
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 217
BACILLUS THURINGIENSIS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 217
Microbial Pest Control Agent
BACILLUS THURINGIENSIS
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Bacillus thuringiensis.
(Environmental health criteria ; 217)
1.Bacillus thuringinesis - pathogenicity 2.Pest control,
Biological - methods 3.Insecticides - chemistry
4.Environmental exposure 5.Occupational exposure
I.Series
ISBN 92 4 157217 5 (NLM Classification: QW 127.5.B2)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS THURINGIENSIS
PREAMBLE
ABBREVIATIONS
1. SUMMARY
1.1. Identity, biological characteristics, and
analytical methods
1.2. Mode of action on target insects
1.3. Habitats
1.4. Commercial products, production and application
1.5. Effects of Bt on non-target organisms
1.6. Exposure and effects of Bt on humans
1.7. Conclusions
2. IDENTITY, BIOLOGICAL PROPERTIES, AND ANALYTICAL METHODS
2.1. Identity
2.1.1. Bacillus thuringiensis (Bt)
2.1.2. Relationship between Bacillus thuringiensis and
Bacillus cereus
2.1.3. Crystal composition and morphology
2.1.4. Classification of Bt subspecies
2.1.5. Genetics of ICP
2.1.6. Beta-exotoxin
2.1.7. Other Bt metabolites
2.2. Bioassays
2.2.1. Spore counts
2.2.2. International bioassay for ICPs
3. MODE OF ACTION ON TARGET INSECTS
3.1. Bioactivity of field isolates
3.2. Mechanism of action of Bt formulations
3.3. Resistance of insect populations
4. NATURAL AND TREATED HABITATS
4.1. Natural occurrence of Bt
4.1.1. Bt in insect hosts
4.1.2. Bt in soil
4.1.3. Bt on plant surfaces
4.2. Treated habitats
4.3. Environmental fate, distribution and movement
4.3.1. Distribution and fate of Bt in terrestrial
habitats
4.3.1.1 Fate of Bt and ICP on plant surfaces
4.3.1.2 Fate of Bt in soil
4.3.2. Distribution and fate of Bt in aquatic habitats
4.3.3. Transport of Bt by non-target organisms
5. COMMERCIAL PRODUCTION
5.1. History of Bt and its commercial applications
5.1.1. Production levels
5.1.2. Production processes, formulations and quality
standards
5.1.3. General patterns of use
5.1.3.1 Applications in agriculture and forestry
5.1.3.2 Applications in vector control
6. EFFECTS ON ANIMALS
6.1. Mammals
6.1.1. Oral exposure
6.1.2. Inhalation exposure
6.1.3. Dermal exposure
6.1.4. Dermal scarification exposure
6.1.5. Subcutaneous inoculation
6.1.6. Ocular exposure
6.1.7. Intraperitoneal exposure
6.1.7.1 Immune-intact animals
6.1.7.2 Immune-suppressed animals
6.1.8. Effects of activated Bt ICP
6.1.9. Studies in wild animals
6.2. Effects on birds
6.3. Effects on aquatic vertebrates
6.4. Effects on invertebrates
6.4.1. Effects on invertebrates other than insects
6.4.2. Effects on non-target insects
6.4.2.1 Aquatic insects
6.4.2.2 Terrestrial insects
6.4.2.3 Honey-bees
6.4.2.4 Parasitoids
7. EXPOSURE AND EFFECTS ON HUMANS
7.1. Bacillus thuringiensis
7.1.1. Experimental exposure of humans
7.1.2. Exposure of workers during manufacture
7.1.3. Exposure of workers in spraying operations
7.1.4. Exposure of human populations by spraying
operations over populated areas.
7.1.5. Clinical case reports
7.1.6. Dietary exposure of the general population
7.2. Bacillus cereus
8. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9. CONCLUSIONS AND RECOMMENDATIONS
10. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANISATIONS
REFERENCES
RÉSUMÉ
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postage
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure
to environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects
of pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As such,
they include and review studies that are of direct relevance for the
evaluation. However, they do not describe every study carried out.
Worldwide data are used and are quoted from original studies, not from
abstracts or reviews. Both published and unpublished reports are
considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant published
data are absent or when they are pivotal to the risk assessment. A
detailed policy statement is available that describes the procedures
used for unpublished proprietary data so that this information can be
used in the evaluation without compromising its confidential nature
(WHO (1990) Revised Guidelines for the Preparation of Environmental
Health Criteria Monographs. PCS/90.69, Geneva, World Health
Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary -- a review of the salient facts and the risk evaluation
of the chemical
* Identity -- physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
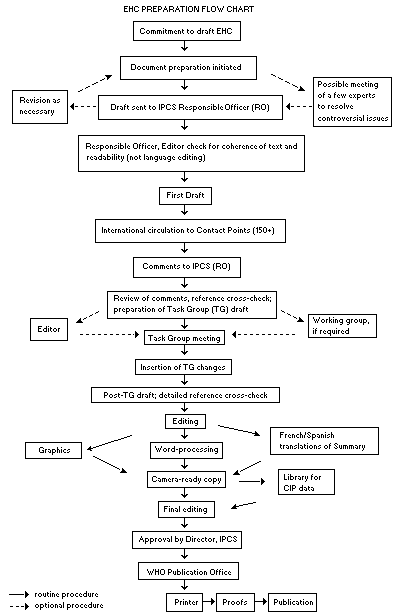
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for layout
and language. The first draft, prepared by consultants or, more
usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS
THURINGIENSIS
Members
Professor D. Calamari, Department of Biology, Structure and Function,
University of Milan, Varese, Italy
Dr C. Cummins, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr N. Gratz, Commugny, Switzerland (Rapporteur)
Dr A. Klier, Unité de Biochimie Microbienne, Département des
Biotechnologies, Institut Pasteur, Paris, France
Professor P. Lüthy, Microbiological Institute ETH, Zurich, Switzerland
Dr C.M. Scanlan, Texas A & M University, Department of Veterinary
Pathobiology, Texas Veterinary Medical Center, Texas, USA (Chairman)
Observers
Dr R.J. Cibulsky, Abbott Laboratories, Chemical and Agricultural
Products, North Chicago, Illinois, USA
Dr E. Cozzi, Clinical Research and Development, Animal Health
Products, Abbott Laboratories, North Chicago, Illinois, USA
Dr M. Ronchin, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Secretariat
Professor M. Maroni, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Dr R. Plestina, Zagreb, Croatia
ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS THURINGIENSIS
A WHO Task Group on Environmental Health Criteria for the
microbial pest control agent Bacillus thuringiensis (Bt) met at the
International Centre for Pesticide Safety in Busto Garolfo, Milan,
Italy, from 27 to 31 October 1997. Professor M. Maroni, Director of
the Centre, welcomed participants on behalf of the Centre, which was
responsible for organizing the meeting. Dr R. Plestina, IPCS temporary
adviser, opened the meeting and welcomed participants on behalf of Dr
M. Mercier, Director of IPCS. The Group reviewed and revised the draft
and made an evaluation of the risks for human health and the
environment from exposure to Bt products. Drs R. Plestina and A. Aitio
of the IPCS Central Unit were responsible for the scientific aspects
of the monograph, and Dr P.G. Jenkins for the editing. The assistance
of manufacturers, notably Abbott Laboratories, in providing
unpublished documentation for the review is greatly appreciated.
Microbial Pest Control Agents (MPCAs), notably products of
various Bt subspecies, are increasingly used in pest management
programmes against the larvae of several insect pests of major
agricultural crops and forests, and several insect vectors of human
diseases, and some nuisance pests. Bt products have been used
worldwide, and their commercial production is about 1% of that of
chemical pesticides. A number of reviews have recently been published
on various aspects of Bt (Entwistle et al., 1983; McClintock et al.,
1995; Cannon, 1996; Dean et al., 1996; Kumar et al., 1996; Schnepf et
al., 1998; Nielsen-LeRoux et al., 1998).
The activities in preparing this document were recommended by an
Informal Consultation on the Safety of Microbial Pest Control Agents
held in Geneva in June 1993. The first draft of the monograph was
prepared by a drafting group in 1994 and was subsequently amended by
Professor C.M. Scanlan (Texas A&M University, Department of Veterinary
Pathobiology, The Texas Veterinary Medical Center, Texas, USA). The
revised draft was circulated to the IPCS contact points. Based on the
comments received, it was amended and updated by Drs B.M. Hansen and
N.B. Hendriksen (National Environmental Research Institute, Roskilde,
Denmark). The document was finally approved by the Task Group members.
ABBREVIATIONS
Bc Bacillus cereus
Bt Bacillus thuringiensis
Bta Bacillus thuringiensis subspecies aizawai
Btd Bacillus thuringiensis subspecies darmstadiensis
Bte Bacillus thuringiensis subspecies entomocidus
Btg Bacillus thuringiensis subspecies galleriae
Bti Bacillus thuringiensis subspecies israelensis
Btk Bacillus thuringiensis subspecies kurstaki
Btko Bacillus thuringiensis subspecies konkukian
Btt Bacillus thuringiensis subspecies thuringiensis
Btte Bacillus thuringiensis subspecies tenebrionis
cfu colony forming unit
GILSP good industrial large-scale practice
GMO genetically modified organism
HPLC high-performance liquid chromatography
ICP insecticidal crystal protein
ITU international toxic unit
IUPAC International Union of Pure and Applied Chemistry
MPCA microbial pest control agent
NTO non-target organism
PCR polymerase chain reaction
SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
1. SUMMARY
This monograph deals with microbial pest control agents (MCPAs)
based on Bacillus thuringiensis (Bt). This bacterium is also a key
source of genes for transgenic expression to provide pest resistance
in plants and microorganisms as pest control agents in so-called
genetically modified organisms (GMOs). The potential effects on human
health and the environment of GMOs involve several aspects that are
only remotely or not at all related to Bt products, and they are
therefore outside the scope of this monograph.
1.1 Identity, biological characteristics and analytical methods
Bt is a facultative anaerobic, gram-positive bacterium that forms
characteristic protein inclusions adjacent to the endospore. Bt
subspecies can synthesize more than one parasporal inclusion. Bt is
genetically indistinguishable from Bc, except for the ability of Bt to
produce parasporal crystalline inclusions, which are toxic for certain
invertebrates, especially species of insect larvae belonging to the
insect orders Coleoptera, Diptera and Lepidoptera. The
parasporal inclusions are formed by different insecticidal crystal
proteins (ICP). The crystals have various shapes (bipyramidal,
cuboidal, flat rhomboid, spherical or composite with two crystal
types), depending on their ICP composition. A partial correlation
between crystal morphology, ICP composition, and bioactivity against
target insects has been established.
The basic phenotypic taxon is the subspecies, identified by the
flagellar (H) serotype. By 1998, 67 subspecies had been described. The
genes that encode the ICPs are mostly on plasmids. Each ICP is the
product of a single gene. Most plasmids with ICP genes are readily
transferred by conjugation between Bt strains and may be transferred
to related species of bacteria. The phenotypic classification has now
been complemented by molecular biological characterization, based on
the sequence of the crystal (cry and cyt) genes rather than target
organism specificity. Different domains of the ICP are responsible for
host susceptibility (receptor recognition) and toxicity (pore
formation).
Techniques commonly used to characterize Bt strains or the ICP
itself include cell wall fatty acid analysis, monoclonal antibodies,
oligonucleotide DNA probes, plasmid profiles, polymerase chain
reaction (PCR) analysis, DNA fingerprinting and SDS-PAGE profiles.
Beta-exotoxin, a heat-stable nucleotide, is produced by some Bt
subspecies during vegetative growth and may contaminate the products.
Beta-exotoxin is toxic for almost all forms of life including humans
and the target insect orders. During vegetative growth, various Bt
strains produce an assortment of antibiotics, enzymes, metabolites and
toxins, including Bc toxins, that may have detrimental effects on both
target organisms and non-target organisms (NTOs). Spore counts do not
accurately reflect the insecticidal activity of a Bt strain or Bt
product. The potency (ITU/mg) of each Bt product is bioassayed using
an international standard that uses a specific test insect.
1.2 Mode of action on target insects
The sporulated Bt with ICP or spore-ICP complexes must be
ingested by a susceptible insect larva. The efficacy of the ICP
depends on the solubilization in the midgut, the conversion of the
protoxin to the biologically active toxin by proteolytic enzymes,
specific membrane receptor binding by the C-terminal domain of the
active toxin, and pore formation by the N-terminal domain with
subsequent lysis of the epithelial cells. Spore germination and
proliferation of the vegetative cells into the haemocoel may result in
a septicaemia, contributing to the cause of death. Receptor binding by
the ICP is the major determinant of host specificity by the different
Bt ICPs.
1.3 Habitats
Many different Bt subspecies have been isolated from dead or
dying insects mostly from the orders Coleoptera, Diptera and
Lepidoptera, but many subspecies have also been isolated from soil,
leaf surfaces and other habitats. The carcasses of dead insects often
contain large quantities of spores and ICPs that may enter the
environment. The coleopteran-active and lepidopteran-active Bt
subspecies are primarily associated with the soil and phylloplane
(leaf surfaces), whereas the dipteran-active Bt subspecies are
commonly found in aquatic environments. In the environment, the spores
persist and vegetative growth may occur when conditions are favourable
and nutrients are available.
1.4 Commercial products, production and application
Conventional Bt products, which utilize naturally-occurring Bt
strains, account for approximately 90% of the world MPCA market. Most
Bt products contain ICP and viable spores, but in some Bti products
the spores are inactivated. Each year some 13 000 tonnes are produced
using aerobic fermentation technology. Conventional Bt products have
been targeted primarily against lepidopteran pests of agricultural and
forestry crops; however in recent years, Bt strains active against
coleopteran pests have also been marketed. Strains of Bti active
against dipteran vectors of parasitic and viral diseases are being
used in public health programmes.
Commercial Bt formulations may be applied as an insecticide to
foliage, soil, water environments or food storage facilities. After
the application of a Bt subspecies to an ecosystem, the vegetative
cells and spores may persist at gradually decreasing concentrations
for weeks, months or years as a component of the natural microflora.
The ICPs, however, are rendered biologically inactive within hours or
days.
1.5 Effects of Bt on non-target organisms
Studies on mammals, particularly those on laboratory animals,
have evaluated possible infectivity and toxicity of various Bt
preparations, which include the ICPs, vegetative cells and spores. The
ICPs, spores and vegetative cells of the Bt subspecies, which were
administered by different routes, were mostly non-pathogenic and
non-toxic to the various animal species tested. The vegetative cells
and/or spores of Bt were demonstrated to persist for weeks without
causing adverse effects. Bt has not been observed to adversely affect
birds, fish or many other non-target aquatic vertebrates tested in a
large number of laboratory and field studies. Relatively few species
of aquatic invertebrates are susceptible to Bt under either laboratory
or field conditions. Bt does not adversely affect earthworms.
The Bt subspecies have generally been shown to be highly specific
in their insecticidal activity for Coleoptera, Diptera and
Lepidoptera and have demonstrated little, if any, direct toxicity to
non-target arthropods. Most of the existing safety data on non-target
arthropods has been generated using the Bt subspecies with activity
against Diptera and Lepidoptera.
Studies of Bti formulations free of toxic contaminants have not
demonstrated deleterious effects on the vast majority of non-target
arthropods. Some midges (Diptera: Chironomidae), which are closely
related to mosquitos, have been shown to be susceptible to high
dosages of Bti, but are not affected by mosquito larvicidal dosages.
In field studies, transient decreases or increases in populations of
some non-target arthropods have been reported.
Many insect orders have been tested in either the laboratory or
field, most of which have shown no effect from Btk.
Mortality has been observed in honey-bees (Apis mellifera)
after exposure to vegetatively growing Btt and Btk, but the effect
does not seem to be related to spores or ICPs. In laboratory and field
studies Btg demonstrated no adverse effect on honey-bees.
Bte strains that produce beta-exotoxin have been shown to have
adverse effects on non-target arthropods.
1.6 Exposure and effects of Bt on humans
The field application of Bt products can result in considerable
aerosol and dermal exposure of workers. Agricultural uses of Bt can
result in Bt contamination of potable water and food. With the
exception of case reports on ocular and dermal irritation, no adverse
health effects have been documented after occupational exposure to Bt
products. Human volunteers ingested and inhaled large quantities of a
Btk formulation but experienced no adverse health effects. Antibody
titres to the vegetative cells, spores and spore-crystal complexes
have been demonstrated in workers who spray Bt products; however, no
adverse health effects were reported. There have been some case
reports on the occurrence of Bt in patients with different infectious
diseases. However, none of these studies unequivocally demonstrates an
actual risk to human health from the use of Bt. Bt has not been
reported to cause adverse effects on human health when present in
drinking-water or food.
1.7 Conclusions
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and biologically active products other than the
ICPs. Bt products may be safely used for the control of insect pests
of agricultural and horticultural crops as well as forests. They are
also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt has the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
2. IDENTITY, BIOLOGICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Commercial Bacillus thuringiensis (Bt) products are microbial
pest control agents (MPCAs) containing specific insecticidal
crystalline proteins (ICPs) and most often living spores as well as
formulating agents. They are processed fermentation products.
2.1.1 Bacillus thuringiensis
Bt is a facultative anaerobic, motile, gram-positive,
spore-forming bacterium. The formation of parasporal crystals adjacent
to the endospore during sporulation stages III to IV distinguishes Bt
from other Bacillus species.
Bt, like other Bacillus species, has been classified on the
basis of its cellular, cultural, biochemical and genetic
characteristics (Baumann et al., 1984; Claus & Berkley, 1986; Slepecky
& Hemphill, 1992; Carlson & Kolsto, 1993; Hansen et al., 1998). In
1958, Heimpel & Angus (1958) introduced a classification scheme to
identify these crystalliferous bacteria based on their morphological
and biochemical characteristics. However recent molecular analysis
shows that several variations can be found within serotypes, and that
specific biochemical characteristics do not always refer to a specific
serotype (Helgason et al., 1998; Hansen et al., 1998).
2.1.2 Relationship between Bacillus thuringiensis and Bacillus cereus
Bt is a member of the Bc group, which also contains Bacillus
cereus (Bc), B. mycoides and B. anthracis. Furthermore, the
psychrotolerant B. weihenstephanensis has recently been proposed as
a new member of the group (Lechner et al., 1998). Bt can only be
distinguished from Bc by the production during the sporulation process
of one or more inclusion bodies, which have been found to be toxic for
invertebrates, primarily insect species in the orders Coleoptera,
Diptera and Lepidoptera (de Barjac, 1981b; Andrews et al., 1987).
Several studies have been dedicated to a comparison of Bt and Bc on
the basis of characters not related to the production of ICPs
(Hendriksen & Hansen, 1998). Phenotypic differentiation of Bt and Bc
is not possible on the basis of morphology or utilization of organic
compounds (Baumann et al., 1984; Logan & Berkeley, 1984; Priest et
al., 1988), characterization of cell content of fatty acids (Väisänen
et al., 1991) or sugars (Wunschel et al., 1994), multilocus enzyme
electrophoresis (Zahner et al., 1989; Carlson et al., 1994),
enterotoxin production (Damgaard et al., 1996a; Hansen & Hendriksen,
1997a), or serological- and phage-typing techniques (Ohba & Aizawa,
1978; 1986; Väisänen et al., 1991; Murakami et al., 1993; Ahmed et
al., 1995). Likewise, genotypic differentiation of Bt and Bc is not
possible by DNA homology analysis (Kaneko et al., 1978), ribotyping
(Priest et al., 1994; Demezas & Bell, 1995), 16S rDNA sequencing (Ash
et al., 1991); analysis of the 16S-23S internal transcribed sequence
(Wunschel et al., 1994; Bourque et al., 1995), PCR analysis of genes
encoding Bc-like toxic products (Damgaard et al., 1996b; Asano et al.,
1997; Hansen & Hendriksen, 1997b) or pulsed field gel electrophoresis
(Carlson & Kolsto, 1993; Carlson et al., 1994). Giffel et al. (1997)
found differences in 16S rDNA sequences between a limited number Bt
and Bc. Beattie et al. (1998) were able to discriminate among members
of the Bc group by Fourier transform infrared spectroscopy, and
Brousseau et al. (1992) were able to distinguish Bt and Bc by random
amplified polymorphic DNA fingerprinting. However, the transfer of ICP
encoding plasmids from Bt to Bc makes the receptor Bc
indistinguishable from Bt, and vice versa (González et al., 1981,
1982).
2.1.3 Crystal composition and morphology
The existence of parasporal inclusions in Bt was first noted in
1915 (Berliner, 1915), but their protein composition was not
delineated until the 1950s (Angus, 1954). Hannay (1953) detected the
crystalline fine structure that is a property of most of the
parasporal inclusions. Bt subspecies can synthesize more than one
inclusion, which may contain different ICPs. ICPs have also been
called delta endotoxins; however, since the term endotoxin usually
refers to toxins associated with the outer membranes of gram-negative
bacteria, comprising a core lipopoplysaccharide, lipid A and somatic
(O) antigens, this term is not used in this monograph. Depending on
their ICP composition, the crystals have various forms (bipyramidal,
cuboidal, flat rhomboid, or a composite with two or more crystal
types). A partial correlation between crystal morphology, ICP
composition, and bioactivity against target insects has been
established (Bulla et al., 1977; Höfte & Whiteley, 1989; Lynch &
Baumann, 1985).
2.1.4 Classification of Bt subspecies
The classification of Bt subspecies based on the serological
analysis of the flagella (H) antigens was introduced in the early
1960s (de Barjac & Bonnefoi, 1962). This classification by serotype
has been supplemented by morphological and biochemical criteria (de
Barjac, 1981a). Until 1977, only 13 Bt subspecies had been described,
and at that time all subspecies were toxic to Lepidopteran larvae
only. The discovery of other subspecies toxic to Diptera (Goldberg &
Margalit, 1977), Coleoptera (Krieg et al., 1983) and apparently
Nematoda (Narva et al., 1991) enlarged the host range and markedly
increased the number of subspecies. Up to the end of 1998, over 67
subspecies based on flagellar H-serovars had been identified (Table
1). Updated lists of the serovarieties can be obtained from the
reference centre of the Pasteur Institute in Paris (Unité des
Bactéries Entomopathogènes, Institut Pasteur, Paris, France).
Table 1. Current classification of 67 Bacillus thuringiensis subspecies based
on their flagellar (H) antigensa
Flagellar antigens B. thuringiensis Flagellar antigens B. thuringiensis
subspecies subspecies
1 thuringiensis 28a, 28c jegathesan
2 finitimus 29 amagiensis
3a, 3c alesti 30 medellin
3a, 3b, 3c kurstaki 31 toguchini
3a, 3d sumiyoshiensis 32 cameroun
3a, 3d, 3e fukuokaensis 33 leesis
4a, 4b sotto 34 konkukian
4a, 4c kenyae 35 seoulensis
5a, 5c galleriae 36 malaysiensis
5a, 5c canadensis 37 anadalousiensis
6 entomocidus 38 oswaldocruzi
7 aizawai 39 brasiliensis
8a, 8b morrisoni 40 huazhongensis
8a, 8c ostriniae 41 sooncheon
8b, 8d nigeriensis 42 jinghongiensis
9 tolworthi 43 guiyanguebsus
10a, 10b darmstadiensis 44 higo
10a, 10c londrina 45 roskildiensis
11a, 11b toumanoffi 46 chanpaisis
11a, 11c kyushuensis 47 wratislaviensis
12 thompsoni 48 balearica
13 pakistani 49 muju
14 israelensis 50 navarrensis
15 dakota 51 xiaguangiensis
16 indiana 52 kim
17 tohokuensis 53 asturiensis
18a, 18b kumamotoensis 54 poloniensis
18a, 18c yosoo 55 palmanyolensis
19 tochigiensis 56 rongseni
20a, 20b yunnanensis 57 pirenaica
20a, 20c pondicheriensis 58 argentinensis
21 colmeri 59 iberica
22 shandongiensis 60 pingluonsis
23 japonensis 61 sylvestriensis
24a, 24b neoleonensis 62 zhaodongensis
24a, 24c novosibirsk 63 bolivia
25 coreanensis 64 azorensis
26 silo 65 pulsiensis
27 mexicanensis 66 gracioensis
28a, 28b monterrey 67 vazensis
a Data provided by the Unité des Bactéries Entomopathogènes, Institut Pasteur,
Paris, France
Crystal serology has shown that a particular crystal type may be
produced by more than one H-serovar (Krywienczyk et al., 1978; Smith,
1987).
2.1.5 Genetics of ICP
In the early 1980s, it was established that most genes coding for
the ICPs reside on large transmissible plasmids, of which most are
readily exchanged between strains by conjugation (González & Carlton,
1980; González et al., 1981). Since these initial studies, numerous
ICP genes have been cloned, sequenced and used to construct Bt strains
with novel insecticidal spectra (Höfte & Whiteley, 1989).
The currently known crystal (cry) gene types encode ICPs that
are specific to either Lepidoptera (cryI), Diptera and
Lepidoptera (cryII), Coleoptera (cryIII), Diptera (cryIV),
or Coleoptera and Lepidoptera (cryV) (Höfte & Whiteley, 1989).
A separate designation is used for the cytolytic (cyt) genes that
encode a nonspecific cytolytic factor, present in Bti ICP and some
other Bt subspecies. However, due to the increasing number of
characterized ICP genes and inconsistencies in the existing cry gene
nomenclature, which is based on insecticidal spectrum, Crickmore et
al. (1998) proposed a new nomenclature based on ICP gene sequences.
The new cry genes are listed in Tables 2 and 3, and Table 4 is a
conversion list from old to new cry gene names. Current
identification of Bt employs both the identity of the cry genes,
which define the host range, and the H-serovars, which define the
subspecies, and recently DNA fingerprinting has been used for further
characterization of subspecies (Hansen et al., 1998).
The ICP gene sequences provided the basis for the construction of
gene-specific probes to screen established Bt strains by hybridization
and PCR analysis for the presence of known nucleotide sequences, and
for characterizing the ICPs from new Bt isolates (Prefontaine et al.,
1987; Juarez-Perez et al., 1997; Bravo et al., 1998; Shevelev et al.,
1998). These studies have permitted the distinction of numerous
subclasses of ICP genes based on sequence homology and toxicity
spectra of the encoded proteins.
All ICPs described to date attack the insect gut upon ingestion
(see chapter 3). To date, each of the proteolytically activated ICP
molecules with insecticidal activity has a variable C-terminal domain,
which is responsible for receptor recognition (host susceptibility),
and a conserved N-terminal domain, which induces pore formation
(toxicity) (Li et al., 1991).
Most naturally occurring Bt strains contain ICPs active against a
single order of insects. However, conjugative transfer between Bt
strains or related species can occur, resulting in new strains with
various plasmid contents. Thus the mobility of the cry genes and the
exchange of plasmids may explain the diverse and complex activity
Table 2. Bacillus thuringiensis crystal protein genes (Crickmore et al., 1998)
Name Acc No Reference Journal Coding
Cry1aa1 M11250 Schnepf et al., 1985 JBC 260 6264-6272 527-4054
Cry1aa2 M10917 Shibano et al., 1985 Gene 34 243-251 153->2955
Cry1aa3 D00348 Shimizu et al., 1988 ABC 52 1565-1573 73-3603
Cry1aa4 X13535 Masson et al., 1989 NAR 17 446-446 1-3528
Cry1aa5 D17518 Udayasuriyan et al., 1994 BBB 58 830-835 81-3611
Cry1aa6 U43605 Masson et al., 1994 Mol Micro 14 851-860 1->1860
Cry1aa7 AF081790 Osman, 1998 unpublished
Cry1aa8 I26149 Liu, 1996 USP 5556784 148-3675
Cry1ab1 M13898 Wabiko et al., 1986 DNA 5 305-314 142-3606
Cry1ab2 M12661 Thorne et al., 1986 J Bact 166 801-811 155-3625
Cry1ab3 M15271 Geiser et al., 1986 Gene 48 109-118 156-3623
Cry1ab4 D00117 Kondo et al., 1987 ABC 51 455-463 163-3630
Cry1ab5 X04698 Hofte et al., 1986 EJB 161 273-280 141-3605
Cry1ab6 M37263 Hefford et al., 1987 J Biotech 6 307-322 73-3540
Cry1ab7 X13233 Haider & Ellar, 1988 NAR 16 10927-10927 1-3465
Cry1ab8 M16463 Oeda et al., 1987 Gene 53 113-119 157-3624
Cry1ab9 X54939 Chak & Jen, 1993 PNSCRC 17 7-14 73-3540
Cry1ab10 A29125 Fischhoff et al., 1987 Bio/technology peptide seq
5 807-813
Cry1ab11 I12419 Ely & Tippett, 1995 USP 5424409 73-
Cry1ab12 AF057670 Silva-Werneck et al., 1998 unpublished 41-3505
Cry1ac1 M11068 Adang et al., 1985 Gene 36 289-300 388-3921
Cry1ac2 M35524 Von Tersch et al., 1991 AEM 57 349-358 239-3772
Cry1ac3 X54159 Dardenne et al., 1990 NAR 18 5546-5546 339->2192
Cry1ac4 M73249 Payne et al., 1991 USP 4990332 1-3537
Cry1ac5 M73248 Payne et al., 1992 USP 5135867 1-3534
Cry1ac6 U43606 Masson et al., 1994 Mol Micro 14 851-860 1->1821
Cry1ac7 U87793 Herrera et al., 1994 AEM 60 682-690 976-4512
Cry1ac8 U87397 Omolo et al., 1997 Curr Micro 34 118-121 153-3686
Cry1ac9 U89872 Gleave et al., 1992 NZJCHS 20 27-36 388-3921
Cry1ac10 AJ002514 Sun & Yu, 1997 unpublished 388-3921
Cry1ac11 AJ130970 Makhdoom & Riazuddin, 1998 unpublished 156-3689
Cry1ac12 I12418 Ely & Tippett, 1995 USP 5424409 81->2990
Cry1ad1 M73250 Payne & Sick, 1993 USP 5246852 1-3537
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1ad2 A27531 Payne & Sick, 1995 AUP 632335 1-3537
Cry1ae1 M65252 Lee & Aronson, 1991 J Bact 173 6635-6638 81-3623
Cry1af1 U82003 Kang et al., 1997 unpublished 172->2905
Cry1ag1 AF081248 Osman, 1998 unpublished
Cry1ba1 X06711 Brizzard & Whiteley, 1988 NAR 16 2723-2724 1-3684
Cry1ba2 X95704 Soetaert, 1996 unpublished 186-3869
Cry1bb1 L32020 Donovan et al., 1994 USP 5322687 67-3753
Cry1bc1 Z46442 Bishop et al., 1994 unpublished 141-3839
Cry1bd1 U70726 Kuo & Chak, 1999 unpublished 842-4534
Cry1be1 Payne et al., 1998 USP 5723758 1-3681
Cry1ca1 X07518 Honee et al., 1988 NAR 16 6240-6240 47-3613
Cry1ca2 X13620 Sanchis et al., 1989 Mol Micro 3 229-238 241->2711
Cry1ca3 M73251 Payne & Sick, 1993 USP 5246852 1-3570
Cry1ca4 A27642 Van Mellaert et al., 1990 EP 0400246 234-3800
Cry1ca5 X96682 Strizhov, 1996 unpublished 1->2286
Cry1cb1 M97880 Kalman et al., 1993 AEM 59 1131-1137 296-3823
Cry1da1 X54160 Hofte et al., 1990 NAR 18 5545-5545 264-3758
Cry1da2 I76415 Payne & Sick, 1997 USP 5691308 1-3495
Cry1db1 Z22511 Lambert, 1993 unpublished 241-3720
Cry1ea1 X53985 Visser et al., 1990 J Bact 172 6783-6788 130-3642
Cry1ea2 X56144 Bosse et al., 1990 NAR 18 7443-7443 1-3516
Cry1ea3 M73252 Payne & Sick, 1991 USP 5039523 1-3516
Cry1ea4 U94323 Barboza-Corona et al., 1998 WJMB 14 437-441 388-3900
Cry1ea5 A15535 Botterman et al., 1994 EP 0358557 54-3566
Cry1eb1 M73253 Payne & Sick, 1993 USP 5206166 1-3522
Cry1fa1 M63897 Chambers et al., 1991 J Bact 173 3966-3976 478-3999
Cry1fa2 M73254 Payne & Sick, 1993 USP 5188960 1-3525
Cry1fb1 Z22512 Lambert, 1993 unpublished 483-4004
Cry1fb2 AB012288 Masuda & Asano, 1998 unpublished 84-3587
Cry1fb3 AF062350 Song & Zhang, 1998 unpublished
Cry1fb4 I73895 Payne et al., 1997 USP 5686069 peptide seq
Cry1ga1 Z22510 Lambert, 1993 unpublished 67-3564
Cry1ga2 Y09326 Shevelev et al., 1997 Febs Lett 404 148-152 692-4210
Cry1gb1 U70725 Kuo & Chak, 1999 unpublished 532-4038
Cry1ha1 Z22513 Lambert, 1993 unpublished 530-4045
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1hb1 U35780 Koo et al., 1995 unpublished 728-4195
Cry1ia1 X62821 Tailor et al., 1992 Mol Micro 6 1211-1217 355-2511
Cry1ia2 M98544 Gleave et al., 1993 AEM 59 1683-1687 1-2160
Cry1ia3 L36338 Shin et al., 1995 AEM 61 2402-2407 279-2438
Cry1ia4 L49391 Kostichka et al., 1996 J Bact 178 2141-2144 61-2217
Cry1ia5 Y08920 Selvapandiyan, 1996 unpublished 524-2680
Cry1ia6 AF076953 Zhong et al., 1998 unpublished 1-2157
Cry1ib1 U07642 Shin et al., 1995 AEM 61 2402-2407 237-2393
Cry1ic1 AF056933 Osman et al., 1998 unpublished 1-2157
Cry1i-like I90732 Payne et al., 1998 See Table 3 peptide seq
Cry1ja1 L32019 Donovan et al., 1994 USP 5322687 99-3519
Cry1jb1 U31527 Von Tersch & Gonzalez, 1994 USP 5356623 177-3686
Cry1jc1 I90730 Payne et al., 1998 USP 5723758 peptide seq
Cry1ka1 U28801 Koo et al., 1995 FEMS 134 159-164 451-4098
Cry1-like I90729 Payne et al., 1998 See Table 3 peptide seq
Cry2aa1 M31738 Donovan et al., 1989 JBC 264 4740-4740 156-2054
Cry2aa2 M23723 Widner & Whiteley, 1989 J Bact 171 965-974 1840-3741
Cry2aa3 D86064 Sasaki et al., 1997 Curr Micro 35 1-8 2007-3911
Cry2aa4 AF047038 Misra et al., 1998 Unpublished 10-1908
Cry2aa5 AJ132464 Yu & Pang, 1999 Unpublished <1-1860
Cry2aa6 AJ132465 Yu & Pang 1999 Unpublished <1-1860
Cry2aa7 AJ132463 Yu & Pang, 1999 Unpublished <1->1611
Cry2ab1 M23724 Widner & Whiteley, 1989 J Bact 171 965-974 1-1899
Cry2ab2 X55416 Dankocsik et al., 1990 Mol Micro 4 2087-2094 874-2775
Cry2ac1 X57252 Wu et al., 1991 FEMS 81 31-36 2125-3990
Cry3aa1 M22472 Herrnstadt et al., 1987 Gene 57 37-46 25-1956
Cry3aa2 J02978 Sekar et al., 1987 PNAS 84 7036-7040 241-2175
Cry3aa3 Y00420 Hofte et al., 1987 NAR 15 7183-7183 566-2497
Cry3aa4 M30503 McPherson et al., 1988 Bio/technology 6 61-66 201-2135
Cry3aa5 M37207 Donovan et al., 1988 MGG 214 365-372 569-2503
Cry3aa6 U10985 Adams et al., 1994 Mol Micro 14 381-389 569-2503
Cry3ba1 X17123 Sick et al., 1990 NAR 18 1305-1305 25-1977
Cry3ba2 A07234 Peferoen et al., 1990 EP 0382990 342-2297
Cry3bb1 M89794 Donovan et al., 1992 AEM 58 3921-3927 202-2157
Cry3bb2 U31633 Donovan et al., 1995 USP 5378625 144-2099
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry3bb3 I15475 Peferoen et al., 1995 USP 5466597 <1->1291
Cry3ca1 X59797 Lambert et al., 1992 Gene 110 131-132 232-2178
Cry4aa1 Y00423 Ward & Ellar, 1987 NAR 15 7195-7195 1-3540
Cry4aa2 D00248 Sen et al., 1988 ABC 52 873-878 393-3935
Cry4ba1 X07423 Chungjatpornchai et al., 1988 EJB 173 9-16 157-3564
Cry4ba2 X07082 Tungpradubkul et al., 1988 NAR 16 1637-1638 151-3558
Cry4ba3 M20242 Yamamoto et al., 1988 Gene 66 107-120 526-3933
Cry4ba4 D00247 Sen et al., 1988 ABC 52 873-878 461-3868
Cry5aa1 L07025 Sick et al., 1994 USP 5281530 1-4155
Cry5ab1 L07026 Narva et al., 1991 EP 0462721 1-3867
Cry5ac1 I34543 Payne et al., 1997 USP 5596071 1-3660
Cry5ba1 U19725 Payne et al., 1997 USP 5596071 1-3735
Cry6aa1 L07022 Narva et al., 1993 USP 5236843 1-1425
Cry6ba1 L07024 Narva et al., 1991 EP 0462721 1-1185
Cry7aa1 M64478 Lambert et al., 1992 AEM 58 2536-2542 184-3597
Cry7ab1 U04367 Payne & Fu, 1994 USP 5286486 1-3414
Cry7ab2 U04368 Payne & Fu, 1994 USP 5286486 1-3414
Cry8aa1 U04364 Foncerrada et al., 1992 EP 0498537 1-3471
Cry8ba1 U04365 Michaels et al., 1993 WO 93/15206 1-3507
Cry8ca1 U04366 Ogiwara et al., 1995 Curr Micro 30 227-235 1-3447
Cry9aa1 X58120 Smulevitch et al., 1991 FEBS 293 25-28 5807-9274
Cry9aa2 X58534 Gleave et al., 1992 JGM 138 55-62 385->3837
Cry9ba1 X75019 Shevelev et al., 1993 FEBS 336 79-82 26-3488
Cry9ca1 Z37527 Lambert et al., 1996 AEM 62 80-86 2096-5569
Cry9da1 D85560 Asano et al., 1997 AEM 63 1054-1057 47-3553
Cry9Da2 AF042733 Wasano & Ohba, 1998 Unpublished <1->1937
Cry9Ea1 AB011496 Midoh & Oyama, 1998 Unpublished 211-3660
Cry9 like AF093107 Wasano & Ohba, 1998 See Table 3 <1->1917
Cry10Aa1 M12662 Thorne et al., 1986 J Bact 166 801-811 941-2965
Cry10Aa2 E00614 Uorufuiirudo, 1996 JP 1986005098 940-2968
Cry11Aa1 M31737 Donovan et al., 1988 J Bact 170 4732-4738 41-1969
Cry11Aa2 M22860 Adams et al., 1989 J Bact 171 521-530 <1-235
Cry11Ba1 X86902 Delecluse, 1995 AEM 61 4230-4235 64-2238
Cry11Bb1 AF017416 Orduz et al., 1998 BBA 1388 267-272 97-2346
Cry12Aa1 L07027 Narva et al., 1991 EP 0462721 1->3771
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry13Aa1 L07023 Narva et al., 1992 WO 92/19739 1-2409
Cry14Aa1 U13955 Narva et al., 1994 WO 94/16079 1-3558
Cry15Aa1 M76442 Brown & Whiteley, 1992 J Bact 174 549-557 1036-2055
Cry16Aa1 X94146 Barloy et al., 1996 J Bact 178 3099-3105 158-1996
Cry17Aa1 X99478 Barloy et al., 1998 Gene 211 293-299 12-1865
Cry18Aa1 X99049 Zhang et al., 1997 J Bact 179 4336-4341 1451-3571
Cry19Aa1 Y07603 Rosso & Delecluse, 1996 AEM 63 4449-4455 719-2662
Cry19Ba1 D88381 Hwang et al., 1998 SAB 21 179-184 626-2671
Cry20Aa1 U82518 Lee & Gill, 1997 AEM 63 4664-4670 60-2318
Cry21Aa1 I32932 Payne et al., 1996 USP 5589382 1-3501
Cry21Aa2 I66477 Feitelson, 1997 USP 5670365 1-3501
Cry22Aa1 I34547 Payne et al., 1997 USP 5596071 1-2169
Cry23Aa1 AF03048 Donovan & Slaney, 1998 WO 98/13498
Cry24Aa1 U88188 Kawalek & Gill, 1998 Unpublished 1-2022
Cry25Aa1 U88189 Kawalek & Gill, 1998 Unpublished 1-2028
Cyt1Aa1 X03182 Waalwijk et al., 1985 NAR 13 8207-8217 140-886
Cyt1Aa2 X04338 Ward & Ellar, 1986 JMB 191 1-11 509-1255
Cyt1Aa3 Y00135 Earp & Ellar, 1987 NAR 15 3619-3619 36-782
Cyt1Aa4 M35968 Galjart et al., 1987 Curr Micro 16 171-177 67-816
Cyt1Ab1 X98793 Thiery et al., 1997 AEM 63 468-473 28-777
Cyt1Ba1 U37196 Payne et al., 1995 USP 5436002 1-795
Cyt2Aa1 Z14147 Koni & Ellar, 1993 JMB 229 319-327 270-1046
Cyt2Ba1 U52043 Guerchicoff et al., 1997 AEM 63 2716-2721 287-655
Cyt2Ba2 AF020789 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba3 AF022884 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba4 AF022885 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba5 AF022886 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->471
Cyt2Ba6 AF034926 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->472
Cyt2Bb1 U82519 Cheong & Gill, 1997 AEM 63 3254-3260 416-1204
spectra observed in Bt (González & Carlton, 1980; González et al.,
1981; González et al., 1982; Reddy et al., 1987; Jarrett & Stephenson,
1990). New Bt strains have been developed by conjugation that are
toxic to two insect orders.
