
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 204
BORON
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms C. Smallwood, US Environmental Protection
Agency, Cincinnati, Ohio, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Boron.
(Environmental health criteria ; 204)
1.Boron 2.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157204 3 (NLM Classification: QD 181.B1)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BORON
PREAMBLE
ABBREVIATIONS
1. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, natural occurrence, and analytical methods
1.1.2. Production, uses, environmental fate, and sources of
exposure
1.1.3. Kinetics and biological monitoring
1.1.4. Effects on experimental animals and humans
1.1.5. Effects on organisms in the environment
1.2. Conclusions
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.3.1. Conversion factors of ppm and mg/m3 for boron
2.3.2. Conversion factors for boron compounds to boron
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Mining and production
3.3. Uses and release
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water and sediment
4.1.3. Soil
4.1.4. Vegetation and wildlife
4.2. Transformation
4.2.1. Biotransformation
4.2.2. Abiotic transformation
4.2.2.1 Air
4.2.2.2 Water
4.2.2.3 Soil
4.2.3. Bioaccumulation
4.2.3.1 Aquatic organisms
4.2.3.2 Terrestrial plants
4.2.3.3 Birds
4.3. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Groundwater
5.1.2.2 Surface water
5.1.2.3 Rainfall
5.1.3. Sewage
5.1.4. Soil
5.1.5. Aquatic biota
5.1.6. Terrestrial biota
5.2. General population exposure
5.2.1. Ambient air
5.2.2. Drinking-water
5.2.3. Soil intake
5.2.4. Dietary intake
5.2.5. Consumer products
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.1.1. Oral
6.1.2. Inhalation
6.1.3. Dermal
6.2. Distribution
6.2.1. Tissue levels
6.2.2. Blood levels
6.3. Metabolism
6.4. Elimination and excretion
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Short-term exposure
7.1.1. Oral route
7.1.2. Inhalation route
7.2. Longer-term exposure
7.2.1. Oral route
7.2.2. Inhalation route
7.3. Dermal and ocular effects
7.4. Reproductive toxicity
7.5. Developmental toxicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Toxicity effects summary
7.9. Physiological effects
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Short-term toxicity and poisoning incidents
8.1.2. Reproductive effects
8.2. Occupational exposure
8.2.1. Short-term irritative effects
8.2.2. Male reproductive and other long-term health effects
8.3. Carcinogenicity
8.4. Physiological effects
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Water
9.1.1.2 Soil
9.1.2. Aquatic organisms
9.1.2.1 Plants
9.1.2.2 Invertebrates
9.1.2.3 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Vertebrates
9.2. Field observations
9.2.1. Aquatic
9.2.2. Terrestrial
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health exposures
10.2. Choice of critical effect and application of uncertainty
factors
10.3. Derivation of the tolerable intake
10.4. Derivation of guidance values
10.5. Evaluation of effects on the environment
10.5.1. Exposure
10.5.2. Effects
10.5.3. Risk evaluation
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations
12. FURTHER RESEARCH
13. EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
RÉSUMÉ, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN, CONCLUSIONES Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
Environmental Health Criteria
PREAMBLE
Objectives
In 1973, the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976, and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g. for genetic, neurotoxic,
teratogenic, and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly, and so
forth.
Since its inauguration, the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently, the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO, and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used, and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effects on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered, and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are used only when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary -- a review of the salient facts and the risk evaluation
of the chemical
* Identity -- physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution, and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g. IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation, or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for the environment; international concern,
i.e. the substance is of major interest to several countries; adequate
data on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the cooperating
organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals and from reference databases such as
Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally,
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government, or industry. Their
function is to evaluate the accuracy, significance, and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can speak
only at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants, or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, the document then goes for language editing, reference
checking, and preparation of camera-ready copy. After approval by the
Director, IPCS, the monograph is submitted to the WHO Office of
Publications for printing. At this time, a copy of the final draft is
sent to the Chairperson and Rapporteur of the Task Group to check for
any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
Members
Dr G.M. Buck, Department of Social and Preventive Medicine, State
University of New York at Buffalo, Buffalo, New York, USA
Dr R. Chapin, Department of Health and Human Services, National
Institutes of Health, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr M.L. Dourson, Toxicology Excellence for Risk Assessment,
Cincinnati, Ohio, USA
Dr P. Foster, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr R.A. Goyer, 6405 Huntingridge Road, Chapel Hill, North
Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Cambs., United Kingdom (Co-Rapporteur)
Dr R. Luoto, National Public Health Institute, Department of
Epidemiology and Health Promotion, Helsinki, Finland
Dr F.H. Nielsen, US Department of Agriculture, Agricultural
Research Service, Grand Forks Human Nutrition Research Center,
Grand Forks, North Dakota, USA
Dr C.J. Price, Center for Life Sciences and Toxicology, Research
Triangle Institute, Research Triangle Park, North Carolina, USA
Dr W.G. Woods, Office of Environmental Health and Safety,
University of California, Riverside, California, USA
Observers
Dr B.D. Culver, University of California, Department of Medicine,
Irvine, California, USA (representing International Commission on
Occupational Health)
Dr S. Dyer, Procter & Gamble, Ecosystems Research Station,
Environmental Science Department, Cincinnati, Ohio, USA
(representing European Centre for Ecotoxicology, Toxicology of
Chemicals)
Dr J.A. Moore, Institute for Evaluating Health Risks, Washington,
DC, USA (representing the American Industrial Health Council)
Dr F.J. Murray, 6611 Northridge Drive, San Jose, California, USA
(representing International Life Sciences Institute)
Mrs M. Richold, Unilever Research ESL, Sharnbrook, Bedford,
United Kingdom (representing International Life Sciences
Institute)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr L. Galvao, Pan American Health Organization, World Health
Organization, Geneva, Switzerland
Dr H. Otterstetter, Pan American Health Organization, World
Health Organization, Geneva, Switzerland
Ms C. Smallwood, US Environmental Protection Agency, National
Center for Environmental Assessment, Cincinnati, Ohio, USA
(Co-Rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
A WHO Task Group on Environmental Health Criteria for Boron met
in Washington, DC, USA, from 18 to 22 November 1996. The meeting was
organized by the WHO Regional Office for the Americas (AMRO) on behalf
of the IPCS. Dr H. Otterstetter, WHO AMRO, opened the meeting and
welcomed the participants. Dr B.H. Chen, IPCS, welcomed the
participants on behalf of the Director of IPCS and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to boron.
The first draft of this monograph was prepared by Ms C. Smallwood
of the US EPA in Cincinnati. The second draft was also prepared by Ms
Smallwood, incorporating comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs. Dr R. Goyer, Chairman of the Task Group,
contributed significantly to the final text of the EHC for Boron.
Dr B.H. Chen, member of the IPCS Central Unit, and Ms M. Sheffer,
Scientific Editor, Ottawa, Canada, were responsible for the overall
scientific content and linguistic editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
Financial support for this Task Group meeting was provided by the
US EPA.
ABBREVIATIONS
BMD Benchmark dose
CAS Chemical Abstracts Service
CL Confidence limit
CNS Central nervous system
EPA Environmental Protection Agency (USA)
FDA Food and Drug Administration (USA)
FSH Follicle stimulating hormone
GLP Good Laboratory Practices
HSDB Hazardous Substances Data Bank
ICP Inductively coupled plasma
ICP-AES Inductively coupled plasma atomic emission spectroscopy
ICP-MS Inductively coupled plasma mass spectroscopy
LH Luteinizing hormone
LOAEL Lowest-observed-adverse-effect level
(human and animal toxicity)
LOEC Lowest-observed-effect concentration
(environmental effects)
MATC Maximum acceptable toxicant concentration
(environmental effects)
MMAD Median mass aerodynamic diameter
NADPH Reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health
NOAEL No-observed-adverse-effect level
(human and animal toxicity)
NOEC No-observed-effect concentration
(environmental effects)
NOHS National Occupational Hazard Survey
RR Rate ratio (or Relative risk)
RTECS Registry of Toxic Effects of Chemical Substances
SBR Standardized birth ratio
SGOT Serum glutamic-oxaloacetic transaminase
SGPT Serum glutamic-pyruvic transaminase
TI Tolerable intake
TLV Threshold limit value
TRI Toxic Release Inventory (US EPA)
1. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, natural occurrence, and analytical methods
Boron is a naturally occurring element that is found in the form
of borates in the oceans, sedimentary rocks, coal, shale, and some
soils. It is widely distributed in nature, with concentrations of
about 10 mg/kg in the Earth's crust (range: 5 mg/kg in basalts to 100
mg/kg in shales) and about 4.5 mg/litre in the ocean.
The most important commercial borate products and minerals are
borax pentahydrate, borax, sodium perborate, boric acid, colemanite,
and ulexite. At the low concentrations and near-neutral pH found in
most biological fluids, monomeric B(OH)3 will be the predominant
species present (with some B(OH)4), regardless of whether the
boron source is boric acid or one of the borates. This is because
boric acid is a very weak acid (p Ka 9.15). Sodium perborate
hydrolyses to give hydrogen peroxide plus metaborate; consequently, it
may exhibit chemical and toxicological properties that are somewhat
different from those of the other borates.
Inductively coupled plasma (ICP) methods are preferred for the
analysis of the low levels of boron found in biological and
environmental samples; colorimetric methods must be used with caution.
1.1.2 Production, uses, environmental fate, and sources of exposure
Economic borate deposits are rare, occurring in arid regions of
Turkey, the USA, Argentina, Chile, Russia, China, and Peru. Total
world production of boron minerals -- mainly colemanite, ulexite,
tincal, and kernite -- was approximately 2 750 000 tonnes in 1994.
About 800 000 tonnes of commercial borate products, expressed as
B2O3, were manufactured from the boron minerals.
Major end uses for borate include insulation- and textile-grade
fibreglass, laundry bleach (sodium perborate), borosilicate glass,
fire retardants, agricultural fertilizers and herbicides (as a trace
element), and enamels, frits, and ceramic glazes, as well as a myriad
of miscellaneous applications.
Boron enters the environment mainly through the weathering of
rocks, boric acid volatilization from seawater, and volcanic activity.
Boron is also released from anthropogenic sources to a lesser extent.
Anthropogenic sources include agricultural, refuse, and fuel wood
burning, power generation using coal and oil, glass product
manufacture, use of borates/perborates in the home and industry,
borate mining/processing, leaching of treated wood/paper, and
sewage/sludge disposal. Many of these sources are difficult to
quantify.
Atmospheric emissions of borates and boric acid in particulate
and vapour form occur as a result of volatilization from the sea,
volcanic activity, and, to a lesser extent, mining operations, glass
and ceramics manufacturing, the application of agricultural chemicals,
and coal-fired power plants. Boron is not present in the atmosphere at
significant levels; however, the total amount present in the
atmosphere at any one time is significant owing to the huge volume of
the atmosphere. Based on their water solubility, borates would not be
expected to persist to a significant degree in the atmosphere.
Boron can be released into water and soil water through
weathering processes and, to a much smaller extent, through
anthropogenic discharges such as sewage outfalls.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water. The
extent of boron adsorption depends on the pH of the water and the
concentration of boron in solution.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported.
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
The octanol/water partition coefficient of boric acid has been
measured as 0.175, indicating a low bioaccumulation potential.
Laboratory experiments with aquatic organisms have confirmed this
potential. Plants accumulate boron; however, uptake is affected by the
pH of the soil solution, temperature, light intensity, and the
concentration of other elements (e.g. calcium and potassium). The
results of studies of boron accumulation in plants, insects, and fish
have shown that boron bioaccumulates in plants but does not biomagnify
in aquatic food-chains.
Boron occurs in soils at concentrations ranging from 10 to 300
mg/kg (average 30 mg/kg), depending on the type of soil, amount of
organic matter, and amount of rainfall. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron in surface water range widely, from 0.001 to as much as 360
mg/litre. However, mean boron concentrations for waters of Europe,
Pakistan, Russia, and Turkey are typically well below 0.6 mg/litre.
Concentrations of boron in water in Japan, South Africa, and South
America are generally below 0.3 mg/litre. Typical boron concentrations
in North American waters are below 0.1 mg/litre, with about 90% at or
below 0.4 mg/litre.
Boron accumulates in aquatic and terrestrial plants but does not
magnify through the food-chain. Concentrations of boron have been
shown to range between 26 and 382 mg/kg in submerged aquatic
freshwater plants, from 11.3 to 57 mg/kg in freshwater emergent
vegetation, and from 2.3 to 94.7 mg/kg dry weight in terrestrial
plants. Based on wet weights, boron concentrations in marine
invertebrates and fish are similar to the levels found in the exposure
media, between 0.5 and 4 mg/kg. The bioconcentration factor for two
freshwater fish species was found to be 0.3.
Boron concentrations in ambient air range from <0.5 to
approximately 80 ng/m3, with an average over the continents of
20 ng/m3.
Close similarity of boron concentrations in groundwater, fresh
surface water, and drinking-water indicates that boron is not removed
in the treatment of groundwater and fresh surface water used for
drinking-water.
Intakes of boron for humans are expected to be 0.44 µg/day from
ambient air, 0.2-0.6 mg/day from drinking-water, and 1.2 mg/day from
the diet. Average boron intake from the soil is considered to be 0.5
µg/day. A reasonable estimate of boron exposure from consumer products
is 0.1 mg/day.
1.1.3 Kinetics and biological monitoring
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in the blood
and body tissues of several mammalian species following
ingestion.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the no-observed-adverse-effect level
(NOAEL) for laboratory studies, reduce the uncertainty for risk
extrapolation between these two species.
1.1.4 Effects on experimental animals and humans
The data regarding developmental and reproductive toxicity show
that lower fetal body weight in rats is the critical effect. The NOAEL
for lower fetal body weight is 9.6 mg boron/kg body weight per day.
The lowest-observed-adverse-effect level (LOAEL), at which rats show
slight (approx. 5%) fetal body weight differences and rib anomalies,
is about 13 mg boron/kg body weight per day. As dose level increases,
the effects that are seen (and the doses at which they are seen) are:
a) further rib effects and testicular pathology in the rat (approx.
25 mg boron/kg body weight per day);
b) decreased fetal body weight and increased fetal cardiovascular
malformations in the rabbit, and severe testicular pathology in
the rat (approx. 40 mg boron/kg body weight per day);
c) testicular atrophy and sterility in the rat (approx. 55 mg
boron/kg body weight per day); and
d) reduced fetal body weight in the mouse (approx. 80 mg boron/kg
body weight per day).
Animal studies on mice and rats showed no evidence of
carcinogenicity of boric acid. Based on the lack of human data and the
limited animal data, boron is not classifiable as to its human
carcinogenicity.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data show that exposure is associated with short-term irritant effects
on the upper respiratory tract, nasopharynx, and eye. These effects,
however, appear to be short-term and reversible. The sole long-term
(7-year) follow-up study failed to identify any long-term health
effects, although a healthy worker effect cannot be entirely ruled out
given the rate of attrition (47%). Two descriptive studies assessed
fertility and secondary sex ratios in relation to exposure. Neither
study reported a detrimental effect on demonstrated fertility for its
select sample. Although an excess percentage of female births has been
suggested, the absence of statistical significance and attention to
other co-variates known to affect sex ratios warrants careful
interpretation of this finding. No studies have been identified that
assess the spectrum of reproductive outcomes, such as
time-to-pregnancy, conception delays, spontaneous abortions, and sperm
analyses in males. The role of other lifestyle or behavioural factors
in relation to health and fertility requires further study to identify
potentially sensitive populations and to evaluate reproductive effects
more fully.
1.1.5 Effects on organisms in the environment
Bacteria are relatively tolerant towards boron. Acute and chronic
effect concentrations range between 8 and 340 mg boron/litre, with
most values greater than 18 mg boron/litre. More sensitive are
protozoa. Tests with Entosiphon and Paramecium yielded 72-h
no-observed-effect concentrations (NOECs) and EC3 values between
0.3 and 18 mg boron/litre.
Boron is an essential micronutrient for cyanobacteria and
diatoms. Standard chronic tests with freshwater green algae resulted
in no-effect concentrations between 10 and 24 mg boron/litre.
Blue-green algae appear to be similar in sensitivity, with an 8-day
EC3 of 20 mg boron/litre.
Based on acute toxicity values, invertebrates are less sensitive
to boron than microorganisms. For several species, 24- to 48-h EC50
values ranged from 95 to 1376 mg boron/litre, with most values in the
100-200 mg boron/litre range. Chronic toxicity studies with
Daphnia magna gave NOECs ranging between 6 and 10 mg boron/litre.
Slightly lower NOEC values were obtained from laboratory and field
biocenosis studies. The 28-day laboratory study consisting of six
trophic stages yielded a NOEC of 2.5 mg boron/litre. Long-term outdoor
pond and field studies (not including fish) yielded NOECs up to 1.52
mg boron/litre.
Acute tests with several fish species yielded toxicity values
ranging from about 10 to nearly 300 mg boron/litre. Rainbow trout
(Oncorhynchus mykiss) and zebra fish (Brachydanio rerio) were the
most sensitive, providing values around 10 mg boron/litre.
The toxicity of boron to early life stages of fish has been
documented for several species in reconstituted water. Embryonic and
early larval stages of rainbow trout, largemouth bass (Micropterus
salmoides), channel catfish (Ictalurus punctatus), and goldfish
(Carassius auratus) were exposed to boron, as boric acid or borax,
from fertilization up to 8 days post-hatch in soft or hard water.
Neither water hardness nor the form of boron consistently affected
embryo-larval survival of fish. Rainbow trout was the most sensitive
species. The NOECs for rainbow trout ranged from 0.009 to 0.103 mg
boron/litre.
The effect of natural dilution water on boron toxicity was
determined by using surface waters collected from three locations,
with boron concentrations of 0.023, 0.091, and 0.75 mg/litre. No
adverse effects were determined up to 0.75 mg boron/litre.
Lowest-observed-effect concentrations (LOECs) ranged from 1.1 to 1.73
mg boron/litre. One test using deep (600 m) well-water, typically used
for aquatic toxicity tests, from a contract laboratory located in
Wareham, Massachusetts, USA, yielded a NOEC of >18.0 mg boron/litre.
Hence, reconstituted water exposures appeared to overestimate the
toxicity determined in natural waters, possibly as a result of
nutrient deficiency in the former.
Boron has been known since the 1920s to be an essential
micronutrient for higher plants, with interspecies differences in the
levels required for optimum growth. Boron plays a role in cell
division, metabolism, and membrane structure and function. Boron in
the form of borates occurs naturally in fruits, nuts, and vegetables.
There is a small range between deficiency and excess uptake (toxicity)
in plants. Boron deficiencies in terrestrial plants have been reported
in many countries. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions because of boron's
susceptibility to leaching. Boron excesses usually occur in soil
solutions from geologically young deposits, arid soils, soils derived
from marine sediments, and soils contaminated by pollutant sources,
such as releases from coal-fired power plants and mining operations.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field.
Mallard (Anas platyrhynchos) duckling growth was adversely
affected at dietary levels of 30 and 300 mg boron/kg, and survival was
reduced at 1000 mg/kg.
1.2 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency in terrestrial plants has been observed in many
countries throughout the world. There is a small range between
deficiency and toxicity in some plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental levels of boron
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre.
For the general population, the greatest boron exposure comes
from the oral intake of food. The mean daily intake of boron in the
diet is about 1.2 mg.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. More than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The tolerable intake (TI) of boron was set as 0.4 mg/kg body
weight per day. The allocation of the TI in various media should be
based on the exposure data of individual countries.
1.3 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
This chapter deals with the identity and physical and chemical
properties of the inorganic borates of importance in commerce, as well
as the analytical methods used to determine boron concentrations in
various media.
2.1 Identity
Elemental boron (B) is a member of Group IIIB of the periodic
table, along with aluminium, gallium, indium, and thallium. It has an
atomic number of 5 and a relative atomic mass of 10.81. Boron is never
found in the elemental form in nature. Its chemistry is complex and
resembles that of silicon (Cotton & Wilkinson, 1988). The Chemical
Abstracts Service (CAS), National Institute for Occupational Safety
and Health (NIOSH) Registry of Toxic Effects of Chemical Substances
(RTECS), and Hazardous Substances Data Bank (HSDB) numbers for boron
are 7440-42-8, ED7350000, and 4482, respectively.
The borates used most widely in commerce are listed in
approximate decreasing order of usage in Table 1, along with their
formulae and CAS numbers. Elemental boron is included, even though its
production is quite small. Throughout this document, the term "borax"
refers to disodium tetraborate decahydrate (see Table 1).
2.2 Physical and chemical properties
Elemental boron exists as a solid at room temperature, either as
black monoclinic crystals or as a yellow or brown amorphous powder
when impure. The amorphous and crystalline forms of boron have
specific gravities of 2.37 and 2.34, respectively. Boron exists as a
mixture of the 10B (19.78%) and 11B (80.22%) isotopes (Budavari et
al., 1989). Boron is a relatively inert metalloid except when in
contact with strong oxidizing agents. Boron dust exposed to air is
flammable and an explosion hazard. It also reacts violently when
ground with lead fluoride and silver fluoride (Lewis, 1992). Physical
and chemical properties of elemental boron and the most important
borates in commerce are provided in Table 2.
Sodium perborates are persalts that are hydrolytically unstable
because they contain characteristic boron-oxygen-oxygen bonds that
react with water to form hydrogen peroxide and stable sodium
metaborate (NaBO2.nH2O). This hydrolysis reaction is the basis of
the use of perborates as bleaches in detergents at high (70-100°C)
temperature. At lower washing temperatures (25-70°C), activators are
needed; these react with peroxide to give peracids, which are stronger
oxidants and give bleaching effects at lower temperatures.
Table 1. Boron compounds of commerce in approximate decreasing order
of usagea
Substance Formula CAS No.
Borax pentahydrate (disodium Na2[B4O5(OH)4].3H2O 3754418
tetraborate pentahydrate) (Na2B4O7.5H2O)
Borax (disodium tetraborate Na2[B4O5(OH)4].8H2O 1303-96-4
decahydrate) (Na2B4O7.10H2O)
Ulexite NaCa[B5O6(OH)6].5H2O 1319-33-1
(Na2O.2CaO.5B2O3.16H2O)
Colemanite Ca[B3O4(OH)3].H2O 1318-33-8
(2CaO.3B2O3.5H2O)
Sodium perborate tetrahydrate Na2[B2O4(OH)4].6H2O 10486-00-7
(NaBO3.4H2O)
Sodium perborate monohydrate Na2[B2O4(OH)4] 10332-33-9
(NaBO3.H2O)
Boric acid B(OH)3 10043-35-3
(H3BO3)
Anhydrous borax Na2B4O7 (amorphous) 1330-43-4
(disodium tetraborate)
Boron oxide B2O3 (amorphous) 1303-86-2
Boronb B 7440-42-8
a US EPA (1991); ATSDR (1992); Culver et al. (1994b).
b Produced in small quantities.
Table 2. Physical and chemical properties of elemental boron and the most important borates in commercea
Substance Relative Colour % boron Relative Water solubility Melting point Boiling point
molecular density (°C) (°C)
mass
Borax pentahydrate 291.35 White 14.85 1.81 3.6 g/100 g @ 20 °C 742 -
Borax 381.37 Colourless 11.34 1.73 5.92 g/100 g @ 25 °C 75, decomposes -
Ulexite 810.6 White 13.33 1.62 Slightly soluble Decomposes -
Colemanite 411.1 White 15.78 2.42 Slightly soluble Decomposes -
Sodium perborate
tetrahydrate 153.9 White 7.03 1.73 23 g/litre @ 20 °C Decomposes -
Sodium perborate
monohydrate 99.8 White 10.83 - 15 g/litre @ 20 °C Decomposes -
Boric acid 61.84 Colourless 17.48 1.435 @ 15 °C 63.5 g/litre @ 30 °C 169 -
Anhydrous borax 201.22 White 21.49 2.367 2.5556 g/100 g @ 25 °C 741 1575
Boron oxide 69.62 Colourless 31.06 2.46 Slightly soluble 450 1860
Boron 10.81 Black crystal or
yellow-brown
amorphous 100 2.3 Insoluble 2300 approx. 3500
a Muetterties (1967); Windholz et al. (1983); Weast et al. (1985); ACGIH (1991); ATSDR (1992); Lewis (1993); US NLM (1993);
Culver et al. (1994b).
Boric acid is a very weak acid, with a p Ka of 9.15, and
therefore boric acid and the sodium borates exist predominantly as
undissociated boric acid [B(OH)3] in dilute aqueous solution below pH
7; above pH 10, the metaborate anion B(OH)4 becomes the main
species in solution. Between pH 6 and pH 11 and at high concentration
(>0.025 mol/litre), highly water soluble polyborate ions such as
B3O3(OH)4, B4O5(OH)42, and B5O6(OH)4 are formed.
The chemical and toxicological properties of borax pentahydrate,
borax, boric acid, and other borates are expected to be similar on a
mol boron/litre equivalent basis when dissolved in water or biological
fluids at the same pH and low concentration. Boric oxide will exhibit
properties identical to those of boric acid, as it is an anhydride
that will hydrolyse to give boric acid. Sodium perborate monohydrate
and tetrahydrate hydrolyse to give hydrogen peroxide and borate. Thus,
they are oxidants and may have chemical and toxicological properties
that are different from those of the other borates.
The chemical properties of sodium metaborate differ from those of
the other sodium borates, in that the metaborate has a much higher
solubility and alkalinity in aqueous solution. Thus, the solubility in
water at 20°C is 41.9 parts sodium metaborate octahydrate (compared
with 4.7 for borax) per hundred parts saturated solution by weight.
The pH of an aqueous solution of the metaborate at 20°C ranges from
10.5 at 0.1% w/w to 12.0 at 18% w/w (compared with pH 9.24 for borax
over a wide range of concentrations).
2.3 Conversion factors
2.3.1 Conversion factors of ppm and mg/m3 for boron
1 ppm = 0.4421 mg/m3
1 mg/m3 = 2.262 ppm
2.3.2 Conversion factors for boron compounds to boron
dose of boric acid × 0.175 = equivalent dose of boron
dose of borax × 0.113 = equivalent dose of boron
dose of anhydrous borax × 0.215 = equivalent dose of boron
dose of sodium perborate tetrahydrate × 0.070 = equivalent dose
of boron
dose of sodium perborate monohydrate × 0.108 = equivalent dose of
boron
dose of metaboric acid × 0.247 = equivalent dose of boron
2.4 Analytical methods
Analyses of environmental and biological samples for boron
content utilize a variety of preparative methods (see Table 3).
Table 3. Preparative methods for analysing boron content in
environmental and biological samples
Media Extraction method Reference
Biological Acid digestion with:
Microwave Pennington et al. (1991)
Dry ashing Wilkner (1986)
Wet ashing Kowalenko (1979)
Banuelos et al. (1992)
Low temperature, wet ashing Hunt & Shuler (1989)
Freeze drying Iyengar et al. (1990)
Smith et al. (1991)
Soil Hot water solubility Odom (1980)
Cumakov (1991)
Water Liquid-liquid extraction from
acidified solutions into
chloroform Aznarez et al. (1985)
Ion exchange column Sekerka & Lechner (1990)
The preferred method for analysis of boron in bone, plasma, and
food is inductively coupled plasma atomic emission spectroscopy
(ICP-AES) (Hunt, 1989). It is also used for tumour, blood, liver,
skin, and cell suspensions (Barth et al., 1991). It also has been used
for wastewater (Huber, 1982) and fish tissues (Hamilton & Wiedmeyer,
1990). Detection limits range from 0.005 to 0.05 mg boron/litre in the
solution analysed.
Inductively coupled plasma mass spectroscopy (ICP-MS) is used to
measure boron concentrations in plant, rat, and human samples. Isotope
ratios (10B/11B) can be measured accurately (Vanderpool et al.,
1994). Using direct nebulization, ICP-MS can give a detection limit of
1 ng/g in human blood, human serum, orchard leaves, and total diet
(Smith et al., 1991).
ICP-MS is the most widely used non-spectrophotometric method for
analysis of boron, as it uses small volumes of sample, is fast, and
applies to a wide range of materials (fresh and saline water, sewage
wastewater, soils, and plant samples, as well as the biological
materials mentioned above). Interferences are minimal or can be
removed (Gregoire, 1990). ICP-MS can detect boron down to 0.15
µg/litre.
The ability to measure the boron isotope ratio accurately allows
studies starting with pure 10B compounds and following the isotopic
dilution in biological systems. This is particularly useful, as no
stable radioactive boron isotopes usable as tracers exist. A number of
boron compounds made with nearly isotopically pure 10B are available
for such studies.
When expensive ICP equipment is not available,
colorimetric/spectrophotometric methods can be utilized. However, many
of these methods are subject to interference and should be used with
caution; they should also preferably be calibrated against an ICP
method.
Azomethine-H has been used to analyse boron in environmental
water samples and is very sensitive, with a detection limit of 0.02
mg/litre (Lopez et al., 1993). The well-known curcumin method is
subject to interference by nitrate, chloride, and fluoride but is
claimed to be applicable to samples with 0.1-1 mg boron/litre (Black
et al., 1993).
A simple, sensitive spectrophotometric method for determination
of boron in soils, plant materials, and water uses Alizarin Red S but
is also subject to interference (Garcia-Campana et al., 1992). Flow
injection analysis utilizing the sorbitol/borate complex and Methyl
Orange indicator for eye lotion samples has a detection limit of
0.02 mg/litre (Nose & Zenki, 1991).
Another method of analysis of boron uses neutron activation and
mass spectrometric analysis. Mass spectrometric assay of 3He from
decay of tritium produced by thermal neutron irradiation of boron to
give 4He has been described by Clarke et al. (1987a). The method,
useful for trace levels of boron in blood and other biological
samples, can detect 108 g boron/g of sample (Clarke et al., 1987b).
Iyengar et al. (1990) used this method to determine boron in citrus
leaves, human erythrocytes, and food items, all with freeze-dried
samples.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Boron, in the form of various inorganic borates, is widely
distributed in low concentrations throughout nature. It constitutes
about 10 mg/kg of the Earth's crust, ranging from 5 mg/kg in basalts
to 100 mg/kg in shales (Woods, 1994). The majority of the boron
resides in the ocean, at an average concentration of about 4.5
mg/litre (Weast et al., 1985). Economic deposits of borate minerals
are rare and are usually found in arid desert regions with a
geological history of volcanic and/or hydrothermal activity (Mellor,
1980). Major world deposits are found in Turkey, the USA, Argentina,
Russia, Chile, China, and Peru (Culver et al., 1994b).
The most abundant boron mineral is tourmaline, an aluminium
borosilicate that contains about 3.1% boron (Muetterties, 1967). It is
not a practicable source of usable boron, as it is widely distributed
as a minor component of rocks. Economic borate minerals include
tincal, kernite, colemanite, and ulexite.
Natural sources of borate released to air are the oceans
(largest), volcanoes, and geothermal steam (Graedel, 1978). Natural
weathering of clay-rich sedimentary rocks on land surfaces accounts
for a large proportion of the boron mobilized into soils and the
aquatic environment, amounting to some 360 000 tonnes of boron
annually (Bertine & Goldberg, 1971). Although few data are available
for quantifying boron released from industrial sources, natural
weathering and seawater evaporation are considered greater sources
than industrial emissions (see chapter 4).
3.2 Mining and production
The total world production of boron minerals in 1994 was
approximately 2 750 000 tonnes (Lyday, 1996). The main commercial
borate minerals are colemanite, kernite, ulexite, and tincal.
Approximately 800 000 tonnes of commercial borate products, expressed
as B2O3, were manufactured from boron minerals in 1994. The two
largest producers are the USA and Turkey. Further mining and
production facilities exist in Argentina, Bolivia, China, Chile, Peru,
and Russia (Lyday, 1996). Most US production of borates occurs in
California, where colemanite, ulexite, tincal, kernite, and brines are
processed. These minerals are also processed elsewhere in the world,
as are ascharite, hydroboracite, datolite, etc.
Disodium tetraborate (borax) containing 5 or 10 molecules of
water is produced mainly from sodium-containing borate ores. The mined
ore is crushed and ground before dissolution in a hot recycled aqueous
solution containing some borax. Insoluble gangue (clay particles)
present in the hot slurry is separated off to produce a clear
concentrated borax solution. Evaporative cooling of this solution to
selected temperatures results in crystallization of the desired
products, which are then separated from the residual liquor and dried
(personal communication from Borax US to the IPCS, 1995).
Boric acid is produced mainly from sodium- or calcium-containing
borate ores. The mined ore is crushed and ground before being reacted
with sulfuric acid in the presence of a hot aqueous recycled liquor
containing some boric acid. The resultant slurry contains insoluble
gangue and either calcium or sodium sulfate by-product. After
separation of unwanted insoluble gangue, recovery of the boric acid
product is similar to that for borax (personal communication from
Borax US to the IPCS, 1995).
3.3 Uses and release
The end uses of boron minerals and of borate products are
diverse. Estimated amounts of borate consumed in the USA for the major
end uses in 1992 are listed in Table 4 (Lyday, 1993). Partial data for
Europe are also included (ECETOC, 1997). It should be noted that
vitreous products such as fibreglass, borosilicate glass, and enamels,
frits, and glazes are not significant sources of potential human
exposure, because the boron is tied up tightly in the glassy
structure. All of the boron from the sodium perborate contained in
detergents ultimately enters the wastewater stream.
Table 4. Estimated amount consumed (as B2O3) for boric acid, borates,
and boron minerals in the USA in 1992a and in Europe in 1993b
Use Consumption (tonnes)
USA Europe
Insulation-grade fibreglass 129 000 44 600c
Textile-grade fibreglass 78 500 27 100c
Soaps and detergents 38 600 142 500
Borosilicate glass 34 400 12 200
Fire retardants 13 400 -d
Agriculture 11 100 -d
Enamels, frits, and ceramic glazes 9 300 3 500
Metallurgy 3 700 -d
Nuclear applications 900 -d
a Lyday (1993).
b ECETOC (1997).
c Does not include minerals.
d No data.
The average market shares for the USA, Europe, and Japan in 1992
were about 23% (fibreglass), 17% (detergents), 11% (enamels/glazes),
and 11% (glass) for major end uses (personal communication from Borax
US to the IPCS, 1995).
Other minor uses include cosmetics and pharmaceuticals (as a pH
buffer), boron neutron capture therapy (for cancer treatment), and
pesticides (personal communication from Borax US to the IPCS, 1995).
The cancer treatment application utilizes a boron compound made with
all 10B isotope, which preferentially accumulates in tumour versus
normal tissue (Barth & Soloway, 1994). Subsequent irradiation of the
patient with thermal neutrons produces 7Li plus alpha particles. The
latter have a destructive path length of about the diameter of a cell,
thereby selectively destroying the cancer. Research in this field is
being pursued in Japan and, to a lesser extent, in the USA.
Boron enters the environment mainly through the weathering of
rocks, volatilization from seawater, agricultural, refuse, and fuel
wood burning, power generators (coal and oil combustion), the
manufacture of glass products and other boron-containing compounds,
the industrial and household use of boron-containing products
(including soaps and detergents), borax mining and processing,
leaching from treated wood and paper, geothermal releases, chemical
plants, and sewage and sludge disposal (Versar, Inc., 1975; Larsen,
1988; ATSDR, 1992; Anderson et al., 1994a). Boron is not present in
the atmosphere at significant levels because of its low volatility,
but the total amount in the air is very significant owing to the huge
volume of the atmosphere (see chapter 4).
Boron releases to water occur from municipal sewage containing
perborates from detergents and also in runoff from areas using
boron-containing herbicides or fertilizers (Waggott, 1969; Nolte,
1988; Butterwick et al., 1989). Boron levels in sewage sludge from 23
cities in the USA ranged from 7.1 to 53.3 mg/kg (Mumma et al., 1984).
It has been estimated that 11 800 tonnes of boron are released yearly
in coal fly ash from coal combustion (Bertine & Goldberg, 1971).
Versar, Inc. (1975) estimated US boron air emissions as 10 500 tonnes
annually from mining, processing, and coal burning. Few quantitative
data on boron releases are available, because boron is not included in
the US Environmental Protection Agency (EPA) Toxic Release Inventory
(TRI) (ATSDR, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Boron is not present in the atmosphere at significant levels
(Sprague, 1972), but the total amount in the air is very significant
owing to the huge volume of the atmosphere. Borates exhibit low
volatility; consequently, boron would not be expected to be present to
a significant degree as a vapour in the atmosphere. Atmospheric
emissions of borates and boric acid in particulate (<1-45 µm in size)
or vapour form occur as a result of volatilization of boric acid from
the sea, volcanic activity, mining operations, glass and ceramics
manufacturing, the application of agricultural chemicals, and
coal-fired power plants. As a particulate, boron would be removed from
the atmosphere either by dry deposition or by wet deposition because
of its relatively high water solubility (Versar, Inc., 1975; Gladney
et al., 1978). Based on analogy with data on general particulate
residence times (Nriagu, 1979), the half-life of airborne boron
particles is expected to be on the order of days, depending on the
size of the particles and atmospheric conditions.
Seawater evaporation is the biggest contribution to boron in air.
The global removal of boron from marine sources has been estimated at
between 800 000 and 4 000 000 tonnes/year and compares with an
estimate of 2 000 000-7 200 000 tonnes/year for the total global
release (Anderson et al., 1994a). Anderson et al. (1994a) estimate
that the total anthropogenic release of boron to the atmosphere is
between 180 000 and 650 000 tonnes/year (9-27% of the total global
release). In spite of all these releases, the atmospheric
concentration of boron is low (mean boron concentrations range from
<0.5 to approximately 80 ng/m3).
4.1.2 Water and sediment
Waterborne boron may be adsorbed by soils and sediments.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water (Rai et
al., 1986). The extent of boron adsorption depends on the pH of the
water and the concentration of boron in solution. The greatest
adsorption is generally observed at pH 7.5-9.0 (Waggott, 1969; Keren &
Mezuman, 1981; Keren et al., 1981).
Simsiman et al. (1987) conducted a field investigation to
determine the leachability and groundwater transport of major and
minor elements, including boron, from ash disposal ponds at the
coal-fired Columbia Power Plant in Portage, Wisconsin, USA. The site
is underlain by sands interspersed with lenses of silt and clay
overlying sandstone (10-20 m below the surface). The soil pH ranged
from 7.1 to 8.8, and the organic matter content was 0.2-0.8%. Boron
plumes were identified in the groundwater at least 120 m down-gradient
of the ponds. The boron plume from the secondary fly ash pond extended
into the sandstone (26-30 m), which suggested rapid downward
infiltration of the leachate. However, attenuation of the boron
occurred at some point between the pond and the aquifer, based on
observed decreases of approximately 40% in the boron concentration.
Barber et al. (1988) monitored the extent of groundwater
contamination emanating from sewage disposal beds near Falmouth,
Massachusetts, USA, by mapping the distribution of boron. Under the pH
conditions of the aquifer (pH 5-7), dissolved boron occurred as the
neutral undissociated orthoboric acid species, which should be
transported with little sorption. The boron plume was 3500 m long,
1100 m wide, and 30 m deep during sampling in 1985.
Deverel & Millard (1988) demonstrated that boron is present in
the oxidized, alkaline, shallow groundwater of the western San Joaquin
Valley, California, USA. Boron was found to be geochemically mobile,
with concentrations significantly correlated (alpha = 0.05) with
groundwater salinity in the alluvial-fan and basin-trough geological
zones.
Corwin (1986) speculated that the adsorption of boron on
sediments provides a means by which boron may persist for long periods
of time in aquatic systems. The desorption (or leaching) of boron from
the sediments would provide a long-term source until a system
equilibrium could be reached, based on differences in the
concentrations of boron in the water column and in the sediment both
at the sediment-water interface and with increasing depth below the
interface. The primary desorption mechanism would be diffusion.
Boron levels (as admixed borate salt) as high as 1900 mg/kg have
been reported in coal fly ash. Cox et al. (1978) reported that
approximately 50% of the boron in 0.5-g samples of fly ash was leached
from the ash into water within 2 h; the leaching rate increased with
increased acidity. In a boron dissolution study, Hollis et al. (1988)
observed that 60% of the boron was removed from 6 g of ash after three
extractions at pH 9, whereas 100% was removed at pH 6 after two
extractions. In a long-term (2-year) leachability study, Dudas (1981)
observed that boron was readily leached, probably as a result of the
moderate solubility of borate salts. Consequently, the disposal of
coal fly ash in lagoons could provide a source of boron contamination
in aquatic systems.
4.1.3 Soil
Boron is adsorbed onto the surfaces of soil particles, with the
degree of adsorption depending on the type of soil, pH, salinity,
organic matter content, iron and aluminium oxide content, iron- and
aluminium-hydroxy content, and clay content (Sprague, 1972). Boron
adsorption can vary from being fully reversible to irreversible (Rai
et al., 1986; Shani et al., 1992). The lack of reversibility may be
the result of solid-phase formation on mineral surfaces (Rai et al.,
1986) and/or the slow release of boron by diffusion from the interior
of clay minerals (Griffin & Burau, 1974).
At acidic pH, boron exists in solutions in the form of
undissociated boric acid; at alkaline pH, it is present as a borate
ion, which reaches maximum adsorption at pH 8.5-9 (Sprague, 1972).
Sims & Bingham (1967) reported that hydroxy iron and aluminium
compounds, present as interlayer-contained materials, coatings on
individual particles, or impurities, resulted in increased boron
retention in layer silicates. Rhoades et al. (1970) observed that in
the silt and sand fractions of arid-zone soils, the sites of boron
adsorption are the magnesium-hydroxy clusters and coatings found on
the weathering surfaces of ferromagnesian minerals and micaceous layer
silicate minerals. Marzadoori et al. (1991) reported that the amount
of boron adsorbed by soil was considerably greater after the organic
matter had been removed from the soil. An increase in
oxalate-extractable iron and aluminium in the soil was observed after
destruction of the organic matter. It was suggested that a portion of
the iron and aluminium oxides as well as other possible adsorption
sites are generally coated or occluded by organic matter and become
active only after its removal.
Couch & Grim (1968) studied the uptake of boron in illite clays
and determined that uptake was enhanced at higher boron soil solution
concentrations in direct relationship to the salinity and temperature
of the solution. Following 30 days of treatment in soils containing
1 mol boric acid/litre at salinities of 0.1, 1.0, or 3.0 mol
CaCl2/litre, boron levels increased by 56, 70, and 98 mg/kg,
respectively. Treatment of illites at 1 mol boric acid/litre for 30
days at 60°C yielded 55 mg boron/kg, whereas the same concentration at
215°C for 12 h yielded 180 mg boron/kg. The investigators also
observed a direct relationship between the specific surface area of
the clay types and boron uptake. Boron uptake in the illite clays was
characterized as initially rapid adsorption, followed by diffusion of
boron into the clay structure, requiring several months to reach
equilibrium.
Several investigators have used either the Langmuir or the
Freundlich adsorption equation to describe the relationship between
adsorption and desorption of boron in soils. The Langmuir equation is
based on the total adsorptive capacity of the soil, the concentrations
of adsorbed boron and boron in solution, and an adsorption equilibrium
constant (K), which represents the bonding energy of the soil. Using
this equation, Hatcher & Bower (1958) determined that an equilibrium
exists between boron in solid and liquid phases. At soil pH values of
6.6-7.7, the predominant boron species in the aqueous phase is
undissociated boric acid, and the principal mechanism of retention is
by reversible, molecular adsorption, which is non-uniform based on the
energy characteristics of the bonding sites. These investigators also
showed that boron desorption was reversible; in other words, boron
that leached into the soil solution could again be adsorbed. However,
based on the Freundlich adsorption isotherms, Elrashidi & O'Connor
(1982) observed incomplete adsorption reversibility in some soils from
New Mexico, USA, at higher boron concentrations.
Biggar & Fireman (1960) determined that the fixation of boron in
soils occurs by one of three mechanisms: physical (molecular)
adsorption, in which the boron is held to the surface of the soil by
van der Waals bonds; anion exchange; or chemical precipitation.
Chemical adsorption involves ionic and covalent bonding. The
investigators speculated that the initial adsorption is probably
molecular in nature, followed by the formation of surface compounds
that result in an increase in adsorption sites, particularly at higher
boron concentrations in the soil solution. At higher concentrations,
chemical bonding of borate ions with hydroxyl ions on the soil surface
results in boron fixation to soluble aluminium, silicon, and iron.
This same mechanism (chemisorption) was observed by Couch & Grim
(1968) for the uptake of borate ions to clay mineral surfaces. The
presence of calcium ions, drying, and high pH values will tend to
increase the amount of fixed boron. Wetting and drying of the soil
will increase the maximum adsorption capacity and bonding energy of
the soil for boron.
Many of the surface boron compounds initially formed by
adsorption mechanisms may be unstable and leached by water. However,
as a result of the equilibrium that exists between adsorbed and
dissolved boron in soils, the adsorbed boron may act as a buffer,
impeding the leaching of excess boron from soils. Wierenga et al.
(1975) conducted a study to determine the downward movement of boron
through a sandstone formation in New Mexico, USA. The experimental
dispersion coefficient was calculated as 1.06 cm2/day, primarily
resulting from diffusion. Assuming an average annual rainfall of 20
cm/year and an average annual recharge of 10% of the annual
precipitation, the investigators determined that it would take 500
years for the boron front to reach a depth of 35 m into the sandstone.
As the groundwater table at this site is at 86 m, Wierenga et al.
(1975) calculated that it would take 1628 years for boron, at a
concentration one-half that of the surface concentration, to reach the
groundwater. A 10-fold increase in annual recharge from precipitation
would reduce the transit time by one-tenth.
Bingham et al. (1971) concluded that the single most important
property of soil that will influence the mobility of boron is the
abundance of amorphous aluminium oxide. Gerritse et al. (1982) showed
that the mobility of boron in sludge-amended sandy and sandy loam
soils was increased as a result of complexation with dissolved organic
compounds, high ionic strengths of the soil solutions, and other
factors.
4.1.4 Vegetation and wildlife
Hingston (1986) investigated the components of the biogeochemical
cycle for boron in two eucalypt forests. The importance of the
biological component of the cycle was indicated by the amount of boron
stored within trees (2.1 and 2.5 kg/ha for the two forests) compared
with the amount of extractable boron in the soils to a depth of 1 m (2
and 7 kg/ha), and by the highly significant correlations between
hot-water-soluble boron and organic carbon for these soils.
4.2 Transformation
4.2.1 Biotransformation
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported in the
literature (personal communication from Borax US to the IPCS, 1995).
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
4.2.2 Abiotic transformation
Inorganic borates such as boric acid, boric oxide, and sodium
borates are stable, except for dehydration at high temperatures.
Organoboron compounds are sufficiently uncommon in nature to be
irrelevant to this document. In aqueous media, the chemical speciation
of boron-oxygen compounds is pH and concentration dependent.
4.2.2.1 Air
No information was available in the current literature concerning
the photolysis, oxidation, or hydrolysis of boron-oxygen compounds in
the atmosphere. The small amount of boron in air is assumed to be in
the form of boric acid.
4.2.2.2 Water
In natural waters, boron exists primarily as undissociated boric
acid with some borate ions. As a group, the boron-oxygen compounds are
sufficiently soluble in water to achieve the levels that have been
observed (Sprague, 1972; see chapter 5).
In seawater, inorganic boron content generally bears a linear
relationship to the amount of chloride ion present; a ratio of
0.000 24 g boron/g of total halogen expressed as chloride ion has been
calculated (Mellor, 1980). Byrne & Kester (1974) demonstrated that
weakly dissociated boric acid is the predominant species but also that
there are weakly associated ion pair neutral and positively charged
borate complexes of sodium, magnesium, and calcium. The metaborate ion
will undergo rapid hydrolysis in seawater to form the borate ion and
the weakly dissociated boric acid. Noakes & Hood (1961) concluded that
organically bound boron contributes very little, if any, to the total
boron content of seawater. Boron associated with organic matter was
found to vary with oxygen content, with the lowest concentrations
occurring in the minimum oxygen zone. Mance et al. (1988) described
boron as a significant constituent of seawater, with an average
concentration of 4.5 mg/litre.
Boric acid is a very weak acid, with a p Ka of 9.15; in fresh
water, therefore, boric acid and sodium borates exist predominantly as
undissociated boric acid below pH 7, but the metaborate anion becomes
the main species in solution above pH 10. Between these two pH bands,
there is also a characteristic presence of complex polyborate anions
in solution when the concentration is increased, leading to enhanced
solubility.
4.2.2.3 Soil
Borates as such cannot degrade, but borate complexes with organic
matter or sod mineral surfaces can be altered by water leaching or pH
change.
4.2.3 Bioaccumulation
4.2.3.1 Aquatic organisms
Highly water soluble materials are unlikely to bioaccumulate to
any significant degree, and borate species are all present essentially
as undissociated boric acid at neutral pH. The octanol/water partition
coefficient for boric acid has been measured as 0.175 (Barres, 1967),
indicating low bioaccumulation potential.
Thompson et al. (1976) studied boron uptake in two saltwater
species, juvenile Pacific oysters (Crassostrea gigas) and
underyearling sockeye salmon (Oncorhynchus nerka), in
continuous-flow systems with 95% solution replacement every 6 h.
Oysters (30/tank) were exposed to two boron levels (1 mg/litre above
background and 10 mg/litre above background) for 47 days, and salmon
(3/tank) were exposed only to the higher concentration for 21 days.
Control tanks received only seawater inflow. The background
concentration of boron in seawater in this study was approximately
3.98 mg/litre. The oysters were sampled on days 0, 8, 16, 36, and 47
of exposure. After this time, the remaining oysters were maintained in
seawater alone for another 24 days and then analysed for boron uptake.
Following the 21-day exposure period, the sockeye salmon were killed
and the boron concentration was determined in gill, liver, kidney,
muscle, and bone tissue. For both species, the tissue levels
approximated the boron concentrations in the test water, indicating
that these species take up boron in relation to its availability.
In the oyster, tissue concentrations returned to background
levels (3.67-4.13 µg/g) by the 71st day of the study, indicating a
fairly rapid clearance of boron with no evidence of long-term
retention. Boron concentrations in sockeye salmon tissues in normal
seawater ranged from 0.5 to 1.5 µg/g wet weight, with concentrations
increasing from muscle to gill and kidney, to liver, and to bone.
Boron levels were elevated in the bone and kidney tissue (5.9-17 µg/g
wet weight and 4.5-11.9 µg/g wet weight, respectively) of the exposed
salmon; however, they were not significantly different from test water
levels. Consequently, there was no evidence for active bioaccumulation
of boron in these species (Thompson et al., 1976).
Suloway et al. (1983) studied the bioaccumulation potential of
the components of coal fly ash extract in fathead minnows
(Pimephales promelas) and green sunfish (Lepomis cyanellus). Five
fish of each species were exposed for 30 days to fly ash extracts
containing boron at concentrations ranging from 1.23 to 91.7 mg/litre.
Whole-body concentrations of boron ranged from 1.16 to 4.15 µg/g in
the exposed fathead minnows and from 1.08 to 4.62 µg/g in the exposed
green sunfish. The reported bioconcentration factor was 0.3 for both
species. These results are consistent with those described above and
indicate that boron does not bioaccumulate significantly in fish.
4.2.3.2 Terrestrial plants
Eaton (1944) investigated growth reaction and boron accumulation
characteristics of plants grown in outdoor sand culture beds where
cultures were supplied with nutrient solutions containing differing
concentrations of boron. The concentrations of boron ranged from 58 to
1804 µg/g dry weight in the leaves of plants grown in 5 mg boron/litre
and from 209 to 3875 µg/g dry weight in the leaves of plants grown in
25 mg boron/litre. The boron concentrations were generally lower in
roots, stems, and fruits than in the leaves. This is consistent with
the fact that boron is absorbed from the soil solution by the roots
and passively carried in the transpiration stream to the leaves, where
the water evaporates and the boron accumulates. The absorption into
the roots usually occurs as active transport against a concentration
gradient (the concentration in the soil solution is generally lower
than in the root tissues); therefore, an expenditure of energy is
required. However, at higher boron soil solution concentrations, which
are toxic to some plant species, the mechanism of uptake is passive
diffusion (Bingham et al., 1970). Boron is relatively immobile in the
phloem; consequently, the accumulated boron does not move out of the
leaf tissues and into the fruit and other tissues (Kohl & Oertli,
1961).
Several factors affect the uptake of boron, including the pH of
the soil solution, temperature, light intensity, and the concentration
of other elements (e.g. calcium and potassium). Uptake is reduced by a
factor of four as soil pH increases from 4 to 9 (Bingham et al., 1970)
and increased by an increase in light intensity (Tanaka, 1966); the
rate of boron absorption rapidly increases at temperatures ranging
from 10 to 30°C and is sharply reduced above 35°C (Reisenauer & Cox,
1971).
4.2.3.3 Birds
Pendleton et al. (1995) exposed adult male mallard ducks to a
dietary concentration of 1600 mg boron/kg for up to 48 days.
Equilibrium levels were reached between days 2 and 15. Boron
concentrations were highest in the blood, followed by the brain and
liver. Boron was rapidly eliminated, with few detectable residues
after 1 day on a "clean diet." The presence of arsenic (300 mg/kg) in
the diet slowed the accumulation of boron.
4.3 Ultimate fate following use
No information was available in the current literature concerning
the disposal of boron or boron compounds. Information was located,
however, regarding the reclamation and revegetation of coal combustion
products (i.e. ash) that contain high concentrations of metals,
including boron. Although the chemical and physical properties of coal
ash tend to be detrimental to plant growth and establishment,
additions of fertilizer and manure provide a more suitable medium
(Schwab et al., 1991). Plant establishment on the site is only the
first phase of the reclamation process. It is also necessary to ensure
that leachate from the ash does not contaminate the surface water and
groundwater in the immediate region and that uptake of metals in the
plant materials does not result in metal concentrations that are toxic
to livestock or wildlife. In a study of the revegetation of several
ash disposal sites in Kansas, USA, Schwab et al. (1991) noted
variations in plant uptake of boron from coal ash owing to differences
in ash type, plant species, and ash treatment. Boron contained in
detergents after use releases to the municipal sewage system. It
should be noted that boron is not removed by the usual water treatment
processes. Landfill will tend to be the ultimate fate of many boron
products.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Boron, as boric acid, is released into the atmosphere during
volcanic eruptions; however, most is captured by the oceans
(Muetterties, 1967). Coal-fired power plants and agricultural burning
are major sources of atmospheric boron contamination; at least 30% of
boron in coal is lost in this manner (Eisler, 1990). Nevertheless,
boron does not appear to be present in ambient air at significant
levels (Sprague, 1972), presumably because of rapid transport to other
media (see section 4.1.1). Although the concentration is low, the
atmosphere carries a significant amount of boron as boric acid vapour.
Mean boron concentrations in emissions from active volcanic sites
range from <2.5 to 31.4 µg/m3 for gaseous boron and are below
4 µg/m3 for particulate boron. Volcanic lake fumes (El Chichon,
Mexico) contained mean boron concentrations of up to 8.5 µg/m3 for
particulate boron and up to 16.1 µg/m3 for gaseous boron (Anderson et
al., 1994a).
Anderson et al. (1994a) monitored atmospheric concentrations of
boron at continental, coastal, and remote marine sites. Mean
particulate boron concentrations ranged from 1.8 to 12.2 ng/m3, from
2.4 to 3.7 ng/m3, and from <0.5 to 2.8 ng/m3 for the three types of
site, respectively. Mean gaseous boron concentrations ranged from
<0.5 to 20.7 ng/m3, from 3.5 to 82.8 ng/m3, and from 0.6 to
25 ng/m3, respectively. Anderson et al. (1994a) assumed 90% of boron
in the air is gaseous and 10% is in particulate form.
5.1.2 Water
5.1.2.1 Groundwater
Naturally occurring boron is present in groundwater primarily as
a result of leaching from rocks and soils containing borates and
borosilicates (i.e. local geology). Concentrations of boron in
groundwater throughout the world range widely, from <0.3 to
>100 mg/litre. Boron levels in European groundwaters are presented in
Table 5. In general, concentrations of boron were greatest in southern
Europe (Italy, Spain, but not Greece) and least in northern Europe
(Denmark, France, Germany, Netherlands, and the United Kingdom). For
Italy and Spain, mean boron concentrations ranged from 0.5 to
1.5 mg/litre. Concentrations ranged up to approximately 0.6 mg
boron/litre in the Netherlands and United Kingdom, and levels in
approximately 90% of samples in Denmark, France, and Germany were
found to be below 0.3, 0.3, and 0.1 mg boron/litre, respectively.
Table 5. Concentrations of boron in European groundwatera
Country Area No. of Boron concentration
samples (mg/litre)
Denmark 525 92.2% < 0.3
7.4% > 0.3
0.4% > 1.0
France 716 99.5 < 0.3
0.5 > 0.3
Germany Baden-Wurttemberg 2574 89% < 0.1
10.7% > 0.1
0.3% > 1.0
Lower Saxony 188 96% < 1.0
4% > 1.0
Greece Patras 10 100% < 0.1
Halkidiki 3 2.3-5.4
Italy North of Rome 423 Mean = 1.0
Sicily 18 Mean = 1.5
Paglia 102 Mean = 0.75
Netherlands Inland 0.08-0.6
Spain Valencia 21 Mean = 0.64
Almeria 17 Mean = 0.98
Murcia 15 Mean = 0.51
United Kingdom London 21 0.02-0.54
Northumbria 164 Mean = 0.31
Dumfries and Galloway Mean = 0.04
Permo-Triassic
(Scotland)
a ECETOC (1997).
Groundwater contaminated with excessive concentrations of boron
from surface water recharge has been noted beneath the Kesterson
Reservoir, California, USA. This reservoir serves as an evaporative
sink for several metalloids, including boron, and receives
agricultural drainage from farmlands within the San Joaquin River
Valley. Benson et al. (1991) reported an average boron concentration
of 15 mg/litre. Concentrations of boron elsewhere within the San
Joaquin River Valley have been shown to range from 0.14 to 120
mg/litre, with a median of 4 mg/litre (Deverel & Millard, 1988;
Butterwick et al., 1989).
5.1.2.2 Surface water
The majority of the Earth's boron occurs in the oceans, with an
average concentration of 4.5 mg/litre (Weast et al., 1985). The amount
of boron in fresh water depends on such factors as the geochemical
nature of the drainage area, proximity to marine coastal regions, and
inputs from industrial and municipal effluents (Butterwick et al.,
1989). Concentrations of boron in fresh surface water are summarized
in Table 6.
Concentrations ranged from 0.001 to 2 mg boron/litre in Europe,
with mean values typically below 0.6 mg/litre. Similar concentrations
have been reported for water bodies within Pakistan, Russia, and
Turkey; concentrations range from <0.01 to 7 mg boron/litre, with
most values below 0.5 mg/litre. Concentrations ranged up to 0.01 mg
boron/litre in Japan and up to 0.3 mg boron/litre in South African
surface waters. Samples taken in surface waters from two South
American rivers (Rio Arenales, Argentina, and Loa River, Chile)
contained boron at concentrations ranging between 4 and 26 mg/litre in
areas rich in boron-containing soils. In other areas, the Rio Arenales
contained less than 0.3 mg boron/litre. Concentrations of boron in
surface waters of North America (Canada, USA) ranged from
0.02 mg/litre to as much as 360 mg/litre, indicative of boron-rich
deposits. However, typical boron concentrations were less than
0.1 mg/litre, with a 90th-percentile boron concentration of
approximately 0.4 mg/litre.
5.1.2.3 Rainfall
The median and mean concentrations of borate in rain and snow at
six sites in western Switzerland were found to be 0.0031 and 0.0056 mg
boron/litre, respectively (Atteia et al., 1993).
5.1.3 Sewage
Concentrations of boron in sewage waters are summarized in Table
7.
The majority of the boron present in sewage occurs primarily as
undissociated boric acid; reported levels of boron in sewage in the
USA range from 0.4 to 1.5 mg/litre and up to 4.05 mg/litre because of
industrial waste discharges (Banerji, 1969). In Europe, sewage from
domestic and industrial sources typically has an average boron
concentration of 2 mg/litre, with levels up to 5 mg/litre (Butterwick
et al., 1989). Calculations by the German Government Environment
Agency attribute 50% of the boron in wastewater to the use of
detergent products (Butterwick et al., 1989). In boron mine drainage
waters in Turkey, the boron concentrations were reported to be 16-390
mg/litre (Okay et al., 1985). Boron levels in sewage sludge in 23 US
cities ranged from 7.1 to 53.3 mg/kg dry weight (Mumma et al., 1984).
Table 6. Concentrations of boron in fresh surface water
Area Boron concentration Reference
(mg/litre)
USA Median = 0.076 ECETOC (1997)
90th percentile = 0.387
Drainage basins, USA 0.019-0.289a Kopp & Kroner (1970)
Coastal drainage waters, 15 (boron-rich deposits) Deverel & Millard (1988)
California, USA
Lakes, California, USA 157-360 (boron-rich Deverel & Millard (1988)
deposits)
Ontario, Canada 0.029-0.086 Sekerka & Lechner (1990)
Cold River drainage 0.0627 Tsui & McCart (1981)
area, western Canada
United Kingdom 0.046-0.822 Mance et al. (1988)
Italy 0.4-1.0 (range of Manfredi et al. (1975)
means)
<0.1-0.5 Tartari & Camusso (1988)
Sweden 0.013 (0.001-1.046) Ahl & Jönsson (1972)
Germany 0.02-2.0 Graffmann et al. (1974)
The Netherlands Range of medians = Unilever (1994)
0.09-0.145
Rivers, Austria <0.02-0.6 (background Schöller & Bolzer (1989)
level)
River Neva, Russia 0.01-0.02 Huber (1994)
Degh Nala, Pakistan <0.01-0.46 (near Tehseen et al. (1994)
effluent discharges)
Simav River, Turkey <0.5 (uncontaminated) Okay et al. (1985)
4 (maximum 7)
(contaminated with
boron mine waste)
Table 6. (continued)
Area Boron concentration Reference
(mg/litre)
Rio Arenales, Argentina <0.3 Bundschuh (1992)
6.9 (near borate plant)
Loa River Basin, Chile 3.99-26 (soil rich in Cáceres et al. (1992)
minerals and natural
salts; low rainfall)
Japan (River Asahi) 0.009-0.0117 Korenaga et al. (1980)
South Africa 0.02-0.33 Reid & Davies (1989)
a Lowest concentration in the western Great Lakes Basin to highest concentration
in the western Gulf Basin.
Table 7. Concentrations of boron in sewage waters
Area/source Boron concentration Reference
(mg/litre)
USA
Industrial waste 0.4-1.5 Banerji (1969)
discharge (maximum 4.05)
Europe
Domestic and industrial 2 (maximum 5) Butterwick et al. (1989)
Egypt
Sewage water 0.32-0.38 El-Hassanin et al. (1993)
Sweden
Effluent 0.34-0.436 Ahl & Jönsson (1972)
Spain, Alicante
Industrial waste 1.45 Navarro et al. (1992)
Spain, Elche
Industrial waste 3 Navarro et al. (1992)
United Kingdom
Municipal 1.21-3.96 Waggott (1969)
(range of means)
5.1.4 Soil
According to Whetstone et al. (1942), boron occurs in soils at
concentrations ranging from 10 to 300 mg/kg (average 30 mg/kg),
depending on the type of soil, amount of soil organic matter, and
amount of rainfall. Background boron levels in US soils were reported
at a geometric mean concentration of 26 mg/kg, with a maximum
concentration of 300 mg/kg (Eckel & Langley, 1988).
5.1.5 Aquatic biota
Concentrations of boron in aquatic biota are summarized in
Table 8.
Little specific information was found concerning the
bioaccumulation of boron in aquatic plants. At Kesterson National
Wildlife Refuge in the San Joaquin River Valley, California, USA (an
evaporative sink that has high concentrations of boron, selenium, and
arsenic and is supplied with subsurface drainage water from
agricultural fields), studies of the aquatic food-chain contamination
have suggested that aquatic plants bioaccumulate high levels of boron,
but boron does not biomagnify in aquatic food-chains. The following
studies report observed concentrations in marine algae and freshwater
aquatic vascular species. Igelsrud et al. (1938) reported boron levels
ranging from 4.2 to 14.9 mg/kg of dried material in marine algae.
Yamamoto et al. (1973) compared the boron content in freshwater and
marine phytoplankton and observed that minor differences occurred
between forms, even though the boron content of seawater averages 460
times that of fresh water.
Adams et al. (1973) conducted a survey to determine the
concentration of 11 potentially polluting ions, including boron, in a
wide variety of aquatic plants from three major watersheds in
Pennsylvania, USA: the Delaware, Susquehanna, and Allegheny rivers.
Sources of pollution in this area are quite diverse, including
lumbering activities, coal strip-mining, recreation, agricultural use,
and urban-industrial centres. Boron constituent levels in 21 species
of submerged and floating aquatic vascular plants ranged from 26.3 to
170 µg/g, and levels in 8 species of emergent aquatic vascular plants
ranged from 11.3 to 57 µg/g.
Tsui & McCart (1981) studied the bioaccumulation of several
elements, including boron, in five freshwater fish species from the
Cold River drainage area in western Canada. Test species were selected
to represent different feeding habits and modes of life. Northern pike
(Esox lucius) and lake trout (Salvelinus namaycush) are primarily
predators; lake herring (Coregonus artedii) is a plankton feeder;
and lake whitefish (Coregonus clupeaformis) and white sucker
(Catostomus commersoni) are primarily bottom-feeders. The fish were
collected during spring and summer of 1978 from seven lakes within
this area, and the muscle tissue was analysed for the presence of
boron. The maximum average concentration of boron in the lakes was
Table 8. Concentrations of boron in aquatic biota
Species Area Tissue Boron concentration Reference
(mg/kg)a
Marine algae 4.2-14.9 dw Igelsrud et al. (1938)
Filamentous algae Lower San Joaquin River and its 3.5-280 dw Saiki et al. (1993)
tributaries, California, USA
Plankton Lower San Joaquin River and its 1.1-46 dw Saiki et al. (1993)
tributaries, California, USA
Aquatic plants Lower San Joaquin River, 382 (270-510) dw Ohlendorf et al. (1986)
California, USA
Submerged and floating Pennsylvania, USA 26.3-170 Adams et al. (1973)
aquatic vascular plants
Emergent aquatic Pennsylvania, USA 11.3-57 Adams et al. (1973)
vascular plants
Various marine shellfish British Columbia, Canada 0.9-5.5 ww Thompson et al. (1976)
Benthic bivalve San Joaquin River and its
(Corbicula fluminea) tributaries, California, USA Soft tissue <2-2 dw Leland & Scudder (1990)
Clam (Elliptio dilitata) Precambrian Shield lake, Soft tissue 2.6 ww Wren et al. (1983)
Ontario, Canada
Chironomid larvae Lower San Joaquin River and its
tributaries, California, USA <1.8-27 dw Saiki et al. (1993)
Amphipods Lower San Joaquin River and its <2.2-23 dw Saiki et al. (1993)
tributaries, California, USA
Crayfish Lower San Joaquin River and its 1.2-23 dw Saiki et al. (1993)
tributaries, California, USA
Table 8. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Freshwater fish Cold River drainage area, Muscle 3.23-12.44 Tsui & McCart (1981)
western Canada (range of means)
Freshwater fish Precambrian Shield lake, Muscle 1.8-2.9 ww Wren et al. (1983)
Ontario, Canada
Bluegill (Lepomis San Joaquin River, California, Whole body 14 dw (3.5 ww) Saiki & May (1988)
macrochirus) USA
Lower San Joaquin River and its <1.8-7.9 dw Saiki et al. (1993)
tributaries, California, USA
Largemouth bass Lower San Joaquin River and its <1.8-2.0 dw Saiki et al. (1993)
(Micropterus salmoides) tributaries, California, USA
Common carp San Joaquin River, California, Whole body 20 dw (5 ww) Saiki & May (1988)
(Cyprinus carpio) USA
San Joaquin River, California, Whole body 0.5-6.2 dwb Klasing & Pilch (1988)
USA
Mosquitofish Lower San Joaquin River and its <1.9-8.4 dw Saiki et al. (1993)
(Gambusia affinis) tributaries, California, USA
Volta, California, USA Whole body mean = 2.8 dw Ohlendorf et al. (1986)
Kesterson, California, USA Whole body mean = 11.1 dw Ohlendorf et al. (1986)
Tilapia spp. Mexicali Valley, Baja California, Muscle 2.9 ww Mora & Anderson (1995)
Mexico
Mugil spp. Mexicali Valley, Baja California, Muscle 1.9 ww Mora & Anderson (1995)
Mexico
Table 8. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Sockeye salmon British Columbia, Canada Gill mean = 0.6 ww Thompson et al. (1976)
(Oncorhynchus nerka) Liver mean = 0.7 ww
Bone mean = 1.5 ww
Atlantic cod (Gadus Northwest Atlantic Ocean Muscle 28 (1-93) ww Hellou et al. (1992)
morhua) Liver 9.7 (5.2-35.4) ww
Ovaries <0.8
Anchoveta Whole body 3.3-3.8 aw Jenkins (1980)
(Cetengraulis
mysticetus)
Aquatic birds Precambrian Shield lake, Muscle 2.5-3.7 ww Wren et al. (1983)
Ontario, Canada
Grassland water district, Liver 1.7-40 dw Paveglio et al. (1992)
California, USA
Double-crested Mexicali Valley, Baja California,
cormorant Mexico Liver 4.2 (2.9-8.2) ww Mora & Anderson (1995)
(Phalacrocorax auritus)
Duck species Grassland water district, Egg 3.07-6.17 dw Hothem & Welsh (1994)
California, USA
Wading bird species Grassland water district, Egg 2.2-3.45 dw Hothem & Welsh (1994)
California, USA
Aquatic mammals Precambrian Shield lake, Muscle 7.9 ww Wren et al. (1983)
Ontario, Canada
a ww = wet weight; dw = dry weight; aw = ash weight; concentrations are given as means or ranges of means; ranges are given in parentheses.
b Exposed to tile drainage water.
0.0627 mg/litre. The mean tissue concentrations of boron in the five
fish species ranged from 3.23 µg/g for lake whitefish to 12.44 µg/g
for white sucker.
Wren et al. (1983) reported boron concentrations in freshwater
fish and clams from a Precambrian Shield lake in Ontario, Canada. The
lake was free from direct human impact. Boron levels in the
undeveloped and protected muscle tissue of the fish were generally
lower than those observed in fish from the Cold River drainage area in
western Canada. The mean concentrations (wet weights) in the fish
ranged from 1.8 to 2.9 µg/g. The boron concentration in the soft
tissue of the clam (Elliptio dilitata) was 2.6 µg/g.
In contrast, boron levels were only slightly elevated in
whole-body samples of bluegill (Lepomis macrochirus) and common carp
(Cyprinus carpio) from the San Joaquin River and two tributaries
that receive agricultural subsurface drainage water. The highest boron
concentrations (dry weights) measured were 14 µg/g (approx. 3.5 µg/g
wet weight) in bluegills and 20 µg/g (approx. 5 µg/g wet weight) in
carp (Saiki & May, 1988). Ohlendorf et al. (1986) reported similar
values for mosquitofish (Gambusia affinis) from the San Joaquin
River Valley. However, Saiki & May (1988) reported that the elevated
boron levels may also result from natural boron deposits in adjacent
soils or from sand-and-gravel mining operations in the area.
Paveglio et al. (1992) analysed boron concentrations in livers of
aquatic birds collected from the Grassland Water District of
California, USA, during 1985-1988. The use of subsurface agricultural
drainage water for marsh management resulted in trace element
contamination of components of the food-chain in this region. During
the breeding and wintering periods, livers of birds from northern and
southern areas of the grasslands contained high concentrations of
boron (1.7-40 mg/kg dry weight).
A number of studies have investigated the accumulation of boron
in aquatic food organisms, such as plants, insects, and fish (Saiki &
May, 1988; Hothem & Ohlendorf, 1989; Smith & Anders, 1989; Paveglio et
al., 1992; Saiki et al., 1993). The results of these studies suggest
that aquatic plants bioaccumulate boron, but that boron does not
biomagnify in aquatic food-chains.
5.1.6 Terrestrial biota
Concentrations of boron in terrestrial biota are summarized in
Table 9.
The studies discussed in section 4.2.3 suggest that plants grown
in boron-rich soil often contain high levels of boron in their
tissues. Another source of boron entry into the food-chain is through
the reclamation and revegetation of areas containing coal combustion
products (i.e. ash). Schwab et al. (1991) reported strong positive
correlations between the concentration of boron in soybean and sorghum
used to revegetate several ash disposal sites in Kansas, USA, and the
level of extractable boron in the coal fly ash. Variations in plant
uptake of boron from coal ash were attributed to differences in ash
type, plant species, and ash treatment. In terrestrial food-chains,
boron accumulates in plants; however, boron is not biomagnified to
animals (Saiki et al., 1993).
5.2 General population exposure
Human exposure to boron could result from the following sources:
the consumption of drinking-water from natural or municipal sources
that contain boron; the consumption of crops grown in boron-enriched
soils, irrigated with boron-enriched waters, or contaminated with
airborne boron particles; the consumption of aquatic organisms
inhabiting natural waters high in boron; the absorption of boron from
cosmetic or medical preparations through mucous membranes or damaged
skin; and the inhalation, dermal absorption, or accidental ingestion
of boron-containing household cleaning products, pesticides, or
fertilizers. The most frequent and appreciable general population
exposures to boron are likely to be from ingestion of food and, to a
lesser extent, from ingestion of drinking-water.
5.2.1 Ambient air
Boron does not appear to be present in ambient air at significant
levels (Sprague, 1972). There are few studies available that estimate
the concentration of boron-containing compounds in ambient air; this
is partly due to the difficulties of analysis at the low levels
involved (ATSDR, 1992). However, Anderson et al. (1994a) have
estimated the continental levels. Using their assumption that
particulate boron constitutes 10% and gaseous boron constitutes 90% of
the total, the range is 0.36-19.9 ng/m3. Therefore, using the average
adult air consumption of 22 m3/day and the maximum value in this
range, a respiratory exposure of 438 ng/day (0.44 µg/day) is
calculated.
5.2.2 Drinking-water
Drinking-water is derived from groundwater and/or surface water
sources. Concentrations of boron found in drinking-waters of Chile,
Germany, the United Kingdom, and the USA are presented in Table 10.
Overall, boron concentrations ranged from 0.01 to 15.0 mg/litre, with
most values below 0.5 mg/litre. These values are consistent with
ranges and means observed for data presented for groundwater (Table 5)
and surface water (Table 6). Further, the consistency is supported by
two factors: 1) boron concentrations in water are largely dependent on
the leaching of boron from the surrounding geology, and 2) boron is
not removed by conventional drinking-water treatment methods. Hence,
when considering the need for allocating a TI of boron via drinking-
water, a mean boron concentration for the world is judged to be
between 0.1 and 0.3 mg/litre.
Table 9. Concentrations of boron in terrestrial biota
Species Area Tissue Boron concentration Reference
(mg/kg)a
Lichen
(Parmelia caperata) Travale-Radicondoli geothermal 12.3 (nd-25.4) dw Loppi & Bargagli (1996)
area, Italy
Cerealsb 2.3-5.0 dw Bergmann (1984)
Legumesc 15.4-41.4 dw Bergmann (1984)
Dandelion
(Taraxacum officinale) 80 dw Bergmann (1984)
Poppy
(Papaver somniferum) 94.7 dw Bergmann (1984)
Moss
(Hylocomium splendens) Norway 3.6 (0.38-21) dw Bergmann et al. (1995)
Omnivorous terrestrial Mexicali Valley, Baja California, Liver 2.3-5.3 (1.2-8.7) ww Mora & Anderson (1995)
bird species Mexico
Mourning dove Mexicali Valley, Baja California, Liver 10 (4.3-28.5) ww Mora & Anderson (1995)
(Zenaida macroura) Mexico
Mule deer
(Odocoileus hemionus) Piceance Creek Basin, Metacarpal 2.3 (1.5-3.1) dw (fawn) Stelter (1980)
Colorado, USA
Piceance Creek Basin, Metacarpal 1.4 (1.1-1.7) dw (adult) Stelter (1980)
Colorado, USA
Table 9. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Deer mice
(Peromyscus maniculatus) Piceance Creek Basin, Colorado, Whole skeleton <2 dw Stelter (1980)
USA
Four species of rodent 4.3-6.3 Wiener et al. (1977)
a dw = dry weight; ww = wet weight; nd = not detected.
b Barley (Hordeum vulgare), rye (Secale cereale), wheat (Triticum vulgare), maize (Zea mays).
c Fieldbean (Vicia faba), pea (Pisum sativum), soya bean (Glycine max), lentil (Lens esculenta).
Table 10. Concentrations of boron in drinking-water
Area Samples Concentration Reference
(mg/litre)
USA 2595 0.8% > 1.0 McCabe et al. (1970);
Choi & Chen (1979)
Germany 240 <0.25 Graffmann et al. (1974)
110 <0.21 Wiecken & Wübbold-Weber
(1993)
United Kingdom 0.05-0.505 J. Bennett
(personal communication)a
200 0.01-0.45 D.E. Wilkinson
(personal communication)b
Chile 0.31-15.0 Barr et al. (1993)
a Boron levels at drinking-water abstraction points of the River
Thames: letter of 22 February 1993 from National Rivers Authority,
Thames Region, United Kingdom, to the IPCS.
b Boron values for Anglican Water Region (1.1.92-31.12.92): letter
of 9 March 1993, ref. DEW/AH/1733CD, from Anglican Water Services
Limited, United Kingdom, to the IPCS.
5.2.3 Soil intake
An older report indicates that boron is found in soils at
concentrations ranging from 10 to 300 mg/kg, with an average value of
30 mg/kg (Whetstone et al., 1942).
A more recent study (Eckel & Langley, 1988) gives a similar upper
boron concentration range (300 mg/kg) and average value (26 mg/kg).
The use of this latter average soil level with an incidental
consumption of 20 mg soil/person per day (IPCS, 1994) yields an
average boron intake of 0.5 µg/day (i.e. 26 mg boron/kg of soil ×
0.000 02 kg of soil consumed per person per day = 0.0005 mg
boron/person per day).
5.2.4 Dietary intake
For the general population, the greatest exposure to boron comes
from food. Most of the concentrations of boron in foods reported
before 1985 are of questionable validity because of inadequate
analytical methods. Two recent reports (Hunt et al., 1991; Anderson et
al., 1994b) provide an adequate indication of the amounts of boron
found in various foods (see Table 11).
Table 11. Boron content of some common foods
Food Boron concentration
(µg/g, fresh weight basis)
Hunt et al. Anderson et al.
(1991) (1994b)
Fruits
Apple, red with peel, raw 2.73 2.38
Apple juice 1.88 2.41
Apple sauce 2.83 1.04
Banana, raw 3.72
Cherries, dark 1.47 0.92
Grape juice 2.02 2.06
Orange juice 0.41 1.59
Peaches, canned 1.87
Pears, canned 1.22
Dried fruits
Dates 9.2
Prunes 27 21.5
Raisins 25 19.0
Vegetables
Beans, green 0.46 1.56
Broccoli, flowers 1.85
Broccoli, stalks 0.89
Lettuce, iceberg <0.015
Carrots, canned 0.75
Nuts
Almonds 23
Hazelnuts 16
Peanuts 18 13.8
Meats
Beef, round, ground, raw <0.015 <0.05
Chicken, breast, ground, raw <0.015 0.09
Turkey breast <0.015
Milk and milk products
Cheese, cream <0.015 0.19
Milk, 2% <0.015 0.23
Cereal grain products
Bread, white, enriched 0.20 0.48
Cornflakes, fortified 0.31 0.92
Flour, wheat, white 0.28
Noodles, egg, dry, enriched 0.37
Rice, white, instant <0.015
Spaghetti, dry, enriched <0.015
Table 11. (continued)
Food Boron concentration
(µg/g, fresh weight basis)
Hunt et al. Anderson et al.
(1991) (1994b)
Miscellaneous
Catsup 0.85 1.39
Eggs, homogenized <0.015 0.12
Honey 7.2 6.07
Jelly, strawberry 0.41
Jelly, grape 1.47 1.86
Sugar, white <0.015 0.29
Beveragesa
Wine 3.5
Beer 1.8 0.13
a Boron concentration in µg/ml.
The richest sources of boron are fruits, vegetables, pulses,
legumes, and nuts. Dairy products, fish, meats, and most grains are
poor sources of boron. Based on the recent analyses of foods and food
products, estimations of daily intakes of various age/sex groups have
been made. Rainey et al. (1996) estimated that the median, mean, and
95th-percentile daily intakes of boron are 0.76, 0.93, and 2.16
mg/day, respectively, for all 27 age/sex groups in the USA; 0.34,
0.50, and 1.49 mg/day, respectively, for infants aged 0-5 months;
1.00, 1.18, and 2.61 mg/day, respectively, for males aged 60-65 years;
and 0.87, 1.0, and 2.17, respectively, for females aged 60-65. Using
food included in US Food and Drug Administration (FDA) Total Diet
Studies, Iyengar et al. (1988) determined the mean adult male daily
intake of boron to be 1.52 mg/day, whereas Anderson et al. (1994b)
determined the intake to be 1.21 mg/day. Based on the United Kingdom
National Food Survey (UK Ministry of Agriculture, Fisheries and Food,
1991), the dietary intake of boron in the United Kingdom ranges from
0.8 to 1.9 mg/day. It should be noted that increased consumption of
specific foods with high boron content will increase boron intake
significantly; for example, one serving of wine or avocado provides
0.42 or 1.11 mg, respectively (Anderson et al., 1994b). Moreover, for
the population obtaining their drinking-water from the 10% of the
public water systems that provide water containing >0.4 mg
boron/litre, water used for drinking and cooking may be the major, or
a significant, source of boron. Based on the preceding values, the
mean daily intake of boron in the diet is judged to be near 1.2
mg/day.
5.2.5 Consumer products
Boric acid, borax, and other borates are used in a great array of
consumer goods. The principal use of boric acid and borax in the USA
is in the manufacture of glass products. The way boron is bound to
glass products should not result in significant releases of boron in
its production or use.
Other consumer products in which boron compounds are used include
soaps and detergents (as a bleaching agent), preservatives, adhesives,
porcelain, enamel, leathers, carpets, artificial gems, high-contrast
photographic materials, wicks, electric condensers, fertilizers,
insecticides, and herbicides (Moore et al., 1997).
Sodium borate and boric acid are also widely used in numerous
cosmetic products, including makeup, skin and hair care preparations,
deodorants, moisturizing creams, breath fresheners, and shaving
creams, with concentrations up to 5% (US FDA, 1981; Beyer et al.,
1983). In 1981, the US FDA limited boric acid concentrations in
consumer goods to 5% (US FDA, 1981). Boric acid exposures from some
personal care products, estimated by the European Union Subcommittee
on "Cosmetic Ingredients," include 0.09-0.46 mg boric acid/kg body
weight per day for oral hygiene products, 0.03 mg boric acid/kg body
weight per day for eye products, and 0.25 mg boric acid/ kg body
weight per day for deodorants (SCC opinion XXIV/1820/95). Exposure is
related to personal practices. Boric acid is used in vaginal products
and contraceptives. Consequently, by topical application, these
compounds may come in contact with body surfaces, including the
ocular, buccal, and vaginal mucosae (Beyer et al., 1983).
Body-building supplements contain 1.5-10 mg boron/serving, with a
median of 4 mg boron/serving. These supplements could result in daily
exposures of 1.5-30 mg boron, as some are taken up to 3 times a day.
Bottled water can contain <0.005-4.35 mg boron/litre, depending on
its origin, with an average boron content of 0.75 mg/litre (Moore et
al., 1997).
The European Union permits boric acid in certain consumer
products (e.g. 3% boric acid in eye products, up to 0.5% in oral
hygiene products) (SCC opinion XXIV/1820/95). Usage data provided by
the industry (EU Technical Guidance Document, 1995) allow the
estimation of total exposure to boric acid from consumer products as
22.8 mg/day per person, equivalent to 4 mg boron/day. If absorption
across the dermis is assumed to range from 1 to 10%, the absorbed
boron dose from the source is 0.04-0.4 mg boron/day. The Task Group
felt that 0.1 mg/day would serve as a reasonable average estimate of
boron exposure from consumer products.
5.3 Occupational exposure
Occupational exposures to boron compounds may be significant
(ATSDR, 1992). Inhalation of dusts is the most significant route of
exposure in occupational settings. Dermal absorption of boron may also
occur if damaged skin is in contact with boron compounds, but this is
considered a minor pathway (Culver et al., 1994a).
Borate dusts have been monitored in workplace air. Reported
concentrations of borax dust in different areas of a large borax
mining and refining plant ranged from 1.1 to 14.6 mg/m3 (Garabrant et
al., 1985; see also section 8.2.1), and the mean boric acid/boric
oxide dust concentration in a boric acid manufacturing plant was
4.1 mg/m3 (Garabrant et al., 1984). Other industries where workers
may be occupationally exposed to boron include manufacture of
fibreglass and other glass products, cleaning and laundry products,
fertilizers, pesticides, and cosmetics (Stokinger, 1981). The range of
normal values of blood boron concentration for non-occupationally
exposed working adults can be found in Culver et al. (1994a). NIOSH
(1989) estimated that the number of workers potentially exposed to
boric acid increased from 6500 in the early 1970s to 35 600 in the
early 1980s. Information about frequency, concentration, and duration
of exposures is not contained in the NOES or National Occupational
Hazard Survey (NOHS) databases. Wegman et al. (1994) reported that
NIOSH estimated that there were 420 000 US workers with potential
occupational exposure to sodium borate.
As part of an epidemiological study of acute respiratory
irritation, Woskie et al. (1994) assessed short-term (time-weighted
average over 0.25 h) and daily (time-weighted average over 6 h) dust
and boron exposures of workers in a sodium borate production facility.
The investigators used personal monitors to measure the boron
concentration in air and the mass concentration of total airborne
dusts. The arithmetic mean for the total dust concentration ranged
from 0.29 to 18.85 mg/m3, and the average concentration of boron
relative to total dust ranged from 5.6 to 10.1%. Because the data
demonstrated that a substantial portion of the total dust was
non-borate material (e.g. cigarette smoke, vehicle exhaust, background
or ambient dust), the authors cautioned against using total dust as an
indicator or surrogate marker for actual exposure to boron compounds.
In a study in which chronic abnormality in pulmonary function and
acute irritant symptoms associated with borate dust in mixing and
processing operations were examined (Wegman et al., 1994; see also
section 8.2.1), personal monitors were used to measure boron
concentrations. The results showed that workers were exposed to dust
concentrations ranging from 0.1 to 205 mg/m3. The arithmetic mean of
daily exposures in the comparison group was 0.45 mg total dust/m3
(0.02 mg total boron/m3), whereas the average daily exposure for the
exposed group was 5.72 mg total dust/m3 (0.44 mg total boron/m3).
Whorton et al. (1994) studied male employees exposed to borate
dusts at a borax mine and production facility in California, USA, from
the 1950s through the 1980s. Men were identified with two or more past
consecutive years of "high" exposure to doses with a range of
17.6-44.8 mg dust/m3 (doses averaged 23.2 mg dust/m3, or 3.3 mg
boron/m3). The average exposure to sodium borate dust was 203 mg/day,
which assumes 7 h/day of actual exposure at an average concentration
of 23.2 mg/m3. The estimated 8-h respiratory volume for light work is
10 m3. The average concentration of boron in sodium borates is 14%;
the daily dose of boron, assuming 100% absorption, is therefore
28.4 mg.
In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine levels from workers
with the amount of boron exposure. Mean daily inspired boron was
calculated for low-, medium-, and high-exposure groups of male
workers; the numbers of workers in these groups were four, five, and
five, respectively. Total daily boron intake was calculated for each
individual, including the dietary contribution. Blood and urine
samples were taken at the start of the work week and at the end of
shifts on Monday, Thursday, and Friday and analysed for boron. An
average blood level of boron of 0.26 µg/ml occurred in the
high-exposure group of workers, where exposure was estimated to be
0.38 mg boron/kg body weight per day. Post-shift blood and urine boron
concentrations did not increase with the days of the work week,
indicating that boron did not accumulate when exposure was up to 0.38
mg/kg body weight per day during the work week.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Numerous studies have shown that boric acid and borax are
absorbed from the gastrointestinal tract and from the respiratory
tract, as indicated by increased levels of boron in the blood,
tissues, or urine or by systemic toxic effects of exposed individuals
or laboratory animals. Blood, tissue, and urine boron levels have
rarely been reported in cases of fatal human exposures to inorganic
borates.
6.1.1 Oral
Pharmacokinetic data indicate that boron, usually administered as
boric acid, is absorbed rapidly and virtually completely from the
gastrointestinal tract, as evidenced by recovery of >90% of the dose
in urine (Jansen et al., 1984). In a study in which human volunteers
were given a single known dose of boric acid (131 mg boron), 94% of
the ingested boron was excreted in the urine over a 96-h period (Schou
et al., 1984). Based on urinary excretion of boron, Job (1973)
observed a similar degree of oral absorption in volunteers who drank
curative spa waters with a daily dose of approximately 100 mg boron
for 2 weeks. Similar results had previously been reported by Kent &
McCance (1941). These investigators also showed that the urinary
excretion of boron ingested solely from dietary sources also reflected
absorption rates ranging from 83 to 98% (Kent & McCance, 1941).
Ingested boron is also readily absorbed by other species, including
rats (Ku et al., 1991), rabbits (Draize & Kelley, 1959), sheep (Brown
et al., 1989), and cattle (Owen, 1944; Weeth et al., 1981), as
indicated by high levels of boron in tissues or urine or systemic
toxicity following oral exposure. A 20-µg dose of 10B fed to fasted
rats resulted in peak liver concentrations at 3 h and a return to
normal isotope ratios within 24 h. Absorption was essentially
complete, as evidenced by 95% recovery of 10B from urine and
4% recovery from faeces within 72 h post-dosing (Vanderpool et al.,
1994).
Barr et al. (1993) studied areas of northern Chile in which the
concentrations of naturally occurring boron in the water supplies
ranged from 0.31 to 15.2 mg/litre. Blood boron levels for residents
from seven geographical regions showed a positive correlation with
boron levels in the local drinking-water supplies. Rough extrapolation
from the plotted data indicates that in three regions with boron
levels in drinking-water below 2.5 mg/litre (i.e. estimated intake of
0.07 mg boron/kg body weight per day for a 70-kg human consuming
2 litres of water per day), blood boron levels were below 0.1 µg/g
blood. In the region with the highest boron concentration in
drinking-water (15.2 mg boron/litre) (i.e. estimated intake of 0.43 mg
boron/kg body weight per day), average blood levels were approximately
0.7 µg boron/g blood. Accuracy of linear fitting for the original data
was limited by the small sample size obtained in each region, as well
as the absence of individual water intake data.
6.1.2 Inhalation
Absorption is indicated by increases in human blood and urine
boron levels after inhalation exposure to borax in the range of 3.3-18
mg/m3 (Culver, 1994a). Mice exposed to amorphous boron at a
concentration of 72.8 mg/m3 for 7 h/day, 5 days/week, for 30 days had
significant concentrations of boron in the liver and kidney (Stokinger
& Spiegl, 1953), indicating that amorphous boron is absorbed from the
respiratory tract. High boron levels were also found in the lungs,
indicating that absorption was not complete. Wilding et al. (1959)
showed that rats exposed to aerosols of boron oxide by inhalation at
77 mg/m3 for 6 h/day, 5 days/week, for 6 weeks excreted boron in
their urine, indicating that boron oxide is absorbed from the
respiratory tract. Ingestion (i.e. swallowing particles cleared from
the respiratory tract by the mucociliary escalator system and
subsequent absorption from the gastrointestinal tract) may have
contributed to systemic uptake.
6.1.3 Dermal
Dermal absorption across intact skin is negligible in all species
evaluated, including human infants (Friis-Hansen et al., 1982), human
adults (Stuttgen et al., 1982), rabbits (Draize & Kelley, 1959), and
rats (Nielsen, 1970). Several investigators have demonstrated that
dermal absorption of boron compounds may be affected by the vehicle
used to dissolve or suspend the compounds (Nielsen, 1970; Stuttgen et
al., 1982). When boric acid is applied to broken or damaged skin,
however, absorption of boric acid through the damaged skin has been
demonstrated (Draize & Kelley, 1959; Nielsen, 1970). In a report by
Vignec & Ellis (1954), infants 1.25-10 months old received
applications of a talcum powder containing 5% boric acid 7-10
times/day for at least 1 month. The authors calculated that the
infants were exposed to an estimated dose of 2.33 g boric acid/day
(408 mg boron/day). The boron concentrations in a test group composed
of 12 infants were 0.04 ± 0.04 mg/100 ml in blood and 0.16 ± 0.14
mg/100 ml in the urine. An additional group of 12 treated infants, who
had developed a mild to moderate diaper rash, had an average blood
boron concentration of 0.03 ± 0.04 mg/100 ml. Although Vignec & Ellis
(1954) did not analyse the data statistically, the range of the values
obtained indicates that, at most, only traces of boric acid penetrated
the skin, even in infants with moderate diaper rash.
6.2 Distribution
6.2.1 Tissue levels
Boron is a normal constituent of blood and tissues in humans and
animals as a result of ingestion in food and (in some locations)
drinking-water. Blood and tissue levels under normal dietary
conditions or with boron supplementation are summarized in Table 12.
Boric acid distributes evenly throughout the body fluids. The
most complete study of boron distribution was conducted in male F-344
rats (30/dose group) fed a control diet (<20 mg boron/kg) or a diet
containing 9000 mg boric acid/kg (approximately 68 mg boron/kg body
weight per day) for up to 7 days (Ku et al., 1991). Six animals/ group
were killed at 1, 2, 3, 4, and 7 days after the start of exposure.
Boron levels were determined in all major tissues and organs. The
authors reported that boron concentrations were comparable in all
tissues examined, including plasma, liver, kidney, muscle, colon,
brain, testis, epididymis, seminal vesicles, prostate, and adrenals.
Most of the tissues appeared to reach steady-state boron levels (12-30
mg boron/kg tissue) by 3-4 days; these levels were 3- to 20-fold above
controls. Adipose tissue accumulated only 20% as much boron as other
tissues (3.78 mg/kg tissue). Bone boron levels (47.4 mg/kg tissue)
indicated greater accumulation in bone than in other tissues; in
addition, bone boron levels continued to increase throughout the
7 days. The higher affinity of boron for bone is indicative of a
second kinetic component in which a small percentage of the boron
absorbed may be sequestered. Elimination kinetics from bone also
differ from those from soft tissue and body fluids (see Chapin et al.,
1997, in section 6.4). Accumulation of boron in bone has also been
observed in humans (Alexander et al., 1951; Forbes et al., 1954) and
other animal species (Forbes & Mitchell, 1957).
6.2.2 Blood levels
O'Sullivan & Taylor (1983) reported the blood boron levels in
three of seven infants (6-16 weeks old) who had ingested borax from
pacifiers dipped in a borax and honey mixture. These three infants
ingested 8-30 g of borax (286-429 mg borax/day) over a 4- to 10-week
time period. Signs of central nervous system (CNS) toxicity (seizures)
were reported in all seven infants. The blood levels ranged from 0.26
to 0.85 mg boron/100 ml blood; these blood values did not correlate
well with the estimated amount of borax ingested per individual, but
were clearly elevated relative to 15 children (ages 2-21 months)
without exposure (average 0.021 mg/100 ml, range 0-0.063 mg/100 ml).
In a review of 782 cases of accidental boric acid exposures (age
range 2 weeks to 98 years), serum levels ranged from 0 to 5.94 mg
boron/100 ml. However, these levels did not correlate with the
estimated amount of boric acid ingested (Litovitz et al., 1988).
Magour et al. (1982) determined the tissue levels of boron in
Wistar rats administered 20 mg boron/kg body weight per day as sodium
borate in drinking-water; exposure was initiated at 3 weeks of age and
continued for 21 days. Blood boron levels continually rose during the
treatment period; the maximum level was approximately 3.2 µg/g or
0.32 mg/100 ml blood. Intraperitoneal administration of 42 mg boron/kg
body weight to 3-week-old or 3-month-old female Wistar rats resulted
in generally higher tissue levels in the older rats and slightly
higher tissue/blood ratios across a 4-h post-administration period
(Magour et al., 1982). These data suggest that boron may be
distributed differently in mature versus immature rats, but the
differences were subtle and transient.
Table 12. Blood and tissue boron levels following ingestion of boron in diet or drinking-water
Species Ingested boron Fluid/organ Concentrationa Reference
Chick 0.465 µg/g diet Plasma 0.077 µg/ml Hunt (1989)
3.465 µg/g diet Plasma 0.152 µg/ml
0.465 µg/g diet Femur 0.251 µg/g dw
3.465 µg/g diet Femur 0.861 µg/g dw
Chick 0.18 µg/g diet Brain 1.05 µg/g dw Bain & Hunt (1996)
+ 3 µg/g diet Brain 1.01 µg/g dw
+ 20 µg/g diet Brain 1.61 µg/g dw
0.18 µg/g diet Heart 0.25 µg/g dw
+ 3 µg/g diet Heart 0.55 µg/g dw
+ 20 µg/g diet Heart 0.88 µg/g dw
0.18 µg/g diet Liver 1.35 µg/g dw
+ 3 µg/g diet Liver 1.01 µg/g dw
+ 20 µg/g diet Liver 1.22 µg/g dw
0.18 µg/g diet Kidney 0.55 µg/g dw
+ 3 µg/g diet Kidney 0.66 µg/g dw
+ 20 µg/g diet Kidney 1.11 µg/g dw
0.18 µg/g diet Spleen 2.22 µg/g dw
+ 3 µg/g diet Spleen 2.02 µg/g dw
+ 20 µg/g diet Spleen 2.38 µg/g dw
0.18 µg/g diet Femur 0.32 µg/g dw
+ 3 µg/g diet Femur 0.53 µg/g dw
+ 20 µg/g diet Femur 1.81 µg/g dw
0.18 µg/g diet Muscle 0.12 µg/g dw
+ 3 µg/g diet Muscle 0.15 µg/g dw
+ 20 µg/g diet Muscle 0.41 µg/g dw
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Chick 0.18 µg/g Femur 1.21 µg/g dw Hunt et al. (1994a)
(Vitamin D-deficient diet)
1.4 µg/g Femur 1.49 µg/g dw
(Vitamin D-deficient diet)
0.18 µg/g Femur 0.74 µg/g dw
(Vitamin D-adequate diet)
1.4 µg/g Femur 0.75 µg/g dw
(Vitamin D-adequate diet)
Human Unknown Brain, normal 0.87 µg/g dw Shuler et al. (1990)
Brain, thalassaemic 1.38 µg/g dw
Heart, normal 0.59 µg/g dw
Heart, thalassaemic 2.21 µg/g dw
Kidney, normal 1.27 µg/g dw
Kidney, thalassaemic 4.57 µg/g dw
Liver, normal 2.25 µg/g dw
Liver, thalassaemic 1.72 µg/g dw
Pancreas, normal 0.51 µg/g dw
Pancreas, thalassaemic 2.94 µg/g dw
Spleen, normal 3.95 µg/g dw
Spleen, thalassaemic 1.14 µg/g dw
Human Unknown Bone, arthritic 0.63 µg/g fw Havercroft & Ward (1991)
Bone, normal 1.57 µg/g fw
Synovial fluid, arthritic 30.4 ng/ml
Synovial fluid, normal 48.1 ng/ml
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Human 27 mg/day Blood 0.659 µg/g Barr et al. (1993)
(drinking-water computed)
21 mg/day Blood 0.450 µg/g
(drinking-water computed)
17 mg/day Blood 0.585 µg/g
(drinking-water computed)
4.5 mg/day Blood 0.347 µg/g
(drinking-water computed)
2.5 mg/day Blood 0.052 µg/g
(drinking-water computed)
1.3 mg/day Blood 0.068 µg/g
(drinking-water computed)
0.56 mg/day Blood 0.022 µg/g
(drinking-water computed)
Human Unknown Blood 28 ng/ml Ward (1993)
Synovial fluid 30 ng/ml
Saliva 4.4 ng/ml
Cerebrospinal fluid 1.5 ng/ml
Hair, scalp 1.05 µg/g fw
Nails 15 µg/g fw
Bone 1.6 µg/g fw
Liver 0.1 µg/g fw
Kidney 0.5 µg/g fw
Brain 0.08 µg/g fw
Human About 1.2 mg/day Plasma 34 ng/ml Nielsen (1996)
About 3.3 mg/day Plasma 53 ng/ml
Human Unknown Pre-term milk 0.5 ng/ml Aquilio et al. (1996)
Term milk 1.2 ng/ml
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Human 0.36 mg/day Red blood cells 0.21 µg/g dw Hunt et al. (1997)
3.23 mg/day Red blood cells 0.19 µg/g dw
0.36 mg/day Plasma 64 ng/ml
3.23 mg/day Plasma 95 ng/ml
Rat 0.17 µg/g diet Plasma 55 ng/ml Nielsen et al. (1992a)
3.17 µg/g diet Plasma 55 ng/ml
0.17 µg/g diet Femur 1.28 µg/g dw
3.17 µg/g diet Femur 1.73 µg/g dw
Rat 0.2 µg/g diet Femur 0.72 µg/g dw Nielsen & Shuler (1992)
3.2 µg/g diet Femur 0.88 µg/g dw
Rat 0.12 µg/g diet Femur 0.83 µg/g dw Penland & Ebehardt (1993)
2.7 µg/g diet Femur 0.95 µg/g dw
Rat 10 µg/g diet Blood 0.11 µg/ml Bai & Hunt (1996)
Brain 0.64 µg/g
Heart 1.0 µg/g
Kidney 2.0 µg/g
Liver 0.51 µg/g
Spleen 3.3 µg/g
Testes 0.91 µg/g
Tibia 1.3 µg/g
Muscle 0.50 µg/g
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Rat (injected 0.15 µg/g diet Muscle 0.12 µg/g dw Bai & Hunt (1996)
with 1.5 µg/g diet Muscle 0.17 µg/g dw
mycobacterium 3.0 µg/g diet Muscle 0.18 µg/g dw
tuberculosis) 10.0 µg/g diet Muscle 0.30 µg/g dw
0.15 µg/g diet Heart 0.37 µg/g dw
1.5 µg/g diet Heart 0.34 µg/g dw
3.0 µg/g diet Heart 0.44 µg/g dw
10.0 µg/g diet Heart 0.57 µg/g dw
0.15 µg/g diet Liver 0.14 µg/g dw
1.5 µg/g diet Liver 0.21 µg/g dw
3.0 µg/g diet Liver 0.17 µg/g dw
10.0 µg/g diet Liver 0.26 µg/g dw
0.15 µg/g diet Kidney 0.81 µg/g dw
1.5 µg/g diet Kidney 0.45 µg/g dw
3.0 µg/g diet Kidney 0.61 µg/g dw
10.0 µg/g diet Kidney 0.99 µg/g dw
a dw = dry weight; fw = fresh weight.
Table 13 shows that blood boron levels generally increase with
the increase in oral dose in both rats and humans. However, procedural
differences among studies currently preclude a definitive
cross-species comparison to determine comparability of blood boron
levels at similar doses. For example, studies may differ in their
approach to estimation of boron intake, duration of exposure,
analytical methodology, or temporal relationship between exposure and
blood collection. Recognizing these limitations, a preliminary
comparison across species has been made based on rats exposed to boron
via diet or drinking-water and humans exposed via diet,
drinking-water, or accidental ingestion (Fig. 1). In this context,
good overlap occurs between rat and human blood boron values in the
dose ranges from 0.01 to 100 mg boron/kg body weight per day. These
data reinforce the perception that boron kinetics are similar in rats
and humans, but they also emphasize the need for additional data to
strengthen the basis for the comparison.
6.3 Metabolism
Metabolism of inorganic borates by biological systems is not
feasible owing to the excessive energy (523 kJ/mol) required to break
the boron-oxygen bond (Emsley, 1989). Inorganic borates, in low
concentrations, convert to boric acid at physiological pH in the
aqueous layer overlying mucosal surfaces prior to absorption. This is
supported by the evidence in both human and animal studies, where more
than 90% of the administered dose of borate is excreted as boric acid.
There is evidence in both in vitro and in vivo systems that boric
acid has an affinity for cis-hydroxyl groups, and this may be the
mechanism that explains the biological effects of boric acid. However,
this attachment is known to be reversible and concentration dependent,
responding to clearance mechanisms.
6.4 Elimination and excretion
Clearance of boron compounds is similar in humans and animals.
Elimination of borates from the blood is largely by excretion of >90%
of the administered dose via the urine, regardless of the route of
administration. Excretion is relatively rapid, occurring over a period
of a few to several days, with a half-life of elimination of 24 h or
less. The kinetics of elimination of boron have been evaluated in
human volunteers given boric acid via the intravenous and oral routes
(Jansen et al., 1984; Schou et al., 1984). The half-life for
elimination was the same by either route in these studies and was
approximately 21 h. This value is corroborated by case reports of
boric acid poisoning. Litovitz et al. (1988) examined the case reports
of almost 800 patients accidentally or intentionally poisoned with
boric acid and found a very comparable elimination half-life: 13.4 h
(range 4-27.8 h). Incomplete or inconsistent patient histories
(especiallywith regard to total dose or dose-to-sample interval)
undoubtedly contributed to the variation in those half-life estimates.
Table 13. Blood boron levels as a function of dose in humans and rats
Boron dose Blood boron level (ng/ml) Comments Reference
(mg/kg body
weight per day) Human Rat
0.0054 64 (plasma) Diet Hunt et al. (1997)
0.01 22 Drinking-water Barr et al. (1993)
0.017 55 (plasma) Diet Nielsen et al. (1992a)
0.02 34 (plasma) Diet Nielsen (1996)
0.02 68 Drinking-water Barr et al. (1993)
0.04 52 Drinking-water Barr et al. (1993)
0.049 95 (plasma) Diet Hunt et al. (1997)
0.05 53 (plasma) Diet Nielsen (1996)
0.08 347 Drinking-water Barr et al. (1993)
0.2 200a Diet Chapin et al. (1997)
0.3 55 (plasma, uncertain) Diet Nielsen et al. (1992a)
0.3 585 Drinking-water Barr et al. (1993)
0.35 229 ± 143b Diet Price et al. (1997)c
0.4 450 Drinking-water Barr et al. (1993)
0.5 659 Drinking-water Barr et al. (1993)
1.4 3 000 Drinking-water Job (1973)
1.7 800a Diet Chapin et al. (1997)
3.3 564 ± 211b Diet Price et al. (1997)
3.5 3 200 (uncertain) Oral Litovitz et al. (1988)
6.3 975 ± 261b Diet Price et al. (1997)
8.4 2 400a Diet Chapin et al. (1997)
9.6 1 270 ± 298b Diet Price et al. (1997)
13 1 530 ± 546b Diet Price et al. (1997)
20 3 100 Drinking-water Magour et al. (1982)
25 2 820 ± 987b Diet Price et al. (1997)
26 6 700 (serum) Diet Ku et al. (1993a)
38 10 300 (serum) Diet Ku et al. (1993a)
40 6 500 Oral O'Sullivan & Taylor (1983)
52 13 300 (serum) Diet Ku et al. (1993a)
56 27 000 (uncertain) Oral Litovitz et al. (1988)
68 17 300 (serum) Diet Ku et al. (1993a)
95 16 000 (plasma) Diet Ku et al. (1991)
Table 13 (continued)
a Developed by R. Chapin. Estimated from measured bone boron concentrations and ratio of bone/blood boron levels, from Chapin
et al. (1997) and Ku et al. (1993a).
b Mean ± SD (n = 27-30 per dose group; Price et al., 1997).
c Price et al. (1997) data are for pregnant rats.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
Members
Dr G.M. Buck, Department of Social and Preventive Medicine, State
University of New York at Buffalo, Buffalo, New York, USA
Dr R. Chapin, Department of Health and Human Services, National
Institutes of Health, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr M.L. Dourson, Toxicology Excellence for Risk Assessment,
Cincinnati, Ohio, USA
Dr P. Foster, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr R.A. Goyer, 6405 Huntingridge Road, Chapel Hill, North
Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Cambs., United Kingdom (Co-Rapporteur)
Dr R. Luoto, National Public Health Institute, Department of
Epidemiology and Health Promotion, Helsinki, Finland
Dr F.H. Nielsen, US Department of Agriculture, Agricultural
Research Service, Grand Forks Human Nutrition Research Center,
Grand Forks, North Dakota, USA
Dr C.J. Price, Center for Life Sciences and Toxicology, Research
Triangle Institute, Research Triangle Park, North Carolina, USA
Dr W.G. Woods, Office of Environmental Health and Safety,
University of California, Riverside, California, USA
Observers
Dr B.D. Culver, University of California, Department of Medicine,
Irvine, California, USA (representing International Commission on
Occupational Health)
Dr S. Dyer, Procter & Gamble, Ecosystems Research Station,
Environmental Science Department, Cincinnati, Ohio, USA
(representing European Centre for Ecotoxicology, Toxicology of
Chemicals)
Dr J.A. Moore, Institute for Evaluating Health Risks, Washington,
DC, USA (representing the American Industrial Health Council)
Dr F.J. Murray, 6611 Northridge Drive, San Jose, California, USA
(representing International Life Sciences Institute)
Mrs M. Richold, Unilever Research ESL, Sharnbrook, Bedford,
United Kingdom (representing International Life Sciences
Institute)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr L. Galvao, Pan American Health Organization, World Health
Organization, Geneva, Switzerland
Dr H. Otterstetter, Pan American Health Organization, World
Health Organization, Geneva, Switzerland
Ms C. Smallwood, US Environmental Protection Agency, National
Center for Environmental Assessment, Cincinnati, Ohio, USA
(Co-Rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
A WHO Task Group on Environmental Health Criteria for Boron met
in Washington, DC, USA, from 18 to 22 November 1996. The meeting was
organized by the WHO Regional Office for the Americas (AMRO) on behalf
of the IPCS. Dr H. Otterstetter, WHO AMRO, opened the meeting and
welcomed the participants. Dr B.H. Chen, IPCS, welcomed the
participants on behalf of the Director of IPCS and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to boron.
The first draft of this monograph was prepared by Ms C. Smallwood
of the US EPA in Cincinnati. The second draft was also prepared by Ms
Smallwood, incorporating comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs. Dr R. Goyer, Chairman of the Task Group,
contributed significantly to the final text of the EHC for Boron.
Dr B.H. Chen, member of the IPCS Central Unit, and Ms M. Sheffer,
Scientific Editor, Ottawa, Canada, were responsible for the overall
scientific content and linguistic editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
Financial support for this Task Group meeting was provided by the
US EPA.
ABBREVIATIONS
BMD Benchmark dose
CAS Chemical Abstracts Service
CL Confidence limit
CNS Central nervous system
EPA Environmental Protection Agency (USA)
FDA Food and Drug Administration (USA)
FSH Follicle stimulating hormone
GLP Good Laboratory Practices
HSDB Hazardous Substances Data Bank
ICP Inductively coupled plasma
ICP-AES Inductively coupled plasma atomic emission spectroscopy
ICP-MS Inductively coupled plasma mass spectroscopy
LH Luteinizing hormone
LOAEL Lowest-observed-adverse-effect level
(human and animal toxicity)
LOEC Lowest-observed-effect concentration
(environmental effects)
MATC Maximum acceptable toxicant concentration
(environmental effects)
MMAD Median mass aerodynamic diameter
NADPH Reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health
NOAEL No-observed-adverse-effect level
(human and animal toxicity)
NOEC No-observed-effect concentration
(environmental effects)
NOHS National Occupational Hazard Survey
RR Rate ratio (or Relative risk)
RTECS Registry of Toxic Effects of Chemical Substances
SBR Standardized birth ratio
SGOT Serum glutamic-oxaloacetic transaminase
SGPT Serum glutamic-pyruvic transaminase
TI Tolerable intake
TLV Threshold limit value
TRI Toxic Release Inventory (US EPA)
1. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, natural occurrence, and analytical methods
Boron is a naturally occurring element that is found in the form
of borates in the oceans, sedimentary rocks, coal, shale, and some
soils. It is widely distributed in nature, with concentrations of
about 10 mg/kg in the Earth's crust (range: 5 mg/kg in basalts to 100
mg/kg in shales) and about 4.5 mg/litre in the ocean.
The most important commercial borate products and minerals are
borax pentahydrate, borax, sodium perborate, boric acid, colemanite,
and ulexite. At the low concentrations and near-neutral pH found in
most biological fluids, monomeric B(OH)3 will be the predominant
species present (with some B(OH)4), regardless of whether the
boron source is boric acid or one of the borates. This is because
boric acid is a very weak acid (p Ka 9.15). Sodium perborate
hydrolyses to give hydrogen peroxide plus metaborate; consequently, it
may exhibit chemical and toxicological properties that are somewhat
different from those of the other borates.
Inductively coupled plasma (ICP) methods are preferred for the
analysis of the low levels of boron found in biological and
environmental samples; colorimetric methods must be used with caution.
1.1.2 Production, uses, environmental fate, and sources of exposure
Economic borate deposits are rare, occurring in arid regions of
Turkey, the USA, Argentina, Chile, Russia, China, and Peru. Total
world production of boron minerals -- mainly colemanite, ulexite,
tincal, and kernite -- was approximately 2 750 000 tonnes in 1994.
About 800 000 tonnes of commercial borate products, expressed as
B2O3, were manufactured from the boron minerals.
Major end uses for borate include insulation- and textile-grade
fibreglass, laundry bleach (sodium perborate), borosilicate glass,
fire retardants, agricultural fertilizers and herbicides (as a trace
element), and enamels, frits, and ceramic glazes, as well as a myriad
of miscellaneous applications.
Boron enters the environment mainly through the weathering of
rocks, boric acid volatilization from seawater, and volcanic activity.
Boron is also released from anthropogenic sources to a lesser extent.
Anthropogenic sources include agricultural, refuse, and fuel wood
burning, power generation using coal and oil, glass product
manufacture, use of borates/perborates in the home and industry,
borate mining/processing, leaching of treated wood/paper, and
sewage/sludge disposal. Many of these sources are difficult to
quantify.
Atmospheric emissions of borates and boric acid in particulate
and vapour form occur as a result of volatilization from the sea,
volcanic activity, and, to a lesser extent, mining operations, glass
and ceramics manufacturing, the application of agricultural chemicals,
and coal-fired power plants. Boron is not present in the atmosphere at
significant levels; however, the total amount present in the
atmosphere at any one time is significant owing to the huge volume of
the atmosphere. Based on their water solubility, borates would not be
expected to persist to a significant degree in the atmosphere.
Boron can be released into water and soil water through
weathering processes and, to a much smaller extent, through
anthropogenic discharges such as sewage outfalls.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water. The
extent of boron adsorption depends on the pH of the water and the
concentration of boron in solution.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported.
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
The octanol/water partition coefficient of boric acid has been
measured as 0.175, indicating a low bioaccumulation potential.
Laboratory experiments with aquatic organisms have confirmed this
potential. Plants accumulate boron; however, uptake is affected by the
pH of the soil solution, temperature, light intensity, and the
concentration of other elements (e.g. calcium and potassium). The
results of studies of boron accumulation in plants, insects, and fish
have shown that boron bioaccumulates in plants but does not biomagnify
in aquatic food-chains.
Boron occurs in soils at concentrations ranging from 10 to 300
mg/kg (average 30 mg/kg), depending on the type of soil, amount of
organic matter, and amount of rainfall. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron in surface water range widely, from 0.001 to as much as 360
mg/litre. However, mean boron concentrations for waters of Europe,
Pakistan, Russia, and Turkey are typically well below 0.6 mg/litre.
Concentrations of boron in water in Japan, South Africa, and South
America are generally below 0.3 mg/litre. Typical boron concentrations
in North American waters are below 0.1 mg/litre, with about 90% at or
below 0.4 mg/litre.
Boron accumulates in aquatic and terrestrial plants but does not
magnify through the food-chain. Concentrations of boron have been
shown to range between 26 and 382 mg/kg in submerged aquatic
freshwater plants, from 11.3 to 57 mg/kg in freshwater emergent
vegetation, and from 2.3 to 94.7 mg/kg dry weight in terrestrial
plants. Based on wet weights, boron concentrations in marine
invertebrates and fish are similar to the levels found in the exposure
media, between 0.5 and 4 mg/kg. The bioconcentration factor for two
freshwater fish species was found to be 0.3.
Boron concentrations in ambient air range from <0.5 to
approximately 80 ng/m3, with an average over the continents of
20 ng/m3.
Close similarity of boron concentrations in groundwater, fresh
surface water, and drinking-water indicates that boron is not removed
in the treatment of groundwater and fresh surface water used for
drinking-water.
Intakes of boron for humans are expected to be 0.44 µg/day from
ambient air, 0.2-0.6 mg/day from drinking-water, and 1.2 mg/day from
the diet. Average boron intake from the soil is considered to be 0.5
µg/day. A reasonable estimate of boron exposure from consumer products
is 0.1 mg/day.
1.1.3 Kinetics and biological monitoring
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in the blood
and body tissues of several mammalian species following
ingestion.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the no-observed-adverse-effect level
(NOAEL) for laboratory studies, reduce the uncertainty for risk
extrapolation between these two species.
1.1.4 Effects on experimental animals and humans
The data regarding developmental and reproductive toxicity show
that lower fetal body weight in rats is the critical effect. The NOAEL
for lower fetal body weight is 9.6 mg boron/kg body weight per day.
The lowest-observed-adverse-effect level (LOAEL), at which rats show
slight (approx. 5%) fetal body weight differences and rib anomalies,
is about 13 mg boron/kg body weight per day. As dose level increases,
the effects that are seen (and the doses at which they are seen) are:
a) further rib effects and testicular pathology in the rat (approx.
25 mg boron/kg body weight per day);
b) decreased fetal body weight and increased fetal cardiovascular
malformations in the rabbit, and severe testicular pathology in
the rat (approx. 40 mg boron/kg body weight per day);
c) testicular atrophy and sterility in the rat (approx. 55 mg
boron/kg body weight per day); and
d) reduced fetal body weight in the mouse (approx. 80 mg boron/kg
body weight per day).
Animal studies on mice and rats showed no evidence of
carcinogenicity of boric acid. Based on the lack of human data and the
limited animal data, boron is not classifiable as to its human
carcinogenicity.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data show that exposure is associated with short-term irritant effects
on the upper respiratory tract, nasopharynx, and eye. These effects,
however, appear to be short-term and reversible. The sole long-term
(7-year) follow-up study failed to identify any long-term health
effects, although a healthy worker effect cannot be entirely ruled out
given the rate of attrition (47%). Two descriptive studies assessed
fertility and secondary sex ratios in relation to exposure. Neither
study reported a detrimental effect on demonstrated fertility for its
select sample. Although an excess percentage of female births has been
suggested, the absence of statistical significance and attention to
other co-variates known to affect sex ratios warrants careful
interpretation of this finding. No studies have been identified that
assess the spectrum of reproductive outcomes, such as
time-to-pregnancy, conception delays, spontaneous abortions, and sperm
analyses in males. The role of other lifestyle or behavioural factors
in relation to health and fertility requires further study to identify
potentially sensitive populations and to evaluate reproductive effects
more fully.
1.1.5 Effects on organisms in the environment
Bacteria are relatively tolerant towards boron. Acute and chronic
effect concentrations range between 8 and 340 mg boron/litre, with
most values greater than 18 mg boron/litre. More sensitive are
protozoa. Tests with Entosiphon and Paramecium yielded 72-h
no-observed-effect concentrations (NOECs) and EC3 values between
0.3 and 18 mg boron/litre.
Boron is an essential micronutrient for cyanobacteria and
diatoms. Standard chronic tests with freshwater green algae resulted
in no-effect concentrations between 10 and 24 mg boron/litre.
Blue-green algae appear to be similar in sensitivity, with an 8-day
EC3 of 20 mg boron/litre.
Based on acute toxicity values, invertebrates are less sensitive
to boron than microorganisms. For several species, 24- to 48-h EC50
values ranged from 95 to 1376 mg boron/litre, with most values in the
100-200 mg boron/litre range. Chronic toxicity studies with
Daphnia magna gave NOECs ranging between 6 and 10 mg boron/litre.
Slightly lower NOEC values were obtained from laboratory and field
biocenosis studies. The 28-day laboratory study consisting of six
trophic stages yielded a NOEC of 2.5 mg boron/litre. Long-term outdoor
pond and field studies (not including fish) yielded NOECs up to 1.52
mg boron/litre.
Acute tests with several fish species yielded toxicity values
ranging from about 10 to nearly 300 mg boron/litre. Rainbow trout
(Oncorhynchus mykiss) and zebra fish (Brachydanio rerio) were the
most sensitive, providing values around 10 mg boron/litre.
The toxicity of boron to early life stages of fish has been
documented for several species in reconstituted water. Embryonic and
early larval stages of rainbow trout, largemouth bass (Micropterus
salmoides), channel catfish (Ictalurus punctatus), and goldfish
(Carassius auratus) were exposed to boron, as boric acid or borax,
from fertilization up to 8 days post-hatch in soft or hard water.
Neither water hardness nor the form of boron consistently affected
embryo-larval survival of fish. Rainbow trout was the most sensitive
species. The NOECs for rainbow trout ranged from 0.009 to 0.103 mg
boron/litre.
The effect of natural dilution water on boron toxicity was
determined by using surface waters collected from three locations,
with boron concentrations of 0.023, 0.091, and 0.75 mg/litre. No
adverse effects were determined up to 0.75 mg boron/litre.
Lowest-observed-effect concentrations (LOECs) ranged from 1.1 to 1.73
mg boron/litre. One test using deep (600 m) well-water, typically used
for aquatic toxicity tests, from a contract laboratory located in
Wareham, Massachusetts, USA, yielded a NOEC of >18.0 mg boron/litre.
Hence, reconstituted water exposures appeared to overestimate the
toxicity determined in natural waters, possibly as a result of
nutrient deficiency in the former.
Boron has been known since the 1920s to be an essential
micronutrient for higher plants, with interspecies differences in the
levels required for optimum growth. Boron plays a role in cell
division, metabolism, and membrane structure and function. Boron in
the form of borates occurs naturally in fruits, nuts, and vegetables.
There is a small range between deficiency and excess uptake (toxicity)
in plants. Boron deficiencies in terrestrial plants have been reported
in many countries. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions because of boron's
susceptibility to leaching. Boron excesses usually occur in soil
solutions from geologically young deposits, arid soils, soils derived
from marine sediments, and soils contaminated by pollutant sources,
such as releases from coal-fired power plants and mining operations.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field.
Mallard (Anas platyrhynchos) duckling growth was adversely
affected at dietary levels of 30 and 300 mg boron/kg, and survival was
reduced at 1000 mg/kg.
1.2 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency in terrestrial plants has been observed in many
countries throughout the world. There is a small range between
deficiency and toxicity in some plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental levels of boron
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre.
For the general population, the greatest boron exposure comes
from the oral intake of food. The mean daily intake of boron in the
diet is about 1.2 mg.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. More than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The tolerable intake (TI) of boron was set as 0.4 mg/kg body
weight per day. The allocation of the TI in various media should be
based on the exposure data of individual countries.
1.3 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
This chapter deals with the identity and physical and chemical
properties of the inorganic borates of importance in commerce, as well
as the analytical methods used to determine boron concentrations in
various media.
2.1 Identity
Elemental boron (B) is a member of Group IIIB of the periodic
table, along with aluminium, gallium, indium, and thallium. It has an
atomic number of 5 and a relative atomic mass of 10.81. Boron is never
found in the elemental form in nature. Its chemistry is complex and
resembles that of silicon (Cotton & Wilkinson, 1988). The Chemical
Abstracts Service (CAS), National Institute for Occupational Safety
and Health (NIOSH) Registry of Toxic Effects of Chemical Substances
(RTECS), and Hazardous Substances Data Bank (HSDB) numbers for boron
are 7440-42-8, ED7350000, and 4482, respectively.
The borates used most widely in commerce are listed in
approximate decreasing order of usage in Table 1, along with their
formulae and CAS numbers. Elemental boron is included, even though its
production is quite small. Throughout this document, the term "borax"
refers to disodium tetraborate decahydrate (see Table 1).
2.2 Physical and chemical properties
Elemental boron exists as a solid at room temperature, either as
black monoclinic crystals or as a yellow or brown amorphous powder
when impure. The amorphous and crystalline forms of boron have
specific gravities of 2.37 and 2.34, respectively. Boron exists as a
mixture of the 10B (19.78%) and 11B (80.22%) isotopes (Budavari et
al., 1989). Boron is a relatively inert metalloid except when in
contact with strong oxidizing agents. Boron dust exposed to air is
flammable and an explosion hazard. It also reacts violently when
ground with lead fluoride and silver fluoride (Lewis, 1992). Physical
and chemical properties of elemental boron and the most important
borates in commerce are provided in Table 2.
Sodium perborates are persalts that are hydrolytically unstable
because they contain characteristic boron-oxygen-oxygen bonds that
react with water to form hydrogen peroxide and stable sodium
metaborate (NaBO2.nH2O). This hydrolysis reaction is the basis of
the use of perborates as bleaches in detergents at high (70-100°C)
temperature. At lower washing temperatures (25-70°C), activators are
needed; these react with peroxide to give peracids, which are stronger
oxidants and give bleaching effects at lower temperatures.
Table 1. Boron compounds of commerce in approximate decreasing order
of usagea
Substance Formula CAS No.
Borax pentahydrate (disodium Na2[B4O5(OH)4].3H2O 3754418
tetraborate pentahydrate) (Na2B4O7.5H2O)
Borax (disodium tetraborate Na2[B4O5(OH)4].8H2O 1303-96-4
decahydrate) (Na2B4O7.10H2O)
Ulexite NaCa[B5O6(OH)6].5H2O 1319-33-1
(Na2O.2CaO.5B2O3.16H2O)
Colemanite Ca[B3O4(OH)3].H2O 1318-33-8
(2CaO.3B2O3.5H2O)
Sodium perborate tetrahydrate Na2[B2O4(OH)4].6H2O 10486-00-7
(NaBO3.4H2O)
Sodium perborate monohydrate Na2[B2O4(OH)4] 10332-33-9
(NaBO3.H2O)
Boric acid B(OH)3 10043-35-3
(H3BO3)
Anhydrous borax Na2B4O7 (amorphous) 1330-43-4
(disodium tetraborate)
Boron oxide B2O3 (amorphous) 1303-86-2
Boronb B 7440-42-8
a US EPA (1991); ATSDR (1992); Culver et al. (1994b).
b Produced in small quantities.
Table 2. Physical and chemical properties of elemental boron and the most important borates in commercea
Substance Relative Colour % boron Relative Water solubility Melting point Boiling point
molecular density (°C) (°C)
mass
Borax pentahydrate 291.35 White 14.85 1.81 3.6 g/100 g @ 20 °C 742 -
Borax 381.37 Colourless 11.34 1.73 5.92 g/100 g @ 25 °C 75, decomposes -
Ulexite 810.6 White 13.33 1.62 Slightly soluble Decomposes -
Colemanite 411.1 White 15.78 2.42 Slightly soluble Decomposes -
Sodium perborate
tetrahydrate 153.9 White 7.03 1.73 23 g/litre @ 20 °C Decomposes -
Sodium perborate
monohydrate 99.8 White 10.83 - 15 g/litre @ 20 °C Decomposes -
Boric acid 61.84 Colourless 17.48 1.435 @ 15 °C 63.5 g/litre @ 30 °C 169 -
Anhydrous borax 201.22 White 21.49 2.367 2.5556 g/100 g @ 25 °C 741 1575
Boron oxide 69.62 Colourless 31.06 2.46 Slightly soluble 450 1860
Boron 10.81 Black crystal or
yellow-brown
amorphous 100 2.3 Insoluble 2300 approx. 3500
a Muetterties (1967); Windholz et al. (1983); Weast et al. (1985); ACGIH (1991); ATSDR (1992); Lewis (1993); US NLM (1993);
Culver et al. (1994b).
Boric acid is a very weak acid, with a p Ka of 9.15, and
therefore boric acid and the sodium borates exist predominantly as
undissociated boric acid [B(OH)3] in dilute aqueous solution below pH
7; above pH 10, the metaborate anion B(OH)4 becomes the main
species in solution. Between pH 6 and pH 11 and at high concentration
(>0.025 mol/litre), highly water soluble polyborate ions such as
B3O3(OH)4, B4O5(OH)42, and B5O6(OH)4 are formed.
The chemical and toxicological properties of borax pentahydrate,
borax, boric acid, and other borates are expected to be similar on a
mol boron/litre equivalent basis when dissolved in water or biological
fluids at the same pH and low concentration. Boric oxide will exhibit
properties identical to those of boric acid, as it is an anhydride
that will hydrolyse to give boric acid. Sodium perborate monohydrate
and tetrahydrate hydrolyse to give hydrogen peroxide and borate. Thus,
they are oxidants and may have chemical and toxicological properties
that are different from those of the other borates.
The chemical properties of sodium metaborate differ from those of
the other sodium borates, in that the metaborate has a much higher
solubility and alkalinity in aqueous solution. Thus, the solubility in
water at 20°C is 41.9 parts sodium metaborate octahydrate (compared
with 4.7 for borax) per hundred parts saturated solution by weight.
The pH of an aqueous solution of the metaborate at 20°C ranges from
10.5 at 0.1% w/w to 12.0 at 18% w/w (compared with pH 9.24 for borax
over a wide range of concentrations).
2.3 Conversion factors
2.3.1 Conversion factors of ppm and mg/m3 for boron
1 ppm = 0.4421 mg/m3
1 mg/m3 = 2.262 ppm
2.3.2 Conversion factors for boron compounds to boron
dose of boric acid × 0.175 = equivalent dose of boron
dose of borax × 0.113 = equivalent dose of boron
dose of anhydrous borax × 0.215 = equivalent dose of boron
dose of sodium perborate tetrahydrate × 0.070 = equivalent dose
of boron
dose of sodium perborate monohydrate × 0.108 = equivalent dose of
boron
dose of metaboric acid × 0.247 = equivalent dose of boron
2.4 Analytical methods
Analyses of environmental and biological samples for boron
content utilize a variety of preparative methods (see Table 3).
Table 3. Preparative methods for analysing boron content in
environmental and biological samples
Media Extraction method Reference
Biological Acid digestion with:
Microwave Pennington et al. (1991)
Dry ashing Wilkner (1986)
Wet ashing Kowalenko (1979)
Banuelos et al. (1992)
Low temperature, wet ashing Hunt & Shuler (1989)
Freeze drying Iyengar et al. (1990)
Smith et al. (1991)
Soil Hot water solubility Odom (1980)
Cumakov (1991)
Water Liquid-liquid extraction from
acidified solutions into
chloroform Aznarez et al. (1985)
Ion exchange column Sekerka & Lechner (1990)
The preferred method for analysis of boron in bone, plasma, and
food is inductively coupled plasma atomic emission spectroscopy
(ICP-AES) (Hunt, 1989). It is also used for tumour, blood, liver,
skin, and cell suspensions (Barth et al., 1991). It also has been used
for wastewater (Huber, 1982) and fish tissues (Hamilton & Wiedmeyer,
1990). Detection limits range from 0.005 to 0.05 mg boron/litre in the
solution analysed.
Inductively coupled plasma mass spectroscopy (ICP-MS) is used to
measure boron concentrations in plant, rat, and human samples. Isotope
ratios (10B/11B) can be measured accurately (Vanderpool et al.,
1994). Using direct nebulization, ICP-MS can give a detection limit of
1 ng/g in human blood, human serum, orchard leaves, and total diet
(Smith et al., 1991).
ICP-MS is the most widely used non-spectrophotometric method for
analysis of boron, as it uses small volumes of sample, is fast, and
applies to a wide range of materials (fresh and saline water, sewage
wastewater, soils, and plant samples, as well as the biological
materials mentioned above). Interferences are minimal or can be
removed (Gregoire, 1990). ICP-MS can detect boron down to 0.15
µg/litre.
The ability to measure the boron isotope ratio accurately allows
studies starting with pure 10B compounds and following the isotopic
dilution in biological systems. This is particularly useful, as no
stable radioactive boron isotopes usable as tracers exist. A number of
boron compounds made with nearly isotopically pure 10B are available
for such studies.
When expensive ICP equipment is not available,
colorimetric/spectrophotometric methods can be utilized. However, many
of these methods are subject to interference and should be used with
caution; they should also preferably be calibrated against an ICP
method.
Azomethine-H has been used to analyse boron in environmental
water samples and is very sensitive, with a detection limit of 0.02
mg/litre (Lopez et al., 1993). The well-known curcumin method is
subject to interference by nitrate, chloride, and fluoride but is
claimed to be applicable to samples with 0.1-1 mg boron/litre (Black
et al., 1993).
A simple, sensitive spectrophotometric method for determination
of boron in soils, plant materials, and water uses Alizarin Red S but
is also subject to interference (Garcia-Campana et al., 1992). Flow
injection analysis utilizing the sorbitol/borate complex and Methyl
Orange indicator for eye lotion samples has a detection limit of
0.02 mg/litre (Nose & Zenki, 1991).
Another method of analysis of boron uses neutron activation and
mass spectrometric analysis. Mass spectrometric assay of 3He from
decay of tritium produced by thermal neutron irradiation of boron to
give 4He has been described by Clarke et al. (1987a). The method,
useful for trace levels of boron in blood and other biological
samples, can detect 108 g boron/g of sample (Clarke et al., 1987b).
Iyengar et al. (1990) used this method to determine boron in citrus
leaves, human erythrocytes, and food items, all with freeze-dried
samples.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Boron, in the form of various inorganic borates, is widely
distributed in low concentrations throughout nature. It constitutes
about 10 mg/kg of the Earth's crust, ranging from 5 mg/kg in basalts
to 100 mg/kg in shales (Woods, 1994). The majority of the boron
resides in the ocean, at an average concentration of about 4.5
mg/litre (Weast et al., 1985). Economic deposits of borate minerals
are rare and are usually found in arid desert regions with a
geological history of volcanic and/or hydrothermal activity (Mellor,
1980). Major world deposits are found in Turkey, the USA, Argentina,
Russia, Chile, China, and Peru (Culver et al., 1994b).
The most abundant boron mineral is tourmaline, an aluminium
borosilicate that contains about 3.1% boron (Muetterties, 1967). It is
not a practicable source of usable boron, as it is widely distributed
as a minor component of rocks. Economic borate minerals include
tincal, kernite, colemanite, and ulexite.
Natural sources of borate released to air are the oceans
(largest), volcanoes, and geothermal steam (Graedel, 1978). Natural
weathering of clay-rich sedimentary rocks on land surfaces accounts
for a large proportion of the boron mobilized into soils and the
aquatic environment, amounting to some 360 000 tonnes of boron
annually (Bertine & Goldberg, 1971). Although few data are available
for quantifying boron released from industrial sources, natural
weathering and seawater evaporation are considered greater sources
than industrial emissions (see chapter 4).
3.2 Mining and production
The total world production of boron minerals in 1994 was
approximately 2 750 000 tonnes (Lyday, 1996). The main commercial
borate minerals are colemanite, kernite, ulexite, and tincal.
Approximately 800 000 tonnes of commercial borate products, expressed
as B2O3, were manufactured from boron minerals in 1994. The two
largest producers are the USA and Turkey. Further mining and
production facilities exist in Argentina, Bolivia, China, Chile, Peru,
and Russia (Lyday, 1996). Most US production of borates occurs in
California, where colemanite, ulexite, tincal, kernite, and brines are
processed. These minerals are also processed elsewhere in the world,
as are ascharite, hydroboracite, datolite, etc.
Disodium tetraborate (borax) containing 5 or 10 molecules of
water is produced mainly from sodium-containing borate ores. The mined
ore is crushed and ground before dissolution in a hot recycled aqueous
solution containing some borax. Insoluble gangue (clay particles)
present in the hot slurry is separated off to produce a clear
concentrated borax solution. Evaporative cooling of this solution to
selected temperatures results in crystallization of the desired
products, which are then separated from the residual liquor and dried
(personal communication from Borax US to the IPCS, 1995).
Boric acid is produced mainly from sodium- or calcium-containing
borate ores. The mined ore is crushed and ground before being reacted
with sulfuric acid in the presence of a hot aqueous recycled liquor
containing some boric acid. The resultant slurry contains insoluble
gangue and either calcium or sodium sulfate by-product. After
separation of unwanted insoluble gangue, recovery of the boric acid
product is similar to that for borax (personal communication from
Borax US to the IPCS, 1995).
3.3 Uses and release
The end uses of boron minerals and of borate products are
diverse. Estimated amounts of borate consumed in the USA for the major
end uses in 1992 are listed in Table 4 (Lyday, 1993). Partial data for
Europe are also included (ECETOC, 1997). It should be noted that
vitreous products such as fibreglass, borosilicate glass, and enamels,
frits, and glazes are not significant sources of potential human
exposure, because the boron is tied up tightly in the glassy
structure. All of the boron from the sodium perborate contained in
detergents ultimately enters the wastewater stream.
Table 4. Estimated amount consumed (as B2O3) for boric acid, borates,
and boron minerals in the USA in 1992a and in Europe in 1993b
Use Consumption (tonnes)
USA Europe
Insulation-grade fibreglass 129 000 44 600c
Textile-grade fibreglass 78 500 27 100c
Soaps and detergents 38 600 142 500
Borosilicate glass 34 400 12 200
Fire retardants 13 400 -d
Agriculture 11 100 -d
Enamels, frits, and ceramic glazes 9 300 3 500
Metallurgy 3 700 -d
Nuclear applications 900 -d
a Lyday (1993).
b ECETOC (1997).
c Does not include minerals.
d No data.
The average market shares for the USA, Europe, and Japan in 1992
were about 23% (fibreglass), 17% (detergents), 11% (enamels/glazes),
and 11% (glass) for major end uses (personal communication from Borax
US to the IPCS, 1995).
Other minor uses include cosmetics and pharmaceuticals (as a pH
buffer), boron neutron capture therapy (for cancer treatment), and
pesticides (personal communication from Borax US to the IPCS, 1995).
The cancer treatment application utilizes a boron compound made with
all 10B isotope, which preferentially accumulates in tumour versus
normal tissue (Barth & Soloway, 1994). Subsequent irradiation of the
patient with thermal neutrons produces 7Li plus alpha particles. The
latter have a destructive path length of about the diameter of a cell,
thereby selectively destroying the cancer. Research in this field is
being pursued in Japan and, to a lesser extent, in the USA.
Boron enters the environment mainly through the weathering of
rocks, volatilization from seawater, agricultural, refuse, and fuel
wood burning, power generators (coal and oil combustion), the
manufacture of glass products and other boron-containing compounds,
the industrial and household use of boron-containing products
(including soaps and detergents), borax mining and processing,
leaching from treated wood and paper, geothermal releases, chemical
plants, and sewage and sludge disposal (Versar, Inc., 1975; Larsen,
1988; ATSDR, 1992; Anderson et al., 1994a). Boron is not present in
the atmosphere at significant levels because of its low volatility,
but the total amount in the air is very significant owing to the huge
volume of the atmosphere (see chapter 4).
Boron releases to water occur from municipal sewage containing
perborates from detergents and also in runoff from areas using
boron-containing herbicides or fertilizers (Waggott, 1969; Nolte,
1988; Butterwick et al., 1989). Boron levels in sewage sludge from 23
cities in the USA ranged from 7.1 to 53.3 mg/kg (Mumma et al., 1984).
It has been estimated that 11 800 tonnes of boron are released yearly
in coal fly ash from coal combustion (Bertine & Goldberg, 1971).
Versar, Inc. (1975) estimated US boron air emissions as 10 500 tonnes
annually from mining, processing, and coal burning. Few quantitative
data on boron releases are available, because boron is not included in
the US Environmental Protection Agency (EPA) Toxic Release Inventory
(TRI) (ATSDR, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Boron is not present in the atmosphere at significant levels
(Sprague, 1972), but the total amount in the air is very significant
owing to the huge volume of the atmosphere. Borates exhibit low
volatility; consequently, boron would not be expected to be present to
a significant degree as a vapour in the atmosphere. Atmospheric
emissions of borates and boric acid in particulate (<1-45 µm in size)
or vapour form occur as a result of volatilization of boric acid from
the sea, volcanic activity, mining operations, glass and ceramics
manufacturing, the application of agricultural chemicals, and
coal-fired power plants. As a particulate, boron would be removed from
the atmosphere either by dry deposition or by wet deposition because
of its relatively high water solubility (Versar, Inc., 1975; Gladney
et al., 1978). Based on analogy with data on general particulate
residence times (Nriagu, 1979), the half-life of airborne boron
particles is expected to be on the order of days, depending on the
size of the particles and atmospheric conditions.
Seawater evaporation is the biggest contribution to boron in air.
The global removal of boron from marine sources has been estimated at
between 800 000 and 4 000 000 tonnes/year and compares with an
estimate of 2 000 000-7 200 000 tonnes/year for the total global
release (Anderson et al., 1994a). Anderson et al. (1994a) estimate
that the total anthropogenic release of boron to the atmosphere is
between 180 000 and 650 000 tonnes/year (9-27% of the total global
release). In spite of all these releases, the atmospheric
concentration of boron is low (mean boron concentrations range from
<0.5 to approximately 80 ng/m3).
4.1.2 Water and sediment
Waterborne boron may be adsorbed by soils and sediments.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water (Rai et
al., 1986). The extent of boron adsorption depends on the pH of the
water and the concentration of boron in solution. The greatest
adsorption is generally observed at pH 7.5-9.0 (Waggott, 1969; Keren &
Mezuman, 1981; Keren et al., 1981).
Simsiman et al. (1987) conducted a field investigation to
determine the leachability and groundwater transport of major and
minor elements, including boron, from ash disposal ponds at the
coal-fired Columbia Power Plant in Portage, Wisconsin, USA. The site
is underlain by sands interspersed with lenses of silt and clay
overlying sandstone (10-20 m below the surface). The soil pH ranged
from 7.1 to 8.8, and the organic matter content was 0.2-0.8%. Boron
plumes were identified in the groundwater at least 120 m down-gradient
of the ponds. The boron plume from the secondary fly ash pond extended
into the sandstone (26-30 m), which suggested rapid downward
infiltration of the leachate. However, attenuation of the boron
occurred at some point between the pond and the aquifer, based on
observed decreases of approximately 40% in the boron concentration.
Barber et al. (1988) monitored the extent of groundwater
contamination emanating from sewage disposal beds near Falmouth,
Massachusetts, USA, by mapping the distribution of boron. Under the pH
conditions of the aquifer (pH 5-7), dissolved boron occurred as the
neutral undissociated orthoboric acid species, which should be
transported with little sorption. The boron plume was 3500 m long,
1100 m wide, and 30 m deep during sampling in 1985.
Deverel & Millard (1988) demonstrated that boron is present in
the oxidized, alkaline, shallow groundwater of the western San Joaquin
Valley, California, USA. Boron was found to be geochemically mobile,
with concentrations significantly correlated (alpha = 0.05) with
groundwater salinity in the alluvial-fan and basin-trough geological
zones.
Corwin (1986) speculated that the adsorption of boron on
sediments provides a means by which boron may persist for long periods
of time in aquatic systems. The desorption (or leaching) of boron from
the sediments would provide a long-term source until a system
equilibrium could be reached, based on differences in the
concentrations of boron in the water column and in the sediment both
at the sediment-water interface and with increasing depth below the
interface. The primary desorption mechanism would be diffusion.
Boron levels (as admixed borate salt) as high as 1900 mg/kg have
been reported in coal fly ash. Cox et al. (1978) reported that
approximately 50% of the boron in 0.5-g samples of fly ash was leached
from the ash into water within 2 h; the leaching rate increased with
increased acidity. In a boron dissolution study, Hollis et al. (1988)
observed that 60% of the boron was removed from 6 g of ash after three
extractions at pH 9, whereas 100% was removed at pH 6 after two
extractions. In a long-term (2-year) leachability study, Dudas (1981)
observed that boron was readily leached, probably as a result of the
moderate solubility of borate salts. Consequently, the disposal of
coal fly ash in lagoons could provide a source of boron contamination
in aquatic systems.
4.1.3 Soil
Boron is adsorbed onto the surfaces of soil particles, with the
degree of adsorption depending on the type of soil, pH, salinity,
organic matter content, iron and aluminium oxide content, iron- and
aluminium-hydroxy content, and clay content (Sprague, 1972). Boron
adsorption can vary from being fully reversible to irreversible (Rai
et al., 1986; Shani et al., 1992). The lack of reversibility may be
the result of solid-phase formation on mineral surfaces (Rai et al.,
1986) and/or the slow release of boron by diffusion from the interior
of clay minerals (Griffin & Burau, 1974).
At acidic pH, boron exists in solutions in the form of
undissociated boric acid; at alkaline pH, it is present as a borate
ion, which reaches maximum adsorption at pH 8.5-9 (Sprague, 1972).
