC, Murphy SR, McLaughlin JE,
& Seymour JL (1988) A 13-week skin irritation study with acrylic acid
in 3 strains of mice. Toxicologist, 8: 127.
Union Carbide Corporation (1977) Toxicology studies: acrylic acid
glacial. New York, Union Carbide Corporation, Industrial and
Toxicology Department.
US EPA (1981) Chemical hazard information profile: Acrylic acid (CAS
No 79-10-7). Washington, DC, US Environmental Protection Agency, TSCA
Assistance Division (EPA 749F9400.6).
US ITC (1977) Synthetic organic chemicals - US production and sales.
Washington, DC, International Trade Commission.
US ITC (1983) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1985) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1986) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1987) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1988) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US National Fire Protection Association (1986) Fire protection guide
on hazardous materials, 9th ed. Boston, Massachusetts, US National
Fire Protection Association, pp 40-42.
Van der Walle HB, Seutter LPC, & Delbressine E (1982) Concomitant
sensitization to hydroquinone and p-methoxyphenol in the guinea
pig; inhibitors in acrylic monomers. Contact Dermatitis, 8: 147-154.
Van der Zande L, Kunnen R, Uijtewaal B, Van Wijk R, & Bisschop A
(1986) Effect on hepatic ornithine decarboxylase of some food
additives and synthetic elastomers. Food Addit Contam, 3(1): 57-62.
Veith GD, DeFoe DL, & Bergstedt BV (1979) Measuring and estimating the
bioconcentration factor of chemicals in fish. J Fish Res Board Can,
36: 1040-1048.
Verschueren K (1983) Handbook of environmental data of organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., p 161.
Vincent WJ & Guient V (1982) The development of air sampling and
analytical methodology for determining occupational exposure. Am Ind
Hyg Assoc J, 43(7): 499-504.
Vodicka P, Gut I, & Frantick E (1990) Effects of inhaled acrylic acid
derivatives in rats. Toxicology, 65(´): 209-222.
Vojtisek B, Hronova B, Hamrik J, Jambor V, Zezula V, Zendulka I,
Dvorak R, & Sisak M (1991) [The effect of clover silage treated with
acrylic acid on selected indicators of metabolism in cows around the
time of parturition and the status of their calves.] Vet Med (Praha),
36: 273-280 (in Czech).
Waegemaekers TH & van der Walle HB (1984) Alpha, beta-
diacryloxypropionic acid, a sensitizing impurity in commercial acrylic
acid. Dermatol Beruf Umwelt, 32: 55-58.
Weast RC & Astle MJ (1985) CRC handbook of data on organic compounds,
Volumes I and II. Boca Raton, Florida, CRC Press Inc., pp V1, 42, 57.
Weast RC, Lide DR, Astle MJ, & Beyer WH ed. (1989) Acrylic acid. In:
CRC handbook of chemistry and physics, 70th ed. Boca Raton, Florida,
CRC Press, p C-57.
Wegner WS, Reeves HC, Rabin R, & Ajl SI (1968) Alternate pathways of
metabolism of short-chain fatty acids. Bacteriol Rev, 32: 1-26.
Whanger PD & Matrone G (1967) Metabolism of lactic, succinic and
acrylic acids by rumen microorganisms from sheep fed sulphur-adequate
and sulphur-deficient diet. Biochem Biophys Acta, 136: 27-35.
Wiegand HJ (1990) Species differences in the metabolism of n-butyl
acrylate. Naunyn- Schmiedebergs Arch Pharmacol, 341: 512 (Abstract
47).
Wiegand HJ, Schiffmann D, & Henschler D (1989) Non-genotoxicity of
acrylic acid and n-butyl acrylate in a mammalian cell system (SHE
cells). Arch Toxicol, 63: 250-251.
Windholz M ed. (1983) Merck index, 10th ed. Rahway, New Yersey, Merck
& Co. Inc.
Winter SM & Sipes IG (1993) The disposition of acrylic acid in the
male Sprague-Dawley rat following oral or topical administration. Food
Chem Toxicol, 31: 615-621.
Winter SM, Weber GL, Gooley PR, MacKenzie NE, & Sipes IG (1992)
Identification and comparison of the urinary metabolites of
[1,2,3-13C3] acrylic acid and [1,2,3-13C3]propionic acid in the rat by
homonuclear 13C nuclear magnetic resonance spectroscopy. Drug Metab
Dispos, 20: 665-672.
Wise HE & Fahrenthold PD (1981) Occurrence and predictability of
priority pollutants in wastewater of the organic chemicals and
plastics/synthetic fibers industrial categories - ACS 1981 National
Meeting, Division of Industrial Chemistry Symposium on Treatability of
Industrial Aqueous Effluents. Washington, DC, American Chemical
Society.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from testing of 255
chemicals. Environ Mutagen, 9: 1-110.
1. RESUME ET RECOMMANDATIONS
L'acide acrylique est un liquide incolore qui dégage une odeur
âcre et irritante à la température et à la pression ordinaires. Le
seuil olfactif de l'acide acrylique est bas (0,20-3,14 mg/m3). Il est
miscible à l'eau et à la plupart des solvants organiques.
L'acide acrylique existe dans le commerce en deux qualités;
l'acide acrylique technique et l'acide acrylique glacial. Il se
polymérise facilement sous l'action de la chaleur, de la lumière ou en
présence de métaux, c'est pourquoi un inhibiteur de polymérisation est
ajouté aux produits du commerce.
La production mondiale d'acide acrylique a été estimée à environ
2 millions de tonnes en 1994. On l'utilise essentiellement comme une
matière première pour la production d'esters acryliques, comme
monomère pour la production de l'acide polyacrylique et de ses sels,
comme comonomère avec l'acrylamide pour la préparation de polymères
utilisés comme floculants, avec l'éthylène pour la préparation de
résines échangeuses d'ions, l'ester méthylique sevant par aillleurs à
la préparation d'un certain nombre d'autres copolymères.
Il est possible de doser les résidus d'acide acrylique présents
dans l'air et dans d'autres milieux par chromatographie en phase
gazeuse, par chromatographie en phase liquide à haute performance ou
encore par polarographie. Les limites de détection de ces méthodes
sont de 14 ppm dans l'air et de 1 ppm dans les autres milieux.
L'acide acrylique existe à l'état naturel dans certaines algues
marines et on en a également trouvé dans les sécrétions du rumen chez
les ovins.
Comme il est miscible à l'eau, l'acide acrylique ne devrait pas
s'adsorber de manière importante aux particules du sol ou aux
sédiments. Dans le sol, les composés chimiques à faible constante de
Henry sont essentiellement non volatils. Toutefois, la tension de
vapeur de l'acide acrylique donne à penser qu'il se volatilise en
surface du sol ou lorsque le sol est sec.
L'acide acrylique libéré dans l'atmosphère réagit sur les
radicaux hydroxyles et sur l'ozone produits par voie photochimique et
se décompose alors rapidement. L'acide acrylique ne risque pas d'être
transporté dans l'atmosphère sur une longue distance car sa durée de
vie atmosphérique est inférieure à un mois.
L'acide acrylique peut se former par hydrolyse de l'acrylamide
monomère présent dans les déchets industriels enfouis dans le sol, en
particulier dans des conditions d'aérobiose.
Lorsqu'il est libéré dans l'eau, l'acide acrylique est facilement
biodégradé. Sa destinée dans l'eau est liée à sa dégradation par voie
chimique ou microbienne. Il est rapidement oxydé et risque donc de
provoquer une désoxygénation des étendues d'eau dans lesquelles il est
déversé en grandes quantités. On a montré que l'acide acrylique
pouvait être décomposé dans des conditions d'aérobiose ou
d'anaérobiose.
On ne dispose d'aucune donnée quantitative sur les concentrations
d'acide acrylique dans l'air ambiant, l'eau de boisson ou le sol.
Toutefois, on sait qu'il est présent dans les effluents liquides
déversés sur les sites où on le prépare par oxydation ménagée du
propylène.
On ne possède aucune donnée sur l'exposition de la population en
général. Toutefois, les consommateurs peuvent être exposés à de
l'acide acrylique libre présent dans des produits ménagers tels que
certaines peintures à l'eau. Les personnes qui vivent à proximité
d'unités de production d'acide acrylique ou de ses esters ou
polymères, peuvent être exposées à l'acide acrylique présent dans
l'air ambiant. L'absorption d'esters acryliques peut constituer une
source potentielle d'exposition interne à l'acide acrylique.
L'inhalation et le contact sont deux voies importantes
d'exposition professionnelle.
Quelle que soit la voie d'exposition, l'acide acrylique est
rapidement résorbé et métabolisé. Il est métabolisé dans une forte
proportion, principalement en acide 3-hydroxypropionique, en CO2 et
en acide mercapturique qui sont éliminés ensuite dans l'air expiré et
dans les urines. Du fait de sa métabolisation et de son élimination
rapides, la demi-vie de l'acide acrylique est brève (quelques minutes)
et il n'a donc aucune tendance à la bioaccumulation.
On a fait état de valeurs très diverses pour la DL50, mais la
plupart des données indiquent que l'acide acrylique présente une
toxicité aiguë modérée à faible par la voie orale et une toxicité
aiguë modérée par la voie respiratoire ou percutanée.
L'acide acrylique est corrosif ou irritant pour la peau et les
yeux. On ne sait pas avec précision au-dessous de quelle concentration
il n'est plus irritant. Il est également très irritant pour les voies
respiratoires.
On a obtenu des résultats positifs et négatifs lors d'épreuves de
sensibilisation cutanée avec de l'acide acrylique, mais il semble que
les résultats positifs obtenus étaient dus à la présence d'une
impureté.
Lors d'études au cours desquelles des rats ont absorbé de l'acide
acrylique dans leur eau de boisson, on a constaté que la dose sans
effet nocif observable (NOAEL) était de 140 mg/kg de poids corporel
par jour, le critère retenu étant la réduction du gain de poids sur
12 mois, et de 240 mg/kg de poids corporel par jour, la critère retenu
dans ce cas étant la présence d'anomalies histologiques au niveau de
l'estomac. Le même type d'étude chronique sur des rats a montré qu'à
la dose la plus élevée étudiée (78 mg/kg de poids corporel et par
jour), il n'y avait aucun effet observable. La dose la plus faible
provoquant un effet observable (LOAEL) a été de 15 mg/m3 (5 ppm) par
la voie respiratoire chez des souris exposées pendant 90 jours à de
l'acide acrylique, le critère retenu étant en présence de lésions
nasales infimes chez les femelles. Des effets au niveau du nez ont
également été observés chez des rats à la dose de 225 mg/m3, soit
75 ppm, mais pas à celle de 15 ou 75 mg/m3 (5 ou 25 ppm).
Les études de reproduction dont on connaît les résultats
indiquent que l'acide acrylique n'est pas tératogène et qu'il n'a
aucun effet sur la reproduction.
Les épreuves de génotoxicité in vitro ont donné des résultats
positifs et des résultats négatifs. Une épreuve pratiquée in vivo, à
la recherche d'aberration chromosomique dans la moelle osseuse, a
donné des résultats négatifs. Aucune conclusion définitive n'a pu être
tirée d'une étude in vivo sur la liaison à l'ADN ni de la recherche
de mutations létales dominantes.
Les données disponibles ne donnent pas de résultats indicatifs
d'un pouvoir cancérogène de l'acide acrylique, toutefois ces données
sont insuffisantes pour conclure qu'il n'y a aucun risque.
On a signalé des cas d'intoxication dans la population générale,
mais aucune étude épidémiologique en milieu professionnel n'a été
publiée.
Comme l'action toxique de l'acide acrylique se manifeste au point
de contact, des valeurs guides distinctes sont recommandées pour
l'exposition par voie orale et l'exposition par la voie respiratoire.
On propose des valeurs guides de 9,9 mg/litre dans l'eau de boisson et
de 54 µg/m3 dans l'air ambiant pour la population générale.
L'acide acrylique est faiblement toxique pour les bactéries et
les micro-organismes terricoles.
Parmi les organismes aquatiques étudiés, ce sont les algues qui
constituent le groupe le plus sensible, avec des valeurs de la CE50
pour la croissance, qui vont de 0,04 à 63 mg/litre et une
concentration sans effet observable (NOEC) chez l'espèce la plus
sensible, qui est de 0,008 mg/litre. Pour la daphnie, les valeurs de
la CE50 (critère : immobilisation) sont de 54 mg/litre sur 24 heures
et de 95 mg/litre sur 48 heures. L'acide acrylique est plus toxique
pour les daphnies que ses sels alcalins. En ce qui concerne la
toxicité aiguë, les études toxicologiques sur des poissons ont donné
les résultats suivants : de 25 mg/litre (CL50 à 96 heures) pour la
truite arc-en-ciel, à 315 mg/litre (CL50 à 72 heures) pour l'orfe. En
se basant sur l'absence de réponse sublétale et comportementale, on a
observé que la concentration d'acide acrylique sans effet observable
était de 6,3 mg/litre pour la truite arc-en-ciel.
Du fait que son faible coefficient de partage entre l'octanol et
l'eau, l'acide acrylique a peu de chances de subir une
bioconcentration chez les organismes aquatiques. Par ailleurs, on n'a
pas signalé de cas de bioamplification le long de la chaîne
alimentaire.
On ne dispose d'aucune donnée concernant les effets de l'acide
acrylique sur les organismes terrestres.
1. RESUMEN Y RECOMENDACIONES
El ácido acrílico es un líquido incoloro que desprende un olor
acreirritante a temperatura y presión ambiente. Su umbral de olor es
bajo (0,20-3,14 mg/m3). Es miscible en agua y en la mayor parte de
los disolventes orgánicos.
El ácido acrílico está disponible en el comercio en dos
calidades: técnica y glacial. Como se polimeriza fácilmente si se
expone al calor, a la luz o a metales, los productos comerciales
contienen un inhibidor de la polimerización.
La producción mundial de ácido acrílico en 1994 se estimó en
aproximadamente 2 millones de toneladas. Principalmente se utiliza
como materia prima en la producción de ésteres acrílicos, como
monómero para ácidos y sales poliacrílicos, y como comonómero con
acrilamida para polímeros que sirven de agentes de floculación, con
etileno para polímeros de resina intercambiadora de iones, con éster
metílico para polímeros y con ácido itacónico para otros copolímeros.
Los residuos de ácido acrílico en el aire y en otros medios
pueden cuantificarse mediante técnicas de cromatografía de gases,
cromatografía líquida de alta resolución y polarografía. Los límites
de detección de esos métodos son de 14 ppm en el aire y 1 ppm en otros
medios.
Se ha notificado la presencia natural de ácido acrílico en algas
marinas, y se le ha encontrado en el líquido ruminal de las ovejas.
Por ser miscible en agua, no cabe prever que el ácido acrílico se
adsorba de manera significativa en el suelo o en los sedimentos. En
el suelo, las sustancias químicas con bajas constantes de Henry son
esencialmente no volátiles. Sin embargo, la presión de vapor del
ácido acrílico indica que éste se volatiliza del suelo superficial y
del suelo seco.
El ácido acrílico liberado en la atmósfera reacciona con los
radicales hidroxilos y el ozono de origen fotoquímico, degradándose
rápidamente. El transporte atmosférico del ácido acrílico a grandes
distancias no es posible, porque su permanencia en la atmósfera es
inferior a un mes.
El ácido acrílico puede formarse por hidrólisis del monómero
acrilamida en desechos industriales presentes en el suelo,
especialmente en condiciones aeróbicas.
Liberado en el agua, el ácido acrílico se biodegrada rápidamente.
Su destino en el agua depende de la degradación química y microbiana.
El ácido acrílico se oxida rápidamente en el agua; descargado en
grandes cantidades en una masa de agua, puede agotar el oxígeno. Se
ha demostrado que la degradación se produce en condiciones aeróbicas y
anaeróbicas.
No se dispone de datos cuantitativos sobre niveles de ácido
acrílico en el aire ambiente, el agua potable o el suelo. Sin
embargo, se sabe que el ácido acrílico está presente en los efluentes
de su producción por oxidación del propileno.
No hay datos sobre la exposición de la población general. No
obstante, los consumidores podrían estar expuestos al ácido acrílico
en productos de uso doméstico tales como las pinturas a base de agua.
La población residente en las cercanías de fábricas productoras de
ácido acrílico o de sus ésteres o polímeros puede estar expuesta al
ácido acrílico en el aire ambiente. Una posible fuente de exposición
interna puede ser el metabolismo de los ésteres del ácido acrílico
absorbidos.
La inhalación y el contacto con la piel son importantes vías de
exposición ocupacional.
Independientemente de la vía de exposición, el ácido acrílico se
absorbe y metaboliza con rapidez. Se metaboliza ampliamente, sobre
todo en ácido 3-hidroxipropiónico, CO2 y ácido mercaptúrico, que se
eliminan en el aire expirado y por la orina. Debido a su rápido
metabolismo y eliminación, la semivida del ácido acrílico es breve
(minutos), por lo que no tiene potencial de bioacumulación.
Aunque se ha notificado una amplia gama de valores de DL50, la
mayor parte de los datos indica que el ácido acrílico tiene una
toxicidad aguda de baja a moderada por vía oral, y moderada por
inhalación o por vía cutánea.
El ácido acrílico es corrosivo o irritante para la piel y los
ojos. No se sabe con certeza a qué concentración no es irritante.
También irrita fuertemente las vías respiratorias.
En cuanto a la sensibilización de la piel al ácido acrílico, se
han notificado resultados positivos y negativos, pero es posible que
los positivos se deban a una impureza.
En estudios con agua potable en ratas, el nivel sin efectos
negativos observados (NOAEL) fue de 140 mg/kg de peso corporal al día
para la reducción del aumento de peso corporal en un estudio de 12
meses de duración, y de 240 mg/kg de peso corporal al día para las
alteraciones histopatológicas en el estómago. Un estudio de
exposición crónica al agua potable en ratas no reveló efectos a la
dosis más alta ensayada (78 mg/kg de peso corporal al día). En
ratones expuestos al ácido acrílico por inhalación durante 90 días se
detectó un nivel inferior con efectos negativos observados (LOAEL) de
15 mg/m3 (5 ppm), sobre la base de lesiones nasales muy ligeras en
las hembras. En las ratas se observaron efectos nasales a 225 mg/m3
(75 ppm), pero no a 15 ni a 75 mg/m3 (5 ó 25 ppm).
Los estudios de reproducción disponibles indican que el ácido
acrílico no es teratogénico, ni tiene efecto alguno en la
reproducción.
En las pruebas de genotoxicidad in vitro se han obtenido
resultados tanto positivos como negativos. Una prueba de aberración
cromosómica en médula ósea in vivo dio resultados negativos. Un
estudio in vivo de unión de ADN y una prueba de dominancia letal no
permitieron sacar conclusiones definitivas.
Los datos disponibles no indican que el ácido acrílico sea
carcinógeno, pero esos datos son insuficientes para concluir que no
existe ningún riesgo de carcinogenicidad.
No se han comunicado casos de intoxicación en la población
general. Tampoco se han notificado estudios epidemiológicos
ocupacionales.
Puesto que la toxicidad del ácido acrílico afecta al lugar en que
se produce el contacto, se recomiendan valores de orientación
distintos para la exposición oral y por inhalación. Para la población
general se proponen valores de orientación de 9,9 mg/litro para el
agua potable y de 54 œg/m3 para el aire ambiente.
La toxicidad del ácido acrílico para las bacterias y los
microorganismos del suelo es baja.
Las algas son el grupo de organismos acuáticos más sensible entre
los estudiados, con unos valores de CE50 , sobre la base del
crecimiento, que van de 0,04 a 63 mg/litro y una concentración sin
efectos observados (NOEC) para la especie más sensible de 0,008
mg/litro. Los valores de CE50 (basados en la inmovilización) para
Daphnia magna son de 54 mg/litro (24 horas) y 95 mg/litro (48
horas). El ácido acrílico es más tóxico para los dáfnidos que la sal
alcalina. Estudios de toxicidad aguda en peces han dado resultados
que oscilan entre 27 mg/litro (CL50 a las 96 horas) para la trucha
arco iris y 315 mg/litro (CL50 a las 72 horas) para Leuciscus idus.
La NOEC a las 96 horas para la toxicidad del ácido acrílico en la
trucha arco iris se ha establecido en 6,3 mg/litro, sobre la base de
la ausencia de respuestas subletales/comportamentales.
Debido a su bajo coeficiente de reparto octanol/agua, es poco
probable que el ácido acrílico sea objeto de bioconcentración en
organismos acuáticos. No se conocen casos de bioamplificación en
cadenas alimentarias.
No se dispone de datos sobre los efectos del ácido acrílico en
organismos terrestres.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of acrylic acid is difficult to ascertain,
owing to the wide range of LD50 values reported (Table 4). However
most data indicate that the substance is of low to moderate toxicity
by the oral and inhalation routes and of moderate toxicity by the
dermal route. It has been proposed that the wide variation in oral
LD50 values may be due to the different forms in which acrylic acid
has been applied, i.e. undiluted, in aqueous solution at various
concentrations or in neutralized solution (BG Chemie, 1991).
Stomach lesions, necrosis and haemorrhage have been reported
following oral dosing of rats with acrylic acid (Ghanayem et al.,
1985; DeBethizy et al., 1987). The lowest dose at which lesions were
seen was 144 mg/kg.
CrL:CDBR rats were exposed (whole body) to aerosol (mean mass
median diameter of 2.3 µm ± 2.3) concentrations of acrylic acid
ranging from 8775 to 14 145 mg/m3 (2925 to 4715 ppm) for 30 min, 8139
to 12 624 mg/m3 (2713 to 4208 ppm) for 60 min, and 3669 to
10 239 mg/m3 (1223 to 3413 ppm) for 120 min. Additionally, rats were
exposed (whole body) to acrylic acid vapour concentrations ranging from
2784 to 6426 mg/m3 (928 to 2142 ppm) for 60 min. Exposure to acrylic
acid produced treatment-related signs of nasal mucosa, upper airway and
lower airway irritation, ocular irritation, corneal opacities and
dermal toxicity in all experimental groups. Deaths, as a function of
both aerosol concentration and exposure duration, were seen in the
30-, 60- and 120-min aerosol exposures. No deaths resulted from the
vapour exposures. Following the 14-day observation period, necropsies
revealed treatment-related alterations of the lungs, eyes and skin
consistent with that of an irritant. However, comparison of LC values
suggested no difference in toxicity between the aerosol and vapour
(Hagan & Emmons, 1991).
Table 4. The acute toxicity (LD50 and LC50) of acrylic acid for experimental animals
Species Route Parameter Dose Reference
Mouse oral LD50 830 mg/kg Klimkina et al., 1969
Mouse oral LD50 1200 mg/kg Zeller, 1958
Rat oral LD50 193 mg/kg IARC, 1979
Rat oral LD50 340 mg/kg Carpenter et al., 1974
Rat oral LD50 1350 mg/kg Majka et al., 1974
Rat oral LD50 1500 mg/kg Zeller, 1958
Rat oral LD50 2520 mg/kg Fassett, 1963
Rat oral LD50 2100-3200 mg/kg Miller, 1964
Rat oral LD50 2500 mg/kg Verschueren, 1983
Mouse subcutaneous LD50 1590 mg/kg Sittig, 1985
Rabbit percutaneous LD50 295 mg/kg Carpenter et al., 1974
Rabbit percutaneous LD50 640 mg/kg Gelbke & Hofman, 1979
Rabbit percutaneous LD50 750 mg/kg IARC, 1979
Rabbit percutaneous LD50 950 mg/kg Fassett, 1963
Mouse inhalation LC50 (2 h) 5300 mg/m3 RTECS, 1989
Rat inhalation LC50 (30 min) 26 000 mg/m3 Hagan & Emmons, 1991
LC50 (60 min) 11 100 mg/m3
LC50 (120 min) 7500 mg/m3
Table 4. (contd.)
Species Route Parameter Dose Reference
Rat inhalation LC50 (4 h) 3600 mg/m3 Majka et al., 1974
Rat inhalation LC50 (4 h) > 5100 mg/m3 Klimisch & Zeller, 1980
Mouse intraperitoneal LD50 17 mg/kg Lawrence et al., 1972
Mouse intraperitoneal LD50 128 mg/kg RTECS, 1989
Mouse intraperitoneal LD50 140 mg/kg Zeller, 1958
Rat intraperitoneal LD50 24 mg/kg Majka et al., 1974
Rat intraperitoneal LD50 24 mg/kg Singh et al., 1972
In a single inhalation study in rats, no deaths occurred when
six animals were exposed to acrylic acid at a concentration of
12 000 mg/m3 (4000 ppm) for 4 h and observed over 14 days (Union
Carbide Corp., 1977).
A single 4-h exposure of six rats to 6000 mg/m3 (2000 ppm) of
acrylic acid caused no death (Carpenter et al., 1974).
One 5-h exposure to an atmosphere saturated with acrylic acid
(6000 ppm or 17 700 mg/m3) given to four rats (2 male, 2 female)
produced nose and eye irritation, respiratory difficulty and
unresponsiveness in all rats. One rat died. Histopathological
examination showed lung haemorrhage and degenerative changes in the
liver and kidney tubules of all rats (Gage, 1970), but these were
possibly secondary changes in dying animals.
Rats exposed for 1 h to acrylic acid concentrations of 300, 900
or 1500 mg/m3 (100, 300 or 500 ppm) exhibited exposure-dependent
decreases in both respiratory frequency and minute volume (Silver et
al., 1981).
In a sensory irritation study, the single exposure to acrylic
acid vapour estimated for a 50% reduction of the respiratory rate
(RD50) was 1539 mg/m3 (513 ppm) in F344/N rats and 2055 mg/m3
(685 ppm) in B6C3F1 mice. During exposure to 225 mg/m3 (75 ppm) of
acrylic acid vapour for 6 h, a 20-30% decrease in minute volume was
observed in both species (Buckley et al., 1984).
7.2 Irritation and sensitization
7.2.1 Eye irritation
Application of acrylic acid in different concentrations (glacial,
10%, 3% and 1%) to rabbit eyes revealed that it is corrosive in high
concentrations, i.e. glacial and 10%; 1% and 3% solution caused eye
irritancy (Majka et al., 1974). There are also other reports of
undiluted acrylic acid causing eye irritation and corneal damage
(Carpenter et al. 1974; BG Chemie, 1991).
7.2.2 Skin irritation and sensitization
7.2.2.1 Skin irritation
Undiluted acrylic acid is corrosive to rabbit skin (Carpenter et
al., 1974; Majka et al., 1974; BG Chemie, 1991). A study with rabbits
reported that a one-minute exposure to a 50% or 20% aqueous solution
caused, respectively, erythema and oedema or slight erythema (BG
Chemie, 1991). Another study reported a 10% solution to be corrosive
when applied to rabbit skin and that a 0.6-5% solution caused
irritation of various severity (Majka et al., 1974).
The irritant effects of repeated dermal exposure have also been
investigated. A 5% acrylic acid solution in acetone caused skin
irritation in the mouse after daily non-occlusive application for 14
days (DePass et al., 1984). No irritation was seen with a 1% solution.
In another study, groups of three strains of mice received dermal
applications of 0.1 ml acrylic acid in acetone 3 times a week for 13
weeks at concentrations of 0, 1 or 4% (Tegeris et al., 1987, 1988). At
4%, there were signs of significant skin irritation (desquamation,
fissures and eschar), with proliferative, degenerative and
inflammatory changes being detected histologically in the epidermis
and dermis, from weeks 1 to 2. At 1%, minimal proliferative changes,
detected histologically, were the only effects seen. No differences
were found between the response of the three strains of mice.
7.2.2.2 Skin sensitization
Acrylic acid has been tested for contact sensitivity in guinea-
pigs. In one study, a 20% aqueous solution of pure unstabilized
acrylic acid was applied to the skin once a day until definite skin
irritation was seen. When challenged topically 11 days later with a 2%
solution, there was no evidence of skin sensitization up to 24 h post-
challenge (BG Chemie, 1991).
In another study, the highest non-irritating concentration of
acrylic acid (not specified) was applied topically four times in 10
days. At the time of the third application, Freund's adjuvant was
injected intradermally. When challenged two weeks later, none of the
10 guinea-pigs showed evidence of skin sensitization (Rao et al.,
1981).
Three out of six guinea-pigs exposed to acrylic acid, said to be
99% pure, showed a skin sensitization response in a Polak test (Parker
& Turk, 1983). Induction was by dermal injections of a total of 1 mg
acrylic acid, together with adjuvant, followed by topical challenge
with 5% acrylic acid. However, the impurities and inhibitors of the
acrylic acid used were not mentioned in the report.
Acrylic acid was found to be an extreme sensitizer by the guinea
pig maximization test and a weak sensitizer by the Landsteiner Draize
test. The compound used for testing was considered pure, but no
analytical data were provided (Magnusson & Kligman, 1969).
Acrylic acid gave a clearly positive result in the Freunds
Complete Adjuvant test in guinea-pigs (Waegemaekers & van der Walle,
1984). Induction was by three intradermal injections of 1.2% followed
by topical application of 2.2 or 7.2%. The positive response was
believed to be due to the historical impurity, alpha,ß-
diacryloxypropionic acid. This impurity was identified in acrylic acid
from just one of three suppliers. Limited testing of acrylic acid from
the other two suppliers gave negative skin sensitization results. It
should be noted that the impurity is not present in acrylic acid
resulting from current production methods involving distillation.
Commercial acrylic acid also contains a small amount of
polymerization inhibitors, usually hydroquinone monomethyl ether
(methoxyphenol). This is a known skin sensitizer in guinea-pigs (van
der Walle et al., 1982). Other inhibitors used with acrylic acid have
also been reported to have skin-sensitizing properties, namely pheno-
thiazine (Costellati et al., 1990) and diphenyl- p-phenylenediamine
(Magnusson et al., 1968; Kalimo et al., 1989). However, it is unclear
whether the small amount of one of these inhibitors present
(0.02-0.1%) could contribute to the skin-sensitizing properties of
commercial acrylic acid.
7.2.3 Upper respiratory tract irritation
Olfactory cell proliferation, as measured by tritiated thymidine
incorporation, was investigated in male F-344 rats and B6C3F1 mice
exposed to 224 mg/m3 (75 ppm) acrylic acid 6 h daily for 5 days. A
17-fold increase in cell proliferation occurred in mice and a 4-fold
increase in rats (Swenberg et al, 1986). Further information on upper
respiratory tract irritation is given in sections 7.1 and 7.3.2.
7.3 Short-term exposure
Results of key studies on the short-term repeated exposure
effects of acrylic acid are presented in Table 5.
Table 5. Key studies on the noncarcinogenic effects of repeated exposures to acrylic acid
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Fisher-344 oral, 250 mg/kg bw/day 83 mg/kg bw/day Decreased body weight, De Pass et al.,
drinking-water, 0, 83, reduced water and food 1983
250, 750 mg/kg body consumption, changes in
weight/day for 3 months organ weights
Rat, Wistar, gavage, 0, 150 mg/kg bw/day 50% mortality in both Hellwig et al.,
150, 375 mg/kg bw/day, treatment groups, dose- 1993
5 times/week for 3 dependent irritation in the
months forestomach and
glandular stomach,
purulent rhinitis, tubular
nephroses
Rat, Wistar, oral,
drinking-water, 0, 9, 61,
140, 331 mg/kg body
weight/day for 3 months 331 mg/kg bw/day 140 mg/kg bw/day Reduced water and food Hellwig et al.,
consumption in males 1993
Rat, Wistar, oral, 140 mg/kg bw/day 61 mg/kg bw/day Reduced water and food Hellwig et al.,
drinking-water, 0, 9, 61, consumption in males 1993
140, 331 mg/kg body
weight/day for 12 months
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Wistar, oral, 78 mg/kg bw/day No treatment-related Hellwig et al.,
drinking-water, 0, 8, 27, toxic effects including 1993
78 mg/kg body tumorogenicity
weight/day for 26
(males) or 28 (females)
months
Rat, inhalation, 80, 300 300 ppm 80 ppm Nose irritation, lethargy Gage, 1970
ppm, 6 h/day 5 (900 mg/m3) (240 mg/m3) reduced body weight gain
days/week,
20 exposures
Rat, Fisher-344 225 ppm (675 75 ppm Decrease of adipose Miller et al.,
inhalation, 0, 25, 75, mg/m3) (225 mg/m3) tissue in females, lesions 1979
225 ppm, 6 h/day, of nasal mucosa
5 days/week for 2 weeks
Rat, Fisher 344 75 ppm 25 ppm Lesions of nasal olfactory Miller et al.,
inhalation, 0, 5, 25, (225 mg/m3) (75 mg/m3) epithelium 1981
75 ppm, 6 h/day,
5 days/week for
13 weeks
Rat, F-344, and mouse 75 ppm Olfactory cell proliferation Swenberg et al.,
B6C3F1 inhalation, (225 mg/m3) 17-fold in mice, 4-fold in 1986
75 ppm, 6 h/day, 5 days rats
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Mouse, B6C3F1 25 ppm Decrease in body weight Miller et al.,
inhalation, 0, 25, 74, (75 mg/m3) gain, lesions in nasal 1979
223 ppm, 6 h/day mucosa
5 days/week for 2 weeks
Mouse, B6C3F inhalation 5 ppm 5 ppm Atrophy, disorganization, Lomax et al.,
0, 5, 25 ppm for 6 or 22 22 h/day 6 h/day necrosis of the olfactory 1994
h/day and 25 ppm for epithelium of nasal
4.4 h/day for 2 weeks, cavity. Recovery after 6
6 weeks recovery period weeks except for mice
exposed to 25 ppm for
22 h/day where
metaplasia was seen
Mouse, B6C3F1 5 ppm Slight focal lesions of Miller et al.,
inhalation, 0, 5, 25, (15 mg/m3) nasal olfactory 1981
75 ppm, 6 h/day 5 epithelium
days/week for 13 weeks
a LOEL = lowest-observed-effect level; NOEL = no-observed-effect level
7.3.1 Oral
Acrylic acid was administered via oral gavage to ten rats for 20
days with doses increasing by 50% every fourth day (range:
135 mg/kg to 684 mg/kg). Reduction in body weight gain and minor
histopathological changes in the stomach were found at higher doses
(Majka et al., 1974).
In a 3-month study (Hellwig et al., 1993), groups of 10 male and
10 female Wistar rats were gavaged, 5 times per week, with acrylic
acid at doses of 150 or 375 mg/kg body weight. A control group of 10
males and 10 females was gavaged with water. A high mortality rate was
observed in experimental groups; 50% of both males and females in the
low-dose group and 60% (males) and 90% (females) in the high-dose
group died. Cyanosis, dyspnoea and irritation ulceration of
forestomach and glandular stomach, purulent rhinitis and lung
emphysema and alveolar hyperaemia were the main findings reported.
Necrotizing tubular nephroses were seen in the animals that died
during the study. The symptoms and histopathological findings were
substantially the same in both groups, but they were less pronounced
and observed in a smaller number of animals given acrylic acid at
150 mg/kg body weight.
Acrylic acid was given to Wistar rats in drinking-water for
3 months as part of a 12-month study (Hellwig et al., 1993). Further
details are given in section 7.4.
In a subchronic study acrylic acid was incorporated into the
drinking-water of rats (15/sex/group) for 3 months, resulting in doses
of 0,83, 250 and 750 mg/kg per day. At the high and intermediate dose
levels, reduction in body weight gain and changes in organ weights
were observed. These effects coincided with a dose-related reduction
in food and water consumption. At the 83 mg/kg dose, the only effect
was a slight reduction in water consumption. No significant treatment-
related histological effects were seen at any dose level (DePass et
al., 1983).
7.3.2 Inhalation
In a short-term inhalation study (Gage, 1970) no adverse effects
were observed in eight rats (four males and four females) exposed to
240 mg/m3 (80 ppm) acrylic acid vapour, 6 h/day, 5 days/week for 20
exposures. Eight rats (four males and four females) exposed at
900 mg/m3 (300 ppm) showed signs of nasal irritation, lethargy and
reduced body weight gain. Histological and haematological examinations
were normal. Higher doses for shorter periods of time, i.e. 4500 mg/m3
(1500 ppm) for 4 × 6 h, resulted in nasal discharge, lethargy,
retarded weight gain and kidney congestion.
In a 2-week subacute inhalation study by Miller et al. (1979a,
1981a), F-344 rats and B6C3F1 mice (5/sex/group) were exposed to
actual concentrations of 0, 75, 225 or 675 mg/m3 (0, 25, 75 or
225 ppm) acrylic acid vapour for 6 h/day, 5 days/week for 2 weeks.
Significant decreases in body weight gain were seen in exposed groups
at 675 mg/m3. Decreased body weight gain in male mice at 75 and
225 mg/m3 was not considered to be exposure-related because of the low
initial weight and unusually large weight gain in the controls. A
reduction of adipose tissue was observed in female rats at 675 mg/m3.
Rats had lesions of the nasal mucosa at 675 mg/m3. In mice lesions of
the nasal mucosa were observed in 2/5 males and 4/5 females at 75 mg/m3
and in all mice exposed to 225 or 675 mg/m3. The lesions were
described as slight focal degeneration in the olfactory epithelium. No
effects on lung or trachea were observed in rats or mice.
Fifteen F-344 rats/sex/group and 15 B6C3F1 mice/sex/group were
exposed to actual concentrations measured by infrared analysis of 0,
15, 75 or 225 mg/m3 (0, 5, 25 or 75 ppm) acrylic acid (Miller et al.,
1979a, 1981a). The exposure was 6 h/day for 5 days/week for 13 weeks.
Animals were observed twice per day. There were no treatment-related
deaths in rats or mice during the study period. Mean body weight gains
of female mice in the 75 and 225 mg/m3 exposure groups after 12 weeks
of exposure were significantly lower than controls. There were no
significant differences in organ weights, clinical chemistry
parameters, urinalysis parameters or gross pathology that could
clearly be related to exposure. Slight focal degeneration of the nasal
olfactory epithelium was observed in rats at 225 mg/m3 but no effects
were seen at 15 or 75 mg/m3. In mice, there was a clear exposure-
related increase in focal degeneration of the olfactory nasal
epithelium at > 25 mg/m3. Lesions of the olfactory epithelium were
detected histopathologically in all male and female mice in the
225 mg/m3 exposure group, in all males and 9/10 females in the
75 mg/m3 group and in 1/10 males and 4/10 females in the
15 mg/m3 group. The lesions were described as very slight at 15 mg/m3,
slight at 75 mg/m3 and slight to moderate at 225 mg/m3.
In a whole-body inhalation study, groups of 10 B6C3F1 female
mice were exposed to 0, 15 or 75 mg/m3 (0, 5 or 25 ppm) acrylic acid
vapour for 6 or 22 h/day for 2 weeks. An additional group of mice was
exposed to 75 mg/m3 for 4.4 h/day for 2 weeks. No histopathological
lesions in the nasal cavity were detected in mice exposed to 0 or 15
mg/m3 for 6 h/day. Histopathological lesions were observed in the
olfactory epithelium of the dorsal meatus of the nasal cavity in all
other groups. These lesions included atrophy, disorganization,
necrosis, desquamation of the epithelium and nasal cell hypertrophy.
Lesions were more severe after the 22 h exposure at 75 mg/m3. After a
6-week recovery period, the olfactory epithelium was normal except for
mice exposed to 75 mg/m3 for 22 h/day where metaplasia was seen
(replacement of olfactory epithelium with respiratory-like epithelium)
(Lomax et al., 1994).
Animals exposed to 720 mg/m3 (240 ppm) for 4 h/day, 6 days/week,
for 5 weeks exhibited decreased body weight gain, increased urinary
secretion of phenol red, reduced urine-concentrating ability, nasal
discharge and increased reticulocyte count. Histopathological
examination revealed lesions of the gastric mucosa after repeated
doses of acrylic acid. Inflammation of the upper respiratory tract was
also seen (Majka et al., 1974).
7.4 Long-term exposure
Results of the studies of long-term repeated exposure effects to
acrylic acid are presented in Table 5.
In a 12-month study (Hellwig et al., 1993), Wistar rats
(20/group/sex) were given drinking-water containing 120, 800, 2000 or
5000 mg/litre (equivalent to doses of 9, 61, 140, 331 mg/kg body
weight/day, respectively). Satellite groups (10 rats/group/sex) were
treated concurrently for 3 months. Both in satellite groups and
12-months exposure groups, a reduction in drinking-water and/or feed
consumption and retarded body weight gain were observed in males at
140 and 331 mg/kg per day (2000 and 5000 mg/litre), probably due to
the unpalatability of acrylic acid at these concentrations.
Differences between groups in the results of clinico-chemical,
haematological and urinalytical examinations as well as in gross
pathological and histopathological findings were not treatment-
related. The no-observed-effect level (NOEL) was 61 mg/kg body weight
(800 mg/litre).
Acrylic acid was given to Wistar rats in drinking-water for 26
(males) and 28 (females) months in a chronic/carcinogenicity study
(Hellwig et al., 1993). Further details are given in section 7.7.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
Results of key studies on the reproductive system are shown in
Table 6.
Table 6. Reproductive effects of acrylic acid exposure in female rats and mice and developmental effects in their offspring
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Reproduction Rat Fischer 250 mg/kg 83 mg/kg 750 mg/kg 250 mg/kg Decreased body Decrease in
and development 344; oral, bw/day bw/day bw/day bw/day weight and food body weight gain
(dePass et al., drinking- consumption of male and
1983) water; 0, 83, female pups,
250, 750 mg/kg decrease in
bw/day for 3 liver, kidney,
months before heart and spleen
mating and weight in male
through-out pups and liver
gestation and weight in female
lactation pups
Two-generation Rat Wistar; 240 mg/kg 53 mg/kg 240 mg/kg 53 mg/kg Fertility not Retarded growth
reproductive oral, drinking- bw/day bw/day bw/day bw/day affected. Reduced of F1 and F2 pups
toxicity water, 0, 500, food and water
(BASF, 1994a) 2500, 5000 ppm consumption,
(53, 240, reduced body
460 mg/kg/day) weights, gross
and
histopathological
changes in stomach
Developmental Rat Sprague- - - 2.4 mg/kg No data Increased
toxicity Dawley, bw resorptions
(Singh et al., intraperitoneal and number
1972) 0, 2.4, 4, 8, of gross and
8.0 mg/kg bw skeletal
i.p. on days 5, abnormalities
10 and 15 of
gestation
Table 6. (contd.)
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Developmental Rat Sprague- 360 mg/m3 - - 1080 Decreased None
toxicity Dawley, (120 ppm) mg/m3 body weight,
(Klimisch & inhalation, (360 ppm) food consumption
Hellwig, 0, 40, 120, uterus-to-body
1991) 360 ppm for weight; eye and
10 days during nose irritation
gestation
(days 6
through
15)
Developmental Rabbit New 180 90 mg/m3 - -- Squamous -
toxicity Zealand white, mg/m3 (30 ppm) metaplasia
(Chun et al., inhalation, 0, (60 ppm) erosions,
1993) 30, 60, 125, ulcerations in
250 ppm (0, olfactory
90, 180, 375, epithelium
750 mg/m3)
during
gestation
(days 10-23)
Developmental Rabbit New 225 mg/m3 75 mg/m3 - 675 Perinasal, None
toxicity Zealand white, (75 ppm) (25 ppm) mg/m3 perioral
(Neeper- inhalation (225 ppm) wetness,
Bradley & 0, 25, 75, nasal
Kubena, 1993) 225 ppm congestion,
(0, 75, 225, blepharospasm
675 mg/m3)
during
gestation
(days 6-18)
A one-generation study was conducted in which 10 male and 20
female rats received acrylic acid orally, in drinking-water, at doses
of 83, 250 or 750 mg/kg body weight per day for 3 months, after which
the animals were mated; this formed part of the subchronic study
reported in section 7.3.1. (DePass et al., 1983). Exposure was
continued throughout gestation and lactation.
Maternal toxicity at the two highest doses included decreased
body weight gain and decreased food consumption. An apparent decrease
in the fertility of females, as well as a reduction in gestation
index, number of live pups/litter and percentage of pups weaned at the
highest dose, was observed, but this difference was not statistically
significant when compared with the control group. Unusually low
fertility in the control group makes interpretation difficult.
In a two-generation reproductive toxicity study (BASF, 1994a),
Wistar rats were exposed to acrylic acid in drinking-water at levels
of 500, 2500 or 5000 mg/litre. Acrylic acid did not impair
reproductive function in either of the parental generations exposed to
any dose level. Clear signs of toxicity, including reduced food and
water consumption, reduced body weight and/or body weight gain, and
gross and histopathological changes in the stomach, were observed in
both parental generations exposed to 5000 mg/litre. At the
2500 mg/litre level, signs of toxicity were present in the second
parental generation animals. No adverse effects were observed in either
parental generation exposed to 500 mg/litre. Signs of developmental
toxicity, including retarded growth of F1 and F2 pups and some
delays in physical development of F2 pups only, were observed at
5000 mg/litre but less so at 2500 mg/litre. It is difficult to evaluate
the role of acrylic acid in the decreased pup weights, because of the
combined effects of reduced water consumption and the poor
palatability of water containing acrylic acid. The greatest effects on
body weight occurred during the period of early life around weaning.
Developmental toxicity was not observed at 500 mg/litre. Thus,
500 mg/litre (equivalent to 53 mg/kg per day) was a NOEL for both
generations of offspring and also a NOAEL for general toxicity in the
second parental generation. The NOEL for general toxicity in the first
parental generation was 2500 mg/litre (equivalent to 240 mg/kg per
day).
In a study by Vojtisek et al. (1991) cows were given 16 kg/day
clover grass silage, treated with 3 litres acrylic acid/tonne, from
day 57.6 ± 21.1 before parturition, for 107 days. Control groups of
eight cows were given the same amount of silage, but treated with 4
litres formic acid/tonne from day 55.5 ± 21.9 before parturition.
Changes in clinical chemistry and haematological parameters observed
in the course of the experiment (on days 0, 39, 65 and 107) were of no
toxicological importance. No differences in colostrum density, body
weight, clinico-chemical or haematological examinations of live calves
were seen.
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Oral
In the experiment of DePass et al., (1983) (see also section
7.5.1) there was a statistically significant decrease in body weight
of the male and female pups at the highest dose (750 mg/kg per day),
which was maternally toxic. The male pups also exhibited significant
decreases in absolute and relative liver weights and in absolute
kidney and heart weights at 750 mg/kg per day. The female pups showed
a significant decrease in absolute and relative spleen weight and in
absolute liver weight at the highest dose. There was an increase in
relative brain weight in both sexes at this dose.
7.5.2.2 Inhalation
Groups of 30 pregnant Sprague-Dawley rats were exposed to nominal
concentrations of 0, 120, 360 or 1080 mg/m3 (0, 40, 120, or 360 ppm)
acrylic acid vapour for 6 h/day for 10 days during gestation (day 6 to
day 15 of gestation) (Klimisch & Hellwig, 1991). There was clear
evidence of maternal toxicity at 1080 mg/m3 consisting of eye and
nose irritation, as well as reduced body weight gain and food
consumption. The latter two effects were also seen at 360 mg/m3 and
there was an indication of minimal maternal toxicity at 40 ppm
(reduced body weight gain). There were no effects on preimplantation
loss, the number of live fetuses and resorption, fetal size or on the
appearance of the soft tissues and skeleton of the fetuses. This study
identified a NOAEL for developmental effects of 1080 mg/m3 and a
LOAEL for maternal toxicity of 1080 mg/m3.
An inhalation developmental study has also been reported in
rabbits (Neeper-Bradley & Kubena, 1993). In the range-finding study
(Chun et al., 1993), groups of eight pregnant New Zealand white
rabbits were exposed to 0, 90, 180, 375 or 750 mg/m3 (0, 30, 60, 125
or 250 ppm) acrylic acid vapour for 6 h/day on days 10-23 of
gestation. Three animals per group were necropsied on day 23 of
gestation, and the remaining animals were examined on day 29.
Exposure-related maternal toxicity at 375 and 750 mg/m3 was observed,
including signs of nasal irritation and reduced body weight. Final
body weight was reduced to a lesser degree in animals exposed to 90
and 180 mg/m3. Histopathological examination of a single section of
the nose showed adverse effects in the olfactory epithelium. The
lesions included squamous metaplasia, epithelial erosion and
ulceration of the epithelium, and increased in severity with
increasing exposure concentration. The effect first appeared in the
90 mg/m3 group at day 23 and in the 180 mg/m3 group at day 29.
In the main developmental study (Neeper-Bradley & Kubena, 1993), groups
of 16 pregnant rabbits were exposed to 0, 75, 225 or 675 mg/m3 (0, 25,
75 or 225 ppm) acrylic acid vapour for 6 h/day on gestation days 6-18.
Maternal toxicity was evident in groups exposed to 225 or 675 mg/m3,
but not to 75 mg/m3 (NOEL). Signs of nasal irritation, including
perinasal wetness and nasal congestion, were observed. Significant
decrements in food consumption and body weight gain were observed
occasionally during exposure, but the body weights at the end of the
exposure were not significantly affected. Histopathological
examination of maternal tissues was not performed. No exposure-
related adverse effects were observed in the number of corpora lutea
and total, viable or nonviable implantations; preimplantation loss;
fetal length or weight; or on morphological abnormalities (skeletal
or soft tissue). This study identified a NOEL for developmental
effects of 675 mg/m3.
7.5.2.3 Intraperitoneal
When acrylic acid was injected intraperitoneally into pregnant
female Sprague Dawley rats at doses of 0, 2.4, 4.8, or 8.0 mg/kg on
days 5, 10 and 15 of gestation, the chemical was both embryotoxic and
teratogenic (Singh et al., 1972). The number of resorptions and the
number of gross and skeletal abnormalities increased with increasing
acrylic acid concentration, and the NOEL was identified at the low
dose (i.e. 2.4 mg/kg). Fetotoxicity (decreased number of live fetuses
and mean fetal weight) was observed at a dose of 2.4 mg/kg. This study
is difficult to interpret as the control groups treated with distilled
water, normal saline and cotton-seed oil also showed gross and
skeleton abnormalities. There was also no information about maternal
toxicity.
7.6 Mutagenicity and related end-points
7.6.1 In vitro and insect studies
Acrylic acid was found to be without mutagenic activity in five
test strains of Salmonella typhimurium with and without activation
by rat and hamster liver microsomal preparations using both plate and
liquid suspension assays (Lijinsky & Andrews, 1980). Although negative
responses were reported in all strains, no cytotoxicity was observed
at the concentrations tested (up to 1000 µg/plate).
Acrylic acid was also evaluated for mutagenicity in the
Salmonella/microsome preincubation assay using the standard protocol
approved by the National Toxicology Program. Acrylic acid was tested
at doses of 0, 10, 33, 100, 333, 1000 and 3333 µg/plate in four
Salmonella typhimurium strains (TA98, TA100, TA1535 and TA1537) in
the presence and absence of Aroclor-1254-induced rat or hamster liver
S9. Acrylic acid was negative in these tests and the highest non-toxic
dose level tested in any Salmonella test strain was 1000 µg/plate. The
3333 µg/plate dose level was toxic and caused a complete clearing of
the background lawn (Zeiger et al., 1987).
In another study (Cameron et al., 1991) acrylic acid was also
found to be negative in the Salmonella assay, both in the presence and
the absence of both Aroclor-1254-induced rat or hamster liver S9 mix.
Three mammalian gene mutation studies have been reported. No
increase in mutation frequency was seen in a CHO/HPRT gene mutation
assay with or without Aroclor 1254-induced rat liver S9 (McCarthy et
al., 1992). The upper dose levels in this single experiment resulted
in a reasonable amount of toxicity (survival reduced by up to 65-76%).
In two mouse lymphoma L5178Y TK+/- studies positive results have been
obtained. A concentration-related increase in mutant frequency was
observed with and without rat liver S9 in association with an
acceptable level of toxicity (Cameron et al., 1991). The authors did
not state if there was any adjustment of pH. In the other study,
conducted without exogenous metabolic activation, there were
concentration-related increases in mutant frequency in two separate
experiments in the presence of marked but not excessive toxicity.
Small colonies predominated, which suggested a clastogenic effect;
this was confirmed by chromosome analysis. Acrylic acid was tested at
concentrations of 300, 450 and 500 µg/ml in this study but the authors
did not state if there was any adjustment of pH (Moore et al., 1988).
A single experiment was conducted with CHO cells exposed to
acrylic acid solutions adjusted to pH 7 at concentrations that reduced
cloning efficiency by up to 58-65% (McCarthy et al., 1992). A
concentration-related increase in the percentage of cells with
chromosome aberrations, primarily chromatid breaks and exchanges, was
observed in the presence and absence of rat liver S9. A positive
result has also been briefly reported for this in vitro chromosome
aberration assay using CHL cells in the absence of exogenous metabolic
activation (Ishidate, 1988).
The effect of acrylic acid on unscheduled DNA synthesis (UDS) in
rat hepatocytes has been investigated in one unreplicated assay
(McCarthy et al., 1992). There was no increase in UDS at
concentrations up to those closely approaching a very toxic level.
Similar dose levels of acrylic acid in a single UDS experiment with
SHE cells gave a negative result (Wiegand et al., 1989).
Negative results have also been obtained in a micronucleus test
and a transformation test, both with SHE cells, as well as in the
Drosophila sex-linked recessive lethal assay (Wiegand et al., 1989;
McCarthy et al., 1992).
In a study by Segal et al. (1987), it was reported that acrylic
acid forms 2-carboxyethyl adducts with adenine, guanine and thymine
following in vitro reaction with calf thymus DNA. These adducts are
identical to those formed with the same bases following in vitro
reaction of carcinogen ß-propionolactone. The relevance of the results
of this study is questionable because appropriate control treatments
were not conducted and the 2-carboxyethyl adducts were formed after
long treatment period.