2.1.6 Beta-exotoxin
Beta-exotoxin is associated with certain Bt subspecies (Btd, Btg,
Btte and Btt), and products made from these Bt subspecies may contain
the toxin (Cantwell et al., 1964; Mohd-Salleh et al., 1980). This
heat-stable nucleotide, which is composed of adenine, glucose and
allaric acid, inhibits RNA polymerase enzymes by acting competitively
with ATP (Faust, 1973; Farkas et al., 1977). Since RNA synthesis is a
vital process in all life, beta-exotoxin exerts its toxicity for
almost all forms of life tested including numerous insect species in
the orders Coleoptera, Diptera and Lepidoptera. The presence of
beta-exotoxin can be assayed using houseflies (Musca domestica) or
high-performance liquid chromatography (HPLC) techniques (Campbell et
al., 1987).
Bt containing beta-exotoxin is used for the control of houseflies
in some countries, but regulatory agencies currently prohibit the use
of beta-exotoxin for other purposes.
2.1.7 Other Bt metabolites
Commercial Bt products do not contain metabolites that are
considered hazardous to humans and the environment. However, Bt, like
other bacteria, may produce during the vegetative growth and
sporulation stages an assortment of antibiotics, enzymes, metabolites
and toxins that are biologically active and may have effects on both
target and non-target organisms (NTOs).
Using a non-quantitative (Lund & Granum, 1997) commercial Bc
enterotoxin immunoassay (Tecra), Damgaard (1995) reported that
vegetative cells grown from spores of commercial Bt products excreted
a diarrhoeal enterotoxin. Damgaard et al. (1996a) found by Vero cell
assay that Bt isolated from food was enterotoxigenic. None of these
investigations estimated the quantity or activity of enterotoxins
produced by the Bt strains. However, Shinagawa (1990) investigated a
number of Bc and Bt isolates with an immunological assay and concluded
that 43% of the Bt isolates had the same level of enterotoxins as
enterotoxic Bc. Tayabali & Seligy (1997) found that vegetative Bt
obtained from commercial products caused extensive damage to
cultivated insect cells.
At least three enterotoxic activities have been described in Bc
(Agata et al., 1995; Lund & Granum, 1997), and some Bc isolates are
known to produce an emetic toxin (Andersson et al., 1998). The emetic
toxin is primarily associated with the Bc H-1 (Kramer & Gilbert, 1992;
Nishikawa et al., 1996) serotype, and has not so far been associated
with Bt isolates.
Table 3. Bt-associated toxins or toxin-like proteins that have not been assigned a name or entered
into the nomenclature for the reasons given
Name Accession Journal Coding region Reason Reference
Cry1i-like I90732 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry1-like I90729 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry9-like AF093107 unpublished <1->1917 Insufficient sequence data Wasano & Ohba, 1998
40kda M76442 J Bact 174 549-557 45-971 No reported toxicity Brown & Whiteley, 1992
Cryc35 X92691 unpublished 1-981 No reported toxicity Juarez-Perez et al., 1995
Crytdk D86346 unpublished 177-2645 No reported toxicity Hashimoto, 1996
Cryc53 X98616 unpublished 1-1005 No reported toxicity Juarez-Perez et al., 1996
p21med X98794 AEM 63 468-473 1-552 No reported toxicity Thiery et al., 1997
ET34 AF038049 WO 98/13498 No reported toxicity Donovan & Slaney, 1998
Vip3a(a) L48811 PNAS 93 5389-5394 739-3105 Not a crystal protein Estruch et al., 1996
Vip3a(b) L48812 PNAS 93 5389-5394 118-2484 Not a crystal protein Estruch et al., 1996
Table 4. Bacillus thuringiensis holotype toxins
Name Old name Name Old name
Cry1Aa CryIA(a) Cry5Ac
Cry1Ab CryIA(b) Cry5Ba
Cry1Ac CryIA(c) Cry6Aa CryVIA
Cry1Ad CryIA(d) Cry6Ba CryVIB
Cry1Ae CryIA(e) Cry7Aa CryIIIC
Cry1Af Cry7Ab CryIIICb
Cry1Ag Cry8Aa CryIIIE
Cry1Ba CryIB Cry8Ba CryIIIG
Cry1Bb ET5 Cry8Ca CryIIIF
Cry1Bc PEG5 Cry9Aa CryIG
Cry1Bd CryE1 Cry9Ba CryIX
Cry1Be Cry9Ca CryIH
Cry1Ca CryIC Cry9Da
Cry1Cb CryIC(b) Cry9Ea
Cry1Da CryID Cry10Aa CryIVC
Cry1Db PrtB Cry11Aa CryIVD
Cry1Ea CryIE Cry11Ba Jeg80
Cry1Eb CryIE(b) Cry11Bb
Cry1Fa CryIF Cry12Aa CryVB
Cry1Fb PrtD Cry13Aa CryVC
Cry1Ga PrtA Cry14Aa CryVD
Cry1Gb CryH2 Cry15Aa 34kDa
Cry1Ha PrtC Cry16Aa cbm71
Cry1Hb Cry17Aa cbm72
Cry1Ia CryV Cry18Aa CryBP1
Cry1Ib CryV Cry19Aa Jeg65
Cry1Ic Cry19Ba
Cry1Ja ET4 Cry20Aa
Cry1Jb ET1 Cry21Aa
Cry1Jc Cry22Aa
Cry1Ka Cry23Aa
Cry2Aa CryIIA Cry24Aa Jeg72
Cry2Ab CryIIB Cry25Aa Jeg74
Cry2Ac CryIIC Cry26Aa
Cry3Aa CryIIIA Cry27Aa
Cry3Ba CryIIIB Cry28Aa
Cry3Bb CryIIIBb Cyt1Aa CytA
Cry3Ca CryIIID Cyt1Ab CytM
Cry4Aa CryIVA Cyt1Ba
Cry4Ba CryIVB Cyt2Aa CytB
Cry5Aa CryVA(a) Cyt2Ba "CytB"
Cry5Ab CryVA(b) Cyt2Bb
Alpha-toxin is a phospholipase C, which primarily affects the
cell membrane phospholipids (Heimpel, 1954; Bonnefoi & Béguin, 1959).
Gamma-toxin is toxic to sawflies (Tenthredinidae), but the mode of
action of this heat-labile toxin has not been determined (Heimpel,
1967).
The so called "water-soluble toxin" paralyses Lepidoptera
(Fast, 1971), and the "mouse factor exotoxin" is toxic to mice as
well as to Lepidoptera (Krieg, 1971). The modes of action of these
toxins have not been delineated.
A novel Bt vegetative insecticidal protein (Vip3A) has been
identified from the culture media of some Bt strains (Estruch et al.,
1996).
Several Bt and Bc enzymes have been described which may play a
role in non-target activity: phospholipase (Damgaard et al., 1996b),
sphingomyelinase (Gilmore et al., 1989), protease (Hotha & Banik,
1997), chitinase (Sampson & Gooday, 1998), and haemolysin (Baida &
Kuzmin, 1995).
2.2 Bioassays
2.2.1 Spore counts
Bacterial spore counts do not necessarily reflect the
insecticidal activity of a Bt strain or Bt product because the number
and amount of ICPs produced per bacterial cell can vary.
2.2.2 International bioassay for ICPs
The final formulation of each Bt product is bioassayed against an
accepted international standard using a specific test insect (Dulmage
et al., 1981; de Barjac & Larget-Thiery, 1984). Its potency is defined
in ITU/mg product. The standardization allows comparison of different
formulations in the laboratory. Currently, the larvicidal activity is
expressed in terms of lethal doses (LD50) or lethal concentrations
(e.g., LC50, LC90) according to the bioassay method used. For
example, when susceptible mosquito larvae are exposed to Bti ICP, they
have an LC50 of approximately 10 ng/ml water. A Bti whole culture
gives an LC50 of approximately 103 cells/ml for susceptible mosquito
larvae while a 109 cells/ml culture does not affect any laboratory
mammals exposed by various routes.
3. MODE OF ACTION ON TARGET INSECTS
3.1 Bioactivity of field isolates
The mode of action of Bt has been reviewed by Schnepf et al.
(1998) and can be summarized in the following stages: 1) ingestion of
sporulated Bt and ICP by an insect larva; 2) solubilization of the
crystalline ICP in the midgut; 3) activation of the ICP by proteases;
4) binding of the activated ICP to specific receptors in the midgut
cell membrane; 5) insertion of the toxin in the cell membrane and
formation of pores and channels in the gut cell membrane, followed by
destruction of the epithelial cells (Cooksey, 1971; Norris 1971; Fast,
1981; Huber & Lüthy, 1981; Lüthy & Ebersold, 1981; Smedley & Ellar,
1996); and 6) subsequent Bt spore germination and septicaemia may
enhance mortality (Fig. 1).
The specific bioactivity of Bt is dominated by the ICPs that are
encoded by the cry genes and are active against susceptible species
in the insect orders Coleoptera, Diptera and Lepidoptera.
Specific Bt activities against other insect orders (Hymenoptera,
Homoptera, Dictyoptera, Mallophaga) and to nematodes
(Strongylida, Tylenchida), mites (Acari), flatworms (Digenea)
and protozoa (Diplomonadida) have been described (Feitelson, 1993;
Zukowski, 1995). The ICP must be ingested to be effective against the
target (Visser et al., 1993).
3.2 Mechanism of action of Bt formulations
The ICP-spore complexes of Bt are ingested by susceptible insect
larvae. In the midgut the parasporal crystalline ICP is dissociated to
the protoxin form, and the protoxin is then activated to a holotoxin
by gut proteases (Warren et al., 1984; Jaquet et al., 1987; Aronson et
al., 1991; Honée & Visser, 1993). Shortly afterwards, the gut becomes
paralysed and the larva ceases to feed.
The ICP structure and function have been reviewed in detail by
Schnepf et al. (1998). Binding of the ICP to putative receptors is a
major determinant of ICP specificity and the formation of pores in the
midgut epithelial cells is a major mechanism of toxicity (Van
Frankenhuyzen, 1993).
The active toxin consists of three distinct domains (Höfte &
Whiteley, 1989; Li et al., 1991; Grochulski et al., 1995). The three
domains interact in a complex manner, but experimental data suggest
that the C-terminal and middle domains of the toxin are involved in
epithelial cell receptor binding and structural functions, while the
N-terminal domain is primarily involved in ion channel and pore
formation (Huber et al., 1981; Schnepf et al., 1998; Dean et al.,
1996).
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS
THURINGIENSIS
Members
Professor D. Calamari, Department of Biology, Structure and Function,
University of Milan, Varese, Italy
Dr C. Cummins, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr N. Gratz, Commugny, Switzerland (Rapporteur)
Dr A. Klier, Unité de Biochimie Microbienne, Département des
Biotechnologies, Institut Pasteur, Paris, France
Professor P. Lüthy, Microbiological Institute ETH, Zurich, Switzerland
Dr C.M. Scanlan, Texas A & M University, Department of Veterinary
Pathobiology, Texas Veterinary Medical Center, Texas, USA (Chairman)
Observers
Dr R.J. Cibulsky, Abbott Laboratories, Chemical and Agricultural
Products, North Chicago, Illinois, USA
Dr E. Cozzi, Clinical Research and Development, Animal Health
Products, Abbott Laboratories, North Chicago, Illinois, USA
Dr M. Ronchin, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Secretariat
Professor M. Maroni, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Dr R. Plestina, Zagreb, Croatia
ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS THURINGIENSIS
A WHO Task Group on Environmental Health Criteria for the
microbial pest control agent Bacillus thuringiensis (Bt) met at the
International Centre for Pesticide Safety in Busto Garolfo, Milan,
Italy, from 27 to 31 October 1997. Professor M. Maroni, Director of
the Centre, welcomed participants on behalf of the Centre, which was
responsible for organizing the meeting. Dr R. Plestina, IPCS temporary
adviser, opened the meeting and welcomed participants on behalf of Dr
M. Mercier, Director of IPCS. The Group reviewed and revised the draft
and made an evaluation of the risks for human health and the
environment from exposure to Bt products. Drs R. Plestina and A. Aitio
of the IPCS Central Unit were responsible for the scientific aspects
of the monograph, and Dr P.G. Jenkins for the editing. The assistance
of manufacturers, notably Abbott Laboratories, in providing
unpublished documentation for the review is greatly appreciated.
Microbial Pest Control Agents (MPCAs), notably products of
various Bt subspecies, are increasingly used in pest management
programmes against the larvae of several insect pests of major
agricultural crops and forests, and several insect vectors of human
diseases, and some nuisance pests. Bt products have been used
worldwide, and their commercial production is about 1% of that of
chemical pesticides. A number of reviews have recently been published
on various aspects of Bt (Entwistle et al., 1983; McClintock et al.,
1995; Cannon, 1996; Dean et al., 1996; Kumar et al., 1996; Schnepf et
al., 1998; Nielsen-LeRoux et al., 1998).
The activities in preparing this document were recommended by an
Informal Consultation on the Safety of Microbial Pest Control Agents
held in Geneva in June 1993. The first draft of the monograph was
prepared by a drafting group in 1994 and was subsequently amended by
Professor C.M. Scanlan (Texas A&M University, Department of Veterinary
Pathobiology, The Texas Veterinary Medical Center, Texas, USA). The
revised draft was circulated to the IPCS contact points. Based on the
comments received, it was amended and updated by Drs B.M. Hansen and
N.B. Hendriksen (National Environmental Research Institute, Roskilde,
Denmark). The document was finally approved by the Task Group members.
ABBREVIATIONS
Bc Bacillus cereus
Bt Bacillus thuringiensis
Bta Bacillus thuringiensis subspecies aizawai
Btd Bacillus thuringiensis subspecies darmstadiensis
Bte Bacillus thuringiensis subspecies entomocidus
Btg Bacillus thuringiensis subspecies galleriae
Bti Bacillus thuringiensis subspecies israelensis
Btk Bacillus thuringiensis subspecies kurstaki
Btko Bacillus thuringiensis subspecies konkukian
Btt Bacillus thuringiensis subspecies thuringiensis
Btte Bacillus thuringiensis subspecies tenebrionis
cfu colony forming unit
GILSP good industrial large-scale practice
GMO genetically modified organism
HPLC high-performance liquid chromatography
ICP insecticidal crystal protein
ITU international toxic unit
IUPAC International Union of Pure and Applied Chemistry
MPCA microbial pest control agent
NTO non-target organism
PCR polymerase chain reaction
SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
1. SUMMARY
This monograph deals with microbial pest control agents (MCPAs)
based on Bacillus thuringiensis (Bt). This bacterium is also a key
source of genes for transgenic expression to provide pest resistance
in plants and microorganisms as pest control agents in so-called
genetically modified organisms (GMOs). The potential effects on human
health and the environment of GMOs involve several aspects that are
only remotely or not at all related to Bt products, and they are
therefore outside the scope of this monograph.
1.1 Identity, biological characteristics and analytical methods
Bt is a facultative anaerobic, gram-positive bacterium that forms
characteristic protein inclusions adjacent to the endospore. Bt
subspecies can synthesize more than one parasporal inclusion. Bt is
genetically indistinguishable from Bc, except for the ability of Bt to
produce parasporal crystalline inclusions, which are toxic for certain
invertebrates, especially species of insect larvae belonging to the
insect orders Coleoptera, Diptera and Lepidoptera. The
parasporal inclusions are formed by different insecticidal crystal
proteins (ICP). The crystals have various shapes (bipyramidal,
cuboidal, flat rhomboid, spherical or composite with two crystal
types), depending on their ICP composition. A partial correlation
between crystal morphology, ICP composition, and bioactivity against
target insects has been established.
The basic phenotypic taxon is the subspecies, identified by the
flagellar (H) serotype. By 1998, 67 subspecies had been described. The
genes that encode the ICPs are mostly on plasmids. Each ICP is the
product of a single gene. Most plasmids with ICP genes are readily
transferred by conjugation between Bt strains and may be transferred
to related species of bacteria. The phenotypic classification has now
been complemented by molecular biological characterization, based on
the sequence of the crystal (cry and cyt) genes rather than target
organism specificity. Different domains of the ICP are responsible for
host susceptibility (receptor recognition) and toxicity (pore
formation).
Techniques commonly used to characterize Bt strains or the ICP
itself include cell wall fatty acid analysis, monoclonal antibodies,
oligonucleotide DNA probes, plasmid profiles, polymerase chain
reaction (PCR) analysis, DNA fingerprinting and SDS-PAGE profiles.
Beta-exotoxin, a heat-stable nucleotide, is produced by some Bt
subspecies during vegetative growth and may contaminate the products.
Beta-exotoxin is toxic for almost all forms of life including humans
and the target insect orders. During vegetative growth, various Bt
strains produce an assortment of antibiotics, enzymes, metabolites and
toxins, including Bc toxins, that may have detrimental effects on both
target organisms and non-target organisms (NTOs). Spore counts do not
accurately reflect the insecticidal activity of a Bt strain or Bt
product. The potency (ITU/mg) of each Bt product is bioassayed using
an international standard that uses a specific test insect.
1.2 Mode of action on target insects
The sporulated Bt with ICP or spore-ICP complexes must be
ingested by a susceptible insect larva. The efficacy of the ICP
depends on the solubilization in the midgut, the conversion of the
protoxin to the biologically active toxin by proteolytic enzymes,
specific membrane receptor binding by the C-terminal domain of the
active toxin, and pore formation by the N-terminal domain with
subsequent lysis of the epithelial cells. Spore germination and
proliferation of the vegetative cells into the haemocoel may result in
a septicaemia, contributing to the cause of death. Receptor binding by
the ICP is the major determinant of host specificity by the different
Bt ICPs.
1.3 Habitats
Many different Bt subspecies have been isolated from dead or
dying insects mostly from the orders Coleoptera, Diptera and
Lepidoptera, but many subspecies have also been isolated from soil,
leaf surfaces and other habitats. The carcasses of dead insects often
contain large quantities of spores and ICPs that may enter the
environment. The coleopteran-active and lepidopteran-active Bt
subspecies are primarily associated with the soil and phylloplane
(leaf surfaces), whereas the dipteran-active Bt subspecies are
commonly found in aquatic environments. In the environment, the spores
persist and vegetative growth may occur when conditions are favourable
and nutrients are available.
1.4 Commercial products, production and application
Conventional Bt products, which utilize naturally-occurring Bt
strains, account for approximately 90% of the world MPCA market. Most
Bt products contain ICP and viable spores, but in some Bti products
the spores are inactivated. Each year some 13 000 tonnes are produced
using aerobic fermentation technology. Conventional Bt products have
been targeted primarily against lepidopteran pests of agricultural and
forestry crops; however in recent years, Bt strains active against
coleopteran pests have also been marketed. Strains of Bti active
against dipteran vectors of parasitic and viral diseases are being
used in public health programmes.
Commercial Bt formulations may be applied as an insecticide to
foliage, soil, water environments or food storage facilities. After
the application of a Bt subspecies to an ecosystem, the vegetative
cells and spores may persist at gradually decreasing concentrations
for weeks, months or years as a component of the natural microflora.
The ICPs, however, are rendered biologically inactive within hours or
days.
1.5 Effects of Bt on non-target organisms
Studies on mammals, particularly those on laboratory animals,
have evaluated possible infectivity and toxicity of various Bt
preparations, which include the ICPs, vegetative cells and spores. The
ICPs, spores and vegetative cells of the Bt subspecies, which were
administered by different routes, were mostly non-pathogenic and
non-toxic to the various animal species tested. The vegetative cells
and/or spores of Bt were demonstrated to persist for weeks without
causing adverse effects. Bt has not been observed to adversely affect
birds, fish or many other non-target aquatic vertebrates tested in a
large number of laboratory and field studies. Relatively few species
of aquatic invertebrates are susceptible to Bt under either laboratory
or field conditions. Bt does not adversely affect earthworms.
The Bt subspecies have generally been shown to be highly specific
in their insecticidal activity for Coleoptera, Diptera and
Lepidoptera and have demonstrated little, if any, direct toxicity to
non-target arthropods. Most of the existing safety data on non-target
arthropods has been generated using the Bt subspecies with activity
against Diptera and Lepidoptera.
Studies of Bti formulations free of toxic contaminants have not
demonstrated deleterious effects on the vast majority of non-target
arthropods. Some midges (Diptera: Chironomidae), which are closely
related to mosquitos, have been shown to be susceptible to high
dosages of Bti, but are not affected by mosquito larvicidal dosages.
In field studies, transient decreases or increases in populations of
some non-target arthropods have been reported.
Many insect orders have been tested in either the laboratory or
field, most of which have shown no effect from Btk.
Mortality has been observed in honey-bees (Apis mellifera)
after exposure to vegetatively growing Btt and Btk, but the effect
does not seem to be related to spores or ICPs. In laboratory and field
studies Btg demonstrated no adverse effect on honey-bees.
Bte strains that produce beta-exotoxin have been shown to have
adverse effects on non-target arthropods.
1.6 Exposure and effects of Bt on humans
The field application of Bt products can result in considerable
aerosol and dermal exposure of workers. Agricultural uses of Bt can
result in Bt contamination of potable water and food. With the
exception of case reports on ocular and dermal irritation, no adverse
health effects have been documented after occupational exposure to Bt
products. Human volunteers ingested and inhaled large quantities of a
Btk formulation but experienced no adverse health effects. Antibody
titres to the vegetative cells, spores and spore-crystal complexes
have been demonstrated in workers who spray Bt products; however, no
adverse health effects were reported. There have been some case
reports on the occurrence of Bt in patients with different infectious
diseases. However, none of these studies unequivocally demonstrates an
actual risk to human health from the use of Bt. Bt has not been
reported to cause adverse effects on human health when present in
drinking-water or food.
1.7 Conclusions
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and biologically active products other than the
ICPs. Bt products may be safely used for the control of insect pests
of agricultural and horticultural crops as well as forests. They are
also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt has the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
2. IDENTITY, BIOLOGICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Commercial Bacillus thuringiensis (Bt) products are microbial
pest control agents (MPCAs) containing specific insecticidal
crystalline proteins (ICPs) and most often living spores as well as
formulating agents. They are processed fermentation products.
2.1.1 Bacillus thuringiensis
Bt is a facultative anaerobic, motile, gram-positive,
spore-forming bacterium. The formation of parasporal crystals adjacent
to the endospore during sporulation stages III to IV distinguishes Bt
from other Bacillus species.
Bt, like other Bacillus species, has been classified on the
basis of its cellular, cultural, biochemical and genetic
characteristics (Baumann et al., 1984; Claus & Berkley, 1986; Slepecky
& Hemphill, 1992; Carlson & Kolsto, 1993; Hansen et al., 1998). In
1958, Heimpel & Angus (1958) introduced a classification scheme to
identify these crystalliferous bacteria based on their morphological
and biochemical characteristics. However recent molecular analysis
shows that several variations can be found within serotypes, and that
specific biochemical characteristics do not always refer to a specific
serotype (Helgason et al., 1998; Hansen et al., 1998).
2.1.2 Relationship between Bacillus thuringiensis and Bacillus cereus
Bt is a member of the Bc group, which also contains Bacillus
cereus (Bc), B. mycoides and B. anthracis. Furthermore, the
psychrotolerant B. weihenstephanensis has recently been proposed as
a new member of the group (Lechner et al., 1998). Bt can only be
distinguished from Bc by the production during the sporulation process
of one or more inclusion bodies, which have been found to be toxic for
invertebrates, primarily insect species in the orders Coleoptera,
Diptera and Lepidoptera (de Barjac, 1981b; Andrews et al., 1987).
Several studies have been dedicated to a comparison of Bt and Bc on
the basis of characters not related to the production of ICPs
(Hendriksen & Hansen, 1998). Phenotypic differentiation of Bt and Bc
is not possible on the basis of morphology or utilization of organic
compounds (Baumann et al., 1984; Logan & Berkeley, 1984; Priest et
al., 1988), characterization of cell content of fatty acids (Väisänen
et al., 1991) or sugars (Wunschel et al., 1994), multilocus enzyme
electrophoresis (Zahner et al., 1989; Carlson et al., 1994),
enterotoxin production (Damgaard et al., 1996a; Hansen & Hendriksen,
1997a), or serological- and phage-typing techniques (Ohba & Aizawa,
1978; 1986; Väisänen et al., 1991; Murakami et al., 1993; Ahmed et
al., 1995). Likewise, genotypic differentiation of Bt and Bc is not
possible by DNA homology analysis (Kaneko et al., 1978), ribotyping
(Priest et al., 1994; Demezas & Bell, 1995), 16S rDNA sequencing (Ash
et al., 1991); analysis of the 16S-23S internal transcribed sequence
(Wunschel et al., 1994; Bourque et al., 1995), PCR analysis of genes
encoding Bc-like toxic products (Damgaard et al., 1996b; Asano et al.,
1997; Hansen & Hendriksen, 1997b) or pulsed field gel electrophoresis
(Carlson & Kolsto, 1993; Carlson et al., 1994). Giffel et al. (1997)
found differences in 16S rDNA sequences between a limited number Bt
and Bc. Beattie et al. (1998) were able to discriminate among members
of the Bc group by Fourier transform infrared spectroscopy, and
Brousseau et al. (1992) were able to distinguish Bt and Bc by random
amplified polymorphic DNA fingerprinting. However, the transfer of ICP
encoding plasmids from Bt to Bc makes the receptor Bc
indistinguishable from Bt, and vice versa (González et al., 1981,
1982).
2.1.3 Crystal composition and morphology
The existence of parasporal inclusions in Bt was first noted in
1915 (Berliner, 1915), but their protein composition was not
delineated until the 1950s (Angus, 1954). Hannay (1953) detected the
crystalline fine structure that is a property of most of the
parasporal inclusions. Bt subspecies can synthesize more than one
inclusion, which may contain different ICPs. ICPs have also been
called delta endotoxins; however, since the term endotoxin usually
refers to toxins associated with the outer membranes of gram-negative
bacteria, comprising a core lipopoplysaccharide, lipid A and somatic
(O) antigens, this term is not used in this monograph. Depending on
their ICP composition, the crystals have various forms (bipyramidal,
cuboidal, flat rhomboid, or a composite with two or more crystal
types). A partial correlation between crystal morphology, ICP
composition, and bioactivity against target insects has been
established (Bulla et al., 1977; Höfte & Whiteley, 1989; Lynch &
Baumann, 1985).
2.1.4 Classification of Bt subspecies
The classification of Bt subspecies based on the serological
analysis of the flagella (H) antigens was introduced in the early
1960s (de Barjac & Bonnefoi, 1962). This classification by serotype
has been supplemented by morphological and biochemical criteria (de
Barjac, 1981a). Until 1977, only 13 Bt subspecies had been described,
and at that time all subspecies were toxic to Lepidopteran larvae
only. The discovery of other subspecies toxic to Diptera (Goldberg &
Margalit, 1977), Coleoptera (Krieg et al., 1983) and apparently
Nematoda (Narva et al., 1991) enlarged the host range and markedly
increased the number of subspecies. Up to the end of 1998, over 67
subspecies based on flagellar H-serovars had been identified (Table
1). Updated lists of the serovarieties can be obtained from the
reference centre of the Pasteur Institute in Paris (Unité des
Bactéries Entomopathogènes, Institut Pasteur, Paris, France).
Table 1. Current classification of 67 Bacillus thuringiensis subspecies based
on their flagellar (H) antigensa
Flagellar antigens B. thuringiensis Flagellar antigens B. thuringiensis
subspecies subspecies
1 thuringiensis 28a, 28c jegathesan
2 finitimus 29 amagiensis
3a, 3c alesti 30 medellin
3a, 3b, 3c kurstaki 31 toguchini
3a, 3d sumiyoshiensis 32 cameroun
3a, 3d, 3e fukuokaensis 33 leesis
4a, 4b sotto 34 konkukian
4a, 4c kenyae 35 seoulensis
5a, 5c galleriae 36 malaysiensis
5a, 5c canadensis 37 anadalousiensis
6 entomocidus 38 oswaldocruzi
7 aizawai 39 brasiliensis
8a, 8b morrisoni 40 huazhongensis
8a, 8c ostriniae 41 sooncheon
8b, 8d nigeriensis 42 jinghongiensis
9 tolworthi 43 guiyanguebsus
10a, 10b darmstadiensis 44 higo
10a, 10c londrina 45 roskildiensis
11a, 11b toumanoffi 46 chanpaisis
11a, 11c kyushuensis 47 wratislaviensis
12 thompsoni 48 balearica
13 pakistani 49 muju
14 israelensis 50 navarrensis
15 dakota 51 xiaguangiensis
16 indiana 52 kim
17 tohokuensis 53 asturiensis
18a, 18b kumamotoensis 54 poloniensis
18a, 18c yosoo 55 palmanyolensis
19 tochigiensis 56 rongseni
20a, 20b yunnanensis 57 pirenaica
20a, 20c pondicheriensis 58 argentinensis
21 colmeri 59 iberica
22 shandongiensis 60 pingluonsis
23 japonensis 61 sylvestriensis
24a, 24b neoleonensis 62 zhaodongensis
24a, 24c novosibirsk 63 bolivia
25 coreanensis 64 azorensis
26 silo 65 pulsiensis
27 mexicanensis 66 gracioensis
28a, 28b monterrey 67 vazensis
a Data provided by the Unité des Bactéries Entomopathogènes, Institut Pasteur,
Paris, France
Crystal serology has shown that a particular crystal type may be
produced by more than one H-serovar (Krywienczyk et al., 1978; Smith,
1987).
2.1.5 Genetics of ICP
In the early 1980s, it was established that most genes coding for
the ICPs reside on large transmissible plasmids, of which most are
readily exchanged between strains by conjugation (González & Carlton,
1980; González et al., 1981). Since these initial studies, numerous
ICP genes have been cloned, sequenced and used to construct Bt strains
with novel insecticidal spectra (Höfte & Whiteley, 1989).
The currently known crystal (cry) gene types encode ICPs that
are specific to either Lepidoptera (cryI), Diptera and
Lepidoptera (cryII), Coleoptera (cryIII), Diptera (cryIV),
or Coleoptera and Lepidoptera (cryV) (Höfte & Whiteley, 1989).
A separate designation is used for the cytolytic (cyt) genes that
encode a nonspecific cytolytic factor, present in Bti ICP and some
other Bt subspecies. However, due to the increasing number of
characterized ICP genes and inconsistencies in the existing cry gene
nomenclature, which is based on insecticidal spectrum, Crickmore et
al. (1998) proposed a new nomenclature based on ICP gene sequences.
The new cry genes are listed in Tables 2 and 3, and Table 4 is a
conversion list from old to new cry gene names. Current
identification of Bt employs both the identity of the cry genes,
which define the host range, and the H-serovars, which define the
subspecies, and recently DNA fingerprinting has been used for further
characterization of subspecies (Hansen et al., 1998).
The ICP gene sequences provided the basis for the construction of
gene-specific probes to screen established Bt strains by hybridization
and PCR analysis for the presence of known nucleotide sequences, and
for characterizing the ICPs from new Bt isolates (Prefontaine et al.,
1987; Juarez-Perez et al., 1997; Bravo et al., 1998; Shevelev et al.,
1998). These studies have permitted the distinction of numerous
subclasses of ICP genes based on sequence homology and toxicity
spectra of the encoded proteins.
All ICPs described to date attack the insect gut upon ingestion
(see chapter 3). To date, each of the proteolytically activated ICP
molecules with insecticidal activity has a variable C-terminal domain,
which is responsible for receptor recognition (host susceptibility),
and a conserved N-terminal domain, which induces pore formation
(toxicity) (Li et al., 1991).
