
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 195
Hexachlorobenzene
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 195
First draft prepared by Mr R. Newhook and Ms W. Dormer,
Health Criteria, Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Hexachlorobenzene.
(Environmental health criteria ; 195)
1. Hexachlorobenzene - toxicity 2.Hexachlorobenzene - adverse effects
3. Environmental exposure I. Series
ISBN 92 4 157195 0 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
PREAMBLE
ABBREVIATIONS
PREFACE
1. SUMMARY AND CONCLUSIONS
1.1. Identity, physical and chemical properties,
and analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and
transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory
animals and humans
1.6. Effects on laboratory animals and in vitro tests
1.7. Effects on humans
1.8. Effects on other organisms in the
laboratory and field
1.9. Evaluation of human health risks and
effects on the environment
1.9.1. Health effects
1.9.2. Environmental effects
1.10. Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Sources, uses and production processes
3.2. World production levels
3.3. Entry into the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Environmental transport and degradation
4.2. Bioaccumulation and biomagnification
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Sediment
5.1.5. Biota
5.1.6. Food and drinking-water
5.2. General population exposure
5.2.1. Human tissues and fluids
5.2.2. Intake from ambient air
5.2.3. Intake from drinking-water
5.2.4. Intake from foods
5.2.5. Apportionment of intakes
5.2.6. Trends in exposure of the general
population over time
5.2.7. Occupational exposure during
manufacture, formulation or use
6. KINETICS AND METABOLISM
6.1. Aquatic and terrestrial biota
6.2. Mammals
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term and subchronic exposure
7.3. Long-term toxicity and carcinogenicity
7.4. Mutagenicity and related end-points
7.5. Reproductive and developmental toxicity
7.6. Immunotoxicity
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Short-term exposure
9.1.1. Aquatic biota
9.1.2. Terrestrial biota
9.2. Long-term exposure
9.2.1. Aquatic biota
9.2.2. Terrestrial biota
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.1.3. Approaches to risk assessment
10.1.3.1 Non-neoplastic effects
10.1.3.2 Neoplastic effects
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND
THE ENVIRONMENT
12. FURTHER RESEARCH
12.1. Environment
12.2. Human health
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RÉSUMÉ ET CONCLUSIONS
RÉSUMEN Y CONCLUSIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission and the Federal Ministry for the Environment,
Nature Conservation and Nuclear Safety, Germany.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
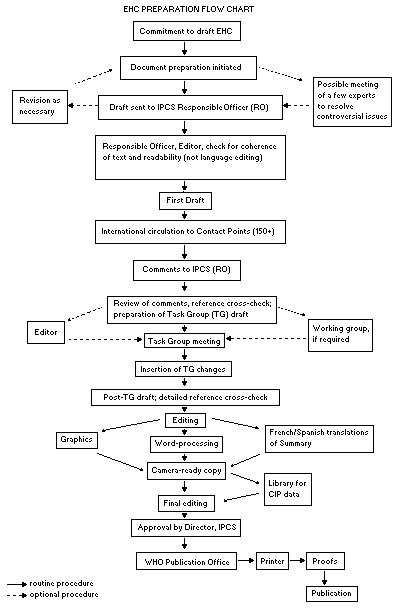
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
Members
Dr D. Arnold, Health Canada, Tunney's Pasture, Ottawa, Ontario Canada
Dr A. Göcmen, Department of Pediatrics, Faculty of Medicine,
Hacettepe University, Hacettepe, Ankara, Turkey
Professor B. Jansson, Institute of Applied Environmental Research,
ITM-Solna, Stockholm University, Stockholm, Sweden
Dr J. Jarrell, Foothills Hospital, Calgary Regional Health
Authority, Calgary, Alberta, Canada
Dr A. Langley, South Australian Health Commission, Rundle Mall,
Australia ( Chairman)
Mr R. Newhook, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Rapporteur)
Dr D. Peakall, Wimbledon, London, United Kingdom
( Vice-chairman)
Dr A.G. Smith, Medical Research Council Toxicology Unit,
Hodgkin Building, University of Leicester, Leicester,
United Kingdom
Dr J. Sunyer, Department of Epidemiology and Public Health,
Institut Municipal d'Investigacio Medica, Barcelona, Spain
Dr A. van Birgelen, National Health and Environmental Effects
Research Laboratory, Pharmacokinetics Branch, US Environmental
Protection Agency, Research Triangle Park, North Carolina, USAa
Dr J. Vos, National Institute of Public Health and the Environment
(RIVM), Hygiene, Bilthoven, The Netherlands
a Dr A. Van Birgelen's present address: National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Observers
Dr J. de Gerlache, Solvay SA, Department of Chemical Safety and
Toxicology, Brussels, Belgium (Representing EURO CHLOR)
Dr Roger Drew, Toxicology Information Section, Safety, Health &
Environment Division, ICI Australia Operations Pty Ltd., ICI
House, Melbourne, Victoria, Australia (Representing European
Centre for Ecotoxicology and Toxicology of Chemicals)
Secretariat
Dr G.C. Becking, Interregional Research Unit, International
Programme on Chemical Safety, Research Triangle Park, North
Carolina USA ( Secretary)
Ms W. Dormer, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Temporary Adviser to Secretariat)
Dr J. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
A WHO Task Group on Environmental Health Criteria for
Hexachlorobenzene met in Geneva from 26 February to 1 March 1996.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft and made an
evaluation of the risks for human health and the environment from
exposure to hexachlorobenzene.
The first draft was prepared by Mr R. Newhook and Ms W. Dormer,
Health Canada, Ottawa, Canada. These authors also prepared the draft
reviewed by the Task Group, which incorporated the comments received
following circulation of the first draft to IPCS Contact Points for
Environmental Health Criteria monographs.
The IPCS gratefully acknowledges the financial and other support
of the Health Protection Branch, Health Canada. This support was
indispensable for the completion of this monograph.
Dr G.C. Becking (IPCS, Central Unit, Inter-regional Research
Unit) and Dr P.G. Jenkins (IPCS, Central Unit, Geneva) were
responsible for the overall scientific content and the technical
editing, respectively, of this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BMF biomagnification factor
DL detection limit
HCB hexachlorobenzene
i.p. intraperitoneal
ND not detectable
PCT porphyria cutanea tarda
p,p'DDE 1,1'-(2,2-dichloroethylidene)-bis[4-chlorobenzene]
SER smooth endoplasmic reticulum
T3 triiodothyronine
T4 thyroxine
PREFACE
The preparation of comprehensive Environmental Health Criteria
(EHC), as outlined in the Preamble of this monograph, is an extremely
time-consuming and resource-intensive procedure. Often countries have
prepared recent comprehensive reviews on chemicals as required by
their national legislation, and the International Programme on
Chemical Safety (IPCS) has been asked by Member States to determine
how best to utilize such national reviews during the preparation of
international EHC. Utilizing such national documents should avoid
duplication of effort and result in the more rapid production of more
concise IPCS EHC monographs.
This monograph on hexachlorobenzene has been prepared using as
background document the review (Supporting Document) prepared under
the Canadian Environmental Protection Act (CEPA), dated June 1993.
From this document, staff of Health Canada have chosen only the most
relevant studies for assessing the human and environmental risks from
exposure to hexachlorobenzene. These have been described from the
original references and supplemented by additional information
published more recently. This has resulted in a concise monograph,
yet one that supplies sufficient information for the reader to
understand the basis for the conclusions reached by the Task Group.
Readers who wish to consult the text of the Canadian Supporting
Document can obtain a copy from the Director, IPCS, World Health
Organization, Geneva, Switzerland.
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, and
analytical methods
Hexachlorobenzene (HCB) is a chlorinated organic compound with
moderate volatility. It is practically insoluble in water, but is
highly lipid-soluble and bioaccumulative. Technical grade HCB contains
up to 2% impurities, most of which is pentachlorobenzene. The
remainder includes the higher chlorinated dibenzo- p-dioxins,
dibenzofurans and biphenyls. Analysis of HCB in environmental media
and biological materials generally involves extraction of the sample
into organic solvents, often followed by a clean-up step, to produce
organic extracts for gas chromatography/mass spectrometry (GC/MS) or
gas chromatography with electron capture detection (GC/ECD).
1.2 Sources of human and environmental exposure
HCB was at one time used extensively as a seed dressing to
prevent fungal disease on grains, but this use was discontinued in
most countries in the 1970s. HCB continues to be released to the
environment from a number of sources, including the use of some
chlorinated pesticides, incomplete combustion, old dump sites and
inappropriate manufacture and disposal of wastes from the manufacture
of chlorinated solvents, chlorinated aromatics and chlorinated
pesticides.
1.3 Environmental transport, distribution and transformation
HCB is distributed throughout the environment because it is
mobile and persistent, although slow photodegradation in air and
microbial degradation in soil do occur. In the troposphere, HCB is
transported long distances and removed from the air phase through
deposition to soil and water. Significant biomagnification of HCB
through the food chain has been reported.
1.4 Environmental levels and human exposure
Low concentrations of HCB are present in ambient air (a few
ng/m3 or less) and in drinking-water and surface water (a few
ng/litre or less) in areas that are distant from point sources around
the world. However, higher levels have been measured near point
sources. HCB is bioaccumulative and has been detected in
invertebrates, fish, reptiles, birds and mammals (including humans)
distant from point sources, particularly in fatty tissues of organisms
at higher trophic levels. Mean levels in adipose tissue of the human
general population in various countries range from tens to hundreds of
ng/g wet weight. Based on representative levels of HCB in air, water
and food, the total intake of HCB by adults in the general population
is estimated to be between 0.0004 and 0.003 µg/kg body weight per day.
This intake is predominantly from the diet. Owing to the presence of
HCB in breast milk, mean intakes by nursing infants have been
estimated to range from < 0.018 to 5.1 µg/kg body weight per day in
various countries. The results of most studies on the levels of HCB in
foods and human tissues over time indicate that exposure of the
general population to HCB declined from the 1970s to the mid-1990s in
many locations. However, this trend has not been evident during the
last decade in some other locations.
1.5 Kinetics and metabolism in laboratory animals and humans
There is a lack of toxicokinetic information for humans. HCB is
readily absorbed by the oral route in experimental animals and poorly
via the skin (there are no data concerning inhalation). In animals and
humans, HCB accumulates in lipid-rich tissues, such as adipose tissue,
adrenal cortex, bone marrow, skin and some endocrine tissues, and can
be transferred to offspring both across the placenta and via mothers'
milk. HCB undergoes limited metabolism, yielding pentachlorophenol,
tetrachlorohydroquinone and pentachlorothiophenol as the major
metabolites in urine. Elimination half-lives for HCB range from
approximately one month in rats and rabbits to 2 or 3 years in
monkeys.
1.6 Effects on laboratory animals and in vitro tests
The acute toxicity of HCB to experimental animals is low (1000 to
10 000 mg/kg body weight). In animal studies, HCB is not a skin or eye
irritant and does not sensitize the guinea-pig.
The available data on the systemic toxicity of HCB indicate that
the pathway for the biosynthesis of haem is a major target of
hexachlorobenzene toxicity. Elevated levels of porphyrins and/or
porphyrin precursors have been found in the liver, other tissues and
excreta of several species of laboratory mammals exposed to HCB.
Porphyria has been reported in a number of studies in rats with
subchronic or chronic oral exposure to between 2.5 and 15 mg HCB/kg
body weight per day. Excretion of coproporphyrins was increased in
pigs ingesting 0.5 mg HCB/kg body weight per day or more (no effects
were observed at 0.05 mg HCB/kg body weight per day in the latter
study). Repeated exposure to HCB has also been shown to affect a wide
range of organ systems (including the liver, lungs, kidneys, thyroid,
skin, and nervous and immune systems), although these have been
reported less frequently than porphyria.
HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
It is known to bind to the Ah receptor.
In chronic studies, mild effects on the liver (histopathological
changes, enzyme induction) occurred in several studies of rats exposed
to between 0.25 and 0.6 mg HCB/kg body weight per day; the NOELs in
these studies were 0.05 to 0.07 mg HCB/kg body weight per day.
Concentrations of neurotransmitters in the hypothalamus were altered
in mink dams with chronic dietary exposure to 0.16 mg HCB/kg body
weight per day, and in their offspring exposed throughout gestation
and nursing. Calcium homoeostasis and bone morphometry were affected
in subchronic studies on rats at 0.7 mg HCB/kg body weight per day,
but not at 0.07 mg/kg body weight per day.
The carcinogenicity of HCB has been assessed in several adequate
bioassays on rodents. In hamsters fed diets yielding average doses of
4, 8 or 16 mg/kg body weight per day for life, there were increases in
the incidence of liver cell tumours (hepatomas) in both sexes at all
doses, haemangioendotheliomas of the liver at 8-16 mg/kg body weight
per day, and adenomas of the thyroid in males at the highest dose.
Dietary exposure of mice to 6, 12 and 24 mg/kg body weight per day for
120 weeks resulted in an increase in the incidence of liver cell
tumours (hepatomas) in both sexes at the two higher doses (not
significant, except for females at the highest dose). In utero,
lactational and oral exposure of rats to HCB in diets yielding average
lifetime doses ranging from 0.01 to 1.5 mg/kg body weight per day
(males) or 1.9 mg/kg body weight per day (females) for up to 130 weeks
post utero produced increased incidences, at the highest dose, of
neoplastic liver nodules and adrenal phaeochromocytomas in females and
of parathyroid adenomas in males. In another long-term study on rats,
exposure for up to 2 years to diets yielding average HCB doses of 4-5
and 8-9 mg/kg body weight per day induced increases in the incidences
of hepatomas and of renal cell adenomas at both doses in both sexes,
and of hepatocellular carcinomas, bile duct adenomas/ carcinomas and
adrenal phaeochromocytomas and adrenal cortical adenomas in females.
High incidences of liver tumours have also been reported in some more
limited studies in which single dietary concentrations were
administered to small groups of female rats. In addition, it has been
reported that, following subchronic dietary exposure to HCB, mice,
hamsters and rats developed tumours in the liver, bile duct, kidney,
thymus, spleen and lymph nodes. Dietary exposure to HCB promoted the
induction of liver tumours by polychlorinated terphenyl in mice and by
diethylnitrosamine in rats.
Except in the case of renal tumours in male rats (which appear at
least in part to be the result of hyaline droplet nephropathy) and
hepatomas in rats (which may result from hyperplastic responses to
hepatocellular necrosis), mechanistic studies that address the
relevance to humans of the tumour types induced by HCB have not been
identified.
HCB has little capability to induce directly gene mutation,
chromosomal damage and DNA repair. It exhibited weak mutagenic
activity in a small number of the available studies on bacteria and
yeast, although it should be noted that each of these studies has
limitations. There is also some evidence of low-level binding to DNA
in vitro and in vivo, but at levels well below those expected for
genotoxic carcinogens.
In studies of reproduction, oral exposure of monkeys to as little
as 0.1 mg HCB/kg body weight per day for 90 days affected the light
microscopic structure and ultrastructure of the surface germinal
epithelium, an unusual target for ovarian toxins. This dose also
caused ultrastructural injury to the primordial germ cells. These
specific target sites, which are damaged further at higher doses, were
associated with otherwise normal follicular, oocyte and embryo
development, suggesting specificity of HCB action within the site of
the ovary. Male reproduction was only affected at much higher doses
(between 30 and 221 mg/kg body weight per day) in studies on several
non-primate species.
Transplacental or lactational exposure of rats and cats to
maternal doses of between 3 and 4 mg/kg body weight per day was found
to be hepatotoxic and/or affected the survival or growth of nursing
offspring. In some cases, these or higher doses reduced litter sizes
and/or increased the number of stillbirths. (Adverse effects on
suckling infants have generally been observed more frequently, and at
lower doses, than embryotoxic or fetotoxic effects). The offspring of
mink with chronic exposure to as little as 1 mg HCB/kg diet
(approximately 0.16 mg/kg body weight per day) had reduced birth
weight and increased mortality to weaning. Although skeletal and renal
abnormalities have been observed in fetuses in some studies of rats
and mice exposed to HCB during gestation, these were either not
clearly related to treatment or occurred at doses that were also
maternally toxic. In two studies, one of which included lactational
and postnatal exposure, neurobehavioural development of rat pups was
affected by in utero exposure to HCB at oral maternal doses of 0.64
to 2.5 mg HCB/kg body weight per day.
The results of a number of studies have indicated that HCB
affects the immune system. Rats or monkeys exposed to between 3 and
120 mg HCB/kg body weight per day had histopathological alterations in
the thymus, spleen, lymph nodes and/or lymphoid tissues of the lung.
Chronic exposure of beagle dogs to 0.12 mg/kg body weight per day
caused nodular hyperplasia of the gastric lymphoid tissue. In a number
of studies on rats, humoral immunity and, to a lesser extent, cell-
mediated immunity were enhanced by several weeks exposure to HCB in
the diet, while macrophage function was unaltered. As little as 4 mg
HCB/kg diet (approximately 0.2 mg/kg body weight per day) during
gestation, through nursing and to 5 weeks of age increased humoral and
cell-mediated immune responses and caused accumulation of macrophages
in the lung tissue of rat pups. In contrast, HCB has been found to be
immunosuppressive in most studies with mice; doses of as little as
0.5-0.6 mg/kg body weight per day for several weeks depressed
resistance to infection by Leishmania or to a challenge with tumour
cells, decreased cytotoxic macrophage activity of the spleen, and
reduced the delayed-type hypersensitivity response in offspring
exposed in utero and through nursing. In a number of studies on
various strains of rats, short-term or subchronic exposure to HCB
affected thyroid function, as indicated by decreased serum levels of
total and free thyroxine (T4) and often, to a lesser extent,
triiodothyronine (T3).
1.7 Effects on humans
Most data on the effects of HCB on humans originate from
accidental poisonings that took place in Turkey in 1955-1959, in which
more than 600 cases of porphyria cutanea tarda (PCT) were identified.
In this incident, disturbances in porphyrin metabolism, dermatological
lesions, hyperpigmentation, hypertrichosis, enlarged liver,
enlargement of the thyroid gland and lymph nodes, and (in roughly half
the cases) osteoporosis or arthritis were observed, primarily in
children. Breast-fed infants of mothers exposed to HCB in this
incident developed a disorder called pembe yara (pink sore), and most
died within a year. There is also limited evidence that PCT occurs in
humans with relatively high exposure to HCB in the workplace or in the
general environment.
The few available epidemiological studies of cancer are limited
by small size, poorly characterized exposures to HCB and exposure to
numerous other agents, and are insufficient to assess the
carcinogenicity of HCB to humans.
1.8 Effects on other organisms in the laboratory and field
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa, and caused mortality in pink shrimp and grass shrimp, but
did not cause mortality in freshwater or marine fish. In longer-term
studies, the growth of sensitive freshwater algae and protozoa was
affected by a concentration of 1 µg/litre, while concentrations of
approximately 3 µg/litre caused mortality in amphipods and liver
necrosis in large-mouth bass.
1.9 Evaluation of human health risks and effects on the environment
1.9.1 Health effects
The Task Group concluded that the available data are sufficient
to develop guidance values for non-neoplastic and neoplastic effects
of HCB.
For non-neoplastic effects, based on the lowest reported NOEL
(0.05 mg HCB/kg body weight per day), for primarily hepatic effects
observed at higher doses in studies on pigs and rats exposed by the
oral route, and incorporating an uncertainty factor of 300 (× 10 for
interspecies variation, × 10 for intraspecies variation, and × 3 for
severity of effect), a TDI of 0.17 µg/kg body weight per day has been
derived.
The approach for neoplastic effects is based on the tumorigenic
dose TD5 i.e., the intake associated with a 5% excess incidence of
tumours in experimental studies in animals. Based on the results of
the two-generation carcinogenicity bioassay in rats and using the
multi-stage model, the TD5 value is 0.81 mg/kg body weight per day
for neoplastic nodules of the liver in females. Based on consideration
of the insufficient mechanistic data, an uncertainty factor of 5000
was used to develop a health-based guidance value of 0.16 µg/kg body
weight per day.
1.9.2 Environmental effects
The Task Group pointed out that there are very few experimental
studies on which an environmental risk assessment can be made. Levels
of HCB in surface water are generally several orders lower than those
expected to present a hazard to aquatic organisms, except in a few
extremely contaminated locations. However, HCB concentrations in the
eggs of sea birds and raptors from a number of locations from around
the world approach those associated with reduced embryo weights in
herring gulls (1500 µg/kg), suggesting that HCB has the potential to
harm embryos of sensitive bird species. Similarly, levels of HCB in
fish at a number of sites worldwide are within an order of magnitude
of the dietary level of 1000 µg/kg associated with reduced birth
weight and increased mortality of offspring in mink. This suggests
that HCB has the potential to cause adverse effects in mink and
perhaps other fish-eating mammals.
1.10 Conclusions
a) HCB is a persistent chemical that bioaccumulates owing to its
lipid solubility and resistance to breakdown.
b) Animal studies have shown that HCB causes cancer and affects a
wide range of organ systems including the liver, lungs, kidneys,
thyroid, reproductive tissues and nervous and immune systems.
c) Clinical toxicity, including porphyria cutanea tarda in children
and adults, and mortality in nursing infants, has been observed
in humans with high accidental exposure.
d) Various measures are warranted to reduce the environmental burden
of HCB.
e) The following health-based guidance values for the total daily
intake (TDI) of HCB in humans have been suggested: for non-cancer
effects, 0.17 µg/kg body weight/day; for neoplastic effects,
0.16 µg/kg body weight/day.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Hexachlorobenzene (HCB) is a chlorinated aromatic hydrocarbon
with the chemical formula C6Cl6. Its CAS registry number is 118-74-1.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
Members
Dr D. Arnold, Health Canada, Tunney's Pasture, Ottawa, Ontario Canada
Dr A. Göcmen, Department of Pediatrics, Faculty of Medicine,
Hacettepe University, Hacettepe, Ankara, Turkey
Professor B. Jansson, Institute of Applied Environmental Research,
ITM-Solna, Stockholm University, Stockholm, Sweden
Dr J. Jarrell, Foothills Hospital, Calgary Regional Health
Authority, Calgary, Alberta, Canada
Dr A. Langley, South Australian Health Commission, Rundle Mall,
Australia ( Chairman)
Mr R. Newhook, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Rapporteur)
Dr D. Peakall, Wimbledon, London, United Kingdom
( Vice-chairman)
Dr A.G. Smith, Medical Research Council Toxicology Unit,
Hodgkin Building, University of Leicester, Leicester,
United Kingdom
Dr J. Sunyer, Department of Epidemiology and Public Health,
Institut Municipal d'Investigacio Medica, Barcelona, Spain
Dr A. van Birgelen, National Health and Environmental Effects
Research Laboratory, Pharmacokinetics Branch, US Environmental
Protection Agency, Research Triangle Park, North Carolina, USAa
Dr J. Vos, National Institute of Public Health and the Environment
(RIVM), Hygiene, Bilthoven, The Netherlands
a Dr A. Van Birgelen's present address: National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Observers
Dr J. de Gerlache, Solvay SA, Department of Chemical Safety and
Toxicology, Brussels, Belgium (Representing EURO CHLOR)
Dr Roger Drew, Toxicology Information Section, Safety, Health &
Environment Division, ICI Australia Operations Pty Ltd., ICI
House, Melbourne, Victoria, Australia (Representing European
Centre for Ecotoxicology and Toxicology of Chemicals)
Secretariat
Dr G.C. Becking, Interregional Research Unit, International
Programme on Chemical Safety, Research Triangle Park, North
Carolina USA ( Secretary)
Ms W. Dormer, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Temporary Adviser to Secretariat)
Dr J. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
A WHO Task Group on Environmental Health Criteria for
Hexachlorobenzene met in Geneva from 26 February to 1 March 1996.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft and made an
evaluation of the risks for human health and the environment from
exposure to hexachlorobenzene.
The first draft was prepared by Mr R. Newhook and Ms W. Dormer,
Health Canada, Ottawa, Canada. These authors also prepared the draft
reviewed by the Task Group, which incorporated the comments received
following circulation of the first draft to IPCS Contact Points for
Environmental Health Criteria monographs.
The IPCS gratefully acknowledges the financial and other support
of the Health Protection Branch, Health Canada. This support was
indispensable for the completion of this monograph.
Dr G.C. Becking (IPCS, Central Unit, Inter-regional Research
Unit) and Dr P.G. Jenkins (IPCS, Central Unit, Geneva) were
responsible for the overall scientific content and the technical
editing, respectively, of this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BMF biomagnification factor
DL detection limit
HCB hexachlorobenzene
i.p. intraperitoneal
ND not detectable
PCT porphyria cutanea tarda
p,p'DDE 1,1'-(2,2-dichloroethylidene)-bis[4-chlorobenzene]
SER smooth endoplasmic reticulum
T3 triiodothyronine
T4 thyroxine
PREFACE
The preparation of comprehensive Environmental Health Criteria
(EHC), as outlined in the Preamble of this monograph, is an extremely
time-consuming and resource-intensive procedure. Often countries have
prepared recent comprehensive reviews on chemicals as required by
their national legislation, and the International Programme on
Chemical Safety (IPCS) has been asked by Member States to determine
how best to utilize such national reviews during the preparation of
international EHC. Utilizing such national documents should avoid
duplication of effort and result in the more rapid production of more
concise IPCS EHC monographs.
This monograph on hexachlorobenzene has been prepared using as
background document the review (Supporting Document) prepared under
the Canadian Environmental Protection Act (CEPA), dated June 1993.
From this document, staff of Health Canada have chosen only the most
relevant studies for assessing the human and environmental risks from
exposure to hexachlorobenzene. These have been described from the
original references and supplemented by additional information
published more recently. This has resulted in a concise monograph,
yet one that supplies sufficient information for the reader to
understand the basis for the conclusions reached by the Task Group.
Readers who wish to consult the text of the Canadian Supporting
Document can obtain a copy from the Director, IPCS, World Health
Organization, Geneva, Switzerland.
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, and
analytical methods
Hexachlorobenzene (HCB) is a chlorinated organic compound with
moderate volatility. It is practically insoluble in water, but is
highly lipid-soluble and bioaccumulative. Technical grade HCB contains
up to 2% impurities, most of which is pentachlorobenzene. The
remainder includes the higher chlorinated dibenzo- p-dioxins,
dibenzofurans and biphenyls. Analysis of HCB in environmental media
and biological materials generally involves extraction of the sample
into organic solvents, often followed by a clean-up step, to produce
organic extracts for gas chromatography/mass spectrometry (GC/MS) or
gas chromatography with electron capture detection (GC/ECD).
1.2 Sources of human and environmental exposure
HCB was at one time used extensively as a seed dressing to
prevent fungal disease on grains, but this use was discontinued in
most countries in the 1970s. HCB continues to be released to the
environment from a number of sources, including the use of some
chlorinated pesticides, incomplete combustion, old dump sites and
inappropriate manufacture and disposal of wastes from the manufacture
of chlorinated solvents, chlorinated aromatics and chlorinated
pesticides.
1.3 Environmental transport, distribution and transformation
HCB is distributed throughout the environment because it is
mobile and persistent, although slow photodegradation in air and
microbial degradation in soil do occur. In the troposphere, HCB is
transported long distances and removed from the air phase through
deposition to soil and water. Significant biomagnification of HCB
through the food chain has been reported.
1.4 Environmental levels and human exposure
Low concentrations of HCB are present in ambient air (a few
ng/m3 or less) and in drinking-water and surface water (a few
ng/litre or less) in areas that are distant from point sources around
the world. However, higher levels have been measured near point
sources. HCB is bioaccumulative and has been detected in
invertebrates, fish, reptiles, birds and mammals (including humans)
distant from point sources, particularly in fatty tissues of organisms
at higher trophic levels. Mean levels in adipose tissue of the human
general population in various countries range from tens to hundreds of
ng/g wet weight. Based on representative levels of HCB in air, water
and food, the total intake of HCB by adults in the general population
is estimated to be between 0.0004 and 0.003 µg/kg body weight per day.
This intake is predominantly from the diet. Owing to the presence of
HCB in breast milk, mean intakes by nursing infants have been
estimated to range from < 0.018 to 5.1 µg/kg body weight per day in
various countries. The results of most studies on the levels of HCB in
foods and human tissues over time indicate that exposure of the
general population to HCB declined from the 1970s to the mid-1990s in
many locations. However, this trend has not been evident during the
last decade in some other locations.
1.5 Kinetics and metabolism in laboratory animals and humans
There is a lack of toxicokinetic information for humans. HCB is
readily absorbed by the oral route in experimental animals and poorly
via the skin (there are no data concerning inhalation). In animals and
humans, HCB accumulates in lipid-rich tissues, such as adipose tissue,
adrenal cortex, bone marrow, skin and some endocrine tissues, and can
be transferred to offspring both across the placenta and via mothers'
milk. HCB undergoes limited metabolism, yielding pentachlorophenol,
tetrachlorohydroquinone and pentachlorothiophenol as the major
metabolites in urine. Elimination half-lives for HCB range from
approximately one month in rats and rabbits to 2 or 3 years in
monkeys.
1.6 Effects on laboratory animals and in vitro tests
The acute toxicity of HCB to experimental animals is low (1000 to
10 000 mg/kg body weight). In animal studies, HCB is not a skin or eye
irritant and does not sensitize the guinea-pig.
The available data on the systemic toxicity of HCB indicate that
the pathway for the biosynthesis of haem is a major target of
hexachlorobenzene toxicity. Elevated levels of porphyrins and/or
porphyrin precursors have been found in the liver, other tissues and
excreta of several species of laboratory mammals exposed to HCB.
Porphyria has been reported in a number of studies in rats with
subchronic or chronic oral exposure to between 2.5 and 15 mg HCB/kg
body weight per day. Excretion of coproporphyrins was increased in
pigs ingesting 0.5 mg HCB/kg body weight per day or more (no effects
were observed at 0.05 mg HCB/kg body weight per day in the latter
study). Repeated exposure to HCB has also been shown to affect a wide
range of organ systems (including the liver, lungs, kidneys, thyroid,
skin, and nervous and immune systems), although these have been
reported less frequently than porphyria.
HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
It is known to bind to the Ah receptor.
In chronic studies, mild effects on the liver (histopathological
changes, enzyme induction) occurred in several studies of rats exposed
to between 0.25 and 0.6 mg HCB/kg body weight per day; the NOELs in
these studies were 0.05 to 0.07 mg HCB/kg body weight per day.
Concentrations of neurotransmitters in the hypothalamus were altered
in mink dams with chronic dietary exposure to 0.16 mg HCB/kg body
weight per day, and in their offspring exposed throughout gestation
and nursing. Calcium homoeostasis and bone morphometry were affected
in subchronic studies on rats at 0.7 mg HCB/kg body weight per day,
but not at 0.07 mg/kg body weight per day.
The carcinogenicity of HCB has been assessed in several adequate
bioassays on rodents. In hamsters fed diets yielding average doses of
4, 8 or 16 mg/kg body weight per day for life, there were increases in
the incidence of liver cell tumours (hepatomas) in both sexes at all
doses, haemangioendotheliomas of the liver at 8-16 mg/kg body weight
per day, and adenomas of the thyroid in males at the highest dose.
Dietary exposure of mice to 6, 12 and 24 mg/kg body weight per day for
120 weeks resulted in an increase in the incidence of liver cell
tumours (hepatomas) in both sexes at the two higher doses (not
significant, except for females at the highest dose). In utero,
lactational and oral exposure of rats to HCB in diets yielding average
lifetime doses ranging from 0.01 to 1.5 mg/kg body weight per day
(males) or 1.9 mg/kg body weight per day (females) for up to 130 weeks
post utero produced increased incidences, at the highest dose, of
neoplastic liver nodules and adrenal phaeochromocytomas in females and
of parathyroid adenomas in males. In another long-term study on rats,
exposure for up to 2 years to diets yielding average HCB doses of 4-5
and 8-9 mg/kg body weight per day induced increases in the incidences
of hepatomas and of renal cell adenomas at both doses in both sexes,
and of hepatocellular carcinomas, bile duct adenomas/ carcinomas and
adrenal phaeochromocytomas and adrenal cortical adenomas in females.
High incidences of liver tumours have also been reported in some more
limited studies in which single dietary concentrations were
administered to small groups of female rats. In addition, it has been
reported that, following subchronic dietary exposure to HCB, mice,
hamsters and rats developed tumours in the liver, bile duct, kidney,
thymus, spleen and lymph nodes. Dietary exposure to HCB promoted the
induction of liver tumours by polychlorinated terphenyl in mice and by
diethylnitrosamine in rats.
Except in the case of renal tumours in male rats (which appear at
least in part to be the result of hyaline droplet nephropathy) and
hepatomas in rats (which may result from hyperplastic responses to
hepatocellular necrosis), mechanistic studies that address the
relevance to humans of the tumour types induced by HCB have not been
identified.
HCB has little capability to induce directly gene mutation,
chromosomal damage and DNA repair. It exhibited weak mutagenic
activity in a small number of the available studies on bacteria and
yeast, although it should be noted that each of these studies has
limitations. There is also some evidence of low-level binding to DNA
in vitro and in vivo, but at levels well below those expected for
genotoxic carcinogens.
In studies of reproduction, oral exposure of monkeys to as little
as 0.1 mg HCB/kg body weight per day for 90 days affected the light
microscopic structure and ultrastructure of the surface germinal
epithelium, an unusual target for ovarian toxins. This dose also
caused ultrastructural injury to the primordial germ cells. These
specific target sites, which are damaged further at higher doses, were
associated with otherwise normal follicular, oocyte and embryo
development, suggesting specificity of HCB action within the site of
the ovary. Male reproduction was only affected at much higher doses
(between 30 and 221 mg/kg body weight per day) in studies on several
non-primate species.
Transplacental or lactational exposure of rats and cats to
maternal doses of between 3 and 4 mg/kg body weight per day was found
to be hepatotoxic and/or affected the survival or growth of nursing
offspring. In some cases, these or higher doses reduced litter sizes
and/or increased the number of stillbirths. (Adverse effects on
suckling infants have generally been observed more frequently, and at
lower doses, than embryotoxic or fetotoxic effects). The offspring of
mink with chronic exposure to as little as 1 mg HCB/kg diet
(approximately 0.16 mg/kg body weight per day) had reduced birth
weight and increased mortality to weaning. Although skeletal and renal
abnormalities have been observed in fetuses in some studies of rats
and mice exposed to HCB during gestation, these were either not
clearly related to treatment or occurred at doses that were also
maternally toxic. In two studies, one of which included lactational
and postnatal exposure, neurobehavioural development of rat pups was
affected by in utero exposure to HCB at oral maternal doses of 0.64
to 2.5 mg HCB/kg body weight per day.
The results of a number of studies have indicated that HCB
affects the immune system. Rats or monkeys exposed to between 3 and
120 mg HCB/kg body weight per day had histopathological alterations in
the thymus, spleen, lymph nodes and/or lymphoid tissues of the lung.
Chronic exposure of beagle dogs to 0.12 mg/kg body weight per day
caused nodular hyperplasia of the gastric lymphoid tissue. In a number
of studies on rats, humoral immunity and, to a lesser extent, cell-
mediated immunity were enhanced by several weeks exposure to HCB in
the diet, while macrophage function was unaltered. As little as 4 mg
HCB/kg diet (approximately 0.2 mg/kg body weight per day) during
gestation, through nursing and to 5 weeks of age increased humoral and
cell-mediated immune responses and caused accumulation of macrophages
in the lung tissue of rat pups. In contrast, HCB has been found to be
immunosuppressive in most studies with mice; doses of as little as
0.5-0.6 mg/kg body weight per day for several weeks depressed
resistance to infection by Leishmania or to a challenge with tumour
cells, decreased cytotoxic macrophage activity of the spleen, and
reduced the delayed-type hypersensitivity response in offspring
exposed in utero and through nursing. In a number of studies on
various strains of rats, short-term or subchronic exposure to HCB
affected thyroid function, as indicated by decreased serum levels of
total and free thyroxine (T4) and often, to a lesser extent,
triiodothyronine (T3).
1.7 Effects on humans
Most data on the effects of HCB on humans originate from
accidental poisonings that took place in Turkey in 1955-1959, in which
more than 600 cases of porphyria cutanea tarda (PCT) were identified.
In this incident, disturbances in porphyrin metabolism, dermatological
lesions, hyperpigmentation, hypertrichosis, enlarged liver,
enlargement of the thyroid gland and lymph nodes, and (in roughly half
the cases) osteoporosis or arthritis were observed, primarily in
children. Breast-fed infants of mothers exposed to HCB in this
incident developed a disorder called pembe yara (pink sore), and most
died within a year. There is also limited evidence that PCT occurs in
humans with relatively high exposure to HCB in the workplace or in the
general environment.
The few available epidemiological studies of cancer are limited
by small size, poorly characterized exposures to HCB and exposure to
numerous other agents, and are insufficient to assess the
carcinogenicity of HCB to humans.
1.8 Effects on other organisms in the laboratory and field
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa, and caused mortality in pink shrimp and grass shrimp, but
did not cause mortality in freshwater or marine fish. In longer-term
studies, the growth of sensitive freshwater algae and protozoa was
affected by a concentration of 1 µg/litre, while concentrations of
approximately 3 µg/litre caused mortality in amphipods and liver
necrosis in large-mouth bass.
1.9 Evaluation of human health risks and effects on the environment
1.9.1 Health effects
The Task Group concluded that the available data are sufficient
to develop guidance values for non-neoplastic and neoplastic effects
of HCB.
For non-neoplastic effects, based on the lowest reported NOEL
(0.05 mg HCB/kg body weight per day), for primarily hepatic effects
observed at higher doses in studies on pigs and rats exposed by the
oral route, and incorporating an uncertainty factor of 300 (× 10 for
interspecies variation, × 10 for intraspecies variation, and × 3 for
severity of effect), a TDI of 0.17 µg/kg body weight per day has been
derived.
The approach for neoplastic effects is based on the tumorigenic
dose TD5 i.e., the intake associated with a 5% excess incidence of
tumours in experimental studies in animals. Based on the results of
the two-generation carcinogenicity bioassay in rats and using the
multi-stage model, the TD5 value is 0.81 mg/kg body weight per day
for neoplastic nodules of the liver in females. Based on consideration
of the insufficient mechanistic data, an uncertainty factor of 5000
was used to develop a health-based guidance value of 0.16 µg/kg body
weight per day.
1.9.2 Environmental effects
The Task Group pointed out that there are very few experimental
studies on which an environmental risk assessment can be made. Levels
of HCB in surface water are generally several orders lower than those
expected to present a hazard to aquatic organisms, except in a few
extremely contaminated locations. However, HCB concentrations in the
eggs of sea birds and raptors from a number of locations from around
the world approach those associated with reduced embryo weights in
herring gulls (1500 µg/kg), suggesting that HCB has the potential to
harm embryos of sensitive bird species. Similarly, levels of HCB in
fish at a number of sites worldwide are within an order of magnitude
of the dietary level of 1000 µg/kg associated with reduced birth
weight and increased mortality of offspring in mink. This suggests
that HCB has the potential to cause adverse effects in mink and
perhaps other fish-eating mammals.
1.10 Conclusions
a) HCB is a persistent chemical that bioaccumulates owing to its
lipid solubility and resistance to breakdown.
b) Animal studies have shown that HCB causes cancer and affects a
wide range of organ systems including the liver, lungs, kidneys,
thyroid, reproductive tissues and nervous and immune systems.
c) Clinical toxicity, including porphyria cutanea tarda in children
and adults, and mortality in nursing infants, has been observed
in humans with high accidental exposure.
d) Various measures are warranted to reduce the environmental burden
of HCB.
e) The following health-based guidance values for the total daily
intake (TDI) of HCB in humans have been suggested: for non-cancer
effects, 0.17 µg/kg body weight/day; for neoplastic effects,
0.16 µg/kg body weight/day.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Hexachlorobenzene (HCB) is a chlorinated aromatic hydrocarbon
with the chemical formula C6Cl6. Its CAS registry number is 118-74-1.
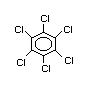 Synonyms: perchlorobenzene, pentachlorophenyl chloride, phenyl
perchloryl
Trade names: Amatin, Anticarie, Bunt Cure, Bunt-No-More, Co-op Hexa,
Granox NM, Julin's Carbon Chloride, No Bunt, No Bunt
40, No Bunt 80, No Bunt Liquid, Sanocide, Smut-Go,
Snieciotox, HexaCB
2.2 Physical and chemical properties
Some physical and chemical properties of HCB are listed in
Table 1. At ambient temperature, HCB is a white crystalline that is
virtually insoluble in water, but is soluble in ether, benzene and
chloroform (NTP, 1994). It has a high octanol/water partition
coefficient, low vapour pressure, moderate Henry's Law constant and
low flammability. Technical grade HCB is available as a wettable
powder, liquid and dust (NTP, 1994). Technical grade HCB contains
about 98% HCB, 1.8% pentachlorobenzene and 0.2% 1,2,4,5-
tetrachlorobenzene (IARC, 1979), and it is known to contain a variety
of impurities, including hepta- and octachlorodibenzofurans,
octachlorodibenzo- p-dioxin and decachlorobiphenyl (Villanueva et
al., 1974; Goldstein et al., 1978).
Table 1. Physical and chemical properties of hexachlorobenzenea
Property Value
Relative molecular mass 284.79
Melting point (°C) 230
Boiling point (°C) 322 (sublimates)
Density (g/cm3 at 20°C 1.5691
Vapour pressure 0.0023
(Pa at 25°C)
Log octanol/water partition coefficient 5.5
Water solubility 0.005
(mg/litre at 25°C)
Henry's Law Constant (caluclated)b 131
(Pa/mol per m3)
Conversion factors 1 ppm = 11.8 mg/m3
1 mg/m3 = 0.08 ppm
a From ATSDR (1990); Mackay et al. (1992)
b The Henry's Law Constant has been calculated using the
tabled values for aqueous solubility and vapour pressure
2.3 Analytical methods
Analytical methods for the determination of HCB in environmental
samples and biological tissues vary depending upon the matrix and
representative methods for various matrices, and are summarized in
Tables 2 and 3.
Table 2. Analytical methods for determining hexachlorobenzene in environmental samplesa
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Water Extract with dichloromethane, GC/ECD 0.05 mg/kg 95 ± 10-20% US EPA (1982)
exchange to hexane,
concentrate; Florisil column
chromatography as a clean-up
Water Extract with dichloromethane GC/MS 1.9 mg/kg No data US EPA (1982)
at pH 11 and 2, concentrate
Air Glass fibre filter and XAD2 HRGC/ 0.18 pg/m3 >99% Hippelein et al. (1993)
traps separated by a PUF LRMS
disk; extraction with toluene
Air Polyurethane foam (PUF) GC/ECD <0.1 µg/m3 94.5±8% Lewis & MacLeod (1982)
sampling cartridge, extraction
with diethyl ether in hexane
Air Polyurethane foam (PUF) GC/ECD low pg/m3 93±1.1% Oehme & Stray (1982)
plugs, extraction with hexane, range (not
fractionation by HPLC specified)
Air Porous polyurethane foam GC/ECD No data Tenax more Billings &
(PUF), or Tenax-GC resin; effective Bidleman (1980)
filters refluxed with than PUF in
dichloromethane and retaining HCB
chlorinated solvents removed
and refluxed with hexane;
clean-up by alumina
chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Air Adsorb on Amberlite XAD-2 GC/PID 0.014 mg/m3 approx Langhorst &
resin separated by a silanized 95 ± 12% Nestrick (1979)
glass wool plug, desorption
with carbon tetrachloride.
Air Trace Atmospheric Gas Analyser approx No data Thomson et al. (1980)
using negative atmospheric 0.35 µg/m3
pressure chemical ionization
for trace gas analysis;
collection from ambient air and
transfer into a carrier of CO2
for analysis
Soil, Hexane extraction GC/ECD 10 mg/kg 78±2.6% to DeLeon et al. (1980)
chemical 96.5±3.6%
waste
disposal
site samples
Soil Extract with dichloromethane GC/MS 18 mg/kg No data US EPA (1986b)
5 mg/kg
Sediment Solvent extraction subjected GC/MS 46% Lopez-Avila et al.
to acid-base fractionation; (1983)
base/neutral fraction subjected
to silica gel chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Wastes, Extract with dichloromethane GC/MS 190 mg/kg No data US EPA (1986b)
non-water 50 mg/kg
miscible
Wastes, soil Extract with dichloromethane GC/MS 20 µg/litrec No data US EPA (1986b)
a Portions of the table were taken from ATSDR (1990)
b GC = gas chromatography; ECD = electron capture detector; MS = mass spectrometry; PID = photoionization detector;
HRGC = high-resolution gas chromatography; LRMS = low-resolution mass spectrometry
c Identification limit; detection limits for actual samples are several orders of magnitude higher depending upon the
sample matrix and extraction procedure employed.
Table 3. Analytical methods for determining hexachlorobenzene in biological materials
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Fish tissue Grind with sodium sulfate, extract GC/ECD approx No data Oliver & Nicol (1982)
with hexane/acetone, clean-up by 0.05 µg/kg
Na2SO4/Alumina/silica gel/Florisil
column followed by a H2SO4 column
on silica gel
Fish tissue Extraction with hexane/isopropanol, GC/ECD No data No data Lunde & Ofstad (1976)
solvent and sulfuric acid
partitioning
Fish tissue Sulfuric acid digestion, silica gel GC/ECD 10-15 µg/kg 93% Lamparski et al. (1980)
column chromatography, methylation,
alumina column chromatography
Oyster Extraction with acetone/acetonitrile, GC/ECD No data No data Murray et al. (1980)
tissue partitioning into petroleum ether,
silica gel chromatography
Adipose Extraction with hexane, subjected GC/ECD No data 87.4-92.6% Watts et al. (1980)
tissue to Florisil clean-up and one-fraction
(chicken) elution
Adipose Extraction (solvent not specified), HRGC/MS 12 µg/kg No data Stanley (1986)
tissue bulk lipid removal, Florisil
fractionation
Adipose Extraction with benzene/acetone, GC/ECD 0.12 µg/kg 79-95% Mes (1992)
tissue Florisil fractionation
Table 3 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Blood/urine Extraction with carbon tetrachloride, GC/PID 4.1 µg/kg 83% Langhorst &
silica gel column chromatography, (urine) Nestrick (1979)
concentrate 16 µg/kg
(blood)
Blood Extraction with hexane, concentrate GC/ECD No data No data US EPA (1980)
Blood Extraction with hexane/isopropanol GC/ECD No data No data Lunde & Bjorseth (1977)
Breast milk Extraction with acetone/benzene, GC/ECD 33 µg/kg 70-82% Mes et al. (1993)
Florisil fractionation
GC = gas chromatography; ECD = electron capture detector; PID = photoionization detector; HRGC = high-resolution gas
chromatography; MS = mass spectrometry
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Sources, uses and production processes
Industrial synthesis of HCB may be achieved through the
chlorination of benzene at 150-200°C using a ferric chloride catalyst
or from the distillation of residues from the production of
tetrachloroethylene (US EPA, 1985a). HCB may also be synthesized by
refluxing hexachlorocyclohexane isomers with sulfuryl chloride or
chlorosulfonic acid in the presence of a ferric chloride or aluminum
catalyst (Brooks & Hunt, 1984).
Historically, HCB had many uses in industry and agriculture. The
major agricultural application for HCB used to be as a seed dressing
for crops such as wheat, barley, oats and rye to prevent growth of
fungi. The use of HCB in such applications was discontinued in many
countries in the 1970s owing to concerns about adverse effects on the
environment and human health. HCB may continue to be used for this
purpose in some countries; for example, HCB was still used in 1986 as
a fungicide, seed-dressing and scabicide in sheep in Tunisia (Jemaa et
al., 1986). However, it is uncertain as to whether HCB is still used
for this purpose.
In industry, HCB has been used directly in the manufacture of
pyrotechnics, tracer bullets and as a fluxing agent in the manufacture
of aluminum. HCB has also been used as a wood-preserving agent, a
porosity-control agent in the manufacture of graphite anodes, and as a
peptizing agent in the production of nitroso and styrene rubber for
tyres (Mumma & Lawless, 1975). It is likely that some of these
applications have been discontinued, although no information is
available.
Although HCB production has ceased in most countries, it is still
being generated inadvertently as a by-product and/or impurity in
several chemical processes. HCB is formed as a reaction by-product of
thermal chlorination, oxychlorination, and pyrolysis operations in the
manufacture of chlorinated solvents (mainly carbon tetrachloride,
trichloroethylene and tetrachloroethylene) (Government of Canada,
1993). The concentrations of HCB in distillation bottoms was estimated
to be 25%, 15% and 5%, respectively, for tetrachloroethylene, carbon
tetrachloride and trichloroethylene (Jacoff et al., 1986). While HCB
could potentially also be a contaminant in the final product, it was
not detected (detection limit 5 mg/litre) in carbon tetrachloride and
tetrachloroethylene in an investigation in Canada (personal
communication to Health Canada by Mr John Schultiess, Dow Chemical
Canada Inc., 1991). Analysis of production lots of tri- and
tetrachloroethylene produced in Europe in 1996 failed to detect HCB at
a detection limit of 2 µg/litre solvent (personal communication to the
IPCS by Mr C. de Rooij, Solvay Corporation Europe, 1996).
HCB is also generated as a waste by-product during the
manufacture of chlorinated solvents, chlorinated aromatics and
pesticides (Jacoff et al., 1986). The waste streams from the
production of pentachloronitrobenzene (PCNB), chlorothalonil and
dacthal are expected to contribute the bulk of HCB released from the
pesticide industry (Brooks & Hunt, 1984), although HCB can also be
generated as a waste by-product from the production of
pentachlorophenol, atrazine, simazine, propazine and maleic hydrazide
(Quinlivan et al., 1975; Mumma & Lawless, 1975). These pesticides are
also known to contain HCB as an impurity in the final product, usually
at levels of less than 1% HCB when appropriate procedures are used for
the synthesis and purification stages (Tobin, 1986). When such
procedures are not met, the level of HCB could be much higher (e.g.,
pentachloronitrobenzene has been reported to contain 1.8-11% HCB
(Tobin, 1986)). However, owing to many voluntary and regulatory
pressures, it is unlikely that such high levels of HCB are present in
today's pesticide formulations, but no information is available to
substantiate this point.
The chlor-alkali industry produces chlorine (Cl2), hydrogen and
caustic soda (NaOH) by electrolysis of purified and concentrated
sodium chloride (NaCl). Processes using graphite anodes are known to
produce HCB as a by-product (Quinlivan et al., 1975; Mumma & Lawless,
1975; Alves & Chevalier, 1980) owing to the reaction of chlorine with
graphite anode materials such as carbon and oils. Depending on the
purification procedures, the final products might also be contaminated
with HCB. In some countries, graphite anodes have been replaced by
dimensionally stabilized anodes (DSA), which do not generate HCB
(Government of Canada, 1993).
Incineration is an important source of HCB in the environment.
Emission levels from incinerators are very site-specific, and
therefore generic levels are difficult to estimate. Earlier
information yielded a crude estimate of the total HCB released from
all municipal incinerators in the USA to be 57-454 kg/year (US EPA,
1986a), but levels currently emitted are not known.
3.2 World production levels
Few recent data on the quantities of HCB produced are available.
Worldwide production of pure HCB was estimated to be 10 000
tonnes/year for the years 1978-1981 (Rippen & Frank, 1986). An
estimated 300 tonnes was produced by three manufacturers in the USA in
1973 (IARC, 1979). HCB was produced/imported in the European Community
at 8000 tonnes/year in 1978 (Rippen & Frank, 1986), and a company in
Spain used to produce an estimated 150 tonnes of HCB annually (IARC,
1979). Approximately 1500 tonnes of HCB were manufactured annually in
Germany for the production of the rubber auxiliary PCTP (BUA, 1994),
but this production was discontinued in 1993. No further centres of
HCB manufacture in Europe or North America have been identified.
Production of HCB has declined as a result of restrictions on its use
starting in the 1970s.
Considerable amounts of HCB are inadvertently produced as a by-
product in the manufacture of chlorinated solvents, chlorinated
aromatics and chlorinated pesticides. Jacoff et al. (1986) estimated
that approximately 4130 tonnes of HCB are generated annually as a
waste product in the USA and that nearly 77% of this is produced from
the manufacture of three chlorinated solvents: carbon tetrachloride,
trichloroethylene and tetrachloroethylene. The remainder is produced
by the chlorinated pesticide industry. In 1977, about 300 tonnes of
HCB were generated in Japan as a waste by-product in the production of
tetrachloroethylene, almost all of which was incinerated (IARC, 1979).
It was estimated that >5000 tonnes HCB/year were produced as a by-
product during tetrachloroethylene production in the Federal Republic
of Germany in 1980 (Rippen & Frank, 1986). However, recent estimates
for Europe from ECSA (European Chlorinated Solvent Association; P.G.
Johnson (1996) personal communication to IPCS) indicate that up to
4000 tonnes/year of HCB are produced as a by-product during certain
tetrachloroethylene production processes and that over 99% of this by-
product was incinerated at high temperatures.
3.3 Entry into the environment
Currently, the principal sources of HCB in the environment are
estimated to be the manufacture of chlorinated solvents, the
manufacture and application of HCB-contaminated pesticides, and
inadequate incineration of chlorine-containing wastes. It should be
noted that only a small fraction of the HCB generated as a by-product
may be released, depending on the process technology and waste-
disposal practices employed. For example, according to the US Toxic
Chemical Release Inventory (TRI), releases of HCB from the ten largest
processing facilities were 460 kg, most of this to air, compared with
almost 542 000 kg transferred offsite as waste. The TRI data are not
comprehensive, since only certain types of facilities are required to
report (ATSDR, 1994). ECSA (P.G. Johnson, personal communication to
IPCS) estimated that European emissions of HCB were about 200 kg/year
in 1993.
As discussed in the previous section, HCB is a contaminant of a
number of chlorinated pesticides. Since most current applications for
these products are dispersive, most HCB from this source will be
released to the environment.
Substantial quantities of HCB are also contained in the wastes
generated through the manufacture of chlorinated solvents and
pesticides. In the mid-1980s in the USA, 81% of these HCB-containing
wastes were disposed of by incineration, compared to 19% via
landfilling (Jacoff et al., 1986). It is likely that the amount of HCB
wastes disposed of by incineration has since increased, although
information has not been found to confirm this point. HCB can be
emitted from incinerators as a result of incomplete thermal
decomposition of these wastes and as a product of incomplete
combustion (PIC) from the thermal decomposition of a variety of
chlorinated organics such as Kepone, mirex, chlorobenzenes,
polychlorinated biphenyls, pentachlorophenol, polyvinyl chloride and
mixtures of chlorinated solvents (Ahling et al., 1978; Dellinger et
al., 1991).
Although only a small proportion of the HCB-containing waste
generated in the USA is landfilled, HCB may continue to leach to
groundwater from previously landfilled HCB waste sites. The
contribution of this route is uncertain, although HCB is not easily
leached, and landfills containing HCB are now designed to prevent
leachate losses into adjacent water systems (Brooks & Hunt, 1984). HCB
emission into the atmosphere from landfills containing HCB wastes
occurs from slow volatilization and from displacement of the
contaminated soil (Brooks & Hunt, 1984).
HCB has been detected in emissions from a number of industries,
including paint manufacturers, coal and steel producers, pulp and
paper mills, textile mills, pyrotechnics producers, aluminum smelters,
soap producers and wood-preservation facilities (Quinlivan et al.,
1975; Gilbertson, 1979; Alves & Chevalier, 1980), probably reflecting
the use of products contaminated with HCB. Municipal and industrial
wastewater facilities may also discharge HCB-contaminated effluents
(Environment Canada/Ontario Ministry of the Environment, 1986; King &
Sherbin, 1986), probably owing to inputs from industrial sources.
Long-range transport plays a significant role as a means of
redistribution of HCB throughout the environment. Wet deposition
(deposition via rain or snowfall) is the primary mechanism for
transport of HCB from the atmosphere to aquatic and terrestrial
systems in Canada (Eisenreich & Strachan, 1992). For example, it is
estimated that long-range transport and total deposition to the
Canadian environment is approximately 510 kg/year, an amount that is
similar to that from all other sources combined (Government of Canada,
1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental transport and degradation
HCB is distributed throughout the environment because it is
mobile and resistant to degradation. Volatilization from water to air
and sedimentation following adsorption to suspended particulates are
the major removal processes from water (Oliver, 1984a; Oliver &
Charlton, 1984). Once in the sediments, HCB will tend to accumulate
and become trapped by overlying sediments (Oliver & Nicol, 1982).
Although HCB is not readily leached from soils and sediments, some
desorption does occur and may be a continuous source of HCB to the
environment, even if inputs to the system cease (Oliver, 1984a; Oliver
et al., 1989). Chemical or biological degradation is not considered to
be important for the removal of HCB from water or sediments (Callahan
et al., 1979; Mansour et al., 1986; Mill & Haag, 1986; Oliver & Carey,
1986). In the troposphere, HCB is transported over long distances by
virtue of its persistence, but does undergo slow photolytic
degradation (the half-life is approximately 80 days; Mill & Haag,
1986), or is removed from the air phase via atmospheric deposition to
water and soil (Bidleman et al., 1986; Ballschmiter & Wittlinger,
1991; Lane et al., 1992a, 1992b). In soil, volatilization is the major
removal process at the surface (Kilzer et al., 1979; Griffin & Chou,
1981; Schwarzenbach et al., 1983; Nash & Gish, 1989), while slow
aerobic (half-life of 2.7-5.7 years) and anaerobic biodegradation
(half-life of 10.6-22.9 years) are the major removal processes at
lower depths (Beck & Hansen, 1974; Howard et al., 1991).
4.2 Bioaccumulation and biomagnification
The bioaccumulative properties of HCB result from the combination
of its physicochemical properties (high octanol/water partition
coefficient) and its slow elimination due to limited metabolism
related to its high chemical stability. Organisms generally accumulate
HCB from water and from food, although benthic organisms may also
accumulate HCB directly from sediment (Oliver, 1984b; Knezovich &
Harrison, 1988; Gobas et al., 1989). The uptake of HCB in benthic
invertebrates has been investigated in a number of laboratory and
field studies. The results demonstrated that some HCB in sediments is
available to infaunal species. Reported bioaccumulation factorsa
(BAF) for invertebrates in HCB-containing sediments range from 0.04 to
0.58 in high-organic-content sediment to 1.95 in low-organic-content
a Defined as tissue concentration (wet weight) divided by sediment
concentration (dry weight). BAFs from Oliver (1984b) were divided
by 6.67 to convert tissue dry weight to wet weight.
sediment (Oliver, 1984b; Knezovich & Harrison, 1988; Gobas et al.,
1989). The bioavailability of sediment-bound HCB is inversely related
to sediment organic carbon content (Knezovich & Harrison, 1988), and
varies with the type and size of the organisms and their feeding
habits (Boese et al., 1990), the extent of contact with sediment pore
and interstitial waters (Landrum, 1989), and the surface area of the
substrate (Swindoll & Applehans, 1987). Landrum (1989) suggested that
the bioavailability of sediment-sorbed chemicals declines as the
contact time between the sediment and a contaminant increases. For
example, Schuytema et al., (1990) observed that addition of HCB-spiked
sediments did not result in a significant increase in the uptake of
HCB by the worm ( Lumbriculus variegatus), amphipods ( Hyalella
azteca and Gammarus lacustris), and fathead minnows ( Pimephales
promelas) in a laboratory recirculating water/sediment system.
However, there was a substantial increase of HCB levels in bed
sediment, suggesting that sediment served as a more effective sink for
HCB than the organisms.
The biomagnification factor (BMF) for HCB in the earthworm
Eisenia andrei after exposure via food was 0.068 on a wet weight
basis (0.071 on a lipid basis) (Belfroid et al., 1994a), the biota
lipid-to-soil accumulation factor, defined as the ratio of the
concentration in the animal to that on the soil, was 215 g soil dry
weight/g lipid (Belfroid et al., 1994b), and the bioconcentration
factors (BCFs) for earthworms kept in water were found to be between
48 × 104 and 62 × 104 ml water/g lipid (Belfroid et al., 1993).
Field studies indicate that exposure via food is important for
organisms at higher trophic levels, as significant biomagnification
has been observed in several studies in natural aquatic ecosystems. In
Lake Ontario, Oliver & Niimi (1988) observed that tissue residue
concentrations increased from plankton (mean = 1.6 ng/g wet weight) to
mysids (mean = 4.0 ng/g wet weight) to alewives (mean = 20 ng/g wet
weight) to salmonids (mean = 38 ng/g wet weight). Braune & Norstrom
(1989) used field data on body burdens of HCB in the herring gull
( Larus argentatus) and one of its principal food items, the alewife
( Alosa pseudoharengus) in a Great Lakes food chain to calculate a
biomagnification factor (whole body, wet weight basis) of 31.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
HCB has been detected in air, water, sediment, soil and biota.
Representative levels reported in various environmental media in many
countries are presented in Tables 4, 5 and 6.
5.1.1 Air
HCB is widely dispersed in ambient air, and is generally present
at low concentrations. Mean concentrations of HCB in air removed from
point sources in Canada, Norway, Sweden, Germany, the USA, the Arctic
and the Antarctic range from 0.04 to 0.6 ng/m3 (Table 4). Levels of
HCB in air are generally similar between urban, rural and remote
sites, reflecting the persistence and long-range transport of this
substance.
Airborne concentrations of HCB measured in the USA near nine
chlorinated solvent and pesticide plants in 1973 and 1976 were much
higher than background levels (Spigarelli et al., 1986).
Concentrations as high as 24 µg/m3 were detected in the immediate
vicinity of one plant, while the maximum concentration of HCB distant
from the site was 0.36 µg/m3. The highest levels were associated with
the production of perchloroethylene, trichloroethylene and carbon
tetrachloride, and with plants where onsite landfill and open pit
waste disposal were practiced. More recently, Grimalt et al. (1994)
reported that airborne concentrations of HCB in a community in the
vicinity of a organochlorine factory built in 1898 in Catalonia,
Spain, averaged 35 ng/m3, compared with 0.3 ng/m3 in Barcelona, the
reference community for this study. It is not known how representative
the data from these studies are, as HCB releases are expected to be
minimized from industries using appropriate modern technology and
waste management practices.
No data are available on the levels of HCB in indoor air.
Table 4. Levels of hexachlorobenzene in ambient air (ng/m3)
Location Year Detection Mean Rangea Reference
limit
Canada (Windsor, Ontario) 1987-1990 0.03 0.13 ND-0.44 Environment Canada (1992)
Canada (Ontario)
- industrial/urban areas 1985-1989 0.007 0.167 0.07-0.31 Lane et al. (1992b)
- rural areas 0.094 0.02-0.31
Canada (Egbert, Ontario) 1988-1989 - >0.054 0.00004-0.64 Hoff et al. (1992)
Canada (Walpole Island) 1988-1989 0.02 0.15 ND-0.34 Environment Canada (1992)
Canadian High Arctic 1987 0.15 ND-0.154 Patton et al. (1989)
(Beaufort Sea)
Bear Island (Arctic)
- summer
- winter 0.001 0.04 0.029-0.045 Oehme & Stray (1982)
0.001 0.111 0.059-0.188
Southern Ocean and 1990 0.06 0.04-0.078 Bidleman et al. (1993)
Antarctica
Enewetak Atoll 1979 0.10 0.095-0.13 Atlas & Giam (1981)
(Pacific Ocean)
Spitzbergen
- summer 0.001 0.071 0.05-0.085 Oehme & Stray (1982)
- winter 0.001 0.086 0.071-0.095
Table 4 contd.
Location Year Detection Mean Rangea Reference
limit
Germany (Hamburg - 1986-1987 0.6 0.3-2.5 Bruckmann et al. (1988)
residential, suburban
and industrial sites)
South Germany 1986-1990 0.21 0.058-0.52 Morosini et al. (1993)
Norway (Lillestrom) 0.001 0.162 0.055-0.234 Oehme & Stray (1982)
Sweden (Aspvreten) 1984 0.067 0.054->0.165 Bidleman et al. (1987)
Sweden (Stockholm) 1983-1985 0.07 0.054->0.130 Bidleman et al. (1987)
Spain
- (near organochlorine 1989 & 1992 - 35 11-44 Grimalt et al. (1994)
compounds factory)
- hospital in Barcelona - 0.3 0.25-0.4
USA (Portland, Oregon) 1984 0.075 0.05-0.11 Ligocki et al. (1985)
USA - chemical production ND-24000 Spigarelli et al. (1986)
plants
USA - urban areas 1975-1979 0.1 0.5 ND-4.4 Carey et al. (1985)
a ND = not detected
5.1.2 Water
Levels of HCB in freshwater in Europe and North America are
generally below 1 ng/litre (Table 5), although higher values have been
reported in aquatic systems that receive industrial discharges and
surface run-off. In the connecting channels to the Great Lakes in
Canada, HCB levels were often found to exceed 1.0 ng/litre,
particularly near point sources. Levels in the St. Clair River near
the Dow Chemical outfall were as high as 87 ng/litre in 1985 and
75 ng/litre in 1986 (Oliver & Kaiser, 1986).
Mean concentrations of HCB in seawater rarely exceed 1 ng/litre
(Table 5) (Ernst, 1986; Burton & Bennett, 1987). In the Nueces Estuary
in Texas, USA, the highest level (0.61 ng/litre) was found near
sewage outfalls (Ray et al., 1983a). Higher concentrations (up to
196 ng/litre) were observed in the Forth Estuary in Scotland, near
domestic and chemical industry discharges (Rogers et al., 1989).
5.1.3 Soil
Data identified on levels of HCB in soil are quite limited and
are summarized in Table 6. The most extensive data are from the 1972
US National Soils Monitoring Program, in which the concentrations of a
variety of pesticides were determined at 1483 sites from 37 states
(Carey et al., 1979). HCB was detected at 11 sites, with a range of
concentrations in positive samples from 10 to 440 µg/kg dry weight. Of
24 samples of agricultural soil in British Columbia, Canada, where HCB
had last been applied as a seed treatment 10-15 years prior to the
survey, 6 had detectable HCB residues of between 1.3 and 2.2 ng/g dry
weight (Wilson & Wan, 1982).
Mean concentrations of HCB reported from uncontaminated soil in
Europe were found to range from 0.3 ng/g in Switzerland (Müller, 1982)
to 5.1 ng/g in a Swedish rural heathland soil (Thomas et al., 1985)
(it was not indicated whether concentrations were on a dry or wet
weight basis). Soil from a farming area in Italy contained 40 ng/g
(dry or wet weight basis not indicated) (Leoni & D'Arca, 1976). HCB
levels were not markedly increased by long-term application of sludge
to land in Germany at a rate of 50 to 500 tons per ha and averaged
2.8 ng/g (dry or wet weight basis not indicated) (Witte et al.,
1988a,b). Monitoring programmes in Germany yielded average levels of
HCB contamination of soil ranging from approximately 1 ng/g dry weight
in the North Rhine-Westphalia (1990) to approximately 6 ng/g dry
weight in Baden-Württemberg (1988) (BUA, 1994).
Table 5. Concentrations of hexachlorobenzene (ng/litre) in drinking-water and surface water
Location Year Detection Mean Rangea Reference
limit
Drinking-water
Canada (Ontario) 1980 0.01 0.1 0.06-0.2 Oliver & Nicol (1982)
Canada (Maritime Provinces) 1985-1988 2.0 ND Environment Canada (1989)
Croatia
- Sisak 1988-1989 0.5 1.0a <1-4 Fingler et al. (1992)
- Zagreb 2.0a 1-3
USA 1977-1981 100 ND US EPA (1985b)
Surface water
Canada
- Lake Superior 1986 0.007 0.026 0.018-0.040 Stevens & Neilson
- Lake Huron 0.033 0.018-0.073 (1989)
- Georgian Bay 0.041 0.032-0.054
- Lake Erie 0.078 0.025-0.260
- Lake Ontario 0.063 0.020-0.113
Canada-St. Clair River 1985 0.30-87 Oliver & Kaiser (1986)
- tributaries to 0.08-0.79
St. Clair R.
Table 5 contd.
Location Year Detection Mean Rangea Reference
limit
Canada (Atlantic Region); 1979-1989 2.0 ND-2.2 Leger 1991
lakes, streams, reservoirs,
estuaries, coastal waters
Germany (Elbe) 1990 - 12 3-62 BUA (1994)
Greece (Strimon River) 1985-1986 - 1.52 0.5-2.8 Kilikidis et al. (1992)
Italy (tributaries to
Adriatic Sea) 1977-1978 1.0 ND Galassi & Provini (1981)
Mediterranean Sea 1982-1983 0.1 2.13 ND-12.6 El-Dib & Badawy (1985)
Netherlands/Belgium 1993 10 <10 <10 RIWA (1993)
Netherlands 1987 - <10 ND-100 De Walle et al. (1995)
North Sea (coastal waters
and estuaries) 1979-1980 2.7 0.03-15 Ernst (1986)
Scotland (Forth Estuary) 1987 0.01 <0.01-196 Rogers et al. (1989)
Scotland (Forth Estuary) 1990 0.7-8.0 Harper et al. (1992)
Spain (Ebre Delta) 1985-1986 0.0005 0.041 ND-1.0 Grimalt et al. (1988)
USA (Texas-estuary) 1980 0.24 <0.01-0.61 Ray et al. (1983a)
USA (coastal, surface <0.1 <0.1-26 Cross et al. (1987)
microlayer)
a median value
Table 6. Levels of hexachlorobenzene in soil (ng/g dry weight)
Source Year Detection Mean Rangea Reference
limit
Canada (British Columbia) 1.0 Wilson & Wan (1982)
- agricultural soils <1.0-2.2
- near a former grain 260 ng/g
treatment plant
Czech/Polish Border - - 3.25 0.47-4.8 Holoubek et al. (1994)
(Giant Mountains)
Germany (contaminated soil) 1989 0.3-339 Hagenmaier et al. (1992)
India 1987 24a 0-165 Nair & Pillai (1989)
Italy (farming area) 1971-1972 40 Leoni & D'Arca (1976)
Netherlands - Ochten 1993 - 18 5.1-66 Hendriks et al. (1995)
- Gelderse Poort 80 73-89
Netherlands 1987 - <10 <80 De Walle et al. (1995)
Sweden 5.1 Thomas et al. (1985)
USA 1968-1973 10-440 Carey et al. (1979)
USA (chemical plants) 0.002 ND-5 700 000 Spigarelli et al. (1986)
USA (hazardous waste sites) 1977-1978 20 000-400 000 Davis & Morgan (1986)
USA (5 locations near Love 0.1 1.04-5.6 0.15-26.3 Ding et al. (1992)
Canal)
a ng/g wet weight
Levels in soil are highest near industrial sources of HCB. Levels
as high as 12 600 ng/g dry weight were reported at one landfill site
in Canada (Wilson & Wan, 1982), and 570 µg/g (dry or wet weight not
indicated) on the grounds of a chlorinated solvent and pesticide
production plant in the USA (Spigarelli et al., 1986). Soils near a
former grain treatment plant in Canada contained 260 ng/g dry weight
of HCB (Wilson & Wan, 1982). Levels of HCB in soils from contaminated
floodplains in the Netherlands ranged from 5.1 to 89 ng/g dry weight
(Hendriks et al., 1995).
5.1.4 Sediment
HCB strongly sorbs to sediment and suspended matter, and
differences in the concentrations in the water as well as in the
composition of the sediments and suspended matter result in a wide
range of concentrations in this medium.
In sediment samples collected from 1979 to 1989 in the Atlantic
provinces of Canada, HCB was reported to be below the limit of
detection of 0.2 ng/g dry weight in 140 of 152 samples (Leger, 1991).
In surveys conducted from 1980 to 1983, HCB levels in sediments from
the Great Lakes ranged from 0.02 to 840 ng/g dry weight (Oliver &
Nicol, 1982; Fox et al., 1983; Kaminsky et al., 1983; Oliver &
Bourbonniere, 1985; Bourbonniere et al., 1986; Oliver et al., 1989;
IJC, 1989). Analyses of sediment cores from Lake Ontario indicated
that levels of HCB have declined from the 1960s to the early 1980s but
more recent data are not available to determine if this downward trend
has continued (Oliver & Nicol, 1982; Oliver et al., 1989). HCB levels
in sediment sampled from eight lakes in northern remote Canada (date
of sampling not specified) ranged from 0.09 to 1.80 ng/g dry weight
(Muir et al., 1995).
Levels as high as 5100 ng/g dry weight were detected in the Rhine
River in Baden-Württemberg, Germany, in 1986 (BUA, 1994). The majority
of sediment samples taken from the rivers Rhine and Elbe between 1980
and 1990 contained levels of HCB between 10 and 500 ng/g dry weight,
although levels below 1 ng/g dry weight were determined in some other
locations (BUA, 1994). A Nordic study on chlorinated compounds in the
Baltic, Kattegat and Skagerrak (œstfeldt et al., 1994) found HCB
concentrations in sediment ranging from 1 to 20 ng/g loi (loss on
ignition), the higher values occurring mainly in the Bothnian Bay. An
extreme value of 63 ng/g loi was found in Öresund between Denmark and
Sweden. Levels of HCB in sediment samples collected near effluent
discharges along a stream in Pakistan ranged from <0.05 to 94.5 ng/g
wet weight (Tehseen et al., 1994).
Higher levels of HCB in sediments were reported in studies
conducted near point sources. As much as 280 000 ng HCB/g dry weight
was detected in 1985 downstream of the Dow Chemical sewer discharges
in the St. Clair River, USA (Oliver & Pugsley, 1986).
5.1.5 Biota
HCB has been detected in invertebrates, fish, reptiles, birds and
mammals from around the world. Following the detection of HCB in
tissues of wild birds by De Vos in 1967, high residues were often
found in predatory birds, whereas minor quantities were detected in
fish, mussels and birds of the aquatic environment (Vos et al., 1968;
Koeman et al., 1969). Based on Canadian data from monitoring studies
in birds, HCB levels declined sharply from the mid-1970s (the earliest
data available) and into the early 1980s, after which they levelled
off (Noble & Elliott, 1986; Environment Canada/Department of Fisheries
and Oceans/Health and Welfare Canada, 1991).
Levels of HCB in freshwater mussels in the Great Lakes and
connecting channels have been found to range from 0.1 ng/g wet weight
to 24 ng/g wet weight (Kauss & Hamdy, 1985; Innes et al., 1988;
Muncaster et al., 1989). A similar range (4.4-26 ng/g wet weight) was
observed in benthic amphipods, the pelagic amphipod Pseudalibrotus
litoralis and brittle stars from the Beaufort Sea (Hargrave et al.,
1989). Lower levels (0.1-1.8 ng/g wet weight) were observed in mussels
( Mytilus galloprovincialis) from the Ebro Delta in the Western
Mediterranean, and these levels were observed to decline from 1980 to
1992 (Solé et al., 1994). Levels in marine species of clams and
oysters from the USA were reported in several studies to be < 1 ng/g
wet weight (Phelps et al., 1986; Eisenberg & Topping, 1984; Ray et
al., 1983b). Similarly, levels in invertebrates, including mussels
( Mytilus edulis), soft clams ( Mya arenaria), lugworms ( Arenicola
marina), and polychaetes ( Nereis diversicolor), were <1 ng/g
fresh weight in the German Wadden Sea (Ernst, 1986). Bjerk & Brevik
(1980) reported higher levels (50-350 ng/g wet weight) of HCB in crabs
( Carcinus maenas, Pagurus sp.), snails ( Littorina littorea),
brittle stars ( Ophiura albida) and sea stars ( Asteroidea) from the
contaminated Frierfjord in Norway, which receives discharge from
various industries located in the region, and HCB and related
compounds were reported to originate from one main source
(unspecified) in the area. œstfeldt et al. (1994) found that mussels
( Mytilus edulis) from the Baltic contain higher levels of HCB
(200-800 ng/g lipid weight) than mussels from Kattegat (11-20 ng/g
lipid weight).
In a 1981-1982 survey of HCB levels in fish from watersheds in
Eastern Canada, whole body concentrations in brook trout ( Salvelinus
fontinalis) and yellow perch ( Perca flavescens) ranged from below
the limit of detection (4.2 ng/g in 1981; 0.2 ng/g in 1982) to 54 ng/g
for trout and 15 ng/g wet weight for perch (Peterson & Ray, 1987).
Relatively high body burdens of HCB have been observed in fish in Lake
Ontario and connecting channels. HCB was not detected (ND) in juvenile
spottail shiners ( Notropis hudsonius) from Lakes Superior and Erie
(detection level = 1 ng/g wet weight) (Suns et al., 1983; Environment
Canada/Department of Fisheries and Oceans/Health and Welfare Canada,
1991), while mean body burdens in shiners in Lake Ontario ranged from
ND to 13 ng/g wet weight, and those in the Detroit, Niagara, and
St Clair rivers averaged 5 ng/g wet weight, ND to 8 ng/g wet weight,
and 231 ng/g wet weight, respectively (Suns et al., 1985). Mean
concentrations of HCB in the muscle tissue of various species of
salmonids from Lake Ontario ranged from 5 to 37 ng/g wet weight (Niimi
& Oliver, 1989).
Levels of HCB measured in whole fish species taken from major
rivers and lakes in the USA (including known contaminated areas)
ranged from <2 to 913 ng/g wet weight (Kuehl et al., 1983; DeVault,
1985; Schmitt et al., 1990; Kuehl & Butterworth, 1994). Levels in
roach ( Rutilus rutilus L.) and perch ( Perca fluviatilis L.) from
the "moderately polluted" Lahn River in Germany ranged from ND to
233 ng/g wet weight, with a mean of 1 ng/g (Schuler et al., 1985).
Concentrations of HCB in the whole bodies of carp ( Cyprinus carpio)
from the mouth of tributaries to Lake Ontario and the Niagara River
ranged from 52 to 1600 ng/g on a lipid basis (6.7 to 205 ng/g on a
fresh weight basis). The highest values were measured near hazardous
waste dumps and industrial facilities (as high as 1600 ng/g fat)
(Jaffe & Hites, 1986). Brunn & Manz (1982) reported a mean whole-body
concentration of HCB in fish (mainly trout) from inland rivers,
streams, and ponds in Germany of 5 ng/g wet weight. The highest levels
were recorded from fish caught in rivers.
HCB levels in seawater are generally lower than those in
freshwater, resulting in lower levels in edible parts of marine fish.
In fish taken from the North Sea (species not reported), HCB levels in
fish muscle tissues averaged 0.3-0.4 ng/g wet weight, with a maximum
of 0.8 ng/g (Ernst, 1986). HCB concentrations in livers averaged
42 ng/g wet weight for cod ( Gadus morhua) and 4 ng/g (range of
0.2-14 ng/g) for flounder ( Platichthis flesus). These levels were
comparable to levels measured in fish near the coast of southwest
Greenland and in the North Atlantic Ocean. Livers of cod from the
coast of southwest Greenland contained 32.4 ng/g on average, and those
of hake ( Merluccius merluccius) from the North Atlantic Ocean
averaged 40.5 ng/g) (Ernst, 1986). Levels of HCB were below the
determination limit (DL) in cod liver (DL = 5 ng/g) and herring muscle
(DL = 1 ng/g) of fish from the Clyde Sea near Scotland (Kelly &
Campbell, 1994). Cod from the Firth of Forth had mean liver levels of
38.7 ng/g wet weight, and levels in herring muscle of 2.0 and 2.3 ng/g
wet weight were observed in fish from the Firth of Forth and North
Sea, respectively (Kelly & Campbell, 1994). In surveillance monitoring
of contaminants in fish from coastal waters near England and Wales,
concentrations of HCB in livers of cod ( Gadus morhua), whiting
( Merlangius merlangus), dab ( Limanda limanda) and flounder
( Platichthys flesus) were 2-290, 5-230, 3-55 and 1-52 ng/g,
respectively (all results on a wet tissue weight basis) (MAFF/HSE,
1994). Levels of HCB in muscle tissues of herring ( Clupea harengus)
from the Baltic Sea ranged from <1 to 39 ng/g (Hansen et al., 1985);
concentrations in whitefish ( Coregonus lavaretus) and trout ( Salmo
trutta) ranged from <1 to 9 ng/g fresh weight in a 1992 survey
(Atuma et al., 1993).
Fish taken from the contaminated waters of the Frierfjord in
Norway contained mean concentrations of HCB in liver of 11 600 ng/g
for saithe ( Pollachius virens), and 16 800 ng/g for cod ( Gadus
morhua) (Bjerk & Brevik, 1980). Levels of HCB from fish taken from
the uncontaminated Sogndalfjord were much lower, averaging 18 ng/g wet
weight in livers of cod ( Gadus morhua), 8 ng/g in haddock
( Melanogrammus aeglefinus) and 1 ng/g in lemon sole ( Microstomus
kitt) and flounder ( Platichthys flesus) (Skåre et al., 1985).
Flounder ( Platichthis flesus) taken from the Elbe Estuary in
Germany, downstream from Hamburg (a highly industrialized area),
contained mean levels of HCB in muscle of 688 ng/g (range 84-1907 ng/g
wet weight). Further downstream, towards the mouth of the river,
levels were lower, averaging 12.5 ng/g (range 2-32 ng/g) (Kohler et
al., 1986).
The mean level of HCB in 15 snapping turtle eggs from Ontario,
Canada was 27.1 ng/g wet weight (Bishop et al., 1995).
The levels of HCB in birds have been similar across the various
regions of Canada since the 1980s, probably as a combined result of
emission reductions and the long-range transport of HCB to remote
locations. Mean concentrations of HCB in herring gull eggs ( Larus
argentatus) in 1991 ranged from 16 to 71 ng/g wet weight at various
colonies in the Great Lakes, and were relatively uniform across lakes
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991). These levels were approximately an order of
magnitude lower than in 1974. The mean level of HCB in herring gull
eggs from Norwegian coastal waters in 1981 was 120 ng/g wet weight
(Moksnes & Norheim, 1986). In a study from the Netherlands, mean
levels in eggs of common terns collected in 1987 were 0.03 µg/g wet
weight and in those of black-headed gulls collected in 1988 were
93 µg/g fat (Stronkhorst et al., 1993). Levels of HCB found in eggs of
sea-bird species ( Haematopus ostralegus, Larus ridibundus, Larus
argentatus and Sterna hirundo) from the banks of a river near an
organochlorine chemical plant in Germany were < 500 ng/g wet weight
(Heidmann, 1986); mean levels of less than 15 ng/g wet weight were
found in eggs of several species of land birds, including rooks
( Corvus frugilerus) and sparrow hawks ( Accipiter nisus) from
agricultural, industrial and rural sites. Recent surveys have
indicated similar levels of HCB in the eggs of five other predatory
bird species across Canada (means ranged from 10 to 53 ng/g wet
weight) (Noble & Elliott, 1986; Pearce et al., 1989; Noble et al.,
1992). However, the mean level of HCB in peregrine falcon ( Falco
peregrinus) eggs collected across Canada from 1980 to 1987 was
279 ng/g wet weight, and concentrations ranged as high as 1060 ng/g
wet weight (Peakall et al., 1990).
HCB has been found to accumulate in lipids of the common
goldeneye duck ( Bucephala clangula) that overwinter in the Niagara
River (mean of 150 ng/g) (Foley & Batcheller, 1988) and the Detroit
River (mean of 1700 ng/g) (Smith et al., 1985a) in the USA. Goldeneye
wintering in the Baltic Sea contained average levels of 250 ng/g lipid
(Falandysz & Szefer, 1982). Levels of HCB in the livers of silver
seagulls taken from estuaries in Germany were lower in 1988 than 1989
(approximately 80 and 150 ng/g fat, respectively, in samples from the
River Ems estuary). Higher levels were observed for both years in
liver samples of birds taken from the River Elbe estuary (>250 ng/g
fat) (BUA 1994).
In breast muscle tissue samples from various species of birds,
HCB concentrations tend to be progressively greater at higher trophic
levels (i.e., piscivores > molluscivores > omnivores > grazers)
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991).
In the blubber of marine mammals in the Canadian Arctic, mean
levels of HCB were 19 ng/g wet weight for ringed seals ( Phoca
hispida) and 491 ng/g wet weight for beluga whales ( Delphinapterus
leucas) (Norstrom et al., 1990), while male belugas sampled in the
Gulf of St. Lawrence contained up to 1340 ng/g (Béland et al., 1991).
Blubber from male and female white-beaked dolphins ( Lagerorhunchus
albirostris) collected near the Newfoundland coast averaged
1110 ng/g and 880 ng/g wet weight, respectively. Lower levels
(290 ng/g and 100 ng/g wet weight) were observed in blubber from male
and female pilot whales ( Globicephala meleana), also collected near
the Newfoundland coast (Muir et al., 1988). The higher levels observed
in the dolphins may reflect greater exposure to HCB because of
overwintering and feeding in the Gulf of St. Lawrence. Blubber of
harbour porpoises ( Phocoena phocoena) collected in Poland between
1989 and 1990 contained an average of 573 ng/g wet weight (Kannan et
al., 1993), and those collected around the coast of Scotland between
1989 and 1991 contained an average of 263 ng/g (Wells et al., 1994).
Levels of HCB in the blubber of bottlenosed dolphins also collected
off the coast of Scotland contained an average of 276 ng/g (Wells et
al., 1994). Levels in the blubber of three species of dolphins from
the Bay of Bengal, southern India, were low, ranging from 1.1 to
13 ng/g wet weight (Tanabe et al., 1993). Harbour seals ( Phoca
vitulina) found sick or dead in Norwegian waters due to a disease
outbreak caused by a morbilli virus had a mean HCB level in the
blubber of 27 ng/g wet weight (range of 5-94 ng/g) (Skaare et al.,
1990).
Limited data were found on levels of HCB in terrestrial mammals.
In a 1973-1974 survey of HCB in the adipose tissue of fox ( Vulpes
vulpes), doe ( Capreolus capreolus) and wild boar ( Sus scrofa) in
Germany, HCB concentrations ranged from <10 to 3110 ng/g. The lowest
levels were observed in the does, presumably because they are
herbivorous, whereas foxes and wild boar feed on small animals and are
therefore more affected by biomagnification of HCB (Koss & Manz,
1976). Similar patterns were evident in a study from Sweden, in which
rabbits ( Oryctolagus cuniculus, muscle), moose ( Alcaes alcaes,
muscle), reindeer ( Rangifer tarandus, suet) and osprey ( Pandion
haliaetus, muscle) were found to contain 9, 15, 51 and 330 ng HCB/g
lipid weight, respectively (Jansson et al., 1993). The mean
concentration in 66 serum samples taken in muskoxen in the Canadian
Northwest Territories in 1989 was 2.8 ng/g (range of 1.1-7.5 ng/g)
(Salisbury et al., 1992). The mean concentration of HCB in fat samples
from 58 caribou from the same region ranged from 32.93 to 129.4 ng/g
(lipid corrected) (Elkin & Bethke, 1995). The mean concentration of
HCB in the livers and lipids of adult river otters ( Lutra
canadensis) in western Canada were 3 ng/g and 30 ng/g wet weight,
respectively, for females and 4 ng/g and 25 ng/g wet weight,
respectively, for males (Somers et al., 1987). Concentrations of HCB
in mink carcasses collected in Ontario in the late 1970s and early
1980s ranged from < 0.5 to 10 ng/g wet weight (Proulx et al., 1987).
In the Canadian north, the mean level of HCB in the fat of polar bears
( Ursus maritimus) hunted between 1982 and 1984 was 296 ng/g wet
weight (Norstrom et al., 1990).
5.1.6 Food and drinking-water
HCB is commonly detected, at low levels, in food (Table 7).
Levels of HCB tend to be highest in fatty foods and/or those that have
been treated with HCB-contaminated pesticides. The most extensive data
identified have been collected through the United States Food and Drug
Administration (US FDA) Total Diet Study. The results of the surveys
from 1982 to 1991 indicate that HCB is detectable (DL = 0.1 ng/g) in a
small fraction of food items, most often dairy products, meats, and
peanuts/peanut butter (KAN-DO Office and Pesticides Team, 1995). In
the most recent surveys, conducted during 1990-1991, mean levels were
less than 1 ng/g for all products.
Table 7. Concentration (µg/kg wet weight unless otherwise specified) of hexachlorobenzene in various foods
Country Food Mean contenta Range Reference
Australia cereals 0.01 < 0.01-0.01 Kannan et al. (1994)
pulses 0.02 0.01-0.05
oils 0.07 0.02-0.11
beverages 0.03 0.02-0.04
vegetables 0.01 < 0.01-0.02
fruits 0.01 < 0.01-0.02
dairy products 0.55 0.14-1.6
meat and fat 0.46 0.01-3.0
fishes 4.2 < 0.01-60
Canada fresh meat & eggs 0.17 Davies (1988)b
root vegetables & potato 0.04
fresh fruit ND(<0.01)
leafy/other above-ground vegetables 0.02
2% milk 0.16
Canada apples ND(<0.2)-2.6 OMAF/OME (1988)
peaches ND(<0.2)
tomatoes ND(<0.2)
potatoes ND(<0.2)
wheat ND(<0.2)
eggs ND(<0.2)
hamburger 0.39 0.2-0.57
prime beef ND(<0.2)-0.21
pork ND(<0.2)
chicken ND(<0.2)
Table 7 contd.
Country Food Mean contenta Range Reference
Germany milk 0.22d 0.088-0.45d Fürst et al. (1992)
cream 0.98d 0.31-1.30d
butter 4.86d 2.32-6.88d
cheese 2.72d 2.16-3.70d
India cereals 0.03 0.01-0.04 Kannan et al. (1992a)
pulses (edible seeds of legumes) 0.07 0.02-0.16
spices 0.22 <0.01-0.54
oils 1.5 0.09-2.8
milk 0.03 0.01-0.10
butter 1.7 0.86-2.4
fishes & prawn 0.07 <0.01-0.55
meat & animal fat 0.61 0.02-4.8
Mexico cheese 16.67d 1 Albert et al. (1990)
Morocco eggs 20.9 0.09-300 Kessabi et al. (1990)
poultry liver 5.1 trace-30.0
bovine liver 21.9 1.2-119.8
bovine kidney 15.1 trace-133.0
Papua New cheese 0.43 Kannan et al. (1994)
Guinea pork fat 0.40
chicken 0.20
striped mullet 0.04
tilapia 0.01 0.02-0.05
mud crab 0.03 < 0.01-0.02
oyster 0.02 < 0.01-0.05
Table 7 contd.
Country Food Mean contenta Range Reference
Solomon Islands pork 0.14
chicken 0.06
greenspotted kingfish 0.03 0.01-0.06 Kannan et al. (1994)
indian mackerel 0.01 0.01
paddletail snapper 0.01 0.01
Southern Baltic canned cod-livers 60 ± 6 50-76 Falandysz et al. (1993)
Spain bologna - fresh 2.57d Ariño et al. (1992)
- cooked 2.48d
Spain pork sausage Ariño et al. (1992)
- before curing 6.63d
- after 30 days curing 6.0d
Spain ham - fresh 3.46d Ariño et al. (1992)
- cured 1.29d
Spain pork 2.86-3.9d Ariño et al. (1993)
Spain lamb - chop, raw 14.67d Conchello et al. (1993)
- chop, grilled 12.06d
- leg, raw 8.53d
- leg, roasted 7.02d
Spain chicken 120 ± 10 To-Figueras et al. (1986)
calf 249 ± 37
rabbit 860 ± 159
pork 169 ± 20
sheep 225 ± 35
butter 315 ± 18
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom bread ND (10) MAFF/HSE (1994)
milk 0.6
butter ND(10)
cheese 3.33d
ewes' cheese ND(10)
pasta ND(10)
beef burgers ND(10)
canned meat 10d
cooked meats 10d
lamb ND(10)
rabbit ND(10)
salami ND(10)
United Kingdom sausages ND(10) MAFF/HSE (1994)
pies and pasties ND(10)
salmon (tinned) 2.0
breaded cod ND(2.0)
fish cakes 2.0
mackerel 20
plaice ND(2.0)
prawn products ND(2.0)
sardines (tinned) ND(2.0)
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom carrot 0.0317 Wang & Jones (1994)
potato 3.35
cabbage 0.0418
cauliflower 0.0729
lettuce 0.108
onion 0.0014
bean 0.0101
pea 0.0039
tomato 0.0139
USA cheese, processed 0.2 ND-0.5 US FDA
cheese, cheddar 0.1 ND-0.5 (unpublished)c
beef, ground (regular) 0.1 ND-0.4
beef, chuck roast 0.3 ND-1.0
beef, round steak 0.2 ND-1.0
beef, loin/sirloin steak 0.2 ND-1.0
USA lamb chop 0.3 ND-1.0 US FDA
frankfurters 0.1 ND-0.6 (unpublished)c
cod/haddock fillet ND(0.1) ND-0.2
eggs, scrambled 0.1 ND-0.3
eggs, fried 0.2 ND-0.7
peanut butter 0.2 ND-0.4
peanuts, dry roasted 0.3 ND-1.0
watermelon 0.1 ND-0.5
butter 0.6 ND-1.0
cream 0.1 ND-0.4
Table 7 contd.
Country Food Mean contenta Range Reference
Viet Nam rice 0.03 <0.01-0.05 Kannan et al. (1992b)
pulses 0.04 <0.01-0.18
oil 1.2
butter 5.0
animal fat 0.41 0.29-0.65
meat 0.11 0.03-0.18
fish 0.05 0.01-0.31
prawn 0.03
shellfish 0.04
crab 0.17
caviar 3.8 1.9-7.2
a ND = not detected (detection limit given in brackets).
b Fresh produce and meats grown in Ontario were purchased from four grocery stores in Toronto when locally grown produce
was available (Ontario freshwater fish were not available and therefore, were excluded from analysis). All food items
were grouped into one of five composites for analysis, with the relative proportions of different food items in each
composite calculated from the estimates of the amounts purchased per person per year by Ontario residents.
c The US Food and Drug Administration Total Diet Study conducted from April 1990 to April 1991; reporting residue levels
in 234 individual food items collected from 3 cities in each of 4 geographical regions of the USA (data available from
US FDA, Washington, DC).
d Originally reported on a fat basis, and subsequently converted to wet weight using percentage fat contents reported in
NHW (1987).
In a number of more limited recent surveys, HCB levels have been
determined in commercial foods available in several countries from
North America, Europe and Asia (Table 7). The results of these studies
are consistent with the USA study described above, in that HCB has
been detected primarily in fatty foods such as meats and dairy
products. In these studies, mean concentrations are generally in the
low ng/g range or less, although substantially higher concentrations
have been reported in some surveys from Europe and Asia.
The effects of cooking, curing and ripening on the HCB residues
in pork meat products were investigated in Spain by Ariño et al.
(1992). Neither cooking at 80-82°C for 100 min nor curing reduced the
HCB content in pork bologna and pork sausage, respectively, whereas
the level of HCB in dry-salted and cured ham declined by 42%
throughout maturation.
HCB has been detected infrequently, and at very low
concentrations in drinking-water supplies (Table 5). Samples of
drinking-water collected in 1980 from Canadian cities in the vicinity
of Lake Ontario contained from 0.06 to 0.20 ng/litre, with a mean of
0.1 ng/litre (Oliver & Nicol, 1982). In other Canadian and USA
surveys, HCB was not detected (US EPA 1985b - DL = 100 ng/litre;
Environment Canada, 1989 - DL = 2 ng/litre). Slightly higher
concentrations of HCB (median of 1-2 ng/litre) were reported in
Croatian drinking-water supplies drawn from a nearby polluted river
(pollution sources were not identified) (Fingler et al., 1992).
5.2 General population exposure
5.2.1 Human tissues and fluids
Owing to its persistence and lipophilicity, HCB is present at low
levels in the fatty tissues of virtually all members of the general
population. Levels of HCB in adipose tissues, breast milk, blood and
follicular fluid of various populations from around the world are
shown in Table 8. It should be noted that the quality of the studies
given in Table 8 varies quite widely, from extensive national surveys
to those with relatively few samples.
Levels of HCB in human adipose tissue from around the world are
generally <1 mg/kg (Table 8). Although available data are limited,
concentrations of HCB reported in fat tissue are generally slightly
higher in samples from European countries than from elsewhere in the
world. The highest levels reported in recent surveys are from Spain
(mean levels of approximately 3-6 mg/kg); the authors suggested that
this reflected contamination of foods caused by its presence as an
impurity in other pesticides (Camps et al., 1989; Gómez-Catalán et
al., 1993, 1995). Concentrations of HCB increased with age in a number
of these surveys, but there were no consistent differences in residue
levels between the sexes (Mes et al., 1982; Williams et al., 1984,
1988; Abbott et al., 1985; Mes, 1990; Mes et al., 1990; Gomez-Catalan
et al., 1993; Kemper, 1993; Ludwicki & Góralczyk, 1994).
In general, concentrations of HCB in breast milk in various
countries or regions (Table 8) range widely, and appear to be related
to the degree of industrialization and/or urbanization within the
survey area. The levels of HCB in breast milk have been expressed on a
whole milk basis, using the fat content reported by the authors or,
where this was not reported, a fat content of 4.2% (NHW, 1987).
Schechter et al. (1989a) reported that concentrations of HCB in breast
milk in the mid-1980s were lowest in samples from Thailand
(0.3 µg/litre whole milk) and Viet Nam (< 0.17 µg/litre ), somewhat
higher in those from a semi-rural area of the USA (0.7-0.8 µg/litre),
and higher still in German samples (12.6 µg/litre ) (numbers of
samples in this study were extremely small, except for the German data
(n=167)). In surveys summarized by Mes et al. (1986), mean HCB levels
were 1 µg/litre whole milk in the USA, 2 µg/litre in Canada,
3 µg/litre in Sweden, 4 µg/litre in Great Britain, and 35µg/litre in
Germany. Still higher levels (48-89 µg/litre whole milk) have been
reported in studies from Spain (Conde et al. 1993). Bates et al.
(1994) reported that the concentrations of HCB in breast milk of
primiparae from New Zealand increased linearly with age, but were not
related to body mass index, fish intake, smoking status, type of
residential water supply or location of residence (urban versus
rural). In a study of body burdens of organochlorines in an indigenous
population, Ayotte et al. (1995) reported that mean concentrations of
HCB in the milk fat of 107 Inuit women from northern Quebec were
several times higher than those in 50 Caucasian women from southern
Quebec (57 and 1.2 µg/litre whole milk, respectively). Levels of
organochlorine compounds in breast milk correlated with levels of
omega-3 fatty acids in plasma phospholipids, indicating that
consumption of marine organisms is an important source of exposure to
these xenobiotics.
In a HCB poisoning incident in Turkey (section 8.1), breast-fed
infants were fatally intoxicated through their mothers' milk. In an
early report of this incident (Peters et al., 1966), HCB was reported
as being present in breast milk, although it was not quantified.
However, elevated levels were measured (mean of 510 ng/g on a fat
basis (approximately 21 ng/g on a wet weight basis) for 56 porphyric
mothers) 20-30 years after the incident, compared with a mean of
70 ng/g fat in 77 milk samples from women of families without
porphyria or from areas outside of the endemic area (Peters et al.,
1982; Gocmen et al., 1989).
Table 8. Levels of hexachlorobenzene in human tissues and fluids
(mg/kg wet weight adipose tissue; mg/kg whole milk; µg/litre blood serum; µg/litre follicular fluid)
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue
Australia 31 0.14a (0.01-1.70) (fat basis) 1990-1991 Stevens et al. (1993)
Canada 108 0.026a (0.0073-0.118) 1985 Mes & Malcolm (1992),
Mes et al. (1990)
Canada 25 0.019a (max. value: 0.087) - Mes (1992)
Canada 141 0.071 (males)
(0.018-0.244)
0.109 (females)
(0.019-0.373) 1984 Williams et al. (1988)
Canada 99 0.095 (0.01-0.667) 1976 Mes et al. (1982)
Canada 168 0.062 (0.001-0.52) 1972 Mes et al. (1977)
Federal Republic of Germany 93 (0.11-21.8) 1971 Leoni & D'Arca (1976)
Federal Republic of Germany 6 0.263 (0.083-0.753) van der Ven et al. (1992)
India 7 0.012 (0-0.064) 1987 Nair & Pillai (1989)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Italy 28 0.491 (0.126-1.36) 1973-1974 Leoni & D'Arca (1976)
Japan 39 0.044 1986-1987 Kashimoto et al. (1989)
Japan 15 0.21 (0.10-0.42) - Morita et al. (1975)
Netherlands average 0.7a (fat basis) 1968-1969 Greve & Van Zoonen (1990)
of 1.2a (fat basis) 1973-1975
51/year 0.86a (fat basis) 1976
0.98a (fat basis) 1977-1978
0.85a (fat basis) 1980
0.80a (fat basis) 1981
0.58a (fat basis) 1982
0.49a (fat basis) 1983
0.42a (fat basis) 1985
0.38a (fat basis) 1986
New Zealand - 0.31 - US EPA (1985a)
Poland 53 0.221 (0.068-0.937) early 1980s Szymczyœski et al. (1986)
Poland 277 0.31 1989-1992 Ludwicki & Góralczyk (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Spain 256 2.99 1985-1988 Gómez-Catalán et al. (1993)
(4 cities)
Spain 86 3.37 (0.42-12.53) 1991 Gómez-Catalán et al. (1995)
(lipid basis)
Spain 171 5.55 1982-1983 To-Figueras et al. (1986)
Spain 168 2.95 (0.2-17.37) 1988-89 Ferrer et al. (1992)
United Republic of Tanzania 9 0.003 (0.0013-0.0076) - van der Ven et al. (1992
United Kingdom 201 0.05 (n.d-0.29) 1969-1971 Abbott et al. (1972)
United Kingdom 236 0.19 (0.02-3.2) 1976-1977 Abbott et al. (1981)
United Kingdom 187 0.11 (0.03-0.32) 1982-1983 Abbott et al. (1985)
USA 10 0.125 (0.03-0.47 - Barquet et al. (1981)
USA 6081 0.037a 1974-1983 Robinson et al. (1990)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
USA 763 0.118 (0.001-0.256) 1982 US EPA (1994)
689 0.043 (0.032-0.054) 1984
671 0.051 (0.043-0.059) 1986
B. Breast milkb
Australia 39 0.042 (rural) 1970 Newton & Greene (1972)
28 0.063 (urban)
Australia 137 0.007 (0.002-0.019) (rural) 1979-1980 Stacey et al. (1985)
130 0.008 (0.002-0.017) (urban)
Australia 60 0.017 (0.0007-0.32) - Quinsey et al. (1995)
Australia 128 0.0036a (<0.01-0.216) 1990-1991 Stevens et al. (1993)
Brazil 30 0.00048 (0.00024-0.0036)c 1987-1988 Beretta & Dick (1994)
Canada 412 0.0008 (max = 0.014) 1986 Mes et al. (1993)
Canada 210 0.002 (max.= 0.009) 1982 Mes et al. (1986)
Canada 100 0.002 (max.= 0.021) 1975 Mes & Davies (1979)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Canada 536 0.0013c 1989-1990 Dewailly et al. (1991)
Canada 127 0.00051 1978 Frank et al. (1988)
15 0.0004 1979
12 0.00028 1980-1981
13 0.00052 1983-1984
18 0.00026 1985
Finland 143c 0.002c 1984-1985 Mussalo-Rauhamaa et al. (1988)
Finland 50 0.0023 (0.0007-0.006) 1982 Wickström et al. (1983)
France 20 0.002 (0.00004-0.008)c 1990-1991 Bordet et al. (1993)
Federal Republic of Germany 144 0.021c 1984 Fürst et al. (1994)
220 0.019c 1985
157 0.015c 1986
144 0.015c 1987
196 0.013c 1988
145 0.01c 1989
286 0.0095c 1990
113 0.0074c 1991
Federal Republic of 167 0.0126c 1985-1987 Schecter et al. (1989a)
Germany
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Federal Republic of 2709 0.048c 1979-1981 BUA (1994)
Germany 3778 0.013c 1986
1897 0.014c 1987
2994 0.011c 1988
3256 0.01c 1989
5340 0.009c 1990
Former German 483 0.007c 1990-1991
Democratic Republic
India 16 0.042 (0-0.25)e 1987 Nair & Pillai (1989)
Israel 100 0.00256 - Weisenberg et al. (1985)
Italy 56 0.058d - Franchi & Focardi (1991)
Italy 64 0.006 (0.004-0.009) 1987 Larsen et al. (1994)
Netherlands 202 0.036a,c 1972-1973 Greve & Van Zoonen (1990)
278 0.008a,c 1983
New Zealand 38 0.0011 1988 Bates et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Norway 28 0.0007 1991 Johansen et al. (1994)
Spain 240 0.089(0.039-0.21)c 1984-1987 Conde et al. (1993)
358 0.048(0.037-0.073)c 1990-1991
Sweden 20 0.0042 (0.002-0.009)c 1978 Norén (1983a)
Sweden 2 0.0007-0.004c - Norén (1983b)
Sweden 227 0.003 (0.002-0.004)c 1972 Norén (1988)
245 0.003 (0.003-0.004)c 1976
340 0.003 (0.003-0.004)c 1980
102 0.001 (0.0008-0.001)c 1984-1985
Sweden 140 0.0012c 1989 Norén (1993)
Sweden 40 0.0017c 1986-1987 Vaz et al. (1993)
Thailand 3 0.0003c 1985-1987 Schecter et al. (1989a)
Turkey 51 0.0035c 1988 Üstünbas et al. (1994)
Turkey 56 0.021c 20-30 years Gocmen et al. (1989)
post-exposure
during
1955-1959
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
United Kingdom 193 0.001 (<0.001-0.005) 1989-1991 MAFF (1992)
USA 40 0.00052 1979 Bush et al. (1985)
USA 8 0.0007-0.0008c 1985-1987 Schecter et al. (1989a)
Viet Nam 12 <0.00017c 1985-1987 Schecter et al. (1989a)
Yugoslavia 10 0.006 (0.002-0.017) 1978 Kodric-Smit et al. (1980)
C. Serum
Canada 25 0.25 1993 Jarrel et al. (1993)
29 0.21
20 0.35
Croatia 15 1a (<0.5-4) 1985 Krauthacker (1993)
24 0.9a (<0.5-3) 1987-1988
26 1a (<0.5-7) 1989-1990
32 <0.5a (<0.5-4) 1990
Germany 6 1.23 (0.33-2.66)f - van der Ven et al. (1992)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
C. Serum (contd)
Spain (near 21 26 (7.5-69) 1992 Grimalt et al. (1994)
organochlorine
compounds
factory)
Spain (hospital 13 4.8 (1.5-15) 1992 Grimalt et al. (1994)
in Barcelona)
Spain 100 11.09 (1.60-94.2) 1986 To-Figueras et al. (1995)
4.13 (0.70-19.7) 1993-1994
United Republic 11 0.01 (0-0.03)f - van der Ven et al. (1992)
of Tanzania
USA 370 0.189a (0.05-3.21) - Needham et al. (1990)
D. Whole blood
Slovakia 50 25.2 (6.1-43.2) 1992 Koœan et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
E. Follicular fluid
Canada 25 0.11 1993 Jarrell et al. (1993b)
29 0.14
20 0.20
Federal Republic 15 2.59 (1.1-5.7) - Trapp et al. (1984)
of Germany
a Median value.
b The most recent data were used for calculation of intakes via breast milk (section 5.2.4).
c Originally expressed as mg/kg milk fat and subsequently converted to a wet weight basis using either the % fat reported,
or if not given, using 4.2% fat (NHW, 1987).
d Number of positive samples
e Originally expressed on a dry weight basis and subsequently converted to wet weight using 88% moisture for conversion
(NHW, 1987).
f Values reported in µg/kg.
HCB is present in a wide range of other tissues and fluids from
humans, but at lower levels than in adipose tissue and breast milk.
For example, concentrations of HCB in serum of 370 subjects from the
general population in the USA studied by Needham et al. (1990)
averaged 0.189 µg/litre, compared to concentrations of 39 ng/g lipid
in 287 adipose tissue samples. Schechter et al. (1989b) reported HCB
levels in various organs in autopsy tissue from three American
patients. HCB levels in adipose tissue ranged from 15 to 24 ng/g wet
tissue, while kidney, muscle, lung, spleen and testis contained 1 ng/g
or less, and adrenals, bone marrow and liver contained intermediate
concentrations.
Median blood serum levels of HCB ranged from <0.5 to
1.0 µg/litre in four population groups (total of 97 samples) from
Zagreb, Croatia (Krauthacker, 1993). The groups included workers
employed in the distribution and packing of seeds treated with
different pesticides, who were expected to have absorbed
organochlorine compounds at levels greater than the general
population. However, levels of HCB in the blood of these workers were
not elevated. Van der Ven et al. (1992) reported the HCB levels in
maternal serum of 6 and 11 full-term pregnant women in Germany and
Tanzania, respectively. Levels in Germany averaged 1.23 µg/kg (0.33-
2.66 µg/kg), whereas those in Tanzania were 0.01 µg/kg (0-0.03 µg/kg).
Analysis of blood plasma samples from a human organ specimen bank in
Germany revealed that median annual concentrations of HCB between 1983
and 1989 ranged between 3.1 and 5.4 µg/litre (Kemper, 1993). Serum and
ovarian follicular fluid have been shown to contain HCB in patients
receiving in vitro fertilization (Jarrell et al., 1993b). Of 72
patients, HCB was detected in the serum of 60 and follicular fluid of
49. There was a significant geographical variation among three major
cities in Canada (Jarrell et al., 1993b). Follicular fluid from
similarly treated patients in Germany has been shown to contain HCB
(Trapp et al., 1994). In some studies, workers exposed to chlorinated
solvents or chlorinated pesticides had elevated levels of HCB in blood
(section 5.2.7).
5.2.2 Intake from ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the range
of mean levels of HCB measured in ambient air in cities from around
the world of approximately 0.1 to 0.6 ng/m3 (Table 4), mean intake of
HCB from ambient air for the general population is estimated to range
from 3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day. Since no
data on levels of HCB in indoor air were found, it has been assumed
that levels indoors are the same as those outdoors. The intake of HCB
via air may be greater in populations residing in the vicinity of
point sources, but this exposure is considered to be too site-specific
to estimate reliably.
5.2.3 Intake from drinking-water
Based on a daily volume of ingestion for adults of 1.4 litres, a
mean body weight for males and females of 64 kg (IPCS, 1994), and the
range of mean concentrations of HCB detected in drinking-water from
cities of approximately 0.1 to 2 ng/litre (Table 5), the estimated
mean daily intake of HCB from drinking water for the general
population ranges from approximately 2.2 × 10-6 to 4.4 × 10-5 µg/kg
body weight per day.
5.2.4 Intake from foods
Based on the average daily consumption of various foodstuffs by
adults from around the worlda, a mean body weight for males and
females of 64 kg (IPCS, 1994), and the mean level of HCB detected in
various foods in the 1990-1991 US FDA Total Diet Study (Table 7), the
estimated daily intake of HCB from food ranges from approximately
0.0004 to 0.0028 µg/kg body weight per day (this range was generated
by assuming, for food groups in which HCB was not detected, that non-
detectable values were zero with the detection limit being 0.1 µg/kg.
These estimates overlap the range of dietary estimates (between 0.001
and 0.027 µg/kg body weight per day) that have been reported for
various countries (Canada, USA., Germany, Finland, Viet Nam, Thailand,
India, Japan, Australia, the Netherlands) (Gartrell et al., 1986; De
Walle et al., 1995; Fujita & Morikawa, 1992; Kannan et al., 1992a,b;
Government of Canada, 1993; Kannan et al., 1994). Intakes via food may
be substantially higher in selected European and Asian countries,
where the content of HCB in a sampling of a limited range of foods was
relatively high (Table 7), or in indigenous populations consuming
large quantities of some wildlife species, such as marine mammals,
that are known to accumulate relatively high tissue levels of
lipophilic contaminants (Government of Canada, 1993; Ayotte et al.,
1995; Kuhnlein et al., 1995).
a Dietary intakes (g/person/day) consist of: cereals, 323, starchy
roots, 225; sugar (excludes syrups and honey), 72; pulses and
nuts, 33; vegetables and fruits, 325; meat, 125; eggs, 19; fish,
23; milk products (excludes butter), 360; fats and oils (includes
butter), 31 (all intakes from IPCS, 1994).
Dietary intakes may also be greater in infants during breast-
feeding, owing to the accumulation of HCB in the mothers' milk. The
mean concentrations of HCB in the most recent surveys found for
various countries range from <0.17 to 48 µg HCB/litre whole milk
(Table 8). Assuming that infants are exclusively breast fed for the
first 6 months, during which they consume an average of 0.75 litres of
breast milk per day and have an average body weight of 7 kg (Health
Canada, 1994), the estimated mean intakes of HCB from breast milk in
various countries range from <0.018 to 5.1 µg/kg body weight per day.
Daily intakes of HCB by breast-feeding infants of Inuit mothers in
northern Quebec, Canada (a population that consumes substantial
quantities of marine organisms that accumulate lipophilic
contaminants) was estimated at 0.45 µg/kg body weight per day, a value
that was several times greater than for a more southerly population in
the same province (Ayotte et al., 1995).
5.2.5 Apportionment of intakes
Total intake of HCB from ambient air, drinking-water and foods is
estimated to range from approximately 0.0004 to 0.003 µg/kg body
weight per day for the general population, the principal route of
exposure being through the diet (92%). The estimated contributions
from air and drinking-water are much smaller (7% and 1%,
respectively). (The contribution from each environmental medium was
calculated based on the mid-point of the intakes estimated in the
previous sections.)
5.2.6 Trends in exposure of the general population over time
The results of most studies of the levels of HCB in foods and
human tissues over time indicate that exposure of the general
population to HCB declined from the 1970s to the mid-1990s in many
locations. However, this trend has not been evident during the last
decade in some other locations.
Routine monitoring of foods in some countries indicates that
exposure to HCB is decreasing. For example, mean concentrations in
grab samples of milk, bovine fat, poultry fat and egg fat collected
from suppliers in Ontario, Canada, decreased by an order of magnitude
or more between the early 1970s and the mid-1980s (Frank et al., 1983,
1985a, 1985b; Frank & Ripley, 1990). Brown et al. (1986) reported that
the frequency of detectable (> 10 ng/g in fat samples, wet weight)
levels of HCB in the USA meat and poultry supply increased
dramatically from 1972 to 1977-1978, but had fallen off sharply up to
1984. More recent data collected through the US FDA Total Diet Study
indicate that this trend had continued. Between 1982-1984 and 1991,
the most recent year for which data are available, both the frequency
of detection of HCB and the estimated average daily intake for people
of various ages decreased by roughly 80% (US FDA 1990, 1991, 1992). A
decline in HCB levels in fish from the Baltic and the Swedish West
Coast was found in the National Swedish Monitoring Programme over the
period 1988 to 1994 (Bignert, 1995). The annual decrease in levels in
herring muscle from different places in the Baltic was 12 to 15% and
in cod liver from the Baltic 21%; from the West Coast it was 12% in
herring muscle, 23% in cod liver and 23% in dab liver. The trend with
higher concentrations in samples from the Baltic as compared to the
West Coast is still seen in the samples.
The results of most studies of temporal trends of HCB levels in
human adipose tissue or milk (summarized in Table 8) indicate that
general population exposures have declined since the 1970s. In routine
monitoring of breast milk contaminants in German mothers, mean
concentrations of HCB declined by more than 50% between 1984 and 1991
(Fürst et al., 1994), and by about 80% between 1979 and 1990 (BUA
1994). The median HCB content in samples of plasma from a human
specimen bank in Germany decreased from 4.8 µg/litre in 1983 to
3.1 µg/litre in 1989, a period of increasing restrictions on indoor
applications of pentachlorophenol, which contains HCB as a contaminant
(Kemper, 1993). Mes (1990) reported that concentrations of HCB in
human adipose tissue from Canadian surveys were significantly lower in
1985 than in 1972; this decrease occurred in all age classes over this
period. An increase in the HCB content of human adipose tissue and
milk was observed in the early 1970s in the Netherlands, and was
attributed to an increase in the HCB concentration in products of
animal origin (Greve & Van Zoonen, 1990). Once measures were taken to
avoid contamination of such products, a gradual decrease in HCB levels
was observed. Johansen et al. (1994) reported that the concentration
of HCB in routine monitoring of milk from Norwegian mothers declined
by 65% between 1982 and 1991. In contrast, in the most extensive study
of levels of HCB in adipose tissues, the US National Human Adipose
Tissue Survey (Robinson et al., 1990), in which data on residues were
collected from a nationally representative sample of 6081 autopsies
and surgical patients from 1974-1983, there was little change in
residue concentrations over the study period, with the national median
level remaining near 30 to 40 ng/g.
5.2.7 Occupational exposure during manufacture, formulation, or use
Workers may be exposed to higher concentrations of HCB than the
general population, particularly in the manufacture of chlorinated
solvents, and in the manufacture and application of pesticides
contaminated with HCB.
In a survey of production industries (perchloroethylene,
trichloroethylene, carbon tetrachloride, chlorine, triazine herbicides
and pentachloronitrobenzene), the highest HCB concentrations were
associated with the production of perchloroethylene and
trichloroethylene (Spigarelli et al., 1986). The highest level of HCB
determined in the air on plant property was 24 µg/m3 at a plant
producing perchloroethylene, carbon tetrachloride and chlorine.
Relatively high HCB levels (maximum concentration of 2.2 µg/m3 in
air) were also detected in samples from the pentachloronitrobenzene
production plant. Lower levels of HCB were measured at triazine
herbicide production plants (ND - 0.02 µg/m3), and, in the one plant
that produced only carbon tetrachloride, HCB was not detected (MDL not
reported). It is not known how representative the data from these
studies are, as the generation and release of HCB would be minimized
in plants using appropriate modern technology and waste management
practices.
Personal breathing-zone samples (54 in all) from workers in a
pentachlorophenol production plant contained HCB concentrations
ranging from <0.1 to 120 µg/m3 (Marlow, 1986), while levels in 112
area samples throughout the plant ranged from <0.1 to 630 µg/m3.
HCB concentrations in the blood of workers in a factory producing
chlorinated solvents ranged from 14 to 233 µg/litre (Burns & Miller,
1975); this compared with a range <1 to 310 µg/litre in the blood of
vegetable spraymen (Burns et al., 1974). Mean levels of HCB in the
blood plasma of workers in a chlorinated solvents plant in the USA
were 311 µg/litre in 1974, and 312 µg/litre in 1975, and levels in
whole blood were 160 µg/litre in 1976, and 170 µg/litre in 1977
(Currier et al., 1980). Concentrations of HCB in blood were positively
correlated with the number of years worked in the plant, but were not
associated with airborne levels of HCB or job-category-based exposure
estimates. Pesticide-exposed vineyard workers in Germany tended to
have higher HCB whole blood levels (median 7 µg/litre, maximum
30 µg/litre) than reference controls (median 3 µg/litre, maximum
17 µg/litre) (Kemper, 1993). Angerer et al. (1992) reported that the
mean plasma level of HCB in 53 workers at a municipal waste
incinerator was 5.0 µg/litre, compared with 4.69 µg/litre in 64
subjects with no known occupational contact.
6. KINETICS AND METABOLISM
6.1 Aquatic and terrestrial biota
Terrestrial plants such as barley, cress and wheat, and algae
such as Oedogonium cardiacum slowly metabolize HCB to polar
metabolites and non-extractable residues (Lu & Metcalf, 1975;
Scheunert et al., 1983, 1985; Topp et al., 1989). For example, of the
total radiolabelled HCB in barley after uptake from soil over one
growing season, 14% was present as polar metabolites, 20% as plant-
bound residues and the remainder as the parent compound (Topp et al.,
1989). In the only available study on HCB depuration rates in plants,
the aquatic macrophyte Myriophyllum spicatum eliminated 95% of HCB
during the first 28 days after exposure ceased (Gobas et al., 1991).
Invertebrates slowly metabolize HCB to compounds such as
pentachlorothioanisole, pentachlorophenol and other polar metabolites
(Lu & Metcalf, 1975; Bauer et al., 1989). In an aquatic model
ecosystem treated with 14C-HCB for 24 h, unchanged HCB accounted for
84% of the total radioactivity in snails ( Physa sp.), 67% in water
fleas ( Daphnia magna) and 65% in mosquito larvae ( Culex pipiens)
(Lu & Metcalf, 1975). Half-lives for the elimination of HCB by
invertebrates were less than 5 days for filter-feeding bivalves
( Elliptio complanata and Mytilus edulis) (Bro-Rasmussen, 1986;
Russell & Gobas, 1989), 16 days for deposit-feeding clams ( Macoma
nasuta) (Boese et al., 1990), and 27 days for oligochaete worms
( Tubifex tubifex and Limnodrilus hoffmeisteri) (Oliver, 1987).
Sanborn et al. (1977) detected pentachlorophenol and at least
four unidentified polar metabolites in green sunfish ( Lepomis
cyanellus) after 28 days of ingesting HCB-contaminated food.
Pentachlorophenol has also been detected in the excreta and tissues of
rainbow trout ( Oncorhynchus mykiss) following an intraperitoneal
dose with HCB (Koss & Koransky, 1978; Koss et al., 1978). Zebra fish
( Brachydanio rerio) did not metabolize HCB after a 48-h exposure in
water (Kasokat et al., 1989). Elimination half-lives of HCB ranged
from 7-21 days for fathead minnows ( Pimephales promelas) after a
waterborne exposure (Kosian et al., 1981) to up to 210 days for
rainbow trout ( Oncorhynchus mykiss) after ingestion of HCB in food
(Niimi & Cho, 1981).
Clark et al. (1987) reported that 63% of the total HCB eliminated
in herring gulls ( Larus argentatus) was found in the egg yolk.
Breslin et al. (1983) found that 50% of total HCB eliminated from
laying bobwhite quail ( Colinus virginianus), a species that lays
many eggs, was accounted for in egg yolk. For most wild species, egg
laying will account for a relatively small loss of HCB, while
depletion of stored fat during energetically costly activities such as
migration and moulting may result in a significant reduction in body
burdens. The half-life for elimination of HCB in birds ranged from
24-35 days for domesticated chickens ( Gallus gallus domesticus) fed
HCB-contaminated diets (Kan & Tuinstra, 1976; Hansen et al., 1978) to
211 days in intraperitoneally dosed juvenile herring gulls (Clark et
al., 1987).
6.2 Mammals
There are few data on the absorption of HCB by humans. By
comparing intake and faecal excretion of HCB in a single breast-fed
infant, Abraham et al (1994) estimated that absorption was virtually
complete (greater than 99.7% at one month of age and greater than 97%
at 5 months). The concentrations of HCB in the diet and faeces of a
single formula-fed infant were too low for reliable estimation of
absorption (Abraham et al., 1994). The results of animal studies
indicate that 80% or more of an oral dose of HCB (between 10 and 180
mg/kg body weight) is absorbed if administered in an oil vehicle
(Albro & Thomas, 1974; Koss & Koransky, 1975; Ingebritsen et al.,
1981; Bleavins et al., 1982). In female rats treated with 14C-HCB in
oil, peak values of radioactivity were reached in 2 to 5 days. The
absorption was poor (2-20%, depending on the dose) when the substance
was given as an aqueous suspension (Koss & Koransky, 1975). Little
information was identified on dermal absorption, although it appears
to be lower. Koizumi (1991) observed that after dermal application of
approximately 2.5 mg 14C-HCB in tetrachloroethylene to Fisher-344
rats for 72 h, only 9.7% of the administered dose was absorbed. No
information on absorption via the lungs has been reported.
There are no experimental studies of tissue distribution of HCB
in humans, although in a small autopsy study of members of the general
population (Schechter et al., 1989b), the highest levels were found in
(in order) adipose tissue, adrenals, bone marrow and liver. Laboratory
studies in a number of animal species also indicate that the highest
concentrations of HCB are accumulated in tissues with a high lipid
content, such as the adipose tissue, adrenal cortex, bone marrow, skin
and some endocrine tissues (thyroid, adrenal and ovary) following
ingestion or injection of HCB (Koss & Koransky, 1975; Yang et al.,
1978; Courtney, 1979; Sundlof et al., 1982; Ingebritsen, 1986; Smith
et al., 1987, 1994; Goldey et al., 1990; Foster et al., 1993; Jarrell
et al., 1993a). No information was found on the tissue distribution
following inhalation or dermal exposure. HCB crosses the placenta, and
is eliminated via the mothers' milk in both animals and humans
(Villeneuve et al., 1974; Mendoza et al., 1975; Courtney & Andrews,
1979, 1985; Courtney et al., 1979; Bailey et al., 1980; Bleavins et
al., 1982; Goldey et al., 1990; section 5.2.1).
Metabolic transformation is not extensive in the wide range of
species examined. The pathways of biotransformation of HCB have been
reviewed by Debets & Strik (1979) and by Renner (1988). The metabolism
of HCB operates via three distinct pathways. These are oxidative
pathways, which give rise to phenolic metabolites including
pentachlorophenol, tetrachlorohydroquinone and tetrachloro-
benzoquinone; a glutathione-conjugation pathway leading to penta-
chlorothiophenol, pentachlorothioanisoles, and several other sulfur-
containing metabolites; and a minor pathway that yields lower
chlorinated benzenes through reductive dechlorination. Metabolism
occurs primarily in the liver, although dechlorination of HCB has also
been demonstrated in vitro in enzyme preparations from the lung,
kidney and small intestine (Mehendale et al., 1975).
The metabolism of HCB has been studied in the rat and guinea-pig
(Mehendale et al., 1975; Rozman et al., 1975; Koss & Koransky, 1976;
Koss et al., 1978; Koss & Koransky, 1978; Courtney, 1979), and in the
monkey (Rozman et al., 1975; Courtney, 1979). Dosing routes included
gastric intubation and the intraperitoneal route, while dosing
vehicles included oil and aqueous media. The monitoring for metabolic
products of HCB has included excretory products and/or tissue residues
for periods ranging from 28 to 40 days post-dosing. Findings were
quite dissimilar among the studies. The most common finding was that
less than 40% of the administered dose was recovered in the excretory
products and a majority of the recovered dose was unchanged HCB.
The major metabolites found in the urine of rats, mice and
guinea-pigs exposed to HCB by various routes in most studies are
pentachlorophenol (PCP), tetrachlorohydroquinone and
pentachlorothiophenol (PCTP) (Koss & Koransky, 1978; Koss et al.,
1978). (There is some question as to whether most of the latter
compound detected in some studies was an analytical artefact from
alkaline hydrolysis of the n-acetyl cysteine conjugate.) Other
metabolites include tetra- and pentachlorobenzenes and thioanisoles,
and tri- and tetrachlorophenols, both in free and conjugated forms. It
has been reported that, after dietary exposure of male and female
Wistar rats to HCB for 13 weeks, N-acetyl- S-(pentachloro-
phenyl)cysteine was the most abundant metabolite via the conjugation
pathway (89-92% of the total urinary metabolites collected over 24 h,
after one week of treatment). Mercaptotetrachlorothioanisole was also
present, excreted as a glucuronide (den Besten et al., 1994). The
excreta from male Wistar rats given 125 mg/kg body weight on day 1 and
6 were collected for 12 days (Jansson & Bergman, 1978). Faeces and/or
urine contained HCB (about 4% of the total does), pentachlorobenzene,
pentachlorophenol, pentachlorobenzenethiol (both as such and as
conjugates), methylthiopentachlorobenzene, tetrachlorobenzenedithiol
and/or methylthiotetrachlorobenzenethiol (both as such and as
conjugates), dichlorotetrakis(methylthio)benzene (trace amounts),
hexakis(methylthio)benzene (trace amounts), bis(methylthio)-
tetrachlorobenzene, tetrachlorobenzenethiol (trace amounts) and
methylthiotetrachlorobenzene (trace amounts). Compounds found
accumulated in adipose tissue were hexachlorobenzene,
pentachlorobenzene, pentachlorobenzenethiol, bis(methylthio)-
tetrachlorobenzene and pentachloroanisole.
Rizzardini & Smith (1982) administered 50 µmoles of HCB/kg body
weight to male and female rats by gavage in arachis oil for 103 days.
Three urinary metabolites were identified, i.e., pentachlorophenol,
2,3,5,6-tetrachlorobenzene, 1,4-diol and pentachlorothiophenol
(derived from mercapturate). The authors reported that female rats
excreted several times more HCB metabolites than males.
PCP and PCTP have been detected in the urine of humans from the
general population of Spain with high body burdens of HCB (To-Figueras
et al., 1992).
No reliable information on the elimination half-life of HCB in
humans was found. Excretion of HCB by laboratory animals occurs mainly
through the faeces regardless of the route of administration (US EPA,
1985a; ATSDR, 1990). Both biliary excretion and non-biliary intestinal
transfer contribute to faecal excretion (Rozman et al., 1981;
Ingebritsen et al., 1981; Richter & Schäfer, 1981; Sundlof et al.,
1982). Reported half-lives for the elimination of an oral dose of HCB
(doses were 3 mg/kg body weight or less in these studies) are
approximately one month in rats and rabbits, 10-18 weeks in sheep,
pigs and dogs, and 2.5 to 3 years in rhesus monkeys (Avrahami &
Steele, 1972; Avrahami, 1975; Rozman et al., 1981; Sundlof et al.,
1982; Scheufler & Rozman, 1984; Yamaguchi et al., 1986). HCB has been
detected in the milk of several species, including humans, and the
results of experiments with mice and ferrets indicate that the
majority of the maternal body burden can be eliminated via the
mother's milk during lactation (Bleavins et al., 1982; Courtney &
Andrews, 1985).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
This section summarizes the extensive literature on the toxicity
of HCB to laboratory mammals, with emphasis on those studies reporting
the lowest-observed-effect levels. Information on the dosage with
respect to body weight was obtained from the original papers, wherever
possible. When doses were not expressed in this way by the
investigators and could not be calculated from the data provided,
approximate doses (given in parentheses) have been estimated based on
the reference values given in NIOSH (1985).
7.1 Single exposure
The acute toxicity of HCB in experimental animals is low;
reported oral LD50 values for various species range from 1700 mg/kg
body weight for the cat to between 3500 and > 10 000 mg/kg body
weight for the rat, with intermediate values for the mouse, rabbit and
guinea-pig. Reported LC50 values for inhalation exposure range from
1600 mg/m3 for the cat to 4000 mg/m3 for the mouse, with
intermediate values reported for the rat and rabbit (IARC, 1979;
Strik, 1986; Lewis, 1992). Acute lethal doses elicit convulsions,
tremors, weakness, ataxia, paralysis and pathological changes in
organs. Strik (1986) reported that HCB has a low skin irritation
score, is not irritating to the eye and does not sensitize the guinea-
pig, although no details were provided. In several studies, single
oral doses of 100-1000 mg/kg body weight produced increases in the
activities of various liver enzymes in rats within 24 h (Strik, 1986).
7.2 Short-term and subchronic exposure
The effects of short-term, repeated exposure to HCB are primarily
hepatotoxic and neurological. In a number of studies, the effects of
HCB on rats exposed to oral doses in the range of 30-250 mg/kg body
weight per day included altered body weight, cutaneous lesions,
tremors and other neurological signs, hepatomegaly, liver damage and,
in some cases, early alterations in porphyrin or haem metabolism
(Courtney, 1979; US EPA, 1985a; Strik, 1986). Short-term exposure
in vivo induced a variety of enzymes, including glutathione- S-
transferases and isozymes of cytochrome P-450, identified as
cytochromes P-450IA1 (CYPIA1), P-450IA2 (CYPIA2) and P-450IIB (CYPIIB)
(Wada et al., 1968; Courtney, 1979; Denomme et al., 1983; US EPA,
1985a; Linko et al., 1986; Strik, 1986; Hahn et al., 1988, 1989; Vos
et al., 1988; Green et al., 1989; Rizzardini et al., 1990; D'Amour &
Charbonneau, 1992; Smith et al., 1993; Goerz et al., 1994). This means
that HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
Enzyme induction has been observed at relatively low doses in some
studies. For instance, in Wistar rats fed HCB in the diet for 14 days,
the low-effect level for induction of microsomal liver enzyme was
50 mg HCB/kg feed (approximately 2.5 mg/kg body weight per day), and
the no-effect level was 20 mg HCB/kg feed (approximately 1 mg/kg body
weight per day) (den Tonkelaar & van Esch, 1974).
The effects produced by subchronic exposure to HCB are similar to
those observed in short-term studies, but are generally evident at
lower doses (Courtney, 1979; US EPA, 1985a; ATSDR, 1990, 1994). At
relatively high doses (32 mg/kg body weight per day or more for
periods from several weeks to 90 days), reported effects have included
death, skin lesions, behavioural and neurological changes, reduced
body weight gain, increased organ weights, and altered thyroid
function and serum levels of thyroid hormones (the latter effect is
discussed later in this section). At lower doses, hepatotoxic effects
have been commonly reported, including histological alterations, the
induction of a variety of hepatic microsomal enzymes and porphyria.
The porphyrinogenic effects of exposure to HCB have been
extensively studied since the seminal reports of Ockner & Schmid
(1961) and De Matteis et al. (1961). These and subsequent earlier
works, many of them conducted at relatively high doses, have been
summarized by Courtney (1979), and much of this research is not
discussed in this report. Porphyria has been observed in several
species of laboratory mammals, most often manifested as increased
levels of porphyrins and/or porphyrin precursors in the liver, other
tissues and excreta. This disturbance in haem synthesis is associated
with the inhibition of uroporphyrinogen decarboxylase activity (this
enzyme converts uroporphyrinogen III to coproporphyrinogen III),
leading to the accumulation of uroporphyrin and other highly
carboxylated porphyrins, and with the induction of ALA synthetase (the
enzyme controlling the rate of haem synthesis) (ATSDR, 1994). There is
a delay before exposed rats become porphyric, which appears to reflect
the time for the animals to receive a sufficient cumulative dose of
HCB (Krishnan et al., 1991, 1992), as well as the time needed for the
porphyrins to accumulate to the level of overt porphyria (Kennedy et
al., 1986; Kennedy & Wigfield, 1990). Although in most studies
porphyria has been associated with longer-term exposure to HCB, rats
exposed to doses of 25-50 mg HCB/kg body weight per day for as little
as several days had increased levels of hepatic and urinary porphyrins
(Krishnan et al., 1991, 1992). In another study, hepatic levels of
highly carboxylated porphyrins were elevated by a single exposure to
50 mg HCB/kg body weight, although the latter result was not
accompanied by clinical porphyria (Kennedy & Wigfield, 1990).
HCB-induced porphyria has been extensively studied in rats, in
which dietary or gavage exposure of various strains to between 2.5 and
15 mg HCB/kg body weight per day for periods of 8 to 15 weeks has
caused hepatic porphyria, and, in some studies, increased levels of
porphyrins in the kidney and spleen (Grant et al., 1975; Kuiper-
Goodman et al., 1977; Goldstein et al., 1978; Mendoza et al., 1979;
Rizzardini & Smith, 1982; Teschke et al., 1983; Smith et al., 1985b;
Green et al., 1989; Van Ommen et al., 1989; Kennedy & Wigfield, 1990;
Smith et al., 1990; Den Besten et al., 1993). A no-observed-effect-
level (NOEL) for HCB-induced porphyria was not determined in these
studies. Although the data on other species are limited, levels of
hepatic or urinary porphyrins were increased in mice of various
strains fed diets containing 200 mg HCB/kg feed (yielding approximate
doses of 24 mg HCB/kg body weight per day) for periods of 7 to 15
weeks in some studies (Smith & Francis, 1983; Rizzardini et al., 1988;
Vincent et al., 1989), and porphyria was induced in Japanese quail
following short-term oral and intraperitoneal exposure to 500 mg
HCB/kg body weight per day (section 9.1.2).
The lowest doses producing porphyrinogenic and other effects on
the liver in a subchronic study were reported by den Tonkelaar et al.
(1978). Groups of five pigs exposed for 90 days to doses of 0.5 mg/kg
body weight per day or more in the diet had increased urinary levels
of coproporphyrin and alterations in liver histology and microsomal
enzyme activities, but no effects were observed at 0.05 mg/kg body
weight per day. However, marked excretion of coproporphyrin alone is
not a characteristic of the inhibition of uroporphyrinogen
decarboxylase in animal systems.
Female rats are more sensitive than males to the porphyrinogenic
effects of exposure to HCB. In various strains of rats exposed to
doses of 5 to 10 mg HCB/kg body weight per day in the diet or by
gavage, for periods of between 3 months or more, females developed a
marked porphyria which was absent or much reduced in males (Grant et
al., 1975; Kuiper-Goodman et al., 1977; Rizzardini & Smith, 1982;
Smith et al., 1985b). In a number of studies, the basis for the
susceptibility to HCB-induced porphyria of female rats compared to
males has been examined. Grant et al. (1975) reported that ovariectomy
decreased, and castration increased, the accumulation of porphyrins in
the livers of female and male Sprague-Dawley rats with subchronic
exposure to HCB, suggesting a role for steroid hormones in the
development of porphyria in this species. In another study, female
Fischer-344 rats with HCB-induced porphyria had higher levels of
cytochrome P-450IA isoenzymes and ethoxyresorufin- O-deethylase
activity than males, whereas males had higher levels of total
cytochrome P-450 and activities of microsomal monooxygenases
associated with cytochrome P-450IIB1 (Smith et al., 1990). In Fischer-
344 rats with HCB-induced porphyria, sex-related differences in
urinary and hepatic porphyrin levels were paralleled by differences in
the excretion of phenolic metabolites, particularly
pentachlorothiophenol (Rizzardini & Smith, 1982). These findings were
further investigated in a short-term study by D'Amour & Charbonneau
(1992), which indicated that male rats may be more resistant to HCB-
induced porphyria than females because hepatic conjugation of HCB with
glutathione is more important in males. Male Sprague-Dawley rats
receiving a porphyrinogenic dose of HCB (100 mg HCB/kg body weight per
day by gavage for 5 days) had significantly lower hepatic gluthione
concentration and higher glutathione transferase activity (to 3,4-
dichloronitrobenzene) than controls, whereas no significant
differences were observed in females. Biliary excretion of PCTP (a
metabolite of glutathione conjugation) and the rate of elimination of
HCB from the liver were greater in males than in females.
Other mechanistic studies have suggested the involvement of
oxidative metabolism of HCB in the development of porphyria, although
the mechanism remains to be elucidated. In female Wistar rats
co-treated with 300 mg HCB/kg in the diet (approximately 15 mg HCB/kg
body weight per day) and triacetyloleandomycin (TAO) (to selectively
inhibit cytochrome P-450IIIA1/2 and thereby prevent the oxidative
biotransformation of HCB) for 10-13 weeks, both the excretion of PCP
and TCHQ (tetrachlorohydroquinone, the reduced analogue of the
reactive tetrachlorobenzoquinone) and the extent of hepatic porphyria
and urinary porphyrin excretion were greatly diminished (Van Ommen et
al., 1989; Den Besten et al., 1993). In 13-week feeding studies on
female Wistar rats exposed to 300 mg HCB/kg diet (approximately 15 mg
HCB/kg body weight per day) in the presence or absence of TAO, the
degree of porphyria was better correlated with excretion of PCP than
TCHQ, and in comparative studies pentachlorobenzene (which is
metabolized to PCP by a different mechanism than for HCB) was not
porphyrinogenic (Den Besten et al., 1993).
In addition, it has been suggested that the aryl hydrocarbon
receptor (Ah receptor) may be involved in the accumulation of hepatic
porphyrins in mice (Linko et al., 1986; Hahn et al., 1988, 1989). Ah-
responsive strains of inbred mice were more sensitive to hepatic
porphyrin accumulation after HCB exposure than non-responsive mice
(Smith & Francis, 1983; Hahn et al., 1988), and HCB has been shown to
be a weak agonist for the Ah receptor (Hahn et al., 1989).
Full discussion of the evidence for a unifying hypothesis of
porphyria induced by HCB and other chemicals that act in a similar
way, as well as for human sporadic porphyria cutanea tarda, is beyond
the scope of this document. There is however, substantial experimental
and human evidence implicating a complex interaction between
hepatocellular iron and oxidative processes leading to the oxidation
of unstable uroporphyrinogen to uroporphyrin, possibly mediated by
induced cytochrome P-450 isozymes (reviewed by Smith & De Matteis,
1990). There is evidence that inhibition of uroporphyrinogen
decarboxylase may occur through formation of an inhibitor of the
enzyme during the oxidation of uroporphyrinogen (Rios de Molina et
al., 1980; Smith & De Matteis, 1990). HCB may act partly through
induction and uncoupling of the cytochrome P-450 system to form
reactive oxygen species, especially in the presence of an increased
available iron pool (Smith & De Matteis, 1990; Den Besten et al.,
1993).
Subchronic exposure to low doses of HCB has also caused changes
in calcium homoeostasis and bone morphometry. Male Fischer-344 rats
administered HCB by gavage in corn oil had elevated serum levels of
1,25-dihydroxy-vitamin-D3 and reduced calcium excretion after 5
weeks, and increased femur density, weight and strength after 15
weeks. These effects were evident at 0.7 mg/kg body weight per day but
not at 0.07 mg/kg body weight per day (Andrews et al., 1989, 1990).
While technical HCB is known to be contaminated with chlorinated
dibenzo- p-dioxins, dibenzofurans and biphenyls (Villanueva et al.,
1974; Goldstein et al., 1978), the effects (primarily hepatic) of
subchronic dietary exposure of rats to either pure or technical HCB
were virtually identical, indicating that the effects observed in this
study were due to the parent compound (Goldstein et al., 1978).
In a number of studies on various strains of rats, short-term or
subchronic exposure to HCB affected the thyroid, as indicated by
decreased serum levels of total and free thyroxine (T4) and often, to
a lesser extent, triiodothyronine (T3). In some instances, these are
accompanied by compensatory increases in thyroid weight, circulating
levels of thyroid-stimulating hormone or iodine uptake by the thyroid
(Rozman et al., 1986; Kleiman de Pisarev et al., 1989, 1990; Van Raaij
et al., 1991a, 1993a, 1993b; Foster et al., 1993; Den Besten et al.,
1993; Sopena de Krakoff et al., 1994). Den Besten et al. (1993)
reported such effects in rats exposed to as little as 9.5 mg/kg body
weight per day following dietary exposures for 13 weeks, although
effect levels were somewhat higher in other studies, which involved
exposure for a shorter duration and/or employed an aqueous vehicle.
Somewhat different effects (decreased levels of T3 in serum and no
change in T4, accompanied by increased uptake of iodine by the
thyroid) were observed in hamsters exposed to 100-200 mg HCB/kg feed
(approximately 12-24 mg/kg body weight per day) for 18-28 weeks (Smith
et al., 1987).
The mechanisms that have been advanced to account for the effects
of HCB on the thyroid include accelerated metabolism of thyroid
hormones by HCB-induced enzymes or accelerated deiodination of
thyroxine, in conjunction with increased biliary excretion (Kleiman de
Pisarev, 1989; Van Raaij et al., 1993b), and interference with plasma
transport of thyroid hormones through displacement of T4 from binding
sites on proteins (Van Raaij et al., 1991a, 1993a). Van Raaij et al.
(1991b, 1993a) reported that intraperitoneal injection of
pentachlorophenol and tetrachlorohydroquinone, but not HCB itself,
decreased serum thyroxine levels in rats, indicating that these
metabolites may be involved in the effects of HCB on the thyroid.
These authors reported that PCP was a more effective competitor for
thyroxine-binding sites of serum carriers in vitro, and more
effective at occupying carrier sites in ex vivo experiments, than
HCB (van Raaij et al., 1991a), and demonstrated that T4 binding sites
were partially occupied in the serum of rats exposed to HCB (Van Raaij
et al., 1993a). In the latter study, it was estimated that competition
for thyroid hormone binding sites, by PCP metabolized from HCB, could
account for almost half of the observed reduction in serum levels of
T4.
7.3 Long-term toxicity and carcinogenicity
A range of non-neoplastic effects from long-term exposure to HCB,
which are primarily hepatotoxic, have been observed at relatively low
doses. In a two-generation study with Sprague-Dawley rats, liver and
heart weights were increased in Fo males exposed to TWA doses of 0.29
and 1.50 mg/kg body weight per day in the diet for 3 months, and
histopathological changes in the liver were observed in F1 animals of
both sexes exposed to maternal doses of 0.29-0.38 and 1.50-1.90 mg
HCB/kg body weight per day in diet in utero, through nursing, and
then continued on the same diet as their parents for their lifetimes.
The no-effect level in this study was 0.06-0.07 mg/kg body weight per
day (Arnold et al., 1985; Arnold & Krewski, 1988). Dietary exposures
of Sprague-Dawley rats to 10 mg/kg and above (approximately 0.5-0.6
mg/kg body weight per day) for 9-10 months induced in vivo mixed-
function oxidase activity, as indicated by reductions in drug-induced
sleeping times (Grant et al., 1974). Exposure of Sprague-Dawley rats
to 5 mg HCB/kg in diet (approximately 0.25-0.30 mg/kg body weight per
day) for 3-12 months caused proliferation of smooth endoplasmic
reticulum, altered mitochondria and increased numbers of storage
vesicles in liver, but these effects were not evident at 1 mg/kg in
diet (approximately 0.05-0.06 mg/kg body weight per day) (Mollenhauer
et al., 1975; 1976). In a study by Böger et al. (1979), oral
administration of 2, 8 or 32 mg HCB to female Wistar rats twice weekly
for 203 days (0.57, 2.3 or 9.1 mg HCB/kg body weight per day) resulted
in hepatocellular enlargement, proliferated smooth endoplasmic
reticulum, increased glycogen and porphyrin deposits, and enlarged
mitochondria, but these effects were not seen at a lower dose (0.5 mg
HCB/kg body weight twice weekly, or 0.14 mg HCB/kg body weight per
day). Bleavins et al. (1984a) reported that exposure of female mink to
a dietary concentration of 1 mg/kg (estimated to yield a dose of
0.16 mg/kg body weight per day) for 47 weeks significantly increased
serotonin concentrations in the hypothalamus of dams, and depressed
hypothalamic dopamine concentrations in kits exposed in utero and
through nursing.
As in subchronic studies, female rats were more sensitive than
males to porphyria induced by chronic exposure to HCB. Grant et al.
(1974) reported that in Sprague-Dawley rats fed diets containing HCB
for 9-10 months, reduced weight gain and porphyria were observed in
females, but not males, receiving 80 or 160 mg HCB/kg feed
(approximately 4 or 8 mg HCB/kg body weight per day). A dose-related
increase in relative liver weights and in the hepatic content of HCB
was noted in both sexes. Hepatic enzyme activities and cytochrome
P-450 activities were increased in males administered 40 mg HCB/kg
feed or more. Exposure to 10 mg HCB/kg feed (approximately 0.5-0.6 mg
HCB/kg body weight per day) induced in vivo mixed-function oxidase
activity, as indicated by reductions in sleeping time for
pentobarbital and zoxazolamine exposure.
The carcinogenicity of HCB has been assessed in several bioassays
in rats, mice and hamsters. The following discussion is limited
principally to the four studies in which adequate numbers of animals
of both sexes were exposed for a sufficient length of time to more
than one dose level.
Cabral et al. (1977) and Cabral & Shubik (1986) reported a
statistically significant increase of liver cell tumours (hepatomas)
in groups of 30-60 male and female Syrian golden hamsters fed 50, 100
or 200 mg HCB/kg (4, 8 or 16 mg/kg body weight per day) HCB in their
diets for life. The incidence of "haemangioendotheliomas" of the liver
was significantly increased in both sexes at 200 mg/kg and in males at
100 mg/kg, and of alveolar adenomas of the thyroid in males at
200 mg/kg. (The latter finding is interesting in the light of reports
of excesses of thyroid neoplasms, or of enlargement of the thyroid, in
human populations with elevated exposures to HCB (section 8.1.)) The
authors reported that three of the hepatic "haemangioendotheliomas"
(which are non-invasive by definition) metastasized. It seems likely,
therefore, that these tumours were malignant, though misclassified.
In another study, HCB was administered in the diet to groups of
30 or 50 outbred male and female Swiss mice at concentrations of 0,
50, 100 and 200 mg/kg (0, 6, 12 and 24 mg/kg body weight per day) for
120 weeks (Cabral et al., 1979; Cabral & Shubik, 1986). In females
exposed to 200 mg/kg, a statistically significant increase in the
incidence of "liver cell tumours (hepatomas)" was noted. "Hepatomas"
were also elevated, though not significantly, in males at this dose
and in both sexes at 100 mg/kg. The number of tumour-bearing animals,
the latent period, and the multiplicity and size of tumours increased
with dose.
Arnold et al. (1985) and Arnold & Krewski (1988) investigated the
potential carcinogenicity to rats of combined in utero, lactational
and oral exposure to analytical grade HCB. Groups of 40 or more
weanling male and female Sprague-Dawley rats were fed diets containing
0, 0.32, 1.6, 8 or 40 mg HCB/kg. (Based on data supplied by the
author, mean doses for males were 0, 0.01, 0.06, 0.29 and 1.50 mg/kg
body weight per day and for females 0, 0.01, 0.07, 0.38 and 1.90 mg/kg
body weight per day). After 3 months, the F0 rats were bred, and 50
F1 pups of each sex were randomly selected from each group. From
weaning, the F1 animals were continued on the same diet for their
lifetimes (up to 130 weeks). In exposed F1 females, increased
incidences of neoplastic liver nodules and adrenal phaeochromocytomas
were noted at the highest dose. A significantly increased incidence of
parathyroid adenomas was noted in males receiving 40 mg HCB/kg in
their diet.
In a study by Lambrecht et al. (1983a,b; Ertürk et al., 1986),
groups of 94 weanling Sprague-Dawley rats were fed diets containing 0,
75 or 150 mg/kg (4 and 8 mg/kg body weight per day for males and 5 and
9 mg/kg body weight per day for females, respectively) for up to 2
years. Statistically significant increases in the incidence of
hepatomas/haemangiomas and of renal cell adenomas were noted at both
doses in animals of both sexes surviving beyond 12 months. Incidences
of hepatocellular carcinomas and bile duct adenomas/carcinomas were
also elevated in females at both doses. In female rats, significant
increases in the incidences of adrenal cortical adenomas at 75 mg/kg
and phaeochromocytomas at both doses were reported. Lambrecht et al.
(1983b) reported a leukaemia involving the thymus, spleen, liver and
kidney in rats exposed to HCB in this study, but did not present any
quantitative data. The results of this study were only reported in
summary form, with few details of the study protocol and results. In
addition, HCB was incorporated into the diet as a powder in this
study, raising the possibility that some of the effects observed may
have been in part attributable to the inhalation of aerosolized HCB.
High incidences of liver tumours have also been reported in some
more limited studies in which single dietary concentrations (100 or
200 mg/kg) were administered to small groups (i.e., between 4 and 15)
of females of three strains of rats (Smith & Cabral, 1980; Smith et
al., 1985b); in one strain (Fischer-344), hepatocellular carcinomas
were observed (Smith et al., 1985b). HCB has not, however, been
carcinogenic in several other studies in various strains of mice
(Theiss et al., 1977; Shirai et al., 1978; Smith et al., 1989),
perhaps as a result of the low doses, short durations of exposure
and/or small group sizes employed. Results were also negative in a
second study by Arnold et al. (1985), in which groups of 50 male
Sprague-Dawley rats were fed diets containing 40 mg HCB/kg in
conjunction with various levels of vitamin A for 119 weeks, indicating
the probable higher sensitivity of the two-generation carcinogenesis
bioassay.
Ertürk et al. (1982, 1986; Lambrecht et al., 1982a,b) examined
the tumorigenic activity of subchronic exposure to HCB in both sexes
of Swiss mice, Syrian golden hamsters and Sprague-Dawley rats at
dietary levels of 0, 100 and 200 mg/kg (mice) and 0, 200 and 400 mg/kg
(hamsters and rats) for 90 days. At day 91, 25 of 50 animals in each
group were sacrificed for histological examination, with the remainder
being sacrificed at 6-week intervals (up to 341, 361 and 424 days for
mice, hamsters and rats, respectively). The results of these studies
were reported in summary form only, and much of the quantitative data
were not presented. The authors reported that, as the experiment
progressed, treated animals developed hepatomas, bile duct adenomas,
renal adenomas and carcinomas, and lymphosarcomas of the thymus,
spleen, and lymph nodes. However, the only tumour and species for
which they presented clear evidence of a treatment-related increase in
incidence was for lymphatic tumours in mice (Ertürk et al., 1982).
Lymphatic and renal neoplasms were observed as early as the end of the
90-day period. It is not clear from these reports which tumours each
species developed or the dietary levels associated with the observed
effects, as well as other experimental details.
Results from a number of studies have indicated that HCB is a
co-carcinogen or promoter of cancer. Concomitant exposure to 50 mg
HCB/kg in diet (approximately 6 mg HCB/kg body weight per day)
enhanced the induction of liver tumours by polychlorinated terphenyl
(at 250 mg/kg diet) in male ICR mice (Shirai et al., 1978). Exposure
to HCB (100-200 mg/kg in diet (approximately 5-10 mg HCB/kg body
weight per day) or 1 mmole/kg i.p. at 1 and 5 weeks) promoted the
development of hepatocellular carcinomas and/or hepatic gamma-
glutamyltranspeptidase-positive foci initiated by diethylnitrosamine
in various strains of rats (Pereira et al., 1982; Herren-Freund &
Pereira, 1986; Stewart et al., 1989).
In some recent studies, the possible mechanisms by which HCB
induces tumours in animals have been investigated.
Bouthillier et al. (1991) presented the results of studies of
Sprague-Dawley rats exposed to 100 mg HCB/kg by gavage for periods of
several weeks, which indicated that the observed increase in renal
tumours in male Sprague-Dawley rats following exposure to HCB
(Lambrecht et al., 1983b; Ertürk et al., 1986) is related to protein
droplet nephropathy. The mechanism by which structurally diverse
hydrocarbons induce hyaline droplet nephropathy in male rats has been
well documented and involves accumulation of alpha-2u-globulin,
resulting in necrosis, regeneration and, in some cases, tumours. This
response is sex- and species-specific, and hence is unlikely to be
relevant to humans. This mechanism does not, however, explain the
increased (but lower) incidence of renal tumours in females also
reported by Lambrecht et al. (1983b).
Carthew & Smith (1994) hypothesized that some HCB-induced hepatic
tumours in rats may be produced by a non-genotoxic mechanism. They
noted that hepatotoxicity of HCB in rodents gives rise to peliosis and
necrosis with haemosiderosis, indicating that vascular damage has
occurred, and confirmed the presence of such damage in the liver of
chronically HCB-exposed rats by the identification of widespread
fibrin deposits, using an antibody to rat fibrin. These deposits
occurred in association with abundant haemosiderosis in hepatocytes
and areas of widened hepatic sinusoids. On this basis, it was
suggested that the formation of hepatomas and haemangiomas with
elements of peliosis could be the result of compensatory hyperplastic
responses to hepatocellular necrosis and the simultaneous loss of
hepatocellular cords, perhaps potentiated by the accumulation of iron
in the liver.
Mechanistic studies that address the relevance to humans of the
remaining tumour types induced in rodents by HCB have not been
identified.
7.4 Mutagenicity and related end-points
HCB has not been found to be genotoxic in most studies conducted
to date. HCB did not cause either frameshift or base pair substitution
mutations in Salmonella typhimurium at doses of as much as 10
mg/plate with or without metabolic activation, with both rat and
hamster liver activation systems, pre-incubation and plate
incorporation methods, and technical and 99.9% pure HCB (Haworth et
al., 1983; Górski et al., 1986; Siekel et al., 1991). A weak positive
response in S. typhimurium strain TA98 at 50 and 100 µg/plate was
reported by Gopalaswamy & Aiyar (1986) and Gopalaswamy & Nair (1992).
However, the authors also reported mutagenic activity for lindane, in
contrast to the results of other studies (e.g., Haworth et al., 1983).
Doses of up to 1000 µg/plate of HCB did not induce tryptophan
reversion or DNA damage in Escherichia coli strains WP2 and WP2uvrA
with or without metabolic activation (Siekel et al., 1991).
There have been reports of mutagenic activity for HCB in
eukaryotic cells in vitro, although these studies have limitations.
Guerzoni et al. (1976) reported a positive finding for methionine
reversion in Saccharomyces cerevisiae strain 632/4 exposed to HCB,
but Brusick (1986) did not consider the observed increase to meet
current standards of a positive response. In addition, only a single
dose level was used in that study, and there was no exogenous
metabolic activation. Kuroda (1986) reported that in cultured Chinese
hamster lung cells (V79), HCB did not induce OUAr mutations, but did
induce 8AGr mutations. However, both the magnitude of the increase
(which was small, roughly 1/105 survivors at the two highest doses)
and uncertain dose-response indicate that this response is open to
question.
Oral administration of as much as 221 mg HCB/kg body weight per
day to male rats for 5 or 10 days failed to induce dominant lethal
effects in two different studies (Khera, 1974; Simon et al., 1979),
although Simon et al. (1979) did observe a slight reduction in male
reproductive performance (numbers of females inseminated and
impregnated). Rumsby et al. (1992) reported that liver neoplasms that
developed in iron-overloaded C57Bl/10ScSn mice exposed for 18 months
to 0.01% HCB in the diet were not associated with a high frequency of
mutations in the Ha-ras proto-oncogene at codon 61. Only two mutations
were observed at different sites, from 23 preneoplastic and neoplastic
lesions examined, indicating that activation of the Ha-ras gene is not
an important event in the hepatocarcinogenicity of HCB in this test
system.
HCB has not been found to be clastogenic in the few available
studies in which this end-point has been examined. The compound did
not increase the frequency of sister chromatid exchanges in the bone
marrow of male mice given as much as 400 mg/kg body weight (by an
unspecified route), although the lack of detail in reporting the test
protocol and results limits the interpretation of this study (Górski
et al., 1986). HCB did not induce chromosomal aberrations in vitro
in cultured Chinese hamster fibroblast cells at concentrations as high
as 12 mg/ml, with or without metabolic activation (Ishidate, 1988), or
in human peripheral blood lymphocytes exposed to up to 0.1 mmol/litre
(Siekel et al., 1991). Treatment of rats with 1000 mg HCB/kg diet for
15 days was hepatotoxic, but did not cause early diploidization in
hepatocytes as measured by flow cytometry (Rizzardini et al., 1990).
The results of less specific assays also indicate that HCB does
not interact strongly with DNA, although there are two reports that
the compound binds, at low levels, to DNA. After incubating
hepatocytes isolated from phenobarbital-treated rats with 14C-HCB
(5 µM) for 20 h, Stewart & Smith (1987) reported the maximum amount of
radioactivity associated with DNA was < 9.9 × 10-5% of the substrate
added, and was only marginally above that of hepatocytes held at 4°C;
the authors considered this to be significantly lower than expected
for hepatocarcinogens. Gopalaswamy & Nair (1992) also reported a low
order of binding of HCB to DNA from the livers of rats exposed to
25 mg HCB/kg. Short-term exposure (<1 day) of rats to oral doses of
700 or 1400 mg/kg body weight (Kitchin & Brown, 1989) or to as much at
300 mg/kg body weight i.p. (Górski et al., 1986) did not cause hepatic
DNA damage, as measured by alkaline elution.