In contrast to the results above, Frederick & Reynolds (1989)
found that incubation of the negatively charged acrylate anion with
two representative nucleophiles, methylamine and imidazole, did not
result in the formation of adducts of the acrylate ion to the
nucleophile.
It is suggested that binding of acrylic acid to cellular
nucleophiles might be due to small amounts of the unionized acid in
the equilibrium between acrylate anion and acrylic acid at cellular
pH. However, this event is considered to be insignificant in vivo,
based upon the rapid metabolism and excretion of acrylic acid
(Frederick & Reynolds, 1989).
7.6.2 In vivo mammalian studies
Preliminary results indicated no DNA adducts in the stomach and
liver of rats after oral dosing (Sagelsdorf et al., 1988). Although
DNA adducts were found in the skin of mice after dermal application,
the investigators concluded that further work was needed to confirm
the significance of these findings. No firm conclusions can therefore
be drawn from these preliminary results.
No increase in the incidence of chromosomal aberrations was
observed in the bone marrow of rats following acute or repeated
exposure to acrylic acid (McCarthy et al., 1992). Rats either received
a single gavage dose of up to 1000 mg/kg acrylic acid or were exposed
to up to 5000 mg/litre in the drinking-water for 5 days. There was no
effect on the mitotic index of the bone marrow but there was evidence
of systemic toxicity.
Negative results were reported in a dominant lethal assay with
mice following acute or repeated exposure to acrylic acid (McCarthy et
al., 1992). Male CD-1 mice received either a single gavage dose of up
to 324 mg/kg or daily gavage doses of up to 162 mg/kg for 5 days. The
fact that the dose levels were selected not to exceed the LD1 for
acute dosing and 0.5 LD1 for repeat dosing limits the value of this
study.
7.7 Carcinogenicity
In a carcinogenicity study (Hellwig et al., 1993), Wistar rats
(50/group/sex) were given acrylic acid in the drinking-water at
concentrations of 0, 120, 400 or 1200 mg/litre (0, 8, 27, or
78 mg/kg body weight per day, respectively) over 26 (males) or 28
(females) months. The highest concentration was selected because of
evidence of palatability problems at 2000 and 5000 mg/litre in an
earlier chronic drinking-water study. At 1200 mg/litre there was some
evidence for slightly reduced water consumption. In comparison with
the controls, mortality was not increased by administering acrylic
acid. No clear toxic effects were revealed in the groups. The few
statistically significant differences in some haematological
parameters between groups were considered to be of an incidental
nature. The extensive histopathological examination revealed no clear
treatment-related non-neoplastic tissue changes. The incidence and
organ distribution of the tumours found in the groups treated with
acrylic acid for 26/28 months did not differ from those of the
controls. In this study the NOEL was 78 mg/kg body weight per day.
It has been reported that when 25 µl of a 1% solution
(i.e. 0.25 mg) of acrylic acid was applied topically in acetone
on the dorsal skin three times a week for their lifetime to 40
male C3H/HeJ mice, no malignancies were observed at the site of
application (De Pass et al., 1984).
When 1 mg (14 µmol) of acrylic acid was applied topically in
acetone 3 times a week to 30 female ICR/HA (currently designated
Hsd:(ICR)Br) mice for 1.5 years, squamous cell carcinomas were
observed in two of the mice (Cote et al., 1986). No conclusion can be
drawn from this study, because only an abstract was issued, it was not
subsequently published in full and an independent review of this study
(Sivak, 1987) uncovered many inconsistencies and hence questioned the
validity of the findings.
Acrylic acid was assayed for carcinogenic activity in female
Hsd:(ICR)bR mice by subcutaneous injection of 20 µmol (approx 1.4 mg)
in 0.05 µl trioctanoin, once a week for 52 weeks. The mice were then
observed for an additional 93 days (total 450 days) when the survivors
were killed. Two mice with sarcomas at the site of application were
observed out of 30 mice (Segal et al., 1987). This would be an
expected finding as an irritant solution was repeatedly given by
subcutaneous injection. Injections for this length of time frequently
result in sarcomas, no matter what the compound, because of repeated
insult.
7.8 Other studies
In contrast to some of its esters, acrylic acid did not
significantly decrease non-protein sulfhydryl content (NPSH) in the
liver, blood or forestomach after oral dosing of rats with up to
400 mg/kg body weight (as 8% acrylic acid solution in 0.5% methyl-
cellulose solution). However, a significant depletion of NPSH was
observed in the glandular stomach at doses above 4 mg/kg (as 0.8%
acrylic acid solution) (Miller et al., 1981b; De Bethizy et al., 1987).
Acrylic acid was found to be ineffective in inducing GSH
depletion, lipid peroxidation and haemolysis (Ferrali et al., 1989).
It was reported that methylethyl- n-butyl and 2-ethyl-hexyl
acrylates, when inhaled by male Wistar rats, induced hyperglycaemia
but that acrylic acid was without effect (Vodicka et al., 1990).
Rats received neutralized acrylic acid (50 mg/kg) intra-
peritoneally for 8 consecutive days and were observed for central and
peripheral nervous system effects. They showed a 25 % decrease in body
weight gain but no signs of neurotoxicity (Kohriyama et al., 1994).
In an in vitro study, acrylic acid was more potent than
acrylamide as an inhibitor of creatinine kenase (CK) activity in rat
brain homogenates. However, when the two chemicals were given
intraperitoneally 50 mg/kg per day for 8 days to rats, only acrylamide
inhibited CK activity in the brain. The 14C level in the brain, 24 h
after the injection of 14C-labelled chemicals, was more than seven
times greater with acrylamide then with acrylic acid (Kohriyama et
al., 1994).
Single intraperitoneal injections of up to 2.2 mg/kg body weight
of acrylic acid to Wistar rats had no effect on the hepatic activity
of ornithine decarboxylase, which was measured for tumour-promoting
activity (van de Zande et al., 1986).
Laparotomies were carried out on pregnant Sprague-Dawley rats on
day 13 of pregnancy under anaesthesia, and half of the developing
fetuses were injected with 10, 100 or 1000 µg acrylic acid per fetus.
One fetus injected with 100 µg acrylic acid showed slight
hydrocephalus and microcephalia. A dose of 1000 µg resulted in 78%
resorption (Slott & Hales, 1985).
It was reported that acrylic acid interferes with incorporation
of thymidine into DNA and uracil into RNA in Staphylococcus aureus
and Escherichia coli (Glombitza & Heyser, 1971).
7.9 Factors modifying toxicity
No studies on factors modifying the toxicity of acrylic acid have
been reported.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Acute toxicity
8.1.1.1 Poisoning accidents
There have been no reports on poisoning accidents in the general
population or on the short- or long-term effects of acrylic acid on
the general population.
8.2 Occupational exposure
8.2.1 Poisoning accidents
There have been no reports on poisoning accidents due to
occupational exposure.
8.2.2 Effects of short- and long-term exposure
Fowler (1990) described the case of a male chemical worker who
developed acute generalized urticaria after working with acrylic acid
and acrylate compounds. Immediate hypersensitivity testing showed a
severe local reaction to 2% acrylic acid (which was apparently not
irritant), but no reaction to other acrylate compounds. The acrylic
acid used for testing was thought to be pure but it was not analysed
for the presence of alpha,ß-diacryloxypropionic acid. Re-exposure of
the worker in the workplace to acrylic acid resulted in generalized
urticaria.
No cross-sensitization to 0.1% acrylic acid was observed in six
patients who had exhibited hypersensitivity to acrylate-based sealants
(Conde-Salazar et al., 1988).
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
9.1 Microorganisms
The toxic threshold (EC3) level for inhibition of growth of the
bacterium, Pseudomonas putida was 41 mg/litre after exposure for
16 h to a neutralized solution of acrylic acid (Bringmann & Kühn,
1980). Stewart et al. (1995) studied the anaerobic degradation of
acrylic acid in 55-day tests in glucose-acetate enrichment culture. At
100 mg/litre of acrylic acid there was minimal effect on acetate
utilization by methanogens; concentrations of 500, 1000 and
1500 mg/litre inhibited acetate utilization for a period of time
before recovery.
In a study of the effect of acrylic acid on the soil carbon
cycle, it was shown that acrylic acid in soil (sandy loam soil), at
levels of up to 100 mg/kg soil, had no effect on the respiration of
the soil microflora. However, a concentration of 1000 mg/kg acrylic
acid completely suppressed respiration (Hossack et al., 1992).
9.2 Aquatic organisms
The toxicity of acrylic acid to aquatic organisms is summarized
in Table 7. Algae appear to be the most sensitive group with EC50
values, based on growth, ranging from 0.04 to 63 mg/litre; and a NOEC
for the most sensitive species of 0.008 mg/litre. Other acute toxicity
studies range from 27 mg/litre (96-h LC50) for the rainbow trout to
315 mg/litre (72-h LC50) for the golden orfe. A 96-h NOEC for glacial
acrylic acid toxicity to rainbow trout was found to be 6.3 mg/litre
based on a lack of sublethal/behavioural responses, e.g., quiescence,
fish on bottom of test vessel, loss of equilibrium and erratic
swimming, at this concentration (Bowman, 1990). Neutralized acrylic
acid was found to be less toxic to daphnids than the non-neutralized
solution (Bringmann & Kühn, 1982).
9.3 Terrestrial organisms
No data on terrestrial organisms have been reported.
Table 7. Toxicity of acrylic acid to aquatic organisms
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Blue-green CMIT Exposure duration 8 days; TT (EC3) 0.15 the toxic threshold Bringmann &
alga temp. 27°C; neutralized with extinction Kühn, 1978
Microcystis pollutant solution; static values > 3% lower
aeruginosa closed flasks shaken once than for controls
a day; the
concentration of the
algae suspension measured
turbidimetrically at 578 nm
Green alga CMIT Exposure duration 7 days; TT (EC3) 18 as above Bringmann &
Scenedesmus temp. 27°C; neutralized Kühn, 1980
quadricauda pollutant solution; static
closed flasks shaken once a
day; the concentration of
the algal suspension measured
turbidi-metrically at 578 nm
Green alga CMIT 96-h static algal assay; EC50 0.17 50% algal Forbis, 1989
Selenastrum acrylic acid exposure growth
capricornutum concentrations ranged from inhibition
(Printz) 0.15 to 1.9 mg/litre
Green alga algal Exposure duration EC50 0.04 50% algal BASF, 1994b
Scenedesmus inhibition 72 h biomass
subspicatus test inhibition
EC50 0.13 50% algal
growth rate
LOEC 0.016 inhibition
NOEC 0.008
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Green alga algal Exposure duration EC50 1.53 50% algal SNF, 1995
Chlorella inhibition 72 h; nominal acrylic biomass
vulgaris test (OECD acid exposure inhibition
No. 201) concentrations
ranged from 0.2 to EC50 63.0 50% algal
4.7 mg/litre for growth rate
biomass test and were inhibition
50 and 100 mg/litre
for growth rate
inhibition test
Protozoan CMIT Exposure duration TT (EC5) 20 the toxic Bringmann &
Entosiphon 72 h; temp. 25°C; threshold with Kühn, 1982
sulcatum pollutant solution extinction
(Stein) adjusted to pH 6.9; values > 5%
static closed flasks; lower than for
the number of controls
protozoans was
determined by
means of cell counter
Water flea Daphnia Exposure duration EC50 765 after 24 h Bringmann &
Daphnia immobilization 24 h; temp. 20°C; 50% of exposed Kühn, 1982
magna test pH 7.8-8.2; organisms were
(Straus) performed in 50 ml immobilized in
beakers covered with neutralized test.
filter paper;
10 organisms/
20 ml test solution;
9 h illumination/
15 h dark
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
EC100 5000 100% immobilized
after 24 h in
neutralized test
Acrylic acid in
non-neutralized
test
EC50 54 in non-neutralized
test
EC100 91
Water flea Daphnia Dynamic test. Acrylic acid EC50 95 after 48 h 50% of Burgess, 1990
Daphnia magna immobilization exposure concentrations exposed organisms
test ranged from 7.9-110 were immobilized
mg/litre.
Exposure duration 48 h; NOEC 23 based on mortality
temp. 19-20°C; pH 6.7-7.7;
dissolved O2 7.9-8.0 mg/litre
Golden orfe L15-Fish test Exposure duration LC0 210 no lethality Juhnke &
Leuciscus idus German 48 h; temp. 19-21°C; pH 7-8; Luedemann,
melanotus standard dissolved O2 1978
method for the > 5 mg/litre; 10-fish LC50 315 death of 50%
contaminants per 10 litre test of exposed
of water, solution; continuous fish
waste water aeration
and sludge
LC100 420 death of all
exposed fish
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Rainbow trout A flow-through Exposure duration 96 h; LC50 27 death of 50% Bowman,
Oncorhynchus toxicity test measured glacial acrylic of exposed 1990
mykiss acid exposure NOEC 6.3 fish
concentrations ranged lack of
from 6.3 to 90 mg/litre; sublethal/
20 fish per group behavioural
responses
Common carp Exposure duration LC100 100 Nishiuchi,
Cyprinus 24 h 1975
carpio
a CMIT = Cell multiplication inhibition test
b TT = Toxicity threshold
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure of the general population
Very limited data are available on general population exposure.
Exposure of the general population to acrylic acid may occur by the
dermal route via contact with unreacted acrylic acid in household
goods such as water-based paints or detergents. Exposure is also
possible from inhalation due to evaporation of acrylic acid from
paint.
Exposure of the general population through drinking-water and via
ambient air might be assumed. Exposure through drinking-water is
possible from surface or groundwater contamination while inhalation
exposure could occur in the vicinity of industrial emissions. However,
there is no data on measured ambient air concentrations in populated
areas and no data are available on acrylic acid concentrations in
drinking-water. It was reported that acrylic acid might occur in
wastewater effluent from industrial facilities at concentrations not
exceeding 0.5 mg/litre. However, effluent levels of 2500 mg/litre were
reported at a methyl acrylate production facility in India. After
treatment acrylic acid concentration was below the limit of detention
(0.1 mg/litre) in wastewater from a production facility in Europe.
Food is an unlikely source of exposure to commercially derived
acrylic acid because it is not known to be used in applications that
involve direct contact with food.
10.1.2 Occupational exposure
The most important means of human exposure to acrylic acid is
occupational exposure via inhalation and skin contact. On the basis of
statistical estimates derived from a NIOSH survey conducted in
1972-1974, 28 600 workers were potentially exposed to acrylic acid in
the USA. More recently, NIOSH estimated that 96 500 workers were
potentially exposed in the USA.
One study conducted at a factory, where a number of chemicals
including acrylic acid and a variety of acrylates and methacrylates
were used, indicated that levels in air of acrylic acid and ethyl
acrylate varied from 0.03 to 168 mg/m3 (0.01 to 56 ppm). However, the
8-h TWA levels of most areas of the plant were well below the hygienic
standard of 30 mg/m3 (10 ppm) for acrylic acid recommended by OSHA
and ACGIH at the time of the study.
Exposures of workers to acrylic acid have been compiled from four
producing companies. Operators had a mean 8 h-exposure value of
0.48 mg/m3 (range of 0.03 to 3 mg/m3); during loading/unloading
the mean 8 h-exposure was 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3).
10.1.3 Toxic effects
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, moderate acute toxicity by the inhalation route and
moderate acute toxicity by the dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. Although a 1% solution has
been reported to irritate rabbit skin and eyes, no externally visible
irritation was seen in one mouse study following repeated dermal
application of 1%. Acrylic acid is also a strong irritant to the
respiratory tract.
Both positive and negative results have been obtained in skin
sensitization tests with acrylic acid, but it appears that the
positive findings may have been due to the impurity alpha,ß-
diacryloxypropionic acid.
10.1.3.1 Carcinogenic and mutagenic effects
Both positive and negative results have been obtained in
in vitro genotoxicity tests. Notably, acrylic acid was negative in
the Ames test and in vitro UDS assay, positive in a chromosome
aberration assay (with pH adjustment) and in the in vitro lymphoma
cell assay. Chromosome damage was also demonstrated in one lymphoma
assay. An in vivo bone marrow chromosome aberration assay was
negative. No firm conclusions can be drawn from an in vivo
DNA-binding study or from a dominant lethal assay. There are no
satisfactory in vivo genotoxicity data for sites of initial contact,
e.g., nasal epithelium or skin.
A single chronic cancer bioassay of acrylic acid, administered in
drinking-water, has been reported. Male and female Wistar rats were
given drinking-water containing 120, 400 or 1200 mg/litre (estimated
doses were 8, 27 and 78 mg/kg body weight per day), after preliminary
3- and 12-month studies showed reduced water consumption and body
weight gain in groups of rats receiving drinking-water containing 2000
or 5000 mg/litre. No systemic effects, including weight change or
histopathology, and no increase in tumour incidence resulted from
acrylic acid exposure. Although the lack of any toxic effects in this
study suggests that the maximum tolerated dose was not achieved, it
appears that the high dose in this study was approaching a maximum
tolerance dose (MTD), based on the mild body weight effects noted at
140 mg/kg per day in a 12-month study and at 240 mg/kg per day in a
two-generation reproductive study. Apart from not reaching the MTD,
the study used adequate number of animals (50), was well documented
and reported complete histopathology.
There has been no cancer bioassay conducted in a second species
by the oral route, nor any inhalation cancer bioassay. Available data
are inadequate to evaluate the carcinogenic potential of acrylic acid
via the dermal route.
There have been several chronic bioassays conducted on related
compounds that are relevant to evaluation of the potential
carcinogenicity of acrylic acid. Chronic inhalation studies have been
reported for methyl acrylate, butyl acrylate and ethyl acrylate. All
of these studies exposed rats to 0, 5, 25 or 75 ppm of the acrylates
for 2 years, which was followed by complete necropsy and histopa-
thological evaluation. The acrylate esters are rapidly metabolized to
acrylic acid and alcohols by nasal, blood and other tissue carboxyl-
esterases. In all of these studies a concentration-dependent increase
in nasal olfactory degeneration was observed, and the response was
very similar to the response observed in a subchronic inhalation study
of acrylic acid. There were no treatment-related increases in tumour
incidence in these studies. Although there may have been quantitative
differences in the tissue doses of acrylic acid, the acrylate ester
studies provide supportive evidence that carcinogenic effects do not
occur in tissues exposed chronically to acrylic acid.
Two cancer bioassays via oral exposure have been conducted with
ethyl acrylate, a gavage study in F-344 rats and B6C3F1 mice (NTP,
1986) and a drinking-water study in Wistar rats. The highest dose was
similar in the two studies and produced lesions of the forestomach.
However, the gavage study resulted in a dose-dependent increase in
tumour incidence in both sexes of rats and mice while the drinking-
water study did not. The gavage study suggests a potential for
carcinogenic effects for ethyl acrylate, and, by extension, for the
other acrylate esters and acrylic acid. The greater potency for this
effect in a gavage study may relate to the contact site toxicity of
these compounds. Gavage may not always be an appropriate exposure
method for direct irritants because of the extremely high local doses
that are achieved, compared to the local doses in a drinking-water
study.
A similar result can be seen in 3-month gavage and drinking-water
studies. The drinking-water study caused mildly reduced body weight
gain compared to controls, while the gavage study, at very similar
doses, resulted in lethality in most of the dosed animals. These
results suggest that the gavage route of exposure should play little
role in the risk assessment of acrylic acid because human exposures
are not likely to result in repeated high-concentration doses.
Current practice requires strong evidence to conclude that a
chemical is not a carcinogenic hazard, usually including adequate
chronic bioassays in two species. A second species chronic bioassay is
not available for acrylic acid, and the acrylate ester inhalation and
drinking-water bioassays described above were carried out on rats. The
existing acrylic acid bioassay is adequate and demonstrated no cancer
effects. No epidemiological evidence regarding potential
carcinogenicity of acrylic acid is available. Based on the available
data, one cannot definitively conclude that acrylic acid does not pose
a carcinogenic hazard to humans, although substantial relevant data do
not suggest a cancer hazard.
10.1.3.2 Non-cancer effects
a) Route-specific effects
Both oral and inhalation exposures show effects at the site of
contact. Inhalation exposures resulted in nasal effects in studies of
2 weeks to 3 months in duration and at various exposure
concentrations. High concentration drinking-water exposure to acrylic
acid resulted in forestomach lesions. No other specific organ
pathology has been consistently demonstrated in either oral or
inhalation studies. Metabolic studies show rapid metabolism and
clearance of acrylic acid that is similar for several routes of
exposure, and there is no evidence of bioaccumulation of this
chemical. The available evidence supports the conclusion that systemic
toxicity is not likely to occur, and that the effects of oral and
inhalation exposure can be treated as independent events with no
additivity of doses received by the different routes of exposure. For
this reason, guidance values will be derived separately for oral and
inhalation routes.
b) Developmental and reproductive effects
The toxicity data base for acrylic acid includes inhalation
developmental studies in rats and rabbits and oral reproductive
studies in rats. No specific reproductive or developmental effects
were observed in any of these studies. The only effects observed
included nasal effects in the inhalation studies and stomach lesions
and reduced body weight gain in the drinking-water studies. Based on
these results and the evidence for specific site-of-contact effects
described above, the potential hazards for developmental and
reproductive effects have been adequately studied and are not critical
to the human health risk assessment.
c) Oral gavage studies
As described above (section 10.1.3.1) the 3-month gavage and
drinking-water studies showed important differences in toxicity,
depending on the method of dosing. The drinking-water study revealed
reduced body weight gain compared to controls, while the gavage study,
at very similar daily doses, showed lethality in most of the dosed
animals. Therefore, the gavage route of exposure should be of little
importance in the risk assessment of acrylic acid because human
exposures are not likely to result in repeated high-concentration oral
doses.
d) Critical oral studies
Potential critical studies include a 3-month drinking-water study
in rats, 3-, 12- and 26- to 28-month drinking-water studies in rats,
and a two-generation reproductive study in rats. Table 8 summarizes
the effects seen in these studies and the determination of which
effects are considered adverse.
Table 8. Summary of adverse effect levels in drinking-water studies on ratsa
LOAEL NOAEL Effectb Reference
750 250 Body weight; 81 and 84% of controls in males and females, DePass et al., 1983
respectively, at 750; 95% of C in females at 250
none 331 Body weight: Hellwig et al., 1993
males: 93% of C at 331; (3-month study)
females: no effect
331 140 Body weight: Hellwig et al., 1993
males: 91% of C at 331, 94% of C at 140; (12-month study)
females; no effect
none 78 no effects Hellwig et al., 1993
(26- to 28-month studies)
460 240 460: stomach irritation, body weight: 91% of C in F0 males, BASF, 1993
65% of C in F1 pups, 85% of C in F1 (both sexes), 68% of C
in F2 pups
240: body weight: 89% of C in F1 pups, 88% of C in F2 pups
a concentrations are given in mg/kg body weight per day
b C = control group; F0 = first parental generation; F1 = second parental generation
A consistent finding throughout the drinking-water studies was
decreased water consumption, probably due to taste aversion and
sometimes accompanied by decreased food consumption. Reductions in
body weight appear to parallel decreased water consumption and were
worse in the pups in the reproductive study. The water consumption
effect makes the body weight changes difficult to interpret as a
direct toxic effect of acrylic acid, but the possibility that the
effect was at least partly a direct result of acrylic-acid-induced
irritation cannot be ruled out. The body weight effects are therefore
considered to be adverse when the magnitude of the effect approaches a
10% reduction. A somewhat larger change in the pup weight would be
required to be considered adverse, because the largest weight
decrements were seen at the end of active nursing and the beginning of
weaning; they may be more severely affected by the reduced water
consumption because of the greater maternal need for water intake
during this time and the unknown effects of taste aversion on weaning
behaviour. Based on these considerations and the presence of stomach
histopathology at 460 mg/kg body weight per day, the NOAEL in the two-
generation reproduction study was 240 mg/kg body weight per day. The
NOAEL based on body weight changes in the 12-month studies was
140 mg/kg body weight per day.
e) Critical inhalation studies
In a subchronic study, which is the only study that is
potentially a critical study for inhalation, lesions in the nasal
epithelium were seen in rats at 225 mg/m3 (75 ppm) and in mice at
15 mg/m3 (5 ppm). The lesion in mice was described as very slight at
15 mg/m3 (5 ppm), slight at 75 mg/m3 (25 ppm), and slight to
moderate at 225 mg/m3 (75 ppm). Despite the mild nature of the
response at 15 mg/m3 (5 ppm), there was a clear increase in incidence
and severity of this lesion with increasing exposure concentration, so
it must be considered adverse.
f) Progression of lesions
There is suggestive evidence from both inhalation and oral routes
of exposure that the effects caused by acrylic acid are largely
determined by the exposure concentration and are relatively less
affected by the duration of exposure in repeated exposure studies.
In the oral studies, even if different LOAEL and NOAEL values
could be defined by the 3-month and 12-month oral drinking studies,
the effects on weight are similar for the two exposure durations. In
inhalation studies in mice, the severity of the lesions in animals
exposed for 6 h/day to 75 mg/m3 (25 ppm) was very similar in the
2-week and the 90-day studies, although limited description of the
lesions in the 2-week study makes this conclusion tenuous. Additional
2-week studies included groups exposed to 15 and 75 mg/m3 (5 and
25 ppm) for 6 h/day. The 75 mg/m3 (25 ppm) group showed effects similar
to those seen in the subchronic study, suggesting limited progression
over this time-frame. The 15 mg/m3 (5 ppm) group exposed for 6 h/day
showed no effect after 2 weeks, so slight progression does occur at
low concentrations, given that lesions were observed in the 90-day
study. The 2-week study also included recovery groups, which showed
that the lesions were completely reversed in animals exposed to
15 mg/m3 (5 ppm) for 22 h and 75 mg/m3 (25 ppm) for 6 h, but
not in animals exposed to 75 mg/m3 (25 ppm) for 22 h/day.
Comparison of the subchronic and chronic inhalation studies of
the acrylate esters also supports the conclusion that there is limited
progression of nasal lesions. Some progression of lesions does occur,
but much less than would be predicted by an assumption of a constant
concentration × time relationship.
10.1.4 Risk evaluation
10.1.4.1 Inhalation exposure
The LOAEL of 15 mg/m3 (5 ppm) from the subchronic study is used
as the basis for the guidance value. The appropriate uncertainty
factors (UF) include 5 for inter-individual differences and 10 as a
composite UF for interspecies, LOAEL to NOAEL and subchronic to
chronic extrapolations. Reduced uncertainty for these aspects of the
guidance value (GV) for inhalation results from the information
discussed previously in this monograph. The inter-individual
uncertainty is reduced because of the direct-acting nature of the
toxicity, which does not involve metabolism. The uncertainty in
extrapolating from a LOAEL to a NOAEL is reduced because the lesion at
the LOAEL is very mild and reversible. The uncertainty in
extrapolating from a subchronic to a chronic exposure is reduced
because of the evidence discussed above showing limited progression.
The interspecies uncertainty is reduced because of the direct-acting
nature of the toxicity of acrylic acid and because the mouse is
apparently very sensitive. The toxicity of acrylic acid is clearly
limited to the site of deposition and is not dependent on metabolic
activation. Since the deposition is controlled by physical
interactions, there is little reason to expect significantly greater
deposition in humans.
Based on these considerations, the GVair for inhalation exposure
of the general population may be calculated as follows:
GVair = 15mg/m3 × 6 × 5 × 1 = 54 µg/m3
24 5 50
where:
15 mg/m3 = LOAEL for mice
6/24 and 5/7 = duration of exposure in h/day and weeks/day,
respectively
50 = total uncertainty factor
10.1.4.2 Oral exposure
For oral exposure the GV can be calculated based on NOAEL values
in the 12-month oral study (140 mg/kg body weight per day), the two-
generation reproductive study (240 mg/kg body weight per day) or the
NOAEL in the chronic study (78 mg/kg body weight day). The chronic
study is used to derive the GV because a lifetime exposure study is
preferred to a short-duration study. The chronic study found no effect
at the highest dose tested. The NOAEL is supported by the finding of
adverse effects on body weight at 331 mg/kg body weight per day in the
12-month study and body weight effects and stomach pathology in the
two-generation reproductive study at 460 mg/kg body weight per day.
With these supporting studies, the NOAEL from the chronic study can be
used with confidence. A total uncertainty factor of 25 is based on a
factor of 5 for inter-individual variability, and a factor of 5 for
interspecies variability and for the lack of an adequate oral study in
a second species. Reduced uncertainty factors are used for
interspecies and inter-individual differences because of the direct-
acting nature of the toxicity, as described in section 10.1.4.1. The
need for a second species is suggested by the apparent differences in
sensitivity between rats and mice in the subchronic inhalation study.
Based on these considerations, the GVoral for drinking-water
exposure may be calculated as follows:
Tolerance intake = 78 = 3.1 mg/kg
25
From the above calculation a guidance value for oral intake of
acrylic acid may be derived according to the following formula:
GVoral = 3.1 × 64 ‰ 2 = 99 mg/litre
where:
3.1 mg/kg body weight per day = tolerable intake (TI)
64 = average weight (kg) of human body
25 = uncertainty factor
2 = average intake of drinking water (litres/day)
However, the Task Group expressed significant concern regarding
the guidance value for oral intake calculated above, because of the
difference in toxicity between drinking-water and gavage exposures.
Exposure levels only 50-fold above the GV calculated above were lethal
to 50% of the animals in the 90-day gavage study, and comparison with
effects in the 90-day drinking-water study revealed a very significant
difference between the two dosing methods. High doses taken over a
short time period have much more severe effects.
The concern is reduced by the fact that the local effects in the
stomach might be determined by both concentration of delivered dose
and total dose, so the same total dose delivered in more dilute
solutions results in lesser effects, as shown for ethyl acrylate. The
concentration in drinking-water needed to deliver the TI dose is
probably far less than that required to cause direct effects, and is
not a public health concern if small amounts are ingested over the
course of the day, approximating to continuous exposure. The
possibility remains that a large part of the TI could be delivered
over a short time via drinking-water.
Because of potential differences in individual drinking
behaviour, the Task Group decided that a risk of effects still exists
at the TI calculated above. For this reason an additional uncertainty
factor of 10 was applied to derive the GV for drinking-water.
Thus, the final guidance value for oral intake of acrylic acid
is:
GVoral = 99 mg/litre = 9.9 mg/litre
10
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Acrylic acid has been found to occur naturally in some species of
marine alga. No quantitative data are available regarding
environmental levels in ambient air or soil. It was reported that
acrylic acid might occur in wastewater effluent from industrial
facilities at concentrations not exceeding 0.5 mg/litre. Effluent
levels of (2500 mg/litre) have been reported at a methyl acrylate
production facility in India. After treatment acrylic acid was below
the limit of detection (0.1 mg/litre) in wastewater from a production
facility in Europe.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Acrylic acid is
eliminated from the environment by both abiotic and biotic
degradation. Photolysis reactions are possible processes, but aerobic
degradation constitutes the major route of breakdown. Microorganisms
are capable of degrading acrylic acid under aerobic and anaerobic
conditions.
In soil, acrylic acid biodegrades very rapidly, the half-life
being less than one day. There is no potential for long-range
atmospheric transport of acrylic acid since it has an atmospheric
lifetime of less than one month.
Bioaccumulation of acrylic acid in organisms, based on the low
octanol-water partition coefficient, is likely to be negligible. There
has been no report of biomagnification of acrylic acid in food chains.
10.2.2 Effects
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values based on growth ranging from 0.04 to 63 mg/litre and
a NOEC for the most sensitive species of 0.008 mg/litre. Acrylic acid
has low to moderate acute toxicity for invertebrates and fish with
LC50 values ranging from 27 to 315 mg/litre. The 96-h NOEC was
6.3 mg/litre for rainbow trout, based on a lack of sublethal/
behavioural responses.
No data are available concerning the effects of acrylic acid on
terrestrial organisms in the environment.
10.2.3 Risk evaluation
If released into the environment acrylic acid will partition to
water where it will be readily degraded. Therefore, it is unlikely to
pose a problem in the general environment. In the case of spills,
acrylic acid is likely to cause localized mortality to aquatic
organisms both from direct toxicity and oxygen depletion. There is
likely to be a problem near to outfalls from plants discharging
acrylic acid if there is inadequate sewage treatment. The toxicity of
acrylic acid to bacteria and soil microorganisms is low. No data are
available concerning the effects of acrylic acid on terrestrial
organisms in the environment; however, data from laboratory mammals
suggest a low risk.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure for the general
population. However, no obvious adverse effects on the general
population have been identified.
Acrylic acid poses minimal risk for the general environment
except in the case of uncontrolled discharge.
11.2 Recommendations for protection of human health
It is recommended that exposure of the general population to
acrylic acid in the ambient air and drinking-water does not exceed the
guidance values given in this monograph.
12. FUTURE RESEARCH
a) Monitoring of acrylic acid concentrations in ambient air, water,
soil and effluent should be carried out. Research should be
undertaken to lower the analytical detection limit in water.
b) Further in vivo studies on the genotoxic potential of acrylic
acid at the initial site of contact are recommended.
c) The carcinogenicity of acrylic acid should be assessed in a well-
controlled two-species bioassay (with both sexes) using proper
exposure levels via inhalation or dermal exposure.
d) Better understanding of the relationship between persistent
tissue damage and tumour formation would aid the risk assessment
for an irritant chemical such as acrylic acid.
e) Additional research on the development of models describing the
disposition and kinetics of acrylic acid is needed. Such models
should focus on species differences in order to allow more
confident extrapolation from animals to humans. In addition,
models allowing comparison of acrylic acid dose for various
acrylate esters would be useful to allow complete use of
available data.
f) Since many workers are exposed to acrylic acid, epidemiological
studies are needed, which should concentrate on the following
end-points:
i) rhinitis, laryngitis and olfactory function;
ii) respiratory tract disease;
iii) skin disease.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer (IARC, 1979, 1987). Data
on the carcinogenicity of the compound for humans were considered
inadequate. There was inadequate evidence for carcinogenicity in
animals. Therefore, IARC considered acrylic acid not classifiable as
to its carcinogenicity to humans.
REFERENCES
AAR (1987) Emergency handling of hazardous materials in surface
transportation. Washington, DC, Association of America Railroads,
Hazardous Systems (BOE), 11 pp.
ACGIH (1988) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1988-1989.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
ACGIH (1990) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1990-1991.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists, p 9.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Andreoni V, Bernasconi S, Sorlini C, & Villa M (1990) Microbial
degradation of acrylic acid. Ann Microbiol, 40: 279-287.
Archer G & Horvath MK (1991) Adsorption and desorption of acrylic acid
to soils. Painesville, Ohio, Ricerca Inc., Department of Environmental
Sciences (Document No. 3193-88-0214-EF-001).
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reactions of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
Atkinson R & Carter WPL (1984) Kinetics and mechanisms of the gas-
phase reactions of ozone with organic compounds under atmospheric
conditions. Chem Rev, 84: 437-470.
Bandara BMR, Gunatilaka AAL, Kumar NS, & Wimalasiri WR (1988)
Antimicrobial activity of some marine algae of Sri Lanka. J Natl Sci
Counc (Sri Lanka), 16(2): 209-221.
BASF (1958) [Report on biological tests for crude and pure acrylic
acid.] Ludwigshafen, Germany, BASF AG, Toxicology Department
(Unpublished report No. VII/365-366) (in German).
BASF (1988) [Determination of the distribution coefficient log Pow of
acrylic acid in 1-octanol/water at room temperature (25°C).]
Ludwigshafen, Germany, BASF AG, Analytical Laboratory, pp 1-4 (in
German).
BASF (1992) Acrylic acid glacial P. Technical information monomers.
Ludwigshafen, Germany, BASF AG.
BASF (1993) [Examination of the biological degradability or the
elimination potential, respectively, of acrylic acid (pure P)
according to the experimental design of Zahn-Wellens (DUU/OM Project
No. 92/2641/10/1).] Ludwigshafen, Germany, BASF AG (in German).
BASF (1994a) Continuous administration in the drinking-water over 2
generations (1 liter in the first and 1 liter in the second
generation) (Project No. 71RO114/92011). Ludwigshafen, Germany, BASF
AG.
BASF (1994b) [Determination of the inhibitory effect of acrylic acid
(pure) on the cell replication of the green algae Scenedesmus
supsicatus.] Ludwingshafen, Germany, BASF AG (Unpublished
experiment No. 94/0840/60/1) (in German).
BG Chemie (1991) Acrylic acid. In: Toxicological evaluations, Volume
2. Berlin, Springer-Verlag, pp 39-73.
Black KA (1993) 14C-Acrylic acid. Comparative bioavailability study
in male mice and rats -analysis of tissues. Spring House,
Pennsylvania, Rohm and Haas Company, Toxicology Department (Report
No. 93R-200).
Black KA, Finch L, & Frederick CB (1993) Metabolism of acrylic acid to
carbon dioxide in mouse tissues. Fundam Appl Toxicol, 21: 97-104.
Black KA, Beskitt JL, Tallant MJ, & Frantz SW (1994) Disposition of
acrylic acid (AA) in C3H mice after the oral, cutaneous and
intravenous routes (Rohm and Haas Company, Spring House, PA and Bushy
Run Research Center, Export, PA). Toxicologist, 14(1): 430 (Abstract
1705).
Black KA, Beskitt JL, Finch L, Tallant MJ, Udinsky JR, & Frantz ST
(1995) Disposition and metabolism of acrylic acid in C3H mice and
Fischer 344 rats after oral or cutaneous administration. J Toxicol
Environ Health, 45: 291-311.
Borzelleca JF, Larson PS, Hennigar GR, Huf EG, Crawford EM, & Smith RB
(1964) Ethyl acrylate and methyl methacrylate: Studies on the chronic
oral toxicity of monomeric ethyl acrylate and methyl methacrylate.
Toxicol Appl Pharmacol, 6: 29-36.
Bowen RL (1979) Adhesive bonding of various materials to hard tooth
tissues. XVIII: Synthesis of a polyfunctional surface-active
comonomer. J Dent Res, 58: 1101-1107.
Bowman JE (1990) Acute flow-through toxicity of glacial acrylic acid
to rainbow trout ( Salmo gairdneri). Columbia, Missouri, Analytical
Bio-Chemistry Laboratories Inc., Aquatic Toxicology Division (Report
No. 37343).
Bretherick L (1985) Handbook of reactive chemical hazards, 3rd ed.
Boston, Massachusetts, Butterworth, 351 pp.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: model organisms Microcytis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Z Wasser-Abwasser-Forsch, 15: 1-6 (in
German).
Brown RK, McMeekin TA, & Balis C (1977) Effect of some unicellular
algae on Escherichia coli populations in sea water and oysters. J
Appl Bacteriol, 43: 129-136.
Buckley LA, James RA, & Barrow CS (1984) Differences in nasal cavity
toxicity between rats and mice exposed to acrylic vapor. Toxicologist,
4(1): 1 (Abstract only).
Burgess D (1990) Acute flow-through toxicity of acrylic acid to
Daphnia magna. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37344).
Bysshe SE (1990) Bioconcentration factor in aquatic organisms. In:
Lyman WJ, Reehle WF, & Rosenblatt DN ed. Handbook of chemical property
estimation methods for environmental behaviour of organic compounds.
Washington, DC, American Chemical Society, vol 15, pp 1-30.
Cameron TP, Rogers-Back AM, Lawlor TE, Harbel JW, Seifried HE, &
Dunkel VC (1991) Genotoxicity of multifunctional acrylates in the
Salmonella/mammalian-microsome assay and mouse lymphoma TK+/- assay.
Environ Mol Mutagen, 17: 264-271.
Carpenter CP, Weil CS, & Smyth HF Jr (1974) Range-finding toxicity
data: List VIII. Toxicol Appl Pharmacol, 28: 313-319.
Casciery T & Clary J (1993) Acrylic acid health effects overview. In:
Taylor TB, Murphy SR, & Hunt EK ed. Health effect assessments of the
basic acrylates. Boca Raton, Florida, CRC Press, p 13.
Chemical Marketing Reporter (1992) Acrylic grows at rapid pace, but so
does the world's capacity. Chem Mark Report, July 20.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation
of petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CHRIS (1985) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado).
CHRIS (1989) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado.
Chun JS, Kubena MF, & Neeper-Bradley TL (1993) Developmental toxicity
dose range-finding study of inhaled acrylic acid vapor in New Zealand
white rabbits. Export, Pennsylvania, Union Carbide Corporation (Bushy
Run Research Center Report No. 92N1007).
CITI (Japanese Chemicals Inspection and Testing Institute) ed. (1992)
Acrylic acid. In: Biodegradation and bioaccumulation: Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology and Information Center, pp 2-83.
Conde-Salazar L, Guimaraens D, & Romero LV (1988) Occupational
allergic contact dermatitis from anaerobic acrylic reactants. Contact
Dermatitis, 18: 129-132.
Corrigan MA & Scott R (1988) Acrylic acid: In vitro absorption
through human and mouse skin. Macclesfield, United Kingdom, Imperial
Chemical Industries PLC, Central Toxicology Laboratory (Report No.
CTL/P/2047).
Costellati L, Guglielmin AM, Vistoli O, Carboni GP, & Rambaldi R
(1990) [Allergic contact dermatitis - evaluation of patch tests in
animal fodder plant workers.] Med Lav, 81: 296-300 (in Italian).
Cote IL, Hochwalt A, Seidman I, Budzilovich G, Solomon JJ, & Segal A
(1986) Acrylic acid: Skin carcinogenesis in ICR/HA mice. Toxicologist,
6: 235.
Dean JA (1987) Handbook of organic chemistry. New York, McGraw-Hill
Book Co., pp 1-76.
De Bethizy ID, Udinsky JR, & Scribner HE (1987) The disposition and
metabolism of acrylic acid and ethyl acrylate in male Sprague-Dawley
rats. Fundam Appl Toxicol, 8(4) 549-561.
DePass LR, Woodside MD, Garman RH, & Weil CS (1983) Subchronic and
reproductive toxicology studies on acrylic acid in the drinking water
of the rat. Drug Chem Toxicol, 6(1): 1-20.
DePass LR, Fowler EH, Meckley DR, & Weil CS (1984) Dermal oncogenicity
bioassays of acrylic acid, ethyl acrylate and butyl acrylate. J
Toxicol Environ Health, 14: 115-120.
Douglas MT & Bell G (1992) Assessment of ready biodegradability of
acrylic acid (Closed bottle test). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 1/91329).
D'Souza RW & Francis WR (1988) Vehicle and pH effects on the dermal
penetration of acrylic acid: in vitro- in vivo correlation.
Toxicologist, 8: 831.
EEC (1988) Methods for the determination of ecotoxicity,
biodegradation. Activated sewage sludge respiration inhibition test.
In: Council Directive of 18 November 1987 adapting to technical
progress for the ninth time Directive 67/548/EEC on the approximation
of the laws, regulations and administrative provisions relating to the
classification, packaging and labelling of dangerous substances
(88/302/EEC). Off J Eur Comm, L133: 118-122.
Ekhina RS & Ampleeva GP (1977) [Combined effect of six acrylates on
the sanitary status of a reservoir.] Vodemov, 1977: 157-164 (in
Russian).
Elf Atochem (1992) Norsocryl AA, acrylic acid specifications. Paris,
Elf Atochem.
Fassett DW (1963) Organic acids, anhydrides, lactones, acid halides
and amides, thioacids. In: Patty FA ed. Industrial hygiene and
toxicology, 2nd ed. New York, Wiley-Interscience, vol 2, p 1794.
Fazzalari FA ed. (1978) Compilation of odor and taste threshold values
data. Philadelphia, Pennsylvania, American Society for Testing and
Materials, p 141.
Ferrali M, Ciccoli L, & Comporti M (1989) Allyl alcohol-induced
hemolysis and its relation to iron release and lipid peroxidation.
Biochem Pharmacol, 38(1): 1819-1826.
Finch L & Frederick CB (1992) Rate and route of oxidation of acrylic
acid to carbon dioxide in rat liver. Fundam Appl Toxicol, 19:
498-504.
Forbis AD (1989) Acute toxicity of acrylic acid to Selenastrum
capricornutum Printz. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc., Aquatic Toxicology Division (Report No. 37345).
Fowler JH Jr (1990) Immediate contact hypersensitivity to acrylic
acid. Dermatol Clin, 8: 193-195.
Frantz SW & Beskitt JL (1993) 14C-Acrylic acid: comparative
bioavailability study in male mice and rats. Export, Pennsylvania,
Bushy Run Research Center (Report No. 92 N 1005).
Frantz SW, Beskitt JL, Tallant MJ, & Black KA (1994) Disposition of
acrylic acid (AA) in Fischer rats after the oral, cutaneous and
intravenous routes. Toxicologist, 14(1): 430 (Abstract 1704).
Frederick CB & Reynolds CH (1989) Modeling the reactivity of acrylic
acid and acrylate anion with biological nucleophiles. Toxicol Lett,
47(3): 241-247.
Frederick CB, Udinsky JR, & Finch L (1994) The regional hydrolysis of
ethyl acrylate to acrylic acid in the rat nasal cavity. Toxicol Lett,
70: 49-56.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Ganou-Parfait B, Fahrasmane L, Celestine-Myrtil DA, Parfait A, & Galzy
P (1988) Micrococcus in rum technology in the French West Indies.
Microbiol Aliments Nutr, 6(3): 273-277.
Gelbke HP & Hofmann HTh (1979) [Report on testing for acute inhalation
risk of acrylic acid (pure) in Sprague-Dawley rats.] Ludwigshafen,
Germany, BASF AG, Department of Toxicology (in German).
Ghanayem BI, Maronpot RR, & Matthews HB (1985) Ethyl acrylate-induced
gastric toxicity: II. Structure-toxicity relationships and mechanism.
Toxicol Appl Pharmacol, 80: 336-344.
Ghanayem BI, Burka LT, & Matthews HB (1987) Ethyl acrylate
distribution, macromolecular binding, excretion and metabolism in male
Fischer 344 rats. Fundam Appl Toxicol, 9: 389-397.
Glombitza KW (1970a) [Antimicrobial contents of algae: 2. Presence of
acrylic acid in different sea algae.] Planta Med, 18(3): 210-221 (in
German).
Glombitza KW (1970b) [Antimicrobiol contents in algae: 3. Quantitative
determination of acrylic acid in sea algae.] Planta Med, 18(4):
231-234 (in German).
Glombitza KW (1979) Antibiotics from algae. In: Hoppe HA, Levring T, &
Tanaka J ed. Marine algae in pharmaceutical science. Berlin, New York,
Walter de Gruyter, pp 303-307.
Glombitza KW & Heyser R (1971) [Antimicrobial constituent compounds in
algae - Part 4: Effect of acrylic acid on respiration and synthesis of
macromolecular in Staphylococcus aureus and Escherichia coli.]
Helgoal Wiss Meeresunters, 22(3/4): 442-453 (in German).
Grudzinski UJ (1988) [Substantiation of single maximum permissible
levels of acrylic acid in the air of populated regions.] Gig I Sanit,
9: 64-65 (in Russian).
Hagan JV & Emmons HF (1991) Acrylic acid: Acute inhalation study in
rats (Protocol No. 87P-240). Spring House, Pennsylvania, Rohm and Haas
Company, Toxicology Department (Report No 87R-106).
Halarnkar PJ & Blomquist GJ (1989) Comparative aspects of propionate
metabolism. Comp Biochem Physiol, 92B: 227-231.
Hansch C & Leo A (1987) The log P database. Claremont, California,
Pomona College, p 391.
Hawkins DR, Kirkpatrick D, Aikens PJ, & Saxton JE (1992) The
metabolism of acrylic acid in soil under aerobic conditions.
Huntingdon, United Kingdom, Huntingdon Research Centre (HRC
Confidential Report No. 93A/920625).
Hellwig J, Deckerdt K, & Freisberg KO (1993) Subchronic and chronic
studies of the effects of oral administration acrylic acid to rats.
Food Chem Toxicol, 31: 1-18.
Herwig N (1978) Nutritious antibiotics. Mar Aquarist, 8(5): 48-50.
Heyser R & Glombitza KW (1972) Effect of acrylic acid on glycolysis
and citric acid cycle in bacteria: 5. Antimicrobiol components in
algae. Arch Pharm, 305(11): 863-870.
Hossack DJN, Thomas FJ, & Ruckman SM (1992) Acrylic acid: Effects on
soil carbon cycle (respiration). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 2/920228).
Howard PH, Boethlings RS, Jarris WF, Meylan WM, & Midalenko EM (1991)
Handbook on environmental degradation rates. Chelsea, Michigan, Lewis
Publishers.
Husain S, Sastry GSR, Prasad PR, & Sarma VR (1991) Differential pulse
polarographic determination of residual acrylic acid in sodium
polyacrylate. Electroanalysis, 3: 71-72.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein. Lyon, International Agency for Research on Cancer, pp 47-71
(IARC Monograph on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 19).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 56 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1992) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M (1988) Data book of chromosomal aberration test in vitro.
Amsterdam, Oxford, New York, Elsevier Science Publishers.
Juhnke I & Luedemann D (1978) [Results of testing of 200 chemical
compounds for acute fish toxicity using the Golden Orfe test.]
Z. Wasser Abwasser Forschung, 11(5): 61-164 (in German).
Kalimo K, Jolanki R, Estlander T, & Kanerva L (1989) Contact allergy
to antioxidants in industrial greases. Contact Dermatitis, 20:
151-152.
Kavaler AR (1987) Chemical profile on acrylic acid. Chem Mark Report,
232(23): 62.
Kirk-Othmer (1978-1984) Kirk-Othmer encyclopedia of chemical
technology, Volumes 1-26, 3rd ed. New York, John Wiley and Sons.
Klimisch HJ & Hellwig J (1991) The prenatal inhalation toxicity of
acrylic acid in rats. Fundam Appl Toxicol, 16(4): 656-666.
Klimisch HJ & Zeller H (1980) [Acute inhalation toxicity in the rat.]
Ludwigshafen/Rhine, Germany, BASF AG, Department of Toxicology (in
German).
Klimkina NV, Bolding ZN, & Sergeev AN (1969) [Experimental basis for
maximum permissible content of acrylic acid in reservoir waters.] Prom
Zag Vodoemov, 9: 171-185 (in Russian, with English abstract).
Kodama M & Ogata T (1983) Acrylic acid, as an antibacterial substance
in scallop. Bull Jpn Soc Sci Fish, 49(7): 1103-1107.
Kohriyama K, Matsuoka M, & Igisu H (1994) Effects of acrylamide and
acrylic acid on creatinine kinase activity in the rat brain. Arch
Toxicol, 68(1): 67-70.
Korenman IM & Lunicheva EV (1972) Distribution of acrylic acid between
organic solvents and water. J Appl Chem (USSR), 45: 1101-1105.
Kostanyan GG, Dadayan AA, & Safuryan GE (1969) [Polarographic
determination of acrylic acid in commercial propionic acid.] Arm Khem,
22: 1044 (in Russian with English abstract).
Kutzman RS, Meyer GI, & Wolf AP (1982) The biodistribution and
metabolic fate of (11C) acrylic acid in the rat after acute inhalation
exposure or stomach intubation. J Toxicol Environ Health, 10(6):
969-980.
Lawrence WH, Bass GE, Purcell WP, & Autian J (1972) Use of
mathematical models in the study of structure-toxicity relationships
of dental compounds: I. Esters of acrylic acid and methacrylic acids.
J Dent Res, 51: 526.
Lijinsky W & Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratog Carcinog Mutagen, 1: 259-267.
Lomax LG, Brown OW, & Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic acid
for either 6 or 22 h per day. Abstract presented at a Meeting on Nasal
Toxicity and Dosimetry of Inhaled Xenobiotics: Implications for human
health, Durham, North Carolina, 20-22 September 1993. Inhal Toxicol,
6: 445-449.
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods: Environmental behavior of organic
compounds. New York, McGraw-Hill Book Co., pp 9-65.
McCarthy KL, Thomas WC, Aardema MJ, Seymour JL, Putman DL, Yang LL,
Curren RD, & Valencia R (1992) Genetic toxicology of acrylic acid.
Food Chem Toxicol, 30: 505-515.
Mackay D & Peterson S (1981) Calculating fugacity. Environ Sci
Technol, 15: 1006-1014.
Magnusson B & Kligman AM (1969) The identification of contact
allergens by animal assay: The Guinea pig maximization test. J Invest
Dermatol, 52: 268-276.
Magnusson B, Blohm S, Fregert S, Hjorth N, Hovding G, Pirila V, & Skog
E (1968) Routine patch testing: IV. Supplementary series of test
substances for Scandinavian countries. Acta Dermato-Venereol, 48:
110-114.
Majka J, Knobloch K, & Stetkiewicz J (1974) [Evaluation of acute and
subacute toxicity of acrylic acid.] Med Prac, 25: 427-35 (in
Polish).
Mao J, Doane R, & Kovacs F Jr (1994) Separation of acrolein and its
possible metabolites using different modes of high performance liquid
chromatography. J Liq Chromatogr, 17: 1811-1819.
Miller ML (1964) Acrylic acid polymers. In: Bikales NM ed.
Encyclopedia of polymer science and technology, plastics, resins,
rubbers, fibers. New York, Interscience Publishers, vol 1, pp 197-226.
Miller RR, Ayres JA, & Jersey GC (1979a) Acrylic acid 90-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1024).
Miller RR, Ayres JA, & Jersey GC (1979b) Acrylic acid 10-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1015).
Miller RR, Ayres JA, Jersey GC, & McKenna MJ (1981a) Inhalation
toxicity of acrylic acid. Fundam Appl Toxicol, 1(3): 271-277.