Sims & Bingham (1967) reported that hydroxy iron and aluminium
compounds, present as interlayer-contained materials, coatings on
individual particles, or impurities, resulted in increased boron
retention in layer silicates. Rhoades et al. (1970) observed that in
the silt and sand fractions of arid-zone soils, the sites of boron
adsorption are the magnesium-hydroxy clusters and coatings found on
the weathering surfaces of ferromagnesian minerals and micaceous layer
silicate minerals. Marzadoori et al. (1991) reported that the amount
of boron adsorbed by soil was considerably greater after the organic
matter had been removed from the soil. An increase in
oxalate-extractable iron and aluminium in the soil was observed after
destruction of the organic matter. It was suggested that a portion of
the iron and aluminium oxides as well as other possible adsorption
sites are generally coated or occluded by organic matter and become
active only after its removal.
Couch & Grim (1968) studied the uptake of boron in illite clays
and determined that uptake was enhanced at higher boron soil solution
concentrations in direct relationship to the salinity and temperature
of the solution. Following 30 days of treatment in soils containing
1 mol boric acid/litre at salinities of 0.1, 1.0, or 3.0 mol
CaCl2/litre, boron levels increased by 56, 70, and 98 mg/kg,
respectively. Treatment of illites at 1 mol boric acid/litre for 30
days at 60°C yielded 55 mg boron/kg, whereas the same concentration at
215°C for 12 h yielded 180 mg boron/kg. The investigators also
observed a direct relationship between the specific surface area of
the clay types and boron uptake. Boron uptake in the illite clays was
characterized as initially rapid adsorption, followed by diffusion of
boron into the clay structure, requiring several months to reach
equilibrium.
Several investigators have used either the Langmuir or the
Freundlich adsorption equation to describe the relationship between
adsorption and desorption of boron in soils. The Langmuir equation is
based on the total adsorptive capacity of the soil, the concentrations
of adsorbed boron and boron in solution, and an adsorption equilibrium
constant (K), which represents the bonding energy of the soil. Using
this equation, Hatcher & Bower (1958) determined that an equilibrium
exists between boron in solid and liquid phases. At soil pH values of
6.6-7.7, the predominant boron species in the aqueous phase is
undissociated boric acid, and the principal mechanism of retention is
by reversible, molecular adsorption, which is non-uniform based on the
energy characteristics of the bonding sites. These investigators also
showed that boron desorption was reversible; in other words, boron
that leached into the soil solution could again be adsorbed. However,
based on the Freundlich adsorption isotherms, Elrashidi & O'Connor
(1982) observed incomplete adsorption reversibility in some soils from
New Mexico, USA, at higher boron concentrations.
Biggar & Fireman (1960) determined that the fixation of boron in
soils occurs by one of three mechanisms: physical (molecular)
adsorption, in which the boron is held to the surface of the soil by
van der Waals bonds; anion exchange; or chemical precipitation.
Chemical adsorption involves ionic and covalent bonding. The
investigators speculated that the initial adsorption is probably
molecular in nature, followed by the formation of surface compounds
that result in an increase in adsorption sites, particularly at higher
boron concentrations in the soil solution. At higher concentrations,
chemical bonding of borate ions with hydroxyl ions on the soil surface
results in boron fixation to soluble aluminium, silicon, and iron.
This same mechanism (chemisorption) was observed by Couch & Grim
(1968) for the uptake of borate ions to clay mineral surfaces. The
presence of calcium ions, drying, and high pH values will tend to
increase the amount of fixed boron. Wetting and drying of the soil
will increase the maximum adsorption capacity and bonding energy of
the soil for boron.
Many of the surface boron compounds initially formed by
adsorption mechanisms may be unstable and leached by water. However,
as a result of the equilibrium that exists between adsorbed and
dissolved boron in soils, the adsorbed boron may act as a buffer,
impeding the leaching of excess boron from soils. Wierenga et al.
(1975) conducted a study to determine the downward movement of boron
through a sandstone formation in New Mexico, USA. The experimental
dispersion coefficient was calculated as 1.06 cm2/day, primarily
resulting from diffusion. Assuming an average annual rainfall of 20
cm/year and an average annual recharge of 10% of the annual
precipitation, the investigators determined that it would take 500
years for the boron front to reach a depth of 35 m into the sandstone.
As the groundwater table at this site is at 86 m, Wierenga et al.
(1975) calculated that it would take 1628 years for boron, at a
concentration one-half that of the surface concentration, to reach the
groundwater. A 10-fold increase in annual recharge from precipitation
would reduce the transit time by one-tenth.
Bingham et al. (1971) concluded that the single most important
property of soil that will influence the mobility of boron is the
abundance of amorphous aluminium oxide. Gerritse et al. (1982) showed
that the mobility of boron in sludge-amended sandy and sandy loam
soils was increased as a result of complexation with dissolved organic
compounds, high ionic strengths of the soil solutions, and other
factors.
4.1.4 Vegetation and wildlife
Hingston (1986) investigated the components of the biogeochemical
cycle for boron in two eucalypt forests. The importance of the
biological component of the cycle was indicated by the amount of boron
stored within trees (2.1 and 2.5 kg/ha for the two forests) compared
with the amount of extractable boron in the soils to a depth of 1 m (2
and 7 kg/ha), and by the highly significant correlations between
hot-water-soluble boron and organic carbon for these soils.
4.2 Transformation
4.2.1 Biotransformation
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported in the
literature (personal communication from Borax US to the IPCS, 1995).
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
4.2.2 Abiotic transformation
Inorganic borates such as boric acid, boric oxide, and sodium
borates are stable, except for dehydration at high temperatures.
Organoboron compounds are sufficiently uncommon in nature to be
irrelevant to this document. In aqueous media, the chemical speciation
of boron-oxygen compounds is pH and concentration dependent.
4.2.2.1 Air
No information was available in the current literature concerning
the photolysis, oxidation, or hydrolysis of boron-oxygen compounds in
the atmosphere. The small amount of boron in air is assumed to be in
the form of boric acid.
4.2.2.2 Water
In natural waters, boron exists primarily as undissociated boric
acid with some borate ions. As a group, the boron-oxygen compounds are
sufficiently soluble in water to achieve the levels that have been
observed (Sprague, 1972; see chapter 5).
In seawater, inorganic boron content generally bears a linear
relationship to the amount of chloride ion present; a ratio of
0.000 24 g boron/g of total halogen expressed as chloride ion has been
calculated (Mellor, 1980). Byrne & Kester (1974) demonstrated that
weakly dissociated boric acid is the predominant species but also that
there are weakly associated ion pair neutral and positively charged
borate complexes of sodium, magnesium, and calcium. The metaborate ion
will undergo rapid hydrolysis in seawater to form the borate ion and
the weakly dissociated boric acid. Noakes & Hood (1961) concluded that
organically bound boron contributes very little, if any, to the total
boron content of seawater. Boron associated with organic matter was
found to vary with oxygen content, with the lowest concentrations
occurring in the minimum oxygen zone. Mance et al. (1988) described
boron as a significant constituent of seawater, with an average
concentration of 4.5 mg/litre.
Boric acid is a very weak acid, with a p Ka of 9.15; in fresh
water, therefore, boric acid and sodium borates exist predominantly as
undissociated boric acid below pH 7, but the metaborate anion becomes
the main species in solution above pH 10. Between these two pH bands,
there is also a characteristic presence of complex polyborate anions
in solution when the concentration is increased, leading to enhanced
solubility.
4.2.2.3 Soil
Borates as such cannot degrade, but borate complexes with organic
matter or sod mineral surfaces can be altered by water leaching or pH
change.
4.2.3 Bioaccumulation
4.2.3.1 Aquatic organisms
Highly water soluble materials are unlikely to bioaccumulate to
any significant degree, and borate species are all present essentially
as undissociated boric acid at neutral pH. The octanol/water partition
coefficient for boric acid has been measured as 0.175 (Barres, 1967),
indicating low bioaccumulation potential.
Thompson et al. (1976) studied boron uptake in two saltwater
species, juvenile Pacific oysters (Crassostrea gigas) and
underyearling sockeye salmon (Oncorhynchus nerka), in
continuous-flow systems with 95% solution replacement every 6 h.
Oysters (30/tank) were exposed to two boron levels (1 mg/litre above
background and 10 mg/litre above background) for 47 days, and salmon
(3/tank) were exposed only to the higher concentration for 21 days.
Control tanks received only seawater inflow. The background
concentration of boron in seawater in this study was approximately
3.98 mg/litre. The oysters were sampled on days 0, 8, 16, 36, and 47
of exposure. After this time, the remaining oysters were maintained in
seawater alone for another 24 days and then analysed for boron uptake.
Following the 21-day exposure period, the sockeye salmon were killed
and the boron concentration was determined in gill, liver, kidney,
muscle, and bone tissue. For both species, the tissue levels
approximated the boron concentrations in the test water, indicating
that these species take up boron in relation to its availability.
In the oyster, tissue concentrations returned to background
levels (3.67-4.13 µg/g) by the 71st day of the study, indicating a
fairly rapid clearance of boron with no evidence of long-term
retention. Boron concentrations in sockeye salmon tissues in normal
seawater ranged from 0.5 to 1.5 µg/g wet weight, with concentrations
increasing from muscle to gill and kidney, to liver, and to bone.
Boron levels were elevated in the bone and kidney tissue (5.9-17 µg/g
wet weight and 4.5-11.9 µg/g wet weight, respectively) of the exposed
salmon; however, they were not significantly different from test water
levels. Consequently, there was no evidence for active bioaccumulation
of boron in these species (Thompson et al., 1976).
Suloway et al. (1983) studied the bioaccumulation potential of
the components of coal fly ash extract in fathead minnows
(Pimephales promelas) and green sunfish (Lepomis cyanellus). Five
fish of each species were exposed for 30 days to fly ash extracts
containing boron at concentrations ranging from 1.23 to 91.7 mg/litre.
Whole-body concentrations of boron ranged from 1.16 to 4.15 µg/g in
the exposed fathead minnows and from 1.08 to 4.62 µg/g in the exposed
green sunfish. The reported bioconcentration factor was 0.3 for both
species. These results are consistent with those described above and
indicate that boron does not bioaccumulate significantly in fish.
4.2.3.2 Terrestrial plants
Eaton (1944) investigated growth reaction and boron accumulation
characteristics of plants grown in outdoor sand culture beds where
cultures were supplied with nutrient solutions containing differing
concentrations of boron. The concentrations of boron ranged from 58 to
1804 µg/g dry weight in the leaves of plants grown in 5 mg boron/litre
and from 209 to 3875 µg/g dry weight in the leaves of plants grown in
25 mg boron/litre. The boron concentrations were generally lower in
roots, stems, and fruits than in the leaves. This is consistent with
the fact that boron is absorbed from the soil solution by the roots
and passively carried in the transpiration stream to the leaves, where
the water evaporates and the boron accumulates. The absorption into
the roots usually occurs as active transport against a concentration
gradient (the concentration in the soil solution is generally lower
than in the root tissues); therefore, an expenditure of energy is
required. However, at higher boron soil solution concentrations, which
are toxic to some plant species, the mechanism of uptake is passive
diffusion (Bingham et al., 1970). Boron is relatively immobile in the
phloem; consequently, the accumulated boron does not move out of the
leaf tissues and into the fruit and other tissues (Kohl & Oertli,
1961).
Several factors affect the uptake of boron, including the pH of
the soil solution, temperature, light intensity, and the concentration
of other elements (e.g. calcium and potassium). Uptake is reduced by a
factor of four as soil pH increases from 4 to 9 (Bingham et al., 1970)
and increased by an increase in light intensity (Tanaka, 1966); the
rate of boron absorption rapidly increases at temperatures ranging
from 10 to 30°C and is sharply reduced above 35°C (Reisenauer & Cox,
1971).
4.2.3.3 Birds
Pendleton et al. (1995) exposed adult male mallard ducks to a
dietary concentration of 1600 mg boron/kg for up to 48 days.
Equilibrium levels were reached between days 2 and 15. Boron
concentrations were highest in the blood, followed by the brain and
liver. Boron was rapidly eliminated, with few detectable residues
after 1 day on a "clean diet." The presence of arsenic (300 mg/kg) in
the diet slowed the accumulation of boron.
4.3 Ultimate fate following use
No information was available in the current literature concerning
the disposal of boron or boron compounds. Information was located,
however, regarding the reclamation and revegetation of coal combustion
products (i.e. ash) that contain high concentrations of metals,
including boron. Although the chemical and physical properties of coal
ash tend to be detrimental to plant growth and establishment,
additions of fertilizer and manure provide a more suitable medium
(Schwab et al., 1991). Plant establishment on the site is only the
first phase of the reclamation process. It is also necessary to ensure
that leachate from the ash does not contaminate the surface water and
groundwater in the immediate region and that uptake of metals in the
plant materials does not result in metal concentrations that are toxic
to livestock or wildlife. In a study of the revegetation of several
ash disposal sites in Kansas, USA, Schwab et al. (1991) noted
variations in plant uptake of boron from coal ash owing to differences
in ash type, plant species, and ash treatment. Boron contained in
detergents after use releases to the municipal sewage system. It
should be noted that boron is not removed by the usual water treatment
processes. Landfill will tend to be the ultimate fate of many boron
products.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Boron, as boric acid, is released into the atmosphere during
volcanic eruptions; however, most is captured by the oceans
(Muetterties, 1967). Coal-fired power plants and agricultural burning
are major sources of atmospheric boron contamination; at least 30% of
boron in coal is lost in this manner (Eisler, 1990). Nevertheless,
boron does not appear to be present in ambient air at significant
levels (Sprague, 1972), presumably because of rapid transport to other
media (see section 4.1.1). Although the concentration is low, the
atmosphere carries a significant amount of boron as boric acid vapour.
Mean boron concentrations in emissions from active volcanic sites
range from <2.5 to 31.4 µg/m3 for gaseous boron and are below
4 µg/m3 for particulate boron. Volcanic lake fumes (El Chichon,
Mexico) contained mean boron concentrations of up to 8.5 µg/m3 for
particulate boron and up to 16.1 µg/m3 for gaseous boron (Anderson et
al., 1994a).
Anderson et al. (1994a) monitored atmospheric concentrations of
boron at continental, coastal, and remote marine sites. Mean
particulate boron concentrations ranged from 1.8 to 12.2 ng/m3, from
2.4 to 3.7 ng/m3, and from <0.5 to 2.8 ng/m3 for the three types of
site, respectively. Mean gaseous boron concentrations ranged from
<0.5 to 20.7 ng/m3, from 3.5 to 82.8 ng/m3, and from 0.6 to
25 ng/m3, respectively. Anderson et al. (1994a) assumed 90% of boron
in the air is gaseous and 10% is in particulate form.
5.1.2 Water
5.1.2.1 Groundwater
Naturally occurring boron is present in groundwater primarily as
a result of leaching from rocks and soils containing borates and
borosilicates (i.e. local geology). Concentrations of boron in
groundwater throughout the world range widely, from <0.3 to
>100 mg/litre. Boron levels in European groundwaters are presented in
Table 5. In general, concentrations of boron were greatest in southern
Europe (Italy, Spain, but not Greece) and least in northern Europe
(Denmark, France, Germany, Netherlands, and the United Kingdom). For
Italy and Spain, mean boron concentrations ranged from 0.5 to
1.5 mg/litre. Concentrations ranged up to approximately 0.6 mg
boron/litre in the Netherlands and United Kingdom, and levels in
approximately 90% of samples in Denmark, France, and Germany were
found to be below 0.3, 0.3, and 0.1 mg boron/litre, respectively.
Table 5. Concentrations of boron in European groundwatera
Country Area No. of Boron concentration
samples (mg/litre)
Denmark 525 92.2% < 0.3
7.4% > 0.3
0.4% > 1.0
France 716 99.5 < 0.3
0.5 > 0.3
Germany Baden-Wurttemberg 2574 89% < 0.1
10.7% > 0.1
0.3% > 1.0
Lower Saxony 188 96% < 1.0
4% > 1.0
Greece Patras 10 100% < 0.1
Halkidiki 3 2.3-5.4
Italy North of Rome 423 Mean = 1.0
Sicily 18 Mean = 1.5
Paglia 102 Mean = 0.75
Netherlands Inland 0.08-0.6
Spain Valencia 21 Mean = 0.64
Almeria 17 Mean = 0.98
Murcia 15 Mean = 0.51
United Kingdom London 21 0.02-0.54
Northumbria 164 Mean = 0.31
Dumfries and Galloway Mean = 0.04
Permo-Triassic
(Scotland)
a ECETOC (1997).
Groundwater contaminated with excessive concentrations of boron
from surface water recharge has been noted beneath the Kesterson
Reservoir, California, USA. This reservoir serves as an evaporative
sink for several metalloids, including boron, and receives
agricultural drainage from farmlands within the San Joaquin River
Valley. Benson et al. (1991) reported an average boron concentration
of 15 mg/litre. Concentrations of boron elsewhere within the San
Joaquin River Valley have been shown to range from 0.14 to 120
mg/litre, with a median of 4 mg/litre (Deverel & Millard, 1988;
Butterwick et al., 1989).
5.1.2.2 Surface water
The majority of the Earth's boron occurs in the oceans, with an
average concentration of 4.5 mg/litre (Weast et al., 1985). The amount
of boron in fresh water depends on such factors as the geochemical
nature of the drainage area, proximity to marine coastal regions, and
inputs from industrial and municipal effluents (Butterwick et al.,
1989). Concentrations of boron in fresh surface water are summarized
in Table 6.
Concentrations ranged from 0.001 to 2 mg boron/litre in Europe,
with mean values typically below 0.6 mg/litre. Similar concentrations
have been reported for water bodies within Pakistan, Russia, and
Turkey; concentrations range from <0.01 to 7 mg boron/litre, with
most values below 0.5 mg/litre. Concentrations ranged up to 0.01 mg
boron/litre in Japan and up to 0.3 mg boron/litre in South African
surface waters. Samples taken in surface waters from two South
American rivers (Rio Arenales, Argentina, and Loa River, Chile)
contained boron at concentrations ranging between 4 and 26 mg/litre in
areas rich in boron-containing soils. In other areas, the Rio Arenales
contained less than 0.3 mg boron/litre. Concentrations of boron in
surface waters of North America (Canada, USA) ranged from
0.02 mg/litre to as much as 360 mg/litre, indicative of boron-rich
deposits. However, typical boron concentrations were less than
0.1 mg/litre, with a 90th-percentile boron concentration of
approximately 0.4 mg/litre.
5.1.2.3 Rainfall
The median and mean concentrations of borate in rain and snow at
six sites in western Switzerland were found to be 0.0031 and 0.0056 mg
boron/litre, respectively (Atteia et al., 1993).
5.1.3 Sewage
Concentrations of boron in sewage waters are summarized in Table
7.
The majority of the boron present in sewage occurs primarily as
undissociated boric acid; reported levels of boron in sewage in the
USA range from 0.4 to 1.5 mg/litre and up to 4.05 mg/litre because of
industrial waste discharges (Banerji, 1969). In Europe, sewage from
domestic and industrial sources typically has an average boron
concentration of 2 mg/litre, with levels up to 5 mg/litre (Butterwick
et al., 1989). Calculations by the German Government Environment
Agency attribute 50% of the boron in wastewater to the use of
detergent products (Butterwick et al., 1989). In boron mine drainage
waters in Turkey, the boron concentrations were reported to be 16-390
mg/litre (Okay et al., 1985). Boron levels in sewage sludge in 23 US
cities ranged from 7.1 to 53.3 mg/kg dry weight (Mumma et al., 1984).
Table 6. Concentrations of boron in fresh surface water
Area Boron concentration Reference
(mg/litre)
USA Median = 0.076 ECETOC (1997)
90th percentile = 0.387
Drainage basins, USA 0.019-0.289a Kopp & Kroner (1970)
Coastal drainage waters, 15 (boron-rich deposits) Deverel & Millard (1988)
California, USA
Lakes, California, USA 157-360 (boron-rich Deverel & Millard (1988)
deposits)
Ontario, Canada 0.029-0.086 Sekerka & Lechner (1990)
Cold River drainage 0.0627 Tsui & McCart (1981)
area, western Canada
United Kingdom 0.046-0.822 Mance et al. (1988)
Italy 0.4-1.0 (range of Manfredi et al. (1975)
means)
<0.1-0.5 Tartari & Camusso (1988)
Sweden 0.013 (0.001-1.046) Ahl & Jönsson (1972)
Germany 0.02-2.0 Graffmann et al. (1974)
The Netherlands Range of medians = Unilever (1994)
0.09-0.145
Rivers, Austria <0.02-0.6 (background Schöller & Bolzer (1989)
level)
River Neva, Russia 0.01-0.02 Huber (1994)
Degh Nala, Pakistan <0.01-0.46 (near Tehseen et al. (1994)
effluent discharges)
Simav River, Turkey <0.5 (uncontaminated) Okay et al. (1985)
4 (maximum 7)
(contaminated with
boron mine waste)
Table 6. (continued)
Area Boron concentration Reference
(mg/litre)
Rio Arenales, Argentina <0.3 Bundschuh (1992)
6.9 (near borate plant)
Loa River Basin, Chile 3.99-26 (soil rich in Cáceres et al. (1992)
minerals and natural
salts; low rainfall)
Japan (River Asahi) 0.009-0.0117 Korenaga et al. (1980)
South Africa 0.02-0.33 Reid & Davies (1989)
a Lowest concentration in the western Great Lakes Basin to highest concentration
in the western Gulf Basin.
Table 7. Concentrations of boron in sewage waters
Area/source Boron concentration Reference
(mg/litre)
USA
Industrial waste 0.4-1.5 Banerji (1969)
discharge (maximum 4.05)
Europe
Domestic and industrial 2 (maximum 5) Butterwick et al. (1989)
Egypt
Sewage water 0.32-0.38 El-Hassanin et al. (1993)
Sweden
Effluent 0.34-0.436 Ahl & Jönsson (1972)
Spain, Alicante
Industrial waste 1.45 Navarro et al. (1992)
Spain, Elche
Industrial waste 3 Navarro et al. (1992)
United Kingdom
Municipal 1.21-3.96 Waggott (1969)
(range of means)
5.1.4 Soil
According to Whetstone et al. (1942), boron occurs in soils at
concentrations ranging from 10 to 300 mg/kg (average 30 mg/kg),
depending on the type of soil, amount of soil organic matter, and
amount of rainfall. Background boron levels in US soils were reported
at a geometric mean concentration of 26 mg/kg, with a maximum
concentration of 300 mg/kg (Eckel & Langley, 1988).
5.1.5 Aquatic biota
Concentrations of boron in aquatic biota are summarized in
Table 8.
Little specific information was found concerning the
bioaccumulation of boron in aquatic plants. At Kesterson National
Wildlife Refuge in the San Joaquin River Valley, California, USA (an
evaporative sink that has high concentrations of boron, selenium, and
arsenic and is supplied with subsurface drainage water from
agricultural fields), studies of the aquatic food-chain contamination
have suggested that aquatic plants bioaccumulate high levels of boron,
but boron does not biomagnify in aquatic food-chains. The following
studies report observed concentrations in marine algae and freshwater
aquatic vascular species. Igelsrud et al. (1938) reported boron levels
ranging from 4.2 to 14.9 mg/kg of dried material in marine algae.
Yamamoto et al. (1973) compared the boron content in freshwater and
marine phytoplankton and observed that minor differences occurred
between forms, even though the boron content of seawater averages 460
times that of fresh water.
Adams et al. (1973) conducted a survey to determine the
concentration of 11 potentially polluting ions, including boron, in a
wide variety of aquatic plants from three major watersheds in
Pennsylvania, USA: the Delaware, Susquehanna, and Allegheny rivers.
Sources of pollution in this area are quite diverse, including
lumbering activities, coal strip-mining, recreation, agricultural use,
and urban-industrial centres. Boron constituent levels in 21 species
of submerged and floating aquatic vascular plants ranged from 26.3 to
170 µg/g, and levels in 8 species of emergent aquatic vascular plants
ranged from 11.3 to 57 µg/g.
Tsui & McCart (1981) studied the bioaccumulation of several
elements, including boron, in five freshwater fish species from the
Cold River drainage area in western Canada. Test species were selected
to represent different feeding habits and modes of life. Northern pike
(Esox lucius) and lake trout (Salvelinus namaycush) are primarily
predators; lake herring (Coregonus artedii) is a plankton feeder;
and lake whitefish (Coregonus clupeaformis) and white sucker
(Catostomus commersoni) are primarily bottom-feeders. The fish were
collected during spring and summer of 1978 from seven lakes within
this area, and the muscle tissue was analysed for the presence of
boron. The maximum average concentration of boron in the lakes was
Table 8. Concentrations of boron in aquatic biota
Species Area Tissue Boron concentration Reference
(mg/kg)a
Marine algae 4.2-14.9 dw Igelsrud et al. (1938)
Filamentous algae Lower San Joaquin River and its 3.5-280 dw Saiki et al. (1993)
tributaries, California, USA
Plankton Lower San Joaquin River and its 1.1-46 dw Saiki et al. (1993)
tributaries, California, USA
Aquatic plants Lower San Joaquin River, 382 (270-510) dw Ohlendorf et al. (1986)
California, USA
Submerged and floating Pennsylvania, USA 26.3-170 Adams et al. (1973)
aquatic vascular plants
Emergent aquatic Pennsylvania, USA 11.3-57 Adams et al. (1973)
vascular plants
Various marine shellfish British Columbia, Canada 0.9-5.5 ww Thompson et al. (1976)
Benthic bivalve San Joaquin River and its
(Corbicula fluminea) tributaries, California, USA Soft tissue <2-2 dw Leland & Scudder (1990)
Clam (Elliptio dilitata) Precambrian Shield lake, Soft tissue 2.6 ww Wren et al. (1983)
Ontario, Canada
Chironomid larvae Lower San Joaquin River and its
tributaries, California, USA <1.8-27 dw Saiki et al. (1993)
Amphipods Lower San Joaquin River and its <2.2-23 dw Saiki et al. (1993)
tributaries, California, USA
Crayfish Lower San Joaquin River and its 1.2-23 dw Saiki et al. (1993)
tributaries, California, USA
Table 8. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Freshwater fish Cold River drainage area, Muscle 3.23-12.44 Tsui & McCart (1981)
western Canada (range of means)
Freshwater fish Precambrian Shield lake, Muscle 1.8-2.9 ww Wren et al. (1983)
Ontario, Canada
Bluegill (Lepomis San Joaquin River, California, Whole body 14 dw (3.5 ww) Saiki & May (1988)
macrochirus) USA
Lower San Joaquin River and its <1.8-7.9 dw Saiki et al. (1993)
tributaries, California, USA
Largemouth bass Lower San Joaquin River and its <1.8-2.0 dw Saiki et al. (1993)
(Micropterus salmoides) tributaries, California, USA
Common carp San Joaquin River, California, Whole body 20 dw (5 ww) Saiki & May (1988)
(Cyprinus carpio) USA
San Joaquin River, California, Whole body 0.5-6.2 dwb Klasing & Pilch (1988)
USA
Mosquitofish Lower San Joaquin River and its <1.9-8.4 dw Saiki et al. (1993)
(Gambusia affinis) tributaries, California, USA
Volta, California, USA Whole body mean = 2.8 dw Ohlendorf et al. (1986)
Kesterson, California, USA Whole body mean = 11.1 dw Ohlendorf et al. (1986)
Tilapia spp. Mexicali Valley, Baja California, Muscle 2.9 ww Mora & Anderson (1995)
Mexico
Mugil spp. Mexicali Valley, Baja California, Muscle 1.9 ww Mora & Anderson (1995)
Mexico
Table 8. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Sockeye salmon British Columbia, Canada Gill mean = 0.6 ww Thompson et al. (1976)
(Oncorhynchus nerka) Liver mean = 0.7 ww
Bone mean = 1.5 ww
Atlantic cod (Gadus Northwest Atlantic Ocean Muscle 28 (1-93) ww Hellou et al. (1992)
morhua) Liver 9.7 (5.2-35.4) ww
Ovaries <0.8
Anchoveta Whole body 3.3-3.8 aw Jenkins (1980)
(Cetengraulis
mysticetus)
Aquatic birds Precambrian Shield lake, Muscle 2.5-3.7 ww Wren et al. (1983)
Ontario, Canada
Grassland water district, Liver 1.7-40 dw Paveglio et al. (1992)
California, USA
Double-crested Mexicali Valley, Baja California,
cormorant Mexico Liver 4.2 (2.9-8.2) ww Mora & Anderson (1995)
(Phalacrocorax auritus)
Duck species Grassland water district, Egg 3.07-6.17 dw Hothem & Welsh (1994)
California, USA
Wading bird species Grassland water district, Egg 2.2-3.45 dw Hothem & Welsh (1994)
California, USA
Aquatic mammals Precambrian Shield lake, Muscle 7.9 ww Wren et al. (1983)
Ontario, Canada
a ww = wet weight; dw = dry weight; aw = ash weight; concentrations are given as means or ranges of means; ranges are given in parentheses.
b Exposed to tile drainage water.
0.0627 mg/litre. The mean tissue concentrations of boron in the five
fish species ranged from 3.23 µg/g for lake whitefish to 12.44 µg/g
for white sucker.
Wren et al. (1983) reported boron concentrations in freshwater
fish and clams from a Precambrian Shield lake in Ontario, Canada. The
lake was free from direct human impact. Boron levels in the
undeveloped and protected muscle tissue of the fish were generally
lower than those observed in fish from the Cold River drainage area in
western Canada. The mean concentrations (wet weights) in the fish
ranged from 1.8 to 2.9 µg/g. The boron concentration in the soft
tissue of the clam (Elliptio dilitata) was 2.6 µg/g.
In contrast, boron levels were only slightly elevated in
whole-body samples of bluegill (Lepomis macrochirus) and common carp
(Cyprinus carpio) from the San Joaquin River and two tributaries
that receive agricultural subsurface drainage water. The highest boron
concentrations (dry weights) measured were 14 µg/g (approx. 3.5 µg/g
wet weight) in bluegills and 20 µg/g (approx. 5 µg/g wet weight) in
carp (Saiki & May, 1988). Ohlendorf et al. (1986) reported similar
values for mosquitofish (Gambusia affinis) from the San Joaquin
River Valley. However, Saiki & May (1988) reported that the elevated
boron levels may also result from natural boron deposits in adjacent
soils or from sand-and-gravel mining operations in the area.
Paveglio et al. (1992) analysed boron concentrations in livers of
aquatic birds collected from the Grassland Water District of
California, USA, during 1985-1988. The use of subsurface agricultural
drainage water for marsh management resulted in trace element
contamination of components of the food-chain in this region. During
the breeding and wintering periods, livers of birds from northern and
southern areas of the grasslands contained high concentrations of
boron (1.7-40 mg/kg dry weight).
A number of studies have investigated the accumulation of boron
in aquatic food organisms, such as plants, insects, and fish (Saiki &
May, 1988; Hothem & Ohlendorf, 1989; Smith & Anders, 1989; Paveglio et
al., 1992; Saiki et al., 1993). The results of these studies suggest
that aquatic plants bioaccumulate boron, but that boron does not
biomagnify in aquatic food-chains.
5.1.6 Terrestrial biota
Concentrations of boron in terrestrial biota are summarized in
Table 9.
The studies discussed in section 4.2.3 suggest that plants grown
in boron-rich soil often contain high levels of boron in their
tissues. Another source of boron entry into the food-chain is through
the reclamation and revegetation of areas containing coal combustion
products (i.e. ash). Schwab et al. (1991) reported strong positive
correlations between the concentration of boron in soybean and sorghum
used to revegetate several ash disposal sites in Kansas, USA, and the
level of extractable boron in the coal fly ash. Variations in plant
uptake of boron from coal ash were attributed to differences in ash
type, plant species, and ash treatment. In terrestrial food-chains,
boron accumulates in plants; however, boron is not biomagnified to
animals (Saiki et al., 1993).
5.2 General population exposure
Human exposure to boron could result from the following sources:
the consumption of drinking-water from natural or municipal sources
that contain boron; the consumption of crops grown in boron-enriched
soils, irrigated with boron-enriched waters, or contaminated with
airborne boron particles; the consumption of aquatic organisms
inhabiting natural waters high in boron; the absorption of boron from
cosmetic or medical preparations through mucous membranes or damaged
skin; and the inhalation, dermal absorption, or accidental ingestion
of boron-containing household cleaning products, pesticides, or
fertilizers. The most frequent and appreciable general population
exposures to boron are likely to be from ingestion of food and, to a
lesser extent, from ingestion of drinking-water.
5.2.1 Ambient air
Boron does not appear to be present in ambient air at significant
levels (Sprague, 1972). There are few studies available that estimate
the concentration of boron-containing compounds in ambient air; this
is partly due to the difficulties of analysis at the low levels
involved (ATSDR, 1992). However, Anderson et al. (1994a) have
estimated the continental levels. Using their assumption that
particulate boron constitutes 10% and gaseous boron constitutes 90% of
the total, the range is 0.36-19.9 ng/m3. Therefore, using the average
adult air consumption of 22 m3/day and the maximum value in this
range, a respiratory exposure of 438 ng/day (0.44 µg/day) is
calculated.
5.2.2 Drinking-water
Drinking-water is derived from groundwater and/or surface water
sources. Concentrations of boron found in drinking-waters of Chile,
Germany, the United Kingdom, and the USA are presented in Table 10.
Overall, boron concentrations ranged from 0.01 to 15.0 mg/litre, with
most values below 0.5 mg/litre. These values are consistent with
ranges and means observed for data presented for groundwater (Table 5)
and surface water (Table 6). Further, the consistency is supported by
two factors: 1) boron concentrations in water are largely dependent on
the leaching of boron from the surrounding geology, and 2) boron is
not removed by conventional drinking-water treatment methods. Hence,
when considering the need for allocating a TI of boron via drinking-
water, a mean boron concentration for the world is judged to be
between 0.1 and 0.3 mg/litre.
Table 9. Concentrations of boron in terrestrial biota
Species Area Tissue Boron concentration Reference
(mg/kg)a
Lichen
(Parmelia caperata) Travale-Radicondoli geothermal 12.3 (nd-25.4) dw Loppi & Bargagli (1996)
area, Italy
Cerealsb 2.3-5.0 dw Bergmann (1984)
Legumesc 15.4-41.4 dw Bergmann (1984)
Dandelion
(Taraxacum officinale) 80 dw Bergmann (1984)
Poppy
(Papaver somniferum) 94.7 dw Bergmann (1984)
Moss
(Hylocomium splendens) Norway 3.6 (0.38-21) dw Bergmann et al. (1995)
Omnivorous terrestrial Mexicali Valley, Baja California, Liver 2.3-5.3 (1.2-8.7) ww Mora & Anderson (1995)
bird species Mexico
Mourning dove Mexicali Valley, Baja California, Liver 10 (4.3-28.5) ww Mora & Anderson (1995)
(Zenaida macroura) Mexico
Mule deer
(Odocoileus hemionus) Piceance Creek Basin, Metacarpal 2.3 (1.5-3.1) dw (fawn) Stelter (1980)
Colorado, USA
Piceance Creek Basin, Metacarpal 1.4 (1.1-1.7) dw (adult) Stelter (1980)
Colorado, USA
Table 9. (continued)
Species Area Tissue Boron concentration Reference
(mg/kg)a
Deer mice
(Peromyscus maniculatus) Piceance Creek Basin, Colorado, Whole skeleton <2 dw Stelter (1980)
USA
Four species of rodent 4.3-6.3 Wiener et al. (1977)
a dw = dry weight; ww = wet weight; nd = not detected.
b Barley (Hordeum vulgare), rye (Secale cereale), wheat (Triticum vulgare), maize (Zea mays).
c Fieldbean (Vicia faba), pea (Pisum sativum), soya bean (Glycine max), lentil (Lens esculenta).
Table 10. Concentrations of boron in drinking-water
Area Samples Concentration Reference
(mg/litre)
USA 2595 0.8% > 1.0 McCabe et al. (1970);
Choi & Chen (1979)
Germany 240 <0.25 Graffmann et al. (1974)
110 <0.21 Wiecken & Wübbold-Weber
(1993)
United Kingdom 0.05-0.505 J. Bennett
(personal communication)a
200 0.01-0.45 D.E. Wilkinson
(personal communication)b
Chile 0.31-15.0 Barr et al. (1993)
a Boron levels at drinking-water abstraction points of the River
Thames: letter of 22 February 1993 from National Rivers Authority,
Thames Region, United Kingdom, to the IPCS.
b Boron values for Anglican Water Region (1.1.92-31.12.92): letter
of 9 March 1993, ref. DEW/AH/1733CD, from Anglican Water Services
Limited, United Kingdom, to the IPCS.
5.2.3 Soil intake
An older report indicates that boron is found in soils at
concentrations ranging from 10 to 300 mg/kg, with an average value of
30 mg/kg (Whetstone et al., 1942).
A more recent study (Eckel & Langley, 1988) gives a similar upper
boron concentration range (300 mg/kg) and average value (26 mg/kg).
The use of this latter average soil level with an incidental
consumption of 20 mg soil/person per day (IPCS, 1994) yields an
average boron intake of 0.5 µg/day (i.e. 26 mg boron/kg of soil ×
0.000 02 kg of soil consumed per person per day = 0.0005 mg
boron/person per day).
5.2.4 Dietary intake
For the general population, the greatest exposure to boron comes
from food. Most of the concentrations of boron in foods reported
before 1985 are of questionable validity because of inadequate
analytical methods. Two recent reports (Hunt et al., 1991; Anderson et
al., 1994b) provide an adequate indication of the amounts of boron
found in various foods (see Table 11).
Table 11. Boron content of some common foods
Food Boron concentration
(µg/g, fresh weight basis)
Hunt et al. Anderson et al.
(1991) (1994b)
Fruits
Apple, red with peel, raw 2.73 2.38
Apple juice 1.88 2.41
Apple sauce 2.83 1.04
Banana, raw 3.72
Cherries, dark 1.47 0.92
Grape juice 2.02 2.06
Orange juice 0.41 1.59
Peaches, canned 1.87
Pears, canned 1.22
Dried fruits
Dates 9.2
Prunes 27 21.5
Raisins 25 19.0
Vegetables
Beans, green 0.46 1.56
Broccoli, flowers 1.85
Broccoli, stalks 0.89
Lettuce, iceberg <0.015
Carrots, canned 0.75
Nuts
Almonds 23
Hazelnuts 16
Peanuts 18 13.8
Meats
Beef, round, ground, raw <0.015 <0.05
Chicken, breast, ground, raw <0.015 0.09
Turkey breast <0.015
Milk and milk products
Cheese, cream <0.015 0.19
Milk, 2% <0.015 0.23
Cereal grain products
Bread, white, enriched 0.20 0.48
Cornflakes, fortified 0.31 0.92
Flour, wheat, white 0.28
Noodles, egg, dry, enriched 0.37
Rice, white, instant <0.015
Spaghetti, dry, enriched <0.015
Table 11. (continued)
Food Boron concentration
(µg/g, fresh weight basis)
Hunt et al. Anderson et al.
(1991) (1994b)
Miscellaneous
Catsup 0.85 1.39
Eggs, homogenized <0.015 0.12
Honey 7.2 6.07
Jelly, strawberry 0.41
Jelly, grape 1.47 1.86
Sugar, white <0.015 0.29
Beveragesa
Wine 3.5
Beer 1.8 0.13
a Boron concentration in µg/ml.
The richest sources of boron are fruits, vegetables, pulses,
legumes, and nuts. Dairy products, fish, meats, and most grains are
poor sources of boron. Based on the recent analyses of foods and food
products, estimations of daily intakes of various age/sex groups have
been made. Rainey et al. (1996) estimated that the median, mean, and
95th-percentile daily intakes of boron are 0.76, 0.93, and 2.16
mg/day, respectively, for all 27 age/sex groups in the USA; 0.34,
0.50, and 1.49 mg/day, respectively, for infants aged 0-5 months;
1.00, 1.18, and 2.61 mg/day, respectively, for males aged 60-65 years;
and 0.87, 1.0, and 2.17, respectively, for females aged 60-65. Using
food included in US Food and Drug Administration (FDA) Total Diet
Studies, Iyengar et al. (1988) determined the mean adult male daily
intake of boron to be 1.52 mg/day, whereas Anderson et al. (1994b)
determined the intake to be 1.21 mg/day. Based on the United Kingdom
National Food Survey (UK Ministry of Agriculture, Fisheries and Food,
1991), the dietary intake of boron in the United Kingdom ranges from
0.8 to 1.9 mg/day. It should be noted that increased consumption of
specific foods with high boron content will increase boron intake
significantly; for example, one serving of wine or avocado provides
0.42 or 1.11 mg, respectively (Anderson et al., 1994b). Moreover, for
the population obtaining their drinking-water from the 10% of the
public water systems that provide water containing >0.4 mg
boron/litre, water used for drinking and cooking may be the major, or
a significant, source of boron. Based on the preceding values, the
mean daily intake of boron in the diet is judged to be near 1.2
mg/day.
5.2.5 Consumer products
Boric acid, borax, and other borates are used in a great array of
consumer goods. The principal use of boric acid and borax in the USA
is in the manufacture of glass products. The way boron is bound to
glass products should not result in significant releases of boron in
its production or use.
Other consumer products in which boron compounds are used include
soaps and detergents (as a bleaching agent), preservatives, adhesives,
porcelain, enamel, leathers, carpets, artificial gems, high-contrast
photographic materials, wicks, electric condensers, fertilizers,
insecticides, and herbicides (Moore et al., 1997).
Sodium borate and boric acid are also widely used in numerous
cosmetic products, including makeup, skin and hair care preparations,
deodorants, moisturizing creams, breath fresheners, and shaving
creams, with concentrations up to 5% (US FDA, 1981; Beyer et al.,
1983). In 1981, the US FDA limited boric acid concentrations in
consumer goods to 5% (US FDA, 1981). Boric acid exposures from some
personal care products, estimated by the European Union Subcommittee
on "Cosmetic Ingredients," include 0.09-0.46 mg boric acid/kg body
weight per day for oral hygiene products, 0.03 mg boric acid/kg body
weight per day for eye products, and 0.25 mg boric acid/ kg body
weight per day for deodorants (SCC opinion XXIV/1820/95). Exposure is
related to personal practices. Boric acid is used in vaginal products
and contraceptives. Consequently, by topical application, these
compounds may come in contact with body surfaces, including the
ocular, buccal, and vaginal mucosae (Beyer et al., 1983).
Body-building supplements contain 1.5-10 mg boron/serving, with a
median of 4 mg boron/serving. These supplements could result in daily
exposures of 1.5-30 mg boron, as some are taken up to 3 times a day.
Bottled water can contain <0.005-4.35 mg boron/litre, depending on
its origin, with an average boron content of 0.75 mg/litre (Moore et
al., 1997).
The European Union permits boric acid in certain consumer
products (e.g. 3% boric acid in eye products, up to 0.5% in oral
hygiene products) (SCC opinion XXIV/1820/95). Usage data provided by
the industry (EU Technical Guidance Document, 1995) allow the
estimation of total exposure to boric acid from consumer products as
22.8 mg/day per person, equivalent to 4 mg boron/day. If absorption
across the dermis is assumed to range from 1 to 10%, the absorbed
boron dose from the source is 0.04-0.4 mg boron/day. The Task Group
felt that 0.1 mg/day would serve as a reasonable average estimate of
boron exposure from consumer products.
5.3 Occupational exposure
Occupational exposures to boron compounds may be significant
(ATSDR, 1992). Inhalation of dusts is the most significant route of
exposure in occupational settings. Dermal absorption of boron may also
occur if damaged skin is in contact with boron compounds, but this is
considered a minor pathway (Culver et al., 1994a).
Borate dusts have been monitored in workplace air. Reported
concentrations of borax dust in different areas of a large borax
mining and refining plant ranged from 1.1 to 14.6 mg/m3 (Garabrant et
al., 1985; see also section 8.2.1), and the mean boric acid/boric
oxide dust concentration in a boric acid manufacturing plant was
4.1 mg/m3 (Garabrant et al., 1984). Other industries where workers
may be occupationally exposed to boron include manufacture of
fibreglass and other glass products, cleaning and laundry products,
fertilizers, pesticides, and cosmetics (Stokinger, 1981). The range of
normal values of blood boron concentration for non-occupationally
exposed working adults can be found in Culver et al. (1994a). NIOSH
(1989) estimated that the number of workers potentially exposed to
boric acid increased from 6500 in the early 1970s to 35 600 in the
early 1980s. Information about frequency, concentration, and duration
of exposures is not contained in the NOES or National Occupational
Hazard Survey (NOHS) databases. Wegman et al. (1994) reported that
NIOSH estimated that there were 420 000 US workers with potential
occupational exposure to sodium borate.
As part of an epidemiological study of acute respiratory
irritation, Woskie et al. (1994) assessed short-term (time-weighted
average over 0.25 h) and daily (time-weighted average over 6 h) dust
and boron exposures of workers in a sodium borate production facility.
The investigators used personal monitors to measure the boron
concentration in air and the mass concentration of total airborne
dusts. The arithmetic mean for the total dust concentration ranged
from 0.29 to 18.85 mg/m3, and the average concentration of boron
relative to total dust ranged from 5.6 to 10.1%. Because the data
demonstrated that a substantial portion of the total dust was
non-borate material (e.g. cigarette smoke, vehicle exhaust, background
or ambient dust), the authors cautioned against using total dust as an
indicator or surrogate marker for actual exposure to boron compounds.
In a study in which chronic abnormality in pulmonary function and
acute irritant symptoms associated with borate dust in mixing and
processing operations were examined (Wegman et al., 1994; see also
section 8.2.1), personal monitors were used to measure boron
concentrations. The results showed that workers were exposed to dust
concentrations ranging from 0.1 to 205 mg/m3. The arithmetic mean of
daily exposures in the comparison group was 0.45 mg total dust/m3
(0.02 mg total boron/m3), whereas the average daily exposure for the
exposed group was 5.72 mg total dust/m3 (0.44 mg total boron/m3).
Whorton et al. (1994) studied male employees exposed to borate
dusts at a borax mine and production facility in California, USA, from
the 1950s through the 1980s. Men were identified with two or more past
consecutive years of "high" exposure to doses with a range of
17.6-44.8 mg dust/m3 (doses averaged 23.2 mg dust/m3, or 3.3 mg
boron/m3). The average exposure to sodium borate dust was 203 mg/day,
which assumes 7 h/day of actual exposure at an average concentration
of 23.2 mg/m3. The estimated 8-h respiratory volume for light work is
10 m3. The average concentration of boron in sodium borates is 14%;
the daily dose of boron, assuming 100% absorption, is therefore
28.4 mg.
In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine levels from workers
with the amount of boron exposure. Mean daily inspired boron was
calculated for low-, medium-, and high-exposure groups of male
workers; the numbers of workers in these groups were four, five, and
five, respectively. Total daily boron intake was calculated for each
individual, including the dietary contribution. Blood and urine
samples were taken at the start of the work week and at the end of
shifts on Monday, Thursday, and Friday and analysed for boron. An
average blood level of boron of 0.26 µg/ml occurred in the
high-exposure group of workers, where exposure was estimated to be
0.38 mg boron/kg body weight per day. Post-shift blood and urine boron
concentrations did not increase with the days of the work week,
indicating that boron did not accumulate when exposure was up to 0.38
mg/kg body weight per day during the work week.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Numerous studies have shown that boric acid and borax are
absorbed from the gastrointestinal tract and from the respiratory
tract, as indicated by increased levels of boron in the blood,
tissues, or urine or by systemic toxic effects of exposed individuals
or laboratory animals. Blood, tissue, and urine boron levels have
rarely been reported in cases of fatal human exposures to inorganic
borates.
6.1.1 Oral
Pharmacokinetic data indicate that boron, usually administered as
boric acid, is absorbed rapidly and virtually completely from the
gastrointestinal tract, as evidenced by recovery of >90% of the dose
in urine (Jansen et al., 1984). In a study in which human volunteers
were given a single known dose of boric acid (131 mg boron), 94% of
the ingested boron was excreted in the urine over a 96-h period (Schou
et al., 1984). Based on urinary excretion of boron, Job (1973)
observed a similar degree of oral absorption in volunteers who drank
curative spa waters with a daily dose of approximately 100 mg boron
for 2 weeks. Similar results had previously been reported by Kent &
McCance (1941). These investigators also showed that the urinary
excretion of boron ingested solely from dietary sources also reflected
absorption rates ranging from 83 to 98% (Kent & McCance, 1941).
Ingested boron is also readily absorbed by other species, including
rats (Ku et al., 1991), rabbits (Draize & Kelley, 1959), sheep (Brown
et al., 1989), and cattle (Owen, 1944; Weeth et al., 1981), as
indicated by high levels of boron in tissues or urine or systemic
toxicity following oral exposure. A 20-µg dose of 10B fed to fasted
rats resulted in peak liver concentrations at 3 h and a return to
normal isotope ratios within 24 h. Absorption was essentially
complete, as evidenced by 95% recovery of 10B from urine and
4% recovery from faeces within 72 h post-dosing (Vanderpool et al.,
1994).
Barr et al. (1993) studied areas of northern Chile in which the
concentrations of naturally occurring boron in the water supplies
ranged from 0.31 to 15.2 mg/litre. Blood boron levels for residents
from seven geographical regions showed a positive correlation with
boron levels in the local drinking-water supplies. Rough extrapolation
from the plotted data indicates that in three regions with boron
levels in drinking-water below 2.5 mg/litre (i.e. estimated intake of
0.07 mg boron/kg body weight per day for a 70-kg human consuming
2 litres of water per day), blood boron levels were below 0.1 µg/g
blood. In the region with the highest boron concentration in
drinking-water (15.2 mg boron/litre) (i.e. estimated intake of 0.43 mg
boron/kg body weight per day), average blood levels were approximately
0.7 µg boron/g blood. Accuracy of linear fitting for the original data
was limited by the small sample size obtained in each region, as well
as the absence of individual water intake data.
6.1.2 Inhalation
Absorption is indicated by increases in human blood and urine
boron levels after inhalation exposure to borax in the range of 3.3-18
mg/m3 (Culver, 1994a). Mice exposed to amorphous boron at a
concentration of 72.8 mg/m3 for 7 h/day, 5 days/week, for 30 days had
significant concentrations of boron in the liver and kidney (Stokinger
& Spiegl, 1953), indicating that amorphous boron is absorbed from the
respiratory tract. High boron levels were also found in the lungs,
indicating that absorption was not complete. Wilding et al. (1959)
showed that rats exposed to aerosols of boron oxide by inhalation at
77 mg/m3 for 6 h/day, 5 days/week, for 6 weeks excreted boron in
their urine, indicating that boron oxide is absorbed from the
respiratory tract. Ingestion (i.e. swallowing particles cleared from
the respiratory tract by the mucociliary escalator system and
subsequent absorption from the gastrointestinal tract) may have
contributed to systemic uptake.
6.1.3 Dermal
Dermal absorption across intact skin is negligible in all species
evaluated, including human infants (Friis-Hansen et al., 1982), human
adults (Stuttgen et al., 1982), rabbits (Draize & Kelley, 1959), and
rats (Nielsen, 1970). Several investigators have demonstrated that
dermal absorption of boron compounds may be affected by the vehicle
used to dissolve or suspend the compounds (Nielsen, 1970; Stuttgen et
al., 1982). When boric acid is applied to broken or damaged skin,
however, absorption of boric acid through the damaged skin has been
demonstrated (Draize & Kelley, 1959; Nielsen, 1970). In a report by
Vignec & Ellis (1954), infants 1.25-10 months old received
applications of a talcum powder containing 5% boric acid 7-10
times/day for at least 1 month. The authors calculated that the
infants were exposed to an estimated dose of 2.33 g boric acid/day
(408 mg boron/day). The boron concentrations in a test group composed
of 12 infants were 0.04 ± 0.04 mg/100 ml in blood and 0.16 ± 0.14
mg/100 ml in the urine. An additional group of 12 treated infants, who
had developed a mild to moderate diaper rash, had an average blood
boron concentration of 0.03 ± 0.04 mg/100 ml. Although Vignec & Ellis
(1954) did not analyse the data statistically, the range of the values
obtained indicates that, at most, only traces of boric acid penetrated
the skin, even in infants with moderate diaper rash.
6.2 Distribution
6.2.1 Tissue levels
Boron is a normal constituent of blood and tissues in humans and
animals as a result of ingestion in food and (in some locations)
drinking-water. Blood and tissue levels under normal dietary
conditions or with boron supplementation are summarized in Table 12.
Boric acid distributes evenly throughout the body fluids. The
most complete study of boron distribution was conducted in male F-344
rats (30/dose group) fed a control diet (<20 mg boron/kg) or a diet
containing 9000 mg boric acid/kg (approximately 68 mg boron/kg body
weight per day) for up to 7 days (Ku et al., 1991). Six animals/ group
were killed at 1, 2, 3, 4, and 7 days after the start of exposure.
Boron levels were determined in all major tissues and organs. The
authors reported that boron concentrations were comparable in all
tissues examined, including plasma, liver, kidney, muscle, colon,
brain, testis, epididymis, seminal vesicles, prostate, and adrenals.
Most of the tissues appeared to reach steady-state boron levels (12-30
mg boron/kg tissue) by 3-4 days; these levels were 3- to 20-fold above
controls. Adipose tissue accumulated only 20% as much boron as other
tissues (3.78 mg/kg tissue). Bone boron levels (47.4 mg/kg tissue)
indicated greater accumulation in bone than in other tissues; in
addition, bone boron levels continued to increase throughout the
7 days. The higher affinity of boron for bone is indicative of a
second kinetic component in which a small percentage of the boron
absorbed may be sequestered. Elimination kinetics from bone also
differ from those from soft tissue and body fluids (see Chapin et al.,
1997, in section 6.4). Accumulation of boron in bone has also been
observed in humans (Alexander et al., 1951; Forbes et al., 1954) and
other animal species (Forbes & Mitchell, 1957).
6.2.2 Blood levels
O'Sullivan & Taylor (1983) reported the blood boron levels in
three of seven infants (6-16 weeks old) who had ingested borax from
pacifiers dipped in a borax and honey mixture. These three infants
ingested 8-30 g of borax (286-429 mg borax/day) over a 4- to 10-week
time period. Signs of central nervous system (CNS) toxicity (seizures)
were reported in all seven infants. The blood levels ranged from 0.26
to 0.85 mg boron/100 ml blood; these blood values did not correlate
well with the estimated amount of borax ingested per individual, but
were clearly elevated relative to 15 children (ages 2-21 months)
without exposure (average 0.021 mg/100 ml, range 0-0.063 mg/100 ml).
In a review of 782 cases of accidental boric acid exposures (age
range 2 weeks to 98 years), serum levels ranged from 0 to 5.94 mg
boron/100 ml. However, these levels did not correlate with the
estimated amount of boric acid ingested (Litovitz et al., 1988).
Magour et al. (1982) determined the tissue levels of boron in
Wistar rats administered 20 mg boron/kg body weight per day as sodium
borate in drinking-water; exposure was initiated at 3 weeks of age and
continued for 21 days. Blood boron levels continually rose during the
treatment period; the maximum level was approximately 3.2 µg/g or
0.32 mg/100 ml blood. Intraperitoneal administration of 42 mg boron/kg
body weight to 3-week-old or 3-month-old female Wistar rats resulted
in generally higher tissue levels in the older rats and slightly
higher tissue/blood ratios across a 4-h post-administration period
(Magour et al., 1982). These data suggest that boron may be
distributed differently in mature versus immature rats, but the
differences were subtle and transient.
Table 12. Blood and tissue boron levels following ingestion of boron in diet or drinking-water
Species Ingested boron Fluid/organ Concentrationa Reference
Chick 0.465 µg/g diet Plasma 0.077 µg/ml Hunt (1989)
3.465 µg/g diet Plasma 0.152 µg/ml
0.465 µg/g diet Femur 0.251 µg/g dw
3.465 µg/g diet Femur 0.861 µg/g dw
Chick 0.18 µg/g diet Brain 1.05 µg/g dw Bain & Hunt (1996)
+ 3 µg/g diet Brain 1.01 µg/g dw
+ 20 µg/g diet Brain 1.61 µg/g dw
0.18 µg/g diet Heart 0.25 µg/g dw
+ 3 µg/g diet Heart 0.55 µg/g dw
+ 20 µg/g diet Heart 0.88 µg/g dw
0.18 µg/g diet Liver 1.35 µg/g dw
+ 3 µg/g diet Liver 1.01 µg/g dw
+ 20 µg/g diet Liver 1.22 µg/g dw
0.18 µg/g diet Kidney 0.55 µg/g dw
+ 3 µg/g diet Kidney 0.66 µg/g dw
+ 20 µg/g diet Kidney 1.11 µg/g dw
0.18 µg/g diet Spleen 2.22 µg/g dw
+ 3 µg/g diet Spleen 2.02 µg/g dw
+ 20 µg/g diet Spleen 2.38 µg/g dw
0.18 µg/g diet Femur 0.32 µg/g dw
+ 3 µg/g diet Femur 0.53 µg/g dw
+ 20 µg/g diet Femur 1.81 µg/g dw
0.18 µg/g diet Muscle 0.12 µg/g dw
+ 3 µg/g diet Muscle 0.15 µg/g dw
+ 20 µg/g diet Muscle 0.41 µg/g dw
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Chick 0.18 µg/g Femur 1.21 µg/g dw Hunt et al. (1994a)
(Vitamin D-deficient diet)
1.4 µg/g Femur 1.49 µg/g dw
(Vitamin D-deficient diet)
0.18 µg/g Femur 0.74 µg/g dw
(Vitamin D-adequate diet)
1.4 µg/g Femur 0.75 µg/g dw
(Vitamin D-adequate diet)
Human Unknown Brain, normal 0.87 µg/g dw Shuler et al. (1990)
Brain, thalassaemic 1.38 µg/g dw
Heart, normal 0.59 µg/g dw
Heart, thalassaemic 2.21 µg/g dw
Kidney, normal 1.27 µg/g dw
Kidney, thalassaemic 4.57 µg/g dw
Liver, normal 2.25 µg/g dw
Liver, thalassaemic 1.72 µg/g dw
Pancreas, normal 0.51 µg/g dw
Pancreas, thalassaemic 2.94 µg/g dw
Spleen, normal 3.95 µg/g dw
Spleen, thalassaemic 1.14 µg/g dw
Human Unknown Bone, arthritic 0.63 µg/g fw Havercroft & Ward (1991)
Bone, normal 1.57 µg/g fw
Synovial fluid, arthritic 30.4 ng/ml
Synovial fluid, normal 48.1 ng/ml
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Human 27 mg/day Blood 0.659 µg/g Barr et al. (1993)
(drinking-water computed)
21 mg/day Blood 0.450 µg/g
(drinking-water computed)
17 mg/day Blood 0.585 µg/g
(drinking-water computed)
4.5 mg/day Blood 0.347 µg/g
(drinking-water computed)
2.5 mg/day Blood 0.052 µg/g
(drinking-water computed)
1.3 mg/day Blood 0.068 µg/g
(drinking-water computed)
0.56 mg/day Blood 0.022 µg/g
(drinking-water computed)
Human Unknown Blood 28 ng/ml Ward (1993)
Synovial fluid 30 ng/ml
Saliva 4.4 ng/ml
Cerebrospinal fluid 1.5 ng/ml
Hair, scalp 1.05 µg/g fw
Nails 15 µg/g fw
Bone 1.6 µg/g fw
Liver 0.1 µg/g fw
Kidney 0.5 µg/g fw
Brain 0.08 µg/g fw
Human About 1.2 mg/day Plasma 34 ng/ml Nielsen (1996)
About 3.3 mg/day Plasma 53 ng/ml
Human Unknown Pre-term milk 0.5 ng/ml Aquilio et al. (1996)
Term milk 1.2 ng/ml
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Human 0.36 mg/day Red blood cells 0.21 µg/g dw Hunt et al. (1997)
3.23 mg/day Red blood cells 0.19 µg/g dw
0.36 mg/day Plasma 64 ng/ml
3.23 mg/day Plasma 95 ng/ml
Rat 0.17 µg/g diet Plasma 55 ng/ml Nielsen et al. (1992a)
3.17 µg/g diet Plasma 55 ng/ml
0.17 µg/g diet Femur 1.28 µg/g dw
3.17 µg/g diet Femur 1.73 µg/g dw
Rat 0.2 µg/g diet Femur 0.72 µg/g dw Nielsen & Shuler (1992)
3.2 µg/g diet Femur 0.88 µg/g dw
Rat 0.12 µg/g diet Femur 0.83 µg/g dw Penland & Ebehardt (1993)
2.7 µg/g diet Femur 0.95 µg/g dw
Rat 10 µg/g diet Blood 0.11 µg/ml Bai & Hunt (1996)
Brain 0.64 µg/g
Heart 1.0 µg/g
Kidney 2.0 µg/g
Liver 0.51 µg/g
Spleen 3.3 µg/g
Testes 0.91 µg/g
Tibia 1.3 µg/g
Muscle 0.50 µg/g
Table 12. (continued)
Species Ingested boron Fluid/organ Concentrationa Reference
Rat (injected 0.15 µg/g diet Muscle 0.12 µg/g dw Bai & Hunt (1996)
with 1.5 µg/g diet Muscle 0.17 µg/g dw
mycobacterium 3.0 µg/g diet Muscle 0.18 µg/g dw
tuberculosis) 10.0 µg/g diet Muscle 0.30 µg/g dw
0.15 µg/g diet Heart 0.37 µg/g dw
1.5 µg/g diet Heart 0.34 µg/g dw
3.0 µg/g diet Heart 0.44 µg/g dw
10.0 µg/g diet Heart 0.57 µg/g dw
0.15 µg/g diet Liver 0.14 µg/g dw
1.5 µg/g diet Liver 0.21 µg/g dw
3.0 µg/g diet Liver 0.17 µg/g dw
10.0 µg/g diet Liver 0.26 µg/g dw
0.15 µg/g diet Kidney 0.81 µg/g dw
1.5 µg/g diet Kidney 0.45 µg/g dw
3.0 µg/g diet Kidney 0.61 µg/g dw
10.0 µg/g diet Kidney 0.99 µg/g dw
a dw = dry weight; fw = fresh weight.
Table 13 shows that blood boron levels generally increase with
the increase in oral dose in both rats and humans. However, procedural
differences among studies currently preclude a definitive
cross-species comparison to determine comparability of blood boron
levels at similar doses. For example, studies may differ in their
approach to estimation of boron intake, duration of exposure,
analytical methodology, or temporal relationship between exposure and
blood collection. Recognizing these limitations, a preliminary
comparison across species has been made based on rats exposed to boron
via diet or drinking-water and humans exposed via diet,
drinking-water, or accidental ingestion (Fig. 1). In this context,
good overlap occurs between rat and human blood boron values in the
dose ranges from 0.01 to 100 mg boron/kg body weight per day. These
data reinforce the perception that boron kinetics are similar in rats
and humans, but they also emphasize the need for additional data to
strengthen the basis for the comparison.
6.3 Metabolism
Metabolism of inorganic borates by biological systems is not
feasible owing to the excessive energy (523 kJ/mol) required to break
the boron-oxygen bond (Emsley, 1989). Inorganic borates, in low
concentrations, convert to boric acid at physiological pH in the
aqueous layer overlying mucosal surfaces prior to absorption. This is
supported by the evidence in both human and animal studies, where more
than 90% of the administered dose of borate is excreted as boric acid.
There is evidence in both in vitro and in vivo systems that boric
acid has an affinity for cis-hydroxyl groups, and this may be the
mechanism that explains the biological effects of boric acid. However,
this attachment is known to be reversible and concentration dependent,
responding to clearance mechanisms.
6.4 Elimination and excretion
Clearance of boron compounds is similar in humans and animals.
Elimination of borates from the blood is largely by excretion of >90%
of the administered dose via the urine, regardless of the route of
administration. Excretion is relatively rapid, occurring over a period
of a few to several days, with a half-life of elimination of 24 h or
less. The kinetics of elimination of boron have been evaluated in
human volunteers given boric acid via the intravenous and oral routes
(Jansen et al., 1984; Schou et al., 1984). The half-life for
elimination was the same by either route in these studies and was
approximately 21 h. This value is corroborated by case reports of
boric acid poisoning. Litovitz et al. (1988) examined the case reports
of almost 800 patients accidentally or intentionally poisoned with
boric acid and found a very comparable elimination half-life: 13.4 h
(range 4-27.8 h). Incomplete or inconsistent patient histories
(especiallywith regard to total dose or dose-to-sample interval)
undoubtedly contributed to the variation in those half-life estimates.
Table 13. Blood boron levels as a function of dose in humans and rats
Boron dose Blood boron level (ng/ml) Comments Reference
(mg/kg body
weight per day) Human Rat
0.0054 64 (plasma) Diet Hunt et al. (1997)
0.01 22 Drinking-water Barr et al. (1993)
0.017 55 (plasma) Diet Nielsen et al. (1992a)
0.02 34 (plasma) Diet Nielsen (1996)
0.02 68 Drinking-water Barr et al. (1993)
0.04 52 Drinking-water Barr et al. (1993)
0.049 95 (plasma) Diet Hunt et al. (1997)
0.05 53 (plasma) Diet Nielsen (1996)
0.08 347 Drinking-water Barr et al. (1993)
0.2 200a Diet Chapin et al. (1997)
0.3 55 (plasma, uncertain) Diet Nielsen et al. (1992a)
0.3 585 Drinking-water Barr et al. (1993)
0.35 229 ± 143b Diet Price et al. (1997)c
0.4 450 Drinking-water Barr et al. (1993)
0.5 659 Drinking-water Barr et al. (1993)
1.4 3 000 Drinking-water Job (1973)
1.7 800a Diet Chapin et al. (1997)
3.3 564 ± 211b Diet Price et al. (1997)
3.5 3 200 (uncertain) Oral Litovitz et al. (1988)
6.3 975 ± 261b Diet Price et al. (1997)
8.4 2 400a Diet Chapin et al. (1997)
9.6 1 270 ± 298b Diet Price et al. (1997)
13 1 530 ± 546b Diet Price et al. (1997)
20 3 100 Drinking-water Magour et al. (1982)
25 2 820 ± 987b Diet Price et al. (1997)
26 6 700 (serum) Diet Ku et al. (1993a)
38 10 300 (serum) Diet Ku et al. (1993a)
40 6 500 Oral O'Sullivan & Taylor (1983)
52 13 300 (serum) Diet Ku et al. (1993a)
56 27 000 (uncertain) Oral Litovitz et al. (1988)
68 17 300 (serum) Diet Ku et al. (1993a)
95 16 000 (plasma) Diet Ku et al. (1991)
Table 13 (continued)
a Developed by R. Chapin. Estimated from measured bone boron concentrations and ratio of bone/blood boron levels, from Chapin
et al. (1997) and Ku et al. (1993a).
b Mean ± SD (n = 27-30 per dose group; Price et al., 1997).
c Price et al. (1997) data are for pregnant rats.
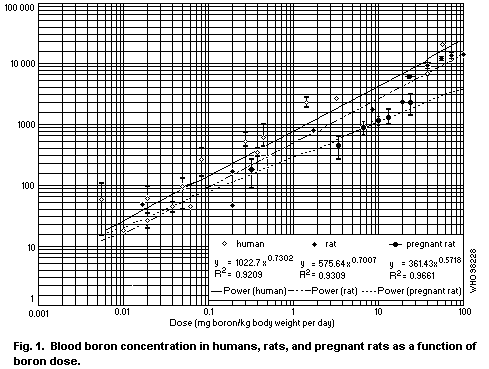 In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine boron levels from
workers with the amount of boron exposure (see section 5.3). An
average boron level of 0.26 µg/ml blood occurred in the highest
exposure group, where exposure was estimated to be 0.38 mg boron/kg
body weight per day. Post-shift blood and urine boron concentrations
did not increase with the days of the work week. This lack of
accumulation over the work week corroborates the relatively short
elimination half-life for boron in humans.
Elimination times for animals have not been explicitly stated in
the literature but can be either calculated or estimated from
published data. Farr & Konikowski (1963) measured urinary boron
concentrations in mice after intravenous injection of sodium
pentaborate. Using their data and assuming first-order kinetics for
elimination, the half-life for elimination in the mouse was on the
order of 1 h. Ku et al. (1991) reported that an apparent steady-state
level of boron is reached in the blood and tissues of rats after 3-4
days of oral dosing; again, assuming first-order kinetics, the
half-life in rats would be on the order of 14-19 h.
Male Fischer-344 rats were exposed to boric acid at 3000-9000
mg/kg in the diet for up to 9 weeks (Ku et al., 1993a). Urinary boron
was elevated (450-600 µg boron/mg creatinine) 24 h after the end of
dosing. By 3-4 days post-treatment, urinary boron in all groups had
returned to average control levels (8.97 ± 1.95 µg boron/mg creatinine
for days 1, 7, and 14 post-treatment). Elimination of boron from bone
followed a different time-course from elimination from serum or soft
tissues. F-344 rats consuming boric acid in the diet for 9 weeks
(approx. 1.4-6.8 mg boron/kg body weight per day) showed dose-related
elevation of bone boron, which declined very gradually during the
post-treatment period. Bone boron levels remained elevated above
controls in all exposed groups at 32 weeks post-treatment (Chapin et
al., 1997). Longer post-treatment intervals, as well as factors
influencing rates of accumulation or mobilization of boron in bone,
remain to be evaluated.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Short-term exposure
7.1.1 Oral route
The oral LD50 values for boric acid and borax in laboratory
animals are given in Table 14. Values for mice and rats are in the
range of approx. 400-700 mg boron/kg body weight (Pfeiffer et al.,
1945; Weir & Fisher, 1972). For guinea-pigs, Verbitskaya (1975)
reported an oral LD50 of 210 mg boron/kg body weight. Acute oral LD50
values in the range of 250-350 mg/kg body weight for boric acid or
borax exposure have also been reported for dogs, rabbits, and cats
(Pfeiffer et al., 1945; Verbitskaya, 1975).
General clinical effects of boric acid or borax in rats, mice,
and guinea-pigs given single large doses orally are depression,
ataxia, occasional convulsions, decreased body temperature, and
violet-red colour of skin and all mucous membranes (Pfeiffer et al.,
1945; Weir & Fisher, 1972). Toxic signs in dogs given boric acid
(0.2-2.0 g/kg body weight) orally in combination with subcutaneous
morphine to prevent vomiting were cyanosis of mucous membranes,
red-violet skin colour, rigidity of legs, convulsion, and shock-like
syndrome (Pfeiffer et al., 1945). Rabbits given boric acid at 800
mg/kg body weight per day for 4 days showed anorexia, weight loss, and
diarrhoea; 850 and 1000 mg/kg body weight per day for 4 days caused
100% mortality (Draize & Kelley, 1959). Cattle receiving 0.8, 150, or
300 mg boron/litre of drinking-water (as boric acid) for 30 days were
lethargic at the highest dose and had swelling and irritation in the
legs and around the dew claws, slight diarrhoea, and decreased food
consumption at the middle and high doses (Green & Weeth, 1977).
Following exposure to boric acid, microscopic changes in tissues
of mice, rats, and dogs involved primarily the kidneys and nervous
system (Pfeiffer et al., 1945). Glomerular and tubular damage were
noted in the kidneys. Glomerular damage consisted of changes in
permeability of the capillaries, and tubular damage consisted of
cellular vacuolization and shedding of cells into the tubular lumen.
Nervous system damage consisted of an increase in small dark cells
(probably microglia) in the spinal cord and in the grey matter of the
brain cortex.
7.1.2 Inhalation route
The LC50 for sodium perborate tetrahydrate by inhalation in rats
was >74 mg/m3 (Silaev, 1984). The 1-h inhalation LC50 values for
boron trichloride are reported as 12.2 g/m3 for male rats and 21.1
g/m3 for female rats; for boron trifluoride, the LC50 values range
from 0.89 to 1.2 g/m3 for rats (Vernot et al., 1977). While these
boron-halogen compounds are not considered in this report because of
apparent anion toxicity, it is appropriate to note in passing the
inhalation studies performed with them (Stokinger & Spiegl, 1953;
Rusch et al., 1986). These studies evaluated rats, mice, and
guinea-pigs and report various dose-related toxic effects beginning at
24 mg/m3.
Table 14. Acute toxicity of boron compounds in laboratory animals
Route Compound Species Compound LD50 Borona LD50
(mg/kg body weight) (mg/kg body weight) Reference
Oral Boric acid Mice 3450 603 Pfeiffer et al. (1945)
Borax Rats 3493 396 Wang et al. (1984)
Borax Rats 4500 510b Weir & Fisher (1972)
Borax Rats 4980 560b Weir & Fisher (1972)
Borax Rats 5660 642 Smyth et al. (1969)
Borax Rats 6080 690b Weir & Fisher (1972)
Boric acid Rats 2660 465 Pfeiffer et al. (1945)
Boric acid Rats 3160 550b Weir & Fisher (1972)
Boric acid Rats 3450 600b Weir & Fisher (1972)
Boric acid Rats 4080 710b Weir & Fisher (1972)
Boric acid Rats 5140 899 Smyth et al. (1969)
Subcutaneous Boric acid Mice 1740c 304 Pfeiffer et al. (1945)
Boric acid Mice 2070 362 Pfeiffer et al. (1945)
Boric acid Guinea-pigs 1200 210 Pfeiffer et al. (1945)
Intravenous Boric acid Mice 1780 311 Pfeiffer et al. (1945)
Boric acid Rats 1330 232 Pfeiffer et al. (1945)
a Calculated by multiplying the dose in mg boron compound/kg by the ratio of the molecular weights of boron/boron compound,
except when noted otherwise.
b Reported by investigators.
c Solution adjusted to pH 7.4 with sodium hydroxide.
7.2 Longer-term exposure
7.2.1 Oral route
The effects of longer-term oral exposure to boron compounds in
animals are summarized in Table 15.
In a 13-week study conducted by the US National Toxicology
Program (NTP, 1987; Dieter, 1994), B6C3F1 mice (10/sex per dose) were
exposed to boric acid in the diet at concentrations sufficient to
produce estimated consumptions of approximately 0, 34, 70, 141, 281,
or 563 mg boron/kg body weight per day for males and 0, 47, 97, 194,
388, or 776 mg boron/kg body weight per day for females. The results
showed that female mice were less sensitive than male mice to the
lethal effects of boric acid; 8/10 high-dose males and 6/10 high-dose
females died, and 1/10 males receiving 281 mg boron/kg body weight per
day died. Clinical signs of toxicity were a thin, hunched appearance,
dehydration, foot lesions, and scaly tails. A dose-related decrease in
body weight gain was observed. Microscopic effects included a
dose-related incidence of minimal to mild extramedullary
haematopoiesis of the spleen in males and females, hyperkeratosis and
acanthosis of the stomach in eight males and three females receiving
the highest dose, and testicular lesions; the testicular lesions are
described in greater detail in section 7.4.
Following this range-finding study, a 2-year study was conducted
in which mice (50/sex per dose) received approximately 0, 275, or 550
mg boric acid/kg body weight per day (0, 48.1, or 96.3 mg boron/kg
body weight per day) in the diet (NTP, 1987; Dieter, 1994). No
clinical signs of toxicity were observed. Body weights were 10-17%
lower in males after 32 weeks and in females after 52 weeks.
Non-accidental mortality at the end of the study was 9/50, 20/50, and
23/50 in control, low-, and high-dose males and 17/50, 15/50, and
12/50 in control, low-, and high-dose females. The increased mortality
rate was statistically significant in males. The only significant
lesions in male mice appeared in the testes; no significant
non-neoplastic lesions appeared in females.
Lee et al. (1978) fed borax in the diet to male Sprague-Dawley
rats (18/dose) at concentrations of 0, 500, 1000, or 2000 mg boron/kg
(equivalent to approximate doses of 0, 30, 60, or 125-131 mg boron/kg
body weight per day) for 30 or 60 days. Body weights were not
consistently affected by treatment; organ weights were not affected in
the 30 mg/kg body weight per day group. At 60 and 125-131 mg/kg body
weight per day, absolute liver weights were significantly lower after
60 days; epididymal weights were significantly lower (37.6% and 34.8%,
respectively) after 60 days, but not after 30 days. Weights of
prostate, spleen, kidney, heart, and lung were not changed at
any dose; reproductive effects observed in the study are discussed in
section 7.4.
Table 15. Effects of longer-term oral exposure to boron compounds in experimental animals
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Mice 0, 34, 70, 141, 281, Diet 13 weeks Over 60% mortality in both sexes NTP (1987)
563 for males; 0, 47, at 563 and 776 mg/kg body weight
97, 194, 388, 776 for per day; 10% in males at 281 mg/kg
femalesb body weight per day. At 141 mg/kg
body weight per day, degeneration
in seminiferous tubules and
decreased tubules in males and
decreased weight gain in males and
females. Extramedullary
haematopoiesis of the spleen in
all dosed groups, hyperkeratosis
and acanthosis of stomach at 563
and 776 mg/kg body weight per day.
Boric acid Mice 0, 48, 96 Diet 103 weeks Decrease in body weight (10-17%) NTP (1987)
in high-dose males after week 32
and in high-dose females after week
52. No clinical toxic signs observed.
Testicular atrophy and interstitial
cell hyperplasia were seen in males
at both levels. Dose-related increase
in incidence of splenic lymphoid
depletion in males. No other
significant increase in
non-neoplastic lesions.
Boric acid Rats 0, 22.7, 57c and Drinking-water 30 days Growth was not inhibited at the Pfeiffer et al.
higher low dose, but it was delayed at (1945)
>57 mg/kg body weight per day.
No haematological effects or
histological alterations.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Borax Rats 0, 0.056, 0.28, 2.8, Drinking-water <198 days Decrease in pancreas-to-body Wang et al.
28d weight ratio at all doses in (1984)
females at day 98. Increase in
pancreas-to-body weight ratio
in males at day 198. No details
given; normal histology.
Boric acid Rats 0.95, 3.65, 5.2, 9.9e Diet 8 weeks Decreased body weight at 5.2 Forbes & Mitchell
and 9.9 mg/kg body weight per (1957)
day. No other toxicity
end-points evaluated.
Borax or Rats 0, 2.6, 8.8, 26.3, Diet 90 days Mortality was 100% at the highest Weir & Fisher
boric acid 87.5, 262.5e dose; testicular atrophy at 87.5 (1972)
and 26.3 mg/kg body weight per
day; decrease in body weight and
in the weight of liver, kidney,
spleen, and testes at 87.5 mg/kg
body weight per day; weight
changes were inconsistent at
lower doses.
Borax or Rats 0, 5.9, 17.5, 58.5e Diet 2 years Both compounds suppressed growth Weir & Fisher
boric acid at 58.5 mg/kg body weight per (1972)
day. Testes weight and
testes-to-body weight ratios
were decreased, and brain-to-body
weight and thyroid-to-body weight
ratios were increased at 58.5
mg/kg body weight per day; also,
atrophy in seminiferous epithelium
and decrease in tubular size; no
effects observed at lower doses.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Rabbits 31 Oral gavage 5 days/week Elevated serum glutamic-oxaloacetic Verbitskaya
or borax for 4 months transaminase (SGOT) and serum (1975)
glutamic-pyruvic transaminase (SGPT)
levels, serum lactate dehydrogenase
and aldolase were transiently
increased, catalase and amylase were
decreased.
Sodium Rats 0, 3 g/litre sodium Drinking-water 14 weeks Increase in RNA concentration Settimi et al.
tetraborate tetraborate (boron and in succinate dehydrogenase (1982)
(not conversion not and acid proteinase activation
specified made due to in the brain. Decrease in
whether uncertainty NADPH-cytochrome reductase and
anhydrous or regarding in the content of cytochrome
decahydrate) compound) b5 and P-450 in the liver. No
effect on body or organ weight.
Sodium Mice 0, 0.95e Drinking-water Lifetime No effects on body weight or Schroeder &
metaborate longevity. Mitchener (1975)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of boron/boron compound.
b Estimated based on feed consumption values of 161 g/kg body weight per day for male and 222 g/kg body weight per day for female controls
at week 4 of treatment.
c Calculated based on water consumption of 0.12-0.14 ml/g per day reported by authors.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
e Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or food factor of
0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for mice.
In a 90-day study, Sprague-Dawley rats (10/sex per dose) received
0, 2.6, 8.8, 26.3, 87.5, or 262.5 mg boron/kg body weight per day in
the diet as boric acid or borax (Weir & Fisher, 1972). Similar effects
were observed with both boric acid and borax. All high-dose animals
died within 3-6 weeks. In animals receiving 87.5 mg boron/kg body
weight per day, body weights in males and females were reduced
43.8-54.9% and 10.1-12.6%, respectively. Absolute organ weights --
including the liver, spleen, kidneys, brain, adrenals, and ovaries --
in this dose group were also significantly decreased. Relative
organ-to-body weights of the adrenals and kidneys were significantly
increased, but relative weights of the liver and ovaries were
decreased. A pronounced reduction in testicular weights in males in
the 87.5 mg boron/kg body weight per day group was also observed;
effects on the male reproductive system are discussed further in
section 7.4.
In a 2-year study, rats (35/sex per dose) were administered
weight-normalized doses of 0, 5.9, 17.5, or 58.5 mg boron/kg body
weight per day in the diet as borax or boric acid (Weir & Fisher,
1972). High-dose animals had coarse hair coats, scaly tails, hunched
posture, swollen and desquamated pads of the paws, abnormally long
toenails, shrunken scrotum, inflamed eyelids, and bloody eye
discharge. These signs became frequent and more pronounced during the
first year but did not change thereafter. Serum chemistry and urine
values were normal; the haematocrit and haemoglobin levels were
significantly lower than in controls. The absolute and relative
weights of the testes were significantly lower, and relative weights
of the brain and thyroid gland were higher, than in controls. In
animals in the mid- and low-dose groups, no significant effects on
general appearance, behaviour, growth, food consumption, haematology,
serum chemistry, or histopathology were observed (Weir & Fisher,
1972).
Weir & Fisher (1972) also fed boric acid or borax to beagle dogs
for 90 days or 2 years. In the 90-day study (weight-normalized doses
of 0, 0.44, 4.38, or 43.75 mg boron/kg body weight per day;
5 animals/sex per dose), testicular effects were observed in males in
the two highest dose groups. In the boric acid study, testis weight
was significantly lower than controls in the middle- and upper-dose
groups (reduced by 25% and 40%, respectively). Although testicular
microscopic structure was not detectably abnormal in the controls and
middle-dose group, 4 of 5 dogs in the high-dose group had complete
atrophy, and the remaining high-dose dog had one-third of tubules
showing some abnormality. In the borax study, testis weights in the
low, middle-, and high-dose groups were 80%, 85%, and 50% of controls,
respectively; only the last was significantly different from controls.
No mention was made of the testicular microscopic structure of the
controls or low-dose animals; middle-dose animals were not detectably
altered (aside from the considerable fixation-induced artifact in the
outer third of the tissue), whereas 4 of 5 high-dose dogs had complete
testicular atrophy, and the remaining high-dose dog had "partial"
atrophy. No other clinical or microscopic signs of toxicity were
reported in any animals. In the 2-year study, the dogs (4/sex per
dose) received the boric acid or borax in the diet at
weight-normalized doses of 1.5, 2.9, or 8.8 mg boron/kg body weight
per day. An additional group received 29 mg boron/kg body weight per
day for 38 weeks. No effects were observed on general appearance, body
weight, food consumption, organ weights, haematology, or serum
chemistry. Changes in testicular morphology occurred in males in the
highest (38-week) dose group; these reproductive changes are described
in section 7.4.
Two reports are available on the toxic effects of boron on bones.
Seffner et al. (1990) exposed growing pigs to boron (4 or 8 mg/kg body
weight per day; source of boron was not specified). They reported a
dose-related thinning of the cortex of the humerus and a reduction
(significant at 8 mg/kg body weight per day) in presumptively
bone-derived serum alkaline phosphatase, suggesting reduced osteoblast
activity. Confidence in this study is moderate because of the
uncertainty of the form/source of boron and the authors' inability to
replicate several previous biochemical findings.
A second report investigated the effects of boric acid
(calculated exposures: <0.2-68 mg boron/kg body weight per day) on
several bone parameters in adult rats (Chapin et al., 1997). This
study found no change in physical bone measures (size, cortical
thickness, etc.) but reported a 5-10% increase in resistance of
vertebrae to a crush force.
7.2.2 Inhalation route
Mice exposed to amorphous elemental boron at 72 mg/m3 for
7 h/day, 5 days/week, for 6 weeks did not exhibit any toxic effects
(Stokinger & Spiegl, 1953). Wilding et al. (1959) conducted numerous
longer-term experiments in rats and dogs exposed to boron oxide
particles (median mass aerodynamic diameter [MMAD] 1.9-2.5 µm). The
exposures took place for 6 h/day and 5 days/week and included rats
exposed at 77 mg boric oxide/m3 for 24 weeks, 175 mg boric oxide/m3
for 12 weeks, or 470 mg boric oxide/m3 for 10 weeks; dogs were
exposed to 57 mg boron oxide/m3 for 23 weeks. No toxic effects were
observed, with normal values obtained for body weight gains, blood
chemistry, haematology, and urinalysis; there were also no microscopic
changes reported.
Subchronic inhalation studies have been performed with boron
trifluoride (Torkelson et al., 1961; Rusch et al., 1986) at
2-18 mg/m3. Reported effects included pneumonitis and reduced body
weight gains and organ weights.
7.3 Dermal and ocular effects
Animal studies show that boron oxide dust can affect the skin and
eyes. When boron oxide dust (50 mg) was applied to the eyes of
rabbits, conjunctivitis resulted (Wilding et al., 1959). Roudabush et
al. (1965) determined that boric acid (5 ml, 10% in water, w/v) and
borax (10 ml, 5% in water, w/v) were mild skin irritants after 24-72 h
following application to abraided skin. Borax was also a mild skin
irritant and boric acid a moderate irritant after 24 and 72 h when
applied to the guinea-pig. Rats fed 88 or 263 mg boron/kg body weight
per day as borax or boric acid had inflamed eyes and skin desquamation
on their paws and tails (Weir & Fisher, 1972).
7.4 Reproductive toxicity
Reproductive and developmental effects of borates in laboratory
animals are summarized in Table 16.
The data regarding subchronic and chronic oral exposure to boric
acid or borax in laboratory animals have unequivocally demonstrated
that the male reproductive tract is a consistent target of toxicity
(Table 16). Testicular lesions have been observed in rats, mice, and
dogs administered boric acid or borax in food or drinking-water
(Truhaut et al., 1964; Weir & Fisher, 1972; Green et al., 1973; Lee et
al., 1978; NTP, 1987; Ku et al., 1993a). The first clinical indication
of testicular toxicity in dogs is shrunken scrota observed during
treatment; significant decreases in absolute and relative testicular
weight are also reported. After subchronic exposure, the
histopathological effects range from inhibited spermiation (the
process of release of mature spermatids from the seminiferous
epithelium) to degeneration of the seminiferous tubules with variable
loss of germ cells, to complete absence of germ cells, resulting in
atrophy and transient or irreversible loss of fertility, but not of
mating behaviour.
Time- and dose-response studies of the Sprague-Dawley male rat
reproductive end-points after acute administration of boric acid
(Linder et al., 1990) revealed adverse effects on spermiation,
epididymal sperm morphology, and caput sperm reserves during
histopathological examinations of the testes and epididymis. Rats were
administered two doses in one day in both experiments, with a total
dose of 0 or 350 mg boron/kg body weight in the time-response
experiment where animals were sacrificed at 2, 14, 28, or 57 days
after exposure and a total dose of 0, 44, 87, 175, or 350 mg boron/kg
body weight in the dose-response experiment with animals sacrificed
after 14 days. In animals receiving 175 or 350 mg boron/kg body
weight, testicular effects apparent at 14 days were enlarged irregular
cytoplasmic lobes of mature spermatids in stage VIII seminiferous
tubules (Leblond & Clermont, 1952) and a substantial increase in the
testicular spermatid head count per testis. The first visible lesion
was inhibited spermiation. Epididymal effects after 14 days included
an increase in abnormal sperm forms, reduced caput epididymal sperm
morphology with head and tail defects, and reduced caput epididymal
sperm reserves. Epididymal sperm parameters had returned to normal and
only minimal testicular changes remained by post-treatment day 57,
indicating that these effects were reversible at these dose levels.
The NOAEL for male reproductive effects in this study was 87 mg
boron/kg body weight.
Table 16. Reproductive and developmental effects of boron compounds in laboratory animals
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid CD-1 0, 19.2, 104.5, Diet 27 weeks None of the high-dose pairs was Fail et al.
mice 220.2 for males; 0, fertile. At the middle dose, (1990, 1991)
31.8, 147.9, 290.2 there was a significant decrease
for females in litters/pair, live pups/litter,
percentage of pups born alive,
and live pup weight. Fertility
index was decreased at the middle
dose. Body weight gain was reduced
in males and females at the high
dose, despite increases in food
and water consumption. Cross-mating
experiments showed that boric acid
affected primarily the male
reproductive system. A dose level
of 220.2 mg/kg body weight per day
significantly reduced reproductive
organ weights in males and altered
sperm motility, concentration, and
morphology. At the high dose,
fertility of the low-dose F1 mice
was not affected, but marginally
reduced sperm concentrations were
seen in males; increases in uterus
and kidney plus adrenal weights
and shorter estrus cycles were
seen in females; and F2 live pup
weights were reduced.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid SD rats 0, 350 Gavage 2-57 days Inhibited sperm release, adverse Linder et al.
changes in sperm morphology; (1990)
reversible by day 57.
Boric acid Rats 0, 60.9b Diet 4-28 days Decreased weight gain; inhibition Treinen
of spermiation starting at day 7 and & Chapin
degeneration in seminiferous tubules (1991)
at day 28; decreased serum
testosterone starting at day 4; no
effects on liver or kidney histology.
Boric acid F-344 0, 26, 38, 52, 68 Feed Weekly to Mild inhibited sperm release at Ku et al.
rats 63 days 26 mg/kg body weight per day; (1993a)
severe inhibited sperm release at
38 mg/kg body weight per day;
progression to testicular atrophy
at 52 and 68 mg/kg body weight per
day, with many other changes
resulting from these primary
effects.
Boric acid Rats 175 Water 15 days Vacuolation and granulation of Silaev et al.
the cytoplasm and absence of (1977)
nuclear chromatin in spermatids
of seminiferous tubules.
Reduction in tubule diameter
and absence of germinative cells.
Borax or Rats 0, 5.9, 17.5, 58.5c Diet Multi- Sterility, lack of spermatozoa, Weir & Fisher
boric acid generation testicular atrophy, decreased (1972)
ovulation at 58.5 mg/kg body
weight per day. No effects at
lower doses.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 0.44, 4.4, 44c Diet 90 days One male dog died at 44 mg/kg Weir & Fisher
boric acid body weight per day; decrease (1972)
in thyroid- and testes-to-body
weight ratios, and severe
testicular atrophy in males at
44 mg/kg body weight per day.
At 0.44 mg/kg body weight per
day, decreased spleen-to-body
weight ratio in males. At
<4.4 mg/kg body weight per day,
no changes in organ weight in
females.
Borax Rats 0, 30, 60, 125-131 Diet 30 or 60 days At the 60 and 125-131 mg/kg Lee et al.
body weight per day doses, liver (1978)
weights were significantly lower
after 60 days; epididymal
weights were significantly lower
(37.6 and 34.8%) after 60 days.
FSH levels were elevated in all
treatment groups after 60 days,
and at the highest dose FSH
levels remained elevated after
12 months.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax Rats 0, 25, 50, 100c Diet 60 days Decrease in weights of liver, Dixon et al.
testes, and epididymis at the (1979)
two highest dietary levels.
Seminiferous tubule diameter
was decreased in a dose-related
manner in all groups. Loss of
germinal cell elements was seen
only in 50 and 100 mg/kg body
weight per day groups. Plasma
levels of FSH were elevated.
Reduced fertility only at two
highest dosage levels.
Borax Rats 0, 23.7, 44.7d Drinking-water 70 days Significant decrease in body Seal & Weeth
weight and decrease in weight (1980)
of testes, seminal vesicles,
spleen at both doses.
Spermato-genesis impaired and
haematocrit decreased slightly
at highest dose.
Borax Rats 0, 0.042, 0.14, 0.84b Drinking-water 90 days No observable reproductive Dixon et al.
effects or changes in serum (1976)
chemistry, body weight, or
weight of testes, prostate,
or seminal vesicles.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 1.5, 2.9, 8.8, 29c Diet 2 years Severe testicular atrophy and Weir & Fisher
boric acid spermatogenic arrest at week (1972)
26 with 29 mg/kg body weight
per day; no effects on body or
organ weights, gross morphology,
or histological parameters at
lower doses.
Boric acid Mice 0, 43.4, 79.0, 175.3 Diet Gestation There was no effect on survival NTP (1989);
days 0-17 or pregnancy rate. Maternal Heindel et al.
effects included mild renal (1992)
lesions at >43.4 mg/kg body
weight per day and increased
water intake, increased relative
kidney weight, and decreased
weight gain during treatment at
175.3 mg/kg body weight per day.
At the high-dose level, there
was a significant increase in
the percentage of resorptions/
litter. Average fetal body
weight/litter was significantly
reduced at >79.0 mg/kg body weight
per day. Malformations included a
missing or shortened XIII rib and
variations on extra lumbar I
rib. Malformations were
significantly increased at
175.3 mg/kg body weight per day.
The percentage of variations/
litter decreased at <79.0 mg/kg
body weight per day and was not
affected at 175.3 mg/kg body
weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats 0, 13.6, 28.5, 57.7, Diet Gestation The highest dose was administered NTP (1990);
94.2 days 0-20 to one group on gestation days Heindel et al.
6-15. There was no effect on (1992)
survival or pregnancy rate.
Maternal rats showed increased
relative liver and kidney weights
at >28.5 mg/kg body weight per
day, increased absolute kidney
weight at 94.2 mg/kg body weight
per day, and a decrease in body
weight gain at >57.7 mg/kg body
weight per day. At low doses,
there was a decrease in extra
lumbar rib I (a variation) and
at high doses an increase in
missing or shortened XIII rib
(a malformation). The 94.2 mg/kg
body weight per day level
increased prenatal mortality.
Fetal body weights were
significantly reduced in all
treated groups in a dose-related
manner. Malformations increased
at >28.5 mg/kg body weight per
day; variations increased at
94.2 mg/kg body weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats Phase 1 Diet Phase 1 Phase 1 -- Fetal body weight was
0, 3.3, 6.3, 9.6, gestation decreased on gestational day 20
13.3, 25 days (gd) in the 13.3 and 25 mg/kg body
0-20 weight per day dose groups. On
Phase 2 Phase 2 gd 20, incidences of short rib
0, 3.3, 6.5, 9.8, postnatal XIII or wavy rib were increased
12.9, 25.4 days (pn) in these dose groups relative
0-21 to controls. The high-dose group
(exposure contained a biologically
limited to relevant but not statistically
gestation significant decrease in
days 0-20) incidence of extra rib on
lumbar I.
Phase 2 -- On pn days 0-21, there Price et al.
were no decreased fetal body (1996a)
weight effects. On pn day 21,
the percentage of pups per
litter with short rib XIII was
elevated in the 25.4 mg/kg body
weight per day dose group. No
wavy rib or extra rib on lumbar
I was found at pn day 21.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rabbits 0, 10.9, 21.9, 43.7 Gavage Exposure on No treatment-related clinical Price et al.
in water gestation signs of toxicity except for (1996b)
days 6-19; vaginal bleeding at 43.7 mg/kg
termination body weight per day. This dose
on gd 30 had no live fetuses on day 30.
Food intake decreased during
treatment (43.7 mg/kg body
weight per day), but increased
on days 25-30 (21.9 and 43.7
mg/kg body weight per day). Body
weight and body weight gain were
decreased during treatment at
43.7 mg/kg body weight per day,
but corrected body weight
increased at 21.9 and 43.7 mg/kg
body weight per day. Gravid
uterine weight and number of
corpora lutea/dam were decreased
at 43.7 mg/kg body weight per
day. Significant developmental
effects were limited to the
high-dose group. Prenatal
mortality was greatly increased.
Malformations were also
increased, primarily owing to
the incidence of fetuses with
cardiovascular defects. The
skeletal variation observed
was sternebrae. Fetal body
weight was slightly reduced
in the high-dose group.
Table 16. (continued)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of
boron/boron compound.
b Estimated by the authors.
c Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or
food factor of 0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for
mice.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
In a 2-year study by Weir & Fisher (1972), groups of 4 male and 4
female beagle dogs were fed diets containing boric acid or borax to
provide doses of 0, 1.45, 2.93, or 8.75 mg boron/kg body weight per
day. No evidence of toxicity was observed. An additional group of dogs
(4 male and 4 female) was fed diets containing boric acid or borax
leading to doses of 0 or 29.3 mg boron/kg body weight per day for 38
weeks. Testicular atrophy was observed in 2 test dogs receiving borax
at 26 weeks. The authors stated that boric acid caused testicular
degeneration in dogs, including spermatogenic arrest and atrophy of
the seminiferous epithelium. This study was terminated at 38 weeks. In
the study, the number of dogs was small and variable (1-2 dogs at each
of three time points) and inadequate to allow statistical analysis. A
common control group was used for both borax and boric acid exposure
groups. Testicular lesions occurred in the controls (1 of 4 controls
had slight to severe seminiferous tubular atrophy, another had
moderate to severe atrophy, whereas a third had a detectable but
insignificant reduction in spermatogenesis and 5% atrophic
seminiferous tubules). This study was conducted before the advent of
Good Laboratory Practices (GLP). Confidence in this study is low, and
it was considered not suitable for inclusion into the risk assessment
because of 1) small and variable numbers of dogs, 2) variable
background lesions in controls leading to uncertainty regarding the
strength of the response to treatment, 3) lack of GLP, and 4) other,
more recent studies of greater scientific quality with findings at a
similar or lower intake level of boron (Ku et al., 1993a; Price et
al., 1996a).
Weir & Fisher (1972) fed Sprague-Dawley rats (35/sex per group) a
diet containing borax or boric acid for 2 years at 0, 5.9, 17.5, or
58.5 mg boron/kg body weight per day. At the high dose, rats receiving
either compound had decreased food consumption during the first 13
weeks of study and decreased growth throughout the study. Testes
weights were significantly decreased at 58.5 mg/kg body weight per
day. The seminiferous epithelium was atrophied, and the tubular size
in the testes was decreased. No treatment-related effects were
observed in rats receiving 5.9 or 17.5 mg boron/kg body weight per
day. The LOAEL in this study was 58.5 mg boron/kg body weight per day
for decreased testes weights, atrophied testes, histopathological
alterations of the testes, and increased brain and thyroid weights.
The NOAEL for this study was 17.5 mg boron/kg body weight per day.
In a companion three-generation reproduction study in rats, Weir
& Fisher (1972) found that 58.5 mg boron/kg body weight per day
produced testicular atrophy and complete suppression of fertility in
rats. Lower doses (17.5 or 5.9 mg boron/kg body weight per day) did
not reduce fertility. The small group size (n = 8), low control
fertility rates (approx. 60%), limited data reported, and
inappropriate statistics all limit the applicability of these data for
risk assessment.
In a multigeneration continuous-breeding experiment (Fail et al.,
1990, 1991), Swiss CD-1 mice (F0 generation) were fed boric acid in
the diet at 0, 1000, 4500, or 9000 mg/kg feed for 27 weeks, which gave
calculated doses of 0, 19.2, 104.7, and 222.1 mg boron/kg body weight
per day for males and 0, 31.9, 148.1, and 290.5 mg boron/kg body
weight per day for females. Treatment with boric acid significantly
impaired fertility: no males or females in the high-dose groups were
fertile. At the middle dose, the number of litters per pair, number of
live pups per litter, proportion of pups born alive, and pup weight
adjusted for litter size were all decreased. The trend towards a lower
fertility index at this dose level started with the first litter and
progressed in severity with subsequent matings. Animals from different
treatment groups were cross-mated to determine the affected sex at
this dose. When mid-dose males were mated with control females, mating
and fertility indices were significantly depressed, with only one pair
in that group producing a live litter; these indices were not affected
when control males were mated with mid-dose females, confirming that
the male was the affected sex. At F0 necropsy, sperm motility was
significantly reduced in all exposed groups (by 12%, 32%, and 47%,
from low- to high-dose groups, respectively). Low-dose and mid-dose
animals from the F1 generation were exposed during gestation and
lactation. The fertility of the low-dose F1 mice was not affected,
but the litter-adjusted body weights of the F2 pups were
significantly decreased (by 3.3%) relative to controls. The low dose
was considered the LOAEL for decreased sperm motility in the F0
males, 26% increased uterine weight and 8% increased kidney/adrenal
weight in F1 females, and a 3.3% reduction in litter-adjusted birth
weight in the F2 pups. This study had no NOAEL, but the magnitude of
the changes at the low dose (a 12% decrease in F0 sperm motility, an
8% increase in F1 kidney/adrenal weights, and a 3% reduction in F2
pup weights) is small and indicates that this dose level is close to
the NOAEL.
Fail et al. (1989) utilized both CD-1 mice and wild deer mice
(Peromyscus maniculatus) to characterize the effects of boric acid
on fertility and to test the reversibility of these effects. Adult
CD-1 mice were exposed to boric acid in the feed for 27 weeks at 0,
1000, 4500, or 9000 mg/kg (consumed doses were not given). The males
at the high and middle doses had testicular atrophy and decreased
spermatogenesis. Fertility was diminished in animals receiving the
middle dose and completely absent in the high-dose group. The same
doses were used to test toxicity and reversibility in the wild mouse.
The ability to produce offspring was significantly reduced by boric
acid: none of the males in the high-dose group sired litters. Weight
gain was similar for the treated and control wild mice. Although body
and organ weights were similar between the mid-dose and control groups
at necropsy, the testis and total accessory sex organ weights were
significantly lower in the high-dose group. These two species appear
to differ in their sensitivity to boric acid. The CD-1 mice had body
weight loss as early as 5 weeks at the mid-dose level, whereas deer
mice did not lose weight even at the high dose. Tissue pathology
findings at the middle dose were significant in CD-1 mice but not in
deer mice. Deer mice were found to recover from boric acid exposure.
The development of the testicular lesion was investigated by
Treinen & Chapin (1991), who fed boric acid at a level of 0 or 60.9 mg
boron/kg body weight per day to male F-344 rats and sacrificed six
treated and four control male rats at intervals from 4 to 28 days
after the start of exposure. In half of the treated rats, there was
inhibition of spermiation in 10-30% of stage IX tubules at 7 days and
inhibition in all stage IX and stage X tubules of exposed rats at 10
days. At 28 days, there was significant loss of spermatocytes and
spermatids from all tubules in exposed rats, and basal serum
testosterone levels were significantly decreased from 4 days on.
Secondary to the loss of germ cells, the activities of enzymes
found primarily in spermatogenic cells were significantly decreased,
and enzyme activities associated with premeiotic spermatogenic cells
were significantly increased in Sprague-Dawley rats exposed to 60 or
125-131 mg boron/kg body weight per day for 30 or 60 days (Lee et al.,
1978). Mean plasma follicle stimulating hormone (FSH) levels were
significantly elevated in a dose-dependent manner in all treatment
groups in this study (30 to 125-131 mg boron/kg body weight per day)
after 60-day exposures. FSH levels in animals exposed to the highest
dose tested (125-131 mg boron/kg body weight per day) were still
elevated 12 months after treatment termination, owing to atrophied
testes and no recovery of spermatogenesis. Plasma luteinizing hormone
(LH) levels were not significantly elevated, and mean plasma
testosterone levels were within the normal range throughout the study
(Lee et al., 1978).
Reversibility of testicular lesions was evaluated by Ku et al.
(1993a) in an experiment in which F-344 rats were dosed at 3000, 4500,
6000, or 9000 mg boric acid/kg (26, 38, 52, and 68 mg boron/kg body
weight per day) in the feed for 9 weeks and assessed for recovery up
to 32 weeks post-treatment. Inhibited spermiation was exhibited at
3000/4500 mg boric acid/kg (5.6 µg boron/mg tissue), whereas inhibited
spermiation progressed to atrophy at 6000/9000 mg boric acid/kg (11.9
µg boron/mg testes), with no boron accumulation in the testes to
levels greater than found in blood during the 9-week period. After
treatment, serum and testis boron levels in all dose groups fell to
background levels. Inhibited spermiation at 4500 mg/kg was reversed by
16 weeks post-treatment, but focal atrophy was detected that did not
recover up to 32 weeks post-treatment.
An unpublished study by W.W. Ku and colleagues (reported in Ku et
al., 1993a) found no detectable treatment-related changes in
testicular structure in rats induced by consumption of 17.5 mg
boron/kg body weight per day for up to 9 weeks.
In a follow-up study to explore/identify the mechanism for this
testicular toxicity of boric acid, Ku et al. (1993b) evaluated several
end-points in cell culture systems following in vitro boric acid
exposure. The data suggest an effect of boric acid on the DNA
synthesis activity of mitotic and meiotic germ cells and, to a lesser
extent, on energy metabolism in Sertoli cells. The effect on DNA
synthesis occurred at boron concentrations that were associated with
atrophy in vivo and shows that boric acid interferes with the
production and/or maturation of early germ cells; this offers an
explanation for atrophy, but not for inhibited spermiation.
Additional mechanistic studies by Ku & Chapin (1994) showed that
testicular toxicity and CNS hormonal effects were not due to selective
boron accumulation in testis or brain/hypothalamus, with testis boron
concentrations at 1-2 mmol/litre. Changes in testis phosphorus,
calcium, and zinc levels did not precede atrophy. In vitro studies
showed no effect on the steroidogenic functions of isolated Leydig
cells, supporting the suggestion of a CNS-mediated hormonal effect.
The authors showed that inhibited spermiation was not due to increased
testicular cyclic adenosine monophosphate or reduced serine protease
plasminogen activators. Also evaluated were effects of boric acid in
Sertoli-germ cell co-cultures on Sertoli cell energy metabolism
(lactate secreted by Sertoli cells is a preferred energy source for
germ cells) and DNA/RNA synthesis (germ cells synthesize DNA/RNA, and
boric acid impairs the synthesis of these nucleic acids in the liver).
The most sensitive in vitro end-point was DNA synthesis in
mitotic/meiotic germ cells; energy metabolism in germ cells was
affected to a lesser extent, which was manifested in vivo as a
decrease in early germ cell/Sertoli cell ratio prior to atrophy in the
testes. The mechanisms of inhibited spermiation are still not defined.
In summary, male reproductive effects from boron have been noted
in mice, rats, and dogs. Reproductive effects observed include
inhibition of spermiation in stage IX and X tubules, followed by germ
cell loss, changes in epididymal sperm morphology and caput sperm
reserves, decreased serum testosterone levels, and testicular atrophy.
Male reproductive effects have been reported in oral exposure studies
at doses as low as 29 mg boron/kg body weight per day in dogs exposed
for 2 years to dietary boric acid or borax (Weir & Fisher, 1972); for
rats in this same study, the LOAEL for reproductive toxicity was 58.5
mg boron/kg body weight per day.
7.5 Developmental toxicity
Developmental toxicity has been demonstrated experimentally in
rats, mice, and rabbits (see Table 16) (Heindel et al., 1992; Price et
al., 1996b).
Sprague-Dawley rats were fed a diet containing 0, 13.6, 28.5, or
57.7 mg boron/kg body weight per day as boric acid from gestation days
0 to 20 (Heindel et al., 1992). An additional group of rats received
boric acid at 94.2 mg boron/kg body weight per day on gestation days
6-15 only. Maternal effects included a significant and dose-related
increase in relative liver and kidney weights at >28.5 mg boron/kg
body weight per day. Treatment with 94.2 mg boron/kg body weight per
day significantly increased prenatal mortality. Average fetal body
weight per litter was significantly reduced in a dose-related manner
in all treated groups compared with controls. The percentage of
malformed fetuses per litter and the percentage of litters with at
least one malformed fetus were significantly increased at >28.5 mg
boron/kg body weight per day. Malformations consisted primarily of
anomalies of the eyes, the CNS, the cardiovascular system, and the
axial skeleton. The most common malformations were enlargement of
lateral ventricles in the brain and agenesis or shortening of rib
XIII. The percentage of fetuses with variations per litter was reduced
relative to controls at 13.6 and 28.5 mg boron/kg body weight per day
(due to a reduction in the incidence of rudimentary or full ribs at
lumbar 1) but was significantly increased in rats exposed to 94.2 mg
boron/kg body weight per day. The variation with the highest incidence
among fetuses was wavy ribs. The LOAEL of 13.6 mg boron/kg body weight
per day (lowest dose tested) for rats occurred in the absence of
maternal toxicity; a NOAEL was not found in this study.
Price et al. (1996a) did a follow-up to the Heindel et al. (1992)
study in Sprague-Dawley (CD) rats to determine a NOAEL for fetal body
weight reduction and to determine whether the offspring would recover
from prenatally reduced body weight during postnatal development.
Skeletal malformations and variations were also studied to further
characterize the low end of the dose-response curve (phase 1) and to
determine whether the incidence of skeletal defects in offspring
changed during postnatal life (phase 2). Boric acid was administered
in the diet to CD rats from gestational day 0 to 20. In phase 1, dams
were terminated and uterine contents examined on gestational day 20.
During phase 1, the intake of boric acid was 0, 3.3, 6.3, 9.6, 13.3,
or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls;
the reduction was significant only in the 13.3 and 25 mg boron/kg body
weight per day dose groups on gestational day 20. During phase 1,
incidences of short rib XIII (a malformation) and wavy rib (a
variation) were increased in the >13.3 mg boron/kg body weight per
day dose groups relative to control litters. There was a decreased
incidence of rudimentary extra rib on lumbar 1 (a variation) in the
high-dose group that was deemed biologically but not statistically
significant. During phase 2, the intake of boric acid during gestation
was 0, 3.3, 6.5, 9.8, 12.9, or 25.3 mg boron/kg body weight per day.
At birth, boric acid exposure stopped and dams were allowed to deliver
and rear their litters until postnatal day 21. On postnatal day 0 of
phase 2, there were no effects of boric acid on offspring body weight,
nor were any differences seen through postnatal day 21. On postnatal
day 21 of phase 2, the percentage of pups per litter with short rib
XIII was elevated only in the 25.3 mg boron/kg body weight per day
dose group, but there was no treatment-related increase in wavy rib or
extra rib (full or rudimentary) on lumbar 1 observed in these pups on
day 21. The NOAEL for phase 1 of this study is 9.6 mg boron/kg body
weight per day based on a decrease in fetal body weight. The LOAEL was
13.3 mg boron/kg body weight per day for phase 1. The NOAEL for phase
2 was 12.9 mg boron/kg body weight per day, and the LOAEL was 25.3 mg
boron/kg body weight per day. The results of this study provide a
NOAEL and LOAEL that complement the rat LOAEL of 13.6 mg/kg body
weight per day in the Heindel et al. (1992) study.
Heindel et al. (1992) also investigated the developmental
toxicity and teratogenicity of boric acid in mice at 0, 43, 79, or 175
mg boron/kg body weight per day in the diet. There was a significant
dose-related decrease in average fetal body weight per litter at 79
and 175 mg boron/kg body weight per day. In offspring of mice exposed
to 79 or 175 mg boron/kg body weight per day during gestation days
0-17, there was an increased incidence of skeletal (rib)
malformations. These changes occurred at doses for which there were
also signs of maternal toxicity (increased kidney weight and
pathology); the LOAEL for developmental effects (decreased fetal body
weight per litter) was 79 mg boron/kg body weight per day, and the
NOAEL for developmental effects was 43 mg boron/kg body weight per
day.
Price et al. (1996b) investigated the developmental toxicity and
teratogenicity of boric acid in rabbits at doses of 0, 10.9, 21.9, or
43.7 mg boron/kg body weight per day given by gavage. Frank
developmental effects in rabbits exposed to 43.7 mg boron/kg body
weight per day included a high rate of prenatal mortality, increased
number of pregnant females with no live fetuses, and fewer live
fetuses per live litter on day 30. Also at 43.7 mg boron/kg body
weight per day, malformed live fetuses per litter increased
significantly, primarily because of the incidence of fetuses with
cardiovascular defects, the most prevalent of which was
interventricular septal defect. Skeletal variations observed were
extra rib on lumbar 1 and misaligned sternebrae. The NOAEL for
maternal and developmental effects was 21.9 mg boron/kg body weight
per day, and the LOAEL for maternal and developmental effects was 43.7
mg boron/kg body weight per day.
7.6 Mutagenicity and related end-points
Boric acid was not mutagenic in Salmonella typhimurium with or
without rat or hamster S9 fraction (Haworth et al., 1983; Benson et
al., 1984; NTP, 1987) or in mouse lymphoma L5178Y/TK+/ cells with or
without rat liver S9 (NTP, 1987; McGregor et al., 1988). Borax was not
mutagenic in Salmonella with or without rat liver S9 (Benson et al.,
1984). Refined borax, crude borax ore, and kermite ore were not
mutagenic in V79 Chinese hamster cells, C3H/1OT1/2 mouse embryo
fibroblasts, or diploid human foreskin fibroblasts (Landolph, 1985).
Sodium perborate (NaBO3) was shown to interact with DNA in the
Escherichia coli Pol A assay, presumably by being converted to
hydrogen peroxide (Rosenkranz, 1973). Other tests showed that boric
acid did not induce chromosomal aberrations or sister chromatid
exchanges in Chinese hamster ovary cells (NTP, 1987). Existing data
suggest that genotoxicity is not an area of concern following exposure
to boron compounds in humans.
7.7 Carcinogenicity
The 2-year feeding study (NTP, 1987; Dieter, 1994) showed no
evidence of carcinogenicity in B6C3F1 mice. The Weir & Fisher (1972)
study showed no evidence of boric acid-related carcinogenicity in
rats, although not all tissues were examined. Based on the lack of
human data and on the data from these two animal tests, boron is
classified by the US EPA as a Group D chemical (not classifiable as to
human carcinogenicity) (US EPA, 1994).
7.8 Toxicity effects summary
The laboratory animal toxicity data show that boric acid (and
other borates) is not a carcinogen and lacks significant mutagenicity.
The crossover mating trial in the Fail et al. (1991) study showed that
female mouse reproduction was unaffected by about 120 mg boron/kg body
weight per day. The NOAELs and LOAELs of reproductive/developmental
effects from various studies are ranked in Table 17. The Weir & Fisher
(1972) dog study is omitted from this summary because of low numbers
of animals and the presence of lesions in the controls. It can be seen
that rat fetal development is more sensitive than other processes to
the adverse effects of elevated boron exposure, with a LOAEL in rats
of 13.3 mg boron/kg body weight per day reducing fetal weight gain and
increasing rib anomalies (reversibly). The current NOAEL for these
effects in rats is approximately 10 mg boron/kg body weight per day.
Increased doses produced sequentially additional effects on sperm
release, increased rabbit cardiovascular defects, reduced epididymal
sperm counts, testicular atrophy, and mouse fetal defects.
The Task Group recognized the lack of a NOAEL in the Fail et al.
(1991) study. The effects of concern in part duplicated the
developmental toxicity findings (reduced pup body weight). However,
the unique effects in the adult F1 female mice (increased uterine
weight, reduced cycle length) are consistent with an effect on female
reproduction that was not seen in the F0 females. The possibility of
unique transgenerational or functional developmental toxicity in the
absence of an available dose-response concerned the Task Group.
Although the current NOAEL is based on rat fetal body weight
effects, the Task Group noted that this could be lowered and a new
"critical effect" could emerge if the significant data gap
(second-generation fertility and necropsy data for multiple doses
including a NOAEL in rats) were filled.
7.9 Physiological effects
Since 1981, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for higher animals;
that is, a dietary deprivation of boron consistently results in
changed biological functions that can be construed as detrimental and
are preventable or reversible by an intake of physiological amounts of
boron. Convincing findings have been reported that show that dietary
boron deprivation affects mineral and energy metabolism in chicks and
Table 17. Ranking of reproductive/developmental effects of boric acid
Species/durationa Dose (mg boron/kg Effect Reference
body weight per day)
SD rat/gd 0-20 9.6 NOAEL for developmental effects immediately pre-term Price et al. (1996a)
SD rat/gd 0-20 12.9 NOAEL for developmental effects measured at weaning Price et al. (1996a)
SD rat/gd 0-20 13.3 LOAEL for reduced fetal weight, increased rib Price et al. (1996a)
13.6 malformations/variations Heindel et al. (1992)
Male SD rat/multigeneration 17.5 NOAEL for male sterility, testicular atrophy Weir & Fisher (1972)
SD rat/gd 0-20 25.4 LOAEL for increased short rib XIII at weaning Price et al. (1996a)
Male SD rat/63 days 26 LOAEL for mild inhibited sperm release Ku et al. (1993a)
Male SD rat/¾63 days 52 LOAEL for testicular atrophy Ku et al. (1993a)
Male beagle dogs/2 years 29 Altered testis weight and histopathology Weir & Fisher (1972)
Male beagle dogs/90 days 44 Altered testis weight and histopathology Weir & Fisher (1972)
CD-1 mouse/multigeneration 19.2 LOAEL for reduced sperm motility, reduced F2 pup weight Fail et al. (1991)
CD-1 mouse/gd 0-17 43 NOAEL for mouse developmental toxicity Heindel et al. (1992)
79 LOAEL for decreased fetal body weight
New Zealand white rabbits/ 21.9/43.7 NOAEL/LOAEL for decreased fetal body weight, Price et al. (1996b)
gd 6-19 increased fetal cardiovascular malformations and
maternal toxicity
a gd = gestational days.
rats (Hunt, 1994); these effects are more marked when a physiological
stressor is present, especially marginal cholecalciferol (vitamin D3)
nutriture. For example, a boron supplement of 3 µg/g to a basal diet
containing 0.465 µg boron/g alleviated the marginal cholecalciferol
deficiency-induced changes in bone, plasma glucose, energy substrate
utilization, and growth (Hunt, 1994). Hunt et al. (1994) have found
that boron deprivation depresses macromineral content in bone and some
indices of the maturation of the cartilage growth plate independently
of vitamin D nutriture. Brain composition and function in rats are
also affected by dietary boron. Boron deprivation was found to
systematically influence brain electrical activity assessed by an
electrocorticogram in mature rats (Penland & Eberhardt, 1993). In this
study, brain copper concentrations were higher in boron-deprived than
in boron-supplemented rats. Furthermore, calcium concentrations in
total brain and in brain cortex, as well as phosphorus concentration
in the cerebellum, were found to be higher in boron-deprived (0.158
µg/g diet) than in boron-supplemented (2.72 µg/g diet) rats fed a
cholecalciferol-deficient diet (Hegsted et al., 1991). This study also
found that the apparent absorption and balance of calcium, magnesium,
and phosphorus were decreased by boron deprivation. Hunt (1988) has
reported that chicks apparently require about 1.0 µg boron/g diet for
normal development. These studies collectively indicate that boron in
physiological amounts is beneficial to, if not essential for, higher
animals (see also section 8.4).
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Short-term toxicity and poisoning incidents
Available human exposure data on boron compounds for routes other
than inhalation focus on boric acid and borax. According to Stokinger
(1981), the lowest lethal dose for humans exposed to boric acid is 640
mg/kg body weight by oral exposure, 8600 mg/kg body weight by dermal
exposure, and 29 mg/kg body weight by intravenous injection. Stokinger
(1981) stated that deaths can occur at doses between 5 and 20 g of
boric acid total for adults and below 5 g total for infants. Litovitz
et al. (1988) stated that potential lethal doses are usually cited as
3-6 g total for infants and 15-20 g total for adults. A case-series
report of seven infants (aged 6-16 weeks) who used pacifiers coated
with a borax and honey mixture for 4-10 weeks reported that exposures
ranged from 4 to 30 g, with an estimated average daily ingestion of
0.143-0.429 g (O'Sullivan & Taylor, 1983). The actual relative doses
are unknown. Toxicity was manifested by generalized or alternating
focal seizure disorders, irritability, and gastrointestinal
disturbances. Other findings included inflammation, congestion,
oedema, exfoliation of the mucosa, cloudy swelling and granular
degeneration of tubular cells, and exfoliative dermatitis. Although
infants appear to be more sensitive than adults to boron compounds,
lethal doses are not well documented in the literature.
A few case reports have been published for poisoning incidents.
Teshima et al. (1992) reported that a 26-year-old woman ingested 21 g
of boric acid. The elimination of boric acid was about 4 times faster
with haemodialysis than with conventional medical treatment. The
patient was discharged from the hospital 12 days following admission.
In another case report, Grella et al. (1976) described transplacental
poisoning. A pregnant woman who had a personal history of diabetes was
accidentally given 70 g of boric acid instead of 70 g of glucose for
the glucose tolerance test at 33 weeks' gestation. She was immediately
treated with gastric lavage and intravenous sodium bicarbonate
fructose. The woman developed contractions, and an emergency caesarean
delivery was scheduled. The infant was born alive weighing 2.5 kg and
had spontaneous respirations. Soon afterwards, the infant developed
cardiac arrest, was resuscitated, and died. Cause of death was
attributed to cardiocirculatory collapse.
Boric acid and borax were widely used in medicine at the
beginning of the century for therapeutic purposes, both locally as
well as orally. Boric acid was used to treat various diseases, such as
epilepsy and infectious diseases. Several case studies reviewed by
Kliegel (1980) describe mild to severe responses to boron compounds.
Among all 19 patients with reported body hair loss as a response to
boron compound treatment, 16 persons had epilepsy and 3 had urinary or
genital infections. Stein et al. (1973) described an extremely unusual
case of a 32-year-old woman who ingested and swallowed several bottles
of mouthwash containing boric acid daily for a minimum of 1 year. The
woman was reported to have pancreatitis resulting from heavy alcohol
ingestion and medications for epilepsy. The woman lost almost all of
her body and scalp hair; other clinical signs included erythema on the
palms of her hands, severe fatigue, anorexia, and mental confusion.
Her blood boric acid level was 3.2 mg/100 ml, corresponding to 0.56 mg
boron/100 ml (the normal value was reported as 0.3 mg boric acid/100
ml). Upon cessation of mouthwash consumption, hair growth returned,
suggesting that the effect was reversible.
Goldbloom & Goldbloom (1953) reported four cases of boric acid
poisoning and reviewed an additional 109 cases in the literature. The
four cases were infants exposed to boric acid by repeated topical
applications of baby powder. Toxicity was manifested by cutaneous
lesions (erythema over the entire body, excoriation of the buttocks,
and desquamation), gastrointestinal disturbances, and seizures. One
patient died, but cause of death was not specifically attributed to
boric acid. Approximately 35% of the 109 other case reports involved
children under 1 year of age. The mortality rate was 70.2% for
children compared with 55.0% for all cases combined. Death occurred in
27/51 (53%) patients exposed by ingestion, in 3/4 (75%) patients
subjected to gastric lavage with boric acid, in 19/28 (68%) patients
exposed by dermal application for treating burns, wounds, and skin
eruptions, and in 14/26 (54%) patients exposed by other routes.
Information on signs and symptoms for 80 patients showed that
gastrointestinal disturbances were prevalent (73%), followed by CNS
effects (67%). Cutaneous lesions were prevalent in 76% of the cases
and in 88% of cases involving children under 2 years of age. Gross and
microscopic findings were reported for 27/60 (45%) fatal cases. In
general, boric acid caused chemical irritation primarily at sites of
application and excretion and in organs with maximum boron
concentrations. The most common CNS findings were oedema and
congestion of the brain and meninges. Other common findings included
liver enlargement, vascular congestion, fatty changes, swelling, and
granular degeneration (n = 13).
In addition to case reports, poison centres have published
case-series reports. Unlike the case reports reviewed by Goldbloom &
Goldbloom (1953), more recent reports suggest that the oral toxicity
of boron in humans is milder than previously thought. Litovitz et al.
(1988) conducted a retrospective review of 784 cases of boric acid
ingestion reported to the National Capital Poison Center in
Washington, DC, USA, during 1981-1985 and the Maryland Poison Center
in Baltimore, MD, USA, during 1984-1985. The amount of boric acid
ingested and clinical manifestations of toxicity were reported; 88.3%
of the cases were asymptomatic. All but two of the cases had acute
(single) ingestion, and 80.2% involved children under 6 years of age.
No severe toxicity or life-threatening effects were noted, although
boric acid levels in blood serum ranged from 0 to 340 µg/ml. The most
frequently occurring symptoms, which involved the gastrointestinal
tract, included vomiting (n = 32), abdominal pain (n = 15),
diarrhoea (n = 13), and nausea (n = 7). Other symptoms (primarily
CNS and cutaneous) occurred in six or fewer cases: lethargy, rash,
headache, light-headedness, fever, irritability, and muscle cramps.
The average dose ingested estimated from 659 cases was 1.4 g (range
0.010-88.8 g). For children under 6 years, the average dose was 0.5 g
(range 0.010-22.2 g), compared with 4.1 g (range 0.030-88.8 g) for
individuals age 6 years or above. The average dose for asymptomatic
cases was 0.9 g (range 0.010-88.8 g), compared with 3.2 g (range
0.10-55.3 g) for symptomatic cases. According to Litovitz et al.
(1988), 21 of the children under 6 years of age, 15 of whom were under
2 years of age, ingested the reported potential lethal dose of 3 g; 8
adults ingested the reported potential lethal dose of 15 g without
evidence of lethal effects.
Linden et al. (1986) published a retrospective review of 364
cases of boric acid exposure reported to the Rocky Mountain Poison and
Drug Center in Denver, CO, USA, between 1983 and 1984. Vomiting,
diarrhoea, and abdominal pain (incidence not reported) were the most
common symptoms given by the 276 cases exposed in 1983. Of the 72
cases reported in 1984 for whom medical records were complete, 79%
were asymptomatic, whereas 20% had mild gastrointestinal symptoms
noted. One 2-year-old child died, presumably from repeated ingestion
of an insecticide containing 99% boric acid.
Overall, owing to the wide variability of data collected from
poisoning centres, the average dose of boric acid required to produce
clinical symptoms is still unclear but is presumably within the range
of 100 mg to 55.5 g, observed by Litovitz et al. (1988).
8.1.2 Reproductive effects
An ecological study assessed boron exposure from drinking-water
and fertility among residents in two geographical regions in
Turkey.a Region I comprised 2368 residents, whereas Region II
comprised 2319 residents. Boron levels in drinking-water were
noticeably higher in Region I (range 2.05-29 mg/litre) than in Region
II (range 0.03-0.40 mg/litre). Ever-married residents from each region
who could provide reproductive histories for three generations of
family members represented the study sample -- i.e. 159 probands (6.7%
of population) in Region I and 154 (6.7%) in Region II. The
percentages of married couples with one or more live births (>90%)
were comparable for the two regions, regardless of generation
assessed. The overall percentage of couples with unresolved
infertility or those without children across three generations was
comparable for the two regions (i.e. 6.0% and 4.6%, respectively).
Secondary sex ratios (ratio of male to female live births) appeared to
be different for the two regions. Region I had a ratio below 1 (0.89),
suggesting an excess of female births; Region II had a ratio slightly
above 1 (1.04), suggesting a slight excess of male births. Statistical
a Sayli BS, Tüccar E, & Ellan AH (1996) An assessment of fertility in
boron-exposed Turkish subpopulations. Unpublished manuscript, Ankara
University Medical Faculty, Ankara, Turkey.
significance was not formally evaluated in any of the above analyses.
The results of this descriptive study suggest that fertility, as
measured by the ability to produce a live birth, is not adversely
affected for residents of this geographical area with high levels of
boron in their drinking-water and soil. The observed reversal of the
secondary sex ratio for Region I requires careful interpretation, as
no attention was given to factors reported to alter sex ratios (e.g.
advancing parental age, elective abortion rates, and multiple births).
Further consideration of potential sources of bias stemming from the
selection of respondents who were largely male and their ability to
accurately recollect and report fertility outcomes is needed. The
extent to which these findings are generalizable, if at all, to other
populations is unknown.
8.2 Occupational exposure
8.2.1 Short-term irritative effects
The majority of toxic effects reported in occupational studies
are acute or short-term in nature and result from the irritant effects
of boron compounds. Several studies have shown that workers exposed to
borax complain of symptoms that are due to respiratory irritation,
which include nosebleeds, eye and nasal irritation, sore throats,
cough, and shortness of breath; dermatitis has also been reported
(Birmingham & Key, 1963; NIOSH, 1978). Generally, people with
hyperreactive respiratory tracts (e.g. asthmatics) may experience more
severe irritant effects following inhalation exposure. However, there
is a lack of data to support the existence of an especially sensitive
high-risk human population for exposure to boron or boron compounds.
A medical survey of 113 workers employed in a borax mining and
refining plant was conducted by Garabrant et al. (1984). The reference
group comprised 214 workers employed in areas with low or minimal
exposure to boric acid and boron oxide; exposed workers had been
employed for a minimum of 5 years or were currently employed in areas
of heavy borax exposure. The average air concentration of particles
<5 µm in diameter measured from samples taken between 1979 and 1981
was 4.1 mg/m3 (range 1.2-8.5 mg/m3). The workers in both groups were
predominantly white males; the mean age of the exposed group was 38.2
years, compared with 42.1 years in the referent group. The mean
duration of employment for exposed and unexposed workers was 11.0 and
12.9 years, respectively. Smoking patterns were similar for the two
groups. Symptoms reported more frequently by exposed workers than by
unexposed workers (p < 0.001) were eye irritation (12.4% and 2.8%,
respectively), dry mouth, nose, or throat (10.6% and 1.9%,
respectively), sore throat (5.3% and 0.5%, respectively), and
productive cough (4.4 and 0%, respectively). No significant
differences were noted between the two groups of workers with respect
to dry cough, shortness of breath, chest tightness, chest pain, and
nosebleeds.
A more detailed analysis of 629 (93% participation) workers in
this plant was presented by Garabrant et al. (1985). This analysis was
based on frequency of acute symptoms in four mean boron dust exposure
categories (1.1, 4.0, 8.4, and 14.6 mg/m3) and persistent symptoms
(presumably chronic effects) in three exposure categories (0.9, 4.5,
and 14.6 mg/m3 of total particulates). The particles were composed
almost entirely of borax. Acute symptoms showing a significant linear
trend (p < 0.0001) in order of decreasing frequency were dryness of
mouth, nose, throat, and eye irritation, dry cough, nosebleeds, sore
throat, productive cough, shortness of breath, and chest tightness.
The frequency of these symptoms in the highest exposure category
ranged from 5% to 33%. The only symptom reported by 5% or more of
workers exposed to 4.0 mg/m3 was eye irritation; no symptoms were
reported by 5% or more of workers exposed to 1.1 mg/m3. The pulmonary
function findings were not significantly affected by exposure to
boron. Chest X-rays did not show abnormal regions indicative of boron
exposure. The authors concluded that borax caused simple respiratory
irritation that produces chronic bronchitis with no impairment of
pulmonary function. Also, borax dust appeared to cause acute and
persistent respiratory irritation at concentrations >4.5 mg/m3.
Therefore, the value for the intermediate exposure category for
persistent symptoms (4.5 mg/m3 as borax) was considered to be the
LOAEL.
Wegman et al. (1994) conducted a prospective cohort study to
examine acute irritative effects as well as chronic pulmonary function
abnormalities in mine and processing plant workers exposed to sodium
borate dust. Exposure-response associations were also examined as
related to acute irritant symptoms associated with sodium borate dust
exposure in mining and processing plants. In comparison with unexposed
workers, exposed workers reported significantly more nasal irritation
(rate ratio [RR] = 8.8), eye irritation (RR = 5.2), throat irritation
(RR = 2.9), cough (RR = 1.7), and breathlessness (RR = 7.1).
Continuous measures of particulate exposure were made throughout the
day and were related to hourly measurements of health outcomes.
Exposure-response relationships were present for each of the specific
symptoms at several symptom intensity levels. Acute irritant effects
did not lead to any undue chronic sequelae. With regard to the
acceptable level of risk, Wegman et al. (1994) concluded that a
threshold limit value (TLV) exposure of 10 mg borate/m3 was
protective of workers' health. This value is consistent with the
nuisance dust standard (ACGIH, 1991).
8.2.2 Male reproductive and other long-term health effects
Effects on the male reproductive system have also been reported,
although data regarding subchronic or chronic exposure on the
population in general are limited.
In the study by Wegman et al. (1994), sodium borate particulate
exposure estimates were used to estimate cumulative exposure in
relation to long-term pulmonary function. Of the 631 workers who
originally underwent pulmonary evaluation 7 years earlier, 336 (53%)
underwent a subsequent evaluation. Ninety (303/336) per cent of
workers were found to have acceptable pulmonary test results. After
the expected smoking-related pulmonary abnormalities were taken into
account, no relation was observed between forced expiratory volume in
1 second (FEV1) and accumulated exposure to sodium borate. People
with hyperreactive respiratory tracts such as those with asthma may
experience more severe irritant effects following inhalation exposure.
However, a sensitive population was not found.
Fertility following occupational exposure to boron was assessed
in a descriptive study. Whorton et al. (1994) estimated the
standardized birth ratio (SBR) to assess fertility in 542/750 (72%
participation) occupational workers in a US borax mine located in the
Mojave Desert, California, USA. These workers are unique, in that
their occupational exposure to sodium borate dust and desert soil is
uncontaminated by other exposures and given their long average length
of employment (mean 18 years). Self-administered questionnaires were
used to ascertain the observed number of live births fathered by male
workers following employment. The US general population adjusted for
age, race, parity, and calendar time period was used to estimate the
expected number of live births. The SBR is simply a ratio of the
observed number of live births to the expected number. An SBR above
100 reflects an excess of live births in relation to the US general
population, whereas an SBR below 100 reflects a deficit. Occupational
boron exposure was based on job titles and categorized into quintiles
of mean exposure levels ranging from <0.82 mg/m3 to >5.05 mg/m3.
SBRs were significantly elevated for workers in the lowest
(<0.82 mg/m3) and highest (>5.05 mg/m3) exposure categories
(i.e. 151 and 125, respectively). No significant trend between
exposure and SBRs was observed. An excess percentage of female live
births in comparison with male births was observed across most
categories of exposure and length of employment. The authors noted
that this female excess did not reflect a deficiency of male births,
as an excess of births for both genders was found. None of the
findings, however, was statistically significant. These findings need
to be carefully interpreted, largely given the selection of the US
general population as the standard. (The authors state that fertility
rates were not available for their target population.) The extent to
which this sample of workers is comparable to the US general
population with respect to factors that affect fecundity and fertility
remains to be established. Further considerations are needed with
respect to the calculation of SBRs, such as the exclusions of multiple
births, and in terms of potential response bias. The authors did
attempt to enhance the validity of self-reported fertility data and
select co-variates by using additional data sources and by efforts to
minimize selection bias. In sum, the findings do not support an
adverse effect of boron on demonstrated fertility for this
occupational sample of male workers in comparison with the US general
population.
8.3 Carcinogenicity
Evidence for carcinogenicity of boron and boron compounds in
humans is not available, largely because it has not been studied.
Based on the evidence from two lifetime studies in mice (NTP, 1987)
and rats (Weir & Fisher, 1972), boron compounds have been classified
by the US EPA in Group D (i.e. not classifiable as to human
carcinogenicity) (US EPA, 1994).
8.4 Physiological effects
Since 1987, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for humans; that is,
a dietary deprivation of boron consistently results in changed
biological functions that could be construed as detrimental and that
are preventable or reversible by an intake of physiological amounts of
boron. Many of these changed functions caused by boron deprivation
have been duplicated in animal models. The major reason boron is not
generally recognized as essential or nutritionally important for
humans is probably that a specific biochemical function for boron has
not been elucidated, or, as demonstrated for plants (also for which no
biochemical function has been elucidated), boron has not been shown
necessary to complete the life cycle. Nonetheless, findings from human
experiments show that boron is a dynamic trace element that can affect
the metabolism or utilization of numerous substances involved in life
processes, including calcium, copper, magnesium, nitrogen, glucose,
triglycerides, reactive oxygen, and estrogen. Through these effects,
boron can affect the function or composition of several body systems,
including blood, brain, and skeleton, in a positive manner, which
demonstrates that boron is a beneficial element, if not an essential
mineral element, at physiological amounts.
Although the first findings involving boron deprivation of humans
appeared in 1987 (Nielsen et al., 1987), the most convincing findings
have come mainly from two studies in which men over the age of 45,
postmenopausal women, and postmenopausal women on estrogen therapy
were fed a low-boron diet (0.25 mg/2000 kcal) for 63 days and then fed
the same diet supplemented with 3 mg boron/day for 49 days (Nielsen,
1989, 1994; Nielsen et al., 1990, 1991, 1992b; Penland, 1994). These
dietary intakes were near the low and high values in the range of
usual dietary boron intakes (see section 5.2.4). The major differences
between the two studies were the intakes of copper and magnesium; in
one experiment they were marginal or inadequate, whereas in the other
they were adequate. Boron deprivation had several effects, regardless
of dietary copper and magnesium. In addition, the marginal or
inadequate copper and magnesium caused apparent detrimental changes
that were more marked during boron deprivation than during boron
repletion. Among the effects of boron supplementation after 63 days of
boron depletion in these experiments were the following: an effect on
macromineral metabolism, evidenced by increased serum
25-hydroxycholecalciferol and a decrease in the elevated calcitonin
that was apparently caused by inadequate copper and magnesium; an
effect on energy metabolism, suggested by decreased serum glucose and
increased serum triglycerides; an effect on nitrogen metabolism,
indicated by decreased blood urea nitrogen and serum creatinine and
increased urinary hydroxyproline excretion; an effect on reactive
oxygen metabolism, indicated by increased serum erythrocyte superoxide
dismutase and serum ceruloplasmin; and an effect on erythropoiesis and
haematopoiesis, suggested by increased blood haemoglobin and mean
corpuscular haemoglobin content but decreased haematocrit, platelet
number, and erythrocyte number. Boron supplementation after depletion
also enhanced the elevation in serum 17ß-estradiol and plasma copper
caused by estrogen therapy, altered electroencephalograms such that
they suggested improved behavioural activation (e.g. less drowsiness)
and mental alertness, and improved processes of attention and memory.
Two hypotheses have appeared to account for the multiple effects
described above. One hypothesis is that boron has a role in cell
membrane function, stability, or structure, such that it influences
the response to hormone action, transmembrane signalling, or
transmembrane movement of regulatory cations or anions (Nielsen,
1991); a similar hypothesis has also appeared for the functional role
of boron in plants (Parr & Loughman, 1983; Blaser-Grill et al., 1990;
Blevins & Lukaszewski, 1994). The other hypothesis is that boron is a
negative regulator that influences a number of metabolic pathways by
competitively inhibiting some key enzyme reactions (Hunt, 1994).
Regardless of the fact that the function of boron remains
undefined, boron is becoming recognized as an element of potential
nutritional importance because of the findings from human and animal
studies. The latest report on trace elements in human nutrition and
health published by the World Health Organization (WHO, 1996)
suggested that an individual mean basal requirement for adults may be
about 0.375 mg/day, and the minimum mean population intake that meets
basal needs could be about 0.75 mg/day for adults. The report also
indicated that an acceptable safe range of population mean intakes for
boron for adults could well be 1-13 mg/day.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Several investigators have studied the effects of borates on
bacteria, protozoa, and algae (see Table 18). Effect concentrations
for the bacterium Pseudomonas putida range widely. No observed
effects were noted after 72 h at 291 mg boron/litre by Schöberl &
Huber (1988), whereas Guhl (1996) found a 16-h EC10 of 7.6 mg
boron/litre. Guhl (1996) and Bringmann & Kuhn (1980) reported 30-min
EC10 and 72-h EC3 values of 340 and 290 mg boron/litre,
respectively. Twenty per cent light loss, compared with controls, from
Photobacterium phosphoreum was determined to occur after 30 min at
18 mg boron/litre (Guhl, 1996). Nitrogen-fixing cyanobacteria require
boron for proper functioning of the heterocyst cell wall (Bonilla et
al., 1990). Mateo et al. (1986) concluded that boron is essential for
nitrogen fixation in Anabaena.
A wide range of effect concentrations was reported for two
protozoan species. Bringmann & Kuhn (1980) reported a 72-h EC3 of 0.3
mg boron/litre for Entosiphon sulcatum, whereas Guhl (1996) showed a
72-h NOEC of >10 mg boron/litre. Reproduction of Paramecium
caudatum was completely inhibited at 70 mg boron/litre (Bringmann &
Kuhn, 1980), and no effects were observed at 18 mg boron/litre by
Sprague (1972).
De Jong (1965) determined the sensitivity of another green alga,
Chlorella vulgaris, to borax concentrations ranging from 0.0001 to
30% of the medium. Inoculated cultures were cultivated at room
temperature in daylight for 3-4 months. The highest concentration of
borax tolerated by C. vulgaris was 0.6 mg boron/litre, and the
lowest concentration inhibiting growth was 1.2 mg boron/litre.
Concentrations above 50 mg boron/litre induce the formation of giant
cells in Chlorella pyrenoidosa as a result of a stronger inhibition
of the formation of daughter cells compared with the synthesis of
biomass. For cells grown in 100 mg boron/litre, cell division was
severely inhibited for the first 72 h of exposure. During this time,
photosynthesis was inhibited by only 47%. The protein and nucleic acid
content of the giant cells increased and nitrate uptake was also
enhanced, whereas the lipid content decreased. The authors therefore
concluded that the delay in cell division is probably a direct effect
of boron on cytokinesis, as nuclear division is unaffected (Maeso et
al., 1985).
Martinez et al. (1986) observed that boric acid caused a decrease in
growth rates, protein content, and chlorophyll content in the
blue-green alga Anacystis nidulans, following exposure to 75 and 100
mg boron/litre as boric acid. The photosynthetic pigments completely
disappeared after 72 h of exposure. Nitrate uptake was also lowered.
Table 18. Borate toxicity to bacteria, protozoa, and algae
Species Duration End-point Parameter Borate concentration Reference
in solution
(mg boron/litre)
Pseudomonas putida 30 min Growth inhibition EC10 340 Guhl (1996)
16 h Growth inhibition EC10 7.6 Guhl (1996)
72 h Growth inhibition NOEC 291 Schöberl & Huber (1988)
72 h Growth inhibition EC3 290 Bringmann & Kuhn (1980)
Photobacterium phosphoreum 30 min Light loss EC20 18 Guhl (1996)
Entosiphon sulcatum 72 h Growth inhibition EC3 0.3 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC >10 Guhl (1996)
Paramecium caudatum 72 h Growth inhibition EC100 <70 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC 18 Sprague (1972)
Algae, Scenedesmus quadricauda 8 days Growth inhibition EC3 0.16 Bringmann & Kuhn (1978)
Algae, Scenedesmus subspicatus 72 h Growth inhibition EC0 10 Guhl (1996)
72 h Growth inhibition EC10 24 Guhl (1996)
72 h Growth inhibition EC50 34 Guhl (1996)
72 h Growth inhibition EC100 100 Guhl (1996)
Algae, Scenedesmus subspicatus Chronic Growth inhibition EC10 24 Kopf & Wilk (1995)
Chronic Growth inhibition EC50 52 Kopf & Wilk (1995)
Chronic Growth inhibition EC90 109 Kopf & Wilk (1995)
Algae, Microcystis aeruginosa 8 days Growth inhibition EC3 20.3 Bringmann & Kuhn (1978)
An accumulation of carbohydrates was observed, probably because the
loss of the photosynthetic pigments inhibited their degradation.
Lewin (1966) concluded that borate is an essential micronutrient for
growth in marine diatoms, whereas Smyth & Dugger (1981) concluded that
boron is essential for Cylindrotheca fusiformis. Antia & Cheng
(1975) studied the effects of boric acid on the growth of 19 species
(10 classes) of marine phytoplankton. Axenic cultures in a
nutrient-enriched, pH-controlled seawater (33% salinity) medium were
exposed to the following concentrations of boric acid: 0, 5, 10, 50,
or 100 mg boron/litre. Growth measurements were taken every 2-4 days
by determining the optical density at 600 nm after vortex mixing.
Concentrations of 5 and 10 mg boron/litre did not inhibit the growth
of any species tested, with 10 mg boron/litre actually stimulating
growth in the blue-green alga Anacystis marina, the diatom
Skeletonema costatum, and the cryptomonad Rhodomonas lens. Growth
was strongly inhibited in 26% of the species at 50 mg boron/litre
(e.g. green alga Tetraselmis maculata; haptophyte Emiliania
huxleyi; diatom Phaeodactylum tricornutum) and in 63% of the
species at 100 mg boron/litre (e.g. chrysophyte Monochrysis
lutheri). The highest concentration was also lethal to 37% of the
species (e.g. diatom Cyclotella cryptica; dinoflagellate
Amphidinium carteri). Some species (e.g. green alga Monallantus
salina) required an adaptation period, with growth imperceptible
until 34-36 days after inoculation. Sequential transfer tests showed
that many of the initially inhibited species recovered after exposure
to 50 mg boron/litre, but not after exposure to 100 mg boron/litre.