7.5 Reproductive and developmental toxicity
Relatively low doses of HCB have been found to affect some
reproductive tissues in female monkeys. Oral exposure of cynomolgus
monkeys to 0.1 mg/kg body weight per day in gelatin capsules for 90
days caused stratification of the ovarian germinal epithelium
(Babineau et al., 1991; Jarrell et al., 1993a). Higher dosages (1.0
and 10.0 mg/kg body weight per day) were associated with cellular
degeneration of this surface epithelium. The low dosage was associated
with ultrastructural as well as light microscopic changes in surface
epithelium (Babineau et al., 1991; Sims et al., 1991).
In ovarian follicles the low dose was associated with an
increased number of lysosomal elements in germ cells (Singh et al.,
1990a). The basal lamina was thickened. Higher dosages were associated
with greater degenerative changes in their cells and granulosa cells
(Singh et al., 1991, 1990b).
These studies demonstrated changes in ovarian tissues with no
other evidence of toxicity. In particular, the induction of
superovulation with human menopausal gondotrophin (HMG) in these
animals was associated with a normal estradiol response, oocyte
recovery, oocyte maturation, in vitro fertilization and early embryo
development (Jarrell et al., 1993a). These studies confirm the
findings of Iatropoulous et al. (1976) in which the administration of
8 to 128 mg/kg body weight (by gavage in 1% methylcellulose) for 60
days induced severe follicular degeneration in primordial germ cells,
pseudostratification of the ovarian surface epithelium, hepatic
degeneration and severe systemic toxicity in Rhesus monkeys.
In subsequent studies of similarly treated animals, the higher
doses were associated with reduced luteal phase progesterone and
blunted estradiol responses to HMG (Foster et al., 1992a,b). Reduction
in adrenal steroidogenesis occurred in ovariectomized rats in response
to exposure to HCB at concentrations of 1, 10 and 100 mg/kg body
weight for 30 days (Foster et al., 1995).
In contrast, the results of studies on a variety of species have
indicated that repeated exposure to HCB can affect male reproduction,
but only at relatively high doses. Mice exposed to 250 mg HCB per kg
feed (approximately 30 mg HCB/kg body weight per day) for 21 days had
reduced serum testosterone levels; based on the results of in vitro
tests, it was suggested that this was due to increased metabolism by
hepatic microsomal enzymes induced by HCB (Elissalde & Clark, 1979).
Histological changes in the testes (retarded sexual maturation) were
noted in pigs fed a diet yielding a dose of 50 mg HCB/kg body weight
per day for 90 days (den Tonkelaar et al., 1978). The mating index for
male rats receiving five consecutive daily gavage doses of 221 mg
HCB/kg body weight in corn oil was decreased compared to those
receiving 0 or 70 mg/kg body weight However, the fertility index for
the mated female rats (sperm positive smears) was not affected (Simon
et al., 1979).
As discussed in the following paragraphs, placental and
lactational transfer of HCB, demonstrated in a number of species, can
adversely affect both the fetus and nursing offspring. The lactational
route appears to be more important than placental transfer. Adverse
effects on suckling infants are generally observed more frequently,
and at lower doses, than are embryotoxic or fetotoxic effects.
Grant et al. (1977) conducted a four-generation study on female
(20/dose level) and male (10/dose level) weanling Sprague-Dawley rats
fed diets containing 0, 10, 20, 40, 80, 160, 320 or 640 mg HCB/kg
feed. The two highest doses caused some deaths in the F0 dams before
first whelping, and reduced the fertility index. Dietary levels of 160
mg/kg or more reduced litter sizes, increased the number of
stillbirths, and adversely affected pup survival. Similar effects were
seen at 80 mg/kg after the first two generations, while 40 mg/kg was
hepatotoxic to the F1a and F3a pups. A dietary level of 20 mg/kg
(approximately 1-1.2 mg/kg body weight per day) was designated as the
no-observed-effect level.
Arnold et al. (1985) fed groups of male and female Sprague-Dawley
rats from weaning on diets containing up to 40 mg HCB/kg. The rats
were then bred at 3 months, and the F1 pups were continued on the
same diet for their lifetimes. HCB had no effect on fertility, but pup
survival was significantly reduced in the 40 mg/kg group (calculated
doses of 1.50 and 1.90 mg/kg body weight per day for males and
females, respectively).
In other studies, maternal doses in the range from 1.4 to 4 mg/kg
given to rats and cats have been found to be hepatotoxic and/or
affected the survival or growth of nursing offspring. In some cases,
these or higher doses reduced litter sizes and/or increased numbers of
stillbirths (Mendoza et al., 1977, 1978, 1979; Hansen et al., 1979;
Kitchin et al., 1982).
Mink are particularly sensitive to the effects of prenatal and
perinatal exposure to HCB; the offspring of mink fed diets containing
concentrations as low as 1 mg/kg (approximately 0.16 mg/kg body weight
per day) for 47 weeks (prior to mating and throughout gestation and
nursing) had reduced birth weights and increased mortality (Rush et
al., 1983; Bleavins et al., 1984b).
The available data on the developmental toxicity of HCB are
limited. CD-1 mice administered 100 mg/kg body weight by gavage on
days 7-16 of gestation had a significantly increased incidence of
abnormal fetuses per litter, and one case of renal agenesis was
reported. Some cleft palates were produced, but they all occurred in
one litter. This dose also increased maternal liver-to-body weight
ratios and decreased fetal body weights (Courtney et al., 1976). In a
series of studies reported by Andrews & Courtney (1986), combined
in utero and lactational exposure of CD-1 mice and CD rats (strain
unclear, probably Sprague-Dawley) to HCB (mouse dams received 10 or
50 mg/kg body weight per day, and rats 10 mg/kg body weight per day,
by gavage during gestation) resulted in increases in body weight and
kidney weights of pups of both species, along with enlarged kidneys
and a few cases of hydronephrosis. Increased liver weights were
observed in rat pups, and the occurrence of abnormal kidneys was
sporadic, with no dose-response relationship in studies with mice.
Khera (1974) reported a significant increase in the incidence of
unilateral or bilateral 14th rib in litters of Wistar rats receiving
doses of 80 and 120 mg HCB/kg body weight during gestation, but
maternal toxicity (loss of body weight and neurological effects) and
reduced fetal weights were noted in animals in these groups. (It
should be noted that, based on the biological half-lives reported for
HCB in mammals (section 6.2), the concentration of HCB in the dams in
these studies would not have reached the maximum that might occur as a
result of intake over a longer period).
Neurobehavioural development was affected in the offspring of
rats exposed to 2.5 or 25 mg/kg body weight per day by gavage 2 weeks
prior to breeding. Pups in both treated groups were hyperactive (based
on tests of negative geotaxic reflex, olfactory discrimination, and
exploratory locomotor activity) at 6-20 days of age. Pups from the
high treatment groups showed reduced acoustic startle response at 23
days of age, but a significantly increased response at 90 days. These
doses did not affect learning (swim T-maze) or motor activity in older
offspring, nor maternal or fetal body weights, length of gestation,
number of pups/litter at birth, or number of days to eye opening
(Goldey & Taylor, 1992).
Lilienthal et al. (1996) recently reported HCB-induced effects on
neurobehavioural development of rat pups exposed both maternally and
through the diet (dams were exposed to 0, 8 or 16 mg HCB/kg diet for
90 days prior to mating and throughout gestation and nursing, after
which the offspring were fed the same levels for 150 days). Exposure
to HCB did not affect the mean body weight of the pups (except males
at 150 days of age), or the number of pups/litter, but did increase
the mean body weight of dam, and their liver-to-body weight ratios.
Schedule-controlled behaviour was affected at 8 and 16 mg HCB/kg diet
(0.64 and 1.28 mg/kg body weight per day), as indicated by a dose-
related decrease in post-reinforcement pause at the end of the
experiment. Exploratory locomotor activity, open field behaviour at 21
days of age, and active avoidance learning at 90 days of age were
unaffected.
7.6 Immunotoxicity
The results of a number of studies have indicated that HCB
affects the immune system, with immunosuppressive effects in mice and
immunostimulatory effects in rats (summarized by Vos, 1986).
Balb/C mice exposed to 5 mg HCB/kg diet (approximately 0.6 mg/kg
body weight per day) for 3 to 18 weeks were more susceptible to
Leishmania infection (Loose, 1982) and had reductions in resistance
to a challenge with tumour cells and in the cytotoxic macrophage
activity of the spleen (Loose et al., 1981). Barnett et al. (1987)
reported that Balb/C mice exposed to maternal doses of 0.5 or 5 mg
HCB/kg body weight per day in utero and through nursing had severe
depression of the delayed-type hypersensitivity response to a contact
allergen (oxazolone). In a number of studies, exposure of mice to
diets containing 167 mg HCB/kg in diet (approximately 20 mg HCB/kg
body weight per day) for several weeks depressed humoral immunity,
cell-mediated immunity and host resistance (Vos, 1986; Carthew et al.,
1990).
In rats or rhesus monkeys with oral exposure to between 3 and
120 mg HCB/kg body weight per day for periods from 3 weeks to 6 months
in various studies, proliferative histopathological effects in the
thymus, spleen, lymph nodes, and/or lymphoid tissues of the lung have
been observed (Kimbrough & Linder, 1974; Iatropoulos et al., 1976;
Goldstein et al., 1978; Vos et al., 1979a,b; Kitchin et al., 1982).
Gralla et al. (1977) observed that long-term exposure to 1 mg HCB/day
(equivalent to a dose at the start of the experiment of roughly
0.12 mg/kg body weight per day) caused nodular hyperplasia of the
gastric lymphoid tissue in beagle dogs.
In rats, prominent changes following dietary exposure to HCB
include elevated IgM levels and an increase in the weights of the
spleen and lymph nodes. Histopathologically, the spleen shows
hyperplasia of B-lymphocytes in the marginal zone and follicles, while
lymph nodes show an increase in proportions of high endothelial
venules, indicative of activation. High endothelial-like venules are
induced in the lung, as are accumulations of macrophages. Functional
tests revealed an increase in cell-mediated immunity, as measured by
DTH reactions, a notable increase in primary and secondary antibody
response to tetanus toxoid, and decreased NK activity in the lung (Vos
et al., 1979a,b). Stimulation of humoral and cell-mediated immunity
occurred even at dietary levels as low as 4 mg HCB/kg (approximately
0.2 mg HCB/kg body weight per day); at such a dose conventional
parameters for hepatotoxicity were unaltered (Vos et al., 1983).
Therefore, the developing immune system of the rat seems to be
particularly vulnerable to the immunotoxic action of HCB.
More recent studies indicate that HCB may cause autoimmune-like
effects in the rat. Wistar rats treated with HCB had elevated levels
of IgM, but not IgG, against the autoantigens single-stranded DNA,
native DNA, rat IgG (representing rheumatoid factor), and bromelain-
treated mouse erythrocytes (that expose phosphatidylcholine as a major
autoantigen). It has been suggested that HCB activates a recently
described B cell subset committed to the production of these
antibodies (Schielen et al., 1993). The role of these autoantibodies
is still a matter of controversy. Increased levels have been
associated with various systemic autoimmune diseases, but a protective
role of these autoantibodies against development of autoimmune disease
has been postulated as well. Interesting in this respect are the
observations that HCB had quite opposite effects in two different
models of autoimmune disease in the Lewis rat. HCB treatment severely
potentiates allergic encephalitis elicited by immunization with myelin
in complete Freund's adjuvant, while it strongly inhibits the
development of arthritic lesions elicited by complete Freund's
adjuvant as such (Van Loveren et al., 1990).
A possible relation between the immunomodulatory properties of
HCB and HCB-induced skin lesions, attributed in the literature to the
porphyrinogenic action of HCB, was recently indicated. In rats treated
with a combination of HCB and triacetyloleandomycin (TAO, a selective
inhibitor of cytochrome P-450IIIa), porphyria was greatly reduced.
Remarkably, combined treatment with HCB and TAO did not substantially
affect the incidence and severity of skin lesions. In addition, TAO
did not influence the immunomodulatory effect of HCB, including the
formation of antibodies. From these findings it has been suggested
that an immunological component underlies, at least in part, the
HCB-induced skin lesions in the rat (Schielen et al., 1995).
8. EFFECTS ON HUMANS
8.1 General population exposure
Numerous reviews have been published of an accidental poisoning
incident in Turkey that occurred in 1955-1959 as a result of
HCB-treated wheat grain (distributed by the Turkish government for
planting purposes) being ground into flour and made into bread
(Schmid, 1960; Cam & Nigogosyan, 1963; Dogramaci, 1964; Peters, 1976;
Courtney, 1979; Peters et al., 1982; US EPA, 1985a; Gocmen et al.,
1989). In this incident, more than 600 cases of porphyria cutanea
tarda (PCT) were clinically identified, and it was estimated that as
many as 3000-5000 persons were affected, with a mortality of 10%. The
condition developed primarily in children 4-14 years of age (roughly
80% of cases), occurring infrequently in adults and rarely in children
under 4 years of age. In a number of reports, it has been suggested
that males developed the condition in higher proportion than females.
However, Dogramaci et al. (1962) demonstrated that the sex ratio was
skewed in favour of males in both the affected and unaffected
populations. In addition to disturbances in porphyrin metabolism
(excretion of porphyrins and porphyrin precursors was greatly
increased), clinical manifestations included skin lesions (erythema,
bullae), ulcerations and resultant scarring, friable skin,
hyperpigmentation, hypertrichosis, enlarged liver, weight loss,
enlargement of the thyroid gland and lymph nodes, neurological
effects, and a characteristic port wine colour of the urine (from
increased excretion of porphyrins). In roughly half the cases,
osteoporosis of extremities, deformation of the fingers or arthritis
was also noted. The dermatological lesions, which occurred on the
exposed parts of the body, particularly the face and hands, were often
precipitated by sunlight. They tended to remit in winter and relapse
during the spring and summer (Peters, 1976; Peters et al., 1982). The
estimated dose was 50-200 mg/day for a number of months before
manifestations of the disease became apparent (Cam & Nigogosyan,
1963); the basis for this estimate was not presented, however, making
exposure calculations unreliable for this population. In 20- to 30-
year follow-ups of exposed individuals, neurological, dermatological
and orthopaedic abnormalities persisted, and there were elevated
levels of porphyrins in excreta of some individuals (Peters et al.,
1982; Peters et al., 1986; Gocmen et al., 1989).
In this incident, a disorder called "pembe yara" or "pink sore"
was described in infants of mothers who either had PCT or had eaten
HCB-contaminated bread. These infants developed characteristic pink
cutaneous lesions, and often had fevers, diarrhea, vomiting, weakness,
convulsions, enlarged livers and progressive wasting. It is noteworthy
that PCT was not observed in these children (Cam, 1960; Peters et al.,
1982). At least 95% of these children died within a year of birth, and
in many villages no children between the ages of 2-5 years survived
during the period 1955-1960. Elevated concentrations of HCB (levels
were not quantified at the time, but the average concentration in milk
from 56 porphyric mothers, 20-30 years after the incident, was
510 ng/g on a fat basis) were found in the mothers' milk and cessation
of breast-feeding slowed the deterioration of infants with this
disorder (Peters et al., 1966; Gocmen et al., 1989).
No adequate epidemiological studies of cancer in populations
exposed to HCB in the environment were found in the literature. In
long-term follow-up of the Turkish poisoning victims with porphyria
(Peters et al., 1982; Cripps et al., 1984; Gocmen et al., 1989) there
was no evidence of increased cancer incidence, although these studies
were not designed to evaluate this end-point, and only a small
fraction of the exposed people was followed up. There was a high
frequency of enlarged thyroids in the Turkish poisoning victims (27%
of men and 60% of women, compared to an average of 5% in the area
(Peters et al., 1982)), but Gocmen et al. (1989) reported that they
observed no malignant tumours of the liver or thyroid in 252 of the
poisoning victims. In three patients who underwent thyroidectomy,
histopathological examination indicated that the enlargement was due
to colloidal goitre.
Grimalt et al. (1994) reported a small ecological study of cancer
incidence (129 cases in all) in the inhabitants of a village in Spain
located near a chlorinated solvents factory. There were statistically
significant excesses of thyroid neoplasms and soft-tissue sarcomas in
males, compared with the province as a whole, although these were
based on only 2 and 3 cases, respectively. The exposures experienced
by this population were somewhat unclear. Levels of HCB in ambient air
and in the sera of volunteers were much higher in the village than in
Barcelona (means of 35 ng/m3 versus 0.3 ng/m3 and 26 µg/litre versus
4.8 µg/litre, respectively), but the authors presented evidence that
historical exposures had been much higher and indicated that all of
the males with cancer for whom there were occupational histories had
worked in the factory. Ambient air monitoring revealed that there were
exposures to a variety of other compounds, including polychlorinated
biphenyls, p,p'DDE, chloroform, carbon tetrachloride,
trichloroethylene and tetrachloroethylene, but at similar or lower
levels than in the reference community.
8.2 Occupational exposure
There have been case reports of workers developing PCT as a
result of direct contact with HCB (Courtney, 1979; Currier et al.,
1980), although there was no association between exposure to HCB and
PCT in three cross-sectional studies of very small populations of
exposed workers (Morley et al., 1973; Burns et al., 1974; Currier et
al., 1980). There was no evidence of cutaneous porphyria in a cross-
sectional study of the general population in Louisiana, USA, exposed
to HCB through the improper transport and disposal of hex waste;
however, plasma concentrations of HCB were significantly correlated
with levels of coproporphyrin in urine and of lactic dehydrogenase in
blood (Burns & Miller, 1975).
Available epidemiological studies on the carcinogenicity of HCB
in occupationally exposed humans are restricted to one study of a
cohort of 2391 magnesium metal production workers in Norway. Although
the incidence of lung cancer was significantly elevated compared to
that of the general population, workers were exposed to numerous other
agents in addition to HCB, including coal tar, asbestos and dust of
metal oxides and chlorides (Heldaas et al., 1989). Selden et al.
(1989) reported a case of hepatocellular carcinoma in a 65-year-old
man who had been employed for 26 years in an aluminum smelting plant,
where he had potential exposure to a range of substances, including
HCB, other chlorobenzenes, chlorophenols, dioxins and furans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Data on the acute and chronic ecotoxicity of HCB are available
for species from a number of trophic levels, including protozoans,
algae, invertebrates and fish, for both the freshwater and marine
environments. With reference to terrestrial organisms, toxicity data
are available only for birds and mammals (the results of studies in
mammals are summarized in chapter 7). Since HCB is nearly insoluble in
water, and tends to partition from water to the atmosphere, the
substance is lost rapidly from open-test solutions. Hence, it is
difficult to maintain test concentrations for a sufficient time to
establish concentration-effects profiles for aquatic organisms.
Furthermore, HCB tends to bind to suspended solids in the water column
and thus may not be bioavailable to test organisms. This discussion of
the toxicity of HCB to aquatic organisms will therefore focus on tests
conducted under flow-through conditions, static renewal conditions, or
using closed vessels with minimal headspace. In addition, no
consideration has been given to tests in which concentrations of HCB
were well above its solubility in water (5 µg/litre at 25°C).
9.1 Short-term exposure
9.1.1 Aquatic biota
Of four freshwater algal species tested, only one, Chlorella
pyrenoidosa, was affected by concentrations of HCB in water at or
below its limit of aqueous solubility. Reduced production of
chlorophyll, dry matter, carbohydrate and nitrogen was observed for
C. pyrenoidosa after exposure to a nominal concentration of
1 µg/litre HCB for 46 h in a static-closed system (Geike & Parasher,
1976a). A no-observed-effect concentration (NOEC) was not determined
in this study.
At concentrations equal to its aqueous solubility in water
(5 µg/litre), HCB was not lethal to the freshwater water flea
Daphnia magna in a flow-through test in which concentrations of HCB
were measured (Nebecker et al., 1989). In 96-h flow-through tests on
marine invertebrates, exposure to HCB caused 13% mortality in pink
shrimp ( Penaeus duorarum) at a measured concentration of 7 µg
HCB/litre, and 10% mortality in grass shrimp ( Palaemonetes pugio) at
17 µg/litre. The NOEC values in these species were 2.3 µg/litre and
6.1 µg/litre, respectively (Parrish et al., 1974). In a static-closed
system, there was a 10% reduction in reproduction of the ciliate
protozoan Euplotes vannus after exposure to a nominal concentration
of 10 µg/litre HCB for 48 h (Persoone & Uyttersprot, 1975).
The available data on freshwater fish species indicated no
harmful effects at concentrations at or near the limit of solubility
of HCB in water during acute exposure (Call et al., 1983; Ahmad et
al., 1984). In the only available study for marine fish species, there
were no effects on mortality in sheepshead minnow ( Cyprinodon
variegatus) after flow-through exposure to a measured concentration
of 13 µg/litre HCB for 96 h (Parrish et al., 1974).
Limited data are available concerning the toxic effects of HCB in
sediment on freshwater and marine biota. In a 96-h sediment toxicity
test on the marine shrimp, Crangon septemspinosa, no mortality was
observed at the highest concentration of HCB tested, 300 µg/litre
(McLeese & Metcalfe, 1980).
Several studies have confirmed that there is a relatively
constant body residue associated with acute lethality in freshwater
fish, invertebrates and algae exposed to mono-to-pentachlorobenzenes
(McCarty et al., 1992a; Ikemoto et al., 1992). The acute LC50
critical body residue for chlorobenzenes is 2 µmol/g wet weight, or
569.6 µg/g wet weight for HCB, assuming that HCB has the same mode of
action as the other chlorobenzenes (McCarty et al., 1992b).
9.1.2 Terrestrial biota
The LD50 for HCB in herring gull ( Larus argentatus) embryos
injected on day 4 and tallied on day 25 was 4.3 µg/g body weight
(Boersma et al., 1986). At a dose of 1.5 µg/g body weight, there were
significant reductions in embryonic weight. Five-day LC50 values
(i.e., 5 days of HCB-containing diet followed by 3 days of untreated
diet) were 617 µg/g diet for 10-day-old ring-necked pheasants
( Phasianus colchicus) and > 5000 µg/g diet for 5-day-old mallards
( Anas platyrhynchos) (Hill et al., 1975). Induction of porphyria has
been observed in studies of Japanese quail following administration of
500 µg HCB/g body weight per day for between 5 and 10 days either in
food or via intraperitoneal injection (Buhler & Carpenter, 1986;
Lambrecht et al., 1988).
9.2 Long-term exposure
9.2.1 Aquatic biota
Growth of cultures of the alga Chlorella pyrenoidosa was
increased by exposure for 3 months to a nominal concentration of 1 µg
HCB/litre (Geike & Parasher, 1976b), while that of the protozoan
Tetrahymena pyriformis was decreased after a 10-day exposure to the
same concentration (Geike & Parasher, 1976b).
After exposure to 5 µg HCB/litre for 10 days in a static-renewal
system, crayfish ( Procambarus clarki) experienced damage to the
hepatopancreas (Laseter et al., 1976). The fertility of Daphnia
magna was reduced by 50% after exposure for 14 days to a measured
concentration of 16 µg/litre HCB in a static-closed system (Calamari
et al., 1983). Significantly increased mortality was observed in
amphipods, Gammarus lacustris, exposed to a measured concentration
of 3.3 µg HCB/litre for 28 days under flow-through conditions
(Nebecker et al., 1989). However, the results of this study indicated
a weak-dose response relationship. In two other flow-through studies,
there were no effects on survival, growth or reproduction of the
amphipod Hyallela azteca and the worm Lumbriculus variegatus at a
measured concentration of 4.7 µg HCB/litre (Nebecker et al., 1989).
In several studies, fathead minnows ( Pimephales promelas) and
rainbow trout ( Oncorhynchus mykiss) experienced no mortality or
effects on growth after exposure to levels of HCB approaching its
aqueous solubility (Ahmad et al., 1984; Carlson & Kosian, 1987; US
EPA, 1988; Nebecker et al., 1989). However, Laseter et al. (1976)
reported liver necrosis in large-mouth bass ( Micropterus salmoides)
after an exposure for 10 days to 3.5 µg HCB/litre under flow-through
conditions.
Guidelines for the protection and management of aquatic sediment
quality in Ontario, Canada (Persaud et al., 1991) have given a no-
observed-effect level (NOEL), a lowest-observed-effect level and a
severe-effect level for a variety of contaminants. The values given
for HCB are 10 ng/g dry weight, 20 ng/g dry weight and 24 000 ng/g
organic carbon. The partitioning approach was used to determine the
lowest-observed-effect level, whereas the severe-effect level was more
dependent on the screening level concentration approach. The
limitation of both approaches is that they are unable to separate the
biological effects that are due to a combination of contaminants; thus
while ecotoxicological effects can be established, these cannot be
attributed to any one chemical contaminant. This is a very serious
limitation since virtually all sediments are contaminated with a wide
variety of pollutants, and there is no indication that HCB was the
dominant pollutant.
Quantitative structure-activity relationships (QSAR) were used to
estimate the narcotic toxicity for 19 species to predict NOELs (Van
Leeuwen et al., 1992). The NOELs for water, sediment and residues in
biota were predicted only on the basis of the octanol/water partition
coefficient and relative molecular mass. The QSAR-derived level for
HCB in sediments was 5814 ng/g dry weight (20.4 nmol/g in the
reference) for sediments with 5% total organic carbon content. The
adjusted value for sediment with 1% total organic carbon content is
1163 ng/g. There is no experimental verification of these
calculations. Thus, no firm evidence is available on the critical
levels of HCB in sediments.
9.2.2 Terrestrial biota
In adult Japanese quail ( Coturnix japonica) fed diets
containing HCB for 90 days, mortality was increased at 100 µg HCB/g
in diet, and hatchability of eggs was significantly reduced at 20 µg/g
(Vos et al., 1971, 1972). At 5 µg/g, increased liver weight, slight
liver damage and increased faecal excretion of coproporphyrin were
observed. Eurasian kestrels ( Falco tinnunculus) fed mice containing
200 µg HCB/g fresh body weight for 65 days had significant weight
loss, ruffling of feathers, tremors, increased liver weight and
decreased heart weight (Vos et al., 1972).
The available long-term toxicity data for mammals are discussed
in section 7.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on estimates of mean exposure from various media (section
5.2), the general population is exposed to HCB principally in food
(mean intakes for adults range from 0.0004 to 0.0028 µg/kg body weight
per day). Intakes are estimated to be considerably less for ambient
air (3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day) and
drinking-water (2.2 × 10-6 to 4.4 × 10-5 µg/kg body weight per day).
Based on these intakes, it is estimated that the total average daily
intake of HCB from food, air and drinking-water is between 0.0004 and
0.003 µg/kg body weight per day.
Data on levels of occupational exposure to HCB are limited but
indicate that workers in some industries may be exposed to higher
levels of HCB than the general population, particularly in the
manufacture of chlorinated solvents, and in the manufacture and
application of chlorinated pesticides contaminated with HCB. In some
instances inappropriate manufacturing and waste management practices
may expose nearby populations to higher levels of HCB than the general
population. Exposures may also be elevated in some indigenous
subsistence populations, particularly those that consume large
quantities of food species near the top of the food chain.
Owing to the elimination of HCB in breast milk, mean intakes by
nursing infants are estimated to range from < 0.018 to 5.1 µg/kg body
weight per day in various countries (see section 5.2.4 and Table 8).
10.1.2 Health effects
Available data on the effects of HCB in humans are limited
principally to those of people exposed in an accidental poisoning
incident that occurred in Turkey between 1955 and 1959. More than 600
cases of porphyria cutanea tarda (PCT) were observed, and infants of
exposed mothers experienced cutaneous lesions, clinical symptoms and
high mortality. It has been estimated that victims were exposed to an
estimated dose of 50-200 mg HCB/day for an undetermined, but extended,
period of time. However, the basis of this estimate was not provided,
making exposure calculations unreliable for this population. Studies
of the carcinogenicity of HCB in humans are limited to two small
epidemiological studies of cancer incidence in populations with poorly
characterized exposure to HCB as well as to numerous other chemicals.
No excesses of neoplasms have been reported in long-term follow-up
studies of the people with porphyria in the incident in Turkey, but
only a small fraction of the population was followed-up, and these
studies were not designed specifically to assess neoplastic end-
points.
Hence, the available data on humans are inadequate to serve as a
basis for assessment of effects from exposure to HCB. The remainder of
this evaluation is, therefore, based on studies in animals.
Based on the studies reviewed in section 7, the critical effects
induced by HCB in experimental animals comprise both non-neoplastic
and neoplastic effects.
With respect to non-neoplastic effects, repeated exposure to HCB
has been found to cause a wide range of non-neoplastic effects in
several species of animals, with similar lowest-observed-effect-levels
(LOELs) and no-observed-effect-levels (NOELs) for a number of end-
points (see Table 9). In these studies, effects reported have included
those on the liver in pigs and rats, on calcium metabolism in rats, on
ovarian histopathology in monkeys, on immune function in mice and
rats, on neurotransmitter levels in the hypothalamus of mink, on
postnatal survival in mink, and on neurobehavioural development in
rats. The range over which the various effects have been observed is
quite narrow; the lowest LOELs compiled in Table 9 range from 0.1 to
0.7 mg/kg body weight per day, while the lowest NOELs range from 0.05
to 0.07 mg/kg body weight per day.
Based on the induction of a variety of tumours in hamsters, rats
and mice exposed by ingestion, there is sufficient evidence that HCB
is carcinogenic in animals. The available evidence indicates that HCB
has little or no genotoxic activity and is therefore unlikely to be a
direct-acting (genotoxic) carcinogen. However, the Task Group noted
that tumours, some of which were malignant, have been induced in
multiple species, at multiple sites, in some instances at doses that
were not overtly toxic in other respects and that are within an order
of magnitude of those that produce more subtle toxicological effects,
or following subchronic exposure. Although there is some evidence to
suggest that HCB may cause cancer by indirect mechanisms, the evidence
is not definitive at this time and does not address all tumour sites.
Table 9. No-observed-effect and lowest-observed-effect levels (NOELs and LOELs) in mammals exposed to HCB
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Mouse Depressed delayed-type hypersensitivity - 0.5a Barnett et al.
response to oxazolone in mice exposed (1987)
to HCB in peanut butter in utero
(throughout gestation) and via nursing
to 45 days of age (section 7.6)
Mouse Increased susceptibility to Leishmania - 0.6 Loose et al. (1981);
infection, and reductions in resistance Loose (1982)
to a challenge with tumour cells and in
the cytotoxic macrophage activity of the
spleen in mice with subchronic exposure
to HCB in diet (section 7.6)
Rat Alterations in Ca metabolism (increased 0.07 0.7 Andrews et al.
serum 1,25-dihydroxy-vitamin-D3 levels, (1989, 1990)
reduced Ca excretion, alterations in
femur density, bone morphometry and
strength), increased liver weights, with
subchronic gavage exposure to HCB
(section 7.2)
Rat Increased cell-mediated and humoral - 0.2a Vos et al. (1983)
immune function, intraalveolar
macrophage accumulation, microsomal
ethoxyresorufin-O-deethylase activity,
in rats exposed to HCB in utero, via
nursing and in the diet to 5 weeks of
age (section 7.6)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Rat Increased organ weights (heart, brain 0.05-0.07 0.27-0.35 Arnold et al. (1985);
and liver) in F0 males, compound- Arnold & Krewski (1988)
related histological changes in liver
of both sexes of F1 rats with long-term
exposure to HCB in diet (section 7.3)
Rat Ultrastructural changes in livers 0.05-0.06 0.25-0.30 Mollenhauer et al.
(proliferation of SER, altered (1975, 1976)
mitochrondria, increase in numbers of
storage vesicles) of rats with long-term
exposure to HCB in diet (section 7.3)
Rat Induction of in vivo mixed-function - 0.5-0.6 Grant et al. (1974)
oxidase activity in rats with long-term
exposure to HCB in diet (section 7.3)
Rat Dose-related decrease in the post- - 0.64 Lilienthal et al.
reinforcement pause (PRP) after schedule- (1996)
controlled operant conditioning of rats
exposed to HCB in utero, through nursing,
and up to post-natal day 150
Mink Increased serotonin concentrations in - 0.16a Rush et al. (1983);
hypothalamus of mink dams with long-term Bleavins et al. (1984a,b)
dietary exposure to HCB, decreased
dopamine levels in hypothalamus, reduced
birth weights, and increased mortality
to weaning in mink kits with in utero
plus lactational exposure to HCB
(sections 7.3, 7.5)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Dog Nodular hyperplasia of gastric lymphoid - 0.12 Gralla et al.
tissue in beagles with long-term (1977)
exposure to HCB in gelatin capsules
(section 7.6)
Pig Increased urinary coproporphyrin and 0.05 0.05 Den Tonkelaar et al.
microsomal liver enzyme activity in (1978)
pigs with subchronic exposure to HCB
in diet (section 7.2)
a Doses reported are those received by dams
10.1.3 Approaches to risk assessment
The following is provided as a potential basis for derivation of
guidance values. Since ingestion is by far the principal route of
exposure and since the toxicological data for other routes of
administration are insufficient for evaluation, only the oral route is
addressed here, though the ultimate objective should be reduction of
total exposure from all routes.
Based on the scientific evaluation of the data for the non-
neoplastic and neoplastic end-points, two possible approaches to
develop health-based guidance values were suggested.
10.1.3.1 Non-neoplastic effects
The approach for non-neoplastic effects assumes a threshold for
these effects and is based on the use of the NOAEL or NOEL and an
uncertainty factor that takes account of interspecies and
interindividual variation in sensitivity to the substance, as well as
the quality of the available studies and the severity of effect.
The available data are sufficient to develop a Tolerable Daily
Intake (TDI) for HCB. The lowest reported NOELs and LOELs for several
different types of effects, such as those on the liver in rats and
pigs, calcium metabolism in rats, ovarian morphology in monkeys,
immune function in rats and mice, neurobehavioural development in rats
and perinatal survival in mink, fall within a very small range (Table
9). Based on the lowest reported NOELs included in the table
(approximately 0.05 mg/kg body weight per day based primarily on
hepatic effects observed in a subchronic study in pigs and in chronic
studies in rats), a TDI of 0.17 µg/kg body weight per day has been
derived for non-neoplastic effects, by incorporating an uncertainty
factor of 300 (x 10 for intraspecies variation; × 10 for interspecies
variation, × 3 for severity of effect). A factor of 3 for severity of
effects was chosen as HCB causes i) multiple non-neoplastic effects in
several species, and ii) LOELs for a number of end-points for which
NOELs have not been determined are very close to the NOEL, from the
critical studies, of 0.05 mg/kg body weight per day. However, it is
fully realized that national authorities may choose other end-points
or uncertainty factors depending upon data evaluation and future
scientific findings.
10.1.3.2 Neoplastic effects
The approach for neoplastic effects is based on the Tumorigenic
Dose5, or TD5 i.e., the intake or exposure associated with a 5%
excess incidence of tumours in experimental studies in animals (IPCS,
1994). This is a benchmark approach in which the TD5 is calculated
directly from the experimental data rather than using the upper or
lower confidence limits. Uncertainty factors are then applied to the
TD5 to obtain a guidance value. The choice of uncertainty factors is
based on the level and nature of mechanistic data available, the
quality of the database, the tumour pattern, the dose-response
relationship, and the experimental model chosen. The final value will
reflect the degree of certainty one has with the available
information.
For the purpose of indicating the magnitude of risk of HCB, the
two-generation study in rats has been selected, owing to its relevance
to the exposure of the general human population, as the design of this
study involved exposure to relatively low concentrations of HCB in the
diet (including in utero and lactational exposure). Moreover, tumour
pathology was inadequately reported in the available studies in
hamsters and mice, and there is some concern that in the other
adequate study in rats, there may also have been exposure by
inhalation to some HCB that was incorporated in the diet as a powder.
The TD5 value was calculated from the results of the two-
generation study in rats using a multistage model (Crump & Howe,
1982). The tumour incidences in the pups were analysed in the same
manner as data from a single-generation study, owing to the lack of
information on individual litters. On this basis, the TD5 values
range from 0.81 mg/kg body weight per day for neoplastic liver nodules
in females to 2.01 mg/kg body weight per day for parathyroid adenomas
in males. The Task Group decided that the most sensitive end-point
(neoplastic nodules of the liver) would be used in its analysis. In
calculating the suggested guidance value, it was agreed to use an
uncertainty factor of 5000, based on consideration of the insufficient
mechanistic data. The TD5 was divided by this uncertainty factor to
arrive at the suggested guidance value of 0.16 µg/kg body weight per
day. However, it is fully realized that national authorities may
choose other end-points or uncertainty factors depending upon data
evaluation and future scientific findings.
Although infants may have a high intake of HCB via breast milk
for a short time, the TD5 and TDI were considered to be protective of
the health of this population (unless there are extreme exposures),
because one of the long-term studies used in deriving these values
included lactational exposure. However, it should be noted that the
TD5 and TDI values derived above should not be compared directly with
intakes from breast milk by nursing infants, since the guidance values
are based on a lifetime intake, whereas the duration of breast-feeding
is relatively short.
10.2 Evaluation of effects on the environment
HCB is widely distributed in the environment, by virtue of its
mobility and resistance to degradation, although slow photodegradation
in air (half-life of approximately 80 days) and microbial degradation
(half-life of several years) do occur. It has been detected in air,
water, sediment, soil and biota from around the world. HCB is a
bioaccumulative substance (BCF values range from 375 to > 35 000),
and biomagnification of HCB through the food chain has been reported.
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa. In longer-term studies, the growth of sensitive freshwater
algae and protozoa was affected by a concentration of 1 µg/litre,
while a concentration of approximately 3 µg/litre caused mortality in
amphipods and liver necrosis in largemouth bass. The concentrations of
HCB in surface waters around the world are much lower than these
effect levels (3 to 5 orders of magnitude lower), except in a few
extremely contaminated localities.
Injection studies in eggs have shown that tissue levels of
1500 ng/g wet weight reduce embryo weights in herring gulls (lowest
dose tested). No studies were available to establish a NOAEL. For many
bird species, reduced embryo weights are associated with lower
survival of chicks. This effect level is within an order of magnitude
of the levels measured in the eggs of sea birds and raptors from a
number of locations from around the world, suggesting that present
levels of HCB in certain locations may harm embryos of bird species.
Experimental studies on mink indicate that they are sensitive to
the toxic effects of HCB; long-term ingestion of diets containing
1000 ng HCB/g (the lowest dose tested) increased mortality, decreased
birth weights of offspring exposed in utero and via lactation, and
altered levels of neurotransmitters in the hypothalamus of dams and
their offspring. No studies were available to establish a NOAEL. This
dietary effect level is only a few times higher than the
concentrations of HCB measured in various species of fish from a
number of industrialized locations from around the world, suggesting
that present levels of HCB in fish species from certain locations may
adversely affect mink and perhaps other fish-eating mammals.
11. RECOMMENDATION FOR PROTECTION OF HUMAN HEALTH AND THE ENVIRONMENT
a) Alternatives should be found for any present uses of HCB.
b) It is important to reduce the environmental burden of HCB by:
(i) identifying remaining sources and quantities of release to
the environment from these sources, including point source
emissions, waste disposal sites and production facilities;
(ii) applying appropriate manufacturing and waste disposal
practices in order to decrease levels of HCB in the
environment.
c) Human monitoring of HCB in blood and breast milk should be
undertaken to develop data representing exposure of the general
population, in order to identify highly exposed populations and
potential sources, and to enable interpretation of individual
results.
d) In order to gauge the efficacy of control measures it would be
valuable to monitor environmental levels and effects in locations
where levels are higher than the global average.
e) Neonatal effects in humans and other species have been associated
with ingestion of high doses of HCB through breast milk. It is
recommended that techniques be developed to assess appropriately
the risk to infant health from exposure to HCB and related
compounds in breast milk.
12. FURTHER RESEARCH
12.1 Environment
a) To improve the database available for environmental risk
assessment, it is considered important to establish a NOEL for
the serious reproductive effects seen in mink at dietary levels
approaching those found in certain locations.
b) Since HCB is persistent in soil and sediment, it would be
valuable to perform biodiversity experiments with HCB-treated
soil and sediment.
12.2 Human health
a) Based on the effects of low doses of HCB on ovarian tissues in
primates, involving disorders of germ cells and the ovarian
surface epithelium, the following is recommended:
(i) exposed populations should be studied for relevant
reproductive human outcomes of interest, particularly, fetal
loss and ovarian cancer;
(ii) reproductive tissues such as ovarian follicular fluid should
be included in human monitoring studies on HCB levels and/or
effects.
b) In order to decrease uncertainty in the risk assessment of HCB
and related compounds, research into the primary mechanism(s) of
action for tumorigenic, thyroid, reproductive, porphyrigenic,
neurotoxic and immunological effects of HCB should be undertaken.
c) Preliminary evidence suggests that HCB acts, at least in part,
through Ah receptor-linked mechanisms. This should be evaluated
more fully and compared to other polyhalogenated aromatic
chemicals for which a wealth of data are already available.
d) Given the toxicity of HCB and the few data for humans,
multicentre longitudinal studies of highly exposed human
populations should be undertaken. End-points of interest should
cover toxicokinetics (e.g., half-life), thyroid function,
porphyrin metabolism, reproductive outcomes (e.g., fetal losses),
and cancer. Nursing infants from these populations should be
followed to assess immunological and neurobehavioural
development.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
HCB as a Group 2B carcinogen (possibly carcinogenic to humans) based
on inadequate evidence for carcinogenicity to humans and sufficient
evidence for carcinogenicity to animals (IARC, 1987).
A drinking-water guideline of 1 µg/litre was developed for HCB
based on an evaluation of the production of liver tumours in female
rats and applying the linearized multistage model to calculate an
excess life-time cancer risk of 10-5 (WHO, 1993).
A conditional acceptable daily intake of 0.6 µg HCB/kg body
weight was developed by the Joint FAO/WHO Joint Meeting on Pesticide
Residues in Food (FAO/WHO, 1975). This recommendation was withdrawn in
1978 (FAO/WHO, 1978).
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the Legal File of
the International Register of Potentially Toxic Chemicals (IRPTC,
1993).
REFERENCES
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in the United Kingdom 1969-71. Br Med J, 2:
533-556.
Abbott DC, Collins GB, Goulding R, & Hoodless RA (1981) Organochlorine
pesticide residues in human fat in the United Kingdom 1976-77. Br Med
J, 283: 1425-1428.
Abbott DC, Goulding R, Holmes DC, & Hoodless RA (1985) Organochlorine
pesticide residues in human fat in the United Kingdom 1982-1983. Hum
Toxicol, 4: 435-445.
Abraham K, Hille A, Ende M, & Helge H (1994) Intake and fecal
excretion of PCDDs, PCDFs, HCB and PCBs (138, 153, 180) in a breast-
fed and a formula-fed infant. Chemosphere, 29(9-11): 2279-2286.
Ahling B, Bjorseth A, & Lunde G (1978) Formation of chlorinated
hydrocarbons during combustion of poly(vinyl chloride). Chemosphere,
10: 799-806.
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, Defoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: A toxicity data summary, Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory (EPA 600/3-84-009).
Albert LA, Alpuche L, Barcenas C, & Rendón J (1990) A survey of
organochlorine pesticide residues in cheese samples from three Mexican
regions. Environ Pollut, 65: 119-126.
Albro PW & Thomas R(1974) Intestinal absorption of hexachlorobenzene
and hexachlorocyclohexane isomers in rats. Bull Environ Contam
Toxicol, 12: 289-294.
Alves HHD & Chevalier M (1980) L'hexachlorobenzène dans
l'environnement québécois : Production, utilisation et présence.
Montréal, Québec, Environnement Canada, Service de la Protection de
l'Environnement (Rapport No EPS-3 QR-80-1).
Andrews JE & Courtney KD (1986) Hexachlorobenzene-induced renal
maldevelopment in CD-1 mice and CD rats. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 381-391 (IARC
Scientific Publications No. 77).
Andrews JE, Courtney KD, Stead AG, & Donaldson WE (1989)
Hexachlorobenzene induced hyperparathyroidism and osteosclerosis in
rats. Fundam Appl Toxicol, 12: 242-251.
Andrews JE, Jackson LD, Stead AG, & Donaldson WE (1990) Morphometric
analysis of osteosclerotic bone resulting from hexachlorobenzene
exposure. J Toxicol Environ Health, 31: 193-201.
Angerer J, Heinzow B, Reimann DO, Knorz W, & Lehnert G (1992)
Internal exposure to organic substances in a municipal waste
incinerator. Int Arch Occup Environ Health, 64: 265-273.
Ariño AA, Herrera A, Conchello MP, & Pérez C (1992) Hexachlorobenzene
residues in Spanish meat products after cooking, curing, and long-term
ripening. J Food Prot, 55(11): 920-923.
Ariño A, Herrera A, Conchello P, & Lazaro R (1993) Hexachlorobenzene
and hexachlorocyclohexane residues in pork as affected by weight and
sex. Bull Environ Contam Toxicol, 51: 647-650.
Arnold DL & Krewski D (1988) Long-term toxicity of hexachlorobenzene.
Food Chem Toxicol, 26: 169-174.
Arnold DL, Moodie CA, Charbonneau SM, Grice HC, McGuire PF, Bryce FR,
Collins BT, Zwadizka ZZ, Krewski DR, Nera EA, & Munro IC (1985) Long-
term toxicity of hexachlorobenzene in the rat and the effect of
dietary vitamin A. Food Chem Toxicol, 23: 779-793.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for hexachlorobenzene. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-90-17).
ATSDR (1994) Toxicological profile for hexachlorobenzene - Update.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry.
Atuma SS, Andersson O, Linder C-E, & Hansson L (1993) Levels of some
organochlorine compounds in sea trout ( Salmo trutta) and whitefish
( Caregonus lavaretus) from the Gulf of Bothnia. Aqua Fenn, 23:
221-226.
Avrahami M (1975) Hexachlorobenzene: IV. Accumulation and elimination
of HCB by pigs after oral dosing. NZ J Exp Agric, 3: 285-287.
Avrahami M & Steele RT (1972) Hexachlorobenzene: I. Accumulation and
elimination of HCB in sheep after oral dosing. NZ J Agric Res, 15:
476-481.
Ayotte P, Dewailly E, Bruneau S, Careau H, & Vézina A (1995) Arctic
air pollution and human health: what effects should be expected? Sci
Total Environ, 160/161: 529-537.
Babineau KA, Singh A, Jarrell JF, & Villeneuve DC (1991) Surface
epithelium of the ovary following oral administration of
hexachlorobenzene to the monkey. J Submicrosc Cytol Pathol, 23:
457-464.
Bailey J, Knauf V, Mueller W, & Hobson W (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
rhesus monkeys; enhanced toxicity. Environ Res, 21: 190-196.
Ballschmiter K, & Wittlinger R (1991) Interhemisphere exchange of
hexachlorocyclohexanes, hexachlorobenzene, polychlorobiphenyls, and
1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane in the lower
troposphere. Environ Sci Technol, 25: 1103-1111.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LSF
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39:
263-274.
Barquet A, Morgade C, & Pfaffenberger CD (1981) Determination of
organochlorine pesticides and metabolites in drinking water, human
blood serum, and adipose tissue. J Toxicol Environ Health, 7:
469-479.
Bates MN, Hannah DJ, Buckland SJ, Taucher JA, & Van Maanen T (1994)
Chlorinated organic contaminants in breast milk of New Zealand women.
Environ Health Perspect, 102 (Suppl 1): 211-217.
Bauer I, Weigelt S, & Ernst W (1989) Biotransformation of
hexachlorobenzene in the blue mussel ( Mytilus edulis). Chemosphere,
19 (10/11): 1701-1707.
Beck J & Hansen KE (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in soil.
Pestic Sci, 5: 41-48.
Béland P, Deguise S, & Plante R (1991) Toxicology and pathology of
St. Lawrence marine mammals - Final report. Rimouski, Québec, Canada,
Wildlife Toxicology Fund Research Grant, St. Lawrence National
Institute of Ecotoxicology, 95 pp.
Belfroid A, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, & Hermens
J (1993) The toxicokinetic behaviour of chlorobenzenes in earthworms
( Eisenia andrei): Experiments in water. Ecotoxicol Environ Saf,
25: 154-165.
Belfroid A, Meiling J, Sijm D, Hermens J, Seinen W, & Van Gestel K
(1994a) Uptake of hydrophobic halogenated aromatic hydrocarbons from
food by earthworms ( Eisenia andrei). Arch Environ Contam Toxicol,
27: 260-265.
Belfroid A, Sikkenk M, Seinen W, Van Gestel K, & Hermens J (1994b) The
toxicokinetic behavior of chlorobenzenes in earthworm ( Eisenia
andrei): Experiments in soil. Environ Toxicol Chem, 13: 93-99.
Beretta M & Dick T (1994) Organochlorine compounds in human milk,
Porto Alegre, Brazil. Bull Environ Contam Toxicol, 53: 357-360.
Biddleman TF, Billings WN, & Foreman WT (1986) Vapor-particle
partitioning of semivolatile organic compounds: Estimates from field
collection. Environ Sci Technol, 20: 1038-1043.
Bidleman TF, Wideqvist U, Jansson B, & Soderlund R (1987)
Organochlorine pesticides and polychlorinated biphenyls in the
atmosphere of southern Sweden. Atmos Environ, 21: 641-654.
Bidleman TF, Walla MD, Roura R, Carr E, & Schmidt S (1993)
Organochlorine pesticides in the atmosphere of the Southern Ocean and
Antarctica, January-March, 1990. Mar Pollut Bull, 26(5): 258-262.
Billings WN & Bidleman TF (1980) Field comparison of polyurethane foam
and Teax-GC resin for high-volume air sampling of chlorinated
hydrocarbons. Environ Sci Technol, 14(6):679-683.
Bishop CA, Lean DRS, Brooks RJ, Carey JH, & Ng P (1995) Chlorinated
hydrocarbons in early life stages of the common snapping turtle
( Chelydra serpentina serpentina) from a coastal wetland on Lake
Ontario, Canada. Environ Toxicol Chem, 14(3): 421-426.
Bjerk JE & Brevik EM (1980) Organochlorine compounds in aquatic
environments. Arch Environ Contam Toxicol, 9: 743-750.
Bleavins MR, Breslin WJ, Aulerich RJ, & Ringer RK (1982) Excretion and
placental and mammary transfer of hexachlorobenzene in the European
ferret ( Mustela putorius furo). J Toxicol Environ Health, 14:
929-940.
Bleavins MR, Bursian SJ, Brewster JS, & Aulerich RJ (1984a) Effects of
dietary hexachlorobenzene exposure on regional brain biogenic amine
concentrations in mink and European ferrets. J Toxicol Environ Health,
14: 363-377.
Bleavins MR, Aulerich RJ, & Ringer RK (1984b) Effects of chronic
dietary hexachlorobenzene exposure on the reproductive performance and
survivability of mink and European ferrets. Arch Environ Contam
Toxicol, 13: 357-365.
Boersma DC, Ellenton JL, & Yagminas A (1986) Investigation of the
hepatic mixed-function oxidase system in herring gull embryos in
relation to environmental contaminants. Environ Toxicol Chem, 5:
309-318.
Boese BL, Winsor M, Lee H II, Specht DT, & Rukavina LC (1990)
Depuration kinetics of hexachlorobenzene in the clam, Macoma nasuta.
Comp Biochem Physiol, C996: 327-331.
Bordet F, Mallet J, Maurice L, Borrel S, & Venant A (1993)
Organochlorine pesticide and PCB congener content of French human
milk. Bull Environ Contam Toxicol, 50: 425-432.
Bourbonniere RA, Van Sickle BL, & Mayer T (1986) The Great Lakes
sediment bank-I (including catalogs of lakes Huron and Ontario
samples). Burlington, Ontario, Environment Canada, National Water
Research Institute (NWRI Contribution No. 86-151).
Bouthillier L, Greselin E, Brodeur J, Viau C, & Charbonneau M (1991)
Male rat specific nephrotoxicity resulting from subchronic
administration of hexachlorobenzene. Toxicol Appl Pharmacol, 110:
315-326.
Braune BM & Norstrom RJ (1989) Dynamics of organochlorine compounds in
herring gulls: III. Tissue distribution and bioaccumulation in Lake
Ontario gulls. Environ Toxicol Chem, 8: 957-968.
Breslin WJ, Bleavins MR, & Ringer RK (1983) Distribution and excretion
of hexachlorobenzene in bobwhite. J Toxicol Environ Health, 11:
885-896.
Brooks GW & Hunt GE (1984) Source assessment for hexachlorobenzene.
Research Triangle Park, North Carolina, Radian Corporation (Prepared
for the US Environmental Protection Agency, Research Triangle Park)
(EPA 68-02-3818).
Bro-Rasmussen F (1986) Hexachlorobenzene: An ecotoxicological profile
of an organochlorine compound. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 231-239 (IARC
Scientific Publications No. 77).
Brown EA, Biddle K, & Spaulding JE (1986) Residue levels of
hexachlorobenzene in meat and poultry in the food supply of the U.S.A.
In: Morris CR & Cabral JRPed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 99-108 (IARC Scientific Publications No. 77).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17: 2363-1380.
Brunn H & Manz D (1982) Contamination of native fish stock by
hexachlorobenzene and polychlorinated biphenyl residues. Bull Environ
Contam Toxicol, 28: 599-604.
Brusick DJ (1986) Genotoxicity of hexachlorobenzene and other
chlorinated benzenes. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 393-397 (IARC
Scientific Publications No. 77).
BUA (German Chemical Society-Advisory Committee on Existing Chemicals
of Environmental Relevance) (1994) Hexachlorobenzene. Stuttgart, S.
Hirzel Wissenschaftliche Verlagsgesellschaft, 257 pp (BUA Report 119).
Buhler DR & Carpenter HM (1986) Japanese quail as a model for the
study of hexachlorobenzene-induced porphyria. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 477-480 (IARC
Scientific Publications No. 77).
Burns JE & Miller FM (1975) Hexachlorobenzene contamination: its
effects in a Louisiana population. Arch Environ Health, 30: 44-48.
Burns JE, Miller FM, Gomes ED, & Albert RA (1974) Hexachlorobenzene
exposure from contaminated DCPA in vegetable spraymen. Arch Environ
Health, 29: 192-194.
Burton MA & Bennett BG (1987) Exposure of man to environmental
hexachlorobenzene (HCB): an exposure commitment assessment. Sci Total
Environ, 66: 137-146.
Bush B, Snow J, Connor S, & Koblintz R (1985) Polychlorinated biphenyl
congeners (PCBs), p,pœ-DDe and hexachlorobenzene in human milk in
three areas of upstate New York. Arch Environ Contam Toxicol, 14:
443-450.
Cabral JRP & Shubik P (1986) Carcinogenic activity of
hexachlorobenzene in mice and hamsters. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 411-416 (IARC
Scientific Publications No. 77).
Cabral JRP, Shubik P, Mollner T, & Raitano F (1977) Carcinogenic
activity of hexachlorobenzene in hamsters. Nature (Lond), 269:
510-511.
Cabral JRP, Mollner T, Raitano F, & Shubik P (1979) Carcinogenesis of
hexachlorobenzene in mice. Int J Cancer, 23: 47-51.
Calamari D, Galassi S, Setti F, & Vighi M (1983) Toxicity of selected
chlorobenzenes to aquatic organisms. Chemosphere, 12: 253-262.
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA priority pollutants and related chemicals
in freshwater organisms. Center for Lake Superior Environmental
Studies, University of Wisconsin-Superior, Environmental Research
Laboratory, Duluth, Minnesota, U.S. Environmental Protection Agency
(EPA 600/3-83-095).
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed R, Jennings P,
Durfee R, Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979)
Water-related environmental fate of 129 priority pollutants, Volume
II. Washington, DC, US Environmental Protection Agency, Office of
Water Planning and Standards/Office of Water and Waste Management (EPA
440/4-79-029b).
Cam C (1960) Une nouvelle dermatose épidémique des enfants. Ann
Dermatol, 87: 393-397.
Cam C & Nigogosyan G (1963) Acquired toxic porphyria cutanea tarda due
to hexachlorobenzene. J Am Med Assoc, 183: 90-93.
Camps M, Planas J, Gómez-Catalán J, Sabroso M, To-Figueras J, &
Corbella J (1989) Organochlorine residues in human adipose tissue in
Spain: study of an agrarian area. Bull Environ Contam Toxicol, 42:
195-201.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1979) Pesticide
residue levels in soils and crops from 37 states, 1972 - National
Soils Monitoring Program (IV). Pest. Monit. J, 12: 209-229.
Carey AE, Dixon TE, & Yang HSC (1985) Environmental exposure to
hexachlorobenzene in the United States. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
Carlson AR & Kosian PA (1987) Toxicity of chlorinated benzenes to
fathead minnows ( Pimephales promelas). Arch Environ Contam
Toxicol, 16: 129-135.
Carthew P & Smith AG (1994) Pathological mechanisms of hepatic tumour
formation in rats exposed chronically to dietary hexachlorobenzene. J
Appl Toxicol, 14: 447-452.
Carthew P, Edwards RE, & Smith AG (1990) Immunotoxic effects of
hexachlorobenzene on the pathogenesis of systemic, pneumonic and
hepatic virus infections in the mouse. Hum Exp Toxicol, 8: 403-411.
Clark TP, Norstrom RJ, Fox GA, & Won HT (1987) Dynamics of
organochlorine compounds in herring gulls ( Larus argentatus): II. A
two-compartment model and data for ten compounds. Environ Toxicol
Chem, 6: 547-559.
Conchello P, Herrera A, Ariño A, Lazaro R, & Pérez-Arquillué C (1993)
Effect of grilling, roasting, and cooking on the natural
hexachlorobenzene content of ovine meat. Bull Environ Contam Toxicol,
50: 828-833.
Conde C, Naluenda C, & Arrabal C (1993) Hexachlorobenzene (HCB) in
human milk in Spain from 1984 to 1991. Bull Environ Contam Toxicol,
51: 827-831.
Courtney KD (1979) Hexachlorobenzene (HCB): a review. Environ Res,
20: 225-266.
Courtney KD & Andrews JE (1979) Mobilization of hexachlorobenzene
(HCB) during gestation. Toxicol Lett, 3: 357-361.
Courtney KD & Andrews JE (1985) Neonatal and maternal body burdens of
hexachlorobenzene (HCB) in mice: gestational exposure and lactational
transfer. Fundam Appl Toxicol, 5: 265-277.
Courtney KD, Copeland MF, & Robbins A (1976) The effects of
pentachloronitrobenzene, hexachlorobenzene, and related compounds on
fetal development. Toxicol Appl Pharmacol, 35: 239-256.
Courtney KD, Andrews JE, & Svendsgaard DJ (1979) Hexachlorobenzene
(HCB) deposition in maternal and fetal tissues of rat and mouse: I.
Chemical quantification of HCB in tissues. Environ Res, 19: 1-13.
Cripps DJ, Peters HA, Gocmen A, & Dogramaci I (1984) Porphyria turcica
due to hexachlorobenzene: a 20 to 30 year follow-up study on 204
patients. Br J Dermatol, 111: 413-422.
Cross JN, Hardy JT, Hose JE, Hershelman GP, Antrim LD, Gossett RW, &
Crecelius EA (1987) Contaminant concentrations and toxicity of sea-
surface microlayer near Los Angeles, California. Mar Environ Res,
23: 307-323.
Crump K & Howe N (1982) Global '82: a computer program to extrapolate
quantal animal toxicity data to low doses (Contract #41USC252C3).
Washington, DC, US Occupational Safety and Health Administration,
Office of Carcinogen Standards.
Currier MF, McClimans CD, & Barna-Lloyd G (1980) Hexachlorobenzene
blood levels and the health status of men employed in the manufacture
of chlorinated solvents. J Toxicol Environ Health, 6: 367-377.
D'Amour M & Charbonneau M (1992) Sex-related differences in hepatic
glutathione conjugation of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 112: 229-234.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17: 263-276.
Davis BD & Morgan RC (1986) Hexachlorobenzene in hazardous waste
sites. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 23-30 (IARC Scientific Publications No. 77).
Debets FMH & Strik JJTWA (1979) An approach to elucidate the mechanism
of hexachlorobenzene-induced hepatic porphyria, as a model for the
hepatotoxicity of polyhalogenated aromatic compounds (PHA's). In:
Strik JJTWA & Koeman JH ed. Chemical porphyria in man. Amsterdam,
Oxford, New York, Elsevier/North Holland Biomedical Press, pp 181-208.
Deleon IR, Maberry MA, Overton EB, Raschke CK, Remele PC, Steele, CF,
Warren VL, & Laseter JL (1980) Rapid gas chromatographic method for
the determination of volatile and semivolatile organochlorine
compounds in soil and chemical waste disposal site samples. J
Chromatogr Sci, 18: 85-88.
Dellinger B, Taylor PH, & Tirey DA (1991) Minimization and control of
hazardous combustion by-products. Cincinnati, Ohio, US Environmental
Protection Agency, Risk Reduction Laboratory (EPA 600/S2-90/039).
De Matteis F, Prior BE, & Rimington C (1961) Nervous and biochemical
disturbances following hexachlorobenzene intoxication. Nature (Lond),
191(4786): 363-366.
Den Besten C, Bennik MM, Van Iersel M, Peters MA, Teunis C, & Van
Bladeren PJ (1994) Comparison of the urinary metabolite profiles of
hexachlorobenzene and pentachlorobenzene in the rat. Chem-Biol
Interact, 90: 121-137.
Den Desten C, Bennik MHJ, Bruggeman I, Schielen P, Kuper F, Brouwer A,
Koeman JH, Vos JG, & Van Bladeren PJ (1993) The role of oxidative
metabolism in hexachlorobenzene-induced porphyria and thyroid hormone
homeostasis: a comparison with pentachlorobenzene in a 13-week feeding
study. Toxicol Appl Pharmacol, 119: 181-194.
Denomme MA, Leece B, Gyorkos J, Homonko K, & Safe S (1983)
Polychlorinated benzene and phenol congeners as inducers of rat
hepatic drug-metabolizing enzymes in immature male Wistar rats. Can J
Physiol Pharmacol, 61: 1063-1070.
Den Tonkelaar EM & Van Esch GJ (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
Den Tonkelaar EM, Verschueren HG, Bankovska J, De Vries T, Kroes R, &
Van Esch G (1978) Hexachlorobenzene toxicity in pigs. Toxicol Appl
Pharmacol, 43: 137-145.
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Dewailly E, Nantel A, Laliberté C, Weber J-P, Gingras S, & Meyer F
(1991) Organochlorines in the breast milk in Québec: a provincial
survey. Poster presentation at the Conference on Measuring,
Understanding, and Predicting Exposures in the 21st Century, Atlanta,
Georgia, 18-22 November 1991.
Ding W-H, Aldous KM, Briggs RG, Valente H, Hilker DR, Connor S, &
Eadon GA (1992) Application of multivariate statistical analysis to
evaluate local sources of chlorobenzene congeners in soil samples.
Chemosphere, 25(5): 675-690.
Dogramaci I (1964) Porphyrias and porphyrin metabolism with special
reference to porphyria in childhood. Adv Pediatr, 13: 11-63.
Dogramaci I, Wray JD, Ergene T, Sezer V, & Müftü Y (1962) Porphyria
turcica: a survey of 592 cases of cutaneous porphyria seen in
southeastern Turkey. Turk J Pediatr, 4: 138-148.
Eisenberg M & Topping JJ (1984) Organochlorine residues in shellfish
from Maryland waters. J Environ Sci Health, B19: 673-688.
Eisenreich SJ & Strachan WMJ (1992) Estimating atmospheric deposition
of toxic substances to the Great Lakes - An update: Proceedings of a
workshop, Burlington, 31 January-2 February 1992. Burlington, Ontario,
Canada Centre for Inland Waters.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCBs in
waste, sediment, and fish from the Mediterranean Sea. Bull Environ
Contam Toxicol, 34: 216-227.
Elissalde MH & Clark DE (1979) Testosterone metabolism by
hexachlorobenzene-induced hepatic microsomal enzymes. Am J Vet Res,
40: 1762-1766.
Elkin BT & Bethke RW (1995) Environmental contaminants in caribou in
the Northwest Territories, Canada. Sci Total Environ, 160/161:
307-321.
Enriquez De Salamanca R, Lopez-Miras A, Munoz JJ, To-Figueras J, &
Conde C (1990) Is hexachlorobenzene human overload related to
porphyria cutanea tarda? A speculative hypothesis. Med Hypotheses,
33: 69-71.
Environment Canada (1989) Atlantic region federal-provincial toxic
chemical survey of municipal drinking water sources 1985-1988 -
Interpretative Report. Moncton, New Brunswick, Environment Canada,
Conservation and Protection, Water Quality Branch, Inland Waters
Directorate.
Environment Canada (1992) Detroit incinerator monitoring program: Data
report No. 6. Ottawa, Ontario, Environment Canada, Conservation and
Protection, River Road Environmental Technology Centre (Report No.
PMD-92-1).
Environment Canada/Department of Fisheries & Oceans/Health & Welfare
Canada (1991) Toxic chemicals in the Great Lakes and associated
effects - Volume 1: Contaminant levels and trends. Ottawa, Ontario,
Minister of Supply and Services.
Environment Canada/Ontario Ministry of the Environment (1986) St.
Clair River pollution investigation (Sarnia area). Toronto, Canada-
Ontario Agreement Respecting Great Lakes Water Quality.
Ernst W (1986) Hexachlorobenzene in the marine environment:
distribution, fate and ecotoxicological aspects. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 211-222 (IARC Scientific Publications No. 77).
Erturk E, Lambrecht RW, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982) Leukemogenicity of hexachlorobenzene (HCB) in Swiss
mice (SM) after subchronic feeding. Proc Am Assoc Cancer Res, 23: 55
(Abstract).
Ertürk E, Lambrecht RW, Peters HA, Cripps DJ, Gocmen A, Morris CR, &
Bryan GT (1986) Oncogenicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 417-423 (IARC Scientific Publications No. 77).
Falandysz J & Szefer P (1982) Chlorinated hydrocarbons in diving ducks
wintering in Gdansk Bay, Baltic Sea. Sci Total Environ, 24: 119-127.
Falandysz J, Kannan K, Tanabe S, & Tatukawa R (1993) Persistent
organochlorine residues in canned cod-livers of the southern Baltic
origin. Bull Environ Contam Toxicol, 50: 929-934.
FAO/WHO (1975) Report of the Joint Meeting of FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 37 pp (WHO
Technical Report Series 574).
FAO/WHO (1978) Pesticide residues in food - 1978. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and
Environment and the WHO Expert Group on Pesticide Residues. Rome, Food
and Agriculture Organization of the United Nations, pp 19-20 (FAO
Plant Production and Protection Paper 15).
Ferrer A, Bona MA, Castellano M, To-Figueras J, & Brunet M (1992)
Organochlorine residues in human adipose tissue of the population of
Zaragoza (Spain). Bull Environ Contam Toxicol, 48: 561-566.
Fingler S, Drevenkar V, Tkalœeviœ B, & œmit Z (1992) Levels of
polychlorinated biphenyls, organochlorine pesticides, and
chlorophenols in the Kupa River water and in drinking waters from
different areas in Croatia. Bull Environ Contam Toxicol, 49:
805-812.
Foley RE & Batcheller GR (1988) Organochlorine contaminants in common
goldeneye wintering on the Niagara River. J Wildl Manage, 52:
441-445.
Foster WG, McMahon A, Jarrell JF, & Villeneuve DC (1992a)
Hexachlorobenzene (HCB) suppresses circulating progesterone
concentrations during the luteal phase in the cynomolgus monkey. J
Appl Toxicol, 12: 13-17.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1992b) Ovarian
toxicity of hexachlorobenzene (HCB) in the superovulated female rat. J
Biochem Toxicol, 7: 1-4.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1993) Body
distribution and endocrine toxicity of hexachlorobenzene (HCB) in the
female rat. J Appl Toxicol, 13: 79-83.
Foster WG, Mertineit C, Yagminas A, McMahon A, & Lecavalier P (1995)
The effects of hexachlorobenzene on circulating levels of adrenal
steroids in the ovariectomized rat. J Biochem Toxicol, 10(3):
129-135.
Fox ME, Carey JH, & Olivier BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the Western Basin
of Lake Ontario. J Great Lakes Res, 9: 287-294.
Franchi E & Focardi S (1991) Polychlorinated biphenyl congeners,
hexachlorobenzene and DDT in human milk in central Italy. Sci Total
Environ, 102: 223-228.
Frank R & Ripley BD (1990) Food residues from pesticides and
environmental pollutants in Ontario. In: Nriagu JO & Simmons MS ed.
Food contamination from environmental sources. New York, Wiley-
Interscience, pp 473-524.
Frank R, Braun HE, & Fleming G (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969 to 1981. J Food Prot, 46:
893-900.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985a)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario - 1983. J Food Prot, 48:
499-504.
Frank R, Rasper J, Braun HE, & Ashton G (1985b) Disappearance of
organochlorine residues from abdominal and egg fats of chickens,
Ontario, Canada, 1969-1982. J Assoc Off Anal Chem, 68: 124-129.
Frank R, Rasper J, Smout MS, & Braun HE (1988) Organochlorine residues
in adipose tissues, blood and milk from Ontario residents, 1976-1985.
Can J Public Health, 79: 150-158.
Fujita M & Morikawa K (1992) Regional differences in dietary intake of
environmental pollutants in Japan. J Expo Anal Environ Epidemiol,
2(2):177-193.
Fürst P, Fürst CHR, & Wilmers K (1992) Survey of dairy products for
PCDD's, PCDF's, PCB's and HCB. Chemosphere, 25(7-10): 1039-1048.
Fürst P, Fürst C, & Wilmers K (1994) Human milk as a bioindicator for
body burden of PCDDs, PCDFs, organochlorine pesticides, and PCBs.
Environ Health Perspect, 102 (Suppl 1): 187-193.
Galassi S & Provini A (1981) Chlorinated pesticides and PCB contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in adult total diet
samples, October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Geike F & Parasher CD (1976a) Effect of hexachlorobenzene on some
growth parameters of Chlorella pyrenoidosa. Bull Environ Contam
Toxicol, 15: 670-677.
Geike F & Parasher CD (1976b) Effect of hexachlorobenzene (HCB) on
growth of Tetrahymena pyriformis. Bull Environ Contam Toxicol, 16:
347-354.
Gilbertson M (1979) Hexachlorobenzene (HCB) in Canada. Ottawa,
Ontario, Environment Canada, Environmental Protection Service
(Unpublished report).
Gobas FAPC, Bedard DC, Ciborowski JJH, & Haffner GD (1989)
Bioaccumulation of chlorinated hydrocarbons by mayfly ( Hexagenia
limbata) in Lake St. Clair. J Great Lakes Res, 15: 581-588.
Gobas FAPC, McNeil EJ, Lovett-Doust L, & Haffner GD (1991)
Bioconcentration of chlorinated aromatic hydrocarbons in aquatic
macrophytes. Environ Sci Technol, 25: 924-929.
Gocmen A, Peters HA, Cripps DJ, Bryan GT, & Morris CR (1989)
Hexachlorobenzene episode in Turkey. Biomed Environ Sci, 2: 36-43.
Goerz G, Bolsen K, Kalofoutis A, & Tsambaos D (1994) Influence of oral
isotrentoin on hepatic and cutaneous P-450-dependent isozyme
activities. Arch Dermatol Res, 286: 104-106.
Goldey ES & Taylor DH (1992) Developmental neurotoxicity following
premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol
Teratol, 14: 15-21.
Goldey ES, Fisher JW, & Taylor DH (1990) Maternal transfer of
hexachlorobenzene in the rat. Neurotoxicol Teratol, 12: 562-563.
Goldstein JA, Friesen M, Scotti TM, Hickman P, Hass JR, & Bergman H
(1978) Assessment of the contribution of chlorinated dibenzo-p-dioxins
and dibenzofurans to hexachlorobenzene-induced toxicity, porphyria,
changes in mixed function oxygenases, and histopathological changes.
Toxicol Appl Pharmacol, 46: 633-649.
Gómez-Catalán J, Planas J, To-Figueras J, Camps M, & Corbella J (1993)
Organochlorine pesticide residues in the population of Catalonia
(Spain). Bull Environ Contam Toxicol, 51: 160-164.
Gómez-Catalán J, Lezaun M, To-Figueras J, & Corbella J (1995)
Organochlorine residues in the adipose tissue of the population of
Navarra (Spain). Bull Environ Contam Toxicol, 54: 534-540.
Gopalaswamy UV & Aiyar AS (1986) Biotransformation and toxicity of
lindane and its metabolite hexachlorobenzene in mammals. In: Morris CR
& Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 267-276 (IARC Scientific Publications No. 77).
Gopalaswamy UV & Nair CKK (1992) DNA binding and mutagenicity of
lindane and its metabolites. Bull Environ Contam Toxicol, 49:
300-305.
Gorski T, Gorska E, Gorecka D, & Sikora M (1986) Hexachlorobenzene is
non-genotoxic in short-term tests. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 399-401 (IARC
Scientific Publications No. 77).
Government of Canada (1993) Canadian Environmental Protection Act
priority substances list assessment report: Hexachlorobenzene. Ottawa,
Ontario, Environment Canada, Health and Welfare Canada, 52 pp.
Gralla EJ, Fleischman RW, Luthra YK, Hagopian M, Baker JR, Esber H, &
Marcus W (1977) Toxic effects of hexachlorobenzene after daily
administration to beagle dogs for one year. Toxicol Appl Pharmacol,
40: 227-239.
Grant DL, Iverson F, Hatina GV, & Villeneuve DC (1974) Effects of
hexachlorobenzene on liver porphyrin levels and microsomal enzymes in
the rat. Environ Physiol Biochem, 4: 159-165.
Grant DL, Shields JB, & Villeneuve DC (1975) Chemical (HCB) porphyria:
effect of removal of sex organs in the rat. Bull Environ Contam
Toxicol, 14: 422-425.
Grant DL, Phillips WEJ, & Hatina GV (1977) Effect of hexachlorobenzene
on reproduction in the rat. Arch Environ Contam Toxicol, 5: 207-216.
Green JA, Francis JE, Wolf CR, Manson MM, & Smith AG (1989) Sexual
dimorphism of cytochrome P-450 induction by hexachlorobenzene in rats.
Biochem Soc Trans, 17: 1016-1017.
Greve PA & Van Zoonen P (1990) Organochlorine pesticides and PCB's in
tissues from Dutch citizens (1968-1986). Int J Environ Anal Chem,
38: 265-277.
Griffin RA & Chou SFJ (1981) Movement of PCB's and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Grimalt JO, Gomez-Belinchon JI, Llop R, & Albaiges J (1988) Water-
phase distribution of hexachlorobenzene in a deltaic environment (Ebre
Delta, Western Mediterranean). Chemosphere, 17: 1893-1903.
Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, Anto JM,
& Albaiges J (1994) Risk excess of soft-tissue and thyroid cancers in
a community exposed to airborne organochlorinated compound mixtures
with a high hexachlorobenzene content. Int J Cancer, 56: 200-203.
Guerzoni ME, Del Cupolo L, & Ponti I (1976) [Mutagenic activity of
pesticides.] Riv Sci Tech Alim Nutr Um, 6: 161-165 (in Italian, with
English summary).
Hagenmaier H, She J, & Lindig C (1992) Persistence of polychlorinated
dibenzo-p-dioxins and polychlorinated dibenzofurans in contaminated
soil at Maulach and Rastatt in southwest Germany. Chemosphere,
25(7/10): 1449-1456.
Hahn ME, Gasiewicz TA, Linko P, & Goldstein JA (1988) The role of the
Ah locus in hexachlorobenzene-induced porphyria. Biochem J, 254:
245-254.
Hahn ME, Goldstein JA, Linko P, & Gasiewicz TA (1989) Interaction of
hexachlorobenzene with the receptor for 2,3,7,8-tetrachlorodibenzo-p-
dioxin in vitro and in vivo. Arch Biochem Biophys, 270(1):
344-355.
Hansen LG, Dorn SB, Sundlof SM, & Vogel RS (1978) Toxicity,
accumulation and depletion of hexachlorobenzene in laying chickens. J
Agric Food Chem, 26: 1369-1374.
Hansen LG, Teske RH, Sundlof SM, & Simon J (1979) Hexachlorobenzene
and feline reproduction: effects of ground pork contaminated by
dietary exposure or spiked with purified HCB. Vet Hum Toxicol, 21:
248-253.
Hansen PD, Von Westernhagen H, & Rosenthal H (1985) Chlorinated
hydrocarbons and hatching success in Baltic herring spring spawners.
Mar Environ Res, 15: 59-76.
Hargrave BT, Vass WP, Erickson PE, & Fowler BR (1989) Distribution of
chlorinated hydrocarbon pesticides and PCBs in the Arctic Ocean.
Dartmouth, NS, Department of Fisheries and Oceans, 224 pp (Canadian
Technical Report of Fisheries and Aquatic Sciences No. 1644).
Harper DJ, Ridgeway IM, & Leatherland TM (1992) Concentrations of
hexachlorobenzene, trichlorobenzenes and chloroform in the waters of
the Forth Estuary, Scotland. Mar Pollut Bull, 24(5): 244-249.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Health Canada (1994) Canadian Environmental Protection Act: Human
health risk assessment for priority substances. Ottawa, Ontario,
Minister of Supply and Services.
Heidmann WA (1986) Hexachlorobenzene residues in selected species of
land and sea birds in northern Germany. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 223-228 (IARC
Scientific Publications No. 77).
Heikes DL (1980) Residues of pentachloronitrobenzene and related
compounds in peanut butter. Bull Environ Contam Toxicol, 24:
338-343.
Heldaas SS, Langard S, & Andersen A (1989) Incidence of cancer in a
cohort of magnesium production workers. Br J Ind Med, 46: 617-623.
Hendriks AJ, Ma W-C, Brouns JJ, De Ruiter-Dijkman EM, & Gast R (1995)
Modelling and monitoring organochlorine and heavy metal accumulation
in soils, earthworms, and shrews in Rhine-Delta floodplains. Arch
Environ Contam Toxicol, 29: 115-127.
Herren-Freund SL & Pereira MA (1986) Carcinogenicity of by-products of
disinfection in mouse and rat liver. Environ Health Perspect, 69:
59-65.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of Interior, Fish and Wildlife Service (Special Scientific
Report œ Wildlife No. 191).
Hippelein M, Kaupp H, Dörr G, & McLachlan MS (1993) Testing of a
sampling system and analytical method for determination of
semivolatile organic compounds in ambient air. Chemosphere, 26:
2255-2263.
Hoff RM, Muir DCG, & Grift NP (1992) Annual cycle of polychlorinated
biphenyls and organohalogen pesticides in air in southern Ontario: 1.
Air concentration data. Environ Sci Technol, 26(2): 266-275.
Holoubek I, œáslavskœ J, Vancura R, Kocan A, Chovancová J, Petrik J,
Drobna B, Cudlin P, & Triska J (1994) Project Tocoen: The fate of
selected organic pollutants in the environment - Part XXIV. The
content of PCBs and PCDDs/Fs in high-mountain soils. Toxicol Environ
Chem, 45: 189-197.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
In: Taup H ed. Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers.
IARC (1979) Hexachlorobenzene. In: Some halogenated hydrocarbons.
Lyon, International Agency for Research on Cancer, pp 155-178 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Human, Volume 20).
IARC (1987) Hexachlorobenzene. In: Overall evaluations of
carcinogenicity: An updating of IARC monographs, volumes 1 to 42.
Lyon, International Agenci for Research on Cancer, pp 219-220 (IARC
Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Iatropoulos MJ, Hobson W, Knauf V, & Adams HP (1976) Morphological
effects of hexachlorobenzene toxicity in female rhesus monkeys.
Toxicol Appl Pharmacol, 37: 433-444.
IJC (International Joint Commission) (1989) In: Rathke DR & McRae G
ed. Great Lakes surveillance: 1987 report on Great Lakes water
quality. Report to the International Joint Commission, Windsor,
Ontario.
Ikemoto Y, Motoba K, Suzuki T, & Uchida M (1992) Quantitative
structure-activity relationships of nonspecific and specific toxicants
in several organism species. Environ Toxicol Chem, 11: 931-939.
Ingebritsen K (1986) Comparative studies on the distribution and
excretion of 14C-hexachlorobenzene by whole-body autoradiography. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 277-285 (IARC Scientific Publications No. 77).
Ingebritsen K, Skaare JU, Nafstad I, & Forde M (1981) Studies on the
biliary excretion and metabolites of hexachlorobenzene in the rat.
Xenobiotica, 11: 795-800.
Innes D, Muncaster B, Lazar R, Haffner D, & Hebert P (1988) Monitoring
of organic contaminants using freshwater mussels. Proceedings of the
Environmental Research Technology Transfer Conference, 30 November-1
December 1987 - Part B: Water quality research. Toronto, Ontario,
Environment Ontario.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: The derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
IRPTC (1993) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M Jr (1988) Data book of chromosomal aberration test
in vitro. Amsterdam, New York, Oxford, Elsevier Science Publishers.
Jacoff FS, Scarberry R, & Rosa D (1986) Source assessment of
hexachlorobenzene from the organic chemical manufacturing industry.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 31-37 (IARC Scientific Publications No. 77). 31-37.
Jaffe R & Hites RA (1986) Anthropogenic, polyhalogenated, organic
compounds in non-migratory fish from the Niagara River area and
tributaries to Lake Ontario. J Great Lakes Res, 12: 63-71.
Jansson B & Bergman A (1978) Sulfur-containing derivatives of
hexachlorobenzene (HCB) - metabolites in the rat. Chemosphere, 3:
257-268.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
Jarrell JF, McMahon A, Villeneuve D, Franklin C, Singh A, Valli VE, &
Bartlett S (1993a) Hexachlorobenzene toxicity in the monkey primordial
germ cell without induced porphyria. Reprod Toxicol, 7: 41-47.
Jarrell JF, Villeneuve D, Franklin C, Bartlett S, Wrixon W, Kohut J, &
Zouves CG (1993b) Contamination of human ovarian follicular fluid and
serum by chlorinated organic compounds in three Canadian cities. Can
Med Assoc J, 148(8): 1321-1327.
Jemaa Z, Sabbah S, Driss MR, & Bouguerra ML (1986) Hexachlorobenzene
in Tunisian mothers' milk, cord blood and foodstuffs. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 139-142 (IARC Scientific Publications No. 77).
Johansen HR, Becher G, Polder A, & Skaare JU (1994) Congener-specific
determination of polychlorinated biphenyls and organochlorine
pesticides in human milk from Norwegian mothers living in Oslo. J
Toxicol Environ Health, 42: 157-171.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites in Lake Ontario. J Great Lakes Res, 9:
183-189.
Kan CA & Tuinstra LGM (1976) Accumulation and excretion of certain
organochloride insecticides in broiler breeder hens. J Agric Food
Chem, 24: 775-778.
Kan-Do Office & Pesticides Team (1995) Accumulated pesticide and
industrial chemical findings from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kannan K, Tanabe S, Ramesh A, Subramanian A, & Tatsukawa R (1992a)
Persistent organochlorine residues in foodstuffs from India and their
implications on human dietary exposure. J Agric Food Chem, 40:
518-524.
Kannan K, Tanabe S, Hoang Trong Quynh, Nguyen Duc Hue, & Tatsukawa R
(1992b) Residue pattern and dietary intake of persistent
organochlorine compounds in foodstuffs from Vietnam. Arch Environ
Contam Toxicol, 22: 367-374.
Kannan K, Falandysz J, Tanabe S, & Tatsukawa R (1993) Persistent
organochlorines in Harbour Porpoises from Puck Bay, Poland. Mar Pollut
Bull, 26(3): 162-165.
Kannan K, Tanabe S, Williams RJ, & Tatsukawa R (1994) Persistant
organochlorine residues in foodstuffs from Australia, Papua New Guinea
and the Solomon Islands: contamination levels and human dietary
exposure. Sci Total Environ, 153: 29-49.
Kashimoto T, Takayama K, Mimura M, Miyata H, Murakami Y, & Matsumoto H
(1989) PCDDs, PCDFs, PCBs, coplanar PCBs and organochlorinated
pesticides in human adipose tissue in Japan. Chemosphere, 19(1/6):
921-926.
Kasokat T, Nagel R, & Urich K (1989) Metabolism of chlorobenzene and
hexachlorobenzene by the zebra fish, Brachydanio rerio. Bull Environ
Contam Toxicol, 42: 254-261.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St. Clair and Detroit Rivers using introduced
clams, Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kelly AG & Campbell A (1994) Organochlorine contaminants in liver of
cod ( Gadus morhua) and muscle of herring ( Clupea harengus) from
Scottish waters. Mar Pollut Bull, 28(2): 103-108.
Kemper FH (1993) Human organ specimen banking-15 years of experience.
Sci Total Environ, 139-140: 13-25.
Kennedy SW & Wigfield DC (1990) Dose-response relationships in
hexachlorobenzene-induced porphyria. Biochem Pharmacol, 40:
1381-1388.
Kennedy SW, Wigfield DC, & Fox GA (1986) The delay in polyhalogenated
aromatic hydrocarbon-induced porphyria: mechanistic reality or
methodological artefact? Toxicol Lett, 31: 235-241.
Kessabi M, Elhraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the Mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kessabi M, Abdennebi E, Laraje R, & Lhafi A (1990) Contamination of
eggs, poultry liver and bovine liver and kidney by chlorinated
pesticides in Morocco. Sci Total Environ, 90: 283-287.
Khera KS (1974) Teratogenicity and dominant lethal studies on
hexachlorobenzene in rats. Food Cosmet Toxicol, 12: 471-477.
Kilikidis SD, Kamarianos AP, & Karamanlis XN (1992) Seasonal
fluctuations of organochlorine compounds in the water of the Strimon
River (N. Greece). Bull Environ Contam Toxicol, 49: 375-380.
Kilzer L, Scheunert I, Geyer H, Klein W, & Korte F (1979) Laboratory
screening of the volatilization rates of organic chemicals from water
and soil. Chemosphere, 10: 751-761.
Kimbrough RD & Linder RE (1974) The toxicity of technical
hexachlorobenzene in the Sherman rat strain. A preliminary study. Res
Commun Chem Pathol Pharmacol, 8: 653-664.
King L & Sherbin G (1986) Point sources of toxic organics to the upper
St. Clair River. Water Pollut Res J Can, 21: 433-446.
Kitchin KT & Brown JL (1989) Biochemical studies of promoters of
carcinogenesis in rat livers. Teratog Carcinog Mutagen, 9: 273-285.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Kleiman de Pisarev DL, Sancovich HA, & Ferramola de Sancovich AM
(1989) Enhanced thyroxine metabolism in hexachlorobenzene-intoxicated
rats. J Endocrinol Invest, 12: 767-772.
Kleiman de Pisarev DL, Del Carmen Rios de Molina M, & San Martin de
Viale LC (1990) Thyroid function and thyroxine metabolism in
hexachlorobenzene-induced porphyria. Biochem Pharmacol,. 39:
817-825.
Knezovich JP & Harrison FL (1988) The bioavailability of sediment-
sorbed chlorobenzenes to larvae of the midge, Chironomus decorus.
Ecotoxicol Environ Saf, 15: 226-241.
Kocan A, Petrík J, Drobná G, & Chovancová J (1994) Levels of PCBs and
some organochlorine pesticides in the human population of selected
areas of the Slovak Republic: I. Blood. Chemosphere, 29(9/11):
2315-2325.
Kodric-Smit M, Smit Z, & Olie K (1980) Organochlorine contaminants in
human milk from Slavonia Province, Yugoslavia, 1978. Pestic Monit J,
14: 1-2.
Koeman JH, Ten Noever de Brauw MC & De Vos RH (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
Kohler A, Harms U, & Lukas B (1986) Accumulation of organochlorines
and mercury in flounder - an approach to pollution assessments. Helgol
Meeresunters 40: 431-440.
Koizumi A (1991) Experimental evidence for the possible exposure of
workers to hexachlorobenzene by skin contamination. Br J Ind Med,
48: 622-628.
Kosian P, Lemke A, Studders K, & Veith G (1981) The precision of the
ASTM bioconcentration test. Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory & Superior,
Wisconsin, University of Wisconsin-Superior, Center for Lake Superior
Environmental Studies, 24 pp (EPA 600/3-81/022).
Koss G & Koransky W (1975) Studies on the toxicology of
hexachlorobenzene: I. Pharmacokinetics. Arch Toxicol, 34: 203-212.
Koss G & Koransky W (1976) Studies on the toxicology of
hexachlorobenzene. II. Identification and determination of
metabolites. Arch Toxicol, 35: 107-114.
Koss G & Koransky W (1978) Pentachlorophenol in different species of
vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. In: Ranga Rao K ed. Pentachlorophenol. New York,
London, Plenum Press, pp 131-137.
Koss G & Manz D (1976) Residues of hexachlorobenzene in wild mammals
of Germany. Bull Environ Contam Toxicol, 15: 189-191.
Koss G, Strik JJTWA, & Kan CA (1978) Metabolites of hexachlorobenzene
in the excreta of different animal species. Excerpta Med Int Congress,
440: 211-212.
Krauthacker B (1993) Organochlorine pesticides and polychlorinated
biphenyls (PCBs) in human serum collected from the general population
from Zagreb (1985-1990). Bull Environ Contam Toxicol, 50: 8-11.
Krishnan K, Brodeur J, & Charbonneau M (1991) Development of an
experimental model for the study of hexachlorobenzene-induced hepatic
porphyria in the rat. Toxicol Appl Pharmacol, 17: 433-441.
Krishnan K, Brodeur J, Plaa GL, & Charbonneau M (1992) Modulation of
hexachlorobenzene-induced hepatic porphyria by methyl isobutyl ketone
in the rat. Toxicol Lett, 61: 167-174.
Kuehl DW & Butterworth B (1994) A national study of chemical residues
in fish: III. Study results. Chemosphere, 29(3): 523-535.
Kuehl DW, Leonard EN, Butterworth BC, & Johnson KL (1983)
Polychlorinated chemical residues in fish from major watersheds near
the Great Lakes, 1979. Environ Int, 9: 293-299.
Kuhnlein HV, Receveur O, Muir DCG, Chan HM, & Soueida R (1995) Arctic
indigenous women consume greater than acceptable levels of
organochlorines. J Nutr, 125: 2501-2510.
Kuiper-Goodman T, Grant DL, Moodle CA, Korsrud GO, & Munro IC (1977)
Subacute toxicity of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 40: 529-549.
Kuroda Y (1986) Genetic and chemical factors affecting chemical
mutagenesis in cultured mammalian cells. In: Antimutagenesis and
anticarcinogenesis mechanisms. New York, London, Plenum Press,
pp 359-375.
Lambrecht RW, Erturk E, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982a) Renal toxicity and tumorigenicity of
hexachlorobenzene (HCB) in rats (R). Proc Am Assoc Cancer Res, 23:
54 (Abstract).
Lambrecht RW, Erturk E, Grunden E, Headley DB, Morris CR, Peters HA, &
Bryan GT (1982b) Hepatotoxicity and tumorigenicity of
hexachlorobenzene (HCB) in Syrian golden hamsters (H) after subchronic
administration. Fed Proc, 41: 329 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983a) Hepatocarcinogenicity of chronically administered
hexachlorobenzene in rats. Fed Proc, 42: 786 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983b) Renal tumors in rats chronically exposed to hexachlorobenzene
(HCB). Proc Am Assoc Cancer Res, 24: 59 (Abstract).
Lambrecht RW, Sinclair PR, Bement WJ, Sinclair JF, Carpenter HM,
Buhler DR, Urquhart AJ, & Elder GH (1988) Hepatic uroporphyrin
accumulation and uroporphyrinogen decarboxylase activity in cultured
chick-embryo hepatocytes and in Japanese quail ( Coturnix coturnix
japonica) and mice treated with polyhalogenated aromatic
hydrocarbons. Biochem J, 253: 131-138.
Lamparski LL, Langhorst ML, Nestrick TJ, & Cutie S (1980) Gas-liquid
chromatographic determination of chlorinated benzenes and phenols in
selected biological matrices. J Assoc Off Anal Chem, 63(1): 27-32.
Landrum PF (1989) Bioavailability and toxicokinetics of polycyclic
aromatic hydrocarbons sorbed to sediment for the amphipod
Pontoporeia hoyi. Environ Sci Technol, 23: 588-595.
Lane DA, Schroeder WH, & Johnson ND (1992a) On the spatial and
temporal variations in atmospheric concentrations of hexachlorobenzene
and hexachlorocyclohexane isomers at several locations in the province
of Ontario, Canada. Atmos Environ, 26A: 31-42.
Lane DA, Johnson ND, Hanley MJJ, Schroeder WH, & Ord DT (1992b) Gas-
and particle-phase concentrations of alpha-hexachlorocyclohexane,
gama-hexachlorocyclohexane, and hexachlorobenzene in Ontario air.
Environ Sci Technol, 26: 126-133.
Langhorst ML & Nestrick TJ (1979) Determination of chlorobenzenes in
air and biological samples by gas chromatography with photoionization
detection. Anal Chem, 51(12): 2018-2025.
Larsen BR, Turrio-Baldassarri L, Nilsson T, Iacovella N, Di Domenico
A, Montagna M, & Facchetti S (1994) Toxic PCB congeners and
organochlorine pesticides in Italian human milk. Ecotoxicol Environ
Saf, 28: 1-13.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW, &
Evans RL (1976) An ecological study of hexachlorobenzene (HCB). New
Orleans, University of New Orleans, Department of Biological Sciences
(Prepared for the US Environmental Protection Agency, Office of Toxic
Substances, Washington) (EPA 560/6-76-009).
Laska AL, Bartell C, & Laseter JL (1976) Distribution of
hexachlorobenzene and hexachlorobutadiene in water, soil, and selected
aquatic organisms along the lower Mississippi River, Louisiana. Bull
Environ Contam Toxicol, 15: 535-542.
Leger DA (1991) Environmental concentrations of hexachlorobenzene in
Atlantic Canada. Moncton, New Brunswick, Environment Canada, Inland
Waters Directorate.
Leoni V & D'Arca SU (1976) Experimental data and critical review of
the occurrence of hexachlorobenzene in the Italian environment. Sci
Total Environ, 5: 253-272.
Lewis RJ (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Company.
Lewis RG & MacLeod KE (1982) Portable sampler for pesticides and
semivolatile industrial organic chemicals in air. Anal Chem, 54:
310-315.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic compounds
in rain: II. Gas scavenging of neutral organic compounds. Atmos
Environ, 19: 1609-1617.
Lilienthal H, Benthe C, Heinzow B, & Winneke G (1996) Impairment of
schedule-controlled behavior by pre- and postnatal exposure to
hexachlorobenzene in rats. Arch Toxicol, 70: 174-181.
Linko P, Yeowell HN, Gasiewicz TA, & Goldstein JA (1986) Induction of
cytochrome P-450 isozymes by hexachlorobenzene in rats and aromatic
hydrocarbon (Ah)-responsive mice. J Biochem Toxicol, 1: 95-107.
Loose LD (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice. Environ Health
Perspect, 43: 89-97.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspect, 39: 79-92.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55(6): 881-889.
Lu P & Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model ecosystem. Environ Health
Perspect, 10: 269-284.
Ludwicki JK & Góralczyk K (1994) Organochlorine pesticides and PCBs in
human adipose tissues in Poland. Bull Environ Contam Toxicol, 52:
400-403.
Lunde G & Bjorseth A (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci
Total Environ, 8(3): 241-246.
Lunde G & Ofstad EB (1976) Determination of fat-soluble chorinated
compounds in fish. Z Anal Chem, 282: 395-399.
McCarty LS, MACKAY D, Smith AD, Ozburn GW, & Dixon DG (1992a) Residue-
based interpretation of toxicity and bioconcentration QSARs from
aquatic bioassays: Neutral narcotic organics. Environ Toxicol Chem,
11: 917-930.
McCarty LS, Ozburn GW, Smith AD, & Dixon DG (1992b) Toxicokinetic
modeling of mixtures of organic chemicals. Environ Toxicol Chem, 11:
1037-1047.
Mack GA & Stanley J (1985) The national human adipose tissue survey.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (Report No. NHATS-ST-01).
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of physical-
chemical properties and environmental fate for organic chemicals -
Volume I: Monoaromatic hydrocarbons, chlorobenzenes, and PCBs.
Chelsea, Michigan, Lewis Publishers.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol, 25: 921-928.
MAFF (Ministry of Agriculture, Fisheries and Food) (1992) Report of
the working party on pesticide residues: 1988-1990. London, Her
Majesty's Stationery Office (Food Surveillance Paper No. 34).
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1994) Report of the working party on pesticide
residues, 1993 - Supplement to the pesticides register. London, Her
Majesty's Stationery Office.
Mansour M, Scheunert I, Viswanathan R, & Korte F (1986) Assessment of
the persistence of hexachlorobenzene in the ecosphere. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 53-59 (IARC Scientific Publications No. 77).
Marlow DA (1986) Hexachlorobenzene exposure in the production of
chlorophenols. In: Morris CR & Cabral JRP ed. Hexachlorobenzene:
Proceedings of an International Symposium. Lyon, International Agency
for Research on Cancer, pp 161-169 (IARC Scientific Publications
No. 77).
Mehendale HM, Fields M, & Matthews HB (1975) Metabolism and effects of
hexachlorobenzene on hepatic microsomal enzymes in the rat. J Agric
Food Chem, 23: 261-265.
Mendoza CE, Grant DL, & Shields JB (1975) Body burden of
hexachlorobenzene in suckling rats and its effects on various organs
and on liver porphyrin accumulation. Environ Physiol Biochem, 5:
460-464.
Mendoza CE, Collins B, Shields JB, & Laver GW (1977) Hexachlorobenzene
residues and effects on esterase activities in pre-weanling rats after
a reciprocal transfer between HCB-treated and control dams. Arch
Toxicol, 38: 191-199.
Mendoza CE, Collins BT, Shields JB, & Laver GW (1978) Effects of
hexachlorobenzene or hexabromobenzene on body and organ weights of
preweanling rats after a reciprocal transfer between the treated and
control dams. J Agric Food Chem, 26: 941-945.
Mendoza CE, Shields JB, & Laver GW (1979) Comparison of the
porphyrinogenic activity of hexabromobenzene and hexachlorobenzene in
primiparous Wistar rats. Bull Environ Contam Toxicol, 21: 358-364.
Mes J (1990) Trends in the levels of some chlorinated hydrocarbon
residues in adipose tissue of Canadians. Environ Pollut, 65:
269-278.
Mes J (1992) Organochlorine residues in human blood and biopsy fat and
their relationship. Bull Environ Contam Toxicol, 48: 815-820.
Mes J & Davies DJ (1979) Presence of polychlorinated biphenyl and
organochlorine pesticide residues and the absence of polychlorinated
terphenyls in Canadian human milk samples. Bull Environ Contam
Toxicol, 21: 381-387.
Mes J & Malcolm S (1992) Comparison of chlorinated hydrocarbon
residues in human populations from the Great Lakes and other regions
of Canada. Chemosphere, 25(3): 417-424.
Mes J, Campbell DS, Robinson RN, & Davies DJA (1977) Polychlorinated
biphenyl and organochlorine pesticide residues in adipose tissue of
Canadians - 1977. Bull Environ Contam Toxicol, 17: 196-203.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and other
chlorinated hydrocarbon residues in adipose tissue of Canadians. Bull
Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, Turton D, & Sun W-F (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mes J, Marchand L, & Davies DJ (1990) Organochlorine residues in
adipose tissue of Canadians. Bull Environ Contam Toxicol, 45:
681-688.
Mes J, Davies DJ, Doucet J, Weber D, & McMullen E (1993) Levels of
chlorinated hydrocarbon residues in Canadian human breast milk and
their relationship to some characteristics of the donors. Food Addit
Contam, 10(4): 429-441.
Mill T & Haag W (1986) The environmental fate of hexachlorobenzene.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 61-66 (IARC Scientific Publications No. 77).
Moksnes MT & Norheim G (1986) Levels of chlorinated hydrocarbons and
composition of PCB in herring gull Larus argentatus eggs collected
in Norway in 1969 compared to 1979-81. Environ Pollut, 11: 109-116.
Mollenhauer HH, Johnson JH, Younger RL, & Clark DE (1975)
Ultrastructural changes in liver of the rat fed hexachlorobenzene. Am
J Vet Res, 36: 1777-1781.
Mollenhauer HH, Johnston JH, Younger RL, & Clark DE (1976) A unique
intracellular aberration related to hexachlorobenzene ingestion. Am J
Vet Res, 37: 847-850.
Morita M, Mimura S, & Yagyu H (1975) A systematic determination of
chlorinated benzenes in human adipose tissue. Environ Pollut, 9:
175-179.
Morley A, Geary D, & Harben F (1973) Hexachlorobenzene pesticides and
porphyria. Med J Aust, 1: 565.
Morosini M, Schreltmüller J, Reuter U, & Ballschmiter K (1993)
Correlation between C-6/C-14 chlorinated hydrocarbons levels in the
vegetation and in the boundary layer of the troposphere. Environ Sci
Technol, 27: 1517-1523.
Muir DCG, Wagemann R, Grift NP, Norstrom RJ, Simon M, & Lien J (1988)
Organochlorine chemical and heavy metal contaminants in white-beaked
dolphins ( Lagenorhynchus albirostris) and pilot whales
( Globicephala melaena) from the coast of Newfoundland, Canada. Arch
Environ Contam Toxicol, 17: 613-629.
Muir DCG, Grift NP, Lockhart WL, Wilkinson P, Billeck BN, & Brunskill
GJ (1995) Spatial trends and historical profiles of organochlorine
pesticides in Arctic lake sediments. Sci Total Environ, 160/161:
447-457.
Müller MD (1982) [Hexachlorobenzene in Switzerland: Measurement and
background of environmental contamination.] Chimia, 36(11): 437-445
(in German).
Mumma CF & Lawless EW (1975) Survey of industrial processing data.
Task I: Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processes. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA 560/3-75-003).
Muncaster BW, Innes DJ, Hebert PDN, & Haffner GD (1989) Patterns of
organic contaminant accumulation by freshwater mussels in the
St. Clair River, Ontario. J Great Lakes Res, 15: 645-653.
Murray HE, Neff GS, Hrung Y, & Giam CS (1980) Determination of
benzo(a)pyrene, hexachlorobenzene and pentachlorophenol in oysters
from Galveston Bay, Texas. Bull Environ Contam Toxicol, 25(4):
663-667.
Mussalo-Rauhamaa H, Pyysalo H, & Antervo K (1988) Relation between the
content of organochlorine compounds in Finnish human milk and
characteristics of the mothers. J Toxicol Environ Health, 25: 1-19.
Nair A & Pillai MKK (1989) Monitoring of hexachlorobenzene residues in
Delhi and Faridabad, India. Bull Environ Contam Toxicol, 42:
682-686.
Nash RG & Gish TJ (1989) Halogenated pesticide volatilization and
dissipation from soil under controlled conditions. Chemosphere, 18:
2353-2362.
Nebecker AV, Griffis WL, Wise CM, Hopkins E, & Barbitta JA (1989)
Survival, reproduction and bioconcentration in invertebrates and fish
exposed to hexachlorobenzene. Environ Toxicol Chem, 8: 601-611.
Needham LL, Burse VW, Head SL, Korver MP, McClure PC, Andrews JS Jr,
Rowley DL, Sung J, & Kahn SE (1990) Adipose tissue/serum partitioning
of chlorinated hydrocarbon pesticides in humans. Chemosphere, 20:
975-980.
Newton KG & Greene NC (1972) Organochlorine pesticide residue levels
in human milk -Victoria, Australia - 1970. Pestic Monit J, 6(1):
4-8.
NHW (National Health and Welfare) (1987) Nutrient value of some common
foods. Ottawa, Ontario, Health Services and Promotion Branch/Health
Protection Branch.
Niimi AJ & Cho CY (1981) Elimination of hexachlorobenzene (HCB) by
rainbow trout ( Salmo gairdneri), and an examination of its kinetics
in Lake Ontario salmonids. Can J Fish Aquat Sci, 38: 1350-1356.
Niimi AJ & Oliver BG (1989) Distribution of polychlorinated biphenyl
congeners and other halocarbons in whole fish and muscle among Lake
Ontario salmonids. Environ Sci Technol, 23: 83-88.
NIOSH (1985) Registry of toxic effects of chemical substances,
1983-1984 - Cumulative supplement to the 1981-1982 edition.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Noble DG & Elliott JE (1986) Environmental contaminants in Canadian
seabirds, 1968-1984: Trends and effects. Ottawa, Ontario, Canadian
Wildlife Service (Technical Report Series No. 13).
Noble DG, Elliott JE, & Shutt L (1992) Contaminants in Canadian
raptors, 1965-1988. Ottawa, Ontario, Canadian Wildlife Service
(Technical Report Series No. 91).
Norén K (1983a) Levels of organochlorine contaminants in human milk in
relation to the dietary habits of the mothers. Acta Paediatr Scand,
72: 811-816.
Norén K (1983b) Some aspects of the determination of organochlorine
contaminants in human milk. Arch Environ Contam Toxicol, 12:
277-283.
Norén K (1988) Changes in the levels of organochlorine pesticides,
polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans in
human milk from Stockholm, 1972-1985. Chemosphere, 17(1): 39-49.
Norén K (1993) Contemporary and retrospective investigations of human
milk in the trend studies of organochlorine contaminants in Sweden.
Sci Total Environ, 139/140: 347-355.
Norstrom RJ, Simon M, & Muir DCG (1990) Polychlorinated dibenzo-p-
dioxins and dibenzofurans in marine mammals in the Canadian north.
Environ Pollut, 66: 1-19.
NTP (1994) Seventh annual report on carcinogens. Summary, 1994.
Research Triangle Park North Carolina, US Department of Health and
Human Services, National Toxicology Program.
Ockner RK & Schmid R (1961) Acquired porphyria in man and rat due to
hexachlorobenzene intoxication. Nature (Lond), 189: 499.
Oehme M & Stray H (1982) Quantitative determination of ultra-traces of
chlorinated compounds in high-volume air samples from the Arctic using
polyurethane foam as collection medium. Fresenius Z Anal Chem, 311:
665-673.
Oliver BG (1984a) Distribution and pathways of some chlorinated
benzenes in the Niagara River and Lake Ontario. Water Pollut Res J
Can, 19: 47-59.
Oliver BG (1984b) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Partitioning relationships for chlorinated organics
between water and particulates in the St. Clair, Detroit and Niagara
Rivers. In: Kaiser LLE ed. QSAR in environmental toxicology - II.
Boston, Massachussetts, D. Reidel Publishing Co., pp 251-260.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St. Clair, and Erie: Implications
regarding sources along the St. Clair and Detroit rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Carey JH (1986) Photodegradation of wastes and pollutants
in aquatic environment. In: Pelizzetti E & Serpo N ed. Homogenous and
heterogenous photocatalysis. Boston, Massachussetts, D. Reidel
Publishing Co., pp 629-650.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Kaiser KLE (1986) Chlorinated organics in nearshore waters
and tributaries of the St. Clair River. Water Pollut Res J Can, 21:
344-350.
Oliver BG & Nicol KD (1982) Chlorobenzenes in sediments, water, and
selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ
Sci Technol, 16: 532-536.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of polychlorinated
biphenyl congeners and other chlorinated hydrocarbons in the Lake
Ontario ecosystem. Environ Sci Technol, 22: 388-397.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21: 368-379.
Oliver BG, Charlton MN, & Durham RW (1989) Distribution,
redistribution, and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
sediments. Environ Sci Technol, 23: 200-208.
OMAF/OME (Ontario Ministry of Agriculture and Food/Ontario Ministry of
the Environment) (1988) Polychlorinated dibenzo-p-dioxins and
polychlorinated dibenzofurans and other organochlorine contaminants in
food. Toronto, Ontario Ministry of Agriculture and Food and Ministry
of the Environment (Report prepared by the Joint OMAF/OME Toxics in
Food Steering Committee).
Ostfeldt P, Gustavson K, Jansson B, Jonsson P, Miettinen V, Ringstad
O, & Wesén C (1994) Halogenated organic compounds in the marine
environment 1989-1990. Copenhagen, Nordic Council of Ministers, 124 pp
(TemaNord 1994:591).
Parrish PR, Cook GH, & Patrick JM Jr (1974) Effects on several
estuarine animals. In: Rogers WA ed. Proceedings of the 28th Annual
Conference, White Sulphur Springs, West Virginia, 17-20 November 1974.
Sulphur Springs, West Virginia, Southeastern Association of Game and
Fish Commissioners.
Patton GW, Hinckley DA, Walla MD, & Bidleman TF (1989) Airborne
organochlorines in the Canadian High Arctic. Tellus, 41B: 243-255.
Peakall DB, Noble DG, Elliott JE, Somers JD, & Erickson G (1990)
Environmental contaminants in Canadian peregrine falcons, Falco
peregrinus: A toxicological assessment. Can Field-Nat, 104:
244-254.
Pearce PA, Elliott JE, Peakall DB, & Norstrom RJ (1989) Organochlorine
contaminants in eggs of seabirds in the Northwest Atlantic, 1968-1984.
Environ Pollut, 56: 217-235.
Pereira MA, Herren SL, Britt AL, & Khoury MM (1982) Sex difference in
enhancement of GGTase-positive foci by hexachlorobenzene and lindane
in rat liver. Cancer Lett, 15: 95-101.
Persoone G & Uyttersprot G (1975) The influence of inorganic and
organic pollutants on the rate of reproduction of a marine
hypotrichous ciliate: Euplotes vannus Muller. Rev Int Océanogr méd,
37-38: 125-151.
Peters HA (1976) Hexachlorobenzene poisoning in Turkey. Fed Proc,
35: 2400-2403.
Peters HA, Johnson SAM, Cam S, Oral S, Müftü Y, & Ergene T (1966)
Hexachlorobenzene-induced porphyria: effect of chelation on the
disease, porphyrin and metal metabolism. Am J Med Sci, 251: 314-322.
Peters HA, Gocmen A, Cripps DJ, Bryan GT, & Dogramaci I (1982)
Epidemiology of hexachlorobenzene-induced porphyria in Turkey:
clinical and laboratory follow-up after 25 years. Arch Neurol, 39:
744-749.
Peters HA, Cripps DJ, Gocmen A, Ertürk E, Bryan GT, & Morris CR (1986)
Neurotoxicity of hexachlorobenzene-induced porphyria turcica. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 575-579 (IARC Scientific Publications No. 77).
Peterson R & Ray S (1987) Organochlorine residues in brook trout and
yellow perch from New Brunswick and Nova Scotia (Canada) lakes. Water
Pollut Res J Can, 22: 352-364.
Phelps DK, Pruell RJ, & Lake JL (1986) Hexachlorobenzene in selected
marine samples: an environmental perspective. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 121-130 (IARC
Scientific Publications No. 77).
Proulx G, Weseloh DV, Elliott JE, Teeple S, Anghern PAM, & Mineau P
(1987) Organochlorine and PCB residues in Lake Erie mink populations.
Bull Environ Contam Toxicol, 39: 939-944.
Quinlivan S, Ghassemi M, & Santy M (1975) Survey of methods used to
control wastes containing hexachlorobenzene. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste Management
Programs (EPA 530/SW-120c).
Quinsey PM, Donohue DC, & Ahokas JT (1995) Persistence of
organochlorines in breast milk of women in Victoria, Australia. Food
Chem Toxicol, 33(1): 49-56.
Ray LE, Murray HR, & Giam CS (1983a) Analysis of water and sediment
from the Nueces Estuary/Corpus Christi Bay (Texas) for selected
organic pollutants. Chemosphere, 12: 1039-1045.
Ray LE, Murray HE, & Giam CS (1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12: 1031-1038.
Renner G (1988) Hexachlorobenzene and its metabolism. Toxicol Environ
Chem, 18: 51-78.
Richter E & Schäfer SG (1981) Intestinal excretion of
hexachlorobenzene. Arch Toxicol, 47: 233-239.
Rios de Molina MC, Wainstok de Calmanovici R, & San Martin de Viale LC
(1980) Investigations on the presence of porphyrinogen carboxy-lyase
inhibitor in the liver of rats intoxicated with hexachlorobenzene. Int
J Biochem, 12: 1027-1032.
Rippen G & Frank R (1986) Estimation of hexachlorobenzene pathways
from the technosphere into the environment. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 45-52 (IARC
Scientific Publications No. 77).
RIWA (1993) Rapport annuel 1993 de l'Association des Services d'Eau du
Rhin et de la Meuse, Annex 11. Amsterdam, Secrétariat de l'Association
des Services d'Eau du Rhin et de la Meuse.
Rizzardini M & Smith AG (1982) Sex differences in the metabolism of
hexachlorobenzene by rats and the development of porphyria in females.
Biochem Pharmacol, 31: 3543-3548.
Rizzardini M, Graziani A, Carugo C, & Cantoni L (1988) Investigations
on the role of free radical processes in hexachlorobenzene-induced
porphyria in mice. J Biochem Toxicol, 3: 33-46.
Rizzardini M, Cantoni L, Villa P, & Ubezio P (1990) Biochemical,
morphological and flow-cytometric evaluation of the effects of
hexachlorobenzene on rat liver. Cell Biol Toxicol, 6: 185-203.
Robinson PE, Mack GA, Remmers J, Levy R, & Mohadjer L (1990) Trends of
PCB, hexachlorobenzene, and ß-benzene hexachloride levels in the
adipose tissue of the U.S. population. Environ Res, 53: 175-192.
Rogers HR, Crathorne B, & Leatherland TM (1989) Occurrence of
chlorobenzene isomers in the water column of a UK estuary. Mar Pollut
Bull, 20: 276-281.
Rozman K, Rozman T, & Greim H (1981) Enhanced fecal elimination of
stored hexachlorobenzene from rats and rhesus monkeys by hexadecane or
mineral oil. Toxicology, 22: 33-44.
Rozman K, Gorski JR, Rozman P, & Parkinson A (1986) Reduced serum
thyroid hormone levels in hexachlorobenzene-induced porphyria. Toxicol
Lett, 30: 71-78.
Rumsby PC, Evans JG, Phillimore HE, Carthew P, & Smith AG (1992)
Search for Ha- ras codon 51 mutations in liver tumours caused by
hexachlorobenzene and Aroclor 1254 in C57BL/10ScSn mice with iron
overload. Carcinogenesis, 13: 1917-1920.
Rush GF, Smith JH, Maita K, Bleavins M, Aulerich RJ, Ringer RK, & Hook
JB (1983) Perinatal hexachlorobenzene toxicity in the mink. Environ
Res, 31: 116-124.
Russell RW & Gobas FAPC (1989) Calibration of the freshwater mussel,
Elliptio complanata, for quantitative biomonitoring of
hexachlorobenzene and octachlorostyrene in aquatic systems. Bull
Environ Contam Toxicol, 43: 576-582.
Salisbury CDC, Fesser ACE, MacNeil JD, & Patterson JR (1992) Trace
metal and pesticide levels in muskoxen from Victoria Island, Northwest
Territories, Canada. Intern. J Environ Chem, 48: 209-215.
Sanborn JR, Childers WF, & Hansen LG (1977) Uptake and elimination of
[14C] hexachlorobenzene (HCB) by the green sunfish, Lepomis
cyanellus Raf., after feeding contaminated food. J Agric Food Chem,
25: 551-553.
Schechter A, Fürst P, Krüger C, Meemken H-A, Groebel W, & Constable JD
(1989a) Levels of polychlorinated dibenzofurans, dibenzodioxins, PCBs,
DDT and DDE, hexachlorobenzene, dieldrin, hexachlorocyclohexanes and
oxychlordane in human breast milk from the United States, Thailand,
Vietnam, and Germany. Chemosphere, 18: 445-454.
Schechter A, Mes J, & Davies D (1989b) Polychlorinated biphenyl (PCB),
DDT, DDE and hexachlorobenzene (HCB) and PCDD/PCDF isomer levels in
various organs in autopsy tissue from North American patients.
Chemosphere, 18: 811-818.
Scheufler E & Rozman KK (1984) Comparative decontamination of
hexachlorobenzene-exposed rats and rabbits by hexadecane. J Toxicol
Environ Health, 14: 353-362.
Scheunert I, Marra C, Viswanatha R, Klein WK, & Korte F (1983) Fate of
hexachlorobenzene-14C in wheat plants and soil under outdoor
conditions. Chemosphere, 12: 843-858.
Scheunert I, Topp E, Schmitzer J, Klein W, & Korte F (1985) Formation
and fate of bound residues of [14C] benzene and [14C] chlorobenzene
in soil and plants. Ecotoxicol Environ Saf, 9: 159-170.
Schielen P, Schoo W, Tekstra J, Oostermeijer HHA, Seinen W, & Bloksma
N (1993) Autoimmmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schielen P, Den Besten C, Vos JG, Van Bladeren PJ, Seinen W, & Bloksma
N (1995) Immune effects of hexachlorobenzene in the rat: role of
metabolism in a 13-week feeding study. Toxicol Appl Pharmacol, 131:
37-43.
Schmid R (1960) Cutaneous porphyria in Turkey. N Engl J Med, 263:
397-398.
Schmitt CJ, Zaijcek JL, & Peterman PH (1990) National Contaminant
Biomonitoring Program: residues of organochlorine chemicals in U.S.
freshwater fish, 1976-1984. Arch. Environ Toxicol, 19: 748-781.
Schuler W, Brunn H, & Manz D (1985) Pesticides and polychlorinated
biphenyls in fish from the Lahn River. Bull Environ Contam Toxicol,
34: 608-616.
Schuytema G, Krawczyk DF, Griffis WL, Nebeker AV, & Robideaux ML
(1990) Hexachlorobenzene uptake by fathead minnows and
macroinvertebrates in recirculating sediment/water systems. Arch
Environ Contam Toxicol, 19: 1-9.
Schwarzenbach RP, Giger W, Hoehn E, & Schneider JK (1983) Behaviour of
organic compounds during infiltration of river water to groundwater.
Field studies. Environ Sci Technol, 17: 472-479.
Selden A, Jacobson G, Berg P, & Axelson O (1989) Hepatocellular
carcinoma and exposure to hexachlorobenzene: a case report. Br J Ind
Med, 46: 138-140.
Shirai T, Miyata Y, Nakanishi K, Murasaki G, & Ito N (1978)
Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in ICR mice
and its enhancement by hexachlorobenzene. Cancer Lett, 4: 271-275.
Siekel P, Chalupa I, Beno J, Blasko M, Novotny J, & Burian J (1991) A
genotoxicological study of hexachlorobenzene and pentachloroanisole.
Teratog Carcinog Mutagen, 11: 55-60.
Simon GS, Tardiff RG, & Borzelleca JF (1979) Failure of
hexachlorobenzene to induce dominant lethal mutations in the rat.
Toxicol Appl Pharmacol, 47: 415-419.
Sims DE, Singh A, Donald A, Jarrell J, & Villeneuve DC (1991)
Alteration of primate ovary surface epithelium by exposure to
hexachlorobenzene: a quantitative study. Histol Histopathol, 6:
525-529.
Singh A, Friesen D, Jarrel J, & Villeneuve DC (1990a)
Hexachlorobenzene toxicity in the monkey ovary: I. Ultrastructure
induced by low (0.1 mg/kg) dose exposure. In: Proceedings of the XIIth
International Congress for Electron Microscopy, Seattle, Washington -
Volume 2: Analytical sciences, San Francisco, California, San
Francisco Press, Inc., pp 282-283.
Singh A, Sims DE, Jarrell J, & Villeneuve DC (1990b) Hexachlorobenzene
toxicity in the monkey ovary: II. Ultrastructure induced by medium
(1.0 mg/kg) dose exposure. Anal Environ Toxicants, 13: 435-438.
Singh A, Dykeman A, Jarrell J, & Villeneuve DC (1991)
Hexachlorobenzene toxicity in the monkey ovary: III. Ultrastructure
induced by high (10 mg/kg) dose exposure. In: Proceedings of the 49th
Annual Meeting of the Electron Microscopy Society of America. San
Francisco, California, San Francisco Press, Inc.
Skåre JU, Stenersen J, Kveseth N, & Polder A (1985) Time trends of
organochlorine chemical residues in seven sedentary marine fish
species from a Norwegian fjord during the period 1972-1982. Arch
Environ Contam Toxicol, 14: 33-41.
Skaare JU, Markussen NH, Norheim G, Haugen S, & Holt G (1990) Levels
of polychlorinated biphenyls, organochlorine pesticides, mercury,
cadmium, copper, selenium, arsenic, and zinc in the harbour seal,
Phoca vitulina, in Norwegian waters. Environ Pollut, 66: 309-324.
Smith AG & Cabral JR (1980) Liver-cell tumours in rats fed
hexachlorobenzene. Cancer Lett, 11: 169-172.
Smith AG & De Matteis F (1990) Oxidative injury mediated by the
hepatic cytochrome P-450 system in conjunction with cellular iron.
Effects on the pathway of haem biosynthesis. Xenobiotica, 29(9):
865-877.
Smith AG & Francis JE (1983) Synergism of iron and hexachlorobenzene
inhibits hepatic uroporphyrinogen decarboxylase in inbred mice.
Biochem J, 214: 909-913.
Smith VE, Spurr JM, Filkins JC, & Jones JJ (1985a) Organochlorine
contaminants of wintering ducks foraging on Detroit River sediments. J
Great Lakes Res, 11(3): 231-246.
Smith AG, Francis JE, Dinsdale D, Manson MM, & Cabral JRP (1985b)
Hepatocarcinogenicity of hexachlorobenzene in rats and the sex
difference in hepatic iron status and development of porphyria.
Carcinogenesis, 6: 631-636.
Smith AG, Dinsdale D, Cabral JRP, & Wright AL (1987) Goitre and
wasting induced in hamsters by hexachlorobenzene. Arch Toxicol, 60:
343-349.
Smith AG, Cabral JRP, Carthew P, Francis JE, & Manson MM (1989)
Carcinogenicity of iron in conjunction with a chlorinated
environmental chemical, hexachlorobenzene, in C57BL/10ScSn mice. Int J
Cancer, 43: 492-496.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM (1990)
Sex-linked hepatic uroporphyria and the induction of cytochromes
P450IA in rats caused by hexachlorobenzene and polyhalogenated
biphenyls. Biochem Pharmacol, 40: 2059-2068.
Smith AG, Carthew P, Francis JE, Cabral JRP, & Manson MM (1993)
Enhancement by iron of hepatic neoplasia in rats caused by
hexachlorobenzene. Carcinogenesis, 14: 1381-1387.
Smith AG, Carthew P, Francis JE, & Ingebritsen K (1994) Influence of
iron on the induction of hepatic tumors and porphyria by
octachlorostyrene in C57BL/10ScSn mice. Cancer Lett, 81: 145-150.
Solé M, Porte C, Pastor D, & Albaigés J (1994) Long-term trends of
polychlorinated biphenyls and organochlorinated pesticides in mussels
from the Western Mediterranean coast. Chemosphere, 28(5): 897-903.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sopena de Kracoff YE, Ferramola de Sancovich AM, Sancovich HA, &
Kleiman de Pisarev DL (1994) Effect of thyroidectomy and thyroxine on
hexachlorobenzene induced porphyria. J Endocrinol Invest, 17:
301-305.
Spigarelli JL, Going JE, & Li R (1986) Hexachlorobenzene levels in
multimedia environmental samples from selected chemical production
plants. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 155-160 (IARC Scientific Publications No. 77).
Stacey CI, Perriman WS, & Whitney S (1985) Organochlorine pesticide
residue levels in human milk: Western Australia, 1979-1980. Arch
Environ Health, 40(2): 102-108.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary. Kansas
City, Missouri, Midwest Research Institute (Prepared for the US
Environmental Protection Agency, Office of Toxic Substances, Field
Studies Branch) (EPA 560/5-86/035).
Stevens RJJ & Neilson MA (1989) Inter- and intralake distribution of
trace contaminants in surface waters of the Great Lakes. J Great Lakes
Res, 15: 377-393.
Stevens MF, Ebell GF, & Psaila-Savona P (1993) Organochlorine
pesticides in Western Australian nursing mothers. Med J Aust, 158:
238-241.
Stewart FP & Smith AG (1987) Metabolism and covalent binding of
hexachlorobenzene by isolated male and female rat hepatocytes. Biochem
Pharmacol, 36: 2232-2234.
Stewart FP, Manson MM, Cabral JRP, & Smith AG (1989) Hexachlorobenzene
as a promoter of diethylnitrosamine-initiated hepatocarcinogenesis in
rats and comparison with induction of porphyria. Carcinogenesis,
10(7): 1225-1230.
Strik JJTWA (1986) Subacute toxicity of hexachlorobenzene. In: Morris
CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 335-342 (IARC Scientific Publications No. 77).
Stronkhorst J, Ysebaert TJ, Smedes F, Meininger PL, Dirksen S, &
Boudewijn TJ (1993) Contaminants in eggs of some waterbird species
from the Scheldt Estuary, SW Netherlands. Mar Pollut Bull, 26(10):
572-578.
Sundlof SF, Hansen LG, Koritz GD, & Sundlof SM (1982) The
pharmacokinetics of hexachlorobenzene in male beagles. Distribution,
excretion and pharmacokinetic model. Drug Metab Dispos, 10: 371-381.
Suns K, Craig GR, Crawford G, Rees GA, Tosine H, & Osborne J (1983)
Organochlorine contaminant residues in spottail shiner ( Notropis
hudsonius) from the Niagara River. J Great Lakes Res, 9: 335-340.
Suns K, Crawford GE, Russell DD, & Clement RE (1985) Temporal trends
and spatial distribution of organochlorine and mercury residues in
Great Lakes spottail shiners (1975-1983). Rexdale, Ontario, Ontario
Ministry of the Environment, Water Resources Branch.
Swindoll CM & Applehans FM (1987) Factors influencing the accumulation
of sediment-sorbed hexachlorobiphenyl by midge larvae. Bull Environ
Contam Toxicol, 39: 1055-1062.
Szymczyœski GA, Waliszewski SM, Tuszewski M, & Pyda P (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznaœ. J Environ Sci Health, A21(1): 5-14.
Tanabe S, Subramanian AN, Ramesh A, Kumaran PL, Miyazaki N, &
Tatsukawa R (1993) Persistent organochlorine residues in dolphins from
the Bay of Bengal, South India. Mar Pollut Bull, 26(6): 311-316.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Teschke R, Bolsen K, Landmann H, & Goerz G (1983) Effect of
hexachlorobenzene on the activities of hepatic alcohol metabolizing
enzymes. Biochem Pharmacol, 32: 1745-1751.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound: Volume 1 (Prepared for Puget Sound Dredged Disposal
Analysis and Puget Sound Estuary Program, Bellevue, Washington).
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in Strain A mice. Cancer Res, 37:
2717-2720.
Thomas W, Simon H, & Rüling Å (1985) Classification of plant species
by their organic (PAH, PCB, BHC) and inorganic (heavy metals) trace
pollutant concentrations. Sci Total Environ, 46: 83-94.
Thomson BA, Davidson WR, & Lovett AM (1980) Applications of a
versatile technique for trace analysis: Atmospheric pressure negative
chemical ionization. Environ Health Perspect, 36: 77-84.
Tobin P (1986) Known and potential sources of hexachlorobenzene. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 3-11 (IARC Scientific Publications No. 77).
To-Figueras J, Rodamilans M, Gómez-Catalán J, & Corbella J (1986)
Hexachlorobenzene residues in the general population of Barcelona,
Spain. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, p 147 (IARC Scientific Publications No. 77).
To-Figueras J, Gómez-Catalán J, Rodamilans M, & Corbella J (1992)
Sulphur derivative of hexachlorobenzene in human urine. Hum Exp
Toxicol, 11: 271-273.
To-Figueras J, Barrot C, Rodamilans M, Gómez-Catalán J, Torra M,
Brunet M, Sabater F, & Corbella J (1995) Accumulation of
hexachlorobenzene in humans: a long standing risk. Hum Exp Toxicol,
14: 20-23.
Topp E, Scheunert I, & Korte K (1989) Kinetics of the uptake of
14C-labelled chlorinated benzenes from soil by plants. Ecotoxicol
Environ Saf, 17: 157-166.
Trapp M, Baukloh V, Bohnet H-G, & Heeschen W (1984) Pollutants in
human follicular fluid. Fertil Steril, 42(1): 146-148.
US EPA (1980) Analysis of pesticide residues in human and
environmental samples: A compilation of methods selected for use in
pesticide monitoring programs. Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Toxicology Division,
Health Effects Research Laboratory (EPA 600/8-80-038).
US EPA (1982) Test methods: Methods of organic chemical analysis of
municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Office of Research and Development,
Environmental Monitoring and Support Laboratory (EPA 600/4-82-057).
US EPA (1985a) Health assessment document for chlorinated benzenes.
Washington, DC, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA 600/8-84-015F).
US EPA (1985b) Drinking water criteria document for hexachlorobenzene.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Criteria and Assessment Office (EPA 600/X-84-179-1).
US EPA (1986a) Exposure assessment for hexachlorobenzene. Washington,
DC, US Environmental Protection Agency, Office of Pesticides and Toxic
Substances (EPA 560/5-86-0(19).
US EPA (1986b) Test methods for evaluating solid waste, 3rd ed.
Washington, DC, US Environmental Protection Agency, Office of Solid
Waste and Emergency Response (SW 846).
US EPA (1988) Ambient aquatic life water quality criteria for
hexachlorobenzene. Duluth, Minnesota, US Environmental Protection
Agency, Office of Research and Development.
US FDA (US Food and Drug Administration) (1990) Residues in foods -
1988. J Assoc Off Anal Chem, 73: 133A-152A.
US FDA (US Food and Drug Administration) (1991) Residues in foods -
1989. J Assoc Off Anal Chem, 74: 127A-146A.
US FDA (US Food and Drug Administration) (1992) Residues in foods -
1990. J Assoc Off Anal Chem, 75: 121A-141A.
Üstünbas HB, Öztürk MA, Hasanoglu E, & Dogan M (1994) Organochlorine
pesticide residues in human milk in Kayseri. Hum Exp Toxicol, 13:
299-302.
Van der Ven K, Van der Ven H, Thibold A, Bauer O, Kaisi M, Mbura J,
Mhaya HN, Wever N, Diedrich K, & Krebs D (1992) Chlorinated
hydrocarbon content of fetal and maternal body tissues and fluids in
full term pregnant women: a comparison of Germany versus Tanzania. Hum
Reprod, 7(1): 95-100.
Van Leeuwen CJ, Van der Zandt PTJ, Aldenberg T, Verhaar HJM, & Hermans
JLM (1992) Application of QSARs, extrapolation and equilibrium
partitioning in aquatic effects assessment: I. Narcotic industrial
pollutants. Environ Toxicol Chem, 11: 267-282.
Van Loveren H, Kranjc EI, Rombout PJA, Blommaert FA, & Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Ommen B, Hendriks W, Bessems JGM, Geesink G, Müller F, & Van
Bladeren PJ (1989) The relation between the oxidative
biotransformation of hexachlorobenzene and its porphyrinogenic
activity. Toxicol Appl Pharmacol, 100: 517-528.
Van Raaij JAGM, Van den Berg KJ, & Notten WRF (1991a)
Hexachlorobenzene and its metabolites pentachlorophenol and
tetrachlorohydroquinone: interaction with thyroxine binding sites of
rat thyroid hormone carriers ex vivo and in vitro. Toxicol Lett,
59: 101-107.
Van Raaij JAGM, Van den Berg KJ, Engel R, Bragt PC, & Notten WRF
(1991b) Effects of hexachlorobenzene and its metabolites
pentachlorophenol and tetrachlorohydroquinone on serum thyroid hormone
levels in rats. Toxicology, 67: 107-116.
Van Raaij JAGM, Frijters CMG, & Van den Berg KJ (1993a)
Hexachlorobenzene-induced hypothyroidism: involvement of different
mechanisms by parent compound and metabolite. Biochem Pharmacol, 46:
1385-1391.
Van Raaij JAGM, Kaptein E, Visser TJ, & Van den Berg KJ (1993b)
Increased glucoronidation of thyroid hormone in hexachlorobenzene-
treated rats. Biochem Pharmacol, 45: 627-631.
Vaz R, Slorach SA, & Hofvander Y (1993) Organochlorine contaminants in
Swedish human milk: studies conducted at the National Food
Administration 1981-1990. Food Addit Contam, 10(4): 407-418.
Villanueva EC, Jennings RW, Burse VW, & Kimbrough RD (1974) Evidence
of chlorodibenzo-p-dioxin and chlorodibenzofuran in hexachlorobenzene.
J Agric Food Chem, 22: 916-917.
Villeneuve DC, Panopio LG, & Grant DL (1974) Placental transfer of
hexachlorobenzene in the rabbit. Environ Physiol Biochem, 4:
112-115.
Vincent SH, Smith AG, & Muller-Eberhard U (1989) Modulation of hepatic
heme-binding Z protein in mice by the porphyrogenic carcinogens
griseofulvin and hexachlorobenzene. Cancer Lett, 45: 109-114.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
ymposium. Lyon, International Agency for Research on Cancer,
pp 347-356 (IARC Scientific Publications No. 77).
Vos JG, Breeman HA, & Benschop H (1968) The occurrence of the
fungicide hexachlorobenzene in wild birds and its toxicological
importance: a preliminary communication. Meded Fac. Landbouwwet
Rijksuniv Gent, 33: 1263-1269.
Vos JG, Van der Maas HL Musch A, & Ram E (1971) Toxicity of
hexachlorobenzene in Japanese quail with special reference to
porphyria, liver damage, reproduction and tissue residues. Toxicol
Appl Pharmacol, 18: 944-957.
Vos JG, Botterweg PF, Strik JJTWA, & Koeman JH (1972) Experimental
studies with HCB in birds. TNO-Nieuws, 27: 599-603.
Vos JG, Van Logten MJ, Kreeftenberg JG, & Kruizinga W (1979a)
Hexachlorobenzene-induced stimulation of the humoral immune response
in rats. Ann NY Acad Sci, 320: 535-550.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979b) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Brouwer GMJ, Van Leeuwen FXR, & Wagenaar SJ (1983) Toxicity of
hexachlorobenzene in the rat following combined pre- and post-natal
exposure: comparison of effects on the immune system, liver and lung.
In: Parke DV, Gibson GG, & Hubbard R ed. Immunotoxicology. New York,
London, Academic Press, pp 219-235.
Vos RME, Snoek MC, Van Berkel WJH, Müller F, & Van Bladeren PJ (1988)
Differential induction of rat hepatic glutathione S-transferase
isoenzymes by hexachlorobenzene and benzyl isothiocyanate: comparison
with induction by phenobarbital and 3-methylcholanthrene. Biochem
Pharmacol, 37: 1077-1082.
Wada O, Yano Y, Urata G, & Nakao K (1968) Behavior of hepatic
microsomal cytochromes after treatment of mice with drugs known to
disturb porphyrin metabolism in liver. Biochem Pharmacol, 17:
595-603.
Wang M-J & Jones KC (1994) Occurrence of chlorobenzenes in nine United
Kingdom retail vegetables. J Agric Food Chem, 42: 2322-2328.
Washington State Department of Ecology (1990) Proposed sediment
management standards rule (September 18, 1990). Washington State
Register, issue 90-19.
Watts RR, Hodgson DW, Christ HL, & Moseman RF (1980) Improved method
for hexachlorobenzene and mirex determination with hexachlorobenzene
confirmation in adipose tissue: Collaborative study. J Assoc Off Anal
Chem, 63(5): 1128-1134.
Weisenberg E, Arad I, Grauer F, & Sahm Z (1985) Polychlorinated
biphenyls and organochlorine insecticides in human milk in Israel.
Arch Environ Contam Toxicol, 14: 517-521.
Wells DE, Campbell LA, Ross HM, Thompson PM, & Lockyer CH (1994)
Organochlorine residues in harbour porpoise and bottlenose dolphins
stranded on the coast of Scotland, 1988-1991. Sci Total Environ,
151: 77-99.
WHO (1993) Guidelines for drinking-water quality - Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization, p 84.
Wickström K, Pyysalo H, & Siimes MA (1983) Levels of chlordane,
hexachlorobenzene, PCB and DDT compounds in Finnish human milk in
1982. Bull Environ Contam Toxicol, 31: 251-256.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71: 410-414.
Wilson DM & Wan MTK (1982) Hexachlorobenzene: Industrial and
agricultural sources in British Columbia. Vancouer, Environment
Canada, Environmental Protection Service (Regional Program Report
No. 82-07).
Witte H, Langenohl T, & Offenbacher SAG (1988a) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part A: Organic pollutant load in sewage
sludge. Korresp Abwasser, 13: 118-125.
Witte H, Langenohl T, & Offenbacher SAG (1988b) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part B: Impact of the application of
sewage sludge on organic matter content in soil and plants. Korresp
Abwasser, 13: 126-136.
Yamaguchi Y, Kawano M, & Tatsukawa R (1986) Tissue distribution and
excretion of hexabromobenzene (HBB) and hexachlorobenzene (HCB)
administered to rats. Chemosphere, 15: 453-459.
Yang RSH, Pittman KA, Rourke DR, & Stein VB (1978) Pharmacokinetics
and metabolism of hexachlorobenzene in the rat and the rhesus monkey.
J Agric Food Chem, 26: 1076-1083.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés chimiques et physiques et méthodes d'analyse
L'hexachlorobenzène (HCB) est un composé organique chloré
modérément volatil. Il est pratiquement insoluble dans l'eau, mais
extrêmement soluble dans les lipides et présente une tendance à la
bioaccumulation. L'hexachlorobenzène de qualité technique contient
jusqu'à 2% d'impuretés, dont la principale est le pentachlorobenzène.
Les autres consistent en dibenzo- p-dioxines, dibenzofuranes et
biphényles fortement substitués par le chlore. L'analyse des
échantillons biologiques ou prélevés dans l'environnement comporte
généralement une extraction préliminaire de la prise d'essai, souvent
suivie d'une purification, après quoi les extraits organiques sont
soumis soit à une chromatographie en phase gazeuse couplée à la
spectrométrie de masse (GC/MS), soit à une chromatographie en phase
gazeuse avec détection par capture d'électrons (GC/ECD).
2. Sources d'exposition humaine et environnementale
L'hexachlorobenzène a été utilisé un temps comme fongicide pour
traiter les semences, mais il n'est plus actuellement utilisé à cet
effet dans la plupart des pays. Il continue néanmoins à être libéré
dans l'environnement à partir d'un certain nombre de sources,
notamment lors de l'épandage de pesticides organochlorés, ou encore
lorsque les sous-produits de la préparation des solvants, composés
aromatiques ou pesticides chlorés sont rejetés sans précautions,
incomplètement brûlés ou s'échappent de décharges anciennes.
3.Transport, distribution et transformation dans l'environnement
L'hexachlorobenzène est réparti dans tout l'environnement du fait
de sa mobilité et de sa persistance, même s'il se décompose lentement
dans l'air sous l'action de la lumière et dans le sol sous l'action
des microorganismes. Dans la troposphère, il est transporté sur de
grandes distances et s'élimine de l'air en se déposant sur le sol et
sur l'eau. On a fait état d'une bioamplification notable le long de la
chaîne alimentaire.
4. Concentrations dans l'environnement et exposition humaine
Un peu partout dans le monde, l'hexachlorobenzène est présent, à
distance de ses sources, sous faible concentration dans l'air ambiant
(quelques ng/m3 ou moins) ainsi que dans l'eau de boisson et les eaux
de surface (quelques ng/litre tout au plus). Cependant, au voisinage
des points d'émission, on a pu mesurer des concentrations plus
élevées. Ce composé s'accumule dans les milieux biologiques et on en a
décelé la présence chez des invertébrés, des poissons, des reptiles,
des oiseaux et des mammifères (y compris l'Homme) à distance des
points d'émission, en particulier dans les tissus adipeux des
organismes situés aux niveaux trophiques supérieurs. Chez la
population humaine de divers pays, on en a mesuré dans les tissus
adipeux des quantités qui vont, en moyenne, de quelques dizaines à
quelques centaines de ng/g de poids humide. En se fondant sur les
quantités représentatives d'hexachlorobenzène présentes dans l'air,
l'eau et les denrées alimentaires, on peut estimer à une valeur
comprise entre 0,0004 et 0,003 µg/kg de poids corporel, la dose
absorbée journalièrement par un adulte de la population générale. Cet
apport se fait principalement par la voie alimentaire. Du fait de la
présence d'hexachlorobenzène dans le lait maternel, on estime que dans
les différents pays les enfants nourris au sein en reçoivent
quotidiennement une quantité comprise entre < 0,018 et 5,1 µg/kg de
poids corporel. Les études consacrées à l'évolution de la quantité
d'hexachlorobenzène présente dans l'organisme humain montrent, pour la
plupart, que l'exposition de la population générale a baissé dans de
nombreux endroits, entre les années 70 et le milieu de la décennie
actuelle.
5. Cinétique et métabolisme chez l'Homme et les animaux de
laboratoire
On manque de données toxicocinétiques chez l'Homme.
L'hexachlorobenzène est facilement résorbé par la voie orale chez
l'animal d'expérience, mais il franchit mal la barrière cutanée (on ne
possède pas de données concernant l'inhalation). Chez l'Homme et
l'animal, il s'accumule dans les tissus riches en lipides, comme les
tissus adipeux, le cortex surrénalien, la moelle osseuse, la peau et
certains tissus endocriniens. En outre, il peut être transmis à la
progéniture par l'intermédiaire du lait maternel ou en traversant la
barrière placentaire. La métabolisation de l'hexachlorobenzène est
limitée et ses principaux métabolites urinaires sont le
pentachlorophénol, la tétrachlorhydroquinone et le
pentachlorothiophénol. La demi-vie d'élimination de
l'hexachlorobenzène va d'environ un mois chez les rats et les lapins à
2 ou 3 ans chez le singe.
6. Effets sur les animaux de laboratoire et dans les épreuves
in vitro
L'hexachlorobenzène présente une faible toxicité aiguë pour les
animaux de laboratoire (1000 à 10 000 mg/kg de poids corporel).
L'expérimentation animale montre en outre que ce composé n'est pas
irritant pour la peau ou les yeux et ne provoque pas de
sensibilisation chez le cobaye.
Les données dont on dispose au sujet de la toxicité générale de
l'hexachlorobenzène indiquent que celle-ci s'exerce notamment au
niveau de la voie de biosynthèse de l'hème. Chez plusieurs espèces de
mammifères de laboratoire exposés à de l'hexachlorobenzène, on a
constaté une élévation des concentrations de porphyrines ou de leurs
précurseurs dans les excréta ainsi que dans divers tissus, notamment
le tissu hépatique. De nombreuses études ont relevé des cas de
porphyrie chez des rats exposés de manière chronique ou subchronique à
de l'hexachlorobenzène administré par voie orale à des doses
quotidiennes comprises entre 2,5 et 15 mg par kg de poids corporel.
Chez des porcs à qui on faisait ingérer ce composé en doses
quotidiennes égales ou supérieures à 0,5 mg par kg de poids corporel,
on a observé une augmentation de l'excrétion des coproporphyrines
(aucun effet n'a été observé dans cette étude à la dose de 0,05
mg/kg). On a également montré que l'exposition à l'hexachlorobenzène
affectait de nombreux organes ou systèmes (comme le foie, les poumons,
les reins, la thyroïde, la peau ainsi que le système nerveux et le
système immunitaire) mais ces effets n'ont pas été aussi souvent
signalés que la porphyrie.
L'hexachlorobenzène est un inducteur du cytochrome P-450 de type
mixte. Il possède des propriétés phénobarbital-inductibles et
3- méthylcholantrène-inductibles. Il se fixe sur le récepteur Ah.
Lors d'études longitudinales sur des rats, on a observé à
plusieurs reprises des effets bénins (modifications
histopathologiques, induction d'enzymes) chez les animaux recevant des
doses quotidiennes comprises entre 0,25 et 0,6 mg de composé par kg de
poids corporel. La dose sans effet observable obtenue dans ces études
se situait entre 0,05 et 0,07 mg d'hexachlorobenzène par kg de poids
corporel et par jour. Chez des visons femelles, on a observé une
modification de la concentration de neurotransmetteurs dans
l'hypothalamus après administration prolongée du composé par la voie
alimentaire à la dose quotidienne de 0,16 mg par kg de poids corporel.
Les mêmes constatations ont été faites dans la progéniture de ces
animaux, qui avait été exposée pendant les périodes gestationnelle et
périnatale. Lors d'études subchroniques sur des rats on a constaté une
modification de l'homéostase calcique et des paramètres
ostéomorphométriques à la dose quotidienne de 0,7 mg/kg de poids
corporel, mais pas à celle de 0,07 mg/kg.
Un certain nombre d'études in vivo ont été effectuées sur des
rongeurs afin de mettre en évidence la cancérogénicité éventuelle de
l'hexachlorobenzène. Chez des hamsters qui avaient reçu une nourriture
contenant de l'hexachlorobenzène à la dose moyenne de 4, 8 ou 16 mg/kg
de poids corporel, on a observé chez les deux sexes et à toutes les
doses un accroissement de l'incidence des carcinomes
hépatocellulaires. Aux doses de 8 et 16 mg/kg, on constatait la
présence d'hémangioendothéliomes et à la dose la plus forte,
d'adénomes de la thyroïde chez les mâles. En exposant pendant 120
semaines des souris à ce composé par la voie alimentaire aux doses
quotidiennes respectives de 6, 12 et 24 mg/kg de poids corporel, on a
provoqué un accroissement de l'incidence des carcinomes
hépatocellulaires chez les deux sexes aux deux doses les plus élevées,
mais cet accroissement n'était pas significatif, sauf chez les
femelles exposées à la dose la plus forte. In utero, l'exposition de
rats par la voie orale ou lactationnelle à des doses alimentaires
quotidiennes d'hexachlorobenzène allant de 0,01 à 1,5 mg/kg de poids
corporel (mâles) ou de 1,9 mg/kg (femelles) pendant des périodes
pouvant durer jusqu'à 130 semaines post utero, c'est-à-dire la durée
de vie moyenne, a entraîné à la dose la plus forte un accroissement de
l'incidence des nodules hépatiques néoplasiques et des
phéochromocytomes surrénaliens chez les femelles et un excès
d'adénomes parathyroïdiens chez les mâles. Lors d'une autre étude
chronique effectuée sur des rats, on a exposé les animaux, par la voie
alimentaire et pendant des durées allant jusqu'à 2 ans, à des doses
journalières moyennes de 4-5 et 8-9 mg/kg de poids corporel. Les
effets constatés consistaient en une augmentation de l'incidence des
hépatomes et des adénomes rénaux aux deux doses et chez les deux
sexes. Chez les femelles, on observait en outre une augmentation de
l'incidence des carcinomes hépatocellulaires, des adénomes et des
carcinomes des voies biliaires, des phéochromocytomes et des adénomes
du cortex surrénalien. On a également signalé une incidence élevée des
tumeurs du foie dans un certain nombre d'études plus limitées au cours
desquelles on avait administré une seule dose d'hexachlorobenzène par
la voie alimentaire à de petits groupes de rates. Par ailleurs, on a
observé qu'après exposition subchronique par voie alimentaire à ce
composé, des souris, des hamsters et des rats avaient présenté des
tumeurs du foie, des voies biliaires, du rein, du thymus, de la rate
et des ganglions lymphatiques. Le même type d'exposition favorise
l'apparition de tumeurs hépatiques chez des souris sous l'action de
terphényles polychlorés et chez des rats, sous l'action de la
diéthylnitrosamine.
Sauf dans le cas des tumeurs rénales chez les rats mâles (qui, du
moins en partie, semblent résulter d'une dégénérescence hyaline) et
des hépatomes chez les rats des deux sexes (qui pourraient résulter de
réactions hyperplasiques à la nécrose hépatocellulaire), on n'a pas pu
trouver d'études mécanistiques concernant les divers types de tumeurs
provoquées par l'hexachlorobenzène et le risque encouru à cet égard
par l'Homme.
L'hexachlorobenzène n'a guère d'aptitude à provoquer directement
des mutations géniques, des lésions chromosomiques ou la réparation de
l'ADN. Il s'est révélé faiblement mutagène lors de quelques-unes des
études portant sur des bactéries et des levures, mais il convient de
noter que chacune de ces études comportait des limitations. Il y a
également des signes d'un faible taux de liaison à l'ADN in vitro et
in vivo, mais dans une proportion très inférieure à celle que l'on
attendrait d'une substance cancérogène génotoxique.
Lors d'études sur la reproduction, des doses d'hexachlorobenzène
ne dépassant pas 0,1 mg par kg de poids corporel qu'on avait fait
ingérer quotidiennement pendant 90 jours à des singes, ont provoqué
des anomalies dans la structure microscopique et l'ultrastructure de
l'épithélium germinatif superficiel, structures qui constituent une
cible inhabituelle pour des toxines ovariennes. Cette dose a également
endommagé l'ultrastructure des cellules germinales primordiales. Alors
même que ces sites étaient spécifiquement attaqués et présentaient des
lésions d'autant plus importantes que la dose était plus forte, le
développement folliculaire, ovocytaire et embryonnaire restait normal,
ce qui semble indiquer que l'hexachlorobenzène a un site d'action à
localisation spécifiquement ovarienne. Chez les mâles, la fonction de
reproduction n'est affectée qu'à des doses beaucoup plus élevées
(entre 30 et 221 mg/kg p.c. par jour), comme l'ont montré un certain
nombre d'études effectuées sur plusieurs espèces n'appartenant pas à
l'ordre des primates.
Des rats et des chats exposés par la voie transplacentaire ou
lactationnelle à des doses quotidiennes d'hexachlorobenzène comprises
entre 3 et 4 mg/kg p.c. ont présenté des signes d'hépatotoxicité et on
a également constaté des effets délétères sur la survie et la
croissance de leur progéniture. Dans certains cas, il y avait à ces
doses - ou à des doses plus élevées - une réduction de l'effectif des
portées et un nombre accru de mortinaissances. (En général, les ratons
et les chatons à la mamelle étaient plus souvent affectés - et à des
doses plus faibles - que les embryons et les foetus). Chez la
progéniture de visons qui recevaient une alimentation ne contenant pas
plus de 1 mg d'hexachlorobenzène par kg de nourriture (soit environ
0,16 mg/kg p.c. par jour), on a constaté une réduction du poids de
naissance et un accroissement de la mortalité au sevrage. Quelques
études ont mis en évidence des anomalies squelettiques ou rénales chez
les foetus de rats et de souris exposés à de l'hexachlorobenzène
pendant la gestation, mais les doses qui produisaient ces anomalies
n'étaient pas toxiques pour les mères. Par ailleurs, le lien de ces
anomalies avec la prise d'hexachlorobenzène n'a pas été formellement
établi. Dans deux études, dont l'une comportait une exposition
transplacentaire et postnatale, on a observé des anomalies du
développement neurocomportemental des ratons après exposition
in utero, les mères ayant reçu par voie orale des doses quotidiennes
d'hexachlorobenzène allant de 0,64 à 2,5 mg/kg de poids corporel.
Selon un certain nombre d'études, l'hexachlorobenzène aurait des
effets délétères sur le système immunitaire. Chez des rats et des
singes exposés à des doses quotidiennes comprises entre 3 et 120 mg
d'hexachlorobenzène par kg de poids corporel, on a constaté des
modifications histopathologiques au niveau du thymus, de la rate, des
ganglions lymphatiques et des tissus lymphoïdes pulmonaires. Chez des
chiens beagle exposés de façon chronique à des doses quotidiennes
correspondant à 0,12 mg de composé par kg p.c., on a observé une
hyperplasie nodulaire du tissu lymphoïde gastrique. Un certain nombre
d'études menées sur des rats ont montré qu'après plusieurs semaines
d'exposition à de l'hexachlorobenzène par la voie alimentaire, il y
avait stimulation de l'immunité humorale, et dans une moindre mesure,
de l'immunité à médiation cellulaire, sans modification de la fonction
des macrophages. A des doses quotidiennes ne dépassant pas 4 mg de
composé par kg de nourriture (environ 0,2 mg par kg p.c.),
administrées pendant la gestation, pendant le maternage et jusqu'à
l'âge de 5 semaines, il y a eu augmentation de la réponse immunitaire
à médiation cellulaire et de la réponse immunitaire humorale ainsi
qu'une accumulation de macrophages dans le tissu pulmonaire des
ratons. Par contre, la plupart des études effectuées sur des souris
ont fait ressortir les propriétés immunosuppressives de
l'hexachlorobenzène; des doses ne dépassant pas 0,5 à 0,6 mg/kg de
poids corporel administrées quotidiennement pendant plusieurs semaines
ont eu les effets suivants: diminution de la résistance à une
infection leishmanienne ou à une épreuve cancérogène par exposition à
des cellules tumorales, réduction de l'activité cytotoxique des
macrophages spléniques et de l'hypersensibilité retardée chez la
progéniture après exposition in utero ou pendant la période de
maternage. Lors d'un certain nombre d'études portant sur diverses
souches de rats, on a constaté qu'une exposition de brève durée ou une
exposition subchronique à de l'hexachlorobenzène modifiait la fonction
thyroïdienne, comme on pouvait en juger d'après la réduction de la
thyroxine sérique libre ou totale (T4) et souvent, mais dans une
moindre mesure, de la triiodothyronine (T3).
7. Effets sur l'Homme
La plupart des données que l'on possède au sujet des effets de
l'hexachlorobenzène sur l'Homme, proviennent d'intoxications
accidentelles qui se sont produites en Turquie en 1955-59, avec plus
de 600 cas répertoriés de porphyrie cutanée tardive. Lors de cet
accident, on a observé des troubles du métabolisme des porphyrines,
des lésions cutanées, des hyperpigmentations, des hypertrichoses, des
hépatomégalies, des hypertrophies de la thyroïde et des ganglions
lymphatiques, avec, dans environ la moitié des cas, une ostéoporose et
une arthrite, principalement d'ailleurs, chez les enfants. Les enfants
nourris au sein dont la mère avait été exposée, présentaient des
lésions appelées pembe yara, c'est-à-dire "lésions roses", et la
plupart d'entre eux sont décédés dans l'année. On dispose de quelques
données concernant des cas de porphyrie cutanée tardive chez des
personnes ayant subi une exposition relativement intense à
l'hexachlorobenzène sur leur lieu de travail ou dans leur
environnement général.
Les quelques études épidémiologiques disponibles concernant le
cancer souffrent d'un certain nombre d'insuffisances: effectif réduit,
exposition à l'hexachlorobenzène mal caractérisée ou exposition
simultanée à de nombreux autres agents, et ne permettent pas d'évaluer
la cancérogénicité de ce composé pour l'Homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Lors d'études sur la toxicité aiguë de l'hexachlorobenzène pour
les organismes aquatiques, on a constaté que l'exposition à des
concentrations de l'ordre de 1 à 17 µg/litre réduisait la production
de chlorophylle chez les algues ainsi que la reproduction chez les
ciliés, et qu'en outre, elle provoquait la mort des crevettes roses et
des crevettes américaines du genre Hippolyte, mais elle n'a pas
provoqué la mort de poissons d'eau douce ou de mer. Lors d'études à
plus long terme, on a constaté que la croissance de certaines algues
et protozoaires dulçaquicoles sensibles étaient affectée à une
concentration d'hexachlorobenzène de 1 µg/litre et que des
concentrations d'environ 3 µg/litre provoquaient la mort d'amphipodes
et de perches appartenant à l'espèce Micropterus salmoides.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
9.1 Effets sur la santé
Le Groupe de travail a conclu que les données disponibles sont
suffisantes pour que l'on puisse formuler des valeurs-guides relatives
aux effets cancérogènes et non cancérogènes de l'hexachlorobenzène.
En ce qui concerne les effets non cancérogènes constatés sur le
foie à dose élevée chez des porcs et des rats exposés par la voie
orale et en se basant sur la dose sans effet observable la plus faible
(0,05 mg/kg de poids corporel par jour), on arrive, compte tenu d'un
facteur d'incertitude de 300 (10× pour les variations
interspécifiques, 10x pour les variations intraspécifiques et 3× pour
la gravité de l'effet), à une TDI de 0,17 µg/kg de poids corporel.
La méthode utilisée pour déterminer la valeur-guide relative aux
effets cancérogènes repose sur la dose tumorigène TD5, c'est-à-dire
la dose ingérée qui provoque une augmentation de 5% de l'incidence
tumorale chez les animaux de laboratoire. D'après les résultats d'une
étude de cancérogénicité portant sur deux générations de rats et en
faisant appel à un modèle multiphasique, on obtient une TD5 de
0,81 mg/kg de poids corporel par jour, l'effet retenu étant la
formation de nodules cancéreux hépatiques chez les femelles. Compte
tenu de l'insuffisance des données mécanistiques, on a appliqué un
facteur d'incertitude de 5000 pour calculer la valeur-guide chiffrée à
0,16 µg/kg de poids corporel par jour.
9.2 Effets sur l'environnement
Le Groupe de travail a remarqué qu'il existe très peu d'études
expérimentales à partir desquelles on puisse procéder à une évaluation
du risque écologique. La concentration d'hexachlorobenzène dans les
eaux de surface est généralement inférieure de plusieurs ordres de
grandeur à celle qui pourrait être dangereuse pour les organismes
aquatiques, sauf dans certains endroits fortement pollués. Toutefois,
les concentrations d'hexachlorobenzène relevées dans les oeufs
d'oiseaux de mer et de rapaces en différents lieux du globe sont
proches de celles qui provoquent une diminution du poids des embryons
chez la mouette argentée (1500 µg/kg), ce qui incite à penser que le
composé pourrait être embryotoxique pour certaines espèces sensibles
d'oiseaux. De même, les concentrations d'hexachlorobenzène dans les
poissons de divers endroits du monde sont du même ordre de grandeur
que la dose de 1000 µg/kg qui entraîne une réduction du poids de
naissance et une augmentation de la mortalité chez la progéniture de
visons. Cela incite à penser que ce composé pourrait avoir des effets
indésirables chez les visons et éventuellement chez d'autres
mammifères piscivores.
10. Conclusions
a) L'hexachlorobenzène est un composé chimique persistant qui subit
une bioaccumulation du fait de sa liposolubilité et de sa résistance à
la décomposition.
b) L'expérimentation animale montre que l'hexachlorobenzène provoque
des cancers et affecte de nombreux organes, tissus et systèmes comme
le foie, les poumons, les reins, la thyroïde, les tissus des gonades,
le système nerveux et le système immunitaire.
c) En ce qui concerne l'Homme, on a pu observer, à l'occasion d'une
forte exposition d'origine accidentelle, les manifestations cliniques
d'une intoxication par l'hexachlorobenzène qui se traduisaient par une
porphyrie cutanée tardive chez les enfants et les adultes et par la
mort chez des nourrissons alimentés au sein.
d) Il est justifié de prendre diverses mesures pour réduire la
quantité d'hexachlorobenzène présente dans l'environnement.
e) On a proposé les valeurs-guides à visée sanitaire suivantes pour
la dose totale ingérée quotidiennement (TDI): en ce qui concerne les
effets non cancérogènes, 0,17 µg/kg de poids corporel par jour ; en ce
qui concerne les effets cancérogènes, 0,16 µg/kg de poids corporel par
jour.
1. RÉSUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El hexaclorobenceno (HCB) es un compuesto orgánico clorado de
volatilidad moderada. Es prácticamente insoluble en el agua, pero es
muy liposoluble y bioacumulativo. El HCB de calidad técnica contiene
hasta un 2% de impurezas, en su mayor parte pentaclorobenceno; el
resto incluye dibenzo- p-dioxinas, dibenzofuranos y bifenilos
altamente clorados. Para determinar el HCB en el medio ambiente y en
material biológico se procede por lo general a extraer la muestra
mediante disolventes orgánicos, a lo que sigue con frecuencia un paso
de limpieza, a fin de obtener extractos orgánicos analizables mediante
cromatografía de gases/espectrometría de masas (GC/MS) o cromatografía
de gases con detección de captura de electrones (GC/ECD).
2. Fuentes de exposición humana y ambiental
Hubo un tiempo en que el HCB se utilizó mucho en la limpieza de
semillas para prevenir las enfermedades micóticas de los cereales,
pero ese uso se abandonó en la mayor parte de los países en los años
setenta. El HCB se sigue liberando en el medio ambiente a partir de
diversas fuentes, que incluyen el uso de algunos plaguicidas clorados,
procesos de combustión incompleta y viejos vertederos, así como los
métodos inapropiados de producción y de eliminación de desechos en la
fabricación de disolventes clorados, compuestos aromáticos clorados y
plaguicidas clorados.
3. Transporte, distribución y transformación en el medio ambiente
El HCB se distribuye por todo el medio ambiente porque es móvil y
persistente, aunque se produce una lenta fotodegradación en el aire y
una degradación microbiana en el suelo. En la troposfera el HCB es
transportado a grandes distancias y es eliminado de la fase aérea por
su depósito en el agua y el suelo. Se ha informado de que se produce
una importante bioamplificación del HCB a través de la cadena trófica.
4. Niveles ambientales y exposición humana
Se encuentran concentraciones bajas de HCB en el aire ambiental
(a lo sumo unos pocos ng/m3), en el agua de bebida y en las aguas
superficiales (a lo sumo unos pocos ng/litro) de zonas alejadas del
punto emisor en todo el mundo. No obstante, se han hallado
concentraciones más altas cerca de los puntos emisores. El HCB es
bioacumulativo y se ha detectado en invertebrados, peces, reptiles,
aves y mamíferos (incluido el hombre) lejos de los puntos emisores,
particularmente en el tejido adiposo de organismos de los niveles
tróficos más altos. Los niveles medios en el tejido adiposo de la
población humana general en diversos países van de decenas a centenas
de ng/g de peso en fresco. Considerando los niveles representativos de
HCB en el aire, el agua y los alimentos, se estima que la ingesta
total de HCB por los adultos de la población general está comprendida
entre 0,0004 y 0,003 mg/kg de peso corporal al día. Esa ingesta se
realiza principalmente a través de los alimentos. Debido a la
presencia de HCB en la leche materna, se ha estimado que la ingesta
media por los lactantes alimentados al pecho en diversos países va de
< 0,018 a 5,1 mg/kg de peso corporal al día. Los resultados de la
mayoría de los estudios realizados acerca de las concentraciones de
HCB en los alimentos y en los tejidos humanos a lo largo del tiempo
indican que la exposición de la población general al HCB disminuyó
desde los años setenta hasta mediados de los noventa en muchos
lugares. Sin embargo, esa tendencia no se ha confirmado con claridad
durante el último decenio en otros lugares.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay suficientes datos sobre la toxicocinética en el hombre. El
HCB es absorbido rápidamente por vía oral por los animales de
experimentación, y escasamente a través de la piel (no existen datos
sobre la inhalación). En los animales y en los seres humanos, el HCB
se acumula en los tejidos ricos en lípidos, como el tejido adiposo, la
corteza suprarrenal, la médula ósea, la piel y algunos tejidos
endocrinos, y puede transmitirse a la descendencia a través tanto de
la placenta como de la leche materna. El HCB sufre un metabolismo
limitado, generando pentaclorofenol, tetraclorohidroquinona y
pentaclorotiofenol como principales metabolitos en la orina. Las
semividas de eliminación del HCB están comprendidas entre
aproximadamente un mes en la rata y el conejo y 2 ó 3 años en el mono.
6. Efectos en animales de laboratorio y en las pruebas in vitro
La toxicidad aguda del HCB en los animales de experimentación es
baja (1000 × 10 000 mg/kg de peso corporal). En los estudios con
animales, el HCB no causa irritación cutánea ni ocular y no tiene
efectos de sensibilización en el cobayo.
Los datos disponibles acerca de la toxicidad sistémica del HCB
indican que las vías de la biosíntesis del grupo hemo son una
importante diana de la toxicidad del hexaclorobenceno. Se han hallado
niveles elevados de porfirinas o de precursores de la porfirina, o de
ambas cosas, en el hígado, en otros tejidos y en las excretas de
varias especies de mamíferos de laboratorio expuestos al HCB. Se ha
informado de la aparición de porfiria en varios estudios realizados
con ratas expuestas por vía oral crónica o subcrónica a dosis entre
2,5 y 15 mg de HCB/kg de peso corporal al día. La excreción de
coproporfirinas aumentó en cerdos que ingirieron 0,5 mg de HCB/kg de
peso corporal al día o más (en el último estudio no se observó ningún
efecto con 0,05 mg de HCB/kg de peso corporal al día). Se ha visto
también que la exposición repetida al HCB afecta a una amplia gama de
sistemas orgánicos (entre ellos el hígado, los pulmones, los riñones,
la tiroides, la piel y los sistemas nervioso e inmunitario), aunque
las referencias a estos efectos son menos frecuentes que las
relacionadas con la porfiria.
El HCB es un inductor de tipo mixto del citocromo P-450, con
propiedades inducibles por el fenobarbital y por el 3-metilcolantreno.
Se sabe que se une al receptor Ah.
Por lo que se refiere a los estudios crónicos, en ratas expuestas
a dosis de 0,25 a 0,6 mg de HCB/kg peso corporal al día se observaron
efectos leves en el hígado (cambios histopatológicos, inducción
enzimática); en dichos estudios los NOEL estaban comprendidos entre
0,05 y 0,07 mg de HCB/kg de peso corporal al día. Las concentraciones
de neurotransmisores en el hipotálamo se vieron alteradas en visones
hembra sometidos a través de los alimentos a una exposición crónica de
0,16 mg de HCB/kg de peso corporal al día, y en su descendencia
expuesta a lo largo de la gestación y la lactancia. En estudios
subcrónicos realizados en ratas la homeostasis del calcio y la
morfometría ósea se vieron afectadas con 0,7 mg de HCB/kg de peso
corporal al día, pero no con 0,07 mg/kg de peso corporal al día.
La carcinogenicidad del HCB ha sido evaluada mediante varios
bioensayos realizados con roedores. En hámsters mantenidos con
alimentos con los que ingerían unas dosis medias de 4, 8 ó 16 mg/kg de
peso corporal al día durante toda la vida, se produjeron aumentos en
la incidencia de tumores de las células del hígado (hepatomas) en los
dos sexos y a todas las dosis, hemangioendoteliomas hepáticos a dosis
de 8-16 mg/kg de peso corporal al día, y adenomas tiroideos de los
machos a la dosis mayor. La exposición alimentaria de ratones a dosis
de 6, 12 y 24 mg/kg de peso corporal al día durante 120 semanas dio
lugar a un aumento de la incidencia de tumores de las células del
hígado (hepatomas) en ambos sexos a las dos dosis mayores (no
significativo, excepto para las hembras a la dosis mayor). En ratas,
la exposición in útero, durante la lactancia y por vía oral al HCB a
través de alimentos que proporcionaban a lo largo de su vida dosis
medias comprendidas entre 0,01 y 1,5 mg/kg de peso corporal al día
(machos) o 1,9 mg/kg de peso corporal al día (hembras) por espacio de
hasta 130 semanas post útero produjo a la mayor de las dosis un
aumento de la incidencia de nódulos hepáticos neoplásicos y de
feocromocitomas suprarrenales en las hembras y de adenomas
paratiroideos en los machos. En otro estudio crónico realizado en la
rata, la exposición por un periodo de hasta dos años a alimentos que
proporcionaban dosis medias de HCB de 4-5 y de 8-9 mg/kg de peso
corporal al día indujo aumentos de la incidencia de hepatomas y de
adenomas de las células renales a ambas dosis en los dos sexos, y de
carcinomas hepatocelulares, adenomas y carcinomas de las vías
biliares, y feocromocitomas suprarrenales y adenomas de la corteza
suprarrenal en las hembras. Se ha informado también de incidencias
elevadas de tumores hepáticos en algunos estudios más limitados en los
que se administraron concentraciones alimentarias únicas a grupos
reducidos de ratas hembra. Además, se ha informado de que, después de
una exposición alimentaria subcrónica al HCB, ratones, hámsters y
ratas desarrollaron tumores en el hígado, las vías biliares, el riñón,
el timo, el bazo y los ganglios linfáticos. La exposición alimentaria
al HCB favoreció la inducción de tumores hepáticos por el terfenilo
policlorado en el ratón y por la dietilnitrosamina en la rata.
Con excepción de los tumores renales en la rata macho
(aparentemente debidos, al menos en parte, a una nefropatía por
acumulación de gotas hialinas) y de los hepatomas en la rata (posible
resultado de la respuesta hiperplásica a una necrosis hepatocelular),
no se conocen estudios mecanísticos que hayan determinado el
significado del tipo de tumores inducidos por el HCB en el caso del
hombre.
El HCB tiene una escasa capacidad de inducción directa de
mutaciones de los genes, lesiones cromosómicas y reparaciones del ADN.
Mostró una leve actividad mutágena en un reducido número de los
estudios realizados en bacterias y levaduras, aunque hay que señalar
que todos esos estudios presentan limitaciones. Existen también
algunos indicios de un cierto grado de unión al ADN in vitro e
in vivo, aunque a niveles muy inferiores a los habituales en los
carcinógenos genotóxicos.
En estudios sobre la reproducción, la exposición oral de monos a
tan sólo 0,1 mg de HCB/kg de peso corporal al día durante 90 días
afectó a la estructura revelada por microscopia óptica y a la
ultraestructura del epitelio germinal superficial, una diana poco
usual para las toxinas que afectan al ovario. Dicha dosis causó
también daños ultraestructurales en las células germinales
primordiales. Estos cambios específicos en tejidos-diana, para los que
dosis mayores son aún más lesivas, se asocian por lo demás a un
desarrollo normal del folículo, el ovocito y el embrión, lo que indica
que el HCB tiene una acción específica en el ovario. La reproducción
masculina sólo se vio afectada a dosis mucho mayores (entre 30 y
221 mg/kg de peso corporal al día) en estudios realizados en varias
especies distintas de los primates.
La exposición de ratas y gatos, a través de la placenta o durante
la lactancia, a dosis maternas comprendidas entre 3 y 4 mg/kg de peso
corporal al día tuvo efectos hepatotóxicos o afectó a la supervivencia
o el crecimiento de la descendencia en período de lactancia. En
algunos casos, dosis iguales o superiores a ésas redujeron el tamaño
de las camadas o aumentaron el número de abortos. (Los efectos nocivos
en los cachorros sin destetar han sido observados más frecuentemente,
y a dosis menores, que los efectos embriotóxicos o fetotóxicos.) La
descendencia de visones expuestos crónicamente a sólo 1 mg de HCB/kg
de alimento (aproximadamente 0,16 mg/kg de peso corporal al día) tuvo
un peso reducido al nacer y presentó una mayor mortalidad hasta el
destete. A pesar de que se han observado trastornos esqueléticos y
renales de los fetos en algunos estudios realizados en ratas y ratones
expuestos al HCB durante la gestación, dichas alteraciones o bien no
estaban claramente relacionadas con el tratamiento o bien ocurrieron a
dosis que eran también tóxicas para las madres. En dos estudios, uno
de los cuales incluía exposición posnatal y durante la lactancia, el
desarrollo neurocomportamental de las crías de rata se vio afectado
por la exposición in útero a dosis maternas orales de 0,64 a 2,5 mg
de HCB/kg de peso corporal al día.
Los resultados de varios estudios indican que el HCB afecta al
sistema inmunitario. Ratas y monos expuestos a dosis entre 3 y 120 mg
de HCB/kg de peso corporal al día sufrieron alteraciones
histopatológicas en el timo, en el bazo y en los ganglios linfáticos o
los tejidos linfoides del pulmón. La exposición crónica de perros
sabuesos a 0,12 mg/kg de peso corporal al día produjo una hiperplasia
nodular del tejido linfoide gástrico. En varios estudios realizados en
la rata, la inmunidad humoral y, en menor grado, la celular se vieron
potenciadas tras varias semanas de exposición alimentaria al HCB,
mientras que la función de los macrófagos no se alteró. Una cantidad
tan pequeña como 4 mg de HCB/kg de alimento (aproximadamente 0,2 mg/kg
de peso corporal al día) durante la gestación, a lo largo de la
lactancia y hasta las 5 semanas de edad incrementó las respuestas
inmunitarias humoral y celular y provocó la acumulación de macrófagos
en el tejido pulmonar de crías de rata. Por el contrario, se ha
observado un efecto inmunodepresor del HCB en la mayor parte de los
estudios llevados a cabo con ratones; dosis de sólo 0,5-0,6 mg/kg de
peso corporal al día durante varias semanas redujeron la resistencia a
la infección por Leishmania o a una provocación con células
tumorales, disminuyeron la actividad citotóxica de los macrófagos del
bazo, y redujeron la respuesta de hipersensibilidad de tipo retardado
en la descendencia expuesta in útero y durante la lactancia. En
varios estudios realizados con diversas cepas de ratas, la exposición
de breve duración o subcrónica al HCB afectó a la función tiroidea, a
juzgar por los reducidos niveles séricos de tiroxina total y tiroxina
libre (T4) y a menudo, en menor grado, de triyodotironina (T3).
7. Efectos en el ser humano
La mayor parte de los datos acerca de los efectos del HCB en el
ser humano provienen de intoxicaciones accidentales que tuvieron lugar
en Turquía en los años 1955-1959, entre las que se identificaron más
de 600 casos de porfiria cutánea tardía (PCT). En esa ocasión se
observaron alteraciones en el metabolismo de la porfirina, lesiones
dermatológicas, hiperpigmentación, hipertricosis, aumento del tamaño
del hígado, de la glándula tiroides y de los ganglios linfáticos; se
observaron también (aproximadamente en la mitad de los casos)
osteoporosis o artritis, sobre todo en los niños. Los niños
amamantados por madres expuestas al HCB como consecuencia de ese
accidente desarrollaron un trastorno conocido como pembe yara
(ulceración rosada), y la mayor parte murieron antes de un año.
Existen también algunos indicios de que la PCT afecta a personas
sometidas a una exposición relativamente alta al HCB en el lugar de
trabajo o en el medio ambiente general.
Los pocos estudios epidemiológicos disponibles acerca de la
incidencia de cáncer tienen un valor limitado, ya sea por lo reducido
de la muestra, por la deficiente caracterización de la exposición al
CHB o por la exposición a otros muchos agentes, y son insuficientes
para evaluar la carcinogenicidad del HCB para el ser humano.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
En los estudios realizados sobre la toxicidad aguda del HCB para
los organismos acuáticos, la exposición a concentraciones comprendidas
entre 1 y 17 mg/litro redujo la producción de clorofila en algas y la
reproducción de protozoos ciliados, y causó mortalidad en el camarón
rosado y en las quisquillas, pero no aumentó la mortalidad de peces de
agua dulce o de mar. En estudios a largo plazo, el crecimiento de
algas y protozoos vulnerables de agua dulce se vio afectado por una
concentración de 1 mg/litro, mientras que concentraciones de
aproximadamente 3 mg/litro provocaron mortalidad en anfípodos y
necrosis hepática en la perca americana.
9. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
9.1 Efectos en la salud
El Grupo Especial llegó a la conclusión de que los datos
disponibles son suficientes para establecer valores indicativos
respecto a los efectos neoplásicos y no neoplásicos del HCB.
En cuanto a los efectos no neoplásicos, considerando el NOEL más
bajo notificado (0,05 mg de HCB/kg de peso corporal al día), referido
sobre todo a los efectos hepáticos observados a dosis mayores en
estudios realizados en cerdos y ratas expuestos por vía oral, e
incorporando un factor de incertidumbre de 300 (× 10 en concepto de
variación interespecíes, × 10 en concepto de variación intraespecie, y
× 3 en concepto de gravedad del efecto), se ha calculado una IDT de
0,17 mg/kg de peso corporal al día.
El criterio seguido en cuanto a los efectos neoplásicos se basa
en la dosis tumorigénica TD5, es decir, la ingesta asociada a un
exceso del 5% en la incidencia de tumores detectada en los
experimentos con animales. Considerando los resultados del bioensayo
de carcinogenicidad en dos generaciones de ratas, y empleando el
modelo polietápico, la TD5 es de 0,81 mg/kg de peso corporal al día
para los nódulos neoplásicos del hígado en las hembras. Habida cuenta
de la insuficiencia de los datos mecanísticos, se utilizó un factor de
incertidumbre de 5000 para establecer un valor indicativo, basado en
criterios de salud, de 0,16 mg/kg de peso corporal al día.
9.2 Efectos en el medio ambiente
El Grupo Especial señaló que existen muy pocos estudios
experimentales con los que llevar a cabo una evaluación de los riesgos
para el medio ambiente. Los niveles de HCB en las aguas superficiales,
excepto en unos pocos lugares extremadamente contaminados, son en
general varios órdenes de magnitud inferiores a los que se supone que
entrañan riesgos para los organismos acuáticos. No obstante, las
concentraciones de HCB en los huevos de las aves marinas y las rapaces
de algunos lugares en distintas zonas del mundo se aproximan a niveles
que en la gaviota argéntea se asocian a una disminución del peso del
embrión (1500 mg/kg), lo que parece indicar que el HCB puede dañar los
embriones de especies de aves vulnerables. Del mismo modo, los niveles
de HCB observados en peces de diversos lugares del mundo se encuentran
a un orden de magnitud del nivel alimentario de 1000 mg/kg, asociado a
una reducción del peso al nacer y a un aumento de la mortalidad de la
descendencia en los visones. Esto parece indicar que el HCB puede
tener efectos nocivos en los visones y, tal vez, en otros mamíferos
que se alimentan de peces.
10. Conclusiones
a) El HCB es un producto químico persistente que se bioacumula debido
a su liposolubilidad y a su resistencia a la degradación.
b) Los estudios realizados con animales han demostrado que el HCB
produce cáncer y afecta a una amplia gama de sistemas de órganos, con
inclusión del hígado, los pulmones, los riñones, la tiroides, los
tejidos reproductivos y los sistemas nervioso e inmunitario.
c) En seres humanos sometidos a una alta exposición accidental se ha
observado toxicidad sintomática, en particular porfiria cutánea tardía
en niños y en adultos y mortalidad en lactantes.
d) Es necesario adoptar diversas medidas para reducir la carga
ambiental de HCB.
e) Se han propuesto los siguientes valores indicativos basados en
criterios de salud para la ingesta diaria total (IDT) de HCB por el
ser humano: efectos no cancerígenos, 0,17 µg/kg de peso corporal/día;
yefectos neoplásicos, 0,16 µ/kg de peso corporal/día.
Synonyms: perchlorobenzene, pentachlorophenyl chloride, phenyl
perchloryl
Trade names: Amatin, Anticarie, Bunt Cure, Bunt-No-More, Co-op Hexa,
Granox NM, Julin's Carbon Chloride, No Bunt, No Bunt
40, No Bunt 80, No Bunt Liquid, Sanocide, Smut-Go,
Snieciotox, HexaCB
2.2 Physical and chemical properties
Some physical and chemical properties of HCB are listed in
Table 1. At ambient temperature, HCB is a white crystalline that is
virtually insoluble in water, but is soluble in ether, benzene and
chloroform (NTP, 1994). It has a high octanol/water partition
coefficient, low vapour pressure, moderate Henry's Law constant and
low flammability. Technical grade HCB is available as a wettable
powder, liquid and dust (NTP, 1994). Technical grade HCB contains
about 98% HCB, 1.8% pentachlorobenzene and 0.2% 1,2,4,5-
tetrachlorobenzene (IARC, 1979), and it is known to contain a variety
of impurities, including hepta- and octachlorodibenzofurans,
octachlorodibenzo- p-dioxin and decachlorobiphenyl (Villanueva et
al., 1974; Goldstein et al., 1978).
Table 1. Physical and chemical properties of hexachlorobenzenea
Property Value
Relative molecular mass 284.79
Melting point (°C) 230
Boiling point (°C) 322 (sublimates)
Density (g/cm3 at 20°C 1.5691
Vapour pressure 0.0023
(Pa at 25°C)
Log octanol/water partition coefficient 5.5
Water solubility 0.005
(mg/litre at 25°C)
Henry's Law Constant (caluclated)b 131
(Pa/mol per m3)
Conversion factors 1 ppm = 11.8 mg/m3
1 mg/m3 = 0.08 ppm
a From ATSDR (1990); Mackay et al. (1992)
b The Henry's Law Constant has been calculated using the
tabled values for aqueous solubility and vapour pressure
2.3 Analytical methods
Analytical methods for the determination of HCB in environmental
samples and biological tissues vary depending upon the matrix and
representative methods for various matrices, and are summarized in
Tables 2 and 3.
Table 2. Analytical methods for determining hexachlorobenzene in environmental samplesa
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Water Extract with dichloromethane, GC/ECD 0.05 mg/kg 95 ± 10-20% US EPA (1982)
exchange to hexane,
concentrate; Florisil column
chromatography as a clean-up
Water Extract with dichloromethane GC/MS 1.9 mg/kg No data US EPA (1982)
at pH 11 and 2, concentrate
Air Glass fibre filter and XAD2 HRGC/ 0.18 pg/m3 >99% Hippelein et al. (1993)
traps separated by a PUF LRMS
disk; extraction with toluene
Air Polyurethane foam (PUF) GC/ECD <0.1 µg/m3 94.5±8% Lewis & MacLeod (1982)
sampling cartridge, extraction
with diethyl ether in hexane
Air Polyurethane foam (PUF) GC/ECD low pg/m3 93±1.1% Oehme & Stray (1982)
plugs, extraction with hexane, range (not
fractionation by HPLC specified)
Air Porous polyurethane foam GC/ECD No data Tenax more Billings &
(PUF), or Tenax-GC resin; effective Bidleman (1980)
filters refluxed with than PUF in
dichloromethane and retaining HCB
chlorinated solvents removed
and refluxed with hexane;
clean-up by alumina
chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Air Adsorb on Amberlite XAD-2 GC/PID 0.014 mg/m3 approx Langhorst &
resin separated by a silanized 95 ± 12% Nestrick (1979)
glass wool plug, desorption
with carbon tetrachloride.
Air Trace Atmospheric Gas Analyser approx No data Thomson et al. (1980)
using negative atmospheric 0.35 µg/m3
pressure chemical ionization
for trace gas analysis;
collection from ambient air and
transfer into a carrier of CO2
for analysis
Soil, Hexane extraction GC/ECD 10 mg/kg 78±2.6% to DeLeon et al. (1980)
chemical 96.5±3.6%
waste
disposal
site samples
Soil Extract with dichloromethane GC/MS 18 mg/kg No data US EPA (1986b)
5 mg/kg
Sediment Solvent extraction subjected GC/MS 46% Lopez-Avila et al.
to acid-base fractionation; (1983)
base/neutral fraction subjected
to silica gel chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Wastes, Extract with dichloromethane GC/MS 190 mg/kg No data US EPA (1986b)
non-water 50 mg/kg
miscible
Wastes, soil Extract with dichloromethane GC/MS 20 µg/litrec No data US EPA (1986b)
a Portions of the table were taken from ATSDR (1990)
b GC = gas chromatography; ECD = electron capture detector; MS = mass spectrometry; PID = photoionization detector;
HRGC = high-resolution gas chromatography; LRMS = low-resolution mass spectrometry
c Identification limit; detection limits for actual samples are several orders of magnitude higher depending upon the
sample matrix and extraction procedure employed.
Table 3. Analytical methods for determining hexachlorobenzene in biological materials
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Fish tissue Grind with sodium sulfate, extract GC/ECD approx No data Oliver & Nicol (1982)
with hexane/acetone, clean-up by 0.05 µg/kg
Na2SO4/Alumina/silica gel/Florisil
column followed by a H2SO4 column
on silica gel
Fish tissue Extraction with hexane/isopropanol, GC/ECD No data No data Lunde & Ofstad (1976)
solvent and sulfuric acid
partitioning
Fish tissue Sulfuric acid digestion, silica gel GC/ECD 10-15 µg/kg 93% Lamparski et al. (1980)
column chromatography, methylation,
alumina column chromatography
Oyster Extraction with acetone/acetonitrile, GC/ECD No data No data Murray et al. (1980)
tissue partitioning into petroleum ether,
silica gel chromatography
Adipose Extraction with hexane, subjected GC/ECD No data 87.4-92.6% Watts et al. (1980)
tissue to Florisil clean-up and one-fraction
(chicken) elution
Adipose Extraction (solvent not specified), HRGC/MS 12 µg/kg No data Stanley (1986)
tissue bulk lipid removal, Florisil
fractionation
Adipose Extraction with benzene/acetone, GC/ECD 0.12 µg/kg 79-95% Mes (1992)
tissue Florisil fractionation
Table 3 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Blood/urine Extraction with carbon tetrachloride, GC/PID 4.1 µg/kg 83% Langhorst &
silica gel column chromatography, (urine) Nestrick (1979)
concentrate 16 µg/kg
(blood)
Blood Extraction with hexane, concentrate GC/ECD No data No data US EPA (1980)
Blood Extraction with hexane/isopropanol GC/ECD No data No data Lunde & Bjorseth (1977)
Breast milk Extraction with acetone/benzene, GC/ECD 33 µg/kg 70-82% Mes et al. (1993)
Florisil fractionation
GC = gas chromatography; ECD = electron capture detector; PID = photoionization detector; HRGC = high-resolution gas
chromatography; MS = mass spectrometry
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Sources, uses and production processes
Industrial synthesis of HCB may be achieved through the
chlorination of benzene at 150-200°C using a ferric chloride catalyst
or from the distillation of residues from the production of
tetrachloroethylene (US EPA, 1985a). HCB may also be synthesized by
refluxing hexachlorocyclohexane isomers with sulfuryl chloride or
chlorosulfonic acid in the presence of a ferric chloride or aluminum
catalyst (Brooks & Hunt, 1984).
Historically, HCB had many uses in industry and agriculture. The
major agricultural application for HCB used to be as a seed dressing
for crops such as wheat, barley, oats and rye to prevent growth of
fungi. The use of HCB in such applications was discontinued in many
countries in the 1970s owing to concerns about adverse effects on the
environment and human health. HCB may continue to be used for this
purpose in some countries; for example, HCB was still used in 1986 as
a fungicide, seed-dressing and scabicide in sheep in Tunisia (Jemaa et
al., 1986). However, it is uncertain as to whether HCB is still used
for this purpose.
In industry, HCB has been used directly in the manufacture of
pyrotechnics, tracer bullets and as a fluxing agent in the manufacture
of aluminum. HCB has also been used as a wood-preserving agent, a
porosity-control agent in the manufacture of graphite anodes, and as a
peptizing agent in the production of nitroso and styrene rubber for
tyres (Mumma & Lawless, 1975). It is likely that some of these
applications have been discontinued, although no information is
available.
Although HCB production has ceased in most countries, it is still
being generated inadvertently as a by-product and/or impurity in
several chemical processes. HCB is formed as a reaction by-product of
thermal chlorination, oxychlorination, and pyrolysis operations in the
manufacture of chlorinated solvents (mainly carbon tetrachloride,
trichloroethylene and tetrachloroethylene) (Government of Canada,
1993). The concentrations of HCB in distillation bottoms was estimated
to be 25%, 15% and 5%, respectively, for tetrachloroethylene, carbon
tetrachloride and trichloroethylene (Jacoff et al., 1986). While HCB
could potentially also be a contaminant in the final product, it was
not detected (detection limit 5 mg/litre) in carbon tetrachloride and
tetrachloroethylene in an investigation in Canada (personal
communication to Health Canada by Mr John Schultiess, Dow Chemical
Canada Inc., 1991). Analysis of production lots of tri- and
tetrachloroethylene produced in Europe in 1996 failed to detect HCB at
a detection limit of 2 µg/litre solvent (personal communication to the
IPCS by Mr C. de Rooij, Solvay Corporation Europe, 1996).
HCB is also generated as a waste by-product during the
manufacture of chlorinated solvents, chlorinated aromatics and
pesticides (Jacoff et al., 1986). The waste streams from the
production of pentachloronitrobenzene (PCNB), chlorothalonil and
dacthal are expected to contribute the bulk of HCB released from the
pesticide industry (Brooks & Hunt, 1984), although HCB can also be
generated as a waste by-product from the production of
pentachlorophenol, atrazine, simazine, propazine and maleic hydrazide
(Quinlivan et al., 1975; Mumma & Lawless, 1975). These pesticides are
also known to contain HCB as an impurity in the final product, usually
at levels of less than 1% HCB when appropriate procedures are used for
the synthesis and purification stages (Tobin, 1986). When such
procedures are not met, the level of HCB could be much higher (e.g.,
pentachloronitrobenzene has been reported to contain 1.8-11% HCB
(Tobin, 1986)). However, owing to many voluntary and regulatory
pressures, it is unlikely that such high levels of HCB are present in
today's pesticide formulations, but no information is available to
substantiate this point.
The chlor-alkali industry produces chlorine (Cl2), hydrogen and
caustic soda (NaOH) by electrolysis of purified and concentrated
sodium chloride (NaCl). Processes using graphite anodes are known to
produce HCB as a by-product (Quinlivan et al., 1975; Mumma & Lawless,
1975; Alves & Chevalier, 1980) owing to the reaction of chlorine with
graphite anode materials such as carbon and oils. Depending on the
purification procedures, the final products might also be contaminated
with HCB. In some countries, graphite anodes have been replaced by
dimensionally stabilized anodes (DSA), which do not generate HCB
(Government of Canada, 1993).
Incineration is an important source of HCB in the environment.
Emission levels from incinerators are very site-specific, and
therefore generic levels are difficult to estimate. Earlier
information yielded a crude estimate of the total HCB released from
all municipal incinerators in the USA to be 57-454 kg/year (US EPA,
1986a), but levels currently emitted are not known.
3.2 World production levels
Few recent data on the quantities of HCB produced are available.
Worldwide production of pure HCB was estimated to be 10 000
tonnes/year for the years 1978-1981 (Rippen & Frank, 1986). An
estimated 300 tonnes was produced by three manufacturers in the USA in
1973 (IARC, 1979). HCB was produced/imported in the European Community
at 8000 tonnes/year in 1978 (Rippen & Frank, 1986), and a company in
Spain used to produce an estimated 150 tonnes of HCB annually (IARC,
1979). Approximately 1500 tonnes of HCB were manufactured annually in
Germany for the production of the rubber auxiliary PCTP (BUA, 1994),
but this production was discontinued in 1993. No further centres of
HCB manufacture in Europe or North America have been identified.
Production of HCB has declined as a result of restrictions on its use
starting in the 1970s.
Considerable amounts of HCB are inadvertently produced as a by-
product in the manufacture of chlorinated solvents, chlorinated
aromatics and chlorinated pesticides. Jacoff et al. (1986) estimated
that approximately 4130 tonnes of HCB are generated annually as a
waste product in the USA and that nearly 77% of this is produced from
the manufacture of three chlorinated solvents: carbon tetrachloride,
trichloroethylene and tetrachloroethylene. The remainder is produced
by the chlorinated pesticide industry. In 1977, about 300 tonnes of
HCB were generated in Japan as a waste by-product in the production of
tetrachloroethylene, almost all of which was incinerated (IARC, 1979).
It was estimated that >5000 tonnes HCB/year were produced as a by-
product during tetrachloroethylene production in the Federal Republic
of Germany in 1980 (Rippen & Frank, 1986). However, recent estimates
for Europe from ECSA (European Chlorinated Solvent Association; P.G.
Johnson (1996) personal communication to IPCS) indicate that up to
4000 tonnes/year of HCB are produced as a by-product during certain
tetrachloroethylene production processes and that over 99% of this by-
product was incinerated at high temperatures.
3.3 Entry into the environment
Currently, the principal sources of HCB in the environment are
estimated to be the manufacture of chlorinated solvents, the
manufacture and application of HCB-contaminated pesticides, and
inadequate incineration of chlorine-containing wastes. It should be
noted that only a small fraction of the HCB generated as a by-product
may be released, depending on the process technology and waste-
disposal practices employed. For example, according to the US Toxic
Chemical Release Inventory (TRI), releases of HCB from the ten largest
processing facilities were 460 kg, most of this to air, compared with
almost 542 000 kg transferred offsite as waste. The TRI data are not
comprehensive, since only certain types of facilities are required to
report (ATSDR, 1994). ECSA (P.G. Johnson, personal communication to
IPCS) estimated that European emissions of HCB were about 200 kg/year
in 1993.
As discussed in the previous section, HCB is a contaminant of a
number of chlorinated pesticides. Since most current applications for
these products are dispersive, most HCB from this source will be
released to the environment.
Substantial quantities of HCB are also contained in the wastes
generated through the manufacture of chlorinated solvents and
pesticides. In the mid-1980s in the USA, 81% of these HCB-containing
wastes were disposed of by incineration, compared to 19% via
landfilling (Jacoff et al., 1986). It is likely that the amount of HCB
wastes disposed of by incineration has since increased, although
information has not been found to confirm this point. HCB can be
emitted from incinerators as a result of incomplete thermal
decomposition of these wastes and as a product of incomplete
combustion (PIC) from the thermal decomposition of a variety of
chlorinated organics such as Kepone, mirex, chlorobenzenes,
polychlorinated biphenyls, pentachlorophenol, polyvinyl chloride and
mixtures of chlorinated solvents (Ahling et al., 1978; Dellinger et
al., 1991).
Although only a small proportion of the HCB-containing waste
generated in the USA is landfilled, HCB may continue to leach to
groundwater from previously landfilled HCB waste sites. The
contribution of this route is uncertain, although HCB is not easily
leached, and landfills containing HCB are now designed to prevent
leachate losses into adjacent water systems (Brooks & Hunt, 1984). HCB
emission into the atmosphere from landfills containing HCB wastes
occurs from slow volatilization and from displacement of the
contaminated soil (Brooks & Hunt, 1984).
HCB has been detected in emissions from a number of industries,
including paint manufacturers, coal and steel producers, pulp and
paper mills, textile mills, pyrotechnics producers, aluminum smelters,
soap producers and wood-preservation facilities (Quinlivan et al.,
1975; Gilbertson, 1979; Alves & Chevalier, 1980), probably reflecting
the use of products contaminated with HCB. Municipal and industrial
wastewater facilities may also discharge HCB-contaminated effluents
(Environment Canada/Ontario Ministry of the Environment, 1986; King &
Sherbin, 1986), probably owing to inputs from industrial sources.
Long-range transport plays a significant role as a means of
redistribution of HCB throughout the environment. Wet deposition
(deposition via rain or snowfall) is the primary mechanism for
transport of HCB from the atmosphere to aquatic and terrestrial
systems in Canada (Eisenreich & Strachan, 1992). For example, it is
estimated that long-range transport and total deposition to the
Canadian environment is approximately 510 kg/year, an amount that is
similar to that from all other sources combined (Government of Canada,
1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental transport and degradation
HCB is distributed throughout the environment because it is
mobile and resistant to degradation. Volatilization from water to air
and sedimentation following adsorption to suspended particulates are
the major removal processes from water (Oliver, 1984a; Oliver &
Charlton, 1984). Once in the sediments, HCB will tend to accumulate
and become trapped by overlying sediments (Oliver & Nicol, 1982).
Although HCB is not readily leached from soils and sediments, some
desorption does occur and may be a continuous source of HCB to the
environment, even if inputs to the system cease (Oliver, 1984a; Oliver
et al., 1989). Chemical or biological degradation is not considered to
be important for the removal of HCB from water or sediments (Callahan
et al., 1979; Mansour et al., 1986; Mill & Haag, 1986; Oliver & Carey,
1986). In the troposphere, HCB is transported over long distances by
virtue of its persistence, but does undergo slow photolytic
degradation (the half-life is approximately 80 days; Mill & Haag,
1986), or is removed from the air phase via atmospheric deposition to
water and soil (Bidleman et al., 1986; Ballschmiter & Wittlinger,
1991; Lane et al., 1992a, 1992b). In soil, volatilization is the major
removal process at the surface (Kilzer et al., 1979; Griffin & Chou,
1981; Schwarzenbach et al., 1983; Nash & Gish, 1989), while slow
aerobic (half-life of 2.7-5.7 years) and anaerobic biodegradation
(half-life of 10.6-22.9 years) are the major removal processes at
lower depths (Beck & Hansen, 1974; Howard et al., 1991).
4.2 Bioaccumulation and biomagnification
The bioaccumulative properties of HCB result from the combination
of its physicochemical properties (high octanol/water partition
coefficient) and its slow elimination due to limited metabolism
related to its high chemical stability. Organisms generally accumulate
HCB from water and from food, although benthic organisms may also
accumulate HCB directly from sediment (Oliver, 1984b; Knezovich &
Harrison, 1988; Gobas et al., 1989). The uptake of HCB in benthic
invertebrates has been investigated in a number of laboratory and
field studies. The results demonstrated that some HCB in sediments is
available to infaunal species. Reported bioaccumulation factorsa
(BAF) for invertebrates in HCB-containing sediments range from 0.04 to
0.58 in high-organic-content sediment to 1.95 in low-organic-content
a Defined as tissue concentration (wet weight) divided by sediment
concentration (dry weight). BAFs from Oliver (1984b) were divided
by 6.67 to convert tissue dry weight to wet weight.
sediment (Oliver, 1984b; Knezovich & Harrison, 1988; Gobas et al.,
1989). The bioavailability of sediment-bound HCB is inversely related
to sediment organic carbon content (Knezovich & Harrison, 1988), and
varies with the type and size of the organisms and their feeding
habits (Boese et al., 1990), the extent of contact with sediment pore
and interstitial waters (Landrum, 1989), and the surface area of the
substrate (Swindoll & Applehans, 1987). Landrum (1989) suggested that
the bioavailability of sediment-sorbed chemicals declines as the
contact time between the sediment and a contaminant increases. For
example, Schuytema et al., (1990) observed that addition of HCB-spiked
sediments did not result in a significant increase in the uptake of
HCB by the worm ( Lumbriculus variegatus), amphipods ( Hyalella
azteca and Gammarus lacustris), and fathead minnows ( Pimephales
promelas) in a laboratory recirculating water/sediment system.
However, there was a substantial increase of HCB levels in bed
sediment, suggesting that sediment served as a more effective sink for
HCB than the organisms.
The biomagnification factor (BMF) for HCB in the earthworm
Eisenia andrei after exposure via food was 0.068 on a wet weight
basis (0.071 on a lipid basis) (Belfroid et al., 1994a), the biota
lipid-to-soil accumulation factor, defined as the ratio of the
concentration in the animal to that on the soil, was 215 g soil dry
weight/g lipid (Belfroid et al., 1994b), and the bioconcentration
factors (BCFs) for earthworms kept in water were found to be between
48 × 104 and 62 × 104 ml water/g lipid (Belfroid et al., 1993).
Field studies indicate that exposure via food is important for
organisms at higher trophic levels, as significant biomagnification
has been observed in several studies in natural aquatic ecosystems. In
Lake Ontario, Oliver & Niimi (1988) observed that tissue residue
concentrations increased from plankton (mean = 1.6 ng/g wet weight) to
mysids (mean = 4.0 ng/g wet weight) to alewives (mean = 20 ng/g wet
weight) to salmonids (mean = 38 ng/g wet weight). Braune & Norstrom
(1989) used field data on body burdens of HCB in the herring gull
( Larus argentatus) and one of its principal food items, the alewife
( Alosa pseudoharengus) in a Great Lakes food chain to calculate a
biomagnification factor (whole body, wet weight basis) of 31.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
HCB has been detected in air, water, sediment, soil and biota.
Representative levels reported in various environmental media in many
countries are presented in Tables 4, 5 and 6.
5.1.1 Air
HCB is widely dispersed in ambient air, and is generally present
at low concentrations. Mean concentrations of HCB in air removed from
point sources in Canada, Norway, Sweden, Germany, the USA, the Arctic
and the Antarctic range from 0.04 to 0.6 ng/m3 (Table 4). Levels of
HCB in air are generally similar between urban, rural and remote
sites, reflecting the persistence and long-range transport of this
substance.
Airborne concentrations of HCB measured in the USA near nine
chlorinated solvent and pesticide plants in 1973 and 1976 were much
higher than background levels (Spigarelli et al., 1986).
Concentrations as high as 24 µg/m3 were detected in the immediate
vicinity of one plant, while the maximum concentration of HCB distant
from the site was 0.36 µg/m3. The highest levels were associated with
the production of perchloroethylene, trichloroethylene and carbon
tetrachloride, and with plants where onsite landfill and open pit
waste disposal were practiced. More recently, Grimalt et al. (1994)
reported that airborne concentrations of HCB in a community in the
vicinity of a organochlorine factory built in 1898 in Catalonia,
Spain, averaged 35 ng/m3, compared with 0.3 ng/m3 in Barcelona, the
reference community for this study. It is not known how representative
the data from these studies are, as HCB releases are expected to be
minimized from industries using appropriate modern technology and
waste management practices.
No data are available on the levels of HCB in indoor air.
Table 4. Levels of hexachlorobenzene in ambient air (ng/m3)
Location Year Detection Mean Rangea Reference
limit
Canada (Windsor, Ontario) 1987-1990 0.03 0.13 ND-0.44 Environment Canada (1992)
Canada (Ontario)
- industrial/urban areas 1985-1989 0.007 0.167 0.07-0.31 Lane et al. (1992b)
- rural areas 0.094 0.02-0.31
Canada (Egbert, Ontario) 1988-1989 - >0.054 0.00004-0.64 Hoff et al. (1992)
Canada (Walpole Island) 1988-1989 0.02 0.15 ND-0.34 Environment Canada (1992)
Canadian High Arctic 1987 0.15 ND-0.154 Patton et al. (1989)
(Beaufort Sea)
Bear Island (Arctic)
- summer
- winter 0.001 0.04 0.029-0.045 Oehme & Stray (1982)
0.001 0.111 0.059-0.188
Southern Ocean and 1990 0.06 0.04-0.078 Bidleman et al. (1993)
Antarctica
Enewetak Atoll 1979 0.10 0.095-0.13 Atlas & Giam (1981)
(Pacific Ocean)
Spitzbergen
- summer 0.001 0.071 0.05-0.085 Oehme & Stray (1982)
- winter 0.001 0.086 0.071-0.095
Table 4 contd.
Location Year Detection Mean Rangea Reference
limit
Germany (Hamburg - 1986-1987 0.6 0.3-2.5 Bruckmann et al. (1988)
residential, suburban
and industrial sites)
South Germany 1986-1990 0.21 0.058-0.52 Morosini et al. (1993)
Norway (Lillestrom) 0.001 0.162 0.055-0.234 Oehme & Stray (1982)
Sweden (Aspvreten) 1984 0.067 0.054->0.165 Bidleman et al. (1987)
Sweden (Stockholm) 1983-1985 0.07 0.054->0.130 Bidleman et al. (1987)
Spain
- (near organochlorine 1989 & 1992 - 35 11-44 Grimalt et al. (1994)
compounds factory)
- hospital in Barcelona - 0.3 0.25-0.4
USA (Portland, Oregon) 1984 0.075 0.05-0.11 Ligocki et al. (1985)
USA - chemical production ND-24000 Spigarelli et al. (1986)
plants
USA - urban areas 1975-1979 0.1 0.5 ND-4.4 Carey et al. (1985)
a ND = not detected
5.1.2 Water
Levels of HCB in freshwater in Europe and North America are
generally below 1 ng/litre (Table 5), although higher values have been
reported in aquatic systems that receive industrial discharges and
surface run-off. In the connecting channels to the Great Lakes in
Canada, HCB levels were often found to exceed 1.0 ng/litre,
particularly near point sources. Levels in the St. Clair River near
the Dow Chemical outfall were as high as 87 ng/litre in 1985 and
75 ng/litre in 1986 (Oliver & Kaiser, 1986).
Mean concentrations of HCB in seawater rarely exceed 1 ng/litre
(Table 5) (Ernst, 1986; Burton & Bennett, 1987). In the Nueces Estuary
in Texas, USA, the highest level (0.61 ng/litre) was found near
sewage outfalls (Ray et al., 1983a). Higher concentrations (up to
196 ng/litre) were observed in the Forth Estuary in Scotland, near
domestic and chemical industry discharges (Rogers et al., 1989).
5.1.3 Soil
Data identified on levels of HCB in soil are quite limited and
are summarized in Table 6. The most extensive data are from the 1972
US National Soils Monitoring Program, in which the concentrations of a
variety of pesticides were determined at 1483 sites from 37 states
(Carey et al., 1979). HCB was detected at 11 sites, with a range of
concentrations in positive samples from 10 to 440 µg/kg dry weight. Of
24 samples of agricultural soil in British Columbia, Canada, where HCB
had last been applied as a seed treatment 10-15 years prior to the
survey, 6 had detectable HCB residues of between 1.3 and 2.2 ng/g dry
weight (Wilson & Wan, 1982).
Mean concentrations of HCB reported from uncontaminated soil in
Europe were found to range from 0.3 ng/g in Switzerland (Müller, 1982)
to 5.1 ng/g in a Swedish rural heathland soil (Thomas et al., 1985)
(it was not indicated whether concentrations were on a dry or wet
weight basis). Soil from a farming area in Italy contained 40 ng/g
(dry or wet weight basis not indicated) (Leoni & D'Arca, 1976). HCB
levels were not markedly increased by long-term application of sludge
to land in Germany at a rate of 50 to 500 tons per ha and averaged
2.8 ng/g (dry or wet weight basis not indicated) (Witte et al.,
1988a,b). Monitoring programmes in Germany yielded average levels of
HCB contamination of soil ranging from approximately 1 ng/g dry weight
in the North Rhine-Westphalia (1990) to approximately 6 ng/g dry
weight in Baden-Württemberg (1988) (BUA, 1994).
Table 5. Concentrations of hexachlorobenzene (ng/litre) in drinking-water and surface water
Location Year Detection Mean Rangea Reference
limit
Drinking-water
Canada (Ontario) 1980 0.01 0.1 0.06-0.2 Oliver & Nicol (1982)
Canada (Maritime Provinces) 1985-1988 2.0 ND Environment Canada (1989)
Croatia
- Sisak 1988-1989 0.5 1.0a <1-4 Fingler et al. (1992)
- Zagreb 2.0a 1-3
USA 1977-1981 100 ND US EPA (1985b)
Surface water
Canada
- Lake Superior 1986 0.007 0.026 0.018-0.040 Stevens & Neilson
- Lake Huron 0.033 0.018-0.073 (1989)
- Georgian Bay 0.041 0.032-0.054
- Lake Erie 0.078 0.025-0.260
- Lake Ontario 0.063 0.020-0.113
Canada-St. Clair River 1985 0.30-87 Oliver & Kaiser (1986)
- tributaries to 0.08-0.79
St. Clair R.
Table 5 contd.
Location Year Detection Mean Rangea Reference
limit
Canada (Atlantic Region); 1979-1989 2.0 ND-2.2 Leger 1991
lakes, streams, reservoirs,
estuaries, coastal waters
Germany (Elbe) 1990 - 12 3-62 BUA (1994)
Greece (Strimon River) 1985-1986 - 1.52 0.5-2.8 Kilikidis et al. (1992)
Italy (tributaries to
Adriatic Sea) 1977-1978 1.0 ND Galassi & Provini (1981)
Mediterranean Sea 1982-1983 0.1 2.13 ND-12.6 El-Dib & Badawy (1985)
Netherlands/Belgium 1993 10 <10 <10 RIWA (1993)
Netherlands 1987 - <10 ND-100 De Walle et al. (1995)
North Sea (coastal waters
and estuaries) 1979-1980 2.7 0.03-15 Ernst (1986)
Scotland (Forth Estuary) 1987 0.01 <0.01-196 Rogers et al. (1989)
Scotland (Forth Estuary) 1990 0.7-8.0 Harper et al. (1992)
Spain (Ebre Delta) 1985-1986 0.0005 0.041 ND-1.0 Grimalt et al. (1988)
USA (Texas-estuary) 1980 0.24 <0.01-0.61 Ray et al. (1983a)
USA (coastal, surface <0.1 <0.1-26 Cross et al. (1987)
microlayer)
a median value
Table 6. Levels of hexachlorobenzene in soil (ng/g dry weight)
Source Year Detection Mean Rangea Reference
limit
Canada (British Columbia) 1.0 Wilson & Wan (1982)
- agricultural soils <1.0-2.2
- near a former grain 260 ng/g
treatment plant
Czech/Polish Border - - 3.25 0.47-4.8 Holoubek et al. (1994)
(Giant Mountains)
Germany (contaminated soil) 1989 0.3-339 Hagenmaier et al. (1992)
India 1987 24a 0-165 Nair & Pillai (1989)
Italy (farming area) 1971-1972 40 Leoni & D'Arca (1976)
Netherlands - Ochten 1993 - 18 5.1-66 Hendriks et al. (1995)
- Gelderse Poort 80 73-89
Netherlands 1987 - <10 <80 De Walle et al. (1995)
Sweden 5.1 Thomas et al. (1985)
USA 1968-1973 10-440 Carey et al. (1979)
USA (chemical plants) 0.002 ND-5 700 000 Spigarelli et al. (1986)
USA (hazardous waste sites) 1977-1978 20 000-400 000 Davis & Morgan (1986)
USA (5 locations near Love 0.1 1.04-5.6 0.15-26.3 Ding et al. (1992)
Canal)
a ng/g wet weight
Levels in soil are highest near industrial sources of HCB. Levels
as high as 12 600 ng/g dry weight were reported at one landfill site
in Canada (Wilson & Wan, 1982), and 570 µg/g (dry or wet weight not
indicated) on the grounds of a chlorinated solvent and pesticide
production plant in the USA (Spigarelli et al., 1986). Soils near a
former grain treatment plant in Canada contained 260 ng/g dry weight
of HCB (Wilson & Wan, 1982). Levels of HCB in soils from contaminated
floodplains in the Netherlands ranged from 5.1 to 89 ng/g dry weight
(Hendriks et al., 1995).
5.1.4 Sediment
HCB strongly sorbs to sediment and suspended matter, and
differences in the concentrations in the water as well as in the
composition of the sediments and suspended matter result in a wide
range of concentrations in this medium.
In sediment samples collected from 1979 to 1989 in the Atlantic
provinces of Canada, HCB was reported to be below the limit of
detection of 0.2 ng/g dry weight in 140 of 152 samples (Leger, 1991).
In surveys conducted from 1980 to 1983, HCB levels in sediments from
the Great Lakes ranged from 0.02 to 840 ng/g dry weight (Oliver &
Nicol, 1982; Fox et al., 1983; Kaminsky et al., 1983; Oliver &
Bourbonniere, 1985; Bourbonniere et al., 1986; Oliver et al., 1989;
IJC, 1989). Analyses of sediment cores from Lake Ontario indicated
that levels of HCB have declined from the 1960s to the early 1980s but
more recent data are not available to determine if this downward trend
has continued (Oliver & Nicol, 1982; Oliver et al., 1989). HCB levels
in sediment sampled from eight lakes in northern remote Canada (date
of sampling not specified) ranged from 0.09 to 1.80 ng/g dry weight
(Muir et al., 1995).
Levels as high as 5100 ng/g dry weight were detected in the Rhine
River in Baden-Württemberg, Germany, in 1986 (BUA, 1994). The majority
of sediment samples taken from the rivers Rhine and Elbe between 1980
and 1990 contained levels of HCB between 10 and 500 ng/g dry weight,
although levels below 1 ng/g dry weight were determined in some other
locations (BUA, 1994). A Nordic study on chlorinated compounds in the
Baltic, Kattegat and Skagerrak (œstfeldt et al., 1994) found HCB
concentrations in sediment ranging from 1 to 20 ng/g loi (loss on
ignition), the higher values occurring mainly in the Bothnian Bay. An
extreme value of 63 ng/g loi was found in Öresund between Denmark and
Sweden. Levels of HCB in sediment samples collected near effluent
discharges along a stream in Pakistan ranged from <0.05 to 94.5 ng/g
wet weight (Tehseen et al., 1994).
Higher levels of HCB in sediments were reported in studies
conducted near point sources. As much as 280 000 ng HCB/g dry weight
was detected in 1985 downstream of the Dow Chemical sewer discharges
in the St. Clair River, USA (Oliver & Pugsley, 1986).
5.1.5 Biota
HCB has been detected in invertebrates, fish, reptiles, birds and
mammals from around the world. Following the detection of HCB in
tissues of wild birds by De Vos in 1967, high residues were often
found in predatory birds, whereas minor quantities were detected in
fish, mussels and birds of the aquatic environment (Vos et al., 1968;
Koeman et al., 1969). Based on Canadian data from monitoring studies
in birds, HCB levels declined sharply from the mid-1970s (the earliest
data available) and into the early 1980s, after which they levelled
off (Noble & Elliott, 1986; Environment Canada/Department of Fisheries
and Oceans/Health and Welfare Canada, 1991).
Levels of HCB in freshwater mussels in the Great Lakes and
connecting channels have been found to range from 0.1 ng/g wet weight
to 24 ng/g wet weight (Kauss & Hamdy, 1985; Innes et al., 1988;
Muncaster et al., 1989). A similar range (4.4-26 ng/g wet weight) was
observed in benthic amphipods, the pelagic amphipod Pseudalibrotus
litoralis and brittle stars from the Beaufort Sea (Hargrave et al.,
1989). Lower levels (0.1-1.8 ng/g wet weight) were observed in mussels
( Mytilus galloprovincialis) from the Ebro Delta in the Western
Mediterranean, and these levels were observed to decline from 1980 to
1992 (Solé et al., 1994). Levels in marine species of clams and
oysters from the USA were reported in several studies to be < 1 ng/g
wet weight (Phelps et al., 1986; Eisenberg & Topping, 1984; Ray et
al., 1983b). Similarly, levels in invertebrates, including mussels
( Mytilus edulis), soft clams ( Mya arenaria), lugworms ( Arenicola
marina), and polychaetes ( Nereis diversicolor), were <1 ng/g
fresh weight in the German Wadden Sea (Ernst, 1986). Bjerk & Brevik
(1980) reported higher levels (50-350 ng/g wet weight) of HCB in crabs
( Carcinus maenas, Pagurus sp.), snails ( Littorina littorea),
brittle stars ( Ophiura albida) and sea stars ( Asteroidea) from the
contaminated Frierfjord in Norway, which receives discharge from
various industries located in the region, and HCB and related
compounds were reported to originate from one main source
(unspecified) in the area. œstfeldt et al. (1994) found that mussels
( Mytilus edulis) from the Baltic contain higher levels of HCB
(200-800 ng/g lipid weight) than mussels from Kattegat (11-20 ng/g
lipid weight).
In a 1981-1982 survey of HCB levels in fish from watersheds in
Eastern Canada, whole body concentrations in brook trout ( Salvelinus
fontinalis) and yellow perch ( Perca flavescens) ranged from below
the limit of detection (4.2 ng/g in 1981; 0.2 ng/g in 1982) to 54 ng/g
for trout and 15 ng/g wet weight for perch (Peterson & Ray, 1987).
Relatively high body burdens of HCB have been observed in fish in Lake
Ontario and connecting channels. HCB was not detected (ND) in juvenile
spottail shiners ( Notropis hudsonius) from Lakes Superior and Erie
(detection level = 1 ng/g wet weight) (Suns et al., 1983; Environment
Canada/Department of Fisheries and Oceans/Health and Welfare Canada,
1991), while mean body burdens in shiners in Lake Ontario ranged from
ND to 13 ng/g wet weight, and those in the Detroit, Niagara, and
St Clair rivers averaged 5 ng/g wet weight, ND to 8 ng/g wet weight,
and 231 ng/g wet weight, respectively (Suns et al., 1985). Mean
concentrations of HCB in the muscle tissue of various species of
salmonids from Lake Ontario ranged from 5 to 37 ng/g wet weight (Niimi
& Oliver, 1989).
Levels of HCB measured in whole fish species taken from major
rivers and lakes in the USA (including known contaminated areas)
ranged from <2 to 913 ng/g wet weight (Kuehl et al., 1983; DeVault,
1985; Schmitt et al., 1990; Kuehl & Butterworth, 1994). Levels in
roach ( Rutilus rutilus L.) and perch ( Perca fluviatilis L.) from
the "moderately polluted" Lahn River in Germany ranged from ND to
233 ng/g wet weight, with a mean of 1 ng/g (Schuler et al., 1985).
Concentrations of HCB in the whole bodies of carp ( Cyprinus carpio)
from the mouth of tributaries to Lake Ontario and the Niagara River
ranged from 52 to 1600 ng/g on a lipid basis (6.7 to 205 ng/g on a
fresh weight basis). The highest values were measured near hazardous
waste dumps and industrial facilities (as high as 1600 ng/g fat)
(Jaffe & Hites, 1986). Brunn & Manz (1982) reported a mean whole-body
concentration of HCB in fish (mainly trout) from inland rivers,
streams, and ponds in Germany of 5 ng/g wet weight. The highest levels
were recorded from fish caught in rivers.
HCB levels in seawater are generally lower than those in
freshwater, resulting in lower levels in edible parts of marine fish.
In fish taken from the North Sea (species not reported), HCB levels in
fish muscle tissues averaged 0.3-0.4 ng/g wet weight, with a maximum
of 0.8 ng/g (Ernst, 1986). HCB concentrations in livers averaged
42 ng/g wet weight for cod ( Gadus morhua) and 4 ng/g (range of
0.2-14 ng/g) for flounder ( Platichthis flesus). These levels were
comparable to levels measured in fish near the coast of southwest
Greenland and in the North Atlantic Ocean. Livers of cod from the
coast of southwest Greenland contained 32.4 ng/g on average, and those
of hake ( Merluccius merluccius) from the North Atlantic Ocean
averaged 40.5 ng/g) (Ernst, 1986). Levels of HCB were below the
determination limit (DL) in cod liver (DL = 5 ng/g) and herring muscle
(DL = 1 ng/g) of fish from the Clyde Sea near Scotland (Kelly &
Campbell, 1994). Cod from the Firth of Forth had mean liver levels of
38.7 ng/g wet weight, and levels in herring muscle of 2.0 and 2.3 ng/g
wet weight were observed in fish from the Firth of Forth and North
Sea, respectively (Kelly & Campbell, 1994). In surveillance monitoring
of contaminants in fish from coastal waters near England and Wales,
concentrations of HCB in livers of cod ( Gadus morhua), whiting
( Merlangius merlangus), dab ( Limanda limanda) and flounder
( Platichthys flesus) were 2-290, 5-230, 3-55 and 1-52 ng/g,
respectively (all results on a wet tissue weight basis) (MAFF/HSE,
1994). Levels of HCB in muscle tissues of herring ( Clupea harengus)
from the Baltic Sea ranged from <1 to 39 ng/g (Hansen et al., 1985);
concentrations in whitefish ( Coregonus lavaretus) and trout ( Salmo
trutta) ranged from <1 to 9 ng/g fresh weight in a 1992 survey
(Atuma et al., 1993).
Fish taken from the contaminated waters of the Frierfjord in
Norway contained mean concentrations of HCB in liver of 11 600 ng/g
for saithe ( Pollachius virens), and 16 800 ng/g for cod ( Gadus
morhua) (Bjerk & Brevik, 1980). Levels of HCB from fish taken from
the uncontaminated Sogndalfjord were much lower, averaging 18 ng/g wet
weight in livers of cod ( Gadus morhua), 8 ng/g in haddock
( Melanogrammus aeglefinus) and 1 ng/g in lemon sole ( Microstomus
kitt) and flounder ( Platichthys flesus) (Skåre et al., 1985).
Flounder ( Platichthis flesus) taken from the Elbe Estuary in
Germany, downstream from Hamburg (a highly industrialized area),
contained mean levels of HCB in muscle of 688 ng/g (range 84-1907 ng/g
wet weight). Further downstream, towards the mouth of the river,
levels were lower, averaging 12.5 ng/g (range 2-32 ng/g) (Kohler et
al., 1986).
The mean level of HCB in 15 snapping turtle eggs from Ontario,
Canada was 27.1 ng/g wet weight (Bishop et al., 1995).
The levels of HCB in birds have been similar across the various
regions of Canada since the 1980s, probably as a combined result of
emission reductions and the long-range transport of HCB to remote
locations. Mean concentrations of HCB in herring gull eggs ( Larus
argentatus) in 1991 ranged from 16 to 71 ng/g wet weight at various
colonies in the Great Lakes, and were relatively uniform across lakes
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991). These levels were approximately an order of
magnitude lower than in 1974. The mean level of HCB in herring gull
eggs from Norwegian coastal waters in 1981 was 120 ng/g wet weight
(Moksnes & Norheim, 1986). In a study from the Netherlands, mean
levels in eggs of common terns collected in 1987 were 0.03 µg/g wet
weight and in those of black-headed gulls collected in 1988 were
93 µg/g fat (Stronkhorst et al., 1993). Levels of HCB found in eggs of
sea-bird species ( Haematopus ostralegus, Larus ridibundus, Larus
argentatus and Sterna hirundo) from the banks of a river near an
organochlorine chemical plant in Germany were < 500 ng/g wet weight
(Heidmann, 1986); mean levels of less than 15 ng/g wet weight were
found in eggs of several species of land birds, including rooks
( Corvus frugilerus) and sparrow hawks ( Accipiter nisus) from
agricultural, industrial and rural sites. Recent surveys have
indicated similar levels of HCB in the eggs of five other predatory
bird species across Canada (means ranged from 10 to 53 ng/g wet
weight) (Noble & Elliott, 1986; Pearce et al., 1989; Noble et al.,
1992). However, the mean level of HCB in peregrine falcon ( Falco
peregrinus) eggs collected across Canada from 1980 to 1987 was
279 ng/g wet weight, and concentrations ranged as high as 1060 ng/g
wet weight (Peakall et al., 1990).
HCB has been found to accumulate in lipids of the common
goldeneye duck ( Bucephala clangula) that overwinter in the Niagara
River (mean of 150 ng/g) (Foley & Batcheller, 1988) and the Detroit
River (mean of 1700 ng/g) (Smith et al., 1985a) in the USA. Goldeneye
wintering in the Baltic Sea contained average levels of 250 ng/g lipid
(Falandysz & Szefer, 1982). Levels of HCB in the livers of silver
seagulls taken from estuaries in Germany were lower in 1988 than 1989
(approximately 80 and 150 ng/g fat, respectively, in samples from the
River Ems estuary). Higher levels were observed for both years in
liver samples of birds taken from the River Elbe estuary (>250 ng/g
fat) (BUA 1994).
In breast muscle tissue samples from various species of birds,
HCB concentrations tend to be progressively greater at higher trophic
levels (i.e., piscivores > molluscivores > omnivores > grazers)
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991).
In the blubber of marine mammals in the Canadian Arctic, mean
levels of HCB were 19 ng/g wet weight for ringed seals ( Phoca
hispida) and 491 ng/g wet weight for beluga whales ( Delphinapterus
leucas) (Norstrom et al., 1990), while male belugas sampled in the
Gulf of St. Lawrence contained up to 1340 ng/g (Béland et al., 1991).
Blubber from male and female white-beaked dolphins ( Lagerorhunchus
albirostris) collected near the Newfoundland coast averaged
1110 ng/g and 880 ng/g wet weight, respectively. Lower levels
(290 ng/g and 100 ng/g wet weight) were observed in blubber from male
and female pilot whales ( Globicephala meleana), also collected near
the Newfoundland coast (Muir et al., 1988). The higher levels observed
in the dolphins may reflect greater exposure to HCB because of
overwintering and feeding in the Gulf of St. Lawrence. Blubber of
harbour porpoises ( Phocoena phocoena) collected in Poland between
1989 and 1990 contained an average of 573 ng/g wet weight (Kannan et
al., 1993), and those collected around the coast of Scotland between
1989 and 1991 contained an average of 263 ng/g (Wells et al., 1994).
Levels of HCB in the blubber of bottlenosed dolphins also collected
off the coast of Scotland contained an average of 276 ng/g (Wells et
al., 1994). Levels in the blubber of three species of dolphins from
the Bay of Bengal, southern India, were low, ranging from 1.1 to
13 ng/g wet weight (Tanabe et al., 1993). Harbour seals ( Phoca
vitulina) found sick or dead in Norwegian waters due to a disease
outbreak caused by a morbilli virus had a mean HCB level in the
blubber of 27 ng/g wet weight (range of 5-94 ng/g) (Skaare et al.,
1990).
Limited data were found on levels of HCB in terrestrial mammals.
In a 1973-1974 survey of HCB in the adipose tissue of fox ( Vulpes
vulpes), doe ( Capreolus capreolus) and wild boar ( Sus scrofa) in
Germany, HCB concentrations ranged from <10 to 3110 ng/g. The lowest
levels were observed in the does, presumably because they are
herbivorous, whereas foxes and wild boar feed on small animals and are
therefore more affected by biomagnification of HCB (Koss & Manz,
1976). Similar patterns were evident in a study from Sweden, in which
rabbits ( Oryctolagus cuniculus, muscle), moose ( Alcaes alcaes,
muscle), reindeer ( Rangifer tarandus, suet) and osprey ( Pandion
haliaetus, muscle) were found to contain 9, 15, 51 and 330 ng HCB/g
lipid weight, respectively (Jansson et al., 1993). The mean
concentration in 66 serum samples taken in muskoxen in the Canadian
Northwest Territories in 1989 was 2.8 ng/g (range of 1.1-7.5 ng/g)
(Salisbury et al., 1992). The mean concentration of HCB in fat samples
from 58 caribou from the same region ranged from 32.93 to 129.4 ng/g
(lipid corrected) (Elkin & Bethke, 1995). The mean concentration of
HCB in the livers and lipids of adult river otters ( Lutra
canadensis) in western Canada were 3 ng/g and 30 ng/g wet weight,
respectively, for females and 4 ng/g and 25 ng/g wet weight,
respectively, for males (Somers et al., 1987). Concentrations of HCB
in mink carcasses collected in Ontario in the late 1970s and early
1980s ranged from < 0.5 to 10 ng/g wet weight (Proulx et al., 1987).
In the Canadian north, the mean level of HCB in the fat of polar bears
( Ursus maritimus) hunted between 1982 and 1984 was 296 ng/g wet
weight (Norstrom et al., 1990).
5.1.6 Food and drinking-water
HCB is commonly detected, at low levels, in food (Table 7).
Levels of HCB tend to be highest in fatty foods and/or those that have
been treated with HCB-contaminated pesticides. The most extensive data
identified have been collected through the United States Food and Drug
Administration (US FDA) Total Diet Study. The results of the surveys
from 1982 to 1991 indicate that HCB is detectable (DL = 0.1 ng/g) in a
small fraction of food items, most often dairy products, meats, and
peanuts/peanut butter (KAN-DO Office and Pesticides Team, 1995). In
the most recent surveys, conducted during 1990-1991, mean levels were
less than 1 ng/g for all products.
Table 7. Concentration (µg/kg wet weight unless otherwise specified) of hexachlorobenzene in various foods
Country Food Mean contenta Range Reference
Australia cereals 0.01 < 0.01-0.01 Kannan et al. (1994)
pulses 0.02 0.01-0.05
oils 0.07 0.02-0.11
beverages 0.03 0.02-0.04
vegetables 0.01 < 0.01-0.02
fruits 0.01 < 0.01-0.02
dairy products 0.55 0.14-1.6
meat and fat 0.46 0.01-3.0
fishes 4.2 < 0.01-60
Canada fresh meat & eggs 0.17 Davies (1988)b
root vegetables & potato 0.04
fresh fruit ND(<0.01)
leafy/other above-ground vegetables 0.02
2% milk 0.16
Canada apples ND(<0.2)-2.6 OMAF/OME (1988)
peaches ND(<0.2)
tomatoes ND(<0.2)
potatoes ND(<0.2)
wheat ND(<0.2)
eggs ND(<0.2)
hamburger 0.39 0.2-0.57
prime beef ND(<0.2)-0.21
pork ND(<0.2)
chicken ND(<0.2)
Table 7 contd.
Country Food Mean contenta Range Reference
Germany milk 0.22d 0.088-0.45d Fürst et al. (1992)
cream 0.98d 0.31-1.30d
butter 4.86d 2.32-6.88d
cheese 2.72d 2.16-3.70d
India cereals 0.03 0.01-0.04 Kannan et al. (1992a)
pulses (edible seeds of legumes) 0.07 0.02-0.16
spices 0.22 <0.01-0.54
oils 1.5 0.09-2.8
milk 0.03 0.01-0.10
butter 1.7 0.86-2.4
fishes & prawn 0.07 <0.01-0.55
meat & animal fat 0.61 0.02-4.8
Mexico cheese 16.67d 1 Albert et al. (1990)
Morocco eggs 20.9 0.09-300 Kessabi et al. (1990)
poultry liver 5.1 trace-30.0
bovine liver 21.9 1.2-119.8
bovine kidney 15.1 trace-133.0
Papua New cheese 0.43 Kannan et al. (1994)
Guinea pork fat 0.40
chicken 0.20
striped mullet 0.04
tilapia 0.01 0.02-0.05
mud crab 0.03 < 0.01-0.02
oyster 0.02 < 0.01-0.05
Table 7 contd.
Country Food Mean contenta Range Reference
Solomon Islands pork 0.14
chicken 0.06
greenspotted kingfish 0.03 0.01-0.06 Kannan et al. (1994)
indian mackerel 0.01 0.01
paddletail snapper 0.01 0.01
Southern Baltic canned cod-livers 60 ± 6 50-76 Falandysz et al. (1993)
Spain bologna - fresh 2.57d Ariño et al. (1992)
- cooked 2.48d
Spain pork sausage Ariño et al. (1992)
- before curing 6.63d
- after 30 days curing 6.0d
Spain ham - fresh 3.46d Ariño et al. (1992)
- cured 1.29d
Spain pork 2.86-3.9d Ariño et al. (1993)
Spain lamb - chop, raw 14.67d Conchello et al. (1993)
- chop, grilled 12.06d
- leg, raw 8.53d
- leg, roasted 7.02d
Spain chicken 120 ± 10 To-Figueras et al. (1986)
calf 249 ± 37
rabbit 860 ± 159
pork 169 ± 20
sheep 225 ± 35
butter 315 ± 18
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom bread ND (10) MAFF/HSE (1994)
milk 0.6
butter ND(10)
cheese 3.33d
ewes' cheese ND(10)
pasta ND(10)
beef burgers ND(10)
canned meat 10d
cooked meats 10d
lamb ND(10)
rabbit ND(10)
salami ND(10)
United Kingdom sausages ND(10) MAFF/HSE (1994)
pies and pasties ND(10)
salmon (tinned) 2.0
breaded cod ND(2.0)
fish cakes 2.0
mackerel 20
plaice ND(2.0)
prawn products ND(2.0)
sardines (tinned) ND(2.0)
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom carrot 0.0317 Wang & Jones (1994)
potato 3.35
cabbage 0.0418
cauliflower 0.0729
lettuce 0.108
onion 0.0014
bean 0.0101
pea 0.0039
tomato 0.0139
USA cheese, processed 0.2 ND-0.5 US FDA
cheese, cheddar 0.1 ND-0.5 (unpublished)c
beef, ground (regular) 0.1 ND-0.4
beef, chuck roast 0.3 ND-1.0
beef, round steak 0.2 ND-1.0
beef, loin/sirloin steak 0.2 ND-1.0
USA lamb chop 0.3 ND-1.0 US FDA
frankfurters 0.1 ND-0.6 (unpublished)c
cod/haddock fillet ND(0.1) ND-0.2
eggs, scrambled 0.1 ND-0.3
eggs, fried 0.2 ND-0.7
peanut butter 0.2 ND-0.4
peanuts, dry roasted 0.3 ND-1.0
watermelon 0.1 ND-0.5
butter 0.6 ND-1.0
cream 0.1 ND-0.4
Table 7 contd.
Country Food Mean contenta Range Reference
Viet Nam rice 0.03 <0.01-0.05 Kannan et al. (1992b)
pulses 0.04 <0.01-0.18
oil 1.2
butter 5.0
animal fat 0.41 0.29-0.65
meat 0.11 0.03-0.18
fish 0.05 0.01-0.31
prawn 0.03
shellfish 0.04
crab 0.17
caviar 3.8 1.9-7.2
a ND = not detected (detection limit given in brackets).
b Fresh produce and meats grown in Ontario were purchased from four grocery stores in Toronto when locally grown produce
was available (Ontario freshwater fish were not available and therefore, were excluded from analysis). All food items
were grouped into one of five composites for analysis, with the relative proportions of different food items in each
composite calculated from the estimates of the amounts purchased per person per year by Ontario residents.
c The US Food and Drug Administration Total Diet Study conducted from April 1990 to April 1991; reporting residue levels
in 234 individual food items collected from 3 cities in each of 4 geographical regions of the USA (data available from
US FDA, Washington, DC).
d Originally reported on a fat basis, and subsequently converted to wet weight using percentage fat contents reported in
NHW (1987).
In a number of more limited recent surveys, HCB levels have been
determined in commercial foods available in several countries from
North America, Europe and Asia (Table 7). The results of these studies
are consistent with the USA study described above, in that HCB has
been detected primarily in fatty foods such as meats and dairy
products. In these studies, mean concentrations are generally in the
low ng/g range or less, although substantially higher concentrations
have been reported in some surveys from Europe and Asia.
The effects of cooking, curing and ripening on the HCB residues
in pork meat products were investigated in Spain by Ariño et al.
(1992). Neither cooking at 80-82°C for 100 min nor curing reduced the
HCB content in pork bologna and pork sausage, respectively, whereas
the level of HCB in dry-salted and cured ham declined by 42%
throughout maturation.
HCB has been detected infrequently, and at very low
concentrations in drinking-water supplies (Table 5). Samples of
drinking-water collected in 1980 from Canadian cities in the vicinity
of Lake Ontario contained from 0.06 to 0.20 ng/litre, with a mean of
0.1 ng/litre (Oliver & Nicol, 1982). In other Canadian and USA
surveys, HCB was not detected (US EPA 1985b - DL = 100 ng/litre;
Environment Canada, 1989 - DL = 2 ng/litre). Slightly higher
concentrations of HCB (median of 1-2 ng/litre) were reported in
Croatian drinking-water supplies drawn from a nearby polluted river
(pollution sources were not identified) (Fingler et al., 1992).
5.2 General population exposure
5.2.1 Human tissues and fluids
Owing to its persistence and lipophilicity, HCB is present at low
levels in the fatty tissues of virtually all members of the general
population. Levels of HCB in adipose tissues, breast milk, blood and
follicular fluid of various populations from around the world are
shown in Table 8. It should be noted that the quality of the studies
given in Table 8 varies quite widely, from extensive national surveys
to those with relatively few samples.
Levels of HCB in human adipose tissue from around the world are
generally <1 mg/kg (Table 8). Although available data are limited,
concentrations of HCB reported in fat tissue are generally slightly
higher in samples from European countries than from elsewhere in the
world. The highest levels reported in recent surveys are from Spain
(mean levels of approximately 3-6 mg/kg); the authors suggested that
this reflected contamination of foods caused by its presence as an
impurity in other pesticides (Camps et al., 1989; Gómez-Catalán et
al., 1993, 1995). Concentrations of HCB increased with age in a number
of these surveys, but there were no consistent differences in residue
levels between the sexes (Mes et al., 1982; Williams et al., 1984,
1988; Abbott et al., 1985; Mes, 1990; Mes et al., 1990; Gomez-Catalan
et al., 1993; Kemper, 1993; Ludwicki & Góralczyk, 1994).
In general, concentrations of HCB in breast milk in various
countries or regions (Table 8) range widely, and appear to be related
to the degree of industrialization and/or urbanization within the
survey area. The levels of HCB in breast milk have been expressed on a
whole milk basis, using the fat content reported by the authors or,
where this was not reported, a fat content of 4.2% (NHW, 1987).
Schechter et al. (1989a) reported that concentrations of HCB in breast
milk in the mid-1980s were lowest in samples from Thailand
(0.3 µg/litre whole milk) and Viet Nam (< 0.17 µg/litre ), somewhat
higher in those from a semi-rural area of the USA (0.7-0.8 µg/litre),
and higher still in German samples (12.6 µg/litre ) (numbers of
samples in this study were extremely small, except for the German data
(n=167)). In surveys summarized by Mes et al. (1986), mean HCB levels
were 1 µg/litre whole milk in the USA, 2 µg/litre in Canada,
3 µg/litre in Sweden, 4 µg/litre in Great Britain, and 35µg/litre in
Germany. Still higher levels (48-89 µg/litre whole milk) have been
reported in studies from Spain (Conde et al. 1993). Bates et al.
(1994) reported that the concentrations of HCB in breast milk of
primiparae from New Zealand increased linearly with age, but were not
related to body mass index, fish intake, smoking status, type of
residential water supply or location of residence (urban versus
rural). In a study of body burdens of organochlorines in an indigenous
population, Ayotte et al. (1995) reported that mean concentrations of
HCB in the milk fat of 107 Inuit women from northern Quebec were
several times higher than those in 50 Caucasian women from southern
Quebec (57 and 1.2 µg/litre whole milk, respectively). Levels of
organochlorine compounds in breast milk correlated with levels of
omega-3 fatty acids in plasma phospholipids, indicating that
consumption of marine organisms is an important source of exposure to
these xenobiotics.
In a HCB poisoning incident in Turkey (section 8.1), breast-fed
infants were fatally intoxicated through their mothers' milk. In an
early report of this incident (Peters et al., 1966), HCB was reported
as being present in breast milk, although it was not quantified.
However, elevated levels were measured (mean of 510 ng/g on a fat
basis (approximately 21 ng/g on a wet weight basis) for 56 porphyric
mothers) 20-30 years after the incident, compared with a mean of
70 ng/g fat in 77 milk samples from women of families without
porphyria or from areas outside of the endemic area (Peters et al.,
1982; Gocmen et al., 1989).
Table 8. Levels of hexachlorobenzene in human tissues and fluids
(mg/kg wet weight adipose tissue; mg/kg whole milk; µg/litre blood serum; µg/litre follicular fluid)
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue
Australia 31 0.14a (0.01-1.70) (fat basis) 1990-1991 Stevens et al. (1993)
Canada 108 0.026a (0.0073-0.118) 1985 Mes & Malcolm (1992),
Mes et al. (1990)
Canada 25 0.019a (max. value: 0.087) - Mes (1992)
Canada 141 0.071 (males)
(0.018-0.244)
0.109 (females)
(0.019-0.373) 1984 Williams et al. (1988)
Canada 99 0.095 (0.01-0.667) 1976 Mes et al. (1982)
Canada 168 0.062 (0.001-0.52) 1972 Mes et al. (1977)
Federal Republic of Germany 93 (0.11-21.8) 1971 Leoni & D'Arca (1976)
Federal Republic of Germany 6 0.263 (0.083-0.753) van der Ven et al. (1992)
India 7 0.012 (0-0.064) 1987 Nair & Pillai (1989)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Italy 28 0.491 (0.126-1.36) 1973-1974 Leoni & D'Arca (1976)
Japan 39 0.044 1986-1987 Kashimoto et al. (1989)
Japan 15 0.21 (0.10-0.42) - Morita et al. (1975)
Netherlands average 0.7a (fat basis) 1968-1969 Greve & Van Zoonen (1990)
of 1.2a (fat basis) 1973-1975
51/year 0.86a (fat basis) 1976
0.98a (fat basis) 1977-1978
0.85a (fat basis) 1980
0.80a (fat basis) 1981
0.58a (fat basis) 1982
0.49a (fat basis) 1983
0.42a (fat basis) 1985
0.38a (fat basis) 1986
New Zealand - 0.31 - US EPA (1985a)
Poland 53 0.221 (0.068-0.937) early 1980s Szymczyœski et al. (1986)
Poland 277 0.31 1989-1992 Ludwicki & Góralczyk (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Spain 256 2.99 1985-1988 Gómez-Catalán et al. (1993)
(4 cities)
Spain 86 3.37 (0.42-12.53) 1991 Gómez-Catalán et al. (1995)
(lipid basis)
Spain 171 5.55 1982-1983 To-Figueras et al. (1986)
Spain 168 2.95 (0.2-17.37) 1988-89 Ferrer et al. (1992)
United Republic of Tanzania 9 0.003 (0.0013-0.0076) - van der Ven et al. (1992
United Kingdom 201 0.05 (n.d-0.29) 1969-1971 Abbott et al. (1972)
United Kingdom 236 0.19 (0.02-3.2) 1976-1977 Abbott et al. (1981)
United Kingdom 187 0.11 (0.03-0.32) 1982-1983 Abbott et al. (1985)
USA 10 0.125 (0.03-0.47 - Barquet et al. (1981)
USA 6081 0.037a 1974-1983 Robinson et al. (1990)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
USA 763 0.118 (0.001-0.256) 1982 US EPA (1994)
689 0.043 (0.032-0.054) 1984
671 0.051 (0.043-0.059) 1986
B. Breast milkb
Australia 39 0.042 (rural) 1970 Newton & Greene (1972)
28 0.063 (urban)
Australia 137 0.007 (0.002-0.019) (rural) 1979-1980 Stacey et al. (1985)
130 0.008 (0.002-0.017) (urban)
Australia 60 0.017 (0.0007-0.32) - Quinsey et al. (1995)
Australia 128 0.0036a (<0.01-0.216) 1990-1991 Stevens et al. (1993)
Brazil 30 0.00048 (0.00024-0.0036)c 1987-1988 Beretta & Dick (1994)
Canada 412 0.0008 (max = 0.014) 1986 Mes et al. (1993)
Canada 210 0.002 (max.= 0.009) 1982 Mes et al. (1986)
Canada 100 0.002 (max.= 0.021) 1975 Mes & Davies (1979)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Canada 536 0.0013c 1989-1990 Dewailly et al. (1991)
Canada 127 0.00051 1978 Frank et al. (1988)
15 0.0004 1979
12 0.00028 1980-1981
13 0.00052 1983-1984
18 0.00026 1985
Finland 143c 0.002c 1984-1985 Mussalo-Rauhamaa et al. (1988)
Finland 50 0.0023 (0.0007-0.006) 1982 Wickström et al. (1983)
France 20 0.002 (0.00004-0.008)c 1990-1991 Bordet et al. (1993)
Federal Republic of Germany 144 0.021c 1984 Fürst et al. (1994)
220 0.019c 1985
157 0.015c 1986
144 0.015c 1987
196 0.013c 1988
145 0.01c 1989
286 0.0095c 1990
113 0.0074c 1991
Federal Republic of 167 0.0126c 1985-1987 Schecter et al. (1989a)
Germany
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Federal Republic of 2709 0.048c 1979-1981 BUA (1994)
Germany 3778 0.013c 1986
1897 0.014c 1987
2994 0.011c 1988
3256 0.01c 1989
5340 0.009c 1990
Former German 483 0.007c 1990-1991
Democratic Republic
India 16 0.042 (0-0.25)e 1987 Nair & Pillai (1989)
Israel 100 0.00256 - Weisenberg et al. (1985)
Italy 56 0.058d - Franchi & Focardi (1991)
Italy 64 0.006 (0.004-0.009) 1987 Larsen et al. (1994)
Netherlands 202 0.036a,c 1972-1973 Greve & Van Zoonen (1990)
278 0.008a,c 1983
New Zealand 38 0.0011 1988 Bates et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Norway 28 0.0007 1991 Johansen et al. (1994)
Spain 240 0.089(0.039-0.21)c 1984-1987 Conde et al. (1993)
358 0.048(0.037-0.073)c 1990-1991
Sweden 20 0.0042 (0.002-0.009)c 1978 Norén (1983a)
Sweden 2 0.0007-0.004c - Norén (1983b)
Sweden 227 0.003 (0.002-0.004)c 1972 Norén (1988)
245 0.003 (0.003-0.004)c 1976
340 0.003 (0.003-0.004)c 1980
102 0.001 (0.0008-0.001)c 1984-1985
Sweden 140 0.0012c 1989 Norén (1993)
Sweden 40 0.0017c 1986-1987 Vaz et al. (1993)
Thailand 3 0.0003c 1985-1987 Schecter et al. (1989a)
Turkey 51 0.0035c 1988 Üstünbas et al. (1994)
Turkey 56 0.021c 20-30 years Gocmen et al. (1989)
post-exposure
during
1955-1959
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
United Kingdom 193 0.001 (<0.001-0.005) 1989-1991 MAFF (1992)
USA 40 0.00052 1979 Bush et al. (1985)
USA 8 0.0007-0.0008c 1985-1987 Schecter et al. (1989a)
Viet Nam 12 <0.00017c 1985-1987 Schecter et al. (1989a)
Yugoslavia 10 0.006 (0.002-0.017) 1978 Kodric-Smit et al. (1980)
C. Serum
Canada 25 0.25 1993 Jarrel et al. (1993)
29 0.21
20 0.35
Croatia 15 1a (<0.5-4) 1985 Krauthacker (1993)
24 0.9a (<0.5-3) 1987-1988
26 1a (<0.5-7) 1989-1990
32 <0.5a (<0.5-4) 1990
Germany 6 1.23 (0.33-2.66)f - van der Ven et al. (1992)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
C. Serum (contd)
Spain (near 21 26 (7.5-69) 1992 Grimalt et al. (1994)
organochlorine
compounds
factory)
Spain (hospital 13 4.8 (1.5-15) 1992 Grimalt et al. (1994)
in Barcelona)
Spain 100 11.09 (1.60-94.2) 1986 To-Figueras et al. (1995)
4.13 (0.70-19.7) 1993-1994
United Republic 11 0.01 (0-0.03)f - van der Ven et al. (1992)
of Tanzania
USA 370 0.189a (0.05-3.21) - Needham et al. (1990)
D. Whole blood
Slovakia 50 25.2 (6.1-43.2) 1992 Koœan et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
E. Follicular fluid
Canada 25 0.11 1993 Jarrell et al. (1993b)
29 0.14
20 0.20
Federal Republic 15 2.59 (1.1-5.7) - Trapp et al. (1984)
of Germany
a Median value.
b The most recent data were used for calculation of intakes via breast milk (section 5.2.4).
c Originally expressed as mg/kg milk fat and subsequently converted to a wet weight basis using either the % fat reported,
or if not given, using 4.2% fat (NHW, 1987).
d Number of positive samples
e Originally expressed on a dry weight basis and subsequently converted to wet weight using 88% moisture for conversion
(NHW, 1987).
f Values reported in µg/kg.
HCB is present in a wide range of other tissues and fluids from
humans, but at lower levels than in adipose tissue and breast milk.
For example, concentrations of HCB in serum of 370 subjects from the
general population in the USA studied by Needham et al. (1990)
averaged 0.189 µg/litre, compared to concentrations of 39 ng/g lipid
in 287 adipose tissue samples. Schechter et al. (1989b) reported HCB
levels in various organs in autopsy tissue from three American
patients. HCB levels in adipose tissue ranged from 15 to 24 ng/g wet
tissue, while kidney, muscle, lung, spleen and testis contained 1 ng/g
or less, and adrenals, bone marrow and liver contained intermediate
concentrations.
Median blood serum levels of HCB ranged from <0.5 to
1.0 µg/litre in four population groups (total of 97 samples) from
Zagreb, Croatia (Krauthacker, 1993). The groups included workers
employed in the distribution and packing of seeds treated with
different pesticides, who were expected to have absorbed
organochlorine compounds at levels greater than the general
population. However, levels of HCB in the blood of these workers were
not elevated. Van der Ven et al. (1992) reported the HCB levels in
maternal serum of 6 and 11 full-term pregnant women in Germany and
Tanzania, respectively. Levels in Germany averaged 1.23 µg/kg (0.33-
2.66 µg/kg), whereas those in Tanzania were 0.01 µg/kg (0-0.03 µg/kg).
Analysis of blood plasma samples from a human organ specimen bank in
Germany revealed that median annual concentrations of HCB between 1983
and 1989 ranged between 3.1 and 5.4 µg/litre (Kemper, 1993). Serum and
ovarian follicular fluid have been shown to contain HCB in patients
receiving in vitro fertilization (Jarrell et al., 1993b). Of 72
patients, HCB was detected in the serum of 60 and follicular fluid of
49. There was a significant geographical variation among three major
cities in Canada (Jarrell et al., 1993b). Follicular fluid from
similarly treated patients in Germany has been shown to contain HCB
(Trapp et al., 1994). In some studies, workers exposed to chlorinated
solvents or chlorinated pesticides had elevated levels of HCB in blood
(section 5.2.7).
5.2.2 Intake from ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the range
of mean levels of HCB measured in ambient air in cities from around
the world of approximately 0.1 to 0.6 ng/m3 (Table 4), mean intake of
HCB from ambient air for the general population is estimated to range
from 3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day. Since no
data on levels of HCB in indoor air were found, it has been assumed
that levels indoors are the same as those outdoors. The intake of HCB
via air may be greater in populations residing in the vicinity of
point sources, but this exposure is considered to be too site-specific
to estimate reliably.
5.2.3 Intake from drinking-water
Based on a daily volume of ingestion for adults of 1.4 litres, a
mean body weight for males and females of 64 kg (IPCS, 1994), and the
range of mean concentrations of HCB detected in drinking-water from
cities of approximately 0.1 to 2 ng/litre (Table 5), the estimated
mean daily intake of HCB from drinking water for the general
population ranges from approximately 2.2 × 10-6 to 4.4 × 10-5 µg/kg
body weight per day.
5.2.4 Intake from foods
Based on the average daily consumption of various foodstuffs by
adults from around the worlda, a mean body weight for males and
females of 64 kg (IPCS, 1994), and the mean level of HCB detected in
various foods in the 1990-1991 US FDA Total Diet Study (Table 7), the
estimated daily intake of HCB from food ranges from approximately
0.0004 to 0.0028 µg/kg body weight per day (this range was generated
by assuming, for food groups in which HCB was not detected, that non-
detectable values were zero with the detection limit being 0.1 µg/kg.
These estimates overlap the range of dietary estimates (between 0.001
and 0.027 µg/kg body weight per day) that have been reported for
various countries (Canada, USA., Germany, Finland, Viet Nam, Thailand,
India, Japan, Australia, the Netherlands) (Gartrell et al., 1986; De
Walle et al., 1995; Fujita & Morikawa, 1992; Kannan et al., 1992a,b;
Government of Canada, 1993; Kannan et al., 1994). Intakes via food may
be substantially higher in selected European and Asian countries,
where the content of HCB in a sampling of a limited range of foods was
relatively high (Table 7), or in indigenous populations consuming
large quantities of some wildlife species, such as marine mammals,
that are known to accumulate relatively high tissue levels of
lipophilic contaminants (Government of Canada, 1993; Ayotte et al.,
1995; Kuhnlein et al., 1995).
a Dietary intakes (g/person/day) consist of: cereals, 323, starchy
roots, 225; sugar (excludes syrups and honey), 72; pulses and
nuts, 33; vegetables and fruits, 325; meat, 125; eggs, 19; fish,
23; milk products (excludes butter), 360; fats and oils (includes
butter), 31 (all intakes from IPCS, 1994).
Dietary intakes may also be greater in infants during breast-
feeding, owing to the accumulation of HCB in the mothers' milk. The
mean concentrations of HCB in the most recent surveys found for
various countries range from <0.17 to 48 µg HCB/litre whole milk
(Table 8). Assuming that infants are exclusively breast fed for the
first 6 months, during which they consume an average of 0.75 litres of
breast milk per day and have an average body weight of 7 kg (Health
Canada, 1994), the estimated mean intakes of HCB from breast milk in
various countries range from <0.018 to 5.1 µg/kg body weight per day.
Daily intakes of HCB by breast-feeding infants of Inuit mothers in
northern Quebec, Canada (a population that consumes substantial
quantities of marine organisms that accumulate lipophilic
contaminants) was estimated at 0.45 µg/kg body weight per day, a value
that was several times greater than for a more southerly population in
the same province (Ayotte et al., 1995).
5.2.5 Apportionment of intakes
Total intake of HCB from ambient air, drinking-water and foods is
estimated to range from approximately 0.0004 to 0.003 µg/kg body
weight per day for the general population, the principal route of
exposure being through the diet (92%). The estimated contributions
from air and drinking-water are much smaller (7% and 1%,
respectively). (The contribution from each environmental medium was
calculated based on the mid-point of the intakes estimated in the
previous sections.)
5.2.6 Trends in exposure of the general population over time
The results of most studies of the levels of HCB in foods and
human tissues over time indicate that exposure of the general
population to HCB declined from the 1970s to the mid-1990s in many
locations. However, this trend has not been evident during the last
decade in some other locations.
Routine monitoring of foods in some countries indicates that
exposure to HCB is decreasing. For example, mean concentrations in
grab samples of milk, bovine fat, poultry fat and egg fat collected
from suppliers in Ontario, Canada, decreased by an order of magnitude
or more between the early 1970s and the mid-1980s (Frank et al., 1983,
1985a, 1985b; Frank & Ripley, 1990). Brown et al. (1986) reported that
the frequency of detectable (> 10 ng/g in fat samples, wet weight)
levels of HCB in the USA meat and poultry supply increased
dramatically from 1972 to 1977-1978, but had fallen off sharply up to
1984. More recent data collected through the US FDA Total Diet Study
indicate that this trend had continued. Between 1982-1984 and 1991,
the most recent year for which data are available, both the frequency
of detection of HCB and the estimated average daily intake for people
of various ages decreased by roughly 80% (US FDA 1990, 1991, 1992). A
decline in HCB levels in fish from the Baltic and the Swedish West
Coast was found in the National Swedish Monitoring Programme over the
period 1988 to 1994 (Bignert, 1995). The annual decrease in levels in
herring muscle from different places in the Baltic was 12 to 15% and
in cod liver from the Baltic 21%; from the West Coast it was 12% in
herring muscle, 23% in cod liver and 23% in dab liver. The trend with
higher concentrations in samples from the Baltic as compared to the
West Coast is still seen in the samples.
The results of most studies of temporal trends of HCB levels in
human adipose tissue or milk (summarized in Table 8) indicate that
general population exposures have declined since the 1970s. In routine
monitoring of breast milk contaminants in German mothers, mean
concentrations of HCB declined by more than 50% between 1984 and 1991
(Fürst et al., 1994), and by about 80% between 1979 and 1990 (BUA
1994). The median HCB content in samples of plasma from a human
specimen bank in Germany decreased from 4.8 µg/litre in 1983 to
3.1 µg/litre in 1989, a period of increasing restrictions on indoor
applications of pentachlorophenol, which contains HCB as a contaminant
(Kemper, 1993). Mes (1990) reported that concentrations of HCB in
human adipose tissue from Canadian surveys were significantly lower in
1985 than in 1972; this decrease occurred in all age classes over this
period. An increase in the HCB content of human adipose tissue and
milk was observed in the early 1970s in the Netherlands, and was
attributed to an increase in the HCB concentration in products of
animal origin (Greve & Van Zoonen, 1990). Once measures were taken to
avoid contamination of such products, a gradual decrease in HCB levels
was observed. Johansen et al. (1994) reported that the concentration
of HCB in routine monitoring of milk from Norwegian mothers declined
by 65% between 1982 and 1991. In contrast, in the most extensive study
of levels of HCB in adipose tissues, the US National Human Adipose
Tissue Survey (Robinson et al., 1990), in which data on residues were
collected from a nationally representative sample of 6081 autopsies
and surgical patients from 1974-1983, there was little change in
residue concentrations over the study period, with the national median
level remaining near 30 to 40 ng/g.
5.2.7 Occupational exposure during manufacture, formulation, or use
Workers may be exposed to higher concentrations of HCB than the
general population, particularly in the manufacture of chlorinated
solvents, and in the manufacture and application of pesticides
contaminated with HCB.
In a survey of production industries (perchloroethylene,
trichloroethylene, carbon tetrachloride, chlorine, triazine herbicides
and pentachloronitrobenzene), the highest HCB concentrations were
associated with the production of perchloroethylene and
trichloroethylene (Spigarelli et al., 1986). The highest level of HCB
determined in the air on plant property was 24 µg/m3 at a plant
producing perchloroethylene, carbon tetrachloride and chlorine.
Relatively high HCB levels (maximum concentration of 2.2 µg/m3 in
air) were also detected in samples from the pentachloronitrobenzene
production plant. Lower levels of HCB were measured at triazine
herbicide production plants (ND - 0.02 µg/m3), and, in the one plant
that produced only carbon tetrachloride, HCB was not detected (MDL not
reported). It is not known how representative the data from these
studies are, as the generation and release of HCB would be minimized
in plants using appropriate modern technology and waste management
practices.
Personal breathing-zone samples (54 in all) from workers in a
pentachlorophenol production plant contained HCB concentrations
ranging from <0.1 to 120 µg/m3 (Marlow, 1986), while levels in 112
area samples throughout the plant ranged from <0.1 to 630 µg/m3.
HCB concentrations in the blood of workers in a factory producing
chlorinated solvents ranged from 14 to 233 µg/litre (Burns & Miller,
1975); this compared with a range <1 to 310 µg/litre in the blood of
vegetable spraymen (Burns et al., 1974). Mean levels of HCB in the
blood plasma of workers in a chlorinated solvents plant in the USA
were 311 µg/litre in 1974, and 312 µg/litre in 1975, and levels in
whole blood were 160 µg/litre in 1976, and 170 µg/litre in 1977
(Currier et al., 1980). Concentrations of HCB in blood were positively
correlated with the number of years worked in the plant, but were not
associated with airborne levels of HCB or job-category-based exposure
estimates. Pesticide-exposed vineyard workers in Germany tended to
have higher HCB whole blood levels (median 7 µg/litre, maximum
30 µg/litre) than reference controls (median 3 µg/litre, maximum
17 µg/litre) (Kemper, 1993). Angerer et al. (1992) reported that the
mean plasma level of HCB in 53 workers at a municipal waste
incinerator was 5.0 µg/litre, compared with 4.69 µg/litre in 64
subjects with no known occupational contact.
6. KINETICS AND METABOLISM
6.1 Aquatic and terrestrial biota
Terrestrial plants such as barley, cress and wheat, and algae
such as Oedogonium cardiacum slowly metabolize HCB to polar
metabolites and non-extractable residues (Lu & Metcalf, 1975;
Scheunert et al., 1983, 1985; Topp et al., 1989). For example, of the
total radiolabelled HCB in barley after uptake from soil over one
growing season, 14% was present as polar metabolites, 20% as plant-
bound residues and the remainder as the parent compound (Topp et al.,
1989). In the only available study on HCB depuration rates in plants,
the aquatic macrophyte Myriophyllum spicatum eliminated 95% of HCB
during the first 28 days after exposure ceased (Gobas et al., 1991).
Invertebrates slowly metabolize HCB to compounds such as
pentachlorothioanisole, pentachlorophenol and other polar metabolites
(Lu & Metcalf, 1975; Bauer et al., 1989). In an aquatic model
ecosystem treated with 14C-HCB for 24 h, unchanged HCB accounted for
84% of the total radioactivity in snails ( Physa sp.), 67% in water
fleas ( Daphnia magna) and 65% in mosquito larvae ( Culex pipiens)
(Lu & Metcalf, 1975). Half-lives for the elimination of HCB by
invertebrates were less than 5 days for filter-feeding bivalves
( Elliptio complanata and Mytilus edulis) (Bro-Rasmussen, 1986;
Russell & Gobas, 1989), 16 days for deposit-feeding clams ( Macoma
nasuta) (Boese et al., 1990), and 27 days for oligochaete worms
( Tubifex tubifex and Limnodrilus hoffmeisteri) (Oliver, 1987).
Sanborn et al. (1977) detected pentachlorophenol and at least
four unidentified polar metabolites in green sunfish ( Lepomis
cyanellus) after 28 days of ingesting HCB-contaminated food.
Pentachlorophenol has also been detected in the excreta and tissues of
rainbow trout ( Oncorhynchus mykiss) following an intraperitoneal
dose with HCB (Koss & Koransky, 1978; Koss et al., 1978). Zebra fish
( Brachydanio rerio) did not metabolize HCB after a 48-h exposure in
water (Kasokat et al., 1989). Elimination half-lives of HCB ranged
from 7-21 days for fathead minnows ( Pimephales promelas) after a
waterborne exposure (Kosian et al., 1981) to up to 210 days for
rainbow trout ( Oncorhynchus mykiss) after ingestion of HCB in food
(Niimi & Cho, 1981).
Clark et al. (1987) reported that 63% of the total HCB eliminated
in herring gulls ( Larus argentatus) was found in the egg yolk.
Breslin et al. (1983) found that 50% of total HCB eliminated from
laying bobwhite quail ( Colinus virginianus), a species that lays
many eggs, was accounted for in egg yolk. For most wild species, egg
laying will account for a relatively small loss of HCB, while
depletion of stored fat during energetically costly activities such as
migration and moulting may result in a significant reduction in body
burdens. The half-life for elimination of HCB in birds ranged from
24-35 days for domesticated chickens ( Gallus gallus domesticus) fed
HCB-contaminated diets (Kan & Tuinstra, 1976; Hansen et al., 1978) to
211 days in intraperitoneally dosed juvenile herring gulls (Clark et
al., 1987).
6.2 Mammals
There are few data on the absorption of HCB by humans. By
comparing intake and faecal excretion of HCB in a single breast-fed
infant, Abraham et al (1994) estimated that absorption was virtually
complete (greater than 99.7% at one month of age and greater than 97%
at 5 months). The concentrations of HCB in the diet and faeces of a
single formula-fed infant were too low for reliable estimation of
absorption (Abraham et al., 1994). The results of animal studies
indicate that 80% or more of an oral dose of HCB (between 10 and 180
mg/kg body weight) is absorbed if administered in an oil vehicle
(Albro & Thomas, 1974; Koss & Koransky, 1975; Ingebritsen et al.,
1981; Bleavins et al., 1982). In female rats treated with 14C-HCB in
oil, peak values of radioactivity were reached in 2 to 5 days. The
absorption was poor (2-20%, depending on the dose) when the substance
was given as an aqueous suspension (Koss & Koransky, 1975). Little
information was identified on dermal absorption, although it appears
to be lower. Koizumi (1991) observed that after dermal application of
approximately 2.5 mg 14C-HCB in tetrachloroethylene to Fisher-344
rats for 72 h, only 9.7% of the administered dose was absorbed. No
information on absorption via the lungs has been reported.
There are no experimental studies of tissue distribution of HCB
in humans, although in a small autopsy study of members of the general
population (Schechter et al., 1989b), the highest levels were found in
(in order) adipose tissue, adrenals, bone marrow and liver. Laboratory
studies in a number of animal species also indicate that the highest
concentrations of HCB are accumulated in tissues with a high lipid
content, such as the adipose tissue, adrenal cortex, bone marrow, skin
and some endocrine tissues (thyroid, adrenal and ovary) following
ingestion or injection of HCB (Koss & Koransky, 1975; Yang et al.,
1978; Courtney, 1979; Sundlof et al., 1982; Ingebritsen, 1986; Smith
et al., 1987, 1994; Goldey et al., 1990; Foster et al., 1993; Jarrell
et al., 1993a). No information was found on the tissue distribution
following inhalation or dermal exposure. HCB crosses the placenta, and
is eliminated via the mothers' milk in both animals and humans
(Villeneuve et al., 1974; Mendoza et al., 1975; Courtney & Andrews,
1979, 1985; Courtney et al., 1979; Bailey et al., 1980; Bleavins et
al., 1982; Goldey et al., 1990; section 5.2.1).
Metabolic transformation is not extensive in the wide range of
species examined. The pathways of biotransformation of HCB have been
reviewed by Debets & Strik (1979) and by Renner (1988). The metabolism
of HCB operates via three distinct pathways. These are oxidative
pathways, which give rise to phenolic metabolites including
pentachlorophenol, tetrachlorohydroquinone and tetrachloro-
benzoquinone; a glutathione-conjugation pathway leading to penta-
chlorothiophenol, pentachlorothioanisoles, and several other sulfur-
containing metabolites; and a minor pathway that yields lower
chlorinated benzenes through reductive dechlorination. Metabolism
occurs primarily in the liver, although dechlorination of HCB has also
been demonstrated in vitro in enzyme preparations from the lung,
kidney and small intestine (Mehendale et al., 1975).
The metabolism of HCB has been studied in the rat and guinea-pig
(Mehendale et al., 1975; Rozman et al., 1975; Koss & Koransky, 1976;
Koss et al., 1978; Koss & Koransky, 1978; Courtney, 1979), and in the
monkey (Rozman et al., 1975; Courtney, 1979). Dosing routes included
gastric intubation and the intraperitoneal route, while dosing
vehicles included oil and aqueous media. The monitoring for metabolic
products of HCB has included excretory products and/or tissue residues
for periods ranging from 28 to 40 days post-dosing. Findings were
quite dissimilar among the studies. The most common finding was that
less than 40% of the administered dose was recovered in the excretory
products and a majority of the recovered dose was unchanged HCB.
The major metabolites found in the urine of rats, mice and
guinea-pigs exposed to HCB by various routes in most studies are
pentachlorophenol (PCP), tetrachlorohydroquinone and
pentachlorothiophenol (PCTP) (Koss & Koransky, 1978; Koss et al.,
1978). (There is some question as to whether most of the latter
compound detected in some studies was an analytical artefact from
alkaline hydrolysis of the n-acetyl cysteine conjugate.) Other
metabolites include tetra- and pentachlorobenzenes and thioanisoles,
and tri- and tetrachlorophenols, both in free and conjugated forms. It
has been reported that, after dietary exposure of male and female
Wistar rats to HCB for 13 weeks, N-acetyl- S-(pentachloro-
phenyl)cysteine was the most abundant metabolite via the conjugation
pathway (89-92% of the total urinary metabolites collected over 24 h,
after one week of treatment). Mercaptotetrachlorothioanisole was also
present, excreted as a glucuronide (den Besten et al., 1994). The
excreta from male Wistar rats given 125 mg/kg body weight on day 1 and
6 were collected for 12 days (Jansson & Bergman, 1978). Faeces and/or
urine contained HCB (about 4% of the total does), pentachlorobenzene,
pentachlorophenol, pentachlorobenzenethiol (both as such and as
conjugates), methylthiopentachlorobenzene, tetrachlorobenzenedithiol
and/or methylthiotetrachlorobenzenethiol (both as such and as
conjugates), dichlorotetrakis(methylthio)benzene (trace amounts),
hexakis(methylthio)benzene (trace amounts), bis(methylthio)-
tetrachlorobenzene, tetrachlorobenzenethiol (trace amounts) and
methylthiotetrachlorobenzene (trace amounts). Compounds found
accumulated in adipose tissue were hexachlorobenzene,
pentachlorobenzene, pentachlorobenzenethiol, bis(methylthio)-
tetrachlorobenzene and pentachloroanisole.
Rizzardini & Smith (1982) administered 50 µmoles of HCB/kg body
weight to male and female rats by gavage in arachis oil for 103 days.
Three urinary metabolites were identified, i.e., pentachlorophenol,
2,3,5,6-tetrachlorobenzene, 1,4-diol and pentachlorothiophenol
(derived from mercapturate). The authors reported that female rats
excreted several times more HCB metabolites than males.
PCP and PCTP have been detected in the urine of humans from the
general population of Spain with high body burdens of HCB (To-Figueras
et al., 1992).
No reliable information on the elimination half-life of HCB in
humans was found. Excretion of HCB by laboratory animals occurs mainly
through the faeces regardless of the route of administration (US EPA,
1985a; ATSDR, 1990). Both biliary excretion and non-biliary intestinal
transfer contribute to faecal excretion (Rozman et al., 1981;
Ingebritsen et al., 1981; Richter & Schäfer, 1981; Sundlof et al.,
1982). Reported half-lives for the elimination of an oral dose of HCB
(doses were 3 mg/kg body weight or less in these studies) are
approximately one month in rats and rabbits, 10-18 weeks in sheep,
pigs and dogs, and 2.5 to 3 years in rhesus monkeys (Avrahami &
Steele, 1972; Avrahami, 1975; Rozman et al., 1981; Sundlof et al.,
1982; Scheufler & Rozman, 1984; Yamaguchi et al., 1986). HCB has been
detected in the milk of several species, including humans, and the
results of experiments with mice and ferrets indicate that the
majority of the maternal body burden can be eliminated via the
mother's milk during lactation (Bleavins et al., 1982; Courtney &
Andrews, 1985).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
This section summarizes the extensive literature on the toxicity
of HCB to laboratory mammals, with emphasis on those studies reporting
the lowest-observed-effect levels. Information on the dosage with
respect to body weight was obtained from the original papers, wherever
possible. When doses were not expressed in this way by the
investigators and could not be calculated from the data provided,
approximate doses (given in parentheses) have been estimated based on
the reference values given in NIOSH (1985).
7.1 Single exposure
The acute toxicity of HCB in experimental animals is low;
reported oral LD50 values for various species range from 1700 mg/kg
body weight for the cat to between 3500 and > 10 000 mg/kg body
weight for the rat, with intermediate values for the mouse, rabbit and
guinea-pig. Reported LC50 values for inhalation exposure range from
1600 mg/m3 for the cat to 4000 mg/m3 for the mouse, with
intermediate values reported for the rat and rabbit (IARC, 1979;
Strik, 1986; Lewis, 1992). Acute lethal doses elicit convulsions,
tremors, weakness, ataxia, paralysis and pathological changes in
organs. Strik (1986) reported that HCB has a low skin irritation
score, is not irritating to the eye and does not sensitize the guinea-
pig, although no details were provided. In several studies, single
oral doses of 100-1000 mg/kg body weight produced increases in the
activities of various liver enzymes in rats within 24 h (Strik, 1986).
7.2 Short-term and subchronic exposure
The effects of short-term, repeated exposure to HCB are primarily
hepatotoxic and neurological. In a number of studies, the effects of
HCB on rats exposed to oral doses in the range of 30-250 mg/kg body
weight per day included altered body weight, cutaneous lesions,
tremors and other neurological signs, hepatomegaly, liver damage and,
in some cases, early alterations in porphyrin or haem metabolism
(Courtney, 1979; US EPA, 1985a; Strik, 1986). Short-term exposure
in vivo induced a variety of enzymes, including glutathione- S-
transferases and isozymes of cytochrome P-450, identified as
cytochromes P-450IA1 (CYPIA1), P-450IA2 (CYPIA2) and P-450IIB (CYPIIB)
(Wada et al., 1968; Courtney, 1979; Denomme et al., 1983; US EPA,
1985a; Linko et al., 1986; Strik, 1986; Hahn et al., 1988, 1989; Vos
et al., 1988; Green et al., 1989; Rizzardini et al., 1990; D'Amour &
Charbonneau, 1992; Smith et al., 1993; Goerz et al., 1994). This means
that HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
Enzyme induction has been observed at relatively low doses in some
studies. For instance, in Wistar rats fed HCB in the diet for 14 days,
the low-effect level for induction of microsomal liver enzyme was
50 mg HCB/kg feed (approximately 2.5 mg/kg body weight per day), and
the no-effect level was 20 mg HCB/kg feed (approximately 1 mg/kg body
weight per day) (den Tonkelaar & van Esch, 1974).
The effects produced by subchronic exposure to HCB are similar to
those observed in short-term studies, but are generally evident at
lower doses (Courtney, 1979; US EPA, 1985a; ATSDR, 1990, 1994). At
relatively high doses (32 mg/kg body weight per day or more for
periods from several weeks to 90 days), reported effects have included
death, skin lesions, behavioural and neurological changes, reduced
body weight gain, increased organ weights, and altered thyroid
function and serum levels of thyroid hormones (the latter effect is
discussed later in this section). At lower doses, hepatotoxic effects
have been commonly reported, including histological alterations, the
induction of a variety of hepatic microsomal enzymes and porphyria.
The porphyrinogenic effects of exposure to HCB have been
extensively studied since the seminal reports of Ockner & Schmid
(1961) and De Matteis et al. (1961). These and subsequent earlier
works, many of them conducted at relatively high doses, have been
summarized by Courtney (1979), and much of this research is not
discussed in this report. Porphyria has been observed in several
species of laboratory mammals, most often manifested as increased
levels of porphyrins and/or porphyrin precursors in the liver, other
tissues and excreta. This disturbance in haem synthesis is associated
with the inhibition of uroporphyrinogen decarboxylase activity (this
enzyme converts uroporphyrinogen III to coproporphyrinogen III),
leading to the accumulation of uroporphyrin and other highly
carboxylated porphyrins, and with the induction of ALA synthetase (the
enzyme controlling the rate of haem synthesis) (ATSDR, 1994). There is
a delay before exposed rats become porphyric, which appears to reflect
the time for the animals to receive a sufficient cumulative dose of
HCB (Krishnan et al., 1991, 1992), as well as the time needed for the
porphyrins to accumulate to the level of overt porphyria (Kennedy et
al., 1986; Kennedy & Wigfield, 1990). Although in most studies
porphyria has been associated with longer-term exposure to HCB, rats
exposed to doses of 25-50 mg HCB/kg body weight per day for as little
as several days had increased levels of hepatic and urinary porphyrins
(Krishnan et al., 1991, 1992). In another study, hepatic levels of
highly carboxylated porphyrins were elevated by a single exposure to
50 mg HCB/kg body weight, although the latter result was not
accompanied by clinical porphyria (Kennedy & Wigfield, 1990).
HCB-induced porphyria has been extensively studied in rats, in
which dietary or gavage exposure of various strains to between 2.5 and
15 mg HCB/kg body weight per day for periods of 8 to 15 weeks has
caused hepatic porphyria, and, in some studies, increased levels of
porphyrins in the kidney and spleen (Grant et al., 1975; Kuiper-
Goodman et al., 1977; Goldstein et al., 1978; Mendoza et al., 1979;
Rizzardini & Smith, 1982; Teschke et al., 1983; Smith et al., 1985b;
Green et al., 1989; Van Ommen et al., 1989; Kennedy & Wigfield, 1990;
Smith et al., 1990; Den Besten et al., 1993). A no-observed-effect-
level (NOEL) for HCB-induced porphyria was not determined in these
studies. Although the data on other species are limited, levels of
hepatic or urinary porphyrins were increased in mice of various
strains fed diets containing 200 mg HCB/kg feed (yielding approximate
doses of 24 mg HCB/kg body weight per day) for periods of 7 to 15
weeks in some studies (Smith & Francis, 1983; Rizzardini et al., 1988;
Vincent et al., 1989), and porphyria was induced in Japanese quail
following short-term oral and intraperitoneal exposure to 500 mg
HCB/kg body weight per day (section 9.1.2).
The lowest doses producing porphyrinogenic and other effects on
the liver in a subchronic study were reported by den Tonkelaar et al.
(1978). Groups of five pigs exposed for 90 days to doses of 0.5 mg/kg
body weight per day or more in the diet had increased urinary levels
of coproporphyrin and alterations in liver histology and microsomal
enzyme activities, but no effects were observed at 0.05 mg/kg body
weight per day. However, marked excretion of coproporphyrin alone is
not a characteristic of the inhibition of uroporphyrinogen
decarboxylase in animal systems.
Female rats are more sensitive than males to the porphyrinogenic
effects of exposure to HCB. In various strains of rats exposed to
doses of 5 to 10 mg HCB/kg body weight per day in the diet or by
gavage, for periods of between 3 months or more, females developed a
marked porphyria which was absent or much reduced in males (Grant et
al., 1975; Kuiper-Goodman et al., 1977; Rizzardini & Smith, 1982;
Smith et al., 1985b). In a number of studies, the basis for the
susceptibility to HCB-induced porphyria of female rats compared to
males has been examined. Grant et al. (1975) reported that ovariectomy
decreased, and castration increased, the accumulation of porphyrins in
the livers of female and male Sprague-Dawley rats with subchronic
exposure to HCB, suggesting a role for steroid hormones in the
development of porphyria in this species. In another study, female
Fischer-344 rats with HCB-induced porphyria had higher levels of
cytochrome P-450IA isoenzymes and ethoxyresorufin- O-deethylase
activity than males, whereas males had higher levels of total
cytochrome P-450 and activities of microsomal monooxygenases
associated with cytochrome P-450IIB1 (Smith et al., 1990). In Fischer-
344 rats with HCB-induced porphyria, sex-related differences in
urinary and hepatic porphyrin levels were paralleled by differences in
the excretion of phenolic metabolites, particularly
pentachlorothiophenol (Rizzardini & Smith, 1982). These findings were
further investigated in a short-term study by D'Amour & Charbonneau
(1992), which indicated that male rats may be more resistant to HCB-
induced porphyria than females because hepatic conjugation of HCB with
glutathione is more important in males. Male Sprague-Dawley rats
receiving a porphyrinogenic dose of HCB (100 mg HCB/kg body weight per
day by gavage for 5 days) had significantly lower hepatic gluthione
concentration and higher glutathione transferase activity (to 3,4-
dichloronitrobenzene) than controls, whereas no significant
differences were observed in females. Biliary excretion of PCTP (a
metabolite of glutathione conjugation) and the rate of elimination of
HCB from the liver were greater in males than in females.
Other mechanistic studies have suggested the involvement of
oxidative metabolism of HCB in the development of porphyria, although
the mechanism remains to be elucidated. In female Wistar rats
co-treated with 300 mg HCB/kg in the diet (approximately 15 mg HCB/kg
body weight per day) and triacetyloleandomycin (TAO) (to selectively
inhibit cytochrome P-450IIIA1/2 and thereby prevent the oxidative
biotransformation of HCB) for 10-13 weeks, both the excretion of PCP
and TCHQ (tetrachlorohydroquinone, the reduced analogue of the
reactive tetrachlorobenzoquinone) and the extent of hepatic porphyria
and urinary porphyrin excretion were greatly diminished (Van Ommen et
al., 1989; Den Besten et al., 1993). In 13-week feeding studies on
female Wistar rats exposed to 300 mg HCB/kg diet (approximately 15 mg
HCB/kg body weight per day) in the presence or absence of TAO, the
degree of porphyria was better correlated with excretion of PCP than
TCHQ, and in comparative studies pentachlorobenzene (which is
metabolized to PCP by a different mechanism than for HCB) was not
porphyrinogenic (Den Besten et al., 1993).
In addition, it has been suggested that the aryl hydrocarbon
receptor (Ah receptor) may be involved in the accumulation of hepatic
porphyrins in mice (Linko et al., 1986; Hahn et al., 1988, 1989). Ah-
responsive strains of inbred mice were more sensitive to hepatic
porphyrin accumulation after HCB exposure than non-responsive mice
(Smith & Francis, 1983; Hahn et al., 1988), and HCB has been shown to
be a weak agonist for the Ah receptor (Hahn et al., 1989).
Full discussion of the evidence for a unifying hypothesis of
porphyria induced by HCB and other chemicals that act in a similar
way, as well as for human sporadic porphyria cutanea tarda, is beyond
the scope of this document. There is however, substantial experimental
and human evidence implicating a complex interaction between
hepatocellular iron and oxidative processes leading to the oxidation
of unstable uroporphyrinogen to uroporphyrin, possibly mediated by
induced cytochrome P-450 isozymes (reviewed by Smith & De Matteis,
1990). There is evidence that inhibition of uroporphyrinogen
decarboxylase may occur through formation of an inhibitor of the
enzyme during the oxidation of uroporphyrinogen (Rios de Molina et
al., 1980; Smith & De Matteis, 1990). HCB may act partly through
induction and uncoupling of the cytochrome P-450 system to form
reactive oxygen species, especially in the presence of an increased
available iron pool (Smith & De Matteis, 1990; Den Besten et al.,
1993).
Subchronic exposure to low doses of HCB has also caused changes
in calcium homoeostasis and bone morphometry. Male Fischer-344 rats
administered HCB by gavage in corn oil had elevated serum levels of
1,25-dihydroxy-vitamin-D3 and reduced calcium excretion after 5
weeks, and increased femur density, weight and strength after 15
weeks. These effects were evident at 0.7 mg/kg body weight per day but
not at 0.07 mg/kg body weight per day (Andrews et al., 1989, 1990).
While technical HCB is known to be contaminated with chlorinated
dibenzo- p-dioxins, dibenzofurans and biphenyls (Villanueva et al.,
1974; Goldstein et al., 1978), the effects (primarily hepatic) of
subchronic dietary exposure of rats to either pure or technical HCB
were virtually identical, indicating that the effects observed in this
study were due to the parent compound (Goldstein et al., 1978).
In a number of studies on various strains of rats, short-term or
subchronic exposure to HCB affected the thyroid, as indicated by
decreased serum levels of total and free thyroxine (T4) and often, to
a lesser extent, triiodothyronine (T3). In some instances, these are
accompanied by compensatory increases in thyroid weight, circulating
levels of thyroid-stimulating hormone or iodine uptake by the thyroid
(Rozman et al., 1986; Kleiman de Pisarev et al., 1989, 1990; Van Raaij
et al., 1991a, 1993a, 1993b; Foster et al., 1993; Den Besten et al.,
1993; Sopena de Krakoff et al., 1994). Den Besten et al. (1993)
reported such effects in rats exposed to as little as 9.5 mg/kg body
weight per day following dietary exposures for 13 weeks, although
effect levels were somewhat higher in other studies, which involved
exposure for a shorter duration and/or employed an aqueous vehicle.
Somewhat different effects (decreased levels of T3 in serum and no
change in T4, accompanied by increased uptake of iodine by the
thyroid) were observed in hamsters exposed to 100-200 mg HCB/kg feed
(approximately 12-24 mg/kg body weight per day) for 18-28 weeks (Smith
et al., 1987).
The mechanisms that have been advanced to account for the effects
of HCB on the thyroid include accelerated metabolism of thyroid
hormones by HCB-induced enzymes or accelerated deiodination of
thyroxine, in conjunction with increased biliary excretion (Kleiman de
Pisarev, 1989; Van Raaij et al., 1993b), and interference with plasma
transport of thyroid hormones through displacement of T4 from binding
sites on proteins (Van Raaij et al., 1991a, 1993a). Van Raaij et al.
(1991b, 1993a) reported that intraperitoneal injection of
pentachlorophenol and tetrachlorohydroquinone, but not HCB itself,
decreased serum thyroxine levels in rats, indicating that these
metabolites may be involved in the effects of HCB on the thyroid.
These authors reported that PCP was a more effective competitor for
thyroxine-binding sites of serum carriers in vitro, and more
effective at occupying carrier sites in ex vivo experiments, than
HCB (van Raaij et al., 1991a), and demonstrated that T4 binding sites
were partially occupied in the serum of rats exposed to HCB (Van Raaij
et al., 1993a). In the latter study, it was estimated that competition
for thyroid hormone binding sites, by PCP metabolized from HCB, could
account for almost half of the observed reduction in serum levels of
T4.
7.3 Long-term toxicity and carcinogenicity
A range of non-neoplastic effects from long-term exposure to HCB,
which are primarily hepatotoxic, have been observed at relatively low
doses. In a two-generation study with Sprague-Dawley rats, liver and
heart weights were increased in Fo males exposed to TWA doses of 0.29
and 1.50 mg/kg body weight per day in the diet for 3 months, and
histopathological changes in the liver were observed in F1 animals of
both sexes exposed to maternal doses of 0.29-0.38 and 1.50-1.90 mg
HCB/kg body weight per day in diet in utero, through nursing, and
then continued on the same diet as their parents for their lifetimes.
The no-effect level in this study was 0.06-0.07 mg/kg body weight per
day (Arnold et al., 1985; Arnold & Krewski, 1988). Dietary exposures
of Sprague-Dawley rats to 10 mg/kg and above (approximately 0.5-0.6
mg/kg body weight per day) for 9-10 months induced in vivo mixed-
function oxidase activity, as indicated by reductions in drug-induced
sleeping times (Grant et al., 1974). Exposure of Sprague-Dawley rats
to 5 mg HCB/kg in diet (approximately 0.25-0.30 mg/kg body weight per
day) for 3-12 months caused proliferation of smooth endoplasmic
reticulum, altered mitochondria and increased numbers of storage
vesicles in liver, but these effects were not evident at 1 mg/kg in
diet (approximately 0.05-0.06 mg/kg body weight per day) (Mollenhauer
et al., 1975; 1976). In a study by Böger et al. (1979), oral
administration of 2, 8 or 32 mg HCB to female Wistar rats twice weekly
for 203 days (0.57, 2.3 or 9.1 mg HCB/kg body weight per day) resulted
in hepatocellular enlargement, proliferated smooth endoplasmic
reticulum, increased glycogen and porphyrin deposits, and enlarged
mitochondria, but these effects were not seen at a lower dose (0.5 mg
HCB/kg body weight twice weekly, or 0.14 mg HCB/kg body weight per
day). Bleavins et al. (1984a) reported that exposure of female mink to
a dietary concentration of 1 mg/kg (estimated to yield a dose of
0.16 mg/kg body weight per day) for 47 weeks significantly increased
serotonin concentrations in the hypothalamus of dams, and depressed
hypothalamic dopamine concentrations in kits exposed in utero and
through nursing.
As in subchronic studies, female rats were more sensitive than
males to porphyria induced by chronic exposure to HCB. Grant et al.
(1974) reported that in Sprague-Dawley rats fed diets containing HCB
for 9-10 months, reduced weight gain and porphyria were observed in
females, but not males, receiving 80 or 160 mg HCB/kg feed
(approximately 4 or 8 mg HCB/kg body weight per day). A dose-related
increase in relative liver weights and in the hepatic content of HCB
was noted in both sexes. Hepatic enzyme activities and cytochrome
P-450 activities were increased in males administered 40 mg HCB/kg
feed or more. Exposure to 10 mg HCB/kg feed (approximately 0.5-0.6 mg
HCB/kg body weight per day) induced in vivo mixed-function oxidase
activity, as indicated by reductions in sleeping time for
pentobarbital and zoxazolamine exposure.
The carcinogenicity of HCB has been assessed in several bioassays
in rats, mice and hamsters. The following discussion is limited
principally to the four studies in which adequate numbers of animals
of both sexes were exposed for a sufficient length of time to more
than one dose level.
Cabral et al. (1977) and Cabral & Shubik (1986) reported a
statistically significant increase of liver cell tumours (hepatomas)
in groups of 30-60 male and female Syrian golden hamsters fed 50, 100
or 200 mg HCB/kg (4, 8 or 16 mg/kg body weight per day) HCB in their
diets for life. The incidence of "haemangioendotheliomas" of the liver
was significantly increased in both sexes at 200 mg/kg and in males at
100 mg/kg, and of alveolar adenomas of the thyroid in males at
200 mg/kg. (The latter finding is interesting in the light of reports
of excesses of thyroid neoplasms, or of enlargement of the thyroid, in
human populations with elevated exposures to HCB (section 8.1.)) The
authors reported that three of the hepatic "haemangioendotheliomas"
(which are non-invasive by definition) metastasized. It seems likely,
therefore, that these tumours were malignant, though misclassified.
In another study, HCB was administered in the diet to groups of
30 or 50 outbred male and female Swiss mice at concentrations of 0,
50, 100 and 200 mg/kg (0, 6, 12 and 24 mg/kg body weight per day) for
120 weeks (Cabral et al., 1979; Cabral & Shubik, 1986). In females
exposed to 200 mg/kg, a statistically significant increase in the
incidence of "liver cell tumours (hepatomas)" was noted. "Hepatomas"
were also elevated, though not significantly, in males at this dose
and in both sexes at 100 mg/kg. The number of tumour-bearing animals,
the latent period, and the multiplicity and size of tumours increased
with dose.
Arnold et al. (1985) and Arnold & Krewski (1988) investigated the
potential carcinogenicity to rats of combined in utero, lactational
and oral exposure to analytical grade HCB. Groups of 40 or more
weanling male and female Sprague-Dawley rats were fed diets containing
0, 0.32, 1.6, 8 or 40 mg HCB/kg. (Based on data supplied by the
author, mean doses for males were 0, 0.01, 0.06, 0.29 and 1.50 mg/kg
body weight per day and for females 0, 0.01, 0.07, 0.38 and 1.90 mg/kg
body weight per day). After 3 months, the F0 rats were bred, and 50
F1 pups of each sex were randomly selected from each group. From
weaning, the F1 animals were continued on the same diet for their
lifetimes (up to 130 weeks). In exposed F1 females, increased
incidences of neoplastic liver nodules and adrenal phaeochromocytomas
were noted at the highest dose. A significantly increased incidence of
parathyroid adenomas was noted in males receiving 40 mg HCB/kg in
their diet.
In a study by Lambrecht et al. (1983a,b; Ertürk et al., 1986),
groups of 94 weanling Sprague-Dawley rats were fed diets containing 0,
75 or 150 mg/kg (4 and 8 mg/kg body weight per day for males and 5 and
9 mg/kg body weight per day for females, respectively) for up to 2
years. Statistically significant increases in the incidence of
hepatomas/haemangiomas and of renal cell adenomas were noted at both
doses in animals of both sexes surviving beyond 12 months. Incidences
of hepatocellular carcinomas and bile duct adenomas/carcinomas were
also elevated in females at both doses. In female rats, significant
increases in the incidences of adrenal cortical adenomas at 75 mg/kg
and phaeochromocytomas at both doses were reported. Lambrecht et al.
(1983b) reported a leukaemia involving the thymus, spleen, liver and
kidney in rats exposed to HCB in this study, but did not present any
quantitative data. The results of this study were only reported in
summary form, with few details of the study protocol and results. In
addition, HCB was incorporated into the diet as a powder in this
study, raising the possibility that some of the effects observed may
have been in part attributable to the inhalation of aerosolized HCB.
High incidences of liver tumours have also been reported in some
more limited studies in which single dietary concentrations (100 or
200 mg/kg) were administered to small groups (i.e., between 4 and 15)
of females of three strains of rats (Smith & Cabral, 1980; Smith et
al., 1985b); in one strain (Fischer-344), hepatocellular carcinomas
were observed (Smith et al., 1985b). HCB has not, however, been
carcinogenic in several other studies in various strains of mice
(Theiss et al., 1977; Shirai et al., 1978; Smith et al., 1989),
perhaps as a result of the low doses, short durations of exposure
and/or small group sizes employed. Results were also negative in a
second study by Arnold et al. (1985), in which groups of 50 male
Sprague-Dawley rats were fed diets containing 40 mg HCB/kg in
conjunction with various levels of vitamin A for 119 weeks, indicating
the probable higher sensitivity of the two-generation carcinogenesis
bioassay.
Ertürk et al. (1982, 1986; Lambrecht et al., 1982a,b) examined
the tumorigenic activity of subchronic exposure to HCB in both sexes
of Swiss mice, Syrian golden hamsters and Sprague-Dawley rats at
dietary levels of 0, 100 and 200 mg/kg (mice) and 0, 200 and 400 mg/kg
(hamsters and rats) for 90 days. At day 91, 25 of 50 animals in each
group were sacrificed for histological examination, with the remainder
being sacrificed at 6-week intervals (up to 341, 361 and 424 days for
mice, hamsters and rats, respectively). The results of these studies
were reported in summary form only, and much of the quantitative data
were not presented. The authors reported that, as the experiment
progressed, treated animals developed hepatomas, bile duct adenomas,
renal adenomas and carcinomas, and lymphosarcomas of the thymus,
spleen, and lymph nodes. However, the only tumour and species for
which they presented clear evidence of a treatment-related increase in
incidence was for lymphatic tumours in mice (Ertürk et al., 1982).
Lymphatic and renal neoplasms were observed as early as the end of the
90-day period. It is not clear from these reports which tumours each
species developed or the dietary levels associated with the observed
effects, as well as other experimental details.
Results from a number of studies have indicated that HCB is a
co-carcinogen or promoter of cancer. Concomitant exposure to 50 mg
HCB/kg in diet (approximately 6 mg HCB/kg body weight per day)
enhanced the induction of liver tumours by polychlorinated terphenyl
(at 250 mg/kg diet) in male ICR mice (Shirai et al., 1978). Exposure
to HCB (100-200 mg/kg in diet (approximately 5-10 mg HCB/kg body
weight per day) or 1 mmole/kg i.p. at 1 and 5 weeks) promoted the
development of hepatocellular carcinomas and/or hepatic gamma-
glutamyltranspeptidase-positive foci initiated by diethylnitrosamine
in various strains of rats (Pereira et al., 1982; Herren-Freund &
Pereira, 1986; Stewart et al., 1989).
In some recent studies, the possible mechanisms by which HCB
induces tumours in animals have been investigated.
Bouthillier et al. (1991) presented the results of studies of
Sprague-Dawley rats exposed to 100 mg HCB/kg by gavage for periods of
several weeks, which indicated that the observed increase in renal
tumours in male Sprague-Dawley rats following exposure to HCB
(Lambrecht et al., 1983b; Ertürk et al., 1986) is related to protein
droplet nephropathy. The mechanism by which structurally diverse
hydrocarbons induce hyaline droplet nephropathy in male rats has been
well documented and involves accumulation of alpha-2u-globulin,
resulting in necrosis, regeneration and, in some cases, tumours. This
response is sex- and species-specific, and hence is unlikely to be
relevant to humans. This mechanism does not, however, explain the
increased (but lower) incidence of renal tumours in females also
reported by Lambrecht et al. (1983b).
Carthew & Smith (1994) hypothesized that some HCB-induced hepatic
tumours in rats may be produced by a non-genotoxic mechanism. They
noted that hepatotoxicity of HCB in rodents gives rise to peliosis and
necrosis with haemosiderosis, indicating that vascular damage has
occurred, and confirmed the presence of such damage in the liver of
chronically HCB-exposed rats by the identification of widespread
fibrin deposits, using an antibody to rat fibrin. These deposits
occurred in association with abundant haemosiderosis in hepatocytes
and areas of widened hepatic sinusoids. On this basis, it was
suggested that the formation of hepatomas and haemangiomas with
elements of peliosis could be the result of compensatory hyperplastic
responses to hepatocellular necrosis and the simultaneous loss of
hepatocellular cords, perhaps potentiated by the accumulation of iron
in the liver.
Mechanistic studies that address the relevance to humans of the
remaining tumour types induced in rodents by HCB have not been
identified.
7.4 Mutagenicity and related end-points
HCB has not been found to be genotoxic in most studies conducted
to date. HCB did not cause either frameshift or base pair substitution
mutations in Salmonella typhimurium at doses of as much as 10
mg/plate with or without metabolic activation, with both rat and
hamster liver activation systems, pre-incubation and plate
incorporation methods, and technical and 99.9% pure HCB (Haworth et
al., 1983; Górski et al., 1986; Siekel et al., 1991). A weak positive
response in S. typhimurium strain TA98 at 50 and 100 µg/plate was
reported by Gopalaswamy & Aiyar (1986) and Gopalaswamy & Nair (1992).
However, the authors also reported mutagenic activity for lindane, in
contrast to the results of other studies (e.g., Haworth et al., 1983).
Doses of up to 1000 µg/plate of HCB did not induce tryptophan
reversion or DNA damage in Escherichia coli strains WP2 and WP2uvrA
with or without metabolic activation (Siekel et al., 1991).
There have been reports of mutagenic activity for HCB in
eukaryotic cells in vitro, although these studies have limitations.
Guerzoni et al. (1976) reported a positive finding for methionine
reversion in Saccharomyces cerevisiae strain 632/4 exposed to HCB,
but Brusick (1986) did not consider the observed increase to meet
current standards of a positive response. In addition, only a single
dose level was used in that study, and there was no exogenous
metabolic activation. Kuroda (1986) reported that in cultured Chinese
hamster lung cells (V79), HCB did not induce OUAr mutations, but did
induce 8AGr mutations. However, both the magnitude of the increase
(which was small, roughly 1/105 survivors at the two highest doses)
and uncertain dose-response indicate that this response is open to
question.
Oral administration of as much as 221 mg HCB/kg body weight per
day to male rats for 5 or 10 days failed to induce dominant lethal
effects in two different studies (Khera, 1974; Simon et al., 1979),
although Simon et al. (1979) did observe a slight reduction in male
reproductive performance (numbers of females inseminated and
impregnated). Rumsby et al. (1992) reported that liver neoplasms that
developed in iron-overloaded C57Bl/10ScSn mice exposed for 18 months
to 0.01% HCB in the diet were not associated with a high frequency of
mutations in the Ha-ras proto-oncogene at codon 61. Only two mutations
were observed at different sites, from 23 preneoplastic and neoplastic
lesions examined, indicating that activation of the Ha-ras gene is not
an important event in the hepatocarcinogenicity of HCB in this test
system.
HCB has not been found to be clastogenic in the few available
studies in which this end-point has been examined. The compound did
not increase the frequency of sister chromatid exchanges in the bone
marrow of male mice given as much as 400 mg/kg body weight (by an
unspecified route), although the lack of detail in reporting the test
protocol and results limits the interpretation of this study (Górski
et al., 1986). HCB did not induce chromosomal aberrations in vitro
in cultured Chinese hamster fibroblast cells at concentrations as high
as 12 mg/ml, with or without metabolic activation (Ishidate, 1988), or
in human peripheral blood lymphocytes exposed to up to 0.1 mmol/litre
(Siekel et al., 1991). Treatment of rats with 1000 mg HCB/kg diet for
15 days was hepatotoxic, but did not cause early diploidization in
hepatocytes as measured by flow cytometry (Rizzardini et al., 1990).
The results of less specific assays also indicate that HCB does
not interact strongly with DNA, although there are two reports that
the compound binds, at low levels, to DNA. After incubating
hepatocytes isolated from phenobarbital-treated rats with 14C-HCB
(5 µM) for 20 h, Stewart & Smith (1987) reported the maximum amount of
radioactivity associated with DNA was < 9.9 × 10-5% of the substrate
added, and was only marginally above that of hepatocytes held at 4°C;
the authors considered this to be significantly lower than expected
for hepatocarcinogens. Gopalaswamy & Nair (1992) also reported a low
order of binding of HCB to DNA from the livers of rats exposed to
25 mg HCB/kg. Short-term exposure (<1 day) of rats to oral doses of
700 or 1400 mg/kg body weight (Kitchin & Brown, 1989) or to as much at
300 mg/kg body weight i.p. (Górski et al., 1986) did not cause hepatic
DNA damage, as measured by alkaline elution.
7.5 Reproductive and developmental toxicity
Relatively low doses of HCB have been found to affect some
reproductive tissues in female monkeys. Oral exposure of cynomolgus
monkeys to 0.1 mg/kg body weight per day in gelatin capsules for 90
days caused stratification of the ovarian germinal epithelium
(Babineau et al., 1991; Jarrell et al., 1993a). Higher dosages (1.0
and 10.0 mg/kg body weight per day) were associated with cellular
degeneration of this surface epithelium. The low dosage was associated
with ultrastructural as well as light microscopic changes in surface
epithelium (Babineau et al., 1991; Sims et al., 1991).
In ovarian follicles the low dose was associated with an
increased number of lysosomal elements in germ cells (Singh et al.,
1990a). The basal lamina was thickened. Higher dosages were associated
with greater degenerative changes in their cells and granulosa cells
(Singh et al., 1991, 1990b).
These studies demonstrated changes in ovarian tissues with no
other evidence of toxicity. In particular, the induction of
superovulation with human menopausal gondotrophin (HMG) in these
animals was associated with a normal estradiol response, oocyte
recovery, oocyte maturation, in vitro fertilization and early embryo
development (Jarrell et al., 1993a). These studies confirm the
findings of Iatropoulous et al. (1976) in which the administration of
8 to 128 mg/kg body weight (by gavage in 1% methylcellulose) for 60
days induced severe follicular degeneration in primordial germ cells,
pseudostratification of the ovarian surface epithelium, hepatic
degeneration and severe systemic toxicity in Rhesus monkeys.
In subsequent studies of similarly treated animals, the higher
doses were associated with reduced luteal phase progesterone and
blunted estradiol responses to HMG (Foster et al., 1992a,b). Reduction
in adrenal steroidogenesis occurred in ovariectomized rats in response
to exposure to HCB at concentrations of 1, 10 and 100 mg/kg body
weight for 30 days (Foster et al., 1995).
In contrast, the results of studies on a variety of species have
indicated that repeated exposure to HCB can affect male reproduction,
but only at relatively high doses. Mice exposed to 250 mg HCB per kg
feed (approximately 30 mg HCB/kg body weight per day) for 21 days had
reduced serum testosterone levels; based on the results of in vitro
tests, it was suggested that this was due to increased metabolism by
hepatic microsomal enzymes induced by HCB (Elissalde & Clark, 1979).
Histological changes in the testes (retarded sexual maturation) were
noted in pigs fed a diet yielding a dose of 50 mg HCB/kg body weight
per day for 90 days (den Tonkelaar et al., 1978). The mating index for
male rats receiving five consecutive daily gavage doses of 221 mg
HCB/kg body weight in corn oil was decreased compared to those
receiving 0 or 70 mg/kg body weight However, the fertility index for
the mated female rats (sperm positive smears) was not affected (Simon
et al., 1979).
As discussed in the following paragraphs, placental and
lactational transfer of HCB, demonstrated in a number of species, can
adversely affect both the fetus and nursing offspring. The lactational
route appears to be more important than placental transfer. Adverse
effects on suckling infants are generally observed more frequently,
and at lower doses, than are embryotoxic or fetotoxic effects.
Grant et al. (1977) conducted a four-generation study on female
(20/dose level) and male (10/dose level) weanling Sprague-Dawley rats
fed diets containing 0, 10, 20, 40, 80, 160, 320 or 640 mg HCB/kg
feed. The two highest doses caused some deaths in the F0 dams before
first whelping, and reduced the fertility index. Dietary levels of 160
mg/kg or more reduced litter sizes, increased the number of
stillbirths, and adversely affected pup survival. Similar effects were
seen at 80 mg/kg after the first two generations, while 40 mg/kg was
hepatotoxic to the F1a and F3a pups. A dietary level of 20 mg/kg
(approximately 1-1.2 mg/kg body weight per day) was designated as the
no-observed-effect level.
Arnold et al. (1985) fed groups of male and female Sprague-Dawley
rats from weaning on diets containing up to 40 mg HCB/kg. The rats
were then bred at 3 months, and the F1 pups were continued on the
same diet for their lifetimes. HCB had no effect on fertility, but pup
survival was significantly reduced in the 40 mg/kg group (calculated
doses of 1.50 and 1.90 mg/kg body weight per day for males and
females, respectively).
In other studies, maternal doses in the range from 1.4 to 4 mg/kg
given to rats and cats have been found to be hepatotoxic and/or
affected the survival or growth of nursing offspring. In some cases,
these or higher doses reduced litter sizes and/or increased numbers of
stillbirths (Mendoza et al., 1977, 1978, 1979; Hansen et al., 1979;
Kitchin et al., 1982).
Mink are particularly sensitive to the effects of prenatal and
perinatal exposure to HCB; the offspring of mink fed diets containing
concentrations as low as 1 mg/kg (approximately 0.16 mg/kg body weight
per day) for 47 weeks (prior to mating and throughout gestation and
nursing) had reduced birth weights and increased mortality (Rush et
al., 1983; Bleavins et al., 1984b).
The available data on the developmental toxicity of HCB are
limited. CD-1 mice administered 100 mg/kg body weight by gavage on
days 7-16 of gestation had a significantly increased incidence of
abnormal fetuses per litter, and one case of renal agenesis was
reported. Some cleft palates were produced, but they all occurred in
one litter. This dose also increased maternal liver-to-body weight
ratios and decreased fetal body weights (Courtney et al., 1976). In a
series of studies reported by Andrews & Courtney (1986), combined
in utero and lactational exposure of CD-1 mice and CD rats (strain
unclear, probably Sprague-Dawley) to HCB (mouse dams received 10 or
50 mg/kg body weight per day, and rats 10 mg/kg body weight per day,
by gavage during gestation) resulted in increases in body weight and
kidney weights of pups of both species, along with enlarged kidneys
and a few cases of hydronephrosis. Increased liver weights were
observed in rat pups, and the occurrence of abnormal kidneys was
sporadic, with no dose-response relationship in studies with mice.
Khera (1974) reported a significant increase in the incidence of
unilateral or bilateral 14th rib in litters of Wistar rats receiving
doses of 80 and 120 mg HCB/kg body weight during gestation, but
maternal toxicity (loss of body weight and neurological effects) and
reduced fetal weights were noted in animals in these groups. (It
should be noted that, based on the biological half-lives reported for
HCB in mammals (section 6.2), the concentration of HCB in the dams in
these studies would not have reached the maximum that might occur as a
result of intake over a longer period).
Neurobehavioural development was affected in the offspring of
rats exposed to 2.5 or 25 mg/kg body weight per day by gavage 2 weeks
prior to breeding. Pups in both treated groups were hyperactive (based
on tests of negative geotaxic reflex, olfactory discrimination, and
exploratory locomotor activity) at 6-20 days of age. Pups from the
high treatment groups showed reduced acoustic startle response at 23
days of age, but a significantly increased response at 90 days. These
doses did not affect learning (swim T-maze) or motor activity in older
offspring, nor maternal or fetal body weights, length of gestation,
number of pups/litter at birth, or number of days to eye opening
(Goldey & Taylor, 1992).
Lilienthal et al. (1996) recently reported HCB-induced effects on
neurobehavioural development of rat pups exposed both maternally and
through the diet (dams were exposed to 0, 8 or 16 mg HCB/kg diet for
90 days prior to mating and throughout gestation and nursing, after
which the offspring were fed the same levels for 150 days). Exposure
to HCB did not affect the mean body weight of the pups (except males
at 150 days of age), or the number of pups/litter, but did increase
the mean body weight of dam, and their liver-to-body weight ratios.
Schedule-controlled behaviour was affected at 8 and 16 mg HCB/kg diet
(0.64 and 1.28 mg/kg body weight per day), as indicated by a dose-
related decrease in post-reinforcement pause at the end of the
experiment. Exploratory locomotor activity, open field behaviour at 21
days of age, and active avoidance learning at 90 days of age were
unaffected.
7.6 Immunotoxicity
The results of a number of studies have indicated that HCB
affects the immune system, with immunosuppressive effects in mice and
immunostimulatory effects in rats (summarized by Vos, 1986).
Balb/C mice exposed to 5 mg HCB/kg diet (approximately 0.6 mg/kg
body weight per day) for 3 to 18 weeks were more susceptible to
Leishmania infection (Loose, 1982) and had reductions in resistance
to a challenge with tumour cells and in the cytotoxic macrophage
activity of the spleen (Loose et al., 1981). Barnett et al. (1987)
reported that Balb/C mice exposed to maternal doses of 0.5 or 5 mg
HCB/kg body weight per day in utero and through nursing had severe
depression of the delayed-type hypersensitivity response to a contact
allergen (oxazolone). In a number of studies, exposure of mice to
diets containing 167 mg HCB/kg in diet (approximately 20 mg HCB/kg
body weight per day) for several weeks depressed humoral immunity,
cell-mediated immunity and host resistance (Vos, 1986; Carthew et al.,
1990).
In rats or rhesus monkeys with oral exposure to between 3 and
120 mg HCB/kg body weight per day for periods from 3 weeks to 6 months
in various studies, proliferative histopathological effects in the
thymus, spleen, lymph nodes, and/or lymphoid tissues of the lung have
been observed (Kimbrough & Linder, 1974; Iatropoulos et al., 1976;
Goldstein et al., 1978; Vos et al., 1979a,b; Kitchin et al., 1982).
Gralla et al. (1977) observed that long-term exposure to 1 mg HCB/day
(equivalent to a dose at the start of the experiment of roughly
0.12 mg/kg body weight per day) caused nodular hyperplasia of the
gastric lymphoid tissue in beagle dogs.
In rats, prominent changes following dietary exposure to HCB
include elevated IgM levels and an increase in the weights of the
spleen and lymph nodes. Histopathologically, the spleen shows
hyperplasia of B-lymphocytes in the marginal zone and follicles, while
lymph nodes show an increase in proportions of high endothelial
venules, indicative of activation. High endothelial-like venules are
induced in the lung, as are accumulations of macrophages. Functional
tests revealed an increase in cell-mediated immunity, as measured by
DTH reactions, a notable increase in primary and secondary antibody
response to tetanus toxoid, and decreased NK activity in the lung (Vos
et al., 1979a,b). Stimulation of humoral and cell-mediated immunity
occurred even at dietary levels as low as 4 mg HCB/kg (approximately
0.2 mg HCB/kg body weight per day); at such a dose conventional
parameters for hepatotoxicity were unaltered (Vos et al., 1983).
Therefore, the developing immune system of the rat seems to be
particularly vulnerable to the immunotoxic action of HCB.
More recent studies indicate that HCB may cause autoimmune-like
effects in the rat. Wistar rats treated with HCB had elevated levels
of IgM, but not IgG, against the autoantigens single-stranded DNA,
native DNA, rat IgG (representing rheumatoid factor), and bromelain-
treated mouse erythrocytes (that expose phosphatidylcholine as a major
autoantigen). It has been suggested that HCB activates a recently
described B cell subset committed to the production of these
antibodies (Schielen et al., 1993). The role of these autoantibodies
is still a matter of controversy. Increased levels have been
associated with various systemic autoimmune diseases, but a protective
role of these autoantibodies against development of autoimmune disease
has been postulated as well. Interesting in this respect are the
observations that HCB had quite opposite effects in two different
models of autoimmune disease in the Lewis rat. HCB treatment severely
potentiates allergic encephalitis elicited by immunization with myelin
in complete Freund's adjuvant, while it strongly inhibits the
development of arthritic lesions elicited by complete Freund's
adjuvant as such (Van Loveren et al., 1990).
A possible relation between the immunomodulatory properties of
HCB and HCB-induced skin lesions, attributed in the literature to the
porphyrinogenic action of HCB, was recently indicated. In rats treated
with a combination of HCB and triacetyloleandomycin (TAO, a selective
inhibitor of cytochrome P-450IIIa), porphyria was greatly reduced.
Remarkably, combined treatment with HCB and TAO did not substantially
affect the incidence and severity of skin lesions. In addition, TAO
did not influence the immunomodulatory effect of HCB, including the
formation of antibodies. From these findings it has been suggested
that an immunological component underlies, at least in part, the
HCB-induced skin lesions in the rat (Schielen et al., 1995).
8. EFFECTS ON HUMANS
8.1 General population exposure
Numerous reviews have been published of an accidental poisoning
incident in Turkey that occurred in 1955-1959 as a result of
HCB-treated wheat grain (distributed by the Turkish government for
planting purposes) being ground into flour and made into bread
(Schmid, 1960; Cam & Nigogosyan, 1963; Dogramaci, 1964; Peters, 1976;
Courtney, 1979; Peters et al., 1982; US EPA, 1985a; Gocmen et al.,
1989). In this incident, more than 600 cases of porphyria cutanea
tarda (PCT) were clinically identified, and it was estimated that as
many as 3000-5000 persons were affected, with a mortality of 10%. The
condition developed primarily in children 4-14 years of age (roughly
80% of cases), occurring infrequently in adults and rarely in children
under 4 years of age. In a number of reports, it has been suggested
that males developed the condition in higher proportion than females.
However, Dogramaci et al. (1962) demonstrated that the sex ratio was
skewed in favour of males in both the affected and unaffected
populations. In addition to disturbances in porphyrin metabolism
(excretion of porphyrins and porphyrin precursors was greatly
increased), clinical manifestations included skin lesions (erythema,
bullae), ulcerations and resultant scarring, friable skin,
hyperpigmentation, hypertrichosis, enlarged liver, weight loss,
enlargement of the thyroid gland and lymph nodes, neurological
effects, and a characteristic port wine colour of the urine (from
increased excretion of porphyrins). In roughly half the cases,
osteoporosis of extremities, deformation of the fingers or arthritis
was also noted. The dermatological lesions, which occurred on the
exposed parts of the body, particularly the face and hands, were often
precipitated by sunlight. They tended to remit in winter and relapse
during the spring and summer (Peters, 1976; Peters et al., 1982). The
estimated dose was 50-200 mg/day for a number of months before
manifestations of the disease became apparent (Cam & Nigogosyan,
1963); the basis for this estimate was not presented, however, making
exposure calculations unreliable for this population. In 20- to 30-
year follow-ups of exposed individuals, neurological, dermatological
and orthopaedic abnormalities persisted, and there were elevated
levels of porphyrins in excreta of some individuals (Peters et al.,
1982; Peters et al., 1986; Gocmen et al., 1989).
In this incident, a disorder called "pembe yara" or "pink sore"
was described in infants of mothers who either had PCT or had eaten
HCB-contaminated bread. These infants developed characteristic pink
cutaneous lesions, and often had fevers, diarrhea, vomiting, weakness,
convulsions, enlarged livers and progressive wasting. It is noteworthy
that PCT was not observed in these children (Cam, 1960; Peters et al.,
1982). At least 95% of these children died within a year of birth, and
in many villages no children between the ages of 2-5 years survived
during the period 1955-1960. Elevated concentrations of HCB (levels
were not quantified at the time, but the average concentration in milk
from 56 porphyric mothers, 20-30 years after the incident, was
510 ng/g on a fat basis) were found in the mothers' milk and cessation
of breast-feeding slowed the deterioration of infants with this
disorder (Peters et al., 1966; Gocmen et al., 1989).
No adequate epidemiological studies of cancer in populations
exposed to HCB in the environment were found in the literature. In
long-term follow-up of the Turkish poisoning victims with porphyria
(Peters et al., 1982; Cripps et al., 1984; Gocmen et al., 1989) there
was no evidence of increased cancer incidence, although these studies
were not designed to evaluate this end-point, and only a small
fraction of the exposed people was followed up. There was a high
frequency of enlarged thyroids in the Turkish poisoning victims (27%
of men and 60% of women, compared to an average of 5% in the area
(Peters et al., 1982)), but Gocmen et al. (1989) reported that they
observed no malignant tumours of the liver or thyroid in 252 of the
poisoning victims. In three patients who underwent thyroidectomy,
histopathological examination indicated that the enlargement was due
to colloidal goitre.
Grimalt et al. (1994) reported a small ecological study of cancer
incidence (129 cases in all) in the inhabitants of a village in Spain
located near a chlorinated solvents factory. There were statistically
significant excesses of thyroid neoplasms and soft-tissue sarcomas in
males, compared with the province as a whole, although these were
based on only 2 and 3 cases, respectively. The exposures experienced
by this population were somewhat unclear. Levels of HCB in ambient air
and in the sera of volunteers were much higher in the village than in
Barcelona (means of 35 ng/m3 versus 0.3 ng/m3 and 26 µg/litre versus
4.8 µg/litre, respectively), but the authors presented evidence that
historical exposures had been much higher and indicated that all of
the males with cancer for whom there were occupational histories had
worked in the factory. Ambient air monitoring revealed that there were
exposures to a variety of other compounds, including polychlorinated
biphenyls, p,p'DDE, chloroform, carbon tetrachloride,
trichloroethylene and tetrachloroethylene, but at similar or lower
levels than in the reference community.
8.2 Occupational exposure
There have been case reports of workers developing PCT as a
result of direct contact with HCB (Courtney, 1979; Currier et al.,
1980), although there was no association between exposure to HCB and
PCT in three cross-sectional studies of very small populations of
exposed workers (Morley et al., 1973; Burns et al., 1974; Currier et
al., 1980). There was no evidence of cutaneous porphyria in a cross-
sectional study of the general population in Louisiana, USA, exposed
to HCB through the improper transport and disposal of hex waste;
however, plasma concentrations of HCB were significantly correlated
with levels of coproporphyrin in urine and of lactic dehydrogenase in
blood (Burns & Miller, 1975).
Available epidemiological studies on the carcinogenicity of HCB
in occupationally exposed humans are restricted to one study of a
cohort of 2391 magnesium metal production workers in Norway. Although
the incidence of lung cancer was significantly elevated compared to
that of the general population, workers were exposed to numerous other
agents in addition to HCB, including coal tar, asbestos and dust of
metal oxides and chlorides (Heldaas et al., 1989). Selden et al.
(1989) reported a case of hepatocellular carcinoma in a 65-year-old
man who had been employed for 26 years in an aluminum smelting plant,
where he had potential exposure to a range of substances, including
HCB, other chlorobenzenes, chlorophenols, dioxins and furans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Data on the acute and chronic ecotoxicity of HCB are available
for species from a number of trophic levels, including protozoans,
algae, invertebrates and fish, for both the freshwater and marine
environments. With reference to terrestrial organisms, toxicity data
are available only for birds and mammals (the results of studies in
mammals are summarized in chapter 7). Since HCB is nearly insoluble in
water, and tends to partition from water to the atmosphere, the
substance is lost rapidly from open-test solutions. Hence, it is
difficult to maintain test concentrations for a sufficient time to
establish concentration-effects profiles for aquatic organisms.
Furthermore, HCB tends to bind to suspended solids in the water column
and thus may not be bioavailable to test organisms. This discussion of
the toxicity of HCB to aquatic organisms will therefore focus on tests
conducted under flow-through conditions, static renewal conditions, or
using closed vessels with minimal headspace. In addition, no
consideration has been given to tests in which concentrations of HCB
were well above its solubility in water (5 µg/litre at 25°C).
9.1 Short-term exposure
9.1.1 Aquatic biota
Of four freshwater algal species tested, only one, Chlorella
pyrenoidosa, was affected by concentrations of HCB in water at or
below its limit of aqueous solubility. Reduced production of
chlorophyll, dry matter, carbohydrate and nitrogen was observed for
C. pyrenoidosa after exposure to a nominal concentration of
1 µg/litre HCB for 46 h in a static-closed system (Geike & Parasher,
1976a). A no-observed-effect concentration (NOEC) was not determined
in this study.
At concentrations equal to its aqueous solubility in water
(5 µg/litre), HCB was not lethal to the freshwater water flea
Daphnia magna in a flow-through test in which concentrations of HCB
were measured (Nebecker et al., 1989). In 96-h flow-through tests on
marine invertebrates, exposure to HCB caused 13% mortality in pink
shrimp ( Penaeus duorarum) at a measured concentration of 7 µg
HCB/litre, and 10% mortality in grass shrimp ( Palaemonetes pugio) at
17 µg/litre. The NOEC values in these species were 2.3 µg/litre and
6.1 µg/litre, respectively (Parrish et al., 1974). In a static-closed
system, there was a 10% reduction in reproduction of the ciliate
protozoan Euplotes vannus after exposure to a nominal concentration
of 10 µg/litre HCB for 48 h (Persoone & Uyttersprot, 1975).