Miller RR, Ayres JA, Rampy LW, & McKenna MJ (1981b) The metabolism of
acrylate esters in rat tissue homogenates. Fundam Appl Toxicol,
1(6): 410-414.
Miller RR, Young JT, Kociba RJ, Keyes DG, Bodner KM, Calhoun LL, &
Ayers JA (1985) Chronic toxicity and oncogenicity bioassay of inhaled
ethyl acrylate in Fischer 344 rats and B6C31 mice. Drug Chem Toxicol,
8: 1-42.
Mitchell DY & Petersen DR (1988) Inhibition of rat liver aldehyde
dehydrogenases by acrolein. Drug Metab Dispos, 16: 37-42.
Moiseeva TN, Kozulin SV, Kulikova LK, & Voronin SP (1991) Screening of
strains decomposing acrylic acid and its derivatives. Soviet-
Biotechnology, 1991(6): 119-125.
Mok WSL (1989) Formation of acrylic acid from lactic acid in
supercritical water. J Org Chem, 54(19): 4596-4602.
Moore MM, Amtower A, Doerr CL, Brock KH, & Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environ Mol Mutagen, 11: 49-63.
Nagasawa T, Nakamura T, & Yamada H (1990) Production of acrylic acid
and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase.
Appl Microbiol Biotechnol, 34(3): 322-324.
Narayanasamy K, Shukla S, & Parekh LJ (1990) Utilization of
acrylonitrile by bacteria isolated from petrochemical waste waters.
Indian J Exp Biol, 28(10): 968-971.
Nawaz MS, Franklin W, & Cerniglia CE (1993) Degradation of acrylamide
by immobilized cells of Xanthomonas maltophilia. Can J Microbiol,
39(2): 207-212.
Nawaz MS, Franklin W, & Cerniglia CE (1994) Degradation of aliphatic
amide mixture by immobilized and nonimmobilized cells of Pseudomonas
sp. Environ Sci Technol, 28(6): 1106-1109.
Neeper-Bradley TL & Kubena MF (1993) Developmental toxicity evaluation
of inhaled acrylic acid vapor in New Zealand white rabbits. Export,
Pennsylvania, Union Carbide Corporation (Bushy Run Research Center
Report No. 92N1008).
NIOSH (1974) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 74-127).
NIOSH (1977) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 77-213).
NIOSH (1989) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (CD-ROM version by Canadian Centre for Occupational Safety and
Health, Hamilton, Ontario).
NIOSH (1990) National occupational exposure survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard, Evaluations, and Field Studies (Unpublished
provisional data as of January 7, 1990).
Nishikawa H, Hosomura H, Sonoda H, & Inagaki M (1979) [Behaviour of
acrylamide in a soil-vegetation system.] Kenkyu Hokoku-Gifu-Ken Kogyo
Gijutsu Senta, 11: 31-34 (in Japanese).
Nishiuchi Y (1975) Toxicity of formulated pesticides to some
freshwater organisms. Suisan Zoshoku, 23: 132.
NLM (1989) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
NLM (1991) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
Noble RC & Czerkawski JW (1973) A gas-chromatographic method for the
determination of low concentrations of acrylic acid in mixtures of C2
to C5 fatty acids in biological materials. Analyst, 98: 122-125.
NTP (1986) Carcinogenesis studies of ethyl acrylates (CAS No.
140-88-5) in F344/N and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program, 224 pp
(NTP Technical Report Series No. 259; NIH Publication No. 87-2515).
OHM/TADS (1989) Oil and hazardous material - Technical assistance
Washington, DC, US Environmental Protection Agency (CD-ROM version by
Micromedex Inc., Denver, Colorado).
OSHA (1981) OSHA analytical methods manual. Part 1 - Organic
substances: Method 28. Washington, DC, US Department of Labor,
Occupational Safety and Health Administration.
OSHA (1989) Department of Labor, Occupational Safety and Health
Administration - 29 CFR, Part 1910: Air Contaminants. Final Rule. Fed
Reg, 54(12): 2332-2983.
Pahren HR & Bloodgood DE (1961) Biological oxidation of several vinyl
compounds. J Water Pollut Control Fed, 33(3): 233-238.
Parker D & Turk JL (1983) Contact sensitivity to acrylate compounds in
guinea pigs. Contact Dermatitis, 9(1): 55-60.
Rao KS, Betso JE, & Olson KJ (1981) A collection of guinea pig
sensitisation test results -grouped by chemical class. Drug Chem
Toxicol, 4: 331-351.
Reininghaus W, Koestner A, & Klimisch HJ (1991) Chronic toxicity and
oncogenicity of inhaled methyl acrylate and n-butyl acrylate in
Sprague-Dawley rats. Food Chem Toxicol, 29(5): 329-339.
Riddick JA, Bunger WB, & Sakano T (1986) Organic solvents physical
properties and methods of purification. In: Techniques of chemistry,
4th ed. New York, Wiley-Interscience, vol 2, p 1325.
Ruth JH (1986) Odour thresholds and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A/143.
Sagelsdorf P, Lutz WK, & Schlatter C (1988) Investigation of the
potential for covalent binding of acrylic acid in vivo after oral
and dermal administration, preliminary results. Schwerzenbach bei
Zurich, Switzerland, Toxicology Institute of the University of
Zurich's Swiss Federal Institute of Technology (Report No.TOXETHZ
1044K).
Sanders JM, Burka LT, & Matthews HB (1988) Metabolism and disposition
of n-butyl acrylate in male Fischer rats. Drug Metab Dispos,
16(3): 429-434.
Sanseverino J, Montenecourt BS, & Sands JA (1989) Detection of acrylic
acid formation in Megasphaera elsdenii in the presence of 3-butynoic
acid. Appl Microbiol Biotechnol, 30(3): 239-242.
Sasaki S (1978) In: Hutizinger O ed. The scientific aspects of the
chemical substance control law in Japan in aquatic pollutants
transformation and biological effects. Oxford, New York, Pergamon
Press, pp 283-298.
Sax NI (1984) Dangerous properties of industrial chemicals, 6th ed.
New York, Van Nostrand Reinhold Co., p 129.
Sax NI & Lewis R (1989) Dangerous properties of industrial chemicals,
7th ed. New York, Van Nostrand Reinhold Co., pp 71-72.
Schultz H (1991) Beta-oxidation of fatty acid. Biochem Biophys,
1081: 109-120.
Schwartz BS, Doty RL, Moroe C, Frye R, & Barker S (1989) Olfactory
function in chemical workers exposed to acrylate and methacrylate
vapors. Am J Public Health, 79(5): 613-618.
Segal A, Fedyk J, Meclchionne S, & Seidman I (1987) The isolation and
characterization of 2-carboxyethyl adducts following in vitro
reaction of acrylic acid with calf thymus DNA and bioassay of acrylic
acid in female Hsd:(ICR)Br mice. Chem-Biol Interact, 61: 189-197.
Shah JF (1990) A hydrolysis study of 14C-acrylic acid. Painesville,
Ohio, Ricerca Inc.
Shanker R, Ramakrishna C, & Seth PK (1990) Microbial degradation of
acrylamide monomer. Arch Microbiol, 154(2): 192-198.
Shelton DR & Tiedje JM (1984) General method for determining anaerobic
biodegradation potential. Appl Environ Microbiol, 47: 850-857.
Sieburth JMN (1960) Acrylic acid, an "antibiotic" principle in
Phaeocystis blooms in Antarctic waters. Science, 132: 676-677.
Silver EH, Leith DE, & Murphy SD (1981) Potentiation by triorthotolyl
phosphate of acrylate ester-induced alterations in respiration.
Toxicology, 22(3): 193-203.
Simon P, Brand F, & Lemacon C (1989) Florisil sorbent sampling and ion
chromatographic determination of airborn aliphatic carboxylic acids. J
Chromatogr, 479: 445-451.
Singh M & Thomas M (1985) Analysis of effluent from a methyl acrylate
plant by gas chromatography. Indian J Environ Health, 27: 361-364.
Singh AR, Lawrence WH, & Autian J (1972) Embryonic-fetal toxicity and
teratogenic effects of a group of methacrylate esters in rats. J Dent
Res, 51(6): 1632-1638.
Singh HB, Jaber HM, & Davenport HE (1984) Reactivity/volatility
classification of selected organic chemicals: Existing data. Menlo
Park, California, SRI International, p 190 (EPA-600/3-84-082).
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Data Corporation, p
43.
Sivak A (1987) Evaluation of acrylic acid mouse skin tumour bioassay
and DNA adduct study performed at New York University Medical Center.
New York, New York University (Report No. ADL/558.46 to the Celanese
Corporation).
Slott VL & Hales BF (1985) Teratogenicity and embryolethality of
acrolein and structurally related compounds in rats. Teratology,
32(1): 65-72.
SNF (1995) Inhibition test (72 h) in freshwater unicellular algae.
Licata-Messana L, SEPC (Société d'Ecotoxicologie et de Physico-
Chimie). Saint Etienne, France, Société Nouvelle Floerger.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17: A416-A427.
SRI International (1981) Production and use profile for CHIP of
acrylic acid. Menlo Park, California, SRI International (US EPA
Technical Directive 5.16).
Staples RE (1993) Evaluation of the environmental fate and
ecotoxicological parameters for several acrylic monomers. Washington,
DC, Assessment Technologies BAMM.
Stewart JM, Bhattacharya SK, Madura RL, Mason SH, & Schonberg JC
(1995) Anaerobic treatability of selected organic toxicants in
petrochemical wastes. Water Res, 29: 2730-2738.
Swenberg JA, Gross EA, & Randall HW (1986) Localization and
quantitation of cell proliferation following exposure to nasal
irritants. In: Barrow CS ed. Toxicology of nasal passages. Washington,
DC, Hemisphere Publishing Corporation, pp 291-300.
Takamizawa K, Horitsu H, Ichikawa T, Tawai K, & Suzuki T (1993) Beta-
hydroxypropionic acid production by Byssochlamys sp grown on acrylic
acid. Appl Microbiol Biotechnol, 40(2/3): 196-200.
Tegeris AS, Balmer MF, Morton MF, Buckley IR, & Garner FM (1987)
13-week mouse comparative skin irritation study with acrylic acid.
Washington, DC, Basic Acrylate Monomer Manufacturers Association
(Unpublished report).
Tegeris AS, Balmer MF, Garner FM, Thomas WC, Murphy SR, McLaughlin JE,
& Seymour JL (1988) A 13-week skin irritation study with acrylic acid
in 3 strains of mice. Toxicologist, 8: 127.
Union Carbide Corporation (1977) Toxicology studies: acrylic acid
glacial. New York, Union Carbide Corporation, Industrial and
Toxicology Department.
US EPA (1981) Chemical hazard information profile: Acrylic acid (CAS
No 79-10-7). Washington, DC, US Environmental Protection Agency, TSCA
Assistance Division (EPA 749F9400.6).
US ITC (1977) Synthetic organic chemicals - US production and sales.
Washington, DC, International Trade Commission.
US ITC (1983) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1985) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1986) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1987) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US ITC (1988) Synthetic organic chemicals - US production and sales.
Washington, DC, US International Trade Commission.
US National Fire Protection Association (1986) Fire protection guide
on hazardous materials, 9th ed. Boston, Massachusetts, US National
Fire Protection Association, pp 40-42.
Van der Walle HB, Seutter LPC, & Delbressine E (1982) Concomitant
sensitization to hydroquinone and p-methoxyphenol in the guinea
pig; inhibitors in acrylic monomers. Contact Dermatitis, 8: 147-154.
Van der Zande L, Kunnen R, Uijtewaal B, Van Wijk R, & Bisschop A
(1986) Effect on hepatic ornithine decarboxylase of some food
additives and synthetic elastomers. Food Addit Contam, 3(1): 57-62.
Veith GD, DeFoe DL, & Bergstedt BV (1979) Measuring and estimating the
bioconcentration factor of chemicals in fish. J Fish Res Board Can,
36: 1040-1048.
Verschueren K (1983) Handbook of environmental data of organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Co., p 161.
Vincent WJ & Guient V (1982) The development of air sampling and
analytical methodology for determining occupational exposure. Am Ind
Hyg Assoc J, 43(7): 499-504.
Vodicka P, Gut I, & Frantick E (1990) Effects of inhaled acrylic acid
derivatives in rats. Toxicology, 65(´): 209-222.
Vojtisek B, Hronova B, Hamrik J, Jambor V, Zezula V, Zendulka I,
Dvorak R, & Sisak M (1991) [The effect of clover silage treated with
acrylic acid on selected indicators of metabolism in cows around the
time of parturition and the status of their calves.] Vet Med (Praha),
36: 273-280 (in Czech).
Waegemaekers TH & van der Walle HB (1984) Alpha, beta-
diacryloxypropionic acid, a sensitizing impurity in commercial acrylic
acid. Dermatol Beruf Umwelt, 32: 55-58.
Weast RC & Astle MJ (1985) CRC handbook of data on organic compounds,
Volumes I and II. Boca Raton, Florida, CRC Press Inc., pp V1, 42, 57.
Weast RC, Lide DR, Astle MJ, & Beyer WH ed. (1989) Acrylic acid. In:
CRC handbook of chemistry and physics, 70th ed. Boca Raton, Florida,
CRC Press, p C-57.
Wegner WS, Reeves HC, Rabin R, & Ajl SI (1968) Alternate pathways of
metabolism of short-chain fatty acids. Bacteriol Rev, 32: 1-26.
Whanger PD & Matrone G (1967) Metabolism of lactic, succinic and
acrylic acids by rumen microorganisms from sheep fed sulphur-adequate
and sulphur-deficient diet. Biochem Biophys Acta, 136: 27-35.
Wiegand HJ (1990) Species differences in the metabolism of n-butyl
acrylate. Naunyn- Schmiedebergs Arch Pharmacol, 341: 512 (Abstract
47).
Wiegand HJ, Schiffmann D, & Henschler D (1989) Non-genotoxicity of
acrylic acid and n-butyl acrylate in a mammalian cell system (SHE
cells). Arch Toxicol, 63: 250-251.
Windholz M ed. (1983) Merck index, 10th ed. Rahway, New Yersey, Merck
& Co. Inc.
Winter SM & Sipes IG (1993) The disposition of acrylic acid in the
male Sprague-Dawley rat following oral or topical administration. Food
Chem Toxicol, 31: 615-621.
Winter SM, Weber GL, Gooley PR, MacKenzie NE, & Sipes IG (1992)
Identification and comparison of the urinary metabolites of
[1,2,3-13C3] acrylic acid and [1,2,3-13C3]propionic acid in the rat by
homonuclear 13C nuclear magnetic resonance spectroscopy. Drug Metab
Dispos, 20: 665-672.
Wise HE & Fahrenthold PD (1981) Occurrence and predictability of
priority pollutants in wastewater of the organic chemicals and
plastics/synthetic fibers industrial categories - ACS 1981 National
Meeting, Division of Industrial Chemistry Symposium on Treatability of
Industrial Aqueous Effluents. Washington, DC, American Chemical
Society.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from testing of 255
chemicals. Environ Mutagen, 9: 1-110.
1. RESUME ET RECOMMANDATIONS
L'acide acrylique est un liquide incolore qui dégage une odeur
âcre et irritante à la température et à la pression ordinaires. Le
seuil olfactif de l'acide acrylique est bas (0,20-3,14 mg/m3). Il est
miscible à l'eau et à la plupart des solvants organiques.
L'acide acrylique existe dans le commerce en deux qualités;
l'acide acrylique technique et l'acide acrylique glacial. Il se
polymérise facilement sous l'action de la chaleur, de la lumière ou en
présence de métaux, c'est pourquoi un inhibiteur de polymérisation est
ajouté aux produits du commerce.
La production mondiale d'acide acrylique a été estimée à environ
2 millions de tonnes en 1994. On l'utilise essentiellement comme une
matière première pour la production d'esters acryliques, comme
monomère pour la production de l'acide polyacrylique et de ses sels,
comme comonomère avec l'acrylamide pour la préparation de polymères
utilisés comme floculants, avec l'éthylène pour la préparation de
résines échangeuses d'ions, l'ester méthylique sevant par aillleurs à
la préparation d'un certain nombre d'autres copolymères.
Il est possible de doser les résidus d'acide acrylique présents
dans l'air et dans d'autres milieux par chromatographie en phase
gazeuse, par chromatographie en phase liquide à haute performance ou
encore par polarographie. Les limites de détection de ces méthodes
sont de 14 ppm dans l'air et de 1 ppm dans les autres milieux.
L'acide acrylique existe à l'état naturel dans certaines algues
marines et on en a également trouvé dans les sécrétions du rumen chez
les ovins.
Comme il est miscible à l'eau, l'acide acrylique ne devrait pas
s'adsorber de manière importante aux particules du sol ou aux
sédiments. Dans le sol, les composés chimiques à faible constante de
Henry sont essentiellement non volatils. Toutefois, la tension de
vapeur de l'acide acrylique donne à penser qu'il se volatilise en
surface du sol ou lorsque le sol est sec.
L'acide acrylique libéré dans l'atmosphère réagit sur les
radicaux hydroxyles et sur l'ozone produits par voie photochimique et
se décompose alors rapidement. L'acide acrylique ne risque pas d'être
transporté dans l'atmosphère sur une longue distance car sa durée de
vie atmosphérique est inférieure à un mois.
L'acide acrylique peut se former par hydrolyse de l'acrylamide
monomère présent dans les déchets industriels enfouis dans le sol, en
particulier dans des conditions d'aérobiose.
Lorsqu'il est libéré dans l'eau, l'acide acrylique est facilement
biodégradé. Sa destinée dans l'eau est liée à sa dégradation par voie
chimique ou microbienne. Il est rapidement oxydé et risque donc de
provoquer une désoxygénation des étendues d'eau dans lesquelles il est
déversé en grandes quantités. On a montré que l'acide acrylique
pouvait être décomposé dans des conditions d'aérobiose ou
d'anaérobiose.
On ne dispose d'aucune donnée quantitative sur les concentrations
d'acide acrylique dans l'air ambiant, l'eau de boisson ou le sol.
Toutefois, on sait qu'il est présent dans les effluents liquides
déversés sur les sites où on le prépare par oxydation ménagée du
propylène.
On ne possède aucune donnée sur l'exposition de la population en
général. Toutefois, les consommateurs peuvent être exposés à de
l'acide acrylique libre présent dans des produits ménagers tels que
certaines peintures à l'eau. Les personnes qui vivent à proximité
d'unités de production d'acide acrylique ou de ses esters ou
polymères, peuvent être exposées à l'acide acrylique présent dans
l'air ambiant. L'absorption d'esters acryliques peut constituer une
source potentielle d'exposition interne à l'acide acrylique.
L'inhalation et le contact sont deux voies importantes
d'exposition professionnelle.
Quelle que soit la voie d'exposition, l'acide acrylique est
rapidement résorbé et métabolisé. Il est métabolisé dans une forte
proportion, principalement en acide 3-hydroxypropionique, en CO2 et
en acide mercapturique qui sont éliminés ensuite dans l'air expiré et
dans les urines. Du fait de sa métabolisation et de son élimination
rapides, la demi-vie de l'acide acrylique est brève (quelques minutes)
et il n'a donc aucune tendance à la bioaccumulation.
On a fait état de valeurs très diverses pour la DL50, mais la
plupart des données indiquent que l'acide acrylique présente une
toxicité aiguë modérée à faible par la voie orale et une toxicité
aiguë modérée par la voie respiratoire ou percutanée.
L'acide acrylique est corrosif ou irritant pour la peau et les
yeux. On ne sait pas avec précision au-dessous de quelle concentration
il n'est plus irritant. Il est également très irritant pour les voies
respiratoires.
On a obtenu des résultats positifs et négatifs lors d'épreuves de
sensibilisation cutanée avec de l'acide acrylique, mais il semble que
les résultats positifs obtenus étaient dus à la présence d'une
impureté.
Lors d'études au cours desquelles des rats ont absorbé de l'acide
acrylique dans leur eau de boisson, on a constaté que la dose sans
effet nocif observable (NOAEL) était de 140 mg/kg de poids corporel
par jour, le critère retenu étant la réduction du gain de poids sur
12 mois, et de 240 mg/kg de poids corporel par jour, la critère retenu
dans ce cas étant la présence d'anomalies histologiques au niveau de
l'estomac. Le même type d'étude chronique sur des rats a montré qu'à
la dose la plus élevée étudiée (78 mg/kg de poids corporel et par
jour), il n'y avait aucun effet observable. La dose la plus faible
provoquant un effet observable (LOAEL) a été de 15 mg/m3 (5 ppm) par
la voie respiratoire chez des souris exposées pendant 90 jours à de
l'acide acrylique, le critère retenu étant en présence de lésions
nasales infimes chez les femelles. Des effets au niveau du nez ont
également été observés chez des rats à la dose de 225 mg/m3, soit
75 ppm, mais pas à celle de 15 ou 75 mg/m3 (5 ou 25 ppm).
Les études de reproduction dont on connaît les résultats
indiquent que l'acide acrylique n'est pas tératogène et qu'il n'a
aucun effet sur la reproduction.
Les épreuves de génotoxicité in vitro ont donné des résultats
positifs et des résultats négatifs. Une épreuve pratiquée in vivo, à
la recherche d'aberration chromosomique dans la moelle osseuse, a
donné des résultats négatifs. Aucune conclusion définitive n'a pu être
tirée d'une étude in vivo sur la liaison à l'ADN ni de la recherche
de mutations létales dominantes.
Les données disponibles ne donnent pas de résultats indicatifs
d'un pouvoir cancérogène de l'acide acrylique, toutefois ces données
sont insuffisantes pour conclure qu'il n'y a aucun risque.
On a signalé des cas d'intoxication dans la population générale,
mais aucune étude épidémiologique en milieu professionnel n'a été
publiée.
Comme l'action toxique de l'acide acrylique se manifeste au point
de contact, des valeurs guides distinctes sont recommandées pour
l'exposition par voie orale et l'exposition par la voie respiratoire.
On propose des valeurs guides de 9,9 mg/litre dans l'eau de boisson et
de 54 µg/m3 dans l'air ambiant pour la population générale.
L'acide acrylique est faiblement toxique pour les bactéries et
les micro-organismes terricoles.
Parmi les organismes aquatiques étudiés, ce sont les algues qui
constituent le groupe le plus sensible, avec des valeurs de la CE50
pour la croissance, qui vont de 0,04 à 63 mg/litre et une
concentration sans effet observable (NOEC) chez l'espèce la plus
sensible, qui est de 0,008 mg/litre. Pour la daphnie, les valeurs de
la CE50 (critère : immobilisation) sont de 54 mg/litre sur 24 heures
et de 95 mg/litre sur 48 heures. L'acide acrylique est plus toxique
pour les daphnies que ses sels alcalins. En ce qui concerne la
toxicité aiguë, les études toxicologiques sur des poissons ont donné
les résultats suivants : de 25 mg/litre (CL50 à 96 heures) pour la
truite arc-en-ciel, à 315 mg/litre (CL50 à 72 heures) pour l'orfe. En
se basant sur l'absence de réponse sublétale et comportementale, on a
observé que la concentration d'acide acrylique sans effet observable
était de 6,3 mg/litre pour la truite arc-en-ciel.
Du fait que son faible coefficient de partage entre l'octanol et
l'eau, l'acide acrylique a peu de chances de subir une
bioconcentration chez les organismes aquatiques. Par ailleurs, on n'a
pas signalé de cas de bioamplification le long de la chaîne
alimentaire.
On ne dispose d'aucune donnée concernant les effets de l'acide
acrylique sur les organismes terrestres.
1. RESUMEN Y RECOMENDACIONES
El ácido acrílico es un líquido incoloro que desprende un olor
acreirritante a temperatura y presión ambiente. Su umbral de olor es
bajo (0,20-3,14 mg/m3). Es miscible en agua y en la mayor parte de
los disolventes orgánicos.
El ácido acrílico está disponible en el comercio en dos
calidades: técnica y glacial. Como se polimeriza fácilmente si se
expone al calor, a la luz o a metales, los productos comerciales
contienen un inhibidor de la polimerización.
La producción mundial de ácido acrílico en 1994 se estimó en
aproximadamente 2 millones de toneladas. Principalmente se utiliza
como materia prima en la producción de ésteres acrílicos, como
monómero para ácidos y sales poliacrílicos, y como comonómero con
acrilamida para polímeros que sirven de agentes de floculación, con
etileno para polímeros de resina intercambiadora de iones, con éster
metílico para polímeros y con ácido itacónico para otros copolímeros.
Los residuos de ácido acrílico en el aire y en otros medios
pueden cuantificarse mediante técnicas de cromatografía de gases,
cromatografía líquida de alta resolución y polarografía. Los límites
de detección de esos métodos son de 14 ppm en el aire y 1 ppm en otros
medios.
Se ha notificado la presencia natural de ácido acrílico en algas
marinas, y se le ha encontrado en el líquido ruminal de las ovejas.
Por ser miscible en agua, no cabe prever que el ácido acrílico se
adsorba de manera significativa en el suelo o en los sedimentos. En
el suelo, las sustancias químicas con bajas constantes de Henry son
esencialmente no volátiles. Sin embargo, la presión de vapor del
ácido acrílico indica que éste se volatiliza del suelo superficial y
del suelo seco.
El ácido acrílico liberado en la atmósfera reacciona con los
radicales hidroxilos y el ozono de origen fotoquímico, degradándose
rápidamente. El transporte atmosférico del ácido acrílico a grandes
distancias no es posible, porque su permanencia en la atmósfera es
inferior a un mes.
El ácido acrílico puede formarse por hidrólisis del monómero
acrilamida en desechos industriales presentes en el suelo,
especialmente en condiciones aeróbicas.
Liberado en el agua, el ácido acrílico se biodegrada rápidamente.
Su destino en el agua depende de la degradación química y microbiana.
El ácido acrílico se oxida rápidamente en el agua; descargado en
grandes cantidades en una masa de agua, puede agotar el oxígeno. Se
ha demostrado que la degradación se produce en condiciones aeróbicas y
anaeróbicas.
No se dispone de datos cuantitativos sobre niveles de ácido
acrílico en el aire ambiente, el agua potable o el suelo. Sin
embargo, se sabe que el ácido acrílico está presente en los efluentes
de su producción por oxidación del propileno.
No hay datos sobre la exposición de la población general. No
obstante, los consumidores podrían estar expuestos al ácido acrílico
en productos de uso doméstico tales como las pinturas a base de agua.
La población residente en las cercanías de fábricas productoras de
ácido acrílico o de sus ésteres o polímeros puede estar expuesta al
ácido acrílico en el aire ambiente. Una posible fuente de exposición
interna puede ser el metabolismo de los ésteres del ácido acrílico
absorbidos.
La inhalación y el contacto con la piel son importantes vías de
exposición ocupacional.
Independientemente de la vía de exposición, el ácido acrílico se
absorbe y metaboliza con rapidez. Se metaboliza ampliamente, sobre
todo en ácido 3-hidroxipropiónico, CO2 y ácido mercaptúrico, que se
eliminan en el aire expirado y por la orina. Debido a su rápido
metabolismo y eliminación, la semivida del ácido acrílico es breve
(minutos), por lo que no tiene potencial de bioacumulación.
Aunque se ha notificado una amplia gama de valores de DL50, la
mayor parte de los datos indica que el ácido acrílico tiene una
toxicidad aguda de baja a moderada por vía oral, y moderada por
inhalación o por vía cutánea.
El ácido acrílico es corrosivo o irritante para la piel y los
ojos. No se sabe con certeza a qué concentración no es irritante.
También irrita fuertemente las vías respiratorias.
En cuanto a la sensibilización de la piel al ácido acrílico, se
han notificado resultados positivos y negativos, pero es posible que
los positivos se deban a una impureza.
En estudios con agua potable en ratas, el nivel sin efectos
negativos observados (NOAEL) fue de 140 mg/kg de peso corporal al día
para la reducción del aumento de peso corporal en un estudio de 12
meses de duración, y de 240 mg/kg de peso corporal al día para las
alteraciones histopatológicas en el estómago. Un estudio de
exposición crónica al agua potable en ratas no reveló efectos a la
dosis más alta ensayada (78 mg/kg de peso corporal al día). En
ratones expuestos al ácido acrílico por inhalación durante 90 días se
detectó un nivel inferior con efectos negativos observados (LOAEL) de
15 mg/m3 (5 ppm), sobre la base de lesiones nasales muy ligeras en
las hembras. En las ratas se observaron efectos nasales a 225 mg/m3
(75 ppm), pero no a 15 ni a 75 mg/m3 (5 ó 25 ppm).
Los estudios de reproducción disponibles indican que el ácido
acrílico no es teratogénico, ni tiene efecto alguno en la
reproducción.
En las pruebas de genotoxicidad in vitro se han obtenido
resultados tanto positivos como negativos. Una prueba de aberración
cromosómica en médula ósea in vivo dio resultados negativos. Un
estudio in vivo de unión de ADN y una prueba de dominancia letal no
permitieron sacar conclusiones definitivas.
Los datos disponibles no indican que el ácido acrílico sea
carcinógeno, pero esos datos son insuficientes para concluir que no
existe ningún riesgo de carcinogenicidad.
No se han comunicado casos de intoxicación en la población
general. Tampoco se han notificado estudios epidemiológicos
ocupacionales.
Puesto que la toxicidad del ácido acrílico afecta al lugar en que
se produce el contacto, se recomiendan valores de orientación
distintos para la exposición oral y por inhalación. Para la población
general se proponen valores de orientación de 9,9 mg/litro para el
agua potable y de 54 œg/m3 para el aire ambiente.
La toxicidad del ácido acrílico para las bacterias y los
microorganismos del suelo es baja.
Las algas son el grupo de organismos acuáticos más sensible entre
los estudiados, con unos valores de CE50 , sobre la base del
crecimiento, que van de 0,04 a 63 mg/litro y una concentración sin
efectos observados (NOEC) para la especie más sensible de 0,008
mg/litro. Los valores de CE50 (basados en la inmovilización) para
Daphnia magna son de 54 mg/litro (24 horas) y 95 mg/litro (48
horas). El ácido acrílico es más tóxico para los dáfnidos que la sal
alcalina. Estudios de toxicidad aguda en peces han dado resultados
que oscilan entre 27 mg/litro (CL50 a las 96 horas) para la trucha
arco iris y 315 mg/litro (CL50 a las 72 horas) para Leuciscus idus.
La NOEC a las 96 horas para la toxicidad del ácido acrílico en la
trucha arco iris se ha establecido en 6,3 mg/litro, sobre la base de
la ausencia de respuestas subletales/comportamentales.
Debido a su bajo coeficiente de reparto octanol/agua, es poco
probable que el ácido acrílico sea objeto de bioconcentración en
organismos acuáticos. No se conocen casos de bioamplificación en
cadenas alimentarias.
No se dispone de datos sobre los efectos del ácido acrílico en
organismos terrestres.
 The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
Members
Dr B.I. Ghanayem, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr D. Guth, Office of Research and Development, National Centre
for Environmental Assessment, Research Triangle Park North
Carolina, USA
Mr L. Heiskanen, Environmental Health and Safety Unit,
Department of Health and Family Services, Canberra, Australia
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, United Kingdom ( Co-rapporteur)
Dr P. Lundberg, Department of Toxicology and Chemistry,
National Institute for Working Life, Sweden ( Chairman)
Dr K. Rydzynski, The Nofer Institute of Occupational Medicine,
Lodz, Poland ( Co-rapporteur)
Dr R.O. Shillaker, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries & Food, United Kingdom
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Observers
Dr M. Wooder, Rohm and Haas Uk, Ltd., Croydon, Surrey, United
Kingdom (representing the American Industrial Health Council)
Dr A. Lombard, Service Hygiène Industrielle Toxicologique, ELF-
ATOCHEM, Paris, France (representing the Centre for Ecotoxicology
and Toxicology of Chemicals)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
A WHO Task Group on Environmental Health Criteria for Acrylic
Acid met at the Institute of Terrestrial Ecology, Monks Wood,
Huntington, United Kingdom, from 16 to 19 April 1996. Dr S. Dobson
opened the meeting and welcomed the participants on behalf of the
Institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to acrylic acid.
Dr K. Rydzynski, the Nofer Institute of Occupational Medicine,
Poland, prepared the first draft of this monograph. Dr R.O.
Shillaker, Pesticides Safety Directorate, Ministry of Agriculture,
Fisheries and Food, United Kingdom, contributed to the preparation of
the first draft. The second draft was prepared by Dr K. Rydzynski
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria.
Dr D. Guth, National Centre for Environmental Protection, USA,
contributed to the preparation of the final text of the evaluation.
The meeting was chaired by Dr P. Lundberg, National Institute for
Working Life, Sweden.
Dr B.H. Chen and Dr P.G. Jenkins, IPCS Central Unit, were
responsible for the overall scientific content and technical editing,
respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial
Hygienists
CHO Chinese hamster ovary
EC50 median effective concentration
FID flame ionization detector
GC gas chromatography
GSH reduced glutathione
GV guidance value
HPLC high performance liquid chromatography
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect concentration
NMR nuclear magnetic resonance
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
TCA tricarboxylic acid cycle
TI tolerable intake
TT toxicity threshold
UDS unscheduled DNA synthesis
1. SUMMARY AND RECOMMENDATIONS
Acrylic acid is a colourless liquid, with an irritating acrid
odour, at room temperature and pressure. The odour threshold of
acrylic acid is low (0.20-3.14 mg/m3). It is miscible with water and
most organic solvents.
Acrylic acid is commercially available in two grades; technical
grade and glacial grade. Acrylic acid polymerizes easily when exposed
to heat, light or metals, and a polymerization inhibitor is therefore
added to commercial products.
The worldwide production of acrylic acid in 1994 was estimated to
be approximately 2 million tonnes. It is used primarily as a starting
material in the production of acrylic esters as a monomer for
polyacrylic acid and salts and as a co-monomer with acrylamide for
polymers used as flocculants, with ethylene for ion-exchange resin
polymers, with methyl ester for polymers, and with itatonic acid for
other co-polymers.
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
are 14 ppm in air and 1 ppm in other media.
Acrylic acid has been reported to occur naturally in marine algae
and has been found in the rumen fluid of sheep.
Being miscible with water, acrylic acid would not be expected to
adsorb significantly to soil or sediment. Under soil conditions,
chemicals with low Henry's Law constants are essentially non-volatile.
However, the vapour pressure of acrylic acid suggests that it
volatilizes from surface and dry soil.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
Acrylic acid may be formed by hydrolysis of acrylamide monomer
from industrial waste in soil, especially under aerobic conditions.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. Acrylic acid is rapidly oxidized in water and can
therefore potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
No quantitative data on levels of acrylic acid in ambient air,
drinking-water or soil are available. However, acrylic acid occurs in
wastewater effluent from its production via the oxidation of
propylene.
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as water-based paints. People living in the vicinity of plants
producing acrylic acid or its esters or polymers may be exposed to
acrylic acid in the ambient air. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters.
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxy propionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route and moderate acute toxicity by the inhalation or
dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats, the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg body weight per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). A lowest-observed-adverse-effect level (LOAEL)
of 15 mg/m3 (5 ppm) by the inhalation route was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in
in vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay gave negative results. No firm conclusions can be
drawn from an in vivo DNA binding study or from a dominant lethal
assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization) for
Daphnia magna are 54 mg/litre (24 h) and 95 mg/litre (48 h). Acrylic
acid is more toxic to daphnids than is the alkaline salt. Acute
toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre (72-h
LC50) for the golden orfe. The 96-h NOEC for acrylic acid toxicity to
rainbow trout was found to be 6.3 mg/litre, based on a lack of
sublethal/behavioural responses.
Because of its low octanol-water partition coefficient, acrylic
acid is unlikely to bioconcentrate in aquatic organisms. There have
been no reports of biomagnification in food chains.
No data are available concerning the effects of acrylic acid on
terrestrial organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: acrylic acid
CAS name: 2-propenoic acid
CAS registry number:
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
Members
Dr B.I. Ghanayem, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr D. Guth, Office of Research and Development, National Centre
for Environmental Assessment, Research Triangle Park North
Carolina, USA
Mr L. Heiskanen, Environmental Health and Safety Unit,
Department of Health and Family Services, Canberra, Australia
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, United Kingdom ( Co-rapporteur)
Dr P. Lundberg, Department of Toxicology and Chemistry,
National Institute for Working Life, Sweden ( Chairman)
Dr K. Rydzynski, The Nofer Institute of Occupational Medicine,
Lodz, Poland ( Co-rapporteur)
Dr R.O. Shillaker, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries & Food, United Kingdom
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Observers
Dr M. Wooder, Rohm and Haas Uk, Ltd., Croydon, Surrey, United
Kingdom (representing the American Industrial Health Council)
Dr A. Lombard, Service Hygiène Industrielle Toxicologique, ELF-
ATOCHEM, Paris, France (representing the Centre for Ecotoxicology
and Toxicology of Chemicals)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLIC ACID
A WHO Task Group on Environmental Health Criteria for Acrylic
Acid met at the Institute of Terrestrial Ecology, Monks Wood,
Huntington, United Kingdom, from 16 to 19 April 1996. Dr S. Dobson
opened the meeting and welcomed the participants on behalf of the
Institute. Dr B.H. Chen, IPCS, welcomed the participants on behalf of
the Director, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to acrylic acid.
Dr K. Rydzynski, the Nofer Institute of Occupational Medicine,
Poland, prepared the first draft of this monograph. Dr R.O.
Shillaker, Pesticides Safety Directorate, Ministry of Agriculture,
Fisheries and Food, United Kingdom, contributed to the preparation of
the first draft. The second draft was prepared by Dr K. Rydzynski
incorporating comments received following the circulation of the first
draft to the IPCS Contact Points for Environmental Health Criteria.
Dr D. Guth, National Centre for Environmental Protection, USA,
contributed to the preparation of the final text of the evaluation.
The meeting was chaired by Dr P. Lundberg, National Institute for
Working Life, Sweden.
Dr B.H. Chen and Dr P.G. Jenkins, IPCS Central Unit, were
responsible for the overall scientific content and technical editing,
respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ACGIH American Conference of Governmental Industrial
Hygienists
CHO Chinese hamster ovary
EC50 median effective concentration
FID flame ionization detector
GC gas chromatography
GSH reduced glutathione
GV guidance value
HPLC high performance liquid chromatography
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect concentration
NMR nuclear magnetic resonance
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
TCA tricarboxylic acid cycle
TI tolerable intake
TT toxicity threshold
UDS unscheduled DNA synthesis
1. SUMMARY AND RECOMMENDATIONS
Acrylic acid is a colourless liquid, with an irritating acrid
odour, at room temperature and pressure. The odour threshold of
acrylic acid is low (0.20-3.14 mg/m3). It is miscible with water and
most organic solvents.
Acrylic acid is commercially available in two grades; technical
grade and glacial grade. Acrylic acid polymerizes easily when exposed
to heat, light or metals, and a polymerization inhibitor is therefore
added to commercial products.
The worldwide production of acrylic acid in 1994 was estimated to
be approximately 2 million tonnes. It is used primarily as a starting
material in the production of acrylic esters as a monomer for
polyacrylic acid and salts and as a co-monomer with acrylamide for
polymers used as flocculants, with ethylene for ion-exchange resin
polymers, with methyl ester for polymers, and with itatonic acid for
other co-polymers.
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
are 14 ppm in air and 1 ppm in other media.
Acrylic acid has been reported to occur naturally in marine algae
and has been found in the rumen fluid of sheep.
Being miscible with water, acrylic acid would not be expected to
adsorb significantly to soil or sediment. Under soil conditions,
chemicals with low Henry's Law constants are essentially non-volatile.
However, the vapour pressure of acrylic acid suggests that it
volatilizes from surface and dry soil.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
Acrylic acid may be formed by hydrolysis of acrylamide monomer
from industrial waste in soil, especially under aerobic conditions.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. Acrylic acid is rapidly oxidized in water and can
therefore potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
No quantitative data on levels of acrylic acid in ambient air,
drinking-water or soil are available. However, acrylic acid occurs in
wastewater effluent from its production via the oxidation of
propylene.
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as water-based paints. People living in the vicinity of plants
producing acrylic acid or its esters or polymers may be exposed to
acrylic acid in the ambient air. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters.
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxy propionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route and moderate acute toxicity by the inhalation or
dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats, the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg body weight per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). A lowest-observed-adverse-effect level (LOAEL)
of 15 mg/m3 (5 ppm) by the inhalation route was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in
in vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay gave negative results. No firm conclusions can be
drawn from an in vivo DNA binding study or from a dominant lethal
assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization) for
Daphnia magna are 54 mg/litre (24 h) and 95 mg/litre (48 h). Acrylic
acid is more toxic to daphnids than is the alkaline salt. Acute
toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre (72-h
LC50) for the golden orfe. The 96-h NOEC for acrylic acid toxicity to
rainbow trout was found to be 6.3 mg/litre, based on a lack of
sublethal/behavioural responses.
Because of its low octanol-water partition coefficient, acrylic
acid is unlikely to bioconcentrate in aquatic organisms. There have
been no reports of biomagnification in food chains.
No data are available concerning the effects of acrylic acid on
terrestrial organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: acrylic acid
CAS name: 2-propenoic acid
CAS registry number: 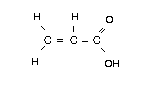 Relative molecular mass: 72.06
2.1.2 Technical product
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins (IARC, 1979; CHRIS, 1989). Acrylic acid polymerizes easily when
exposed to heat, light or metals, and so a polymerization inhibitor is
added to commercial acrylic acid to prevent the strong exothermic
polymerization (NLM, 1989). The inhibitors that are usually used in
acrylic acid preparations are the monomethyl ether of hydroquinone
(methoxyphenol) at 200 ± 20 ppm, phenothiazine at 0.1% and
hydroquinone at 0.1%. Methylene blue at 0.5 to 1.0% and N,N'-
diphenyl- p-phenylenediamine at 0.05% can also be used (IARC, 1979;
CHRIS, 1989; OHM/TADS, 1989; BASF, 1993).
2.2 Physical and chemical properties
2.2.1 Physical properties
Acrylic acid is a colourless liquid at room temperature and
pressure (IARC, 1979; Windholz, 1983; CHRIS, 1985). It has an
irritating acrid odour and it is totally miscible with water and most
organic solvents. Some of the most important physical properties of
acrylic acid are summarized in Table 1.
Table 1. Physical and chemical properties of acrylic acid
Property Value References
Odour threshold concentration (mg/m3) 0.20-3.14 Fazzalari, 1978; Amoore & Hautala, 1983;
Ruth, 1986; Grudzinski, 1988; HSDB, 1989
Melting point (°C at 1 atm) 12.3-14.0 CHRIS, 1989; Weast et al., 1989
Boiling point (°C at 1 atm) 141.3-141.6 CHRIS, 1989; Weast et al., 1989
Flash point (°C)
open cup
closed cup 54.0-68.3 IARC, 1979; Kirk-Othmer, 1984; Sax & Lewis 1989;
46-48.5 Elf Atochem, 1992; BASF, 1994a
Autoignition temperature (°C) 390-446 IARC, 1979; HSDB, 1989; BASF, 1992; Elf Atochem, 1992
Flammable limits (%)
lower 28 HSDB, 1989
upper
Burning rate (mm/min) 1.6 CHRIS, 1989
Specific gravity (g/ml at 20°C) 1.0497-1.0511 IARC, 1979; CHRIS, 1989; Weast et al., 1989
Relative vapour density (air =1 at 20°C) 2.5 HSDB, 1989
Viscosity (mPa.s at 20°C) 1.22-1.30 BASF, 1992; Elf Atochem, 1992
Saturated concentration in air
(g/m3 at 20°C) 22.8 Verschueren, 1983
Volatility (mmHg at 20°C) 3.1; 7.76 Riddick et al., 1986
Vapour pressure (mmHg)
at 39°C 10 OHM/TADS, 1989
at 75°C 60
Table 1. (contd)
Property Value References
Henry's law constant (atm.m3/mol) 3.2 × 10-7 Singh et al., 1984
Surface tension (dyne/cm) 28.1 at 30°C Dean, 1987
Heat of fusion (cal/g) 30.03-37.03 CHRIS, 1989; Weast et al., 1989
Heat of polymerization (cal/g) -257 CHRIS, 1989
Heat of combustion (cal/g) -327 at 25°C Weast et al., 1989
Heat of vaporization (cal/g) 10.955 Weast et al., 1989
Activated carbon absorbability (g/g) 0.129 Verschueren, 1983
Partition coefficient (log Kow at 20-25°C) 0.161-0.46 Korenman & Lunicheva, 1972; GEMS, 1983; Hansch & Leo, 1987;
BASF, 1988
Dissociation constant (pKa at 25°C) 4.25 Weast et al., 1989
Critical temperature (°C) 342 CHRIS, 1985
Critical pressure (atm) 57 CHRIS, 1985
Solubility: in water and most organic completely Dean, 1987; Sax & Lewis, 1989; Weast et al., 1989
solvents (alcohol, chloroform, benzene) miscible
Refractive index (nD20-25) 1.4224-1.4185 Kirk-Othmer, 1984
Maximum absorption (nm, in methanol) 252 Weast et al., 1989
2.2.2 Chemical properties
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according to
supplier's recommendations. Heating can cause vigorous polymerization
in some circumstances. Acrylic acid reacts readily with free radicals
and electrophilic or nucleophilic agents (Kirk-Othmer, 1984). It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of an inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C (CHRIS, 1989). The mechanism of auto-
accelerating polymerization of acrylic acid in hexane-methanol
solution, which can become explosive, has been studied by Bretherick
(1985).
Acrylic acid rapidly decomposes in the atmosphere by
photochemical attack on the double bond (NLM, 1989; OHM/TADS, 1989).
Acrylic acid is corrosive to many metals but not to stainless
steel or aluminium (Kirk-Othmer, 1984; AAR, 1987).
2.3 Conversion factors
In air:
1 ppm = 3.0 mg/m3 (NLM, 1989)
1 mg/m3 = 0.33 ppm (NLM, 1989)
2.4 Analytical methods
2.4.1 In air
A summary of methods for the detection of acrylic acid in air is
given in Table 2.
Table 2. Methods for the analysis of acrylic acid in air
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples absorbed GC on a glass column FID 33 mg/ml acetone The method is Vincent &
on silica gel treated packed with 1% FFAP (lower) to significantly Guient, 1982
with p-methoxyphenol on Chromosorb T 2084 mg/ml acetone affected by high
followed by desorption (upper); this is humidity. Samples
with acetone (94% equivalent to can be stored for
recovery) concentrations ranging up to 11 days at
from 0.5 ppm to 30 ppm room temperature or
(1.5-90 mg/m3) of under refrigeration
acrylic acid in a without affecting
48-litre sample volume recovery. Recommended
as useful for
determining acrylic
acid in the
occupational
environment
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples Reverse phase UV detector 1 g per sample; The sensitivity of OSHA, 1981
collected by HPLC 210 nm assuming 24 litre the analytical
drawing a known sample volume, this method permits
volume of air column: 25 cm × is equivalent to sampling times
through two 4.6 mm i.d. 0.042 mg/m3 as short as
XAD-8 sampling stainless steel (0.014 ppm) 15 min. Under
tubes connected column packed conditions of
in series, with Zorbax 8 m this procedure,
followed by ODS-bound, spherical the possibility
desorption with silica particles of interference
methanol/water from acetaldehyde,
(1:1) mobile phase: acetic acid,
96:4 (V/V) acrylamide,
water/acetonitrile acrolein,
containing 0.1% by acrylonitrile,
volume phosphoric methacrylic
acid; flow rate: acid is excluded.
1 ml/min; injection Method recommended
volume: 25 litre and fully validated
retention by OSHA for acrylic
time:6 min acid determinations
in workplace air
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air is pumped HPLC equipped Conductivity 1 mg/m3 air The method is Simon et
through a florisil with Aminex HPX detector (10 litre rapid, easy and al., 1989
tube at a rate of OFH organic acid sample volume) appears suitable for
1 litre/min. The analysis column the determination
sorbent is mixed (300 mm × 7.8 mm). of acrylic acid
with water (5 ml) Eluent, 2.5 × 10-4 when present in
and 1N H2SO4 (10 µl) M benzoic acid industrial emissions
prior to injection is pumped at containing other
to the chromatographic 0.8 ml/min aliphatic acids
system
a FID = Flame ionisation detector
Air samples are collected on silica gel treated with
p-methoxyhydroquinone used as an inhibitor of polymerization
(Vincent & Guient 1982) or on XAD-8 sampling tubes (OSHA, 1981). XAD-8
sampling tubes contain solid sorbent, i.e. acrylic ester polymer, of
16-50 mesh (OSHA, 1981).
After separation with gas chromatographic (GC) technique or
reverse phase high performance liquid chromatography (HPLC), flame
ionization detection (Vincent & Guient 1982) or UV detection at 210 nm
(OSHA, 1981) are utilized, respectively. The latter method was
modified and recommended by OSHA as a fully validated method for the
determination of acrylic acid in workplace air (OSHA, 1981). This
method, when coupled with an ion suppression technique, proved
successful for the retention and separation of acrylic acid.
A retention time of approximately 6 min is obtained with a Dupont
Zorbax ODS 8-µm silica packed column and a water/acetonitrile (96:4)
mobile phase containing 0.1% (by volume) phosphoric acid. The
phosphoric acid serves to suppress the ionization of acrylic acid
resulting in the retention of the undissociated form of the molecule.
Under these conditions acrylic acid is separated from potential
interfering substances: methacrylic acid, acrylamide, acrolein,
acrylonitrile and acetic acid. Propanoic acid, a saturated precursor
of acrylic acid, can be resolved from acrylic acid in a 13-min
analysis at 1 ml/min flow rate using a 0.1% aqueous phosphoric acid
mobile phase. Detection of acrylic acid at 210 nm is approximately 100
times more sensitive than that of propanoic acid, owing to the
unsaturated nature of acrylic acid. This method permits the detection
of acrylic acid in the presence of very high levels of propanoic acid.
A third method utilizes high-performance ion-exclusion
chromatography with conductimetric detection (Simon et al., 1989). The
use of 2.5 × 10-4 M benzoic acid as the mobile phase in this method
allows the separation of acrylic acid from propionic acid and other
aliphatic acids.
2.4.2 In industrial effluents
A gas chromatographic method has been developed for the analysis
of acrylic acid and some other related pollutants present in small
quantities in the effluent from a methyl acrylate plant in India
(Singh & Thomas, 1985). In this method, effluent samples were injected
directly to the GC system without prior extraction or concentration. A
Porapak Q (4 feet × 1/8 inch I.D.) column and a FID were utilized in
this method. The experimental parameters for the analysis are: column
temperature, 165°C; injector and detector temperature, 250°C; carrier
gas, N2 at 50 ml/min; hydrogen pressure, 1.3 kg/cm2; air pressure,
2.2 kg/cm2 and injection volume, 1-10 µl. The method was found to be
sensitive for detecting acrylic acid at concentrations as low as
1 ppm.
2.4.3 In polyacrylate materials
A differential pulse polarographic method was used for the
determination of residual acrylic acid in sodium polyacrylate
polymeric systems (Husain et al., 1991). The method has the advantage
of analysing acrylic acid in trace quantities directly without
resorting to separation techniques. Sample solutions of the tested
polymers were extracted with N,N-dimethylformamide several times and
the extraction mixture was made up to 25 ml, with the solvent
tert-butyl ammonium iodide (0.02 M) in N,N-dimethylformamide
serving as the supporting electrolyte. The polarographic measurements
were performed with a Metrohm E-506 Polarecord equipped with a three-
electrode system (a dropping mercury electrode (DME), Ag/AgCl
(saturated KCl) reference electrode, and an auxiliary platinum
electrode). Using this method, free acrylic acid in polymers at levels
of 10-100 ppm can be measured with a precision of ± 3%.
2.4.4 In biological samples
Methods for the analysis of acrylic acid in aqueous samples and
tissues extracts in metabolic studies have been reported (Mao et al.,
1994; Mitchell & Petersen, 1988; Black et al., 1995). In these
methods, high-performance ion-exclusion chromatography and/or reverse
phase HPLC with radiometric, refractive index, photo diode-array and
UV detectors were used for the separation and quantification of
acrylic acid.
In another study, residues of acrylic acid in an anaerobic
degradation mixture were quantified using a gas chromatographic
technique with a flame ionization detector (FID) (Stewart et al.,
1995). The column was an 80/120 Carbopak B-DA/4% Carbowax 20 M. The
column and FID temperatures were 175 and 200°C, respectively. The
carrier gas was helium at a flow rate of 24 ml/min. The detection
limit was 1 mg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acrylic acid has been reported to occur naturally in the
following species of marine algae: 9 species of Chlorophyceae, 10
species of Rhodophyceae and 11 species of Phaeophyceae (Sieburth,
1960, Glombitza, 1970a,b, 1979). It is also produced in
Phaeodactylum tricornutum, Phaeocystis spp. and Polysiphonia
lanosa (Brown et al., 1977), as a result of hydrolysis of dimethyl-ß-
propiothetin (Verschueren, 1983).
Acrylic acid has been identified as an antibacterial substance in
oysters (Brown et al., 1977), scallops, ( Patinopecten yessoensis)
(Kodama & Ogata, 1983), and the digestive tract of penguins (Sieburth,
1960; Herwig 1978). It is thought to originate from the phytoplankton
Protogonyaulax (Kodama & Ogata, 1983), Phaeocystis spp (Sieburth,
1960) and Phaeodactylum tricornutum (Brown et al., 1977) on which
the molluscs and penguins fed. It has also been shown that under
natural conditions acrylic acid is generated by certain species of
algae and acts as a microbiocide (Glombitza, 1970a,b, 1979; Heyser &
Glombitza, 1972). It has also been identified as the agent responsible
for the antimicrobial activity of the marine algae Gracilaria
corticata and Ulva lactuca (Bandara et al., 1988).