Most naturally occurring Bt strains contain ICPs active against a
single order of insects. However, conjugative transfer between Bt
strains or related species can occur, resulting in new strains with
various plasmid contents. Thus the mobility of the cry genes and the
exchange of plasmids may explain the diverse and complex activity
Table 2. Bacillus thuringiensis crystal protein genes (Crickmore et al., 1998)
Name Acc No Reference Journal Coding
Cry1aa1 M11250 Schnepf et al., 1985 JBC 260 6264-6272 527-4054
Cry1aa2 M10917 Shibano et al., 1985 Gene 34 243-251 153->2955
Cry1aa3 D00348 Shimizu et al., 1988 ABC 52 1565-1573 73-3603
Cry1aa4 X13535 Masson et al., 1989 NAR 17 446-446 1-3528
Cry1aa5 D17518 Udayasuriyan et al., 1994 BBB 58 830-835 81-3611
Cry1aa6 U43605 Masson et al., 1994 Mol Micro 14 851-860 1->1860
Cry1aa7 AF081790 Osman, 1998 unpublished
Cry1aa8 I26149 Liu, 1996 USP 5556784 148-3675
Cry1ab1 M13898 Wabiko et al., 1986 DNA 5 305-314 142-3606
Cry1ab2 M12661 Thorne et al., 1986 J Bact 166 801-811 155-3625
Cry1ab3 M15271 Geiser et al., 1986 Gene 48 109-118 156-3623
Cry1ab4 D00117 Kondo et al., 1987 ABC 51 455-463 163-3630
Cry1ab5 X04698 Hofte et al., 1986 EJB 161 273-280 141-3605
Cry1ab6 M37263 Hefford et al., 1987 J Biotech 6 307-322 73-3540
Cry1ab7 X13233 Haider & Ellar, 1988 NAR 16 10927-10927 1-3465
Cry1ab8 M16463 Oeda et al., 1987 Gene 53 113-119 157-3624
Cry1ab9 X54939 Chak & Jen, 1993 PNSCRC 17 7-14 73-3540
Cry1ab10 A29125 Fischhoff et al., 1987 Bio/technology peptide seq
5 807-813
Cry1ab11 I12419 Ely & Tippett, 1995 USP 5424409 73-
Cry1ab12 AF057670 Silva-Werneck et al., 1998 unpublished 41-3505
Cry1ac1 M11068 Adang et al., 1985 Gene 36 289-300 388-3921
Cry1ac2 M35524 Von Tersch et al., 1991 AEM 57 349-358 239-3772
Cry1ac3 X54159 Dardenne et al., 1990 NAR 18 5546-5546 339->2192
Cry1ac4 M73249 Payne et al., 1991 USP 4990332 1-3537
Cry1ac5 M73248 Payne et al., 1992 USP 5135867 1-3534
Cry1ac6 U43606 Masson et al., 1994 Mol Micro 14 851-860 1->1821
Cry1ac7 U87793 Herrera et al., 1994 AEM 60 682-690 976-4512
Cry1ac8 U87397 Omolo et al., 1997 Curr Micro 34 118-121 153-3686
Cry1ac9 U89872 Gleave et al., 1992 NZJCHS 20 27-36 388-3921
Cry1ac10 AJ002514 Sun & Yu, 1997 unpublished 388-3921
Cry1ac11 AJ130970 Makhdoom & Riazuddin, 1998 unpublished 156-3689
Cry1ac12 I12418 Ely & Tippett, 1995 USP 5424409 81->2990
Cry1ad1 M73250 Payne & Sick, 1993 USP 5246852 1-3537
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1ad2 A27531 Payne & Sick, 1995 AUP 632335 1-3537
Cry1ae1 M65252 Lee & Aronson, 1991 J Bact 173 6635-6638 81-3623
Cry1af1 U82003 Kang et al., 1997 unpublished 172->2905
Cry1ag1 AF081248 Osman, 1998 unpublished
Cry1ba1 X06711 Brizzard & Whiteley, 1988 NAR 16 2723-2724 1-3684
Cry1ba2 X95704 Soetaert, 1996 unpublished 186-3869
Cry1bb1 L32020 Donovan et al., 1994 USP 5322687 67-3753
Cry1bc1 Z46442 Bishop et al., 1994 unpublished 141-3839
Cry1bd1 U70726 Kuo & Chak, 1999 unpublished 842-4534
Cry1be1 Payne et al., 1998 USP 5723758 1-3681
Cry1ca1 X07518 Honee et al., 1988 NAR 16 6240-6240 47-3613
Cry1ca2 X13620 Sanchis et al., 1989 Mol Micro 3 229-238 241->2711
Cry1ca3 M73251 Payne & Sick, 1993 USP 5246852 1-3570
Cry1ca4 A27642 Van Mellaert et al., 1990 EP 0400246 234-3800
Cry1ca5 X96682 Strizhov, 1996 unpublished 1->2286
Cry1cb1 M97880 Kalman et al., 1993 AEM 59 1131-1137 296-3823
Cry1da1 X54160 Hofte et al., 1990 NAR 18 5545-5545 264-3758
Cry1da2 I76415 Payne & Sick, 1997 USP 5691308 1-3495
Cry1db1 Z22511 Lambert, 1993 unpublished 241-3720
Cry1ea1 X53985 Visser et al., 1990 J Bact 172 6783-6788 130-3642
Cry1ea2 X56144 Bosse et al., 1990 NAR 18 7443-7443 1-3516
Cry1ea3 M73252 Payne & Sick, 1991 USP 5039523 1-3516
Cry1ea4 U94323 Barboza-Corona et al., 1998 WJMB 14 437-441 388-3900
Cry1ea5 A15535 Botterman et al., 1994 EP 0358557 54-3566
Cry1eb1 M73253 Payne & Sick, 1993 USP 5206166 1-3522
Cry1fa1 M63897 Chambers et al., 1991 J Bact 173 3966-3976 478-3999
Cry1fa2 M73254 Payne & Sick, 1993 USP 5188960 1-3525
Cry1fb1 Z22512 Lambert, 1993 unpublished 483-4004
Cry1fb2 AB012288 Masuda & Asano, 1998 unpublished 84-3587
Cry1fb3 AF062350 Song & Zhang, 1998 unpublished
Cry1fb4 I73895 Payne et al., 1997 USP 5686069 peptide seq
Cry1ga1 Z22510 Lambert, 1993 unpublished 67-3564
Cry1ga2 Y09326 Shevelev et al., 1997 Febs Lett 404 148-152 692-4210
Cry1gb1 U70725 Kuo & Chak, 1999 unpublished 532-4038
Cry1ha1 Z22513 Lambert, 1993 unpublished 530-4045
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1hb1 U35780 Koo et al., 1995 unpublished 728-4195
Cry1ia1 X62821 Tailor et al., 1992 Mol Micro 6 1211-1217 355-2511
Cry1ia2 M98544 Gleave et al., 1993 AEM 59 1683-1687 1-2160
Cry1ia3 L36338 Shin et al., 1995 AEM 61 2402-2407 279-2438
Cry1ia4 L49391 Kostichka et al., 1996 J Bact 178 2141-2144 61-2217
Cry1ia5 Y08920 Selvapandiyan, 1996 unpublished 524-2680
Cry1ia6 AF076953 Zhong et al., 1998 unpublished 1-2157
Cry1ib1 U07642 Shin et al., 1995 AEM 61 2402-2407 237-2393
Cry1ic1 AF056933 Osman et al., 1998 unpublished 1-2157
Cry1i-like I90732 Payne et al., 1998 See Table 3 peptide seq
Cry1ja1 L32019 Donovan et al., 1994 USP 5322687 99-3519
Cry1jb1 U31527 Von Tersch & Gonzalez, 1994 USP 5356623 177-3686
Cry1jc1 I90730 Payne et al., 1998 USP 5723758 peptide seq
Cry1ka1 U28801 Koo et al., 1995 FEMS 134 159-164 451-4098
Cry1-like I90729 Payne et al., 1998 See Table 3 peptide seq
Cry2aa1 M31738 Donovan et al., 1989 JBC 264 4740-4740 156-2054
Cry2aa2 M23723 Widner & Whiteley, 1989 J Bact 171 965-974 1840-3741
Cry2aa3 D86064 Sasaki et al., 1997 Curr Micro 35 1-8 2007-3911
Cry2aa4 AF047038 Misra et al., 1998 Unpublished 10-1908
Cry2aa5 AJ132464 Yu & Pang, 1999 Unpublished <1-1860
Cry2aa6 AJ132465 Yu & Pang 1999 Unpublished <1-1860
Cry2aa7 AJ132463 Yu & Pang, 1999 Unpublished <1->1611
Cry2ab1 M23724 Widner & Whiteley, 1989 J Bact 171 965-974 1-1899
Cry2ab2 X55416 Dankocsik et al., 1990 Mol Micro 4 2087-2094 874-2775
Cry2ac1 X57252 Wu et al., 1991 FEMS 81 31-36 2125-3990
Cry3aa1 M22472 Herrnstadt et al., 1987 Gene 57 37-46 25-1956
Cry3aa2 J02978 Sekar et al., 1987 PNAS 84 7036-7040 241-2175
Cry3aa3 Y00420 Hofte et al., 1987 NAR 15 7183-7183 566-2497
Cry3aa4 M30503 McPherson et al., 1988 Bio/technology 6 61-66 201-2135
Cry3aa5 M37207 Donovan et al., 1988 MGG 214 365-372 569-2503
Cry3aa6 U10985 Adams et al., 1994 Mol Micro 14 381-389 569-2503
Cry3ba1 X17123 Sick et al., 1990 NAR 18 1305-1305 25-1977
Cry3ba2 A07234 Peferoen et al., 1990 EP 0382990 342-2297
Cry3bb1 M89794 Donovan et al., 1992 AEM 58 3921-3927 202-2157
Cry3bb2 U31633 Donovan et al., 1995 USP 5378625 144-2099
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry3bb3 I15475 Peferoen et al., 1995 USP 5466597 <1->1291
Cry3ca1 X59797 Lambert et al., 1992 Gene 110 131-132 232-2178
Cry4aa1 Y00423 Ward & Ellar, 1987 NAR 15 7195-7195 1-3540
Cry4aa2 D00248 Sen et al., 1988 ABC 52 873-878 393-3935
Cry4ba1 X07423 Chungjatpornchai et al., 1988 EJB 173 9-16 157-3564
Cry4ba2 X07082 Tungpradubkul et al., 1988 NAR 16 1637-1638 151-3558
Cry4ba3 M20242 Yamamoto et al., 1988 Gene 66 107-120 526-3933
Cry4ba4 D00247 Sen et al., 1988 ABC 52 873-878 461-3868
Cry5aa1 L07025 Sick et al., 1994 USP 5281530 1-4155
Cry5ab1 L07026 Narva et al., 1991 EP 0462721 1-3867
Cry5ac1 I34543 Payne et al., 1997 USP 5596071 1-3660
Cry5ba1 U19725 Payne et al., 1997 USP 5596071 1-3735
Cry6aa1 L07022 Narva et al., 1993 USP 5236843 1-1425
Cry6ba1 L07024 Narva et al., 1991 EP 0462721 1-1185
Cry7aa1 M64478 Lambert et al., 1992 AEM 58 2536-2542 184-3597
Cry7ab1 U04367 Payne & Fu, 1994 USP 5286486 1-3414
Cry7ab2 U04368 Payne & Fu, 1994 USP 5286486 1-3414
Cry8aa1 U04364 Foncerrada et al., 1992 EP 0498537 1-3471
Cry8ba1 U04365 Michaels et al., 1993 WO 93/15206 1-3507
Cry8ca1 U04366 Ogiwara et al., 1995 Curr Micro 30 227-235 1-3447
Cry9aa1 X58120 Smulevitch et al., 1991 FEBS 293 25-28 5807-9274
Cry9aa2 X58534 Gleave et al., 1992 JGM 138 55-62 385->3837
Cry9ba1 X75019 Shevelev et al., 1993 FEBS 336 79-82 26-3488
Cry9ca1 Z37527 Lambert et al., 1996 AEM 62 80-86 2096-5569
Cry9da1 D85560 Asano et al., 1997 AEM 63 1054-1057 47-3553
Cry9Da2 AF042733 Wasano & Ohba, 1998 Unpublished <1->1937
Cry9Ea1 AB011496 Midoh & Oyama, 1998 Unpublished 211-3660
Cry9 like AF093107 Wasano & Ohba, 1998 See Table 3 <1->1917
Cry10Aa1 M12662 Thorne et al., 1986 J Bact 166 801-811 941-2965
Cry10Aa2 E00614 Uorufuiirudo, 1996 JP 1986005098 940-2968
Cry11Aa1 M31737 Donovan et al., 1988 J Bact 170 4732-4738 41-1969
Cry11Aa2 M22860 Adams et al., 1989 J Bact 171 521-530 <1-235
Cry11Ba1 X86902 Delecluse, 1995 AEM 61 4230-4235 64-2238
Cry11Bb1 AF017416 Orduz et al., 1998 BBA 1388 267-272 97-2346
Cry12Aa1 L07027 Narva et al., 1991 EP 0462721 1->3771
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry13Aa1 L07023 Narva et al., 1992 WO 92/19739 1-2409
Cry14Aa1 U13955 Narva et al., 1994 WO 94/16079 1-3558
Cry15Aa1 M76442 Brown & Whiteley, 1992 J Bact 174 549-557 1036-2055
Cry16Aa1 X94146 Barloy et al., 1996 J Bact 178 3099-3105 158-1996
Cry17Aa1 X99478 Barloy et al., 1998 Gene 211 293-299 12-1865
Cry18Aa1 X99049 Zhang et al., 1997 J Bact 179 4336-4341 1451-3571
Cry19Aa1 Y07603 Rosso & Delecluse, 1996 AEM 63 4449-4455 719-2662
Cry19Ba1 D88381 Hwang et al., 1998 SAB 21 179-184 626-2671
Cry20Aa1 U82518 Lee & Gill, 1997 AEM 63 4664-4670 60-2318
Cry21Aa1 I32932 Payne et al., 1996 USP 5589382 1-3501
Cry21Aa2 I66477 Feitelson, 1997 USP 5670365 1-3501
Cry22Aa1 I34547 Payne et al., 1997 USP 5596071 1-2169
Cry23Aa1 AF03048 Donovan & Slaney, 1998 WO 98/13498
Cry24Aa1 U88188 Kawalek & Gill, 1998 Unpublished 1-2022
Cry25Aa1 U88189 Kawalek & Gill, 1998 Unpublished 1-2028
Cyt1Aa1 X03182 Waalwijk et al., 1985 NAR 13 8207-8217 140-886
Cyt1Aa2 X04338 Ward & Ellar, 1986 JMB 191 1-11 509-1255
Cyt1Aa3 Y00135 Earp & Ellar, 1987 NAR 15 3619-3619 36-782
Cyt1Aa4 M35968 Galjart et al., 1987 Curr Micro 16 171-177 67-816
Cyt1Ab1 X98793 Thiery et al., 1997 AEM 63 468-473 28-777
Cyt1Ba1 U37196 Payne et al., 1995 USP 5436002 1-795
Cyt2Aa1 Z14147 Koni & Ellar, 1993 JMB 229 319-327 270-1046
Cyt2Ba1 U52043 Guerchicoff et al., 1997 AEM 63 2716-2721 287-655
Cyt2Ba2 AF020789 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba3 AF022884 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba4 AF022885 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba5 AF022886 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->471
Cyt2Ba6 AF034926 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->472
Cyt2Bb1 U82519 Cheong & Gill, 1997 AEM 63 3254-3260 416-1204
spectra observed in Bt (González & Carlton, 1980; González et al.,
1981; González et al., 1982; Reddy et al., 1987; Jarrett & Stephenson,
1990). New Bt strains have been developed by conjugation that are
toxic to two insect orders.
2.1.6 Beta-exotoxin
Beta-exotoxin is associated with certain Bt subspecies (Btd, Btg,
Btte and Btt), and products made from these Bt subspecies may contain
the toxin (Cantwell et al., 1964; Mohd-Salleh et al., 1980). This
heat-stable nucleotide, which is composed of adenine, glucose and
allaric acid, inhibits RNA polymerase enzymes by acting competitively
with ATP (Faust, 1973; Farkas et al., 1977). Since RNA synthesis is a
vital process in all life, beta-exotoxin exerts its toxicity for
almost all forms of life tested including numerous insect species in
the orders Coleoptera, Diptera and Lepidoptera. The presence of
beta-exotoxin can be assayed using houseflies (Musca domestica) or
high-performance liquid chromatography (HPLC) techniques (Campbell et
al., 1987).
Bt containing beta-exotoxin is used for the control of houseflies
in some countries, but regulatory agencies currently prohibit the use
of beta-exotoxin for other purposes.
2.1.7 Other Bt metabolites
Commercial Bt products do not contain metabolites that are
considered hazardous to humans and the environment. However, Bt, like
other bacteria, may produce during the vegetative growth and
sporulation stages an assortment of antibiotics, enzymes, metabolites
and toxins that are biologically active and may have effects on both
target and non-target organisms (NTOs).
Using a non-quantitative (Lund & Granum, 1997) commercial Bc
enterotoxin immunoassay (Tecra), Damgaard (1995) reported that
vegetative cells grown from spores of commercial Bt products excreted
a diarrhoeal enterotoxin. Damgaard et al. (1996a) found by Vero cell
assay that Bt isolated from food was enterotoxigenic. None of these
investigations estimated the quantity or activity of enterotoxins
produced by the Bt strains. However, Shinagawa (1990) investigated a
number of Bc and Bt isolates with an immunological assay and concluded
that 43% of the Bt isolates had the same level of enterotoxins as
enterotoxic Bc. Tayabali & Seligy (1997) found that vegetative Bt
obtained from commercial products caused extensive damage to
cultivated insect cells.
At least three enterotoxic activities have been described in Bc
(Agata et al., 1995; Lund & Granum, 1997), and some Bc isolates are
known to produce an emetic toxin (Andersson et al., 1998). The emetic
toxin is primarily associated with the Bc H-1 (Kramer & Gilbert, 1992;
Nishikawa et al., 1996) serotype, and has not so far been associated
with Bt isolates.
Table 3. Bt-associated toxins or toxin-like proteins that have not been assigned a name or entered
into the nomenclature for the reasons given
Name Accession Journal Coding region Reason Reference
Cry1i-like I90732 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry1-like I90729 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry9-like AF093107 unpublished <1->1917 Insufficient sequence data Wasano & Ohba, 1998
40kda M76442 J Bact 174 549-557 45-971 No reported toxicity Brown & Whiteley, 1992
Cryc35 X92691 unpublished 1-981 No reported toxicity Juarez-Perez et al., 1995
Crytdk D86346 unpublished 177-2645 No reported toxicity Hashimoto, 1996
Cryc53 X98616 unpublished 1-1005 No reported toxicity Juarez-Perez et al., 1996
p21med X98794 AEM 63 468-473 1-552 No reported toxicity Thiery et al., 1997
ET34 AF038049 WO 98/13498 No reported toxicity Donovan & Slaney, 1998
Vip3a(a) L48811 PNAS 93 5389-5394 739-3105 Not a crystal protein Estruch et al., 1996
Vip3a(b) L48812 PNAS 93 5389-5394 118-2484 Not a crystal protein Estruch et al., 1996
Table 4. Bacillus thuringiensis holotype toxins
Name Old name Name Old name
Cry1Aa CryIA(a) Cry5Ac
Cry1Ab CryIA(b) Cry5Ba
Cry1Ac CryIA(c) Cry6Aa CryVIA
Cry1Ad CryIA(d) Cry6Ba CryVIB
Cry1Ae CryIA(e) Cry7Aa CryIIIC
Cry1Af Cry7Ab CryIIICb
Cry1Ag Cry8Aa CryIIIE
Cry1Ba CryIB Cry8Ba CryIIIG
Cry1Bb ET5 Cry8Ca CryIIIF
Cry1Bc PEG5 Cry9Aa CryIG
Cry1Bd CryE1 Cry9Ba CryIX
Cry1Be Cry9Ca CryIH
Cry1Ca CryIC Cry9Da
Cry1Cb CryIC(b) Cry9Ea
Cry1Da CryID Cry10Aa CryIVC
Cry1Db PrtB Cry11Aa CryIVD
Cry1Ea CryIE Cry11Ba Jeg80
Cry1Eb CryIE(b) Cry11Bb
Cry1Fa CryIF Cry12Aa CryVB
Cry1Fb PrtD Cry13Aa CryVC
Cry1Ga PrtA Cry14Aa CryVD
Cry1Gb CryH2 Cry15Aa 34kDa
Cry1Ha PrtC Cry16Aa cbm71
Cry1Hb Cry17Aa cbm72
Cry1Ia CryV Cry18Aa CryBP1
Cry1Ib CryV Cry19Aa Jeg65
Cry1Ic Cry19Ba
Cry1Ja ET4 Cry20Aa
Cry1Jb ET1 Cry21Aa
Cry1Jc Cry22Aa
Cry1Ka Cry23Aa
Cry2Aa CryIIA Cry24Aa Jeg72
Cry2Ab CryIIB Cry25Aa Jeg74
Cry2Ac CryIIC Cry26Aa
Cry3Aa CryIIIA Cry27Aa
Cry3Ba CryIIIB Cry28Aa
Cry3Bb CryIIIBb Cyt1Aa CytA
Cry3Ca CryIIID Cyt1Ab CytM
Cry4Aa CryIVA Cyt1Ba
Cry4Ba CryIVB Cyt2Aa CytB
Cry5Aa CryVA(a) Cyt2Ba "CytB"
Cry5Ab CryVA(b) Cyt2Bb
Alpha-toxin is a phospholipase C, which primarily affects the
cell membrane phospholipids (Heimpel, 1954; Bonnefoi & Béguin, 1959).
Gamma-toxin is toxic to sawflies (Tenthredinidae), but the mode of
action of this heat-labile toxin has not been determined (Heimpel,
1967).
The so called "water-soluble toxin" paralyses Lepidoptera
(Fast, 1971), and the "mouse factor exotoxin" is toxic to mice as
well as to Lepidoptera (Krieg, 1971). The modes of action of these
toxins have not been delineated.
A novel Bt vegetative insecticidal protein (Vip3A) has been
identified from the culture media of some Bt strains (Estruch et al.,
1996).
Several Bt and Bc enzymes have been described which may play a
role in non-target activity: phospholipase (Damgaard et al., 1996b),
sphingomyelinase (Gilmore et al., 1989), protease (Hotha & Banik,
1997), chitinase (Sampson & Gooday, 1998), and haemolysin (Baida &
Kuzmin, 1995).
2.2 Bioassays
2.2.1 Spore counts
Bacterial spore counts do not necessarily reflect the
insecticidal activity of a Bt strain or Bt product because the number
and amount of ICPs produced per bacterial cell can vary.
2.2.2 International bioassay for ICPs
The final formulation of each Bt product is bioassayed against an
accepted international standard using a specific test insect (Dulmage
et al., 1981; de Barjac & Larget-Thiery, 1984). Its potency is defined
in ITU/mg product. The standardization allows comparison of different
formulations in the laboratory. Currently, the larvicidal activity is
expressed in terms of lethal doses (LD50) or lethal concentrations
(e.g., LC50, LC90) according to the bioassay method used. For
example, when susceptible mosquito larvae are exposed to Bti ICP, they
have an LC50 of approximately 10 ng/ml water. A Bti whole culture
gives an LC50 of approximately 103 cells/ml for susceptible mosquito
larvae while a 109 cells/ml culture does not affect any laboratory
mammals exposed by various routes.
3. MODE OF ACTION ON TARGET INSECTS
3.1 Bioactivity of field isolates
The mode of action of Bt has been reviewed by Schnepf et al.
(1998) and can be summarized in the following stages: 1) ingestion of
sporulated Bt and ICP by an insect larva; 2) solubilization of the
crystalline ICP in the midgut; 3) activation of the ICP by proteases;
4) binding of the activated ICP to specific receptors in the midgut
cell membrane; 5) insertion of the toxin in the cell membrane and
formation of pores and channels in the gut cell membrane, followed by
destruction of the epithelial cells (Cooksey, 1971; Norris 1971; Fast,
1981; Huber & Lüthy, 1981; Lüthy & Ebersold, 1981; Smedley & Ellar,
1996); and 6) subsequent Bt spore germination and septicaemia may
enhance mortality (Fig. 1).
The specific bioactivity of Bt is dominated by the ICPs that are
encoded by the cry genes and are active against susceptible species
in the insect orders Coleoptera, Diptera and Lepidoptera.
Specific Bt activities against other insect orders (Hymenoptera,
Homoptera, Dictyoptera, Mallophaga) and to nematodes
(Strongylida, Tylenchida), mites (Acari), flatworms (Digenea)
and protozoa (Diplomonadida) have been described (Feitelson, 1993;
Zukowski, 1995). The ICP must be ingested to be effective against the
target (Visser et al., 1993).
3.2 Mechanism of action of Bt formulations
The ICP-spore complexes of Bt are ingested by susceptible insect
larvae. In the midgut the parasporal crystalline ICP is dissociated to
the protoxin form, and the protoxin is then activated to a holotoxin
by gut proteases (Warren et al., 1984; Jaquet et al., 1987; Aronson et
al., 1991; Honée & Visser, 1993). Shortly afterwards, the gut becomes
paralysed and the larva ceases to feed.
The ICP structure and function have been reviewed in detail by
Schnepf et al. (1998). Binding of the ICP to putative receptors is a
major determinant of ICP specificity and the formation of pores in the
midgut epithelial cells is a major mechanism of toxicity (Van
Frankenhuyzen, 1993).
The active toxin consists of three distinct domains (Höfte &
Whiteley, 1989; Li et al., 1991; Grochulski et al., 1995). The three
domains interact in a complex manner, but experimental data suggest
that the C-terminal and middle domains of the toxin are involved in
epithelial cell receptor binding and structural functions, while the
N-terminal domain is primarily involved in ion channel and pore
formation (Huber et al., 1981; Schnepf et al., 1998; Dean et al.,
1996).
 Binding to specific receptors has been demonstrated to be closely
related to the insecticidal spectrum of the ICPs (Denolf et al.,
1997). Van Rie et al. (1989) found the affinity of these toxins
similar for the tobacco budworm (Heliothis virescens) and the tomato
hornworm (Manduca sexta) brush border membrane vesicles, but the
number of binding sites differed and reflected varied bioactivity.
However, the toxin affinity for binding sites does not appear constant
for all insects.
Pore or ion channel formation occurs after the binding to the
receptor and insertion of the N-terminal domain into the membrane,
whereby the regulation of the trans-membrane electric potential is
disturbed. This can result in colloid-osmotic lysis of the cells,
which is the main cytolytic mechanism that is common to all ICPs
(Knowles & Ellar, 1987; Slatin et al., 1990; Schwartz et al., 1991;
Schnepf et al., 1998). When the midgut epithelium of the larva is
damaged, the haemolymph and gut contents can mix. This results in
favourable conditions for the Bt spores to germinate. The resulting
vegetative cells of Bt and the pre-existing microorganisms in the gut
proliferate in the haemocoel causing septicaemia, and may thus
contribute to the mortality of the insect larva.
3.3 Resistance of insect populations
A number of insect populations of several different species with
different levels of resistance to Bt have been obtained by laboratory
selection experiments during the last 15 years (Schnepf et al., 1998).
The species include Plodia interpunctella, Cadra cautella,
Leptinotarsa decemlineata, Chrysomela scripta, Tricholplusia ni,
Spodoptera littoralis, Spodoptera exigua, Heliothis virescens,
Ostrinia nubilalis and Culex quinquefasciatus (Schnepf et al.,
1998) and resistance is shown to either Btk, Bti, Btte or other Bt
subspecies.
During the last few years populations of the diamondback moth,
Plutella xylostella, resistant to Btk and Bta have been found in
heavily treated areas in numerous geographically isolated regions in
the world, including Hawaii, Phillippines, Indonesia, Malaysia,
Central America and some USA states (Schnepf et al., 1998). It is
clear that this widespread appearance of resistance to Bt presents a
cautionary tale for the way of using Bt and Bt toxin genes in pest
management. Schnepf et al. (1998) have reviewed resistance management
of Bt.
4. NATURAL AND TREATED HABITATS
The Bt subspecies represents a group of organisms that occur
naturally and can be added to an ecosystem to achieve insect control
(Andrews et al., 1987; Stahly et al., 1991). In this monograph, a
natural habitat is considered to be one where Bt can be isolated when
there has been no previous history of application of the organism to
that ecosystem, whereas a treated habitat is one where Bt can be
isolated after a previous history of application of the organism for
insect control.
Insecticides formulated with Bt are being manufactured and used
worldwide. These commercial Bt products may be applied as an
insecticide to foliage, soil, water environments and food storage
facilities. After application of Bt to an ecosystem, the organism may
persist as a component of the natural microflora.
4.1 Natural occurrence of Bt
Members of the Bacillus cereus group can be found in most
ecological niches. Hansen et al. (1996) reviewed the occurrence of Bt
in the environment. Although the early Bt isolates were pathogenic for
insects, it is now apparent that several Bt isolates have no known
target (Ohba & Aizawa, 1986; Ohba et al., 1988; Hansen et al., 1996,
1998; Damgaard et al., 1997b). This lack of insecticidal activity may
be attributed to the loss of ability to produce ICPs (Gordon, 1977),
which may be due to a mutation in the ICP gene that could prevent
expression (Klier & Lecadet, 1976; Stahly et al., 1978; Dean, 1984) or
to the loss of ICP encoding sequences. Finally, the lack of known
activity of a Bt crystalline toxin might simply be explained by a
failure to test against the actual target organism. The list of Bt
targets is still increasing. Although our knowledge of the activity of
Bt populations in the environment is limited, a certain level of
turn-over and vegetative growth must occur, as annual and seasonal
variations in numbers and subspecies diversity of Bt populations have
been observed (Damgaard et al., 1997b; Kim et al., 1998).
4.1.1 Bt in insect hosts
Numerous Bt subspecies have been isolated from dead or dying
insect larvae and in most cases the isolate has toxic activity to the
insect from which it was isolated (Goldberg & Margalit, 1977; de
Barjac, 1981b; Hansen et al., 1996). These organisms have a narrow
host range in the orders Coleoptera, Diptera and Lepidoptera and
can proliferate within the bodies of their host insects. When the
infected insect larva dies, the dead insect carcass usually contains
relatively large quantities of spores and crystals that may be
released into the environment (Prasertphon et al., 1973; Grassi &
Deseö, 1984; Aly, 1985; Aly et al., 1985). Growth of Bt in non-target
organisms has also been described. Eilenberg et al. (in press) found
that Bt multiplication had occurred in non-target insects, which were
also infected by insect pathogenic fungi.
Akiba (1986) reported recycling of naturally occurring Bt in
insect cadavers when competitive microorganisms were at a low density.
Outbreaks of Bt in susceptible insect populations occur relatively
infrequently; most outbreaks have been limited to situations where the
insect density is relatively high, providing better opportunity for
establishing the disease within the insect population (Lynch et al.,
1976; Burges & Hurst, 1977; Vaòková & Purrini, 1979; Margalit & Dean,
1985).
4.1.2 Bt in soil
The spores of Bt persist in soil, and vegetative growth occurs
when nutrients are available (DeLucca et al., 1981; Akiba, 1986; Ohba
& Aizawa, 1986; Travers et al., 1987; Martin & Travers, 1989).
DeLucca et al. (1981) found that Bt represented between 0.5% and
0.005% of all Bacillus species isolated from soil samples in the
USA. Martin & Travers (1989) recovered Bt from soils globally. Meadows
(1993) isolated Bt from 785 of 1115 soil samples, and the percentage
of samples that contained Bt ranged from 56% in New Zealand to 94% in
samples from Asia and central and southern Africa. Ohba & Aizawa
(1986) isolated Bt from 136 out of 189 soil samples in Japan.
4.1.3 Bt on plant surfaces
Bt has been found extensively in the phylloplane. Numerous Bt
subspecies have been recovered from coniferous trees, deciduous trees
and vegetables, as well as from other herbs (Smith & Couche, 1991;
Damgaard et al., 1997b). The Bt isolates have demonstrated a broad
diversity both with specific activities to insects from the orders
Coleoptera and Lepidoptera and with unknown activities (Smith &
Couche, 1991; Damgaard et al., 1997b; Hansen et al., 1998). The
bacterium has also been isolated from stored grain products (Meadows
et al., 1992).
4.2 Treated habitats
Treated habitats are the locations where Bt insecticides (usually
a mixture of spores and crystals) have been applied.
In Canada, Meadows (1993) estimated that approximately 1015
viable Btk spores per ha were released in a typical spray operation to
control spruce budworm (Choristoneura fumiferana).
4.3 Environmental fate, distribution and movement
Bt, like other members of the genus Bacillus, has the ability
to form endospores that are resistant to inactivation by heat and
desiccation and that persist in the environment under adverse
conditions (Stahly et al., 1991). When considering the degradation of
Bt in the environment, it is important to distinguish between changes
in the numbers of viable spores and changes in biocidal activity. The
survival and activity in the environment has been reviewed by Hansen
et al. (1996).
The distribution and environmental transport of applied Bt
formulations are influenced by the type of application and various
environmental factors (Bulla et al., 1985; Andrews et al., 1987). Bt
formulations are used in agriculture and forestry against coleopteran
and lepidopteran pests and are usually directed towards the surface of
plants, while the Bt formulations for control of dipteran pests
(mosquitos and blackflies) are applied to their aquatic, larval
habitats. Many Bt insecticides exhibit poor stability under field
conditions, and so frequent reapplication is required (Griego &
Spence, 1978; Sorenson & Falcon, 1980; Beegle et al., 1981).
4.3.1 Distribution and fate of Bt in terrestrial habitats
4.3.1.1 Fate of Bt and ICP on plant surfaces
Solar radiation appears to be the environmental factor most
damaging to the stability of Bt ICP (Pinnock et al., 1974; Pinnock et
al., 1977; Griego & Spence, 1978; Pusztai et al., 1991).
Griego & Spence (1978) demonstrated that Bt spores are
inactivated rapidly when exposed to UV radiation, while Pusztai et al.
(1991) demonstrated that the tryptophan residues of the Bt protoxin
are damaged by solar radiation in the 300-380 nm range.
The combined effect of sunlight, leaf temperature and vapour
pressure deficit appeared to contribute more to the reduction of
bioactivity than any other single factor (Leong et al., 1980). Residue
bioactivity may be detected up to 2 weeks after treatment with
formulations containing UV protectants (Hostetter et al., 1975). Other
studies on the effect of environmental exposure to Bt spores revealed
that spore survival can be affected by the surface to which the
material is applied.
Pinnock et al. (1974) reported that the half-life of Bt spores on
leaves of California live oak (Quercus agrifolia) was 3.9 days, as
compared to a half-life of 0.63 days when applied to leaves of western
redbud (Cercis occidentalis).
Ignoffo (1992) summarized data for the reduction of spore
viability and ICP activity on leaves of various plants in sunlight: Bt
spore viability was reduced 80% in one day on red cedar leaves and 8%
on soy bean leaves, while the ICP activity declined by 20% on red
cedar leaves but 65% on soy bean leaves.
Dent (1993) reported that Bt formulations on foliage frequently
have half-lives of up to 10 days. However, unformulated Bt may have a
half-life of only a few hours. Pedersen et al. (1995) found that the
initial spore half-life was 16 h during the first week after spraying
cabbage with unformulated Btk.
There is also evidence that plant chemicals can inactivate Bt or
influence infectivity. Lüthy (1986) demonstrated that extracts
prepared from cotton leaves could inactivate ICPs.
Commercially applied Bt may persist at low levels for
considerable periods of time. Reardon & Haissig (1983) reported that
Btk was still present on balsam fir (Abies balsamea) one year after
applications to control spruce budworm. The proliferation of spores
through infection of susceptible insects should not be discounted as a
source of low levels of Bt in treated areas. Several studies have
demonstrated that Bt is able to grow and sporulate in insect cadavers
(Meadows, 1993). From dead Egyptian cotton leafworm (Spodoptera
littoralis), Jarrett & Stephenson (1990) isolated between 5.0 × 105
and 9.2 × 107 spores per larva.
Bt may be lost to the soil by overspray during application or by
the action of rain on plant surfaces. Further losses arise from in
situ degradation by environmental factors, such as ultraviolet (UV)
radiation and microbial activity (Griego & Spence, 1978; Sorenson &
Falcon, 1980; Beegle et al., 1981; West et al., 1984a,b). Pedersen et
al. (1995) found that Bt was dispersed by rain splash from the soil to
the lower leaves of cabbage.
4.3.1.2 Fate of Bt in soil
Petras & Casida (1985) reported that Bt spore counts in soil
declined by a factor of ten in the first 2 weeks after application and
then remained constant for 8 months. The response was similar in
spores from commercial and laboratory cultures. In contrast,
vegetative cells introduced into the soil persisted for only a short
time. Soil pH had little effect on their survival. Spore persistence
for more than 2 weeks apparently resulted from the inability of the
spores to germinate in the soil.
Pedersen et al. (1995) sprayed unformulated Btk (1.2 5 104 cfu
per g soil, spontaneous rifampicin-resistant mutant) on soil in 1993,
and 2.3 × 103 cfu per g soil remained after 336 days. The field was
left undisturbed, and 5´ years later spots with 1.5 × 103 Btk per g
soil were found (Hansen & Hendriksen, 1999), but spots with very low
Btk numbers were also recorded. These data indicate that the Btk had
multiplied.
West et al. (1984a,b) reported that vegetative cells in soil
disappeared at a rapid, exponential rate, whereas parasporal crystals
disappeared at a slower, non-exponential rate, and spore numbers
remained unaltered through 91 days of incubation at 25°C, with no
detectable germination. The proteinaceous crystalline protoxin of Bt
has been shown to be degraded by soil microorganisms at an exponential
rate with a half-life of about 3-6 days.
Saleh et al. (1970) reported that Bt spores could remain viable
for several months in the soil and germinate when soil conditions
favoured bacterial growth.
Bt spores do not appear to germinate readily in most soils and
the crystalline protoxins are metabolized by other microorganisms.
West et al. (1984a) reported that Bta in soil showed an exponential
loss of insecticidal activity. The rate of loss was greater in
non-sterile soil than in autoclaved soil. There was an initial rapid
decrease, which stabilized at approximately 10% of the original
inoculum level after 250 days incubation, until the cessation of
sampling after two years. No loss of insecticidal activity was
observed in autoclaved soil, which suggests that soil microorganisms
were responsible for the loss of insecticidal activity in the natural,
non-sterilized soil.
Several studies determined that Bt did not grow under most
natural soil conditions (West et al., 1984a,b; Akiba, 1986). The data
suggested that this was attributable to a failure of Bt spores to
germinate in soil under these conditions. The spore is the only state
in which Bt persists in natural soils.
An environmental fate study demonstrated no significant spore
accumulation in either the organic or the mineral layers of soil over
an 11-month period when Bt was applied aerially at 100 times the
concentration used for operational programmes (Bernier et al., 1990).
Studies have indicated that Bt is relatively immobile in soil.
Martin & Reichelderfer (1989) found no vertical movement beyond a 6-cm
deep zone in soil and less than 10 m lateral movement, even along
drainage courses.
Akiba (1991) reported a one-month irrigation study simulating the
summer rainy season in Japan. There was no translocation of Bt below a
depth of 10 cm. In soils receiving 45 cm simulated rainfall, Bt was
detected to a depth of 3-6 cm. In tests using soil columns, Bt did not
pass through a column of volcanic ash soil but a few spores were
detected in flow-through water from an alluvial sand column. Results
suggested that the major factor causing a decrease in the level of Bt
was not a physical dilution due to the rainwater, but possibly an
affinity of the spores for the soil particles.
Venkateswerlu & Stotsky (1992) reported that adsorption and
binding of Bt toxin proteins to soil particles were greater on
montmorillonite than on kaolinite clays. Maximum adsorption occurred
within 30 min, and adsorption was not significantly affected by
temperature between 7°C and 50°C.
4.3.2 Distribution and fate of Bt in aquatic habitats
Bti is often applied directly to water for the control of
mosquitos and blackflies. Rapid sedimentation in all but the fastest
flowing streams is regarded as an important limitation on the efficacy
of such applications.
Sheeran & Fisher (1992) demonstrated that the sedimentation of
Bti is facilitated by adsorption onto particulate material.
Ohana et al. (1987) found that spores may persist for at least
22 days in sediments, and the spores may be mobilized with such
sediments during floods.
Btk has been reported to survive in fresh water and in seawater
for more than 70 and 40 days, respectively, at 20°C (Menon & De
Mestral, 1985). A higher percentage of Btk was found to survive for
extended periods in lake water than in tap and distilled water,
presumably due to the presence of more nutrients in lake water. Bt has
not been isolated from any drinking-water supplies.
Spores of Bti remained viable for shorter periods when suspended
in moving water than when in static bottles, indicating that static
laboratory trials may overestimate the longevity of these spores in
the environment (Yousten et al., 1992).
Carcases of mosquito larvae killed by Bti have been shown to
allow for the complete growth cycle (germination, vegetative growth
and sporulation), thus becoming toxic themselves to scavenging yellow
fever mosquito (Aedes aegypti) larvae (Khawaled et al., 1990).
Contact of Bti with mud can result in an immediate disappearance
of larvicidal activity, but it has little influence on spore viability
(Ohana et al., 1987). The cessation of toxicity was found to be caused
by bacterial adsorption to soil particles, but the inactivation could
be reversed by washing the mud away.
Special Bti formulations have been developed to prolong residence
time of Bt at the surface or in the water column, where target insects
feed.
Manasherob et al. (1998) found germination, growth and
sporulation of Bti in excreted food vacuoles of a protozoan.
4.3.3 Transport of Bt by non-target organisms
In a field trial where Btk was sprayed on cabbage and soil,
Pedersen et al. (1995) found that the Btk could be transported by
non-target insects. Up to 103 cfu per g were found on surface-active
insects, and carabid beetles carrying Btk were found up to 135 m from
the Btk-treated area.
In a study of interactions between Bti and fathead minnows
(Pimephales promelas), ingestion of Bt resulted in a high number of
recoverable spores in the gastrointestinal tract and faeces (Snarski,
1990). Bti spore counts decreased rapidly after test fish were
transferred to clean water, but spores were detected in low numbers in
faeces for over 2 weeks. The data indicated that minnows could
disperse Bt spores in the freshwater environment.
Meadows (1993) reported that, after the application of Bt on
land, it may be dispersed by birds and mammals feeding on infected
target insects. Some Bt-infected insect larvae may contain 6.6 × 108
to 4.2 × 109 spores per gram dry mass (Burges & Hurst 1977).
5. COMMERCIAL PRODUCTION
5.1 History of Bt and its commercial applications
5.1.1 Production levels
Conventional Bt products, which utilize naturally occurring or
modified Bt strains, account for approximately 90% of the world MPCA
market (Bernhard & Utz, 1993). Current annual production of Bt has
been estimated at 3000 or more tonnes in developed countries. In
China, up to 10 000 tons are produced annually (personal communication
by Guan Xiong, Fujian Agricultural, University, 1997).
5.1.2 Production processes, formulations and quality standards
Bt products are produced using fermentation technology (Bernhard
& Utz, 1993). Most commercial products contain ICP and viable Bt
spores, but the spores are inactivated in some Bti products. During
commercial-scale production of Bt products, there is little loss of
bioactive components to the environment. The type of loss incurred is
a function of the recovery method involved. No significant amount of
bioactive component is lost from fermenter harvests if filtration is
used to separate the insoluble solids (active ingredients) from the
soluble liquid (inert) fraction of the harvest liquor, as shown by
complete lack of bioactivity in the resulting liquid fraction. The
liquid waste fractions may contain a low concentration of insoluble
active component, but this is typically inactivated by processing in
on-site waste treatment facilities. Although it is not a physical
loss, measurable bioactivity is diminished if the recovered active
material is processed through a dryer, due to the exposure of the
bioactive components to the high temperatures required for drying.
Guidelines for the handling of microorganisms during manufacture have
been reviewed by Frommer et al. (1989).
Commercial Bt formulations include wettable powders, suspension
concentrates, water dispersible granules, oil miscible suspensions,
capsule suspensions and granules (Tomlin, 1997).
Quality standards for Bt fermentation products have been accepted
by IUPAC (Quinlan, 1990). These standards include limits on the
concentration of microbial contaminants and metabolites (Table 5).
In most cases strains of Bt that produce beta-exotoxins are not
approved for commercial application, although some commercial use has
been approved for control of certain fly species that are not
susceptible to ICPs (Carlberg et al., 1985).
Table 5. Maximum allowable levels of microbial contamination in
bacterial insecticides (IUPAC Recommendation; Quinlan, 1990)
Types of microorganisms Maximum concentrations
Viable mesophiles <1 × 105/g
Viable yeasts and moulds <100/g
Coliforms <10/g
Staphylococcus aureus <1/g
Salmonella <1/10g
Lancefield Group D Streptococci <1 × 104/g
5.1.3 General patterns of use
Commercial applications of Bt have been directed mainly against
lepidopteran pests of agricultural and forest crops; however, in
recent years strains active against coleopteran pests have also been
marketed (Table 6).
Strains of Bti active against dipteran vectors of parasitic
disease organisms have been used in public health programmes.
5.1.3.1 Applications in agriculture and forestry
Commercial use of Bt on agricultural and forest crops dates back
nearly 30 years, when it became available in France. Use of Bt has
increased greatly in recent years and the number of companies with a
commercial interest in Bt products has increased from four in 1980 to
at least 18 (Van Frankenhuyzen, 1993). Several commercial Bt products
with Bta, Btk or Btte have been applied to crops using conventional
spraying technology (Table 6). Various formulations have been used on
major crops such as cotton, maize, soybeans, potatoes, tomatoes,
various crop trees and stored grains. Formulations have ranged from
ultralow-volume oil to high-volume, wettable powder and aqueous
suspensions. In the main, naturally occurring Bt strains have been
used, but transgenic microorganisms expressing Bt toxins have been
developed by conjugation and by genetic manipulation, and in some
cases, these have reached the commercial market. These modified
organisms have been developed in order to increase host range, prolong
field activity or improve delivery of toxins to target organisms. For
example, the coleopteran-active cryIIIA gene has been transferred to
a lepidopteran-active Btk (Carlton et al., 1990). A plasmid bearing an
ICP gene has been transferred from Bt to a non-pathogenic
leaf-colonizing isolate of Pseudomonas fluorescens; fixation of the
transgenic cells produces ICP contained within a membrane which
prolongs persistence (Gelernter, 1990). The gene expressing cryIA(c)
ICP has been inserted in Clavibacter xyli subspecies cynodontis, a
bacterium that colonizes plant vascular systems. This has been shown
to deliver the ICP effectively to European corn borer (Ostrinia
nubilalis) feeding within plant stems (Beach, 1990). Improvements in
performance arising from such modifications are such that transgenic
organisms and their products are likely to be used much more widely in
the future.
5.1.3.2 Applications in vector control
Bti has been used to control both mosquitos and blackflies in
large-scale programmes (Lacey et al., 1982; Chilcott et al., 1983;
Car, 1984; Car & de Moor, 1984; Cibulsky & Fusco, 1987; Becker &
Margalit, 1993; Bernhard & Utz, 1993). For example, in Germany 23
tonnes of Bti wettable powder and 19 000 litres of liquid concentrate
were used to control mosquitos (Anopheles and Culex species)
between 1981 and 1991 in the Upper Rhine Valley (Becker & Margalit,
1993). In China, approximately 10 tonnes of Bti have been used in
recent years to control the malarial vector, Anopheles sinensis.
The Onchocerciasis Control Programme of West Africa used more
than five million litres of Bti from 1982 to 1997 to control
blackflies (Simulium damnosum), the vector of the onchocerciasis
filarial worm (Onchocerca volvulus), on the Upper Volta River
System. The Programme was initially based solely on the control of the
vector, Simulium damnosum sensu lato, by spraying the insecticide
at breeding sites on river systems, where larval stages develop. At
the peak of larvicidal activities about 50 000 km of rivers were
treated in an area of over one million km2. The purpose was to
interrupt the transmission of the parasite Onchocerca volvulus. The
interruption of the cycle is achieved by destroying larval stages
through aerial application of insecticides to breeding sites.
Insecticide application was undertaken weekly (Moulinier et al.,
1995). In order to assess the environmental impact of such treatments
a network of sampling stations throughout the programme area were
established.