The available data on freshwater fish species indicated no
harmful effects at concentrations at or near the limit of solubility
of HCB in water during acute exposure (Call et al., 1983; Ahmad et
al., 1984). In the only available study for marine fish species, there
were no effects on mortality in sheepshead minnow ( Cyprinodon
variegatus) after flow-through exposure to a measured concentration
of 13 µg/litre HCB for 96 h (Parrish et al., 1974).
Limited data are available concerning the toxic effects of HCB in
sediment on freshwater and marine biota. In a 96-h sediment toxicity
test on the marine shrimp, Crangon septemspinosa, no mortality was
observed at the highest concentration of HCB tested, 300 µg/litre
(McLeese & Metcalfe, 1980).
Several studies have confirmed that there is a relatively
constant body residue associated with acute lethality in freshwater
fish, invertebrates and algae exposed to mono-to-pentachlorobenzenes
(McCarty et al., 1992a; Ikemoto et al., 1992). The acute LC50
critical body residue for chlorobenzenes is 2 µmol/g wet weight, or
569.6 µg/g wet weight for HCB, assuming that HCB has the same mode of
action as the other chlorobenzenes (McCarty et al., 1992b).
9.1.2 Terrestrial biota
The LD50 for HCB in herring gull ( Larus argentatus) embryos
injected on day 4 and tallied on day 25 was 4.3 µg/g body weight
(Boersma et al., 1986). At a dose of 1.5 µg/g body weight, there were
significant reductions in embryonic weight. Five-day LC50 values
(i.e., 5 days of HCB-containing diet followed by 3 days of untreated
diet) were 617 µg/g diet for 10-day-old ring-necked pheasants
( Phasianus colchicus) and > 5000 µg/g diet for 5-day-old mallards
( Anas platyrhynchos) (Hill et al., 1975). Induction of porphyria has
been observed in studies of Japanese quail following administration of
500 µg HCB/g body weight per day for between 5 and 10 days either in
food or via intraperitoneal injection (Buhler & Carpenter, 1986;
Lambrecht et al., 1988).
9.2 Long-term exposure
9.2.1 Aquatic biota
Growth of cultures of the alga Chlorella pyrenoidosa was
increased by exposure for 3 months to a nominal concentration of 1 µg
HCB/litre (Geike & Parasher, 1976b), while that of the protozoan
Tetrahymena pyriformis was decreased after a 10-day exposure to the
same concentration (Geike & Parasher, 1976b).
After exposure to 5 µg HCB/litre for 10 days in a static-renewal
system, crayfish ( Procambarus clarki) experienced damage to the
hepatopancreas (Laseter et al., 1976). The fertility of Daphnia
magna was reduced by 50% after exposure for 14 days to a measured
concentration of 16 µg/litre HCB in a static-closed system (Calamari
et al., 1983). Significantly increased mortality was observed in
amphipods, Gammarus lacustris, exposed to a measured concentration
of 3.3 µg HCB/litre for 28 days under flow-through conditions
(Nebecker et al., 1989). However, the results of this study indicated
a weak-dose response relationship. In two other flow-through studies,
there were no effects on survival, growth or reproduction of the
amphipod Hyallela azteca and the worm Lumbriculus variegatus at a
measured concentration of 4.7 µg HCB/litre (Nebecker et al., 1989).
In several studies, fathead minnows ( Pimephales promelas) and
rainbow trout ( Oncorhynchus mykiss) experienced no mortality or
effects on growth after exposure to levels of HCB approaching its
aqueous solubility (Ahmad et al., 1984; Carlson & Kosian, 1987; US
EPA, 1988; Nebecker et al., 1989). However, Laseter et al. (1976)
reported liver necrosis in large-mouth bass ( Micropterus salmoides)
after an exposure for 10 days to 3.5 µg HCB/litre under flow-through
conditions.
Guidelines for the protection and management of aquatic sediment
quality in Ontario, Canada (Persaud et al., 1991) have given a no-
observed-effect level (NOEL), a lowest-observed-effect level and a
severe-effect level for a variety of contaminants. The values given
for HCB are 10 ng/g dry weight, 20 ng/g dry weight and 24 000 ng/g
organic carbon. The partitioning approach was used to determine the
lowest-observed-effect level, whereas the severe-effect level was more
dependent on the screening level concentration approach. The
limitation of both approaches is that they are unable to separate the
biological effects that are due to a combination of contaminants; thus
while ecotoxicological effects can be established, these cannot be
attributed to any one chemical contaminant. This is a very serious
limitation since virtually all sediments are contaminated with a wide
variety of pollutants, and there is no indication that HCB was the
dominant pollutant.
Quantitative structure-activity relationships (QSAR) were used to
estimate the narcotic toxicity for 19 species to predict NOELs (Van
Leeuwen et al., 1992). The NOELs for water, sediment and residues in
biota were predicted only on the basis of the octanol/water partition
coefficient and relative molecular mass. The QSAR-derived level for
HCB in sediments was 5814 ng/g dry weight (20.4 nmol/g in the
reference) for sediments with 5% total organic carbon content. The
adjusted value for sediment with 1% total organic carbon content is
1163 ng/g. There is no experimental verification of these
calculations. Thus, no firm evidence is available on the critical
levels of HCB in sediments.
9.2.2 Terrestrial biota
In adult Japanese quail ( Coturnix japonica) fed diets
containing HCB for 90 days, mortality was increased at 100 µg HCB/g
in diet, and hatchability of eggs was significantly reduced at 20 µg/g
(Vos et al., 1971, 1972). At 5 µg/g, increased liver weight, slight
liver damage and increased faecal excretion of coproporphyrin were
observed. Eurasian kestrels ( Falco tinnunculus) fed mice containing
200 µg HCB/g fresh body weight for 65 days had significant weight
loss, ruffling of feathers, tremors, increased liver weight and
decreased heart weight (Vos et al., 1972).
The available long-term toxicity data for mammals are discussed
in section 7.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on estimates of mean exposure from various media (section
5.2), the general population is exposed to HCB principally in food
(mean intakes for adults range from 0.0004 to 0.0028 µg/kg body weight
per day). Intakes are estimated to be considerably less for ambient
air (3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day) and
drinking-water (2.2 × 10-6 to 4.4 × 10-5 µg/kg body weight per day).
Based on these intakes, it is estimated that the total average daily
intake of HCB from food, air and drinking-water is between 0.0004 and
0.003 µg/kg body weight per day.
Data on levels of occupational exposure to HCB are limited but
indicate that workers in some industries may be exposed to higher
levels of HCB than the general population, particularly in the
manufacture of chlorinated solvents, and in the manufacture and
application of chlorinated pesticides contaminated with HCB. In some
instances inappropriate manufacturing and waste management practices
may expose nearby populations to higher levels of HCB than the general
population. Exposures may also be elevated in some indigenous
subsistence populations, particularly those that consume large
quantities of food species near the top of the food chain.
Owing to the elimination of HCB in breast milk, mean intakes by
nursing infants are estimated to range from < 0.018 to 5.1 µg/kg body
weight per day in various countries (see section 5.2.4 and Table 8).
10.1.2 Health effects
Available data on the effects of HCB in humans are limited
principally to those of people exposed in an accidental poisoning
incident that occurred in Turkey between 1955 and 1959. More than 600
cases of porphyria cutanea tarda (PCT) were observed, and infants of
exposed mothers experienced cutaneous lesions, clinical symptoms and
high mortality. It has been estimated that victims were exposed to an
estimated dose of 50-200 mg HCB/day for an undetermined, but extended,
period of time. However, the basis of this estimate was not provided,
making exposure calculations unreliable for this population. Studies
of the carcinogenicity of HCB in humans are limited to two small
epidemiological studies of cancer incidence in populations with poorly
characterized exposure to HCB as well as to numerous other chemicals.
No excesses of neoplasms have been reported in long-term follow-up
studies of the people with porphyria in the incident in Turkey, but
only a small fraction of the population was followed-up, and these
studies were not designed specifically to assess neoplastic end-
points.
Hence, the available data on humans are inadequate to serve as a
basis for assessment of effects from exposure to HCB. The remainder of
this evaluation is, therefore, based on studies in animals.
Based on the studies reviewed in section 7, the critical effects
induced by HCB in experimental animals comprise both non-neoplastic
and neoplastic effects.
With respect to non-neoplastic effects, repeated exposure to HCB
has been found to cause a wide range of non-neoplastic effects in
several species of animals, with similar lowest-observed-effect-levels
(LOELs) and no-observed-effect-levels (NOELs) for a number of end-
points (see Table 9). In these studies, effects reported have included
those on the liver in pigs and rats, on calcium metabolism in rats, on
ovarian histopathology in monkeys, on immune function in mice and
rats, on neurotransmitter levels in the hypothalamus of mink, on
postnatal survival in mink, and on neurobehavioural development in
rats. The range over which the various effects have been observed is
quite narrow; the lowest LOELs compiled in Table 9 range from 0.1 to
0.7 mg/kg body weight per day, while the lowest NOELs range from 0.05
to 0.07 mg/kg body weight per day.
Based on the induction of a variety of tumours in hamsters, rats
and mice exposed by ingestion, there is sufficient evidence that HCB
is carcinogenic in animals. The available evidence indicates that HCB
has little or no genotoxic activity and is therefore unlikely to be a
direct-acting (genotoxic) carcinogen. However, the Task Group noted
that tumours, some of which were malignant, have been induced in
multiple species, at multiple sites, in some instances at doses that
were not overtly toxic in other respects and that are within an order
of magnitude of those that produce more subtle toxicological effects,
or following subchronic exposure. Although there is some evidence to
suggest that HCB may cause cancer by indirect mechanisms, the evidence
is not definitive at this time and does not address all tumour sites.
Table 9. No-observed-effect and lowest-observed-effect levels (NOELs and LOELs) in mammals exposed to HCB
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Mouse Depressed delayed-type hypersensitivity - 0.5a Barnett et al.
response to oxazolone in mice exposed (1987)
to HCB in peanut butter in utero
(throughout gestation) and via nursing
to 45 days of age (section 7.6)
Mouse Increased susceptibility to Leishmania - 0.6 Loose et al. (1981);
infection, and reductions in resistance Loose (1982)
to a challenge with tumour cells and in
the cytotoxic macrophage activity of the
spleen in mice with subchronic exposure
to HCB in diet (section 7.6)
Rat Alterations in Ca metabolism (increased 0.07 0.7 Andrews et al.
serum 1,25-dihydroxy-vitamin-D3 levels, (1989, 1990)
reduced Ca excretion, alterations in
femur density, bone morphometry and
strength), increased liver weights, with
subchronic gavage exposure to HCB
(section 7.2)
Rat Increased cell-mediated and humoral - 0.2a Vos et al. (1983)
immune function, intraalveolar
macrophage accumulation, microsomal
ethoxyresorufin-O-deethylase activity,
in rats exposed to HCB in utero, via
nursing and in the diet to 5 weeks of
age (section 7.6)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Rat Increased organ weights (heart, brain 0.05-0.07 0.27-0.35 Arnold et al. (1985);
and liver) in F0 males, compound- Arnold & Krewski (1988)
related histological changes in liver
of both sexes of F1 rats with long-term
exposure to HCB in diet (section 7.3)
Rat Ultrastructural changes in livers 0.05-0.06 0.25-0.30 Mollenhauer et al.
(proliferation of SER, altered (1975, 1976)
mitochrondria, increase in numbers of
storage vesicles) of rats with long-term
exposure to HCB in diet (section 7.3)
Rat Induction of in vivo mixed-function - 0.5-0.6 Grant et al. (1974)
oxidase activity in rats with long-term
exposure to HCB in diet (section 7.3)
Rat Dose-related decrease in the post- - 0.64 Lilienthal et al.
reinforcement pause (PRP) after schedule- (1996)
controlled operant conditioning of rats
exposed to HCB in utero, through nursing,
and up to post-natal day 150
Mink Increased serotonin concentrations in - 0.16a Rush et al. (1983);
hypothalamus of mink dams with long-term Bleavins et al. (1984a,b)
dietary exposure to HCB, decreased
dopamine levels in hypothalamus, reduced
birth weights, and increased mortality
to weaning in mink kits with in utero
plus lactational exposure to HCB
(sections 7.3, 7.5)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Dog Nodular hyperplasia of gastric lymphoid - 0.12 Gralla et al.
tissue in beagles with long-term (1977)
exposure to HCB in gelatin capsules
(section 7.6)
Pig Increased urinary coproporphyrin and 0.05 0.05 Den Tonkelaar et al.
microsomal liver enzyme activity in (1978)
pigs with subchronic exposure to HCB
in diet (section 7.2)
a Doses reported are those received by dams
10.1.3 Approaches to risk assessment
The following is provided as a potential basis for derivation of
guidance values. Since ingestion is by far the principal route of
exposure and since the toxicological data for other routes of
administration are insufficient for evaluation, only the oral route is
addressed here, though the ultimate objective should be reduction of
total exposure from all routes.
Based on the scientific evaluation of the data for the non-
neoplastic and neoplastic end-points, two possible approaches to
develop health-based guidance values were suggested.
10.1.3.1 Non-neoplastic effects
The approach for non-neoplastic effects assumes a threshold for
these effects and is based on the use of the NOAEL or NOEL and an
uncertainty factor that takes account of interspecies and
interindividual variation in sensitivity to the substance, as well as
the quality of the available studies and the severity of effect.
The available data are sufficient to develop a Tolerable Daily
Intake (TDI) for HCB. The lowest reported NOELs and LOELs for several
different types of effects, such as those on the liver in rats and
pigs, calcium metabolism in rats, ovarian morphology in monkeys,
immune function in rats and mice, neurobehavioural development in rats
and perinatal survival in mink, fall within a very small range (Table
9). Based on the lowest reported NOELs included in the table
(approximately 0.05 mg/kg body weight per day based primarily on
hepatic effects observed in a subchronic study in pigs and in chronic
studies in rats), a TDI of 0.17 µg/kg body weight per day has been
derived for non-neoplastic effects, by incorporating an uncertainty
factor of 300 (x 10 for intraspecies variation; × 10 for interspecies
variation, × 3 for severity of effect). A factor of 3 for severity of
effects was chosen as HCB causes i) multiple non-neoplastic effects in
several species, and ii) LOELs for a number of end-points for which
NOELs have not been determined are very close to the NOEL, from the
critical studies, of 0.05 mg/kg body weight per day. However, it is
fully realized that national authorities may choose other end-points
or uncertainty factors depending upon data evaluation and future
scientific findings.
10.1.3.2 Neoplastic effects
The approach for neoplastic effects is based on the Tumorigenic
Dose5, or TD5 i.e., the intake or exposure associated with a 5%
excess incidence of tumours in experimental studies in animals (IPCS,
1994). This is a benchmark approach in which the TD5 is calculated
directly from the experimental data rather than using the upper or
lower confidence limits. Uncertainty factors are then applied to the
TD5 to obtain a guidance value. The choice of uncertainty factors is
based on the level and nature of mechanistic data available, the
quality of the database, the tumour pattern, the dose-response
relationship, and the experimental model chosen. The final value will
reflect the degree of certainty one has with the available
information.
For the purpose of indicating the magnitude of risk of HCB, the
two-generation study in rats has been selected, owing to its relevance
to the exposure of the general human population, as the design of this
study involved exposure to relatively low concentrations of HCB in the
diet (including in utero and lactational exposure). Moreover, tumour
pathology was inadequately reported in the available studies in
hamsters and mice, and there is some concern that in the other
adequate study in rats, there may also have been exposure by
inhalation to some HCB that was incorporated in the diet as a powder.
The TD5 value was calculated from the results of the two-
generation study in rats using a multistage model (Crump & Howe,
1982). The tumour incidences in the pups were analysed in the same
manner as data from a single-generation study, owing to the lack of
information on individual litters. On this basis, the TD5 values
range from 0.81 mg/kg body weight per day for neoplastic liver nodules
in females to 2.01 mg/kg body weight per day for parathyroid adenomas
in males. The Task Group decided that the most sensitive end-point
(neoplastic nodules of the liver) would be used in its analysis. In
calculating the suggested guidance value, it was agreed to use an
uncertainty factor of 5000, based on consideration of the insufficient
mechanistic data. The TD5 was divided by this uncertainty factor to
arrive at the suggested guidance value of 0.16 µg/kg body weight per
day. However, it is fully realized that national authorities may
choose other end-points or uncertainty factors depending upon data
evaluation and future scientific findings.
Although infants may have a high intake of HCB via breast milk
for a short time, the TD5 and TDI were considered to be protective of
the health of this population (unless there are extreme exposures),
because one of the long-term studies used in deriving these values
included lactational exposure. However, it should be noted that the
TD5 and TDI values derived above should not be compared directly with
intakes from breast milk by nursing infants, since the guidance values
are based on a lifetime intake, whereas the duration of breast-feeding
is relatively short.
10.2 Evaluation of effects on the environment
HCB is widely distributed in the environment, by virtue of its
mobility and resistance to degradation, although slow photodegradation
in air (half-life of approximately 80 days) and microbial degradation
(half-life of several years) do occur. It has been detected in air,
water, sediment, soil and biota from around the world. HCB is a
bioaccumulative substance (BCF values range from 375 to > 35 000),
and biomagnification of HCB through the food chain has been reported.
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa. In longer-term studies, the growth of sensitive freshwater
algae and protozoa was affected by a concentration of 1 µg/litre,
while a concentration of approximately 3 µg/litre caused mortality in
amphipods and liver necrosis in largemouth bass. The concentrations of
HCB in surface waters around the world are much lower than these
effect levels (3 to 5 orders of magnitude lower), except in a few
extremely contaminated localities.
Injection studies in eggs have shown that tissue levels of
1500 ng/g wet weight reduce embryo weights in herring gulls (lowest
dose tested). No studies were available to establish a NOAEL. For many
bird species, reduced embryo weights are associated with lower
survival of chicks. This effect level is within an order of magnitude
of the levels measured in the eggs of sea birds and raptors from a
number of locations from around the world, suggesting that present
levels of HCB in certain locations may harm embryos of bird species.
Experimental studies on mink indicate that they are sensitive to
the toxic effects of HCB; long-term ingestion of diets containing
1000 ng HCB/g (the lowest dose tested) increased mortality, decreased
birth weights of offspring exposed in utero and via lactation, and
altered levels of neurotransmitters in the hypothalamus of dams and
their offspring. No studies were available to establish a NOAEL. This
dietary effect level is only a few times higher than the
concentrations of HCB measured in various species of fish from a
number of industrialized locations from around the world, suggesting
that present levels of HCB in fish species from certain locations may
adversely affect mink and perhaps other fish-eating mammals.
11. RECOMMENDATION FOR PROTECTION OF HUMAN HEALTH AND THE ENVIRONMENT
a) Alternatives should be found for any present uses of HCB.
b) It is important to reduce the environmental burden of HCB by:
(i) identifying remaining sources and quantities of release to
the environment from these sources, including point source
emissions, waste disposal sites and production facilities;
(ii) applying appropriate manufacturing and waste disposal
practices in order to decrease levels of HCB in the
environment.
c) Human monitoring of HCB in blood and breast milk should be
undertaken to develop data representing exposure of the general
population, in order to identify highly exposed populations and
potential sources, and to enable interpretation of individual
results.
d) In order to gauge the efficacy of control measures it would be
valuable to monitor environmental levels and effects in locations
where levels are higher than the global average.
e) Neonatal effects in humans and other species have been associated
with ingestion of high doses of HCB through breast milk. It is
recommended that techniques be developed to assess appropriately
the risk to infant health from exposure to HCB and related
compounds in breast milk.
12. FURTHER RESEARCH
12.1 Environment
a) To improve the database available for environmental risk
assessment, it is considered important to establish a NOEL for
the serious reproductive effects seen in mink at dietary levels
approaching those found in certain locations.
b) Since HCB is persistent in soil and sediment, it would be
valuable to perform biodiversity experiments with HCB-treated
soil and sediment.
12.2 Human health
a) Based on the effects of low doses of HCB on ovarian tissues in
primates, involving disorders of germ cells and the ovarian
surface epithelium, the following is recommended:
(i) exposed populations should be studied for relevant
reproductive human outcomes of interest, particularly, fetal
loss and ovarian cancer;
(ii) reproductive tissues such as ovarian follicular fluid should
be included in human monitoring studies on HCB levels and/or
effects.
b) In order to decrease uncertainty in the risk assessment of HCB
and related compounds, research into the primary mechanism(s) of
action for tumorigenic, thyroid, reproductive, porphyrigenic,
neurotoxic and immunological effects of HCB should be undertaken.
c) Preliminary evidence suggests that HCB acts, at least in part,
through Ah receptor-linked mechanisms. This should be evaluated
more fully and compared to other polyhalogenated aromatic
chemicals for which a wealth of data are already available.
d) Given the toxicity of HCB and the few data for humans,
multicentre longitudinal studies of highly exposed human
populations should be undertaken. End-points of interest should
cover toxicokinetics (e.g., half-life), thyroid function,
porphyrin metabolism, reproductive outcomes (e.g., fetal losses),
and cancer. Nursing infants from these populations should be
followed to assess immunological and neurobehavioural
development.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
HCB as a Group 2B carcinogen (possibly carcinogenic to humans) based
on inadequate evidence for carcinogenicity to humans and sufficient
evidence for carcinogenicity to animals (IARC, 1987).
A drinking-water guideline of 1 µg/litre was developed for HCB
based on an evaluation of the production of liver tumours in female
rats and applying the linearized multistage model to calculate an
excess life-time cancer risk of 10-5 (WHO, 1993).
A conditional acceptable daily intake of 0.6 µg HCB/kg body
weight was developed by the Joint FAO/WHO Joint Meeting on Pesticide
Residues in Food (FAO/WHO, 1975). This recommendation was withdrawn in
1978 (FAO/WHO, 1978).
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the Legal File of
the International Register of Potentially Toxic Chemicals (IRPTC,
1993).
REFERENCES
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in the United Kingdom 1969-71. Br Med J, 2:
533-556.
Abbott DC, Collins GB, Goulding R, & Hoodless RA (1981) Organochlorine
pesticide residues in human fat in the United Kingdom 1976-77. Br Med
J, 283: 1425-1428.
Abbott DC, Goulding R, Holmes DC, & Hoodless RA (1985) Organochlorine
pesticide residues in human fat in the United Kingdom 1982-1983. Hum
Toxicol, 4: 435-445.
Abraham K, Hille A, Ende M, & Helge H (1994) Intake and fecal
excretion of PCDDs, PCDFs, HCB and PCBs (138, 153, 180) in a breast-
fed and a formula-fed infant. Chemosphere, 29(9-11): 2279-2286.
Ahling B, Bjorseth A, & Lunde G (1978) Formation of chlorinated
hydrocarbons during combustion of poly(vinyl chloride). Chemosphere,
10: 799-806.
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, Defoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: A toxicity data summary, Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory (EPA 600/3-84-009).
Albert LA, Alpuche L, Barcenas C, & Rendón J (1990) A survey of
organochlorine pesticide residues in cheese samples from three Mexican
regions. Environ Pollut, 65: 119-126.
Albro PW & Thomas R(1974) Intestinal absorption of hexachlorobenzene
and hexachlorocyclohexane isomers in rats. Bull Environ Contam
Toxicol, 12: 289-294.
Alves HHD & Chevalier M (1980) L'hexachlorobenzène dans
l'environnement québécois : Production, utilisation et présence.
Montréal, Québec, Environnement Canada, Service de la Protection de
l'Environnement (Rapport No EPS-3 QR-80-1).
Andrews JE & Courtney KD (1986) Hexachlorobenzene-induced renal
maldevelopment in CD-1 mice and CD rats. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 381-391 (IARC
Scientific Publications No. 77).
Andrews JE, Courtney KD, Stead AG, & Donaldson WE (1989)
Hexachlorobenzene induced hyperparathyroidism and osteosclerosis in
rats. Fundam Appl Toxicol, 12: 242-251.
Andrews JE, Jackson LD, Stead AG, & Donaldson WE (1990) Morphometric
analysis of osteosclerotic bone resulting from hexachlorobenzene
exposure. J Toxicol Environ Health, 31: 193-201.
Angerer J, Heinzow B, Reimann DO, Knorz W, & Lehnert G (1992)
Internal exposure to organic substances in a municipal waste
incinerator. Int Arch Occup Environ Health, 64: 265-273.
Ariño AA, Herrera A, Conchello MP, & Pérez C (1992) Hexachlorobenzene
residues in Spanish meat products after cooking, curing, and long-term
ripening. J Food Prot, 55(11): 920-923.
Ariño A, Herrera A, Conchello P, & Lazaro R (1993) Hexachlorobenzene
and hexachlorocyclohexane residues in pork as affected by weight and
sex. Bull Environ Contam Toxicol, 51: 647-650.
Arnold DL & Krewski D (1988) Long-term toxicity of hexachlorobenzene.
Food Chem Toxicol, 26: 169-174.
Arnold DL, Moodie CA, Charbonneau SM, Grice HC, McGuire PF, Bryce FR,
Collins BT, Zwadizka ZZ, Krewski DR, Nera EA, & Munro IC (1985) Long-
term toxicity of hexachlorobenzene in the rat and the effect of
dietary vitamin A. Food Chem Toxicol, 23: 779-793.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for hexachlorobenzene. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-90-17).
ATSDR (1994) Toxicological profile for hexachlorobenzene - Update.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry.
Atuma SS, Andersson O, Linder C-E, & Hansson L (1993) Levels of some
organochlorine compounds in sea trout ( Salmo trutta) and whitefish
( Caregonus lavaretus) from the Gulf of Bothnia. Aqua Fenn, 23:
221-226.
Avrahami M (1975) Hexachlorobenzene: IV. Accumulation and elimination
of HCB by pigs after oral dosing. NZ J Exp Agric, 3: 285-287.
Avrahami M & Steele RT (1972) Hexachlorobenzene: I. Accumulation and
elimination of HCB in sheep after oral dosing. NZ J Agric Res, 15:
476-481.
Ayotte P, Dewailly E, Bruneau S, Careau H, & Vézina A (1995) Arctic
air pollution and human health: what effects should be expected? Sci
Total Environ, 160/161: 529-537.
Babineau KA, Singh A, Jarrell JF, & Villeneuve DC (1991) Surface
epithelium of the ovary following oral administration of
hexachlorobenzene to the monkey. J Submicrosc Cytol Pathol, 23:
457-464.
Bailey J, Knauf V, Mueller W, & Hobson W (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
rhesus monkeys; enhanced toxicity. Environ Res, 21: 190-196.
Ballschmiter K, & Wittlinger R (1991) Interhemisphere exchange of
hexachlorocyclohexanes, hexachlorobenzene, polychlorobiphenyls, and
1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane in the lower
troposphere. Environ Sci Technol, 25: 1103-1111.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LSF
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39:
263-274.
Barquet A, Morgade C, & Pfaffenberger CD (1981) Determination of
organochlorine pesticides and metabolites in drinking water, human
blood serum, and adipose tissue. J Toxicol Environ Health, 7:
469-479.
Bates MN, Hannah DJ, Buckland SJ, Taucher JA, & Van Maanen T (1994)
Chlorinated organic contaminants in breast milk of New Zealand women.
Environ Health Perspect, 102 (Suppl 1): 211-217.
Bauer I, Weigelt S, & Ernst W (1989) Biotransformation of
hexachlorobenzene in the blue mussel ( Mytilus edulis). Chemosphere,
19 (10/11): 1701-1707.
Beck J & Hansen KE (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in soil.
Pestic Sci, 5: 41-48.
Béland P, Deguise S, & Plante R (1991) Toxicology and pathology of
St. Lawrence marine mammals - Final report. Rimouski, Québec, Canada,
Wildlife Toxicology Fund Research Grant, St. Lawrence National
Institute of Ecotoxicology, 95 pp.
Belfroid A, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, & Hermens
J (1993) The toxicokinetic behaviour of chlorobenzenes in earthworms
( Eisenia andrei): Experiments in water. Ecotoxicol Environ Saf,
25: 154-165.
Belfroid A, Meiling J, Sijm D, Hermens J, Seinen W, & Van Gestel K
(1994a) Uptake of hydrophobic halogenated aromatic hydrocarbons from
food by earthworms ( Eisenia andrei). Arch Environ Contam Toxicol,
27: 260-265.
Belfroid A, Sikkenk M, Seinen W, Van Gestel K, & Hermens J (1994b) The
toxicokinetic behavior of chlorobenzenes in earthworm ( Eisenia
andrei): Experiments in soil. Environ Toxicol Chem, 13: 93-99.
Beretta M & Dick T (1994) Organochlorine compounds in human milk,
Porto Alegre, Brazil. Bull Environ Contam Toxicol, 53: 357-360.
Biddleman TF, Billings WN, & Foreman WT (1986) Vapor-particle
partitioning of semivolatile organic compounds: Estimates from field
collection. Environ Sci Technol, 20: 1038-1043.
Bidleman TF, Wideqvist U, Jansson B, & Soderlund R (1987)
Organochlorine pesticides and polychlorinated biphenyls in the
atmosphere of southern Sweden. Atmos Environ, 21: 641-654.
Bidleman TF, Walla MD, Roura R, Carr E, & Schmidt S (1993)
Organochlorine pesticides in the atmosphere of the Southern Ocean and
Antarctica, January-March, 1990. Mar Pollut Bull, 26(5): 258-262.
Billings WN & Bidleman TF (1980) Field comparison of polyurethane foam
and Teax-GC resin for high-volume air sampling of chlorinated
hydrocarbons. Environ Sci Technol, 14(6):679-683.
Bishop CA, Lean DRS, Brooks RJ, Carey JH, & Ng P (1995) Chlorinated
hydrocarbons in early life stages of the common snapping turtle
( Chelydra serpentina serpentina) from a coastal wetland on Lake
Ontario, Canada. Environ Toxicol Chem, 14(3): 421-426.
Bjerk JE & Brevik EM (1980) Organochlorine compounds in aquatic
environments. Arch Environ Contam Toxicol, 9: 743-750.
Bleavins MR, Breslin WJ, Aulerich RJ, & Ringer RK (1982) Excretion and
placental and mammary transfer of hexachlorobenzene in the European
ferret ( Mustela putorius furo). J Toxicol Environ Health, 14:
929-940.
Bleavins MR, Bursian SJ, Brewster JS, & Aulerich RJ (1984a) Effects of
dietary hexachlorobenzene exposure on regional brain biogenic amine
concentrations in mink and European ferrets. J Toxicol Environ Health,
14: 363-377.
Bleavins MR, Aulerich RJ, & Ringer RK (1984b) Effects of chronic
dietary hexachlorobenzene exposure on the reproductive performance and
survivability of mink and European ferrets. Arch Environ Contam
Toxicol, 13: 357-365.
Boersma DC, Ellenton JL, & Yagminas A (1986) Investigation of the
hepatic mixed-function oxidase system in herring gull embryos in
relation to environmental contaminants. Environ Toxicol Chem, 5:
309-318.
Boese BL, Winsor M, Lee H II, Specht DT, & Rukavina LC (1990)
Depuration kinetics of hexachlorobenzene in the clam, Macoma nasuta.
Comp Biochem Physiol, C996: 327-331.
Bordet F, Mallet J, Maurice L, Borrel S, & Venant A (1993)
Organochlorine pesticide and PCB congener content of French human
milk. Bull Environ Contam Toxicol, 50: 425-432.
Bourbonniere RA, Van Sickle BL, & Mayer T (1986) The Great Lakes
sediment bank-I (including catalogs of lakes Huron and Ontario
samples). Burlington, Ontario, Environment Canada, National Water
Research Institute (NWRI Contribution No. 86-151).
Bouthillier L, Greselin E, Brodeur J, Viau C, & Charbonneau M (1991)
Male rat specific nephrotoxicity resulting from subchronic
administration of hexachlorobenzene. Toxicol Appl Pharmacol, 110:
315-326.
Braune BM & Norstrom RJ (1989) Dynamics of organochlorine compounds in
herring gulls: III. Tissue distribution and bioaccumulation in Lake
Ontario gulls. Environ Toxicol Chem, 8: 957-968.
Breslin WJ, Bleavins MR, & Ringer RK (1983) Distribution and excretion
of hexachlorobenzene in bobwhite. J Toxicol Environ Health, 11:
885-896.
Brooks GW & Hunt GE (1984) Source assessment for hexachlorobenzene.
Research Triangle Park, North Carolina, Radian Corporation (Prepared
for the US Environmental Protection Agency, Research Triangle Park)
(EPA 68-02-3818).
Bro-Rasmussen F (1986) Hexachlorobenzene: An ecotoxicological profile
of an organochlorine compound. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 231-239 (IARC
Scientific Publications No. 77).
Brown EA, Biddle K, & Spaulding JE (1986) Residue levels of
hexachlorobenzene in meat and poultry in the food supply of the U.S.A.
In: Morris CR & Cabral JRPed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 99-108 (IARC Scientific Publications No. 77).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17: 2363-1380.
Brunn H & Manz D (1982) Contamination of native fish stock by
hexachlorobenzene and polychlorinated biphenyl residues. Bull Environ
Contam Toxicol, 28: 599-604.
Brusick DJ (1986) Genotoxicity of hexachlorobenzene and other
chlorinated benzenes. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 393-397 (IARC
Scientific Publications No. 77).
BUA (German Chemical Society-Advisory Committee on Existing Chemicals
of Environmental Relevance) (1994) Hexachlorobenzene. Stuttgart, S.
Hirzel Wissenschaftliche Verlagsgesellschaft, 257 pp (BUA Report 119).
Buhler DR & Carpenter HM (1986) Japanese quail as a model for the
study of hexachlorobenzene-induced porphyria. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 477-480 (IARC
Scientific Publications No. 77).
Burns JE & Miller FM (1975) Hexachlorobenzene contamination: its
effects in a Louisiana population. Arch Environ Health, 30: 44-48.
Burns JE, Miller FM, Gomes ED, & Albert RA (1974) Hexachlorobenzene
exposure from contaminated DCPA in vegetable spraymen. Arch Environ
Health, 29: 192-194.
Burton MA & Bennett BG (1987) Exposure of man to environmental
hexachlorobenzene (HCB): an exposure commitment assessment. Sci Total
Environ, 66: 137-146.
Bush B, Snow J, Connor S, & Koblintz R (1985) Polychlorinated biphenyl
congeners (PCBs), p,pœ-DDe and hexachlorobenzene in human milk in
three areas of upstate New York. Arch Environ Contam Toxicol, 14:
443-450.
Cabral JRP & Shubik P (1986) Carcinogenic activity of
hexachlorobenzene in mice and hamsters. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 411-416 (IARC
Scientific Publications No. 77).
Cabral JRP, Shubik P, Mollner T, & Raitano F (1977) Carcinogenic
activity of hexachlorobenzene in hamsters. Nature (Lond), 269:
510-511.
Cabral JRP, Mollner T, Raitano F, & Shubik P (1979) Carcinogenesis of
hexachlorobenzene in mice. Int J Cancer, 23: 47-51.
Calamari D, Galassi S, Setti F, & Vighi M (1983) Toxicity of selected
chlorobenzenes to aquatic organisms. Chemosphere, 12: 253-262.
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA priority pollutants and related chemicals
in freshwater organisms. Center for Lake Superior Environmental
Studies, University of Wisconsin-Superior, Environmental Research
Laboratory, Duluth, Minnesota, U.S. Environmental Protection Agency
(EPA 600/3-83-095).
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed R, Jennings P,
Durfee R, Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979)
Water-related environmental fate of 129 priority pollutants, Volume
II. Washington, DC, US Environmental Protection Agency, Office of
Water Planning and Standards/Office of Water and Waste Management (EPA
440/4-79-029b).
Cam C (1960) Une nouvelle dermatose épidémique des enfants. Ann
Dermatol, 87: 393-397.
Cam C & Nigogosyan G (1963) Acquired toxic porphyria cutanea tarda due
to hexachlorobenzene. J Am Med Assoc, 183: 90-93.
Camps M, Planas J, Gómez-Catalán J, Sabroso M, To-Figueras J, &
Corbella J (1989) Organochlorine residues in human adipose tissue in
Spain: study of an agrarian area. Bull Environ Contam Toxicol, 42:
195-201.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1979) Pesticide
residue levels in soils and crops from 37 states, 1972 - National
Soils Monitoring Program (IV). Pest. Monit. J, 12: 209-229.
Carey AE, Dixon TE, & Yang HSC (1985) Environmental exposure to
hexachlorobenzene in the United States. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
Carlson AR & Kosian PA (1987) Toxicity of chlorinated benzenes to
fathead minnows ( Pimephales promelas). Arch Environ Contam
Toxicol, 16: 129-135.
Carthew P & Smith AG (1994) Pathological mechanisms of hepatic tumour
formation in rats exposed chronically to dietary hexachlorobenzene. J
Appl Toxicol, 14: 447-452.
Carthew P, Edwards RE, & Smith AG (1990) Immunotoxic effects of
hexachlorobenzene on the pathogenesis of systemic, pneumonic and
hepatic virus infections in the mouse. Hum Exp Toxicol, 8: 403-411.
Clark TP, Norstrom RJ, Fox GA, & Won HT (1987) Dynamics of
organochlorine compounds in herring gulls ( Larus argentatus): II. A
two-compartment model and data for ten compounds. Environ Toxicol
Chem, 6: 547-559.
Conchello P, Herrera A, Ariño A, Lazaro R, & Pérez-Arquillué C (1993)
Effect of grilling, roasting, and cooking on the natural
hexachlorobenzene content of ovine meat. Bull Environ Contam Toxicol,
50: 828-833.
Conde C, Naluenda C, & Arrabal C (1993) Hexachlorobenzene (HCB) in
human milk in Spain from 1984 to 1991. Bull Environ Contam Toxicol,
51: 827-831.
Courtney KD (1979) Hexachlorobenzene (HCB): a review. Environ Res,
20: 225-266.
Courtney KD & Andrews JE (1979) Mobilization of hexachlorobenzene
(HCB) during gestation. Toxicol Lett, 3: 357-361.
Courtney KD & Andrews JE (1985) Neonatal and maternal body burdens of
hexachlorobenzene (HCB) in mice: gestational exposure and lactational
transfer. Fundam Appl Toxicol, 5: 265-277.
Courtney KD, Copeland MF, & Robbins A (1976) The effects of
pentachloronitrobenzene, hexachlorobenzene, and related compounds on
fetal development. Toxicol Appl Pharmacol, 35: 239-256.
Courtney KD, Andrews JE, & Svendsgaard DJ (1979) Hexachlorobenzene
(HCB) deposition in maternal and fetal tissues of rat and mouse: I.
Chemical quantification of HCB in tissues. Environ Res, 19: 1-13.
Cripps DJ, Peters HA, Gocmen A, & Dogramaci I (1984) Porphyria turcica
due to hexachlorobenzene: a 20 to 30 year follow-up study on 204
patients. Br J Dermatol, 111: 413-422.
Cross JN, Hardy JT, Hose JE, Hershelman GP, Antrim LD, Gossett RW, &
Crecelius EA (1987) Contaminant concentrations and toxicity of sea-
surface microlayer near Los Angeles, California. Mar Environ Res,
23: 307-323.
Crump K & Howe N (1982) Global '82: a computer program to extrapolate
quantal animal toxicity data to low doses (Contract #41USC252C3).
Washington, DC, US Occupational Safety and Health Administration,
Office of Carcinogen Standards.
Currier MF, McClimans CD, & Barna-Lloyd G (1980) Hexachlorobenzene
blood levels and the health status of men employed in the manufacture
of chlorinated solvents. J Toxicol Environ Health, 6: 367-377.
D'Amour M & Charbonneau M (1992) Sex-related differences in hepatic
glutathione conjugation of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 112: 229-234.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17: 263-276.
Davis BD & Morgan RC (1986) Hexachlorobenzene in hazardous waste
sites. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 23-30 (IARC Scientific Publications No. 77).
Debets FMH & Strik JJTWA (1979) An approach to elucidate the mechanism
of hexachlorobenzene-induced hepatic porphyria, as a model for the
hepatotoxicity of polyhalogenated aromatic compounds (PHA's). In:
Strik JJTWA & Koeman JH ed. Chemical porphyria in man. Amsterdam,
Oxford, New York, Elsevier/North Holland Biomedical Press, pp 181-208.
Deleon IR, Maberry MA, Overton EB, Raschke CK, Remele PC, Steele, CF,
Warren VL, & Laseter JL (1980) Rapid gas chromatographic method for
the determination of volatile and semivolatile organochlorine
compounds in soil and chemical waste disposal site samples. J
Chromatogr Sci, 18: 85-88.
Dellinger B, Taylor PH, & Tirey DA (1991) Minimization and control of
hazardous combustion by-products. Cincinnati, Ohio, US Environmental
Protection Agency, Risk Reduction Laboratory (EPA 600/S2-90/039).
De Matteis F, Prior BE, & Rimington C (1961) Nervous and biochemical
disturbances following hexachlorobenzene intoxication. Nature (Lond),
191(4786): 363-366.
Den Besten C, Bennik MM, Van Iersel M, Peters MA, Teunis C, & Van
Bladeren PJ (1994) Comparison of the urinary metabolite profiles of
hexachlorobenzene and pentachlorobenzene in the rat. Chem-Biol
Interact, 90: 121-137.
Den Desten C, Bennik MHJ, Bruggeman I, Schielen P, Kuper F, Brouwer A,
Koeman JH, Vos JG, & Van Bladeren PJ (1993) The role of oxidative
metabolism in hexachlorobenzene-induced porphyria and thyroid hormone
homeostasis: a comparison with pentachlorobenzene in a 13-week feeding
study. Toxicol Appl Pharmacol, 119: 181-194.
Denomme MA, Leece B, Gyorkos J, Homonko K, & Safe S (1983)
Polychlorinated benzene and phenol congeners as inducers of rat
hepatic drug-metabolizing enzymes in immature male Wistar rats. Can J
Physiol Pharmacol, 61: 1063-1070.
Den Tonkelaar EM & Van Esch GJ (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
Den Tonkelaar EM, Verschueren HG, Bankovska J, De Vries T, Kroes R, &
Van Esch G (1978) Hexachlorobenzene toxicity in pigs. Toxicol Appl
Pharmacol, 43: 137-145.
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Dewailly E, Nantel A, Laliberté C, Weber J-P, Gingras S, & Meyer F
(1991) Organochlorines in the breast milk in Québec: a provincial
survey. Poster presentation at the Conference on Measuring,
Understanding, and Predicting Exposures in the 21st Century, Atlanta,
Georgia, 18-22 November 1991.
Ding W-H, Aldous KM, Briggs RG, Valente H, Hilker DR, Connor S, &
Eadon GA (1992) Application of multivariate statistical analysis to
evaluate local sources of chlorobenzene congeners in soil samples.
Chemosphere, 25(5): 675-690.
Dogramaci I (1964) Porphyrias and porphyrin metabolism with special
reference to porphyria in childhood. Adv Pediatr, 13: 11-63.
Dogramaci I, Wray JD, Ergene T, Sezer V, & Müftü Y (1962) Porphyria
turcica: a survey of 592 cases of cutaneous porphyria seen in
southeastern Turkey. Turk J Pediatr, 4: 138-148.
Eisenberg M & Topping JJ (1984) Organochlorine residues in shellfish
from Maryland waters. J Environ Sci Health, B19: 673-688.
Eisenreich SJ & Strachan WMJ (1992) Estimating atmospheric deposition
of toxic substances to the Great Lakes - An update: Proceedings of a
workshop, Burlington, 31 January-2 February 1992. Burlington, Ontario,
Canada Centre for Inland Waters.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCBs in
waste, sediment, and fish from the Mediterranean Sea. Bull Environ
Contam Toxicol, 34: 216-227.
Elissalde MH & Clark DE (1979) Testosterone metabolism by
hexachlorobenzene-induced hepatic microsomal enzymes. Am J Vet Res,
40: 1762-1766.
Elkin BT & Bethke RW (1995) Environmental contaminants in caribou in
the Northwest Territories, Canada. Sci Total Environ, 160/161:
307-321.
Enriquez De Salamanca R, Lopez-Miras A, Munoz JJ, To-Figueras J, &
Conde C (1990) Is hexachlorobenzene human overload related to
porphyria cutanea tarda? A speculative hypothesis. Med Hypotheses,
33: 69-71.
Environment Canada (1989) Atlantic region federal-provincial toxic
chemical survey of municipal drinking water sources 1985-1988 -
Interpretative Report. Moncton, New Brunswick, Environment Canada,
Conservation and Protection, Water Quality Branch, Inland Waters
Directorate.
Environment Canada (1992) Detroit incinerator monitoring program: Data
report No. 6. Ottawa, Ontario, Environment Canada, Conservation and
Protection, River Road Environmental Technology Centre (Report No.
PMD-92-1).
Environment Canada/Department of Fisheries & Oceans/Health & Welfare
Canada (1991) Toxic chemicals in the Great Lakes and associated
effects - Volume 1: Contaminant levels and trends. Ottawa, Ontario,
Minister of Supply and Services.
Environment Canada/Ontario Ministry of the Environment (1986) St.
Clair River pollution investigation (Sarnia area). Toronto, Canada-
Ontario Agreement Respecting Great Lakes Water Quality.
Ernst W (1986) Hexachlorobenzene in the marine environment:
distribution, fate and ecotoxicological aspects. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 211-222 (IARC Scientific Publications No. 77).
Erturk E, Lambrecht RW, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982) Leukemogenicity of hexachlorobenzene (HCB) in Swiss
mice (SM) after subchronic feeding. Proc Am Assoc Cancer Res, 23: 55
(Abstract).
Ertürk E, Lambrecht RW, Peters HA, Cripps DJ, Gocmen A, Morris CR, &
Bryan GT (1986) Oncogenicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 417-423 (IARC Scientific Publications No. 77).
Falandysz J & Szefer P (1982) Chlorinated hydrocarbons in diving ducks
wintering in Gdansk Bay, Baltic Sea. Sci Total Environ, 24: 119-127.
Falandysz J, Kannan K, Tanabe S, & Tatukawa R (1993) Persistent
organochlorine residues in canned cod-livers of the southern Baltic
origin. Bull Environ Contam Toxicol, 50: 929-934.
FAO/WHO (1975) Report of the Joint Meeting of FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 37 pp (WHO
Technical Report Series 574).
FAO/WHO (1978) Pesticide residues in food - 1978. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and
Environment and the WHO Expert Group on Pesticide Residues. Rome, Food
and Agriculture Organization of the United Nations, pp 19-20 (FAO
Plant Production and Protection Paper 15).
Ferrer A, Bona MA, Castellano M, To-Figueras J, & Brunet M (1992)
Organochlorine residues in human adipose tissue of the population of
Zaragoza (Spain). Bull Environ Contam Toxicol, 48: 561-566.
Fingler S, Drevenkar V, Tkalœeviœ B, & œmit Z (1992) Levels of
polychlorinated biphenyls, organochlorine pesticides, and
chlorophenols in the Kupa River water and in drinking waters from
different areas in Croatia. Bull Environ Contam Toxicol, 49:
805-812.
Foley RE & Batcheller GR (1988) Organochlorine contaminants in common
goldeneye wintering on the Niagara River. J Wildl Manage, 52:
441-445.
Foster WG, McMahon A, Jarrell JF, & Villeneuve DC (1992a)
Hexachlorobenzene (HCB) suppresses circulating progesterone
concentrations during the luteal phase in the cynomolgus monkey. J
Appl Toxicol, 12: 13-17.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1992b) Ovarian
toxicity of hexachlorobenzene (HCB) in the superovulated female rat. J
Biochem Toxicol, 7: 1-4.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1993) Body
distribution and endocrine toxicity of hexachlorobenzene (HCB) in the
female rat. J Appl Toxicol, 13: 79-83.
Foster WG, Mertineit C, Yagminas A, McMahon A, & Lecavalier P (1995)
The effects of hexachlorobenzene on circulating levels of adrenal
steroids in the ovariectomized rat. J Biochem Toxicol, 10(3):
129-135.
Fox ME, Carey JH, & Olivier BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the Western Basin
of Lake Ontario. J Great Lakes Res, 9: 287-294.
Franchi E & Focardi S (1991) Polychlorinated biphenyl congeners,
hexachlorobenzene and DDT in human milk in central Italy. Sci Total
Environ, 102: 223-228.
Frank R & Ripley BD (1990) Food residues from pesticides and
environmental pollutants in Ontario. In: Nriagu JO & Simmons MS ed.
Food contamination from environmental sources. New York, Wiley-
Interscience, pp 473-524.
Frank R, Braun HE, & Fleming G (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969 to 1981. J Food Prot, 46:
893-900.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985a)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario - 1983. J Food Prot, 48:
499-504.
Frank R, Rasper J, Braun HE, & Ashton G (1985b) Disappearance of
organochlorine residues from abdominal and egg fats of chickens,
Ontario, Canada, 1969-1982. J Assoc Off Anal Chem, 68: 124-129.
Frank R, Rasper J, Smout MS, & Braun HE (1988) Organochlorine residues
in adipose tissues, blood and milk from Ontario residents, 1976-1985.
Can J Public Health, 79: 150-158.
Fujita M & Morikawa K (1992) Regional differences in dietary intake of
environmental pollutants in Japan. J Expo Anal Environ Epidemiol,
2(2):177-193.
Fürst P, Fürst CHR, & Wilmers K (1992) Survey of dairy products for
PCDD's, PCDF's, PCB's and HCB. Chemosphere, 25(7-10): 1039-1048.
Fürst P, Fürst C, & Wilmers K (1994) Human milk as a bioindicator for
body burden of PCDDs, PCDFs, organochlorine pesticides, and PCBs.
Environ Health Perspect, 102 (Suppl 1): 187-193.
Galassi S & Provini A (1981) Chlorinated pesticides and PCB contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in adult total diet
samples, October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Geike F & Parasher CD (1976a) Effect of hexachlorobenzene on some
growth parameters of Chlorella pyrenoidosa. Bull Environ Contam
Toxicol, 15: 670-677.
Geike F & Parasher CD (1976b) Effect of hexachlorobenzene (HCB) on
growth of Tetrahymena pyriformis. Bull Environ Contam Toxicol, 16:
347-354.
Gilbertson M (1979) Hexachlorobenzene (HCB) in Canada. Ottawa,
Ontario, Environment Canada, Environmental Protection Service
(Unpublished report).
Gobas FAPC, Bedard DC, Ciborowski JJH, & Haffner GD (1989)
Bioaccumulation of chlorinated hydrocarbons by mayfly ( Hexagenia
limbata) in Lake St. Clair. J Great Lakes Res, 15: 581-588.
Gobas FAPC, McNeil EJ, Lovett-Doust L, & Haffner GD (1991)
Bioconcentration of chlorinated aromatic hydrocarbons in aquatic
macrophytes. Environ Sci Technol, 25: 924-929.
Gocmen A, Peters HA, Cripps DJ, Bryan GT, & Morris CR (1989)
Hexachlorobenzene episode in Turkey. Biomed Environ Sci, 2: 36-43.
Goerz G, Bolsen K, Kalofoutis A, & Tsambaos D (1994) Influence of oral
isotrentoin on hepatic and cutaneous P-450-dependent isozyme
activities. Arch Dermatol Res, 286: 104-106.
Goldey ES & Taylor DH (1992) Developmental neurotoxicity following
premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol
Teratol, 14: 15-21.
Goldey ES, Fisher JW, & Taylor DH (1990) Maternal transfer of
hexachlorobenzene in the rat. Neurotoxicol Teratol, 12: 562-563.
Goldstein JA, Friesen M, Scotti TM, Hickman P, Hass JR, & Bergman H
(1978) Assessment of the contribution of chlorinated dibenzo-p-dioxins
and dibenzofurans to hexachlorobenzene-induced toxicity, porphyria,
changes in mixed function oxygenases, and histopathological changes.
Toxicol Appl Pharmacol, 46: 633-649.
Gómez-Catalán J, Planas J, To-Figueras J, Camps M, & Corbella J (1993)
Organochlorine pesticide residues in the population of Catalonia
(Spain). Bull Environ Contam Toxicol, 51: 160-164.
Gómez-Catalán J, Lezaun M, To-Figueras J, & Corbella J (1995)
Organochlorine residues in the adipose tissue of the population of
Navarra (Spain). Bull Environ Contam Toxicol, 54: 534-540.
Gopalaswamy UV & Aiyar AS (1986) Biotransformation and toxicity of
lindane and its metabolite hexachlorobenzene in mammals. In: Morris CR
& Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 267-276 (IARC Scientific Publications No. 77).
Gopalaswamy UV & Nair CKK (1992) DNA binding and mutagenicity of
lindane and its metabolites. Bull Environ Contam Toxicol, 49:
300-305.
Gorski T, Gorska E, Gorecka D, & Sikora M (1986) Hexachlorobenzene is
non-genotoxic in short-term tests. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 399-401 (IARC
Scientific Publications No. 77).
Government of Canada (1993) Canadian Environmental Protection Act
priority substances list assessment report: Hexachlorobenzene. Ottawa,
Ontario, Environment Canada, Health and Welfare Canada, 52 pp.
Gralla EJ, Fleischman RW, Luthra YK, Hagopian M, Baker JR, Esber H, &
Marcus W (1977) Toxic effects of hexachlorobenzene after daily
administration to beagle dogs for one year. Toxicol Appl Pharmacol,
40: 227-239.
Grant DL, Iverson F, Hatina GV, & Villeneuve DC (1974) Effects of
hexachlorobenzene on liver porphyrin levels and microsomal enzymes in
the rat. Environ Physiol Biochem, 4: 159-165.
Grant DL, Shields JB, & Villeneuve DC (1975) Chemical (HCB) porphyria:
effect of removal of sex organs in the rat. Bull Environ Contam
Toxicol, 14: 422-425.
Grant DL, Phillips WEJ, & Hatina GV (1977) Effect of hexachlorobenzene
on reproduction in the rat. Arch Environ Contam Toxicol, 5: 207-216.
Green JA, Francis JE, Wolf CR, Manson MM, & Smith AG (1989) Sexual
dimorphism of cytochrome P-450 induction by hexachlorobenzene in rats.
Biochem Soc Trans, 17: 1016-1017.
Greve PA & Van Zoonen P (1990) Organochlorine pesticides and PCB's in
tissues from Dutch citizens (1968-1986). Int J Environ Anal Chem,
38: 265-277.
Griffin RA & Chou SFJ (1981) Movement of PCB's and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Grimalt JO, Gomez-Belinchon JI, Llop R, & Albaiges J (1988) Water-
phase distribution of hexachlorobenzene in a deltaic environment (Ebre
Delta, Western Mediterranean). Chemosphere, 17: 1893-1903.
Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, Anto JM,
& Albaiges J (1994) Risk excess of soft-tissue and thyroid cancers in
a community exposed to airborne organochlorinated compound mixtures
with a high hexachlorobenzene content. Int J Cancer, 56: 200-203.
Guerzoni ME, Del Cupolo L, & Ponti I (1976) [Mutagenic activity of
pesticides.] Riv Sci Tech Alim Nutr Um, 6: 161-165 (in Italian, with
English summary).
Hagenmaier H, She J, & Lindig C (1992) Persistence of polychlorinated
dibenzo-p-dioxins and polychlorinated dibenzofurans in contaminated
soil at Maulach and Rastatt in southwest Germany. Chemosphere,
25(7/10): 1449-1456.
Hahn ME, Gasiewicz TA, Linko P, & Goldstein JA (1988) The role of the
Ah locus in hexachlorobenzene-induced porphyria. Biochem J, 254:
245-254.
Hahn ME, Goldstein JA, Linko P, & Gasiewicz TA (1989) Interaction of
hexachlorobenzene with the receptor for 2,3,7,8-tetrachlorodibenzo-p-
dioxin in vitro and in vivo. Arch Biochem Biophys, 270(1):
344-355.
Hansen LG, Dorn SB, Sundlof SM, & Vogel RS (1978) Toxicity,
accumulation and depletion of hexachlorobenzene in laying chickens. J
Agric Food Chem, 26: 1369-1374.
Hansen LG, Teske RH, Sundlof SM, & Simon J (1979) Hexachlorobenzene
and feline reproduction: effects of ground pork contaminated by
dietary exposure or spiked with purified HCB. Vet Hum Toxicol, 21:
248-253.
Hansen PD, Von Westernhagen H, & Rosenthal H (1985) Chlorinated
hydrocarbons and hatching success in Baltic herring spring spawners.
Mar Environ Res, 15: 59-76.
Hargrave BT, Vass WP, Erickson PE, & Fowler BR (1989) Distribution of
chlorinated hydrocarbon pesticides and PCBs in the Arctic Ocean.
Dartmouth, NS, Department of Fisheries and Oceans, 224 pp (Canadian
Technical Report of Fisheries and Aquatic Sciences No. 1644).
Harper DJ, Ridgeway IM, & Leatherland TM (1992) Concentrations of
hexachlorobenzene, trichlorobenzenes and chloroform in the waters of
the Forth Estuary, Scotland. Mar Pollut Bull, 24(5): 244-249.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Health Canada (1994) Canadian Environmental Protection Act: Human
health risk assessment for priority substances. Ottawa, Ontario,
Minister of Supply and Services.
Heidmann WA (1986) Hexachlorobenzene residues in selected species of
land and sea birds in northern Germany. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 223-228 (IARC
Scientific Publications No. 77).
Heikes DL (1980) Residues of pentachloronitrobenzene and related
compounds in peanut butter. Bull Environ Contam Toxicol, 24:
338-343.
Heldaas SS, Langard S, & Andersen A (1989) Incidence of cancer in a
cohort of magnesium production workers. Br J Ind Med, 46: 617-623.
Hendriks AJ, Ma W-C, Brouns JJ, De Ruiter-Dijkman EM, & Gast R (1995)
Modelling and monitoring organochlorine and heavy metal accumulation
in soils, earthworms, and shrews in Rhine-Delta floodplains. Arch
Environ Contam Toxicol, 29: 115-127.
Herren-Freund SL & Pereira MA (1986) Carcinogenicity of by-products of
disinfection in mouse and rat liver. Environ Health Perspect, 69:
59-65.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of Interior, Fish and Wildlife Service (Special Scientific
Report œ Wildlife No. 191).
Hippelein M, Kaupp H, Dörr G, & McLachlan MS (1993) Testing of a
sampling system and analytical method for determination of
semivolatile organic compounds in ambient air. Chemosphere, 26:
2255-2263.
Hoff RM, Muir DCG, & Grift NP (1992) Annual cycle of polychlorinated
biphenyls and organohalogen pesticides in air in southern Ontario: 1.
Air concentration data. Environ Sci Technol, 26(2): 266-275.
Holoubek I, œáslavskœ J, Vancura R, Kocan A, Chovancová J, Petrik J,
Drobna B, Cudlin P, & Triska J (1994) Project Tocoen: The fate of
selected organic pollutants in the environment - Part XXIV. The
content of PCBs and PCDDs/Fs in high-mountain soils. Toxicol Environ
Chem, 45: 189-197.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
In: Taup H ed. Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers.
IARC (1979) Hexachlorobenzene. In: Some halogenated hydrocarbons.
Lyon, International Agency for Research on Cancer, pp 155-178 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Human, Volume 20).
IARC (1987) Hexachlorobenzene. In: Overall evaluations of
carcinogenicity: An updating of IARC monographs, volumes 1 to 42.
Lyon, International Agenci for Research on Cancer, pp 219-220 (IARC
Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Iatropoulos MJ, Hobson W, Knauf V, & Adams HP (1976) Morphological
effects of hexachlorobenzene toxicity in female rhesus monkeys.
Toxicol Appl Pharmacol, 37: 433-444.
IJC (International Joint Commission) (1989) In: Rathke DR & McRae G
ed. Great Lakes surveillance: 1987 report on Great Lakes water
quality. Report to the International Joint Commission, Windsor,
Ontario.
Ikemoto Y, Motoba K, Suzuki T, & Uchida M (1992) Quantitative
structure-activity relationships of nonspecific and specific toxicants
in several organism species. Environ Toxicol Chem, 11: 931-939.
Ingebritsen K (1986) Comparative studies on the distribution and
excretion of 14C-hexachlorobenzene by whole-body autoradiography. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 277-285 (IARC Scientific Publications No. 77).
Ingebritsen K, Skaare JU, Nafstad I, & Forde M (1981) Studies on the
biliary excretion and metabolites of hexachlorobenzene in the rat.
Xenobiotica, 11: 795-800.
Innes D, Muncaster B, Lazar R, Haffner D, & Hebert P (1988) Monitoring
of organic contaminants using freshwater mussels. Proceedings of the
Environmental Research Technology Transfer Conference, 30 November-1
December 1987 - Part B: Water quality research. Toronto, Ontario,
Environment Ontario.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: The derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
IRPTC (1993) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M Jr (1988) Data book of chromosomal aberration test
in vitro. Amsterdam, New York, Oxford, Elsevier Science Publishers.
Jacoff FS, Scarberry R, & Rosa D (1986) Source assessment of
hexachlorobenzene from the organic chemical manufacturing industry.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 31-37 (IARC Scientific Publications No. 77). 31-37.
Jaffe R & Hites RA (1986) Anthropogenic, polyhalogenated, organic
compounds in non-migratory fish from the Niagara River area and
tributaries to Lake Ontario. J Great Lakes Res, 12: 63-71.
Jansson B & Bergman A (1978) Sulfur-containing derivatives of
hexachlorobenzene (HCB) - metabolites in the rat. Chemosphere, 3:
257-268.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
Jarrell JF, McMahon A, Villeneuve D, Franklin C, Singh A, Valli VE, &
Bartlett S (1993a) Hexachlorobenzene toxicity in the monkey primordial
germ cell without induced porphyria. Reprod Toxicol, 7: 41-47.
Jarrell JF, Villeneuve D, Franklin C, Bartlett S, Wrixon W, Kohut J, &
Zouves CG (1993b) Contamination of human ovarian follicular fluid and
serum by chlorinated organic compounds in three Canadian cities. Can
Med Assoc J, 148(8): 1321-1327.
Jemaa Z, Sabbah S, Driss MR, & Bouguerra ML (1986) Hexachlorobenzene
in Tunisian mothers' milk, cord blood and foodstuffs. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 139-142 (IARC Scientific Publications No. 77).
Johansen HR, Becher G, Polder A, & Skaare JU (1994) Congener-specific
determination of polychlorinated biphenyls and organochlorine
pesticides in human milk from Norwegian mothers living in Oslo. J
Toxicol Environ Health, 42: 157-171.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites in Lake Ontario. J Great Lakes Res, 9:
183-189.
Kan CA & Tuinstra LGM (1976) Accumulation and excretion of certain
organochloride insecticides in broiler breeder hens. J Agric Food
Chem, 24: 775-778.
Kan-Do Office & Pesticides Team (1995) Accumulated pesticide and
industrial chemical findings from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kannan K, Tanabe S, Ramesh A, Subramanian A, & Tatsukawa R (1992a)
Persistent organochlorine residues in foodstuffs from India and their
implications on human dietary exposure. J Agric Food Chem, 40:
518-524.
Kannan K, Tanabe S, Hoang Trong Quynh, Nguyen Duc Hue, & Tatsukawa R
(1992b) Residue pattern and dietary intake of persistent
organochlorine compounds in foodstuffs from Vietnam. Arch Environ
Contam Toxicol, 22: 367-374.
Kannan K, Falandysz J, Tanabe S, & Tatsukawa R (1993) Persistent
organochlorines in Harbour Porpoises from Puck Bay, Poland. Mar Pollut
Bull, 26(3): 162-165.
Kannan K, Tanabe S, Williams RJ, & Tatsukawa R (1994) Persistant
organochlorine residues in foodstuffs from Australia, Papua New Guinea
and the Solomon Islands: contamination levels and human dietary
exposure. Sci Total Environ, 153: 29-49.
Kashimoto T, Takayama K, Mimura M, Miyata H, Murakami Y, & Matsumoto H
(1989) PCDDs, PCDFs, PCBs, coplanar PCBs and organochlorinated
pesticides in human adipose tissue in Japan. Chemosphere, 19(1/6):
921-926.
Kasokat T, Nagel R, & Urich K (1989) Metabolism of chlorobenzene and
hexachlorobenzene by the zebra fish, Brachydanio rerio. Bull Environ
Contam Toxicol, 42: 254-261.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St. Clair and Detroit Rivers using introduced
clams, Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kelly AG & Campbell A (1994) Organochlorine contaminants in liver of
cod ( Gadus morhua) and muscle of herring ( Clupea harengus) from
Scottish waters. Mar Pollut Bull, 28(2): 103-108.
Kemper FH (1993) Human organ specimen banking-15 years of experience.
Sci Total Environ, 139-140: 13-25.
Kennedy SW & Wigfield DC (1990) Dose-response relationships in
hexachlorobenzene-induced porphyria. Biochem Pharmacol, 40:
1381-1388.
Kennedy SW, Wigfield DC, & Fox GA (1986) The delay in polyhalogenated
aromatic hydrocarbon-induced porphyria: mechanistic reality or
methodological artefact? Toxicol Lett, 31: 235-241.
Kessabi M, Elhraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the Mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kessabi M, Abdennebi E, Laraje R, & Lhafi A (1990) Contamination of
eggs, poultry liver and bovine liver and kidney by chlorinated
pesticides in Morocco. Sci Total Environ, 90: 283-287.
Khera KS (1974) Teratogenicity and dominant lethal studies on
hexachlorobenzene in rats. Food Cosmet Toxicol, 12: 471-477.
Kilikidis SD, Kamarianos AP, & Karamanlis XN (1992) Seasonal
fluctuations of organochlorine compounds in the water of the Strimon
River (N. Greece). Bull Environ Contam Toxicol, 49: 375-380.
Kilzer L, Scheunert I, Geyer H, Klein W, & Korte F (1979) Laboratory
screening of the volatilization rates of organic chemicals from water
and soil. Chemosphere, 10: 751-761.
Kimbrough RD & Linder RE (1974) The toxicity of technical
hexachlorobenzene in the Sherman rat strain. A preliminary study. Res
Commun Chem Pathol Pharmacol, 8: 653-664.
King L & Sherbin G (1986) Point sources of toxic organics to the upper
St. Clair River. Water Pollut Res J Can, 21: 433-446.
Kitchin KT & Brown JL (1989) Biochemical studies of promoters of
carcinogenesis in rat livers. Teratog Carcinog Mutagen, 9: 273-285.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Kleiman de Pisarev DL, Sancovich HA, & Ferramola de Sancovich AM
(1989) Enhanced thyroxine metabolism in hexachlorobenzene-intoxicated
rats. J Endocrinol Invest, 12: 767-772.
Kleiman de Pisarev DL, Del Carmen Rios de Molina M, & San Martin de
Viale LC (1990) Thyroid function and thyroxine metabolism in
hexachlorobenzene-induced porphyria. Biochem Pharmacol,. 39:
817-825.
Knezovich JP & Harrison FL (1988) The bioavailability of sediment-
sorbed chlorobenzenes to larvae of the midge, Chironomus decorus.
Ecotoxicol Environ Saf, 15: 226-241.
Kocan A, Petrík J, Drobná G, & Chovancová J (1994) Levels of PCBs and
some organochlorine pesticides in the human population of selected
areas of the Slovak Republic: I. Blood. Chemosphere, 29(9/11):
2315-2325.
Kodric-Smit M, Smit Z, & Olie K (1980) Organochlorine contaminants in
human milk from Slavonia Province, Yugoslavia, 1978. Pestic Monit J,
14: 1-2.
Koeman JH, Ten Noever de Brauw MC & De Vos RH (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
Kohler A, Harms U, & Lukas B (1986) Accumulation of organochlorines
and mercury in flounder - an approach to pollution assessments. Helgol
Meeresunters 40: 431-440.
Koizumi A (1991) Experimental evidence for the possible exposure of
workers to hexachlorobenzene by skin contamination. Br J Ind Med,
48: 622-628.
Kosian P, Lemke A, Studders K, & Veith G (1981) The precision of the
ASTM bioconcentration test. Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory & Superior,
Wisconsin, University of Wisconsin-Superior, Center for Lake Superior
Environmental Studies, 24 pp (EPA 600/3-81/022).
Koss G & Koransky W (1975) Studies on the toxicology of
hexachlorobenzene: I. Pharmacokinetics. Arch Toxicol, 34: 203-212.
Koss G & Koransky W (1976) Studies on the toxicology of
hexachlorobenzene. II. Identification and determination of
metabolites. Arch Toxicol, 35: 107-114.
Koss G & Koransky W (1978) Pentachlorophenol in different species of
vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. In: Ranga Rao K ed. Pentachlorophenol. New York,
London, Plenum Press, pp 131-137.
Koss G & Manz D (1976) Residues of hexachlorobenzene in wild mammals
of Germany. Bull Environ Contam Toxicol, 15: 189-191.
Koss G, Strik JJTWA, & Kan CA (1978) Metabolites of hexachlorobenzene
in the excreta of different animal species. Excerpta Med Int Congress,
440: 211-212.
Krauthacker B (1993) Organochlorine pesticides and polychlorinated
biphenyls (PCBs) in human serum collected from the general population
from Zagreb (1985-1990). Bull Environ Contam Toxicol, 50: 8-11.
Krishnan K, Brodeur J, & Charbonneau M (1991) Development of an
experimental model for the study of hexachlorobenzene-induced hepatic
porphyria in the rat. Toxicol Appl Pharmacol, 17: 433-441.
Krishnan K, Brodeur J, Plaa GL, & Charbonneau M (1992) Modulation of
hexachlorobenzene-induced hepatic porphyria by methyl isobutyl ketone
in the rat. Toxicol Lett, 61: 167-174.
Kuehl DW & Butterworth B (1994) A national study of chemical residues
in fish: III. Study results. Chemosphere, 29(3): 523-535.
Kuehl DW, Leonard EN, Butterworth BC, & Johnson KL (1983)
Polychlorinated chemical residues in fish from major watersheds near
the Great Lakes, 1979. Environ Int, 9: 293-299.
Kuhnlein HV, Receveur O, Muir DCG, Chan HM, & Soueida R (1995) Arctic
indigenous women consume greater than acceptable levels of
organochlorines. J Nutr, 125: 2501-2510.
Kuiper-Goodman T, Grant DL, Moodle CA, Korsrud GO, & Munro IC (1977)
Subacute toxicity of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 40: 529-549.
Kuroda Y (1986) Genetic and chemical factors affecting chemical
mutagenesis in cultured mammalian cells. In: Antimutagenesis and
anticarcinogenesis mechanisms. New York, London, Plenum Press,
pp 359-375.
Lambrecht RW, Erturk E, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982a) Renal toxicity and tumorigenicity of
hexachlorobenzene (HCB) in rats (R). Proc Am Assoc Cancer Res, 23:
54 (Abstract).
Lambrecht RW, Erturk E, Grunden E, Headley DB, Morris CR, Peters HA, &
Bryan GT (1982b) Hepatotoxicity and tumorigenicity of
hexachlorobenzene (HCB) in Syrian golden hamsters (H) after subchronic
administration. Fed Proc, 41: 329 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983a) Hepatocarcinogenicity of chronically administered
hexachlorobenzene in rats. Fed Proc, 42: 786 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983b) Renal tumors in rats chronically exposed to hexachlorobenzene
(HCB). Proc Am Assoc Cancer Res, 24: 59 (Abstract).
Lambrecht RW, Sinclair PR, Bement WJ, Sinclair JF, Carpenter HM,
Buhler DR, Urquhart AJ, & Elder GH (1988) Hepatic uroporphyrin
accumulation and uroporphyrinogen decarboxylase activity in cultured
chick-embryo hepatocytes and in Japanese quail ( Coturnix coturnix
japonica) and mice treated with polyhalogenated aromatic
hydrocarbons. Biochem J, 253: 131-138.
Lamparski LL, Langhorst ML, Nestrick TJ, & Cutie S (1980) Gas-liquid
chromatographic determination of chlorinated benzenes and phenols in
selected biological matrices. J Assoc Off Anal Chem, 63(1): 27-32.
Landrum PF (1989) Bioavailability and toxicokinetics of polycyclic
aromatic hydrocarbons sorbed to sediment for the amphipod
Pontoporeia hoyi. Environ Sci Technol, 23: 588-595.
Lane DA, Schroeder WH, & Johnson ND (1992a) On the spatial and
temporal variations in atmospheric concentrations of hexachlorobenzene
and hexachlorocyclohexane isomers at several locations in the province
of Ontario, Canada. Atmos Environ, 26A: 31-42.
Lane DA, Johnson ND, Hanley MJJ, Schroeder WH, & Ord DT (1992b) Gas-
and particle-phase concentrations of alpha-hexachlorocyclohexane,
gama-hexachlorocyclohexane, and hexachlorobenzene in Ontario air.
Environ Sci Technol, 26: 126-133.
Langhorst ML & Nestrick TJ (1979) Determination of chlorobenzenes in
air and biological samples by gas chromatography with photoionization
detection. Anal Chem, 51(12): 2018-2025.
Larsen BR, Turrio-Baldassarri L, Nilsson T, Iacovella N, Di Domenico
A, Montagna M, & Facchetti S (1994) Toxic PCB congeners and
organochlorine pesticides in Italian human milk. Ecotoxicol Environ
Saf, 28: 1-13.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW, &
Evans RL (1976) An ecological study of hexachlorobenzene (HCB). New
Orleans, University of New Orleans, Department of Biological Sciences
(Prepared for the US Environmental Protection Agency, Office of Toxic
Substances, Washington) (EPA 560/6-76-009).
Laska AL, Bartell C, & Laseter JL (1976) Distribution of
hexachlorobenzene and hexachlorobutadiene in water, soil, and selected
aquatic organisms along the lower Mississippi River, Louisiana. Bull
Environ Contam Toxicol, 15: 535-542.
Leger DA (1991) Environmental concentrations of hexachlorobenzene in
Atlantic Canada. Moncton, New Brunswick, Environment Canada, Inland
Waters Directorate.
Leoni V & D'Arca SU (1976) Experimental data and critical review of
the occurrence of hexachlorobenzene in the Italian environment. Sci
Total Environ, 5: 253-272.
Lewis RJ (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Company.
Lewis RG & MacLeod KE (1982) Portable sampler for pesticides and
semivolatile industrial organic chemicals in air. Anal Chem, 54:
310-315.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic compounds
in rain: II. Gas scavenging of neutral organic compounds. Atmos
Environ, 19: 1609-1617.
Lilienthal H, Benthe C, Heinzow B, & Winneke G (1996) Impairment of
schedule-controlled behavior by pre- and postnatal exposure to
hexachlorobenzene in rats. Arch Toxicol, 70: 174-181.
Linko P, Yeowell HN, Gasiewicz TA, & Goldstein JA (1986) Induction of
cytochrome P-450 isozymes by hexachlorobenzene in rats and aromatic
hydrocarbon (Ah)-responsive mice. J Biochem Toxicol, 1: 95-107.
Loose LD (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice. Environ Health
Perspect, 43: 89-97.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspect, 39: 79-92.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55(6): 881-889.
Lu P & Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model ecosystem. Environ Health
Perspect, 10: 269-284.
Ludwicki JK & Góralczyk K (1994) Organochlorine pesticides and PCBs in
human adipose tissues in Poland. Bull Environ Contam Toxicol, 52:
400-403.
Lunde G & Bjorseth A (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci
Total Environ, 8(3): 241-246.
Lunde G & Ofstad EB (1976) Determination of fat-soluble chorinated
compounds in fish. Z Anal Chem, 282: 395-399.
McCarty LS, MACKAY D, Smith AD, Ozburn GW, & Dixon DG (1992a) Residue-
based interpretation of toxicity and bioconcentration QSARs from
aquatic bioassays: Neutral narcotic organics. Environ Toxicol Chem,
11: 917-930.
McCarty LS, Ozburn GW, Smith AD, & Dixon DG (1992b) Toxicokinetic
modeling of mixtures of organic chemicals. Environ Toxicol Chem, 11:
1037-1047.
Mack GA & Stanley J (1985) The national human adipose tissue survey.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (Report No. NHATS-ST-01).
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of physical-
chemical properties and environmental fate for organic chemicals -
Volume I: Monoaromatic hydrocarbons, chlorobenzenes, and PCBs.
Chelsea, Michigan, Lewis Publishers.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol, 25: 921-928.
MAFF (Ministry of Agriculture, Fisheries and Food) (1992) Report of
the working party on pesticide residues: 1988-1990. London, Her
Majesty's Stationery Office (Food Surveillance Paper No. 34).
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1994) Report of the working party on pesticide
residues, 1993 - Supplement to the pesticides register. London, Her
Majesty's Stationery Office.
Mansour M, Scheunert I, Viswanathan R, & Korte F (1986) Assessment of
the persistence of hexachlorobenzene in the ecosphere. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 53-59 (IARC Scientific Publications No. 77).
Marlow DA (1986) Hexachlorobenzene exposure in the production of
chlorophenols. In: Morris CR & Cabral JRP ed. Hexachlorobenzene:
Proceedings of an International Symposium. Lyon, International Agency
for Research on Cancer, pp 161-169 (IARC Scientific Publications
No. 77).
Mehendale HM, Fields M, & Matthews HB (1975) Metabolism and effects of
hexachlorobenzene on hepatic microsomal enzymes in the rat. J Agric
Food Chem, 23: 261-265.
Mendoza CE, Grant DL, & Shields JB (1975) Body burden of
hexachlorobenzene in suckling rats and its effects on various organs
and on liver porphyrin accumulation. Environ Physiol Biochem, 5:
460-464.
Mendoza CE, Collins B, Shields JB, & Laver GW (1977) Hexachlorobenzene
residues and effects on esterase activities in pre-weanling rats after
a reciprocal transfer between HCB-treated and control dams. Arch
Toxicol, 38: 191-199.
Mendoza CE, Collins BT, Shields JB, & Laver GW (1978) Effects of
hexachlorobenzene or hexabromobenzene on body and organ weights of
preweanling rats after a reciprocal transfer between the treated and
control dams. J Agric Food Chem, 26: 941-945.
Mendoza CE, Shields JB, & Laver GW (1979) Comparison of the
porphyrinogenic activity of hexabromobenzene and hexachlorobenzene in
primiparous Wistar rats. Bull Environ Contam Toxicol, 21: 358-364.
Mes J (1990) Trends in the levels of some chlorinated hydrocarbon
residues in adipose tissue of Canadians. Environ Pollut, 65:
269-278.
Mes J (1992) Organochlorine residues in human blood and biopsy fat and
their relationship. Bull Environ Contam Toxicol, 48: 815-820.
Mes J & Davies DJ (1979) Presence of polychlorinated biphenyl and
organochlorine pesticide residues and the absence of polychlorinated
terphenyls in Canadian human milk samples. Bull Environ Contam
Toxicol, 21: 381-387.
Mes J & Malcolm S (1992) Comparison of chlorinated hydrocarbon
residues in human populations from the Great Lakes and other regions
of Canada. Chemosphere, 25(3): 417-424.
Mes J, Campbell DS, Robinson RN, & Davies DJA (1977) Polychlorinated
biphenyl and organochlorine pesticide residues in adipose tissue of
Canadians - 1977. Bull Environ Contam Toxicol, 17: 196-203.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and other
chlorinated hydrocarbon residues in adipose tissue of Canadians. Bull
Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, Turton D, & Sun W-F (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mes J, Marchand L, & Davies DJ (1990) Organochlorine residues in
adipose tissue of Canadians. Bull Environ Contam Toxicol, 45:
681-688.
Mes J, Davies DJ, Doucet J, Weber D, & McMullen E (1993) Levels of
chlorinated hydrocarbon residues in Canadian human breast milk and
their relationship to some characteristics of the donors. Food Addit
Contam, 10(4): 429-441.
Mill T & Haag W (1986) The environmental fate of hexachlorobenzene.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 61-66 (IARC Scientific Publications No. 77).
Moksnes MT & Norheim G (1986) Levels of chlorinated hydrocarbons and
composition of PCB in herring gull Larus argentatus eggs collected
in Norway in 1969 compared to 1979-81. Environ Pollut, 11: 109-116.
Mollenhauer HH, Johnson JH, Younger RL, & Clark DE (1975)
Ultrastructural changes in liver of the rat fed hexachlorobenzene. Am
J Vet Res, 36: 1777-1781.
Mollenhauer HH, Johnston JH, Younger RL, & Clark DE (1976) A unique
intracellular aberration related to hexachlorobenzene ingestion. Am J
Vet Res, 37: 847-850.
Morita M, Mimura S, & Yagyu H (1975) A systematic determination of
chlorinated benzenes in human adipose tissue. Environ Pollut, 9:
175-179.
Morley A, Geary D, & Harben F (1973) Hexachlorobenzene pesticides and
porphyria. Med J Aust, 1: 565.
Morosini M, Schreltmüller J, Reuter U, & Ballschmiter K (1993)
Correlation between C-6/C-14 chlorinated hydrocarbons levels in the
vegetation and in the boundary layer of the troposphere. Environ Sci
Technol, 27: 1517-1523.
Muir DCG, Wagemann R, Grift NP, Norstrom RJ, Simon M, & Lien J (1988)
Organochlorine chemical and heavy metal contaminants in white-beaked
dolphins ( Lagenorhynchus albirostris) and pilot whales
( Globicephala melaena) from the coast of Newfoundland, Canada. Arch
Environ Contam Toxicol, 17: 613-629.
Muir DCG, Grift NP, Lockhart WL, Wilkinson P, Billeck BN, & Brunskill
GJ (1995) Spatial trends and historical profiles of organochlorine
pesticides in Arctic lake sediments. Sci Total Environ, 160/161:
447-457.
Müller MD (1982) [Hexachlorobenzene in Switzerland: Measurement and
background of environmental contamination.] Chimia, 36(11): 437-445
(in German).
Mumma CF & Lawless EW (1975) Survey of industrial processing data.
Task I: Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processes. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA 560/3-75-003).
Muncaster BW, Innes DJ, Hebert PDN, & Haffner GD (1989) Patterns of
organic contaminant accumulation by freshwater mussels in the
St. Clair River, Ontario. J Great Lakes Res, 15: 645-653.
Murray HE, Neff GS, Hrung Y, & Giam CS (1980) Determination of
benzo(a)pyrene, hexachlorobenzene and pentachlorophenol in oysters
from Galveston Bay, Texas. Bull Environ Contam Toxicol, 25(4):
663-667.
Mussalo-Rauhamaa H, Pyysalo H, & Antervo K (1988) Relation between the
content of organochlorine compounds in Finnish human milk and
characteristics of the mothers. J Toxicol Environ Health, 25: 1-19.
Nair A & Pillai MKK (1989) Monitoring of hexachlorobenzene residues in
Delhi and Faridabad, India. Bull Environ Contam Toxicol, 42:
682-686.
Nash RG & Gish TJ (1989) Halogenated pesticide volatilization and
dissipation from soil under controlled conditions. Chemosphere, 18:
2353-2362.
Nebecker AV, Griffis WL, Wise CM, Hopkins E, & Barbitta JA (1989)
Survival, reproduction and bioconcentration in invertebrates and fish
exposed to hexachlorobenzene. Environ Toxicol Chem, 8: 601-611.
Needham LL, Burse VW, Head SL, Korver MP, McClure PC, Andrews JS Jr,
Rowley DL, Sung J, & Kahn SE (1990) Adipose tissue/serum partitioning
of chlorinated hydrocarbon pesticides in humans. Chemosphere, 20:
975-980.
Newton KG & Greene NC (1972) Organochlorine pesticide residue levels
in human milk -Victoria, Australia - 1970. Pestic Monit J, 6(1):
4-8.
NHW (National Health and Welfare) (1987) Nutrient value of some common
foods. Ottawa, Ontario, Health Services and Promotion Branch/Health
Protection Branch.
Niimi AJ & Cho CY (1981) Elimination of hexachlorobenzene (HCB) by
rainbow trout ( Salmo gairdneri), and an examination of its kinetics
in Lake Ontario salmonids. Can J Fish Aquat Sci, 38: 1350-1356.
Niimi AJ & Oliver BG (1989) Distribution of polychlorinated biphenyl
congeners and other halocarbons in whole fish and muscle among Lake
Ontario salmonids. Environ Sci Technol, 23: 83-88.
NIOSH (1985) Registry of toxic effects of chemical substances,
1983-1984 - Cumulative supplement to the 1981-1982 edition.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Noble DG & Elliott JE (1986) Environmental contaminants in Canadian
seabirds, 1968-1984: Trends and effects. Ottawa, Ontario, Canadian
Wildlife Service (Technical Report Series No. 13).
Noble DG, Elliott JE, & Shutt L (1992) Contaminants in Canadian
raptors, 1965-1988. Ottawa, Ontario, Canadian Wildlife Service
(Technical Report Series No. 91).
Norén K (1983a) Levels of organochlorine contaminants in human milk in
relation to the dietary habits of the mothers. Acta Paediatr Scand,
72: 811-816.
Norén K (1983b) Some aspects of the determination of organochlorine
contaminants in human milk. Arch Environ Contam Toxicol, 12:
277-283.
Norén K (1988) Changes in the levels of organochlorine pesticides,
polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans in
human milk from Stockholm, 1972-1985. Chemosphere, 17(1): 39-49.
Norén K (1993) Contemporary and retrospective investigations of human
milk in the trend studies of organochlorine contaminants in Sweden.
Sci Total Environ, 139/140: 347-355.
Norstrom RJ, Simon M, & Muir DCG (1990) Polychlorinated dibenzo-p-
dioxins and dibenzofurans in marine mammals in the Canadian north.
Environ Pollut, 66: 1-19.
NTP (1994) Seventh annual report on carcinogens. Summary, 1994.
Research Triangle Park North Carolina, US Department of Health and
Human Services, National Toxicology Program.
Ockner RK & Schmid R (1961) Acquired porphyria in man and rat due to
hexachlorobenzene intoxication. Nature (Lond), 189: 499.
Oehme M & Stray H (1982) Quantitative determination of ultra-traces of
chlorinated compounds in high-volume air samples from the Arctic using
polyurethane foam as collection medium. Fresenius Z Anal Chem, 311:
665-673.
Oliver BG (1984a) Distribution and pathways of some chlorinated
benzenes in the Niagara River and Lake Ontario. Water Pollut Res J
Can, 19: 47-59.
Oliver BG (1984b) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Partitioning relationships for chlorinated organics
between water and particulates in the St. Clair, Detroit and Niagara
Rivers. In: Kaiser LLE ed. QSAR in environmental toxicology - II.
Boston, Massachussetts, D. Reidel Publishing Co., pp 251-260.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St. Clair, and Erie: Implications
regarding sources along the St. Clair and Detroit rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Carey JH (1986) Photodegradation of wastes and pollutants
in aquatic environment. In: Pelizzetti E & Serpo N ed. Homogenous and
heterogenous photocatalysis. Boston, Massachussetts, D. Reidel
Publishing Co., pp 629-650.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Kaiser KLE (1986) Chlorinated organics in nearshore waters
and tributaries of the St. Clair River. Water Pollut Res J Can, 21:
344-350.
Oliver BG & Nicol KD (1982) Chlorobenzenes in sediments, water, and
selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ
Sci Technol, 16: 532-536.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of polychlorinated
biphenyl congeners and other chlorinated hydrocarbons in the Lake
Ontario ecosystem. Environ Sci Technol, 22: 388-397.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21: 368-379.
Oliver BG, Charlton MN, & Durham RW (1989) Distribution,
redistribution, and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
sediments. Environ Sci Technol, 23: 200-208.
OMAF/OME (Ontario Ministry of Agriculture and Food/Ontario Ministry of
the Environment) (1988) Polychlorinated dibenzo-p-dioxins and
polychlorinated dibenzofurans and other organochlorine contaminants in
food. Toronto, Ontario Ministry of Agriculture and Food and Ministry
of the Environment (Report prepared by the Joint OMAF/OME Toxics in
Food Steering Committee).
Ostfeldt P, Gustavson K, Jansson B, Jonsson P, Miettinen V, Ringstad
O, & Wesén C (1994) Halogenated organic compounds in the marine
environment 1989-1990. Copenhagen, Nordic Council of Ministers, 124 pp
(TemaNord 1994:591).
Parrish PR, Cook GH, & Patrick JM Jr (1974) Effects on several
estuarine animals. In: Rogers WA ed. Proceedings of the 28th Annual
Conference, White Sulphur Springs, West Virginia, 17-20 November 1974.
Sulphur Springs, West Virginia, Southeastern Association of Game and
Fish Commissioners.
Patton GW, Hinckley DA, Walla MD, & Bidleman TF (1989) Airborne
organochlorines in the Canadian High Arctic. Tellus, 41B: 243-255.
Peakall DB, Noble DG, Elliott JE, Somers JD, & Erickson G (1990)
Environmental contaminants in Canadian peregrine falcons, Falco
peregrinus: A toxicological assessment. Can Field-Nat, 104:
244-254.
Pearce PA, Elliott JE, Peakall DB, & Norstrom RJ (1989) Organochlorine
contaminants in eggs of seabirds in the Northwest Atlantic, 1968-1984.
Environ Pollut, 56: 217-235.
Pereira MA, Herren SL, Britt AL, & Khoury MM (1982) Sex difference in
enhancement of GGTase-positive foci by hexachlorobenzene and lindane
in rat liver. Cancer Lett, 15: 95-101.
Persoone G & Uyttersprot G (1975) The influence of inorganic and
organic pollutants on the rate of reproduction of a marine
hypotrichous ciliate: Euplotes vannus Muller. Rev Int Océanogr méd,
37-38: 125-151.
Peters HA (1976) Hexachlorobenzene poisoning in Turkey. Fed Proc,
35: 2400-2403.
Peters HA, Johnson SAM, Cam S, Oral S, Müftü Y, & Ergene T (1966)
Hexachlorobenzene-induced porphyria: effect of chelation on the
disease, porphyrin and metal metabolism. Am J Med Sci, 251: 314-322.
Peters HA, Gocmen A, Cripps DJ, Bryan GT, & Dogramaci I (1982)
Epidemiology of hexachlorobenzene-induced porphyria in Turkey:
clinical and laboratory follow-up after 25 years. Arch Neurol, 39:
744-749.
Peters HA, Cripps DJ, Gocmen A, Ertürk E, Bryan GT, & Morris CR (1986)
Neurotoxicity of hexachlorobenzene-induced porphyria turcica. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 575-579 (IARC Scientific Publications No. 77).
Peterson R & Ray S (1987) Organochlorine residues in brook trout and
yellow perch from New Brunswick and Nova Scotia (Canada) lakes. Water
Pollut Res J Can, 22: 352-364.
Phelps DK, Pruell RJ, & Lake JL (1986) Hexachlorobenzene in selected
marine samples: an environmental perspective. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 121-130 (IARC
Scientific Publications No. 77).
Proulx G, Weseloh DV, Elliott JE, Teeple S, Anghern PAM, & Mineau P
(1987) Organochlorine and PCB residues in Lake Erie mink populations.
Bull Environ Contam Toxicol, 39: 939-944.
Quinlivan S, Ghassemi M, & Santy M (1975) Survey of methods used to
control wastes containing hexachlorobenzene. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste Management
Programs (EPA 530/SW-120c).
Quinsey PM, Donohue DC, & Ahokas JT (1995) Persistence of
organochlorines in breast milk of women in Victoria, Australia. Food
Chem Toxicol, 33(1): 49-56.
Ray LE, Murray HR, & Giam CS (1983a) Analysis of water and sediment
from the Nueces Estuary/Corpus Christi Bay (Texas) for selected
organic pollutants. Chemosphere, 12: 1039-1045.
Ray LE, Murray HE, & Giam CS (1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12: 1031-1038.
Renner G (1988) Hexachlorobenzene and its metabolism. Toxicol Environ
Chem, 18: 51-78.
Richter E & Schäfer SG (1981) Intestinal excretion of
hexachlorobenzene. Arch Toxicol, 47: 233-239.
Rios de Molina MC, Wainstok de Calmanovici R, & San Martin de Viale LC
(1980) Investigations on the presence of porphyrinogen carboxy-lyase
inhibitor in the liver of rats intoxicated with hexachlorobenzene. Int
J Biochem, 12: 1027-1032.
Rippen G & Frank R (1986) Estimation of hexachlorobenzene pathways
from the technosphere into the environment. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 45-52 (IARC
Scientific Publications No. 77).
RIWA (1993) Rapport annuel 1993 de l'Association des Services d'Eau du
Rhin et de la Meuse, Annex 11. Amsterdam, Secrétariat de l'Association
des Services d'Eau du Rhin et de la Meuse.
Rizzardini M & Smith AG (1982) Sex differences in the metabolism of
hexachlorobenzene by rats and the development of porphyria in females.
Biochem Pharmacol, 31: 3543-3548.
Rizzardini M, Graziani A, Carugo C, & Cantoni L (1988) Investigations
on the role of free radical processes in hexachlorobenzene-induced
porphyria in mice. J Biochem Toxicol, 3: 33-46.
Rizzardini M, Cantoni L, Villa P, & Ubezio P (1990) Biochemical,
morphological and flow-cytometric evaluation of the effects of
hexachlorobenzene on rat liver. Cell Biol Toxicol, 6: 185-203.
Robinson PE, Mack GA, Remmers J, Levy R, & Mohadjer L (1990) Trends of
PCB, hexachlorobenzene, and ß-benzene hexachloride levels in the
adipose tissue of the U.S. population. Environ Res, 53: 175-192.
Rogers HR, Crathorne B, & Leatherland TM (1989) Occurrence of
chlorobenzene isomers in the water column of a UK estuary. Mar Pollut
Bull, 20: 276-281.
Rozman K, Rozman T, & Greim H (1981) Enhanced fecal elimination of
stored hexachlorobenzene from rats and rhesus monkeys by hexadecane or
mineral oil. Toxicology, 22: 33-44.
Rozman K, Gorski JR, Rozman P, & Parkinson A (1986) Reduced serum
thyroid hormone levels in hexachlorobenzene-induced porphyria. Toxicol
Lett, 30: 71-78.
Rumsby PC, Evans JG, Phillimore HE, Carthew P, & Smith AG (1992)
Search for Ha- ras codon 51 mutations in liver tumours caused by
hexachlorobenzene and Aroclor 1254 in C57BL/10ScSn mice with iron
overload. Carcinogenesis, 13: 1917-1920.
Rush GF, Smith JH, Maita K, Bleavins M, Aulerich RJ, Ringer RK, & Hook
JB (1983) Perinatal hexachlorobenzene toxicity in the mink. Environ
Res, 31: 116-124.
Russell RW & Gobas FAPC (1989) Calibration of the freshwater mussel,
Elliptio complanata, for quantitative biomonitoring of
hexachlorobenzene and octachlorostyrene in aquatic systems. Bull
Environ Contam Toxicol, 43: 576-582.
Salisbury CDC, Fesser ACE, MacNeil JD, & Patterson JR (1992) Trace
metal and pesticide levels in muskoxen from Victoria Island, Northwest
Territories, Canada. Intern. J Environ Chem, 48: 209-215.
Sanborn JR, Childers WF, & Hansen LG (1977) Uptake and elimination of
[14C] hexachlorobenzene (HCB) by the green sunfish, Lepomis
cyanellus Raf., after feeding contaminated food. J Agric Food Chem,
25: 551-553.
Schechter A, Fürst P, Krüger C, Meemken H-A, Groebel W, & Constable JD
(1989a) Levels of polychlorinated dibenzofurans, dibenzodioxins, PCBs,
DDT and DDE, hexachlorobenzene, dieldrin, hexachlorocyclohexanes and
oxychlordane in human breast milk from the United States, Thailand,
Vietnam, and Germany. Chemosphere, 18: 445-454.
Schechter A, Mes J, & Davies D (1989b) Polychlorinated biphenyl (PCB),
DDT, DDE and hexachlorobenzene (HCB) and PCDD/PCDF isomer levels in
various organs in autopsy tissue from North American patients.
Chemosphere, 18: 811-818.
Scheufler E & Rozman KK (1984) Comparative decontamination of
hexachlorobenzene-exposed rats and rabbits by hexadecane. J Toxicol
Environ Health, 14: 353-362.
Scheunert I, Marra C, Viswanatha R, Klein WK, & Korte F (1983) Fate of
hexachlorobenzene-14C in wheat plants and soil under outdoor
conditions. Chemosphere, 12: 843-858.
Scheunert I, Topp E, Schmitzer J, Klein W, & Korte F (1985) Formation
and fate of bound residues of [14C] benzene and [14C] chlorobenzene
in soil and plants. Ecotoxicol Environ Saf, 9: 159-170.
Schielen P, Schoo W, Tekstra J, Oostermeijer HHA, Seinen W, & Bloksma
N (1993) Autoimmmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schielen P, Den Besten C, Vos JG, Van Bladeren PJ, Seinen W, & Bloksma
N (1995) Immune effects of hexachlorobenzene in the rat: role of
metabolism in a 13-week feeding study. Toxicol Appl Pharmacol, 131:
37-43.
Schmid R (1960) Cutaneous porphyria in Turkey. N Engl J Med, 263:
397-398.
Schmitt CJ, Zaijcek JL, & Peterman PH (1990) National Contaminant
Biomonitoring Program: residues of organochlorine chemicals in U.S.
freshwater fish, 1976-1984. Arch. Environ Toxicol, 19: 748-781.
Schuler W, Brunn H, & Manz D (1985) Pesticides and polychlorinated
biphenyls in fish from the Lahn River. Bull Environ Contam Toxicol,
34: 608-616.
Schuytema G, Krawczyk DF, Griffis WL, Nebeker AV, & Robideaux ML
(1990) Hexachlorobenzene uptake by fathead minnows and
macroinvertebrates in recirculating sediment/water systems. Arch
Environ Contam Toxicol, 19: 1-9.
Schwarzenbach RP, Giger W, Hoehn E, & Schneider JK (1983) Behaviour of
organic compounds during infiltration of river water to groundwater.
Field studies. Environ Sci Technol, 17: 472-479.
Selden A, Jacobson G, Berg P, & Axelson O (1989) Hepatocellular
carcinoma and exposure to hexachlorobenzene: a case report. Br J Ind
Med, 46: 138-140.
Shirai T, Miyata Y, Nakanishi K, Murasaki G, & Ito N (1978)
Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in ICR mice
and its enhancement by hexachlorobenzene. Cancer Lett, 4: 271-275.
Siekel P, Chalupa I, Beno J, Blasko M, Novotny J, & Burian J (1991) A
genotoxicological study of hexachlorobenzene and pentachloroanisole.
Teratog Carcinog Mutagen, 11: 55-60.
Simon GS, Tardiff RG, & Borzelleca JF (1979) Failure of
hexachlorobenzene to induce dominant lethal mutations in the rat.
Toxicol Appl Pharmacol, 47: 415-419.
Sims DE, Singh A, Donald A, Jarrell J, & Villeneuve DC (1991)
Alteration of primate ovary surface epithelium by exposure to
hexachlorobenzene: a quantitative study. Histol Histopathol, 6:
525-529.
Singh A, Friesen D, Jarrel J, & Villeneuve DC (1990a)
Hexachlorobenzene toxicity in the monkey ovary: I. Ultrastructure
induced by low (0.1 mg/kg) dose exposure. In: Proceedings of the XIIth
International Congress for Electron Microscopy, Seattle, Washington -
Volume 2: Analytical sciences, San Francisco, California, San
Francisco Press, Inc., pp 282-283.
Singh A, Sims DE, Jarrell J, & Villeneuve DC (1990b) Hexachlorobenzene
toxicity in the monkey ovary: II. Ultrastructure induced by medium
(1.0 mg/kg) dose exposure. Anal Environ Toxicants, 13: 435-438.
Singh A, Dykeman A, Jarrell J, & Villeneuve DC (1991)
Hexachlorobenzene toxicity in the monkey ovary: III. Ultrastructure
induced by high (10 mg/kg) dose exposure. In: Proceedings of the 49th
Annual Meeting of the Electron Microscopy Society of America. San
Francisco, California, San Francisco Press, Inc.
Skåre JU, Stenersen J, Kveseth N, & Polder A (1985) Time trends of
organochlorine chemical residues in seven sedentary marine fish
species from a Norwegian fjord during the period 1972-1982. Arch
Environ Contam Toxicol, 14: 33-41.
Skaare JU, Markussen NH, Norheim G, Haugen S, & Holt G (1990) Levels
of polychlorinated biphenyls, organochlorine pesticides, mercury,
cadmium, copper, selenium, arsenic, and zinc in the harbour seal,
Phoca vitulina, in Norwegian waters. Environ Pollut, 66: 309-324.
Smith AG & Cabral JR (1980) Liver-cell tumours in rats fed
hexachlorobenzene. Cancer Lett, 11: 169-172.
Smith AG & De Matteis F (1990) Oxidative injury mediated by the
hepatic cytochrome P-450 system in conjunction with cellular iron.
Effects on the pathway of haem biosynthesis. Xenobiotica, 29(9):
865-877.
Smith AG & Francis JE (1983) Synergism of iron and hexachlorobenzene
inhibits hepatic uroporphyrinogen decarboxylase in inbred mice.
Biochem J, 214: 909-913.
Smith VE, Spurr JM, Filkins JC, & Jones JJ (1985a) Organochlorine
contaminants of wintering ducks foraging on Detroit River sediments. J
Great Lakes Res, 11(3): 231-246.
Smith AG, Francis JE, Dinsdale D, Manson MM, & Cabral JRP (1985b)
Hepatocarcinogenicity of hexachlorobenzene in rats and the sex
difference in hepatic iron status and development of porphyria.
Carcinogenesis, 6: 631-636.
Smith AG, Dinsdale D, Cabral JRP, & Wright AL (1987) Goitre and
wasting induced in hamsters by hexachlorobenzene. Arch Toxicol, 60:
343-349.
Smith AG, Cabral JRP, Carthew P, Francis JE, & Manson MM (1989)
Carcinogenicity of iron in conjunction with a chlorinated
environmental chemical, hexachlorobenzene, in C57BL/10ScSn mice. Int J
Cancer, 43: 492-496.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM (1990)
Sex-linked hepatic uroporphyria and the induction of cytochromes
P450IA in rats caused by hexachlorobenzene and polyhalogenated
biphenyls. Biochem Pharmacol, 40: 2059-2068.
Smith AG, Carthew P, Francis JE, Cabral JRP, & Manson MM (1993)
Enhancement by iron of hepatic neoplasia in rats caused by
hexachlorobenzene. Carcinogenesis, 14: 1381-1387.
Smith AG, Carthew P, Francis JE, & Ingebritsen K (1994) Influence of
iron on the induction of hepatic tumors and porphyria by
octachlorostyrene in C57BL/10ScSn mice. Cancer Lett, 81: 145-150.
Solé M, Porte C, Pastor D, & Albaigés J (1994) Long-term trends of
polychlorinated biphenyls and organochlorinated pesticides in mussels
from the Western Mediterranean coast. Chemosphere, 28(5): 897-903.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sopena de Kracoff YE, Ferramola de Sancovich AM, Sancovich HA, &
Kleiman de Pisarev DL (1994) Effect of thyroidectomy and thyroxine on
hexachlorobenzene induced porphyria. J Endocrinol Invest, 17:
301-305.
Spigarelli JL, Going JE, & Li R (1986) Hexachlorobenzene levels in
multimedia environmental samples from selected chemical production
plants. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 155-160 (IARC Scientific Publications No. 77).
Stacey CI, Perriman WS, & Whitney S (1985) Organochlorine pesticide
residue levels in human milk: Western Australia, 1979-1980. Arch
Environ Health, 40(2): 102-108.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary. Kansas
City, Missouri, Midwest Research Institute (Prepared for the US
Environmental Protection Agency, Office of Toxic Substances, Field
Studies Branch) (EPA 560/5-86/035).
Stevens RJJ & Neilson MA (1989) Inter- and intralake distribution of
trace contaminants in surface waters of the Great Lakes. J Great Lakes
Res, 15: 377-393.
Stevens MF, Ebell GF, & Psaila-Savona P (1993) Organochlorine
pesticides in Western Australian nursing mothers. Med J Aust, 158:
238-241.
Stewart FP & Smith AG (1987) Metabolism and covalent binding of
hexachlorobenzene by isolated male and female rat hepatocytes. Biochem
Pharmacol, 36: 2232-2234.
Stewart FP, Manson MM, Cabral JRP, & Smith AG (1989) Hexachlorobenzene
as a promoter of diethylnitrosamine-initiated hepatocarcinogenesis in
rats and comparison with induction of porphyria. Carcinogenesis,
10(7): 1225-1230.
Strik JJTWA (1986) Subacute toxicity of hexachlorobenzene. In: Morris
CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 335-342 (IARC Scientific Publications No. 77).
Stronkhorst J, Ysebaert TJ, Smedes F, Meininger PL, Dirksen S, &
Boudewijn TJ (1993) Contaminants in eggs of some waterbird species
from the Scheldt Estuary, SW Netherlands. Mar Pollut Bull, 26(10):
572-578.
Sundlof SF, Hansen LG, Koritz GD, & Sundlof SM (1982) The
pharmacokinetics of hexachlorobenzene in male beagles. Distribution,
excretion and pharmacokinetic model. Drug Metab Dispos, 10: 371-381.
Suns K, Craig GR, Crawford G, Rees GA, Tosine H, & Osborne J (1983)
Organochlorine contaminant residues in spottail shiner ( Notropis
hudsonius) from the Niagara River. J Great Lakes Res, 9: 335-340.
Suns K, Crawford GE, Russell DD, & Clement RE (1985) Temporal trends
and spatial distribution of organochlorine and mercury residues in
Great Lakes spottail shiners (1975-1983). Rexdale, Ontario, Ontario
Ministry of the Environment, Water Resources Branch.
Swindoll CM & Applehans FM (1987) Factors influencing the accumulation
of sediment-sorbed hexachlorobiphenyl by midge larvae. Bull Environ
Contam Toxicol, 39: 1055-1062.
Szymczyœski GA, Waliszewski SM, Tuszewski M, & Pyda P (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznaœ. J Environ Sci Health, A21(1): 5-14.
Tanabe S, Subramanian AN, Ramesh A, Kumaran PL, Miyazaki N, &
Tatsukawa R (1993) Persistent organochlorine residues in dolphins from
the Bay of Bengal, South India. Mar Pollut Bull, 26(6): 311-316.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Teschke R, Bolsen K, Landmann H, & Goerz G (1983) Effect of
hexachlorobenzene on the activities of hepatic alcohol metabolizing
enzymes. Biochem Pharmacol, 32: 1745-1751.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound: Volume 1 (Prepared for Puget Sound Dredged Disposal
Analysis and Puget Sound Estuary Program, Bellevue, Washington).
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in Strain A mice. Cancer Res, 37:
2717-2720.
Thomas W, Simon H, & Rüling Å (1985) Classification of plant species
by their organic (PAH, PCB, BHC) and inorganic (heavy metals) trace
pollutant concentrations. Sci Total Environ, 46: 83-94.
Thomson BA, Davidson WR, & Lovett AM (1980) Applications of a
versatile technique for trace analysis: Atmospheric pressure negative
chemical ionization. Environ Health Perspect, 36: 77-84.
Tobin P (1986) Known and potential sources of hexachlorobenzene. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 3-11 (IARC Scientific Publications No. 77).
To-Figueras J, Rodamilans M, Gómez-Catalán J, & Corbella J (1986)
Hexachlorobenzene residues in the general population of Barcelona,
Spain. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, p 147 (IARC Scientific Publications No. 77).
To-Figueras J, Gómez-Catalán J, Rodamilans M, & Corbella J (1992)
Sulphur derivative of hexachlorobenzene in human urine. Hum Exp
Toxicol, 11: 271-273.
To-Figueras J, Barrot C, Rodamilans M, Gómez-Catalán J, Torra M,
Brunet M, Sabater F, & Corbella J (1995) Accumulation of
hexachlorobenzene in humans: a long standing risk. Hum Exp Toxicol,
14: 20-23.
Topp E, Scheunert I, & Korte K (1989) Kinetics of the uptake of
14C-labelled chlorinated benzenes from soil by plants. Ecotoxicol
Environ Saf, 17: 157-166.
Trapp M, Baukloh V, Bohnet H-G, & Heeschen W (1984) Pollutants in
human follicular fluid. Fertil Steril, 42(1): 146-148.
US EPA (1980) Analysis of pesticide residues in human and
environmental samples: A compilation of methods selected for use in
pesticide monitoring programs. Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Toxicology Division,
Health Effects Research Laboratory (EPA 600/8-80-038).
US EPA (1982) Test methods: Methods of organic chemical analysis of
municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Office of Research and Development,
Environmental Monitoring and Support Laboratory (EPA 600/4-82-057).
US EPA (1985a) Health assessment document for chlorinated benzenes.
Washington, DC, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA 600/8-84-015F).
US EPA (1985b) Drinking water criteria document for hexachlorobenzene.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Criteria and Assessment Office (EPA 600/X-84-179-1).
US EPA (1986a) Exposure assessment for hexachlorobenzene. Washington,
DC, US Environmental Protection Agency, Office of Pesticides and Toxic
Substances (EPA 560/5-86-0(19).
US EPA (1986b) Test methods for evaluating solid waste, 3rd ed.
Washington, DC, US Environmental Protection Agency, Office of Solid
Waste and Emergency Response (SW 846).
US EPA (1988) Ambient aquatic life water quality criteria for
hexachlorobenzene. Duluth, Minnesota, US Environmental Protection
Agency, Office of Research and Development.
US FDA (US Food and Drug Administration) (1990) Residues in foods -
1988. J Assoc Off Anal Chem, 73: 133A-152A.
US FDA (US Food and Drug Administration) (1991) Residues in foods -
1989. J Assoc Off Anal Chem, 74: 127A-146A.
US FDA (US Food and Drug Administration) (1992) Residues in foods -
1990. J Assoc Off Anal Chem, 75: 121A-141A.
Üstünbas HB, Öztürk MA, Hasanoglu E, & Dogan M (1994) Organochlorine
pesticide residues in human milk in Kayseri. Hum Exp Toxicol, 13:
299-302.
Van der Ven K, Van der Ven H, Thibold A, Bauer O, Kaisi M, Mbura J,
Mhaya HN, Wever N, Diedrich K, & Krebs D (1992) Chlorinated
hydrocarbon content of fetal and maternal body tissues and fluids in
full term pregnant women: a comparison of Germany versus Tanzania. Hum
Reprod, 7(1): 95-100.
Van Leeuwen CJ, Van der Zandt PTJ, Aldenberg T, Verhaar HJM, & Hermans
JLM (1992) Application of QSARs, extrapolation and equilibrium
partitioning in aquatic effects assessment: I. Narcotic industrial
pollutants. Environ Toxicol Chem, 11: 267-282.
Van Loveren H, Kranjc EI, Rombout PJA, Blommaert FA, & Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Ommen B, Hendriks W, Bessems JGM, Geesink G, Müller F, & Van
Bladeren PJ (1989) The relation between the oxidative
biotransformation of hexachlorobenzene and its porphyrinogenic
activity. Toxicol Appl Pharmacol, 100: 517-528.
Van Raaij JAGM, Van den Berg KJ, & Notten WRF (1991a)
Hexachlorobenzene and its metabolites pentachlorophenol and
tetrachlorohydroquinone: interaction with thyroxine binding sites of
rat thyroid hormone carriers ex vivo and in vitro. Toxicol Lett,
59: 101-107.
Van Raaij JAGM, Van den Berg KJ, Engel R, Bragt PC, & Notten WRF
(1991b) Effects of hexachlorobenzene and its metabolites
pentachlorophenol and tetrachlorohydroquinone on serum thyroid hormone
levels in rats. Toxicology, 67: 107-116.
Van Raaij JAGM, Frijters CMG, & Van den Berg KJ (1993a)
Hexachlorobenzene-induced hypothyroidism: involvement of different
mechanisms by parent compound and metabolite. Biochem Pharmacol, 46:
1385-1391.
Van Raaij JAGM, Kaptein E, Visser TJ, & Van den Berg KJ (1993b)
Increased glucoronidation of thyroid hormone in hexachlorobenzene-
treated rats. Biochem Pharmacol, 45: 627-631.
Vaz R, Slorach SA, & Hofvander Y (1993) Organochlorine contaminants in
Swedish human milk: studies conducted at the National Food
Administration 1981-1990. Food Addit Contam, 10(4): 407-418.
Villanueva EC, Jennings RW, Burse VW, & Kimbrough RD (1974) Evidence
of chlorodibenzo-p-dioxin and chlorodibenzofuran in hexachlorobenzene.
J Agric Food Chem, 22: 916-917.
Villeneuve DC, Panopio LG, & Grant DL (1974) Placental transfer of
hexachlorobenzene in the rabbit. Environ Physiol Biochem, 4:
112-115.
Vincent SH, Smith AG, & Muller-Eberhard U (1989) Modulation of hepatic
heme-binding Z protein in mice by the porphyrogenic carcinogens
griseofulvin and hexachlorobenzene. Cancer Lett, 45: 109-114.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
ymposium. Lyon, International Agency for Research on Cancer,
pp 347-356 (IARC Scientific Publications No. 77).
Vos JG, Breeman HA, & Benschop H (1968) The occurrence of the
fungicide hexachlorobenzene in wild birds and its toxicological
importance: a preliminary communication. Meded Fac. Landbouwwet
Rijksuniv Gent, 33: 1263-1269.
Vos JG, Van der Maas HL Musch A, & Ram E (1971) Toxicity of
hexachlorobenzene in Japanese quail with special reference to
porphyria, liver damage, reproduction and tissue residues. Toxicol
Appl Pharmacol, 18: 944-957.
Vos JG, Botterweg PF, Strik JJTWA, & Koeman JH (1972) Experimental
studies with HCB in birds. TNO-Nieuws, 27: 599-603.
Vos JG, Van Logten MJ, Kreeftenberg JG, & Kruizinga W (1979a)
Hexachlorobenzene-induced stimulation of the humoral immune response
in rats. Ann NY Acad Sci, 320: 535-550.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979b) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Brouwer GMJ, Van Leeuwen FXR, & Wagenaar SJ (1983) Toxicity of
hexachlorobenzene in the rat following combined pre- and post-natal
exposure: comparison of effects on the immune system, liver and lung.
In: Parke DV, Gibson GG, & Hubbard R ed. Immunotoxicology. New York,
London, Academic Press, pp 219-235.
Vos RME, Snoek MC, Van Berkel WJH, Müller F, & Van Bladeren PJ (1988)
Differential induction of rat hepatic glutathione S-transferase
isoenzymes by hexachlorobenzene and benzyl isothiocyanate: comparison
with induction by phenobarbital and 3-methylcholanthrene. Biochem
Pharmacol, 37: 1077-1082.
Wada O, Yano Y, Urata G, & Nakao K (1968) Behavior of hepatic
microsomal cytochromes after treatment of mice with drugs known to
disturb porphyrin metabolism in liver. Biochem Pharmacol, 17:
595-603.
Wang M-J & Jones KC (1994) Occurrence of chlorobenzenes in nine United
Kingdom retail vegetables. J Agric Food Chem, 42: 2322-2328.
Washington State Department of Ecology (1990) Proposed sediment
management standards rule (September 18, 1990). Washington State
Register, issue 90-19.
Watts RR, Hodgson DW, Christ HL, & Moseman RF (1980) Improved method
for hexachlorobenzene and mirex determination with hexachlorobenzene
confirmation in adipose tissue: Collaborative study. J Assoc Off Anal
Chem, 63(5): 1128-1134.
Weisenberg E, Arad I, Grauer F, & Sahm Z (1985) Polychlorinated
biphenyls and organochlorine insecticides in human milk in Israel.
Arch Environ Contam Toxicol, 14: 517-521.
Wells DE, Campbell LA, Ross HM, Thompson PM, & Lockyer CH (1994)
Organochlorine residues in harbour porpoise and bottlenose dolphins
stranded on the coast of Scotland, 1988-1991. Sci Total Environ,
151: 77-99.
WHO (1993) Guidelines for drinking-water quality - Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization, p 84.
Wickström K, Pyysalo H, & Siimes MA (1983) Levels of chlordane,
hexachlorobenzene, PCB and DDT compounds in Finnish human milk in
1982. Bull Environ Contam Toxicol, 31: 251-256.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71: 410-414.
Wilson DM & Wan MTK (1982) Hexachlorobenzene: Industrial and
agricultural sources in British Columbia. Vancouer, Environment
Canada, Environmental Protection Service (Regional Program Report
No. 82-07).
Witte H, Langenohl T, & Offenbacher SAG (1988a) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part A: Organic pollutant load in sewage
sludge. Korresp Abwasser, 13: 118-125.
Witte H, Langenohl T, & Offenbacher SAG (1988b) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part B: Impact of the application of
sewage sludge on organic matter content in soil and plants. Korresp
Abwasser, 13: 126-136.
Yamaguchi Y, Kawano M, & Tatsukawa R (1986) Tissue distribution and
excretion of hexabromobenzene (HBB) and hexachlorobenzene (HCB)
administered to rats. Chemosphere, 15: 453-459.
Yang RSH, Pittman KA, Rourke DR, & Stein VB (1978) Pharmacokinetics
and metabolism of hexachlorobenzene in the rat and the rhesus monkey.
J Agric Food Chem, 26: 1076-1083.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés chimiques et physiques et méthodes d'analyse
L'hexachlorobenzène (HCB) est un composé organique chloré
modérément volatil. Il est pratiquement insoluble dans l'eau, mais
extrêmement soluble dans les lipides et présente une tendance à la
bioaccumulation. L'hexachlorobenzène de qualité technique contient
jusqu'à 2% d'impuretés, dont la principale est le pentachlorobenzène.
Les autres consistent en dibenzo- p-dioxines, dibenzofuranes et
biphényles fortement substitués par le chlore. L'analyse des
échantillons biologiques ou prélevés dans l'environnement comporte
généralement une extraction préliminaire de la prise d'essai, souvent
suivie d'une purification, après quoi les extraits organiques sont
soumis soit à une chromatographie en phase gazeuse couplée à la
spectrométrie de masse (GC/MS), soit à une chromatographie en phase
gazeuse avec détection par capture d'électrons (GC/ECD).
2. Sources d'exposition humaine et environnementale
L'hexachlorobenzène a été utilisé un temps comme fongicide pour
traiter les semences, mais il n'est plus actuellement utilisé à cet
effet dans la plupart des pays. Il continue néanmoins à être libéré
dans l'environnement à partir d'un certain nombre de sources,
notamment lors de l'épandage de pesticides organochlorés, ou encore
lorsque les sous-produits de la préparation des solvants, composés
aromatiques ou pesticides chlorés sont rejetés sans précautions,
incomplètement brûlés ou s'échappent de décharges anciennes.
3.Transport, distribution et transformation dans l'environnement
L'hexachlorobenzène est réparti dans tout l'environnement du fait
de sa mobilité et de sa persistance, même s'il se décompose lentement
dans l'air sous l'action de la lumière et dans le sol sous l'action
des microorganismes. Dans la troposphère, il est transporté sur de
grandes distances et s'élimine de l'air en se déposant sur le sol et
sur l'eau. On a fait état d'une bioamplification notable le long de la
chaîne alimentaire.
4. Concentrations dans l'environnement et exposition humaine
Un peu partout dans le monde, l'hexachlorobenzène est présent, à
distance de ses sources, sous faible concentration dans l'air ambiant
(quelques ng/m3 ou moins) ainsi que dans l'eau de boisson et les eaux
de surface (quelques ng/litre tout au plus). Cependant, au voisinage
des points d'émission, on a pu mesurer des concentrations plus
élevées. Ce composé s'accumule dans les milieux biologiques et on en a
décelé la présence chez des invertébrés, des poissons, des reptiles,
des oiseaux et des mammifères (y compris l'Homme) à distance des
points d'émission, en particulier dans les tissus adipeux des
organismes situés aux niveaux trophiques supérieurs. Chez la
population humaine de divers pays, on en a mesuré dans les tissus
adipeux des quantités qui vont, en moyenne, de quelques dizaines à
quelques centaines de ng/g de poids humide. En se fondant sur les
quantités représentatives d'hexachlorobenzène présentes dans l'air,
l'eau et les denrées alimentaires, on peut estimer à une valeur
comprise entre 0,0004 et 0,003 µg/kg de poids corporel, la dose
absorbée journalièrement par un adulte de la population générale. Cet
apport se fait principalement par la voie alimentaire. Du fait de la
présence d'hexachlorobenzène dans le lait maternel, on estime que dans
les différents pays les enfants nourris au sein en reçoivent
quotidiennement une quantité comprise entre < 0,018 et 5,1 µg/kg de
poids corporel. Les études consacrées à l'évolution de la quantité
d'hexachlorobenzène présente dans l'organisme humain montrent, pour la
plupart, que l'exposition de la population générale a baissé dans de
nombreux endroits, entre les années 70 et le milieu de la décennie
actuelle.
5. Cinétique et métabolisme chez l'Homme et les animaux de
laboratoire
On manque de données toxicocinétiques chez l'Homme.
L'hexachlorobenzène est facilement résorbé par la voie orale chez
l'animal d'expérience, mais il franchit mal la barrière cutanée (on ne
possède pas de données concernant l'inhalation). Chez l'Homme et
l'animal, il s'accumule dans les tissus riches en lipides, comme les
tissus adipeux, le cortex surrénalien, la moelle osseuse, la peau et
certains tissus endocriniens. En outre, il peut être transmis à la
progéniture par l'intermédiaire du lait maternel ou en traversant la
barrière placentaire. La métabolisation de l'hexachlorobenzène est
limitée et ses principaux métabolites urinaires sont le
pentachlorophénol, la tétrachlorhydroquinone et le
pentachlorothiophénol. La demi-vie d'élimination de
l'hexachlorobenzène va d'environ un mois chez les rats et les lapins à
2 ou 3 ans chez le singe.
6. Effets sur les animaux de laboratoire et dans les épreuves
in vitro
L'hexachlorobenzène présente une faible toxicité aiguë pour les
animaux de laboratoire (1000 à 10 000 mg/kg de poids corporel).
L'expérimentation animale montre en outre que ce composé n'est pas
irritant pour la peau ou les yeux et ne provoque pas de
sensibilisation chez le cobaye.
Les données dont on dispose au sujet de la toxicité générale de
l'hexachlorobenzène indiquent que celle-ci s'exerce notamment au
niveau de la voie de biosynthèse de l'hème. Chez plusieurs espèces de
mammifères de laboratoire exposés à de l'hexachlorobenzène, on a
constaté une élévation des concentrations de porphyrines ou de leurs
précurseurs dans les excréta ainsi que dans divers tissus, notamment
le tissu hépatique. De nombreuses études ont relevé des cas de
porphyrie chez des rats exposés de manière chronique ou subchronique à
de l'hexachlorobenzène administré par voie orale à des doses
quotidiennes comprises entre 2,5 et 15 mg par kg de poids corporel.
Chez des porcs à qui on faisait ingérer ce composé en doses
quotidiennes égales ou supérieures à 0,5 mg par kg de poids corporel,
on a observé une augmentation de l'excrétion des coproporphyrines
(aucun effet n'a été observé dans cette étude à la dose de 0,05
mg/kg). On a également montré que l'exposition à l'hexachlorobenzène
affectait de nombreux organes ou systèmes (comme le foie, les poumons,
les reins, la thyroïde, la peau ainsi que le système nerveux et le
système immunitaire) mais ces effets n'ont pas été aussi souvent
signalés que la porphyrie.
L'hexachlorobenzène est un inducteur du cytochrome P-450 de type
mixte. Il possède des propriétés phénobarbital-inductibles et
3- méthylcholantrène-inductibles. Il se fixe sur le récepteur Ah.
Lors d'études longitudinales sur des rats, on a observé à
plusieurs reprises des effets bénins (modifications
histopathologiques, induction d'enzymes) chez les animaux recevant des
doses quotidiennes comprises entre 0,25 et 0,6 mg de composé par kg de
poids corporel. La dose sans effet observable obtenue dans ces études
se situait entre 0,05 et 0,07 mg d'hexachlorobenzène par kg de poids
corporel et par jour. Chez des visons femelles, on a observé une
modification de la concentration de neurotransmetteurs dans
l'hypothalamus après administration prolongée du composé par la voie
alimentaire à la dose quotidienne de 0,16 mg par kg de poids corporel.
Les mêmes constatations ont été faites dans la progéniture de ces
animaux, qui avait été exposée pendant les périodes gestationnelle et
périnatale. Lors d'études subchroniques sur des rats on a constaté une
modification de l'homéostase calcique et des paramètres
ostéomorphométriques à la dose quotidienne de 0,7 mg/kg de poids
corporel, mais pas à celle de 0,07 mg/kg.
Un certain nombre d'études in vivo ont été effectuées sur des
rongeurs afin de mettre en évidence la cancérogénicité éventuelle de
l'hexachlorobenzène. Chez des hamsters qui avaient reçu une nourriture
contenant de l'hexachlorobenzène à la dose moyenne de 4, 8 ou 16 mg/kg
de poids corporel, on a observé chez les deux sexes et à toutes les
doses un accroissement de l'incidence des carcinomes
hépatocellulaires. Aux doses de 8 et 16 mg/kg, on constatait la
présence d'hémangioendothéliomes et à la dose la plus forte,
d'adénomes de la thyroïde chez les mâles. En exposant pendant 120
semaines des souris à ce composé par la voie alimentaire aux doses
quotidiennes respectives de 6, 12 et 24 mg/kg de poids corporel, on a
provoqué un accroissement de l'incidence des carcinomes
hépatocellulaires chez les deux sexes aux deux doses les plus élevées,
mais cet accroissement n'était pas significatif, sauf chez les
femelles exposées à la dose la plus forte. In utero, l'exposition de
rats par la voie orale ou lactationnelle à des doses alimentaires
quotidiennes d'hexachlorobenzène allant de 0,01 à 1,5 mg/kg de poids
corporel (mâles) ou de 1,9 mg/kg (femelles) pendant des périodes
pouvant durer jusqu'à 130 semaines post utero, c'est-à-dire la durée
de vie moyenne, a entraîné à la dose la plus forte un accroissement de
l'incidence des nodules hépatiques néoplasiques et des
phéochromocytomes surrénaliens chez les femelles et un excès
d'adénomes parathyroïdiens chez les mâles. Lors d'une autre étude
chronique effectuée sur des rats, on a exposé les animaux, par la voie
alimentaire et pendant des durées allant jusqu'à 2 ans, à des doses
journalières moyennes de 4-5 et 8-9 mg/kg de poids corporel. Les
effets constatés consistaient en une augmentation de l'incidence des
hépatomes et des adénomes rénaux aux deux doses et chez les deux
sexes. Chez les femelles, on observait en outre une augmentation de
l'incidence des carcinomes hépatocellulaires, des adénomes et des
carcinomes des voies biliaires, des phéochromocytomes et des adénomes
du cortex surrénalien. On a également signalé une incidence élevée des
tumeurs du foie dans un certain nombre d'études plus limitées au cours
desquelles on avait administré une seule dose d'hexachlorobenzène par
la voie alimentaire à de petits groupes de rates. Par ailleurs, on a
observé qu'après exposition subchronique par voie alimentaire à ce
composé, des souris, des hamsters et des rats avaient présenté des
tumeurs du foie, des voies biliaires, du rein, du thymus, de la rate
et des ganglions lymphatiques. Le même type d'exposition favorise
l'apparition de tumeurs hépatiques chez des souris sous l'action de
terphényles polychlorés et chez des rats, sous l'action de la
diéthylnitrosamine.
Sauf dans le cas des tumeurs rénales chez les rats mâles (qui, du
moins en partie, semblent résulter d'une dégénérescence hyaline) et
des hépatomes chez les rats des deux sexes (qui pourraient résulter de
réactions hyperplasiques à la nécrose hépatocellulaire), on n'a pas pu
trouver d'études mécanistiques concernant les divers types de tumeurs
provoquées par l'hexachlorobenzène et le risque encouru à cet égard
par l'Homme.
L'hexachlorobenzène n'a guère d'aptitude à provoquer directement
des mutations géniques, des lésions chromosomiques ou la réparation de
l'ADN. Il s'est révélé faiblement mutagène lors de quelques-unes des
études portant sur des bactéries et des levures, mais il convient de
noter que chacune de ces études comportait des limitations. Il y a
également des signes d'un faible taux de liaison à l'ADN in vitro et
in vivo, mais dans une proportion très inférieure à celle que l'on
attendrait d'une substance cancérogène génotoxique.
Lors d'études sur la reproduction, des doses d'hexachlorobenzène
ne dépassant pas 0,1 mg par kg de poids corporel qu'on avait fait
ingérer quotidiennement pendant 90 jours à des singes, ont provoqué
des anomalies dans la structure microscopique et l'ultrastructure de
l'épithélium germinatif superficiel, structures qui constituent une
cible inhabituelle pour des toxines ovariennes. Cette dose a également
endommagé l'ultrastructure des cellules germinales primordiales. Alors
même que ces sites étaient spécifiquement attaqués et présentaient des
lésions d'autant plus importantes que la dose était plus forte, le
développement folliculaire, ovocytaire et embryonnaire restait normal,
ce qui semble indiquer que l'hexachlorobenzène a un site d'action à
localisation spécifiquement ovarienne. Chez les mâles, la fonction de
reproduction n'est affectée qu'à des doses beaucoup plus élevées
(entre 30 et 221 mg/kg p.c. par jour), comme l'ont montré un certain
nombre d'études effectuées sur plusieurs espèces n'appartenant pas à
l'ordre des primates.
Des rats et des chats exposés par la voie transplacentaire ou
lactationnelle à des doses quotidiennes d'hexachlorobenzène comprises
entre 3 et 4 mg/kg p.c. ont présenté des signes d'hépatotoxicité et on
a également constaté des effets délétères sur la survie et la
croissance de leur progéniture. Dans certains cas, il y avait à ces
doses - ou à des doses plus élevées - une réduction de l'effectif des
portées et un nombre accru de mortinaissances. (En général, les ratons
et les chatons à la mamelle étaient plus souvent affectés - et à des
doses plus faibles - que les embryons et les foetus). Chez la
progéniture de visons qui recevaient une alimentation ne contenant pas
plus de 1 mg d'hexachlorobenzène par kg de nourriture (soit environ
0,16 mg/kg p.c. par jour), on a constaté une réduction du poids de
naissance et un accroissement de la mortalité au sevrage. Quelques
études ont mis en évidence des anomalies squelettiques ou rénales chez
les foetus de rats et de souris exposés à de l'hexachlorobenzène
pendant la gestation, mais les doses qui produisaient ces anomalies
n'étaient pas toxiques pour les mères. Par ailleurs, le lien de ces
anomalies avec la prise d'hexachlorobenzène n'a pas été formellement
établi. Dans deux études, dont l'une comportait une exposition
transplacentaire et postnatale, on a observé des anomalies du
développement neurocomportemental des ratons après exposition
in utero, les mères ayant reçu par voie orale des doses quotidiennes
d'hexachlorobenzène allant de 0,64 à 2,5 mg/kg de poids corporel.
Selon un certain nombre d'études, l'hexachlorobenzène aurait des
effets délétères sur le système immunitaire. Chez des rats et des
singes exposés à des doses quotidiennes comprises entre 3 et 120 mg
d'hexachlorobenzène par kg de poids corporel, on a constaté des
modifications histopathologiques au niveau du thymus, de la rate, des
ganglions lymphatiques et des tissus lymphoïdes pulmonaires. Chez des
chiens beagle exposés de façon chronique à des doses quotidiennes
correspondant à 0,12 mg de composé par kg p.c., on a observé une
hyperplasie nodulaire du tissu lymphoïde gastrique. Un certain nombre
d'études menées sur des rats ont montré qu'après plusieurs semaines
d'exposition à de l'hexachlorobenzène par la voie alimentaire, il y
avait stimulation de l'immunité humorale, et dans une moindre mesure,
de l'immunité à médiation cellulaire, sans modification de la fonction
des macrophages. A des doses quotidiennes ne dépassant pas 4 mg de
composé par kg de nourriture (environ 0,2 mg par kg p.c.),
administrées pendant la gestation, pendant le maternage et jusqu'à
l'âge de 5 semaines, il y a eu augmentation de la réponse immunitaire
à médiation cellulaire et de la réponse immunitaire humorale ainsi
qu'une accumulation de macrophages dans le tissu pulmonaire des
ratons. Par contre, la plupart des études effectuées sur des souris
ont fait ressortir les propriétés immunosuppressives de
l'hexachlorobenzène; des doses ne dépassant pas 0,5 à 0,6 mg/kg de
poids corporel administrées quotidiennement pendant plusieurs semaines
ont eu les effets suivants: diminution de la résistance à une
infection leishmanienne ou à une épreuve cancérogène par exposition à
des cellules tumorales, réduction de l'activité cytotoxique des
macrophages spléniques et de l'hypersensibilité retardée chez la
progéniture après exposition in utero ou pendant la période de
maternage. Lors d'un certain nombre d'études portant sur diverses
souches de rats, on a constaté qu'une exposition de brève durée ou une
exposition subchronique à de l'hexachlorobenzène modifiait la fonction
thyroïdienne, comme on pouvait en juger d'après la réduction de la
thyroxine sérique libre ou totale (T4) et souvent, mais dans une
moindre mesure, de la triiodothyronine (T3).
7. Effets sur l'Homme
La plupart des données que l'on possède au sujet des effets de
l'hexachlorobenzène sur l'Homme, proviennent d'intoxications
accidentelles qui se sont produites en Turquie en 1955-59, avec plus
de 600 cas répertoriés de porphyrie cutanée tardive. Lors de cet
accident, on a observé des troubles du métabolisme des porphyrines,
des lésions cutanées, des hyperpigmentations, des hypertrichoses, des
hépatomégalies, des hypertrophies de la thyroïde et des ganglions
lymphatiques, avec, dans environ la moitié des cas, une ostéoporose et
une arthrite, principalement d'ailleurs, chez les enfants. Les enfants
nourris au sein dont la mère avait été exposée, présentaient des
lésions appelées pembe yara, c'est-à-dire "lésions roses", et la
plupart d'entre eux sont décédés dans l'année. On dispose de quelques
données concernant des cas de porphyrie cutanée tardive chez des
personnes ayant subi une exposition relativement intense à
l'hexachlorobenzène sur leur lieu de travail ou dans leur
environnement général.
Les quelques études épidémiologiques disponibles concernant le
cancer souffrent d'un certain nombre d'insuffisances: effectif réduit,
exposition à l'hexachlorobenzène mal caractérisée ou exposition
simultanée à de nombreux autres agents, et ne permettent pas d'évaluer
la cancérogénicité de ce composé pour l'Homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Lors d'études sur la toxicité aiguë de l'hexachlorobenzène pour
les organismes aquatiques, on a constaté que l'exposition à des
concentrations de l'ordre de 1 à 17 µg/litre réduisait la production
de chlorophylle chez les algues ainsi que la reproduction chez les
ciliés, et qu'en outre, elle provoquait la mort des crevettes roses et
des crevettes américaines du genre Hippolyte, mais elle n'a pas
provoqué la mort de poissons d'eau douce ou de mer. Lors d'études à
plus long terme, on a constaté que la croissance de certaines algues
et protozoaires dulçaquicoles sensibles étaient affectée à une
concentration d'hexachlorobenzène de 1 µg/litre et que des
concentrations d'environ 3 µg/litre provoquaient la mort d'amphipodes
et de perches appartenant à l'espèce Micropterus salmoides.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
9.1 Effets sur la santé
Le Groupe de travail a conclu que les données disponibles sont
suffisantes pour que l'on puisse formuler des valeurs-guides relatives
aux effets cancérogènes et non cancérogènes de l'hexachlorobenzène.
En ce qui concerne les effets non cancérogènes constatés sur le
foie à dose élevée chez des porcs et des rats exposés par la voie
orale et en se basant sur la dose sans effet observable la plus faible
(0,05 mg/kg de poids corporel par jour), on arrive, compte tenu d'un
facteur d'incertitude de 300 (10× pour les variations
interspécifiques, 10x pour les variations intraspécifiques et 3× pour
la gravité de l'effet), à une TDI de 0,17 µg/kg de poids corporel.
La méthode utilisée pour déterminer la valeur-guide relative aux
effets cancérogènes repose sur la dose tumorigène TD5, c'est-à-dire
la dose ingérée qui provoque une augmentation de 5% de l'incidence
tumorale chez les animaux de laboratoire. D'après les résultats d'une
étude de cancérogénicité portant sur deux générations de rats et en
faisant appel à un modèle multiphasique, on obtient une TD5 de
0,81 mg/kg de poids corporel par jour, l'effet retenu étant la
formation de nodules cancéreux hépatiques chez les femelles. Compte
tenu de l'insuffisance des données mécanistiques, on a appliqué un
facteur d'incertitude de 5000 pour calculer la valeur-guide chiffrée à
0,16 µg/kg de poids corporel par jour.
9.2 Effets sur l'environnement
Le Groupe de travail a remarqué qu'il existe très peu d'études
expérimentales à partir desquelles on puisse procéder à une évaluation
du risque écologique. La concentration d'hexachlorobenzène dans les
eaux de surface est généralement inférieure de plusieurs ordres de
grandeur à celle qui pourrait être dangereuse pour les organismes
aquatiques, sauf dans certains endroits fortement pollués. Toutefois,
les concentrations d'hexachlorobenzène relevées dans les oeufs
d'oiseaux de mer et de rapaces en différents lieux du globe sont
proches de celles qui provoquent une diminution du poids des embryons
chez la mouette argentée (1500 µg/kg), ce qui incite à penser que le
composé pourrait être embryotoxique pour certaines espèces sensibles
d'oiseaux. De même, les concentrations d'hexachlorobenzène dans les
poissons de divers endroits du monde sont du même ordre de grandeur
que la dose de 1000 µg/kg qui entraîne une réduction du poids de
naissance et une augmentation de la mortalité chez la progéniture de
visons. Cela incite à penser que ce composé pourrait avoir des effets
indésirables chez les visons et éventuellement chez d'autres
mammifères piscivores.
10. Conclusions
a) L'hexachlorobenzène est un composé chimique persistant qui subit
une bioaccumulation du fait de sa liposolubilité et de sa résistance à
la décomposition.
b) L'expérimentation animale montre que l'hexachlorobenzène provoque
des cancers et affecte de nombreux organes, tissus et systèmes comme
le foie, les poumons, les reins, la thyroïde, les tissus des gonades,
le système nerveux et le système immunitaire.
c) En ce qui concerne l'Homme, on a pu observer, à l'occasion d'une
forte exposition d'origine accidentelle, les manifestations cliniques
d'une intoxication par l'hexachlorobenzène qui se traduisaient par une
porphyrie cutanée tardive chez les enfants et les adultes et par la
mort chez des nourrissons alimentés au sein.
d) Il est justifié de prendre diverses mesures pour réduire la
quantité d'hexachlorobenzène présente dans l'environnement.
e) On a proposé les valeurs-guides à visée sanitaire suivantes pour
la dose totale ingérée quotidiennement (TDI): en ce qui concerne les
effets non cancérogènes, 0,17 µg/kg de poids corporel par jour ; en ce
qui concerne les effets cancérogènes, 0,16 µg/kg de poids corporel par
jour.
1. RÉSUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El hexaclorobenceno (HCB) es un compuesto orgánico clorado de
volatilidad moderada. Es prácticamente insoluble en el agua, pero es
muy liposoluble y bioacumulativo. El HCB de calidad técnica contiene
hasta un 2% de impurezas, en su mayor parte pentaclorobenceno; el
resto incluye dibenzo- p-dioxinas, dibenzofuranos y bifenilos
altamente clorados. Para determinar el HCB en el medio ambiente y en
material biológico se procede por lo general a extraer la muestra
mediante disolventes orgánicos, a lo que sigue con frecuencia un paso
de limpieza, a fin de obtener extractos orgánicos analizables mediante
cromatografía de gases/espectrometría de masas (GC/MS) o cromatografía
de gases con detección de captura de electrones (GC/ECD).
2. Fuentes de exposición humana y ambiental
Hubo un tiempo en que el HCB se utilizó mucho en la limpieza de
semillas para prevenir las enfermedades micóticas de los cereales,
pero ese uso se abandonó en la mayor parte de los países en los años
setenta. El HCB se sigue liberando en el medio ambiente a partir de
diversas fuentes, que incluyen el uso de algunos plaguicidas clorados,
procesos de combustión incompleta y viejos vertederos, así como los
métodos inapropiados de producción y de eliminación de desechos en la
fabricación de disolventes clorados, compuestos aromáticos clorados y
plaguicidas clorados.
3. Transporte, distribución y transformación en el medio ambiente
El HCB se distribuye por todo el medio ambiente porque es móvil y
persistente, aunque se produce una lenta fotodegradación en el aire y
una degradación microbiana en el suelo. En la troposfera el HCB es
transportado a grandes distancias y es eliminado de la fase aérea por
su depósito en el agua y el suelo. Se ha informado de que se produce
una importante bioamplificación del HCB a través de la cadena trófica.
4. Niveles ambientales y exposición humana
Se encuentran concentraciones bajas de HCB en el aire ambiental
(a lo sumo unos pocos ng/m3), en el agua de bebida y en las aguas
superficiales (a lo sumo unos pocos ng/litro) de zonas alejadas del
punto emisor en todo el mundo. No obstante, se han hallado
concentraciones más altas cerca de los puntos emisores. El HCB es
bioacumulativo y se ha detectado en invertebrados, peces, reptiles,
aves y mamíferos (incluido el hombre) lejos de los puntos emisores,
particularmente en el tejido adiposo de organismos de los niveles
tróficos más altos. Los niveles medios en el tejido adiposo de la
población humana general en diversos países van de decenas a centenas
de ng/g de peso en fresco. Considerando los niveles representativos de
HCB en el aire, el agua y los alimentos, se estima que la ingesta
total de HCB por los adultos de la población general está comprendida
entre 0,0004 y 0,003 mg/kg de peso corporal al día. Esa ingesta se
realiza principalmente a través de los alimentos. Debido a la
presencia de HCB en la leche materna, se ha estimado que la ingesta
media por los lactantes alimentados al pecho en diversos países va de
< 0,018 a 5,1 mg/kg de peso corporal al día. Los resultados de la
mayoría de los estudios realizados acerca de las concentraciones de
HCB en los alimentos y en los tejidos humanos a lo largo del tiempo
indican que la exposición de la población general al HCB disminuyó
desde los años setenta hasta mediados de los noventa en muchos
lugares. Sin embargo, esa tendencia no se ha confirmado con claridad
durante el último decenio en otros lugares.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay suficientes datos sobre la toxicocinética en el hombre. El
HCB es absorbido rápidamente por vía oral por los animales de
experimentación, y escasamente a través de la piel (no existen datos
sobre la inhalación). En los animales y en los seres humanos, el HCB
se acumula en los tejidos ricos en lípidos, como el tejido adiposo, la
corteza suprarrenal, la médula ósea, la piel y algunos tejidos
endocrinos, y puede transmitirse a la descendencia a través tanto de
la placenta como de la leche materna. El HCB sufre un metabolismo
limitado, generando pentaclorofenol, tetraclorohidroquinona y
pentaclorotiofenol como principales metabolitos en la orina. Las
semividas de eliminación del HCB están comprendidas entre
aproximadamente un mes en la rata y el conejo y 2 ó 3 años en el mono.
6. Efectos en animales de laboratorio y en las pruebas in vitro
La toxicidad aguda del HCB en los animales de experimentación es
baja (1000 × 10 000 mg/kg de peso corporal). En los estudios con
animales, el HCB no causa irritación cutánea ni ocular y no tiene
efectos de sensibilización en el cobayo.
Los datos disponibles acerca de la toxicidad sistémica del HCB
indican que las vías de la biosíntesis del grupo hemo son una
importante diana de la toxicidad del hexaclorobenceno. Se han hallado
niveles elevados de porfirinas o de precursores de la porfirina, o de
ambas cosas, en el hígado, en otros tejidos y en las excretas de
varias especies de mamíferos de laboratorio expuestos al HCB. Se ha
informado de la aparición de porfiria en varios estudios realizados
con ratas expuestas por vía oral crónica o subcrónica a dosis entre
2,5 y 15 mg de HCB/kg de peso corporal al día. La excreción de
coproporfirinas aumentó en cerdos que ingirieron 0,5 mg de HCB/kg de
peso corporal al día o más (en el último estudio no se observó ningún
efecto con 0,05 mg de HCB/kg de peso corporal al día). Se ha visto
también que la exposición repetida al HCB afecta a una amplia gama de
sistemas orgánicos (entre ellos el hígado, los pulmones, los riñones,
la tiroides, la piel y los sistemas nervioso e inmunitario), aunque
las referencias a estos efectos son menos frecuentes que las
relacionadas con la porfiria.
El HCB es un inductor de tipo mixto del citocromo P-450, con
propiedades inducibles por el fenobarbital y por el 3-metilcolantreno.
Se sabe que se une al receptor Ah.
Por lo que se refiere a los estudios crónicos, en ratas expuestas
a dosis de 0,25 a 0,6 mg de HCB/kg peso corporal al día se observaron
efectos leves en el hígado (cambios histopatológicos, inducción
enzimática); en dichos estudios los NOEL estaban comprendidos entre
0,05 y 0,07 mg de HCB/kg de peso corporal al día. Las concentraciones
de neurotransmisores en el hipotálamo se vieron alteradas en visones
hembra sometidos a través de los alimentos a una exposición crónica de
0,16 mg de HCB/kg de peso corporal al día, y en su descendencia
expuesta a lo largo de la gestación y la lactancia. En estudios
subcrónicos realizados en ratas la homeostasis del calcio y la
morfometría ósea se vieron afectadas con 0,7 mg de HCB/kg de peso
corporal al día, pero no con 0,07 mg/kg de peso corporal al día.
La carcinogenicidad del HCB ha sido evaluada mediante varios
bioensayos realizados con roedores. En hámsters mantenidos con
alimentos con los que ingerían unas dosis medias de 4, 8 ó 16 mg/kg de
peso corporal al día durante toda la vida, se produjeron aumentos en
la incidencia de tumores de las células del hígado (hepatomas) en los
dos sexos y a todas las dosis, hemangioendoteliomas hepáticos a dosis
de 8-16 mg/kg de peso corporal al día, y adenomas tiroideos de los
machos a la dosis mayor. La exposición alimentaria de ratones a dosis
de 6, 12 y 24 mg/kg de peso corporal al día durante 120 semanas dio
lugar a un aumento de la incidencia de tumores de las células del
hígado (hepatomas) en ambos sexos a las dos dosis mayores (no
significativo, excepto para las hembras a la dosis mayor). En ratas,
la exposición in útero, durante la lactancia y por vía oral al HCB a
través de alimentos que proporcionaban a lo largo de su vida dosis
medias comprendidas entre 0,01 y 1,5 mg/kg de peso corporal al día
(machos) o 1,9 mg/kg de peso corporal al día (hembras) por espacio de
hasta 130 semanas post útero produjo a la mayor de las dosis un
aumento de la incidencia de nódulos hepáticos neoplásicos y de
feocromocitomas suprarrenales en las hembras y de adenomas
paratiroideos en los machos. En otro estudio crónico realizado en la
rata, la exposición por un periodo de hasta dos años a alimentos que
proporcionaban dosis medias de HCB de 4-5 y de 8-9 mg/kg de peso
corporal al día indujo aumentos de la incidencia de hepatomas y de
adenomas de las células renales a ambas dosis en los dos sexos, y de
carcinomas hepatocelulares, adenomas y carcinomas de las vías
biliares, y feocromocitomas suprarrenales y adenomas de la corteza
suprarrenal en las hembras. Se ha informado también de incidencias
elevadas de tumores hepáticos en algunos estudios más limitados en los
que se administraron concentraciones alimentarias únicas a grupos
reducidos de ratas hembra. Además, se ha informado de que, después de
una exposición alimentaria subcrónica al HCB, ratones, hámsters y
ratas desarrollaron tumores en el hígado, las vías biliares, el riñón,
el timo, el bazo y los ganglios linfáticos. La exposición alimentaria
al HCB favoreció la inducción de tumores hepáticos por el terfenilo
policlorado en el ratón y por la dietilnitrosamina en la rata.
Con excepción de los tumores renales en la rata macho
(aparentemente debidos, al menos en parte, a una nefropatía por
acumulación de gotas hialinas) y de los hepatomas en la rata (posible
resultado de la respuesta hiperplásica a una necrosis hepatocelular),
no se conocen estudios mecanísticos que hayan determinado el
significado del tipo de tumores inducidos por el HCB en el caso del
hombre.
El HCB tiene una escasa capacidad de inducción directa de
mutaciones de los genes, lesiones cromosómicas y reparaciones del ADN.
Mostró una leve actividad mutágena en un reducido número de los
estudios realizados en bacterias y levaduras, aunque hay que señalar
que todos esos estudios presentan limitaciones. Existen también
algunos indicios de un cierto grado de unión al ADN in vitro e
in vivo, aunque a niveles muy inferiores a los habituales en los
carcinógenos genotóxicos.
En estudios sobre la reproducción, la exposición oral de monos a
tan sólo 0,1 mg de HCB/kg de peso corporal al día durante 90 días
afectó a la estructura revelada por microscopia óptica y a la
ultraestructura del epitelio germinal superficial, una diana poco
usual para las toxinas que afectan al ovario. Dicha dosis causó
también daños ultraestructurales en las células germinales
primordiales. Estos cambios específicos en tejidos-diana, para los que
dosis mayores son aún más lesivas, se asocian por lo demás a un
desarrollo normal del folículo, el ovocito y el embrión, lo que indica
que el HCB tiene una acción específica en el ovario. La reproducción
masculina sólo se vio afectada a dosis mucho mayores (entre 30 y
221 mg/kg de peso corporal al día) en estudios realizados en varias
especies distintas de los primates.
La exposición de ratas y gatos, a través de la placenta o durante
la lactancia, a dosis maternas comprendidas entre 3 y 4 mg/kg de peso
corporal al día tuvo efectos hepatotóxicos o afectó a la supervivencia
o el crecimiento de la descendencia en período de lactancia. En
algunos casos, dosis iguales o superiores a ésas redujeron el tamaño
de las camadas o aumentaron el número de abortos. (Los efectos nocivos
en los cachorros sin destetar han sido observados más frecuentemente,
y a dosis menores, que los efectos embriotóxicos o fetotóxicos.) La
descendencia de visones expuestos crónicamente a sólo 1 mg de HCB/kg
de alimento (aproximadamente 0,16 mg/kg de peso corporal al día) tuvo
un peso reducido al nacer y presentó una mayor mortalidad hasta el
destete. A pesar de que se han observado trastornos esqueléticos y
renales de los fetos en algunos estudios realizados en ratas y ratones
expuestos al HCB durante la gestación, dichas alteraciones o bien no
estaban claramente relacionadas con el tratamiento o bien ocurrieron a
dosis que eran también tóxicas para las madres. En dos estudios, uno
de los cuales incluía exposición posnatal y durante la lactancia, el
desarrollo neurocomportamental de las crías de rata se vio afectado
por la exposición in útero a dosis maternas orales de 0,64 a 2,5 mg
de HCB/kg de peso corporal al día.
Los resultados de varios estudios indican que el HCB afecta al
sistema inmunitario. Ratas y monos expuestos a dosis entre 3 y 120 mg
de HCB/kg de peso corporal al día sufrieron alteraciones
histopatológicas en el timo, en el bazo y en los ganglios linfáticos o
los tejidos linfoides del pulmón. La exposición crónica de perros
sabuesos a 0,12 mg/kg de peso corporal al día produjo una hiperplasia
nodular del tejido linfoide gástrico. En varios estudios realizados en
la rata, la inmunidad humoral y, en menor grado, la celular se vieron
potenciadas tras varias semanas de exposición alimentaria al HCB,
mientras que la función de los macrófagos no se alteró. Una cantidad
tan pequeña como 4 mg de HCB/kg de alimento (aproximadamente 0,2 mg/kg
de peso corporal al día) durante la gestación, a lo largo de la
lactancia y hasta las 5 semanas de edad incrementó las respuestas
inmunitarias humoral y celular y provocó la acumulación de macrófagos
en el tejido pulmonar de crías de rata. Por el contrario, se ha
observado un efecto inmunodepresor del HCB en la mayor parte de los
estudios llevados a cabo con ratones; dosis de sólo 0,5-0,6 mg/kg de
peso corporal al día durante varias semanas redujeron la resistencia a
la infección por Leishmania o a una provocación con células
tumorales, disminuyeron la actividad citotóxica de los macrófagos del
bazo, y redujeron la respuesta de hipersensibilidad de tipo retardado
en la descendencia expuesta in útero y durante la lactancia. En
varios estudios realizados con diversas cepas de ratas, la exposición
de breve duración o subcrónica al HCB afectó a la función tiroidea, a
juzgar por los reducidos niveles séricos de tiroxina total y tiroxina
libre (T4) y a menudo, en menor grado, de triyodotironina (T3).
7. Efectos en el ser humano
La mayor parte de los datos acerca de los efectos del HCB en el
ser humano provienen de intoxicaciones accidentales que tuvieron lugar
en Turquía en los años 1955-1959, entre las que se identificaron más
de 600 casos de porfiria cutánea tardía (PCT). En esa ocasión se
observaron alteraciones en el metabolismo de la porfirina, lesiones
dermatológicas, hiperpigmentación, hipertricosis, aumento del tamaño
del hígado, de la glándula tiroides y de los ganglios linfáticos; se
observaron también (aproximadamente en la mitad de los casos)
osteoporosis o artritis, sobre todo en los niños. Los niños
amamantados por madres expuestas al HCB como consecuencia de ese
accidente desarrollaron un trastorno conocido como pembe yara
(ulceración rosada), y la mayor parte murieron antes de un año.
Existen también algunos indicios de que la PCT afecta a personas
sometidas a una exposición relativamente alta al HCB en el lugar de
trabajo o en el medio ambiente general.
Los pocos estudios epidemiológicos disponibles acerca de la
incidencia de cáncer tienen un valor limitado, ya sea por lo reducido
de la muestra, por la deficiente caracterización de la exposición al
CHB o por la exposición a otros muchos agentes, y son insuficientes
para evaluar la carcinogenicidad del HCB para el ser humano.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
En los estudios realizados sobre la toxicidad aguda del HCB para
los organismos acuáticos, la exposición a concentraciones comprendidas
entre 1 y 17 mg/litro redujo la producción de clorofila en algas y la
reproducción de protozoos ciliados, y causó mortalidad en el camarón
rosado y en las quisquillas, pero no aumentó la mortalidad de peces de
agua dulce o de mar. En estudios a largo plazo, el crecimiento de
algas y protozoos vulnerables de agua dulce se vio afectado por una
concentración de 1 mg/litro, mientras que concentraciones de
aproximadamente 3 mg/litro provocaron mortalidad en anfípodos y
necrosis hepática en la perca americana.
9. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
9.1 Efectos en la salud
El Grupo Especial llegó a la conclusión de que los datos
disponibles son suficientes para establecer valores indicativos
respecto a los efectos neoplásicos y no neoplásicos del HCB.
En cuanto a los efectos no neoplásicos, considerando el NOEL más
bajo notificado (0,05 mg de HCB/kg de peso corporal al día), referido
sobre todo a los efectos hepáticos observados a dosis mayores en
estudios realizados en cerdos y ratas expuestos por vía oral, e
incorporando un factor de incertidumbre de 300 (× 10 en concepto de
variación interespecíes, × 10 en concepto de variación intraespecie, y
× 3 en concepto de gravedad del efecto), se ha calculado una IDT de
0,17 mg/kg de peso corporal al día.
El criterio seguido en cuanto a los efectos neoplásicos se basa
en la dosis tumorigénica TD5, es decir, la ingesta asociada a un
exceso del 5% en la incidencia de tumores detectada en los
experimentos con animales. Considerando los resultados del bioensayo
de carcinogenicidad en dos generaciones de ratas, y empleando el
modelo polietápico, la TD5 es de 0,81 mg/kg de peso corporal al día
para los nódulos neoplásicos del hígado en las hembras. Habida cuenta
de la insuficiencia de los datos mecanísticos, se utilizó un factor de
incertidumbre de 5000 para establecer un valor indicativo, basado en
criterios de salud, de 0,16 mg/kg de peso corporal al día.
9.2 Efectos en el medio ambiente
El Grupo Especial señaló que existen muy pocos estudios
experimentales con los que llevar a cabo una evaluación de los riesgos
para el medio ambiente. Los niveles de HCB en las aguas superficiales,
excepto en unos pocos lugares extremadamente contaminados, son en
general varios órdenes de magnitud inferiores a los que se supone que
entrañan riesgos para los organismos acuáticos. No obstante, las
concentraciones de HCB en los huevos de las aves marinas y las rapaces
de algunos lugares en distintas zonas del mundo se aproximan a niveles
que en la gaviota argéntea se asocian a una disminución del peso del
embrión (1500 mg/kg), lo que parece indicar que el HCB puede dañar los
embriones de especies de aves vulnerables. Del mismo modo, los niveles
de HCB observados en peces de diversos lugares del mundo se encuentran
a un orden de magnitud del nivel alimentario de 1000 mg/kg, asociado a
una reducción del peso al nacer y a un aumento de la mortalidad de la
descendencia en los visones. Esto parece indicar que el HCB puede
tener efectos nocivos en los visones y, tal vez, en otros mamíferos
que se alimentan de peces.
10. Conclusiones
a) El HCB es un producto químico persistente que se bioacumula debido
a su liposolubilidad y a su resistencia a la degradación.
b) Los estudios realizados con animales han demostrado que el HCB
produce cáncer y afecta a una amplia gama de sistemas de órganos, con
inclusión del hígado, los pulmones, los riñones, la tiroides, los
tejidos reproductivos y los sistemas nervioso e inmunitario.
c) En seres humanos sometidos a una alta exposición accidental se ha
observado toxicidad sintomática, en particular porfiria cutánea tardía
en niños y en adultos y mortalidad en lactantes.
d) Es necesario adoptar diversas medidas para reducir la carga
ambiental de HCB.
e) Se han propuesto los siguientes valores indicativos basados en
criterios de salud para la ingesta diaria total (IDT) de HCB por el
ser humano: efectos no cancerígenos, 0,17 µg/kg de peso corporal/día;
yefectos neoplásicos, 0,16 µ/kg de peso corporal/día.