Acrylic acid has been found in the rumen fluid of sheep as a
result of bacterial fermentation of carbohydrates (Noble & Czerkawski,
1973), where it is converted by rumen microorganisms to propionic acid
(Whanger & Matrone, 1967). It can also be produced from lactic acid by
the anaerobic rumen bacterium Megasphaera elsdenii in the presence
of 3-butynoic acid (Sanseverino et al., 1989).
Acrylic acid has been found in agricultural rum obtained by
fermentation of sugarcane juice by the action of Micrococci spp
(Ganou-Parfait et al., 1988).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
The first commercial process for the manufacture of acrylic acid
and its esters involved hydrolysis of ethylene cyanohydrin in sulfuric
acid. This route is no longer commercially significant (Kirk-Othmer
1984).
Most commercial acrylic acid is now produced via a process in
which propylene is vapour-oxidized to acrolein, which is in turn
oxidized at 300°C with molybdenum-vanadium catalyst to acrylic acid
(NLM, 1989). Other methods of production are as follows:
* a modification of the Reppe process by the reaction of acetylene,
carbon monoxide and alcohol with a nickel catalyst;
* by hydrolysis of acrylonitrile;
* condensation of ethylene oxide with hydrocyanic acid followed by
reaction with sulfuric acid at 160°C;
* a process in which formaldehyde undergoes a type of aldol
reaction with a large molar excess of acetic acid in the vapour
phase in a catalyst tube containing calcium Decalso (Kirk-
Othmer, 1984);
* a heterolytic dehydration pathway of lactic acid in supercritical
water (Mok et al., 1989).
3.2.1.2 Impurities
Commercial acrylic acid is available in two grades: technical and
glacial. Glacial grade is 98-99.5% acrylic acid (NLM, 1989). This may
contain, as impurities, water up to 0.3% w/w and acrylic acid dimer up
to 0.1% w/w (BASF, 1992; Elf Atochem, 1992).
3.2.1.3 Other sources
Acrylic acid has also been detected in trace amounts in
commercial propionic acid (Kostanyan et al., 1969).
3.2.1.4 Production data
Available data on the production of acrylic acid are shown in
Table 3.
Table 3. Production data
Country Year Production of Reference
acrylic acid
(in kilotonnes)
China 1994 105 CEFICA (1995)a
European Community 1975 155 IARC (1979)
1994 665 CEFIC (1995)a
Japan 1976 70 IARC (1979)
1994 420 CEFIC (1995)a
Korea 1994 60 CEFIC (1995)a
Taiwan 1994 50 CEFIC (1995)a
USA 1993 332 US ITC (1983)
1985 361 US ITC (1985)
1986 348 US ITC (1986)
1987 499 US ITC (1987)
1988 480 US ITC (1988)
1991 554 NLM (1991)
1994 685 CEFIC (1995)a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union Chemicals risk
assessment document.
The worldwide production of acrylic acid was approximately
1.13 million tonnes in 1991 (Chemical Marketing Reporter, 1992).
Worldwide capacity for acrylic acid production was reported to be
2 million tonnes in 1994 (CEFIC, 1995).
3.2.2 Experimental production of acrylic acid by bacterial isolates
The following bacterial species have been utilized in
experimental systems to produce acrylic acid:
* from acrylonitrile: (1) by the action of epsilon-caprolactum-
induced Rhodococcus rhodochrous J1 (Nagasawa et al., 1990);
with a periodic substrate feeding system the highest accumulation
(390 g/litre) was obtained; (2) by Arthrobacter sp. isolated
from petrochemical industry waste (Narayanasamy et al., 1990).
* from acrylamide by the action of Pseudomonas sp. and
Xanthomonas maltophilia isolated from herbicide-contaminated
soils (Nawaz et al., 1993, 1994); batch culture of these bacteria
completely degraded 62.8 mM acrylamide to acrylic acid and
ammonia in 24 and 48 h, respectively.
3.2.3 Uses
Acrylic acid is used primarily: as a starting chemical for ethyl
acrylate, n-butyl acrylate, methyl acrylate, 2-ethylhexyl acrylate;
as a monomer for polyacrylic acid and salts, cross-linked high (and
low) molecular weight polymers; as a co-monomer with acrylamide for
polymers used as flocculants; with ethylene for ion-exchange resin
polymers; with methyl ester for polymers; and with methylene succinic
acid (itaconic acid) for other co-polymers (SRI, 1981; NLM, 1989).
In 1987, 25% of the acrylic acid produced in the USA was used for
surface coatings; 20% for polyacrylic acid and salts, including super-
absorbent polymers, detergents, water treatment and dispersants; 13%
for textiles and non-wovens; and 9% for adhesives and sealants
(Kavaler, 1987).
Until 1979, in the European Union countries more than 80% of
acrylic acid was used for the production of polyacrylates and in Japan
90% was used in the production of acrylic esters (IARC, 1979). In
1988, European use of acrylic acid was 69% for esters, 10% for
detergents, 8.5% for flocculants and dispersants and 6.5% for super-
absorbers (CEFIC, 1995).a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Other uses are in the production of copolymers for dental
adhesives (Bowen, 1979), in the production of hydrogels used for
contact lenses (Kirk-Othmer, 1984), in surface coating formulations
(Kirk-Othmer, 1984), and in latex applications to increase stability
in order to prevent premature coagulation (Kirk-Othmer, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Acrylic acid is miscible with water (Riddick et al., 1986) and
therefore would not be expected to adsorb significantly to soil or
sediment (Lyman et al., 1982). The Henry's Law constant for acrylic
acid is reported to be 3.2 × 10-7 atm m3/mol (Singh et al., 1984).
Under soil conditions, chemicals with such low Henry's Law constants
are essentially non-volatile (Lyman et al., 1982). However, the vapour
pressure of acrylic acid suggests that it volatilizes from surface and
dry soil (Howard, 1991).
The adsorption and desorption of acrylic acid were examined in
five different soils: an aquatic sandy loam sediment, a loamy sand, a
clay loam and two loams. The average Koc for the adsorption of
acrylic acid to soil was 43, and ranged from 23 to 63. The Koc values
for the desorption data were widely scattered with values ranging from
18 to 837. This indicates that the degree of adsorption is not
correlated to the organic carbon content (OC), which ranged from 0.46%
for the loamy sand to 4.58% for one of the loams. The results of this
study indicate a high mobility of acrylic acid through soil (Archer &
Horvath, 1991).
Using the fugacity model of Mackay & Peterson (1981) the
theoretical distribution of acrylic acid has been estimated. About 97%
of acrylic acid released to the environment should be associated with
the aquatic environment (the water phase), approximately 1.6% in air,
1% in sediment and < 1% in soils, suspended solids and biota
(Staples, 1993).
Since the atmospheric lifetime of acrylic acid is less than one
month (Atkinson, 1987), there is no potential for long-range transport
of this compound.
4.2 Transformation
4.2.1 Abiotic degradation
The UV absorption band of acrylic acid extends to about 320 nm
(Weast & Astle, 1985). Vapour phase acrylic acid reacts with
photochemically produced hydroxyl radicals primarily by addition to
the double bond and with atmospheric ozone, resulting in an estimated
overall half-life of 6.6 h to 6.5 days (Atkinson & Carter, 1984).
Based upon the estimated rate constant for vapour phase reactions and
assuming hydroxyl radical concentrations of 5 × 105 radicals per cm3
and an ozone concentrations of 7 × 1011 molecules per cm3 (Atkinson
and Carter, 1984; Atkinson, 1987), a half-life of 2.5-23.8 h was
estimated by Howard et al. (1991).
Acrylic acid was found to be stable to hydrolysis at pH values
between 3.7 and 11 (Shah, 1990).
4.2.2 Biodegradation
4.2.2.1 Aerobic biodegradation
When added to water, acrylic acid is rapidly oxidized, and
wastewater containing the compound can deplete reservoirs of oxygen
(Ekhina & Ampleeva, 1977).
Several biodegradability studies show that acrylic acid will
readily biodegrade (Lyman et al., 1982; Keystone Environmental
Resources, 1989a; Douglas & Bell, 1992). The BOD5 (biological
oxygen demand, 5 days) for glacial acrylic acid, using acclimated,
fresh dilution water and raw sewage from a local treatment plant as
the inoculum, was determined to be 0.315 g of oxygen consumed per gram
of product. The COD (chemical oxygen demand) under the same conditions
was 1.48 g/g (Keystone Environmental Resources, 1989)a; therefore,
the BOD5/COD ratio was 0.21. A BOD5/COD ratio of 0.26 was also
reported by Lyman et al. (1982). Biodegradation of acrylic acid in a
14-day BOD test was up to 68% (CITI, 1992). Acrylic acid at a
concentration of 3 mg/litre attained 81% biodegradation within 28 days
in a closed-bottle test based on the consumption of oxygen (Douglas &
Bell, 1992). The pass level of 60% was reached within 10 days of
exceeding the 10% level, and so acrylic acid is considered to be
"readily biodegradable" according to EC classification criteria (EEC,
1988).
The metabolism of 14C-acrylic acid in sandy loam soil has been
studied under aerobic conditions for up to 28 days after treatment at
a rate of 100 mg/kg. Acrylic acid was rapidly metabolized; after 3
days no acrylic acid was detected in soil extracts. Carbon dioxide
evolution accounted for 72.9% of applied radioactivity by day 3 and a
total of 81.1% over the 28-day study period. The half-life for acrylic
acid under these conditions was estimated to be less than 1 day
(Hawkins et al., 1992).
Acrylic acid formed from hydrolysis of acrylamide added to soil
was totally degraded within 15 days of its formation (Nishikawa et
al., 1979). In a 42-day screening study using a sewage seed inoculum,
71% of acrylic acid was mineralized under aerobic conditions. After
previous acclimatization, 81% of acrylic acid degraded to carbon
dioxide in 22 days (Pahren & Bloodgood 1961; Chou et al., 1978).
a Report sent by J.M. Flaherty to J. McLanghlin, Rohm and Haas
Spring House (work order numbers M8903002 and M8902005).
A collection of strains utilizing acrylonitrile, acrylamide and
acrylic acid as sole carbon and/or nitrogen source was isolated from
environmental samples. Strains with maximum decomposing activity were
identified as Pseudomonas pseudoalcaligenes 6p; P.alkaligenes 5g
and Brevibacterium spp. 13 PA (Moiseeva et al., 1991).
An aerobic gram-negative bacterium ( Pseudomonas sp.) isolated
from tropical garden soil was found to be able to degrade a high
concentration of acrylamide (4 mg/litre) to acrylic acid and ammonia,
which were utilized as sole carbon and nitrogen sources, respectively,
for growth (Shanker et al., 1990).
A strain of Byssochlamys sp. produced ß-hydroxypropionic acid
(ß-HPA) when grown on media containing high concentrations of acrylic
acid. The maximal production of ß-HPA was 4.8% when the initial
culture medium contained 7% acrylic acid and 2% glucose and the
initial culture pH was adjusted to 7.0 (Takamizawa et al., 1993).
Acrylic acid has been reported to be significantly degraded
(> 30%) in the MITI test, a biodegradability screening test of the
Japanese Ministry of International Trade and Industry (Sasaki, 1978).
Acrylic acid was completely degraded in a standard Zahn-Wellens test
and the authors concluded that it is biodegradable (BASF, 1993).
Acrylic acid has been found to be degraded by a strain of
Alcaligenes denitrificans isolated from a landfill soil. The
bacterium degraded acrylic acid through the intermediate formation of
L-(+)-lactic and acetic acids, which were further metabolized
(Andreoni et al., 1990).
4.2.2.2 Anaerobic biodegradation
Speece (1983) reported that acrylic acid can undergo ultimate
anaerobic biodegradation. In an anaerobic screening study utilizing
10% sludge from a secondary digester as an inoculum, acrylic acid was
judged to be degradable, with over 75% of theoretical methane being
produced within 8 weeks of incubation (Shelton & Tiedje, 1984).
In another study, acrylic acid was toxic to unacclimated
anaerobic acetate-enriched cultures and was poorly utilized (21%) in a
completely mixed anaerobic reactor with a 20-day hydraulic retention
time after a 90-day acclimatization period (Chou et al., 1978). A
possible explanation for the conflicting results of anaerobic
degradation is the observation that acetate cultures have to exhaust
the acetic acid as carbon and energy source before they can utilize a
cross-fed compound (Chou et al., 1978).
The biodegradability of acrylic acid using methanogenic acetate
enrichment culture was studied by Stewart et al. (1995). Acrylic acid
was degraded with almost no effect on methanogens with spikes up to
100 mg/litre. However, concentrations of 500, 1000 and 1500 mg/litre
were found to inhibit the methanogens for several days before
recovery. Acrylic acid was eventually degraded to less than 1 mg/litre
(> 99% of initial concentration) in all cases by the end of the
study (55 days).
4.2.3 Bioaccumulation and biomagnification
From the low value for log Kow, ranging from 0.161 to 0.46
(Hansch & Leo, 1987; BASF, 1988), one would expect the
bioconcentration of acrylic acid in organisms to be negligible Bysshe
(1990) using a regression equation calculated theoretical
bioconcentration factors ranging from 0.78 to 1.3. Veith et al. (1979)
estimated the bioconcentration factor to be in the range of 1.6 to
2.4.
There have been no reports of biomagnification of acrylic acid in
the food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
No quantitative data are available for environmental levels of
acrylic acid in ambient air, water or soil. Acrylic acid has been
found to occur naturally in some marine algae (Sieburth, 1960; Brown
et al., 1977) and some molluscs (Kodama & Ogata, 1983). The acrylic
acid content of Phaeocystis spp. can be 7.4% of dry weight
(Sieburth, 1960). Other marine algae have been found to contain
acrylic acid: Chlorophyceae, 0.124-16.5 mg/g dry weight;
Rhodophyceae, 0-0.131 mg/kg dry weight; and Phaeophyceae 0-0.02 mg/g
dry weight (Glombitza, 1970a, 1979).
5.2 General population exposure
No data are available for general population exposure. However,
consumers may be exposed to unreacted acrylic acid in the following
household goods: polishes, paints and coatings, adhesives, rug
backing, plastics, textiles and paper finishes (USEPA, 1981).
Information on the typical content of unreacted acrylic acid in these
kinds of products is unavailable.
Populations living in the vicinity of plants producing acrylic
acid or manufacturing its esters or polymers may be exposed to acrylic
acid in the ambient air. The concentrations of emitted vapours of
acrylic acid in the plume from such plants were found to vary from 22
to 183 mg/m3 (Grudzinski, 1988). However, there are no data on
concentrations of acrylic acid in the ambient air of populated areas.
Acrylic acid occurs in wastewater effluents from its production
by the oxidation of propylene at concentrations not exceeding
0.5 mg/litre (Wise & Fahrentholdt, 1981). After treatment of
wastewater from a production facility in Europe, acrylic acid levels
were below the limit of detection (0.1 mg/litre) (CEFIC, 1995)a.
However, effluent from a methyl acrylate plant in India was found to
contain 2500 mg/litre as acrylic acid (Singh & Thomas, 1985).
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Since there is evidence that acrylic acid esters are hydrolysed
to acrylic acid in laboratory animals (Ghanayen et al., 1987) and in
human tissues in vitro (Wiegand, 1990), a potential source of
internal exposure to acrylic acid may result from metabolism of
absorbed acrylic acid esters (Frederick et al., 1994; Sanders et al.,
1988).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure is the most important means of human
exposure to acrylic acid. Inhalation and contact with skin are
important routes of exposure.
The National Institute of Occupational Safety and Health (NIOSH)
conducted two observational nationwide surveys, a decade apart, to
determine the extent of exposure of workers to a variety of substances
in their work environment. The National Occupational Hazard Survey
(NOHS) was conducted during 1972-1974 using a stratified probability
sample of 4636 businesses in 67 metropolitan areas throughout the USA
employing nearly 900 000 workers (NIOSH 1974, 1977). According to the
NOHS, an estimated 28 600 workers were potentially exposed to acrylic
acid, approximately 10% of whom were exposed to acrylic acid and 90%
to trade-name products. However, this estimation excluded the exposure
of the general population to trade-name chemicals possibly containing
acrylic acid. Acrylic acid was seen in 16 major industry groups and in
41 occupational groups in the NOHS.
During 1981-1983 NIOSH conducted the National Occupational
Exposure Survey (NOES), using a stratified probability sample of 4490
businesses in 98 different geographic locations of the USA employing
nearly 1.8 million workers (NIOSH, 1990). According to the NOES, an
estimated 96 500 workers were potentially exposed to acrylic acid,
approximately 10% of whom were exposed to acrylic acid and 90% to
trade-name products. Acrylic acid was seen in 25 major industry groups
and in 67 occupational groups (NIOSH, 1990).
One study conducted at a large manufacturing facility of the Rohm
and Haas Company in the USA, where several chemicals including acrylic
acid and a variety of acrylates and methacrylates were used, indicated
that ethyl acrylate and acrylic acid levels varied from 0.01 to
56 ppm. Most areas of the plant had levels (as 8-h time-weighted
averages) well below the hygiene standards recommended at that time by
the OSHA and the ACGIH of 10 ppm for acrylic acid and 5 ppm for ethyl
acrylate (ACGIH, 1988, OSHA, 1989). Many of the available industrial
hygiene data were specific for high, short-term exposure tasks
(5-40 min samples) when chemicals were pumped into containers for
shipping or when lines were open for new connections, or to obtain
samples. They revealed levels at the high end of the above-mentioned
range (Schwartz et al., 1989).
Exposures of workers to acrylic acid for short periods of time of
less than 15 min and for full shift expressed as time-weighted average
(TWA) concentration have been compiled from four producing companies.
Operators had a mean short-term exposure limit (STEL) value of
8.4 mg/m3 (range < 0.3 to 189 mg/m3); loading/unloading operations
a mean of 3.9 mg/m3 (range 1.2 to 12 mg/m3); and those engaged in
quality assurance a mean of 0.3 mg/m3 (range of less than 0.3 to
0.6 mg/m3). Concerning the 8-h TWA, the operators showed levels of
0.48 mg/m3 (range of 0.03 to 3 mg/m3) and loading/unloading
operations a mean of 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3)
(Casciery & Clary, 1993).
No such data are available from other countries.
6. KINETICS AND METABOLISM
6.1 Human studies
Apart from in vitro skin absorption studies, no data are
available on kinetics, metabolism or elimination or acrylic acid in
humans.
The absorption of 14C-acrylic acid (site of label unspecified)
dissolved in acetone, water or phosphate buffer (pH 6.5) was tested
using samples of excised (postmortem) human and mouse skin in vitro
(Corrigan & Scott, 1988). Acrylic acid concentrations of 0.01, 0.1,
1.0 and 4.0% were applied at 100 µl/cm2 under occlusive conditions.
Samples were taken from the receptor fluid up to 32 h. Rates of
absorption decreased in the order of magnitude as follows: acetone >
water > phosphate buffer. Independent of the vehicle, the absorption
rate increased as a function of acrylic acid concentration.
Permeability coefficients, which ideally are concentration-independent
expressions of absorption rate, for human skin ranged from 0.37 to
0.72 × 10-3 cm/h for water and from 0.47 to 1.81 × 10-4 cm/h for
phosphate buffer. Permeability coefficients were not calculated for
acetone because of evaporation of this volatile vehicle during the
course of the experiments (Corrigan & Scott, 1988).
A briefly reported in vitro percutaneous penetration study
using excised human cadaver skin indicated that 14C-acrylic acid
absorption can vary significantly as a function of pH and delivery
vehicle. In vitro flux, estimated after a 1 mg dose was applied,
varied by 600 times within the treatments studied and decreased in the
order: acetone (600 µg/cm2 per h) > phosphate buffer pH 6.0
(23 µg/cm2 per h) > ethylene glycol (15 µg/cm2 per h) > phosphate
buffer pH 7.4 (1 µg/cm2 per h) (D'Souza and Francis, 1988).
6.2 Studies on experimental animals
6.2.1. Absorption, distribution and excretion
6.2.1.1 Oral exposure
After oral gavage administration of an aqueous solution of
(1-11C)-acrylic acid (26 µg/kg body weight) to female Sprague-Dawley
rats, it was rapidly absorbed and expired mainly as 11CO2 within 1 h
post-administration. The uptake appeared biphasic. The short alpha-
phase had an apparent first-order absorption constant (Ka) of 19% of
the available dose per minute (biological half-time = 3.6 min) and the
Ka of the ß-phase was 30% (biological half-time = 23 min). Relative
retention of radiolabel (dpm per g tissue versus dpm per g body
weight) after 65 min was above unity in liver (2.6), adipose tissue
(1.9), small intestine (1.5), kidneys (1.2) and spleen (1.0).
Approximately 6% of the radiolabel was excreted in the urine within
65 min (Kutzman et al., 1982).
In another study, single gavage doses of 4, 40 or 400 mg/kg body
weight of (2,3-14C)-acrylic acid in 0.5% aqueous methylcellulose
solution were administered to male Sprague-Dawley rats. Approximately
35, 55 and 60%, respectively, of the administrated dose were
eliminated, mostly as 14CO2, within 8 h. By 24 h 50-65% of the dosed
radioactivity was eliminated and the excretion of radioactivity had
virtually ceased. After 72 h, 44-65% of the administrated
radioactivity had been eliminated as 14CO2; 2.9-4.3% in urine, 2.4-
3.6% in the faeces and 18.9-24.6% remained in the tissues examined
(liver, stomach, muscle, blood, plasma, adipose tissue). The residual
radioactivity was highest in the adipose tissue (9-15%), followed by
muscle (6.5-7.5%) and liver (1.7-2.2%) (De Bethizy et al., 1987).
The disposition of (1-14C)-acrylic acid was also determined in
male Sprague-Dawley rats following oral administration by gavage in
water at 400 mg/kg body weight. Excretion of acrylic acid-derived
radioactivity was determined by collection of urine, faeces and
expired air for 72 h following administration. The predominant route
of excretion was in the expired air with approximately 80% of the
radioactivity exhaled as 14CO2 within 24 h and 83.2% after 72 h.
Elimination of radioactivity as exhaled volatile organic compounds was
negligible (less than 0.5% of the radiolabel). Within 24 h of dose
administration, excretion of radioactivity accounted for 5.0% in the
urine and 8.8% of the radiolabel in faeces. Tissue concentrations of
radioactivity after 72 h were generally low: 0.4% of the total dose in
the liver, 0.39% in muscle and 0.18% in skin (Winter & Sipes, 1993).
A comparative bioavailability and disposition study in male
Fischer-344 rats and male C3H mice after a single administration of
(1-14C)-acrylic acid (40 or 150 mg/kg body weight in water) by gavage
has been conducted. This study confirmed that acrylic acid is rapidly
absorbed and metabolized. In rats and mice about 80-90% of the dose
was exhaled as 14CO2 within 24 h (Black et al., 1995). In rats,
excretion of radiolabel in urine and faeces within 72 h accounted for
< 5% and < 1% of the dose, respectively. Elimination of
radioactivity in rats as exhaled organic volatile compounds was less
than 0.5% of the radiolabel. Similar patterns were observed in male
mice (Black et al., 1995).
6.2.1.2 Inhalation exposure
A tissue distribution study has been conducted in 39 female
Sprague-Dawley rats nose-exposed to (1-11C)-acrylic acid vapour for
1 min (concentration not indicated). Radioactivity was widely
distributed; 90 seconds after exposure 18.3% of the delivered dose
remained in the rats. Approximately 28.0% of this radioactivity was
associated with the snout and 42.9% of the radioactivity was found in
the head; this was considered to be solubilized in the mucous of the
turbinates and the nasopharynx. After 65 min, the activity in the
snout was reduced to 8.1% and approximately 60% of the label was
expired as 11CO2. The elimination of labelled CO2 appeared to be
biphasic, with a half-time of approximately 30.6 min during the
alpha-phase. The amount of radioactivity retained in liver and fat
increased markedly between 1.5 and 65 min post-exposure (Kutzman et
al., 1982).
6.2.1.3 Dermal exposure
In one in vitro experiment, the dermal penetration capacity of
(1-14C)-acrylic acid was tested using excised mouse skin. Skin slices
were treated in a diffusion chamber with 0.01, 0.1, 1 and 4% (w/v)
100 µl/cm2 of acrylic acid dissolved in acetone, water or phosphate
buffer pH 6.5. The results were comparable with the study performed on
excised human skin (section 6.1). Permeability coefficients for mouse
skin were 0.96-1.73 × 10-3 cm/h for water and 1.91-3.1 × 10-4 cm/h
for phosphate buffer. The permeability coefficients and steady-state
absorption rate data indicate that mouse skin is approximately three
times more permeable than human skin to acrylic acid (Corrigan &
Scott, 1988). This difference may not be biologically significant.
In a briefly reported study, male Sprague-Dawley rats were
administered dermally 5 mg 14C-acrylic acid per kg body weight
(D'Souza & Francis, 1988). Phosphate buffer of pH 6 or 7.4 or acetone
was used as a formulating agent. In each case the formulation was
applied to the shaved back of the rats and covered with a glass
chamber. The rate of appearance of 14CO2 measured at 0.5, 1, 2, 4,
8, 16 and 24 h after application was used as a measure of the
absorption rate of acrylic acid. The absorption rate was dependent on
the vehicle and decreased in the following order, acetone > phosphate
buffer of pH 6 > phosphate buffer of pH 7.4. Cumulative absorption
after 24 h was 22% from acetone, approximately 19% from phosphate
buffer of pH 6, and 9% from phosphate buffer of pH 7.4. The results of
the in vivo investigations were comparable to those of the in
vitro studies obtained by the same authors (D'Souza & Francis, 1988).
The disposition of (1-14C)-acrylic acid was determined in male
Sprague-Dawley rats after topical application of 100 µl of a 4% (v/v)
solution of acrylic acid in acetone to an area of 8.4 cm2 of the skin
(501 µg/cm2) using a skin-mounted, charcoal-containing trap covered
with fixed aluminium discs to ensure complete recovery of the label.
Excretion of acrylic-acid-derived radioactivity was determined by
collection of urine, faeces and expired air for 72 h following
administration of acrylic acid. Approximately 73% of the radioactivity
volatilized from the skin and was trapped in the charcoal sorbent.
After 72 h, 6% of radioactivity was detected at the site of
application in the skin or on the skin surface. Approximately 75% of
the absorbed dose, representing about 16% of the applied dose, was
exhaled as 14CO2 within 12 h. Excretion of radioactivity in the
urine accounted for approximately 9% of the applied radioactivity
(approximately 4% of the absorbed dose), the faeces containing only
negligible amounts of radioactivity. After 72 h, less than 0.4% of the
applied dose was retained in tissues other than skin (Winter & Sipes,
1993).
In another study, 1% (v/v) acetone solutions of 14C-acrylic acid
at doses of 10 or 40 mg/kg were applied to the clipped skin of the
shoulder region of male F-344 rats or male C3H/HeN Crl BR mice. A non-
occlusive "frame" device was cemented to the skin surface of animals
to allow for free evaporation of acrylic acid, which was trapped using
on-line volatile organic traps. Since this technique was inefficient,
activated-charcoal-impregnated filter paper sheets were placed
occlusively on the treated skin surface of a second high dose group of
animals to provide for absorption of evaporating acrylic acid (Black
et al., 1995). In rats, the reported 72-h recovery was low and ranged
from 50 to 60% of the applied dose. Evaporation accounted for most of
the applied acrylic acid, but approximately 26 and 19% of the applied
high and low doses were absorbed in rats within 72 h, respectively.
The major route of elimination of absorbed acrylic acid was via
exhalation of 14CO2 and accounted for 69.5 and 77% of the absorbed
low and high doses, respectively. Minimal faecal elimination of
absorbed acrylic-acid-derived radioactivity was reported (< 1%), and
tissues and carcasses contained approximately 2-3% of the absorbed
chemical at 72 h.
In mice, the 72-h recovery ranged from 61.5 to 84.0% of the
applied acrylic acid dose. As in the rat experiments, while most of
the applied acrylic acid was lost to evaporation, absorption accounted
for 11-12% of the applied dose. Exhalation of 14CO2 accounted for
83.5 and 77.7% of the absorbed high and low doses, respectively.
Elimination via other routes was negligible, and less than 1% of the
absorbed dose remained in the tissues and carcasses at 72 h (Black et
al., 1995).
6.2.1.4 Intravenous administration
Single i.v. doses of (1-14C)-labelled acrylic acid (10 mg/kg
body weight in phosphate-buffered saline) were given to male F-344
rats and male C3H/HeNCrlBR mice into the tail veins. In rats 63% of
the 14C-dose was eliminated as 14CO2 after 4 h and 68% after 72 h,
while almost no 14C was recovered as exhaled organic volatiles.
Tissue samples (liver, kidney and fat) and plasma contained 1.9% at 1
h, 0.4% at 8 h, and 0.2% at 72 h of the recovered dose. Overall the
recovery was 72.8 ± 10.8%. In mice, 51% of the radioactivity was
exhaled as 14CO2 over the 72-h collection period, the majority
exhaled in the first 4 h. The volatile radioactive fractions were
about 0.6% of the total dose. Overall, 55.7 ± 6.6% of this intravenous
10 mg/kg dose was recovered in mice (Frantz & Beskitt, 1993).
6.2.2 Metabolism
6.2.2.1 In vitro investigations
Oxidation of (2,3-14C)-acrylic acid was studied by incubating
acrylic acid with hepatic microsomal preparations obtained from male
Sprague-Dawley rats. No metabolites were detected by HPLC and acrylic
acid was recovered unchanged from the incubation mixture (De Bethizy
et al., 1987).
Results of the in vitro metabolism of (1-14C)-acrylic acid
incubated with freshly isolated hepatocytes and liver homogenates of
male F-344 rats or mitochondria isolated from liver homogenates of
male F-344 rats indicate that acrylic acid is rapidly metabolized to
14CO2. Addition of equimolar amounts of propionic acid, 3-hydroxy-
propionic acid or 3-mercaptopropionic acid caused a significant
inhibition of the oxidation of acrylic acid by isolated mitochondria.
A single major metabolite co-eluting with 3-hydroxypropionic acid was
found by HPLC analysis in the mitochondrial incubation mixtures. The
authors suggested that acrylic acid is metabolized in vitro by
mammalian enzymes to CO2 via 3-hydroxypropionate by the non-vitamin-
B12 - dependent pathway for propionate metabolism (Finch & Frederick,
1992).
The oxidation rate of acrylic acid in 13 different tissues
(liver, kidney, forestomach, glandular stomach, small and large
intestine, spleen, brain, heart, lung, skeletal muscle, fat and skin)
of male and female C3H/HeNCrlBR mice was measured by incubating tissue
slices with (1-14C)-acrylic acid and collecting 14CO2. All the
tissues studied oxidized acrylic acid to a certain extent, but
activity in kidney, followed by liver, was much higher than in other
tissues. Oxidation of acrylic acid followed pseudo-Michaelis-Menten
kinetics in the liver, kidney and skin, with a Km for all these
tissues of approximately 0.67 mM. Marked differences were observed in
the Vmax values, 2890 ± 436 nmol/h per g for kidney, 616 ± 62 nmol/h
per g for liver and 47.9 ± 5.8 nmol/h per g for skin. Half-lives in
these tissues were 0.13, 0.867 and 10.2 h, respectively. Lung,
glandular stomach, heart, spleen, fat and large intestine preparations
oxidized acrylic acid at rates from 10 to 40% of the rate determined
in the liver; in the remaining tissues reaction rates were less than
10% of those in the liver. Rates of metabolism in tissues from male
and female mice were similar.3-Hydroxypropionic acid was the only
metabolite detected by HPLC analysis following incubation of tissues
with (1-14C)-acrylic acid. To determine if CO2 was formed from the
C1 carbon, and if acetyl-CoA was derived from carbons 2 and 3 of
acrylic acid, the authors incubated (2,3-14C)-acrylic acid and
(1-14C)-acetate with liver and kidney slices and measured the rate of
14CO2 formation. It was concluded that CO2 originated from C1, but
that acetyl-CoA was derived from carbons 2 and 3 of acrylic acid. Both
substrates were oxidized well by the tissues, thus providing for the
complete metabolism of acrylic acid to CO2. The results demonstrate
that the rate of acrylic acid metabolism varies significantly among
mouse tissues and suggested that the kidneys and liver are major sites
of acrylic acid metabolism (Black et al., 1993).
6.2.2.2 In vivo investigations
After oral administration of (2,3-14C)-acrylic acid (4, 40 or
400 mg/kg body weight in 0.5% methylcellulose) to male Sprague-Dawley
rats, the major portion of the radioactivity (up to 65%) was exhaled
as 14CO2 within 24 h. In urine four metabolites were identified by
HPLC analysis. One of the two major metabolites eluted very near to
the solvent front and did not co-elute with acetic acid pyruvic acid
or lactic acid. The second metabolite co-eluted with 3-hydroxypro-
pionic acid. Traces of two other unidentified residues were also
detected. Radioactivity could not be detected at the retention times
corresponding to that of 2,3-epoxypropionic acid, glyceric acid or
N-acetyl- S-(2-carboxy-2-hydroxyethyl)-cysteine, suggesting that
acrylic acid is not epoxidized to 2,3-epoxypropionic acid in vivo.
It was suggested that acrylic acid was metabolized by the non-vitamin-
B12-dependent pathway for propionic acid metabolism, with degradation
to CO2 being the main route of elimination. Residual radioactivity in
tissues may be due to incorporation of 14C from acrylic acid into
acetyl-CoA (De Bethizy et al., 1987).
Using HPLC and NMR analysis, 3-hydroxypropionic acid, N-acetyl-
S-2-(2-carboxyethyl)-cysteine and N-acetyl- S-(2-carboxyethyl)-
cysteine-S-oxide were identified as urinary metabolites after oral
administration of (2,3-14C)-acrylic acid (400 mg/kg body weight in
water by gavage) to male Sprague-Dawley rats. According to the
authors, the detection of mercapturates may be a consequence of the
high dose used in this study (Winter et al., 1992).
HPLC analysis for acrylic acid and its metabolites in rats
revealed that a metabolite that coeluted with 3-hydroxypropionic acid
was found in the urine, plasma and liver of rats that had received
acrylic acid by gavage. Furthermore, a material that co-eluted with
authentic acrylic acid was detected in the urine and liver, but not in
the plasma, of these rats. Acrylic acid, but not 3-hydroxypropionic
acid, was also detected in the urine of rats after cutaneous
application (Black et al., 1995). In mice, 3-hydroxypropionic acid was
identified in the liver after gavage administration of acrylic acid.
No acrylic acid was detected in the liver of these animals (Black et
al., 1995).
6.2.2.3 Metabolic pathways
Acrylic acid is rapidly metabolized to CO2, a major metabolite
formed via acrylyl-CoA by the non-vitamin-B12-dependent pathway of
mammalian propionate catabolism (Finch & Frederick, 1992; Winter et
al., 1992; Black et al., 1993; Winter & Sipes, 1993). This pathway
occurs in the mitochondrion (Finch & Frederick, 1992) and consist of
reactions analogous to fatty acid ß-oxidation (Schultz, 1991).
ß-oxidation is the major route of propionate catabolism in many
invertebrates and plants (Wegner et al., 1968; Halarnkar & Blomquist,
1989); however the primary pathway of propionate catabolism in mammals
is that involving the vitamin-B12-dependent enzyme, methyl-malonyl-
CoA mutase (Black et al., 1993). A small amount of 3-hydroxypropionic
acid was identified as the major urinary metabolite of acrylic acid
(De Bethizy et al., 1987; Winter et al., 1992). There is no evidence
to suggest that epoxide intermediates are formed during the metabolism
of acrylic acid (De Bethizy et al., 1987). N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl) cysteine-
S-oxide were identified in the urine of rats that had received
400 mg/kg (2,3,-14C)-acrylic acid by gavage (Winter et al., 1992),
suggesting a direct reaction between acrylic acid and reduced
glutathione.
The major route of metabolism for acrylic acid esters has been
shown to involve the rapid cleavage of the ester bond by carboxyl
esterases (see Fig. 1) (Ghanayem et al., 1987; Sanders et al., 1988;
Frederik et al., 1994). Thus exposure to acrylic acid esters may
constitute a significant internal exposure to acrylic acid. A
secondary metabolic pathway involves conjugation of the acrylic acid
ester with glutathione to yield acetyl- S-(2-carboxyethyl) cysteine
alkylesters. (Ghanayem et al., 1987; Sanders et al., 1988). This
intermediate may be further metabolized to N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl)-cysteine-
S-oxide. However, it is currently uncertain what proportion of N-
acetyl- S-(2-carboxyethyl) cysteine, or its oxide, formed from the
metabolism of the acrylic acid esters originates from the reaction of
the intact ester with glutathione and what proportion originates from
the conjugation of the released acrylic acid with glutathione (see Fig
1).
On the basis of available information, proposed metabolic
pathways for acrylic acid are summarized in Fig. 1. The proposed
scheme also includes relationships between metabolism of acrylic acid
and its esters (e.g., ethyl acrylate) and metabolism of propionate via
the major vitamin-B12-dependent pathway.
Relative molecular mass: 72.06
2.1.2 Technical product
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins (IARC, 1979; CHRIS, 1989). Acrylic acid polymerizes easily when
exposed to heat, light or metals, and so a polymerization inhibitor is
added to commercial acrylic acid to prevent the strong exothermic
polymerization (NLM, 1989). The inhibitors that are usually used in
acrylic acid preparations are the monomethyl ether of hydroquinone
(methoxyphenol) at 200 ± 20 ppm, phenothiazine at 0.1% and
hydroquinone at 0.1%. Methylene blue at 0.5 to 1.0% and N,N'-
diphenyl- p-phenylenediamine at 0.05% can also be used (IARC, 1979;
CHRIS, 1989; OHM/TADS, 1989; BASF, 1993).
2.2 Physical and chemical properties
2.2.1 Physical properties
Acrylic acid is a colourless liquid at room temperature and
pressure (IARC, 1979; Windholz, 1983; CHRIS, 1985). It has an
irritating acrid odour and it is totally miscible with water and most
organic solvents. Some of the most important physical properties of
acrylic acid are summarized in Table 1.
Table 1. Physical and chemical properties of acrylic acid
Property Value References
Odour threshold concentration (mg/m3) 0.20-3.14 Fazzalari, 1978; Amoore & Hautala, 1983;
Ruth, 1986; Grudzinski, 1988; HSDB, 1989
Melting point (°C at 1 atm) 12.3-14.0 CHRIS, 1989; Weast et al., 1989
Boiling point (°C at 1 atm) 141.3-141.6 CHRIS, 1989; Weast et al., 1989
Flash point (°C)
open cup
closed cup 54.0-68.3 IARC, 1979; Kirk-Othmer, 1984; Sax & Lewis 1989;
46-48.5 Elf Atochem, 1992; BASF, 1994a
Autoignition temperature (°C) 390-446 IARC, 1979; HSDB, 1989; BASF, 1992; Elf Atochem, 1992
Flammable limits (%)
lower 28 HSDB, 1989
upper
Burning rate (mm/min) 1.6 CHRIS, 1989
Specific gravity (g/ml at 20°C) 1.0497-1.0511 IARC, 1979; CHRIS, 1989; Weast et al., 1989
Relative vapour density (air =1 at 20°C) 2.5 HSDB, 1989
Viscosity (mPa.s at 20°C) 1.22-1.30 BASF, 1992; Elf Atochem, 1992
Saturated concentration in air
(g/m3 at 20°C) 22.8 Verschueren, 1983
Volatility (mmHg at 20°C) 3.1; 7.76 Riddick et al., 1986
Vapour pressure (mmHg)
at 39°C 10 OHM/TADS, 1989
at 75°C 60
Table 1. (contd)
Property Value References
Henry's law constant (atm.m3/mol) 3.2 × 10-7 Singh et al., 1984
Surface tension (dyne/cm) 28.1 at 30°C Dean, 1987
Heat of fusion (cal/g) 30.03-37.03 CHRIS, 1989; Weast et al., 1989
Heat of polymerization (cal/g) -257 CHRIS, 1989
Heat of combustion (cal/g) -327 at 25°C Weast et al., 1989
Heat of vaporization (cal/g) 10.955 Weast et al., 1989
Activated carbon absorbability (g/g) 0.129 Verschueren, 1983
Partition coefficient (log Kow at 20-25°C) 0.161-0.46 Korenman & Lunicheva, 1972; GEMS, 1983; Hansch & Leo, 1987;
BASF, 1988
Dissociation constant (pKa at 25°C) 4.25 Weast et al., 1989
Critical temperature (°C) 342 CHRIS, 1985
Critical pressure (atm) 57 CHRIS, 1985
Solubility: in water and most organic completely Dean, 1987; Sax & Lewis, 1989; Weast et al., 1989
solvents (alcohol, chloroform, benzene) miscible
Refractive index (nD20-25) 1.4224-1.4185 Kirk-Othmer, 1984
Maximum absorption (nm, in methanol) 252 Weast et al., 1989
2.2.2 Chemical properties
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according to
supplier's recommendations. Heating can cause vigorous polymerization
in some circumstances. Acrylic acid reacts readily with free radicals
and electrophilic or nucleophilic agents (Kirk-Othmer, 1984). It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of an inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C (CHRIS, 1989). The mechanism of auto-
accelerating polymerization of acrylic acid in hexane-methanol
solution, which can become explosive, has been studied by Bretherick
(1985).
Acrylic acid rapidly decomposes in the atmosphere by
photochemical attack on the double bond (NLM, 1989; OHM/TADS, 1989).
Acrylic acid is corrosive to many metals but not to stainless
steel or aluminium (Kirk-Othmer, 1984; AAR, 1987).
2.3 Conversion factors
In air:
1 ppm = 3.0 mg/m3 (NLM, 1989)
1 mg/m3 = 0.33 ppm (NLM, 1989)
2.4 Analytical methods
2.4.1 In air
A summary of methods for the detection of acrylic acid in air is
given in Table 2.
Table 2. Methods for the analysis of acrylic acid in air
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples absorbed GC on a glass column FID 33 mg/ml acetone The method is Vincent &
on silica gel treated packed with 1% FFAP (lower) to significantly Guient, 1982
with p-methoxyphenol on Chromosorb T 2084 mg/ml acetone affected by high
followed by desorption (upper); this is humidity. Samples
with acetone (94% equivalent to can be stored for
recovery) concentrations ranging up to 11 days at
from 0.5 ppm to 30 ppm room temperature or
(1.5-90 mg/m3) of under refrigeration
acrylic acid in a without affecting
48-litre sample volume recovery. Recommended
as useful for
determining acrylic
acid in the
occupational
environment
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air samples Reverse phase UV detector 1 g per sample; The sensitivity of OSHA, 1981
collected by HPLC 210 nm assuming 24 litre the analytical
drawing a known sample volume, this method permits
volume of air column: 25 cm × is equivalent to sampling times
through two 4.6 mm i.d. 0.042 mg/m3 as short as
XAD-8 sampling stainless steel (0.014 ppm) 15 min. Under
tubes connected column packed conditions of
in series, with Zorbax 8 m this procedure,
followed by ODS-bound, spherical the possibility
desorption with silica particles of interference
methanol/water from acetaldehyde,
(1:1) mobile phase: acetic acid,
96:4 (V/V) acrylamide,
water/acetonitrile acrolein,
containing 0.1% by acrylonitrile,
volume phosphoric methacrylic
acid; flow rate: acid is excluded.
1 ml/min; injection Method recommended
volume: 25 litre and fully validated
retention by OSHA for acrylic
time:6 min acid determinations
in workplace air
Table 2. (contd)
Sampling Analytical methods Detectiona Detection limit Comment Reference
methods
Air is pumped HPLC equipped Conductivity 1 mg/m3 air The method is Simon et
through a florisil with Aminex HPX detector (10 litre rapid, easy and al., 1989
tube at a rate of OFH organic acid sample volume) appears suitable for
1 litre/min. The analysis column the determination
sorbent is mixed (300 mm × 7.8 mm). of acrylic acid
with water (5 ml) Eluent, 2.5 × 10-4 when present in
and 1N H2SO4 (10 µl) M benzoic acid industrial emissions
prior to injection is pumped at containing other
to the chromatographic 0.8 ml/min aliphatic acids
system
a FID = Flame ionisation detector
Air samples are collected on silica gel treated with
p-methoxyhydroquinone used as an inhibitor of polymerization
(Vincent & Guient 1982) or on XAD-8 sampling tubes (OSHA, 1981). XAD-8
sampling tubes contain solid sorbent, i.e. acrylic ester polymer, of
16-50 mesh (OSHA, 1981).
After separation with gas chromatographic (GC) technique or
reverse phase high performance liquid chromatography (HPLC), flame
ionization detection (Vincent & Guient 1982) or UV detection at 210 nm
(OSHA, 1981) are utilized, respectively. The latter method was
modified and recommended by OSHA as a fully validated method for the
determination of acrylic acid in workplace air (OSHA, 1981). This
method, when coupled with an ion suppression technique, proved
successful for the retention and separation of acrylic acid.
A retention time of approximately 6 min is obtained with a Dupont
Zorbax ODS 8-µm silica packed column and a water/acetonitrile (96:4)
mobile phase containing 0.1% (by volume) phosphoric acid. The
phosphoric acid serves to suppress the ionization of acrylic acid
resulting in the retention of the undissociated form of the molecule.
Under these conditions acrylic acid is separated from potential
interfering substances: methacrylic acid, acrylamide, acrolein,
acrylonitrile and acetic acid. Propanoic acid, a saturated precursor
of acrylic acid, can be resolved from acrylic acid in a 13-min
analysis at 1 ml/min flow rate using a 0.1% aqueous phosphoric acid
mobile phase. Detection of acrylic acid at 210 nm is approximately 100
times more sensitive than that of propanoic acid, owing to the
unsaturated nature of acrylic acid. This method permits the detection
of acrylic acid in the presence of very high levels of propanoic acid.
A third method utilizes high-performance ion-exclusion
chromatography with conductimetric detection (Simon et al., 1989). The
use of 2.5 × 10-4 M benzoic acid as the mobile phase in this method
allows the separation of acrylic acid from propionic acid and other
aliphatic acids.
2.4.2 In industrial effluents
A gas chromatographic method has been developed for the analysis
of acrylic acid and some other related pollutants present in small
quantities in the effluent from a methyl acrylate plant in India
(Singh & Thomas, 1985). In this method, effluent samples were injected
directly to the GC system without prior extraction or concentration. A
Porapak Q (4 feet × 1/8 inch I.D.) column and a FID were utilized in
this method. The experimental parameters for the analysis are: column
temperature, 165°C; injector and detector temperature, 250°C; carrier
gas, N2 at 50 ml/min; hydrogen pressure, 1.3 kg/cm2; air pressure,
2.2 kg/cm2 and injection volume, 1-10 µl. The method was found to be
sensitive for detecting acrylic acid at concentrations as low as
1 ppm.
2.4.3 In polyacrylate materials
A differential pulse polarographic method was used for the
determination of residual acrylic acid in sodium polyacrylate
polymeric systems (Husain et al., 1991). The method has the advantage
of analysing acrylic acid in trace quantities directly without
resorting to separation techniques. Sample solutions of the tested
polymers were extracted with N,N-dimethylformamide several times and
the extraction mixture was made up to 25 ml, with the solvent
tert-butyl ammonium iodide (0.02 M) in N,N-dimethylformamide
serving as the supporting electrolyte. The polarographic measurements
were performed with a Metrohm E-506 Polarecord equipped with a three-
electrode system (a dropping mercury electrode (DME), Ag/AgCl
(saturated KCl) reference electrode, and an auxiliary platinum
electrode). Using this method, free acrylic acid in polymers at levels
of 10-100 ppm can be measured with a precision of ± 3%.
2.4.4 In biological samples
Methods for the analysis of acrylic acid in aqueous samples and
tissues extracts in metabolic studies have been reported (Mao et al.,
1994; Mitchell & Petersen, 1988; Black et al., 1995). In these
methods, high-performance ion-exclusion chromatography and/or reverse
phase HPLC with radiometric, refractive index, photo diode-array and
UV detectors were used for the separation and quantification of
acrylic acid.
In another study, residues of acrylic acid in an anaerobic
degradation mixture were quantified using a gas chromatographic
technique with a flame ionization detector (FID) (Stewart et al.,
1995). The column was an 80/120 Carbopak B-DA/4% Carbowax 20 M. The
column and FID temperatures were 175 and 200°C, respectively. The
carrier gas was helium at a flow rate of 24 ml/min. The detection
limit was 1 mg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acrylic acid has been reported to occur naturally in the
following species of marine algae: 9 species of Chlorophyceae, 10
species of Rhodophyceae and 11 species of Phaeophyceae (Sieburth,
1960, Glombitza, 1970a,b, 1979). It is also produced in
Phaeodactylum tricornutum, Phaeocystis spp. and Polysiphonia
lanosa (Brown et al., 1977), as a result of hydrolysis of dimethyl-ß-
propiothetin (Verschueren, 1983).
Acrylic acid has been identified as an antibacterial substance in
oysters (Brown et al., 1977), scallops, ( Patinopecten yessoensis)
(Kodama & Ogata, 1983), and the digestive tract of penguins (Sieburth,
1960; Herwig 1978). It is thought to originate from the phytoplankton
Protogonyaulax (Kodama & Ogata, 1983), Phaeocystis spp (Sieburth,
1960) and Phaeodactylum tricornutum (Brown et al., 1977) on which
the molluscs and penguins fed. It has also been shown that under
natural conditions acrylic acid is generated by certain species of
algae and acts as a microbiocide (Glombitza, 1970a,b, 1979; Heyser &
Glombitza, 1972). It has also been identified as the agent responsible
for the antimicrobial activity of the marine algae Gracilaria
corticata and Ulva lactuca (Bandara et al., 1988).
Acrylic acid has been found in the rumen fluid of sheep as a
result of bacterial fermentation of carbohydrates (Noble & Czerkawski,
1973), where it is converted by rumen microorganisms to propionic acid
(Whanger & Matrone, 1967). It can also be produced from lactic acid by
the anaerobic rumen bacterium Megasphaera elsdenii in the presence
of 3-butynoic acid (Sanseverino et al., 1989).
Acrylic acid has been found in agricultural rum obtained by
fermentation of sugarcane juice by the action of Micrococci spp
(Ganou-Parfait et al., 1988).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
The first commercial process for the manufacture of acrylic acid
and its esters involved hydrolysis of ethylene cyanohydrin in sulfuric
acid. This route is no longer commercially significant (Kirk-Othmer
1984).
Most commercial acrylic acid is now produced via a process in
which propylene is vapour-oxidized to acrolein, which is in turn
oxidized at 300°C with molybdenum-vanadium catalyst to acrylic acid
(NLM, 1989). Other methods of production are as follows:
* a modification of the Reppe process by the reaction of acetylene,
carbon monoxide and alcohol with a nickel catalyst;
* by hydrolysis of acrylonitrile;
* condensation of ethylene oxide with hydrocyanic acid followed by
reaction with sulfuric acid at 160°C;
* a process in which formaldehyde undergoes a type of aldol
reaction with a large molar excess of acetic acid in the vapour
phase in a catalyst tube containing calcium Decalso (Kirk-
Othmer, 1984);
* a heterolytic dehydration pathway of lactic acid in supercritical
water (Mok et al., 1989).
3.2.1.2 Impurities
Commercial acrylic acid is available in two grades: technical and
glacial. Glacial grade is 98-99.5% acrylic acid (NLM, 1989). This may
contain, as impurities, water up to 0.3% w/w and acrylic acid dimer up
to 0.1% w/w (BASF, 1992; Elf Atochem, 1992).
3.2.1.3 Other sources
Acrylic acid has also been detected in trace amounts in
commercial propionic acid (Kostanyan et al., 1969).
3.2.1.4 Production data
Available data on the production of acrylic acid are shown in
Table 3.
Table 3. Production data
Country Year Production of Reference
acrylic acid
(in kilotonnes)
China 1994 105 CEFICA (1995)a
European Community 1975 155 IARC (1979)
1994 665 CEFIC (1995)a
Japan 1976 70 IARC (1979)
1994 420 CEFIC (1995)a
Korea 1994 60 CEFIC (1995)a
Taiwan 1994 50 CEFIC (1995)a
USA 1993 332 US ITC (1983)
1985 361 US ITC (1985)
1986 348 US ITC (1986)
1987 499 US ITC (1987)
1988 480 US ITC (1988)
1991 554 NLM (1991)
1994 685 CEFIC (1995)a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union Chemicals risk
assessment document.
The worldwide production of acrylic acid was approximately
1.13 million tonnes in 1991 (Chemical Marketing Reporter, 1992).
Worldwide capacity for acrylic acid production was reported to be
2 million tonnes in 1994 (CEFIC, 1995).
3.2.2 Experimental production of acrylic acid by bacterial isolates
The following bacterial species have been utilized in
experimental systems to produce acrylic acid:
* from acrylonitrile: (1) by the action of epsilon-caprolactum-
induced Rhodococcus rhodochrous J1 (Nagasawa et al., 1990);
with a periodic substrate feeding system the highest accumulation
(390 g/litre) was obtained; (2) by Arthrobacter sp. isolated
from petrochemical industry waste (Narayanasamy et al., 1990).
* from acrylamide by the action of Pseudomonas sp. and
Xanthomonas maltophilia isolated from herbicide-contaminated
soils (Nawaz et al., 1993, 1994); batch culture of these bacteria
completely degraded 62.8 mM acrylamide to acrylic acid and
ammonia in 24 and 48 h, respectively.