The authors concluded that higher concentrations would be expected to
cause species redistribution, favouring growth of some forms and
suppressing growth of others.
A few studies have investigated the effects of boron on microorganisms
in sewage treatment plants. A boron concentration of 20 mg/litre had
no effect on activated sewage treatment (Gerike et al., 1976), and
boron levels below 200 mg/litre resulted in no significant inhibition
of anaerobic sludge digestion (Butterwick et al., 1989).
9.1.1.2 Soil
Information concerning the effects of boron on soil microorganisms is
scarce. Butterwick et al. (1989) reported results of a study in which
actinomycetes were found to undergo genetic transformations at boron
concentrations of 1000-6000 mg/litre. Bowen & Gauch (1966) exposed the
fungi Saccharomyces cerevisiae, Neurospora crassa, Aspergillus
niger, and Penicillium chrysogenum to boron. They found that
growth was significantly reduced at boron concentrations of 50, 250,
1300, and 4000 mg/litre for the four species, respectively. The
authors concluded that boron was not essential for any of the species
tested.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Nobel (1981) studied the effect of several boron compounds on
photosynthesis in submerged macrophytes, watermilfoil (Myriophyllum
alterniflorum), buttercup (Ranunculus penicillatus), and waterweed
(Elodea canadensis). The watermilfoil and buttercup (four
plants/concentration) were exposed to the following concentrations of
boric acid for 28 days: 0, 1, 2, 5, or 10 mg boron/litre. The
waterweed (eight plants/concentration) was exposed to the following
concentrations of boric acid for 21 days -- 0, 1, 2, 5, 10, or 250 mg
boron/litre -- and also to the following boron compounds at a
concentration of 2 mg boron/litre: boron trioxide, sodium perborate,
sodium metaborate, and borax. The test water was characterized as an
oligotrophic calcium-deficient nutritive medium. Net photosynthesis
was measured weekly as a function of the dissolved oxygen content.
Significant reductions (p = 0.01) in photosynthesis compared with
controls were observed at 2 mg boron/litre in the buttercup and
waterweed and at 5 mg boron/litre in the watermilfoil. The LC50
values for each species were as follows: 5 mg boron/litre for the
waterweed and the watermilfoil and 10 mg boron/litre for the
buttercup. Boron trioxide did not adversely affect photosynthesis in
the waterweed. The toxicity of the other boron compounds in the
waterweed at 2 mg boron/litre exhibited the following trend: borax >
metaborate > perborate > boric acid. Because calcium is known to
inhibit the uptake of boron in plants and, therefore, to mitigate the
damaging effects (Reeve & Shive, 1944), the authors concluded that the
toxic effects of boron in macrophytes are more pronounced in soft
(low-calcium) water.
In phytoassays conducted by Wang (1986), boron at concentrations up to
60 mg/litre did not significantly affect the growth of duckweed
(Lemna minor), expressed by the increase in frond number. In
addition, a concentration of 40.3 mg boron/litre added as a
tetraborate salt led to 50% inhibition of root growth in the
freshwater Eurasian watermilfoil (Myriophyllum spicatum) after 32
days of treatment (Stanley, 1974).
A pot study was carried out on the reed Phragmites australis during
the growth seasons of 1992 and 1993. Both soil culture in the presence
of free water and a gravel hydroculture with graduated repeated
additions of boron (as boric acid) were used during the growth seasons
to maintain the water phase at 0, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and,
in the case of the hydroculture, even 32.0 mg boron/litre. The number
of stalks per pot, height growth of the plants, and the yield of dry
substance of leaves, stalks, and roots at post-harvest time were
determined. The boron content in leaves, stalks, and roots, the
appearance of boron toxicity symptoms in leaves, and differences in
growth at each of the exposure concentrations were monitored. It was
concluded from the study that Phragmites australis can tolerate a
relatively high boron content (up to 4 mg boron/litre) in the liquid
nutrient substrate; for a period of 2-3 months, even 8 mg/litre was
tolerated without noticeable damage. Boron toxicity symptoms in the
leaves of Phragmites australis arose first at leaf boron
concentrations of around 150-180 mg/kg dry weight; however, no adverse
effects on growth, development, or dry substance yields of the plants
could be established at these leaf boron concentrations. Long-term
exposure to 8 mg boron/litre in waters would lead to symptoms of
damage, growth reductions, and yield reductions of plants (Marks et
al., 1994; Bergmann et al., 1995).
9.1.2.2 Invertebrates
1) Freshwater
Acute toxicity
Summaries of the median response toxicity data for aquatic
invertebrates are given in Table 19. These studies are also described
in detail in the following text.
Gersich (1984) studied the acute toxicity of boric acid to Daphnia
magna. Static (48-h) tests were conducted following the methodology
approved by the American Society for Testing and Materials. Three
replicates of 10 neonates each were exposed to seven nominal
concentrations: 0 (control), 54, 91, 151, 252, 420, or 700 mg
boron/litre. The 48-h LC50 for D. magna was 133 mg/litre as boron,
with a 95% confidence interval of 115-153 mg/litre, calculated by
probit analysis. All test organisms died at 420 mg/litre, 0% mortality
was observed at 54 mg/litre, and control mortality averaged 7%.
Lewis & Valentine (1981) also investigated the toxicity of boric acid
to D. magna. The investigators followed US EPA-approved procedures
and exposed <24-h-old neonates to five nominal test concentrations
(not given in paper). The 48-h LC50, calculated using probit
analysis, was determined to be 226 mg/litre as boron (95% confidence
interval of 200-246 mg/litre). The no-kill (0% mortality)
concentration was <200 mg/litre. Comparison of these results with
those of Gersich (1984) indicates a factor of 1.7 difference in the
48-h LC50 values; however, Canton & Adema (1978) report that a
difference of 2.0 may be normal in LC50 values not determined at the
same time or under similar conditions.
A few studies have been conducted to assess the toxicity of the sodium
borates to D. magna. As part of the study conducted by NAPM (1974),
the effects of sodium tetraborate pentahydrate on D. magna were
investigated. Static (96-h) tests were performed in which 10-20
organisms per test were exposed to the following nominal
concentrations of sodium tetraborate pentahydrate: 0, 18, 33, 58, 102,
or 182 mg/litre. The highest test concentration resulted in only 26.7%
mortality at 96 h; consequently, the 96-h LC50 for D. magna was
>182 mg sodium tetraborate pentahydrate/litre (>27 mg boron/litre).
Table 19. Median response concentrations for aquatic invertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
ARTHROPODA
Crustacea - Daphnidae
Daphnia magna Boric acid S, N 133.0 Gersich (1984)
Daphnia magna Boric acid S, N 226.0 Lewis & Valentine
(1981)
Daphnia magna Borax S, N 73.0 Bringmann & Kuhn
(1977)
Daphnia magna Anhydrous borax S, N <52.0 Threshold concentration,
based on immobilization Anderson (1946)
Daphnia magna Sodium tetraborate S, N >182.0 Mortality 26.7% at 96 h NAPM (1974)
pentahydrate
Daphnia magna Sodium tetraborate S, N 141 Maier & Knight (1991)
Crustacea - Limnoriidae
Limnoria lignorum Borax S, N 28.35 After 5-day recovery period Robinson & Perkins
(1977)
Insecta - Chironomidae
Chironomus decorus Sodium tetraborate S, N 1376 Maier & Knight (1991)
MOLLUSCA
Gastropida - Onchidiidae
Onchidoris fusca Borax S, N <28.35 After 5-day recovery period Robinson & Perkins
(1977)
a S = static test; N = nominal concentration.
Bringmann & Kuhn (1977) reported a 24-h LC50 of 340 mg disodium
tetraborate (anhydrous borax)/litre (73 mg boron/litre) for
D. magna in static tests using tap-water free of chlorine. The LC0
was 61 mg disodium tetraborate/litre (13 mg boron/litre), and the
LC100 was 1930 mg disodium tetraborate/litre (415 mg boron/litre).
Anderson (1946) obtained similar results with D. magna, reporting a
threshold concentration based on immobilization of <240 mg anhydrous
borax/litre (<52 mg boron/litre) at 48 h. Maier & Knight (1991)
reported a 48-h LC50 value of 141 mg boron/litre (95% confidence
limits 123-159 mg boron/litre) for D. magna exposed to sodium
tetraborate. Increasing water hardness and sulfate concentrations did
not affect the toxicity of boron to D. magna. In this study, boron
was much more toxic to daphnids than to Chironomus decorus
4th-instar larvae, for which the 48-h LC50 was 1376 mg boron/litre
(95% confidence limits 1298-1453 mg boron/litre) (Maier & Knight,
1991).
Tubificid worms (Tubifex sp.) appear to be less sensitive than
D. magna to sodium borate, with concentrations of sodium borate
decahydrate (borax) as high as 750 mg/litre (85 mg boron/litre)
causing no effect after a 24-h exposure. The 24-h LC100 was reported
as 2000 mg/litre (227 mg boron/litre). The tubificid also exhibited
less sensitivity to boric acid, with a no-effect level at 24 h of
exposure of 7500 mg/litre (1311 mg boron/litre) and an LC100 of 10 000
mg/litre (1748 mg boron/litre) (Mann, 1973).
In an early study, Fay (1959) determined the effects of metaboric acid
(B2O3.H2O) on various life stages of three species of mosquitos:
Aedes aegypti, Anopheles quadrimaculatus, and Culex
quinquefasciatus. A concentration of 8000 mg/litre (1973 mg
boron/litre) did not affect hatching of A. aegypti eggs following
72 h of exposure. However, newly hatched larvae of
C. quinquefasciatus and A. quadrimaculatus were quite sensitive to
metaboric acid. Complete mortality of C. quinquefasciatus larvae was
observed after 24 h of exposure to 2000 mg metaboric acid/litre (493
mg boron/litre). Complete mortality of A. aegypti was observed after
24 h of exposure to 4000 mg/litre (986 mg boron/litre), with complete
mortality occurring in A. quadrimaculatus only at the 8000 mg/litre
(1973 mg boron/litre) level. In contrast, at the 2nd- and 3rd-instar
stage, A. quadrimaculatus was more sensitive than the other species.
In 2nd-instar larvae, 100% mortality occurred at 500 mg/litre (123 mg
boron/litre) in A. quadrimaculatus, 96% mortality occurred at
1500 mg/litre (370 mg boron/litre) in A. aegypti, and 100% mortality
occurred at 2000 mg/litre (493 mg boron/litre) in
C. quinquefasciatus. The 3rd-instar larvae were less sensitive than
the 2nd-instar larvae, with 100% mortality of A. quadrimaculatus at
2500 mg/litre (617 mg boron/litre), 88% mortality of A. aegypti at
3000 mg/litre (740 mg boron/litre), and 48% mortality of
C. quinquefasciatus at 4000 mg/litre (986 mg boron/ litre). Pupae of
the three species were even less sensitive to metaboric acid,
requiring concentrations ranging from 6000 mg/litre (1480 mg
boron/litre) to 16 000 mg/litre (3946 mg boron/litre) to prevent
emergence to the adult stage. From the information provided in this
study, it appears that the 2nd-instar larva was the life stage that
was most sensitive to exposure to metaboric acid.
Fay (1959) also studied the effects of prolonged exposure to boron
trioxide (B2O3.H2O) on the immature stages of the same three
species of mosquitos. Newly hatched larvae were reared in the
following concentrations of metaboric acid to determine the percentage
of successful adult emergence: 10, 25, 50, 100, or 250 mg/litre. No
significant reduction in maturation was observed in A. aegypti or
C. quinquefasciatus at any test concentration <100 mg/litre.
However, at 50 mg/litre, only 2% of the larvae of A.
quadrimaculatus reached the adult stage. At 250 mg metaboric
acid/litre, only 1% of A. aegypti and only 3% of
C. quinquefasciatus reached the adult stage.
Maier & Knight (1991) evaluated the chronic sublethal effects of boron
to Chironomus decorus larvae. A significantly decreased growth rate
over a 96-h period was observed at 20 mg boron/litre.
Chronic toxicity
Gersich (1984) conducted a 21-day static renewal chronic toxicity test
with D. magna. Daphnids (20 organisms/concentration, or four
replicates with 5 organisms/replicate) were exposed to the following
nominal concentrations of boric acid: 0, 7, 14, 28, 56, or 105
mg/litre as boron. The test concentrations remained stable during
testing; the mean measured concentrations ranged from 91.4 to 106% of
the nominal test concentrations. The 21-day LC50, calculated using
the moving average method, was 52.2 mg boron/litre, and the 95%
confidence interval was 42.6-66.7 mg boron/litre. No mortality was
observed in the control group. The mean number of broods per daphnid,
mean total young per daphnid, mean brood size per daphnid, and mean
size differed significantly (alpha = 0.05) from the control at the
13.6 mg boron/litre concentration. Therefore, the maximum acceptable
toxicant concentration (MATC) was determined based on the most
biologically and statistically significant end-points, reproduction
and growth. The MATC for boric acid was estimated to be between 6.4
and 13.6 mg boron/litre, or 9.3 mg boron/litre (the geometric mean of
these two values).
Lewis & Valentine (1981) also conducted a 21-day static renewal
chronic toxicity test with D. magna. The test was conducted
similarly to that of Gersich (1984), except for the exposure regimen.
Ten test chambers were set up for each test concentration: three
beakers contained five daphnids each for obtaining data on survival,
and seven beakers each contained one daphnid for obtaining data on
survival, growth, and reproduction. Twenty control chambers were used:
6 chambers contained five daphnids each, and 14 chambers each
contained one daphnid. Daphnids were exposed to the following nominal
concentrations of boric acid: 0, 6, 13, 27, 53, or 106 mg/litre as
boron. The concentration of the test solution remained stable during
testing, with mean measured concentrations exceeding 95% of the
nominal concentration. The 21-day LC50, calculated by probit
analysis, was 53.2 mg boron/litre, with a 95% confidence interval of
44.1-64.5 mg boron/litre. Mortality was 9% in the control group. The
mean brood size and total number of young produced were significantly
reduced (p < 0.05) compared with controls at concentrations of
>13 mg boron/litre. Mean length of daphnids was significantly reduced
(p < 0.05) compared with controls at 53 mg boron/litre. The NOEC
was 6 mg boron/litre. Therefore, based on the most sensitive
parameters, the MATC was >6 and <13 mg boron/litre, or 8.83 mg
boron/litre (geometric mean of these values).
2) Marine
In static tests, Mann (1973) determined 24-h LC100 values for the
amphipod Gammarus tigrinus exposed to boric acid, borax, and sodium
perborate at two salinities (32.23% and 17.66%). The LC100, 10 000
mg/litre, was the same for all three compounds at both salinities. The
no-effect concentration for all of the compounds was 7500 mg/litre. In
contrast, Robinson & Perkins (1977) reported toxicity values for borax
in the isopod Limnoria lignorum and the sea slug Onchidoris fusca
that are roughly two orders of magnitude lower than those reported by
Mann (1973) for the amphipod Gammarus tigrinus. The salinity of the
test water was 30%. The 24-h static LC50 values, after a 5-day
recovery period, were 250 mg/litre (28.35 mg boron/litre) for the
isopod and >250 mg/litre (>28.35 mg boron/litre) for the sea slug.
In studies with sea urchin (Anthocidaris crassispina) embryos,
exposure to boric acid at a concentration of 37 mg boron/litre had no
effect on development, whereas exposure to 75 mg boron/litre was fatal
(Eisler, 1990).
3) Biocenosis studies
Three biocenosis studies (also known as microcosms, mesocosms, and
experimental ecosystems) have been conducted (summarized in ECETOC,
1997). A 28-day laboratory microcosm test with microorganisms
consisting of six trophic stages yielded a NOEC and LOEC of 2.5 and
5.0 mg boron/litre, respectively. Studies with outdoor ponds
containing up to 29 species and treated with 0.7 mg boron/litre over a
2-year period resulted in no significant differences compared with
untreated control ponds. Further, field studies carried out in seven
different water bodies subjected to different levels of anthropogenic
boron inputs yielded no toxic effects of boron at concentrations
between 0.16 and 1.52 mg/litre.
9.1.2.3 Vertebrates
1) Freshwater
Acute toxicity
Summaries of the acute toxicity data for aquatic vertebrates are given
in Table 20. These studies are also described in detail in the
following text.
For boron compounds, three studies are available that provide
information concerning the 96-h LC50 values, the toxicity value
established by the US EPA (Stephan et al., 1985) for use in the
derivation of water quality criteria for the protection of aquatic
life and its uses. Wallen et al. (1957) conducted 96-h static toxicity
tests with mosquitofish. The test water, turbid pond water, was
selected to simulate the commonly turbid wastewater from oil refinery
operations; however, it was determined that the highest turbidities
used in the study should not produce direct effects on the fish during
the exposure time. Adult female fish (10/concentration) were exposed
to the following nominal concentrations of boric acid or borax: 10,
18, 32, 56, or 100 mg/litre. If no deaths occurred at 96 h, the tests
were rerun at concentrations of 100, 180, 320, 560, or 1000 mg/litre
and, subsequently, at concentrations 10 times these values
(1000-10 000 mg/litre). The 96-h LC50 for mosquito-fish exposed to
boric acid was 5600 mg/litre (979 mg boron/litre), and the 96-h LC50
for borax was 3600 mg/litre (408 mg boron/litre).
As part of the study conducted by NAPM (1974), static bioassays were
conducted to determine the toxicity of sodium tetraborate pentahydrate
to fathead minnows. The tests were conducted using standard methods
and filtered tap-water. Fish (15/treatment) were exposed to the
following nominal concentrations: 0, 200, 360, 640, 1120, or 2000
mg/litre. The 96-h LC50 was 1900 mg/litre (332 mg boron/litre).
Hamilton & Buhl (1990) reported the results of 96-h static toxicity
tests with two life stages of chinook salmon (Oncorhynchus
tshawyts-cha) and coho salmon (O. kisutch). Swim-up fry
(8-12 weeks old) were tested in fresh water, and advanced fry (15-21
weeks old) were tested in brackish water. The 96-h LC50 values for
chinook salmon swim-up fry and advanced fry were 725 mg/litre (127 mg
boron/litre) and 600 mg/litre (105 mg boron/litre), respectively. The
96-h LC50 values for coho salmon swim-up fry and advanced fry were
447 mg/litre (78.1 mg boron/litre) and 600 mg/litre (105 mg
boron/litre), respectively.
Results of 24- and 48-h static acute tests with rainbow trout,
guppy (Poecilia reticulata), and mosquitofish clearly show the
difference in the degree of toxicity resulting from exposure to boric
acid versus sodium borate. Mann (1973) reported the following 24-h
LC100 values for rain-bow trout and guppy, respectively: boric acid,
10 000 mg/litre (1748 mg boron/litre) and 7500 mg/litre (1311 mg
boron/litre); borax, 5000 mg/litre (567 mg boron/litre) for both
species. Static 24-h LC50 values determined by Wallen et al. (1957)
for the mosquitofish are 18 000 mg/litre (3146 mg boron/litre) for
boric acid and 12 000 mg/litre (1361 mg boron/litre) for borax. The
48-h LC50 values determined in this study were 10 500 mg/litre (1835
mg boron/litre) for boric acid and 8200 mg/litre (930 mg boron/litre)
for borax. Rainbow trout are more sensitive to borax than
mosquitofish, with 24- and 48-h static LC50 values of 2800 mg/litre
(602 mg boron/ litre) and 1800 mg/litre (387 mg boron/litre),
respectively (Alabaster, 1969). Juhnke & Ludemann (1978) reported an
LC50 of 807 mg/litre as boron for golden orfe (Leuciscus idus)
Table 20. Acute toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Coho salmon Boric acid S, N 78.1 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus kisutch) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Chinook salmon Boric acid S, N 127 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus tshawytscha) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Rainbow trout Borax S, N 602 387 Alabaster (1969)
(Oncorhynchus mykiss) Borax FT, N 65 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 150 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Borax FT, N 88 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 100 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Fathead minnow Sodium S, N 332 NAPM (1974)
(Pimephales promelas) tetraborate
pentahydrate
Mosquitofish Borax S, N 1361 930 408 Wallen et al. (1957)
(Gambusia affinis) Boric acid S, N 3146 1835 979 Wallen et al. (1957)
Golden orfe 173 Schöberl & Huber (1988)
(Leuciscus idus)
Table 20. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Zebra fish Borax 14.2 Guhl (1996)
(Brachydanio rerio)
Bluegill Borax S, N 4.6 Turnbull et al. (1954)
(Lepomis macrochirus)
Dab Anhydrous borax Semi-static, 88.3 74 Salinity 34.62 ± 0.2% Taylor et
(Limanda limanda) N al. (1985)
Colorado squaw-fish Boric acid 279b Hamilton (1995)
(Ptychocheilus lucius) >100c
527d
Razorback sucker Boric acid 233b Hamilton (1995)
(Xyrauchen texanus) 279c
>100d
Bonytail Boric acid 280b Hamilton (1995)
(Gila elegans) >100c
552d
a S = static test; FT = flow-through test; N = nominal concentration.
b Swim-up fry.
c 1-g juvenile.
d 2-g juvenile.
exposed to anhydrous borax in static acute tests (exposure time not
given).
Mann (1973) reported 24-h LC100 values for rainbow trout and
guppies exposed to sodium perborate (500 mg/litre or 66.1 mg
boron/litre) that were considerably lower than those reported for
boric acid and borax. The authors attributed this increased toxicity
to a pH shift into the alkaline range (up to 9.1), which resulted in
increased mucilage formation, with the fish exhibiting a crippling
behaviour.
The treatment of embryos of the toad Bufo vulgaris with 0.5%
boric acid for 24 h from the two-cell stage to the tail-bud stage
resulted in several developmental malformations, including a reduction
or lack of external gills, short tail, bifurcation of the epiphysis,
suppression of forebrain development, and inhibition of sensory organ
development. Several of these effects were attributed to the direct
action of boric acid on the ectoderm (Takeuchi, 1958).
Chronic toxicity
Toxicity of borate to early life stages of fish has been
documented for several species (Birge & Black, 1977; Black et al.,
1993). Embryonic and early larval stages of rainbow trout, largemouth
bass, channel catfish, and goldfish were exposed to boron, as boric
acid or borax, from fertilization up to 8 days post-hatch. All
exposures were in soft (50 mg CaCO3/litre) or hard (approx. 200 mg
CaCO3/litre) reconstituted water. Test responses included embryonic
mortality, teratogenesis, and larval mortality. Gross debilitating
anomalies of survivors were classified as mortalities. Effect
concentrations (LC50, NOEC, and LOEC) for each species are presented
in Table 21. Neither water hardness nor the form of boron (boric acid,
borax) added to the test aquaria consistently affected embryo-larval
survival of rainbow trout, channel catfish, and goldfish (Birge &
Black, 1977).
On the basis of median lethal concentrations (LC50), no species
was found to be especially sensitive. The range of LC50s for all
species was 12.2-235 mg boron/litre. In addition, Birge & Black (1977)
reported LC1s ranging from 0.001 to 0.1 mg boron/litre for rainbow
trout, from 0.2 to 5.5 mg boron/litre for channel catfish, and from
0.2 to 1.4 mg boron/litre for goldfish. The LC1 showed rainbow trout
to be the most sensitive species. The NOEC ranged from 0.009 to 0.103
mg boron/litre for rainbow trout and was 1.39 mg boron/litre for
large-mouth bass. These were consistent with the acute toxicity
results that indicated rainbow trout and zebra fish as the most
sensitive species (see Table 20).
The effect of natural dilution water on boron toxicity was
reported by Black et al. (1993). Natural surface waters were collected
from three US locations: the Erwin National Fish Hatchery in
Tennessee, Brookville Lake in Indiana, and Firehole River in
Yellowstone National Park, Wyoming. Surface water control boron
Table 21. Chronic toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Coho salmon Sodium metaborate S, N 113 Alevins, 283-h exposure Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate
Coho salmon Sodium metaborate S, N 12.2 Underyearlings, 283-h exposure, Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate salinity 28 ± 1%
Rainbow trout Borax FT, N 27 Alevin, 28-day exposure, Birge & Black (1977)
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre
Rainbow trout Boric acid FT, N 100 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Borax FT, N 54 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 79 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 138 Embryo/larva, 32-day exposure Black et al. (1993)
(Oncorhynchus mykiss)
Rainbow trout Boric acid 138 Hardness of 200 mg CaCO3/ litre;
(Oncorhynchus mykiss) duration 32 days
NOEC = 0.009 mg boron/litre
LOEC = 0.1 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid 92 Hardness of 200 mg CaCO3/ litre;
(Micropterus salmoides) duration 11 days
NOEC = >1.39 mg boron/litre
LOEC = 12.17 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid FT, N 92 Embryo/larva, 11-day exposure Black et al. (1993)
(Micropterus salmoides)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Goldfish Borax FT, N 71.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 178.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 68.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 170.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 65.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 46.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 59.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 75.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 235.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 220.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 120.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Channel catfish Boric acid FT, N 102.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 71.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 22.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 54 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 157 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 60 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 145 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 47 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 130 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Leopard frog Borax FT, N 54 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 135 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 148 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 135 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 145 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 123 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
a S = static test; FT = flow-through test; N = nominal concentration.
concentrations were 0.023, 0.091, and 0.75 mg/litre for Erwin,
Brookville, and Yellowstone waters, respectively. Total organic carbon
for the three surface waters ranged from 0.8 to 1.9 mg/litre.
Hardness levels for these natural waters ranged from 24 to 209 mg
CaCO3/litre. In the surface water tests, three nominal treatments
were used: surface water control, control plus 1.0 mg boron/litre
added (as boric acid), and control plus 10 mg boron/litre added. Total
and dissolved boron levels were measured for each of the surface water
treatments. No statistically significant differences were noted
between total and dissolved boron concentrations, except for
Yellowstone (1.0 mg boron/litre) and Erwin (10 mg boron/litre)
treatments. The true significance of this, however, is minimal, as
total versus dissolved concentrations for Yellowstone and Erwin waters
were 1.61 vs. 1.52 mg boron/litre and 9.91 vs. 9.48 mg boron/litre,
respectively. Hence, it is reasonable to assume that all boron was in
solution. Boron did not elicit toxicity to embryo-larval stages of
rainbow trout exposed to surface water control boron levels up to 0.75
mg/litre. LOECs for Erwin, Brookville, and Yellowstone treatments were
1.10, 1.24, and 1.73 mg boron/litre, respectively, indicating that the
threshold for no effects is approximately 1 mg boron/litre. In
addition, deep (600 m) well-water, typically used for aquatic toxicity
tests, from a contract laboratory located in Wareham, Massachusetts,
USA, was also used. Boron was spiked into well-water to obtain an
exponential series of nominal concentrations: 0.0017, 0.017, 0.17,
1.7, and 17 mg boron/ litre, respectively. Analytical confirmation of
exposure was obtained for the two highest concentrations, 2.1 and
18.0 mg boron/litre. No effects were observed, even at the highest
boron concentration.
The effect concentrations generated in natural waters were
greater than those from the reconstituted water experiments. The cause
of these differences is not readily apparent, particularly as
bioavailability was not reduced by natural water. However, some
investigators (Belanger et al., 1989; Farris et al., 1994) have shown
that organisms cultured in natural water often grow and reproduce
better than their counterparts in reconstituted or laboratory
dechlorinated water. Therefore, a reasonable hypothesis that may
explain, in part, the differences in reconstituted versus natural
water responses is the better health experienced by organisms exposed
to boron in natural water. The causative agents for this lack of
health in laboratory water are not certain but may include nutrient
deficiencies (Keating & Dagbusan, 1984; Belanger et al., 1989).
Early life stage tests have been conducted with fathead minnows
to determine effects of chronic exposure (30-60 days) to boric acid.
The 30-day NOEC and LOEC were 14 and 24 mg boron/litre, respectively,
based on growth reduction; the 60-day NOEC and LOEC were 24 and 88 mg
boron/litre, respectively, based on reduction in fry survival
(Butterwick et al., 1989).
Birge & Black (1977) also studied the effect of boric acid and
borax on embryos and larvae of the leopard frog (Rana pipiens) and
the effect of boric acid on embryos and larvae of the Fowler's toad
(Bufo fowleri). The embryos were exposed for 3.5 days and the larvae
for 7.5 days to concentrations of boric acid and borax ranging from
0.05 to 300.00 mg boron/litre in hard (200 mg CaCO3/litre) and soft
(50 mg CaCO3/litre) water (as described above for fish exposures).
The leopard frog and Fowler's toad were equisensitive to borax and
boric acid. For the leopard frog, LC1 and LC50 values were 13 and
130 mg boron/litre (dosed as boric acid) in soft water and 22 and 135
mg boron/litre in hard water. Lower toxicity values were observed via
borax exposures, with LC1 and LC50 values of 5 and 47 mg boron/litre
in soft water and 3 and 54 mg boron/litre in hard water, respectively.
The LC1 and LC50 values for Fowler's toad exposed to boric acid were
25 and 145 mg boron/litre in soft water and 5 and 123 mg boron/litre
in hard water, respectively.
2) Marine
Taylor et al. (1985) studied the toxicity of several metals,
including boron (as anhydrous borax), to a saltwater fish (dab,
Limanda limanda). The tests were conducted following standard
procedures under semi-static conditions, in which the test solution
was replaced at 24-h intervals. The salinity of the test water was
34.62 ± 0.2%. Adult fish obtained from wild populations
(20/concentration) were exposed to a series of five concentrations
(not given in the paper) for 96 h. The LC50, calculated by probit
analysis, was 74.0 mg boron/litre, with a 95% confidence interval of
66.4-83.0 mg boron/litre. Pre-death symptoms included minor
respiratory problems.
Similar results were observed in bioassays conducted by Thompson
et al. (1976) with two life stages of coho salmon. Coho alevins
(0.19-0.7 g) were tested in aerated well-water (fresh water), and coho
underyearlings (1.8-3.8 g) were tested in aerated seawater with a
salinity of 28 ± 1%. Test solutions were prepared using sodium
metaborate tetrahydrate (Na2B2O4.4H2O), and exposure lasted in
excess of 12 days. The 283-h LC50 for freshwater coho alevins was 113
mg boron/litre, with 95% confidence limits of 104-123 mg boron/litre.
The 283-h LC50 for saltwater coho underyearlings was 12.2 mg
boron/litre, with 95% confidence limits of 10.89-14.56 mg boron/litre.
The authors attributed the difference in toxicity in fresh water and
salt water to differences in fish age, test temperature (11°C in fresh
water; 8°C in salt water), and available boron levels. Seawater also
has higher background levels of boron than fresh water. The results
reported by Hamilton & Buhl (1990), discussed above, do not indicate
significant differences in acute toxicity between different life
stages of chinook and coho salmon.
In static tests, Mann (1973) observed differing sensitivities in
the common eel (Anguilla anguilla) to both boric acid and borax at
two salinities: 12.39 and 25.05%. At the lower salinity, a 24-h LC100
of >10 000 mg/litre was reported for both borax and boric acid; at
the higher salinity, the 24-h LC100 was 7500 mg/litre for both
compounds. The authors speculated that differences in pH could
contribute to the toxicity of the boron compounds at the higher
salinity. At a boric acid concentration of 7500 mg/litre, the pH in
the lower-salinity test water was 7.2; the pH in the higher-salinity
test water was 6.6. As was observed with rainbow trout and
mosquitofish, Mann (1973) also observed greater toxicity in the common
eel following exposure to sodium perborate. The LC100 at both
salinities was 750 mg/litre (99.2 mg boron/litre), resulting from the
damaging effects of an alkaline pH shift.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
1) Essentiality
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron plays a role in carbohydrate metabolism, sugar translocation,
pollen germination, hormone action, normal growth and functioning of
the apical meristem, nucleic acid synthesis, and membrane structure
and function (Lovatt & Dugger, 1984). Recent work shows important
involvement of boron in cell wall cross-linking, involving
complexation with specific pectin components (Loomis & Durst, 1992; Hu
et al., 1996).
Early investigation of the effects of boric acid and borax on the
fieldbean (Vicia faba) and other plants indicated the role of boron
in plant nutrition (Warrington, 1923). The first comprehensive study
of the effects of boron on plant growth was conducted by Eaton (1944).
Fifty species of plants were grown in sand cultures with standard
nutrient solutions containing the following boron concentrations:
trace (0.03-0.04), 1, 5, 10, 15, or 75 mg/litre. For each species,
Eaton (1944) recorded the boron concentration resulting in the best
growth, the lowest concentration resulting in injury, and the symptoms
of deficiency with trace boron levels. Approximately 82% of the plants
exhibited the best growth at concentrations from trace to 5 mg/litre,
and 35% of the plants grown in trace boron levels developed
morphological symptoms of deficiency. Eaton (1944) concluded that
there was overlap of the beneficial and injurious effects of boron
between species; therefore, three broad categories of tolerance
(sensitive, semi-tolerant, and tolerant) were established. The
tolerant plants endure a wide range of boron concentrations with
little effect, and the sensitive plants exhibit a strong reaction to
either too much or too little boron.
Boron generally occurs in soils at concentrations ranging from 10
to 30 mg/kg, depending on such factors as soil type, amount of
rainfall, and irrigation type (Sprague, 1972). Boron deficiencies
usually occur in soil solutions from sandy soils or acid peat soils,
soils derived from igneous rocks, soils low in organic matter, and
irrigated soils (Sprague, 1972).
The symptoms of boron deficiency in plants include cessation of
root and leaf growth, necrosis of leaf primordia and primary root
tips, necrosis of stem and leaf phloem, bark splitting, retardation of
enzyme reactions, reduced pollen germination, and even death (Versar,
Inc., 1975; Wells & Whitton, 1977). Normal growth will usually resume
if boron is added to the growth medium.
Hudak (1973) found that a boron-deficient nutrient solution
inhibited mitosis in the root tip of the fieldbean within 72 h. A 10
mg boron/litre solution produced optimum cell division and elongation
of the root tip; however, 50 mg boron/litre caused a reduction in
mitosis within 24 h.
Ludbrook (1942) studied the effects of boron deficiency and
toxicity in Monterey pine (Pinus radiata) seedlings grown in water
culture. The suboptimal concentrations of boron added to the nutrient
solution were 0, 0.005, 0.01, or 0.05 mg/litre, and the potentially
toxic concentrations of boron were 0.5, 2.5, 5.0, 10.0, 20.0, or 40.0
mg/litre. Seedlings exposed to the toxic levels were initially grown
for 2 months in a nutrient solution containing added boron at 0.5
mg/litre. Marked reduction in root growth was observed at the two
lowest suboptimal concentrations; however, improved root growth was
observed at 0.05 mg/litre, with the development of small lateral
roots. At 10.0 mg boron/litre, a slight yellowing of the tips of the
needles developed, followed by browning and drying at 20.0 mg/litre,
until all plants died at 40.0 mg/litre. Ludbrook (1942) concluded that
the optimum growth range for Monterey pine was 0.05-5.0 mg
boron/litre.
Recent work has shown that boron in a number of plant species is
tightly bound in the polysaccharide cell wall. Boron deficiency leads
to profound changes in cell wall morphology, suggesting that boron is
critical to cell wall expansion. It has been proposed that this
structural, cross-linking function of boron is involved with the
pectin fraction, which contains apiose and other hydroxylated
fragments amenable to complexation by borate (Loomis & Durst, 1992).
Fourteen species of crop plants were studied, and it was concluded
that high pectin content requires more boron for forming cell walls or
that pectin forms a tightly held boron complex that depletes boron
availability for other critical functions, thereby increasing the
overall demand for boron (Hu et al., 1996). Kobayashi et al. (1996)
have isolated and characterized a rhamnogalacturonan II/borate complex
from enzyme-digested cell wall pectin.
Toxicity
Boron excesses usually occur in soil solutions from geologically
young deposits, arid soils, soils derived from marine sediments, and
soils contaminated by pollutant sources, such as releases from
coal-fired power plants and mining operations (Sprague, 1972).
The initial symptom of boron toxicity in plants is chlorosis
(yellowing) of the leaf tip, progressing along the leaf margin and
into the blade. Necrosis of the chlorotic tissue occurs, followed by
leaf abscission. Necrosis of the leaf tissue results in a loss of
photosynthetic capacity, which reduces plant productivity (Lovatt &
Dugger, 1984). Pollen germination and pollen tube growth may also be
inhibited (Versar, Inc., 1975).
Several investigators have shown a direct relationship between
the boron content in leaves (foliar) and the severity of the symptoms
of toxicity. Gilliam & Watson (1981) conducted an experiment in which
Anderson yews (Taxus media) were grown in soil at four boron
concentrations (0.5, 5.0, 25.0, or 50 mg/kg). Symptoms of toxicity
were observed when foliar boron accumulation reached concentrations
ranging from 85 to 100 µg/g of dry tissue. The observed symptoms
included leaf tip yellowing, followed by necrosis and premature
defoliation. Suppression of shoot and root growth was observed at 50
mg boron/kg soil.
Shopova et al. (1981) found that concentrations of 16, 24, and
32 mg boron/kg soil resulted in a decline in plant development,
yellowing of leaves, late flowering, reduction of mitotic frequency in
root tip cells, and abnormalities during meiosis in the poppy
(Papaver somniferum).
Kluge & Podlesak (1985) found that symptoms due to boron excess
begin to develop on the leaves (leaf tip necroses) of pot-grown spring
barley (Hordeum vulgare) as soon as the boron content of the leaf
tissue reaches 60-80 mg/kg dry weight.
Gestring & Soltanpour (1987) grew alfalfa (Medicago sativa) in
three soil types amended with sodium borate at rates of 0, 10, 20, and
40 mg boron/kg. Alfalfa yield was significantly reduced by boron
application in both the sandy loam and loam soils; however, no yield
reduction was observed in the silt loam soil. Soil extractable boron
did not adequately assess boron toxicity, whereas plant boron levels
were a more reliable index of toxicity. The critical range of plant
boron resulting in a yield reduction was 850-975 mg boron/kg plant
tissue (dry weight). The significant difference between the soils
shows the importance of soil pH, organic matter content, and per cent
clay to the toxicity of boron to plants.
Sage et al. (1989) exposed the rare serpentine plant
(Streptanthus morrisonii) to boron (0, 20, 60, 240, 650, 1200, or
2400 µmol/litre) via watering. Plants showed mild to moderate toxicity
symptoms (older leaves exhibiting chlorosis and necrosis) at boron
concentrations of 240 and 650 µmol/litre. Severe toxicity symptoms
(significant leaf loss) were apparent at 1200 and 2400 µmol
boron/litre. Boron levels in the leaves of plants showing severe
toxicity were an order of magnitude higher than those in the leaves of
plants in the field.
Glaubig & Bingham (1985) reported significant linear
relationships between both soil and leaf tissue boron concentrations
and foliar damage in four tree species endemic to California (digger
pine, Pinus sabiniana; California laurel, Umbellularia
californica; madrone, Arbutus menziesii; bigleaf maple,
Acer macrophyllum). Foliar damage over 25% of the leaf/needle area
occurred at saturated soil concentrations ranging from 0.08 to
1.2 mmol boron/litre for the four species and at leaf/needle
concentrations ranging from 30 to 115 mmol boron/kg. The bigleaf maple
was the most sensitive of the four species, and the digger pine was
the most tolerant species.
Under experimental conditions, Shann & Adriano (1988)
demonstrated that chronic foliar aerosol exposures of boron produced
phytotoxicity in relation to boron accumulation in the leaves. Five
crop species (swiss chard, beet, radish, tomato, and cucumber) were
exposed to aerosol concentrations of 0, 1.55, or 3.09 µg boron/cm2
leaf area. The authors concluded that the visual damage (leaf tip
necrosis) resulting from aerosol exposure was identical to that
observed from root boron toxicity for all crops tested.
9.1.3.2 Invertebrates
Boric acid is an effective stomach poison for several species of
insects, including the German cockroach (Blattella germanica).
Pretreatment of wood and other substrates with boric acid or borax can
prevent insect infestation. Baits and aerosols containing boron
compounds (e.g. borax and boric acid) have been shown to control fruit
flies, cockroaches, houseflies, and termites (Butterwick et al.,
1989).
Boron in the diet at concentrations of 0.025-5% caused
sterilization of house flies (Musca domestica) when both sexes were
fed the diet (Borkovec et al., 1969). Boron toxicity in honey bees has
also been observed; a concentration of 50 mg boric acid/litre (8.7 mg
boron/litre) in syrup had no effect on survival, whereas 100 mg boric
acid/litre (17.5 mg boron/litre) caused 50% mortality (Ostrovskij,
1955).
Lang & Treece (1972) conducted a study to determine if boric acid
would induce sterility in the face fly (Musca autumnalis). Adult
male and female flies (50 each/concentration) were exposed to boric
acid concentrations of 0.5%, 0.8%, or 1.0% in each of three media
(water, malt, and dry food) for the first 4 days after eclosion
(hatching from the pupal stage). Complete sterility (100%) was
observed in flies fed the two highest concentrations in dietary malt;
however, a high degree of recovery was observed within 5 days after
cessation of treatment. Many eggs from the females fed 1.0% boric acid
were transparent and watery in appearance; this was possibly due to
the interference of boric acid with egg chorion formation. Almost 100%
mortality occurred in the flies exposed to boric acid concentrations
of 0.5% and 0.8% in water within the first 4 days of treatment. In a
subsequent test, erratic sterility with recovery of fertility was
observed in face flies exposed to boric acid concentrations of 0.1%
and 0.3% in water.
9.1.3.3 Vertebrates
In order to determine the impact of high levels of boron on
water-fowl in Kesterson Reservoir (located within the Kesterson
National Wildlife Refuge in the San Joaquin Valley of California,
USA), a number of investigators have studied the effects of controlled
boron exposure to mallard ducks. Smith & Anders (1989) studied the
effects on reproduction in mallards fed diets supplemented with 0, 30,
300, or 1000 mg boron/kg as boric acid (concentrations known to be
found in the field). The adults were fed this diet prior to mating and
during egg incubation, and the ducklings were fed this diet after
hatching. In the 1000 mg/kg group, hatching success of fertile eggs
and duckling survival were significantly reduced, and embryo mortality
was significantly higher. The body weight of hatchlings was lower in
the 300 and 1000 mg/kg groups than in controls. Growth rate of
ducklings was significantly reduced at all three dose levels. No
effect on egg fertility, eggshell thickness, or eggshell quality was
observed at any dose level. In addition, boron did not produce any
overt signs of toxicity in young or adult mallards. The researchers
were unable to determine whether duckling mortality and impaired
growth were due to pre-hatching or post-hatching exposure or the
combined effect.
Hoffman et al. (1990) examined the effect of dietary boron
exposure on the survival, growth, and physiology of ducklings hatched
from uncontaminated eggs. Day-old mallards were exposed to 0, 100,
400, or 1600 mg boron/kg diet as boric acid for 10 weeks. No
significant effect on survival was found. The highest dietary
concentration of boron significantly decreased overall growth and the
rate of growth (sexes combined), whereas lower concentrations altered
growth only in female ducklings. Significantly decreased food
consumption was observed in the high-dose group during the first
3 weeks and in all dose groups during the 2nd week. Effects on blood,
brain, and liver biochemistry were also observed in the high-dose
group. Overall, dietary boric acid proved to be less toxic to
ducklings hatched from untreated eggs than to ducklings hatched from
boron-contaminated eggs.
Boron has also been shown to affect the behaviour of mallard
ducklings. Whitworth et al. (1991) analysed the activity schedules and
behaviour durations of day-old ducklings that received diets
containing 0, 100, 400, or 1600 mg boron/kg as boric acid. The highest
dose level had significant effects on the activity schedules of the
ducklings, including increased time at rest. Ducklings exposed to 1600
mg/kg in the diet spent less time in alert behaviours and in the water
compared with controls.
These studies suggest that high dietary levels of boron can
adversely affect normal duckling development and survival. In
addition, interactive effects have been demonstrated in mallard
ducklings given both boron and selenium in the diet. Hoffman et al.
(1991) noted further growth reduction in ducklings fed diets with 60
mg selenium/kg (as seleno-methionine) and 1000 mg boron/kg (as boric
acid) compared with ducklings fed diets with only 1000 mg boron/kg (as
boric acid) over a 4-week period. These effects were magnified when
dietary protein was restricted. Reduction of the dietary protein
content from 22% to 7% in combination with the same selenium and boron
exposure scenario as above resulted in significant duckling mortality.
These findings suggest that selenium and boron may interact to cause
more severe toxicological effects in waterfowl than boron causes
alone, especially in cases where dietary protein is restricted.
The liver boron content of mallards fed with dietary boron at 0,
30, 300, or 1000 mg/kg was found to range from 0 to 51 µg/g dry
weight. The level of 33-51 µg boron/g in liver was associated with
reproductive impairment in the 1000 mg/kg exposed group (Smith &
Anders, 1989).
Boron levels in the livers of aquatic birds from the Grassland
Water District of California, USA, were also examined during
1985-1988. The levels detected ranged from 1.7 to 40 µg boron/g dry
weight; the highest level detected is in a range associated with
reproductive impairment (Paveglio, 1992).
9.2 Field observations
9.2.1 Aquatic
Concentrations of boron in trout hatcheries of Germany, the
United Kingdom, and the USA (California) have been determined. Mean
concentrations of boron in feed waters to 20 United Kingdom rainbow
trout hatcheries were 0.009-0.021 mg/litre (ECETOC, 1997). A range of
0.002-0.107 mg boron/litre was noted in 18 hatcheries in Germany. A
range of higher concentrations, 0.02-1.0 mg boron/litre, was reported
for the 10 largest hatcheries in California (Bingham, 1982; EA, 1994).
Concentrations of boron in streams and lakes of the United
Kingdom and USA capable of sustaining trout species were found to be
similar to concentrations found in hatcheries. Median boron
concentrations from nine different water regions within the United
Kingdom ranged from 0.007 to 0.272 mg/litre, with maximum values in
each of the regions ranging from 0.113 to 2.3 mg boron/litre (ECETOC,
1997). Boron concentrations in California surface waters that
supported viable populations of wild rainbow trout ranged from <0.01
to 13.1 mg/litre (Bingham, 1982). An update to the Bingham report was
provided by a survey of 37 fisheries biologists in the western USA
(EA, 1994). No instances were found where rainbow trout populations
were limited by boron. Several locations were found to have successful
trout populations with aqueous boron concentrations near or above
1 mg/litre. Specific examples include East and Paulina lakes in Oregon
(>0.9 mg boron/litre), Firehole River in Wyoming (>0.9 mg
boron/litre), Napa River in California (>1.2 mg boron/litre), and
Little Warm Springs in California (>3.2 mg boron/ litre). The first
two sites are renowned trophy trout waters. Important fish species
other than trout, such as northern pike, sturgeon, and catfish, were
reported to live in streams with higher boron concentrations, up to
approximately 2 mg/litre, such as in the Poplar River in Montana and
Souris River in North Dakota. Neither of these latter rivers was
reported to contain rainbow trout owing to excessive temperatures and
unsuitable habitat (EA, 1994).
9.2.2 Terrestrial
Boron deficiencies in terrestrial plants have been reported in
many countries of the world (Kabata-Pendias & Pendias, 1984). For
example, deficiency has been reported in Canada, New Zealand, Sweden,
Nigeria, the United Kingdom, the USA, and some arid regions of India
and Pakistan. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions, because of boron's
susceptibility to leaching. Boron deficiency may also occur in
heavy-textured soils with high pH, because boron is readily adsorbed
under these conditions. Boron deficiency is more widespread than the
deficiency for any other micronutrient (Gupta et al., 1985).
When deficiency exists, boron applications increase yield and
improve quality of many crops (Gupta et al., 1985). Hopmans & Flinn
(1984) observed severe dieback in Monterey pine plantations during dry
years in south-eastern Australia owing to boron deficiency. Borax
applied in the spring at rates of 50, 100, or 150 kg/ha resulted in a
significant improvement in height growth of fertilized trees.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field. Few irrigation waters contain
enough boron to injure plants directly. However, it is the continued
use and concentration in the soil as a result of evapotranspiration
that lead to the eventual toxicity problems (Gupta et al., 1985). In
waters used for irrigation, Wilcox (1958) reported a critical boron
concentration of 0.3-1.0 mg/litre for sensitive crops, such as citrus
and other fruits. Semi-tolerant crops, such as potatoes, tomatoes, and
oats, tolerate concentrations of 1-2 mg/litre; tolerant crops, such as
sugar beets, onions, and carrots, can withstand concentrations of
2-4 mg/litre.
The symptoms of boron toxicity are predominantly manifested in
the leaves and roots; however, Eaton et al. (1941) observed that these
effects were frequently absent in stone fruit trees, with boron
accumulation occurring in the bark and fruits rather than the leaves.
Dye et al. (1984) reported that boron concentrations in bark of 31-120
µg/g dry matter and in fruit of 90-311 µg/g dry matter resulted in
bud-drop and branch die-back in peach trees. In nectarine trees with
boron tissue concentrations of 380-457 µg/g dry matter, the buds
failed to swell and subsequently dropped in the spring. Dye et al.
(1983) observed the same symptoms in apricot trees given about 2.4 g
of borax per tree during planting. Buds on unhealthy trees that failed
to break contained 40-100 µg boron/g dry matter.
Pollutant sources of boron, in the form of either airborne
emissions or leachates from soil application, have been shown to
produce toxic symptoms in endemic species. Smidt & Whitton (1975)
studied the toxic responses in a stand of Monterey pine adjacent to a
boiler house. The trees were exposed to boron as a result of dumping
of furnace ash into an adjacent gully and from airborne ash emitted
from the furnace chimney. The average boron concentration in the ash
was 1900 mg/kg. Chlorosis and necrosis of the needles were most severe
closest to the furnace chimney, with the effects decreasing with
increased distance from the furnace. No evidence of toxicity was
observed at needle boron concentrations of 13-70 µg/g, whereas toxic
symptoms were observed at needle boron concentrations of approximately
200 µg/g. The authors concluded that foliar absorption of airborne
boron, as well as root absorption from boron in soil solution,
resulted in the injury to this stand of trees.
Temple & Linzon (1976) reported that atmospheric boron emissions
from an appliance manufacturing plant and a fibreglass manufacturing
plant resulted in >35% foliar necrosis on vegetation within 700 m of
the plant, with 1-15% foliar necrosis occurring within 500 m in all
directions from the plant. The amount of leaf damage was proportional
to the boron concentration in the leaves. Species of maple
(Acer sp.) appeared to be the most sensitive, with slight necrosis
occurring at a concentration of 432 µg/g in unwashed foliage, moderate
necrosis at foliar concentrations ranging from 707 to 1013 µg/g, and
severe necrosis at foliar concentrations of 1207-1560 µg/g.
Lang et al. (1986) found that boron thresholds for detectable
levels (10%) of visible injury due to boron toxicity were about 500
mg/kg of foliage for both bigleaf maple and digger pine growing near
geothermal generating units.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health exposures
Boron is a naturally occurring element found combined with other
elements (primarily oxygen) throughout the environment. It is found in
the Earth's crust, with the majority of readily available forms
occurring in the ocean. It is estimated that more boron is released
into the environment by natural weathering than from anthropogenic
sources. Boron is not present in the atmosphere at significant levels.
Boron is present in surface water and groundwater and is readily
adsorbed on the surfaces of soil particles. Boron is present in food,
beverages, and drinking-water.
Boron has a wide variety of uses in the manufacture of glass,
glass products, antiseptics, cleaning products, pharmaceuticals,
biological growth control agents, wood and leather preservatives, and
insecticides. It is also used in photographic chemicals, enamels and
frits, and fire retardants.
Exposure to boron compounds can occur by inhalation, ingestion,
and dermal contact. Boron compounds are absorbed from the respiratory
and gastrointestinal tracts, as indicated by increased levels of boron
in the blood, tissues, and urine. Dermal absorption of boron across
intact skin is negligible.
Specific information on population exposures from different
countries was discussed in chapter 5. This information was used to
determine mean intakes in ambient air, drinking-water, soil, food, and
consumer products. From these mean intakes, per cent allocations of
the TIs can be developed, as shown in Table 22. In general, food and
drinking-water form the greatest contributions to boron intake in
humans, at about 65% and 30%, respectively.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data suggest that exposure is associated with short-term and
reversible irritant effects on the upper respiratory tract,
nasopharynx, and eye. The sole long-term (7-year) follow-up study
failed to identify any long-term health effects, although a healthy
worker effect cannot be entirely ruled out, given the rate of
attrition (47%). In two descriptive studies that assessed fertility
and secondary sex ratios in relation to exposure, no detrimental
effects on demonstrated fertility for the study samples were reported.
Although an excess percentage of female births has been suggested, the
absence of statistical significance and attention to other co-variates
known to affect sex ratios warrants careful interpretation of this
finding. No studies have been identified that assess the spectrum of
reproductive outcomes, such as time-to-pregnancy, conception delays,
spontaneous abortions, or semen quality. The role of other lifestyle
or behavioural factors in relation to health and fertility requires
further study to identify potentially sensitive populations and to
evaluate reproductive effects more fully.
Table 22. Estimation of exposures based on ranges of mean
concentrations in several environmental media on a global basis,
and allocation of the TI among media
Environmental media Mean intakea % allocation of TI
(mg/day)
Ambient air 0.000 44 Nil
Drinking-water 0.6b approx. 30
Soil 0.000 5 Nil
Food 1.2 approx. 65
Consumer products 0.1 5
Total 1.9 100
a Mean intakes are estimated in chapter 5.
b Assuming an intake of 2 litres of water per day.
10.2 Choice of critical effect and application of uncertainty factors
The critical effect appears to lie within the area of
developmental toxicity. Other effects, such as reproductive toxicity,
also occur at slightly higher doses and were considered in the choice
of critical effects through a comparison of LOAELs (Table 17) or
benchmark doses (BMDs) (Moore et al., 1997).a Several developmental
effects from boron exposure have been noted in rats, mice, and
rabbits. Again, these effects are the most sensitive in a larger
toxicity database that includes sub-chronic and chronic bioassays. The
specific critical effect within these developmental toxicity studies
is a decreased average fetal body weight per litter in the rat
(Heindel et al., 1992; Price et al., 1996a).
a The Weir & Fisher (1972) 2-year dog studies were not used directly
for risk assessment for several reasons. The NOAEL and LOAEL were
taken from two studies; one was 2 years long, and the high-dose group
was a supplemental group terminated at 38 weeks. There were a limited
number of animals (4/dose) per test group. One control group was used
for both borax and boric acid, so that there were never more than 2
control animals sacrificed at one time. Some of the control animals
showed some form of testicular damage.
In the Price et al. (1996a) study, boric acid was administered in
the diet to CD rats from gestational day 0 to 20 at 3.3, 6.3, 9.6,
13.3, or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls.
Fetal body weight was statistically significantly decreased only in
the 13.3 and 25 mg/kg body weight per day dose groups on gestational
day 20. The NOAEL for decreased fetal body weight was 9.6 mg boron/kg
body weight per day, and the LOAEL was 13.3 mg boron/kg body weight
per day. The results of this study provide a NOAEL and LOAEL that
complement the LOAEL of 13.6 mg boron/kg body weight per day in the
Heindel et al. (1992) study.b This NOAEL is also similar to BMDs for
developmental toxicity (see appendix).
For interspecies variation, a default value of 10 has often been
applied in the absence of actual data showing less than the usual
variation between species. For inter-individual (intraspecies)
variation, a default value of 10 has often been applied in the absence
of actual data within a species. A scheme has been adopted by IPCS
(1994) that allows for subdivision of each of these defaults to
incorporate appropriate data on toxicodynamics or toxicokinetics.
Where appropriate data exist, this scheme improves the extrapolation
process and uses correction factors for toxicodynamic and
toxicokinetic data instead of 10-fold uncertainty factors for inter-
and intraspecies variation. For interspecies differences, the 10-fold
factor is divided into a default factor of 100.4 for toxicodynamics
and 100.6 for toxicokinetics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.4 × 100.6 = 10).
For inter-individual (intraspecies) differences, the 10-fold
factor is divided into a default factor of 100.5 each for
toxicokinetics and toxicodynamics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.5 × 100.5 = 10).
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in blood and
body tissues of several mammalian species following ingestion.
b In fact, the Price et al. (1996a) study was conducted specifically
to address the lack of a NOAEL from the Heindel et al. (1992) study.
Similarity in study designs and results from these two studies greatly
strengthens the dose-response relationship for developmental toxicity
as the critical effect.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the NOAEL for laboratory studies, reduce
the uncertainty for risk extrapolation between these two species.
10.3 Derivation of the tolerable intake
A TI is defined as an estimate of the intake of a substance that
can occur over a lifetime without appreciable health risks. The TI is
derived on the basis of the NOAEL of the critical effect, the adverse
effects judged to be the most appropriate for determining the TI,
using appropriate uncertainty factors. The use of the critical effect,
judged here to be developmental toxicity, is expected to protect
against other effects such as reproductive toxicity occurring at
higher doses.
9.6 mg/kg body weight per day
TI =
[100.4 × 100.1] × [100.5 × 100.4]
= 0.4 mg/kg body weight per day
= 400 µg/kg body weight per day
where:
* 9.6 mg/kg body weight per day is the NOAEL from a well conducted
developmental study with decreased fetal body weight occurring in
a dose-related manner
* 100.4 × 100.1 (=100.5) is the uncertainty factor for interspecies
differences:
Dynamics: Data do not exist to support a factor other than the
default value of 100.4. Moreover, differences in LOAELs among
rats, rabbits, and mice for the critical effect (Table 16)
support the default value. The Task Group judged that 100.4 was
appropriate.
Kinetics: Oral absorption of boron in rats and humans is
quantitatively similar, with values of 94% for humans and 95% for
rats (section 6.1.1). The ratio of these absorption rates is
approximately 1.
Distribution of boron in rats and humans appears quantitatively
similar, as determined by a comparison of blood boron levels
after doses in either diet or drinking-water (Fig. 1, section
6.2.2). Ratios of rat blood boron values to a regression line for
human blood boron values are as low as 0.7 and as high as 6, with
the majority of values in the range of 2-3. Comparative
regression techniques should be pursued to confirm this trend.
Pregnant rats appear to have lower blood boron values than non-
pregnant rats when given similar doses. Comparative data for
pregnant and non-pregnant humans are lacking.
Metabolism of boron is thought not to occur in humans or animals
owing to the excessive energy required to break the boron-oxygen
bond (section 6.3). As it is unlikely that there are any
differences between species in the metabolism of boron, the ratio
of metabolic parameters is 1.
Elimination of boron in rats and humans appears quantitatively
similar, with half-life values in humans of 21 h in volunteers
and approximately 13 h in poisonings, and values in rats of
14=19 h (section 6.4). The ratio of these elimination half-life
values is approximately 1.3 when human volunteer data are
compared with the rat data, or 0.8 when human poisoning data are
compared with the rat data. The mean of these two ratios is
about 1.
As a result, the Task Group judged that the default value of
100.6 could be reduced to 100.1.
* 100.5 × 100.4 (=100.9) is the uncertainty factor for
inter-individual (intraspecies) differences:
Dynamics: Data in humans do not exist to support a value
different from the default of 100.5. Animal studies suggest that
intraspecies variability in toxicodynamics exists. The Task Group
judged that 100.5 was appropriate.
Kinetics: Data exist in humans to suggest some limited
variability in boron absorption and/or distribution (Nielsen,
1995). However, the lack of boron metabolism in humans and
experimental animals suggests some reduction in the default value
of 100.5. The Task Group judged that 100.4 was appropriate.
Other uncertainty factors for adequacy of database are not
considered necessary, because the overall database includes subchronic
and chronic studies on several species and several reproductive
studies.
The total uncertainty factor,a,b is therefore:
100.5 × 100.9 = 101.4 (or 25)
Data are inadequate to allow the development of a TI for
inhalation exposure (TIi).
10.4 Derivation of guidance values
Exposure to boron from various media varies with the locality. In
areas where data on exposure are available, specific guidance values
that are tailored to the local circumstances should be developed from
the TI presented in this document.
For example, guidance values for specific media could be
developed by first using the allocations shown in Table 22 with the TI
derived in section 10.3. Table 23 shows the resulting allocation of
the TI by media in mg/kg body weight per day.
The second step in the development of a guidance value would be
to pick appropriate body weight and exposure assumptions. Two examples
are given using allocations found in Table 23. The resulting guidance
value (GV) for example A in drinking-water would be:
a After closure of the Task Group meeting, Dr. M. Dourson, after
additional consideration, dissented from the Task Group's
recommendation on the uncertainty factor.
b Subsequent to the Task Group meeting, a provisional guideline for
boron in drinking-water was recommended by the Working Group Meeting
on Chemical Substances in Drinking-Water, using a different
uncertainty factor, based upon a different weighting attached to some
toxico-kinetic studies for that area of application (WHO, 1998 a,b).
Example A
0.12 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 4 mg/litre
where:
* 64 kg is the average human body weight (IPCS, 1994)
* 2 litres/day is one assumption of water intake (IPCS, 1994).
The resulting guidance value (GV) for example B would be:
Example B
0.04 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 1.3 mg/litre
Assumptions of food intake and consumer product use could be used
with allocations given in Table 23 to generate tolerances or other
risk management standards. Individual countries are encouraged to
develop guidance values using allocations, body weights, and exposure
assumptions that are appropriate for their populations.
Table 23. Examples of allocation of TI by media
Environmental Example A Example B
media
Per centa mg/kg body Per cent mg/kg body
weight per day weight per day
Ambient air Nil 0 Nil 0
Drinking-water 30 0.12 10 0.04
Soil Nil 0 Nil 0
Food 65 0.26 85 0.34
Consumer products 5 0.02 5 0.02
Total 100 0.4 100 0.4
a Values from Table 22.
10.5 Evaluation of effects on the environment
10.5.1 Exposure
Boron is not present in the atmosphere at significant levels;
however, the total amount present in the atmosphere at any one time is
significant owing to the huge volume of the atmosphere. Boron
concentrations in air range from <0.5 to approximately 80 ng/m3,
with an average of 20 ng/m3.
Boron occurs naturally in rocks, with concentrations ranging from
5 mg/kg in basalts to 100 mg/kg in shales. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron range widely, from 0.001 to as much as 360 mg/litre. However,
mean concentrations for waters of Europe, Pakistan, Russia, and Turkey
are typically well below 0.6 mg boron/litre. Concentrations of boron
in waters in Japan, South Africa, and South America are generally
below 0.3 mg/litre. Typical concentrations in North American waters
are below 0.1 mg boron/litre, with about 90% at 0.4 mg boron/litre or
below.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Boron occurs in soils at concentrations ranging from 10 to 300 mg/kg
(average 30 mg/kg), depending on the type of soil, amount of organic
matter, and amount of rainfall.
Boron does not bioaccumulate in aquatic invertebrates and fish.
Based on wet weights, boron concentrations in marine invertebrates and
fish are similar to the levels found in the exposure media, between
0.5 and 4 mg/kg. Bioconcentration factors for two freshwater fish
species were found to be 0.3. The results of studies of boron
accumulation in plants, insects, and fish have shown that boron
bioaccumulates in plants but does not biomagnify in aquatic
food-chains. Concentrations of boron have been shown to range between
26 and 382 mg/kg in submerged aquatic freshwater plants, from 11.3 to
57 mg/kg in freshwater emergent vegetation, and from 2.3 to 94.7 mg/kg
dry weight in terrestrial plants.
10.5.2 Effects
Boron is an essential micronutrient for cyanobacteria and
diatoms. Considering all microorganisms, algae and protozoa appear to
be equisensitive to boron and bacteria the most tolerant. Both algae
and protozoa provide NOEC (including EC3) values between 0.3 and 20
mg boron/litre.
Chronic studies with Daphnia magna provided consistent NOECs
between 6 and 10 mg boron/litre. Long-term outdoor pond and field
studies with microorganisms and invertebrates provided NOEC values up
to 1.52 mg boron/litre.
Via acute tests, the rainbow trout and zebra fish were determined
to be the most sensitive taxa, with acute values around 10 mg
boron/litre. Further tests with embryo-larval stages of several fish
species showed rainbow trout to be the most sensitive to boron. Tests
conducted with early life stages of rainbow trout in reconstituted
water overpredicted toxicity compared with tests conducted with
natural water. No adverse effects were found in natural water
exposures containing up to 0.75 mg boron/litre. Consistent LOECs were
between 1.1 and 1.7 mg boron/litre. These laboratory values were
confirmed by several observations of successful trout populations
thriving in hatcheries, streams, and lakes containing boron
concentrations up to 1 mg/litre.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
The symptoms of boron deficiency in plants include cessation of root
and leaf growth, necrosis, retardation of enzyme reactions, and
reduced pollen germination. The initial symptoms of boron toxicity in
plants are chlorosis, necrosis, and a subsequent loss of
photosynthetic capacity, which reduces plant productivity.
Mallard duckling growth was adversely affected at dietary levels
of 30 and 300 mg/kg, and survival was reduced at 1000 mg/kg.
10.5.3 Risk evaluation
Risk characterization is based on the comparison of the
environmental concentration with a concentration protective for an
ecosystem. Ambient concentrations of boron in surface waters have been
reported for several countries covering four continents. The data
indicate that mean or median levels are approximately 0.1 mg
boron/litre and that 90th-percentile or greater values are
approximately 0.5 mg boron/litre. Considerable data exist on the
toxicity of boron to freshwater organisms and ecosystems. Acute tests
with invertebrates and fish showed that rainbow trout and zebra fish
were most sensitive to borate (LC50 approx. 10 mg boron/litre).
Chronic results for algae, higher plants, and fish clearly showed that
the rainbow trout was the most sensitive species, with consistent
NOECs and LOECs of about 1 mg boron/litre. Confirmed were several
observations of healthy rainbow trout populations in US hatcheries and
streams at or above 1 mg boron/litre. Not surprisingly, biocenoses
(model ecosystems) covering all stages of the aquatic food-web
(excluding fish) were more tolerant of boron than were rainbow trout
(NOEC up to 1.52 mg boron/litre). Given all this information, the
comparison of the environmental effects concentration with the general
ambient environmental levels indicates that the risk of adverse
effects of boron on the aquatic ecosystem is low. In a few boron-rich
environments, natural levels will be higher. It is reasonable to
assume that aquatic organisms in such habitats may be adapted to the
local conditions.
Boron deficiencies in terrestrial plants have been reported in
many countries. Boron deficiency is more likely to occur in
light-textured, acid soil in humid regions, because of boron's
susceptibility to leaching. In general, there is a small range between
deficiency and toxicity. However, considerable variation exists
between species in their resistance to boron. Species sensitive to
boron are known to include citrus, stone fruits, and nut trees;
semi-tolerant species include tubers and cereals; and tolerant species
include most vegetables. Toxicity due to excess boron is much less
common in the environment than boron deficiency. Irrigation water is
one of the main sources of high boron levels that result in toxicity
in the field. However, few irrigation waters contain enough boron to
injure plants directly; it is the continued use and concentration in
the soil resulting from evapotranspiration that lead to the eventual
toxicity problems. Pollutant sources of boron, in the form of either
airborne emissions or leachates from soil application, have also been
shown to produce toxic symptoms in endemic plant species growing in
the immediate vicinity.
Levels of boron measured in aquatic plants from Kesterson
National Wildlife Refuge, California, USA (an evapotranspiration sink
that receives boron-rich run-off from irrigated agricultural fields)
are higher than those found to cause effects on mallard ducklings in
the laboratory. Therefore, there appears to be a risk to waterfowl in
this area. However, the bioavailability of such boron levels is
uncertain. The extent of risk to waterfowl in most geographical areas
is expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency has been observed in many countries throughout the
world. There is a small range between deficiency and toxicity in some
plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental boron levels
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre. For the
general population, the greatest boron exposure comes from the oral
intake of food. The mean daily intake of boron in the diet is
estimated to be near 1.2 mg/day.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. Greater than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The TI of boron was set as 0.4 mg/kg body weight per day. The
allocation of the TI in various media should be based on the exposure
data of individual countries.
11.2 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
12. FURTHER RESEARCH
a) Further studies regarding global cycling of boron.
b) Determine whether boron is required for normal fetal and post-
natal development for higher animals.
c) Define a biochemical function that confirms the essentiality of
boron for higher animals.
d) Determine the homeostatic mechanism through which boron
concentrations are maintained during low dietary intakes.
e) Further studies on the absorption, distribution, and clearance of
boron at oral doses in the range of 0.01-1 mg boron/kg body
weight per day.
f) A modern rat reproduction study to determine the potential for
developmental effects of borates in succeeding generations in a
sensitive animal species.
g) Analytical epidemiology studies to assess the spectrum of
reproductive and developmental outcomes to assess boron toxicity
in targeted human populations.
h) Epidemiological studies that incorporate toxicological and
physiological components to enhance an understanding of the
toxicokinetics and physiology of boron in populations, including
potentially sensitive ones.
i) Further quantitative definition of the maximum tolerable
concentration for sensitive aquatic species.
j) A refined understanding of the relationship of water and dietary
uptake of boron into waterfowl.
k) Development of an approach that integrates essentiality and
toxicity for the establishment of acceptable boron concentrations
in aquatic and terrestrial environments.
13. EVALUATIONS BY INTERNATIONAL BODIES
The EC Cosmetics Directive 76/768/EEC (and its subsequent
amendment) sets an upper limit of boric acid in cosmetic products,
which must not contain more than 5% in talc, 0.5% in oral hygiene
products, and 3% in other products. Only the talc containing boric
acid must, in addition, not be used on children less than 3 years of
age.
In 1997, subsequent to the Task Group meeting, a provisional
guideline for boron in drinking-water was recommended by the Working
Group on Chemical Substances in Drinking-Water, which updated the
Guidelines for drinking-water quality (WHO, 1998a,b).
REFERENCES
ACGIH (1991) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, pp 141-148.
Adams FS, Cole H Jr, & Massie LB (1973) Element constitution of
selected aquatic vascular plants from Pennsylvania: Submersed and
floating leaved species and rooted emergent species. Environ Pollut,
5: 117-147.
Ahl T & Jönsson E (1972) Boron in Swedish and Norwegian fresh waters.
Ambio, 1(2): 66-70.
Alabaster JS (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int Pest
Control, 11: 29-35.
Alexander GV, Nusbaum RE, & MacDonald NS (1951) The boron and lithium
content of human bones. J Biol Chem, 192: 489-496.
Allen BC, Strong PL, Price CJ, Hubbard SA, & Daston GP (1996)
Benchmark dose analysis of developmental toxicity in rats exposed to
boric acid. Fundam Appl Toxicol, 32: 194-204.
Anderson BG (1946) Industrial wastes: The toxicity thresholds of
various sodium salts determined by the use of Daphnia magna. Sewage
Works J, 18: 82-87.
Anderson DL, Kitto ME, McCarthy L, & Zollar WH (1994a) Sources of
atmospheric distribution of particulate and gas phase boron. Atmos
Environ, 28: 1401-1410.
Anderson DL, Cunningham WC, & Lindstrom TR (1994b) Concentrations and
intakes of H, B, S, K, Na, Cl and NaCl in foods. J Food Compos Anal,
7: 59-82.
Antia NJ & Cheng JY (1975) Culture studies on the effects from borate
pollution on the growth of marine phytoplankters. J Fish Res Board
Can, 32: 2487-2494.
Aquilio E, Spagnoli R, Seri S, Bottone G, & Spennati G (1996) Trace
element content in human milk during lactation of preterm newborns.
Biol Trace Elem Res, 51: 63-70.
ATSDR (1992) Toxicological profile for boron. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry.
Atteia O, Védy J-C, & Parriaux A (1993) Trace elements dynamics in
soils and aquifers of Western Switzerland. Stud Environ Sci Environ
Contam, 55: 79-101.
Aznarez J, Ferrer A, Rabadan JM, & Marco L (1985) Extractive
spectrophotometric and fluorimetric determination of boron with
2,2,4-trimethyl-1,3-pentanediol and carminic acid. Talanta, 32(12):
1156-1158.
Bai Y & Hunt CD (1996) Absorption and distribution of boron in rats
following a single oral administration of boron. Proc North Dakota
Acad Sci, 50: 53.
Banerji SK (1969) Boron adsorption on soils and biological sludges and
its effects on endogenous respiration. In: Proceedings of the 24th
Industrial Waste Conference, Lafayette, 6-8 May 1969. Lafayette,
Indiana, Purdue University (Engineering Extension Series No. 135).
Banuelos GS, Cardon G, Pflaum T, & Akohoue S (1992) Comparison of dry
ashing and wet acid digestion on the determination of boron in plant
tissue. Commun Soil Sci Plant Anal, 23(17-20): 2383-2397.
Barber LB, Thurman EM, Schroeder MP, & Le Blanc DR (1988) Long-term
fate of organic micropollutants in sewage-contaminated groundwater.
Environ Sci Technol, 22: 205-211.
Barnes DG, Datson GP, Evans JS, Jarabek AM, Kavlock RJ, Kimmel CA,
Park C, & Spitzer HL (1995) Benchmark dose workshop: Criteria for use
of a benchmark dose to estimate a reference dose. Regul Toxicol
Pharmacol, 21: 296-301.
Barr RD, Clarke WB, Clarke RM, Venturelli J, Norman GR, & Downing RG
(1993) Regulation of lithium and boron levels in normal human blood:
Environmental and genetic considerations. J Lab Clin Med,
121: 614-619.
Barres M (1967) Contribution à l'étude de l'isopoly--condensation des
borates alcalins par électrométrie et partages. Rev Chem Minér,
4: 803-838.
Barth RF & Soloway AH (1994) Boron neutron capture therapy of primary
and metastatic brain tumors. Mol Chem Neuropathol, 21(2-3): 139-154.
Barth RF, Adams DM, Soloway AH, Mechetner EB, Alam F, & Anisuzzaman KM
(1991) Determination of boron in tissues and cells using
direct-current plasma atomic emission spectroscopy. Anal Chem,
63: 890-893.
Belanger SE, Farris JL, & Cherry DS (1989) Effects of diet, water
hardness and population source on acute and chronic copper toxicity to
Ceriodaphnia dubia. Arch Environ Contam Toxicol, 18: 601-611.
Benson WH, Birge WJ, & Dorough HW (1984) Absence of mutagenic activity
of sodium borate (borax) and boric acid in the Salmonella
preincubation test. Environ Toxicol Chem, 3: 209-214.
Benson SM, White AF, Halfman S, Flexser S, & Alavi M (1991)
Groundwater contamination at the Kesterson Reservoir, California: 1.
Hydrogeologic setting and conservative solute transport. Water Resour
Res, 27: 1071-1084.
Bergmann W (1984) The significance of the micronutrient boron in
agriculture. London, Borax Holdings Limited, 26 pp.
Bergmann W, Bruchlos P, & Marks G (1995) [A contribution to the
question of the limit value for boron in water for Ogragnutes
australis seed.] Tenside Deterg, 32: 229-237 (in German).
Bertine KK & Goldberg ED (1971) Fossil fuel combustion and the major
sedimentary cycle. Science, 173: 233-235.
Beyer KH, Bergfeld WF, Berndt WO, Boutwell RK, Carlton WW, Hoffmann
DK, & Schroeter AL (1983) Final report on the safety assessment of
sodium borate and boric acid. J Am Coll Toxicol, 2: 87-125.
Biggar JW & Fireman M (1960) Boron adsorption and release by soils.
Soil Sci Soc Am Proc, 24: 115-120.
Bingham FT (1982) The boron concentration of wild trout streams in
California. Riverside, California, University of California,
Department of Soil Science (Unpublished document).
Bingham FT, Elseewi A, & Oertli JJ (1970) Characteristics of boron
absorption by excised barley roots. Soil Sci Soc Am Proc, 34: 613-617.
Bingham FT, Page AL, Coleman NT, & Flach K (1971) Boron adsorption
characteristics of selected amorphous soils from Mexico and Hawaii.
Soil Sci Soc Am Proc, 35: 546-550.
Birge WJ & Black JA (1977) Sensitivity of vertebrate embryos to boron
compounds. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Prepared for US EPA by the University of
Kentucky, Lexington) (EPA-560/1-76-008).
Birge WJ & Black JA (1981) Toxicity of boron to embryonic and larval
stages of largemouth bass (Micropterus salmoides) and rainbow trout
(Salmo gairdneri)--Completion report. Cincinnati, Ohio, Procter &
Gamble Company.
Birmingham DJ & Key MM (1963) Preliminary survey: U.S. Borax plant,
California. Cincinnati, Ohio, Occupational Health Research and
Training Facility, Division of Occupational Health.
Black JA, Barnum JB, & Birge WJ (1993) An integrated assessment of the
biological effects of boron to the rainbow trout. Chemosphere, 26:
1383-1413.
Blasser-Grill J, Knoppik D, Amberger A, & Goldbach H (1990) Influence
of boron on the membrane potential in Elodea densa and Helianthus
annus roots and H+ extrusion of suspension cultured Daucus
carota cells. Plant Physiol, 90: 280-284.
Blevins DG & Luk-aszewski KM (1994) Proposed physiologic functions of
boron in plants pertinent to animal and human metabolism. Environ
Health Perspect, 102(Suppl 7): 31-33.
Bodek I, Ehntholt EJ, Glazer AE, Loreti CP, & Lyman W (1988) Chapter
10: Chemical classes, Section 10.9: Boron-containing compounds. In:
Bodek I, Lyman WJ, Reehl WF, Rosenblatt DH, Walton BT, & Conway RA ed.
Environmental inorganic chemistry: Properties, processes, and
estimation methods. Oxford, New York, Pergamon Press,
pp 10.9/1-10.9/10.
Bonilla I, Garcia-Gomez M, & Mates P (1990) Boron requirements in
cyanobacteria: Possible role in the early evolution of photosynthetic
organisms. Plant Physiol, 94: 1554-1560.
Borkovec AB, Settepani JA, LaBrecque GC, & Fye RL (1969) Boron
compounds as chemosterilants for house flies. J Econ Entomol, 62:
1472-1480.
Bowen JE & Gauch HG (1966) Nonessentiality of boron in fungi and the
nature of its toxicity. Plant Physiol, 41: 319-324.
Boyd CE & Walley WW (1972) Studies of the biogeochemistry of boron. 1.
Concentrations in surface waters, rainfall and aquatic plants. Am Mid
Nat, 88: 1-14.
Bringmann G & Kuhn R (1977) [The toxicity of waterborne contaminants
towards Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in
German with English summary).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kuhn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G, Kuhn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa: III. Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German with
English summary).
Brown TF, McCormick ME, Morris DR, & Zeringue LK (1989) Effects of
dietary boron on mineral balance in sheep. Nutr Res, 9: 503-512.
Budavari S, O'Neil MJ, Smith A, & Heckelman PE ed. (1989) The Merck
index, 11th ed. Rahway, New Jersey, Merck and Co., Inc.
Bundschuh J (1992) Boron contamination of the ground- and surface
waters of Lerma Valley, Argentina. Aqua (Lond), 41(1): 13-17.
Butterwick L, De Oude N, & Raymond K (1989) Safety assessment of boron
in aquatic and terrestrial environments. Ecotoxicol Environ Saf, 17:
339-371.
Byrne RH Jr & Kester DR (1974) Inorganic speciation of boron in
seawater. J Mar Res, 32: 119-127.
Cáceres LV, Gruttner ED, & Contreras RN (1992) Water recycling in arid
regions: Chilean case. Ambio, 21(2): 138-144.
Canton JH & Adema DMM (1978) Reproducibility of short-term and
reproduction toxicity experiments with Daphnia magna and comparison
of the sensitivity of Daphnia magna with Daphnia pulex and
Daphnia cucullata in short-term experiments. Hydrobiologia,
59: 135-140.
Chapin RE, Ku WW, Kenney MA, McCoy H, Gladen B, Wine RN, Wilson R, &
Elwell MR (1997) The effects of dietary boron on bone strength in
rats. Fundam Appl Toxicol, 35: 205-215.
Choi WW & Chen KY (1979) Evaluation of boron removal by adsorption on
solids. Environ Sci Technol, 13: 189-196.
Clarke WB, Koekebakker K, Barr RD, Downing RG, & Fleming RF (1987a)
Analysis of ultratrace lithium and boron by neutron activation and
mass spectrometric measurement of 3He and 4He. Appl Rad Isot, 38(9):
735-743.
Clarke WB, Webber CE, & Koekebakker K (1987b) Lithium and boron in
human blood. J Lab Clin Med, 109: 155-158.
Corwin DL (1986) A one-dimensional model of chemical diffusion and
sorption in saturated soil and aquatic systems. J Environ Qual,
15: 173-182.
Cotton FA & Wilkinson G (1988) Advanced inorganic chemistry, 5th ed.
New York, John Wiley and Sons, pp 162-165.
Couch EL & Grim RE (1968) Boron fixation by illites. Clays Clay Miner,
16: 249-256.
Cox JA, Lundquist GL, Przyjazny A, & Schmulbach CD (1978) Leaching of
boron from coal ash. Environ Sci Technol, 12: 722-723.
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, &
Strong PL (1994a) The relationship of blood- and urine-boron to boron
exposure in borax-workers and the usefulness of urine-boron as an
exposure marker. Environ Health Perspect, 102(Suppl 7): 133-137.
Culver BD, Smith RG, Brotherton RJ, Strong PL, & Gray TM (1994b)
Chapter 44: Boron. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology. New York, John Wiley and Sons, vol 2, part 1.
Cumakov A (1991) Chemical methods of fractionation of soil trace
elements: 1. Boron. Chem Abstr, 115: 182048v.
De Jong LEDD (1965) Tolerance of Chlorella vulgaris for metallic and
non-metallic ions. Antonie van Leeuwenhoek, 31: 301-313.
Deverel SJ & Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, California. Environ Sci Technol, 22: 697-702.
Dieter MP (1994) Toxicity and carcinogenicity studies of boric acid in
male and female B6C3F1 mice. Environ Health Perspect, 102(Suppl 7):
93-97.
Dixon RL, Lee IP, & Sherins RJ (1976) Methods to assess reproductive
effects of environmental chemicals: Studies of cadmium and boron
administered orally. Environ Health Perspect, 13: 59-67.
Dixon RL, Sherins RJ, & Lee IP (1979) Assessment of environmental
factors affecting male fertility. Environ Health Perspect, 30: 53-68.
Doonan DJ & Lower LD (1978) Boron compounds (oxides, acid, borates).
In: Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New
York, John Wiley and Sons, vol 4, pp 67-110.
Draize JH & Kelley EA (1959) The urinary excretion of boric acid
preparations following oral administration and topical applications to
intact and damaged skin of rabbits. Toxicol Appl Pharmacol, 1:
267-276.
Dudas MJ (1981) Long-term leachability of selected elements from fly
ash. Environ Sci Technol, 15: 840-843.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1983) Die-back of
apricot trees following soil application of boron. NZ J Exp Agric, 11:
331-342.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1984) Boron toxicity
in peach and nectarine trees in Otaga. NZ J Exp Agric, 12: 303-313.
EA (1994) Boron concentrations and rainbow trout populations in seven
states in the western United States. Corvallis, Oregon, EA
Engineering, Science and Technology (Unpublished report prepared for
the Procter & Gamble Company, Cincinnati).
Eaton FM (1944) Deficiency, toxicity, and accumulation of boron in
plants. J Agric Res, 69: 237-277.
Eaton FM, McCallum RD, & Mayhugh MS (1941) Quality of irrigation
waters of the Hollister area of California with special reference to
boron content and its effect on apricots and prunes. US Dept Agric
Tech Bull, 746: 1-59.
ECETOC (1995) Reproductive and general toxicology of some inorganic
borates and risk assessment for human beings. Brussels, European
Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 63).
ECETOC (1997) Ecotoxicology of some inorganic borates - Interim
report. Brussels, European Centre for Ecotoxicology and Toxicology of
Chemicals, 97 pp (Special Report No. 11).
Eckel WP & Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
Superfund '88: Proceedings of the 9th national conference. Washington,
DC, US Hazardous Materials Control Research Institute, pp 282-286.
Eisler R (1990) Boron hazards to fish, wildlife, and invertebrates: A
synoptic review. Washington, DC, US Department of Interior, Fish and
Wildlife Service.
El-Hassanin AS, Labib TM, & Dobal AT (1993) Potential Pb, Cd, Zn and B
contamination of sandy soils after different irrigation periods with
sewage effluent. Water Air Soil Pollut, 66: 239-249.
Elrashidi MA & O'Connor GA (1982) Boron sorption and desorption in
soils. Soil Sci Soc Am J, 46: 27-31.
Emsley J (1989) The elements. Oxford, Clarendon Press, p 32.
Fail PA, George JD, Sauls SW, Dennis SW, & Seely JC (1989) Effect of
boric acid on reproduction and fertility of rodents. Adv Contracept
Deliv Syst, 5: 323-333.
Fail PA, George JD, Grizzle TB, Heindel JJ, & Chapin RE (1990) Final
report on the reproductive toxicity of boric acid (CAS No. 10043-35-3)
in CD-1 Swiss mice. Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NTP Report No. 90-105).
Fail PA, George JD, Seely JC, Grizzle TB, & Heindel JJ (1991)
Reproductive toxicity of boric acid in Swiss (CD-1) mice: Assessment
using the continuous breeding protocol. Fundam Appl Toxicol, E17:
225-239.
Farr LE & Konikowski T (1963) The renal clearance of sodium
pentaborate in mice and men. Clin Chem, 9: 717-726.
Farris JL, Grudzien JL, Belanger SE, Cherry DS, & Cairns J (1994)
Molluscan cellulolytic activity responses to zinc exposure in
laboratory and field comparisons. Hydrobiologia, 287: 161-178.
Fay RW (1959) Toxic effects of boron trioxide against immature stages
of Aedes aegypti, Anopheles quadrimaculatus, and
Culex quinquefasciatus. J Econ Entomol, 52: 1027-1028.
Forbes RM & Mitchell HH (1957) Accumulation of dietary boron and
strontium in young and adult albino rats. Arch Ind Health, 16:
489-492.
Forbes RM, Cooper AR, & Mitchell HH (1954) On the occurrence of
beryllium, boron, cobalt and mercury in human tissues. J Biol Chem,
209: 857-865.
Friis-Hansen B, Aggerbeck B, & Jansen JA (1982) Unaffected blood boron
levels in newborn infants treated with a boric acid ointment. Food
Chem Toxicol, 20: 451-454.
Garabrant DH, Bernstein L, Peters JM, & Smith TJ (1984) Respiratory
and eye irritation from boron oxide and boric acid dusts. J Occup Med,
26: 584-586.
Garabrant DH, Bernstein L, Peters JM, Smith TJ, & Wright WE (1985)
Respiratory effects of borax dust. Br J Ind Med, 42: 831-837.
Garcia-Campana A, Barrero FA, & Ceba MR (1992) Spectrofluorimetric
determination of boron in soils: Plant and natural waters with
alizarin red S. Analyst, 117: 1189-1191.
Gerike P, Fischer WK, & Holtmann W (1976) [The influence of boron on
aerobic biological waste water purification.] Tenside Deterg, 13:
249-252 (in German).
Gerritse RG, Vriesema R, Dalenberg JW, & DeRoos HP (1982) Effect of
sewage sludge on trace element mobility in soils. J Environ Qual, 11:
359-364.
Gersich FM (1984) Evaluation of a static renewal chronic toxicity test
method for Daphnia magna Straus using boric acid. Environ Toxicol
Chem, 3: 89-94.
Gestring WD & Soltanpour PN (1987) Comparison of soil tests for
assessing boron toxicity to alfalfa. Soil Sci Soc Am J, 51: 1214-1219.
Gilliam CH & Watson ME (1981) Boron accumulation in Taxus media.
Hortic Sci, 16: 340-341.
Gladney ES, Wangen LE, Curtis DB, & Jurney ET (1978) Observations on
boron release from coal-fired power plants. Environ Sci Technol, 12:
1084-1085.
Glaubig BA & Bingham FL (1985) Boron toxicity characteristics of four
northern California endemic tree species. J Environ Qual, 14: 72-77.
Goldbloom RB & Goldbloom A (1953) Boric acid poisoning: Report of four
cases and a review of 109 cases from the world literature. J Pediatr,
43: 631-643.
Graedel TE (1978) Inorganic elements, hydrides, oxides, and
carbonates. In: Chemical compounds in the atmosphere. New York,
London, San Francisco, Academic Press, pp 35-49.
Graffmann G, Kuzel P, Nösler H, & Nonnenmarcher G (1974)
[Determination of traces of boron in fresh and drinking-water.] Chem
Ztg, 98: 499-504 (in German).
Green NR & Ferrando AA (1994) Plasma boron and the effects of boron
supplementation in males. Environ Health Perspect, 102(Suppl 7):
73-77.
Green GH & Weeth HJ (1977) Responses of heifers ingesting boron in
water. J Anim Sci, 46: 812-818.
Green GH, Lott MD, & Weeth HJ (1973) Effects of boron water on rats.
Proc West Sect Am Soc Anim Sci, 24: 254-258.
Gregoire DC (1990) Determination of boron in fresh and saline water by
inductively coupled plasma mass spectrometry. J Anal Atom Spectrom,
5: 623-626.
Gregory J, Foster K, Tyler H, & Wiseman M (1990) The dietary and
nutritional survey of British adults--Table on consumption of wine and
fortified wine. London, Her Majesty's Stationary Office.
Grella P, Tambuscio B, & Suma V (1976) [Boric acid and poisoning
during pregnancy: Description of one case.] Acta Anaesthesiol Ital,
27: 745-748 (in Italian).
Griffin RA & Burau RG (1974) Kinetic equilibrium studies of boron
desorption from soil. Soil Sci Soc Am Proc, 38: 892-897.
Guhl W (1996) [Ecological aspects of boron.] SÖFW-Journal, 118(18/92):
1159-1168 (in German).
Gupta UC, Jane YW, Campbell CA, Leyshon AJ, & Nicholaichuk W (1985)
Boron toxicity and deficiency: A review. Can J Soil Sci, 65: 381-409.
Hamilton SJ (1995) Hazard assessment of inorganics to three endangered
fish in the Green River, Utah. Ecotoxicol Environ Saf, 30: 134-142.
Hamilton SJ & Buhl KJ (1990) Acute toxicity of boron, molybdenum, and
selenium to fry of chinook salmon and coho salmon. Arch Environ Contam
Toxicol, 19: 366-373.
Hamilton SJ & Wiedmeyer RH (1990) Concentrations of boron, molybdenum,
and selenium in chinook salmon. Trans Am Fish Soc, 119: 500-510.
Hatcher JT & Bower CA (1958) Equilibria and dynamics of boron
adsorption by soils. Soil Sci, 85: 319-323.
Havercroft JM & Ward NI (1991) Boron and other elements in relation to
rheumatoid arthritis. In: Momeïlovie B ed. Trace elements in man and
animals. Zagreb, Croatia, IMI, pp 8/2-8/3.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Hegsted M, Keenan MJ, Siver F, & Wozniak P (1991) Effect of boron on
vitamin D deficient rats. Biol Trace Elem Res, 26: 243-255.
Heindel JJ, Price CJ, Field EA, Marr MC, Myers CB, Morrissey RE, &
Schwetz BA (1992) Developmental toxicity of boric acid in mice and
rats. Fundam Appl Toxicol, 18: 266-277.
Hellou J, Warren WG, Payne JF, Belkhode S, & Lobel P (1992) Heavy
metals and other elements in three tissues of cod, Gadus morhua from
the Northwest Atlantic. Mar Pollut Bull, 24(9): 452-458.
Hingston FJ (1986) Biogeochemical cycling of boron in native eucalypt
forests of south-western Australia. Aust For Res, 16: 73-83.
Hoffman DJ, Camardese MB, Lecaptain LJ, & Pendleton GW (1990) Effects
of boron growth and physiology in mallard ducklings. Environ Toxicol
Chem, 9: 335-346.
Hoffman DJ, Sanderson CJ, Lecaptain LJ, Cromartie E, & Pendleton GW
(1991) Interactive effects of boron, selenium, and dietary protein on
survival, growth, and physiology in mallard ducklings. Arch Environ
Contam Toxicol, 20: 288-294.
Hollis JF, Keren R, & Gal M (1988) Boron release and sorption by fly
ash as affected by pH and particle size. J Environ Qual, 17: 181-184.
Hopmans P & Flinn DW (1984) Boron deficiency in Pinus radiata D. Don
and the effect of applied boron on height growth and nutrient uptake.
Plant Soil, 79: 295-298.
Hothem RL & Ohlendorf HM (1989) Contaminants in foods of aquatic birds
at Kesterson Reservoir, California, 1985. Arch Environ Contam Toxicol,
18: 773-786.
Hothem RL & Welsh D (1994) Contaminants in eggs of aquatic birds from
the grasslands of central California. Arch Environ Contam Toxicol,
27: 180-185.
Hu H, Brown PH, & Labavitch JM (1996) Species variability in boron
requirement is correlated with cell wall pectin. J Exp Bot, 295:
227-232.
Huber L (1982) [ICP-AES, a new technique for multi-elemental
determination in water, waste water and sludge.] Wasser, 58: 173-185
(in German).
Huber L (1994) Boron concentrations in the river Neva, Russia. Munich,
Germany, Bavarian State Office for Water, Institute of Water Research
(Unpublished report).
Hudak J (1973) Effect of boric acid on the division and elongation of
cells in root tip of Vicia faba (L.) cv. Povazsky Acta Fac Rerum Nat
Univ Comenianae Physiol Plant, 6: 119-126.
Hunt CD (1988) Boron homeostasis in the cholecalciferol-deficient
chick. Proc Natl Acad Sci (USA), 42: 60.
Hunt CD (1989) Dietary boron modified the effects of magnesium and
molybdenum on mineral metabolism in the cholecalciferol-deficient
chick. Biol Trace Elem Res, 22: 201-220.
Hunt CD (1994) The biochemical effects of physiologic amount of
dietary boron in animal nutrition models. Environ Heath Perspect,
102(Suppl 7): 35-43.
Hunt CD & Herbel JL (1993) Trace elements in man and animals--TEMA 8.
Proceedings of the Eighth International Symposium on Trace Elements in
Man and Animals, Gersdorf, Germany, Verlag Media Touristik, pp
714-718.
Hunt CD & Shuler TR (1990) Open vessel, wet ash, low-temperature
digestion of biological materials for inductively-coupled organ plasma
spectrometry (ICAP) analysis of boron and other elements. J Micronutr
Anal, 6: 161-174.
Hunt CD, Shuler TR, & Mullen LM (1991) Concentration of boron and
other elements in human foods and personal-care products. J Am Diet
Assoc, 91: 558-568.
Hunt CD, Herbel JL, & Idso JP (1994) Dietary boron modifies the
effects of vitamin D3 nutrition on indices of energy substrate
utilisation and mineral metabolism in the chick. J Bone Min Metab,
9: 171-182.
Hunt CD, Herbel JL, & Nielsen FH (1997) Metabolic responses of
postmenopausal women to supplemental dietary boron and aluminium
during usual and low magnesium intake: boron, calcium and magnesium
absorption and retention and blood mineral concentrations. Am J Clin
Nutr, 65: 803-813.
Igelsrud I, Thompson TG, & Zwicker BMG (1938) The boron content of sea
water and of marine organisms. Am J Sci, 35: 47-63.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
Iyengar GV, Clarke WB, Downing RG, & Tanner JT (1988) Lithium in
biological and dietary materials. In: Trace element analytical
chemistry in medicine and biology: Proceedings of the 5th
international workshop. Berlin, New York, Walter de Gruyter & Co.,
pp 267-269.
Iyengar GV, Clarke WB, Downing RG, & Tanner JT (1990) Determination of
boron and lithium in diverse biological matrices using neutron
activation-mass spectrometry (NA-MS). Fresenius J Anal Chem,
338: 562-566.
Jansen JA, Schou JS, & Aggerbeck A (1984) Gastrointestinal absorption
and in vitro release of boric acid from water-emulsifying ointments.
Food Chem Toxicol, 22: 49-53.
Jenkins DW (1980) Biological monitoring of toxic trace metals--Volume
2: Toxic trace metals in plants and animals of the world. Washington,
DC, US Environmental Protection Agency (EPA-600/3-80-090).
Jeshima D, Morishta K, Ueda Y, Zutagami K, Higuchi S, Komoda T,
Nanishi F, Janeyama J, Yoshitake J, & Aoyama T (1992) Clinical
management of boric acid ingestion: pharmacokinetic assessment of
efficacy of haemodialysis for treatment of acute boric acid poisoning.
J Pharmacobio-Dyn, 15: 287-294.
Job C (1973) Resorption and excretion of orally administered boron. Z
Angew Bader-Klimahelik, 20: 137-142.
Juhnke I & Ludemann D (1978) [Results of the investigation of 200
chemical compounds for acute fish toxicity with the golden orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German with English
summary).
Kabata-Pendias A & Pendias H (1984) Trace elements in soil and plants.
Boca Raton, Florida, CRC Press, Inc.
Keating KI & Dagbusan BC (1984) Effect of selenium deficiency on
cuticle integrity in the Cladocera (crustacea). Proc Natl Acad Sci
(USA), 81: 3433-3437.
Kent NL & McCance RA (1941) The absorption and excretion of "minor"
elements by man: I. Silver, gold, lithium, boron and vanadium. Biochem
J, 35: 837-844.
Keren R & Mezuman U (1981) Boron adsorption by clay minerals using a
phenomenological equation. Clays Clay Miner, 29: 198-204.
Keren R, Gast RG, & Bar-Yosef B (1981) pH-dependent boron adsorption
by Na-montmorillonite. Soil Sci Soc Am J, 45: 45-48.
King N, Odom TW, Sampson HW, & Pardue SL (1991a) In ovo
administration of boron alters bone mineralization of the chicken
embryo. Biol Trace Elem Res, 30: 47-58.
King N, Odom T, Sampson HW, & Yersin AG (1991b) The effect of
in ovo boron supplementation on bone mineralization of the vitamin
D-deficient chicken embryo. Biol Trace Elem Res, 31: 223-233.
Klasing SA & Pilch SM (1988) Agricultural drainage water contamination
in the San Joaquin Valley: a public health perspective for selenium,
boron and molybdenum. Sacramento, California, San Joaquin Valley
Drainage Program.
Kliegel W (1980) [Boron in biology, medicine and pharmacy:
Physiological effects and use of boron compounds.] Berlin, Germany,
Springer-Verlag (in German).
Kluge R & Podlesak W (1985) Plant critical levels for the evaluation
of boron toxicity in spring barley (Hordeum vulgare L.). Plant Soil,
83: 381-388.
Kobayashi M, Matoh T, & Azuma J (1996) Two chains of
rhamnogalacturonan II are cross-linked by borate-diol ester bonds in
higher plant cell walls. Plant Physiol, 110: 1017-1020.
Kohl HC & Oertli JJ (1961) Distribution of boron in leaves. Plant
Physiol, 36: 420-424.
Kopf W & Wilk A (1995) Investigation on aquatic toxicity against plant
organisms. Munich, Germany, Institute for Water Research (Unpublished
report).
Kopp JF & Kroner RC (1970) Trace elements in waters of the United
States. Cincinnati, Ohio, US Department of the Interior, Federal Water
Pollution Control Administration.
Korenaga T, Motomizu S, & Toei K (1980) Improved extraction method for
the spectrophotometric determination of trace amounts of boron in
river water with 1,8-dihydroxynaphthalene-4-sulphonic acid and removal
of the excess of reagent. Analyst, 105: 955-964.
Kowalenko CG (1979) Sodium hypobromite digestion for boron analysis of
plant and soil materials. Comun Soil Sci Plant Anal, 10(11):
1421-1434.
Ku WW & Chapin RE (1994) Mechanism of the testicular toxicity of boric
acid in rats: In vivo and in vitro studies. Environ Health
Perspect, 102(Suppl 7): 99-105.
Ku WW, Chapin RE, Moseman RF, Brink RE, Pierce KD, & Adams KY (1991)
Tissue disposition of boron in Fischer rats. Toxicol Appl Pharmacol,
111: 145-151.
Ku WW, Chapin RE, Wine RN, & Gladen BC (1993a) Testicular toxicity of
boric acid (BA): Relationship of dose to lesion development and
recovery in the F344 rat. Reprod Toxicol, 7: 305-319.
Ku WW, Shih LM, & Chapin RE (1993b) The effects of boric acid (BA) on
testicular cells in culture. Reprod Toxicol, 7: 321-331.
Landolph JR (1985) Cytotoxicity and negligible genotoxicity of borax
and borax ores to cultured mammalian cells. Am J Ind Med, 7: 31-43.
Lang JT & Treece RE (1972) Boric acid effects on face fly fecundity. J
Econ Entomol, 65: 740-741.
Lang FJ, Bingham FT, Hendrix FF, & Crane NL (1986) Boron deposition on
soil and native vegetation from geothermal emissions. J Environ Qual,
15(3): 260-265.
Larsen LA (1988) Boron. In: Seiler HG & Sigel H ed. Handbook on
toxicity of inorganic compounds. New York, Basel, Marcel Dekker, Inc.,
pp 129-141.
Leblond CP & Clermont Y (1952) Definition of the stages of the
seminiferous epithelium of the rat. Ann NY Acad Sci, 55: 548-573.
Lee IP, Sherins RJ, & Dixon RL (1978) Evidence for induction of
germinal aplasia in male rats by environmental exposure to boron.
Toxicol Appl Pharmacol, 45: 577-590.
Leland HV & Scudder BC (1990) Trace elements in Corbicula fluminea
from the San Joaquin River, California. Sci Total Environ, 97/98:
641-672.
Lewin JC (1966) Boron as a growth requirement for diatoms. J Phycol,
2: 160-163.
Lewis RJ Sr (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Co., vol II, pp 528-533.
Lewis RJ Sr (1993) Hawley's condensed chemical dictionary, 12th ed.
New York, Van Nostrand Reinhold Co., pp 161-165.
Lewis MA & Valentine LC (1981) Acute and chronic toxicities of boric
acid to Daphnia magna Straus. Bull Environ Contam Toxicol, 27:
309-315.
Linden CH, Hall AH, Kulig KW, & Rumack BH (1986) Acute ingestion of
boric acid. J Toxicol Clin Toxicol, 24: 269-279.
Linder RE, Strader LF, & Rehnberg GL (1990) Effect of acute exposure
to boric acid on the male reproductive system of the rat. J Toxicol
Environ Health, 31: 133-146.
Litovitz TL, Klein-Schwartz W, Oderda GM, & Schmitz BF (1988) Clinical
manifestation of toxicity in a series of 784 boric acid ingestions. Am
J Emerg Med, 6: 209-213.
Loomis WD & Durst RW (1992) Chemistry and biology of boron.
Biofactors, 3: 229-239.
Lopez FJ, Gimenez E, & Hernandez F (1993) Analytical study on the
determination of boron in environmental water samples. Fresenius J
Anal Chem, 346: 984-987.
Loppi S & Bargagli R (1996) Lichen biomonitoring of trace elements in
a geothermal area (central Italy). Water Air Soil Pollut, 88: 177-187.
Lovatt CJ & Dugger WM (1984) Boron. In: Frieden E ed. Biochemistry of
the essential ultratrace elements. New York, London, Plenum Press, pp
389-421.
Ludbrook WV (1942) The effects of various concentrations of boron on
the growth of pine seedlings in water culture. J Aust Inst Agric Sci,
8: 112-114.
Lyday P (1993) Boron. Washington, DC, US Department of the Interior,
p 16.
Lyday P (1996) Boron: Annual review, 1995. Boston, Massachusetts, US
Geological Survey, pp 1-2 (Mineral Industry Surveys series).
McCabe LJ, Symons JM, Lee RD, & Robeck GG (1970) Survey of community
water supply systems. J Am Water Works Assoc, 62: 670-687.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspari WJ (1988) Responses of the L5178Y tk-/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Maeso ES, Fernandez-Valiente E, Bonilla I, & Mateo P (1985)
Accumulation of proteins in giant cells, induced by high boron
concentrations in Chlorella pyrenoidosa. J Plant Physiol, 121:
301-311.
Magour S, Schramel P, Ovcar J, & Maser H (1982) Uptake and
distribution of boron in rats: Interaction with ethanol and
hexobarbital in the brain. Arch Environ Contam Toxicol, 11: 521-525.
Maier KJ & Knight AW (1991) The toxicity of waterborne boron to
Daphnia magna and Chironomus decorus and the effects of water
hardness and sulfate on boron toxicity. Arch Environ Contam Toxicol,
20: 282-287.
Mance G, O'Donnell AR, & Smith PR (1988) Proposed environmental
quality standards for List II substances in water: Boron. Water
Research Centre (Technical Report No. 256).
Manfredi F, Galleri A, & Cenci P (1975) [Concentrations of boron in
Imperia Province.] Ig Mod, 68: 466-476 (in Italian).
Mann H (1973) [Study of the effect of boron compounds on fish and
other aquatic organisms.] Arch Fisch Wiss, 24: 171-175 (in German with
English summary).
Marks G, Bruchlos P, & Bergmann W (1994) [Experimental results on the
tolerance of Phragmites australis seed.] In: [Proceedings of VDLUFA
Congress, Jena, Germany, 19-24 September 1994], pp 705-708 (Report No.
38/1994) (in German).
Martinez F, Mateo P, Bonilla I, & Fernandez-Valiente E (1986) Cellular
changes due to boron toxicity in the blue-green alga Anacystis
nidulans. Int J Exp Bot, 46: 145-152.
Marzadoori C, Antisari LV, Ciavatta C, & Sequi P (1991) Soil organic
matter influence on adsorption and desorption of boron. Soil Sci Soc
Am J, 55: 1582-1585.
Mateo P, Bonilla I, Fernandez-Valiente E, & Sanchez-Maeso E (1986)
Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC
7119. Plant Physiol, 81: 17-21.
Meacham SL, Japer LJ, & Valpe SL (1995) Effects of boron
supplementation on blood and urinary calcium, magnesium and phosphorus
and urinary boron in athletic and sedentary women. Am J Clin Nutr,
61: 341-345.
Mellor (1980) Mellor's comprehensive treatise on inorganic and
theoretical chemistry, Volume V. Boron, Part A: Boron-oxygen
compounds. London, New York, Longman.
Moore JA and an Expert Scientific Committee (1997) An assessment of
boric acid and borax using the IEHR evaluative process for assessing
human developmental and reproductive toxicity of agents. Reprod
Toxicol, 11(1): 123-160.
Mora MA & Anderson DW (1995) Selenium, boron and heavy metals in birds
from Mexicali Valley, Baja California, Mexico. Bull Environ Contam
Toxicol, 54: 198-206.
Muetterties EL (1967) The chemistry of boron and its compounds. New
York, John Wiley and Sons, pp 1-2, 329.
Mumma RO, Raupach DC, & Waldman JP (1984) National survey of elements
and other constituents in municipal sewage sludges. Arch Environ
Contam Toxicol, 13: 75-83.
Murray FJ (1995) A human health risk assessment of boron (boric acid
and borax) in drinking water. Regul Toxicol Pharmacol, 22: 221-230.
NAPM (1974) Environmental effect of photoprocessing chemicals: Volume
1. Prepared by NAPM, Inc., in cooperation with Hydroscience, Inc.
Harrison, NY, National Association of Photographic Manufacturers, Inc.
Navarro J, Mansour M, & Ivorra R (1992) Use of waste water in
Mediterranean crops. Fresenius Environ Bull, 1: 423-427.
Newnhan (1994a) The role of boron in human nutrition. J Appl Nutr,
46(3): 81-85.
Nielsen GH (1970) Percutaneous absorption of boric acid from
boron-containing preparations in rats. Acta Pharmacol Toxicol, 20:
413-424.
Nielsen FH (1989) Dietary boron affects variables associated with
copper metabolism in humans. In: Aulse M, Baumann W, Bröunlich H,
Brückner C, Groppel B, & Grün M ed. Tenth International Trace Element
Symposium, 1989. Jena, Friedrich-Schiller University, vol 4, pp
1106-1111.
Nielsen FH (1991) Nutritional requirements for boron, silicon,
vanadium, nickel and arsenic: Current knowledge and speculation. FASEB
J, 5: 2661-2667.
Nielsen GH (1992) Facts and fallacies about boron. Nutr Today,
27: 6-12.
Nielsen FH (1994) Biochemical and physiologic consequences of boron
deprivation in humans. Environ Health Perspect, 102(Suppl 7): 59-73.
Nielsen FH (1995) Beneficial, unavoidable essential? Defining boron's
nutritional importance. Borax Pioneer, 4.
Nielsen FH (1996) Dietary supplementation of physiological amounts of
boron increases plasma and urinary boron of perimenopausal women.
(Professional Communication). Proc North Dakota Acad Sci, 50: 52.
Nielsen FH & Shuler TR (1992) Studies of the interaction between boron
and calcium and its modification by magnesium and potassium in rats:
Effects on growth, blood variables and bone mineral composition. Biol
Trace Elem Res, 35: 225-237.
Nielsen FH, Hunt CD, Mullen LM, & Hunt JR (1987) Effect of dietary
boron on mineral, estrogen and testosterone metabolism in
postmenopausal women. FASEB J, 1: 394-397.
Nielsen FH, Mullen LM, & Gallagher SK (1990) Effect of boron depletion
and repletion on blood indicators of calcium status in humans fed a
magnesium-low diet. J Trace Elem Exp Med, 3: 45-54.
Nielsen FH, Mullen LM, & Nielsen EJ (1991) Dietary boron affects blood
cell counts and hemoglobin concentrations in humans. J Trace Elem Exp
Med, 4: 211-223.
Nielsen FH, Shuler TR, & Uthus EO (1992a) Dietary arginine and
methionine effects and their modification by dietary boron and
potassium on the mineral element composition of plasma and bone in the
rat. J Trace Elem Exp Med, 5: 247-259.
Nielsen FH, Gallagher SK, Johnson LK, & Nielsen EJ (1992b) Boron
enhances and mimics some effects of estrogen therapy in postmenopausal
women. J Trace Elem Exp Med, 5: 237-246.
NIOSH (1978) Health hazard evaluation determination: Kerr-McGee
Chemical Corporation, Trona, CA. Cincinnati, Ohio, National Institute
for Occupational Safety and Health (Publication No. 77-59-496).
NIOSH (1989) National occupational exposure survey. Cincinnati, Ohio,
National Institute of Occupational Safety and Health (Publications Nos
89-101, 89-102, 89-103).
Noakes JE & Hood DW (1961) Boron-boric acid complexes in seawater.
Deep-Sea Res, 8: 121-129.
Nobel W (1981) [The effect of boron on submerged soft-water
macrophytes.] Angew Bot, 55: 501-514 (in German with English summary).
Nolte J (1988) Pollution source analysis of river water and sewage
sludge. Environ Technol Lett, 9: 857-868.
Nose K & Zenki M (1991) Flow injection spectrophotometric
determination of boron with D-sorbitol using methyl orange as an
indicator. Analyst, 116: 711-714.
Nriagu JO (1979) Copper in the atmosphere and precipitation. In:
Nriagu JO ed. Copper in the environment, Part I: Ecological cycling.
New York, John Wiley and Sons, pp 43-75.
NTP (1987) Toxicology and carcinogenesis studies of boric acid (CAS
No. 10043-35-3) in B6C3F1 mice (feed studies). Research Triangle
Park, North Carolina, US Department of Health and Human Services,
National Toxicology Program (NTP TR No. 324).
NTP (1989) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in CD-1-Swiss mice. Research Triangle Park, North
Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP Report No. 89-250).
NTP (1990) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in Sprague-Dawley rats. Research Triangle Park,
North Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP Report No. 90-105).
NTP (1991) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in New Zealand white rabbits. Research Triangle
Park, North Carolina, US Department of Health and Human Services,
National Toxicology Program (NTP TER-90003).
Odom JW (1980) Kinetics of the hot water soluble boron soil test.
Commun Soil Sci Plant Anal, 11(7): 759-765.
Ohlendorf HM, Hoffman DJ, Saiki MK, & Aldrich TW (1986) Embryonic
mortality and abnormalities of aquatic birds: Apparent impacts of
selenium from irrigation drainwater. Sci Total Environ, 52: 49-63.
Okay O, Güçlü H, Soner E, & Balka T (1985) Boron pollution in the
Simav River, Turkey and various methods of boron removal. Water Res,
19(7): 857-862.
Ostrovskij NI (1955) [Toxic effect on honey bees by contact with
herbicides and foliar fertilizer.] Dokl Vses Akad Skh Nauk im V I
Lenina, 20: 32-34 (in Russian).
O'Sullivan K & Taylor M (1983) Chronic boric acid poisoning in
infants. Arch Dis Child, 58: 737-739.
Owen EC (1944) The excretion of borate by the dairy cow. J Dairy Res,
13: 243-248.
Parr AJ & Loughman PC (1983) Boron and membrane function in plants.
Annu Proc Phytochem Soc Eur, 21: 87-107.
Paveglio FL, Bunck CM, & Heinz GH (1992) Selenium and boron in aquatic
birds from central California. J Wildl Manage, 56: 31-42.
Pendleton GW, Whitworth MR, & Olsen GH (1995) Accumulation and loss of
arsenic and boron, alone and in combination, in mallard ducks. Environ
Toxicol Chem, 14(8): 1357-1364.
Penland JG (1994) Dietary boron, brain function and cognitive
performance. Environ Health Perspect, 102(Suppl 7): 65-72.
Penland JG & Eberhardt MJ (1993) Effects of dietary boron and
magnesium on brain function of mature male and female Long-Evans rats.
J Trace Elem Exp Med, 6: 53-64.
Pennington HD, Finch CR, Lyons CC, & Littau SA (1991) Microwave
digestion of plant samples for boron analysis. Hort Science, 26(12):
1496-1497.
Pfeiffer CC, Hallman LF, & Gersh I (1945) Boric acid ointment: A study
of possible intoxication in the treatment of burns. J Am Med Assoc,
128: 266-274.
Price CJ, Strong PL, Marr MC, Myers CB, & Murray FJ (1996a)
Developmental toxicity NOAEL and postnatal recovery in rats fed boric
acid during gestation. Fundam Appl Toxicol, 32: 179-193.
Price CJ, Marr MC, Myeos CB, Seely JC, Heindel JJ, & Schwetz BA
(1996b) The developmental toxicity of boric acid in rabbits. Fundam
Appl Toxicol, 34: 176-187.
Price CJ, Strong PL, Murray FJ, & Goldberg MM (1997) Blood boron
concentrations in pregnant rats fed boric acid throughout gestation.
Reprod Toxicol, 11(6): 833-842.
Rai D, Zachara JM, Schwab AP, Schmidt RL, Girvin DC, & Rogers JE
(1986) Chemical attenuation rates, coefficients, and constants in
leachate migration--Volume 1: A critical review (Research Project
2198-1). Richland, Washington, Battelle, Pacific Northwest
Laboratories (Report to Electric Power Research Institute, Palo Alto,
California).
Rainey CJ, Christensen RE, & Nyquist LA (1996) The dietary consumption
of boron in the United States. Irvine, California, Nutrition Research
Group (Unpublished report).
Reeve E & Shive JW (1944) Potassium-boron and calcium-boron
relationships in plant nutrition. Soil Sci, 57: 1-14.
Reid PC & Davies E (1989) Boron content of South African surface
waters: Preliminary assessment for irrigation. Water SA, 15(4):
261-264.
Reisenauer HM & Cox WJ (1971) Boron uptake by plants from boron
containing waters. Presented at 162nd National American Chemical
Society Meeting, 12-17 September 1971. Washington, DC, American
Chemical Society, Division of Water, Air and Waste Chemistry.
Rhoades JD, Ingvalson RD, & Hatcher JT (1970) Adsorption of boron by
ferromagnesium minerals and magnesium hydroxide. Soil Sci Soc Am Proc,
34: 938-941.
Robinson DJS & Perkins EJ (1977) The toxicity of some wood pulp
effluent constituents. Carlisle, UK, Cumbria Sea Fisheries Committee,
22 pp (Scientific Report No. 74/1).
Rosenkranz HS (1973) Sodium hypochlorite and sodium perborate:
Preferential inhibitors of DNA polymerase-deficient bacteria. Mutat
Res, 21: 171-174.
Roudabush RL, Terhaar CJ, Fassett DW, & Dziuba SP (1965) Comparative
acute effects of some chemicals on the skin of rabbits and guinea
pigs. Toxicol Appl Pharmacol, 7: 559-565.
Rusch GM, Hoffman GM, McConnell RF, & Rinehart WE (1986) Inhalation
toxicity studies with boron trifluoride. Toxicol Appl Pharmacol,
83: 69-78.
Sage RF, Ustin SL, & Manning SJ (1989) Boron toxicity in the rare
serpentine plant, Streptanthus morrisonii. Environ Pollut, 61:
77-93.
Saiki MK & May TW (1988) Trace element residues in bluegills and
common carp from the lower San Joaquin River, California and its
tributaries. Sci Total Environ, 74: 199-217.
Saiki MK, Jennings MR, & Brumbaugh WG (1993) Boron, molybdenum, and
selenium in aquatic food chains from the lower San Joaquin River and
its tributaries, California. Arch Environ Contam Toxicol, 24: 307-319.
Schöberl P & Huber L (1988) [Ecologically relevant data of non-tenside
compounds in detergents and cleaners.] Tenside Deterg, 25: 99-107 (in
German).
Schöller F & Bolzer W (1989) Boron--a substance of problem in the
water field. Water Supply, 7: 169-177.
Schöller F, Ollram F, & Putre R (1981) [Boron content of lower
Austria's waters.] Österr Wasserwirtsch, 33: 9-15 (in German).
Schou JS, Jansen JA, & Aggerbeck B (1984) Human pharmacokinetics and
safety of boric acid. Arch Toxicol, 7(Suppl): 232-235.
Schroeder HA & Mitchener M (1975) Life-term effects of mercury, methyl
mercury, and nine other trace metals on mice. J Nutr, 105: 452-458.
Schwab AP, Tomecek MB, & Ohlenbusch PD (1991) Plant availability of
lead, cadmium, and boron in amended coal ash. Water Air Soil Pollut,
57-58: 297-306.
Seal BS & Weeth HJ (1980) Effect of boron in drinking water on the
male laboratory rat. Bull Environ Contam Toxicol, 25: 782-789.
Seffner W, Teubener W, Runde H, Wiedner H, Vogt J, Otto G, Zschau E,
Geinitz D, & Franke J (1990) Boron as an antidote to fluorosis? II.
Studies on various organs of pigs. Fluoride, 23(2): 68-79.
Sekerka I & Lechner JF (1990) Automated method for the determination
of boron in water by flow-injection analysis with in-line
preconcentration and spectrophotometric detection. Anal Chim Acta,
234: 199-206.
Settimi L, Elovaara E, & Savolainen H (1982) Effects of extended
peroral borate ingestion on rat liver and brain. Toxicol Lett,
10: 219-223.
Shani U, Dudley LM, & Hanks RJ (1992) Model of boron movement in
soils. Soil Sci Soc Am J, 56: 1365-1370.
Shann JR & Adriano DC (1988) The chronic exposure of selected crop
species to boron aerosols. Environ Exp Bot, 28: 289-299.
Shopova M, Petrovska D, Musalevski A, Najcevska C, & Sekovski Z (1981)
Cytogenetical and morphological effect of general toxicity caused with
different concentrations of boron. Mutat Res, 85: 229.
Shuler TR, Pootrakul P, Yarnsukon P, & Nielsen FH (1990) Effect of
thalassaemia/immunoglobin E disease on macro, trace and ultratrace
element concentrations in human tissues. J Trace Elem Exp Med, 3:
31-43.
Silaev AA (1984) [Experimental determination of the maximum
permissible concentration of sodium perborate in workplace air.] Gig
Tr Prof Zabol, 6: 44 (in Russian with French translation).
Silaev AA, Kasparov AA, Korolev VV, & Nebstrueva VV (1977)
Electron-microscopic investigation of the effect of boric acid on the
seminiferous tubules of albino rats. Bull Exp Biol Med (USSR), 83:
588-591.
Sims JR & Bingham FT (1967) Retention of boron by layer silicates,
sesquioxides and soil materials: I. Layer silicates. Soil Sci Soc Am
Proc, 31: 728-731.
Simsiman GV, Chesters G, & Andren AW (1987) Effect of ash disposal
ponds on groundwater quality at a coal-fired power plant. Water Res,
21: 417-426.
Smidt RE & Whitton JS (1975) Note on boron toxicity in a stand of
radiata pine in Hawkes Bay. NZ J Sci, 18: 109-113.
Smith GJ & Anders VP (1989) Toxic effects of boron on mallard
reproduction. Environ Toxicol Chem, 8: 943-950.
Smith FG, Wiederim DR, Houk RS, Egan CB, & Serfass RE (1991)
Measurement of boron concentration and isotope ratios in biological
samples by inductively coupled plasma mass spectrometry with direct
injection nebulization. Anal Chem Acta, 248: 229-234.
Smyth DA & Dugger WM (1981) Changes during boron deficient culture of
the diatom Cylindrotheca fusiformis. Plant Physiol, 51: 111-117.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, & Nycum
JS (1969) Range finding toxicity data: List VII. Am Ind Hyg Assoc J,
30: 470-476.
Sprague RW (1972) The ecological significance of boron. Anaheim,
California, US Borax Research Corporation, 58 pp.
Stanley RA (1974) Toxicity of heavy metals and salts to Eurasian
watermilfoil (Myriophyllum spicatum L.). Arch Environ Contam
Toxicol, 2(4): 331-341.
Stein KM, Odom RB, Justice GR, & Martin GC (1973) Toxic alopecia from
ingestion of boric acid. Arch Dermatol, 108: 95-97.
Stelter LH (1980) Baseline levels of selected trace elements in
Colorado oil shale region animals. J Wildl Dis, 16: 175-182.
Stephan CE, Mount DI, Hansen DJ, Gentile JH, Chapman GA, & Brungs WA
(1985) Guidelines for deriving numerical national water quality
criteria for the protection of aquatic organisms and their uses--Final
Report. Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication PB85-227049).
Stokinger HE (1981) Boron. In: Clayton GD & Clayton FE ed. Patty's
industrial hygiene and toxicology--Volume 2B: Toxicology, 3rd ed. New
York, John Wiley and Sons, pp 2978-3005.
Stokinger HE & Spiegl CJ (1953) Special materials, Part A:
Inhalation-toxicity studies of boron halides and certain fluorinated
hydrocarbons. In: Voegtlin C & Hodge HC ed. Pharmacology and
toxicology of uranium compounds: Chronic inhalation and other studies.
New York, McGraw-Hill, pp 2291-2321.
Stuttgen G, Siebel TH, & Aggerbeck B (1982) Absorption of boric acid
through human skin depending on the type of vehicle. Arch Dermatol
Res, 272: 21-29.
Suloway JJ, Roy WR, Skelly TM, Dickerson DR, Schuller RM, & Griffin RA
(1983) Chemical and toxicological properties of coal fly ash.
Champaign, Illinois, Illinois State Geological Survey (Publication
NTIS PB84-116110).
Takeuchi T (1958) Effects of boric acid on the development of the eggs
of the toad, Bufo vulgaris Formosus. Sci Rep Tohoku Univ Ser R Biol,
24: 33-43.
Tanaka H (1966) Response of Lemna pausicostata to boron as affected
by light intensity. Plant Soil, 25: 425-434.
Tartari G & Camusso M (1988) Boron content in freshwaters of northern
Italy. Water Air Soil Pollut, 38: 409-417.
Taylor D, Maddock BG, & Mance G (1985) The acute toxicity of nine
"grey list" metals (arsenic, boron, chromium, copper, lead, nickel,
tin, vanadium and zinc) to two marine fish species: Dab (Limanda
limanda) and grey mullet (Chelon labrosus). Aquat Toxicol, 7:
135-144.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the Province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Temple PJ & Linzon SN (1976) Boron as a phytotoxic air pollutant. J
Air Pollut Control Assoc, 26: 498-499.
Teshima D, Morishita K, Ueda Y, Futagami K, Higuchi S, Komoda T,
Nanishi F, Taniyama T, Yoshitake J, & Aoyama T (1992) Clinical
management of boric acid ingestion: Pharmacokinetic assessment of
efficacy of hemodialysis for treatment of acute boric acid poisoning.
J Pharmacobio-Dyn, 15: 287-294.
Thompson JAJ, Davis JC, & Drew RE (1976) Toxicity, uptake and survey
studies of boron in the marine environment. Water Res, 10: 869-875.
Torkelson TR, Sadek SE, & Rowe VK (1961) The toxicity of boron
trifluoride when inhaled by laboratory animals. Am Ind Hyg Assoc J,
22: 263-270.
Treinen KA & Chapin RE (1991) Development of testicular lesions in
F344 rats after treatment with boric acid. Toxicol Appl Pharmacol,
107: 325-335.
Truhaut R, Phu-Lich N, & Loisillier F (1964) Effects of the repeated
ingestion of small doses of boron derivatives on the reproductive
functions of the rat. C R Acad Sci (Paris), 258: 5099-5102.
Tsui PTP & McCart PJ (1981) Chlorinated hydrocarbon residues and heavy
metals in several fish species from the Cold Lake area in Alberta,
Canada. Int J Environ Anal Chem, 10: 277-285.
Turnbull H, Demann JG, & Weston RF (1954) Toxicity of various refinery
materials to fresh water fish. Ind Eng Chem, 46: 324-333.
UK Ministry of Agriculture, Fisheries and Food (1991) Household food
consumption and expenditure 1991--Annual Report of the National Food
Survey Committee. London, Her Majesty's Stationary Office, Table B1.
Unilever (1994) Summary of external research project on environmental
levels of boron, especially in trout-sustaining waters. Unilever
Research, Port Sunlight Laboratory, 3 pp (Unpublished report).
US EPA (1991) Health and environmental effects document for boron and
boron compounds. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (EPA/600/8-91/015).
US EPA (1994) Integrated risk information system--Online. Cincinnati,
Ohio, US Environmental Protection Agency, Environmental Criteria and
Assessment Office.
US FDA (1981) Product formulation data--Computer printout. Washington,
DC, US Food and Drug Administration.
US NLM (1993) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine, National Toxicology Information Program.
Vanderpool RA, Haff D, & Johnson PE (1994) Use of inductively coupled
plasma-mass spectrometry in boron--10 stable isotope experiments with
plants, rats and humans. Environ Health Perspect, 102(Suppl 7): 13-20.
Verbitskaya GV (1975) [Experimental and field investigations
concerning the hygienic evaluation of boron-containing drinking
water.] Gig i Sanit, 7: 49-53 (in Russian with English summary).
Vernot EH, MacEwen JD, Haun CC, & Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicol Appl Pharmacol, 42: 417-424.
Versar Inc. (1975) Preliminary investigation of effects on the
environment of boron, indium, nickel, selenium, tin, vanadium and
their compounds--Volume 1: Boron. Springfield, Virginia, Versar, Inc.
(Prepared for the US Environmental Protection Agency, Washington) (EPA
560/2-75-005a).
Vignec AJ & Ellis R (1954) Inabsorbability of boric acid in infant
powder. Am J Dis Child, 88: 72-80.
Waggott A (1969) An investigation of the potential problem of
increasing boron concentrations in rivers and water courses. Water
Res, 3: 749-765.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia
affinis of certain pure chemicals in turbid waters. Sewage Ind
Wastes, 29: 695-711.
Wang W (1986) Toxicity tests of aquatic pollutants by using common
duckweed. Environ Pollut, B11: 1-14.
Wang E, Xun X, Wang H, Chen Y, Meng X, Zhang S, Huang J, Cong S, Tao
K, Yu B, Xiao J, He D, Yang X, & Jiang J (1984) Toxicological effect
of boron in laboratory rats. Zhonghua Yufangyixue Zazhi, 18(1): 20-22
(in Chinese, with English abstract).
Ward NI (1993) Boron levels in human tissues and fluids. In: Meissner
MAD & Mills C-F ed. Trace elements in man and animals--TEMA 8.
Proceedings of the Eighth International Symposium on Trace Elements in
Man and Animals. Gersford, Germany, Verlag Media Touristik, pp
724-728.
Warrington K (1923) The effects of boric acid and borax on the bread
bean and certain other plants. Ann Bot, 37: 629-672.
Weast RC, Astle MJ, & Beyer WH ed. (1985) CRC handbook of chemistry
and physics, 69th ed. Boca Raton, Florida, CRC Press, Inc., pp B/77,
B/129.
Weeth HJ, Speth CF, & Hanks DR (1981) Boron content of plasma and
urine as indicators of boron intake in cattle. Am J Vet Res, 42:
474-477.
Wegman DH, Eisen EA, Hu X, Woskie SR, Smith RG, & Garabrant DH (1994)
Acute and chronic respiratory effects of sodium borate particulate
exposures. Environ Health Perspect, 102(Suppl 7): 119-128.
Weir RJ & Fisher RS (1972) Toxicologic studies on borax and boric
acid. Toxicol Appl Pharmacol, 23: 351-364.
Wells N & Whitton JS (1977) A pedochemical survey: 3. Boron. NZ J Sci,
20: 317-332.
Whetstone RR, Robinson WO, & Byers HG (1942) Boron distribution in
soils and related data. Washington, DC, US Department of Agriculture
(Technical Bulletin No. 797).
Whitworth MR, Pendleton GW, Hoffman DJ, & Camardese MB (1991) Effects
of dietary boron and arsenic on the behavior of mallard ducklings.
Environ Toxicol Chem, 10: 911-916.
WHO (1993) Boron. In: Guidelines for drinking-water quality--Volume 1:
Recommendations. Geneva, World Health Organization, pp 43-44.
WHO (1996) Trace elements in human nutrition and health. Geneva, World
Health Organization, pp 175-179.
WHO (1998a) Guidelines for drinking-water quality. Second edition.
Addendum to Volume 1: Recommendations. Geneva, World Health
Organization.
WHO (1998b) Guidelines for drinking-water quality. Second edition.
Addendum to Volume 2: Health criteria and other supporting
information. Geneva, World Health Organization (WHO/EOS/98.1).
Whorton MD, Haas JL, Trent L, & Wong O (1994) Reproductive effects of
sodium borates on male employees: Birth rate assessment. Occup Environ
Med, 51: 761-767.
Wiecken B & Wübbold-Weber S (1993) [Final report on boron monitoring
in drinking-water.] Degussa AG (Unpublished report) (in German).
Wiener JG, Kaufman DW, Kaufman GA, Gentry JB, Smith MH, & Ramsey PR
(1977) Chemical composition of rodents: use of whole body
concentrations for estimation of standing crops of elements. Southwest
Nat, 22: 77-88.
Wierenga PJ, van Genuchten MTH, & Boyle FW (1975) Transfer of boron
and tritiated water through sandstone. J Environ Qual, 4: 83-87.
Wikner B (1986) Pretreatment of plant and soil samples--A problem in
boron analysis: Part I. Plants. Commun Soil Sci Plant Anal, 17(1):
1-25.
Wilcox LV (1958) Water quality from the standpoint of irrigation. J Am
Water Works Assoc, 50: 650-654.
Wilding JL, Smith WJ, Yevich P, Sicks ME, Ryan SG, & Punte CL (1959)
The toxicity of boron oxide. Am Ind Hyg Assoc J, 20: 284-289.
Windholz M, Budavari S, Blemetti RF, & Otterbein ES ed. (1983) Merck
index, 10th ed. Rahway, New Jersey, Merck and Co., Inc., pp 185-187,
1099, 1231, 1237, 1239.
Woods WG (1994) An introduction to boron: history, sources, users and
chemistry. Environ Health Perspect, 102(Suppl 7): 5-11.
Woskie SR, Shen P, Eisen EA, Finkel MH, Smith TJ, Smith R, & Wegman,
DH (1994) The real-time dust exposures of sodium borate workers:
Examination of exposure variability. Am Ind Hyg Assoc J, 55: 207-217.
Wren CD, Maccrimmon HR, & Loescher BR (1983) Examination of
bioaccumulation and biomagnification of metals in a Precambrian Shield
lake. Water Air Soil Pollut, 19: 277-291.
Yamamoto T, Tamaoka T, Fujita T, & Isoda C (1973) Boron content in
marine plankton. Rec Oceanogr Works Jpn, 12: 13-21.
APPENDIX
Alternative Approaches -- Benchmark Dose
A suggested alternative to the use of a NOAEL in the development
of the TI is to use a BMD. Some advantages in using a BMD are as
follows: 1) more data from the dose-response curve are used; 2)
influence by background incidence rate is lessened; and 3) sensitivity
to the spacing of doses in the assay is reduced. A BMD is derived by
first calculating or estimating a dose that causes a critical effect
in a small percentage (e.g. 5%) of test animals (effective dose). A
BMD is then determined as the lower confidence limit of this effective
dose (Barnes et al., 1995).
Using data from the Heindel et al. (1992) study in rats and a
decreased fetal body weight as the critical effect, a BMD for boron of
9.3 mg/kg body weight per day was estimated using a polynomial model
(see Fig. 2). This BMD was based on the 95% confidence limit on the
dose that causes a 5% decrease in fetal body weight. Dividing by an
uncertainty factor of 25 gives a TI of 0.4 mg/kg body weight per day.
Another model (Weibull) was used (Allen et al., 1996) with study data
from Heindel et al. (1992) and Price et al. (1996a) and with decreased
fetal weight as the critical effect to derive a BMD of 9.8 mg/kg body
weight per day based on the 95% confidence limit on the dose that
causes a 5% decrease in fetal body weight. Dividing by an uncertainty
factor of 25 also gives a TI of 0.4 mg/kg body weight per day. Both
BMD-based TIs are identical to the one developed with the traditional
approach. Although use of a BMD to derive a TI does have some
advantages over the NOAEL method, there is as yet no consensus on the
incidence of effect to be used as a basis for deriving the BMD.
In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine boron levels from
workers with the amount of boron exposure (see section 5.3). An
average boron level of 0.26 µg/ml blood occurred in the highest
exposure group, where exposure was estimated to be 0.38 mg boron/kg
body weight per day. Post-shift blood and urine boron concentrations
did not increase with the days of the work week. This lack of
accumulation over the work week corroborates the relatively short
elimination half-life for boron in humans.
Elimination times for animals have not been explicitly stated in
the literature but can be either calculated or estimated from
published data. Farr & Konikowski (1963) measured urinary boron
concentrations in mice after intravenous injection of sodium
pentaborate. Using their data and assuming first-order kinetics for
elimination, the half-life for elimination in the mouse was on the
order of 1 h. Ku et al. (1991) reported that an apparent steady-state
level of boron is reached in the blood and tissues of rats after 3-4
days of oral dosing; again, assuming first-order kinetics, the
half-life in rats would be on the order of 14-19 h.
Male Fischer-344 rats were exposed to boric acid at 3000-9000
mg/kg in the diet for up to 9 weeks (Ku et al., 1993a). Urinary boron
was elevated (450-600 µg boron/mg creatinine) 24 h after the end of
dosing. By 3-4 days post-treatment, urinary boron in all groups had
returned to average control levels (8.97 ± 1.95 µg boron/mg creatinine
for days 1, 7, and 14 post-treatment). Elimination of boron from bone
followed a different time-course from elimination from serum or soft
tissues. F-344 rats consuming boric acid in the diet for 9 weeks
(approx. 1.4-6.8 mg boron/kg body weight per day) showed dose-related
elevation of bone boron, which declined very gradually during the
post-treatment period. Bone boron levels remained elevated above
controls in all exposed groups at 32 weeks post-treatment (Chapin et
al., 1997). Longer post-treatment intervals, as well as factors
influencing rates of accumulation or mobilization of boron in bone,
remain to be evaluated.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Short-term exposure
7.1.1 Oral route
The oral LD50 values for boric acid and borax in laboratory
animals are given in Table 14. Values for mice and rats are in the
range of approx. 400-700 mg boron/kg body weight (Pfeiffer et al.,
1945; Weir & Fisher, 1972). For guinea-pigs, Verbitskaya (1975)
reported an oral LD50 of 210 mg boron/kg body weight. Acute oral LD50
values in the range of 250-350 mg/kg body weight for boric acid or
borax exposure have also been reported for dogs, rabbits, and cats
(Pfeiffer et al., 1945; Verbitskaya, 1975).
General clinical effects of boric acid or borax in rats, mice,
and guinea-pigs given single large doses orally are depression,
ataxia, occasional convulsions, decreased body temperature, and
violet-red colour of skin and all mucous membranes (Pfeiffer et al.,
1945; Weir & Fisher, 1972). Toxic signs in dogs given boric acid
(0.2-2.0 g/kg body weight) orally in combination with subcutaneous
morphine to prevent vomiting were cyanosis of mucous membranes,
red-violet skin colour, rigidity of legs, convulsion, and shock-like
syndrome (Pfeiffer et al., 1945). Rabbits given boric acid at 800
mg/kg body weight per day for 4 days showed anorexia, weight loss, and
diarrhoea; 850 and 1000 mg/kg body weight per day for 4 days caused
100% mortality (Draize & Kelley, 1959). Cattle receiving 0.8, 150, or
300 mg boron/litre of drinking-water (as boric acid) for 30 days were
lethargic at the highest dose and had swelling and irritation in the
legs and around the dew claws, slight diarrhoea, and decreased food
consumption at the middle and high doses (Green & Weeth, 1977).
Following exposure to boric acid, microscopic changes in tissues
of mice, rats, and dogs involved primarily the kidneys and nervous
system (Pfeiffer et al., 1945). Glomerular and tubular damage were
noted in the kidneys. Glomerular damage consisted of changes in
permeability of the capillaries, and tubular damage consisted of
cellular vacuolization and shedding of cells into the tubular lumen.
Nervous system damage consisted of an increase in small dark cells
(probably microglia) in the spinal cord and in the grey matter of the
brain cortex.
7.1.2 Inhalation route
The LC50 for sodium perborate tetrahydrate by inhalation in rats
was >74 mg/m3 (Silaev, 1984). The 1-h inhalation LC50 values for
boron trichloride are reported as 12.2 g/m3 for male rats and 21.1
g/m3 for female rats; for boron trifluoride, the LC50 values range
from 0.89 to 1.2 g/m3 for rats (Vernot et al., 1977). While these
boron-halogen compounds are not considered in this report because of
apparent anion toxicity, it is appropriate to note in passing the
inhalation studies performed with them (Stokinger & Spiegl, 1953;
Rusch et al., 1986). These studies evaluated rats, mice, and
guinea-pigs and report various dose-related toxic effects beginning at
24 mg/m3.
Table 14. Acute toxicity of boron compounds in laboratory animals
Route Compound Species Compound LD50 Borona LD50
(mg/kg body weight) (mg/kg body weight) Reference
Oral Boric acid Mice 3450 603 Pfeiffer et al. (1945)
Borax Rats 3493 396 Wang et al. (1984)
Borax Rats 4500 510b Weir & Fisher (1972)
Borax Rats 4980 560b Weir & Fisher (1972)
Borax Rats 5660 642 Smyth et al. (1969)
Borax Rats 6080 690b Weir & Fisher (1972)
Boric acid Rats 2660 465 Pfeiffer et al. (1945)
Boric acid Rats 3160 550b Weir & Fisher (1972)
Boric acid Rats 3450 600b Weir & Fisher (1972)
Boric acid Rats 4080 710b Weir & Fisher (1972)
Boric acid Rats 5140 899 Smyth et al. (1969)
Subcutaneous Boric acid Mice 1740c 304 Pfeiffer et al. (1945)
Boric acid Mice 2070 362 Pfeiffer et al. (1945)
Boric acid Guinea-pigs 1200 210 Pfeiffer et al. (1945)
Intravenous Boric acid Mice 1780 311 Pfeiffer et al. (1945)
Boric acid Rats 1330 232 Pfeiffer et al. (1945)
a Calculated by multiplying the dose in mg boron compound/kg by the ratio of the molecular weights of boron/boron compound,
except when noted otherwise.
b Reported by investigators.
c Solution adjusted to pH 7.4 with sodium hydroxide.
7.2 Longer-term exposure
7.2.1 Oral route
The effects of longer-term oral exposure to boron compounds in
animals are summarized in Table 15.
In a 13-week study conducted by the US National Toxicology
Program (NTP, 1987; Dieter, 1994), B6C3F1 mice (10/sex per dose) were
exposed to boric acid in the diet at concentrations sufficient to
produce estimated consumptions of approximately 0, 34, 70, 141, 281,
or 563 mg boron/kg body weight per day for males and 0, 47, 97, 194,
388, or 776 mg boron/kg body weight per day for females. The results
showed that female mice were less sensitive than male mice to the
lethal effects of boric acid; 8/10 high-dose males and 6/10 high-dose
females died, and 1/10 males receiving 281 mg boron/kg body weight per
day died. Clinical signs of toxicity were a thin, hunched appearance,
dehydration, foot lesions, and scaly tails. A dose-related decrease in
body weight gain was observed. Microscopic effects included a
dose-related incidence of minimal to mild extramedullary
haematopoiesis of the spleen in males and females, hyperkeratosis and
acanthosis of the stomach in eight males and three females receiving
the highest dose, and testicular lesions; the testicular lesions are
described in greater detail in section 7.4.
Following this range-finding study, a 2-year study was conducted
in which mice (50/sex per dose) received approximately 0, 275, or 550
mg boric acid/kg body weight per day (0, 48.1, or 96.3 mg boron/kg
body weight per day) in the diet (NTP, 1987; Dieter, 1994). No
clinical signs of toxicity were observed. Body weights were 10-17%
lower in males after 32 weeks and in females after 52 weeks.
Non-accidental mortality at the end of the study was 9/50, 20/50, and
23/50 in control, low-, and high-dose males and 17/50, 15/50, and
12/50 in control, low-, and high-dose females. The increased mortality
rate was statistically significant in males. The only significant
lesions in male mice appeared in the testes; no significant
non-neoplastic lesions appeared in females.
Lee et al. (1978) fed borax in the diet to male Sprague-Dawley
rats (18/dose) at concentrations of 0, 500, 1000, or 2000 mg boron/kg
(equivalent to approximate doses of 0, 30, 60, or 125-131 mg boron/kg
body weight per day) for 30 or 60 days. Body weights were not
consistently affected by treatment; organ weights were not affected in
the 30 mg/kg body weight per day group. At 60 and 125-131 mg/kg body
weight per day, absolute liver weights were significantly lower after
60 days; epididymal weights were significantly lower (37.6% and 34.8%,
respectively) after 60 days, but not after 30 days. Weights of
prostate, spleen, kidney, heart, and lung were not changed at
any dose; reproductive effects observed in the study are discussed in
section 7.4.
Table 15. Effects of longer-term oral exposure to boron compounds in experimental animals
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Mice 0, 34, 70, 141, 281, Diet 13 weeks Over 60% mortality in both sexes NTP (1987)
563 for males; 0, 47, at 563 and 776 mg/kg body weight
97, 194, 388, 776 for per day; 10% in males at 281 mg/kg
femalesb body weight per day. At 141 mg/kg
body weight per day, degeneration
in seminiferous tubules and
decreased tubules in males and
decreased weight gain in males and
females. Extramedullary
haematopoiesis of the spleen in
all dosed groups, hyperkeratosis
and acanthosis of stomach at 563
and 776 mg/kg body weight per day.
Boric acid Mice 0, 48, 96 Diet 103 weeks Decrease in body weight (10-17%) NTP (1987)
in high-dose males after week 32
and in high-dose females after week
52. No clinical toxic signs observed.
Testicular atrophy and interstitial
cell hyperplasia were seen in males
at both levels. Dose-related increase
in incidence of splenic lymphoid
depletion in males. No other
significant increase in
non-neoplastic lesions.
Boric acid Rats 0, 22.7, 57c and Drinking-water 30 days Growth was not inhibited at the Pfeiffer et al.
higher low dose, but it was delayed at (1945)
>57 mg/kg body weight per day.
No haematological effects or
histological alterations.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Borax Rats 0, 0.056, 0.28, 2.8, Drinking-water <198 days Decrease in pancreas-to-body Wang et al.
28d weight ratio at all doses in (1984)
females at day 98. Increase in
pancreas-to-body weight ratio
in males at day 198. No details
given; normal histology.
Boric acid Rats 0.95, 3.65, 5.2, 9.9e Diet 8 weeks Decreased body weight at 5.2 Forbes & Mitchell
and 9.9 mg/kg body weight per (1957)
day. No other toxicity
end-points evaluated.
Borax or Rats 0, 2.6, 8.8, 26.3, Diet 90 days Mortality was 100% at the highest Weir & Fisher
boric acid 87.5, 262.5e dose; testicular atrophy at 87.5 (1972)
and 26.3 mg/kg body weight per
day; decrease in body weight and
in the weight of liver, kidney,
spleen, and testes at 87.5 mg/kg
body weight per day; weight
changes were inconsistent at
lower doses.