See Also:
Toxicological Abbreviations
Hexachlorobenzene (HSG 107, 1998)
Hexachlorobenzene (ICSC)
Hexachlorobenzene (PIM 256)
Hexachlorobenzene (FAO/PL:1969/M/17/1)
Hexachlorobenzene (WHO Pesticide Residues Series 4)
Hexachlorobenzene (IARC Summary & Evaluation, Supplement7, 1987)
Hexachlorobenzene (IARC Summary & Evaluation, Volume 20, 1979)
Hexachlorobenzene (IARC Summary & Evaluation, Volume 79, 2001)
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
Members
Dr D. Arnold, Health Canada, Tunney's Pasture, Ottawa, Ontario Canada
Dr A. Göcmen, Department of Pediatrics, Faculty of Medicine,
Hacettepe University, Hacettepe, Ankara, Turkey
Professor B. Jansson, Institute of Applied Environmental Research,
ITM-Solna, Stockholm University, Stockholm, Sweden
Dr J. Jarrell, Foothills Hospital, Calgary Regional Health
Authority, Calgary, Alberta, Canada
Dr A. Langley, South Australian Health Commission, Rundle Mall,
Australia ( Chairman)
Mr R. Newhook, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Rapporteur)
Dr D. Peakall, Wimbledon, London, United Kingdom
( Vice-chairman)
Dr A.G. Smith, Medical Research Council Toxicology Unit,
Hodgkin Building, University of Leicester, Leicester,
United Kingdom
Dr J. Sunyer, Department of Epidemiology and Public Health,
Institut Municipal d'Investigacio Medica, Barcelona, Spain
Dr A. van Birgelen, National Health and Environmental Effects
Research Laboratory, Pharmacokinetics Branch, US Environmental
Protection Agency, Research Triangle Park, North Carolina, USAa
Dr J. Vos, National Institute of Public Health and the Environment
(RIVM), Hygiene, Bilthoven, The Netherlands
a Dr A. Van Birgelen's present address: National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Observers
Dr J. de Gerlache, Solvay SA, Department of Chemical Safety and
Toxicology, Brussels, Belgium (Representing EURO CHLOR)
Dr Roger Drew, Toxicology Information Section, Safety, Health &
Environment Division, ICI Australia Operations Pty Ltd., ICI
House, Melbourne, Victoria, Australia (Representing European
Centre for Ecotoxicology and Toxicology of Chemicals)
Secretariat
Dr G.C. Becking, Interregional Research Unit, International
Programme on Chemical Safety, Research Triangle Park, North
Carolina USA ( Secretary)
Ms W. Dormer, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Temporary Adviser to Secretariat)
Dr J. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
A WHO Task Group on Environmental Health Criteria for
Hexachlorobenzene met in Geneva from 26 February to 1 March 1996.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft and made an
evaluation of the risks for human health and the environment from
exposure to hexachlorobenzene.
The first draft was prepared by Mr R. Newhook and Ms W. Dormer,
Health Canada, Ottawa, Canada. These authors also prepared the draft
reviewed by the Task Group, which incorporated the comments received
following circulation of the first draft to IPCS Contact Points for
Environmental Health Criteria monographs.
The IPCS gratefully acknowledges the financial and other support
of the Health Protection Branch, Health Canada. This support was
indispensable for the completion of this monograph.
Dr G.C. Becking (IPCS, Central Unit, Inter-regional Research
Unit) and Dr P.G. Jenkins (IPCS, Central Unit, Geneva) were
responsible for the overall scientific content and the technical
editing, respectively, of this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BMF biomagnification factor
DL detection limit
HCB hexachlorobenzene
i.p. intraperitoneal
ND not detectable
PCT porphyria cutanea tarda
p,p'DDE 1,1'-(2,2-dichloroethylidene)-bis[4-chlorobenzene]
SER smooth endoplasmic reticulum
T3 triiodothyronine
T4 thyroxine
PREFACE
The preparation of comprehensive Environmental Health Criteria
(EHC), as outlined in the Preamble of this monograph, is an extremely
time-consuming and resource-intensive procedure. Often countries have
prepared recent comprehensive reviews on chemicals as required by
their national legislation, and the International Programme on
Chemical Safety (IPCS) has been asked by Member States to determine
how best to utilize such national reviews during the preparation of
international EHC. Utilizing such national documents should avoid
duplication of effort and result in the more rapid production of more
concise IPCS EHC monographs.
This monograph on hexachlorobenzene has been prepared using as
background document the review (Supporting Document) prepared under
the Canadian Environmental Protection Act (CEPA), dated June 1993.
From this document, staff of Health Canada have chosen only the most
relevant studies for assessing the human and environmental risks from
exposure to hexachlorobenzene. These have been described from the
original references and supplemented by additional information
published more recently. This has resulted in a concise monograph,
yet one that supplies sufficient information for the reader to
understand the basis for the conclusions reached by the Task Group.
Readers who wish to consult the text of the Canadian Supporting
Document can obtain a copy from the Director, IPCS, World Health
Organization, Geneva, Switzerland.
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, and
analytical methods
Hexachlorobenzene (HCB) is a chlorinated organic compound with
moderate volatility. It is practically insoluble in water, but is
highly lipid-soluble and bioaccumulative. Technical grade HCB contains
up to 2% impurities, most of which is pentachlorobenzene. The
remainder includes the higher chlorinated dibenzo- p-dioxins,
dibenzofurans and biphenyls. Analysis of HCB in environmental media
and biological materials generally involves extraction of the sample
into organic solvents, often followed by a clean-up step, to produce
organic extracts for gas chromatography/mass spectrometry (GC/MS) or
gas chromatography with electron capture detection (GC/ECD).
1.2 Sources of human and environmental exposure
HCB was at one time used extensively as a seed dressing to
prevent fungal disease on grains, but this use was discontinued in
most countries in the 1970s. HCB continues to be released to the
environment from a number of sources, including the use of some
chlorinated pesticides, incomplete combustion, old dump sites and
inappropriate manufacture and disposal of wastes from the manufacture
of chlorinated solvents, chlorinated aromatics and chlorinated
pesticides.
1.3 Environmental transport, distribution and transformation
HCB is distributed throughout the environment because it is
mobile and persistent, although slow photodegradation in air and
microbial degradation in soil do occur. In the troposphere, HCB is
transported long distances and removed from the air phase through
deposition to soil and water. Significant biomagnification of HCB
through the food chain has been reported.
1.4 Environmental levels and human exposure
Low concentrations of HCB are present in ambient air (a few
ng/m3 or less) and in drinking-water and surface water (a few
ng/litre or less) in areas that are distant from point sources around
the world. However, higher levels have been measured near point
sources. HCB is bioaccumulative and has been detected in
invertebrates, fish, reptiles, birds and mammals (including humans)
distant from point sources, particularly in fatty tissues of organisms
at higher trophic levels. Mean levels in adipose tissue of the human
general population in various countries range from tens to hundreds of
ng/g wet weight. Based on representative levels of HCB in air, water
and food, the total intake of HCB by adults in the general population
is estimated to be between 0.0004 and 0.003 µg/kg body weight per day.
This intake is predominantly from the diet. Owing to the presence of
HCB in breast milk, mean intakes by nursing infants have been
estimated to range from < 0.018 to 5.1 µg/kg body weight per day in
various countries. The results of most studies on the levels of HCB in
foods and human tissues over time indicate that exposure of the
general population to HCB declined from the 1970s to the mid-1990s in
many locations. However, this trend has not been evident during the
last decade in some other locations.
1.5 Kinetics and metabolism in laboratory animals and humans
There is a lack of toxicokinetic information for humans. HCB is
readily absorbed by the oral route in experimental animals and poorly
via the skin (there are no data concerning inhalation). In animals and
humans, HCB accumulates in lipid-rich tissues, such as adipose tissue,
adrenal cortex, bone marrow, skin and some endocrine tissues, and can
be transferred to offspring both across the placenta and via mothers'
milk. HCB undergoes limited metabolism, yielding pentachlorophenol,
tetrachlorohydroquinone and pentachlorothiophenol as the major
metabolites in urine. Elimination half-lives for HCB range from
approximately one month in rats and rabbits to 2 or 3 years in
monkeys.
1.6 Effects on laboratory animals and in vitro tests
The acute toxicity of HCB to experimental animals is low (1000 to
10 000 mg/kg body weight). In animal studies, HCB is not a skin or eye
irritant and does not sensitize the guinea-pig.
The available data on the systemic toxicity of HCB indicate that
the pathway for the biosynthesis of haem is a major target of
hexachlorobenzene toxicity. Elevated levels of porphyrins and/or
porphyrin precursors have been found in the liver, other tissues and
excreta of several species of laboratory mammals exposed to HCB.
Porphyria has been reported in a number of studies in rats with
subchronic or chronic oral exposure to between 2.5 and 15 mg HCB/kg
body weight per day. Excretion of coproporphyrins was increased in
pigs ingesting 0.5 mg HCB/kg body weight per day or more (no effects
were observed at 0.05 mg HCB/kg body weight per day in the latter
study). Repeated exposure to HCB has also been shown to affect a wide
range of organ systems (including the liver, lungs, kidneys, thyroid,
skin, and nervous and immune systems), although these have been
reported less frequently than porphyria.
HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
It is known to bind to the Ah receptor.
In chronic studies, mild effects on the liver (histopathological
changes, enzyme induction) occurred in several studies of rats exposed
to between 0.25 and 0.6 mg HCB/kg body weight per day; the NOELs in
these studies were 0.05 to 0.07 mg HCB/kg body weight per day.
Concentrations of neurotransmitters in the hypothalamus were altered
in mink dams with chronic dietary exposure to 0.16 mg HCB/kg body
weight per day, and in their offspring exposed throughout gestation
and nursing. Calcium homoeostasis and bone morphometry were affected
in subchronic studies on rats at 0.7 mg HCB/kg body weight per day,
but not at 0.07 mg/kg body weight per day.
The carcinogenicity of HCB has been assessed in several adequate
bioassays on rodents. In hamsters fed diets yielding average doses of
4, 8 or 16 mg/kg body weight per day for life, there were increases in
the incidence of liver cell tumours (hepatomas) in both sexes at all
doses, haemangioendotheliomas of the liver at 8-16 mg/kg body weight
per day, and adenomas of the thyroid in males at the highest dose.
Dietary exposure of mice to 6, 12 and 24 mg/kg body weight per day for
120 weeks resulted in an increase in the incidence of liver cell
tumours (hepatomas) in both sexes at the two higher doses (not
significant, except for females at the highest dose). In utero,
lactational and oral exposure of rats to HCB in diets yielding average
lifetime doses ranging from 0.01 to 1.5 mg/kg body weight per day
(males) or 1.9 mg/kg body weight per day (females) for up to 130 weeks
post utero produced increased incidences, at the highest dose, of
neoplastic liver nodules and adrenal phaeochromocytomas in females and
of parathyroid adenomas in males. In another long-term study on rats,
exposure for up to 2 years to diets yielding average HCB doses of 4-5
and 8-9 mg/kg body weight per day induced increases in the incidences
of hepatomas and of renal cell adenomas at both doses in both sexes,
and of hepatocellular carcinomas, bile duct adenomas/ carcinomas and
adrenal phaeochromocytomas and adrenal cortical adenomas in females.
High incidences of liver tumours have also been reported in some more
limited studies in which single dietary concentrations were
administered to small groups of female rats. In addition, it has been
reported that, following subchronic dietary exposure to HCB, mice,
hamsters and rats developed tumours in the liver, bile duct, kidney,
thymus, spleen and lymph nodes. Dietary exposure to HCB promoted the
induction of liver tumours by polychlorinated terphenyl in mice and by
diethylnitrosamine in rats.
Except in the case of renal tumours in male rats (which appear at
least in part to be the result of hyaline droplet nephropathy) and
hepatomas in rats (which may result from hyperplastic responses to
hepatocellular necrosis), mechanistic studies that address the
relevance to humans of the tumour types induced by HCB have not been
identified.
HCB has little capability to induce directly gene mutation,
chromosomal damage and DNA repair. It exhibited weak mutagenic
activity in a small number of the available studies on bacteria and
yeast, although it should be noted that each of these studies has
limitations. There is also some evidence of low-level binding to DNA
in vitro and in vivo, but at levels well below those expected for
genotoxic carcinogens.
In studies of reproduction, oral exposure of monkeys to as little
as 0.1 mg HCB/kg body weight per day for 90 days affected the light
microscopic structure and ultrastructure of the surface germinal
epithelium, an unusual target for ovarian toxins. This dose also
caused ultrastructural injury to the primordial germ cells. These
specific target sites, which are damaged further at higher doses, were
associated with otherwise normal follicular, oocyte and embryo
development, suggesting specificity of HCB action within the site of
the ovary. Male reproduction was only affected at much higher doses
(between 30 and 221 mg/kg body weight per day) in studies on several
non-primate species.
Transplacental or lactational exposure of rats and cats to
maternal doses of between 3 and 4 mg/kg body weight per day was found
to be hepatotoxic and/or affected the survival or growth of nursing
offspring. In some cases, these or higher doses reduced litter sizes
and/or increased the number of stillbirths. (Adverse effects on
suckling infants have generally been observed more frequently, and at
lower doses, than embryotoxic or fetotoxic effects). The offspring of
mink with chronic exposure to as little as 1 mg HCB/kg diet
(approximately 0.16 mg/kg body weight per day) had reduced birth
weight and increased mortality to weaning. Although skeletal and renal
abnormalities have been observed in fetuses in some studies of rats
and mice exposed to HCB during gestation, these were either not
clearly related to treatment or occurred at doses that were also
maternally toxic. In two studies, one of which included lactational
and postnatal exposure, neurobehavioural development of rat pups was
affected by in utero exposure to HCB at oral maternal doses of 0.64
to 2.5 mg HCB/kg body weight per day.
The results of a number of studies have indicated that HCB
affects the immune system. Rats or monkeys exposed to between 3 and
120 mg HCB/kg body weight per day had histopathological alterations in
the thymus, spleen, lymph nodes and/or lymphoid tissues of the lung.
Chronic exposure of beagle dogs to 0.12 mg/kg body weight per day
caused nodular hyperplasia of the gastric lymphoid tissue. In a number
of studies on rats, humoral immunity and, to a lesser extent, cell-
mediated immunity were enhanced by several weeks exposure to HCB in
the diet, while macrophage function was unaltered. As little as 4 mg
HCB/kg diet (approximately 0.2 mg/kg body weight per day) during
gestation, through nursing and to 5 weeks of age increased humoral and
cell-mediated immune responses and caused accumulation of macrophages
in the lung tissue of rat pups. In contrast, HCB has been found to be
immunosuppressive in most studies with mice; doses of as little as
0.5-0.6 mg/kg body weight per day for several weeks depressed
resistance to infection by Leishmania or to a challenge with tumour
cells, decreased cytotoxic macrophage activity of the spleen, and
reduced the delayed-type hypersensitivity response in offspring
exposed in utero and through nursing. In a number of studies on
various strains of rats, short-term or subchronic exposure to HCB
affected thyroid function, as indicated by decreased serum levels of
total and free thyroxine (T4) and often, to a lesser extent,
triiodothyronine (T3).
1.7 Effects on humans
Most data on the effects of HCB on humans originate from
accidental poisonings that took place in Turkey in 1955-1959, in which
more than 600 cases of porphyria cutanea tarda (PCT) were identified.
In this incident, disturbances in porphyrin metabolism, dermatological
lesions, hyperpigmentation, hypertrichosis, enlarged liver,
enlargement of the thyroid gland and lymph nodes, and (in roughly half
the cases) osteoporosis or arthritis were observed, primarily in
children. Breast-fed infants of mothers exposed to HCB in this
incident developed a disorder called pembe yara (pink sore), and most
died within a year. There is also limited evidence that PCT occurs in
humans with relatively high exposure to HCB in the workplace or in the
general environment.
The few available epidemiological studies of cancer are limited
by small size, poorly characterized exposures to HCB and exposure to
numerous other agents, and are insufficient to assess the
carcinogenicity of HCB to humans.
1.8 Effects on other organisms in the laboratory and field
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa, and caused mortality in pink shrimp and grass shrimp, but
did not cause mortality in freshwater or marine fish. In longer-term
studies, the growth of sensitive freshwater algae and protozoa was
affected by a concentration of 1 µg/litre, while concentrations of
approximately 3 µg/litre caused mortality in amphipods and liver
necrosis in large-mouth bass.
1.9 Evaluation of human health risks and effects on the environment
1.9.1 Health effects
The Task Group concluded that the available data are sufficient
to develop guidance values for non-neoplastic and neoplastic effects
of HCB.
For non-neoplastic effects, based on the lowest reported NOEL
(0.05 mg HCB/kg body weight per day), for primarily hepatic effects
observed at higher doses in studies on pigs and rats exposed by the
oral route, and incorporating an uncertainty factor of 300 (× 10 for
interspecies variation, × 10 for intraspecies variation, and × 3 for
severity of effect), a TDI of 0.17 µg/kg body weight per day has been
derived.
The approach for neoplastic effects is based on the tumorigenic
dose TD5 i.e., the intake associated with a 5% excess incidence of
tumours in experimental studies in animals. Based on the results of
the two-generation carcinogenicity bioassay in rats and using the
multi-stage model, the TD5 value is 0.81 mg/kg body weight per day
for neoplastic nodules of the liver in females. Based on consideration
of the insufficient mechanistic data, an uncertainty factor of 5000
was used to develop a health-based guidance value of 0.16 µg/kg body
weight per day.
1.9.2 Environmental effects
The Task Group pointed out that there are very few experimental
studies on which an environmental risk assessment can be made. Levels
of HCB in surface water are generally several orders lower than those
expected to present a hazard to aquatic organisms, except in a few
extremely contaminated locations. However, HCB concentrations in the
eggs of sea birds and raptors from a number of locations from around
the world approach those associated with reduced embryo weights in
herring gulls (1500 µg/kg), suggesting that HCB has the potential to
harm embryos of sensitive bird species. Similarly, levels of HCB in
fish at a number of sites worldwide are within an order of magnitude
of the dietary level of 1000 µg/kg associated with reduced birth
weight and increased mortality of offspring in mink. This suggests
that HCB has the potential to cause adverse effects in mink and
perhaps other fish-eating mammals.
1.10 Conclusions
a) HCB is a persistent chemical that bioaccumulates owing to its
lipid solubility and resistance to breakdown.
b) Animal studies have shown that HCB causes cancer and affects a
wide range of organ systems including the liver, lungs, kidneys,
thyroid, reproductive tissues and nervous and immune systems.
c) Clinical toxicity, including porphyria cutanea tarda in children
and adults, and mortality in nursing infants, has been observed
in humans with high accidental exposure.
d) Various measures are warranted to reduce the environmental burden
of HCB.
e) The following health-based guidance values for the total daily
intake (TDI) of HCB in humans have been suggested: for non-cancer
effects, 0.17 µg/kg body weight/day; for neoplastic effects,
0.16 µg/kg body weight/day.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Hexachlorobenzene (HCB) is a chlorinated aromatic hydrocarbon
with the chemical formula C6Cl6. Its CAS registry number is
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
Members
Dr D. Arnold, Health Canada, Tunney's Pasture, Ottawa, Ontario Canada
Dr A. Göcmen, Department of Pediatrics, Faculty of Medicine,
Hacettepe University, Hacettepe, Ankara, Turkey
Professor B. Jansson, Institute of Applied Environmental Research,
ITM-Solna, Stockholm University, Stockholm, Sweden
Dr J. Jarrell, Foothills Hospital, Calgary Regional Health
Authority, Calgary, Alberta, Canada
Dr A. Langley, South Australian Health Commission, Rundle Mall,
Australia ( Chairman)
Mr R. Newhook, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Rapporteur)
Dr D. Peakall, Wimbledon, London, United Kingdom
( Vice-chairman)
Dr A.G. Smith, Medical Research Council Toxicology Unit,
Hodgkin Building, University of Leicester, Leicester,
United Kingdom
Dr J. Sunyer, Department of Epidemiology and Public Health,
Institut Municipal d'Investigacio Medica, Barcelona, Spain
Dr A. van Birgelen, National Health and Environmental Effects
Research Laboratory, Pharmacokinetics Branch, US Environmental
Protection Agency, Research Triangle Park, North Carolina, USAa
Dr J. Vos, National Institute of Public Health and the Environment
(RIVM), Hygiene, Bilthoven, The Netherlands
a Dr A. Van Birgelen's present address: National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA
Observers
Dr J. de Gerlache, Solvay SA, Department of Chemical Safety and
Toxicology, Brussels, Belgium (Representing EURO CHLOR)
Dr Roger Drew, Toxicology Information Section, Safety, Health &
Environment Division, ICI Australia Operations Pty Ltd., ICI
House, Melbourne, Victoria, Australia (Representing European
Centre for Ecotoxicology and Toxicology of Chemicals)
Secretariat
Dr G.C. Becking, Interregional Research Unit, International
Programme on Chemical Safety, Research Triangle Park, North
Carolina USA ( Secretary)
Ms W. Dormer, Bureau of Chemical Hazards, Environmental
Substances Division, Health Canada, Tunney's Pasture, Ottawa,
Ontario, Canada ( Temporary Adviser to Secretariat)
Dr J. Wilbourn, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBENZENE
A WHO Task Group on Environmental Health Criteria for
Hexachlorobenzene met in Geneva from 26 February to 1 March 1996.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft and made an
evaluation of the risks for human health and the environment from
exposure to hexachlorobenzene.
The first draft was prepared by Mr R. Newhook and Ms W. Dormer,
Health Canada, Ottawa, Canada. These authors also prepared the draft
reviewed by the Task Group, which incorporated the comments received
following circulation of the first draft to IPCS Contact Points for
Environmental Health Criteria monographs.
The IPCS gratefully acknowledges the financial and other support
of the Health Protection Branch, Health Canada. This support was
indispensable for the completion of this monograph.
Dr G.C. Becking (IPCS, Central Unit, Inter-regional Research
Unit) and Dr P.G. Jenkins (IPCS, Central Unit, Geneva) were
responsible for the overall scientific content and the technical
editing, respectively, of this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BMF biomagnification factor
DL detection limit
HCB hexachlorobenzene
i.p. intraperitoneal
ND not detectable
PCT porphyria cutanea tarda
p,p'DDE 1,1'-(2,2-dichloroethylidene)-bis[4-chlorobenzene]
SER smooth endoplasmic reticulum
T3 triiodothyronine
T4 thyroxine
PREFACE
The preparation of comprehensive Environmental Health Criteria
(EHC), as outlined in the Preamble of this monograph, is an extremely
time-consuming and resource-intensive procedure. Often countries have
prepared recent comprehensive reviews on chemicals as required by
their national legislation, and the International Programme on
Chemical Safety (IPCS) has been asked by Member States to determine
how best to utilize such national reviews during the preparation of
international EHC. Utilizing such national documents should avoid
duplication of effort and result in the more rapid production of more
concise IPCS EHC monographs.
This monograph on hexachlorobenzene has been prepared using as
background document the review (Supporting Document) prepared under
the Canadian Environmental Protection Act (CEPA), dated June 1993.
From this document, staff of Health Canada have chosen only the most
relevant studies for assessing the human and environmental risks from
exposure to hexachlorobenzene. These have been described from the
original references and supplemented by additional information
published more recently. This has resulted in a concise monograph,
yet one that supplies sufficient information for the reader to
understand the basis for the conclusions reached by the Task Group.
Readers who wish to consult the text of the Canadian Supporting
Document can obtain a copy from the Director, IPCS, World Health
Organization, Geneva, Switzerland.
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, and
analytical methods
Hexachlorobenzene (HCB) is a chlorinated organic compound with
moderate volatility. It is practically insoluble in water, but is
highly lipid-soluble and bioaccumulative. Technical grade HCB contains
up to 2% impurities, most of which is pentachlorobenzene. The
remainder includes the higher chlorinated dibenzo- p-dioxins,
dibenzofurans and biphenyls. Analysis of HCB in environmental media
and biological materials generally involves extraction of the sample
into organic solvents, often followed by a clean-up step, to produce
organic extracts for gas chromatography/mass spectrometry (GC/MS) or
gas chromatography with electron capture detection (GC/ECD).
1.2 Sources of human and environmental exposure
HCB was at one time used extensively as a seed dressing to
prevent fungal disease on grains, but this use was discontinued in
most countries in the 1970s. HCB continues to be released to the
environment from a number of sources, including the use of some
chlorinated pesticides, incomplete combustion, old dump sites and
inappropriate manufacture and disposal of wastes from the manufacture
of chlorinated solvents, chlorinated aromatics and chlorinated
pesticides.
1.3 Environmental transport, distribution and transformation
HCB is distributed throughout the environment because it is
mobile and persistent, although slow photodegradation in air and
microbial degradation in soil do occur. In the troposphere, HCB is
transported long distances and removed from the air phase through
deposition to soil and water. Significant biomagnification of HCB
through the food chain has been reported.
1.4 Environmental levels and human exposure
Low concentrations of HCB are present in ambient air (a few
ng/m3 or less) and in drinking-water and surface water (a few
ng/litre or less) in areas that are distant from point sources around
the world. However, higher levels have been measured near point
sources. HCB is bioaccumulative and has been detected in
invertebrates, fish, reptiles, birds and mammals (including humans)
distant from point sources, particularly in fatty tissues of organisms
at higher trophic levels. Mean levels in adipose tissue of the human
general population in various countries range from tens to hundreds of
ng/g wet weight. Based on representative levels of HCB in air, water
and food, the total intake of HCB by adults in the general population
is estimated to be between 0.0004 and 0.003 µg/kg body weight per day.
This intake is predominantly from the diet. Owing to the presence of
HCB in breast milk, mean intakes by nursing infants have been
estimated to range from < 0.018 to 5.1 µg/kg body weight per day in
various countries. The results of most studies on the levels of HCB in
foods and human tissues over time indicate that exposure of the
general population to HCB declined from the 1970s to the mid-1990s in
many locations. However, this trend has not been evident during the
last decade in some other locations.
1.5 Kinetics and metabolism in laboratory animals and humans
There is a lack of toxicokinetic information for humans. HCB is
readily absorbed by the oral route in experimental animals and poorly
via the skin (there are no data concerning inhalation). In animals and
humans, HCB accumulates in lipid-rich tissues, such as adipose tissue,
adrenal cortex, bone marrow, skin and some endocrine tissues, and can
be transferred to offspring both across the placenta and via mothers'
milk. HCB undergoes limited metabolism, yielding pentachlorophenol,
tetrachlorohydroquinone and pentachlorothiophenol as the major
metabolites in urine. Elimination half-lives for HCB range from
approximately one month in rats and rabbits to 2 or 3 years in
monkeys.
1.6 Effects on laboratory animals and in vitro tests
The acute toxicity of HCB to experimental animals is low (1000 to
10 000 mg/kg body weight). In animal studies, HCB is not a skin or eye
irritant and does not sensitize the guinea-pig.
The available data on the systemic toxicity of HCB indicate that
the pathway for the biosynthesis of haem is a major target of
hexachlorobenzene toxicity. Elevated levels of porphyrins and/or
porphyrin precursors have been found in the liver, other tissues and
excreta of several species of laboratory mammals exposed to HCB.
Porphyria has been reported in a number of studies in rats with
subchronic or chronic oral exposure to between 2.5 and 15 mg HCB/kg
body weight per day. Excretion of coproporphyrins was increased in
pigs ingesting 0.5 mg HCB/kg body weight per day or more (no effects
were observed at 0.05 mg HCB/kg body weight per day in the latter
study). Repeated exposure to HCB has also been shown to affect a wide
range of organ systems (including the liver, lungs, kidneys, thyroid,
skin, and nervous and immune systems), although these have been
reported less frequently than porphyria.
HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
It is known to bind to the Ah receptor.
In chronic studies, mild effects on the liver (histopathological
changes, enzyme induction) occurred in several studies of rats exposed
to between 0.25 and 0.6 mg HCB/kg body weight per day; the NOELs in
these studies were 0.05 to 0.07 mg HCB/kg body weight per day.
Concentrations of neurotransmitters in the hypothalamus were altered
in mink dams with chronic dietary exposure to 0.16 mg HCB/kg body
weight per day, and in their offspring exposed throughout gestation
and nursing. Calcium homoeostasis and bone morphometry were affected
in subchronic studies on rats at 0.7 mg HCB/kg body weight per day,
but not at 0.07 mg/kg body weight per day.
The carcinogenicity of HCB has been assessed in several adequate
bioassays on rodents. In hamsters fed diets yielding average doses of
4, 8 or 16 mg/kg body weight per day for life, there were increases in
the incidence of liver cell tumours (hepatomas) in both sexes at all
doses, haemangioendotheliomas of the liver at 8-16 mg/kg body weight
per day, and adenomas of the thyroid in males at the highest dose.
Dietary exposure of mice to 6, 12 and 24 mg/kg body weight per day for
120 weeks resulted in an increase in the incidence of liver cell
tumours (hepatomas) in both sexes at the two higher doses (not
significant, except for females at the highest dose). In utero,
lactational and oral exposure of rats to HCB in diets yielding average
lifetime doses ranging from 0.01 to 1.5 mg/kg body weight per day
(males) or 1.9 mg/kg body weight per day (females) for up to 130 weeks
post utero produced increased incidences, at the highest dose, of
neoplastic liver nodules and adrenal phaeochromocytomas in females and
of parathyroid adenomas in males. In another long-term study on rats,
exposure for up to 2 years to diets yielding average HCB doses of 4-5
and 8-9 mg/kg body weight per day induced increases in the incidences
of hepatomas and of renal cell adenomas at both doses in both sexes,
and of hepatocellular carcinomas, bile duct adenomas/ carcinomas and
adrenal phaeochromocytomas and adrenal cortical adenomas in females.
High incidences of liver tumours have also been reported in some more
limited studies in which single dietary concentrations were
administered to small groups of female rats. In addition, it has been
reported that, following subchronic dietary exposure to HCB, mice,
hamsters and rats developed tumours in the liver, bile duct, kidney,
thymus, spleen and lymph nodes. Dietary exposure to HCB promoted the
induction of liver tumours by polychlorinated terphenyl in mice and by
diethylnitrosamine in rats.
Except in the case of renal tumours in male rats (which appear at
least in part to be the result of hyaline droplet nephropathy) and
hepatomas in rats (which may result from hyperplastic responses to
hepatocellular necrosis), mechanistic studies that address the
relevance to humans of the tumour types induced by HCB have not been
identified.
HCB has little capability to induce directly gene mutation,
chromosomal damage and DNA repair. It exhibited weak mutagenic
activity in a small number of the available studies on bacteria and
yeast, although it should be noted that each of these studies has
limitations. There is also some evidence of low-level binding to DNA
in vitro and in vivo, but at levels well below those expected for
genotoxic carcinogens.
In studies of reproduction, oral exposure of monkeys to as little
as 0.1 mg HCB/kg body weight per day for 90 days affected the light
microscopic structure and ultrastructure of the surface germinal
epithelium, an unusual target for ovarian toxins. This dose also
caused ultrastructural injury to the primordial germ cells. These
specific target sites, which are damaged further at higher doses, were
associated with otherwise normal follicular, oocyte and embryo
development, suggesting specificity of HCB action within the site of
the ovary. Male reproduction was only affected at much higher doses
(between 30 and 221 mg/kg body weight per day) in studies on several
non-primate species.
Transplacental or lactational exposure of rats and cats to
maternal doses of between 3 and 4 mg/kg body weight per day was found
to be hepatotoxic and/or affected the survival or growth of nursing
offspring. In some cases, these or higher doses reduced litter sizes
and/or increased the number of stillbirths. (Adverse effects on
suckling infants have generally been observed more frequently, and at
lower doses, than embryotoxic or fetotoxic effects). The offspring of
mink with chronic exposure to as little as 1 mg HCB/kg diet
(approximately 0.16 mg/kg body weight per day) had reduced birth
weight and increased mortality to weaning. Although skeletal and renal
abnormalities have been observed in fetuses in some studies of rats
and mice exposed to HCB during gestation, these were either not
clearly related to treatment or occurred at doses that were also
maternally toxic. In two studies, one of which included lactational
and postnatal exposure, neurobehavioural development of rat pups was
affected by in utero exposure to HCB at oral maternal doses of 0.64
to 2.5 mg HCB/kg body weight per day.
The results of a number of studies have indicated that HCB
affects the immune system. Rats or monkeys exposed to between 3 and
120 mg HCB/kg body weight per day had histopathological alterations in
the thymus, spleen, lymph nodes and/or lymphoid tissues of the lung.
Chronic exposure of beagle dogs to 0.12 mg/kg body weight per day
caused nodular hyperplasia of the gastric lymphoid tissue. In a number
of studies on rats, humoral immunity and, to a lesser extent, cell-
mediated immunity were enhanced by several weeks exposure to HCB in
the diet, while macrophage function was unaltered. As little as 4 mg
HCB/kg diet (approximately 0.2 mg/kg body weight per day) during
gestation, through nursing and to 5 weeks of age increased humoral and
cell-mediated immune responses and caused accumulation of macrophages
in the lung tissue of rat pups. In contrast, HCB has been found to be
immunosuppressive in most studies with mice; doses of as little as
0.5-0.6 mg/kg body weight per day for several weeks depressed
resistance to infection by Leishmania or to a challenge with tumour
cells, decreased cytotoxic macrophage activity of the spleen, and
reduced the delayed-type hypersensitivity response in offspring
exposed in utero and through nursing. In a number of studies on
various strains of rats, short-term or subchronic exposure to HCB
affected thyroid function, as indicated by decreased serum levels of
total and free thyroxine (T4) and often, to a lesser extent,
triiodothyronine (T3).
1.7 Effects on humans
Most data on the effects of HCB on humans originate from
accidental poisonings that took place in Turkey in 1955-1959, in which
more than 600 cases of porphyria cutanea tarda (PCT) were identified.
In this incident, disturbances in porphyrin metabolism, dermatological
lesions, hyperpigmentation, hypertrichosis, enlarged liver,
enlargement of the thyroid gland and lymph nodes, and (in roughly half
the cases) osteoporosis or arthritis were observed, primarily in
children. Breast-fed infants of mothers exposed to HCB in this
incident developed a disorder called pembe yara (pink sore), and most
died within a year. There is also limited evidence that PCT occurs in
humans with relatively high exposure to HCB in the workplace or in the
general environment.
The few available epidemiological studies of cancer are limited
by small size, poorly characterized exposures to HCB and exposure to
numerous other agents, and are insufficient to assess the
carcinogenicity of HCB to humans.
1.8 Effects on other organisms in the laboratory and field
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa, and caused mortality in pink shrimp and grass shrimp, but
did not cause mortality in freshwater or marine fish. In longer-term
studies, the growth of sensitive freshwater algae and protozoa was
affected by a concentration of 1 µg/litre, while concentrations of
approximately 3 µg/litre caused mortality in amphipods and liver
necrosis in large-mouth bass.
1.9 Evaluation of human health risks and effects on the environment
1.9.1 Health effects
The Task Group concluded that the available data are sufficient
to develop guidance values for non-neoplastic and neoplastic effects
of HCB.
For non-neoplastic effects, based on the lowest reported NOEL
(0.05 mg HCB/kg body weight per day), for primarily hepatic effects
observed at higher doses in studies on pigs and rats exposed by the
oral route, and incorporating an uncertainty factor of 300 (× 10 for
interspecies variation, × 10 for intraspecies variation, and × 3 for
severity of effect), a TDI of 0.17 µg/kg body weight per day has been
derived.
The approach for neoplastic effects is based on the tumorigenic
dose TD5 i.e., the intake associated with a 5% excess incidence of
tumours in experimental studies in animals. Based on the results of
the two-generation carcinogenicity bioassay in rats and using the
multi-stage model, the TD5 value is 0.81 mg/kg body weight per day
for neoplastic nodules of the liver in females. Based on consideration
of the insufficient mechanistic data, an uncertainty factor of 5000
was used to develop a health-based guidance value of 0.16 µg/kg body
weight per day.
1.9.2 Environmental effects
The Task Group pointed out that there are very few experimental
studies on which an environmental risk assessment can be made. Levels
of HCB in surface water are generally several orders lower than those
expected to present a hazard to aquatic organisms, except in a few
extremely contaminated locations. However, HCB concentrations in the
eggs of sea birds and raptors from a number of locations from around
the world approach those associated with reduced embryo weights in
herring gulls (1500 µg/kg), suggesting that HCB has the potential to
harm embryos of sensitive bird species. Similarly, levels of HCB in
fish at a number of sites worldwide are within an order of magnitude
of the dietary level of 1000 µg/kg associated with reduced birth
weight and increased mortality of offspring in mink. This suggests
that HCB has the potential to cause adverse effects in mink and
perhaps other fish-eating mammals.
1.10 Conclusions
a) HCB is a persistent chemical that bioaccumulates owing to its
lipid solubility and resistance to breakdown.
b) Animal studies have shown that HCB causes cancer and affects a
wide range of organ systems including the liver, lungs, kidneys,
thyroid, reproductive tissues and nervous and immune systems.
c) Clinical toxicity, including porphyria cutanea tarda in children
and adults, and mortality in nursing infants, has been observed
in humans with high accidental exposure.
d) Various measures are warranted to reduce the environmental burden
of HCB.
e) The following health-based guidance values for the total daily
intake (TDI) of HCB in humans have been suggested: for non-cancer
effects, 0.17 µg/kg body weight/day; for neoplastic effects,
0.16 µg/kg body weight/day.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Hexachlorobenzene (HCB) is a chlorinated aromatic hydrocarbon
with the chemical formula C6Cl6. Its CAS registry number is  Synonyms: perchlorobenzene, pentachlorophenyl chloride, phenyl
perchloryl
Trade names: Amatin, Anticarie, Bunt Cure, Bunt-No-More, Co-op Hexa,
Granox NM, Julin's Carbon Chloride, No Bunt, No Bunt
40, No Bunt 80, No Bunt Liquid, Sanocide, Smut-Go,
Snieciotox, HexaCB
2.2 Physical and chemical properties
Some physical and chemical properties of HCB are listed in
Table 1. At ambient temperature, HCB is a white crystalline that is
virtually insoluble in water, but is soluble in ether, benzene and
chloroform (NTP, 1994). It has a high octanol/water partition
coefficient, low vapour pressure, moderate Henry's Law constant and
low flammability. Technical grade HCB is available as a wettable
powder, liquid and dust (NTP, 1994). Technical grade HCB contains
about 98% HCB, 1.8% pentachlorobenzene and 0.2% 1,2,4,5-
tetrachlorobenzene (IARC, 1979), and it is known to contain a variety
of impurities, including hepta- and octachlorodibenzofurans,
octachlorodibenzo- p-dioxin and decachlorobiphenyl (Villanueva et
al., 1974; Goldstein et al., 1978).
Table 1. Physical and chemical properties of hexachlorobenzenea
Property Value
Relative molecular mass 284.79
Melting point (°C) 230
Boiling point (°C) 322 (sublimates)
Density (g/cm3 at 20°C 1.5691
Vapour pressure 0.0023
(Pa at 25°C)
Log octanol/water partition coefficient 5.5
Water solubility 0.005
(mg/litre at 25°C)
Henry's Law Constant (caluclated)b 131
(Pa/mol per m3)
Conversion factors 1 ppm = 11.8 mg/m3
1 mg/m3 = 0.08 ppm
a From ATSDR (1990); Mackay et al. (1992)
b The Henry's Law Constant has been calculated using the
tabled values for aqueous solubility and vapour pressure
2.3 Analytical methods
Analytical methods for the determination of HCB in environmental
samples and biological tissues vary depending upon the matrix and
representative methods for various matrices, and are summarized in
Tables 2 and 3.
Table 2. Analytical methods for determining hexachlorobenzene in environmental samplesa
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Water Extract with dichloromethane, GC/ECD 0.05 mg/kg 95 ± 10-20% US EPA (1982)
exchange to hexane,
concentrate; Florisil column
chromatography as a clean-up
Water Extract with dichloromethane GC/MS 1.9 mg/kg No data US EPA (1982)
at pH 11 and 2, concentrate
Air Glass fibre filter and XAD2 HRGC/ 0.18 pg/m3 >99% Hippelein et al. (1993)
traps separated by a PUF LRMS
disk; extraction with toluene
Air Polyurethane foam (PUF) GC/ECD <0.1 µg/m3 94.5±8% Lewis & MacLeod (1982)
sampling cartridge, extraction
with diethyl ether in hexane
Air Polyurethane foam (PUF) GC/ECD low pg/m3 93±1.1% Oehme & Stray (1982)
plugs, extraction with hexane, range (not
fractionation by HPLC specified)
Air Porous polyurethane foam GC/ECD No data Tenax more Billings &
(PUF), or Tenax-GC resin; effective Bidleman (1980)
filters refluxed with than PUF in
dichloromethane and retaining HCB
chlorinated solvents removed
and refluxed with hexane;
clean-up by alumina
chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Air Adsorb on Amberlite XAD-2 GC/PID 0.014 mg/m3 approx Langhorst &
resin separated by a silanized 95 ± 12% Nestrick (1979)
glass wool plug, desorption
with carbon tetrachloride.
Air Trace Atmospheric Gas Analyser approx No data Thomson et al. (1980)
using negative atmospheric 0.35 µg/m3
pressure chemical ionization
for trace gas analysis;
collection from ambient air and
transfer into a carrier of CO2
for analysis
Soil, Hexane extraction GC/ECD 10 mg/kg 78±2.6% to DeLeon et al. (1980)
chemical 96.5±3.6%
waste
disposal
site samples
Soil Extract with dichloromethane GC/MS 18 mg/kg No data US EPA (1986b)
5 mg/kg
Sediment Solvent extraction subjected GC/MS 46% Lopez-Avila et al.
to acid-base fractionation; (1983)
base/neutral fraction subjected
to silica gel chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Wastes, Extract with dichloromethane GC/MS 190 mg/kg No data US EPA (1986b)
non-water 50 mg/kg
miscible
Wastes, soil Extract with dichloromethane GC/MS 20 µg/litrec No data US EPA (1986b)
a Portions of the table were taken from ATSDR (1990)
b GC = gas chromatography; ECD = electron capture detector; MS = mass spectrometry; PID = photoionization detector;
HRGC = high-resolution gas chromatography; LRMS = low-resolution mass spectrometry
c Identification limit; detection limits for actual samples are several orders of magnitude higher depending upon the
sample matrix and extraction procedure employed.
Table 3. Analytical methods for determining hexachlorobenzene in biological materials
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Fish tissue Grind with sodium sulfate, extract GC/ECD approx No data Oliver & Nicol (1982)
with hexane/acetone, clean-up by 0.05 µg/kg
Na2SO4/Alumina/silica gel/Florisil
column followed by a H2SO4 column
on silica gel
Fish tissue Extraction with hexane/isopropanol, GC/ECD No data No data Lunde & Ofstad (1976)
solvent and sulfuric acid
partitioning
Fish tissue Sulfuric acid digestion, silica gel GC/ECD 10-15 µg/kg 93% Lamparski et al. (1980)
column chromatography, methylation,
alumina column chromatography
Oyster Extraction with acetone/acetonitrile, GC/ECD No data No data Murray et al. (1980)
tissue partitioning into petroleum ether,
silica gel chromatography
Adipose Extraction with hexane, subjected GC/ECD No data 87.4-92.6% Watts et al. (1980)
tissue to Florisil clean-up and one-fraction
(chicken) elution
Adipose Extraction (solvent not specified), HRGC/MS 12 µg/kg No data Stanley (1986)
tissue bulk lipid removal, Florisil
fractionation
Adipose Extraction with benzene/acetone, GC/ECD 0.12 µg/kg 79-95% Mes (1992)
tissue Florisil fractionation
Table 3 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Blood/urine Extraction with carbon tetrachloride, GC/PID 4.1 µg/kg 83% Langhorst &
silica gel column chromatography, (urine) Nestrick (1979)
concentrate 16 µg/kg
(blood)
Blood Extraction with hexane, concentrate GC/ECD No data No data US EPA (1980)
Blood Extraction with hexane/isopropanol GC/ECD No data No data Lunde & Bjorseth (1977)
Breast milk Extraction with acetone/benzene, GC/ECD 33 µg/kg 70-82% Mes et al. (1993)
Florisil fractionation
GC = gas chromatography; ECD = electron capture detector; PID = photoionization detector; HRGC = high-resolution gas
chromatography; MS = mass spectrometry
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Sources, uses and production processes
Industrial synthesis of HCB may be achieved through the
chlorination of benzene at 150-200°C using a ferric chloride catalyst
or from the distillation of residues from the production of
tetrachloroethylene (US EPA, 1985a). HCB may also be synthesized by
refluxing hexachlorocyclohexane isomers with sulfuryl chloride or
chlorosulfonic acid in the presence of a ferric chloride or aluminum
catalyst (Brooks & Hunt, 1984).
Historically, HCB had many uses in industry and agriculture. The
major agricultural application for HCB used to be as a seed dressing
for crops such as wheat, barley, oats and rye to prevent growth of
fungi. The use of HCB in such applications was discontinued in many
countries in the 1970s owing to concerns about adverse effects on the
environment and human health. HCB may continue to be used for this
purpose in some countries; for example, HCB was still used in 1986 as
a fungicide, seed-dressing and scabicide in sheep in Tunisia (Jemaa et
al., 1986). However, it is uncertain as to whether HCB is still used
for this purpose.
In industry, HCB has been used directly in the manufacture of
pyrotechnics, tracer bullets and as a fluxing agent in the manufacture
of aluminum. HCB has also been used as a wood-preserving agent, a
porosity-control agent in the manufacture of graphite anodes, and as a
peptizing agent in the production of nitroso and styrene rubber for
tyres (Mumma & Lawless, 1975). It is likely that some of these
applications have been discontinued, although no information is
available.
Although HCB production has ceased in most countries, it is still
being generated inadvertently as a by-product and/or impurity in
several chemical processes. HCB is formed as a reaction by-product of
thermal chlorination, oxychlorination, and pyrolysis operations in the
manufacture of chlorinated solvents (mainly carbon tetrachloride,
trichloroethylene and tetrachloroethylene) (Government of Canada,
1993). The concentrations of HCB in distillation bottoms was estimated
to be 25%, 15% and 5%, respectively, for tetrachloroethylene, carbon
tetrachloride and trichloroethylene (Jacoff et al., 1986). While HCB
could potentially also be a contaminant in the final product, it was
not detected (detection limit 5 mg/litre) in carbon tetrachloride and
tetrachloroethylene in an investigation in Canada (personal
communication to Health Canada by Mr John Schultiess, Dow Chemical
Canada Inc., 1991). Analysis of production lots of tri- and
tetrachloroethylene produced in Europe in 1996 failed to detect HCB at
a detection limit of 2 µg/litre solvent (personal communication to the
IPCS by Mr C. de Rooij, Solvay Corporation Europe, 1996).
HCB is also generated as a waste by-product during the
manufacture of chlorinated solvents, chlorinated aromatics and
pesticides (Jacoff et al., 1986). The waste streams from the
production of pentachloronitrobenzene (PCNB), chlorothalonil and
dacthal are expected to contribute the bulk of HCB released from the
pesticide industry (Brooks & Hunt, 1984), although HCB can also be
generated as a waste by-product from the production of
pentachlorophenol, atrazine, simazine, propazine and maleic hydrazide
(Quinlivan et al., 1975; Mumma & Lawless, 1975). These pesticides are
also known to contain HCB as an impurity in the final product, usually
at levels of less than 1% HCB when appropriate procedures are used for
the synthesis and purification stages (Tobin, 1986). When such
procedures are not met, the level of HCB could be much higher (e.g.,
pentachloronitrobenzene has been reported to contain 1.8-11% HCB
(Tobin, 1986)). However, owing to many voluntary and regulatory
pressures, it is unlikely that such high levels of HCB are present in
today's pesticide formulations, but no information is available to
substantiate this point.
The chlor-alkali industry produces chlorine (Cl2), hydrogen and
caustic soda (NaOH) by electrolysis of purified and concentrated
sodium chloride (NaCl). Processes using graphite anodes are known to
produce HCB as a by-product (Quinlivan et al., 1975; Mumma & Lawless,
1975; Alves & Chevalier, 1980) owing to the reaction of chlorine with
graphite anode materials such as carbon and oils. Depending on the
purification procedures, the final products might also be contaminated
with HCB. In some countries, graphite anodes have been replaced by
dimensionally stabilized anodes (DSA), which do not generate HCB
(Government of Canada, 1993).
Incineration is an important source of HCB in the environment.
Emission levels from incinerators are very site-specific, and
therefore generic levels are difficult to estimate. Earlier
information yielded a crude estimate of the total HCB released from
all municipal incinerators in the USA to be 57-454 kg/year (US EPA,
1986a), but levels currently emitted are not known.
3.2 World production levels
Few recent data on the quantities of HCB produced are available.
Worldwide production of pure HCB was estimated to be 10 000
tonnes/year for the years 1978-1981 (Rippen & Frank, 1986). An
estimated 300 tonnes was produced by three manufacturers in the USA in
1973 (IARC, 1979). HCB was produced/imported in the European Community
at 8000 tonnes/year in 1978 (Rippen & Frank, 1986), and a company in
Spain used to produce an estimated 150 tonnes of HCB annually (IARC,
1979). Approximately 1500 tonnes of HCB were manufactured annually in
Germany for the production of the rubber auxiliary PCTP (BUA, 1994),
but this production was discontinued in 1993. No further centres of
HCB manufacture in Europe or North America have been identified.
Production of HCB has declined as a result of restrictions on its use
starting in the 1970s.
Considerable amounts of HCB are inadvertently produced as a by-
product in the manufacture of chlorinated solvents, chlorinated
aromatics and chlorinated pesticides. Jacoff et al. (1986) estimated
that approximately 4130 tonnes of HCB are generated annually as a
waste product in the USA and that nearly 77% of this is produced from
the manufacture of three chlorinated solvents: carbon tetrachloride,
trichloroethylene and tetrachloroethylene. The remainder is produced
by the chlorinated pesticide industry. In 1977, about 300 tonnes of
HCB were generated in Japan as a waste by-product in the production of
tetrachloroethylene, almost all of which was incinerated (IARC, 1979).
It was estimated that >5000 tonnes HCB/year were produced as a by-
product during tetrachloroethylene production in the Federal Republic
of Germany in 1980 (Rippen & Frank, 1986). However, recent estimates
for Europe from ECSA (European Chlorinated Solvent Association; P.G.
Johnson (1996) personal communication to IPCS) indicate that up to
4000 tonnes/year of HCB are produced as a by-product during certain
tetrachloroethylene production processes and that over 99% of this by-
product was incinerated at high temperatures.
3.3 Entry into the environment
Currently, the principal sources of HCB in the environment are
estimated to be the manufacture of chlorinated solvents, the
manufacture and application of HCB-contaminated pesticides, and
inadequate incineration of chlorine-containing wastes. It should be
noted that only a small fraction of the HCB generated as a by-product
may be released, depending on the process technology and waste-
disposal practices employed. For example, according to the US Toxic
Chemical Release Inventory (TRI), releases of HCB from the ten largest
processing facilities were 460 kg, most of this to air, compared with
almost 542 000 kg transferred offsite as waste. The TRI data are not
comprehensive, since only certain types of facilities are required to
report (ATSDR, 1994). ECSA (P.G. Johnson, personal communication to
IPCS) estimated that European emissions of HCB were about 200 kg/year
in 1993.
As discussed in the previous section, HCB is a contaminant of a
number of chlorinated pesticides. Since most current applications for
these products are dispersive, most HCB from this source will be
released to the environment.
Substantial quantities of HCB are also contained in the wastes
generated through the manufacture of chlorinated solvents and
pesticides. In the mid-1980s in the USA, 81% of these HCB-containing
wastes were disposed of by incineration, compared to 19% via
landfilling (Jacoff et al., 1986). It is likely that the amount of HCB
wastes disposed of by incineration has since increased, although
information has not been found to confirm this point. HCB can be
emitted from incinerators as a result of incomplete thermal
decomposition of these wastes and as a product of incomplete
combustion (PIC) from the thermal decomposition of a variety of
chlorinated organics such as Kepone, mirex, chlorobenzenes,
polychlorinated biphenyls, pentachlorophenol, polyvinyl chloride and
mixtures of chlorinated solvents (Ahling et al., 1978; Dellinger et
al., 1991).
Although only a small proportion of the HCB-containing waste
generated in the USA is landfilled, HCB may continue to leach to
groundwater from previously landfilled HCB waste sites. The
contribution of this route is uncertain, although HCB is not easily
leached, and landfills containing HCB are now designed to prevent
leachate losses into adjacent water systems (Brooks & Hunt, 1984). HCB
emission into the atmosphere from landfills containing HCB wastes
occurs from slow volatilization and from displacement of the
contaminated soil (Brooks & Hunt, 1984).
HCB has been detected in emissions from a number of industries,
including paint manufacturers, coal and steel producers, pulp and
paper mills, textile mills, pyrotechnics producers, aluminum smelters,
soap producers and wood-preservation facilities (Quinlivan et al.,
1975; Gilbertson, 1979; Alves & Chevalier, 1980), probably reflecting
the use of products contaminated with HCB. Municipal and industrial
wastewater facilities may also discharge HCB-contaminated effluents
(Environment Canada/Ontario Ministry of the Environment, 1986; King &
Sherbin, 1986), probably owing to inputs from industrial sources.
Long-range transport plays a significant role as a means of
redistribution of HCB throughout the environment. Wet deposition
(deposition via rain or snowfall) is the primary mechanism for
transport of HCB from the atmosphere to aquatic and terrestrial
systems in Canada (Eisenreich & Strachan, 1992). For example, it is
estimated that long-range transport and total deposition to the
Canadian environment is approximately 510 kg/year, an amount that is
similar to that from all other sources combined (Government of Canada,
1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental transport and degradation
HCB is distributed throughout the environment because it is
mobile and resistant to degradation. Volatilization from water to air
and sedimentation following adsorption to suspended particulates are
the major removal processes from water (Oliver, 1984a; Oliver &
Charlton, 1984). Once in the sediments, HCB will tend to accumulate
and become trapped by overlying sediments (Oliver & Nicol, 1982).
Although HCB is not readily leached from soils and sediments, some
desorption does occur and may be a continuous source of HCB to the
environment, even if inputs to the system cease (Oliver, 1984a; Oliver
et al., 1989). Chemical or biological degradation is not considered to
be important for the removal of HCB from water or sediments (Callahan
et al., 1979; Mansour et al., 1986; Mill & Haag, 1986; Oliver & Carey,
1986). In the troposphere, HCB is transported over long distances by
virtue of its persistence, but does undergo slow photolytic
degradation (the half-life is approximately 80 days; Mill & Haag,
1986), or is removed from the air phase via atmospheric deposition to
water and soil (Bidleman et al., 1986; Ballschmiter & Wittlinger,
1991; Lane et al., 1992a, 1992b). In soil, volatilization is the major
removal process at the surface (Kilzer et al., 1979; Griffin & Chou,
1981; Schwarzenbach et al., 1983; Nash & Gish, 1989), while slow
aerobic (half-life of 2.7-5.7 years) and anaerobic biodegradation
(half-life of 10.6-22.9 years) are the major removal processes at
lower depths (Beck & Hansen, 1974; Howard et al., 1991).
4.2 Bioaccumulation and biomagnification
The bioaccumulative properties of HCB result from the combination
of its physicochemical properties (high octanol/water partition
coefficient) and its slow elimination due to limited metabolism
related to its high chemical stability. Organisms generally accumulate
HCB from water and from food, although benthic organisms may also
accumulate HCB directly from sediment (Oliver, 1984b; Knezovich &
Harrison, 1988; Gobas et al., 1989). The uptake of HCB in benthic
invertebrates has been investigated in a number of laboratory and
field studies. The results demonstrated that some HCB in sediments is
available to infaunal species. Reported bioaccumulation factorsa
(BAF) for invertebrates in HCB-containing sediments range from 0.04 to
0.58 in high-organic-content sediment to 1.95 in low-organic-content
a Defined as tissue concentration (wet weight) divided by sediment
concentration (dry weight). BAFs from Oliver (1984b) were divided
by 6.67 to convert tissue dry weight to wet weight.
sediment (Oliver, 1984b; Knezovich & Harrison, 1988; Gobas et al.,
1989). The bioavailability of sediment-bound HCB is inversely related
to sediment organic carbon content (Knezovich & Harrison, 1988), and
varies with the type and size of the organisms and their feeding
habits (Boese et al., 1990), the extent of contact with sediment pore
and interstitial waters (Landrum, 1989), and the surface area of the
substrate (Swindoll & Applehans, 1987). Landrum (1989) suggested that
the bioavailability of sediment-sorbed chemicals declines as the
contact time between the sediment and a contaminant increases. For
example, Schuytema et al., (1990) observed that addition of HCB-spiked
sediments did not result in a significant increase in the uptake of
HCB by the worm ( Lumbriculus variegatus), amphipods ( Hyalella
azteca and Gammarus lacustris), and fathead minnows ( Pimephales
promelas) in a laboratory recirculating water/sediment system.
However, there was a substantial increase of HCB levels in bed
sediment, suggesting that sediment served as a more effective sink for
HCB than the organisms.
The biomagnification factor (BMF) for HCB in the earthworm
Eisenia andrei after exposure via food was 0.068 on a wet weight
basis (0.071 on a lipid basis) (Belfroid et al., 1994a), the biota
lipid-to-soil accumulation factor, defined as the ratio of the
concentration in the animal to that on the soil, was 215 g soil dry
weight/g lipid (Belfroid et al., 1994b), and the bioconcentration
factors (BCFs) for earthworms kept in water were found to be between
48 × 104 and 62 × 104 ml water/g lipid (Belfroid et al., 1993).
Field studies indicate that exposure via food is important for
organisms at higher trophic levels, as significant biomagnification
has been observed in several studies in natural aquatic ecosystems. In
Lake Ontario, Oliver & Niimi (1988) observed that tissue residue
concentrations increased from plankton (mean = 1.6 ng/g wet weight) to
mysids (mean = 4.0 ng/g wet weight) to alewives (mean = 20 ng/g wet
weight) to salmonids (mean = 38 ng/g wet weight). Braune & Norstrom
(1989) used field data on body burdens of HCB in the herring gull
( Larus argentatus) and one of its principal food items, the alewife
( Alosa pseudoharengus) in a Great Lakes food chain to calculate a
biomagnification factor (whole body, wet weight basis) of 31.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
HCB has been detected in air, water, sediment, soil and biota.
Representative levels reported in various environmental media in many
countries are presented in Tables 4, 5 and 6.
5.1.1 Air
HCB is widely dispersed in ambient air, and is generally present
at low concentrations. Mean concentrations of HCB in air removed from
point sources in Canada, Norway, Sweden, Germany, the USA, the Arctic
and the Antarctic range from 0.04 to 0.6 ng/m3 (Table 4). Levels of
HCB in air are generally similar between urban, rural and remote
sites, reflecting the persistence and long-range transport of this
substance.
Airborne concentrations of HCB measured in the USA near nine
chlorinated solvent and pesticide plants in 1973 and 1976 were much
higher than background levels (Spigarelli et al., 1986).
Concentrations as high as 24 µg/m3 were detected in the immediate
vicinity of one plant, while the maximum concentration of HCB distant
from the site was 0.36 µg/m3. The highest levels were associated with
the production of perchloroethylene, trichloroethylene and carbon
tetrachloride, and with plants where onsite landfill and open pit
waste disposal were practiced. More recently, Grimalt et al. (1994)
reported that airborne concentrations of HCB in a community in the
vicinity of a organochlorine factory built in 1898 in Catalonia,
Spain, averaged 35 ng/m3, compared with 0.3 ng/m3 in Barcelona, the
reference community for this study. It is not known how representative
the data from these studies are, as HCB releases are expected to be
minimized from industries using appropriate modern technology and
waste management practices.
No data are available on the levels of HCB in indoor air.
Table 4. Levels of hexachlorobenzene in ambient air (ng/m3)
Location Year Detection Mean Rangea Reference
limit
Canada (Windsor, Ontario) 1987-1990 0.03 0.13 ND-0.44 Environment Canada (1992)
Canada (Ontario)
- industrial/urban areas 1985-1989 0.007 0.167 0.07-0.31 Lane et al. (1992b)
- rural areas 0.094 0.02-0.31
Canada (Egbert, Ontario) 1988-1989 - >0.054 0.00004-0.64 Hoff et al. (1992)
Canada (Walpole Island) 1988-1989 0.02 0.15 ND-0.34 Environment Canada (1992)
Canadian High Arctic 1987 0.15 ND-0.154 Patton et al. (1989)
(Beaufort Sea)
Bear Island (Arctic)
- summer
- winter 0.001 0.04 0.029-0.045 Oehme & Stray (1982)
0.001 0.111 0.059-0.188
Southern Ocean and 1990 0.06 0.04-0.078 Bidleman et al. (1993)
Antarctica
Enewetak Atoll 1979 0.10 0.095-0.13 Atlas & Giam (1981)
(Pacific Ocean)
Spitzbergen
- summer 0.001 0.071 0.05-0.085 Oehme & Stray (1982)
- winter 0.001 0.086 0.071-0.095
Table 4 contd.
Location Year Detection Mean Rangea Reference
limit
Germany (Hamburg - 1986-1987 0.6 0.3-2.5 Bruckmann et al. (1988)
residential, suburban
and industrial sites)
South Germany 1986-1990 0.21 0.058-0.52 Morosini et al. (1993)
Norway (Lillestrom) 0.001 0.162 0.055-0.234 Oehme & Stray (1982)
Sweden (Aspvreten) 1984 0.067 0.054->0.165 Bidleman et al. (1987)
Sweden (Stockholm) 1983-1985 0.07 0.054->0.130 Bidleman et al. (1987)
Spain
- (near organochlorine 1989 & 1992 - 35 11-44 Grimalt et al. (1994)
compounds factory)
- hospital in Barcelona - 0.3 0.25-0.4
USA (Portland, Oregon) 1984 0.075 0.05-0.11 Ligocki et al. (1985)
USA - chemical production ND-24000 Spigarelli et al. (1986)
plants
USA - urban areas 1975-1979 0.1 0.5 ND-4.4 Carey et al. (1985)
a ND = not detected
5.1.2 Water
Levels of HCB in freshwater in Europe and North America are
generally below 1 ng/litre (Table 5), although higher values have been
reported in aquatic systems that receive industrial discharges and
surface run-off. In the connecting channels to the Great Lakes in
Canada, HCB levels were often found to exceed 1.0 ng/litre,
particularly near point sources. Levels in the St. Clair River near
the Dow Chemical outfall were as high as 87 ng/litre in 1985 and
75 ng/litre in 1986 (Oliver & Kaiser, 1986).
Mean concentrations of HCB in seawater rarely exceed 1 ng/litre
(Table 5) (Ernst, 1986; Burton & Bennett, 1987). In the Nueces Estuary
in Texas, USA, the highest level (0.61 ng/litre) was found near
sewage outfalls (Ray et al., 1983a). Higher concentrations (up to
196 ng/litre) were observed in the Forth Estuary in Scotland, near
domestic and chemical industry discharges (Rogers et al., 1989).
5.1.3 Soil
Data identified on levels of HCB in soil are quite limited and
are summarized in Table 6. The most extensive data are from the 1972
US National Soils Monitoring Program, in which the concentrations of a
variety of pesticides were determined at 1483 sites from 37 states
(Carey et al., 1979). HCB was detected at 11 sites, with a range of
concentrations in positive samples from 10 to 440 µg/kg dry weight. Of
24 samples of agricultural soil in British Columbia, Canada, where HCB
had last been applied as a seed treatment 10-15 years prior to the
survey, 6 had detectable HCB residues of between 1.3 and 2.2 ng/g dry
weight (Wilson & Wan, 1982).
Mean concentrations of HCB reported from uncontaminated soil in
Europe were found to range from 0.3 ng/g in Switzerland (Müller, 1982)
to 5.1 ng/g in a Swedish rural heathland soil (Thomas et al., 1985)
(it was not indicated whether concentrations were on a dry or wet
weight basis). Soil from a farming area in Italy contained 40 ng/g
(dry or wet weight basis not indicated) (Leoni & D'Arca, 1976). HCB
levels were not markedly increased by long-term application of sludge
to land in Germany at a rate of 50 to 500 tons per ha and averaged
2.8 ng/g (dry or wet weight basis not indicated) (Witte et al.,
1988a,b). Monitoring programmes in Germany yielded average levels of
HCB contamination of soil ranging from approximately 1 ng/g dry weight
in the North Rhine-Westphalia (1990) to approximately 6 ng/g dry
weight in Baden-Württemberg (1988) (BUA, 1994).
Table 5. Concentrations of hexachlorobenzene (ng/litre) in drinking-water and surface water
Location Year Detection Mean Rangea Reference
limit
Drinking-water
Canada (Ontario) 1980 0.01 0.1 0.06-0.2 Oliver & Nicol (1982)
Canada (Maritime Provinces) 1985-1988 2.0 ND Environment Canada (1989)
Croatia
- Sisak 1988-1989 0.5 1.0a <1-4 Fingler et al. (1992)
- Zagreb 2.0a 1-3
USA 1977-1981 100 ND US EPA (1985b)
Surface water
Canada
- Lake Superior 1986 0.007 0.026 0.018-0.040 Stevens & Neilson
- Lake Huron 0.033 0.018-0.073 (1989)
- Georgian Bay 0.041 0.032-0.054
- Lake Erie 0.078 0.025-0.260
- Lake Ontario 0.063 0.020-0.113
Canada-St. Clair River 1985 0.30-87 Oliver & Kaiser (1986)
- tributaries to 0.08-0.79
St. Clair R.
Table 5 contd.
Location Year Detection Mean Rangea Reference
limit
Canada (Atlantic Region); 1979-1989 2.0 ND-2.2 Leger 1991
lakes, streams, reservoirs,
estuaries, coastal waters
Germany (Elbe) 1990 - 12 3-62 BUA (1994)
Greece (Strimon River) 1985-1986 - 1.52 0.5-2.8 Kilikidis et al. (1992)
Italy (tributaries to
Adriatic Sea) 1977-1978 1.0 ND Galassi & Provini (1981)
Mediterranean Sea 1982-1983 0.1 2.13 ND-12.6 El-Dib & Badawy (1985)
Netherlands/Belgium 1993 10 <10 <10 RIWA (1993)
Netherlands 1987 - <10 ND-100 De Walle et al. (1995)
North Sea (coastal waters
and estuaries) 1979-1980 2.7 0.03-15 Ernst (1986)
Scotland (Forth Estuary) 1987 0.01 <0.01-196 Rogers et al. (1989)
Scotland (Forth Estuary) 1990 0.7-8.0 Harper et al. (1992)
Spain (Ebre Delta) 1985-1986 0.0005 0.041 ND-1.0 Grimalt et al. (1988)
USA (Texas-estuary) 1980 0.24 <0.01-0.61 Ray et al. (1983a)
USA (coastal, surface <0.1 <0.1-26 Cross et al. (1987)
microlayer)
a median value
Table 6. Levels of hexachlorobenzene in soil (ng/g dry weight)
Source Year Detection Mean Rangea Reference
limit
Canada (British Columbia) 1.0 Wilson & Wan (1982)
- agricultural soils <1.0-2.2
- near a former grain 260 ng/g
treatment plant
Czech/Polish Border - - 3.25 0.47-4.8 Holoubek et al. (1994)
(Giant Mountains)
Germany (contaminated soil) 1989 0.3-339 Hagenmaier et al. (1992)
India 1987 24a 0-165 Nair & Pillai (1989)
Italy (farming area) 1971-1972 40 Leoni & D'Arca (1976)
Netherlands - Ochten 1993 - 18 5.1-66 Hendriks et al. (1995)
- Gelderse Poort 80 73-89
Netherlands 1987 - <10 <80 De Walle et al. (1995)
Sweden 5.1 Thomas et al. (1985)
USA 1968-1973 10-440 Carey et al. (1979)
USA (chemical plants) 0.002 ND-5 700 000 Spigarelli et al. (1986)
USA (hazardous waste sites) 1977-1978 20 000-400 000 Davis & Morgan (1986)
USA (5 locations near Love 0.1 1.04-5.6 0.15-26.3 Ding et al. (1992)
Canal)
a ng/g wet weight
Levels in soil are highest near industrial sources of HCB. Levels
as high as 12 600 ng/g dry weight were reported at one landfill site
in Canada (Wilson & Wan, 1982), and 570 µg/g (dry or wet weight not
indicated) on the grounds of a chlorinated solvent and pesticide
production plant in the USA (Spigarelli et al., 1986). Soils near a
former grain treatment plant in Canada contained 260 ng/g dry weight
of HCB (Wilson & Wan, 1982). Levels of HCB in soils from contaminated
floodplains in the Netherlands ranged from 5.1 to 89 ng/g dry weight
(Hendriks et al., 1995).
5.1.4 Sediment
HCB strongly sorbs to sediment and suspended matter, and
differences in the concentrations in the water as well as in the
composition of the sediments and suspended matter result in a wide
range of concentrations in this medium.
In sediment samples collected from 1979 to 1989 in the Atlantic
provinces of Canada, HCB was reported to be below the limit of
detection of 0.2 ng/g dry weight in 140 of 152 samples (Leger, 1991).
In surveys conducted from 1980 to 1983, HCB levels in sediments from
the Great Lakes ranged from 0.02 to 840 ng/g dry weight (Oliver &
Nicol, 1982; Fox et al., 1983; Kaminsky et al., 1983; Oliver &
Bourbonniere, 1985; Bourbonniere et al., 1986; Oliver et al., 1989;
IJC, 1989). Analyses of sediment cores from Lake Ontario indicated
that levels of HCB have declined from the 1960s to the early 1980s but
more recent data are not available to determine if this downward trend
has continued (Oliver & Nicol, 1982; Oliver et al., 1989). HCB levels
in sediment sampled from eight lakes in northern remote Canada (date
of sampling not specified) ranged from 0.09 to 1.80 ng/g dry weight
(Muir et al., 1995).
Levels as high as 5100 ng/g dry weight were detected in the Rhine
River in Baden-Württemberg, Germany, in 1986 (BUA, 1994). The majority
of sediment samples taken from the rivers Rhine and Elbe between 1980
and 1990 contained levels of HCB between 10 and 500 ng/g dry weight,
although levels below 1 ng/g dry weight were determined in some other
locations (BUA, 1994). A Nordic study on chlorinated compounds in the
Baltic, Kattegat and Skagerrak (œstfeldt et al., 1994) found HCB
concentrations in sediment ranging from 1 to 20 ng/g loi (loss on
ignition), the higher values occurring mainly in the Bothnian Bay. An
extreme value of 63 ng/g loi was found in Öresund between Denmark and
Sweden. Levels of HCB in sediment samples collected near effluent
discharges along a stream in Pakistan ranged from <0.05 to 94.5 ng/g
wet weight (Tehseen et al., 1994).
Higher levels of HCB in sediments were reported in studies
conducted near point sources. As much as 280 000 ng HCB/g dry weight
was detected in 1985 downstream of the Dow Chemical sewer discharges
in the St. Clair River, USA (Oliver & Pugsley, 1986).
5.1.5 Biota
HCB has been detected in invertebrates, fish, reptiles, birds and
mammals from around the world. Following the detection of HCB in
tissues of wild birds by De Vos in 1967, high residues were often
found in predatory birds, whereas minor quantities were detected in
fish, mussels and birds of the aquatic environment (Vos et al., 1968;
Koeman et al., 1969). Based on Canadian data from monitoring studies
in birds, HCB levels declined sharply from the mid-1970s (the earliest
data available) and into the early 1980s, after which they levelled
off (Noble & Elliott, 1986; Environment Canada/Department of Fisheries
and Oceans/Health and Welfare Canada, 1991).
Levels of HCB in freshwater mussels in the Great Lakes and
connecting channels have been found to range from 0.1 ng/g wet weight
to 24 ng/g wet weight (Kauss & Hamdy, 1985; Innes et al., 1988;
Muncaster et al., 1989). A similar range (4.4-26 ng/g wet weight) was
observed in benthic amphipods, the pelagic amphipod Pseudalibrotus
litoralis and brittle stars from the Beaufort Sea (Hargrave et al.,
1989). Lower levels (0.1-1.8 ng/g wet weight) were observed in mussels
( Mytilus galloprovincialis) from the Ebro Delta in the Western
Mediterranean, and these levels were observed to decline from 1980 to
1992 (Solé et al., 1994). Levels in marine species of clams and
oysters from the USA were reported in several studies to be < 1 ng/g
wet weight (Phelps et al., 1986; Eisenberg & Topping, 1984; Ray et
al., 1983b). Similarly, levels in invertebrates, including mussels
( Mytilus edulis), soft clams ( Mya arenaria), lugworms ( Arenicola
marina), and polychaetes ( Nereis diversicolor), were <1 ng/g
fresh weight in the German Wadden Sea (Ernst, 1986). Bjerk & Brevik
(1980) reported higher levels (50-350 ng/g wet weight) of HCB in crabs
( Carcinus maenas, Pagurus sp.), snails ( Littorina littorea),
brittle stars ( Ophiura albida) and sea stars ( Asteroidea) from the
contaminated Frierfjord in Norway, which receives discharge from
various industries located in the region, and HCB and related
compounds were reported to originate from one main source
(unspecified) in the area. œstfeldt et al. (1994) found that mussels
( Mytilus edulis) from the Baltic contain higher levels of HCB
(200-800 ng/g lipid weight) than mussels from Kattegat (11-20 ng/g
lipid weight).
In a 1981-1982 survey of HCB levels in fish from watersheds in
Eastern Canada, whole body concentrations in brook trout ( Salvelinus
fontinalis) and yellow perch ( Perca flavescens) ranged from below
the limit of detection (4.2 ng/g in 1981; 0.2 ng/g in 1982) to 54 ng/g
for trout and 15 ng/g wet weight for perch (Peterson & Ray, 1987).
Relatively high body burdens of HCB have been observed in fish in Lake
Ontario and connecting channels. HCB was not detected (ND) in juvenile
spottail shiners ( Notropis hudsonius) from Lakes Superior and Erie
(detection level = 1 ng/g wet weight) (Suns et al., 1983; Environment
Canada/Department of Fisheries and Oceans/Health and Welfare Canada,
1991), while mean body burdens in shiners in Lake Ontario ranged from
ND to 13 ng/g wet weight, and those in the Detroit, Niagara, and
St Clair rivers averaged 5 ng/g wet weight, ND to 8 ng/g wet weight,
and 231 ng/g wet weight, respectively (Suns et al., 1985). Mean
concentrations of HCB in the muscle tissue of various species of
salmonids from Lake Ontario ranged from 5 to 37 ng/g wet weight (Niimi
& Oliver, 1989).
Levels of HCB measured in whole fish species taken from major
rivers and lakes in the USA (including known contaminated areas)
ranged from <2 to 913 ng/g wet weight (Kuehl et al., 1983; DeVault,
1985; Schmitt et al., 1990; Kuehl & Butterworth, 1994). Levels in
roach ( Rutilus rutilus L.) and perch ( Perca fluviatilis L.) from
the "moderately polluted" Lahn River in Germany ranged from ND to
233 ng/g wet weight, with a mean of 1 ng/g (Schuler et al., 1985).
Concentrations of HCB in the whole bodies of carp ( Cyprinus carpio)
from the mouth of tributaries to Lake Ontario and the Niagara River
ranged from 52 to 1600 ng/g on a lipid basis (6.7 to 205 ng/g on a
fresh weight basis). The highest values were measured near hazardous
waste dumps and industrial facilities (as high as 1600 ng/g fat)
(Jaffe & Hites, 1986). Brunn & Manz (1982) reported a mean whole-body
concentration of HCB in fish (mainly trout) from inland rivers,
streams, and ponds in Germany of 5 ng/g wet weight. The highest levels
were recorded from fish caught in rivers.
HCB levels in seawater are generally lower than those in
freshwater, resulting in lower levels in edible parts of marine fish.
In fish taken from the North Sea (species not reported), HCB levels in
fish muscle tissues averaged 0.3-0.4 ng/g wet weight, with a maximum
of 0.8 ng/g (Ernst, 1986). HCB concentrations in livers averaged
42 ng/g wet weight for cod ( Gadus morhua) and 4 ng/g (range of
0.2-14 ng/g) for flounder ( Platichthis flesus). These levels were
comparable to levels measured in fish near the coast of southwest
Greenland and in the North Atlantic Ocean. Livers of cod from the
coast of southwest Greenland contained 32.4 ng/g on average, and those
of hake ( Merluccius merluccius) from the North Atlantic Ocean
averaged 40.5 ng/g) (Ernst, 1986). Levels of HCB were below the
determination limit (DL) in cod liver (DL = 5 ng/g) and herring muscle
(DL = 1 ng/g) of fish from the Clyde Sea near Scotland (Kelly &
Campbell, 1994). Cod from the Firth of Forth had mean liver levels of
38.7 ng/g wet weight, and levels in herring muscle of 2.0 and 2.3 ng/g
wet weight were observed in fish from the Firth of Forth and North
Sea, respectively (Kelly & Campbell, 1994). In surveillance monitoring
of contaminants in fish from coastal waters near England and Wales,
concentrations of HCB in livers of cod ( Gadus morhua), whiting
( Merlangius merlangus), dab ( Limanda limanda) and flounder
( Platichthys flesus) were 2-290, 5-230, 3-55 and 1-52 ng/g,
respectively (all results on a wet tissue weight basis) (MAFF/HSE,
1994). Levels of HCB in muscle tissues of herring ( Clupea harengus)
from the Baltic Sea ranged from <1 to 39 ng/g (Hansen et al., 1985);
concentrations in whitefish ( Coregonus lavaretus) and trout ( Salmo
trutta) ranged from <1 to 9 ng/g fresh weight in a 1992 survey
(Atuma et al., 1993).
Fish taken from the contaminated waters of the Frierfjord in
Norway contained mean concentrations of HCB in liver of 11 600 ng/g
for saithe ( Pollachius virens), and 16 800 ng/g for cod ( Gadus
morhua) (Bjerk & Brevik, 1980). Levels of HCB from fish taken from
the uncontaminated Sogndalfjord were much lower, averaging 18 ng/g wet
weight in livers of cod ( Gadus morhua), 8 ng/g in haddock
( Melanogrammus aeglefinus) and 1 ng/g in lemon sole ( Microstomus
kitt) and flounder ( Platichthys flesus) (Skåre et al., 1985).
Flounder ( Platichthis flesus) taken from the Elbe Estuary in
Germany, downstream from Hamburg (a highly industrialized area),
contained mean levels of HCB in muscle of 688 ng/g (range 84-1907 ng/g
wet weight). Further downstream, towards the mouth of the river,
levels were lower, averaging 12.5 ng/g (range 2-32 ng/g) (Kohler et
al., 1986).
The mean level of HCB in 15 snapping turtle eggs from Ontario,
Canada was 27.1 ng/g wet weight (Bishop et al., 1995).
The levels of HCB in birds have been similar across the various
regions of Canada since the 1980s, probably as a combined result of
emission reductions and the long-range transport of HCB to remote
locations. Mean concentrations of HCB in herring gull eggs ( Larus
argentatus) in 1991 ranged from 16 to 71 ng/g wet weight at various
colonies in the Great Lakes, and were relatively uniform across lakes
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991). These levels were approximately an order of
magnitude lower than in 1974. The mean level of HCB in herring gull
eggs from Norwegian coastal waters in 1981 was 120 ng/g wet weight
(Moksnes & Norheim, 1986). In a study from the Netherlands, mean
levels in eggs of common terns collected in 1987 were 0.03 µg/g wet
weight and in those of black-headed gulls collected in 1988 were
93 µg/g fat (Stronkhorst et al., 1993). Levels of HCB found in eggs of
sea-bird species ( Haematopus ostralegus, Larus ridibundus, Larus
argentatus and Sterna hirundo) from the banks of a river near an
organochlorine chemical plant in Germany were < 500 ng/g wet weight
(Heidmann, 1986); mean levels of less than 15 ng/g wet weight were
found in eggs of several species of land birds, including rooks
( Corvus frugilerus) and sparrow hawks ( Accipiter nisus) from
agricultural, industrial and rural sites. Recent surveys have
indicated similar levels of HCB in the eggs of five other predatory
bird species across Canada (means ranged from 10 to 53 ng/g wet
weight) (Noble & Elliott, 1986; Pearce et al., 1989; Noble et al.,
1992). However, the mean level of HCB in peregrine falcon ( Falco
peregrinus) eggs collected across Canada from 1980 to 1987 was
279 ng/g wet weight, and concentrations ranged as high as 1060 ng/g
wet weight (Peakall et al., 1990).
HCB has been found to accumulate in lipids of the common
goldeneye duck ( Bucephala clangula) that overwinter in the Niagara
River (mean of 150 ng/g) (Foley & Batcheller, 1988) and the Detroit
River (mean of 1700 ng/g) (Smith et al., 1985a) in the USA. Goldeneye
wintering in the Baltic Sea contained average levels of 250 ng/g lipid
(Falandysz & Szefer, 1982). Levels of HCB in the livers of silver
seagulls taken from estuaries in Germany were lower in 1988 than 1989
(approximately 80 and 150 ng/g fat, respectively, in samples from the
River Ems estuary). Higher levels were observed for both years in
liver samples of birds taken from the River Elbe estuary (>250 ng/g
fat) (BUA 1994).
In breast muscle tissue samples from various species of birds,
HCB concentrations tend to be progressively greater at higher trophic
levels (i.e., piscivores > molluscivores > omnivores > grazers)
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991).
In the blubber of marine mammals in the Canadian Arctic, mean
levels of HCB were 19 ng/g wet weight for ringed seals ( Phoca
hispida) and 491 ng/g wet weight for beluga whales ( Delphinapterus
leucas) (Norstrom et al., 1990), while male belugas sampled in the
Gulf of St. Lawrence contained up to 1340 ng/g (Béland et al., 1991).
Blubber from male and female white-beaked dolphins ( Lagerorhunchus
albirostris) collected near the Newfoundland coast averaged
1110 ng/g and 880 ng/g wet weight, respectively. Lower levels
(290 ng/g and 100 ng/g wet weight) were observed in blubber from male
and female pilot whales ( Globicephala meleana), also collected near
the Newfoundland coast (Muir et al., 1988). The higher levels observed
in the dolphins may reflect greater exposure to HCB because of
overwintering and feeding in the Gulf of St. Lawrence. Blubber of
harbour porpoises ( Phocoena phocoena) collected in Poland between
1989 and 1990 contained an average of 573 ng/g wet weight (Kannan et
al., 1993), and those collected around the coast of Scotland between
1989 and 1991 contained an average of 263 ng/g (Wells et al., 1994).
Levels of HCB in the blubber of bottlenosed dolphins also collected
off the coast of Scotland contained an average of 276 ng/g (Wells et
al., 1994). Levels in the blubber of three species of dolphins from
the Bay of Bengal, southern India, were low, ranging from 1.1 to
13 ng/g wet weight (Tanabe et al., 1993). Harbour seals ( Phoca
vitulina) found sick or dead in Norwegian waters due to a disease
outbreak caused by a morbilli virus had a mean HCB level in the
blubber of 27 ng/g wet weight (range of 5-94 ng/g) (Skaare et al.,
1990).
Limited data were found on levels of HCB in terrestrial mammals.
In a 1973-1974 survey of HCB in the adipose tissue of fox ( Vulpes
vulpes), doe ( Capreolus capreolus) and wild boar ( Sus scrofa) in
Germany, HCB concentrations ranged from <10 to 3110 ng/g. The lowest
levels were observed in the does, presumably because they are
herbivorous, whereas foxes and wild boar feed on small animals and are
therefore more affected by biomagnification of HCB (Koss & Manz,
1976). Similar patterns were evident in a study from Sweden, in which
rabbits ( Oryctolagus cuniculus, muscle), moose ( Alcaes alcaes,
muscle), reindeer ( Rangifer tarandus, suet) and osprey ( Pandion
haliaetus, muscle) were found to contain 9, 15, 51 and 330 ng HCB/g
lipid weight, respectively (Jansson et al., 1993). The mean
concentration in 66 serum samples taken in muskoxen in the Canadian
Northwest Territories in 1989 was 2.8 ng/g (range of 1.1-7.5 ng/g)
(Salisbury et al., 1992). The mean concentration of HCB in fat samples
from 58 caribou from the same region ranged from 32.93 to 129.4 ng/g
(lipid corrected) (Elkin & Bethke, 1995). The mean concentration of
HCB in the livers and lipids of adult river otters ( Lutra
canadensis) in western Canada were 3 ng/g and 30 ng/g wet weight,
respectively, for females and 4 ng/g and 25 ng/g wet weight,
respectively, for males (Somers et al., 1987). Concentrations of HCB
in mink carcasses collected in Ontario in the late 1970s and early
1980s ranged from < 0.5 to 10 ng/g wet weight (Proulx et al., 1987).
In the Canadian north, the mean level of HCB in the fat of polar bears
( Ursus maritimus) hunted between 1982 and 1984 was 296 ng/g wet
weight (Norstrom et al., 1990).
5.1.6 Food and drinking-water
HCB is commonly detected, at low levels, in food (Table 7).
Levels of HCB tend to be highest in fatty foods and/or those that have
been treated with HCB-contaminated pesticides. The most extensive data
identified have been collected through the United States Food and Drug
Administration (US FDA) Total Diet Study. The results of the surveys
from 1982 to 1991 indicate that HCB is detectable (DL = 0.1 ng/g) in a
small fraction of food items, most often dairy products, meats, and
peanuts/peanut butter (KAN-DO Office and Pesticides Team, 1995). In
the most recent surveys, conducted during 1990-1991, mean levels were
less than 1 ng/g for all products.
Table 7. Concentration (µg/kg wet weight unless otherwise specified) of hexachlorobenzene in various foods
Country Food Mean contenta Range Reference
Australia cereals 0.01 < 0.01-0.01 Kannan et al. (1994)
pulses 0.02 0.01-0.05
oils 0.07 0.02-0.11
beverages 0.03 0.02-0.04
vegetables 0.01 < 0.01-0.02
fruits 0.01 < 0.01-0.02
dairy products 0.55 0.14-1.6
meat and fat 0.46 0.01-3.0
fishes 4.2 < 0.01-60
Canada fresh meat & eggs 0.17 Davies (1988)b
root vegetables & potato 0.04
fresh fruit ND(<0.01)
leafy/other above-ground vegetables 0.02
2% milk 0.16
Canada apples ND(<0.2)-2.6 OMAF/OME (1988)
peaches ND(<0.2)
tomatoes ND(<0.2)
potatoes ND(<0.2)
wheat ND(<0.2)
eggs ND(<0.2)
hamburger 0.39 0.2-0.57
prime beef ND(<0.2)-0.21
pork ND(<0.2)
chicken ND(<0.2)
Table 7 contd.
Country Food Mean contenta Range Reference
Germany milk 0.22d 0.088-0.45d Fürst et al. (1992)
cream 0.98d 0.31-1.30d
butter 4.86d 2.32-6.88d
cheese 2.72d 2.16-3.70d
India cereals 0.03 0.01-0.04 Kannan et al. (1992a)
pulses (edible seeds of legumes) 0.07 0.02-0.16
spices 0.22 <0.01-0.54
oils 1.5 0.09-2.8
milk 0.03 0.01-0.10
butter 1.7 0.86-2.4
fishes & prawn 0.07 <0.01-0.55
meat & animal fat 0.61 0.02-4.8
Mexico cheese 16.67d 1 Albert et al. (1990)
Morocco eggs 20.9 0.09-300 Kessabi et al. (1990)
poultry liver 5.1 trace-30.0
bovine liver 21.9 1.2-119.8
bovine kidney 15.1 trace-133.0
Papua New cheese 0.43 Kannan et al. (1994)
Guinea pork fat 0.40
chicken 0.20
striped mullet 0.04
tilapia 0.01 0.02-0.05
mud crab 0.03 < 0.01-0.02
oyster 0.02 < 0.01-0.05
Table 7 contd.
Country Food Mean contenta Range Reference
Solomon Islands pork 0.14
chicken 0.06
greenspotted kingfish 0.03 0.01-0.06 Kannan et al. (1994)
indian mackerel 0.01 0.01
paddletail snapper 0.01 0.01
Southern Baltic canned cod-livers 60 ± 6 50-76 Falandysz et al. (1993)
Spain bologna - fresh 2.57d Ariño et al. (1992)
- cooked 2.48d
Spain pork sausage Ariño et al. (1992)
- before curing 6.63d
- after 30 days curing 6.0d
Spain ham - fresh 3.46d Ariño et al. (1992)
- cured 1.29d
Spain pork 2.86-3.9d Ariño et al. (1993)
Spain lamb - chop, raw 14.67d Conchello et al. (1993)
- chop, grilled 12.06d
- leg, raw 8.53d
- leg, roasted 7.02d
Spain chicken 120 ± 10 To-Figueras et al. (1986)
calf 249 ± 37
rabbit 860 ± 159
pork 169 ± 20
sheep 225 ± 35
butter 315 ± 18
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom bread ND (10) MAFF/HSE (1994)
milk 0.6
butter ND(10)
cheese 3.33d
ewes' cheese ND(10)
pasta ND(10)
beef burgers ND(10)
canned meat 10d
cooked meats 10d
lamb ND(10)
rabbit ND(10)
salami ND(10)
United Kingdom sausages ND(10) MAFF/HSE (1994)
pies and pasties ND(10)
salmon (tinned) 2.0
breaded cod ND(2.0)
fish cakes 2.0
mackerel 20
plaice ND(2.0)
prawn products ND(2.0)
sardines (tinned) ND(2.0)
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom carrot 0.0317 Wang & Jones (1994)
potato 3.35
cabbage 0.0418
cauliflower 0.0729
lettuce 0.108
onion 0.0014
bean 0.0101
pea 0.0039
tomato 0.0139
USA cheese, processed 0.2 ND-0.5 US FDA
cheese, cheddar 0.1 ND-0.5 (unpublished)c
beef, ground (regular) 0.1 ND-0.4
beef, chuck roast 0.3 ND-1.0
beef, round steak 0.2 ND-1.0
beef, loin/sirloin steak 0.2 ND-1.0
USA lamb chop 0.3 ND-1.0 US FDA
frankfurters 0.1 ND-0.6 (unpublished)c
cod/haddock fillet ND(0.1) ND-0.2
eggs, scrambled 0.1 ND-0.3
eggs, fried 0.2 ND-0.7
peanut butter 0.2 ND-0.4
peanuts, dry roasted 0.3 ND-1.0
watermelon 0.1 ND-0.5
butter 0.6 ND-1.0
cream 0.1 ND-0.4
Table 7 contd.
Country Food Mean contenta Range Reference
Viet Nam rice 0.03 <0.01-0.05 Kannan et al. (1992b)
pulses 0.04 <0.01-0.18
oil 1.2
butter 5.0
animal fat 0.41 0.29-0.65
meat 0.11 0.03-0.18
fish 0.05 0.01-0.31
prawn 0.03
shellfish 0.04
crab 0.17
caviar 3.8 1.9-7.2
a ND = not detected (detection limit given in brackets).
b Fresh produce and meats grown in Ontario were purchased from four grocery stores in Toronto when locally grown produce
was available (Ontario freshwater fish were not available and therefore, were excluded from analysis). All food items
were grouped into one of five composites for analysis, with the relative proportions of different food items in each
composite calculated from the estimates of the amounts purchased per person per year by Ontario residents.
c The US Food and Drug Administration Total Diet Study conducted from April 1990 to April 1991; reporting residue levels
in 234 individual food items collected from 3 cities in each of 4 geographical regions of the USA (data available from
US FDA, Washington, DC).
d Originally reported on a fat basis, and subsequently converted to wet weight using percentage fat contents reported in
NHW (1987).
In a number of more limited recent surveys, HCB levels have been
determined in commercial foods available in several countries from
North America, Europe and Asia (Table 7). The results of these studies
are consistent with the USA study described above, in that HCB has
been detected primarily in fatty foods such as meats and dairy
products. In these studies, mean concentrations are generally in the
low ng/g range or less, although substantially higher concentrations
have been reported in some surveys from Europe and Asia.
The effects of cooking, curing and ripening on the HCB residues
in pork meat products were investigated in Spain by Ariño et al.
(1992). Neither cooking at 80-82°C for 100 min nor curing reduced the
HCB content in pork bologna and pork sausage, respectively, whereas
the level of HCB in dry-salted and cured ham declined by 42%
throughout maturation.
HCB has been detected infrequently, and at very low
concentrations in drinking-water supplies (Table 5). Samples of
drinking-water collected in 1980 from Canadian cities in the vicinity
of Lake Ontario contained from 0.06 to 0.20 ng/litre, with a mean of
0.1 ng/litre (Oliver & Nicol, 1982). In other Canadian and USA
surveys, HCB was not detected (US EPA 1985b - DL = 100 ng/litre;
Environment Canada, 1989 - DL = 2 ng/litre). Slightly higher
concentrations of HCB (median of 1-2 ng/litre) were reported in
Croatian drinking-water supplies drawn from a nearby polluted river
(pollution sources were not identified) (Fingler et al., 1992).
5.2 General population exposure
5.2.1 Human tissues and fluids
Owing to its persistence and lipophilicity, HCB is present at low
levels in the fatty tissues of virtually all members of the general
population. Levels of HCB in adipose tissues, breast milk, blood and
follicular fluid of various populations from around the world are
shown in Table 8. It should be noted that the quality of the studies
given in Table 8 varies quite widely, from extensive national surveys
to those with relatively few samples.
Levels of HCB in human adipose tissue from around the world are
generally <1 mg/kg (Table 8). Although available data are limited,
concentrations of HCB reported in fat tissue are generally slightly
higher in samples from European countries than from elsewhere in the
world. The highest levels reported in recent surveys are from Spain
(mean levels of approximately 3-6 mg/kg); the authors suggested that
this reflected contamination of foods caused by its presence as an
impurity in other pesticides (Camps et al., 1989; Gómez-Catalán et
al., 1993, 1995). Concentrations of HCB increased with age in a number
of these surveys, but there were no consistent differences in residue
levels between the sexes (Mes et al., 1982; Williams et al., 1984,
1988; Abbott et al., 1985; Mes, 1990; Mes et al., 1990; Gomez-Catalan
et al., 1993; Kemper, 1993; Ludwicki & Góralczyk, 1994).
In general, concentrations of HCB in breast milk in various
countries or regions (Table 8) range widely, and appear to be related
to the degree of industrialization and/or urbanization within the
survey area. The levels of HCB in breast milk have been expressed on a
whole milk basis, using the fat content reported by the authors or,
where this was not reported, a fat content of 4.2% (NHW, 1987).
Schechter et al. (1989a) reported that concentrations of HCB in breast
milk in the mid-1980s were lowest in samples from Thailand
(0.3 µg/litre whole milk) and Viet Nam (< 0.17 µg/litre ), somewhat
higher in those from a semi-rural area of the USA (0.7-0.8 µg/litre),
and higher still in German samples (12.6 µg/litre ) (numbers of
samples in this study were extremely small, except for the German data
(n=167)). In surveys summarized by Mes et al. (1986), mean HCB levels
were 1 µg/litre whole milk in the USA, 2 µg/litre in Canada,
3 µg/litre in Sweden, 4 µg/litre in Great Britain, and 35µg/litre in
Germany. Still higher levels (48-89 µg/litre whole milk) have been
reported in studies from Spain (Conde et al. 1993). Bates et al.
(1994) reported that the concentrations of HCB in breast milk of
primiparae from New Zealand increased linearly with age, but were not
related to body mass index, fish intake, smoking status, type of
residential water supply or location of residence (urban versus
rural). In a study of body burdens of organochlorines in an indigenous
population, Ayotte et al. (1995) reported that mean concentrations of
HCB in the milk fat of 107 Inuit women from northern Quebec were
several times higher than those in 50 Caucasian women from southern
Quebec (57 and 1.2 µg/litre whole milk, respectively). Levels of
organochlorine compounds in breast milk correlated with levels of
omega-3 fatty acids in plasma phospholipids, indicating that
consumption of marine organisms is an important source of exposure to
these xenobiotics.
In a HCB poisoning incident in Turkey (section 8.1), breast-fed
infants were fatally intoxicated through their mothers' milk. In an
early report of this incident (Peters et al., 1966), HCB was reported
as being present in breast milk, although it was not quantified.
However, elevated levels were measured (mean of 510 ng/g on a fat
basis (approximately 21 ng/g on a wet weight basis) for 56 porphyric
mothers) 20-30 years after the incident, compared with a mean of
70 ng/g fat in 77 milk samples from women of families without
porphyria or from areas outside of the endemic area (Peters et al.,
1982; Gocmen et al., 1989).
Table 8. Levels of hexachlorobenzene in human tissues and fluids
(mg/kg wet weight adipose tissue; mg/kg whole milk; µg/litre blood serum; µg/litre follicular fluid)
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue
Australia 31 0.14a (0.01-1.70) (fat basis) 1990-1991 Stevens et al. (1993)
Canada 108 0.026a (0.0073-0.118) 1985 Mes & Malcolm (1992),
Mes et al. (1990)
Canada 25 0.019a (max. value: 0.087) - Mes (1992)
Canada 141 0.071 (males)
(0.018-0.244)
0.109 (females)
(0.019-0.373) 1984 Williams et al. (1988)
Canada 99 0.095 (0.01-0.667) 1976 Mes et al. (1982)
Canada 168 0.062 (0.001-0.52) 1972 Mes et al. (1977)
Federal Republic of Germany 93 (0.11-21.8) 1971 Leoni & D'Arca (1976)
Federal Republic of Germany 6 0.263 (0.083-0.753) van der Ven et al. (1992)
India 7 0.012 (0-0.064) 1987 Nair & Pillai (1989)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Italy 28 0.491 (0.126-1.36) 1973-1974 Leoni & D'Arca (1976)
Japan 39 0.044 1986-1987 Kashimoto et al. (1989)
Japan 15 0.21 (0.10-0.42) - Morita et al. (1975)
Netherlands average 0.7a (fat basis) 1968-1969 Greve & Van Zoonen (1990)
of 1.2a (fat basis) 1973-1975
51/year 0.86a (fat basis) 1976
0.98a (fat basis) 1977-1978
0.85a (fat basis) 1980
0.80a (fat basis) 1981
0.58a (fat basis) 1982
0.49a (fat basis) 1983
0.42a (fat basis) 1985
0.38a (fat basis) 1986
New Zealand - 0.31 - US EPA (1985a)
Poland 53 0.221 (0.068-0.937) early 1980s Szymczyœski et al. (1986)
Poland 277 0.31 1989-1992 Ludwicki & Góralczyk (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Spain 256 2.99 1985-1988 Gómez-Catalán et al. (1993)
(4 cities)
Spain 86 3.37 (0.42-12.53) 1991 Gómez-Catalán et al. (1995)
(lipid basis)
Spain 171 5.55 1982-1983 To-Figueras et al. (1986)
Spain 168 2.95 (0.2-17.37) 1988-89 Ferrer et al. (1992)
United Republic of Tanzania 9 0.003 (0.0013-0.0076) - van der Ven et al. (1992
United Kingdom 201 0.05 (n.d-0.29) 1969-1971 Abbott et al. (1972)
United Kingdom 236 0.19 (0.02-3.2) 1976-1977 Abbott et al. (1981)
United Kingdom 187 0.11 (0.03-0.32) 1982-1983 Abbott et al. (1985)
USA 10 0.125 (0.03-0.47 - Barquet et al. (1981)
USA 6081 0.037a 1974-1983 Robinson et al. (1990)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
USA 763 0.118 (0.001-0.256) 1982 US EPA (1994)
689 0.043 (0.032-0.054) 1984
671 0.051 (0.043-0.059) 1986
B. Breast milkb
Australia 39 0.042 (rural) 1970 Newton & Greene (1972)
28 0.063 (urban)
Australia 137 0.007 (0.002-0.019) (rural) 1979-1980 Stacey et al. (1985)
130 0.008 (0.002-0.017) (urban)
Australia 60 0.017 (0.0007-0.32) - Quinsey et al. (1995)
Australia 128 0.0036a (<0.01-0.216) 1990-1991 Stevens et al. (1993)
Brazil 30 0.00048 (0.00024-0.0036)c 1987-1988 Beretta & Dick (1994)
Canada 412 0.0008 (max = 0.014) 1986 Mes et al. (1993)
Canada 210 0.002 (max.= 0.009) 1982 Mes et al. (1986)
Canada 100 0.002 (max.= 0.021) 1975 Mes & Davies (1979)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Canada 536 0.0013c 1989-1990 Dewailly et al. (1991)
Canada 127 0.00051 1978 Frank et al. (1988)
15 0.0004 1979
12 0.00028 1980-1981
13 0.00052 1983-1984
18 0.00026 1985
Finland 143c 0.002c 1984-1985 Mussalo-Rauhamaa et al. (1988)
Finland 50 0.0023 (0.0007-0.006) 1982 Wickström et al. (1983)
France 20 0.002 (0.00004-0.008)c 1990-1991 Bordet et al. (1993)
Federal Republic of Germany 144 0.021c 1984 Fürst et al. (1994)
220 0.019c 1985
157 0.015c 1986
144 0.015c 1987
196 0.013c 1988
145 0.01c 1989
286 0.0095c 1990
113 0.0074c 1991
Federal Republic of 167 0.0126c 1985-1987 Schecter et al. (1989a)
Germany
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Federal Republic of 2709 0.048c 1979-1981 BUA (1994)
Germany 3778 0.013c 1986
1897 0.014c 1987
2994 0.011c 1988
3256 0.01c 1989
5340 0.009c 1990
Former German 483 0.007c 1990-1991
Democratic Republic
India 16 0.042 (0-0.25)e 1987 Nair & Pillai (1989)
Israel 100 0.00256 - Weisenberg et al. (1985)
Italy 56 0.058d - Franchi & Focardi (1991)
Italy 64 0.006 (0.004-0.009) 1987 Larsen et al. (1994)
Netherlands 202 0.036a,c 1972-1973 Greve & Van Zoonen (1990)
278 0.008a,c 1983
New Zealand 38 0.0011 1988 Bates et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Norway 28 0.0007 1991 Johansen et al. (1994)
Spain 240 0.089(0.039-0.21)c 1984-1987 Conde et al. (1993)
358 0.048(0.037-0.073)c 1990-1991
Sweden 20 0.0042 (0.002-0.009)c 1978 Norén (1983a)
Sweden 2 0.0007-0.004c - Norén (1983b)
Sweden 227 0.003 (0.002-0.004)c 1972 Norén (1988)
245 0.003 (0.003-0.004)c 1976
340 0.003 (0.003-0.004)c 1980
102 0.001 (0.0008-0.001)c 1984-1985
Sweden 140 0.0012c 1989 Norén (1993)
Sweden 40 0.0017c 1986-1987 Vaz et al. (1993)
Thailand 3 0.0003c 1985-1987 Schecter et al. (1989a)
Turkey 51 0.0035c 1988 Üstünbas et al. (1994)
Turkey 56 0.021c 20-30 years Gocmen et al. (1989)
post-exposure
during
1955-1959
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
United Kingdom 193 0.001 (<0.001-0.005) 1989-1991 MAFF (1992)
USA 40 0.00052 1979 Bush et al. (1985)
USA 8 0.0007-0.0008c 1985-1987 Schecter et al. (1989a)
Viet Nam 12 <0.00017c 1985-1987 Schecter et al. (1989a)
Yugoslavia 10 0.006 (0.002-0.017) 1978 Kodric-Smit et al. (1980)
C. Serum
Canada 25 0.25 1993 Jarrel et al. (1993)
29 0.21
20 0.35
Croatia 15 1a (<0.5-4) 1985 Krauthacker (1993)
24 0.9a (<0.5-3) 1987-1988
26 1a (<0.5-7) 1989-1990
32 <0.5a (<0.5-4) 1990
Germany 6 1.23 (0.33-2.66)f - van der Ven et al. (1992)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
C. Serum (contd)
Spain (near 21 26 (7.5-69) 1992 Grimalt et al. (1994)
organochlorine
compounds
factory)
Spain (hospital 13 4.8 (1.5-15) 1992 Grimalt et al. (1994)
in Barcelona)
Spain 100 11.09 (1.60-94.2) 1986 To-Figueras et al. (1995)
4.13 (0.70-19.7) 1993-1994
United Republic 11 0.01 (0-0.03)f - van der Ven et al. (1992)
of Tanzania
USA 370 0.189a (0.05-3.21) - Needham et al. (1990)
D. Whole blood
Slovakia 50 25.2 (6.1-43.2) 1992 Koœan et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
E. Follicular fluid
Canada 25 0.11 1993 Jarrell et al. (1993b)
29 0.14
20 0.20
Federal Republic 15 2.59 (1.1-5.7) - Trapp et al. (1984)
of Germany
a Median value.
b The most recent data were used for calculation of intakes via breast milk (section 5.2.4).
c Originally expressed as mg/kg milk fat and subsequently converted to a wet weight basis using either the % fat reported,
or if not given, using 4.2% fat (NHW, 1987).
d Number of positive samples
e Originally expressed on a dry weight basis and subsequently converted to wet weight using 88% moisture for conversion
(NHW, 1987).
f Values reported in µg/kg.
HCB is present in a wide range of other tissues and fluids from
humans, but at lower levels than in adipose tissue and breast milk.
For example, concentrations of HCB in serum of 370 subjects from the
general population in the USA studied by Needham et al. (1990)
averaged 0.189 µg/litre, compared to concentrations of 39 ng/g lipid
in 287 adipose tissue samples. Schechter et al. (1989b) reported HCB
levels in various organs in autopsy tissue from three American
patients. HCB levels in adipose tissue ranged from 15 to 24 ng/g wet
tissue, while kidney, muscle, lung, spleen and testis contained 1 ng/g
or less, and adrenals, bone marrow and liver contained intermediate
concentrations.
Median blood serum levels of HCB ranged from <0.5 to
1.0 µg/litre in four population groups (total of 97 samples) from
Zagreb, Croatia (Krauthacker, 1993). The groups included workers
employed in the distribution and packing of seeds treated with
different pesticides, who were expected to have absorbed
organochlorine compounds at levels greater than the general
population. However, levels of HCB in the blood of these workers were
not elevated. Van der Ven et al. (1992) reported the HCB levels in
maternal serum of 6 and 11 full-term pregnant women in Germany and
Tanzania, respectively. Levels in Germany averaged 1.23 µg/kg (0.33-
2.66 µg/kg), whereas those in Tanzania were 0.01 µg/kg (0-0.03 µg/kg).
Analysis of blood plasma samples from a human organ specimen bank in
Germany revealed that median annual concentrations of HCB between 1983
and 1989 ranged between 3.1 and 5.4 µg/litre (Kemper, 1993). Serum and
ovarian follicular fluid have been shown to contain HCB in patients
receiving in vitro fertilization (Jarrell et al., 1993b). Of 72
patients, HCB was detected in the serum of 60 and follicular fluid of
49. There was a significant geographical variation among three major
cities in Canada (Jarrell et al., 1993b). Follicular fluid from
similarly treated patients in Germany has been shown to contain HCB
(Trapp et al., 1994). In some studies, workers exposed to chlorinated
solvents or chlorinated pesticides had elevated levels of HCB in blood
(section 5.2.7).
5.2.2 Intake from ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the range
of mean levels of HCB measured in ambient air in cities from around
the world of approximately 0.1 to 0.6 ng/m3 (Table 4), mean intake of
HCB from ambient air for the general population is estimated to range
from 3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day. Since no
data on levels of HCB in indoor air were found, it has been assumed
that levels indoors are the same as those outdoors. The intake of HCB
via air may be greater in populations residing in the vicinity of
point sources, but this exposure is considered to be too site-specific
to estimate reliably.
5.2.3 Intake from drinking-water
Based on a daily volume of ingestion for adults of 1.4 litres, a
mean body weight for males and females of 64 kg (IPCS, 1994), and the
range of mean concentrations of HCB detected in drinking-water from
cities of approximately 0.1 to 2 ng/litre (Table 5), the estimated
mean daily intake of HCB from drinking water for the general
population ranges from approximately 2.2 × 10-6 to 4.4 × 10-5 µg/kg
body weight per day.
5.2.4 Intake from foods
Based on the average daily consumption of various foodstuffs by
adults from around the worlda, a mean body weight for males and
females of 64 kg (IPCS, 1994), and the mean level of HCB detected in
various foods in the 1990-1991 US FDA Total Diet Study (Table 7), the
estimated daily intake of HCB from food ranges from approximately
0.0004 to 0.0028 µg/kg body weight per day (this range was generated
by assuming, for food groups in which HCB was not detected, that non-
detectable values were zero with the detection limit being 0.1 µg/kg.
These estimates overlap the range of dietary estimates (between 0.001
and 0.027 µg/kg body weight per day) that have been reported for
various countries (Canada, USA., Germany, Finland, Viet Nam, Thailand,
India, Japan, Australia, the Netherlands) (Gartrell et al., 1986; De
Walle et al., 1995; Fujita & Morikawa, 1992; Kannan et al., 1992a,b;
Government of Canada, 1993; Kannan et al., 1994). Intakes via food may
be substantially higher in selected European and Asian countries,
where the content of HCB in a sampling of a limited range of foods was
relatively high (Table 7), or in indigenous populations consuming
large quantities of some wildlife species, such as marine mammals,
that are known to accumulate relatively high tissue levels of
lipophilic contaminants (Government of Canada, 1993; Ayotte et al.,
1995; Kuhnlein et al., 1995).
a Dietary intakes (g/person/day) consist of: cereals, 323, starchy
roots, 225; sugar (excludes syrups and honey), 72; pulses and
nuts, 33; vegetables and fruits, 325; meat, 125; eggs, 19; fish,
23; milk products (excludes butter), 360; fats and oils (includes
butter), 31 (all intakes from IPCS, 1994).
Dietary intakes may also be greater in infants during breast-
feeding, owing to the accumulation of HCB in the mothers' milk. The
mean concentrations of HCB in the most recent surveys found for
various countries range from <0.17 to 48 µg HCB/litre whole milk
(Table 8). Assuming that infants are exclusively breast fed for the
first 6 months, during which they consume an average of 0.75 litres of
breast milk per day and have an average body weight of 7 kg (Health
Canada, 1994), the estimated mean intakes of HCB from breast milk in
various countries range from <0.018 to 5.1 µg/kg body weight per day.
Daily intakes of HCB by breast-feeding infants of Inuit mothers in
northern Quebec, Canada (a population that consumes substantial
quantities of marine organisms that accumulate lipophilic
contaminants) was estimated at 0.45 µg/kg body weight per day, a value
that was several times greater than for a more southerly population in
the same province (Ayotte et al., 1995).
5.2.5 Apportionment of intakes
Total intake of HCB from ambient air, drinking-water and foods is
estimated to range from approximately 0.0004 to 0.003 µg/kg body
weight per day for the general population, the principal route of
exposure being through the diet (92%). The estimated contributions
from air and drinking-water are much smaller (7% and 1%,
respectively). (The contribution from each environmental medium was
calculated based on the mid-point of the intakes estimated in the
previous sections.)
5.2.6 Trends in exposure of the general population over time
The results of most studies of the levels of HCB in foods and
human tissues over time indicate that exposure of the general
population to HCB declined from the 1970s to the mid-1990s in many
locations. However, this trend has not been evident during the last
decade in some other locations.
Routine monitoring of foods in some countries indicates that
exposure to HCB is decreasing. For example, mean concentrations in
grab samples of milk, bovine fat, poultry fat and egg fat collected
from suppliers in Ontario, Canada, decreased by an order of magnitude
or more between the early 1970s and the mid-1980s (Frank et al., 1983,
1985a, 1985b; Frank & Ripley, 1990). Brown et al. (1986) reported that
the frequency of detectable (> 10 ng/g in fat samples, wet weight)
levels of HCB in the USA meat and poultry supply increased
dramatically from 1972 to 1977-1978, but had fallen off sharply up to
1984. More recent data collected through the US FDA Total Diet Study
indicate that this trend had continued. Between 1982-1984 and 1991,
the most recent year for which data are available, both the frequency
of detection of HCB and the estimated average daily intake for people
of various ages decreased by roughly 80% (US FDA 1990, 1991, 1992). A
decline in HCB levels in fish from the Baltic and the Swedish West
Coast was found in the National Swedish Monitoring Programme over the
period 1988 to 1994 (Bignert, 1995). The annual decrease in levels in
herring muscle from different places in the Baltic was 12 to 15% and
in cod liver from the Baltic 21%; from the West Coast it was 12% in
herring muscle, 23% in cod liver and 23% in dab liver. The trend with
higher concentrations in samples from the Baltic as compared to the
West Coast is still seen in the samples.
The results of most studies of temporal trends of HCB levels in
human adipose tissue or milk (summarized in Table 8) indicate that
general population exposures have declined since the 1970s. In routine
monitoring of breast milk contaminants in German mothers, mean
concentrations of HCB declined by more than 50% between 1984 and 1991
(Fürst et al., 1994), and by about 80% between 1979 and 1990 (BUA
1994). The median HCB content in samples of plasma from a human
specimen bank in Germany decreased from 4.8 µg/litre in 1983 to
3.1 µg/litre in 1989, a period of increasing restrictions on indoor
applications of pentachlorophenol, which contains HCB as a contaminant
(Kemper, 1993). Mes (1990) reported that concentrations of HCB in
human adipose tissue from Canadian surveys were significantly lower in
1985 than in 1972; this decrease occurred in all age classes over this
period. An increase in the HCB content of human adipose tissue and
milk was observed in the early 1970s in the Netherlands, and was
attributed to an increase in the HCB concentration in products of
animal origin (Greve & Van Zoonen, 1990). Once measures were taken to
avoid contamination of such products, a gradual decrease in HCB levels
was observed. Johansen et al. (1994) reported that the concentration
of HCB in routine monitoring of milk from Norwegian mothers declined
by 65% between 1982 and 1991. In contrast, in the most extensive study
of levels of HCB in adipose tissues, the US National Human Adipose
Tissue Survey (Robinson et al., 1990), in which data on residues were
collected from a nationally representative sample of 6081 autopsies
and surgical patients from 1974-1983, there was little change in
residue concentrations over the study period, with the national median
level remaining near 30 to 40 ng/g.
5.2.7 Occupational exposure during manufacture, formulation, or use
Workers may be exposed to higher concentrations of HCB than the
general population, particularly in the manufacture of chlorinated
solvents, and in the manufacture and application of pesticides
contaminated with HCB.
In a survey of production industries (perchloroethylene,
trichloroethylene, carbon tetrachloride, chlorine, triazine herbicides
and pentachloronitrobenzene), the highest HCB concentrations were
associated with the production of perchloroethylene and
trichloroethylene (Spigarelli et al., 1986). The highest level of HCB
determined in the air on plant property was 24 µg/m3 at a plant
producing perchloroethylene, carbon tetrachloride and chlorine.
Relatively high HCB levels (maximum concentration of 2.2 µg/m3 in
air) were also detected in samples from the pentachloronitrobenzene
production plant. Lower levels of HCB were measured at triazine
herbicide production plants (ND - 0.02 µg/m3), and, in the one plant
that produced only carbon tetrachloride, HCB was not detected (MDL not
reported). It is not known how representative the data from these
studies are, as the generation and release of HCB would be minimized
in plants using appropriate modern technology and waste management
practices.
Personal breathing-zone samples (54 in all) from workers in a
pentachlorophenol production plant contained HCB concentrations
ranging from <0.1 to 120 µg/m3 (Marlow, 1986), while levels in 112
area samples throughout the plant ranged from <0.1 to 630 µg/m3.
HCB concentrations in the blood of workers in a factory producing
chlorinated solvents ranged from 14 to 233 µg/litre (Burns & Miller,
1975); this compared with a range <1 to 310 µg/litre in the blood of
vegetable spraymen (Burns et al., 1974). Mean levels of HCB in the
blood plasma of workers in a chlorinated solvents plant in the USA
were 311 µg/litre in 1974, and 312 µg/litre in 1975, and levels in
whole blood were 160 µg/litre in 1976, and 170 µg/litre in 1977
(Currier et al., 1980). Concentrations of HCB in blood were positively
correlated with the number of years worked in the plant, but were not
associated with airborne levels of HCB or job-category-based exposure
estimates. Pesticide-exposed vineyard workers in Germany tended to
have higher HCB whole blood levels (median 7 µg/litre, maximum
30 µg/litre) than reference controls (median 3 µg/litre, maximum
17 µg/litre) (Kemper, 1993). Angerer et al. (1992) reported that the
mean plasma level of HCB in 53 workers at a municipal waste
incinerator was 5.0 µg/litre, compared with 4.69 µg/litre in 64
subjects with no known occupational contact.
6. KINETICS AND METABOLISM
6.1 Aquatic and terrestrial biota
Terrestrial plants such as barley, cress and wheat, and algae
such as Oedogonium cardiacum slowly metabolize HCB to polar
metabolites and non-extractable residues (Lu & Metcalf, 1975;
Scheunert et al., 1983, 1985; Topp et al., 1989). For example, of the
total radiolabelled HCB in barley after uptake from soil over one
growing season, 14% was present as polar metabolites, 20% as plant-
bound residues and the remainder as the parent compound (Topp et al.,
1989). In the only available study on HCB depuration rates in plants,
the aquatic macrophyte Myriophyllum spicatum eliminated 95% of HCB
during the first 28 days after exposure ceased (Gobas et al., 1991).
Invertebrates slowly metabolize HCB to compounds such as
pentachlorothioanisole, pentachlorophenol and other polar metabolites
(Lu & Metcalf, 1975; Bauer et al., 1989). In an aquatic model
ecosystem treated with 14C-HCB for 24 h, unchanged HCB accounted for
84% of the total radioactivity in snails ( Physa sp.), 67% in water
fleas ( Daphnia magna) and 65% in mosquito larvae ( Culex pipiens)
(Lu & Metcalf, 1975). Half-lives for the elimination of HCB by
invertebrates were less than 5 days for filter-feeding bivalves
( Elliptio complanata and Mytilus edulis) (Bro-Rasmussen, 1986;
Russell & Gobas, 1989), 16 days for deposit-feeding clams ( Macoma
nasuta) (Boese et al., 1990), and 27 days for oligochaete worms
( Tubifex tubifex and Limnodrilus hoffmeisteri) (Oliver, 1987).
Sanborn et al. (1977) detected pentachlorophenol and at least
four unidentified polar metabolites in green sunfish ( Lepomis
cyanellus) after 28 days of ingesting HCB-contaminated food.
Pentachlorophenol has also been detected in the excreta and tissues of
rainbow trout ( Oncorhynchus mykiss) following an intraperitoneal
dose with HCB (Koss & Koransky, 1978; Koss et al., 1978). Zebra fish
( Brachydanio rerio) did not metabolize HCB after a 48-h exposure in
water (Kasokat et al., 1989). Elimination half-lives of HCB ranged
from 7-21 days for fathead minnows ( Pimephales promelas) after a
waterborne exposure (Kosian et al., 1981) to up to 210 days for
rainbow trout ( Oncorhynchus mykiss) after ingestion of HCB in food
(Niimi & Cho, 1981).
Clark et al. (1987) reported that 63% of the total HCB eliminated
in herring gulls ( Larus argentatus) was found in the egg yolk.
Breslin et al. (1983) found that 50% of total HCB eliminated from
laying bobwhite quail ( Colinus virginianus), a species that lays
many eggs, was accounted for in egg yolk. For most wild species, egg
laying will account for a relatively small loss of HCB, while
depletion of stored fat during energetically costly activities such as
migration and moulting may result in a significant reduction in body
burdens. The half-life for elimination of HCB in birds ranged from
24-35 days for domesticated chickens ( Gallus gallus domesticus) fed
HCB-contaminated diets (Kan & Tuinstra, 1976; Hansen et al., 1978) to
211 days in intraperitoneally dosed juvenile herring gulls (Clark et
al., 1987).
6.2 Mammals
There are few data on the absorption of HCB by humans. By
comparing intake and faecal excretion of HCB in a single breast-fed
infant, Abraham et al (1994) estimated that absorption was virtually
complete (greater than 99.7% at one month of age and greater than 97%
at 5 months). The concentrations of HCB in the diet and faeces of a
single formula-fed infant were too low for reliable estimation of
absorption (Abraham et al., 1994). The results of animal studies
indicate that 80% or more of an oral dose of HCB (between 10 and 180
mg/kg body weight) is absorbed if administered in an oil vehicle
(Albro & Thomas, 1974; Koss & Koransky, 1975; Ingebritsen et al.,
1981; Bleavins et al., 1982). In female rats treated with 14C-HCB in
oil, peak values of radioactivity were reached in 2 to 5 days. The
absorption was poor (2-20%, depending on the dose) when the substance
was given as an aqueous suspension (Koss & Koransky, 1975). Little
information was identified on dermal absorption, although it appears
to be lower. Koizumi (1991) observed that after dermal application of
approximately 2.5 mg 14C-HCB in tetrachloroethylene to Fisher-344
rats for 72 h, only 9.7% of the administered dose was absorbed. No
information on absorption via the lungs has been reported.
There are no experimental studies of tissue distribution of HCB
in humans, although in a small autopsy study of members of the general
population (Schechter et al., 1989b), the highest levels were found in
(in order) adipose tissue, adrenals, bone marrow and liver. Laboratory
studies in a number of animal species also indicate that the highest
concentrations of HCB are accumulated in tissues with a high lipid
content, such as the adipose tissue, adrenal cortex, bone marrow, skin
and some endocrine tissues (thyroid, adrenal and ovary) following
ingestion or injection of HCB (Koss & Koransky, 1975; Yang et al.,
1978; Courtney, 1979; Sundlof et al., 1982; Ingebritsen, 1986; Smith
et al., 1987, 1994; Goldey et al., 1990; Foster et al., 1993; Jarrell
et al., 1993a). No information was found on the tissue distribution
following inhalation or dermal exposure. HCB crosses the placenta, and
is eliminated via the mothers' milk in both animals and humans
(Villeneuve et al., 1974; Mendoza et al., 1975; Courtney & Andrews,
1979, 1985; Courtney et al., 1979; Bailey et al., 1980; Bleavins et
al., 1982; Goldey et al., 1990; section 5.2.1).
Metabolic transformation is not extensive in the wide range of
species examined. The pathways of biotransformation of HCB have been
reviewed by Debets & Strik (1979) and by Renner (1988). The metabolism
of HCB operates via three distinct pathways. These are oxidative
pathways, which give rise to phenolic metabolites including
pentachlorophenol, tetrachlorohydroquinone and tetrachloro-
benzoquinone; a glutathione-conjugation pathway leading to penta-
chlorothiophenol, pentachlorothioanisoles, and several other sulfur-
containing metabolites; and a minor pathway that yields lower
chlorinated benzenes through reductive dechlorination. Metabolism
occurs primarily in the liver, although dechlorination of HCB has also
been demonstrated in vitro in enzyme preparations from the lung,
kidney and small intestine (Mehendale et al., 1975).
The metabolism of HCB has been studied in the rat and guinea-pig
(Mehendale et al., 1975; Rozman et al., 1975; Koss & Koransky, 1976;
Koss et al., 1978; Koss & Koransky, 1978; Courtney, 1979), and in the
monkey (Rozman et al., 1975; Courtney, 1979). Dosing routes included
gastric intubation and the intraperitoneal route, while dosing
vehicles included oil and aqueous media. The monitoring for metabolic
products of HCB has included excretory products and/or tissue residues
for periods ranging from 28 to 40 days post-dosing. Findings were
quite dissimilar among the studies. The most common finding was that
less than 40% of the administered dose was recovered in the excretory
products and a majority of the recovered dose was unchanged HCB.
The major metabolites found in the urine of rats, mice and
guinea-pigs exposed to HCB by various routes in most studies are
pentachlorophenol (PCP), tetrachlorohydroquinone and
pentachlorothiophenol (PCTP) (Koss & Koransky, 1978; Koss et al.,
1978). (There is some question as to whether most of the latter
compound detected in some studies was an analytical artefact from
alkaline hydrolysis of the n-acetyl cysteine conjugate.) Other
metabolites include tetra- and pentachlorobenzenes and thioanisoles,
and tri- and tetrachlorophenols, both in free and conjugated forms. It
has been reported that, after dietary exposure of male and female
Wistar rats to HCB for 13 weeks, N-acetyl- S-(pentachloro-
phenyl)cysteine was the most abundant metabolite via the conjugation
pathway (89-92% of the total urinary metabolites collected over 24 h,
after one week of treatment). Mercaptotetrachlorothioanisole was also
present, excreted as a glucuronide (den Besten et al., 1994). The
excreta from male Wistar rats given 125 mg/kg body weight on day 1 and
6 were collected for 12 days (Jansson & Bergman, 1978). Faeces and/or
urine contained HCB (about 4% of the total does), pentachlorobenzene,
pentachlorophenol, pentachlorobenzenethiol (both as such and as
conjugates), methylthiopentachlorobenzene, tetrachlorobenzenedithiol
and/or methylthiotetrachlorobenzenethiol (both as such and as
conjugates), dichlorotetrakis(methylthio)benzene (trace amounts),
hexakis(methylthio)benzene (trace amounts), bis(methylthio)-
tetrachlorobenzene, tetrachlorobenzenethiol (trace amounts) and
methylthiotetrachlorobenzene (trace amounts). Compounds found
accumulated in adipose tissue were hexachlorobenzene,
pentachlorobenzene, pentachlorobenzenethiol, bis(methylthio)-
tetrachlorobenzene and pentachloroanisole.
Rizzardini & Smith (1982) administered 50 µmoles of HCB/kg body
weight to male and female rats by gavage in arachis oil for 103 days.
Three urinary metabolites were identified, i.e., pentachlorophenol,
2,3,5,6-tetrachlorobenzene, 1,4-diol and pentachlorothiophenol
(derived from mercapturate). The authors reported that female rats
excreted several times more HCB metabolites than males.
PCP and PCTP have been detected in the urine of humans from the
general population of Spain with high body burdens of HCB (To-Figueras
et al., 1992).
No reliable information on the elimination half-life of HCB in
humans was found. Excretion of HCB by laboratory animals occurs mainly
through the faeces regardless of the route of administration (US EPA,
1985a; ATSDR, 1990). Both biliary excretion and non-biliary intestinal
transfer contribute to faecal excretion (Rozman et al., 1981;
Ingebritsen et al., 1981; Richter & Schäfer, 1981; Sundlof et al.,
1982). Reported half-lives for the elimination of an oral dose of HCB
(doses were 3 mg/kg body weight or less in these studies) are
approximately one month in rats and rabbits, 10-18 weeks in sheep,
pigs and dogs, and 2.5 to 3 years in rhesus monkeys (Avrahami &
Steele, 1972; Avrahami, 1975; Rozman et al., 1981; Sundlof et al.,
1982; Scheufler & Rozman, 1984; Yamaguchi et al., 1986). HCB has been
detected in the milk of several species, including humans, and the
results of experiments with mice and ferrets indicate that the
majority of the maternal body burden can be eliminated via the
mother's milk during lactation (Bleavins et al., 1982; Courtney &
Andrews, 1985).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
This section summarizes the extensive literature on the toxicity
of HCB to laboratory mammals, with emphasis on those studies reporting
the lowest-observed-effect levels. Information on the dosage with
respect to body weight was obtained from the original papers, wherever
possible. When doses were not expressed in this way by the
investigators and could not be calculated from the data provided,
approximate doses (given in parentheses) have been estimated based on
the reference values given in NIOSH (1985).
7.1 Single exposure
The acute toxicity of HCB in experimental animals is low;
reported oral LD50 values for various species range from 1700 mg/kg
body weight for the cat to between 3500 and > 10 000 mg/kg body
weight for the rat, with intermediate values for the mouse, rabbit and
guinea-pig. Reported LC50 values for inhalation exposure range from
1600 mg/m3 for the cat to 4000 mg/m3 for the mouse, with
intermediate values reported for the rat and rabbit (IARC, 1979;
Strik, 1986; Lewis, 1992). Acute lethal doses elicit convulsions,
tremors, weakness, ataxia, paralysis and pathological changes in
organs. Strik (1986) reported that HCB has a low skin irritation
score, is not irritating to the eye and does not sensitize the guinea-
pig, although no details were provided. In several studies, single
oral doses of 100-1000 mg/kg body weight produced increases in the
activities of various liver enzymes in rats within 24 h (Strik, 1986).
7.2 Short-term and subchronic exposure
The effects of short-term, repeated exposure to HCB are primarily
hepatotoxic and neurological. In a number of studies, the effects of
HCB on rats exposed to oral doses in the range of 30-250 mg/kg body
weight per day included altered body weight, cutaneous lesions,
tremors and other neurological signs, hepatomegaly, liver damage and,
in some cases, early alterations in porphyrin or haem metabolism
(Courtney, 1979; US EPA, 1985a; Strik, 1986). Short-term exposure
in vivo induced a variety of enzymes, including glutathione- S-
transferases and isozymes of cytochrome P-450, identified as
cytochromes P-450IA1 (CYPIA1), P-450IA2 (CYPIA2) and P-450IIB (CYPIIB)
(Wada et al., 1968; Courtney, 1979; Denomme et al., 1983; US EPA,
1985a; Linko et al., 1986; Strik, 1986; Hahn et al., 1988, 1989; Vos
et al., 1988; Green et al., 1989; Rizzardini et al., 1990; D'Amour &
Charbonneau, 1992; Smith et al., 1993; Goerz et al., 1994). This means
that HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
Enzyme induction has been observed at relatively low doses in some
studies. For instance, in Wistar rats fed HCB in the diet for 14 days,
the low-effect level for induction of microsomal liver enzyme was
50 mg HCB/kg feed (approximately 2.5 mg/kg body weight per day), and
the no-effect level was 20 mg HCB/kg feed (approximately 1 mg/kg body
weight per day) (den Tonkelaar & van Esch, 1974).
The effects produced by subchronic exposure to HCB are similar to
those observed in short-term studies, but are generally evident at
lower doses (Courtney, 1979; US EPA, 1985a; ATSDR, 1990, 1994). At
relatively high doses (32 mg/kg body weight per day or more for
periods from several weeks to 90 days), reported effects have included
death, skin lesions, behavioural and neurological changes, reduced
body weight gain, increased organ weights, and altered thyroid
function and serum levels of thyroid hormones (the latter effect is
discussed later in this section). At lower doses, hepatotoxic effects
have been commonly reported, including histological alterations, the
induction of a variety of hepatic microsomal enzymes and porphyria.
The porphyrinogenic effects of exposure to HCB have been
extensively studied since the seminal reports of Ockner & Schmid
(1961) and De Matteis et al. (1961). These and subsequent earlier
works, many of them conducted at relatively high doses, have been
summarized by Courtney (1979), and much of this research is not
discussed in this report. Porphyria has been observed in several
species of laboratory mammals, most often manifested as increased
levels of porphyrins and/or porphyrin precursors in the liver, other
tissues and excreta. This disturbance in haem synthesis is associated
with the inhibition of uroporphyrinogen decarboxylase activity (this
enzyme converts uroporphyrinogen III to coproporphyrinogen III),
leading to the accumulation of uroporphyrin and other highly
carboxylated porphyrins, and with the induction of ALA synthetase (the
enzyme controlling the rate of haem synthesis) (ATSDR, 1994). There is
a delay before exposed rats become porphyric, which appears to reflect
the time for the animals to receive a sufficient cumulative dose of
HCB (Krishnan et al., 1991, 1992), as well as the time needed for the
porphyrins to accumulate to the level of overt porphyria (Kennedy et
al., 1986; Kennedy & Wigfield, 1990). Although in most studies
porphyria has been associated with longer-term exposure to HCB, rats
exposed to doses of 25-50 mg HCB/kg body weight per day for as little
as several days had increased levels of hepatic and urinary porphyrins
(Krishnan et al., 1991, 1992). In another study, hepatic levels of
highly carboxylated porphyrins were elevated by a single exposure to
50 mg HCB/kg body weight, although the latter result was not
accompanied by clinical porphyria (Kennedy & Wigfield, 1990).
HCB-induced porphyria has been extensively studied in rats, in
which dietary or gavage exposure of various strains to between 2.5 and
15 mg HCB/kg body weight per day for periods of 8 to 15 weeks has
caused hepatic porphyria, and, in some studies, increased levels of
porphyrins in the kidney and spleen (Grant et al., 1975; Kuiper-
Goodman et al., 1977; Goldstein et al., 1978; Mendoza et al., 1979;
Rizzardini & Smith, 1982; Teschke et al., 1983; Smith et al., 1985b;
Green et al., 1989; Van Ommen et al., 1989; Kennedy & Wigfield, 1990;
Smith et al., 1990; Den Besten et al., 1993). A no-observed-effect-
level (NOEL) for HCB-induced porphyria was not determined in these
studies. Although the data on other species are limited, levels of
hepatic or urinary porphyrins were increased in mice of various
strains fed diets containing 200 mg HCB/kg feed (yielding approximate
doses of 24 mg HCB/kg body weight per day) for periods of 7 to 15
weeks in some studies (Smith & Francis, 1983; Rizzardini et al., 1988;
Vincent et al., 1989), and porphyria was induced in Japanese quail
following short-term oral and intraperitoneal exposure to 500 mg
HCB/kg body weight per day (section 9.1.2).
The lowest doses producing porphyrinogenic and other effects on
the liver in a subchronic study were reported by den Tonkelaar et al.
(1978). Groups of five pigs exposed for 90 days to doses of 0.5 mg/kg
body weight per day or more in the diet had increased urinary levels
of coproporphyrin and alterations in liver histology and microsomal
enzyme activities, but no effects were observed at 0.05 mg/kg body
weight per day. However, marked excretion of coproporphyrin alone is
not a characteristic of the inhibition of uroporphyrinogen
decarboxylase in animal systems.
Female rats are more sensitive than males to the porphyrinogenic
effects of exposure to HCB. In various strains of rats exposed to
doses of 5 to 10 mg HCB/kg body weight per day in the diet or by
gavage, for periods of between 3 months or more, females developed a
marked porphyria which was absent or much reduced in males (Grant et
al., 1975; Kuiper-Goodman et al., 1977; Rizzardini & Smith, 1982;
Smith et al., 1985b). In a number of studies, the basis for the
susceptibility to HCB-induced porphyria of female rats compared to
males has been examined. Grant et al. (1975) reported that ovariectomy
decreased, and castration increased, the accumulation of porphyrins in
the livers of female and male Sprague-Dawley rats with subchronic
exposure to HCB, suggesting a role for steroid hormones in the
development of porphyria in this species. In another study, female
Fischer-344 rats with HCB-induced porphyria had higher levels of
cytochrome P-450IA isoenzymes and ethoxyresorufin- O-deethylase
activity than males, whereas males had higher levels of total
cytochrome P-450 and activities of microsomal monooxygenases
associated with cytochrome P-450IIB1 (Smith et al., 1990). In Fischer-
344 rats with HCB-induced porphyria, sex-related differences in
urinary and hepatic porphyrin levels were paralleled by differences in
the excretion of phenolic metabolites, particularly
pentachlorothiophenol (Rizzardini & Smith, 1982). These findings were
further investigated in a short-term study by D'Amour & Charbonneau
(1992), which indicated that male rats may be more resistant to HCB-
induced porphyria than females because hepatic conjugation of HCB with
glutathione is more important in males. Male Sprague-Dawley rats
receiving a porphyrinogenic dose of HCB (100 mg HCB/kg body weight per
day by gavage for 5 days) had significantly lower hepatic gluthione
concentration and higher glutathione transferase activity (to 3,4-
dichloronitrobenzene) than controls, whereas no significant
differences were observed in females. Biliary excretion of PCTP (a
metabolite of glutathione conjugation) and the rate of elimination of
HCB from the liver were greater in males than in females.
Other mechanistic studies have suggested the involvement of
oxidative metabolism of HCB in the development of porphyria, although
the mechanism remains to be elucidated. In female Wistar rats
co-treated with 300 mg HCB/kg in the diet (approximately 15 mg HCB/kg
body weight per day) and triacetyloleandomycin (TAO) (to selectively
inhibit cytochrome P-450IIIA1/2 and thereby prevent the oxidative
biotransformation of HCB) for 10-13 weeks, both the excretion of PCP
and TCHQ (tetrachlorohydroquinone, the reduced analogue of the
reactive tetrachlorobenzoquinone) and the extent of hepatic porphyria
and urinary porphyrin excretion were greatly diminished (Van Ommen et
al., 1989; Den Besten et al., 1993). In 13-week feeding studies on
female Wistar rats exposed to 300 mg HCB/kg diet (approximately 15 mg
HCB/kg body weight per day) in the presence or absence of TAO, the
degree of porphyria was better correlated with excretion of PCP than
TCHQ, and in comparative studies pentachlorobenzene (which is
metabolized to PCP by a different mechanism than for HCB) was not
porphyrinogenic (Den Besten et al., 1993).
In addition, it has been suggested that the aryl hydrocarbon
receptor (Ah receptor) may be involved in the accumulation of hepatic
porphyrins in mice (Linko et al., 1986; Hahn et al., 1988, 1989). Ah-
responsive strains of inbred mice were more sensitive to hepatic
porphyrin accumulation after HCB exposure than non-responsive mice
(Smith & Francis, 1983; Hahn et al., 1988), and HCB has been shown to
be a weak agonist for the Ah receptor (Hahn et al., 1989).
Full discussion of the evidence for a unifying hypothesis of
porphyria induced by HCB and other chemicals that act in a similar
way, as well as for human sporadic porphyria cutanea tarda, is beyond
the scope of this document. There is however, substantial experimental
and human evidence implicating a complex interaction between
hepatocellular iron and oxidative processes leading to the oxidation
of unstable uroporphyrinogen to uroporphyrin, possibly mediated by
induced cytochrome P-450 isozymes (reviewed by Smith & De Matteis,
1990). There is evidence that inhibition of uroporphyrinogen
decarboxylase may occur through formation of an inhibitor of the
enzyme during the oxidation of uroporphyrinogen (Rios de Molina et
al., 1980; Smith & De Matteis, 1990). HCB may act partly through
induction and uncoupling of the cytochrome P-450 system to form
reactive oxygen species, especially in the presence of an increased
available iron pool (Smith & De Matteis, 1990; Den Besten et al.,
1993).
Subchronic exposure to low doses of HCB has also caused changes
in calcium homoeostasis and bone morphometry. Male Fischer-344 rats
administered HCB by gavage in corn oil had elevated serum levels of
1,25-dihydroxy-vitamin-D3 and reduced calcium excretion after 5
weeks, and increased femur density, weight and strength after 15
weeks. These effects were evident at 0.7 mg/kg body weight per day but
not at 0.07 mg/kg body weight per day (Andrews et al., 1989, 1990).
While technical HCB is known to be contaminated with chlorinated
dibenzo- p-dioxins, dibenzofurans and biphenyls (Villanueva et al.,
1974; Goldstein et al., 1978), the effects (primarily hepatic) of
subchronic dietary exposure of rats to either pure or technical HCB
were virtually identical, indicating that the effects observed in this
study were due to the parent compound (Goldstein et al., 1978).
In a number of studies on various strains of rats, short-term or
subchronic exposure to HCB affected the thyroid, as indicated by
decreased serum levels of total and free thyroxine (T4) and often, to
a lesser extent, triiodothyronine (T3). In some instances, these are
accompanied by compensatory increases in thyroid weight, circulating
levels of thyroid-stimulating hormone or iodine uptake by the thyroid
(Rozman et al., 1986; Kleiman de Pisarev et al., 1989, 1990; Van Raaij
et al., 1991a, 1993a, 1993b; Foster et al., 1993; Den Besten et al.,
1993; Sopena de Krakoff et al., 1994). Den Besten et al. (1993)
reported such effects in rats exposed to as little as 9.5 mg/kg body
weight per day following dietary exposures for 13 weeks, although
effect levels were somewhat higher in other studies, which involved
exposure for a shorter duration and/or employed an aqueous vehicle.
Somewhat different effects (decreased levels of T3 in serum and no
change in T4, accompanied by increased uptake of iodine by the
thyroid) were observed in hamsters exposed to 100-200 mg HCB/kg feed
(approximately 12-24 mg/kg body weight per day) for 18-28 weeks (Smith
et al., 1987).
The mechanisms that have been advanced to account for the effects
of HCB on the thyroid include accelerated metabolism of thyroid
hormones by HCB-induced enzymes or accelerated deiodination of
thyroxine, in conjunction with increased biliary excretion (Kleiman de
Pisarev, 1989; Van Raaij et al., 1993b), and interference with plasma
transport of thyroid hormones through displacement of T4 from binding
sites on proteins (Van Raaij et al., 1991a, 1993a). Van Raaij et al.
(1991b, 1993a) reported that intraperitoneal injection of
pentachlorophenol and tetrachlorohydroquinone, but not HCB itself,
decreased serum thyroxine levels in rats, indicating that these
metabolites may be involved in the effects of HCB on the thyroid.
These authors reported that PCP was a more effective competitor for
thyroxine-binding sites of serum carriers in vitro, and more
effective at occupying carrier sites in ex vivo experiments, than
HCB (van Raaij et al., 1991a), and demonstrated that T4 binding sites
were partially occupied in the serum of rats exposed to HCB (Van Raaij
et al., 1993a). In the latter study, it was estimated that competition
for thyroid hormone binding sites, by PCP metabolized from HCB, could
account for almost half of the observed reduction in serum levels of
T4.
7.3 Long-term toxicity and carcinogenicity
A range of non-neoplastic effects from long-term exposure to HCB,
which are primarily hepatotoxic, have been observed at relatively low
doses. In a two-generation study with Sprague-Dawley rats, liver and
heart weights were increased in Fo males exposed to TWA doses of 0.29
and 1.50 mg/kg body weight per day in the diet for 3 months, and
histopathological changes in the liver were observed in F1 animals of
both sexes exposed to maternal doses of 0.29-0.38 and 1.50-1.90 mg
HCB/kg body weight per day in diet in utero, through nursing, and
then continued on the same diet as their parents for their lifetimes.
The no-effect level in this study was 0.06-0.07 mg/kg body weight per
day (Arnold et al., 1985; Arnold & Krewski, 1988). Dietary exposures
of Sprague-Dawley rats to 10 mg/kg and above (approximately 0.5-0.6
mg/kg body weight per day) for 9-10 months induced in vivo mixed-
function oxidase activity, as indicated by reductions in drug-induced
sleeping times (Grant et al., 1974). Exposure of Sprague-Dawley rats
to 5 mg HCB/kg in diet (approximately 0.25-0.30 mg/kg body weight per
day) for 3-12 months caused proliferation of smooth endoplasmic
reticulum, altered mitochondria and increased numbers of storage
vesicles in liver, but these effects were not evident at 1 mg/kg in
diet (approximately 0.05-0.06 mg/kg body weight per day) (Mollenhauer
et al., 1975; 1976). In a study by Böger et al. (1979), oral
administration of 2, 8 or 32 mg HCB to female Wistar rats twice weekly
for 203 days (0.57, 2.3 or 9.1 mg HCB/kg body weight per day) resulted
in hepatocellular enlargement, proliferated smooth endoplasmic
reticulum, increased glycogen and porphyrin deposits, and enlarged
mitochondria, but these effects were not seen at a lower dose (0.5 mg
HCB/kg body weight twice weekly, or 0.14 mg HCB/kg body weight per
day). Bleavins et al. (1984a) reported that exposure of female mink to
a dietary concentration of 1 mg/kg (estimated to yield a dose of
0.16 mg/kg body weight per day) for 47 weeks significantly increased
serotonin concentrations in the hypothalamus of dams, and depressed
hypothalamic dopamine concentrations in kits exposed in utero and
through nursing.
As in subchronic studies, female rats were more sensitive than
males to porphyria induced by chronic exposure to HCB. Grant et al.
(1974) reported that in Sprague-Dawley rats fed diets containing HCB
for 9-10 months, reduced weight gain and porphyria were observed in
females, but not males, receiving 80 or 160 mg HCB/kg feed
(approximately 4 or 8 mg HCB/kg body weight per day). A dose-related
increase in relative liver weights and in the hepatic content of HCB
was noted in both sexes. Hepatic enzyme activities and cytochrome
P-450 activities were increased in males administered 40 mg HCB/kg
feed or more. Exposure to 10 mg HCB/kg feed (approximately 0.5-0.6 mg
HCB/kg body weight per day) induced in vivo mixed-function oxidase
activity, as indicated by reductions in sleeping time for
pentobarbital and zoxazolamine exposure.
The carcinogenicity of HCB has been assessed in several bioassays
in rats, mice and hamsters. The following discussion is limited
principally to the four studies in which adequate numbers of animals
of both sexes were exposed for a sufficient length of time to more
than one dose level.
Cabral et al. (1977) and Cabral & Shubik (1986) reported a
statistically significant increase of liver cell tumours (hepatomas)
in groups of 30-60 male and female Syrian golden hamsters fed 50, 100
or 200 mg HCB/kg (4, 8 or 16 mg/kg body weight per day) HCB in their
diets for life. The incidence of "haemangioendotheliomas" of the liver
was significantly increased in both sexes at 200 mg/kg and in males at
100 mg/kg, and of alveolar adenomas of the thyroid in males at
200 mg/kg. (The latter finding is interesting in the light of reports
of excesses of thyroid neoplasms, or of enlargement of the thyroid, in
human populations with elevated exposures to HCB (section 8.1.)) The
authors reported that three of the hepatic "haemangioendotheliomas"
(which are non-invasive by definition) metastasized. It seems likely,
therefore, that these tumours were malignant, though misclassified.
In another study, HCB was administered in the diet to groups of
30 or 50 outbred male and female Swiss mice at concentrations of 0,
50, 100 and 200 mg/kg (0, 6, 12 and 24 mg/kg body weight per day) for
120 weeks (Cabral et al., 1979; Cabral & Shubik, 1986). In females
exposed to 200 mg/kg, a statistically significant increase in the
incidence of "liver cell tumours (hepatomas)" was noted. "Hepatomas"
were also elevated, though not significantly, in males at this dose
and in both sexes at 100 mg/kg. The number of tumour-bearing animals,
the latent period, and the multiplicity and size of tumours increased
with dose.
Arnold et al. (1985) and Arnold & Krewski (1988) investigated the
potential carcinogenicity to rats of combined in utero, lactational
and oral exposure to analytical grade HCB. Groups of 40 or more
weanling male and female Sprague-Dawley rats were fed diets containing
0, 0.32, 1.6, 8 or 40 mg HCB/kg. (Based on data supplied by the
author, mean doses for males were 0, 0.01, 0.06, 0.29 and 1.50 mg/kg
body weight per day and for females 0, 0.01, 0.07, 0.38 and 1.90 mg/kg
body weight per day). After 3 months, the F0 rats were bred, and 50
F1 pups of each sex were randomly selected from each group. From
weaning, the F1 animals were continued on the same diet for their
lifetimes (up to 130 weeks). In exposed F1 females, increased
incidences of neoplastic liver nodules and adrenal phaeochromocytomas
were noted at the highest dose. A significantly increased incidence of
parathyroid adenomas was noted in males receiving 40 mg HCB/kg in
their diet.
In a study by Lambrecht et al. (1983a,b; Ertürk et al., 1986),
groups of 94 weanling Sprague-Dawley rats were fed diets containing 0,
75 or 150 mg/kg (4 and 8 mg/kg body weight per day for males and 5 and
9 mg/kg body weight per day for females, respectively) for up to 2
years. Statistically significant increases in the incidence of
hepatomas/haemangiomas and of renal cell adenomas were noted at both
doses in animals of both sexes surviving beyond 12 months. Incidences
of hepatocellular carcinomas and bile duct adenomas/carcinomas were
also elevated in females at both doses. In female rats, significant
increases in the incidences of adrenal cortical adenomas at 75 mg/kg
and phaeochromocytomas at both doses were reported. Lambrecht et al.
(1983b) reported a leukaemia involving the thymus, spleen, liver and
kidney in rats exposed to HCB in this study, but did not present any
quantitative data. The results of this study were only reported in
summary form, with few details of the study protocol and results. In
addition, HCB was incorporated into the diet as a powder in this
study, raising the possibility that some of the effects observed may
have been in part attributable to the inhalation of aerosolized HCB.
High incidences of liver tumours have also been reported in some
more limited studies in which single dietary concentrations (100 or
200 mg/kg) were administered to small groups (i.e., between 4 and 15)
of females of three strains of rats (Smith & Cabral, 1980; Smith et
al., 1985b); in one strain (Fischer-344), hepatocellular carcinomas
were observed (Smith et al., 1985b). HCB has not, however, been
carcinogenic in several other studies in various strains of mice
(Theiss et al., 1977; Shirai et al., 1978; Smith et al., 1989),
perhaps as a result of the low doses, short durations of exposure
and/or small group sizes employed. Results were also negative in a
second study by Arnold et al. (1985), in which groups of 50 male
Sprague-Dawley rats were fed diets containing 40 mg HCB/kg in
conjunction with various levels of vitamin A for 119 weeks, indicating
the probable higher sensitivity of the two-generation carcinogenesis
bioassay.
Ertürk et al. (1982, 1986; Lambrecht et al., 1982a,b) examined
the tumorigenic activity of subchronic exposure to HCB in both sexes
of Swiss mice, Syrian golden hamsters and Sprague-Dawley rats at
dietary levels of 0, 100 and 200 mg/kg (mice) and 0, 200 and 400 mg/kg
(hamsters and rats) for 90 days. At day 91, 25 of 50 animals in each
group were sacrificed for histological examination, with the remainder
being sacrificed at 6-week intervals (up to 341, 361 and 424 days for
mice, hamsters and rats, respectively). The results of these studies
were reported in summary form only, and much of the quantitative data
were not presented. The authors reported that, as the experiment
progressed, treated animals developed hepatomas, bile duct adenomas,
renal adenomas and carcinomas, and lymphosarcomas of the thymus,
spleen, and lymph nodes. However, the only tumour and species for
which they presented clear evidence of a treatment-related increase in
incidence was for lymphatic tumours in mice (Ertürk et al., 1982).
Lymphatic and renal neoplasms were observed as early as the end of the
90-day period. It is not clear from these reports which tumours each
species developed or the dietary levels associated with the observed
effects, as well as other experimental details.
Results from a number of studies have indicated that HCB is a
co-carcinogen or promoter of cancer. Concomitant exposure to 50 mg
HCB/kg in diet (approximately 6 mg HCB/kg body weight per day)
enhanced the induction of liver tumours by polychlorinated terphenyl
(at 250 mg/kg diet) in male ICR mice (Shirai et al., 1978). Exposure
to HCB (100-200 mg/kg in diet (approximately 5-10 mg HCB/kg body
weight per day) or 1 mmole/kg i.p. at 1 and 5 weeks) promoted the
development of hepatocellular carcinomas and/or hepatic gamma-
glutamyltranspeptidase-positive foci initiated by diethylnitrosamine
in various strains of rats (Pereira et al., 1982; Herren-Freund &
Pereira, 1986; Stewart et al., 1989).
In some recent studies, the possible mechanisms by which HCB
induces tumours in animals have been investigated.
Bouthillier et al. (1991) presented the results of studies of
Sprague-Dawley rats exposed to 100 mg HCB/kg by gavage for periods of
several weeks, which indicated that the observed increase in renal
tumours in male Sprague-Dawley rats following exposure to HCB
(Lambrecht et al., 1983b; Ertürk et al., 1986) is related to protein
droplet nephropathy. The mechanism by which structurally diverse
hydrocarbons induce hyaline droplet nephropathy in male rats has been
well documented and involves accumulation of alpha-2u-globulin,
resulting in necrosis, regeneration and, in some cases, tumours. This
response is sex- and species-specific, and hence is unlikely to be
relevant to humans. This mechanism does not, however, explain the
increased (but lower) incidence of renal tumours in females also
reported by Lambrecht et al. (1983b).
Carthew & Smith (1994) hypothesized that some HCB-induced hepatic
tumours in rats may be produced by a non-genotoxic mechanism. They
noted that hepatotoxicity of HCB in rodents gives rise to peliosis and
necrosis with haemosiderosis, indicating that vascular damage has
occurred, and confirmed the presence of such damage in the liver of
chronically HCB-exposed rats by the identification of widespread
fibrin deposits, using an antibody to rat fibrin. These deposits
occurred in association with abundant haemosiderosis in hepatocytes
and areas of widened hepatic sinusoids. On this basis, it was
suggested that the formation of hepatomas and haemangiomas with
elements of peliosis could be the result of compensatory hyperplastic
responses to hepatocellular necrosis and the simultaneous loss of
hepatocellular cords, perhaps potentiated by the accumulation of iron
in the liver.
Mechanistic studies that address the relevance to humans of the
remaining tumour types induced in rodents by HCB have not been
identified.
7.4 Mutagenicity and related end-points
HCB has not been found to be genotoxic in most studies conducted
to date. HCB did not cause either frameshift or base pair substitution
mutations in Salmonella typhimurium at doses of as much as 10
mg/plate with or without metabolic activation, with both rat and
hamster liver activation systems, pre-incubation and plate
incorporation methods, and technical and 99.9% pure HCB (Haworth et
al., 1983; Górski et al., 1986; Siekel et al., 1991). A weak positive
response in S. typhimurium strain TA98 at 50 and 100 µg/plate was
reported by Gopalaswamy & Aiyar (1986) and Gopalaswamy & Nair (1992).
However, the authors also reported mutagenic activity for lindane, in
contrast to the results of other studies (e.g., Haworth et al., 1983).
Doses of up to 1000 µg/plate of HCB did not induce tryptophan
reversion or DNA damage in Escherichia coli strains WP2 and WP2uvrA
with or without metabolic activation (Siekel et al., 1991).
There have been reports of mutagenic activity for HCB in
eukaryotic cells in vitro, although these studies have limitations.
Guerzoni et al. (1976) reported a positive finding for methionine
reversion in Saccharomyces cerevisiae strain 632/4 exposed to HCB,
but Brusick (1986) did not consider the observed increase to meet
current standards of a positive response. In addition, only a single
dose level was used in that study, and there was no exogenous
metabolic activation. Kuroda (1986) reported that in cultured Chinese
hamster lung cells (V79), HCB did not induce OUAr mutations, but did
induce 8AGr mutations. However, both the magnitude of the increase
(which was small, roughly 1/105 survivors at the two highest doses)
and uncertain dose-response indicate that this response is open to
question.
Oral administration of as much as 221 mg HCB/kg body weight per
day to male rats for 5 or 10 days failed to induce dominant lethal
effects in two different studies (Khera, 1974; Simon et al., 1979),
although Simon et al. (1979) did observe a slight reduction in male
reproductive performance (numbers of females inseminated and
impregnated). Rumsby et al. (1992) reported that liver neoplasms that
developed in iron-overloaded C57Bl/10ScSn mice exposed for 18 months
to 0.01% HCB in the diet were not associated with a high frequency of
mutations in the Ha-ras proto-oncogene at codon 61. Only two mutations
were observed at different sites, from 23 preneoplastic and neoplastic
lesions examined, indicating that activation of the Ha-ras gene is not
an important event in the hepatocarcinogenicity of HCB in this test
system.
HCB has not been found to be clastogenic in the few available
studies in which this end-point has been examined. The compound did
not increase the frequency of sister chromatid exchanges in the bone
marrow of male mice given as much as 400 mg/kg body weight (by an
unspecified route), although the lack of detail in reporting the test
protocol and results limits the interpretation of this study (Górski
et al., 1986). HCB did not induce chromosomal aberrations in vitro
in cultured Chinese hamster fibroblast cells at concentrations as high
as 12 mg/ml, with or without metabolic activation (Ishidate, 1988), or
in human peripheral blood lymphocytes exposed to up to 0.1 mmol/litre
(Siekel et al., 1991). Treatment of rats with 1000 mg HCB/kg diet for
15 days was hepatotoxic, but did not cause early diploidization in
hepatocytes as measured by flow cytometry (Rizzardini et al., 1990).
The results of less specific assays also indicate that HCB does
not interact strongly with DNA, although there are two reports that
the compound binds, at low levels, to DNA. After incubating
hepatocytes isolated from phenobarbital-treated rats with 14C-HCB
(5 µM) for 20 h, Stewart & Smith (1987) reported the maximum amount of
radioactivity associated with DNA was < 9.9 × 10-5% of the substrate
added, and was only marginally above that of hepatocytes held at 4°C;
the authors considered this to be significantly lower than expected
for hepatocarcinogens. Gopalaswamy & Nair (1992) also reported a low
order of binding of HCB to DNA from the livers of rats exposed to
25 mg HCB/kg. Short-term exposure (<1 day) of rats to oral doses of
700 or 1400 mg/kg body weight (Kitchin & Brown, 1989) or to as much at
300 mg/kg body weight i.p. (Górski et al., 1986) did not cause hepatic
DNA damage, as measured by alkaline elution.
7.5 Reproductive and developmental toxicity
Relatively low doses of HCB have been found to affect some
reproductive tissues in female monkeys. Oral exposure of cynomolgus
monkeys to 0.1 mg/kg body weight per day in gelatin capsules for 90
days caused stratification of the ovarian germinal epithelium
(Babineau et al., 1991; Jarrell et al., 1993a). Higher dosages (1.0
and 10.0 mg/kg body weight per day) were associated with cellular
degeneration of this surface epithelium. The low dosage was associated
with ultrastructural as well as light microscopic changes in surface
epithelium (Babineau et al., 1991; Sims et al., 1991).
In ovarian follicles the low dose was associated with an
increased number of lysosomal elements in germ cells (Singh et al.,
1990a). The basal lamina was thickened. Higher dosages were associated
with greater degenerative changes in their cells and granulosa cells
(Singh et al., 1991, 1990b).
These studies demonstrated changes in ovarian tissues with no
other evidence of toxicity. In particular, the induction of
superovulation with human menopausal gondotrophin (HMG) in these
animals was associated with a normal estradiol response, oocyte
recovery, oocyte maturation, in vitro fertilization and early embryo
development (Jarrell et al., 1993a). These studies confirm the
findings of Iatropoulous et al. (1976) in which the administration of
8 to 128 mg/kg body weight (by gavage in 1% methylcellulose) for 60
days induced severe follicular degeneration in primordial germ cells,
pseudostratification of the ovarian surface epithelium, hepatic
degeneration and severe systemic toxicity in Rhesus monkeys.
In subsequent studies of similarly treated animals, the higher
doses were associated with reduced luteal phase progesterone and
blunted estradiol responses to HMG (Foster et al., 1992a,b). Reduction
in adrenal steroidogenesis occurred in ovariectomized rats in response
to exposure to HCB at concentrations of 1, 10 and 100 mg/kg body
weight for 30 days (Foster et al., 1995).
In contrast, the results of studies on a variety of species have
indicated that repeated exposure to HCB can affect male reproduction,
but only at relatively high doses. Mice exposed to 250 mg HCB per kg
feed (approximately 30 mg HCB/kg body weight per day) for 21 days had
reduced serum testosterone levels; based on the results of in vitro
tests, it was suggested that this was due to increased metabolism by
hepatic microsomal enzymes induced by HCB (Elissalde & Clark, 1979).
Histological changes in the testes (retarded sexual maturation) were
noted in pigs fed a diet yielding a dose of 50 mg HCB/kg body weight
per day for 90 days (den Tonkelaar et al., 1978). The mating index for
male rats receiving five consecutive daily gavage doses of 221 mg
HCB/kg body weight in corn oil was decreased compared to those
receiving 0 or 70 mg/kg body weight However, the fertility index for
the mated female rats (sperm positive smears) was not affected (Simon
et al., 1979).