3.2.3 Uses
Acrylic acid is used primarily: as a starting chemical for ethyl
acrylate, n-butyl acrylate, methyl acrylate, 2-ethylhexyl acrylate;
as a monomer for polyacrylic acid and salts, cross-linked high (and
low) molecular weight polymers; as a co-monomer with acrylamide for
polymers used as flocculants; with ethylene for ion-exchange resin
polymers; with methyl ester for polymers; and with methylene succinic
acid (itaconic acid) for other co-polymers (SRI, 1981; NLM, 1989).
In 1987, 25% of the acrylic acid produced in the USA was used for
surface coatings; 20% for polyacrylic acid and salts, including super-
absorbent polymers, detergents, water treatment and dispersants; 13%
for textiles and non-wovens; and 9% for adhesives and sealants
(Kavaler, 1987).
Until 1979, in the European Union countries more than 80% of
acrylic acid was used for the production of polyacrylates and in Japan
90% was used in the production of acrylic esters (IARC, 1979). In
1988, European use of acrylic acid was 69% for esters, 10% for
detergents, 8.5% for flocculants and dispersants and 6.5% for super-
absorbers (CEFIC, 1995).a
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Other uses are in the production of copolymers for dental
adhesives (Bowen, 1979), in the production of hydrogels used for
contact lenses (Kirk-Othmer, 1984), in surface coating formulations
(Kirk-Othmer, 1984), and in latex applications to increase stability
in order to prevent premature coagulation (Kirk-Othmer, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Acrylic acid is miscible with water (Riddick et al., 1986) and
therefore would not be expected to adsorb significantly to soil or
sediment (Lyman et al., 1982). The Henry's Law constant for acrylic
acid is reported to be 3.2 × 10-7 atm m3/mol (Singh et al., 1984).
Under soil conditions, chemicals with such low Henry's Law constants
are essentially non-volatile (Lyman et al., 1982). However, the vapour
pressure of acrylic acid suggests that it volatilizes from surface and
dry soil (Howard, 1991).
The adsorption and desorption of acrylic acid were examined in
five different soils: an aquatic sandy loam sediment, a loamy sand, a
clay loam and two loams. The average Koc for the adsorption of
acrylic acid to soil was 43, and ranged from 23 to 63. The Koc values
for the desorption data were widely scattered with values ranging from
18 to 837. This indicates that the degree of adsorption is not
correlated to the organic carbon content (OC), which ranged from 0.46%
for the loamy sand to 4.58% for one of the loams. The results of this
study indicate a high mobility of acrylic acid through soil (Archer &
Horvath, 1991).
Using the fugacity model of Mackay & Peterson (1981) the
theoretical distribution of acrylic acid has been estimated. About 97%
of acrylic acid released to the environment should be associated with
the aquatic environment (the water phase), approximately 1.6% in air,
1% in sediment and < 1% in soils, suspended solids and biota
(Staples, 1993).
Since the atmospheric lifetime of acrylic acid is less than one
month (Atkinson, 1987), there is no potential for long-range transport
of this compound.
4.2 Transformation
4.2.1 Abiotic degradation
The UV absorption band of acrylic acid extends to about 320 nm
(Weast & Astle, 1985). Vapour phase acrylic acid reacts with
photochemically produced hydroxyl radicals primarily by addition to
the double bond and with atmospheric ozone, resulting in an estimated
overall half-life of 6.6 h to 6.5 days (Atkinson & Carter, 1984).
Based upon the estimated rate constant for vapour phase reactions and
assuming hydroxyl radical concentrations of 5 × 105 radicals per cm3
and an ozone concentrations of 7 × 1011 molecules per cm3 (Atkinson
and Carter, 1984; Atkinson, 1987), a half-life of 2.5-23.8 h was
estimated by Howard et al. (1991).
Acrylic acid was found to be stable to hydrolysis at pH values
between 3.7 and 11 (Shah, 1990).
4.2.2 Biodegradation
4.2.2.1 Aerobic biodegradation
When added to water, acrylic acid is rapidly oxidized, and
wastewater containing the compound can deplete reservoirs of oxygen
(Ekhina & Ampleeva, 1977).
Several biodegradability studies show that acrylic acid will
readily biodegrade (Lyman et al., 1982; Keystone Environmental
Resources, 1989a; Douglas & Bell, 1992). The BOD5 (biological
oxygen demand, 5 days) for glacial acrylic acid, using acclimated,
fresh dilution water and raw sewage from a local treatment plant as
the inoculum, was determined to be 0.315 g of oxygen consumed per gram
of product. The COD (chemical oxygen demand) under the same conditions
was 1.48 g/g (Keystone Environmental Resources, 1989)a; therefore,
the BOD5/COD ratio was 0.21. A BOD5/COD ratio of 0.26 was also
reported by Lyman et al. (1982). Biodegradation of acrylic acid in a
14-day BOD test was up to 68% (CITI, 1992). Acrylic acid at a
concentration of 3 mg/litre attained 81% biodegradation within 28 days
in a closed-bottle test based on the consumption of oxygen (Douglas &
Bell, 1992). The pass level of 60% was reached within 10 days of
exceeding the 10% level, and so acrylic acid is considered to be
"readily biodegradable" according to EC classification criteria (EEC,
1988).
The metabolism of 14C-acrylic acid in sandy loam soil has been
studied under aerobic conditions for up to 28 days after treatment at
a rate of 100 mg/kg. Acrylic acid was rapidly metabolized; after 3
days no acrylic acid was detected in soil extracts. Carbon dioxide
evolution accounted for 72.9% of applied radioactivity by day 3 and a
total of 81.1% over the 28-day study period. The half-life for acrylic
acid under these conditions was estimated to be less than 1 day
(Hawkins et al., 1992).
Acrylic acid formed from hydrolysis of acrylamide added to soil
was totally degraded within 15 days of its formation (Nishikawa et
al., 1979). In a 42-day screening study using a sewage seed inoculum,
71% of acrylic acid was mineralized under aerobic conditions. After
previous acclimatization, 81% of acrylic acid degraded to carbon
dioxide in 22 days (Pahren & Bloodgood 1961; Chou et al., 1978).
a Report sent by J.M. Flaherty to J. McLanghlin, Rohm and Haas
Spring House (work order numbers M8903002 and M8902005).
A collection of strains utilizing acrylonitrile, acrylamide and
acrylic acid as sole carbon and/or nitrogen source was isolated from
environmental samples. Strains with maximum decomposing activity were
identified as Pseudomonas pseudoalcaligenes 6p; P.alkaligenes 5g
and Brevibacterium spp. 13 PA (Moiseeva et al., 1991).
An aerobic gram-negative bacterium ( Pseudomonas sp.) isolated
from tropical garden soil was found to be able to degrade a high
concentration of acrylamide (4 mg/litre) to acrylic acid and ammonia,
which were utilized as sole carbon and nitrogen sources, respectively,
for growth (Shanker et al., 1990).
A strain of Byssochlamys sp. produced ß-hydroxypropionic acid
(ß-HPA) when grown on media containing high concentrations of acrylic
acid. The maximal production of ß-HPA was 4.8% when the initial
culture medium contained 7% acrylic acid and 2% glucose and the
initial culture pH was adjusted to 7.0 (Takamizawa et al., 1993).
Acrylic acid has been reported to be significantly degraded
(> 30%) in the MITI test, a biodegradability screening test of the
Japanese Ministry of International Trade and Industry (Sasaki, 1978).
Acrylic acid was completely degraded in a standard Zahn-Wellens test
and the authors concluded that it is biodegradable (BASF, 1993).
Acrylic acid has been found to be degraded by a strain of
Alcaligenes denitrificans isolated from a landfill soil. The
bacterium degraded acrylic acid through the intermediate formation of
L-(+)-lactic and acetic acids, which were further metabolized
(Andreoni et al., 1990).
4.2.2.2 Anaerobic biodegradation
Speece (1983) reported that acrylic acid can undergo ultimate
anaerobic biodegradation. In an anaerobic screening study utilizing
10% sludge from a secondary digester as an inoculum, acrylic acid was
judged to be degradable, with over 75% of theoretical methane being
produced within 8 weeks of incubation (Shelton & Tiedje, 1984).
In another study, acrylic acid was toxic to unacclimated
anaerobic acetate-enriched cultures and was poorly utilized (21%) in a
completely mixed anaerobic reactor with a 20-day hydraulic retention
time after a 90-day acclimatization period (Chou et al., 1978). A
possible explanation for the conflicting results of anaerobic
degradation is the observation that acetate cultures have to exhaust
the acetic acid as carbon and energy source before they can utilize a
cross-fed compound (Chou et al., 1978).
The biodegradability of acrylic acid using methanogenic acetate
enrichment culture was studied by Stewart et al. (1995). Acrylic acid
was degraded with almost no effect on methanogens with spikes up to
100 mg/litre. However, concentrations of 500, 1000 and 1500 mg/litre
were found to inhibit the methanogens for several days before
recovery. Acrylic acid was eventually degraded to less than 1 mg/litre
(> 99% of initial concentration) in all cases by the end of the
study (55 days).
4.2.3 Bioaccumulation and biomagnification
From the low value for log Kow, ranging from 0.161 to 0.46
(Hansch & Leo, 1987; BASF, 1988), one would expect the
bioconcentration of acrylic acid in organisms to be negligible Bysshe
(1990) using a regression equation calculated theoretical
bioconcentration factors ranging from 0.78 to 1.3. Veith et al. (1979)
estimated the bioconcentration factor to be in the range of 1.6 to
2.4.
There have been no reports of biomagnification of acrylic acid in
the food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
No quantitative data are available for environmental levels of
acrylic acid in ambient air, water or soil. Acrylic acid has been
found to occur naturally in some marine algae (Sieburth, 1960; Brown
et al., 1977) and some molluscs (Kodama & Ogata, 1983). The acrylic
acid content of Phaeocystis spp. can be 7.4% of dry weight
(Sieburth, 1960). Other marine algae have been found to contain
acrylic acid: Chlorophyceae, 0.124-16.5 mg/g dry weight;
Rhodophyceae, 0-0.131 mg/kg dry weight; and Phaeophyceae 0-0.02 mg/g
dry weight (Glombitza, 1970a, 1979).
5.2 General population exposure
No data are available for general population exposure. However,
consumers may be exposed to unreacted acrylic acid in the following
household goods: polishes, paints and coatings, adhesives, rug
backing, plastics, textiles and paper finishes (USEPA, 1981).
Information on the typical content of unreacted acrylic acid in these
kinds of products is unavailable.
Populations living in the vicinity of plants producing acrylic
acid or manufacturing its esters or polymers may be exposed to acrylic
acid in the ambient air. The concentrations of emitted vapours of
acrylic acid in the plume from such plants were found to vary from 22
to 183 mg/m3 (Grudzinski, 1988). However, there are no data on
concentrations of acrylic acid in the ambient air of populated areas.
Acrylic acid occurs in wastewater effluents from its production
by the oxidation of propylene at concentrations not exceeding
0.5 mg/litre (Wise & Fahrentholdt, 1981). After treatment of
wastewater from a production facility in Europe, acrylic acid levels
were below the limit of detection (0.1 mg/litre) (CEFIC, 1995)a.
However, effluent from a methyl acrylate plant in India was found to
contain 2500 mg/litre as acrylic acid (Singh & Thomas, 1985).
a Submission of the European Council of Chemical Industry
Federations (CEFIC) to the European Union chemicals risk
assessment document.
Since there is evidence that acrylic acid esters are hydrolysed
to acrylic acid in laboratory animals (Ghanayen et al., 1987) and in
human tissues in vitro (Wiegand, 1990), a potential source of
internal exposure to acrylic acid may result from metabolism of
absorbed acrylic acid esters (Frederick et al., 1994; Sanders et al.,
1988).
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure is the most important means of human
exposure to acrylic acid. Inhalation and contact with skin are
important routes of exposure.
The National Institute of Occupational Safety and Health (NIOSH)
conducted two observational nationwide surveys, a decade apart, to
determine the extent of exposure of workers to a variety of substances
in their work environment. The National Occupational Hazard Survey
(NOHS) was conducted during 1972-1974 using a stratified probability
sample of 4636 businesses in 67 metropolitan areas throughout the USA
employing nearly 900 000 workers (NIOSH 1974, 1977). According to the
NOHS, an estimated 28 600 workers were potentially exposed to acrylic
acid, approximately 10% of whom were exposed to acrylic acid and 90%
to trade-name products. However, this estimation excluded the exposure
of the general population to trade-name chemicals possibly containing
acrylic acid. Acrylic acid was seen in 16 major industry groups and in
41 occupational groups in the NOHS.
During 1981-1983 NIOSH conducted the National Occupational
Exposure Survey (NOES), using a stratified probability sample of 4490
businesses in 98 different geographic locations of the USA employing
nearly 1.8 million workers (NIOSH, 1990). According to the NOES, an
estimated 96 500 workers were potentially exposed to acrylic acid,
approximately 10% of whom were exposed to acrylic acid and 90% to
trade-name products. Acrylic acid was seen in 25 major industry groups
and in 67 occupational groups (NIOSH, 1990).
One study conducted at a large manufacturing facility of the Rohm
and Haas Company in the USA, where several chemicals including acrylic
acid and a variety of acrylates and methacrylates were used, indicated
that ethyl acrylate and acrylic acid levels varied from 0.01 to
56 ppm. Most areas of the plant had levels (as 8-h time-weighted
averages) well below the hygiene standards recommended at that time by
the OSHA and the ACGIH of 10 ppm for acrylic acid and 5 ppm for ethyl
acrylate (ACGIH, 1988, OSHA, 1989). Many of the available industrial
hygiene data were specific for high, short-term exposure tasks
(5-40 min samples) when chemicals were pumped into containers for
shipping or when lines were open for new connections, or to obtain
samples. They revealed levels at the high end of the above-mentioned
range (Schwartz et al., 1989).
Exposures of workers to acrylic acid for short periods of time of
less than 15 min and for full shift expressed as time-weighted average
(TWA) concentration have been compiled from four producing companies.
Operators had a mean short-term exposure limit (STEL) value of
8.4 mg/m3 (range < 0.3 to 189 mg/m3); loading/unloading operations
a mean of 3.9 mg/m3 (range 1.2 to 12 mg/m3); and those engaged in
quality assurance a mean of 0.3 mg/m3 (range of less than 0.3 to
0.6 mg/m3). Concerning the 8-h TWA, the operators showed levels of
0.48 mg/m3 (range of 0.03 to 3 mg/m3) and loading/unloading
operations a mean of 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3)
(Casciery & Clary, 1993).
No such data are available from other countries.
6. KINETICS AND METABOLISM
6.1 Human studies
Apart from in vitro skin absorption studies, no data are
available on kinetics, metabolism or elimination or acrylic acid in
humans.
The absorption of 14C-acrylic acid (site of label unspecified)
dissolved in acetone, water or phosphate buffer (pH 6.5) was tested
using samples of excised (postmortem) human and mouse skin in vitro
(Corrigan & Scott, 1988). Acrylic acid concentrations of 0.01, 0.1,
1.0 and 4.0% were applied at 100 µl/cm2 under occlusive conditions.
Samples were taken from the receptor fluid up to 32 h. Rates of
absorption decreased in the order of magnitude as follows: acetone >
water > phosphate buffer. Independent of the vehicle, the absorption
rate increased as a function of acrylic acid concentration.
Permeability coefficients, which ideally are concentration-independent
expressions of absorption rate, for human skin ranged from 0.37 to
0.72 × 10-3 cm/h for water and from 0.47 to 1.81 × 10-4 cm/h for
phosphate buffer. Permeability coefficients were not calculated for
acetone because of evaporation of this volatile vehicle during the
course of the experiments (Corrigan & Scott, 1988).
A briefly reported in vitro percutaneous penetration study
using excised human cadaver skin indicated that 14C-acrylic acid
absorption can vary significantly as a function of pH and delivery
vehicle. In vitro flux, estimated after a 1 mg dose was applied,
varied by 600 times within the treatments studied and decreased in the
order: acetone (600 µg/cm2 per h) > phosphate buffer pH 6.0
(23 µg/cm2 per h) > ethylene glycol (15 µg/cm2 per h) > phosphate
buffer pH 7.4 (1 µg/cm2 per h) (D'Souza and Francis, 1988).
6.2 Studies on experimental animals
6.2.1. Absorption, distribution and excretion
6.2.1.1 Oral exposure
After oral gavage administration of an aqueous solution of
(1-11C)-acrylic acid (26 µg/kg body weight) to female Sprague-Dawley
rats, it was rapidly absorbed and expired mainly as 11CO2 within 1 h
post-administration. The uptake appeared biphasic. The short alpha-
phase had an apparent first-order absorption constant (Ka) of 19% of
the available dose per minute (biological half-time = 3.6 min) and the
Ka of the ß-phase was 30% (biological half-time = 23 min). Relative
retention of radiolabel (dpm per g tissue versus dpm per g body
weight) after 65 min was above unity in liver (2.6), adipose tissue
(1.9), small intestine (1.5), kidneys (1.2) and spleen (1.0).
Approximately 6% of the radiolabel was excreted in the urine within
65 min (Kutzman et al., 1982).
In another study, single gavage doses of 4, 40 or 400 mg/kg body
weight of (2,3-14C)-acrylic acid in 0.5% aqueous methylcellulose
solution were administered to male Sprague-Dawley rats. Approximately
35, 55 and 60%, respectively, of the administrated dose were
eliminated, mostly as 14CO2, within 8 h. By 24 h 50-65% of the dosed
radioactivity was eliminated and the excretion of radioactivity had
virtually ceased. After 72 h, 44-65% of the administrated
radioactivity had been eliminated as 14CO2; 2.9-4.3% in urine, 2.4-
3.6% in the faeces and 18.9-24.6% remained in the tissues examined
(liver, stomach, muscle, blood, plasma, adipose tissue). The residual
radioactivity was highest in the adipose tissue (9-15%), followed by
muscle (6.5-7.5%) and liver (1.7-2.2%) (De Bethizy et al., 1987).
The disposition of (1-14C)-acrylic acid was also determined in
male Sprague-Dawley rats following oral administration by gavage in
water at 400 mg/kg body weight. Excretion of acrylic acid-derived
radioactivity was determined by collection of urine, faeces and
expired air for 72 h following administration. The predominant route
of excretion was in the expired air with approximately 80% of the
radioactivity exhaled as 14CO2 within 24 h and 83.2% after 72 h.
Elimination of radioactivity as exhaled volatile organic compounds was
negligible (less than 0.5% of the radiolabel). Within 24 h of dose
administration, excretion of radioactivity accounted for 5.0% in the
urine and 8.8% of the radiolabel in faeces. Tissue concentrations of
radioactivity after 72 h were generally low: 0.4% of the total dose in
the liver, 0.39% in muscle and 0.18% in skin (Winter & Sipes, 1993).
A comparative bioavailability and disposition study in male
Fischer-344 rats and male C3H mice after a single administration of
(1-14C)-acrylic acid (40 or 150 mg/kg body weight in water) by gavage
has been conducted. This study confirmed that acrylic acid is rapidly
absorbed and metabolized. In rats and mice about 80-90% of the dose
was exhaled as 14CO2 within 24 h (Black et al., 1995). In rats,
excretion of radiolabel in urine and faeces within 72 h accounted for
< 5% and < 1% of the dose, respectively. Elimination of
radioactivity in rats as exhaled organic volatile compounds was less
than 0.5% of the radiolabel. Similar patterns were observed in male
mice (Black et al., 1995).
6.2.1.2 Inhalation exposure
A tissue distribution study has been conducted in 39 female
Sprague-Dawley rats nose-exposed to (1-11C)-acrylic acid vapour for
1 min (concentration not indicated). Radioactivity was widely
distributed; 90 seconds after exposure 18.3% of the delivered dose
remained in the rats. Approximately 28.0% of this radioactivity was
associated with the snout and 42.9% of the radioactivity was found in
the head; this was considered to be solubilized in the mucous of the
turbinates and the nasopharynx. After 65 min, the activity in the
snout was reduced to 8.1% and approximately 60% of the label was
expired as 11CO2. The elimination of labelled CO2 appeared to be
biphasic, with a half-time of approximately 30.6 min during the
alpha-phase. The amount of radioactivity retained in liver and fat
increased markedly between 1.5 and 65 min post-exposure (Kutzman et
al., 1982).
6.2.1.3 Dermal exposure
In one in vitro experiment, the dermal penetration capacity of
(1-14C)-acrylic acid was tested using excised mouse skin. Skin slices
were treated in a diffusion chamber with 0.01, 0.1, 1 and 4% (w/v)
100 µl/cm2 of acrylic acid dissolved in acetone, water or phosphate
buffer pH 6.5. The results were comparable with the study performed on
excised human skin (section 6.1). Permeability coefficients for mouse
skin were 0.96-1.73 × 10-3 cm/h for water and 1.91-3.1 × 10-4 cm/h
for phosphate buffer. The permeability coefficients and steady-state
absorption rate data indicate that mouse skin is approximately three
times more permeable than human skin to acrylic acid (Corrigan &
Scott, 1988). This difference may not be biologically significant.
In a briefly reported study, male Sprague-Dawley rats were
administered dermally 5 mg 14C-acrylic acid per kg body weight
(D'Souza & Francis, 1988). Phosphate buffer of pH 6 or 7.4 or acetone
was used as a formulating agent. In each case the formulation was
applied to the shaved back of the rats and covered with a glass
chamber. The rate of appearance of 14CO2 measured at 0.5, 1, 2, 4,
8, 16 and 24 h after application was used as a measure of the
absorption rate of acrylic acid. The absorption rate was dependent on
the vehicle and decreased in the following order, acetone > phosphate
buffer of pH 6 > phosphate buffer of pH 7.4. Cumulative absorption
after 24 h was 22% from acetone, approximately 19% from phosphate
buffer of pH 6, and 9% from phosphate buffer of pH 7.4. The results of
the in vivo investigations were comparable to those of the in
vitro studies obtained by the same authors (D'Souza & Francis, 1988).
The disposition of (1-14C)-acrylic acid was determined in male
Sprague-Dawley rats after topical application of 100 µl of a 4% (v/v)
solution of acrylic acid in acetone to an area of 8.4 cm2 of the skin
(501 µg/cm2) using a skin-mounted, charcoal-containing trap covered
with fixed aluminium discs to ensure complete recovery of the label.
Excretion of acrylic-acid-derived radioactivity was determined by
collection of urine, faeces and expired air for 72 h following
administration of acrylic acid. Approximately 73% of the radioactivity
volatilized from the skin and was trapped in the charcoal sorbent.
After 72 h, 6% of radioactivity was detected at the site of
application in the skin or on the skin surface. Approximately 75% of
the absorbed dose, representing about 16% of the applied dose, was
exhaled as 14CO2 within 12 h. Excretion of radioactivity in the
urine accounted for approximately 9% of the applied radioactivity
(approximately 4% of the absorbed dose), the faeces containing only
negligible amounts of radioactivity. After 72 h, less than 0.4% of the
applied dose was retained in tissues other than skin (Winter & Sipes,
1993).
In another study, 1% (v/v) acetone solutions of 14C-acrylic acid
at doses of 10 or 40 mg/kg were applied to the clipped skin of the
shoulder region of male F-344 rats or male C3H/HeN Crl BR mice. A non-
occlusive "frame" device was cemented to the skin surface of animals
to allow for free evaporation of acrylic acid, which was trapped using
on-line volatile organic traps. Since this technique was inefficient,
activated-charcoal-impregnated filter paper sheets were placed
occlusively on the treated skin surface of a second high dose group of
animals to provide for absorption of evaporating acrylic acid (Black
et al., 1995). In rats, the reported 72-h recovery was low and ranged
from 50 to 60% of the applied dose. Evaporation accounted for most of
the applied acrylic acid, but approximately 26 and 19% of the applied
high and low doses were absorbed in rats within 72 h, respectively.
The major route of elimination of absorbed acrylic acid was via
exhalation of 14CO2 and accounted for 69.5 and 77% of the absorbed
low and high doses, respectively. Minimal faecal elimination of
absorbed acrylic-acid-derived radioactivity was reported (< 1%), and
tissues and carcasses contained approximately 2-3% of the absorbed
chemical at 72 h.
In mice, the 72-h recovery ranged from 61.5 to 84.0% of the
applied acrylic acid dose. As in the rat experiments, while most of
the applied acrylic acid was lost to evaporation, absorption accounted
for 11-12% of the applied dose. Exhalation of 14CO2 accounted for
83.5 and 77.7% of the absorbed high and low doses, respectively.
Elimination via other routes was negligible, and less than 1% of the
absorbed dose remained in the tissues and carcasses at 72 h (Black et
al., 1995).
6.2.1.4 Intravenous administration
Single i.v. doses of (1-14C)-labelled acrylic acid (10 mg/kg
body weight in phosphate-buffered saline) were given to male F-344
rats and male C3H/HeNCrlBR mice into the tail veins. In rats 63% of
the 14C-dose was eliminated as 14CO2 after 4 h and 68% after 72 h,
while almost no 14C was recovered as exhaled organic volatiles.
Tissue samples (liver, kidney and fat) and plasma contained 1.9% at 1
h, 0.4% at 8 h, and 0.2% at 72 h of the recovered dose. Overall the
recovery was 72.8 ± 10.8%. In mice, 51% of the radioactivity was
exhaled as 14CO2 over the 72-h collection period, the majority
exhaled in the first 4 h. The volatile radioactive fractions were
about 0.6% of the total dose. Overall, 55.7 ± 6.6% of this intravenous
10 mg/kg dose was recovered in mice (Frantz & Beskitt, 1993).
6.2.2 Metabolism
6.2.2.1 In vitro investigations
Oxidation of (2,3-14C)-acrylic acid was studied by incubating
acrylic acid with hepatic microsomal preparations obtained from male
Sprague-Dawley rats. No metabolites were detected by HPLC and acrylic
acid was recovered unchanged from the incubation mixture (De Bethizy
et al., 1987).
Results of the in vitro metabolism of (1-14C)-acrylic acid
incubated with freshly isolated hepatocytes and liver homogenates of
male F-344 rats or mitochondria isolated from liver homogenates of
male F-344 rats indicate that acrylic acid is rapidly metabolized to
14CO2. Addition of equimolar amounts of propionic acid, 3-hydroxy-
propionic acid or 3-mercaptopropionic acid caused a significant
inhibition of the oxidation of acrylic acid by isolated mitochondria.
A single major metabolite co-eluting with 3-hydroxypropionic acid was
found by HPLC analysis in the mitochondrial incubation mixtures. The
authors suggested that acrylic acid is metabolized in vitro by
mammalian enzymes to CO2 via 3-hydroxypropionate by the non-vitamin-
B12 - dependent pathway for propionate metabolism (Finch & Frederick,
1992).
The oxidation rate of acrylic acid in 13 different tissues
(liver, kidney, forestomach, glandular stomach, small and large
intestine, spleen, brain, heart, lung, skeletal muscle, fat and skin)
of male and female C3H/HeNCrlBR mice was measured by incubating tissue
slices with (1-14C)-acrylic acid and collecting 14CO2. All the
tissues studied oxidized acrylic acid to a certain extent, but
activity in kidney, followed by liver, was much higher than in other
tissues. Oxidation of acrylic acid followed pseudo-Michaelis-Menten
kinetics in the liver, kidney and skin, with a Km for all these
tissues of approximately 0.67 mM. Marked differences were observed in
the Vmax values, 2890 ± 436 nmol/h per g for kidney, 616 ± 62 nmol/h
per g for liver and 47.9 ± 5.8 nmol/h per g for skin. Half-lives in
these tissues were 0.13, 0.867 and 10.2 h, respectively. Lung,
glandular stomach, heart, spleen, fat and large intestine preparations
oxidized acrylic acid at rates from 10 to 40% of the rate determined
in the liver; in the remaining tissues reaction rates were less than
10% of those in the liver. Rates of metabolism in tissues from male
and female mice were similar.3-Hydroxypropionic acid was the only
metabolite detected by HPLC analysis following incubation of tissues
with (1-14C)-acrylic acid. To determine if CO2 was formed from the
C1 carbon, and if acetyl-CoA was derived from carbons 2 and 3 of
acrylic acid, the authors incubated (2,3-14C)-acrylic acid and
(1-14C)-acetate with liver and kidney slices and measured the rate of
14CO2 formation. It was concluded that CO2 originated from C1, but
that acetyl-CoA was derived from carbons 2 and 3 of acrylic acid. Both
substrates were oxidized well by the tissues, thus providing for the
complete metabolism of acrylic acid to CO2. The results demonstrate
that the rate of acrylic acid metabolism varies significantly among
mouse tissues and suggested that the kidneys and liver are major sites
of acrylic acid metabolism (Black et al., 1993).
6.2.2.2 In vivo investigations
After oral administration of (2,3-14C)-acrylic acid (4, 40 or
400 mg/kg body weight in 0.5% methylcellulose) to male Sprague-Dawley
rats, the major portion of the radioactivity (up to 65%) was exhaled
as 14CO2 within 24 h. In urine four metabolites were identified by
HPLC analysis. One of the two major metabolites eluted very near to
the solvent front and did not co-elute with acetic acid pyruvic acid
or lactic acid. The second metabolite co-eluted with 3-hydroxypro-
pionic acid. Traces of two other unidentified residues were also
detected. Radioactivity could not be detected at the retention times
corresponding to that of 2,3-epoxypropionic acid, glyceric acid or
N-acetyl- S-(2-carboxy-2-hydroxyethyl)-cysteine, suggesting that
acrylic acid is not epoxidized to 2,3-epoxypropionic acid in vivo.
It was suggested that acrylic acid was metabolized by the non-vitamin-
B12-dependent pathway for propionic acid metabolism, with degradation
to CO2 being the main route of elimination. Residual radioactivity in
tissues may be due to incorporation of 14C from acrylic acid into
acetyl-CoA (De Bethizy et al., 1987).
Using HPLC and NMR analysis, 3-hydroxypropionic acid, N-acetyl-
S-2-(2-carboxyethyl)-cysteine and N-acetyl- S-(2-carboxyethyl)-
cysteine-S-oxide were identified as urinary metabolites after oral
administration of (2,3-14C)-acrylic acid (400 mg/kg body weight in
water by gavage) to male Sprague-Dawley rats. According to the
authors, the detection of mercapturates may be a consequence of the
high dose used in this study (Winter et al., 1992).
HPLC analysis for acrylic acid and its metabolites in rats
revealed that a metabolite that coeluted with 3-hydroxypropionic acid
was found in the urine, plasma and liver of rats that had received
acrylic acid by gavage. Furthermore, a material that co-eluted with
authentic acrylic acid was detected in the urine and liver, but not in
the plasma, of these rats. Acrylic acid, but not 3-hydroxypropionic
acid, was also detected in the urine of rats after cutaneous
application (Black et al., 1995). In mice, 3-hydroxypropionic acid was
identified in the liver after gavage administration of acrylic acid.
No acrylic acid was detected in the liver of these animals (Black et
al., 1995).
6.2.2.3 Metabolic pathways
Acrylic acid is rapidly metabolized to CO2, a major metabolite
formed via acrylyl-CoA by the non-vitamin-B12-dependent pathway of
mammalian propionate catabolism (Finch & Frederick, 1992; Winter et
al., 1992; Black et al., 1993; Winter & Sipes, 1993). This pathway
occurs in the mitochondrion (Finch & Frederick, 1992) and consist of
reactions analogous to fatty acid ß-oxidation (Schultz, 1991).
ß-oxidation is the major route of propionate catabolism in many
invertebrates and plants (Wegner et al., 1968; Halarnkar & Blomquist,
1989); however the primary pathway of propionate catabolism in mammals
is that involving the vitamin-B12-dependent enzyme, methyl-malonyl-
CoA mutase (Black et al., 1993). A small amount of 3-hydroxypropionic
acid was identified as the major urinary metabolite of acrylic acid
(De Bethizy et al., 1987; Winter et al., 1992). There is no evidence
to suggest that epoxide intermediates are formed during the metabolism
of acrylic acid (De Bethizy et al., 1987). N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl) cysteine-
S-oxide were identified in the urine of rats that had received
400 mg/kg (2,3,-14C)-acrylic acid by gavage (Winter et al., 1992),
suggesting a direct reaction between acrylic acid and reduced
glutathione.
The major route of metabolism for acrylic acid esters has been
shown to involve the rapid cleavage of the ester bond by carboxyl
esterases (see Fig. 1) (Ghanayem et al., 1987; Sanders et al., 1988;
Frederik et al., 1994). Thus exposure to acrylic acid esters may
constitute a significant internal exposure to acrylic acid. A
secondary metabolic pathway involves conjugation of the acrylic acid
ester with glutathione to yield acetyl- S-(2-carboxyethyl) cysteine
alkylesters. (Ghanayem et al., 1987; Sanders et al., 1988). This
intermediate may be further metabolized to N-acetyl- S-(2-
carboxyethyl) cysteine and N-acetyl- S-(2-carboxyethyl)-cysteine-
S-oxide. However, it is currently uncertain what proportion of N-
acetyl- S-(2-carboxyethyl) cysteine, or its oxide, formed from the
metabolism of the acrylic acid esters originates from the reaction of
the intact ester with glutathione and what proportion originates from
the conjugation of the released acrylic acid with glutathione (see Fig
1).
On the basis of available information, proposed metabolic
pathways for acrylic acid are summarized in Fig. 1. The proposed
scheme also includes relationships between metabolism of acrylic acid
and its esters (e.g., ethyl acrylate) and metabolism of propionate via
the major vitamin-B12-dependent pathway.
 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of acrylic acid is difficult to ascertain,
owing to the wide range of LD50 values reported (Table 4). However
most data indicate that the substance is of low to moderate toxicity
by the oral and inhalation routes and of moderate toxicity by the
dermal route. It has been proposed that the wide variation in oral
LD50 values may be due to the different forms in which acrylic acid
has been applied, i.e. undiluted, in aqueous solution at various
concentrations or in neutralized solution (BG Chemie, 1991).
Stomach lesions, necrosis and haemorrhage have been reported
following oral dosing of rats with acrylic acid (Ghanayem et al.,
1985; DeBethizy et al., 1987). The lowest dose at which lesions were
seen was 144 mg/kg.
CrL:CDBR rats were exposed (whole body) to aerosol (mean mass
median diameter of 2.3 µm ± 2.3) concentrations of acrylic acid
ranging from 8775 to 14 145 mg/m3 (2925 to 4715 ppm) for 30 min, 8139
to 12 624 mg/m3 (2713 to 4208 ppm) for 60 min, and 3669 to
10 239 mg/m3 (1223 to 3413 ppm) for 120 min. Additionally, rats were
exposed (whole body) to acrylic acid vapour concentrations ranging from
2784 to 6426 mg/m3 (928 to 2142 ppm) for 60 min. Exposure to acrylic
acid produced treatment-related signs of nasal mucosa, upper airway and
lower airway irritation, ocular irritation, corneal opacities and
dermal toxicity in all experimental groups. Deaths, as a function of
both aerosol concentration and exposure duration, were seen in the
30-, 60- and 120-min aerosol exposures. No deaths resulted from the
vapour exposures. Following the 14-day observation period, necropsies
revealed treatment-related alterations of the lungs, eyes and skin
consistent with that of an irritant. However, comparison of LC values
suggested no difference in toxicity between the aerosol and vapour
(Hagan & Emmons, 1991).
Table 4. The acute toxicity (LD50 and LC50) of acrylic acid for experimental animals
Species Route Parameter Dose Reference
Mouse oral LD50 830 mg/kg Klimkina et al., 1969
Mouse oral LD50 1200 mg/kg Zeller, 1958
Rat oral LD50 193 mg/kg IARC, 1979
Rat oral LD50 340 mg/kg Carpenter et al., 1974
Rat oral LD50 1350 mg/kg Majka et al., 1974
Rat oral LD50 1500 mg/kg Zeller, 1958
Rat oral LD50 2520 mg/kg Fassett, 1963
Rat oral LD50 2100-3200 mg/kg Miller, 1964
Rat oral LD50 2500 mg/kg Verschueren, 1983
Mouse subcutaneous LD50 1590 mg/kg Sittig, 1985
Rabbit percutaneous LD50 295 mg/kg Carpenter et al., 1974
Rabbit percutaneous LD50 640 mg/kg Gelbke & Hofman, 1979
Rabbit percutaneous LD50 750 mg/kg IARC, 1979
Rabbit percutaneous LD50 950 mg/kg Fassett, 1963
Mouse inhalation LC50 (2 h) 5300 mg/m3 RTECS, 1989
Rat inhalation LC50 (30 min) 26 000 mg/m3 Hagan & Emmons, 1991
LC50 (60 min) 11 100 mg/m3
LC50 (120 min) 7500 mg/m3
Table 4. (contd.)
Species Route Parameter Dose Reference
Rat inhalation LC50 (4 h) 3600 mg/m3 Majka et al., 1974
Rat inhalation LC50 (4 h) > 5100 mg/m3 Klimisch & Zeller, 1980
Mouse intraperitoneal LD50 17 mg/kg Lawrence et al., 1972
Mouse intraperitoneal LD50 128 mg/kg RTECS, 1989
Mouse intraperitoneal LD50 140 mg/kg Zeller, 1958
Rat intraperitoneal LD50 24 mg/kg Majka et al., 1974
Rat intraperitoneal LD50 24 mg/kg Singh et al., 1972
In a single inhalation study in rats, no deaths occurred when
six animals were exposed to acrylic acid at a concentration of
12 000 mg/m3 (4000 ppm) for 4 h and observed over 14 days (Union
Carbide Corp., 1977).
A single 4-h exposure of six rats to 6000 mg/m3 (2000 ppm) of
acrylic acid caused no death (Carpenter et al., 1974).
One 5-h exposure to an atmosphere saturated with acrylic acid
(6000 ppm or 17 700 mg/m3) given to four rats (2 male, 2 female)
produced nose and eye irritation, respiratory difficulty and
unresponsiveness in all rats. One rat died. Histopathological
examination showed lung haemorrhage and degenerative changes in the
liver and kidney tubules of all rats (Gage, 1970), but these were
possibly secondary changes in dying animals.
Rats exposed for 1 h to acrylic acid concentrations of 300, 900
or 1500 mg/m3 (100, 300 or 500 ppm) exhibited exposure-dependent
decreases in both respiratory frequency and minute volume (Silver et
al., 1981).
In a sensory irritation study, the single exposure to acrylic
acid vapour estimated for a 50% reduction of the respiratory rate
(RD50) was 1539 mg/m3 (513 ppm) in F344/N rats and 2055 mg/m3
(685 ppm) in B6C3F1 mice. During exposure to 225 mg/m3 (75 ppm) of
acrylic acid vapour for 6 h, a 20-30% decrease in minute volume was
observed in both species (Buckley et al., 1984).
7.2 Irritation and sensitization
7.2.1 Eye irritation
Application of acrylic acid in different concentrations (glacial,
10%, 3% and 1%) to rabbit eyes revealed that it is corrosive in high
concentrations, i.e. glacial and 10%; 1% and 3% solution caused eye
irritancy (Majka et al., 1974). There are also other reports of
undiluted acrylic acid causing eye irritation and corneal damage
(Carpenter et al. 1974; BG Chemie, 1991).
7.2.2 Skin irritation and sensitization
7.2.2.1 Skin irritation
Undiluted acrylic acid is corrosive to rabbit skin (Carpenter et
al., 1974; Majka et al., 1974; BG Chemie, 1991). A study with rabbits
reported that a one-minute exposure to a 50% or 20% aqueous solution
caused, respectively, erythema and oedema or slight erythema (BG
Chemie, 1991). Another study reported a 10% solution to be corrosive
when applied to rabbit skin and that a 0.6-5% solution caused
irritation of various severity (Majka et al., 1974).
The irritant effects of repeated dermal exposure have also been
investigated. A 5% acrylic acid solution in acetone caused skin
irritation in the mouse after daily non-occlusive application for 14
days (DePass et al., 1984). No irritation was seen with a 1% solution.
In another study, groups of three strains of mice received dermal
applications of 0.1 ml acrylic acid in acetone 3 times a week for 13
weeks at concentrations of 0, 1 or 4% (Tegeris et al., 1987, 1988). At
4%, there were signs of significant skin irritation (desquamation,
fissures and eschar), with proliferative, degenerative and
inflammatory changes being detected histologically in the epidermis
and dermis, from weeks 1 to 2. At 1%, minimal proliferative changes,
detected histologically, were the only effects seen. No differences
were found between the response of the three strains of mice.
7.2.2.2 Skin sensitization
Acrylic acid has been tested for contact sensitivity in guinea-
pigs. In one study, a 20% aqueous solution of pure unstabilized
acrylic acid was applied to the skin once a day until definite skin
irritation was seen. When challenged topically 11 days later with a 2%
solution, there was no evidence of skin sensitization up to 24 h post-
challenge (BG Chemie, 1991).
In another study, the highest non-irritating concentration of
acrylic acid (not specified) was applied topically four times in 10
days. At the time of the third application, Freund's adjuvant was
injected intradermally. When challenged two weeks later, none of the
10 guinea-pigs showed evidence of skin sensitization (Rao et al.,
1981).
Three out of six guinea-pigs exposed to acrylic acid, said to be
99% pure, showed a skin sensitization response in a Polak test (Parker
& Turk, 1983). Induction was by dermal injections of a total of 1 mg
acrylic acid, together with adjuvant, followed by topical challenge
with 5% acrylic acid. However, the impurities and inhibitors of the
acrylic acid used were not mentioned in the report.
Acrylic acid was found to be an extreme sensitizer by the guinea
pig maximization test and a weak sensitizer by the Landsteiner Draize
test. The compound used for testing was considered pure, but no
analytical data were provided (Magnusson & Kligman, 1969).
Acrylic acid gave a clearly positive result in the Freunds
Complete Adjuvant test in guinea-pigs (Waegemaekers & van der Walle,
1984). Induction was by three intradermal injections of 1.2% followed
by topical application of 2.2 or 7.2%. The positive response was
believed to be due to the historical impurity, alpha,ß-
diacryloxypropionic acid. This impurity was identified in acrylic acid
from just one of three suppliers. Limited testing of acrylic acid from
the other two suppliers gave negative skin sensitization results. It
should be noted that the impurity is not present in acrylic acid
resulting from current production methods involving distillation.
Commercial acrylic acid also contains a small amount of
polymerization inhibitors, usually hydroquinone monomethyl ether
(methoxyphenol). This is a known skin sensitizer in guinea-pigs (van
der Walle et al., 1982). Other inhibitors used with acrylic acid have
also been reported to have skin-sensitizing properties, namely pheno-
thiazine (Costellati et al., 1990) and diphenyl- p-phenylenediamine
(Magnusson et al., 1968; Kalimo et al., 1989). However, it is unclear
whether the small amount of one of these inhibitors present
(0.02-0.1%) could contribute to the skin-sensitizing properties of
commercial acrylic acid.
7.2.3 Upper respiratory tract irritation
Olfactory cell proliferation, as measured by tritiated thymidine
incorporation, was investigated in male F-344 rats and B6C3F1 mice
exposed to 224 mg/m3 (75 ppm) acrylic acid 6 h daily for 5 days. A
17-fold increase in cell proliferation occurred in mice and a 4-fold
increase in rats (Swenberg et al, 1986). Further information on upper
respiratory tract irritation is given in sections 7.1 and 7.3.2.
7.3 Short-term exposure
Results of key studies on the short-term repeated exposure
effects of acrylic acid are presented in Table 5.
Table 5. Key studies on the noncarcinogenic effects of repeated exposures to acrylic acid
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Fisher-344 oral, 250 mg/kg bw/day 83 mg/kg bw/day Decreased body weight, De Pass et al.,
drinking-water, 0, 83, reduced water and food 1983
250, 750 mg/kg body consumption, changes in
weight/day for 3 months organ weights
Rat, Wistar, gavage, 0, 150 mg/kg bw/day 50% mortality in both Hellwig et al.,
150, 375 mg/kg bw/day, treatment groups, dose- 1993
5 times/week for 3 dependent irritation in the
months forestomach and
glandular stomach,
purulent rhinitis, tubular
nephroses
Rat, Wistar, oral,
drinking-water, 0, 9, 61,
140, 331 mg/kg body
weight/day for 3 months 331 mg/kg bw/day 140 mg/kg bw/day Reduced water and food Hellwig et al.,
consumption in males 1993
Rat, Wistar, oral, 140 mg/kg bw/day 61 mg/kg bw/day Reduced water and food Hellwig et al.,
drinking-water, 0, 9, 61, consumption in males 1993
140, 331 mg/kg body
weight/day for 12 months
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Wistar, oral, 78 mg/kg bw/day No treatment-related Hellwig et al.,
drinking-water, 0, 8, 27, toxic effects including 1993
78 mg/kg body tumorogenicity
weight/day for 26
(males) or 28 (females)
months
Rat, inhalation, 80, 300 300 ppm 80 ppm Nose irritation, lethargy Gage, 1970
ppm, 6 h/day 5 (900 mg/m3) (240 mg/m3) reduced body weight gain
days/week,
20 exposures
Rat, Fisher-344 225 ppm (675 75 ppm Decrease of adipose Miller et al.,
inhalation, 0, 25, 75, mg/m3) (225 mg/m3) tissue in females, lesions 1979
225 ppm, 6 h/day, of nasal mucosa
5 days/week for 2 weeks
Rat, Fisher 344 75 ppm 25 ppm Lesions of nasal olfactory Miller et al.,
inhalation, 0, 5, 25, (225 mg/m3) (75 mg/m3) epithelium 1981
75 ppm, 6 h/day,
5 days/week for
13 weeks
Rat, F-344, and mouse 75 ppm Olfactory cell proliferation Swenberg et al.,
B6C3F1 inhalation, (225 mg/m3) 17-fold in mice, 4-fold in 1986
75 ppm, 6 h/day, 5 days rats
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Mouse, B6C3F1 25 ppm Decrease in body weight Miller et al.,
inhalation, 0, 25, 74, (75 mg/m3) gain, lesions in nasal 1979
223 ppm, 6 h/day mucosa
5 days/week for 2 weeks
Mouse, B6C3F inhalation 5 ppm 5 ppm Atrophy, disorganization, Lomax et al.,
0, 5, 25 ppm for 6 or 22 22 h/day 6 h/day necrosis of the olfactory 1994
h/day and 25 ppm for epithelium of nasal
4.4 h/day for 2 weeks, cavity. Recovery after 6
6 weeks recovery period weeks except for mice
exposed to 25 ppm for
22 h/day where
metaplasia was seen
Mouse, B6C3F1 5 ppm Slight focal lesions of Miller et al.,
inhalation, 0, 5, 25, (15 mg/m3) nasal olfactory 1981
75 ppm, 6 h/day 5 epithelium
days/week for 13 weeks
a LOEL = lowest-observed-effect level; NOEL = no-observed-effect level
7.3.1 Oral
Acrylic acid was administered via oral gavage to ten rats for 20
days with doses increasing by 50% every fourth day (range:
135 mg/kg to 684 mg/kg). Reduction in body weight gain and minor
histopathological changes in the stomach were found at higher doses
(Majka et al., 1974).
In a 3-month study (Hellwig et al., 1993), groups of 10 male and
10 female Wistar rats were gavaged, 5 times per week, with acrylic
acid at doses of 150 or 375 mg/kg body weight. A control group of 10
males and 10 females was gavaged with water. A high mortality rate was
observed in experimental groups; 50% of both males and females in the
low-dose group and 60% (males) and 90% (females) in the high-dose
group died. Cyanosis, dyspnoea and irritation ulceration of
forestomach and glandular stomach, purulent rhinitis and lung
emphysema and alveolar hyperaemia were the main findings reported.
Necrotizing tubular nephroses were seen in the animals that died
during the study. The symptoms and histopathological findings were
substantially the same in both groups, but they were less pronounced
and observed in a smaller number of animals given acrylic acid at
150 mg/kg body weight.
Acrylic acid was given to Wistar rats in drinking-water for
3 months as part of a 12-month study (Hellwig et al., 1993). Further
details are given in section 7.4.
In a subchronic study acrylic acid was incorporated into the
drinking-water of rats (15/sex/group) for 3 months, resulting in doses
of 0,83, 250 and 750 mg/kg per day. At the high and intermediate dose
levels, reduction in body weight gain and changes in organ weights
were observed. These effects coincided with a dose-related reduction
in food and water consumption. At the 83 mg/kg dose, the only effect
was a slight reduction in water consumption. No significant treatment-
related histological effects were seen at any dose level (DePass et
al., 1983).
7.3.2 Inhalation
In a short-term inhalation study (Gage, 1970) no adverse effects
were observed in eight rats (four males and four females) exposed to
240 mg/m3 (80 ppm) acrylic acid vapour, 6 h/day, 5 days/week for 20
exposures. Eight rats (four males and four females) exposed at
900 mg/m3 (300 ppm) showed signs of nasal irritation, lethargy and
reduced body weight gain. Histological and haematological examinations
were normal. Higher doses for shorter periods of time, i.e. 4500 mg/m3
(1500 ppm) for 4 × 6 h, resulted in nasal discharge, lethargy,
retarded weight gain and kidney congestion.
In a 2-week subacute inhalation study by Miller et al. (1979a,
1981a), F-344 rats and B6C3F1 mice (5/sex/group) were exposed to
actual concentrations of 0, 75, 225 or 675 mg/m3 (0, 25, 75 or
225 ppm) acrylic acid vapour for 6 h/day, 5 days/week for 2 weeks.
Significant decreases in body weight gain were seen in exposed groups
at 675 mg/m3. Decreased body weight gain in male mice at 75 and
225 mg/m3 was not considered to be exposure-related because of the low
initial weight and unusually large weight gain in the controls. A
reduction of adipose tissue was observed in female rats at 675 mg/m3.
Rats had lesions of the nasal mucosa at 675 mg/m3. In mice lesions of
the nasal mucosa were observed in 2/5 males and 4/5 females at 75 mg/m3
and in all mice exposed to 225 or 675 mg/m3. The lesions were
described as slight focal degeneration in the olfactory epithelium. No
effects on lung or trachea were observed in rats or mice.
Fifteen F-344 rats/sex/group and 15 B6C3F1 mice/sex/group were
exposed to actual concentrations measured by infrared analysis of 0,
15, 75 or 225 mg/m3 (0, 5, 25 or 75 ppm) acrylic acid (Miller et al.,
1979a, 1981a). The exposure was 6 h/day for 5 days/week for 13 weeks.
Animals were observed twice per day. There were no treatment-related
deaths in rats or mice during the study period. Mean body weight gains
of female mice in the 75 and 225 mg/m3 exposure groups after 12 weeks
of exposure were significantly lower than controls. There were no
significant differences in organ weights, clinical chemistry
parameters, urinalysis parameters or gross pathology that could
clearly be related to exposure. Slight focal degeneration of the nasal
olfactory epithelium was observed in rats at 225 mg/m3 but no effects
were seen at 15 or 75 mg/m3. In mice, there was a clear exposure-
related increase in focal degeneration of the olfactory nasal
epithelium at > 25 mg/m3. Lesions of the olfactory epithelium were
detected histopathologically in all male and female mice in the
225 mg/m3 exposure group, in all males and 9/10 females in the
75 mg/m3 group and in 1/10 males and 4/10 females in the
15 mg/m3 group. The lesions were described as very slight at 15 mg/m3,
slight at 75 mg/m3 and slight to moderate at 225 mg/m3.
In a whole-body inhalation study, groups of 10 B6C3F1 female
mice were exposed to 0, 15 or 75 mg/m3 (0, 5 or 25 ppm) acrylic acid
vapour for 6 or 22 h/day for 2 weeks. An additional group of mice was
exposed to 75 mg/m3 for 4.4 h/day for 2 weeks. No histopathological
lesions in the nasal cavity were detected in mice exposed to 0 or 15
mg/m3 for 6 h/day. Histopathological lesions were observed in the
olfactory epithelium of the dorsal meatus of the nasal cavity in all
other groups. These lesions included atrophy, disorganization,
necrosis, desquamation of the epithelium and nasal cell hypertrophy.
Lesions were more severe after the 22 h exposure at 75 mg/m3. After a
6-week recovery period, the olfactory epithelium was normal except for
mice exposed to 75 mg/m3 for 22 h/day where metaplasia was seen
(replacement of olfactory epithelium with respiratory-like epithelium)
(Lomax et al., 1994).
Animals exposed to 720 mg/m3 (240 ppm) for 4 h/day, 6 days/week,
for 5 weeks exhibited decreased body weight gain, increased urinary
secretion of phenol red, reduced urine-concentrating ability, nasal
discharge and increased reticulocyte count. Histopathological
examination revealed lesions of the gastric mucosa after repeated
doses of acrylic acid. Inflammation of the upper respiratory tract was
also seen (Majka et al., 1974).
7.4 Long-term exposure
Results of the studies of long-term repeated exposure effects to
acrylic acid are presented in Table 5.
In a 12-month study (Hellwig et al., 1993), Wistar rats
(20/group/sex) were given drinking-water containing 120, 800, 2000 or
5000 mg/litre (equivalent to doses of 9, 61, 140, 331 mg/kg body
weight/day, respectively). Satellite groups (10 rats/group/sex) were
treated concurrently for 3 months. Both in satellite groups and
12-months exposure groups, a reduction in drinking-water and/or feed
consumption and retarded body weight gain were observed in males at
140 and 331 mg/kg per day (2000 and 5000 mg/litre), probably due to
the unpalatability of acrylic acid at these concentrations.
Differences between groups in the results of clinico-chemical,
haematological and urinalytical examinations as well as in gross
pathological and histopathological findings were not treatment-
related. The no-observed-effect level (NOEL) was 61 mg/kg body weight
(800 mg/litre).