Borax or Rats 0, 5.9, 17.5, 58.5e Diet 2 years Both compounds suppressed growth Weir & Fisher
boric acid at 58.5 mg/kg body weight per (1972)
day. Testes weight and
testes-to-body weight ratios
were decreased, and brain-to-body
weight and thyroid-to-body weight
ratios were increased at 58.5
mg/kg body weight per day; also,
atrophy in seminiferous epithelium
and decrease in tubular size; no
effects observed at lower doses.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Rabbits 31 Oral gavage 5 days/week Elevated serum glutamic-oxaloacetic Verbitskaya
or borax for 4 months transaminase (SGOT) and serum (1975)
glutamic-pyruvic transaminase (SGPT)
levels, serum lactate dehydrogenase
and aldolase were transiently
increased, catalase and amylase were
decreased.
Sodium Rats 0, 3 g/litre sodium Drinking-water 14 weeks Increase in RNA concentration Settimi et al.
tetraborate tetraborate (boron and in succinate dehydrogenase (1982)
(not conversion not and acid proteinase activation
specified made due to in the brain. Decrease in
whether uncertainty NADPH-cytochrome reductase and
anhydrous or regarding in the content of cytochrome
decahydrate) compound) b5 and P-450 in the liver. No
effect on body or organ weight.
Sodium Mice 0, 0.95e Drinking-water Lifetime No effects on body weight or Schroeder &
metaborate longevity. Mitchener (1975)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of boron/boron compound.
b Estimated based on feed consumption values of 161 g/kg body weight per day for male and 222 g/kg body weight per day for female controls
at week 4 of treatment.
c Calculated based on water consumption of 0.12-0.14 ml/g per day reported by authors.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
e Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or food factor of
0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for mice.
In a 90-day study, Sprague-Dawley rats (10/sex per dose) received
0, 2.6, 8.8, 26.3, 87.5, or 262.5 mg boron/kg body weight per day in
the diet as boric acid or borax (Weir & Fisher, 1972). Similar effects
were observed with both boric acid and borax. All high-dose animals
died within 3-6 weeks. In animals receiving 87.5 mg boron/kg body
weight per day, body weights in males and females were reduced
43.8-54.9% and 10.1-12.6%, respectively. Absolute organ weights --
including the liver, spleen, kidneys, brain, adrenals, and ovaries --
in this dose group were also significantly decreased. Relative
organ-to-body weights of the adrenals and kidneys were significantly
increased, but relative weights of the liver and ovaries were
decreased. A pronounced reduction in testicular weights in males in
the 87.5 mg boron/kg body weight per day group was also observed;
effects on the male reproductive system are discussed further in
section 7.4.
In a 2-year study, rats (35/sex per dose) were administered
weight-normalized doses of 0, 5.9, 17.5, or 58.5 mg boron/kg body
weight per day in the diet as borax or boric acid (Weir & Fisher,
1972). High-dose animals had coarse hair coats, scaly tails, hunched
posture, swollen and desquamated pads of the paws, abnormally long
toenails, shrunken scrotum, inflamed eyelids, and bloody eye
discharge. These signs became frequent and more pronounced during the
first year but did not change thereafter. Serum chemistry and urine
values were normal; the haematocrit and haemoglobin levels were
significantly lower than in controls. The absolute and relative
weights of the testes were significantly lower, and relative weights
of the brain and thyroid gland were higher, than in controls. In
animals in the mid- and low-dose groups, no significant effects on
general appearance, behaviour, growth, food consumption, haematology,
serum chemistry, or histopathology were observed (Weir & Fisher,
1972).
Weir & Fisher (1972) also fed boric acid or borax to beagle dogs
for 90 days or 2 years. In the 90-day study (weight-normalized doses
of 0, 0.44, 4.38, or 43.75 mg boron/kg body weight per day;
5 animals/sex per dose), testicular effects were observed in males in
the two highest dose groups. In the boric acid study, testis weight
was significantly lower than controls in the middle- and upper-dose
groups (reduced by 25% and 40%, respectively). Although testicular
microscopic structure was not detectably abnormal in the controls and
middle-dose group, 4 of 5 dogs in the high-dose group had complete
atrophy, and the remaining high-dose dog had one-third of tubules
showing some abnormality. In the borax study, testis weights in the
low, middle-, and high-dose groups were 80%, 85%, and 50% of controls,
respectively; only the last was significantly different from controls.
No mention was made of the testicular microscopic structure of the
controls or low-dose animals; middle-dose animals were not detectably
altered (aside from the considerable fixation-induced artifact in the
outer third of the tissue), whereas 4 of 5 high-dose dogs had complete
testicular atrophy, and the remaining high-dose dog had "partial"
atrophy. No other clinical or microscopic signs of toxicity were
reported in any animals. In the 2-year study, the dogs (4/sex per
dose) received the boric acid or borax in the diet at
weight-normalized doses of 1.5, 2.9, or 8.8 mg boron/kg body weight
per day. An additional group received 29 mg boron/kg body weight per
day for 38 weeks. No effects were observed on general appearance, body
weight, food consumption, organ weights, haematology, or serum
chemistry. Changes in testicular morphology occurred in males in the
highest (38-week) dose group; these reproductive changes are described
in section 7.4.
Two reports are available on the toxic effects of boron on bones.
Seffner et al. (1990) exposed growing pigs to boron (4 or 8 mg/kg body
weight per day; source of boron was not specified). They reported a
dose-related thinning of the cortex of the humerus and a reduction
(significant at 8 mg/kg body weight per day) in presumptively
bone-derived serum alkaline phosphatase, suggesting reduced osteoblast
activity. Confidence in this study is moderate because of the
uncertainty of the form/source of boron and the authors' inability to
replicate several previous biochemical findings.
A second report investigated the effects of boric acid
(calculated exposures: <0.2-68 mg boron/kg body weight per day) on
several bone parameters in adult rats (Chapin et al., 1997). This
study found no change in physical bone measures (size, cortical
thickness, etc.) but reported a 5-10% increase in resistance of
vertebrae to a crush force.
7.2.2 Inhalation route
Mice exposed to amorphous elemental boron at 72 mg/m3 for
7 h/day, 5 days/week, for 6 weeks did not exhibit any toxic effects
(Stokinger & Spiegl, 1953). Wilding et al. (1959) conducted numerous
longer-term experiments in rats and dogs exposed to boron oxide
particles (median mass aerodynamic diameter [MMAD] 1.9-2.5 µm). The
exposures took place for 6 h/day and 5 days/week and included rats
exposed at 77 mg boric oxide/m3 for 24 weeks, 175 mg boric oxide/m3
for 12 weeks, or 470 mg boric oxide/m3 for 10 weeks; dogs were
exposed to 57 mg boron oxide/m3 for 23 weeks. No toxic effects were
observed, with normal values obtained for body weight gains, blood
chemistry, haematology, and urinalysis; there were also no microscopic
changes reported.
Subchronic inhalation studies have been performed with boron
trifluoride (Torkelson et al., 1961; Rusch et al., 1986) at
2-18 mg/m3. Reported effects included pneumonitis and reduced body
weight gains and organ weights.
7.3 Dermal and ocular effects
Animal studies show that boron oxide dust can affect the skin and
eyes. When boron oxide dust (50 mg) was applied to the eyes of
rabbits, conjunctivitis resulted (Wilding et al., 1959). Roudabush et
al. (1965) determined that boric acid (5 ml, 10% in water, w/v) and
borax (10 ml, 5% in water, w/v) were mild skin irritants after 24-72 h
following application to abraided skin. Borax was also a mild skin
irritant and boric acid a moderate irritant after 24 and 72 h when
applied to the guinea-pig. Rats fed 88 or 263 mg boron/kg body weight
per day as borax or boric acid had inflamed eyes and skin desquamation
on their paws and tails (Weir & Fisher, 1972).
7.4 Reproductive toxicity
Reproductive and developmental effects of borates in laboratory
animals are summarized in Table 16.
The data regarding subchronic and chronic oral exposure to boric
acid or borax in laboratory animals have unequivocally demonstrated
that the male reproductive tract is a consistent target of toxicity
(Table 16). Testicular lesions have been observed in rats, mice, and
dogs administered boric acid or borax in food or drinking-water
(Truhaut et al., 1964; Weir & Fisher, 1972; Green et al., 1973; Lee et
al., 1978; NTP, 1987; Ku et al., 1993a). The first clinical indication
of testicular toxicity in dogs is shrunken scrota observed during
treatment; significant decreases in absolute and relative testicular
weight are also reported. After subchronic exposure, the
histopathological effects range from inhibited spermiation (the
process of release of mature spermatids from the seminiferous
epithelium) to degeneration of the seminiferous tubules with variable
loss of germ cells, to complete absence of germ cells, resulting in
atrophy and transient or irreversible loss of fertility, but not of
mating behaviour.
Time- and dose-response studies of the Sprague-Dawley male rat
reproductive end-points after acute administration of boric acid
(Linder et al., 1990) revealed adverse effects on spermiation,
epididymal sperm morphology, and caput sperm reserves during
histopathological examinations of the testes and epididymis. Rats were
administered two doses in one day in both experiments, with a total
dose of 0 or 350 mg boron/kg body weight in the time-response
experiment where animals were sacrificed at 2, 14, 28, or 57 days
after exposure and a total dose of 0, 44, 87, 175, or 350 mg boron/kg
body weight in the dose-response experiment with animals sacrificed
after 14 days. In animals receiving 175 or 350 mg boron/kg body
weight, testicular effects apparent at 14 days were enlarged irregular
cytoplasmic lobes of mature spermatids in stage VIII seminiferous
tubules (Leblond & Clermont, 1952) and a substantial increase in the
testicular spermatid head count per testis. The first visible lesion
was inhibited spermiation. Epididymal effects after 14 days included
an increase in abnormal sperm forms, reduced caput epididymal sperm
morphology with head and tail defects, and reduced caput epididymal
sperm reserves. Epididymal sperm parameters had returned to normal and
only minimal testicular changes remained by post-treatment day 57,
indicating that these effects were reversible at these dose levels.
The NOAEL for male reproductive effects in this study was 87 mg
boron/kg body weight.
Table 16. Reproductive and developmental effects of boron compounds in laboratory animals
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid CD-1 0, 19.2, 104.5, Diet 27 weeks None of the high-dose pairs was Fail et al.
mice 220.2 for males; 0, fertile. At the middle dose, (1990, 1991)
31.8, 147.9, 290.2 there was a significant decrease
for females in litters/pair, live pups/litter,
percentage of pups born alive,
and live pup weight. Fertility
index was decreased at the middle
dose. Body weight gain was reduced
in males and females at the high
dose, despite increases in food
and water consumption. Cross-mating
experiments showed that boric acid
affected primarily the male
reproductive system. A dose level
of 220.2 mg/kg body weight per day
significantly reduced reproductive
organ weights in males and altered
sperm motility, concentration, and
morphology. At the high dose,
fertility of the low-dose F1 mice
was not affected, but marginally
reduced sperm concentrations were
seen in males; increases in uterus
and kidney plus adrenal weights
and shorter estrus cycles were
seen in females; and F2 live pup
weights were reduced.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid SD rats 0, 350 Gavage 2-57 days Inhibited sperm release, adverse Linder et al.
changes in sperm morphology; (1990)
reversible by day 57.
Boric acid Rats 0, 60.9b Diet 4-28 days Decreased weight gain; inhibition Treinen
of spermiation starting at day 7 and & Chapin
degeneration in seminiferous tubules (1991)
at day 28; decreased serum
testosterone starting at day 4; no
effects on liver or kidney histology.
Boric acid F-344 0, 26, 38, 52, 68 Feed Weekly to Mild inhibited sperm release at Ku et al.
rats 63 days 26 mg/kg body weight per day; (1993a)
severe inhibited sperm release at
38 mg/kg body weight per day;
progression to testicular atrophy
at 52 and 68 mg/kg body weight per
day, with many other changes
resulting from these primary
effects.
Boric acid Rats 175 Water 15 days Vacuolation and granulation of Silaev et al.
the cytoplasm and absence of (1977)
nuclear chromatin in spermatids
of seminiferous tubules.
Reduction in tubule diameter
and absence of germinative cells.
Borax or Rats 0, 5.9, 17.5, 58.5c Diet Multi- Sterility, lack of spermatozoa, Weir & Fisher
boric acid generation testicular atrophy, decreased (1972)
ovulation at 58.5 mg/kg body
weight per day. No effects at
lower doses.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 0.44, 4.4, 44c Diet 90 days One male dog died at 44 mg/kg Weir & Fisher
boric acid body weight per day; decrease (1972)
in thyroid- and testes-to-body
weight ratios, and severe
testicular atrophy in males at
44 mg/kg body weight per day.
At 0.44 mg/kg body weight per
day, decreased spleen-to-body
weight ratio in males. At
<4.4 mg/kg body weight per day,
no changes in organ weight in
females.
Borax Rats 0, 30, 60, 125-131 Diet 30 or 60 days At the 60 and 125-131 mg/kg Lee et al.
body weight per day doses, liver (1978)
weights were significantly lower
after 60 days; epididymal
weights were significantly lower
(37.6 and 34.8%) after 60 days.
FSH levels were elevated in all
treatment groups after 60 days,
and at the highest dose FSH
levels remained elevated after
12 months.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax Rats 0, 25, 50, 100c Diet 60 days Decrease in weights of liver, Dixon et al.
testes, and epididymis at the (1979)
two highest dietary levels.
Seminiferous tubule diameter
was decreased in a dose-related
manner in all groups. Loss of
germinal cell elements was seen
only in 50 and 100 mg/kg body
weight per day groups. Plasma
levels of FSH were elevated.
Reduced fertility only at two
highest dosage levels.
Borax Rats 0, 23.7, 44.7d Drinking-water 70 days Significant decrease in body Seal & Weeth
weight and decrease in weight (1980)
of testes, seminal vesicles,
spleen at both doses.
Spermato-genesis impaired and
haematocrit decreased slightly
at highest dose.
Borax Rats 0, 0.042, 0.14, 0.84b Drinking-water 90 days No observable reproductive Dixon et al.
effects or changes in serum (1976)
chemistry, body weight, or
weight of testes, prostate,
or seminal vesicles.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 1.5, 2.9, 8.8, 29c Diet 2 years Severe testicular atrophy and Weir & Fisher
boric acid spermatogenic arrest at week (1972)
26 with 29 mg/kg body weight
per day; no effects on body or
organ weights, gross morphology,
or histological parameters at
lower doses.
Boric acid Mice 0, 43.4, 79.0, 175.3 Diet Gestation There was no effect on survival NTP (1989);
days 0-17 or pregnancy rate. Maternal Heindel et al.
effects included mild renal (1992)
lesions at >43.4 mg/kg body
weight per day and increased
water intake, increased relative
kidney weight, and decreased
weight gain during treatment at
175.3 mg/kg body weight per day.
At the high-dose level, there
was a significant increase in
the percentage of resorptions/
litter. Average fetal body
weight/litter was significantly
reduced at >79.0 mg/kg body weight
per day. Malformations included a
missing or shortened XIII rib and
variations on extra lumbar I
rib. Malformations were
significantly increased at
175.3 mg/kg body weight per day.
The percentage of variations/
litter decreased at <79.0 mg/kg
body weight per day and was not
affected at 175.3 mg/kg body
weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats 0, 13.6, 28.5, 57.7, Diet Gestation The highest dose was administered NTP (1990);
94.2 days 0-20 to one group on gestation days Heindel et al.
6-15. There was no effect on (1992)
survival or pregnancy rate.
Maternal rats showed increased
relative liver and kidney weights
at >28.5 mg/kg body weight per
day, increased absolute kidney
weight at 94.2 mg/kg body weight
per day, and a decrease in body
weight gain at >57.7 mg/kg body
weight per day. At low doses,
there was a decrease in extra
lumbar rib I (a variation) and
at high doses an increase in
missing or shortened XIII rib
(a malformation). The 94.2 mg/kg
body weight per day level
increased prenatal mortality.
Fetal body weights were
significantly reduced in all
treated groups in a dose-related
manner. Malformations increased
at >28.5 mg/kg body weight per
day; variations increased at
94.2 mg/kg body weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats Phase 1 Diet Phase 1 Phase 1 -- Fetal body weight was
0, 3.3, 6.3, 9.6, gestation decreased on gestational day 20
13.3, 25 days (gd) in the 13.3 and 25 mg/kg body
0-20 weight per day dose groups. On
Phase 2 Phase 2 gd 20, incidences of short rib
0, 3.3, 6.5, 9.8, postnatal XIII or wavy rib were increased
12.9, 25.4 days (pn) in these dose groups relative
0-21 to controls. The high-dose group
(exposure contained a biologically
limited to relevant but not statistically
gestation significant decrease in
days 0-20) incidence of extra rib on
lumbar I.
Phase 2 -- On pn days 0-21, there Price et al.
were no decreased fetal body (1996a)
weight effects. On pn day 21,
the percentage of pups per
litter with short rib XIII was
elevated in the 25.4 mg/kg body
weight per day dose group. No
wavy rib or extra rib on lumbar
I was found at pn day 21.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rabbits 0, 10.9, 21.9, 43.7 Gavage Exposure on No treatment-related clinical Price et al.
in water gestation signs of toxicity except for (1996b)
days 6-19; vaginal bleeding at 43.7 mg/kg
termination body weight per day. This dose
on gd 30 had no live fetuses on day 30.
Food intake decreased during
treatment (43.7 mg/kg body
weight per day), but increased
on days 25-30 (21.9 and 43.7
mg/kg body weight per day). Body
weight and body weight gain were
decreased during treatment at
43.7 mg/kg body weight per day,
but corrected body weight
increased at 21.9 and 43.7 mg/kg
body weight per day. Gravid
uterine weight and number of
corpora lutea/dam were decreased
at 43.7 mg/kg body weight per
day. Significant developmental
effects were limited to the
high-dose group. Prenatal
mortality was greatly increased.
Malformations were also
increased, primarily owing to
the incidence of fetuses with
cardiovascular defects. The
skeletal variation observed
was sternebrae. Fetal body
weight was slightly reduced
in the high-dose group.
Table 16. (continued)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of
boron/boron compound.
b Estimated by the authors.
c Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or
food factor of 0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for
mice.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
In a 2-year study by Weir & Fisher (1972), groups of 4 male and 4
female beagle dogs were fed diets containing boric acid or borax to
provide doses of 0, 1.45, 2.93, or 8.75 mg boron/kg body weight per
day. No evidence of toxicity was observed. An additional group of dogs
(4 male and 4 female) was fed diets containing boric acid or borax
leading to doses of 0 or 29.3 mg boron/kg body weight per day for 38
weeks. Testicular atrophy was observed in 2 test dogs receiving borax
at 26 weeks. The authors stated that boric acid caused testicular
degeneration in dogs, including spermatogenic arrest and atrophy of
the seminiferous epithelium. This study was terminated at 38 weeks. In
the study, the number of dogs was small and variable (1-2 dogs at each
of three time points) and inadequate to allow statistical analysis. A
common control group was used for both borax and boric acid exposure
groups. Testicular lesions occurred in the controls (1 of 4 controls
had slight to severe seminiferous tubular atrophy, another had
moderate to severe atrophy, whereas a third had a detectable but
insignificant reduction in spermatogenesis and 5% atrophic
seminiferous tubules). This study was conducted before the advent of
Good Laboratory Practices (GLP). Confidence in this study is low, and
it was considered not suitable for inclusion into the risk assessment
because of 1) small and variable numbers of dogs, 2) variable
background lesions in controls leading to uncertainty regarding the
strength of the response to treatment, 3) lack of GLP, and 4) other,
more recent studies of greater scientific quality with findings at a
similar or lower intake level of boron (Ku et al., 1993a; Price et
al., 1996a).
Weir & Fisher (1972) fed Sprague-Dawley rats (35/sex per group) a
diet containing borax or boric acid for 2 years at 0, 5.9, 17.5, or
58.5 mg boron/kg body weight per day. At the high dose, rats receiving
either compound had decreased food consumption during the first 13
weeks of study and decreased growth throughout the study. Testes
weights were significantly decreased at 58.5 mg/kg body weight per
day. The seminiferous epithelium was atrophied, and the tubular size
in the testes was decreased. No treatment-related effects were
observed in rats receiving 5.9 or 17.5 mg boron/kg body weight per
day. The LOAEL in this study was 58.5 mg boron/kg body weight per day
for decreased testes weights, atrophied testes, histopathological
alterations of the testes, and increased brain and thyroid weights.
The NOAEL for this study was 17.5 mg boron/kg body weight per day.
In a companion three-generation reproduction study in rats, Weir
& Fisher (1972) found that 58.5 mg boron/kg body weight per day
produced testicular atrophy and complete suppression of fertility in
rats. Lower doses (17.5 or 5.9 mg boron/kg body weight per day) did
not reduce fertility. The small group size (n = 8), low control
fertility rates (approx. 60%), limited data reported, and
inappropriate statistics all limit the applicability of these data for
risk assessment.
In a multigeneration continuous-breeding experiment (Fail et al.,
1990, 1991), Swiss CD-1 mice (F0 generation) were fed boric acid in
the diet at 0, 1000, 4500, or 9000 mg/kg feed for 27 weeks, which gave
calculated doses of 0, 19.2, 104.7, and 222.1 mg boron/kg body weight
per day for males and 0, 31.9, 148.1, and 290.5 mg boron/kg body
weight per day for females. Treatment with boric acid significantly
impaired fertility: no males or females in the high-dose groups were
fertile. At the middle dose, the number of litters per pair, number of
live pups per litter, proportion of pups born alive, and pup weight
adjusted for litter size were all decreased. The trend towards a lower
fertility index at this dose level started with the first litter and
progressed in severity with subsequent matings. Animals from different
treatment groups were cross-mated to determine the affected sex at
this dose. When mid-dose males were mated with control females, mating
and fertility indices were significantly depressed, with only one pair
in that group producing a live litter; these indices were not affected
when control males were mated with mid-dose females, confirming that
the male was the affected sex. At F0 necropsy, sperm motility was
significantly reduced in all exposed groups (by 12%, 32%, and 47%,
from low- to high-dose groups, respectively). Low-dose and mid-dose
animals from the F1 generation were exposed during gestation and
lactation. The fertility of the low-dose F1 mice was not affected,
but the litter-adjusted body weights of the F2 pups were
significantly decreased (by 3.3%) relative to controls. The low dose
was considered the LOAEL for decreased sperm motility in the F0
males, 26% increased uterine weight and 8% increased kidney/adrenal
weight in F1 females, and a 3.3% reduction in litter-adjusted birth
weight in the F2 pups. This study had no NOAEL, but the magnitude of
the changes at the low dose (a 12% decrease in F0 sperm motility, an
8% increase in F1 kidney/adrenal weights, and a 3% reduction in F2
pup weights) is small and indicates that this dose level is close to
the NOAEL.
Fail et al. (1989) utilized both CD-1 mice and wild deer mice
(Peromyscus maniculatus) to characterize the effects of boric acid
on fertility and to test the reversibility of these effects. Adult
CD-1 mice were exposed to boric acid in the feed for 27 weeks at 0,
1000, 4500, or 9000 mg/kg (consumed doses were not given). The males
at the high and middle doses had testicular atrophy and decreased
spermatogenesis. Fertility was diminished in animals receiving the
middle dose and completely absent in the high-dose group. The same
doses were used to test toxicity and reversibility in the wild mouse.
The ability to produce offspring was significantly reduced by boric
acid: none of the males in the high-dose group sired litters. Weight
gain was similar for the treated and control wild mice. Although body
and organ weights were similar between the mid-dose and control groups
at necropsy, the testis and total accessory sex organ weights were
significantly lower in the high-dose group. These two species appear
to differ in their sensitivity to boric acid. The CD-1 mice had body
weight loss as early as 5 weeks at the mid-dose level, whereas deer
mice did not lose weight even at the high dose. Tissue pathology
findings at the middle dose were significant in CD-1 mice but not in
deer mice. Deer mice were found to recover from boric acid exposure.
The development of the testicular lesion was investigated by
Treinen & Chapin (1991), who fed boric acid at a level of 0 or 60.9 mg
boron/kg body weight per day to male F-344 rats and sacrificed six
treated and four control male rats at intervals from 4 to 28 days
after the start of exposure. In half of the treated rats, there was
inhibition of spermiation in 10-30% of stage IX tubules at 7 days and
inhibition in all stage IX and stage X tubules of exposed rats at 10
days. At 28 days, there was significant loss of spermatocytes and
spermatids from all tubules in exposed rats, and basal serum
testosterone levels were significantly decreased from 4 days on.
Secondary to the loss of germ cells, the activities of enzymes
found primarily in spermatogenic cells were significantly decreased,
and enzyme activities associated with premeiotic spermatogenic cells
were significantly increased in Sprague-Dawley rats exposed to 60 or
125-131 mg boron/kg body weight per day for 30 or 60 days (Lee et al.,
1978). Mean plasma follicle stimulating hormone (FSH) levels were
significantly elevated in a dose-dependent manner in all treatment
groups in this study (30 to 125-131 mg boron/kg body weight per day)
after 60-day exposures. FSH levels in animals exposed to the highest
dose tested (125-131 mg boron/kg body weight per day) were still
elevated 12 months after treatment termination, owing to atrophied
testes and no recovery of spermatogenesis. Plasma luteinizing hormone
(LH) levels were not significantly elevated, and mean plasma
testosterone levels were within the normal range throughout the study
(Lee et al., 1978).
Reversibility of testicular lesions was evaluated by Ku et al.
(1993a) in an experiment in which F-344 rats were dosed at 3000, 4500,
6000, or 9000 mg boric acid/kg (26, 38, 52, and 68 mg boron/kg body
weight per day) in the feed for 9 weeks and assessed for recovery up
to 32 weeks post-treatment. Inhibited spermiation was exhibited at
3000/4500 mg boric acid/kg (5.6 µg boron/mg tissue), whereas inhibited
spermiation progressed to atrophy at 6000/9000 mg boric acid/kg (11.9
µg boron/mg testes), with no boron accumulation in the testes to
levels greater than found in blood during the 9-week period. After
treatment, serum and testis boron levels in all dose groups fell to
background levels. Inhibited spermiation at 4500 mg/kg was reversed by
16 weeks post-treatment, but focal atrophy was detected that did not
recover up to 32 weeks post-treatment.
An unpublished study by W.W. Ku and colleagues (reported in Ku et
al., 1993a) found no detectable treatment-related changes in
testicular structure in rats induced by consumption of 17.5 mg
boron/kg body weight per day for up to 9 weeks.
In a follow-up study to explore/identify the mechanism for this
testicular toxicity of boric acid, Ku et al. (1993b) evaluated several
end-points in cell culture systems following in vitro boric acid
exposure. The data suggest an effect of boric acid on the DNA
synthesis activity of mitotic and meiotic germ cells and, to a lesser
extent, on energy metabolism in Sertoli cells. The effect on DNA
synthesis occurred at boron concentrations that were associated with
atrophy in vivo and shows that boric acid interferes with the
production and/or maturation of early germ cells; this offers an
explanation for atrophy, but not for inhibited spermiation.
Additional mechanistic studies by Ku & Chapin (1994) showed that
testicular toxicity and CNS hormonal effects were not due to selective
boron accumulation in testis or brain/hypothalamus, with testis boron
concentrations at 1-2 mmol/litre. Changes in testis phosphorus,
calcium, and zinc levels did not precede atrophy. In vitro studies
showed no effect on the steroidogenic functions of isolated Leydig
cells, supporting the suggestion of a CNS-mediated hormonal effect.
The authors showed that inhibited spermiation was not due to increased
testicular cyclic adenosine monophosphate or reduced serine protease
plasminogen activators. Also evaluated were effects of boric acid in
Sertoli-germ cell co-cultures on Sertoli cell energy metabolism
(lactate secreted by Sertoli cells is a preferred energy source for
germ cells) and DNA/RNA synthesis (germ cells synthesize DNA/RNA, and
boric acid impairs the synthesis of these nucleic acids in the liver).
The most sensitive in vitro end-point was DNA synthesis in
mitotic/meiotic germ cells; energy metabolism in germ cells was
affected to a lesser extent, which was manifested in vivo as a
decrease in early germ cell/Sertoli cell ratio prior to atrophy in the
testes. The mechanisms of inhibited spermiation are still not defined.
In summary, male reproductive effects from boron have been noted
in mice, rats, and dogs. Reproductive effects observed include
inhibition of spermiation in stage IX and X tubules, followed by germ
cell loss, changes in epididymal sperm morphology and caput sperm
reserves, decreased serum testosterone levels, and testicular atrophy.
Male reproductive effects have been reported in oral exposure studies
at doses as low as 29 mg boron/kg body weight per day in dogs exposed
for 2 years to dietary boric acid or borax (Weir & Fisher, 1972); for
rats in this same study, the LOAEL for reproductive toxicity was 58.5
mg boron/kg body weight per day.
7.5 Developmental toxicity
Developmental toxicity has been demonstrated experimentally in
rats, mice, and rabbits (see Table 16) (Heindel et al., 1992; Price et
al., 1996b).
Sprague-Dawley rats were fed a diet containing 0, 13.6, 28.5, or
57.7 mg boron/kg body weight per day as boric acid from gestation days
0 to 20 (Heindel et al., 1992). An additional group of rats received
boric acid at 94.2 mg boron/kg body weight per day on gestation days
6-15 only. Maternal effects included a significant and dose-related
increase in relative liver and kidney weights at >28.5 mg boron/kg
body weight per day. Treatment with 94.2 mg boron/kg body weight per
day significantly increased prenatal mortality. Average fetal body
weight per litter was significantly reduced in a dose-related manner
in all treated groups compared with controls. The percentage of
malformed fetuses per litter and the percentage of litters with at
least one malformed fetus were significantly increased at >28.5 mg
boron/kg body weight per day. Malformations consisted primarily of
anomalies of the eyes, the CNS, the cardiovascular system, and the
axial skeleton. The most common malformations were enlargement of
lateral ventricles in the brain and agenesis or shortening of rib
XIII. The percentage of fetuses with variations per litter was reduced
relative to controls at 13.6 and 28.5 mg boron/kg body weight per day
(due to a reduction in the incidence of rudimentary or full ribs at
lumbar 1) but was significantly increased in rats exposed to 94.2 mg
boron/kg body weight per day. The variation with the highest incidence
among fetuses was wavy ribs. The LOAEL of 13.6 mg boron/kg body weight
per day (lowest dose tested) for rats occurred in the absence of
maternal toxicity; a NOAEL was not found in this study.
Price et al. (1996a) did a follow-up to the Heindel et al. (1992)
study in Sprague-Dawley (CD) rats to determine a NOAEL for fetal body
weight reduction and to determine whether the offspring would recover
from prenatally reduced body weight during postnatal development.
Skeletal malformations and variations were also studied to further
characterize the low end of the dose-response curve (phase 1) and to
determine whether the incidence of skeletal defects in offspring
changed during postnatal life (phase 2). Boric acid was administered
in the diet to CD rats from gestational day 0 to 20. In phase 1, dams
were terminated and uterine contents examined on gestational day 20.
During phase 1, the intake of boric acid was 0, 3.3, 6.3, 9.6, 13.3,
or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls;
the reduction was significant only in the 13.3 and 25 mg boron/kg body
weight per day dose groups on gestational day 20. During phase 1,
incidences of short rib XIII (a malformation) and wavy rib (a
variation) were increased in the >13.3 mg boron/kg body weight per
day dose groups relative to control litters. There was a decreased
incidence of rudimentary extra rib on lumbar 1 (a variation) in the
high-dose group that was deemed biologically but not statistically
significant. During phase 2, the intake of boric acid during gestation
was 0, 3.3, 6.5, 9.8, 12.9, or 25.3 mg boron/kg body weight per day.
At birth, boric acid exposure stopped and dams were allowed to deliver
and rear their litters until postnatal day 21. On postnatal day 0 of
phase 2, there were no effects of boric acid on offspring body weight,
nor were any differences seen through postnatal day 21. On postnatal
day 21 of phase 2, the percentage of pups per litter with short rib
XIII was elevated only in the 25.3 mg boron/kg body weight per day
dose group, but there was no treatment-related increase in wavy rib or
extra rib (full or rudimentary) on lumbar 1 observed in these pups on
day 21. The NOAEL for phase 1 of this study is 9.6 mg boron/kg body
weight per day based on a decrease in fetal body weight. The LOAEL was
13.3 mg boron/kg body weight per day for phase 1. The NOAEL for phase
2 was 12.9 mg boron/kg body weight per day, and the LOAEL was 25.3 mg
boron/kg body weight per day. The results of this study provide a
NOAEL and LOAEL that complement the rat LOAEL of 13.6 mg/kg body
weight per day in the Heindel et al. (1992) study.
Heindel et al. (1992) also investigated the developmental
toxicity and teratogenicity of boric acid in mice at 0, 43, 79, or 175
mg boron/kg body weight per day in the diet. There was a significant
dose-related decrease in average fetal body weight per litter at 79
and 175 mg boron/kg body weight per day. In offspring of mice exposed
to 79 or 175 mg boron/kg body weight per day during gestation days
0-17, there was an increased incidence of skeletal (rib)
malformations. These changes occurred at doses for which there were
also signs of maternal toxicity (increased kidney weight and
pathology); the LOAEL for developmental effects (decreased fetal body
weight per litter) was 79 mg boron/kg body weight per day, and the
NOAEL for developmental effects was 43 mg boron/kg body weight per
day.
Price et al. (1996b) investigated the developmental toxicity and
teratogenicity of boric acid in rabbits at doses of 0, 10.9, 21.9, or
43.7 mg boron/kg body weight per day given by gavage. Frank
developmental effects in rabbits exposed to 43.7 mg boron/kg body
weight per day included a high rate of prenatal mortality, increased
number of pregnant females with no live fetuses, and fewer live
fetuses per live litter on day 30. Also at 43.7 mg boron/kg body
weight per day, malformed live fetuses per litter increased
significantly, primarily because of the incidence of fetuses with
cardiovascular defects, the most prevalent of which was
interventricular septal defect. Skeletal variations observed were
extra rib on lumbar 1 and misaligned sternebrae. The NOAEL for
maternal and developmental effects was 21.9 mg boron/kg body weight
per day, and the LOAEL for maternal and developmental effects was 43.7
mg boron/kg body weight per day.
7.6 Mutagenicity and related end-points
Boric acid was not mutagenic in Salmonella typhimurium with or
without rat or hamster S9 fraction (Haworth et al., 1983; Benson et
al., 1984; NTP, 1987) or in mouse lymphoma L5178Y/TK+/ cells with or
without rat liver S9 (NTP, 1987; McGregor et al., 1988). Borax was not
mutagenic in Salmonella with or without rat liver S9 (Benson et al.,
1984). Refined borax, crude borax ore, and kermite ore were not
mutagenic in V79 Chinese hamster cells, C3H/1OT1/2 mouse embryo
fibroblasts, or diploid human foreskin fibroblasts (Landolph, 1985).
Sodium perborate (NaBO3) was shown to interact with DNA in the
Escherichia coli Pol A assay, presumably by being converted to
hydrogen peroxide (Rosenkranz, 1973). Other tests showed that boric
acid did not induce chromosomal aberrations or sister chromatid
exchanges in Chinese hamster ovary cells (NTP, 1987). Existing data
suggest that genotoxicity is not an area of concern following exposure
to boron compounds in humans.
7.7 Carcinogenicity
The 2-year feeding study (NTP, 1987; Dieter, 1994) showed no
evidence of carcinogenicity in B6C3F1 mice. The Weir & Fisher (1972)
study showed no evidence of boric acid-related carcinogenicity in
rats, although not all tissues were examined. Based on the lack of
human data and on the data from these two animal tests, boron is
classified by the US EPA as a Group D chemical (not classifiable as to
human carcinogenicity) (US EPA, 1994).
7.8 Toxicity effects summary
The laboratory animal toxicity data show that boric acid (and
other borates) is not a carcinogen and lacks significant mutagenicity.
The crossover mating trial in the Fail et al. (1991) study showed that
female mouse reproduction was unaffected by about 120 mg boron/kg body
weight per day. The NOAELs and LOAELs of reproductive/developmental
effects from various studies are ranked in Table 17. The Weir & Fisher
(1972) dog study is omitted from this summary because of low numbers
of animals and the presence of lesions in the controls. It can be seen
that rat fetal development is more sensitive than other processes to
the adverse effects of elevated boron exposure, with a LOAEL in rats
of 13.3 mg boron/kg body weight per day reducing fetal weight gain and
increasing rib anomalies (reversibly). The current NOAEL for these
effects in rats is approximately 10 mg boron/kg body weight per day.
Increased doses produced sequentially additional effects on sperm
release, increased rabbit cardiovascular defects, reduced epididymal
sperm counts, testicular atrophy, and mouse fetal defects.
The Task Group recognized the lack of a NOAEL in the Fail et al.
(1991) study. The effects of concern in part duplicated the
developmental toxicity findings (reduced pup body weight). However,
the unique effects in the adult F1 female mice (increased uterine
weight, reduced cycle length) are consistent with an effect on female
reproduction that was not seen in the F0 females. The possibility of
unique transgenerational or functional developmental toxicity in the
absence of an available dose-response concerned the Task Group.
Although the current NOAEL is based on rat fetal body weight
effects, the Task Group noted that this could be lowered and a new
"critical effect" could emerge if the significant data gap
(second-generation fertility and necropsy data for multiple doses
including a NOAEL in rats) were filled.
7.9 Physiological effects
Since 1981, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for higher animals;
that is, a dietary deprivation of boron consistently results in
changed biological functions that can be construed as detrimental and
are preventable or reversible by an intake of physiological amounts of
boron. Convincing findings have been reported that show that dietary
boron deprivation affects mineral and energy metabolism in chicks and
Table 17. Ranking of reproductive/developmental effects of boric acid
Species/durationa Dose (mg boron/kg Effect Reference
body weight per day)
SD rat/gd 0-20 9.6 NOAEL for developmental effects immediately pre-term Price et al. (1996a)
SD rat/gd 0-20 12.9 NOAEL for developmental effects measured at weaning Price et al. (1996a)
SD rat/gd 0-20 13.3 LOAEL for reduced fetal weight, increased rib Price et al. (1996a)
13.6 malformations/variations Heindel et al. (1992)
Male SD rat/multigeneration 17.5 NOAEL for male sterility, testicular atrophy Weir & Fisher (1972)
SD rat/gd 0-20 25.4 LOAEL for increased short rib XIII at weaning Price et al. (1996a)
Male SD rat/63 days 26 LOAEL for mild inhibited sperm release Ku et al. (1993a)
Male SD rat/¾63 days 52 LOAEL for testicular atrophy Ku et al. (1993a)
Male beagle dogs/2 years 29 Altered testis weight and histopathology Weir & Fisher (1972)
Male beagle dogs/90 days 44 Altered testis weight and histopathology Weir & Fisher (1972)
CD-1 mouse/multigeneration 19.2 LOAEL for reduced sperm motility, reduced F2 pup weight Fail et al. (1991)
CD-1 mouse/gd 0-17 43 NOAEL for mouse developmental toxicity Heindel et al. (1992)
79 LOAEL for decreased fetal body weight
New Zealand white rabbits/ 21.9/43.7 NOAEL/LOAEL for decreased fetal body weight, Price et al. (1996b)
gd 6-19 increased fetal cardiovascular malformations and
maternal toxicity
a gd = gestational days.
rats (Hunt, 1994); these effects are more marked when a physiological
stressor is present, especially marginal cholecalciferol (vitamin D3)
nutriture. For example, a boron supplement of 3 µg/g to a basal diet
containing 0.465 µg boron/g alleviated the marginal cholecalciferol
deficiency-induced changes in bone, plasma glucose, energy substrate
utilization, and growth (Hunt, 1994). Hunt et al. (1994) have found
that boron deprivation depresses macromineral content in bone and some
indices of the maturation of the cartilage growth plate independently
of vitamin D nutriture. Brain composition and function in rats are
also affected by dietary boron. Boron deprivation was found to
systematically influence brain electrical activity assessed by an
electrocorticogram in mature rats (Penland & Eberhardt, 1993). In this
study, brain copper concentrations were higher in boron-deprived than
in boron-supplemented rats. Furthermore, calcium concentrations in
total brain and in brain cortex, as well as phosphorus concentration
in the cerebellum, were found to be higher in boron-deprived (0.158
µg/g diet) than in boron-supplemented (2.72 µg/g diet) rats fed a
cholecalciferol-deficient diet (Hegsted et al., 1991). This study also
found that the apparent absorption and balance of calcium, magnesium,
and phosphorus were decreased by boron deprivation. Hunt (1988) has
reported that chicks apparently require about 1.0 µg boron/g diet for
normal development. These studies collectively indicate that boron in
physiological amounts is beneficial to, if not essential for, higher
animals (see also section 8.4).
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Short-term toxicity and poisoning incidents
Available human exposure data on boron compounds for routes other
than inhalation focus on boric acid and borax. According to Stokinger
(1981), the lowest lethal dose for humans exposed to boric acid is 640
mg/kg body weight by oral exposure, 8600 mg/kg body weight by dermal
exposure, and 29 mg/kg body weight by intravenous injection. Stokinger
(1981) stated that deaths can occur at doses between 5 and 20 g of
boric acid total for adults and below 5 g total for infants. Litovitz
et al. (1988) stated that potential lethal doses are usually cited as
3-6 g total for infants and 15-20 g total for adults. A case-series
report of seven infants (aged 6-16 weeks) who used pacifiers coated
with a borax and honey mixture for 4-10 weeks reported that exposures
ranged from 4 to 30 g, with an estimated average daily ingestion of
0.143-0.429 g (O'Sullivan & Taylor, 1983). The actual relative doses
are unknown. Toxicity was manifested by generalized or alternating
focal seizure disorders, irritability, and gastrointestinal
disturbances. Other findings included inflammation, congestion,
oedema, exfoliation of the mucosa, cloudy swelling and granular
degeneration of tubular cells, and exfoliative dermatitis. Although
infants appear to be more sensitive than adults to boron compounds,
lethal doses are not well documented in the literature.
A few case reports have been published for poisoning incidents.
Teshima et al. (1992) reported that a 26-year-old woman ingested 21 g
of boric acid. The elimination of boric acid was about 4 times faster
with haemodialysis than with conventional medical treatment. The
patient was discharged from the hospital 12 days following admission.
In another case report, Grella et al. (1976) described transplacental
poisoning. A pregnant woman who had a personal history of diabetes was
accidentally given 70 g of boric acid instead of 70 g of glucose for
the glucose tolerance test at 33 weeks' gestation. She was immediately
treated with gastric lavage and intravenous sodium bicarbonate
fructose. The woman developed contractions, and an emergency caesarean
delivery was scheduled. The infant was born alive weighing 2.5 kg and
had spontaneous respirations. Soon afterwards, the infant developed
cardiac arrest, was resuscitated, and died. Cause of death was
attributed to cardiocirculatory collapse.
Boric acid and borax were widely used in medicine at the
beginning of the century for therapeutic purposes, both locally as
well as orally. Boric acid was used to treat various diseases, such as
epilepsy and infectious diseases. Several case studies reviewed by
Kliegel (1980) describe mild to severe responses to boron compounds.
Among all 19 patients with reported body hair loss as a response to
boron compound treatment, 16 persons had epilepsy and 3 had urinary or
genital infections. Stein et al. (1973) described an extremely unusual
case of a 32-year-old woman who ingested and swallowed several bottles
of mouthwash containing boric acid daily for a minimum of 1 year. The
woman was reported to have pancreatitis resulting from heavy alcohol
ingestion and medications for epilepsy. The woman lost almost all of
her body and scalp hair; other clinical signs included erythema on the
palms of her hands, severe fatigue, anorexia, and mental confusion.
Her blood boric acid level was 3.2 mg/100 ml, corresponding to 0.56 mg
boron/100 ml (the normal value was reported as 0.3 mg boric acid/100
ml). Upon cessation of mouthwash consumption, hair growth returned,
suggesting that the effect was reversible.
Goldbloom & Goldbloom (1953) reported four cases of boric acid
poisoning and reviewed an additional 109 cases in the literature. The
four cases were infants exposed to boric acid by repeated topical
applications of baby powder. Toxicity was manifested by cutaneous
lesions (erythema over the entire body, excoriation of the buttocks,
and desquamation), gastrointestinal disturbances, and seizures. One
patient died, but cause of death was not specifically attributed to
boric acid. Approximately 35% of the 109 other case reports involved
children under 1 year of age. The mortality rate was 70.2% for
children compared with 55.0% for all cases combined. Death occurred in
27/51 (53%) patients exposed by ingestion, in 3/4 (75%) patients
subjected to gastric lavage with boric acid, in 19/28 (68%) patients
exposed by dermal application for treating burns, wounds, and skin
eruptions, and in 14/26 (54%) patients exposed by other routes.
Information on signs and symptoms for 80 patients showed that
gastrointestinal disturbances were prevalent (73%), followed by CNS
effects (67%). Cutaneous lesions were prevalent in 76% of the cases
and in 88% of cases involving children under 2 years of age. Gross and
microscopic findings were reported for 27/60 (45%) fatal cases. In
general, boric acid caused chemical irritation primarily at sites of
application and excretion and in organs with maximum boron
concentrations. The most common CNS findings were oedema and
congestion of the brain and meninges. Other common findings included
liver enlargement, vascular congestion, fatty changes, swelling, and
granular degeneration (n = 13).
In addition to case reports, poison centres have published
case-series reports. Unlike the case reports reviewed by Goldbloom &
Goldbloom (1953), more recent reports suggest that the oral toxicity
of boron in humans is milder than previously thought. Litovitz et al.
(1988) conducted a retrospective review of 784 cases of boric acid
ingestion reported to the National Capital Poison Center in
Washington, DC, USA, during 1981-1985 and the Maryland Poison Center
in Baltimore, MD, USA, during 1984-1985. The amount of boric acid
ingested and clinical manifestations of toxicity were reported; 88.3%
of the cases were asymptomatic. All but two of the cases had acute
(single) ingestion, and 80.2% involved children under 6 years of age.
No severe toxicity or life-threatening effects were noted, although
boric acid levels in blood serum ranged from 0 to 340 µg/ml. The most
frequently occurring symptoms, which involved the gastrointestinal
tract, included vomiting (n = 32), abdominal pain (n = 15),
diarrhoea (n = 13), and nausea (n = 7). Other symptoms (primarily
CNS and cutaneous) occurred in six or fewer cases: lethargy, rash,
headache, light-headedness, fever, irritability, and muscle cramps.
The average dose ingested estimated from 659 cases was 1.4 g (range
0.010-88.8 g). For children under 6 years, the average dose was 0.5 g
(range 0.010-22.2 g), compared with 4.1 g (range 0.030-88.8 g) for
individuals age 6 years or above. The average dose for asymptomatic
cases was 0.9 g (range 0.010-88.8 g), compared with 3.2 g (range
0.10-55.3 g) for symptomatic cases. According to Litovitz et al.
(1988), 21 of the children under 6 years of age, 15 of whom were under
2 years of age, ingested the reported potential lethal dose of 3 g; 8
adults ingested the reported potential lethal dose of 15 g without
evidence of lethal effects.
Linden et al. (1986) published a retrospective review of 364
cases of boric acid exposure reported to the Rocky Mountain Poison and
Drug Center in Denver, CO, USA, between 1983 and 1984. Vomiting,
diarrhoea, and abdominal pain (incidence not reported) were the most
common symptoms given by the 276 cases exposed in 1983. Of the 72
cases reported in 1984 for whom medical records were complete, 79%
were asymptomatic, whereas 20% had mild gastrointestinal symptoms
noted. One 2-year-old child died, presumably from repeated ingestion
of an insecticide containing 99% boric acid.
Overall, owing to the wide variability of data collected from
poisoning centres, the average dose of boric acid required to produce
clinical symptoms is still unclear but is presumably within the range
of 100 mg to 55.5 g, observed by Litovitz et al. (1988).
8.1.2 Reproductive effects
An ecological study assessed boron exposure from drinking-water
and fertility among residents in two geographical regions in
Turkey.a Region I comprised 2368 residents, whereas Region II
comprised 2319 residents. Boron levels in drinking-water were
noticeably higher in Region I (range 2.05-29 mg/litre) than in Region
II (range 0.03-0.40 mg/litre). Ever-married residents from each region
who could provide reproductive histories for three generations of
family members represented the study sample -- i.e. 159 probands (6.7%
of population) in Region I and 154 (6.7%) in Region II. The
percentages of married couples with one or more live births (>90%)
were comparable for the two regions, regardless of generation
assessed. The overall percentage of couples with unresolved
infertility or those without children across three generations was
comparable for the two regions (i.e. 6.0% and 4.6%, respectively).
Secondary sex ratios (ratio of male to female live births) appeared to
be different for the two regions. Region I had a ratio below 1 (0.89),
suggesting an excess of female births; Region II had a ratio slightly
above 1 (1.04), suggesting a slight excess of male births. Statistical
a Sayli BS, Tüccar E, & Ellan AH (1996) An assessment of fertility in
boron-exposed Turkish subpopulations. Unpublished manuscript, Ankara
University Medical Faculty, Ankara, Turkey.
significance was not formally evaluated in any of the above analyses.
The results of this descriptive study suggest that fertility, as
measured by the ability to produce a live birth, is not adversely
affected for residents of this geographical area with high levels of
boron in their drinking-water and soil. The observed reversal of the
secondary sex ratio for Region I requires careful interpretation, as
no attention was given to factors reported to alter sex ratios (e.g.
advancing parental age, elective abortion rates, and multiple births).
Further consideration of potential sources of bias stemming from the
selection of respondents who were largely male and their ability to
accurately recollect and report fertility outcomes is needed. The
extent to which these findings are generalizable, if at all, to other
populations is unknown.
8.2 Occupational exposure
8.2.1 Short-term irritative effects
The majority of toxic effects reported in occupational studies
are acute or short-term in nature and result from the irritant effects
of boron compounds. Several studies have shown that workers exposed to
borax complain of symptoms that are due to respiratory irritation,
which include nosebleeds, eye and nasal irritation, sore throats,
cough, and shortness of breath; dermatitis has also been reported
(Birmingham & Key, 1963; NIOSH, 1978). Generally, people with
hyperreactive respiratory tracts (e.g. asthmatics) may experience more
severe irritant effects following inhalation exposure. However, there
is a lack of data to support the existence of an especially sensitive
high-risk human population for exposure to boron or boron compounds.
A medical survey of 113 workers employed in a borax mining and
refining plant was conducted by Garabrant et al. (1984). The reference
group comprised 214 workers employed in areas with low or minimal
exposure to boric acid and boron oxide; exposed workers had been
employed for a minimum of 5 years or were currently employed in areas
of heavy borax exposure. The average air concentration of particles
<5 µm in diameter measured from samples taken between 1979 and 1981
was 4.1 mg/m3 (range 1.2-8.5 mg/m3). The workers in both groups were
predominantly white males; the mean age of the exposed group was 38.2
years, compared with 42.1 years in the referent group. The mean
duration of employment for exposed and unexposed workers was 11.0 and
12.9 years, respectively. Smoking patterns were similar for the two
groups. Symptoms reported more frequently by exposed workers than by
unexposed workers (p < 0.001) were eye irritation (12.4% and 2.8%,
respectively), dry mouth, nose, or throat (10.6% and 1.9%,
respectively), sore throat (5.3% and 0.5%, respectively), and
productive cough (4.4 and 0%, respectively). No significant
differences were noted between the two groups of workers with respect
to dry cough, shortness of breath, chest tightness, chest pain, and
nosebleeds.
A more detailed analysis of 629 (93% participation) workers in
this plant was presented by Garabrant et al. (1985). This analysis was
based on frequency of acute symptoms in four mean boron dust exposure
categories (1.1, 4.0, 8.4, and 14.6 mg/m3) and persistent symptoms
(presumably chronic effects) in three exposure categories (0.9, 4.5,
and 14.6 mg/m3 of total particulates). The particles were composed
almost entirely of borax. Acute symptoms showing a significant linear
trend (p < 0.0001) in order of decreasing frequency were dryness of
mouth, nose, throat, and eye irritation, dry cough, nosebleeds, sore
throat, productive cough, shortness of breath, and chest tightness.
The frequency of these symptoms in the highest exposure category
ranged from 5% to 33%. The only symptom reported by 5% or more of
workers exposed to 4.0 mg/m3 was eye irritation; no symptoms were
reported by 5% or more of workers exposed to 1.1 mg/m3. The pulmonary
function findings were not significantly affected by exposure to
boron. Chest X-rays did not show abnormal regions indicative of boron
exposure. The authors concluded that borax caused simple respiratory
irritation that produces chronic bronchitis with no impairment of
pulmonary function. Also, borax dust appeared to cause acute and
persistent respiratory irritation at concentrations >4.5 mg/m3.
Therefore, the value for the intermediate exposure category for
persistent symptoms (4.5 mg/m3 as borax) was considered to be the
LOAEL.
Wegman et al. (1994) conducted a prospective cohort study to
examine acute irritative effects as well as chronic pulmonary function
abnormalities in mine and processing plant workers exposed to sodium
borate dust. Exposure-response associations were also examined as
related to acute irritant symptoms associated with sodium borate dust
exposure in mining and processing plants. In comparison with unexposed
workers, exposed workers reported significantly more nasal irritation
(rate ratio [RR] = 8.8), eye irritation (RR = 5.2), throat irritation
(RR = 2.9), cough (RR = 1.7), and breathlessness (RR = 7.1).
Continuous measures of particulate exposure were made throughout the
day and were related to hourly measurements of health outcomes.
Exposure-response relationships were present for each of the specific
symptoms at several symptom intensity levels. Acute irritant effects
did not lead to any undue chronic sequelae. With regard to the
acceptable level of risk, Wegman et al. (1994) concluded that a
threshold limit value (TLV) exposure of 10 mg borate/m3 was
protective of workers' health. This value is consistent with the
nuisance dust standard (ACGIH, 1991).
8.2.2 Male reproductive and other long-term health effects
Effects on the male reproductive system have also been reported,
although data regarding subchronic or chronic exposure on the
population in general are limited.
In the study by Wegman et al. (1994), sodium borate particulate
exposure estimates were used to estimate cumulative exposure in
relation to long-term pulmonary function. Of the 631 workers who
originally underwent pulmonary evaluation 7 years earlier, 336 (53%)
underwent a subsequent evaluation. Ninety (303/336) per cent of
workers were found to have acceptable pulmonary test results. After
the expected smoking-related pulmonary abnormalities were taken into
account, no relation was observed between forced expiratory volume in
1 second (FEV1) and accumulated exposure to sodium borate. People
with hyperreactive respiratory tracts such as those with asthma may
experience more severe irritant effects following inhalation exposure.
However, a sensitive population was not found.
Fertility following occupational exposure to boron was assessed
in a descriptive study. Whorton et al. (1994) estimated the
standardized birth ratio (SBR) to assess fertility in 542/750 (72%
participation) occupational workers in a US borax mine located in the
Mojave Desert, California, USA. These workers are unique, in that
their occupational exposure to sodium borate dust and desert soil is
uncontaminated by other exposures and given their long average length
of employment (mean 18 years). Self-administered questionnaires were
used to ascertain the observed number of live births fathered by male
workers following employment. The US general population adjusted for
age, race, parity, and calendar time period was used to estimate the
expected number of live births. The SBR is simply a ratio of the
observed number of live births to the expected number. An SBR above
100 reflects an excess of live births in relation to the US general
population, whereas an SBR below 100 reflects a deficit. Occupational
boron exposure was based on job titles and categorized into quintiles
of mean exposure levels ranging from <0.82 mg/m3 to >5.05 mg/m3.
SBRs were significantly elevated for workers in the lowest
(<0.82 mg/m3) and highest (>5.05 mg/m3) exposure categories
(i.e. 151 and 125, respectively). No significant trend between
exposure and SBRs was observed. An excess percentage of female live
births in comparison with male births was observed across most
categories of exposure and length of employment. The authors noted
that this female excess did not reflect a deficiency of male births,
as an excess of births for both genders was found. None of the
findings, however, was statistically significant. These findings need
to be carefully interpreted, largely given the selection of the US
general population as the standard. (The authors state that fertility
rates were not available for their target population.) The extent to
which this sample of workers is comparable to the US general
population with respect to factors that affect fecundity and fertility
remains to be established. Further considerations are needed with
respect to the calculation of SBRs, such as the exclusions of multiple
births, and in terms of potential response bias. The authors did
attempt to enhance the validity of self-reported fertility data and
select co-variates by using additional data sources and by efforts to
minimize selection bias. In sum, the findings do not support an
adverse effect of boron on demonstrated fertility for this
occupational sample of male workers in comparison with the US general
population.
8.3 Carcinogenicity
Evidence for carcinogenicity of boron and boron compounds in
humans is not available, largely because it has not been studied.
Based on the evidence from two lifetime studies in mice (NTP, 1987)
and rats (Weir & Fisher, 1972), boron compounds have been classified
by the US EPA in Group D (i.e. not classifiable as to human
carcinogenicity) (US EPA, 1994).
8.4 Physiological effects
Since 1987, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for humans; that is,
a dietary deprivation of boron consistently results in changed
biological functions that could be construed as detrimental and that
are preventable or reversible by an intake of physiological amounts of
boron. Many of these changed functions caused by boron deprivation
have been duplicated in animal models. The major reason boron is not
generally recognized as essential or nutritionally important for
humans is probably that a specific biochemical function for boron has
not been elucidated, or, as demonstrated for plants (also for which no
biochemical function has been elucidated), boron has not been shown
necessary to complete the life cycle. Nonetheless, findings from human
experiments show that boron is a dynamic trace element that can affect
the metabolism or utilization of numerous substances involved in life
processes, including calcium, copper, magnesium, nitrogen, glucose,
triglycerides, reactive oxygen, and estrogen. Through these effects,
boron can affect the function or composition of several body systems,
including blood, brain, and skeleton, in a positive manner, which
demonstrates that boron is a beneficial element, if not an essential
mineral element, at physiological amounts.
Although the first findings involving boron deprivation of humans
appeared in 1987 (Nielsen et al., 1987), the most convincing findings
have come mainly from two studies in which men over the age of 45,
postmenopausal women, and postmenopausal women on estrogen therapy
were fed a low-boron diet (0.25 mg/2000 kcal) for 63 days and then fed
the same diet supplemented with 3 mg boron/day for 49 days (Nielsen,
1989, 1994; Nielsen et al., 1990, 1991, 1992b; Penland, 1994). These
dietary intakes were near the low and high values in the range of
usual dietary boron intakes (see section 5.2.4). The major differences
between the two studies were the intakes of copper and magnesium; in
one experiment they were marginal or inadequate, whereas in the other
they were adequate. Boron deprivation had several effects, regardless
of dietary copper and magnesium. In addition, the marginal or
inadequate copper and magnesium caused apparent detrimental changes
that were more marked during boron deprivation than during boron
repletion. Among the effects of boron supplementation after 63 days of
boron depletion in these experiments were the following: an effect on
macromineral metabolism, evidenced by increased serum
25-hydroxycholecalciferol and a decrease in the elevated calcitonin
that was apparently caused by inadequate copper and magnesium; an
effect on energy metabolism, suggested by decreased serum glucose and
increased serum triglycerides; an effect on nitrogen metabolism,
indicated by decreased blood urea nitrogen and serum creatinine and
increased urinary hydroxyproline excretion; an effect on reactive
oxygen metabolism, indicated by increased serum erythrocyte superoxide
dismutase and serum ceruloplasmin; and an effect on erythropoiesis and
haematopoiesis, suggested by increased blood haemoglobin and mean
corpuscular haemoglobin content but decreased haematocrit, platelet
number, and erythrocyte number. Boron supplementation after depletion
also enhanced the elevation in serum 17ß-estradiol and plasma copper
caused by estrogen therapy, altered electroencephalograms such that
they suggested improved behavioural activation (e.g. less drowsiness)
and mental alertness, and improved processes of attention and memory.
Two hypotheses have appeared to account for the multiple effects
described above. One hypothesis is that boron has a role in cell
membrane function, stability, or structure, such that it influences
the response to hormone action, transmembrane signalling, or
transmembrane movement of regulatory cations or anions (Nielsen,
1991); a similar hypothesis has also appeared for the functional role
of boron in plants (Parr & Loughman, 1983; Blaser-Grill et al., 1990;
Blevins & Lukaszewski, 1994). The other hypothesis is that boron is a
negative regulator that influences a number of metabolic pathways by
competitively inhibiting some key enzyme reactions (Hunt, 1994).
Regardless of the fact that the function of boron remains
undefined, boron is becoming recognized as an element of potential
nutritional importance because of the findings from human and animal
studies. The latest report on trace elements in human nutrition and
health published by the World Health Organization (WHO, 1996)
suggested that an individual mean basal requirement for adults may be
about 0.375 mg/day, and the minimum mean population intake that meets
basal needs could be about 0.75 mg/day for adults. The report also
indicated that an acceptable safe range of population mean intakes for
boron for adults could well be 1-13 mg/day.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Several investigators have studied the effects of borates on
bacteria, protozoa, and algae (see Table 18). Effect concentrations
for the bacterium Pseudomonas putida range widely. No observed
effects were noted after 72 h at 291 mg boron/litre by Schöberl &
Huber (1988), whereas Guhl (1996) found a 16-h EC10 of 7.6 mg
boron/litre. Guhl (1996) and Bringmann & Kuhn (1980) reported 30-min
EC10 and 72-h EC3 values of 340 and 290 mg boron/litre,
respectively. Twenty per cent light loss, compared with controls, from
Photobacterium phosphoreum was determined to occur after 30 min at
18 mg boron/litre (Guhl, 1996). Nitrogen-fixing cyanobacteria require
boron for proper functioning of the heterocyst cell wall (Bonilla et
al., 1990). Mateo et al. (1986) concluded that boron is essential for
nitrogen fixation in Anabaena.
A wide range of effect concentrations was reported for two
protozoan species. Bringmann & Kuhn (1980) reported a 72-h EC3 of 0.3
mg boron/litre for Entosiphon sulcatum, whereas Guhl (1996) showed a
72-h NOEC of >10 mg boron/litre. Reproduction of Paramecium
caudatum was completely inhibited at 70 mg boron/litre (Bringmann &
Kuhn, 1980), and no effects were observed at 18 mg boron/litre by
Sprague (1972).
De Jong (1965) determined the sensitivity of another green alga,
Chlorella vulgaris, to borax concentrations ranging from 0.0001 to
30% of the medium. Inoculated cultures were cultivated at room
temperature in daylight for 3-4 months. The highest concentration of
borax tolerated by C. vulgaris was 0.6 mg boron/litre, and the
lowest concentration inhibiting growth was 1.2 mg boron/litre.
Concentrations above 50 mg boron/litre induce the formation of giant
cells in Chlorella pyrenoidosa as a result of a stronger inhibition
of the formation of daughter cells compared with the synthesis of
biomass. For cells grown in 100 mg boron/litre, cell division was
severely inhibited for the first 72 h of exposure. During this time,
photosynthesis was inhibited by only 47%. The protein and nucleic acid
content of the giant cells increased and nitrate uptake was also
enhanced, whereas the lipid content decreased. The authors therefore
concluded that the delay in cell division is probably a direct effect
of boron on cytokinesis, as nuclear division is unaffected (Maeso et
al., 1985).
Martinez et al. (1986) observed that boric acid caused a decrease in
growth rates, protein content, and chlorophyll content in the
blue-green alga Anacystis nidulans, following exposure to 75 and 100
mg boron/litre as boric acid. The photosynthetic pigments completely
disappeared after 72 h of exposure. Nitrate uptake was also lowered.
Table 18. Borate toxicity to bacteria, protozoa, and algae
Species Duration End-point Parameter Borate concentration Reference
in solution
(mg boron/litre)
Pseudomonas putida 30 min Growth inhibition EC10 340 Guhl (1996)
16 h Growth inhibition EC10 7.6 Guhl (1996)
72 h Growth inhibition NOEC 291 Schöberl & Huber (1988)
72 h Growth inhibition EC3 290 Bringmann & Kuhn (1980)
Photobacterium phosphoreum 30 min Light loss EC20 18 Guhl (1996)
Entosiphon sulcatum 72 h Growth inhibition EC3 0.3 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC >10 Guhl (1996)
Paramecium caudatum 72 h Growth inhibition EC100 <70 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC 18 Sprague (1972)
Algae, Scenedesmus quadricauda 8 days Growth inhibition EC3 0.16 Bringmann & Kuhn (1978)
Algae, Scenedesmus subspicatus 72 h Growth inhibition EC0 10 Guhl (1996)
72 h Growth inhibition EC10 24 Guhl (1996)
72 h Growth inhibition EC50 34 Guhl (1996)
72 h Growth inhibition EC100 100 Guhl (1996)
Algae, Scenedesmus subspicatus Chronic Growth inhibition EC10 24 Kopf & Wilk (1995)
Chronic Growth inhibition EC50 52 Kopf & Wilk (1995)
Chronic Growth inhibition EC90 109 Kopf & Wilk (1995)
Algae, Microcystis aeruginosa 8 days Growth inhibition EC3 20.3 Bringmann & Kuhn (1978)
An accumulation of carbohydrates was observed, probably because the
loss of the photosynthetic pigments inhibited their degradation.
Lewin (1966) concluded that borate is an essential micronutrient for
growth in marine diatoms, whereas Smyth & Dugger (1981) concluded that
boron is essential for Cylindrotheca fusiformis. Antia & Cheng
(1975) studied the effects of boric acid on the growth of 19 species
(10 classes) of marine phytoplankton. Axenic cultures in a
nutrient-enriched, pH-controlled seawater (33% salinity) medium were
exposed to the following concentrations of boric acid: 0, 5, 10, 50,
or 100 mg boron/litre. Growth measurements were taken every 2-4 days
by determining the optical density at 600 nm after vortex mixing.
Concentrations of 5 and 10 mg boron/litre did not inhibit the growth
of any species tested, with 10 mg boron/litre actually stimulating
growth in the blue-green alga Anacystis marina, the diatom
Skeletonema costatum, and the cryptomonad Rhodomonas lens. Growth
was strongly inhibited in 26% of the species at 50 mg boron/litre
(e.g. green alga Tetraselmis maculata; haptophyte Emiliania
huxleyi; diatom Phaeodactylum tricornutum) and in 63% of the
species at 100 mg boron/litre (e.g. chrysophyte Monochrysis
lutheri). The highest concentration was also lethal to 37% of the
species (e.g. diatom Cyclotella cryptica; dinoflagellate
Amphidinium carteri). Some species (e.g. green alga Monallantus
salina) required an adaptation period, with growth imperceptible
until 34-36 days after inoculation. Sequential transfer tests showed
that many of the initially inhibited species recovered after exposure
to 50 mg boron/litre, but not after exposure to 100 mg boron/litre.
The authors concluded that higher concentrations would be expected to
cause species redistribution, favouring growth of some forms and
suppressing growth of others.
A few studies have investigated the effects of boron on microorganisms
in sewage treatment plants. A boron concentration of 20 mg/litre had
no effect on activated sewage treatment (Gerike et al., 1976), and
boron levels below 200 mg/litre resulted in no significant inhibition
of anaerobic sludge digestion (Butterwick et al., 1989).
9.1.1.2 Soil
Information concerning the effects of boron on soil microorganisms is
scarce. Butterwick et al. (1989) reported results of a study in which
actinomycetes were found to undergo genetic transformations at boron
concentrations of 1000-6000 mg/litre. Bowen & Gauch (1966) exposed the
fungi Saccharomyces cerevisiae, Neurospora crassa, Aspergillus
niger, and Penicillium chrysogenum to boron. They found that
growth was significantly reduced at boron concentrations of 50, 250,
1300, and 4000 mg/litre for the four species, respectively. The
authors concluded that boron was not essential for any of the species
tested.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Nobel (1981) studied the effect of several boron compounds on
photosynthesis in submerged macrophytes, watermilfoil (Myriophyllum
alterniflorum), buttercup (Ranunculus penicillatus), and waterweed
(Elodea canadensis). The watermilfoil and buttercup (four
plants/concentration) were exposed to the following concentrations of
boric acid for 28 days: 0, 1, 2, 5, or 10 mg boron/litre. The
waterweed (eight plants/concentration) was exposed to the following
concentrations of boric acid for 21 days -- 0, 1, 2, 5, 10, or 250 mg
boron/litre -- and also to the following boron compounds at a
concentration of 2 mg boron/litre: boron trioxide, sodium perborate,
sodium metaborate, and borax. The test water was characterized as an
oligotrophic calcium-deficient nutritive medium. Net photosynthesis
was measured weekly as a function of the dissolved oxygen content.
Significant reductions (p = 0.01) in photosynthesis compared with
controls were observed at 2 mg boron/litre in the buttercup and
waterweed and at 5 mg boron/litre in the watermilfoil. The LC50
values for each species were as follows: 5 mg boron/litre for the
waterweed and the watermilfoil and 10 mg boron/litre for the
buttercup. Boron trioxide did not adversely affect photosynthesis in
the waterweed. The toxicity of the other boron compounds in the
waterweed at 2 mg boron/litre exhibited the following trend: borax >
metaborate > perborate > boric acid. Because calcium is known to
inhibit the uptake of boron in plants and, therefore, to mitigate the
damaging effects (Reeve & Shive, 1944), the authors concluded that the
toxic effects of boron in macrophytes are more pronounced in soft
(low-calcium) water.
In phytoassays conducted by Wang (1986), boron at concentrations up to
60 mg/litre did not significantly affect the growth of duckweed
(Lemna minor), expressed by the increase in frond number. In
addition, a concentration of 40.3 mg boron/litre added as a
tetraborate salt led to 50% inhibition of root growth in the
freshwater Eurasian watermilfoil (Myriophyllum spicatum) after 32
days of treatment (Stanley, 1974).
A pot study was carried out on the reed Phragmites australis during
the growth seasons of 1992 and 1993. Both soil culture in the presence
of free water and a gravel hydroculture with graduated repeated
additions of boron (as boric acid) were used during the growth seasons
to maintain the water phase at 0, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and,
in the case of the hydroculture, even 32.0 mg boron/litre. The number
of stalks per pot, height growth of the plants, and the yield of dry
substance of leaves, stalks, and roots at post-harvest time were
determined. The boron content in leaves, stalks, and roots, the
appearance of boron toxicity symptoms in leaves, and differences in
growth at each of the exposure concentrations were monitored. It was
concluded from the study that Phragmites australis can tolerate a
relatively high boron content (up to 4 mg boron/litre) in the liquid
nutrient substrate; for a period of 2-3 months, even 8 mg/litre was
tolerated without noticeable damage. Boron toxicity symptoms in the
leaves of Phragmites australis arose first at leaf boron
concentrations of around 150-180 mg/kg dry weight; however, no adverse
effects on growth, development, or dry substance yields of the plants
could be established at these leaf boron concentrations. Long-term
exposure to 8 mg boron/litre in waters would lead to symptoms of
damage, growth reductions, and yield reductions of plants (Marks et
al., 1994; Bergmann et al., 1995).
9.1.2.2 Invertebrates
1) Freshwater
Acute toxicity
Summaries of the median response toxicity data for aquatic
invertebrates are given in Table 19. These studies are also described
in detail in the following text.
Gersich (1984) studied the acute toxicity of boric acid to Daphnia
magna. Static (48-h) tests were conducted following the methodology
approved by the American Society for Testing and Materials. Three
replicates of 10 neonates each were exposed to seven nominal
concentrations: 0 (control), 54, 91, 151, 252, 420, or 700 mg
boron/litre. The 48-h LC50 for D. magna was 133 mg/litre as boron,
with a 95% confidence interval of 115-153 mg/litre, calculated by
probit analysis. All test organisms died at 420 mg/litre, 0% mortality
was observed at 54 mg/litre, and control mortality averaged 7%.
Lewis & Valentine (1981) also investigated the toxicity of boric acid
to D. magna. The investigators followed US EPA-approved procedures
and exposed <24-h-old neonates to five nominal test concentrations
(not given in paper). The 48-h LC50, calculated using probit
analysis, was determined to be 226 mg/litre as boron (95% confidence
interval of 200-246 mg/litre). The no-kill (0% mortality)
concentration was <200 mg/litre. Comparison of these results with
those of Gersich (1984) indicates a factor of 1.7 difference in the
48-h LC50 values; however, Canton & Adema (1978) report that a
difference of 2.0 may be normal in LC50 values not determined at the
same time or under similar conditions.
A few studies have been conducted to assess the toxicity of the sodium
borates to D. magna. As part of the study conducted by NAPM (1974),
the effects of sodium tetraborate pentahydrate on D. magna were
investigated. Static (96-h) tests were performed in which 10-20
organisms per test were exposed to the following nominal
concentrations of sodium tetraborate pentahydrate: 0, 18, 33, 58, 102,
or 182 mg/litre. The highest test concentration resulted in only 26.7%
mortality at 96 h; consequently, the 96-h LC50 for D. magna was
>182 mg sodium tetraborate pentahydrate/litre (>27 mg boron/litre).
Table 19. Median response concentrations for aquatic invertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
ARTHROPODA
Crustacea - Daphnidae
Daphnia magna Boric acid S, N 133.0 Gersich (1984)
Daphnia magna Boric acid S, N 226.0 Lewis & Valentine
(1981)
Daphnia magna Borax S, N 73.0 Bringmann & Kuhn
(1977)
Daphnia magna Anhydrous borax S, N <52.0 Threshold concentration,
based on immobilization Anderson (1946)
Daphnia magna Sodium tetraborate S, N >182.0 Mortality 26.7% at 96 h NAPM (1974)
pentahydrate
Daphnia magna Sodium tetraborate S, N 141 Maier & Knight (1991)
Crustacea - Limnoriidae
Limnoria lignorum Borax S, N 28.35 After 5-day recovery period Robinson & Perkins
(1977)
Insecta - Chironomidae
Chironomus decorus Sodium tetraborate S, N 1376 Maier & Knight (1991)
MOLLUSCA
Gastropida - Onchidiidae
Onchidoris fusca Borax S, N <28.35 After 5-day recovery period Robinson & Perkins
(1977)
a S = static test; N = nominal concentration.
Bringmann & Kuhn (1977) reported a 24-h LC50 of 340 mg disodium
tetraborate (anhydrous borax)/litre (73 mg boron/litre) for
D. magna in static tests using tap-water free of chlorine. The LC0
was 61 mg disodium tetraborate/litre (13 mg boron/litre), and the
LC100 was 1930 mg disodium tetraborate/litre (415 mg boron/litre).
Anderson (1946) obtained similar results with D. magna, reporting a
threshold concentration based on immobilization of <240 mg anhydrous
borax/litre (<52 mg boron/litre) at 48 h. Maier & Knight (1991)
reported a 48-h LC50 value of 141 mg boron/litre (95% confidence
limits 123-159 mg boron/litre) for D. magna exposed to sodium
tetraborate. Increasing water hardness and sulfate concentrations did
not affect the toxicity of boron to D. magna. In this study, boron
was much more toxic to daphnids than to Chironomus decorus
4th-instar larvae, for which the 48-h LC50 was 1376 mg boron/litre
(95% confidence limits 1298-1453 mg boron/litre) (Maier & Knight,
1991).
Tubificid worms (Tubifex sp.) appear to be less sensitive than
D. magna to sodium borate, with concentrations of sodium borate
decahydrate (borax) as high as 750 mg/litre (85 mg boron/litre)
causing no effect after a 24-h exposure. The 24-h LC100 was reported
as 2000 mg/litre (227 mg boron/litre). The tubificid also exhibited
less sensitivity to boric acid, with a no-effect level at 24 h of
exposure of 7500 mg/litre (1311 mg boron/litre) and an LC100 of 10 000
mg/litre (1748 mg boron/litre) (Mann, 1973).
In an early study, Fay (1959) determined the effects of metaboric acid
(B2O3.H2O) on various life stages of three species of mosquitos:
Aedes aegypti, Anopheles quadrimaculatus, and Culex
quinquefasciatus. A concentration of 8000 mg/litre (1973 mg
boron/litre) did not affect hatching of A. aegypti eggs following
72 h of exposure. However, newly hatched larvae of
C. quinquefasciatus and A. quadrimaculatus were quite sensitive to
metaboric acid. Complete mortality of C. quinquefasciatus larvae was
observed after 24 h of exposure to 2000 mg metaboric acid/litre (493
mg boron/litre). Complete mortality of A. aegypti was observed after
24 h of exposure to 4000 mg/litre (986 mg boron/litre), with complete
mortality occurring in A. quadrimaculatus only at the 8000 mg/litre
(1973 mg boron/litre) level. In contrast, at the 2nd- and 3rd-instar
stage, A. quadrimaculatus was more sensitive than the other species.
In 2nd-instar larvae, 100% mortality occurred at 500 mg/litre (123 mg
boron/litre) in A. quadrimaculatus, 96% mortality occurred at
1500 mg/litre (370 mg boron/litre) in A. aegypti, and 100% mortality
occurred at 2000 mg/litre (493 mg boron/litre) in
C. quinquefasciatus. The 3rd-instar larvae were less sensitive than
the 2nd-instar larvae, with 100% mortality of A. quadrimaculatus at
2500 mg/litre (617 mg boron/litre), 88% mortality of A. aegypti at
3000 mg/litre (740 mg boron/litre), and 48% mortality of
C. quinquefasciatus at 4000 mg/litre (986 mg boron/ litre). Pupae of
the three species were even less sensitive to metaboric acid,
requiring concentrations ranging from 6000 mg/litre (1480 mg
boron/litre) to 16 000 mg/litre (3946 mg boron/litre) to prevent
emergence to the adult stage. From the information provided in this
study, it appears that the 2nd-instar larva was the life stage that
was most sensitive to exposure to metaboric acid.
Fay (1959) also studied the effects of prolonged exposure to boron
trioxide (B2O3.H2O) on the immature stages of the same three
species of mosquitos. Newly hatched larvae were reared in the
following concentrations of metaboric acid to determine the percentage
of successful adult emergence: 10, 25, 50, 100, or 250 mg/litre. No
significant reduction in maturation was observed in A. aegypti or
C. quinquefasciatus at any test concentration <100 mg/litre.
However, at 50 mg/litre, only 2% of the larvae of A.
quadrimaculatus reached the adult stage. At 250 mg metaboric
acid/litre, only 1% of A. aegypti and only 3% of
C. quinquefasciatus reached the adult stage.
Maier & Knight (1991) evaluated the chronic sublethal effects of boron
to Chironomus decorus larvae. A significantly decreased growth rate
over a 96-h period was observed at 20 mg boron/litre.
Chronic toxicity
Gersich (1984) conducted a 21-day static renewal chronic toxicity test
with D. magna. Daphnids (20 organisms/concentration, or four
replicates with 5 organisms/replicate) were exposed to the following
nominal concentrations of boric acid: 0, 7, 14, 28, 56, or 105
mg/litre as boron. The test concentrations remained stable during
testing; the mean measured concentrations ranged from 91.4 to 106% of
the nominal test concentrations. The 21-day LC50, calculated using
the moving average method, was 52.2 mg boron/litre, and the 95%
confidence interval was 42.6-66.7 mg boron/litre. No mortality was
observed in the control group. The mean number of broods per daphnid,
mean total young per daphnid, mean brood size per daphnid, and mean
size differed significantly (alpha = 0.05) from the control at the
13.6 mg boron/litre concentration. Therefore, the maximum acceptable
toxicant concentration (MATC) was determined based on the most
biologically and statistically significant end-points, reproduction
and growth. The MATC for boric acid was estimated to be between 6.4
and 13.6 mg boron/litre, or 9.3 mg boron/litre (the geometric mean of
these two values).
Lewis & Valentine (1981) also conducted a 21-day static renewal
chronic toxicity test with D. magna. The test was conducted
similarly to that of Gersich (1984), except for the exposure regimen.
Ten test chambers were set up for each test concentration: three
beakers contained five daphnids each for obtaining data on survival,
and seven beakers each contained one daphnid for obtaining data on
survival, growth, and reproduction. Twenty control chambers were used:
6 chambers contained five daphnids each, and 14 chambers each
contained one daphnid. Daphnids were exposed to the following nominal
concentrations of boric acid: 0, 6, 13, 27, 53, or 106 mg/litre as
boron. The concentration of the test solution remained stable during
testing, with mean measured concentrations exceeding 95% of the
nominal concentration. The 21-day LC50, calculated by probit
analysis, was 53.2 mg boron/litre, with a 95% confidence interval of
44.1-64.5 mg boron/litre. Mortality was 9% in the control group. The
mean brood size and total number of young produced were significantly
reduced (p < 0.05) compared with controls at concentrations of
>13 mg boron/litre. Mean length of daphnids was significantly reduced
(p < 0.05) compared with controls at 53 mg boron/litre. The NOEC
was 6 mg boron/litre. Therefore, based on the most sensitive
parameters, the MATC was >6 and <13 mg boron/litre, or 8.83 mg
boron/litre (geometric mean of these values).
2) Marine
In static tests, Mann (1973) determined 24-h LC100 values for the
amphipod Gammarus tigrinus exposed to boric acid, borax, and sodium
perborate at two salinities (32.23% and 17.66%). The LC100, 10 000
mg/litre, was the same for all three compounds at both salinities. The
no-effect concentration for all of the compounds was 7500 mg/litre. In
contrast, Robinson & Perkins (1977) reported toxicity values for borax
in the isopod Limnoria lignorum and the sea slug Onchidoris fusca
that are roughly two orders of magnitude lower than those reported by
Mann (1973) for the amphipod Gammarus tigrinus. The salinity of the
test water was 30%. The 24-h static LC50 values, after a 5-day
recovery period, were 250 mg/litre (28.35 mg boron/litre) for the
isopod and >250 mg/litre (>28.35 mg boron/litre) for the sea slug.
In studies with sea urchin (Anthocidaris crassispina) embryos,
exposure to boric acid at a concentration of 37 mg boron/litre had no
effect on development, whereas exposure to 75 mg boron/litre was fatal
(Eisler, 1990).
3) Biocenosis studies
Three biocenosis studies (also known as microcosms, mesocosms, and
experimental ecosystems) have been conducted (summarized in ECETOC,
1997). A 28-day laboratory microcosm test with microorganisms
consisting of six trophic stages yielded a NOEC and LOEC of 2.5 and
5.0 mg boron/litre, respectively. Studies with outdoor ponds
containing up to 29 species and treated with 0.7 mg boron/litre over a
2-year period resulted in no significant differences compared with
untreated control ponds. Further, field studies carried out in seven
different water bodies subjected to different levels of anthropogenic
boron inputs yielded no toxic effects of boron at concentrations
between 0.16 and 1.52 mg/litre.
9.1.2.3 Vertebrates
1) Freshwater
Acute toxicity
Summaries of the acute toxicity data for aquatic vertebrates are given
in Table 20. These studies are also described in detail in the
following text.
For boron compounds, three studies are available that provide
information concerning the 96-h LC50 values, the toxicity value
established by the US EPA (Stephan et al., 1985) for use in the
derivation of water quality criteria for the protection of aquatic
life and its uses. Wallen et al. (1957) conducted 96-h static toxicity
tests with mosquitofish. The test water, turbid pond water, was
selected to simulate the commonly turbid wastewater from oil refinery
operations; however, it was determined that the highest turbidities
used in the study should not produce direct effects on the fish during
the exposure time. Adult female fish (10/concentration) were exposed
to the following nominal concentrations of boric acid or borax: 10,
18, 32, 56, or 100 mg/litre. If no deaths occurred at 96 h, the tests
were rerun at concentrations of 100, 180, 320, 560, or 1000 mg/litre
and, subsequently, at concentrations 10 times these values
(1000-10 000 mg/litre). The 96-h LC50 for mosquito-fish exposed to
boric acid was 5600 mg/litre (979 mg boron/litre), and the 96-h LC50
for borax was 3600 mg/litre (408 mg boron/litre).
As part of the study conducted by NAPM (1974), static bioassays were
conducted to determine the toxicity of sodium tetraborate pentahydrate
to fathead minnows. The tests were conducted using standard methods
and filtered tap-water. Fish (15/treatment) were exposed to the
following nominal concentrations: 0, 200, 360, 640, 1120, or 2000
mg/litre. The 96-h LC50 was 1900 mg/litre (332 mg boron/litre).
Hamilton & Buhl (1990) reported the results of 96-h static toxicity
tests with two life stages of chinook salmon (Oncorhynchus
tshawyts-cha) and coho salmon (O. kisutch). Swim-up fry
(8-12 weeks old) were tested in fresh water, and advanced fry (15-21
weeks old) were tested in brackish water. The 96-h LC50 values for
chinook salmon swim-up fry and advanced fry were 725 mg/litre (127 mg
boron/litre) and 600 mg/litre (105 mg boron/litre), respectively. The
96-h LC50 values for coho salmon swim-up fry and advanced fry were
447 mg/litre (78.1 mg boron/litre) and 600 mg/litre (105 mg
boron/litre), respectively.
Results of 24- and 48-h static acute tests with rainbow trout,
guppy (Poecilia reticulata), and mosquitofish clearly show the
difference in the degree of toxicity resulting from exposure to boric
acid versus sodium borate. Mann (1973) reported the following 24-h
LC100 values for rain-bow trout and guppy, respectively: boric acid,
10 000 mg/litre (1748 mg boron/litre) and 7500 mg/litre (1311 mg
boron/litre); borax, 5000 mg/litre (567 mg boron/litre) for both
species. Static 24-h LC50 values determined by Wallen et al. (1957)
for the mosquitofish are 18 000 mg/litre (3146 mg boron/litre) for
boric acid and 12 000 mg/litre (1361 mg boron/litre) for borax. The
48-h LC50 values determined in this study were 10 500 mg/litre (1835
mg boron/litre) for boric acid and 8200 mg/litre (930 mg boron/litre)
for borax. Rainbow trout are more sensitive to borax than
mosquitofish, with 24- and 48-h static LC50 values of 2800 mg/litre
(602 mg boron/ litre) and 1800 mg/litre (387 mg boron/litre),
respectively (Alabaster, 1969). Juhnke & Ludemann (1978) reported an
LC50 of 807 mg/litre as boron for golden orfe (Leuciscus idus)
Table 20. Acute toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Coho salmon Boric acid S, N 78.1 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus kisutch) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Chinook salmon Boric acid S, N 127 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus tshawytscha) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Rainbow trout Borax S, N 602 387 Alabaster (1969)
(Oncorhynchus mykiss) Borax FT, N 65 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 150 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Borax FT, N 88 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 100 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Fathead minnow Sodium S, N 332 NAPM (1974)
(Pimephales promelas) tetraborate
pentahydrate
Mosquitofish Borax S, N 1361 930 408 Wallen et al. (1957)
(Gambusia affinis) Boric acid S, N 3146 1835 979 Wallen et al. (1957)
Golden orfe 173 Schöberl & Huber (1988)
(Leuciscus idus)
Table 20. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Zebra fish Borax 14.2 Guhl (1996)
(Brachydanio rerio)
Bluegill Borax S, N 4.6 Turnbull et al. (1954)
(Lepomis macrochirus)
Dab Anhydrous borax Semi-static, 88.3 74 Salinity 34.62 ± 0.2% Taylor et
(Limanda limanda) N al. (1985)
Colorado squaw-fish Boric acid 279b Hamilton (1995)
(Ptychocheilus lucius) >100c
527d
Razorback sucker Boric acid 233b Hamilton (1995)
(Xyrauchen texanus) 279c
>100d
Bonytail Boric acid 280b Hamilton (1995)
(Gila elegans) >100c
552d
a S = static test; FT = flow-through test; N = nominal concentration.
b Swim-up fry.
c 1-g juvenile.
d 2-g juvenile.
exposed to anhydrous borax in static acute tests (exposure time not
given).
Mann (1973) reported 24-h LC100 values for rainbow trout and
guppies exposed to sodium perborate (500 mg/litre or 66.1 mg
boron/litre) that were considerably lower than those reported for
boric acid and borax. The authors attributed this increased toxicity
to a pH shift into the alkaline range (up to 9.1), which resulted in
increased mucilage formation, with the fish exhibiting a crippling
behaviour.
The treatment of embryos of the toad Bufo vulgaris with 0.5%
boric acid for 24 h from the two-cell stage to the tail-bud stage
resulted in several developmental malformations, including a reduction
or lack of external gills, short tail, bifurcation of the epiphysis,
suppression of forebrain development, and inhibition of sensory organ
development. Several of these effects were attributed to the direct
action of boric acid on the ectoderm (Takeuchi, 1958).
Chronic toxicity
Toxicity of borate to early life stages of fish has been
documented for several species (Birge & Black, 1977; Black et al.,
1993). Embryonic and early larval stages of rainbow trout, largemouth
bass, channel catfish, and goldfish were exposed to boron, as boric
acid or borax, from fertilization up to 8 days post-hatch. All
exposures were in soft (50 mg CaCO3/litre) or hard (approx. 200 mg
CaCO3/litre) reconstituted water. Test responses included embryonic
mortality, teratogenesis, and larval mortality. Gross debilitating
anomalies of survivors were classified as mortalities. Effect
concentrations (LC50, NOEC, and LOEC) for each species are presented
in Table 21. Neither water hardness nor the form of boron (boric acid,
borax) added to the test aquaria consistently affected embryo-larval
survival of rainbow trout, channel catfish, and goldfish (Birge &
Black, 1977).
On the basis of median lethal concentrations (LC50), no species
was found to be especially sensitive. The range of LC50s for all
species was 12.2-235 mg boron/litre. In addition, Birge & Black (1977)
reported LC1s ranging from 0.001 to 0.1 mg boron/litre for rainbow
trout, from 0.2 to 5.5 mg boron/litre for channel catfish, and from
0.2 to 1.4 mg boron/litre for goldfish. The LC1 showed rainbow trout
to be the most sensitive species. The NOEC ranged from 0.009 to 0.103
mg boron/litre for rainbow trout and was 1.39 mg boron/litre for
large-mouth bass. These were consistent with the acute toxicity
results that indicated rainbow trout and zebra fish as the most
sensitive species (see Table 20).
The effect of natural dilution water on boron toxicity was
reported by Black et al. (1993). Natural surface waters were collected
from three US locations: the Erwin National Fish Hatchery in
Tennessee, Brookville Lake in Indiana, and Firehole River in
Yellowstone National Park, Wyoming. Surface water control boron
Table 21. Chronic toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Coho salmon Sodium metaborate S, N 113 Alevins, 283-h exposure Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate
Coho salmon Sodium metaborate S, N 12.2 Underyearlings, 283-h exposure, Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate salinity 28 ± 1%
Rainbow trout Borax FT, N 27 Alevin, 28-day exposure, Birge & Black (1977)
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre
Rainbow trout Boric acid FT, N 100 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Borax FT, N 54 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 79 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 138 Embryo/larva, 32-day exposure Black et al. (1993)
(Oncorhynchus mykiss)
Rainbow trout Boric acid 138 Hardness of 200 mg CaCO3/ litre;
(Oncorhynchus mykiss) duration 32 days
NOEC = 0.009 mg boron/litre
LOEC = 0.1 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid 92 Hardness of 200 mg CaCO3/ litre;
(Micropterus salmoides) duration 11 days
NOEC = >1.39 mg boron/litre
LOEC = 12.17 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid FT, N 92 Embryo/larva, 11-day exposure Black et al. (1993)
(Micropterus salmoides)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Goldfish Borax FT, N 71.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 178.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 68.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 170.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 65.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 46.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 59.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 75.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 235.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 220.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 120.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Channel catfish Boric acid FT, N 102.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 71.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 22.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 54 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 157 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 60 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 145 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 47 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 130 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Leopard frog Borax FT, N 54 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 135 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 148 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 135 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 145 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 123 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
a S = static test; FT = flow-through test; N = nominal concentration.
concentrations were 0.023, 0.091, and 0.75 mg/litre for Erwin,
Brookville, and Yellowstone waters, respectively. Total organic carbon
for the three surface waters ranged from 0.8 to 1.9 mg/litre.
Hardness levels for these natural waters ranged from 24 to 209 mg
CaCO3/litre. In the surface water tests, three nominal treatments
were used: surface water control, control plus 1.0 mg boron/litre
added (as boric acid), and control plus 10 mg boron/litre added. Total
and dissolved boron levels were measured for each of the surface water
treatments. No statistically significant differences were noted
between total and dissolved boron concentrations, except for
Yellowstone (1.0 mg boron/litre) and Erwin (10 mg boron/litre)
treatments. The true significance of this, however, is minimal, as
total versus dissolved concentrations for Yellowstone and Erwin waters
were 1.61 vs. 1.52 mg boron/litre and 9.91 vs. 9.48 mg boron/litre,
respectively. Hence, it is reasonable to assume that all boron was in
solution. Boron did not elicit toxicity to embryo-larval stages of
rainbow trout exposed to surface water control boron levels up to 0.75
mg/litre. LOECs for Erwin, Brookville, and Yellowstone treatments were
1.10, 1.24, and 1.73 mg boron/litre, respectively, indicating that the
threshold for no effects is approximately 1 mg boron/litre. In
addition, deep (600 m) well-water, typically used for aquatic toxicity
tests, from a contract laboratory located in Wareham, Massachusetts,
USA, was also used. Boron was spiked into well-water to obtain an
exponential series of nominal concentrations: 0.0017, 0.017, 0.17,
1.7, and 17 mg boron/ litre, respectively. Analytical confirmation of
exposure was obtained for the two highest concentrations, 2.1 and
18.0 mg boron/litre. No effects were observed, even at the highest
boron concentration.
The effect concentrations generated in natural waters were
greater than those from the reconstituted water experiments. The cause
of these differences is not readily apparent, particularly as
bioavailability was not reduced by natural water. However, some
investigators (Belanger et al., 1989; Farris et al., 1994) have shown
that organisms cultured in natural water often grow and reproduce
better than their counterparts in reconstituted or laboratory
dechlorinated water. Therefore, a reasonable hypothesis that may
explain, in part, the differences in reconstituted versus natural
water responses is the better health experienced by organisms exposed
to boron in natural water. The causative agents for this lack of
health in laboratory water are not certain but may include nutrient
deficiencies (Keating & Dagbusan, 1984; Belanger et al., 1989).
Early life stage tests have been conducted with fathead minnows
to determine effects of chronic exposure (30-60 days) to boric acid.
The 30-day NOEC and LOEC were 14 and 24 mg boron/litre, respectively,
based on growth reduction; the 60-day NOEC and LOEC were 24 and 88 mg
boron/litre, respectively, based on reduction in fry survival
(Butterwick et al., 1989).
Birge & Black (1977) also studied the effect of boric acid and
borax on embryos and larvae of the leopard frog (Rana pipiens) and
the effect of boric acid on embryos and larvae of the Fowler's toad
(Bufo fowleri). The embryos were exposed for 3.5 days and the larvae
for 7.5 days to concentrations of boric acid and borax ranging from
0.05 to 300.00 mg boron/litre in hard (200 mg CaCO3/litre) and soft
(50 mg CaCO3/litre) water (as described above for fish exposures).
The leopard frog and Fowler's toad were equisensitive to borax and
boric acid. For the leopard frog, LC1 and LC50 values were 13 and
130 mg boron/litre (dosed as boric acid) in soft water and 22 and 135
mg boron/litre in hard water. Lower toxicity values were observed via
borax exposures, with LC1 and LC50 values of 5 and 47 mg boron/litre
in soft water and 3 and 54 mg boron/litre in hard water, respectively.
The LC1 and LC50 values for Fowler's toad exposed to boric acid were
25 and 145 mg boron/litre in soft water and 5 and 123 mg boron/litre
in hard water, respectively.
2) Marine
Taylor et al. (1985) studied the toxicity of several metals,
including boron (as anhydrous borax), to a saltwater fish (dab,
Limanda limanda). The tests were conducted following standard
procedures under semi-static conditions, in which the test solution
was replaced at 24-h intervals. The salinity of the test water was
34.62 ± 0.2%. Adult fish obtained from wild populations
(20/concentration) were exposed to a series of five concentrations
(not given in the paper) for 96 h. The LC50, calculated by probit
analysis, was 74.0 mg boron/litre, with a 95% confidence interval of
66.4-83.0 mg boron/litre. Pre-death symptoms included minor
respiratory problems.
Similar results were observed in bioassays conducted by Thompson
et al. (1976) with two life stages of coho salmon. Coho alevins
(0.19-0.7 g) were tested in aerated well-water (fresh water), and coho
underyearlings (1.8-3.8 g) were tested in aerated seawater with a
salinity of 28 ± 1%. Test solutions were prepared using sodium
metaborate tetrahydrate (Na2B2O4.4H2O), and exposure lasted in
excess of 12 days. The 283-h LC50 for freshwater coho alevins was 113
mg boron/litre, with 95% confidence limits of 104-123 mg boron/litre.
The 283-h LC50 for saltwater coho underyearlings was 12.2 mg
boron/litre, with 95% confidence limits of 10.89-14.56 mg boron/litre.
The authors attributed the difference in toxicity in fresh water and
salt water to differences in fish age, test temperature (11°C in fresh
water; 8°C in salt water), and available boron levels. Seawater also
has higher background levels of boron than fresh water. The results
reported by Hamilton & Buhl (1990), discussed above, do not indicate
significant differences in acute toxicity between different life
stages of chinook and coho salmon.
In static tests, Mann (1973) observed differing sensitivities in
the common eel (Anguilla anguilla) to both boric acid and borax at
two salinities: 12.39 and 25.05%. At the lower salinity, a 24-h LC100
of >10 000 mg/litre was reported for both borax and boric acid; at
the higher salinity, the 24-h LC100 was 7500 mg/litre for both
compounds. The authors speculated that differences in pH could
contribute to the toxicity of the boron compounds at the higher
salinity. At a boric acid concentration of 7500 mg/litre, the pH in
the lower-salinity test water was 7.2; the pH in the higher-salinity
test water was 6.6. As was observed with rainbow trout and
mosquitofish, Mann (1973) also observed greater toxicity in the common
eel following exposure to sodium perborate. The LC100 at both
salinities was 750 mg/litre (99.2 mg boron/litre), resulting from the
damaging effects of an alkaline pH shift.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
1) Essentiality
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron plays a role in carbohydrate metabolism, sugar translocation,
pollen germination, hormone action, normal growth and functioning of
the apical meristem, nucleic acid synthesis, and membrane structure
and function (Lovatt & Dugger, 1984). Recent work shows important
involvement of boron in cell wall cross-linking, involving
complexation with specific pectin components (Loomis & Durst, 1992; Hu
et al., 1996).
Early investigation of the effects of boric acid and borax on the
fieldbean (Vicia faba) and other plants indicated the role of boron
in plant nutrition (Warrington, 1923). The first comprehensive study
of the effects of boron on plant growth was conducted by Eaton (1944).
Fifty species of plants were grown in sand cultures with standard
nutrient solutions containing the following boron concentrations:
trace (0.03-0.04), 1, 5, 10, 15, or 75 mg/litre. For each species,
Eaton (1944) recorded the boron concentration resulting in the best
growth, the lowest concentration resulting in injury, and the symptoms
of deficiency with trace boron levels. Approximately 82% of the plants
exhibited the best growth at concentrations from trace to 5 mg/litre,
and 35% of the plants grown in trace boron levels developed
morphological symptoms of deficiency. Eaton (1944) concluded that
there was overlap of the beneficial and injurious effects of boron
between species; therefore, three broad categories of tolerance
(sensitive, semi-tolerant, and tolerant) were established. The
tolerant plants endure a wide range of boron concentrations with
little effect, and the sensitive plants exhibit a strong reaction to
either too much or too little boron.
Boron generally occurs in soils at concentrations ranging from 10
to 30 mg/kg, depending on such factors as soil type, amount of
rainfall, and irrigation type (Sprague, 1972). Boron deficiencies
usually occur in soil solutions from sandy soils or acid peat soils,
soils derived from igneous rocks, soils low in organic matter, and
irrigated soils (Sprague, 1972).
The symptoms of boron deficiency in plants include cessation of
root and leaf growth, necrosis of leaf primordia and primary root
tips, necrosis of stem and leaf phloem, bark splitting, retardation of
enzyme reactions, reduced pollen germination, and even death (Versar,
Inc., 1975; Wells & Whitton, 1977). Normal growth will usually resume
if boron is added to the growth medium.
Hudak (1973) found that a boron-deficient nutrient solution
inhibited mitosis in the root tip of the fieldbean within 72 h. A 10
mg boron/litre solution produced optimum cell division and elongation
of the root tip; however, 50 mg boron/litre caused a reduction in
mitosis within 24 h.
Ludbrook (1942) studied the effects of boron deficiency and
toxicity in Monterey pine (Pinus radiata) seedlings grown in water
culture. The suboptimal concentrations of boron added to the nutrient
solution were 0, 0.005, 0.01, or 0.05 mg/litre, and the potentially
toxic concentrations of boron were 0.5, 2.5, 5.0, 10.0, 20.0, or 40.0
mg/litre. Seedlings exposed to the toxic levels were initially grown
for 2 months in a nutrient solution containing added boron at 0.5
mg/litre. Marked reduction in root growth was observed at the two
lowest suboptimal concentrations; however, improved root growth was
observed at 0.05 mg/litre, with the development of small lateral
roots. At 10.0 mg boron/litre, a slight yellowing of the tips of the
needles developed, followed by browning and drying at 20.0 mg/litre,
until all plants died at 40.0 mg/litre. Ludbrook (1942) concluded that
the optimum growth range for Monterey pine was 0.05-5.0 mg
boron/litre.
Recent work has shown that boron in a number of plant species is
tightly bound in the polysaccharide cell wall. Boron deficiency leads
to profound changes in cell wall morphology, suggesting that boron is
critical to cell wall expansion. It has been proposed that this
structural, cross-linking function of boron is involved with the
pectin fraction, which contains apiose and other hydroxylated
fragments amenable to complexation by borate (Loomis & Durst, 1992).
Fourteen species of crop plants were studied, and it was concluded
that high pectin content requires more boron for forming cell walls or
that pectin forms a tightly held boron complex that depletes boron
availability for other critical functions, thereby increasing the
overall demand for boron (Hu et al., 1996). Kobayashi et al. (1996)
have isolated and characterized a rhamnogalacturonan II/borate complex
from enzyme-digested cell wall pectin.
Toxicity
Boron excesses usually occur in soil solutions from geologically
young deposits, arid soils, soils derived from marine sediments, and
soils contaminated by pollutant sources, such as releases from
coal-fired power plants and mining operations (Sprague, 1972).
The initial symptom of boron toxicity in plants is chlorosis
(yellowing) of the leaf tip, progressing along the leaf margin and
into the blade. Necrosis of the chlorotic tissue occurs, followed by
leaf abscission. Necrosis of the leaf tissue results in a loss of
photosynthetic capacity, which reduces plant productivity (Lovatt &
Dugger, 1984). Pollen germination and pollen tube growth may also be
inhibited (Versar, Inc., 1975).
Several investigators have shown a direct relationship between
the boron content in leaves (foliar) and the severity of the symptoms
of toxicity. Gilliam & Watson (1981) conducted an experiment in which
Anderson yews (Taxus media) were grown in soil at four boron
concentrations (0.5, 5.0, 25.0, or 50 mg/kg). Symptoms of toxicity
were observed when foliar boron accumulation reached concentrations
ranging from 85 to 100 µg/g of dry tissue. The observed symptoms
included leaf tip yellowing, followed by necrosis and premature
defoliation. Suppression of shoot and root growth was observed at 50
mg boron/kg soil.
Shopova et al. (1981) found that concentrations of 16, 24, and
32 mg boron/kg soil resulted in a decline in plant development,
yellowing of leaves, late flowering, reduction of mitotic frequency in
root tip cells, and abnormalities during meiosis in the poppy
(Papaver somniferum).
Kluge & Podlesak (1985) found that symptoms due to boron excess
begin to develop on the leaves (leaf tip necroses) of pot-grown spring
barley (Hordeum vulgare) as soon as the boron content of the leaf
tissue reaches 60-80 mg/kg dry weight.
Gestring & Soltanpour (1987) grew alfalfa (Medicago sativa) in
three soil types amended with sodium borate at rates of 0, 10, 20, and
40 mg boron/kg. Alfalfa yield was significantly reduced by boron
application in both the sandy loam and loam soils; however, no yield
reduction was observed in the silt loam soil. Soil extractable boron
did not adequately assess boron toxicity, whereas plant boron levels
were a more reliable index of toxicity. The critical range of plant
boron resulting in a yield reduction was 850-975 mg boron/kg plant
tissue (dry weight). The significant difference between the soils
shows the importance of soil pH, organic matter content, and per cent
clay to the toxicity of boron to plants.
Sage et al. (1989) exposed the rare serpentine plant
(Streptanthus morrisonii) to boron (0, 20, 60, 240, 650, 1200, or
2400 µmol/litre) via watering. Plants showed mild to moderate toxicity
symptoms (older leaves exhibiting chlorosis and necrosis) at boron
concentrations of 240 and 650 µmol/litre. Severe toxicity symptoms
(significant leaf loss) were apparent at 1200 and 2400 µmol
boron/litre. Boron levels in the leaves of plants showing severe
toxicity were an order of magnitude higher than those in the leaves of
plants in the field.
Glaubig & Bingham (1985) reported significant linear
relationships between both soil and leaf tissue boron concentrations
and foliar damage in four tree species endemic to California (digger
pine, Pinus sabiniana; California laurel, Umbellularia
californica; madrone, Arbutus menziesii; bigleaf maple,
Acer macrophyllum). Foliar damage over 25% of the leaf/needle area
occurred at saturated soil concentrations ranging from 0.08 to
1.2 mmol boron/litre for the four species and at leaf/needle
concentrations ranging from 30 to 115 mmol boron/kg. The bigleaf maple
was the most sensitive of the four species, and the digger pine was
the most tolerant species.
Under experimental conditions, Shann & Adriano (1988)
demonstrated that chronic foliar aerosol exposures of boron produced
phytotoxicity in relation to boron accumulation in the leaves. Five
crop species (swiss chard, beet, radish, tomato, and cucumber) were
exposed to aerosol concentrations of 0, 1.55, or 3.09 µg boron/cm2
leaf area. The authors concluded that the visual damage (leaf tip
necrosis) resulting from aerosol exposure was identical to that
observed from root boron toxicity for all crops tested.
9.1.3.2 Invertebrates
Boric acid is an effective stomach poison for several species of
insects, including the German cockroach (Blattella germanica).
Pretreatment of wood and other substrates with boric acid or borax can
prevent insect infestation. Baits and aerosols containing boron
compounds (e.g. borax and boric acid) have been shown to control fruit
flies, cockroaches, houseflies, and termites (Butterwick et al.,
1989).
Boron in the diet at concentrations of 0.025-5% caused
sterilization of house flies (Musca domestica) when both sexes were
fed the diet (Borkovec et al., 1969). Boron toxicity in honey bees has
also been observed; a concentration of 50 mg boric acid/litre (8.7 mg
boron/litre) in syrup had no effect on survival, whereas 100 mg boric
acid/litre (17.5 mg boron/litre) caused 50% mortality (Ostrovskij,
1955).
Lang & Treece (1972) conducted a study to determine if boric acid
would induce sterility in the face fly (Musca autumnalis). Adult
male and female flies (50 each/concentration) were exposed to boric
acid concentrations of 0.5%, 0.8%, or 1.0% in each of three media
(water, malt, and dry food) for the first 4 days after eclosion
(hatching from the pupal stage). Complete sterility (100%) was
observed in flies fed the two highest concentrations in dietary malt;
however, a high degree of recovery was observed within 5 days after
cessation of treatment. Many eggs from the females fed 1.0% boric acid
were transparent and watery in appearance; this was possibly due to
the interference of boric acid with egg chorion formation. Almost 100%
mortality occurred in the flies exposed to boric acid concentrations
of 0.5% and 0.8% in water within the first 4 days of treatment. In a
subsequent test, erratic sterility with recovery of fertility was
observed in face flies exposed to boric acid concentrations of 0.1%
and 0.3% in water.
9.1.3.3 Vertebrates
In order to determine the impact of high levels of boron on
water-fowl in Kesterson Reservoir (located within the Kesterson
National Wildlife Refuge in the San Joaquin Valley of California,
USA), a number of investigators have studied the effects of controlled
boron exposure to mallard ducks. Smith & Anders (1989) studied the
effects on reproduction in mallards fed diets supplemented with 0, 30,
300, or 1000 mg boron/kg as boric acid (concentrations known to be
found in the field). The adults were fed this diet prior to mating and
during egg incubation, and the ducklings were fed this diet after
hatching. In the 1000 mg/kg group, hatching success of fertile eggs
and duckling survival were significantly reduced, and embryo mortality
was significantly higher. The body weight of hatchlings was lower in
the 300 and 1000 mg/kg groups than in controls. Growth rate of
ducklings was significantly reduced at all three dose levels. No
effect on egg fertility, eggshell thickness, or eggshell quality was
observed at any dose level. In addition, boron did not produce any
overt signs of toxicity in young or adult mallards. The researchers
were unable to determine whether duckling mortality and impaired
growth were due to pre-hatching or post-hatching exposure or the
combined effect.
Hoffman et al. (1990) examined the effect of dietary boron
exposure on the survival, growth, and physiology of ducklings hatched
from uncontaminated eggs. Day-old mallards were exposed to 0, 100,
400, or 1600 mg boron/kg diet as boric acid for 10 weeks. No
significant effect on survival was found. The highest dietary
concentration of boron significantly decreased overall growth and the
rate of growth (sexes combined), whereas lower concentrations altered
growth only in female ducklings. Significantly decreased food
consumption was observed in the high-dose group during the first
3 weeks and in all dose groups during the 2nd week. Effects on blood,
brain, and liver biochemistry were also observed in the high-dose
group. Overall, dietary boric acid proved to be less toxic to
ducklings hatched from untreated eggs than to ducklings hatched from
boron-contaminated eggs.
Boron has also been shown to affect the behaviour of mallard
ducklings. Whitworth et al. (1991) analysed the activity schedules and
behaviour durations of day-old ducklings that received diets
containing 0, 100, 400, or 1600 mg boron/kg as boric acid. The highest
dose level had significant effects on the activity schedules of the
ducklings, including increased time at rest. Ducklings exposed to 1600
mg/kg in the diet spent less time in alert behaviours and in the water
compared with controls.
These studies suggest that high dietary levels of boron can
adversely affect normal duckling development and survival. In
addition, interactive effects have been demonstrated in mallard
ducklings given both boron and selenium in the diet. Hoffman et al.
(1991) noted further growth reduction in ducklings fed diets with 60
mg selenium/kg (as seleno-methionine) and 1000 mg boron/kg (as boric
acid) compared with ducklings fed diets with only 1000 mg boron/kg (as
boric acid) over a 4-week period. These effects were magnified when
dietary protein was restricted. Reduction of the dietary protein
content from 22% to 7% in combination with the same selenium and boron
exposure scenario as above resulted in significant duckling mortality.
These findings suggest that selenium and boron may interact to cause
more severe toxicological effects in waterfowl than boron causes
alone, especially in cases where dietary protein is restricted.
The liver boron content of mallards fed with dietary boron at 0,
30, 300, or 1000 mg/kg was found to range from 0 to 51 µg/g dry
weight. The level of 33-51 µg boron/g in liver was associated with
reproductive impairment in the 1000 mg/kg exposed group (Smith &
Anders, 1989).
Boron levels in the livers of aquatic birds from the Grassland
Water District of California, USA, were also examined during
1985-1988. The levels detected ranged from 1.7 to 40 µg boron/g dry
weight; the highest level detected is in a range associated with
reproductive impairment (Paveglio, 1992).
9.2 Field observations
9.2.1 Aquatic
Concentrations of boron in trout hatcheries of Germany, the
United Kingdom, and the USA (California) have been determined. Mean
concentrations of boron in feed waters to 20 United Kingdom rainbow
trout hatcheries were 0.009-0.021 mg/litre (ECETOC, 1997). A range of
0.002-0.107 mg boron/litre was noted in 18 hatcheries in Germany. A
range of higher concentrations, 0.02-1.0 mg boron/litre, was reported
for the 10 largest hatcheries in California (Bingham, 1982; EA, 1994).
Concentrations of boron in streams and lakes of the United
Kingdom and USA capable of sustaining trout species were found to be
similar to concentrations found in hatcheries. Median boron
concentrations from nine different water regions within the United
Kingdom ranged from 0.007 to 0.272 mg/litre, with maximum values in
each of the regions ranging from 0.113 to 2.3 mg boron/litre (ECETOC,
1997). Boron concentrations in California surface waters that
supported viable populations of wild rainbow trout ranged from <0.01
to 13.1 mg/litre (Bingham, 1982). An update to the Bingham report was
provided by a survey of 37 fisheries biologists in the western USA
(EA, 1994). No instances were found where rainbow trout populations
were limited by boron. Several locations were found to have successful
trout populations with aqueous boron concentrations near or above
1 mg/litre. Specific examples include East and Paulina lakes in Oregon
(>0.9 mg boron/litre), Firehole River in Wyoming (>0.9 mg
boron/litre), Napa River in California (>1.2 mg boron/litre), and
Little Warm Springs in California (>3.2 mg boron/ litre). The first
two sites are renowned trophy trout waters. Important fish species
other than trout, such as northern pike, sturgeon, and catfish, were
reported to live in streams with higher boron concentrations, up to
approximately 2 mg/litre, such as in the Poplar River in Montana and
Souris River in North Dakota. Neither of these latter rivers was
reported to contain rainbow trout owing to excessive temperatures and
unsuitable habitat (EA, 1994).
9.2.2 Terrestrial
Boron deficiencies in terrestrial plants have been reported in
many countries of the world (Kabata-Pendias & Pendias, 1984). For
example, deficiency has been reported in Canada, New Zealand, Sweden,
Nigeria, the United Kingdom, the USA, and some arid regions of India
and Pakistan. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions, because of boron's
susceptibility to leaching. Boron deficiency may also occur in
heavy-textured soils with high pH, because boron is readily adsorbed
under these conditions. Boron deficiency is more widespread than the
deficiency for any other micronutrient (Gupta et al., 1985).
When deficiency exists, boron applications increase yield and
improve quality of many crops (Gupta et al., 1985). Hopmans & Flinn
(1984) observed severe dieback in Monterey pine plantations during dry
years in south-eastern Australia owing to boron deficiency. Borax
applied in the spring at rates of 50, 100, or 150 kg/ha resulted in a
significant improvement in height growth of fertilized trees.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field. Few irrigation waters contain
enough boron to injure plants directly. However, it is the continued
use and concentration in the soil as a result of evapotranspiration
that lead to the eventual toxicity problems (Gupta et al., 1985). In
waters used for irrigation, Wilcox (1958) reported a critical boron
concentration of 0.3-1.0 mg/litre for sensitive crops, such as citrus
and other fruits. Semi-tolerant crops, such as potatoes, tomatoes, and
oats, tolerate concentrations of 1-2 mg/litre; tolerant crops, such as
sugar beets, onions, and carrots, can withstand concentrations of
2-4 mg/litre.
The symptoms of boron toxicity are predominantly manifested in
the leaves and roots; however, Eaton et al. (1941) observed that these
effects were frequently absent in stone fruit trees, with boron
accumulation occurring in the bark and fruits rather than the leaves.
Dye et al. (1984) reported that boron concentrations in bark of 31-120
µg/g dry matter and in fruit of 90-311 µg/g dry matter resulted in
bud-drop and branch die-back in peach trees. In nectarine trees with
boron tissue concentrations of 380-457 µg/g dry matter, the buds
failed to swell and subsequently dropped in the spring. Dye et al.
(1983) observed the same symptoms in apricot trees given about 2.4 g
of borax per tree during planting. Buds on unhealthy trees that failed
to break contained 40-100 µg boron/g dry matter.
Pollutant sources of boron, in the form of either airborne
emissions or leachates from soil application, have been shown to
produce toxic symptoms in endemic species. Smidt & Whitton (1975)
studied the toxic responses in a stand of Monterey pine adjacent to a
boiler house. The trees were exposed to boron as a result of dumping
of furnace ash into an adjacent gully and from airborne ash emitted
from the furnace chimney. The average boron concentration in the ash
was 1900 mg/kg. Chlorosis and necrosis of the needles were most severe
closest to the furnace chimney, with the effects decreasing with
increased distance from the furnace. No evidence of toxicity was
observed at needle boron concentrations of 13-70 µg/g, whereas toxic
symptoms were observed at needle boron concentrations of approximately
200 µg/g. The authors concluded that foliar absorption of airborne
boron, as well as root absorption from boron in soil solution,
resulted in the injury to this stand of trees.
Temple & Linzon (1976) reported that atmospheric boron emissions
from an appliance manufacturing plant and a fibreglass manufacturing
plant resulted in >35% foliar necrosis on vegetation within 700 m of
the plant, with 1-15% foliar necrosis occurring within 500 m in all
directions from the plant. The amount of leaf damage was proportional
to the boron concentration in the leaves. Species of maple
(Acer sp.) appeared to be the most sensitive, with slight necrosis
occurring at a concentration of 432 µg/g in unwashed foliage, moderate
necrosis at foliar concentrations ranging from 707 to 1013 µg/g, and
severe necrosis at foliar concentrations of 1207-1560 µg/g.
Lang et al. (1986) found that boron thresholds for detectable
levels (10%) of visible injury due to boron toxicity were about 500
mg/kg of foliage for both bigleaf maple and digger pine growing near
geothermal generating units.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health exposures
Boron is a naturally occurring element found combined with other
elements (primarily oxygen) throughout the environment. It is found in
the Earth's crust, with the majority of readily available forms
occurring in the ocean. It is estimated that more boron is released
into the environment by natural weathering than from anthropogenic
sources. Boron is not present in the atmosphere at significant levels.
Boron is present in surface water and groundwater and is readily
adsorbed on the surfaces of soil particles. Boron is present in food,
beverages, and drinking-water.
Boron has a wide variety of uses in the manufacture of glass,
glass products, antiseptics, cleaning products, pharmaceuticals,
biological growth control agents, wood and leather preservatives, and
insecticides. It is also used in photographic chemicals, enamels and
frits, and fire retardants.
Exposure to boron compounds can occur by inhalation, ingestion,
and dermal contact. Boron compounds are absorbed from the respiratory
and gastrointestinal tracts, as indicated by increased levels of boron
in the blood, tissues, and urine. Dermal absorption of boron across
intact skin is negligible.
Specific information on population exposures from different
countries was discussed in chapter 5. This information was used to
determine mean intakes in ambient air, drinking-water, soil, food, and
consumer products. From these mean intakes, per cent allocations of
the TIs can be developed, as shown in Table 22. In general, food and
drinking-water form the greatest contributions to boron intake in
humans, at about 65% and 30%, respectively.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data suggest that exposure is associated with short-term and
reversible irritant effects on the upper respiratory tract,
nasopharynx, and eye. The sole long-term (7-year) follow-up study
failed to identify any long-term health effects, although a healthy
worker effect cannot be entirely ruled out, given the rate of
attrition (47%). In two descriptive studies that assessed fertility
and secondary sex ratios in relation to exposure, no detrimental
effects on demonstrated fertility for the study samples were reported.
Although an excess percentage of female births has been suggested, the
absence of statistical significance and attention to other co-variates
known to affect sex ratios warrants careful interpretation of this
finding. No studies have been identified that assess the spectrum of
reproductive outcomes, such as time-to-pregnancy, conception delays,
spontaneous abortions, or semen quality. The role of other lifestyle
or behavioural factors in relation to health and fertility requires
further study to identify potentially sensitive populations and to
evaluate reproductive effects more fully.
Table 22. Estimation of exposures based on ranges of mean
concentrations in several environmental media on a global basis,
and allocation of the TI among media
Environmental media Mean intakea % allocation of TI
(mg/day)
Ambient air 0.000 44 Nil
Drinking-water 0.6b approx. 30
Soil 0.000 5 Nil
Food 1.2 approx. 65
Consumer products 0.1 5
Total 1.9 100
a Mean intakes are estimated in chapter 5.
b Assuming an intake of 2 litres of water per day.
10.2 Choice of critical effect and application of uncertainty factors
The critical effect appears to lie within the area of
developmental toxicity. Other effects, such as reproductive toxicity,
also occur at slightly higher doses and were considered in the choice
of critical effects through a comparison of LOAELs (Table 17) or
benchmark doses (BMDs) (Moore et al., 1997).a Several developmental
effects from boron exposure have been noted in rats, mice, and
rabbits. Again, these effects are the most sensitive in a larger
toxicity database that includes sub-chronic and chronic bioassays. The
specific critical effect within these developmental toxicity studies
is a decreased average fetal body weight per litter in the rat
(Heindel et al., 1992; Price et al., 1996a).
a The Weir & Fisher (1972) 2-year dog studies were not used directly
for risk assessment for several reasons. The NOAEL and LOAEL were
taken from two studies; one was 2 years long, and the high-dose group
was a supplemental group terminated at 38 weeks. There were a limited
number of animals (4/dose) per test group. One control group was used
for both borax and boric acid, so that there were never more than 2
control animals sacrificed at one time. Some of the control animals
showed some form of testicular damage.
In the Price et al. (1996a) study, boric acid was administered in
the diet to CD rats from gestational day 0 to 20 at 3.3, 6.3, 9.6,
13.3, or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls.
Fetal body weight was statistically significantly decreased only in
the 13.3 and 25 mg/kg body weight per day dose groups on gestational
day 20. The NOAEL for decreased fetal body weight was 9.6 mg boron/kg
body weight per day, and the LOAEL was 13.3 mg boron/kg body weight
per day. The results of this study provide a NOAEL and LOAEL that
complement the LOAEL of 13.6 mg boron/kg body weight per day in the
Heindel et al. (1992) study.b This NOAEL is also similar to BMDs for
developmental toxicity (see appendix).
For interspecies variation, a default value of 10 has often been
applied in the absence of actual data showing less than the usual
variation between species. For inter-individual (intraspecies)
variation, a default value of 10 has often been applied in the absence
of actual data within a species. A scheme has been adopted by IPCS
(1994) that allows for subdivision of each of these defaults to
incorporate appropriate data on toxicodynamics or toxicokinetics.
Where appropriate data exist, this scheme improves the extrapolation
process and uses correction factors for toxicodynamic and
toxicokinetic data instead of 10-fold uncertainty factors for inter-
and intraspecies variation. For interspecies differences, the 10-fold
factor is divided into a default factor of 100.4 for toxicodynamics
and 100.6 for toxicokinetics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.4 × 100.6 = 10).
For inter-individual (intraspecies) differences, the 10-fold
factor is divided into a default factor of 100.5 each for
toxicokinetics and toxicodynamics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.5 × 100.5 = 10).
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in blood and
body tissues of several mammalian species following ingestion.
b In fact, the Price et al. (1996a) study was conducted specifically
to address the lack of a NOAEL from the Heindel et al. (1992) study.
Similarity in study designs and results from these two studies greatly
strengthens the dose-response relationship for developmental toxicity
as the critical effect.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the NOAEL for laboratory studies, reduce
the uncertainty for risk extrapolation between these two species.
10.3 Derivation of the tolerable intake
A TI is defined as an estimate of the intake of a substance that
can occur over a lifetime without appreciable health risks. The TI is
derived on the basis of the NOAEL of the critical effect, the adverse
effects judged to be the most appropriate for determining the TI,
using appropriate uncertainty factors. The use of the critical effect,
judged here to be developmental toxicity, is expected to protect
against other effects such as reproductive toxicity occurring at
higher doses.
9.6 mg/kg body weight per day
TI =
[100.4 × 100.1] × [100.5 × 100.4]
= 0.4 mg/kg body weight per day
= 400 µg/kg body weight per day
where:
* 9.6 mg/kg body weight per day is the NOAEL from a well conducted
developmental study with decreased fetal body weight occurring in
a dose-related manner
* 100.4 × 100.1 (=100.5) is the uncertainty factor for interspecies
differences:
Dynamics: Data do not exist to support a factor other than the
default value of 100.4. Moreover, differences in LOAELs among
rats, rabbits, and mice for the critical effect (Table 16)
support the default value. The Task Group judged that 100.4 was
appropriate.
Kinetics: Oral absorption of boron in rats and humans is
quantitatively similar, with values of 94% for humans and 95% for
rats (section 6.1.1). The ratio of these absorption rates is
approximately 1.
Distribution of boron in rats and humans appears quantitatively
similar, as determined by a comparison of blood boron levels
after doses in either diet or drinking-water (Fig. 1, section
6.2.2). Ratios of rat blood boron values to a regression line for
human blood boron values are as low as 0.7 and as high as 6, with
the majority of values in the range of 2-3. Comparative
regression techniques should be pursued to confirm this trend.
Pregnant rats appear to have lower blood boron values than non-
pregnant rats when given similar doses. Comparative data for
pregnant and non-pregnant humans are lacking.
Metabolism of boron is thought not to occur in humans or animals
owing to the excessive energy required to break the boron-oxygen
bond (section 6.3). As it is unlikely that there are any
differences between species in the metabolism of boron, the ratio
of metabolic parameters is 1.
Elimination of boron in rats and humans appears quantitatively
similar, with half-life values in humans of 21 h in volunteers
and approximately 13 h in poisonings, and values in rats of
14=19 h (section 6.4). The ratio of these elimination half-life
values is approximately 1.3 when human volunteer data are
compared with the rat data, or 0.8 when human poisoning data are
compared with the rat data. The mean of these two ratios is
about 1.
As a result, the Task Group judged that the default value of
100.6 could be reduced to 100.1.
* 100.5 × 100.4 (=100.9) is the uncertainty factor for
inter-individual (intraspecies) differences:
Dynamics: Data in humans do not exist to support a value
different from the default of 100.5. Animal studies suggest that
intraspecies variability in toxicodynamics exists. The Task Group
judged that 100.5 was appropriate.
Kinetics: Data exist in humans to suggest some limited
variability in boron absorption and/or distribution (Nielsen,
1995). However, the lack of boron metabolism in humans and
experimental animals suggests some reduction in the default value
of 100.5. The Task Group judged that 100.4 was appropriate.
Other uncertainty factors for adequacy of database are not
considered necessary, because the overall database includes subchronic
and chronic studies on several species and several reproductive
studies.
The total uncertainty factor,a,b is therefore:
100.5 × 100.9 = 101.4 (or 25)
Data are inadequate to allow the development of a TI for
inhalation exposure (TIi).
10.4 Derivation of guidance values
Exposure to boron from various media varies with the locality. In
areas where data on exposure are available, specific guidance values
that are tailored to the local circumstances should be developed from
the TI presented in this document.
For example, guidance values for specific media could be
developed by first using the allocations shown in Table 22 with the TI
derived in section 10.3. Table 23 shows the resulting allocation of
the TI by media in mg/kg body weight per day.
The second step in the development of a guidance value would be
to pick appropriate body weight and exposure assumptions. Two examples
are given using allocations found in Table 23. The resulting guidance
value (GV) for example A in drinking-water would be:
a After closure of the Task Group meeting, Dr. M. Dourson, after
additional consideration, dissented from the Task Group's
recommendation on the uncertainty factor.
b Subsequent to the Task Group meeting, a provisional guideline for
boron in drinking-water was recommended by the Working Group Meeting
on Chemical Substances in Drinking-Water, using a different
uncertainty factor, based upon a different weighting attached to some
toxico-kinetic studies for that area of application (WHO, 1998 a,b).
Example A
0.12 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 4 mg/litre
where:
* 64 kg is the average human body weight (IPCS, 1994)
* 2 litres/day is one assumption of water intake (IPCS, 1994).
The resulting guidance value (GV) for example B would be:
Example B
0.04 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 1.3 mg/litre
Assumptions of food intake and consumer product use could be used
with allocations given in Table 23 to generate tolerances or other
risk management standards. Individual countries are encouraged to
develop guidance values using allocations, body weights, and exposure
assumptions that are appropriate for their populations.
Table 23. Examples of allocation of TI by media
Environmental Example A Example B
media
Per centa mg/kg body Per cent mg/kg body
weight per day weight per day
Ambient air Nil 0 Nil 0
Drinking-water 30 0.12 10 0.04
Soil Nil 0 Nil 0
Food 65 0.26 85 0.34
Consumer products 5 0.02 5 0.02
Total 100 0.4 100 0.4
a Values from Table 22.
10.5 Evaluation of effects on the environment
10.5.1 Exposure
Boron is not present in the atmosphere at significant levels;
however, the total amount present in the atmosphere at any one time is
significant owing to the huge volume of the atmosphere. Boron
concentrations in air range from <0.5 to approximately 80 ng/m3,
with an average of 20 ng/m3.
Boron occurs naturally in rocks, with concentrations ranging from
5 mg/kg in basalts to 100 mg/kg in shales. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron range widely, from 0.001 to as much as 360 mg/litre. However,
mean concentrations for waters of Europe, Pakistan, Russia, and Turkey
are typically well below 0.6 mg boron/litre. Concentrations of boron
in waters in Japan, South Africa, and South America are generally
below 0.3 mg/litre. Typical concentrations in North American waters
are below 0.1 mg boron/litre, with about 90% at 0.4 mg boron/litre or
below.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Boron occurs in soils at concentrations ranging from 10 to 300 mg/kg
(average 30 mg/kg), depending on the type of soil, amount of organic
matter, and amount of rainfall.
Boron does not bioaccumulate in aquatic invertebrates and fish.
Based on wet weights, boron concentrations in marine invertebrates and
fish are similar to the levels found in the exposure media, between
0.5 and 4 mg/kg. Bioconcentration factors for two freshwater fish
species were found to be 0.3. The results of studies of boron
accumulation in plants, insects, and fish have shown that boron
bioaccumulates in plants but does not biomagnify in aquatic
food-chains. Concentrations of boron have been shown to range between
26 and 382 mg/kg in submerged aquatic freshwater plants, from 11.3 to
57 mg/kg in freshwater emergent vegetation, and from 2.3 to 94.7 mg/kg
dry weight in terrestrial plants.
10.5.2 Effects
Boron is an essential micronutrient for cyanobacteria and
diatoms. Considering all microorganisms, algae and protozoa appear to
be equisensitive to boron and bacteria the most tolerant. Both algae
and protozoa provide NOEC (including EC3) values between 0.3 and 20
mg boron/litre.
Chronic studies with Daphnia magna provided consistent NOECs
between 6 and 10 mg boron/litre. Long-term outdoor pond and field
studies with microorganisms and invertebrates provided NOEC values up
to 1.52 mg boron/litre.
Via acute tests, the rainbow trout and zebra fish were determined
to be the most sensitive taxa, with acute values around 10 mg
boron/litre. Further tests with embryo-larval stages of several fish
species showed rainbow trout to be the most sensitive to boron. Tests
conducted with early life stages of rainbow trout in reconstituted
water overpredicted toxicity compared with tests conducted with
natural water. No adverse effects were found in natural water
exposures containing up to 0.75 mg boron/litre. Consistent LOECs were
between 1.1 and 1.7 mg boron/litre. These laboratory values were
confirmed by several observations of successful trout populations
thriving in hatcheries, streams, and lakes containing boron
concentrations up to 1 mg/litre.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
The symptoms of boron deficiency in plants include cessation of root
and leaf growth, necrosis, retardation of enzyme reactions, and
reduced pollen germination. The initial symptoms of boron toxicity in
plants are chlorosis, necrosis, and a subsequent loss of
photosynthetic capacity, which reduces plant productivity.
Mallard duckling growth was adversely affected at dietary levels
of 30 and 300 mg/kg, and survival was reduced at 1000 mg/kg.
10.5.3 Risk evaluation
Risk characterization is based on the comparison of the
environmental concentration with a concentration protective for an
ecosystem. Ambient concentrations of boron in surface waters have been
reported for several countries covering four continents. The data
indicate that mean or median levels are approximately 0.1 mg
boron/litre and that 90th-percentile or greater values are
approximately 0.5 mg boron/litre. Considerable data exist on the
toxicity of boron to freshwater organisms and ecosystems. Acute tests
with invertebrates and fish showed that rainbow trout and zebra fish
were most sensitive to borate (LC50 approx. 10 mg boron/litre).
Chronic results for algae, higher plants, and fish clearly showed that
the rainbow trout was the most sensitive species, with consistent
NOECs and LOECs of about 1 mg boron/litre. Confirmed were several
observations of healthy rainbow trout populations in US hatcheries and
streams at or above 1 mg boron/litre. Not surprisingly, biocenoses
(model ecosystems) covering all stages of the aquatic food-web
(excluding fish) were more tolerant of boron than were rainbow trout
(NOEC up to 1.52 mg boron/litre). Given all this information, the
comparison of the environmental effects concentration with the general
ambient environmental levels indicates that the risk of adverse
effects of boron on the aquatic ecosystem is low. In a few boron-rich
environments, natural levels will be higher. It is reasonable to
assume that aquatic organisms in such habitats may be adapted to the
local conditions.
Boron deficiencies in terrestrial plants have been reported in
many countries. Boron deficiency is more likely to occur in
light-textured, acid soil in humid regions, because of boron's
susceptibility to leaching. In general, there is a small range between
deficiency and toxicity. However, considerable variation exists
between species in their resistance to boron. Species sensitive to
boron are known to include citrus, stone fruits, and nut trees;
semi-tolerant species include tubers and cereals; and tolerant species
include most vegetables. Toxicity due to excess boron is much less
common in the environment than boron deficiency. Irrigation water is
one of the main sources of high boron levels that result in toxicity
in the field. However, few irrigation waters contain enough boron to
injure plants directly; it is the continued use and concentration in
the soil resulting from evapotranspiration that lead to the eventual
toxicity problems. Pollutant sources of boron, in the form of either
airborne emissions or leachates from soil application, have also been
shown to produce toxic symptoms in endemic plant species growing in
the immediate vicinity.
Levels of boron measured in aquatic plants from Kesterson
National Wildlife Refuge, California, USA (an evapotranspiration sink
that receives boron-rich run-off from irrigated agricultural fields)
are higher than those found to cause effects on mallard ducklings in
the laboratory. Therefore, there appears to be a risk to waterfowl in
this area. However, the bioavailability of such boron levels is
uncertain. The extent of risk to waterfowl in most geographical areas
is expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency has been observed in many countries throughout the
world. There is a small range between deficiency and toxicity in some
plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental boron levels
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre. For the
general population, the greatest boron exposure comes from the oral
intake of food. The mean daily intake of boron in the diet is
estimated to be near 1.2 mg/day.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. Greater than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The TI of boron was set as 0.4 mg/kg body weight per day. The
allocation of the TI in various media should be based on the exposure
data of individual countries.
11.2 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
12. FURTHER RESEARCH
a) Further studies regarding global cycling of boron.
b) Determine whether boron is required for normal fetal and post-
natal development for higher animals.
c) Define a biochemical function that confirms the essentiality of
boron for higher animals.
d) Determine the homeostatic mechanism through which boron
concentrations are maintained during low dietary intakes.
e) Further studies on the absorption, distribution, and clearance of
boron at oral doses in the range of 0.01-1 mg boron/kg body
weight per day.
f) A modern rat reproduction study to determine the potential for
developmental effects of borates in succeeding generations in a
sensitive animal species.
g) Analytical epidemiology studies to assess the spectrum of
reproductive and developmental outcomes to assess boron toxicity
in targeted human populations.
h) Epidemiological studies that incorporate toxicological and
physiological components to enhance an understanding of the
toxicokinetics and physiology of boron in populations, including
potentially sensitive ones.
i) Further quantitative definition of the maximum tolerable
concentration for sensitive aquatic species.
j) A refined understanding of the relationship of water and dietary
uptake of boron into waterfowl.
k) Development of an approach that integrates essentiality and
toxicity for the establishment of acceptable boron concentrations
in aquatic and terrestrial environments.
13. EVALUATIONS BY INTERNATIONAL BODIES
The EC Cosmetics Directive 76/768/EEC (and its subsequent
amendment) sets an upper limit of boric acid in cosmetic products,
which must not contain more than 5% in talc, 0.5% in oral hygiene
products, and 3% in other products. Only the talc containing boric
acid must, in addition, not be used on children less than 3 years of
age.
In 1997, subsequent to the Task Group meeting, a provisional
guideline for boron in drinking-water was recommended by the Working
Group on Chemical Substances in Drinking-Water, which updated the
Guidelines for drinking-water quality (WHO, 1998a,b).
REFERENCES
ACGIH (1991) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, pp 141-148.
Adams FS, Cole H Jr, & Massie LB (1973) Element constitution of
selected aquatic vascular plants from Pennsylvania: Submersed and
floating leaved species and rooted emergent species. Environ Pollut,
5: 117-147.
Ahl T & Jönsson E (1972) Boron in Swedish and Norwegian fresh waters.
Ambio, 1(2): 66-70.
Alabaster JS (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int Pest
Control, 11: 29-35.
Alexander GV, Nusbaum RE, & MacDonald NS (1951) The boron and lithium
content of human bones. J Biol Chem, 192: 489-496.
Allen BC, Strong PL, Price CJ, Hubbard SA, & Daston GP (1996)
Benchmark dose analysis of developmental toxicity in rats exposed to
boric acid. Fundam Appl Toxicol, 32: 194-204.
Anderson BG (1946) Industrial wastes: The toxicity thresholds of
various sodium salts determined by the use of Daphnia magna. Sewage
Works J, 18: 82-87.
Anderson DL, Kitto ME, McCarthy L, & Zollar WH (1994a) Sources of
atmospheric distribution of particulate and gas phase boron. Atmos
Environ, 28: 1401-1410.
Anderson DL, Cunningham WC, & Lindstrom TR (1994b) Concentrations and
intakes of H, B, S, K, Na, Cl and NaCl in foods. J Food Compos Anal,
7: 59-82.
Antia NJ & Cheng JY (1975) Culture studies on the effects from borate
pollution on the growth of marine phytoplankters. J Fish Res Board
Can, 32: 2487-2494.
Aquilio E, Spagnoli R, Seri S, Bottone G, & Spennati G (1996) Trace
element content in human milk during lactation of preterm newborns.
Biol Trace Elem Res, 51: 63-70.
ATSDR (1992) Toxicological profile for boron. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry.
Atteia O, Védy J-C, & Parriaux A (1993) Trace elements dynamics in
soils and aquifers of Western Switzerland. Stud Environ Sci Environ
Contam, 55: 79-101.
Aznarez J, Ferrer A, Rabadan JM, & Marco L (1985) Extractive
spectrophotometric and fluorimetric determination of boron with
2,2,4-trimethyl-1,3-pentanediol and carminic acid. Talanta, 32(12):
1156-1158.
Bai Y & Hunt CD (1996) Absorption and distribution of boron in rats
following a single oral administration of boron. Proc North Dakota
Acad Sci, 50: 53.
Banerji SK (1969) Boron adsorption on soils and biological sludges and
its effects on endogenous respiration. In: Proceedings of the 24th
Industrial Waste Conference, Lafayette, 6-8 May 1969. Lafayette,
Indiana, Purdue University (Engineering Extension Series No. 135).
Banuelos GS, Cardon G, Pflaum T, & Akohoue S (1992) Comparison of dry
ashing and wet acid digestion on the determination of boron in plant
tissue. Commun Soil Sci Plant Anal, 23(17-20): 2383-2397.
Barber LB, Thurman EM, Schroeder MP, & Le Blanc DR (1988) Long-term
fate of organic micropollutants in sewage-contaminated groundwater.
Environ Sci Technol, 22: 205-211.
Barnes DG, Datson GP, Evans JS, Jarabek AM, Kavlock RJ, Kimmel CA,
Park C, & Spitzer HL (1995) Benchmark dose workshop: Criteria for use
of a benchmark dose to estimate a reference dose. Regul Toxicol
Pharmacol, 21: 296-301.
Barr RD, Clarke WB, Clarke RM, Venturelli J, Norman GR, & Downing RG
(1993) Regulation of lithium and boron levels in normal human blood:
Environmental and genetic considerations. J Lab Clin Med,
121: 614-619.
Barres M (1967) Contribution à l'étude de l'isopoly--condensation des
borates alcalins par électrométrie et partages. Rev Chem Minér,
4: 803-838.
Barth RF & Soloway AH (1994) Boron neutron capture therapy of primary
and metastatic brain tumors. Mol Chem Neuropathol, 21(2-3): 139-154.
Barth RF, Adams DM, Soloway AH, Mechetner EB, Alam F, & Anisuzzaman KM
(1991) Determination of boron in tissues and cells using
direct-current plasma atomic emission spectroscopy. Anal Chem,
63: 890-893.
Belanger SE, Farris JL, & Cherry DS (1989) Effects of diet, water
hardness and population source on acute and chronic copper toxicity to
Ceriodaphnia dubia. Arch Environ Contam Toxicol, 18: 601-611.
Benson WH, Birge WJ, & Dorough HW (1984) Absence of mutagenic activity
of sodium borate (borax) and boric acid in the Salmonella
preincubation test. Environ Toxicol Chem, 3: 209-214.
Benson SM, White AF, Halfman S, Flexser S, & Alavi M (1991)
Groundwater contamination at the Kesterson Reservoir, California: 1.
Hydrogeologic setting and conservative solute transport. Water Resour
Res, 27: 1071-1084.
Bergmann W (1984) The significance of the micronutrient boron in
agriculture. London, Borax Holdings Limited, 26 pp.
Bergmann W, Bruchlos P, & Marks G (1995) [A contribution to the
question of the limit value for boron in water for Ogragnutes
australis seed.] Tenside Deterg, 32: 229-237 (in German).
Bertine KK & Goldberg ED (1971) Fossil fuel combustion and the major
sedimentary cycle. Science, 173: 233-235.
Beyer KH, Bergfeld WF, Berndt WO, Boutwell RK, Carlton WW, Hoffmann
DK, & Schroeter AL (1983) Final report on the safety assessment of
sodium borate and boric acid. J Am Coll Toxicol, 2: 87-125.
Biggar JW & Fireman M (1960) Boron adsorption and release by soils.
Soil Sci Soc Am Proc, 24: 115-120.
Bingham FT (1982) The boron concentration of wild trout streams in
California. Riverside, California, University of California,
Department of Soil Science (Unpublished document).
Bingham FT, Elseewi A, & Oertli JJ (1970) Characteristics of boron
absorption by excised barley roots. Soil Sci Soc Am Proc, 34: 613-617.
Bingham FT, Page AL, Coleman NT, & Flach K (1971) Boron adsorption
characteristics of selected amorphous soils from Mexico and Hawaii.
Soil Sci Soc Am Proc, 35: 546-550.
Birge WJ & Black JA (1977) Sensitivity of vertebrate embryos to boron
compounds. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Prepared for US EPA by the University of
Kentucky, Lexington) (EPA-560/1-76-008).
Birge WJ & Black JA (1981) Toxicity of boron to embryonic and larval
stages of largemouth bass (Micropterus salmoides) and rainbow trout
(Salmo gairdneri)--Completion report. Cincinnati, Ohio, Procter &
Gamble Company.
Birmingham DJ & Key MM (1963) Preliminary survey: U.S. Borax plant,
California. Cincinnati, Ohio, Occupational Health Research and
Training Facility, Division of Occupational Health.
Black JA, Barnum JB, & Birge WJ (1993) An integrated assessment of the
biological effects of boron to the rainbow trout. Chemosphere, 26:
1383-1413.
Blasser-Grill J, Knoppik D, Amberger A, & Goldbach H (1990) Influence
of boron on the membrane potential in Elodea densa and Helianthus
annus roots and H+ extrusion of suspension cultured Daucus
carota cells. Plant Physiol, 90: 280-284.
Blevins DG & Luk-aszewski KM (1994) Proposed physiologic functions of
boron in plants pertinent to animal and human metabolism. Environ
Health Perspect, 102(Suppl 7): 31-33.
Bodek I, Ehntholt EJ, Glazer AE, Loreti CP, & Lyman W (1988) Chapter
10: Chemical classes, Section 10.9: Boron-containing compounds. In:
Bodek I, Lyman WJ, Reehl WF, Rosenblatt DH, Walton BT, & Conway RA ed.
Environmental inorganic chemistry: Properties, processes, and
estimation methods. Oxford, New York, Pergamon Press,
pp 10.9/1-10.9/10.
Bonilla I, Garcia-Gomez M, & Mates P (1990) Boron requirements in
cyanobacteria: Possible role in the early evolution of photosynthetic
organisms. Plant Physiol, 94: 1554-1560.
Borkovec AB, Settepani JA, LaBrecque GC, & Fye RL (1969) Boron
compounds as chemosterilants for house flies. J Econ Entomol, 62:
1472-1480.
Bowen JE & Gauch HG (1966) Nonessentiality of boron in fungi and the
nature of its toxicity. Plant Physiol, 41: 319-324.
Boyd CE & Walley WW (1972) Studies of the biogeochemistry of boron. 1.
Concentrations in surface waters, rainfall and aquatic plants. Am Mid
Nat, 88: 1-14.
Bringmann G & Kuhn R (1977) [The toxicity of waterborne contaminants
towards Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in
German with English summary).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kuhn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G, Kuhn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa: III. Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German with
English summary).
Brown TF, McCormick ME, Morris DR, & Zeringue LK (1989) Effects of
dietary boron on mineral balance in sheep. Nutr Res, 9: 503-512.
Budavari S, O'Neil MJ, Smith A, & Heckelman PE ed. (1989) The Merck
index, 11th ed. Rahway, New Jersey, Merck and Co., Inc.
Bundschuh J (1992) Boron contamination of the ground- and surface
waters of Lerma Valley, Argentina. Aqua (Lond), 41(1): 13-17.