As discussed in the following paragraphs, placental and
lactational transfer of HCB, demonstrated in a number of species, can
adversely affect both the fetus and nursing offspring. The lactational
route appears to be more important than placental transfer. Adverse
effects on suckling infants are generally observed more frequently,
and at lower doses, than are embryotoxic or fetotoxic effects.
Grant et al. (1977) conducted a four-generation study on female
(20/dose level) and male (10/dose level) weanling Sprague-Dawley rats
fed diets containing 0, 10, 20, 40, 80, 160, 320 or 640 mg HCB/kg
feed. The two highest doses caused some deaths in the F0 dams before
first whelping, and reduced the fertility index. Dietary levels of 160
mg/kg or more reduced litter sizes, increased the number of
stillbirths, and adversely affected pup survival. Similar effects were
seen at 80 mg/kg after the first two generations, while 40 mg/kg was
hepatotoxic to the F1a and F3a pups. A dietary level of 20 mg/kg
(approximately 1-1.2 mg/kg body weight per day) was designated as the
no-observed-effect level.
Arnold et al. (1985) fed groups of male and female Sprague-Dawley
rats from weaning on diets containing up to 40 mg HCB/kg. The rats
were then bred at 3 months, and the F1 pups were continued on the
same diet for their lifetimes. HCB had no effect on fertility, but pup
survival was significantly reduced in the 40 mg/kg group (calculated
doses of 1.50 and 1.90 mg/kg body weight per day for males and
females, respectively).
In other studies, maternal doses in the range from 1.4 to 4 mg/kg
given to rats and cats have been found to be hepatotoxic and/or
affected the survival or growth of nursing offspring. In some cases,
these or higher doses reduced litter sizes and/or increased numbers of
stillbirths (Mendoza et al., 1977, 1978, 1979; Hansen et al., 1979;
Kitchin et al., 1982).
Mink are particularly sensitive to the effects of prenatal and
perinatal exposure to HCB; the offspring of mink fed diets containing
concentrations as low as 1 mg/kg (approximately 0.16 mg/kg body weight
per day) for 47 weeks (prior to mating and throughout gestation and
nursing) had reduced birth weights and increased mortality (Rush et
al., 1983; Bleavins et al., 1984b).
The available data on the developmental toxicity of HCB are
limited. CD-1 mice administered 100 mg/kg body weight by gavage on
days 7-16 of gestation had a significantly increased incidence of
abnormal fetuses per litter, and one case of renal agenesis was
reported. Some cleft palates were produced, but they all occurred in
one litter. This dose also increased maternal liver-to-body weight
ratios and decreased fetal body weights (Courtney et al., 1976). In a
series of studies reported by Andrews & Courtney (1986), combined
in utero and lactational exposure of CD-1 mice and CD rats (strain
unclear, probably Sprague-Dawley) to HCB (mouse dams received 10 or
50 mg/kg body weight per day, and rats 10 mg/kg body weight per day,
by gavage during gestation) resulted in increases in body weight and
kidney weights of pups of both species, along with enlarged kidneys
and a few cases of hydronephrosis. Increased liver weights were
observed in rat pups, and the occurrence of abnormal kidneys was
sporadic, with no dose-response relationship in studies with mice.
Khera (1974) reported a significant increase in the incidence of
unilateral or bilateral 14th rib in litters of Wistar rats receiving
doses of 80 and 120 mg HCB/kg body weight during gestation, but
maternal toxicity (loss of body weight and neurological effects) and
reduced fetal weights were noted in animals in these groups. (It
should be noted that, based on the biological half-lives reported for
HCB in mammals (section 6.2), the concentration of HCB in the dams in
these studies would not have reached the maximum that might occur as a
result of intake over a longer period).
Neurobehavioural development was affected in the offspring of
rats exposed to 2.5 or 25 mg/kg body weight per day by gavage 2 weeks
prior to breeding. Pups in both treated groups were hyperactive (based
on tests of negative geotaxic reflex, olfactory discrimination, and
exploratory locomotor activity) at 6-20 days of age. Pups from the
high treatment groups showed reduced acoustic startle response at 23
days of age, but a significantly increased response at 90 days. These
doses did not affect learning (swim T-maze) or motor activity in older
offspring, nor maternal or fetal body weights, length of gestation,
number of pups/litter at birth, or number of days to eye opening
(Goldey & Taylor, 1992).
Lilienthal et al. (1996) recently reported HCB-induced effects on
neurobehavioural development of rat pups exposed both maternally and
through the diet (dams were exposed to 0, 8 or 16 mg HCB/kg diet for
90 days prior to mating and throughout gestation and nursing, after
which the offspring were fed the same levels for 150 days). Exposure
to HCB did not affect the mean body weight of the pups (except males
at 150 days of age), or the number of pups/litter, but did increase
the mean body weight of dam, and their liver-to-body weight ratios.
Schedule-controlled behaviour was affected at 8 and 16 mg HCB/kg diet
(0.64 and 1.28 mg/kg body weight per day), as indicated by a dose-
related decrease in post-reinforcement pause at the end of the
experiment. Exploratory locomotor activity, open field behaviour at 21
days of age, and active avoidance learning at 90 days of age were
unaffected.
7.6 Immunotoxicity
The results of a number of studies have indicated that HCB
affects the immune system, with immunosuppressive effects in mice and
immunostimulatory effects in rats (summarized by Vos, 1986).
Balb/C mice exposed to 5 mg HCB/kg diet (approximately 0.6 mg/kg
body weight per day) for 3 to 18 weeks were more susceptible to
Leishmania infection (Loose, 1982) and had reductions in resistance
to a challenge with tumour cells and in the cytotoxic macrophage
activity of the spleen (Loose et al., 1981). Barnett et al. (1987)
reported that Balb/C mice exposed to maternal doses of 0.5 or 5 mg
HCB/kg body weight per day in utero and through nursing had severe
depression of the delayed-type hypersensitivity response to a contact
allergen (oxazolone). In a number of studies, exposure of mice to
diets containing 167 mg HCB/kg in diet (approximately 20 mg HCB/kg
body weight per day) for several weeks depressed humoral immunity,
cell-mediated immunity and host resistance (Vos, 1986; Carthew et al.,
1990).
In rats or rhesus monkeys with oral exposure to between 3 and
120 mg HCB/kg body weight per day for periods from 3 weeks to 6 months
in various studies, proliferative histopathological effects in the
thymus, spleen, lymph nodes, and/or lymphoid tissues of the lung have
been observed (Kimbrough & Linder, 1974; Iatropoulos et al., 1976;
Goldstein et al., 1978; Vos et al., 1979a,b; Kitchin et al., 1982).
Gralla et al. (1977) observed that long-term exposure to 1 mg HCB/day
(equivalent to a dose at the start of the experiment of roughly
0.12 mg/kg body weight per day) caused nodular hyperplasia of the
gastric lymphoid tissue in beagle dogs.
In rats, prominent changes following dietary exposure to HCB
include elevated IgM levels and an increase in the weights of the
spleen and lymph nodes. Histopathologically, the spleen shows
hyperplasia of B-lymphocytes in the marginal zone and follicles, while
lymph nodes show an increase in proportions of high endothelial
venules, indicative of activation. High endothelial-like venules are
induced in the lung, as are accumulations of macrophages. Functional
tests revealed an increase in cell-mediated immunity, as measured by
DTH reactions, a notable increase in primary and secondary antibody
response to tetanus toxoid, and decreased NK activity in the lung (Vos
et al., 1979a,b). Stimulation of humoral and cell-mediated immunity
occurred even at dietary levels as low as 4 mg HCB/kg (approximately
0.2 mg HCB/kg body weight per day); at such a dose conventional
parameters for hepatotoxicity were unaltered (Vos et al., 1983).
Therefore, the developing immune system of the rat seems to be
particularly vulnerable to the immunotoxic action of HCB.
More recent studies indicate that HCB may cause autoimmune-like
effects in the rat. Wistar rats treated with HCB had elevated levels
of IgM, but not IgG, against the autoantigens single-stranded DNA,
native DNA, rat IgG (representing rheumatoid factor), and bromelain-
treated mouse erythrocytes (that expose phosphatidylcholine as a major
autoantigen). It has been suggested that HCB activates a recently
described B cell subset committed to the production of these
antibodies (Schielen et al., 1993). The role of these autoantibodies
is still a matter of controversy. Increased levels have been
associated with various systemic autoimmune diseases, but a protective
role of these autoantibodies against development of autoimmune disease
has been postulated as well. Interesting in this respect are the
observations that HCB had quite opposite effects in two different
models of autoimmune disease in the Lewis rat. HCB treatment severely
potentiates allergic encephalitis elicited by immunization with myelin
in complete Freund's adjuvant, while it strongly inhibits the
development of arthritic lesions elicited by complete Freund's
adjuvant as such (Van Loveren et al., 1990).
A possible relation between the immunomodulatory properties of
HCB and HCB-induced skin lesions, attributed in the literature to the
porphyrinogenic action of HCB, was recently indicated. In rats treated
with a combination of HCB and triacetyloleandomycin (TAO, a selective
inhibitor of cytochrome P-450IIIa), porphyria was greatly reduced.
Remarkably, combined treatment with HCB and TAO did not substantially
affect the incidence and severity of skin lesions. In addition, TAO
did not influence the immunomodulatory effect of HCB, including the
formation of antibodies. From these findings it has been suggested
that an immunological component underlies, at least in part, the
HCB-induced skin lesions in the rat (Schielen et al., 1995).
8. EFFECTS ON HUMANS
8.1 General population exposure
Numerous reviews have been published of an accidental poisoning
incident in Turkey that occurred in 1955-1959 as a result of
HCB-treated wheat grain (distributed by the Turkish government for
planting purposes) being ground into flour and made into bread
(Schmid, 1960; Cam & Nigogosyan, 1963; Dogramaci, 1964; Peters, 1976;
Courtney, 1979; Peters et al., 1982; US EPA, 1985a; Gocmen et al.,
1989). In this incident, more than 600 cases of porphyria cutanea
tarda (PCT) were clinically identified, and it was estimated that as
many as 3000-5000 persons were affected, with a mortality of 10%. The
condition developed primarily in children 4-14 years of age (roughly
80% of cases), occurring infrequently in adults and rarely in children
under 4 years of age. In a number of reports, it has been suggested
that males developed the condition in higher proportion than females.
However, Dogramaci et al. (1962) demonstrated that the sex ratio was
skewed in favour of males in both the affected and unaffected
populations. In addition to disturbances in porphyrin metabolism
(excretion of porphyrins and porphyrin precursors was greatly
increased), clinical manifestations included skin lesions (erythema,
bullae), ulcerations and resultant scarring, friable skin,
hyperpigmentation, hypertrichosis, enlarged liver, weight loss,
enlargement of the thyroid gland and lymph nodes, neurological
effects, and a characteristic port wine colour of the urine (from
increased excretion of porphyrins). In roughly half the cases,
osteoporosis of extremities, deformation of the fingers or arthritis
was also noted. The dermatological lesions, which occurred on the
exposed parts of the body, particularly the face and hands, were often
precipitated by sunlight. They tended to remit in winter and relapse
during the spring and summer (Peters, 1976; Peters et al., 1982). The
estimated dose was 50-200 mg/day for a number of months before
manifestations of the disease became apparent (Cam & Nigogosyan,
1963); the basis for this estimate was not presented, however, making
exposure calculations unreliable for this population. In 20- to 30-
year follow-ups of exposed individuals, neurological, dermatological
and orthopaedic abnormalities persisted, and there were elevated
levels of porphyrins in excreta of some individuals (Peters et al.,
1982; Peters et al., 1986; Gocmen et al., 1989).
In this incident, a disorder called "pembe yara" or "pink sore"
was described in infants of mothers who either had PCT or had eaten
HCB-contaminated bread. These infants developed characteristic pink
cutaneous lesions, and often had fevers, diarrhea, vomiting, weakness,
convulsions, enlarged livers and progressive wasting. It is noteworthy
that PCT was not observed in these children (Cam, 1960; Peters et al.,
1982). At least 95% of these children died within a year of birth, and
in many villages no children between the ages of 2-5 years survived
during the period 1955-1960. Elevated concentrations of HCB (levels
were not quantified at the time, but the average concentration in milk
from 56 porphyric mothers, 20-30 years after the incident, was
510 ng/g on a fat basis) were found in the mothers' milk and cessation
of breast-feeding slowed the deterioration of infants with this
disorder (Peters et al., 1966; Gocmen et al., 1989).
No adequate epidemiological studies of cancer in populations
exposed to HCB in the environment were found in the literature. In
long-term follow-up of the Turkish poisoning victims with porphyria
(Peters et al., 1982; Cripps et al., 1984; Gocmen et al., 1989) there
was no evidence of increased cancer incidence, although these studies
were not designed to evaluate this end-point, and only a small
fraction of the exposed people was followed up. There was a high
frequency of enlarged thyroids in the Turkish poisoning victims (27%
of men and 60% of women, compared to an average of 5% in the area
(Peters et al., 1982)), but Gocmen et al. (1989) reported that they
observed no malignant tumours of the liver or thyroid in 252 of the
poisoning victims. In three patients who underwent thyroidectomy,
histopathological examination indicated that the enlargement was due
to colloidal goitre.
Grimalt et al. (1994) reported a small ecological study of cancer
incidence (129 cases in all) in the inhabitants of a village in Spain
located near a chlorinated solvents factory. There were statistically
significant excesses of thyroid neoplasms and soft-tissue sarcomas in
males, compared with the province as a whole, although these were
based on only 2 and 3 cases, respectively. The exposures experienced
by this population were somewhat unclear. Levels of HCB in ambient air
and in the sera of volunteers were much higher in the village than in
Barcelona (means of 35 ng/m3 versus 0.3 ng/m3 and 26 µg/litre versus
4.8 µg/litre, respectively), but the authors presented evidence that
historical exposures had been much higher and indicated that all of
the males with cancer for whom there were occupational histories had
worked in the factory. Ambient air monitoring revealed that there were
exposures to a variety of other compounds, including polychlorinated
biphenyls, p,p'DDE, chloroform, carbon tetrachloride,
trichloroethylene and tetrachloroethylene, but at similar or lower
levels than in the reference community.
8.2 Occupational exposure
There have been case reports of workers developing PCT as a
result of direct contact with HCB (Courtney, 1979; Currier et al.,
1980), although there was no association between exposure to HCB and
PCT in three cross-sectional studies of very small populations of
exposed workers (Morley et al., 1973; Burns et al., 1974; Currier et
al., 1980). There was no evidence of cutaneous porphyria in a cross-
sectional study of the general population in Louisiana, USA, exposed
to HCB through the improper transport and disposal of hex waste;
however, plasma concentrations of HCB were significantly correlated
with levels of coproporphyrin in urine and of lactic dehydrogenase in
blood (Burns & Miller, 1975).
Available epidemiological studies on the carcinogenicity of HCB
in occupationally exposed humans are restricted to one study of a
cohort of 2391 magnesium metal production workers in Norway. Although
the incidence of lung cancer was significantly elevated compared to
that of the general population, workers were exposed to numerous other
agents in addition to HCB, including coal tar, asbestos and dust of
metal oxides and chlorides (Heldaas et al., 1989). Selden et al.
(1989) reported a case of hepatocellular carcinoma in a 65-year-old
man who had been employed for 26 years in an aluminum smelting plant,
where he had potential exposure to a range of substances, including
HCB, other chlorobenzenes, chlorophenols, dioxins and furans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Data on the acute and chronic ecotoxicity of HCB are available
for species from a number of trophic levels, including protozoans,
algae, invertebrates and fish, for both the freshwater and marine
environments. With reference to terrestrial organisms, toxicity data
are available only for birds and mammals (the results of studies in
mammals are summarized in chapter 7). Since HCB is nearly insoluble in
water, and tends to partition from water to the atmosphere, the
substance is lost rapidly from open-test solutions. Hence, it is
difficult to maintain test concentrations for a sufficient time to
establish concentration-effects profiles for aquatic organisms.
Furthermore, HCB tends to bind to suspended solids in the water column
and thus may not be bioavailable to test organisms. This discussion of
the toxicity of HCB to aquatic organisms will therefore focus on tests
conducted under flow-through conditions, static renewal conditions, or
using closed vessels with minimal headspace. In addition, no
consideration has been given to tests in which concentrations of HCB
were well above its solubility in water (5 µg/litre at 25°C).
9.1 Short-term exposure
9.1.1 Aquatic biota
Of four freshwater algal species tested, only one, Chlorella
pyrenoidosa, was affected by concentrations of HCB in water at or
below its limit of aqueous solubility. Reduced production of
chlorophyll, dry matter, carbohydrate and nitrogen was observed for
C. pyrenoidosa after exposure to a nominal concentration of
1 µg/litre HCB for 46 h in a static-closed system (Geike & Parasher,
1976a). A no-observed-effect concentration (NOEC) was not determined
in this study.
At concentrations equal to its aqueous solubility in water
(5 µg/litre), HCB was not lethal to the freshwater water flea
Daphnia magna in a flow-through test in which concentrations of HCB
were measured (Nebecker et al., 1989). In 96-h flow-through tests on
marine invertebrates, exposure to HCB caused 13% mortality in pink
shrimp ( Penaeus duorarum) at a measured concentration of 7 µg
HCB/litre, and 10% mortality in grass shrimp ( Palaemonetes pugio) at
17 µg/litre. The NOEC values in these species were 2.3 µg/litre and
6.1 µg/litre, respectively (Parrish et al., 1974). In a static-closed
system, there was a 10% reduction in reproduction of the ciliate
protozoan Euplotes vannus after exposure to a nominal concentration
of 10 µg/litre HCB for 48 h (Persoone & Uyttersprot, 1975).
The available data on freshwater fish species indicated no
harmful effects at concentrations at or near the limit of solubility
of HCB in water during acute exposure (Call et al., 1983; Ahmad et
al., 1984). In the only available study for marine fish species, there
were no effects on mortality in sheepshead minnow ( Cyprinodon
variegatus) after flow-through exposure to a measured concentration
of 13 µg/litre HCB for 96 h (Parrish et al., 1974).
Limited data are available concerning the toxic effects of HCB in
sediment on freshwater and marine biota. In a 96-h sediment toxicity
test on the marine shrimp, Crangon septemspinosa, no mortality was
observed at the highest concentration of HCB tested, 300 µg/litre
(McLeese & Metcalfe, 1980).
Several studies have confirmed that there is a relatively
constant body residue associated with acute lethality in freshwater
fish, invertebrates and algae exposed to mono-to-pentachlorobenzenes
(McCarty et al., 1992a; Ikemoto et al., 1992). The acute LC50
critical body residue for chlorobenzenes is 2 µmol/g wet weight, or
569.6 µg/g wet weight for HCB, assuming that HCB has the same mode of
action as the other chlorobenzenes (McCarty et al., 1992b).
9.1.2 Terrestrial biota
The LD50 for HCB in herring gull ( Larus argentatus) embryos
injected on day 4 and tallied on day 25 was 4.3 µg/g body weight
(Boersma et al., 1986). At a dose of 1.5 µg/g body weight, there were
significant reductions in embryonic weight. Five-day LC50 values
(i.e., 5 days of HCB-containing diet followed by 3 days of untreated
diet) were 617 µg/g diet for 10-day-old ring-necked pheasants
( Phasianus colchicus) and > 5000 µg/g diet for 5-day-old mallards
( Anas platyrhynchos) (Hill et al., 1975). Induction of porphyria has
been observed in studies of Japanese quail following administration of
500 µg HCB/g body weight per day for between 5 and 10 days either in
food or via intraperitoneal injection (Buhler & Carpenter, 1986;
Lambrecht et al., 1988).
9.2 Long-term exposure
9.2.1 Aquatic biota
Growth of cultures of the alga Chlorella pyrenoidosa was
increased by exposure for 3 months to a nominal concentration of 1 µg
HCB/litre (Geike & Parasher, 1976b), while that of the protozoan
Tetrahymena pyriformis was decreased after a 10-day exposure to the
same concentration (Geike & Parasher, 1976b).
After exposure to 5 µg HCB/litre for 10 days in a static-renewal
system, crayfish ( Procambarus clarki) experienced damage to the
hepatopancreas (Laseter et al., 1976). The fertility of Daphnia
magna was reduced by 50% after exposure for 14 days to a measured
concentration of 16 µg/litre HCB in a static-closed system (Calamari
et al., 1983). Significantly increased mortality was observed in
amphipods, Gammarus lacustris, exposed to a measured concentration
of 3.3 µg HCB/litre for 28 days under flow-through conditions
(Nebecker et al., 1989). However, the results of this study indicated
a weak-dose response relationship. In two other flow-through studies,
there were no effects on survival, growth or reproduction of the
amphipod Hyallela azteca and the worm Lumbriculus variegatus at a
measured concentration of 4.7 µg HCB/litre (Nebecker et al., 1989).
In several studies, fathead minnows ( Pimephales promelas) and
rainbow trout ( Oncorhynchus mykiss) experienced no mortality or
effects on growth after exposure to levels of HCB approaching its
aqueous solubility (Ahmad et al., 1984; Carlson & Kosian, 1987; US
EPA, 1988; Nebecker et al., 1989). However, Laseter et al. (1976)
reported liver necrosis in large-mouth bass ( Micropterus salmoides)
after an exposure for 10 days to 3.5 µg HCB/litre under flow-through
conditions.
Guidelines for the protection and management of aquatic sediment
quality in Ontario, Canada (Persaud et al., 1991) have given a no-
observed-effect level (NOEL), a lowest-observed-effect level and a
severe-effect level for a variety of contaminants. The values given
for HCB are 10 ng/g dry weight, 20 ng/g dry weight and 24 000 ng/g
organic carbon. The partitioning approach was used to determine the
lowest-observed-effect level, whereas the severe-effect level was more
dependent on the screening level concentration approach. The
limitation of both approaches is that they are unable to separate the
biological effects that are due to a combination of contaminants; thus
while ecotoxicological effects can be established, these cannot be
attributed to any one chemical contaminant. This is a very serious
limitation since virtually all sediments are contaminated with a wide
variety of pollutants, and there is no indication that HCB was the
dominant pollutant.
Quantitative structure-activity relationships (QSAR) were used to
estimate the narcotic toxicity for 19 species to predict NOELs (Van
Leeuwen et al., 1992). The NOELs for water, sediment and residues in
biota were predicted only on the basis of the octanol/water partition
coefficient and relative molecular mass. The QSAR-derived level for
HCB in sediments was 5814 ng/g dry weight (20.4 nmol/g in the
reference) for sediments with 5% total organic carbon content. The
adjusted value for sediment with 1% total organic carbon content is
1163 ng/g. There is no experimental verification of these
calculations. Thus, no firm evidence is available on the critical
levels of HCB in sediments.
9.2.2 Terrestrial biota
In adult Japanese quail ( Coturnix japonica) fed diets
containing HCB for 90 days, mortality was increased at 100 µg HCB/g
in diet, and hatchability of eggs was significantly reduced at 20 µg/g
(Vos et al., 1971, 1972). At 5 µg/g, increased liver weight, slight
liver damage and increased faecal excretion of coproporphyrin were
observed. Eurasian kestrels ( Falco tinnunculus) fed mice containing
200 µg HCB/g fresh body weight for 65 days had significant weight
loss, ruffling of feathers, tremors, increased liver weight and
decreased heart weight (Vos et al., 1972).
The available long-term toxicity data for mammals are discussed
in section 7.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on estimates of mean exposure from various media (section
5.2), the general population is exposed to HCB principally in food
(mean intakes for adults range from 0.0004 to 0.0028 µg/kg body weight
per day). Intakes are estimated to be considerably less for ambient
air (3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day) and
drinking-water (2.2 × 10-6 to 4.4 × 10-5 µg/kg body weight per day).
Based on these intakes, it is estimated that the total average daily
intake of HCB from food, air and drinking-water is between 0.0004 and
0.003 µg/kg body weight per day.
Data on levels of occupational exposure to HCB are limited but
indicate that workers in some industries may be exposed to higher
levels of HCB than the general population, particularly in the
manufacture of chlorinated solvents, and in the manufacture and
application of chlorinated pesticides contaminated with HCB. In some
instances inappropriate manufacturing and waste management practices
may expose nearby populations to higher levels of HCB than the general
population. Exposures may also be elevated in some indigenous
subsistence populations, particularly those that consume large
quantities of food species near the top of the food chain.
Owing to the elimination of HCB in breast milk, mean intakes by
nursing infants are estimated to range from < 0.018 to 5.1 µg/kg body
weight per day in various countries (see section 5.2.4 and Table 8).
10.1.2 Health effects
Available data on the effects of HCB in humans are limited
principally to those of people exposed in an accidental poisoning
incident that occurred in Turkey between 1955 and 1959. More than 600
cases of porphyria cutanea tarda (PCT) were observed, and infants of
exposed mothers experienced cutaneous lesions, clinical symptoms and
high mortality. It has been estimated that victims were exposed to an
estimated dose of 50-200 mg HCB/day for an undetermined, but extended,
period of time. However, the basis of this estimate was not provided,
making exposure calculations unreliable for this population. Studies
of the carcinogenicity of HCB in humans are limited to two small
epidemiological studies of cancer incidence in populations with poorly
characterized exposure to HCB as well as to numerous other chemicals.
No excesses of neoplasms have been reported in long-term follow-up
studies of the people with porphyria in the incident in Turkey, but
only a small fraction of the population was followed-up, and these
studies were not designed specifically to assess neoplastic end-
points.
Hence, the available data on humans are inadequate to serve as a
basis for assessment of effects from exposure to HCB. The remainder of
this evaluation is, therefore, based on studies in animals.
Based on the studies reviewed in section 7, the critical effects
induced by HCB in experimental animals comprise both non-neoplastic
and neoplastic effects.
With respect to non-neoplastic effects, repeated exposure to HCB
has been found to cause a wide range of non-neoplastic effects in
several species of animals, with similar lowest-observed-effect-levels
(LOELs) and no-observed-effect-levels (NOELs) for a number of end-
points (see Table 9). In these studies, effects reported have included
those on the liver in pigs and rats, on calcium metabolism in rats, on
ovarian histopathology in monkeys, on immune function in mice and
rats, on neurotransmitter levels in the hypothalamus of mink, on
postnatal survival in mink, and on neurobehavioural development in
rats. The range over which the various effects have been observed is
quite narrow; the lowest LOELs compiled in Table 9 range from 0.1 to
0.7 mg/kg body weight per day, while the lowest NOELs range from 0.05
to 0.07 mg/kg body weight per day.
Based on the induction of a variety of tumours in hamsters, rats
and mice exposed by ingestion, there is sufficient evidence that HCB
is carcinogenic in animals. The available evidence indicates that HCB
has little or no genotoxic activity and is therefore unlikely to be a
direct-acting (genotoxic) carcinogen. However, the Task Group noted
that tumours, some of which were malignant, have been induced in
multiple species, at multiple sites, in some instances at doses that
were not overtly toxic in other respects and that are within an order
of magnitude of those that produce more subtle toxicological effects,
or following subchronic exposure. Although there is some evidence to
suggest that HCB may cause cancer by indirect mechanisms, the evidence
is not definitive at this time and does not address all tumour sites.
Table 9. No-observed-effect and lowest-observed-effect levels (NOELs and LOELs) in mammals exposed to HCB
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Mouse Depressed delayed-type hypersensitivity - 0.5a Barnett et al.
response to oxazolone in mice exposed (1987)
to HCB in peanut butter in utero
(throughout gestation) and via nursing
to 45 days of age (section 7.6)
Mouse Increased susceptibility to Leishmania - 0.6 Loose et al. (1981);
infection, and reductions in resistance Loose (1982)
to a challenge with tumour cells and in
the cytotoxic macrophage activity of the
spleen in mice with subchronic exposure
to HCB in diet (section 7.6)
Rat Alterations in Ca metabolism (increased 0.07 0.7 Andrews et al.
serum 1,25-dihydroxy-vitamin-D3 levels, (1989, 1990)
reduced Ca excretion, alterations in
femur density, bone morphometry and
strength), increased liver weights, with
subchronic gavage exposure to HCB
(section 7.2)
Rat Increased cell-mediated and humoral - 0.2a Vos et al. (1983)
immune function, intraalveolar
macrophage accumulation, microsomal
ethoxyresorufin-O-deethylase activity,
in rats exposed to HCB in utero, via
nursing and in the diet to 5 weeks of
age (section 7.6)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Rat Increased organ weights (heart, brain 0.05-0.07 0.27-0.35 Arnold et al. (1985);
and liver) in F0 males, compound- Arnold & Krewski (1988)
related histological changes in liver
of both sexes of F1 rats with long-term
exposure to HCB in diet (section 7.3)
Rat Ultrastructural changes in livers 0.05-0.06 0.25-0.30 Mollenhauer et al.
(proliferation of SER, altered (1975, 1976)
mitochrondria, increase in numbers of
storage vesicles) of rats with long-term
exposure to HCB in diet (section 7.3)
Rat Induction of in vivo mixed-function - 0.5-0.6 Grant et al. (1974)
oxidase activity in rats with long-term
exposure to HCB in diet (section 7.3)
Rat Dose-related decrease in the post- - 0.64 Lilienthal et al.
reinforcement pause (PRP) after schedule- (1996)
controlled operant conditioning of rats
exposed to HCB in utero, through nursing,
and up to post-natal day 150
Mink Increased serotonin concentrations in - 0.16a Rush et al. (1983);
hypothalamus of mink dams with long-term Bleavins et al. (1984a,b)
dietary exposure to HCB, decreased
dopamine levels in hypothalamus, reduced
birth weights, and increased mortality
to weaning in mink kits with in utero
plus lactational exposure to HCB
(sections 7.3, 7.5)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Dog Nodular hyperplasia of gastric lymphoid - 0.12 Gralla et al.
tissue in beagles with long-term (1977)
exposure to HCB in gelatin capsules
(section 7.6)
Pig Increased urinary coproporphyrin and 0.05 0.05 Den Tonkelaar et al.
microsomal liver enzyme activity in (1978)
pigs with subchronic exposure to HCB
in diet (section 7.2)
a Doses reported are those received by dams
10.1.3 Approaches to risk assessment
The following is provided as a potential basis for derivation of
guidance values. Since ingestion is by far the principal route of
exposure and since the toxicological data for other routes of
administration are insufficient for evaluation, only the oral route is
addressed here, though the ultimate objective should be reduction of
total exposure from all routes.
Based on the scientific evaluation of the data for the non-
neoplastic and neoplastic end-points, two possible approaches to
develop health-based guidance values were suggested.
10.1.3.1 Non-neoplastic effects
The approach for non-neoplastic effects assumes a threshold for
these effects and is based on the use of the NOAEL or NOEL and an
uncertainty factor that takes account of interspecies and
interindividual variation in sensitivity to the substance, as well as
the quality of the available studies and the severity of effect.
The available data are sufficient to develop a Tolerable Daily
Intake (TDI) for HCB. The lowest reported NOELs and LOELs for several
different types of effects, such as those on the liver in rats and
pigs, calcium metabolism in rats, ovarian morphology in monkeys,
immune function in rats and mice, neurobehavioural development in rats
and perinatal survival in mink, fall within a very small range (Table
9). Based on the lowest reported NOELs included in the table
(approximately 0.05 mg/kg body weight per day based primarily on
hepatic effects observed in a subchronic study in pigs and in chronic
studies in rats), a TDI of 0.17 µg/kg body weight per day has been
derived for non-neoplastic effects, by incorporating an uncertainty
factor of 300 (x 10 for intraspecies variation; × 10 for interspecies
variation, × 3 for severity of effect). A factor of 3 for severity of
effects was chosen as HCB causes i) multiple non-neoplastic effects in
several species, and ii) LOELs for a number of end-points for which
NOELs have not been determined are very close to the NOEL, from the
critical studies, of 0.05 mg/kg body weight per day. However, it is
fully realized that national authorities may choose other end-points
or uncertainty factors depending upon data evaluation and future
scientific findings.
10.1.3.2 Neoplastic effects
The approach for neoplastic effects is based on the Tumorigenic
Dose5, or TD5 i.e., the intake or exposure associated with a 5%
excess incidence of tumours in experimental studies in animals (IPCS,
1994). This is a benchmark approach in which the TD5 is calculated
directly from the experimental data rather than using the upper or
lower confidence limits. Uncertainty factors are then applied to the
TD5 to obtain a guidance value. The choice of uncertainty factors is
based on the level and nature of mechanistic data available, the
quality of the database, the tumour pattern, the dose-response
relationship, and the experimental model chosen. The final value will
reflect the degree of certainty one has with the available
information.
For the purpose of indicating the magnitude of risk of HCB, the
two-generation study in rats has been selected, owing to its relevance
to the exposure of the general human population, as the design of this
study involved exposure to relatively low concentrations of HCB in the
diet (including in utero and lactational exposure). Moreover, tumour
pathology was inadequately reported in the available studies in
hamsters and mice, and there is some concern that in the other
adequate study in rats, there may also have been exposure by
inhalation to some HCB that was incorporated in the diet as a powder.
The TD5 value was calculated from the results of the two-
generation study in rats using a multistage model (Crump & Howe,
1982). The tumour incidences in the pups were analysed in the same
manner as data from a single-generation study, owing to the lack of
information on individual litters. On this basis, the TD5 values
range from 0.81 mg/kg body weight per day for neoplastic liver nodules
in females to 2.01 mg/kg body weight per day for parathyroid adenomas
in males. The Task Group decided that the most sensitive end-point
(neoplastic nodules of the liver) would be used in its analysis. In
calculating the suggested guidance value, it was agreed to use an
uncertainty factor of 5000, based on consideration of the insufficient
mechanistic data. The TD5 was divided by this uncertainty factor to
arrive at the suggested guidance value of 0.16 µg/kg body weight per
day. However, it is fully realized that national authorities may
choose other end-points or uncertainty factors depending upon data
evaluation and future scientific findings.
Although infants may have a high intake of HCB via breast milk
for a short time, the TD5 and TDI were considered to be protective of
the health of this population (unless there are extreme exposures),
because one of the long-term studies used in deriving these values
included lactational exposure. However, it should be noted that the
TD5 and TDI values derived above should not be compared directly with
intakes from breast milk by nursing infants, since the guidance values
are based on a lifetime intake, whereas the duration of breast-feeding
is relatively short.
10.2 Evaluation of effects on the environment
HCB is widely distributed in the environment, by virtue of its
mobility and resistance to degradation, although slow photodegradation
in air (half-life of approximately 80 days) and microbial degradation
(half-life of several years) do occur. It has been detected in air,
water, sediment, soil and biota from around the world. HCB is a
bioaccumulative substance (BCF values range from 375 to > 35 000),
and biomagnification of HCB through the food chain has been reported.
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa. In longer-term studies, the growth of sensitive freshwater
algae and protozoa was affected by a concentration of 1 µg/litre,
while a concentration of approximately 3 µg/litre caused mortality in
amphipods and liver necrosis in largemouth bass. The concentrations of
HCB in surface waters around the world are much lower than these
effect levels (3 to 5 orders of magnitude lower), except in a few
extremely contaminated localities.
Injection studies in eggs have shown that tissue levels of
1500 ng/g wet weight reduce embryo weights in herring gulls (lowest
dose tested). No studies were available to establish a NOAEL. For many
bird species, reduced embryo weights are associated with lower
survival of chicks. This effect level is within an order of magnitude
of the levels measured in the eggs of sea birds and raptors from a
number of locations from around the world, suggesting that present
levels of HCB in certain locations may harm embryos of bird species.
Experimental studies on mink indicate that they are sensitive to
the toxic effects of HCB; long-term ingestion of diets containing
1000 ng HCB/g (the lowest dose tested) increased mortality, decreased
birth weights of offspring exposed in utero and via lactation, and
altered levels of neurotransmitters in the hypothalamus of dams and
their offspring. No studies were available to establish a NOAEL. This
dietary effect level is only a few times higher than the
concentrations of HCB measured in various species of fish from a
number of industrialized locations from around the world, suggesting
that present levels of HCB in fish species from certain locations may
adversely affect mink and perhaps other fish-eating mammals.
11. RECOMMENDATION FOR PROTECTION OF HUMAN HEALTH AND THE ENVIRONMENT
a) Alternatives should be found for any present uses of HCB.
b) It is important to reduce the environmental burden of HCB by:
(i) identifying remaining sources and quantities of release to
the environment from these sources, including point source
emissions, waste disposal sites and production facilities;
(ii) applying appropriate manufacturing and waste disposal
practices in order to decrease levels of HCB in the
environment.
c) Human monitoring of HCB in blood and breast milk should be
undertaken to develop data representing exposure of the general
population, in order to identify highly exposed populations and
potential sources, and to enable interpretation of individual
results.
d) In order to gauge the efficacy of control measures it would be
valuable to monitor environmental levels and effects in locations
where levels are higher than the global average.
e) Neonatal effects in humans and other species have been associated
with ingestion of high doses of HCB through breast milk. It is
recommended that techniques be developed to assess appropriately
the risk to infant health from exposure to HCB and related
compounds in breast milk.
12. FURTHER RESEARCH
12.1 Environment
a) To improve the database available for environmental risk
assessment, it is considered important to establish a NOEL for
the serious reproductive effects seen in mink at dietary levels
approaching those found in certain locations.
b) Since HCB is persistent in soil and sediment, it would be
valuable to perform biodiversity experiments with HCB-treated
soil and sediment.
12.2 Human health
a) Based on the effects of low doses of HCB on ovarian tissues in
primates, involving disorders of germ cells and the ovarian
surface epithelium, the following is recommended:
(i) exposed populations should be studied for relevant
reproductive human outcomes of interest, particularly, fetal
loss and ovarian cancer;
(ii) reproductive tissues such as ovarian follicular fluid should
be included in human monitoring studies on HCB levels and/or
effects.
b) In order to decrease uncertainty in the risk assessment of HCB
and related compounds, research into the primary mechanism(s) of
action for tumorigenic, thyroid, reproductive, porphyrigenic,
neurotoxic and immunological effects of HCB should be undertaken.
c) Preliminary evidence suggests that HCB acts, at least in part,
through Ah receptor-linked mechanisms. This should be evaluated
more fully and compared to other polyhalogenated aromatic
chemicals for which a wealth of data are already available.
d) Given the toxicity of HCB and the few data for humans,
multicentre longitudinal studies of highly exposed human
populations should be undertaken. End-points of interest should
cover toxicokinetics (e.g., half-life), thyroid function,
porphyrin metabolism, reproductive outcomes (e.g., fetal losses),
and cancer. Nursing infants from these populations should be
followed to assess immunological and neurobehavioural
development.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
HCB as a Group 2B carcinogen (possibly carcinogenic to humans) based
on inadequate evidence for carcinogenicity to humans and sufficient
evidence for carcinogenicity to animals (IARC, 1987).
A drinking-water guideline of 1 µg/litre was developed for HCB
based on an evaluation of the production of liver tumours in female
rats and applying the linearized multistage model to calculate an
excess life-time cancer risk of 10-5 (WHO, 1993).
A conditional acceptable daily intake of 0.6 µg HCB/kg body
weight was developed by the Joint FAO/WHO Joint Meeting on Pesticide
Residues in Food (FAO/WHO, 1975). This recommendation was withdrawn in
1978 (FAO/WHO, 1978).
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the Legal File of
the International Register of Potentially Toxic Chemicals (IRPTC,
1993).
REFERENCES
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in the United Kingdom 1969-71. Br Med J, 2:
533-556.
Abbott DC, Collins GB, Goulding R, & Hoodless RA (1981) Organochlorine
pesticide residues in human fat in the United Kingdom 1976-77. Br Med
J, 283: 1425-1428.
Abbott DC, Goulding R, Holmes DC, & Hoodless RA (1985) Organochlorine
pesticide residues in human fat in the United Kingdom 1982-1983. Hum
Toxicol, 4: 435-445.
Abraham K, Hille A, Ende M, & Helge H (1994) Intake and fecal
excretion of PCDDs, PCDFs, HCB and PCBs (138, 153, 180) in a breast-
fed and a formula-fed infant. Chemosphere, 29(9-11): 2279-2286.
Ahling B, Bjorseth A, & Lunde G (1978) Formation of chlorinated
hydrocarbons during combustion of poly(vinyl chloride). Chemosphere,
10: 799-806.
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, Defoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: A toxicity data summary, Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory (EPA 600/3-84-009).
Albert LA, Alpuche L, Barcenas C, & Rendón J (1990) A survey of
organochlorine pesticide residues in cheese samples from three Mexican
regions. Environ Pollut, 65: 119-126.
Albro PW & Thomas R(1974) Intestinal absorption of hexachlorobenzene
and hexachlorocyclohexane isomers in rats. Bull Environ Contam
Toxicol, 12: 289-294.
Alves HHD & Chevalier M (1980) L'hexachlorobenzène dans
l'environnement québécois : Production, utilisation et présence.
Montréal, Québec, Environnement Canada, Service de la Protection de
l'Environnement (Rapport No EPS-3 QR-80-1).
Andrews JE & Courtney KD (1986) Hexachlorobenzene-induced renal
maldevelopment in CD-1 mice and CD rats. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 381-391 (IARC
Scientific Publications No. 77).
Andrews JE, Courtney KD, Stead AG, & Donaldson WE (1989)
Hexachlorobenzene induced hyperparathyroidism and osteosclerosis in
rats. Fundam Appl Toxicol, 12: 242-251.
Andrews JE, Jackson LD, Stead AG, & Donaldson WE (1990) Morphometric
analysis of osteosclerotic bone resulting from hexachlorobenzene
exposure. J Toxicol Environ Health, 31: 193-201.
Angerer J, Heinzow B, Reimann DO, Knorz W, & Lehnert G (1992)
Internal exposure to organic substances in a municipal waste
incinerator. Int Arch Occup Environ Health, 64: 265-273.
Ariño AA, Herrera A, Conchello MP, & Pérez C (1992) Hexachlorobenzene
residues in Spanish meat products after cooking, curing, and long-term
ripening. J Food Prot, 55(11): 920-923.
Ariño A, Herrera A, Conchello P, & Lazaro R (1993) Hexachlorobenzene
and hexachlorocyclohexane residues in pork as affected by weight and
sex. Bull Environ Contam Toxicol, 51: 647-650.
Arnold DL & Krewski D (1988) Long-term toxicity of hexachlorobenzene.
Food Chem Toxicol, 26: 169-174.
Arnold DL, Moodie CA, Charbonneau SM, Grice HC, McGuire PF, Bryce FR,
Collins BT, Zwadizka ZZ, Krewski DR, Nera EA, & Munro IC (1985) Long-
term toxicity of hexachlorobenzene in the rat and the effect of
dietary vitamin A. Food Chem Toxicol, 23: 779-793.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for hexachlorobenzene. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-90-17).
ATSDR (1994) Toxicological profile for hexachlorobenzene - Update.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry.
Atuma SS, Andersson O, Linder C-E, & Hansson L (1993) Levels of some
organochlorine compounds in sea trout ( Salmo trutta) and whitefish
( Caregonus lavaretus) from the Gulf of Bothnia. Aqua Fenn, 23:
221-226.
Avrahami M (1975) Hexachlorobenzene: IV. Accumulation and elimination
of HCB by pigs after oral dosing. NZ J Exp Agric, 3: 285-287.
Avrahami M & Steele RT (1972) Hexachlorobenzene: I. Accumulation and
elimination of HCB in sheep after oral dosing. NZ J Agric Res, 15:
476-481.
Ayotte P, Dewailly E, Bruneau S, Careau H, & Vézina A (1995) Arctic
air pollution and human health: what effects should be expected? Sci
Total Environ, 160/161: 529-537.
Babineau KA, Singh A, Jarrell JF, & Villeneuve DC (1991) Surface
epithelium of the ovary following oral administration of
hexachlorobenzene to the monkey. J Submicrosc Cytol Pathol, 23:
457-464.
Bailey J, Knauf V, Mueller W, & Hobson W (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
rhesus monkeys; enhanced toxicity. Environ Res, 21: 190-196.
Ballschmiter K, & Wittlinger R (1991) Interhemisphere exchange of
hexachlorocyclohexanes, hexachlorobenzene, polychlorobiphenyls, and
1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane in the lower
troposphere. Environ Sci Technol, 25: 1103-1111.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LSF
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39:
263-274.
Barquet A, Morgade C, & Pfaffenberger CD (1981) Determination of
organochlorine pesticides and metabolites in drinking water, human
blood serum, and adipose tissue. J Toxicol Environ Health, 7:
469-479.
Bates MN, Hannah DJ, Buckland SJ, Taucher JA, & Van Maanen T (1994)
Chlorinated organic contaminants in breast milk of New Zealand women.
Environ Health Perspect, 102 (Suppl 1): 211-217.
Bauer I, Weigelt S, & Ernst W (1989) Biotransformation of
hexachlorobenzene in the blue mussel ( Mytilus edulis). Chemosphere,
19 (10/11): 1701-1707.
Beck J & Hansen KE (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in soil.
Pestic Sci, 5: 41-48.
Béland P, Deguise S, & Plante R (1991) Toxicology and pathology of
St. Lawrence marine mammals - Final report. Rimouski, Québec, Canada,
Wildlife Toxicology Fund Research Grant, St. Lawrence National
Institute of Ecotoxicology, 95 pp.
Belfroid A, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, & Hermens
J (1993) The toxicokinetic behaviour of chlorobenzenes in earthworms
( Eisenia andrei): Experiments in water. Ecotoxicol Environ Saf,
25: 154-165.
Belfroid A, Meiling J, Sijm D, Hermens J, Seinen W, & Van Gestel K
(1994a) Uptake of hydrophobic halogenated aromatic hydrocarbons from
food by earthworms ( Eisenia andrei). Arch Environ Contam Toxicol,
27: 260-265.
Belfroid A, Sikkenk M, Seinen W, Van Gestel K, & Hermens J (1994b) The
toxicokinetic behavior of chlorobenzenes in earthworm ( Eisenia
andrei): Experiments in soil. Environ Toxicol Chem, 13: 93-99.
Beretta M & Dick T (1994) Organochlorine compounds in human milk,
Porto Alegre, Brazil. Bull Environ Contam Toxicol, 53: 357-360.
Biddleman TF, Billings WN, & Foreman WT (1986) Vapor-particle
partitioning of semivolatile organic compounds: Estimates from field
collection. Environ Sci Technol, 20: 1038-1043.
Bidleman TF, Wideqvist U, Jansson B, & Soderlund R (1987)
Organochlorine pesticides and polychlorinated biphenyls in the
atmosphere of southern Sweden. Atmos Environ, 21: 641-654.
Bidleman TF, Walla MD, Roura R, Carr E, & Schmidt S (1993)
Organochlorine pesticides in the atmosphere of the Southern Ocean and
Antarctica, January-March, 1990. Mar Pollut Bull, 26(5): 258-262.
Billings WN & Bidleman TF (1980) Field comparison of polyurethane foam
and Teax-GC resin for high-volume air sampling of chlorinated
hydrocarbons. Environ Sci Technol, 14(6):679-683.
Bishop CA, Lean DRS, Brooks RJ, Carey JH, & Ng P (1995) Chlorinated
hydrocarbons in early life stages of the common snapping turtle
( Chelydra serpentina serpentina) from a coastal wetland on Lake
Ontario, Canada. Environ Toxicol Chem, 14(3): 421-426.
Bjerk JE & Brevik EM (1980) Organochlorine compounds in aquatic
environments. Arch Environ Contam Toxicol, 9: 743-750.
Bleavins MR, Breslin WJ, Aulerich RJ, & Ringer RK (1982) Excretion and
placental and mammary transfer of hexachlorobenzene in the European
ferret ( Mustela putorius furo). J Toxicol Environ Health, 14:
929-940.
Bleavins MR, Bursian SJ, Brewster JS, & Aulerich RJ (1984a) Effects of
dietary hexachlorobenzene exposure on regional brain biogenic amine
concentrations in mink and European ferrets. J Toxicol Environ Health,
14: 363-377.
Bleavins MR, Aulerich RJ, & Ringer RK (1984b) Effects of chronic
dietary hexachlorobenzene exposure on the reproductive performance and
survivability of mink and European ferrets. Arch Environ Contam
Toxicol, 13: 357-365.
Boersma DC, Ellenton JL, & Yagminas A (1986) Investigation of the
hepatic mixed-function oxidase system in herring gull embryos in
relation to environmental contaminants. Environ Toxicol Chem, 5:
309-318.
Boese BL, Winsor M, Lee H II, Specht DT, & Rukavina LC (1990)
Depuration kinetics of hexachlorobenzene in the clam, Macoma nasuta.
Comp Biochem Physiol, C996: 327-331.
Bordet F, Mallet J, Maurice L, Borrel S, & Venant A (1993)
Organochlorine pesticide and PCB congener content of French human
milk. Bull Environ Contam Toxicol, 50: 425-432.
Bourbonniere RA, Van Sickle BL, & Mayer T (1986) The Great Lakes
sediment bank-I (including catalogs of lakes Huron and Ontario
samples). Burlington, Ontario, Environment Canada, National Water
Research Institute (NWRI Contribution No. 86-151).
Bouthillier L, Greselin E, Brodeur J, Viau C, & Charbonneau M (1991)
Male rat specific nephrotoxicity resulting from subchronic
administration of hexachlorobenzene. Toxicol Appl Pharmacol, 110:
315-326.
Braune BM & Norstrom RJ (1989) Dynamics of organochlorine compounds in
herring gulls: III. Tissue distribution and bioaccumulation in Lake
Ontario gulls. Environ Toxicol Chem, 8: 957-968.
Breslin WJ, Bleavins MR, & Ringer RK (1983) Distribution and excretion
of hexachlorobenzene in bobwhite. J Toxicol Environ Health, 11:
885-896.
Brooks GW & Hunt GE (1984) Source assessment for hexachlorobenzene.
Research Triangle Park, North Carolina, Radian Corporation (Prepared
for the US Environmental Protection Agency, Research Triangle Park)
(EPA 68-02-3818).
Bro-Rasmussen F (1986) Hexachlorobenzene: An ecotoxicological profile
of an organochlorine compound. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 231-239 (IARC
Scientific Publications No. 77).
Brown EA, Biddle K, & Spaulding JE (1986) Residue levels of
hexachlorobenzene in meat and poultry in the food supply of the U.S.A.
In: Morris CR & Cabral JRPed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 99-108 (IARC Scientific Publications No. 77).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17: 2363-1380.
Brunn H & Manz D (1982) Contamination of native fish stock by
hexachlorobenzene and polychlorinated biphenyl residues. Bull Environ
Contam Toxicol, 28: 599-604.
Brusick DJ (1986) Genotoxicity of hexachlorobenzene and other
chlorinated benzenes. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 393-397 (IARC
Scientific Publications No. 77).
BUA (German Chemical Society-Advisory Committee on Existing Chemicals
of Environmental Relevance) (1994) Hexachlorobenzene. Stuttgart, S.
Hirzel Wissenschaftliche Verlagsgesellschaft, 257 pp (BUA Report 119).
Buhler DR & Carpenter HM (1986) Japanese quail as a model for the
study of hexachlorobenzene-induced porphyria. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 477-480 (IARC
Scientific Publications No. 77).
Burns JE & Miller FM (1975) Hexachlorobenzene contamination: its
effects in a Louisiana population. Arch Environ Health, 30: 44-48.
Burns JE, Miller FM, Gomes ED, & Albert RA (1974) Hexachlorobenzene
exposure from contaminated DCPA in vegetable spraymen. Arch Environ
Health, 29: 192-194.
Burton MA & Bennett BG (1987) Exposure of man to environmental
hexachlorobenzene (HCB): an exposure commitment assessment. Sci Total
Environ, 66: 137-146.
Bush B, Snow J, Connor S, & Koblintz R (1985) Polychlorinated biphenyl
congeners (PCBs), p,pœ-DDe and hexachlorobenzene in human milk in
three areas of upstate New York. Arch Environ Contam Toxicol, 14:
443-450.
Cabral JRP & Shubik P (1986) Carcinogenic activity of
hexachlorobenzene in mice and hamsters. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 411-416 (IARC
Scientific Publications No. 77).
Cabral JRP, Shubik P, Mollner T, & Raitano F (1977) Carcinogenic
activity of hexachlorobenzene in hamsters. Nature (Lond), 269:
510-511.
Cabral JRP, Mollner T, Raitano F, & Shubik P (1979) Carcinogenesis of
hexachlorobenzene in mice. Int J Cancer, 23: 47-51.
Calamari D, Galassi S, Setti F, & Vighi M (1983) Toxicity of selected
chlorobenzenes to aquatic organisms. Chemosphere, 12: 253-262.
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA priority pollutants and related chemicals
in freshwater organisms. Center for Lake Superior Environmental
Studies, University of Wisconsin-Superior, Environmental Research
Laboratory, Duluth, Minnesota, U.S. Environmental Protection Agency
(EPA 600/3-83-095).
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed R, Jennings P,
Durfee R, Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979)
Water-related environmental fate of 129 priority pollutants, Volume
II. Washington, DC, US Environmental Protection Agency, Office of
Water Planning and Standards/Office of Water and Waste Management (EPA
440/4-79-029b).
Cam C (1960) Une nouvelle dermatose épidémique des enfants. Ann
Dermatol, 87: 393-397.
Cam C & Nigogosyan G (1963) Acquired toxic porphyria cutanea tarda due
to hexachlorobenzene. J Am Med Assoc, 183: 90-93.
Camps M, Planas J, Gómez-Catalán J, Sabroso M, To-Figueras J, &
Corbella J (1989) Organochlorine residues in human adipose tissue in
Spain: study of an agrarian area. Bull Environ Contam Toxicol, 42:
195-201.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1979) Pesticide
residue levels in soils and crops from 37 states, 1972 - National
Soils Monitoring Program (IV). Pest. Monit. J, 12: 209-229.
Carey AE, Dixon TE, & Yang HSC (1985) Environmental exposure to
hexachlorobenzene in the United States. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
Carlson AR & Kosian PA (1987) Toxicity of chlorinated benzenes to
fathead minnows ( Pimephales promelas). Arch Environ Contam
Toxicol, 16: 129-135.
Carthew P & Smith AG (1994) Pathological mechanisms of hepatic tumour
formation in rats exposed chronically to dietary hexachlorobenzene. J
Appl Toxicol, 14: 447-452.
Carthew P, Edwards RE, & Smith AG (1990) Immunotoxic effects of
hexachlorobenzene on the pathogenesis of systemic, pneumonic and
hepatic virus infections in the mouse. Hum Exp Toxicol, 8: 403-411.
Clark TP, Norstrom RJ, Fox GA, & Won HT (1987) Dynamics of
organochlorine compounds in herring gulls ( Larus argentatus): II. A
two-compartment model and data for ten compounds. Environ Toxicol
Chem, 6: 547-559.
Conchello P, Herrera A, Ariño A, Lazaro R, & Pérez-Arquillué C (1993)
Effect of grilling, roasting, and cooking on the natural
hexachlorobenzene content of ovine meat. Bull Environ Contam Toxicol,
50: 828-833.
Conde C, Naluenda C, & Arrabal C (1993) Hexachlorobenzene (HCB) in
human milk in Spain from 1984 to 1991. Bull Environ Contam Toxicol,
51: 827-831.
Courtney KD (1979) Hexachlorobenzene (HCB): a review. Environ Res,
20: 225-266.
Courtney KD & Andrews JE (1979) Mobilization of hexachlorobenzene
(HCB) during gestation. Toxicol Lett, 3: 357-361.
Courtney KD & Andrews JE (1985) Neonatal and maternal body burdens of
hexachlorobenzene (HCB) in mice: gestational exposure and lactational
transfer. Fundam Appl Toxicol, 5: 265-277.
Courtney KD, Copeland MF, & Robbins A (1976) The effects of
pentachloronitrobenzene, hexachlorobenzene, and related compounds on
fetal development. Toxicol Appl Pharmacol, 35: 239-256.
Courtney KD, Andrews JE, & Svendsgaard DJ (1979) Hexachlorobenzene
(HCB) deposition in maternal and fetal tissues of rat and mouse: I.
Chemical quantification of HCB in tissues. Environ Res, 19: 1-13.
Cripps DJ, Peters HA, Gocmen A, & Dogramaci I (1984) Porphyria turcica
due to hexachlorobenzene: a 20 to 30 year follow-up study on 204
patients. Br J Dermatol, 111: 413-422.
Cross JN, Hardy JT, Hose JE, Hershelman GP, Antrim LD, Gossett RW, &
Crecelius EA (1987) Contaminant concentrations and toxicity of sea-
surface microlayer near Los Angeles, California. Mar Environ Res,
23: 307-323.
Crump K & Howe N (1982) Global '82: a computer program to extrapolate
quantal animal toxicity data to low doses (Contract #41USC252C3).
Washington, DC, US Occupational Safety and Health Administration,
Office of Carcinogen Standards.
Currier MF, McClimans CD, & Barna-Lloyd G (1980) Hexachlorobenzene
blood levels and the health status of men employed in the manufacture
of chlorinated solvents. J Toxicol Environ Health, 6: 367-377.
D'Amour M & Charbonneau M (1992) Sex-related differences in hepatic
glutathione conjugation of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 112: 229-234.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17: 263-276.
Davis BD & Morgan RC (1986) Hexachlorobenzene in hazardous waste
sites. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 23-30 (IARC Scientific Publications No. 77).
Debets FMH & Strik JJTWA (1979) An approach to elucidate the mechanism
of hexachlorobenzene-induced hepatic porphyria, as a model for the
hepatotoxicity of polyhalogenated aromatic compounds (PHA's). In:
Strik JJTWA & Koeman JH ed. Chemical porphyria in man. Amsterdam,
Oxford, New York, Elsevier/North Holland Biomedical Press, pp 181-208.
Deleon IR, Maberry MA, Overton EB, Raschke CK, Remele PC, Steele, CF,
Warren VL, & Laseter JL (1980) Rapid gas chromatographic method for
the determination of volatile and semivolatile organochlorine
compounds in soil and chemical waste disposal site samples. J
Chromatogr Sci, 18: 85-88.
Dellinger B, Taylor PH, & Tirey DA (1991) Minimization and control of
hazardous combustion by-products. Cincinnati, Ohio, US Environmental
Protection Agency, Risk Reduction Laboratory (EPA 600/S2-90/039).
De Matteis F, Prior BE, & Rimington C (1961) Nervous and biochemical
disturbances following hexachlorobenzene intoxication. Nature (Lond),
191(4786): 363-366.
Den Besten C, Bennik MM, Van Iersel M, Peters MA, Teunis C, & Van
Bladeren PJ (1994) Comparison of the urinary metabolite profiles of
hexachlorobenzene and pentachlorobenzene in the rat. Chem-Biol
Interact, 90: 121-137.
Den Desten C, Bennik MHJ, Bruggeman I, Schielen P, Kuper F, Brouwer A,
Koeman JH, Vos JG, & Van Bladeren PJ (1993) The role of oxidative
metabolism in hexachlorobenzene-induced porphyria and thyroid hormone
homeostasis: a comparison with pentachlorobenzene in a 13-week feeding
study. Toxicol Appl Pharmacol, 119: 181-194.
Denomme MA, Leece B, Gyorkos J, Homonko K, & Safe S (1983)
Polychlorinated benzene and phenol congeners as inducers of rat
hepatic drug-metabolizing enzymes in immature male Wistar rats. Can J
Physiol Pharmacol, 61: 1063-1070.
Den Tonkelaar EM & Van Esch GJ (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
Den Tonkelaar EM, Verschueren HG, Bankovska J, De Vries T, Kroes R, &
Van Esch G (1978) Hexachlorobenzene toxicity in pigs. Toxicol Appl
Pharmacol, 43: 137-145.
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Dewailly E, Nantel A, Laliberté C, Weber J-P, Gingras S, & Meyer F
(1991) Organochlorines in the breast milk in Québec: a provincial
survey. Poster presentation at the Conference on Measuring,
Understanding, and Predicting Exposures in the 21st Century, Atlanta,
Georgia, 18-22 November 1991.
Ding W-H, Aldous KM, Briggs RG, Valente H, Hilker DR, Connor S, &
Eadon GA (1992) Application of multivariate statistical analysis to
evaluate local sources of chlorobenzene congeners in soil samples.
Chemosphere, 25(5): 675-690.
Dogramaci I (1964) Porphyrias and porphyrin metabolism with special
reference to porphyria in childhood. Adv Pediatr, 13: 11-63.
Dogramaci I, Wray JD, Ergene T, Sezer V, & Müftü Y (1962) Porphyria
turcica: a survey of 592 cases of cutaneous porphyria seen in
southeastern Turkey. Turk J Pediatr, 4: 138-148.
Eisenberg M & Topping JJ (1984) Organochlorine residues in shellfish
from Maryland waters. J Environ Sci Health, B19: 673-688.
Eisenreich SJ & Strachan WMJ (1992) Estimating atmospheric deposition
of toxic substances to the Great Lakes - An update: Proceedings of a
workshop, Burlington, 31 January-2 February 1992. Burlington, Ontario,
Canada Centre for Inland Waters.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCBs in
waste, sediment, and fish from the Mediterranean Sea. Bull Environ
Contam Toxicol, 34: 216-227.
Elissalde MH & Clark DE (1979) Testosterone metabolism by
hexachlorobenzene-induced hepatic microsomal enzymes. Am J Vet Res,
40: 1762-1766.
Elkin BT & Bethke RW (1995) Environmental contaminants in caribou in
the Northwest Territories, Canada. Sci Total Environ, 160/161:
307-321.
Enriquez De Salamanca R, Lopez-Miras A, Munoz JJ, To-Figueras J, &
Conde C (1990) Is hexachlorobenzene human overload related to
porphyria cutanea tarda? A speculative hypothesis. Med Hypotheses,
33: 69-71.
Environment Canada (1989) Atlantic region federal-provincial toxic
chemical survey of municipal drinking water sources 1985-1988 -
Interpretative Report. Moncton, New Brunswick, Environment Canada,
Conservation and Protection, Water Quality Branch, Inland Waters
Directorate.
Environment Canada (1992) Detroit incinerator monitoring program: Data
report No. 6. Ottawa, Ontario, Environment Canada, Conservation and
Protection, River Road Environmental Technology Centre (Report No.
PMD-92-1).
Environment Canada/Department of Fisheries & Oceans/Health & Welfare
Canada (1991) Toxic chemicals in the Great Lakes and associated
effects - Volume 1: Contaminant levels and trends. Ottawa, Ontario,
Minister of Supply and Services.
Environment Canada/Ontario Ministry of the Environment (1986) St.
Clair River pollution investigation (Sarnia area). Toronto, Canada-
Ontario Agreement Respecting Great Lakes Water Quality.
Ernst W (1986) Hexachlorobenzene in the marine environment:
distribution, fate and ecotoxicological aspects. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 211-222 (IARC Scientific Publications No. 77).
Erturk E, Lambrecht RW, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982) Leukemogenicity of hexachlorobenzene (HCB) in Swiss
mice (SM) after subchronic feeding. Proc Am Assoc Cancer Res, 23: 55
(Abstract).
Ertürk E, Lambrecht RW, Peters HA, Cripps DJ, Gocmen A, Morris CR, &
Bryan GT (1986) Oncogenicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 417-423 (IARC Scientific Publications No. 77).
Falandysz J & Szefer P (1982) Chlorinated hydrocarbons in diving ducks
wintering in Gdansk Bay, Baltic Sea. Sci Total Environ, 24: 119-127.
Falandysz J, Kannan K, Tanabe S, & Tatukawa R (1993) Persistent
organochlorine residues in canned cod-livers of the southern Baltic
origin. Bull Environ Contam Toxicol, 50: 929-934.
FAO/WHO (1975) Report of the Joint Meeting of FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 37 pp (WHO
Technical Report Series 574).
FAO/WHO (1978) Pesticide residues in food - 1978. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and
Environment and the WHO Expert Group on Pesticide Residues. Rome, Food
and Agriculture Organization of the United Nations, pp 19-20 (FAO
Plant Production and Protection Paper 15).
Ferrer A, Bona MA, Castellano M, To-Figueras J, & Brunet M (1992)
Organochlorine residues in human adipose tissue of the population of
Zaragoza (Spain). Bull Environ Contam Toxicol, 48: 561-566.
Fingler S, Drevenkar V, Tkalœeviœ B, & œmit Z (1992) Levels of
polychlorinated biphenyls, organochlorine pesticides, and
chlorophenols in the Kupa River water and in drinking waters from
different areas in Croatia. Bull Environ Contam Toxicol, 49:
805-812.
Foley RE & Batcheller GR (1988) Organochlorine contaminants in common
goldeneye wintering on the Niagara River. J Wildl Manage, 52:
441-445.
Foster WG, McMahon A, Jarrell JF, & Villeneuve DC (1992a)
Hexachlorobenzene (HCB) suppresses circulating progesterone
concentrations during the luteal phase in the cynomolgus monkey. J
Appl Toxicol, 12: 13-17.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1992b) Ovarian
toxicity of hexachlorobenzene (HCB) in the superovulated female rat. J
Biochem Toxicol, 7: 1-4.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1993) Body
distribution and endocrine toxicity of hexachlorobenzene (HCB) in the
female rat. J Appl Toxicol, 13: 79-83.
Foster WG, Mertineit C, Yagminas A, McMahon A, & Lecavalier P (1995)
The effects of hexachlorobenzene on circulating levels of adrenal
steroids in the ovariectomized rat. J Biochem Toxicol, 10(3):
129-135.
Fox ME, Carey JH, & Olivier BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the Western Basin
of Lake Ontario. J Great Lakes Res, 9: 287-294.
Franchi E & Focardi S (1991) Polychlorinated biphenyl congeners,
hexachlorobenzene and DDT in human milk in central Italy. Sci Total
Environ, 102: 223-228.
Frank R & Ripley BD (1990) Food residues from pesticides and
environmental pollutants in Ontario. In: Nriagu JO & Simmons MS ed.
Food contamination from environmental sources. New York, Wiley-
Interscience, pp 473-524.
Frank R, Braun HE, & Fleming G (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969 to 1981. J Food Prot, 46:
893-900.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985a)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario - 1983. J Food Prot, 48:
499-504.
Frank R, Rasper J, Braun HE, & Ashton G (1985b) Disappearance of
organochlorine residues from abdominal and egg fats of chickens,
Ontario, Canada, 1969-1982. J Assoc Off Anal Chem, 68: 124-129.
Frank R, Rasper J, Smout MS, & Braun HE (1988) Organochlorine residues
in adipose tissues, blood and milk from Ontario residents, 1976-1985.
Can J Public Health, 79: 150-158.
Fujita M & Morikawa K (1992) Regional differences in dietary intake of
environmental pollutants in Japan. J Expo Anal Environ Epidemiol,
2(2):177-193.
Fürst P, Fürst CHR, & Wilmers K (1992) Survey of dairy products for
PCDD's, PCDF's, PCB's and HCB. Chemosphere, 25(7-10): 1039-1048.
Fürst P, Fürst C, & Wilmers K (1994) Human milk as a bioindicator for
body burden of PCDDs, PCDFs, organochlorine pesticides, and PCBs.
Environ Health Perspect, 102 (Suppl 1): 187-193.
Galassi S & Provini A (1981) Chlorinated pesticides and PCB contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in adult total diet
samples, October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Geike F & Parasher CD (1976a) Effect of hexachlorobenzene on some
growth parameters of Chlorella pyrenoidosa. Bull Environ Contam
Toxicol, 15: 670-677.
Geike F & Parasher CD (1976b) Effect of hexachlorobenzene (HCB) on
growth of Tetrahymena pyriformis. Bull Environ Contam Toxicol, 16:
347-354.
Gilbertson M (1979) Hexachlorobenzene (HCB) in Canada. Ottawa,
Ontario, Environment Canada, Environmental Protection Service
(Unpublished report).
Gobas FAPC, Bedard DC, Ciborowski JJH, & Haffner GD (1989)
Bioaccumulation of chlorinated hydrocarbons by mayfly ( Hexagenia
limbata) in Lake St. Clair. J Great Lakes Res, 15: 581-588.
Gobas FAPC, McNeil EJ, Lovett-Doust L, & Haffner GD (1991)
Bioconcentration of chlorinated aromatic hydrocarbons in aquatic
macrophytes. Environ Sci Technol, 25: 924-929.
Gocmen A, Peters HA, Cripps DJ, Bryan GT, & Morris CR (1989)
Hexachlorobenzene episode in Turkey. Biomed Environ Sci, 2: 36-43.
Goerz G, Bolsen K, Kalofoutis A, & Tsambaos D (1994) Influence of oral
isotrentoin on hepatic and cutaneous P-450-dependent isozyme
activities. Arch Dermatol Res, 286: 104-106.
Goldey ES & Taylor DH (1992) Developmental neurotoxicity following
premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol
Teratol, 14: 15-21.
Goldey ES, Fisher JW, & Taylor DH (1990) Maternal transfer of
hexachlorobenzene in the rat. Neurotoxicol Teratol, 12: 562-563.
Goldstein JA, Friesen M, Scotti TM, Hickman P, Hass JR, & Bergman H
(1978) Assessment of the contribution of chlorinated dibenzo-p-dioxins
and dibenzofurans to hexachlorobenzene-induced toxicity, porphyria,
changes in mixed function oxygenases, and histopathological changes.
Toxicol Appl Pharmacol, 46: 633-649.
Gómez-Catalán J, Planas J, To-Figueras J, Camps M, & Corbella J (1993)
Organochlorine pesticide residues in the population of Catalonia
(Spain). Bull Environ Contam Toxicol, 51: 160-164.
Gómez-Catalán J, Lezaun M, To-Figueras J, & Corbella J (1995)
Organochlorine residues in the adipose tissue of the population of
Navarra (Spain). Bull Environ Contam Toxicol, 54: 534-540.
Gopalaswamy UV & Aiyar AS (1986) Biotransformation and toxicity of
lindane and its metabolite hexachlorobenzene in mammals. In: Morris CR
& Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 267-276 (IARC Scientific Publications No. 77).
Gopalaswamy UV & Nair CKK (1992) DNA binding and mutagenicity of
lindane and its metabolites. Bull Environ Contam Toxicol, 49:
300-305.
Gorski T, Gorska E, Gorecka D, & Sikora M (1986) Hexachlorobenzene is
non-genotoxic in short-term tests. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 399-401 (IARC
Scientific Publications No. 77).
Government of Canada (1993) Canadian Environmental Protection Act
priority substances list assessment report: Hexachlorobenzene. Ottawa,
Ontario, Environment Canada, Health and Welfare Canada, 52 pp.
Gralla EJ, Fleischman RW, Luthra YK, Hagopian M, Baker JR, Esber H, &
Marcus W (1977) Toxic effects of hexachlorobenzene after daily
administration to beagle dogs for one year. Toxicol Appl Pharmacol,
40: 227-239.
Grant DL, Iverson F, Hatina GV, & Villeneuve DC (1974) Effects of
hexachlorobenzene on liver porphyrin levels and microsomal enzymes in
the rat. Environ Physiol Biochem, 4: 159-165.
Grant DL, Shields JB, & Villeneuve DC (1975) Chemical (HCB) porphyria:
effect of removal of sex organs in the rat. Bull Environ Contam
Toxicol, 14: 422-425.
Grant DL, Phillips WEJ, & Hatina GV (1977) Effect of hexachlorobenzene
on reproduction in the rat. Arch Environ Contam Toxicol, 5: 207-216.
Green JA, Francis JE, Wolf CR, Manson MM, & Smith AG (1989) Sexual
dimorphism of cytochrome P-450 induction by hexachlorobenzene in rats.
Biochem Soc Trans, 17: 1016-1017.
Greve PA & Van Zoonen P (1990) Organochlorine pesticides and PCB's in
tissues from Dutch citizens (1968-1986). Int J Environ Anal Chem,
38: 265-277.
Griffin RA & Chou SFJ (1981) Movement of PCB's and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Grimalt JO, Gomez-Belinchon JI, Llop R, & Albaiges J (1988) Water-
phase distribution of hexachlorobenzene in a deltaic environment (Ebre
Delta, Western Mediterranean). Chemosphere, 17: 1893-1903.
Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, Anto JM,
& Albaiges J (1994) Risk excess of soft-tissue and thyroid cancers in
a community exposed to airborne organochlorinated compound mixtures
with a high hexachlorobenzene content. Int J Cancer, 56: 200-203.
Guerzoni ME, Del Cupolo L, & Ponti I (1976) [Mutagenic activity of
pesticides.] Riv Sci Tech Alim Nutr Um, 6: 161-165 (in Italian, with
English summary).
Hagenmaier H, She J, & Lindig C (1992) Persistence of polychlorinated
dibenzo-p-dioxins and polychlorinated dibenzofurans in contaminated
soil at Maulach and Rastatt in southwest Germany. Chemosphere,
25(7/10): 1449-1456.
Hahn ME, Gasiewicz TA, Linko P, & Goldstein JA (1988) The role of the
Ah locus in hexachlorobenzene-induced porphyria. Biochem J, 254:
245-254.
Hahn ME, Goldstein JA, Linko P, & Gasiewicz TA (1989) Interaction of
hexachlorobenzene with the receptor for 2,3,7,8-tetrachlorodibenzo-p-
dioxin in vitro and in vivo. Arch Biochem Biophys, 270(1):
344-355.
Hansen LG, Dorn SB, Sundlof SM, & Vogel RS (1978) Toxicity,
accumulation and depletion of hexachlorobenzene in laying chickens. J
Agric Food Chem, 26: 1369-1374.
Hansen LG, Teske RH, Sundlof SM, & Simon J (1979) Hexachlorobenzene
and feline reproduction: effects of ground pork contaminated by
dietary exposure or spiked with purified HCB. Vet Hum Toxicol, 21:
248-253.
Hansen PD, Von Westernhagen H, & Rosenthal H (1985) Chlorinated
hydrocarbons and hatching success in Baltic herring spring spawners.
Mar Environ Res, 15: 59-76.
Hargrave BT, Vass WP, Erickson PE, & Fowler BR (1989) Distribution of
chlorinated hydrocarbon pesticides and PCBs in the Arctic Ocean.
Dartmouth, NS, Department of Fisheries and Oceans, 224 pp (Canadian
Technical Report of Fisheries and Aquatic Sciences No. 1644).
Harper DJ, Ridgeway IM, & Leatherland TM (1992) Concentrations of
hexachlorobenzene, trichlorobenzenes and chloroform in the waters of
the Forth Estuary, Scotland. Mar Pollut Bull, 24(5): 244-249.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Health Canada (1994) Canadian Environmental Protection Act: Human
health risk assessment for priority substances. Ottawa, Ontario,
Minister of Supply and Services.
Heidmann WA (1986) Hexachlorobenzene residues in selected species of
land and sea birds in northern Germany. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 223-228 (IARC
Scientific Publications No. 77).
Heikes DL (1980) Residues of pentachloronitrobenzene and related
compounds in peanut butter. Bull Environ Contam Toxicol, 24:
338-343.
Heldaas SS, Langard S, & Andersen A (1989) Incidence of cancer in a
cohort of magnesium production workers. Br J Ind Med, 46: 617-623.
Hendriks AJ, Ma W-C, Brouns JJ, De Ruiter-Dijkman EM, & Gast R (1995)
Modelling and monitoring organochlorine and heavy metal accumulation
in soils, earthworms, and shrews in Rhine-Delta floodplains. Arch
Environ Contam Toxicol, 29: 115-127.
Herren-Freund SL & Pereira MA (1986) Carcinogenicity of by-products of
disinfection in mouse and rat liver. Environ Health Perspect, 69:
59-65.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of Interior, Fish and Wildlife Service (Special Scientific
Report œ Wildlife No. 191).
Hippelein M, Kaupp H, Dörr G, & McLachlan MS (1993) Testing of a
sampling system and analytical method for determination of
semivolatile organic compounds in ambient air. Chemosphere, 26:
2255-2263.
Hoff RM, Muir DCG, & Grift NP (1992) Annual cycle of polychlorinated
biphenyls and organohalogen pesticides in air in southern Ontario: 1.
Air concentration data. Environ Sci Technol, 26(2): 266-275.
Holoubek I, œáslavskœ J, Vancura R, Kocan A, Chovancová J, Petrik J,
Drobna B, Cudlin P, & Triska J (1994) Project Tocoen: The fate of
selected organic pollutants in the environment - Part XXIV. The
content of PCBs and PCDDs/Fs in high-mountain soils. Toxicol Environ
Chem, 45: 189-197.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
In: Taup H ed. Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers.
IARC (1979) Hexachlorobenzene. In: Some halogenated hydrocarbons.
Lyon, International Agency for Research on Cancer, pp 155-178 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Human, Volume 20).
IARC (1987) Hexachlorobenzene. In: Overall evaluations of
carcinogenicity: An updating of IARC monographs, volumes 1 to 42.
Lyon, International Agenci for Research on Cancer, pp 219-220 (IARC
Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Iatropoulos MJ, Hobson W, Knauf V, & Adams HP (1976) Morphological
effects of hexachlorobenzene toxicity in female rhesus monkeys.
Toxicol Appl Pharmacol, 37: 433-444.
IJC (International Joint Commission) (1989) In: Rathke DR & McRae G
ed. Great Lakes surveillance: 1987 report on Great Lakes water
quality. Report to the International Joint Commission, Windsor,
Ontario.
Ikemoto Y, Motoba K, Suzuki T, & Uchida M (1992) Quantitative
structure-activity relationships of nonspecific and specific toxicants
in several organism species. Environ Toxicol Chem, 11: 931-939.
Ingebritsen K (1986) Comparative studies on the distribution and
excretion of 14C-hexachlorobenzene by whole-body autoradiography. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 277-285 (IARC Scientific Publications No. 77).
Ingebritsen K, Skaare JU, Nafstad I, & Forde M (1981) Studies on the
biliary excretion and metabolites of hexachlorobenzene in the rat.
Xenobiotica, 11: 795-800.
Innes D, Muncaster B, Lazar R, Haffner D, & Hebert P (1988) Monitoring
of organic contaminants using freshwater mussels. Proceedings of the
Environmental Research Technology Transfer Conference, 30 November-1
December 1987 - Part B: Water quality research. Toronto, Ontario,
Environment Ontario.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: The derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
IRPTC (1993) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M Jr (1988) Data book of chromosomal aberration test
in vitro. Amsterdam, New York, Oxford, Elsevier Science Publishers.
Jacoff FS, Scarberry R, & Rosa D (1986) Source assessment of
hexachlorobenzene from the organic chemical manufacturing industry.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 31-37 (IARC Scientific Publications No. 77). 31-37.
Jaffe R & Hites RA (1986) Anthropogenic, polyhalogenated, organic
compounds in non-migratory fish from the Niagara River area and
tributaries to Lake Ontario. J Great Lakes Res, 12: 63-71.
Jansson B & Bergman A (1978) Sulfur-containing derivatives of
hexachlorobenzene (HCB) - metabolites in the rat. Chemosphere, 3:
257-268.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
Jarrell JF, McMahon A, Villeneuve D, Franklin C, Singh A, Valli VE, &
Bartlett S (1993a) Hexachlorobenzene toxicity in the monkey primordial
germ cell without induced porphyria. Reprod Toxicol, 7: 41-47.
Jarrell JF, Villeneuve D, Franklin C, Bartlett S, Wrixon W, Kohut J, &
Zouves CG (1993b) Contamination of human ovarian follicular fluid and
serum by chlorinated organic compounds in three Canadian cities. Can
Med Assoc J, 148(8): 1321-1327.
Jemaa Z, Sabbah S, Driss MR, & Bouguerra ML (1986) Hexachlorobenzene
in Tunisian mothers' milk, cord blood and foodstuffs. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 139-142 (IARC Scientific Publications No. 77).
Johansen HR, Becher G, Polder A, & Skaare JU (1994) Congener-specific
determination of polychlorinated biphenyls and organochlorine
pesticides in human milk from Norwegian mothers living in Oslo. J
Toxicol Environ Health, 42: 157-171.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites in Lake Ontario. J Great Lakes Res, 9:
183-189.
Kan CA & Tuinstra LGM (1976) Accumulation and excretion of certain
organochloride insecticides in broiler breeder hens. J Agric Food
Chem, 24: 775-778.
Kan-Do Office & Pesticides Team (1995) Accumulated pesticide and
industrial chemical findings from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kannan K, Tanabe S, Ramesh A, Subramanian A, & Tatsukawa R (1992a)
Persistent organochlorine residues in foodstuffs from India and their
implications on human dietary exposure. J Agric Food Chem, 40:
518-524.
Kannan K, Tanabe S, Hoang Trong Quynh, Nguyen Duc Hue, & Tatsukawa R
(1992b) Residue pattern and dietary intake of persistent
organochlorine compounds in foodstuffs from Vietnam. Arch Environ
Contam Toxicol, 22: 367-374.
Kannan K, Falandysz J, Tanabe S, & Tatsukawa R (1993) Persistent
organochlorines in Harbour Porpoises from Puck Bay, Poland. Mar Pollut
Bull, 26(3): 162-165.
Kannan K, Tanabe S, Williams RJ, & Tatsukawa R (1994) Persistant
organochlorine residues in foodstuffs from Australia, Papua New Guinea
and the Solomon Islands: contamination levels and human dietary
exposure. Sci Total Environ, 153: 29-49.
Kashimoto T, Takayama K, Mimura M, Miyata H, Murakami Y, & Matsumoto H
(1989) PCDDs, PCDFs, PCBs, coplanar PCBs and organochlorinated
pesticides in human adipose tissue in Japan. Chemosphere, 19(1/6):
921-926.
Kasokat T, Nagel R, & Urich K (1989) Metabolism of chlorobenzene and
hexachlorobenzene by the zebra fish, Brachydanio rerio. Bull Environ
Contam Toxicol, 42: 254-261.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St. Clair and Detroit Rivers using introduced
clams, Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kelly AG & Campbell A (1994) Organochlorine contaminants in liver of
cod ( Gadus morhua) and muscle of herring ( Clupea harengus) from
Scottish waters. Mar Pollut Bull, 28(2): 103-108.
Kemper FH (1993) Human organ specimen banking-15 years of experience.
Sci Total Environ, 139-140: 13-25.
Kennedy SW & Wigfield DC (1990) Dose-response relationships in
hexachlorobenzene-induced porphyria. Biochem Pharmacol, 40:
1381-1388.
Kennedy SW, Wigfield DC, & Fox GA (1986) The delay in polyhalogenated
aromatic hydrocarbon-induced porphyria: mechanistic reality or
methodological artefact? Toxicol Lett, 31: 235-241.
Kessabi M, Elhraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the Mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kessabi M, Abdennebi E, Laraje R, & Lhafi A (1990) Contamination of
eggs, poultry liver and bovine liver and kidney by chlorinated
pesticides in Morocco. Sci Total Environ, 90: 283-287.
Khera KS (1974) Teratogenicity and dominant lethal studies on
hexachlorobenzene in rats. Food Cosmet Toxicol, 12: 471-477.
Kilikidis SD, Kamarianos AP, & Karamanlis XN (1992) Seasonal
fluctuations of organochlorine compounds in the water of the Strimon
River (N. Greece). Bull Environ Contam Toxicol, 49: 375-380.
Kilzer L, Scheunert I, Geyer H, Klein W, & Korte F (1979) Laboratory
screening of the volatilization rates of organic chemicals from water
and soil. Chemosphere, 10: 751-761.
Kimbrough RD & Linder RE (1974) The toxicity of technical
hexachlorobenzene in the Sherman rat strain. A preliminary study. Res
Commun Chem Pathol Pharmacol, 8: 653-664.
King L & Sherbin G (1986) Point sources of toxic organics to the upper
St. Clair River. Water Pollut Res J Can, 21: 433-446.
Kitchin KT & Brown JL (1989) Biochemical studies of promoters of
carcinogenesis in rat livers. Teratog Carcinog Mutagen, 9: 273-285.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Kleiman de Pisarev DL, Sancovich HA, & Ferramola de Sancovich AM
(1989) Enhanced thyroxine metabolism in hexachlorobenzene-intoxicated
rats. J Endocrinol Invest, 12: 767-772.
Kleiman de Pisarev DL, Del Carmen Rios de Molina M, & San Martin de
Viale LC (1990) Thyroid function and thyroxine metabolism in
hexachlorobenzene-induced porphyria. Biochem Pharmacol,. 39:
817-825.
Knezovich JP & Harrison FL (1988) The bioavailability of sediment-
sorbed chlorobenzenes to larvae of the midge, Chironomus decorus.
Ecotoxicol Environ Saf, 15: 226-241.
Kocan A, Petrík J, Drobná G, & Chovancová J (1994) Levels of PCBs and
some organochlorine pesticides in the human population of selected
areas of the Slovak Republic: I. Blood. Chemosphere, 29(9/11):
2315-2325.
Kodric-Smit M, Smit Z, & Olie K (1980) Organochlorine contaminants in
human milk from Slavonia Province, Yugoslavia, 1978. Pestic Monit J,
14: 1-2.
Koeman JH, Ten Noever de Brauw MC & De Vos RH (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
Kohler A, Harms U, & Lukas B (1986) Accumulation of organochlorines
and mercury in flounder - an approach to pollution assessments. Helgol
Meeresunters 40: 431-440.
Koizumi A (1991) Experimental evidence for the possible exposure of
workers to hexachlorobenzene by skin contamination. Br J Ind Med,
48: 622-628.
Kosian P, Lemke A, Studders K, & Veith G (1981) The precision of the
ASTM bioconcentration test. Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory & Superior,
Wisconsin, University of Wisconsin-Superior, Center for Lake Superior
Environmental Studies, 24 pp (EPA 600/3-81/022).
Koss G & Koransky W (1975) Studies on the toxicology of
hexachlorobenzene: I. Pharmacokinetics. Arch Toxicol, 34: 203-212.
Koss G & Koransky W (1976) Studies on the toxicology of
hexachlorobenzene. II. Identification and determination of
metabolites. Arch Toxicol, 35: 107-114.
Koss G & Koransky W (1978) Pentachlorophenol in different species of
vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. In: Ranga Rao K ed. Pentachlorophenol. New York,
London, Plenum Press, pp 131-137.
Koss G & Manz D (1976) Residues of hexachlorobenzene in wild mammals
of Germany. Bull Environ Contam Toxicol, 15: 189-191.
Koss G, Strik JJTWA, & Kan CA (1978) Metabolites of hexachlorobenzene
in the excreta of different animal species. Excerpta Med Int Congress,
440: 211-212.
Krauthacker B (1993) Organochlorine pesticides and polychlorinated
biphenyls (PCBs) in human serum collected from the general population
from Zagreb (1985-1990). Bull Environ Contam Toxicol, 50: 8-11.
Krishnan K, Brodeur J, & Charbonneau M (1991) Development of an
experimental model for the study of hexachlorobenzene-induced hepatic
porphyria in the rat. Toxicol Appl Pharmacol, 17: 433-441.
Krishnan K, Brodeur J, Plaa GL, & Charbonneau M (1992) Modulation of
hexachlorobenzene-induced hepatic porphyria by methyl isobutyl ketone
in the rat. Toxicol Lett, 61: 167-174.
Kuehl DW & Butterworth B (1994) A national study of chemical residues
in fish: III. Study results. Chemosphere, 29(3): 523-535.
Kuehl DW, Leonard EN, Butterworth BC, & Johnson KL (1983)
Polychlorinated chemical residues in fish from major watersheds near
the Great Lakes, 1979. Environ Int, 9: 293-299.
Kuhnlein HV, Receveur O, Muir DCG, Chan HM, & Soueida R (1995) Arctic
indigenous women consume greater than acceptable levels of
organochlorines. J Nutr, 125: 2501-2510.
Kuiper-Goodman T, Grant DL, Moodle CA, Korsrud GO, & Munro IC (1977)
Subacute toxicity of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 40: 529-549.
Kuroda Y (1986) Genetic and chemical factors affecting chemical
mutagenesis in cultured mammalian cells. In: Antimutagenesis and
anticarcinogenesis mechanisms. New York, London, Plenum Press,
pp 359-375.
Lambrecht RW, Erturk E, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982a) Renal toxicity and tumorigenicity of
hexachlorobenzene (HCB) in rats (R). Proc Am Assoc Cancer Res, 23:
54 (Abstract).
Lambrecht RW, Erturk E, Grunden E, Headley DB, Morris CR, Peters HA, &
Bryan GT (1982b) Hepatotoxicity and tumorigenicity of
hexachlorobenzene (HCB) in Syrian golden hamsters (H) after subchronic
administration. Fed Proc, 41: 329 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983a) Hepatocarcinogenicity of chronically administered
hexachlorobenzene in rats. Fed Proc, 42: 786 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983b) Renal tumors in rats chronically exposed to hexachlorobenzene
(HCB). Proc Am Assoc Cancer Res, 24: 59 (Abstract).
Lambrecht RW, Sinclair PR, Bement WJ, Sinclair JF, Carpenter HM,
Buhler DR, Urquhart AJ, & Elder GH (1988) Hepatic uroporphyrin
accumulation and uroporphyrinogen decarboxylase activity in cultured
chick-embryo hepatocytes and in Japanese quail ( Coturnix coturnix
japonica) and mice treated with polyhalogenated aromatic
hydrocarbons. Biochem J, 253: 131-138.
Lamparski LL, Langhorst ML, Nestrick TJ, & Cutie S (1980) Gas-liquid
chromatographic determination of chlorinated benzenes and phenols in
selected biological matrices. J Assoc Off Anal Chem, 63(1): 27-32.
Landrum PF (1989) Bioavailability and toxicokinetics of polycyclic
aromatic hydrocarbons sorbed to sediment for the amphipod
Pontoporeia hoyi. Environ Sci Technol, 23: 588-595.
Lane DA, Schroeder WH, & Johnson ND (1992a) On the spatial and
temporal variations in atmospheric concentrations of hexachlorobenzene
and hexachlorocyclohexane isomers at several locations in the province
of Ontario, Canada. Atmos Environ, 26A: 31-42.
Lane DA, Johnson ND, Hanley MJJ, Schroeder WH, & Ord DT (1992b) Gas-
and particle-phase concentrations of alpha-hexachlorocyclohexane,
gama-hexachlorocyclohexane, and hexachlorobenzene in Ontario air.
Environ Sci Technol, 26: 126-133.
Langhorst ML & Nestrick TJ (1979) Determination of chlorobenzenes in
air and biological samples by gas chromatography with photoionization
detection. Anal Chem, 51(12): 2018-2025.
Larsen BR, Turrio-Baldassarri L, Nilsson T, Iacovella N, Di Domenico
A, Montagna M, & Facchetti S (1994) Toxic PCB congeners and
organochlorine pesticides in Italian human milk. Ecotoxicol Environ
Saf, 28: 1-13.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW, &
Evans RL (1976) An ecological study of hexachlorobenzene (HCB). New
Orleans, University of New Orleans, Department of Biological Sciences
(Prepared for the US Environmental Protection Agency, Office of Toxic
Substances, Washington) (EPA 560/6-76-009).
Laska AL, Bartell C, & Laseter JL (1976) Distribution of
hexachlorobenzene and hexachlorobutadiene in water, soil, and selected
aquatic organisms along the lower Mississippi River, Louisiana. Bull
Environ Contam Toxicol, 15: 535-542.
Leger DA (1991) Environmental concentrations of hexachlorobenzene in
Atlantic Canada. Moncton, New Brunswick, Environment Canada, Inland
Waters Directorate.
Leoni V & D'Arca SU (1976) Experimental data and critical review of
the occurrence of hexachlorobenzene in the Italian environment. Sci
Total Environ, 5: 253-272.
Lewis RJ (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Company.
Lewis RG & MacLeod KE (1982) Portable sampler for pesticides and
semivolatile industrial organic chemicals in air. Anal Chem, 54:
310-315.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic compounds
in rain: II. Gas scavenging of neutral organic compounds. Atmos
Environ, 19: 1609-1617.
Lilienthal H, Benthe C, Heinzow B, & Winneke G (1996) Impairment of
schedule-controlled behavior by pre- and postnatal exposure to
hexachlorobenzene in rats. Arch Toxicol, 70: 174-181.
Linko P, Yeowell HN, Gasiewicz TA, & Goldstein JA (1986) Induction of
cytochrome P-450 isozymes by hexachlorobenzene in rats and aromatic
hydrocarbon (Ah)-responsive mice. J Biochem Toxicol, 1: 95-107.
Loose LD (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice. Environ Health
Perspect, 43: 89-97.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspect, 39: 79-92.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55(6): 881-889.
Lu P & Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model ecosystem. Environ Health
Perspect, 10: 269-284.
Ludwicki JK & Góralczyk K (1994) Organochlorine pesticides and PCBs in
human adipose tissues in Poland. Bull Environ Contam Toxicol, 52:
400-403.
Lunde G & Bjorseth A (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci
Total Environ, 8(3): 241-246.
Lunde G & Ofstad EB (1976) Determination of fat-soluble chorinated
compounds in fish. Z Anal Chem, 282: 395-399.
McCarty LS, MACKAY D, Smith AD, Ozburn GW, & Dixon DG (1992a) Residue-
based interpretation of toxicity and bioconcentration QSARs from
aquatic bioassays: Neutral narcotic organics. Environ Toxicol Chem,
11: 917-930.
McCarty LS, Ozburn GW, Smith AD, & Dixon DG (1992b) Toxicokinetic
modeling of mixtures of organic chemicals. Environ Toxicol Chem, 11:
1037-1047.
Mack GA & Stanley J (1985) The national human adipose tissue survey.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (Report No. NHATS-ST-01).
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of physical-
chemical properties and environmental fate for organic chemicals -
Volume I: Monoaromatic hydrocarbons, chlorobenzenes, and PCBs.
Chelsea, Michigan, Lewis Publishers.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol, 25: 921-928.
MAFF (Ministry of Agriculture, Fisheries and Food) (1992) Report of
the working party on pesticide residues: 1988-1990. London, Her
Majesty's Stationery Office (Food Surveillance Paper No. 34).
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1994) Report of the working party on pesticide
residues, 1993 - Supplement to the pesticides register. London, Her
Majesty's Stationery Office.
Mansour M, Scheunert I, Viswanathan R, & Korte F (1986) Assessment of
the persistence of hexachlorobenzene in the ecosphere. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 53-59 (IARC Scientific Publications No. 77).
Marlow DA (1986) Hexachlorobenzene exposure in the production of
chlorophenols. In: Morris CR & Cabral JRP ed. Hexachlorobenzene:
Proceedings of an International Symposium. Lyon, International Agency
for Research on Cancer, pp 161-169 (IARC Scientific Publications
No. 77).
Mehendale HM, Fields M, & Matthews HB (1975) Metabolism and effects of
hexachlorobenzene on hepatic microsomal enzymes in the rat. J Agric
Food Chem, 23: 261-265.
Mendoza CE, Grant DL, & Shields JB (1975) Body burden of
hexachlorobenzene in suckling rats and its effects on various organs
and on liver porphyrin accumulation. Environ Physiol Biochem, 5:
460-464.
Mendoza CE, Collins B, Shields JB, & Laver GW (1977) Hexachlorobenzene
residues and effects on esterase activities in pre-weanling rats after
a reciprocal transfer between HCB-treated and control dams. Arch
Toxicol, 38: 191-199.
Mendoza CE, Collins BT, Shields JB, & Laver GW (1978) Effects of
hexachlorobenzene or hexabromobenzene on body and organ weights of
preweanling rats after a reciprocal transfer between the treated and
control dams. J Agric Food Chem, 26: 941-945.
Mendoza CE, Shields JB, & Laver GW (1979) Comparison of the
porphyrinogenic activity of hexabromobenzene and hexachlorobenzene in
primiparous Wistar rats. Bull Environ Contam Toxicol, 21: 358-364.
Mes J (1990) Trends in the levels of some chlorinated hydrocarbon
residues in adipose tissue of Canadians. Environ Pollut, 65:
269-278.
Mes J (1992) Organochlorine residues in human blood and biopsy fat and
their relationship. Bull Environ Contam Toxicol, 48: 815-820.
Mes J & Davies DJ (1979) Presence of polychlorinated biphenyl and
organochlorine pesticide residues and the absence of polychlorinated
terphenyls in Canadian human milk samples. Bull Environ Contam
Toxicol, 21: 381-387.
Mes J & Malcolm S (1992) Comparison of chlorinated hydrocarbon
residues in human populations from the Great Lakes and other regions
of Canada. Chemosphere, 25(3): 417-424.
Mes J, Campbell DS, Robinson RN, & Davies DJA (1977) Polychlorinated
biphenyl and organochlorine pesticide residues in adipose tissue of
Canadians - 1977. Bull Environ Contam Toxicol, 17: 196-203.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and other
chlorinated hydrocarbon residues in adipose tissue of Canadians. Bull
Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, Turton D, & Sun W-F (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mes J, Marchand L, & Davies DJ (1990) Organochlorine residues in
adipose tissue of Canadians. Bull Environ Contam Toxicol, 45:
681-688.
Mes J, Davies DJ, Doucet J, Weber D, & McMullen E (1993) Levels of
chlorinated hydrocarbon residues in Canadian human breast milk and
their relationship to some characteristics of the donors. Food Addit
Contam, 10(4): 429-441.
Mill T & Haag W (1986) The environmental fate of hexachlorobenzene.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 61-66 (IARC Scientific Publications No. 77).
Moksnes MT & Norheim G (1986) Levels of chlorinated hydrocarbons and
composition of PCB in herring gull Larus argentatus eggs collected
in Norway in 1969 compared to 1979-81. Environ Pollut, 11: 109-116.
Mollenhauer HH, Johnson JH, Younger RL, & Clark DE (1975)
Ultrastructural changes in liver of the rat fed hexachlorobenzene. Am
J Vet Res, 36: 1777-1781.
Mollenhauer HH, Johnston JH, Younger RL, & Clark DE (1976) A unique
intracellular aberration related to hexachlorobenzene ingestion. Am J
Vet Res, 37: 847-850.
Morita M, Mimura S, & Yagyu H (1975) A systematic determination of
chlorinated benzenes in human adipose tissue. Environ Pollut, 9:
175-179.
Morley A, Geary D, & Harben F (1973) Hexachlorobenzene pesticides and
porphyria. Med J Aust, 1: 565.
Morosini M, Schreltmüller J, Reuter U, & Ballschmiter K (1993)
Correlation between C-6/C-14 chlorinated hydrocarbons levels in the
vegetation and in the boundary layer of the troposphere. Environ Sci
Technol, 27: 1517-1523.
Muir DCG, Wagemann R, Grift NP, Norstrom RJ, Simon M, & Lien J (1988)
Organochlorine chemical and heavy metal contaminants in white-beaked
dolphins ( Lagenorhynchus albirostris) and pilot whales
( Globicephala melaena) from the coast of Newfoundland, Canada. Arch
Environ Contam Toxicol, 17: 613-629.
Muir DCG, Grift NP, Lockhart WL, Wilkinson P, Billeck BN, & Brunskill
GJ (1995) Spatial trends and historical profiles of organochlorine
pesticides in Arctic lake sediments. Sci Total Environ, 160/161:
447-457.
Müller MD (1982) [Hexachlorobenzene in Switzerland: Measurement and
background of environmental contamination.] Chimia, 36(11): 437-445
(in German).
Mumma CF & Lawless EW (1975) Survey of industrial processing data.
Task I: Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processes. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA 560/3-75-003).
Muncaster BW, Innes DJ, Hebert PDN, & Haffner GD (1989) Patterns of
organic contaminant accumulation by freshwater mussels in the
St. Clair River, Ontario. J Great Lakes Res, 15: 645-653.
Murray HE, Neff GS, Hrung Y, & Giam CS (1980) Determination of
benzo(a)pyrene, hexachlorobenzene and pentachlorophenol in oysters
from Galveston Bay, Texas. Bull Environ Contam Toxicol, 25(4):
663-667.
Mussalo-Rauhamaa H, Pyysalo H, & Antervo K (1988) Relation between the
content of organochlorine compounds in Finnish human milk and
characteristics of the mothers. J Toxicol Environ Health, 25: 1-19.
Nair A & Pillai MKK (1989) Monitoring of hexachlorobenzene residues in
Delhi and Faridabad, India. Bull Environ Contam Toxicol, 42:
682-686.
Nash RG & Gish TJ (1989) Halogenated pesticide volatilization and
dissipation from soil under controlled conditions. Chemosphere, 18:
2353-2362.
Nebecker AV, Griffis WL, Wise CM, Hopkins E, & Barbitta JA (1989)
Survival, reproduction and bioconcentration in invertebrates and fish
exposed to hexachlorobenzene. Environ Toxicol Chem, 8: 601-611.
Needham LL, Burse VW, Head SL, Korver MP, McClure PC, Andrews JS Jr,
Rowley DL, Sung J, & Kahn SE (1990) Adipose tissue/serum partitioning
of chlorinated hydrocarbon pesticides in humans. Chemosphere, 20:
975-980.
Newton KG & Greene NC (1972) Organochlorine pesticide residue levels
in human milk -Victoria, Australia - 1970. Pestic Monit J, 6(1):
4-8.
NHW (National Health and Welfare) (1987) Nutrient value of some common
foods. Ottawa, Ontario, Health Services and Promotion Branch/Health
Protection Branch.
Niimi AJ & Cho CY (1981) Elimination of hexachlorobenzene (HCB) by
rainbow trout ( Salmo gairdneri), and an examination of its kinetics
in Lake Ontario salmonids. Can J Fish Aquat Sci, 38: 1350-1356.
Niimi AJ & Oliver BG (1989) Distribution of polychlorinated biphenyl
congeners and other halocarbons in whole fish and muscle among Lake
Ontario salmonids. Environ Sci Technol, 23: 83-88.
NIOSH (1985) Registry of toxic effects of chemical substances,
1983-1984 - Cumulative supplement to the 1981-1982 edition.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Noble DG & Elliott JE (1986) Environmental contaminants in Canadian
seabirds, 1968-1984: Trends and effects. Ottawa, Ontario, Canadian
Wildlife Service (Technical Report Series No. 13).
Noble DG, Elliott JE, & Shutt L (1992) Contaminants in Canadian
raptors, 1965-1988. Ottawa, Ontario, Canadian Wildlife Service
(Technical Report Series No. 91).
Norén K (1983a) Levels of organochlorine contaminants in human milk in
relation to the dietary habits of the mothers. Acta Paediatr Scand,
72: 811-816.
Norén K (1983b) Some aspects of the determination of organochlorine
contaminants in human milk. Arch Environ Contam Toxicol, 12:
277-283.
Norén K (1988) Changes in the levels of organochlorine pesticides,
polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans in
human milk from Stockholm, 1972-1985. Chemosphere, 17(1): 39-49.
Norén K (1993) Contemporary and retrospective investigations of human
milk in the trend studies of organochlorine contaminants in Sweden.
Sci Total Environ, 139/140: 347-355.
Norstrom RJ, Simon M, & Muir DCG (1990) Polychlorinated dibenzo-p-
dioxins and dibenzofurans in marine mammals in the Canadian north.
Environ Pollut, 66: 1-19.
NTP (1994) Seventh annual report on carcinogens. Summary, 1994.
Research Triangle Park North Carolina, US Department of Health and
Human Services, National Toxicology Program.
Ockner RK & Schmid R (1961) Acquired porphyria in man and rat due to
hexachlorobenzene intoxication. Nature (Lond), 189: 499.
Oehme M & Stray H (1982) Quantitative determination of ultra-traces of
chlorinated compounds in high-volume air samples from the Arctic using
polyurethane foam as collection medium. Fresenius Z Anal Chem, 311:
665-673.
Oliver BG (1984a) Distribution and pathways of some chlorinated
benzenes in the Niagara River and Lake Ontario. Water Pollut Res J
Can, 19: 47-59.
Oliver BG (1984b) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Partitioning relationships for chlorinated organics
between water and particulates in the St. Clair, Detroit and Niagara
Rivers. In: Kaiser LLE ed. QSAR in environmental toxicology - II.
Boston, Massachussetts, D. Reidel Publishing Co., pp 251-260.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St. Clair, and Erie: Implications
regarding sources along the St. Clair and Detroit rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Carey JH (1986) Photodegradation of wastes and pollutants
in aquatic environment. In: Pelizzetti E & Serpo N ed. Homogenous and
heterogenous photocatalysis. Boston, Massachussetts, D. Reidel
Publishing Co., pp 629-650.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Kaiser KLE (1986) Chlorinated organics in nearshore waters
and tributaries of the St. Clair River. Water Pollut Res J Can, 21:
344-350.
Oliver BG & Nicol KD (1982) Chlorobenzenes in sediments, water, and
selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ
Sci Technol, 16: 532-536.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of polychlorinated
biphenyl congeners and other chlorinated hydrocarbons in the Lake
Ontario ecosystem. Environ Sci Technol, 22: 388-397.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21: 368-379.
Oliver BG, Charlton MN, & Durham RW (1989) Distribution,
redistribution, and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
sediments. Environ Sci Technol, 23: 200-208.
OMAF/OME (Ontario Ministry of Agriculture and Food/Ontario Ministry of
the Environment) (1988) Polychlorinated dibenzo-p-dioxins and
polychlorinated dibenzofurans and other organochlorine contaminants in
food. Toronto, Ontario Ministry of Agriculture and Food and Ministry
of the Environment (Report prepared by the Joint OMAF/OME Toxics in
Food Steering Committee).
Ostfeldt P, Gustavson K, Jansson B, Jonsson P, Miettinen V, Ringstad
O, & Wesén C (1994) Halogenated organic compounds in the marine
environment 1989-1990. Copenhagen, Nordic Council of Ministers, 124 pp
(TemaNord 1994:591).
Parrish PR, Cook GH, & Patrick JM Jr (1974) Effects on several
estuarine animals. In: Rogers WA ed. Proceedings of the 28th Annual
Conference, White Sulphur Springs, West Virginia, 17-20 November 1974.
Sulphur Springs, West Virginia, Southeastern Association of Game and
Fish Commissioners.
Patton GW, Hinckley DA, Walla MD, & Bidleman TF (1989) Airborne
organochlorines in the Canadian High Arctic. Tellus, 41B: 243-255.
Peakall DB, Noble DG, Elliott JE, Somers JD, & Erickson G (1990)
Environmental contaminants in Canadian peregrine falcons, Falco
peregrinus: A toxicological assessment. Can Field-Nat, 104:
244-254.
Pearce PA, Elliott JE, Peakall DB, & Norstrom RJ (1989) Organochlorine
contaminants in eggs of seabirds in the Northwest Atlantic, 1968-1984.
Environ Pollut, 56: 217-235.
Pereira MA, Herren SL, Britt AL, & Khoury MM (1982) Sex difference in
enhancement of GGTase-positive foci by hexachlorobenzene and lindane
in rat liver. Cancer Lett, 15: 95-101.
Persoone G & Uyttersprot G (1975) The influence of inorganic and
organic pollutants on the rate of reproduction of a marine
hypotrichous ciliate: Euplotes vannus Muller. Rev Int Océanogr méd,
37-38: 125-151.
Peters HA (1976) Hexachlorobenzene poisoning in Turkey. Fed Proc,
35: 2400-2403.
Peters HA, Johnson SAM, Cam S, Oral S, Müftü Y, & Ergene T (1966)
Hexachlorobenzene-induced porphyria: effect of chelation on the
disease, porphyrin and metal metabolism. Am J Med Sci, 251: 314-322.
Peters HA, Gocmen A, Cripps DJ, Bryan GT, & Dogramaci I (1982)
Epidemiology of hexachlorobenzene-induced porphyria in Turkey:
clinical and laboratory follow-up after 25 years. Arch Neurol, 39:
744-749.
Peters HA, Cripps DJ, Gocmen A, Ertürk E, Bryan GT, & Morris CR (1986)
Neurotoxicity of hexachlorobenzene-induced porphyria turcica. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 575-579 (IARC Scientific Publications No. 77).
Peterson R & Ray S (1987) Organochlorine residues in brook trout and
yellow perch from New Brunswick and Nova Scotia (Canada) lakes. Water
Pollut Res J Can, 22: 352-364.
Phelps DK, Pruell RJ, & Lake JL (1986) Hexachlorobenzene in selected
marine samples: an environmental perspective. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 121-130 (IARC
Scientific Publications No. 77).
Proulx G, Weseloh DV, Elliott JE, Teeple S, Anghern PAM, & Mineau P
(1987) Organochlorine and PCB residues in Lake Erie mink populations.
Bull Environ Contam Toxicol, 39: 939-944.
Quinlivan S, Ghassemi M, & Santy M (1975) Survey of methods used to
control wastes containing hexachlorobenzene. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste Management
Programs (EPA 530/SW-120c).
Quinsey PM, Donohue DC, & Ahokas JT (1995) Persistence of
organochlorines in breast milk of women in Victoria, Australia. Food
Chem Toxicol, 33(1): 49-56.
Ray LE, Murray HR, & Giam CS (1983a) Analysis of water and sediment
from the Nueces Estuary/Corpus Christi Bay (Texas) for selected
organic pollutants. Chemosphere, 12: 1039-1045.
Ray LE, Murray HE, & Giam CS (1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12: 1031-1038.
Renner G (1988) Hexachlorobenzene and its metabolism. Toxicol Environ
Chem, 18: 51-78.
Richter E & Schäfer SG (1981) Intestinal excretion of
hexachlorobenzene. Arch Toxicol, 47: 233-239.
Rios de Molina MC, Wainstok de Calmanovici R, & San Martin de Viale LC
(1980) Investigations on the presence of porphyrinogen carboxy-lyase
inhibitor in the liver of rats intoxicated with hexachlorobenzene. Int
J Biochem, 12: 1027-1032.
Rippen G & Frank R (1986) Estimation of hexachlorobenzene pathways
from the technosphere into the environment. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 45-52 (IARC
Scientific Publications No. 77).
RIWA (1993) Rapport annuel 1993 de l'Association des Services d'Eau du
Rhin et de la Meuse, Annex 11. Amsterdam, Secrétariat de l'Association
des Services d'Eau du Rhin et de la Meuse.
Rizzardini M & Smith AG (1982) Sex differences in the metabolism of
hexachlorobenzene by rats and the development of porphyria in females.
Biochem Pharmacol, 31: 3543-3548.
Rizzardini M, Graziani A, Carugo C, & Cantoni L (1988) Investigations
on the role of free radical processes in hexachlorobenzene-induced
porphyria in mice. J Biochem Toxicol, 3: 33-46.
Rizzardini M, Cantoni L, Villa P, & Ubezio P (1990) Biochemical,
morphological and flow-cytometric evaluation of the effects of
hexachlorobenzene on rat liver. Cell Biol Toxicol, 6: 185-203.
Robinson PE, Mack GA, Remmers J, Levy R, & Mohadjer L (1990) Trends of
PCB, hexachlorobenzene, and ß-benzene hexachloride levels in the
adipose tissue of the U.S. population. Environ Res, 53: 175-192.
Rogers HR, Crathorne B, & Leatherland TM (1989) Occurrence of
chlorobenzene isomers in the water column of a UK estuary. Mar Pollut
Bull, 20: 276-281.
Rozman K, Rozman T, & Greim H (1981) Enhanced fecal elimination of
stored hexachlorobenzene from rats and rhesus monkeys by hexadecane or
mineral oil. Toxicology, 22: 33-44.
Rozman K, Gorski JR, Rozman P, & Parkinson A (1986) Reduced serum
thyroid hormone levels in hexachlorobenzene-induced porphyria. Toxicol
Lett, 30: 71-78.
Rumsby PC, Evans JG, Phillimore HE, Carthew P, & Smith AG (1992)
Search for Ha- ras codon 51 mutations in liver tumours caused by
hexachlorobenzene and Aroclor 1254 in C57BL/10ScSn mice with iron
overload. Carcinogenesis, 13: 1917-1920.
Rush GF, Smith JH, Maita K, Bleavins M, Aulerich RJ, Ringer RK, & Hook
JB (1983) Perinatal hexachlorobenzene toxicity in the mink. Environ
Res, 31: 116-124.
Russell RW & Gobas FAPC (1989) Calibration of the freshwater mussel,
Elliptio complanata, for quantitative biomonitoring of
hexachlorobenzene and octachlorostyrene in aquatic systems. Bull
Environ Contam Toxicol, 43: 576-582.
Salisbury CDC, Fesser ACE, MacNeil JD, & Patterson JR (1992) Trace
metal and pesticide levels in muskoxen from Victoria Island, Northwest
Territories, Canada. Intern. J Environ Chem, 48: 209-215.
Sanborn JR, Childers WF, & Hansen LG (1977) Uptake and elimination of
[14C] hexachlorobenzene (HCB) by the green sunfish, Lepomis
cyanellus Raf., after feeding contaminated food. J Agric Food Chem,
25: 551-553.
Schechter A, Fürst P, Krüger C, Meemken H-A, Groebel W, & Constable JD
(1989a) Levels of polychlorinated dibenzofurans, dibenzodioxins, PCBs,
DDT and DDE, hexachlorobenzene, dieldrin, hexachlorocyclohexanes and
oxychlordane in human breast milk from the United States, Thailand,
Vietnam, and Germany. Chemosphere, 18: 445-454.
Schechter A, Mes J, & Davies D (1989b) Polychlorinated biphenyl (PCB),
DDT, DDE and hexachlorobenzene (HCB) and PCDD/PCDF isomer levels in
various organs in autopsy tissue from North American patients.
Chemosphere, 18: 811-818.
Scheufler E & Rozman KK (1984) Comparative decontamination of
hexachlorobenzene-exposed rats and rabbits by hexadecane. J Toxicol
Environ Health, 14: 353-362.
Scheunert I, Marra C, Viswanatha R, Klein WK, & Korte F (1983) Fate of
hexachlorobenzene-14C in wheat plants and soil under outdoor
conditions. Chemosphere, 12: 843-858.
Scheunert I, Topp E, Schmitzer J, Klein W, & Korte F (1985) Formation
and fate of bound residues of [14C] benzene and [14C] chlorobenzene
in soil and plants. Ecotoxicol Environ Saf, 9: 159-170.
Schielen P, Schoo W, Tekstra J, Oostermeijer HHA, Seinen W, & Bloksma
N (1993) Autoimmmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schielen P, Den Besten C, Vos JG, Van Bladeren PJ, Seinen W, & Bloksma
N (1995) Immune effects of hexachlorobenzene in the rat: role of
metabolism in a 13-week feeding study. Toxicol Appl Pharmacol, 131:
37-43.
Schmid R (1960) Cutaneous porphyria in Turkey. N Engl J Med, 263:
397-398.
Schmitt CJ, Zaijcek JL, & Peterman PH (1990) National Contaminant
Biomonitoring Program: residues of organochlorine chemicals in U.S.
freshwater fish, 1976-1984. Arch. Environ Toxicol, 19: 748-781.
Schuler W, Brunn H, & Manz D (1985) Pesticides and polychlorinated
biphenyls in fish from the Lahn River. Bull Environ Contam Toxicol,
34: 608-616.
Schuytema G, Krawczyk DF, Griffis WL, Nebeker AV, & Robideaux ML
(1990) Hexachlorobenzene uptake by fathead minnows and
macroinvertebrates in recirculating sediment/water systems. Arch
Environ Contam Toxicol, 19: 1-9.
Schwarzenbach RP, Giger W, Hoehn E, & Schneider JK (1983) Behaviour of
organic compounds during infiltration of river water to groundwater.
Field studies. Environ Sci Technol, 17: 472-479.
Selden A, Jacobson G, Berg P, & Axelson O (1989) Hepatocellular
carcinoma and exposure to hexachlorobenzene: a case report. Br J Ind
Med, 46: 138-140.
Shirai T, Miyata Y, Nakanishi K, Murasaki G, & Ito N (1978)
Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in ICR mice
and its enhancement by hexachlorobenzene. Cancer Lett, 4: 271-275.
Siekel P, Chalupa I, Beno J, Blasko M, Novotny J, & Burian J (1991) A
genotoxicological study of hexachlorobenzene and pentachloroanisole.
Teratog Carcinog Mutagen, 11: 55-60.
Simon GS, Tardiff RG, & Borzelleca JF (1979) Failure of
hexachlorobenzene to induce dominant lethal mutations in the rat.
Toxicol Appl Pharmacol, 47: 415-419.
Sims DE, Singh A, Donald A, Jarrell J, & Villeneuve DC (1991)
Alteration of primate ovary surface epithelium by exposure to
hexachlorobenzene: a quantitative study. Histol Histopathol, 6:
525-529.
Singh A, Friesen D, Jarrel J, & Villeneuve DC (1990a)
Hexachlorobenzene toxicity in the monkey ovary: I. Ultrastructure
induced by low (0.1 mg/kg) dose exposure. In: Proceedings of the XIIth
International Congress for Electron Microscopy, Seattle, Washington -
Volume 2: Analytical sciences, San Francisco, California, San
Francisco Press, Inc., pp 282-283.
Singh A, Sims DE, Jarrell J, & Villeneuve DC (1990b) Hexachlorobenzene
toxicity in the monkey ovary: II. Ultrastructure induced by medium
(1.0 mg/kg) dose exposure. Anal Environ Toxicants, 13: 435-438.
Singh A, Dykeman A, Jarrell J, & Villeneuve DC (1991)
Hexachlorobenzene toxicity in the monkey ovary: III. Ultrastructure
induced by high (10 mg/kg) dose exposure. In: Proceedings of the 49th
Annual Meeting of the Electron Microscopy Society of America. San
Francisco, California, San Francisco Press, Inc.
Skåre JU, Stenersen J, Kveseth N, & Polder A (1985) Time trends of
organochlorine chemical residues in seven sedentary marine fish
species from a Norwegian fjord during the period 1972-1982. Arch
Environ Contam Toxicol, 14: 33-41.
Skaare JU, Markussen NH, Norheim G, Haugen S, & Holt G (1990) Levels
of polychlorinated biphenyls, organochlorine pesticides, mercury,
cadmium, copper, selenium, arsenic, and zinc in the harbour seal,
Phoca vitulina, in Norwegian waters. Environ Pollut, 66: 309-324.
Smith AG & Cabral JR (1980) Liver-cell tumours in rats fed
hexachlorobenzene. Cancer Lett, 11: 169-172.
Smith AG & De Matteis F (1990) Oxidative injury mediated by the
hepatic cytochrome P-450 system in conjunction with cellular iron.
Effects on the pathway of haem biosynthesis. Xenobiotica, 29(9):
865-877.
Smith AG & Francis JE (1983) Synergism of iron and hexachlorobenzene
inhibits hepatic uroporphyrinogen decarboxylase in inbred mice.
Biochem J, 214: 909-913.
Smith VE, Spurr JM, Filkins JC, & Jones JJ (1985a) Organochlorine
contaminants of wintering ducks foraging on Detroit River sediments. J
Great Lakes Res, 11(3): 231-246.
Smith AG, Francis JE, Dinsdale D, Manson MM, & Cabral JRP (1985b)
Hepatocarcinogenicity of hexachlorobenzene in rats and the sex
difference in hepatic iron status and development of porphyria.
Carcinogenesis, 6: 631-636.
Smith AG, Dinsdale D, Cabral JRP, & Wright AL (1987) Goitre and
wasting induced in hamsters by hexachlorobenzene. Arch Toxicol, 60:
343-349.
Smith AG, Cabral JRP, Carthew P, Francis JE, & Manson MM (1989)
Carcinogenicity of iron in conjunction with a chlorinated
environmental chemical, hexachlorobenzene, in C57BL/10ScSn mice. Int J
Cancer, 43: 492-496.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM (1990)
Sex-linked hepatic uroporphyria and the induction of cytochromes
P450IA in rats caused by hexachlorobenzene and polyhalogenated
biphenyls. Biochem Pharmacol, 40: 2059-2068.
Smith AG, Carthew P, Francis JE, Cabral JRP, & Manson MM (1993)
Enhancement by iron of hepatic neoplasia in rats caused by
hexachlorobenzene. Carcinogenesis, 14: 1381-1387.
Smith AG, Carthew P, Francis JE, & Ingebritsen K (1994) Influence of
iron on the induction of hepatic tumors and porphyria by
octachlorostyrene in C57BL/10ScSn mice. Cancer Lett, 81: 145-150.
Solé M, Porte C, Pastor D, & Albaigés J (1994) Long-term trends of
polychlorinated biphenyls and organochlorinated pesticides in mussels
from the Western Mediterranean coast. Chemosphere, 28(5): 897-903.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sopena de Kracoff YE, Ferramola de Sancovich AM, Sancovich HA, &
Kleiman de Pisarev DL (1994) Effect of thyroidectomy and thyroxine on
hexachlorobenzene induced porphyria. J Endocrinol Invest, 17:
301-305.
Spigarelli JL, Going JE, & Li R (1986) Hexachlorobenzene levels in
multimedia environmental samples from selected chemical production
plants. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 155-160 (IARC Scientific Publications No. 77).
Stacey CI, Perriman WS, & Whitney S (1985) Organochlorine pesticide
residue levels in human milk: Western Australia, 1979-1980. Arch
Environ Health, 40(2): 102-108.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary. Kansas
City, Missouri, Midwest Research Institute (Prepared for the US
Environmental Protection Agency, Office of Toxic Substances, Field
Studies Branch) (EPA 560/5-86/035).
Stevens RJJ & Neilson MA (1989) Inter- and intralake distribution of
trace contaminants in surface waters of the Great Lakes. J Great Lakes
Res, 15: 377-393.
Stevens MF, Ebell GF, & Psaila-Savona P (1993) Organochlorine
pesticides in Western Australian nursing mothers. Med J Aust, 158:
238-241.
Stewart FP & Smith AG (1987) Metabolism and covalent binding of
hexachlorobenzene by isolated male and female rat hepatocytes. Biochem
Pharmacol, 36: 2232-2234.
Stewart FP, Manson MM, Cabral JRP, & Smith AG (1989) Hexachlorobenzene
as a promoter of diethylnitrosamine-initiated hepatocarcinogenesis in
rats and comparison with induction of porphyria. Carcinogenesis,
10(7): 1225-1230.
Strik JJTWA (1986) Subacute toxicity of hexachlorobenzene. In: Morris
CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 335-342 (IARC Scientific Publications No. 77).
Stronkhorst J, Ysebaert TJ, Smedes F, Meininger PL, Dirksen S, &
Boudewijn TJ (1993) Contaminants in eggs of some waterbird species
from the Scheldt Estuary, SW Netherlands. Mar Pollut Bull, 26(10):
572-578.
Sundlof SF, Hansen LG, Koritz GD, & Sundlof SM (1982) The
pharmacokinetics of hexachlorobenzene in male beagles. Distribution,
excretion and pharmacokinetic model. Drug Metab Dispos, 10: 371-381.
Suns K, Craig GR, Crawford G, Rees GA, Tosine H, & Osborne J (1983)
Organochlorine contaminant residues in spottail shiner ( Notropis
hudsonius) from the Niagara River. J Great Lakes Res, 9: 335-340.
Suns K, Crawford GE, Russell DD, & Clement RE (1985) Temporal trends
and spatial distribution of organochlorine and mercury residues in
Great Lakes spottail shiners (1975-1983). Rexdale, Ontario, Ontario
Ministry of the Environment, Water Resources Branch.
Swindoll CM & Applehans FM (1987) Factors influencing the accumulation
of sediment-sorbed hexachlorobiphenyl by midge larvae. Bull Environ
Contam Toxicol, 39: 1055-1062.
Szymczyœski GA, Waliszewski SM, Tuszewski M, & Pyda P (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznaœ. J Environ Sci Health, A21(1): 5-14.
Tanabe S, Subramanian AN, Ramesh A, Kumaran PL, Miyazaki N, &
Tatsukawa R (1993) Persistent organochlorine residues in dolphins from
the Bay of Bengal, South India. Mar Pollut Bull, 26(6): 311-316.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Teschke R, Bolsen K, Landmann H, & Goerz G (1983) Effect of
hexachlorobenzene on the activities of hepatic alcohol metabolizing
enzymes. Biochem Pharmacol, 32: 1745-1751.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound: Volume 1 (Prepared for Puget Sound Dredged Disposal
Analysis and Puget Sound Estuary Program, Bellevue, Washington).
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in Strain A mice. Cancer Res, 37:
2717-2720.
Thomas W, Simon H, & Rüling Å (1985) Classification of plant species
by their organic (PAH, PCB, BHC) and inorganic (heavy metals) trace
pollutant concentrations. Sci Total Environ, 46: 83-94.
Thomson BA, Davidson WR, & Lovett AM (1980) Applications of a
versatile technique for trace analysis: Atmospheric pressure negative
chemical ionization. Environ Health Perspect, 36: 77-84.
Tobin P (1986) Known and potential sources of hexachlorobenzene. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 3-11 (IARC Scientific Publications No. 77).
To-Figueras J, Rodamilans M, Gómez-Catalán J, & Corbella J (1986)
Hexachlorobenzene residues in the general population of Barcelona,
Spain. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, p 147 (IARC Scientific Publications No. 77).
To-Figueras J, Gómez-Catalán J, Rodamilans M, & Corbella J (1992)
Sulphur derivative of hexachlorobenzene in human urine. Hum Exp
Toxicol, 11: 271-273.
To-Figueras J, Barrot C, Rodamilans M, Gómez-Catalán J, Torra M,
Brunet M, Sabater F, & Corbella J (1995) Accumulation of
hexachlorobenzene in humans: a long standing risk. Hum Exp Toxicol,
14: 20-23.
Topp E, Scheunert I, & Korte K (1989) Kinetics of the uptake of
14C-labelled chlorinated benzenes from soil by plants. Ecotoxicol
Environ Saf, 17: 157-166.
Trapp M, Baukloh V, Bohnet H-G, & Heeschen W (1984) Pollutants in
human follicular fluid. Fertil Steril, 42(1): 146-148.
US EPA (1980) Analysis of pesticide residues in human and
environmental samples: A compilation of methods selected for use in
pesticide monitoring programs. Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Toxicology Division,
Health Effects Research Laboratory (EPA 600/8-80-038).
US EPA (1982) Test methods: Methods of organic chemical analysis of
municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Office of Research and Development,
Environmental Monitoring and Support Laboratory (EPA 600/4-82-057).
US EPA (1985a) Health assessment document for chlorinated benzenes.
Washington, DC, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA 600/8-84-015F).
US EPA (1985b) Drinking water criteria document for hexachlorobenzene.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Criteria and Assessment Office (EPA 600/X-84-179-1).
US EPA (1986a) Exposure assessment for hexachlorobenzene. Washington,
DC, US Environmental Protection Agency, Office of Pesticides and Toxic
Substances (EPA 560/5-86-0(19).
US EPA (1986b) Test methods for evaluating solid waste, 3rd ed.
Washington, DC, US Environmental Protection Agency, Office of Solid
Waste and Emergency Response (SW 846).
US EPA (1988) Ambient aquatic life water quality criteria for
hexachlorobenzene. Duluth, Minnesota, US Environmental Protection
Agency, Office of Research and Development.
US FDA (US Food and Drug Administration) (1990) Residues in foods -
1988. J Assoc Off Anal Chem, 73: 133A-152A.
US FDA (US Food and Drug Administration) (1991) Residues in foods -
1989. J Assoc Off Anal Chem, 74: 127A-146A.
US FDA (US Food and Drug Administration) (1992) Residues in foods -
1990. J Assoc Off Anal Chem, 75: 121A-141A.
Üstünbas HB, Öztürk MA, Hasanoglu E, & Dogan M (1994) Organochlorine
pesticide residues in human milk in Kayseri. Hum Exp Toxicol, 13:
299-302.
Van der Ven K, Van der Ven H, Thibold A, Bauer O, Kaisi M, Mbura J,
Mhaya HN, Wever N, Diedrich K, & Krebs D (1992) Chlorinated
hydrocarbon content of fetal and maternal body tissues and fluids in
full term pregnant women: a comparison of Germany versus Tanzania. Hum
Reprod, 7(1): 95-100.
Van Leeuwen CJ, Van der Zandt PTJ, Aldenberg T, Verhaar HJM, & Hermans
JLM (1992) Application of QSARs, extrapolation and equilibrium
partitioning in aquatic effects assessment: I. Narcotic industrial
pollutants. Environ Toxicol Chem, 11: 267-282.
Van Loveren H, Kranjc EI, Rombout PJA, Blommaert FA, & Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Ommen B, Hendriks W, Bessems JGM, Geesink G, Müller F, & Van
Bladeren PJ (1989) The relation between the oxidative
biotransformation of hexachlorobenzene and its porphyrinogenic
activity. Toxicol Appl Pharmacol, 100: 517-528.
Van Raaij JAGM, Van den Berg KJ, & Notten WRF (1991a)
Hexachlorobenzene and its metabolites pentachlorophenol and
tetrachlorohydroquinone: interaction with thyroxine binding sites of
rat thyroid hormone carriers ex vivo and in vitro. Toxicol Lett,
59: 101-107.
Van Raaij JAGM, Van den Berg KJ, Engel R, Bragt PC, & Notten WRF
(1991b) Effects of hexachlorobenzene and its metabolites
pentachlorophenol and tetrachlorohydroquinone on serum thyroid hormone
levels in rats. Toxicology, 67: 107-116.
Van Raaij JAGM, Frijters CMG, & Van den Berg KJ (1993a)
Hexachlorobenzene-induced hypothyroidism: involvement of different
mechanisms by parent compound and metabolite. Biochem Pharmacol, 46:
1385-1391.
Van Raaij JAGM, Kaptein E, Visser TJ, & Van den Berg KJ (1993b)
Increased glucoronidation of thyroid hormone in hexachlorobenzene-
treated rats. Biochem Pharmacol, 45: 627-631.
Vaz R, Slorach SA, & Hofvander Y (1993) Organochlorine contaminants in
Swedish human milk: studies conducted at the National Food
Administration 1981-1990. Food Addit Contam, 10(4): 407-418.
Villanueva EC, Jennings RW, Burse VW, & Kimbrough RD (1974) Evidence
of chlorodibenzo-p-dioxin and chlorodibenzofuran in hexachlorobenzene.
J Agric Food Chem, 22: 916-917.
Villeneuve DC, Panopio LG, & Grant DL (1974) Placental transfer of
hexachlorobenzene in the rabbit. Environ Physiol Biochem, 4:
112-115.
Vincent SH, Smith AG, & Muller-Eberhard U (1989) Modulation of hepatic
heme-binding Z protein in mice by the porphyrogenic carcinogens
griseofulvin and hexachlorobenzene. Cancer Lett, 45: 109-114.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
ymposium. Lyon, International Agency for Research on Cancer,
pp 347-356 (IARC Scientific Publications No. 77).
Vos JG, Breeman HA, & Benschop H (1968) The occurrence of the
fungicide hexachlorobenzene in wild birds and its toxicological
importance: a preliminary communication. Meded Fac. Landbouwwet
Rijksuniv Gent, 33: 1263-1269.
Vos JG, Van der Maas HL Musch A, & Ram E (1971) Toxicity of
hexachlorobenzene in Japanese quail with special reference to
porphyria, liver damage, reproduction and tissue residues. Toxicol
Appl Pharmacol, 18: 944-957.
Vos JG, Botterweg PF, Strik JJTWA, & Koeman JH (1972) Experimental
studies with HCB in birds. TNO-Nieuws, 27: 599-603.
Vos JG, Van Logten MJ, Kreeftenberg JG, & Kruizinga W (1979a)
Hexachlorobenzene-induced stimulation of the humoral immune response
in rats. Ann NY Acad Sci, 320: 535-550.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979b) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Brouwer GMJ, Van Leeuwen FXR, & Wagenaar SJ (1983) Toxicity of
hexachlorobenzene in the rat following combined pre- and post-natal
exposure: comparison of effects on the immune system, liver and lung.
In: Parke DV, Gibson GG, & Hubbard R ed. Immunotoxicology. New York,
London, Academic Press, pp 219-235.
Vos RME, Snoek MC, Van Berkel WJH, Müller F, & Van Bladeren PJ (1988)
Differential induction of rat hepatic glutathione S-transferase
isoenzymes by hexachlorobenzene and benzyl isothiocyanate: comparison
with induction by phenobarbital and 3-methylcholanthrene. Biochem
Pharmacol, 37: 1077-1082.
Wada O, Yano Y, Urata G, & Nakao K (1968) Behavior of hepatic
microsomal cytochromes after treatment of mice with drugs known to
disturb porphyrin metabolism in liver. Biochem Pharmacol, 17:
595-603.
Wang M-J & Jones KC (1994) Occurrence of chlorobenzenes in nine United
Kingdom retail vegetables. J Agric Food Chem, 42: 2322-2328.
Washington State Department of Ecology (1990) Proposed sediment
management standards rule (September 18, 1990). Washington State
Register, issue 90-19.
Watts RR, Hodgson DW, Christ HL, & Moseman RF (1980) Improved method
for hexachlorobenzene and mirex determination with hexachlorobenzene
confirmation in adipose tissue: Collaborative study. J Assoc Off Anal
Chem, 63(5): 1128-1134.
Weisenberg E, Arad I, Grauer F, & Sahm Z (1985) Polychlorinated
biphenyls and organochlorine insecticides in human milk in Israel.
Arch Environ Contam Toxicol, 14: 517-521.
Wells DE, Campbell LA, Ross HM, Thompson PM, & Lockyer CH (1994)
Organochlorine residues in harbour porpoise and bottlenose dolphins
stranded on the coast of Scotland, 1988-1991. Sci Total Environ,
151: 77-99.
WHO (1993) Guidelines for drinking-water quality - Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization, p 84.
Wickström K, Pyysalo H, & Siimes MA (1983) Levels of chlordane,
hexachlorobenzene, PCB and DDT compounds in Finnish human milk in
1982. Bull Environ Contam Toxicol, 31: 251-256.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71: 410-414.
Wilson DM & Wan MTK (1982) Hexachlorobenzene: Industrial and
agricultural sources in British Columbia. Vancouer, Environment
Canada, Environmental Protection Service (Regional Program Report
No. 82-07).
Witte H, Langenohl T, & Offenbacher SAG (1988a) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part A: Organic pollutant load in sewage
sludge. Korresp Abwasser, 13: 118-125.
Witte H, Langenohl T, & Offenbacher SAG (1988b) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part B: Impact of the application of
sewage sludge on organic matter content in soil and plants. Korresp
Abwasser, 13: 126-136.
Yamaguchi Y, Kawano M, & Tatsukawa R (1986) Tissue distribution and
excretion of hexabromobenzene (HBB) and hexachlorobenzene (HCB)
administered to rats. Chemosphere, 15: 453-459.
Yang RSH, Pittman KA, Rourke DR, & Stein VB (1978) Pharmacokinetics
and metabolism of hexachlorobenzene in the rat and the rhesus monkey.
J Agric Food Chem, 26: 1076-1083.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés chimiques et physiques et méthodes d'analyse
L'hexachlorobenzène (HCB) est un composé organique chloré
modérément volatil. Il est pratiquement insoluble dans l'eau, mais
extrêmement soluble dans les lipides et présente une tendance à la
bioaccumulation. L'hexachlorobenzène de qualité technique contient
jusqu'à 2% d'impuretés, dont la principale est le pentachlorobenzène.
Les autres consistent en dibenzo- p-dioxines, dibenzofuranes et
biphényles fortement substitués par le chlore. L'analyse des
échantillons biologiques ou prélevés dans l'environnement comporte
généralement une extraction préliminaire de la prise d'essai, souvent
suivie d'une purification, après quoi les extraits organiques sont
soumis soit à une chromatographie en phase gazeuse couplée à la
spectrométrie de masse (GC/MS), soit à une chromatographie en phase
gazeuse avec détection par capture d'électrons (GC/ECD).
2. Sources d'exposition humaine et environnementale
L'hexachlorobenzène a été utilisé un temps comme fongicide pour
traiter les semences, mais il n'est plus actuellement utilisé à cet
effet dans la plupart des pays. Il continue néanmoins à être libéré
dans l'environnement à partir d'un certain nombre de sources,
notamment lors de l'épandage de pesticides organochlorés, ou encore
lorsque les sous-produits de la préparation des solvants, composés
aromatiques ou pesticides chlorés sont rejetés sans précautions,
incomplètement brûlés ou s'échappent de décharges anciennes.
3.Transport, distribution et transformation dans l'environnement
L'hexachlorobenzène est réparti dans tout l'environnement du fait
de sa mobilité et de sa persistance, même s'il se décompose lentement
dans l'air sous l'action de la lumière et dans le sol sous l'action
des microorganismes. Dans la troposphère, il est transporté sur de
grandes distances et s'élimine de l'air en se déposant sur le sol et
sur l'eau. On a fait état d'une bioamplification notable le long de la
chaîne alimentaire.
4. Concentrations dans l'environnement et exposition humaine
Un peu partout dans le monde, l'hexachlorobenzène est présent, à
distance de ses sources, sous faible concentration dans l'air ambiant
(quelques ng/m3 ou moins) ainsi que dans l'eau de boisson et les eaux
de surface (quelques ng/litre tout au plus). Cependant, au voisinage
des points d'émission, on a pu mesurer des concentrations plus
élevées. Ce composé s'accumule dans les milieux biologiques et on en a
décelé la présence chez des invertébrés, des poissons, des reptiles,
des oiseaux et des mammifères (y compris l'Homme) à distance des
points d'émission, en particulier dans les tissus adipeux des
organismes situés aux niveaux trophiques supérieurs. Chez la
population humaine de divers pays, on en a mesuré dans les tissus
adipeux des quantités qui vont, en moyenne, de quelques dizaines à
quelques centaines de ng/g de poids humide. En se fondant sur les
quantités représentatives d'hexachlorobenzène présentes dans l'air,
l'eau et les denrées alimentaires, on peut estimer à une valeur
comprise entre 0,0004 et 0,003 µg/kg de poids corporel, la dose
absorbée journalièrement par un adulte de la population générale. Cet
apport se fait principalement par la voie alimentaire. Du fait de la
présence d'hexachlorobenzène dans le lait maternel, on estime que dans
les différents pays les enfants nourris au sein en reçoivent
quotidiennement une quantité comprise entre < 0,018 et 5,1 µg/kg de
poids corporel. Les études consacrées à l'évolution de la quantité
d'hexachlorobenzène présente dans l'organisme humain montrent, pour la
plupart, que l'exposition de la population générale a baissé dans de
nombreux endroits, entre les années 70 et le milieu de la décennie
actuelle.
5. Cinétique et métabolisme chez l'Homme et les animaux de
laboratoire
On manque de données toxicocinétiques chez l'Homme.
L'hexachlorobenzène est facilement résorbé par la voie orale chez
l'animal d'expérience, mais il franchit mal la barrière cutanée (on ne
possède pas de données concernant l'inhalation). Chez l'Homme et
l'animal, il s'accumule dans les tissus riches en lipides, comme les
tissus adipeux, le cortex surrénalien, la moelle osseuse, la peau et
certains tissus endocriniens. En outre, il peut être transmis à la
progéniture par l'intermédiaire du lait maternel ou en traversant la
barrière placentaire. La métabolisation de l'hexachlorobenzène est
limitée et ses principaux métabolites urinaires sont le
pentachlorophénol, la tétrachlorhydroquinone et le
pentachlorothiophénol. La demi-vie d'élimination de
l'hexachlorobenzène va d'environ un mois chez les rats et les lapins à
2 ou 3 ans chez le singe.
6. Effets sur les animaux de laboratoire et dans les épreuves
in vitro
L'hexachlorobenzène présente une faible toxicité aiguë pour les
animaux de laboratoire (1000 à 10 000 mg/kg de poids corporel).
L'expérimentation animale montre en outre que ce composé n'est pas
irritant pour la peau ou les yeux et ne provoque pas de
sensibilisation chez le cobaye.
Les données dont on dispose au sujet de la toxicité générale de
l'hexachlorobenzène indiquent que celle-ci s'exerce notamment au
niveau de la voie de biosynthèse de l'hème. Chez plusieurs espèces de
mammifères de laboratoire exposés à de l'hexachlorobenzène, on a
constaté une élévation des concentrations de porphyrines ou de leurs
précurseurs dans les excréta ainsi que dans divers tissus, notamment
le tissu hépatique. De nombreuses études ont relevé des cas de
porphyrie chez des rats exposés de manière chronique ou subchronique à
de l'hexachlorobenzène administré par voie orale à des doses
quotidiennes comprises entre 2,5 et 15 mg par kg de poids corporel.
Chez des porcs à qui on faisait ingérer ce composé en doses
quotidiennes égales ou supérieures à 0,5 mg par kg de poids corporel,
on a observé une augmentation de l'excrétion des coproporphyrines
(aucun effet n'a été observé dans cette étude à la dose de 0,05
mg/kg). On a également montré que l'exposition à l'hexachlorobenzène
affectait de nombreux organes ou systèmes (comme le foie, les poumons,
les reins, la thyroïde, la peau ainsi que le système nerveux et le
système immunitaire) mais ces effets n'ont pas été aussi souvent
signalés que la porphyrie.
L'hexachlorobenzène est un inducteur du cytochrome P-450 de type
mixte. Il possède des propriétés phénobarbital-inductibles et
3- méthylcholantrène-inductibles. Il se fixe sur le récepteur Ah.
Lors d'études longitudinales sur des rats, on a observé à
plusieurs reprises des effets bénins (modifications
histopathologiques, induction d'enzymes) chez les animaux recevant des
doses quotidiennes comprises entre 0,25 et 0,6 mg de composé par kg de
poids corporel. La dose sans effet observable obtenue dans ces études
se situait entre 0,05 et 0,07 mg d'hexachlorobenzène par kg de poids
corporel et par jour. Chez des visons femelles, on a observé une
modification de la concentration de neurotransmetteurs dans
l'hypothalamus après administration prolongée du composé par la voie
alimentaire à la dose quotidienne de 0,16 mg par kg de poids corporel.
Les mêmes constatations ont été faites dans la progéniture de ces
animaux, qui avait été exposée pendant les périodes gestationnelle et
périnatale. Lors d'études subchroniques sur des rats on a constaté une
modification de l'homéostase calcique et des paramètres
ostéomorphométriques à la dose quotidienne de 0,7 mg/kg de poids
corporel, mais pas à celle de 0,07 mg/kg.
Un certain nombre d'études in vivo ont été effectuées sur des
rongeurs afin de mettre en évidence la cancérogénicité éventuelle de
l'hexachlorobenzène. Chez des hamsters qui avaient reçu une nourriture
contenant de l'hexachlorobenzène à la dose moyenne de 4, 8 ou 16 mg/kg
de poids corporel, on a observé chez les deux sexes et à toutes les
doses un accroissement de l'incidence des carcinomes
hépatocellulaires. Aux doses de 8 et 16 mg/kg, on constatait la
présence d'hémangioendothéliomes et à la dose la plus forte,
d'adénomes de la thyroïde chez les mâles. En exposant pendant 120
semaines des souris à ce composé par la voie alimentaire aux doses
quotidiennes respectives de 6, 12 et 24 mg/kg de poids corporel, on a
provoqué un accroissement de l'incidence des carcinomes
hépatocellulaires chez les deux sexes aux deux doses les plus élevées,
mais cet accroissement n'était pas significatif, sauf chez les
femelles exposées à la dose la plus forte. In utero, l'exposition de
rats par la voie orale ou lactationnelle à des doses alimentaires
quotidiennes d'hexachlorobenzène allant de 0,01 à 1,5 mg/kg de poids
corporel (mâles) ou de 1,9 mg/kg (femelles) pendant des périodes
pouvant durer jusqu'à 130 semaines post utero, c'est-à-dire la durée
de vie moyenne, a entraîné à la dose la plus forte un accroissement de
l'incidence des nodules hépatiques néoplasiques et des
phéochromocytomes surrénaliens chez les femelles et un excès
d'adénomes parathyroïdiens chez les mâles. Lors d'une autre étude
chronique effectuée sur des rats, on a exposé les animaux, par la voie
alimentaire et pendant des durées allant jusqu'à 2 ans, à des doses
journalières moyennes de 4-5 et 8-9 mg/kg de poids corporel. Les
effets constatés consistaient en une augmentation de l'incidence des
hépatomes et des adénomes rénaux aux deux doses et chez les deux
sexes. Chez les femelles, on observait en outre une augmentation de
l'incidence des carcinomes hépatocellulaires, des adénomes et des
carcinomes des voies biliaires, des phéochromocytomes et des adénomes
du cortex surrénalien. On a également signalé une incidence élevée des
tumeurs du foie dans un certain nombre d'études plus limitées au cours
desquelles on avait administré une seule dose d'hexachlorobenzène par
la voie alimentaire à de petits groupes de rates. Par ailleurs, on a
observé qu'après exposition subchronique par voie alimentaire à ce
composé, des souris, des hamsters et des rats avaient présenté des
tumeurs du foie, des voies biliaires, du rein, du thymus, de la rate
et des ganglions lymphatiques. Le même type d'exposition favorise
l'apparition de tumeurs hépatiques chez des souris sous l'action de
terphényles polychlorés et chez des rats, sous l'action de la
diéthylnitrosamine.
Sauf dans le cas des tumeurs rénales chez les rats mâles (qui, du
moins en partie, semblent résulter d'une dégénérescence hyaline) et
des hépatomes chez les rats des deux sexes (qui pourraient résulter de
réactions hyperplasiques à la nécrose hépatocellulaire), on n'a pas pu
trouver d'études mécanistiques concernant les divers types de tumeurs
provoquées par l'hexachlorobenzène et le risque encouru à cet égard
par l'Homme.
L'hexachlorobenzène n'a guère d'aptitude à provoquer directement
des mutations géniques, des lésions chromosomiques ou la réparation de
l'ADN. Il s'est révélé faiblement mutagène lors de quelques-unes des
études portant sur des bactéries et des levures, mais il convient de
noter que chacune de ces études comportait des limitations. Il y a
également des signes d'un faible taux de liaison à l'ADN in vitro et
in vivo, mais dans une proportion très inférieure à celle que l'on
attendrait d'une substance cancérogène génotoxique.
Lors d'études sur la reproduction, des doses d'hexachlorobenzène
ne dépassant pas 0,1 mg par kg de poids corporel qu'on avait fait
ingérer quotidiennement pendant 90 jours à des singes, ont provoqué
des anomalies dans la structure microscopique et l'ultrastructure de
l'épithélium germinatif superficiel, structures qui constituent une
cible inhabituelle pour des toxines ovariennes. Cette dose a également
endommagé l'ultrastructure des cellules germinales primordiales. Alors
même que ces sites étaient spécifiquement attaqués et présentaient des
lésions d'autant plus importantes que la dose était plus forte, le
développement folliculaire, ovocytaire et embryonnaire restait normal,
ce qui semble indiquer que l'hexachlorobenzène a un site d'action à
localisation spécifiquement ovarienne. Chez les mâles, la fonction de
reproduction n'est affectée qu'à des doses beaucoup plus élevées
(entre 30 et 221 mg/kg p.c. par jour), comme l'ont montré un certain
nombre d'études effectuées sur plusieurs espèces n'appartenant pas à
l'ordre des primates.
Des rats et des chats exposés par la voie transplacentaire ou
lactationnelle à des doses quotidiennes d'hexachlorobenzène comprises
entre 3 et 4 mg/kg p.c. ont présenté des signes d'hépatotoxicité et on
a également constaté des effets délétères sur la survie et la
croissance de leur progéniture. Dans certains cas, il y avait à ces
doses - ou à des doses plus élevées - une réduction de l'effectif des
portées et un nombre accru de mortinaissances. (En général, les ratons
et les chatons à la mamelle étaient plus souvent affectés - et à des
doses plus faibles - que les embryons et les foetus). Chez la
progéniture de visons qui recevaient une alimentation ne contenant pas
plus de 1 mg d'hexachlorobenzène par kg de nourriture (soit environ
0,16 mg/kg p.c. par jour), on a constaté une réduction du poids de
naissance et un accroissement de la mortalité au sevrage. Quelques
études ont mis en évidence des anomalies squelettiques ou rénales chez
les foetus de rats et de souris exposés à de l'hexachlorobenzène
pendant la gestation, mais les doses qui produisaient ces anomalies
n'étaient pas toxiques pour les mères. Par ailleurs, le lien de ces
anomalies avec la prise d'hexachlorobenzène n'a pas été formellement
établi. Dans deux études, dont l'une comportait une exposition
transplacentaire et postnatale, on a observé des anomalies du
développement neurocomportemental des ratons après exposition
in utero, les mères ayant reçu par voie orale des doses quotidiennes
d'hexachlorobenzène allant de 0,64 à 2,5 mg/kg de poids corporel.
Selon un certain nombre d'études, l'hexachlorobenzène aurait des
effets délétères sur le système immunitaire. Chez des rats et des
singes exposés à des doses quotidiennes comprises entre 3 et 120 mg
d'hexachlorobenzène par kg de poids corporel, on a constaté des
modifications histopathologiques au niveau du thymus, de la rate, des
ganglions lymphatiques et des tissus lymphoïdes pulmonaires. Chez des
chiens beagle exposés de façon chronique à des doses quotidiennes
correspondant à 0,12 mg de composé par kg p.c., on a observé une
hyperplasie nodulaire du tissu lymphoïde gastrique. Un certain nombre
d'études menées sur des rats ont montré qu'après plusieurs semaines
d'exposition à de l'hexachlorobenzène par la voie alimentaire, il y
avait stimulation de l'immunité humorale, et dans une moindre mesure,
de l'immunité à médiation cellulaire, sans modification de la fonction
des macrophages. A des doses quotidiennes ne dépassant pas 4 mg de
composé par kg de nourriture (environ 0,2 mg par kg p.c.),
administrées pendant la gestation, pendant le maternage et jusqu'à
l'âge de 5 semaines, il y a eu augmentation de la réponse immunitaire
à médiation cellulaire et de la réponse immunitaire humorale ainsi
qu'une accumulation de macrophages dans le tissu pulmonaire des
ratons. Par contre, la plupart des études effectuées sur des souris
ont fait ressortir les propriétés immunosuppressives de
l'hexachlorobenzène; des doses ne dépassant pas 0,5 à 0,6 mg/kg de
poids corporel administrées quotidiennement pendant plusieurs semaines
ont eu les effets suivants: diminution de la résistance à une
infection leishmanienne ou à une épreuve cancérogène par exposition à
des cellules tumorales, réduction de l'activité cytotoxique des
macrophages spléniques et de l'hypersensibilité retardée chez la
progéniture après exposition in utero ou pendant la période de
maternage. Lors d'un certain nombre d'études portant sur diverses
souches de rats, on a constaté qu'une exposition de brève durée ou une
exposition subchronique à de l'hexachlorobenzène modifiait la fonction
thyroïdienne, comme on pouvait en juger d'après la réduction de la
thyroxine sérique libre ou totale (T4) et souvent, mais dans une
moindre mesure, de la triiodothyronine (T3).
7. Effets sur l'Homme
La plupart des données que l'on possède au sujet des effets de
l'hexachlorobenzène sur l'Homme, proviennent d'intoxications
accidentelles qui se sont produites en Turquie en 1955-59, avec plus
de 600 cas répertoriés de porphyrie cutanée tardive. Lors de cet
accident, on a observé des troubles du métabolisme des porphyrines,
des lésions cutanées, des hyperpigmentations, des hypertrichoses, des
hépatomégalies, des hypertrophies de la thyroïde et des ganglions
lymphatiques, avec, dans environ la moitié des cas, une ostéoporose et
une arthrite, principalement d'ailleurs, chez les enfants. Les enfants
nourris au sein dont la mère avait été exposée, présentaient des
lésions appelées pembe yara, c'est-à-dire "lésions roses", et la
plupart d'entre eux sont décédés dans l'année. On dispose de quelques
données concernant des cas de porphyrie cutanée tardive chez des
personnes ayant subi une exposition relativement intense à
l'hexachlorobenzène sur leur lieu de travail ou dans leur
environnement général.
Les quelques études épidémiologiques disponibles concernant le
cancer souffrent d'un certain nombre d'insuffisances: effectif réduit,
exposition à l'hexachlorobenzène mal caractérisée ou exposition
simultanée à de nombreux autres agents, et ne permettent pas d'évaluer
la cancérogénicité de ce composé pour l'Homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Lors d'études sur la toxicité aiguë de l'hexachlorobenzène pour
les organismes aquatiques, on a constaté que l'exposition à des
concentrations de l'ordre de 1 à 17 µg/litre réduisait la production
de chlorophylle chez les algues ainsi que la reproduction chez les
ciliés, et qu'en outre, elle provoquait la mort des crevettes roses et
des crevettes américaines du genre Hippolyte, mais elle n'a pas
provoqué la mort de poissons d'eau douce ou de mer. Lors d'études à
plus long terme, on a constaté que la croissance de certaines algues
et protozoaires dulçaquicoles sensibles étaient affectée à une
concentration d'hexachlorobenzène de 1 µg/litre et que des
concentrations d'environ 3 µg/litre provoquaient la mort d'amphipodes
et de perches appartenant à l'espèce Micropterus salmoides.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
9.1 Effets sur la santé
Le Groupe de travail a conclu que les données disponibles sont
suffisantes pour que l'on puisse formuler des valeurs-guides relatives
aux effets cancérogènes et non cancérogènes de l'hexachlorobenzène.
En ce qui concerne les effets non cancérogènes constatés sur le
foie à dose élevée chez des porcs et des rats exposés par la voie
orale et en se basant sur la dose sans effet observable la plus faible
(0,05 mg/kg de poids corporel par jour), on arrive, compte tenu d'un
facteur d'incertitude de 300 (10× pour les variations
interspécifiques, 10x pour les variations intraspécifiques et 3× pour
la gravité de l'effet), à une TDI de 0,17 µg/kg de poids corporel.
La méthode utilisée pour déterminer la valeur-guide relative aux
effets cancérogènes repose sur la dose tumorigène TD5, c'est-à-dire
la dose ingérée qui provoque une augmentation de 5% de l'incidence
tumorale chez les animaux de laboratoire. D'après les résultats d'une
étude de cancérogénicité portant sur deux générations de rats et en
faisant appel à un modèle multiphasique, on obtient une TD5 de
0,81 mg/kg de poids corporel par jour, l'effet retenu étant la
formation de nodules cancéreux hépatiques chez les femelles. Compte
tenu de l'insuffisance des données mécanistiques, on a appliqué un
facteur d'incertitude de 5000 pour calculer la valeur-guide chiffrée à
0,16 µg/kg de poids corporel par jour.
9.2 Effets sur l'environnement
Le Groupe de travail a remarqué qu'il existe très peu d'études
expérimentales à partir desquelles on puisse procéder à une évaluation
du risque écologique. La concentration d'hexachlorobenzène dans les
eaux de surface est généralement inférieure de plusieurs ordres de
grandeur à celle qui pourrait être dangereuse pour les organismes
aquatiques, sauf dans certains endroits fortement pollués. Toutefois,
les concentrations d'hexachlorobenzène relevées dans les oeufs
d'oiseaux de mer et de rapaces en différents lieux du globe sont
proches de celles qui provoquent une diminution du poids des embryons
chez la mouette argentée (1500 µg/kg), ce qui incite à penser que le
composé pourrait être embryotoxique pour certaines espèces sensibles
d'oiseaux. De même, les concentrations d'hexachlorobenzène dans les
poissons de divers endroits du monde sont du même ordre de grandeur
que la dose de 1000 µg/kg qui entraîne une réduction du poids de
naissance et une augmentation de la mortalité chez la progéniture de
visons. Cela incite à penser que ce composé pourrait avoir des effets
indésirables chez les visons et éventuellement chez d'autres
mammifères piscivores.
10. Conclusions
a) L'hexachlorobenzène est un composé chimique persistant qui subit
une bioaccumulation du fait de sa liposolubilité et de sa résistance à
la décomposition.
b) L'expérimentation animale montre que l'hexachlorobenzène provoque
des cancers et affecte de nombreux organes, tissus et systèmes comme
le foie, les poumons, les reins, la thyroïde, les tissus des gonades,
le système nerveux et le système immunitaire.
c) En ce qui concerne l'Homme, on a pu observer, à l'occasion d'une
forte exposition d'origine accidentelle, les manifestations cliniques
d'une intoxication par l'hexachlorobenzène qui se traduisaient par une
porphyrie cutanée tardive chez les enfants et les adultes et par la
mort chez des nourrissons alimentés au sein.
d) Il est justifié de prendre diverses mesures pour réduire la
quantité d'hexachlorobenzène présente dans l'environnement.
e) On a proposé les valeurs-guides à visée sanitaire suivantes pour
la dose totale ingérée quotidiennement (TDI): en ce qui concerne les
effets non cancérogènes, 0,17 µg/kg de poids corporel par jour ; en ce
qui concerne les effets cancérogènes, 0,16 µg/kg de poids corporel par
jour.
1. RÉSUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El hexaclorobenceno (HCB) es un compuesto orgánico clorado de
volatilidad moderada. Es prácticamente insoluble en el agua, pero es
muy liposoluble y bioacumulativo. El HCB de calidad técnica contiene
hasta un 2% de impurezas, en su mayor parte pentaclorobenceno; el
resto incluye dibenzo- p-dioxinas, dibenzofuranos y bifenilos
altamente clorados. Para determinar el HCB en el medio ambiente y en
material biológico se procede por lo general a extraer la muestra
mediante disolventes orgánicos, a lo que sigue con frecuencia un paso
de limpieza, a fin de obtener extractos orgánicos analizables mediante
cromatografía de gases/espectrometría de masas (GC/MS) o cromatografía
de gases con detección de captura de electrones (GC/ECD).
2. Fuentes de exposición humana y ambiental
Hubo un tiempo en que el HCB se utilizó mucho en la limpieza de
semillas para prevenir las enfermedades micóticas de los cereales,
pero ese uso se abandonó en la mayor parte de los países en los años
setenta. El HCB se sigue liberando en el medio ambiente a partir de
diversas fuentes, que incluyen el uso de algunos plaguicidas clorados,
procesos de combustión incompleta y viejos vertederos, así como los
métodos inapropiados de producción y de eliminación de desechos en la
fabricación de disolventes clorados, compuestos aromáticos clorados y
plaguicidas clorados.
3. Transporte, distribución y transformación en el medio ambiente
El HCB se distribuye por todo el medio ambiente porque es móvil y
persistente, aunque se produce una lenta fotodegradación en el aire y
una degradación microbiana en el suelo. En la troposfera el HCB es
transportado a grandes distancias y es eliminado de la fase aérea por
su depósito en el agua y el suelo. Se ha informado de que se produce
una importante bioamplificación del HCB a través de la cadena trófica.