Acrylic acid was given to Wistar rats in drinking-water for 26
(males) and 28 (females) months in a chronic/carcinogenicity study
(Hellwig et al., 1993). Further details are given in section 7.7.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
Results of key studies on the reproductive system are shown in
Table 6.
Table 6. Reproductive effects of acrylic acid exposure in female rats and mice and developmental effects in their offspring
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Reproduction Rat Fischer 250 mg/kg 83 mg/kg 750 mg/kg 250 mg/kg Decreased body Decrease in
and development 344; oral, bw/day bw/day bw/day bw/day weight and food body weight gain
(dePass et al., drinking- consumption of male and
1983) water; 0, 83, female pups,
250, 750 mg/kg decrease in
bw/day for 3 liver, kidney,
months before heart and spleen
mating and weight in male
through-out pups and liver
gestation and weight in female
lactation pups
Two-generation Rat Wistar; 240 mg/kg 53 mg/kg 240 mg/kg 53 mg/kg Fertility not Retarded growth
reproductive oral, drinking- bw/day bw/day bw/day bw/day affected. Reduced of F1 and F2 pups
toxicity water, 0, 500, food and water
(BASF, 1994a) 2500, 5000 ppm consumption,
(53, 240, reduced body
460 mg/kg/day) weights, gross
and
histopathological
changes in stomach
Developmental Rat Sprague- - - 2.4 mg/kg No data Increased
toxicity Dawley, bw resorptions
(Singh et al., intraperitoneal and number
1972) 0, 2.4, 4, 8, of gross and
8.0 mg/kg bw skeletal
i.p. on days 5, abnormalities
10 and 15 of
gestation
Table 6. (contd.)
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Developmental Rat Sprague- 360 mg/m3 - - 1080 Decreased None
toxicity Dawley, (120 ppm) mg/m3 body weight,
(Klimisch & inhalation, (360 ppm) food consumption
Hellwig, 0, 40, 120, uterus-to-body
1991) 360 ppm for weight; eye and
10 days during nose irritation
gestation
(days 6
through
15)
Developmental Rabbit New 180 90 mg/m3 - -- Squamous -
toxicity Zealand white, mg/m3 (30 ppm) metaplasia
(Chun et al., inhalation, 0, (60 ppm) erosions,
1993) 30, 60, 125, ulcerations in
250 ppm (0, olfactory
90, 180, 375, epithelium
750 mg/m3)
during
gestation
(days 10-23)
Developmental Rabbit New 225 mg/m3 75 mg/m3 - 675 Perinasal, None
toxicity Zealand white, (75 ppm) (25 ppm) mg/m3 perioral
(Neeper- inhalation (225 ppm) wetness,
Bradley & 0, 25, 75, nasal
Kubena, 1993) 225 ppm congestion,
(0, 75, 225, blepharospasm
675 mg/m3)
during
gestation
(days 6-18)
A one-generation study was conducted in which 10 male and 20
female rats received acrylic acid orally, in drinking-water, at doses
of 83, 250 or 750 mg/kg body weight per day for 3 months, after which
the animals were mated; this formed part of the subchronic study
reported in section 7.3.1. (DePass et al., 1983). Exposure was
continued throughout gestation and lactation.
Maternal toxicity at the two highest doses included decreased
body weight gain and decreased food consumption. An apparent decrease
in the fertility of females, as well as a reduction in gestation
index, number of live pups/litter and percentage of pups weaned at the
highest dose, was observed, but this difference was not statistically
significant when compared with the control group. Unusually low
fertility in the control group makes interpretation difficult.
In a two-generation reproductive toxicity study (BASF, 1994a),
Wistar rats were exposed to acrylic acid in drinking-water at levels
of 500, 2500 or 5000 mg/litre. Acrylic acid did not impair
reproductive function in either of the parental generations exposed to
any dose level. Clear signs of toxicity, including reduced food and
water consumption, reduced body weight and/or body weight gain, and
gross and histopathological changes in the stomach, were observed in
both parental generations exposed to 5000 mg/litre. At the
2500 mg/litre level, signs of toxicity were present in the second
parental generation animals. No adverse effects were observed in either
parental generation exposed to 500 mg/litre. Signs of developmental
toxicity, including retarded growth of F1 and F2 pups and some
delays in physical development of F2 pups only, were observed at
5000 mg/litre but less so at 2500 mg/litre. It is difficult to evaluate
the role of acrylic acid in the decreased pup weights, because of the
combined effects of reduced water consumption and the poor
palatability of water containing acrylic acid. The greatest effects on
body weight occurred during the period of early life around weaning.
Developmental toxicity was not observed at 500 mg/litre. Thus,
500 mg/litre (equivalent to 53 mg/kg per day) was a NOEL for both
generations of offspring and also a NOAEL for general toxicity in the
second parental generation. The NOEL for general toxicity in the first
parental generation was 2500 mg/litre (equivalent to 240 mg/kg per
day).
In a study by Vojtisek et al. (1991) cows were given 16 kg/day
clover grass silage, treated with 3 litres acrylic acid/tonne, from
day 57.6 ± 21.1 before parturition, for 107 days. Control groups of
eight cows were given the same amount of silage, but treated with 4
litres formic acid/tonne from day 55.5 ± 21.9 before parturition.
Changes in clinical chemistry and haematological parameters observed
in the course of the experiment (on days 0, 39, 65 and 107) were of no
toxicological importance. No differences in colostrum density, body
weight, clinico-chemical or haematological examinations of live calves
were seen.
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Oral
In the experiment of DePass et al., (1983) (see also section
7.5.1) there was a statistically significant decrease in body weight
of the male and female pups at the highest dose (750 mg/kg per day),
which was maternally toxic. The male pups also exhibited significant
decreases in absolute and relative liver weights and in absolute
kidney and heart weights at 750 mg/kg per day. The female pups showed
a significant decrease in absolute and relative spleen weight and in
absolute liver weight at the highest dose. There was an increase in
relative brain weight in both sexes at this dose.
7.5.2.2 Inhalation
Groups of 30 pregnant Sprague-Dawley rats were exposed to nominal
concentrations of 0, 120, 360 or 1080 mg/m3 (0, 40, 120, or 360 ppm)
acrylic acid vapour for 6 h/day for 10 days during gestation (day 6 to
day 15 of gestation) (Klimisch & Hellwig, 1991). There was clear
evidence of maternal toxicity at 1080 mg/m3 consisting of eye and
nose irritation, as well as reduced body weight gain and food
consumption. The latter two effects were also seen at 360 mg/m3 and
there was an indication of minimal maternal toxicity at 40 ppm
(reduced body weight gain). There were no effects on preimplantation
loss, the number of live fetuses and resorption, fetal size or on the
appearance of the soft tissues and skeleton of the fetuses. This study
identified a NOAEL for developmental effects of 1080 mg/m3 and a
LOAEL for maternal toxicity of 1080 mg/m3.
An inhalation developmental study has also been reported in
rabbits (Neeper-Bradley & Kubena, 1993). In the range-finding study
(Chun et al., 1993), groups of eight pregnant New Zealand white
rabbits were exposed to 0, 90, 180, 375 or 750 mg/m3 (0, 30, 60, 125
or 250 ppm) acrylic acid vapour for 6 h/day on days 10-23 of
gestation. Three animals per group were necropsied on day 23 of
gestation, and the remaining animals were examined on day 29.
Exposure-related maternal toxicity at 375 and 750 mg/m3 was observed,
including signs of nasal irritation and reduced body weight. Final
body weight was reduced to a lesser degree in animals exposed to 90
and 180 mg/m3. Histopathological examination of a single section of
the nose showed adverse effects in the olfactory epithelium. The
lesions included squamous metaplasia, epithelial erosion and
ulceration of the epithelium, and increased in severity with
increasing exposure concentration. The effect first appeared in the
90 mg/m3 group at day 23 and in the 180 mg/m3 group at day 29.
In the main developmental study (Neeper-Bradley & Kubena, 1993), groups
of 16 pregnant rabbits were exposed to 0, 75, 225 or 675 mg/m3 (0, 25,
75 or 225 ppm) acrylic acid vapour for 6 h/day on gestation days 6-18.
Maternal toxicity was evident in groups exposed to 225 or 675 mg/m3,
but not to 75 mg/m3 (NOEL). Signs of nasal irritation, including
perinasal wetness and nasal congestion, were observed. Significant
decrements in food consumption and body weight gain were observed
occasionally during exposure, but the body weights at the end of the
exposure were not significantly affected. Histopathological
examination of maternal tissues was not performed. No exposure-
related adverse effects were observed in the number of corpora lutea
and total, viable or nonviable implantations; preimplantation loss;
fetal length or weight; or on morphological abnormalities (skeletal
or soft tissue). This study identified a NOEL for developmental
effects of 675 mg/m3.
7.5.2.3 Intraperitoneal
When acrylic acid was injected intraperitoneally into pregnant
female Sprague Dawley rats at doses of 0, 2.4, 4.8, or 8.0 mg/kg on
days 5, 10 and 15 of gestation, the chemical was both embryotoxic and
teratogenic (Singh et al., 1972). The number of resorptions and the
number of gross and skeletal abnormalities increased with increasing
acrylic acid concentration, and the NOEL was identified at the low
dose (i.e. 2.4 mg/kg). Fetotoxicity (decreased number of live fetuses
and mean fetal weight) was observed at a dose of 2.4 mg/kg. This study
is difficult to interpret as the control groups treated with distilled
water, normal saline and cotton-seed oil also showed gross and
skeleton abnormalities. There was also no information about maternal
toxicity.
7.6 Mutagenicity and related end-points
7.6.1 In vitro and insect studies
Acrylic acid was found to be without mutagenic activity in five
test strains of Salmonella typhimurium with and without activation
by rat and hamster liver microsomal preparations using both plate and
liquid suspension assays (Lijinsky & Andrews, 1980). Although negative
responses were reported in all strains, no cytotoxicity was observed
at the concentrations tested (up to 1000 µg/plate).
Acrylic acid was also evaluated for mutagenicity in the
Salmonella/microsome preincubation assay using the standard protocol
approved by the National Toxicology Program. Acrylic acid was tested
at doses of 0, 10, 33, 100, 333, 1000 and 3333 µg/plate in four
Salmonella typhimurium strains (TA98, TA100, TA1535 and TA1537) in
the presence and absence of Aroclor-1254-induced rat or hamster liver
S9. Acrylic acid was negative in these tests and the highest non-toxic
dose level tested in any Salmonella test strain was 1000 µg/plate. The
3333 µg/plate dose level was toxic and caused a complete clearing of
the background lawn (Zeiger et al., 1987).
In another study (Cameron et al., 1991) acrylic acid was also
found to be negative in the Salmonella assay, both in the presence and
the absence of both Aroclor-1254-induced rat or hamster liver S9 mix.
Three mammalian gene mutation studies have been reported. No
increase in mutation frequency was seen in a CHO/HPRT gene mutation
assay with or without Aroclor 1254-induced rat liver S9 (McCarthy et
al., 1992). The upper dose levels in this single experiment resulted
in a reasonable amount of toxicity (survival reduced by up to 65-76%).
In two mouse lymphoma L5178Y TK+/- studies positive results have been
obtained. A concentration-related increase in mutant frequency was
observed with and without rat liver S9 in association with an
acceptable level of toxicity (Cameron et al., 1991). The authors did
not state if there was any adjustment of pH. In the other study,
conducted without exogenous metabolic activation, there were
concentration-related increases in mutant frequency in two separate
experiments in the presence of marked but not excessive toxicity.
Small colonies predominated, which suggested a clastogenic effect;
this was confirmed by chromosome analysis. Acrylic acid was tested at
concentrations of 300, 450 and 500 µg/ml in this study but the authors
did not state if there was any adjustment of pH (Moore et al., 1988).
A single experiment was conducted with CHO cells exposed to
acrylic acid solutions adjusted to pH 7 at concentrations that reduced
cloning efficiency by up to 58-65% (McCarthy et al., 1992). A
concentration-related increase in the percentage of cells with
chromosome aberrations, primarily chromatid breaks and exchanges, was
observed in the presence and absence of rat liver S9. A positive
result has also been briefly reported for this in vitro chromosome
aberration assay using CHL cells in the absence of exogenous metabolic
activation (Ishidate, 1988).
The effect of acrylic acid on unscheduled DNA synthesis (UDS) in
rat hepatocytes has been investigated in one unreplicated assay
(McCarthy et al., 1992). There was no increase in UDS at
concentrations up to those closely approaching a very toxic level.
Similar dose levels of acrylic acid in a single UDS experiment with
SHE cells gave a negative result (Wiegand et al., 1989).
Negative results have also been obtained in a micronucleus test
and a transformation test, both with SHE cells, as well as in the
Drosophila sex-linked recessive lethal assay (Wiegand et al., 1989;
McCarthy et al., 1992).
In a study by Segal et al. (1987), it was reported that acrylic
acid forms 2-carboxyethyl adducts with adenine, guanine and thymine
following in vitro reaction with calf thymus DNA. These adducts are
identical to those formed with the same bases following in vitro
reaction of carcinogen ß-propionolactone. The relevance of the results
of this study is questionable because appropriate control treatments
were not conducted and the 2-carboxyethyl adducts were formed after
long treatment period.
In contrast to the results above, Frederick & Reynolds (1989)
found that incubation of the negatively charged acrylate anion with
two representative nucleophiles, methylamine and imidazole, did not
result in the formation of adducts of the acrylate ion to the
nucleophile.
It is suggested that binding of acrylic acid to cellular
nucleophiles might be due to small amounts of the unionized acid in
the equilibrium between acrylate anion and acrylic acid at cellular
pH. However, this event is considered to be insignificant in vivo,
based upon the rapid metabolism and excretion of acrylic acid
(Frederick & Reynolds, 1989).
7.6.2 In vivo mammalian studies
Preliminary results indicated no DNA adducts in the stomach and
liver of rats after oral dosing (Sagelsdorf et al., 1988). Although
DNA adducts were found in the skin of mice after dermal application,
the investigators concluded that further work was needed to confirm
the significance of these findings. No firm conclusions can therefore
be drawn from these preliminary results.
No increase in the incidence of chromosomal aberrations was
observed in the bone marrow of rats following acute or repeated
exposure to acrylic acid (McCarthy et al., 1992). Rats either received
a single gavage dose of up to 1000 mg/kg acrylic acid or were exposed
to up to 5000 mg/litre in the drinking-water for 5 days. There was no
effect on the mitotic index of the bone marrow but there was evidence
of systemic toxicity.
Negative results were reported in a dominant lethal assay with
mice following acute or repeated exposure to acrylic acid (McCarthy et
al., 1992). Male CD-1 mice received either a single gavage dose of up
to 324 mg/kg or daily gavage doses of up to 162 mg/kg for 5 days. The
fact that the dose levels were selected not to exceed the LD1 for
acute dosing and 0.5 LD1 for repeat dosing limits the value of this
study.
7.7 Carcinogenicity
In a carcinogenicity study (Hellwig et al., 1993), Wistar rats
(50/group/sex) were given acrylic acid in the drinking-water at
concentrations of 0, 120, 400 or 1200 mg/litre (0, 8, 27, or
78 mg/kg body weight per day, respectively) over 26 (males) or 28
(females) months. The highest concentration was selected because of
evidence of palatability problems at 2000 and 5000 mg/litre in an
earlier chronic drinking-water study. At 1200 mg/litre there was some
evidence for slightly reduced water consumption. In comparison with
the controls, mortality was not increased by administering acrylic
acid. No clear toxic effects were revealed in the groups. The few
statistically significant differences in some haematological
parameters between groups were considered to be of an incidental
nature. The extensive histopathological examination revealed no clear
treatment-related non-neoplastic tissue changes. The incidence and
organ distribution of the tumours found in the groups treated with
acrylic acid for 26/28 months did not differ from those of the
controls. In this study the NOEL was 78 mg/kg body weight per day.
It has been reported that when 25 µl of a 1% solution
(i.e. 0.25 mg) of acrylic acid was applied topically in acetone
on the dorsal skin three times a week for their lifetime to 40
male C3H/HeJ mice, no malignancies were observed at the site of
application (De Pass et al., 1984).
When 1 mg (14 µmol) of acrylic acid was applied topically in
acetone 3 times a week to 30 female ICR/HA (currently designated
Hsd:(ICR)Br) mice for 1.5 years, squamous cell carcinomas were
observed in two of the mice (Cote et al., 1986). No conclusion can be
drawn from this study, because only an abstract was issued, it was not
subsequently published in full and an independent review of this study
(Sivak, 1987) uncovered many inconsistencies and hence questioned the
validity of the findings.
Acrylic acid was assayed for carcinogenic activity in female
Hsd:(ICR)bR mice by subcutaneous injection of 20 µmol (approx 1.4 mg)
in 0.05 µl trioctanoin, once a week for 52 weeks. The mice were then
observed for an additional 93 days (total 450 days) when the survivors
were killed. Two mice with sarcomas at the site of application were
observed out of 30 mice (Segal et al., 1987). This would be an
expected finding as an irritant solution was repeatedly given by
subcutaneous injection. Injections for this length of time frequently
result in sarcomas, no matter what the compound, because of repeated
insult.
7.8 Other studies
In contrast to some of its esters, acrylic acid did not
significantly decrease non-protein sulfhydryl content (NPSH) in the
liver, blood or forestomach after oral dosing of rats with up to
400 mg/kg body weight (as 8% acrylic acid solution in 0.5% methyl-
cellulose solution). However, a significant depletion of NPSH was
observed in the glandular stomach at doses above 4 mg/kg (as 0.8%
acrylic acid solution) (Miller et al., 1981b; De Bethizy et al., 1987).
Acrylic acid was found to be ineffective in inducing GSH
depletion, lipid peroxidation and haemolysis (Ferrali et al., 1989).
It was reported that methylethyl- n-butyl and 2-ethyl-hexyl
acrylates, when inhaled by male Wistar rats, induced hyperglycaemia
but that acrylic acid was without effect (Vodicka et al., 1990).
Rats received neutralized acrylic acid (50 mg/kg) intra-
peritoneally for 8 consecutive days and were observed for central and
peripheral nervous system effects. They showed a 25 % decrease in body
weight gain but no signs of neurotoxicity (Kohriyama et al., 1994).
In an in vitro study, acrylic acid was more potent than
acrylamide as an inhibitor of creatinine kenase (CK) activity in rat
brain homogenates. However, when the two chemicals were given
intraperitoneally 50 mg/kg per day for 8 days to rats, only acrylamide
inhibited CK activity in the brain. The 14C level in the brain, 24 h
after the injection of 14C-labelled chemicals, was more than seven
times greater with acrylamide then with acrylic acid (Kohriyama et
al., 1994).
Single intraperitoneal injections of up to 2.2 mg/kg body weight
of acrylic acid to Wistar rats had no effect on the hepatic activity
of ornithine decarboxylase, which was measured for tumour-promoting
activity (van de Zande et al., 1986).
Laparotomies were carried out on pregnant Sprague-Dawley rats on
day 13 of pregnancy under anaesthesia, and half of the developing
fetuses were injected with 10, 100 or 1000 µg acrylic acid per fetus.
One fetus injected with 100 µg acrylic acid showed slight
hydrocephalus and microcephalia. A dose of 1000 µg resulted in 78%
resorption (Slott & Hales, 1985).
It was reported that acrylic acid interferes with incorporation
of thymidine into DNA and uracil into RNA in Staphylococcus aureus
and Escherichia coli (Glombitza & Heyser, 1971).
7.9 Factors modifying toxicity
No studies on factors modifying the toxicity of acrylic acid have
been reported.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Acute toxicity
8.1.1.1 Poisoning accidents
There have been no reports on poisoning accidents in the general
population or on the short- or long-term effects of acrylic acid on
the general population.
8.2 Occupational exposure
8.2.1 Poisoning accidents
There have been no reports on poisoning accidents due to
occupational exposure.
8.2.2 Effects of short- and long-term exposure
Fowler (1990) described the case of a male chemical worker who
developed acute generalized urticaria after working with acrylic acid
and acrylate compounds. Immediate hypersensitivity testing showed a
severe local reaction to 2% acrylic acid (which was apparently not
irritant), but no reaction to other acrylate compounds. The acrylic
acid used for testing was thought to be pure but it was not analysed
for the presence of alpha,ß-diacryloxypropionic acid. Re-exposure of
the worker in the workplace to acrylic acid resulted in generalized
urticaria.
No cross-sensitization to 0.1% acrylic acid was observed in six
patients who had exhibited hypersensitivity to acrylate-based sealants
(Conde-Salazar et al., 1988).
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
9.1 Microorganisms
The toxic threshold (EC3) level for inhibition of growth of the
bacterium, Pseudomonas putida was 41 mg/litre after exposure for
16 h to a neutralized solution of acrylic acid (Bringmann & Kühn,
1980). Stewart et al. (1995) studied the anaerobic degradation of
acrylic acid in 55-day tests in glucose-acetate enrichment culture. At
100 mg/litre of acrylic acid there was minimal effect on acetate
utilization by methanogens; concentrations of 500, 1000 and
1500 mg/litre inhibited acetate utilization for a period of time
before recovery.
In a study of the effect of acrylic acid on the soil carbon
cycle, it was shown that acrylic acid in soil (sandy loam soil), at
levels of up to 100 mg/kg soil, had no effect on the respiration of
the soil microflora. However, a concentration of 1000 mg/kg acrylic
acid completely suppressed respiration (Hossack et al., 1992).
9.2 Aquatic organisms
The toxicity of acrylic acid to aquatic organisms is summarized
in Table 7. Algae appear to be the most sensitive group with EC50
values, based on growth, ranging from 0.04 to 63 mg/litre; and a NOEC
for the most sensitive species of 0.008 mg/litre. Other acute toxicity
studies range from 27 mg/litre (96-h LC50) for the rainbow trout to
315 mg/litre (72-h LC50) for the golden orfe. A 96-h NOEC for glacial
acrylic acid toxicity to rainbow trout was found to be 6.3 mg/litre
based on a lack of sublethal/behavioural responses, e.g., quiescence,
fish on bottom of test vessel, loss of equilibrium and erratic
swimming, at this concentration (Bowman, 1990). Neutralized acrylic
acid was found to be less toxic to daphnids than the non-neutralized
solution (Bringmann & Kühn, 1982).
9.3 Terrestrial organisms
No data on terrestrial organisms have been reported.
Table 7. Toxicity of acrylic acid to aquatic organisms
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Blue-green CMIT Exposure duration 8 days; TT (EC3) 0.15 the toxic threshold Bringmann &
alga temp. 27°C; neutralized with extinction Kühn, 1978
Microcystis pollutant solution; static values > 3% lower
aeruginosa closed flasks shaken once than for controls
a day; the
concentration of the
algae suspension measured
turbidimetrically at 578 nm
Green alga CMIT Exposure duration 7 days; TT (EC3) 18 as above Bringmann &
Scenedesmus temp. 27°C; neutralized Kühn, 1980
quadricauda pollutant solution; static
closed flasks shaken once a
day; the concentration of
the algal suspension measured
turbidi-metrically at 578 nm
Green alga CMIT 96-h static algal assay; EC50 0.17 50% algal Forbis, 1989
Selenastrum acrylic acid exposure growth
capricornutum concentrations ranged from inhibition
(Printz) 0.15 to 1.9 mg/litre
Green alga algal Exposure duration EC50 0.04 50% algal BASF, 1994b
Scenedesmus inhibition 72 h biomass
subspicatus test inhibition
EC50 0.13 50% algal
growth rate
LOEC 0.016 inhibition
NOEC 0.008
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Green alga algal Exposure duration EC50 1.53 50% algal SNF, 1995
Chlorella inhibition 72 h; nominal acrylic biomass
vulgaris test (OECD acid exposure inhibition
No. 201) concentrations
ranged from 0.2 to EC50 63.0 50% algal
4.7 mg/litre for growth rate
biomass test and were inhibition
50 and 100 mg/litre
for growth rate
inhibition test
Protozoan CMIT Exposure duration TT (EC5) 20 the toxic Bringmann &
Entosiphon 72 h; temp. 25°C; threshold with Kühn, 1982
sulcatum pollutant solution extinction
(Stein) adjusted to pH 6.9; values > 5%
static closed flasks; lower than for
the number of controls
protozoans was
determined by
means of cell counter
Water flea Daphnia Exposure duration EC50 765 after 24 h Bringmann &
Daphnia immobilization 24 h; temp. 20°C; 50% of exposed Kühn, 1982
magna test pH 7.8-8.2; organisms were
(Straus) performed in 50 ml immobilized in
beakers covered with neutralized test.
filter paper;
10 organisms/
20 ml test solution;
9 h illumination/
15 h dark
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
EC100 5000 100% immobilized
after 24 h in
neutralized test
Acrylic acid in
non-neutralized
test
EC50 54 in non-neutralized
test
EC100 91
Water flea Daphnia Dynamic test. Acrylic acid EC50 95 after 48 h 50% of Burgess, 1990
Daphnia magna immobilization exposure concentrations exposed organisms
test ranged from 7.9-110 were immobilized
mg/litre.
Exposure duration 48 h; NOEC 23 based on mortality
temp. 19-20°C; pH 6.7-7.7;
dissolved O2 7.9-8.0 mg/litre
Golden orfe L15-Fish test Exposure duration LC0 210 no lethality Juhnke &
Leuciscus idus German 48 h; temp. 19-21°C; pH 7-8; Luedemann,
melanotus standard dissolved O2 1978
method for the > 5 mg/litre; 10-fish LC50 315 death of 50%
contaminants per 10 litre test of exposed
of water, solution; continuous fish
waste water aeration
and sludge
LC100 420 death of all
exposed fish
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Rainbow trout A flow-through Exposure duration 96 h; LC50 27 death of 50% Bowman,
Oncorhynchus toxicity test measured glacial acrylic of exposed 1990
mykiss acid exposure NOEC 6.3 fish
concentrations ranged lack of
from 6.3 to 90 mg/litre; sublethal/
20 fish per group behavioural
responses
Common carp Exposure duration LC100 100 Nishiuchi,
Cyprinus 24 h 1975
carpio
a CMIT = Cell multiplication inhibition test
b TT = Toxicity threshold
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure of the general population
Very limited data are available on general population exposure.
Exposure of the general population to acrylic acid may occur by the
dermal route via contact with unreacted acrylic acid in household
goods such as water-based paints or detergents. Exposure is also
possible from inhalation due to evaporation of acrylic acid from
paint.
Exposure of the general population through drinking-water and via
ambient air might be assumed. Exposure through drinking-water is
possible from surface or groundwater contamination while inhalation
exposure could occur in the vicinity of industrial emissions. However,
there is no data on measured ambient air concentrations in populated
areas and no data are available on acrylic acid concentrations in
drinking-water. It was reported that acrylic acid might occur in
wastewater effluent from industrial facilities at concentrations not
exceeding 0.5 mg/litre. However, effluent levels of 2500 mg/litre were
reported at a methyl acrylate production facility in India. After
treatment acrylic acid concentration was below the limit of detention
(0.1 mg/litre) in wastewater from a production facility in Europe.
Food is an unlikely source of exposure to commercially derived
acrylic acid because it is not known to be used in applications that
involve direct contact with food.
10.1.2 Occupational exposure
The most important means of human exposure to acrylic acid is
occupational exposure via inhalation and skin contact. On the basis of
statistical estimates derived from a NIOSH survey conducted in
1972-1974, 28 600 workers were potentially exposed to acrylic acid in
the USA. More recently, NIOSH estimated that 96 500 workers were
potentially exposed in the USA.
One study conducted at a factory, where a number of chemicals
including acrylic acid and a variety of acrylates and methacrylates
were used, indicated that levels in air of acrylic acid and ethyl
acrylate varied from 0.03 to 168 mg/m3 (0.01 to 56 ppm). However, the
8-h TWA levels of most areas of the plant were well below the hygienic
standard of 30 mg/m3 (10 ppm) for acrylic acid recommended by OSHA
and ACGIH at the time of the study.
Exposures of workers to acrylic acid have been compiled from four
producing companies. Operators had a mean 8 h-exposure value of
0.48 mg/m3 (range of 0.03 to 3 mg/m3); during loading/unloading
the mean 8 h-exposure was 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3).
10.1.3 Toxic effects
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, moderate acute toxicity by the inhalation route and
moderate acute toxicity by the dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. Although a 1% solution has
been reported to irritate rabbit skin and eyes, no externally visible
irritation was seen in one mouse study following repeated dermal
application of 1%. Acrylic acid is also a strong irritant to the
respiratory tract.
Both positive and negative results have been obtained in skin
sensitization tests with acrylic acid, but it appears that the
positive findings may have been due to the impurity alpha,ß-
diacryloxypropionic acid.
10.1.3.1 Carcinogenic and mutagenic effects
Both positive and negative results have been obtained in
in vitro genotoxicity tests. Notably, acrylic acid was negative in
the Ames test and in vitro UDS assay, positive in a chromosome
aberration assay (with pH adjustment) and in the in vitro lymphoma
cell assay. Chromosome damage was also demonstrated in one lymphoma
assay. An in vivo bone marrow chromosome aberration assay was
negative. No firm conclusions can be drawn from an in vivo
DNA-binding study or from a dominant lethal assay. There are no
satisfactory in vivo genotoxicity data for sites of initial contact,
e.g., nasal epithelium or skin.
A single chronic cancer bioassay of acrylic acid, administered in
drinking-water, has been reported. Male and female Wistar rats were
given drinking-water containing 120, 400 or 1200 mg/litre (estimated
doses were 8, 27 and 78 mg/kg body weight per day), after preliminary
3- and 12-month studies showed reduced water consumption and body
weight gain in groups of rats receiving drinking-water containing 2000
or 5000 mg/litre. No systemic effects, including weight change or
histopathology, and no increase in tumour incidence resulted from
acrylic acid exposure. Although the lack of any toxic effects in this
study suggests that the maximum tolerated dose was not achieved, it
appears that the high dose in this study was approaching a maximum
tolerance dose (MTD), based on the mild body weight effects noted at
140 mg/kg per day in a 12-month study and at 240 mg/kg per day in a
two-generation reproductive study. Apart from not reaching the MTD,
the study used adequate number of animals (50), was well documented
and reported complete histopathology.
There has been no cancer bioassay conducted in a second species
by the oral route, nor any inhalation cancer bioassay. Available data
are inadequate to evaluate the carcinogenic potential of acrylic acid
via the dermal route.
There have been several chronic bioassays conducted on related
compounds that are relevant to evaluation of the potential
carcinogenicity of acrylic acid. Chronic inhalation studies have been
reported for methyl acrylate, butyl acrylate and ethyl acrylate. All
of these studies exposed rats to 0, 5, 25 or 75 ppm of the acrylates
for 2 years, which was followed by complete necropsy and histopa-
thological evaluation. The acrylate esters are rapidly metabolized to
acrylic acid and alcohols by nasal, blood and other tissue carboxyl-
esterases. In all of these studies a concentration-dependent increase
in nasal olfactory degeneration was observed, and the response was
very similar to the response observed in a subchronic inhalation study
of acrylic acid. There were no treatment-related increases in tumour
incidence in these studies. Although there may have been quantitative
differences in the tissue doses of acrylic acid, the acrylate ester
studies provide supportive evidence that carcinogenic effects do not
occur in tissues exposed chronically to acrylic acid.
Two cancer bioassays via oral exposure have been conducted with
ethyl acrylate, a gavage study in F-344 rats and B6C3F1 mice (NTP,
1986) and a drinking-water study in Wistar rats. The highest dose was
similar in the two studies and produced lesions of the forestomach.
However, the gavage study resulted in a dose-dependent increase in
tumour incidence in both sexes of rats and mice while the drinking-
water study did not. The gavage study suggests a potential for
carcinogenic effects for ethyl acrylate, and, by extension, for the
other acrylate esters and acrylic acid. The greater potency for this
effect in a gavage study may relate to the contact site toxicity of
these compounds. Gavage may not always be an appropriate exposure
method for direct irritants because of the extremely high local doses
that are achieved, compared to the local doses in a drinking-water
study.
A similar result can be seen in 3-month gavage and drinking-water
studies. The drinking-water study caused mildly reduced body weight
gain compared to controls, while the gavage study, at very similar
doses, resulted in lethality in most of the dosed animals. These
results suggest that the gavage route of exposure should play little
role in the risk assessment of acrylic acid because human exposures
are not likely to result in repeated high-concentration doses.
Current practice requires strong evidence to conclude that a
chemical is not a carcinogenic hazard, usually including adequate
chronic bioassays in two species. A second species chronic bioassay is
not available for acrylic acid, and the acrylate ester inhalation and
drinking-water bioassays described above were carried out on rats. The
existing acrylic acid bioassay is adequate and demonstrated no cancer
effects. No epidemiological evidence regarding potential
carcinogenicity of acrylic acid is available. Based on the available
data, one cannot definitively conclude that acrylic acid does not pose
a carcinogenic hazard to humans, although substantial relevant data do
not suggest a cancer hazard.
10.1.3.2 Non-cancer effects
a) Route-specific effects
Both oral and inhalation exposures show effects at the site of
contact. Inhalation exposures resulted in nasal effects in studies of
2 weeks to 3 months in duration and at various exposure
concentrations. High concentration drinking-water exposure to acrylic
acid resulted in forestomach lesions. No other specific organ
pathology has been consistently demonstrated in either oral or
inhalation studies. Metabolic studies show rapid metabolism and
clearance of acrylic acid that is similar for several routes of
exposure, and there is no evidence of bioaccumulation of this
chemical. The available evidence supports the conclusion that systemic
toxicity is not likely to occur, and that the effects of oral and
inhalation exposure can be treated as independent events with no
additivity of doses received by the different routes of exposure. For
this reason, guidance values will be derived separately for oral and
inhalation routes.
b) Developmental and reproductive effects
The toxicity data base for acrylic acid includes inhalation
developmental studies in rats and rabbits and oral reproductive
studies in rats. No specific reproductive or developmental effects
were observed in any of these studies. The only effects observed
included nasal effects in the inhalation studies and stomach lesions
and reduced body weight gain in the drinking-water studies. Based on
these results and the evidence for specific site-of-contact effects
described above, the potential hazards for developmental and
reproductive effects have been adequately studied and are not critical
to the human health risk assessment.
c) Oral gavage studies
As described above (section 10.1.3.1) the 3-month gavage and
drinking-water studies showed important differences in toxicity,
depending on the method of dosing. The drinking-water study revealed
reduced body weight gain compared to controls, while the gavage study,
at very similar daily doses, showed lethality in most of the dosed
animals. Therefore, the gavage route of exposure should be of little
importance in the risk assessment of acrylic acid because human
exposures are not likely to result in repeated high-concentration oral
doses.
d) Critical oral studies
Potential critical studies include a 3-month drinking-water study
in rats, 3-, 12- and 26- to 28-month drinking-water studies in rats,
and a two-generation reproductive study in rats. Table 8 summarizes
the effects seen in these studies and the determination of which
effects are considered adverse.
Table 8. Summary of adverse effect levels in drinking-water studies on ratsa
LOAEL NOAEL Effectb Reference
750 250 Body weight; 81 and 84% of controls in males and females, DePass et al., 1983
respectively, at 750; 95% of C in females at 250
none 331 Body weight: Hellwig et al., 1993
males: 93% of C at 331; (3-month study)
females: no effect
331 140 Body weight: Hellwig et al., 1993
males: 91% of C at 331, 94% of C at 140; (12-month study)
females; no effect
none 78 no effects Hellwig et al., 1993
(26- to 28-month studies)
460 240 460: stomach irritation, body weight: 91% of C in F0 males, BASF, 1993
65% of C in F1 pups, 85% of C in F1 (both sexes), 68% of C
in F2 pups
240: body weight: 89% of C in F1 pups, 88% of C in F2 pups
a concentrations are given in mg/kg body weight per day
b C = control group; F0 = first parental generation; F1 = second parental generation
A consistent finding throughout the drinking-water studies was
decreased water consumption, probably due to taste aversion and
sometimes accompanied by decreased food consumption. Reductions in
body weight appear to parallel decreased water consumption and were
worse in the pups in the reproductive study. The water consumption
effect makes the body weight changes difficult to interpret as a
direct toxic effect of acrylic acid, but the possibility that the
effect was at least partly a direct result of acrylic-acid-induced
irritation cannot be ruled out. The body weight effects are therefore
considered to be adverse when the magnitude of the effect approaches a
10% reduction. A somewhat larger change in the pup weight would be
required to be considered adverse, because the largest weight
decrements were seen at the end of active nursing and the beginning of
weaning; they may be more severely affected by the reduced water
consumption because of the greater maternal need for water intake
during this time and the unknown effects of taste aversion on weaning
behaviour. Based on these considerations and the presence of stomach
histopathology at 460 mg/kg body weight per day, the NOAEL in the two-
generation reproduction study was 240 mg/kg body weight per day. The
NOAEL based on body weight changes in the 12-month studies was
140 mg/kg body weight per day.
e) Critical inhalation studies
In a subchronic study, which is the only study that is
potentially a critical study for inhalation, lesions in the nasal
epithelium were seen in rats at 225 mg/m3 (75 ppm) and in mice at
15 mg/m3 (5 ppm). The lesion in mice was described as very slight at
15 mg/m3 (5 ppm), slight at 75 mg/m3 (25 ppm), and slight to
moderate at 225 mg/m3 (75 ppm). Despite the mild nature of the
response at 15 mg/m3 (5 ppm), there was a clear increase in incidence
and severity of this lesion with increasing exposure concentration, so
it must be considered adverse.
f) Progression of lesions
There is suggestive evidence from both inhalation and oral routes
of exposure that the effects caused by acrylic acid are largely
determined by the exposure concentration and are relatively less
affected by the duration of exposure in repeated exposure studies.
In the oral studies, even if different LOAEL and NOAEL values
could be defined by the 3-month and 12-month oral drinking studies,
the effects on weight are similar for the two exposure durations. In
inhalation studies in mice, the severity of the lesions in animals
exposed for 6 h/day to 75 mg/m3 (25 ppm) was very similar in the
2-week and the 90-day studies, although limited description of the
lesions in the 2-week study makes this conclusion tenuous. Additional
2-week studies included groups exposed to 15 and 75 mg/m3 (5 and
25 ppm) for 6 h/day. The 75 mg/m3 (25 ppm) group showed effects similar
to those seen in the subchronic study, suggesting limited progression
over this time-frame. The 15 mg/m3 (5 ppm) group exposed for 6 h/day
showed no effect after 2 weeks, so slight progression does occur at
low concentrations, given that lesions were observed in the 90-day
study. The 2-week study also included recovery groups, which showed
that the lesions were completely reversed in animals exposed to
15 mg/m3 (5 ppm) for 22 h and 75 mg/m3 (25 ppm) for 6 h, but
not in animals exposed to 75 mg/m3 (25 ppm) for 22 h/day.
Comparison of the subchronic and chronic inhalation studies of
the acrylate esters also supports the conclusion that there is limited
progression of nasal lesions. Some progression of lesions does occur,
but much less than would be predicted by an assumption of a constant
concentration × time relationship.
10.1.4 Risk evaluation
10.1.4.1 Inhalation exposure
The LOAEL of 15 mg/m3 (5 ppm) from the subchronic study is used
as the basis for the guidance value. The appropriate uncertainty
factors (UF) include 5 for inter-individual differences and 10 as a
composite UF for interspecies, LOAEL to NOAEL and subchronic to
chronic extrapolations. Reduced uncertainty for these aspects of the
guidance value (GV) for inhalation results from the information
discussed previously in this monograph. The inter-individual
uncertainty is reduced because of the direct-acting nature of the
toxicity, which does not involve metabolism. The uncertainty in
extrapolating from a LOAEL to a NOAEL is reduced because the lesion at
the LOAEL is very mild and reversible. The uncertainty in
extrapolating from a subchronic to a chronic exposure is reduced
because of the evidence discussed above showing limited progression.
The interspecies uncertainty is reduced because of the direct-acting
nature of the toxicity of acrylic acid and because the mouse is
apparently very sensitive. The toxicity of acrylic acid is clearly
limited to the site of deposition and is not dependent on metabolic
activation. Since the deposition is controlled by physical
interactions, there is little reason to expect significantly greater
deposition in humans.
Based on these considerations, the GVair for inhalation exposure
of the general population may be calculated as follows:
GVair = 15mg/m3 × 6 × 5 × 1 = 54 µg/m3
24 5 50
where:
15 mg/m3 = LOAEL for mice
6/24 and 5/7 = duration of exposure in h/day and weeks/day,
respectively
50 = total uncertainty factor
10.1.4.2 Oral exposure
For oral exposure the GV can be calculated based on NOAEL values
in the 12-month oral study (140 mg/kg body weight per day), the two-
generation reproductive study (240 mg/kg body weight per day) or the
NOAEL in the chronic study (78 mg/kg body weight day). The chronic
study is used to derive the GV because a lifetime exposure study is
preferred to a short-duration study. The chronic study found no effect
at the highest dose tested. The NOAEL is supported by the finding of
adverse effects on body weight at 331 mg/kg body weight per day in the
12-month study and body weight effects and stomach pathology in the
two-generation reproductive study at 460 mg/kg body weight per day.
With these supporting studies, the NOAEL from the chronic study can be
used with confidence. A total uncertainty factor of 25 is based on a
factor of 5 for inter-individual variability, and a factor of 5 for
interspecies variability and for the lack of an adequate oral study in
a second species. Reduced uncertainty factors are used for
interspecies and inter-individual differences because of the direct-
acting nature of the toxicity, as described in section 10.1.4.1. The
need for a second species is suggested by the apparent differences in
sensitivity between rats and mice in the subchronic inhalation study.
Based on these considerations, the GVoral for drinking-water
exposure may be calculated as follows:
Tolerance intake = 78 = 3.1 mg/kg
25
From the above calculation a guidance value for oral intake of
acrylic acid may be derived according to the following formula:
GVoral = 3.1 × 64 ‰ 2 = 99 mg/litre
where:
3.1 mg/kg body weight per day = tolerable intake (TI)
64 = average weight (kg) of human body
25 = uncertainty factor
2 = average intake of drinking water (litres/day)
However, the Task Group expressed significant concern regarding
the guidance value for oral intake calculated above, because of the
difference in toxicity between drinking-water and gavage exposures.
Exposure levels only 50-fold above the GV calculated above were lethal
to 50% of the animals in the 90-day gavage study, and comparison with
effects in the 90-day drinking-water study revealed a very significant
difference between the two dosing methods. High doses taken over a
short time period have much more severe effects.
The concern is reduced by the fact that the local effects in the
stomach might be determined by both concentration of delivered dose
and total dose, so the same total dose delivered in more dilute
solutions results in lesser effects, as shown for ethyl acrylate. The
concentration in drinking-water needed to deliver the TI dose is
probably far less than that required to cause direct effects, and is
not a public health concern if small amounts are ingested over the
course of the day, approximating to continuous exposure. The
possibility remains that a large part of the TI could be delivered
over a short time via drinking-water.
Because of potential differences in individual drinking
behaviour, the Task Group decided that a risk of effects still exists
at the TI calculated above. For this reason an additional uncertainty
factor of 10 was applied to derive the GV for drinking-water.
Thus, the final guidance value for oral intake of acrylic acid
is:
GVoral = 99 mg/litre = 9.9 mg/litre
10
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Acrylic acid has been found to occur naturally in some species of
marine alga. No quantitative data are available regarding
environmental levels in ambient air or soil. It was reported that
acrylic acid might occur in wastewater effluent from industrial
facilities at concentrations not exceeding 0.5 mg/litre. Effluent
levels of (2500 mg/litre) have been reported at a methyl acrylate
production facility in India. After treatment acrylic acid was below
the limit of detection (0.1 mg/litre) in wastewater from a production
facility in Europe.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Acrylic acid is
eliminated from the environment by both abiotic and biotic
degradation. Photolysis reactions are possible processes, but aerobic
degradation constitutes the major route of breakdown. Microorganisms
are capable of degrading acrylic acid under aerobic and anaerobic
conditions.
In soil, acrylic acid biodegrades very rapidly, the half-life
being less than one day. There is no potential for long-range
atmospheric transport of acrylic acid since it has an atmospheric
lifetime of less than one month.
Bioaccumulation of acrylic acid in organisms, based on the low
octanol-water partition coefficient, is likely to be negligible. There
has been no report of biomagnification of acrylic acid in food chains.
10.2.2 Effects
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values based on growth ranging from 0.04 to 63 mg/litre and
a NOEC for the most sensitive species of 0.008 mg/litre. Acrylic acid
has low to moderate acute toxicity for invertebrates and fish with
LC50 values ranging from 27 to 315 mg/litre. The 96-h NOEC was
6.3 mg/litre for rainbow trout, based on a lack of sublethal/
behavioural responses.
No data are available concerning the effects of acrylic acid on
terrestrial organisms in the environment.
10.2.3 Risk evaluation
If released into the environment acrylic acid will partition to
water where it will be readily degraded. Therefore, it is unlikely to
pose a problem in the general environment. In the case of spills,
acrylic acid is likely to cause localized mortality to aquatic
organisms both from direct toxicity and oxygen depletion. There is
likely to be a problem near to outfalls from plants discharging
acrylic acid if there is inadequate sewage treatment. The toxicity of
acrylic acid to bacteria and soil microorganisms is low. No data are
available concerning the effects of acrylic acid on terrestrial
organisms in the environment; however, data from laboratory mammals
suggest a low risk.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure for the general
population. However, no obvious adverse effects on the general
population have been identified.
Acrylic acid poses minimal risk for the general environment
except in the case of uncontrolled discharge.
11.2 Recommendations for protection of human health
It is recommended that exposure of the general population to
acrylic acid in the ambient air and drinking-water does not exceed the
guidance values given in this monograph.
12. FUTURE RESEARCH
a) Monitoring of acrylic acid concentrations in ambient air, water,
soil and effluent should be carried out. Research should be
undertaken to lower the analytical detection limit in water.
b) Further in vivo studies on the genotoxic potential of acrylic
acid at the initial site of contact are recommended.
c) The carcinogenicity of acrylic acid should be assessed in a well-
controlled two-species bioassay (with both sexes) using proper
exposure levels via inhalation or dermal exposure.
d) Better understanding of the relationship between persistent
tissue damage and tumour formation would aid the risk assessment
for an irritant chemical such as acrylic acid.
e) Additional research on the development of models describing the
disposition and kinetics of acrylic acid is needed. Such models
should focus on species differences in order to allow more
confident extrapolation from animals to humans. In addition,
models allowing comparison of acrylic acid dose for various
acrylate esters would be useful to allow complete use of
available data.
f) Since many workers are exposed to acrylic acid, epidemiological
studies are needed, which should concentrate on the following
end-points:
i) rhinitis, laryngitis and olfactory function;
ii) respiratory tract disease;
iii) skin disease.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer (IARC, 1979, 1987). Data
on the carcinogenicity of the compound for humans were considered
inadequate. There was inadequate evidence for carcinogenicity in
animals. Therefore, IARC considered acrylic acid not classifiable as
to its carcinogenicity to humans.
REFERENCES
AAR (1987) Emergency handling of hazardous materials in surface
transportation. Washington, DC, Association of America Railroads,
Hazardous Systems (BOE), 11 pp.
ACGIH (1988) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1988-1989.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
ACGIH (1990) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1990-1991.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists, p 9.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Andreoni V, Bernasconi S, Sorlini C, & Villa M (1990) Microbial
degradation of acrylic acid. Ann Microbiol, 40: 279-287.
Archer G & Horvath MK (1991) Adsorption and desorption of acrylic acid
to soils. Painesville, Ohio, Ricerca Inc., Department of Environmental
Sciences (Document No. 3193-88-0214-EF-001).
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reactions of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
Atkinson R & Carter WPL (1984) Kinetics and mechanisms of the gas-
phase reactions of ozone with organic compounds under atmospheric
conditions. Chem Rev, 84: 437-470.
Bandara BMR, Gunatilaka AAL, Kumar NS, & Wimalasiri WR (1988)
Antimicrobial activity of some marine algae of Sri Lanka. J Natl Sci
Counc (Sri Lanka), 16(2): 209-221.
BASF (1958) [Report on biological tests for crude and pure acrylic
acid.] Ludwigshafen, Germany, BASF AG, Toxicology Department
(Unpublished report No. VII/365-366) (in German).
BASF (1988) [Determination of the distribution coefficient log Pow of
acrylic acid in 1-octanol/water at room temperature (25°C).]
Ludwigshafen, Germany, BASF AG, Analytical Laboratory, pp 1-4 (in
German).
BASF (1992) Acrylic acid glacial P. Technical information monomers.
Ludwigshafen, Germany, BASF AG.
BASF (1993) [Examination of the biological degradability or the
elimination potential, respectively, of acrylic acid (pure P)
according to the experimental design of Zahn-Wellens (DUU/OM Project
No. 92/2641/10/1).] Ludwigshafen, Germany, BASF AG (in German).
BASF (1994a) Continuous administration in the drinking-water over 2
generations (1 liter in the first and 1 liter in the second
generation) (Project No. 71RO114/92011). Ludwigshafen, Germany, BASF
AG.
BASF (1994b) [Determination of the inhibitory effect of acrylic acid
(pure) on the cell replication of the green algae Scenedesmus
supsicatus.] Ludwingshafen, Germany, BASF AG (Unpublished
experiment No. 94/0840/60/1) (in German).
BG Chemie (1991) Acrylic acid. In: Toxicological evaluations, Volume
2. Berlin, Springer-Verlag, pp 39-73.
Black KA (1993) 14C-Acrylic acid. Comparative bioavailability study
in male mice and rats -analysis of tissues. Spring House,
Pennsylvania, Rohm and Haas Company, Toxicology Department (Report
No. 93R-200).
Black KA, Finch L, & Frederick CB (1993) Metabolism of acrylic acid to
carbon dioxide in mouse tissues. Fundam Appl Toxicol, 21: 97-104.
Black KA, Beskitt JL, Tallant MJ, & Frantz SW (1994) Disposition of
acrylic acid (AA) in C3H mice after the oral, cutaneous and
intravenous routes (Rohm and Haas Company, Spring House, PA and Bushy
Run Research Center, Export, PA). Toxicologist, 14(1): 430 (Abstract
1705).
Black KA, Beskitt JL, Finch L, Tallant MJ, Udinsky JR, & Frantz ST
(1995) Disposition and metabolism of acrylic acid in C3H mice and
Fischer 344 rats after oral or cutaneous administration. J Toxicol
Environ Health, 45: 291-311.
Borzelleca JF, Larson PS, Hennigar GR, Huf EG, Crawford EM, & Smith RB
(1964) Ethyl acrylate and methyl methacrylate: Studies on the chronic
oral toxicity of monomeric ethyl acrylate and methyl methacrylate.
Toxicol Appl Pharmacol, 6: 29-36.
Bowen RL (1979) Adhesive bonding of various materials to hard tooth
tissues. XVIII: Synthesis of a polyfunctional surface-active
comonomer. J Dent Res, 58: 1101-1107.
Bowman JE (1990) Acute flow-through toxicity of glacial acrylic acid
to rainbow trout ( Salmo gairdneri). Columbia, Missouri, Analytical
Bio-Chemistry Laboratories Inc., Aquatic Toxicology Division (Report
No. 37343).
Bretherick L (1985) Handbook of reactive chemical hazards, 3rd ed.
Boston, Massachusetts, Butterworth, 351 pp.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: model organisms Microcytis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Z Wasser-Abwasser-Forsch, 15: 1-6 (in
German).
Brown RK, McMeekin TA, & Balis C (1977) Effect of some unicellular
algae on Escherichia coli populations in sea water and oysters. J
Appl Bacteriol, 43: 129-136.
Buckley LA, James RA, & Barrow CS (1984) Differences in nasal cavity
toxicity between rats and mice exposed to acrylic vapor. Toxicologist,
4(1): 1 (Abstract only).
Burgess D (1990) Acute flow-through toxicity of acrylic acid to
Daphnia magna. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37344).
Bysshe SE (1990) Bioconcentration factor in aquatic organisms. In:
Lyman WJ, Reehle WF, & Rosenblatt DN ed. Handbook of chemical property
estimation methods for environmental behaviour of organic compounds.
Washington, DC, American Chemical Society, vol 15, pp 1-30.
Cameron TP, Rogers-Back AM, Lawlor TE, Harbel JW, Seifried HE, &
Dunkel VC (1991) Genotoxicity of multifunctional acrylates in the
Salmonella/mammalian-microsome assay and mouse lymphoma TK+/- assay.
Environ Mol Mutagen, 17: 264-271.
Carpenter CP, Weil CS, & Smyth HF Jr (1974) Range-finding toxicity
data: List VIII. Toxicol Appl Pharmacol, 28: 313-319.
Casciery T & Clary J (1993) Acrylic acid health effects overview. In:
Taylor TB, Murphy SR, & Hunt EK ed. Health effect assessments of the
basic acrylates. Boca Raton, Florida, CRC Press, p 13.
Chemical Marketing Reporter (1992) Acrylic grows at rapid pace, but so
does the world's capacity. Chem Mark Report, July 20.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation
of petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CHRIS (1985) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado).
CHRIS (1989) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado.
Chun JS, Kubena MF, & Neeper-Bradley TL (1993) Developmental toxicity
dose range-finding study of inhaled acrylic acid vapor in New Zealand
white rabbits. Export, Pennsylvania, Union Carbide Corporation (Bushy
Run Research Center Report No. 92N1007).
CITI (Japanese Chemicals Inspection and Testing Institute) ed. (1992)
Acrylic acid. In: Biodegradation and bioaccumulation: Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology and Information Center, pp 2-83.
Conde-Salazar L, Guimaraens D, & Romero LV (1988) Occupational
allergic contact dermatitis from anaerobic acrylic reactants. Contact
Dermatitis, 18: 129-132.