Formulations of Bti range from wettable powder and fluid
concentrates applied via conventional spray equipment from ground and
air to slow-release briquet and tablet formulations. Examples of
commercial Bti products are listed in Table 6.
Table 6. Examples of commercial Bt products used against agricultural, forestry and public health pests
(Tomlin, 1997; see also the Internet site http://www.sipweb.org/bacteria.htm)
Bt sub-species Commercial products Producer
Bt (not defined) Rijin Scientific & Technological Development
Bt (not defined) Bitayon Jewin-Joffe Industry Ltd
Bt (not defined) Delfin, Thuricide SDS Biotech KK
Btk Bactospeine, Biobit, Dipel, Foray Abbott (USA)
Bollgard Ecogen/Crop Care
Bactucide Caffaro
Baturad Cequisa
Condor, Crymax, Cutlass, Ecogen
Foil, Jackpot, Lepinox,
Rapax, Raven
Jackpot, Lepinox, Rapax Intrachem
Cordalene Agrichem
Larvo Troy
Costar, Delfin, Design, Novartis/Thermo Trilogy Co
Javelin, Steward, Thuricide,
Vault
Ecotech Bio, Ecotech Pro Ecogen/Roussel-Uclaf
Halt Wockhardt
Bta Xentari, Florbac Abbott
Certan Novartis
Btte Novodor Abbott
Bti Bactimos, Gnatrol, Vectobac Abbott
Acrobe Cyanamid
JieJueLing, MieJueLing Huazhong Agricultural University
Teknar Novartis/ThermoTrilogy Co
Bt Ybt-1520 Mianfeng pesticide Huazhong Agricultural University
Bt chinesensis Shuangdo preparation Huazhong Agricultural University
Btg Spicturin Tuticorin Alkali Chemicals and
Fertilisers Ltd
6. EFFECTS ON ANIMALS
6.1 Mammals
Microbial pest control agents (MPCA) can, in principle, cause
harmful effects via toxicity, inflammation, or a combination of these
effects. The presence of bacteria in a specimen derived from tissues
does not necessarily mean infection. Colonization refers to the
multiplication of MPCA either on the surface or within an animal/human
organism without causing any tissue damage. Persistence refers to
the ability to recover the inoculum of the MPCA over time. Persistence
and transient disturbances of the normal microbial flora are to be
expected after exposure of experimental animals to MPCA, since
clearance of the inoculum is not instantaneous. Persistence may not be
equated with infection (Siegel & Shadduck, 1990). Infection by a
MPCA means that there is evidence of the establishment and
proliferation of the MPCA in tissues, coupled with tissue damage.
Evidence of multiplication includes a measurable increase in the total
amount of MPCA, recovery of vegetative stages when spores were
administered, and failure of the inoculum to clear. It cannot be
determined solely on the basis of lesions since injection of foreign
material can elicit an inflammatory process (Siegel et al., 1987).
A classification of MPCA toxicity and infectivity has been
proposed, in which MPCA is classified as toxic if an oral dose
< 106 cfu per mouse causes mortality or clinical or pathological
changes (Burges, 1980). However, any classification is very difficult
because of the complexicity of the issue when dealing with living
organisms (Ignoffo, 1973; Shadduck, 1983).
Older reports do not discriminate between different strains of Bt
but modern molecular techniques have proven that variability exists
within strains with the same serotype (Helgason et al., 1998; Hansen &
Hendriksen, 1997a,b).
Mammalian toxicity studies on Bt-containing pesticides
demonstrate that the tested isolates are not toxic or pathogenic
(McClintock et al., 1995), as they occur in the products. Toxicity
studies submitted to the US Environmental Protection Agency to support
registration of Bt subspecies, and reviewed by McClintock et al.
(1995), failed to show any significant adverse effects on body weight
gain, clinical observations or upon necropsy.
Infectivity/pathogenicity studies have shown that the intact rodent
system responds as expected to eliminate Bt gradually from the body
after oral, pulmonary or intravenous challenge. However, clearance of
Bti and Btk is not instantaneous. An intact immune system is not a
prerequisite for clearance of Bti and Btk.
6.1.1 Oral exposure
In studies conducted with a single oral dose of laboratory grown
Bt and commercial Bt formulations, there was no mortality associated
with ingestion of Bti or Btk in mice and rats (Fisher & Rosner, 1959;
de Barjac et al., 1980; Shadduck, 1980; Siegel et al., 1987)
(Table 7).
Additionally, in data summarized by McClintock et al. (1995), no
toxicity or infectivity was observed following oral administration of
various Bt subspecies at doses of up to 4.7 × 1011 cfu/kg in rats.
In a study involving repeated oral exposure of mice and rats for
21 days with laboratory grown Bti, there was no mortality associated
with ingestion of Bti and normal weight gain was observed in all
treated rodents (de Barjac et al., 1980) (Table 8).
Hadley et al. (1987) conducted a study in which sheep were
repeatedly treated with two commercial Btk formulations for 60 days.
The only clinical sign was loose stools in sheep exposed to one Btk
formulation. There was also microscopic evidence of moderate to marked
lymphoid hyperplasia of the Peyer's patches in the caecum and colon of
two out of six sheep treated with one Btk formulation and in one out
of six sheep treated with the other Btk formulation. The authors did
not consider these findings clinically significant.
Other multiple dose studies with Bt were summarized by McClintock
et al. (1995). In rats, no toxicity or infectivity was associated with
dietary exposure to Bti (4 g/kg per day ) for 3 months. Administration
of 1.3 × 109 Btk spores/kg per day to rats by oral gavage was not
toxic or infectious. McClintock et al. (1995) also reported the
results of a 2-year study in which a commercial Btk preparation was
fed to rats at 8400 mg/kg per day in the diet. Despite this excessive
dose, the only effect observed was a decrease in body weight of
females during weeks 10-104 of the study.
6.1.2 Inhalation exposure
These tests primarily address the potential infectivity of a
MPCA. Inhalation is a likely route by which humans and animals may be
exposed to Bt during application.
De Barjac et al. (1980) exposed 10 female Swiss mice for 12 min
to 2 × 108 Bti spores (48-h laboratory grown whole culture). The mice
were monitored for clinical signs for 15 days, and then killed. The
lungs were removed aseptically and cultured for bacteria, but no Bti
was recovered.
Siegel et al. (1987) exposed 27 female Sprague-Dawley rats to
2 × 106 spores of a commercial Bti formulation for 30 min. Rats were
serially killed over a 27-day period and the lungs were cultured.
Table 7. Acute toxicity (single oral exposure) of Bt in experimental animals
Subspecies Material tested Test animal Dosea Mortalityb Reference
tested
Btk Washed cells, 24-h culturec Rat, female, Sprague-Dawley 1.4 × 107cfu 0/6 Shadduck, 1980
Btk Commercial product Ratd 2 × 1011cfu 0/10 Fisher & Rosner, 1959
Bti 48-h culturec Mouse, female, Swiss 1.7 × 108cfu 0/20 de Barjac et al., 1980
Bti 48-h culturec Rat, female, Wistar 3.4 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellsc, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rat, female, Sprague-Dawley 4 × 107cfu 0/10 Siegel et al., 1987
a All doses given are per animal
b Number dead/number treated
c Laboratory grown culture
d Breed and sex unknown
Table 8. Repeated dose (oral exposure) toxicity of Bt in mice, rats and sheep
Subspecies Material tested Test animal Dose Exposure Mortalitya Reference
tested time
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Bti 48-h cultureb Mouse, female, Swiss 4.7 × 1010cfu 21 days 0/20 de Barjac et al., 1980
Bti 48-h cultureb Rat, female, Wistar 1.2 × 1011cfu 21 days 0/10 de Barjac et al., 1980
a Number dead/number exposed
b Laboratory grown culture
Recovery of Bti declined from 5.92 × 103 cfu/g lung tissue at 3 h
after exposure to none detected at 7 days after exposure. No gross
lung lesions were observed.
Fisher & Rosner (1959) exposed 10 mice to 3 × 1010 spores of a
commercial Btk product 4 times in a 6-day period. The Btk-treated mice
exhibited no clinical signs during the treatment period and no gross
pathological changes at necropsy.
6.1.3 Dermal exposure
This test is similar to the dermal exposure tests used in
chemical toxicology. Bt does not have any external contact toxicity
due to its mode of action, as shown in the following studies.
De Barjac et al. (1980) applied 5.1 × 107 cfu Bti of a 48-h
laboratory grown culture to the skin of 20 female Swiss mice. No
mortality was observed and there was no evidence of skin inflammation.
Other studies, reviewed by McClintock et al. (1995), indicate
that Bt was not toxic or pathogenic to rabbits following dermal
exposure to various Bt subspecies at doses of up to 2500 mg/kg. In
some cases, mild irritation was observed.
6.1.4 Dermal scarification exposure
This test evaluates both the potential toxicity and infectivity
of a MPCA. In the case of Bt, toxicity is unlikely due to its mode of
action. However, this test also evaluates the importance of intact
skin in preventing infection by Bt.
Fisher & Rosner (1959) scarified the skin of 4 rabbits, then
applied 2.2 × 106 cfu of a commercial Btk formulation. No skin
inflammation or sign of infection was observed.
De Barjac et al. (1980) applied 3.3 × 1013 cfu of a commercial
Bti formulation to the skin of 6 male New Zealand White rabbits. No
skin inflammation or sign of infection was observed.
6.1.5 Subcutaneous inoculation
This route of exposure is considered a more challenging test of
potential infectivity than oral or dermal exposure, because the
barrier of the skin is breached. However, subcutaneous exposure may
take place only if the skin is damaged by the spraying or is already
otherwise damaged.
De Barjac et al. (1980) subcutaneously inoculated 20 female Swiss
mice and 10 tricolour guinea-pigs, respectively, with 8.5 × 107 cfu
and 1.7 × 108 cfu of a 48-h laboratory-grown Bti culture. There was
no evidence of infection and no mortality was observed.
Siegel et al. (1987) subcutaneously inoculated 15 female BALB/c
mice with 1 × 109 cfu of a commercial Bti formulation. Abscesses
occurred at the injection site but these were attributed to the
introduction of high concentrations of heat-stable foreign material,
since they also occurred when autoclaved Bti was injected. There was
no evidence of infection and no mortality was observed.
6.1.6 Ocular exposure
The primary purpose of this procedure is to test for the
irritancy of a MPCA, although this test also evaluates potential
infectivity as well. In these tests, Bt may persist for days in rabbit
eyes because of the depth of the conjunctival sac coupled with limited
tear production by rabbits.
De Barjac et al. (1980) inoculated the eyes of six male New
Zealand White rabbits with 3.7 × 107 cfu of a 48-h laboratory-grown
Bti culture. No conjunctivitis or ocular irritation was observed.
Siegel & Shadduck (1990) inoculated 12 female New Zealand White
rabbits with 5.4 × 106 cfu of a commercial Bti formulation. No ocular
irritation or conjunctivitis was observed and no Bti was recovered by
swabbing after one week.
In data reviewed by McClintock et al. (1995), only mild
irritation was observed following ocular administration of certain Bt
subspecies to rabbits.
Siegel et al. (1987) inoculated the eyes of 6 male New Zealand
White rabbits with 50 mg of a dry powder-commercial Bti formulation
for 9 days, and another 6 male New Zealand White rabbits were treated
with 50 mg of a laboratory-grown Bti culture for 9 days. No ocular
irritation or conjunctivitis was observed in the rabbits that received
the commercial powder. The rabbits that received the laboratory
culture experienced severe conjunctival congestion and discharge. This
was not attributed to Bti but rather to the nature of the inoculum.
The laboratory strain was a dry paste with hard clumps while the
commercial formulation was a soft powder.
6.1.7 Intraperitoneal exposure
The administration of a MPCA by this route is considered a highly
challenging route of exposure. Human and animal exposure to Bt by this
route is very unlikely to occur during the course of normal
application of Bt. This route evaluates the ability of Bt to cause
infection or produce toxic metabolites in the peritoneal cavity. Some
of the safety studies that utilized this route of exposure also
evaluated the clearance of Bt over time (Table 9).
Additional studies employing mice have been conducted using this
route of exposure, which evaluates the role played by an intact immune
system in preventing infection by Bt. These studies were deemed
necessary to assess the risk posed by Bt to humans undergoing
immunosuppressive chemotherapy and the risk posed by Bt to humans
infected with the human immunodeficiency viruses. Immune suppression
in mice was accomplished either by use of corticosteroids, which
inhibited helper T-cells and selectively damaged B-cell activity, or
through the use of athymic mice, which lack the functional T
lymphocyte component of their immune system.
6.1.7.1 Immune-intact animals
De Barjac et al. (1980) intraperitoneally injected 100 female
Swiss mice with 3.4 × 107 cfu of a 48-h laboratory-grown Bti culture
and killed groups of 10 mice daily (Table 9). Blood samples were taken
by cardiac puncture and Bt was recovered until day 8. No mortality was
observed.
Fisher & Rosner (1959) intraperitoneally injected 30 mice of
unspecified sex and strain with a laboratory-grown Btk culture and
withdrew cardiac blood samples 24, 48 and 72 h after injection. There
was no mortality and Btk was recovered as late as 48 h after injection
from heart blood.
Siegel & Shadduck (1990) conducted three clearance studies using
female CD-1 mice. In one experiment, 33 females were injected with
2.7 × 107 cfu of a washed commercial Bti formulation and serially
killed over 80 days. Bti did not clear and was recovered from the
heart blood on days 67 and 80. The investigators noted that the
initial inoculum was composed of approximately 95% vegetative cells
and that vegetative cells take longer to clear than spores. This was
confirmed in a follow-up experiment in which two groups of 16 females
each were injected with inocula containing 1.5 × 107 cfu of spores
only or a 25% vegetative cell and 75% spore mixture. Both inocula
cleared exponentially from the spleens of the mice but the 100% spore
inoculum cleared sooner than did the inoculum that contained
vegetative cells. These experiments demonstrated that Bti and Btk
persist for a variable length of time in mice following injection but
that they are cleared over time. These studies also suggest that the
nature of the inoculum may play a role in the speed by which it
is cleared.
Data summarized by McClintock et al. (1995) indicate that
toxicity (100% mortality in extreme cases) may be observed following
injection of >108 cfu of certain Bt subspecies intraperitoneally
in mice. Lower doses (< 107 cfu/mouse) were non-toxic. Death
generally occurred shortly after injection, indicating that an
infectious process had not occurred. Although the basis for the
toxicity observed at doses >108 cfu is not understood, these
findings are not considered as evidence of a hazard associated with Bt
products, since the route of administration is not relevant to human
and animal exposure conditions.
Table 9. Acute toxicity of Bt after intraperitoneal injection of guinea-pigs, mice and rats
Subspecies Material tested Test animal Dose Mortalitya Reference
tested
Btk Washed cell, 24-h cultureb Rat, female, Sprague-Dawley 1.4 × 109cfu 0/6 Shadduck, 1980
Btk Commercial product Mouse 3 × 109cfu 0/5 Fisher & Rosner, 1959
Bti 48-h cultureb Mouse, female, Swiss 6.8 × 107cfu 0/20 de Barjac et al., 1980
Bti 48-h cultureb Guinea-pig, female, tricolour 1.7 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellb, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rats, male and female Sprague-Dawley 4 × 107cfu 1/20 Siegel et al., 1987
a Number dead/number treated
b Laboratory grown culture
6.1.7.2 Immune-suppressed animals
Siegel et al. (1987) injected 42 female BALB/c mice with 1.25 mg
of a cortisone acetate twice weekly in order to suppress their immune
system and subsequently injected them with 3.4 × 107 cfu of a washed
commercial Bti formulation. Three cortisone-treated mice but none of
the non-cortisone-treated mice died but this mortality was attributed
to injury caused by injection. In the remaining 39 mice Bti was still
recovered in the spleen 49 days after injection. In a companion
experiment, 42 athymic mice were injected with the same dose of a
washed commercial Bti formulation. Twenty-six of the 42 died within 5
to 10 h after injection; autopsy did not reveal the cause of death. In
the surviving mice, Bti was recovered in the spleen 49 days after the
injection. In a follow-up experiment, 30 athymic mice were injected
with 2.6 × 107 cfu of another (washed) commercial Bti formulation and
serially killed over a 36-day period. No mortality occurred. Bti was
still recovered on day 36 after injection.
Siegel & Shadduck (1990) injected 24 athymic mice with 2.7 × 107
cfu of a washed commercial Bti formulation and evaluated clearance
over a 27-day period. No mortality was observed and clearance was
faster in the euthymic than athymic mice. Bti was still recovered 27
days after injection.
These experiments demonstrated that an intact immune system is
not essential to prevent infection by Bti and Btk, but the kinetics of
clearance differ between athymic and euthymic mice as well as between
corticosteroid-treated and untreated euthymic mice. Based on these
data, immune-suppressed individuals do not face any increased risk of
infection by Bt.
6.1.8 Effects of activated Bt ICP
It has been demonstrated that the alkali-solubilized ICP from Bti
is lethal when injected into mice (Thomas & Ellar, 1983).
Alkali-solubilized Bti ICP was also cytolytic to human erythrocytes,
mouse fibroblasts, and primary pig lymphocytes in vitro (Thomas &
Ellar, 1983; Gill et al., 1987). This activity is attributed to a
cytolytic factor encoded by Cyt A gene of Bti. Most other Bt
subspecies lack this gene. Human exposure to activated Bti ICP is most
unlikely.
6.1.9 Studies in wild animals
Numerous studies have been conducted on wild animals as part of
the registration process. Most of the data are proprietary and not
publicly available. No adverse effects have been reported.
In Canada, Innes & Bendell (1989) studied the effect of a
commercial Btk formulation on small mammal populations in woodland.
Populations of eight species of rodents (Clethrionomys gapperi,
Eutamius minimus, Microtus chrotorrhinus, Napaeozapus insignis,
Peromyscus maniculatus, Phenacomys intermedius, Tamias striatus
and Zapus hudsonius) and four species of shrew (Blarina
brevicauda, Sorex cinereus, Sorex fumeus and Sorex hoyii)
were studied by trapping over a 3-month period and shown to be
unaffected when compared to populations from untreated areas. This
suggests that the ingestion of infected insects by shrews had no
immediate effects on their populations.
6.2 Effects on birds
In a number of studies (Table 10), the acute toxicity and
pathogenicity of commercial Bta, Bti, Btk and Btte formulations for
young bobwhite quail (Colinus virginianus) and young mallards (Anas
platyrhynchus), when administered daily by oral gavage at high
dosages, were evaluated (Beavers et al., 1989a,b; Lattin et al.,
1990a,b,c,d; Beavers, 1991a,b). The Bt-treated birds showed no
apparent toxicity or pathogenicity. In those studies which also
evaluated feed consumption and weight gain, the Bt-treated birds
showed no effect when compared with the non-treated controls.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
breeding bird populations (13-14 families, 33-34 species) during a
field trial for spruce budworm control. The bird populations in 10-ha
control and treated plots were measured before and daily for 3 weeks
after application. No differences were detected between the
populations in the control and treated plots.
In the USA, Gaddis & Corkran (1986) evaluated the effect of a Bt
spray programme on the reproductive performance of the chestnut-backed
chickadee (Parus rufescens). This study was undertaken to determine
if secondary effects on the chickadees would result from the possible
reduction of lepidopteran species, which contribute to the diet of
this species. The data showed no treatment-related effect on the
number of eggs per nest, percentage of eggs hatched, percentage of
young fledged, percentage of nests fledging at least one young, or the
body weight of the nestlings.
6.3 Effects on aquatic vertebrates
The World Health Organization (WHO, 1982) reviewed laboratory and
field studies, performed by that time, that examined the impact of Bt
on frogs (Hyla regilla, Rana temporaria), goldfish (Carassius
auratus), mosquito fish (Gambusia affinis), newts (Taricha
torosa, Triturus vulgaris), rainwater killifish (Lucania parva)
and toads (Bufo species). No adverse effects were reported.
Under static renewal conditions, Boeri (1991) exposed rainbow
trout (Oncorhynchus mykiss) to high concentrations (100 mg/litre) of
a commercial Bta formulation for 96 h and observed no adverse effects
(Table 11).
Table 10. Effects of oral 5-day exposure of Bt on birds
Materials Species Dose Results Reference
testeda
Bta Colinus virginianus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991b
pathogenicity observed
Anas platyrhynchus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991a
pathogenicity observed
Bti Colinus virginianus 3077 mg (3.4 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990d
pathogenicity observed
Anas platyrhynchus 3077 mg (6.2 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990b
pathogenicity observed
Btk Colinus virginianus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990a
pathogenicity observed
Anas platyrhynchus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990c
pathogenicity observed
Btte Colinus virginianus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989a
pathogenicity observed
Anas platyrhynchus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989b
pathogenicity observed
a commercial preparation
Under static renewal conditions, Surprenant (1989) exposed
rainbow trout (Oncorhynchus mykiss) to high concentrations (100
mg/litre) of a commercial Btte formulation for 96 h and observed no
adverse effects (Table 11).
During 30- or 32-day static renewal tests, bluegill sunfish
(Lepomis macrochirus), sheepshead minnow (Cyprinodon variegatus)
and rainbow trout (Oncorhynchus mykiss) were exposed to commercial
Bti, Btk or Btte formulations at aqueous and dietary concentrations
from 100 to 500 times the expected environmental concentrations
(Table 11) (Christensen, 1990a,b,c,d,e,f,g,h). The results of these
studies indicated that exposure to very high concentrations of Bti,
Btk and Btte did not adversely affect the survival of these fish, nor
did it produce lesions. In the Btk study, the rainbow trout had a 20%
mortality during the last 4 days of the study (Christensen,1990b).
This effect was attributed to the excessive competition for food that
resulted from poor visibility due to the turbidity and the presence of
suspended solids encountered in the water.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
brook trout (Salvelinus fontinalis Mitchell), common white suckers
(Catostomus commersoni Lacepede) and smallmouth bass (Micropterus
dolomieui Lacepede) during a field trial for spruce budworm control.
The fish populations were assessed visually in underwater surveys
before and after the spray programme. No effect on their populations
was seen.
Two analyses of surveys of the impact of the larvicidal campaign
in the Onchocerciasis Control Programme of West Africa, which compared
fish populations during the programme with the normal yearly
fluctuation, observed little or no effects on the non-target
populations. However, few details were provided (Yameogo et al., 1988;
Levêque et al., 1988; Calamari et al., 1998).
6.4. Effects on invertebrates
6.4.1 Effects on invertebrates other than insects
The World Health Organization (WHO, 1982), reviewed laboratory
and field studies performed up to that time that examined the impact
of Bt on aquatic invertebrates, which included bivalve mollusks
(oyster larvae, Crassostrea gigas, Ostrea edulis), copepods,
decapods, flatworms, isopods, gastropods and ostracods. Of these
organisms, only a few demonstrated any adverse effects.
In Canada, Buckner et al. (1974) evaluated the impact of Btk on a
number of aquatic invertebrates during a field trial for control of
the spruce budworm. Populations of Amphipoda (amphipods), Decapoda
(crayfish), Hydracarina (water-mites), Hirudinea (leeches),
Table 11. Effects of Bt on fish
Material Species Concentration Duration Results Reference
testeda
Bta Oncorhynchus mykiss 100 mg/litre water 96 h No-observed-effect level Boeri, 1991
Btk Lepomis macrochirus 2.9 × 109cfu/litre waterb 32 days No significant toxicity Christensen, 1990a
1.2 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 2.9 × 109cfu/litre waterb 32 days 20% mortality but Christensen, 1990b
1.1 × 1010cfu/g dietc not infectivity
Cyprinodon variegatus 2.6 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990c
3.3 × 109cfu/g dietc or pathology
Bti Lepomis macrochirus 1.2 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990f
1.3 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 1.1 × 1010cfu/litre waterc 32 days No significant toxicity Christensen, 1990g
1.7 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 1.3 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990h
2.1 × 1010cfu/g dietc or pathology
Btte Salmo gairdneri 100 mg/litre water 96 h No-observed-effect level Surprenant, 1989
Oncorhynchus mykiss 1.6 × 1010 cfu/litre waterc 30 days No significant toxicity Christensen, 1990d
1.34 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 9.94 × 109cfu/g diet 30 days No significant toxicity Christensen, 1990e
or pathology
a commercial formulations
b nominal concentration
c measured average concentration
Hydrozoa (freshwater hydra), Nematoda (roundworms), Oligochaeta
(segmented worms), Porifera (freshwater sponges), Pulmonata
(freshwater snails) and Turbellaria (flatworms) were determined by
sampling 14 days prior to and up to 28 days after treatment. The
populations of these aquatic invertebrates were not affected by the
Btk treatment.
Benz & Altwegg (1975) studied the impact of Bt treatment at 100
times the recommended rate on populations of the earthworm Lumbricus
terrestris and found no effect.
Horsburgh & Cobb (1981) reported that populations of the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi
were not affected by biweekly sprays with a commercial Btk product.
Weires & Smith (1977) determined that sprays of a commercial Btk
product on apples during a 4-month season had no effect on the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi,
or on two predatory mites (Amblyseius fallacis and Zetzellia
mali).
6.4.2 Effects on non-target insects
An extensive literature exists on the consequences of exposure of
NTOs to Bt, including reports of several long-term field studies. The
data have been reviewed periodically (e.g., WHO, 1982; Lacey & Mulla,
1990; Melin & Cozzi, 1990; Molloy, 1992; Otvos & Vanderveen, 1993).
The range of non-target species that have been found to be susceptible
to direct toxic action of Bt has remained small. A list of non-target
species found to be insensitive to Btte was issued by Keller &
Langenbruch (1993). In more than 30 years of commercial use, no
serious, direct effects on NTOs have been reported as arising from
Bt-based MPCAs. Several studies which identified effects of Bt on
predators or parasitoids of susceptible insect species are listed by
Navon (1993), but the effects have been small. Mortality in bees has
been observed after exposure to vegetatively growing Bt but the effect
does not seem to be related to spores or ICPs.
6.4.2.1 Aquatic insects
Bti is specific in its toxicity to dipterans. Nevertheless, many
studies have tested the effect of Bti applications on a wide range of
aquatic insects.
Lacey & Mulla (1990) summarized a number of studies of the
effects of Bti on certain non-target arthropod species and arthropod
populations in the laboratory and field (Table 12). The results of
representative studies are summarized below.
Table 12. Effects of Bti on non-target arthropodsa
Arthropod order Type of study Resulta References
Coleoptera Laboratory - Schnetter et al., 1981
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982; Mulla, 1988
Diptera Laboratory + Garcia et al., 1980; Ali, 1981;
(Chironomidae) Ali & Baggs, 1981; Schnetter et al., 1981
Field + Mulla et al., 1971; Ali, 1981;
Mulligan & Schaefer, 1982;
Rogatin & Baizhanov, 1984
Field - Miura et al., 1980
Ephemeroptera Laboratory - Ali, 1980; Schnetter et al., 1981;
Mulligan & Schaefer, 1982
Field - Schnetter et al., 1981; Mulla et al., 1982,
Mulligan & Schaefer, 1982; Mulla, 1988
Heteroptera Field - Schnetter et al., 1981;
(Corixidae) Mulligan & Schaefer, 1982
Heteroptera Laboratory - Schnetter et al., 1981;
(Notonectidae) Olejnicek & Maryskova, 1986;
Aly & Mulla, 1987
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982;
Mulla, 1988
Field + Purcell, 1981
Odonata Laboratory - Mulla & Khasawinah, 1969;
Mulligan & Schaefer, 1982; Aly, 1985;
Aly & Mulla, 1987
Field - Mulla, 1988
a - = no effect reported; + = an effect was reported, but does not necessarily imply that either
individual arthropods or populations of arthropods were adversely affected.
Four species of chironomid larvae (Chironomus crassicaudatus,
Chironomus decorus, Glyptotendipes paripes, Tanytarsus species)
were tested with four Bti preparations. The chironomid larvae were
less susceptible to Bti, being 13- to 75-fold more tolerant than
mosquito larvae to the various Bti preparations (Ali, 1981; Ali &
Baggs 1981). Garcia et al. (1980) induced low to high levels of
mortality in some nematocerous Diptera, including a variety of taxa
in the families Ceratopogonidae, Chironomidae and Dixidae, using
dosages of Bti that were 50 to several hundredfold
higher than concentrations used for mosquito control. Schnetter et al.
(1981) reported complete mortality in chironomid larvae ( Chironomus
thummi ) exposed to high levels of Bti for 48 h without food.
Field-collected adult aquatic beetles exposed to Bti suffered little
or no mortality (Schnetter et al., 1981). Ali (1980) tested a Bti
formulation at 20 times the larvicidal dosage for mosquitos and
reported no adverse effects against larval mayflies (Baetis
species). Schnetter et al. (1981) reported that mayflies (Cloeon
species) suffered no mortality when fed Bti at high dosages.
Aly & Mulla (1987) fed Bti intoxicated mosquito larvae (Culex
quinquefasciatus) to field-collected fourth to fifth instar
backswimmers (Notonecta undulata). The predators were fed at the
rate of 10 larvae per predator per day for 4 days, then the predators
were fed unintoxicated mosquito larvae and observed for 15 to 17 days.
The nymph and adult notonectids exhibited no adverse effects.
Olejnicek & Maryskova (1986) observed no marked mortality in
backswimmers (Notonecta glauca) that were fed Bti intoxicated
mosquito larvae. Schnetter et al. (1981) found no mortality in
backswimmers (Notonecta glauca) exposed for 48 h to high levels of
Bti.
Mosquito larvae intoxicated with extremely high dosages of Bti
were fed to naiads of the dragonfly Tarnetrum corruptum and
damselfly Enallagma civile; the duration of development of the
dragonfly and damselfly naiads, from the time of exposure to
emergence, was not affected (Aly, 1985; Aly & Mulla, 1987)
Merritt et al. (1989) reported no evident of effects on the drift
of aquatic invertebrates, or on the numbers of these invertebrates in
benthic Surber samples, during a blackfly (Simulium species) control
programme. In the USA, Molloy (1992) reviewed ten field trials where
Bti was used against blackfly (Simulium species) larvae. He
concluded that, although there was a potential for adverse impact of
Bti on filter-feeding chironomids, the impact on stream insect
communities overall was very small.
Over a three-season period, Bti administered at mosquito
larvicidal rates had no adverse effects on the larvae of diving
beetles (Dytiscidae) or water scavengers (Hydrophylidae) (Mulla et
al., 1982; Mulla, 1988).
The application of a Bti formulation in a wildlife marsh showed
no adverse effects on beetle larvae (Mulligan & Schaefer, 1982).
Ali (1981) evaluated the efficacy of various levels of a Bti
formulation against chironomids in the families Chironominae and
Tanytarsinae and obtained mortality at dosages higher than those
employed to control mosquito larvae. Miura et al. (1980), using
mosquito larvicidal dosages of a commercial Bti product, showed no
reduction in the field populations of chironomids following treatment.
Mulla et al. (1971) reported marked reductions in some chironomid
populations, using a commercial Bti product at rates of 20 to 40 times
the mosquitocidal rates. Mulligan & Schaefer (1982) reported a 40 to
70% reduction in some chironomid species after application of a Bti
formulation to a wildlife marsh. Rogatin & Baizhanov (1984) noted a
significant reduction of chironomids after Bti exposure.
Extensive quantitative observations were made on mayfly nymphs,
mostly Callibaetis pacificus, but no notable effects were observed
when Bti was applied against mosquito larvae (Mulla et al., 1982;
Mulla, 1988). Mulligan & Schaefer (1982) found that Bti did not
adversely affect mayfly nymphs (Callibaetis species). Schnetter et
al. (1981) reported that mayflies (Cloeon species) were not affected
when Bti was used in floodwater mosquito (Aedes vexans) larval
habitats. Schnetter et al. (1981) collected water boatmen (Corisella
species) from mosquito larval habitats on the upper Rhine river in
Germany. The water boatman population was not affected after exposure
to Bti for 48 h. Adverse effects were noted on backswimmers (Buenoa
species, Notonecta undulata, and Notonecta unifasciata) during
field trials with Bti (Mulla et al., 1982; Mulla, 1988). Mulligan &
Schaefer (1982) reported that the backswimmer (Notonecta species)
populations in a wetland marsh were not adversely affected by the
application of a Bti formulation. Purcell (1981) noted reductions in
populations of backswimmers (Buenoa elegans, Notonecta indica)
after application of Bti, but attributed this to the flying activity
of these predators.
No adverse effects on naiads of the dragonfly (Tarnetrum
corruptum) and damselfly (Enallagma civile) were reported when Bti
was used against larval mosquito populations (Mulla & Khasawinah,
1969; Mulla, 1988).
No notable reduction in the number of nymphs of several species
of dragonfly (Anisoptera) and damselfly (Zygoptera) occurred when
Bti was applied in a wetland marsh (Mulligan & Schaefer, 1982).
In the follow-up to the Onchocerciasis Control Programme of West
Africa (section 5.1.3.2), little or no effect on the non-target
populations was observed. However, few details were provided (Yameogo
et al., 1988; Levêque et al., 1988; Calamari et al., 1998).
6.4.2.2 Terrestrial insects
Melin & Cozzi (1990) summarized a number of studies on the
effects of Btk, Btg, Btt and Bte on non-target arthropod species and
arthropod populations in the laboratory and field. Representative
studies on Btk, Btg, Btt and Bte are listed in Tables 13 and 14.
Obadofin & Finlayson (1977) determined that a commercial Btk
product had a minimal effect on the ground beetle ( Bembidion
lampros ). Wilkinson et al. (1975) evaluated the contact activity of
a commercial Btk product for 5 days at levels equivalent to field
rates on an adult ladybird beetle (Hippodamia convergens) and found
no adverse effects.
Workman (1977) exposed earwigs (Labidura riparia) to a
commercial Btk product at rates equivalent to 10 times the normal
field application rate. No mortality was observed in these predators.
Hamed (1978-1979) found that two tachinid species (Bessa fugax
and Zenilla dolosa) were not affected after being fed suspensions
of a commercial Btk product. Horn (1983) observed a reduction in the
number of syrphid larvae on collards sprayed with a commercial Btk
product. This effect was attributed to a repellent effect on the
syrphid adults.
Hamed (1978-1979) found that Picromerus bidens was not
adversely affected after feeding upon lepidopteran larvae (Yponomeuta
evonymellus) that had fed upon leaves treated with commercial Btk
products.
Hassan (1983) determined a commercial Btk product to be harmless
to adult lacewings (Chrysopa carnea) when they were exposed at
normal field rate concentrations. Wilkinson et al. (1975) found
negligible mortality in larval or adult lacewings (Chrysopa carnea)
when a commercial Btk product was applied as a contact spray at
recommended field rates.
Yousten (1973) fed lethal quantities of Btk to larval cabbage
loopers (Trichoplusia ni) and just prior to death offered these
larvae to young Chinese praying mantids (Tenodera aridifolia
subspecies sinensis). The mantids were not susceptible to the
spore-crystal mixtures in the intact insect host.
Asquith (1975) found that black ladybird beetles (Stethorus
punctum) on apple trees were not affected by treatment with a
commercial Btk product. Buckner et al. (1974) monitored populations of
ground beetles following aerial spraying of spruce with two commercial
Btk products and found no effect on these predators. Harding et al.
(1972) detected no reduction in population levels of ladybird beetles
(coccinellids), rove beetles (staphyllinids), or checkered beetles
(clerids) in plots treated with a commercial Btk product. Johnson
(1974) evaluated several commercial Btk products as both sprays and
baits on tobacco. During the 2-year study, the populations of two
coccinellids (Hippodamia convergens and Colemegilla maculata)
were not affected by the microbial treatments. Wallner & Surgeoner
(1974) found no effects on coccinellids (Cycloneda munda,
Chilocorus bivulnerus and Adalia bipuncta) following forest
sprays with a commercial Btk product.
While evaluating a commercial Btk product for the control of
gypsy moth (Lymantria dispar) and elm spanworm ( Ennomos
subsignacius ), Dunbar et al. (1972) found no adverse effect on
Table 13. Effects of Btk on non-target arthropods
Arthropod order Type of study Resultsa References
Acarina Field - Weires & Smith, 1977;
Horsburgh & Cobb, 1981
Coleoptera Laboratory - Wilkinson et al., 1975; Obadofin & Finlayson, 1977
Field - Harding et al., 1972; Buckner et al., 1974;
Johnson, 1974; Wallner & Surgeoner, 1974;
Asquith, 1975
Dermaptera Laboratory - Workman, 1977
Diptera Laboratory - Hamed, 1978-1979
Laboratory - Horn, 1983
Field - Dunbar et al., 1972; Fusco, 1980
Heteroptera Laboratory - Hamed, 1978-1979
Field - Harding et al., 1972; Elsey, 1973;
Jensen, 1974; Wallner & Surgeoner, 1974
Hymenoptera Laboratory - Krieg, 1973
(Honey-bees) Laboratory - Krieg et al., 1980
Field - Buckner et al., 1974
Hymenoptera Laboratory - Wallner & Surgeoner, 1974;
(Parasitoids) Laboratory + Hassan & Krieg, 1975; Krieg et al., 1980
Dunbar & Johnson, 1975;
Mück et al., 1981; Weseloh &
Andreadis, 1982; Wallner et al., 1983;
Thomas & Watson, 1986
Field - Dunbar et al., 1972; Buckner et al., 1974;
Wanller & Surgeoner, 1974; Hamel, 1977;
Morris et al., 1977; Morris et al., 1980;
Fusco, 1980
Field + Weseloh et al., 1983
Table 13. (cont'd)
Arthropod order Type of study Resultsa References
Neuroptera Laboratory - Wilkinson et al., 1975; Hassan, 1983
Dictyoptera Laboratory - Yousten, 1973
(mantis)
a - = no effect reported; + an effect was reported, but does not imply that either
individual arthropods or populations of arthropods were adversely affected.
Table 14. Effects of different Bt strains on non-target arthropods
Arthropod order Type of study Resulta References
Btg
Hymenoptera Laboratory - Cantwell & Shieh, 1981
(Honey-bees) Field - Burges, 1977
Field - Burges & Bailey, 1968
Btt
Coleoptera Field + Kazakova & Dzhunusov, 1977
Hymenoptera Laboratory + Krieg & Herfs, 1963; Krieg, 1973
(Honey-bees) Laboratory - Martouret & Euverte, 1964;
Cantwell et al., 1966
Hymenoptera Laboratory + Hassan & Krieg, 1975;
(Parasitoids) Salama et al., 1982; Hassan, 1983;
Salama & Zaki, 1983
Laboratory - Krieg et al., 1980
Table 14. (cont'd)
Arthropod order Type of study Resulta References
Bte
Coleoptera Laboratory - Salama & Zaki, 1983
Laboratory + Salama et al., 1982
Hymenoptera Laboratory + Salama & Zaki, 1983
(Parasitoids)
Neuroptera Laboratory + Salama et al., 1982
a - = no effect reported; + = an effect was reported, but does not imply
that either individual arthropods or populations of arthropods were adversely affected.
two tachinids (Blepharipa scutellata and Parasitigena agilis).
Fusco (1980) reported an increased incidence of parasitism by two
tachinids (Blepharipa pratensis and Compsilura concinnata) when
Btk was applied in a field study.
Elsey (1973) reported no detrimental effect on spined stiltbug
nymphs or adults (Jalysus spinosus) during a 2-month field study
with a commercial Btk product. Harding et al. (1972) conducted a 2
year study to evaluate the effects of Btk on the natural enemies of
the bollworm (Helicoverpa zea) on cotton (Gossypium hirsutum).
Following applications of Btk against this pest, they reported no
detectable effects on Anthocoridae (minute pirate bugs, Orius
species), Lygaediae (bigeyed bugs, Geocoris species), Nabidae
(damsel bugs, Nabis species), or Reduviidae (assassin bugs).
Jensen (1974) used a commercial Btk product on soybeans (Glycine
canescens) to control the green cloverworm (Plathypena scabra) and
the velvetbean caterpillar (Anticarsis gemmitalis). No adverse
effect was observed on Lygaediae (bigeyed bugs, Geocoris species)
or Nabidae (damsel bugs, Nabis species).
Wallner & Surgeoner (1974) found no effect on the spined soldier
bug (Podisus maculiventris), following forest sprays of commercial
Btk products to control the oakleaf caterpillar (Heterocampa
manteo).
Although many data exist, in a review of the effects of the use
of Btk in Canada, Addison (1993) concluded that few studies on NTOs
had used soil invertebrate species and soil conditions relevant to
field conditions in Canadian forests.
Salama & Zaki (1983) reared cotton leafworm larvae (Spodoptera
littoralis) on a diet containing Bte and then fed these larvae to
adult staphylinid beetles (Paederus alferii). Predator longevity was
not significantly affected and no difference was seen in acceptability
to predators between untreated larvae and those exposed to Bte.