4. Niveles ambientales y exposición humana
Se encuentran concentraciones bajas de HCB en el aire ambiental
(a lo sumo unos pocos ng/m3), en el agua de bebida y en las aguas
superficiales (a lo sumo unos pocos ng/litro) de zonas alejadas del
punto emisor en todo el mundo. No obstante, se han hallado
concentraciones más altas cerca de los puntos emisores. El HCB es
bioacumulativo y se ha detectado en invertebrados, peces, reptiles,
aves y mamíferos (incluido el hombre) lejos de los puntos emisores,
particularmente en el tejido adiposo de organismos de los niveles
tróficos más altos. Los niveles medios en el tejido adiposo de la
población humana general en diversos países van de decenas a centenas
de ng/g de peso en fresco. Considerando los niveles representativos de
HCB en el aire, el agua y los alimentos, se estima que la ingesta
total de HCB por los adultos de la población general está comprendida
entre 0,0004 y 0,003 mg/kg de peso corporal al día. Esa ingesta se
realiza principalmente a través de los alimentos. Debido a la
presencia de HCB en la leche materna, se ha estimado que la ingesta
media por los lactantes alimentados al pecho en diversos países va de
< 0,018 a 5,1 mg/kg de peso corporal al día. Los resultados de la
mayoría de los estudios realizados acerca de las concentraciones de
HCB en los alimentos y en los tejidos humanos a lo largo del tiempo
indican que la exposición de la población general al HCB disminuyó
desde los años setenta hasta mediados de los noventa en muchos
lugares. Sin embargo, esa tendencia no se ha confirmado con claridad
durante el último decenio en otros lugares.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay suficientes datos sobre la toxicocinética en el hombre. El
HCB es absorbido rápidamente por vía oral por los animales de
experimentación, y escasamente a través de la piel (no existen datos
sobre la inhalación). En los animales y en los seres humanos, el HCB
se acumula en los tejidos ricos en lípidos, como el tejido adiposo, la
corteza suprarrenal, la médula ósea, la piel y algunos tejidos
endocrinos, y puede transmitirse a la descendencia a través tanto de
la placenta como de la leche materna. El HCB sufre un metabolismo
limitado, generando pentaclorofenol, tetraclorohidroquinona y
pentaclorotiofenol como principales metabolitos en la orina. Las
semividas de eliminación del HCB están comprendidas entre
aproximadamente un mes en la rata y el conejo y 2 ó 3 años en el mono.
6. Efectos en animales de laboratorio y en las pruebas in vitro
La toxicidad aguda del HCB en los animales de experimentación es
baja (1000 × 10 000 mg/kg de peso corporal). En los estudios con
animales, el HCB no causa irritación cutánea ni ocular y no tiene
efectos de sensibilización en el cobayo.
Los datos disponibles acerca de la toxicidad sistémica del HCB
indican que las vías de la biosíntesis del grupo hemo son una
importante diana de la toxicidad del hexaclorobenceno. Se han hallado
niveles elevados de porfirinas o de precursores de la porfirina, o de
ambas cosas, en el hígado, en otros tejidos y en las excretas de
varias especies de mamíferos de laboratorio expuestos al HCB. Se ha
informado de la aparición de porfiria en varios estudios realizados
con ratas expuestas por vía oral crónica o subcrónica a dosis entre
2,5 y 15 mg de HCB/kg de peso corporal al día. La excreción de
coproporfirinas aumentó en cerdos que ingirieron 0,5 mg de HCB/kg de
peso corporal al día o más (en el último estudio no se observó ningún
efecto con 0,05 mg de HCB/kg de peso corporal al día). Se ha visto
también que la exposición repetida al HCB afecta a una amplia gama de
sistemas orgánicos (entre ellos el hígado, los pulmones, los riñones,
la tiroides, la piel y los sistemas nervioso e inmunitario), aunque
las referencias a estos efectos son menos frecuentes que las
relacionadas con la porfiria.
El HCB es un inductor de tipo mixto del citocromo P-450, con
propiedades inducibles por el fenobarbital y por el 3-metilcolantreno.
Se sabe que se une al receptor Ah.
Por lo que se refiere a los estudios crónicos, en ratas expuestas
a dosis de 0,25 a 0,6 mg de HCB/kg peso corporal al día se observaron
efectos leves en el hígado (cambios histopatológicos, inducción
enzimática); en dichos estudios los NOEL estaban comprendidos entre
0,05 y 0,07 mg de HCB/kg de peso corporal al día. Las concentraciones
de neurotransmisores en el hipotálamo se vieron alteradas en visones
hembra sometidos a través de los alimentos a una exposición crónica de
0,16 mg de HCB/kg de peso corporal al día, y en su descendencia
expuesta a lo largo de la gestación y la lactancia. En estudios
subcrónicos realizados en ratas la homeostasis del calcio y la
morfometría ósea se vieron afectadas con 0,7 mg de HCB/kg de peso
corporal al día, pero no con 0,07 mg/kg de peso corporal al día.
La carcinogenicidad del HCB ha sido evaluada mediante varios
bioensayos realizados con roedores. En hámsters mantenidos con
alimentos con los que ingerían unas dosis medias de 4, 8 ó 16 mg/kg de
peso corporal al día durante toda la vida, se produjeron aumentos en
la incidencia de tumores de las células del hígado (hepatomas) en los
dos sexos y a todas las dosis, hemangioendoteliomas hepáticos a dosis
de 8-16 mg/kg de peso corporal al día, y adenomas tiroideos de los
machos a la dosis mayor. La exposición alimentaria de ratones a dosis
de 6, 12 y 24 mg/kg de peso corporal al día durante 120 semanas dio
lugar a un aumento de la incidencia de tumores de las células del
hígado (hepatomas) en ambos sexos a las dos dosis mayores (no
significativo, excepto para las hembras a la dosis mayor). En ratas,
la exposición in útero, durante la lactancia y por vía oral al HCB a
través de alimentos que proporcionaban a lo largo de su vida dosis
medias comprendidas entre 0,01 y 1,5 mg/kg de peso corporal al día
(machos) o 1,9 mg/kg de peso corporal al día (hembras) por espacio de
hasta 130 semanas post útero produjo a la mayor de las dosis un
aumento de la incidencia de nódulos hepáticos neoplásicos y de
feocromocitomas suprarrenales en las hembras y de adenomas
paratiroideos en los machos. En otro estudio crónico realizado en la
rata, la exposición por un periodo de hasta dos años a alimentos que
proporcionaban dosis medias de HCB de 4-5 y de 8-9 mg/kg de peso
corporal al día indujo aumentos de la incidencia de hepatomas y de
adenomas de las células renales a ambas dosis en los dos sexos, y de
carcinomas hepatocelulares, adenomas y carcinomas de las vías
biliares, y feocromocitomas suprarrenales y adenomas de la corteza
suprarrenal en las hembras. Se ha informado también de incidencias
elevadas de tumores hepáticos en algunos estudios más limitados en los
que se administraron concentraciones alimentarias únicas a grupos
reducidos de ratas hembra. Además, se ha informado de que, después de
una exposición alimentaria subcrónica al HCB, ratones, hámsters y
ratas desarrollaron tumores en el hígado, las vías biliares, el riñón,
el timo, el bazo y los ganglios linfáticos. La exposición alimentaria
al HCB favoreció la inducción de tumores hepáticos por el terfenilo
policlorado en el ratón y por la dietilnitrosamina en la rata.
Con excepción de los tumores renales en la rata macho
(aparentemente debidos, al menos en parte, a una nefropatía por
acumulación de gotas hialinas) y de los hepatomas en la rata (posible
resultado de la respuesta hiperplásica a una necrosis hepatocelular),
no se conocen estudios mecanísticos que hayan determinado el
significado del tipo de tumores inducidos por el HCB en el caso del
hombre.
El HCB tiene una escasa capacidad de inducción directa de
mutaciones de los genes, lesiones cromosómicas y reparaciones del ADN.
Mostró una leve actividad mutágena en un reducido número de los
estudios realizados en bacterias y levaduras, aunque hay que señalar
que todos esos estudios presentan limitaciones. Existen también
algunos indicios de un cierto grado de unión al ADN in vitro e
in vivo, aunque a niveles muy inferiores a los habituales en los
carcinógenos genotóxicos.
En estudios sobre la reproducción, la exposición oral de monos a
tan sólo 0,1 mg de HCB/kg de peso corporal al día durante 90 días
afectó a la estructura revelada por microscopia óptica y a la
ultraestructura del epitelio germinal superficial, una diana poco
usual para las toxinas que afectan al ovario. Dicha dosis causó
también daños ultraestructurales en las células germinales
primordiales. Estos cambios específicos en tejidos-diana, para los que
dosis mayores son aún más lesivas, se asocian por lo demás a un
desarrollo normal del folículo, el ovocito y el embrión, lo que indica
que el HCB tiene una acción específica en el ovario. La reproducción
masculina sólo se vio afectada a dosis mucho mayores (entre 30 y
221 mg/kg de peso corporal al día) en estudios realizados en varias
especies distintas de los primates.
La exposición de ratas y gatos, a través de la placenta o durante
la lactancia, a dosis maternas comprendidas entre 3 y 4 mg/kg de peso
corporal al día tuvo efectos hepatotóxicos o afectó a la supervivencia
o el crecimiento de la descendencia en período de lactancia. En
algunos casos, dosis iguales o superiores a ésas redujeron el tamaño
de las camadas o aumentaron el número de abortos. (Los efectos nocivos
en los cachorros sin destetar han sido observados más frecuentemente,
y a dosis menores, que los efectos embriotóxicos o fetotóxicos.) La
descendencia de visones expuestos crónicamente a sólo 1 mg de HCB/kg
de alimento (aproximadamente 0,16 mg/kg de peso corporal al día) tuvo
un peso reducido al nacer y presentó una mayor mortalidad hasta el
destete. A pesar de que se han observado trastornos esqueléticos y
renales de los fetos en algunos estudios realizados en ratas y ratones
expuestos al HCB durante la gestación, dichas alteraciones o bien no
estaban claramente relacionadas con el tratamiento o bien ocurrieron a
dosis que eran también tóxicas para las madres. En dos estudios, uno
de los cuales incluía exposición posnatal y durante la lactancia, el
desarrollo neurocomportamental de las crías de rata se vio afectado
por la exposición in útero a dosis maternas orales de 0,64 a 2,5 mg
de HCB/kg de peso corporal al día.
Los resultados de varios estudios indican que el HCB afecta al
sistema inmunitario. Ratas y monos expuestos a dosis entre 3 y 120 mg
de HCB/kg de peso corporal al día sufrieron alteraciones
histopatológicas en el timo, en el bazo y en los ganglios linfáticos o
los tejidos linfoides del pulmón. La exposición crónica de perros
sabuesos a 0,12 mg/kg de peso corporal al día produjo una hiperplasia
nodular del tejido linfoide gástrico. En varios estudios realizados en
la rata, la inmunidad humoral y, en menor grado, la celular se vieron
potenciadas tras varias semanas de exposición alimentaria al HCB,
mientras que la función de los macrófagos no se alteró. Una cantidad
tan pequeña como 4 mg de HCB/kg de alimento (aproximadamente 0,2 mg/kg
de peso corporal al día) durante la gestación, a lo largo de la
lactancia y hasta las 5 semanas de edad incrementó las respuestas
inmunitarias humoral y celular y provocó la acumulación de macrófagos
en el tejido pulmonar de crías de rata. Por el contrario, se ha
observado un efecto inmunodepresor del HCB en la mayor parte de los
estudios llevados a cabo con ratones; dosis de sólo 0,5-0,6 mg/kg de
peso corporal al día durante varias semanas redujeron la resistencia a
la infección por Leishmania o a una provocación con células
tumorales, disminuyeron la actividad citotóxica de los macrófagos del
bazo, y redujeron la respuesta de hipersensibilidad de tipo retardado
en la descendencia expuesta in útero y durante la lactancia. En
varios estudios realizados con diversas cepas de ratas, la exposición
de breve duración o subcrónica al HCB afectó a la función tiroidea, a
juzgar por los reducidos niveles séricos de tiroxina total y tiroxina
libre (T4) y a menudo, en menor grado, de triyodotironina (T3).
7. Efectos en el ser humano
La mayor parte de los datos acerca de los efectos del HCB en el
ser humano provienen de intoxicaciones accidentales que tuvieron lugar
en Turquía en los años 1955-1959, entre las que se identificaron más
de 600 casos de porfiria cutánea tardía (PCT). En esa ocasión se
observaron alteraciones en el metabolismo de la porfirina, lesiones
dermatológicas, hiperpigmentación, hipertricosis, aumento del tamaño
del hígado, de la glándula tiroides y de los ganglios linfáticos; se
observaron también (aproximadamente en la mitad de los casos)
osteoporosis o artritis, sobre todo en los niños. Los niños
amamantados por madres expuestas al HCB como consecuencia de ese
accidente desarrollaron un trastorno conocido como pembe yara
(ulceración rosada), y la mayor parte murieron antes de un año.
Existen también algunos indicios de que la PCT afecta a personas
sometidas a una exposición relativamente alta al HCB en el lugar de
trabajo o en el medio ambiente general.
Los pocos estudios epidemiológicos disponibles acerca de la
incidencia de cáncer tienen un valor limitado, ya sea por lo reducido
de la muestra, por la deficiente caracterización de la exposición al
CHB o por la exposición a otros muchos agentes, y son insuficientes
para evaluar la carcinogenicidad del HCB para el ser humano.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
En los estudios realizados sobre la toxicidad aguda del HCB para
los organismos acuáticos, la exposición a concentraciones comprendidas
entre 1 y 17 mg/litro redujo la producción de clorofila en algas y la
reproducción de protozoos ciliados, y causó mortalidad en el camarón
rosado y en las quisquillas, pero no aumentó la mortalidad de peces de
agua dulce o de mar. En estudios a largo plazo, el crecimiento de
algas y protozoos vulnerables de agua dulce se vio afectado por una
concentración de 1 mg/litro, mientras que concentraciones de
aproximadamente 3 mg/litro provocaron mortalidad en anfípodos y
necrosis hepática en la perca americana.
9. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
9.1 Efectos en la salud
El Grupo Especial llegó a la conclusión de que los datos
disponibles son suficientes para establecer valores indicativos
respecto a los efectos neoplásicos y no neoplásicos del HCB.
En cuanto a los efectos no neoplásicos, considerando el NOEL más
bajo notificado (0,05 mg de HCB/kg de peso corporal al día), referido
sobre todo a los efectos hepáticos observados a dosis mayores en
estudios realizados en cerdos y ratas expuestos por vía oral, e
incorporando un factor de incertidumbre de 300 (× 10 en concepto de
variación interespecíes, × 10 en concepto de variación intraespecie, y
× 3 en concepto de gravedad del efecto), se ha calculado una IDT de
0,17 mg/kg de peso corporal al día.
El criterio seguido en cuanto a los efectos neoplásicos se basa
en la dosis tumorigénica TD5, es decir, la ingesta asociada a un
exceso del 5% en la incidencia de tumores detectada en los
experimentos con animales. Considerando los resultados del bioensayo
de carcinogenicidad en dos generaciones de ratas, y empleando el
modelo polietápico, la TD5 es de 0,81 mg/kg de peso corporal al día
para los nódulos neoplásicos del hígado en las hembras. Habida cuenta
de la insuficiencia de los datos mecanísticos, se utilizó un factor de
incertidumbre de 5000 para establecer un valor indicativo, basado en
criterios de salud, de 0,16 mg/kg de peso corporal al día.
9.2 Efectos en el medio ambiente
El Grupo Especial señaló que existen muy pocos estudios
experimentales con los que llevar a cabo una evaluación de los riesgos
para el medio ambiente. Los niveles de HCB en las aguas superficiales,
excepto en unos pocos lugares extremadamente contaminados, son en
general varios órdenes de magnitud inferiores a los que se supone que
entrañan riesgos para los organismos acuáticos. No obstante, las
concentraciones de HCB en los huevos de las aves marinas y las rapaces
de algunos lugares en distintas zonas del mundo se aproximan a niveles
que en la gaviota argéntea se asocian a una disminución del peso del
embrión (1500 mg/kg), lo que parece indicar que el HCB puede dañar los
embriones de especies de aves vulnerables. Del mismo modo, los niveles
de HCB observados en peces de diversos lugares del mundo se encuentran
a un orden de magnitud del nivel alimentario de 1000 mg/kg, asociado a
una reducción del peso al nacer y a un aumento de la mortalidad de la
descendencia en los visones. Esto parece indicar que el HCB puede
tener efectos nocivos en los visones y, tal vez, en otros mamíferos
que se alimentan de peces.
10. Conclusiones
a) El HCB es un producto químico persistente que se bioacumula debido
a su liposolubilidad y a su resistencia a la degradación.
b) Los estudios realizados con animales han demostrado que el HCB
produce cáncer y afecta a una amplia gama de sistemas de órganos, con
inclusión del hígado, los pulmones, los riñones, la tiroides, los
tejidos reproductivos y los sistemas nervioso e inmunitario.
c) En seres humanos sometidos a una alta exposición accidental se ha
observado toxicidad sintomática, en particular porfiria cutánea tardía
en niños y en adultos y mortalidad en lactantes.
d) Es necesario adoptar diversas medidas para reducir la carga
ambiental de HCB.
e) Se han propuesto los siguientes valores indicativos basados en
criterios de salud para la ingesta diaria total (IDT) de HCB por el
ser humano: efectos no cancerígenos, 0,17 µg/kg de peso corporal/día;
yefectos neoplásicos, 0,16 µ/kg de peso corporal/día.
Synonyms: perchlorobenzene, pentachlorophenyl chloride, phenyl
perchloryl
Trade names: Amatin, Anticarie, Bunt Cure, Bunt-No-More, Co-op Hexa,
Granox NM, Julin's Carbon Chloride, No Bunt, No Bunt
40, No Bunt 80, No Bunt Liquid, Sanocide, Smut-Go,
Snieciotox, HexaCB
2.2 Physical and chemical properties
Some physical and chemical properties of HCB are listed in
Table 1. At ambient temperature, HCB is a white crystalline that is
virtually insoluble in water, but is soluble in ether, benzene and
chloroform (NTP, 1994). It has a high octanol/water partition
coefficient, low vapour pressure, moderate Henry's Law constant and
low flammability. Technical grade HCB is available as a wettable
powder, liquid and dust (NTP, 1994). Technical grade HCB contains
about 98% HCB, 1.8% pentachlorobenzene and 0.2% 1,2,4,5-
tetrachlorobenzene (IARC, 1979), and it is known to contain a variety
of impurities, including hepta- and octachlorodibenzofurans,
octachlorodibenzo- p-dioxin and decachlorobiphenyl (Villanueva et
al., 1974; Goldstein et al., 1978).
Table 1. Physical and chemical properties of hexachlorobenzenea
Property Value
Relative molecular mass 284.79
Melting point (°C) 230
Boiling point (°C) 322 (sublimates)
Density (g/cm3 at 20°C 1.5691
Vapour pressure 0.0023
(Pa at 25°C)
Log octanol/water partition coefficient 5.5
Water solubility 0.005
(mg/litre at 25°C)
Henry's Law Constant (caluclated)b 131
(Pa/mol per m3)
Conversion factors 1 ppm = 11.8 mg/m3
1 mg/m3 = 0.08 ppm
a From ATSDR (1990); Mackay et al. (1992)
b The Henry's Law Constant has been calculated using the
tabled values for aqueous solubility and vapour pressure
2.3 Analytical methods
Analytical methods for the determination of HCB in environmental
samples and biological tissues vary depending upon the matrix and
representative methods for various matrices, and are summarized in
Tables 2 and 3.
Table 2. Analytical methods for determining hexachlorobenzene in environmental samplesa
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Water Extract with dichloromethane, GC/ECD 0.05 mg/kg 95 ± 10-20% US EPA (1982)
exchange to hexane,
concentrate; Florisil column
chromatography as a clean-up
Water Extract with dichloromethane GC/MS 1.9 mg/kg No data US EPA (1982)
at pH 11 and 2, concentrate
Air Glass fibre filter and XAD2 HRGC/ 0.18 pg/m3 >99% Hippelein et al. (1993)
traps separated by a PUF LRMS
disk; extraction with toluene
Air Polyurethane foam (PUF) GC/ECD <0.1 µg/m3 94.5±8% Lewis & MacLeod (1982)
sampling cartridge, extraction
with diethyl ether in hexane
Air Polyurethane foam (PUF) GC/ECD low pg/m3 93±1.1% Oehme & Stray (1982)
plugs, extraction with hexane, range (not
fractionation by HPLC specified)
Air Porous polyurethane foam GC/ECD No data Tenax more Billings &
(PUF), or Tenax-GC resin; effective Bidleman (1980)
filters refluxed with than PUF in
dichloromethane and retaining HCB
chlorinated solvents removed
and refluxed with hexane;
clean-up by alumina
chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Air Adsorb on Amberlite XAD-2 GC/PID 0.014 mg/m3 approx Langhorst &
resin separated by a silanized 95 ± 12% Nestrick (1979)
glass wool plug, desorption
with carbon tetrachloride.
Air Trace Atmospheric Gas Analyser approx No data Thomson et al. (1980)
using negative atmospheric 0.35 µg/m3
pressure chemical ionization
for trace gas analysis;
collection from ambient air and
transfer into a carrier of CO2
for analysis
Soil, Hexane extraction GC/ECD 10 mg/kg 78±2.6% to DeLeon et al. (1980)
chemical 96.5±3.6%
waste
disposal
site samples
Soil Extract with dichloromethane GC/MS 18 mg/kg No data US EPA (1986b)
5 mg/kg
Sediment Solvent extraction subjected GC/MS 46% Lopez-Avila et al.
to acid-base fractionation; (1983)
base/neutral fraction subjected
to silica gel chromatography
Table 2 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix methodb detection
limit
Wastes, Extract with dichloromethane GC/MS 190 mg/kg No data US EPA (1986b)
non-water 50 mg/kg
miscible
Wastes, soil Extract with dichloromethane GC/MS 20 µg/litrec No data US EPA (1986b)
a Portions of the table were taken from ATSDR (1990)
b GC = gas chromatography; ECD = electron capture detector; MS = mass spectrometry; PID = photoionization detector;
HRGC = high-resolution gas chromatography; LRMS = low-resolution mass spectrometry
c Identification limit; detection limits for actual samples are several orders of magnitude higher depending upon the
sample matrix and extraction procedure employed.
Table 3. Analytical methods for determining hexachlorobenzene in biological materials
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Fish tissue Grind with sodium sulfate, extract GC/ECD approx No data Oliver & Nicol (1982)
with hexane/acetone, clean-up by 0.05 µg/kg
Na2SO4/Alumina/silica gel/Florisil
column followed by a H2SO4 column
on silica gel
Fish tissue Extraction with hexane/isopropanol, GC/ECD No data No data Lunde & Ofstad (1976)
solvent and sulfuric acid
partitioning
Fish tissue Sulfuric acid digestion, silica gel GC/ECD 10-15 µg/kg 93% Lamparski et al. (1980)
column chromatography, methylation,
alumina column chromatography
Oyster Extraction with acetone/acetonitrile, GC/ECD No data No data Murray et al. (1980)
tissue partitioning into petroleum ether,
silica gel chromatography
Adipose Extraction with hexane, subjected GC/ECD No data 87.4-92.6% Watts et al. (1980)
tissue to Florisil clean-up and one-fraction
(chicken) elution
Adipose Extraction (solvent not specified), HRGC/MS 12 µg/kg No data Stanley (1986)
tissue bulk lipid removal, Florisil
fractionation
Adipose Extraction with benzene/acetone, GC/ECD 0.12 µg/kg 79-95% Mes (1992)
tissue Florisil fractionation
Table 3 contd.
Sample Sample preparation Analytical Sample Recovery Reference
matrix method detection
limit
Blood/urine Extraction with carbon tetrachloride, GC/PID 4.1 µg/kg 83% Langhorst &
silica gel column chromatography, (urine) Nestrick (1979)
concentrate 16 µg/kg
(blood)
Blood Extraction with hexane, concentrate GC/ECD No data No data US EPA (1980)
Blood Extraction with hexane/isopropanol GC/ECD No data No data Lunde & Bjorseth (1977)
Breast milk Extraction with acetone/benzene, GC/ECD 33 µg/kg 70-82% Mes et al. (1993)
Florisil fractionation
GC = gas chromatography; ECD = electron capture detector; PID = photoionization detector; HRGC = high-resolution gas
chromatography; MS = mass spectrometry
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Sources, uses and production processes
Industrial synthesis of HCB may be achieved through the
chlorination of benzene at 150-200°C using a ferric chloride catalyst
or from the distillation of residues from the production of
tetrachloroethylene (US EPA, 1985a). HCB may also be synthesized by
refluxing hexachlorocyclohexane isomers with sulfuryl chloride or
chlorosulfonic acid in the presence of a ferric chloride or aluminum
catalyst (Brooks & Hunt, 1984).
Historically, HCB had many uses in industry and agriculture. The
major agricultural application for HCB used to be as a seed dressing
for crops such as wheat, barley, oats and rye to prevent growth of
fungi. The use of HCB in such applications was discontinued in many
countries in the 1970s owing to concerns about adverse effects on the
environment and human health. HCB may continue to be used for this
purpose in some countries; for example, HCB was still used in 1986 as
a fungicide, seed-dressing and scabicide in sheep in Tunisia (Jemaa et
al., 1986). However, it is uncertain as to whether HCB is still used
for this purpose.
In industry, HCB has been used directly in the manufacture of
pyrotechnics, tracer bullets and as a fluxing agent in the manufacture
of aluminum. HCB has also been used as a wood-preserving agent, a
porosity-control agent in the manufacture of graphite anodes, and as a
peptizing agent in the production of nitroso and styrene rubber for
tyres (Mumma & Lawless, 1975). It is likely that some of these
applications have been discontinued, although no information is
available.
Although HCB production has ceased in most countries, it is still
being generated inadvertently as a by-product and/or impurity in
several chemical processes. HCB is formed as a reaction by-product of
thermal chlorination, oxychlorination, and pyrolysis operations in the
manufacture of chlorinated solvents (mainly carbon tetrachloride,
trichloroethylene and tetrachloroethylene) (Government of Canada,
1993). The concentrations of HCB in distillation bottoms was estimated
to be 25%, 15% and 5%, respectively, for tetrachloroethylene, carbon
tetrachloride and trichloroethylene (Jacoff et al., 1986). While HCB
could potentially also be a contaminant in the final product, it was
not detected (detection limit 5 mg/litre) in carbon tetrachloride and
tetrachloroethylene in an investigation in Canada (personal
communication to Health Canada by Mr John Schultiess, Dow Chemical
Canada Inc., 1991). Analysis of production lots of tri- and
tetrachloroethylene produced in Europe in 1996 failed to detect HCB at
a detection limit of 2 µg/litre solvent (personal communication to the
IPCS by Mr C. de Rooij, Solvay Corporation Europe, 1996).
HCB is also generated as a waste by-product during the
manufacture of chlorinated solvents, chlorinated aromatics and
pesticides (Jacoff et al., 1986). The waste streams from the
production of pentachloronitrobenzene (PCNB), chlorothalonil and
dacthal are expected to contribute the bulk of HCB released from the
pesticide industry (Brooks & Hunt, 1984), although HCB can also be
generated as a waste by-product from the production of
pentachlorophenol, atrazine, simazine, propazine and maleic hydrazide
(Quinlivan et al., 1975; Mumma & Lawless, 1975). These pesticides are
also known to contain HCB as an impurity in the final product, usually
at levels of less than 1% HCB when appropriate procedures are used for
the synthesis and purification stages (Tobin, 1986). When such
procedures are not met, the level of HCB could be much higher (e.g.,
pentachloronitrobenzene has been reported to contain 1.8-11% HCB
(Tobin, 1986)). However, owing to many voluntary and regulatory
pressures, it is unlikely that such high levels of HCB are present in
today's pesticide formulations, but no information is available to
substantiate this point.
The chlor-alkali industry produces chlorine (Cl2), hydrogen and
caustic soda (NaOH) by electrolysis of purified and concentrated
sodium chloride (NaCl). Processes using graphite anodes are known to
produce HCB as a by-product (Quinlivan et al., 1975; Mumma & Lawless,
1975; Alves & Chevalier, 1980) owing to the reaction of chlorine with
graphite anode materials such as carbon and oils. Depending on the
purification procedures, the final products might also be contaminated
with HCB. In some countries, graphite anodes have been replaced by
dimensionally stabilized anodes (DSA), which do not generate HCB
(Government of Canada, 1993).
Incineration is an important source of HCB in the environment.
Emission levels from incinerators are very site-specific, and
therefore generic levels are difficult to estimate. Earlier
information yielded a crude estimate of the total HCB released from
all municipal incinerators in the USA to be 57-454 kg/year (US EPA,
1986a), but levels currently emitted are not known.
3.2 World production levels
Few recent data on the quantities of HCB produced are available.
Worldwide production of pure HCB was estimated to be 10 000
tonnes/year for the years 1978-1981 (Rippen & Frank, 1986). An
estimated 300 tonnes was produced by three manufacturers in the USA in
1973 (IARC, 1979). HCB was produced/imported in the European Community
at 8000 tonnes/year in 1978 (Rippen & Frank, 1986), and a company in
Spain used to produce an estimated 150 tonnes of HCB annually (IARC,
1979). Approximately 1500 tonnes of HCB were manufactured annually in
Germany for the production of the rubber auxiliary PCTP (BUA, 1994),
but this production was discontinued in 1993. No further centres of
HCB manufacture in Europe or North America have been identified.
Production of HCB has declined as a result of restrictions on its use
starting in the 1970s.
Considerable amounts of HCB are inadvertently produced as a by-
product in the manufacture of chlorinated solvents, chlorinated
aromatics and chlorinated pesticides. Jacoff et al. (1986) estimated
that approximately 4130 tonnes of HCB are generated annually as a
waste product in the USA and that nearly 77% of this is produced from
the manufacture of three chlorinated solvents: carbon tetrachloride,
trichloroethylene and tetrachloroethylene. The remainder is produced
by the chlorinated pesticide industry. In 1977, about 300 tonnes of
HCB were generated in Japan as a waste by-product in the production of
tetrachloroethylene, almost all of which was incinerated (IARC, 1979).
It was estimated that >5000 tonnes HCB/year were produced as a by-
product during tetrachloroethylene production in the Federal Republic
of Germany in 1980 (Rippen & Frank, 1986). However, recent estimates
for Europe from ECSA (European Chlorinated Solvent Association; P.G.
Johnson (1996) personal communication to IPCS) indicate that up to
4000 tonnes/year of HCB are produced as a by-product during certain
tetrachloroethylene production processes and that over 99% of this by-
product was incinerated at high temperatures.
3.3 Entry into the environment
Currently, the principal sources of HCB in the environment are
estimated to be the manufacture of chlorinated solvents, the
manufacture and application of HCB-contaminated pesticides, and
inadequate incineration of chlorine-containing wastes. It should be
noted that only a small fraction of the HCB generated as a by-product
may be released, depending on the process technology and waste-
disposal practices employed. For example, according to the US Toxic
Chemical Release Inventory (TRI), releases of HCB from the ten largest
processing facilities were 460 kg, most of this to air, compared with
almost 542 000 kg transferred offsite as waste. The TRI data are not
comprehensive, since only certain types of facilities are required to
report (ATSDR, 1994). ECSA (P.G. Johnson, personal communication to
IPCS) estimated that European emissions of HCB were about 200 kg/year
in 1993.
As discussed in the previous section, HCB is a contaminant of a
number of chlorinated pesticides. Since most current applications for
these products are dispersive, most HCB from this source will be
released to the environment.
Substantial quantities of HCB are also contained in the wastes
generated through the manufacture of chlorinated solvents and
pesticides. In the mid-1980s in the USA, 81% of these HCB-containing
wastes were disposed of by incineration, compared to 19% via
landfilling (Jacoff et al., 1986). It is likely that the amount of HCB
wastes disposed of by incineration has since increased, although
information has not been found to confirm this point. HCB can be
emitted from incinerators as a result of incomplete thermal
decomposition of these wastes and as a product of incomplete
combustion (PIC) from the thermal decomposition of a variety of
chlorinated organics such as Kepone, mirex, chlorobenzenes,
polychlorinated biphenyls, pentachlorophenol, polyvinyl chloride and
mixtures of chlorinated solvents (Ahling et al., 1978; Dellinger et
al., 1991).
Although only a small proportion of the HCB-containing waste
generated in the USA is landfilled, HCB may continue to leach to
groundwater from previously landfilled HCB waste sites. The
contribution of this route is uncertain, although HCB is not easily
leached, and landfills containing HCB are now designed to prevent
leachate losses into adjacent water systems (Brooks & Hunt, 1984). HCB
emission into the atmosphere from landfills containing HCB wastes
occurs from slow volatilization and from displacement of the
contaminated soil (Brooks & Hunt, 1984).
HCB has been detected in emissions from a number of industries,
including paint manufacturers, coal and steel producers, pulp and
paper mills, textile mills, pyrotechnics producers, aluminum smelters,
soap producers and wood-preservation facilities (Quinlivan et al.,
1975; Gilbertson, 1979; Alves & Chevalier, 1980), probably reflecting
the use of products contaminated with HCB. Municipal and industrial
wastewater facilities may also discharge HCB-contaminated effluents
(Environment Canada/Ontario Ministry of the Environment, 1986; King &
Sherbin, 1986), probably owing to inputs from industrial sources.
Long-range transport plays a significant role as a means of
redistribution of HCB throughout the environment. Wet deposition
(deposition via rain or snowfall) is the primary mechanism for
transport of HCB from the atmosphere to aquatic and terrestrial
systems in Canada (Eisenreich & Strachan, 1992). For example, it is
estimated that long-range transport and total deposition to the
Canadian environment is approximately 510 kg/year, an amount that is
similar to that from all other sources combined (Government of Canada,
1993).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Environmental transport and degradation
HCB is distributed throughout the environment because it is
mobile and resistant to degradation. Volatilization from water to air
and sedimentation following adsorption to suspended particulates are
the major removal processes from water (Oliver, 1984a; Oliver &
Charlton, 1984). Once in the sediments, HCB will tend to accumulate
and become trapped by overlying sediments (Oliver & Nicol, 1982).
Although HCB is not readily leached from soils and sediments, some
desorption does occur and may be a continuous source of HCB to the
environment, even if inputs to the system cease (Oliver, 1984a; Oliver
et al., 1989). Chemical or biological degradation is not considered to
be important for the removal of HCB from water or sediments (Callahan
et al., 1979; Mansour et al., 1986; Mill & Haag, 1986; Oliver & Carey,
1986). In the troposphere, HCB is transported over long distances by
virtue of its persistence, but does undergo slow photolytic
degradation (the half-life is approximately 80 days; Mill & Haag,
1986), or is removed from the air phase via atmospheric deposition to
water and soil (Bidleman et al., 1986; Ballschmiter & Wittlinger,
1991; Lane et al., 1992a, 1992b). In soil, volatilization is the major
removal process at the surface (Kilzer et al., 1979; Griffin & Chou,
1981; Schwarzenbach et al., 1983; Nash & Gish, 1989), while slow
aerobic (half-life of 2.7-5.7 years) and anaerobic biodegradation
(half-life of 10.6-22.9 years) are the major removal processes at
lower depths (Beck & Hansen, 1974; Howard et al., 1991).
4.2 Bioaccumulation and biomagnification
The bioaccumulative properties of HCB result from the combination
of its physicochemical properties (high octanol/water partition
coefficient) and its slow elimination due to limited metabolism
related to its high chemical stability. Organisms generally accumulate
HCB from water and from food, although benthic organisms may also
accumulate HCB directly from sediment (Oliver, 1984b; Knezovich &
Harrison, 1988; Gobas et al., 1989). The uptake of HCB in benthic
invertebrates has been investigated in a number of laboratory and
field studies. The results demonstrated that some HCB in sediments is
available to infaunal species. Reported bioaccumulation factorsa
(BAF) for invertebrates in HCB-containing sediments range from 0.04 to
0.58 in high-organic-content sediment to 1.95 in low-organic-content
a Defined as tissue concentration (wet weight) divided by sediment
concentration (dry weight). BAFs from Oliver (1984b) were divided
by 6.67 to convert tissue dry weight to wet weight.
sediment (Oliver, 1984b; Knezovich & Harrison, 1988; Gobas et al.,
1989). The bioavailability of sediment-bound HCB is inversely related
to sediment organic carbon content (Knezovich & Harrison, 1988), and
varies with the type and size of the organisms and their feeding
habits (Boese et al., 1990), the extent of contact with sediment pore
and interstitial waters (Landrum, 1989), and the surface area of the
substrate (Swindoll & Applehans, 1987). Landrum (1989) suggested that
the bioavailability of sediment-sorbed chemicals declines as the
contact time between the sediment and a contaminant increases. For
example, Schuytema et al., (1990) observed that addition of HCB-spiked
sediments did not result in a significant increase in the uptake of
HCB by the worm ( Lumbriculus variegatus), amphipods ( Hyalella
azteca and Gammarus lacustris), and fathead minnows ( Pimephales
promelas) in a laboratory recirculating water/sediment system.
However, there was a substantial increase of HCB levels in bed
sediment, suggesting that sediment served as a more effective sink for
HCB than the organisms.
The biomagnification factor (BMF) for HCB in the earthworm
Eisenia andrei after exposure via food was 0.068 on a wet weight
basis (0.071 on a lipid basis) (Belfroid et al., 1994a), the biota
lipid-to-soil accumulation factor, defined as the ratio of the
concentration in the animal to that on the soil, was 215 g soil dry
weight/g lipid (Belfroid et al., 1994b), and the bioconcentration
factors (BCFs) for earthworms kept in water were found to be between
48 × 104 and 62 × 104 ml water/g lipid (Belfroid et al., 1993).
Field studies indicate that exposure via food is important for
organisms at higher trophic levels, as significant biomagnification
has been observed in several studies in natural aquatic ecosystems. In
Lake Ontario, Oliver & Niimi (1988) observed that tissue residue
concentrations increased from plankton (mean = 1.6 ng/g wet weight) to
mysids (mean = 4.0 ng/g wet weight) to alewives (mean = 20 ng/g wet
weight) to salmonids (mean = 38 ng/g wet weight). Braune & Norstrom
(1989) used field data on body burdens of HCB in the herring gull
( Larus argentatus) and one of its principal food items, the alewife
( Alosa pseudoharengus) in a Great Lakes food chain to calculate a
biomagnification factor (whole body, wet weight basis) of 31.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
HCB has been detected in air, water, sediment, soil and biota.
Representative levels reported in various environmental media in many
countries are presented in Tables 4, 5 and 6.
5.1.1 Air
HCB is widely dispersed in ambient air, and is generally present
at low concentrations. Mean concentrations of HCB in air removed from
point sources in Canada, Norway, Sweden, Germany, the USA, the Arctic
and the Antarctic range from 0.04 to 0.6 ng/m3 (Table 4). Levels of
HCB in air are generally similar between urban, rural and remote
sites, reflecting the persistence and long-range transport of this
substance.
Airborne concentrations of HCB measured in the USA near nine
chlorinated solvent and pesticide plants in 1973 and 1976 were much
higher than background levels (Spigarelli et al., 1986).
Concentrations as high as 24 µg/m3 were detected in the immediate
vicinity of one plant, while the maximum concentration of HCB distant
from the site was 0.36 µg/m3. The highest levels were associated with
the production of perchloroethylene, trichloroethylene and carbon
tetrachloride, and with plants where onsite landfill and open pit
waste disposal were practiced. More recently, Grimalt et al. (1994)
reported that airborne concentrations of HCB in a community in the
vicinity of a organochlorine factory built in 1898 in Catalonia,
Spain, averaged 35 ng/m3, compared with 0.3 ng/m3 in Barcelona, the
reference community for this study. It is not known how representative
the data from these studies are, as HCB releases are expected to be
minimized from industries using appropriate modern technology and
waste management practices.
No data are available on the levels of HCB in indoor air.
Table 4. Levels of hexachlorobenzene in ambient air (ng/m3)
Location Year Detection Mean Rangea Reference
limit
Canada (Windsor, Ontario) 1987-1990 0.03 0.13 ND-0.44 Environment Canada (1992)
Canada (Ontario)
- industrial/urban areas 1985-1989 0.007 0.167 0.07-0.31 Lane et al. (1992b)
- rural areas 0.094 0.02-0.31
Canada (Egbert, Ontario) 1988-1989 - >0.054 0.00004-0.64 Hoff et al. (1992)
Canada (Walpole Island) 1988-1989 0.02 0.15 ND-0.34 Environment Canada (1992)
Canadian High Arctic 1987 0.15 ND-0.154 Patton et al. (1989)
(Beaufort Sea)
Bear Island (Arctic)
- summer
- winter 0.001 0.04 0.029-0.045 Oehme & Stray (1982)
0.001 0.111 0.059-0.188
Southern Ocean and 1990 0.06 0.04-0.078 Bidleman et al. (1993)
Antarctica
Enewetak Atoll 1979 0.10 0.095-0.13 Atlas & Giam (1981)
(Pacific Ocean)
Spitzbergen
- summer 0.001 0.071 0.05-0.085 Oehme & Stray (1982)
- winter 0.001 0.086 0.071-0.095
Table 4 contd.
Location Year Detection Mean Rangea Reference
limit
Germany (Hamburg - 1986-1987 0.6 0.3-2.5 Bruckmann et al. (1988)
residential, suburban
and industrial sites)
South Germany 1986-1990 0.21 0.058-0.52 Morosini et al. (1993)
Norway (Lillestrom) 0.001 0.162 0.055-0.234 Oehme & Stray (1982)
Sweden (Aspvreten) 1984 0.067 0.054->0.165 Bidleman et al. (1987)
Sweden (Stockholm) 1983-1985 0.07 0.054->0.130 Bidleman et al. (1987)
Spain
- (near organochlorine 1989 & 1992 - 35 11-44 Grimalt et al. (1994)
compounds factory)
- hospital in Barcelona - 0.3 0.25-0.4
USA (Portland, Oregon) 1984 0.075 0.05-0.11 Ligocki et al. (1985)
USA - chemical production ND-24000 Spigarelli et al. (1986)
plants
USA - urban areas 1975-1979 0.1 0.5 ND-4.4 Carey et al. (1985)
a ND = not detected
5.1.2 Water
Levels of HCB in freshwater in Europe and North America are
generally below 1 ng/litre (Table 5), although higher values have been
reported in aquatic systems that receive industrial discharges and
surface run-off. In the connecting channels to the Great Lakes in
Canada, HCB levels were often found to exceed 1.0 ng/litre,
particularly near point sources. Levels in the St. Clair River near
the Dow Chemical outfall were as high as 87 ng/litre in 1985 and
75 ng/litre in 1986 (Oliver & Kaiser, 1986).
Mean concentrations of HCB in seawater rarely exceed 1 ng/litre
(Table 5) (Ernst, 1986; Burton & Bennett, 1987). In the Nueces Estuary
in Texas, USA, the highest level (0.61 ng/litre) was found near
sewage outfalls (Ray et al., 1983a). Higher concentrations (up to
196 ng/litre) were observed in the Forth Estuary in Scotland, near
domestic and chemical industry discharges (Rogers et al., 1989).
5.1.3 Soil
Data identified on levels of HCB in soil are quite limited and
are summarized in Table 6. The most extensive data are from the 1972
US National Soils Monitoring Program, in which the concentrations of a
variety of pesticides were determined at 1483 sites from 37 states
(Carey et al., 1979). HCB was detected at 11 sites, with a range of
concentrations in positive samples from 10 to 440 µg/kg dry weight. Of
24 samples of agricultural soil in British Columbia, Canada, where HCB
had last been applied as a seed treatment 10-15 years prior to the
survey, 6 had detectable HCB residues of between 1.3 and 2.2 ng/g dry
weight (Wilson & Wan, 1982).
Mean concentrations of HCB reported from uncontaminated soil in
Europe were found to range from 0.3 ng/g in Switzerland (Müller, 1982)
to 5.1 ng/g in a Swedish rural heathland soil (Thomas et al., 1985)
(it was not indicated whether concentrations were on a dry or wet
weight basis). Soil from a farming area in Italy contained 40 ng/g
(dry or wet weight basis not indicated) (Leoni & D'Arca, 1976). HCB
levels were not markedly increased by long-term application of sludge
to land in Germany at a rate of 50 to 500 tons per ha and averaged
2.8 ng/g (dry or wet weight basis not indicated) (Witte et al.,
1988a,b). Monitoring programmes in Germany yielded average levels of
HCB contamination of soil ranging from approximately 1 ng/g dry weight
in the North Rhine-Westphalia (1990) to approximately 6 ng/g dry
weight in Baden-Württemberg (1988) (BUA, 1994).
Table 5. Concentrations of hexachlorobenzene (ng/litre) in drinking-water and surface water
Location Year Detection Mean Rangea Reference
limit
Drinking-water
Canada (Ontario) 1980 0.01 0.1 0.06-0.2 Oliver & Nicol (1982)
Canada (Maritime Provinces) 1985-1988 2.0 ND Environment Canada (1989)
Croatia
- Sisak 1988-1989 0.5 1.0a <1-4 Fingler et al. (1992)
- Zagreb 2.0a 1-3
USA 1977-1981 100 ND US EPA (1985b)
Surface water
Canada
- Lake Superior 1986 0.007 0.026 0.018-0.040 Stevens & Neilson
- Lake Huron 0.033 0.018-0.073 (1989)
- Georgian Bay 0.041 0.032-0.054
- Lake Erie 0.078 0.025-0.260
- Lake Ontario 0.063 0.020-0.113
Canada-St. Clair River 1985 0.30-87 Oliver & Kaiser (1986)
- tributaries to 0.08-0.79
St. Clair R.
Table 5 contd.
Location Year Detection Mean Rangea Reference
limit
Canada (Atlantic Region); 1979-1989 2.0 ND-2.2 Leger 1991
lakes, streams, reservoirs,
estuaries, coastal waters
Germany (Elbe) 1990 - 12 3-62 BUA (1994)
Greece (Strimon River) 1985-1986 - 1.52 0.5-2.8 Kilikidis et al. (1992)
Italy (tributaries to
Adriatic Sea) 1977-1978 1.0 ND Galassi & Provini (1981)
Mediterranean Sea 1982-1983 0.1 2.13 ND-12.6 El-Dib & Badawy (1985)
Netherlands/Belgium 1993 10 <10 <10 RIWA (1993)
Netherlands 1987 - <10 ND-100 De Walle et al. (1995)
North Sea (coastal waters
and estuaries) 1979-1980 2.7 0.03-15 Ernst (1986)
Scotland (Forth Estuary) 1987 0.01 <0.01-196 Rogers et al. (1989)
Scotland (Forth Estuary) 1990 0.7-8.0 Harper et al. (1992)
Spain (Ebre Delta) 1985-1986 0.0005 0.041 ND-1.0 Grimalt et al. (1988)
USA (Texas-estuary) 1980 0.24 <0.01-0.61 Ray et al. (1983a)
USA (coastal, surface <0.1 <0.1-26 Cross et al. (1987)
microlayer)
a median value
Table 6. Levels of hexachlorobenzene in soil (ng/g dry weight)
Source Year Detection Mean Rangea Reference
limit
Canada (British Columbia) 1.0 Wilson & Wan (1982)
- agricultural soils <1.0-2.2
- near a former grain 260 ng/g
treatment plant
Czech/Polish Border - - 3.25 0.47-4.8 Holoubek et al. (1994)
(Giant Mountains)
Germany (contaminated soil) 1989 0.3-339 Hagenmaier et al. (1992)
India 1987 24a 0-165 Nair & Pillai (1989)
Italy (farming area) 1971-1972 40 Leoni & D'Arca (1976)
Netherlands - Ochten 1993 - 18 5.1-66 Hendriks et al. (1995)
- Gelderse Poort 80 73-89
Netherlands 1987 - <10 <80 De Walle et al. (1995)
Sweden 5.1 Thomas et al. (1985)
USA 1968-1973 10-440 Carey et al. (1979)
USA (chemical plants) 0.002 ND-5 700 000 Spigarelli et al. (1986)
USA (hazardous waste sites) 1977-1978 20 000-400 000 Davis & Morgan (1986)
USA (5 locations near Love 0.1 1.04-5.6 0.15-26.3 Ding et al. (1992)
Canal)
a ng/g wet weight
Levels in soil are highest near industrial sources of HCB. Levels
as high as 12 600 ng/g dry weight were reported at one landfill site
in Canada (Wilson & Wan, 1982), and 570 µg/g (dry or wet weight not
indicated) on the grounds of a chlorinated solvent and pesticide
production plant in the USA (Spigarelli et al., 1986). Soils near a
former grain treatment plant in Canada contained 260 ng/g dry weight
of HCB (Wilson & Wan, 1982). Levels of HCB in soils from contaminated
floodplains in the Netherlands ranged from 5.1 to 89 ng/g dry weight
(Hendriks et al., 1995).
5.1.4 Sediment
HCB strongly sorbs to sediment and suspended matter, and
differences in the concentrations in the water as well as in the
composition of the sediments and suspended matter result in a wide
range of concentrations in this medium.
In sediment samples collected from 1979 to 1989 in the Atlantic
provinces of Canada, HCB was reported to be below the limit of
detection of 0.2 ng/g dry weight in 140 of 152 samples (Leger, 1991).
In surveys conducted from 1980 to 1983, HCB levels in sediments from
the Great Lakes ranged from 0.02 to 840 ng/g dry weight (Oliver &
Nicol, 1982; Fox et al., 1983; Kaminsky et al., 1983; Oliver &
Bourbonniere, 1985; Bourbonniere et al., 1986; Oliver et al., 1989;
IJC, 1989). Analyses of sediment cores from Lake Ontario indicated
that levels of HCB have declined from the 1960s to the early 1980s but
more recent data are not available to determine if this downward trend
has continued (Oliver & Nicol, 1982; Oliver et al., 1989). HCB levels
in sediment sampled from eight lakes in northern remote Canada (date
of sampling not specified) ranged from 0.09 to 1.80 ng/g dry weight
(Muir et al., 1995).
Levels as high as 5100 ng/g dry weight were detected in the Rhine
River in Baden-Württemberg, Germany, in 1986 (BUA, 1994). The majority
of sediment samples taken from the rivers Rhine and Elbe between 1980
and 1990 contained levels of HCB between 10 and 500 ng/g dry weight,
although levels below 1 ng/g dry weight were determined in some other
locations (BUA, 1994). A Nordic study on chlorinated compounds in the
Baltic, Kattegat and Skagerrak (œstfeldt et al., 1994) found HCB
concentrations in sediment ranging from 1 to 20 ng/g loi (loss on
ignition), the higher values occurring mainly in the Bothnian Bay. An
extreme value of 63 ng/g loi was found in Öresund between Denmark and
Sweden. Levels of HCB in sediment samples collected near effluent
discharges along a stream in Pakistan ranged from <0.05 to 94.5 ng/g
wet weight (Tehseen et al., 1994).
Higher levels of HCB in sediments were reported in studies
conducted near point sources. As much as 280 000 ng HCB/g dry weight
was detected in 1985 downstream of the Dow Chemical sewer discharges
in the St. Clair River, USA (Oliver & Pugsley, 1986).
5.1.5 Biota
HCB has been detected in invertebrates, fish, reptiles, birds and
mammals from around the world. Following the detection of HCB in
tissues of wild birds by De Vos in 1967, high residues were often
found in predatory birds, whereas minor quantities were detected in
fish, mussels and birds of the aquatic environment (Vos et al., 1968;
Koeman et al., 1969). Based on Canadian data from monitoring studies
in birds, HCB levels declined sharply from the mid-1970s (the earliest
data available) and into the early 1980s, after which they levelled
off (Noble & Elliott, 1986; Environment Canada/Department of Fisheries
and Oceans/Health and Welfare Canada, 1991).
Levels of HCB in freshwater mussels in the Great Lakes and
connecting channels have been found to range from 0.1 ng/g wet weight
to 24 ng/g wet weight (Kauss & Hamdy, 1985; Innes et al., 1988;
Muncaster et al., 1989). A similar range (4.4-26 ng/g wet weight) was
observed in benthic amphipods, the pelagic amphipod Pseudalibrotus
litoralis and brittle stars from the Beaufort Sea (Hargrave et al.,
1989). Lower levels (0.1-1.8 ng/g wet weight) were observed in mussels
( Mytilus galloprovincialis) from the Ebro Delta in the Western
Mediterranean, and these levels were observed to decline from 1980 to
1992 (Solé et al., 1994). Levels in marine species of clams and
oysters from the USA were reported in several studies to be < 1 ng/g
wet weight (Phelps et al., 1986; Eisenberg & Topping, 1984; Ray et
al., 1983b). Similarly, levels in invertebrates, including mussels
( Mytilus edulis), soft clams ( Mya arenaria), lugworms ( Arenicola
marina), and polychaetes ( Nereis diversicolor), were <1 ng/g
fresh weight in the German Wadden Sea (Ernst, 1986). Bjerk & Brevik
(1980) reported higher levels (50-350 ng/g wet weight) of HCB in crabs
( Carcinus maenas, Pagurus sp.), snails ( Littorina littorea),
brittle stars ( Ophiura albida) and sea stars ( Asteroidea) from the
contaminated Frierfjord in Norway, which receives discharge from
various industries located in the region, and HCB and related
compounds were reported to originate from one main source
(unspecified) in the area. œstfeldt et al. (1994) found that mussels
( Mytilus edulis) from the Baltic contain higher levels of HCB
(200-800 ng/g lipid weight) than mussels from Kattegat (11-20 ng/g
lipid weight).
In a 1981-1982 survey of HCB levels in fish from watersheds in
Eastern Canada, whole body concentrations in brook trout ( Salvelinus
fontinalis) and yellow perch ( Perca flavescens) ranged from below
the limit of detection (4.2 ng/g in 1981; 0.2 ng/g in 1982) to 54 ng/g
for trout and 15 ng/g wet weight for perch (Peterson & Ray, 1987).
Relatively high body burdens of HCB have been observed in fish in Lake
Ontario and connecting channels. HCB was not detected (ND) in juvenile
spottail shiners ( Notropis hudsonius) from Lakes Superior and Erie
(detection level = 1 ng/g wet weight) (Suns et al., 1983; Environment
Canada/Department of Fisheries and Oceans/Health and Welfare Canada,
1991), while mean body burdens in shiners in Lake Ontario ranged from
ND to 13 ng/g wet weight, and those in the Detroit, Niagara, and
St Clair rivers averaged 5 ng/g wet weight, ND to 8 ng/g wet weight,
and 231 ng/g wet weight, respectively (Suns et al., 1985). Mean
concentrations of HCB in the muscle tissue of various species of
salmonids from Lake Ontario ranged from 5 to 37 ng/g wet weight (Niimi
& Oliver, 1989).
Levels of HCB measured in whole fish species taken from major
rivers and lakes in the USA (including known contaminated areas)
ranged from <2 to 913 ng/g wet weight (Kuehl et al., 1983; DeVault,
1985; Schmitt et al., 1990; Kuehl & Butterworth, 1994). Levels in
roach ( Rutilus rutilus L.) and perch ( Perca fluviatilis L.) from
the "moderately polluted" Lahn River in Germany ranged from ND to
233 ng/g wet weight, with a mean of 1 ng/g (Schuler et al., 1985).
Concentrations of HCB in the whole bodies of carp ( Cyprinus carpio)
from the mouth of tributaries to Lake Ontario and the Niagara River
ranged from 52 to 1600 ng/g on a lipid basis (6.7 to 205 ng/g on a
fresh weight basis). The highest values were measured near hazardous
waste dumps and industrial facilities (as high as 1600 ng/g fat)
(Jaffe & Hites, 1986). Brunn & Manz (1982) reported a mean whole-body
concentration of HCB in fish (mainly trout) from inland rivers,
streams, and ponds in Germany of 5 ng/g wet weight. The highest levels
were recorded from fish caught in rivers.
HCB levels in seawater are generally lower than those in
freshwater, resulting in lower levels in edible parts of marine fish.
In fish taken from the North Sea (species not reported), HCB levels in
fish muscle tissues averaged 0.3-0.4 ng/g wet weight, with a maximum
of 0.8 ng/g (Ernst, 1986). HCB concentrations in livers averaged
42 ng/g wet weight for cod ( Gadus morhua) and 4 ng/g (range of
0.2-14 ng/g) for flounder ( Platichthis flesus). These levels were
comparable to levels measured in fish near the coast of southwest
Greenland and in the North Atlantic Ocean. Livers of cod from the
coast of southwest Greenland contained 32.4 ng/g on average, and those
of hake ( Merluccius merluccius) from the North Atlantic Ocean
averaged 40.5 ng/g) (Ernst, 1986). Levels of HCB were below the
determination limit (DL) in cod liver (DL = 5 ng/g) and herring muscle
(DL = 1 ng/g) of fish from the Clyde Sea near Scotland (Kelly &
Campbell, 1994). Cod from the Firth of Forth had mean liver levels of
38.7 ng/g wet weight, and levels in herring muscle of 2.0 and 2.3 ng/g
wet weight were observed in fish from the Firth of Forth and North
Sea, respectively (Kelly & Campbell, 1994). In surveillance monitoring
of contaminants in fish from coastal waters near England and Wales,
concentrations of HCB in livers of cod ( Gadus morhua), whiting
( Merlangius merlangus), dab ( Limanda limanda) and flounder
( Platichthys flesus) were 2-290, 5-230, 3-55 and 1-52 ng/g,
respectively (all results on a wet tissue weight basis) (MAFF/HSE,
1994). Levels of HCB in muscle tissues of herring ( Clupea harengus)
from the Baltic Sea ranged from <1 to 39 ng/g (Hansen et al., 1985);
concentrations in whitefish ( Coregonus lavaretus) and trout ( Salmo
trutta) ranged from <1 to 9 ng/g fresh weight in a 1992 survey
(Atuma et al., 1993).
Fish taken from the contaminated waters of the Frierfjord in
Norway contained mean concentrations of HCB in liver of 11 600 ng/g
for saithe ( Pollachius virens), and 16 800 ng/g for cod ( Gadus
morhua) (Bjerk & Brevik, 1980). Levels of HCB from fish taken from
the uncontaminated Sogndalfjord were much lower, averaging 18 ng/g wet
weight in livers of cod ( Gadus morhua), 8 ng/g in haddock
( Melanogrammus aeglefinus) and 1 ng/g in lemon sole ( Microstomus
kitt) and flounder ( Platichthys flesus) (Skåre et al., 1985).
Flounder ( Platichthis flesus) taken from the Elbe Estuary in
Germany, downstream from Hamburg (a highly industrialized area),
contained mean levels of HCB in muscle of 688 ng/g (range 84-1907 ng/g
wet weight). Further downstream, towards the mouth of the river,
levels were lower, averaging 12.5 ng/g (range 2-32 ng/g) (Kohler et
al., 1986).
The mean level of HCB in 15 snapping turtle eggs from Ontario,
Canada was 27.1 ng/g wet weight (Bishop et al., 1995).
The levels of HCB in birds have been similar across the various
regions of Canada since the 1980s, probably as a combined result of
emission reductions and the long-range transport of HCB to remote
locations. Mean concentrations of HCB in herring gull eggs ( Larus
argentatus) in 1991 ranged from 16 to 71 ng/g wet weight at various
colonies in the Great Lakes, and were relatively uniform across lakes
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991). These levels were approximately an order of
magnitude lower than in 1974. The mean level of HCB in herring gull
eggs from Norwegian coastal waters in 1981 was 120 ng/g wet weight
(Moksnes & Norheim, 1986). In a study from the Netherlands, mean
levels in eggs of common terns collected in 1987 were 0.03 µg/g wet
weight and in those of black-headed gulls collected in 1988 were
93 µg/g fat (Stronkhorst et al., 1993). Levels of HCB found in eggs of
sea-bird species ( Haematopus ostralegus, Larus ridibundus, Larus
argentatus and Sterna hirundo) from the banks of a river near an
organochlorine chemical plant in Germany were < 500 ng/g wet weight
(Heidmann, 1986); mean levels of less than 15 ng/g wet weight were
found in eggs of several species of land birds, including rooks
( Corvus frugilerus) and sparrow hawks ( Accipiter nisus) from
agricultural, industrial and rural sites. Recent surveys have
indicated similar levels of HCB in the eggs of five other predatory
bird species across Canada (means ranged from 10 to 53 ng/g wet
weight) (Noble & Elliott, 1986; Pearce et al., 1989; Noble et al.,
1992). However, the mean level of HCB in peregrine falcon ( Falco
peregrinus) eggs collected across Canada from 1980 to 1987 was
279 ng/g wet weight, and concentrations ranged as high as 1060 ng/g
wet weight (Peakall et al., 1990).
HCB has been found to accumulate in lipids of the common
goldeneye duck ( Bucephala clangula) that overwinter in the Niagara
River (mean of 150 ng/g) (Foley & Batcheller, 1988) and the Detroit
River (mean of 1700 ng/g) (Smith et al., 1985a) in the USA. Goldeneye
wintering in the Baltic Sea contained average levels of 250 ng/g lipid
(Falandysz & Szefer, 1982). Levels of HCB in the livers of silver
seagulls taken from estuaries in Germany were lower in 1988 than 1989
(approximately 80 and 150 ng/g fat, respectively, in samples from the
River Ems estuary). Higher levels were observed for both years in
liver samples of birds taken from the River Elbe estuary (>250 ng/g
fat) (BUA 1994).
In breast muscle tissue samples from various species of birds,
HCB concentrations tend to be progressively greater at higher trophic
levels (i.e., piscivores > molluscivores > omnivores > grazers)
(Environment Canada/Department of Fisheries and Oceans/Health and
Welfare Canada, 1991).
In the blubber of marine mammals in the Canadian Arctic, mean
levels of HCB were 19 ng/g wet weight for ringed seals ( Phoca
hispida) and 491 ng/g wet weight for beluga whales ( Delphinapterus
leucas) (Norstrom et al., 1990), while male belugas sampled in the
Gulf of St. Lawrence contained up to 1340 ng/g (Béland et al., 1991).
Blubber from male and female white-beaked dolphins ( Lagerorhunchus
albirostris) collected near the Newfoundland coast averaged
1110 ng/g and 880 ng/g wet weight, respectively. Lower levels
(290 ng/g and 100 ng/g wet weight) were observed in blubber from male
and female pilot whales ( Globicephala meleana), also collected near
the Newfoundland coast (Muir et al., 1988). The higher levels observed
in the dolphins may reflect greater exposure to HCB because of
overwintering and feeding in the Gulf of St. Lawrence. Blubber of
harbour porpoises ( Phocoena phocoena) collected in Poland between
1989 and 1990 contained an average of 573 ng/g wet weight (Kannan et
al., 1993), and those collected around the coast of Scotland between
1989 and 1991 contained an average of 263 ng/g (Wells et al., 1994).
Levels of HCB in the blubber of bottlenosed dolphins also collected
off the coast of Scotland contained an average of 276 ng/g (Wells et
al., 1994). Levels in the blubber of three species of dolphins from
the Bay of Bengal, southern India, were low, ranging from 1.1 to
13 ng/g wet weight (Tanabe et al., 1993). Harbour seals ( Phoca
vitulina) found sick or dead in Norwegian waters due to a disease
outbreak caused by a morbilli virus had a mean HCB level in the
blubber of 27 ng/g wet weight (range of 5-94 ng/g) (Skaare et al.,
1990).
Limited data were found on levels of HCB in terrestrial mammals.
In a 1973-1974 survey of HCB in the adipose tissue of fox ( Vulpes
vulpes), doe ( Capreolus capreolus) and wild boar ( Sus scrofa) in
Germany, HCB concentrations ranged from <10 to 3110 ng/g. The lowest
levels were observed in the does, presumably because they are
herbivorous, whereas foxes and wild boar feed on small animals and are
therefore more affected by biomagnification of HCB (Koss & Manz,
1976). Similar patterns were evident in a study from Sweden, in which
rabbits ( Oryctolagus cuniculus, muscle), moose ( Alcaes alcaes,
muscle), reindeer ( Rangifer tarandus, suet) and osprey ( Pandion
haliaetus, muscle) were found to contain 9, 15, 51 and 330 ng HCB/g
lipid weight, respectively (Jansson et al., 1993). The mean
concentration in 66 serum samples taken in muskoxen in the Canadian
Northwest Territories in 1989 was 2.8 ng/g (range of 1.1-7.5 ng/g)
(Salisbury et al., 1992). The mean concentration of HCB in fat samples
from 58 caribou from the same region ranged from 32.93 to 129.4 ng/g
(lipid corrected) (Elkin & Bethke, 1995). The mean concentration of
HCB in the livers and lipids of adult river otters ( Lutra
canadensis) in western Canada were 3 ng/g and 30 ng/g wet weight,
respectively, for females and 4 ng/g and 25 ng/g wet weight,
respectively, for males (Somers et al., 1987). Concentrations of HCB
in mink carcasses collected in Ontario in the late 1970s and early
1980s ranged from < 0.5 to 10 ng/g wet weight (Proulx et al., 1987).
In the Canadian north, the mean level of HCB in the fat of polar bears
( Ursus maritimus) hunted between 1982 and 1984 was 296 ng/g wet
weight (Norstrom et al., 1990).
5.1.6 Food and drinking-water
HCB is commonly detected, at low levels, in food (Table 7).
Levels of HCB tend to be highest in fatty foods and/or those that have
been treated with HCB-contaminated pesticides. The most extensive data
identified have been collected through the United States Food and Drug
Administration (US FDA) Total Diet Study. The results of the surveys
from 1982 to 1991 indicate that HCB is detectable (DL = 0.1 ng/g) in a
small fraction of food items, most often dairy products, meats, and
peanuts/peanut butter (KAN-DO Office and Pesticides Team, 1995). In
the most recent surveys, conducted during 1990-1991, mean levels were
less than 1 ng/g for all products.
Table 7. Concentration (µg/kg wet weight unless otherwise specified) of hexachlorobenzene in various foods
Country Food Mean contenta Range Reference
Australia cereals 0.01 < 0.01-0.01 Kannan et al. (1994)
pulses 0.02 0.01-0.05
oils 0.07 0.02-0.11
beverages 0.03 0.02-0.04
vegetables 0.01 < 0.01-0.02
fruits 0.01 < 0.01-0.02
dairy products 0.55 0.14-1.6
meat and fat 0.46 0.01-3.0
fishes 4.2 < 0.01-60
Canada fresh meat & eggs 0.17 Davies (1988)b
root vegetables & potato 0.04
fresh fruit ND(<0.01)
leafy/other above-ground vegetables 0.02
2% milk 0.16
Canada apples ND(<0.2)-2.6 OMAF/OME (1988)
peaches ND(<0.2)
tomatoes ND(<0.2)
potatoes ND(<0.2)
wheat ND(<0.2)
eggs ND(<0.2)
hamburger 0.39 0.2-0.57
prime beef ND(<0.2)-0.21
pork ND(<0.2)
chicken ND(<0.2)
Table 7 contd.
Country Food Mean contenta Range Reference
Germany milk 0.22d 0.088-0.45d Fürst et al. (1992)
cream 0.98d 0.31-1.30d
butter 4.86d 2.32-6.88d
cheese 2.72d 2.16-3.70d
India cereals 0.03 0.01-0.04 Kannan et al. (1992a)
pulses (edible seeds of legumes) 0.07 0.02-0.16
spices 0.22 <0.01-0.54
oils 1.5 0.09-2.8
milk 0.03 0.01-0.10
butter 1.7 0.86-2.4
fishes & prawn 0.07 <0.01-0.55
meat & animal fat 0.61 0.02-4.8
Mexico cheese 16.67d 1 Albert et al. (1990)
Morocco eggs 20.9 0.09-300 Kessabi et al. (1990)
poultry liver 5.1 trace-30.0
bovine liver 21.9 1.2-119.8
bovine kidney 15.1 trace-133.0
Papua New cheese 0.43 Kannan et al. (1994)
Guinea pork fat 0.40
chicken 0.20
striped mullet 0.04
tilapia 0.01 0.02-0.05
mud crab 0.03 < 0.01-0.02
oyster 0.02 < 0.01-0.05
Table 7 contd.
Country Food Mean contenta Range Reference
Solomon Islands pork 0.14
chicken 0.06
greenspotted kingfish 0.03 0.01-0.06 Kannan et al. (1994)
indian mackerel 0.01 0.01
paddletail snapper 0.01 0.01
Southern Baltic canned cod-livers 60 ± 6 50-76 Falandysz et al. (1993)
Spain bologna - fresh 2.57d Ariño et al. (1992)
- cooked 2.48d
Spain pork sausage Ariño et al. (1992)
- before curing 6.63d
- after 30 days curing 6.0d
Spain ham - fresh 3.46d Ariño et al. (1992)
- cured 1.29d
Spain pork 2.86-3.9d Ariño et al. (1993)
Spain lamb - chop, raw 14.67d Conchello et al. (1993)
- chop, grilled 12.06d
- leg, raw 8.53d
- leg, roasted 7.02d
Spain chicken 120 ± 10 To-Figueras et al. (1986)
calf 249 ± 37
rabbit 860 ± 159
pork 169 ± 20
sheep 225 ± 35
butter 315 ± 18
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom bread ND (10) MAFF/HSE (1994)
milk 0.6
butter ND(10)
cheese 3.33d
ewes' cheese ND(10)
pasta ND(10)
beef burgers ND(10)
canned meat 10d
cooked meats 10d
lamb ND(10)
rabbit ND(10)
salami ND(10)
United Kingdom sausages ND(10) MAFF/HSE (1994)
pies and pasties ND(10)
salmon (tinned) 2.0
breaded cod ND(2.0)
fish cakes 2.0
mackerel 20
plaice ND(2.0)
prawn products ND(2.0)
sardines (tinned) ND(2.0)
Table 7 contd.
Country Food Mean contenta Range Reference
United Kingdom carrot 0.0317 Wang & Jones (1994)
potato 3.35
cabbage 0.0418
cauliflower 0.0729
lettuce 0.108
onion 0.0014
bean 0.0101
pea 0.0039
tomato 0.0139
USA cheese, processed 0.2 ND-0.5 US FDA
cheese, cheddar 0.1 ND-0.5 (unpublished)c
beef, ground (regular) 0.1 ND-0.4
beef, chuck roast 0.3 ND-1.0
beef, round steak 0.2 ND-1.0
beef, loin/sirloin steak 0.2 ND-1.0
USA lamb chop 0.3 ND-1.0 US FDA
frankfurters 0.1 ND-0.6 (unpublished)c
cod/haddock fillet ND(0.1) ND-0.2
eggs, scrambled 0.1 ND-0.3
eggs, fried 0.2 ND-0.7
peanut butter 0.2 ND-0.4
peanuts, dry roasted 0.3 ND-1.0
watermelon 0.1 ND-0.5
butter 0.6 ND-1.0
cream 0.1 ND-0.4
Table 7 contd.
Country Food Mean contenta Range Reference
Viet Nam rice 0.03 <0.01-0.05 Kannan et al. (1992b)
pulses 0.04 <0.01-0.18
oil 1.2
butter 5.0
animal fat 0.41 0.29-0.65
meat 0.11 0.03-0.18
fish 0.05 0.01-0.31
prawn 0.03
shellfish 0.04
crab 0.17
caviar 3.8 1.9-7.2
a ND = not detected (detection limit given in brackets).
b Fresh produce and meats grown in Ontario were purchased from four grocery stores in Toronto when locally grown produce
was available (Ontario freshwater fish were not available and therefore, were excluded from analysis). All food items
were grouped into one of five composites for analysis, with the relative proportions of different food items in each
composite calculated from the estimates of the amounts purchased per person per year by Ontario residents.
c The US Food and Drug Administration Total Diet Study conducted from April 1990 to April 1991; reporting residue levels
in 234 individual food items collected from 3 cities in each of 4 geographical regions of the USA (data available from
US FDA, Washington, DC).
d Originally reported on a fat basis, and subsequently converted to wet weight using percentage fat contents reported in
NHW (1987).
In a number of more limited recent surveys, HCB levels have been
determined in commercial foods available in several countries from
North America, Europe and Asia (Table 7). The results of these studies
are consistent with the USA study described above, in that HCB has
been detected primarily in fatty foods such as meats and dairy
products. In these studies, mean concentrations are generally in the
low ng/g range or less, although substantially higher concentrations
have been reported in some surveys from Europe and Asia.
The effects of cooking, curing and ripening on the HCB residues
in pork meat products were investigated in Spain by Ariño et al.
(1992). Neither cooking at 80-82°C for 100 min nor curing reduced the
HCB content in pork bologna and pork sausage, respectively, whereas
the level of HCB in dry-salted and cured ham declined by 42%
throughout maturation.
HCB has been detected infrequently, and at very low
concentrations in drinking-water supplies (Table 5). Samples of
drinking-water collected in 1980 from Canadian cities in the vicinity
of Lake Ontario contained from 0.06 to 0.20 ng/litre, with a mean of
0.1 ng/litre (Oliver & Nicol, 1982). In other Canadian and USA
surveys, HCB was not detected (US EPA 1985b - DL = 100 ng/litre;
Environment Canada, 1989 - DL = 2 ng/litre). Slightly higher
concentrations of HCB (median of 1-2 ng/litre) were reported in
Croatian drinking-water supplies drawn from a nearby polluted river
(pollution sources were not identified) (Fingler et al., 1992).
5.2 General population exposure
5.2.1 Human tissues and fluids
Owing to its persistence and lipophilicity, HCB is present at low
levels in the fatty tissues of virtually all members of the general
population. Levels of HCB in adipose tissues, breast milk, blood and
follicular fluid of various populations from around the world are
shown in Table 8. It should be noted that the quality of the studies
given in Table 8 varies quite widely, from extensive national surveys
to those with relatively few samples.
Levels of HCB in human adipose tissue from around the world are
generally <1 mg/kg (Table 8). Although available data are limited,
concentrations of HCB reported in fat tissue are generally slightly
higher in samples from European countries than from elsewhere in the
world. The highest levels reported in recent surveys are from Spain
(mean levels of approximately 3-6 mg/kg); the authors suggested that
this reflected contamination of foods caused by its presence as an
impurity in other pesticides (Camps et al., 1989; Gómez-Catalán et
al., 1993, 1995). Concentrations of HCB increased with age in a number
of these surveys, but there were no consistent differences in residue
levels between the sexes (Mes et al., 1982; Williams et al., 1984,
1988; Abbott et al., 1985; Mes, 1990; Mes et al., 1990; Gomez-Catalan
et al., 1993; Kemper, 1993; Ludwicki & Góralczyk, 1994).
In general, concentrations of HCB in breast milk in various
countries or regions (Table 8) range widely, and appear to be related
to the degree of industrialization and/or urbanization within the
survey area. The levels of HCB in breast milk have been expressed on a
whole milk basis, using the fat content reported by the authors or,
where this was not reported, a fat content of 4.2% (NHW, 1987).
Schechter et al. (1989a) reported that concentrations of HCB in breast
milk in the mid-1980s were lowest in samples from Thailand
(0.3 µg/litre whole milk) and Viet Nam (< 0.17 µg/litre ), somewhat
higher in those from a semi-rural area of the USA (0.7-0.8 µg/litre),
and higher still in German samples (12.6 µg/litre ) (numbers of
samples in this study were extremely small, except for the German data
(n=167)). In surveys summarized by Mes et al. (1986), mean HCB levels
were 1 µg/litre whole milk in the USA, 2 µg/litre in Canada,
3 µg/litre in Sweden, 4 µg/litre in Great Britain, and 35µg/litre in
Germany. Still higher levels (48-89 µg/litre whole milk) have been
reported in studies from Spain (Conde et al. 1993). Bates et al.
(1994) reported that the concentrations of HCB in breast milk of
primiparae from New Zealand increased linearly with age, but were not
related to body mass index, fish intake, smoking status, type of
residential water supply or location of residence (urban versus
rural). In a study of body burdens of organochlorines in an indigenous
population, Ayotte et al. (1995) reported that mean concentrations of
HCB in the milk fat of 107 Inuit women from northern Quebec were
several times higher than those in 50 Caucasian women from southern
Quebec (57 and 1.2 µg/litre whole milk, respectively). Levels of
organochlorine compounds in breast milk correlated with levels of
omega-3 fatty acids in plasma phospholipids, indicating that
consumption of marine organisms is an important source of exposure to
these xenobiotics.
In a HCB poisoning incident in Turkey (section 8.1), breast-fed
infants were fatally intoxicated through their mothers' milk. In an
early report of this incident (Peters et al., 1966), HCB was reported
as being present in breast milk, although it was not quantified.
However, elevated levels were measured (mean of 510 ng/g on a fat
basis (approximately 21 ng/g on a wet weight basis) for 56 porphyric
mothers) 20-30 years after the incident, compared with a mean of
70 ng/g fat in 77 milk samples from women of families without
porphyria or from areas outside of the endemic area (Peters et al.,
1982; Gocmen et al., 1989).
Table 8. Levels of hexachlorobenzene in human tissues and fluids
(mg/kg wet weight adipose tissue; mg/kg whole milk; µg/litre blood serum; µg/litre follicular fluid)
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue
Australia 31 0.14a (0.01-1.70) (fat basis) 1990-1991 Stevens et al. (1993)
Canada 108 0.026a (0.0073-0.118) 1985 Mes & Malcolm (1992),
Mes et al. (1990)
Canada 25 0.019a (max. value: 0.087) - Mes (1992)
Canada 141 0.071 (males)
(0.018-0.244)
0.109 (females)
(0.019-0.373) 1984 Williams et al. (1988)
Canada 99 0.095 (0.01-0.667) 1976 Mes et al. (1982)
Canada 168 0.062 (0.001-0.52) 1972 Mes et al. (1977)
Federal Republic of Germany 93 (0.11-21.8) 1971 Leoni & D'Arca (1976)
Federal Republic of Germany 6 0.263 (0.083-0.753) van der Ven et al. (1992)
India 7 0.012 (0-0.064) 1987 Nair & Pillai (1989)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Italy 28 0.491 (0.126-1.36) 1973-1974 Leoni & D'Arca (1976)
Japan 39 0.044 1986-1987 Kashimoto et al. (1989)
Japan 15 0.21 (0.10-0.42) - Morita et al. (1975)
Netherlands average 0.7a (fat basis) 1968-1969 Greve & Van Zoonen (1990)
of 1.2a (fat basis) 1973-1975
51/year 0.86a (fat basis) 1976
0.98a (fat basis) 1977-1978
0.85a (fat basis) 1980
0.80a (fat basis) 1981
0.58a (fat basis) 1982
0.49a (fat basis) 1983
0.42a (fat basis) 1985
0.38a (fat basis) 1986
New Zealand - 0.31 - US EPA (1985a)
Poland 53 0.221 (0.068-0.937) early 1980s Szymczyœski et al. (1986)
Poland 277 0.31 1989-1992 Ludwicki & Góralczyk (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
Spain 256 2.99 1985-1988 Gómez-Catalán et al. (1993)
(4 cities)
Spain 86 3.37 (0.42-12.53) 1991 Gómez-Catalán et al. (1995)
(lipid basis)
Spain 171 5.55 1982-1983 To-Figueras et al. (1986)
Spain 168 2.95 (0.2-17.37) 1988-89 Ferrer et al. (1992)
United Republic of Tanzania 9 0.003 (0.0013-0.0076) - van der Ven et al. (1992
United Kingdom 201 0.05 (n.d-0.29) 1969-1971 Abbott et al. (1972)
United Kingdom 236 0.19 (0.02-3.2) 1976-1977 Abbott et al. (1981)
United Kingdom 187 0.11 (0.03-0.32) 1982-1983 Abbott et al. (1985)
USA 10 0.125 (0.03-0.47 - Barquet et al. (1981)
USA 6081 0.037a 1974-1983 Robinson et al. (1990)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
A. Adipose tissue (contd)
USA 763 0.118 (0.001-0.256) 1982 US EPA (1994)
689 0.043 (0.032-0.054) 1984
671 0.051 (0.043-0.059) 1986
B. Breast milkb
Australia 39 0.042 (rural) 1970 Newton & Greene (1972)
28 0.063 (urban)
Australia 137 0.007 (0.002-0.019) (rural) 1979-1980 Stacey et al. (1985)
130 0.008 (0.002-0.017) (urban)
Australia 60 0.017 (0.0007-0.32) - Quinsey et al. (1995)
Australia 128 0.0036a (<0.01-0.216) 1990-1991 Stevens et al. (1993)
Brazil 30 0.00048 (0.00024-0.0036)c 1987-1988 Beretta & Dick (1994)
Canada 412 0.0008 (max = 0.014) 1986 Mes et al. (1993)
Canada 210 0.002 (max.= 0.009) 1982 Mes et al. (1986)
Canada 100 0.002 (max.= 0.021) 1975 Mes & Davies (1979)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Canada 536 0.0013c 1989-1990 Dewailly et al. (1991)
Canada 127 0.00051 1978 Frank et al. (1988)
15 0.0004 1979
12 0.00028 1980-1981
13 0.00052 1983-1984
18 0.00026 1985
Finland 143c 0.002c 1984-1985 Mussalo-Rauhamaa et al. (1988)
Finland 50 0.0023 (0.0007-0.006) 1982 Wickström et al. (1983)
France 20 0.002 (0.00004-0.008)c 1990-1991 Bordet et al. (1993)
Federal Republic of Germany 144 0.021c 1984 Fürst et al. (1994)
220 0.019c 1985
157 0.015c 1986
144 0.015c 1987
196 0.013c 1988
145 0.01c 1989
286 0.0095c 1990
113 0.0074c 1991
Federal Republic of 167 0.0126c 1985-1987 Schecter et al. (1989a)
Germany
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Federal Republic of 2709 0.048c 1979-1981 BUA (1994)
Germany 3778 0.013c 1986
1897 0.014c 1987
2994 0.011c 1988
3256 0.01c 1989
5340 0.009c 1990
Former German 483 0.007c 1990-1991
Democratic Republic
India 16 0.042 (0-0.25)e 1987 Nair & Pillai (1989)
Israel 100 0.00256 - Weisenberg et al. (1985)
Italy 56 0.058d - Franchi & Focardi (1991)
Italy 64 0.006 (0.004-0.009) 1987 Larsen et al. (1994)
Netherlands 202 0.036a,c 1972-1973 Greve & Van Zoonen (1990)
278 0.008a,c 1983
New Zealand 38 0.0011 1988 Bates et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
Norway 28 0.0007 1991 Johansen et al. (1994)
Spain 240 0.089(0.039-0.21)c 1984-1987 Conde et al. (1993)
358 0.048(0.037-0.073)c 1990-1991
Sweden 20 0.0042 (0.002-0.009)c 1978 Norén (1983a)
Sweden 2 0.0007-0.004c - Norén (1983b)
Sweden 227 0.003 (0.002-0.004)c 1972 Norén (1988)
245 0.003 (0.003-0.004)c 1976
340 0.003 (0.003-0.004)c 1980
102 0.001 (0.0008-0.001)c 1984-1985
Sweden 140 0.0012c 1989 Norén (1993)
Sweden 40 0.0017c 1986-1987 Vaz et al. (1993)
Thailand 3 0.0003c 1985-1987 Schecter et al. (1989a)
Turkey 51 0.0035c 1988 Üstünbas et al. (1994)
Turkey 56 0.021c 20-30 years Gocmen et al. (1989)
post-exposure
during
1955-1959
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
B. Breast milkb (contd)
United Kingdom 193 0.001 (<0.001-0.005) 1989-1991 MAFF (1992)
USA 40 0.00052 1979 Bush et al. (1985)
USA 8 0.0007-0.0008c 1985-1987 Schecter et al. (1989a)
Viet Nam 12 <0.00017c 1985-1987 Schecter et al. (1989a)
Yugoslavia 10 0.006 (0.002-0.017) 1978 Kodric-Smit et al. (1980)
C. Serum
Canada 25 0.25 1993 Jarrel et al. (1993)
29 0.21
20 0.35
Croatia 15 1a (<0.5-4) 1985 Krauthacker (1993)
24 0.9a (<0.5-3) 1987-1988
26 1a (<0.5-7) 1989-1990
32 <0.5a (<0.5-4) 1990
Germany 6 1.23 (0.33-2.66)f - van der Ven et al. (1992)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
C. Serum (contd)
Spain (near 21 26 (7.5-69) 1992 Grimalt et al. (1994)
organochlorine
compounds
factory)
Spain (hospital 13 4.8 (1.5-15) 1992 Grimalt et al. (1994)
in Barcelona)
Spain 100 11.09 (1.60-94.2) 1986 To-Figueras et al. (1995)
4.13 (0.70-19.7) 1993-1994
United Republic 11 0.01 (0-0.03)f - van der Ven et al. (1992)
of Tanzania
USA 370 0.189a (0.05-3.21) - Needham et al. (1990)
D. Whole blood
Slovakia 50 25.2 (6.1-43.2) 1992 Koœan et al. (1994)
Table 8 contd.
Country Sample Mean tissue concentration Year Reference
size (range)
E. Follicular fluid
Canada 25 0.11 1993 Jarrell et al. (1993b)
29 0.14
20 0.20
Federal Republic 15 2.59 (1.1-5.7) - Trapp et al. (1984)
of Germany
a Median value.
b The most recent data were used for calculation of intakes via breast milk (section 5.2.4).
c Originally expressed as mg/kg milk fat and subsequently converted to a wet weight basis using either the % fat reported,
or if not given, using 4.2% fat (NHW, 1987).
d Number of positive samples
e Originally expressed on a dry weight basis and subsequently converted to wet weight using 88% moisture for conversion
(NHW, 1987).
f Values reported in µg/kg.
HCB is present in a wide range of other tissues and fluids from
humans, but at lower levels than in adipose tissue and breast milk.
For example, concentrations of HCB in serum of 370 subjects from the
general population in the USA studied by Needham et al. (1990)
averaged 0.189 µg/litre, compared to concentrations of 39 ng/g lipid
in 287 adipose tissue samples. Schechter et al. (1989b) reported HCB
levels in various organs in autopsy tissue from three American
patients. HCB levels in adipose tissue ranged from 15 to 24 ng/g wet
tissue, while kidney, muscle, lung, spleen and testis contained 1 ng/g
or less, and adrenals, bone marrow and liver contained intermediate
concentrations.
Median blood serum levels of HCB ranged from <0.5 to
1.0 µg/litre in four population groups (total of 97 samples) from
Zagreb, Croatia (Krauthacker, 1993). The groups included workers
employed in the distribution and packing of seeds treated with
different pesticides, who were expected to have absorbed
organochlorine compounds at levels greater than the general
population. However, levels of HCB in the blood of these workers were
not elevated. Van der Ven et al. (1992) reported the HCB levels in
maternal serum of 6 and 11 full-term pregnant women in Germany and
Tanzania, respectively. Levels in Germany averaged 1.23 µg/kg (0.33-
2.66 µg/kg), whereas those in Tanzania were 0.01 µg/kg (0-0.03 µg/kg).
Analysis of blood plasma samples from a human organ specimen bank in
Germany revealed that median annual concentrations of HCB between 1983
and 1989 ranged between 3.1 and 5.4 µg/litre (Kemper, 1993). Serum and
ovarian follicular fluid have been shown to contain HCB in patients
receiving in vitro fertilization (Jarrell et al., 1993b). Of 72
patients, HCB was detected in the serum of 60 and follicular fluid of
49. There was a significant geographical variation among three major
cities in Canada (Jarrell et al., 1993b). Follicular fluid from
similarly treated patients in Germany has been shown to contain HCB
(Trapp et al., 1994). In some studies, workers exposed to chlorinated
solvents or chlorinated pesticides had elevated levels of HCB in blood
(section 5.2.7).
5.2.2 Intake from ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the range
of mean levels of HCB measured in ambient air in cities from around
the world of approximately 0.1 to 0.6 ng/m3 (Table 4), mean intake of
HCB from ambient air for the general population is estimated to range
from 3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day. Since no
data on levels of HCB in indoor air were found, it has been assumed
that levels indoors are the same as those outdoors. The intake of HCB
via air may be greater in populations residing in the vicinity of
point sources, but this exposure is considered to be too site-specific
to estimate reliably.
5.2.3 Intake from drinking-water
Based on a daily volume of ingestion for adults of 1.4 litres, a
mean body weight for males and females of 64 kg (IPCS, 1994), and the
range of mean concentrations of HCB detected in drinking-water from
cities of approximately 0.1 to 2 ng/litre (Table 5), the estimated
mean daily intake of HCB from drinking water for the general
population ranges from approximately 2.2 × 10-6 to 4.4 × 10-5 µg/kg
body weight per day.
5.2.4 Intake from foods
Based on the average daily consumption of various foodstuffs by
adults from around the worlda, a mean body weight for males and
females of 64 kg (IPCS, 1994), and the mean level of HCB detected in
various foods in the 1990-1991 US FDA Total Diet Study (Table 7), the
estimated daily intake of HCB from food ranges from approximately
0.0004 to 0.0028 µg/kg body weight per day (this range was generated
by assuming, for food groups in which HCB was not detected, that non-
detectable values were zero with the detection limit being 0.1 µg/kg.
These estimates overlap the range of dietary estimates (between 0.001
and 0.027 µg/kg body weight per day) that have been reported for
various countries (Canada, USA., Germany, Finland, Viet Nam, Thailand,
India, Japan, Australia, the Netherlands) (Gartrell et al., 1986; De
Walle et al., 1995; Fujita & Morikawa, 1992; Kannan et al., 1992a,b;
Government of Canada, 1993; Kannan et al., 1994). Intakes via food may
be substantially higher in selected European and Asian countries,
where the content of HCB in a sampling of a limited range of foods was
relatively high (Table 7), or in indigenous populations consuming
large quantities of some wildlife species, such as marine mammals,
that are known to accumulate relatively high tissue levels of
lipophilic contaminants (Government of Canada, 1993; Ayotte et al.,
1995; Kuhnlein et al., 1995).
a Dietary intakes (g/person/day) consist of: cereals, 323, starchy
roots, 225; sugar (excludes syrups and honey), 72; pulses and
nuts, 33; vegetables and fruits, 325; meat, 125; eggs, 19; fish,
23; milk products (excludes butter), 360; fats and oils (includes
butter), 31 (all intakes from IPCS, 1994).
Dietary intakes may also be greater in infants during breast-
feeding, owing to the accumulation of HCB in the mothers' milk. The
mean concentrations of HCB in the most recent surveys found for
various countries range from <0.17 to 48 µg HCB/litre whole milk
(Table 8). Assuming that infants are exclusively breast fed for the
first 6 months, during which they consume an average of 0.75 litres of
breast milk per day and have an average body weight of 7 kg (Health
Canada, 1994), the estimated mean intakes of HCB from breast milk in
various countries range from <0.018 to 5.1 µg/kg body weight per day.
Daily intakes of HCB by breast-feeding infants of Inuit mothers in
northern Quebec, Canada (a population that consumes substantial
quantities of marine organisms that accumulate lipophilic
contaminants) was estimated at 0.45 µg/kg body weight per day, a value
that was several times greater than for a more southerly population in
the same province (Ayotte et al., 1995).
5.2.5 Apportionment of intakes
Total intake of HCB from ambient air, drinking-water and foods is
estimated to range from approximately 0.0004 to 0.003 µg/kg body
weight per day for the general population, the principal route of
exposure being through the diet (92%). The estimated contributions
from air and drinking-water are much smaller (7% and 1%,
respectively). (The contribution from each environmental medium was
calculated based on the mid-point of the intakes estimated in the
previous sections.)
5.2.6 Trends in exposure of the general population over time
The results of most studies of the levels of HCB in foods and
human tissues over time indicate that exposure of the general
population to HCB declined from the 1970s to the mid-1990s in many
locations. However, this trend has not been evident during the last
decade in some other locations.
Routine monitoring of foods in some countries indicates that
exposure to HCB is decreasing. For example, mean concentrations in
grab samples of milk, bovine fat, poultry fat and egg fat collected
from suppliers in Ontario, Canada, decreased by an order of magnitude
or more between the early 1970s and the mid-1980s (Frank et al., 1983,
1985a, 1985b; Frank & Ripley, 1990). Brown et al. (1986) reported that
the frequency of detectable (> 10 ng/g in fat samples, wet weight)
levels of HCB in the USA meat and poultry supply increased
dramatically from 1972 to 1977-1978, but had fallen off sharply up to
1984. More recent data collected through the US FDA Total Diet Study
indicate that this trend had continued. Between 1982-1984 and 1991,
the most recent year for which data are available, both the frequency
of detection of HCB and the estimated average daily intake for people
of various ages decreased by roughly 80% (US FDA 1990, 1991, 1992). A
decline in HCB levels in fish from the Baltic and the Swedish West
Coast was found in the National Swedish Monitoring Programme over the
period 1988 to 1994 (Bignert, 1995). The annual decrease in levels in
herring muscle from different places in the Baltic was 12 to 15% and
in cod liver from the Baltic 21%; from the West Coast it was 12% in
herring muscle, 23% in cod liver and 23% in dab liver. The trend with
higher concentrations in samples from the Baltic as compared to the
West Coast is still seen in the samples.
The results of most studies of temporal trends of HCB levels in
human adipose tissue or milk (summarized in Table 8) indicate that
general population exposures have declined since the 1970s. In routine
monitoring of breast milk contaminants in German mothers, mean
concentrations of HCB declined by more than 50% between 1984 and 1991
(Fürst et al., 1994), and by about 80% between 1979 and 1990 (BUA
1994). The median HCB content in samples of plasma from a human
specimen bank in Germany decreased from 4.8 µg/litre in 1983 to
3.1 µg/litre in 1989, a period of increasing restrictions on indoor
applications of pentachlorophenol, which contains HCB as a contaminant
(Kemper, 1993). Mes (1990) reported that concentrations of HCB in
human adipose tissue from Canadian surveys were significantly lower in
1985 than in 1972; this decrease occurred in all age classes over this
period. An increase in the HCB content of human adipose tissue and
milk was observed in the early 1970s in the Netherlands, and was
attributed to an increase in the HCB concentration in products of
animal origin (Greve & Van Zoonen, 1990). Once measures were taken to
avoid contamination of such products, a gradual decrease in HCB levels
was observed. Johansen et al. (1994) reported that the concentration
of HCB in routine monitoring of milk from Norwegian mothers declined
by 65% between 1982 and 1991. In contrast, in the most extensive study
of levels of HCB in adipose tissues, the US National Human Adipose
Tissue Survey (Robinson et al., 1990), in which data on residues were
collected from a nationally representative sample of 6081 autopsies
and surgical patients from 1974-1983, there was little change in
residue concentrations over the study period, with the national median
level remaining near 30 to 40 ng/g.
5.2.7 Occupational exposure during manufacture, formulation, or use
Workers may be exposed to higher concentrations of HCB than the
general population, particularly in the manufacture of chlorinated
solvents, and in the manufacture and application of pesticides
contaminated with HCB.
In a survey of production industries (perchloroethylene,
trichloroethylene, carbon tetrachloride, chlorine, triazine herbicides
and pentachloronitrobenzene), the highest HCB concentrations were
associated with the production of perchloroethylene and
trichloroethylene (Spigarelli et al., 1986). The highest level of HCB
determined in the air on plant property was 24 µg/m3 at a plant
producing perchloroethylene, carbon tetrachloride and chlorine.
Relatively high HCB levels (maximum concentration of 2.2 µg/m3 in
air) were also detected in samples from the pentachloronitrobenzene
production plant. Lower levels of HCB were measured at triazine
herbicide production plants (ND - 0.02 µg/m3), and, in the one plant
that produced only carbon tetrachloride, HCB was not detected (MDL not
reported). It is not known how representative the data from these
studies are, as the generation and release of HCB would be minimized
in plants using appropriate modern technology and waste management
practices.
Personal breathing-zone samples (54 in all) from workers in a
pentachlorophenol production plant contained HCB concentrations
ranging from <0.1 to 120 µg/m3 (Marlow, 1986), while levels in 112
area samples throughout the plant ranged from <0.1 to 630 µg/m3.
HCB concentrations in the blood of workers in a factory producing
chlorinated solvents ranged from 14 to 233 µg/litre (Burns & Miller,
1975); this compared with a range <1 to 310 µg/litre in the blood of
vegetable spraymen (Burns et al., 1974). Mean levels of HCB in the
blood plasma of workers in a chlorinated solvents plant in the USA
were 311 µg/litre in 1974, and 312 µg/litre in 1975, and levels in
whole blood were 160 µg/litre in 1976, and 170 µg/litre in 1977
(Currier et al., 1980). Concentrations of HCB in blood were positively
correlated with the number of years worked in the plant, but were not
associated with airborne levels of HCB or job-category-based exposure
estimates. Pesticide-exposed vineyard workers in Germany tended to
have higher HCB whole blood levels (median 7 µg/litre, maximum
30 µg/litre) than reference controls (median 3 µg/litre, maximum
17 µg/litre) (Kemper, 1993). Angerer et al. (1992) reported that the
mean plasma level of HCB in 53 workers at a municipal waste
incinerator was 5.0 µg/litre, compared with 4.69 µg/litre in 64
subjects with no known occupational contact.
6. KINETICS AND METABOLISM
6.1 Aquatic and terrestrial biota
Terrestrial plants such as barley, cress and wheat, and algae
such as Oedogonium cardiacum slowly metabolize HCB to polar
metabolites and non-extractable residues (Lu & Metcalf, 1975;
Scheunert et al., 1983, 1985; Topp et al., 1989). For example, of the
total radiolabelled HCB in barley after uptake from soil over one
growing season, 14% was present as polar metabolites, 20% as plant-
bound residues and the remainder as the parent compound (Topp et al.,
1989). In the only available study on HCB depuration rates in plants,
the aquatic macrophyte Myriophyllum spicatum eliminated 95% of HCB
during the first 28 days after exposure ceased (Gobas et al., 1991).
Invertebrates slowly metabolize HCB to compounds such as
pentachlorothioanisole, pentachlorophenol and other polar metabolites
(Lu & Metcalf, 1975; Bauer et al., 1989). In an aquatic model
ecosystem treated with 14C-HCB for 24 h, unchanged HCB accounted for
84% of the total radioactivity in snails ( Physa sp.), 67% in water
fleas ( Daphnia magna) and 65% in mosquito larvae ( Culex pipiens)
(Lu & Metcalf, 1975). Half-lives for the elimination of HCB by
invertebrates were less than 5 days for filter-feeding bivalves
( Elliptio complanata and Mytilus edulis) (Bro-Rasmussen, 1986;
Russell & Gobas, 1989), 16 days for deposit-feeding clams ( Macoma
nasuta) (Boese et al., 1990), and 27 days for oligochaete worms
( Tubifex tubifex and Limnodrilus hoffmeisteri) (Oliver, 1987).
Sanborn et al. (1977) detected pentachlorophenol and at least
four unidentified polar metabolites in green sunfish ( Lepomis
cyanellus) after 28 days of ingesting HCB-contaminated food.
Pentachlorophenol has also been detected in the excreta and tissues of
rainbow trout ( Oncorhynchus mykiss) following an intraperitoneal
dose with HCB (Koss & Koransky, 1978; Koss et al., 1978). Zebra fish
( Brachydanio rerio) did not metabolize HCB after a 48-h exposure in
water (Kasokat et al., 1989). Elimination half-lives of HCB ranged
from 7-21 days for fathead minnows ( Pimephales promelas) after a
waterborne exposure (Kosian et al., 1981) to up to 210 days for
rainbow trout ( Oncorhynchus mykiss) after ingestion of HCB in food
(Niimi & Cho, 1981).
Clark et al. (1987) reported that 63% of the total HCB eliminated
in herring gulls ( Larus argentatus) was found in the egg yolk.
Breslin et al. (1983) found that 50% of total HCB eliminated from
laying bobwhite quail ( Colinus virginianus), a species that lays
many eggs, was accounted for in egg yolk. For most wild species, egg
laying will account for a relatively small loss of HCB, while
depletion of stored fat during energetically costly activities such as
migration and moulting may result in a significant reduction in body
burdens. The half-life for elimination of HCB in birds ranged from
24-35 days for domesticated chickens ( Gallus gallus domesticus) fed
HCB-contaminated diets (Kan & Tuinstra, 1976; Hansen et al., 1978) to
211 days in intraperitoneally dosed juvenile herring gulls (Clark et
al., 1987).
6.2 Mammals
There are few data on the absorption of HCB by humans. By
comparing intake and faecal excretion of HCB in a single breast-fed
infant, Abraham et al (1994) estimated that absorption was virtually
complete (greater than 99.7% at one month of age and greater than 97%
at 5 months). The concentrations of HCB in the diet and faeces of a
single formula-fed infant were too low for reliable estimation of
absorption (Abraham et al., 1994). The results of animal studies
indicate that 80% or more of an oral dose of HCB (between 10 and 180
mg/kg body weight) is absorbed if administered in an oil vehicle
(Albro & Thomas, 1974; Koss & Koransky, 1975; Ingebritsen et al.,
1981; Bleavins et al., 1982). In female rats treated with 14C-HCB in
oil, peak values of radioactivity were reached in 2 to 5 days. The
absorption was poor (2-20%, depending on the dose) when the substance
was given as an aqueous suspension (Koss & Koransky, 1975). Little
information was identified on dermal absorption, although it appears
to be lower. Koizumi (1991) observed that after dermal application of
approximately 2.5 mg 14C-HCB in tetrachloroethylene to Fisher-344
rats for 72 h, only 9.7% of the administered dose was absorbed. No
information on absorption via the lungs has been reported.
There are no experimental studies of tissue distribution of HCB
in humans, although in a small autopsy study of members of the general
population (Schechter et al., 1989b), the highest levels were found in
(in order) adipose tissue, adrenals, bone marrow and liver. Laboratory
studies in a number of animal species also indicate that the highest
concentrations of HCB are accumulated in tissues with a high lipid
content, such as the adipose tissue, adrenal cortex, bone marrow, skin
and some endocrine tissues (thyroid, adrenal and ovary) following
ingestion or injection of HCB (Koss & Koransky, 1975; Yang et al.,
1978; Courtney, 1979; Sundlof et al., 1982; Ingebritsen, 1986; Smith
et al., 1987, 1994; Goldey et al., 1990; Foster et al., 1993; Jarrell
et al., 1993a). No information was found on the tissue distribution
following inhalation or dermal exposure. HCB crosses the placenta, and
is eliminated via the mothers' milk in both animals and humans
(Villeneuve et al., 1974; Mendoza et al., 1975; Courtney & Andrews,
1979, 1985; Courtney et al., 1979; Bailey et al., 1980; Bleavins et
al., 1982; Goldey et al., 1990; section 5.2.1).
Metabolic transformation is not extensive in the wide range of
species examined. The pathways of biotransformation of HCB have been
reviewed by Debets & Strik (1979) and by Renner (1988). The metabolism
of HCB operates via three distinct pathways. These are oxidative
pathways, which give rise to phenolic metabolites including
pentachlorophenol, tetrachlorohydroquinone and tetrachloro-
benzoquinone; a glutathione-conjugation pathway leading to penta-
chlorothiophenol, pentachlorothioanisoles, and several other sulfur-
containing metabolites; and a minor pathway that yields lower
chlorinated benzenes through reductive dechlorination. Metabolism
occurs primarily in the liver, although dechlorination of HCB has also
been demonstrated in vitro in enzyme preparations from the lung,
kidney and small intestine (Mehendale et al., 1975).
The metabolism of HCB has been studied in the rat and guinea-pig
(Mehendale et al., 1975; Rozman et al., 1975; Koss & Koransky, 1976;
Koss et al., 1978; Koss & Koransky, 1978; Courtney, 1979), and in the
monkey (Rozman et al., 1975; Courtney, 1979). Dosing routes included
gastric intubation and the intraperitoneal route, while dosing
vehicles included oil and aqueous media. The monitoring for metabolic
products of HCB has included excretory products and/or tissue residues
for periods ranging from 28 to 40 days post-dosing. Findings were
quite dissimilar among the studies. The most common finding was that
less than 40% of the administered dose was recovered in the excretory
products and a majority of the recovered dose was unchanged HCB.
The major metabolites found in the urine of rats, mice and
guinea-pigs exposed to HCB by various routes in most studies are
pentachlorophenol (PCP), tetrachlorohydroquinone and
pentachlorothiophenol (PCTP) (Koss & Koransky, 1978; Koss et al.,
1978). (There is some question as to whether most of the latter
compound detected in some studies was an analytical artefact from
alkaline hydrolysis of the n-acetyl cysteine conjugate.) Other
metabolites include tetra- and pentachlorobenzenes and thioanisoles,
and tri- and tetrachlorophenols, both in free and conjugated forms. It
has been reported that, after dietary exposure of male and female
Wistar rats to HCB for 13 weeks, N-acetyl- S-(pentachloro-
phenyl)cysteine was the most abundant metabolite via the conjugation
pathway (89-92% of the total urinary metabolites collected over 24 h,
after one week of treatment). Mercaptotetrachlorothioanisole was also
present, excreted as a glucuronide (den Besten et al., 1994). The
excreta from male Wistar rats given 125 mg/kg body weight on day 1 and
6 were collected for 12 days (Jansson & Bergman, 1978). Faeces and/or
urine contained HCB (about 4% of the total does), pentachlorobenzene,
pentachlorophenol, pentachlorobenzenethiol (both as such and as
conjugates), methylthiopentachlorobenzene, tetrachlorobenzenedithiol
and/or methylthiotetrachlorobenzenethiol (both as such and as
conjugates), dichlorotetrakis(methylthio)benzene (trace amounts),
hexakis(methylthio)benzene (trace amounts), bis(methylthio)-
tetrachlorobenzene, tetrachlorobenzenethiol (trace amounts) and
methylthiotetrachlorobenzene (trace amounts). Compounds found
accumulated in adipose tissue were hexachlorobenzene,
pentachlorobenzene, pentachlorobenzenethiol, bis(methylthio)-
tetrachlorobenzene and pentachloroanisole.
Rizzardini & Smith (1982) administered 50 µmoles of HCB/kg body
weight to male and female rats by gavage in arachis oil for 103 days.
Three urinary metabolites were identified, i.e., pentachlorophenol,
2,3,5,6-tetrachlorobenzene, 1,4-diol and pentachlorothiophenol
(derived from mercapturate). The authors reported that female rats
excreted several times more HCB metabolites than males.
PCP and PCTP have been detected in the urine of humans from the
general population of Spain with high body burdens of HCB (To-Figueras
et al., 1992).
No reliable information on the elimination half-life of HCB in
humans was found. Excretion of HCB by laboratory animals occurs mainly
through the faeces regardless of the route of administration (US EPA,
1985a; ATSDR, 1990). Both biliary excretion and non-biliary intestinal
transfer contribute to faecal excretion (Rozman et al., 1981;
Ingebritsen et al., 1981; Richter & Schäfer, 1981; Sundlof et al.,
1982). Reported half-lives for the elimination of an oral dose of HCB
(doses were 3 mg/kg body weight or less in these studies) are
approximately one month in rats and rabbits, 10-18 weeks in sheep,
pigs and dogs, and 2.5 to 3 years in rhesus monkeys (Avrahami &
Steele, 1972; Avrahami, 1975; Rozman et al., 1981; Sundlof et al.,
1982; Scheufler & Rozman, 1984; Yamaguchi et al., 1986). HCB has been
detected in the milk of several species, including humans, and the
results of experiments with mice and ferrets indicate that the
majority of the maternal body burden can be eliminated via the
mother's milk during lactation (Bleavins et al., 1982; Courtney &
Andrews, 1985).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
This section summarizes the extensive literature on the toxicity
of HCB to laboratory mammals, with emphasis on those studies reporting
the lowest-observed-effect levels. Information on the dosage with
respect to body weight was obtained from the original papers, wherever
possible. When doses were not expressed in this way by the
investigators and could not be calculated from the data provided,
approximate doses (given in parentheses) have been estimated based on
the reference values given in NIOSH (1985).
7.1 Single exposure
The acute toxicity of HCB in experimental animals is low;
reported oral LD50 values for various species range from 1700 mg/kg
body weight for the cat to between 3500 and > 10 000 mg/kg body
weight for the rat, with intermediate values for the mouse, rabbit and
guinea-pig. Reported LC50 values for inhalation exposure range from
1600 mg/m3 for the cat to 4000 mg/m3 for the mouse, with
intermediate values reported for the rat and rabbit (IARC, 1979;
Strik, 1986; Lewis, 1992). Acute lethal doses elicit convulsions,
tremors, weakness, ataxia, paralysis and pathological changes in
organs. Strik (1986) reported that HCB has a low skin irritation
score, is not irritating to the eye and does not sensitize the guinea-
pig, although no details were provided. In several studies, single
oral doses of 100-1000 mg/kg body weight produced increases in the
activities of various liver enzymes in rats within 24 h (Strik, 1986).
7.2 Short-term and subchronic exposure
The effects of short-term, repeated exposure to HCB are primarily
hepatotoxic and neurological. In a number of studies, the effects of
HCB on rats exposed to oral doses in the range of 30-250 mg/kg body
weight per day included altered body weight, cutaneous lesions,
tremors and other neurological signs, hepatomegaly, liver damage and,
in some cases, early alterations in porphyrin or haem metabolism
(Courtney, 1979; US EPA, 1985a; Strik, 1986). Short-term exposure
in vivo induced a variety of enzymes, including glutathione- S-
transferases and isozymes of cytochrome P-450, identified as
cytochromes P-450IA1 (CYPIA1), P-450IA2 (CYPIA2) and P-450IIB (CYPIIB)
(Wada et al., 1968; Courtney, 1979; Denomme et al., 1983; US EPA,
1985a; Linko et al., 1986; Strik, 1986; Hahn et al., 1988, 1989; Vos
et al., 1988; Green et al., 1989; Rizzardini et al., 1990; D'Amour &
Charbonneau, 1992; Smith et al., 1993; Goerz et al., 1994). This means
that HCB is a mixed-type cytochrome-P-450-inducing compound, with
phenobarbital-inducible and 3-methylcholanthrene-inducible properties.
Enzyme induction has been observed at relatively low doses in some
studies. For instance, in Wistar rats fed HCB in the diet for 14 days,
the low-effect level for induction of microsomal liver enzyme was
50 mg HCB/kg feed (approximately 2.5 mg/kg body weight per day), and
the no-effect level was 20 mg HCB/kg feed (approximately 1 mg/kg body
weight per day) (den Tonkelaar & van Esch, 1974).
The effects produced by subchronic exposure to HCB are similar to
those observed in short-term studies, but are generally evident at
lower doses (Courtney, 1979; US EPA, 1985a; ATSDR, 1990, 1994). At
relatively high doses (32 mg/kg body weight per day or more for
periods from several weeks to 90 days), reported effects have included
death, skin lesions, behavioural and neurological changes, reduced
body weight gain, increased organ weights, and altered thyroid
function and serum levels of thyroid hormones (the latter effect is
discussed later in this section). At lower doses, hepatotoxic effects
have been commonly reported, including histological alterations, the
induction of a variety of hepatic microsomal enzymes and porphyria.
The porphyrinogenic effects of exposure to HCB have been
extensively studied since the seminal reports of Ockner & Schmid
(1961) and De Matteis et al. (1961). These and subsequent earlier
works, many of them conducted at relatively high doses, have been
summarized by Courtney (1979), and much of this research is not
discussed in this report. Porphyria has been observed in several
species of laboratory mammals, most often manifested as increased
levels of porphyrins and/or porphyrin precursors in the liver, other
tissues and excreta. This disturbance in haem synthesis is associated
with the inhibition of uroporphyrinogen decarboxylase activity (this
enzyme converts uroporphyrinogen III to coproporphyrinogen III),
leading to the accumulation of uroporphyrin and other highly
carboxylated porphyrins, and with the induction of ALA synthetase (the
enzyme controlling the rate of haem synthesis) (ATSDR, 1994). There is
a delay before exposed rats become porphyric, which appears to reflect
the time for the animals to receive a sufficient cumulative dose of
HCB (Krishnan et al., 1991, 1992), as well as the time needed for the
porphyrins to accumulate to the level of overt porphyria (Kennedy et
al., 1986; Kennedy & Wigfield, 1990). Although in most studies
porphyria has been associated with longer-term exposure to HCB, rats
exposed to doses of 25-50 mg HCB/kg body weight per day for as little
as several days had increased levels of hepatic and urinary porphyrins
(Krishnan et al., 1991, 1992). In another study, hepatic levels of
highly carboxylated porphyrins were elevated by a single exposure to
50 mg HCB/kg body weight, although the latter result was not
accompanied by clinical porphyria (Kennedy & Wigfield, 1990).
HCB-induced porphyria has been extensively studied in rats, in
which dietary or gavage exposure of various strains to between 2.5 and
15 mg HCB/kg body weight per day for periods of 8 to 15 weeks has
caused hepatic porphyria, and, in some studies, increased levels of
porphyrins in the kidney and spleen (Grant et al., 1975; Kuiper-
Goodman et al., 1977; Goldstein et al., 1978; Mendoza et al., 1979;
Rizzardini & Smith, 1982; Teschke et al., 1983; Smith et al., 1985b;
Green et al., 1989; Van Ommen et al., 1989; Kennedy & Wigfield, 1990;
Smith et al., 1990; Den Besten et al., 1993). A no-observed-effect-
level (NOEL) for HCB-induced porphyria was not determined in these
studies. Although the data on other species are limited, levels of
hepatic or urinary porphyrins were increased in mice of various
strains fed diets containing 200 mg HCB/kg feed (yielding approximate
doses of 24 mg HCB/kg body weight per day) for periods of 7 to 15
weeks in some studies (Smith & Francis, 1983; Rizzardini et al., 1988;
Vincent et al., 1989), and porphyria was induced in Japanese quail
following short-term oral and intraperitoneal exposure to 500 mg
HCB/kg body weight per day (section 9.1.2).
The lowest doses producing porphyrinogenic and other effects on
the liver in a subchronic study were reported by den Tonkelaar et al.
(1978). Groups of five pigs exposed for 90 days to doses of 0.5 mg/kg
body weight per day or more in the diet had increased urinary levels
of coproporphyrin and alterations in liver histology and microsomal
enzyme activities, but no effects were observed at 0.05 mg/kg body
weight per day. However, marked excretion of coproporphyrin alone is
not a characteristic of the inhibition of uroporphyrinogen
decarboxylase in animal systems.
Female rats are more sensitive than males to the porphyrinogenic
effects of exposure to HCB. In various strains of rats exposed to
doses of 5 to 10 mg HCB/kg body weight per day in the diet or by
gavage, for periods of between 3 months or more, females developed a
marked porphyria which was absent or much reduced in males (Grant et
al., 1975; Kuiper-Goodman et al., 1977; Rizzardini & Smith, 1982;
Smith et al., 1985b). In a number of studies, the basis for the
susceptibility to HCB-induced porphyria of female rats compared to
males has been examined. Grant et al. (1975) reported that ovariectomy
decreased, and castration increased, the accumulation of porphyrins in
the livers of female and male Sprague-Dawley rats with subchronic
exposure to HCB, suggesting a role for steroid hormones in the
development of porphyria in this species. In another study, female
Fischer-344 rats with HCB-induced porphyria had higher levels of
cytochrome P-450IA isoenzymes and ethoxyresorufin- O-deethylase
activity than males, whereas males had higher levels of total
cytochrome P-450 and activities of microsomal monooxygenases
associated with cytochrome P-450IIB1 (Smith et al., 1990). In Fischer-
344 rats with HCB-induced porphyria, sex-related differences in
urinary and hepatic porphyrin levels were paralleled by differences in
the excretion of phenolic metabolites, particularly
pentachlorothiophenol (Rizzardini & Smith, 1982). These findings were
further investigated in a short-term study by D'Amour & Charbonneau
(1992), which indicated that male rats may be more resistant to HCB-
induced porphyria than females because hepatic conjugation of HCB with
glutathione is more important in males. Male Sprague-Dawley rats
receiving a porphyrinogenic dose of HCB (100 mg HCB/kg body weight per
day by gavage for 5 days) had significantly lower hepatic gluthione
concentration and higher glutathione transferase activity (to 3,4-
dichloronitrobenzene) than controls, whereas no significant
differences were observed in females. Biliary excretion of PCTP (a
metabolite of glutathione conjugation) and the rate of elimination of
HCB from the liver were greater in males than in females.
Other mechanistic studies have suggested the involvement of
oxidative metabolism of HCB in the development of porphyria, although
the mechanism remains to be elucidated. In female Wistar rats
co-treated with 300 mg HCB/kg in the diet (approximately 15 mg HCB/kg
body weight per day) and triacetyloleandomycin (TAO) (to selectively
inhibit cytochrome P-450IIIA1/2 and thereby prevent the oxidative
biotransformation of HCB) for 10-13 weeks, both the excretion of PCP
and TCHQ (tetrachlorohydroquinone, the reduced analogue of the
reactive tetrachlorobenzoquinone) and the extent of hepatic porphyria
and urinary porphyrin excretion were greatly diminished (Van Ommen et
al., 1989; Den Besten et al., 1993). In 13-week feeding studies on
female Wistar rats exposed to 300 mg HCB/kg diet (approximately 15 mg
HCB/kg body weight per day) in the presence or absence of TAO, the
degree of porphyria was better correlated with excretion of PCP than
TCHQ, and in comparative studies pentachlorobenzene (which is
metabolized to PCP by a different mechanism than for HCB) was not
porphyrinogenic (Den Besten et al., 1993).
In addition, it has been suggested that the aryl hydrocarbon
receptor (Ah receptor) may be involved in the accumulation of hepatic
porphyrins in mice (Linko et al., 1986; Hahn et al., 1988, 1989). Ah-
responsive strains of inbred mice were more sensitive to hepatic
porphyrin accumulation after HCB exposure than non-responsive mice
(Smith & Francis, 1983; Hahn et al., 1988), and HCB has been shown to
be a weak agonist for the Ah receptor (Hahn et al., 1989).
Full discussion of the evidence for a unifying hypothesis of
porphyria induced by HCB and other chemicals that act in a similar
way, as well as for human sporadic porphyria cutanea tarda, is beyond
the scope of this document. There is however, substantial experimental
and human evidence implicating a complex interaction between
hepatocellular iron and oxidative processes leading to the oxidation
of unstable uroporphyrinogen to uroporphyrin, possibly mediated by
induced cytochrome P-450 isozymes (reviewed by Smith & De Matteis,
1990). There is evidence that inhibition of uroporphyrinogen
decarboxylase may occur through formation of an inhibitor of the
enzyme during the oxidation of uroporphyrinogen (Rios de Molina et
al., 1980; Smith & De Matteis, 1990). HCB may act partly through
induction and uncoupling of the cytochrome P-450 system to form
reactive oxygen species, especially in the presence of an increased
available iron pool (Smith & De Matteis, 1990; Den Besten et al.,
1993).
Subchronic exposure to low doses of HCB has also caused changes
in calcium homoeostasis and bone morphometry. Male Fischer-344 rats
administered HCB by gavage in corn oil had elevated serum levels of
1,25-dihydroxy-vitamin-D3 and reduced calcium excretion after 5
weeks, and increased femur density, weight and strength after 15
weeks. These effects were evident at 0.7 mg/kg body weight per day but
not at 0.07 mg/kg body weight per day (Andrews et al., 1989, 1990).
While technical HCB is known to be contaminated with chlorinated
dibenzo- p-dioxins, dibenzofurans and biphenyls (Villanueva et al.,
1974; Goldstein et al., 1978), the effects (primarily hepatic) of
subchronic dietary exposure of rats to either pure or technical HCB
were virtually identical, indicating that the effects observed in this
study were due to the parent compound (Goldstein et al., 1978).
In a number of studies on various strains of rats, short-term or
subchronic exposure to HCB affected the thyroid, as indicated by
decreased serum levels of total and free thyroxine (T4) and often, to
a lesser extent, triiodothyronine (T3). In some instances, these are
accompanied by compensatory increases in thyroid weight, circulating
levels of thyroid-stimulating hormone or iodine uptake by the thyroid
(Rozman et al., 1986; Kleiman de Pisarev et al., 1989, 1990; Van Raaij
et al., 1991a, 1993a, 1993b; Foster et al., 1993; Den Besten et al.,
1993; Sopena de Krakoff et al., 1994). Den Besten et al. (1993)
reported such effects in rats exposed to as little as 9.5 mg/kg body
weight per day following dietary exposures for 13 weeks, although
effect levels were somewhat higher in other studies, which involved
exposure for a shorter duration and/or employed an aqueous vehicle.
Somewhat different effects (decreased levels of T3 in serum and no
change in T4, accompanied by increased uptake of iodine by the
thyroid) were observed in hamsters exposed to 100-200 mg HCB/kg feed
(approximately 12-24 mg/kg body weight per day) for 18-28 weeks (Smith
et al., 1987).
The mechanisms that have been advanced to account for the effects
of HCB on the thyroid include accelerated metabolism of thyroid
hormones by HCB-induced enzymes or accelerated deiodination of
thyroxine, in conjunction with increased biliary excretion (Kleiman de
Pisarev, 1989; Van Raaij et al., 1993b), and interference with plasma
transport of thyroid hormones through displacement of T4 from binding
sites on proteins (Van Raaij et al., 1991a, 1993a). Van Raaij et al.
(1991b, 1993a) reported that intraperitoneal injection of
pentachlorophenol and tetrachlorohydroquinone, but not HCB itself,
decreased serum thyroxine levels in rats, indicating that these
metabolites may be involved in the effects of HCB on the thyroid.
These authors reported that PCP was a more effective competitor for
thyroxine-binding sites of serum carriers in vitro, and more
effective at occupying carrier sites in ex vivo experiments, than
HCB (van Raaij et al., 1991a), and demonstrated that T4 binding sites
were partially occupied in the serum of rats exposed to HCB (Van Raaij
et al., 1993a). In the latter study, it was estimated that competition
for thyroid hormone binding sites, by PCP metabolized from HCB, could
account for almost half of the observed reduction in serum levels of
T4.
7.3 Long-term toxicity and carcinogenicity
A range of non-neoplastic effects from long-term exposure to HCB,
which are primarily hepatotoxic, have been observed at relatively low
doses. In a two-generation study with Sprague-Dawley rats, liver and
heart weights were increased in Fo males exposed to TWA doses of 0.29
and 1.50 mg/kg body weight per day in the diet for 3 months, and
histopathological changes in the liver were observed in F1 animals of
both sexes exposed to maternal doses of 0.29-0.38 and 1.50-1.90 mg
HCB/kg body weight per day in diet in utero, through nursing, and
then continued on the same diet as their parents for their lifetimes.
The no-effect level in this study was 0.06-0.07 mg/kg body weight per
day (Arnold et al., 1985; Arnold & Krewski, 1988). Dietary exposures
of Sprague-Dawley rats to 10 mg/kg and above (approximately 0.5-0.6
mg/kg body weight per day) for 9-10 months induced in vivo mixed-
function oxidase activity, as indicated by reductions in drug-induced
sleeping times (Grant et al., 1974). Exposure of Sprague-Dawley rats
to 5 mg HCB/kg in diet (approximately 0.25-0.30 mg/kg body weight per
day) for 3-12 months caused proliferation of smooth endoplasmic
reticulum, altered mitochondria and increased numbers of storage
vesicles in liver, but these effects were not evident at 1 mg/kg in
diet (approximately 0.05-0.06 mg/kg body weight per day) (Mollenhauer
et al., 1975; 1976). In a study by Böger et al. (1979), oral
administration of 2, 8 or 32 mg HCB to female Wistar rats twice weekly
for 203 days (0.57, 2.3 or 9.1 mg HCB/kg body weight per day) resulted
in hepatocellular enlargement, proliferated smooth endoplasmic
reticulum, increased glycogen and porphyrin deposits, and enlarged
mitochondria, but these effects were not seen at a lower dose (0.5 mg
HCB/kg body weight twice weekly, or 0.14 mg HCB/kg body weight per
day). Bleavins et al. (1984a) reported that exposure of female mink to
a dietary concentration of 1 mg/kg (estimated to yield a dose of
0.16 mg/kg body weight per day) for 47 weeks significantly increased
serotonin concentrations in the hypothalamus of dams, and depressed
hypothalamic dopamine concentrations in kits exposed in utero and
through nursing.
As in subchronic studies, female rats were more sensitive than
males to porphyria induced by chronic exposure to HCB. Grant et al.
(1974) reported that in Sprague-Dawley rats fed diets containing HCB
for 9-10 months, reduced weight gain and porphyria were observed in
females, but not males, receiving 80 or 160 mg HCB/kg feed
(approximately 4 or 8 mg HCB/kg body weight per day). A dose-related
increase in relative liver weights and in the hepatic content of HCB
was noted in both sexes. Hepatic enzyme activities and cytochrome
P-450 activities were increased in males administered 40 mg HCB/kg
feed or more. Exposure to 10 mg HCB/kg feed (approximately 0.5-0.6 mg
HCB/kg body weight per day) induced in vivo mixed-function oxidase
activity, as indicated by reductions in sleeping time for
pentobarbital and zoxazolamine exposure.
The carcinogenicity of HCB has been assessed in several bioassays
in rats, mice and hamsters. The following discussion is limited
principally to the four studies in which adequate numbers of animals
of both sexes were exposed for a sufficient length of time to more
than one dose level.
Cabral et al. (1977) and Cabral & Shubik (1986) reported a
statistically significant increase of liver cell tumours (hepatomas)
in groups of 30-60 male and female Syrian golden hamsters fed 50, 100
or 200 mg HCB/kg (4, 8 or 16 mg/kg body weight per day) HCB in their
diets for life. The incidence of "haemangioendotheliomas" of the liver
was significantly increased in both sexes at 200 mg/kg and in males at
100 mg/kg, and of alveolar adenomas of the thyroid in males at
200 mg/kg. (The latter finding is interesting in the light of reports
of excesses of thyroid neoplasms, or of enlargement of the thyroid, in
human populations with elevated exposures to HCB (section 8.1.)) The
authors reported that three of the hepatic "haemangioendotheliomas"
(which are non-invasive by definition) metastasized. It seems likely,
therefore, that these tumours were malignant, though misclassified.
In another study, HCB was administered in the diet to groups of
30 or 50 outbred male and female Swiss mice at concentrations of 0,
50, 100 and 200 mg/kg (0, 6, 12 and 24 mg/kg body weight per day) for
120 weeks (Cabral et al., 1979; Cabral & Shubik, 1986). In females
exposed to 200 mg/kg, a statistically significant increase in the
incidence of "liver cell tumours (hepatomas)" was noted. "Hepatomas"
were also elevated, though not significantly, in males at this dose
and in both sexes at 100 mg/kg. The number of tumour-bearing animals,
the latent period, and the multiplicity and size of tumours increased
with dose.
Arnold et al. (1985) and Arnold & Krewski (1988) investigated the
potential carcinogenicity to rats of combined in utero, lactational
and oral exposure to analytical grade HCB. Groups of 40 or more
weanling male and female Sprague-Dawley rats were fed diets containing
0, 0.32, 1.6, 8 or 40 mg HCB/kg. (Based on data supplied by the
author, mean doses for males were 0, 0.01, 0.06, 0.29 and 1.50 mg/kg
body weight per day and for females 0, 0.01, 0.07, 0.38 and 1.90 mg/kg
body weight per day). After 3 months, the F0 rats were bred, and 50
F1 pups of each sex were randomly selected from each group. From
weaning, the F1 animals were continued on the same diet for their
lifetimes (up to 130 weeks). In exposed F1 females, increased
incidences of neoplastic liver nodules and adrenal phaeochromocytomas
were noted at the highest dose. A significantly increased incidence of
parathyroid adenomas was noted in males receiving 40 mg HCB/kg in
their diet.
In a study by Lambrecht et al. (1983a,b; Ertürk et al., 1986),
groups of 94 weanling Sprague-Dawley rats were fed diets containing 0,
75 or 150 mg/kg (4 and 8 mg/kg body weight per day for males and 5 and
9 mg/kg body weight per day for females, respectively) for up to 2
years. Statistically significant increases in the incidence of
hepatomas/haemangiomas and of renal cell adenomas were noted at both
doses in animals of both sexes surviving beyond 12 months. Incidences
of hepatocellular carcinomas and bile duct adenomas/carcinomas were
also elevated in females at both doses. In female rats, significant
increases in the incidences of adrenal cortical adenomas at 75 mg/kg
and phaeochromocytomas at both doses were reported. Lambrecht et al.
(1983b) reported a leukaemia involving the thymus, spleen, liver and
kidney in rats exposed to HCB in this study, but did not present any
quantitative data. The results of this study were only reported in
summary form, with few details of the study protocol and results. In
addition, HCB was incorporated into the diet as a powder in this
study, raising the possibility that some of the effects observed may
have been in part attributable to the inhalation of aerosolized HCB.
High incidences of liver tumours have also been reported in some
more limited studies in which single dietary concentrations (100 or
200 mg/kg) were administered to small groups (i.e., between 4 and 15)
of females of three strains of rats (Smith & Cabral, 1980; Smith et
al., 1985b); in one strain (Fischer-344), hepatocellular carcinomas
were observed (Smith et al., 1985b). HCB has not, however, been
carcinogenic in several other studies in various strains of mice
(Theiss et al., 1977; Shirai et al., 1978; Smith et al., 1989),
perhaps as a result of the low doses, short durations of exposure
and/or small group sizes employed. Results were also negative in a
second study by Arnold et al. (1985), in which groups of 50 male
Sprague-Dawley rats were fed diets containing 40 mg HCB/kg in
conjunction with various levels of vitamin A for 119 weeks, indicating
the probable higher sensitivity of the two-generation carcinogenesis
bioassay.
Ertürk et al. (1982, 1986; Lambrecht et al., 1982a,b) examined
the tumorigenic activity of subchronic exposure to HCB in both sexes
of Swiss mice, Syrian golden hamsters and Sprague-Dawley rats at
dietary levels of 0, 100 and 200 mg/kg (mice) and 0, 200 and 400 mg/kg
(hamsters and rats) for 90 days. At day 91, 25 of 50 animals in each
group were sacrificed for histological examination, with the remainder
being sacrificed at 6-week intervals (up to 341, 361 and 424 days for
mice, hamsters and rats, respectively). The results of these studies
were reported in summary form only, and much of the quantitative data
were not presented. The authors reported that, as the experiment
progressed, treated animals developed hepatomas, bile duct adenomas,
renal adenomas and carcinomas, and lymphosarcomas of the thymus,
spleen, and lymph nodes. However, the only tumour and species for
which they presented clear evidence of a treatment-related increase in
incidence was for lymphatic tumours in mice (Ertürk et al., 1982).
Lymphatic and renal neoplasms were observed as early as the end of the
90-day period. It is not clear from these reports which tumours each
species developed or the dietary levels associated with the observed
effects, as well as other experimental details.
Results from a number of studies have indicated that HCB is a
co-carcinogen or promoter of cancer. Concomitant exposure to 50 mg
HCB/kg in diet (approximately 6 mg HCB/kg body weight per day)
enhanced the induction of liver tumours by polychlorinated terphenyl
(at 250 mg/kg diet) in male ICR mice (Shirai et al., 1978). Exposure
to HCB (100-200 mg/kg in diet (approximately 5-10 mg HCB/kg body
weight per day) or 1 mmole/kg i.p. at 1 and 5 weeks) promoted the
development of hepatocellular carcinomas and/or hepatic gamma-
glutamyltranspeptidase-positive foci initiated by diethylnitrosamine
in various strains of rats (Pereira et al., 1982; Herren-Freund &
Pereira, 1986; Stewart et al., 1989).
In some recent studies, the possible mechanisms by which HCB
induces tumours in animals have been investigated.
Bouthillier et al. (1991) presented the results of studies of
Sprague-Dawley rats exposed to 100 mg HCB/kg by gavage for periods of
several weeks, which indicated that the observed increase in renal
tumours in male Sprague-Dawley rats following exposure to HCB
(Lambrecht et al., 1983b; Ertürk et al., 1986) is related to protein
droplet nephropathy. The mechanism by which structurally diverse
hydrocarbons induce hyaline droplet nephropathy in male rats has been
well documented and involves accumulation of alpha-2u-globulin,
resulting in necrosis, regeneration and, in some cases, tumours. This
response is sex- and species-specific, and hence is unlikely to be
relevant to humans. This mechanism does not, however, explain the
increased (but lower) incidence of renal tumours in females also
reported by Lambrecht et al. (1983b).
Carthew & Smith (1994) hypothesized that some HCB-induced hepatic
tumours in rats may be produced by a non-genotoxic mechanism. They
noted that hepatotoxicity of HCB in rodents gives rise to peliosis and
necrosis with haemosiderosis, indicating that vascular damage has
occurred, and confirmed the presence of such damage in the liver of
chronically HCB-exposed rats by the identification of widespread
fibrin deposits, using an antibody to rat fibrin. These deposits
occurred in association with abundant haemosiderosis in hepatocytes
and areas of widened hepatic sinusoids. On this basis, it was
suggested that the formation of hepatomas and haemangiomas with
elements of peliosis could be the result of compensatory hyperplastic
responses to hepatocellular necrosis and the simultaneous loss of
hepatocellular cords, perhaps potentiated by the accumulation of iron
in the liver.
Mechanistic studies that address the relevance to humans of the
remaining tumour types induced in rodents by HCB have not been
identified.
7.4 Mutagenicity and related end-points
HCB has not been found to be genotoxic in most studies conducted
to date. HCB did not cause either frameshift or base pair substitution
mutations in Salmonella typhimurium at doses of as much as 10
mg/plate with or without metabolic activation, with both rat and
hamster liver activation systems, pre-incubation and plate
incorporation methods, and technical and 99.9% pure HCB (Haworth et
al., 1983; Górski et al., 1986; Siekel et al., 1991). A weak positive
response in S. typhimurium strain TA98 at 50 and 100 µg/plate was
reported by Gopalaswamy & Aiyar (1986) and Gopalaswamy & Nair (1992).
However, the authors also reported mutagenic activity for lindane, in
contrast to the results of other studies (e.g., Haworth et al., 1983).
Doses of up to 1000 µg/plate of HCB did not induce tryptophan
reversion or DNA damage in Escherichia coli strains WP2 and WP2uvrA
with or without metabolic activation (Siekel et al., 1991).
There have been reports of mutagenic activity for HCB in
eukaryotic cells in vitro, although these studies have limitations.
Guerzoni et al. (1976) reported a positive finding for methionine
reversion in Saccharomyces cerevisiae strain 632/4 exposed to HCB,
but Brusick (1986) did not consider the observed increase to meet
current standards of a positive response. In addition, only a single
dose level was used in that study, and there was no exogenous
metabolic activation. Kuroda (1986) reported that in cultured Chinese
hamster lung cells (V79), HCB did not induce OUAr mutations, but did
induce 8AGr mutations. However, both the magnitude of the increase
(which was small, roughly 1/105 survivors at the two highest doses)
and uncertain dose-response indicate that this response is open to
question.
Oral administration of as much as 221 mg HCB/kg body weight per
day to male rats for 5 or 10 days failed to induce dominant lethal
effects in two different studies (Khera, 1974; Simon et al., 1979),
although Simon et al. (1979) did observe a slight reduction in male
reproductive performance (numbers of females inseminated and
impregnated). Rumsby et al. (1992) reported that liver neoplasms that
developed in iron-overloaded C57Bl/10ScSn mice exposed for 18 months
to 0.01% HCB in the diet were not associated with a high frequency of
mutations in the Ha-ras proto-oncogene at codon 61. Only two mutations
were observed at different sites, from 23 preneoplastic and neoplastic
lesions examined, indicating that activation of the Ha-ras gene is not
an important event in the hepatocarcinogenicity of HCB in this test
system.
HCB has not been found to be clastogenic in the few available
studies in which this end-point has been examined. The compound did
not increase the frequency of sister chromatid exchanges in the bone
marrow of male mice given as much as 400 mg/kg body weight (by an
unspecified route), although the lack of detail in reporting the test
protocol and results limits the interpretation of this study (Górski
et al., 1986). HCB did not induce chromosomal aberrations in vitro
in cultured Chinese hamster fibroblast cells at concentrations as high
as 12 mg/ml, with or without metabolic activation (Ishidate, 1988), or
in human peripheral blood lymphocytes exposed to up to 0.1 mmol/litre
(Siekel et al., 1991). Treatment of rats with 1000 mg HCB/kg diet for
15 days was hepatotoxic, but did not cause early diploidization in
hepatocytes as measured by flow cytometry (Rizzardini et al., 1990).
The results of less specific assays also indicate that HCB does
not interact strongly with DNA, although there are two reports that
the compound binds, at low levels, to DNA. After incubating
hepatocytes isolated from phenobarbital-treated rats with 14C-HCB
(5 µM) for 20 h, Stewart & Smith (1987) reported the maximum amount of
radioactivity associated with DNA was < 9.9 × 10-5% of the substrate
added, and was only marginally above that of hepatocytes held at 4°C;
the authors considered this to be significantly lower than expected
for hepatocarcinogens. Gopalaswamy & Nair (1992) also reported a low
order of binding of HCB to DNA from the livers of rats exposed to
25 mg HCB/kg. Short-term exposure (<1 day) of rats to oral doses of
700 or 1400 mg/kg body weight (Kitchin & Brown, 1989) or to as much at
300 mg/kg body weight i.p. (Górski et al., 1986) did not cause hepatic
DNA damage, as measured by alkaline elution.
7.5 Reproductive and developmental toxicity
Relatively low doses of HCB have been found to affect some
reproductive tissues in female monkeys. Oral exposure of cynomolgus
monkeys to 0.1 mg/kg body weight per day in gelatin capsules for 90
days caused stratification of the ovarian germinal epithelium
(Babineau et al., 1991; Jarrell et al., 1993a). Higher dosages (1.0
and 10.0 mg/kg body weight per day) were associated with cellular
degeneration of this surface epithelium. The low dosage was associated
with ultrastructural as well as light microscopic changes in surface
epithelium (Babineau et al., 1991; Sims et al., 1991).
In ovarian follicles the low dose was associated with an
increased number of lysosomal elements in germ cells (Singh et al.,
1990a). The basal lamina was thickened. Higher dosages were associated
with greater degenerative changes in their cells and granulosa cells
(Singh et al., 1991, 1990b).
These studies demonstrated changes in ovarian tissues with no
other evidence of toxicity. In particular, the induction of
superovulation with human menopausal gondotrophin (HMG) in these
animals was associated with a normal estradiol response, oocyte
recovery, oocyte maturation, in vitro fertilization and early embryo
development (Jarrell et al., 1993a). These studies confirm the
findings of Iatropoulous et al. (1976) in which the administration of
8 to 128 mg/kg body weight (by gavage in 1% methylcellulose) for 60
days induced severe follicular degeneration in primordial germ cells,
pseudostratification of the ovarian surface epithelium, hepatic
degeneration and severe systemic toxicity in Rhesus monkeys.
In subsequent studies of similarly treated animals, the higher
doses were associated with reduced luteal phase progesterone and
blunted estradiol responses to HMG (Foster et al., 1992a,b). Reduction
in adrenal steroidogenesis occurred in ovariectomized rats in response
to exposure to HCB at concentrations of 1, 10 and 100 mg/kg body
weight for 30 days (Foster et al., 1995).
In contrast, the results of studies on a variety of species have
indicated that repeated exposure to HCB can affect male reproduction,
but only at relatively high doses. Mice exposed to 250 mg HCB per kg
feed (approximately 30 mg HCB/kg body weight per day) for 21 days had
reduced serum testosterone levels; based on the results of in vitro
tests, it was suggested that this was due to increased metabolism by
hepatic microsomal enzymes induced by HCB (Elissalde & Clark, 1979).
Histological changes in the testes (retarded sexual maturation) were
noted in pigs fed a diet yielding a dose of 50 mg HCB/kg body weight
per day for 90 days (den Tonkelaar et al., 1978). The mating index for
male rats receiving five consecutive daily gavage doses of 221 mg
HCB/kg body weight in corn oil was decreased compared to those
receiving 0 or 70 mg/kg body weight However, the fertility index for
the mated female rats (sperm positive smears) was not affected (Simon
et al., 1979).
As discussed in the following paragraphs, placental and
lactational transfer of HCB, demonstrated in a number of species, can
adversely affect both the fetus and nursing offspring. The lactational
route appears to be more important than placental transfer. Adverse
effects on suckling infants are generally observed more frequently,
and at lower doses, than are embryotoxic or fetotoxic effects.
Grant et al. (1977) conducted a four-generation study on female
(20/dose level) and male (10/dose level) weanling Sprague-Dawley rats
fed diets containing 0, 10, 20, 40, 80, 160, 320 or 640 mg HCB/kg
feed. The two highest doses caused some deaths in the F0 dams before
first whelping, and reduced the fertility index. Dietary levels of 160
mg/kg or more reduced litter sizes, increased the number of
stillbirths, and adversely affected pup survival. Similar effects were
seen at 80 mg/kg after the first two generations, while 40 mg/kg was
hepatotoxic to the F1a and F3a pups. A dietary level of 20 mg/kg
(approximately 1-1.2 mg/kg body weight per day) was designated as the
no-observed-effect level.
Arnold et al. (1985) fed groups of male and female Sprague-Dawley
rats from weaning on diets containing up to 40 mg HCB/kg. The rats
were then bred at 3 months, and the F1 pups were continued on the
same diet for their lifetimes. HCB had no effect on fertility, but pup
survival was significantly reduced in the 40 mg/kg group (calculated
doses of 1.50 and 1.90 mg/kg body weight per day for males and
females, respectively).
In other studies, maternal doses in the range from 1.4 to 4 mg/kg
given to rats and cats have been found to be hepatotoxic and/or
affected the survival or growth of nursing offspring. In some cases,
these or higher doses reduced litter sizes and/or increased numbers of
stillbirths (Mendoza et al., 1977, 1978, 1979; Hansen et al., 1979;
Kitchin et al., 1982).
Mink are particularly sensitive to the effects of prenatal and
perinatal exposure to HCB; the offspring of mink fed diets containing
concentrations as low as 1 mg/kg (approximately 0.16 mg/kg body weight
per day) for 47 weeks (prior to mating and throughout gestation and
nursing) had reduced birth weights and increased mortality (Rush et
al., 1983; Bleavins et al., 1984b).
The available data on the developmental toxicity of HCB are
limited. CD-1 mice administered 100 mg/kg body weight by gavage on
days 7-16 of gestation had a significantly increased incidence of
abnormal fetuses per litter, and one case of renal agenesis was
reported. Some cleft palates were produced, but they all occurred in
one litter. This dose also increased maternal liver-to-body weight
ratios and decreased fetal body weights (Courtney et al., 1976). In a
series of studies reported by Andrews & Courtney (1986), combined
in utero and lactational exposure of CD-1 mice and CD rats (strain
unclear, probably Sprague-Dawley) to HCB (mouse dams received 10 or
50 mg/kg body weight per day, and rats 10 mg/kg body weight per day,
by gavage during gestation) resulted in increases in body weight and
kidney weights of pups of both species, along with enlarged kidneys
and a few cases of hydronephrosis. Increased liver weights were
observed in rat pups, and the occurrence of abnormal kidneys was
sporadic, with no dose-response relationship in studies with mice.
Khera (1974) reported a significant increase in the incidence of
unilateral or bilateral 14th rib in litters of Wistar rats receiving
doses of 80 and 120 mg HCB/kg body weight during gestation, but
maternal toxicity (loss of body weight and neurological effects) and
reduced fetal weights were noted in animals in these groups. (It
should be noted that, based on the biological half-lives reported for
HCB in mammals (section 6.2), the concentration of HCB in the dams in
these studies would not have reached the maximum that might occur as a
result of intake over a longer period).
Neurobehavioural development was affected in the offspring of
rats exposed to 2.5 or 25 mg/kg body weight per day by gavage 2 weeks
prior to breeding. Pups in both treated groups were hyperactive (based
on tests of negative geotaxic reflex, olfactory discrimination, and
exploratory locomotor activity) at 6-20 days of age. Pups from the
high treatment groups showed reduced acoustic startle response at 23
days of age, but a significantly increased response at 90 days. These
doses did not affect learning (swim T-maze) or motor activity in older
offspring, nor maternal or fetal body weights, length of gestation,
number of pups/litter at birth, or number of days to eye opening
(Goldey & Taylor, 1992).
Lilienthal et al. (1996) recently reported HCB-induced effects on
neurobehavioural development of rat pups exposed both maternally and
through the diet (dams were exposed to 0, 8 or 16 mg HCB/kg diet for
90 days prior to mating and throughout gestation and nursing, after
which the offspring were fed the same levels for 150 days). Exposure
to HCB did not affect the mean body weight of the pups (except males
at 150 days of age), or the number of pups/litter, but did increase
the mean body weight of dam, and their liver-to-body weight ratios.
Schedule-controlled behaviour was affected at 8 and 16 mg HCB/kg diet
(0.64 and 1.28 mg/kg body weight per day), as indicated by a dose-
related decrease in post-reinforcement pause at the end of the
experiment. Exploratory locomotor activity, open field behaviour at 21
days of age, and active avoidance learning at 90 days of age were
unaffected.
7.6 Immunotoxicity
The results of a number of studies have indicated that HCB
affects the immune system, with immunosuppressive effects in mice and
immunostimulatory effects in rats (summarized by Vos, 1986).
Balb/C mice exposed to 5 mg HCB/kg diet (approximately 0.6 mg/kg
body weight per day) for 3 to 18 weeks were more susceptible to
Leishmania infection (Loose, 1982) and had reductions in resistance
to a challenge with tumour cells and in the cytotoxic macrophage
activity of the spleen (Loose et al., 1981). Barnett et al. (1987)
reported that Balb/C mice exposed to maternal doses of 0.5 or 5 mg
HCB/kg body weight per day in utero and through nursing had severe
depression of the delayed-type hypersensitivity response to a contact
allergen (oxazolone). In a number of studies, exposure of mice to
diets containing 167 mg HCB/kg in diet (approximately 20 mg HCB/kg
body weight per day) for several weeks depressed humoral immunity,
cell-mediated immunity and host resistance (Vos, 1986; Carthew et al.,
1990).
In rats or rhesus monkeys with oral exposure to between 3 and
120 mg HCB/kg body weight per day for periods from 3 weeks to 6 months
in various studies, proliferative histopathological effects in the
thymus, spleen, lymph nodes, and/or lymphoid tissues of the lung have
been observed (Kimbrough & Linder, 1974; Iatropoulos et al., 1976;
Goldstein et al., 1978; Vos et al., 1979a,b; Kitchin et al., 1982).
Gralla et al. (1977) observed that long-term exposure to 1 mg HCB/day
(equivalent to a dose at the start of the experiment of roughly
0.12 mg/kg body weight per day) caused nodular hyperplasia of the
gastric lymphoid tissue in beagle dogs.
In rats, prominent changes following dietary exposure to HCB
include elevated IgM levels and an increase in the weights of the
spleen and lymph nodes. Histopathologically, the spleen shows
hyperplasia of B-lymphocytes in the marginal zone and follicles, while
lymph nodes show an increase in proportions of high endothelial
venules, indicative of activation. High endothelial-like venules are
induced in the lung, as are accumulations of macrophages. Functional
tests revealed an increase in cell-mediated immunity, as measured by
DTH reactions, a notable increase in primary and secondary antibody
response to tetanus toxoid, and decreased NK activity in the lung (Vos
et al., 1979a,b). Stimulation of humoral and cell-mediated immunity
occurred even at dietary levels as low as 4 mg HCB/kg (approximately
0.2 mg HCB/kg body weight per day); at such a dose conventional
parameters for hepatotoxicity were unaltered (Vos et al., 1983).
Therefore, the developing immune system of the rat seems to be
particularly vulnerable to the immunotoxic action of HCB.
More recent studies indicate that HCB may cause autoimmune-like
effects in the rat. Wistar rats treated with HCB had elevated levels
of IgM, but not IgG, against the autoantigens single-stranded DNA,
native DNA, rat IgG (representing rheumatoid factor), and bromelain-
treated mouse erythrocytes (that expose phosphatidylcholine as a major
autoantigen). It has been suggested that HCB activates a recently
described B cell subset committed to the production of these
antibodies (Schielen et al., 1993). The role of these autoantibodies
is still a matter of controversy. Increased levels have been
associated with various systemic autoimmune diseases, but a protective
role of these autoantibodies against development of autoimmune disease
has been postulated as well. Interesting in this respect are the
observations that HCB had quite opposite effects in two different
models of autoimmune disease in the Lewis rat. HCB treatment severely
potentiates allergic encephalitis elicited by immunization with myelin
in complete Freund's adjuvant, while it strongly inhibits the
development of arthritic lesions elicited by complete Freund's
adjuvant as such (Van Loveren et al., 1990).
A possible relation between the immunomodulatory properties of
HCB and HCB-induced skin lesions, attributed in the literature to the
porphyrinogenic action of HCB, was recently indicated. In rats treated
with a combination of HCB and triacetyloleandomycin (TAO, a selective
inhibitor of cytochrome P-450IIIa), porphyria was greatly reduced.
Remarkably, combined treatment with HCB and TAO did not substantially
affect the incidence and severity of skin lesions. In addition, TAO
did not influence the immunomodulatory effect of HCB, including the
formation of antibodies. From these findings it has been suggested
that an immunological component underlies, at least in part, the
HCB-induced skin lesions in the rat (Schielen et al., 1995).
8. EFFECTS ON HUMANS
8.1 General population exposure
Numerous reviews have been published of an accidental poisoning
incident in Turkey that occurred in 1955-1959 as a result of
HCB-treated wheat grain (distributed by the Turkish government for
planting purposes) being ground into flour and made into bread
(Schmid, 1960; Cam & Nigogosyan, 1963; Dogramaci, 1964; Peters, 1976;
Courtney, 1979; Peters et al., 1982; US EPA, 1985a; Gocmen et al.,
1989). In this incident, more than 600 cases of porphyria cutanea
tarda (PCT) were clinically identified, and it was estimated that as
many as 3000-5000 persons were affected, with a mortality of 10%. The
condition developed primarily in children 4-14 years of age (roughly
80% of cases), occurring infrequently in adults and rarely in children
under 4 years of age. In a number of reports, it has been suggested
that males developed the condition in higher proportion than females.
However, Dogramaci et al. (1962) demonstrated that the sex ratio was
skewed in favour of males in both the affected and unaffected
populations. In addition to disturbances in porphyrin metabolism
(excretion of porphyrins and porphyrin precursors was greatly
increased), clinical manifestations included skin lesions (erythema,
bullae), ulcerations and resultant scarring, friable skin,
hyperpigmentation, hypertrichosis, enlarged liver, weight loss,
enlargement of the thyroid gland and lymph nodes, neurological
effects, and a characteristic port wine colour of the urine (from
increased excretion of porphyrins). In roughly half the cases,
osteoporosis of extremities, deformation of the fingers or arthritis
was also noted. The dermatological lesions, which occurred on the
exposed parts of the body, particularly the face and hands, were often
precipitated by sunlight. They tended to remit in winter and relapse
during the spring and summer (Peters, 1976; Peters et al., 1982). The
estimated dose was 50-200 mg/day for a number of months before
manifestations of the disease became apparent (Cam & Nigogosyan,
1963); the basis for this estimate was not presented, however, making
exposure calculations unreliable for this population. In 20- to 30-
year follow-ups of exposed individuals, neurological, dermatological
and orthopaedic abnormalities persisted, and there were elevated
levels of porphyrins in excreta of some individuals (Peters et al.,
1982; Peters et al., 1986; Gocmen et al., 1989).
In this incident, a disorder called "pembe yara" or "pink sore"
was described in infants of mothers who either had PCT or had eaten
HCB-contaminated bread. These infants developed characteristic pink
cutaneous lesions, and often had fevers, diarrhea, vomiting, weakness,
convulsions, enlarged livers and progressive wasting. It is noteworthy
that PCT was not observed in these children (Cam, 1960; Peters et al.,
1982). At least 95% of these children died within a year of birth, and
in many villages no children between the ages of 2-5 years survived
during the period 1955-1960. Elevated concentrations of HCB (levels
were not quantified at the time, but the average concentration in milk
from 56 porphyric mothers, 20-30 years after the incident, was
510 ng/g on a fat basis) were found in the mothers' milk and cessation
of breast-feeding slowed the deterioration of infants with this
disorder (Peters et al., 1966; Gocmen et al., 1989).
No adequate epidemiological studies of cancer in populations
exposed to HCB in the environment were found in the literature. In
long-term follow-up of the Turkish poisoning victims with porphyria
(Peters et al., 1982; Cripps et al., 1984; Gocmen et al., 1989) there
was no evidence of increased cancer incidence, although these studies
were not designed to evaluate this end-point, and only a small
fraction of the exposed people was followed up. There was a high
frequency of enlarged thyroids in the Turkish poisoning victims (27%
of men and 60% of women, compared to an average of 5% in the area
(Peters et al., 1982)), but Gocmen et al. (1989) reported that they
observed no malignant tumours of the liver or thyroid in 252 of the
poisoning victims. In three patients who underwent thyroidectomy,
histopathological examination indicated that the enlargement was due
to colloidal goitre.
Grimalt et al. (1994) reported a small ecological study of cancer
incidence (129 cases in all) in the inhabitants of a village in Spain
located near a chlorinated solvents factory. There were statistically
significant excesses of thyroid neoplasms and soft-tissue sarcomas in
males, compared with the province as a whole, although these were
based on only 2 and 3 cases, respectively. The exposures experienced
by this population were somewhat unclear. Levels of HCB in ambient air
and in the sera of volunteers were much higher in the village than in
Barcelona (means of 35 ng/m3 versus 0.3 ng/m3 and 26 µg/litre versus
4.8 µg/litre, respectively), but the authors presented evidence that
historical exposures had been much higher and indicated that all of
the males with cancer for whom there were occupational histories had
worked in the factory. Ambient air monitoring revealed that there were
exposures to a variety of other compounds, including polychlorinated
biphenyls, p,p'DDE, chloroform, carbon tetrachloride,
trichloroethylene and tetrachloroethylene, but at similar or lower
levels than in the reference community.
8.2 Occupational exposure
There have been case reports of workers developing PCT as a
result of direct contact with HCB (Courtney, 1979; Currier et al.,
1980), although there was no association between exposure to HCB and
PCT in three cross-sectional studies of very small populations of
exposed workers (Morley et al., 1973; Burns et al., 1974; Currier et
al., 1980). There was no evidence of cutaneous porphyria in a cross-
sectional study of the general population in Louisiana, USA, exposed
to HCB through the improper transport and disposal of hex waste;
however, plasma concentrations of HCB were significantly correlated
with levels of coproporphyrin in urine and of lactic dehydrogenase in
blood (Burns & Miller, 1975).
Available epidemiological studies on the carcinogenicity of HCB
in occupationally exposed humans are restricted to one study of a
cohort of 2391 magnesium metal production workers in Norway. Although
the incidence of lung cancer was significantly elevated compared to
that of the general population, workers were exposed to numerous other
agents in addition to HCB, including coal tar, asbestos and dust of
metal oxides and chlorides (Heldaas et al., 1989). Selden et al.
(1989) reported a case of hepatocellular carcinoma in a 65-year-old
man who had been employed for 26 years in an aluminum smelting plant,
where he had potential exposure to a range of substances, including
HCB, other chlorobenzenes, chlorophenols, dioxins and furans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Data on the acute and chronic ecotoxicity of HCB are available
for species from a number of trophic levels, including protozoans,
algae, invertebrates and fish, for both the freshwater and marine
environments. With reference to terrestrial organisms, toxicity data
are available only for birds and mammals (the results of studies in
mammals are summarized in chapter 7). Since HCB is nearly insoluble in
water, and tends to partition from water to the atmosphere, the
substance is lost rapidly from open-test solutions. Hence, it is
difficult to maintain test concentrations for a sufficient time to
establish concentration-effects profiles for aquatic organisms.
Furthermore, HCB tends to bind to suspended solids in the water column
and thus may not be bioavailable to test organisms. This discussion of
the toxicity of HCB to aquatic organisms will therefore focus on tests
conducted under flow-through conditions, static renewal conditions, or
using closed vessels with minimal headspace. In addition, no
consideration has been given to tests in which concentrations of HCB
were well above its solubility in water (5 µg/litre at 25°C).
9.1 Short-term exposure
9.1.1 Aquatic biota
Of four freshwater algal species tested, only one, Chlorella
pyrenoidosa, was affected by concentrations of HCB in water at or
below its limit of aqueous solubility. Reduced production of
chlorophyll, dry matter, carbohydrate and nitrogen was observed for
C. pyrenoidosa after exposure to a nominal concentration of
1 µg/litre HCB for 46 h in a static-closed system (Geike & Parasher,
1976a). A no-observed-effect concentration (NOEC) was not determined
in this study.
At concentrations equal to its aqueous solubility in water
(5 µg/litre), HCB was not lethal to the freshwater water flea
Daphnia magna in a flow-through test in which concentrations of HCB
were measured (Nebecker et al., 1989). In 96-h flow-through tests on
marine invertebrates, exposure to HCB caused 13% mortality in pink
shrimp ( Penaeus duorarum) at a measured concentration of 7 µg
HCB/litre, and 10% mortality in grass shrimp ( Palaemonetes pugio) at
17 µg/litre. The NOEC values in these species were 2.3 µg/litre and
6.1 µg/litre, respectively (Parrish et al., 1974). In a static-closed
system, there was a 10% reduction in reproduction of the ciliate
protozoan Euplotes vannus after exposure to a nominal concentration
of 10 µg/litre HCB for 48 h (Persoone & Uyttersprot, 1975).
The available data on freshwater fish species indicated no
harmful effects at concentrations at or near the limit of solubility
of HCB in water during acute exposure (Call et al., 1983; Ahmad et
al., 1984). In the only available study for marine fish species, there
were no effects on mortality in sheepshead minnow ( Cyprinodon
variegatus) after flow-through exposure to a measured concentration
of 13 µg/litre HCB for 96 h (Parrish et al., 1974).
Limited data are available concerning the toxic effects of HCB in
sediment on freshwater and marine biota. In a 96-h sediment toxicity
test on the marine shrimp, Crangon septemspinosa, no mortality was
observed at the highest concentration of HCB tested, 300 µg/litre
(McLeese & Metcalfe, 1980).
Several studies have confirmed that there is a relatively
constant body residue associated with acute lethality in freshwater
fish, invertebrates and algae exposed to mono-to-pentachlorobenzenes
(McCarty et al., 1992a; Ikemoto et al., 1992). The acute LC50
critical body residue for chlorobenzenes is 2 µmol/g wet weight, or
569.6 µg/g wet weight for HCB, assuming that HCB has the same mode of
action as the other chlorobenzenes (McCarty et al., 1992b).
9.1.2 Terrestrial biota
The LD50 for HCB in herring gull ( Larus argentatus) embryos
injected on day 4 and tallied on day 25 was 4.3 µg/g body weight
(Boersma et al., 1986). At a dose of 1.5 µg/g body weight, there were
significant reductions in embryonic weight. Five-day LC50 values
(i.e., 5 days of HCB-containing diet followed by 3 days of untreated
diet) were 617 µg/g diet for 10-day-old ring-necked pheasants
( Phasianus colchicus) and > 5000 µg/g diet for 5-day-old mallards
( Anas platyrhynchos) (Hill et al., 1975). Induction of porphyria has
been observed in studies of Japanese quail following administration of
500 µg HCB/g body weight per day for between 5 and 10 days either in
food or via intraperitoneal injection (Buhler & Carpenter, 1986;
Lambrecht et al., 1988).
9.2 Long-term exposure
9.2.1 Aquatic biota
Growth of cultures of the alga Chlorella pyrenoidosa was
increased by exposure for 3 months to a nominal concentration of 1 µg
HCB/litre (Geike & Parasher, 1976b), while that of the protozoan
Tetrahymena pyriformis was decreased after a 10-day exposure to the
same concentration (Geike & Parasher, 1976b).