Butterwick L, De Oude N, & Raymond K (1989) Safety assessment of boron
in aquatic and terrestrial environments. Ecotoxicol Environ Saf, 17:
339-371.
Byrne RH Jr & Kester DR (1974) Inorganic speciation of boron in
seawater. J Mar Res, 32: 119-127.
Cáceres LV, Gruttner ED, & Contreras RN (1992) Water recycling in arid
regions: Chilean case. Ambio, 21(2): 138-144.
Canton JH & Adema DMM (1978) Reproducibility of short-term and
reproduction toxicity experiments with Daphnia magna and comparison
of the sensitivity of Daphnia magna with Daphnia pulex and
Daphnia cucullata in short-term experiments. Hydrobiologia,
59: 135-140.
Chapin RE, Ku WW, Kenney MA, McCoy H, Gladen B, Wine RN, Wilson R, &
Elwell MR (1997) The effects of dietary boron on bone strength in
rats. Fundam Appl Toxicol, 35: 205-215.
Choi WW & Chen KY (1979) Evaluation of boron removal by adsorption on
solids. Environ Sci Technol, 13: 189-196.
Clarke WB, Koekebakker K, Barr RD, Downing RG, & Fleming RF (1987a)
Analysis of ultratrace lithium and boron by neutron activation and
mass spectrometric measurement of 3He and 4He. Appl Rad Isot, 38(9):
735-743.
Clarke WB, Webber CE, & Koekebakker K (1987b) Lithium and boron in
human blood. J Lab Clin Med, 109: 155-158.
Corwin DL (1986) A one-dimensional model of chemical diffusion and
sorption in saturated soil and aquatic systems. J Environ Qual,
15: 173-182.
Cotton FA & Wilkinson G (1988) Advanced inorganic chemistry, 5th ed.
New York, John Wiley and Sons, pp 162-165.
Couch EL & Grim RE (1968) Boron fixation by illites. Clays Clay Miner,
16: 249-256.
Cox JA, Lundquist GL, Przyjazny A, & Schmulbach CD (1978) Leaching of
boron from coal ash. Environ Sci Technol, 12: 722-723.
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, &
Strong PL (1994a) The relationship of blood- and urine-boron to boron
exposure in borax-workers and the usefulness of urine-boron as an
exposure marker. Environ Health Perspect, 102(Suppl 7): 133-137.
Culver BD, Smith RG, Brotherton RJ, Strong PL, & Gray TM (1994b)
Chapter 44: Boron. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology. New York, John Wiley and Sons, vol 2, part 1.
Cumakov A (1991) Chemical methods of fractionation of soil trace
elements: 1. Boron. Chem Abstr, 115: 182048v.
De Jong LEDD (1965) Tolerance of Chlorella vulgaris for metallic and
non-metallic ions. Antonie van Leeuwenhoek, 31: 301-313.
Deverel SJ & Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, California. Environ Sci Technol, 22: 697-702.
Dieter MP (1994) Toxicity and carcinogenicity studies of boric acid in
male and female B6C3F1 mice. Environ Health Perspect, 102(Suppl 7):
93-97.
Dixon RL, Lee IP, & Sherins RJ (1976) Methods to assess reproductive
effects of environmental chemicals: Studies of cadmium and boron
administered orally. Environ Health Perspect, 13: 59-67.
Dixon RL, Sherins RJ, & Lee IP (1979) Assessment of environmental
factors affecting male fertility. Environ Health Perspect, 30: 53-68.
Doonan DJ & Lower LD (1978) Boron compounds (oxides, acid, borates).
In: Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New
York, John Wiley and Sons, vol 4, pp 67-110.
Draize JH & Kelley EA (1959) The urinary excretion of boric acid
preparations following oral administration and topical applications to
intact and damaged skin of rabbits. Toxicol Appl Pharmacol, 1:
267-276.
Dudas MJ (1981) Long-term leachability of selected elements from fly
ash. Environ Sci Technol, 15: 840-843.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1983) Die-back of
apricot trees following soil application of boron. NZ J Exp Agric, 11:
331-342.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1984) Boron toxicity
in peach and nectarine trees in Otaga. NZ J Exp Agric, 12: 303-313.
EA (1994) Boron concentrations and rainbow trout populations in seven
states in the western United States. Corvallis, Oregon, EA
Engineering, Science and Technology (Unpublished report prepared for
the Procter & Gamble Company, Cincinnati).
Eaton FM (1944) Deficiency, toxicity, and accumulation of boron in
plants. J Agric Res, 69: 237-277.
Eaton FM, McCallum RD, & Mayhugh MS (1941) Quality of irrigation
waters of the Hollister area of California with special reference to
boron content and its effect on apricots and prunes. US Dept Agric
Tech Bull, 746: 1-59.
ECETOC (1995) Reproductive and general toxicology of some inorganic
borates and risk assessment for human beings. Brussels, European
Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 63).
ECETOC (1997) Ecotoxicology of some inorganic borates - Interim
report. Brussels, European Centre for Ecotoxicology and Toxicology of
Chemicals, 97 pp (Special Report No. 11).
Eckel WP & Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
Superfund '88: Proceedings of the 9th national conference. Washington,
DC, US Hazardous Materials Control Research Institute, pp 282-286.
Eisler R (1990) Boron hazards to fish, wildlife, and invertebrates: A
synoptic review. Washington, DC, US Department of Interior, Fish and
Wildlife Service.
El-Hassanin AS, Labib TM, & Dobal AT (1993) Potential Pb, Cd, Zn and B
contamination of sandy soils after different irrigation periods with
sewage effluent. Water Air Soil Pollut, 66: 239-249.
Elrashidi MA & O'Connor GA (1982) Boron sorption and desorption in
soils. Soil Sci Soc Am J, 46: 27-31.
Emsley J (1989) The elements. Oxford, Clarendon Press, p 32.
Fail PA, George JD, Sauls SW, Dennis SW, & Seely JC (1989) Effect of
boric acid on reproduction and fertility of rodents. Adv Contracept
Deliv Syst, 5: 323-333.
Fail PA, George JD, Grizzle TB, Heindel JJ, & Chapin RE (1990) Final
report on the reproductive toxicity of boric acid (CAS No. 10043-35-3)
in CD-1 Swiss mice. Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology Program
(NTP Report No. 90-105).
Fail PA, George JD, Seely JC, Grizzle TB, & Heindel JJ (1991)
Reproductive toxicity of boric acid in Swiss (CD-1) mice: Assessment
using the continuous breeding protocol. Fundam Appl Toxicol, E17:
225-239.
Farr LE & Konikowski T (1963) The renal clearance of sodium
pentaborate in mice and men. Clin Chem, 9: 717-726.
Farris JL, Grudzien JL, Belanger SE, Cherry DS, & Cairns J (1994)
Molluscan cellulolytic activity responses to zinc exposure in
laboratory and field comparisons. Hydrobiologia, 287: 161-178.
Fay RW (1959) Toxic effects of boron trioxide against immature stages
of Aedes aegypti, Anopheles quadrimaculatus, and
Culex quinquefasciatus. J Econ Entomol, 52: 1027-1028.
Forbes RM & Mitchell HH (1957) Accumulation of dietary boron and
strontium in young and adult albino rats. Arch Ind Health, 16:
489-492.
Forbes RM, Cooper AR, & Mitchell HH (1954) On the occurrence of
beryllium, boron, cobalt and mercury in human tissues. J Biol Chem,
209: 857-865.
Friis-Hansen B, Aggerbeck B, & Jansen JA (1982) Unaffected blood boron
levels in newborn infants treated with a boric acid ointment. Food
Chem Toxicol, 20: 451-454.
Garabrant DH, Bernstein L, Peters JM, & Smith TJ (1984) Respiratory
and eye irritation from boron oxide and boric acid dusts. J Occup Med,
26: 584-586.
Garabrant DH, Bernstein L, Peters JM, Smith TJ, & Wright WE (1985)
Respiratory effects of borax dust. Br J Ind Med, 42: 831-837.
Garcia-Campana A, Barrero FA, & Ceba MR (1992) Spectrofluorimetric
determination of boron in soils: Plant and natural waters with
alizarin red S. Analyst, 117: 1189-1191.
Gerike P, Fischer WK, & Holtmann W (1976) [The influence of boron on
aerobic biological waste water purification.] Tenside Deterg, 13:
249-252 (in German).
Gerritse RG, Vriesema R, Dalenberg JW, & DeRoos HP (1982) Effect of
sewage sludge on trace element mobility in soils. J Environ Qual, 11:
359-364.
Gersich FM (1984) Evaluation of a static renewal chronic toxicity test
method for Daphnia magna Straus using boric acid. Environ Toxicol
Chem, 3: 89-94.
Gestring WD & Soltanpour PN (1987) Comparison of soil tests for
assessing boron toxicity to alfalfa. Soil Sci Soc Am J, 51: 1214-1219.
Gilliam CH & Watson ME (1981) Boron accumulation in Taxus media.
Hortic Sci, 16: 340-341.
Gladney ES, Wangen LE, Curtis DB, & Jurney ET (1978) Observations on
boron release from coal-fired power plants. Environ Sci Technol, 12:
1084-1085.
Glaubig BA & Bingham FL (1985) Boron toxicity characteristics of four
northern California endemic tree species. J Environ Qual, 14: 72-77.
Goldbloom RB & Goldbloom A (1953) Boric acid poisoning: Report of four
cases and a review of 109 cases from the world literature. J Pediatr,
43: 631-643.
Graedel TE (1978) Inorganic elements, hydrides, oxides, and
carbonates. In: Chemical compounds in the atmosphere. New York,
London, San Francisco, Academic Press, pp 35-49.
Graffmann G, Kuzel P, Nösler H, & Nonnenmarcher G (1974)
[Determination of traces of boron in fresh and drinking-water.] Chem
Ztg, 98: 499-504 (in German).
Green NR & Ferrando AA (1994) Plasma boron and the effects of boron
supplementation in males. Environ Health Perspect, 102(Suppl 7):
73-77.
Green GH & Weeth HJ (1977) Responses of heifers ingesting boron in
water. J Anim Sci, 46: 812-818.
Green GH, Lott MD, & Weeth HJ (1973) Effects of boron water on rats.
Proc West Sect Am Soc Anim Sci, 24: 254-258.
Gregoire DC (1990) Determination of boron in fresh and saline water by
inductively coupled plasma mass spectrometry. J Anal Atom Spectrom,
5: 623-626.
Gregory J, Foster K, Tyler H, & Wiseman M (1990) The dietary and
nutritional survey of British adults--Table on consumption of wine and
fortified wine. London, Her Majesty's Stationary Office.
Grella P, Tambuscio B, & Suma V (1976) [Boric acid and poisoning
during pregnancy: Description of one case.] Acta Anaesthesiol Ital,
27: 745-748 (in Italian).
Griffin RA & Burau RG (1974) Kinetic equilibrium studies of boron
desorption from soil. Soil Sci Soc Am Proc, 38: 892-897.
Guhl W (1996) [Ecological aspects of boron.] SÖFW-Journal, 118(18/92):
1159-1168 (in German).
Gupta UC, Jane YW, Campbell CA, Leyshon AJ, & Nicholaichuk W (1985)
Boron toxicity and deficiency: A review. Can J Soil Sci, 65: 381-409.
Hamilton SJ (1995) Hazard assessment of inorganics to three endangered
fish in the Green River, Utah. Ecotoxicol Environ Saf, 30: 134-142.
Hamilton SJ & Buhl KJ (1990) Acute toxicity of boron, molybdenum, and
selenium to fry of chinook salmon and coho salmon. Arch Environ Contam
Toxicol, 19: 366-373.
Hamilton SJ & Wiedmeyer RH (1990) Concentrations of boron, molybdenum,
and selenium in chinook salmon. Trans Am Fish Soc, 119: 500-510.
Hatcher JT & Bower CA (1958) Equilibria and dynamics of boron
adsorption by soils. Soil Sci, 85: 319-323.
Havercroft JM & Ward NI (1991) Boron and other elements in relation to
rheumatoid arthritis. In: Momeïlovie B ed. Trace elements in man and
animals. Zagreb, Croatia, IMI, pp 8/2-8/3.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Hegsted M, Keenan MJ, Siver F, & Wozniak P (1991) Effect of boron on
vitamin D deficient rats. Biol Trace Elem Res, 26: 243-255.
Heindel JJ, Price CJ, Field EA, Marr MC, Myers CB, Morrissey RE, &
Schwetz BA (1992) Developmental toxicity of boric acid in mice and
rats. Fundam Appl Toxicol, 18: 266-277.
Hellou J, Warren WG, Payne JF, Belkhode S, & Lobel P (1992) Heavy
metals and other elements in three tissues of cod, Gadus morhua from
the Northwest Atlantic. Mar Pollut Bull, 24(9): 452-458.
Hingston FJ (1986) Biogeochemical cycling of boron in native eucalypt
forests of south-western Australia. Aust For Res, 16: 73-83.
Hoffman DJ, Camardese MB, Lecaptain LJ, & Pendleton GW (1990) Effects
of boron growth and physiology in mallard ducklings. Environ Toxicol
Chem, 9: 335-346.
Hoffman DJ, Sanderson CJ, Lecaptain LJ, Cromartie E, & Pendleton GW
(1991) Interactive effects of boron, selenium, and dietary protein on
survival, growth, and physiology in mallard ducklings. Arch Environ
Contam Toxicol, 20: 288-294.
Hollis JF, Keren R, & Gal M (1988) Boron release and sorption by fly
ash as affected by pH and particle size. J Environ Qual, 17: 181-184.
Hopmans P & Flinn DW (1984) Boron deficiency in Pinus radiata D. Don
and the effect of applied boron on height growth and nutrient uptake.
Plant Soil, 79: 295-298.
Hothem RL & Ohlendorf HM (1989) Contaminants in foods of aquatic birds
at Kesterson Reservoir, California, 1985. Arch Environ Contam Toxicol,
18: 773-786.
Hothem RL & Welsh D (1994) Contaminants in eggs of aquatic birds from
the grasslands of central California. Arch Environ Contam Toxicol,
27: 180-185.
Hu H, Brown PH, & Labavitch JM (1996) Species variability in boron
requirement is correlated with cell wall pectin. J Exp Bot, 295:
227-232.
Huber L (1982) [ICP-AES, a new technique for multi-elemental
determination in water, waste water and sludge.] Wasser, 58: 173-185
(in German).
Huber L (1994) Boron concentrations in the river Neva, Russia. Munich,
Germany, Bavarian State Office for Water, Institute of Water Research
(Unpublished report).
Hudak J (1973) Effect of boric acid on the division and elongation of
cells in root tip of Vicia faba (L.) cv. Povazsky Acta Fac Rerum Nat
Univ Comenianae Physiol Plant, 6: 119-126.
Hunt CD (1988) Boron homeostasis in the cholecalciferol-deficient
chick. Proc Natl Acad Sci (USA), 42: 60.
Hunt CD (1989) Dietary boron modified the effects of magnesium and
molybdenum on mineral metabolism in the cholecalciferol-deficient
chick. Biol Trace Elem Res, 22: 201-220.
Hunt CD (1994) The biochemical effects of physiologic amount of
dietary boron in animal nutrition models. Environ Heath Perspect,
102(Suppl 7): 35-43.
Hunt CD & Herbel JL (1993) Trace elements in man and animals--TEMA 8.
Proceedings of the Eighth International Symposium on Trace Elements in
Man and Animals, Gersdorf, Germany, Verlag Media Touristik, pp
714-718.
Hunt CD & Shuler TR (1990) Open vessel, wet ash, low-temperature
digestion of biological materials for inductively-coupled organ plasma
spectrometry (ICAP) analysis of boron and other elements. J Micronutr
Anal, 6: 161-174.
Hunt CD, Shuler TR, & Mullen LM (1991) Concentration of boron and
other elements in human foods and personal-care products. J Am Diet
Assoc, 91: 558-568.
Hunt CD, Herbel JL, & Idso JP (1994) Dietary boron modifies the
effects of vitamin D3 nutrition on indices of energy substrate
utilisation and mineral metabolism in the chick. J Bone Min Metab,
9: 171-182.
Hunt CD, Herbel JL, & Nielsen FH (1997) Metabolic responses of
postmenopausal women to supplemental dietary boron and aluminium
during usual and low magnesium intake: boron, calcium and magnesium
absorption and retention and blood mineral concentrations. Am J Clin
Nutr, 65: 803-813.
Igelsrud I, Thompson TG, & Zwicker BMG (1938) The boron content of sea
water and of marine organisms. Am J Sci, 35: 47-63.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety.
Iyengar GV, Clarke WB, Downing RG, & Tanner JT (1988) Lithium in
biological and dietary materials. In: Trace element analytical
chemistry in medicine and biology: Proceedings of the 5th
international workshop. Berlin, New York, Walter de Gruyter & Co.,
pp 267-269.
Iyengar GV, Clarke WB, Downing RG, & Tanner JT (1990) Determination of
boron and lithium in diverse biological matrices using neutron
activation-mass spectrometry (NA-MS). Fresenius J Anal Chem,
338: 562-566.
Jansen JA, Schou JS, & Aggerbeck A (1984) Gastrointestinal absorption
and in vitro release of boric acid from water-emulsifying ointments.
Food Chem Toxicol, 22: 49-53.
Jenkins DW (1980) Biological monitoring of toxic trace metals--Volume
2: Toxic trace metals in plants and animals of the world. Washington,
DC, US Environmental Protection Agency (EPA-600/3-80-090).
Jeshima D, Morishta K, Ueda Y, Zutagami K, Higuchi S, Komoda T,
Nanishi F, Janeyama J, Yoshitake J, & Aoyama T (1992) Clinical
management of boric acid ingestion: pharmacokinetic assessment of
efficacy of haemodialysis for treatment of acute boric acid poisoning.
J Pharmacobio-Dyn, 15: 287-294.
Job C (1973) Resorption and excretion of orally administered boron. Z
Angew Bader-Klimahelik, 20: 137-142.
Juhnke I & Ludemann D (1978) [Results of the investigation of 200
chemical compounds for acute fish toxicity with the golden orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German with English
summary).
Kabata-Pendias A & Pendias H (1984) Trace elements in soil and plants.
Boca Raton, Florida, CRC Press, Inc.
Keating KI & Dagbusan BC (1984) Effect of selenium deficiency on
cuticle integrity in the Cladocera (crustacea). Proc Natl Acad Sci
(USA), 81: 3433-3437.
Kent NL & McCance RA (1941) The absorption and excretion of "minor"
elements by man: I. Silver, gold, lithium, boron and vanadium. Biochem
J, 35: 837-844.
Keren R & Mezuman U (1981) Boron adsorption by clay minerals using a
phenomenological equation. Clays Clay Miner, 29: 198-204.
Keren R, Gast RG, & Bar-Yosef B (1981) pH-dependent boron adsorption
by Na-montmorillonite. Soil Sci Soc Am J, 45: 45-48.
King N, Odom TW, Sampson HW, & Pardue SL (1991a) In ovo
administration of boron alters bone mineralization of the chicken
embryo. Biol Trace Elem Res, 30: 47-58.
King N, Odom T, Sampson HW, & Yersin AG (1991b) The effect of
in ovo boron supplementation on bone mineralization of the vitamin
D-deficient chicken embryo. Biol Trace Elem Res, 31: 223-233.
Klasing SA & Pilch SM (1988) Agricultural drainage water contamination
in the San Joaquin Valley: a public health perspective for selenium,
boron and molybdenum. Sacramento, California, San Joaquin Valley
Drainage Program.
Kliegel W (1980) [Boron in biology, medicine and pharmacy:
Physiological effects and use of boron compounds.] Berlin, Germany,
Springer-Verlag (in German).
Kluge R & Podlesak W (1985) Plant critical levels for the evaluation
of boron toxicity in spring barley (Hordeum vulgare L.). Plant Soil,
83: 381-388.
Kobayashi M, Matoh T, & Azuma J (1996) Two chains of
rhamnogalacturonan II are cross-linked by borate-diol ester bonds in
higher plant cell walls. Plant Physiol, 110: 1017-1020.
Kohl HC & Oertli JJ (1961) Distribution of boron in leaves. Plant
Physiol, 36: 420-424.
Kopf W & Wilk A (1995) Investigation on aquatic toxicity against plant
organisms. Munich, Germany, Institute for Water Research (Unpublished
report).
Kopp JF & Kroner RC (1970) Trace elements in waters of the United
States. Cincinnati, Ohio, US Department of the Interior, Federal Water
Pollution Control Administration.
Korenaga T, Motomizu S, & Toei K (1980) Improved extraction method for
the spectrophotometric determination of trace amounts of boron in
river water with 1,8-dihydroxynaphthalene-4-sulphonic acid and removal
of the excess of reagent. Analyst, 105: 955-964.
Kowalenko CG (1979) Sodium hypobromite digestion for boron analysis of
plant and soil materials. Comun Soil Sci Plant Anal, 10(11):
1421-1434.
Ku WW & Chapin RE (1994) Mechanism of the testicular toxicity of boric
acid in rats: In vivo and in vitro studies. Environ Health
Perspect, 102(Suppl 7): 99-105.
Ku WW, Chapin RE, Moseman RF, Brink RE, Pierce KD, & Adams KY (1991)
Tissue disposition of boron in Fischer rats. Toxicol Appl Pharmacol,
111: 145-151.
Ku WW, Chapin RE, Wine RN, & Gladen BC (1993a) Testicular toxicity of
boric acid (BA): Relationship of dose to lesion development and
recovery in the F344 rat. Reprod Toxicol, 7: 305-319.
Ku WW, Shih LM, & Chapin RE (1993b) The effects of boric acid (BA) on
testicular cells in culture. Reprod Toxicol, 7: 321-331.
Landolph JR (1985) Cytotoxicity and negligible genotoxicity of borax
and borax ores to cultured mammalian cells. Am J Ind Med, 7: 31-43.
Lang JT & Treece RE (1972) Boric acid effects on face fly fecundity. J
Econ Entomol, 65: 740-741.
Lang FJ, Bingham FT, Hendrix FF, & Crane NL (1986) Boron deposition on
soil and native vegetation from geothermal emissions. J Environ Qual,
15(3): 260-265.
Larsen LA (1988) Boron. In: Seiler HG & Sigel H ed. Handbook on
toxicity of inorganic compounds. New York, Basel, Marcel Dekker, Inc.,
pp 129-141.
Leblond CP & Clermont Y (1952) Definition of the stages of the
seminiferous epithelium of the rat. Ann NY Acad Sci, 55: 548-573.
Lee IP, Sherins RJ, & Dixon RL (1978) Evidence for induction of
germinal aplasia in male rats by environmental exposure to boron.
Toxicol Appl Pharmacol, 45: 577-590.
Leland HV & Scudder BC (1990) Trace elements in Corbicula fluminea
from the San Joaquin River, California. Sci Total Environ, 97/98:
641-672.
Lewin JC (1966) Boron as a growth requirement for diatoms. J Phycol,
2: 160-163.
Lewis RJ Sr (1992) Sax's dangerous properties of industrial materials,
8th ed. New York, Van Nostrand Reinhold Co., vol II, pp 528-533.
Lewis RJ Sr (1993) Hawley's condensed chemical dictionary, 12th ed.
New York, Van Nostrand Reinhold Co., pp 161-165.
Lewis MA & Valentine LC (1981) Acute and chronic toxicities of boric
acid to Daphnia magna Straus. Bull Environ Contam Toxicol, 27:
309-315.
Linden CH, Hall AH, Kulig KW, & Rumack BH (1986) Acute ingestion of
boric acid. J Toxicol Clin Toxicol, 24: 269-279.
Linder RE, Strader LF, & Rehnberg GL (1990) Effect of acute exposure
to boric acid on the male reproductive system of the rat. J Toxicol
Environ Health, 31: 133-146.
Litovitz TL, Klein-Schwartz W, Oderda GM, & Schmitz BF (1988) Clinical
manifestation of toxicity in a series of 784 boric acid ingestions. Am
J Emerg Med, 6: 209-213.
Loomis WD & Durst RW (1992) Chemistry and biology of boron.
Biofactors, 3: 229-239.
Lopez FJ, Gimenez E, & Hernandez F (1993) Analytical study on the
determination of boron in environmental water samples. Fresenius J
Anal Chem, 346: 984-987.
Loppi S & Bargagli R (1996) Lichen biomonitoring of trace elements in
a geothermal area (central Italy). Water Air Soil Pollut, 88: 177-187.
Lovatt CJ & Dugger WM (1984) Boron. In: Frieden E ed. Biochemistry of
the essential ultratrace elements. New York, London, Plenum Press, pp
389-421.
Ludbrook WV (1942) The effects of various concentrations of boron on
the growth of pine seedlings in water culture. J Aust Inst Agric Sci,
8: 112-114.
Lyday P (1993) Boron. Washington, DC, US Department of the Interior,
p 16.
Lyday P (1996) Boron: Annual review, 1995. Boston, Massachusetts, US
Geological Survey, pp 1-2 (Mineral Industry Surveys series).
McCabe LJ, Symons JM, Lee RD, & Robeck GG (1970) Survey of community
water supply systems. J Am Water Works Assoc, 62: 670-687.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, &
Caspari WJ (1988) Responses of the L5178Y tk-/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen,
12: 85-154.
Maeso ES, Fernandez-Valiente E, Bonilla I, & Mateo P (1985)
Accumulation of proteins in giant cells, induced by high boron
concentrations in Chlorella pyrenoidosa. J Plant Physiol, 121:
301-311.
Magour S, Schramel P, Ovcar J, & Maser H (1982) Uptake and
distribution of boron in rats: Interaction with ethanol and
hexobarbital in the brain. Arch Environ Contam Toxicol, 11: 521-525.
Maier KJ & Knight AW (1991) The toxicity of waterborne boron to
Daphnia magna and Chironomus decorus and the effects of water
hardness and sulfate on boron toxicity. Arch Environ Contam Toxicol,
20: 282-287.
Mance G, O'Donnell AR, & Smith PR (1988) Proposed environmental
quality standards for List II substances in water: Boron. Water
Research Centre (Technical Report No. 256).
Manfredi F, Galleri A, & Cenci P (1975) [Concentrations of boron in
Imperia Province.] Ig Mod, 68: 466-476 (in Italian).
Mann H (1973) [Study of the effect of boron compounds on fish and
other aquatic organisms.] Arch Fisch Wiss, 24: 171-175 (in German with
English summary).
Marks G, Bruchlos P, & Bergmann W (1994) [Experimental results on the
tolerance of Phragmites australis seed.] In: [Proceedings of VDLUFA
Congress, Jena, Germany, 19-24 September 1994], pp 705-708 (Report No.
38/1994) (in German).
Martinez F, Mateo P, Bonilla I, & Fernandez-Valiente E (1986) Cellular
changes due to boron toxicity in the blue-green alga Anacystis
nidulans. Int J Exp Bot, 46: 145-152.
Marzadoori C, Antisari LV, Ciavatta C, & Sequi P (1991) Soil organic
matter influence on adsorption and desorption of boron. Soil Sci Soc
Am J, 55: 1582-1585.
Mateo P, Bonilla I, Fernandez-Valiente E, & Sanchez-Maeso E (1986)
Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC
7119. Plant Physiol, 81: 17-21.
Meacham SL, Japer LJ, & Valpe SL (1995) Effects of boron
supplementation on blood and urinary calcium, magnesium and phosphorus
and urinary boron in athletic and sedentary women. Am J Clin Nutr,
61: 341-345.
Mellor (1980) Mellor's comprehensive treatise on inorganic and
theoretical chemistry, Volume V. Boron, Part A: Boron-oxygen
compounds. London, New York, Longman.
Moore JA and an Expert Scientific Committee (1997) An assessment of
boric acid and borax using the IEHR evaluative process for assessing
human developmental and reproductive toxicity of agents. Reprod
Toxicol, 11(1): 123-160.
Mora MA & Anderson DW (1995) Selenium, boron and heavy metals in birds
from Mexicali Valley, Baja California, Mexico. Bull Environ Contam
Toxicol, 54: 198-206.
Muetterties EL (1967) The chemistry of boron and its compounds. New
York, John Wiley and Sons, pp 1-2, 329.
Mumma RO, Raupach DC, & Waldman JP (1984) National survey of elements
and other constituents in municipal sewage sludges. Arch Environ
Contam Toxicol, 13: 75-83.
Murray FJ (1995) A human health risk assessment of boron (boric acid
and borax) in drinking water. Regul Toxicol Pharmacol, 22: 221-230.
NAPM (1974) Environmental effect of photoprocessing chemicals: Volume
1. Prepared by NAPM, Inc., in cooperation with Hydroscience, Inc.
Harrison, NY, National Association of Photographic Manufacturers, Inc.
Navarro J, Mansour M, & Ivorra R (1992) Use of waste water in
Mediterranean crops. Fresenius Environ Bull, 1: 423-427.
Newnhan (1994a) The role of boron in human nutrition. J Appl Nutr,
46(3): 81-85.
Nielsen GH (1970) Percutaneous absorption of boric acid from
boron-containing preparations in rats. Acta Pharmacol Toxicol, 20:
413-424.
Nielsen FH (1989) Dietary boron affects variables associated with
copper metabolism in humans. In: Aulse M, Baumann W, Bröunlich H,
Brückner C, Groppel B, & Grün M ed. Tenth International Trace Element
Symposium, 1989. Jena, Friedrich-Schiller University, vol 4, pp
1106-1111.
Nielsen FH (1991) Nutritional requirements for boron, silicon,
vanadium, nickel and arsenic: Current knowledge and speculation. FASEB
J, 5: 2661-2667.
Nielsen GH (1992) Facts and fallacies about boron. Nutr Today,
27: 6-12.
Nielsen FH (1994) Biochemical and physiologic consequences of boron
deprivation in humans. Environ Health Perspect, 102(Suppl 7): 59-73.
Nielsen FH (1995) Beneficial, unavoidable essential? Defining boron's
nutritional importance. Borax Pioneer, 4.
Nielsen FH (1996) Dietary supplementation of physiological amounts of
boron increases plasma and urinary boron of perimenopausal women.
(Professional Communication). Proc North Dakota Acad Sci, 50: 52.
Nielsen FH & Shuler TR (1992) Studies of the interaction between boron
and calcium and its modification by magnesium and potassium in rats:
Effects on growth, blood variables and bone mineral composition. Biol
Trace Elem Res, 35: 225-237.
Nielsen FH, Hunt CD, Mullen LM, & Hunt JR (1987) Effect of dietary
boron on mineral, estrogen and testosterone metabolism in
postmenopausal women. FASEB J, 1: 394-397.
Nielsen FH, Mullen LM, & Gallagher SK (1990) Effect of boron depletion
and repletion on blood indicators of calcium status in humans fed a
magnesium-low diet. J Trace Elem Exp Med, 3: 45-54.
Nielsen FH, Mullen LM, & Nielsen EJ (1991) Dietary boron affects blood
cell counts and hemoglobin concentrations in humans. J Trace Elem Exp
Med, 4: 211-223.
Nielsen FH, Shuler TR, & Uthus EO (1992a) Dietary arginine and
methionine effects and their modification by dietary boron and
potassium on the mineral element composition of plasma and bone in the
rat. J Trace Elem Exp Med, 5: 247-259.
Nielsen FH, Gallagher SK, Johnson LK, & Nielsen EJ (1992b) Boron
enhances and mimics some effects of estrogen therapy in postmenopausal
women. J Trace Elem Exp Med, 5: 237-246.
NIOSH (1978) Health hazard evaluation determination: Kerr-McGee
Chemical Corporation, Trona, CA. Cincinnati, Ohio, National Institute
for Occupational Safety and Health (Publication No. 77-59-496).
NIOSH (1989) National occupational exposure survey. Cincinnati, Ohio,
National Institute of Occupational Safety and Health (Publications Nos
89-101, 89-102, 89-103).
Noakes JE & Hood DW (1961) Boron-boric acid complexes in seawater.
Deep-Sea Res, 8: 121-129.
Nobel W (1981) [The effect of boron on submerged soft-water
macrophytes.] Angew Bot, 55: 501-514 (in German with English summary).
Nolte J (1988) Pollution source analysis of river water and sewage
sludge. Environ Technol Lett, 9: 857-868.
Nose K & Zenki M (1991) Flow injection spectrophotometric
determination of boron with D-sorbitol using methyl orange as an
indicator. Analyst, 116: 711-714.
Nriagu JO (1979) Copper in the atmosphere and precipitation. In:
Nriagu JO ed. Copper in the environment, Part I: Ecological cycling.
New York, John Wiley and Sons, pp 43-75.
NTP (1987) Toxicology and carcinogenesis studies of boric acid (CAS
No. 10043-35-3) in B6C3F1 mice (feed studies). Research Triangle
Park, North Carolina, US Department of Health and Human Services,
National Toxicology Program (NTP TR No. 324).
NTP (1989) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in CD-1-Swiss mice. Research Triangle Park, North
Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP Report No. 89-250).
NTP (1990) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in Sprague-Dawley rats. Research Triangle Park,
North Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP Report No. 90-105).
NTP (1991) Final report on the developmental toxicity of boric acid
(CAS No. 10043-35-3) in New Zealand white rabbits. Research Triangle
Park, North Carolina, US Department of Health and Human Services,
National Toxicology Program (NTP TER-90003).
Odom JW (1980) Kinetics of the hot water soluble boron soil test.
Commun Soil Sci Plant Anal, 11(7): 759-765.
Ohlendorf HM, Hoffman DJ, Saiki MK, & Aldrich TW (1986) Embryonic
mortality and abnormalities of aquatic birds: Apparent impacts of
selenium from irrigation drainwater. Sci Total Environ, 52: 49-63.
Okay O, Güçlü H, Soner E, & Balka T (1985) Boron pollution in the
Simav River, Turkey and various methods of boron removal. Water Res,
19(7): 857-862.
Ostrovskij NI (1955) [Toxic effect on honey bees by contact with
herbicides and foliar fertilizer.] Dokl Vses Akad Skh Nauk im V I
Lenina, 20: 32-34 (in Russian).
O'Sullivan K & Taylor M (1983) Chronic boric acid poisoning in
infants. Arch Dis Child, 58: 737-739.
Owen EC (1944) The excretion of borate by the dairy cow. J Dairy Res,
13: 243-248.
Parr AJ & Loughman PC (1983) Boron and membrane function in plants.
Annu Proc Phytochem Soc Eur, 21: 87-107.
Paveglio FL, Bunck CM, & Heinz GH (1992) Selenium and boron in aquatic
birds from central California. J Wildl Manage, 56: 31-42.
Pendleton GW, Whitworth MR, & Olsen GH (1995) Accumulation and loss of
arsenic and boron, alone and in combination, in mallard ducks. Environ
Toxicol Chem, 14(8): 1357-1364.
Penland JG (1994) Dietary boron, brain function and cognitive
performance. Environ Health Perspect, 102(Suppl 7): 65-72.
Penland JG & Eberhardt MJ (1993) Effects of dietary boron and
magnesium on brain function of mature male and female Long-Evans rats.
J Trace Elem Exp Med, 6: 53-64.
Pennington HD, Finch CR, Lyons CC, & Littau SA (1991) Microwave
digestion of plant samples for boron analysis. Hort Science, 26(12):
1496-1497.
Pfeiffer CC, Hallman LF, & Gersh I (1945) Boric acid ointment: A study
of possible intoxication in the treatment of burns. J Am Med Assoc,
128: 266-274.
Price CJ, Strong PL, Marr MC, Myers CB, & Murray FJ (1996a)
Developmental toxicity NOAEL and postnatal recovery in rats fed boric
acid during gestation. Fundam Appl Toxicol, 32: 179-193.
Price CJ, Marr MC, Myeos CB, Seely JC, Heindel JJ, & Schwetz BA
(1996b) The developmental toxicity of boric acid in rabbits. Fundam
Appl Toxicol, 34: 176-187.
Price CJ, Strong PL, Murray FJ, & Goldberg MM (1997) Blood boron
concentrations in pregnant rats fed boric acid throughout gestation.
Reprod Toxicol, 11(6): 833-842.
Rai D, Zachara JM, Schwab AP, Schmidt RL, Girvin DC, & Rogers JE
(1986) Chemical attenuation rates, coefficients, and constants in
leachate migration--Volume 1: A critical review (Research Project
2198-1). Richland, Washington, Battelle, Pacific Northwest
Laboratories (Report to Electric Power Research Institute, Palo Alto,
California).
Rainey CJ, Christensen RE, & Nyquist LA (1996) The dietary consumption
of boron in the United States. Irvine, California, Nutrition Research
Group (Unpublished report).
Reeve E & Shive JW (1944) Potassium-boron and calcium-boron
relationships in plant nutrition. Soil Sci, 57: 1-14.
Reid PC & Davies E (1989) Boron content of South African surface
waters: Preliminary assessment for irrigation. Water SA, 15(4):
261-264.
Reisenauer HM & Cox WJ (1971) Boron uptake by plants from boron
containing waters. Presented at 162nd National American Chemical
Society Meeting, 12-17 September 1971. Washington, DC, American
Chemical Society, Division of Water, Air and Waste Chemistry.
Rhoades JD, Ingvalson RD, & Hatcher JT (1970) Adsorption of boron by
ferromagnesium minerals and magnesium hydroxide. Soil Sci Soc Am Proc,
34: 938-941.
Robinson DJS & Perkins EJ (1977) The toxicity of some wood pulp
effluent constituents. Carlisle, UK, Cumbria Sea Fisheries Committee,
22 pp (Scientific Report No. 74/1).
Rosenkranz HS (1973) Sodium hypochlorite and sodium perborate:
Preferential inhibitors of DNA polymerase-deficient bacteria. Mutat
Res, 21: 171-174.
Roudabush RL, Terhaar CJ, Fassett DW, & Dziuba SP (1965) Comparative
acute effects of some chemicals on the skin of rabbits and guinea
pigs. Toxicol Appl Pharmacol, 7: 559-565.
Rusch GM, Hoffman GM, McConnell RF, & Rinehart WE (1986) Inhalation
toxicity studies with boron trifluoride. Toxicol Appl Pharmacol,
83: 69-78.
Sage RF, Ustin SL, & Manning SJ (1989) Boron toxicity in the rare
serpentine plant, Streptanthus morrisonii. Environ Pollut, 61:
77-93.
Saiki MK & May TW (1988) Trace element residues in bluegills and
common carp from the lower San Joaquin River, California and its
tributaries. Sci Total Environ, 74: 199-217.
Saiki MK, Jennings MR, & Brumbaugh WG (1993) Boron, molybdenum, and
selenium in aquatic food chains from the lower San Joaquin River and
its tributaries, California. Arch Environ Contam Toxicol, 24: 307-319.
Schöberl P & Huber L (1988) [Ecologically relevant data of non-tenside
compounds in detergents and cleaners.] Tenside Deterg, 25: 99-107 (in
German).
Schöller F & Bolzer W (1989) Boron--a substance of problem in the
water field. Water Supply, 7: 169-177.
Schöller F, Ollram F, & Putre R (1981) [Boron content of lower
Austria's waters.] Österr Wasserwirtsch, 33: 9-15 (in German).
Schou JS, Jansen JA, & Aggerbeck B (1984) Human pharmacokinetics and
safety of boric acid. Arch Toxicol, 7(Suppl): 232-235.
Schroeder HA & Mitchener M (1975) Life-term effects of mercury, methyl
mercury, and nine other trace metals on mice. J Nutr, 105: 452-458.
Schwab AP, Tomecek MB, & Ohlenbusch PD (1991) Plant availability of
lead, cadmium, and boron in amended coal ash. Water Air Soil Pollut,
57-58: 297-306.
Seal BS & Weeth HJ (1980) Effect of boron in drinking water on the
male laboratory rat. Bull Environ Contam Toxicol, 25: 782-789.
Seffner W, Teubener W, Runde H, Wiedner H, Vogt J, Otto G, Zschau E,
Geinitz D, & Franke J (1990) Boron as an antidote to fluorosis? II.
Studies on various organs of pigs. Fluoride, 23(2): 68-79.
Sekerka I & Lechner JF (1990) Automated method for the determination
of boron in water by flow-injection analysis with in-line
preconcentration and spectrophotometric detection. Anal Chim Acta,
234: 199-206.
Settimi L, Elovaara E, & Savolainen H (1982) Effects of extended
peroral borate ingestion on rat liver and brain. Toxicol Lett,
10: 219-223.
Shani U, Dudley LM, & Hanks RJ (1992) Model of boron movement in
soils. Soil Sci Soc Am J, 56: 1365-1370.
Shann JR & Adriano DC (1988) The chronic exposure of selected crop
species to boron aerosols. Environ Exp Bot, 28: 289-299.
Shopova M, Petrovska D, Musalevski A, Najcevska C, & Sekovski Z (1981)
Cytogenetical and morphological effect of general toxicity caused with
different concentrations of boron. Mutat Res, 85: 229.
Shuler TR, Pootrakul P, Yarnsukon P, & Nielsen FH (1990) Effect of
thalassaemia/immunoglobin E disease on macro, trace and ultratrace
element concentrations in human tissues. J Trace Elem Exp Med, 3:
31-43.
Silaev AA (1984) [Experimental determination of the maximum
permissible concentration of sodium perborate in workplace air.] Gig
Tr Prof Zabol, 6: 44 (in Russian with French translation).
Silaev AA, Kasparov AA, Korolev VV, & Nebstrueva VV (1977)
Electron-microscopic investigation of the effect of boric acid on the
seminiferous tubules of albino rats. Bull Exp Biol Med (USSR), 83:
588-591.
Sims JR & Bingham FT (1967) Retention of boron by layer silicates,
sesquioxides and soil materials: I. Layer silicates. Soil Sci Soc Am
Proc, 31: 728-731.
Simsiman GV, Chesters G, & Andren AW (1987) Effect of ash disposal
ponds on groundwater quality at a coal-fired power plant. Water Res,
21: 417-426.
Smidt RE & Whitton JS (1975) Note on boron toxicity in a stand of
radiata pine in Hawkes Bay. NZ J Sci, 18: 109-113.
Smith GJ & Anders VP (1989) Toxic effects of boron on mallard
reproduction. Environ Toxicol Chem, 8: 943-950.
Smith FG, Wiederim DR, Houk RS, Egan CB, & Serfass RE (1991)
Measurement of boron concentration and isotope ratios in biological
samples by inductively coupled plasma mass spectrometry with direct
injection nebulization. Anal Chem Acta, 248: 229-234.
Smyth DA & Dugger WM (1981) Changes during boron deficient culture of
the diatom Cylindrotheca fusiformis. Plant Physiol, 51: 111-117.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, & Nycum
JS (1969) Range finding toxicity data: List VII. Am Ind Hyg Assoc J,
30: 470-476.
Sprague RW (1972) The ecological significance of boron. Anaheim,
California, US Borax Research Corporation, 58 pp.
Stanley RA (1974) Toxicity of heavy metals and salts to Eurasian
watermilfoil (Myriophyllum spicatum L.). Arch Environ Contam
Toxicol, 2(4): 331-341.
Stein KM, Odom RB, Justice GR, & Martin GC (1973) Toxic alopecia from
ingestion of boric acid. Arch Dermatol, 108: 95-97.
Stelter LH (1980) Baseline levels of selected trace elements in
Colorado oil shale region animals. J Wildl Dis, 16: 175-182.
Stephan CE, Mount DI, Hansen DJ, Gentile JH, Chapman GA, & Brungs WA
(1985) Guidelines for deriving numerical national water quality
criteria for the protection of aquatic organisms and their uses--Final
Report. Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication PB85-227049).
Stokinger HE (1981) Boron. In: Clayton GD & Clayton FE ed. Patty's
industrial hygiene and toxicology--Volume 2B: Toxicology, 3rd ed. New
York, John Wiley and Sons, pp 2978-3005.
Stokinger HE & Spiegl CJ (1953) Special materials, Part A:
Inhalation-toxicity studies of boron halides and certain fluorinated
hydrocarbons. In: Voegtlin C & Hodge HC ed. Pharmacology and
toxicology of uranium compounds: Chronic inhalation and other studies.
New York, McGraw-Hill, pp 2291-2321.
Stuttgen G, Siebel TH, & Aggerbeck B (1982) Absorption of boric acid
through human skin depending on the type of vehicle. Arch Dermatol
Res, 272: 21-29.
Suloway JJ, Roy WR, Skelly TM, Dickerson DR, Schuller RM, & Griffin RA
(1983) Chemical and toxicological properties of coal fly ash.
Champaign, Illinois, Illinois State Geological Survey (Publication
NTIS PB84-116110).
Takeuchi T (1958) Effects of boric acid on the development of the eggs
of the toad, Bufo vulgaris Formosus. Sci Rep Tohoku Univ Ser R Biol,
24: 33-43.
Tanaka H (1966) Response of Lemna pausicostata to boron as affected
by light intensity. Plant Soil, 25: 425-434.
Tartari G & Camusso M (1988) Boron content in freshwaters of northern
Italy. Water Air Soil Pollut, 38: 409-417.
Taylor D, Maddock BG, & Mance G (1985) The acute toxicity of nine
"grey list" metals (arsenic, boron, chromium, copper, lead, nickel,
tin, vanadium and zinc) to two marine fish species: Dab (Limanda
limanda) and grey mullet (Chelon labrosus). Aquat Toxicol, 7:
135-144.
Tehseen WM, Hansen LG, Wood SG, & Hanif M (1994) Assessment of
chemical contaminants in water and sediment samples from Degh Nala in
the Province of Punjab, Pakistan. Arch Environ Contam Toxicol, 26:
79-89.
Temple PJ & Linzon SN (1976) Boron as a phytotoxic air pollutant. J
Air Pollut Control Assoc, 26: 498-499.
Teshima D, Morishita K, Ueda Y, Futagami K, Higuchi S, Komoda T,
Nanishi F, Taniyama T, Yoshitake J, & Aoyama T (1992) Clinical
management of boric acid ingestion: Pharmacokinetic assessment of
efficacy of hemodialysis for treatment of acute boric acid poisoning.
J Pharmacobio-Dyn, 15: 287-294.
Thompson JAJ, Davis JC, & Drew RE (1976) Toxicity, uptake and survey
studies of boron in the marine environment. Water Res, 10: 869-875.
Torkelson TR, Sadek SE, & Rowe VK (1961) The toxicity of boron
trifluoride when inhaled by laboratory animals. Am Ind Hyg Assoc J,
22: 263-270.
Treinen KA & Chapin RE (1991) Development of testicular lesions in
F344 rats after treatment with boric acid. Toxicol Appl Pharmacol,
107: 325-335.
Truhaut R, Phu-Lich N, & Loisillier F (1964) Effects of the repeated
ingestion of small doses of boron derivatives on the reproductive
functions of the rat. C R Acad Sci (Paris), 258: 5099-5102.
Tsui PTP & McCart PJ (1981) Chlorinated hydrocarbon residues and heavy
metals in several fish species from the Cold Lake area in Alberta,
Canada. Int J Environ Anal Chem, 10: 277-285.
Turnbull H, Demann JG, & Weston RF (1954) Toxicity of various refinery
materials to fresh water fish. Ind Eng Chem, 46: 324-333.
UK Ministry of Agriculture, Fisheries and Food (1991) Household food
consumption and expenditure 1991--Annual Report of the National Food
Survey Committee. London, Her Majesty's Stationary Office, Table B1.
Unilever (1994) Summary of external research project on environmental
levels of boron, especially in trout-sustaining waters. Unilever
Research, Port Sunlight Laboratory, 3 pp (Unpublished report).
US EPA (1991) Health and environmental effects document for boron and
boron compounds. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (EPA/600/8-91/015).
US EPA (1994) Integrated risk information system--Online. Cincinnati,
Ohio, US Environmental Protection Agency, Environmental Criteria and
Assessment Office.
US FDA (1981) Product formulation data--Computer printout. Washington,
DC, US Food and Drug Administration.
US NLM (1993) Hazardous substances data bank. Bethesda, Maryland,
National Library of Medicine, National Toxicology Information Program.
Vanderpool RA, Haff D, & Johnson PE (1994) Use of inductively coupled
plasma-mass spectrometry in boron--10 stable isotope experiments with
plants, rats and humans. Environ Health Perspect, 102(Suppl 7): 13-20.
Verbitskaya GV (1975) [Experimental and field investigations
concerning the hygienic evaluation of boron-containing drinking
water.] Gig i Sanit, 7: 49-53 (in Russian with English summary).
Vernot EH, MacEwen JD, Haun CC, & Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicol Appl Pharmacol, 42: 417-424.
Versar Inc. (1975) Preliminary investigation of effects on the
environment of boron, indium, nickel, selenium, tin, vanadium and
their compounds--Volume 1: Boron. Springfield, Virginia, Versar, Inc.
(Prepared for the US Environmental Protection Agency, Washington) (EPA
560/2-75-005a).
Vignec AJ & Ellis R (1954) Inabsorbability of boric acid in infant
powder. Am J Dis Child, 88: 72-80.
Waggott A (1969) An investigation of the potential problem of
increasing boron concentrations in rivers and water courses. Water
Res, 3: 749-765.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia
affinis of certain pure chemicals in turbid waters. Sewage Ind
Wastes, 29: 695-711.
Wang W (1986) Toxicity tests of aquatic pollutants by using common
duckweed. Environ Pollut, B11: 1-14.
Wang E, Xun X, Wang H, Chen Y, Meng X, Zhang S, Huang J, Cong S, Tao
K, Yu B, Xiao J, He D, Yang X, & Jiang J (1984) Toxicological effect
of boron in laboratory rats. Zhonghua Yufangyixue Zazhi, 18(1): 20-22
(in Chinese, with English abstract).
Ward NI (1993) Boron levels in human tissues and fluids. In: Meissner
MAD & Mills C-F ed. Trace elements in man and animals--TEMA 8.
Proceedings of the Eighth International Symposium on Trace Elements in
Man and Animals. Gersford, Germany, Verlag Media Touristik, pp
724-728.
Warrington K (1923) The effects of boric acid and borax on the bread
bean and certain other plants. Ann Bot, 37: 629-672.
Weast RC, Astle MJ, & Beyer WH ed. (1985) CRC handbook of chemistry
and physics, 69th ed. Boca Raton, Florida, CRC Press, Inc., pp B/77,
B/129.
Weeth HJ, Speth CF, & Hanks DR (1981) Boron content of plasma and
urine as indicators of boron intake in cattle. Am J Vet Res, 42:
474-477.
Wegman DH, Eisen EA, Hu X, Woskie SR, Smith RG, & Garabrant DH (1994)
Acute and chronic respiratory effects of sodium borate particulate
exposures. Environ Health Perspect, 102(Suppl 7): 119-128.
Weir RJ & Fisher RS (1972) Toxicologic studies on borax and boric
acid. Toxicol Appl Pharmacol, 23: 351-364.
Wells N & Whitton JS (1977) A pedochemical survey: 3. Boron. NZ J Sci,
20: 317-332.
Whetstone RR, Robinson WO, & Byers HG (1942) Boron distribution in
soils and related data. Washington, DC, US Department of Agriculture
(Technical Bulletin No. 797).
Whitworth MR, Pendleton GW, Hoffman DJ, & Camardese MB (1991) Effects
of dietary boron and arsenic on the behavior of mallard ducklings.
Environ Toxicol Chem, 10: 911-916.
WHO (1993) Boron. In: Guidelines for drinking-water quality--Volume 1:
Recommendations. Geneva, World Health Organization, pp 43-44.
WHO (1996) Trace elements in human nutrition and health. Geneva, World
Health Organization, pp 175-179.
WHO (1998a) Guidelines for drinking-water quality. Second edition.
Addendum to Volume 1: Recommendations. Geneva, World Health
Organization.
WHO (1998b) Guidelines for drinking-water quality. Second edition.
Addendum to Volume 2: Health criteria and other supporting
information. Geneva, World Health Organization (WHO/EOS/98.1).
Whorton MD, Haas JL, Trent L, & Wong O (1994) Reproductive effects of
sodium borates on male employees: Birth rate assessment. Occup Environ
Med, 51: 761-767.
Wiecken B & Wübbold-Weber S (1993) [Final report on boron monitoring
in drinking-water.] Degussa AG (Unpublished report) (in German).
Wiener JG, Kaufman DW, Kaufman GA, Gentry JB, Smith MH, & Ramsey PR
(1977) Chemical composition of rodents: use of whole body
concentrations for estimation of standing crops of elements. Southwest
Nat, 22: 77-88.
Wierenga PJ, van Genuchten MTH, & Boyle FW (1975) Transfer of boron
and tritiated water through sandstone. J Environ Qual, 4: 83-87.
Wikner B (1986) Pretreatment of plant and soil samples--A problem in
boron analysis: Part I. Plants. Commun Soil Sci Plant Anal, 17(1):
1-25.
Wilcox LV (1958) Water quality from the standpoint of irrigation. J Am
Water Works Assoc, 50: 650-654.
Wilding JL, Smith WJ, Yevich P, Sicks ME, Ryan SG, & Punte CL (1959)
The toxicity of boron oxide. Am Ind Hyg Assoc J, 20: 284-289.
Windholz M, Budavari S, Blemetti RF, & Otterbein ES ed. (1983) Merck
index, 10th ed. Rahway, New Jersey, Merck and Co., Inc., pp 185-187,
1099, 1231, 1237, 1239.
Woods WG (1994) An introduction to boron: history, sources, users and
chemistry. Environ Health Perspect, 102(Suppl 7): 5-11.
Woskie SR, Shen P, Eisen EA, Finkel MH, Smith TJ, Smith R, & Wegman,
DH (1994) The real-time dust exposures of sodium borate workers:
Examination of exposure variability. Am Ind Hyg Assoc J, 55: 207-217.
Wren CD, Maccrimmon HR, & Loescher BR (1983) Examination of
bioaccumulation and biomagnification of metals in a Precambrian Shield
lake. Water Air Soil Pollut, 19: 277-291.
Yamamoto T, Tamaoka T, Fujita T, & Isoda C (1973) Boron content in
marine plankton. Rec Oceanogr Works Jpn, 12: 13-21.
APPENDIX
Alternative Approaches -- Benchmark Dose
A suggested alternative to the use of a NOAEL in the development
of the TI is to use a BMD. Some advantages in using a BMD are as
follows: 1) more data from the dose-response curve are used; 2)
influence by background incidence rate is lessened; and 3) sensitivity
to the spacing of doses in the assay is reduced. A BMD is derived by
first calculating or estimating a dose that causes a critical effect
in a small percentage (e.g. 5%) of test animals (effective dose). A
BMD is then determined as the lower confidence limit of this effective
dose (Barnes et al., 1995).
Using data from the Heindel et al. (1992) study in rats and a
decreased fetal body weight as the critical effect, a BMD for boron of
9.3 mg/kg body weight per day was estimated using a polynomial model
(see Fig. 2). This BMD was based on the 95% confidence limit on the
dose that causes a 5% decrease in fetal body weight. Dividing by an
uncertainty factor of 25 gives a TI of 0.4 mg/kg body weight per day.
Another model (Weibull) was used (Allen et al., 1996) with study data
from Heindel et al. (1992) and Price et al. (1996a) and with decreased
fetal weight as the critical effect to derive a BMD of 9.8 mg/kg body
weight per day based on the 95% confidence limit on the dose that
causes a 5% decrease in fetal body weight. Dividing by an uncertainty
factor of 25 also gives a TI of 0.4 mg/kg body weight per day. Both
BMD-based TIs are identical to the one developed with the traditional
approach. Although use of a BMD to derive a TI does have some
advantages over the NOAEL method, there is as yet no consensus on the
incidence of effect to be used as a basis for deriving the BMD.
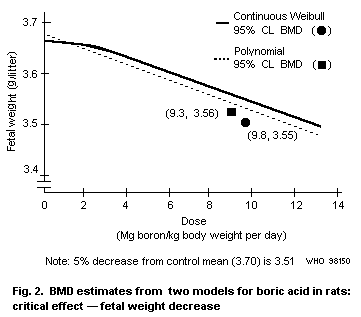 RÉSUMÉ, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé
1.1 Identité, état naturel et méthodes d'analyse
Le bore est un élément naturel qui se trouve sous la forme de
borates dans les océans, les roches sédimentaires, la houille, le
schiste et certaines huiles minérales. Il est très répandu dans la
nature, avec des concentrations de l'ordre de 10 mg/kg dans l'écorce
terrestre (elles vont de 5 mg/kg dans les basaltes à 100 mg/kg dans
les schistes) et d'environ 4,5 mg/litre dans les océans.
Les dérivés boriques les plus importants, qu'il s'agisse de
minéraux ou de produits du commerce, sont le borax à cinq molécules
d'eau, le borax, le perborate de sodium, l'acide borique, la
colemanite et l'ulexite. Aux faibles concentrations et au pH
pratiquement neutre qui caractérisent la plupart des liquides
biologiques, c'est le monomère B(OH)3 qui est l'espèce prédominante
(avec un peu de B(OH)4-), que la source de bore soit l'acide borique
ou un borate. Cela tient au fait que l'acide borique est un acide très
faible (pKa = 9,15).Le perborate de sodium s'hydrolyse pour donner du
peroxyde d'hydrogène et du métaborate; il peut donc présenter des
propriétés chimiques et toxicologiques un peu différentes de celles
des autres borates.
Les méthodes utilisant un plasma à couplage inductif (ICP) sont
les méthodes de choix pour le dosage des faibles quantités de bore
présentes dans les échantillons biologiques et environnementaux; les
méthodes colorimétriques doivent être utilisées avec prudence.
1.2 Production, usages, destinée dans l'environnement et sources d'exposition
Les dépôts de borates économiquement exploitables sont rares et
se trouvent dans des zones arides de Turquie, des Etats-Unis, du
Chili, de Russie, de Chine et du Pérou. La production mondiale totale
de minéraux contenant du bore--principalement de la colemanite, de
l'ulexite, du tincal et de la kernite--a été d'environ 2 750 000
tonnes en 1994. Environ 800 000 tonnes (en équivalents de B2O3) de
dérivés boriques commerciaux ont été produits à partir des minerais.
Les borates sont, entre autres, principalement utilisés pour
fabriquer des produits isolants, des fibres de verre de qualité
textile, des agents de blanchiment (perborate de sodium), des verres
au borosilicate, des retardateurs de flamme, des engrais et des
herbicides (à l'état de traces), des émaux, des vernis pour
céramiques, des frittes ainsi qu'une myriade d'applications diverses.
La pénétration du bore dans l'environnement se fait
principalement par l'action des agents météorologiques sur les roches,
la volatilisation de l'acide borique présent dans l'eau de mer et
l'activité volcanique. L'apport de bore dû aux activités humaines est
moindre. Parmi ces dernières sources figurent les brûlis agricoles,
l'incinération des déchets, la combustion du bois de feu, la
production d'énergie à partir du charbon et du pétrole, l'industrie du
verre, les borates et perborates utilisés comme produits industriels
et ménagers, l'exploitation des mines de borates, le traitement des
borates et des bois et papiers imprégnés et enfin le rejet des
effluents et des boues. Il est souvent difficile de chiffrer l'apport
de ces sources.
L'émission de borates et d'acide borique dans l'atmosphère se
fait sous la forme de particules et de vapeurs par suite de la
volatilisation de l'eau de mer, de l'activité volcanique et, dans une
moindre mesure, de l'exploitation des mines, de la fabrication de
verre et de céramique, de l'usage agricole de certains dérivés et des
rejets des centrales thermiques fonctionnant au charbon. Le bore n'est
pas très abondant dans l'atmosphère, mais la quantité totale qui s'y
trouve à un moment donné n'est pas négligeable du fait du volume
énorme de l'atmosphère. Compte tenu de la solubilité des borates dans
l'eau, ils ne devraient pas séjourner longtemps dans l'atmosphère en
concentrations importantes.
L'action des phénomènes météorologiques peut provoquer la
libération de bore dans le sol et dans l'eau de même que, encore qu'en
proportion bien moindre, les déversements d'origine humaine tels que
les rejets d'effluents. On pense que les phénomènes
d'adsorption-désorption sont les seuls mécanismes importants qui
soient susceptibles d'influer sur la destinée du bore dans
l'environnement. Le taux d'adsorption du bore dépend de sa
concentration en solution et du pH de l'eau.
Le bore s'adsorbe sur les particules du sol, le degré
d'adsorption étant fonction du type de sol, du pH, de la salinité, de
la teneur en matières organiques, en oxyde et hydroxyde d'aluminium,
en hydroxyde de fer et en argile. Le phénomène peut être parfaitement
réversible ou au contraire complètement irréversible, selon la nature
et l'état du sol.
Les ions borate présents en solution aqueuse s'y trouvent
essentiellement au degré d'oxydation maximum. Il n'y a pas de
processus aérobie qui soit susceptible d'influer sur la formation des
différentes espèces chimiques et on n'a pas fait état de
biotransformations. Il ne devrait donc pas y avoir de différence dans
les différentes espèces chimiques qui soit due à une
biotransformation.
Le coefficient de partage octanol/eau de l'acide borique est de
0, 175, ce qui indique que ce composé a un faible potentiel de
bioaccumulation. Les expériences de laboratoire effectuées sur des
organismes aquatiques ont confirmé l'existence d'un faible potentiel
de bioaccumulation. Les végétaux ont tendance à accumuler du bore;
toutefois la fixation du bore par les plantes dépend du pH de la
solution de sol, de la température, de l'intensité lumineuse et de la
concentration d'autres éléments (par ex. le calcium et le potassium).
Les études relatives à l'accumulation du bore par les plantes, les
insectes et les poissons montrent que cet élément s'accumule dans les
végétaux mais qu'il ne s'amplifie pas de long de la chaîne alimentaire
aquatique.
Le bore est présent dans les différents sols à des concentrations
qui vont de 10 à 300 mg/kg (la moyenne est de 30 mg/kg) selon la
nature du sol, sa teneur en matières organiques et l'importance des
précipitations. Sa concentration dans les eaux superficielles dépend
de plusieurs facteurs tels que la nature géochimique du bassin de
drainage, la proximité de zones littorales et les apports dus aux
décharges industrielles et municipales. Les valeurs sont très
variables, allant de 0,001 à 360 mg/litre. Toutefois les
concentrations moyennes dans les eaux de l'Europe, du Pakistan, de la
Russie et de la Turquie se situent nettement en dessous de 0,6
mg/litre. Au Japon, en Afrique du Sud et en Amérique du sud elles sont
généralement inférieures à 0,3 mg/kg. En Amérique du Nord, elles se
caractérisent par des valeurs inférieures à 0,1 mg/litre, dont 90%
inférieures ou égales à 0,4 mg/litre.
Le bore s'accumule dans les plantes aquatiques et terrestres mais
il ne s'amplifie pas le long de la chaîne alimentaire. On a trouvé des
concentrations de bore comprises entre 26 et 382 mg/kg dans des
plantes d'eau douces immergées, entre 11,3 et 57 mg/kg dans des
plantes d'eau douce semi-immergées et entre 2,3 et 94,7 mg/kg dans des
plantes terrestres. En se basant sur le poids frais, on constate que
les concentrations de bore trouvées dans les invertébrés marins et les
poissons sont analogues à celles que l'on mesure dans les milieux
correspondants, soit entre 0,5 et 4 mg/kg. Chez deux espèces de
poissons, on a trouvé un facteur de bioconcentration de 0,3.
Dans l'air ambiant, la concentration du bore varie de < 0,5 à
environ 80 ng/m3, avec une moyenne de 20 ng/m3 au-dessus des
continents.
Le fait que la concentration du bore dans les eaux souterraines
et les eaux douces de surface soit très voisine de sa concentration
dans l'eau de boisson, indique que ce élément n'est pas éliminé par
les traitements auxquels sont soumises les eaux souterraines et les
eaux de surface destinées à la boisson.
L'apport de bore chez l'Homme devrait être de 0,44 µg/jour à
partir de l'air ambiant, de 0,2-0,6 mg/jour à partir de l'eau de
boisson et de 1,2 mg/jour à partir de l'alimentation. On estime que
l'apport moyen de bore à partir du sol est de 0,5 µg/jour. On peut
raisonnablement estimer que l'apport de bore par les produits de
consommation est de 0,1 mg/jour.
1.3 Cinétique et suivi biologique
La pharmacocinétique du bore se révèle assez semblable d'une
espèce à l'autre, notamment en ce qui concerne les aspects suivants:
a) Absorption. L'absorption des borates est pratiquement complète
(environ 95% chez l'Homme et le rat) et après ingestion, le bore
apparaît rapidement dans le sang et les tissus de plusieurs
espèces de mammifères.
b) Distribution. Chez les mammifères, elle se produit selon un
mécanisme de diffusion passive par les liquides biologiques.
Contrairement aux tissus mous et au sang, les os sont capables de
fixer sélectivement le bore (teneur supérieure ou égale à 4 fois
celle du sérum) et de le retenir sensiblement plus longtemps.
c) Métabolisme. Le métabolisme de l'acide borique est
thermodynamiquement défavorable dans les systèmes biologiques.
Les espèces ioniques présentes dans le courant sanguin devraient
donc être les mêmes chez tous les mammifères. Dans ces
conditions, on risque beaucoup moins de se tromper en procédant à
des extrapolations, puisqu'il n'est pas nécessaire de prendre en
considération les différences interspécifiques qui existent
généralement au niveau des voies enzymatiques et de la vitesse de
métabolisation.
d) Elimination. La cinétique d'élimination (en particulier la voie
d'élimination et la demi-vie terminale) se révèle également
analogue chez l'Homme et le rat.
Les analogies qui existent entre les paramètres
pharmacocinétiques relevés chez l'Homme et chez le rat, l'espèce qui
sert à définir la dose sans effet nocif observable (NOAEL) dans les
études de laboratoire, permettent de réduire l'incertitude que
comporte l'extrapolation à l'Homme des résultats obtenus sur le rat.
1.4 Effets sur l'Homme et les animaux de laboratoire
Les données relatives aux effets toxiques sur le développement et
la reproduction montrent que l'effet déterminant consiste en une
réduction du poids des foetus. On a estimé à 9, 6 mg de bore par jour
et par kg de poids corporel, la NOAEL relative à cet effet. La dose la
plus faible produisant un effet nocif observable (LOAEL) est, chez le
rat, d'environ 13 mg/kg de poids corporel par jour, l'effet observé
étant une légère différence dans le poids des foetus (approx. 5%) et
des anomalies costales. A mesure que l'on augmente la dose, on observe
les effets suivants, selon la dose:
a) autres anomalies costales et testiculaires (approx. 25 mg de bore
par jour et par kg de poids corporel);
b) réduction du poids des foetus et accroissement des malformations
cardiovasculaires chez le lapin; graves anomalies testiculaires
chez le rat (approx. 40 mg de bore par jour et par kg de poids
corporel);
c) atrophie testiculaire et stérilité chez le rat (approx. 55 mg de
bore par jour et par kg de poids corporel);
d) réduction du poids des foetus chez la souris (approx. 80 mg de
bore par jour et par kg de poids corporel).
Les études effectuées sur des rats et des souris ne révèlent
aucun signe de cancérogénicité de l'acide borique. Comme les données
humaines font défaut et que les données animales sont également
limitées, on ne peut pas ranger le bore dans une classe précise de
cancérogénicité.
Seules quelques études ont été consacrées à l'évaluation des
effets résultant d'une exposition humaine aux dérivés du bore. Les
données disponibles montrent que l'exposition à ces composés peut se
traduire à brève échéance par une irritation des voies respiratoires
supérieures, du rhinopharynx et des yeux. Ces effets sont cependant de
brève durée et réversibles. La seule étude de suivi à long terme (7
ans) qui ait été consacrée à l'action toxique du bore n'a pas permis
de mettre en évidence d'effets à long terme, encore que l'on ne puisse
pas totalement exclure que ce résultat soit dû à la bonne santé des
travailleurs étudiés, compte tenu du taux élevé d'attrition. Deux
études descriptives ont porté sur la fécondité et le sex ratio
secondaire en rapport avec une exposition. Aucune des deux études n'a
révélé d'effet indésirable sur la fécondité observée dans
l'échantillon. Il y aurait bien eu un excès de naissances féminines,
mais il n'était pas statistiquement significatif et le fait qu'il
existait d'autres facteurs connus pour influer sur le sex ratio
incite à une interprétation prudente de ce résultat. On n'a pas
retrouvé d'études portant sur l'ensemble des paramètres de la
reproduction, par exemple le temps écoulé jusqu'à la grossesse, les
retards dans la conception, les avortements spontanés et le
comportement des spermatozoïdes. Il faut étudier plus à fond les
autres styles de vie et facteurs comportementaux dans leurs rapports
avec la santé et la fécondité afin de mettre en évidence les
populations potentiellement sensibles et évaluer dans le détail les
effets sur la reproduction.
1.5 Effets sur les êtres vivants dans leur milieu naturel
Les bactéries ont une tolérance au bore relativement élevée. Les
concentrations produisant des effets aigus ou chroniques vont de 8 à
340 mg de bore par litre, la plupart des valeurs se situant autour de
18 mg/litre. Ce sont les protozoaires qui sont les plus sensibles. Les
épreuves effectuées sur des protozoaires du genre Entosiphon et
Paramecium ont donné, pour la concentration sans effet observable
sur 72 h (NOEC) et pour la CE3, des valeurs comprises entre 0,3 et 18
mg de bore par litre.
Le bore est un micronutriment essentiel des cyanobactéries et des
diatomées. Les épreuves habituelles pour l'évaluation de la toxicité
chronique, ont donné une concentration sans effet observable comprise
entre 10 et 24 mg de bore par litre pour les algues vertes
dulçaquicoles. Les algues bleues ont une sensibilité analogue, avec
une CE3 à 8 jours de 20 mg de B par litre.
Si l'on se base sur les valeurs de la toxicité aiguë, les
invertébrés sont moins sensibles au bore que les microorganismes. Pour
un certain nombre d'espèces, les valeurs de la CE50 à 24 et 48 h
allaient de 95 à 1376 g de B par litre, la plupart des valeurs se
situant dans l'intervalle 100-200 mg/litre. Les études de toxicité
chronique effectuées sur Daphnia magna ont donné une NOEC allant de
6 à 10 mg de B par litre. Des valeurs un peu plus faibles ont été
obtenues lors d'études en laboratoire ou dans des biocénoses
naturelles. Une étude de 28 jours en laboratoire comportant six stades
trophiques a donné une NOEC de 2,5 mg de B par litre. Des études à
long terme sur des étangs et autres études en milieu naturel (à
l'exclusion des poissons) ont donné des NOEC allant jusqu'à 1,52 mg de
B par litre.
Des épreuves de toxicité aiguë portant sur plusieurs espèces de
poissons ont donné des valeurs comprises entre 10 et près de 300 mg de
B par litre. Ce sont des espèces telles que la truite arc-en-ciel
(Oncorhynchus mykiss) et Brachydanio rerio qui se sont révélées
les plus sensibles, avec des valeurs d'environ 10 mg de B par litre.
La toxicité du bore pour les stades juvéniles des poissons est
attestée par des études effectuées en eau reconstituée sur plusieurs
espèces. C'est ainsi que l'on a exposé à du bore (acide borique ou
borax) les embryons et les premiers stades larvaires de truites
arc-en-ciel, de certaines perches (Micropterus salmoides), de
poissons-chats (Ictalurus punctatus) et de poissons rouges
(Carassius auratus), depuis le moment de la fécondation jusqu'à 8
jours après l'éclosion en eau douce ou dure. Ni la dureté de l'eau, ni
la forme sous laquelle se trouvait le bore n'ont eu d'effets
systématiques sur la survie embryo-larvaire des poissons. C'est la
truite arc-en-ciel qui s'est révélée l'espèce la plus sensible. Pour
ce poisson, la NOEC se situait entre 0,009 et 0,103 mg de B par litre.
L'effet de la dilution naturelle de l'eau sur la toxicité du bore
a été déterminé en utilisant des eaux de surface prélevées en trois
endroits, avec des concentrations de bore de 0,023, de 0,091 et de
0,75 mg/litre. Aucun effet indésirable n'a été noté jusqu'à la dose de
0,75 mg/litre. Les valeurs de la concentration la plus faible
produisant des effets observables (LOEC) allaient de 1,1 à 1,73
mg/litre. L'une des épreuves, effectuée sur de l'eau de puits prélevée
à grande profondeur (600 m), utilisée systématiquement pour les essais
de toxicité en milieu aquatique et fournie par un laboratoire de
Wareham (USA), a donné une NOEC > 18 mg de B par litre. Il semble
donc que les épreuves effectuées en eau reconstituée surestiment la
toxicité des eaux naturelles, peut-être du fait que les premières sont
pauvres en certains nutriments.
On sait depuis les années 20 que le bore est un micronutriment
essentiel pour les végétaux supérieurs, la quantité minimale
nécessaire à la croissance dépendant de l'espèce. Le bore intervient
dans la division cellulaire et le métabolisme ainsi que dans la
structure et la fonction de la membrane. Il existe à l'état naturel
sous forme de borates dans les fruits, les noix et les légumes. Chez
les plantes, l'intervalle entre carence et fixation excessive
(toxicité) est étroit. Dans de nombreux pays, on a constaté que les
plantes présentaient une carence en bore. Cet déficit a plus de
chances de se rencontrer dans les sols acides à texture légère des
régions humides, car le bore est facilement lessivé. En revanche, on
trouve un excès de bore dans les solutions de sols provenant de dépôts
géologiques récents, dans les sols arides, les sols issus de sédiments
marins et ceux qui sont contaminés par diverses sources de pollution,
comme les émissions des centrales thermiques à charbon et celles qui
proviennent des exploitations minières. L'eau d'irrigation est l'une
des principales causes des fortes teneurs en bore qui contaminent les
terrains agricoles.
On a observé qu'à des doses dans l'alimentation de 30 et 300 mg
de bore par kg, des colverts (Anas platyrhyncos) présentaient des
troubles et qu'à la dose de 1000 mg/kg leur survie était réduite.
2. Conclusions
Le bore est un élément naturel qui existe à l'état de borates
dans les océans, les roches sédimentaires, la houille, le schiste et
certains sols. Certaines sources naturelles sont à l'origine de rejets
de bore dans l'environnement comme les océans, la vapeur géothermique
et l'action des agents climatiques sur les roches sédimentaires riches
en argile. Les activités humaines libèrent également du bore dans
l'environnement, mais dans une moindre proportion.
Le bore est un micronutriment essentiel des plantes supérieures,
la concentration nécessaire à une croissance optimale étant variable
suivant les espèces. Pour certaines plantes, il y a une faible marge
entre carence et toxicité.
Si l'on compare la concentration environnementale qui ne produit
pas d'effet (1 mg/litre) et les teneurs en bore généralement
rencontrées dans le milieu, on peut en déduire que le risque d'effets
indésirables sur l'écosystème aquatique est faible. Il est possible
toutefois que dans certains milieux riches en bore, les teneurs
naturelles soient plus élevées. On peut raisonnablement penser qu'en
pareil cas, les organismes aquatiques se sont adaptés aux conditions
locales.
Chez l'Homme, l'exposition au bore provient principalement de
l'alimentation et de l'eau de boisson. On estime que la concentration
du bore dans l'eau de boisson est, en moyenne mondiale, de l'ordre de
0,1 à 0,3 mg/litre. Pour la population générale, c'est la nourriture
qui apporte le plus de bore. La dose moyenne ingérée avec la
nourriture est d'environ 1,2 mg par jour.
Chez l'Homme et l'animal, l'acide borique et les borates sont
absorbés au niveau des voies digestives et respiratoires. La
résorption dépasse 90% de la dose administrée et on peut s'en rendre
compte en mesurant l'excrétion urinaire, qui est rapide, puisqu'elle
s'effectue en quelques jours.
L'expérimentation animale montre que sous forme d'acide borique
et de borate, le bore a des effets toxiques sur le développement et la
reproduction à des concentrations qui sont 100 à 1000 fois supérieures
à celles que l'on rencontre dans l'environnement. On ne possède pas
suffisamment de données toxicologiques sur l'Homme. On a fixé à 0,4 mg
par kg de poids corporel la dose journalière tolérable de bore. Pour
tenir compte des divers milieux lors de la fixation de cette dose, il
faut connaître l'exposition dans les différents pays.
3. Recommandations
a) Les valeurs guides pour l'eau et les aliments doivent être basées
sur la dose tolérable indiquée dans le présent document.
b) Dans l'application de la dose tolérable, il faut tenir compte du
fait que le bore peut avoir un effet physiologiquement bénéfique
pour l'Homme.
c) Dans l'application des normes, on tiendra compte du fait que le
bore est essentiel pour certains composants de l'environnement
(par exemple, le bore est un micronutriment essentiel pour les
plantes supérieures).
d) Les suppléments alimentaires qui dépassent la dose tolérable sont
à éviter.
RESUMEN, CONCLUSIONES Y RECOMENDACIONES
1. Resumen
1.1 Identidad, presencia en la naturaleza y métodos analíticos
El boro es un elemento presente en la naturaleza que se encuentra
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Está ampliamente distribuido en
la naturaleza, con concentraciones de alrededor de 10 mg/kg en la
corteza terrestre (margen de variación: de 5 mg/kg en los basaltos a
100 mg/kg en los esquistos) y de unos 4,5 mg/litro en el océano.
Los productos y minerales comerciales de borato más importantes
son el pentahidrato de bórax, el bórax, el perborato de sodio, el
ácido bórico, la colemanita y la ulexita. En las bajas concentraciones
y el pH casi neutro en que está presente en la mayoría de los fluidos
biológicos, la especie predominante es el B(OH)3 (con algo de
B(OH)4-), con independencia de que la fuente de boro sea el ácido
bórico o uno de los boratos. Esto se debe a que el ácido bórico es un
ácido muy débil (p Ka 9,15). El perborato de sodio se hidroliza para
dar peróxido de hidrógeno y metaborato; por consiguiente, puede
mostrar propiedades químicas y toxicológicas algo distintas de las de
los otros boratos.
Para el análisis de los bajos niveles de boro presente en las
muestras biológicas y del medio ambiente se prefieren los métodos de
plasma acoplado por inducción (PAI); los métodos colorimétricos se
deben utilizar con cautela.
1.2 Producción, aplicaciones, destino en el medio ambiente y fuentes
de exposición
Los depósitos económicos de borato son raros y se encuentran en
regiones áridas de Turquía, los Estados Unidos, la Argentina, Chile,
Rusia, China y el Perú. La producción mundial total de minerales de
boro--sobre todo colemanita, ulexita, tíncal y kernita--fue de
alrededor de 2 750 000 toneladas en 1994. Unas 800 000 toneladas de
productos comerciales de borato, expresados como B2O3, se fabricaron
a partir de minerales del boro.
Las principales aplicaciones finales del borato son la fibra de
vidrio de calidad de aislamiento o textil, la lejía para lavar
(perborato de sodio), el vidrio de borosilicato, pirorretardantes,
fertilizantes y herbicidas agrícolas (como elemento traza) y esmaltes,
fritas y vidriados cerámicos, así como innumerables aplicaciones
diversas.
El boro entra en el medio ambiente sobre todo mediante la
meteorización de las rocas, la volatilización de ácido bórico del agua
del mar y la actividad volcánica. También se desprende boro de fuentes
antropogénicas en menor medida. Entre las fuentes antropo-génicas
figuran la quema de productos agrícolas, de basuras y de leña, la
producción de energía utilizando carbón y petróleo, la fabricación de
productos de vidrio, la utilización de boratos/perboratos en el hogar
y en la industria, la extracción/elaboración de borato, la lixiviación
de madera/papel tratados y la eliminación de aguas residuales/fangos
de alcantarillado. Muchas de estas fuentes son difíciles de
cuantificar.
Se producen emisiones a la atmósfera de boratos y de ácido bórico
en forma de partículas y de vapor como consecuencia de la
volatilización desde el agua del mar, la actividad volcánica y, en
menor medida, las operaciones de extracción, la fabricación de vidrio
y cerámica, la aplicación de productos químicos agrícolas y las
centrales eléctricas de carbón. El boro no está presente en la
atmósfera en concentraciones significativas; sin embrago, la cantidad
total que hay en ella en cualquier momento es significativa debido al
enorme volumen de la atmósfera. Teniendo en cuenta su solubilidad en
el agua, no es previsible que los boratos persistan en la atmósfera en
una medida significativa.
Se puede incorporar boro al agua libre y a la del suelo mediante
procesos de meteorización, y en una medida mucho menor por vertidos
antropogénicos, como desagües de aguas residuales. Se supone que las
reacciones de adsorción-desorción son el único mecanismo importante
que influye en el destino del boro en el agua. El grado de adsorción
de boro depende del pH del agua y de su concentración en la solución.
El boro se adsorbe en las partículas del suelo, dependiendo el
grado del tipo de suelo, el pH, la salinidad, el contenido de materia
orgánica, el contenido de óxido de hierro y de aluminio, el contenido
de hidróxido de hierro y de aluminio y el contenido de arcilla. La
adsorción de boro puede variar entre totalmente reversible e
irreversible, en función del tipo y de la condición del suelo.
Los iones borato presentes en solución acuosa están básicamente
en estado totalmente oxidado. No es probable que ningún proceso
aeróbico influya en su especiación y no se han notificado procesos de
biotransformación. Por consiguiente, no es probable que haya ninguna
diferencia en las especies de boro debida a la biotransformación.
El coeficiente de reparto octanol/agua del ácido bórico medido es
de 0,175, lo cual indica un potencial de bioacumulación bajo. Los
experimentos de laboratorio con organismos acuáticos han confirmado
este potencial. Las plantas acumulan boro; sin embargo, la absorción
se ve afectada por el pH de la solución del suelo, la temperatura, la
intensidad de la luz y la concentración de otros elementos (por
ejemplo, calcio y potasio). Los estudios de la acumulación de boro en
plantas, insectos y peces han puesto de manifiesto que el boro se
bioacumula en las plantas, pero no se bioamplifica en la cadena
alimentaria de los organismos acuáticos.
El boro está presente en los suelos en concentraciones que van de
10 a 300 mg/kg (promedio de 30 mg/kg), en función del tipo de suelo,
la cantidad de materia orgánica y la cantidad de precipitación. Las
concentraciones de boro en el agua superficial dependen de factores
como la naturaleza geoquímica de la superficie de drenaje, la
proximidad a regiones costeras marinas y la incorporación de vertidos
de efluentes industriales y urbanos. Las concentraciones de boro en el
agua superficial varían ampliamente, desde 0,001 hasta llegar a
360 mg/litro. Sin embargo, las concentraciones medias en las aguas de
Europa, el Pakistán, Rusia y Turquía suelen ser inferiores a
0,6 mg/litro. Las concentraciones de boro en el agua del Japón,
Sudáfrica y América del Sur están en general por debajo de
0,3 mg/litro. En las aguas de América del Norte son normalmente
menores de 0,1 mg/litro, con 0,4 mg/litro o menos en alrededor del
90%.
El boro se acumula en las plantas acuáticas y terrestres, pero no
se amplifica a través de la cadena alimentaria. Se ha comprobado que
las concentraciones de boro oscilan entre 26 y 382 mg/kg en las
plantas acuáticas de agua dulce sumergidas, entre 11,3 y 57 mg/kg en
la vegetación de agua dulce que crece fuera del agua y entre 2,3 y
94,7 mg/kg de peso seco en las plantas terrestres. Tomando como base
el peso fresco, las concentraciones de boro en los invertebrados y los
peces marinos son semejantes a los niveles presentes en los medios de
exposición, entre 0,5 y 4 mg/kg. Para dos especies de peces de agua
dulce se observó un factor de bioconcentración de 0,3.
Las concentraciones de boro en el aire del medio ambiente oscilan
entre <0,5 y alrededor de 80 ng/m3, con un promedio en todos los
continentes de 20 ng/m3.
La estrecha semejanza entre las concentraciones de boro en el
agua fréatica, el agua dulce superficial y el agua potable indica que
el boro no se elimina en el tratamiento del agua fréatica y del agua
dulce superficial utilizadas para beber.
Se supone que la ingesta de boro por el ser humano es de
0,44 µg/día a partir del medio ambiente, de 0,2-0,6 mg/día con el agua
de beber y de 1,2 mg/día en la alimentación. Se considera que la
ingesta media de boro a partir del suelo es de 0,5 µg/día. Una
estimación razonable de la exposición al boro en los productos de
consumo es de 0,1 mg/día.
1.3 Cinética y vigilancia biológica
La farmacocinética del boro parece ser bastante parecida en todas
las especies en los siguientes aspectos:
a) La absorción de boratos es básicamente completa (alrededor del
95% en el ser humano y en ratas), y tras la ingestión aparece con
rapidez boro en la sangre y los tejidos corporales de varias
especies de mamíferos.
b) La distribución del boro en los mamíferos parece producirse por
difusión pasiva en todos los líquidos del cuerpo. Al contrario
que los tejidos blandos y la sangre, los huesos muestran una
absorción selectiva de boro (>4 veces mayor que en el suero) y
tiempos de retención considerablemente más largos.
c) El metabolismo del ácido bórico es termodinámicamente
desfavorable en los sistemas biológicos. Por consiguiente, se
supone que las especies iónicas en la circulación sistémica son
equivalentes en todos los mamíferos. Así se elimina una fuente
importante de posible incertidumbre para la extrapolación del
riesgo, puesto que no es necesario tener en cuenta diferencias
interespecíficas en las rutas enzimáticas y/o las tasas
metabólicas.
d) La cinética de la eliminación (especialmente la vía de
eliminación y la semivida terminal) también parece ser semejante
para el ser humano y para las ratas.
Las semejanzas de los parámetros farmacocinéticos entre el ser
humano y las ratas, especie que define la concentración sin efectos
adversos observados (NOAEL) para estudios de laboratorio, reducen la
incertidumbre en cuanto a la extrapolación entre estas dos especies.
1.4 Efectos en animales de laboratorio y en el ser humano
Los datos relativos a la toxicidad en el desarrollo y
reproductiva indican que el efecto crítico en las ratas es el menor
peso corporal del feto. La NOAEL para el menor peso corporal del feto
es de 9,6 mg de boro/kg de peso corporal al día. La menor
concentración con efectos adversos observados (LOAEL), en la que las
ratas muestran ligeras (approx. 5%) diferencias en el peso corporal
del feto y anomalías en las costillas, es de unos 13 mg de boro/kg de
peso corporal al día. A medida que aumenta la dosis, los efectos que
se observan (y las dosis a las que se ven) son los siguientes:
a) nuevos efectos en las costillas y patología testicular en ratas
(approx. 25 mg de boro/kg de peso corporal al día);
b) disminución del peso corporal del feto y aumento de las
malformaciones cardiovasculares fetales en conejos, y patología
testicular grave en ratas (approx. 40 mg de boro/kg de peso
corporal al día);
c) atrofia testicular y esterilidad en ratas (approx. 55 mg de
boro/kg de peso corporal al día); y
d) reducción del peso corporal del feto en ratones (approx. 80 mg de
boro/kg de peso corporal al día).
En los estudios de animales con ratones y ratas no se observaron
pruebas de carcinogenicidad del ácido bórico. Debido a la falta de
datos humanos y a lo limitado de los obtenidos de animales, el boro no
se puede clasificar en cuanto a su carcinogenicidad humana.
Son pocos los estudios que se han realizado en el ser humano de
evaluación de los efectos para la salud asociados con la exposición a
compuestos de boro. Los datos disponibles indican que la exposición
está asociada con efectos irritantes de corta duración en las vías
respiratorias superiores, la nasofaringe y los ojos. Sin embargo,
parece que esos efectos son breves y reversibles. En el único estudio
de seguimiento de larga duración (siete años) no se consiguió
identificar ningún efecto prolongado para la salud, aunque no se puede
excluir del todo un efecto en trabajadores sanos debido a la tasa de
desgaste (47%). En dos estudios descriptivos se evaluó la fecundidad y
la razón secundaria de sexos en relación con la exposición. En ninguno
de los dos se notificó un efecto perjudicial demostrado en la
fecundidad de la muestra seleccionada. Aunque se ha indicado la
posibilidad de un porcentaje de nacimientos de hembras superior al
normal, la ausencia de significación estadística y la atención a otras
covariantes que se saben que influyen en el sexo obliga a una
interpretación cauta de este resultado. No se conoce ningún estudio en
el que se evalúe el espectro de resultados reproductivos, como el
tiempo hasta la gestación, los retrasos en la concepción, los abortos
espontáneos y los análisis del esperma en los machos. La función de
otros factores relativos al tipo de vida y al comportamiento en
relación con la salud y la fecundidad requiere un ulterior estudio
para identificar poblaciones posiblemente sensibles y evaluar los
efectos reproductivos de manera más completa.
1.5 Efectos en los organismos del medio ambiente
Las bacterias tienen una tolerancia hacia el boro relativamente
grande. Las concentraciones con efectos agudos y crónicos oscilan
entre 8 y 340 mg de boro/litro, siendo la mayoría de los valores
superiores a 18 mg de boro/litro. Los protozoos son más sensibles. En
pruebas realizadas con Entosiphon y con Paramecium se obtuvieron
concentraciones sin efectos observados (NOEC) en 72 horas y valores de
CE3 de 0,3 a 18 mg de boro/litro.
El boro es un micronutriente esencial para las cianobacterias y
las diatomeas. En pruebas crónicas normales con algas verdes de agua
dulce se observaron concentraciones sin efectos comprendidas entre 10
y 24 mg de boro/litro. Las algas verdeazuladas parecen tener una
sensibilidad semejante, con una CE3 en ocho días de 20 mg de
boro/litro.
Tomando como base los valores de la toxicidad, los invertebrados
son menos sensibles al boro que los microorganismos. Para varias
especies, los valores de la CE50 en 24-48 horas oscilaron entre 95 y
1376 mg de boro/litro, con la mayoría de los valores del orden de
100-200 mg de boro/litro. En estudios de toxicidad crónica con
Daphnia magna, los valores de la NOEC oscilaron entre 6 y 10 mg de
boro/litro. Se obtuvieron valores de la NOEC ligeramente más bajos en
estudios de biocenosis de laboratorio y de campo. En el estudio de
laboratorio de 28 días, consistente en seis etapas tróficas, se obtuvo
una NOEC de 2,5 mg de boro/litro. En estudios prolongados realizados
en un estanque al aire libre y en el campo (sin incluir peces) las
NOEC fueron de hasta 1,52 mg de boro/litro.
En pruebas de toxicidad aguda con varias especies de peces se
obtuvieron valores comprendidos entre alrededor de 10 y cerca de
300 mg de boro/litro. La trucha irisada (Oncorhynchus mykiss) y
Brachydanio rerio fueron los más sensibles, con valores aproximados
de 10 mg de boro/litro.
La toxicidad del boro en las primeras fases de la vida de los
peces está documentada para varias especies en agua reconstituida. Se
expusieron a boro, en forma de ácido bórico o de bórax, fases
embrionarias o larvarias iniciales de trucha irisada, perca atruchada
(Micropterus salmoides), coto punteado (Ictalurus punctatus) y
Carassius auratus desde la fecundación hasta ocho días después de la
eclosión en agua blanda o dura. Ni la dureza del agua ni la forma del
boro influyeron de manera uniforme en la supervivencia de los
embriones-larvas de los peces. La trucha irisada fue la especie más
sensible. Las NOEC para la trucha irisada oscilaron entre 0,009 y
0,103 mg de boro/litro.
El efecto de la dilución natural en el agua sobre la toxicidad
del boro se determinó utilizando agua superficial recogida en tres
lugares, con concentraciones de boro de 0,023, 0,091 y 0,75 mg/litro.
No se detectó ningún efecto adverso hasta los 0,75 mg de boro/litro.
Las concentraciones más bajas con efectos observados (LOEC) oscilaron
entre 1,1 y 1,73 mg de boro/litro. En una prueba con agua de un pozo
profundo (600 m), utilizada habitualmente para pruebas de toxicidad
acuática en virtud de un contrato con un laboratorio situado en
Wareham, Massachusetts, Estados Unidos, se obtuvo una NOEC de
>18,0 mg de boro/litro. Así pues, al parecer en la exposición a agua
reconstituida se sobreestimaba la toxicidad determinada en aguas
naturales, posiblemente como consecuencia de la deficiencia de
nutrientes en la primera.
Desde los años veinte se sabe que el boro es un micronutriente
esencial para las plantas superiores, con diferencias interespecíficas
en cuanto a las concentraciones necesarias para un crecimiento óptimo.
El boro interviene en la división, el metabolismo y la estructura y la
función de las membranas de las células. En forma de borato está
presente en las frutas, las nueces y las hortalizas. La diferencia
entre la deficiencia y la absorción excesiva (toxicidad) es pequeña en
las plantas. Se ha notificado deficiencia de boro en plantas
terrestres en muchos países. Es más probable que se produzca
deficiencia de boro en suelos ácidos de textura ligera en las regiones
húmedas, debido a su susceptibilidad a la lixiviación. Suele haber
exceso de boro en soluciones del suelo procedentes de depósitos
jóvenes desde el punto de vista geológico, en suelos áridos, en los
derivados de sedimentos marinos y en los afectados por fuentes de
contaminación como los vertidos de centrales termoeléctricas de carbón
y de operaciones de extracción. El agua de riego es una de las
principales fuentes de concentraciones altas de boro que provocan
toxicidad en el campo.
El crecimiento del pato real (Anas platyrhynchos) se vio
afectado negativamente por concentraciones de 30 y 300 mg de boro/kg
en los alimentos y con 1000 mg/kg se redujo la supervivencia.
2. Conclusiones
El boro es un elemento que se encuentra presente en la naturaleza
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Las fuentes naturales de los
boratos que se liberan en el medio ambiente son los océanos, el vapor
geotérmico y la meteorización natural de las rocas sedimentarias ricas
en arcilla. También se desprende boro en menor medida de fuentes
antropogénicas.
El boro es un micronutriente esencial para las plantas
superiores, con diferencias interespecíficas en cuanto a las
concentraciones necesarias para un crecimiento óptimo. Se ha observado
deficiencia de boro en plantas terrestres en muchos países de todo el
mundo. La diferencia entre la deficiencia y toxicidad es pequeña en
algunas plantas.
La comparación de la concentración sin efectos en el medio
ambiente (1 mg/litro) con los niveles generales en el medio ambiente
indica que el riesgo de efectos adversos del boro en el ecosistema
acuático es bajo. Los niveles naturales son más elevados en algunos
medios ricos en boro. Es razonable suponer que los organismos
acuáticos de dichos hábitats pueden adaptarse a las condiciones
locales.
La exposición del ser humano al boro se produce sobre todo por
medio de los alimentos y el agua potable. Se consideró que la
concentración media mundial de boro en el agua potable estaba
comprendida entre 0,1 y 0,3 mg de boro/litro.
Para la población general, la mayor exposición al boro procede de
la ingesta oral con los alimentos. La ingesta diaria media de boro con
los alimentos es de alrededor de 1,2 mg.
En las personas y en los animales, el ácido bórico y el borato se
absorben del tracto gastrointestinal y de las vías respiratorias. Se
absorbe más del 90% de las dosis administradas de estos compuestos,
como se pone de manifiesto en la excreción en la orina, que es rápida,
en unos pocos días.
En experimentos con animales se ha demostrado que el boro en
forma de ácido bórico y de borato tiene toxicidad reproductiva y en el
desarrollo en concentraciones de 100 a 1000 veces superiores a los
niveles normales de exposición. Se carece de datos suficientes acerca
de la toxicidad en el ser humano. La ingesta tolerable (IT) de boro se
fijó en 0,4 mg/kg de peso corporal al día. La asignación de la IT en
diversos medios debe basarse en los datos relativos a la exposición de
cada país.
3. Recomendaciones
a) Los valores guía del agua y los alimentos deben basarse en la IT
proporcionada por este documento.
b) La IT se debe aplicar quedando entendido que el boro puede
aportar beneficios fisiológicos para la salud humana.
c) Hay que reconocer al aplicar las normas que el boro es esencial
para algunos elementos constituyentes del medio ambiente (por
ejemplo, el boro es un micronutriente esencial para las plantas
superiores).
d) Se deben evitar los complementos de la alimentación que superen
la IT.
RÉSUMÉ, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé
1.1 Identité, état naturel et méthodes d'analyse
Le bore est un élément naturel qui se trouve sous la forme de
borates dans les océans, les roches sédimentaires, la houille, le
schiste et certaines huiles minérales. Il est très répandu dans la
nature, avec des concentrations de l'ordre de 10 mg/kg dans l'écorce
terrestre (elles vont de 5 mg/kg dans les basaltes à 100 mg/kg dans
les schistes) et d'environ 4,5 mg/litre dans les océans.
Les dérivés boriques les plus importants, qu'il s'agisse de
minéraux ou de produits du commerce, sont le borax à cinq molécules
d'eau, le borax, le perborate de sodium, l'acide borique, la
colemanite et l'ulexite. Aux faibles concentrations et au pH
pratiquement neutre qui caractérisent la plupart des liquides
biologiques, c'est le monomère B(OH)3 qui est l'espèce prédominante
(avec un peu de B(OH)4-), que la source de bore soit l'acide borique
ou un borate. Cela tient au fait que l'acide borique est un acide très
faible (pKa = 9,15).Le perborate de sodium s'hydrolyse pour donner du
peroxyde d'hydrogène et du métaborate; il peut donc présenter des
propriétés chimiques et toxicologiques un peu différentes de celles
des autres borates.
Les méthodes utilisant un plasma à couplage inductif (ICP) sont
les méthodes de choix pour le dosage des faibles quantités de bore
présentes dans les échantillons biologiques et environnementaux; les
méthodes colorimétriques doivent être utilisées avec prudence.
1.2 Production, usages, destinée dans l'environnement et sources d'exposition
Les dépôts de borates économiquement exploitables sont rares et
se trouvent dans des zones arides de Turquie, des Etats-Unis, du
Chili, de Russie, de Chine et du Pérou. La production mondiale totale
de minéraux contenant du bore--principalement de la colemanite, de
l'ulexite, du tincal et de la kernite--a été d'environ 2 750 000
tonnes en 1994. Environ 800 000 tonnes (en équivalents de B2O3) de
dérivés boriques commerciaux ont été produits à partir des minerais.
Les borates sont, entre autres, principalement utilisés pour
fabriquer des produits isolants, des fibres de verre de qualité
textile, des agents de blanchiment (perborate de sodium), des verres
au borosilicate, des retardateurs de flamme, des engrais et des
herbicides (à l'état de traces), des émaux, des vernis pour
céramiques, des frittes ainsi qu'une myriade d'applications diverses.
La pénétration du bore dans l'environnement se fait
principalement par l'action des agents météorologiques sur les roches,
la volatilisation de l'acide borique présent dans l'eau de mer et
l'activité volcanique. L'apport de bore dû aux activités humaines est
moindre. Parmi ces dernières sources figurent les brûlis agricoles,
l'incinération des déchets, la combustion du bois de feu, la
production d'énergie à partir du charbon et du pétrole, l'industrie du
verre, les borates et perborates utilisés comme produits industriels
et ménagers, l'exploitation des mines de borates, le traitement des
borates et des bois et papiers imprégnés et enfin le rejet des
effluents et des boues. Il est souvent difficile de chiffrer l'apport
de ces sources.
L'émission de borates et d'acide borique dans l'atmosphère se
fait sous la forme de particules et de vapeurs par suite de la
volatilisation de l'eau de mer, de l'activité volcanique et, dans une
moindre mesure, de l'exploitation des mines, de la fabrication de
verre et de céramique, de l'usage agricole de certains dérivés et des
rejets des centrales thermiques fonctionnant au charbon. Le bore n'est
pas très abondant dans l'atmosphère, mais la quantité totale qui s'y
trouve à un moment donné n'est pas négligeable du fait du volume
énorme de l'atmosphère. Compte tenu de la solubilité des borates dans
l'eau, ils ne devraient pas séjourner longtemps dans l'atmosphère en
concentrations importantes.
L'action des phénomènes météorologiques peut provoquer la
libération de bore dans le sol et dans l'eau de même que, encore qu'en
proportion bien moindre, les déversements d'origine humaine tels que
les rejets d'effluents. On pense que les phénomènes
d'adsorption-désorption sont les seuls mécanismes importants qui
soient susceptibles d'influer sur la destinée du bore dans
l'environnement. Le taux d'adsorption du bore dépend de sa
concentration en solution et du pH de l'eau.
Le bore s'adsorbe sur les particules du sol, le degré
d'adsorption étant fonction du type de sol, du pH, de la salinité, de
la teneur en matières organiques, en oxyde et hydroxyde d'aluminium,
en hydroxyde de fer et en argile. Le phénomène peut être parfaitement
réversible ou au contraire complètement irréversible, selon la nature
et l'état du sol.
Les ions borate présents en solution aqueuse s'y trouvent
essentiellement au degré d'oxydation maximum. Il n'y a pas de
processus aérobie qui soit susceptible d'influer sur la formation des
différentes espèces chimiques et on n'a pas fait état de
biotransformations. Il ne devrait donc pas y avoir de différence dans
les différentes espèces chimiques qui soit due à une
biotransformation.
Le coefficient de partage octanol/eau de l'acide borique est de
0, 175, ce qui indique que ce composé a un faible potentiel de
bioaccumulation. Les expériences de laboratoire effectuées sur des
organismes aquatiques ont confirmé l'existence d'un faible potentiel
de bioaccumulation. Les végétaux ont tendance à accumuler du bore;
toutefois la fixation du bore par les plantes dépend du pH de la
solution de sol, de la température, de l'intensité lumineuse et de la
concentration d'autres éléments (par ex. le calcium et le potassium).
Les études relatives à l'accumulation du bore par les plantes, les
insectes et les poissons montrent que cet élément s'accumule dans les
végétaux mais qu'il ne s'amplifie pas de long de la chaîne alimentaire
aquatique.
Le bore est présent dans les différents sols à des concentrations
qui vont de 10 à 300 mg/kg (la moyenne est de 30 mg/kg) selon la
nature du sol, sa teneur en matières organiques et l'importance des
précipitations. Sa concentration dans les eaux superficielles dépend
de plusieurs facteurs tels que la nature géochimique du bassin de
drainage, la proximité de zones littorales et les apports dus aux
décharges industrielles et municipales. Les valeurs sont très
variables, allant de 0,001 à 360 mg/litre. Toutefois les
concentrations moyennes dans les eaux de l'Europe, du Pakistan, de la
Russie et de la Turquie se situent nettement en dessous de 0,6
mg/litre. Au Japon, en Afrique du Sud et en Amérique du sud elles sont
généralement inférieures à 0,3 mg/kg. En Amérique du Nord, elles se
caractérisent par des valeurs inférieures à 0,1 mg/litre, dont 90%
inférieures ou égales à 0,4 mg/litre.
Le bore s'accumule dans les plantes aquatiques et terrestres mais
il ne s'amplifie pas le long de la chaîne alimentaire. On a trouvé des
concentrations de bore comprises entre 26 et 382 mg/kg dans des
plantes d'eau douces immergées, entre 11,3 et 57 mg/kg dans des
plantes d'eau douce semi-immergées et entre 2,3 et 94,7 mg/kg dans des
plantes terrestres. En se basant sur le poids frais, on constate que
les concentrations de bore trouvées dans les invertébrés marins et les
poissons sont analogues à celles que l'on mesure dans les milieux
correspondants, soit entre 0,5 et 4 mg/kg. Chez deux espèces de
poissons, on a trouvé un facteur de bioconcentration de 0,3.
Dans l'air ambiant, la concentration du bore varie de < 0,5 à
environ 80 ng/m3, avec une moyenne de 20 ng/m3 au-dessus des
continents.
Le fait que la concentration du bore dans les eaux souterraines
et les eaux douces de surface soit très voisine de sa concentration
dans l'eau de boisson, indique que ce élément n'est pas éliminé par
les traitements auxquels sont soumises les eaux souterraines et les
eaux de surface destinées à la boisson.
L'apport de bore chez l'Homme devrait être de 0,44 µg/jour à
partir de l'air ambiant, de 0,2-0,6 mg/jour à partir de l'eau de
boisson et de 1,2 mg/jour à partir de l'alimentation. On estime que
l'apport moyen de bore à partir du sol est de 0,5 µg/jour. On peut
raisonnablement estimer que l'apport de bore par les produits de
consommation est de 0,1 mg/jour.
1.3 Cinétique et suivi biologique
La pharmacocinétique du bore se révèle assez semblable d'une
espèce à l'autre, notamment en ce qui concerne les aspects suivants:
a) Absorption. L'absorption des borates est pratiquement complète
(environ 95% chez l'Homme et le rat) et après ingestion, le bore
apparaît rapidement dans le sang et les tissus de plusieurs
espèces de mammifères.
b) Distribution. Chez les mammifères, elle se produit selon un
mécanisme de diffusion passive par les liquides biologiques.
Contrairement aux tissus mous et au sang, les os sont capables de
fixer sélectivement le bore (teneur supérieure ou égale à 4 fois
celle du sérum) et de le retenir sensiblement plus longtemps.
c) Métabolisme. Le métabolisme de l'acide borique est
thermodynamiquement défavorable dans les systèmes biologiques.
Les espèces ioniques présentes dans le courant sanguin devraient
donc être les mêmes chez tous les mammifères. Dans ces
conditions, on risque beaucoup moins de se tromper en procédant à
des extrapolations, puisqu'il n'est pas nécessaire de prendre en
considération les différences interspécifiques qui existent
généralement au niveau des voies enzymatiques et de la vitesse de
métabolisation.
d) Elimination. La cinétique d'élimination (en particulier la voie
d'élimination et la demi-vie terminale) se révèle également
analogue chez l'Homme et le rat.
Les analogies qui existent entre les paramètres
pharmacocinétiques relevés chez l'Homme et chez le rat, l'espèce qui
sert à définir la dose sans effet nocif observable (NOAEL) dans les
études de laboratoire, permettent de réduire l'incertitude que
comporte l'extrapolation à l'Homme des résultats obtenus sur le rat.
1.4 Effets sur l'Homme et les animaux de laboratoire
Les données relatives aux effets toxiques sur le développement et
la reproduction montrent que l'effet déterminant consiste en une
réduction du poids des foetus. On a estimé à 9, 6 mg de bore par jour
et par kg de poids corporel, la NOAEL relative à cet effet. La dose la
plus faible produisant un effet nocif observable (LOAEL) est, chez le
rat, d'environ 13 mg/kg de poids corporel par jour, l'effet observé
étant une légère différence dans le poids des foetus (approx. 5%) et
des anomalies costales. A mesure que l'on augmente la dose, on observe
les effets suivants, selon la dose:
a) autres anomalies costales et testiculaires (approx. 25 mg de bore
par jour et par kg de poids corporel);
b) réduction du poids des foetus et accroissement des malformations
cardiovasculaires chez le lapin; graves anomalies testiculaires
chez le rat (approx. 40 mg de bore par jour et par kg de poids
corporel);
c) atrophie testiculaire et stérilité chez le rat (approx. 55 mg de
bore par jour et par kg de poids corporel);
d) réduction du poids des foetus chez la souris (approx. 80 mg de
bore par jour et par kg de poids corporel).
Les études effectuées sur des rats et des souris ne révèlent
aucun signe de cancérogénicité de l'acide borique. Comme les données
humaines font défaut et que les données animales sont également
limitées, on ne peut pas ranger le bore dans une classe précise de
cancérogénicité.
Seules quelques études ont été consacrées à l'évaluation des
effets résultant d'une exposition humaine aux dérivés du bore. Les
données disponibles montrent que l'exposition à ces composés peut se
traduire à brève échéance par une irritation des voies respiratoires
supérieures, du rhinopharynx et des yeux. Ces effets sont cependant de
brève durée et réversibles. La seule étude de suivi à long terme (7
ans) qui ait été consacrée à l'action toxique du bore n'a pas permis
de mettre en évidence d'effets à long terme, encore que l'on ne puisse
pas totalement exclure que ce résultat soit dû à la bonne santé des
travailleurs étudiés, compte tenu du taux élevé d'attrition. Deux
études descriptives ont porté sur la fécondité et le sex ratio
secondaire en rapport avec une exposition. Aucune des deux études n'a
révélé d'effet indésirable sur la fécondité observée dans
l'échantillon. Il y aurait bien eu un excès de naissances féminines,
mais il n'était pas statistiquement significatif et le fait qu'il
existait d'autres facteurs connus pour influer sur le sex ratio
incite à une interprétation prudente de ce résultat. On n'a pas
retrouvé d'études portant sur l'ensemble des paramètres de la
reproduction, par exemple le temps écoulé jusqu'à la grossesse, les
retards dans la conception, les avortements spontanés et le
comportement des spermatozoïdes. Il faut étudier plus à fond les
autres styles de vie et facteurs comportementaux dans leurs rapports
avec la santé et la fécondité afin de mettre en évidence les
populations potentiellement sensibles et évaluer dans le détail les
effets sur la reproduction.
1.5 Effets sur les êtres vivants dans leur milieu naturel
Les bactéries ont une tolérance au bore relativement élevée. Les
concentrations produisant des effets aigus ou chroniques vont de 8 à
340 mg de bore par litre, la plupart des valeurs se situant autour de
18 mg/litre. Ce sont les protozoaires qui sont les plus sensibles. Les
épreuves effectuées sur des protozoaires du genre Entosiphon et
Paramecium ont donné, pour la concentration sans effet observable
sur 72 h (NOEC) et pour la CE3, des valeurs comprises entre 0,3 et 18
mg de bore par litre.
Le bore est un micronutriment essentiel des cyanobactéries et des
diatomées. Les épreuves habituelles pour l'évaluation de la toxicité
chronique, ont donné une concentration sans effet observable comprise
entre 10 et 24 mg de bore par litre pour les algues vertes
dulçaquicoles. Les algues bleues ont une sensibilité analogue, avec
une CE3 à 8 jours de 20 mg de B par litre.
Si l'on se base sur les valeurs de la toxicité aiguë, les
invertébrés sont moins sensibles au bore que les microorganismes. Pour
un certain nombre d'espèces, les valeurs de la CE50 à 24 et 48 h
allaient de 95 à 1376 g de B par litre, la plupart des valeurs se
situant dans l'intervalle 100-200 mg/litre. Les études de toxicité
chronique effectuées sur Daphnia magna ont donné une NOEC allant de
6 à 10 mg de B par litre. Des valeurs un peu plus faibles ont été
obtenues lors d'études en laboratoire ou dans des biocénoses
naturelles. Une étude de 28 jours en laboratoire comportant six stades
trophiques a donné une NOEC de 2,5 mg de B par litre. Des études à
long terme sur des étangs et autres études en milieu naturel (à
l'exclusion des poissons) ont donné des NOEC allant jusqu'à 1,52 mg de
B par litre.
Des épreuves de toxicité aiguë portant sur plusieurs espèces de
poissons ont donné des valeurs comprises entre 10 et près de 300 mg de
B par litre. Ce sont des espèces telles que la truite arc-en-ciel
(Oncorhynchus mykiss) et Brachydanio rerio qui se sont révélées
les plus sensibles, avec des valeurs d'environ 10 mg de B par litre.
La toxicité du bore pour les stades juvéniles des poissons est
attestée par des études effectuées en eau reconstituée sur plusieurs
espèces. C'est ainsi que l'on a exposé à du bore (acide borique ou
borax) les embryons et les premiers stades larvaires de truites
arc-en-ciel, de certaines perches (Micropterus salmoides), de
poissons-chats (Ictalurus punctatus) et de poissons rouges
(Carassius auratus), depuis le moment de la fécondation jusqu'à 8
jours après l'éclosion en eau douce ou dure. Ni la dureté de l'eau, ni
la forme sous laquelle se trouvait le bore n'ont eu d'effets
systématiques sur la survie embryo-larvaire des poissons. C'est la
truite arc-en-ciel qui s'est révélée l'espèce la plus sensible. Pour
ce poisson, la NOEC se situait entre 0,009 et 0,103 mg de B par litre.
L'effet de la dilution naturelle de l'eau sur la toxicité du bore
a été déterminé en utilisant des eaux de surface prélevées en trois
endroits, avec des concentrations de bore de 0,023, de 0,091 et de
0,75 mg/litre. Aucun effet indésirable n'a été noté jusqu'à la dose de
0,75 mg/litre. Les valeurs de la concentration la plus faible
produisant des effets observables (LOEC) allaient de 1,1 à 1,73
mg/litre. L'une des épreuves, effectuée sur de l'eau de puits prélevée
à grande profondeur (600 m), utilisée systématiquement pour les essais
de toxicité en milieu aquatique et fournie par un laboratoire de
Wareham (USA), a donné une NOEC > 18 mg de B par litre. Il semble
donc que les épreuves effectuées en eau reconstituée surestiment la
toxicité des eaux naturelles, peut-être du fait que les premières sont
pauvres en certains nutriments.
On sait depuis les années 20 que le bore est un micronutriment
essentiel pour les végétaux supérieurs, la quantité minimale
nécessaire à la croissance dépendant de l'espèce. Le bore intervient
dans la division cellulaire et le métabolisme ainsi que dans la
structure et la fonction de la membrane. Il existe à l'état naturel
sous forme de borates dans les fruits, les noix et les légumes. Chez
les plantes, l'intervalle entre carence et fixation excessive
(toxicité) est étroit. Dans de nombreux pays, on a constaté que les
plantes présentaient une carence en bore. Cet déficit a plus de
chances de se rencontrer dans les sols acides à texture légère des
régions humides, car le bore est facilement lessivé. En revanche, on
trouve un excès de bore dans les solutions de sols provenant de dépôts
géologiques récents, dans les sols arides, les sols issus de sédiments
marins et ceux qui sont contaminés par diverses sources de pollution,
comme les émissions des centrales thermiques à charbon et celles qui
proviennent des exploitations minières. L'eau d'irrigation est l'une
des principales causes des fortes teneurs en bore qui contaminent les
terrains agricoles.
On a observé qu'à des doses dans l'alimentation de 30 et 300 mg
de bore par kg, des colverts (Anas platyrhyncos) présentaient des
troubles et qu'à la dose de 1000 mg/kg leur survie était réduite.
2. Conclusions
Le bore est un élément naturel qui existe à l'état de borates
dans les océans, les roches sédimentaires, la houille, le schiste et
certains sols. Certaines sources naturelles sont à l'origine de rejets
de bore dans l'environnement comme les océans, la vapeur géothermique
et l'action des agents climatiques sur les roches sédimentaires riches
en argile. Les activités humaines libèrent également du bore dans
l'environnement, mais dans une moindre proportion.
Le bore est un micronutriment essentiel des plantes supérieures,
la concentration nécessaire à une croissance optimale étant variable
suivant les espèces. Pour certaines plantes, il y a une faible marge
entre carence et toxicité.
Si l'on compare la concentration environnementale qui ne produit
pas d'effet (1 mg/litre) et les teneurs en bore généralement
rencontrées dans le milieu, on peut en déduire que le risque d'effets
indésirables sur l'écosystème aquatique est faible. Il est possible
toutefois que dans certains milieux riches en bore, les teneurs
naturelles soient plus élevées. On peut raisonnablement penser qu'en
pareil cas, les organismes aquatiques se sont adaptés aux conditions
locales.
Chez l'Homme, l'exposition au bore provient principalement de
l'alimentation et de l'eau de boisson. On estime que la concentration
du bore dans l'eau de boisson est, en moyenne mondiale, de l'ordre de
0,1 à 0,3 mg/litre. Pour la population générale, c'est la nourriture
qui apporte le plus de bore. La dose moyenne ingérée avec la
nourriture est d'environ 1,2 mg par jour.
Chez l'Homme et l'animal, l'acide borique et les borates sont
absorbés au niveau des voies digestives et respiratoires. La
résorption dépasse 90% de la dose administrée et on peut s'en rendre
compte en mesurant l'excrétion urinaire, qui est rapide, puisqu'elle
s'effectue en quelques jours.
L'expérimentation animale montre que sous forme d'acide borique
et de borate, le bore a des effets toxiques sur le développement et la
reproduction à des concentrations qui sont 100 à 1000 fois supérieures
à celles que l'on rencontre dans l'environnement. On ne possède pas
suffisamment de données toxicologiques sur l'Homme. On a fixé à 0,4 mg
par kg de poids corporel la dose journalière tolérable de bore. Pour
tenir compte des divers milieux lors de la fixation de cette dose, il
faut connaître l'exposition dans les différents pays.
3. Recommandations
a) Les valeurs guides pour l'eau et les aliments doivent être basées
sur la dose tolérable indiquée dans le présent document.
b) Dans l'application de la dose tolérable, il faut tenir compte du
fait que le bore peut avoir un effet physiologiquement bénéfique
pour l'Homme.
c) Dans l'application des normes, on tiendra compte du fait que le
bore est essentiel pour certains composants de l'environnement
(par exemple, le bore est un micronutriment essentiel pour les
plantes supérieures).
d) Les suppléments alimentaires qui dépassent la dose tolérable sont
à éviter.
RESUMEN, CONCLUSIONES Y RECOMENDACIONES
1. Resumen
1.1 Identidad, presencia en la naturaleza y métodos analíticos
El boro es un elemento presente en la naturaleza que se encuentra
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Está ampliamente distribuido en
la naturaleza, con concentraciones de alrededor de 10 mg/kg en la
corteza terrestre (margen de variación: de 5 mg/kg en los basaltos a
100 mg/kg en los esquistos) y de unos 4,5 mg/litro en el océano.
Los productos y minerales comerciales de borato más importantes
son el pentahidrato de bórax, el bórax, el perborato de sodio, el
ácido bórico, la colemanita y la ulexita. En las bajas concentraciones
y el pH casi neutro en que está presente en la mayoría de los fluidos
biológicos, la especie predominante es el B(OH)3 (con algo de
B(OH)4-), con independencia de que la fuente de boro sea el ácido
bórico o uno de los boratos. Esto se debe a que el ácido bórico es un
ácido muy débil (p Ka 9,15). El perborato de sodio se hidroliza para
dar peróxido de hidrógeno y metaborato; por consiguiente, puede
mostrar propiedades químicas y toxicológicas algo distintas de las de
los otros boratos.
Para el análisis de los bajos niveles de boro presente en las
muestras biológicas y del medio ambiente se prefieren los métodos de
plasma acoplado por inducción (PAI); los métodos colorimétricos se
deben utilizar con cautela.
1.2 Producción, aplicaciones, destino en el medio ambiente y fuentes
de exposición
Los depósitos económicos de borato son raros y se encuentran en
regiones áridas de Turquía, los Estados Unidos, la Argentina, Chile,
Rusia, China y el Perú. La producción mundial total de minerales de
boro--sobre todo colemanita, ulexita, tíncal y kernita--fue de
alrededor de 2 750 000 toneladas en 1994. Unas 800 000 toneladas de
productos comerciales de borato, expresados como B2O3, se fabricaron
a partir de minerales del boro.
Las principales aplicaciones finales del borato son la fibra de
vidrio de calidad de aislamiento o textil, la lejía para lavar
(perborato de sodio), el vidrio de borosilicato, pirorretardantes,
fertilizantes y herbicidas agrícolas (como elemento traza) y esmaltes,
fritas y vidriados cerámicos, así como innumerables aplicaciones
diversas.
El boro entra en el medio ambiente sobre todo mediante la
meteorización de las rocas, la volatilización de ácido bórico del agua
del mar y la actividad volcánica. También se desprende boro de fuentes
antropogénicas en menor medida. Entre las fuentes antropo-génicas
figuran la quema de productos agrícolas, de basuras y de leña, la
producción de energía utilizando carbón y petróleo, la fabricación de
productos de vidrio, la utilización de boratos/perboratos en el hogar
y en la industria, la extracción/elaboración de borato, la lixiviación
de madera/papel tratados y la eliminación de aguas residuales/fangos
de alcantarillado. Muchas de estas fuentes son difíciles de
cuantificar.
Se producen emisiones a la atmósfera de boratos y de ácido bórico
en forma de partículas y de vapor como consecuencia de la
volatilización desde el agua del mar, la actividad volcánica y, en
menor medida, las operaciones de extracción, la fabricación de vidrio
y cerámica, la aplicación de productos químicos agrícolas y las
centrales eléctricas de carbón. El boro no está presente en la
atmósfera en concentraciones significativas; sin embrago, la cantidad
total que hay en ella en cualquier momento es significativa debido al
enorme volumen de la atmósfera. Teniendo en cuenta su solubilidad en
el agua, no es previsible que los boratos persistan en la atmósfera en
una medida significativa.
Se puede incorporar boro al agua libre y a la del suelo mediante
procesos de meteorización, y en una medida mucho menor por vertidos
antropogénicos, como desagües de aguas residuales. Se supone que las
reacciones de adsorción-desorción son el único mecanismo importante
que influye en el destino del boro en el agua. El grado de adsorción
de boro depende del pH del agua y de su concentración en la solución.
El boro se adsorbe en las partículas del suelo, dependiendo el
grado del tipo de suelo, el pH, la salinidad, el contenido de materia
orgánica, el contenido de óxido de hierro y de aluminio, el contenido
de hidróxido de hierro y de aluminio y el contenido de arcilla. La
adsorción de boro puede variar entre totalmente reversible e
irreversible, en función del tipo y de la condición del suelo.
Los iones borato presentes en solución acuosa están básicamente
en estado totalmente oxidado. No es probable que ningún proceso
aeróbico influya en su especiación y no se han notificado procesos de
biotransformación. Por consiguiente, no es probable que haya ninguna
diferencia en las especies de boro debida a la biotransformación.
El coeficiente de reparto octanol/agua del ácido bórico medido es
de 0,175, lo cual indica un potencial de bioacumulación bajo. Los
experimentos de laboratorio con organismos acuáticos han confirmado
este potencial. Las plantas acumulan boro; sin embargo, la absorción
se ve afectada por el pH de la solución del suelo, la temperatura, la
intensidad de la luz y la concentración de otros elementos (por
ejemplo, calcio y potasio). Los estudios de la acumulación de boro en
plantas, insectos y peces han puesto de manifiesto que el boro se
bioacumula en las plantas, pero no se bioamplifica en la cadena
alimentaria de los organismos acuáticos.
El boro está presente en los suelos en concentraciones que van de
10 a 300 mg/kg (promedio de 30 mg/kg), en función del tipo de suelo,
la cantidad de materia orgánica y la cantidad de precipitación. Las
concentraciones de boro en el agua superficial dependen de factores
como la naturaleza geoquímica de la superficie de drenaje, la
proximidad a regiones costeras marinas y la incorporación de vertidos
de efluentes industriales y urbanos. Las concentraciones de boro en el
agua superficial varían ampliamente, desde 0,001 hasta llegar a
360 mg/litro. Sin embargo, las concentraciones medias en las aguas de
Europa, el Pakistán, Rusia y Turquía suelen ser inferiores a
0,6 mg/litro. Las concentraciones de boro en el agua del Japón,
Sudáfrica y América del Sur están en general por debajo de
0,3 mg/litro. En las aguas de América del Norte son normalmente
menores de 0,1 mg/litro, con 0,4 mg/litro o menos en alrededor del
90%.
El boro se acumula en las plantas acuáticas y terrestres, pero no
se amplifica a través de la cadena alimentaria. Se ha comprobado que
las concentraciones de boro oscilan entre 26 y 382 mg/kg en las
plantas acuáticas de agua dulce sumergidas, entre 11,3 y 57 mg/kg en
la vegetación de agua dulce que crece fuera del agua y entre 2,3 y
94,7 mg/kg de peso seco en las plantas terrestres. Tomando como base
el peso fresco, las concentraciones de boro en los invertebrados y los
peces marinos son semejantes a los niveles presentes en los medios de
exposición, entre 0,5 y 4 mg/kg. Para dos especies de peces de agua
dulce se observó un factor de bioconcentración de 0,3.
Las concentraciones de boro en el aire del medio ambiente oscilan
entre <0,5 y alrededor de 80 ng/m3, con un promedio en todos los
continentes de 20 ng/m3.
La estrecha semejanza entre las concentraciones de boro en el
agua fréatica, el agua dulce superficial y el agua potable indica que
el boro no se elimina en el tratamiento del agua fréatica y del agua
dulce superficial utilizadas para beber.
Se supone que la ingesta de boro por el ser humano es de
0,44 µg/día a partir del medio ambiente, de 0,2-0,6 mg/día con el agua
de beber y de 1,2 mg/día en la alimentación. Se considera que la
ingesta media de boro a partir del suelo es de 0,5 µg/día. Una
estimación razonable de la exposición al boro en los productos de
consumo es de 0,1 mg/día.
1.3 Cinética y vigilancia biológica
La farmacocinética del boro parece ser bastante parecida en todas
las especies en los siguientes aspectos:
a) La absorción de boratos es básicamente completa (alrededor del
95% en el ser humano y en ratas), y tras la ingestión aparece con
rapidez boro en la sangre y los tejidos corporales de varias
especies de mamíferos.
b) La distribución del boro en los mamíferos parece producirse por
difusión pasiva en todos los líquidos del cuerpo. Al contrario
que los tejidos blandos y la sangre, los huesos muestran una
absorción selectiva de boro (>4 veces mayor que en el suero) y
tiempos de retención considerablemente más largos.
c) El metabolismo del ácido bórico es termodinámicamente
desfavorable en los sistemas biológicos. Por consiguiente, se
supone que las especies iónicas en la circulación sistémica son
equivalentes en todos los mamíferos. Así se elimina una fuente
importante de posible incertidumbre para la extrapolación del
riesgo, puesto que no es necesario tener en cuenta diferencias
interespecíficas en las rutas enzimáticas y/o las tasas
metabólicas.
d) La cinética de la eliminación (especialmente la vía de
eliminación y la semivida terminal) también parece ser semejante
para el ser humano y para las ratas.
Las semejanzas de los parámetros farmacocinéticos entre el ser
humano y las ratas, especie que define la concentración sin efectos
adversos observados (NOAEL) para estudios de laboratorio, reducen la
incertidumbre en cuanto a la extrapolación entre estas dos especies.
1.4 Efectos en animales de laboratorio y en el ser humano
Los datos relativos a la toxicidad en el desarrollo y
reproductiva indican que el efecto crítico en las ratas es el menor
peso corporal del feto. La NOAEL para el menor peso corporal del feto
es de 9,6 mg de boro/kg de peso corporal al día. La menor
concentración con efectos adversos observados (LOAEL), en la que las
ratas muestran ligeras (approx. 5%) diferencias en el peso corporal
del feto y anomalías en las costillas, es de unos 13 mg de boro/kg de
peso corporal al día. A medida que aumenta la dosis, los efectos que
se observan (y las dosis a las que se ven) son los siguientes:
a) nuevos efectos en las costillas y patología testicular en ratas
(approx. 25 mg de boro/kg de peso corporal al día);
b) disminución del peso corporal del feto y aumento de las
malformaciones cardiovasculares fetales en conejos, y patología
testicular grave en ratas (approx. 40 mg de boro/kg de peso
corporal al día);
c) atrofia testicular y esterilidad en ratas (approx. 55 mg de
boro/kg de peso corporal al día); y
d) reducción del peso corporal del feto en ratones (approx. 80 mg de
boro/kg de peso corporal al día).
En los estudios de animales con ratones y ratas no se observaron
pruebas de carcinogenicidad del ácido bórico. Debido a la falta de
datos humanos y a lo limitado de los obtenidos de animales, el boro no
se puede clasificar en cuanto a su carcinogenicidad humana.
Son pocos los estudios que se han realizado en el ser humano de
evaluación de los efectos para la salud asociados con la exposición a
compuestos de boro. Los datos disponibles indican que la exposición
está asociada con efectos irritantes de corta duración en las vías
respiratorias superiores, la nasofaringe y los ojos. Sin embargo,
parece que esos efectos son breves y reversibles. En el único estudio
de seguimiento de larga duración (siete años) no se consiguió
identificar ningún efecto prolongado para la salud, aunque no se puede
excluir del todo un efecto en trabajadores sanos debido a la tasa de
desgaste (47%). En dos estudios descriptivos se evaluó la fecundidad y
la razón secundaria de sexos en relación con la exposición. En ninguno
de los dos se notificó un efecto perjudicial demostrado en la
fecundidad de la muestra seleccionada. Aunque se ha indicado la
posibilidad de un porcentaje de nacimientos de hembras superior al
normal, la ausencia de significación estadística y la atención a otras
covariantes que se saben que influyen en el sexo obliga a una
interpretación cauta de este resultado. No se conoce ningún estudio en
el que se evalúe el espectro de resultados reproductivos, como el
tiempo hasta la gestación, los retrasos en la concepción, los abortos
espontáneos y los análisis del esperma en los machos. La función de
otros factores relativos al tipo de vida y al comportamiento en
relación con la salud y la fecundidad requiere un ulterior estudio
para identificar poblaciones posiblemente sensibles y evaluar los
efectos reproductivos de manera más completa.
1.5 Efectos en los organismos del medio ambiente
Las bacterias tienen una tolerancia hacia el boro relativamente
grande. Las concentraciones con efectos agudos y crónicos oscilan
entre 8 y 340 mg de boro/litro, siendo la mayoría de los valores
superiores a 18 mg de boro/litro. Los protozoos son más sensibles. En
pruebas realizadas con Entosiphon y con Paramecium se obtuvieron
concentraciones sin efectos observados (NOEC) en 72 horas y valores de
CE3 de 0,3 a 18 mg de boro/litro.
El boro es un micronutriente esencial para las cianobacterias y
las diatomeas. En pruebas crónicas normales con algas verdes de agua
dulce se observaron concentraciones sin efectos comprendidas entre 10
y 24 mg de boro/litro. Las algas verdeazuladas parecen tener una
sensibilidad semejante, con una CE3 en ocho días de 20 mg de
boro/litro.
Tomando como base los valores de la toxicidad, los invertebrados
son menos sensibles al boro que los microorganismos. Para varias
especies, los valores de la CE50 en 24-48 horas oscilaron entre 95 y
1376 mg de boro/litro, con la mayoría de los valores del orden de
100-200 mg de boro/litro. En estudios de toxicidad crónica con
Daphnia magna, los valores de la NOEC oscilaron entre 6 y 10 mg de
boro/litro. Se obtuvieron valores de la NOEC ligeramente más bajos en
estudios de biocenosis de laboratorio y de campo. En el estudio de
laboratorio de 28 días, consistente en seis etapas tróficas, se obtuvo
una NOEC de 2,5 mg de boro/litro. En estudios prolongados realizados
en un estanque al aire libre y en el campo (sin incluir peces) las
NOEC fueron de hasta 1,52 mg de boro/litro.
En pruebas de toxicidad aguda con varias especies de peces se
obtuvieron valores comprendidos entre alrededor de 10 y cerca de
300 mg de boro/litro. La trucha irisada (Oncorhynchus mykiss) y
Brachydanio rerio fueron los más sensibles, con valores aproximados
de 10 mg de boro/litro.
La toxicidad del boro en las primeras fases de la vida de los
peces está documentada para varias especies en agua reconstituida. Se
expusieron a boro, en forma de ácido bórico o de bórax, fases
embrionarias o larvarias iniciales de trucha irisada, perca atruchada
(Micropterus salmoides), coto punteado (Ictalurus punctatus) y
Carassius auratus desde la fecundación hasta ocho días después de la
eclosión en agua blanda o dura. Ni la dureza del agua ni la forma del
boro influyeron de manera uniforme en la supervivencia de los
embriones-larvas de los peces. La trucha irisada fue la especie más
sensible. Las NOEC para la trucha irisada oscilaron entre 0,009 y
0,103 mg de boro/litro.
El efecto de la dilución natural en el agua sobre la toxicidad
del boro se determinó utilizando agua superficial recogida en tres
lugares, con concentraciones de boro de 0,023, 0,091 y 0,75 mg/litro.
No se detectó ningún efecto adverso hasta los 0,75 mg de boro/litro.
Las concentraciones más bajas con efectos observados (LOEC) oscilaron
entre 1,1 y 1,73 mg de boro/litro. En una prueba con agua de un pozo
profundo (600 m), utilizada habitualmente para pruebas de toxicidad
acuática en virtud de un contrato con un laboratorio situado en
Wareham, Massachusetts, Estados Unidos, se obtuvo una NOEC de
>18,0 mg de boro/litro. Así pues, al parecer en la exposición a agua
reconstituida se sobreestimaba la toxicidad determinada en aguas
naturales, posiblemente como consecuencia de la deficiencia de
nutrientes en la primera.
Desde los años veinte se sabe que el boro es un micronutriente
esencial para las plantas superiores, con diferencias interespecíficas
en cuanto a las concentraciones necesarias para un crecimiento óptimo.
El boro interviene en la división, el metabolismo y la estructura y la
función de las membranas de las células. En forma de borato está
presente en las frutas, las nueces y las hortalizas. La diferencia
entre la deficiencia y la absorción excesiva (toxicidad) es pequeña en
las plantas. Se ha notificado deficiencia de boro en plantas
terrestres en muchos países. Es más probable que se produzca
deficiencia de boro en suelos ácidos de textura ligera en las regiones
húmedas, debido a su susceptibilidad a la lixiviación. Suele haber
exceso de boro en soluciones del suelo procedentes de depósitos
jóvenes desde el punto de vista geológico, en suelos áridos, en los
derivados de sedimentos marinos y en los afectados por fuentes de
contaminación como los vertidos de centrales termoeléctricas de carbón
y de operaciones de extracción. El agua de riego es una de las
principales fuentes de concentraciones altas de boro que provocan
toxicidad en el campo.
El crecimiento del pato real (Anas platyrhynchos) se vio
afectado negativamente por concentraciones de 30 y 300 mg de boro/kg
en los alimentos y con 1000 mg/kg se redujo la supervivencia.
2. Conclusiones
El boro es un elemento que se encuentra presente en la naturaleza
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Las fuentes naturales de los
boratos que se liberan en el medio ambiente son los océanos, el vapor
geotérmico y la meteorización natural de las rocas sedimentarias ricas
en arcilla. También se desprende boro en menor medida de fuentes
antropogénicas.
El boro es un micronutriente esencial para las plantas
superiores, con diferencias interespecíficas en cuanto a las
concentraciones necesarias para un crecimiento óptimo. Se ha observado
deficiencia de boro en plantas terrestres en muchos países de todo el
mundo. La diferencia entre la deficiencia y toxicidad es pequeña en
algunas plantas.
La comparación de la concentración sin efectos en el medio
ambiente (1 mg/litro) con los niveles generales en el medio ambiente
indica que el riesgo de efectos adversos del boro en el ecosistema
acuático es bajo. Los niveles naturales son más elevados en algunos
medios ricos en boro. Es razonable suponer que los organismos
acuáticos de dichos hábitats pueden adaptarse a las condiciones
locales.
La exposición del ser humano al boro se produce sobre todo por
medio de los alimentos y el agua potable. Se consideró que la
concentración media mundial de boro en el agua potable estaba
comprendida entre 0,1 y 0,3 mg de boro/litro.
Para la población general, la mayor exposición al boro procede de
la ingesta oral con los alimentos. La ingesta diaria media de boro con
los alimentos es de alrededor de 1,2 mg.
En las personas y en los animales, el ácido bórico y el borato se
absorben del tracto gastrointestinal y de las vías respiratorias. Se
absorbe más del 90% de las dosis administradas de estos compuestos,
como se pone de manifiesto en la excreción en la orina, que es rápida,
en unos pocos días.
En experimentos con animales se ha demostrado que el boro en
forma de ácido bórico y de borato tiene toxicidad reproductiva y en el
desarrollo en concentraciones de 100 a 1000 veces superiores a los
niveles normales de exposición. Se carece de datos suficientes acerca
de la toxicidad en el ser humano. La ingesta tolerable (IT) de boro se
fijó en 0,4 mg/kg de peso corporal al día. La asignación de la IT en
diversos medios debe basarse en los datos relativos a la exposición de
cada país.
3. Recomendaciones
a) Los valores guía del agua y los alimentos deben basarse en la IT
proporcionada por este documento.
b) La IT se debe aplicar quedando entendido que el boro puede
aportar beneficios fisiológicos para la salud humana.
c) Hay que reconocer al aplicar las normas que el boro es esencial
para algunos elementos constituyentes del medio ambiente (por
ejemplo, el boro es un micronutriente esencial para las plantas
superiores).
d) Se deben evitar los complementos de la alimentación que superen
la IT.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
Members
Dr G.M. Buck, Department of Social and Preventive Medicine, State
University of New York at Buffalo, Buffalo, New York, USA
Dr R. Chapin, Department of Health and Human Services, National
Institutes of Health, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr M.L. Dourson, Toxicology Excellence for Risk Assessment,
Cincinnati, Ohio, USA
Dr P. Foster, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr R.A. Goyer, 6405 Huntingridge Road, Chapel Hill, North
Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Cambs., United Kingdom (Co-Rapporteur)
Dr R. Luoto, National Public Health Institute, Department of
Epidemiology and Health Promotion, Helsinki, Finland
Dr F.H. Nielsen, US Department of Agriculture, Agricultural
Research Service, Grand Forks Human Nutrition Research Center,
Grand Forks, North Dakota, USA
Dr C.J. Price, Center for Life Sciences and Toxicology, Research
Triangle Institute, Research Triangle Park, North Carolina, USA
Dr W.G. Woods, Office of Environmental Health and Safety,
University of California, Riverside, California, USA
Observers
Dr B.D. Culver, University of California, Department of Medicine,
Irvine, California, USA (representing International Commission on
Occupational Health)
Dr S. Dyer, Procter & Gamble, Ecosystems Research Station,
Environmental Science Department, Cincinnati, Ohio, USA
(representing European Centre for Ecotoxicology, Toxicology of
Chemicals)
Dr J.A. Moore, Institute for Evaluating Health Risks, Washington,
DC, USA (representing the American Industrial Health Council)
Dr F.J. Murray, 6611 Northridge Drive, San Jose, California, USA
(representing International Life Sciences Institute)
Mrs M. Richold, Unilever Research ESL, Sharnbrook, Bedford,
United Kingdom (representing International Life Sciences
Institute)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr L. Galvao, Pan American Health Organization, World Health
Organization, Geneva, Switzerland
Dr H. Otterstetter, Pan American Health Organization, World
Health Organization, Geneva, Switzerland
Ms C. Smallwood, US Environmental Protection Agency, National
Center for Environmental Assessment, Cincinnati, Ohio, USA
(Co-Rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
A WHO Task Group on Environmental Health Criteria for Boron met
in Washington, DC, USA, from 18 to 22 November 1996. The meeting was
organized by the WHO Regional Office for the Americas (AMRO) on behalf
of the IPCS. Dr H. Otterstetter, WHO AMRO, opened the meeting and
welcomed the participants. Dr B.H. Chen, IPCS, welcomed the
participants on behalf of the Director of IPCS and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to boron.
The first draft of this monograph was prepared by Ms C. Smallwood
of the US EPA in Cincinnati. The second draft was also prepared by Ms
Smallwood, incorporating comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs. Dr R. Goyer, Chairman of the Task Group,
contributed significantly to the final text of the EHC for Boron.
Dr B.H. Chen, member of the IPCS Central Unit, and Ms M. Sheffer,
Scientific Editor, Ottawa, Canada, were responsible for the overall
scientific content and linguistic editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
Financial support for this Task Group meeting was provided by the
US EPA.
ABBREVIATIONS
BMD Benchmark dose
CAS Chemical Abstracts Service
CL Confidence limit
CNS Central nervous system
EPA Environmental Protection Agency (USA)
FDA Food and Drug Administration (USA)
FSH Follicle stimulating hormone
GLP Good Laboratory Practices
HSDB Hazardous Substances Data Bank
ICP Inductively coupled plasma
ICP-AES Inductively coupled plasma atomic emission spectroscopy
ICP-MS Inductively coupled plasma mass spectroscopy
LH Luteinizing hormone
LOAEL Lowest-observed-adverse-effect level
(human and animal toxicity)
LOEC Lowest-observed-effect concentration
(environmental effects)
MATC Maximum acceptable toxicant concentration
(environmental effects)
MMAD Median mass aerodynamic diameter
NADPH Reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health
NOAEL No-observed-adverse-effect level
(human and animal toxicity)
NOEC No-observed-effect concentration
(environmental effects)
NOHS National Occupational Hazard Survey
RR Rate ratio (or Relative risk)
RTECS Registry of Toxic Effects of Chemical Substances
SBR Standardized birth ratio
SGOT Serum glutamic-oxaloacetic transaminase
SGPT Serum glutamic-pyruvic transaminase
TI Tolerable intake
TLV Threshold limit value
TRI Toxic Release Inventory (US EPA)
1. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, natural occurrence, and analytical methods
Boron is a naturally occurring element that is found in the form
of borates in the oceans, sedimentary rocks, coal, shale, and some
soils. It is widely distributed in nature, with concentrations of
about 10 mg/kg in the Earth's crust (range: 5 mg/kg in basalts to 100
mg/kg in shales) and about 4.5 mg/litre in the ocean.
The most important commercial borate products and minerals are
borax pentahydrate, borax, sodium perborate, boric acid, colemanite,
and ulexite. At the low concentrations and near-neutral pH found in
most biological fluids, monomeric B(OH)3 will be the predominant
species present (with some B(OH)4), regardless of whether the
boron source is boric acid or one of the borates. This is because
boric acid is a very weak acid (p Ka 9.15). Sodium perborate
hydrolyses to give hydrogen peroxide plus metaborate; consequently, it
may exhibit chemical and toxicological properties that are somewhat
different from those of the other borates.
Inductively coupled plasma (ICP) methods are preferred for the
analysis of the low levels of boron found in biological and
environmental samples; colorimetric methods must be used with caution.
1.1.2 Production, uses, environmental fate, and sources of exposure
Economic borate deposits are rare, occurring in arid regions of
Turkey, the USA, Argentina, Chile, Russia, China, and Peru. Total
world production of boron minerals -- mainly colemanite, ulexite,
tincal, and kernite -- was approximately 2 750 000 tonnes in 1994.
About 800 000 tonnes of commercial borate products, expressed as
B2O3, were manufactured from the boron minerals.
Major end uses for borate include insulation- and textile-grade
fibreglass, laundry bleach (sodium perborate), borosilicate glass,
fire retardants, agricultural fertilizers and herbicides (as a trace
element), and enamels, frits, and ceramic glazes, as well as a myriad
of miscellaneous applications.
Boron enters the environment mainly through the weathering of
rocks, boric acid volatilization from seawater, and volcanic activity.
Boron is also released from anthropogenic sources to a lesser extent.
Anthropogenic sources include agricultural, refuse, and fuel wood
burning, power generation using coal and oil, glass product
manufacture, use of borates/perborates in the home and industry,
borate mining/processing, leaching of treated wood/paper, and
sewage/sludge disposal. Many of these sources are difficult to
quantify.
Atmospheric emissions of borates and boric acid in particulate
and vapour form occur as a result of volatilization from the sea,
volcanic activity, and, to a lesser extent, mining operations, glass
and ceramics manufacturing, the application of agricultural chemicals,
and coal-fired power plants. Boron is not present in the atmosphere at
significant levels; however, the total amount present in the
atmosphere at any one time is significant owing to the huge volume of
the atmosphere. Based on their water solubility, borates would not be
expected to persist to a significant degree in the atmosphere.
Boron can be released into water and soil water through
weathering processes and, to a much smaller extent, through
anthropogenic discharges such as sewage outfalls.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water. The
extent of boron adsorption depends on the pH of the water and the
concentration of boron in solution.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported.
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
The octanol/water partition coefficient of boric acid has been
measured as 0.175, indicating a low bioaccumulation potential.
Laboratory experiments with aquatic organisms have confirmed this
potential. Plants accumulate boron; however, uptake is affected by the
pH of the soil solution, temperature, light intensity, and the
concentration of other elements (e.g. calcium and potassium). The
results of studies of boron accumulation in plants, insects, and fish
have shown that boron bioaccumulates in plants but does not biomagnify
in aquatic food-chains.
Boron occurs in soils at concentrations ranging from 10 to 300
mg/kg (average 30 mg/kg), depending on the type of soil, amount of
organic matter, and amount of rainfall. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron in surface water range widely, from 0.001 to as much as 360
mg/litre. However, mean boron concentrations for waters of Europe,
Pakistan, Russia, and Turkey are typically well below 0.6 mg/litre.
Concentrations of boron in water in Japan, South Africa, and South
America are generally below 0.3 mg/litre. Typical boron concentrations
in North American waters are below 0.1 mg/litre, with about 90% at or
below 0.4 mg/litre.
Boron accumulates in aquatic and terrestrial plants but does not
magnify through the food-chain. Concentrations of boron have been
shown to range between 26 and 382 mg/kg in submerged aquatic
freshwater plants, from 11.3 to 57 mg/kg in freshwater emergent
vegetation, and from 2.3 to 94.7 mg/kg dry weight in terrestrial
plants. Based on wet weights, boron concentrations in marine
invertebrates and fish are similar to the levels found in the exposure
media, between 0.5 and 4 mg/kg. The bioconcentration factor for two
freshwater fish species was found to be 0.3.
Boron concentrations in ambient air range from <0.5 to
approximately 80 ng/m3, with an average over the continents of
20 ng/m3.
Close similarity of boron concentrations in groundwater, fresh
surface water, and drinking-water indicates that boron is not removed
in the treatment of groundwater and fresh surface water used for
drinking-water.