Salama et al. (1982) treated aphids with sprays of Bte and
provided these treated insects to newly hatched coccinellid larvae
(Coccinella undecimpunctata). The survival of larvae of predators
was not affected by feeding on the treated prey. However, the duration
of predator larval development was increased in the group treated with
Bte and there was a definite reduction in prey consumption.
Salama et al. (1982) evaluated the effect of Bte on the
development of lacewing larvae (Chrysopa carnea) by presenting them
with either sprayed aphids or treated cotton leafworm larvae
(Spodoptera littoralis). When fed either the sprayed aphids or the
treated cotton leafworms, the duration of larval development was
significantly extended and prey consumption was significantly reduced.
6.4.2.3 Honey-bees
Krieg (1973) observed mortality in adult honey-bees (Apis
mellifera) that were fed non-sporulated broth cultures of Btk. The
mortalities were attributed to the thermolabile alpha-toxin. Since
alpha-toxin is inactivated during sporulation, the toxin would not
present a problem in sporulated commercial Btk products. When Krieg et
al. (1980) fed fully sporulated cultures of Btk to adult honey-bees at
concentrations of 1 × 108 spores and crystal per bee over a 7-day
period, no harmful effects were observed.
Cantwell & Shieh (1981) fed a 1:20 solution of Btg in a sucrose
solution to newly emerged adult honey-bees. After 14 days, there was
no difference in mortality between treated and untreated groups.
Treatment of hives resulted in no adverse effect on the adult workers
or colony life as determined by egg laying, brood production, brood
capping, or honey production.
Buckner et al. (1974) observed no adverse effects on honey-bees
following aerial spraying of spruce (Picea species) with commercial
Btk products.
Cantwell et al. (1966) fed honey-bees sugar solutions containing
Btt spores, Btt culture supernatant with beta-exotoxin, and Btt
crystals. The crystals did not harm the bees, but the supernatant
caused nearly 100% mortality at day 7. Significant mortality was seen
in the spore-treated bees at 8 days and was attributed to bacterial
septicaemia. It should be noted that the dosages of each treatment
were many times higher than the bees would be exposed to in the course
of a lepidopteran control programme. Krieg (1973) reported mortality
in honey-bees fed whole nonsporulated cultures of Btt, which was
attributed to the presence of beta-exotoxin. Krieg & Herfs (1963)
reported that vegetative cells of Btt did not harm honey-bees;
however, they reported toxicity in Btt preparations containing the
beta-exotoxin. Martouret & Euverte (1964) fed worker honey-bees
cultures of Btt incorporated into mixtures of sugar, honey and clay.
Complete mortality was seen at 7 days for the spore-crystal-exotoxin
preparation and at 14 days for the spore-crystal complex.
6.4.2.4 Parasitoids
a) Btk
Dunbar & Johnson (1975) collected adult parasitoids
(Cardiochiles nigriceps) in the field and fed them suspensions of a
commercial Btk product. In the group fed Btk, shorter life spans were
reported. Since the investigators could not be sure whether feeding
actually took place, starvation may have been the cause of death.
Hassan & Krieg (1975) observed no adverse effects on adult
chalcid wasps (Trichogramma cacoeciae) that were fed suspensions of
a commercial Btk product. Krieg et al. (1980) fed washed spores and
crystals of Btk (5 × 107 spores and crystals) for 7 days to adult
chalcid wasps (Trichogramma cacoeciae) and observed no mortality or
reduced capacity to parasitize.
Mück et al. (1981) reported significant mortality in adult
braconids (Cotesia glomerata) that were fed a commercial Btk product
at rates of 108 and 109 spores per ml, but observed little effect on
the adult parasitoids (Pimpla turionellae). They reported midgut
epithelial damage in the Pimpla turionellae, which resulted from the
ICP.
Thomas & Watson (1986) found lower survival in adult ichneumonids
(Hyposoter exiguae) fed suspensions of a commercial Btk product.
They concluded the mortality was due to the spore-crystal complex.
Wallner & Surgeoner (1974) observed no effect on parasitoids
following treatments with commercial Btk products for control of the
notodontid moth (Heterocampa manteo).
Wallner et al. (1983) reported an indirect effect on the braconid
Rogas lymantriae when it parasitized gypsy moth (Lymantria dispar)
hosts fed Btk. The sex ratio of the parasitoid offspring was skewed
towards males in the treated larvae, as the female parasitoids lay
more fertilized eggs in larger, untreated host larvae.
Weseloh & Andreadis (1982) reported synergism in laboratory tests
with gypsy moth larvae (Lymantria dispar) fed a commercial Btk
product and exposed to the braconid (Cotesia melanoscelus). The
percentage of parasitism was increased in Btk-intoxicated larvae since
these grew more slowly and were at the approximate size suitable for
parasitism for a longer time.
Buckner et al. (1974) reported no detrimental effects on
parasitoid populations following field application of a commercial Btk
product.
Dunbar et al. (1972) reported an increase in the percentage of
parasitism of gypsy moth (Lymantria dispar) and elm spanworm
(Ennomos subsignarius) larvae in forestry plots treated with a
commercial Btk product.
Fusco (1980) reported an increase in the percentage of parasitism
of gypsy moth (Lymantria dispar) larvae by the braconids Cotesia
melanoscelus and Phobocampe unicincta following aerial sprays with
a commercial Btk product.
Hamel (1977) found that parasitoids attacking early instar
western spruce budworm larvae (Choristoneura occidentalis) increased
in number following aerial application of a commercial Btk product,
while older budworm larvae were reduced in number.
In two field studies, commercial Btk products showed no
detrimental effects on parasitoid populations (Morris et al., 1977,
1980).
Wallner & Surgeoner (1974) demonstrated 6- to 12-fold increases
in the percentage of parasitism in gypsy moth larvae (Lymantria
dispar) by the braconid Cotesia melanoscelus in forestry plots
treated with a commercial Btk product.
b) Btt
Hassan (1983) observed the chalcid Trichogramma cacoeciae was
not affected by exposure to dried surface films of Btt.
Hassan & Krieg (1975) fed a suspension of three different
commercial Bt products to adult chalcids (Trichogramma cacoeciae)
and reported a minor reduction in the capacity to parasitize with
Btt, but none with the other Bt products. The effect of the Btt
product may have been due to the beta-exotoxin.
Krieg et al. (1980) fed washed spores and crystals of Btt to
adult chalcids (Trichogramma cacoeciae) for 7 days and observed no
mortality or reduced capacity to parasitize.
Lowered reproductive potential was observed for both the
braconids Microplitis demolitor and Zele chlorophthalma
following exposure to Btt (Salama et al., 1982; Salama & Zaki, 1983).
Salama & Zaki (1983) reported increased development times for
Zele chlorophthalma parasitizing the cotton leafworm Spodoptera
littoralis treated with Bte.
7. EXPOSURE AND EFFECTS ON HUMANS
There are some case reports on the occurrence of Bt in patients
with different infectious diseases. However, none of these studies
demonstrate an actual risk to human health by the use of Bt. They
emphasize the need for further research on the production of toxins,
knowledge of factors causing the genes of the toxins to be expressed,
and knowledge on the natural occurrence of Bt and Bc. The medical
practice does not discriminate between Bt and Bc as causative agents
in infectious diseases. Therefore, the true proportion of Bt in Bc-
induced disease is not known.
7.1 Bacillus thuringiensis
For aeons, humans have been exposed to Bt in their natural
habitats, particularly from soil, water and the phylloplane. However
in the recorded scientific literature, only few adverse effects to
these environmental Bt levels have been documented.
The manufacture and field application of Bt products can result
in aerosol and dermal exposure of workers and the human population,
especially by spraying programmes in populated areas. Agricultural and
horticultural uses of Bt can also result in dietary exposure.
7.1.1 Experimental exposure of humans
Eight human volunteers ingested 1 gram of a Btk formulation
(3 × 109 spores/g of powder) daily for 5 days. Of the eight
volunteers, five also inhaled 100 mg of the Btk powder daily for five
days. Comprehensive medical examinations immediately before, after,
and 4 to 5 weeks later failed to demonstrate any adverse health
effects, and all the blood chemistry and urinalysis tests were
negative (Fisher & Rosner, 1959).
Pivovarov et al. (1977) reported that ingestion of foods
contaminated with Btg at concentrations of 105 to 109 cells/g
caused nausea, vomiting, diarrhoea and tenesmus, colic-like pains in
the abdomen, and fever in three of the four volunteers studied. The
toxicity of the Btg strain may have been due to beta-exotoxin (Ray,
1990).
7.1.2. Exposure of workers during manufacture
Many manufacturers of Bt products monitor the exposure and the
associated health risks of their workers. Over a period of 30 years of
production, there have been no reports of such workers having been
adversely affected (RJ Cibulsky, personal communication, 1997).
7.1.3 Exposure of workers in spraying operations
Noble et al. (1992) studied aerosol Btk exposure and subsequent
nose and throat carriage of Bt by workers during a major spray
programme for gypsy moth (Lymantria dispar) control. Spraying down
from high lifts, spraying low foliage or spraying with prevailing
breezes resulted in lower exposures of spray operators than did
spraying upwards into trees. The mean exposure values ranged from
3.0 × 103 to 5.9 × 106 Bt spores/m3 sampled air. Individuals working
most shifts during the spray period were exposed to 5.4 × 106 to
7.2 × 107 organisms. Nearly all the workers exposed to higher
concentrations for several shifts (5 to 20) were culture-positive for
Bt, and the majority of the workers remained culture-positive for 14
to 30 days. Of those who were culture-positive, eight workers reverted
to a culture-negative status during the project or within 30 days of
project completion. During the spray programme, some workers
experienced chapped lips, dry skin, eye irritation, and nasal drip and
stuffiness, but no serious health problems resulted. These symptoms
were transient and frequently occurred during the beginning of a spray
run and when Bt spray concentrations were increased. No significant
differences were found with respect to gender or smoking status.
In the same study, Noble et al. (1992) evaluated the health
records of the general population in the county where the Btk spray
programme was conducted. After examining the records of 3500 hospital
emergency room admissions, 1140 family practice patients, and over 400
bacterial cultures from 10 hospitals, no evidence for community
illness or infections attributed to Btk could be documented.
Laferrière et al. (1987) demonstrated antibody titres in 11 of
107 workers exposed to Btk during a 2-year spraying period. By the
middle of the spray operation, seven had developed titres to
spore-crystal complexes, six to vegetative cells, and one to spores.
Their titres tended to be low, but were higher in those exposed for a
second year. Two months after the exposure ended, nine workers were
retested. Of these workers, five had no detectable antibodies to the
spore-crystal complexes, and four who had been among those with the
highest titres against vegetative cells had significantly lower
titres.
Elliott et al. (1988) measured the exposure of individual workers
and other individuals within the spraying area on the day of
application during an aerial Btk spray programme for gypsy moth
(Lymantria dispar) control. Concentrations of spores were measured
using personal air sampling devices. The concentration of spores
ranged from 0 to 1.1 × 104 cfu/m3 for individual workers, the
highest concentration being incurred by a spray card checker who was
in brief contact with the material. For non-working individuals, the
average Bt exposure was 1.3 × 103 cfu/m3. In the spray area, a
general survey showed concentrations of 0 to 4.2 × 103 cfu/m3.
7.1.4 Exposure of human populations by spraying operations over
populated areas
Btk and Bti have been sprayed over populated areas in several
countries, including the USA, Canada and New Zealand. Some of these
applications have been followed by public health surveillance
programmes. In general, no (or very few) harmful effects have been
reported among residents of the sprayed communities.
7.1.5 Clinical case reports
Commercial Bt products have been used for over two decades, but
Bt has been isolated in only a few cases of human bacterial infection.
Samples & Buettner (1983) reported that a farm worker developed a
corneal ulcer in one eye. It had been accidentally splashed with a
commercial Btk product and Bt was subsequently isolated from the
affected eye. The eye was treated with a topical antibiotic and
corticosteroid and the corneal ulcer resolved 14 days after treatment.
The report attributed the corneal ulcer to Bt infection. However, the
possibility that Bt may have been a non-pathogenic contaminant of the
ulcer was not considered. There are no other reports of Bt being
associated with ocular infections in workers.
During the investigation of a gastroenteritis outbreak in a
chronic care institution, bacteria were isolated from four individuals
and were identified as B. thuringiensis. The Bt isolates showed
cytotoxic effects characteristic of B. cereus (Jackson et al.,
1995).
Damgaard et al. (1997a) isolated Bt in burn wounds in two
patients. None of the isolates showed any toxicity to Vero cells.
Hernandez et al. (1998) isolated Bt from a war wound; this strain (Bt
konkukian) could infect immunosuppressed mice after cutaneous
application.
Warren et al. (1984) reported that a research worker developed a
marked local reaction and lymphadenitis following a needle stick
injury when handling Bti. Acinetobacter calcoaceticus and Bt were
cultured from the exudate. The condition responded to penicillin.
Green et al. (1990) reported that Bt was isolated from body
fluids of 55 patients with different infectious diseases. In 52 of
them, it was considered a contaminant, while in three cases with
pre-existing medical problems, no firm conclusion was established
concerning a causal relationship between the infection and Bt.
Furthermore, Bt was isolated from the conjunctiva of a worker
presenting with conjunctivitis, and with a history of a splash with a
Bt product.
Despite the widespread use of Bt-based products, only two
incidents of possible allergic reactions have been reported to the US
EPA (McClintock et al., 1995). After detailed analysis, neither of
these was considered to be causally related to Bt.
7.1.6 Dietary exposure of the general population
In some Asian countries, Bti has been added to domestic
containers of drinking-water for mosquito control. From these high Bt
exposures in drinking-water, no adverse effects in humans have been
reported. In Africa, some rivers have been dosed with Bti at weekly
intervals for blackfly control. No adverse effects in the human
populations that drink the river water have been reported. Btk has
been reported to survive for 1 to 2 months in fresh water and in
seawater. However, viable Bt cultures have not been isolated from
drinking-water supplies (Menon & De Mestral, 1985).
There is little information on levels of Bt to be found in food,
but it is possible that, in view of the widespread prevalence of Bt,
its presence in food is common and is not always related to its use on
food products. Bt spores have been shown to be unable to germinate in
mammalian digestive systems; however, Bt has been isolated from faecal
and urinary samples in occupational studies.
Noble et al. (1992) reported that 5 out of 10 vegetable samples
were positive for Btk. The positive samples were obtained from both
supermarkets and from organically grown products. Such results may
account for the recovery of Bt from faecal and urinary samples during
the occupational studies and may reflect community exposure through
food.
7.2 Bacillus cereus
The close affiliation between Bt and Bc raises the question of
whether strains of Bt can cause human illness during vegetative
growth. During vegetative growth Bc can produce different kinds of
toxins; these toxins can cause gastrointestinal diseases in humans
after ingestion. The emetic toxin is an enzymatically synthesized
peptide that causes vomiting (Granum, 1997) a few hours after
ingestion. Most Bc strains producing this toxin seem to belong to the
same serotype (Mikami et al., 1995; Nishikawa et al., 1996). The
enterotoxins are a group of proteins causing abdominal pain and
diarrhoea after an incubation period of 8-16 h. The enterotoxins
causing gastrointestinal disease are most likely produced in the small
intestine. Characteristics of the two types of disease caused by Bc
are shown Table 15. Based on analysis of outbreaks the infective dose
is believed to vary between 105 and 108 vegetative cells or
activated spores per gram, but it may be so low as 104 (Granum,
1997). In addition to the two toxins, Bc can produce different lytic
enzymes, e.g., haemolysins, which most likely are involved in the
gastrointestinal diseases. In addition to gastrointestinal diseases Bc
can cause various diseases, notably in immunosuppressed individuals
(Drobniewski, 1993).
Analysis of reported foodborne diseases reveals that Bc is
frequently diagnosed as the cause of gastrointestinal disorders
(Notermans & Batt, 1998) in many countries. Several food-borne disease
outbreaks caused by Bc have been reported by Notermans & Batt (1998).
However, Bc gastrointestinal diseases are highly under-reported, as
both types of illness are relatively mild and usually last less than
24 h (Granum, 1997; Notermans & Batt, 1998). The incidence of Bc in
foods varies between 101 and 107, the highest concentrations being
found in herbs/spices and boiled rice (Notermans & Batt, 1998). The
degree of toxicity of enterotoxins varies from Bc strain to strain,
probably due to differences in toxin components (Lund & Granum, 1997).
Hassan & Nabbut (1996) found that clinical Bc isolates from human
diarrhoeal faeces were strong producers of diarrhoeal enterotoxin,
while isolates from blood, wounds, normal faeces, milk and rice were
weak producers of diarrhoeal enterotoxin (Hassan & Nabbut, 1996). This
variation is reflected in the variable numbers (105-108 viable cells
or spores per g) of Bc reported to cause symptoms in humans, and it
has been suggested that foods containing more than 104 Bc per g may
not be safe for consumption (Granum, 1997). Several European countries
have a critical level of 104-105 Bc per g for acceptance of food
products (Notermans & Batt, 1998). This critical level will include
Bt, as the methods used do not discriminate between Bc and Bt.
Table 15. Characteristics of the two types of disease caused by B. cereus
(from Granum, 1997)
Characteristic Emetic syndrome Diarrhoeal
Infective dose (cells/g) 105-108 104-107
Toxin produced Preformed in food In the small intestine
Type of toxin Cyclic peptide Protein
Incubation period (h) 0.5-5 8-16 (occasionally >24)
Duration of illness 6-24 12-24 (occasionally many days)
Symptoms Vomiting, nausea, malaise Abdominal pain, diarrhoea
8. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and from biologically active products other than
the ICPs. Bt products may be safely used for the control of insect
pests of agricultural and horticultural crops as well as forests. Bt
is also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt have the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
9. CONCLUSIONS AND RECOMMENDATIONS
* Bt may be safely used for the control of insect pests of
agricultural crops and forests.
* Bti is safe for use in aquatic environments, including
drinking-water reservoirs, for the control of mosquito, blackfly
and nuisance insect larvae.
* Bt products should contain the ICPs and be free from other
microorganisms and biologically active metabolites.
* New Bt products based on either new Bt strains and/or new ICPs
require appropriate assessment.
* FAO and WHO should develop standard specifications for Bt
preparations as is done for chemical pesticides.
* Good industrial large-scale practice (GILSP) standards should be
employed for the production of Bt products.
* Standardized valid methods for the assessment of gastrointestinal
consequences of vegetatively produced agents should be developed.
* The occurrence of resistant insect populations underscores the
need for research on the relationships between cry-toxins and
the ecology of Bt.
* More research on the fate of Bt spores and ICPs in the
environment is needed. This should cover the natural occurrence
of Bt and Bc in foods and its relationship to exposure to Bt from
its pesticide use.
* Research into dose-response analysis and the consequent
acceptable daily intake levels of Bt in the diet and beverages is
a high priority.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANISATIONS
WHO (1985) considered the safe use of MPCA at the Ninth Meeting
of the WHO Expert Committee on Vector Control in 1984. The report
considered that addition of live microorganisms to drinking-water is
undesirable and recommended that the use of Bt H-14 for the control of
Aedes aegypti in drinking-water should be restricted to the
asporogenic form. At the 1990 meeting, WHO (1991), after reviewing new
research data, stated that its previous recommendation was unduly
restrictive, provided that properly designed formulations were used.
REFERENCES
Addison JA (1993) Persistence and nontarget effects of Bacillus
thuringiensis in soil: a review. Can J Forensic Res, 23: 2329-2342.
Agata N, Ohta M, Arakawa Y, & Mori M (1995) The bceT gene of
Bacillus cereus encodes an enterotoxic protein. Microbiology, 141:
983-988.
Ahmed R, Sankar-Mistry P, Jackson S, Ackermann H-W, & Kasatiya SS
(1995) Bacillus cereus phage typing as an epidemiological tool in
outbreaks of food poisoning. J Clin Microbiol, 33: 636-640.
Akiba Y (1986) Microbial ecology of Bacillus thuringiensis: VI.
Germination of Bacillus thuringiensis spores in the soil. Appl
Entomol Zool, 21: 76-80.
Akiba Y (1991) Assessment of rainwater-mediated dispersion of
field-sprayed Bacillus thuringiensis in the soil. Appl Entomol Zool,
26: 477-483.
Ali A (1980) Nuisance chironomids and their control: a review. Bull
Entomol Soc Am, 26: 3-16.
Ali A (1981) Bacillus thuringiensis serovar. israelensis
(ABG-6108) against chironomids and some nontarget aquatic
invertebrates. J Invertebr Pathol, 38: 264-272.
Ali A & Baggs RD (1981) Susceptibility of some Florida chironomids and
mosquitoes to various formulations of Bacillus thuringiensis serovar
israelensis de Barjac. J Econ Entomol, 74: 672-677.
Aly C (1985) Germination of Bacillus thuringiensis var. israelensis
spores in the gut of Aedes larvae (Diptera: Culicidae). J Invertebr
Pathol, 45: 1-8.
Aly C & Mulla MS (1987) Effect of two microbial insecticides on
aquatic predators of mosquitoes. J Appl Entomol, 103: 113-118.
Aly C, Mulla MS, & Federici BA (1985) Sporulation and toxin production
by Bacillus thuringiensis var. israelensis in cadavers of mosquito
larvae (Diptera: Culicidae). J Invertebr Pathol, 46: 251-258.
Andersson MA, Mikkola R, Helin J, Andersson MC, & Salkinoja-Salonen M
(1998) A novel sensitive bioassay for detection of Bacillus cereus
emetic toxin and related depsipeptide ionophores. Appl Environ
Microbiol, 64: 1338-1343.
Andrews RE Jr, Faust RM, Wabiko H, Raymond KC, & Bulla LA Jr (1987)
The biotechnology of Bacillus thuringiensis. CRC Crit Rev
Biotechnol, 6: 163-232.
Angus TA (1954) A bacterial toxin paralysing silkworm larvae. Nature
(Lond), 173: 545-546.
Aronson AI, Han ES, McGaughey W, & Johnson D (1991) The solubility of
inclusion proteins from Bacillus thuringiensis is dependent upon
protoxin composition and is a factor in toxicity to insects. Appl
Environ Microbiol, 57: 981-986.
Asano S-I, Nukumizu Y, Bando H, Hzuka T, & Yamamoto T (1997) Cloning
of novel enterotoxin genes from Bacillus cereus and Bacillus
thuringiensis. Appl Environ Microbiol, 63: 1054-1057.
Ash C, Farrow JA, Dorsch M, Stackebrandt E, & Collins MD (1991)
Comparative analysis of Bacillus anthracis, Bacillus cereus, and
related species on the basis of reverse transcriptase sequencing of
16S rRNA. Int J System Bacteriol, 41: 343-346.
Asquith D (1975) Response of the predaceous black lady beetle
Stethorus punctum (DeConte) to apple orchard insecticide treatments
(Experiment No. D986-4007). Chicago, Illinois, Abbott Laboratories.
Baida GE & Kuzmin NP (1995) Cloning and primary structure of a new
hemolysin gene from Bacillus cereus. Biochim Biophys Acta, 1264:
151-154.
Baumann L, Okamoto K, Unterman BM, Lynch MJ, & Bauman P (1984)
Phenotypic characterization of Bacillus thuringiensis and Bacillus
cereus. J Invertebr Pathol, 44: 329-341.
Beach RM (1990) Application for an experimental use permit to ship and
use a pesticide for experimental purposes only -- Permit No.
58788-EUP-4 for InCideM 586. Hanover, Maryland, Crop Genetics
International.
Beattie SH, Holt C, Hirst D, & Williams AG (1998) Discrimination among
Bacillus cereus, B. mycoides and B. thuringiensis and some other
species of the genus Bacillus by Fourier transform infrared
spectroscopy. FEMS Microbiol Lett, 164: 201-206.
Beavers JB (1991a) ABG-6305: An avian oral pathogenicity and toxicity
study in the mallard (Project No. 161-118). Easton, Maryland, Wildlife
International Ltd, pp 1-20 (Unpublished Abbott document).
Beavers JB (1991b) ABG-6305: An avian oral pathogenicity and toxicity
study in the bobwhite (Project No. 161-117). Easton, Maryland,
Wildlife International Ltd, pp 1-21 (Unpublished Abbott document).
Beavers JB, Larsen AC, & Jaber M (1989a) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the bobwhite (Project No. 161-109). Eston, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Beavers JB, Clauss BS, & Jaber M (1989b) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the mallard (Project No. 161-108). Easton, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Becker N & Margalit J (1993) Use of Bacillus thuringiensis
israelensis against mosquitoes and blackflies. In: Entwistle PF, Cory
JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 147-170.
Beegle CC, Dulmage HT, Wolfenbarger DA, & Martinez E (1981)
Persistence of Bacillus thuringiensis Berliner insecticidal activity
on cotton foliage. Environ Entomol, 10: 400-401.
Benz G & Altwegg A (1975) Safety of Bacillus thuringiensis for
earthworms. J Invertebr Pathol, 26: 125-126.
Berliner E (1915) [About the sleep sickness of the Ephestia kühniella
Zell. and its vector Bacillus thuringiensis.] Z Angew Entomol, 2:
29-56 (in German).
Bernhard K & Utz R (1993) Production of Bacillus thuringiensis
insecticides for experimental and commercial uses. In: Entwistle PF,
Cory JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 255-267.
Bernier RL Jr, Gannon DJ, Moser GP, Mazzarello M, Griffiths MM, &
Guest PJ (1990) Development of a novel Bt strain for the control of
forestry pests. In: Brighton Crop Protection Conference -- Pests and
Diseases -- 1990: Proceedings. Farnham, Surrey, British Crop
Protection Council, pp 245-252.
Boeri RL (1991) Acute toxicity of ABG-6305 to the rainbow trout
(Oncorhynchus mykiss) (Project No. 9107-A). Hampton, New Hampshire,
Resource Analysts Inc, Enviro Systems Division, pp 1-26 (Unpublished
Abbott document).
Bonnefoi A & Béguin S (1959) Recherches sur l'action des cristaux de
Bacillus thuringiensis souche "anduze". Entomophaga, 4: 193-199.
Bourque SN, Valero JR, Lavoie MC, & Levesque RC (1995) Comparative
analysis of the 16S-23S ribosomal intergenic spacer sequences of
Bacillus thuringiensis strains and subspecies and of closely related
species. Appl Environ Microbiol, 61: 1623-1626.
Bravo A, Sarabia S, Lopez L, Ontiveros H, Abarca C, Ortiz M, Lina L,
Villalobos FJ, Pena G, Nunez-Valdez M-E, Soberón M, & Quintero R
(1998) Characterization of cry genes in a Mexican Bacillus
thuringienis strain collection. Appl Environ Microbiol, 64:
4965-4972.
Brousseau R, Saint-Onge A, Prefontaine G, Masson L, & Cabana J (1992)
Arbitrary primer polymerase chain reaction, a powerful method to
identify Bacillus thuringiensis serovars and strains. Appl Environ
Microbiol, 59: 114-119.
Buckner CH, Kingsbury PD, McLeod BB, Mortensen KL, & Ray DGH (1974)
Impact of aerial treatment on non-target organisms, Algonquin Park,
Ontario, and Spruce Woods, Manitoba, Section F. In: Evaluation of
commercial preparations of Bacillus thuringiensis with and without
chitinase against spruce budworm. Ottawa, Ontario, Canadian Forestry
Service, Chemical Control Research Institute, pp 1-72 (Information
report CC-X-59).
Bulla LA Jr, Kramer KJ, & Davidson LI (1977) Characterization of the
entomocidal parasporal crystal of Bacillus thuringiensis. J
Bacteriol, 130: 375-383.
Bulla LA Jr, Faust RM, Andrews R, & Goodman N (1985) Insecticidal
bacilli. In: Dubnau DA ed. The molecular biology of the bacilli. New
York, London, Academic Press Inc., vol 2, pp 185-209.
Burges HD (1977) Control of the wax moth Galleria mellonella on
beecomb by H-serotype V Bacillus thuringiensis and the effect of
chemical additives. Apidologie, 8: 155-168.
Burges HD (1980) Safety, safety testing and quality control of
microbial pesticides. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
738-767.
Burges HD & Bailey L (1968) Control of the greater and lesser wax
moths (Galleria mellonella and Achroia griesella) with Bacillus
thuringiensis. J Invertebr Pathol, 11: 184-195.
Burges HD & Hurst JA (1977) Ecology of Bacillus thuringiensis in
storage moths. J Invertebr Pathol, 30: 131-139.
Calamari D, Yameogo L, Hougard J-M, & Levêque C (1998) Environmental
assessment of larvicide use in the onchocerciasis control programme.
Parasitol Today, 14: 485-489.
Campbell DP, Dieball DE, & Brackett JM (1987) Rapid HPLC assay for the
ß-exotoxin of Bacillus thuringiensis. J Agric Food Chem, 35:
156-158.
Cannon RJC (1996) Bacillus thuringiensis use in agriculture: a
molecular perspective. Biol Rev Cambridge Philos Soc, 71: 561-636.
Cantwell GE & Shieh TR (1981) CertanTM: A new bacterial insecticide
against the greater wax moth. Am Bee J, 121: 424-431.
Cantwell GE, Heimpel AM, & Thompson MJ (1964) The production of an
exotoxin by various crystal-forming bacteria related to Bacillus
thuringiensis var. thuringiensis Berliner. J Insect Pathol, 6:
466-480.
Cantwell GE, Knox DA, Lehnert T, & Michael AS (1966) Mortality of the
honey bee, Apis mellifera, in colonies treated with certain
biological insecticides. J Invertebr Pathol, 8: 228-233.
Car M (1984) Laboratory and field trials with two Bacillus
thuringiensis var. israelensis products for Simulium (Diptera:
Nematocera) control in a small polluted river in South Africa.
Onderstepoort J Vet Res, 51: 141-144.
Car M & de Moor FC (1984) The response of Vaal River drift and benthos
to Simulium (Diptera: Nematocera) control using Bacillus
thuringiensis var. israelensis (H-14). Onderstepoort J Vet Res, 51:
155-160.
Carlberg G, Kihamia CM, & Minhar J (1985) Microbial control of flies
in latrines in Dar es Salaam with a Bacillus thuringiensis (serotype
1) preparation, Muscabac. J Appl Microbiol Biotechnol, 1: 33-44.
Carlson CR & Kolsto A-B (1993) A complete physical map of a Bacillus
thuringiensis chromosome. J Bacteriol, 175: 1053-1060.
Carlson CR, Caugant DA, & Kolstr A-B (1994) Genotypic diversity among
Bacillus cereus and Bacillus thuringiensis strains. Appl Environ
Microbiol, 60: 1719-1725.
Carlton BC, Gawron-Burke C, & Johnson TB (1990) Exploiting the genetic
diversity of Bacillus thuringiensis for the creation of new
bioinsecticides. In: Proceedings of the 5th International Colloquium
on Invertebrate Pathology and Microbial Control. Adelaide, Australia,
Society for Invertebrate Pathology, pp 18-22.
Chilcott CN, Pillai JS, & Kalmakoff J (1983) Efficacy of Bacillus
thuringiensis var. israelensis as a biocontrol agent against
larvae of Simuliidae (Diptera) in New Zealand. NZ J Zool, 10: 319-326.
Christensen KP (1990a) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 32-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-53 (Unpublished Abbott document No. 90-1-3211).
Christensen KP (1990b) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-57
(Unpublished Abbott document No. 90-2-3219).
Christensen KP (1990c) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc.,
vol 2, pp 253-308 (Unpublished Abbott document No. 90-5-3317).
Christensen KP (1990d) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to rainbow trout (Oncorhynchus mykiss)
during a 30-day static renewal test. Wareham, Massachusetts,
Springborn Laboratories Inc., pp 1-54 (Unpublished Abbott document No.
90-3-3263).
Christensen KP (1990e) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to sheepshead minnow (Cyprinodon
variegatus) during a 30-day static renewal test. Wareham,
Massachusetts, Springborn Laboratories Inc., pp 1-50 (Unpublished
Abbott document No. 90-6-3348).
Christensen KP (1990f) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-55 (Unpublished Abbott document No. 90-2-3228).
Christensen KP (1990g) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-55
(Unpublished Abbott document No. 90-2-3242).
Christensen KP (1990h) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-57 (Unpublished Abbott document No. 90-4-3288).
Cibulsky RJ & Fusco RA (1987) Recent experiences with Vectobac for
black fly control: An industrial perspective on future developments.
In: Kim KC & Merritt RW ed. Black flies: Ecology, population
management, and annotated world list. University Park, Pennsylvania,
Pennsylvania State University Press, pp 419-424.
Claus D & Berkeley RCW (1986) Genus Bacillus Cohn 1872. In: Sneath
PHA, Mair NS, Sharp ME, & Holt JG ed. Bergey's manual of systematic
bacteriology. Baltimore, Maryland, Williams & Wilkins, vol 2, pp
1105-1139.
Cooksey KE (1971) The protein crystal toxin of Bacillus
thuringiensis: Biochemistry and mode of action. In: Burges HD &
Hussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc., pp 247-274.
Crickmore N, Zeigler DR, Feitelson J, Schnepf E, van Rie J, Lereclus
D, Baum J, & Dean DH (1998) Revision of the nomenclature for the
Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol
Biol Rev, 62: 807-813.
Damgaard PH (1995) Diarrhoeal enterotoxin production by strains of
Bacillus thuringiensis isolated from commercial Bacillus
thuringiensis -- based insecticides. FEMS Immunol Med Microbiol, 12:
245-250.
Damgaard PH, Granum PE, Bresciani J, Torregrossa MV, Eilenberg J, &
Valentino L (1997a) Characterization of Bacillus thuringiensis
isolated from infections in burn wounds. FEMS Immunol Med Microbiol,
18: 47-53.
Damgaard PH, Hansen BM, Pedersen JC, & Eilenberg J (1997b) Natural
occurrence of B. thuringiensis on cabbage foliage and in insects
associated with cabbage crops. J Appl Bacteriol, 82: 253-258.
Damgaard PH, Larsen HD, Hansen BM, Bresciani J, & Jorgensen K (1996a)
Enterotoxin-producing strains of Bacillus thuringiensis isolated
from food. Lett Appl Microbiol, 23: 146-150.
Damgaard PH, Jacobsen CS, & Sorensen J (1996b) Development and
application of a primer set for specific detection of Bacillus
thuringiensis and Bacillus cereus in soil using magnetic
capture-hybridization and PCR amplification. System Appl Microbiol,
19: 436-441.
de Barjac H (1981a) Identification of H-serotypes of Bacillus
thuringiensis. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
35-43.
de Barjac H (1981b) Insect pathogens in the genus Bacillus. In:
Berkley RCW & Goodfellow M ed. The aerobic endospore-forming bacteria:
Classification and identification. New York, London, Academic Press
Inc., pp 241-250.
de Barjac H & Bonnefoi A (1962) Essai de classification biochimique et
sérologique de 24 souches de Bacillus de type thuringiensis.
Entomophaga, 7: 5-31.
de Barjac H & Larget-Thiery I (1984) Characteristics of IPS 82 as
standard for biological assays of Bacillus thuringiensis H-14
preparations. Geneva, World Health Organization (WHO/VBC/84.892).
de Barjac H, Larget I, Bénichou L, Cosmao V, Viviani G, Ripouteau H, &
Papion S (1980) Test d'innocuité sur mammifères avec du sérotype H 14
de Bacillus thuringiensis. Geneva, World Health Organization
(WHO/VBC/80.761).
Dean DH (1984) Biochemical genetics of the bacterial insect-control
agent Bacillus thuringiensis: Basic principles and prospects for
genetic engineering. Biotechnol Genet Eng Rev, 2: 341-363.
Dean DH, Rajamohan F, Lee MK, Wu SJ, Chen XJ, Alcantara E, & Hussain
SR (1996) Probing the mechanism of action of Bacillus thuringiensis
insecticidal proteins by site-directed mutagenesis - a minireview.
Gene, 179: 111-117.
DeLucca AJ II, Simonson JG, & Larson AD (1981) Bacillus thuringiensis
distribution in soils of the United States. Can J Microbiol, 27:
865-870.
Demezas DH & Bell J (1995) Evaluation of low molecular weight RNA
profiles and ribotyping to differentiate some Bacillus species.
System Appl Microbiol, 18: 582-589.
Denolf P, Hendrickx K, Vandamme J, Jansens S, Peferoen M, Degheele D,
& Vanrie J (1997) Cloning and characterization of Manduca sexta and
Plutella xylostella midgut aminopeptidase N enzymes related to
Bacillus thuringiensis toxin-binding proteins. Eur J Biochem, 248:
748-761.
Dent DR (1993) The use of Bacillus thuringiensis as an insecticide.
In: Jones DG ed. Exploitation of microorganisms. London, Chapman &
Hall, pp 19-44.
Drobniewski FA (1993) Bacillus cereus and related species. Clin
Microbiol Rev, 6: 324-338.
Dulmage HT & cooperators (1981) Insecticidal activity of isolates of
Bacillus thuringiensis and their potential for pest control. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 193-223.
Dunbar JP & Johnson AW (1975) Bacillus thuringiensis: Effects on the
survival of a tobacco budworm parasitoid and predator in the
laboratory. Environ Entomol, 4: 352-354.
Dunbar DM, Kaya HK, Doane CC, Anderson JF, & Weseloh RM (1972) Aerial
application of Bacillus thuringiensis against larvae of the elm
spanworm and gypsy moth and effects on parasitoids of the gypsy moth.
Connecticut Experiment Station (Bulletin No. 735).
Eilenberg J, Damgaard PH, Hansen BM, Pedersen JC, Bresciani J, &
Larsson R (in press) Natural interactions between Strongwellsea
castrans, Cystosporogenes deliaradicae and Bacillus thuringiensis
in the host, Delia radicum. J Invertebr Pathol.
Elliott LJ, Sokolow R, Heumann M, & Elefant SL (1988), An exposure
characterization of a large scale application of a biological
insecticide, Bacillus thuringiensis. Appl Ind Hyg, 3: 119-122.
Elsey KD (1973) Jalysus spinosus effect of insecticide treatments on
this predator of tobacco pests. Environ Entomol, 2: 240-243.
Entwistle PF, Cory JS, Bailey MJ, & Higgs S (1983) Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, John Wiley & Sons, 311 pp.
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, & Koziel MG
(1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal
protein with a wide spectrum of activities against lepidopteran
insects. Proc Natl Acad Sci (USA), 93: 5389-5394.
Farkas J, Sebesta K, Horská K, Samek Z, Dolejs L, & Sorm F (1977)
Structure of thuringiensin, the thermostable exotoxin from Bacillus
thuringiensis. Coll Czech Chem Commun, 42: 909-929.
Fast PG (1971) Isolation of a water-soluble toxin from a commercial
microbial insecticide based on Bacillus thuringiensis. J Invertebr
Pathol, 17: 301.
Fast PG (1981) The crystal toxin of Bacillus thuringiensis. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 223-248.
Faust RM (1973) The Bacillus thuringiensis ß-exotoxin: Current
status. Bull Entomol Soc Am, 19: 153-156.
Feitelson JS (1993) The Bacillus thuringiensis family tree. In: Kim
L ed. Advanced engineered pesticides. New York, Basel, Marcel Dekker
Inc., pp 63-71.
Fisher R & Rosner L (1959) Toxicology of the microbial insecticide,
Thuricide. J Agric Food Chem, 7: 686-688.
Frommer W, Ager B, Archer L, Brunius G, Collins CH, Donikian R,
Frontali C, Hamp S, Houwink EH, Kuenzi MT, Krämer P, Lagast H, Lund S,
Mahler JL, Normand-Plessier F, Sargeant K, Tuijnenburg-Muijs G, Vranch
SP, & Werner RG (1989) Safe biotechnology: III. Safety precautions for
handling microorganisms of different risk classes. Appl Microbiol
Biotechnol, 30: 541-552.
Fusco RA (1980) Field evaluation of a commercial preparation of
Bacillus thuringiensis, DIPEL 4L: Progress report. Pennsylvania
Bureau of Forestry, Gypsy Moth Pest Management Methods Development
Project.
Gaddis PK & Corkran CC (1986) Secondary effects of Bt spray on avian
predators: the reproductive success of chestnut-backed chickadees.
Northwest Ecological Research Institute (Unpublished Research Report
No. 86-03).
Garcia R, Des Rochers B, & Tozer W (1980) Studies on the toxicity of
Bacillus thuringiensis var. israelensis against organisms found in
association with mosquito larvae. Berkeley, California, University of
California, Division of Biological Control.
Gelernter WD (1990) Targeting insecticide-resistant markets: New
developments in microbial-based products. In: Green MB, Moberg WK, &
LeBaron H ed. Managing resistance to agrochemicals: From fundamental
research to practical strategies. Washington, DC, American Chemical
Society, pp 105-117 (ACS Series 421).