Corrigan MA & Scott R (1988) Acrylic acid: In vitro absorption
through human and mouse skin. Macclesfield, United Kingdom, Imperial
Chemical Industries PLC, Central Toxicology Laboratory (Report No.
CTL/P/2047).
Costellati L, Guglielmin AM, Vistoli O, Carboni GP, & Rambaldi R
(1990) [Allergic contact dermatitis - evaluation of patch tests in
animal fodder plant workers.] Med Lav, 81: 296-300 (in Italian).
Cote IL, Hochwalt A, Seidman I, Budzilovich G, Solomon JJ, & Segal A
(1986) Acrylic acid: Skin carcinogenesis in ICR/HA mice. Toxicologist,
6: 235.
Dean JA (1987) Handbook of organic chemistry. New York, McGraw-Hill
Book Co., pp 1-76.
De Bethizy ID, Udinsky JR, & Scribner HE (1987) The disposition and
metabolism of acrylic acid and ethyl acrylate in male Sprague-Dawley
rats. Fundam Appl Toxicol, 8(4) 549-561.
DePass LR, Woodside MD, Garman RH, & Weil CS (1983) Subchronic and
reproductive toxicology studies on acrylic acid in the drinking water
of the rat. Drug Chem Toxicol, 6(1): 1-20.
DePass LR, Fowler EH, Meckley DR, & Weil CS (1984) Dermal oncogenicity
bioassays of acrylic acid, ethyl acrylate and butyl acrylate. J
Toxicol Environ Health, 14: 115-120.
Douglas MT & Bell G (1992) Assessment of ready biodegradability of
acrylic acid (Closed bottle test). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 1/91329).
D'Souza RW & Francis WR (1988) Vehicle and pH effects on the dermal
penetration of acrylic acid: in vitro- in vivo correlation.
Toxicologist, 8: 831.
EEC (1988) Methods for the determination of ecotoxicity,
biodegradation. Activated sewage sludge respiration inhibition test.
In: Council Directive of 18 November 1987 adapting to technical
progress for the ninth time Directive 67/548/EEC on the approximation
of the laws, regulations and administrative provisions relating to the
classification, packaging and labelling of dangerous substances
(88/302/EEC). Off J Eur Comm, L133: 118-122.
Ekhina RS & Ampleeva GP (1977) [Combined effect of six acrylates on
the sanitary status of a reservoir.] Vodemov, 1977: 157-164 (in
Russian).
Elf Atochem (1992) Norsocryl AA, acrylic acid specifications. Paris,
Elf Atochem.
Fassett DW (1963) Organic acids, anhydrides, lactones, acid halides
and amides, thioacids. In: Patty FA ed. Industrial hygiene and
toxicology, 2nd ed. New York, Wiley-Interscience, vol 2, p 1794.
Fazzalari FA ed. (1978) Compilation of odor and taste threshold values
data. Philadelphia, Pennsylvania, American Society for Testing and
Materials, p 141.
Ferrali M, Ciccoli L, & Comporti M (1989) Allyl alcohol-induced
hemolysis and its relation to iron release and lipid peroxidation.
Biochem Pharmacol, 38(1): 1819-1826.
Finch L & Frederick CB (1992) Rate and route of oxidation of acrylic
acid to carbon dioxide in rat liver. Fundam Appl Toxicol, 19:
498-504.
Forbis AD (1989) Acute toxicity of acrylic acid to Selenastrum
capricornutum Printz. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc., Aquatic Toxicology Division (Report No. 37345).
Fowler JH Jr (1990) Immediate contact hypersensitivity to acrylic
acid. Dermatol Clin, 8: 193-195.
Frantz SW & Beskitt JL (1993) 14C-Acrylic acid: comparative
bioavailability study in male mice and rats. Export, Pennsylvania,
Bushy Run Research Center (Report No. 92 N 1005).
Frantz SW, Beskitt JL, Tallant MJ, & Black KA (1994) Disposition of
acrylic acid (AA) in Fischer rats after the oral, cutaneous and
intravenous routes. Toxicologist, 14(1): 430 (Abstract 1704).
Frederick CB & Reynolds CH (1989) Modeling the reactivity of acrylic
acid and acrylate anion with biological nucleophiles. Toxicol Lett,
47(3): 241-247.
Frederick CB, Udinsky JR, & Finch L (1994) The regional hydrolysis of
ethyl acrylate to acrylic acid in the rat nasal cavity. Toxicol Lett,
70: 49-56.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Ganou-Parfait B, Fahrasmane L, Celestine-Myrtil DA, Parfait A, & Galzy
P (1988) Micrococcus in rum technology in the French West Indies.
Microbiol Aliments Nutr, 6(3): 273-277.
Gelbke HP & Hofmann HTh (1979) [Report on testing for acute inhalation
risk of acrylic acid (pure) in Sprague-Dawley rats.] Ludwigshafen,
Germany, BASF AG, Department of Toxicology (in German).
Ghanayem BI, Maronpot RR, & Matthews HB (1985) Ethyl acrylate-induced
gastric toxicity: II. Structure-toxicity relationships and mechanism.
Toxicol Appl Pharmacol, 80: 336-344.
Ghanayem BI, Burka LT, & Matthews HB (1987) Ethyl acrylate
distribution, macromolecular binding, excretion and metabolism in male
Fischer 344 rats. Fundam Appl Toxicol, 9: 389-397.
Glombitza KW (1970a) [Antimicrobial contents of algae: 2. Presence of
acrylic acid in different sea algae.] Planta Med, 18(3): 210-221 (in
German).
Glombitza KW (1970b) [Antimicrobiol contents in algae: 3. Quantitative
determination of acrylic acid in sea algae.] Planta Med, 18(4):
231-234 (in German).
Glombitza KW (1979) Antibiotics from algae. In: Hoppe HA, Levring T, &
Tanaka J ed. Marine algae in pharmaceutical science. Berlin, New York,
Walter de Gruyter, pp 303-307.
Glombitza KW & Heyser R (1971) [Antimicrobial constituent compounds in
algae - Part 4: Effect of acrylic acid on respiration and synthesis of
macromolecular in Staphylococcus aureus and Escherichia coli.]
Helgoal Wiss Meeresunters, 22(3/4): 442-453 (in German).
Grudzinski UJ (1988) [Substantiation of single maximum permissible
levels of acrylic acid in the air of populated regions.] Gig I Sanit,
9: 64-65 (in Russian).
Hagan JV & Emmons HF (1991) Acrylic acid: Acute inhalation study in
rats (Protocol No. 87P-240). Spring House, Pennsylvania, Rohm and Haas
Company, Toxicology Department (Report No 87R-106).
Halarnkar PJ & Blomquist GJ (1989) Comparative aspects of propionate
metabolism. Comp Biochem Physiol, 92B: 227-231.
Hansch C & Leo A (1987) The log P database. Claremont, California,
Pomona College, p 391.
Hawkins DR, Kirkpatrick D, Aikens PJ, & Saxton JE (1992) The
metabolism of acrylic acid in soil under aerobic conditions.
Huntingdon, United Kingdom, Huntingdon Research Centre (HRC
Confidential Report No. 93A/920625).
Hellwig J, Deckerdt K, & Freisberg KO (1993) Subchronic and chronic
studies of the effects of oral administration acrylic acid to rats.
Food Chem Toxicol, 31: 1-18.
Herwig N (1978) Nutritious antibiotics. Mar Aquarist, 8(5): 48-50.
Heyser R & Glombitza KW (1972) Effect of acrylic acid on glycolysis
and citric acid cycle in bacteria: 5. Antimicrobiol components in
algae. Arch Pharm, 305(11): 863-870.
Hossack DJN, Thomas FJ, & Ruckman SM (1992) Acrylic acid: Effects on
soil carbon cycle (respiration). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 2/920228).
Howard PH, Boethlings RS, Jarris WF, Meylan WM, & Midalenko EM (1991)
Handbook on environmental degradation rates. Chelsea, Michigan, Lewis
Publishers.
Husain S, Sastry GSR, Prasad PR, & Sarma VR (1991) Differential pulse
polarographic determination of residual acrylic acid in sodium
polyacrylate. Electroanalysis, 3: 71-72.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein. Lyon, International Agency for Research on Cancer, pp 47-71
(IARC Monograph on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 19).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 56 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1992) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M (1988) Data book of chromosomal aberration test in vitro.
Amsterdam, Oxford, New York, Elsevier Science Publishers.
Juhnke I & Luedemann D (1978) [Results of testing of 200 chemical
compounds for acute fish toxicity using the Golden Orfe test.]
Z. Wasser Abwasser Forschung, 11(5): 61-164 (in German).
Kalimo K, Jolanki R, Estlander T, & Kanerva L (1989) Contact allergy
to antioxidants in industrial greases. Contact Dermatitis, 20:
151-152.
Kavaler AR (1987) Chemical profile on acrylic acid. Chem Mark Report,
232(23): 62.
Kirk-Othmer (1978-1984) Kirk-Othmer encyclopedia of chemical
technology, Volumes 1-26, 3rd ed. New York, John Wiley and Sons.
Klimisch HJ & Hellwig J (1991) The prenatal inhalation toxicity of
acrylic acid in rats. Fundam Appl Toxicol, 16(4): 656-666.
Klimisch HJ & Zeller H (1980) [Acute inhalation toxicity in the rat.]
Ludwigshafen/Rhine, Germany, BASF AG, Department of Toxicology (in
German).
Klimkina NV, Bolding ZN, & Sergeev AN (1969) [Experimental basis for
maximum permissible content of acrylic acid in reservoir waters.] Prom
Zag Vodoemov, 9: 171-185 (in Russian, with English abstract).
Kodama M & Ogata T (1983) Acrylic acid, as an antibacterial substance
in scallop. Bull Jpn Soc Sci Fish, 49(7): 1103-1107.
Kohriyama K, Matsuoka M, & Igisu H (1994) Effects of acrylamide and
acrylic acid on creatinine kinase activity in the rat brain. Arch
Toxicol, 68(1): 67-70.
Korenman IM & Lunicheva EV (1972) Distribution of acrylic acid between
organic solvents and water. J Appl Chem (USSR), 45: 1101-1105.
Kostanyan GG, Dadayan AA, & Safuryan GE (1969) [Polarographic
determination of acrylic acid in commercial propionic acid.] Arm Khem,
22: 1044 (in Russian with English abstract).
Kutzman RS, Meyer GI, & Wolf AP (1982) The biodistribution and
metabolic fate of (11C) acrylic acid in the rat after acute inhalation
exposure or stomach intubation. J Toxicol Environ Health, 10(6):
969-980.
Lawrence WH, Bass GE, Purcell WP, & Autian J (1972) Use of
mathematical models in the study of structure-toxicity relationships
of dental compounds: I. Esters of acrylic acid and methacrylic acids.
J Dent Res, 51: 526.
Lijinsky W & Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratog Carcinog Mutagen, 1: 259-267.
Lomax LG, Brown OW, & Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic acid
for either 6 or 22 h per day. Abstract presented at a Meeting on Nasal
Toxicity and Dosimetry of Inhaled Xenobiotics: Implications for human
health, Durham, North Carolina, 20-22 September 1993. Inhal Toxicol,
6: 445-449.
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods: Environmental behavior of organic
compounds. New York, McGraw-Hill Book Co., pp 9-65.
McCarthy KL, Thomas WC, Aardema MJ, Seymour JL, Putman DL, Yang LL,
Curren RD, & Valencia R (1992) Genetic toxicology of acrylic acid.
Food Chem Toxicol, 30: 505-515.
Mackay D & Peterson S (1981) Calculating fugacity. Environ Sci
Technol, 15: 1006-1014.
Magnusson B & Kligman AM (1969) The identification of contact
allergens by animal assay: The Guinea pig maximization test. J Invest
Dermatol, 52: 268-276.
Magnusson B, Blohm S, Fregert S, Hjorth N, Hovding G, Pirila V, & Skog
E (1968) Routine patch testing: IV. Supplementary series of test
substances for Scandinavian countries. Acta Dermato-Venereol, 48:
110-114.
Majka J, Knobloch K, & Stetkiewicz J (1974) [Evaluation of acute and
subacute toxicity of acrylic acid.] Med Prac, 25: 427-35 (in
Polish).
Mao J, Doane R, & Kovacs F Jr (1994) Separation of acrolein and its
possible metabolites using different modes of high performance liquid
chromatography. J Liq Chromatogr, 17: 1811-1819.
Miller ML (1964) Acrylic acid polymers. In: Bikales NM ed.
Encyclopedia of polymer science and technology, plastics, resins,
rubbers, fibers. New York, Interscience Publishers, vol 1, pp 197-226.
Miller RR, Ayres JA, & Jersey GC (1979a) Acrylic acid 90-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1024).
Miller RR, Ayres JA, & Jersey GC (1979b) Acrylic acid 10-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1015).
Miller RR, Ayres JA, Jersey GC, & McKenna MJ (1981a) Inhalation
toxicity of acrylic acid. Fundam Appl Toxicol, 1(3): 271-277.
Miller RR, Ayres JA, Rampy LW, & McKenna MJ (1981b) The metabolism of
acrylate esters in rat tissue homogenates. Fundam Appl Toxicol,
1(6): 410-414.
Miller RR, Young JT, Kociba RJ, Keyes DG, Bodner KM, Calhoun LL, &
Ayers JA (1985) Chronic toxicity and oncogenicity bioassay of inhaled
ethyl acrylate in Fischer 344 rats and B6C31 mice. Drug Chem Toxicol,
8: 1-42.
Mitchell DY & Petersen DR (1988) Inhibition of rat liver aldehyde
dehydrogenases by acrolein. Drug Metab Dispos, 16: 37-42.
Moiseeva TN, Kozulin SV, Kulikova LK, & Voronin SP (1991) Screening of
strains decomposing acrylic acid and its derivatives. Soviet-
Biotechnology, 1991(6): 119-125.
Mok WSL (1989) Formation of acrylic acid from lactic acid in
supercritical water. J Org Chem, 54(19): 4596-4602.
Moore MM, Amtower A, Doerr CL, Brock KH, & Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environ Mol Mutagen, 11: 49-63.
Nagasawa T, Nakamura T, & Yamada H (1990) Production of acrylic acid
and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase.
Appl Microbiol Biotechnol, 34(3): 322-324.
Narayanasamy K, Shukla S, & Parekh LJ (1990) Utilization of
acrylonitrile by bacteria isolated from petrochemical waste waters.
Indian J Exp Biol, 28(10): 968-971.
Nawaz MS, Franklin W, & Cerniglia CE (1993) Degradation of acrylamide
by immobilized cells of Xanthomonas maltophilia. Can J Microbiol,
39(2): 207-212.
Nawaz MS, Franklin W, & Cerniglia CE (1994) Degradation of aliphatic
amide mixture by immobilized and nonimmobilized cells of Pseudomonas
sp. Environ Sci Technol, 28(6): 1106-1109.
Neeper-Bradley TL & Kubena MF (1993) Developmental toxicity evaluation
of inhaled acrylic acid vapor in New Zealand white rabbits. Export,
Pennsylvania, Union Carbide Corporation (Bushy Run Research Center
Report No. 92N1008).
NIOSH (1974) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 74-127).
NIOSH (1977) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 77-213).
NIOSH (1989) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (CD-ROM version by Canadian Centre for Occupational Safety and
Health, Hamilton, Ontario).
NIOSH (1990) National occupational exposure survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard, Evaluations, and Field Studies (Unpublished
provisional data as of January 7, 1990).
Nishikawa H, Hosomura H, Sonoda H, & Inagaki M (1979) [Behaviour of
acrylamide in a soil-vegetation system.] Kenkyu Hokoku-Gifu-Ken Kogyo
Gijutsu Senta, 11: 31-34 (in Japanese).
Nishiuchi Y (1975) Toxicity of formulated pesticides to some
freshwater organisms. Suisan Zoshoku, 23: 132.
NLM (1989) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
NLM (1991) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
Noble RC & Czerkawski JW (1973) A gas-chromatographic method for the
determination of low concentrations of acrylic acid in mixtures of C2
to C5 fatty acids in biological materials. Analyst, 98: 122-125.
NTP (1986) Carcinogenesis studies of ethyl acrylates (CAS No.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of acrylic acid is difficult to ascertain,
owing to the wide range of LD50 values reported (Table 4). However
most data indicate that the substance is of low to moderate toxicity
by the oral and inhalation routes and of moderate toxicity by the
dermal route. It has been proposed that the wide variation in oral
LD50 values may be due to the different forms in which acrylic acid
has been applied, i.e. undiluted, in aqueous solution at various
concentrations or in neutralized solution (BG Chemie, 1991).
Stomach lesions, necrosis and haemorrhage have been reported
following oral dosing of rats with acrylic acid (Ghanayem et al.,
1985; DeBethizy et al., 1987). The lowest dose at which lesions were
seen was 144 mg/kg.
CrL:CDBR rats were exposed (whole body) to aerosol (mean mass
median diameter of 2.3 µm ± 2.3) concentrations of acrylic acid
ranging from 8775 to 14 145 mg/m3 (2925 to 4715 ppm) for 30 min, 8139
to 12 624 mg/m3 (2713 to 4208 ppm) for 60 min, and 3669 to
10 239 mg/m3 (1223 to 3413 ppm) for 120 min. Additionally, rats were
exposed (whole body) to acrylic acid vapour concentrations ranging from
2784 to 6426 mg/m3 (928 to 2142 ppm) for 60 min. Exposure to acrylic
acid produced treatment-related signs of nasal mucosa, upper airway and
lower airway irritation, ocular irritation, corneal opacities and
dermal toxicity in all experimental groups. Deaths, as a function of
both aerosol concentration and exposure duration, were seen in the
30-, 60- and 120-min aerosol exposures. No deaths resulted from the
vapour exposures. Following the 14-day observation period, necropsies
revealed treatment-related alterations of the lungs, eyes and skin
consistent with that of an irritant. However, comparison of LC values
suggested no difference in toxicity between the aerosol and vapour
(Hagan & Emmons, 1991).
Table 4. The acute toxicity (LD50 and LC50) of acrylic acid for experimental animals
Species Route Parameter Dose Reference
Mouse oral LD50 830 mg/kg Klimkina et al., 1969
Mouse oral LD50 1200 mg/kg Zeller, 1958
Rat oral LD50 193 mg/kg IARC, 1979
Rat oral LD50 340 mg/kg Carpenter et al., 1974
Rat oral LD50 1350 mg/kg Majka et al., 1974
Rat oral LD50 1500 mg/kg Zeller, 1958
Rat oral LD50 2520 mg/kg Fassett, 1963
Rat oral LD50 2100-3200 mg/kg Miller, 1964
Rat oral LD50 2500 mg/kg Verschueren, 1983
Mouse subcutaneous LD50 1590 mg/kg Sittig, 1985
Rabbit percutaneous LD50 295 mg/kg Carpenter et al., 1974
Rabbit percutaneous LD50 640 mg/kg Gelbke & Hofman, 1979
Rabbit percutaneous LD50 750 mg/kg IARC, 1979
Rabbit percutaneous LD50 950 mg/kg Fassett, 1963
Mouse inhalation LC50 (2 h) 5300 mg/m3 RTECS, 1989
Rat inhalation LC50 (30 min) 26 000 mg/m3 Hagan & Emmons, 1991
LC50 (60 min) 11 100 mg/m3
LC50 (120 min) 7500 mg/m3
Table 4. (contd.)
Species Route Parameter Dose Reference
Rat inhalation LC50 (4 h) 3600 mg/m3 Majka et al., 1974
Rat inhalation LC50 (4 h) > 5100 mg/m3 Klimisch & Zeller, 1980
Mouse intraperitoneal LD50 17 mg/kg Lawrence et al., 1972
Mouse intraperitoneal LD50 128 mg/kg RTECS, 1989
Mouse intraperitoneal LD50 140 mg/kg Zeller, 1958
Rat intraperitoneal LD50 24 mg/kg Majka et al., 1974
Rat intraperitoneal LD50 24 mg/kg Singh et al., 1972
In a single inhalation study in rats, no deaths occurred when
six animals were exposed to acrylic acid at a concentration of
12 000 mg/m3 (4000 ppm) for 4 h and observed over 14 days (Union
Carbide Corp., 1977).
A single 4-h exposure of six rats to 6000 mg/m3 (2000 ppm) of
acrylic acid caused no death (Carpenter et al., 1974).
One 5-h exposure to an atmosphere saturated with acrylic acid
(6000 ppm or 17 700 mg/m3) given to four rats (2 male, 2 female)
produced nose and eye irritation, respiratory difficulty and
unresponsiveness in all rats. One rat died. Histopathological
examination showed lung haemorrhage and degenerative changes in the
liver and kidney tubules of all rats (Gage, 1970), but these were
possibly secondary changes in dying animals.
Rats exposed for 1 h to acrylic acid concentrations of 300, 900
or 1500 mg/m3 (100, 300 or 500 ppm) exhibited exposure-dependent
decreases in both respiratory frequency and minute volume (Silver et
al., 1981).
In a sensory irritation study, the single exposure to acrylic
acid vapour estimated for a 50% reduction of the respiratory rate
(RD50) was 1539 mg/m3 (513 ppm) in F344/N rats and 2055 mg/m3
(685 ppm) in B6C3F1 mice. During exposure to 225 mg/m3 (75 ppm) of
acrylic acid vapour for 6 h, a 20-30% decrease in minute volume was
observed in both species (Buckley et al., 1984).
7.2 Irritation and sensitization
7.2.1 Eye irritation
Application of acrylic acid in different concentrations (glacial,
10%, 3% and 1%) to rabbit eyes revealed that it is corrosive in high
concentrations, i.e. glacial and 10%; 1% and 3% solution caused eye
irritancy (Majka et al., 1974). There are also other reports of
undiluted acrylic acid causing eye irritation and corneal damage
(Carpenter et al. 1974; BG Chemie, 1991).
7.2.2 Skin irritation and sensitization
7.2.2.1 Skin irritation
Undiluted acrylic acid is corrosive to rabbit skin (Carpenter et
al., 1974; Majka et al., 1974; BG Chemie, 1991). A study with rabbits
reported that a one-minute exposure to a 50% or 20% aqueous solution
caused, respectively, erythema and oedema or slight erythema (BG
Chemie, 1991). Another study reported a 10% solution to be corrosive
when applied to rabbit skin and that a 0.6-5% solution caused
irritation of various severity (Majka et al., 1974).
The irritant effects of repeated dermal exposure have also been
investigated. A 5% acrylic acid solution in acetone caused skin
irritation in the mouse after daily non-occlusive application for 14
days (DePass et al., 1984). No irritation was seen with a 1% solution.
In another study, groups of three strains of mice received dermal
applications of 0.1 ml acrylic acid in acetone 3 times a week for 13
weeks at concentrations of 0, 1 or 4% (Tegeris et al., 1987, 1988). At
4%, there were signs of significant skin irritation (desquamation,
fissures and eschar), with proliferative, degenerative and
inflammatory changes being detected histologically in the epidermis
and dermis, from weeks 1 to 2. At 1%, minimal proliferative changes,
detected histologically, were the only effects seen. No differences
were found between the response of the three strains of mice.
7.2.2.2 Skin sensitization
Acrylic acid has been tested for contact sensitivity in guinea-
pigs. In one study, a 20% aqueous solution of pure unstabilized
acrylic acid was applied to the skin once a day until definite skin
irritation was seen. When challenged topically 11 days later with a 2%
solution, there was no evidence of skin sensitization up to 24 h post-
challenge (BG Chemie, 1991).
In another study, the highest non-irritating concentration of
acrylic acid (not specified) was applied topically four times in 10
days. At the time of the third application, Freund's adjuvant was
injected intradermally. When challenged two weeks later, none of the
10 guinea-pigs showed evidence of skin sensitization (Rao et al.,
1981).
Three out of six guinea-pigs exposed to acrylic acid, said to be
99% pure, showed a skin sensitization response in a Polak test (Parker
& Turk, 1983). Induction was by dermal injections of a total of 1 mg
acrylic acid, together with adjuvant, followed by topical challenge
with 5% acrylic acid. However, the impurities and inhibitors of the
acrylic acid used were not mentioned in the report.
Acrylic acid was found to be an extreme sensitizer by the guinea
pig maximization test and a weak sensitizer by the Landsteiner Draize
test. The compound used for testing was considered pure, but no
analytical data were provided (Magnusson & Kligman, 1969).
Acrylic acid gave a clearly positive result in the Freunds
Complete Adjuvant test in guinea-pigs (Waegemaekers & van der Walle,
1984). Induction was by three intradermal injections of 1.2% followed
by topical application of 2.2 or 7.2%. The positive response was
believed to be due to the historical impurity, alpha,ß-
diacryloxypropionic acid. This impurity was identified in acrylic acid
from just one of three suppliers. Limited testing of acrylic acid from
the other two suppliers gave negative skin sensitization results. It
should be noted that the impurity is not present in acrylic acid
resulting from current production methods involving distillation.
Commercial acrylic acid also contains a small amount of
polymerization inhibitors, usually hydroquinone monomethyl ether
(methoxyphenol). This is a known skin sensitizer in guinea-pigs (van
der Walle et al., 1982). Other inhibitors used with acrylic acid have
also been reported to have skin-sensitizing properties, namely pheno-
thiazine (Costellati et al., 1990) and diphenyl- p-phenylenediamine
(Magnusson et al., 1968; Kalimo et al., 1989). However, it is unclear
whether the small amount of one of these inhibitors present
(0.02-0.1%) could contribute to the skin-sensitizing properties of
commercial acrylic acid.
7.2.3 Upper respiratory tract irritation
Olfactory cell proliferation, as measured by tritiated thymidine
incorporation, was investigated in male F-344 rats and B6C3F1 mice
exposed to 224 mg/m3 (75 ppm) acrylic acid 6 h daily for 5 days. A
17-fold increase in cell proliferation occurred in mice and a 4-fold
increase in rats (Swenberg et al, 1986). Further information on upper
respiratory tract irritation is given in sections 7.1 and 7.3.2.
7.3 Short-term exposure
Results of key studies on the short-term repeated exposure
effects of acrylic acid are presented in Table 5.
Table 5. Key studies on the noncarcinogenic effects of repeated exposures to acrylic acid
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Fisher-344 oral, 250 mg/kg bw/day 83 mg/kg bw/day Decreased body weight, De Pass et al.,
drinking-water, 0, 83, reduced water and food 1983
250, 750 mg/kg body consumption, changes in
weight/day for 3 months organ weights
Rat, Wistar, gavage, 0, 150 mg/kg bw/day 50% mortality in both Hellwig et al.,
150, 375 mg/kg bw/day, treatment groups, dose- 1993
5 times/week for 3 dependent irritation in the
months forestomach and
glandular stomach,
purulent rhinitis, tubular
nephroses
Rat, Wistar, oral,
drinking-water, 0, 9, 61,
140, 331 mg/kg body
weight/day for 3 months 331 mg/kg bw/day 140 mg/kg bw/day Reduced water and food Hellwig et al.,
consumption in males 1993
Rat, Wistar, oral, 140 mg/kg bw/day 61 mg/kg bw/day Reduced water and food Hellwig et al.,
drinking-water, 0, 9, 61, consumption in males 1993
140, 331 mg/kg body
weight/day for 12 months
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Rat, Wistar, oral, 78 mg/kg bw/day No treatment-related Hellwig et al.,
drinking-water, 0, 8, 27, toxic effects including 1993
78 mg/kg body tumorogenicity
weight/day for 26
(males) or 28 (females)
months
Rat, inhalation, 80, 300 300 ppm 80 ppm Nose irritation, lethargy Gage, 1970
ppm, 6 h/day 5 (900 mg/m3) (240 mg/m3) reduced body weight gain
days/week,
20 exposures
Rat, Fisher-344 225 ppm (675 75 ppm Decrease of adipose Miller et al.,
inhalation, 0, 25, 75, mg/m3) (225 mg/m3) tissue in females, lesions 1979
225 ppm, 6 h/day, of nasal mucosa
5 days/week for 2 weeks
Rat, Fisher 344 75 ppm 25 ppm Lesions of nasal olfactory Miller et al.,
inhalation, 0, 5, 25, (225 mg/m3) (75 mg/m3) epithelium 1981
75 ppm, 6 h/day,
5 days/week for
13 weeks
Rat, F-344, and mouse 75 ppm Olfactory cell proliferation Swenberg et al.,
B6C3F1 inhalation, (225 mg/m3) 17-fold in mice, 4-fold in 1986
75 ppm, 6 h/day, 5 days rats
Table 5. (contd.)
Species, route and dosage LOELa NOELa Observed effects Reference
Mouse, B6C3F1 25 ppm Decrease in body weight Miller et al.,
inhalation, 0, 25, 74, (75 mg/m3) gain, lesions in nasal 1979
223 ppm, 6 h/day mucosa
5 days/week for 2 weeks
Mouse, B6C3F inhalation 5 ppm 5 ppm Atrophy, disorganization, Lomax et al.,
0, 5, 25 ppm for 6 or 22 22 h/day 6 h/day necrosis of the olfactory 1994
h/day and 25 ppm for epithelium of nasal
4.4 h/day for 2 weeks, cavity. Recovery after 6
6 weeks recovery period weeks except for mice
exposed to 25 ppm for
22 h/day where
metaplasia was seen
Mouse, B6C3F1 5 ppm Slight focal lesions of Miller et al.,
inhalation, 0, 5, 25, (15 mg/m3) nasal olfactory 1981
75 ppm, 6 h/day 5 epithelium
days/week for 13 weeks
a LOEL = lowest-observed-effect level; NOEL = no-observed-effect level
7.3.1 Oral
Acrylic acid was administered via oral gavage to ten rats for 20
days with doses increasing by 50% every fourth day (range:
135 mg/kg to 684 mg/kg). Reduction in body weight gain and minor
histopathological changes in the stomach were found at higher doses
(Majka et al., 1974).
In a 3-month study (Hellwig et al., 1993), groups of 10 male and
10 female Wistar rats were gavaged, 5 times per week, with acrylic
acid at doses of 150 or 375 mg/kg body weight. A control group of 10
males and 10 females was gavaged with water. A high mortality rate was
observed in experimental groups; 50% of both males and females in the
low-dose group and 60% (males) and 90% (females) in the high-dose
group died. Cyanosis, dyspnoea and irritation ulceration of
forestomach and glandular stomach, purulent rhinitis and lung
emphysema and alveolar hyperaemia were the main findings reported.
Necrotizing tubular nephroses were seen in the animals that died
during the study. The symptoms and histopathological findings were
substantially the same in both groups, but they were less pronounced
and observed in a smaller number of animals given acrylic acid at
150 mg/kg body weight.
Acrylic acid was given to Wistar rats in drinking-water for
3 months as part of a 12-month study (Hellwig et al., 1993). Further
details are given in section 7.4.
In a subchronic study acrylic acid was incorporated into the
drinking-water of rats (15/sex/group) for 3 months, resulting in doses
of 0,83, 250 and 750 mg/kg per day. At the high and intermediate dose
levels, reduction in body weight gain and changes in organ weights
were observed. These effects coincided with a dose-related reduction
in food and water consumption. At the 83 mg/kg dose, the only effect
was a slight reduction in water consumption. No significant treatment-
related histological effects were seen at any dose level (DePass et
al., 1983).
7.3.2 Inhalation
In a short-term inhalation study (Gage, 1970) no adverse effects
were observed in eight rats (four males and four females) exposed to
240 mg/m3 (80 ppm) acrylic acid vapour, 6 h/day, 5 days/week for 20
exposures. Eight rats (four males and four females) exposed at
900 mg/m3 (300 ppm) showed signs of nasal irritation, lethargy and
reduced body weight gain. Histological and haematological examinations
were normal. Higher doses for shorter periods of time, i.e. 4500 mg/m3
(1500 ppm) for 4 × 6 h, resulted in nasal discharge, lethargy,
retarded weight gain and kidney congestion.
In a 2-week subacute inhalation study by Miller et al. (1979a,
1981a), F-344 rats and B6C3F1 mice (5/sex/group) were exposed to
actual concentrations of 0, 75, 225 or 675 mg/m3 (0, 25, 75 or
225 ppm) acrylic acid vapour for 6 h/day, 5 days/week for 2 weeks.
Significant decreases in body weight gain were seen in exposed groups
at 675 mg/m3. Decreased body weight gain in male mice at 75 and
225 mg/m3 was not considered to be exposure-related because of the low
initial weight and unusually large weight gain in the controls. A
reduction of adipose tissue was observed in female rats at 675 mg/m3.
Rats had lesions of the nasal mucosa at 675 mg/m3. In mice lesions of
the nasal mucosa were observed in 2/5 males and 4/5 females at 75 mg/m3
and in all mice exposed to 225 or 675 mg/m3. The lesions were
described as slight focal degeneration in the olfactory epithelium. No
effects on lung or trachea were observed in rats or mice.
Fifteen F-344 rats/sex/group and 15 B6C3F1 mice/sex/group were
exposed to actual concentrations measured by infrared analysis of 0,
15, 75 or 225 mg/m3 (0, 5, 25 or 75 ppm) acrylic acid (Miller et al.,
1979a, 1981a). The exposure was 6 h/day for 5 days/week for 13 weeks.
Animals were observed twice per day. There were no treatment-related
deaths in rats or mice during the study period. Mean body weight gains
of female mice in the 75 and 225 mg/m3 exposure groups after 12 weeks
of exposure were significantly lower than controls. There were no
significant differences in organ weights, clinical chemistry
parameters, urinalysis parameters or gross pathology that could
clearly be related to exposure. Slight focal degeneration of the nasal
olfactory epithelium was observed in rats at 225 mg/m3 but no effects
were seen at 15 or 75 mg/m3. In mice, there was a clear exposure-
related increase in focal degeneration of the olfactory nasal
epithelium at > 25 mg/m3. Lesions of the olfactory epithelium were
detected histopathologically in all male and female mice in the
225 mg/m3 exposure group, in all males and 9/10 females in the
75 mg/m3 group and in 1/10 males and 4/10 females in the
15 mg/m3 group. The lesions were described as very slight at 15 mg/m3,
slight at 75 mg/m3 and slight to moderate at 225 mg/m3.
In a whole-body inhalation study, groups of 10 B6C3F1 female
mice were exposed to 0, 15 or 75 mg/m3 (0, 5 or 25 ppm) acrylic acid
vapour for 6 or 22 h/day for 2 weeks. An additional group of mice was
exposed to 75 mg/m3 for 4.4 h/day for 2 weeks. No histopathological
lesions in the nasal cavity were detected in mice exposed to 0 or 15
mg/m3 for 6 h/day. Histopathological lesions were observed in the
olfactory epithelium of the dorsal meatus of the nasal cavity in all
other groups. These lesions included atrophy, disorganization,
necrosis, desquamation of the epithelium and nasal cell hypertrophy.
Lesions were more severe after the 22 h exposure at 75 mg/m3. After a
6-week recovery period, the olfactory epithelium was normal except for
mice exposed to 75 mg/m3 for 22 h/day where metaplasia was seen
(replacement of olfactory epithelium with respiratory-like epithelium)
(Lomax et al., 1994).
Animals exposed to 720 mg/m3 (240 ppm) for 4 h/day, 6 days/week,
for 5 weeks exhibited decreased body weight gain, increased urinary
secretion of phenol red, reduced urine-concentrating ability, nasal
discharge and increased reticulocyte count. Histopathological
examination revealed lesions of the gastric mucosa after repeated
doses of acrylic acid. Inflammation of the upper respiratory tract was
also seen (Majka et al., 1974).
7.4 Long-term exposure
Results of the studies of long-term repeated exposure effects to
acrylic acid are presented in Table 5.
In a 12-month study (Hellwig et al., 1993), Wistar rats
(20/group/sex) were given drinking-water containing 120, 800, 2000 or
5000 mg/litre (equivalent to doses of 9, 61, 140, 331 mg/kg body
weight/day, respectively). Satellite groups (10 rats/group/sex) were
treated concurrently for 3 months. Both in satellite groups and
12-months exposure groups, a reduction in drinking-water and/or feed
consumption and retarded body weight gain were observed in males at
140 and 331 mg/kg per day (2000 and 5000 mg/litre), probably due to
the unpalatability of acrylic acid at these concentrations.
Differences between groups in the results of clinico-chemical,
haematological and urinalytical examinations as well as in gross
pathological and histopathological findings were not treatment-
related. The no-observed-effect level (NOEL) was 61 mg/kg body weight
(800 mg/litre).
Acrylic acid was given to Wistar rats in drinking-water for 26
(males) and 28 (females) months in a chronic/carcinogenicity study
(Hellwig et al., 1993). Further details are given in section 7.7.
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
Results of key studies on the reproductive system are shown in
Table 6.
Table 6. Reproductive effects of acrylic acid exposure in female rats and mice and developmental effects in their offspring
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Reproduction Rat Fischer 250 mg/kg 83 mg/kg 750 mg/kg 250 mg/kg Decreased body Decrease in
and development 344; oral, bw/day bw/day bw/day bw/day weight and food body weight gain
(dePass et al., drinking- consumption of male and
1983) water; 0, 83, female pups,
250, 750 mg/kg decrease in
bw/day for 3 liver, kidney,
months before heart and spleen
mating and weight in male
through-out pups and liver
gestation and weight in female
lactation pups
Two-generation Rat Wistar; 240 mg/kg 53 mg/kg 240 mg/kg 53 mg/kg Fertility not Retarded growth
reproductive oral, drinking- bw/day bw/day bw/day bw/day affected. Reduced of F1 and F2 pups
toxicity water, 0, 500, food and water
(BASF, 1994a) 2500, 5000 ppm consumption,
(53, 240, reduced body
460 mg/kg/day) weights, gross
and
histopathological
changes in stomach
Developmental Rat Sprague- - - 2.4 mg/kg No data Increased
toxicity Dawley, bw resorptions
(Singh et al., intraperitoneal and number
1972) 0, 2.4, 4, 8, of gross and
8.0 mg/kg bw skeletal
i.p. on days 5, abnormalities
10 and 15 of
gestation
Table 6. (contd.)
Type of study Species, route Parental Developmental Observed effects
and reference and dosage
LOEL NOEL LOEL NOEL Parental Developmental
Developmental Rat Sprague- 360 mg/m3 - - 1080 Decreased None
toxicity Dawley, (120 ppm) mg/m3 body weight,
(Klimisch & inhalation, (360 ppm) food consumption
Hellwig, 0, 40, 120, uterus-to-body
1991) 360 ppm for weight; eye and
10 days during nose irritation
gestation
(days 6
through
15)
Developmental Rabbit New 180 90 mg/m3 - -- Squamous -
toxicity Zealand white, mg/m3 (30 ppm) metaplasia
(Chun et al., inhalation, 0, (60 ppm) erosions,
1993) 30, 60, 125, ulcerations in
250 ppm (0, olfactory
90, 180, 375, epithelium
750 mg/m3)
during
gestation
(days 10-23)
Developmental Rabbit New 225 mg/m3 75 mg/m3 - 675 Perinasal, None
toxicity Zealand white, (75 ppm) (25 ppm) mg/m3 perioral
(Neeper- inhalation (225 ppm) wetness,
Bradley & 0, 25, 75, nasal
Kubena, 1993) 225 ppm congestion,
(0, 75, 225, blepharospasm
675 mg/m3)
during
gestation
(days 6-18)
A one-generation study was conducted in which 10 male and 20
female rats received acrylic acid orally, in drinking-water, at doses
of 83, 250 or 750 mg/kg body weight per day for 3 months, after which
the animals were mated; this formed part of the subchronic study
reported in section 7.3.1. (DePass et al., 1983). Exposure was
continued throughout gestation and lactation.
Maternal toxicity at the two highest doses included decreased
body weight gain and decreased food consumption. An apparent decrease
in the fertility of females, as well as a reduction in gestation
index, number of live pups/litter and percentage of pups weaned at the
highest dose, was observed, but this difference was not statistically
significant when compared with the control group. Unusually low
fertility in the control group makes interpretation difficult.
In a two-generation reproductive toxicity study (BASF, 1994a),
Wistar rats were exposed to acrylic acid in drinking-water at levels
of 500, 2500 or 5000 mg/litre. Acrylic acid did not impair
reproductive function in either of the parental generations exposed to
any dose level. Clear signs of toxicity, including reduced food and
water consumption, reduced body weight and/or body weight gain, and
gross and histopathological changes in the stomach, were observed in
both parental generations exposed to 5000 mg/litre. At the
2500 mg/litre level, signs of toxicity were present in the second
parental generation animals. No adverse effects were observed in either
parental generation exposed to 500 mg/litre. Signs of developmental
toxicity, including retarded growth of F1 and F2 pups and some
delays in physical development of F2 pups only, were observed at
5000 mg/litre but less so at 2500 mg/litre. It is difficult to evaluate
the role of acrylic acid in the decreased pup weights, because of the
combined effects of reduced water consumption and the poor
palatability of water containing acrylic acid. The greatest effects on
body weight occurred during the period of early life around weaning.
Developmental toxicity was not observed at 500 mg/litre. Thus,
500 mg/litre (equivalent to 53 mg/kg per day) was a NOEL for both
generations of offspring and also a NOAEL for general toxicity in the
second parental generation. The NOEL for general toxicity in the first
parental generation was 2500 mg/litre (equivalent to 240 mg/kg per
day).
In a study by Vojtisek et al. (1991) cows were given 16 kg/day
clover grass silage, treated with 3 litres acrylic acid/tonne, from
day 57.6 ± 21.1 before parturition, for 107 days. Control groups of
eight cows were given the same amount of silage, but treated with 4
litres formic acid/tonne from day 55.5 ± 21.9 before parturition.
Changes in clinical chemistry and haematological parameters observed
in the course of the experiment (on days 0, 39, 65 and 107) were of no
toxicological importance. No differences in colostrum density, body
weight, clinico-chemical or haematological examinations of live calves
were seen.
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Oral
In the experiment of DePass et al., (1983) (see also section
7.5.1) there was a statistically significant decrease in body weight
of the male and female pups at the highest dose (750 mg/kg per day),
which was maternally toxic. The male pups also exhibited significant
decreases in absolute and relative liver weights and in absolute
kidney and heart weights at 750 mg/kg per day. The female pups showed
a significant decrease in absolute and relative spleen weight and in
absolute liver weight at the highest dose. There was an increase in
relative brain weight in both sexes at this dose.
7.5.2.2 Inhalation
Groups of 30 pregnant Sprague-Dawley rats were exposed to nominal
concentrations of 0, 120, 360 or 1080 mg/m3 (0, 40, 120, or 360 ppm)
acrylic acid vapour for 6 h/day for 10 days during gestation (day 6 to
day 15 of gestation) (Klimisch & Hellwig, 1991). There was clear
evidence of maternal toxicity at 1080 mg/m3 consisting of eye and
nose irritation, as well as reduced body weight gain and food
consumption. The latter two effects were also seen at 360 mg/m3 and
there was an indication of minimal maternal toxicity at 40 ppm
(reduced body weight gain). There were no effects on preimplantation
loss, the number of live fetuses and resorption, fetal size or on the
appearance of the soft tissues and skeleton of the fetuses. This study
identified a NOAEL for developmental effects of 1080 mg/m3 and a
LOAEL for maternal toxicity of 1080 mg/m3.
An inhalation developmental study has also been reported in
rabbits (Neeper-Bradley & Kubena, 1993). In the range-finding study
(Chun et al., 1993), groups of eight pregnant New Zealand white
rabbits were exposed to 0, 90, 180, 375 or 750 mg/m3 (0, 30, 60, 125
or 250 ppm) acrylic acid vapour for 6 h/day on days 10-23 of
gestation. Three animals per group were necropsied on day 23 of
gestation, and the remaining animals were examined on day 29.
Exposure-related maternal toxicity at 375 and 750 mg/m3 was observed,
including signs of nasal irritation and reduced body weight. Final
body weight was reduced to a lesser degree in animals exposed to 90
and 180 mg/m3. Histopathological examination of a single section of
the nose showed adverse effects in the olfactory epithelium. The
lesions included squamous metaplasia, epithelial erosion and
ulceration of the epithelium, and increased in severity with
increasing exposure concentration. The effect first appeared in the
90 mg/m3 group at day 23 and in the 180 mg/m3 group at day 29.
In the main developmental study (Neeper-Bradley & Kubena, 1993), groups
of 16 pregnant rabbits were exposed to 0, 75, 225 or 675 mg/m3 (0, 25,
75 or 225 ppm) acrylic acid vapour for 6 h/day on gestation days 6-18.
Maternal toxicity was evident in groups exposed to 225 or 675 mg/m3,
but not to 75 mg/m3 (NOEL). Signs of nasal irritation, including
perinasal wetness and nasal congestion, were observed. Significant
decrements in food consumption and body weight gain were observed
occasionally during exposure, but the body weights at the end of the
exposure were not significantly affected. Histopathological
examination of maternal tissues was not performed. No exposure-
related adverse effects were observed in the number of corpora lutea
and total, viable or nonviable implantations; preimplantation loss;
fetal length or weight; or on morphological abnormalities (skeletal
or soft tissue). This study identified a NOEL for developmental
effects of 675 mg/m3.
7.5.2.3 Intraperitoneal
When acrylic acid was injected intraperitoneally into pregnant
female Sprague Dawley rats at doses of 0, 2.4, 4.8, or 8.0 mg/kg on
days 5, 10 and 15 of gestation, the chemical was both embryotoxic and
teratogenic (Singh et al., 1972). The number of resorptions and the
number of gross and skeletal abnormalities increased with increasing
acrylic acid concentration, and the NOEL was identified at the low
dose (i.e. 2.4 mg/kg). Fetotoxicity (decreased number of live fetuses
and mean fetal weight) was observed at a dose of 2.4 mg/kg. This study
is difficult to interpret as the control groups treated with distilled
water, normal saline and cotton-seed oil also showed gross and
skeleton abnormalities. There was also no information about maternal
toxicity.
7.6 Mutagenicity and related end-points
7.6.1 In vitro and insect studies
Acrylic acid was found to be without mutagenic activity in five
test strains of Salmonella typhimurium with and without activation
by rat and hamster liver microsomal preparations using both plate and
liquid suspension assays (Lijinsky & Andrews, 1980). Although negative
responses were reported in all strains, no cytotoxicity was observed
at the concentrations tested (up to 1000 µg/plate).
Acrylic acid was also evaluated for mutagenicity in the
Salmonella/microsome preincubation assay using the standard protocol
approved by the National Toxicology Program. Acrylic acid was tested
at doses of 0, 10, 33, 100, 333, 1000 and 3333 µg/plate in four
Salmonella typhimurium strains (TA98, TA100, TA1535 and TA1537) in
the presence and absence of Aroclor-1254-induced rat or hamster liver
S9. Acrylic acid was negative in these tests and the highest non-toxic
dose level tested in any Salmonella test strain was 1000 µg/plate. The
3333 µg/plate dose level was toxic and caused a complete clearing of
the background lawn (Zeiger et al., 1987).
In another study (Cameron et al., 1991) acrylic acid was also
found to be negative in the Salmonella assay, both in the presence and
the absence of both Aroclor-1254-induced rat or hamster liver S9 mix.
Three mammalian gene mutation studies have been reported. No
increase in mutation frequency was seen in a CHO/HPRT gene mutation
assay with or without Aroclor 1254-induced rat liver S9 (McCarthy et
al., 1992). The upper dose levels in this single experiment resulted
in a reasonable amount of toxicity (survival reduced by up to 65-76%).
In two mouse lymphoma L5178Y TK+/- studies positive results have been
obtained. A concentration-related increase in mutant frequency was
observed with and without rat liver S9 in association with an
acceptable level of toxicity (Cameron et al., 1991). The authors did
not state if there was any adjustment of pH. In the other study,
conducted without exogenous metabolic activation, there were
concentration-related increases in mutant frequency in two separate
experiments in the presence of marked but not excessive toxicity.
Small colonies predominated, which suggested a clastogenic effect;
this was confirmed by chromosome analysis. Acrylic acid was tested at
concentrations of 300, 450 and 500 µg/ml in this study but the authors
did not state if there was any adjustment of pH (Moore et al., 1988).
A single experiment was conducted with CHO cells exposed to
acrylic acid solutions adjusted to pH 7 at concentrations that reduced
cloning efficiency by up to 58-65% (McCarthy et al., 1992). A
concentration-related increase in the percentage of cells with
chromosome aberrations, primarily chromatid breaks and exchanges, was
observed in the presence and absence of rat liver S9. A positive
result has also been briefly reported for this in vitro chromosome
aberration assay using CHL cells in the absence of exogenous metabolic
activation (Ishidate, 1988).
The effect of acrylic acid on unscheduled DNA synthesis (UDS) in
rat hepatocytes has been investigated in one unreplicated assay
(McCarthy et al., 1992). There was no increase in UDS at
concentrations up to those closely approaching a very toxic level.
Similar dose levels of acrylic acid in a single UDS experiment with
SHE cells gave a negative result (Wiegand et al., 1989).
Negative results have also been obtained in a micronucleus test
and a transformation test, both with SHE cells, as well as in the
Drosophila sex-linked recessive lethal assay (Wiegand et al., 1989;
McCarthy et al., 1992).
In a study by Segal et al. (1987), it was reported that acrylic
acid forms 2-carboxyethyl adducts with adenine, guanine and thymine
following in vitro reaction with calf thymus DNA. These adducts are
identical to those formed with the same bases following in vitro
reaction of carcinogen ß-propionolactone. The relevance of the results
of this study is questionable because appropriate control treatments
were not conducted and the 2-carboxyethyl adducts were formed after
long treatment period.
In contrast to the results above, Frederick & Reynolds (1989)
found that incubation of the negatively charged acrylate anion with
two representative nucleophiles, methylamine and imidazole, did not
result in the formation of adducts of the acrylate ion to the
nucleophile.
It is suggested that binding of acrylic acid to cellular
nucleophiles might be due to small amounts of the unionized acid in
the equilibrium between acrylate anion and acrylic acid at cellular
pH. However, this event is considered to be insignificant in vivo,
based upon the rapid metabolism and excretion of acrylic acid
(Frederick & Reynolds, 1989).
7.6.2 In vivo mammalian studies
Preliminary results indicated no DNA adducts in the stomach and
liver of rats after oral dosing (Sagelsdorf et al., 1988). Although
DNA adducts were found in the skin of mice after dermal application,
the investigators concluded that further work was needed to confirm
the significance of these findings. No firm conclusions can therefore
be drawn from these preliminary results.
No increase in the incidence of chromosomal aberrations was
observed in the bone marrow of rats following acute or repeated
exposure to acrylic acid (McCarthy et al., 1992). Rats either received
a single gavage dose of up to 1000 mg/kg acrylic acid or were exposed
to up to 5000 mg/litre in the drinking-water for 5 days. There was no
effect on the mitotic index of the bone marrow but there was evidence
of systemic toxicity.
Negative results were reported in a dominant lethal assay with
mice following acute or repeated exposure to acrylic acid (McCarthy et
al., 1992). Male CD-1 mice received either a single gavage dose of up
to 324 mg/kg or daily gavage doses of up to 162 mg/kg for 5 days. The
fact that the dose levels were selected not to exceed the LD1 for
acute dosing and 0.5 LD1 for repeat dosing limits the value of this
study.
7.7 Carcinogenicity
In a carcinogenicity study (Hellwig et al., 1993), Wistar rats
(50/group/sex) were given acrylic acid in the drinking-water at
concentrations of 0, 120, 400 or 1200 mg/litre (0, 8, 27, or
78 mg/kg body weight per day, respectively) over 26 (males) or 28
(females) months. The highest concentration was selected because of
evidence of palatability problems at 2000 and 5000 mg/litre in an
earlier chronic drinking-water study. At 1200 mg/litre there was some
evidence for slightly reduced water consumption. In comparison with
the controls, mortality was not increased by administering acrylic
acid. No clear toxic effects were revealed in the groups. The few
statistically significant differences in some haematological
parameters between groups were considered to be of an incidental
nature. The extensive histopathological examination revealed no clear
treatment-related non-neoplastic tissue changes. The incidence and
organ distribution of the tumours found in the groups treated with
acrylic acid for 26/28 months did not differ from those of the
controls. In this study the NOEL was 78 mg/kg body weight per day.
It has been reported that when 25 µl of a 1% solution
(i.e. 0.25 mg) of acrylic acid was applied topically in acetone
on the dorsal skin three times a week for their lifetime to 40
male C3H/HeJ mice, no malignancies were observed at the site of
application (De Pass et al., 1984).
When 1 mg (14 µmol) of acrylic acid was applied topically in
acetone 3 times a week to 30 female ICR/HA (currently designated
Hsd:(ICR)Br) mice for 1.5 years, squamous cell carcinomas were
observed in two of the mice (Cote et al., 1986). No conclusion can be
drawn from this study, because only an abstract was issued, it was not
subsequently published in full and an independent review of this study
(Sivak, 1987) uncovered many inconsistencies and hence questioned the
validity of the findings.
Acrylic acid was assayed for carcinogenic activity in female
Hsd:(ICR)bR mice by subcutaneous injection of 20 µmol (approx 1.4 mg)
in 0.05 µl trioctanoin, once a week for 52 weeks. The mice were then
observed for an additional 93 days (total 450 days) when the survivors
were killed. Two mice with sarcomas at the site of application were
observed out of 30 mice (Segal et al., 1987). This would be an
expected finding as an irritant solution was repeatedly given by
subcutaneous injection. Injections for this length of time frequently
result in sarcomas, no matter what the compound, because of repeated
insult.