Intakes of boron for humans are expected to be 0.44 µg/day from
ambient air, 0.2-0.6 mg/day from drinking-water, and 1.2 mg/day from
the diet. Average boron intake from the soil is considered to be 0.5
µg/day. A reasonable estimate of boron exposure from consumer products
is 0.1 mg/day.
1.1.3 Kinetics and biological monitoring
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in the blood
and body tissues of several mammalian species following
ingestion.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the no-observed-adverse-effect level
(NOAEL) for laboratory studies, reduce the uncertainty for risk
extrapolation between these two species.
1.1.4 Effects on experimental animals and humans
The data regarding developmental and reproductive toxicity show
that lower fetal body weight in rats is the critical effect. The NOAEL
for lower fetal body weight is 9.6 mg boron/kg body weight per day.
The lowest-observed-adverse-effect level (LOAEL), at which rats show
slight (approx. 5%) fetal body weight differences and rib anomalies,
is about 13 mg boron/kg body weight per day. As dose level increases,
the effects that are seen (and the doses at which they are seen) are:
a) further rib effects and testicular pathology in the rat (approx.
25 mg boron/kg body weight per day);
b) decreased fetal body weight and increased fetal cardiovascular
malformations in the rabbit, and severe testicular pathology in
the rat (approx. 40 mg boron/kg body weight per day);
c) testicular atrophy and sterility in the rat (approx. 55 mg
boron/kg body weight per day); and
d) reduced fetal body weight in the mouse (approx. 80 mg boron/kg
body weight per day).
Animal studies on mice and rats showed no evidence of
carcinogenicity of boric acid. Based on the lack of human data and the
limited animal data, boron is not classifiable as to its human
carcinogenicity.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data show that exposure is associated with short-term irritant effects
on the upper respiratory tract, nasopharynx, and eye. These effects,
however, appear to be short-term and reversible. The sole long-term
(7-year) follow-up study failed to identify any long-term health
effects, although a healthy worker effect cannot be entirely ruled out
given the rate of attrition (47%). Two descriptive studies assessed
fertility and secondary sex ratios in relation to exposure. Neither
study reported a detrimental effect on demonstrated fertility for its
select sample. Although an excess percentage of female births has been
suggested, the absence of statistical significance and attention to
other co-variates known to affect sex ratios warrants careful
interpretation of this finding. No studies have been identified that
assess the spectrum of reproductive outcomes, such as
time-to-pregnancy, conception delays, spontaneous abortions, and sperm
analyses in males. The role of other lifestyle or behavioural factors
in relation to health and fertility requires further study to identify
potentially sensitive populations and to evaluate reproductive effects
more fully.
1.1.5 Effects on organisms in the environment
Bacteria are relatively tolerant towards boron. Acute and chronic
effect concentrations range between 8 and 340 mg boron/litre, with
most values greater than 18 mg boron/litre. More sensitive are
protozoa. Tests with Entosiphon and Paramecium yielded 72-h
no-observed-effect concentrations (NOECs) and EC3 values between
0.3 and 18 mg boron/litre.
Boron is an essential micronutrient for cyanobacteria and
diatoms. Standard chronic tests with freshwater green algae resulted
in no-effect concentrations between 10 and 24 mg boron/litre.
Blue-green algae appear to be similar in sensitivity, with an 8-day
EC3 of 20 mg boron/litre.
Based on acute toxicity values, invertebrates are less sensitive
to boron than microorganisms. For several species, 24- to 48-h EC50
values ranged from 95 to 1376 mg boron/litre, with most values in the
100-200 mg boron/litre range. Chronic toxicity studies with
Daphnia magna gave NOECs ranging between 6 and 10 mg boron/litre.
Slightly lower NOEC values were obtained from laboratory and field
biocenosis studies. The 28-day laboratory study consisting of six
trophic stages yielded a NOEC of 2.5 mg boron/litre. Long-term outdoor
pond and field studies (not including fish) yielded NOECs up to 1.52
mg boron/litre.
Acute tests with several fish species yielded toxicity values
ranging from about 10 to nearly 300 mg boron/litre. Rainbow trout
(Oncorhynchus mykiss) and zebra fish (Brachydanio rerio) were the
most sensitive, providing values around 10 mg boron/litre.
The toxicity of boron to early life stages of fish has been
documented for several species in reconstituted water. Embryonic and
early larval stages of rainbow trout, largemouth bass (Micropterus
salmoides), channel catfish (Ictalurus punctatus), and goldfish
(Carassius auratus) were exposed to boron, as boric acid or borax,
from fertilization up to 8 days post-hatch in soft or hard water.
Neither water hardness nor the form of boron consistently affected
embryo-larval survival of fish. Rainbow trout was the most sensitive
species. The NOECs for rainbow trout ranged from 0.009 to 0.103 mg
boron/litre.
The effect of natural dilution water on boron toxicity was
determined by using surface waters collected from three locations,
with boron concentrations of 0.023, 0.091, and 0.75 mg/litre. No
adverse effects were determined up to 0.75 mg boron/litre.
Lowest-observed-effect concentrations (LOECs) ranged from 1.1 to 1.73
mg boron/litre. One test using deep (600 m) well-water, typically used
for aquatic toxicity tests, from a contract laboratory located in
Wareham, Massachusetts, USA, yielded a NOEC of >18.0 mg boron/litre.
Hence, reconstituted water exposures appeared to overestimate the
toxicity determined in natural waters, possibly as a result of
nutrient deficiency in the former.
Boron has been known since the 1920s to be an essential
micronutrient for higher plants, with interspecies differences in the
levels required for optimum growth. Boron plays a role in cell
division, metabolism, and membrane structure and function. Boron in
the form of borates occurs naturally in fruits, nuts, and vegetables.
There is a small range between deficiency and excess uptake (toxicity)
in plants. Boron deficiencies in terrestrial plants have been reported
in many countries. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions because of boron's
susceptibility to leaching. Boron excesses usually occur in soil
solutions from geologically young deposits, arid soils, soils derived
from marine sediments, and soils contaminated by pollutant sources,
such as releases from coal-fired power plants and mining operations.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field.
Mallard (Anas platyrhynchos) duckling growth was adversely
affected at dietary levels of 30 and 300 mg boron/kg, and survival was
reduced at 1000 mg/kg.
1.2 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency in terrestrial plants has been observed in many
countries throughout the world. There is a small range between
deficiency and toxicity in some plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental levels of boron
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre.
For the general population, the greatest boron exposure comes
from the oral intake of food. The mean daily intake of boron in the
diet is about 1.2 mg.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. More than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The tolerable intake (TI) of boron was set as 0.4 mg/kg body
weight per day. The allocation of the TI in various media should be
based on the exposure data of individual countries.
1.3 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
This chapter deals with the identity and physical and chemical
properties of the inorganic borates of importance in commerce, as well
as the analytical methods used to determine boron concentrations in
various media.
2.1 Identity
Elemental boron (B) is a member of Group IIIB of the periodic
table, along with aluminium, gallium, indium, and thallium. It has an
atomic number of 5 and a relative atomic mass of 10.81. Boron is never
found in the elemental form in nature. Its chemistry is complex and
resembles that of silicon (Cotton & Wilkinson, 1988). The Chemical
Abstracts Service (CAS), National Institute for Occupational Safety
and Health (NIOSH) Registry of Toxic Effects of Chemical Substances
(RTECS), and Hazardous Substances Data Bank (HSDB) numbers for boron
are
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
Members
Dr G.M. Buck, Department of Social and Preventive Medicine, State
University of New York at Buffalo, Buffalo, New York, USA
Dr R. Chapin, Department of Health and Human Services, National
Institutes of Health, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr M.L. Dourson, Toxicology Excellence for Risk Assessment,
Cincinnati, Ohio, USA
Dr P. Foster, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr R.A. Goyer, 6405 Huntingridge Road, Chapel Hill, North
Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Cambs., United Kingdom (Co-Rapporteur)
Dr R. Luoto, National Public Health Institute, Department of
Epidemiology and Health Promotion, Helsinki, Finland
Dr F.H. Nielsen, US Department of Agriculture, Agricultural
Research Service, Grand Forks Human Nutrition Research Center,
Grand Forks, North Dakota, USA
Dr C.J. Price, Center for Life Sciences and Toxicology, Research
Triangle Institute, Research Triangle Park, North Carolina, USA
Dr W.G. Woods, Office of Environmental Health and Safety,
University of California, Riverside, California, USA
Observers
Dr B.D. Culver, University of California, Department of Medicine,
Irvine, California, USA (representing International Commission on
Occupational Health)
Dr S. Dyer, Procter & Gamble, Ecosystems Research Station,
Environmental Science Department, Cincinnati, Ohio, USA
(representing European Centre for Ecotoxicology, Toxicology of
Chemicals)
Dr J.A. Moore, Institute for Evaluating Health Risks, Washington,
DC, USA (representing the American Industrial Health Council)
Dr F.J. Murray, 6611 Northridge Drive, San Jose, California, USA
(representing International Life Sciences Institute)
Mrs M. Richold, Unilever Research ESL, Sharnbrook, Bedford,
United Kingdom (representing International Life Sciences
Institute)
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr L. Galvao, Pan American Health Organization, World Health
Organization, Geneva, Switzerland
Dr H. Otterstetter, Pan American Health Organization, World
Health Organization, Geneva, Switzerland
Ms C. Smallwood, US Environmental Protection Agency, National
Center for Environmental Assessment, Cincinnati, Ohio, USA
(Co-Rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BORON
A WHO Task Group on Environmental Health Criteria for Boron met
in Washington, DC, USA, from 18 to 22 November 1996. The meeting was
organized by the WHO Regional Office for the Americas (AMRO) on behalf
of the IPCS. Dr H. Otterstetter, WHO AMRO, opened the meeting and
welcomed the participants. Dr B.H. Chen, IPCS, welcomed the
participants on behalf of the Director of IPCS and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to boron.
The first draft of this monograph was prepared by Ms C. Smallwood
of the US EPA in Cincinnati. The second draft was also prepared by Ms
Smallwood, incorporating comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs. Dr R. Goyer, Chairman of the Task Group,
contributed significantly to the final text of the EHC for Boron.
Dr B.H. Chen, member of the IPCS Central Unit, and Ms M. Sheffer,
Scientific Editor, Ottawa, Canada, were responsible for the overall
scientific content and linguistic editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
Financial support for this Task Group meeting was provided by the
US EPA.
ABBREVIATIONS
BMD Benchmark dose
CAS Chemical Abstracts Service
CL Confidence limit
CNS Central nervous system
EPA Environmental Protection Agency (USA)
FDA Food and Drug Administration (USA)
FSH Follicle stimulating hormone
GLP Good Laboratory Practices
HSDB Hazardous Substances Data Bank
ICP Inductively coupled plasma
ICP-AES Inductively coupled plasma atomic emission spectroscopy
ICP-MS Inductively coupled plasma mass spectroscopy
LH Luteinizing hormone
LOAEL Lowest-observed-adverse-effect level
(human and animal toxicity)
LOEC Lowest-observed-effect concentration
(environmental effects)
MATC Maximum acceptable toxicant concentration
(environmental effects)
MMAD Median mass aerodynamic diameter
NADPH Reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health
NOAEL No-observed-adverse-effect level
(human and animal toxicity)
NOEC No-observed-effect concentration
(environmental effects)
NOHS National Occupational Hazard Survey
RR Rate ratio (or Relative risk)
RTECS Registry of Toxic Effects of Chemical Substances
SBR Standardized birth ratio
SGOT Serum glutamic-oxaloacetic transaminase
SGPT Serum glutamic-pyruvic transaminase
TI Tolerable intake
TLV Threshold limit value
TRI Toxic Release Inventory (US EPA)
1. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, natural occurrence, and analytical methods
Boron is a naturally occurring element that is found in the form
of borates in the oceans, sedimentary rocks, coal, shale, and some
soils. It is widely distributed in nature, with concentrations of
about 10 mg/kg in the Earth's crust (range: 5 mg/kg in basalts to 100
mg/kg in shales) and about 4.5 mg/litre in the ocean.
The most important commercial borate products and minerals are
borax pentahydrate, borax, sodium perborate, boric acid, colemanite,
and ulexite. At the low concentrations and near-neutral pH found in
most biological fluids, monomeric B(OH)3 will be the predominant
species present (with some B(OH)4), regardless of whether the
boron source is boric acid or one of the borates. This is because
boric acid is a very weak acid (p Ka 9.15). Sodium perborate
hydrolyses to give hydrogen peroxide plus metaborate; consequently, it
may exhibit chemical and toxicological properties that are somewhat
different from those of the other borates.
Inductively coupled plasma (ICP) methods are preferred for the
analysis of the low levels of boron found in biological and
environmental samples; colorimetric methods must be used with caution.
1.1.2 Production, uses, environmental fate, and sources of exposure
Economic borate deposits are rare, occurring in arid regions of
Turkey, the USA, Argentina, Chile, Russia, China, and Peru. Total
world production of boron minerals -- mainly colemanite, ulexite,
tincal, and kernite -- was approximately 2 750 000 tonnes in 1994.
About 800 000 tonnes of commercial borate products, expressed as
B2O3, were manufactured from the boron minerals.
Major end uses for borate include insulation- and textile-grade
fibreglass, laundry bleach (sodium perborate), borosilicate glass,
fire retardants, agricultural fertilizers and herbicides (as a trace
element), and enamels, frits, and ceramic glazes, as well as a myriad
of miscellaneous applications.
Boron enters the environment mainly through the weathering of
rocks, boric acid volatilization from seawater, and volcanic activity.
Boron is also released from anthropogenic sources to a lesser extent.
Anthropogenic sources include agricultural, refuse, and fuel wood
burning, power generation using coal and oil, glass product
manufacture, use of borates/perborates in the home and industry,
borate mining/processing, leaching of treated wood/paper, and
sewage/sludge disposal. Many of these sources are difficult to
quantify.
Atmospheric emissions of borates and boric acid in particulate
and vapour form occur as a result of volatilization from the sea,
volcanic activity, and, to a lesser extent, mining operations, glass
and ceramics manufacturing, the application of agricultural chemicals,
and coal-fired power plants. Boron is not present in the atmosphere at
significant levels; however, the total amount present in the
atmosphere at any one time is significant owing to the huge volume of
the atmosphere. Based on their water solubility, borates would not be
expected to persist to a significant degree in the atmosphere.
Boron can be released into water and soil water through
weathering processes and, to a much smaller extent, through
anthropogenic discharges such as sewage outfalls.
Adsorption-desorption reactions are expected to be the only
significant mechanism influencing the fate of boron in water. The
extent of boron adsorption depends on the pH of the water and the
concentration of boron in solution.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Borate ions present in aqueous solution are essentially in their
fully oxidized state. No aerobic processes are likely to affect their
speciation, and no biotransformation processes are reported.
Therefore, there are unlikely to be any differences in boron species
due to biotransformation.
The octanol/water partition coefficient of boric acid has been
measured as 0.175, indicating a low bioaccumulation potential.
Laboratory experiments with aquatic organisms have confirmed this
potential. Plants accumulate boron; however, uptake is affected by the
pH of the soil solution, temperature, light intensity, and the
concentration of other elements (e.g. calcium and potassium). The
results of studies of boron accumulation in plants, insects, and fish
have shown that boron bioaccumulates in plants but does not biomagnify
in aquatic food-chains.
Boron occurs in soils at concentrations ranging from 10 to 300
mg/kg (average 30 mg/kg), depending on the type of soil, amount of
organic matter, and amount of rainfall. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron in surface water range widely, from 0.001 to as much as 360
mg/litre. However, mean boron concentrations for waters of Europe,
Pakistan, Russia, and Turkey are typically well below 0.6 mg/litre.
Concentrations of boron in water in Japan, South Africa, and South
America are generally below 0.3 mg/litre. Typical boron concentrations
in North American waters are below 0.1 mg/litre, with about 90% at or
below 0.4 mg/litre.
Boron accumulates in aquatic and terrestrial plants but does not
magnify through the food-chain. Concentrations of boron have been
shown to range between 26 and 382 mg/kg in submerged aquatic
freshwater plants, from 11.3 to 57 mg/kg in freshwater emergent
vegetation, and from 2.3 to 94.7 mg/kg dry weight in terrestrial
plants. Based on wet weights, boron concentrations in marine
invertebrates and fish are similar to the levels found in the exposure
media, between 0.5 and 4 mg/kg. The bioconcentration factor for two
freshwater fish species was found to be 0.3.
Boron concentrations in ambient air range from <0.5 to
approximately 80 ng/m3, with an average over the continents of
20 ng/m3.
Close similarity of boron concentrations in groundwater, fresh
surface water, and drinking-water indicates that boron is not removed
in the treatment of groundwater and fresh surface water used for
drinking-water.
Intakes of boron for humans are expected to be 0.44 µg/day from
ambient air, 0.2-0.6 mg/day from drinking-water, and 1.2 mg/day from
the diet. Average boron intake from the soil is considered to be 0.5
µg/day. A reasonable estimate of boron exposure from consumer products
is 0.1 mg/day.
1.1.3 Kinetics and biological monitoring
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in the blood
and body tissues of several mammalian species following
ingestion.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the no-observed-adverse-effect level
(NOAEL) for laboratory studies, reduce the uncertainty for risk
extrapolation between these two species.
1.1.4 Effects on experimental animals and humans
The data regarding developmental and reproductive toxicity show
that lower fetal body weight in rats is the critical effect. The NOAEL
for lower fetal body weight is 9.6 mg boron/kg body weight per day.
The lowest-observed-adverse-effect level (LOAEL), at which rats show
slight (approx. 5%) fetal body weight differences and rib anomalies,
is about 13 mg boron/kg body weight per day. As dose level increases,
the effects that are seen (and the doses at which they are seen) are:
a) further rib effects and testicular pathology in the rat (approx.
25 mg boron/kg body weight per day);
b) decreased fetal body weight and increased fetal cardiovascular
malformations in the rabbit, and severe testicular pathology in
the rat (approx. 40 mg boron/kg body weight per day);
c) testicular atrophy and sterility in the rat (approx. 55 mg
boron/kg body weight per day); and
d) reduced fetal body weight in the mouse (approx. 80 mg boron/kg
body weight per day).
Animal studies on mice and rats showed no evidence of
carcinogenicity of boric acid. Based on the lack of human data and the
limited animal data, boron is not classifiable as to its human
carcinogenicity.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data show that exposure is associated with short-term irritant effects
on the upper respiratory tract, nasopharynx, and eye. These effects,
however, appear to be short-term and reversible. The sole long-term
(7-year) follow-up study failed to identify any long-term health
effects, although a healthy worker effect cannot be entirely ruled out
given the rate of attrition (47%). Two descriptive studies assessed
fertility and secondary sex ratios in relation to exposure. Neither
study reported a detrimental effect on demonstrated fertility for its
select sample. Although an excess percentage of female births has been
suggested, the absence of statistical significance and attention to
other co-variates known to affect sex ratios warrants careful
interpretation of this finding. No studies have been identified that
assess the spectrum of reproductive outcomes, such as
time-to-pregnancy, conception delays, spontaneous abortions, and sperm
analyses in males. The role of other lifestyle or behavioural factors
in relation to health and fertility requires further study to identify
potentially sensitive populations and to evaluate reproductive effects
more fully.
1.1.5 Effects on organisms in the environment
Bacteria are relatively tolerant towards boron. Acute and chronic
effect concentrations range between 8 and 340 mg boron/litre, with
most values greater than 18 mg boron/litre. More sensitive are
protozoa. Tests with Entosiphon and Paramecium yielded 72-h
no-observed-effect concentrations (NOECs) and EC3 values between
0.3 and 18 mg boron/litre.
Boron is an essential micronutrient for cyanobacteria and
diatoms. Standard chronic tests with freshwater green algae resulted
in no-effect concentrations between 10 and 24 mg boron/litre.
Blue-green algae appear to be similar in sensitivity, with an 8-day
EC3 of 20 mg boron/litre.
Based on acute toxicity values, invertebrates are less sensitive
to boron than microorganisms. For several species, 24- to 48-h EC50
values ranged from 95 to 1376 mg boron/litre, with most values in the
100-200 mg boron/litre range. Chronic toxicity studies with
Daphnia magna gave NOECs ranging between 6 and 10 mg boron/litre.
Slightly lower NOEC values were obtained from laboratory and field
biocenosis studies. The 28-day laboratory study consisting of six
trophic stages yielded a NOEC of 2.5 mg boron/litre. Long-term outdoor
pond and field studies (not including fish) yielded NOECs up to 1.52
mg boron/litre.
Acute tests with several fish species yielded toxicity values
ranging from about 10 to nearly 300 mg boron/litre. Rainbow trout
(Oncorhynchus mykiss) and zebra fish (Brachydanio rerio) were the
most sensitive, providing values around 10 mg boron/litre.
The toxicity of boron to early life stages of fish has been
documented for several species in reconstituted water. Embryonic and
early larval stages of rainbow trout, largemouth bass (Micropterus
salmoides), channel catfish (Ictalurus punctatus), and goldfish
(Carassius auratus) were exposed to boron, as boric acid or borax,
from fertilization up to 8 days post-hatch in soft or hard water.
Neither water hardness nor the form of boron consistently affected
embryo-larval survival of fish. Rainbow trout was the most sensitive
species. The NOECs for rainbow trout ranged from 0.009 to 0.103 mg
boron/litre.
The effect of natural dilution water on boron toxicity was
determined by using surface waters collected from three locations,
with boron concentrations of 0.023, 0.091, and 0.75 mg/litre. No
adverse effects were determined up to 0.75 mg boron/litre.
Lowest-observed-effect concentrations (LOECs) ranged from 1.1 to 1.73
mg boron/litre. One test using deep (600 m) well-water, typically used
for aquatic toxicity tests, from a contract laboratory located in
Wareham, Massachusetts, USA, yielded a NOEC of >18.0 mg boron/litre.
Hence, reconstituted water exposures appeared to overestimate the
toxicity determined in natural waters, possibly as a result of
nutrient deficiency in the former.
Boron has been known since the 1920s to be an essential
micronutrient for higher plants, with interspecies differences in the
levels required for optimum growth. Boron plays a role in cell
division, metabolism, and membrane structure and function. Boron in
the form of borates occurs naturally in fruits, nuts, and vegetables.
There is a small range between deficiency and excess uptake (toxicity)
in plants. Boron deficiencies in terrestrial plants have been reported
in many countries. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions because of boron's
susceptibility to leaching. Boron excesses usually occur in soil
solutions from geologically young deposits, arid soils, soils derived
from marine sediments, and soils contaminated by pollutant sources,
such as releases from coal-fired power plants and mining operations.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field.
Mallard (Anas platyrhynchos) duckling growth was adversely
affected at dietary levels of 30 and 300 mg boron/kg, and survival was
reduced at 1000 mg/kg.
1.2 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency in terrestrial plants has been observed in many
countries throughout the world. There is a small range between
deficiency and toxicity in some plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental levels of boron
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre.
For the general population, the greatest boron exposure comes
from the oral intake of food. The mean daily intake of boron in the
diet is about 1.2 mg.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. More than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The tolerable intake (TI) of boron was set as 0.4 mg/kg body
weight per day. The allocation of the TI in various media should be
based on the exposure data of individual countries.
1.3 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
This chapter deals with the identity and physical and chemical
properties of the inorganic borates of importance in commerce, as well
as the analytical methods used to determine boron concentrations in
various media.
2.1 Identity
Elemental boron (B) is a member of Group IIIB of the periodic
table, along with aluminium, gallium, indium, and thallium. It has an
atomic number of 5 and a relative atomic mass of 10.81. Boron is never
found in the elemental form in nature. Its chemistry is complex and
resembles that of silicon (Cotton & Wilkinson, 1988). The Chemical
Abstracts Service (CAS), National Institute for Occupational Safety
and Health (NIOSH) Registry of Toxic Effects of Chemical Substances
(RTECS), and Hazardous Substances Data Bank (HSDB) numbers for boron
are  In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine boron levels from
workers with the amount of boron exposure (see section 5.3). An
average boron level of 0.26 µg/ml blood occurred in the highest
exposure group, where exposure was estimated to be 0.38 mg boron/kg
body weight per day. Post-shift blood and urine boron concentrations
did not increase with the days of the work week. This lack of
accumulation over the work week corroborates the relatively short
elimination half-life for boron in humans.
Elimination times for animals have not been explicitly stated in
the literature but can be either calculated or estimated from
published data. Farr & Konikowski (1963) measured urinary boron
concentrations in mice after intravenous injection of sodium
pentaborate. Using their data and assuming first-order kinetics for
elimination, the half-life for elimination in the mouse was on the
order of 1 h. Ku et al. (1991) reported that an apparent steady-state
level of boron is reached in the blood and tissues of rats after 3-4
days of oral dosing; again, assuming first-order kinetics, the
half-life in rats would be on the order of 14-19 h.
Male Fischer-344 rats were exposed to boric acid at 3000-9000
mg/kg in the diet for up to 9 weeks (Ku et al., 1993a). Urinary boron
was elevated (450-600 µg boron/mg creatinine) 24 h after the end of
dosing. By 3-4 days post-treatment, urinary boron in all groups had
returned to average control levels (8.97 ± 1.95 µg boron/mg creatinine
for days 1, 7, and 14 post-treatment). Elimination of boron from bone
followed a different time-course from elimination from serum or soft
tissues. F-344 rats consuming boric acid in the diet for 9 weeks
(approx. 1.4-6.8 mg boron/kg body weight per day) showed dose-related
elevation of bone boron, which declined very gradually during the
post-treatment period. Bone boron levels remained elevated above
controls in all exposed groups at 32 weeks post-treatment (Chapin et
al., 1997). Longer post-treatment intervals, as well as factors
influencing rates of accumulation or mobilization of boron in bone,
remain to be evaluated.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Short-term exposure
7.1.1 Oral route
The oral LD50 values for boric acid and borax in laboratory
animals are given in Table 14. Values for mice and rats are in the
range of approx. 400-700 mg boron/kg body weight (Pfeiffer et al.,
1945; Weir & Fisher, 1972). For guinea-pigs, Verbitskaya (1975)
reported an oral LD50 of 210 mg boron/kg body weight. Acute oral LD50
values in the range of 250-350 mg/kg body weight for boric acid or
borax exposure have also been reported for dogs, rabbits, and cats
(Pfeiffer et al., 1945; Verbitskaya, 1975).
General clinical effects of boric acid or borax in rats, mice,
and guinea-pigs given single large doses orally are depression,
ataxia, occasional convulsions, decreased body temperature, and
violet-red colour of skin and all mucous membranes (Pfeiffer et al.,
1945; Weir & Fisher, 1972). Toxic signs in dogs given boric acid
(0.2-2.0 g/kg body weight) orally in combination with subcutaneous
morphine to prevent vomiting were cyanosis of mucous membranes,
red-violet skin colour, rigidity of legs, convulsion, and shock-like
syndrome (Pfeiffer et al., 1945). Rabbits given boric acid at 800
mg/kg body weight per day for 4 days showed anorexia, weight loss, and
diarrhoea; 850 and 1000 mg/kg body weight per day for 4 days caused
100% mortality (Draize & Kelley, 1959). Cattle receiving 0.8, 150, or
300 mg boron/litre of drinking-water (as boric acid) for 30 days were
lethargic at the highest dose and had swelling and irritation in the
legs and around the dew claws, slight diarrhoea, and decreased food
consumption at the middle and high doses (Green & Weeth, 1977).
Following exposure to boric acid, microscopic changes in tissues
of mice, rats, and dogs involved primarily the kidneys and nervous
system (Pfeiffer et al., 1945). Glomerular and tubular damage were
noted in the kidneys. Glomerular damage consisted of changes in
permeability of the capillaries, and tubular damage consisted of
cellular vacuolization and shedding of cells into the tubular lumen.
Nervous system damage consisted of an increase in small dark cells
(probably microglia) in the spinal cord and in the grey matter of the
brain cortex.
7.1.2 Inhalation route
The LC50 for sodium perborate tetrahydrate by inhalation in rats
was >74 mg/m3 (Silaev, 1984). The 1-h inhalation LC50 values for
boron trichloride are reported as 12.2 g/m3 for male rats and 21.1
g/m3 for female rats; for boron trifluoride, the LC50 values range
from 0.89 to 1.2 g/m3 for rats (Vernot et al., 1977). While these
boron-halogen compounds are not considered in this report because of
apparent anion toxicity, it is appropriate to note in passing the
inhalation studies performed with them (Stokinger & Spiegl, 1953;
Rusch et al., 1986). These studies evaluated rats, mice, and
guinea-pigs and report various dose-related toxic effects beginning at
24 mg/m3.
Table 14. Acute toxicity of boron compounds in laboratory animals
Route Compound Species Compound LD50 Borona LD50
(mg/kg body weight) (mg/kg body weight) Reference
Oral Boric acid Mice 3450 603 Pfeiffer et al. (1945)
Borax Rats 3493 396 Wang et al. (1984)
Borax Rats 4500 510b Weir & Fisher (1972)
Borax Rats 4980 560b Weir & Fisher (1972)
Borax Rats 5660 642 Smyth et al. (1969)
Borax Rats 6080 690b Weir & Fisher (1972)
Boric acid Rats 2660 465 Pfeiffer et al. (1945)
Boric acid Rats 3160 550b Weir & Fisher (1972)
Boric acid Rats 3450 600b Weir & Fisher (1972)
Boric acid Rats 4080 710b Weir & Fisher (1972)
Boric acid Rats 5140 899 Smyth et al. (1969)
Subcutaneous Boric acid Mice 1740c 304 Pfeiffer et al. (1945)
Boric acid Mice 2070 362 Pfeiffer et al. (1945)
Boric acid Guinea-pigs 1200 210 Pfeiffer et al. (1945)
Intravenous Boric acid Mice 1780 311 Pfeiffer et al. (1945)
Boric acid Rats 1330 232 Pfeiffer et al. (1945)
a Calculated by multiplying the dose in mg boron compound/kg by the ratio of the molecular weights of boron/boron compound,
except when noted otherwise.
b Reported by investigators.
c Solution adjusted to pH 7.4 with sodium hydroxide.
7.2 Longer-term exposure
7.2.1 Oral route
The effects of longer-term oral exposure to boron compounds in
animals are summarized in Table 15.
In a 13-week study conducted by the US National Toxicology
Program (NTP, 1987; Dieter, 1994), B6C3F1 mice (10/sex per dose) were
exposed to boric acid in the diet at concentrations sufficient to
produce estimated consumptions of approximately 0, 34, 70, 141, 281,
or 563 mg boron/kg body weight per day for males and 0, 47, 97, 194,
388, or 776 mg boron/kg body weight per day for females. The results
showed that female mice were less sensitive than male mice to the
lethal effects of boric acid; 8/10 high-dose males and 6/10 high-dose
females died, and 1/10 males receiving 281 mg boron/kg body weight per
day died. Clinical signs of toxicity were a thin, hunched appearance,
dehydration, foot lesions, and scaly tails. A dose-related decrease in
body weight gain was observed. Microscopic effects included a
dose-related incidence of minimal to mild extramedullary
haematopoiesis of the spleen in males and females, hyperkeratosis and
acanthosis of the stomach in eight males and three females receiving
the highest dose, and testicular lesions; the testicular lesions are
described in greater detail in section 7.4.
Following this range-finding study, a 2-year study was conducted
in which mice (50/sex per dose) received approximately 0, 275, or 550
mg boric acid/kg body weight per day (0, 48.1, or 96.3 mg boron/kg
body weight per day) in the diet (NTP, 1987; Dieter, 1994). No
clinical signs of toxicity were observed. Body weights were 10-17%
lower in males after 32 weeks and in females after 52 weeks.
Non-accidental mortality at the end of the study was 9/50, 20/50, and
23/50 in control, low-, and high-dose males and 17/50, 15/50, and
12/50 in control, low-, and high-dose females. The increased mortality
rate was statistically significant in males. The only significant
lesions in male mice appeared in the testes; no significant
non-neoplastic lesions appeared in females.
Lee et al. (1978) fed borax in the diet to male Sprague-Dawley
rats (18/dose) at concentrations of 0, 500, 1000, or 2000 mg boron/kg
(equivalent to approximate doses of 0, 30, 60, or 125-131 mg boron/kg
body weight per day) for 30 or 60 days. Body weights were not
consistently affected by treatment; organ weights were not affected in
the 30 mg/kg body weight per day group. At 60 and 125-131 mg/kg body
weight per day, absolute liver weights were significantly lower after
60 days; epididymal weights were significantly lower (37.6% and 34.8%,
respectively) after 60 days, but not after 30 days. Weights of
prostate, spleen, kidney, heart, and lung were not changed at
any dose; reproductive effects observed in the study are discussed in
section 7.4.
Table 15. Effects of longer-term oral exposure to boron compounds in experimental animals
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Mice 0, 34, 70, 141, 281, Diet 13 weeks Over 60% mortality in both sexes NTP (1987)
563 for males; 0, 47, at 563 and 776 mg/kg body weight
97, 194, 388, 776 for per day; 10% in males at 281 mg/kg
femalesb body weight per day. At 141 mg/kg
body weight per day, degeneration
in seminiferous tubules and
decreased tubules in males and
decreased weight gain in males and
females. Extramedullary
haematopoiesis of the spleen in
all dosed groups, hyperkeratosis
and acanthosis of stomach at 563
and 776 mg/kg body weight per day.
Boric acid Mice 0, 48, 96 Diet 103 weeks Decrease in body weight (10-17%) NTP (1987)
in high-dose males after week 32
and in high-dose females after week
52. No clinical toxic signs observed.
Testicular atrophy and interstitial
cell hyperplasia were seen in males
at both levels. Dose-related increase
in incidence of splenic lymphoid
depletion in males. No other
significant increase in
non-neoplastic lesions.
Boric acid Rats 0, 22.7, 57c and Drinking-water 30 days Growth was not inhibited at the Pfeiffer et al.
higher low dose, but it was delayed at (1945)
>57 mg/kg body weight per day.
No haematological effects or
histological alterations.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Borax Rats 0, 0.056, 0.28, 2.8, Drinking-water <198 days Decrease in pancreas-to-body Wang et al.
28d weight ratio at all doses in (1984)
females at day 98. Increase in
pancreas-to-body weight ratio
in males at day 198. No details
given; normal histology.
Boric acid Rats 0.95, 3.65, 5.2, 9.9e Diet 8 weeks Decreased body weight at 5.2 Forbes & Mitchell
and 9.9 mg/kg body weight per (1957)
day. No other toxicity
end-points evaluated.
Borax or Rats 0, 2.6, 8.8, 26.3, Diet 90 days Mortality was 100% at the highest Weir & Fisher
boric acid 87.5, 262.5e dose; testicular atrophy at 87.5 (1972)
and 26.3 mg/kg body weight per
day; decrease in body weight and
in the weight of liver, kidney,
spleen, and testes at 87.5 mg/kg
body weight per day; weight
changes were inconsistent at
lower doses.
Borax or Rats 0, 5.9, 17.5, 58.5e Diet 2 years Both compounds suppressed growth Weir & Fisher
boric acid at 58.5 mg/kg body weight per (1972)
day. Testes weight and
testes-to-body weight ratios
were decreased, and brain-to-body
weight and thyroid-to-body weight
ratios were increased at 58.5
mg/kg body weight per day; also,
atrophy in seminiferous epithelium
and decrease in tubular size; no
effects observed at lower doses.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Rabbits 31 Oral gavage 5 days/week Elevated serum glutamic-oxaloacetic Verbitskaya
or borax for 4 months transaminase (SGOT) and serum (1975)
glutamic-pyruvic transaminase (SGPT)
levels, serum lactate dehydrogenase
and aldolase were transiently
increased, catalase and amylase were
decreased.
Sodium Rats 0, 3 g/litre sodium Drinking-water 14 weeks Increase in RNA concentration Settimi et al.
tetraborate tetraborate (boron and in succinate dehydrogenase (1982)
(not conversion not and acid proteinase activation
specified made due to in the brain. Decrease in
whether uncertainty NADPH-cytochrome reductase and
anhydrous or regarding in the content of cytochrome
decahydrate) compound) b5 and P-450 in the liver. No
effect on body or organ weight.
Sodium Mice 0, 0.95e Drinking-water Lifetime No effects on body weight or Schroeder &
metaborate longevity. Mitchener (1975)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of boron/boron compound.
b Estimated based on feed consumption values of 161 g/kg body weight per day for male and 222 g/kg body weight per day for female controls
at week 4 of treatment.
c Calculated based on water consumption of 0.12-0.14 ml/g per day reported by authors.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
e Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or food factor of
0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for mice.
In a 90-day study, Sprague-Dawley rats (10/sex per dose) received
0, 2.6, 8.8, 26.3, 87.5, or 262.5 mg boron/kg body weight per day in
the diet as boric acid or borax (Weir & Fisher, 1972). Similar effects
were observed with both boric acid and borax. All high-dose animals
died within 3-6 weeks. In animals receiving 87.5 mg boron/kg body
weight per day, body weights in males and females were reduced
43.8-54.9% and 10.1-12.6%, respectively. Absolute organ weights --
including the liver, spleen, kidneys, brain, adrenals, and ovaries --
in this dose group were also significantly decreased. Relative
organ-to-body weights of the adrenals and kidneys were significantly
increased, but relative weights of the liver and ovaries were
decreased. A pronounced reduction in testicular weights in males in
the 87.5 mg boron/kg body weight per day group was also observed;
effects on the male reproductive system are discussed further in
section 7.4.
In a 2-year study, rats (35/sex per dose) were administered
weight-normalized doses of 0, 5.9, 17.5, or 58.5 mg boron/kg body
weight per day in the diet as borax or boric acid (Weir & Fisher,
1972). High-dose animals had coarse hair coats, scaly tails, hunched
posture, swollen and desquamated pads of the paws, abnormally long
toenails, shrunken scrotum, inflamed eyelids, and bloody eye
discharge. These signs became frequent and more pronounced during the
first year but did not change thereafter. Serum chemistry and urine
values were normal; the haematocrit and haemoglobin levels were
significantly lower than in controls. The absolute and relative
weights of the testes were significantly lower, and relative weights
of the brain and thyroid gland were higher, than in controls. In
animals in the mid- and low-dose groups, no significant effects on
general appearance, behaviour, growth, food consumption, haematology,
serum chemistry, or histopathology were observed (Weir & Fisher,
1972).
Weir & Fisher (1972) also fed boric acid or borax to beagle dogs
for 90 days or 2 years. In the 90-day study (weight-normalized doses
of 0, 0.44, 4.38, or 43.75 mg boron/kg body weight per day;
5 animals/sex per dose), testicular effects were observed in males in
the two highest dose groups. In the boric acid study, testis weight
was significantly lower than controls in the middle- and upper-dose
groups (reduced by 25% and 40%, respectively). Although testicular
microscopic structure was not detectably abnormal in the controls and
middle-dose group, 4 of 5 dogs in the high-dose group had complete
atrophy, and the remaining high-dose dog had one-third of tubules
showing some abnormality. In the borax study, testis weights in the
low, middle-, and high-dose groups were 80%, 85%, and 50% of controls,
respectively; only the last was significantly different from controls.
No mention was made of the testicular microscopic structure of the
controls or low-dose animals; middle-dose animals were not detectably
altered (aside from the considerable fixation-induced artifact in the
outer third of the tissue), whereas 4 of 5 high-dose dogs had complete
testicular atrophy, and the remaining high-dose dog had "partial"
atrophy. No other clinical or microscopic signs of toxicity were
reported in any animals. In the 2-year study, the dogs (4/sex per
dose) received the boric acid or borax in the diet at
weight-normalized doses of 1.5, 2.9, or 8.8 mg boron/kg body weight
per day. An additional group received 29 mg boron/kg body weight per
day for 38 weeks. No effects were observed on general appearance, body
weight, food consumption, organ weights, haematology, or serum
chemistry. Changes in testicular morphology occurred in males in the
highest (38-week) dose group; these reproductive changes are described
in section 7.4.
Two reports are available on the toxic effects of boron on bones.
Seffner et al. (1990) exposed growing pigs to boron (4 or 8 mg/kg body
weight per day; source of boron was not specified). They reported a
dose-related thinning of the cortex of the humerus and a reduction
(significant at 8 mg/kg body weight per day) in presumptively
bone-derived serum alkaline phosphatase, suggesting reduced osteoblast
activity. Confidence in this study is moderate because of the
uncertainty of the form/source of boron and the authors' inability to
replicate several previous biochemical findings.
A second report investigated the effects of boric acid
(calculated exposures: <0.2-68 mg boron/kg body weight per day) on
several bone parameters in adult rats (Chapin et al., 1997). This
study found no change in physical bone measures (size, cortical
thickness, etc.) but reported a 5-10% increase in resistance of
vertebrae to a crush force.
7.2.2 Inhalation route
Mice exposed to amorphous elemental boron at 72 mg/m3 for
7 h/day, 5 days/week, for 6 weeks did not exhibit any toxic effects
(Stokinger & Spiegl, 1953). Wilding et al. (1959) conducted numerous
longer-term experiments in rats and dogs exposed to boron oxide
particles (median mass aerodynamic diameter [MMAD] 1.9-2.5 µm). The
exposures took place for 6 h/day and 5 days/week and included rats
exposed at 77 mg boric oxide/m3 for 24 weeks, 175 mg boric oxide/m3
for 12 weeks, or 470 mg boric oxide/m3 for 10 weeks; dogs were
exposed to 57 mg boron oxide/m3 for 23 weeks. No toxic effects were
observed, with normal values obtained for body weight gains, blood
chemistry, haematology, and urinalysis; there were also no microscopic
changes reported.
Subchronic inhalation studies have been performed with boron
trifluoride (Torkelson et al., 1961; Rusch et al., 1986) at
2-18 mg/m3. Reported effects included pneumonitis and reduced body
weight gains and organ weights.
7.3 Dermal and ocular effects
Animal studies show that boron oxide dust can affect the skin and
eyes. When boron oxide dust (50 mg) was applied to the eyes of
rabbits, conjunctivitis resulted (Wilding et al., 1959). Roudabush et
al. (1965) determined that boric acid (5 ml, 10% in water, w/v) and
borax (10 ml, 5% in water, w/v) were mild skin irritants after 24-72 h
following application to abraided skin. Borax was also a mild skin
irritant and boric acid a moderate irritant after 24 and 72 h when
applied to the guinea-pig. Rats fed 88 or 263 mg boron/kg body weight
per day as borax or boric acid had inflamed eyes and skin desquamation
on their paws and tails (Weir & Fisher, 1972).
7.4 Reproductive toxicity
Reproductive and developmental effects of borates in laboratory
animals are summarized in Table 16.
The data regarding subchronic and chronic oral exposure to boric
acid or borax in laboratory animals have unequivocally demonstrated
that the male reproductive tract is a consistent target of toxicity
(Table 16). Testicular lesions have been observed in rats, mice, and
dogs administered boric acid or borax in food or drinking-water
(Truhaut et al., 1964; Weir & Fisher, 1972; Green et al., 1973; Lee et
al., 1978; NTP, 1987; Ku et al., 1993a). The first clinical indication
of testicular toxicity in dogs is shrunken scrota observed during
treatment; significant decreases in absolute and relative testicular
weight are also reported. After subchronic exposure, the
histopathological effects range from inhibited spermiation (the
process of release of mature spermatids from the seminiferous
epithelium) to degeneration of the seminiferous tubules with variable
loss of germ cells, to complete absence of germ cells, resulting in
atrophy and transient or irreversible loss of fertility, but not of
mating behaviour.
Time- and dose-response studies of the Sprague-Dawley male rat
reproductive end-points after acute administration of boric acid
(Linder et al., 1990) revealed adverse effects on spermiation,
epididymal sperm morphology, and caput sperm reserves during
histopathological examinations of the testes and epididymis. Rats were
administered two doses in one day in both experiments, with a total
dose of 0 or 350 mg boron/kg body weight in the time-response
experiment where animals were sacrificed at 2, 14, 28, or 57 days
after exposure and a total dose of 0, 44, 87, 175, or 350 mg boron/kg
body weight in the dose-response experiment with animals sacrificed
after 14 days. In animals receiving 175 or 350 mg boron/kg body
weight, testicular effects apparent at 14 days were enlarged irregular
cytoplasmic lobes of mature spermatids in stage VIII seminiferous
tubules (Leblond & Clermont, 1952) and a substantial increase in the
testicular spermatid head count per testis. The first visible lesion
was inhibited spermiation. Epididymal effects after 14 days included
an increase in abnormal sperm forms, reduced caput epididymal sperm
morphology with head and tail defects, and reduced caput epididymal
sperm reserves. Epididymal sperm parameters had returned to normal and
only minimal testicular changes remained by post-treatment day 57,
indicating that these effects were reversible at these dose levels.
The NOAEL for male reproductive effects in this study was 87 mg
boron/kg body weight.
Table 16. Reproductive and developmental effects of boron compounds in laboratory animals
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid CD-1 0, 19.2, 104.5, Diet 27 weeks None of the high-dose pairs was Fail et al.
mice 220.2 for males; 0, fertile. At the middle dose, (1990, 1991)
31.8, 147.9, 290.2 there was a significant decrease
for females in litters/pair, live pups/litter,
percentage of pups born alive,
and live pup weight. Fertility
index was decreased at the middle
dose. Body weight gain was reduced
in males and females at the high
dose, despite increases in food
and water consumption. Cross-mating
experiments showed that boric acid
affected primarily the male
reproductive system. A dose level
of 220.2 mg/kg body weight per day
significantly reduced reproductive
organ weights in males and altered
sperm motility, concentration, and
morphology. At the high dose,
fertility of the low-dose F1 mice
was not affected, but marginally
reduced sperm concentrations were
seen in males; increases in uterus
and kidney plus adrenal weights
and shorter estrus cycles were
seen in females; and F2 live pup
weights were reduced.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid SD rats 0, 350 Gavage 2-57 days Inhibited sperm release, adverse Linder et al.
changes in sperm morphology; (1990)
reversible by day 57.
Boric acid Rats 0, 60.9b Diet 4-28 days Decreased weight gain; inhibition Treinen
of spermiation starting at day 7 and & Chapin
degeneration in seminiferous tubules (1991)
at day 28; decreased serum
testosterone starting at day 4; no
effects on liver or kidney histology.
Boric acid F-344 0, 26, 38, 52, 68 Feed Weekly to Mild inhibited sperm release at Ku et al.
rats 63 days 26 mg/kg body weight per day; (1993a)
severe inhibited sperm release at
38 mg/kg body weight per day;
progression to testicular atrophy
at 52 and 68 mg/kg body weight per
day, with many other changes
resulting from these primary
effects.
Boric acid Rats 175 Water 15 days Vacuolation and granulation of Silaev et al.
the cytoplasm and absence of (1977)
nuclear chromatin in spermatids
of seminiferous tubules.
Reduction in tubule diameter
and absence of germinative cells.
Borax or Rats 0, 5.9, 17.5, 58.5c Diet Multi- Sterility, lack of spermatozoa, Weir & Fisher
boric acid generation testicular atrophy, decreased (1972)
ovulation at 58.5 mg/kg body
weight per day. No effects at
lower doses.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 0.44, 4.4, 44c Diet 90 days One male dog died at 44 mg/kg Weir & Fisher
boric acid body weight per day; decrease (1972)
in thyroid- and testes-to-body
weight ratios, and severe
testicular atrophy in males at
44 mg/kg body weight per day.
At 0.44 mg/kg body weight per
day, decreased spleen-to-body
weight ratio in males. At
<4.4 mg/kg body weight per day,
no changes in organ weight in
females.
Borax Rats 0, 30, 60, 125-131 Diet 30 or 60 days At the 60 and 125-131 mg/kg Lee et al.
body weight per day doses, liver (1978)
weights were significantly lower
after 60 days; epididymal
weights were significantly lower
(37.6 and 34.8%) after 60 days.
FSH levels were elevated in all
treatment groups after 60 days,
and at the highest dose FSH
levels remained elevated after
12 months.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax Rats 0, 25, 50, 100c Diet 60 days Decrease in weights of liver, Dixon et al.
testes, and epididymis at the (1979)
two highest dietary levels.
Seminiferous tubule diameter
was decreased in a dose-related
manner in all groups. Loss of
germinal cell elements was seen
only in 50 and 100 mg/kg body
weight per day groups. Plasma
levels of FSH were elevated.
Reduced fertility only at two
highest dosage levels.
Borax Rats 0, 23.7, 44.7d Drinking-water 70 days Significant decrease in body Seal & Weeth
weight and decrease in weight (1980)
of testes, seminal vesicles,
spleen at both doses.
Spermato-genesis impaired and
haematocrit decreased slightly
at highest dose.
Borax Rats 0, 0.042, 0.14, 0.84b Drinking-water 90 days No observable reproductive Dixon et al.
effects or changes in serum (1976)
chemistry, body weight, or
weight of testes, prostate,
or seminal vesicles.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 1.5, 2.9, 8.8, 29c Diet 2 years Severe testicular atrophy and Weir & Fisher
boric acid spermatogenic arrest at week (1972)
26 with 29 mg/kg body weight
per day; no effects on body or
organ weights, gross morphology,
or histological parameters at
lower doses.
Boric acid Mice 0, 43.4, 79.0, 175.3 Diet Gestation There was no effect on survival NTP (1989);
days 0-17 or pregnancy rate. Maternal Heindel et al.
effects included mild renal (1992)
lesions at >43.4 mg/kg body
weight per day and increased
water intake, increased relative
kidney weight, and decreased
weight gain during treatment at
175.3 mg/kg body weight per day.
At the high-dose level, there
was a significant increase in
the percentage of resorptions/
litter. Average fetal body
weight/litter was significantly
reduced at >79.0 mg/kg body weight
per day. Malformations included a
missing or shortened XIII rib and
variations on extra lumbar I
rib. Malformations were
significantly increased at
175.3 mg/kg body weight per day.
The percentage of variations/
litter decreased at <79.0 mg/kg
body weight per day and was not
affected at 175.3 mg/kg body
weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats 0, 13.6, 28.5, 57.7, Diet Gestation The highest dose was administered NTP (1990);
94.2 days 0-20 to one group on gestation days Heindel et al.
6-15. There was no effect on (1992)
survival or pregnancy rate.
Maternal rats showed increased
relative liver and kidney weights
at >28.5 mg/kg body weight per
day, increased absolute kidney
weight at 94.2 mg/kg body weight
per day, and a decrease in body
weight gain at >57.7 mg/kg body
weight per day. At low doses,
there was a decrease in extra
lumbar rib I (a variation) and
at high doses an increase in
missing or shortened XIII rib
(a malformation). The 94.2 mg/kg
body weight per day level
increased prenatal mortality.
Fetal body weights were
significantly reduced in all
treated groups in a dose-related
manner. Malformations increased
at >28.5 mg/kg body weight per
day; variations increased at
94.2 mg/kg body weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats Phase 1 Diet Phase 1 Phase 1 -- Fetal body weight was
0, 3.3, 6.3, 9.6, gestation decreased on gestational day 20
13.3, 25 days (gd) in the 13.3 and 25 mg/kg body
0-20 weight per day dose groups. On
Phase 2 Phase 2 gd 20, incidences of short rib
0, 3.3, 6.5, 9.8, postnatal XIII or wavy rib were increased
12.9, 25.4 days (pn) in these dose groups relative
0-21 to controls. The high-dose group
(exposure contained a biologically
limited to relevant but not statistically
gestation significant decrease in
days 0-20) incidence of extra rib on
lumbar I.
Phase 2 -- On pn days 0-21, there Price et al.
were no decreased fetal body (1996a)
weight effects. On pn day 21,
the percentage of pups per
litter with short rib XIII was
elevated in the 25.4 mg/kg body
weight per day dose group. No
wavy rib or extra rib on lumbar
I was found at pn day 21.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rabbits 0, 10.9, 21.9, 43.7 Gavage Exposure on No treatment-related clinical Price et al.
in water gestation signs of toxicity except for (1996b)
days 6-19; vaginal bleeding at 43.7 mg/kg
termination body weight per day. This dose
on gd 30 had no live fetuses on day 30.
Food intake decreased during
treatment (43.7 mg/kg body
weight per day), but increased
on days 25-30 (21.9 and 43.7
mg/kg body weight per day). Body
weight and body weight gain were
decreased during treatment at
43.7 mg/kg body weight per day,
but corrected body weight
increased at 21.9 and 43.7 mg/kg
body weight per day. Gravid
uterine weight and number of
corpora lutea/dam were decreased
at 43.7 mg/kg body weight per
day. Significant developmental
effects were limited to the
high-dose group. Prenatal
mortality was greatly increased.
Malformations were also
increased, primarily owing to
the incidence of fetuses with
cardiovascular defects. The
skeletal variation observed
was sternebrae. Fetal body
weight was slightly reduced
in the high-dose group.
Table 16. (continued)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of
boron/boron compound.
b Estimated by the authors.
c Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or
food factor of 0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for
mice.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
In a 2-year study by Weir & Fisher (1972), groups of 4 male and 4
female beagle dogs were fed diets containing boric acid or borax to
provide doses of 0, 1.45, 2.93, or 8.75 mg boron/kg body weight per
day. No evidence of toxicity was observed. An additional group of dogs
(4 male and 4 female) was fed diets containing boric acid or borax
leading to doses of 0 or 29.3 mg boron/kg body weight per day for 38
weeks. Testicular atrophy was observed in 2 test dogs receiving borax
at 26 weeks. The authors stated that boric acid caused testicular
degeneration in dogs, including spermatogenic arrest and atrophy of
the seminiferous epithelium. This study was terminated at 38 weeks. In
the study, the number of dogs was small and variable (1-2 dogs at each
of three time points) and inadequate to allow statistical analysis. A
common control group was used for both borax and boric acid exposure
groups. Testicular lesions occurred in the controls (1 of 4 controls
had slight to severe seminiferous tubular atrophy, another had
moderate to severe atrophy, whereas a third had a detectable but
insignificant reduction in spermatogenesis and 5% atrophic
seminiferous tubules). This study was conducted before the advent of
Good Laboratory Practices (GLP). Confidence in this study is low, and
it was considered not suitable for inclusion into the risk assessment
because of 1) small and variable numbers of dogs, 2) variable
background lesions in controls leading to uncertainty regarding the
strength of the response to treatment, 3) lack of GLP, and 4) other,
more recent studies of greater scientific quality with findings at a
similar or lower intake level of boron (Ku et al., 1993a; Price et
al., 1996a).
Weir & Fisher (1972) fed Sprague-Dawley rats (35/sex per group) a
diet containing borax or boric acid for 2 years at 0, 5.9, 17.5, or
58.5 mg boron/kg body weight per day. At the high dose, rats receiving
either compound had decreased food consumption during the first 13
weeks of study and decreased growth throughout the study. Testes
weights were significantly decreased at 58.5 mg/kg body weight per
day. The seminiferous epithelium was atrophied, and the tubular size
in the testes was decreased. No treatment-related effects were
observed in rats receiving 5.9 or 17.5 mg boron/kg body weight per
day. The LOAEL in this study was 58.5 mg boron/kg body weight per day
for decreased testes weights, atrophied testes, histopathological
alterations of the testes, and increased brain and thyroid weights.
The NOAEL for this study was 17.5 mg boron/kg body weight per day.
In a companion three-generation reproduction study in rats, Weir
& Fisher (1972) found that 58.5 mg boron/kg body weight per day
produced testicular atrophy and complete suppression of fertility in
rats. Lower doses (17.5 or 5.9 mg boron/kg body weight per day) did
not reduce fertility. The small group size (n = 8), low control
fertility rates (approx. 60%), limited data reported, and
inappropriate statistics all limit the applicability of these data for
risk assessment.
In a multigeneration continuous-breeding experiment (Fail et al.,
1990, 1991), Swiss CD-1 mice (F0 generation) were fed boric acid in
the diet at 0, 1000, 4500, or 9000 mg/kg feed for 27 weeks, which gave
calculated doses of 0, 19.2, 104.7, and 222.1 mg boron/kg body weight
per day for males and 0, 31.9, 148.1, and 290.5 mg boron/kg body
weight per day for females. Treatment with boric acid significantly
impaired fertility: no males or females in the high-dose groups were
fertile. At the middle dose, the number of litters per pair, number of
live pups per litter, proportion of pups born alive, and pup weight
adjusted for litter size were all decreased. The trend towards a lower
fertility index at this dose level started with the first litter and
progressed in severity with subsequent matings. Animals from different
treatment groups were cross-mated to determine the affected sex at
this dose. When mid-dose males were mated with control females, mating
and fertility indices were significantly depressed, with only one pair
in that group producing a live litter; these indices were not affected
when control males were mated with mid-dose females, confirming that
the male was the affected sex. At F0 necropsy, sperm motility was
significantly reduced in all exposed groups (by 12%, 32%, and 47%,
from low- to high-dose groups, respectively). Low-dose and mid-dose
animals from the F1 generation were exposed during gestation and
lactation. The fertility of the low-dose F1 mice was not affected,
but the litter-adjusted body weights of the F2 pups were
significantly decreased (by 3.3%) relative to controls. The low dose
was considered the LOAEL for decreased sperm motility in the F0
males, 26% increased uterine weight and 8% increased kidney/adrenal
weight in F1 females, and a 3.3% reduction in litter-adjusted birth
weight in the F2 pups. This study had no NOAEL, but the magnitude of
the changes at the low dose (a 12% decrease in F0 sperm motility, an
8% increase in F1 kidney/adrenal weights, and a 3% reduction in F2
pup weights) is small and indicates that this dose level is close to
the NOAEL.
Fail et al. (1989) utilized both CD-1 mice and wild deer mice
(Peromyscus maniculatus) to characterize the effects of boric acid
on fertility and to test the reversibility of these effects. Adult
CD-1 mice were exposed to boric acid in the feed for 27 weeks at 0,
1000, 4500, or 9000 mg/kg (consumed doses were not given). The males
at the high and middle doses had testicular atrophy and decreased
spermatogenesis. Fertility was diminished in animals receiving the
middle dose and completely absent in the high-dose group. The same
doses were used to test toxicity and reversibility in the wild mouse.
The ability to produce offspring was significantly reduced by boric
acid: none of the males in the high-dose group sired litters. Weight
gain was similar for the treated and control wild mice. Although body
and organ weights were similar between the mid-dose and control groups
at necropsy, the testis and total accessory sex organ weights were
significantly lower in the high-dose group. These two species appear
to differ in their sensitivity to boric acid. The CD-1 mice had body
weight loss as early as 5 weeks at the mid-dose level, whereas deer
mice did not lose weight even at the high dose. Tissue pathology
findings at the middle dose were significant in CD-1 mice but not in
deer mice. Deer mice were found to recover from boric acid exposure.
The development of the testicular lesion was investigated by
Treinen & Chapin (1991), who fed boric acid at a level of 0 or 60.9 mg
boron/kg body weight per day to male F-344 rats and sacrificed six
treated and four control male rats at intervals from 4 to 28 days
after the start of exposure. In half of the treated rats, there was
inhibition of spermiation in 10-30% of stage IX tubules at 7 days and
inhibition in all stage IX and stage X tubules of exposed rats at 10
days. At 28 days, there was significant loss of spermatocytes and
spermatids from all tubules in exposed rats, and basal serum
testosterone levels were significantly decreased from 4 days on.
Secondary to the loss of germ cells, the activities of enzymes
found primarily in spermatogenic cells were significantly decreased,
and enzyme activities associated with premeiotic spermatogenic cells
were significantly increased in Sprague-Dawley rats exposed to 60 or
125-131 mg boron/kg body weight per day for 30 or 60 days (Lee et al.,
1978). Mean plasma follicle stimulating hormone (FSH) levels were
significantly elevated in a dose-dependent manner in all treatment
groups in this study (30 to 125-131 mg boron/kg body weight per day)
after 60-day exposures. FSH levels in animals exposed to the highest
dose tested (125-131 mg boron/kg body weight per day) were still
elevated 12 months after treatment termination, owing to atrophied
testes and no recovery of spermatogenesis. Plasma luteinizing hormone
(LH) levels were not significantly elevated, and mean plasma
testosterone levels were within the normal range throughout the study
(Lee et al., 1978).
Reversibility of testicular lesions was evaluated by Ku et al.
(1993a) in an experiment in which F-344 rats were dosed at 3000, 4500,
6000, or 9000 mg boric acid/kg (26, 38, 52, and 68 mg boron/kg body
weight per day) in the feed for 9 weeks and assessed for recovery up
to 32 weeks post-treatment. Inhibited spermiation was exhibited at
3000/4500 mg boric acid/kg (5.6 µg boron/mg tissue), whereas inhibited
spermiation progressed to atrophy at 6000/9000 mg boric acid/kg (11.9
µg boron/mg testes), with no boron accumulation in the testes to
levels greater than found in blood during the 9-week period. After
treatment, serum and testis boron levels in all dose groups fell to
background levels. Inhibited spermiation at 4500 mg/kg was reversed by
16 weeks post-treatment, but focal atrophy was detected that did not
recover up to 32 weeks post-treatment.
An unpublished study by W.W. Ku and colleagues (reported in Ku et
al., 1993a) found no detectable treatment-related changes in
testicular structure in rats induced by consumption of 17.5 mg
boron/kg body weight per day for up to 9 weeks.
In a follow-up study to explore/identify the mechanism for this
testicular toxicity of boric acid, Ku et al. (1993b) evaluated several
end-points in cell culture systems following in vitro boric acid
exposure. The data suggest an effect of boric acid on the DNA
synthesis activity of mitotic and meiotic germ cells and, to a lesser
extent, on energy metabolism in Sertoli cells. The effect on DNA
synthesis occurred at boron concentrations that were associated with
atrophy in vivo and shows that boric acid interferes with the
production and/or maturation of early germ cells; this offers an
explanation for atrophy, but not for inhibited spermiation.
Additional mechanistic studies by Ku & Chapin (1994) showed that
testicular toxicity and CNS hormonal effects were not due to selective
boron accumulation in testis or brain/hypothalamus, with testis boron
concentrations at 1-2 mmol/litre. Changes in testis phosphorus,
calcium, and zinc levels did not precede atrophy. In vitro studies
showed no effect on the steroidogenic functions of isolated Leydig
cells, supporting the suggestion of a CNS-mediated hormonal effect.
The authors showed that inhibited spermiation was not due to increased
testicular cyclic adenosine monophosphate or reduced serine protease
plasminogen activators. Also evaluated were effects of boric acid in
Sertoli-germ cell co-cultures on Sertoli cell energy metabolism
(lactate secreted by Sertoli cells is a preferred energy source for
germ cells) and DNA/RNA synthesis (germ cells synthesize DNA/RNA, and
boric acid impairs the synthesis of these nucleic acids in the liver).
The most sensitive in vitro end-point was DNA synthesis in
mitotic/meiotic germ cells; energy metabolism in germ cells was
affected to a lesser extent, which was manifested in vivo as a
decrease in early germ cell/Sertoli cell ratio prior to atrophy in the
testes. The mechanisms of inhibited spermiation are still not defined.
In summary, male reproductive effects from boron have been noted
in mice, rats, and dogs. Reproductive effects observed include
inhibition of spermiation in stage IX and X tubules, followed by germ
cell loss, changes in epididymal sperm morphology and caput sperm
reserves, decreased serum testosterone levels, and testicular atrophy.
Male reproductive effects have been reported in oral exposure studies
at doses as low as 29 mg boron/kg body weight per day in dogs exposed
for 2 years to dietary boric acid or borax (Weir & Fisher, 1972); for
rats in this same study, the LOAEL for reproductive toxicity was 58.5
mg boron/kg body weight per day.
7.5 Developmental toxicity
Developmental toxicity has been demonstrated experimentally in
rats, mice, and rabbits (see Table 16) (Heindel et al., 1992; Price et
al., 1996b).
Sprague-Dawley rats were fed a diet containing 0, 13.6, 28.5, or
57.7 mg boron/kg body weight per day as boric acid from gestation days
0 to 20 (Heindel et al., 1992). An additional group of rats received
boric acid at 94.2 mg boron/kg body weight per day on gestation days
6-15 only. Maternal effects included a significant and dose-related
increase in relative liver and kidney weights at >28.5 mg boron/kg
body weight per day. Treatment with 94.2 mg boron/kg body weight per
day significantly increased prenatal mortality. Average fetal body
weight per litter was significantly reduced in a dose-related manner
in all treated groups compared with controls. The percentage of
malformed fetuses per litter and the percentage of litters with at
least one malformed fetus were significantly increased at >28.5 mg
boron/kg body weight per day. Malformations consisted primarily of
anomalies of the eyes, the CNS, the cardiovascular system, and the
axial skeleton. The most common malformations were enlargement of
lateral ventricles in the brain and agenesis or shortening of rib
XIII. The percentage of fetuses with variations per litter was reduced
relative to controls at 13.6 and 28.5 mg boron/kg body weight per day
(due to a reduction in the incidence of rudimentary or full ribs at
lumbar 1) but was significantly increased in rats exposed to 94.2 mg
boron/kg body weight per day. The variation with the highest incidence
among fetuses was wavy ribs. The LOAEL of 13.6 mg boron/kg body weight
per day (lowest dose tested) for rats occurred in the absence of
maternal toxicity; a NOAEL was not found in this study.
Price et al. (1996a) did a follow-up to the Heindel et al. (1992)
study in Sprague-Dawley (CD) rats to determine a NOAEL for fetal body
weight reduction and to determine whether the offspring would recover
from prenatally reduced body weight during postnatal development.
Skeletal malformations and variations were also studied to further
characterize the low end of the dose-response curve (phase 1) and to
determine whether the incidence of skeletal defects in offspring
changed during postnatal life (phase 2). Boric acid was administered
in the diet to CD rats from gestational day 0 to 20. In phase 1, dams
were terminated and uterine contents examined on gestational day 20.
During phase 1, the intake of boric acid was 0, 3.3, 6.3, 9.6, 13.3,
or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls;
the reduction was significant only in the 13.3 and 25 mg boron/kg body
weight per day dose groups on gestational day 20. During phase 1,
incidences of short rib XIII (a malformation) and wavy rib (a
variation) were increased in the >13.3 mg boron/kg body weight per
day dose groups relative to control litters. There was a decreased
incidence of rudimentary extra rib on lumbar 1 (a variation) in the
high-dose group that was deemed biologically but not statistically
significant. During phase 2, the intake of boric acid during gestation
was 0, 3.3, 6.5, 9.8, 12.9, or 25.3 mg boron/kg body weight per day.
At birth, boric acid exposure stopped and dams were allowed to deliver
and rear their litters until postnatal day 21. On postnatal day 0 of
phase 2, there were no effects of boric acid on offspring body weight,
nor were any differences seen through postnatal day 21. On postnatal
day 21 of phase 2, the percentage of pups per litter with short rib
XIII was elevated only in the 25.3 mg boron/kg body weight per day
dose group, but there was no treatment-related increase in wavy rib or
extra rib (full or rudimentary) on lumbar 1 observed in these pups on
day 21. The NOAEL for phase 1 of this study is 9.6 mg boron/kg body
weight per day based on a decrease in fetal body weight. The LOAEL was
13.3 mg boron/kg body weight per day for phase 1. The NOAEL for phase
2 was 12.9 mg boron/kg body weight per day, and the LOAEL was 25.3 mg
boron/kg body weight per day. The results of this study provide a
NOAEL and LOAEL that complement the rat LOAEL of 13.6 mg/kg body
weight per day in the Heindel et al. (1992) study.
Heindel et al. (1992) also investigated the developmental
toxicity and teratogenicity of boric acid in mice at 0, 43, 79, or 175
mg boron/kg body weight per day in the diet. There was a significant
dose-related decrease in average fetal body weight per litter at 79
and 175 mg boron/kg body weight per day. In offspring of mice exposed
to 79 or 175 mg boron/kg body weight per day during gestation days
0-17, there was an increased incidence of skeletal (rib)
malformations. These changes occurred at doses for which there were
also signs of maternal toxicity (increased kidney weight and
pathology); the LOAEL for developmental effects (decreased fetal body
weight per litter) was 79 mg boron/kg body weight per day, and the
NOAEL for developmental effects was 43 mg boron/kg body weight per
day.
Price et al. (1996b) investigated the developmental toxicity and
teratogenicity of boric acid in rabbits at doses of 0, 10.9, 21.9, or
43.7 mg boron/kg body weight per day given by gavage. Frank
developmental effects in rabbits exposed to 43.7 mg boron/kg body
weight per day included a high rate of prenatal mortality, increased
number of pregnant females with no live fetuses, and fewer live
fetuses per live litter on day 30. Also at 43.7 mg boron/kg body
weight per day, malformed live fetuses per litter increased
significantly, primarily because of the incidence of fetuses with
cardiovascular defects, the most prevalent of which was
interventricular septal defect. Skeletal variations observed were
extra rib on lumbar 1 and misaligned sternebrae. The NOAEL for
maternal and developmental effects was 21.9 mg boron/kg body weight
per day, and the LOAEL for maternal and developmental effects was 43.7
mg boron/kg body weight per day.
7.6 Mutagenicity and related end-points
Boric acid was not mutagenic in Salmonella typhimurium with or
without rat or hamster S9 fraction (Haworth et al., 1983; Benson et
al., 1984; NTP, 1987) or in mouse lymphoma L5178Y/TK+/ cells with or
without rat liver S9 (NTP, 1987; McGregor et al., 1988). Borax was not
mutagenic in Salmonella with or without rat liver S9 (Benson et al.,
1984). Refined borax, crude borax ore, and kermite ore were not
mutagenic in V79 Chinese hamster cells, C3H/1OT1/2 mouse embryo
fibroblasts, or diploid human foreskin fibroblasts (Landolph, 1985).
Sodium perborate (NaBO3) was shown to interact with DNA in the
Escherichia coli Pol A assay, presumably by being converted to
hydrogen peroxide (Rosenkranz, 1973). Other tests showed that boric
acid did not induce chromosomal aberrations or sister chromatid
exchanges in Chinese hamster ovary cells (NTP, 1987). Existing data
suggest that genotoxicity is not an area of concern following exposure
to boron compounds in humans.
7.7 Carcinogenicity
The 2-year feeding study (NTP, 1987; Dieter, 1994) showed no
evidence of carcinogenicity in B6C3F1 mice. The Weir & Fisher (1972)
study showed no evidence of boric acid-related carcinogenicity in
rats, although not all tissues were examined. Based on the lack of
human data and on the data from these two animal tests, boron is
classified by the US EPA as a Group D chemical (not classifiable as to
human carcinogenicity) (US EPA, 1994).
7.8 Toxicity effects summary
The laboratory animal toxicity data show that boric acid (and
other borates) is not a carcinogen and lacks significant mutagenicity.
The crossover mating trial in the Fail et al. (1991) study showed that
female mouse reproduction was unaffected by about 120 mg boron/kg body
weight per day. The NOAELs and LOAELs of reproductive/developmental
effects from various studies are ranked in Table 17. The Weir & Fisher
(1972) dog study is omitted from this summary because of low numbers
of animals and the presence of lesions in the controls. It can be seen
that rat fetal development is more sensitive than other processes to
the adverse effects of elevated boron exposure, with a LOAEL in rats
of 13.3 mg boron/kg body weight per day reducing fetal weight gain and
increasing rib anomalies (reversibly). The current NOAEL for these
effects in rats is approximately 10 mg boron/kg body weight per day.
Increased doses produced sequentially additional effects on sperm
release, increased rabbit cardiovascular defects, reduced epididymal
sperm counts, testicular atrophy, and mouse fetal defects.
The Task Group recognized the lack of a NOAEL in the Fail et al.
(1991) study. The effects of concern in part duplicated the
developmental toxicity findings (reduced pup body weight). However,
the unique effects in the adult F1 female mice (increased uterine
weight, reduced cycle length) are consistent with an effect on female
reproduction that was not seen in the F0 females. The possibility of
unique transgenerational or functional developmental toxicity in the
absence of an available dose-response concerned the Task Group.
Although the current NOAEL is based on rat fetal body weight
effects, the Task Group noted that this could be lowered and a new
"critical effect" could emerge if the significant data gap
(second-generation fertility and necropsy data for multiple doses
including a NOAEL in rats) were filled.
7.9 Physiological effects
Since 1981, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for higher animals;
that is, a dietary deprivation of boron consistently results in
changed biological functions that can be construed as detrimental and
are preventable or reversible by an intake of physiological amounts of
boron. Convincing findings have been reported that show that dietary
boron deprivation affects mineral and energy metabolism in chicks and
Table 17. Ranking of reproductive/developmental effects of boric acid
Species/durationa Dose (mg boron/kg Effect Reference
body weight per day)
SD rat/gd 0-20 9.6 NOAEL for developmental effects immediately pre-term Price et al. (1996a)
SD rat/gd 0-20 12.9 NOAEL for developmental effects measured at weaning Price et al. (1996a)
SD rat/gd 0-20 13.3 LOAEL for reduced fetal weight, increased rib Price et al. (1996a)
13.6 malformations/variations Heindel et al. (1992)
Male SD rat/multigeneration 17.5 NOAEL for male sterility, testicular atrophy Weir & Fisher (1972)
SD rat/gd 0-20 25.4 LOAEL for increased short rib XIII at weaning Price et al. (1996a)
Male SD rat/63 days 26 LOAEL for mild inhibited sperm release Ku et al. (1993a)
Male SD rat/¾63 days 52 LOAEL for testicular atrophy Ku et al. (1993a)
Male beagle dogs/2 years 29 Altered testis weight and histopathology Weir & Fisher (1972)
Male beagle dogs/90 days 44 Altered testis weight and histopathology Weir & Fisher (1972)
CD-1 mouse/multigeneration 19.2 LOAEL for reduced sperm motility, reduced F2 pup weight Fail et al. (1991)
CD-1 mouse/gd 0-17 43 NOAEL for mouse developmental toxicity Heindel et al. (1992)
79 LOAEL for decreased fetal body weight
New Zealand white rabbits/ 21.9/43.7 NOAEL/LOAEL for decreased fetal body weight, Price et al. (1996b)
gd 6-19 increased fetal cardiovascular malformations and
maternal toxicity
a gd = gestational days.
rats (Hunt, 1994); these effects are more marked when a physiological
stressor is present, especially marginal cholecalciferol (vitamin D3)
nutriture. For example, a boron supplement of 3 µg/g to a basal diet
containing 0.465 µg boron/g alleviated the marginal cholecalciferol
deficiency-induced changes in bone, plasma glucose, energy substrate
utilization, and growth (Hunt, 1994). Hunt et al. (1994) have found
that boron deprivation depresses macromineral content in bone and some
indices of the maturation of the cartilage growth plate independently
of vitamin D nutriture. Brain composition and function in rats are
also affected by dietary boron. Boron deprivation was found to
systematically influence brain electrical activity assessed by an
electrocorticogram in mature rats (Penland & Eberhardt, 1993). In this
study, brain copper concentrations were higher in boron-deprived than
in boron-supplemented rats. Furthermore, calcium concentrations in
total brain and in brain cortex, as well as phosphorus concentration
in the cerebellum, were found to be higher in boron-deprived (0.158
µg/g diet) than in boron-supplemented (2.72 µg/g diet) rats fed a
cholecalciferol-deficient diet (Hegsted et al., 1991). This study also
found that the apparent absorption and balance of calcium, magnesium,
and phosphorus were decreased by boron deprivation. Hunt (1988) has
reported that chicks apparently require about 1.0 µg boron/g diet for
normal development. These studies collectively indicate that boron in
physiological amounts is beneficial to, if not essential for, higher
animals (see also section 8.4).
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Short-term toxicity and poisoning incidents
Available human exposure data on boron compounds for routes other
than inhalation focus on boric acid and borax. According to Stokinger
(1981), the lowest lethal dose for humans exposed to boric acid is 640
mg/kg body weight by oral exposure, 8600 mg/kg body weight by dermal
exposure, and 29 mg/kg body weight by intravenous injection. Stokinger
(1981) stated that deaths can occur at doses between 5 and 20 g of
boric acid total for adults and below 5 g total for infants. Litovitz
et al. (1988) stated that potential lethal doses are usually cited as
3-6 g total for infants and 15-20 g total for adults. A case-series
report of seven infants (aged 6-16 weeks) who used pacifiers coated
with a borax and honey mixture for 4-10 weeks reported that exposures
ranged from 4 to 30 g, with an estimated average daily ingestion of
0.143-0.429 g (O'Sullivan & Taylor, 1983). The actual relative doses
are unknown. Toxicity was manifested by generalized or alternating
focal seizure disorders, irritability, and gastrointestinal
disturbances. Other findings included inflammation, congestion,
oedema, exfoliation of the mucosa, cloudy swelling and granular
degeneration of tubular cells, and exfoliative dermatitis. Although
infants appear to be more sensitive than adults to boron compounds,
lethal doses are not well documented in the literature.
A few case reports have been published for poisoning incidents.
Teshima et al. (1992) reported that a 26-year-old woman ingested 21 g
of boric acid. The elimination of boric acid was about 4 times faster
with haemodialysis than with conventional medical treatment. The
patient was discharged from the hospital 12 days following admission.
In another case report, Grella et al. (1976) described transplacental
poisoning. A pregnant woman who had a personal history of diabetes was
accidentally given 70 g of boric acid instead of 70 g of glucose for
the glucose tolerance test at 33 weeks' gestation. She was immediately
treated with gastric lavage and intravenous sodium bicarbonate
fructose. The woman developed contractions, and an emergency caesarean
delivery was scheduled. The infant was born alive weighing 2.5 kg and
had spontaneous respirations. Soon afterwards, the infant developed
cardiac arrest, was resuscitated, and died. Cause of death was
attributed to cardiocirculatory collapse.
Boric acid and borax were widely used in medicine at the
beginning of the century for therapeutic purposes, both locally as
well as orally. Boric acid was used to treat various diseases, such as
epilepsy and infectious diseases. Several case studies reviewed by
Kliegel (1980) describe mild to severe responses to boron compounds.
Among all 19 patients with reported body hair loss as a response to
boron compound treatment, 16 persons had epilepsy and 3 had urinary or
genital infections. Stein et al. (1973) described an extremely unusual
case of a 32-year-old woman who ingested and swallowed several bottles
of mouthwash containing boric acid daily for a minimum of 1 year. The
woman was reported to have pancreatitis resulting from heavy alcohol
ingestion and medications for epilepsy. The woman lost almost all of
her body and scalp hair; other clinical signs included erythema on the
palms of her hands, severe fatigue, anorexia, and mental confusion.
Her blood boric acid level was 3.2 mg/100 ml, corresponding to 0.56 mg
boron/100 ml (the normal value was reported as 0.3 mg boric acid/100
ml). Upon cessation of mouthwash consumption, hair growth returned,
suggesting that the effect was reversible.
Goldbloom & Goldbloom (1953) reported four cases of boric acid
poisoning and reviewed an additional 109 cases in the literature. The
four cases were infants exposed to boric acid by repeated topical
applications of baby powder. Toxicity was manifested by cutaneous
lesions (erythema over the entire body, excoriation of the buttocks,
and desquamation), gastrointestinal disturbances, and seizures. One
patient died, but cause of death was not specifically attributed to
boric acid. Approximately 35% of the 109 other case reports involved
children under 1 year of age. The mortality rate was 70.2% for
children compared with 55.0% for all cases combined. Death occurred in
27/51 (53%) patients exposed by ingestion, in 3/4 (75%) patients
subjected to gastric lavage with boric acid, in 19/28 (68%) patients
exposed by dermal application for treating burns, wounds, and skin
eruptions, and in 14/26 (54%) patients exposed by other routes.
Information on signs and symptoms for 80 patients showed that
gastrointestinal disturbances were prevalent (73%), followed by CNS
effects (67%). Cutaneous lesions were prevalent in 76% of the cases
and in 88% of cases involving children under 2 years of age. Gross and
microscopic findings were reported for 27/60 (45%) fatal cases. In
general, boric acid caused chemical irritation primarily at sites of
application and excretion and in organs with maximum boron
concentrations. The most common CNS findings were oedema and
congestion of the brain and meninges. Other common findings included
liver enlargement, vascular congestion, fatty changes, swelling, and
granular degeneration (n = 13).
In addition to case reports, poison centres have published
case-series reports. Unlike the case reports reviewed by Goldbloom &
Goldbloom (1953), more recent reports suggest that the oral toxicity
of boron in humans is milder than previously thought. Litovitz et al.
(1988) conducted a retrospective review of 784 cases of boric acid
ingestion reported to the National Capital Poison Center in
Washington, DC, USA, during 1981-1985 and the Maryland Poison Center
in Baltimore, MD, USA, during 1984-1985. The amount of boric acid
ingested and clinical manifestations of toxicity were reported; 88.3%
of the cases were asymptomatic. All but two of the cases had acute
(single) ingestion, and 80.2% involved children under 6 years of age.
No severe toxicity or life-threatening effects were noted, although
boric acid levels in blood serum ranged from 0 to 340 µg/ml. The most
frequently occurring symptoms, which involved the gastrointestinal
tract, included vomiting (n = 32), abdominal pain (n = 15),
diarrhoea (n = 13), and nausea (n = 7). Other symptoms (primarily
CNS and cutaneous) occurred in six or fewer cases: lethargy, rash,
headache, light-headedness, fever, irritability, and muscle cramps.
The average dose ingested estimated from 659 cases was 1.4 g (range
0.010-88.8 g). For children under 6 years, the average dose was 0.5 g
(range 0.010-22.2 g), compared with 4.1 g (range 0.030-88.8 g) for
individuals age 6 years or above. The average dose for asymptomatic
cases was 0.9 g (range 0.010-88.8 g), compared with 3.2 g (range
0.10-55.3 g) for symptomatic cases. According to Litovitz et al.
(1988), 21 of the children under 6 years of age, 15 of whom were under
2 years of age, ingested the reported potential lethal dose of 3 g; 8
adults ingested the reported potential lethal dose of 15 g without
evidence of lethal effects.
Linden et al. (1986) published a retrospective review of 364
cases of boric acid exposure reported to the Rocky Mountain Poison and
Drug Center in Denver, CO, USA, between 1983 and 1984. Vomiting,
diarrhoea, and abdominal pain (incidence not reported) were the most
common symptoms given by the 276 cases exposed in 1983. Of the 72
cases reported in 1984 for whom medical records were complete, 79%
were asymptomatic, whereas 20% had mild gastrointestinal symptoms
noted. One 2-year-old child died, presumably from repeated ingestion
of an insecticide containing 99% boric acid.
Overall, owing to the wide variability of data collected from
poisoning centres, the average dose of boric acid required to produce
clinical symptoms is still unclear but is presumably within the range
of 100 mg to 55.5 g, observed by Litovitz et al. (1988).
8.1.2 Reproductive effects
An ecological study assessed boron exposure from drinking-water
and fertility among residents in two geographical regions in
Turkey.a Region I comprised 2368 residents, whereas Region II
comprised 2319 residents. Boron levels in drinking-water were
noticeably higher in Region I (range 2.05-29 mg/litre) than in Region
II (range 0.03-0.40 mg/litre). Ever-married residents from each region
who could provide reproductive histories for three generations of
family members represented the study sample -- i.e. 159 probands (6.7%
of population) in Region I and 154 (6.7%) in Region II. The
percentages of married couples with one or more live births (>90%)
were comparable for the two regions, regardless of generation
assessed. The overall percentage of couples with unresolved
infertility or those without children across three generations was
comparable for the two regions (i.e. 6.0% and 4.6%, respectively).
Secondary sex ratios (ratio of male to female live births) appeared to
be different for the two regions. Region I had a ratio below 1 (0.89),
suggesting an excess of female births; Region II had a ratio slightly
above 1 (1.04), suggesting a slight excess of male births. Statistical
a Sayli BS, Tüccar E, & Ellan AH (1996) An assessment of fertility in
boron-exposed Turkish subpopulations. Unpublished manuscript, Ankara
University Medical Faculty, Ankara, Turkey.
significance was not formally evaluated in any of the above analyses.
The results of this descriptive study suggest that fertility, as
measured by the ability to produce a live birth, is not adversely
affected for residents of this geographical area with high levels of
boron in their drinking-water and soil. The observed reversal of the
secondary sex ratio for Region I requires careful interpretation, as
no attention was given to factors reported to alter sex ratios (e.g.
advancing parental age, elective abortion rates, and multiple births).
Further consideration of potential sources of bias stemming from the
selection of respondents who were largely male and their ability to
accurately recollect and report fertility outcomes is needed. The
extent to which these findings are generalizable, if at all, to other
populations is unknown.
8.2 Occupational exposure
8.2.1 Short-term irritative effects
The majority of toxic effects reported in occupational studies
are acute or short-term in nature and result from the irritant effects
of boron compounds. Several studies have shown that workers exposed to
borax complain of symptoms that are due to respiratory irritation,
which include nosebleeds, eye and nasal irritation, sore throats,
cough, and shortness of breath; dermatitis has also been reported
(Birmingham & Key, 1963; NIOSH, 1978). Generally, people with
hyperreactive respiratory tracts (e.g. asthmatics) may experience more
severe irritant effects following inhalation exposure. However, there
is a lack of data to support the existence of an especially sensitive
high-risk human population for exposure to boron or boron compounds.
A medical survey of 113 workers employed in a borax mining and
refining plant was conducted by Garabrant et al. (1984). The reference
group comprised 214 workers employed in areas with low or minimal
exposure to boric acid and boron oxide; exposed workers had been
employed for a minimum of 5 years or were currently employed in areas
of heavy borax exposure. The average air concentration of particles
<5 µm in diameter measured from samples taken between 1979 and 1981
was 4.1 mg/m3 (range 1.2-8.5 mg/m3). The workers in both groups were
predominantly white males; the mean age of the exposed group was 38.2
years, compared with 42.1 years in the referent group. The mean
duration of employment for exposed and unexposed workers was 11.0 and
12.9 years, respectively. Smoking patterns were similar for the two
groups. Symptoms reported more frequently by exposed workers than by
unexposed workers (p < 0.001) were eye irritation (12.4% and 2.8%,
respectively), dry mouth, nose, or throat (10.6% and 1.9%,
respectively), sore throat (5.3% and 0.5%, respectively), and
productive cough (4.4 and 0%, respectively). No significant
differences were noted between the two groups of workers with respect
to dry cough, shortness of breath, chest tightness, chest pain, and
nosebleeds.
A more detailed analysis of 629 (93% participation) workers in
this plant was presented by Garabrant et al. (1985). This analysis was
based on frequency of acute symptoms in four mean boron dust exposure
categories (1.1, 4.0, 8.4, and 14.6 mg/m3) and persistent symptoms
(presumably chronic effects) in three exposure categories (0.9, 4.5,
and 14.6 mg/m3 of total particulates). The particles were composed
almost entirely of borax. Acute symptoms showing a significant linear
trend (p < 0.0001) in order of decreasing frequency were dryness of
mouth, nose, throat, and eye irritation, dry cough, nosebleeds, sore
throat, productive cough, shortness of breath, and chest tightness.
The frequency of these symptoms in the highest exposure category
ranged from 5% to 33%. The only symptom reported by 5% or more of
workers exposed to 4.0 mg/m3 was eye irritation; no symptoms were
reported by 5% or more of workers exposed to 1.1 mg/m3. The pulmonary
function findings were not significantly affected by exposure to
boron. Chest X-rays did not show abnormal regions indicative of boron
exposure. The authors concluded that borax caused simple respiratory
irritation that produces chronic bronchitis with no impairment of
pulmonary function. Also, borax dust appeared to cause acute and
persistent respiratory irritation at concentrations >4.5 mg/m3.
Therefore, the value for the intermediate exposure category for
persistent symptoms (4.5 mg/m3 as borax) was considered to be the
LOAEL.
Wegman et al. (1994) conducted a prospective cohort study to
examine acute irritative effects as well as chronic pulmonary function
abnormalities in mine and processing plant workers exposed to sodium
borate dust. Exposure-response associations were also examined as
related to acute irritant symptoms associated with sodium borate dust
exposure in mining and processing plants. In comparison with unexposed
workers, exposed workers reported significantly more nasal irritation
(rate ratio [RR] = 8.8), eye irritation (RR = 5.2), throat irritation
(RR = 2.9), cough (RR = 1.7), and breathlessness (RR = 7.1).
Continuous measures of particulate exposure were made throughout the
day and were related to hourly measurements of health outcomes.
Exposure-response relationships were present for each of the specific
symptoms at several symptom intensity levels. Acute irritant effects
did not lead to any undue chronic sequelae. With regard to the
acceptable level of risk, Wegman et al. (1994) concluded that a
threshold limit value (TLV) exposure of 10 mg borate/m3 was
protective of workers' health. This value is consistent with the
nuisance dust standard (ACGIH, 1991).
8.2.2 Male reproductive and other long-term health effects
Effects on the male reproductive system have also been reported,
although data regarding subchronic or chronic exposure on the
population in general are limited.
In the study by Wegman et al. (1994), sodium borate particulate
exposure estimates were used to estimate cumulative exposure in
relation to long-term pulmonary function. Of the 631 workers who
originally underwent pulmonary evaluation 7 years earlier, 336 (53%)
underwent a subsequent evaluation. Ninety (303/336) per cent of
workers were found to have acceptable pulmonary test results. After
the expected smoking-related pulmonary abnormalities were taken into
account, no relation was observed between forced expiratory volume in
1 second (FEV1) and accumulated exposure to sodium borate. People
with hyperreactive respiratory tracts such as those with asthma may
experience more severe irritant effects following inhalation exposure.
However, a sensitive population was not found.
Fertility following occupational exposure to boron was assessed
in a descriptive study. Whorton et al. (1994) estimated the
standardized birth ratio (SBR) to assess fertility in 542/750 (72%
participation) occupational workers in a US borax mine located in the
Mojave Desert, California, USA. These workers are unique, in that
their occupational exposure to sodium borate dust and desert soil is
uncontaminated by other exposures and given their long average length
of employment (mean 18 years). Self-administered questionnaires were
used to ascertain the observed number of live births fathered by male
workers following employment. The US general population adjusted for
age, race, parity, and calendar time period was used to estimate the
expected number of live births. The SBR is simply a ratio of the
observed number of live births to the expected number. An SBR above
100 reflects an excess of live births in relation to the US general
population, whereas an SBR below 100 reflects a deficit. Occupational
boron exposure was based on job titles and categorized into quintiles
of mean exposure levels ranging from <0.82 mg/m3 to >5.05 mg/m3.
SBRs were significantly elevated for workers in the lowest
(<0.82 mg/m3) and highest (>5.05 mg/m3) exposure categories
(i.e. 151 and 125, respectively). No significant trend between
exposure and SBRs was observed. An excess percentage of female live
births in comparison with male births was observed across most
categories of exposure and length of employment. The authors noted
that this female excess did not reflect a deficiency of male births,
as an excess of births for both genders was found. None of the
findings, however, was statistically significant. These findings need
to be carefully interpreted, largely given the selection of the US
general population as the standard. (The authors state that fertility
rates were not available for their target population.) The extent to
which this sample of workers is comparable to the US general
population with respect to factors that affect fecundity and fertility
remains to be established. Further considerations are needed with
respect to the calculation of SBRs, such as the exclusions of multiple
births, and in terms of potential response bias. The authors did
attempt to enhance the validity of self-reported fertility data and
select co-variates by using additional data sources and by efforts to
minimize selection bias. In sum, the findings do not support an
adverse effect of boron on demonstrated fertility for this
occupational sample of male workers in comparison with the US general
population.
8.3 Carcinogenicity
Evidence for carcinogenicity of boron and boron compounds in
humans is not available, largely because it has not been studied.
Based on the evidence from two lifetime studies in mice (NTP, 1987)
and rats (Weir & Fisher, 1972), boron compounds have been classified
by the US EPA in Group D (i.e. not classifiable as to human
carcinogenicity) (US EPA, 1994).
8.4 Physiological effects
Since 1987, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for humans; that is,
a dietary deprivation of boron consistently results in changed
biological functions that could be construed as detrimental and that
are preventable or reversible by an intake of physiological amounts of
boron. Many of these changed functions caused by boron deprivation
have been duplicated in animal models. The major reason boron is not
generally recognized as essential or nutritionally important for
humans is probably that a specific biochemical function for boron has
not been elucidated, or, as demonstrated for plants (also for which no
biochemical function has been elucidated), boron has not been shown
necessary to complete the life cycle. Nonetheless, findings from human
experiments show that boron is a dynamic trace element that can affect
the metabolism or utilization of numerous substances involved in life
processes, including calcium, copper, magnesium, nitrogen, glucose,
triglycerides, reactive oxygen, and estrogen. Through these effects,
boron can affect the function or composition of several body systems,
including blood, brain, and skeleton, in a positive manner, which
demonstrates that boron is a beneficial element, if not an essential
mineral element, at physiological amounts.
Although the first findings involving boron deprivation of humans
appeared in 1987 (Nielsen et al., 1987), the most convincing findings
have come mainly from two studies in which men over the age of 45,
postmenopausal women, and postmenopausal women on estrogen therapy
were fed a low-boron diet (0.25 mg/2000 kcal) for 63 days and then fed
the same diet supplemented with 3 mg boron/day for 49 days (Nielsen,
1989, 1994; Nielsen et al., 1990, 1991, 1992b; Penland, 1994). These
dietary intakes were near the low and high values in the range of
usual dietary boron intakes (see section 5.2.4). The major differences
between the two studies were the intakes of copper and magnesium; in
one experiment they were marginal or inadequate, whereas in the other
they were adequate. Boron deprivation had several effects, regardless
of dietary copper and magnesium. In addition, the marginal or
inadequate copper and magnesium caused apparent detrimental changes
that were more marked during boron deprivation than during boron
repletion. Among the effects of boron supplementation after 63 days of
boron depletion in these experiments were the following: an effect on
macromineral metabolism, evidenced by increased serum
25-hydroxycholecalciferol and a decrease in the elevated calcitonin
that was apparently caused by inadequate copper and magnesium; an
effect on energy metabolism, suggested by decreased serum glucose and
increased serum triglycerides; an effect on nitrogen metabolism,
indicated by decreased blood urea nitrogen and serum creatinine and
increased urinary hydroxyproline excretion; an effect on reactive
oxygen metabolism, indicated by increased serum erythrocyte superoxide
dismutase and serum ceruloplasmin; and an effect on erythropoiesis and
haematopoiesis, suggested by increased blood haemoglobin and mean
corpuscular haemoglobin content but decreased haematocrit, platelet
number, and erythrocyte number. Boron supplementation after depletion
also enhanced the elevation in serum 17ß-estradiol and plasma copper
caused by estrogen therapy, altered electroencephalograms such that
they suggested improved behavioural activation (e.g. less drowsiness)
and mental alertness, and improved processes of attention and memory.
Two hypotheses have appeared to account for the multiple effects
described above. One hypothesis is that boron has a role in cell
membrane function, stability, or structure, such that it influences
the response to hormone action, transmembrane signalling, or
transmembrane movement of regulatory cations or anions (Nielsen,
1991); a similar hypothesis has also appeared for the functional role
of boron in plants (Parr & Loughman, 1983; Blaser-Grill et al., 1990;
Blevins & Lukaszewski, 1994). The other hypothesis is that boron is a
negative regulator that influences a number of metabolic pathways by
competitively inhibiting some key enzyme reactions (Hunt, 1994).
Regardless of the fact that the function of boron remains
undefined, boron is becoming recognized as an element of potential
nutritional importance because of the findings from human and animal
studies. The latest report on trace elements in human nutrition and
health published by the World Health Organization (WHO, 1996)
suggested that an individual mean basal requirement for adults may be
about 0.375 mg/day, and the minimum mean population intake that meets
basal needs could be about 0.75 mg/day for adults. The report also
indicated that an acceptable safe range of population mean intakes for
boron for adults could well be 1-13 mg/day.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Several investigators have studied the effects of borates on
bacteria, protozoa, and algae (see Table 18). Effect concentrations
for the bacterium Pseudomonas putida range widely. No observed
effects were noted after 72 h at 291 mg boron/litre by Schöberl &
Huber (1988), whereas Guhl (1996) found a 16-h EC10 of 7.6 mg
boron/litre. Guhl (1996) and Bringmann & Kuhn (1980) reported 30-min
EC10 and 72-h EC3 values of 340 and 290 mg boron/litre,
respectively. Twenty per cent light loss, compared with controls, from
Photobacterium phosphoreum was determined to occur after 30 min at
18 mg boron/litre (Guhl, 1996). Nitrogen-fixing cyanobacteria require
boron for proper functioning of the heterocyst cell wall (Bonilla et
al., 1990). Mateo et al. (1986) concluded that boron is essential for
nitrogen fixation in Anabaena.
A wide range of effect concentrations was reported for two
protozoan species. Bringmann & Kuhn (1980) reported a 72-h EC3 of 0.3
mg boron/litre for Entosiphon sulcatum, whereas Guhl (1996) showed a
72-h NOEC of >10 mg boron/litre. Reproduction of Paramecium
caudatum was completely inhibited at 70 mg boron/litre (Bringmann &
Kuhn, 1980), and no effects were observed at 18 mg boron/litre by
Sprague (1972).
De Jong (1965) determined the sensitivity of another green alga,
Chlorella vulgaris, to borax concentrations ranging from 0.0001 to
30% of the medium. Inoculated cultures were cultivated at room
temperature in daylight for 3-4 months. The highest concentration of
borax tolerated by C. vulgaris was 0.6 mg boron/litre, and the
lowest concentration inhibiting growth was 1.2 mg boron/litre.
Concentrations above 50 mg boron/litre induce the formation of giant
cells in Chlorella pyrenoidosa as a result of a stronger inhibition
of the formation of daughter cells compared with the synthesis of
biomass. For cells grown in 100 mg boron/litre, cell division was
severely inhibited for the first 72 h of exposure. During this time,
photosynthesis was inhibited by only 47%. The protein and nucleic acid
content of the giant cells increased and nitrate uptake was also
enhanced, whereas the lipid content decreased. The authors therefore
concluded that the delay in cell division is probably a direct effect
of boron on cytokinesis, as nuclear division is unaffected (Maeso et
al., 1985).
Martinez et al. (1986) observed that boric acid caused a decrease in
growth rates, protein content, and chlorophyll content in the
blue-green alga Anacystis nidulans, following exposure to 75 and 100
mg boron/litre as boric acid. The photosynthetic pigments completely
disappeared after 72 h of exposure. Nitrate uptake was also lowered.
Table 18. Borate toxicity to bacteria, protozoa, and algae
Species Duration End-point Parameter Borate concentration Reference
in solution
(mg boron/litre)
Pseudomonas putida 30 min Growth inhibition EC10 340 Guhl (1996)
16 h Growth inhibition EC10 7.6 Guhl (1996)
72 h Growth inhibition NOEC 291 Schöberl & Huber (1988)
72 h Growth inhibition EC3 290 Bringmann & Kuhn (1980)
Photobacterium phosphoreum 30 min Light loss EC20 18 Guhl (1996)
Entosiphon sulcatum 72 h Growth inhibition EC3 0.3 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC >10 Guhl (1996)
Paramecium caudatum 72 h Growth inhibition EC100 <70 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC 18 Sprague (1972)
Algae, Scenedesmus quadricauda 8 days Growth inhibition EC3 0.16 Bringmann & Kuhn (1978)
Algae, Scenedesmus subspicatus 72 h Growth inhibition EC0 10 Guhl (1996)
72 h Growth inhibition EC10 24 Guhl (1996)
72 h Growth inhibition EC50 34 Guhl (1996)
72 h Growth inhibition EC100 100 Guhl (1996)
Algae, Scenedesmus subspicatus Chronic Growth inhibition EC10 24 Kopf & Wilk (1995)
Chronic Growth inhibition EC50 52 Kopf & Wilk (1995)
Chronic Growth inhibition EC90 109 Kopf & Wilk (1995)
Algae, Microcystis aeruginosa 8 days Growth inhibition EC3 20.3 Bringmann & Kuhn (1978)
An accumulation of carbohydrates was observed, probably because the
loss of the photosynthetic pigments inhibited their degradation.
Lewin (1966) concluded that borate is an essential micronutrient for
growth in marine diatoms, whereas Smyth & Dugger (1981) concluded that
boron is essential for Cylindrotheca fusiformis. Antia & Cheng
(1975) studied the effects of boric acid on the growth of 19 species
(10 classes) of marine phytoplankton. Axenic cultures in a
nutrient-enriched, pH-controlled seawater (33% salinity) medium were
exposed to the following concentrations of boric acid: 0, 5, 10, 50,
or 100 mg boron/litre. Growth measurements were taken every 2-4 days
by determining the optical density at 600 nm after vortex mixing.
Concentrations of 5 and 10 mg boron/litre did not inhibit the growth
of any species tested, with 10 mg boron/litre actually stimulating
growth in the blue-green alga Anacystis marina, the diatom
Skeletonema costatum, and the cryptomonad Rhodomonas lens. Growth
was strongly inhibited in 26% of the species at 50 mg boron/litre
(e.g. green alga Tetraselmis maculata; haptophyte Emiliania
huxleyi; diatom Phaeodactylum tricornutum) and in 63% of the
species at 100 mg boron/litre (e.g. chrysophyte Monochrysis
lutheri). The highest concentration was also lethal to 37% of the
species (e.g. diatom Cyclotella cryptica; dinoflagellate
Amphidinium carteri). Some species (e.g. green alga Monallantus
salina) required an adaptation period, with growth imperceptible
until 34-36 days after inoculation. Sequential transfer tests showed
that many of the initially inhibited species recovered after exposure
to 50 mg boron/litre, but not after exposure to 100 mg boron/litre.
The authors concluded that higher concentrations would be expected to
cause species redistribution, favouring growth of some forms and
suppressing growth of others.
A few studies have investigated the effects of boron on microorganisms
in sewage treatment plants. A boron concentration of 20 mg/litre had
no effect on activated sewage treatment (Gerike et al., 1976), and
boron levels below 200 mg/litre resulted in no significant inhibition
of anaerobic sludge digestion (Butterwick et al., 1989).
9.1.1.2 Soil
Information concerning the effects of boron on soil microorganisms is
scarce. Butterwick et al. (1989) reported results of a study in which
actinomycetes were found to undergo genetic transformations at boron
concentrations of 1000-6000 mg/litre. Bowen & Gauch (1966) exposed the
fungi Saccharomyces cerevisiae, Neurospora crassa, Aspergillus
niger, and Penicillium chrysogenum to boron. They found that
growth was significantly reduced at boron concentrations of 50, 250,
1300, and 4000 mg/litre for the four species, respectively. The
authors concluded that boron was not essential for any of the species
tested.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Nobel (1981) studied the effect of several boron compounds on
photosynthesis in submerged macrophytes, watermilfoil (Myriophyllum
alterniflorum), buttercup (Ranunculus penicillatus), and waterweed
(Elodea canadensis). The watermilfoil and buttercup (four
plants/concentration) were exposed to the following concentrations of
boric acid for 28 days: 0, 1, 2, 5, or 10 mg boron/litre. The
waterweed (eight plants/concentration) was exposed to the following
concentrations of boric acid for 21 days -- 0, 1, 2, 5, 10, or 250 mg
boron/litre -- and also to the following boron compounds at a
concentration of 2 mg boron/litre: boron trioxide, sodium perborate,
sodium metaborate, and borax. The test water was characterized as an
oligotrophic calcium-deficient nutritive medium. Net photosynthesis
was measured weekly as a function of the dissolved oxygen content.
Significant reductions (p = 0.01) in photosynthesis compared with
controls were observed at 2 mg boron/litre in the buttercup and
waterweed and at 5 mg boron/litre in the watermilfoil. The LC50
values for each species were as follows: 5 mg boron/litre for the
waterweed and the watermilfoil and 10 mg boron/litre for the
buttercup. Boron trioxide did not adversely affect photosynthesis in
the waterweed. The toxicity of the other boron compounds in the
waterweed at 2 mg boron/litre exhibited the following trend: borax >
metaborate > perborate > boric acid. Because calcium is known to
inhibit the uptake of boron in plants and, therefore, to mitigate the
damaging effects (Reeve & Shive, 1944), the authors concluded that the
toxic effects of boron in macrophytes are more pronounced in soft
(low-calcium) water.
In phytoassays conducted by Wang (1986), boron at concentrations up to
60 mg/litre did not significantly affect the growth of duckweed
(Lemna minor), expressed by the increase in frond number. In
addition, a concentration of 40.3 mg boron/litre added as a
tetraborate salt led to 50% inhibition of root growth in the
freshwater Eurasian watermilfoil (Myriophyllum spicatum) after 32
days of treatment (Stanley, 1974).
A pot study was carried out on the reed Phragmites australis during
the growth seasons of 1992 and 1993. Both soil culture in the presence
of free water and a gravel hydroculture with graduated repeated
additions of boron (as boric acid) were used during the growth seasons
to maintain the water phase at 0, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and,
in the case of the hydroculture, even 32.0 mg boron/litre. The number
of stalks per pot, height growth of the plants, and the yield of dry
substance of leaves, stalks, and roots at post-harvest time were
determined. The boron content in leaves, stalks, and roots, the
appearance of boron toxicity symptoms in leaves, and differences in
growth at each of the exposure concentrations were monitored. It was
concluded from the study that Phragmites australis can tolerate a
relatively high boron content (up to 4 mg boron/litre) in the liquid
nutrient substrate; for a period of 2-3 months, even 8 mg/litre was
tolerated without noticeable damage. Boron toxicity symptoms in the
leaves of Phragmites australis arose first at leaf boron
concentrations of around 150-180 mg/kg dry weight; however, no adverse
effects on growth, development, or dry substance yields of the plants
could be established at these leaf boron concentrations. Long-term
exposure to 8 mg boron/litre in waters would lead to symptoms of
damage, growth reductions, and yield reductions of plants (Marks et
al., 1994; Bergmann et al., 1995).
9.1.2.2 Invertebrates
1) Freshwater
Acute toxicity
Summaries of the median response toxicity data for aquatic
invertebrates are given in Table 19. These studies are also described
in detail in the following text.
Gersich (1984) studied the acute toxicity of boric acid to Daphnia
magna. Static (48-h) tests were conducted following the methodology
approved by the American Society for Testing and Materials. Three
replicates of 10 neonates each were exposed to seven nominal
concentrations: 0 (control), 54, 91, 151, 252, 420, or 700 mg
boron/litre. The 48-h LC50 for D. magna was 133 mg/litre as boron,
with a 95% confidence interval of 115-153 mg/litre, calculated by
probit analysis. All test organisms died at 420 mg/litre, 0% mortality
was observed at 54 mg/litre, and control mortality averaged 7%.
Lewis & Valentine (1981) also investigated the toxicity of boric acid
to D. magna. The investigators followed US EPA-approved procedures
and exposed <24-h-old neonates to five nominal test concentrations
(not given in paper). The 48-h LC50, calculated using probit
analysis, was determined to be 226 mg/litre as boron (95% confidence
interval of 200-246 mg/litre). The no-kill (0% mortality)
concentration was <200 mg/litre. Comparison of these results with
those of Gersich (1984) indicates a factor of 1.7 difference in the
48-h LC50 values; however, Canton & Adema (1978) report that a
difference of 2.0 may be normal in LC50 values not determined at the
same time or under similar conditions.
A few studies have been conducted to assess the toxicity of the sodium
borates to D. magna. As part of the study conducted by NAPM (1974),
the effects of sodium tetraborate pentahydrate on D. magna were
investigated. Static (96-h) tests were performed in which 10-20
organisms per test were exposed to the following nominal
concentrations of sodium tetraborate pentahydrate: 0, 18, 33, 58, 102,
or 182 mg/litre. The highest test concentration resulted in only 26.7%
mortality at 96 h; consequently, the 96-h LC50 for D. magna was
>182 mg sodium tetraborate pentahydrate/litre (>27 mg boron/litre).
Table 19. Median response concentrations for aquatic invertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
ARTHROPODA
Crustacea - Daphnidae
Daphnia magna Boric acid S, N 133.0 Gersich (1984)
Daphnia magna Boric acid S, N 226.0 Lewis & Valentine
(1981)
Daphnia magna Borax S, N 73.0 Bringmann & Kuhn
(1977)
Daphnia magna Anhydrous borax S, N <52.0 Threshold concentration,
based on immobilization Anderson (1946)
Daphnia magna Sodium tetraborate S, N >182.0 Mortality 26.7% at 96 h NAPM (1974)
pentahydrate
Daphnia magna Sodium tetraborate S, N 141 Maier & Knight (1991)
Crustacea - Limnoriidae
Limnoria lignorum Borax S, N 28.35 After 5-day recovery period Robinson & Perkins
(1977)
Insecta - Chironomidae
Chironomus decorus Sodium tetraborate S, N 1376 Maier & Knight (1991)
MOLLUSCA
Gastropida - Onchidiidae
Onchidoris fusca Borax S, N <28.35 After 5-day recovery period Robinson & Perkins
(1977)
a S = static test; N = nominal concentration.
Bringmann & Kuhn (1977) reported a 24-h LC50 of 340 mg disodium
tetraborate (anhydrous borax)/litre (73 mg boron/litre) for
D. magna in static tests using tap-water free of chlorine. The LC0
was 61 mg disodium tetraborate/litre (13 mg boron/litre), and the
LC100 was 1930 mg disodium tetraborate/litre (415 mg boron/litre).
Anderson (1946) obtained similar results with D. magna, reporting a
threshold concentration based on immobilization of <240 mg anhydrous
borax/litre (<52 mg boron/litre) at 48 h. Maier & Knight (1991)
reported a 48-h LC50 value of 141 mg boron/litre (95% confidence
limits 123-159 mg boron/litre) for D. magna exposed to sodium
tetraborate. Increasing water hardness and sulfate concentrations did
not affect the toxicity of boron to D. magna. In this study, boron
was much more toxic to daphnids than to Chironomus decorus
4th-instar larvae, for which the 48-h LC50 was 1376 mg boron/litre
(95% confidence limits 1298-1453 mg boron/litre) (Maier & Knight,
1991).
Tubificid worms (Tubifex sp.) appear to be less sensitive than
D. magna to sodium borate, with concentrations of sodium borate
decahydrate (borax) as high as 750 mg/litre (85 mg boron/litre)
causing no effect after a 24-h exposure. The 24-h LC100 was reported
as 2000 mg/litre (227 mg boron/litre). The tubificid also exhibited
less sensitivity to boric acid, with a no-effect level at 24 h of
exposure of 7500 mg/litre (1311 mg boron/litre) and an LC100 of 10 000
mg/litre (1748 mg boron/litre) (Mann, 1973).
In an early study, Fay (1959) determined the effects of metaboric acid
(B2O3.H2O) on various life stages of three species of mosquitos:
Aedes aegypti, Anopheles quadrimaculatus, and Culex
quinquefasciatus. A concentration of 8000 mg/litre (1973 mg
boron/litre) did not affect hatching of A. aegypti eggs following
72 h of exposure. However, newly hatched larvae of
C. quinquefasciatus and A. quadrimaculatus were quite sensitive to
metaboric acid. Complete mortality of C. quinquefasciatus larvae was
observed after 24 h of exposure to 2000 mg metaboric acid/litre (493
mg boron/litre). Complete mortality of A. aegypti was observed after
24 h of exposure to 4000 mg/litre (986 mg boron/litre), with complete
mortality occurring in A. quadrimaculatus only at the 8000 mg/litre
(1973 mg boron/litre) level. In contrast, at the 2nd- and 3rd-instar
stage, A. quadrimaculatus was more sensitive than the other species.
In 2nd-instar larvae, 100% mortality occurred at 500 mg/litre (123 mg
boron/litre) in A. quadrimaculatus, 96% mortality occurred at
1500 mg/litre (370 mg boron/litre) in A. aegypti, and 100% mortality
occurred at 2000 mg/litre (493 mg boron/litre) in
C. quinquefasciatus. The 3rd-instar larvae were less sensitive than
the 2nd-instar larvae, with 100% mortality of A. quadrimaculatus at
2500 mg/litre (617 mg boron/litre), 88% mortality of A. aegypti at
3000 mg/litre (740 mg boron/litre), and 48% mortality of
C. quinquefasciatus at 4000 mg/litre (986 mg boron/ litre). Pupae of
the three species were even less sensitive to metaboric acid,
requiring concentrations ranging from 6000 mg/litre (1480 mg
boron/litre) to 16 000 mg/litre (3946 mg boron/litre) to prevent
emergence to the adult stage. From the information provided in this
study, it appears that the 2nd-instar larva was the life stage that
was most sensitive to exposure to metaboric acid.
Fay (1959) also studied the effects of prolonged exposure to boron
trioxide (B2O3.H2O) on the immature stages of the same three
species of mosquitos. Newly hatched larvae were reared in the
following concentrations of metaboric acid to determine the percentage
of successful adult emergence: 10, 25, 50, 100, or 250 mg/litre. No
significant reduction in maturation was observed in A. aegypti or
C. quinquefasciatus at any test concentration <100 mg/litre.
However, at 50 mg/litre, only 2% of the larvae of A.
quadrimaculatus reached the adult stage. At 250 mg metaboric
acid/litre, only 1% of A. aegypti and only 3% of
C. quinquefasciatus reached the adult stage.
Maier & Knight (1991) evaluated the chronic sublethal effects of boron
to Chironomus decorus larvae. A significantly decreased growth rate
over a 96-h period was observed at 20 mg boron/litre.
Chronic toxicity
Gersich (1984) conducted a 21-day static renewal chronic toxicity test
with D. magna. Daphnids (20 organisms/concentration, or four
replicates with 5 organisms/replicate) were exposed to the following
nominal concentrations of boric acid: 0, 7, 14, 28, 56, or 105
mg/litre as boron. The test concentrations remained stable during
testing; the mean measured concentrations ranged from 91.4 to 106% of
the nominal test concentrations. The 21-day LC50, calculated using
the moving average method, was 52.2 mg boron/litre, and the 95%
confidence interval was 42.6-66.7 mg boron/litre. No mortality was
observed in the control group. The mean number of broods per daphnid,
mean total young per daphnid, mean brood size per daphnid, and mean
size differed significantly (alpha = 0.05) from the control at the
13.6 mg boron/litre concentration. Therefore, the maximum acceptable
toxicant concentration (MATC) was determined based on the most
biologically and statistically significant end-points, reproduction
and growth. The MATC for boric acid was estimated to be between 6.4
and 13.6 mg boron/litre, or 9.3 mg boron/litre (the geometric mean of
these two values).
Lewis & Valentine (1981) also conducted a 21-day static renewal
chronic toxicity test with D. magna. The test was conducted
similarly to that of Gersich (1984), except for the exposure regimen.
Ten test chambers were set up for each test concentration: three
beakers contained five daphnids each for obtaining data on survival,
and seven beakers each contained one daphnid for obtaining data on
survival, growth, and reproduction. Twenty control chambers were used:
6 chambers contained five daphnids each, and 14 chambers each
contained one daphnid. Daphnids were exposed to the following nominal
concentrations of boric acid: 0, 6, 13, 27, 53, or 106 mg/litre as
boron. The concentration of the test solution remained stable during
testing, with mean measured concentrations exceeding 95% of the
nominal concentration. The 21-day LC50, calculated by probit
analysis, was 53.2 mg boron/litre, with a 95% confidence interval of
44.1-64.5 mg boron/litre. Mortality was 9% in the control group. The
mean brood size and total number of young produced were significantly
reduced (p < 0.05) compared with controls at concentrations of
>13 mg boron/litre. Mean length of daphnids was significantly reduced
(p < 0.05) compared with controls at 53 mg boron/litre. The NOEC
was 6 mg boron/litre. Therefore, based on the most sensitive
parameters, the MATC was >6 and <13 mg boron/litre, or 8.83 mg
boron/litre (geometric mean of these values).
2) Marine
In static tests, Mann (1973) determined 24-h LC100 values for the
amphipod Gammarus tigrinus exposed to boric acid, borax, and sodium
perborate at two salinities (32.23% and 17.66%). The LC100, 10 000
mg/litre, was the same for all three compounds at both salinities. The
no-effect concentration for all of the compounds was 7500 mg/litre. In
contrast, Robinson & Perkins (1977) reported toxicity values for borax
in the isopod Limnoria lignorum and the sea slug Onchidoris fusca
that are roughly two orders of magnitude lower than those reported by
Mann (1973) for the amphipod Gammarus tigrinus. The salinity of the
test water was 30%. The 24-h static LC50 values, after a 5-day
recovery period, were 250 mg/litre (28.35 mg boron/litre) for the
isopod and >250 mg/litre (>28.35 mg boron/litre) for the sea slug.
In studies with sea urchin (Anthocidaris crassispina) embryos,
exposure to boric acid at a concentration of 37 mg boron/litre had no
effect on development, whereas exposure to 75 mg boron/litre was fatal
(Eisler, 1990).
3) Biocenosis studies
Three biocenosis studies (also known as microcosms, mesocosms, and
experimental ecosystems) have been conducted (summarized in ECETOC,
1997). A 28-day laboratory microcosm test with microorganisms
consisting of six trophic stages yielded a NOEC and LOEC of 2.5 and
5.0 mg boron/litre, respectively. Studies with outdoor ponds
containing up to 29 species and treated with 0.7 mg boron/litre over a
2-year period resulted in no significant differences compared with
untreated control ponds. Further, field studies carried out in seven
different water bodies subjected to different levels of anthropogenic
boron inputs yielded no toxic effects of boron at concentrations
between 0.16 and 1.52 mg/litre.
9.1.2.3 Vertebrates
1) Freshwater
Acute toxicity
Summaries of the acute toxicity data for aquatic vertebrates are given
in Table 20. These studies are also described in detail in the
following text.
For boron compounds, three studies are available that provide
information concerning the 96-h LC50 values, the toxicity value
established by the US EPA (Stephan et al., 1985) for use in the
derivation of water quality criteria for the protection of aquatic
life and its uses. Wallen et al. (1957) conducted 96-h static toxicity
tests with mosquitofish. The test water, turbid pond water, was
selected to simulate the commonly turbid wastewater from oil refinery
operations; however, it was determined that the highest turbidities
used in the study should not produce direct effects on the fish during
the exposure time. Adult female fish (10/concentration) were exposed
to the following nominal concentrations of boric acid or borax: 10,
18, 32, 56, or 100 mg/litre. If no deaths occurred at 96 h, the tests
were rerun at concentrations of 100, 180, 320, 560, or 1000 mg/litre
and, subsequently, at concentrations 10 times these values
(1000-10 000 mg/litre). The 96-h LC50 for mosquito-fish exposed to
boric acid was 5600 mg/litre (979 mg boron/litre), and the 96-h LC50
for borax was 3600 mg/litre (408 mg boron/litre).
As part of the study conducted by NAPM (1974), static bioassays were
conducted to determine the toxicity of sodium tetraborate pentahydrate
to fathead minnows. The tests were conducted using standard methods
and filtered tap-water. Fish (15/treatment) were exposed to the
following nominal concentrations: 0, 200, 360, 640, 1120, or 2000
mg/litre. The 96-h LC50 was 1900 mg/litre (332 mg boron/litre).
Hamilton & Buhl (1990) reported the results of 96-h static toxicity
tests with two life stages of chinook salmon (Oncorhynchus
tshawyts-cha) and coho salmon (O. kisutch). Swim-up fry
(8-12 weeks old) were tested in fresh water, and advanced fry (15-21
weeks old) were tested in brackish water. The 96-h LC50 values for
chinook salmon swim-up fry and advanced fry were 725 mg/litre (127 mg
boron/litre) and 600 mg/litre (105 mg boron/litre), respectively. The
96-h LC50 values for coho salmon swim-up fry and advanced fry were
447 mg/litre (78.1 mg boron/litre) and 600 mg/litre (105 mg
boron/litre), respectively.
Results of 24- and 48-h static acute tests with rainbow trout,
guppy (Poecilia reticulata), and mosquitofish clearly show the
difference in the degree of toxicity resulting from exposure to boric
acid versus sodium borate. Mann (1973) reported the following 24-h
LC100 values for rain-bow trout and guppy, respectively: boric acid,
10 000 mg/litre (1748 mg boron/litre) and 7500 mg/litre (1311 mg
boron/litre); borax, 5000 mg/litre (567 mg boron/litre) for both
species. Static 24-h LC50 values determined by Wallen et al. (1957)
for the mosquitofish are 18 000 mg/litre (3146 mg boron/litre) for
boric acid and 12 000 mg/litre (1361 mg boron/litre) for borax. The
48-h LC50 values determined in this study were 10 500 mg/litre (1835
mg boron/litre) for boric acid and 8200 mg/litre (930 mg boron/litre)
for borax. Rainbow trout are more sensitive to borax than
mosquitofish, with 24- and 48-h static LC50 values of 2800 mg/litre
(602 mg boron/ litre) and 1800 mg/litre (387 mg boron/litre),
respectively (Alabaster, 1969). Juhnke & Ludemann (1978) reported an
LC50 of 807 mg/litre as boron for golden orfe (Leuciscus idus)
Table 20. Acute toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Coho salmon Boric acid S, N 78.1 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus kisutch) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Chinook salmon Boric acid S, N 127 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus tshawytscha) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Rainbow trout Borax S, N 602 387 Alabaster (1969)
(Oncorhynchus mykiss) Borax FT, N 65 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 150 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Borax FT, N 88 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 100 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Fathead minnow Sodium S, N 332 NAPM (1974)
(Pimephales promelas) tetraborate
pentahydrate
Mosquitofish Borax S, N 1361 930 408 Wallen et al. (1957)
(Gambusia affinis) Boric acid S, N 3146 1835 979 Wallen et al. (1957)
Golden orfe 173 Schöberl & Huber (1988)
(Leuciscus idus)
Table 20. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Zebra fish Borax 14.2 Guhl (1996)
(Brachydanio rerio)
Bluegill Borax S, N 4.6 Turnbull et al. (1954)
(Lepomis macrochirus)
Dab Anhydrous borax Semi-static, 88.3 74 Salinity 34.62 ± 0.2% Taylor et
(Limanda limanda) N al. (1985)
Colorado squaw-fish Boric acid 279b Hamilton (1995)
(Ptychocheilus lucius) >100c
527d
Razorback sucker Boric acid 233b Hamilton (1995)
(Xyrauchen texanus) 279c
>100d
Bonytail Boric acid 280b Hamilton (1995)
(Gila elegans) >100c
552d
a S = static test; FT = flow-through test; N = nominal concentration.
b Swim-up fry.
c 1-g juvenile.
d 2-g juvenile.
exposed to anhydrous borax in static acute tests (exposure time not
given).
Mann (1973) reported 24-h LC100 values for rainbow trout and
guppies exposed to sodium perborate (500 mg/litre or 66.1 mg
boron/litre) that were considerably lower than those reported for
boric acid and borax. The authors attributed this increased toxicity
to a pH shift into the alkaline range (up to 9.1), which resulted in
increased mucilage formation, with the fish exhibiting a crippling
behaviour.
The treatment of embryos of the toad Bufo vulgaris with 0.5%
boric acid for 24 h from the two-cell stage to the tail-bud stage
resulted in several developmental malformations, including a reduction
or lack of external gills, short tail, bifurcation of the epiphysis,
suppression of forebrain development, and inhibition of sensory organ
development. Several of these effects were attributed to the direct
action of boric acid on the ectoderm (Takeuchi, 1958).
Chronic toxicity
Toxicity of borate to early life stages of fish has been
documented for several species (Birge & Black, 1977; Black et al.,
1993). Embryonic and early larval stages of rainbow trout, largemouth
bass, channel catfish, and goldfish were exposed to boron, as boric
acid or borax, from fertilization up to 8 days post-hatch. All
exposures were in soft (50 mg CaCO3/litre) or hard (approx. 200 mg
CaCO3/litre) reconstituted water. Test responses included embryonic
mortality, teratogenesis, and larval mortality. Gross debilitating
anomalies of survivors were classified as mortalities. Effect
concentrations (LC50, NOEC, and LOEC) for each species are presented
in Table 21. Neither water hardness nor the form of boron (boric acid,
borax) added to the test aquaria consistently affected embryo-larval
survival of rainbow trout, channel catfish, and goldfish (Birge &
Black, 1977).
On the basis of median lethal concentrations (LC50), no species
was found to be especially sensitive. The range of LC50s for all
species was 12.2-235 mg boron/litre. In addition, Birge & Black (1977)
reported LC1s ranging from 0.001 to 0.1 mg boron/litre for rainbow
trout, from 0.2 to 5.5 mg boron/litre for channel catfish, and from
0.2 to 1.4 mg boron/litre for goldfish. The LC1 showed rainbow trout
to be the most sensitive species. The NOEC ranged from 0.009 to 0.103
mg boron/litre for rainbow trout and was 1.39 mg boron/litre for
large-mouth bass. These were consistent with the acute toxicity
results that indicated rainbow trout and zebra fish as the most
sensitive species (see Table 20).
The effect of natural dilution water on boron toxicity was
reported by Black et al. (1993). Natural surface waters were collected
from three US locations: the Erwin National Fish Hatchery in
Tennessee, Brookville Lake in Indiana, and Firehole River in
Yellowstone National Park, Wyoming. Surface water control boron
Table 21. Chronic toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Coho salmon Sodium metaborate S, N 113 Alevins, 283-h exposure Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate
Coho salmon Sodium metaborate S, N 12.2 Underyearlings, 283-h exposure, Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate salinity 28 ± 1%
Rainbow trout Borax FT, N 27 Alevin, 28-day exposure, Birge & Black (1977)
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre
Rainbow trout Boric acid FT, N 100 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Borax FT, N 54 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 79 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 138 Embryo/larva, 32-day exposure Black et al. (1993)
(Oncorhynchus mykiss)
Rainbow trout Boric acid 138 Hardness of 200 mg CaCO3/ litre;
(Oncorhynchus mykiss) duration 32 days
NOEC = 0.009 mg boron/litre
LOEC = 0.1 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid 92 Hardness of 200 mg CaCO3/ litre;
(Micropterus salmoides) duration 11 days
NOEC = >1.39 mg boron/litre
LOEC = 12.17 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid FT, N 92 Embryo/larva, 11-day exposure Black et al. (1993)
(Micropterus salmoides)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Goldfish Borax FT, N 71.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 178.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 68.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 170.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 65.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 46.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 59.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 75.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 235.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 220.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 120.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Channel catfish Boric acid FT, N 102.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 71.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 22.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 54 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 157 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 60 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 145 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 47 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 130 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Leopard frog Borax FT, N 54 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 135 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 148 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 135 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 145 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 123 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
a S = static test; FT = flow-through test; N = nominal concentration.
concentrations were 0.023, 0.091, and 0.75 mg/litre for Erwin,
Brookville, and Yellowstone waters, respectively. Total organic carbon
for the three surface waters ranged from 0.8 to 1.9 mg/litre.
Hardness levels for these natural waters ranged from 24 to 209 mg
CaCO3/litre. In the surface water tests, three nominal treatments
were used: surface water control, control plus 1.0 mg boron/litre
added (as boric acid), and control plus 10 mg boron/litre added. Total
and dissolved boron levels were measured for each of the surface water
treatments. No statistically significant differences were noted
between total and dissolved boron concentrations, except for
Yellowstone (1.0 mg boron/litre) and Erwin (10 mg boron/litre)
treatments. The true significance of this, however, is minimal, as
total versus dissolved concentrations for Yellowstone and Erwin waters
were 1.61 vs. 1.52 mg boron/litre and 9.91 vs. 9.48 mg boron/litre,
respectively. Hence, it is reasonable to assume that all boron was in
solution. Boron did not elicit toxicity to embryo-larval stages of
rainbow trout exposed to surface water control boron levels up to 0.75
mg/litre. LOECs for Erwin, Brookville, and Yellowstone treatments were
1.10, 1.24, and 1.73 mg boron/litre, respectively, indicating that the
threshold for no effects is approximately 1 mg boron/litre. In
addition, deep (600 m) well-water, typically used for aquatic toxicity
tests, from a contract laboratory located in Wareham, Massachusetts,
USA, was also used. Boron was spiked into well-water to obtain an
exponential series of nominal concentrations: 0.0017, 0.017, 0.17,
1.7, and 17 mg boron/ litre, respectively. Analytical confirmation of
exposure was obtained for the two highest concentrations, 2.1 and
18.0 mg boron/litre. No effects were observed, even at the highest
boron concentration.
The effect concentrations generated in natural waters were
greater than those from the reconstituted water experiments. The cause
of these differences is not readily apparent, particularly as
bioavailability was not reduced by natural water. However, some
investigators (Belanger et al., 1989; Farris et al., 1994) have shown
that organisms cultured in natural water often grow and reproduce
better than their counterparts in reconstituted or laboratory
dechlorinated water. Therefore, a reasonable hypothesis that may
explain, in part, the differences in reconstituted versus natural
water responses is the better health experienced by organisms exposed
to boron in natural water. The causative agents for this lack of
health in laboratory water are not certain but may include nutrient
deficiencies (Keating & Dagbusan, 1984; Belanger et al., 1989).
Early life stage tests have been conducted with fathead minnows
to determine effects of chronic exposure (30-60 days) to boric acid.
The 30-day NOEC and LOEC were 14 and 24 mg boron/litre, respectively,
based on growth reduction; the 60-day NOEC and LOEC were 24 and 88 mg
boron/litre, respectively, based on reduction in fry survival
(Butterwick et al., 1989).
Birge & Black (1977) also studied the effect of boric acid and
borax on embryos and larvae of the leopard frog (Rana pipiens) and
the effect of boric acid on embryos and larvae of the Fowler's toad
(Bufo fowleri). The embryos were exposed for 3.5 days and the larvae
for 7.5 days to concentrations of boric acid and borax ranging from
0.05 to 300.00 mg boron/litre in hard (200 mg CaCO3/litre) and soft
(50 mg CaCO3/litre) water (as described above for fish exposures).
The leopard frog and Fowler's toad were equisensitive to borax and
boric acid. For the leopard frog, LC1 and LC50 values were 13 and
130 mg boron/litre (dosed as boric acid) in soft water and 22 and 135
mg boron/litre in hard water. Lower toxicity values were observed via
borax exposures, with LC1 and LC50 values of 5 and 47 mg boron/litre
in soft water and 3 and 54 mg boron/litre in hard water, respectively.
The LC1 and LC50 values for Fowler's toad exposed to boric acid were
25 and 145 mg boron/litre in soft water and 5 and 123 mg boron/litre
in hard water, respectively.
2) Marine
Taylor et al. (1985) studied the toxicity of several metals,
including boron (as anhydrous borax), to a saltwater fish (dab,
Limanda limanda). The tests were conducted following standard
procedures under semi-static conditions, in which the test solution
was replaced at 24-h intervals. The salinity of the test water was
34.62 ± 0.2%. Adult fish obtained from wild populations
(20/concentration) were exposed to a series of five concentrations
(not given in the paper) for 96 h. The LC50, calculated by probit
analysis, was 74.0 mg boron/litre, with a 95% confidence interval of
66.4-83.0 mg boron/litre. Pre-death symptoms included minor
respiratory problems.
Similar results were observed in bioassays conducted by Thompson
et al. (1976) with two life stages of coho salmon. Coho alevins
(0.19-0.7 g) were tested in aerated well-water (fresh water), and coho
underyearlings (1.8-3.8 g) were tested in aerated seawater with a
salinity of 28 ± 1%. Test solutions were prepared using sodium
metaborate tetrahydrate (Na2B2O4.4H2O), and exposure lasted in
excess of 12 days. The 283-h LC50 for freshwater coho alevins was 113
mg boron/litre, with 95% confidence limits of 104-123 mg boron/litre.
The 283-h LC50 for saltwater coho underyearlings was 12.2 mg
boron/litre, with 95% confidence limits of 10.89-14.56 mg boron/litre.
The authors attributed the difference in toxicity in fresh water and
salt water to differences in fish age, test temperature (11°C in fresh
water; 8°C in salt water), and available boron levels. Seawater also
has higher background levels of boron than fresh water. The results
reported by Hamilton & Buhl (1990), discussed above, do not indicate
significant differences in acute toxicity between different life
stages of chinook and coho salmon.
In static tests, Mann (1973) observed differing sensitivities in
the common eel (Anguilla anguilla) to both boric acid and borax at
two salinities: 12.39 and 25.05%. At the lower salinity, a 24-h LC100
of >10 000 mg/litre was reported for both borax and boric acid; at
the higher salinity, the 24-h LC100 was 7500 mg/litre for both
compounds. The authors speculated that differences in pH could
contribute to the toxicity of the boron compounds at the higher
salinity. At a boric acid concentration of 7500 mg/litre, the pH in
the lower-salinity test water was 7.2; the pH in the higher-salinity
test water was 6.6. As was observed with rainbow trout and
mosquitofish, Mann (1973) also observed greater toxicity in the common
eel following exposure to sodium perborate. The LC100 at both
salinities was 750 mg/litre (99.2 mg boron/litre), resulting from the
damaging effects of an alkaline pH shift.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
1) Essentiality
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron plays a role in carbohydrate metabolism, sugar translocation,
pollen germination, hormone action, normal growth and functioning of
the apical meristem, nucleic acid synthesis, and membrane structure
and function (Lovatt & Dugger, 1984). Recent work shows important
involvement of boron in cell wall cross-linking, involving
complexation with specific pectin components (Loomis & Durst, 1992; Hu
et al., 1996).
Early investigation of the effects of boric acid and borax on the
fieldbean (Vicia faba) and other plants indicated the role of boron
in plant nutrition (Warrington, 1923). The first comprehensive study
of the effects of boron on plant growth was conducted by Eaton (1944).
Fifty species of plants were grown in sand cultures with standard
nutrient solutions containing the following boron concentrations:
trace (0.03-0.04), 1, 5, 10, 15, or 75 mg/litre. For each species,
Eaton (1944) recorded the boron concentration resulting in the best
growth, the lowest concentration resulting in injury, and the symptoms
of deficiency with trace boron levels. Approximately 82% of the plants
exhibited the best growth at concentrations from trace to 5 mg/litre,
and 35% of the plants grown in trace boron levels developed
morphological symptoms of deficiency. Eaton (1944) concluded that
there was overlap of the beneficial and injurious effects of boron
between species; therefore, three broad categories of tolerance
(sensitive, semi-tolerant, and tolerant) were established. The
tolerant plants endure a wide range of boron concentrations with
little effect, and the sensitive plants exhibit a strong reaction to
either too much or too little boron.
Boron generally occurs in soils at concentrations ranging from 10
to 30 mg/kg, depending on such factors as soil type, amount of
rainfall, and irrigation type (Sprague, 1972). Boron deficiencies
usually occur in soil solutions from sandy soils or acid peat soils,
soils derived from igneous rocks, soils low in organic matter, and
irrigated soils (Sprague, 1972).
The symptoms of boron deficiency in plants include cessation of
root and leaf growth, necrosis of leaf primordia and primary root
tips, necrosis of stem and leaf phloem, bark splitting, retardation of
enzyme reactions, reduced pollen germination, and even death (Versar,
Inc., 1975; Wells & Whitton, 1977). Normal growth will usually resume
if boron is added to the growth medium.
Hudak (1973) found that a boron-deficient nutrient solution
inhibited mitosis in the root tip of the fieldbean within 72 h. A 10
mg boron/litre solution produced optimum cell division and elongation
of the root tip; however, 50 mg boron/litre caused a reduction in
mitosis within 24 h.
Ludbrook (1942) studied the effects of boron deficiency and
toxicity in Monterey pine (Pinus radiata) seedlings grown in water
culture. The suboptimal concentrations of boron added to the nutrient
solution were 0, 0.005, 0.01, or 0.05 mg/litre, and the potentially
toxic concentrations of boron were 0.5, 2.5, 5.0, 10.0, 20.0, or 40.0
mg/litre. Seedlings exposed to the toxic levels were initially grown
for 2 months in a nutrient solution containing added boron at 0.5
mg/litre. Marked reduction in root growth was observed at the two
lowest suboptimal concentrations; however, improved root growth was
observed at 0.05 mg/litre, with the development of small lateral
roots. At 10.0 mg boron/litre, a slight yellowing of the tips of the
needles developed, followed by browning and drying at 20.0 mg/litre,
until all plants died at 40.0 mg/litre. Ludbrook (1942) concluded that
the optimum growth range for Monterey pine was 0.05-5.0 mg
boron/litre.
Recent work has shown that boron in a number of plant species is
tightly bound in the polysaccharide cell wall. Boron deficiency leads
to profound changes in cell wall morphology, suggesting that boron is
critical to cell wall expansion. It has been proposed that this
structural, cross-linking function of boron is involved with the
pectin fraction, which contains apiose and other hydroxylated
fragments amenable to complexation by borate (Loomis & Durst, 1992).
Fourteen species of crop plants were studied, and it was concluded
that high pectin content requires more boron for forming cell walls or
that pectin forms a tightly held boron complex that depletes boron
availability for other critical functions, thereby increasing the
overall demand for boron (Hu et al., 1996). Kobayashi et al. (1996)
have isolated and characterized a rhamnogalacturonan II/borate complex
from enzyme-digested cell wall pectin.
Toxicity
Boron excesses usually occur in soil solutions from geologically
young deposits, arid soils, soils derived from marine sediments, and
soils contaminated by pollutant sources, such as releases from
coal-fired power plants and mining operations (Sprague, 1972).
The initial symptom of boron toxicity in plants is chlorosis
(yellowing) of the leaf tip, progressing along the leaf margin and
into the blade. Necrosis of the chlorotic tissue occurs, followed by
leaf abscission. Necrosis of the leaf tissue results in a loss of
photosynthetic capacity, which reduces plant productivity (Lovatt &
Dugger, 1984). Pollen germination and pollen tube growth may also be
inhibited (Versar, Inc., 1975).
Several investigators have shown a direct relationship between
the boron content in leaves (foliar) and the severity of the symptoms
of toxicity. Gilliam & Watson (1981) conducted an experiment in which
Anderson yews (Taxus media) were grown in soil at four boron
concentrations (0.5, 5.0, 25.0, or 50 mg/kg). Symptoms of toxicity
were observed when foliar boron accumulation reached concentrations
ranging from 85 to 100 µg/g of dry tissue. The observed symptoms
included leaf tip yellowing, followed by necrosis and premature
defoliation. Suppression of shoot and root growth was observed at 50
mg boron/kg soil.
Shopova et al. (1981) found that concentrations of 16, 24, and
32 mg boron/kg soil resulted in a decline in plant development,
yellowing of leaves, late flowering, reduction of mitotic frequency in
root tip cells, and abnormalities during meiosis in the poppy
(Papaver somniferum).
Kluge & Podlesak (1985) found that symptoms due to boron excess
begin to develop on the leaves (leaf tip necroses) of pot-grown spring
barley (Hordeum vulgare) as soon as the boron content of the leaf
tissue reaches 60-80 mg/kg dry weight.
Gestring & Soltanpour (1987) grew alfalfa (Medicago sativa) in
three soil types amended with sodium borate at rates of 0, 10, 20, and
40 mg boron/kg. Alfalfa yield was significantly reduced by boron
application in both the sandy loam and loam soils; however, no yield
reduction was observed in the silt loam soil. Soil extractable boron
did not adequately assess boron toxicity, whereas plant boron levels
were a more reliable index of toxicity. The critical range of plant
boron resulting in a yield reduction was 850-975 mg boron/kg plant
tissue (dry weight). The significant difference between the soils
shows the importance of soil pH, organic matter content, and per cent
clay to the toxicity of boron to plants.
Sage et al. (1989) exposed the rare serpentine plant
(Streptanthus morrisonii) to boron (0, 20, 60, 240, 650, 1200, or
2400 µmol/litre) via watering. Plants showed mild to moderate toxicity
symptoms (older leaves exhibiting chlorosis and necrosis) at boron
concentrations of 240 and 650 µmol/litre. Severe toxicity symptoms
(significant leaf loss) were apparent at 1200 and 2400 µmol
boron/litre. Boron levels in the leaves of plants showing severe
toxicity were an order of magnitude higher than those in the leaves of
plants in the field.
Glaubig & Bingham (1985) reported significant linear
relationships between both soil and leaf tissue boron concentrations
and foliar damage in four tree species endemic to California (digger
pine, Pinus sabiniana; California laurel, Umbellularia
californica; madrone, Arbutus menziesii; bigleaf maple,
Acer macrophyllum). Foliar damage over 25% of the leaf/needle area
occurred at saturated soil concentrations ranging from 0.08 to
1.2 mmol boron/litre for the four species and at leaf/needle
concentrations ranging from 30 to 115 mmol boron/kg. The bigleaf maple
was the most sensitive of the four species, and the digger pine was
the most tolerant species.
Under experimental conditions, Shann & Adriano (1988)
demonstrated that chronic foliar aerosol exposures of boron produced
phytotoxicity in relation to boron accumulation in the leaves. Five
crop species (swiss chard, beet, radish, tomato, and cucumber) were
exposed to aerosol concentrations of 0, 1.55, or 3.09 µg boron/cm2
leaf area. The authors concluded that the visual damage (leaf tip
necrosis) resulting from aerosol exposure was identical to that
observed from root boron toxicity for all crops tested.
9.1.3.2 Invertebrates
Boric acid is an effective stomach poison for several species of
insects, including the German cockroach (Blattella germanica).
Pretreatment of wood and other substrates with boric acid or borax can
prevent insect infestation. Baits and aerosols containing boron
compounds (e.g. borax and boric acid) have been shown to control fruit
flies, cockroaches, houseflies, and termites (Butterwick et al.,
1989).
Boron in the diet at concentrations of 0.025-5% caused
sterilization of house flies (Musca domestica) when both sexes were
fed the diet (Borkovec et al., 1969). Boron toxicity in honey bees has
also been observed; a concentration of 50 mg boric acid/litre (8.7 mg
boron/litre) in syrup had no effect on survival, whereas 100 mg boric
acid/litre (17.5 mg boron/litre) caused 50% mortality (Ostrovskij,
1955).
Lang & Treece (1972) conducted a study to determine if boric acid
would induce sterility in the face fly (Musca autumnalis). Adult
male and female flies (50 each/concentration) were exposed to boric
acid concentrations of 0.5%, 0.8%, or 1.0% in each of three media
(water, malt, and dry food) for the first 4 days after eclosion
(hatching from the pupal stage). Complete sterility (100%) was
observed in flies fed the two highest concentrations in dietary malt;
however, a high degree of recovery was observed within 5 days after
cessation of treatment. Many eggs from the females fed 1.0% boric acid
were transparent and watery in appearance; this was possibly due to
the interference of boric acid with egg chorion formation. Almost 100%
mortality occurred in the flies exposed to boric acid concentrations
of 0.5% and 0.8% in water within the first 4 days of treatment. In a
subsequent test, erratic sterility with recovery of fertility was
observed in face flies exposed to boric acid concentrations of 0.1%
and 0.3% in water.
9.1.3.3 Vertebrates
In order to determine the impact of high levels of boron on
water-fowl in Kesterson Reservoir (located within the Kesterson
National Wildlife Refuge in the San Joaquin Valley of California,
USA), a number of investigators have studied the effects of controlled
boron exposure to mallard ducks. Smith & Anders (1989) studied the
effects on reproduction in mallards fed diets supplemented with 0, 30,
300, or 1000 mg boron/kg as boric acid (concentrations known to be
found in the field). The adults were fed this diet prior to mating and
during egg incubation, and the ducklings were fed this diet after
hatching. In the 1000 mg/kg group, hatching success of fertile eggs
and duckling survival were significantly reduced, and embryo mortality
was significantly higher. The body weight of hatchlings was lower in
the 300 and 1000 mg/kg groups than in controls. Growth rate of
ducklings was significantly reduced at all three dose levels. No
effect on egg fertility, eggshell thickness, or eggshell quality was
observed at any dose level. In addition, boron did not produce any
overt signs of toxicity in young or adult mallards. The researchers
were unable to determine whether duckling mortality and impaired
growth were due to pre-hatching or post-hatching exposure or the
combined effect.
Hoffman et al. (1990) examined the effect of dietary boron
exposure on the survival, growth, and physiology of ducklings hatched
from uncontaminated eggs. Day-old mallards were exposed to 0, 100,
400, or 1600 mg boron/kg diet as boric acid for 10 weeks. No
significant effect on survival was found. The highest dietary
concentration of boron significantly decreased overall growth and the
rate of growth (sexes combined), whereas lower concentrations altered
growth only in female ducklings. Significantly decreased food
consumption was observed in the high-dose group during the first
3 weeks and in all dose groups during the 2nd week. Effects on blood,
brain, and liver biochemistry were also observed in the high-dose
group. Overall, dietary boric acid proved to be less toxic to
ducklings hatched from untreated eggs than to ducklings hatched from
boron-contaminated eggs.
Boron has also been shown to affect the behaviour of mallard
ducklings. Whitworth et al. (1991) analysed the activity schedules and
behaviour durations of day-old ducklings that received diets
containing 0, 100, 400, or 1600 mg boron/kg as boric acid. The highest
dose level had significant effects on the activity schedules of the
ducklings, including increased time at rest. Ducklings exposed to 1600
mg/kg in the diet spent less time in alert behaviours and in the water
compared with controls.
These studies suggest that high dietary levels of boron can
adversely affect normal duckling development and survival. In
addition, interactive effects have been demonstrated in mallard
ducklings given both boron and selenium in the diet. Hoffman et al.