Giffel MC, Beumer RR, Klijn N, Wagendorp A, & Rombouts FM (1997)
Discrimination between Bacillus cereus and Bacillus thuringiensis
using specific DNA probes based on variable regions of 16S rRNA. FEMS
Microbiol Lett, 146: 47-51.
Gill SS, Hornung JM, Ibarra JE, Singh GJP, & Federici BA (1987)
Cytolytic activity and immunological similarity of Bacillus
thuringiensis subsp. israelensis and Bacillus thuringiensis
subsp. morrisoni isolate PG-14 toxins. Appl Environ Microbiol, 53:
1251-1256.
Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, & Goebel W
(1989) A Bacillus cereus cytolytic determinant, cereolysin AB, which
comprises the phospholipase C and sphingomyelinase genes: Nucleotide
sequence and genetic linkage. J Bacteriol, 171: 744-753.
Goldberg LJ & Margalit J (1977) A bacterial spore demonstrating rapid
larvicidal activity against Anopheles sergentii, Uranotaenia
unguiculata, Culex univitattus, Aedes aegypti and Culex
pipiens. Mosq News, 37: 355-358.
González JM Jr & Carlton BC (1980) Patterns of plasmid DNA in
crystalliferous and acrystalliferous strains of Bacillus
thuringiensis. Plasmid, 3: 92-98.
González JM Jr, Dulmage HT, & Carlton BC (1981) Correlation between
specific plasmids and delta-endotoxin production in Bacillus
thuringiensis. Plasmid, 5: 351-365.
González JM, Jr Brown BJ, & Carlton BC (1982) Transfer of Bacillus
thuringiensis plasmids coding for delta-endotoxin among strains of
B. thuringiensis and B. cereus. Proc Natl Acad Sci (USA), 79:
6951-6955.
Gordon RE (1977) Some taxonomic observations on the genus Bacillus.
In: Briggs JB ed. Biological regulations of vectors: The saprophytic
and aerobic bacteria and fungi. Washington, DC, US Department of
Health, Education and Welfare, pp 67-82 (Publication NIH 77-1180).
Granum PE (1997) Bacillus cereus. In: Doyle MP, Beuchat LR, &
Montville TJ ed. Food microbiology fundamentals and frontiers.
Washington, DC, ASM Press, pp 327-336.
Grassi S & Deseö KV (1984) [The natural occurrence of Bacillus
thuringiensis Berl. And its importance in the plant protection.] In:
[Proceedings of Seminar on Phytopathology, Sorrento, Italy, 26-29
March 1984.] Bologna, Italy, Clueb Publishing Co., vol 2, pp 424-433
(in Italian).
Green M, Heumann M, Sokolow R, Foster LR, Bryant R, & Skeels M (1990)
Public health implications of the microbial pesticide Bacillus
thuringiensis: An epidemiological study, Oregon, 1985-1986. Am J
Publ Health, 80: 848-852.
Griego VM & Spence KD (1978) Inactivation of Bacillus thuringiensis
spores by ultraviolet and visible light. Appl Environ Microbiol, 35:
906-910.
Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL,
Brousseau R, & Cygler M (1995) Bacillus thuringiensis CryIA(a)
insecticidal toxin: crystal structure and channel formation. J Mol
Biol, 254: 447-464.
Hadley WM, Burchiel SW, McDowell TD, Thilsted JP, Hibbs CM, Whorton
JA, Day PW, Friedman MB, & Stoll RE (1987) Five-month oral (diet)
toxicity/ infectivity study of Bacillus thuringiensis insecticides
in sheep. Fundam Appl Toxicol, 8: 236-242.
Hamed AR (1978-79) [Effects of Bacillus thuringiensis on parasites
and predators of Yponomeuta evenymellus (Lep., Yponomeutidae).] Z
Angew Entomol, 87: 294-311 (in German).
Hamel DR (1977) The effects of Bacillus thuringiensis on parasitoids
of the western spruce budworm, Choristoneura occidentalis
(Lepidoptera: Tortricidae), and the spruce coneworm, Dioryctria
reniculelloides (Lepidoptera: Pyralidae), in Montana. Can Entomol,
109: 1409-1415.
Hannay CL (1953) Crystalline inclusions in aerobic spore-forming
bacteria. Nature (Lond), 172: 1004.
Hansen BM & Hendriksen NB (1997a) Bacillus thuringiensis and B.
cereus enterotoxins: Proceedings of the 6th European Meeting of the
International Organization for Biological and Integrated Control of
Noxious Animals and Plants/West Paleaearctic Regional Section,
Copenhagen, 10-15 August 1997. Copenhagen, Denmark, Royal Veterinary
and Agricultural University, Department of Ecology and Molecular
Biology.
Hansen BM & Hendriksen NB (1997b) Comparative PCR analysis of B.
thuringiensis and B. cereus: Abstract for the International
Workshop on Molecular Biology of B. cereus, B. anthracis and
B. thuringiensis, Soria Moria, Oslo, 23-25 May 1997.
Hansen BM & Hendriksen NB (1999) Ecological aspects of the survival in
soil of spray released Bacillus thuringiensis subsp. kurstaki. In:
Abstract for the IOBC Symposium on Indirect Ecological Effects of
Biological Control, Montpelier, 17-20 October 1999. Montpelier,
France, Agropolis International.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1996) Bacillus
thuringiensis, ecology and environmental effects of its use for
microbial pest control (Environmental Project No. 316). Copenhagen,
Denmark, Ministry of Environment and Energy, Danish Environmental
Protection Agency.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1998) Molecular
and phenotypic characterization of Bacillus thuringiensis isolated
from leaves and insects. J Invertebr Pathol, 71: 106-114.
Harding J, Wolfenbarger D, Dupnik T, & Fuchs T (1972) Large scale
tests comparing Bacillus thuringiensis with methyl-parathion for
cotton insect control, field test report. Chicago, Illinois, Abbott
Laboratories (Unpublished document).
Hassan SA (1983) Results of laboratory testing of a series of
pesticides on egg parasites of the genus Trichogramma (Hymenoptera,
Trichogrammatidae). Nachr.bl Dtsch Pflanzenschutzdienstes
(Braunschweig), 35: 21.
Hassan S & Krieg A (1975) [ Bacillus thuringiensis preparations
harmless to the parasite Trichogramma cacoeciae (Hym.:
Trichogrammatidae).] Z Pflanzenkr Pflanzenchutz, 82: 515-521 (in
German).
Hassan G & Nabbut N (1996) Prevalence and characterization of
Bacillus cereus isolates from clinical and natural sources. J Food
Prot, 59: 193-196.
Heimpel AM (1954) Investigations of the mode of action of strains of
Bacillus cereus Frankland and Frankland pathogenic for the larch
sawfly, Pristiphora chrichsonii (Htg.). Kingston, Canada, Queen's
University, 155 pp (Doctoral dissertation).
Heimpel AM (1967) A critical review of Bacillus thuringiensis var.
thuringiensis Berliner and other crystalliferous bacteria. Annu Rev
Entomol, 12: 287-322.
Heimpel AM & Angus TA (1958) The taxonomy of insect pathogens related
to Bacillus cereus Frankland and Frankland. Can J Microbiol, 4:
531-541.
Helgason E, Caugant DA, Lecadet M-M, Chen Y, Mahillon J, Lövgren A,
Hegna I, Kvaloy K, & Kolsto A-B (1998) Genetic diversity of Bacillus
cereus/ B. thuringiensis isolates from natural sources. Curr
Microbiol, 37: 80-87.
Hendriksen NB & Hansen BM (1998) Phylogenetic relations of Bacillus
thuringiensis: Implications for risks associated to its use as a
microbiological pest control agent. IOBC Bull, 21: 5-8.
Hernandez E, Ramisse F, Ducoureau J-P, Cruel T, & Cavallo J-D (1998)
Bacillus thuringiensis subsp. konkukian (serotype H34)
superinfection: Case report and experimental evidence of pathogenicity
in immunosuppressed mice. J Clin Microbiol, 36: 2138-2139.
Höfte H & Whiteley HR (1989) Insecticidal crystal proteins of
Bacillus thuringiensis. Microbiol Rev, 53: 242-255.
Honée G & Visser B (1993) The mode of action of Bacillus
thuringiensis crystal proteins. Entomol Exp Appl, 69: 145-155.
Horn DJ (1983) Selective mortality of parasitoids and predators of
Myzus persicae on collards treated with malathion, carbaryl, or
Bacillus thuringiensis. Entomol Exp Appl, 34: 208-211.
Horsburgh R & Cobb L (1981) Effect of Dipel WP and Dipel + Guthion
tank mix combination for control of variegated leafroller, turfed
apple bud moth, and red-banded leafroller larvae on apples in a full
season and late season program in Virginia (Experiment No.
D-986-4240). Chicago, Illinois, Abbott Laboratories Inc.
Hostetter DL, Ignoffo CM, & Kearby WH (1975) Persistence of
formulations of Bacillus thuringiensis spores and crystals on
eastern red cedar foliage in Missouri. J Kansas Entomol Soc, 48:
189-193.
Hotha S & Banik RM (1997) Production of alkaline protease by Bacillus
thuringiensis H 14 in aqueous two-phase systems. J Chem Technol
Biotechnol, 69: 5-10.
Huber HE & Lüthy P (1981) Bacillus thuringiensis delta-endotoxin:
Composition and activation. In: Davidson EW ed. Pathogenesis of
invertebrate microbial diseases. Totowa, New Jersey, Allanheld-Osmun
Publishers, pp 209-234.
Huber HE, Lüthy P, Ebersold HR, & Cordier JL (1981) The subunits of
the parasporal crystal of Bacillus thuringiensis: size, linkage and
toxicity. Arch Microbiol, 129: 14-18.
Ignoffo CM (1973) Effects of entomopathogens on vertebrates. Ann NY
Acad Sci, 217: 141-164.
Ignoffo CM (1992) Environmental factors affecting persistence of
entomopathogens. Fla Entomol, 75: 516-525.
Innes DGL & Bendell JF (1989) The effects on small-mammal populations
of aerial applications of Bacillus thuringiensis, fenitrothion, and
Matacil(R) used against jack pine budworm in Ontario. Can J Zool,
67: 1318-1323.
Jackson SG, Goodbrand RB, Ahmed R, & Kasatiya S (1995) Bacillus
cereus and Bacillus thuringiensis isolated in a gastroenteritis
outbreak investigation. Lett Appl Microbiol, 21: 103-105.
Jaquet F, Hütter R, & Lüthy P (1987) Specificity of Bacillus
thuringiensis delta-endotoxin. Appl Environ Microbiol, 53: 500-504.
Jarrett P & Stephenson M (1990) Plasmid transfer between strains of
Bacillus thuringiensis infecting Galleria mellonella and
Spodoptera littoralis. Appl Environ Microbiol, 56: 1608-1614.
Jensen R (1974) Comparison of various insecticides including DIPEL WP
for control of the velvetbean caterpillar (Anticarsia gemmatalis)
and the green cloverworm (Plathypena scabra) on soybeans and the
effect on non-target species (Experiment No D911-1582). Chicago,
Illinois, Abbott Laboratories Inc.
Johnson AW (1974) Bacillus thuringiensis and tobacco budworm control
on flue-cured tobacco. J Econ Entomol, 67: 755-759.
Juarez-Perez VM, Ferrandis MD, & Frutos R (1997) PCR-based approach
for detection of novel Bacillus thuringiensis cry genes. Appl
Environ Microbiol, 63: 2997-3002.
Kaneko T, Nozaki R, & Aizawa K (1978) Deoxyribonucleic acid
relatedness between Bacillus anthracis, Bacillus cereus and
Bacillus thuringiensis. Microbiol Immunol, 22: 639-641.
Kazakova SB & Dzhunusov KK (1977) The effect of Bitoxibacillin-202 on
certain orchard insects in the Issyk-Kul' depression. Rev Appl
Entomol, A65: 5987.
Keller B & Langenbruch G-A (1993) Control of coleopteran pests by
Bacillus thuringiensis. In: Entwistle PF, Cory JS, Bailey MJ, &
Higgs S ed. Bacillus thuringiensis, an environmental biopesticide:
Theory and practice. Chichester, New York, Toronto, Wiley & Sons, pp
171-191.
Khawaled K, Ben-Dov E, Zaritsky A, & Barak Z (1990) The fate of
Bacillus thuringiensis var. israelensis in B. thuringiensis
var. israelensis-killed pupae of Aedes aegypti. J Invertebr
Pathol, 56: 312-316.
Kim HS, Lee DW, Woo SD, Yu YM, & Kang SK (1998) Seasonal distribution
and characterization of Bacillus thuringiensis isolated from
sericultural environments in Korea. J Gen Appl Microbiol, 44: 133-138.
Klier AF & Lecadet MM (1976) Arguments based on
hybridization-competition experiments in favor of the in vitro
synthesis of sporulation-specific mRNAs by the RNA polymerase of
Bacillus thuringiensis. Biochem Biophys Res Commun, 73: 263-270.
Knowles BH & Ellar DJ (1987) Colloid-osmotic lysis is a general
feature of the mechanisms of action of Bacillus thuringiensis
delta-endotoxins with different insect specificity. Biochim Biophys
Acta, 924: 509-518.
Krieg A (1971) Concerning alpha-exotoxin produced by vegetative cells
of Bacillus thuringiensis and Bacillus cereus. J Invertebr Pathol,
17: 134-135.
Krieg A (1973) About toxic effects of cultures of Bacillus cereus
and Bacillus thuringiensis on honey bees (Apis mellifera).
Z Pflanzenkr Pflanzenschutz, 80: 483-486.
Krieg A & Herfs W (1963) The effects of Bacillus thuringiensis on
honey bees. Entomol Exp Appl, 6: 1-9.
Krieg A, Hassan S, & Pinsdorf W (1980) Comparison of the effects of
the variety israelensis with other varieties of B. thuringiensis
on non-target organisms of the order Hymenoptera: Trichogramma
cacoeciae and Apis mellifera. Anz Schädlingsk Pflanz Umweltschutz,
53: 81-83.
Krieg A, Huger AM, Langenbruch GA, & Schnetter W (1983) Bacillus
thuringiensis var. tenebrionis, a new pathotype effective against
larvae of Coleoptera .. Z Angew Entomol, 96(5): 500-508.
Krywienczyk J, Dulmage HT, & Fast PG (1978) Occurrence of two
serologically distinct groups within Bacillus thuringiensis serotype
3ab variety kurstaki. J Invertebr Pathol, 37: 372-375.
Kumar PA, Sharma RP, & Malik VS (1996) The insecticidal protein of
Bacillus thuringiensis. Adv Appl Microbiol, 42: 1-43.
Lacey LA & Mulla MS (1990) Safety of Bacillus thuringiensis ssp.
israelensis and Bacillus sphaericus to nontarget organisms in the
aquatic environment. In: Laird M, Lacey LA, & Davidson EW ed. Safety
of microbial pesticides. Boca Raton, Florida, CRC Press, pp 169-188.
Lacey LA, Escaffre H, Philippon B, Sékétéli A, & Guillet P (1982)
Large river treatment with Bacillus thuringiensis (H-14) for the
control of Simulium damnosum s.l. in the Onchocerciasis Control
Programme. Tropenmed Parasitol, 33: 97-101.
Laferrière M, Bastille A, & Nadeau A (1987) Etude immunologique
impliquant les composantes de l'insecticide biologique Bacillus
thuringiensis var. kurstaki. St. Henri Rivière-du-Loup, Québec,
Department of Public Health, Grand-Portage Regional Hospital Center,
pp 1-19 (Unpublished report).
Lattin A, Grimes J, Hoxter KA, & Smith GJ (1990a) Dipel technical
material (Bacillus thuringiensis var. kurstaki): An avian oral
toxicity and pathogenicity study in the bobwhite (Project No.
161-112). Easton, Maryland, Wildlife International Ltd, pp 1-25
(Unpublished Abbott document).
Lattin A, Grimes J, Hoxter K, & Smith GJ (1990b) VectoBac technical
material (Bacillus thuringiensis var. israelensis): An avian oral
toxicity and pathogenicity study in the mallard (Project No. 161-115).
Easton, Maryland, Wildlife International Ltd, pp 1-24 (Unpublished
Abbott document).
Lattin A, Hoxter K, Driscoll C, Grimes J, & Jaber M (1990c) Dipel
technical material (Bacillus thuringiensis var. kurstaki): An
avian oral toxicity and pathogenicity study in the mallard (Project
No. 161-113). Easton, Maryland, Wildlife International Ltd, pp 1-28
(Unpublished Abbott document).
Lattin A, Hoxter K, & Smith GJ (1990d) VectoBac technical material
(Bacillus thuringiensis var. israelensis): An avian oral toxicity
and pathogenicity study in the bobwhite (Project No. 161-114). Easton,
Maryland, Wildlife International Ltd, pp 1-27 (Unpublished Abbott
document).
Lechner S, Mayr R, Francis KP, Prüß BM, Kaplan T, Wießner-Gunkel E,
Stewart GSAB, & Scherer S (1998) Bacillus weihenstephanensis sp.
nov. is a new psychrotolerant species of the Bacillus cereus group.
Int J System Bacteriol, 48: 1373-1378.
Leong KLH, Cano RJ, & Kubinski AM (1980) Factors affecting Bacillus
thuringiensis total field persistence. Environ Entomol, 9: 593-599.
Levêque C, Fairhurst CP, Abbau K, Pangy D, Curtis MS, & Traoré K
(1988) Onchocerciasis control programme in West Africa: ten years of
monitoring fish populations. Chemosphere, 17: 421-440.
Li J, Carroll J, & Ellar DJ (1991) Crystal structure of insecticidal
(delta)-endotoxin from Bacillus thuringiensis at 2.5 Å resolution.
Nature (Lond), 353: 815-821.
Logan NA & Berkeley CW (1984) Identification of Bacillus strains
using the API system. J Gen Microbiol, 130: 1871-1882.
Lund T & Granum PE (1997) Comparison of biological effect of the two
different enterotoxin complexes isolated from three different strains
of Bacillus cereus. Microbiology, 143: 3329-3336.
Lüthy P (1986) Insect pathogenic bacteria as pest control agents.
Fortschr Zool, 32: 201-216.
Lüthy P & Ebersold HR (1981) The entomocidal toxins of Bacillus
thuringiensis. Pharmacol Ther, 13: 257-283.
Lynch MJ & Baumann P (1985) Immunological comparisons of the crystal
protein from strains of Bacillus thuringiensis. J Invertebr Pathol,
46: 47-57.
Lynch RE, Lewis LC, & Brindley TA (1976) Bacteria associated with eggs
and first-instar larvae of the European corn borer: Isolation
techniques and pathogenicity. J Invertebr Pathol, 27: 325-331.
McClintock JT, Schaffer CR, & Sjoblad RD (1995) A comparative review
of the mammalian toxicity of Bacillus thuringiensis-based
pesticides. Pestic Sci, 45: 95-105.
Manasherob R, Ben-Dov E, Zaritsky A, & Barak Z (1998) Germination,
growth, and sporulation of Bacillus thuringiensis subsp.
israelensis in excreted food vacuoles of the protozoan Tetrahymena
pyriformis. Appl Environ Microbiol, 64: 1750-1758.
Margalit J & Dean D (1985) The story of Bacillus thuringiensis var.
israelensis. J Am Mosq Control Assoc, 1: 1-7.
Martin PAW & Travers RS (1989) Worldwide abundance and distribution of
Bacillus thuringiensis isolates. Appl Environ Microbiol, 55(10):
2437-2442.
Martin WF & Reichelderfer CF (1989) Bacillus thuringiensis:
Persistence and movement in field crops. In: Abstracts of the SIP
XXIInd Annual Meeting, Centre for Adult Education, University of
Maryland, College Park, Maryland, 20-24 August 1989. Society for
Invertebrate Pathology, p 25.
Martouret D & Euverte G (1964) The effect of Bacillus thuringiensis
Berliner preparations on the honey bee under conditions of forced
feeding. J Insect Pathol, 6: 198-203.
Meadows MP (1993) Bacillus thuringiensis in the environment: Ecology
and risk assessment. In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S
ed. Bacillus thuringiensis, an environmental biopesticide: Theory
and practice. Chichester, New York, Toronto, Wiley & Sons, pp 193-220.
Meadows MP, Ellis DJ, Butt J, Jarrett P, & Burges HD (1992)
Distribution, frequency and diversity of Bacillus thuringiensis in
an animal feed mill. Appl Environ Microbiol, 58(4): 1344-1350.
Melin BE & Cozzi EM (1990) Safety to nontarget invertebrates of
Lepidopteran strains of Bacillus thuringiensis and their
(ß)-exotoxins. In: Laird M, Lacey LA, & Davidson EW ed. Safety of
microbial insecticides. Boca Raton, Florida, CRC Press, pp 149-167.
Menon AS & De Mestral J (1985) Survival of Bacillus thuringiensis
var. kurstaki in waters. Water Air Soil Pollut, 25: 265-274.
Merritt RW, Walker ED, Wilzbach MA, Cummins KW, & Morgan WT (1989) A
broad evaluation of Bti for black fly (Diptera:Simuliidae) control in
a Michigan river: efficacy, carry and nontarget effects on
invertebrates and fish. J Am Mosq Control Assoc, 5: 397-415.
Mikami T, Horikawa T, Murakami T, Sato N, Ono Y, Matsumoto T, Yamakawa
A, Murayama S, Katagiri S, & Suzuki M (1995) Examination of toxin
production from environmental Bacillus cereus and Bacillus
thuringiensis. J Pharm Soc Jpn, 115: 742-748.
Miura T, Takahashi RM, & Mulligan FS III (1980) Effects of the
bacterial mosquito larvicide, Bacillus thuringiensis serotype H-14
on selected aquatic organisms. Mosq News, 40: 619-622.
Mohd-Salleh MB, Beegle CC, & Lewis LC (1980) Fermentation media and
production of exotoxin by three varieties of Bacillus thuringiensis.
J Invertebr Pathol, 35: 75-83.
Molloy DP (1992) Impact of the black fly (Diptera: Simuliidae) control
agent Bacillus thuringiensis var. israelensis on Chironomids
(Diptera: Chronomidae) and other non-target insects: results of ten
field trials. J Am Mosq Control Assoc, 8: 24-31.
Morris ON, Armstrong JA, & Hildebrand MJ (1977) Aerial field trials
with a new formulation of Bacillus thuringiensis against the spruce
budworm, Choristoneura fumiferana (Clem.). Ottawa, Canada, Chemical
Control Research Institute (Report CC-X-144).
Morris ON, Hildebrand MJ, & Armstrong JA (1980) Preliminary field
studies on the use of additives to improve deposition rate and
efficacy of commercial formulations of Bacillus thuringiensis
applied against the spruce budworm, Choristoneura fumiferana
(Lepidoptera: Tortricidae). Sault Ste. Marie, Ontario, Canada, Forest
Pest Management Institute, pp 1-23 (Report FPM-X-32).
Moulinier CL, Mas JP, Moulinier Y, De Barjac H, Giap G, & Couprie B
(1995) Étude de l'innocuité de Bacillus thuringiensis var.
israelensis pour les larves d'huitre. Bull Soc Pathol Exot, 74(4):
381-391.
Mück O, Hassan S, Huger AM, & Krieg A (1981) Effects of Bacillus
thuringiensis Berliner on the parasitic hymenoptera Apanteles
glomeratus L. (Braconidae) and Pimpla turionellae (L.)
(Ichneumonidiae). Z Angew Entomol, 92: 303-314.
Mulla MS (1988) Activity, field efficacy and use of Bacillus
thuringiensis (H-14) against mosquitoes. In: de Barjac H &
Sutherland DJ ed. Bacterial control of mosquitoes and blackflies. New
Brunswick, New Jersey, Rutgers University Press.
Mulla MS & Khasawinah AM (1969) Laboratory and field evaluation of
larvicides against chironomid midges. J Econ Entomol, 62: 37-41.
Mulla MS, Norland RL, Fanara DM, Darwazeh HA, & McKean DW (1971)
Control of chironomid midges in recreational lakes. J Econ Entomol
64(1): 300-307.
Mulla MS, Federici BA, & Darwazeh HA (1982) Larvicidal efficacy of
Bacillus thuringiensis serotype H-14 against stagnant-water
mosquitoes and its effects on nontarget organisms. Environ Entomol,
11: 788-795.
Mulligan FS III & Schaefer CH (1982) Integration of a selective
mosquito control agent Bacillus thuringiensis serotype H-14, with
natural predator populations in pesticide-sensitive habitats. Proc
Calif Mosq Vector Control Assoc, 49: 19-22.
Murakami T, Hiraoka T, Matsumoto T, Katagiri S, Shinagawa K, & Suzuki
M (1993) Analysis of common antigen of flagella in Bacillus cereus
and Bacillus thuringiensis. FEMS Microbiol Lett, 107: 179-184.
Narva KE, Payne JM, Schwab GE, Hickle LA, Galasan T, & Sick AJ (1991)
Novel Bacillus thuringiensis microbes active against nematodes, and
genes encoding novel nematode-active toxins cloned from Bacillus
thuringiensis isolates: European patent application EP0462 721A2.
Munich, Germany, European Patent Office.
Navon A (1993) Control of Lepidopteran pests with Bacillus
thuringiensis. In: Bacillus thuringiensis, An Environmental
Biopesticide: Theory and Practice. Eds, Entwistle PF, Cory JS, Bailey
MJ & Higgs S. Chichester, New York, Toronto, Wiley & Sons, pp 126-146.
Nielsen-LeRoux C, Hansen BM, Henriksen NB (1998) Safety of Bacillus
thuringiensis. IOBC Bull, 21: 269-272.
Nishikawa Y, Kramer JM, Hanaoka M, & Yasukawa A (1996) Evaluation of
serotyping, biotyping, plasmid banding pattern analysis, and HEp-2
vacuolation factor assay in the epidemiological investigation of
Bacillus cereus emetic-syndrome food poisoning. Int J Food
Microbiol, 31: 149-159.
Noble MA, Riben PD, & Cook GJ (1992) Microbiological and
epidemiological surveillance programme to monitor the health effects
of Foray 48B BTK spray. Vancouver, Canada, Ministry of Forests of the
Province of British Columbia, pp 1-63.
Norris JR (1971) The protein crystal toxin of Bacillus
thuringiensis: biosynthesis and physical structure. In: Burges HD &
Mussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc, pp 229-246.
Notermans S & Batt CA (1998) A risk assessment approach for food-borne
Bacillus cereus and its toxins. J Appl Environ Microbiol Symp,
84(suppl): 51S-61S.
Obadofin AA & Finlayson DG (1977) Interactions of several insecticides
and a carabid predator (Bembidion lampros (Hrbst)) and their effects
on Hylemya brassicae (Bouché). Can J Plant Sci, 57: 1121-1126.
Ohana B, Margalit J, & Barak Z (1987) Fate of Bacillus thuringiensis
subsp. israelensis under simulated field conditions. Appl Environ
Microbiol, 53: 828-831.
Ohba M & Aizawa K (1978) Serological identification of Bacillus
thuringiensis and related bacteria isolated in Japan. J Invertebr
Pathol, 32: 303-309.
Ohba M & Aizawa K (1986) Distribution of Bacillus thuringiensis in
soils of Japan. J Invertebr Pathol, 47: 277-282.
Ohba M, Yu YM, & Aizawa K (1988) Occurrence of noninsecticidal
Bacillus thuringiensis flagellar serotype 14 in the soil of Japan.
Appl Microbiol, 11: 85-89.
Olejnicek J & Maryskova B (1986), The influence of Bacillus
thuringiensis on the mosquito predator Notonecta glauca. Folia
Parasitol (Prague), 37: 279.
Otvos IS & Vanderveen S (1993) Environmental report and current status
of Bacillus thuringiensis var. kurstaki use for control of forest
and agricultural insect pests. Victoria, British Columbia, Ministry of
Forests, Forestry Canada, pp 1-81.
Pedersen JC, Damgaard PH, Eilenberg E, & Hansen BM (1995) Dispersal of
Bacillus thuringiensis var. kurstaki in an experimental cabbage
field. Can J Microbiol, 41: 118-125.
Petras SF & Casida LE Jr (1985) Survival of Bacillus thuringiensis
spores in soil. Appl Environ Microbiol, 50: 1496-1501.
Pinnock DE, Brand RJ, Jackson KL, & Milstead JE (1974) The field
persistence of Bacillus thuringiensis spores on Cercis occidentalis
leaves. J Invertebr Pathol, 23: 341-346.
Pinnock DE, Milstead JE, Kirby ME, & Nelson BJ (1977) Stability of
entomopathogenic bacteria. In: Environmental stability of microbial
insecticides: Miscellaneous publications. Lanham, Maryland,
Entomological Society of America, vol 10, pp 77-97.
Pivovarov Yu P, Ivashina SA, & Padalkin VP (1977) [Hygienic assessment
of investigation of insecticide preparations.] Gig i Sanit, 8: 45-48
(in Russian).
Prasertphon S, Areekul P, & Tanada Y (1973) Sporulation of Bacillus
thuringiensis in host cadavers. J Invertebr Pathol, 21: 205-207.
Prefontaine G, Fast P, Lau PCK, Hefford MA, Hanna Z, & Brousseau R
(1987) Use of oligonucleotide probes to study the relatedness of
delta-endotoxin genes among Bacillus thuringiensis subspecies and
strains. Appl Environ Microbiol, 53: 2808-2814.
Priest FG, Goodfellow M, & Todd C (1988) A numerical classification of
the genus Bacillus. J Gen Microbiol, 134: 1847-1882.
Priest FG, Kaji DA, Rosato YB, & Canhos VP (1994) Characterization of
Bacillus thuringiensis and related bacteria by ribosomal RNA gene
restriction fragment length polymorphisms. Microbiology, 140:
1015-1022.
Purcell BH (1981) Effects of Bacillus thuringiensis var.
israelensis on Aedes taeniorhynchus and some non-target organisms
in the salt marsh. Mosq News, 41(3): 476-484.
Pusztai M, Fast P, Gringorten L, Kaplan H, Lessard T, & Carey PR
(1991) The mechanism of sunlight-mediated inactivation of Bacillus
thuringiensis crystals. Biochem J, 273: 43-47.
Quinlan RJ (1990) Registration requirements and safety considerations
for microbial pest control agents in the European Economic Community.
In: Laird M, Lacey LA, & Davidson EW ed. Safety of microbial
pesticides. Boca Raton, Florida, CRC Press, pp 11-18.
Ray DE (1990) Pesticides derived from plants and other organisms. In:
Hayes WJ & Laws ER ed. Handbook of pesticide toxicology. New York,
London, Academic Press, pp 585-636.
Reardon RC & Haissig K (1983) Spruce budworm (Lepidoptera:
tortricidae) larval populations and field persistence of Bacillus
thuringiensis after treatment in Wisconsin. J Econ Entomol, 76:
1139-1143.
Reddy A, Battisti L, & Thorne CB (1987) Identification of
self-transmissible plasmids in four Bacillus thuringiensis
subspecies. J Bacteriol 169(11): 5263-5270.
Rogatin AB & Baizhanov M (1984) [Laboratory study of Bacillus
thuringiensis israelensis serotype H-14 on various groups of
hydrobionts.] Izv Akad Nauk Kaz SSR Ser Biol, D6: 22-25 (in Russian).
Salama HS & Zaki FN (1983) Interaction between Bacillus thuringiensis
Berliner and the parasites and predators of Spodoptera littoralis
in Egypt. Z Angew Entomol, 95: 425-429.
Salama HS, Zaki FN, & Sharaby AF (1982) Effect of Bacillus
thuringiensis Berl. on parasites and predators of the cotton
leafworm Spodoptera littoralis (Boisd). Z Angew Entomol, 94:
498-504.
Saleh SM, Harris RF, & Allen ON (1970) Fate of Bacillus thuringiensis
in soil: Effect of soil pH and organic amendment. Can J Microbiol,
16: 677-680.
Samples JR & Buettner H (1983) Corneal ulcer caused by a biologic
insecticide (Bacillus thuringiensis). Am J Ophthalmol, 95(2):
258-260.
Sampson MN & Gooday GW (1998) Involvement of chitinases of Bacillus
thuringiensis during pathogenesis in insects. Microbiology, 144:
2189-2194.
Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J,
Zeigler DR, & Dean DH (1998) Bacillus thuringiensis and its
pesticidal crystal proteins. Microbiol Mol Biol Rev, 62: 775-806.
Schnetter W, Engler S, Morawcsik J, & Becker N (1981) [Efficacy of
Bacillus thurigiensis var. israelensis against mosquito larvae
and non-target organisms.] Mitt Dtsch Ges Allg Angew Entomol, 2:
195-202 (in German).
Schwartz JL, Garneau L, Masson L, & Brousseau R (1991) Early response
of cultured lepidopteran cells to exposure to delta-endotoxin from
Bacillus thuringiensis: involvement of calcium and anionic channels.
Biochem Biophys Acta, 1065: 250-260.
Shadduck JA (1980) Bacillus thuringiensis serotype H-14 maximum
challenge and eye irritation safety tests in mammals. Geneva, World
Health Organization, pp 1-21 (WHO/VBC/80.763).
Shadduck JA (1983) Some considerations on the safety evaluation of
nonviral microbial insecticides. WHO Bull, 6(1): 117-128.
Sheeran W & Fisher SW (1992) The effects of agitation, sediment and
competition on the persistence and efficacy of Bacillus thuringiensis
var. israelensis (Bti). Ecotoxicol Environ Saf, 24: 338-346.
Shevelev AB, Lewitin E, Novikova SI, Wojciechowska YaA, Usacheva EA,
Chestukhina GG, & Stepanov (1998) A new PCR-based approach to a fast
search of a wide spectrum of cry genes from Bacillus thuringiensis
strains. Biochem Mol Biol Int, 45: 1265-1271.
Shinagawa K (1990) Purification and characterization of Bacillus
cereus enterotoxin and its application to diagnosis. In: Pohland AE,
Dowell VR Jr, & Richard JL ed. Microbial toxins in foods and feeds:
Cellular and molecular modes of action. New York, Plenum Press, pp
181-193.
Siegel JP & Shadduck JA (1990) Clearance of Bacillus sphaericus and
Bacillus thuringiensis ssp. israelensis from mammals. J Econ
Entomol, 83: 347-355.
Siegel JP, Shadduck JA, & Szabo J (1987) Safety of the entomopathogen
Bacillus thuringiensis var. israelensis for mammals. J Econ
Entomol, 80: 717-723.
Slatin SL, Abrams CK, & English L (1990) Delta-endotoxins form
cation-selective channels in planar lipid bilayers. Biochem Biophys
Res Commun, 169: 765-772.
Slepecky RA & Hemphill HE (1992) The genus Bacillus-nonmedical. In:
Balows A, Truper HG, Dworkin M, Harder W, & Schleifer K-H ed. The
prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol II, pp
1663-1696.
Smedley DP & Ellar DJ (1996) Mutagenesis of 3 surface-exposed loops of
a Bacillus thuringiensis insecticidal toxin reveals residues
important for toxicity, recognition and possibly membrane insertion.
Microbiology, 142: 1617-1624.
Smith RA (1987) Use of crystal serology to differentiate among
varieties of Bacillus thuringiensis. J Invertebr Pathol, 50: 1-8.
Smith RA & Couche GA (1991) The phylloplane as a source of Bacillus
thuringiensis variants. Appl Environ Microbiol, 57: 311-315.
Snarski VM (1990) Interactions between Bacillus thuringiensis subsp.
israelensis and fathead minnows, Pimephales promelas Rafinesque,
under laboratory conditions. Appl Environ Microbiol, 56: 2618-2622.
Sorenson AA & Falcon LA (1980) Comparison of microdroplet and high
volume application of Bacillus thuringiensis on pear suppression of
fruit tree leaf roller Acrhips argyrospilus and coverage on foliage
and fruit. Environ Entomol, 9: 350.
Stahly DP, Dingman DW, Bulla LA Jr, & Aronson AI (1978) Possible
origin and function of the parasporal crystals in Bacillus
thuringiensis. Biochem Biophys Res Commun, 84(3): 581-588.
Stahly DP, Andrews RE, & Yousten AA (1991) The genus Bacillus-insect
pathogens. In: Balows A, Truper HG, Dworkin M, Harder W, & Schleifer
K-H ed. The prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol
II, pp 1697-1745.
Surprenant DC (1989) Acute toxicity of Bacillus thuringiensis var.
tenebrionis technical material to rainbow trout (Salmo gairdneri)
under static renewal conditions. Wareham, Massachusetts, Springborn
Life Sciences Inc., pp 1-19 (Unpublished Abbott document).
Tayabali AF & Seligy VL (1997) Cell integrity markers for in vitro
evaluation of cytotoxic responses to bacteria-containing commercial
insecticides. Ecotoxicol Environ Saf, 37: 152-162.
Thomas WE & Ellar DJ (1983) Bacillus thuringiensis var. israelensis
crystal delta-endotoxin: effects on insect and mammalian cells in
vitro and in vivo. J Cell Sci, 60: 181-197.
Thomas EM & Watson TF (1986) Effect of Dipel (Bacillus thuringiensis)
on the survival of immature and adult Hyposoter exiguae
(Hymenoptera: Ichneumonidae). J Invertebr Pathol, 47: 178-183.
Tomlin CDS ed. (1997) The pesticide manual, 11th ed. Farnham, Surrey,
British Crop Protection Council, 1606 pp.
Travers RS, Martin PAW, & Reichelderfer CF (1987) Selective process
for efficient isolation of soil Bacillus spp. Appl Environ
Microbiol, 53: 1263-1266.
Väisänen OM, Mwaisumo NJ, & Salkinoja-Salonen MS (1991)
Differentiation of dairy strains of the Bacillus cereus group by
phage typing, minimum growth temperature, and fatty acid analysis. J
Appl Bacteriol, 70: 315-324.
Van Frankenhuyzen K (1993) The challenge of Bacillus thuringiensis.
In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 1-35.
Vaòková J & Purrini K (1979) Natural epizooties caused by bacilli of
the species Bacillus thuringiensis and Bacillus cereus. Z Angew
Entomol, 88: 216-221.
Van Rie J, Jansens S, Höfte H, Degheele D, & Van Mellaert H (1989),
Specificity of Bacillus thuringiensis delta-endotoxins: Importance
of specific receptors on the brush border membrane of the mid-gut of
target insects. Eur J Biochem, 186: 239-247.
Venkateswerlu G & Stotzky G (1992) Binding of the protoxin and toxin
proteins of Bacillus thuringiensis subsp. kurstaki on clay
minerals. Curr Microbiol, 25: 225-233.
Visser B, Bosch D, & Honée G (1993) Domain-function studies of
Bacillus thuringiensis crystal proteins: a genetic approach. In:
Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental piopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 71-88.
Wallner W & Surgeoner G (1974) Control of oakleaf caterpillar,
Heterocampa manteo, and the impact of controls on nontarget
organisms. Chicago, Illinois, Abbott Laboratories (Unpublished
document).
Wallner WE, Dubois NR, & Grinberg PS (1983) Alteration of parasitism
by Rogas lymantriae (Hymenoptera: Braconidae) in Bacillus
thuringiensis-stressed gypsy moth (Lepidoptera: Lymantriidae) hosts.
J Econ Entomol, 76: 275-277.
Warren RE, Rubenstein D, Ellar DJ, Kramer JM, & Gilbert RJ (1984)
Bacillus thuringiensis var. israelensis: Protoxin activation and
safety. Lancet: March 24: 678-679.
Weires RW & Smith GL (1977) Apple mite control. Hudson Valley, New
York, New York State Agricultural Experimental Station.
Weseloh RM & Andreadis TG (1982) Possible mechanism for synergism
between Bacillus thuringiensis and the gypsy moth (Lepidoptera:
Lymantriidae) parasitoid, Apanteles melanoscelus (Hymenoptera:
Braconidae). Ann Entomol Soc Am, 75: 435-438.
Weseloh RM, Andreadis TG, Moore REB, & Anderson JF (1983) Field
confirmation of a mechanism causing synergism between Bacillus
thuringiensis and the gypsy moth parasitoid, Apanteles
melanoscelus. J Invertebr Pathol, 41: 99-103.
West AW, Burges HD, & Wyborn CH (1984a) Effect of incubation in
natural and autoclaved soil upon potency and viability of Bacillus
thuringiensis. J Invertebr Pathol, 44: 121-127.
West AW, Burges HD, White JR, & Wyborn CH (1984b) Persistence of
Bacillus thuringiensis parasporal crystal insecticidal activity in
soil. J Invertebr Pathol, 44: 128-133.