7.8 Other studies
In contrast to some of its esters, acrylic acid did not
significantly decrease non-protein sulfhydryl content (NPSH) in the
liver, blood or forestomach after oral dosing of rats with up to
400 mg/kg body weight (as 8% acrylic acid solution in 0.5% methyl-
cellulose solution). However, a significant depletion of NPSH was
observed in the glandular stomach at doses above 4 mg/kg (as 0.8%
acrylic acid solution) (Miller et al., 1981b; De Bethizy et al., 1987).
Acrylic acid was found to be ineffective in inducing GSH
depletion, lipid peroxidation and haemolysis (Ferrali et al., 1989).
It was reported that methylethyl- n-butyl and 2-ethyl-hexyl
acrylates, when inhaled by male Wistar rats, induced hyperglycaemia
but that acrylic acid was without effect (Vodicka et al., 1990).
Rats received neutralized acrylic acid (50 mg/kg) intra-
peritoneally for 8 consecutive days and were observed for central and
peripheral nervous system effects. They showed a 25 % decrease in body
weight gain but no signs of neurotoxicity (Kohriyama et al., 1994).
In an in vitro study, acrylic acid was more potent than
acrylamide as an inhibitor of creatinine kenase (CK) activity in rat
brain homogenates. However, when the two chemicals were given
intraperitoneally 50 mg/kg per day for 8 days to rats, only acrylamide
inhibited CK activity in the brain. The 14C level in the brain, 24 h
after the injection of 14C-labelled chemicals, was more than seven
times greater with acrylamide then with acrylic acid (Kohriyama et
al., 1994).
Single intraperitoneal injections of up to 2.2 mg/kg body weight
of acrylic acid to Wistar rats had no effect on the hepatic activity
of ornithine decarboxylase, which was measured for tumour-promoting
activity (van de Zande et al., 1986).
Laparotomies were carried out on pregnant Sprague-Dawley rats on
day 13 of pregnancy under anaesthesia, and half of the developing
fetuses were injected with 10, 100 or 1000 µg acrylic acid per fetus.
One fetus injected with 100 µg acrylic acid showed slight
hydrocephalus and microcephalia. A dose of 1000 µg resulted in 78%
resorption (Slott & Hales, 1985).
It was reported that acrylic acid interferes with incorporation
of thymidine into DNA and uracil into RNA in Staphylococcus aureus
and Escherichia coli (Glombitza & Heyser, 1971).
7.9 Factors modifying toxicity
No studies on factors modifying the toxicity of acrylic acid have
been reported.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Acute toxicity
8.1.1.1 Poisoning accidents
There have been no reports on poisoning accidents in the general
population or on the short- or long-term effects of acrylic acid on
the general population.
8.2 Occupational exposure
8.2.1 Poisoning accidents
There have been no reports on poisoning accidents due to
occupational exposure.
8.2.2 Effects of short- and long-term exposure
Fowler (1990) described the case of a male chemical worker who
developed acute generalized urticaria after working with acrylic acid
and acrylate compounds. Immediate hypersensitivity testing showed a
severe local reaction to 2% acrylic acid (which was apparently not
irritant), but no reaction to other acrylate compounds. The acrylic
acid used for testing was thought to be pure but it was not analysed
for the presence of alpha,ß-diacryloxypropionic acid. Re-exposure of
the worker in the workplace to acrylic acid resulted in generalized
urticaria.
No cross-sensitization to 0.1% acrylic acid was observed in six
patients who had exhibited hypersensitivity to acrylate-based sealants
(Conde-Salazar et al., 1988).
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
9.1 Microorganisms
The toxic threshold (EC3) level for inhibition of growth of the
bacterium, Pseudomonas putida was 41 mg/litre after exposure for
16 h to a neutralized solution of acrylic acid (Bringmann & Kühn,
1980). Stewart et al. (1995) studied the anaerobic degradation of
acrylic acid in 55-day tests in glucose-acetate enrichment culture. At
100 mg/litre of acrylic acid there was minimal effect on acetate
utilization by methanogens; concentrations of 500, 1000 and
1500 mg/litre inhibited acetate utilization for a period of time
before recovery.
In a study of the effect of acrylic acid on the soil carbon
cycle, it was shown that acrylic acid in soil (sandy loam soil), at
levels of up to 100 mg/kg soil, had no effect on the respiration of
the soil microflora. However, a concentration of 1000 mg/kg acrylic
acid completely suppressed respiration (Hossack et al., 1992).
9.2 Aquatic organisms
The toxicity of acrylic acid to aquatic organisms is summarized
in Table 7. Algae appear to be the most sensitive group with EC50
values, based on growth, ranging from 0.04 to 63 mg/litre; and a NOEC
for the most sensitive species of 0.008 mg/litre. Other acute toxicity
studies range from 27 mg/litre (96-h LC50) for the rainbow trout to
315 mg/litre (72-h LC50) for the golden orfe. A 96-h NOEC for glacial
acrylic acid toxicity to rainbow trout was found to be 6.3 mg/litre
based on a lack of sublethal/behavioural responses, e.g., quiescence,
fish on bottom of test vessel, loss of equilibrium and erratic
swimming, at this concentration (Bowman, 1990). Neutralized acrylic
acid was found to be less toxic to daphnids than the non-neutralized
solution (Bringmann & Kühn, 1982).
9.3 Terrestrial organisms
No data on terrestrial organisms have been reported.
Table 7. Toxicity of acrylic acid to aquatic organisms
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Blue-green CMIT Exposure duration 8 days; TT (EC3) 0.15 the toxic threshold Bringmann &
alga temp. 27°C; neutralized with extinction Kühn, 1978
Microcystis pollutant solution; static values > 3% lower
aeruginosa closed flasks shaken once than for controls
a day; the
concentration of the
algae suspension measured
turbidimetrically at 578 nm
Green alga CMIT Exposure duration 7 days; TT (EC3) 18 as above Bringmann &
Scenedesmus temp. 27°C; neutralized Kühn, 1980
quadricauda pollutant solution; static
closed flasks shaken once a
day; the concentration of
the algal suspension measured
turbidi-metrically at 578 nm
Green alga CMIT 96-h static algal assay; EC50 0.17 50% algal Forbis, 1989
Selenastrum acrylic acid exposure growth
capricornutum concentrations ranged from inhibition
(Printz) 0.15 to 1.9 mg/litre
Green alga algal Exposure duration EC50 0.04 50% algal BASF, 1994b
Scenedesmus inhibition 72 h biomass
subspicatus test inhibition
EC50 0.13 50% algal
growth rate
LOEC 0.016 inhibition
NOEC 0.008
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Green alga algal Exposure duration EC50 1.53 50% algal SNF, 1995
Chlorella inhibition 72 h; nominal acrylic biomass
vulgaris test (OECD acid exposure inhibition
No. 201) concentrations
ranged from 0.2 to EC50 63.0 50% algal
4.7 mg/litre for growth rate
biomass test and were inhibition
50 and 100 mg/litre
for growth rate
inhibition test
Protozoan CMIT Exposure duration TT (EC5) 20 the toxic Bringmann &
Entosiphon 72 h; temp. 25°C; threshold with Kühn, 1982
sulcatum pollutant solution extinction
(Stein) adjusted to pH 6.9; values > 5%
static closed flasks; lower than for
the number of controls
protozoans was
determined by
means of cell counter
Water flea Daphnia Exposure duration EC50 765 after 24 h Bringmann &
Daphnia immobilization 24 h; temp. 20°C; 50% of exposed Kühn, 1982
magna test pH 7.8-8.2; organisms were
(Straus) performed in 50 ml immobilized in
beakers covered with neutralized test.
filter paper;
10 organisms/
20 ml test solution;
9 h illumination/
15 h dark
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
EC100 5000 100% immobilized
after 24 h in
neutralized test
Acrylic acid in
non-neutralized
test
EC50 54 in non-neutralized
test
EC100 91
Water flea Daphnia Dynamic test. Acrylic acid EC50 95 after 48 h 50% of Burgess, 1990
Daphnia magna immobilization exposure concentrations exposed organisms
test ranged from 7.9-110 were immobilized
mg/litre.
Exposure duration 48 h; NOEC 23 based on mortality
temp. 19-20°C; pH 6.7-7.7;
dissolved O2 7.9-8.0 mg/litre
Golden orfe L15-Fish test Exposure duration LC0 210 no lethality Juhnke &
Leuciscus idus German 48 h; temp. 19-21°C; pH 7-8; Luedemann,
melanotus standard dissolved O2 1978
method for the > 5 mg/litre; 10-fish LC50 315 death of 50%
contaminants per 10 litre test of exposed
of water, solution; continuous fish
waste water aeration
and sludge
LC100 420 death of all
exposed fish
Table 7. (contd)
Species Testa Experimental Criticalb Critical Comments Reference
conditions end-point acrylic acid
concentration
(mg/litre)
Rainbow trout A flow-through Exposure duration 96 h; LC50 27 death of 50% Bowman,
Oncorhynchus toxicity test measured glacial acrylic of exposed 1990
mykiss acid exposure NOEC 6.3 fish
concentrations ranged lack of
from 6.3 to 90 mg/litre; sublethal/
20 fish per group behavioural
responses
Common carp Exposure duration LC100 100 Nishiuchi,
Cyprinus 24 h 1975
carpio
a CMIT = Cell multiplication inhibition test
b TT = Toxicity threshold
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure of the general population
Very limited data are available on general population exposure.
Exposure of the general population to acrylic acid may occur by the
dermal route via contact with unreacted acrylic acid in household
goods such as water-based paints or detergents. Exposure is also
possible from inhalation due to evaporation of acrylic acid from
paint.
Exposure of the general population through drinking-water and via
ambient air might be assumed. Exposure through drinking-water is
possible from surface or groundwater contamination while inhalation
exposure could occur in the vicinity of industrial emissions. However,
there is no data on measured ambient air concentrations in populated
areas and no data are available on acrylic acid concentrations in
drinking-water. It was reported that acrylic acid might occur in
wastewater effluent from industrial facilities at concentrations not
exceeding 0.5 mg/litre. However, effluent levels of 2500 mg/litre were
reported at a methyl acrylate production facility in India. After
treatment acrylic acid concentration was below the limit of detention
(0.1 mg/litre) in wastewater from a production facility in Europe.
Food is an unlikely source of exposure to commercially derived
acrylic acid because it is not known to be used in applications that
involve direct contact with food.
10.1.2 Occupational exposure
The most important means of human exposure to acrylic acid is
occupational exposure via inhalation and skin contact. On the basis of
statistical estimates derived from a NIOSH survey conducted in
1972-1974, 28 600 workers were potentially exposed to acrylic acid in
the USA. More recently, NIOSH estimated that 96 500 workers were
potentially exposed in the USA.
One study conducted at a factory, where a number of chemicals
including acrylic acid and a variety of acrylates and methacrylates
were used, indicated that levels in air of acrylic acid and ethyl
acrylate varied from 0.03 to 168 mg/m3 (0.01 to 56 ppm). However, the
8-h TWA levels of most areas of the plant were well below the hygienic
standard of 30 mg/m3 (10 ppm) for acrylic acid recommended by OSHA
and ACGIH at the time of the study.
Exposures of workers to acrylic acid have been compiled from four
producing companies. Operators had a mean 8 h-exposure value of
0.48 mg/m3 (range of 0.03 to 3 mg/m3); during loading/unloading
the mean 8 h-exposure was 0.39 mg/m3 (range of 0.27 to 1.98 mg/m3).
10.1.3 Toxic effects
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, moderate acute toxicity by the inhalation route and
moderate acute toxicity by the dermal route.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. Although a 1% solution has
been reported to irritate rabbit skin and eyes, no externally visible
irritation was seen in one mouse study following repeated dermal
application of 1%. Acrylic acid is also a strong irritant to the
respiratory tract.
Both positive and negative results have been obtained in skin
sensitization tests with acrylic acid, but it appears that the
positive findings may have been due to the impurity alpha,ß-
diacryloxypropionic acid.
10.1.3.1 Carcinogenic and mutagenic effects
Both positive and negative results have been obtained in
in vitro genotoxicity tests. Notably, acrylic acid was negative in
the Ames test and in vitro UDS assay, positive in a chromosome
aberration assay (with pH adjustment) and in the in vitro lymphoma
cell assay. Chromosome damage was also demonstrated in one lymphoma
assay. An in vivo bone marrow chromosome aberration assay was
negative. No firm conclusions can be drawn from an in vivo
DNA-binding study or from a dominant lethal assay. There are no
satisfactory in vivo genotoxicity data for sites of initial contact,
e.g., nasal epithelium or skin.
A single chronic cancer bioassay of acrylic acid, administered in
drinking-water, has been reported. Male and female Wistar rats were
given drinking-water containing 120, 400 or 1200 mg/litre (estimated
doses were 8, 27 and 78 mg/kg body weight per day), after preliminary
3- and 12-month studies showed reduced water consumption and body
weight gain in groups of rats receiving drinking-water containing 2000
or 5000 mg/litre. No systemic effects, including weight change or
histopathology, and no increase in tumour incidence resulted from
acrylic acid exposure. Although the lack of any toxic effects in this
study suggests that the maximum tolerated dose was not achieved, it
appears that the high dose in this study was approaching a maximum
tolerance dose (MTD), based on the mild body weight effects noted at
140 mg/kg per day in a 12-month study and at 240 mg/kg per day in a
two-generation reproductive study. Apart from not reaching the MTD,
the study used adequate number of animals (50), was well documented
and reported complete histopathology.
There has been no cancer bioassay conducted in a second species
by the oral route, nor any inhalation cancer bioassay. Available data
are inadequate to evaluate the carcinogenic potential of acrylic acid
via the dermal route.
There have been several chronic bioassays conducted on related
compounds that are relevant to evaluation of the potential
carcinogenicity of acrylic acid. Chronic inhalation studies have been
reported for methyl acrylate, butyl acrylate and ethyl acrylate. All
of these studies exposed rats to 0, 5, 25 or 75 ppm of the acrylates
for 2 years, which was followed by complete necropsy and histopa-
thological evaluation. The acrylate esters are rapidly metabolized to
acrylic acid and alcohols by nasal, blood and other tissue carboxyl-
esterases. In all of these studies a concentration-dependent increase
in nasal olfactory degeneration was observed, and the response was
very similar to the response observed in a subchronic inhalation study
of acrylic acid. There were no treatment-related increases in tumour
incidence in these studies. Although there may have been quantitative
differences in the tissue doses of acrylic acid, the acrylate ester
studies provide supportive evidence that carcinogenic effects do not
occur in tissues exposed chronically to acrylic acid.
Two cancer bioassays via oral exposure have been conducted with
ethyl acrylate, a gavage study in F-344 rats and B6C3F1 mice (NTP,
1986) and a drinking-water study in Wistar rats. The highest dose was
similar in the two studies and produced lesions of the forestomach.
However, the gavage study resulted in a dose-dependent increase in
tumour incidence in both sexes of rats and mice while the drinking-
water study did not. The gavage study suggests a potential for
carcinogenic effects for ethyl acrylate, and, by extension, for the
other acrylate esters and acrylic acid. The greater potency for this
effect in a gavage study may relate to the contact site toxicity of
these compounds. Gavage may not always be an appropriate exposure
method for direct irritants because of the extremely high local doses
that are achieved, compared to the local doses in a drinking-water
study.
A similar result can be seen in 3-month gavage and drinking-water
studies. The drinking-water study caused mildly reduced body weight
gain compared to controls, while the gavage study, at very similar
doses, resulted in lethality in most of the dosed animals. These
results suggest that the gavage route of exposure should play little
role in the risk assessment of acrylic acid because human exposures
are not likely to result in repeated high-concentration doses.
Current practice requires strong evidence to conclude that a
chemical is not a carcinogenic hazard, usually including adequate
chronic bioassays in two species. A second species chronic bioassay is
not available for acrylic acid, and the acrylate ester inhalation and
drinking-water bioassays described above were carried out on rats. The
existing acrylic acid bioassay is adequate and demonstrated no cancer
effects. No epidemiological evidence regarding potential
carcinogenicity of acrylic acid is available. Based on the available
data, one cannot definitively conclude that acrylic acid does not pose
a carcinogenic hazard to humans, although substantial relevant data do
not suggest a cancer hazard.
10.1.3.2 Non-cancer effects
a) Route-specific effects
Both oral and inhalation exposures show effects at the site of
contact. Inhalation exposures resulted in nasal effects in studies of
2 weeks to 3 months in duration and at various exposure
concentrations. High concentration drinking-water exposure to acrylic
acid resulted in forestomach lesions. No other specific organ
pathology has been consistently demonstrated in either oral or
inhalation studies. Metabolic studies show rapid metabolism and
clearance of acrylic acid that is similar for several routes of
exposure, and there is no evidence of bioaccumulation of this
chemical. The available evidence supports the conclusion that systemic
toxicity is not likely to occur, and that the effects of oral and
inhalation exposure can be treated as independent events with no
additivity of doses received by the different routes of exposure. For
this reason, guidance values will be derived separately for oral and
inhalation routes.
b) Developmental and reproductive effects
The toxicity data base for acrylic acid includes inhalation
developmental studies in rats and rabbits and oral reproductive
studies in rats. No specific reproductive or developmental effects
were observed in any of these studies. The only effects observed
included nasal effects in the inhalation studies and stomach lesions
and reduced body weight gain in the drinking-water studies. Based on
these results and the evidence for specific site-of-contact effects
described above, the potential hazards for developmental and
reproductive effects have been adequately studied and are not critical
to the human health risk assessment.
c) Oral gavage studies
As described above (section 10.1.3.1) the 3-month gavage and
drinking-water studies showed important differences in toxicity,
depending on the method of dosing. The drinking-water study revealed
reduced body weight gain compared to controls, while the gavage study,
at very similar daily doses, showed lethality in most of the dosed
animals. Therefore, the gavage route of exposure should be of little
importance in the risk assessment of acrylic acid because human
exposures are not likely to result in repeated high-concentration oral
doses.
d) Critical oral studies
Potential critical studies include a 3-month drinking-water study
in rats, 3-, 12- and 26- to 28-month drinking-water studies in rats,
and a two-generation reproductive study in rats. Table 8 summarizes
the effects seen in these studies and the determination of which
effects are considered adverse.
Table 8. Summary of adverse effect levels in drinking-water studies on ratsa
LOAEL NOAEL Effectb Reference
750 250 Body weight; 81 and 84% of controls in males and females, DePass et al., 1983
respectively, at 750; 95% of C in females at 250
none 331 Body weight: Hellwig et al., 1993
males: 93% of C at 331; (3-month study)
females: no effect
331 140 Body weight: Hellwig et al., 1993
males: 91% of C at 331, 94% of C at 140; (12-month study)
females; no effect
none 78 no effects Hellwig et al., 1993
(26- to 28-month studies)
460 240 460: stomach irritation, body weight: 91% of C in F0 males, BASF, 1993
65% of C in F1 pups, 85% of C in F1 (both sexes), 68% of C
in F2 pups
240: body weight: 89% of C in F1 pups, 88% of C in F2 pups
a concentrations are given in mg/kg body weight per day
b C = control group; F0 = first parental generation; F1 = second parental generation
A consistent finding throughout the drinking-water studies was
decreased water consumption, probably due to taste aversion and
sometimes accompanied by decreased food consumption. Reductions in
body weight appear to parallel decreased water consumption and were
worse in the pups in the reproductive study. The water consumption
effect makes the body weight changes difficult to interpret as a
direct toxic effect of acrylic acid, but the possibility that the
effect was at least partly a direct result of acrylic-acid-induced
irritation cannot be ruled out. The body weight effects are therefore
considered to be adverse when the magnitude of the effect approaches a
10% reduction. A somewhat larger change in the pup weight would be
required to be considered adverse, because the largest weight
decrements were seen at the end of active nursing and the beginning of
weaning; they may be more severely affected by the reduced water
consumption because of the greater maternal need for water intake
during this time and the unknown effects of taste aversion on weaning
behaviour. Based on these considerations and the presence of stomach
histopathology at 460 mg/kg body weight per day, the NOAEL in the two-
generation reproduction study was 240 mg/kg body weight per day. The
NOAEL based on body weight changes in the 12-month studies was
140 mg/kg body weight per day.
e) Critical inhalation studies
In a subchronic study, which is the only study that is
potentially a critical study for inhalation, lesions in the nasal
epithelium were seen in rats at 225 mg/m3 (75 ppm) and in mice at
15 mg/m3 (5 ppm). The lesion in mice was described as very slight at
15 mg/m3 (5 ppm), slight at 75 mg/m3 (25 ppm), and slight to
moderate at 225 mg/m3 (75 ppm). Despite the mild nature of the
response at 15 mg/m3 (5 ppm), there was a clear increase in incidence
and severity of this lesion with increasing exposure concentration, so
it must be considered adverse.
f) Progression of lesions
There is suggestive evidence from both inhalation and oral routes
of exposure that the effects caused by acrylic acid are largely
determined by the exposure concentration and are relatively less
affected by the duration of exposure in repeated exposure studies.
In the oral studies, even if different LOAEL and NOAEL values
could be defined by the 3-month and 12-month oral drinking studies,
the effects on weight are similar for the two exposure durations. In
inhalation studies in mice, the severity of the lesions in animals
exposed for 6 h/day to 75 mg/m3 (25 ppm) was very similar in the
2-week and the 90-day studies, although limited description of the
lesions in the 2-week study makes this conclusion tenuous. Additional
2-week studies included groups exposed to 15 and 75 mg/m3 (5 and
25 ppm) for 6 h/day. The 75 mg/m3 (25 ppm) group showed effects similar
to those seen in the subchronic study, suggesting limited progression
over this time-frame. The 15 mg/m3 (5 ppm) group exposed for 6 h/day
showed no effect after 2 weeks, so slight progression does occur at
low concentrations, given that lesions were observed in the 90-day
study. The 2-week study also included recovery groups, which showed
that the lesions were completely reversed in animals exposed to
15 mg/m3 (5 ppm) for 22 h and 75 mg/m3 (25 ppm) for 6 h, but
not in animals exposed to 75 mg/m3 (25 ppm) for 22 h/day.
Comparison of the subchronic and chronic inhalation studies of
the acrylate esters also supports the conclusion that there is limited
progression of nasal lesions. Some progression of lesions does occur,
but much less than would be predicted by an assumption of a constant
concentration × time relationship.
10.1.4 Risk evaluation
10.1.4.1 Inhalation exposure
The LOAEL of 15 mg/m3 (5 ppm) from the subchronic study is used
as the basis for the guidance value. The appropriate uncertainty
factors (UF) include 5 for inter-individual differences and 10 as a
composite UF for interspecies, LOAEL to NOAEL and subchronic to
chronic extrapolations. Reduced uncertainty for these aspects of the
guidance value (GV) for inhalation results from the information
discussed previously in this monograph. The inter-individual
uncertainty is reduced because of the direct-acting nature of the
toxicity, which does not involve metabolism. The uncertainty in
extrapolating from a LOAEL to a NOAEL is reduced because the lesion at
the LOAEL is very mild and reversible. The uncertainty in
extrapolating from a subchronic to a chronic exposure is reduced
because of the evidence discussed above showing limited progression.
The interspecies uncertainty is reduced because of the direct-acting
nature of the toxicity of acrylic acid and because the mouse is
apparently very sensitive. The toxicity of acrylic acid is clearly
limited to the site of deposition and is not dependent on metabolic
activation. Since the deposition is controlled by physical
interactions, there is little reason to expect significantly greater
deposition in humans.
Based on these considerations, the GVair for inhalation exposure
of the general population may be calculated as follows:
GVair = 15mg/m3 × 6 × 5 × 1 = 54 µg/m3
24 5 50
where:
15 mg/m3 = LOAEL for mice
6/24 and 5/7 = duration of exposure in h/day and weeks/day,
respectively
50 = total uncertainty factor
10.1.4.2 Oral exposure
For oral exposure the GV can be calculated based on NOAEL values
in the 12-month oral study (140 mg/kg body weight per day), the two-
generation reproductive study (240 mg/kg body weight per day) or the
NOAEL in the chronic study (78 mg/kg body weight day). The chronic
study is used to derive the GV because a lifetime exposure study is
preferred to a short-duration study. The chronic study found no effect
at the highest dose tested. The NOAEL is supported by the finding of
adverse effects on body weight at 331 mg/kg body weight per day in the
12-month study and body weight effects and stomach pathology in the
two-generation reproductive study at 460 mg/kg body weight per day.
With these supporting studies, the NOAEL from the chronic study can be
used with confidence. A total uncertainty factor of 25 is based on a
factor of 5 for inter-individual variability, and a factor of 5 for
interspecies variability and for the lack of an adequate oral study in
a second species. Reduced uncertainty factors are used for
interspecies and inter-individual differences because of the direct-
acting nature of the toxicity, as described in section 10.1.4.1. The
need for a second species is suggested by the apparent differences in
sensitivity between rats and mice in the subchronic inhalation study.
Based on these considerations, the GVoral for drinking-water
exposure may be calculated as follows:
Tolerance intake = 78 = 3.1 mg/kg
25
From the above calculation a guidance value for oral intake of
acrylic acid may be derived according to the following formula:
GVoral = 3.1 × 64 ‰ 2 = 99 mg/litre
where:
3.1 mg/kg body weight per day = tolerable intake (TI)
64 = average weight (kg) of human body
25 = uncertainty factor
2 = average intake of drinking water (litres/day)
However, the Task Group expressed significant concern regarding
the guidance value for oral intake calculated above, because of the
difference in toxicity between drinking-water and gavage exposures.
Exposure levels only 50-fold above the GV calculated above were lethal
to 50% of the animals in the 90-day gavage study, and comparison with
effects in the 90-day drinking-water study revealed a very significant
difference between the two dosing methods. High doses taken over a
short time period have much more severe effects.
The concern is reduced by the fact that the local effects in the
stomach might be determined by both concentration of delivered dose
and total dose, so the same total dose delivered in more dilute
solutions results in lesser effects, as shown for ethyl acrylate. The
concentration in drinking-water needed to deliver the TI dose is
probably far less than that required to cause direct effects, and is
not a public health concern if small amounts are ingested over the
course of the day, approximating to continuous exposure. The
possibility remains that a large part of the TI could be delivered
over a short time via drinking-water.
Because of potential differences in individual drinking
behaviour, the Task Group decided that a risk of effects still exists
at the TI calculated above. For this reason an additional uncertainty
factor of 10 was applied to derive the GV for drinking-water.
Thus, the final guidance value for oral intake of acrylic acid
is:
GVoral = 99 mg/litre = 9.9 mg/litre
10
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Acrylic acid has been found to occur naturally in some species of
marine alga. No quantitative data are available regarding
environmental levels in ambient air or soil. It was reported that
acrylic acid might occur in wastewater effluent from industrial
facilities at concentrations not exceeding 0.5 mg/litre. Effluent
levels of (2500 mg/litre) have been reported at a methyl acrylate
production facility in India. After treatment acrylic acid was below
the limit of detection (0.1 mg/litre) in wastewater from a production
facility in Europe.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Acrylic acid is
eliminated from the environment by both abiotic and biotic
degradation. Photolysis reactions are possible processes, but aerobic
degradation constitutes the major route of breakdown. Microorganisms
are capable of degrading acrylic acid under aerobic and anaerobic
conditions.
In soil, acrylic acid biodegrades very rapidly, the half-life
being less than one day. There is no potential for long-range
atmospheric transport of acrylic acid since it has an atmospheric
lifetime of less than one month.
Bioaccumulation of acrylic acid in organisms, based on the low
octanol-water partition coefficient, is likely to be negligible. There
has been no report of biomagnification of acrylic acid in food chains.
10.2.2 Effects
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values based on growth ranging from 0.04 to 63 mg/litre and
a NOEC for the most sensitive species of 0.008 mg/litre. Acrylic acid
has low to moderate acute toxicity for invertebrates and fish with
LC50 values ranging from 27 to 315 mg/litre. The 96-h NOEC was
6.3 mg/litre for rainbow trout, based on a lack of sublethal/
behavioural responses.
No data are available concerning the effects of acrylic acid on
terrestrial organisms in the environment.
10.2.3 Risk evaluation
If released into the environment acrylic acid will partition to
water where it will be readily degraded. Therefore, it is unlikely to
pose a problem in the general environment. In the case of spills,
acrylic acid is likely to cause localized mortality to aquatic
organisms both from direct toxicity and oxygen depletion. There is
likely to be a problem near to outfalls from plants discharging
acrylic acid if there is inadequate sewage treatment. The toxicity of
acrylic acid to bacteria and soil microorganisms is low. No data are
available concerning the effects of acrylic acid on terrestrial
organisms in the environment; however, data from laboratory mammals
suggest a low risk.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11.1 Conclusions
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure for the general
population. However, no obvious adverse effects on the general
population have been identified.
Acrylic acid poses minimal risk for the general environment
except in the case of uncontrolled discharge.
11.2 Recommendations for protection of human health
It is recommended that exposure of the general population to
acrylic acid in the ambient air and drinking-water does not exceed the
guidance values given in this monograph.
12. FUTURE RESEARCH
a) Monitoring of acrylic acid concentrations in ambient air, water,
soil and effluent should be carried out. Research should be
undertaken to lower the analytical detection limit in water.
b) Further in vivo studies on the genotoxic potential of acrylic
acid at the initial site of contact are recommended.
c) The carcinogenicity of acrylic acid should be assessed in a well-
controlled two-species bioassay (with both sexes) using proper
exposure levels via inhalation or dermal exposure.
d) Better understanding of the relationship between persistent
tissue damage and tumour formation would aid the risk assessment
for an irritant chemical such as acrylic acid.
e) Additional research on the development of models describing the
disposition and kinetics of acrylic acid is needed. Such models
should focus on species differences in order to allow more
confident extrapolation from animals to humans. In addition,
models allowing comparison of acrylic acid dose for various
acrylate esters would be useful to allow complete use of
available data.
f) Since many workers are exposed to acrylic acid, epidemiological
studies are needed, which should concentrate on the following
end-points:
i) rhinitis, laryngitis and olfactory function;
ii) respiratory tract disease;
iii) skin disease.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer (IARC, 1979, 1987). Data
on the carcinogenicity of the compound for humans were considered
inadequate. There was inadequate evidence for carcinogenicity in
animals. Therefore, IARC considered acrylic acid not classifiable as
to its carcinogenicity to humans.
REFERENCES
AAR (1987) Emergency handling of hazardous materials in surface
transportation. Washington, DC, Association of America Railroads,
Hazardous Systems (BOE), 11 pp.
ACGIH (1988) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1988-1989.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
ACGIH (1990) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1990-1991.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists, p 9.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Andreoni V, Bernasconi S, Sorlini C, & Villa M (1990) Microbial
degradation of acrylic acid. Ann Microbiol, 40: 279-287.
Archer G & Horvath MK (1991) Adsorption and desorption of acrylic acid
to soils. Painesville, Ohio, Ricerca Inc., Department of Environmental
Sciences (Document No. 3193-88-0214-EF-001).
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reactions of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
Atkinson R & Carter WPL (1984) Kinetics and mechanisms of the gas-
phase reactions of ozone with organic compounds under atmospheric
conditions. Chem Rev, 84: 437-470.
Bandara BMR, Gunatilaka AAL, Kumar NS, & Wimalasiri WR (1988)
Antimicrobial activity of some marine algae of Sri Lanka. J Natl Sci
Counc (Sri Lanka), 16(2): 209-221.
BASF (1958) [Report on biological tests for crude and pure acrylic
acid.] Ludwigshafen, Germany, BASF AG, Toxicology Department
(Unpublished report No. VII/365-366) (in German).
BASF (1988) [Determination of the distribution coefficient log Pow of
acrylic acid in 1-octanol/water at room temperature (25°C).]
Ludwigshafen, Germany, BASF AG, Analytical Laboratory, pp 1-4 (in
German).
BASF (1992) Acrylic acid glacial P. Technical information monomers.
Ludwigshafen, Germany, BASF AG.
BASF (1993) [Examination of the biological degradability or the
elimination potential, respectively, of acrylic acid (pure P)
according to the experimental design of Zahn-Wellens (DUU/OM Project
No. 92/2641/10/1).] Ludwigshafen, Germany, BASF AG (in German).
BASF (1994a) Continuous administration in the drinking-water over 2
generations (1 liter in the first and 1 liter in the second
generation) (Project No. 71RO114/92011). Ludwigshafen, Germany, BASF
AG.
BASF (1994b) [Determination of the inhibitory effect of acrylic acid
(pure) on the cell replication of the green algae Scenedesmus
supsicatus.] Ludwingshafen, Germany, BASF AG (Unpublished
experiment No. 94/0840/60/1) (in German).
BG Chemie (1991) Acrylic acid. In: Toxicological evaluations, Volume
2. Berlin, Springer-Verlag, pp 39-73.
Black KA (1993) 14C-Acrylic acid. Comparative bioavailability study
in male mice and rats -analysis of tissues. Spring House,
Pennsylvania, Rohm and Haas Company, Toxicology Department (Report
No. 93R-200).
Black KA, Finch L, & Frederick CB (1993) Metabolism of acrylic acid to
carbon dioxide in mouse tissues. Fundam Appl Toxicol, 21: 97-104.
Black KA, Beskitt JL, Tallant MJ, & Frantz SW (1994) Disposition of
acrylic acid (AA) in C3H mice after the oral, cutaneous and
intravenous routes (Rohm and Haas Company, Spring House, PA and Bushy
Run Research Center, Export, PA). Toxicologist, 14(1): 430 (Abstract
1705).
Black KA, Beskitt JL, Finch L, Tallant MJ, Udinsky JR, & Frantz ST
(1995) Disposition and metabolism of acrylic acid in C3H mice and
Fischer 344 rats after oral or cutaneous administration. J Toxicol
Environ Health, 45: 291-311.
Borzelleca JF, Larson PS, Hennigar GR, Huf EG, Crawford EM, & Smith RB
(1964) Ethyl acrylate and methyl methacrylate: Studies on the chronic
oral toxicity of monomeric ethyl acrylate and methyl methacrylate.
Toxicol Appl Pharmacol, 6: 29-36.
Bowen RL (1979) Adhesive bonding of various materials to hard tooth
tissues. XVIII: Synthesis of a polyfunctional surface-active
comonomer. J Dent Res, 58: 1101-1107.
Bowman JE (1990) Acute flow-through toxicity of glacial acrylic acid
to rainbow trout ( Salmo gairdneri). Columbia, Missouri, Analytical
Bio-Chemistry Laboratories Inc., Aquatic Toxicology Division (Report
No. 37343).
Bretherick L (1985) Handbook of reactive chemical hazards, 3rd ed.
Boston, Massachusetts, Butterworth, 351 pp.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: model organisms Microcytis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Z Wasser-Abwasser-Forsch, 15: 1-6 (in
German).
Brown RK, McMeekin TA, & Balis C (1977) Effect of some unicellular
algae on Escherichia coli populations in sea water and oysters. J
Appl Bacteriol, 43: 129-136.
Buckley LA, James RA, & Barrow CS (1984) Differences in nasal cavity
toxicity between rats and mice exposed to acrylic vapor. Toxicologist,
4(1): 1 (Abstract only).
Burgess D (1990) Acute flow-through toxicity of acrylic acid to
Daphnia magna. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37344).
Bysshe SE (1990) Bioconcentration factor in aquatic organisms. In:
Lyman WJ, Reehle WF, & Rosenblatt DN ed. Handbook of chemical property
estimation methods for environmental behaviour of organic compounds.
Washington, DC, American Chemical Society, vol 15, pp 1-30.
Cameron TP, Rogers-Back AM, Lawlor TE, Harbel JW, Seifried HE, &
Dunkel VC (1991) Genotoxicity of multifunctional acrylates in the
Salmonella/mammalian-microsome assay and mouse lymphoma TK+/- assay.
Environ Mol Mutagen, 17: 264-271.
Carpenter CP, Weil CS, & Smyth HF Jr (1974) Range-finding toxicity
data: List VIII. Toxicol Appl Pharmacol, 28: 313-319.
Casciery T & Clary J (1993) Acrylic acid health effects overview. In:
Taylor TB, Murphy SR, & Hunt EK ed. Health effect assessments of the
basic acrylates. Boca Raton, Florida, CRC Press, p 13.
Chemical Marketing Reporter (1992) Acrylic grows at rapid pace, but so
does the world's capacity. Chem Mark Report, July 20.
Chou WL, Speece RE, & Siddiqi RH (1978) Acclimation and degradation
of petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
CHRIS (1985) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado).
CHRIS (1989) CHRIS hazardous chemical data. Washington, DC, US
Department of Transportation, US Coast Guard (CD-ROM version by
Micromedex Inc., Denver, Colorado.
Chun JS, Kubena MF, & Neeper-Bradley TL (1993) Developmental toxicity
dose range-finding study of inhaled acrylic acid vapor in New Zealand
white rabbits. Export, Pennsylvania, Union Carbide Corporation (Bushy
Run Research Center Report No. 92N1007).
CITI (Japanese Chemicals Inspection and Testing Institute) ed. (1992)
Acrylic acid. In: Biodegradation and bioaccumulation: Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology and Information Center, pp 2-83.
Conde-Salazar L, Guimaraens D, & Romero LV (1988) Occupational
allergic contact dermatitis from anaerobic acrylic reactants. Contact
Dermatitis, 18: 129-132.
Corrigan MA & Scott R (1988) Acrylic acid: In vitro absorption
through human and mouse skin. Macclesfield, United Kingdom, Imperial
Chemical Industries PLC, Central Toxicology Laboratory (Report No.
CTL/P/2047).
Costellati L, Guglielmin AM, Vistoli O, Carboni GP, & Rambaldi R
(1990) [Allergic contact dermatitis - evaluation of patch tests in
animal fodder plant workers.] Med Lav, 81: 296-300 (in Italian).
Cote IL, Hochwalt A, Seidman I, Budzilovich G, Solomon JJ, & Segal A
(1986) Acrylic acid: Skin carcinogenesis in ICR/HA mice. Toxicologist,
6: 235.
Dean JA (1987) Handbook of organic chemistry. New York, McGraw-Hill
Book Co., pp 1-76.
De Bethizy ID, Udinsky JR, & Scribner HE (1987) The disposition and
metabolism of acrylic acid and ethyl acrylate in male Sprague-Dawley
rats. Fundam Appl Toxicol, 8(4) 549-561.
DePass LR, Woodside MD, Garman RH, & Weil CS (1983) Subchronic and
reproductive toxicology studies on acrylic acid in the drinking water
of the rat. Drug Chem Toxicol, 6(1): 1-20.
DePass LR, Fowler EH, Meckley DR, & Weil CS (1984) Dermal oncogenicity
bioassays of acrylic acid, ethyl acrylate and butyl acrylate. J
Toxicol Environ Health, 14: 115-120.
Douglas MT & Bell G (1992) Assessment of ready biodegradability of
acrylic acid (Closed bottle test). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 1/91329).
D'Souza RW & Francis WR (1988) Vehicle and pH effects on the dermal
penetration of acrylic acid: in vitro- in vivo correlation.
Toxicologist, 8: 831.
EEC (1988) Methods for the determination of ecotoxicity,
biodegradation. Activated sewage sludge respiration inhibition test.
In: Council Directive of 18 November 1987 adapting to technical
progress for the ninth time Directive 67/548/EEC on the approximation
of the laws, regulations and administrative provisions relating to the
classification, packaging and labelling of dangerous substances
(88/302/EEC). Off J Eur Comm, L133: 118-122.
Ekhina RS & Ampleeva GP (1977) [Combined effect of six acrylates on
the sanitary status of a reservoir.] Vodemov, 1977: 157-164 (in
Russian).
Elf Atochem (1992) Norsocryl AA, acrylic acid specifications. Paris,
Elf Atochem.
Fassett DW (1963) Organic acids, anhydrides, lactones, acid halides
and amides, thioacids. In: Patty FA ed. Industrial hygiene and
toxicology, 2nd ed. New York, Wiley-Interscience, vol 2, p 1794.
Fazzalari FA ed. (1978) Compilation of odor and taste threshold values
data. Philadelphia, Pennsylvania, American Society for Testing and
Materials, p 141.
Ferrali M, Ciccoli L, & Comporti M (1989) Allyl alcohol-induced
hemolysis and its relation to iron release and lipid peroxidation.
Biochem Pharmacol, 38(1): 1819-1826.
Finch L & Frederick CB (1992) Rate and route of oxidation of acrylic
acid to carbon dioxide in rat liver. Fundam Appl Toxicol, 19:
498-504.
Forbis AD (1989) Acute toxicity of acrylic acid to Selenastrum
capricornutum Printz. Columbia, Missouri, Analytical Bio-Chemistry
Laboratories Inc., Aquatic Toxicology Division (Report No. 37345).
Fowler JH Jr (1990) Immediate contact hypersensitivity to acrylic
acid. Dermatol Clin, 8: 193-195.
Frantz SW & Beskitt JL (1993) 14C-Acrylic acid: comparative
bioavailability study in male mice and rats. Export, Pennsylvania,
Bushy Run Research Center (Report No. 92 N 1005).
Frantz SW, Beskitt JL, Tallant MJ, & Black KA (1994) Disposition of
acrylic acid (AA) in Fischer rats after the oral, cutaneous and
intravenous routes. Toxicologist, 14(1): 430 (Abstract 1704).
Frederick CB & Reynolds CH (1989) Modeling the reactivity of acrylic
acid and acrylate anion with biological nucleophiles. Toxicol Lett,
47(3): 241-247.
Frederick CB, Udinsky JR, & Finch L (1994) The regional hydrolysis of
ethyl acrylate to acrylic acid in the rat nasal cavity. Toxicol Lett,
70: 49-56.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Ganou-Parfait B, Fahrasmane L, Celestine-Myrtil DA, Parfait A, & Galzy
P (1988) Micrococcus in rum technology in the French West Indies.
Microbiol Aliments Nutr, 6(3): 273-277.
Gelbke HP & Hofmann HTh (1979) [Report on testing for acute inhalation
risk of acrylic acid (pure) in Sprague-Dawley rats.] Ludwigshafen,
Germany, BASF AG, Department of Toxicology (in German).
Ghanayem BI, Maronpot RR, & Matthews HB (1985) Ethyl acrylate-induced
gastric toxicity: II. Structure-toxicity relationships and mechanism.
Toxicol Appl Pharmacol, 80: 336-344.
Ghanayem BI, Burka LT, & Matthews HB (1987) Ethyl acrylate
distribution, macromolecular binding, excretion and metabolism in male
Fischer 344 rats. Fundam Appl Toxicol, 9: 389-397.
Glombitza KW (1970a) [Antimicrobial contents of algae: 2. Presence of
acrylic acid in different sea algae.] Planta Med, 18(3): 210-221 (in
German).
Glombitza KW (1970b) [Antimicrobiol contents in algae: 3. Quantitative
determination of acrylic acid in sea algae.] Planta Med, 18(4):
231-234 (in German).
Glombitza KW (1979) Antibiotics from algae. In: Hoppe HA, Levring T, &
Tanaka J ed. Marine algae in pharmaceutical science. Berlin, New York,
Walter de Gruyter, pp 303-307.
Glombitza KW & Heyser R (1971) [Antimicrobial constituent compounds in
algae - Part 4: Effect of acrylic acid on respiration and synthesis of
macromolecular in Staphylococcus aureus and Escherichia coli.]
Helgoal Wiss Meeresunters, 22(3/4): 442-453 (in German).
Grudzinski UJ (1988) [Substantiation of single maximum permissible
levels of acrylic acid in the air of populated regions.] Gig I Sanit,
9: 64-65 (in Russian).
Hagan JV & Emmons HF (1991) Acrylic acid: Acute inhalation study in
rats (Protocol No. 87P-240). Spring House, Pennsylvania, Rohm and Haas
Company, Toxicology Department (Report No 87R-106).
Halarnkar PJ & Blomquist GJ (1989) Comparative aspects of propionate
metabolism. Comp Biochem Physiol, 92B: 227-231.
Hansch C & Leo A (1987) The log P database. Claremont, California,
Pomona College, p 391.
Hawkins DR, Kirkpatrick D, Aikens PJ, & Saxton JE (1992) The
metabolism of acrylic acid in soil under aerobic conditions.
Huntingdon, United Kingdom, Huntingdon Research Centre (HRC
Confidential Report No. 93A/920625).
Hellwig J, Deckerdt K, & Freisberg KO (1993) Subchronic and chronic
studies of the effects of oral administration acrylic acid to rats.
Food Chem Toxicol, 31: 1-18.
Herwig N (1978) Nutritious antibiotics. Mar Aquarist, 8(5): 48-50.
Heyser R & Glombitza KW (1972) Effect of acrylic acid on glycolysis
and citric acid cycle in bacteria: 5. Antimicrobiol components in
algae. Arch Pharm, 305(11): 863-870.
Hossack DJN, Thomas FJ, & Ruckman SM (1992) Acrylic acid: Effects on
soil carbon cycle (respiration). Huntingdon, United Kingdom,
Huntingdon Research Centre (HRC Confidential Report No. BMM 2/920228).
Howard PH, Boethlings RS, Jarris WF, Meylan WM, & Midalenko EM (1991)
Handbook on environmental degradation rates. Chelsea, Michigan, Lewis
Publishers.
Husain S, Sastry GSR, Prasad PR, & Sarma VR (1991) Differential pulse
polarographic determination of residual acrylic acid in sodium
polyacrylate. Electroanalysis, 3: 71-72.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein. Lyon, International Agency for Research on Cancer, pp 47-71
(IARC Monograph on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 19).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 56 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IRPTC (1992) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M (1988) Data book of chromosomal aberration test in vitro.
Amsterdam, Oxford, New York, Elsevier Science Publishers.
Juhnke I & Luedemann D (1978) [Results of testing of 200 chemical
compounds for acute fish toxicity using the Golden Orfe test.]
Z. Wasser Abwasser Forschung, 11(5): 61-164 (in German).
Kalimo K, Jolanki R, Estlander T, & Kanerva L (1989) Contact allergy
to antioxidants in industrial greases. Contact Dermatitis, 20:
151-152.
Kavaler AR (1987) Chemical profile on acrylic acid. Chem Mark Report,
232(23): 62.
Kirk-Othmer (1978-1984) Kirk-Othmer encyclopedia of chemical
technology, Volumes 1-26, 3rd ed. New York, John Wiley and Sons.
Klimisch HJ & Hellwig J (1991) The prenatal inhalation toxicity of
acrylic acid in rats. Fundam Appl Toxicol, 16(4): 656-666.
Klimisch HJ & Zeller H (1980) [Acute inhalation toxicity in the rat.]
Ludwigshafen/Rhine, Germany, BASF AG, Department of Toxicology (in
German).
Klimkina NV, Bolding ZN, & Sergeev AN (1969) [Experimental basis for
maximum permissible content of acrylic acid in reservoir waters.] Prom
Zag Vodoemov, 9: 171-185 (in Russian, with English abstract).
Kodama M & Ogata T (1983) Acrylic acid, as an antibacterial substance
in scallop. Bull Jpn Soc Sci Fish, 49(7): 1103-1107.
Kohriyama K, Matsuoka M, & Igisu H (1994) Effects of acrylamide and
acrylic acid on creatinine kinase activity in the rat brain. Arch
Toxicol, 68(1): 67-70.
Korenman IM & Lunicheva EV (1972) Distribution of acrylic acid between
organic solvents and water. J Appl Chem (USSR), 45: 1101-1105.
Kostanyan GG, Dadayan AA, & Safuryan GE (1969) [Polarographic
determination of acrylic acid in commercial propionic acid.] Arm Khem,
22: 1044 (in Russian with English abstract).
Kutzman RS, Meyer GI, & Wolf AP (1982) The biodistribution and
metabolic fate of (11C) acrylic acid in the rat after acute inhalation
exposure or stomach intubation. J Toxicol Environ Health, 10(6):
969-980.
Lawrence WH, Bass GE, Purcell WP, & Autian J (1972) Use of
mathematical models in the study of structure-toxicity relationships
of dental compounds: I. Esters of acrylic acid and methacrylic acids.
J Dent Res, 51: 526.
Lijinsky W & Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratog Carcinog Mutagen, 1: 259-267.
Lomax LG, Brown OW, & Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic acid
for either 6 or 22 h per day. Abstract presented at a Meeting on Nasal
Toxicity and Dosimetry of Inhaled Xenobiotics: Implications for human
health, Durham, North Carolina, 20-22 September 1993. Inhal Toxicol,
6: 445-449.
Lyman WJ, Reehl WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods: Environmental behavior of organic
compounds. New York, McGraw-Hill Book Co., pp 9-65.
McCarthy KL, Thomas WC, Aardema MJ, Seymour JL, Putman DL, Yang LL,
Curren RD, & Valencia R (1992) Genetic toxicology of acrylic acid.
Food Chem Toxicol, 30: 505-515.
Mackay D & Peterson S (1981) Calculating fugacity. Environ Sci
Technol, 15: 1006-1014.
Magnusson B & Kligman AM (1969) The identification of contact
allergens by animal assay: The Guinea pig maximization test. J Invest
Dermatol, 52: 268-276.
Magnusson B, Blohm S, Fregert S, Hjorth N, Hovding G, Pirila V, & Skog
E (1968) Routine patch testing: IV. Supplementary series of test
substances for Scandinavian countries. Acta Dermato-Venereol, 48:
110-114.
Majka J, Knobloch K, & Stetkiewicz J (1974) [Evaluation of acute and
subacute toxicity of acrylic acid.] Med Prac, 25: 427-35 (in
Polish).
Mao J, Doane R, & Kovacs F Jr (1994) Separation of acrolein and its
possible metabolites using different modes of high performance liquid
chromatography. J Liq Chromatogr, 17: 1811-1819.
Miller ML (1964) Acrylic acid polymers. In: Bikales NM ed.
Encyclopedia of polymer science and technology, plastics, resins,
rubbers, fibers. New York, Interscience Publishers, vol 1, pp 197-226.
Miller RR, Ayres JA, & Jersey GC (1979a) Acrylic acid 90-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1024).
Miller RR, Ayres JA, & Jersey GC (1979b) Acrylic acid 10-day vapor
inhalation study with rats and mice - Final report. Midland, Michigan,
Dow Chemical Company, Toxicology Research Laboratory, Health and
Environmental Science (Report No. 79RC-1015).
Miller RR, Ayres JA, Jersey GC, & McKenna MJ (1981a) Inhalation
toxicity of acrylic acid. Fundam Appl Toxicol, 1(3): 271-277.
Miller RR, Ayres JA, Rampy LW, & McKenna MJ (1981b) The metabolism of
acrylate esters in rat tissue homogenates. Fundam Appl Toxicol,
1(6): 410-414.
Miller RR, Young JT, Kociba RJ, Keyes DG, Bodner KM, Calhoun LL, &
Ayers JA (1985) Chronic toxicity and oncogenicity bioassay of inhaled
ethyl acrylate in Fischer 344 rats and B6C31 mice. Drug Chem Toxicol,
8: 1-42.
Mitchell DY & Petersen DR (1988) Inhibition of rat liver aldehyde
dehydrogenases by acrolein. Drug Metab Dispos, 16: 37-42.
Moiseeva TN, Kozulin SV, Kulikova LK, & Voronin SP (1991) Screening of
strains decomposing acrylic acid and its derivatives. Soviet-
Biotechnology, 1991(6): 119-125.
Mok WSL (1989) Formation of acrylic acid from lactic acid in
supercritical water. J Org Chem, 54(19): 4596-4602.
Moore MM, Amtower A, Doerr CL, Brock KH, & Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environ Mol Mutagen, 11: 49-63.
Nagasawa T, Nakamura T, & Yamada H (1990) Production of acrylic acid
and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase.
Appl Microbiol Biotechnol, 34(3): 322-324.
Narayanasamy K, Shukla S, & Parekh LJ (1990) Utilization of
acrylonitrile by bacteria isolated from petrochemical waste waters.
Indian J Exp Biol, 28(10): 968-971.
Nawaz MS, Franklin W, & Cerniglia CE (1993) Degradation of acrylamide
by immobilized cells of Xanthomonas maltophilia. Can J Microbiol,
39(2): 207-212.
Nawaz MS, Franklin W, & Cerniglia CE (1994) Degradation of aliphatic
amide mixture by immobilized and nonimmobilized cells of Pseudomonas
sp. Environ Sci Technol, 28(6): 1106-1109.
Neeper-Bradley TL & Kubena MF (1993) Developmental toxicity evaluation
of inhaled acrylic acid vapor in New Zealand white rabbits. Export,
Pennsylvania, Union Carbide Corporation (Bushy Run Research Center
Report No. 92N1008).
NIOSH (1974) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 74-127).
NIOSH (1977) National occupational hazard survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations, and Field Studies (NIOSH
Publications No. 77-213).
NIOSH (1989) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (CD-ROM version by Canadian Centre for Occupational Safety and
Health, Hamilton, Ontario).
NIOSH (1990) National occupational exposure survey (NOES). Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard, Evaluations, and Field Studies (Unpublished
provisional data as of January 7, 1990).
Nishikawa H, Hosomura H, Sonoda H, & Inagaki M (1979) [Behaviour of
acrylamide in a soil-vegetation system.] Kenkyu Hokoku-Gifu-Ken Kogyo
Gijutsu Senta, 11: 31-34 (in Japanese).
Nishiuchi Y (1975) Toxicity of formulated pesticides to some
freshwater organisms. Suisan Zoshoku, 23: 132.
NLM (1989) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
NLM (1991) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine (CD-ROM version by Micromedex Inc.,
Denver, Colorado).
Noble RC & Czerkawski JW (1973) A gas-chromatographic method for the
determination of low concentrations of acrylic acid in mixtures of C2
to C5 fatty acids in biological materials. Analyst, 98: 122-125.
NTP (1986) Carcinogenesis studies of ethyl acrylates (CAS No.