After exposure to 5 µg HCB/litre for 10 days in a static-renewal
system, crayfish ( Procambarus clarki) experienced damage to the
hepatopancreas (Laseter et al., 1976). The fertility of Daphnia
magna was reduced by 50% after exposure for 14 days to a measured
concentration of 16 µg/litre HCB in a static-closed system (Calamari
et al., 1983). Significantly increased mortality was observed in
amphipods, Gammarus lacustris, exposed to a measured concentration
of 3.3 µg HCB/litre for 28 days under flow-through conditions
(Nebecker et al., 1989). However, the results of this study indicated
a weak-dose response relationship. In two other flow-through studies,
there were no effects on survival, growth or reproduction of the
amphipod Hyallela azteca and the worm Lumbriculus variegatus at a
measured concentration of 4.7 µg HCB/litre (Nebecker et al., 1989).
In several studies, fathead minnows ( Pimephales promelas) and
rainbow trout ( Oncorhynchus mykiss) experienced no mortality or
effects on growth after exposure to levels of HCB approaching its
aqueous solubility (Ahmad et al., 1984; Carlson & Kosian, 1987; US
EPA, 1988; Nebecker et al., 1989). However, Laseter et al. (1976)
reported liver necrosis in large-mouth bass ( Micropterus salmoides)
after an exposure for 10 days to 3.5 µg HCB/litre under flow-through
conditions.
Guidelines for the protection and management of aquatic sediment
quality in Ontario, Canada (Persaud et al., 1991) have given a no-
observed-effect level (NOEL), a lowest-observed-effect level and a
severe-effect level for a variety of contaminants. The values given
for HCB are 10 ng/g dry weight, 20 ng/g dry weight and 24 000 ng/g
organic carbon. The partitioning approach was used to determine the
lowest-observed-effect level, whereas the severe-effect level was more
dependent on the screening level concentration approach. The
limitation of both approaches is that they are unable to separate the
biological effects that are due to a combination of contaminants; thus
while ecotoxicological effects can be established, these cannot be
attributed to any one chemical contaminant. This is a very serious
limitation since virtually all sediments are contaminated with a wide
variety of pollutants, and there is no indication that HCB was the
dominant pollutant.
Quantitative structure-activity relationships (QSAR) were used to
estimate the narcotic toxicity for 19 species to predict NOELs (Van
Leeuwen et al., 1992). The NOELs for water, sediment and residues in
biota were predicted only on the basis of the octanol/water partition
coefficient and relative molecular mass. The QSAR-derived level for
HCB in sediments was 5814 ng/g dry weight (20.4 nmol/g in the
reference) for sediments with 5% total organic carbon content. The
adjusted value for sediment with 1% total organic carbon content is
1163 ng/g. There is no experimental verification of these
calculations. Thus, no firm evidence is available on the critical
levels of HCB in sediments.
9.2.2 Terrestrial biota
In adult Japanese quail ( Coturnix japonica) fed diets
containing HCB for 90 days, mortality was increased at 100 µg HCB/g
in diet, and hatchability of eggs was significantly reduced at 20 µg/g
(Vos et al., 1971, 1972). At 5 µg/g, increased liver weight, slight
liver damage and increased faecal excretion of coproporphyrin were
observed. Eurasian kestrels ( Falco tinnunculus) fed mice containing
200 µg HCB/g fresh body weight for 65 days had significant weight
loss, ruffling of feathers, tremors, increased liver weight and
decreased heart weight (Vos et al., 1972).
The available long-term toxicity data for mammals are discussed
in section 7.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on estimates of mean exposure from various media (section
5.2), the general population is exposed to HCB principally in food
(mean intakes for adults range from 0.0004 to 0.0028 µg/kg body weight
per day). Intakes are estimated to be considerably less for ambient
air (3.4 × 10-5 to 2.1 × 10-4 µg/kg body weight per day) and
drinking-water (2.2 × 10-6 to 4.4 × 10-5 µg/kg body weight per day).
Based on these intakes, it is estimated that the total average daily
intake of HCB from food, air and drinking-water is between 0.0004 and
0.003 µg/kg body weight per day.
Data on levels of occupational exposure to HCB are limited but
indicate that workers in some industries may be exposed to higher
levels of HCB than the general population, particularly in the
manufacture of chlorinated solvents, and in the manufacture and
application of chlorinated pesticides contaminated with HCB. In some
instances inappropriate manufacturing and waste management practices
may expose nearby populations to higher levels of HCB than the general
population. Exposures may also be elevated in some indigenous
subsistence populations, particularly those that consume large
quantities of food species near the top of the food chain.
Owing to the elimination of HCB in breast milk, mean intakes by
nursing infants are estimated to range from < 0.018 to 5.1 µg/kg body
weight per day in various countries (see section 5.2.4 and Table 8).
10.1.2 Health effects
Available data on the effects of HCB in humans are limited
principally to those of people exposed in an accidental poisoning
incident that occurred in Turkey between 1955 and 1959. More than 600
cases of porphyria cutanea tarda (PCT) were observed, and infants of
exposed mothers experienced cutaneous lesions, clinical symptoms and
high mortality. It has been estimated that victims were exposed to an
estimated dose of 50-200 mg HCB/day for an undetermined, but extended,
period of time. However, the basis of this estimate was not provided,
making exposure calculations unreliable for this population. Studies
of the carcinogenicity of HCB in humans are limited to two small
epidemiological studies of cancer incidence in populations with poorly
characterized exposure to HCB as well as to numerous other chemicals.
No excesses of neoplasms have been reported in long-term follow-up
studies of the people with porphyria in the incident in Turkey, but
only a small fraction of the population was followed-up, and these
studies were not designed specifically to assess neoplastic end-
points.
Hence, the available data on humans are inadequate to serve as a
basis for assessment of effects from exposure to HCB. The remainder of
this evaluation is, therefore, based on studies in animals.
Based on the studies reviewed in section 7, the critical effects
induced by HCB in experimental animals comprise both non-neoplastic
and neoplastic effects.
With respect to non-neoplastic effects, repeated exposure to HCB
has been found to cause a wide range of non-neoplastic effects in
several species of animals, with similar lowest-observed-effect-levels
(LOELs) and no-observed-effect-levels (NOELs) for a number of end-
points (see Table 9). In these studies, effects reported have included
those on the liver in pigs and rats, on calcium metabolism in rats, on
ovarian histopathology in monkeys, on immune function in mice and
rats, on neurotransmitter levels in the hypothalamus of mink, on
postnatal survival in mink, and on neurobehavioural development in
rats. The range over which the various effects have been observed is
quite narrow; the lowest LOELs compiled in Table 9 range from 0.1 to
0.7 mg/kg body weight per day, while the lowest NOELs range from 0.05
to 0.07 mg/kg body weight per day.
Based on the induction of a variety of tumours in hamsters, rats
and mice exposed by ingestion, there is sufficient evidence that HCB
is carcinogenic in animals. The available evidence indicates that HCB
has little or no genotoxic activity and is therefore unlikely to be a
direct-acting (genotoxic) carcinogen. However, the Task Group noted
that tumours, some of which were malignant, have been induced in
multiple species, at multiple sites, in some instances at doses that
were not overtly toxic in other respects and that are within an order
of magnitude of those that produce more subtle toxicological effects,
or following subchronic exposure. Although there is some evidence to
suggest that HCB may cause cancer by indirect mechanisms, the evidence
is not definitive at this time and does not address all tumour sites.
Table 9. No-observed-effect and lowest-observed-effect levels (NOELs and LOELs) in mammals exposed to HCB
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Mouse Depressed delayed-type hypersensitivity - 0.5a Barnett et al.
response to oxazolone in mice exposed (1987)
to HCB in peanut butter in utero
(throughout gestation) and via nursing
to 45 days of age (section 7.6)
Mouse Increased susceptibility to Leishmania - 0.6 Loose et al. (1981);
infection, and reductions in resistance Loose (1982)
to a challenge with tumour cells and in
the cytotoxic macrophage activity of the
spleen in mice with subchronic exposure
to HCB in diet (section 7.6)
Rat Alterations in Ca metabolism (increased 0.07 0.7 Andrews et al.
serum 1,25-dihydroxy-vitamin-D3 levels, (1989, 1990)
reduced Ca excretion, alterations in
femur density, bone morphometry and
strength), increased liver weights, with
subchronic gavage exposure to HCB
(section 7.2)
Rat Increased cell-mediated and humoral - 0.2a Vos et al. (1983)
immune function, intraalveolar
macrophage accumulation, microsomal
ethoxyresorufin-O-deethylase activity,
in rats exposed to HCB in utero, via
nursing and in the diet to 5 weeks of
age (section 7.6)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Rat Increased organ weights (heart, brain 0.05-0.07 0.27-0.35 Arnold et al. (1985);
and liver) in F0 males, compound- Arnold & Krewski (1988)
related histological changes in liver
of both sexes of F1 rats with long-term
exposure to HCB in diet (section 7.3)
Rat Ultrastructural changes in livers 0.05-0.06 0.25-0.30 Mollenhauer et al.
(proliferation of SER, altered (1975, 1976)
mitochrondria, increase in numbers of
storage vesicles) of rats with long-term
exposure to HCB in diet (section 7.3)
Rat Induction of in vivo mixed-function - 0.5-0.6 Grant et al. (1974)
oxidase activity in rats with long-term
exposure to HCB in diet (section 7.3)
Rat Dose-related decrease in the post- - 0.64 Lilienthal et al.
reinforcement pause (PRP) after schedule- (1996)
controlled operant conditioning of rats
exposed to HCB in utero, through nursing,
and up to post-natal day 150
Mink Increased serotonin concentrations in - 0.16a Rush et al. (1983);
hypothalamus of mink dams with long-term Bleavins et al. (1984a,b)
dietary exposure to HCB, decreased
dopamine levels in hypothalamus, reduced
birth weights, and increased mortality
to weaning in mink kits with in utero
plus lactational exposure to HCB
(sections 7.3, 7.5)
Table 9 contd.
Species Effect NOEL LOEL Reference
(mg/kg body (mg/kg body
weight per day) weight per day)
Dog Nodular hyperplasia of gastric lymphoid - 0.12 Gralla et al.
tissue in beagles with long-term (1977)
exposure to HCB in gelatin capsules
(section 7.6)
Pig Increased urinary coproporphyrin and 0.05 0.05 Den Tonkelaar et al.
microsomal liver enzyme activity in (1978)
pigs with subchronic exposure to HCB
in diet (section 7.2)
a Doses reported are those received by dams
10.1.3 Approaches to risk assessment
The following is provided as a potential basis for derivation of
guidance values. Since ingestion is by far the principal route of
exposure and since the toxicological data for other routes of
administration are insufficient for evaluation, only the oral route is
addressed here, though the ultimate objective should be reduction of
total exposure from all routes.
Based on the scientific evaluation of the data for the non-
neoplastic and neoplastic end-points, two possible approaches to
develop health-based guidance values were suggested.
10.1.3.1 Non-neoplastic effects
The approach for non-neoplastic effects assumes a threshold for
these effects and is based on the use of the NOAEL or NOEL and an
uncertainty factor that takes account of interspecies and
interindividual variation in sensitivity to the substance, as well as
the quality of the available studies and the severity of effect.
The available data are sufficient to develop a Tolerable Daily
Intake (TDI) for HCB. The lowest reported NOELs and LOELs for several
different types of effects, such as those on the liver in rats and
pigs, calcium metabolism in rats, ovarian morphology in monkeys,
immune function in rats and mice, neurobehavioural development in rats
and perinatal survival in mink, fall within a very small range (Table
9). Based on the lowest reported NOELs included in the table
(approximately 0.05 mg/kg body weight per day based primarily on
hepatic effects observed in a subchronic study in pigs and in chronic
studies in rats), a TDI of 0.17 µg/kg body weight per day has been
derived for non-neoplastic effects, by incorporating an uncertainty
factor of 300 (x 10 for intraspecies variation; × 10 for interspecies
variation, × 3 for severity of effect). A factor of 3 for severity of
effects was chosen as HCB causes i) multiple non-neoplastic effects in
several species, and ii) LOELs for a number of end-points for which
NOELs have not been determined are very close to the NOEL, from the
critical studies, of 0.05 mg/kg body weight per day. However, it is
fully realized that national authorities may choose other end-points
or uncertainty factors depending upon data evaluation and future
scientific findings.
10.1.3.2 Neoplastic effects
The approach for neoplastic effects is based on the Tumorigenic
Dose5, or TD5 i.e., the intake or exposure associated with a 5%
excess incidence of tumours in experimental studies in animals (IPCS,
1994). This is a benchmark approach in which the TD5 is calculated
directly from the experimental data rather than using the upper or
lower confidence limits. Uncertainty factors are then applied to the
TD5 to obtain a guidance value. The choice of uncertainty factors is
based on the level and nature of mechanistic data available, the
quality of the database, the tumour pattern, the dose-response
relationship, and the experimental model chosen. The final value will
reflect the degree of certainty one has with the available
information.
For the purpose of indicating the magnitude of risk of HCB, the
two-generation study in rats has been selected, owing to its relevance
to the exposure of the general human population, as the design of this
study involved exposure to relatively low concentrations of HCB in the
diet (including in utero and lactational exposure). Moreover, tumour
pathology was inadequately reported in the available studies in
hamsters and mice, and there is some concern that in the other
adequate study in rats, there may also have been exposure by
inhalation to some HCB that was incorporated in the diet as a powder.
The TD5 value was calculated from the results of the two-
generation study in rats using a multistage model (Crump & Howe,
1982). The tumour incidences in the pups were analysed in the same
manner as data from a single-generation study, owing to the lack of
information on individual litters. On this basis, the TD5 values
range from 0.81 mg/kg body weight per day for neoplastic liver nodules
in females to 2.01 mg/kg body weight per day for parathyroid adenomas
in males. The Task Group decided that the most sensitive end-point
(neoplastic nodules of the liver) would be used in its analysis. In
calculating the suggested guidance value, it was agreed to use an
uncertainty factor of 5000, based on consideration of the insufficient
mechanistic data. The TD5 was divided by this uncertainty factor to
arrive at the suggested guidance value of 0.16 µg/kg body weight per
day. However, it is fully realized that national authorities may
choose other end-points or uncertainty factors depending upon data
evaluation and future scientific findings.
Although infants may have a high intake of HCB via breast milk
for a short time, the TD5 and TDI were considered to be protective of
the health of this population (unless there are extreme exposures),
because one of the long-term studies used in deriving these values
included lactational exposure. However, it should be noted that the
TD5 and TDI values derived above should not be compared directly with
intakes from breast milk by nursing infants, since the guidance values
are based on a lifetime intake, whereas the duration of breast-feeding
is relatively short.
10.2 Evaluation of effects on the environment
HCB is widely distributed in the environment, by virtue of its
mobility and resistance to degradation, although slow photodegradation
in air (half-life of approximately 80 days) and microbial degradation
(half-life of several years) do occur. It has been detected in air,
water, sediment, soil and biota from around the world. HCB is a
bioaccumulative substance (BCF values range from 375 to > 35 000),
and biomagnification of HCB through the food chain has been reported.
In studies of the acute toxicity of HCB to aquatic organisms,
exposure to concentrations in the range of 1 to 17 µg/litre reduced
production of chlorophyll in algae and reproduction in ciliate
protozoa. In longer-term studies, the growth of sensitive freshwater
algae and protozoa was affected by a concentration of 1 µg/litre,
while a concentration of approximately 3 µg/litre caused mortality in
amphipods and liver necrosis in largemouth bass. The concentrations of
HCB in surface waters around the world are much lower than these
effect levels (3 to 5 orders of magnitude lower), except in a few
extremely contaminated localities.
Injection studies in eggs have shown that tissue levels of
1500 ng/g wet weight reduce embryo weights in herring gulls (lowest
dose tested). No studies were available to establish a NOAEL. For many
bird species, reduced embryo weights are associated with lower
survival of chicks. This effect level is within an order of magnitude
of the levels measured in the eggs of sea birds and raptors from a
number of locations from around the world, suggesting that present
levels of HCB in certain locations may harm embryos of bird species.
Experimental studies on mink indicate that they are sensitive to
the toxic effects of HCB; long-term ingestion of diets containing
1000 ng HCB/g (the lowest dose tested) increased mortality, decreased
birth weights of offspring exposed in utero and via lactation, and
altered levels of neurotransmitters in the hypothalamus of dams and
their offspring. No studies were available to establish a NOAEL. This
dietary effect level is only a few times higher than the
concentrations of HCB measured in various species of fish from a
number of industrialized locations from around the world, suggesting
that present levels of HCB in fish species from certain locations may
adversely affect mink and perhaps other fish-eating mammals.
11. RECOMMENDATION FOR PROTECTION OF HUMAN HEALTH AND THE ENVIRONMENT
a) Alternatives should be found for any present uses of HCB.
b) It is important to reduce the environmental burden of HCB by:
(i) identifying remaining sources and quantities of release to
the environment from these sources, including point source
emissions, waste disposal sites and production facilities;
(ii) applying appropriate manufacturing and waste disposal
practices in order to decrease levels of HCB in the
environment.
c) Human monitoring of HCB in blood and breast milk should be
undertaken to develop data representing exposure of the general
population, in order to identify highly exposed populations and
potential sources, and to enable interpretation of individual
results.
d) In order to gauge the efficacy of control measures it would be
valuable to monitor environmental levels and effects in locations
where levels are higher than the global average.
e) Neonatal effects in humans and other species have been associated
with ingestion of high doses of HCB through breast milk. It is
recommended that techniques be developed to assess appropriately
the risk to infant health from exposure to HCB and related
compounds in breast milk.
12. FURTHER RESEARCH
12.1 Environment
a) To improve the database available for environmental risk
assessment, it is considered important to establish a NOEL for
the serious reproductive effects seen in mink at dietary levels
approaching those found in certain locations.
b) Since HCB is persistent in soil and sediment, it would be
valuable to perform biodiversity experiments with HCB-treated
soil and sediment.
12.2 Human health
a) Based on the effects of low doses of HCB on ovarian tissues in
primates, involving disorders of germ cells and the ovarian
surface epithelium, the following is recommended:
(i) exposed populations should be studied for relevant
reproductive human outcomes of interest, particularly, fetal
loss and ovarian cancer;
(ii) reproductive tissues such as ovarian follicular fluid should
be included in human monitoring studies on HCB levels and/or
effects.
b) In order to decrease uncertainty in the risk assessment of HCB
and related compounds, research into the primary mechanism(s) of
action for tumorigenic, thyroid, reproductive, porphyrigenic,
neurotoxic and immunological effects of HCB should be undertaken.
c) Preliminary evidence suggests that HCB acts, at least in part,
through Ah receptor-linked mechanisms. This should be evaluated
more fully and compared to other polyhalogenated aromatic
chemicals for which a wealth of data are already available.
d) Given the toxicity of HCB and the few data for humans,
multicentre longitudinal studies of highly exposed human
populations should be undertaken. End-points of interest should
cover toxicokinetics (e.g., half-life), thyroid function,
porphyrin metabolism, reproductive outcomes (e.g., fetal losses),
and cancer. Nursing infants from these populations should be
followed to assess immunological and neurobehavioural
development.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
HCB as a Group 2B carcinogen (possibly carcinogenic to humans) based
on inadequate evidence for carcinogenicity to humans and sufficient
evidence for carcinogenicity to animals (IARC, 1987).
A drinking-water guideline of 1 µg/litre was developed for HCB
based on an evaluation of the production of liver tumours in female
rats and applying the linearized multistage model to calculate an
excess life-time cancer risk of 10-5 (WHO, 1993).
A conditional acceptable daily intake of 0.6 µg HCB/kg body
weight was developed by the Joint FAO/WHO Joint Meeting on Pesticide
Residues in Food (FAO/WHO, 1975). This recommendation was withdrawn in
1978 (FAO/WHO, 1978).
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the Legal File of
the International Register of Potentially Toxic Chemicals (IRPTC,
1993).
REFERENCES
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in the United Kingdom 1969-71. Br Med J, 2:
533-556.
Abbott DC, Collins GB, Goulding R, & Hoodless RA (1981) Organochlorine
pesticide residues in human fat in the United Kingdom 1976-77. Br Med
J, 283: 1425-1428.
Abbott DC, Goulding R, Holmes DC, & Hoodless RA (1985) Organochlorine
pesticide residues in human fat in the United Kingdom 1982-1983. Hum
Toxicol, 4: 435-445.
Abraham K, Hille A, Ende M, & Helge H (1994) Intake and fecal
excretion of PCDDs, PCDFs, HCB and PCBs (138, 153, 180) in a breast-
fed and a formula-fed infant. Chemosphere, 29(9-11): 2279-2286.
Ahling B, Bjorseth A, & Lunde G (1978) Formation of chlorinated
hydrocarbons during combustion of poly(vinyl chloride). Chemosphere,
10: 799-806.
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, Defoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: A toxicity data summary, Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Environmental Research
Laboratory (EPA 600/3-84-009).
Albert LA, Alpuche L, Barcenas C, & Rendón J (1990) A survey of
organochlorine pesticide residues in cheese samples from three Mexican
regions. Environ Pollut, 65: 119-126.
Albro PW & Thomas R(1974) Intestinal absorption of hexachlorobenzene
and hexachlorocyclohexane isomers in rats. Bull Environ Contam
Toxicol, 12: 289-294.
Alves HHD & Chevalier M (1980) L'hexachlorobenzène dans
l'environnement québécois : Production, utilisation et présence.
Montréal, Québec, Environnement Canada, Service de la Protection de
l'Environnement (Rapport No EPS-3 QR-80-1).
Andrews JE & Courtney KD (1986) Hexachlorobenzene-induced renal
maldevelopment in CD-1 mice and CD rats. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 381-391 (IARC
Scientific Publications No. 77).
Andrews JE, Courtney KD, Stead AG, & Donaldson WE (1989)
Hexachlorobenzene induced hyperparathyroidism and osteosclerosis in
rats. Fundam Appl Toxicol, 12: 242-251.
Andrews JE, Jackson LD, Stead AG, & Donaldson WE (1990) Morphometric
analysis of osteosclerotic bone resulting from hexachlorobenzene
exposure. J Toxicol Environ Health, 31: 193-201.
Angerer J, Heinzow B, Reimann DO, Knorz W, & Lehnert G (1992)
Internal exposure to organic substances in a municipal waste
incinerator. Int Arch Occup Environ Health, 64: 265-273.
Ariño AA, Herrera A, Conchello MP, & Pérez C (1992) Hexachlorobenzene
residues in Spanish meat products after cooking, curing, and long-term
ripening. J Food Prot, 55(11): 920-923.
Ariño A, Herrera A, Conchello P, & Lazaro R (1993) Hexachlorobenzene
and hexachlorocyclohexane residues in pork as affected by weight and
sex. Bull Environ Contam Toxicol, 51: 647-650.
Arnold DL & Krewski D (1988) Long-term toxicity of hexachlorobenzene.
Food Chem Toxicol, 26: 169-174.
Arnold DL, Moodie CA, Charbonneau SM, Grice HC, McGuire PF, Bryce FR,
Collins BT, Zwadizka ZZ, Krewski DR, Nera EA, & Munro IC (1985) Long-
term toxicity of hexachlorobenzene in the rat and the effect of
dietary vitamin A. Food Chem Toxicol, 23: 779-793.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for hexachlorobenzene. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-90-17).
ATSDR (1994) Toxicological profile for hexachlorobenzene - Update.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry.
Atuma SS, Andersson O, Linder C-E, & Hansson L (1993) Levels of some
organochlorine compounds in sea trout ( Salmo trutta) and whitefish
( Caregonus lavaretus) from the Gulf of Bothnia. Aqua Fenn, 23:
221-226.
Avrahami M (1975) Hexachlorobenzene: IV. Accumulation and elimination
of HCB by pigs after oral dosing. NZ J Exp Agric, 3: 285-287.
Avrahami M & Steele RT (1972) Hexachlorobenzene: I. Accumulation and
elimination of HCB in sheep after oral dosing. NZ J Agric Res, 15:
476-481.
Ayotte P, Dewailly E, Bruneau S, Careau H, & Vézina A (1995) Arctic
air pollution and human health: what effects should be expected? Sci
Total Environ, 160/161: 529-537.
Babineau KA, Singh A, Jarrell JF, & Villeneuve DC (1991) Surface
epithelium of the ovary following oral administration of
hexachlorobenzene to the monkey. J Submicrosc Cytol Pathol, 23:
457-464.
Bailey J, Knauf V, Mueller W, & Hobson W (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
rhesus monkeys; enhanced toxicity. Environ Res, 21: 190-196.
Ballschmiter K, & Wittlinger R (1991) Interhemisphere exchange of
hexachlorocyclohexanes, hexachlorobenzene, polychlorobiphenyls, and
1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane in the lower
troposphere. Environ Sci Technol, 25: 1103-1111.
Barnett JB, Barfield L, Walls R, Joyner R, Owens R, & Soderberg LSF
(1987) The effect of in utero exposure to hexachlorobenzene on the
developing immune response of BALB/c mice. Toxicol Lett, 39:
263-274.
Barquet A, Morgade C, & Pfaffenberger CD (1981) Determination of
organochlorine pesticides and metabolites in drinking water, human
blood serum, and adipose tissue. J Toxicol Environ Health, 7:
469-479.
Bates MN, Hannah DJ, Buckland SJ, Taucher JA, & Van Maanen T (1994)
Chlorinated organic contaminants in breast milk of New Zealand women.
Environ Health Perspect, 102 (Suppl 1): 211-217.
Bauer I, Weigelt S, & Ernst W (1989) Biotransformation of
hexachlorobenzene in the blue mussel ( Mytilus edulis). Chemosphere,
19 (10/11): 1701-1707.
Beck J & Hansen KE (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in soil.
Pestic Sci, 5: 41-48.
Béland P, Deguise S, & Plante R (1991) Toxicology and pathology of
St. Lawrence marine mammals - Final report. Rimouski, Québec, Canada,
Wildlife Toxicology Fund Research Grant, St. Lawrence National
Institute of Ecotoxicology, 95 pp.
Belfroid A, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, & Hermens
J (1993) The toxicokinetic behaviour of chlorobenzenes in earthworms
( Eisenia andrei): Experiments in water. Ecotoxicol Environ Saf,
25: 154-165.
Belfroid A, Meiling J, Sijm D, Hermens J, Seinen W, & Van Gestel K
(1994a) Uptake of hydrophobic halogenated aromatic hydrocarbons from
food by earthworms ( Eisenia andrei). Arch Environ Contam Toxicol,
27: 260-265.
Belfroid A, Sikkenk M, Seinen W, Van Gestel K, & Hermens J (1994b) The
toxicokinetic behavior of chlorobenzenes in earthworm ( Eisenia
andrei): Experiments in soil. Environ Toxicol Chem, 13: 93-99.
Beretta M & Dick T (1994) Organochlorine compounds in human milk,
Porto Alegre, Brazil. Bull Environ Contam Toxicol, 53: 357-360.
Biddleman TF, Billings WN, & Foreman WT (1986) Vapor-particle
partitioning of semivolatile organic compounds: Estimates from field
collection. Environ Sci Technol, 20: 1038-1043.
Bidleman TF, Wideqvist U, Jansson B, & Soderlund R (1987)
Organochlorine pesticides and polychlorinated biphenyls in the
atmosphere of southern Sweden. Atmos Environ, 21: 641-654.
Bidleman TF, Walla MD, Roura R, Carr E, & Schmidt S (1993)
Organochlorine pesticides in the atmosphere of the Southern Ocean and
Antarctica, January-March, 1990. Mar Pollut Bull, 26(5): 258-262.
Billings WN & Bidleman TF (1980) Field comparison of polyurethane foam
and Teax-GC resin for high-volume air sampling of chlorinated
hydrocarbons. Environ Sci Technol, 14(6):679-683.
Bishop CA, Lean DRS, Brooks RJ, Carey JH, & Ng P (1995) Chlorinated
hydrocarbons in early life stages of the common snapping turtle
( Chelydra serpentina serpentina) from a coastal wetland on Lake
Ontario, Canada. Environ Toxicol Chem, 14(3): 421-426.
Bjerk JE & Brevik EM (1980) Organochlorine compounds in aquatic
environments. Arch Environ Contam Toxicol, 9: 743-750.
Bleavins MR, Breslin WJ, Aulerich RJ, & Ringer RK (1982) Excretion and
placental and mammary transfer of hexachlorobenzene in the European
ferret ( Mustela putorius furo). J Toxicol Environ Health, 14:
929-940.
Bleavins MR, Bursian SJ, Brewster JS, & Aulerich RJ (1984a) Effects of
dietary hexachlorobenzene exposure on regional brain biogenic amine
concentrations in mink and European ferrets. J Toxicol Environ Health,
14: 363-377.
Bleavins MR, Aulerich RJ, & Ringer RK (1984b) Effects of chronic
dietary hexachlorobenzene exposure on the reproductive performance and
survivability of mink and European ferrets. Arch Environ Contam
Toxicol, 13: 357-365.
Boersma DC, Ellenton JL, & Yagminas A (1986) Investigation of the
hepatic mixed-function oxidase system in herring gull embryos in
relation to environmental contaminants. Environ Toxicol Chem, 5:
309-318.
Boese BL, Winsor M, Lee H II, Specht DT, & Rukavina LC (1990)
Depuration kinetics of hexachlorobenzene in the clam, Macoma nasuta.
Comp Biochem Physiol, C996: 327-331.
Bordet F, Mallet J, Maurice L, Borrel S, & Venant A (1993)
Organochlorine pesticide and PCB congener content of French human
milk. Bull Environ Contam Toxicol, 50: 425-432.
Bourbonniere RA, Van Sickle BL, & Mayer T (1986) The Great Lakes
sediment bank-I (including catalogs of lakes Huron and Ontario
samples). Burlington, Ontario, Environment Canada, National Water
Research Institute (NWRI Contribution No. 86-151).
Bouthillier L, Greselin E, Brodeur J, Viau C, & Charbonneau M (1991)
Male rat specific nephrotoxicity resulting from subchronic
administration of hexachlorobenzene. Toxicol Appl Pharmacol, 110:
315-326.
Braune BM & Norstrom RJ (1989) Dynamics of organochlorine compounds in
herring gulls: III. Tissue distribution and bioaccumulation in Lake
Ontario gulls. Environ Toxicol Chem, 8: 957-968.
Breslin WJ, Bleavins MR, & Ringer RK (1983) Distribution and excretion
of hexachlorobenzene in bobwhite. J Toxicol Environ Health, 11:
885-896.
Brooks GW & Hunt GE (1984) Source assessment for hexachlorobenzene.
Research Triangle Park, North Carolina, Radian Corporation (Prepared
for the US Environmental Protection Agency, Research Triangle Park)
(EPA 68-02-3818).
Bro-Rasmussen F (1986) Hexachlorobenzene: An ecotoxicological profile
of an organochlorine compound. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 231-239 (IARC
Scientific Publications No. 77).
Brown EA, Biddle K, & Spaulding JE (1986) Residue levels of
hexachlorobenzene in meat and poultry in the food supply of the U.S.A.
In: Morris CR & Cabral JRPed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 99-108 (IARC Scientific Publications No. 77).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17: 2363-1380.
Brunn H & Manz D (1982) Contamination of native fish stock by
hexachlorobenzene and polychlorinated biphenyl residues. Bull Environ
Contam Toxicol, 28: 599-604.
Brusick DJ (1986) Genotoxicity of hexachlorobenzene and other
chlorinated benzenes. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 393-397 (IARC
Scientific Publications No. 77).
BUA (German Chemical Society-Advisory Committee on Existing Chemicals
of Environmental Relevance) (1994) Hexachlorobenzene. Stuttgart, S.
Hirzel Wissenschaftliche Verlagsgesellschaft, 257 pp (BUA Report 119).
Buhler DR & Carpenter HM (1986) Japanese quail as a model for the
study of hexachlorobenzene-induced porphyria. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 477-480 (IARC
Scientific Publications No. 77).
Burns JE & Miller FM (1975) Hexachlorobenzene contamination: its
effects in a Louisiana population. Arch Environ Health, 30: 44-48.
Burns JE, Miller FM, Gomes ED, & Albert RA (1974) Hexachlorobenzene
exposure from contaminated DCPA in vegetable spraymen. Arch Environ
Health, 29: 192-194.
Burton MA & Bennett BG (1987) Exposure of man to environmental
hexachlorobenzene (HCB): an exposure commitment assessment. Sci Total
Environ, 66: 137-146.
Bush B, Snow J, Connor S, & Koblintz R (1985) Polychlorinated biphenyl
congeners (PCBs), p,pœ-DDe and hexachlorobenzene in human milk in
three areas of upstate New York. Arch Environ Contam Toxicol, 14:
443-450.
Cabral JRP & Shubik P (1986) Carcinogenic activity of
hexachlorobenzene in mice and hamsters. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 411-416 (IARC
Scientific Publications No. 77).
Cabral JRP, Shubik P, Mollner T, & Raitano F (1977) Carcinogenic
activity of hexachlorobenzene in hamsters. Nature (Lond), 269:
510-511.
Cabral JRP, Mollner T, Raitano F, & Shubik P (1979) Carcinogenesis of
hexachlorobenzene in mice. Int J Cancer, 23: 47-51.
Calamari D, Galassi S, Setti F, & Vighi M (1983) Toxicity of selected
chlorobenzenes to aquatic organisms. Chemosphere, 12: 253-262.
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA priority pollutants and related chemicals
in freshwater organisms. Center for Lake Superior Environmental
Studies, University of Wisconsin-Superior, Environmental Research
Laboratory, Duluth, Minnesota, U.S. Environmental Protection Agency
(EPA 600/3-83-095).
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed R, Jennings P,
Durfee R, Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979)
Water-related environmental fate of 129 priority pollutants, Volume
II. Washington, DC, US Environmental Protection Agency, Office of
Water Planning and Standards/Office of Water and Waste Management (EPA
440/4-79-029b).
Cam C (1960) Une nouvelle dermatose épidémique des enfants. Ann
Dermatol, 87: 393-397.
Cam C & Nigogosyan G (1963) Acquired toxic porphyria cutanea tarda due
to hexachlorobenzene. J Am Med Assoc, 183: 90-93.
Camps M, Planas J, Gómez-Catalán J, Sabroso M, To-Figueras J, &
Corbella J (1989) Organochlorine residues in human adipose tissue in
Spain: study of an agrarian area. Bull Environ Contam Toxicol, 42:
195-201.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1979) Pesticide
residue levels in soils and crops from 37 states, 1972 - National
Soils Monitoring Program (IV). Pest. Monit. J, 12: 209-229.
Carey AE, Dixon TE, & Yang HSC (1985) Environmental exposure to
hexachlorobenzene in the United States. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances.
Carlson AR & Kosian PA (1987) Toxicity of chlorinated benzenes to
fathead minnows ( Pimephales promelas). Arch Environ Contam
Toxicol, 16: 129-135.
Carthew P & Smith AG (1994) Pathological mechanisms of hepatic tumour
formation in rats exposed chronically to dietary hexachlorobenzene. J
Appl Toxicol, 14: 447-452.
Carthew P, Edwards RE, & Smith AG (1990) Immunotoxic effects of
hexachlorobenzene on the pathogenesis of systemic, pneumonic and
hepatic virus infections in the mouse. Hum Exp Toxicol, 8: 403-411.
Clark TP, Norstrom RJ, Fox GA, & Won HT (1987) Dynamics of
organochlorine compounds in herring gulls ( Larus argentatus): II. A
two-compartment model and data for ten compounds. Environ Toxicol
Chem, 6: 547-559.
Conchello P, Herrera A, Ariño A, Lazaro R, & Pérez-Arquillué C (1993)
Effect of grilling, roasting, and cooking on the natural
hexachlorobenzene content of ovine meat. Bull Environ Contam Toxicol,
50: 828-833.
Conde C, Naluenda C, & Arrabal C (1993) Hexachlorobenzene (HCB) in
human milk in Spain from 1984 to 1991. Bull Environ Contam Toxicol,
51: 827-831.
Courtney KD (1979) Hexachlorobenzene (HCB): a review. Environ Res,
20: 225-266.
Courtney KD & Andrews JE (1979) Mobilization of hexachlorobenzene
(HCB) during gestation. Toxicol Lett, 3: 357-361.
Courtney KD & Andrews JE (1985) Neonatal and maternal body burdens of
hexachlorobenzene (HCB) in mice: gestational exposure and lactational
transfer. Fundam Appl Toxicol, 5: 265-277.
Courtney KD, Copeland MF, & Robbins A (1976) The effects of
pentachloronitrobenzene, hexachlorobenzene, and related compounds on
fetal development. Toxicol Appl Pharmacol, 35: 239-256.
Courtney KD, Andrews JE, & Svendsgaard DJ (1979) Hexachlorobenzene
(HCB) deposition in maternal and fetal tissues of rat and mouse: I.
Chemical quantification of HCB in tissues. Environ Res, 19: 1-13.
Cripps DJ, Peters HA, Gocmen A, & Dogramaci I (1984) Porphyria turcica
due to hexachlorobenzene: a 20 to 30 year follow-up study on 204
patients. Br J Dermatol, 111: 413-422.
Cross JN, Hardy JT, Hose JE, Hershelman GP, Antrim LD, Gossett RW, &
Crecelius EA (1987) Contaminant concentrations and toxicity of sea-
surface microlayer near Los Angeles, California. Mar Environ Res,
23: 307-323.
Crump K & Howe N (1982) Global '82: a computer program to extrapolate
quantal animal toxicity data to low doses (Contract #41USC252C3).
Washington, DC, US Occupational Safety and Health Administration,
Office of Carcinogen Standards.
Currier MF, McClimans CD, & Barna-Lloyd G (1980) Hexachlorobenzene
blood levels and the health status of men employed in the manufacture
of chlorinated solvents. J Toxicol Environ Health, 6: 367-377.
D'Amour M & Charbonneau M (1992) Sex-related differences in hepatic
glutathione conjugation of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 112: 229-234.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17: 263-276.
Davis BD & Morgan RC (1986) Hexachlorobenzene in hazardous waste
sites. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 23-30 (IARC Scientific Publications No. 77).
Debets FMH & Strik JJTWA (1979) An approach to elucidate the mechanism
of hexachlorobenzene-induced hepatic porphyria, as a model for the
hepatotoxicity of polyhalogenated aromatic compounds (PHA's). In:
Strik JJTWA & Koeman JH ed. Chemical porphyria in man. Amsterdam,
Oxford, New York, Elsevier/North Holland Biomedical Press, pp 181-208.
Deleon IR, Maberry MA, Overton EB, Raschke CK, Remele PC, Steele, CF,
Warren VL, & Laseter JL (1980) Rapid gas chromatographic method for
the determination of volatile and semivolatile organochlorine
compounds in soil and chemical waste disposal site samples. J
Chromatogr Sci, 18: 85-88.
Dellinger B, Taylor PH, & Tirey DA (1991) Minimization and control of
hazardous combustion by-products. Cincinnati, Ohio, US Environmental
Protection Agency, Risk Reduction Laboratory (EPA 600/S2-90/039).
De Matteis F, Prior BE, & Rimington C (1961) Nervous and biochemical
disturbances following hexachlorobenzene intoxication. Nature (Lond),
191(4786): 363-366.
Den Besten C, Bennik MM, Van Iersel M, Peters MA, Teunis C, & Van
Bladeren PJ (1994) Comparison of the urinary metabolite profiles of
hexachlorobenzene and pentachlorobenzene in the rat. Chem-Biol
Interact, 90: 121-137.
Den Desten C, Bennik MHJ, Bruggeman I, Schielen P, Kuper F, Brouwer A,
Koeman JH, Vos JG, & Van Bladeren PJ (1993) The role of oxidative
metabolism in hexachlorobenzene-induced porphyria and thyroid hormone
homeostasis: a comparison with pentachlorobenzene in a 13-week feeding
study. Toxicol Appl Pharmacol, 119: 181-194.
Denomme MA, Leece B, Gyorkos J, Homonko K, & Safe S (1983)
Polychlorinated benzene and phenol congeners as inducers of rat
hepatic drug-metabolizing enzymes in immature male Wistar rats. Can J
Physiol Pharmacol, 61: 1063-1070.
Den Tonkelaar EM & Van Esch GJ (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
Den Tonkelaar EM, Verschueren HG, Bankovska J, De Vries T, Kroes R, &
Van Esch G (1978) Hexachlorobenzene toxicity in pigs. Toxicol Appl
Pharmacol, 43: 137-145.
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Dewailly E, Nantel A, Laliberté C, Weber J-P, Gingras S, & Meyer F
(1991) Organochlorines in the breast milk in Québec: a provincial
survey. Poster presentation at the Conference on Measuring,
Understanding, and Predicting Exposures in the 21st Century, Atlanta,
Georgia, 18-22 November 1991.
Ding W-H, Aldous KM, Briggs RG, Valente H, Hilker DR, Connor S, &
Eadon GA (1992) Application of multivariate statistical analysis to
evaluate local sources of chlorobenzene congeners in soil samples.
Chemosphere, 25(5): 675-690.
Dogramaci I (1964) Porphyrias and porphyrin metabolism with special
reference to porphyria in childhood. Adv Pediatr, 13: 11-63.
Dogramaci I, Wray JD, Ergene T, Sezer V, & Müftü Y (1962) Porphyria
turcica: a survey of 592 cases of cutaneous porphyria seen in
southeastern Turkey. Turk J Pediatr, 4: 138-148.
Eisenberg M & Topping JJ (1984) Organochlorine residues in shellfish
from Maryland waters. J Environ Sci Health, B19: 673-688.
Eisenreich SJ & Strachan WMJ (1992) Estimating atmospheric deposition
of toxic substances to the Great Lakes - An update: Proceedings of a
workshop, Burlington, 31 January-2 February 1992. Burlington, Ontario,
Canada Centre for Inland Waters.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCBs in
waste, sediment, and fish from the Mediterranean Sea. Bull Environ
Contam Toxicol, 34: 216-227.
Elissalde MH & Clark DE (1979) Testosterone metabolism by
hexachlorobenzene-induced hepatic microsomal enzymes. Am J Vet Res,
40: 1762-1766.
Elkin BT & Bethke RW (1995) Environmental contaminants in caribou in
the Northwest Territories, Canada. Sci Total Environ, 160/161:
307-321.
Enriquez De Salamanca R, Lopez-Miras A, Munoz JJ, To-Figueras J, &
Conde C (1990) Is hexachlorobenzene human overload related to
porphyria cutanea tarda? A speculative hypothesis. Med Hypotheses,
33: 69-71.
Environment Canada (1989) Atlantic region federal-provincial toxic
chemical survey of municipal drinking water sources 1985-1988 -
Interpretative Report. Moncton, New Brunswick, Environment Canada,
Conservation and Protection, Water Quality Branch, Inland Waters
Directorate.
Environment Canada (1992) Detroit incinerator monitoring program: Data
report No. 6. Ottawa, Ontario, Environment Canada, Conservation and
Protection, River Road Environmental Technology Centre (Report No.
PMD-92-1).
Environment Canada/Department of Fisheries & Oceans/Health & Welfare
Canada (1991) Toxic chemicals in the Great Lakes and associated
effects - Volume 1: Contaminant levels and trends. Ottawa, Ontario,
Minister of Supply and Services.
Environment Canada/Ontario Ministry of the Environment (1986) St.
Clair River pollution investigation (Sarnia area). Toronto, Canada-
Ontario Agreement Respecting Great Lakes Water Quality.
Ernst W (1986) Hexachlorobenzene in the marine environment:
distribution, fate and ecotoxicological aspects. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 211-222 (IARC Scientific Publications No. 77).
Erturk E, Lambrecht RW, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982) Leukemogenicity of hexachlorobenzene (HCB) in Swiss
mice (SM) after subchronic feeding. Proc Am Assoc Cancer Res, 23: 55
(Abstract).
Ertürk E, Lambrecht RW, Peters HA, Cripps DJ, Gocmen A, Morris CR, &
Bryan GT (1986) Oncogenicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 417-423 (IARC Scientific Publications No. 77).
Falandysz J & Szefer P (1982) Chlorinated hydrocarbons in diving ducks
wintering in Gdansk Bay, Baltic Sea. Sci Total Environ, 24: 119-127.
Falandysz J, Kannan K, Tanabe S, & Tatukawa R (1993) Persistent
organochlorine residues in canned cod-livers of the southern Baltic
origin. Bull Environ Contam Toxicol, 50: 929-934.
FAO/WHO (1975) Report of the Joint Meeting of FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 37 pp (WHO
Technical Report Series 574).
FAO/WHO (1978) Pesticide residues in food - 1978. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and
Environment and the WHO Expert Group on Pesticide Residues. Rome, Food
and Agriculture Organization of the United Nations, pp 19-20 (FAO
Plant Production and Protection Paper 15).
Ferrer A, Bona MA, Castellano M, To-Figueras J, & Brunet M (1992)
Organochlorine residues in human adipose tissue of the population of
Zaragoza (Spain). Bull Environ Contam Toxicol, 48: 561-566.
Fingler S, Drevenkar V, Tkalœeviœ B, & œmit Z (1992) Levels of
polychlorinated biphenyls, organochlorine pesticides, and
chlorophenols in the Kupa River water and in drinking waters from
different areas in Croatia. Bull Environ Contam Toxicol, 49:
805-812.
Foley RE & Batcheller GR (1988) Organochlorine contaminants in common
goldeneye wintering on the Niagara River. J Wildl Manage, 52:
441-445.
Foster WG, McMahon A, Jarrell JF, & Villeneuve DC (1992a)
Hexachlorobenzene (HCB) suppresses circulating progesterone
concentrations during the luteal phase in the cynomolgus monkey. J
Appl Toxicol, 12: 13-17.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1992b) Ovarian
toxicity of hexachlorobenzene (HCB) in the superovulated female rat. J
Biochem Toxicol, 7: 1-4.
Foster WG, Pentick JA, McMahon A, & Lecavalier PR (1993) Body
distribution and endocrine toxicity of hexachlorobenzene (HCB) in the
female rat. J Appl Toxicol, 13: 79-83.
Foster WG, Mertineit C, Yagminas A, McMahon A, & Lecavalier P (1995)
The effects of hexachlorobenzene on circulating levels of adrenal
steroids in the ovariectomized rat. J Biochem Toxicol, 10(3):
129-135.
Fox ME, Carey JH, & Olivier BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the Western Basin
of Lake Ontario. J Great Lakes Res, 9: 287-294.
Franchi E & Focardi S (1991) Polychlorinated biphenyl congeners,
hexachlorobenzene and DDT in human milk in central Italy. Sci Total
Environ, 102: 223-228.
Frank R & Ripley BD (1990) Food residues from pesticides and
environmental pollutants in Ontario. In: Nriagu JO & Simmons MS ed.
Food contamination from environmental sources. New York, Wiley-
Interscience, pp 473-524.
Frank R, Braun HE, & Fleming G (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969 to 1981. J Food Prot, 46:
893-900.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985a)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario - 1983. J Food Prot, 48:
499-504.
Frank R, Rasper J, Braun HE, & Ashton G (1985b) Disappearance of
organochlorine residues from abdominal and egg fats of chickens,
Ontario, Canada, 1969-1982. J Assoc Off Anal Chem, 68: 124-129.
Frank R, Rasper J, Smout MS, & Braun HE (1988) Organochlorine residues
in adipose tissues, blood and milk from Ontario residents, 1976-1985.
Can J Public Health, 79: 150-158.
Fujita M & Morikawa K (1992) Regional differences in dietary intake of
environmental pollutants in Japan. J Expo Anal Environ Epidemiol,
2(2):177-193.
Fürst P, Fürst CHR, & Wilmers K (1992) Survey of dairy products for
PCDD's, PCDF's, PCB's and HCB. Chemosphere, 25(7-10): 1039-1048.
Fürst P, Fürst C, & Wilmers K (1994) Human milk as a bioindicator for
body burden of PCDDs, PCDFs, organochlorine pesticides, and PCBs.
Environ Health Perspect, 102 (Suppl 1): 187-193.
Galassi S & Provini A (1981) Chlorinated pesticides and PCB contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in adult total diet
samples, October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Geike F & Parasher CD (1976a) Effect of hexachlorobenzene on some
growth parameters of Chlorella pyrenoidosa. Bull Environ Contam
Toxicol, 15: 670-677.
Geike F & Parasher CD (1976b) Effect of hexachlorobenzene (HCB) on
growth of Tetrahymena pyriformis. Bull Environ Contam Toxicol, 16:
347-354.
Gilbertson M (1979) Hexachlorobenzene (HCB) in Canada. Ottawa,
Ontario, Environment Canada, Environmental Protection Service
(Unpublished report).
Gobas FAPC, Bedard DC, Ciborowski JJH, & Haffner GD (1989)
Bioaccumulation of chlorinated hydrocarbons by mayfly ( Hexagenia
limbata) in Lake St. Clair. J Great Lakes Res, 15: 581-588.
Gobas FAPC, McNeil EJ, Lovett-Doust L, & Haffner GD (1991)
Bioconcentration of chlorinated aromatic hydrocarbons in aquatic
macrophytes. Environ Sci Technol, 25: 924-929.
Gocmen A, Peters HA, Cripps DJ, Bryan GT, & Morris CR (1989)
Hexachlorobenzene episode in Turkey. Biomed Environ Sci, 2: 36-43.
Goerz G, Bolsen K, Kalofoutis A, & Tsambaos D (1994) Influence of oral
isotrentoin on hepatic and cutaneous P-450-dependent isozyme
activities. Arch Dermatol Res, 286: 104-106.
Goldey ES & Taylor DH (1992) Developmental neurotoxicity following
premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol
Teratol, 14: 15-21.
Goldey ES, Fisher JW, & Taylor DH (1990) Maternal transfer of
hexachlorobenzene in the rat. Neurotoxicol Teratol, 12: 562-563.
Goldstein JA, Friesen M, Scotti TM, Hickman P, Hass JR, & Bergman H
(1978) Assessment of the contribution of chlorinated dibenzo-p-dioxins
and dibenzofurans to hexachlorobenzene-induced toxicity, porphyria,
changes in mixed function oxygenases, and histopathological changes.
Toxicol Appl Pharmacol, 46: 633-649.
Gómez-Catalán J, Planas J, To-Figueras J, Camps M, & Corbella J (1993)
Organochlorine pesticide residues in the population of Catalonia
(Spain). Bull Environ Contam Toxicol, 51: 160-164.
Gómez-Catalán J, Lezaun M, To-Figueras J, & Corbella J (1995)
Organochlorine residues in the adipose tissue of the population of
Navarra (Spain). Bull Environ Contam Toxicol, 54: 534-540.
Gopalaswamy UV & Aiyar AS (1986) Biotransformation and toxicity of
lindane and its metabolite hexachlorobenzene in mammals. In: Morris CR
& Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 267-276 (IARC Scientific Publications No. 77).
Gopalaswamy UV & Nair CKK (1992) DNA binding and mutagenicity of
lindane and its metabolites. Bull Environ Contam Toxicol, 49:
300-305.
Gorski T, Gorska E, Gorecka D, & Sikora M (1986) Hexachlorobenzene is
non-genotoxic in short-term tests. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 399-401 (IARC
Scientific Publications No. 77).
Government of Canada (1993) Canadian Environmental Protection Act
priority substances list assessment report: Hexachlorobenzene. Ottawa,
Ontario, Environment Canada, Health and Welfare Canada, 52 pp.
Gralla EJ, Fleischman RW, Luthra YK, Hagopian M, Baker JR, Esber H, &
Marcus W (1977) Toxic effects of hexachlorobenzene after daily
administration to beagle dogs for one year. Toxicol Appl Pharmacol,
40: 227-239.
Grant DL, Iverson F, Hatina GV, & Villeneuve DC (1974) Effects of
hexachlorobenzene on liver porphyrin levels and microsomal enzymes in
the rat. Environ Physiol Biochem, 4: 159-165.
Grant DL, Shields JB, & Villeneuve DC (1975) Chemical (HCB) porphyria:
effect of removal of sex organs in the rat. Bull Environ Contam
Toxicol, 14: 422-425.
Grant DL, Phillips WEJ, & Hatina GV (1977) Effect of hexachlorobenzene
on reproduction in the rat. Arch Environ Contam Toxicol, 5: 207-216.
Green JA, Francis JE, Wolf CR, Manson MM, & Smith AG (1989) Sexual
dimorphism of cytochrome P-450 induction by hexachlorobenzene in rats.
Biochem Soc Trans, 17: 1016-1017.
Greve PA & Van Zoonen P (1990) Organochlorine pesticides and PCB's in
tissues from Dutch citizens (1968-1986). Int J Environ Anal Chem,
38: 265-277.
Griffin RA & Chou SFJ (1981) Movement of PCB's and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Grimalt JO, Gomez-Belinchon JI, Llop R, & Albaiges J (1988) Water-
phase distribution of hexachlorobenzene in a deltaic environment (Ebre
Delta, Western Mediterranean). Chemosphere, 17: 1893-1903.
Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, Anto JM,
& Albaiges J (1994) Risk excess of soft-tissue and thyroid cancers in
a community exposed to airborne organochlorinated compound mixtures
with a high hexachlorobenzene content. Int J Cancer, 56: 200-203.
Guerzoni ME, Del Cupolo L, & Ponti I (1976) [Mutagenic activity of
pesticides.] Riv Sci Tech Alim Nutr Um, 6: 161-165 (in Italian, with
English summary).
Hagenmaier H, She J, & Lindig C (1992) Persistence of polychlorinated
dibenzo-p-dioxins and polychlorinated dibenzofurans in contaminated
soil at Maulach and Rastatt in southwest Germany. Chemosphere,
25(7/10): 1449-1456.
Hahn ME, Gasiewicz TA, Linko P, & Goldstein JA (1988) The role of the
Ah locus in hexachlorobenzene-induced porphyria. Biochem J, 254:
245-254.
Hahn ME, Goldstein JA, Linko P, & Gasiewicz TA (1989) Interaction of
hexachlorobenzene with the receptor for 2,3,7,8-tetrachlorodibenzo-p-
dioxin in vitro and in vivo. Arch Biochem Biophys, 270(1):
344-355.
Hansen LG, Dorn SB, Sundlof SM, & Vogel RS (1978) Toxicity,
accumulation and depletion of hexachlorobenzene in laying chickens. J
Agric Food Chem, 26: 1369-1374.
Hansen LG, Teske RH, Sundlof SM, & Simon J (1979) Hexachlorobenzene
and feline reproduction: effects of ground pork contaminated by
dietary exposure or spiked with purified HCB. Vet Hum Toxicol, 21:
248-253.
Hansen PD, Von Westernhagen H, & Rosenthal H (1985) Chlorinated
hydrocarbons and hatching success in Baltic herring spring spawners.
Mar Environ Res, 15: 59-76.
Hargrave BT, Vass WP, Erickson PE, & Fowler BR (1989) Distribution of
chlorinated hydrocarbon pesticides and PCBs in the Arctic Ocean.
Dartmouth, NS, Department of Fisheries and Oceans, 224 pp (Canadian
Technical Report of Fisheries and Aquatic Sciences No. 1644).
Harper DJ, Ridgeway IM, & Leatherland TM (1992) Concentrations of
hexachlorobenzene, trichlorobenzenes and chloroform in the waters of
the Forth Estuary, Scotland. Mar Pollut Bull, 24(5): 244-249.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Health Canada (1994) Canadian Environmental Protection Act: Human
health risk assessment for priority substances. Ottawa, Ontario,
Minister of Supply and Services.
Heidmann WA (1986) Hexachlorobenzene residues in selected species of
land and sea birds in northern Germany. In: Morris CR & Cabral JRP ed.
Hexachlorobenzene: Proceedings of an International Symposium. Lyon,
International Agency for Research on Cancer, pp 223-228 (IARC
Scientific Publications No. 77).
Heikes DL (1980) Residues of pentachloronitrobenzene and related
compounds in peanut butter. Bull Environ Contam Toxicol, 24:
338-343.
Heldaas SS, Langard S, & Andersen A (1989) Incidence of cancer in a
cohort of magnesium production workers. Br J Ind Med, 46: 617-623.
Hendriks AJ, Ma W-C, Brouns JJ, De Ruiter-Dijkman EM, & Gast R (1995)
Modelling and monitoring organochlorine and heavy metal accumulation
in soils, earthworms, and shrews in Rhine-Delta floodplains. Arch
Environ Contam Toxicol, 29: 115-127.
Herren-Freund SL & Pereira MA (1986) Carcinogenicity of by-products of
disinfection in mouse and rat liver. Environ Health Perspect, 69:
59-65.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of Interior, Fish and Wildlife Service (Special Scientific
Report œ Wildlife No. 191).
Hippelein M, Kaupp H, Dörr G, & McLachlan MS (1993) Testing of a
sampling system and analytical method for determination of
semivolatile organic compounds in ambient air. Chemosphere, 26:
2255-2263.
Hoff RM, Muir DCG, & Grift NP (1992) Annual cycle of polychlorinated
biphenyls and organohalogen pesticides in air in southern Ontario: 1.
Air concentration data. Environ Sci Technol, 26(2): 266-275.
Holoubek I, œáslavskœ J, Vancura R, Kocan A, Chovancová J, Petrik J,
Drobna B, Cudlin P, & Triska J (1994) Project Tocoen: The fate of
selected organic pollutants in the environment - Part XXIV. The
content of PCBs and PCDDs/Fs in high-mountain soils. Toxicol Environ
Chem, 45: 189-197.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
In: Taup H ed. Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers.
IARC (1979) Hexachlorobenzene. In: Some halogenated hydrocarbons.
Lyon, International Agency for Research on Cancer, pp 155-178 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Human, Volume 20).
IARC (1987) Hexachlorobenzene. In: Overall evaluations of
carcinogenicity: An updating of IARC monographs, volumes 1 to 42.
Lyon, International Agenci for Research on Cancer, pp 219-220 (IARC
Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Iatropoulos MJ, Hobson W, Knauf V, & Adams HP (1976) Morphological
effects of hexachlorobenzene toxicity in female rhesus monkeys.
Toxicol Appl Pharmacol, 37: 433-444.
IJC (International Joint Commission) (1989) In: Rathke DR & McRae G
ed. Great Lakes surveillance: 1987 report on Great Lakes water
quality. Report to the International Joint Commission, Windsor,
Ontario.
Ikemoto Y, Motoba K, Suzuki T, & Uchida M (1992) Quantitative
structure-activity relationships of nonspecific and specific toxicants
in several organism species. Environ Toxicol Chem, 11: 931-939.
Ingebritsen K (1986) Comparative studies on the distribution and
excretion of 14C-hexachlorobenzene by whole-body autoradiography. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 277-285 (IARC Scientific Publications No. 77).
Ingebritsen K, Skaare JU, Nafstad I, & Forde M (1981) Studies on the
biliary excretion and metabolites of hexachlorobenzene in the rat.
Xenobiotica, 11: 795-800.
Innes D, Muncaster B, Lazar R, Haffner D, & Hebert P (1988) Monitoring
of organic contaminants using freshwater mussels. Proceedings of the
Environmental Research Technology Transfer Conference, 30 November-1
December 1987 - Part B: Water quality research. Toronto, Ontario,
Environment Ontario.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: The derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
IRPTC (1993) IRPTC legal file. Geneva, United Nations Environment
Programme, International Register of Potentially Toxic Chemicals.
Ishidate M Jr (1988) Data book of chromosomal aberration test
in vitro. Amsterdam, New York, Oxford, Elsevier Science Publishers.
Jacoff FS, Scarberry R, & Rosa D (1986) Source assessment of
hexachlorobenzene from the organic chemical manufacturing industry.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 31-37 (IARC Scientific Publications No. 77). 31-37.
Jaffe R & Hites RA (1986) Anthropogenic, polyhalogenated, organic
compounds in non-migratory fish from the Niagara River area and
tributaries to Lake Ontario. J Great Lakes Res, 12: 63-71.
Jansson B & Bergman A (1978) Sulfur-containing derivatives of
hexachlorobenzene (HCB) - metabolites in the rat. Chemosphere, 3:
257-268.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Environ Toxicol Chem, 12: 1163-1174.
Jarrell JF, McMahon A, Villeneuve D, Franklin C, Singh A, Valli VE, &
Bartlett S (1993a) Hexachlorobenzene toxicity in the monkey primordial
germ cell without induced porphyria. Reprod Toxicol, 7: 41-47.
Jarrell JF, Villeneuve D, Franklin C, Bartlett S, Wrixon W, Kohut J, &
Zouves CG (1993b) Contamination of human ovarian follicular fluid and
serum by chlorinated organic compounds in three Canadian cities. Can
Med Assoc J, 148(8): 1321-1327.
Jemaa Z, Sabbah S, Driss MR, & Bouguerra ML (1986) Hexachlorobenzene
in Tunisian mothers' milk, cord blood and foodstuffs. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 139-142 (IARC Scientific Publications No. 77).
Johansen HR, Becher G, Polder A, & Skaare JU (1994) Congener-specific
determination of polychlorinated biphenyls and organochlorine
pesticides in human milk from Norwegian mothers living in Oslo. J
Toxicol Environ Health, 42: 157-171.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites in Lake Ontario. J Great Lakes Res, 9:
183-189.
Kan CA & Tuinstra LGM (1976) Accumulation and excretion of certain
organochloride insecticides in broiler breeder hens. J Agric Food
Chem, 24: 775-778.
Kan-Do Office & Pesticides Team (1995) Accumulated pesticide and
industrial chemical findings from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kannan K, Tanabe S, Ramesh A, Subramanian A, & Tatsukawa R (1992a)
Persistent organochlorine residues in foodstuffs from India and their
implications on human dietary exposure. J Agric Food Chem, 40:
518-524.
Kannan K, Tanabe S, Hoang Trong Quynh, Nguyen Duc Hue, & Tatsukawa R
(1992b) Residue pattern and dietary intake of persistent
organochlorine compounds in foodstuffs from Vietnam. Arch Environ
Contam Toxicol, 22: 367-374.
Kannan K, Falandysz J, Tanabe S, & Tatsukawa R (1993) Persistent
organochlorines in Harbour Porpoises from Puck Bay, Poland. Mar Pollut
Bull, 26(3): 162-165.
Kannan K, Tanabe S, Williams RJ, & Tatsukawa R (1994) Persistant
organochlorine residues in foodstuffs from Australia, Papua New Guinea
and the Solomon Islands: contamination levels and human dietary
exposure. Sci Total Environ, 153: 29-49.
Kashimoto T, Takayama K, Mimura M, Miyata H, Murakami Y, & Matsumoto H
(1989) PCDDs, PCDFs, PCBs, coplanar PCBs and organochlorinated
pesticides in human adipose tissue in Japan. Chemosphere, 19(1/6):
921-926.
Kasokat T, Nagel R, & Urich K (1989) Metabolism of chlorobenzene and
hexachlorobenzene by the zebra fish, Brachydanio rerio. Bull Environ
Contam Toxicol, 42: 254-261.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St. Clair and Detroit Rivers using introduced
clams, Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kelly AG & Campbell A (1994) Organochlorine contaminants in liver of
cod ( Gadus morhua) and muscle of herring ( Clupea harengus) from
Scottish waters. Mar Pollut Bull, 28(2): 103-108.
Kemper FH (1993) Human organ specimen banking-15 years of experience.
Sci Total Environ, 139-140: 13-25.
Kennedy SW & Wigfield DC (1990) Dose-response relationships in
hexachlorobenzene-induced porphyria. Biochem Pharmacol, 40:
1381-1388.
Kennedy SW, Wigfield DC, & Fox GA (1986) The delay in polyhalogenated
aromatic hydrocarbon-induced porphyria: mechanistic reality or
methodological artefact? Toxicol Lett, 31: 235-241.
Kessabi M, Elhraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the Mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kessabi M, Abdennebi E, Laraje R, & Lhafi A (1990) Contamination of
eggs, poultry liver and bovine liver and kidney by chlorinated
pesticides in Morocco. Sci Total Environ, 90: 283-287.
Khera KS (1974) Teratogenicity and dominant lethal studies on
hexachlorobenzene in rats. Food Cosmet Toxicol, 12: 471-477.
Kilikidis SD, Kamarianos AP, & Karamanlis XN (1992) Seasonal
fluctuations of organochlorine compounds in the water of the Strimon
River (N. Greece). Bull Environ Contam Toxicol, 49: 375-380.
Kilzer L, Scheunert I, Geyer H, Klein W, & Korte F (1979) Laboratory
screening of the volatilization rates of organic chemicals from water
and soil. Chemosphere, 10: 751-761.
Kimbrough RD & Linder RE (1974) The toxicity of technical
hexachlorobenzene in the Sherman rat strain. A preliminary study. Res
Commun Chem Pathol Pharmacol, 8: 653-664.
King L & Sherbin G (1986) Point sources of toxic organics to the upper
St. Clair River. Water Pollut Res J Can, 21: 433-446.
Kitchin KT & Brown JL (1989) Biochemical studies of promoters of
carcinogenesis in rat livers. Teratog Carcinog Mutagen, 9: 273-285.
Kitchin KT, Linder RE, Scotti TM, Walsh D, Curley AO, & Svendsgaard D
(1982) Offspring mortality and maternal lung pathology in female rats
fed hexachlorobenzene. Toxicology, 23: 33-39.
Kleiman de Pisarev DL, Sancovich HA, & Ferramola de Sancovich AM
(1989) Enhanced thyroxine metabolism in hexachlorobenzene-intoxicated
rats. J Endocrinol Invest, 12: 767-772.
Kleiman de Pisarev DL, Del Carmen Rios de Molina M, & San Martin de
Viale LC (1990) Thyroid function and thyroxine metabolism in
hexachlorobenzene-induced porphyria. Biochem Pharmacol,. 39:
817-825.
Knezovich JP & Harrison FL (1988) The bioavailability of sediment-
sorbed chlorobenzenes to larvae of the midge, Chironomus decorus.
Ecotoxicol Environ Saf, 15: 226-241.
Kocan A, Petrík J, Drobná G, & Chovancová J (1994) Levels of PCBs and
some organochlorine pesticides in the human population of selected
areas of the Slovak Republic: I. Blood. Chemosphere, 29(9/11):
2315-2325.
Kodric-Smit M, Smit Z, & Olie K (1980) Organochlorine contaminants in
human milk from Slavonia Province, Yugoslavia, 1978. Pestic Monit J,
14: 1-2.
Koeman JH, Ten Noever de Brauw MC & De Vos RH (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
Kohler A, Harms U, & Lukas B (1986) Accumulation of organochlorines
and mercury in flounder - an approach to pollution assessments. Helgol
Meeresunters 40: 431-440.
Koizumi A (1991) Experimental evidence for the possible exposure of
workers to hexachlorobenzene by skin contamination. Br J Ind Med,
48: 622-628.
Kosian P, Lemke A, Studders K, & Veith G (1981) The precision of the
ASTM bioconcentration test. Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory & Superior,
Wisconsin, University of Wisconsin-Superior, Center for Lake Superior
Environmental Studies, 24 pp (EPA 600/3-81/022).
Koss G & Koransky W (1975) Studies on the toxicology of
hexachlorobenzene: I. Pharmacokinetics. Arch Toxicol, 34: 203-212.
Koss G & Koransky W (1976) Studies on the toxicology of
hexachlorobenzene. II. Identification and determination of
metabolites. Arch Toxicol, 35: 107-114.
Koss G & Koransky W (1978) Pentachlorophenol in different species of
vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. In: Ranga Rao K ed. Pentachlorophenol. New York,
London, Plenum Press, pp 131-137.
Koss G & Manz D (1976) Residues of hexachlorobenzene in wild mammals
of Germany. Bull Environ Contam Toxicol, 15: 189-191.
Koss G, Strik JJTWA, & Kan CA (1978) Metabolites of hexachlorobenzene
in the excreta of different animal species. Excerpta Med Int Congress,
440: 211-212.
Krauthacker B (1993) Organochlorine pesticides and polychlorinated
biphenyls (PCBs) in human serum collected from the general population
from Zagreb (1985-1990). Bull Environ Contam Toxicol, 50: 8-11.
Krishnan K, Brodeur J, & Charbonneau M (1991) Development of an
experimental model for the study of hexachlorobenzene-induced hepatic
porphyria in the rat. Toxicol Appl Pharmacol, 17: 433-441.
Krishnan K, Brodeur J, Plaa GL, & Charbonneau M (1992) Modulation of
hexachlorobenzene-induced hepatic porphyria by methyl isobutyl ketone
in the rat. Toxicol Lett, 61: 167-174.
Kuehl DW & Butterworth B (1994) A national study of chemical residues
in fish: III. Study results. Chemosphere, 29(3): 523-535.
Kuehl DW, Leonard EN, Butterworth BC, & Johnson KL (1983)
Polychlorinated chemical residues in fish from major watersheds near
the Great Lakes, 1979. Environ Int, 9: 293-299.
Kuhnlein HV, Receveur O, Muir DCG, Chan HM, & Soueida R (1995) Arctic
indigenous women consume greater than acceptable levels of
organochlorines. J Nutr, 125: 2501-2510.
Kuiper-Goodman T, Grant DL, Moodle CA, Korsrud GO, & Munro IC (1977)
Subacute toxicity of hexachlorobenzene in the rat. Toxicol Appl
Pharmacol, 40: 529-549.
Kuroda Y (1986) Genetic and chemical factors affecting chemical
mutagenesis in cultured mammalian cells. In: Antimutagenesis and
anticarcinogenesis mechanisms. New York, London, Plenum Press,
pp 359-375.
Lambrecht RW, Erturk E, Grunden EE, Headley DB, Peters HA, Morris CR,
& Bryan GT (1982a) Renal toxicity and tumorigenicity of
hexachlorobenzene (HCB) in rats (R). Proc Am Assoc Cancer Res, 23:
54 (Abstract).
Lambrecht RW, Erturk E, Grunden E, Headley DB, Morris CR, Peters HA, &
Bryan GT (1982b) Hepatotoxicity and tumorigenicity of
hexachlorobenzene (HCB) in Syrian golden hamsters (H) after subchronic
administration. Fed Proc, 41: 329 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983a) Hepatocarcinogenicity of chronically administered
hexachlorobenzene in rats. Fed Proc, 42: 786 (Abstract).
Lambrecht RW, Ertürk E, Grunden EE, Peters HA, Morris CR, & Bryan GT
(1983b) Renal tumors in rats chronically exposed to hexachlorobenzene
(HCB). Proc Am Assoc Cancer Res, 24: 59 (Abstract).
Lambrecht RW, Sinclair PR, Bement WJ, Sinclair JF, Carpenter HM,
Buhler DR, Urquhart AJ, & Elder GH (1988) Hepatic uroporphyrin
accumulation and uroporphyrinogen decarboxylase activity in cultured
chick-embryo hepatocytes and in Japanese quail ( Coturnix coturnix
japonica) and mice treated with polyhalogenated aromatic
hydrocarbons. Biochem J, 253: 131-138.
Lamparski LL, Langhorst ML, Nestrick TJ, & Cutie S (1980) Gas-liquid
chromatographic determination of chlorinated benzenes and phenols in
selected biological matrices. J Assoc Off Anal Chem, 63(1): 27-32.
Landrum PF (1989) Bioavailability and toxicokinetics of polycyclic
aromatic hydrocarbons sorbed to sediment for the amphipod
Pontoporeia hoyi. Environ Sci Technol, 23: 588-595.
Lane DA, Schroeder WH, & Johnson ND (1992a) On the spatial and
temporal variations in atmospheric concentrations of hexachlorobenzene
and hexachlorocyclohexane isomers at several locations in the province
of Ontario, Canada. Atmos Environ, 26A: 31-42.
Lane DA, Johnson ND, Hanley MJJ, Schroeder WH, & Ord DT (1992b) Gas-
and particle-phase concentrations of alpha-hexachlorocyclohexane,
gama-hexachlorocyclohexane, and hexachlorobenzene in Ontario air.
Environ Sci Technol, 26: 126-133.
Langhorst ML & Nestrick TJ (1979) Determination of chlorobenzenes in
air and biological samples by gas chromatography with photoionization
detection. Anal Chem, 51(12): 2018-2025.
Larsen BR, Turrio-Baldassarri L, Nilsson T, Iacovella N, Di Domenico
A, Montagna M, & Facchetti S (1994) Toxic PCB congeners and
organochlorine pesticides in Italian human milk. Ecotoxicol Environ
Saf, 28: 1-13.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW, &
Evans RL (1976) An ecological study of hexachlorobenzene (HCB). New
Orleans, University of New Orleans, Department of Biological Sciences
(Prepared for the US Environmental Protection Agency, Office of Toxic
Substances, Washington) (EPA 560/6-76-009).
Laska AL, Bartell C, & Laseter JL (1976) Distribution of
hexachlorobenzene and hexachlorobutadiene in water, soil, and selected
aquatic organisms along the lower Mississippi River, Louisiana. Bull
Environ Contam Toxicol, 15: 535-542.
Leger DA (1991) Environmental concentrations of hexachlorobenzene in
Atlantic Canada. Moncton, New Brunswick, Environment Canada, Inland
Waters Directorate.
Leoni V & D'Arca SU (1976) Experimental data and critical review of
the occurrence of hexachlorobenzene in the Italian environment. Sci
Total Environ, 5: 253-272.
Lewis RJ (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Company.
Lewis RG & MacLeod KE (1982) Portable sampler for pesticides and
semivolatile industrial organic chemicals in air. Anal Chem, 54:
310-315.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic compounds
in rain: II. Gas scavenging of neutral organic compounds. Atmos
Environ, 19: 1609-1617.
Lilienthal H, Benthe C, Heinzow B, & Winneke G (1996) Impairment of
schedule-controlled behavior by pre- and postnatal exposure to
hexachlorobenzene in rats. Arch Toxicol, 70: 174-181.
Linko P, Yeowell HN, Gasiewicz TA, & Goldstein JA (1986) Induction of
cytochrome P-450 isozymes by hexachlorobenzene in rats and aromatic
hydrocarbon (Ah)-responsive mice. J Biochem Toxicol, 1: 95-107.
Loose LD (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice. Environ Health
Perspect, 43: 89-97.
Loose LD, Silkworth JB, Charbonneau T, & Blumenstock F (1981)
Environmental chemical-induced macrophage dysfunction. Environ Health
Perspect, 39: 79-92.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55(6): 881-889.
Lu P & Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model ecosystem. Environ Health
Perspect, 10: 269-284.
Ludwicki JK & Góralczyk K (1994) Organochlorine pesticides and PCBs in
human adipose tissues in Poland. Bull Environ Contam Toxicol, 52:
400-403.
Lunde G & Bjorseth A (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci
Total Environ, 8(3): 241-246.
Lunde G & Ofstad EB (1976) Determination of fat-soluble chorinated
compounds in fish. Z Anal Chem, 282: 395-399.
McCarty LS, MACKAY D, Smith AD, Ozburn GW, & Dixon DG (1992a) Residue-
based interpretation of toxicity and bioconcentration QSARs from
aquatic bioassays: Neutral narcotic organics. Environ Toxicol Chem,
11: 917-930.
McCarty LS, Ozburn GW, Smith AD, & Dixon DG (1992b) Toxicokinetic
modeling of mixtures of organic chemicals. Environ Toxicol Chem, 11:
1037-1047.
Mack GA & Stanley J (1985) The national human adipose tissue survey.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (Report No. NHATS-ST-01).
Mackay D, Shiu WY, & Ma KC (1992) Illustrated handbook of physical-
chemical properties and environmental fate for organic chemicals -
Volume I: Monoaromatic hydrocarbons, chlorobenzenes, and PCBs.
Chelsea, Michigan, Lewis Publishers.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol, 25: 921-928.
MAFF (Ministry of Agriculture, Fisheries and Food) (1992) Report of
the working party on pesticide residues: 1988-1990. London, Her
Majesty's Stationery Office (Food Surveillance Paper No. 34).
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1994) Report of the working party on pesticide
residues, 1993 - Supplement to the pesticides register. London, Her
Majesty's Stationery Office.
Mansour M, Scheunert I, Viswanathan R, & Korte F (1986) Assessment of
the persistence of hexachlorobenzene in the ecosphere. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 53-59 (IARC Scientific Publications No. 77).
Marlow DA (1986) Hexachlorobenzene exposure in the production of
chlorophenols. In: Morris CR & Cabral JRP ed. Hexachlorobenzene:
Proceedings of an International Symposium. Lyon, International Agency
for Research on Cancer, pp 161-169 (IARC Scientific Publications
No. 77).
Mehendale HM, Fields M, & Matthews HB (1975) Metabolism and effects of
hexachlorobenzene on hepatic microsomal enzymes in the rat. J Agric
Food Chem, 23: 261-265.
Mendoza CE, Grant DL, & Shields JB (1975) Body burden of
hexachlorobenzene in suckling rats and its effects on various organs
and on liver porphyrin accumulation. Environ Physiol Biochem, 5:
460-464.
Mendoza CE, Collins B, Shields JB, & Laver GW (1977) Hexachlorobenzene
residues and effects on esterase activities in pre-weanling rats after
a reciprocal transfer between HCB-treated and control dams. Arch
Toxicol, 38: 191-199.
Mendoza CE, Collins BT, Shields JB, & Laver GW (1978) Effects of
hexachlorobenzene or hexabromobenzene on body and organ weights of
preweanling rats after a reciprocal transfer between the treated and
control dams. J Agric Food Chem, 26: 941-945.
Mendoza CE, Shields JB, & Laver GW (1979) Comparison of the
porphyrinogenic activity of hexabromobenzene and hexachlorobenzene in
primiparous Wistar rats. Bull Environ Contam Toxicol, 21: 358-364.
Mes J (1990) Trends in the levels of some chlorinated hydrocarbon
residues in adipose tissue of Canadians. Environ Pollut, 65:
269-278.
Mes J (1992) Organochlorine residues in human blood and biopsy fat and
their relationship. Bull Environ Contam Toxicol, 48: 815-820.
Mes J & Davies DJ (1979) Presence of polychlorinated biphenyl and
organochlorine pesticide residues and the absence of polychlorinated
terphenyls in Canadian human milk samples. Bull Environ Contam
Toxicol, 21: 381-387.
Mes J & Malcolm S (1992) Comparison of chlorinated hydrocarbon
residues in human populations from the Great Lakes and other regions
of Canada. Chemosphere, 25(3): 417-424.
Mes J, Campbell DS, Robinson RN, & Davies DJA (1977) Polychlorinated
biphenyl and organochlorine pesticide residues in adipose tissue of
Canadians - 1977. Bull Environ Contam Toxicol, 17: 196-203.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and other
chlorinated hydrocarbon residues in adipose tissue of Canadians. Bull
Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, Turton D, & Sun W-F (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mes J, Marchand L, & Davies DJ (1990) Organochlorine residues in
adipose tissue of Canadians. Bull Environ Contam Toxicol, 45:
681-688.
Mes J, Davies DJ, Doucet J, Weber D, & McMullen E (1993) Levels of
chlorinated hydrocarbon residues in Canadian human breast milk and
their relationship to some characteristics of the donors. Food Addit
Contam, 10(4): 429-441.
Mill T & Haag W (1986) The environmental fate of hexachlorobenzene.
In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 61-66 (IARC Scientific Publications No. 77).
Moksnes MT & Norheim G (1986) Levels of chlorinated hydrocarbons and
composition of PCB in herring gull Larus argentatus eggs collected
in Norway in 1969 compared to 1979-81. Environ Pollut, 11: 109-116.
Mollenhauer HH, Johnson JH, Younger RL, & Clark DE (1975)
Ultrastructural changes in liver of the rat fed hexachlorobenzene. Am
J Vet Res, 36: 1777-1781.
Mollenhauer HH, Johnston JH, Younger RL, & Clark DE (1976) A unique
intracellular aberration related to hexachlorobenzene ingestion. Am J
Vet Res, 37: 847-850.
Morita M, Mimura S, & Yagyu H (1975) A systematic determination of
chlorinated benzenes in human adipose tissue. Environ Pollut, 9:
175-179.
Morley A, Geary D, & Harben F (1973) Hexachlorobenzene pesticides and
porphyria. Med J Aust, 1: 565.
Morosini M, Schreltmüller J, Reuter U, & Ballschmiter K (1993)
Correlation between C-6/C-14 chlorinated hydrocarbons levels in the
vegetation and in the boundary layer of the troposphere. Environ Sci
Technol, 27: 1517-1523.
Muir DCG, Wagemann R, Grift NP, Norstrom RJ, Simon M, & Lien J (1988)
Organochlorine chemical and heavy metal contaminants in white-beaked
dolphins ( Lagenorhynchus albirostris) and pilot whales
( Globicephala melaena) from the coast of Newfoundland, Canada. Arch
Environ Contam Toxicol, 17: 613-629.
Muir DCG, Grift NP, Lockhart WL, Wilkinson P, Billeck BN, & Brunskill
GJ (1995) Spatial trends and historical profiles of organochlorine
pesticides in Arctic lake sediments. Sci Total Environ, 160/161:
447-457.
Müller MD (1982) [Hexachlorobenzene in Switzerland: Measurement and
background of environmental contamination.] Chimia, 36(11): 437-445
(in German).
Mumma CF & Lawless EW (1975) Survey of industrial processing data.
Task I: Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processes. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA 560/3-75-003).
Muncaster BW, Innes DJ, Hebert PDN, & Haffner GD (1989) Patterns of
organic contaminant accumulation by freshwater mussels in the
St. Clair River, Ontario. J Great Lakes Res, 15: 645-653.
Murray HE, Neff GS, Hrung Y, & Giam CS (1980) Determination of
benzo(a)pyrene, hexachlorobenzene and pentachlorophenol in oysters
from Galveston Bay, Texas. Bull Environ Contam Toxicol, 25(4):
663-667.
Mussalo-Rauhamaa H, Pyysalo H, & Antervo K (1988) Relation between the
content of organochlorine compounds in Finnish human milk and
characteristics of the mothers. J Toxicol Environ Health, 25: 1-19.
Nair A & Pillai MKK (1989) Monitoring of hexachlorobenzene residues in
Delhi and Faridabad, India. Bull Environ Contam Toxicol, 42:
682-686.
Nash RG & Gish TJ (1989) Halogenated pesticide volatilization and
dissipation from soil under controlled conditions. Chemosphere, 18:
2353-2362.
Nebecker AV, Griffis WL, Wise CM, Hopkins E, & Barbitta JA (1989)
Survival, reproduction and bioconcentration in invertebrates and fish
exposed to hexachlorobenzene. Environ Toxicol Chem, 8: 601-611.
Needham LL, Burse VW, Head SL, Korver MP, McClure PC, Andrews JS Jr,
Rowley DL, Sung J, & Kahn SE (1990) Adipose tissue/serum partitioning
of chlorinated hydrocarbon pesticides in humans. Chemosphere, 20:
975-980.
Newton KG & Greene NC (1972) Organochlorine pesticide residue levels
in human milk -Victoria, Australia - 1970. Pestic Monit J, 6(1):
4-8.
NHW (National Health and Welfare) (1987) Nutrient value of some common
foods. Ottawa, Ontario, Health Services and Promotion Branch/Health
Protection Branch.
Niimi AJ & Cho CY (1981) Elimination of hexachlorobenzene (HCB) by
rainbow trout ( Salmo gairdneri), and an examination of its kinetics
in Lake Ontario salmonids. Can J Fish Aquat Sci, 38: 1350-1356.
Niimi AJ & Oliver BG (1989) Distribution of polychlorinated biphenyl
congeners and other halocarbons in whole fish and muscle among Lake
Ontario salmonids. Environ Sci Technol, 23: 83-88.
NIOSH (1985) Registry of toxic effects of chemical substances,
1983-1984 - Cumulative supplement to the 1981-1982 edition.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
Noble DG & Elliott JE (1986) Environmental contaminants in Canadian
seabirds, 1968-1984: Trends and effects. Ottawa, Ontario, Canadian
Wildlife Service (Technical Report Series No. 13).
Noble DG, Elliott JE, & Shutt L (1992) Contaminants in Canadian
raptors, 1965-1988. Ottawa, Ontario, Canadian Wildlife Service
(Technical Report Series No. 91).
Norén K (1983a) Levels of organochlorine contaminants in human milk in
relation to the dietary habits of the mothers. Acta Paediatr Scand,
72: 811-816.
Norén K (1983b) Some aspects of the determination of organochlorine
contaminants in human milk. Arch Environ Contam Toxicol, 12:
277-283.
Norén K (1988) Changes in the levels of organochlorine pesticides,
polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans in
human milk from Stockholm, 1972-1985. Chemosphere, 17(1): 39-49.
Norén K (1993) Contemporary and retrospective investigations of human
milk in the trend studies of organochlorine contaminants in Sweden.
Sci Total Environ, 139/140: 347-355.
Norstrom RJ, Simon M, & Muir DCG (1990) Polychlorinated dibenzo-p-
dioxins and dibenzofurans in marine mammals in the Canadian north.
Environ Pollut, 66: 1-19.
NTP (1994) Seventh annual report on carcinogens. Summary, 1994.
Research Triangle Park North Carolina, US Department of Health and
Human Services, National Toxicology Program.
Ockner RK & Schmid R (1961) Acquired porphyria in man and rat due to
hexachlorobenzene intoxication. Nature (Lond), 189: 499.
Oehme M & Stray H (1982) Quantitative determination of ultra-traces of
chlorinated compounds in high-volume air samples from the Arctic using
polyurethane foam as collection medium. Fresenius Z Anal Chem, 311:
665-673.
Oliver BG (1984a) Distribution and pathways of some chlorinated
benzenes in the Niagara River and Lake Ontario. Water Pollut Res J
Can, 19: 47-59.
Oliver BG (1984b) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Partitioning relationships for chlorinated organics
between water and particulates in the St. Clair, Detroit and Niagara
Rivers. In: Kaiser LLE ed. QSAR in environmental toxicology - II.
Boston, Massachussetts, D. Reidel Publishing Co., pp 251-260.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St. Clair, and Erie: Implications
regarding sources along the St. Clair and Detroit rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Carey JH (1986) Photodegradation of wastes and pollutants
in aquatic environment. In: Pelizzetti E & Serpo N ed. Homogenous and
heterogenous photocatalysis. Boston, Massachussetts, D. Reidel
Publishing Co., pp 629-650.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Kaiser KLE (1986) Chlorinated organics in nearshore waters
and tributaries of the St. Clair River. Water Pollut Res J Can, 21:
344-350.
Oliver BG & Nicol KD (1982) Chlorobenzenes in sediments, water, and
selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ
Sci Technol, 16: 532-536.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of polychlorinated
biphenyl congeners and other chlorinated hydrocarbons in the Lake
Ontario ecosystem. Environ Sci Technol, 22: 388-397.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21: 368-379.
Oliver BG, Charlton MN, & Durham RW (1989) Distribution,
redistribution, and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
sediments. Environ Sci Technol, 23: 200-208.
OMAF/OME (Ontario Ministry of Agriculture and Food/Ontario Ministry of
the Environment) (1988) Polychlorinated dibenzo-p-dioxins and
polychlorinated dibenzofurans and other organochlorine contaminants in
food. Toronto, Ontario Ministry of Agriculture and Food and Ministry
of the Environment (Report prepared by the Joint OMAF/OME Toxics in
Food Steering Committee).
Ostfeldt P, Gustavson K, Jansson B, Jonsson P, Miettinen V, Ringstad
O, & Wesén C (1994) Halogenated organic compounds in the marine
environment 1989-1990. Copenhagen, Nordic Council of Ministers, 124 pp
(TemaNord 1994:591).
Parrish PR, Cook GH, & Patrick JM Jr (1974) Effects on several
estuarine animals. In: Rogers WA ed. Proceedings of the 28th Annual
Conference, White Sulphur Springs, West Virginia, 17-20 November 1974.
Sulphur Springs, West Virginia, Southeastern Association of Game and
Fish Commissioners.
Patton GW, Hinckley DA, Walla MD, & Bidleman TF (1989) Airborne
organochlorines in the Canadian High Arctic. Tellus, 41B: 243-255.
Peakall DB, Noble DG, Elliott JE, Somers JD, & Erickson G (1990)
Environmental contaminants in Canadian peregrine falcons, Falco
peregrinus: A toxicological assessment. Can Field-Nat, 104:
244-254.
Pearce PA, Elliott JE, Peakall DB, & Norstrom RJ (1989) Organochlorine
contaminants in eggs of seabirds in the Northwest Atlantic, 1968-1984.
Environ Pollut, 56: 217-235.
Pereira MA, Herren SL, Britt AL, & Khoury MM (1982) Sex difference in
enhancement of GGTase-positive foci by hexachlorobenzene and lindane
in rat liver. Cancer Lett, 15: 95-101.
Persoone G & Uyttersprot G (1975) The influence of inorganic and
organic pollutants on the rate of reproduction of a marine
hypotrichous ciliate: Euplotes vannus Muller. Rev Int Océanogr méd,
37-38: 125-151.
Peters HA (1976) Hexachlorobenzene poisoning in Turkey. Fed Proc,
35: 2400-2403.
Peters HA, Johnson SAM, Cam S, Oral S, Müftü Y, & Ergene T (1966)
Hexachlorobenzene-induced porphyria: effect of chelation on the
disease, porphyrin and metal metabolism. Am J Med Sci, 251: 314-322.
Peters HA, Gocmen A, Cripps DJ, Bryan GT, & Dogramaci I (1982)
Epidemiology of hexachlorobenzene-induced porphyria in Turkey:
clinical and laboratory follow-up after 25 years. Arch Neurol, 39:
744-749.
Peters HA, Cripps DJ, Gocmen A, Ertürk E, Bryan GT, & Morris CR (1986)
Neurotoxicity of hexachlorobenzene-induced porphyria turcica. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 575-579 (IARC Scientific Publications No. 77).
Peterson R & Ray S (1987) Organochlorine residues in brook trout and
yellow perch from New Brunswick and Nova Scotia (Canada) lakes. Water
Pollut Res J Can, 22: 352-364.
Phelps DK, Pruell RJ, & Lake JL (1986) Hexachlorobenzene in selected
marine samples: an environmental perspective. In: Morris CR & Cabral
JRP ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 121-130 (IARC
Scientific Publications No. 77).
Proulx G, Weseloh DV, Elliott JE, Teeple S, Anghern PAM, & Mineau P
(1987) Organochlorine and PCB residues in Lake Erie mink populations.
Bull Environ Contam Toxicol, 39: 939-944.
Quinlivan S, Ghassemi M, & Santy M (1975) Survey of methods used to
control wastes containing hexachlorobenzene. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste Management
Programs (EPA 530/SW-120c).
Quinsey PM, Donohue DC, & Ahokas JT (1995) Persistence of
organochlorines in breast milk of women in Victoria, Australia. Food
Chem Toxicol, 33(1): 49-56.
Ray LE, Murray HR, & Giam CS (1983a) Analysis of water and sediment
from the Nueces Estuary/Corpus Christi Bay (Texas) for selected
organic pollutants. Chemosphere, 12: 1039-1045.
Ray LE, Murray HE, & Giam CS (1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12: 1031-1038.
Renner G (1988) Hexachlorobenzene and its metabolism. Toxicol Environ
Chem, 18: 51-78.
Richter E & Schäfer SG (1981) Intestinal excretion of
hexachlorobenzene. Arch Toxicol, 47: 233-239.
Rios de Molina MC, Wainstok de Calmanovici R, & San Martin de Viale LC
(1980) Investigations on the presence of porphyrinogen carboxy-lyase
inhibitor in the liver of rats intoxicated with hexachlorobenzene. Int
J Biochem, 12: 1027-1032.
Rippen G & Frank R (1986) Estimation of hexachlorobenzene pathways
from the technosphere into the environment. In: Morris CR & Cabral JRP
ed. Hexachlorobenzene: Proceedings of an International Symposium.
Lyon, International Agency for Research on Cancer, pp 45-52 (IARC
Scientific Publications No. 77).
RIWA (1993) Rapport annuel 1993 de l'Association des Services d'Eau du
Rhin et de la Meuse, Annex 11. Amsterdam, Secrétariat de l'Association
des Services d'Eau du Rhin et de la Meuse.
Rizzardini M & Smith AG (1982) Sex differences in the metabolism of
hexachlorobenzene by rats and the development of porphyria in females.
Biochem Pharmacol, 31: 3543-3548.
Rizzardini M, Graziani A, Carugo C, & Cantoni L (1988) Investigations
on the role of free radical processes in hexachlorobenzene-induced
porphyria in mice. J Biochem Toxicol, 3: 33-46.
Rizzardini M, Cantoni L, Villa P, & Ubezio P (1990) Biochemical,
morphological and flow-cytometric evaluation of the effects of
hexachlorobenzene on rat liver. Cell Biol Toxicol, 6: 185-203.
Robinson PE, Mack GA, Remmers J, Levy R, & Mohadjer L (1990) Trends of
PCB, hexachlorobenzene, and ß-benzene hexachloride levels in the
adipose tissue of the U.S. population. Environ Res, 53: 175-192.
Rogers HR, Crathorne B, & Leatherland TM (1989) Occurrence of
chlorobenzene isomers in the water column of a UK estuary. Mar Pollut
Bull, 20: 276-281.
Rozman K, Rozman T, & Greim H (1981) Enhanced fecal elimination of
stored hexachlorobenzene from rats and rhesus monkeys by hexadecane or
mineral oil. Toxicology, 22: 33-44.
Rozman K, Gorski JR, Rozman P, & Parkinson A (1986) Reduced serum
thyroid hormone levels in hexachlorobenzene-induced porphyria. Toxicol
Lett, 30: 71-78.
Rumsby PC, Evans JG, Phillimore HE, Carthew P, & Smith AG (1992)
Search for Ha- ras codon 51 mutations in liver tumours caused by
hexachlorobenzene and Aroclor 1254 in C57BL/10ScSn mice with iron
overload. Carcinogenesis, 13: 1917-1920.
Rush GF, Smith JH, Maita K, Bleavins M, Aulerich RJ, Ringer RK, & Hook
JB (1983) Perinatal hexachlorobenzene toxicity in the mink. Environ
Res, 31: 116-124.
Russell RW & Gobas FAPC (1989) Calibration of the freshwater mussel,
Elliptio complanata, for quantitative biomonitoring of
hexachlorobenzene and octachlorostyrene in aquatic systems. Bull
Environ Contam Toxicol, 43: 576-582.
Salisbury CDC, Fesser ACE, MacNeil JD, & Patterson JR (1992) Trace
metal and pesticide levels in muskoxen from Victoria Island, Northwest
Territories, Canada. Intern. J Environ Chem, 48: 209-215.
Sanborn JR, Childers WF, & Hansen LG (1977) Uptake and elimination of
[14C] hexachlorobenzene (HCB) by the green sunfish, Lepomis
cyanellus Raf., after feeding contaminated food. J Agric Food Chem,
25: 551-553.
Schechter A, Fürst P, Krüger C, Meemken H-A, Groebel W, & Constable JD
(1989a) Levels of polychlorinated dibenzofurans, dibenzodioxins, PCBs,
DDT and DDE, hexachlorobenzene, dieldrin, hexachlorocyclohexanes and
oxychlordane in human breast milk from the United States, Thailand,
Vietnam, and Germany. Chemosphere, 18: 445-454.
Schechter A, Mes J, & Davies D (1989b) Polychlorinated biphenyl (PCB),
DDT, DDE and hexachlorobenzene (HCB) and PCDD/PCDF isomer levels in
various organs in autopsy tissue from North American patients.
Chemosphere, 18: 811-818.
Scheufler E & Rozman KK (1984) Comparative decontamination of
hexachlorobenzene-exposed rats and rabbits by hexadecane. J Toxicol
Environ Health, 14: 353-362.
Scheunert I, Marra C, Viswanatha R, Klein WK, & Korte F (1983) Fate of
hexachlorobenzene-14C in wheat plants and soil under outdoor
conditions. Chemosphere, 12: 843-858.
Scheunert I, Topp E, Schmitzer J, Klein W, & Korte F (1985) Formation
and fate of bound residues of [14C] benzene and [14C] chlorobenzene
in soil and plants. Ecotoxicol Environ Saf, 9: 159-170.
Schielen P, Schoo W, Tekstra J, Oostermeijer HHA, Seinen W, & Bloksma
N (1993) Autoimmmune effects of hexachlorobenzene in the rat. Toxicol
Appl Pharmacol, 122: 233-243.
Schielen P, Den Besten C, Vos JG, Van Bladeren PJ, Seinen W, & Bloksma
N (1995) Immune effects of hexachlorobenzene in the rat: role of
metabolism in a 13-week feeding study. Toxicol Appl Pharmacol, 131:
37-43.
Schmid R (1960) Cutaneous porphyria in Turkey. N Engl J Med, 263:
397-398.
Schmitt CJ, Zaijcek JL, & Peterman PH (1990) National Contaminant
Biomonitoring Program: residues of organochlorine chemicals in U.S.
freshwater fish, 1976-1984. Arch. Environ Toxicol, 19: 748-781.
Schuler W, Brunn H, & Manz D (1985) Pesticides and polychlorinated
biphenyls in fish from the Lahn River. Bull Environ Contam Toxicol,
34: 608-616.
Schuytema G, Krawczyk DF, Griffis WL, Nebeker AV, & Robideaux ML
(1990) Hexachlorobenzene uptake by fathead minnows and
macroinvertebrates in recirculating sediment/water systems. Arch
Environ Contam Toxicol, 19: 1-9.
Schwarzenbach RP, Giger W, Hoehn E, & Schneider JK (1983) Behaviour of
organic compounds during infiltration of river water to groundwater.
Field studies. Environ Sci Technol, 17: 472-479.
Selden A, Jacobson G, Berg P, & Axelson O (1989) Hepatocellular
carcinoma and exposure to hexachlorobenzene: a case report. Br J Ind
Med, 46: 138-140.
Shirai T, Miyata Y, Nakanishi K, Murasaki G, & Ito N (1978)
Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in ICR mice
and its enhancement by hexachlorobenzene. Cancer Lett, 4: 271-275.
Siekel P, Chalupa I, Beno J, Blasko M, Novotny J, & Burian J (1991) A
genotoxicological study of hexachlorobenzene and pentachloroanisole.
Teratog Carcinog Mutagen, 11: 55-60.
Simon GS, Tardiff RG, & Borzelleca JF (1979) Failure of
hexachlorobenzene to induce dominant lethal mutations in the rat.
Toxicol Appl Pharmacol, 47: 415-419.
Sims DE, Singh A, Donald A, Jarrell J, & Villeneuve DC (1991)
Alteration of primate ovary surface epithelium by exposure to
hexachlorobenzene: a quantitative study. Histol Histopathol, 6:
525-529.
Singh A, Friesen D, Jarrel J, & Villeneuve DC (1990a)
Hexachlorobenzene toxicity in the monkey ovary: I. Ultrastructure
induced by low (0.1 mg/kg) dose exposure. In: Proceedings of the XIIth
International Congress for Electron Microscopy, Seattle, Washington -
Volume 2: Analytical sciences, San Francisco, California, San
Francisco Press, Inc., pp 282-283.
Singh A, Sims DE, Jarrell J, & Villeneuve DC (1990b) Hexachlorobenzene
toxicity in the monkey ovary: II. Ultrastructure induced by medium
(1.0 mg/kg) dose exposure. Anal Environ Toxicants, 13: 435-438.
Singh A, Dykeman A, Jarrell J, & Villeneuve DC (1991)
Hexachlorobenzene toxicity in the monkey ovary: III. Ultrastructure
induced by high (10 mg/kg) dose exposure. In: Proceedings of the 49th
Annual Meeting of the Electron Microscopy Society of America. San
Francisco, California, San Francisco Press, Inc.
Skåre JU, Stenersen J, Kveseth N, & Polder A (1985) Time trends of
organochlorine chemical residues in seven sedentary marine fish
species from a Norwegian fjord during the period 1972-1982. Arch
Environ Contam Toxicol, 14: 33-41.
Skaare JU, Markussen NH, Norheim G, Haugen S, & Holt G (1990) Levels
of polychlorinated biphenyls, organochlorine pesticides, mercury,
cadmium, copper, selenium, arsenic, and zinc in the harbour seal,
Phoca vitulina, in Norwegian waters. Environ Pollut, 66: 309-324.
Smith AG & Cabral JR (1980) Liver-cell tumours in rats fed
hexachlorobenzene. Cancer Lett, 11: 169-172.
Smith AG & De Matteis F (1990) Oxidative injury mediated by the
hepatic cytochrome P-450 system in conjunction with cellular iron.
Effects on the pathway of haem biosynthesis. Xenobiotica, 29(9):
865-877.
Smith AG & Francis JE (1983) Synergism of iron and hexachlorobenzene
inhibits hepatic uroporphyrinogen decarboxylase in inbred mice.
Biochem J, 214: 909-913.
Smith VE, Spurr JM, Filkins JC, & Jones JJ (1985a) Organochlorine
contaminants of wintering ducks foraging on Detroit River sediments. J
Great Lakes Res, 11(3): 231-246.
Smith AG, Francis JE, Dinsdale D, Manson MM, & Cabral JRP (1985b)
Hepatocarcinogenicity of hexachlorobenzene in rats and the sex
difference in hepatic iron status and development of porphyria.
Carcinogenesis, 6: 631-636.
Smith AG, Dinsdale D, Cabral JRP, & Wright AL (1987) Goitre and
wasting induced in hamsters by hexachlorobenzene. Arch Toxicol, 60:
343-349.
Smith AG, Cabral JRP, Carthew P, Francis JE, & Manson MM (1989)
Carcinogenicity of iron in conjunction with a chlorinated
environmental chemical, hexachlorobenzene, in C57BL/10ScSn mice. Int J
Cancer, 43: 492-496.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM (1990)
Sex-linked hepatic uroporphyria and the induction of cytochromes
P450IA in rats caused by hexachlorobenzene and polyhalogenated
biphenyls. Biochem Pharmacol, 40: 2059-2068.
Smith AG, Carthew P, Francis JE, Cabral JRP, & Manson MM (1993)
Enhancement by iron of hepatic neoplasia in rats caused by
hexachlorobenzene. Carcinogenesis, 14: 1381-1387.
Smith AG, Carthew P, Francis JE, & Ingebritsen K (1994) Influence of
iron on the induction of hepatic tumors and porphyria by
octachlorostyrene in C57BL/10ScSn mice. Cancer Lett, 81: 145-150.
Solé M, Porte C, Pastor D, & Albaigés J (1994) Long-term trends of
polychlorinated biphenyls and organochlorinated pesticides in mussels
from the Western Mediterranean coast. Chemosphere, 28(5): 897-903.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sopena de Kracoff YE, Ferramola de Sancovich AM, Sancovich HA, &
Kleiman de Pisarev DL (1994) Effect of thyroidectomy and thyroxine on
hexachlorobenzene induced porphyria. J Endocrinol Invest, 17:
301-305.
Spigarelli JL, Going JE, & Li R (1986) Hexachlorobenzene levels in
multimedia environmental samples from selected chemical production
plants. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, pp 155-160 (IARC Scientific Publications No. 77).
Stacey CI, Perriman WS, & Whitney S (1985) Organochlorine pesticide
residue levels in human milk: Western Australia, 1979-1980. Arch
Environ Health, 40(2): 102-108.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary. Kansas
City, Missouri, Midwest Research Institute (Prepared for the US
Environmental Protection Agency, Office of Toxic Substances, Field
Studies Branch) (EPA 560/5-86/035).
Stevens RJJ & Neilson MA (1989) Inter- and intralake distribution of
trace contaminants in surface waters of the Great Lakes. J Great Lakes
Res, 15: 377-393.
Stevens MF, Ebell GF, & Psaila-Savona P (1993) Organochlorine
pesticides in Western Australian nursing mothers. Med J Aust, 158:
238-241.
Stewart FP & Smith AG (1987) Metabolism and covalent binding of
hexachlorobenzene by isolated male and female rat hepatocytes. Biochem
Pharmacol, 36: 2232-2234.
Stewart FP, Manson MM, Cabral JRP, & Smith AG (1989) Hexachlorobenzene
as a promoter of diethylnitrosamine-initiated hepatocarcinogenesis in
rats and comparison with induction of porphyria. Carcinogenesis,
10(7): 1225-1230.
Strik JJTWA (1986) Subacute toxicity of hexachlorobenzene. In: Morris
CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
Symposium. Lyon, International Agency for Research on Cancer,
pp 335-342 (IARC Scientific Publications No. 77).
Stronkhorst J, Ysebaert TJ, Smedes F, Meininger PL, Dirksen S, &
Boudewijn TJ (1993) Contaminants in eggs of some waterbird species
from the Scheldt Estuary, SW Netherlands. Mar Pollut Bull, 26(10):
572-578.
Sundlof SF, Hansen LG, Koritz GD, & Sundlof SM (1982) The
pharmacokinetics of hexachlorobenzene in male beagles. Distribution,
excretion and pharmacokinetic model. Drug Metab Dispos, 10: 371-381.
Suns K, Craig GR, Crawford G, Rees GA, Tosine H, & Osborne J (1983)
Organochlorine contaminant residues in spottail shiner ( Notropis
hudsonius) from the Niagara River. J Great Lakes Res, 9: 335-340.
Suns K, Crawford GE, Russell DD, & Clement RE (1985) Temporal trends
and spatial distribution of organochlorine and mercury residues in
Great Lakes spottail shiners (1975-1983). Rexdale, Ontario, Ontario
Ministry of the Environment, Water Resources Branch.
Swindoll CM & Applehans FM (1987) Factors influencing the accumulation
of sediment-sorbed hexachlorobiphenyl by midge larvae. Bull Environ
Contam Toxicol, 39: 1055-1062.
Szymczyœski GA, Waliszewski SM, Tuszewski M, & Pyda P (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznaœ. J Environ Sci Health, A21(1): 5-14.
Tanabe S, Subramanian AN, Ramesh A, Kumaran PL, Miyazaki N, &
Tatsukawa R (1993) Persistent organochlorine residues in dolphins from
the Bay of Bengal, South India. Mar Pollut Bull, 26(6): 311-316.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Teschke R, Bolsen K, Landmann H, & Goerz G (1983) Effect of
hexachlorobenzene on the activities of hepatic alcohol metabolizing
enzymes. Biochem Pharmacol, 32: 1745-1751.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound: Volume 1 (Prepared for Puget Sound Dredged Disposal
Analysis and Puget Sound Estuary Program, Bellevue, Washington).
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in Strain A mice. Cancer Res, 37:
2717-2720.
Thomas W, Simon H, & Rüling Å (1985) Classification of plant species
by their organic (PAH, PCB, BHC) and inorganic (heavy metals) trace
pollutant concentrations. Sci Total Environ, 46: 83-94.
Thomson BA, Davidson WR, & Lovett AM (1980) Applications of a
versatile technique for trace analysis: Atmospheric pressure negative
chemical ionization. Environ Health Perspect, 36: 77-84.
Tobin P (1986) Known and potential sources of hexachlorobenzene. In:
Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings of an
International Symposium. Lyon, International Agency for Research on
Cancer, pp 3-11 (IARC Scientific Publications No. 77).
To-Figueras J, Rodamilans M, Gómez-Catalán J, & Corbella J (1986)
Hexachlorobenzene residues in the general population of Barcelona,
Spain. In: Morris CR & Cabral JRP ed. Hexachlorobenzene: Proceedings
of an International Symposium. Lyon, International Agency for Research
on Cancer, p 147 (IARC Scientific Publications No. 77).
To-Figueras J, Gómez-Catalán J, Rodamilans M, & Corbella J (1992)
Sulphur derivative of hexachlorobenzene in human urine. Hum Exp
Toxicol, 11: 271-273.
To-Figueras J, Barrot C, Rodamilans M, Gómez-Catalán J, Torra M,
Brunet M, Sabater F, & Corbella J (1995) Accumulation of
hexachlorobenzene in humans: a long standing risk. Hum Exp Toxicol,
14: 20-23.
Topp E, Scheunert I, & Korte K (1989) Kinetics of the uptake of
14C-labelled chlorinated benzenes from soil by plants. Ecotoxicol
Environ Saf, 17: 157-166.
Trapp M, Baukloh V, Bohnet H-G, & Heeschen W (1984) Pollutants in
human follicular fluid. Fertil Steril, 42(1): 146-148.
US EPA (1980) Analysis of pesticide residues in human and
environmental samples: A compilation of methods selected for use in
pesticide monitoring programs. Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Toxicology Division,
Health Effects Research Laboratory (EPA 600/8-80-038).
US EPA (1982) Test methods: Methods of organic chemical analysis of
municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Office of Research and Development,
Environmental Monitoring and Support Laboratory (EPA 600/4-82-057).
US EPA (1985a) Health assessment document for chlorinated benzenes.
Washington, DC, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA 600/8-84-015F).
US EPA (1985b) Drinking water criteria document for hexachlorobenzene.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Criteria and Assessment Office (EPA 600/X-84-179-1).
US EPA (1986a) Exposure assessment for hexachlorobenzene. Washington,
DC, US Environmental Protection Agency, Office of Pesticides and Toxic
Substances (EPA 560/5-86-0(19).
US EPA (1986b) Test methods for evaluating solid waste, 3rd ed.
Washington, DC, US Environmental Protection Agency, Office of Solid
Waste and Emergency Response (SW 846).
US EPA (1988) Ambient aquatic life water quality criteria for
hexachlorobenzene. Duluth, Minnesota, US Environmental Protection
Agency, Office of Research and Development.
US FDA (US Food and Drug Administration) (1990) Residues in foods -
1988. J Assoc Off Anal Chem, 73: 133A-152A.
US FDA (US Food and Drug Administration) (1991) Residues in foods -
1989. J Assoc Off Anal Chem, 74: 127A-146A.
US FDA (US Food and Drug Administration) (1992) Residues in foods -
1990. J Assoc Off Anal Chem, 75: 121A-141A.
Üstünbas HB, Öztürk MA, Hasanoglu E, & Dogan M (1994) Organochlorine
pesticide residues in human milk in Kayseri. Hum Exp Toxicol, 13:
299-302.
Van der Ven K, Van der Ven H, Thibold A, Bauer O, Kaisi M, Mbura J,
Mhaya HN, Wever N, Diedrich K, & Krebs D (1992) Chlorinated
hydrocarbon content of fetal and maternal body tissues and fluids in
full term pregnant women: a comparison of Germany versus Tanzania. Hum
Reprod, 7(1): 95-100.
Van Leeuwen CJ, Van der Zandt PTJ, Aldenberg T, Verhaar HJM, & Hermans
JLM (1992) Application of QSARs, extrapolation and equilibrium
partitioning in aquatic effects assessment: I. Narcotic industrial
pollutants. Environ Toxicol Chem, 11: 267-282.
Van Loveren H, Kranjc EI, Rombout PJA, Blommaert FA, & Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicol Appl Pharmacol,
102: 21-33.
Van Ommen B, Hendriks W, Bessems JGM, Geesink G, Müller F, & Van
Bladeren PJ (1989) The relation between the oxidative
biotransformation of hexachlorobenzene and its porphyrinogenic
activity. Toxicol Appl Pharmacol, 100: 517-528.
Van Raaij JAGM, Van den Berg KJ, & Notten WRF (1991a)
Hexachlorobenzene and its metabolites pentachlorophenol and
tetrachlorohydroquinone: interaction with thyroxine binding sites of
rat thyroid hormone carriers ex vivo and in vitro. Toxicol Lett,
59: 101-107.
Van Raaij JAGM, Van den Berg KJ, Engel R, Bragt PC, & Notten WRF
(1991b) Effects of hexachlorobenzene and its metabolites
pentachlorophenol and tetrachlorohydroquinone on serum thyroid hormone
levels in rats. Toxicology, 67: 107-116.
Van Raaij JAGM, Frijters CMG, & Van den Berg KJ (1993a)
Hexachlorobenzene-induced hypothyroidism: involvement of different
mechanisms by parent compound and metabolite. Biochem Pharmacol, 46:
1385-1391.
Van Raaij JAGM, Kaptein E, Visser TJ, & Van den Berg KJ (1993b)
Increased glucoronidation of thyroid hormone in hexachlorobenzene-
treated rats. Biochem Pharmacol, 45: 627-631.
Vaz R, Slorach SA, & Hofvander Y (1993) Organochlorine contaminants in
Swedish human milk: studies conducted at the National Food
Administration 1981-1990. Food Addit Contam, 10(4): 407-418.
Villanueva EC, Jennings RW, Burse VW, & Kimbrough RD (1974) Evidence
of chlorodibenzo-p-dioxin and chlorodibenzofuran in hexachlorobenzene.
J Agric Food Chem, 22: 916-917.
Villeneuve DC, Panopio LG, & Grant DL (1974) Placental transfer of
hexachlorobenzene in the rabbit. Environ Physiol Biochem, 4:
112-115.
Vincent SH, Smith AG, & Muller-Eberhard U (1989) Modulation of hepatic
heme-binding Z protein in mice by the porphyrogenic carcinogens
griseofulvin and hexachlorobenzene. Cancer Lett, 45: 109-114.
Vos JG (1986) Immunotoxicity of hexachlorobenzene. In: Morris CR &
Cabral JRP ed. Hexachlorobenzene: Proceedings of an International
ymposium. Lyon, International Agency for Research on Cancer,
pp 347-356 (IARC Scientific Publications No. 77).
Vos JG, Breeman HA, & Benschop H (1968) The occurrence of the
fungicide hexachlorobenzene in wild birds and its toxicological
importance: a preliminary communication. Meded Fac. Landbouwwet
Rijksuniv Gent, 33: 1263-1269.
Vos JG, Van der Maas HL Musch A, & Ram E (1971) Toxicity of
hexachlorobenzene in Japanese quail with special reference to
porphyria, liver damage, reproduction and tissue residues. Toxicol
Appl Pharmacol, 18: 944-957.
Vos JG, Botterweg PF, Strik JJTWA, & Koeman JH (1972) Experimental
studies with HCB in birds. TNO-Nieuws, 27: 599-603.
Vos JG, Van Logten MJ, Kreeftenberg JG, & Kruizinga W (1979a)
Hexachlorobenzene-induced stimulation of the humoral immune response
in rats. Ann NY Acad Sci, 320: 535-550.
Vos JG, Van Logten MJ, Kreeftenberg JG, Steerenberg PA, & Kruizinga W
(1979b) Effect of hexachlorobenzene on the immune system of rats
following combined pre- and postnatal exposure. Drug Chem Toxicol,
2: 61-76.
Vos JG, Brouwer GMJ, Van Leeuwen FXR, & Wagenaar SJ (1983) Toxicity of
hexachlorobenzene in the rat following combined pre- and post-natal
exposure: comparison of effects on the immune system, liver and lung.
In: Parke DV, Gibson GG, & Hubbard R ed. Immunotoxicology. New York,
London, Academic Press, pp 219-235.
Vos RME, Snoek MC, Van Berkel WJH, Müller F, & Van Bladeren PJ (1988)
Differential induction of rat hepatic glutathione S-transferase
isoenzymes by hexachlorobenzene and benzyl isothiocyanate: comparison
with induction by phenobarbital and 3-methylcholanthrene. Biochem
Pharmacol, 37: 1077-1082.
Wada O, Yano Y, Urata G, & Nakao K (1968) Behavior of hepatic
microsomal cytochromes after treatment of mice with drugs known to
disturb porphyrin metabolism in liver. Biochem Pharmacol, 17:
595-603.
Wang M-J & Jones KC (1994) Occurrence of chlorobenzenes in nine United
Kingdom retail vegetables. J Agric Food Chem, 42: 2322-2328.
Washington State Department of Ecology (1990) Proposed sediment
management standards rule (September 18, 1990). Washington State
Register, issue 90-19.
Watts RR, Hodgson DW, Christ HL, & Moseman RF (1980) Improved method
for hexachlorobenzene and mirex determination with hexachlorobenzene
confirmation in adipose tissue: Collaborative study. J Assoc Off Anal
Chem, 63(5): 1128-1134.
Weisenberg E, Arad I, Grauer F, & Sahm Z (1985) Polychlorinated
biphenyls and organochlorine insecticides in human milk in Israel.
Arch Environ Contam Toxicol, 14: 517-521.
Wells DE, Campbell LA, Ross HM, Thompson PM, & Lockyer CH (1994)
Organochlorine residues in harbour porpoise and bottlenose dolphins
stranded on the coast of Scotland, 1988-1991. Sci Total Environ,
151: 77-99.
WHO (1993) Guidelines for drinking-water quality - Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization, p 84.
Wickström K, Pyysalo H, & Siimes MA (1983) Levels of chlordane,
hexachlorobenzene, PCB and DDT compounds in Finnish human milk in
1982. Bull Environ Contam Toxicol, 31: 251-256.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71: 410-414.
Wilson DM & Wan MTK (1982) Hexachlorobenzene: Industrial and
agricultural sources in British Columbia. Vancouer, Environment
Canada, Environmental Protection Service (Regional Program Report
No. 82-07).
Witte H, Langenohl T, & Offenbacher SAG (1988a) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part A: Organic pollutant load in sewage
sludge. Korresp Abwasser, 13: 118-125.
Witte H, Langenohl T, & Offenbacher SAG (1988b) Investigation of the
entry of organic pollutants into soils and plants through the use of
sewage sludge in agriculture. Part B: Impact of the application of
sewage sludge on organic matter content in soil and plants. Korresp
Abwasser, 13: 126-136.
Yamaguchi Y, Kawano M, & Tatsukawa R (1986) Tissue distribution and
excretion of hexabromobenzene (HBB) and hexachlorobenzene (HCB)
administered to rats. Chemosphere, 15: 453-459.
Yang RSH, Pittman KA, Rourke DR, & Stein VB (1978) Pharmacokinetics
and metabolism of hexachlorobenzene in the rat and the rhesus monkey.
J Agric Food Chem, 26: 1076-1083.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés chimiques et physiques et méthodes d'analyse
L'hexachlorobenzène (HCB) est un composé organique chloré
modérément volatil. Il est pratiquement insoluble dans l'eau, mais
extrêmement soluble dans les lipides et présente une tendance à la
bioaccumulation. L'hexachlorobenzène de qualité technique contient
jusqu'à 2% d'impuretés, dont la principale est le pentachlorobenzène.
Les autres consistent en dibenzo- p-dioxines, dibenzofuranes et
biphényles fortement substitués par le chlore. L'analyse des
échantillons biologiques ou prélevés dans l'environnement comporte
généralement une extraction préliminaire de la prise d'essai, souvent
suivie d'une purification, après quoi les extraits organiques sont
soumis soit à une chromatographie en phase gazeuse couplée à la
spectrométrie de masse (GC/MS), soit à une chromatographie en phase
gazeuse avec détection par capture d'électrons (GC/ECD).
2. Sources d'exposition humaine et environnementale
L'hexachlorobenzène a été utilisé un temps comme fongicide pour
traiter les semences, mais il n'est plus actuellement utilisé à cet
effet dans la plupart des pays. Il continue néanmoins à être libéré
dans l'environnement à partir d'un certain nombre de sources,
notamment lors de l'épandage de pesticides organochlorés, ou encore
lorsque les sous-produits de la préparation des solvants, composés
aromatiques ou pesticides chlorés sont rejetés sans précautions,
incomplètement brûlés ou s'échappent de décharges anciennes.
3.Transport, distribution et transformation dans l'environnement
L'hexachlorobenzène est réparti dans tout l'environnement du fait
de sa mobilité et de sa persistance, même s'il se décompose lentement
dans l'air sous l'action de la lumière et dans le sol sous l'action
des microorganismes. Dans la troposphère, il est transporté sur de
grandes distances et s'élimine de l'air en se déposant sur le sol et
sur l'eau. On a fait état d'une bioamplification notable le long de la
chaîne alimentaire.
4. Concentrations dans l'environnement et exposition humaine
Un peu partout dans le monde, l'hexachlorobenzène est présent, à
distance de ses sources, sous faible concentration dans l'air ambiant
(quelques ng/m3 ou moins) ainsi que dans l'eau de boisson et les eaux
de surface (quelques ng/litre tout au plus). Cependant, au voisinage
des points d'émission, on a pu mesurer des concentrations plus
élevées. Ce composé s'accumule dans les milieux biologiques et on en a
décelé la présence chez des invertébrés, des poissons, des reptiles,
des oiseaux et des mammifères (y compris l'Homme) à distance des
points d'émission, en particulier dans les tissus adipeux des
organismes situés aux niveaux trophiques supérieurs. Chez la
population humaine de divers pays, on en a mesuré dans les tissus
adipeux des quantités qui vont, en moyenne, de quelques dizaines à
quelques centaines de ng/g de poids humide. En se fondant sur les
quantités représentatives d'hexachlorobenzène présentes dans l'air,
l'eau et les denrées alimentaires, on peut estimer à une valeur
comprise entre 0,0004 et 0,003 µg/kg de poids corporel, la dose
absorbée journalièrement par un adulte de la population générale. Cet
apport se fait principalement par la voie alimentaire. Du fait de la
présence d'hexachlorobenzène dans le lait maternel, on estime que dans
les différents pays les enfants nourris au sein en reçoivent
quotidiennement une quantité comprise entre < 0,018 et 5,1 µg/kg de
poids corporel. Les études consacrées à l'évolution de la quantité
d'hexachlorobenzène présente dans l'organisme humain montrent, pour la
plupart, que l'exposition de la population générale a baissé dans de
nombreux endroits, entre les années 70 et le milieu de la décennie
actuelle.
5. Cinétique et métabolisme chez l'Homme et les animaux de
laboratoire
On manque de données toxicocinétiques chez l'Homme.
L'hexachlorobenzène est facilement résorbé par la voie orale chez
l'animal d'expérience, mais il franchit mal la barrière cutanée (on ne
possède pas de données concernant l'inhalation). Chez l'Homme et
l'animal, il s'accumule dans les tissus riches en lipides, comme les
tissus adipeux, le cortex surrénalien, la moelle osseuse, la peau et
certains tissus endocriniens. En outre, il peut être transmis à la
progéniture par l'intermédiaire du lait maternel ou en traversant la
barrière placentaire. La métabolisation de l'hexachlorobenzène est
limitée et ses principaux métabolites urinaires sont le
pentachlorophénol, la tétrachlorhydroquinone et le
pentachlorothiophénol. La demi-vie d'élimination de
l'hexachlorobenzène va d'environ un mois chez les rats et les lapins à
2 ou 3 ans chez le singe.
6. Effets sur les animaux de laboratoire et dans les épreuves
in vitro
L'hexachlorobenzène présente une faible toxicité aiguë pour les
animaux de laboratoire (1000 à 10 000 mg/kg de poids corporel).
L'expérimentation animale montre en outre que ce composé n'est pas
irritant pour la peau ou les yeux et ne provoque pas de
sensibilisation chez le cobaye.
Les données dont on dispose au sujet de la toxicité générale de
l'hexachlorobenzène indiquent que celle-ci s'exerce notamment au
niveau de la voie de biosynthèse de l'hème. Chez plusieurs espèces de
mammifères de laboratoire exposés à de l'hexachlorobenzène, on a
constaté une élévation des concentrations de porphyrines ou de leurs
précurseurs dans les excréta ainsi que dans divers tissus, notamment
le tissu hépatique. De nombreuses études ont relevé des cas de
porphyrie chez des rats exposés de manière chronique ou subchronique à
de l'hexachlorobenzène administré par voie orale à des doses
quotidiennes comprises entre 2,5 et 15 mg par kg de poids corporel.
Chez des porcs à qui on faisait ingérer ce composé en doses
quotidiennes égales ou supérieures à 0,5 mg par kg de poids corporel,
on a observé une augmentation de l'excrétion des coproporphyrines
(aucun effet n'a été observé dans cette étude à la dose de 0,05
mg/kg). On a également montré que l'exposition à l'hexachlorobenzène
affectait de nombreux organes ou systèmes (comme le foie, les poumons,
les reins, la thyroïde, la peau ainsi que le système nerveux et le
système immunitaire) mais ces effets n'ont pas été aussi souvent
signalés que la porphyrie.
L'hexachlorobenzène est un inducteur du cytochrome P-450 de type
mixte. Il possède des propriétés phénobarbital-inductibles et
3- méthylcholantrène-inductibles. Il se fixe sur le récepteur Ah.
Lors d'études longitudinales sur des rats, on a observé à
plusieurs reprises des effets bénins (modifications
histopathologiques, induction d'enzymes) chez les animaux recevant des
doses quotidiennes comprises entre 0,25 et 0,6 mg de composé par kg de
poids corporel. La dose sans effet observable obtenue dans ces études
se situait entre 0,05 et 0,07 mg d'hexachlorobenzène par kg de poids
corporel et par jour. Chez des visons femelles, on a observé une
modification de la concentration de neurotransmetteurs dans
l'hypothalamus après administration prolongée du composé par la voie
alimentaire à la dose quotidienne de 0,16 mg par kg de poids corporel.
Les mêmes constatations ont été faites dans la progéniture de ces
animaux, qui avait été exposée pendant les périodes gestationnelle et
périnatale. Lors d'études subchroniques sur des rats on a constaté une
modification de l'homéostase calcique et des paramètres
ostéomorphométriques à la dose quotidienne de 0,7 mg/kg de poids
corporel, mais pas à celle de 0,07 mg/kg.
Un certain nombre d'études in vivo ont été effectuées sur des
rongeurs afin de mettre en évidence la cancérogénicité éventuelle de
l'hexachlorobenzène. Chez des hamsters qui avaient reçu une nourriture
contenant de l'hexachlorobenzène à la dose moyenne de 4, 8 ou 16 mg/kg
de poids corporel, on a observé chez les deux sexes et à toutes les
doses un accroissement de l'incidence des carcinomes
hépatocellulaires. Aux doses de 8 et 16 mg/kg, on constatait la
présence d'hémangioendothéliomes et à la dose la plus forte,
d'adénomes de la thyroïde chez les mâles. En exposant pendant 120
semaines des souris à ce composé par la voie alimentaire aux doses
quotidiennes respectives de 6, 12 et 24 mg/kg de poids corporel, on a
provoqué un accroissement de l'incidence des carcinomes
hépatocellulaires chez les deux sexes aux deux doses les plus élevées,
mais cet accroissement n'était pas significatif, sauf chez les
femelles exposées à la dose la plus forte. In utero, l'exposition de
rats par la voie orale ou lactationnelle à des doses alimentaires
quotidiennes d'hexachlorobenzène allant de 0,01 à 1,5 mg/kg de poids
corporel (mâles) ou de 1,9 mg/kg (femelles) pendant des périodes
pouvant durer jusqu'à 130 semaines post utero, c'est-à-dire la durée
de vie moyenne, a entraîné à la dose la plus forte un accroissement de
l'incidence des nodules hépatiques néoplasiques et des
phéochromocytomes surrénaliens chez les femelles et un excès
d'adénomes parathyroïdiens chez les mâles. Lors d'une autre étude
chronique effectuée sur des rats, on a exposé les animaux, par la voie
alimentaire et pendant des durées allant jusqu'à 2 ans, à des doses
journalières moyennes de 4-5 et 8-9 mg/kg de poids corporel. Les
effets constatés consistaient en une augmentation de l'incidence des
hépatomes et des adénomes rénaux aux deux doses et chez les deux
sexes. Chez les femelles, on observait en outre une augmentation de
l'incidence des carcinomes hépatocellulaires, des adénomes et des
carcinomes des voies biliaires, des phéochromocytomes et des adénomes
du cortex surrénalien. On a également signalé une incidence élevée des
tumeurs du foie dans un certain nombre d'études plus limitées au cours
desquelles on avait administré une seule dose d'hexachlorobenzène par
la voie alimentaire à de petits groupes de rates. Par ailleurs, on a
observé qu'après exposition subchronique par voie alimentaire à ce
composé, des souris, des hamsters et des rats avaient présenté des
tumeurs du foie, des voies biliaires, du rein, du thymus, de la rate
et des ganglions lymphatiques. Le même type d'exposition favorise
l'apparition de tumeurs hépatiques chez des souris sous l'action de
terphényles polychlorés et chez des rats, sous l'action de la
diéthylnitrosamine.
Sauf dans le cas des tumeurs rénales chez les rats mâles (qui, du
moins en partie, semblent résulter d'une dégénérescence hyaline) et
des hépatomes chez les rats des deux sexes (qui pourraient résulter de
réactions hyperplasiques à la nécrose hépatocellulaire), on n'a pas pu
trouver d'études mécanistiques concernant les divers types de tumeurs
provoquées par l'hexachlorobenzène et le risque encouru à cet égard
par l'Homme.
L'hexachlorobenzène n'a guère d'aptitude à provoquer directement
des mutations géniques, des lésions chromosomiques ou la réparation de
l'ADN. Il s'est révélé faiblement mutagène lors de quelques-unes des
études portant sur des bactéries et des levures, mais il convient de
noter que chacune de ces études comportait des limitations. Il y a
également des signes d'un faible taux de liaison à l'ADN in vitro et
in vivo, mais dans une proportion très inférieure à celle que l'on
attendrait d'une substance cancérogène génotoxique.
Lors d'études sur la reproduction, des doses d'hexachlorobenzène
ne dépassant pas 0,1 mg par kg de poids corporel qu'on avait fait
ingérer quotidiennement pendant 90 jours à des singes, ont provoqué
des anomalies dans la structure microscopique et l'ultrastructure de
l'épithélium germinatif superficiel, structures qui constituent une
cible inhabituelle pour des toxines ovariennes. Cette dose a également
endommagé l'ultrastructure des cellules germinales primordiales. Alors
même que ces sites étaient spécifiquement attaqués et présentaient des
lésions d'autant plus importantes que la dose était plus forte, le
développement folliculaire, ovocytaire et embryonnaire restait normal,
ce qui semble indiquer que l'hexachlorobenzène a un site d'action à
localisation spécifiquement ovarienne. Chez les mâles, la fonction de
reproduction n'est affectée qu'à des doses beaucoup plus élevées
(entre 30 et 221 mg/kg p.c. par jour), comme l'ont montré un certain
nombre d'études effectuées sur plusieurs espèces n'appartenant pas à
l'ordre des primates.
Des rats et des chats exposés par la voie transplacentaire ou
lactationnelle à des doses quotidiennes d'hexachlorobenzène comprises
entre 3 et 4 mg/kg p.c. ont présenté des signes d'hépatotoxicité et on
a également constaté des effets délétères sur la survie et la
croissance de leur progéniture. Dans certains cas, il y avait à ces
doses - ou à des doses plus élevées - une réduction de l'effectif des
portées et un nombre accru de mortinaissances. (En général, les ratons
et les chatons à la mamelle étaient plus souvent affectés - et à des
doses plus faibles - que les embryons et les foetus). Chez la
progéniture de visons qui recevaient une alimentation ne contenant pas
plus de 1 mg d'hexachlorobenzène par kg de nourriture (soit environ
0,16 mg/kg p.c. par jour), on a constaté une réduction du poids de
naissance et un accroissement de la mortalité au sevrage. Quelques
études ont mis en évidence des anomalies squelettiques ou rénales chez
les foetus de rats et de souris exposés à de l'hexachlorobenzène
pendant la gestation, mais les doses qui produisaient ces anomalies
n'étaient pas toxiques pour les mères. Par ailleurs, le lien de ces
anomalies avec la prise d'hexachlorobenzène n'a pas été formellement
établi. Dans deux études, dont l'une comportait une exposition
transplacentaire et postnatale, on a observé des anomalies du
développement neurocomportemental des ratons après exposition
in utero, les mères ayant reçu par voie orale des doses quotidiennes
d'hexachlorobenzène allant de 0,64 à 2,5 mg/kg de poids corporel.
Selon un certain nombre d'études, l'hexachlorobenzène aurait des
effets délétères sur le système immunitaire. Chez des rats et des
singes exposés à des doses quotidiennes comprises entre 3 et 120 mg
d'hexachlorobenzène par kg de poids corporel, on a constaté des
modifications histopathologiques au niveau du thymus, de la rate, des
ganglions lymphatiques et des tissus lymphoïdes pulmonaires. Chez des
chiens beagle exposés de façon chronique à des doses quotidiennes
correspondant à 0,12 mg de composé par kg p.c., on a observé une
hyperplasie nodulaire du tissu lymphoïde gastrique. Un certain nombre
d'études menées sur des rats ont montré qu'après plusieurs semaines
d'exposition à de l'hexachlorobenzène par la voie alimentaire, il y
avait stimulation de l'immunité humorale, et dans une moindre mesure,
de l'immunité à médiation cellulaire, sans modification de la fonction
des macrophages. A des doses quotidiennes ne dépassant pas 4 mg de
composé par kg de nourriture (environ 0,2 mg par kg p.c.),
administrées pendant la gestation, pendant le maternage et jusqu'à
l'âge de 5 semaines, il y a eu augmentation de la réponse immunitaire
à médiation cellulaire et de la réponse immunitaire humorale ainsi
qu'une accumulation de macrophages dans le tissu pulmonaire des
ratons. Par contre, la plupart des études effectuées sur des souris
ont fait ressortir les propriétés immunosuppressives de
l'hexachlorobenzène; des doses ne dépassant pas 0,5 à 0,6 mg/kg de
poids corporel administrées quotidiennement pendant plusieurs semaines
ont eu les effets suivants: diminution de la résistance à une
infection leishmanienne ou à une épreuve cancérogène par exposition à
des cellules tumorales, réduction de l'activité cytotoxique des
macrophages spléniques et de l'hypersensibilité retardée chez la
progéniture après exposition in utero ou pendant la période de
maternage. Lors d'un certain nombre d'études portant sur diverses
souches de rats, on a constaté qu'une exposition de brève durée ou une
exposition subchronique à de l'hexachlorobenzène modifiait la fonction
thyroïdienne, comme on pouvait en juger d'après la réduction de la
thyroxine sérique libre ou totale (T4) et souvent, mais dans une
moindre mesure, de la triiodothyronine (T3).
7. Effets sur l'Homme
La plupart des données que l'on possède au sujet des effets de
l'hexachlorobenzène sur l'Homme, proviennent d'intoxications
accidentelles qui se sont produites en Turquie en 1955-59, avec plus
de 600 cas répertoriés de porphyrie cutanée tardive. Lors de cet
accident, on a observé des troubles du métabolisme des porphyrines,
des lésions cutanées, des hyperpigmentations, des hypertrichoses, des
hépatomégalies, des hypertrophies de la thyroïde et des ganglions
lymphatiques, avec, dans environ la moitié des cas, une ostéoporose et
une arthrite, principalement d'ailleurs, chez les enfants. Les enfants
nourris au sein dont la mère avait été exposée, présentaient des
lésions appelées pembe yara, c'est-à-dire "lésions roses", et la
plupart d'entre eux sont décédés dans l'année. On dispose de quelques
données concernant des cas de porphyrie cutanée tardive chez des
personnes ayant subi une exposition relativement intense à
l'hexachlorobenzène sur leur lieu de travail ou dans leur
environnement général.
Les quelques études épidémiologiques disponibles concernant le
cancer souffrent d'un certain nombre d'insuffisances: effectif réduit,
exposition à l'hexachlorobenzène mal caractérisée ou exposition
simultanée à de nombreux autres agents, et ne permettent pas d'évaluer
la cancérogénicité de ce composé pour l'Homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Lors d'études sur la toxicité aiguë de l'hexachlorobenzène pour
les organismes aquatiques, on a constaté que l'exposition à des
concentrations de l'ordre de 1 à 17 µg/litre réduisait la production
de chlorophylle chez les algues ainsi que la reproduction chez les
ciliés, et qu'en outre, elle provoquait la mort des crevettes roses et
des crevettes américaines du genre Hippolyte, mais elle n'a pas
provoqué la mort de poissons d'eau douce ou de mer. Lors d'études à
plus long terme, on a constaté que la croissance de certaines algues
et protozoaires dulçaquicoles sensibles étaient affectée à une
concentration d'hexachlorobenzène de 1 µg/litre et que des
concentrations d'environ 3 µg/litre provoquaient la mort d'amphipodes
et de perches appartenant à l'espèce Micropterus salmoides.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
9.1 Effets sur la santé
Le Groupe de travail a conclu que les données disponibles sont
suffisantes pour que l'on puisse formuler des valeurs-guides relatives
aux effets cancérogènes et non cancérogènes de l'hexachlorobenzène.
En ce qui concerne les effets non cancérogènes constatés sur le
foie à dose élevée chez des porcs et des rats exposés par la voie
orale et en se basant sur la dose sans effet observable la plus faible
(0,05 mg/kg de poids corporel par jour), on arrive, compte tenu d'un
facteur d'incertitude de 300 (10× pour les variations
interspécifiques, 10x pour les variations intraspécifiques et 3× pour
la gravité de l'effet), à une TDI de 0,17 µg/kg de poids corporel.
La méthode utilisée pour déterminer la valeur-guide relative aux
effets cancérogènes repose sur la dose tumorigène TD5, c'est-à-dire
la dose ingérée qui provoque une augmentation de 5% de l'incidence
tumorale chez les animaux de laboratoire. D'après les résultats d'une
étude de cancérogénicité portant sur deux générations de rats et en
faisant appel à un modèle multiphasique, on obtient une TD5 de
0,81 mg/kg de poids corporel par jour, l'effet retenu étant la
formation de nodules cancéreux hépatiques chez les femelles. Compte
tenu de l'insuffisance des données mécanistiques, on a appliqué un
facteur d'incertitude de 5000 pour calculer la valeur-guide chiffrée à
0,16 µg/kg de poids corporel par jour.
9.2 Effets sur l'environnement
Le Groupe de travail a remarqué qu'il existe très peu d'études
expérimentales à partir desquelles on puisse procéder à une évaluation
du risque écologique. La concentration d'hexachlorobenzène dans les
eaux de surface est généralement inférieure de plusieurs ordres de
grandeur à celle qui pourrait être dangereuse pour les organismes
aquatiques, sauf dans certains endroits fortement pollués. Toutefois,
les concentrations d'hexachlorobenzène relevées dans les oeufs
d'oiseaux de mer et de rapaces en différents lieux du globe sont
proches de celles qui provoquent une diminution du poids des embryons
chez la mouette argentée (1500 µg/kg), ce qui incite à penser que le
composé pourrait être embryotoxique pour certaines espèces sensibles
d'oiseaux. De même, les concentrations d'hexachlorobenzène dans les
poissons de divers endroits du monde sont du même ordre de grandeur
que la dose de 1000 µg/kg qui entraîne une réduction du poids de
naissance et une augmentation de la mortalité chez la progéniture de
visons. Cela incite à penser que ce composé pourrait avoir des effets
indésirables chez les visons et éventuellement chez d'autres
mammifères piscivores.
10. Conclusions
a) L'hexachlorobenzène est un composé chimique persistant qui subit
une bioaccumulation du fait de sa liposolubilité et de sa résistance à
la décomposition.
b) L'expérimentation animale montre que l'hexachlorobenzène provoque
des cancers et affecte de nombreux organes, tissus et systèmes comme
le foie, les poumons, les reins, la thyroïde, les tissus des gonades,
le système nerveux et le système immunitaire.
c) En ce qui concerne l'Homme, on a pu observer, à l'occasion d'une
forte exposition d'origine accidentelle, les manifestations cliniques
d'une intoxication par l'hexachlorobenzène qui se traduisaient par une
porphyrie cutanée tardive chez les enfants et les adultes et par la
mort chez des nourrissons alimentés au sein.
d) Il est justifié de prendre diverses mesures pour réduire la
quantité d'hexachlorobenzène présente dans l'environnement.
e) On a proposé les valeurs-guides à visée sanitaire suivantes pour
la dose totale ingérée quotidiennement (TDI): en ce qui concerne les
effets non cancérogènes, 0,17 µg/kg de poids corporel par jour ; en ce
qui concerne les effets cancérogènes, 0,16 µg/kg de poids corporel par
jour.
1. RÉSUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El hexaclorobenceno (HCB) es un compuesto orgánico clorado de
volatilidad moderada. Es prácticamente insoluble en el agua, pero es
muy liposoluble y bioacumulativo. El HCB de calidad técnica contiene
hasta un 2% de impurezas, en su mayor parte pentaclorobenceno; el
resto incluye dibenzo- p-dioxinas, dibenzofuranos y bifenilos
altamente clorados. Para determinar el HCB en el medio ambiente y en
material biológico se procede por lo general a extraer la muestra
mediante disolventes orgánicos, a lo que sigue con frecuencia un paso
de limpieza, a fin de obtener extractos orgánicos analizables mediante
cromatografía de gases/espectrometría de masas (GC/MS) o cromatografía
de gases con detección de captura de electrones (GC/ECD).
2. Fuentes de exposición humana y ambiental
Hubo un tiempo en que el HCB se utilizó mucho en la limpieza de
semillas para prevenir las enfermedades micóticas de los cereales,
pero ese uso se abandonó en la mayor parte de los países en los años
setenta. El HCB se sigue liberando en el medio ambiente a partir de
diversas fuentes, que incluyen el uso de algunos plaguicidas clorados,
procesos de combustión incompleta y viejos vertederos, así como los
métodos inapropiados de producción y de eliminación de desechos en la
fabricación de disolventes clorados, compuestos aromáticos clorados y
plaguicidas clorados.
3. Transporte, distribución y transformación en el medio ambiente
El HCB se distribuye por todo el medio ambiente porque es móvil y
persistente, aunque se produce una lenta fotodegradación en el aire y
una degradación microbiana en el suelo. En la troposfera el HCB es
transportado a grandes distancias y es eliminado de la fase aérea por
su depósito en el agua y el suelo. Se ha informado de que se produce
una importante bioamplificación del HCB a través de la cadena trófica.
4. Niveles ambientales y exposición humana
Se encuentran concentraciones bajas de HCB en el aire ambiental
(a lo sumo unos pocos ng/m3), en el agua de bebida y en las aguas
superficiales (a lo sumo unos pocos ng/litro) de zonas alejadas del
punto emisor en todo el mundo. No obstante, se han hallado
concentraciones más altas cerca de los puntos emisores. El HCB es
bioacumulativo y se ha detectado en invertebrados, peces, reptiles,
aves y mamíferos (incluido el hombre) lejos de los puntos emisores,
particularmente en el tejido adiposo de organismos de los niveles
tróficos más altos. Los niveles medios en el tejido adiposo de la
población humana general en diversos países van de decenas a centenas
de ng/g de peso en fresco. Considerando los niveles representativos de
HCB en el aire, el agua y los alimentos, se estima que la ingesta
total de HCB por los adultos de la población general está comprendida
entre 0,0004 y 0,003 mg/kg de peso corporal al día. Esa ingesta se
realiza principalmente a través de los alimentos. Debido a la
presencia de HCB en la leche materna, se ha estimado que la ingesta
media por los lactantes alimentados al pecho en diversos países va de
< 0,018 a 5,1 mg/kg de peso corporal al día. Los resultados de la
mayoría de los estudios realizados acerca de las concentraciones de
HCB en los alimentos y en los tejidos humanos a lo largo del tiempo
indican que la exposición de la población general al HCB disminuyó
desde los años setenta hasta mediados de los noventa en muchos
lugares. Sin embargo, esa tendencia no se ha confirmado con claridad
durante el último decenio en otros lugares.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay suficientes datos sobre la toxicocinética en el hombre. El
HCB es absorbido rápidamente por vía oral por los animales de
experimentación, y escasamente a través de la piel (no existen datos
sobre la inhalación). En los animales y en los seres humanos, el HCB
se acumula en los tejidos ricos en lípidos, como el tejido adiposo, la
corteza suprarrenal, la médula ósea, la piel y algunos tejidos
endocrinos, y puede transmitirse a la descendencia a través tanto de
la placenta como de la leche materna. El HCB sufre un metabolismo
limitado, generando pentaclorofenol, tetraclorohidroquinona y
pentaclorotiofenol como principales metabolitos en la orina. Las
semividas de eliminación del HCB están comprendidas entre
aproximadamente un mes en la rata y el conejo y 2 ó 3 años en el mono.
6. Efectos en animales de laboratorio y en las pruebas in vitro
La toxicidad aguda del HCB en los animales de experimentación es
baja (1000 × 10 000 mg/kg de peso corporal). En los estudios con
animales, el HCB no causa irritación cutánea ni ocular y no tiene
efectos de sensibilización en el cobayo.
Los datos disponibles acerca de la toxicidad sistémica del HCB
indican que las vías de la biosíntesis del grupo hemo son una
importante diana de la toxicidad del hexaclorobenceno. Se han hallado
niveles elevados de porfirinas o de precursores de la porfirina, o de
ambas cosas, en el hígado, en otros tejidos y en las excretas de
varias especies de mamíferos de laboratorio expuestos al HCB. Se ha
informado de la aparición de porfiria en varios estudios realizados
con ratas expuestas por vía oral crónica o subcrónica a dosis entre
2,5 y 15 mg de HCB/kg de peso corporal al día. La excreción de
coproporfirinas aumentó en cerdos que ingirieron 0,5 mg de HCB/kg de
peso corporal al día o más (en el último estudio no se observó ningún
efecto con 0,05 mg de HCB/kg de peso corporal al día). Se ha visto
también que la exposición repetida al HCB afecta a una amplia gama de
sistemas orgánicos (entre ellos el hígado, los pulmones, los riñones,
la tiroides, la piel y los sistemas nervioso e inmunitario), aunque
las referencias a estos efectos son menos frecuentes que las
relacionadas con la porfiria.
El HCB es un inductor de tipo mixto del citocromo P-450, con
propiedades inducibles por el fenobarbital y por el 3-metilcolantreno.
Se sabe que se une al receptor Ah.
Por lo que se refiere a los estudios crónicos, en ratas expuestas
a dosis de 0,25 a 0,6 mg de HCB/kg peso corporal al día se observaron
efectos leves en el hígado (cambios histopatológicos, inducción
enzimática); en dichos estudios los NOEL estaban comprendidos entre
0,05 y 0,07 mg de HCB/kg de peso corporal al día. Las concentraciones
de neurotransmisores en el hipotálamo se vieron alteradas en visones
hembra sometidos a través de los alimentos a una exposición crónica de
0,16 mg de HCB/kg de peso corporal al día, y en su descendencia
expuesta a lo largo de la gestación y la lactancia. En estudios
subcrónicos realizados en ratas la homeostasis del calcio y la
morfometría ósea se vieron afectadas con 0,7 mg de HCB/kg de peso
corporal al día, pero no con 0,07 mg/kg de peso corporal al día.
La carcinogenicidad del HCB ha sido evaluada mediante varios
bioensayos realizados con roedores. En hámsters mantenidos con
alimentos con los que ingerían unas dosis medias de 4, 8 ó 16 mg/kg de
peso corporal al día durante toda la vida, se produjeron aumentos en
la incidencia de tumores de las células del hígado (hepatomas) en los
dos sexos y a todas las dosis, hemangioendoteliomas hepáticos a dosis
de 8-16 mg/kg de peso corporal al día, y adenomas tiroideos de los
machos a la dosis mayor. La exposición alimentaria de ratones a dosis
de 6, 12 y 24 mg/kg de peso corporal al día durante 120 semanas dio
lugar a un aumento de la incidencia de tumores de las células del
hígado (hepatomas) en ambos sexos a las dos dosis mayores (no
significativo, excepto para las hembras a la dosis mayor). En ratas,
la exposición in útero, durante la lactancia y por vía oral al HCB a
través de alimentos que proporcionaban a lo largo de su vida dosis
medias comprendidas entre 0,01 y 1,5 mg/kg de peso corporal al día
(machos) o 1,9 mg/kg de peso corporal al día (hembras) por espacio de
hasta 130 semanas post útero produjo a la mayor de las dosis un
aumento de la incidencia de nódulos hepáticos neoplásicos y de
feocromocitomas suprarrenales en las hembras y de adenomas
paratiroideos en los machos. En otro estudio crónico realizado en la
rata, la exposición por un periodo de hasta dos años a alimentos que
proporcionaban dosis medias de HCB de 4-5 y de 8-9 mg/kg de peso
corporal al día indujo aumentos de la incidencia de hepatomas y de
adenomas de las células renales a ambas dosis en los dos sexos, y de
carcinomas hepatocelulares, adenomas y carcinomas de las vías
biliares, y feocromocitomas suprarrenales y adenomas de la corteza
suprarrenal en las hembras. Se ha informado también de incidencias
elevadas de tumores hepáticos en algunos estudios más limitados en los
que se administraron concentraciones alimentarias únicas a grupos
reducidos de ratas hembra. Además, se ha informado de que, después de
una exposición alimentaria subcrónica al HCB, ratones, hámsters y
ratas desarrollaron tumores en el hígado, las vías biliares, el riñón,
el timo, el bazo y los ganglios linfáticos. La exposición alimentaria
al HCB favoreció la inducción de tumores hepáticos por el terfenilo
policlorado en el ratón y por la dietilnitrosamina en la rata.
Con excepción de los tumores renales en la rata macho
(aparentemente debidos, al menos en parte, a una nefropatía por
acumulación de gotas hialinas) y de los hepatomas en la rata (posible
resultado de la respuesta hiperplásica a una necrosis hepatocelular),
no se conocen estudios mecanísticos que hayan determinado el
significado del tipo de tumores inducidos por el HCB en el caso del
hombre.
El HCB tiene una escasa capacidad de inducción directa de
mutaciones de los genes, lesiones cromosómicas y reparaciones del ADN.
Mostró una leve actividad mutágena en un reducido número de los
estudios realizados en bacterias y levaduras, aunque hay que señalar
que todos esos estudios presentan limitaciones. Existen también
algunos indicios de un cierto grado de unión al ADN in vitro e
in vivo, aunque a niveles muy inferiores a los habituales en los
carcinógenos genotóxicos.
En estudios sobre la reproducción, la exposición oral de monos a
tan sólo 0,1 mg de HCB/kg de peso corporal al día durante 90 días
afectó a la estructura revelada por microscopia óptica y a la
ultraestructura del epitelio germinal superficial, una diana poco
usual para las toxinas que afectan al ovario. Dicha dosis causó
también daños ultraestructurales en las células germinales
primordiales. Estos cambios específicos en tejidos-diana, para los que
dosis mayores son aún más lesivas, se asocian por lo demás a un
desarrollo normal del folículo, el ovocito y el embrión, lo que indica
que el HCB tiene una acción específica en el ovario. La reproducción
masculina sólo se vio afectada a dosis mucho mayores (entre 30 y
221 mg/kg de peso corporal al día) en estudios realizados en varias
especies distintas de los primates.
La exposición de ratas y gatos, a través de la placenta o durante
la lactancia, a dosis maternas comprendidas entre 3 y 4 mg/kg de peso
corporal al día tuvo efectos hepatotóxicos o afectó a la supervivencia
o el crecimiento de la descendencia en período de lactancia. En
algunos casos, dosis iguales o superiores a ésas redujeron el tamaño
de las camadas o aumentaron el número de abortos. (Los efectos nocivos
en los cachorros sin destetar han sido observados más frecuentemente,
y a dosis menores, que los efectos embriotóxicos o fetotóxicos.) La
descendencia de visones expuestos crónicamente a sólo 1 mg de HCB/kg
de alimento (aproximadamente 0,16 mg/kg de peso corporal al día) tuvo
un peso reducido al nacer y presentó una mayor mortalidad hasta el
destete. A pesar de que se han observado trastornos esqueléticos y
renales de los fetos en algunos estudios realizados en ratas y ratones
expuestos al HCB durante la gestación, dichas alteraciones o bien no
estaban claramente relacionadas con el tratamiento o bien ocurrieron a
dosis que eran también tóxicas para las madres. En dos estudios, uno
de los cuales incluía exposición posnatal y durante la lactancia, el
desarrollo neurocomportamental de las crías de rata se vio afectado
por la exposición in útero a dosis maternas orales de 0,64 a 2,5 mg
de HCB/kg de peso corporal al día.
Los resultados de varios estudios indican que el HCB afecta al
sistema inmunitario. Ratas y monos expuestos a dosis entre 3 y 120 mg
de HCB/kg de peso corporal al día sufrieron alteraciones
histopatológicas en el timo, en el bazo y en los ganglios linfáticos o
los tejidos linfoides del pulmón. La exposición crónica de perros
sabuesos a 0,12 mg/kg de peso corporal al día produjo una hiperplasia
nodular del tejido linfoide gástrico. En varios estudios realizados en
la rata, la inmunidad humoral y, en menor grado, la celular se vieron
potenciadas tras varias semanas de exposición alimentaria al HCB,
mientras que la función de los macrófagos no se alteró. Una cantidad
tan pequeña como 4 mg de HCB/kg de alimento (aproximadamente 0,2 mg/kg
de peso corporal al día) durante la gestación, a lo largo de la
lactancia y hasta las 5 semanas de edad incrementó las respuestas
inmunitarias humoral y celular y provocó la acumulación de macrófagos
en el tejido pulmonar de crías de rata. Por el contrario, se ha
observado un efecto inmunodepresor del HCB en la mayor parte de los
estudios llevados a cabo con ratones; dosis de sólo 0,5-0,6 mg/kg de
peso corporal al día durante varias semanas redujeron la resistencia a
la infección por Leishmania o a una provocación con células
tumorales, disminuyeron la actividad citotóxica de los macrófagos del
bazo, y redujeron la respuesta de hipersensibilidad de tipo retardado
en la descendencia expuesta in útero y durante la lactancia. En
varios estudios realizados con diversas cepas de ratas, la exposición
de breve duración o subcrónica al HCB afectó a la función tiroidea, a
juzgar por los reducidos niveles séricos de tiroxina total y tiroxina
libre (T4) y a menudo, en menor grado, de triyodotironina (T3).
7. Efectos en el ser humano
La mayor parte de los datos acerca de los efectos del HCB en el
ser humano provienen de intoxicaciones accidentales que tuvieron lugar
en Turquía en los años 1955-1959, entre las que se identificaron más
de 600 casos de porfiria cutánea tardía (PCT). En esa ocasión se
observaron alteraciones en el metabolismo de la porfirina, lesiones
dermatológicas, hiperpigmentación, hipertricosis, aumento del tamaño
del hígado, de la glándula tiroides y de los ganglios linfáticos; se
observaron también (aproximadamente en la mitad de los casos)
osteoporosis o artritis, sobre todo en los niños. Los niños
amamantados por madres expuestas al HCB como consecuencia de ese
accidente desarrollaron un trastorno conocido como pembe yara
(ulceración rosada), y la mayor parte murieron antes de un año.
Existen también algunos indicios de que la PCT afecta a personas
sometidas a una exposición relativamente alta al HCB en el lugar de
trabajo o en el medio ambiente general.
Los pocos estudios epidemiológicos disponibles acerca de la
incidencia de cáncer tienen un valor limitado, ya sea por lo reducido
de la muestra, por la deficiente caracterización de la exposición al
CHB o por la exposición a otros muchos agentes, y son insuficientes
para evaluar la carcinogenicidad del HCB para el ser humano.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
En los estudios realizados sobre la toxicidad aguda del HCB para
los organismos acuáticos, la exposición a concentraciones comprendidas
entre 1 y 17 mg/litro redujo la producción de clorofila en algas y la
reproducción de protozoos ciliados, y causó mortalidad en el camarón
rosado y en las quisquillas, pero no aumentó la mortalidad de peces de
agua dulce o de mar. En estudios a largo plazo, el crecimiento de
algas y protozoos vulnerables de agua dulce se vio afectado por una
concentración de 1 mg/litro, mientras que concentraciones de
aproximadamente 3 mg/litro provocaron mortalidad en anfípodos y
necrosis hepática en la perca americana.
9. Evaluación de los riesgos para la salud humana y de los efectos
en el medio ambiente
9.1 Efectos en la salud
El Grupo Especial llegó a la conclusión de que los datos
disponibles son suficientes para establecer valores indicativos
respecto a los efectos neoplásicos y no neoplásicos del HCB.
En cuanto a los efectos no neoplásicos, considerando el NOEL más
bajo notificado (0,05 mg de HCB/kg de peso corporal al día), referido
sobre todo a los efectos hepáticos observados a dosis mayores en
estudios realizados en cerdos y ratas expuestos por vía oral, e
incorporando un factor de incertidumbre de 300 (× 10 en concepto de
variación interespecíes, × 10 en concepto de variación intraespecie, y
× 3 en concepto de gravedad del efecto), se ha calculado una IDT de
0,17 mg/kg de peso corporal al día.
El criterio seguido en cuanto a los efectos neoplásicos se basa
en la dosis tumorigénica TD5, es decir, la ingesta asociada a un
exceso del 5% en la incidencia de tumores detectada en los
experimentos con animales. Considerando los resultados del bioensayo
de carcinogenicidad en dos generaciones de ratas, y empleando el
modelo polietápico, la TD5 es de 0,81 mg/kg de peso corporal al día
para los nódulos neoplásicos del hígado en las hembras. Habida cuenta
de la insuficiencia de los datos mecanísticos, se utilizó un factor de
incertidumbre de 5000 para establecer un valor indicativo, basado en
criterios de salud, de 0,16 mg/kg de peso corporal al día.
9.2 Efectos en el medio ambiente
El Grupo Especial señaló que existen muy pocos estudios
experimentales con los que llevar a cabo una evaluación de los riesgos
para el medio ambiente. Los niveles de HCB en las aguas superficiales,
excepto en unos pocos lugares extremadamente contaminados, son en
general varios órdenes de magnitud inferiores a los que se supone que
entrañan riesgos para los organismos acuáticos. No obstante, las
concentraciones de HCB en los huevos de las aves marinas y las rapaces
de algunos lugares en distintas zonas del mundo se aproximan a niveles
que en la gaviota argéntea se asocian a una disminución del peso del
embrión (1500 mg/kg), lo que parece indicar que el HCB puede dañar los
embriones de especies de aves vulnerables. Del mismo modo, los niveles
de HCB observados en peces de diversos lugares del mundo se encuentran
a un orden de magnitud del nivel alimentario de 1000 mg/kg, asociado a
una reducción del peso al nacer y a un aumento de la mortalidad de la
descendencia en los visones. Esto parece indicar que el HCB puede
tener efectos nocivos en los visones y, tal vez, en otros mamíferos
que se alimentan de peces.
10. Conclusiones
a) El HCB es un producto químico persistente que se bioacumula debido
a su liposolubilidad y a su resistencia a la degradación.
b) Los estudios realizados con animales han demostrado que el HCB
produce cáncer y afecta a una amplia gama de sistemas de órganos, con
inclusión del hígado, los pulmones, los riñones, la tiroides, los
tejidos reproductivos y los sistemas nervioso e inmunitario.
c) En seres humanos sometidos a una alta exposición accidental se ha
observado toxicidad sintomática, en particular porfiria cutánea tardía
en niños y en adultos y mortalidad en lactantes.
d) Es necesario adoptar diversas medidas para reducir la carga
ambiental de HCB.
e) Se han propuesto los siguientes valores indicativos basados en
criterios de salud para la ingesta diaria total (IDT) de HCB por el
ser humano: efectos no cancerígenos, 0,17 µg/kg de peso corporal/día;
yefectos neoplásicos, 0,16 µ/kg de peso corporal/día.