WHO (1982) Data sheet on the biological control agent Bacillus
thuringiensis serotype H-14 (de Barjac 1978). Geneva, World Health
Organization (WHO/VBC/79.750 Rev. 1).
WHO (1985) Ninth report of the WHO Expert Committee on Vector Biology
and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 720).
WHO (1991) Fourteenth report of the WHO Expert Committee on Vector
Biology and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 813).
Wilkinson JD, Biever KD, & Ignoffo CM (1975) Contact toxicity of some
chemical and biological pesticides to several insect parasitoids and
predators. Entomophaga, 20: 113-120.
Workman RB (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc Fla State Hortic Soc, 90: 401-402.
Wunschel D, Fox KF, Black GE, & Fox A (1994) Discrimination among the
B. cereus group, in comparison to B. subtilis, by structural
carbohydrate profiles and ribosomal RNA spacer region PCR. System Appl
Microbiol, 17: 625-635.
Yameogo L, Levêque C, Traoré K, & Fairhurst CP (1988) Dix ans de
surveillance de la faune aquatique des rivières d'Afrique de l'Ouest
traitées contre les Simulides (Diptera: Simuliidae), agents vecteurs
de l'onchocercose humaine. Nat Can, 115: 287-298.
Yousten AA (1973) Effect of the Bacillus thuringiensis
delta-endotoxin on an insect predator which has consumed intoxicated
cabbage looper larvae. J Invertebr Pathol, 21: 312-314.
Yousten AA, Genthner FJ, & Benfield EF (1992) Fate of Bacillus
sphaericus and Bacillus thuringiensis serovar israelensis in the
aquatic environment. J Am Mosq Control Assoc, 8: 143-148.
Zahner V, Momen H, Salles CA, & Rabinovitch L (1989) A comparative
study of enzyme variation in Bacillus cereus and Bacillus
thuringiensis. J Appl Bacteriol, 67: 275-282.
Zukowski K (1995) [Laboratory examination of the effectiveness of new
biological preparations for reducing populations of cockroaches
(Blattella germanica L.)]. Rocz Panstw Zakl Hig, 46: 293-297 (in
Polish).
RÉSUMÉ
La présente monographie traite des agents microbiens de lutte
contre les nuisibles utilisant le bacille Bacillus thuringiensis
(Bt). Ce bacille, qui est aussi appelé bacille de Thuringe, est
également une source très importante de gènes utilisée pour conférer
aux plantes et aux microorganismes transgéniques (ou organismes
génétiquement modifiés, OGM) la faculté de résister aux ravageurs et
autres nuisibles. Les effets que pourraient avoir ces OGM sur la santé
humaine et l'environnement présentent divers aspects qui sont sans
rapport ou tout au plus en rapport lointain avec les produits à base
de Bt et n'entrent pas, par conséquent, dans le cadre de la présente
monographie.
1. Identité, caractéristiques biologiques et méthodes d'analyse
Bacillus thuringiensis est une bactérie anaérobie facultative,
gram-positif, qui forme des inclusions protéiques caractéristiques
adjacentes à l'endospore. Certaines sous-espèces de Bt peuvent
synthétiser plusieurs inclusions parasporales. Le Bt est génétiquement
indiscernable du Bc, exception faite de son aptitude à former des
inclusions cristallines parasporales qui sont toxiques pour certains
invertébrés, notamment les larves d'insectes appartenant aux ordres
suivants: coléoptères, diptères et lépidoptères. Les inclusions
parasporales sont constituées de diverses protéines cristallisées
insecticides (ICP). Ces cristaux sont de forme variable (bipyramidale,
cuboïdale, rhomboïdale plane, sphérique ou composite, c'est-à-dire
comportant deux types de cristaux) selon la nature des protéines qui
les composent. On a établi une corrélation partielle entre la
morphologie des cristaux, la composition en protéines cristallisées et
l'activité biologique vis-à-vis des insectes.
Le taxon phénotypique fondamental est la sous-espèce,
caractérisée par son sérotype flagellaire (H). En 1998, on avait déjà
décrit 67 sous-espèces. Les gènes qui codent pour les ICP sont pour la
plupart situés sur les plasmides. Chacune de ces protéines n'est le
produit que d'un seul gène. La plupart des plasmides porteurs de gènes
ICP se transmettent facilement d'une souche bactérienne à l'autre par
conjugaison et peuvent aussi passer à une espèce bactérienne voisine.
La classification phénotypique est maintenant complétée par une
caractérisation basée sur la biologie moléculaire et plus précisément
sur la séquence des gènes codant pour les cristaux (cry et cyt)
plutôt que sur la spécificité vis-à-vis des insectes cibles. Divers
domaines des ICP sont responsables de la sensibilité de l'hôte
(reconnaissance des récepteurs) et de la toxicité (formation de
pores).
Parmi les techniques couramment utilisées pour caractériser les
souches de Bt ou les inclusions protéiques elles-mêmes, on peut citer
l'analyse des acides gras pariétaux, les anticorps monoclonaux, les
sondes d'ADN oligonucléotidiques, les profils plasmidiques, l'analyse
par amplification génique (PCR), la technique des empreintes
génétiques et les profils électrophorétiques SDS-PAGE (électrophorèse
en gel de polyacrylamide en présence de dodécylsulfate de sodium).
Certaines sous-espèces de Bt produisent pendant leur croissance
une bêta-exotoxine constitué d'un nucléotide thermostable qui peut
contaminer les produits. Cette bêta-exotoxine est toxique pour presque
toutes les formes de vie, y compris l'Homme et les insectes cibles. Au
cours de leur croissance, les diverses souches de Bt produisent toutes
sortes d'antibiotiques, d'enzymes, de métabolites et de toxines, y
compris des toxines Bc, qui peuvent avoir des effets nocifs sur les
organismes visés ou non visés. La numération des spores n'est pas le
reflet fidèle de l'activité insecticide d'une souche de Bt ou d'un
produit qui en dérive. Pour mesurer l'activité (en unités
toxicologiques internationales (ITU) par mg), on procède à un test
biologique sur insecte au moyen d'un étalon international.
2. Mode d'action sur les insectes cibles
Les Bt sporulés ou les complexes spores-ICP doivent être ingérés
par les larves d'insectes appartenant aux espèces sensibles.
L'efficacité des ICP dépend de plusieurs facteurs: solubilisation dans
l'intestin moyen, conversion de la protoxine en toxine biologiquement
active sous l'action des enzymes protéolytiques, fixation de la toxine
active aux récepteurs membranaires spécifiques par sa région
C-terminale et formation de pores par la région N-terminale entraînant
la lyse des cellules épithéliales. La germination des spores et la
prolifération de cellules bactériennes végétatives dans l'hémocèle
peut entraîner une septicémie qui contribue également à la mort. La
spécificité d'hôte des différentes ICP est essentiellement déterminée
par leur fixation aux récepteurs.
3. Habitats
On a isolé de nombreuses sous-espèces de Bt sur des insectes
morts ou mourants appartenant principalement à l'ordre des
coléoptères, des diptères et des lépidoptères, mais nombreuses sont
également celles qui ont été isolées du sol, de la surface des
feuilles ou d'autres habitats. Les cadavres d'insectes contiennent
souvent de grandes quantités de spores et d'ICP susceptibles de
pénétrer dans l'environnement. Les sous-espèces actives contre les
coléoptères et les lépidoptères sont principalement associées au sol
et aux surfaces foliaires, alors que les sous-espèces actives contre
les diptères se rencontrent communément dans les milieux aquatiques.
Dans l'environnement, les spores sont capables de persister et de se
développer en présence de conditions favorables et de nutriments
appropriés.
4. Produits du commerce. Production et épandage
Les produits commerciaux classiques à base de bacille de
Thuringe, qui utilisent des souches naturelles, représentent environ
90% du marché mondial des agents microbiologiques de lutte contre les
nuisibles. La plupart de ces produits contiennent des spores viables
et des ICP, mais dans certains d'entre eux, les spores sont inactivées
(Bti). Chaque année, on en produit quelque 13 000 tonnes par la
technique de fermentation aérobie. Les produits classiques à base de
Bt sont principalement destinés à lutter contre les lépidoptères qui
ravagent les cultures et les plantations forestières; toutefois, ces
dernières années, on a également commercialisé des souches actives
contre les coléoptères. Les programmes de santé publique utilisent
également des souches de Bti actives contre les diptères vecteurs de
maladies virales ou parasitaires.
Les formulations commerciales de bacille de Thuringe peuvent être
épandues sur le feuillage, le sol, les étendues d'eaux ou dans les
entrepôts de denrées alimentaires pour combattre les insectes. Une
fois le produit épandu dans l'écosystème, les cellules bactériennes
végétatives et les spores peuvent persister à des concentrations
progressivement décroissantes pendant des semaines, des mois ou des
années en tant que constituants de la microflore naturelle. Par
contre, les ICP perdent leur activité biologique au bout de quelques
heures ou de quelques jours.
5. Effets du Bt sur les organismes non visés
Les études effectuées sur des mammifères et notamment celles qui
ont porté sur des animaux de laboratoire ont consisté a évaluer
l'infectiosité et la toxicité éventuelles de diverses préparations à
base de Bt contenant notamment des ICP, des spores et des cellules
bactériennes en phase végétative. Sous ces trois formes, les
différentes sous-espèces de Bt se sont révélées pour la plupart non
pathogènes et non toxiques pour les diverses espèces animales
utilisées. On a montré que les cellules bactériennes en phase
végétative et les spores persistaient pendant plusieurs semaines sans
causer d'effets nocifs. En particulier, on a constaté que le Bt
n'avait pas d'effets indésirables sur les oiseaux, les poissons et de
nombreux autres vertébrés aquatiques non visés, lors d'études en
laboratoire ou sur le terrain portant sur un grand nombre de
spécimens. Il n'y a que relativement peu d'invertébrés aquatiques qui
se révèlent sensibles au Bt en laboratoire ou sur le terrain. Par
ailleurs, le bacille de Thuringe n'exerce pas non plus d'effets nocifs
sur les lombrics.
L'activité insecticide des différentes sous-espèces de Bt
présente en général une spécificité d'hôte très marquée vis-à-vis des
coléoptères, des diptères et des lépidoptères et on a montré qu'elle
n'avait pratiquement aucun effet toxique direct sur les arthropodes
non visés. La plupart des données relatives à l'innocuité de ces
produits pour les arthropodes non visés concernent les sous-espèces de
Bt actives contre les diptères et les lépidoptères.
Les études consacrées aux formulations de Bti exemptes de
contaminants toxiques ont montré qu'elles étaient sans danger pour la
plupart des arthropodes non visés. Certains moucherons (chironomides
appartenant à l'ordre des diptères) très proches des moustiques se
sont révélés sensibles à de fortes doses de Bti mais ne sont nullement
affectés aux doses utilisées pour la destruction des larves de
moustiques. Des études sur le terrain ont mis en évidence des cas de
réduction ou au contraire d'augmentation de certaines populations
d'arthropodes non visés.
Les études toxicologiques auxquelles ont été soumis de nombreux
ordres d'insectes n'ont, pour la plupart d'entre eux, révélé aucun
effet toxique imputable au Btk.
On a observé une certaine mortalité chez des abeilles
(Apis mellifera) qui avaient été soumises à des bacilles des
sous-espèces Btt et Btk en phase végétative, mais il ne semble pas que
les spores ou les ICP soient capables de produire un tel effet. En
laboratoire et sur le terrain, le Btg n'a aucun effet toxique sur les
abeilles.
Les souches de Bte productrices de bêta-exotoxine se sont
révélées capables d'exercer des effets toxiques sur les arthropodes
non visés.
6. Exposition humaine et effets du bacille de Thuringe sur l'Homme
Les ouvriers qui épandent des produits à base de Bt peuvent être
fortement exposés à ces produits par contact cutané ou par inhalation
d'aérosols. L'usage du Bt en agriculture peut entraîner la
contamination de l'eau potable et des denrées alimentaires par le
bacille. Toutefois, à l'exception de quelques cas d'irritation des
yeux ou de la peau, on n'a pas connaissance d'effets nocifs attestés
qui résulteraient d'une exposition professionnelle à des produits à
base de Bt. Des volontaires qui avaient ingéré ou inhalé de grandes
quantités de diverses formulations de Btk, n'ont ressenti aucun effet
indésirable. On a mis en évidence des anticorps dirigés contre les
cellules bactériennes, les spores et les complexes spores-cristaux
chez des ouvriers chargés de l'épandage de produits à base de Bt;
aucun effet indésirable n'a cependant été observé. On connaît le cas
d'un certain nombre de patients atteints de maladies infectieuses chez
lesquels la présence de Bt a été mise en évidence. Toutefois, aucune
des études qui leur ont été consacrées n'a permis de conclure de façon
certaine que l'utilisation du Bt comporte effectivement un risque pour
la santé humaine. Il ne semble pas non plus que la présence de Bt dans
l'eau destinée à la consommation ou dans les denrées alimentaires soit
à l'origine d'effets indésirables chez l'Homme.
7. Conclusions
Compte tenu de la spécificité de leur mode d'action, il est
improbable que les produits à base de Bt constituent un danger
quelconque pour l'Homme et les vertébrés ni pour la très grande
majorité des invertébrés non visés, pour autant qu'ils ne contiennent
pas d'autres microorganismes ou de substances biologiquement actives
autres que les ICP. On peut utiliser ces produits en toute sécurité
pour détruire les insectes qui ravagent les domaines agricoles et
horticoles ainsi que les forêts. Ils sont également sans danger pour
le milieu aquatique et on peut notamment les épandre dans les
réservoirs d'eau potable pour lutter contre les moustiques, les
simulies et les larves d'insectes incommodants. Il convient cependant
de noter qu'en phase végétative, le Bt est capable de produire des
toxines de type Bc dont on ignore si elles sont susceptibles de
provoquer des maladies chez l'Homme.
1. RESUMEN
Esta monografía trata sobre los plaguicidas microbianos (PM)
basados en Bacillus thuringiensis (Bt). Esta bacteria es también una
fuente clave de genes cuya expresión transgénica confiere resistencia
frente a plagas a plantas y microorganismos, actuando como plaguicida
en los denominados organismos modificados genéticamente (OMG). Los
posibles efectos de los OMG sobre la salud humana y el medio están
poco o nada relacionados con los productos basados en Bt, por lo que
quedan fuera del ámbito de esta monografía.
1. Identidad, características biológicas y métodos de laboratorio
Bt es una bacteria gram-positiva y anaerobia facultativa que
forma inclusiones proteicas características junto a la endospora. Las
subespecies de Bt pueden sintetizar más de una inclusión parasporal.
Desde el punto de vista genético, Bt es indistinguible de Bc,
exceptuando la capacidad de Bt para producir inclusiones parasporales
cristalinas que son tóxicas para ciertos invertebrados, en particular
para las larvas de insectos pertenecientes a los órdenes Coleóptera,
Díptera y Lepidóptera. Dichas inclusiones parasporales están
formadas por distintas proteínas cristalinas insecticidas (PCI). Los
cristales tienen formas diversas (bipiramidales, cuboides, romboides
planos, esféricos o compuestos por dos tipos de cristales),
dependiendo de su composición en PCI. Se ha comprobado que existe una
correlación parcial entre la morfología del cristal, la composición en
PCI y la bioactividad frente a los insectos diana.
El taxón fenotípico básico es la subespecie, identificada por el
serotipo flagelar (H). Hasta 1998 se habían descrito 67 subespecies.
Los genes que codifican las PCI se encuentran fundamentalmente en los
plásmidos. Cada PCI es el producto de un solo gen. La mayoría de los
plásmidos con genes de PCI se transfieren fácilmente por conjugación
entre cepas de Bt y pueden transferirse a especies bacterianas
emparentadas. La clasificación fenotípica se ha complementado en la
actualidad con la caracterización biomolecular, basada en la secuencia
de los genes de las proteínas cristalinas (cry y cyt), no en la
especificidad para las especies diana. En las PCI, la susceptibilidad
del huésped (reconocimiento de receptores) y la toxicidad (formación
de poros) son responsabilidad de dominios distintos de la molécula.
Las técnicas utilizadas habitualmente para caracterizar las cepas
de Bt o la propia PCI consisten en análisis de los ácidos grasos de la
pared celular, anticuerpos monoclonales, sondas de oligonucleótidos de
ADN, perfiles de plásmidos, análisis por reacción en cadena de la
polimerasa (PCR), estudios del ADN (huella genética) y perfiles de
SDS-PAGE (dodecil sulfato sódico -- electroforesis en gel de
poliacrilamida).
La beta-exotoxina, un nucleótido termoestable, es sintetizada por
algunas subespecies de Bt durante el crecimiento vegetativo y puede
contaminar los productos. Es tóxica para casi todas las formas de
vida, incluidos los seres humanos y los órdenes de insectos diana.
Durante el crecimiento vegetativo, varias cepas de Bt producen una
gama de antibióticos, enzimas, metabolitos y toxinas, incluidas
toxinas de Bc, que pueden tener efectos nocivos tanto en las especies
que son objetivo del plaguicida como en las que no lo son. Los
recuentos de esporas no reflejan con exactitud la actividad
insecticida de una cepa o un preparado de Bt. Se mide la potencia
(UTI/mg) de cada producto mediante ensayos biológicos para los que se
utiliza un patrón internacional basado en un insecto concreto.
2. Modo de acción en los insectos diana
Es preciso que las larvas de los insectos susceptibles ingieran
Bt esporulado con PCI o con complejos espora-PCI. La eficacia de la
PCI depende de su solubilización en el intestino medio, de la
conversión de la protoxina en la toxina biológicamente activa por la
acción de enzimas proteolíticas, de la unión específica del dominio
C-terminal de la toxina activa al receptor de membrana y de la
formación de poros por parte del dominio N-terminal, con la
consiguiente lisis de las células epiteliales. La germinación de
esporas y la proliferación de células vegetativas en el hemocele puede
ocasionar una septicemia y contribuir a la muerte. La unión de la PCI
al receptor es el determinante principal de la especificidad de
huésped para las distintas PCI de Bt.
3. Hábitats
Se han aislado muchas subespecies de Bt a partir de insectos
muertos o moribundos, la mayoría pertenecientes a los órdenes
Coleóptera, Díptera y Lepidóptera, pero también del suelo, de
superficies foliares y de otros hábitats. Los exoesqueletos de
insectos muertos contienen a menudo grandes cantidades de esporas y
PCI que pueden incorporarse al medio. Las subespecies de Bt activas
frente a coleópteros y lepidópteros se asocian fundamentalmente con el
suelo y el filoplano (superficies foliares), mientras que las activas
frente a dípteros se hallan generalmente en medios acuáticos. En el
ambiente, las esporas persisten y pueden entrar en crecimiento
vegetativo cuando las condiciones son favorables y hay nutrientes
disponibles.
4. Productos comerciales, producción y aplicación
Los preparados convencionales de Bt, que utilizan cepas de Bt que
aparecen de forma espontánea en la naturaleza, representan
aproximadamente el 90% del mercado mundial de los PM. La mayoría de
los preparados de Bt contienen PCI y esporas viables, pero en algunos
productos de Bti las esporas están inactivadas. Cada año se producen
aproximadamente 13.000 toneladas utilizando tecnología de fermentación
aerobia. Los preparados convencionales de Bt tienen como objetivos
primarios las plagas de lepidópteros que afectan a los cultivos
agrícolas y forestales; sin embargo, en los últimos años también se
han comercializado cepas de Bt activas frente a plagas de coleópteros.
Se están utilizando en programas de salud pública cepas de Bti activas
frente a dípteros vectores de enfermedades parasitarias y víricas.
Las formulaciones comerciales de Bt pueden aplicarse como
insecticidas al follaje, el suelo, el medio acuático o instalaciones
de almacenamiento de alimentos. Tras aplicar una subespecie de Bt a un
ecosistema, las células vegetativas y las esporas pueden persistir en
concentraciones gradualmente decrecientes durante semanas, meses o
años como un componente de la microflora natural. Sin embargo, las PCI
pierden su actividad biológica en horas o días.
5. Efectos de Bt sobre especies que no son objetivo del plaguicida
En estudios con mamíferos, en particular con animales de
laboratorio, se ha evaluado la posible infecciosidad y toxicidad de
diversos preparados de Bt, que comprenden las PCI, células vegetativas
y esporas. Las PCI, las esporas y las células vegetativas de las
subespecies de Bt, que se administraron por distintas vías, carecían
en su mayoría de patogenicidad y toxicidad para las diversas especies
animales estudiadas. Se comprobó que las células vegetativas o las
esporas de Bt persistían durante semanas sin causar efectos adversos.
No se ha observado que Bt afecte a pájaros, peces o muchas otras
especies de vertebrados acuáticos que no son objetivo del plaguicida y
se han estudiado en gran número de trabajos de laboratorio y de campo.
Son relativamente pocas las especies de invertebrados acuáticos
susceptibles a Bt, tanto en condiciones de laboratorio como de campo.
Bt no afecta a las lombrices de tierra.
En general, las subespecies de Bt muestran gran especificidad en
su actividad insecticida frente a Coleóptera, Díptera y
Lepidóptera, así como una toxicidad directa escasa, si no nula,
frente a los artrópodos que no son su objetivo. La mayor parte de los
datos disponibles sobre inocuidad en éstos se han obtenido con las
subespecies de Bt activas frente a Díptera y Lepidóptera.
Los estudios sobre formulaciones de Bti sin contaminantes tóxicos
no han puesto de manifiesto efectos nocivos en la gran mayoría de los
artrópodos que no son objetivo del plaguicida. Se ha comprobado que
algunas moscas enanas (Díptera: Chironomidae), estrechamente
emparentadas con los mosquitos, son sensibles a dosis altas de Bti,
pero no se ven afectadas por dosis letales para larvas de mosquito. En
estudios de campo se han descrito disminuciones o aumentos
transitorios de las poblaciones de algunos artrópodos que no son
objetivo del plaguicida.
Se han estudiado muchos órdenes de insectos, tanto en el
laboratorio como en trabajos de campo, y se ha comprobado que en la
mayoría de ellos Btk no tiene efecto.
Se ha observado mortalidad en abejas melíferas (Apis mellifera)
tras la exposición a Btt y Btk en crecimiento vegetativo, pero el
efecto no parece guardar relación con las esporas o las PCI. En los
estudios de laboratorio y de campo, Btg no mostró efectos adversos
sobre las abejas melíferas.
Se ha comprobado que cepas de Bte productoras de beta-exotoxina
tienen efectos adversos sobre artrópodos que no son objetivo del
plaguicida.
6. Exposición a Bt y efectos sobre los seres humanos
La aplicación agrícola de preparados de Bt puede suponer una
considerable exposición de los trabajadores, tanto en aerosol como
dérmica. Puede, asimismo, causar la contaminación del agua potable y
los alimentos por la bacteria. Salvo casos notificados de irritación
ocular y dérmica, no se han documentado efectos adversos sobre la
salud tras la exposición laboral a preparados de Bt. Individuos
voluntarios ingirieron e inhalaron grandes cantidades de una
formulación de Btk sin sufrir efectos adversos. Se detectaron títulos
de anticuerpos frente a las células vegetativas, las esporas y los
complejos espora-cristal en trabajadores que pulverizaban preparados
de Bt, pero no se registraron efectos adversos. Se han descrito
algunos casos de presencia de Bt en pacientes con diversas
enfermedades infecciosas. Sin embargo, ninguno de estos estudios
demuestra de forma inequívoca que el uso de Bt entrañe un riesgo real
para la salud humana. No se ha demostrado que Bt tenga efectos
adversos en seres humanos cuando está presente en el agua potable o
los alimentos.
7. Conclusiones
Debido a la especificidad de su modo de acción, es improbable que
los preparados de Bt entrañen peligro alguno para los seres humanos u
otros vertebrados, o para la gran mayoría de los invertebrados que no
constituyen su objetivo, siempre y cuando no contengan microorganismos
distintos de Bt y productos biológicamente activos distintos de las
PCI. Los preparados de Bt pueden utilizarse con seguridad para
controlar las plagas de insectos de los cultivos agrícolas y
hortícolas, así como las forestales. También es seguro su uso en
medios acuáticos, incluidos los depósitos de agua potable, para
controlar el mosquito, la mosca negra y las larvas de insectos
dañinos. Sin embargo, es preciso señalar que las formas vegetativas de
Bt pueden sintetizar toxinas del tipo de las producidas por Bc, cuya
importancia como causa de enfermedades humanas se desconoce.
Binding to specific receptors has been demonstrated to be closely
related to the insecticidal spectrum of the ICPs (Denolf et al.,
1997). Van Rie et al. (1989) found the affinity of these toxins
similar for the tobacco budworm (Heliothis virescens) and the tomato
hornworm (Manduca sexta) brush border membrane vesicles, but the
number of binding sites differed and reflected varied bioactivity.
However, the toxin affinity for binding sites does not appear constant
for all insects.
Pore or ion channel formation occurs after the binding to the
receptor and insertion of the N-terminal domain into the membrane,
whereby the regulation of the trans-membrane electric potential is
disturbed. This can result in colloid-osmotic lysis of the cells,
which is the main cytolytic mechanism that is common to all ICPs
(Knowles & Ellar, 1987; Slatin et al., 1990; Schwartz et al., 1991;
Schnepf et al., 1998). When the midgut epithelium of the larva is
damaged, the haemolymph and gut contents can mix. This results in
favourable conditions for the Bt spores to germinate. The resulting
vegetative cells of Bt and the pre-existing microorganisms in the gut
proliferate in the haemocoel causing septicaemia, and may thus
contribute to the mortality of the insect larva.
3.3 Resistance of insect populations
A number of insect populations of several different species with
different levels of resistance to Bt have been obtained by laboratory
selection experiments during the last 15 years (Schnepf et al., 1998).
The species include Plodia interpunctella, Cadra cautella,
Leptinotarsa decemlineata, Chrysomela scripta, Tricholplusia ni,
Spodoptera littoralis, Spodoptera exigua, Heliothis virescens,
Ostrinia nubilalis and Culex quinquefasciatus (Schnepf et al.,
1998) and resistance is shown to either Btk, Bti, Btte or other Bt
subspecies.
During the last few years populations of the diamondback moth,
Plutella xylostella, resistant to Btk and Bta have been found in
heavily treated areas in numerous geographically isolated regions in
the world, including Hawaii, Phillippines, Indonesia, Malaysia,
Central America and some USA states (Schnepf et al., 1998). It is
clear that this widespread appearance of resistance to Bt presents a
cautionary tale for the way of using Bt and Bt toxin genes in pest
management. Schnepf et al. (1998) have reviewed resistance management
of Bt.
4. NATURAL AND TREATED HABITATS
The Bt subspecies represents a group of organisms that occur
naturally and can be added to an ecosystem to achieve insect control
(Andrews et al., 1987; Stahly et al., 1991). In this monograph, a
natural habitat is considered to be one where Bt can be isolated when
there has been no previous history of application of the organism to
that ecosystem, whereas a treated habitat is one where Bt can be
isolated after a previous history of application of the organism for
insect control.
Insecticides formulated with Bt are being manufactured and used
worldwide. These commercial Bt products may be applied as an
insecticide to foliage, soil, water environments and food storage
facilities. After application of Bt to an ecosystem, the organism may
persist as a component of the natural microflora.
4.1 Natural occurrence of Bt
Members of the Bacillus cereus group can be found in most
ecological niches. Hansen et al. (1996) reviewed the occurrence of Bt
in the environment. Although the early Bt isolates were pathogenic for
insects, it is now apparent that several Bt isolates have no known
target (Ohba & Aizawa, 1986; Ohba et al., 1988; Hansen et al., 1996,
1998; Damgaard et al., 1997b). This lack of insecticidal activity may
be attributed to the loss of ability to produce ICPs (Gordon, 1977),
which may be due to a mutation in the ICP gene that could prevent
expression (Klier & Lecadet, 1976; Stahly et al., 1978; Dean, 1984) or
to the loss of ICP encoding sequences. Finally, the lack of known
activity of a Bt crystalline toxin might simply be explained by a
failure to test against the actual target organism. The list of Bt
targets is still increasing. Although our knowledge of the activity of
Bt populations in the environment is limited, a certain level of
turn-over and vegetative growth must occur, as annual and seasonal
variations in numbers and subspecies diversity of Bt populations have
been observed (Damgaard et al., 1997b; Kim et al., 1998).
4.1.1 Bt in insect hosts
Numerous Bt subspecies have been isolated from dead or dying
insect larvae and in most cases the isolate has toxic activity to the
insect from which it was isolated (Goldberg & Margalit, 1977; de
Barjac, 1981b; Hansen et al., 1996). These organisms have a narrow
host range in the orders Coleoptera, Diptera and Lepidoptera and
can proliferate within the bodies of their host insects. When the
infected insect larva dies, the dead insect carcass usually contains
relatively large quantities of spores and crystals that may be
released into the environment (Prasertphon et al., 1973; Grassi &
Deseö, 1984; Aly, 1985; Aly et al., 1985). Growth of Bt in non-target
organisms has also been described. Eilenberg et al. (in press) found
that Bt multiplication had occurred in non-target insects, which were
also infected by insect pathogenic fungi.
Akiba (1986) reported recycling of naturally occurring Bt in
insect cadavers when competitive microorganisms were at a low density.
Outbreaks of Bt in susceptible insect populations occur relatively
infrequently; most outbreaks have been limited to situations where the
insect density is relatively high, providing better opportunity for
establishing the disease within the insect population (Lynch et al.,
1976; Burges & Hurst, 1977; Vaòková & Purrini, 1979; Margalit & Dean,
1985).
4.1.2 Bt in soil
The spores of Bt persist in soil, and vegetative growth occurs
when nutrients are available (DeLucca et al., 1981; Akiba, 1986; Ohba
& Aizawa, 1986; Travers et al., 1987; Martin & Travers, 1989).
DeLucca et al. (1981) found that Bt represented between 0.5% and
0.005% of all Bacillus species isolated from soil samples in the
USA. Martin & Travers (1989) recovered Bt from soils globally. Meadows
(1993) isolated Bt from 785 of 1115 soil samples, and the percentage
of samples that contained Bt ranged from 56% in New Zealand to 94% in
samples from Asia and central and southern Africa. Ohba & Aizawa
(1986) isolated Bt from 136 out of 189 soil samples in Japan.
4.1.3 Bt on plant surfaces
Bt has been found extensively in the phylloplane. Numerous Bt
subspecies have been recovered from coniferous trees, deciduous trees
and vegetables, as well as from other herbs (Smith & Couche, 1991;
Damgaard et al., 1997b). The Bt isolates have demonstrated a broad
diversity both with specific activities to insects from the orders
Coleoptera and Lepidoptera and with unknown activities (Smith &
Couche, 1991; Damgaard et al., 1997b; Hansen et al., 1998). The
bacterium has also been isolated from stored grain products (Meadows
et al., 1992).
4.2 Treated habitats
Treated habitats are the locations where Bt insecticides (usually
a mixture of spores and crystals) have been applied.
In Canada, Meadows (1993) estimated that approximately 1015
viable Btk spores per ha were released in a typical spray operation to
control spruce budworm (Choristoneura fumiferana).
4.3 Environmental fate, distribution and movement
Bt, like other members of the genus Bacillus, has the ability
to form endospores that are resistant to inactivation by heat and
desiccation and that persist in the environment under adverse
conditions (Stahly et al., 1991). When considering the degradation of
Bt in the environment, it is important to distinguish between changes
in the numbers of viable spores and changes in biocidal activity. The
survival and activity in the environment has been reviewed by Hansen
et al. (1996).
The distribution and environmental transport of applied Bt
formulations are influenced by the type of application and various
environmental factors (Bulla et al., 1985; Andrews et al., 1987). Bt
formulations are used in agriculture and forestry against coleopteran
and lepidopteran pests and are usually directed towards the surface of
plants, while the Bt formulations for control of dipteran pests
(mosquitos and blackflies) are applied to their aquatic, larval
habitats. Many Bt insecticides exhibit poor stability under field
conditions, and so frequent reapplication is required (Griego &
Spence, 1978; Sorenson & Falcon, 1980; Beegle et al., 1981).
4.3.1 Distribution and fate of Bt in terrestrial habitats
4.3.1.1 Fate of Bt and ICP on plant surfaces
Solar radiation appears to be the environmental factor most
damaging to the stability of Bt ICP (Pinnock et al., 1974; Pinnock et
al., 1977; Griego & Spence, 1978; Pusztai et al., 1991).
Griego & Spence (1978) demonstrated that Bt spores are
inactivated rapidly when exposed to UV radiation, while Pusztai et al.
(1991) demonstrated that the tryptophan residues of the Bt protoxin
are damaged by solar radiation in the 300-380 nm range.
The combined effect of sunlight, leaf temperature and vapour
pressure deficit appeared to contribute more to the reduction of
bioactivity than any other single factor (Leong et al., 1980). Residue
bioactivity may be detected up to 2 weeks after treatment with
formulations containing UV protectants (Hostetter et al., 1975). Other
studies on the effect of environmental exposure to Bt spores revealed
that spore survival can be affected by the surface to which the
material is applied.
Pinnock et al. (1974) reported that the half-life of Bt spores on
leaves of California live oak (Quercus agrifolia) was 3.9 days, as
compared to a half-life of 0.63 days when applied to leaves of western
redbud (Cercis occidentalis).
Ignoffo (1992) summarized data for the reduction of spore
viability and ICP activity on leaves of various plants in sunlight: Bt
spore viability was reduced 80% in one day on red cedar leaves and 8%
on soy bean leaves, while the ICP activity declined by 20% on red
cedar leaves but 65% on soy bean leaves.
Dent (1993) reported that Bt formulations on foliage frequently
have half-lives of up to 10 days. However, unformulated Bt may have a
half-life of only a few hours. Pedersen et al. (1995) found that the
initial spore half-life was 16 h during the first week after spraying
cabbage with unformulated Btk.
There is also evidence that plant chemicals can inactivate Bt or
influence infectivity. Lüthy (1986) demonstrated that extracts
prepared from cotton leaves could inactivate ICPs.
Commercially applied Bt may persist at low levels for
considerable periods of time. Reardon & Haissig (1983) reported that
Btk was still present on balsam fir (Abies balsamea) one year after
applications to control spruce budworm. The proliferation of spores
through infection of susceptible insects should not be discounted as a
source of low levels of Bt in treated areas. Several studies have
demonstrated that Bt is able to grow and sporulate in insect cadavers
(Meadows, 1993). From dead Egyptian cotton leafworm (Spodoptera
littoralis), Jarrett & Stephenson (1990) isolated between 5.0 × 105
and 9.2 × 107 spores per larva.
Bt may be lost to the soil by overspray during application or by
the action of rain on plant surfaces. Further losses arise from in
situ degradation by environmental factors, such as ultraviolet (UV)
radiation and microbial activity (Griego & Spence, 1978; Sorenson &
Falcon, 1980; Beegle et al., 1981; West et al., 1984a,b). Pedersen et
al. (1995) found that Bt was dispersed by rain splash from the soil to
the lower leaves of cabbage.
4.3.1.2 Fate of Bt in soil
Petras & Casida (1985) reported that Bt spore counts in soil
declined by a factor of ten in the first 2 weeks after application and
then remained constant for 8 months. The response was similar in
spores from commercial and laboratory cultures. In contrast,
vegetative cells introduced into the soil persisted for only a short
time. Soil pH had little effect on their survival. Spore persistence
for more than 2 weeks apparently resulted from the inability of the
spores to germinate in the soil.
Pedersen et al. (1995) sprayed unformulated Btk (1.2 5 104 cfu
per g soil, spontaneous rifampicin-resistant mutant) on soil in 1993,
and 2.3 × 103 cfu per g soil remained after 336 days. The field was
left undisturbed, and 5´ years later spots with 1.5 × 103 Btk per g
soil were found (Hansen & Hendriksen, 1999), but spots with very low
Btk numbers were also recorded. These data indicate that the Btk had
multiplied.
West et al. (1984a,b) reported that vegetative cells in soil
disappeared at a rapid, exponential rate, whereas parasporal crystals
disappeared at a slower, non-exponential rate, and spore numbers
remained unaltered through 91 days of incubation at 25°C, with no
detectable germination. The proteinaceous crystalline protoxin of Bt
has been shown to be degraded by soil microorganisms at an exponential
rate with a half-life of about 3-6 days.
Saleh et al. (1970) reported that Bt spores could remain viable
for several months in the soil and germinate when soil conditions
favoured bacterial growth.
Bt spores do not appear to germinate readily in most soils and
the crystalline protoxins are metabolized by other microorganisms.
West et al. (1984a) reported that Bta in soil showed an exponential
loss of insecticidal activity. The rate of loss was greater in
non-sterile soil than in autoclaved soil. There was an initial rapid
decrease, which stabilized at approximately 10% of the original
inoculum level after 250 days incubation, until the cessation of
sampling after two years. No loss of insecticidal activity was
observed in autoclaved soil, which suggests that soil microorganisms
were responsible for the loss of insecticidal activity in the natural,
non-sterilized soil.
Several studies determined that Bt did not grow under most
natural soil conditions (West et al., 1984a,b; Akiba, 1986). The data
suggested that this was attributable to a failure of Bt spores to
germinate in soil under these conditions. The spore is the only state
in which Bt persists in natural soils.
An environmental fate study demonstrated no significant spore
accumulation in either the organic or the mineral layers of soil over
an 11-month period when Bt was applied aerially at 100 times the
concentration used for operational programmes (Bernier et al., 1990).
Studies have indicated that Bt is relatively immobile in soil.
Martin & Reichelderfer (1989) found no vertical movement beyond a 6-cm
deep zone in soil and less than 10 m lateral movement, even along
drainage courses.
Akiba (1991) reported a one-month irrigation study simulating the
summer rainy season in Japan. There was no translocation of Bt below a
depth of 10 cm. In soils receiving 45 cm simulated rainfall, Bt was
detected to a depth of 3-6 cm. In tests using soil columns, Bt did not
pass through a column of volcanic ash soil but a few spores were
detected in flow-through water from an alluvial sand column. Results
suggested that the major factor causing a decrease in the level of Bt
was not a physical dilution due to the rainwater, but possibly an
affinity of the spores for the soil particles.
Venkateswerlu & Stotsky (1992) reported that adsorption and
binding of Bt toxin proteins to soil particles were greater on
montmorillonite than on kaolinite clays. Maximum adsorption occurred
within 30 min, and adsorption was not significantly affected by
temperature between 7°C and 50°C.
4.3.2 Distribution and fate of Bt in aquatic habitats
Bti is often applied directly to water for the control of
mosquitos and blackflies. Rapid sedimentation in all but the fastest
flowing streams is regarded as an important limitation on the efficacy
of such applications.
Sheeran & Fisher (1992) demonstrated that the sedimentation of
Bti is facilitated by adsorption onto particulate material.
Ohana et al. (1987) found that spores may persist for at least
22 days in sediments, and the spores may be mobilized with such
sediments during floods.
Btk has been reported to survive in fresh water and in seawater
for more than 70 and 40 days, respectively, at 20°C (Menon & De
Mestral, 1985). A higher percentage of Btk was found to survive for
extended periods in lake water than in tap and distilled water,
presumably due to the presence of more nutrients in lake water. Bt has
not been isolated from any drinking-water supplies.
Spores of Bti remained viable for shorter periods when suspended
in moving water than when in static bottles, indicating that static
laboratory trials may overestimate the longevity of these spores in
the environment (Yousten et al., 1992).
Carcases of mosquito larvae killed by Bti have been shown to
allow for the complete growth cycle (germination, vegetative growth
and sporulation), thus becoming toxic themselves to scavenging yellow
fever mosquito (Aedes aegypti) larvae (Khawaled et al., 1990).
Contact of Bti with mud can result in an immediate disappearance
of larvicidal activity, but it has little influence on spore viability
(Ohana et al., 1987). The cessation of toxicity was found to be caused
by bacterial adsorption to soil particles, but the inactivation could
be reversed by washing the mud away.
Special Bti formulations have been developed to prolong residence
time of Bt at the surface or in the water column, where target insects
feed.
Manasherob et al. (1998) found germination, growth and
sporulation of Bti in excreted food vacuoles of a protozoan.
4.3.3 Transport of Bt by non-target organisms
In a field trial where Btk was sprayed on cabbage and soil,
Pedersen et al. (1995) found that the Btk could be transported by
non-target insects. Up to 103 cfu per g were found on surface-active
insects, and carabid beetles carrying Btk were found up to 135 m from
the Btk-treated area.
In a study of interactions between Bti and fathead minnows
(Pimephales promelas), ingestion of Bt resulted in a high number of
recoverable spores in the gastrointestinal tract and faeces (Snarski,
1990). Bti spore counts decreased rapidly after test fish were
transferred to clean water, but spores were detected in low numbers in
faeces for over 2 weeks. The data indicated that minnows could
disperse Bt spores in the freshwater environment.