(1991) noted further growth reduction in ducklings fed diets with 60
mg selenium/kg (as seleno-methionine) and 1000 mg boron/kg (as boric
acid) compared with ducklings fed diets with only 1000 mg boron/kg (as
boric acid) over a 4-week period. These effects were magnified when
dietary protein was restricted. Reduction of the dietary protein
content from 22% to 7% in combination with the same selenium and boron
exposure scenario as above resulted in significant duckling mortality.
These findings suggest that selenium and boron may interact to cause
more severe toxicological effects in waterfowl than boron causes
alone, especially in cases where dietary protein is restricted.
The liver boron content of mallards fed with dietary boron at 0,
30, 300, or 1000 mg/kg was found to range from 0 to 51 µg/g dry
weight. The level of 33-51 µg boron/g in liver was associated with
reproductive impairment in the 1000 mg/kg exposed group (Smith &
Anders, 1989).
Boron levels in the livers of aquatic birds from the Grassland
Water District of California, USA, were also examined during
1985-1988. The levels detected ranged from 1.7 to 40 µg boron/g dry
weight; the highest level detected is in a range associated with
reproductive impairment (Paveglio, 1992).
9.2 Field observations
9.2.1 Aquatic
Concentrations of boron in trout hatcheries of Germany, the
United Kingdom, and the USA (California) have been determined. Mean
concentrations of boron in feed waters to 20 United Kingdom rainbow
trout hatcheries were 0.009-0.021 mg/litre (ECETOC, 1997). A range of
0.002-0.107 mg boron/litre was noted in 18 hatcheries in Germany. A
range of higher concentrations, 0.02-1.0 mg boron/litre, was reported
for the 10 largest hatcheries in California (Bingham, 1982; EA, 1994).
Concentrations of boron in streams and lakes of the United
Kingdom and USA capable of sustaining trout species were found to be
similar to concentrations found in hatcheries. Median boron
concentrations from nine different water regions within the United
Kingdom ranged from 0.007 to 0.272 mg/litre, with maximum values in
each of the regions ranging from 0.113 to 2.3 mg boron/litre (ECETOC,
1997). Boron concentrations in California surface waters that
supported viable populations of wild rainbow trout ranged from <0.01
to 13.1 mg/litre (Bingham, 1982). An update to the Bingham report was
provided by a survey of 37 fisheries biologists in the western USA
(EA, 1994). No instances were found where rainbow trout populations
were limited by boron. Several locations were found to have successful
trout populations with aqueous boron concentrations near or above
1 mg/litre. Specific examples include East and Paulina lakes in Oregon
(>0.9 mg boron/litre), Firehole River in Wyoming (>0.9 mg
boron/litre), Napa River in California (>1.2 mg boron/litre), and
Little Warm Springs in California (>3.2 mg boron/ litre). The first
two sites are renowned trophy trout waters. Important fish species
other than trout, such as northern pike, sturgeon, and catfish, were
reported to live in streams with higher boron concentrations, up to
approximately 2 mg/litre, such as in the Poplar River in Montana and
Souris River in North Dakota. Neither of these latter rivers was
reported to contain rainbow trout owing to excessive temperatures and
unsuitable habitat (EA, 1994).
9.2.2 Terrestrial
Boron deficiencies in terrestrial plants have been reported in
many countries of the world (Kabata-Pendias & Pendias, 1984). For
example, deficiency has been reported in Canada, New Zealand, Sweden,
Nigeria, the United Kingdom, the USA, and some arid regions of India
and Pakistan. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions, because of boron's
susceptibility to leaching. Boron deficiency may also occur in
heavy-textured soils with high pH, because boron is readily adsorbed
under these conditions. Boron deficiency is more widespread than the
deficiency for any other micronutrient (Gupta et al., 1985).
When deficiency exists, boron applications increase yield and
improve quality of many crops (Gupta et al., 1985). Hopmans & Flinn
(1984) observed severe dieback in Monterey pine plantations during dry
years in south-eastern Australia owing to boron deficiency. Borax
applied in the spring at rates of 50, 100, or 150 kg/ha resulted in a
significant improvement in height growth of fertilized trees.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field. Few irrigation waters contain
enough boron to injure plants directly. However, it is the continued
use and concentration in the soil as a result of evapotranspiration
that lead to the eventual toxicity problems (Gupta et al., 1985). In
waters used for irrigation, Wilcox (1958) reported a critical boron
concentration of 0.3-1.0 mg/litre for sensitive crops, such as citrus
and other fruits. Semi-tolerant crops, such as potatoes, tomatoes, and
oats, tolerate concentrations of 1-2 mg/litre; tolerant crops, such as
sugar beets, onions, and carrots, can withstand concentrations of
2-4 mg/litre.
The symptoms of boron toxicity are predominantly manifested in
the leaves and roots; however, Eaton et al. (1941) observed that these
effects were frequently absent in stone fruit trees, with boron
accumulation occurring in the bark and fruits rather than the leaves.
Dye et al. (1984) reported that boron concentrations in bark of 31-120
µg/g dry matter and in fruit of 90-311 µg/g dry matter resulted in
bud-drop and branch die-back in peach trees. In nectarine trees with
boron tissue concentrations of 380-457 µg/g dry matter, the buds
failed to swell and subsequently dropped in the spring. Dye et al.
(1983) observed the same symptoms in apricot trees given about 2.4 g
of borax per tree during planting. Buds on unhealthy trees that failed
to break contained 40-100 µg boron/g dry matter.
Pollutant sources of boron, in the form of either airborne
emissions or leachates from soil application, have been shown to
produce toxic symptoms in endemic species. Smidt & Whitton (1975)
studied the toxic responses in a stand of Monterey pine adjacent to a
boiler house. The trees were exposed to boron as a result of dumping
of furnace ash into an adjacent gully and from airborne ash emitted
from the furnace chimney. The average boron concentration in the ash
was 1900 mg/kg. Chlorosis and necrosis of the needles were most severe
closest to the furnace chimney, with the effects decreasing with
increased distance from the furnace. No evidence of toxicity was
observed at needle boron concentrations of 13-70 µg/g, whereas toxic
symptoms were observed at needle boron concentrations of approximately
200 µg/g. The authors concluded that foliar absorption of airborne
boron, as well as root absorption from boron in soil solution,
resulted in the injury to this stand of trees.
Temple & Linzon (1976) reported that atmospheric boron emissions
from an appliance manufacturing plant and a fibreglass manufacturing
plant resulted in >35% foliar necrosis on vegetation within 700 m of
the plant, with 1-15% foliar necrosis occurring within 500 m in all
directions from the plant. The amount of leaf damage was proportional
to the boron concentration in the leaves. Species of maple
(Acer sp.) appeared to be the most sensitive, with slight necrosis
occurring at a concentration of 432 µg/g in unwashed foliage, moderate
necrosis at foliar concentrations ranging from 707 to 1013 µg/g, and
severe necrosis at foliar concentrations of 1207-1560 µg/g.
Lang et al. (1986) found that boron thresholds for detectable
levels (10%) of visible injury due to boron toxicity were about 500
mg/kg of foliage for both bigleaf maple and digger pine growing near
geothermal generating units.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health exposures
Boron is a naturally occurring element found combined with other
elements (primarily oxygen) throughout the environment. It is found in
the Earth's crust, with the majority of readily available forms
occurring in the ocean. It is estimated that more boron is released
into the environment by natural weathering than from anthropogenic
sources. Boron is not present in the atmosphere at significant levels.
Boron is present in surface water and groundwater and is readily
adsorbed on the surfaces of soil particles. Boron is present in food,
beverages, and drinking-water.
Boron has a wide variety of uses in the manufacture of glass,
glass products, antiseptics, cleaning products, pharmaceuticals,
biological growth control agents, wood and leather preservatives, and
insecticides. It is also used in photographic chemicals, enamels and
frits, and fire retardants.
Exposure to boron compounds can occur by inhalation, ingestion,
and dermal contact. Boron compounds are absorbed from the respiratory
and gastrointestinal tracts, as indicated by increased levels of boron
in the blood, tissues, and urine. Dermal absorption of boron across
intact skin is negligible.
Specific information on population exposures from different
countries was discussed in chapter 5. This information was used to
determine mean intakes in ambient air, drinking-water, soil, food, and
consumer products. From these mean intakes, per cent allocations of
the TIs can be developed, as shown in Table 22. In general, food and
drinking-water form the greatest contributions to boron intake in
humans, at about 65% and 30%, respectively.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data suggest that exposure is associated with short-term and
reversible irritant effects on the upper respiratory tract,
nasopharynx, and eye. The sole long-term (7-year) follow-up study
failed to identify any long-term health effects, although a healthy
worker effect cannot be entirely ruled out, given the rate of
attrition (47%). In two descriptive studies that assessed fertility
and secondary sex ratios in relation to exposure, no detrimental
effects on demonstrated fertility for the study samples were reported.
Although an excess percentage of female births has been suggested, the
absence of statistical significance and attention to other co-variates
known to affect sex ratios warrants careful interpretation of this
finding. No studies have been identified that assess the spectrum of
reproductive outcomes, such as time-to-pregnancy, conception delays,
spontaneous abortions, or semen quality. The role of other lifestyle
or behavioural factors in relation to health and fertility requires
further study to identify potentially sensitive populations and to
evaluate reproductive effects more fully.
Table 22. Estimation of exposures based on ranges of mean
concentrations in several environmental media on a global basis,
and allocation of the TI among media
Environmental media Mean intakea % allocation of TI
(mg/day)
Ambient air 0.000 44 Nil
Drinking-water 0.6b approx. 30
Soil 0.000 5 Nil
Food 1.2 approx. 65
Consumer products 0.1 5
Total 1.9 100
a Mean intakes are estimated in chapter 5.
b Assuming an intake of 2 litres of water per day.
10.2 Choice of critical effect and application of uncertainty factors
The critical effect appears to lie within the area of
developmental toxicity. Other effects, such as reproductive toxicity,
also occur at slightly higher doses and were considered in the choice
of critical effects through a comparison of LOAELs (Table 17) or
benchmark doses (BMDs) (Moore et al., 1997).a Several developmental
effects from boron exposure have been noted in rats, mice, and
rabbits. Again, these effects are the most sensitive in a larger
toxicity database that includes sub-chronic and chronic bioassays. The
specific critical effect within these developmental toxicity studies
is a decreased average fetal body weight per litter in the rat
(Heindel et al., 1992; Price et al., 1996a).
a The Weir & Fisher (1972) 2-year dog studies were not used directly
for risk assessment for several reasons. The NOAEL and LOAEL were
taken from two studies; one was 2 years long, and the high-dose group
was a supplemental group terminated at 38 weeks. There were a limited
number of animals (4/dose) per test group. One control group was used
for both borax and boric acid, so that there were never more than 2
control animals sacrificed at one time. Some of the control animals
showed some form of testicular damage.
In the Price et al. (1996a) study, boric acid was administered in
the diet to CD rats from gestational day 0 to 20 at 3.3, 6.3, 9.6,
13.3, or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls.
Fetal body weight was statistically significantly decreased only in
the 13.3 and 25 mg/kg body weight per day dose groups on gestational
day 20. The NOAEL for decreased fetal body weight was 9.6 mg boron/kg
body weight per day, and the LOAEL was 13.3 mg boron/kg body weight
per day. The results of this study provide a NOAEL and LOAEL that
complement the LOAEL of 13.6 mg boron/kg body weight per day in the
Heindel et al. (1992) study.b This NOAEL is also similar to BMDs for
developmental toxicity (see appendix).
For interspecies variation, a default value of 10 has often been
applied in the absence of actual data showing less than the usual
variation between species. For inter-individual (intraspecies)
variation, a default value of 10 has often been applied in the absence
of actual data within a species. A scheme has been adopted by IPCS
(1994) that allows for subdivision of each of these defaults to
incorporate appropriate data on toxicodynamics or toxicokinetics.
Where appropriate data exist, this scheme improves the extrapolation
process and uses correction factors for toxicodynamic and
toxicokinetic data instead of 10-fold uncertainty factors for inter-
and intraspecies variation. For interspecies differences, the 10-fold
factor is divided into a default factor of 100.4 for toxicodynamics
and 100.6 for toxicokinetics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.4 × 100.6 = 10).
For inter-individual (intraspecies) differences, the 10-fold
factor is divided into a default factor of 100.5 each for
toxicokinetics and toxicodynamics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.5 × 100.5 = 10).
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in blood and
body tissues of several mammalian species following ingestion.
b In fact, the Price et al. (1996a) study was conducted specifically
to address the lack of a NOAEL from the Heindel et al. (1992) study.
Similarity in study designs and results from these two studies greatly
strengthens the dose-response relationship for developmental toxicity
as the critical effect.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the NOAEL for laboratory studies, reduce
the uncertainty for risk extrapolation between these two species.
10.3 Derivation of the tolerable intake
A TI is defined as an estimate of the intake of a substance that
can occur over a lifetime without appreciable health risks. The TI is
derived on the basis of the NOAEL of the critical effect, the adverse
effects judged to be the most appropriate for determining the TI,
using appropriate uncertainty factors. The use of the critical effect,
judged here to be developmental toxicity, is expected to protect
against other effects such as reproductive toxicity occurring at
higher doses.
9.6 mg/kg body weight per day
TI =
[100.4 × 100.1] × [100.5 × 100.4]
= 0.4 mg/kg body weight per day
= 400 µg/kg body weight per day
where:
* 9.6 mg/kg body weight per day is the NOAEL from a well conducted
developmental study with decreased fetal body weight occurring in
a dose-related manner
* 100.4 × 100.1 (=100.5) is the uncertainty factor for interspecies
differences:
Dynamics: Data do not exist to support a factor other than the
default value of 100.4. Moreover, differences in LOAELs among
rats, rabbits, and mice for the critical effect (Table 16)
support the default value. The Task Group judged that 100.4 was
appropriate.
Kinetics: Oral absorption of boron in rats and humans is
quantitatively similar, with values of 94% for humans and 95% for
rats (section 6.1.1). The ratio of these absorption rates is
approximately 1.
Distribution of boron in rats and humans appears quantitatively
similar, as determined by a comparison of blood boron levels
after doses in either diet or drinking-water (Fig. 1, section
6.2.2). Ratios of rat blood boron values to a regression line for
human blood boron values are as low as 0.7 and as high as 6, with
the majority of values in the range of 2-3. Comparative
regression techniques should be pursued to confirm this trend.
Pregnant rats appear to have lower blood boron values than non-
pregnant rats when given similar doses. Comparative data for
pregnant and non-pregnant humans are lacking.
Metabolism of boron is thought not to occur in humans or animals
owing to the excessive energy required to break the boron-oxygen
bond (section 6.3). As it is unlikely that there are any
differences between species in the metabolism of boron, the ratio
of metabolic parameters is 1.
Elimination of boron in rats and humans appears quantitatively
similar, with half-life values in humans of 21 h in volunteers
and approximately 13 h in poisonings, and values in rats of
14=19 h (section 6.4). The ratio of these elimination half-life
values is approximately 1.3 when human volunteer data are
compared with the rat data, or 0.8 when human poisoning data are
compared with the rat data. The mean of these two ratios is
about 1.
As a result, the Task Group judged that the default value of
100.6 could be reduced to 100.1.
* 100.5 × 100.4 (=100.9) is the uncertainty factor for
inter-individual (intraspecies) differences:
Dynamics: Data in humans do not exist to support a value
different from the default of 100.5. Animal studies suggest that
intraspecies variability in toxicodynamics exists. The Task Group
judged that 100.5 was appropriate.
Kinetics: Data exist in humans to suggest some limited
variability in boron absorption and/or distribution (Nielsen,
1995). However, the lack of boron metabolism in humans and
experimental animals suggests some reduction in the default value
of 100.5. The Task Group judged that 100.4 was appropriate.
Other uncertainty factors for adequacy of database are not
considered necessary, because the overall database includes subchronic
and chronic studies on several species and several reproductive
studies.
The total uncertainty factor,a,b is therefore:
100.5 × 100.9 = 101.4 (or 25)
Data are inadequate to allow the development of a TI for
inhalation exposure (TIi).
10.4 Derivation of guidance values
Exposure to boron from various media varies with the locality. In
areas where data on exposure are available, specific guidance values
that are tailored to the local circumstances should be developed from
the TI presented in this document.
For example, guidance values for specific media could be
developed by first using the allocations shown in Table 22 with the TI
derived in section 10.3. Table 23 shows the resulting allocation of
the TI by media in mg/kg body weight per day.
The second step in the development of a guidance value would be
to pick appropriate body weight and exposure assumptions. Two examples
are given using allocations found in Table 23. The resulting guidance
value (GV) for example A in drinking-water would be:
a After closure of the Task Group meeting, Dr. M. Dourson, after
additional consideration, dissented from the Task Group's
recommendation on the uncertainty factor.
b Subsequent to the Task Group meeting, a provisional guideline for
boron in drinking-water was recommended by the Working Group Meeting
on Chemical Substances in Drinking-Water, using a different
uncertainty factor, based upon a different weighting attached to some
toxico-kinetic studies for that area of application (WHO, 1998 a,b).
Example A
0.12 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 4 mg/litre
where:
* 64 kg is the average human body weight (IPCS, 1994)
* 2 litres/day is one assumption of water intake (IPCS, 1994).
The resulting guidance value (GV) for example B would be:
Example B
0.04 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 1.3 mg/litre
Assumptions of food intake and consumer product use could be used
with allocations given in Table 23 to generate tolerances or other
risk management standards. Individual countries are encouraged to
develop guidance values using allocations, body weights, and exposure
assumptions that are appropriate for their populations.
Table 23. Examples of allocation of TI by media
Environmental Example A Example B
media
Per centa mg/kg body Per cent mg/kg body
weight per day weight per day
Ambient air Nil 0 Nil 0
Drinking-water 30 0.12 10 0.04
Soil Nil 0 Nil 0
Food 65 0.26 85 0.34
Consumer products 5 0.02 5 0.02
Total 100 0.4 100 0.4
a Values from Table 22.
10.5 Evaluation of effects on the environment
10.5.1 Exposure
Boron is not present in the atmosphere at significant levels;
however, the total amount present in the atmosphere at any one time is
significant owing to the huge volume of the atmosphere. Boron
concentrations in air range from <0.5 to approximately 80 ng/m3,
with an average of 20 ng/m3.
Boron occurs naturally in rocks, with concentrations ranging from
5 mg/kg in basalts to 100 mg/kg in shales. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron range widely, from 0.001 to as much as 360 mg/litre. However,
mean concentrations for waters of Europe, Pakistan, Russia, and Turkey
are typically well below 0.6 mg boron/litre. Concentrations of boron
in waters in Japan, South Africa, and South America are generally
below 0.3 mg/litre. Typical concentrations in North American waters
are below 0.1 mg boron/litre, with about 90% at 0.4 mg boron/litre or
below.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Boron occurs in soils at concentrations ranging from 10 to 300 mg/kg
(average 30 mg/kg), depending on the type of soil, amount of organic
matter, and amount of rainfall.
Boron does not bioaccumulate in aquatic invertebrates and fish.
Based on wet weights, boron concentrations in marine invertebrates and
fish are similar to the levels found in the exposure media, between
0.5 and 4 mg/kg. Bioconcentration factors for two freshwater fish
species were found to be 0.3. The results of studies of boron
accumulation in plants, insects, and fish have shown that boron
bioaccumulates in plants but does not biomagnify in aquatic
food-chains. Concentrations of boron have been shown to range between
26 and 382 mg/kg in submerged aquatic freshwater plants, from 11.3 to
57 mg/kg in freshwater emergent vegetation, and from 2.3 to 94.7 mg/kg
dry weight in terrestrial plants.
10.5.2 Effects
Boron is an essential micronutrient for cyanobacteria and
diatoms. Considering all microorganisms, algae and protozoa appear to
be equisensitive to boron and bacteria the most tolerant. Both algae
and protozoa provide NOEC (including EC3) values between 0.3 and 20
mg boron/litre.
Chronic studies with Daphnia magna provided consistent NOECs
between 6 and 10 mg boron/litre. Long-term outdoor pond and field
studies with microorganisms and invertebrates provided NOEC values up
to 1.52 mg boron/litre.
Via acute tests, the rainbow trout and zebra fish were determined
to be the most sensitive taxa, with acute values around 10 mg
boron/litre. Further tests with embryo-larval stages of several fish
species showed rainbow trout to be the most sensitive to boron. Tests
conducted with early life stages of rainbow trout in reconstituted
water overpredicted toxicity compared with tests conducted with
natural water. No adverse effects were found in natural water
exposures containing up to 0.75 mg boron/litre. Consistent LOECs were
between 1.1 and 1.7 mg boron/litre. These laboratory values were
confirmed by several observations of successful trout populations
thriving in hatcheries, streams, and lakes containing boron
concentrations up to 1 mg/litre.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
The symptoms of boron deficiency in plants include cessation of root
and leaf growth, necrosis, retardation of enzyme reactions, and
reduced pollen germination. The initial symptoms of boron toxicity in
plants are chlorosis, necrosis, and a subsequent loss of
photosynthetic capacity, which reduces plant productivity.
Mallard duckling growth was adversely affected at dietary levels
of 30 and 300 mg/kg, and survival was reduced at 1000 mg/kg.
10.5.3 Risk evaluation
Risk characterization is based on the comparison of the
environmental concentration with a concentration protective for an
ecosystem. Ambient concentrations of boron in surface waters have been
reported for several countries covering four continents. The data
indicate that mean or median levels are approximately 0.1 mg
boron/litre and that 90th-percentile or greater values are
approximately 0.5 mg boron/litre. Considerable data exist on the
toxicity of boron to freshwater organisms and ecosystems. Acute tests
with invertebrates and fish showed that rainbow trout and zebra fish
were most sensitive to borate (LC50 approx. 10 mg boron/litre).
Chronic results for algae, higher plants, and fish clearly showed that
the rainbow trout was the most sensitive species, with consistent
NOECs and LOECs of about 1 mg boron/litre. Confirmed were several
observations of healthy rainbow trout populations in US hatcheries and
streams at or above 1 mg boron/litre. Not surprisingly, biocenoses
(model ecosystems) covering all stages of the aquatic food-web
(excluding fish) were more tolerant of boron than were rainbow trout
(NOEC up to 1.52 mg boron/litre). Given all this information, the
comparison of the environmental effects concentration with the general
ambient environmental levels indicates that the risk of adverse
effects of boron on the aquatic ecosystem is low. In a few boron-rich
environments, natural levels will be higher. It is reasonable to
assume that aquatic organisms in such habitats may be adapted to the
local conditions.
Boron deficiencies in terrestrial plants have been reported in
many countries. Boron deficiency is more likely to occur in
light-textured, acid soil in humid regions, because of boron's
susceptibility to leaching. In general, there is a small range between
deficiency and toxicity. However, considerable variation exists
between species in their resistance to boron. Species sensitive to
boron are known to include citrus, stone fruits, and nut trees;
semi-tolerant species include tubers and cereals; and tolerant species
include most vegetables. Toxicity due to excess boron is much less
common in the environment than boron deficiency. Irrigation water is
one of the main sources of high boron levels that result in toxicity
in the field. However, few irrigation waters contain enough boron to
injure plants directly; it is the continued use and concentration in
the soil resulting from evapotranspiration that lead to the eventual
toxicity problems. Pollutant sources of boron, in the form of either
airborne emissions or leachates from soil application, have also been
shown to produce toxic symptoms in endemic plant species growing in
the immediate vicinity.
Levels of boron measured in aquatic plants from Kesterson
National Wildlife Refuge, California, USA (an evapotranspiration sink
that receives boron-rich run-off from irrigated agricultural fields)
are higher than those found to cause effects on mallard ducklings in
the laboratory. Therefore, there appears to be a risk to waterfowl in
this area. However, the bioavailability of such boron levels is
uncertain. The extent of risk to waterfowl in most geographical areas
is expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency has been observed in many countries throughout the
world. There is a small range between deficiency and toxicity in some
plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental boron levels
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre. For the
general population, the greatest boron exposure comes from the oral
intake of food. The mean daily intake of boron in the diet is
estimated to be near 1.2 mg/day.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. Greater than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The TI of boron was set as 0.4 mg/kg body weight per day. The
allocation of the TI in various media should be based on the exposure
data of individual countries.
11.2 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
12. FURTHER RESEARCH
a) Further studies regarding global cycling of boron.
b) Determine whether boron is required for normal fetal and post-
natal development for higher animals.
c) Define a biochemical function that confirms the essentiality of
boron for higher animals.
d) Determine the homeostatic mechanism through which boron
concentrations are maintained during low dietary intakes.
e) Further studies on the absorption, distribution, and clearance of
boron at oral doses in the range of 0.01-1 mg boron/kg body
weight per day.
f) A modern rat reproduction study to determine the potential for
developmental effects of borates in succeeding generations in a
sensitive animal species.
g) Analytical epidemiology studies to assess the spectrum of
reproductive and developmental outcomes to assess boron toxicity
in targeted human populations.
h) Epidemiological studies that incorporate toxicological and
physiological components to enhance an understanding of the
toxicokinetics and physiology of boron in populations, including
potentially sensitive ones.
i) Further quantitative definition of the maximum tolerable
concentration for sensitive aquatic species.
j) A refined understanding of the relationship of water and dietary
uptake of boron into waterfowl.
k) Development of an approach that integrates essentiality and
toxicity for the establishment of acceptable boron concentrations
in aquatic and terrestrial environments.
13. EVALUATIONS BY INTERNATIONAL BODIES
The EC Cosmetics Directive 76/768/EEC (and its subsequent
amendment) sets an upper limit of boric acid in cosmetic products,
which must not contain more than 5% in talc, 0.5% in oral hygiene
products, and 3% in other products. Only the talc containing boric
acid must, in addition, not be used on children less than 3 years of
age.
In 1997, subsequent to the Task Group meeting, a provisional
guideline for boron in drinking-water was recommended by the Working
Group on Chemical Substances in Drinking-Water, which updated the
Guidelines for drinking-water quality (WHO, 1998a,b).
REFERENCES
ACGIH (1991) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, pp 141-148.
Adams FS, Cole H Jr, & Massie LB (1973) Element constitution of
selected aquatic vascular plants from Pennsylvania: Submersed and
floating leaved species and rooted emergent species. Environ Pollut,
5: 117-147.
Ahl T & Jönsson E (1972) Boron in Swedish and Norwegian fresh waters.
Ambio, 1(2): 66-70.
Alabaster JS (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int Pest
Control, 11: 29-35.
Alexander GV, Nusbaum RE, & MacDonald NS (1951) The boron and lithium
content of human bones. J Biol Chem, 192: 489-496.
Allen BC, Strong PL, Price CJ, Hubbard SA, & Daston GP (1996)
Benchmark dose analysis of developmental toxicity in rats exposed to
boric acid. Fundam Appl Toxicol, 32: 194-204.
Anderson BG (1946) Industrial wastes: The toxicity thresholds of
various sodium salts determined by the use of Daphnia magna. Sewage
Works J, 18: 82-87.
Anderson DL, Kitto ME, McCarthy L, & Zollar WH (1994a) Sources of
atmospheric distribution of particulate and gas phase boron. Atmos
Environ, 28: 1401-1410.
Anderson DL, Cunningham WC, & Lindstrom TR (1994b) Concentrations and
intakes of H, B, S, K, Na, Cl and NaCl in foods. J Food Compos Anal,
7: 59-82.
Antia NJ & Cheng JY (1975) Culture studies on the effects from borate
pollution on the growth of marine phytoplankters. J Fish Res Board
Can, 32: 2487-2494.
Aquilio E, Spagnoli R, Seri S, Bottone G, & Spennati G (1996) Trace
element content in human milk during lactation of preterm newborns.
Biol Trace Elem Res, 51: 63-70.
ATSDR (1992) Toxicological profile for boron. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry.
Atteia O, Védy J-C, & Parriaux A (1993) Trace elements dynamics in
soils and aquifers of Western Switzerland. Stud Environ Sci Environ
Contam, 55: 79-101.
Aznarez J, Ferrer A, Rabadan JM, & Marco L (1985) Extractive
spectrophotometric and fluorimetric determination of boron with
2,2,4-trimethyl-1,3-pentanediol and carminic acid. Talanta, 32(12):
1156-1158.
Bai Y & Hunt CD (1996) Absorption and distribution of boron in rats
following a single oral administration of boron. Proc North Dakota
Acad Sci, 50: 53.
Banerji SK (1969) Boron adsorption on soils and biological sludges and
its effects on endogenous respiration. In: Proceedings of the 24th
Industrial Waste Conference, Lafayette, 6-8 May 1969. Lafayette,
Indiana, Purdue University (Engineering Extension Series No. 135).
Banuelos GS, Cardon G, Pflaum T, & Akohoue S (1992) Comparison of dry
ashing and wet acid digestion on the determination of boron in plant
tissue. Commun Soil Sci Plant Anal, 23(17-20): 2383-2397.
Barber LB, Thurman EM, Schroeder MP, & Le Blanc DR (1988) Long-term
fate of organic micropollutants in sewage-contaminated groundwater.
Environ Sci Technol, 22: 205-211.
Barnes DG, Datson GP, Evans JS, Jarabek AM, Kavlock RJ, Kimmel CA,
Park C, & Spitzer HL (1995) Benchmark dose workshop: Criteria for use
of a benchmark dose to estimate a reference dose. Regul Toxicol
Pharmacol, 21: 296-301.
Barr RD, Clarke WB, Clarke RM, Venturelli J, Norman GR, & Downing RG
(1993) Regulation of lithium and boron levels in normal human blood:
Environmental and genetic considerations. J Lab Clin Med,
121: 614-619.
Barres M (1967) Contribution à l'étude de l'isopoly--condensation des
borates alcalins par électrométrie et partages. Rev Chem Minér,
4: 803-838.
Barth RF & Soloway AH (1994) Boron neutron capture therapy of primary
and metastatic brain tumors. Mol Chem Neuropathol, 21(2-3): 139-154.
Barth RF, Adams DM, Soloway AH, Mechetner EB, Alam F, & Anisuzzaman KM
(1991) Determination of boron in tissues and cells using
direct-current plasma atomic emission spectroscopy. Anal Chem,
63: 890-893.
Belanger SE, Farris JL, & Cherry DS (1989) Effects of diet, water
hardness and population source on acute and chronic copper toxicity to
Ceriodaphnia dubia. Arch Environ Contam Toxicol, 18: 601-611.
Benson WH, Birge WJ, & Dorough HW (1984) Absence of mutagenic activity
of sodium borate (borax) and boric acid in the Salmonella
preincubation test. Environ Toxicol Chem, 3: 209-214.
Benson SM, White AF, Halfman S, Flexser S, & Alavi M (1991)
Groundwater contamination at the Kesterson Reservoir, California: 1.
Hydrogeologic setting and conservative solute transport. Water Resour
Res, 27: 1071-1084.
Bergmann W (1984) The significance of the micronutrient boron in
agriculture. London, Borax Holdings Limited, 26 pp.
Bergmann W, Bruchlos P, & Marks G (1995) [A contribution to the
question of the limit value for boron in water for Ogragnutes
australis seed.] Tenside Deterg, 32: 229-237 (in German).
Bertine KK & Goldberg ED (1971) Fossil fuel combustion and the major
sedimentary cycle. Science, 173: 233-235.
Beyer KH, Bergfeld WF, Berndt WO, Boutwell RK, Carlton WW, Hoffmann
DK, & Schroeter AL (1983) Final report on the safety assessment of
sodium borate and boric acid. J Am Coll Toxicol, 2: 87-125.
Biggar JW & Fireman M (1960) Boron adsorption and release by soils.
Soil Sci Soc Am Proc, 24: 115-120.
Bingham FT (1982) The boron concentration of wild trout streams in
California. Riverside, California, University of California,
Department of Soil Science (Unpublished document).
Bingham FT, Elseewi A, & Oertli JJ (1970) Characteristics of boron
absorption by excised barley roots. Soil Sci Soc Am Proc, 34: 613-617.
Bingham FT, Page AL, Coleman NT, & Flach K (1971) Boron adsorption
characteristics of selected amorphous soils from Mexico and Hawaii.
Soil Sci Soc Am Proc, 35: 546-550.
Birge WJ & Black JA (1977) Sensitivity of vertebrate embryos to boron
compounds. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Prepared for US EPA by the University of
Kentucky, Lexington) (EPA-560/1-76-008).
Birge WJ & Black JA (1981) Toxicity of boron to embryonic and larval
stages of largemouth bass (Micropterus salmoides) and rainbow trout
(Salmo gairdneri)--Completion report. Cincinnati, Ohio, Procter &
Gamble Company.
Birmingham DJ & Key MM (1963) Preliminary survey: U.S. Borax plant,
California. Cincinnati, Ohio, Occupational Health Research and
Training Facility, Division of Occupational Health.
Black JA, Barnum JB, & Birge WJ (1993) An integrated assessment of the
biological effects of boron to the rainbow trout. Chemosphere, 26:
1383-1413.
Blasser-Grill J, Knoppik D, Amberger A, & Goldbach H (1990) Influence
of boron on the membrane potential in Elodea densa and Helianthus
annus roots and H+ extrusion of suspension cultured Daucus
carota cells. Plant Physiol, 90: 280-284.
Blevins DG & Luk-aszewski KM (1994) Proposed physiologic functions of
boron in plants pertinent to animal and human metabolism. Environ
Health Perspect, 102(Suppl 7): 31-33.
Bodek I, Ehntholt EJ, Glazer AE, Loreti CP, & Lyman W (1988) Chapter
10: Chemical classes, Section 10.9: Boron-containing compounds. In:
Bodek I, Lyman WJ, Reehl WF, Rosenblatt DH, Walton BT, & Conway RA ed.
Environmental inorganic chemistry: Properties, processes, and
estimation methods. Oxford, New York, Pergamon Press,
pp 10.9/1-10.9/10.
Bonilla I, Garcia-Gomez M, & Mates P (1990) Boron requirements in
cyanobacteria: Possible role in the early evolution of photosynthetic
organisms. Plant Physiol, 94: 1554-1560.
Borkovec AB, Settepani JA, LaBrecque GC, & Fye RL (1969) Boron
compounds as chemosterilants for house flies. J Econ Entomol, 62:
1472-1480.
Bowen JE & Gauch HG (1966) Nonessentiality of boron in fungi and the
nature of its toxicity. Plant Physiol, 41: 319-324.
Boyd CE & Walley WW (1972) Studies of the biogeochemistry of boron. 1.
Concentrations in surface waters, rainfall and aquatic plants. Am Mid
Nat, 88: 1-14.
Bringmann G & Kuhn R (1977) [The toxicity of waterborne contaminants
towards Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in
German with English summary).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kuhn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G, Kuhn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa: III. Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German with
English summary).
Brown TF, McCormick ME, Morris DR, & Zeringue LK (1989) Effects of
dietary boron on mineral balance in sheep. Nutr Res, 9: 503-512.
Budavari S, O'Neil MJ, Smith A, & Heckelman PE ed. (1989) The Merck
index, 11th ed. Rahway, New Jersey, Merck and Co., Inc.
Bundschuh J (1992) Boron contamination of the ground- and surface
waters of Lerma Valley, Argentina. Aqua (Lond), 41(1): 13-17.
Butterwick L, De Oude N, & Raymond K (1989) Safety assessment of boron
in aquatic and terrestrial environments. Ecotoxicol Environ Saf, 17:
339-371.
Byrne RH Jr & Kester DR (1974) Inorganic speciation of boron in
seawater. J Mar Res, 32: 119-127.
Cáceres LV, Gruttner ED, & Contreras RN (1992) Water recycling in arid
regions: Chilean case. Ambio, 21(2): 138-144.
Canton JH & Adema DMM (1978) Reproducibility of short-term and
reproduction toxicity experiments with Daphnia magna and comparison
of the sensitivity of Daphnia magna with Daphnia pulex and
Daphnia cucullata in short-term experiments. Hydrobiologia,
59: 135-140.
Chapin RE, Ku WW, Kenney MA, McCoy H, Gladen B, Wine RN, Wilson R, &
Elwell MR (1997) The effects of dietary boron on bone strength in
rats. Fundam Appl Toxicol, 35: 205-215.
Choi WW & Chen KY (1979) Evaluation of boron removal by adsorption on
solids. Environ Sci Technol, 13: 189-196.
Clarke WB, Koekebakker K, Barr RD, Downing RG, & Fleming RF (1987a)
Analysis of ultratrace lithium and boron by neutron activation and
mass spectrometric measurement of 3He and 4He. Appl Rad Isot, 38(9):
735-743.
Clarke WB, Webber CE, & Koekebakker K (1987b) Lithium and boron in
human blood. J Lab Clin Med, 109: 155-158.
Corwin DL (1986) A one-dimensional model of chemical diffusion and
sorption in saturated soil and aquatic systems. J Environ Qual,
15: 173-182.
Cotton FA & Wilkinson G (1988) Advanced inorganic chemistry, 5th ed.
New York, John Wiley and Sons, pp 162-165.
Couch EL & Grim RE (1968) Boron fixation by illites. Clays Clay Miner,
16: 249-256.
Cox JA, Lundquist GL, Przyjazny A, & Schmulbach CD (1978) Leaching of
boron from coal ash. Environ Sci Technol, 12: 722-723.
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, &
Strong PL (1994a) The relationship of blood- and urine-boron to boron
exposure in borax-workers and the usefulness of urine-boron as an
exposure marker. Environ Health Perspect, 102(Suppl 7): 133-137.
Culver BD, Smith RG, Brotherton RJ, Strong PL, & Gray TM (1994b)
Chapter 44: Boron. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology. New York, John Wiley and Sons, vol 2, part 1.
Cumakov A (1991) Chemical methods of fractionation of soil trace
elements: 1. Boron. Chem Abstr, 115: 182048v.
De Jong LEDD (1965) Tolerance of Chlorella vulgaris for metallic and
non-metallic ions. Antonie van Leeuwenhoek, 31: 301-313.
Deverel SJ & Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, California. Environ Sci Technol, 22: 697-702.
Dieter MP (1994) Toxicity and carcinogenicity studies of boric acid in
male and female B6C3F1 mice. Environ Health Perspect, 102(Suppl 7):
93-97.
Dixon RL, Lee IP, & Sherins RJ (1976) Methods to assess reproductive
effects of environmental chemicals: Studies of cadmium and boron
administered orally. Environ Health Perspect, 13: 59-67.
Dixon RL, Sherins RJ, & Lee IP (1979) Assessment of environmental
factors affecting male fertility. Environ Health Perspect, 30: 53-68.
Doonan DJ & Lower LD (1978) Boron compounds (oxides, acid, borates).
In: Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New
York, John Wiley and Sons, vol 4, pp 67-110.
Draize JH & Kelley EA (1959) The urinary excretion of boric acid
preparations following oral administration and topical applications to
intact and damaged skin of rabbits. Toxicol Appl Pharmacol, 1:
267-276.
Dudas MJ (1981) Long-term leachability of selected elements from fly
ash. Environ Sci Technol, 15: 840-843.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1983) Die-back of
apricot trees following soil application of boron. NZ J Exp Agric, 11:
331-342.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1984) Boron toxicity
in peach and nectarine trees in Otaga. NZ J Exp Agric, 12: 303-313.
EA (1994) Boron concentrations and rainbow trout populations in seven
states in the western United States. Corvallis, Oregon, EA
Engineering, Science and Technology (Unpublished report prepared for
the Procter & Gamble Company, Cincinnati).
Eaton FM (1944) Deficiency, toxicity, and accumulation of boron in
plants. J Agric Res, 69: 237-277.
Eaton FM, McCallum RD, & Mayhugh MS (1941) Quality of irrigation
waters of the Hollister area of California with special reference to
boron content and its effect on apricots and prunes. US Dept Agric
Tech Bull, 746: 1-59.
ECETOC (1995) Reproductive and general toxicology of some inorganic
borates and risk assessment for human beings. Brussels, European
Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 63).
ECETOC (1997) Ecotoxicology of some inorganic borates - Interim
report. Brussels, European Centre for Ecotoxicology and Toxicology of
Chemicals, 97 pp (Special Report No. 11).
Eckel WP & Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
Superfund '88: Proceedings of the 9th national conference. Washington,
DC, US Hazardous Materials Control Research Institute, pp 282-286.
Eisler R (1990) Boron hazards to fish, wildlife, and invertebrates: A
synoptic review. Washington, DC, US Department of Interior, Fish and
Wildlife Service.
El-Hassanin AS, Labib TM, & Dobal AT (1993) Potential Pb, Cd, Zn and B
contamination of sandy soils after different irrigation periods with
sewage effluent. Water Air Soil Pollut, 66: 239-249.
Elrashidi MA & O'Connor GA (1982) Boron sorption and desorption in
soils. Soil Sci Soc Am J, 46: 27-31.
Emsley J (1989) The elements. Oxford, Clarendon Press, p 32.
Fail PA, George JD, Sauls SW, Dennis SW, & Seely JC (1989) Effect of
boric acid on reproduction and fertility of rodents. Adv Contracept
Deliv Syst, 5: 323-333.
Fail PA, George JD, Grizzle TB, Heindel JJ, & Chapin RE (1990) Final
report on the reproductive toxicity of boric acid (CAS No.
In a study of workers occupationally exposed to borate dust,
Culver et al. (1994a) compared the blood and urine boron levels from
workers with the amount of boron exposure (see section 5.3). An
average boron level of 0.26 µg/ml blood occurred in the highest
exposure group, where exposure was estimated to be 0.38 mg boron/kg
body weight per day. Post-shift blood and urine boron concentrations
did not increase with the days of the work week. This lack of
accumulation over the work week corroborates the relatively short
elimination half-life for boron in humans.
Elimination times for animals have not been explicitly stated in
the literature but can be either calculated or estimated from
published data. Farr & Konikowski (1963) measured urinary boron
concentrations in mice after intravenous injection of sodium
pentaborate. Using their data and assuming first-order kinetics for
elimination, the half-life for elimination in the mouse was on the
order of 1 h. Ku et al. (1991) reported that an apparent steady-state
level of boron is reached in the blood and tissues of rats after 3-4
days of oral dosing; again, assuming first-order kinetics, the
half-life in rats would be on the order of 14-19 h.
Male Fischer-344 rats were exposed to boric acid at 3000-9000
mg/kg in the diet for up to 9 weeks (Ku et al., 1993a). Urinary boron
was elevated (450-600 µg boron/mg creatinine) 24 h after the end of
dosing. By 3-4 days post-treatment, urinary boron in all groups had
returned to average control levels (8.97 ± 1.95 µg boron/mg creatinine
for days 1, 7, and 14 post-treatment). Elimination of boron from bone
followed a different time-course from elimination from serum or soft
tissues. F-344 rats consuming boric acid in the diet for 9 weeks
(approx. 1.4-6.8 mg boron/kg body weight per day) showed dose-related
elevation of bone boron, which declined very gradually during the
post-treatment period. Bone boron levels remained elevated above
controls in all exposed groups at 32 weeks post-treatment (Chapin et
al., 1997). Longer post-treatment intervals, as well as factors
influencing rates of accumulation or mobilization of boron in bone,
remain to be evaluated.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Short-term exposure
7.1.1 Oral route
The oral LD50 values for boric acid and borax in laboratory
animals are given in Table 14. Values for mice and rats are in the
range of approx. 400-700 mg boron/kg body weight (Pfeiffer et al.,
1945; Weir & Fisher, 1972). For guinea-pigs, Verbitskaya (1975)
reported an oral LD50 of 210 mg boron/kg body weight. Acute oral LD50
values in the range of 250-350 mg/kg body weight for boric acid or
borax exposure have also been reported for dogs, rabbits, and cats
(Pfeiffer et al., 1945; Verbitskaya, 1975).
General clinical effects of boric acid or borax in rats, mice,
and guinea-pigs given single large doses orally are depression,
ataxia, occasional convulsions, decreased body temperature, and
violet-red colour of skin and all mucous membranes (Pfeiffer et al.,
1945; Weir & Fisher, 1972). Toxic signs in dogs given boric acid
(0.2-2.0 g/kg body weight) orally in combination with subcutaneous
morphine to prevent vomiting were cyanosis of mucous membranes,
red-violet skin colour, rigidity of legs, convulsion, and shock-like
syndrome (Pfeiffer et al., 1945). Rabbits given boric acid at 800
mg/kg body weight per day for 4 days showed anorexia, weight loss, and
diarrhoea; 850 and 1000 mg/kg body weight per day for 4 days caused
100% mortality (Draize & Kelley, 1959). Cattle receiving 0.8, 150, or
300 mg boron/litre of drinking-water (as boric acid) for 30 days were
lethargic at the highest dose and had swelling and irritation in the
legs and around the dew claws, slight diarrhoea, and decreased food
consumption at the middle and high doses (Green & Weeth, 1977).
Following exposure to boric acid, microscopic changes in tissues
of mice, rats, and dogs involved primarily the kidneys and nervous
system (Pfeiffer et al., 1945). Glomerular and tubular damage were
noted in the kidneys. Glomerular damage consisted of changes in
permeability of the capillaries, and tubular damage consisted of
cellular vacuolization and shedding of cells into the tubular lumen.
Nervous system damage consisted of an increase in small dark cells
(probably microglia) in the spinal cord and in the grey matter of the
brain cortex.
7.1.2 Inhalation route
The LC50 for sodium perborate tetrahydrate by inhalation in rats
was >74 mg/m3 (Silaev, 1984). The 1-h inhalation LC50 values for
boron trichloride are reported as 12.2 g/m3 for male rats and 21.1
g/m3 for female rats; for boron trifluoride, the LC50 values range
from 0.89 to 1.2 g/m3 for rats (Vernot et al., 1977). While these
boron-halogen compounds are not considered in this report because of
apparent anion toxicity, it is appropriate to note in passing the
inhalation studies performed with them (Stokinger & Spiegl, 1953;
Rusch et al., 1986). These studies evaluated rats, mice, and
guinea-pigs and report various dose-related toxic effects beginning at
24 mg/m3.
Table 14. Acute toxicity of boron compounds in laboratory animals
Route Compound Species Compound LD50 Borona LD50
(mg/kg body weight) (mg/kg body weight) Reference
Oral Boric acid Mice 3450 603 Pfeiffer et al. (1945)
Borax Rats 3493 396 Wang et al. (1984)
Borax Rats 4500 510b Weir & Fisher (1972)
Borax Rats 4980 560b Weir & Fisher (1972)
Borax Rats 5660 642 Smyth et al. (1969)
Borax Rats 6080 690b Weir & Fisher (1972)
Boric acid Rats 2660 465 Pfeiffer et al. (1945)
Boric acid Rats 3160 550b Weir & Fisher (1972)
Boric acid Rats 3450 600b Weir & Fisher (1972)
Boric acid Rats 4080 710b Weir & Fisher (1972)
Boric acid Rats 5140 899 Smyth et al. (1969)
Subcutaneous Boric acid Mice 1740c 304 Pfeiffer et al. (1945)
Boric acid Mice 2070 362 Pfeiffer et al. (1945)
Boric acid Guinea-pigs 1200 210 Pfeiffer et al. (1945)
Intravenous Boric acid Mice 1780 311 Pfeiffer et al. (1945)
Boric acid Rats 1330 232 Pfeiffer et al. (1945)
a Calculated by multiplying the dose in mg boron compound/kg by the ratio of the molecular weights of boron/boron compound,
except when noted otherwise.
b Reported by investigators.
c Solution adjusted to pH 7.4 with sodium hydroxide.
7.2 Longer-term exposure
7.2.1 Oral route
The effects of longer-term oral exposure to boron compounds in
animals are summarized in Table 15.
In a 13-week study conducted by the US National Toxicology
Program (NTP, 1987; Dieter, 1994), B6C3F1 mice (10/sex per dose) were
exposed to boric acid in the diet at concentrations sufficient to
produce estimated consumptions of approximately 0, 34, 70, 141, 281,
or 563 mg boron/kg body weight per day for males and 0, 47, 97, 194,
388, or 776 mg boron/kg body weight per day for females. The results
showed that female mice were less sensitive than male mice to the
lethal effects of boric acid; 8/10 high-dose males and 6/10 high-dose
females died, and 1/10 males receiving 281 mg boron/kg body weight per
day died. Clinical signs of toxicity were a thin, hunched appearance,
dehydration, foot lesions, and scaly tails. A dose-related decrease in
body weight gain was observed. Microscopic effects included a
dose-related incidence of minimal to mild extramedullary
haematopoiesis of the spleen in males and females, hyperkeratosis and
acanthosis of the stomach in eight males and three females receiving
the highest dose, and testicular lesions; the testicular lesions are
described in greater detail in section 7.4.
Following this range-finding study, a 2-year study was conducted
in which mice (50/sex per dose) received approximately 0, 275, or 550
mg boric acid/kg body weight per day (0, 48.1, or 96.3 mg boron/kg
body weight per day) in the diet (NTP, 1987; Dieter, 1994). No
clinical signs of toxicity were observed. Body weights were 10-17%
lower in males after 32 weeks and in females after 52 weeks.
Non-accidental mortality at the end of the study was 9/50, 20/50, and
23/50 in control, low-, and high-dose males and 17/50, 15/50, and
12/50 in control, low-, and high-dose females. The increased mortality
rate was statistically significant in males. The only significant
lesions in male mice appeared in the testes; no significant
non-neoplastic lesions appeared in females.
Lee et al. (1978) fed borax in the diet to male Sprague-Dawley
rats (18/dose) at concentrations of 0, 500, 1000, or 2000 mg boron/kg
(equivalent to approximate doses of 0, 30, 60, or 125-131 mg boron/kg
body weight per day) for 30 or 60 days. Body weights were not
consistently affected by treatment; organ weights were not affected in
the 30 mg/kg body weight per day group. At 60 and 125-131 mg/kg body
weight per day, absolute liver weights were significantly lower after
60 days; epididymal weights were significantly lower (37.6% and 34.8%,
respectively) after 60 days, but not after 30 days. Weights of
prostate, spleen, kidney, heart, and lung were not changed at
any dose; reproductive effects observed in the study are discussed in
section 7.4.
Table 15. Effects of longer-term oral exposure to boron compounds in experimental animals
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Mice 0, 34, 70, 141, 281, Diet 13 weeks Over 60% mortality in both sexes NTP (1987)
563 for males; 0, 47, at 563 and 776 mg/kg body weight
97, 194, 388, 776 for per day; 10% in males at 281 mg/kg
femalesb body weight per day. At 141 mg/kg
body weight per day, degeneration
in seminiferous tubules and
decreased tubules in males and
decreased weight gain in males and
females. Extramedullary
haematopoiesis of the spleen in
all dosed groups, hyperkeratosis
and acanthosis of stomach at 563
and 776 mg/kg body weight per day.
Boric acid Mice 0, 48, 96 Diet 103 weeks Decrease in body weight (10-17%) NTP (1987)
in high-dose males after week 32
and in high-dose females after week
52. No clinical toxic signs observed.
Testicular atrophy and interstitial
cell hyperplasia were seen in males
at both levels. Dose-related increase
in incidence of splenic lymphoid
depletion in males. No other
significant increase in
non-neoplastic lesions.
Boric acid Rats 0, 22.7, 57c and Drinking-water 30 days Growth was not inhibited at the Pfeiffer et al.
higher low dose, but it was delayed at (1945)
>57 mg/kg body weight per day.
No haematological effects or
histological alterations.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Borax Rats 0, 0.056, 0.28, 2.8, Drinking-water <198 days Decrease in pancreas-to-body Wang et al.
28d weight ratio at all doses in (1984)
females at day 98. Increase in
pancreas-to-body weight ratio
in males at day 198. No details
given; normal histology.
Boric acid Rats 0.95, 3.65, 5.2, 9.9e Diet 8 weeks Decreased body weight at 5.2 Forbes & Mitchell
and 9.9 mg/kg body weight per (1957)
day. No other toxicity
end-points evaluated.
Borax or Rats 0, 2.6, 8.8, 26.3, Diet 90 days Mortality was 100% at the highest Weir & Fisher
boric acid 87.5, 262.5e dose; testicular atrophy at 87.5 (1972)
and 26.3 mg/kg body weight per
day; decrease in body weight and
in the weight of liver, kidney,
spleen, and testes at 87.5 mg/kg
body weight per day; weight
changes were inconsistent at
lower doses.
Borax or Rats 0, 5.9, 17.5, 58.5e Diet 2 years Both compounds suppressed growth Weir & Fisher
boric acid at 58.5 mg/kg body weight per (1972)
day. Testes weight and
testes-to-body weight ratios
were decreased, and brain-to-body
weight and thyroid-to-body weight
ratios were increased at 58.5
mg/kg body weight per day; also,
atrophy in seminiferous epithelium
and decrease in tubular size; no
effects observed at lower doses.
Table 15. (continued)
Compound Species Dosea (mg boron/kg Vehicle Duration Effects Reference
body weight per day)
Boric acid Rabbits 31 Oral gavage 5 days/week Elevated serum glutamic-oxaloacetic Verbitskaya
or borax for 4 months transaminase (SGOT) and serum (1975)
glutamic-pyruvic transaminase (SGPT)
levels, serum lactate dehydrogenase
and aldolase were transiently
increased, catalase and amylase were
decreased.
Sodium Rats 0, 3 g/litre sodium Drinking-water 14 weeks Increase in RNA concentration Settimi et al.
tetraborate tetraborate (boron and in succinate dehydrogenase (1982)
(not conversion not and acid proteinase activation
specified made due to in the brain. Decrease in
whether uncertainty NADPH-cytochrome reductase and
anhydrous or regarding in the content of cytochrome
decahydrate) compound) b5 and P-450 in the liver. No
effect on body or organ weight.
Sodium Mice 0, 0.95e Drinking-water Lifetime No effects on body weight or Schroeder &
metaborate longevity. Mitchener (1975)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of boron/boron compound.
b Estimated based on feed consumption values of 161 g/kg body weight per day for male and 222 g/kg body weight per day for female controls
at week 4 of treatment.
c Calculated based on water consumption of 0.12-0.14 ml/g per day reported by authors.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
e Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or food factor of
0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for mice.
In a 90-day study, Sprague-Dawley rats (10/sex per dose) received
0, 2.6, 8.8, 26.3, 87.5, or 262.5 mg boron/kg body weight per day in
the diet as boric acid or borax (Weir & Fisher, 1972). Similar effects
were observed with both boric acid and borax. All high-dose animals
died within 3-6 weeks. In animals receiving 87.5 mg boron/kg body
weight per day, body weights in males and females were reduced
43.8-54.9% and 10.1-12.6%, respectively. Absolute organ weights --
including the liver, spleen, kidneys, brain, adrenals, and ovaries --
in this dose group were also significantly decreased. Relative
organ-to-body weights of the adrenals and kidneys were significantly
increased, but relative weights of the liver and ovaries were
decreased. A pronounced reduction in testicular weights in males in
the 87.5 mg boron/kg body weight per day group was also observed;
effects on the male reproductive system are discussed further in
section 7.4.
In a 2-year study, rats (35/sex per dose) were administered
weight-normalized doses of 0, 5.9, 17.5, or 58.5 mg boron/kg body
weight per day in the diet as borax or boric acid (Weir & Fisher,
1972). High-dose animals had coarse hair coats, scaly tails, hunched
posture, swollen and desquamated pads of the paws, abnormally long
toenails, shrunken scrotum, inflamed eyelids, and bloody eye
discharge. These signs became frequent and more pronounced during the
first year but did not change thereafter. Serum chemistry and urine
values were normal; the haematocrit and haemoglobin levels were
significantly lower than in controls. The absolute and relative
weights of the testes were significantly lower, and relative weights
of the brain and thyroid gland were higher, than in controls. In
animals in the mid- and low-dose groups, no significant effects on
general appearance, behaviour, growth, food consumption, haematology,
serum chemistry, or histopathology were observed (Weir & Fisher,
1972).
Weir & Fisher (1972) also fed boric acid or borax to beagle dogs
for 90 days or 2 years. In the 90-day study (weight-normalized doses
of 0, 0.44, 4.38, or 43.75 mg boron/kg body weight per day;
5 animals/sex per dose), testicular effects were observed in males in
the two highest dose groups. In the boric acid study, testis weight
was significantly lower than controls in the middle- and upper-dose
groups (reduced by 25% and 40%, respectively). Although testicular
microscopic structure was not detectably abnormal in the controls and
middle-dose group, 4 of 5 dogs in the high-dose group had complete
atrophy, and the remaining high-dose dog had one-third of tubules
showing some abnormality. In the borax study, testis weights in the
low, middle-, and high-dose groups were 80%, 85%, and 50% of controls,
respectively; only the last was significantly different from controls.
No mention was made of the testicular microscopic structure of the
controls or low-dose animals; middle-dose animals were not detectably
altered (aside from the considerable fixation-induced artifact in the
outer third of the tissue), whereas 4 of 5 high-dose dogs had complete
testicular atrophy, and the remaining high-dose dog had "partial"
atrophy. No other clinical or microscopic signs of toxicity were
reported in any animals. In the 2-year study, the dogs (4/sex per
dose) received the boric acid or borax in the diet at
weight-normalized doses of 1.5, 2.9, or 8.8 mg boron/kg body weight
per day. An additional group received 29 mg boron/kg body weight per
day for 38 weeks. No effects were observed on general appearance, body
weight, food consumption, organ weights, haematology, or serum
chemistry. Changes in testicular morphology occurred in males in the
highest (38-week) dose group; these reproductive changes are described
in section 7.4.
Two reports are available on the toxic effects of boron on bones.
Seffner et al. (1990) exposed growing pigs to boron (4 or 8 mg/kg body
weight per day; source of boron was not specified). They reported a
dose-related thinning of the cortex of the humerus and a reduction
(significant at 8 mg/kg body weight per day) in presumptively
bone-derived serum alkaline phosphatase, suggesting reduced osteoblast
activity. Confidence in this study is moderate because of the
uncertainty of the form/source of boron and the authors' inability to
replicate several previous biochemical findings.
A second report investigated the effects of boric acid
(calculated exposures: <0.2-68 mg boron/kg body weight per day) on
several bone parameters in adult rats (Chapin et al., 1997). This
study found no change in physical bone measures (size, cortical
thickness, etc.) but reported a 5-10% increase in resistance of
vertebrae to a crush force.
7.2.2 Inhalation route
Mice exposed to amorphous elemental boron at 72 mg/m3 for
7 h/day, 5 days/week, for 6 weeks did not exhibit any toxic effects
(Stokinger & Spiegl, 1953). Wilding et al. (1959) conducted numerous
longer-term experiments in rats and dogs exposed to boron oxide
particles (median mass aerodynamic diameter [MMAD] 1.9-2.5 µm). The
exposures took place for 6 h/day and 5 days/week and included rats
exposed at 77 mg boric oxide/m3 for 24 weeks, 175 mg boric oxide/m3
for 12 weeks, or 470 mg boric oxide/m3 for 10 weeks; dogs were
exposed to 57 mg boron oxide/m3 for 23 weeks. No toxic effects were
observed, with normal values obtained for body weight gains, blood
chemistry, haematology, and urinalysis; there were also no microscopic
changes reported.
Subchronic inhalation studies have been performed with boron
trifluoride (Torkelson et al., 1961; Rusch et al., 1986) at
2-18 mg/m3. Reported effects included pneumonitis and reduced body
weight gains and organ weights.
7.3 Dermal and ocular effects
Animal studies show that boron oxide dust can affect the skin and
eyes. When boron oxide dust (50 mg) was applied to the eyes of
rabbits, conjunctivitis resulted (Wilding et al., 1959). Roudabush et
al. (1965) determined that boric acid (5 ml, 10% in water, w/v) and
borax (10 ml, 5% in water, w/v) were mild skin irritants after 24-72 h
following application to abraided skin. Borax was also a mild skin
irritant and boric acid a moderate irritant after 24 and 72 h when
applied to the guinea-pig. Rats fed 88 or 263 mg boron/kg body weight
per day as borax or boric acid had inflamed eyes and skin desquamation
on their paws and tails (Weir & Fisher, 1972).
7.4 Reproductive toxicity
Reproductive and developmental effects of borates in laboratory
animals are summarized in Table 16.
The data regarding subchronic and chronic oral exposure to boric
acid or borax in laboratory animals have unequivocally demonstrated
that the male reproductive tract is a consistent target of toxicity
(Table 16). Testicular lesions have been observed in rats, mice, and
dogs administered boric acid or borax in food or drinking-water
(Truhaut et al., 1964; Weir & Fisher, 1972; Green et al., 1973; Lee et
al., 1978; NTP, 1987; Ku et al., 1993a). The first clinical indication
of testicular toxicity in dogs is shrunken scrota observed during
treatment; significant decreases in absolute and relative testicular
weight are also reported. After subchronic exposure, the
histopathological effects range from inhibited spermiation (the
process of release of mature spermatids from the seminiferous
epithelium) to degeneration of the seminiferous tubules with variable
loss of germ cells, to complete absence of germ cells, resulting in
atrophy and transient or irreversible loss of fertility, but not of
mating behaviour.
Time- and dose-response studies of the Sprague-Dawley male rat
reproductive end-points after acute administration of boric acid
(Linder et al., 1990) revealed adverse effects on spermiation,
epididymal sperm morphology, and caput sperm reserves during
histopathological examinations of the testes and epididymis. Rats were
administered two doses in one day in both experiments, with a total
dose of 0 or 350 mg boron/kg body weight in the time-response
experiment where animals were sacrificed at 2, 14, 28, or 57 days
after exposure and a total dose of 0, 44, 87, 175, or 350 mg boron/kg
body weight in the dose-response experiment with animals sacrificed
after 14 days. In animals receiving 175 or 350 mg boron/kg body
weight, testicular effects apparent at 14 days were enlarged irregular
cytoplasmic lobes of mature spermatids in stage VIII seminiferous
tubules (Leblond & Clermont, 1952) and a substantial increase in the
testicular spermatid head count per testis. The first visible lesion
was inhibited spermiation. Epididymal effects after 14 days included
an increase in abnormal sperm forms, reduced caput epididymal sperm
morphology with head and tail defects, and reduced caput epididymal
sperm reserves. Epididymal sperm parameters had returned to normal and
only minimal testicular changes remained by post-treatment day 57,
indicating that these effects were reversible at these dose levels.
The NOAEL for male reproductive effects in this study was 87 mg
boron/kg body weight.
Table 16. Reproductive and developmental effects of boron compounds in laboratory animals
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid CD-1 0, 19.2, 104.5, Diet 27 weeks None of the high-dose pairs was Fail et al.
mice 220.2 for males; 0, fertile. At the middle dose, (1990, 1991)
31.8, 147.9, 290.2 there was a significant decrease
for females in litters/pair, live pups/litter,
percentage of pups born alive,
and live pup weight. Fertility
index was decreased at the middle
dose. Body weight gain was reduced
in males and females at the high
dose, despite increases in food
and water consumption. Cross-mating
experiments showed that boric acid
affected primarily the male
reproductive system. A dose level
of 220.2 mg/kg body weight per day
significantly reduced reproductive
organ weights in males and altered
sperm motility, concentration, and
morphology. At the high dose,
fertility of the low-dose F1 mice
was not affected, but marginally
reduced sperm concentrations were
seen in males; increases in uterus
and kidney plus adrenal weights
and shorter estrus cycles were
seen in females; and F2 live pup
weights were reduced.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid SD rats 0, 350 Gavage 2-57 days Inhibited sperm release, adverse Linder et al.
changes in sperm morphology; (1990)
reversible by day 57.
Boric acid Rats 0, 60.9b Diet 4-28 days Decreased weight gain; inhibition Treinen
of spermiation starting at day 7 and & Chapin
degeneration in seminiferous tubules (1991)
at day 28; decreased serum
testosterone starting at day 4; no
effects on liver or kidney histology.
Boric acid F-344 0, 26, 38, 52, 68 Feed Weekly to Mild inhibited sperm release at Ku et al.
rats 63 days 26 mg/kg body weight per day; (1993a)
severe inhibited sperm release at
38 mg/kg body weight per day;
progression to testicular atrophy
at 52 and 68 mg/kg body weight per
day, with many other changes
resulting from these primary
effects.
Boric acid Rats 175 Water 15 days Vacuolation and granulation of Silaev et al.
the cytoplasm and absence of (1977)
nuclear chromatin in spermatids
of seminiferous tubules.
Reduction in tubule diameter
and absence of germinative cells.
Borax or Rats 0, 5.9, 17.5, 58.5c Diet Multi- Sterility, lack of spermatozoa, Weir & Fisher
boric acid generation testicular atrophy, decreased (1972)
ovulation at 58.5 mg/kg body
weight per day. No effects at
lower doses.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 0.44, 4.4, 44c Diet 90 days One male dog died at 44 mg/kg Weir & Fisher
boric acid body weight per day; decrease (1972)
in thyroid- and testes-to-body
weight ratios, and severe
testicular atrophy in males at
44 mg/kg body weight per day.
At 0.44 mg/kg body weight per
day, decreased spleen-to-body
weight ratio in males. At
<4.4 mg/kg body weight per day,
no changes in organ weight in
females.
Borax Rats 0, 30, 60, 125-131 Diet 30 or 60 days At the 60 and 125-131 mg/kg Lee et al.
body weight per day doses, liver (1978)
weights were significantly lower
after 60 days; epididymal
weights were significantly lower
(37.6 and 34.8%) after 60 days.
FSH levels were elevated in all
treatment groups after 60 days,
and at the highest dose FSH
levels remained elevated after
12 months.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax Rats 0, 25, 50, 100c Diet 60 days Decrease in weights of liver, Dixon et al.
testes, and epididymis at the (1979)
two highest dietary levels.
Seminiferous tubule diameter
was decreased in a dose-related
manner in all groups. Loss of
germinal cell elements was seen
only in 50 and 100 mg/kg body
weight per day groups. Plasma
levels of FSH were elevated.
Reduced fertility only at two
highest dosage levels.
Borax Rats 0, 23.7, 44.7d Drinking-water 70 days Significant decrease in body Seal & Weeth
weight and decrease in weight (1980)
of testes, seminal vesicles,
spleen at both doses.
Spermato-genesis impaired and
haematocrit decreased slightly
at highest dose.
Borax Rats 0, 0.042, 0.14, 0.84b Drinking-water 90 days No observable reproductive Dixon et al.
effects or changes in serum (1976)
chemistry, body weight, or
weight of testes, prostate,
or seminal vesicles.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Borax or Dogs 0, 1.5, 2.9, 8.8, 29c Diet 2 years Severe testicular atrophy and Weir & Fisher
boric acid spermatogenic arrest at week (1972)
26 with 29 mg/kg body weight
per day; no effects on body or
organ weights, gross morphology,
or histological parameters at
lower doses.
Boric acid Mice 0, 43.4, 79.0, 175.3 Diet Gestation There was no effect on survival NTP (1989);
days 0-17 or pregnancy rate. Maternal Heindel et al.
effects included mild renal (1992)
lesions at >43.4 mg/kg body
weight per day and increased
water intake, increased relative
kidney weight, and decreased
weight gain during treatment at
175.3 mg/kg body weight per day.
At the high-dose level, there
was a significant increase in
the percentage of resorptions/
litter. Average fetal body
weight/litter was significantly
reduced at >79.0 mg/kg body weight
per day. Malformations included a
missing or shortened XIII rib and
variations on extra lumbar I
rib. Malformations were
significantly increased at
175.3 mg/kg body weight per day.
The percentage of variations/
litter decreased at <79.0 mg/kg
body weight per day and was not
affected at 175.3 mg/kg body
weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats 0, 13.6, 28.5, 57.7, Diet Gestation The highest dose was administered NTP (1990);
94.2 days 0-20 to one group on gestation days Heindel et al.
6-15. There was no effect on (1992)
survival or pregnancy rate.
Maternal rats showed increased
relative liver and kidney weights
at >28.5 mg/kg body weight per
day, increased absolute kidney
weight at 94.2 mg/kg body weight
per day, and a decrease in body
weight gain at >57.7 mg/kg body
weight per day. At low doses,
there was a decrease in extra
lumbar rib I (a variation) and
at high doses an increase in
missing or shortened XIII rib
(a malformation). The 94.2 mg/kg
body weight per day level
increased prenatal mortality.
Fetal body weights were
significantly reduced in all
treated groups in a dose-related
manner. Malformations increased
at >28.5 mg/kg body weight per
day; variations increased at
94.2 mg/kg body weight per day.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rats Phase 1 Diet Phase 1 Phase 1 -- Fetal body weight was
0, 3.3, 6.3, 9.6, gestation decreased on gestational day 20
13.3, 25 days (gd) in the 13.3 and 25 mg/kg body
0-20 weight per day dose groups. On
Phase 2 Phase 2 gd 20, incidences of short rib
0, 3.3, 6.5, 9.8, postnatal XIII or wavy rib were increased
12.9, 25.4 days (pn) in these dose groups relative
0-21 to controls. The high-dose group
(exposure contained a biologically
limited to relevant but not statistically
gestation significant decrease in
days 0-20) incidence of extra rib on
lumbar I.
Phase 2 -- On pn days 0-21, there Price et al.
were no decreased fetal body (1996a)
weight effects. On pn day 21,
the percentage of pups per
litter with short rib XIII was
elevated in the 25.4 mg/kg body
weight per day dose group. No
wavy rib or extra rib on lumbar
I was found at pn day 21.
Table 16. (continued)
Compound Species Dosea Vehicle Duration Effects Reference
(mg boron/kg body
weight per day)
Boric acid Rabbits 0, 10.9, 21.9, 43.7 Gavage Exposure on No treatment-related clinical Price et al.
in water gestation signs of toxicity except for (1996b)
days 6-19; vaginal bleeding at 43.7 mg/kg
termination body weight per day. This dose
on gd 30 had no live fetuses on day 30.
Food intake decreased during
treatment (43.7 mg/kg body
weight per day), but increased
on days 25-30 (21.9 and 43.7
mg/kg body weight per day). Body
weight and body weight gain were
decreased during treatment at
43.7 mg/kg body weight per day,
but corrected body weight
increased at 21.9 and 43.7 mg/kg
body weight per day. Gravid
uterine weight and number of
corpora lutea/dam were decreased
at 43.7 mg/kg body weight per
day. Significant developmental
effects were limited to the
high-dose group. Prenatal
mortality was greatly increased.
Malformations were also
increased, primarily owing to
the incidence of fetuses with
cardiovascular defects. The
skeletal variation observed
was sternebrae. Fetal body
weight was slightly reduced
in the high-dose group.
Table 16. (continued)
a Calculated by multiplying the dose in mg boron compound/kg body weight per day by the ratio of molecular weights of
boron/boron compound.
b Estimated by the authors.
c Calculated by assuming reference values of 0.35 kg body weight and daily water consumption of 0.049 litre for rats, or
food factor of 0.05 for rats and 0.025 for dogs, or 0.03 kg body weight and daily water consumption of 0.0057 litre for
mice.
d Calculated by using assumed body weight of 0.35 kg and reported daily water consumption of 19.5 ml.
In a 2-year study by Weir & Fisher (1972), groups of 4 male and 4
female beagle dogs were fed diets containing boric acid or borax to
provide doses of 0, 1.45, 2.93, or 8.75 mg boron/kg body weight per
day. No evidence of toxicity was observed. An additional group of dogs
(4 male and 4 female) was fed diets containing boric acid or borax
leading to doses of 0 or 29.3 mg boron/kg body weight per day for 38
weeks. Testicular atrophy was observed in 2 test dogs receiving borax
at 26 weeks. The authors stated that boric acid caused testicular
degeneration in dogs, including spermatogenic arrest and atrophy of
the seminiferous epithelium. This study was terminated at 38 weeks. In
the study, the number of dogs was small and variable (1-2 dogs at each
of three time points) and inadequate to allow statistical analysis. A
common control group was used for both borax and boric acid exposure
groups. Testicular lesions occurred in the controls (1 of 4 controls
had slight to severe seminiferous tubular atrophy, another had
moderate to severe atrophy, whereas a third had a detectable but
insignificant reduction in spermatogenesis and 5% atrophic
seminiferous tubules). This study was conducted before the advent of
Good Laboratory Practices (GLP). Confidence in this study is low, and
it was considered not suitable for inclusion into the risk assessment
because of 1) small and variable numbers of dogs, 2) variable
background lesions in controls leading to uncertainty regarding the
strength of the response to treatment, 3) lack of GLP, and 4) other,
more recent studies of greater scientific quality with findings at a
similar or lower intake level of boron (Ku et al., 1993a; Price et
al., 1996a).
Weir & Fisher (1972) fed Sprague-Dawley rats (35/sex per group) a
diet containing borax or boric acid for 2 years at 0, 5.9, 17.5, or
58.5 mg boron/kg body weight per day. At the high dose, rats receiving
either compound had decreased food consumption during the first 13
weeks of study and decreased growth throughout the study. Testes
weights were significantly decreased at 58.5 mg/kg body weight per
day. The seminiferous epithelium was atrophied, and the tubular size
in the testes was decreased. No treatment-related effects were
observed in rats receiving 5.9 or 17.5 mg boron/kg body weight per
day. The LOAEL in this study was 58.5 mg boron/kg body weight per day
for decreased testes weights, atrophied testes, histopathological
alterations of the testes, and increased brain and thyroid weights.
The NOAEL for this study was 17.5 mg boron/kg body weight per day.
In a companion three-generation reproduction study in rats, Weir
& Fisher (1972) found that 58.5 mg boron/kg body weight per day
produced testicular atrophy and complete suppression of fertility in
rats. Lower doses (17.5 or 5.9 mg boron/kg body weight per day) did
not reduce fertility. The small group size (n = 8), low control
fertility rates (approx. 60%), limited data reported, and
inappropriate statistics all limit the applicability of these data for
risk assessment.
In a multigeneration continuous-breeding experiment (Fail et al.,
1990, 1991), Swiss CD-1 mice (F0 generation) were fed boric acid in
the diet at 0, 1000, 4500, or 9000 mg/kg feed for 27 weeks, which gave
calculated doses of 0, 19.2, 104.7, and 222.1 mg boron/kg body weight
per day for males and 0, 31.9, 148.1, and 290.5 mg boron/kg body
weight per day for females. Treatment with boric acid significantly
impaired fertility: no males or females in the high-dose groups were
fertile. At the middle dose, the number of litters per pair, number of
live pups per litter, proportion of pups born alive, and pup weight
adjusted for litter size were all decreased. The trend towards a lower
fertility index at this dose level started with the first litter and
progressed in severity with subsequent matings. Animals from different
treatment groups were cross-mated to determine the affected sex at
this dose. When mid-dose males were mated with control females, mating
and fertility indices were significantly depressed, with only one pair
in that group producing a live litter; these indices were not affected
when control males were mated with mid-dose females, confirming that
the male was the affected sex. At F0 necropsy, sperm motility was
significantly reduced in all exposed groups (by 12%, 32%, and 47%,
from low- to high-dose groups, respectively). Low-dose and mid-dose
animals from the F1 generation were exposed during gestation and
lactation. The fertility of the low-dose F1 mice was not affected,
but the litter-adjusted body weights of the F2 pups were
significantly decreased (by 3.3%) relative to controls. The low dose
was considered the LOAEL for decreased sperm motility in the F0
males, 26% increased uterine weight and 8% increased kidney/adrenal
weight in F1 females, and a 3.3% reduction in litter-adjusted birth
weight in the F2 pups. This study had no NOAEL, but the magnitude of
the changes at the low dose (a 12% decrease in F0 sperm motility, an
8% increase in F1 kidney/adrenal weights, and a 3% reduction in F2
pup weights) is small and indicates that this dose level is close to
the NOAEL.
Fail et al. (1989) utilized both CD-1 mice and wild deer mice
(Peromyscus maniculatus) to characterize the effects of boric acid
on fertility and to test the reversibility of these effects. Adult
CD-1 mice were exposed to boric acid in the feed for 27 weeks at 0,
1000, 4500, or 9000 mg/kg (consumed doses were not given). The males
at the high and middle doses had testicular atrophy and decreased
spermatogenesis. Fertility was diminished in animals receiving the
middle dose and completely absent in the high-dose group. The same
doses were used to test toxicity and reversibility in the wild mouse.
The ability to produce offspring was significantly reduced by boric
acid: none of the males in the high-dose group sired litters. Weight
gain was similar for the treated and control wild mice. Although body
and organ weights were similar between the mid-dose and control groups
at necropsy, the testis and total accessory sex organ weights were
significantly lower in the high-dose group. These two species appear
to differ in their sensitivity to boric acid. The CD-1 mice had body
weight loss as early as 5 weeks at the mid-dose level, whereas deer
mice did not lose weight even at the high dose. Tissue pathology
findings at the middle dose were significant in CD-1 mice but not in
deer mice. Deer mice were found to recover from boric acid exposure.
The development of the testicular lesion was investigated by
Treinen & Chapin (1991), who fed boric acid at a level of 0 or 60.9 mg
boron/kg body weight per day to male F-344 rats and sacrificed six
treated and four control male rats at intervals from 4 to 28 days
after the start of exposure. In half of the treated rats, there was
inhibition of spermiation in 10-30% of stage IX tubules at 7 days and
inhibition in all stage IX and stage X tubules of exposed rats at 10
days. At 28 days, there was significant loss of spermatocytes and
spermatids from all tubules in exposed rats, and basal serum
testosterone levels were significantly decreased from 4 days on.
Secondary to the loss of germ cells, the activities of enzymes
found primarily in spermatogenic cells were significantly decreased,
and enzyme activities associated with premeiotic spermatogenic cells
were significantly increased in Sprague-Dawley rats exposed to 60 or
125-131 mg boron/kg body weight per day for 30 or 60 days (Lee et al.,
1978). Mean plasma follicle stimulating hormone (FSH) levels were
significantly elevated in a dose-dependent manner in all treatment
groups in this study (30 to 125-131 mg boron/kg body weight per day)
after 60-day exposures. FSH levels in animals exposed to the highest
dose tested (125-131 mg boron/kg body weight per day) were still
elevated 12 months after treatment termination, owing to atrophied
testes and no recovery of spermatogenesis. Plasma luteinizing hormone
(LH) levels were not significantly elevated, and mean plasma
testosterone levels were within the normal range throughout the study
(Lee et al., 1978).
Reversibility of testicular lesions was evaluated by Ku et al.
(1993a) in an experiment in which F-344 rats were dosed at 3000, 4500,
6000, or 9000 mg boric acid/kg (26, 38, 52, and 68 mg boron/kg body
weight per day) in the feed for 9 weeks and assessed for recovery up
to 32 weeks post-treatment. Inhibited spermiation was exhibited at
3000/4500 mg boric acid/kg (5.6 µg boron/mg tissue), whereas inhibited
spermiation progressed to atrophy at 6000/9000 mg boric acid/kg (11.9
µg boron/mg testes), with no boron accumulation in the testes to
levels greater than found in blood during the 9-week period. After
treatment, serum and testis boron levels in all dose groups fell to
background levels. Inhibited spermiation at 4500 mg/kg was reversed by
16 weeks post-treatment, but focal atrophy was detected that did not
recover up to 32 weeks post-treatment.
An unpublished study by W.W. Ku and colleagues (reported in Ku et
al., 1993a) found no detectable treatment-related changes in
testicular structure in rats induced by consumption of 17.5 mg
boron/kg body weight per day for up to 9 weeks.
In a follow-up study to explore/identify the mechanism for this
testicular toxicity of boric acid, Ku et al. (1993b) evaluated several
end-points in cell culture systems following in vitro boric acid
exposure. The data suggest an effect of boric acid on the DNA
synthesis activity of mitotic and meiotic germ cells and, to a lesser
extent, on energy metabolism in Sertoli cells. The effect on DNA
synthesis occurred at boron concentrations that were associated with
atrophy in vivo and shows that boric acid interferes with the
production and/or maturation of early germ cells; this offers an
explanation for atrophy, but not for inhibited spermiation.
Additional mechanistic studies by Ku & Chapin (1994) showed that
testicular toxicity and CNS hormonal effects were not due to selective
boron accumulation in testis or brain/hypothalamus, with testis boron
concentrations at 1-2 mmol/litre. Changes in testis phosphorus,
calcium, and zinc levels did not precede atrophy. In vitro studies
showed no effect on the steroidogenic functions of isolated Leydig
cells, supporting the suggestion of a CNS-mediated hormonal effect.
The authors showed that inhibited spermiation was not due to increased
testicular cyclic adenosine monophosphate or reduced serine protease
plasminogen activators. Also evaluated were effects of boric acid in
Sertoli-germ cell co-cultures on Sertoli cell energy metabolism
(lactate secreted by Sertoli cells is a preferred energy source for
germ cells) and DNA/RNA synthesis (germ cells synthesize DNA/RNA, and
boric acid impairs the synthesis of these nucleic acids in the liver).
The most sensitive in vitro end-point was DNA synthesis in
mitotic/meiotic germ cells; energy metabolism in germ cells was
affected to a lesser extent, which was manifested in vivo as a
decrease in early germ cell/Sertoli cell ratio prior to atrophy in the
testes. The mechanisms of inhibited spermiation are still not defined.
In summary, male reproductive effects from boron have been noted
in mice, rats, and dogs. Reproductive effects observed include
inhibition of spermiation in stage IX and X tubules, followed by germ
cell loss, changes in epididymal sperm morphology and caput sperm
reserves, decreased serum testosterone levels, and testicular atrophy.
Male reproductive effects have been reported in oral exposure studies
at doses as low as 29 mg boron/kg body weight per day in dogs exposed
for 2 years to dietary boric acid or borax (Weir & Fisher, 1972); for
rats in this same study, the LOAEL for reproductive toxicity was 58.5
mg boron/kg body weight per day.
7.5 Developmental toxicity
Developmental toxicity has been demonstrated experimentally in
rats, mice, and rabbits (see Table 16) (Heindel et al., 1992; Price et
al., 1996b).
Sprague-Dawley rats were fed a diet containing 0, 13.6, 28.5, or
57.7 mg boron/kg body weight per day as boric acid from gestation days
0 to 20 (Heindel et al., 1992). An additional group of rats received
boric acid at 94.2 mg boron/kg body weight per day on gestation days
6-15 only. Maternal effects included a significant and dose-related
increase in relative liver and kidney weights at >28.5 mg boron/kg
body weight per day. Treatment with 94.2 mg boron/kg body weight per
day significantly increased prenatal mortality. Average fetal body
weight per litter was significantly reduced in a dose-related manner
in all treated groups compared with controls. The percentage of
malformed fetuses per litter and the percentage of litters with at
least one malformed fetus were significantly increased at >28.5 mg
boron/kg body weight per day. Malformations consisted primarily of
anomalies of the eyes, the CNS, the cardiovascular system, and the
axial skeleton. The most common malformations were enlargement of
lateral ventricles in the brain and agenesis or shortening of rib
XIII. The percentage of fetuses with variations per litter was reduced
relative to controls at 13.6 and 28.5 mg boron/kg body weight per day
(due to a reduction in the incidence of rudimentary or full ribs at
lumbar 1) but was significantly increased in rats exposed to 94.2 mg
boron/kg body weight per day. The variation with the highest incidence
among fetuses was wavy ribs. The LOAEL of 13.6 mg boron/kg body weight
per day (lowest dose tested) for rats occurred in the absence of
maternal toxicity; a NOAEL was not found in this study.
Price et al. (1996a) did a follow-up to the Heindel et al. (1992)
study in Sprague-Dawley (CD) rats to determine a NOAEL for fetal body
weight reduction and to determine whether the offspring would recover
from prenatally reduced body weight during postnatal development.
Skeletal malformations and variations were also studied to further
characterize the low end of the dose-response curve (phase 1) and to
determine whether the incidence of skeletal defects in offspring
changed during postnatal life (phase 2). Boric acid was administered
in the diet to CD rats from gestational day 0 to 20. In phase 1, dams
were terminated and uterine contents examined on gestational day 20.
During phase 1, the intake of boric acid was 0, 3.3, 6.3, 9.6, 13.3,
or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls;
the reduction was significant only in the 13.3 and 25 mg boron/kg body
weight per day dose groups on gestational day 20. During phase 1,
incidences of short rib XIII (a malformation) and wavy rib (a
variation) were increased in the >13.3 mg boron/kg body weight per
day dose groups relative to control litters. There was a decreased
incidence of rudimentary extra rib on lumbar 1 (a variation) in the
high-dose group that was deemed biologically but not statistically
significant. During phase 2, the intake of boric acid during gestation
was 0, 3.3, 6.5, 9.8, 12.9, or 25.3 mg boron/kg body weight per day.
At birth, boric acid exposure stopped and dams were allowed to deliver
and rear their litters until postnatal day 21. On postnatal day 0 of
phase 2, there were no effects of boric acid on offspring body weight,
nor were any differences seen through postnatal day 21. On postnatal
day 21 of phase 2, the percentage of pups per litter with short rib
XIII was elevated only in the 25.3 mg boron/kg body weight per day
dose group, but there was no treatment-related increase in wavy rib or
extra rib (full or rudimentary) on lumbar 1 observed in these pups on
day 21. The NOAEL for phase 1 of this study is 9.6 mg boron/kg body
weight per day based on a decrease in fetal body weight. The LOAEL was
13.3 mg boron/kg body weight per day for phase 1. The NOAEL for phase
2 was 12.9 mg boron/kg body weight per day, and the LOAEL was 25.3 mg
boron/kg body weight per day. The results of this study provide a
NOAEL and LOAEL that complement the rat LOAEL of 13.6 mg/kg body
weight per day in the Heindel et al. (1992) study.
Heindel et al. (1992) also investigated the developmental
toxicity and teratogenicity of boric acid in mice at 0, 43, 79, or 175
mg boron/kg body weight per day in the diet. There was a significant
dose-related decrease in average fetal body weight per litter at 79
and 175 mg boron/kg body weight per day. In offspring of mice exposed
to 79 or 175 mg boron/kg body weight per day during gestation days
0-17, there was an increased incidence of skeletal (rib)
malformations. These changes occurred at doses for which there were
also signs of maternal toxicity (increased kidney weight and
pathology); the LOAEL for developmental effects (decreased fetal body
weight per litter) was 79 mg boron/kg body weight per day, and the
NOAEL for developmental effects was 43 mg boron/kg body weight per
day.
Price et al. (1996b) investigated the developmental toxicity and
teratogenicity of boric acid in rabbits at doses of 0, 10.9, 21.9, or
43.7 mg boron/kg body weight per day given by gavage. Frank
developmental effects in rabbits exposed to 43.7 mg boron/kg body
weight per day included a high rate of prenatal mortality, increased
number of pregnant females with no live fetuses, and fewer live
fetuses per live litter on day 30. Also at 43.7 mg boron/kg body
weight per day, malformed live fetuses per litter increased
significantly, primarily because of the incidence of fetuses with
cardiovascular defects, the most prevalent of which was
interventricular septal defect. Skeletal variations observed were
extra rib on lumbar 1 and misaligned sternebrae. The NOAEL for
maternal and developmental effects was 21.9 mg boron/kg body weight
per day, and the LOAEL for maternal and developmental effects was 43.7
mg boron/kg body weight per day.
7.6 Mutagenicity and related end-points
Boric acid was not mutagenic in Salmonella typhimurium with or
without rat or hamster S9 fraction (Haworth et al., 1983; Benson et
al., 1984; NTP, 1987) or in mouse lymphoma L5178Y/TK+/ cells with or
without rat liver S9 (NTP, 1987; McGregor et al., 1988). Borax was not
mutagenic in Salmonella with or without rat liver S9 (Benson et al.,
1984). Refined borax, crude borax ore, and kermite ore were not
mutagenic in V79 Chinese hamster cells, C3H/1OT1/2 mouse embryo
fibroblasts, or diploid human foreskin fibroblasts (Landolph, 1985).
Sodium perborate (NaBO3) was shown to interact with DNA in the
Escherichia coli Pol A assay, presumably by being converted to
hydrogen peroxide (Rosenkranz, 1973). Other tests showed that boric
acid did not induce chromosomal aberrations or sister chromatid
exchanges in Chinese hamster ovary cells (NTP, 1987). Existing data
suggest that genotoxicity is not an area of concern following exposure
to boron compounds in humans.
7.7 Carcinogenicity
The 2-year feeding study (NTP, 1987; Dieter, 1994) showed no
evidence of carcinogenicity in B6C3F1 mice. The Weir & Fisher (1972)
study showed no evidence of boric acid-related carcinogenicity in
rats, although not all tissues were examined. Based on the lack of
human data and on the data from these two animal tests, boron is
classified by the US EPA as a Group D chemical (not classifiable as to
human carcinogenicity) (US EPA, 1994).
7.8 Toxicity effects summary
The laboratory animal toxicity data show that boric acid (and
other borates) is not a carcinogen and lacks significant mutagenicity.
The crossover mating trial in the Fail et al. (1991) study showed that
female mouse reproduction was unaffected by about 120 mg boron/kg body
weight per day. The NOAELs and LOAELs of reproductive/developmental
effects from various studies are ranked in Table 17. The Weir & Fisher
(1972) dog study is omitted from this summary because of low numbers
of animals and the presence of lesions in the controls. It can be seen
that rat fetal development is more sensitive than other processes to
the adverse effects of elevated boron exposure, with a LOAEL in rats
of 13.3 mg boron/kg body weight per day reducing fetal weight gain and
increasing rib anomalies (reversibly). The current NOAEL for these
effects in rats is approximately 10 mg boron/kg body weight per day.
Increased doses produced sequentially additional effects on sperm
release, increased rabbit cardiovascular defects, reduced epididymal
sperm counts, testicular atrophy, and mouse fetal defects.
The Task Group recognized the lack of a NOAEL in the Fail et al.
(1991) study. The effects of concern in part duplicated the
developmental toxicity findings (reduced pup body weight). However,
the unique effects in the adult F1 female mice (increased uterine
weight, reduced cycle length) are consistent with an effect on female
reproduction that was not seen in the F0 females. The possibility of
unique transgenerational or functional developmental toxicity in the
absence of an available dose-response concerned the Task Group.
Although the current NOAEL is based on rat fetal body weight
effects, the Task Group noted that this could be lowered and a new
"critical effect" could emerge if the significant data gap
(second-generation fertility and necropsy data for multiple doses
including a NOAEL in rats) were filled.
7.9 Physiological effects
Since 1981, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for higher animals;
that is, a dietary deprivation of boron consistently results in
changed biological functions that can be construed as detrimental and
are preventable or reversible by an intake of physiological amounts of
boron. Convincing findings have been reported that show that dietary
boron deprivation affects mineral and energy metabolism in chicks and
Table 17. Ranking of reproductive/developmental effects of boric acid
Species/durationa Dose (mg boron/kg Effect Reference
body weight per day)
SD rat/gd 0-20 9.6 NOAEL for developmental effects immediately pre-term Price et al. (1996a)
SD rat/gd 0-20 12.9 NOAEL for developmental effects measured at weaning Price et al. (1996a)
SD rat/gd 0-20 13.3 LOAEL for reduced fetal weight, increased rib Price et al. (1996a)
13.6 malformations/variations Heindel et al. (1992)
Male SD rat/multigeneration 17.5 NOAEL for male sterility, testicular atrophy Weir & Fisher (1972)
SD rat/gd 0-20 25.4 LOAEL for increased short rib XIII at weaning Price et al. (1996a)
Male SD rat/63 days 26 LOAEL for mild inhibited sperm release Ku et al. (1993a)
Male SD rat/¾63 days 52 LOAEL for testicular atrophy Ku et al. (1993a)
Male beagle dogs/2 years 29 Altered testis weight and histopathology Weir & Fisher (1972)
Male beagle dogs/90 days 44 Altered testis weight and histopathology Weir & Fisher (1972)
CD-1 mouse/multigeneration 19.2 LOAEL for reduced sperm motility, reduced F2 pup weight Fail et al. (1991)
CD-1 mouse/gd 0-17 43 NOAEL for mouse developmental toxicity Heindel et al. (1992)
79 LOAEL for decreased fetal body weight
New Zealand white rabbits/ 21.9/43.7 NOAEL/LOAEL for decreased fetal body weight, Price et al. (1996b)
gd 6-19 increased fetal cardiovascular malformations and
maternal toxicity
a gd = gestational days.
rats (Hunt, 1994); these effects are more marked when a physiological
stressor is present, especially marginal cholecalciferol (vitamin D3)
nutriture. For example, a boron supplement of 3 µg/g to a basal diet
containing 0.465 µg boron/g alleviated the marginal cholecalciferol
deficiency-induced changes in bone, plasma glucose, energy substrate
utilization, and growth (Hunt, 1994). Hunt et al. (1994) have found
that boron deprivation depresses macromineral content in bone and some
indices of the maturation of the cartilage growth plate independently
of vitamin D nutriture. Brain composition and function in rats are
also affected by dietary boron. Boron deprivation was found to
systematically influence brain electrical activity assessed by an
electrocorticogram in mature rats (Penland & Eberhardt, 1993). In this
study, brain copper concentrations were higher in boron-deprived than
in boron-supplemented rats. Furthermore, calcium concentrations in
total brain and in brain cortex, as well as phosphorus concentration
in the cerebellum, were found to be higher in boron-deprived (0.158
µg/g diet) than in boron-supplemented (2.72 µg/g diet) rats fed a
cholecalciferol-deficient diet (Hegsted et al., 1991). This study also
found that the apparent absorption and balance of calcium, magnesium,
and phosphorus were decreased by boron deprivation. Hunt (1988) has
reported that chicks apparently require about 1.0 µg boron/g diet for
normal development. These studies collectively indicate that boron in
physiological amounts is beneficial to, if not essential for, higher
animals (see also section 8.4).
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Short-term toxicity and poisoning incidents
Available human exposure data on boron compounds for routes other
than inhalation focus on boric acid and borax. According to Stokinger
(1981), the lowest lethal dose for humans exposed to boric acid is 640
mg/kg body weight by oral exposure, 8600 mg/kg body weight by dermal
exposure, and 29 mg/kg body weight by intravenous injection. Stokinger
(1981) stated that deaths can occur at doses between 5 and 20 g of
boric acid total for adults and below 5 g total for infants. Litovitz
et al. (1988) stated that potential lethal doses are usually cited as
3-6 g total for infants and 15-20 g total for adults. A case-series
report of seven infants (aged 6-16 weeks) who used pacifiers coated
with a borax and honey mixture for 4-10 weeks reported that exposures
ranged from 4 to 30 g, with an estimated average daily ingestion of
0.143-0.429 g (O'Sullivan & Taylor, 1983). The actual relative doses
are unknown. Toxicity was manifested by generalized or alternating
focal seizure disorders, irritability, and gastrointestinal
disturbances. Other findings included inflammation, congestion,
oedema, exfoliation of the mucosa, cloudy swelling and granular
degeneration of tubular cells, and exfoliative dermatitis. Although
infants appear to be more sensitive than adults to boron compounds,
lethal doses are not well documented in the literature.
A few case reports have been published for poisoning incidents.
Teshima et al. (1992) reported that a 26-year-old woman ingested 21 g
of boric acid. The elimination of boric acid was about 4 times faster
with haemodialysis than with conventional medical treatment. The
patient was discharged from the hospital 12 days following admission.
In another case report, Grella et al. (1976) described transplacental
poisoning. A pregnant woman who had a personal history of diabetes was
accidentally given 70 g of boric acid instead of 70 g of glucose for
the glucose tolerance test at 33 weeks' gestation. She was immediately
treated with gastric lavage and intravenous sodium bicarbonate
fructose. The woman developed contractions, and an emergency caesarean
delivery was scheduled. The infant was born alive weighing 2.5 kg and
had spontaneous respirations. Soon afterwards, the infant developed
cardiac arrest, was resuscitated, and died. Cause of death was
attributed to cardiocirculatory collapse.
Boric acid and borax were widely used in medicine at the
beginning of the century for therapeutic purposes, both locally as
well as orally. Boric acid was used to treat various diseases, such as
epilepsy and infectious diseases. Several case studies reviewed by
Kliegel (1980) describe mild to severe responses to boron compounds.
Among all 19 patients with reported body hair loss as a response to
boron compound treatment, 16 persons had epilepsy and 3 had urinary or
genital infections. Stein et al. (1973) described an extremely unusual
case of a 32-year-old woman who ingested and swallowed several bottles
of mouthwash containing boric acid daily for a minimum of 1 year. The
woman was reported to have pancreatitis resulting from heavy alcohol
ingestion and medications for epilepsy. The woman lost almost all of
her body and scalp hair; other clinical signs included erythema on the
palms of her hands, severe fatigue, anorexia, and mental confusion.
Her blood boric acid level was 3.2 mg/100 ml, corresponding to 0.56 mg
boron/100 ml (the normal value was reported as 0.3 mg boric acid/100
ml). Upon cessation of mouthwash consumption, hair growth returned,
suggesting that the effect was reversible.
Goldbloom & Goldbloom (1953) reported four cases of boric acid
poisoning and reviewed an additional 109 cases in the literature. The
four cases were infants exposed to boric acid by repeated topical
applications of baby powder. Toxicity was manifested by cutaneous
lesions (erythema over the entire body, excoriation of the buttocks,
and desquamation), gastrointestinal disturbances, and seizures. One
patient died, but cause of death was not specifically attributed to
boric acid. Approximately 35% of the 109 other case reports involved
children under 1 year of age. The mortality rate was 70.2% for
children compared with 55.0% for all cases combined. Death occurred in
27/51 (53%) patients exposed by ingestion, in 3/4 (75%) patients
subjected to gastric lavage with boric acid, in 19/28 (68%) patients
exposed by dermal application for treating burns, wounds, and skin
eruptions, and in 14/26 (54%) patients exposed by other routes.
Information on signs and symptoms for 80 patients showed that
gastrointestinal disturbances were prevalent (73%), followed by CNS
effects (67%). Cutaneous lesions were prevalent in 76% of the cases
and in 88% of cases involving children under 2 years of age. Gross and
microscopic findings were reported for 27/60 (45%) fatal cases. In
general, boric acid caused chemical irritation primarily at sites of
application and excretion and in organs with maximum boron
concentrations. The most common CNS findings were oedema and
congestion of the brain and meninges. Other common findings included
liver enlargement, vascular congestion, fatty changes, swelling, and
granular degeneration (n = 13).
In addition to case reports, poison centres have published
case-series reports. Unlike the case reports reviewed by Goldbloom &
Goldbloom (1953), more recent reports suggest that the oral toxicity
of boron in humans is milder than previously thought. Litovitz et al.
(1988) conducted a retrospective review of 784 cases of boric acid
ingestion reported to the National Capital Poison Center in
Washington, DC, USA, during 1981-1985 and the Maryland Poison Center
in Baltimore, MD, USA, during 1984-1985. The amount of boric acid
ingested and clinical manifestations of toxicity were reported; 88.3%
of the cases were asymptomatic. All but two of the cases had acute
(single) ingestion, and 80.2% involved children under 6 years of age.
No severe toxicity or life-threatening effects were noted, although
boric acid levels in blood serum ranged from 0 to 340 µg/ml. The most
frequently occurring symptoms, which involved the gastrointestinal
tract, included vomiting (n = 32), abdominal pain (n = 15),
diarrhoea (n = 13), and nausea (n = 7). Other symptoms (primarily
CNS and cutaneous) occurred in six or fewer cases: lethargy, rash,
headache, light-headedness, fever, irritability, and muscle cramps.
The average dose ingested estimated from 659 cases was 1.4 g (range
0.010-88.8 g). For children under 6 years, the average dose was 0.5 g
(range 0.010-22.2 g), compared with 4.1 g (range 0.030-88.8 g) for
individuals age 6 years or above. The average dose for asymptomatic
cases was 0.9 g (range 0.010-88.8 g), compared with 3.2 g (range
0.10-55.3 g) for symptomatic cases. According to Litovitz et al.
(1988), 21 of the children under 6 years of age, 15 of whom were under
2 years of age, ingested the reported potential lethal dose of 3 g; 8
adults ingested the reported potential lethal dose of 15 g without
evidence of lethal effects.
Linden et al. (1986) published a retrospective review of 364
cases of boric acid exposure reported to the Rocky Mountain Poison and
Drug Center in Denver, CO, USA, between 1983 and 1984. Vomiting,
diarrhoea, and abdominal pain (incidence not reported) were the most
common symptoms given by the 276 cases exposed in 1983. Of the 72
cases reported in 1984 for whom medical records were complete, 79%
were asymptomatic, whereas 20% had mild gastrointestinal symptoms
noted. One 2-year-old child died, presumably from repeated ingestion
of an insecticide containing 99% boric acid.
Overall, owing to the wide variability of data collected from
poisoning centres, the average dose of boric acid required to produce
clinical symptoms is still unclear but is presumably within the range
of 100 mg to 55.5 g, observed by Litovitz et al. (1988).
8.1.2 Reproductive effects
An ecological study assessed boron exposure from drinking-water
and fertility among residents in two geographical regions in
Turkey.a Region I comprised 2368 residents, whereas Region II
comprised 2319 residents. Boron levels in drinking-water were
noticeably higher in Region I (range 2.05-29 mg/litre) than in Region
II (range 0.03-0.40 mg/litre). Ever-married residents from each region
who could provide reproductive histories for three generations of
family members represented the study sample -- i.e. 159 probands (6.7%
of population) in Region I and 154 (6.7%) in Region II. The
percentages of married couples with one or more live births (>90%)
were comparable for the two regions, regardless of generation
assessed. The overall percentage of couples with unresolved
infertility or those without children across three generations was
comparable for the two regions (i.e. 6.0% and 4.6%, respectively).
Secondary sex ratios (ratio of male to female live births) appeared to
be different for the two regions. Region I had a ratio below 1 (0.89),
suggesting an excess of female births; Region II had a ratio slightly
above 1 (1.04), suggesting a slight excess of male births. Statistical
a Sayli BS, Tüccar E, & Ellan AH (1996) An assessment of fertility in
boron-exposed Turkish subpopulations. Unpublished manuscript, Ankara
University Medical Faculty, Ankara, Turkey.
significance was not formally evaluated in any of the above analyses.
The results of this descriptive study suggest that fertility, as
measured by the ability to produce a live birth, is not adversely
affected for residents of this geographical area with high levels of
boron in their drinking-water and soil. The observed reversal of the
secondary sex ratio for Region I requires careful interpretation, as
no attention was given to factors reported to alter sex ratios (e.g.
advancing parental age, elective abortion rates, and multiple births).
Further consideration of potential sources of bias stemming from the
selection of respondents who were largely male and their ability to
accurately recollect and report fertility outcomes is needed. The
extent to which these findings are generalizable, if at all, to other
populations is unknown.
8.2 Occupational exposure
8.2.1 Short-term irritative effects
The majority of toxic effects reported in occupational studies
are acute or short-term in nature and result from the irritant effects
of boron compounds. Several studies have shown that workers exposed to
borax complain of symptoms that are due to respiratory irritation,
which include nosebleeds, eye and nasal irritation, sore throats,
cough, and shortness of breath; dermatitis has also been reported
(Birmingham & Key, 1963; NIOSH, 1978). Generally, people with
hyperreactive respiratory tracts (e.g. asthmatics) may experience more
severe irritant effects following inhalation exposure. However, there
is a lack of data to support the existence of an especially sensitive
high-risk human population for exposure to boron or boron compounds.
A medical survey of 113 workers employed in a borax mining and
refining plant was conducted by Garabrant et al. (1984). The reference
group comprised 214 workers employed in areas with low or minimal
exposure to boric acid and boron oxide; exposed workers had been
employed for a minimum of 5 years or were currently employed in areas
of heavy borax exposure. The average air concentration of particles
<5 µm in diameter measured from samples taken between 1979 and 1981
was 4.1 mg/m3 (range 1.2-8.5 mg/m3). The workers in both groups were
predominantly white males; the mean age of the exposed group was 38.2
years, compared with 42.1 years in the referent group. The mean
duration of employment for exposed and unexposed workers was 11.0 and
12.9 years, respectively. Smoking patterns were similar for the two
groups. Symptoms reported more frequently by exposed workers than by
unexposed workers (p < 0.001) were eye irritation (12.4% and 2.8%,
respectively), dry mouth, nose, or throat (10.6% and 1.9%,
respectively), sore throat (5.3% and 0.5%, respectively), and
productive cough (4.4 and 0%, respectively). No significant
differences were noted between the two groups of workers with respect
to dry cough, shortness of breath, chest tightness, chest pain, and
nosebleeds.
A more detailed analysis of 629 (93% participation) workers in
this plant was presented by Garabrant et al. (1985). This analysis was
based on frequency of acute symptoms in four mean boron dust exposure
categories (1.1, 4.0, 8.4, and 14.6 mg/m3) and persistent symptoms
(presumably chronic effects) in three exposure categories (0.9, 4.5,
and 14.6 mg/m3 of total particulates). The particles were composed
almost entirely of borax. Acute symptoms showing a significant linear
trend (p < 0.0001) in order of decreasing frequency were dryness of
mouth, nose, throat, and eye irritation, dry cough, nosebleeds, sore
throat, productive cough, shortness of breath, and chest tightness.
The frequency of these symptoms in the highest exposure category
ranged from 5% to 33%. The only symptom reported by 5% or more of
workers exposed to 4.0 mg/m3 was eye irritation; no symptoms were
reported by 5% or more of workers exposed to 1.1 mg/m3. The pulmonary
function findings were not significantly affected by exposure to
boron. Chest X-rays did not show abnormal regions indicative of boron
exposure. The authors concluded that borax caused simple respiratory
irritation that produces chronic bronchitis with no impairment of
pulmonary function. Also, borax dust appeared to cause acute and
persistent respiratory irritation at concentrations >4.5 mg/m3.
Therefore, the value for the intermediate exposure category for
persistent symptoms (4.5 mg/m3 as borax) was considered to be the
LOAEL.
Wegman et al. (1994) conducted a prospective cohort study to
examine acute irritative effects as well as chronic pulmonary function
abnormalities in mine and processing plant workers exposed to sodium
borate dust. Exposure-response associations were also examined as
related to acute irritant symptoms associated with sodium borate dust
exposure in mining and processing plants. In comparison with unexposed
workers, exposed workers reported significantly more nasal irritation
(rate ratio [RR] = 8.8), eye irritation (RR = 5.2), throat irritation
(RR = 2.9), cough (RR = 1.7), and breathlessness (RR = 7.1).
Continuous measures of particulate exposure were made throughout the
day and were related to hourly measurements of health outcomes.
Exposure-response relationships were present for each of the specific
symptoms at several symptom intensity levels. Acute irritant effects
did not lead to any undue chronic sequelae. With regard to the
acceptable level of risk, Wegman et al. (1994) concluded that a
threshold limit value (TLV) exposure of 10 mg borate/m3 was
protective of workers' health. This value is consistent with the
nuisance dust standard (ACGIH, 1991).
8.2.2 Male reproductive and other long-term health effects
Effects on the male reproductive system have also been reported,
although data regarding subchronic or chronic exposure on the
population in general are limited.
In the study by Wegman et al. (1994), sodium borate particulate
exposure estimates were used to estimate cumulative exposure in
relation to long-term pulmonary function. Of the 631 workers who
originally underwent pulmonary evaluation 7 years earlier, 336 (53%)
underwent a subsequent evaluation. Ninety (303/336) per cent of
workers were found to have acceptable pulmonary test results. After
the expected smoking-related pulmonary abnormalities were taken into
account, no relation was observed between forced expiratory volume in
1 second (FEV1) and accumulated exposure to sodium borate. People
with hyperreactive respiratory tracts such as those with asthma may
experience more severe irritant effects following inhalation exposure.
However, a sensitive population was not found.
Fertility following occupational exposure to boron was assessed
in a descriptive study. Whorton et al. (1994) estimated the
standardized birth ratio (SBR) to assess fertility in 542/750 (72%
participation) occupational workers in a US borax mine located in the
Mojave Desert, California, USA. These workers are unique, in that
their occupational exposure to sodium borate dust and desert soil is
uncontaminated by other exposures and given their long average length
of employment (mean 18 years). Self-administered questionnaires were
used to ascertain the observed number of live births fathered by male
workers following employment. The US general population adjusted for
age, race, parity, and calendar time period was used to estimate the
expected number of live births. The SBR is simply a ratio of the
observed number of live births to the expected number. An SBR above
100 reflects an excess of live births in relation to the US general
population, whereas an SBR below 100 reflects a deficit. Occupational
boron exposure was based on job titles and categorized into quintiles
of mean exposure levels ranging from <0.82 mg/m3 to >5.05 mg/m3.
SBRs were significantly elevated for workers in the lowest
(<0.82 mg/m3) and highest (>5.05 mg/m3) exposure categories
(i.e. 151 and 125, respectively). No significant trend between
exposure and SBRs was observed. An excess percentage of female live
births in comparison with male births was observed across most
categories of exposure and length of employment. The authors noted
that this female excess did not reflect a deficiency of male births,
as an excess of births for both genders was found. None of the
findings, however, was statistically significant. These findings need
to be carefully interpreted, largely given the selection of the US
general population as the standard. (The authors state that fertility
rates were not available for their target population.) The extent to
which this sample of workers is comparable to the US general
population with respect to factors that affect fecundity and fertility
remains to be established. Further considerations are needed with
respect to the calculation of SBRs, such as the exclusions of multiple
births, and in terms of potential response bias. The authors did
attempt to enhance the validity of self-reported fertility data and
select co-variates by using additional data sources and by efforts to
minimize selection bias. In sum, the findings do not support an
adverse effect of boron on demonstrated fertility for this
occupational sample of male workers in comparison with the US general
population.
8.3 Carcinogenicity
Evidence for carcinogenicity of boron and boron compounds in
humans is not available, largely because it has not been studied.
Based on the evidence from two lifetime studies in mice (NTP, 1987)
and rats (Weir & Fisher, 1972), boron compounds have been classified
by the US EPA in Group D (i.e. not classifiable as to human
carcinogenicity) (US EPA, 1994).
8.4 Physiological effects
Since 1987, circumstantial evidence has been accumulating that
suggests that boron may be an essential nutrient for humans; that is,
a dietary deprivation of boron consistently results in changed
biological functions that could be construed as detrimental and that
are preventable or reversible by an intake of physiological amounts of
boron. Many of these changed functions caused by boron deprivation
have been duplicated in animal models. The major reason boron is not
generally recognized as essential or nutritionally important for
humans is probably that a specific biochemical function for boron has
not been elucidated, or, as demonstrated for plants (also for which no
biochemical function has been elucidated), boron has not been shown
necessary to complete the life cycle. Nonetheless, findings from human
experiments show that boron is a dynamic trace element that can affect
the metabolism or utilization of numerous substances involved in life
processes, including calcium, copper, magnesium, nitrogen, glucose,
triglycerides, reactive oxygen, and estrogen. Through these effects,
boron can affect the function or composition of several body systems,
including blood, brain, and skeleton, in a positive manner, which
demonstrates that boron is a beneficial element, if not an essential
mineral element, at physiological amounts.
Although the first findings involving boron deprivation of humans
appeared in 1987 (Nielsen et al., 1987), the most convincing findings
have come mainly from two studies in which men over the age of 45,
postmenopausal women, and postmenopausal women on estrogen therapy
were fed a low-boron diet (0.25 mg/2000 kcal) for 63 days and then fed
the same diet supplemented with 3 mg boron/day for 49 days (Nielsen,
1989, 1994; Nielsen et al., 1990, 1991, 1992b; Penland, 1994). These
dietary intakes were near the low and high values in the range of
usual dietary boron intakes (see section 5.2.4). The major differences
between the two studies were the intakes of copper and magnesium; in
one experiment they were marginal or inadequate, whereas in the other
they were adequate. Boron deprivation had several effects, regardless
of dietary copper and magnesium. In addition, the marginal or
inadequate copper and magnesium caused apparent detrimental changes
that were more marked during boron deprivation than during boron
repletion. Among the effects of boron supplementation after 63 days of
boron depletion in these experiments were the following: an effect on
macromineral metabolism, evidenced by increased serum
25-hydroxycholecalciferol and a decrease in the elevated calcitonin
that was apparently caused by inadequate copper and magnesium; an
effect on energy metabolism, suggested by decreased serum glucose and
increased serum triglycerides; an effect on nitrogen metabolism,
indicated by decreased blood urea nitrogen and serum creatinine and
increased urinary hydroxyproline excretion; an effect on reactive
oxygen metabolism, indicated by increased serum erythrocyte superoxide
dismutase and serum ceruloplasmin; and an effect on erythropoiesis and
haematopoiesis, suggested by increased blood haemoglobin and mean
corpuscular haemoglobin content but decreased haematocrit, platelet
number, and erythrocyte number. Boron supplementation after depletion
also enhanced the elevation in serum 17ß-estradiol and plasma copper
caused by estrogen therapy, altered electroencephalograms such that
they suggested improved behavioural activation (e.g. less drowsiness)
and mental alertness, and improved processes of attention and memory.
Two hypotheses have appeared to account for the multiple effects
described above. One hypothesis is that boron has a role in cell
membrane function, stability, or structure, such that it influences
the response to hormone action, transmembrane signalling, or
transmembrane movement of regulatory cations or anions (Nielsen,
1991); a similar hypothesis has also appeared for the functional role
of boron in plants (Parr & Loughman, 1983; Blaser-Grill et al., 1990;
Blevins & Lukaszewski, 1994). The other hypothesis is that boron is a
negative regulator that influences a number of metabolic pathways by
competitively inhibiting some key enzyme reactions (Hunt, 1994).
Regardless of the fact that the function of boron remains
undefined, boron is becoming recognized as an element of potential
nutritional importance because of the findings from human and animal
studies. The latest report on trace elements in human nutrition and
health published by the World Health Organization (WHO, 1996)
suggested that an individual mean basal requirement for adults may be
about 0.375 mg/day, and the minimum mean population intake that meets
basal needs could be about 0.75 mg/day for adults. The report also
indicated that an acceptable safe range of population mean intakes for
boron for adults could well be 1-13 mg/day.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Several investigators have studied the effects of borates on
bacteria, protozoa, and algae (see Table 18). Effect concentrations
for the bacterium Pseudomonas putida range widely. No observed
effects were noted after 72 h at 291 mg boron/litre by Schöberl &
Huber (1988), whereas Guhl (1996) found a 16-h EC10 of 7.6 mg
boron/litre. Guhl (1996) and Bringmann & Kuhn (1980) reported 30-min
EC10 and 72-h EC3 values of 340 and 290 mg boron/litre,
respectively. Twenty per cent light loss, compared with controls, from
Photobacterium phosphoreum was determined to occur after 30 min at
18 mg boron/litre (Guhl, 1996). Nitrogen-fixing cyanobacteria require
boron for proper functioning of the heterocyst cell wall (Bonilla et
al., 1990). Mateo et al. (1986) concluded that boron is essential for
nitrogen fixation in Anabaena.
A wide range of effect concentrations was reported for two
protozoan species. Bringmann & Kuhn (1980) reported a 72-h EC3 of 0.3
mg boron/litre for Entosiphon sulcatum, whereas Guhl (1996) showed a
72-h NOEC of >10 mg boron/litre. Reproduction of Paramecium
caudatum was completely inhibited at 70 mg boron/litre (Bringmann &
Kuhn, 1980), and no effects were observed at 18 mg boron/litre by
Sprague (1972).
De Jong (1965) determined the sensitivity of another green alga,
Chlorella vulgaris, to borax concentrations ranging from 0.0001 to
30% of the medium. Inoculated cultures were cultivated at room
temperature in daylight for 3-4 months. The highest concentration of
borax tolerated by C. vulgaris was 0.6 mg boron/litre, and the
lowest concentration inhibiting growth was 1.2 mg boron/litre.
Concentrations above 50 mg boron/litre induce the formation of giant
cells in Chlorella pyrenoidosa as a result of a stronger inhibition
of the formation of daughter cells compared with the synthesis of
biomass. For cells grown in 100 mg boron/litre, cell division was
severely inhibited for the first 72 h of exposure. During this time,
photosynthesis was inhibited by only 47%. The protein and nucleic acid
content of the giant cells increased and nitrate uptake was also
enhanced, whereas the lipid content decreased. The authors therefore
concluded that the delay in cell division is probably a direct effect
of boron on cytokinesis, as nuclear division is unaffected (Maeso et
al., 1985).
Martinez et al. (1986) observed that boric acid caused a decrease in
growth rates, protein content, and chlorophyll content in the
blue-green alga Anacystis nidulans, following exposure to 75 and 100
mg boron/litre as boric acid. The photosynthetic pigments completely
disappeared after 72 h of exposure. Nitrate uptake was also lowered.
Table 18. Borate toxicity to bacteria, protozoa, and algae
Species Duration End-point Parameter Borate concentration Reference
in solution
(mg boron/litre)
Pseudomonas putida 30 min Growth inhibition EC10 340 Guhl (1996)
16 h Growth inhibition EC10 7.6 Guhl (1996)
72 h Growth inhibition NOEC 291 Schöberl & Huber (1988)
72 h Growth inhibition EC3 290 Bringmann & Kuhn (1980)
Photobacterium phosphoreum 30 min Light loss EC20 18 Guhl (1996)
Entosiphon sulcatum 72 h Growth inhibition EC3 0.3 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC >10 Guhl (1996)
Paramecium caudatum 72 h Growth inhibition EC100 <70 Bringmann & Kuhn (1980)
72 h Growth inhibition NOEC 18 Sprague (1972)
Algae, Scenedesmus quadricauda 8 days Growth inhibition EC3 0.16 Bringmann & Kuhn (1978)
Algae, Scenedesmus subspicatus 72 h Growth inhibition EC0 10 Guhl (1996)
72 h Growth inhibition EC10 24 Guhl (1996)
72 h Growth inhibition EC50 34 Guhl (1996)
72 h Growth inhibition EC100 100 Guhl (1996)
Algae, Scenedesmus subspicatus Chronic Growth inhibition EC10 24 Kopf & Wilk (1995)
Chronic Growth inhibition EC50 52 Kopf & Wilk (1995)
Chronic Growth inhibition EC90 109 Kopf & Wilk (1995)
Algae, Microcystis aeruginosa 8 days Growth inhibition EC3 20.3 Bringmann & Kuhn (1978)
An accumulation of carbohydrates was observed, probably because the
loss of the photosynthetic pigments inhibited their degradation.
Lewin (1966) concluded that borate is an essential micronutrient for
growth in marine diatoms, whereas Smyth & Dugger (1981) concluded that
boron is essential for Cylindrotheca fusiformis. Antia & Cheng
(1975) studied the effects of boric acid on the growth of 19 species
(10 classes) of marine phytoplankton. Axenic cultures in a
nutrient-enriched, pH-controlled seawater (33% salinity) medium were
exposed to the following concentrations of boric acid: 0, 5, 10, 50,
or 100 mg boron/litre. Growth measurements were taken every 2-4 days
by determining the optical density at 600 nm after vortex mixing.
Concentrations of 5 and 10 mg boron/litre did not inhibit the growth
of any species tested, with 10 mg boron/litre actually stimulating
growth in the blue-green alga Anacystis marina, the diatom
Skeletonema costatum, and the cryptomonad Rhodomonas lens. Growth
was strongly inhibited in 26% of the species at 50 mg boron/litre
(e.g. green alga Tetraselmis maculata; haptophyte Emiliania
huxleyi; diatom Phaeodactylum tricornutum) and in 63% of the
species at 100 mg boron/litre (e.g. chrysophyte Monochrysis
lutheri). The highest concentration was also lethal to 37% of the
species (e.g. diatom Cyclotella cryptica; dinoflagellate
Amphidinium carteri). Some species (e.g. green alga Monallantus
salina) required an adaptation period, with growth imperceptible
until 34-36 days after inoculation. Sequential transfer tests showed
that many of the initially inhibited species recovered after exposure
to 50 mg boron/litre, but not after exposure to 100 mg boron/litre.
The authors concluded that higher concentrations would be expected to
cause species redistribution, favouring growth of some forms and
suppressing growth of others.
A few studies have investigated the effects of boron on microorganisms
in sewage treatment plants. A boron concentration of 20 mg/litre had
no effect on activated sewage treatment (Gerike et al., 1976), and
boron levels below 200 mg/litre resulted in no significant inhibition
of anaerobic sludge digestion (Butterwick et al., 1989).
9.1.1.2 Soil
Information concerning the effects of boron on soil microorganisms is
scarce. Butterwick et al. (1989) reported results of a study in which
actinomycetes were found to undergo genetic transformations at boron
concentrations of 1000-6000 mg/litre. Bowen & Gauch (1966) exposed the
fungi Saccharomyces cerevisiae, Neurospora crassa, Aspergillus
niger, and Penicillium chrysogenum to boron. They found that
growth was significantly reduced at boron concentrations of 50, 250,
1300, and 4000 mg/litre for the four species, respectively. The
authors concluded that boron was not essential for any of the species
tested.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Nobel (1981) studied the effect of several boron compounds on
photosynthesis in submerged macrophytes, watermilfoil (Myriophyllum
alterniflorum), buttercup (Ranunculus penicillatus), and waterweed
(Elodea canadensis). The watermilfoil and buttercup (four
plants/concentration) were exposed to the following concentrations of
boric acid for 28 days: 0, 1, 2, 5, or 10 mg boron/litre. The
waterweed (eight plants/concentration) was exposed to the following
concentrations of boric acid for 21 days -- 0, 1, 2, 5, 10, or 250 mg
boron/litre -- and also to the following boron compounds at a
concentration of 2 mg boron/litre: boron trioxide, sodium perborate,
sodium metaborate, and borax. The test water was characterized as an
oligotrophic calcium-deficient nutritive medium. Net photosynthesis
was measured weekly as a function of the dissolved oxygen content.
Significant reductions (p = 0.01) in photosynthesis compared with
controls were observed at 2 mg boron/litre in the buttercup and
waterweed and at 5 mg boron/litre in the watermilfoil. The LC50
values for each species were as follows: 5 mg boron/litre for the
waterweed and the watermilfoil and 10 mg boron/litre for the
buttercup. Boron trioxide did not adversely affect photosynthesis in
the waterweed. The toxicity of the other boron compounds in the
waterweed at 2 mg boron/litre exhibited the following trend: borax >
metaborate > perborate > boric acid. Because calcium is known to
inhibit the uptake of boron in plants and, therefore, to mitigate the
damaging effects (Reeve & Shive, 1944), the authors concluded that the
toxic effects of boron in macrophytes are more pronounced in soft
(low-calcium) water.
In phytoassays conducted by Wang (1986), boron at concentrations up to
60 mg/litre did not significantly affect the growth of duckweed
(Lemna minor), expressed by the increase in frond number. In
addition, a concentration of 40.3 mg boron/litre added as a
tetraborate salt led to 50% inhibition of root growth in the
freshwater Eurasian watermilfoil (Myriophyllum spicatum) after 32
days of treatment (Stanley, 1974).
A pot study was carried out on the reed Phragmites australis during
the growth seasons of 1992 and 1993. Both soil culture in the presence
of free water and a gravel hydroculture with graduated repeated
additions of boron (as boric acid) were used during the growth seasons
to maintain the water phase at 0, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and,
in the case of the hydroculture, even 32.0 mg boron/litre. The number
of stalks per pot, height growth of the plants, and the yield of dry
substance of leaves, stalks, and roots at post-harvest time were
determined. The boron content in leaves, stalks, and roots, the
appearance of boron toxicity symptoms in leaves, and differences in
growth at each of the exposure concentrations were monitored. It was
concluded from the study that Phragmites australis can tolerate a
relatively high boron content (up to 4 mg boron/litre) in the liquid
nutrient substrate; for a period of 2-3 months, even 8 mg/litre was
tolerated without noticeable damage. Boron toxicity symptoms in the
leaves of Phragmites australis arose first at leaf boron
concentrations of around 150-180 mg/kg dry weight; however, no adverse
effects on growth, development, or dry substance yields of the plants
could be established at these leaf boron concentrations. Long-term
exposure to 8 mg boron/litre in waters would lead to symptoms of
damage, growth reductions, and yield reductions of plants (Marks et
al., 1994; Bergmann et al., 1995).
9.1.2.2 Invertebrates
1) Freshwater
Acute toxicity
Summaries of the median response toxicity data for aquatic
invertebrates are given in Table 19. These studies are also described
in detail in the following text.
Gersich (1984) studied the acute toxicity of boric acid to Daphnia
magna. Static (48-h) tests were conducted following the methodology
approved by the American Society for Testing and Materials. Three
replicates of 10 neonates each were exposed to seven nominal
concentrations: 0 (control), 54, 91, 151, 252, 420, or 700 mg
boron/litre. The 48-h LC50 for D. magna was 133 mg/litre as boron,
with a 95% confidence interval of 115-153 mg/litre, calculated by
probit analysis. All test organisms died at 420 mg/litre, 0% mortality
was observed at 54 mg/litre, and control mortality averaged 7%.
Lewis & Valentine (1981) also investigated the toxicity of boric acid
to D. magna. The investigators followed US EPA-approved procedures
and exposed <24-h-old neonates to five nominal test concentrations
(not given in paper). The 48-h LC50, calculated using probit
analysis, was determined to be 226 mg/litre as boron (95% confidence
interval of 200-246 mg/litre). The no-kill (0% mortality)
concentration was <200 mg/litre. Comparison of these results with
those of Gersich (1984) indicates a factor of 1.7 difference in the
48-h LC50 values; however, Canton & Adema (1978) report that a
difference of 2.0 may be normal in LC50 values not determined at the
same time or under similar conditions.
A few studies have been conducted to assess the toxicity of the sodium
borates to D. magna. As part of the study conducted by NAPM (1974),
the effects of sodium tetraborate pentahydrate on D. magna were
investigated. Static (96-h) tests were performed in which 10-20
organisms per test were exposed to the following nominal
concentrations of sodium tetraborate pentahydrate: 0, 18, 33, 58, 102,
or 182 mg/litre. The highest test concentration resulted in only 26.7%
mortality at 96 h; consequently, the 96-h LC50 for D. magna was
>182 mg sodium tetraborate pentahydrate/litre (>27 mg boron/litre).
Table 19. Median response concentrations for aquatic invertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
ARTHROPODA
Crustacea - Daphnidae
Daphnia magna Boric acid S, N 133.0 Gersich (1984)
Daphnia magna Boric acid S, N 226.0 Lewis & Valentine
(1981)
Daphnia magna Borax S, N 73.0 Bringmann & Kuhn
(1977)
Daphnia magna Anhydrous borax S, N <52.0 Threshold concentration,
based on immobilization Anderson (1946)
Daphnia magna Sodium tetraborate S, N >182.0 Mortality 26.7% at 96 h NAPM (1974)
pentahydrate
Daphnia magna Sodium tetraborate S, N 141 Maier & Knight (1991)
Crustacea - Limnoriidae
Limnoria lignorum Borax S, N 28.35 After 5-day recovery period Robinson & Perkins
(1977)
Insecta - Chironomidae
Chironomus decorus Sodium tetraborate S, N 1376 Maier & Knight (1991)
MOLLUSCA
Gastropida - Onchidiidae
Onchidoris fusca Borax S, N <28.35 After 5-day recovery period Robinson & Perkins
(1977)
a S = static test; N = nominal concentration.
Bringmann & Kuhn (1977) reported a 24-h LC50 of 340 mg disodium
tetraborate (anhydrous borax)/litre (73 mg boron/litre) for
D. magna in static tests using tap-water free of chlorine. The LC0
was 61 mg disodium tetraborate/litre (13 mg boron/litre), and the
LC100 was 1930 mg disodium tetraborate/litre (415 mg boron/litre).
Anderson (1946) obtained similar results with D. magna, reporting a
threshold concentration based on immobilization of <240 mg anhydrous
borax/litre (<52 mg boron/litre) at 48 h. Maier & Knight (1991)
reported a 48-h LC50 value of 141 mg boron/litre (95% confidence
limits 123-159 mg boron/litre) for D. magna exposed to sodium
tetraborate. Increasing water hardness and sulfate concentrations did
not affect the toxicity of boron to D. magna. In this study, boron
was much more toxic to daphnids than to Chironomus decorus
4th-instar larvae, for which the 48-h LC50 was 1376 mg boron/litre
(95% confidence limits 1298-1453 mg boron/litre) (Maier & Knight,
1991).
Tubificid worms (Tubifex sp.) appear to be less sensitive than
D. magna to sodium borate, with concentrations of sodium borate
decahydrate (borax) as high as 750 mg/litre (85 mg boron/litre)
causing no effect after a 24-h exposure. The 24-h LC100 was reported
as 2000 mg/litre (227 mg boron/litre). The tubificid also exhibited
less sensitivity to boric acid, with a no-effect level at 24 h of
exposure of 7500 mg/litre (1311 mg boron/litre) and an LC100 of 10 000
mg/litre (1748 mg boron/litre) (Mann, 1973).
In an early study, Fay (1959) determined the effects of metaboric acid
(B2O3.H2O) on various life stages of three species of mosquitos:
Aedes aegypti, Anopheles quadrimaculatus, and Culex
quinquefasciatus. A concentration of 8000 mg/litre (1973 mg
boron/litre) did not affect hatching of A. aegypti eggs following
72 h of exposure. However, newly hatched larvae of
C. quinquefasciatus and A. quadrimaculatus were quite sensitive to
metaboric acid. Complete mortality of C. quinquefasciatus larvae was
observed after 24 h of exposure to 2000 mg metaboric acid/litre (493
mg boron/litre). Complete mortality of A. aegypti was observed after
24 h of exposure to 4000 mg/litre (986 mg boron/litre), with complete
mortality occurring in A. quadrimaculatus only at the 8000 mg/litre
(1973 mg boron/litre) level. In contrast, at the 2nd- and 3rd-instar
stage, A. quadrimaculatus was more sensitive than the other species.
In 2nd-instar larvae, 100% mortality occurred at 500 mg/litre (123 mg
boron/litre) in A. quadrimaculatus, 96% mortality occurred at
1500 mg/litre (370 mg boron/litre) in A. aegypti, and 100% mortality
occurred at 2000 mg/litre (493 mg boron/litre) in
C. quinquefasciatus. The 3rd-instar larvae were less sensitive than
the 2nd-instar larvae, with 100% mortality of A. quadrimaculatus at
2500 mg/litre (617 mg boron/litre), 88% mortality of A. aegypti at
3000 mg/litre (740 mg boron/litre), and 48% mortality of
C. quinquefasciatus at 4000 mg/litre (986 mg boron/ litre). Pupae of
the three species were even less sensitive to metaboric acid,
requiring concentrations ranging from 6000 mg/litre (1480 mg
boron/litre) to 16 000 mg/litre (3946 mg boron/litre) to prevent
emergence to the adult stage. From the information provided in this
study, it appears that the 2nd-instar larva was the life stage that
was most sensitive to exposure to metaboric acid.
Fay (1959) also studied the effects of prolonged exposure to boron
trioxide (B2O3.H2O) on the immature stages of the same three
species of mosquitos. Newly hatched larvae were reared in the
following concentrations of metaboric acid to determine the percentage
of successful adult emergence: 10, 25, 50, 100, or 250 mg/litre. No
significant reduction in maturation was observed in A. aegypti or
C. quinquefasciatus at any test concentration <100 mg/litre.
However, at 50 mg/litre, only 2% of the larvae of A.
quadrimaculatus reached the adult stage. At 250 mg metaboric
acid/litre, only 1% of A. aegypti and only 3% of
C. quinquefasciatus reached the adult stage.
Maier & Knight (1991) evaluated the chronic sublethal effects of boron
to Chironomus decorus larvae. A significantly decreased growth rate
over a 96-h period was observed at 20 mg boron/litre.
Chronic toxicity
Gersich (1984) conducted a 21-day static renewal chronic toxicity test
with D. magna. Daphnids (20 organisms/concentration, or four
replicates with 5 organisms/replicate) were exposed to the following
nominal concentrations of boric acid: 0, 7, 14, 28, 56, or 105
mg/litre as boron. The test concentrations remained stable during
testing; the mean measured concentrations ranged from 91.4 to 106% of
the nominal test concentrations. The 21-day LC50, calculated using
the moving average method, was 52.2 mg boron/litre, and the 95%
confidence interval was 42.6-66.7 mg boron/litre. No mortality was
observed in the control group. The mean number of broods per daphnid,
mean total young per daphnid, mean brood size per daphnid, and mean
size differed significantly (alpha = 0.05) from the control at the
13.6 mg boron/litre concentration. Therefore, the maximum acceptable
toxicant concentration (MATC) was determined based on the most
biologically and statistically significant end-points, reproduction
and growth. The MATC for boric acid was estimated to be between 6.4
and 13.6 mg boron/litre, or 9.3 mg boron/litre (the geometric mean of
these two values).
Lewis & Valentine (1981) also conducted a 21-day static renewal
chronic toxicity test with D. magna. The test was conducted
similarly to that of Gersich (1984), except for the exposure regimen.
Ten test chambers were set up for each test concentration: three
beakers contained five daphnids each for obtaining data on survival,
and seven beakers each contained one daphnid for obtaining data on
survival, growth, and reproduction. Twenty control chambers were used:
6 chambers contained five daphnids each, and 14 chambers each
contained one daphnid. Daphnids were exposed to the following nominal
concentrations of boric acid: 0, 6, 13, 27, 53, or 106 mg/litre as
boron. The concentration of the test solution remained stable during
testing, with mean measured concentrations exceeding 95% of the
nominal concentration. The 21-day LC50, calculated by probit
analysis, was 53.2 mg boron/litre, with a 95% confidence interval of
44.1-64.5 mg boron/litre. Mortality was 9% in the control group. The
mean brood size and total number of young produced were significantly
reduced (p < 0.05) compared with controls at concentrations of
>13 mg boron/litre. Mean length of daphnids was significantly reduced
(p < 0.05) compared with controls at 53 mg boron/litre. The NOEC
was 6 mg boron/litre. Therefore, based on the most sensitive
parameters, the MATC was >6 and <13 mg boron/litre, or 8.83 mg
boron/litre (geometric mean of these values).
2) Marine
In static tests, Mann (1973) determined 24-h LC100 values for the
amphipod Gammarus tigrinus exposed to boric acid, borax, and sodium
perborate at two salinities (32.23% and 17.66%). The LC100, 10 000
mg/litre, was the same for all three compounds at both salinities. The
no-effect concentration for all of the compounds was 7500 mg/litre. In
contrast, Robinson & Perkins (1977) reported toxicity values for borax
in the isopod Limnoria lignorum and the sea slug Onchidoris fusca
that are roughly two orders of magnitude lower than those reported by
Mann (1973) for the amphipod Gammarus tigrinus. The salinity of the
test water was 30%. The 24-h static LC50 values, after a 5-day
recovery period, were 250 mg/litre (28.35 mg boron/litre) for the
isopod and >250 mg/litre (>28.35 mg boron/litre) for the sea slug.
In studies with sea urchin (Anthocidaris crassispina) embryos,
exposure to boric acid at a concentration of 37 mg boron/litre had no
effect on development, whereas exposure to 75 mg boron/litre was fatal
(Eisler, 1990).
3) Biocenosis studies
Three biocenosis studies (also known as microcosms, mesocosms, and
experimental ecosystems) have been conducted (summarized in ECETOC,
1997). A 28-day laboratory microcosm test with microorganisms
consisting of six trophic stages yielded a NOEC and LOEC of 2.5 and
5.0 mg boron/litre, respectively. Studies with outdoor ponds
containing up to 29 species and treated with 0.7 mg boron/litre over a
2-year period resulted in no significant differences compared with
untreated control ponds. Further, field studies carried out in seven
different water bodies subjected to different levels of anthropogenic
boron inputs yielded no toxic effects of boron at concentrations
between 0.16 and 1.52 mg/litre.
9.1.2.3 Vertebrates
1) Freshwater
Acute toxicity
Summaries of the acute toxicity data for aquatic vertebrates are given
in Table 20. These studies are also described in detail in the
following text.
For boron compounds, three studies are available that provide
information concerning the 96-h LC50 values, the toxicity value
established by the US EPA (Stephan et al., 1985) for use in the
derivation of water quality criteria for the protection of aquatic
life and its uses. Wallen et al. (1957) conducted 96-h static toxicity
tests with mosquitofish. The test water, turbid pond water, was
selected to simulate the commonly turbid wastewater from oil refinery
operations; however, it was determined that the highest turbidities
used in the study should not produce direct effects on the fish during
the exposure time. Adult female fish (10/concentration) were exposed
to the following nominal concentrations of boric acid or borax: 10,
18, 32, 56, or 100 mg/litre. If no deaths occurred at 96 h, the tests
were rerun at concentrations of 100, 180, 320, 560, or 1000 mg/litre
and, subsequently, at concentrations 10 times these values
(1000-10 000 mg/litre). The 96-h LC50 for mosquito-fish exposed to
boric acid was 5600 mg/litre (979 mg boron/litre), and the 96-h LC50
for borax was 3600 mg/litre (408 mg boron/litre).
As part of the study conducted by NAPM (1974), static bioassays were
conducted to determine the toxicity of sodium tetraborate pentahydrate
to fathead minnows. The tests were conducted using standard methods
and filtered tap-water. Fish (15/treatment) were exposed to the
following nominal concentrations: 0, 200, 360, 640, 1120, or 2000
mg/litre. The 96-h LC50 was 1900 mg/litre (332 mg boron/litre).
Hamilton & Buhl (1990) reported the results of 96-h static toxicity
tests with two life stages of chinook salmon (Oncorhynchus
tshawyts-cha) and coho salmon (O. kisutch). Swim-up fry
(8-12 weeks old) were tested in fresh water, and advanced fry (15-21
weeks old) were tested in brackish water. The 96-h LC50 values for
chinook salmon swim-up fry and advanced fry were 725 mg/litre (127 mg
boron/litre) and 600 mg/litre (105 mg boron/litre), respectively. The
96-h LC50 values for coho salmon swim-up fry and advanced fry were
447 mg/litre (78.1 mg boron/litre) and 600 mg/litre (105 mg
boron/litre), respectively.
Results of 24- and 48-h static acute tests with rainbow trout,
guppy (Poecilia reticulata), and mosquitofish clearly show the
difference in the degree of toxicity resulting from exposure to boric
acid versus sodium borate. Mann (1973) reported the following 24-h
LC100 values for rain-bow trout and guppy, respectively: boric acid,
10 000 mg/litre (1748 mg boron/litre) and 7500 mg/litre (1311 mg
boron/litre); borax, 5000 mg/litre (567 mg boron/litre) for both
species. Static 24-h LC50 values determined by Wallen et al. (1957)
for the mosquitofish are 18 000 mg/litre (3146 mg boron/litre) for
boric acid and 12 000 mg/litre (1361 mg boron/litre) for borax. The
48-h LC50 values determined in this study were 10 500 mg/litre (1835
mg boron/litre) for boric acid and 8200 mg/litre (930 mg boron/litre)
for borax. Rainbow trout are more sensitive to borax than
mosquitofish, with 24- and 48-h static LC50 values of 2800 mg/litre
(602 mg boron/ litre) and 1800 mg/litre (387 mg boron/litre),
respectively (Alabaster, 1969). Juhnke & Ludemann (1978) reported an
LC50 of 807 mg/litre as boron for golden orfe (Leuciscus idus)
Table 20. Acute toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Coho salmon Boric acid S, N 78.1 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus kisutch) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Chinook salmon Boric acid S, N 127 Swim-up fry Hamilton & Buhl (1990)
(Oncorhynchus tshawytscha) Boric acid S, N 105 Advanced fry Hamilton & Buhl (1990)
Rainbow trout Borax S, N 602 387 Alabaster (1969)
(Oncorhynchus mykiss) Borax FT, N 65 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 150 Embryo, hardness of
50 mg CaCO3/litre Birge & Black (1977)
Borax FT, N 88 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Boric acid FT, N 100 Embryo, hardness of
200 mg CaCO3/litre Birge & Black (1977)
Fathead minnow Sodium S, N 332 NAPM (1974)
(Pimephales promelas) tetraborate
pentahydrate
Mosquitofish Borax S, N 1361 930 408 Wallen et al. (1957)
(Gambusia affinis) Boric acid S, N 3146 1835 979 Wallen et al. (1957)
Golden orfe 173 Schöberl & Huber (1988)
(Leuciscus idus)
Table 20. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration
(as mg boron/litre)
24 h 48 h 96 h
Zebra fish Borax 14.2 Guhl (1996)
(Brachydanio rerio)
Bluegill Borax S, N 4.6 Turnbull et al. (1954)
(Lepomis macrochirus)
Dab Anhydrous borax Semi-static, 88.3 74 Salinity 34.62 ± 0.2% Taylor et
(Limanda limanda) N al. (1985)
Colorado squaw-fish Boric acid 279b Hamilton (1995)
(Ptychocheilus lucius) >100c
527d
Razorback sucker Boric acid 233b Hamilton (1995)
(Xyrauchen texanus) 279c
>100d
Bonytail Boric acid 280b Hamilton (1995)
(Gila elegans) >100c
552d
a S = static test; FT = flow-through test; N = nominal concentration.
b Swim-up fry.
c 1-g juvenile.
d 2-g juvenile.
exposed to anhydrous borax in static acute tests (exposure time not
given).
Mann (1973) reported 24-h LC100 values for rainbow trout and
guppies exposed to sodium perborate (500 mg/litre or 66.1 mg
boron/litre) that were considerably lower than those reported for
boric acid and borax. The authors attributed this increased toxicity
to a pH shift into the alkaline range (up to 9.1), which resulted in
increased mucilage formation, with the fish exhibiting a crippling
behaviour.
The treatment of embryos of the toad Bufo vulgaris with 0.5%
boric acid for 24 h from the two-cell stage to the tail-bud stage
resulted in several developmental malformations, including a reduction
or lack of external gills, short tail, bifurcation of the epiphysis,
suppression of forebrain development, and inhibition of sensory organ
development. Several of these effects were attributed to the direct
action of boric acid on the ectoderm (Takeuchi, 1958).
Chronic toxicity
Toxicity of borate to early life stages of fish has been
documented for several species (Birge & Black, 1977; Black et al.,
1993). Embryonic and early larval stages of rainbow trout, largemouth
bass, channel catfish, and goldfish were exposed to boron, as boric
acid or borax, from fertilization up to 8 days post-hatch. All
exposures were in soft (50 mg CaCO3/litre) or hard (approx. 200 mg
CaCO3/litre) reconstituted water. Test responses included embryonic
mortality, teratogenesis, and larval mortality. Gross debilitating
anomalies of survivors were classified as mortalities. Effect
concentrations (LC50, NOEC, and LOEC) for each species are presented
in Table 21. Neither water hardness nor the form of boron (boric acid,
borax) added to the test aquaria consistently affected embryo-larval
survival of rainbow trout, channel catfish, and goldfish (Birge &
Black, 1977).
On the basis of median lethal concentrations (LC50), no species
was found to be especially sensitive. The range of LC50s for all
species was 12.2-235 mg boron/litre. In addition, Birge & Black (1977)
reported LC1s ranging from 0.001 to 0.1 mg boron/litre for rainbow
trout, from 0.2 to 5.5 mg boron/litre for channel catfish, and from
0.2 to 1.4 mg boron/litre for goldfish. The LC1 showed rainbow trout
to be the most sensitive species. The NOEC ranged from 0.009 to 0.103
mg boron/litre for rainbow trout and was 1.39 mg boron/litre for
large-mouth bass. These were consistent with the acute toxicity
results that indicated rainbow trout and zebra fish as the most
sensitive species (see Table 20).
The effect of natural dilution water on boron toxicity was
reported by Black et al. (1993). Natural surface waters were collected
from three US locations: the Erwin National Fish Hatchery in
Tennessee, Brookville Lake in Indiana, and Firehole River in
Yellowstone National Park, Wyoming. Surface water control boron
Table 21. Chronic toxicity for aquatic vertebrates exposed to boron compounds
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Coho salmon Sodium metaborate S, N 113 Alevins, 283-h exposure Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate
Coho salmon Sodium metaborate S, N 12.2 Underyearlings, 283-h exposure, Thompson et al. (1976)
(Oncorhynchus kisutch) tetrahydrate salinity 28 ± 1%
Rainbow trout Borax FT, N 27 Alevin, 28-day exposure, Birge & Black (1977)
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre
Rainbow trout Boric acid FT, N 100 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Borax FT, N 54 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 79 Alevin, 28-day exposure,
(Oncorhynchus mykiss) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Rainbow trout Boric acid FT, N 138 Embryo/larva, 32-day exposure Black et al. (1993)
(Oncorhynchus mykiss)
Rainbow trout Boric acid 138 Hardness of 200 mg CaCO3/ litre;
(Oncorhynchus mykiss) duration 32 days
NOEC = 0.009 mg boron/litre
LOEC = 0.1 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid 92 Hardness of 200 mg CaCO3/ litre;
(Micropterus salmoides) duration 11 days
NOEC = >1.39 mg boron/litre
LOEC = 12.17 mg boron/litre Birge & Black (1981)
Largemouth bass Boric acid FT, N 92 Embryo/larva, 11-day exposure Black et al. (1993)
(Micropterus salmoides)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Goldfish Borax FT, N 71.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 178.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 68.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 170.0 Embryo, 72-h exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 65.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 46.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Goldfish Borax FT, N 59.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Goldfish Boric acid FT, N 75.0 Fry, 7-day exposure,
(Carassius auratus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 235.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 220.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 120.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Channel catfish Boric acid FT, N 102.0 Embryo, 120-h exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 155.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Channel catfish Borax FT, N 71.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Channel catfish Boric acid FT, N 22.0 Fry, 9-day exposure,
(Ictalurus punctatus) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 54 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 157 Embryo, 84-h exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 60 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 145 Embryo, 84-h exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Borax FT, N 47 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 130 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Table 21. (continued)
Organism Chemical Test Median response Comments Reference
methoda concentration (as
mg boron/litre)
Leopard frog Borax FT, N 54 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Leopard frog Boric acid FT, N 135 Larvae, 7.5-day exposure,
(Rana pipiens) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 148 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 135 Embryo, 84-h exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 145 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 50 mg CaCO3/litre Birge & Black (1977)
Fowler's toad Boric acid FT, N 123 Larvae, 7.5-day exposure,
(Bufo fowleri) hardness of 200 mg CaCO3/litre Birge & Black (1977)
a S = static test; FT = flow-through test; N = nominal concentration.
concentrations were 0.023, 0.091, and 0.75 mg/litre for Erwin,
Brookville, and Yellowstone waters, respectively. Total organic carbon
for the three surface waters ranged from 0.8 to 1.9 mg/litre.
Hardness levels for these natural waters ranged from 24 to 209 mg
CaCO3/litre. In the surface water tests, three nominal treatments
were used: surface water control, control plus 1.0 mg boron/litre
added (as boric acid), and control plus 10 mg boron/litre added. Total
and dissolved boron levels were measured for each of the surface water
treatments. No statistically significant differences were noted
between total and dissolved boron concentrations, except for
Yellowstone (1.0 mg boron/litre) and Erwin (10 mg boron/litre)
treatments. The true significance of this, however, is minimal, as
total versus dissolved concentrations for Yellowstone and Erwin waters
were 1.61 vs. 1.52 mg boron/litre and 9.91 vs. 9.48 mg boron/litre,
respectively. Hence, it is reasonable to assume that all boron was in
solution. Boron did not elicit toxicity to embryo-larval stages of
rainbow trout exposed to surface water control boron levels up to 0.75
mg/litre. LOECs for Erwin, Brookville, and Yellowstone treatments were
1.10, 1.24, and 1.73 mg boron/litre, respectively, indicating that the
threshold for no effects is approximately 1 mg boron/litre. In
addition, deep (600 m) well-water, typically used for aquatic toxicity
tests, from a contract laboratory located in Wareham, Massachusetts,
USA, was also used. Boron was spiked into well-water to obtain an
exponential series of nominal concentrations: 0.0017, 0.017, 0.17,
1.7, and 17 mg boron/ litre, respectively. Analytical confirmation of
exposure was obtained for the two highest concentrations, 2.1 and
18.0 mg boron/litre. No effects were observed, even at the highest
boron concentration.
The effect concentrations generated in natural waters were
greater than those from the reconstituted water experiments. The cause
of these differences is not readily apparent, particularly as
bioavailability was not reduced by natural water. However, some
investigators (Belanger et al., 1989; Farris et al., 1994) have shown
that organisms cultured in natural water often grow and reproduce
better than their counterparts in reconstituted or laboratory
dechlorinated water. Therefore, a reasonable hypothesis that may
explain, in part, the differences in reconstituted versus natural
water responses is the better health experienced by organisms exposed
to boron in natural water. The causative agents for this lack of
health in laboratory water are not certain but may include nutrient
deficiencies (Keating & Dagbusan, 1984; Belanger et al., 1989).
Early life stage tests have been conducted with fathead minnows
to determine effects of chronic exposure (30-60 days) to boric acid.
The 30-day NOEC and LOEC were 14 and 24 mg boron/litre, respectively,
based on growth reduction; the 60-day NOEC and LOEC were 24 and 88 mg
boron/litre, respectively, based on reduction in fry survival
(Butterwick et al., 1989).
Birge & Black (1977) also studied the effect of boric acid and
borax on embryos and larvae of the leopard frog (Rana pipiens) and
the effect of boric acid on embryos and larvae of the Fowler's toad
(Bufo fowleri). The embryos were exposed for 3.5 days and the larvae
for 7.5 days to concentrations of boric acid and borax ranging from
0.05 to 300.00 mg boron/litre in hard (200 mg CaCO3/litre) and soft
(50 mg CaCO3/litre) water (as described above for fish exposures).
The leopard frog and Fowler's toad were equisensitive to borax and
boric acid. For the leopard frog, LC1 and LC50 values were 13 and
130 mg boron/litre (dosed as boric acid) in soft water and 22 and 135
mg boron/litre in hard water. Lower toxicity values were observed via
borax exposures, with LC1 and LC50 values of 5 and 47 mg boron/litre
in soft water and 3 and 54 mg boron/litre in hard water, respectively.
The LC1 and LC50 values for Fowler's toad exposed to boric acid were
25 and 145 mg boron/litre in soft water and 5 and 123 mg boron/litre
in hard water, respectively.
2) Marine
Taylor et al. (1985) studied the toxicity of several metals,
including boron (as anhydrous borax), to a saltwater fish (dab,
Limanda limanda). The tests were conducted following standard
procedures under semi-static conditions, in which the test solution
was replaced at 24-h intervals. The salinity of the test water was
34.62 ± 0.2%. Adult fish obtained from wild populations
(20/concentration) were exposed to a series of five concentrations
(not given in the paper) for 96 h. The LC50, calculated by probit
analysis, was 74.0 mg boron/litre, with a 95% confidence interval of
66.4-83.0 mg boron/litre. Pre-death symptoms included minor
respiratory problems.
Similar results were observed in bioassays conducted by Thompson
et al. (1976) with two life stages of coho salmon. Coho alevins
(0.19-0.7 g) were tested in aerated well-water (fresh water), and coho
underyearlings (1.8-3.8 g) were tested in aerated seawater with a
salinity of 28 ± 1%. Test solutions were prepared using sodium
metaborate tetrahydrate (Na2B2O4.4H2O), and exposure lasted in
excess of 12 days. The 283-h LC50 for freshwater coho alevins was 113
mg boron/litre, with 95% confidence limits of 104-123 mg boron/litre.
The 283-h LC50 for saltwater coho underyearlings was 12.2 mg
boron/litre, with 95% confidence limits of 10.89-14.56 mg boron/litre.
The authors attributed the difference in toxicity in fresh water and
salt water to differences in fish age, test temperature (11°C in fresh
water; 8°C in salt water), and available boron levels. Seawater also
has higher background levels of boron than fresh water. The results
reported by Hamilton & Buhl (1990), discussed above, do not indicate
significant differences in acute toxicity between different life
stages of chinook and coho salmon.
In static tests, Mann (1973) observed differing sensitivities in
the common eel (Anguilla anguilla) to both boric acid and borax at
two salinities: 12.39 and 25.05%. At the lower salinity, a 24-h LC100
of >10 000 mg/litre was reported for both borax and boric acid; at
the higher salinity, the 24-h LC100 was 7500 mg/litre for both
compounds. The authors speculated that differences in pH could
contribute to the toxicity of the boron compounds at the higher
salinity. At a boric acid concentration of 7500 mg/litre, the pH in
the lower-salinity test water was 7.2; the pH in the higher-salinity
test water was 6.6. As was observed with rainbow trout and
mosquitofish, Mann (1973) also observed greater toxicity in the common
eel following exposure to sodium perborate. The LC100 at both
salinities was 750 mg/litre (99.2 mg boron/litre), resulting from the
damaging effects of an alkaline pH shift.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
1) Essentiality
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron plays a role in carbohydrate metabolism, sugar translocation,
pollen germination, hormone action, normal growth and functioning of
the apical meristem, nucleic acid synthesis, and membrane structure
and function (Lovatt & Dugger, 1984). Recent work shows important
involvement of boron in cell wall cross-linking, involving
complexation with specific pectin components (Loomis & Durst, 1992; Hu
et al., 1996).
Early investigation of the effects of boric acid and borax on the
fieldbean (Vicia faba) and other plants indicated the role of boron
in plant nutrition (Warrington, 1923). The first comprehensive study
of the effects of boron on plant growth was conducted by Eaton (1944).
Fifty species of plants were grown in sand cultures with standard
nutrient solutions containing the following boron concentrations:
trace (0.03-0.04), 1, 5, 10, 15, or 75 mg/litre. For each species,
Eaton (1944) recorded the boron concentration resulting in the best
growth, the lowest concentration resulting in injury, and the symptoms
of deficiency with trace boron levels. Approximately 82% of the plants
exhibited the best growth at concentrations from trace to 5 mg/litre,
and 35% of the plants grown in trace boron levels developed
morphological symptoms of deficiency. Eaton (1944) concluded that
there was overlap of the beneficial and injurious effects of boron
between species; therefore, three broad categories of tolerance
(sensitive, semi-tolerant, and tolerant) were established. The
tolerant plants endure a wide range of boron concentrations with
little effect, and the sensitive plants exhibit a strong reaction to
either too much or too little boron.
Boron generally occurs in soils at concentrations ranging from 10
to 30 mg/kg, depending on such factors as soil type, amount of
rainfall, and irrigation type (Sprague, 1972). Boron deficiencies
usually occur in soil solutions from sandy soils or acid peat soils,
soils derived from igneous rocks, soils low in organic matter, and
irrigated soils (Sprague, 1972).
The symptoms of boron deficiency in plants include cessation of
root and leaf growth, necrosis of leaf primordia and primary root
tips, necrosis of stem and leaf phloem, bark splitting, retardation of
enzyme reactions, reduced pollen germination, and even death (Versar,
Inc., 1975; Wells & Whitton, 1977). Normal growth will usually resume
if boron is added to the growth medium.
Hudak (1973) found that a boron-deficient nutrient solution
inhibited mitosis in the root tip of the fieldbean within 72 h. A 10
mg boron/litre solution produced optimum cell division and elongation
of the root tip; however, 50 mg boron/litre caused a reduction in
mitosis within 24 h.
Ludbrook (1942) studied the effects of boron deficiency and
toxicity in Monterey pine (Pinus radiata) seedlings grown in water
culture. The suboptimal concentrations of boron added to the nutrient
solution were 0, 0.005, 0.01, or 0.05 mg/litre, and the potentially
toxic concentrations of boron were 0.5, 2.5, 5.0, 10.0, 20.0, or 40.0
mg/litre. Seedlings exposed to the toxic levels were initially grown
for 2 months in a nutrient solution containing added boron at 0.5
mg/litre. Marked reduction in root growth was observed at the two
lowest suboptimal concentrations; however, improved root growth was
observed at 0.05 mg/litre, with the development of small lateral
roots. At 10.0 mg boron/litre, a slight yellowing of the tips of the
needles developed, followed by browning and drying at 20.0 mg/litre,
until all plants died at 40.0 mg/litre. Ludbrook (1942) concluded that
the optimum growth range for Monterey pine was 0.05-5.0 mg
boron/litre.
Recent work has shown that boron in a number of plant species is
tightly bound in the polysaccharide cell wall. Boron deficiency leads
to profound changes in cell wall morphology, suggesting that boron is
critical to cell wall expansion. It has been proposed that this
structural, cross-linking function of boron is involved with the
pectin fraction, which contains apiose and other hydroxylated
fragments amenable to complexation by borate (Loomis & Durst, 1992).
Fourteen species of crop plants were studied, and it was concluded
that high pectin content requires more boron for forming cell walls or
that pectin forms a tightly held boron complex that depletes boron
availability for other critical functions, thereby increasing the
overall demand for boron (Hu et al., 1996). Kobayashi et al. (1996)
have isolated and characterized a rhamnogalacturonan II/borate complex
from enzyme-digested cell wall pectin.
Toxicity
Boron excesses usually occur in soil solutions from geologically
young deposits, arid soils, soils derived from marine sediments, and
soils contaminated by pollutant sources, such as releases from
coal-fired power plants and mining operations (Sprague, 1972).
The initial symptom of boron toxicity in plants is chlorosis
(yellowing) of the leaf tip, progressing along the leaf margin and
into the blade. Necrosis of the chlorotic tissue occurs, followed by
leaf abscission. Necrosis of the leaf tissue results in a loss of
photosynthetic capacity, which reduces plant productivity (Lovatt &
Dugger, 1984). Pollen germination and pollen tube growth may also be
inhibited (Versar, Inc., 1975).
Several investigators have shown a direct relationship between
the boron content in leaves (foliar) and the severity of the symptoms
of toxicity. Gilliam & Watson (1981) conducted an experiment in which
Anderson yews (Taxus media) were grown in soil at four boron
concentrations (0.5, 5.0, 25.0, or 50 mg/kg). Symptoms of toxicity
were observed when foliar boron accumulation reached concentrations
ranging from 85 to 100 µg/g of dry tissue. The observed symptoms
included leaf tip yellowing, followed by necrosis and premature
defoliation. Suppression of shoot and root growth was observed at 50
mg boron/kg soil.
Shopova et al. (1981) found that concentrations of 16, 24, and
32 mg boron/kg soil resulted in a decline in plant development,
yellowing of leaves, late flowering, reduction of mitotic frequency in
root tip cells, and abnormalities during meiosis in the poppy
(Papaver somniferum).
Kluge & Podlesak (1985) found that symptoms due to boron excess
begin to develop on the leaves (leaf tip necroses) of pot-grown spring
barley (Hordeum vulgare) as soon as the boron content of the leaf
tissue reaches 60-80 mg/kg dry weight.
Gestring & Soltanpour (1987) grew alfalfa (Medicago sativa) in
three soil types amended with sodium borate at rates of 0, 10, 20, and
40 mg boron/kg. Alfalfa yield was significantly reduced by boron
application in both the sandy loam and loam soils; however, no yield
reduction was observed in the silt loam soil. Soil extractable boron
did not adequately assess boron toxicity, whereas plant boron levels
were a more reliable index of toxicity. The critical range of plant
boron resulting in a yield reduction was 850-975 mg boron/kg plant
tissue (dry weight). The significant difference between the soils
shows the importance of soil pH, organic matter content, and per cent
clay to the toxicity of boron to plants.
Sage et al. (1989) exposed the rare serpentine plant
(Streptanthus morrisonii) to boron (0, 20, 60, 240, 650, 1200, or
2400 µmol/litre) via watering. Plants showed mild to moderate toxicity
symptoms (older leaves exhibiting chlorosis and necrosis) at boron
concentrations of 240 and 650 µmol/litre. Severe toxicity symptoms
(significant leaf loss) were apparent at 1200 and 2400 µmol
boron/litre. Boron levels in the leaves of plants showing severe
toxicity were an order of magnitude higher than those in the leaves of
plants in the field.
Glaubig & Bingham (1985) reported significant linear
relationships between both soil and leaf tissue boron concentrations
and foliar damage in four tree species endemic to California (digger
pine, Pinus sabiniana; California laurel, Umbellularia
californica; madrone, Arbutus menziesii; bigleaf maple,
Acer macrophyllum). Foliar damage over 25% of the leaf/needle area
occurred at saturated soil concentrations ranging from 0.08 to
1.2 mmol boron/litre for the four species and at leaf/needle
concentrations ranging from 30 to 115 mmol boron/kg. The bigleaf maple
was the most sensitive of the four species, and the digger pine was
the most tolerant species.
Under experimental conditions, Shann & Adriano (1988)
demonstrated that chronic foliar aerosol exposures of boron produced
phytotoxicity in relation to boron accumulation in the leaves. Five
crop species (swiss chard, beet, radish, tomato, and cucumber) were
exposed to aerosol concentrations of 0, 1.55, or 3.09 µg boron/cm2
leaf area. The authors concluded that the visual damage (leaf tip
necrosis) resulting from aerosol exposure was identical to that
observed from root boron toxicity for all crops tested.
9.1.3.2 Invertebrates
Boric acid is an effective stomach poison for several species of
insects, including the German cockroach (Blattella germanica).
Pretreatment of wood and other substrates with boric acid or borax can
prevent insect infestation. Baits and aerosols containing boron
compounds (e.g. borax and boric acid) have been shown to control fruit
flies, cockroaches, houseflies, and termites (Butterwick et al.,
1989).
Boron in the diet at concentrations of 0.025-5% caused
sterilization of house flies (Musca domestica) when both sexes were
fed the diet (Borkovec et al., 1969). Boron toxicity in honey bees has
also been observed; a concentration of 50 mg boric acid/litre (8.7 mg
boron/litre) in syrup had no effect on survival, whereas 100 mg boric
acid/litre (17.5 mg boron/litre) caused 50% mortality (Ostrovskij,
1955).
Lang & Treece (1972) conducted a study to determine if boric acid
would induce sterility in the face fly (Musca autumnalis). Adult
male and female flies (50 each/concentration) were exposed to boric
acid concentrations of 0.5%, 0.8%, or 1.0% in each of three media
(water, malt, and dry food) for the first 4 days after eclosion
(hatching from the pupal stage). Complete sterility (100%) was
observed in flies fed the two highest concentrations in dietary malt;
however, a high degree of recovery was observed within 5 days after
cessation of treatment. Many eggs from the females fed 1.0% boric acid
were transparent and watery in appearance; this was possibly due to
the interference of boric acid with egg chorion formation. Almost 100%
mortality occurred in the flies exposed to boric acid concentrations
of 0.5% and 0.8% in water within the first 4 days of treatment. In a
subsequent test, erratic sterility with recovery of fertility was
observed in face flies exposed to boric acid concentrations of 0.1%
and 0.3% in water.
9.1.3.3 Vertebrates
In order to determine the impact of high levels of boron on
water-fowl in Kesterson Reservoir (located within the Kesterson
National Wildlife Refuge in the San Joaquin Valley of California,
USA), a number of investigators have studied the effects of controlled
boron exposure to mallard ducks. Smith & Anders (1989) studied the
effects on reproduction in mallards fed diets supplemented with 0, 30,
300, or 1000 mg boron/kg as boric acid (concentrations known to be
found in the field). The adults were fed this diet prior to mating and
during egg incubation, and the ducklings were fed this diet after
hatching. In the 1000 mg/kg group, hatching success of fertile eggs
and duckling survival were significantly reduced, and embryo mortality
was significantly higher. The body weight of hatchlings was lower in
the 300 and 1000 mg/kg groups than in controls. Growth rate of
ducklings was significantly reduced at all three dose levels. No
effect on egg fertility, eggshell thickness, or eggshell quality was
observed at any dose level. In addition, boron did not produce any
overt signs of toxicity in young or adult mallards. The researchers
were unable to determine whether duckling mortality and impaired
growth were due to pre-hatching or post-hatching exposure or the
combined effect.
Hoffman et al. (1990) examined the effect of dietary boron
exposure on the survival, growth, and physiology of ducklings hatched
from uncontaminated eggs. Day-old mallards were exposed to 0, 100,
400, or 1600 mg boron/kg diet as boric acid for 10 weeks. No
significant effect on survival was found. The highest dietary
concentration of boron significantly decreased overall growth and the
rate of growth (sexes combined), whereas lower concentrations altered
growth only in female ducklings. Significantly decreased food
consumption was observed in the high-dose group during the first
3 weeks and in all dose groups during the 2nd week. Effects on blood,
brain, and liver biochemistry were also observed in the high-dose
group. Overall, dietary boric acid proved to be less toxic to
ducklings hatched from untreated eggs than to ducklings hatched from
boron-contaminated eggs.
Boron has also been shown to affect the behaviour of mallard
ducklings. Whitworth et al. (1991) analysed the activity schedules and
behaviour durations of day-old ducklings that received diets
containing 0, 100, 400, or 1600 mg boron/kg as boric acid. The highest
dose level had significant effects on the activity schedules of the
ducklings, including increased time at rest. Ducklings exposed to 1600
mg/kg in the diet spent less time in alert behaviours and in the water
compared with controls.
These studies suggest that high dietary levels of boron can
adversely affect normal duckling development and survival. In
addition, interactive effects have been demonstrated in mallard
ducklings given both boron and selenium in the diet. Hoffman et al.
(1991) noted further growth reduction in ducklings fed diets with 60
mg selenium/kg (as seleno-methionine) and 1000 mg boron/kg (as boric
acid) compared with ducklings fed diets with only 1000 mg boron/kg (as
boric acid) over a 4-week period. These effects were magnified when
dietary protein was restricted. Reduction of the dietary protein
content from 22% to 7% in combination with the same selenium and boron
exposure scenario as above resulted in significant duckling mortality.
These findings suggest that selenium and boron may interact to cause
more severe toxicological effects in waterfowl than boron causes
alone, especially in cases where dietary protein is restricted.
The liver boron content of mallards fed with dietary boron at 0,
30, 300, or 1000 mg/kg was found to range from 0 to 51 µg/g dry
weight. The level of 33-51 µg boron/g in liver was associated with
reproductive impairment in the 1000 mg/kg exposed group (Smith &
Anders, 1989).
Boron levels in the livers of aquatic birds from the Grassland
Water District of California, USA, were also examined during
1985-1988. The levels detected ranged from 1.7 to 40 µg boron/g dry
weight; the highest level detected is in a range associated with
reproductive impairment (Paveglio, 1992).
9.2 Field observations
9.2.1 Aquatic
Concentrations of boron in trout hatcheries of Germany, the
United Kingdom, and the USA (California) have been determined. Mean
concentrations of boron in feed waters to 20 United Kingdom rainbow
trout hatcheries were 0.009-0.021 mg/litre (ECETOC, 1997). A range of
0.002-0.107 mg boron/litre was noted in 18 hatcheries in Germany. A
range of higher concentrations, 0.02-1.0 mg boron/litre, was reported
for the 10 largest hatcheries in California (Bingham, 1982; EA, 1994).
Concentrations of boron in streams and lakes of the United
Kingdom and USA capable of sustaining trout species were found to be
similar to concentrations found in hatcheries. Median boron
concentrations from nine different water regions within the United
Kingdom ranged from 0.007 to 0.272 mg/litre, with maximum values in
each of the regions ranging from 0.113 to 2.3 mg boron/litre (ECETOC,
1997). Boron concentrations in California surface waters that
supported viable populations of wild rainbow trout ranged from <0.01
to 13.1 mg/litre (Bingham, 1982). An update to the Bingham report was
provided by a survey of 37 fisheries biologists in the western USA
(EA, 1994). No instances were found where rainbow trout populations
were limited by boron. Several locations were found to have successful
trout populations with aqueous boron concentrations near or above
1 mg/litre. Specific examples include East and Paulina lakes in Oregon
(>0.9 mg boron/litre), Firehole River in Wyoming (>0.9 mg
boron/litre), Napa River in California (>1.2 mg boron/litre), and
Little Warm Springs in California (>3.2 mg boron/ litre). The first
two sites are renowned trophy trout waters. Important fish species
other than trout, such as northern pike, sturgeon, and catfish, were
reported to live in streams with higher boron concentrations, up to
approximately 2 mg/litre, such as in the Poplar River in Montana and
Souris River in North Dakota. Neither of these latter rivers was
reported to contain rainbow trout owing to excessive temperatures and
unsuitable habitat (EA, 1994).
9.2.2 Terrestrial
Boron deficiencies in terrestrial plants have been reported in
many countries of the world (Kabata-Pendias & Pendias, 1984). For
example, deficiency has been reported in Canada, New Zealand, Sweden,
Nigeria, the United Kingdom, the USA, and some arid regions of India
and Pakistan. Boron deficiency is more likely to occur in
light-textured, acid soils in humid regions, because of boron's
susceptibility to leaching. Boron deficiency may also occur in
heavy-textured soils with high pH, because boron is readily adsorbed
under these conditions. Boron deficiency is more widespread than the
deficiency for any other micronutrient (Gupta et al., 1985).
When deficiency exists, boron applications increase yield and
improve quality of many crops (Gupta et al., 1985). Hopmans & Flinn
(1984) observed severe dieback in Monterey pine plantations during dry
years in south-eastern Australia owing to boron deficiency. Borax
applied in the spring at rates of 50, 100, or 150 kg/ha resulted in a
significant improvement in height growth of fertilized trees.
Irrigation water is one of the main sources of high boron levels
resulting in toxicity in the field. Few irrigation waters contain
enough boron to injure plants directly. However, it is the continued
use and concentration in the soil as a result of evapotranspiration
that lead to the eventual toxicity problems (Gupta et al., 1985). In
waters used for irrigation, Wilcox (1958) reported a critical boron
concentration of 0.3-1.0 mg/litre for sensitive crops, such as citrus
and other fruits. Semi-tolerant crops, such as potatoes, tomatoes, and
oats, tolerate concentrations of 1-2 mg/litre; tolerant crops, such as
sugar beets, onions, and carrots, can withstand concentrations of
2-4 mg/litre.
The symptoms of boron toxicity are predominantly manifested in
the leaves and roots; however, Eaton et al. (1941) observed that these
effects were frequently absent in stone fruit trees, with boron
accumulation occurring in the bark and fruits rather than the leaves.
Dye et al. (1984) reported that boron concentrations in bark of 31-120
µg/g dry matter and in fruit of 90-311 µg/g dry matter resulted in
bud-drop and branch die-back in peach trees. In nectarine trees with
boron tissue concentrations of 380-457 µg/g dry matter, the buds
failed to swell and subsequently dropped in the spring. Dye et al.
(1983) observed the same symptoms in apricot trees given about 2.4 g
of borax per tree during planting. Buds on unhealthy trees that failed
to break contained 40-100 µg boron/g dry matter.
Pollutant sources of boron, in the form of either airborne
emissions or leachates from soil application, have been shown to
produce toxic symptoms in endemic species. Smidt & Whitton (1975)
studied the toxic responses in a stand of Monterey pine adjacent to a
boiler house. The trees were exposed to boron as a result of dumping
of furnace ash into an adjacent gully and from airborne ash emitted
from the furnace chimney. The average boron concentration in the ash
was 1900 mg/kg. Chlorosis and necrosis of the needles were most severe
closest to the furnace chimney, with the effects decreasing with
increased distance from the furnace. No evidence of toxicity was
observed at needle boron concentrations of 13-70 µg/g, whereas toxic
symptoms were observed at needle boron concentrations of approximately
200 µg/g. The authors concluded that foliar absorption of airborne
boron, as well as root absorption from boron in soil solution,
resulted in the injury to this stand of trees.
Temple & Linzon (1976) reported that atmospheric boron emissions
from an appliance manufacturing plant and a fibreglass manufacturing
plant resulted in >35% foliar necrosis on vegetation within 700 m of
the plant, with 1-15% foliar necrosis occurring within 500 m in all
directions from the plant. The amount of leaf damage was proportional
to the boron concentration in the leaves. Species of maple
(Acer sp.) appeared to be the most sensitive, with slight necrosis
occurring at a concentration of 432 µg/g in unwashed foliage, moderate
necrosis at foliar concentrations ranging from 707 to 1013 µg/g, and
severe necrosis at foliar concentrations of 1207-1560 µg/g.
Lang et al. (1986) found that boron thresholds for detectable
levels (10%) of visible injury due to boron toxicity were about 500
mg/kg of foliage for both bigleaf maple and digger pine growing near
geothermal generating units.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health exposures
Boron is a naturally occurring element found combined with other
elements (primarily oxygen) throughout the environment. It is found in
the Earth's crust, with the majority of readily available forms
occurring in the ocean. It is estimated that more boron is released
into the environment by natural weathering than from anthropogenic
sources. Boron is not present in the atmosphere at significant levels.
Boron is present in surface water and groundwater and is readily
adsorbed on the surfaces of soil particles. Boron is present in food,
beverages, and drinking-water.
Boron has a wide variety of uses in the manufacture of glass,
glass products, antiseptics, cleaning products, pharmaceuticals,
biological growth control agents, wood and leather preservatives, and
insecticides. It is also used in photographic chemicals, enamels and
frits, and fire retardants.
Exposure to boron compounds can occur by inhalation, ingestion,
and dermal contact. Boron compounds are absorbed from the respiratory
and gastrointestinal tracts, as indicated by increased levels of boron
in the blood, tissues, and urine. Dermal absorption of boron across
intact skin is negligible.
Specific information on population exposures from different
countries was discussed in chapter 5. This information was used to
determine mean intakes in ambient air, drinking-water, soil, food, and
consumer products. From these mean intakes, per cent allocations of
the TIs can be developed, as shown in Table 22. In general, food and
drinking-water form the greatest contributions to boron intake in
humans, at about 65% and 30%, respectively.
Only a few human studies have been conducted to assess health
effects associated with exposure to boron compounds. The available
data suggest that exposure is associated with short-term and
reversible irritant effects on the upper respiratory tract,
nasopharynx, and eye. The sole long-term (7-year) follow-up study
failed to identify any long-term health effects, although a healthy
worker effect cannot be entirely ruled out, given the rate of
attrition (47%). In two descriptive studies that assessed fertility
and secondary sex ratios in relation to exposure, no detrimental
effects on demonstrated fertility for the study samples were reported.
Although an excess percentage of female births has been suggested, the
absence of statistical significance and attention to other co-variates
known to affect sex ratios warrants careful interpretation of this
finding. No studies have been identified that assess the spectrum of
reproductive outcomes, such as time-to-pregnancy, conception delays,
spontaneous abortions, or semen quality. The role of other lifestyle
or behavioural factors in relation to health and fertility requires
further study to identify potentially sensitive populations and to
evaluate reproductive effects more fully.
Table 22. Estimation of exposures based on ranges of mean
concentrations in several environmental media on a global basis,
and allocation of the TI among media
Environmental media Mean intakea % allocation of TI
(mg/day)
Ambient air 0.000 44 Nil
Drinking-water 0.6b approx. 30
Soil 0.000 5 Nil
Food 1.2 approx. 65
Consumer products 0.1 5
Total 1.9 100
a Mean intakes are estimated in chapter 5.
b Assuming an intake of 2 litres of water per day.
10.2 Choice of critical effect and application of uncertainty factors
The critical effect appears to lie within the area of
developmental toxicity. Other effects, such as reproductive toxicity,
also occur at slightly higher doses and were considered in the choice
of critical effects through a comparison of LOAELs (Table 17) or
benchmark doses (BMDs) (Moore et al., 1997).a Several developmental
effects from boron exposure have been noted in rats, mice, and
rabbits. Again, these effects are the most sensitive in a larger
toxicity database that includes sub-chronic and chronic bioassays. The
specific critical effect within these developmental toxicity studies
is a decreased average fetal body weight per litter in the rat
(Heindel et al., 1992; Price et al., 1996a).
a The Weir & Fisher (1972) 2-year dog studies were not used directly
for risk assessment for several reasons. The NOAEL and LOAEL were
taken from two studies; one was 2 years long, and the high-dose group
was a supplemental group terminated at 38 weeks. There were a limited
number of animals (4/dose) per test group. One control group was used
for both borax and boric acid, so that there were never more than 2
control animals sacrificed at one time. Some of the control animals
showed some form of testicular damage.
In the Price et al. (1996a) study, boric acid was administered in
the diet to CD rats from gestational day 0 to 20 at 3.3, 6.3, 9.6,
13.3, or 25 mg boron/kg body weight per day. For the low- to high-dose
groups, fetal body weights were 99, 98, 97, 94, and 88% of controls.
Fetal body weight was statistically significantly decreased only in
the 13.3 and 25 mg/kg body weight per day dose groups on gestational
day 20. The NOAEL for decreased fetal body weight was 9.6 mg boron/kg
body weight per day, and the LOAEL was 13.3 mg boron/kg body weight
per day. The results of this study provide a NOAEL and LOAEL that
complement the LOAEL of 13.6 mg boron/kg body weight per day in the
Heindel et al. (1992) study.b This NOAEL is also similar to BMDs for
developmental toxicity (see appendix).
For interspecies variation, a default value of 10 has often been
applied in the absence of actual data showing less than the usual
variation between species. For inter-individual (intraspecies)
variation, a default value of 10 has often been applied in the absence
of actual data within a species. A scheme has been adopted by IPCS
(1994) that allows for subdivision of each of these defaults to
incorporate appropriate data on toxicodynamics or toxicokinetics.
Where appropriate data exist, this scheme improves the extrapolation
process and uses correction factors for toxicodynamic and
toxicokinetic data instead of 10-fold uncertainty factors for inter-
and intraspecies variation. For interspecies differences, the 10-fold
factor is divided into a default factor of 100.4 for toxicodynamics
and 100.6 for toxicokinetics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.4 × 100.6 = 10).
For inter-individual (intraspecies) differences, the 10-fold
factor is divided into a default factor of 100.5 each for
toxicokinetics and toxicodynamics in the absence of toxicodynamic or
toxicokinetic data. Multiplying these subdivided default factors gives
the default 10-fold uncertainty factor (100.5 × 100.5 = 10).
The pharmacokinetics of boron appear to be quite similar across
species in the following respects:
a) Absorption of borates is essentially complete (approximately
95% in humans and rats), and boron appears rapidly in blood and
body tissues of several mammalian species following ingestion.
b In fact, the Price et al. (1996a) study was conducted specifically
to address the lack of a NOAEL from the Heindel et al. (1992) study.
Similarity in study designs and results from these two studies greatly
strengthens the dose-response relationship for developmental toxicity
as the critical effect.
b) Distribution of boron in mammals appears to occur by passive
diffusion throughout the body fluids. In contrast to soft tissues
and blood, bone shows selective uptake of boron (>4 times
higher than serum) and significantly longer retention times.
c) Metabolism of boric acid is thermodynamically unfavourable in
biological systems. Thus, the ionic species in systemic
circulation are expected to be equivalent across mammals. This
eliminates a major source of potential uncertainty for risk
extrapolation, as interspecies differences in enzymatic pathways
and/or metabolic rates do not need to be taken into
consideration.
d) Elimination kinetics (especially route of elimination and
terminal half-life) also appear to be similar for humans and
rats.
The similarities in pharmacokinetic parameters between humans and
rats, the species defining the NOAEL for laboratory studies, reduce
the uncertainty for risk extrapolation between these two species.
10.3 Derivation of the tolerable intake
A TI is defined as an estimate of the intake of a substance that
can occur over a lifetime without appreciable health risks. The TI is
derived on the basis of the NOAEL of the critical effect, the adverse
effects judged to be the most appropriate for determining the TI,
using appropriate uncertainty factors. The use of the critical effect,
judged here to be developmental toxicity, is expected to protect
against other effects such as reproductive toxicity occurring at
higher doses.
9.6 mg/kg body weight per day
TI =
[100.4 × 100.1] × [100.5 × 100.4]
= 0.4 mg/kg body weight per day
= 400 µg/kg body weight per day
where:
* 9.6 mg/kg body weight per day is the NOAEL from a well conducted
developmental study with decreased fetal body weight occurring in
a dose-related manner
* 100.4 × 100.1 (=100.5) is the uncertainty factor for interspecies
differences:
Dynamics: Data do not exist to support a factor other than the
default value of 100.4. Moreover, differences in LOAELs among
rats, rabbits, and mice for the critical effect (Table 16)
support the default value. The Task Group judged that 100.4 was
appropriate.
Kinetics: Oral absorption of boron in rats and humans is
quantitatively similar, with values of 94% for humans and 95% for
rats (section 6.1.1). The ratio of these absorption rates is
approximately 1.
Distribution of boron in rats and humans appears quantitatively
similar, as determined by a comparison of blood boron levels
after doses in either diet or drinking-water (Fig. 1, section
6.2.2). Ratios of rat blood boron values to a regression line for
human blood boron values are as low as 0.7 and as high as 6, with
the majority of values in the range of 2-3. Comparative
regression techniques should be pursued to confirm this trend.
Pregnant rats appear to have lower blood boron values than non-
pregnant rats when given similar doses. Comparative data for
pregnant and non-pregnant humans are lacking.
Metabolism of boron is thought not to occur in humans or animals
owing to the excessive energy required to break the boron-oxygen
bond (section 6.3). As it is unlikely that there are any
differences between species in the metabolism of boron, the ratio
of metabolic parameters is 1.
Elimination of boron in rats and humans appears quantitatively
similar, with half-life values in humans of 21 h in volunteers
and approximately 13 h in poisonings, and values in rats of
14=19 h (section 6.4). The ratio of these elimination half-life
values is approximately 1.3 when human volunteer data are
compared with the rat data, or 0.8 when human poisoning data are
compared with the rat data. The mean of these two ratios is
about 1.
As a result, the Task Group judged that the default value of
100.6 could be reduced to 100.1.
* 100.5 × 100.4 (=100.9) is the uncertainty factor for
inter-individual (intraspecies) differences:
Dynamics: Data in humans do not exist to support a value
different from the default of 100.5. Animal studies suggest that
intraspecies variability in toxicodynamics exists. The Task Group
judged that 100.5 was appropriate.
Kinetics: Data exist in humans to suggest some limited
variability in boron absorption and/or distribution (Nielsen,
1995). However, the lack of boron metabolism in humans and
experimental animals suggests some reduction in the default value
of 100.5. The Task Group judged that 100.4 was appropriate.
Other uncertainty factors for adequacy of database are not
considered necessary, because the overall database includes subchronic
and chronic studies on several species and several reproductive
studies.
The total uncertainty factor,a,b is therefore:
100.5 × 100.9 = 101.4 (or 25)
Data are inadequate to allow the development of a TI for
inhalation exposure (TIi).
10.4 Derivation of guidance values
Exposure to boron from various media varies with the locality. In
areas where data on exposure are available, specific guidance values
that are tailored to the local circumstances should be developed from
the TI presented in this document.
For example, guidance values for specific media could be
developed by first using the allocations shown in Table 22 with the TI
derived in section 10.3. Table 23 shows the resulting allocation of
the TI by media in mg/kg body weight per day.
The second step in the development of a guidance value would be
to pick appropriate body weight and exposure assumptions. Two examples
are given using allocations found in Table 23. The resulting guidance
value (GV) for example A in drinking-water would be:
a After closure of the Task Group meeting, Dr. M. Dourson, after
additional consideration, dissented from the Task Group's
recommendation on the uncertainty factor.
b Subsequent to the Task Group meeting, a provisional guideline for
boron in drinking-water was recommended by the Working Group Meeting
on Chemical Substances in Drinking-Water, using a different
uncertainty factor, based upon a different weighting attached to some
toxico-kinetic studies for that area of application (WHO, 1998 a,b).
Example A
0.12 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 4 mg/litre
where:
* 64 kg is the average human body weight (IPCS, 1994)
* 2 litres/day is one assumption of water intake (IPCS, 1994).
The resulting guidance value (GV) for example B would be:
Example B
0.04 mg/kg body weight per day × 64 kg body weight
GV =
2 litres/day
approx. 1.3 mg/litre
Assumptions of food intake and consumer product use could be used
with allocations given in Table 23 to generate tolerances or other
risk management standards. Individual countries are encouraged to
develop guidance values using allocations, body weights, and exposure
assumptions that are appropriate for their populations.
Table 23. Examples of allocation of TI by media
Environmental Example A Example B
media
Per centa mg/kg body Per cent mg/kg body
weight per day weight per day
Ambient air Nil 0 Nil 0
Drinking-water 30 0.12 10 0.04
Soil Nil 0 Nil 0
Food 65 0.26 85 0.34
Consumer products 5 0.02 5 0.02
Total 100 0.4 100 0.4
a Values from Table 22.
10.5 Evaluation of effects on the environment
10.5.1 Exposure
Boron is not present in the atmosphere at significant levels;
however, the total amount present in the atmosphere at any one time is
significant owing to the huge volume of the atmosphere. Boron
concentrations in air range from <0.5 to approximately 80 ng/m3,
with an average of 20 ng/m3.
Boron occurs naturally in rocks, with concentrations ranging from
5 mg/kg in basalts to 100 mg/kg in shales. Concentrations of boron in
surface water are dependent on such factors as the geochemical nature
of the drainage area, proximity to marine coastal regions, and inputs
from industrial and municipal effluent discharges. Concentrations of
boron range widely, from 0.001 to as much as 360 mg/litre. However,
mean concentrations for waters of Europe, Pakistan, Russia, and Turkey
are typically well below 0.6 mg boron/litre. Concentrations of boron
in waters in Japan, South Africa, and South America are generally
below 0.3 mg/litre. Typical concentrations in North American waters
are below 0.1 mg boron/litre, with about 90% at 0.4 mg boron/litre or
below.
Boron is adsorbed onto soil particles, with the degree of
adsorption depending on the type of soil, pH, salinity, organic matter
content, iron and aluminium oxide content, iron- and aluminium-hydroxy
content, and clay content. Boron adsorption can vary from being fully
reversible to irreversible, depending on the soil type and condition.
Boron occurs in soils at concentrations ranging from 10 to 300 mg/kg
(average 30 mg/kg), depending on the type of soil, amount of organic
matter, and amount of rainfall.
Boron does not bioaccumulate in aquatic invertebrates and fish.
Based on wet weights, boron concentrations in marine invertebrates and
fish are similar to the levels found in the exposure media, between
0.5 and 4 mg/kg. Bioconcentration factors for two freshwater fish
species were found to be 0.3. The results of studies of boron
accumulation in plants, insects, and fish have shown that boron
bioaccumulates in plants but does not biomagnify in aquatic
food-chains. Concentrations of boron have been shown to range between
26 and 382 mg/kg in submerged aquatic freshwater plants, from 11.3 to
57 mg/kg in freshwater emergent vegetation, and from 2.3 to 94.7 mg/kg
dry weight in terrestrial plants.
10.5.2 Effects
Boron is an essential micronutrient for cyanobacteria and
diatoms. Considering all microorganisms, algae and protozoa appear to
be equisensitive to boron and bacteria the most tolerant. Both algae
and protozoa provide NOEC (including EC3) values between 0.3 and 20
mg boron/litre.
Chronic studies with Daphnia magna provided consistent NOECs
between 6 and 10 mg boron/litre. Long-term outdoor pond and field
studies with microorganisms and invertebrates provided NOEC values up
to 1.52 mg boron/litre.
Via acute tests, the rainbow trout and zebra fish were determined
to be the most sensitive taxa, with acute values around 10 mg
boron/litre. Further tests with embryo-larval stages of several fish
species showed rainbow trout to be the most sensitive to boron. Tests
conducted with early life stages of rainbow trout in reconstituted
water overpredicted toxicity compared with tests conducted with
natural water. No adverse effects were found in natural water
exposures containing up to 0.75 mg boron/litre. Consistent LOECs were
between 1.1 and 1.7 mg boron/litre. These laboratory values were
confirmed by several observations of successful trout populations
thriving in hatcheries, streams, and lakes containing boron
concentrations up to 1 mg/litre.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
The symptoms of boron deficiency in plants include cessation of root
and leaf growth, necrosis, retardation of enzyme reactions, and
reduced pollen germination. The initial symptoms of boron toxicity in
plants are chlorosis, necrosis, and a subsequent loss of
photosynthetic capacity, which reduces plant productivity.
Mallard duckling growth was adversely affected at dietary levels
of 30 and 300 mg/kg, and survival was reduced at 1000 mg/kg.
10.5.3 Risk evaluation
Risk characterization is based on the comparison of the
environmental concentration with a concentration protective for an
ecosystem. Ambient concentrations of boron in surface waters have been
reported for several countries covering four continents. The data
indicate that mean or median levels are approximately 0.1 mg
boron/litre and that 90th-percentile or greater values are
approximately 0.5 mg boron/litre. Considerable data exist on the
toxicity of boron to freshwater organisms and ecosystems. Acute tests
with invertebrates and fish showed that rainbow trout and zebra fish
were most sensitive to borate (LC50 approx. 10 mg boron/litre).
Chronic results for algae, higher plants, and fish clearly showed that
the rainbow trout was the most sensitive species, with consistent
NOECs and LOECs of about 1 mg boron/litre. Confirmed were several
observations of healthy rainbow trout populations in US hatcheries and
streams at or above 1 mg boron/litre. Not surprisingly, biocenoses
(model ecosystems) covering all stages of the aquatic food-web
(excluding fish) were more tolerant of boron than were rainbow trout
(NOEC up to 1.52 mg boron/litre). Given all this information, the
comparison of the environmental effects concentration with the general
ambient environmental levels indicates that the risk of adverse
effects of boron on the aquatic ecosystem is low. In a few boron-rich
environments, natural levels will be higher. It is reasonable to
assume that aquatic organisms in such habitats may be adapted to the
local conditions.
Boron deficiencies in terrestrial plants have been reported in
many countries. Boron deficiency is more likely to occur in
light-textured, acid soil in humid regions, because of boron's
susceptibility to leaching. In general, there is a small range between
deficiency and toxicity. However, considerable variation exists
between species in their resistance to boron. Species sensitive to
boron are known to include citrus, stone fruits, and nut trees;
semi-tolerant species include tubers and cereals; and tolerant species
include most vegetables. Toxicity due to excess boron is much less
common in the environment than boron deficiency. Irrigation water is
one of the main sources of high boron levels that result in toxicity
in the field. However, few irrigation waters contain enough boron to
injure plants directly; it is the continued use and concentration in
the soil resulting from evapotranspiration that lead to the eventual
toxicity problems. Pollutant sources of boron, in the form of either
airborne emissions or leachates from soil application, have also been
shown to produce toxic symptoms in endemic plant species growing in
the immediate vicinity.
Levels of boron measured in aquatic plants from Kesterson
National Wildlife Refuge, California, USA (an evapotranspiration sink
that receives boron-rich run-off from irrigated agricultural fields)
are higher than those found to cause effects on mallard ducklings in
the laboratory. Therefore, there appears to be a risk to waterfowl in
this area. However, the bioavailability of such boron levels is
uncertain. The extent of risk to waterfowl in most geographical areas
is expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Boron is a naturally occurring element that is found in nature in
the form of borates in the oceans, sedimentary rocks, coal, shale, and
some soils. Natural sources of borates released into the environment
are the oceans, geothermal steam, and natural weathering of clay-rich
sedimentary rocks. Boron is also released from anthropogenic sources
to a lesser extent.
Boron is an essential micronutrient for higher plants, with
interspecies differences in the levels required for optimum growth.
Boron deficiency has been observed in many countries throughout the
world. There is a small range between deficiency and toxicity in some
plants.
Comparison of the environmental no-effect concentration
(1 mg/litre) with the general ambient environmental boron levels
indicates that the risk of adverse effects of boron on the aquatic
ecosystem is low. In a few boron-rich environments, natural levels
will be higher. It is reasonable to assume that aquatic organisms in
such habitats may be adapted to the local conditions.
For humans, boron exposure occurs primarily through the diet and
drinking-water. The mean global boron concentration in drinking-water
was considered to be between 0.1 and 0.3 mg boron/litre. For the
general population, the greatest boron exposure comes from the oral
intake of food. The mean daily intake of boron in the diet is
estimated to be near 1.2 mg/day.
In humans and animals, boric acid and borate are absorbed from
the gastrointestinal and respiratory tracts. Greater than 90% of
administered doses of these compounds are absorbed, as evidenced by
excretion in the urine, which is rapid, occurring over a few to
several days.
Animal experiments have shown that boron in the form of boric
acid and borate demonstrates reproductive and developmental toxicity
at levels that are approximately 100- to 1000-fold greater than normal
exposure levels. There is a lack of sufficient toxicity data on
humans. The TI of boron was set as 0.4 mg/kg body weight per day. The
allocation of the TI in various media should be based on the exposure
data of individual countries.
11.2 Recommendations
a) Water and food guideline values should be based on the TI
provided by this document.
b) The TI should be applied with the understanding that boron may
provide a physiological benefit for human health.
c) It should be recognized in applying standards that boron is
essential for some constituents of the environment (e.g. boron is
an essential micronutrient for higher plants).
d) Dietary supplements that exceed the TI should be avoided.
12. FURTHER RESEARCH
a) Further studies regarding global cycling of boron.
b) Determine whether boron is required for normal fetal and post-
natal development for higher animals.
c) Define a biochemical function that confirms the essentiality of
boron for higher animals.
d) Determine the homeostatic mechanism through which boron
concentrations are maintained during low dietary intakes.
e) Further studies on the absorption, distribution, and clearance of
boron at oral doses in the range of 0.01-1 mg boron/kg body
weight per day.
f) A modern rat reproduction study to determine the potential for
developmental effects of borates in succeeding generations in a
sensitive animal species.
g) Analytical epidemiology studies to assess the spectrum of
reproductive and developmental outcomes to assess boron toxicity
in targeted human populations.
h) Epidemiological studies that incorporate toxicological and
physiological components to enhance an understanding of the
toxicokinetics and physiology of boron in populations, including
potentially sensitive ones.
i) Further quantitative definition of the maximum tolerable
concentration for sensitive aquatic species.
j) A refined understanding of the relationship of water and dietary
uptake of boron into waterfowl.
k) Development of an approach that integrates essentiality and
toxicity for the establishment of acceptable boron concentrations
in aquatic and terrestrial environments.
13. EVALUATIONS BY INTERNATIONAL BODIES
The EC Cosmetics Directive 76/768/EEC (and its subsequent
amendment) sets an upper limit of boric acid in cosmetic products,
which must not contain more than 5% in talc, 0.5% in oral hygiene
products, and 3% in other products. Only the talc containing boric
acid must, in addition, not be used on children less than 3 years of
age.
In 1997, subsequent to the Task Group meeting, a provisional
guideline for boron in drinking-water was recommended by the Working
Group on Chemical Substances in Drinking-Water, which updated the
Guidelines for drinking-water quality (WHO, 1998a,b).
REFERENCES
ACGIH (1991) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, pp 141-148.
Adams FS, Cole H Jr, & Massie LB (1973) Element constitution of
selected aquatic vascular plants from Pennsylvania: Submersed and
floating leaved species and rooted emergent species. Environ Pollut,
5: 117-147.
Ahl T & Jönsson E (1972) Boron in Swedish and Norwegian fresh waters.
Ambio, 1(2): 66-70.
Alabaster JS (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int Pest
Control, 11: 29-35.
Alexander GV, Nusbaum RE, & MacDonald NS (1951) The boron and lithium
content of human bones. J Biol Chem, 192: 489-496.
Allen BC, Strong PL, Price CJ, Hubbard SA, & Daston GP (1996)
Benchmark dose analysis of developmental toxicity in rats exposed to
boric acid. Fundam Appl Toxicol, 32: 194-204.
Anderson BG (1946) Industrial wastes: The toxicity thresholds of
various sodium salts determined by the use of Daphnia magna. Sewage
Works J, 18: 82-87.
Anderson DL, Kitto ME, McCarthy L, & Zollar WH (1994a) Sources of
atmospheric distribution of particulate and gas phase boron. Atmos
Environ, 28: 1401-1410.
Anderson DL, Cunningham WC, & Lindstrom TR (1994b) Concentrations and
intakes of H, B, S, K, Na, Cl and NaCl in foods. J Food Compos Anal,
7: 59-82.
Antia NJ & Cheng JY (1975) Culture studies on the effects from borate
pollution on the growth of marine phytoplankters. J Fish Res Board
Can, 32: 2487-2494.
Aquilio E, Spagnoli R, Seri S, Bottone G, & Spennati G (1996) Trace
element content in human milk during lactation of preterm newborns.
Biol Trace Elem Res, 51: 63-70.
ATSDR (1992) Toxicological profile for boron. Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry.
Atteia O, Védy J-C, & Parriaux A (1993) Trace elements dynamics in
soils and aquifers of Western Switzerland. Stud Environ Sci Environ
Contam, 55: 79-101.
Aznarez J, Ferrer A, Rabadan JM, & Marco L (1985) Extractive
spectrophotometric and fluorimetric determination of boron with
2,2,4-trimethyl-1,3-pentanediol and carminic acid. Talanta, 32(12):
1156-1158.
Bai Y & Hunt CD (1996) Absorption and distribution of boron in rats
following a single oral administration of boron. Proc North Dakota
Acad Sci, 50: 53.
Banerji SK (1969) Boron adsorption on soils and biological sludges and
its effects on endogenous respiration. In: Proceedings of the 24th
Industrial Waste Conference, Lafayette, 6-8 May 1969. Lafayette,
Indiana, Purdue University (Engineering Extension Series No. 135).
Banuelos GS, Cardon G, Pflaum T, & Akohoue S (1992) Comparison of dry
ashing and wet acid digestion on the determination of boron in plant
tissue. Commun Soil Sci Plant Anal, 23(17-20): 2383-2397.
Barber LB, Thurman EM, Schroeder MP, & Le Blanc DR (1988) Long-term
fate of organic micropollutants in sewage-contaminated groundwater.
Environ Sci Technol, 22: 205-211.
Barnes DG, Datson GP, Evans JS, Jarabek AM, Kavlock RJ, Kimmel CA,
Park C, & Spitzer HL (1995) Benchmark dose workshop: Criteria for use
of a benchmark dose to estimate a reference dose. Regul Toxicol
Pharmacol, 21: 296-301.
Barr RD, Clarke WB, Clarke RM, Venturelli J, Norman GR, & Downing RG
(1993) Regulation of lithium and boron levels in normal human blood:
Environmental and genetic considerations. J Lab Clin Med,
121: 614-619.
Barres M (1967) Contribution à l'étude de l'isopoly--condensation des
borates alcalins par électrométrie et partages. Rev Chem Minér,
4: 803-838.
Barth RF & Soloway AH (1994) Boron neutron capture therapy of primary
and metastatic brain tumors. Mol Chem Neuropathol, 21(2-3): 139-154.
Barth RF, Adams DM, Soloway AH, Mechetner EB, Alam F, & Anisuzzaman KM
(1991) Determination of boron in tissues and cells using
direct-current plasma atomic emission spectroscopy. Anal Chem,
63: 890-893.
Belanger SE, Farris JL, & Cherry DS (1989) Effects of diet, water
hardness and population source on acute and chronic copper toxicity to
Ceriodaphnia dubia. Arch Environ Contam Toxicol, 18: 601-611.
Benson WH, Birge WJ, & Dorough HW (1984) Absence of mutagenic activity
of sodium borate (borax) and boric acid in the Salmonella
preincubation test. Environ Toxicol Chem, 3: 209-214.
Benson SM, White AF, Halfman S, Flexser S, & Alavi M (1991)
Groundwater contamination at the Kesterson Reservoir, California: 1.
Hydrogeologic setting and conservative solute transport. Water Resour
Res, 27: 1071-1084.
Bergmann W (1984) The significance of the micronutrient boron in
agriculture. London, Borax Holdings Limited, 26 pp.
Bergmann W, Bruchlos P, & Marks G (1995) [A contribution to the
question of the limit value for boron in water for Ogragnutes
australis seed.] Tenside Deterg, 32: 229-237 (in German).
Bertine KK & Goldberg ED (1971) Fossil fuel combustion and the major
sedimentary cycle. Science, 173: 233-235.
Beyer KH, Bergfeld WF, Berndt WO, Boutwell RK, Carlton WW, Hoffmann
DK, & Schroeter AL (1983) Final report on the safety assessment of
sodium borate and boric acid. J Am Coll Toxicol, 2: 87-125.
Biggar JW & Fireman M (1960) Boron adsorption and release by soils.
Soil Sci Soc Am Proc, 24: 115-120.
Bingham FT (1982) The boron concentration of wild trout streams in
California. Riverside, California, University of California,
Department of Soil Science (Unpublished document).
Bingham FT, Elseewi A, & Oertli JJ (1970) Characteristics of boron
absorption by excised barley roots. Soil Sci Soc Am Proc, 34: 613-617.
Bingham FT, Page AL, Coleman NT, & Flach K (1971) Boron adsorption
characteristics of selected amorphous soils from Mexico and Hawaii.
Soil Sci Soc Am Proc, 35: 546-550.
Birge WJ & Black JA (1977) Sensitivity of vertebrate embryos to boron
compounds. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Prepared for US EPA by the University of
Kentucky, Lexington) (EPA-560/1-76-008).
Birge WJ & Black JA (1981) Toxicity of boron to embryonic and larval
stages of largemouth bass (Micropterus salmoides) and rainbow trout
(Salmo gairdneri)--Completion report. Cincinnati, Ohio, Procter &
Gamble Company.
Birmingham DJ & Key MM (1963) Preliminary survey: U.S. Borax plant,
California. Cincinnati, Ohio, Occupational Health Research and
Training Facility, Division of Occupational Health.
Black JA, Barnum JB, & Birge WJ (1993) An integrated assessment of the
biological effects of boron to the rainbow trout. Chemosphere, 26:
1383-1413.
Blasser-Grill J, Knoppik D, Amberger A, & Goldbach H (1990) Influence
of boron on the membrane potential in Elodea densa and Helianthus
annus roots and H+ extrusion of suspension cultured Daucus
carota cells. Plant Physiol, 90: 280-284.
Blevins DG & Luk-aszewski KM (1994) Proposed physiologic functions of
boron in plants pertinent to animal and human metabolism. Environ
Health Perspect, 102(Suppl 7): 31-33.
Bodek I, Ehntholt EJ, Glazer AE, Loreti CP, & Lyman W (1988) Chapter
10: Chemical classes, Section 10.9: Boron-containing compounds. In:
Bodek I, Lyman WJ, Reehl WF, Rosenblatt DH, Walton BT, & Conway RA ed.
Environmental inorganic chemistry: Properties, processes, and
estimation methods. Oxford, New York, Pergamon Press,
pp 10.9/1-10.9/10.
Bonilla I, Garcia-Gomez M, & Mates P (1990) Boron requirements in
cyanobacteria: Possible role in the early evolution of photosynthetic
organisms. Plant Physiol, 94: 1554-1560.
Borkovec AB, Settepani JA, LaBrecque GC, & Fye RL (1969) Boron
compounds as chemosterilants for house flies. J Econ Entomol, 62:
1472-1480.
Bowen JE & Gauch HG (1966) Nonessentiality of boron in fungi and the
nature of its toxicity. Plant Physiol, 41: 319-324.
Boyd CE & Walley WW (1972) Studies of the biogeochemistry of boron. 1.
Concentrations in surface waters, rainfall and aquatic plants. Am Mid
Nat, 88: 1-14.
Bringmann G & Kuhn R (1977) [The toxicity of waterborne contaminants
towards Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in
German with English summary).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kuhn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G, Kuhn R, & Winter A (1980) [Determination of the
biological effect of water pollutants in protozoa: III. Saprozoic
flagellates.] Z Wasser Abwasser Forsch, 13: 170-173 (in German with
English summary).
Brown TF, McCormick ME, Morris DR, & Zeringue LK (1989) Effects of
dietary boron on mineral balance in sheep. Nutr Res, 9: 503-512.
Budavari S, O'Neil MJ, Smith A, & Heckelman PE ed. (1989) The Merck
index, 11th ed. Rahway, New Jersey, Merck and Co., Inc.
Bundschuh J (1992) Boron contamination of the ground- and surface
waters of Lerma Valley, Argentina. Aqua (Lond), 41(1): 13-17.
Butterwick L, De Oude N, & Raymond K (1989) Safety assessment of boron
in aquatic and terrestrial environments. Ecotoxicol Environ Saf, 17:
339-371.
Byrne RH Jr & Kester DR (1974) Inorganic speciation of boron in
seawater. J Mar Res, 32: 119-127.
Cáceres LV, Gruttner ED, & Contreras RN (1992) Water recycling in arid
regions: Chilean case. Ambio, 21(2): 138-144.
Canton JH & Adema DMM (1978) Reproducibility of short-term and
reproduction toxicity experiments with Daphnia magna and comparison
of the sensitivity of Daphnia magna with Daphnia pulex and
Daphnia cucullata in short-term experiments. Hydrobiologia,
59: 135-140.
Chapin RE, Ku WW, Kenney MA, McCoy H, Gladen B, Wine RN, Wilson R, &
Elwell MR (1997) The effects of dietary boron on bone strength in
rats. Fundam Appl Toxicol, 35: 205-215.
Choi WW & Chen KY (1979) Evaluation of boron removal by adsorption on
solids. Environ Sci Technol, 13: 189-196.
Clarke WB, Koekebakker K, Barr RD, Downing RG, & Fleming RF (1987a)
Analysis of ultratrace lithium and boron by neutron activation and
mass spectrometric measurement of 3He and 4He. Appl Rad Isot, 38(9):
735-743.
Clarke WB, Webber CE, & Koekebakker K (1987b) Lithium and boron in
human blood. J Lab Clin Med, 109: 155-158.
Corwin DL (1986) A one-dimensional model of chemical diffusion and
sorption in saturated soil and aquatic systems. J Environ Qual,
15: 173-182.
Cotton FA & Wilkinson G (1988) Advanced inorganic chemistry, 5th ed.
New York, John Wiley and Sons, pp 162-165.
Couch EL & Grim RE (1968) Boron fixation by illites. Clays Clay Miner,
16: 249-256.
Cox JA, Lundquist GL, Przyjazny A, & Schmulbach CD (1978) Leaching of
boron from coal ash. Environ Sci Technol, 12: 722-723.
Culver BD, Shen PT, Taylor TH, Lee-Feldstein A, Anton-Culver H, &
Strong PL (1994a) The relationship of blood- and urine-boron to boron
exposure in borax-workers and the usefulness of urine-boron as an
exposure marker. Environ Health Perspect, 102(Suppl 7): 133-137.
Culver BD, Smith RG, Brotherton RJ, Strong PL, & Gray TM (1994b)
Chapter 44: Boron. In: Clayton GD & Clayton FE ed. Patty's industrial
hygiene and toxicology. New York, John Wiley and Sons, vol 2, part 1.
Cumakov A (1991) Chemical methods of fractionation of soil trace
elements: 1. Boron. Chem Abstr, 115: 182048v.
De Jong LEDD (1965) Tolerance of Chlorella vulgaris for metallic and
non-metallic ions. Antonie van Leeuwenhoek, 31: 301-313.
Deverel SJ & Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, California. Environ Sci Technol, 22: 697-702.
Dieter MP (1994) Toxicity and carcinogenicity studies of boric acid in
male and female B6C3F1 mice. Environ Health Perspect, 102(Suppl 7):
93-97.
Dixon RL, Lee IP, & Sherins RJ (1976) Methods to assess reproductive
effects of environmental chemicals: Studies of cadmium and boron
administered orally. Environ Health Perspect, 13: 59-67.
Dixon RL, Sherins RJ, & Lee IP (1979) Assessment of environmental
factors affecting male fertility. Environ Health Perspect, 30: 53-68.
Doonan DJ & Lower LD (1978) Boron compounds (oxides, acid, borates).
In: Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New
York, John Wiley and Sons, vol 4, pp 67-110.
Draize JH & Kelley EA (1959) The urinary excretion of boric acid
preparations following oral administration and topical applications to
intact and damaged skin of rabbits. Toxicol Appl Pharmacol, 1:
267-276.
Dudas MJ (1981) Long-term leachability of selected elements from fly
ash. Environ Sci Technol, 15: 840-843.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1983) Die-back of
apricot trees following soil application of boron. NZ J Exp Agric, 11:
331-342.
Dye MH, Buchanan L, Dorofaeff FD, & Beecroft FG (1984) Boron toxicity
in peach and nectarine trees in Otaga. NZ J Exp Agric, 12: 303-313.
EA (1994) Boron concentrations and rainbow trout populations in seven
states in the western United States. Corvallis, Oregon, EA
Engineering, Science and Technology (Unpublished report prepared for
the Procter & Gamble Company, Cincinnati).
Eaton FM (1944) Deficiency, toxicity, and accumulation of boron in
plants. J Agric Res, 69: 237-277.
Eaton FM, McCallum RD, & Mayhugh MS (1941) Quality of irrigation
waters of the Hollister area of California with special reference to
boron content and its effect on apricots and prunes. US Dept Agric
Tech Bull, 746: 1-59.
ECETOC (1995) Reproductive and general toxicology of some inorganic
borates and risk assessment for human beings. Brussels, European
Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 63).
ECETOC (1997) Ecotoxicology of some inorganic borates - Interim
report. Brussels, European Centre for Ecotoxicology and Toxicology of
Chemicals, 97 pp (Special Report No. 11).
Eckel WP & Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
Superfund '88: Proceedings of the 9th national conference. Washington,
DC, US Hazardous Materials Control Research Institute, pp 282-286.
Eisler R (1990) Boron hazards to fish, wildlife, and invertebrates: A
synoptic review. Washington, DC, US Department of Interior, Fish and
Wildlife Service.
El-Hassanin AS, Labib TM, & Dobal AT (1993) Potential Pb, Cd, Zn and B
contamination of sandy soils after different irrigation periods with
sewage effluent. Water Air Soil Pollut, 66: 239-249.
Elrashidi MA & O'Connor GA (1982) Boron sorption and desorption in
soils. Soil Sci Soc Am J, 46: 27-31.
Emsley J (1989) The elements. Oxford, Clarendon Press, p 32.
Fail PA, George JD, Sauls SW, Dennis SW, & Seely JC (1989) Effect of
boric acid on reproduction and fertility of rodents. Adv Contracept
Deliv Syst, 5: 323-333.
Fail PA, George JD, Grizzle TB, Heindel JJ, & Chapin RE (1990) Final
report on the reproductive toxicity of boric acid (CAS No.  RÉSUMÉ, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé
1.1 Identité, état naturel et méthodes d'analyse
Le bore est un élément naturel qui se trouve sous la forme de
borates dans les océans, les roches sédimentaires, la houille, le
schiste et certaines huiles minérales. Il est très répandu dans la
nature, avec des concentrations de l'ordre de 10 mg/kg dans l'écorce
terrestre (elles vont de 5 mg/kg dans les basaltes à 100 mg/kg dans
les schistes) et d'environ 4,5 mg/litre dans les océans.
Les dérivés boriques les plus importants, qu'il s'agisse de
minéraux ou de produits du commerce, sont le borax à cinq molécules
d'eau, le borax, le perborate de sodium, l'acide borique, la
colemanite et l'ulexite. Aux faibles concentrations et au pH
pratiquement neutre qui caractérisent la plupart des liquides
biologiques, c'est le monomère B(OH)3 qui est l'espèce prédominante
(avec un peu de B(OH)4-), que la source de bore soit l'acide borique
ou un borate. Cela tient au fait que l'acide borique est un acide très
faible (pKa = 9,15).Le perborate de sodium s'hydrolyse pour donner du
peroxyde d'hydrogène et du métaborate; il peut donc présenter des
propriétés chimiques et toxicologiques un peu différentes de celles
des autres borates.
Les méthodes utilisant un plasma à couplage inductif (ICP) sont
les méthodes de choix pour le dosage des faibles quantités de bore
présentes dans les échantillons biologiques et environnementaux; les
méthodes colorimétriques doivent être utilisées avec prudence.
1.2 Production, usages, destinée dans l'environnement et sources d'exposition
Les dépôts de borates économiquement exploitables sont rares et
se trouvent dans des zones arides de Turquie, des Etats-Unis, du
Chili, de Russie, de Chine et du Pérou. La production mondiale totale
de minéraux contenant du bore--principalement de la colemanite, de
l'ulexite, du tincal et de la kernite--a été d'environ 2 750 000
tonnes en 1994. Environ 800 000 tonnes (en équivalents de B2O3) de
dérivés boriques commerciaux ont été produits à partir des minerais.
Les borates sont, entre autres, principalement utilisés pour
fabriquer des produits isolants, des fibres de verre de qualité
textile, des agents de blanchiment (perborate de sodium), des verres
au borosilicate, des retardateurs de flamme, des engrais et des
herbicides (à l'état de traces), des émaux, des vernis pour
céramiques, des frittes ainsi qu'une myriade d'applications diverses.
La pénétration du bore dans l'environnement se fait
principalement par l'action des agents météorologiques sur les roches,
la volatilisation de l'acide borique présent dans l'eau de mer et
l'activité volcanique. L'apport de bore dû aux activités humaines est
moindre. Parmi ces dernières sources figurent les brûlis agricoles,
l'incinération des déchets, la combustion du bois de feu, la
production d'énergie à partir du charbon et du pétrole, l'industrie du
verre, les borates et perborates utilisés comme produits industriels
et ménagers, l'exploitation des mines de borates, le traitement des
borates et des bois et papiers imprégnés et enfin le rejet des
effluents et des boues. Il est souvent difficile de chiffrer l'apport
de ces sources.
L'émission de borates et d'acide borique dans l'atmosphère se
fait sous la forme de particules et de vapeurs par suite de la
volatilisation de l'eau de mer, de l'activité volcanique et, dans une
moindre mesure, de l'exploitation des mines, de la fabrication de
verre et de céramique, de l'usage agricole de certains dérivés et des
rejets des centrales thermiques fonctionnant au charbon. Le bore n'est
pas très abondant dans l'atmosphère, mais la quantité totale qui s'y
trouve à un moment donné n'est pas négligeable du fait du volume
énorme de l'atmosphère. Compte tenu de la solubilité des borates dans
l'eau, ils ne devraient pas séjourner longtemps dans l'atmosphère en
concentrations importantes.
L'action des phénomènes météorologiques peut provoquer la
libération de bore dans le sol et dans l'eau de même que, encore qu'en
proportion bien moindre, les déversements d'origine humaine tels que
les rejets d'effluents. On pense que les phénomènes
d'adsorption-désorption sont les seuls mécanismes importants qui
soient susceptibles d'influer sur la destinée du bore dans
l'environnement. Le taux d'adsorption du bore dépend de sa
concentration en solution et du pH de l'eau.
Le bore s'adsorbe sur les particules du sol, le degré
d'adsorption étant fonction du type de sol, du pH, de la salinité, de
la teneur en matières organiques, en oxyde et hydroxyde d'aluminium,
en hydroxyde de fer et en argile. Le phénomène peut être parfaitement
réversible ou au contraire complètement irréversible, selon la nature
et l'état du sol.
Les ions borate présents en solution aqueuse s'y trouvent
essentiellement au degré d'oxydation maximum. Il n'y a pas de
processus aérobie qui soit susceptible d'influer sur la formation des
différentes espèces chimiques et on n'a pas fait état de
biotransformations. Il ne devrait donc pas y avoir de différence dans
les différentes espèces chimiques qui soit due à une
biotransformation.
Le coefficient de partage octanol/eau de l'acide borique est de
0, 175, ce qui indique que ce composé a un faible potentiel de
bioaccumulation. Les expériences de laboratoire effectuées sur des
organismes aquatiques ont confirmé l'existence d'un faible potentiel
de bioaccumulation. Les végétaux ont tendance à accumuler du bore;
toutefois la fixation du bore par les plantes dépend du pH de la
solution de sol, de la température, de l'intensité lumineuse et de la
concentration d'autres éléments (par ex. le calcium et le potassium).
Les études relatives à l'accumulation du bore par les plantes, les
insectes et les poissons montrent que cet élément s'accumule dans les
végétaux mais qu'il ne s'amplifie pas de long de la chaîne alimentaire
aquatique.
Le bore est présent dans les différents sols à des concentrations
qui vont de 10 à 300 mg/kg (la moyenne est de 30 mg/kg) selon la
nature du sol, sa teneur en matières organiques et l'importance des
précipitations. Sa concentration dans les eaux superficielles dépend
de plusieurs facteurs tels que la nature géochimique du bassin de
drainage, la proximité de zones littorales et les apports dus aux
décharges industrielles et municipales. Les valeurs sont très
variables, allant de 0,001 à 360 mg/litre. Toutefois les
concentrations moyennes dans les eaux de l'Europe, du Pakistan, de la
Russie et de la Turquie se situent nettement en dessous de 0,6
mg/litre. Au Japon, en Afrique du Sud et en Amérique du sud elles sont
généralement inférieures à 0,3 mg/kg. En Amérique du Nord, elles se
caractérisent par des valeurs inférieures à 0,1 mg/litre, dont 90%
inférieures ou égales à 0,4 mg/litre.
Le bore s'accumule dans les plantes aquatiques et terrestres mais
il ne s'amplifie pas le long de la chaîne alimentaire. On a trouvé des
concentrations de bore comprises entre 26 et 382 mg/kg dans des
plantes d'eau douces immergées, entre 11,3 et 57 mg/kg dans des
plantes d'eau douce semi-immergées et entre 2,3 et 94,7 mg/kg dans des
plantes terrestres. En se basant sur le poids frais, on constate que
les concentrations de bore trouvées dans les invertébrés marins et les
poissons sont analogues à celles que l'on mesure dans les milieux
correspondants, soit entre 0,5 et 4 mg/kg. Chez deux espèces de
poissons, on a trouvé un facteur de bioconcentration de 0,3.
Dans l'air ambiant, la concentration du bore varie de < 0,5 à
environ 80 ng/m3, avec une moyenne de 20 ng/m3 au-dessus des
continents.
Le fait que la concentration du bore dans les eaux souterraines
et les eaux douces de surface soit très voisine de sa concentration
dans l'eau de boisson, indique que ce élément n'est pas éliminé par
les traitements auxquels sont soumises les eaux souterraines et les
eaux de surface destinées à la boisson.
L'apport de bore chez l'Homme devrait être de 0,44 µg/jour à
partir de l'air ambiant, de 0,2-0,6 mg/jour à partir de l'eau de
boisson et de 1,2 mg/jour à partir de l'alimentation. On estime que
l'apport moyen de bore à partir du sol est de 0,5 µg/jour. On peut
raisonnablement estimer que l'apport de bore par les produits de
consommation est de 0,1 mg/jour.
1.3 Cinétique et suivi biologique
La pharmacocinétique du bore se révèle assez semblable d'une
espèce à l'autre, notamment en ce qui concerne les aspects suivants:
a) Absorption. L'absorption des borates est pratiquement complète
(environ 95% chez l'Homme et le rat) et après ingestion, le bore
apparaît rapidement dans le sang et les tissus de plusieurs
espèces de mammifères.
b) Distribution. Chez les mammifères, elle se produit selon un
mécanisme de diffusion passive par les liquides biologiques.
Contrairement aux tissus mous et au sang, les os sont capables de
fixer sélectivement le bore (teneur supérieure ou égale à 4 fois
celle du sérum) et de le retenir sensiblement plus longtemps.
c) Métabolisme. Le métabolisme de l'acide borique est
thermodynamiquement défavorable dans les systèmes biologiques.
Les espèces ioniques présentes dans le courant sanguin devraient
donc être les mêmes chez tous les mammifères. Dans ces
conditions, on risque beaucoup moins de se tromper en procédant à
des extrapolations, puisqu'il n'est pas nécessaire de prendre en
considération les différences interspécifiques qui existent
généralement au niveau des voies enzymatiques et de la vitesse de
métabolisation.
d) Elimination. La cinétique d'élimination (en particulier la voie
d'élimination et la demi-vie terminale) se révèle également
analogue chez l'Homme et le rat.
Les analogies qui existent entre les paramètres
pharmacocinétiques relevés chez l'Homme et chez le rat, l'espèce qui
sert à définir la dose sans effet nocif observable (NOAEL) dans les
études de laboratoire, permettent de réduire l'incertitude que
comporte l'extrapolation à l'Homme des résultats obtenus sur le rat.
1.4 Effets sur l'Homme et les animaux de laboratoire
Les données relatives aux effets toxiques sur le développement et
la reproduction montrent que l'effet déterminant consiste en une
réduction du poids des foetus. On a estimé à 9, 6 mg de bore par jour
et par kg de poids corporel, la NOAEL relative à cet effet. La dose la
plus faible produisant un effet nocif observable (LOAEL) est, chez le
rat, d'environ 13 mg/kg de poids corporel par jour, l'effet observé
étant une légère différence dans le poids des foetus (approx. 5%) et
des anomalies costales. A mesure que l'on augmente la dose, on observe
les effets suivants, selon la dose:
a) autres anomalies costales et testiculaires (approx. 25 mg de bore
par jour et par kg de poids corporel);
b) réduction du poids des foetus et accroissement des malformations
cardiovasculaires chez le lapin; graves anomalies testiculaires
chez le rat (approx. 40 mg de bore par jour et par kg de poids
corporel);
c) atrophie testiculaire et stérilité chez le rat (approx. 55 mg de
bore par jour et par kg de poids corporel);
d) réduction du poids des foetus chez la souris (approx. 80 mg de
bore par jour et par kg de poids corporel).
Les études effectuées sur des rats et des souris ne révèlent
aucun signe de cancérogénicité de l'acide borique. Comme les données
humaines font défaut et que les données animales sont également
limitées, on ne peut pas ranger le bore dans une classe précise de
cancérogénicité.
Seules quelques études ont été consacrées à l'évaluation des
effets résultant d'une exposition humaine aux dérivés du bore. Les
données disponibles montrent que l'exposition à ces composés peut se
traduire à brève échéance par une irritation des voies respiratoires
supérieures, du rhinopharynx et des yeux. Ces effets sont cependant de
brève durée et réversibles. La seule étude de suivi à long terme (7
ans) qui ait été consacrée à l'action toxique du bore n'a pas permis
de mettre en évidence d'effets à long terme, encore que l'on ne puisse
pas totalement exclure que ce résultat soit dû à la bonne santé des
travailleurs étudiés, compte tenu du taux élevé d'attrition. Deux
études descriptives ont porté sur la fécondité et le sex ratio
secondaire en rapport avec une exposition. Aucune des deux études n'a
révélé d'effet indésirable sur la fécondité observée dans
l'échantillon. Il y aurait bien eu un excès de naissances féminines,
mais il n'était pas statistiquement significatif et le fait qu'il
existait d'autres facteurs connus pour influer sur le sex ratio
incite à une interprétation prudente de ce résultat. On n'a pas
retrouvé d'études portant sur l'ensemble des paramètres de la
reproduction, par exemple le temps écoulé jusqu'à la grossesse, les
retards dans la conception, les avortements spontanés et le
comportement des spermatozoïdes. Il faut étudier plus à fond les
autres styles de vie et facteurs comportementaux dans leurs rapports
avec la santé et la fécondité afin de mettre en évidence les
populations potentiellement sensibles et évaluer dans le détail les
effets sur la reproduction.
1.5 Effets sur les êtres vivants dans leur milieu naturel
Les bactéries ont une tolérance au bore relativement élevée. Les
concentrations produisant des effets aigus ou chroniques vont de 8 à
340 mg de bore par litre, la plupart des valeurs se situant autour de
18 mg/litre. Ce sont les protozoaires qui sont les plus sensibles. Les
épreuves effectuées sur des protozoaires du genre Entosiphon et
Paramecium ont donné, pour la concentration sans effet observable
sur 72 h (NOEC) et pour la CE3, des valeurs comprises entre 0,3 et 18
mg de bore par litre.
Le bore est un micronutriment essentiel des cyanobactéries et des
diatomées. Les épreuves habituelles pour l'évaluation de la toxicité
chronique, ont donné une concentration sans effet observable comprise
entre 10 et 24 mg de bore par litre pour les algues vertes
dulçaquicoles. Les algues bleues ont une sensibilité analogue, avec
une CE3 à 8 jours de 20 mg de B par litre.
Si l'on se base sur les valeurs de la toxicité aiguë, les
invertébrés sont moins sensibles au bore que les microorganismes. Pour
un certain nombre d'espèces, les valeurs de la CE50 à 24 et 48 h
allaient de 95 à 1376 g de B par litre, la plupart des valeurs se
situant dans l'intervalle 100-200 mg/litre. Les études de toxicité
chronique effectuées sur Daphnia magna ont donné une NOEC allant de
6 à 10 mg de B par litre. Des valeurs un peu plus faibles ont été
obtenues lors d'études en laboratoire ou dans des biocénoses
naturelles. Une étude de 28 jours en laboratoire comportant six stades
trophiques a donné une NOEC de 2,5 mg de B par litre. Des études à
long terme sur des étangs et autres études en milieu naturel (à
l'exclusion des poissons) ont donné des NOEC allant jusqu'à 1,52 mg de
B par litre.
Des épreuves de toxicité aiguë portant sur plusieurs espèces de
poissons ont donné des valeurs comprises entre 10 et près de 300 mg de
B par litre. Ce sont des espèces telles que la truite arc-en-ciel
(Oncorhynchus mykiss) et Brachydanio rerio qui se sont révélées
les plus sensibles, avec des valeurs d'environ 10 mg de B par litre.
La toxicité du bore pour les stades juvéniles des poissons est
attestée par des études effectuées en eau reconstituée sur plusieurs
espèces. C'est ainsi que l'on a exposé à du bore (acide borique ou
borax) les embryons et les premiers stades larvaires de truites
arc-en-ciel, de certaines perches (Micropterus salmoides), de
poissons-chats (Ictalurus punctatus) et de poissons rouges
(Carassius auratus), depuis le moment de la fécondation jusqu'à 8
jours après l'éclosion en eau douce ou dure. Ni la dureté de l'eau, ni
la forme sous laquelle se trouvait le bore n'ont eu d'effets
systématiques sur la survie embryo-larvaire des poissons. C'est la
truite arc-en-ciel qui s'est révélée l'espèce la plus sensible. Pour
ce poisson, la NOEC se situait entre 0,009 et 0,103 mg de B par litre.
L'effet de la dilution naturelle de l'eau sur la toxicité du bore
a été déterminé en utilisant des eaux de surface prélevées en trois
endroits, avec des concentrations de bore de 0,023, de 0,091 et de
0,75 mg/litre. Aucun effet indésirable n'a été noté jusqu'à la dose de
0,75 mg/litre. Les valeurs de la concentration la plus faible
produisant des effets observables (LOEC) allaient de 1,1 à 1,73
mg/litre. L'une des épreuves, effectuée sur de l'eau de puits prélevée
à grande profondeur (600 m), utilisée systématiquement pour les essais
de toxicité en milieu aquatique et fournie par un laboratoire de
Wareham (USA), a donné une NOEC > 18 mg de B par litre. Il semble
donc que les épreuves effectuées en eau reconstituée surestiment la
toxicité des eaux naturelles, peut-être du fait que les premières sont
pauvres en certains nutriments.
On sait depuis les années 20 que le bore est un micronutriment
essentiel pour les végétaux supérieurs, la quantité minimale
nécessaire à la croissance dépendant de l'espèce. Le bore intervient
dans la division cellulaire et le métabolisme ainsi que dans la
structure et la fonction de la membrane. Il existe à l'état naturel
sous forme de borates dans les fruits, les noix et les légumes. Chez
les plantes, l'intervalle entre carence et fixation excessive
(toxicité) est étroit. Dans de nombreux pays, on a constaté que les
plantes présentaient une carence en bore. Cet déficit a plus de
chances de se rencontrer dans les sols acides à texture légère des
régions humides, car le bore est facilement lessivé. En revanche, on
trouve un excès de bore dans les solutions de sols provenant de dépôts
géologiques récents, dans les sols arides, les sols issus de sédiments
marins et ceux qui sont contaminés par diverses sources de pollution,
comme les émissions des centrales thermiques à charbon et celles qui
proviennent des exploitations minières. L'eau d'irrigation est l'une
des principales causes des fortes teneurs en bore qui contaminent les
terrains agricoles.
On a observé qu'à des doses dans l'alimentation de 30 et 300 mg
de bore par kg, des colverts (Anas platyrhyncos) présentaient des
troubles et qu'à la dose de 1000 mg/kg leur survie était réduite.
2. Conclusions
Le bore est un élément naturel qui existe à l'état de borates
dans les océans, les roches sédimentaires, la houille, le schiste et
certains sols. Certaines sources naturelles sont à l'origine de rejets
de bore dans l'environnement comme les océans, la vapeur géothermique
et l'action des agents climatiques sur les roches sédimentaires riches
en argile. Les activités humaines libèrent également du bore dans
l'environnement, mais dans une moindre proportion.
Le bore est un micronutriment essentiel des plantes supérieures,
la concentration nécessaire à une croissance optimale étant variable
suivant les espèces. Pour certaines plantes, il y a une faible marge
entre carence et toxicité.
Si l'on compare la concentration environnementale qui ne produit
pas d'effet (1 mg/litre) et les teneurs en bore généralement
rencontrées dans le milieu, on peut en déduire que le risque d'effets
indésirables sur l'écosystème aquatique est faible. Il est possible
toutefois que dans certains milieux riches en bore, les teneurs
naturelles soient plus élevées. On peut raisonnablement penser qu'en
pareil cas, les organismes aquatiques se sont adaptés aux conditions
locales.
Chez l'Homme, l'exposition au bore provient principalement de
l'alimentation et de l'eau de boisson. On estime que la concentration
du bore dans l'eau de boisson est, en moyenne mondiale, de l'ordre de
0,1 à 0,3 mg/litre. Pour la population générale, c'est la nourriture
qui apporte le plus de bore. La dose moyenne ingérée avec la
nourriture est d'environ 1,2 mg par jour.
Chez l'Homme et l'animal, l'acide borique et les borates sont
absorbés au niveau des voies digestives et respiratoires. La
résorption dépasse 90% de la dose administrée et on peut s'en rendre
compte en mesurant l'excrétion urinaire, qui est rapide, puisqu'elle
s'effectue en quelques jours.
L'expérimentation animale montre que sous forme d'acide borique
et de borate, le bore a des effets toxiques sur le développement et la
reproduction à des concentrations qui sont 100 à 1000 fois supérieures
à celles que l'on rencontre dans l'environnement. On ne possède pas
suffisamment de données toxicologiques sur l'Homme. On a fixé à 0,4 mg
par kg de poids corporel la dose journalière tolérable de bore. Pour
tenir compte des divers milieux lors de la fixation de cette dose, il
faut connaître l'exposition dans les différents pays.
3. Recommandations
a) Les valeurs guides pour l'eau et les aliments doivent être basées
sur la dose tolérable indiquée dans le présent document.
b) Dans l'application de la dose tolérable, il faut tenir compte du
fait que le bore peut avoir un effet physiologiquement bénéfique
pour l'Homme.
c) Dans l'application des normes, on tiendra compte du fait que le
bore est essentiel pour certains composants de l'environnement
(par exemple, le bore est un micronutriment essentiel pour les
plantes supérieures).
d) Les suppléments alimentaires qui dépassent la dose tolérable sont
à éviter.
RESUMEN, CONCLUSIONES Y RECOMENDACIONES
1. Resumen
1.1 Identidad, presencia en la naturaleza y métodos analíticos
El boro es un elemento presente en la naturaleza que se encuentra
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Está ampliamente distribuido en
la naturaleza, con concentraciones de alrededor de 10 mg/kg en la
corteza terrestre (margen de variación: de 5 mg/kg en los basaltos a
100 mg/kg en los esquistos) y de unos 4,5 mg/litro en el océano.
Los productos y minerales comerciales de borato más importantes
son el pentahidrato de bórax, el bórax, el perborato de sodio, el
ácido bórico, la colemanita y la ulexita. En las bajas concentraciones
y el pH casi neutro en que está presente en la mayoría de los fluidos
biológicos, la especie predominante es el B(OH)3 (con algo de
B(OH)4-), con independencia de que la fuente de boro sea el ácido
bórico o uno de los boratos. Esto se debe a que el ácido bórico es un
ácido muy débil (p Ka 9,15). El perborato de sodio se hidroliza para
dar peróxido de hidrógeno y metaborato; por consiguiente, puede
mostrar propiedades químicas y toxicológicas algo distintas de las de
los otros boratos.
Para el análisis de los bajos niveles de boro presente en las
muestras biológicas y del medio ambiente se prefieren los métodos de
plasma acoplado por inducción (PAI); los métodos colorimétricos se
deben utilizar con cautela.
1.2 Producción, aplicaciones, destino en el medio ambiente y fuentes
de exposición
Los depósitos económicos de borato son raros y se encuentran en
regiones áridas de Turquía, los Estados Unidos, la Argentina, Chile,
Rusia, China y el Perú. La producción mundial total de minerales de
boro--sobre todo colemanita, ulexita, tíncal y kernita--fue de
alrededor de 2 750 000 toneladas en 1994. Unas 800 000 toneladas de
productos comerciales de borato, expresados como B2O3, se fabricaron
a partir de minerales del boro.
Las principales aplicaciones finales del borato son la fibra de
vidrio de calidad de aislamiento o textil, la lejía para lavar
(perborato de sodio), el vidrio de borosilicato, pirorretardantes,
fertilizantes y herbicidas agrícolas (como elemento traza) y esmaltes,
fritas y vidriados cerámicos, así como innumerables aplicaciones
diversas.
El boro entra en el medio ambiente sobre todo mediante la
meteorización de las rocas, la volatilización de ácido bórico del agua
del mar y la actividad volcánica. También se desprende boro de fuentes
antropogénicas en menor medida. Entre las fuentes antropo-génicas
figuran la quema de productos agrícolas, de basuras y de leña, la
producción de energía utilizando carbón y petróleo, la fabricación de
productos de vidrio, la utilización de boratos/perboratos en el hogar
y en la industria, la extracción/elaboración de borato, la lixiviación
de madera/papel tratados y la eliminación de aguas residuales/fangos
de alcantarillado. Muchas de estas fuentes son difíciles de
cuantificar.
Se producen emisiones a la atmósfera de boratos y de ácido bórico
en forma de partículas y de vapor como consecuencia de la
volatilización desde el agua del mar, la actividad volcánica y, en
menor medida, las operaciones de extracción, la fabricación de vidrio
y cerámica, la aplicación de productos químicos agrícolas y las
centrales eléctricas de carbón. El boro no está presente en la
atmósfera en concentraciones significativas; sin embrago, la cantidad
total que hay en ella en cualquier momento es significativa debido al
enorme volumen de la atmósfera. Teniendo en cuenta su solubilidad en
el agua, no es previsible que los boratos persistan en la atmósfera en
una medida significativa.
Se puede incorporar boro al agua libre y a la del suelo mediante
procesos de meteorización, y en una medida mucho menor por vertidos
antropogénicos, como desagües de aguas residuales. Se supone que las
reacciones de adsorción-desorción son el único mecanismo importante
que influye en el destino del boro en el agua. El grado de adsorción
de boro depende del pH del agua y de su concentración en la solución.
El boro se adsorbe en las partículas del suelo, dependiendo el
grado del tipo de suelo, el pH, la salinidad, el contenido de materia
orgánica, el contenido de óxido de hierro y de aluminio, el contenido
de hidróxido de hierro y de aluminio y el contenido de arcilla. La
adsorción de boro puede variar entre totalmente reversible e
irreversible, en función del tipo y de la condición del suelo.
Los iones borato presentes en solución acuosa están básicamente
en estado totalmente oxidado. No es probable que ningún proceso
aeróbico influya en su especiación y no se han notificado procesos de
biotransformación. Por consiguiente, no es probable que haya ninguna
diferencia en las especies de boro debida a la biotransformación.
El coeficiente de reparto octanol/agua del ácido bórico medido es
de 0,175, lo cual indica un potencial de bioacumulación bajo. Los
experimentos de laboratorio con organismos acuáticos han confirmado
este potencial. Las plantas acumulan boro; sin embargo, la absorción
se ve afectada por el pH de la solución del suelo, la temperatura, la
intensidad de la luz y la concentración de otros elementos (por
ejemplo, calcio y potasio). Los estudios de la acumulación de boro en
plantas, insectos y peces han puesto de manifiesto que el boro se
bioacumula en las plantas, pero no se bioamplifica en la cadena
alimentaria de los organismos acuáticos.
El boro está presente en los suelos en concentraciones que van de
10 a 300 mg/kg (promedio de 30 mg/kg), en función del tipo de suelo,
la cantidad de materia orgánica y la cantidad de precipitación. Las
concentraciones de boro en el agua superficial dependen de factores
como la naturaleza geoquímica de la superficie de drenaje, la
proximidad a regiones costeras marinas y la incorporación de vertidos
de efluentes industriales y urbanos. Las concentraciones de boro en el
agua superficial varían ampliamente, desde 0,001 hasta llegar a
360 mg/litro. Sin embargo, las concentraciones medias en las aguas de
Europa, el Pakistán, Rusia y Turquía suelen ser inferiores a
0,6 mg/litro. Las concentraciones de boro en el agua del Japón,
Sudáfrica y América del Sur están en general por debajo de
0,3 mg/litro. En las aguas de América del Norte son normalmente
menores de 0,1 mg/litro, con 0,4 mg/litro o menos en alrededor del
90%.
El boro se acumula en las plantas acuáticas y terrestres, pero no
se amplifica a través de la cadena alimentaria. Se ha comprobado que
las concentraciones de boro oscilan entre 26 y 382 mg/kg en las
plantas acuáticas de agua dulce sumergidas, entre 11,3 y 57 mg/kg en
la vegetación de agua dulce que crece fuera del agua y entre 2,3 y
94,7 mg/kg de peso seco en las plantas terrestres. Tomando como base
el peso fresco, las concentraciones de boro en los invertebrados y los
peces marinos son semejantes a los niveles presentes en los medios de
exposición, entre 0,5 y 4 mg/kg. Para dos especies de peces de agua
dulce se observó un factor de bioconcentración de 0,3.
Las concentraciones de boro en el aire del medio ambiente oscilan
entre <0,5 y alrededor de 80 ng/m3, con un promedio en todos los
continentes de 20 ng/m3.
La estrecha semejanza entre las concentraciones de boro en el
agua fréatica, el agua dulce superficial y el agua potable indica que
el boro no se elimina en el tratamiento del agua fréatica y del agua
dulce superficial utilizadas para beber.
Se supone que la ingesta de boro por el ser humano es de
0,44 µg/día a partir del medio ambiente, de 0,2-0,6 mg/día con el agua
de beber y de 1,2 mg/día en la alimentación. Se considera que la
ingesta media de boro a partir del suelo es de 0,5 µg/día. Una
estimación razonable de la exposición al boro en los productos de
consumo es de 0,1 mg/día.
1.3 Cinética y vigilancia biológica
La farmacocinética del boro parece ser bastante parecida en todas
las especies en los siguientes aspectos:
a) La absorción de boratos es básicamente completa (alrededor del
95% en el ser humano y en ratas), y tras la ingestión aparece con
rapidez boro en la sangre y los tejidos corporales de varias
especies de mamíferos.
b) La distribución del boro en los mamíferos parece producirse por
difusión pasiva en todos los líquidos del cuerpo. Al contrario
que los tejidos blandos y la sangre, los huesos muestran una
absorción selectiva de boro (>4 veces mayor que en el suero) y
tiempos de retención considerablemente más largos.
c) El metabolismo del ácido bórico es termodinámicamente
desfavorable en los sistemas biológicos. Por consiguiente, se
supone que las especies iónicas en la circulación sistémica son
equivalentes en todos los mamíferos. Así se elimina una fuente
importante de posible incertidumbre para la extrapolación del
riesgo, puesto que no es necesario tener en cuenta diferencias
interespecíficas en las rutas enzimáticas y/o las tasas
metabólicas.
d) La cinética de la eliminación (especialmente la vía de
eliminación y la semivida terminal) también parece ser semejante
para el ser humano y para las ratas.
Las semejanzas de los parámetros farmacocinéticos entre el ser
humano y las ratas, especie que define la concentración sin efectos
adversos observados (NOAEL) para estudios de laboratorio, reducen la
incertidumbre en cuanto a la extrapolación entre estas dos especies.
1.4 Efectos en animales de laboratorio y en el ser humano
Los datos relativos a la toxicidad en el desarrollo y
reproductiva indican que el efecto crítico en las ratas es el menor
peso corporal del feto. La NOAEL para el menor peso corporal del feto
es de 9,6 mg de boro/kg de peso corporal al día. La menor
concentración con efectos adversos observados (LOAEL), en la que las
ratas muestran ligeras (approx. 5%) diferencias en el peso corporal
del feto y anomalías en las costillas, es de unos 13 mg de boro/kg de
peso corporal al día. A medida que aumenta la dosis, los efectos que
se observan (y las dosis a las que se ven) son los siguientes:
a) nuevos efectos en las costillas y patología testicular en ratas
(approx. 25 mg de boro/kg de peso corporal al día);
b) disminución del peso corporal del feto y aumento de las
malformaciones cardiovasculares fetales en conejos, y patología
testicular grave en ratas (approx. 40 mg de boro/kg de peso
corporal al día);
c) atrofia testicular y esterilidad en ratas (approx. 55 mg de
boro/kg de peso corporal al día); y
d) reducción del peso corporal del feto en ratones (approx. 80 mg de
boro/kg de peso corporal al día).
En los estudios de animales con ratones y ratas no se observaron
pruebas de carcinogenicidad del ácido bórico. Debido a la falta de
datos humanos y a lo limitado de los obtenidos de animales, el boro no
se puede clasificar en cuanto a su carcinogenicidad humana.
Son pocos los estudios que se han realizado en el ser humano de
evaluación de los efectos para la salud asociados con la exposición a
compuestos de boro. Los datos disponibles indican que la exposición
está asociada con efectos irritantes de corta duración en las vías
respiratorias superiores, la nasofaringe y los ojos. Sin embargo,
parece que esos efectos son breves y reversibles. En el único estudio
de seguimiento de larga duración (siete años) no se consiguió
identificar ningún efecto prolongado para la salud, aunque no se puede
excluir del todo un efecto en trabajadores sanos debido a la tasa de
desgaste (47%). En dos estudios descriptivos se evaluó la fecundidad y
la razón secundaria de sexos en relación con la exposición. En ninguno
de los dos se notificó un efecto perjudicial demostrado en la
fecundidad de la muestra seleccionada. Aunque se ha indicado la
posibilidad de un porcentaje de nacimientos de hembras superior al
normal, la ausencia de significación estadística y la atención a otras
covariantes que se saben que influyen en el sexo obliga a una
interpretación cauta de este resultado. No se conoce ningún estudio en
el que se evalúe el espectro de resultados reproductivos, como el
tiempo hasta la gestación, los retrasos en la concepción, los abortos
espontáneos y los análisis del esperma en los machos. La función de
otros factores relativos al tipo de vida y al comportamiento en
relación con la salud y la fecundidad requiere un ulterior estudio
para identificar poblaciones posiblemente sensibles y evaluar los
efectos reproductivos de manera más completa.
1.5 Efectos en los organismos del medio ambiente
Las bacterias tienen una tolerancia hacia el boro relativamente
grande. Las concentraciones con efectos agudos y crónicos oscilan
entre 8 y 340 mg de boro/litro, siendo la mayoría de los valores
superiores a 18 mg de boro/litro. Los protozoos son más sensibles. En
pruebas realizadas con Entosiphon y con Paramecium se obtuvieron
concentraciones sin efectos observados (NOEC) en 72 horas y valores de
CE3 de 0,3 a 18 mg de boro/litro.
El boro es un micronutriente esencial para las cianobacterias y
las diatomeas. En pruebas crónicas normales con algas verdes de agua
dulce se observaron concentraciones sin efectos comprendidas entre 10
y 24 mg de boro/litro. Las algas verdeazuladas parecen tener una
sensibilidad semejante, con una CE3 en ocho días de 20 mg de
boro/litro.
Tomando como base los valores de la toxicidad, los invertebrados
son menos sensibles al boro que los microorganismos. Para varias
especies, los valores de la CE50 en 24-48 horas oscilaron entre 95 y
1376 mg de boro/litro, con la mayoría de los valores del orden de
100-200 mg de boro/litro. En estudios de toxicidad crónica con
Daphnia magna, los valores de la NOEC oscilaron entre 6 y 10 mg de
boro/litro. Se obtuvieron valores de la NOEC ligeramente más bajos en
estudios de biocenosis de laboratorio y de campo. En el estudio de
laboratorio de 28 días, consistente en seis etapas tróficas, se obtuvo
una NOEC de 2,5 mg de boro/litro. En estudios prolongados realizados
en un estanque al aire libre y en el campo (sin incluir peces) las
NOEC fueron de hasta 1,52 mg de boro/litro.
En pruebas de toxicidad aguda con varias especies de peces se
obtuvieron valores comprendidos entre alrededor de 10 y cerca de
300 mg de boro/litro. La trucha irisada (Oncorhynchus mykiss) y
Brachydanio rerio fueron los más sensibles, con valores aproximados
de 10 mg de boro/litro.
La toxicidad del boro en las primeras fases de la vida de los
peces está documentada para varias especies en agua reconstituida. Se
expusieron a boro, en forma de ácido bórico o de bórax, fases
embrionarias o larvarias iniciales de trucha irisada, perca atruchada
(Micropterus salmoides), coto punteado (Ictalurus punctatus) y
Carassius auratus desde la fecundación hasta ocho días después de la
eclosión en agua blanda o dura. Ni la dureza del agua ni la forma del
boro influyeron de manera uniforme en la supervivencia de los
embriones-larvas de los peces. La trucha irisada fue la especie más
sensible. Las NOEC para la trucha irisada oscilaron entre 0,009 y
0,103 mg de boro/litro.
El efecto de la dilución natural en el agua sobre la toxicidad
del boro se determinó utilizando agua superficial recogida en tres
lugares, con concentraciones de boro de 0,023, 0,091 y 0,75 mg/litro.
No se detectó ningún efecto adverso hasta los 0,75 mg de boro/litro.
Las concentraciones más bajas con efectos observados (LOEC) oscilaron
entre 1,1 y 1,73 mg de boro/litro. En una prueba con agua de un pozo
profundo (600 m), utilizada habitualmente para pruebas de toxicidad
acuática en virtud de un contrato con un laboratorio situado en
Wareham, Massachusetts, Estados Unidos, se obtuvo una NOEC de
>18,0 mg de boro/litro. Así pues, al parecer en la exposición a agua
reconstituida se sobreestimaba la toxicidad determinada en aguas
naturales, posiblemente como consecuencia de la deficiencia de
nutrientes en la primera.
Desde los años veinte se sabe que el boro es un micronutriente
esencial para las plantas superiores, con diferencias interespecíficas
en cuanto a las concentraciones necesarias para un crecimiento óptimo.
El boro interviene en la división, el metabolismo y la estructura y la
función de las membranas de las células. En forma de borato está
presente en las frutas, las nueces y las hortalizas. La diferencia
entre la deficiencia y la absorción excesiva (toxicidad) es pequeña en
las plantas. Se ha notificado deficiencia de boro en plantas
terrestres en muchos países. Es más probable que se produzca
deficiencia de boro en suelos ácidos de textura ligera en las regiones
húmedas, debido a su susceptibilidad a la lixiviación. Suele haber
exceso de boro en soluciones del suelo procedentes de depósitos
jóvenes desde el punto de vista geológico, en suelos áridos, en los
derivados de sedimentos marinos y en los afectados por fuentes de
contaminación como los vertidos de centrales termoeléctricas de carbón
y de operaciones de extracción. El agua de riego es una de las
principales fuentes de concentraciones altas de boro que provocan
toxicidad en el campo.
El crecimiento del pato real (Anas platyrhynchos) se vio
afectado negativamente por concentraciones de 30 y 300 mg de boro/kg
en los alimentos y con 1000 mg/kg se redujo la supervivencia.
2. Conclusiones
El boro es un elemento que se encuentra presente en la naturaleza
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Las fuentes naturales de los
boratos que se liberan en el medio ambiente son los océanos, el vapor
geotérmico y la meteorización natural de las rocas sedimentarias ricas
en arcilla. También se desprende boro en menor medida de fuentes
antropogénicas.
El boro es un micronutriente esencial para las plantas
superiores, con diferencias interespecíficas en cuanto a las
concentraciones necesarias para un crecimiento óptimo. Se ha observado
deficiencia de boro en plantas terrestres en muchos países de todo el
mundo. La diferencia entre la deficiencia y toxicidad es pequeña en
algunas plantas.
La comparación de la concentración sin efectos en el medio
ambiente (1 mg/litro) con los niveles generales en el medio ambiente
indica que el riesgo de efectos adversos del boro en el ecosistema
acuático es bajo. Los niveles naturales son más elevados en algunos
medios ricos en boro. Es razonable suponer que los organismos
acuáticos de dichos hábitats pueden adaptarse a las condiciones
locales.
La exposición del ser humano al boro se produce sobre todo por
medio de los alimentos y el agua potable. Se consideró que la
concentración media mundial de boro en el agua potable estaba
comprendida entre 0,1 y 0,3 mg de boro/litro.
Para la población general, la mayor exposición al boro procede de
la ingesta oral con los alimentos. La ingesta diaria media de boro con
los alimentos es de alrededor de 1,2 mg.
En las personas y en los animales, el ácido bórico y el borato se
absorben del tracto gastrointestinal y de las vías respiratorias. Se
absorbe más del 90% de las dosis administradas de estos compuestos,
como se pone de manifiesto en la excreción en la orina, que es rápida,
en unos pocos días.
En experimentos con animales se ha demostrado que el boro en
forma de ácido bórico y de borato tiene toxicidad reproductiva y en el
desarrollo en concentraciones de 100 a 1000 veces superiores a los
niveles normales de exposición. Se carece de datos suficientes acerca
de la toxicidad en el ser humano. La ingesta tolerable (IT) de boro se
fijó en 0,4 mg/kg de peso corporal al día. La asignación de la IT en
diversos medios debe basarse en los datos relativos a la exposición de
cada país.
3. Recomendaciones
a) Los valores guía del agua y los alimentos deben basarse en la IT
proporcionada por este documento.
b) La IT se debe aplicar quedando entendido que el boro puede
aportar beneficios fisiológicos para la salud humana.
c) Hay que reconocer al aplicar las normas que el boro es esencial
para algunos elementos constituyentes del medio ambiente (por
ejemplo, el boro es un micronutriente esencial para las plantas
superiores).
d) Se deben evitar los complementos de la alimentación que superen
la IT.
RÉSUMÉ, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé
1.1 Identité, état naturel et méthodes d'analyse
Le bore est un élément naturel qui se trouve sous la forme de
borates dans les océans, les roches sédimentaires, la houille, le
schiste et certaines huiles minérales. Il est très répandu dans la
nature, avec des concentrations de l'ordre de 10 mg/kg dans l'écorce
terrestre (elles vont de 5 mg/kg dans les basaltes à 100 mg/kg dans
les schistes) et d'environ 4,5 mg/litre dans les océans.
Les dérivés boriques les plus importants, qu'il s'agisse de
minéraux ou de produits du commerce, sont le borax à cinq molécules
d'eau, le borax, le perborate de sodium, l'acide borique, la
colemanite et l'ulexite. Aux faibles concentrations et au pH
pratiquement neutre qui caractérisent la plupart des liquides
biologiques, c'est le monomère B(OH)3 qui est l'espèce prédominante
(avec un peu de B(OH)4-), que la source de bore soit l'acide borique
ou un borate. Cela tient au fait que l'acide borique est un acide très
faible (pKa = 9,15).Le perborate de sodium s'hydrolyse pour donner du
peroxyde d'hydrogène et du métaborate; il peut donc présenter des
propriétés chimiques et toxicologiques un peu différentes de celles
des autres borates.
Les méthodes utilisant un plasma à couplage inductif (ICP) sont
les méthodes de choix pour le dosage des faibles quantités de bore
présentes dans les échantillons biologiques et environnementaux; les
méthodes colorimétriques doivent être utilisées avec prudence.
1.2 Production, usages, destinée dans l'environnement et sources d'exposition
Les dépôts de borates économiquement exploitables sont rares et
se trouvent dans des zones arides de Turquie, des Etats-Unis, du
Chili, de Russie, de Chine et du Pérou. La production mondiale totale
de minéraux contenant du bore--principalement de la colemanite, de
l'ulexite, du tincal et de la kernite--a été d'environ 2 750 000
tonnes en 1994. Environ 800 000 tonnes (en équivalents de B2O3) de
dérivés boriques commerciaux ont été produits à partir des minerais.
Les borates sont, entre autres, principalement utilisés pour
fabriquer des produits isolants, des fibres de verre de qualité
textile, des agents de blanchiment (perborate de sodium), des verres
au borosilicate, des retardateurs de flamme, des engrais et des
herbicides (à l'état de traces), des émaux, des vernis pour
céramiques, des frittes ainsi qu'une myriade d'applications diverses.
La pénétration du bore dans l'environnement se fait
principalement par l'action des agents météorologiques sur les roches,
la volatilisation de l'acide borique présent dans l'eau de mer et
l'activité volcanique. L'apport de bore dû aux activités humaines est
moindre. Parmi ces dernières sources figurent les brûlis agricoles,
l'incinération des déchets, la combustion du bois de feu, la
production d'énergie à partir du charbon et du pétrole, l'industrie du
verre, les borates et perborates utilisés comme produits industriels
et ménagers, l'exploitation des mines de borates, le traitement des
borates et des bois et papiers imprégnés et enfin le rejet des
effluents et des boues. Il est souvent difficile de chiffrer l'apport
de ces sources.
L'émission de borates et d'acide borique dans l'atmosphère se
fait sous la forme de particules et de vapeurs par suite de la
volatilisation de l'eau de mer, de l'activité volcanique et, dans une
moindre mesure, de l'exploitation des mines, de la fabrication de
verre et de céramique, de l'usage agricole de certains dérivés et des
rejets des centrales thermiques fonctionnant au charbon. Le bore n'est
pas très abondant dans l'atmosphère, mais la quantité totale qui s'y
trouve à un moment donné n'est pas négligeable du fait du volume
énorme de l'atmosphère. Compte tenu de la solubilité des borates dans
l'eau, ils ne devraient pas séjourner longtemps dans l'atmosphère en
concentrations importantes.
L'action des phénomènes météorologiques peut provoquer la
libération de bore dans le sol et dans l'eau de même que, encore qu'en
proportion bien moindre, les déversements d'origine humaine tels que
les rejets d'effluents. On pense que les phénomènes
d'adsorption-désorption sont les seuls mécanismes importants qui
soient susceptibles d'influer sur la destinée du bore dans
l'environnement. Le taux d'adsorption du bore dépend de sa
concentration en solution et du pH de l'eau.
Le bore s'adsorbe sur les particules du sol, le degré
d'adsorption étant fonction du type de sol, du pH, de la salinité, de
la teneur en matières organiques, en oxyde et hydroxyde d'aluminium,
en hydroxyde de fer et en argile. Le phénomène peut être parfaitement
réversible ou au contraire complètement irréversible, selon la nature
et l'état du sol.
Les ions borate présents en solution aqueuse s'y trouvent
essentiellement au degré d'oxydation maximum. Il n'y a pas de
processus aérobie qui soit susceptible d'influer sur la formation des
différentes espèces chimiques et on n'a pas fait état de
biotransformations. Il ne devrait donc pas y avoir de différence dans
les différentes espèces chimiques qui soit due à une
biotransformation.
Le coefficient de partage octanol/eau de l'acide borique est de
0, 175, ce qui indique que ce composé a un faible potentiel de
bioaccumulation. Les expériences de laboratoire effectuées sur des
organismes aquatiques ont confirmé l'existence d'un faible potentiel
de bioaccumulation. Les végétaux ont tendance à accumuler du bore;
toutefois la fixation du bore par les plantes dépend du pH de la
solution de sol, de la température, de l'intensité lumineuse et de la
concentration d'autres éléments (par ex. le calcium et le potassium).
Les études relatives à l'accumulation du bore par les plantes, les
insectes et les poissons montrent que cet élément s'accumule dans les
végétaux mais qu'il ne s'amplifie pas de long de la chaîne alimentaire
aquatique.
Le bore est présent dans les différents sols à des concentrations
qui vont de 10 à 300 mg/kg (la moyenne est de 30 mg/kg) selon la
nature du sol, sa teneur en matières organiques et l'importance des
précipitations. Sa concentration dans les eaux superficielles dépend
de plusieurs facteurs tels que la nature géochimique du bassin de
drainage, la proximité de zones littorales et les apports dus aux
décharges industrielles et municipales. Les valeurs sont très
variables, allant de 0,001 à 360 mg/litre. Toutefois les
concentrations moyennes dans les eaux de l'Europe, du Pakistan, de la
Russie et de la Turquie se situent nettement en dessous de 0,6
mg/litre. Au Japon, en Afrique du Sud et en Amérique du sud elles sont
généralement inférieures à 0,3 mg/kg. En Amérique du Nord, elles se
caractérisent par des valeurs inférieures à 0,1 mg/litre, dont 90%
inférieures ou égales à 0,4 mg/litre.
Le bore s'accumule dans les plantes aquatiques et terrestres mais
il ne s'amplifie pas le long de la chaîne alimentaire. On a trouvé des
concentrations de bore comprises entre 26 et 382 mg/kg dans des
plantes d'eau douces immergées, entre 11,3 et 57 mg/kg dans des
plantes d'eau douce semi-immergées et entre 2,3 et 94,7 mg/kg dans des
plantes terrestres. En se basant sur le poids frais, on constate que
les concentrations de bore trouvées dans les invertébrés marins et les
poissons sont analogues à celles que l'on mesure dans les milieux
correspondants, soit entre 0,5 et 4 mg/kg. Chez deux espèces de
poissons, on a trouvé un facteur de bioconcentration de 0,3.
Dans l'air ambiant, la concentration du bore varie de < 0,5 à
environ 80 ng/m3, avec une moyenne de 20 ng/m3 au-dessus des
continents.
Le fait que la concentration du bore dans les eaux souterraines
et les eaux douces de surface soit très voisine de sa concentration
dans l'eau de boisson, indique que ce élément n'est pas éliminé par
les traitements auxquels sont soumises les eaux souterraines et les
eaux de surface destinées à la boisson.
L'apport de bore chez l'Homme devrait être de 0,44 µg/jour à
partir de l'air ambiant, de 0,2-0,6 mg/jour à partir de l'eau de
boisson et de 1,2 mg/jour à partir de l'alimentation. On estime que
l'apport moyen de bore à partir du sol est de 0,5 µg/jour. On peut
raisonnablement estimer que l'apport de bore par les produits de
consommation est de 0,1 mg/jour.
1.3 Cinétique et suivi biologique
La pharmacocinétique du bore se révèle assez semblable d'une
espèce à l'autre, notamment en ce qui concerne les aspects suivants:
a) Absorption. L'absorption des borates est pratiquement complète
(environ 95% chez l'Homme et le rat) et après ingestion, le bore
apparaît rapidement dans le sang et les tissus de plusieurs
espèces de mammifères.
b) Distribution. Chez les mammifères, elle se produit selon un
mécanisme de diffusion passive par les liquides biologiques.
Contrairement aux tissus mous et au sang, les os sont capables de
fixer sélectivement le bore (teneur supérieure ou égale à 4 fois
celle du sérum) et de le retenir sensiblement plus longtemps.
c) Métabolisme. Le métabolisme de l'acide borique est
thermodynamiquement défavorable dans les systèmes biologiques.
Les espèces ioniques présentes dans le courant sanguin devraient
donc être les mêmes chez tous les mammifères. Dans ces
conditions, on risque beaucoup moins de se tromper en procédant à
des extrapolations, puisqu'il n'est pas nécessaire de prendre en
considération les différences interspécifiques qui existent
généralement au niveau des voies enzymatiques et de la vitesse de
métabolisation.
d) Elimination. La cinétique d'élimination (en particulier la voie
d'élimination et la demi-vie terminale) se révèle également
analogue chez l'Homme et le rat.
Les analogies qui existent entre les paramètres
pharmacocinétiques relevés chez l'Homme et chez le rat, l'espèce qui
sert à définir la dose sans effet nocif observable (NOAEL) dans les
études de laboratoire, permettent de réduire l'incertitude que
comporte l'extrapolation à l'Homme des résultats obtenus sur le rat.
1.4 Effets sur l'Homme et les animaux de laboratoire
Les données relatives aux effets toxiques sur le développement et
la reproduction montrent que l'effet déterminant consiste en une
réduction du poids des foetus. On a estimé à 9, 6 mg de bore par jour
et par kg de poids corporel, la NOAEL relative à cet effet. La dose la
plus faible produisant un effet nocif observable (LOAEL) est, chez le
rat, d'environ 13 mg/kg de poids corporel par jour, l'effet observé
étant une légère différence dans le poids des foetus (approx. 5%) et
des anomalies costales. A mesure que l'on augmente la dose, on observe
les effets suivants, selon la dose:
a) autres anomalies costales et testiculaires (approx. 25 mg de bore
par jour et par kg de poids corporel);
b) réduction du poids des foetus et accroissement des malformations
cardiovasculaires chez le lapin; graves anomalies testiculaires
chez le rat (approx. 40 mg de bore par jour et par kg de poids
corporel);
c) atrophie testiculaire et stérilité chez le rat (approx. 55 mg de
bore par jour et par kg de poids corporel);
d) réduction du poids des foetus chez la souris (approx. 80 mg de
bore par jour et par kg de poids corporel).
Les études effectuées sur des rats et des souris ne révèlent
aucun signe de cancérogénicité de l'acide borique. Comme les données
humaines font défaut et que les données animales sont également
limitées, on ne peut pas ranger le bore dans une classe précise de
cancérogénicité.
Seules quelques études ont été consacrées à l'évaluation des
effets résultant d'une exposition humaine aux dérivés du bore. Les
données disponibles montrent que l'exposition à ces composés peut se
traduire à brève échéance par une irritation des voies respiratoires
supérieures, du rhinopharynx et des yeux. Ces effets sont cependant de
brève durée et réversibles. La seule étude de suivi à long terme (7
ans) qui ait été consacrée à l'action toxique du bore n'a pas permis
de mettre en évidence d'effets à long terme, encore que l'on ne puisse
pas totalement exclure que ce résultat soit dû à la bonne santé des
travailleurs étudiés, compte tenu du taux élevé d'attrition. Deux
études descriptives ont porté sur la fécondité et le sex ratio
secondaire en rapport avec une exposition. Aucune des deux études n'a
révélé d'effet indésirable sur la fécondité observée dans
l'échantillon. Il y aurait bien eu un excès de naissances féminines,
mais il n'était pas statistiquement significatif et le fait qu'il
existait d'autres facteurs connus pour influer sur le sex ratio
incite à une interprétation prudente de ce résultat. On n'a pas
retrouvé d'études portant sur l'ensemble des paramètres de la
reproduction, par exemple le temps écoulé jusqu'à la grossesse, les
retards dans la conception, les avortements spontanés et le
comportement des spermatozoïdes. Il faut étudier plus à fond les
autres styles de vie et facteurs comportementaux dans leurs rapports
avec la santé et la fécondité afin de mettre en évidence les
populations potentiellement sensibles et évaluer dans le détail les
effets sur la reproduction.
1.5 Effets sur les êtres vivants dans leur milieu naturel
Les bactéries ont une tolérance au bore relativement élevée. Les
concentrations produisant des effets aigus ou chroniques vont de 8 à
340 mg de bore par litre, la plupart des valeurs se situant autour de
18 mg/litre. Ce sont les protozoaires qui sont les plus sensibles. Les
épreuves effectuées sur des protozoaires du genre Entosiphon et
Paramecium ont donné, pour la concentration sans effet observable
sur 72 h (NOEC) et pour la CE3, des valeurs comprises entre 0,3 et 18
mg de bore par litre.
Le bore est un micronutriment essentiel des cyanobactéries et des
diatomées. Les épreuves habituelles pour l'évaluation de la toxicité
chronique, ont donné une concentration sans effet observable comprise
entre 10 et 24 mg de bore par litre pour les algues vertes
dulçaquicoles. Les algues bleues ont une sensibilité analogue, avec
une CE3 à 8 jours de 20 mg de B par litre.
Si l'on se base sur les valeurs de la toxicité aiguë, les
invertébrés sont moins sensibles au bore que les microorganismes. Pour
un certain nombre d'espèces, les valeurs de la CE50 à 24 et 48 h
allaient de 95 à 1376 g de B par litre, la plupart des valeurs se
situant dans l'intervalle 100-200 mg/litre. Les études de toxicité
chronique effectuées sur Daphnia magna ont donné une NOEC allant de
6 à 10 mg de B par litre. Des valeurs un peu plus faibles ont été
obtenues lors d'études en laboratoire ou dans des biocénoses
naturelles. Une étude de 28 jours en laboratoire comportant six stades
trophiques a donné une NOEC de 2,5 mg de B par litre. Des études à
long terme sur des étangs et autres études en milieu naturel (à
l'exclusion des poissons) ont donné des NOEC allant jusqu'à 1,52 mg de
B par litre.
Des épreuves de toxicité aiguë portant sur plusieurs espèces de
poissons ont donné des valeurs comprises entre 10 et près de 300 mg de
B par litre. Ce sont des espèces telles que la truite arc-en-ciel
(Oncorhynchus mykiss) et Brachydanio rerio qui se sont révélées
les plus sensibles, avec des valeurs d'environ 10 mg de B par litre.
La toxicité du bore pour les stades juvéniles des poissons est
attestée par des études effectuées en eau reconstituée sur plusieurs
espèces. C'est ainsi que l'on a exposé à du bore (acide borique ou
borax) les embryons et les premiers stades larvaires de truites
arc-en-ciel, de certaines perches (Micropterus salmoides), de
poissons-chats (Ictalurus punctatus) et de poissons rouges
(Carassius auratus), depuis le moment de la fécondation jusqu'à 8
jours après l'éclosion en eau douce ou dure. Ni la dureté de l'eau, ni
la forme sous laquelle se trouvait le bore n'ont eu d'effets
systématiques sur la survie embryo-larvaire des poissons. C'est la
truite arc-en-ciel qui s'est révélée l'espèce la plus sensible. Pour
ce poisson, la NOEC se situait entre 0,009 et 0,103 mg de B par litre.
L'effet de la dilution naturelle de l'eau sur la toxicité du bore
a été déterminé en utilisant des eaux de surface prélevées en trois
endroits, avec des concentrations de bore de 0,023, de 0,091 et de
0,75 mg/litre. Aucun effet indésirable n'a été noté jusqu'à la dose de
0,75 mg/litre. Les valeurs de la concentration la plus faible
produisant des effets observables (LOEC) allaient de 1,1 à 1,73
mg/litre. L'une des épreuves, effectuée sur de l'eau de puits prélevée
à grande profondeur (600 m), utilisée systématiquement pour les essais
de toxicité en milieu aquatique et fournie par un laboratoire de
Wareham (USA), a donné une NOEC > 18 mg de B par litre. Il semble
donc que les épreuves effectuées en eau reconstituée surestiment la
toxicité des eaux naturelles, peut-être du fait que les premières sont
pauvres en certains nutriments.
On sait depuis les années 20 que le bore est un micronutriment
essentiel pour les végétaux supérieurs, la quantité minimale
nécessaire à la croissance dépendant de l'espèce. Le bore intervient
dans la division cellulaire et le métabolisme ainsi que dans la
structure et la fonction de la membrane. Il existe à l'état naturel
sous forme de borates dans les fruits, les noix et les légumes. Chez
les plantes, l'intervalle entre carence et fixation excessive
(toxicité) est étroit. Dans de nombreux pays, on a constaté que les
plantes présentaient une carence en bore. Cet déficit a plus de
chances de se rencontrer dans les sols acides à texture légère des
régions humides, car le bore est facilement lessivé. En revanche, on
trouve un excès de bore dans les solutions de sols provenant de dépôts
géologiques récents, dans les sols arides, les sols issus de sédiments
marins et ceux qui sont contaminés par diverses sources de pollution,
comme les émissions des centrales thermiques à charbon et celles qui
proviennent des exploitations minières. L'eau d'irrigation est l'une
des principales causes des fortes teneurs en bore qui contaminent les
terrains agricoles.
On a observé qu'à des doses dans l'alimentation de 30 et 300 mg
de bore par kg, des colverts (Anas platyrhyncos) présentaient des
troubles et qu'à la dose de 1000 mg/kg leur survie était réduite.
2. Conclusions
Le bore est un élément naturel qui existe à l'état de borates
dans les océans, les roches sédimentaires, la houille, le schiste et
certains sols. Certaines sources naturelles sont à l'origine de rejets
de bore dans l'environnement comme les océans, la vapeur géothermique
et l'action des agents climatiques sur les roches sédimentaires riches
en argile. Les activités humaines libèrent également du bore dans
l'environnement, mais dans une moindre proportion.
Le bore est un micronutriment essentiel des plantes supérieures,
la concentration nécessaire à une croissance optimale étant variable
suivant les espèces. Pour certaines plantes, il y a une faible marge
entre carence et toxicité.
Si l'on compare la concentration environnementale qui ne produit
pas d'effet (1 mg/litre) et les teneurs en bore généralement
rencontrées dans le milieu, on peut en déduire que le risque d'effets
indésirables sur l'écosystème aquatique est faible. Il est possible
toutefois que dans certains milieux riches en bore, les teneurs
naturelles soient plus élevées. On peut raisonnablement penser qu'en
pareil cas, les organismes aquatiques se sont adaptés aux conditions
locales.
Chez l'Homme, l'exposition au bore provient principalement de
l'alimentation et de l'eau de boisson. On estime que la concentration
du bore dans l'eau de boisson est, en moyenne mondiale, de l'ordre de
0,1 à 0,3 mg/litre. Pour la population générale, c'est la nourriture
qui apporte le plus de bore. La dose moyenne ingérée avec la
nourriture est d'environ 1,2 mg par jour.
Chez l'Homme et l'animal, l'acide borique et les borates sont
absorbés au niveau des voies digestives et respiratoires. La
résorption dépasse 90% de la dose administrée et on peut s'en rendre
compte en mesurant l'excrétion urinaire, qui est rapide, puisqu'elle
s'effectue en quelques jours.
L'expérimentation animale montre que sous forme d'acide borique
et de borate, le bore a des effets toxiques sur le développement et la
reproduction à des concentrations qui sont 100 à 1000 fois supérieures
à celles que l'on rencontre dans l'environnement. On ne possède pas
suffisamment de données toxicologiques sur l'Homme. On a fixé à 0,4 mg
par kg de poids corporel la dose journalière tolérable de bore. Pour
tenir compte des divers milieux lors de la fixation de cette dose, il
faut connaître l'exposition dans les différents pays.
3. Recommandations
a) Les valeurs guides pour l'eau et les aliments doivent être basées
sur la dose tolérable indiquée dans le présent document.
b) Dans l'application de la dose tolérable, il faut tenir compte du
fait que le bore peut avoir un effet physiologiquement bénéfique
pour l'Homme.
c) Dans l'application des normes, on tiendra compte du fait que le
bore est essentiel pour certains composants de l'environnement
(par exemple, le bore est un micronutriment essentiel pour les
plantes supérieures).
d) Les suppléments alimentaires qui dépassent la dose tolérable sont
à éviter.
RESUMEN, CONCLUSIONES Y RECOMENDACIONES
1. Resumen
1.1 Identidad, presencia en la naturaleza y métodos analíticos
El boro es un elemento presente en la naturaleza que se encuentra
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Está ampliamente distribuido en
la naturaleza, con concentraciones de alrededor de 10 mg/kg en la
corteza terrestre (margen de variación: de 5 mg/kg en los basaltos a
100 mg/kg en los esquistos) y de unos 4,5 mg/litro en el océano.
Los productos y minerales comerciales de borato más importantes
son el pentahidrato de bórax, el bórax, el perborato de sodio, el
ácido bórico, la colemanita y la ulexita. En las bajas concentraciones
y el pH casi neutro en que está presente en la mayoría de los fluidos
biológicos, la especie predominante es el B(OH)3 (con algo de
B(OH)4-), con independencia de que la fuente de boro sea el ácido
bórico o uno de los boratos. Esto se debe a que el ácido bórico es un
ácido muy débil (p Ka 9,15). El perborato de sodio se hidroliza para
dar peróxido de hidrógeno y metaborato; por consiguiente, puede
mostrar propiedades químicas y toxicológicas algo distintas de las de
los otros boratos.
Para el análisis de los bajos niveles de boro presente en las
muestras biológicas y del medio ambiente se prefieren los métodos de
plasma acoplado por inducción (PAI); los métodos colorimétricos se
deben utilizar con cautela.
1.2 Producción, aplicaciones, destino en el medio ambiente y fuentes
de exposición
Los depósitos económicos de borato son raros y se encuentran en
regiones áridas de Turquía, los Estados Unidos, la Argentina, Chile,
Rusia, China y el Perú. La producción mundial total de minerales de
boro--sobre todo colemanita, ulexita, tíncal y kernita--fue de
alrededor de 2 750 000 toneladas en 1994. Unas 800 000 toneladas de
productos comerciales de borato, expresados como B2O3, se fabricaron
a partir de minerales del boro.
Las principales aplicaciones finales del borato son la fibra de
vidrio de calidad de aislamiento o textil, la lejía para lavar
(perborato de sodio), el vidrio de borosilicato, pirorretardantes,
fertilizantes y herbicidas agrícolas (como elemento traza) y esmaltes,
fritas y vidriados cerámicos, así como innumerables aplicaciones
diversas.
El boro entra en el medio ambiente sobre todo mediante la
meteorización de las rocas, la volatilización de ácido bórico del agua
del mar y la actividad volcánica. También se desprende boro de fuentes
antropogénicas en menor medida. Entre las fuentes antropo-génicas
figuran la quema de productos agrícolas, de basuras y de leña, la
producción de energía utilizando carbón y petróleo, la fabricación de
productos de vidrio, la utilización de boratos/perboratos en el hogar
y en la industria, la extracción/elaboración de borato, la lixiviación
de madera/papel tratados y la eliminación de aguas residuales/fangos
de alcantarillado. Muchas de estas fuentes son difíciles de
cuantificar.
Se producen emisiones a la atmósfera de boratos y de ácido bórico
en forma de partículas y de vapor como consecuencia de la
volatilización desde el agua del mar, la actividad volcánica y, en
menor medida, las operaciones de extracción, la fabricación de vidrio
y cerámica, la aplicación de productos químicos agrícolas y las
centrales eléctricas de carbón. El boro no está presente en la
atmósfera en concentraciones significativas; sin embrago, la cantidad
total que hay en ella en cualquier momento es significativa debido al
enorme volumen de la atmósfera. Teniendo en cuenta su solubilidad en
el agua, no es previsible que los boratos persistan en la atmósfera en
una medida significativa.
Se puede incorporar boro al agua libre y a la del suelo mediante
procesos de meteorización, y en una medida mucho menor por vertidos
antropogénicos, como desagües de aguas residuales. Se supone que las
reacciones de adsorción-desorción son el único mecanismo importante
que influye en el destino del boro en el agua. El grado de adsorción
de boro depende del pH del agua y de su concentración en la solución.
El boro se adsorbe en las partículas del suelo, dependiendo el
grado del tipo de suelo, el pH, la salinidad, el contenido de materia
orgánica, el contenido de óxido de hierro y de aluminio, el contenido
de hidróxido de hierro y de aluminio y el contenido de arcilla. La
adsorción de boro puede variar entre totalmente reversible e
irreversible, en función del tipo y de la condición del suelo.
Los iones borato presentes en solución acuosa están básicamente
en estado totalmente oxidado. No es probable que ningún proceso
aeróbico influya en su especiación y no se han notificado procesos de
biotransformación. Por consiguiente, no es probable que haya ninguna
diferencia en las especies de boro debida a la biotransformación.
El coeficiente de reparto octanol/agua del ácido bórico medido es
de 0,175, lo cual indica un potencial de bioacumulación bajo. Los
experimentos de laboratorio con organismos acuáticos han confirmado
este potencial. Las plantas acumulan boro; sin embargo, la absorción
se ve afectada por el pH de la solución del suelo, la temperatura, la
intensidad de la luz y la concentración de otros elementos (por
ejemplo, calcio y potasio). Los estudios de la acumulación de boro en
plantas, insectos y peces han puesto de manifiesto que el boro se
bioacumula en las plantas, pero no se bioamplifica en la cadena
alimentaria de los organismos acuáticos.
El boro está presente en los suelos en concentraciones que van de
10 a 300 mg/kg (promedio de 30 mg/kg), en función del tipo de suelo,
la cantidad de materia orgánica y la cantidad de precipitación. Las
concentraciones de boro en el agua superficial dependen de factores
como la naturaleza geoquímica de la superficie de drenaje, la
proximidad a regiones costeras marinas y la incorporación de vertidos
de efluentes industriales y urbanos. Las concentraciones de boro en el
agua superficial varían ampliamente, desde 0,001 hasta llegar a
360 mg/litro. Sin embargo, las concentraciones medias en las aguas de
Europa, el Pakistán, Rusia y Turquía suelen ser inferiores a
0,6 mg/litro. Las concentraciones de boro en el agua del Japón,
Sudáfrica y América del Sur están en general por debajo de
0,3 mg/litro. En las aguas de América del Norte son normalmente
menores de 0,1 mg/litro, con 0,4 mg/litro o menos en alrededor del
90%.
El boro se acumula en las plantas acuáticas y terrestres, pero no
se amplifica a través de la cadena alimentaria. Se ha comprobado que
las concentraciones de boro oscilan entre 26 y 382 mg/kg en las
plantas acuáticas de agua dulce sumergidas, entre 11,3 y 57 mg/kg en
la vegetación de agua dulce que crece fuera del agua y entre 2,3 y
94,7 mg/kg de peso seco en las plantas terrestres. Tomando como base
el peso fresco, las concentraciones de boro en los invertebrados y los
peces marinos son semejantes a los niveles presentes en los medios de
exposición, entre 0,5 y 4 mg/kg. Para dos especies de peces de agua
dulce se observó un factor de bioconcentración de 0,3.
Las concentraciones de boro en el aire del medio ambiente oscilan
entre <0,5 y alrededor de 80 ng/m3, con un promedio en todos los
continentes de 20 ng/m3.
La estrecha semejanza entre las concentraciones de boro en el
agua fréatica, el agua dulce superficial y el agua potable indica que
el boro no se elimina en el tratamiento del agua fréatica y del agua
dulce superficial utilizadas para beber.
Se supone que la ingesta de boro por el ser humano es de
0,44 µg/día a partir del medio ambiente, de 0,2-0,6 mg/día con el agua
de beber y de 1,2 mg/día en la alimentación. Se considera que la
ingesta media de boro a partir del suelo es de 0,5 µg/día. Una
estimación razonable de la exposición al boro en los productos de
consumo es de 0,1 mg/día.
1.3 Cinética y vigilancia biológica
La farmacocinética del boro parece ser bastante parecida en todas
las especies en los siguientes aspectos:
a) La absorción de boratos es básicamente completa (alrededor del
95% en el ser humano y en ratas), y tras la ingestión aparece con
rapidez boro en la sangre y los tejidos corporales de varias
especies de mamíferos.
b) La distribución del boro en los mamíferos parece producirse por
difusión pasiva en todos los líquidos del cuerpo. Al contrario
que los tejidos blandos y la sangre, los huesos muestran una
absorción selectiva de boro (>4 veces mayor que en el suero) y
tiempos de retención considerablemente más largos.
c) El metabolismo del ácido bórico es termodinámicamente
desfavorable en los sistemas biológicos. Por consiguiente, se
supone que las especies iónicas en la circulación sistémica son
equivalentes en todos los mamíferos. Así se elimina una fuente
importante de posible incertidumbre para la extrapolación del
riesgo, puesto que no es necesario tener en cuenta diferencias
interespecíficas en las rutas enzimáticas y/o las tasas
metabólicas.
d) La cinética de la eliminación (especialmente la vía de
eliminación y la semivida terminal) también parece ser semejante
para el ser humano y para las ratas.
Las semejanzas de los parámetros farmacocinéticos entre el ser
humano y las ratas, especie que define la concentración sin efectos
adversos observados (NOAEL) para estudios de laboratorio, reducen la
incertidumbre en cuanto a la extrapolación entre estas dos especies.
1.4 Efectos en animales de laboratorio y en el ser humano
Los datos relativos a la toxicidad en el desarrollo y
reproductiva indican que el efecto crítico en las ratas es el menor
peso corporal del feto. La NOAEL para el menor peso corporal del feto
es de 9,6 mg de boro/kg de peso corporal al día. La menor
concentración con efectos adversos observados (LOAEL), en la que las
ratas muestran ligeras (approx. 5%) diferencias en el peso corporal
del feto y anomalías en las costillas, es de unos 13 mg de boro/kg de
peso corporal al día. A medida que aumenta la dosis, los efectos que
se observan (y las dosis a las que se ven) son los siguientes:
a) nuevos efectos en las costillas y patología testicular en ratas
(approx. 25 mg de boro/kg de peso corporal al día);
b) disminución del peso corporal del feto y aumento de las
malformaciones cardiovasculares fetales en conejos, y patología
testicular grave en ratas (approx. 40 mg de boro/kg de peso
corporal al día);
c) atrofia testicular y esterilidad en ratas (approx. 55 mg de
boro/kg de peso corporal al día); y
d) reducción del peso corporal del feto en ratones (approx. 80 mg de
boro/kg de peso corporal al día).
En los estudios de animales con ratones y ratas no se observaron
pruebas de carcinogenicidad del ácido bórico. Debido a la falta de
datos humanos y a lo limitado de los obtenidos de animales, el boro no
se puede clasificar en cuanto a su carcinogenicidad humana.
Son pocos los estudios que se han realizado en el ser humano de
evaluación de los efectos para la salud asociados con la exposición a
compuestos de boro. Los datos disponibles indican que la exposición
está asociada con efectos irritantes de corta duración en las vías
respiratorias superiores, la nasofaringe y los ojos. Sin embargo,
parece que esos efectos son breves y reversibles. En el único estudio
de seguimiento de larga duración (siete años) no se consiguió
identificar ningún efecto prolongado para la salud, aunque no se puede
excluir del todo un efecto en trabajadores sanos debido a la tasa de
desgaste (47%). En dos estudios descriptivos se evaluó la fecundidad y
la razón secundaria de sexos en relación con la exposición. En ninguno
de los dos se notificó un efecto perjudicial demostrado en la
fecundidad de la muestra seleccionada. Aunque se ha indicado la
posibilidad de un porcentaje de nacimientos de hembras superior al
normal, la ausencia de significación estadística y la atención a otras
covariantes que se saben que influyen en el sexo obliga a una
interpretación cauta de este resultado. No se conoce ningún estudio en
el que se evalúe el espectro de resultados reproductivos, como el
tiempo hasta la gestación, los retrasos en la concepción, los abortos
espontáneos y los análisis del esperma en los machos. La función de
otros factores relativos al tipo de vida y al comportamiento en
relación con la salud y la fecundidad requiere un ulterior estudio
para identificar poblaciones posiblemente sensibles y evaluar los
efectos reproductivos de manera más completa.
1.5 Efectos en los organismos del medio ambiente
Las bacterias tienen una tolerancia hacia el boro relativamente
grande. Las concentraciones con efectos agudos y crónicos oscilan
entre 8 y 340 mg de boro/litro, siendo la mayoría de los valores
superiores a 18 mg de boro/litro. Los protozoos son más sensibles. En
pruebas realizadas con Entosiphon y con Paramecium se obtuvieron
concentraciones sin efectos observados (NOEC) en 72 horas y valores de
CE3 de 0,3 a 18 mg de boro/litro.
El boro es un micronutriente esencial para las cianobacterias y
las diatomeas. En pruebas crónicas normales con algas verdes de agua
dulce se observaron concentraciones sin efectos comprendidas entre 10
y 24 mg de boro/litro. Las algas verdeazuladas parecen tener una
sensibilidad semejante, con una CE3 en ocho días de 20 mg de
boro/litro.
Tomando como base los valores de la toxicidad, los invertebrados
son menos sensibles al boro que los microorganismos. Para varias
especies, los valores de la CE50 en 24-48 horas oscilaron entre 95 y
1376 mg de boro/litro, con la mayoría de los valores del orden de
100-200 mg de boro/litro. En estudios de toxicidad crónica con
Daphnia magna, los valores de la NOEC oscilaron entre 6 y 10 mg de
boro/litro. Se obtuvieron valores de la NOEC ligeramente más bajos en
estudios de biocenosis de laboratorio y de campo. En el estudio de
laboratorio de 28 días, consistente en seis etapas tróficas, se obtuvo
una NOEC de 2,5 mg de boro/litro. En estudios prolongados realizados
en un estanque al aire libre y en el campo (sin incluir peces) las
NOEC fueron de hasta 1,52 mg de boro/litro.
En pruebas de toxicidad aguda con varias especies de peces se
obtuvieron valores comprendidos entre alrededor de 10 y cerca de
300 mg de boro/litro. La trucha irisada (Oncorhynchus mykiss) y
Brachydanio rerio fueron los más sensibles, con valores aproximados
de 10 mg de boro/litro.
La toxicidad del boro en las primeras fases de la vida de los
peces está documentada para varias especies en agua reconstituida. Se
expusieron a boro, en forma de ácido bórico o de bórax, fases
embrionarias o larvarias iniciales de trucha irisada, perca atruchada
(Micropterus salmoides), coto punteado (Ictalurus punctatus) y
Carassius auratus desde la fecundación hasta ocho días después de la
eclosión en agua blanda o dura. Ni la dureza del agua ni la forma del
boro influyeron de manera uniforme en la supervivencia de los
embriones-larvas de los peces. La trucha irisada fue la especie más
sensible. Las NOEC para la trucha irisada oscilaron entre 0,009 y
0,103 mg de boro/litro.
El efecto de la dilución natural en el agua sobre la toxicidad
del boro se determinó utilizando agua superficial recogida en tres
lugares, con concentraciones de boro de 0,023, 0,091 y 0,75 mg/litro.
No se detectó ningún efecto adverso hasta los 0,75 mg de boro/litro.
Las concentraciones más bajas con efectos observados (LOEC) oscilaron
entre 1,1 y 1,73 mg de boro/litro. En una prueba con agua de un pozo
profundo (600 m), utilizada habitualmente para pruebas de toxicidad
acuática en virtud de un contrato con un laboratorio situado en
Wareham, Massachusetts, Estados Unidos, se obtuvo una NOEC de
>18,0 mg de boro/litro. Así pues, al parecer en la exposición a agua
reconstituida se sobreestimaba la toxicidad determinada en aguas
naturales, posiblemente como consecuencia de la deficiencia de
nutrientes en la primera.
Desde los años veinte se sabe que el boro es un micronutriente
esencial para las plantas superiores, con diferencias interespecíficas
en cuanto a las concentraciones necesarias para un crecimiento óptimo.
El boro interviene en la división, el metabolismo y la estructura y la
función de las membranas de las células. En forma de borato está
presente en las frutas, las nueces y las hortalizas. La diferencia
entre la deficiencia y la absorción excesiva (toxicidad) es pequeña en
las plantas. Se ha notificado deficiencia de boro en plantas
terrestres en muchos países. Es más probable que se produzca
deficiencia de boro en suelos ácidos de textura ligera en las regiones
húmedas, debido a su susceptibilidad a la lixiviación. Suele haber
exceso de boro en soluciones del suelo procedentes de depósitos
jóvenes desde el punto de vista geológico, en suelos áridos, en los
derivados de sedimentos marinos y en los afectados por fuentes de
contaminación como los vertidos de centrales termoeléctricas de carbón
y de operaciones de extracción. El agua de riego es una de las
principales fuentes de concentraciones altas de boro que provocan
toxicidad en el campo.
El crecimiento del pato real (Anas platyrhynchos) se vio
afectado negativamente por concentraciones de 30 y 300 mg de boro/kg
en los alimentos y con 1000 mg/kg se redujo la supervivencia.
2. Conclusiones
El boro es un elemento que se encuentra presente en la naturaleza
en forma de boratos en los océanos, las rocas sedimentarias, el
carbón, el esquisto y algunos suelos. Las fuentes naturales de los
boratos que se liberan en el medio ambiente son los océanos, el vapor
geotérmico y la meteorización natural de las rocas sedimentarias ricas
en arcilla. También se desprende boro en menor medida de fuentes
antropogénicas.
El boro es un micronutriente esencial para las plantas
superiores, con diferencias interespecíficas en cuanto a las
concentraciones necesarias para un crecimiento óptimo. Se ha observado
deficiencia de boro en plantas terrestres en muchos países de todo el
mundo. La diferencia entre la deficiencia y toxicidad es pequeña en
algunas plantas.
La comparación de la concentración sin efectos en el medio
ambiente (1 mg/litro) con los niveles generales en el medio ambiente
indica que el riesgo de efectos adversos del boro en el ecosistema
acuático es bajo. Los niveles naturales son más elevados en algunos
medios ricos en boro. Es razonable suponer que los organismos
acuáticos de dichos hábitats pueden adaptarse a las condiciones
locales.
La exposición del ser humano al boro se produce sobre todo por
medio de los alimentos y el agua potable. Se consideró que la
concentración media mundial de boro en el agua potable estaba
comprendida entre 0,1 y 0,3 mg de boro/litro.
Para la población general, la mayor exposición al boro procede de
la ingesta oral con los alimentos. La ingesta diaria media de boro con
los alimentos es de alrededor de 1,2 mg.
En las personas y en los animales, el ácido bórico y el borato se
absorben del tracto gastrointestinal y de las vías respiratorias. Se
absorbe más del 90% de las dosis administradas de estos compuestos,
como se pone de manifiesto en la excreción en la orina, que es rápida,
en unos pocos días.
En experimentos con animales se ha demostrado que el boro en
forma de ácido bórico y de borato tiene toxicidad reproductiva y en el
desarrollo en concentraciones de 100 a 1000 veces superiores a los
niveles normales de exposición. Se carece de datos suficientes acerca
de la toxicidad en el ser humano. La ingesta tolerable (IT) de boro se
fijó en 0,4 mg/kg de peso corporal al día. La asignación de la IT en
diversos medios debe basarse en los datos relativos a la exposición de
cada país.
3. Recomendaciones
a) Los valores guía del agua y los alimentos deben basarse en la IT
proporcionada por este documento.
b) La IT se debe aplicar quedando entendido que el boro puede
aportar beneficios fisiológicos para la salud humana.
c) Hay que reconocer al aplicar las normas que el boro es esencial
para algunos elementos constituyentes del medio ambiente (por
ejemplo, el boro es un micronutriente esencial para las plantas
superiores).
d) Se deben evitar los complementos de la alimentación que superen
la IT.