Meadows (1993) reported that, after the application of Bt on
land, it may be dispersed by birds and mammals feeding on infected
target insects. Some Bt-infected insect larvae may contain 6.6 × 108
to 4.2 × 109 spores per gram dry mass (Burges & Hurst 1977).
5. COMMERCIAL PRODUCTION
5.1 History of Bt and its commercial applications
5.1.1 Production levels
Conventional Bt products, which utilize naturally occurring or
modified Bt strains, account for approximately 90% of the world MPCA
market (Bernhard & Utz, 1993). Current annual production of Bt has
been estimated at 3000 or more tonnes in developed countries. In
China, up to 10 000 tons are produced annually (personal communication
by Guan Xiong, Fujian Agricultural, University, 1997).
5.1.2 Production processes, formulations and quality standards
Bt products are produced using fermentation technology (Bernhard
& Utz, 1993). Most commercial products contain ICP and viable Bt
spores, but the spores are inactivated in some Bti products. During
commercial-scale production of Bt products, there is little loss of
bioactive components to the environment. The type of loss incurred is
a function of the recovery method involved. No significant amount of
bioactive component is lost from fermenter harvests if filtration is
used to separate the insoluble solids (active ingredients) from the
soluble liquid (inert) fraction of the harvest liquor, as shown by
complete lack of bioactivity in the resulting liquid fraction. The
liquid waste fractions may contain a low concentration of insoluble
active component, but this is typically inactivated by processing in
on-site waste treatment facilities. Although it is not a physical
loss, measurable bioactivity is diminished if the recovered active
material is processed through a dryer, due to the exposure of the
bioactive components to the high temperatures required for drying.
Guidelines for the handling of microorganisms during manufacture have
been reviewed by Frommer et al. (1989).
Commercial Bt formulations include wettable powders, suspension
concentrates, water dispersible granules, oil miscible suspensions,
capsule suspensions and granules (Tomlin, 1997).
Quality standards for Bt fermentation products have been accepted
by IUPAC (Quinlan, 1990). These standards include limits on the
concentration of microbial contaminants and metabolites (Table 5).
In most cases strains of Bt that produce beta-exotoxins are not
approved for commercial application, although some commercial use has
been approved for control of certain fly species that are not
susceptible to ICPs (Carlberg et al., 1985).
Table 5. Maximum allowable levels of microbial contamination in
bacterial insecticides (IUPAC Recommendation; Quinlan, 1990)
Types of microorganisms Maximum concentrations
Viable mesophiles <1 × 105/g
Viable yeasts and moulds <100/g
Coliforms <10/g
Staphylococcus aureus <1/g
Salmonella <1/10g
Lancefield Group D Streptococci <1 × 104/g
5.1.3 General patterns of use
Commercial applications of Bt have been directed mainly against
lepidopteran pests of agricultural and forest crops; however, in
recent years strains active against coleopteran pests have also been
marketed (Table 6).
Strains of Bti active against dipteran vectors of parasitic
disease organisms have been used in public health programmes.
5.1.3.1 Applications in agriculture and forestry
Commercial use of Bt on agricultural and forest crops dates back
nearly 30 years, when it became available in France. Use of Bt has
increased greatly in recent years and the number of companies with a
commercial interest in Bt products has increased from four in 1980 to
at least 18 (Van Frankenhuyzen, 1993). Several commercial Bt products
with Bta, Btk or Btte have been applied to crops using conventional
spraying technology (Table 6). Various formulations have been used on
major crops such as cotton, maize, soybeans, potatoes, tomatoes,
various crop trees and stored grains. Formulations have ranged from
ultralow-volume oil to high-volume, wettable powder and aqueous
suspensions. In the main, naturally occurring Bt strains have been
used, but transgenic microorganisms expressing Bt toxins have been
developed by conjugation and by genetic manipulation, and in some
cases, these have reached the commercial market. These modified
organisms have been developed in order to increase host range, prolong
field activity or improve delivery of toxins to target organisms. For
example, the coleopteran-active cryIIIA gene has been transferred to
a lepidopteran-active Btk (Carlton et al., 1990). A plasmid bearing an
ICP gene has been transferred from Bt to a non-pathogenic
leaf-colonizing isolate of Pseudomonas fluorescens; fixation of the
transgenic cells produces ICP contained within a membrane which
prolongs persistence (Gelernter, 1990). The gene expressing cryIA(c)
ICP has been inserted in Clavibacter xyli subspecies cynodontis, a
bacterium that colonizes plant vascular systems. This has been shown
to deliver the ICP effectively to European corn borer (Ostrinia
nubilalis) feeding within plant stems (Beach, 1990). Improvements in
performance arising from such modifications are such that transgenic
organisms and their products are likely to be used much more widely in
the future.
5.1.3.2 Applications in vector control
Bti has been used to control both mosquitos and blackflies in
large-scale programmes (Lacey et al., 1982; Chilcott et al., 1983;
Car, 1984; Car & de Moor, 1984; Cibulsky & Fusco, 1987; Becker &
Margalit, 1993; Bernhard & Utz, 1993). For example, in Germany 23
tonnes of Bti wettable powder and 19 000 litres of liquid concentrate
were used to control mosquitos (Anopheles and Culex species)
between 1981 and 1991 in the Upper Rhine Valley (Becker & Margalit,
1993). In China, approximately 10 tonnes of Bti have been used in
recent years to control the malarial vector, Anopheles sinensis.
The Onchocerciasis Control Programme of West Africa used more
than five million litres of Bti from 1982 to 1997 to control
blackflies (Simulium damnosum), the vector of the onchocerciasis
filarial worm (Onchocerca volvulus), on the Upper Volta River
System. The Programme was initially based solely on the control of the
vector, Simulium damnosum sensu lato, by spraying the insecticide
at breeding sites on river systems, where larval stages develop. At
the peak of larvicidal activities about 50 000 km of rivers were
treated in an area of over one million km2. The purpose was to
interrupt the transmission of the parasite Onchocerca volvulus. The
interruption of the cycle is achieved by destroying larval stages
through aerial application of insecticides to breeding sites.
Insecticide application was undertaken weekly (Moulinier et al.,
1995). In order to assess the environmental impact of such treatments
a network of sampling stations throughout the programme area were
established.
Formulations of Bti range from wettable powder and fluid
concentrates applied via conventional spray equipment from ground and
air to slow-release briquet and tablet formulations. Examples of
commercial Bti products are listed in Table 6.
Table 6. Examples of commercial Bt products used against agricultural, forestry and public health pests
(Tomlin, 1997; see also the Internet site http://www.sipweb.org/bacteria.htm)
Bt sub-species Commercial products Producer
Bt (not defined) Rijin Scientific & Technological Development
Bt (not defined) Bitayon Jewin-Joffe Industry Ltd
Bt (not defined) Delfin, Thuricide SDS Biotech KK
Btk Bactospeine, Biobit, Dipel, Foray Abbott (USA)
Bollgard Ecogen/Crop Care
Bactucide Caffaro
Baturad Cequisa
Condor, Crymax, Cutlass, Ecogen
Foil, Jackpot, Lepinox,
Rapax, Raven
Jackpot, Lepinox, Rapax Intrachem
Cordalene Agrichem
Larvo Troy
Costar, Delfin, Design, Novartis/Thermo Trilogy Co
Javelin, Steward, Thuricide,
Vault
Ecotech Bio, Ecotech Pro Ecogen/Roussel-Uclaf
Halt Wockhardt
Bta Xentari, Florbac Abbott
Certan Novartis
Btte Novodor Abbott
Bti Bactimos, Gnatrol, Vectobac Abbott
Acrobe Cyanamid
JieJueLing, MieJueLing Huazhong Agricultural University
Teknar Novartis/ThermoTrilogy Co
Bt Ybt-1520 Mianfeng pesticide Huazhong Agricultural University
Bt chinesensis Shuangdo preparation Huazhong Agricultural University
Btg Spicturin Tuticorin Alkali Chemicals and
Fertilisers Ltd
6. EFFECTS ON ANIMALS
6.1 Mammals
Microbial pest control agents (MPCA) can, in principle, cause
harmful effects via toxicity, inflammation, or a combination of these
effects. The presence of bacteria in a specimen derived from tissues
does not necessarily mean infection. Colonization refers to the
multiplication of MPCA either on the surface or within an animal/human
organism without causing any tissue damage. Persistence refers to
the ability to recover the inoculum of the MPCA over time. Persistence
and transient disturbances of the normal microbial flora are to be
expected after exposure of experimental animals to MPCA, since
clearance of the inoculum is not instantaneous. Persistence may not be
equated with infection (Siegel & Shadduck, 1990). Infection by a
MPCA means that there is evidence of the establishment and
proliferation of the MPCA in tissues, coupled with tissue damage.
Evidence of multiplication includes a measurable increase in the total
amount of MPCA, recovery of vegetative stages when spores were
administered, and failure of the inoculum to clear. It cannot be
determined solely on the basis of lesions since injection of foreign
material can elicit an inflammatory process (Siegel et al., 1987).
A classification of MPCA toxicity and infectivity has been
proposed, in which MPCA is classified as toxic if an oral dose
< 106 cfu per mouse causes mortality or clinical or pathological
changes (Burges, 1980). However, any classification is very difficult
because of the complexicity of the issue when dealing with living
organisms (Ignoffo, 1973; Shadduck, 1983).
Older reports do not discriminate between different strains of Bt
but modern molecular techniques have proven that variability exists
within strains with the same serotype (Helgason et al., 1998; Hansen &
Hendriksen, 1997a,b).
Mammalian toxicity studies on Bt-containing pesticides
demonstrate that the tested isolates are not toxic or pathogenic
(McClintock et al., 1995), as they occur in the products. Toxicity
studies submitted to the US Environmental Protection Agency to support
registration of Bt subspecies, and reviewed by McClintock et al.
(1995), failed to show any significant adverse effects on body weight
gain, clinical observations or upon necropsy.
Infectivity/pathogenicity studies have shown that the intact rodent
system responds as expected to eliminate Bt gradually from the body
after oral, pulmonary or intravenous challenge. However, clearance of
Bti and Btk is not instantaneous. An intact immune system is not a
prerequisite for clearance of Bti and Btk.
6.1.1 Oral exposure
In studies conducted with a single oral dose of laboratory grown
Bt and commercial Bt formulations, there was no mortality associated
with ingestion of Bti or Btk in mice and rats (Fisher & Rosner, 1959;
de Barjac et al., 1980; Shadduck, 1980; Siegel et al., 1987)
(Table 7).
Additionally, in data summarized by McClintock et al. (1995), no
toxicity or infectivity was observed following oral administration of
various Bt subspecies at doses of up to 4.7 × 1011 cfu/kg in rats.
In a study involving repeated oral exposure of mice and rats for
21 days with laboratory grown Bti, there was no mortality associated
with ingestion of Bti and normal weight gain was observed in all
treated rodents (de Barjac et al., 1980) (Table 8).
Hadley et al. (1987) conducted a study in which sheep were
repeatedly treated with two commercial Btk formulations for 60 days.
The only clinical sign was loose stools in sheep exposed to one Btk
formulation. There was also microscopic evidence of moderate to marked
lymphoid hyperplasia of the Peyer's patches in the caecum and colon of
two out of six sheep treated with one Btk formulation and in one out
of six sheep treated with the other Btk formulation. The authors did
not consider these findings clinically significant.
Other multiple dose studies with Bt were summarized by McClintock
et al. (1995). In rats, no toxicity or infectivity was associated with
dietary exposure to Bti (4 g/kg per day ) for 3 months. Administration
of 1.3 × 109 Btk spores/kg per day to rats by oral gavage was not
toxic or infectious. McClintock et al. (1995) also reported the
results of a 2-year study in which a commercial Btk preparation was
fed to rats at 8400 mg/kg per day in the diet. Despite this excessive
dose, the only effect observed was a decrease in body weight of
females during weeks 10-104 of the study.
6.1.2 Inhalation exposure
These tests primarily address the potential infectivity of a
MPCA. Inhalation is a likely route by which humans and animals may be
exposed to Bt during application.
De Barjac et al. (1980) exposed 10 female Swiss mice for 12 min
to 2 × 108 Bti spores (48-h laboratory grown whole culture). The mice
were monitored for clinical signs for 15 days, and then killed. The
lungs were removed aseptically and cultured for bacteria, but no Bti
was recovered.
Siegel et al. (1987) exposed 27 female Sprague-Dawley rats to
2 × 106 spores of a commercial Bti formulation for 30 min. Rats were
serially killed over a 27-day period and the lungs were cultured.
Table 7. Acute toxicity (single oral exposure) of Bt in experimental animals
Subspecies Material tested Test animal Dosea Mortalityb Reference
tested
Btk Washed cells, 24-h culturec Rat, female, Sprague-Dawley 1.4 × 107cfu 0/6 Shadduck, 1980
Btk Commercial product Ratd 2 × 1011cfu 0/10 Fisher & Rosner, 1959
Bti 48-h culturec Mouse, female, Swiss 1.7 × 108cfu 0/20 de Barjac et al., 1980
Bti 48-h culturec Rat, female, Wistar 3.4 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellsc, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rat, female, Sprague-Dawley 4 × 107cfu 0/10 Siegel et al., 1987
a All doses given are per animal
b Number dead/number treated
c Laboratory grown culture
d Breed and sex unknown
Table 8. Repeated dose (oral exposure) toxicity of Bt in mice, rats and sheep
Subspecies Material tested Test animal Dose Exposure Mortalitya Reference
tested time
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Bti 48-h cultureb Mouse, female, Swiss 4.7 × 1010cfu 21 days 0/20 de Barjac et al., 1980
Bti 48-h cultureb Rat, female, Wistar 1.2 × 1011cfu 21 days 0/10 de Barjac et al., 1980
a Number dead/number exposed
b Laboratory grown culture
Recovery of Bti declined from 5.92 × 103 cfu/g lung tissue at 3 h
after exposure to none detected at 7 days after exposure. No gross
lung lesions were observed.
Fisher & Rosner (1959) exposed 10 mice to 3 × 1010 spores of a
commercial Btk product 4 times in a 6-day period. The Btk-treated mice
exhibited no clinical signs during the treatment period and no gross
pathological changes at necropsy.
6.1.3 Dermal exposure
This test is similar to the dermal exposure tests used in
chemical toxicology. Bt does not have any external contact toxicity
due to its mode of action, as shown in the following studies.
De Barjac et al. (1980) applied 5.1 × 107 cfu Bti of a 48-h
laboratory grown culture to the skin of 20 female Swiss mice. No
mortality was observed and there was no evidence of skin inflammation.
Other studies, reviewed by McClintock et al. (1995), indicate
that Bt was not toxic or pathogenic to rabbits following dermal
exposure to various Bt subspecies at doses of up to 2500 mg/kg. In
some cases, mild irritation was observed.
6.1.4 Dermal scarification exposure
This test evaluates both the potential toxicity and infectivity
of a MPCA. In the case of Bt, toxicity is unlikely due to its mode of
action. However, this test also evaluates the importance of intact
skin in preventing infection by Bt.
Fisher & Rosner (1959) scarified the skin of 4 rabbits, then
applied 2.2 × 106 cfu of a commercial Btk formulation. No skin
inflammation or sign of infection was observed.
De Barjac et al. (1980) applied 3.3 × 1013 cfu of a commercial
Bti formulation to the skin of 6 male New Zealand White rabbits. No
skin inflammation or sign of infection was observed.
6.1.5 Subcutaneous inoculation
This route of exposure is considered a more challenging test of
potential infectivity than oral or dermal exposure, because the
barrier of the skin is breached. However, subcutaneous exposure may
take place only if the skin is damaged by the spraying or is already
otherwise damaged.
De Barjac et al. (1980) subcutaneously inoculated 20 female Swiss
mice and 10 tricolour guinea-pigs, respectively, with 8.5 × 107 cfu
and 1.7 × 108 cfu of a 48-h laboratory-grown Bti culture. There was
no evidence of infection and no mortality was observed.
Siegel et al. (1987) subcutaneously inoculated 15 female BALB/c
mice with 1 × 109 cfu of a commercial Bti formulation. Abscesses
occurred at the injection site but these were attributed to the
introduction of high concentrations of heat-stable foreign material,
since they also occurred when autoclaved Bti was injected. There was
no evidence of infection and no mortality was observed.
6.1.6 Ocular exposure
The primary purpose of this procedure is to test for the
irritancy of a MPCA, although this test also evaluates potential
infectivity as well. In these tests, Bt may persist for days in rabbit
eyes because of the depth of the conjunctival sac coupled with limited
tear production by rabbits.
De Barjac et al. (1980) inoculated the eyes of six male New
Zealand White rabbits with 3.7 × 107 cfu of a 48-h laboratory-grown
Bti culture. No conjunctivitis or ocular irritation was observed.
Siegel & Shadduck (1990) inoculated 12 female New Zealand White
rabbits with 5.4 × 106 cfu of a commercial Bti formulation. No ocular
irritation or conjunctivitis was observed and no Bti was recovered by
swabbing after one week.
In data reviewed by McClintock et al. (1995), only mild
irritation was observed following ocular administration of certain Bt
subspecies to rabbits.
Siegel et al. (1987) inoculated the eyes of 6 male New Zealand
White rabbits with 50 mg of a dry powder-commercial Bti formulation
for 9 days, and another 6 male New Zealand White rabbits were treated
with 50 mg of a laboratory-grown Bti culture for 9 days. No ocular
irritation or conjunctivitis was observed in the rabbits that received
the commercial powder. The rabbits that received the laboratory
culture experienced severe conjunctival congestion and discharge. This
was not attributed to Bti but rather to the nature of the inoculum.
The laboratory strain was a dry paste with hard clumps while the
commercial formulation was a soft powder.
6.1.7 Intraperitoneal exposure
The administration of a MPCA by this route is considered a highly
challenging route of exposure. Human and animal exposure to Bt by this
route is very unlikely to occur during the course of normal
application of Bt. This route evaluates the ability of Bt to cause
infection or produce toxic metabolites in the peritoneal cavity. Some
of the safety studies that utilized this route of exposure also
evaluated the clearance of Bt over time (Table 9).
Additional studies employing mice have been conducted using this
route of exposure, which evaluates the role played by an intact immune
system in preventing infection by Bt. These studies were deemed
necessary to assess the risk posed by Bt to humans undergoing
immunosuppressive chemotherapy and the risk posed by Bt to humans
infected with the human immunodeficiency viruses. Immune suppression
in mice was accomplished either by use of corticosteroids, which
inhibited helper T-cells and selectively damaged B-cell activity, or
through the use of athymic mice, which lack the functional T
lymphocyte component of their immune system.
6.1.7.1 Immune-intact animals
De Barjac et al. (1980) intraperitoneally injected 100 female
Swiss mice with 3.4 × 107 cfu of a 48-h laboratory-grown Bti culture
and killed groups of 10 mice daily (Table 9). Blood samples were taken
by cardiac puncture and Bt was recovered until day 8. No mortality was
observed.
Fisher & Rosner (1959) intraperitoneally injected 30 mice of
unspecified sex and strain with a laboratory-grown Btk culture and
withdrew cardiac blood samples 24, 48 and 72 h after injection. There
was no mortality and Btk was recovered as late as 48 h after injection
from heart blood.
Siegel & Shadduck (1990) conducted three clearance studies using
female CD-1 mice. In one experiment, 33 females were injected with
2.7 × 107 cfu of a washed commercial Bti formulation and serially
killed over 80 days. Bti did not clear and was recovered from the
heart blood on days 67 and 80. The investigators noted that the
initial inoculum was composed of approximately 95% vegetative cells
and that vegetative cells take longer to clear than spores. This was
confirmed in a follow-up experiment in which two groups of 16 females
each were injected with inocula containing 1.5 × 107 cfu of spores
only or a 25% vegetative cell and 75% spore mixture. Both inocula
cleared exponentially from the spleens of the mice but the 100% spore
inoculum cleared sooner than did the inoculum that contained
vegetative cells. These experiments demonstrated that Bti and Btk
persist for a variable length of time in mice following injection but
that they are cleared over time. These studies also suggest that the
nature of the inoculum may play a role in the speed by which it
is cleared.
Data summarized by McClintock et al. (1995) indicate that
toxicity (100% mortality in extreme cases) may be observed following
injection of >108 cfu of certain Bt subspecies intraperitoneally
in mice. Lower doses (< 107 cfu/mouse) were non-toxic. Death
generally occurred shortly after injection, indicating that an
infectious process had not occurred. Although the basis for the
toxicity observed at doses >108 cfu is not understood, these
findings are not considered as evidence of a hazard associated with Bt
products, since the route of administration is not relevant to human
and animal exposure conditions.
Table 9. Acute toxicity of Bt after intraperitoneal injection of guinea-pigs, mice and rats
Subspecies Material tested Test animal Dose Mortalitya Reference
tested
Btk Washed cell, 24-h cultureb Rat, female, Sprague-Dawley 1.4 × 109cfu 0/6 Shadduck, 1980
Btk Commercial product Mouse 3 × 109cfu 0/5 Fisher & Rosner, 1959
Bti 48-h cultureb Mouse, female, Swiss 6.8 × 107cfu 0/20 de Barjac et al., 1980
Bti 48-h cultureb Guinea-pig, female, tricolour 1.7 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellb, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rats, male and female Sprague-Dawley 4 × 107cfu 1/20 Siegel et al., 1987
a Number dead/number treated
b Laboratory grown culture
6.1.7.2 Immune-suppressed animals
Siegel et al. (1987) injected 42 female BALB/c mice with 1.25 mg
of a cortisone acetate twice weekly in order to suppress their immune
system and subsequently injected them with 3.4 × 107 cfu of a washed
commercial Bti formulation. Three cortisone-treated mice but none of
the non-cortisone-treated mice died but this mortality was attributed
to injury caused by injection. In the remaining 39 mice Bti was still
recovered in the spleen 49 days after injection. In a companion
experiment, 42 athymic mice were injected with the same dose of a
washed commercial Bti formulation. Twenty-six of the 42 died within 5
to 10 h after injection; autopsy did not reveal the cause of death. In
the surviving mice, Bti was recovered in the spleen 49 days after the
injection. In a follow-up experiment, 30 athymic mice were injected
with 2.6 × 107 cfu of another (washed) commercial Bti formulation and
serially killed over a 36-day period. No mortality occurred. Bti was
still recovered on day 36 after injection.
Siegel & Shadduck (1990) injected 24 athymic mice with 2.7 × 107
cfu of a washed commercial Bti formulation and evaluated clearance
over a 27-day period. No mortality was observed and clearance was
faster in the euthymic than athymic mice. Bti was still recovered 27
days after injection.
These experiments demonstrated that an intact immune system is
not essential to prevent infection by Bti and Btk, but the kinetics of
clearance differ between athymic and euthymic mice as well as between
corticosteroid-treated and untreated euthymic mice. Based on these
data, immune-suppressed individuals do not face any increased risk of
infection by Bt.
6.1.8 Effects of activated Bt ICP
It has been demonstrated that the alkali-solubilized ICP from Bti
is lethal when injected into mice (Thomas & Ellar, 1983).
Alkali-solubilized Bti ICP was also cytolytic to human erythrocytes,
mouse fibroblasts, and primary pig lymphocytes in vitro (Thomas &
Ellar, 1983; Gill et al., 1987). This activity is attributed to a
cytolytic factor encoded by Cyt A gene of Bti. Most other Bt
subspecies lack this gene. Human exposure to activated Bti ICP is most
unlikely.
6.1.9 Studies in wild animals
Numerous studies have been conducted on wild animals as part of
the registration process. Most of the data are proprietary and not
publicly available. No adverse effects have been reported.
In Canada, Innes & Bendell (1989) studied the effect of a
commercial Btk formulation on small mammal populations in woodland.
Populations of eight species of rodents (Clethrionomys gapperi,
Eutamius minimus, Microtus chrotorrhinus, Napaeozapus insignis,
Peromyscus maniculatus, Phenacomys intermedius, Tamias striatus
and Zapus hudsonius) and four species of shrew (Blarina
brevicauda, Sorex cinereus, Sorex fumeus and Sorex hoyii)
were studied by trapping over a 3-month period and shown to be
unaffected when compared to populations from untreated areas. This
suggests that the ingestion of infected insects by shrews had no
immediate effects on their populations.
6.2 Effects on birds
In a number of studies (Table 10), the acute toxicity and
pathogenicity of commercial Bta, Bti, Btk and Btte formulations for
young bobwhite quail (Colinus virginianus) and young mallards (Anas
platyrhynchus), when administered daily by oral gavage at high
dosages, were evaluated (Beavers et al., 1989a,b; Lattin et al.,
1990a,b,c,d; Beavers, 1991a,b). The Bt-treated birds showed no
apparent toxicity or pathogenicity. In those studies which also
evaluated feed consumption and weight gain, the Bt-treated birds
showed no effect when compared with the non-treated controls.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
breeding bird populations (13-14 families, 33-34 species) during a
field trial for spruce budworm control. The bird populations in 10-ha
control and treated plots were measured before and daily for 3 weeks
after application. No differences were detected between the
populations in the control and treated plots.
In the USA, Gaddis & Corkran (1986) evaluated the effect of a Bt
spray programme on the reproductive performance of the chestnut-backed
chickadee (Parus rufescens). This study was undertaken to determine
if secondary effects on the chickadees would result from the possible
reduction of lepidopteran species, which contribute to the diet of
this species. The data showed no treatment-related effect on the
number of eggs per nest, percentage of eggs hatched, percentage of
young fledged, percentage of nests fledging at least one young, or the
body weight of the nestlings.
6.3 Effects on aquatic vertebrates
The World Health Organization (WHO, 1982) reviewed laboratory and
field studies, performed by that time, that examined the impact of Bt
on frogs (Hyla regilla, Rana temporaria), goldfish (Carassius
auratus), mosquito fish (Gambusia affinis), newts (Taricha
torosa, Triturus vulgaris), rainwater killifish (Lucania parva)
and toads (Bufo species). No adverse effects were reported.
Under static renewal conditions, Boeri (1991) exposed rainbow
trout (Oncorhynchus mykiss) to high concentrations (100 mg/litre) of
a commercial Bta formulation for 96 h and observed no adverse effects
(Table 11).
Table 10. Effects of oral 5-day exposure of Bt on birds
Materials Species Dose Results Reference
testeda
Bta Colinus virginianus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991b
pathogenicity observed
Anas platyrhynchus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991a
pathogenicity observed
Bti Colinus virginianus 3077 mg (3.4 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990d
pathogenicity observed
Anas platyrhynchus 3077 mg (6.2 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990b
pathogenicity observed
Btk Colinus virginianus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990a
pathogenicity observed
Anas platyrhynchus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990c
pathogenicity observed
Btte Colinus virginianus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989a
pathogenicity observed
Anas platyrhynchus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989b
pathogenicity observed
a commercial preparation
Under static renewal conditions, Surprenant (1989) exposed
rainbow trout (Oncorhynchus mykiss) to high concentrations (100
mg/litre) of a commercial Btte formulation for 96 h and observed no
adverse effects (Table 11).
During 30- or 32-day static renewal tests, bluegill sunfish
(Lepomis macrochirus), sheepshead minnow (Cyprinodon variegatus)
and rainbow trout (Oncorhynchus mykiss) were exposed to commercial
Bti, Btk or Btte formulations at aqueous and dietary concentrations
from 100 to 500 times the expected environmental concentrations
(Table 11) (Christensen, 1990a,b,c,d,e,f,g,h). The results of these
studies indicated that exposure to very high concentrations of Bti,
Btk and Btte did not adversely affect the survival of these fish, nor
did it produce lesions. In the Btk study, the rainbow trout had a 20%
mortality during the last 4 days of the study (Christensen,1990b).
This effect was attributed to the excessive competition for food that
resulted from poor visibility due to the turbidity and the presence of
suspended solids encountered in the water.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
brook trout (Salvelinus fontinalis Mitchell), common white suckers
(Catostomus commersoni Lacepede) and smallmouth bass (Micropterus
dolomieui Lacepede) during a field trial for spruce budworm control.
The fish populations were assessed visually in underwater surveys
before and after the spray programme. No effect on their populations
was seen.
Two analyses of surveys of the impact of the larvicidal campaign
in the Onchocerciasis Control Programme of West Africa, which compared
fish populations during the programme with the normal yearly
fluctuation, observed little or no effects on the non-target
populations. However, few details were provided (Yameogo et al., 1988;
Levêque et al., 1988; Calamari et al., 1998).
6.4. Effects on invertebrates
6.4.1 Effects on invertebrates other than insects
The World Health Organization (WHO, 1982), reviewed laboratory
and field studies performed up to that time that examined the impact
of Bt on aquatic invertebrates, which included bivalve mollusks
(oyster larvae, Crassostrea gigas, Ostrea edulis), copepods,
decapods, flatworms, isopods, gastropods and ostracods. Of these
organisms, only a few demonstrated any adverse effects.
In Canada, Buckner et al. (1974) evaluated the impact of Btk on a
number of aquatic invertebrates during a field trial for control of
the spruce budworm. Populations of Amphipoda (amphipods), Decapoda
(crayfish), Hydracarina (water-mites), Hirudinea (leeches),
Table 11. Effects of Bt on fish
Material Species Concentration Duration Results Reference
testeda
Bta Oncorhynchus mykiss 100 mg/litre water 96 h No-observed-effect level Boeri, 1991
Btk Lepomis macrochirus 2.9 × 109cfu/litre waterb 32 days No significant toxicity Christensen, 1990a
1.2 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 2.9 × 109cfu/litre waterb 32 days 20% mortality but Christensen, 1990b
1.1 × 1010cfu/g dietc not infectivity
Cyprinodon variegatus 2.6 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990c
3.3 × 109cfu/g dietc or pathology
Bti Lepomis macrochirus 1.2 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990f
1.3 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 1.1 × 1010cfu/litre waterc 32 days No significant toxicity Christensen, 1990g
1.7 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 1.3 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990h
2.1 × 1010cfu/g dietc or pathology
Btte Salmo gairdneri 100 mg/litre water 96 h No-observed-effect level Surprenant, 1989
Oncorhynchus mykiss 1.6 × 1010 cfu/litre waterc 30 days No significant toxicity Christensen, 1990d
1.34 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 9.94 × 109cfu/g diet 30 days No significant toxicity Christensen, 1990e
or pathology
a commercial formulations
b nominal concentration
c measured average concentration
Hydrozoa (freshwater hydra), Nematoda (roundworms), Oligochaeta
(segmented worms), Porifera (freshwater sponges), Pulmonata
(freshwater snails) and Turbellaria (flatworms) were determined by
sampling 14 days prior to and up to 28 days after treatment. The
populations of these aquatic invertebrates were not affected by the
Btk treatment.
Benz & Altwegg (1975) studied the impact of Bt treatment at 100
times the recommended rate on populations of the earthworm Lumbricus
terrestris and found no effect.
Horsburgh & Cobb (1981) reported that populations of the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi
were not affected by biweekly sprays with a commercial Btk product.
Weires & Smith (1977) determined that sprays of a commercial Btk
product on apples during a 4-month season had no effect on the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi,
or on two predatory mites (Amblyseius fallacis and Zetzellia
mali).
6.4.2 Effects on non-target insects
An extensive literature exists on the consequences of exposure of
NTOs to Bt, including reports of several long-term field studies. The
data have been reviewed periodically (e.g., WHO, 1982; Lacey & Mulla,
1990; Melin & Cozzi, 1990; Molloy, 1992; Otvos & Vanderveen, 1993).
The range of non-target species that have been found to be susceptible
to direct toxic action of Bt has remained small. A list of non-target
species found to be insensitive to Btte was issued by Keller &
Langenbruch (1993). In more than 30 years of commercial use, no
serious, direct effects on NTOs have been reported as arising from
Bt-based MPCAs. Several studies which identified effects of Bt on
predators or parasitoids of susceptible insect species are listed by
Navon (1993), but the effects have been small. Mortality in bees has
been observed after exposure to vegetatively growing Bt but the effect
does not seem to be related to spores or ICPs.
6.4.2.1 Aquatic insects
Bti is specific in its toxicity to dipterans. Nevertheless, many
studies have tested the effect of Bti applications on a wide range of
aquatic insects.
Lacey & Mulla (1990) summarized a number of studies of the
effects of Bti on certain non-target arthropod species and arthropod
populations in the laboratory and field (Table 12). The results of
representative studies are summarized below.
Table 12. Effects of Bti on non-target arthropodsa
Arthropod order Type of study Resulta References
Coleoptera Laboratory - Schnetter et al., 1981
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982; Mulla, 1988
Diptera Laboratory + Garcia et al., 1980; Ali, 1981;
(Chironomidae) Ali & Baggs, 1981; Schnetter et al., 1981
Field + Mulla et al., 1971; Ali, 1981;
Mulligan & Schaefer, 1982;
Rogatin & Baizhanov, 1984
Field - Miura et al., 1980
Ephemeroptera Laboratory - Ali, 1980; Schnetter et al., 1981;
Mulligan & Schaefer, 1982
Field - Schnetter et al., 1981; Mulla et al., 1982,
Mulligan & Schaefer, 1982; Mulla, 1988
Heteroptera Field - Schnetter et al., 1981;
(Corixidae) Mulligan & Schaefer, 1982
Heteroptera Laboratory - Schnetter et al., 1981;
(Notonectidae) Olejnicek & Maryskova, 1986;
Aly & Mulla, 1987
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982;
Mulla, 1988
Field + Purcell, 1981
Odonata Laboratory - Mulla & Khasawinah, 1969;
Mulligan & Schaefer, 1982; Aly, 1985;
Aly & Mulla, 1987
Field - Mulla, 1988
a - = no effect reported; + = an effect was reported, but does not necessarily imply that either
individual arthropods or populations of arthropods were adversely affected.
Four species of chironomid larvae (Chironomus crassicaudatus,
Chironomus decorus, Glyptotendipes paripes, Tanytarsus species)
were tested with four Bti preparations. The chironomid larvae were
less susceptible to Bti, being 13- to 75-fold more tolerant than
mosquito larvae to the various Bti preparations (Ali, 1981; Ali &
Baggs 1981). Garcia et al. (1980) induced low to high levels of
mortality in some nematocerous Diptera, including a variety of taxa
in the families Ceratopogonidae, Chironomidae and Dixidae, using
dosages of Bti that were 50 to several hundredfold
higher than concentrations used for mosquito control. Schnetter et al.
(1981) reported complete mortality in chironomid larvae ( Chironomus
thummi ) exposed to high levels of Bti for 48 h without food.
Field-collected adult aquatic beetles exposed to Bti suffered little
or no mortality (Schnetter et al., 1981). Ali (1980) tested a Bti
formulation at 20 times the larvicidal dosage for mosquitos and
reported no adverse effects against larval mayflies (Baetis
species). Schnetter et al. (1981) reported that mayflies (Cloeon
species) suffered no mortality when fed Bti at high dosages.
Aly & Mulla (1987) fed Bti intoxicated mosquito larvae (Culex
quinquefasciatus) to field-collected fourth to fifth instar
backswimmers (Notonecta undulata). The predators were fed at the
rate of 10 larvae per predator per day for 4 days, then the predators
were fed unintoxicated mosquito larvae and observed for 15 to 17 days.
The nymph and adult notonectids exhibited no adverse effects.
Olejnicek & Maryskova (1986) observed no marked mortality in
backswimmers (Notonecta glauca) that were fed Bti intoxicated
mosquito larvae. Schnetter et al. (1981) found no mortality in
backswimmers (Notonecta glauca) exposed for 48 h to high levels of
Bti.
Mosquito larvae intoxicated with extremely high dosages of Bti
were fed to naiads of the dragonfly Tarnetrum corruptum and
damselfly Enallagma civile; the duration of development of the
dragonfly and damselfly naiads, from the time of exposure to
emergence, was not affected (Aly, 1985; Aly & Mulla, 1987)
Merritt et al. (1989) reported no evident of effects on the drift
of aquatic invertebrates, or on the numbers of these invertebrates in
benthic Surber samples, during a blackfly (Simulium species) control
programme. In the USA, Molloy (1992) reviewed ten field trials where
Bti was used against blackfly (Simulium species) larvae. He
concluded that, although there was a potential for adverse impact of
Bti on filter-feeding chironomids, the impact on stream insect
communities overall was very small.
Over a three-season period, Bti administered at mosquito
larvicidal rates had no adverse effects on the larvae of diving
beetles (Dytiscidae) or water scavengers (Hydrophylidae) (Mulla et
al., 1982; Mulla, 1988).
The application of a Bti formulation in a wildlife marsh showed
no adverse effects on beetle larvae (Mulligan & Schaefer, 1982).
Ali (1981) evaluated the efficacy of various levels of a Bti
formulation against chironomids in the families Chironominae and
Tanytarsinae and obtained mortality at dosages higher than those
employed to control mosquito larvae. Miura et al. (1980), using
mosquito larvicidal dosages of a commercial Bti product, showed no
reduction in the field populations of chironomids following treatment.
Mulla et al. (1971) reported marked reductions in some chironomid
populations, using a commercial Bti product at rates of 20 to 40 times
the mosquitocidal rates. Mulligan & Schaefer (1982) reported a 40 to
70% reduction in some chironomid species after application of a Bti
formulation to a wildlife marsh. Rogatin & Baizhanov (1984) noted a
significant reduction of chironomids after Bti exposure.
Extensive quantitative observations were made on mayfly nymphs,
mostly Callibaetis pacificus, but no notable effects were observed
when Bti was applied against mosquito larvae (Mulla et al., 1982;
Mulla, 1988). Mulligan & Schaefer (1982) found that Bti did not
adversely affect mayfly nymphs (Callibaetis species). Schnetter et
al. (1981) reported that mayflies (Cloeon species) were not affected
when Bti was used in floodwater mosquito (Aedes vexans) larval
habitats. Schnetter et al. (1981) collected water boatmen (Corisella
species) from mosquito larval habitats on the upper Rhine river in
Germany. The water boatman population was not affected after exposure
to Bti for 48 h. Adverse effects were noted on backswimmers (Buenoa
species, Notonecta undulata, and Notonecta unifasciata) during
field trials with Bti (Mulla et al., 1982; Mulla, 1988). Mulligan &
Schaefer (1982) reported that the backswimmer (Notonecta species)
populations in a wetland marsh were not adversely affected by the
application of a Bti formulation. Purcell (1981) noted reductions in
populations of backswimmers (Buenoa elegans, Notonecta indica)
after application of Bti, but attributed this to the flying activity
of these predators.
No adverse effects on naiads of the dragonfly (Tarnetrum
corruptum) and damselfly (Enallagma civile) were reported when Bti
was used against larval mosquito populations (Mulla & Khasawinah,
1969; Mulla, 1988).
No notable reduction in the number of nymphs of several species
of dragonfly (Anisoptera) and damselfly (Zygoptera) occurred when
Bti was applied in a wetland marsh (Mulligan & Schaefer, 1982).
In the follow-up to the Onchocerciasis Control Programme of West
Africa (section 5.1.3.2), little or no effect on the non-target
populations was observed. However, few details were provided (Yameogo
et al., 1988; Levêque et al., 1988; Calamari et al., 1998).
6.4.2.2 Terrestrial insects
Melin & Cozzi (1990) summarized a number of studies on the
effects of Btk, Btg, Btt and Bte on non-target arthropod species and
arthropod populations in the laboratory and field. Representative
studies on Btk, Btg, Btt and Bte are listed in Tables 13 and 14.
Obadofin & Finlayson (1977) determined that a commercial Btk
product had a minimal effect on the ground beetle ( Bembidion
lampros ). Wilkinson et al. (1975) evaluated the contact activity of
a commercial Btk product for 5 days at levels equivalent to field
rates on an adult ladybird beetle (Hippodamia convergens) and found
no adverse effects.
Workman (1977) exposed earwigs (Labidura riparia) to a
commercial Btk product at rates equivalent to 10 times the normal
field application rate. No mortality was observed in these predators.
Hamed (1978-1979) found that two tachinid species (Bessa fugax
and Zenilla dolosa) were not affected after being fed suspensions
of a commercial Btk product. Horn (1983) observed a reduction in the
number of syrphid larvae on collards sprayed with a commercial Btk
product. This effect was attributed to a repellent effect on the
syrphid adults.
Hamed (1978-1979) found that Picromerus bidens was not
adversely affected after feeding upon lepidopteran larvae (Yponomeuta
evonymellus) that had fed upon leaves treated with commercial Btk
products.
Hassan (1983) determined a commercial Btk product to be harmless
to adult lacewings (Chrysopa carnea) when they were exposed at
normal field rate concentrations. Wilkinson et al. (1975) found
negligible mortality in larval or adult lacewings (Chrysopa carnea)
when a commercial Btk product was applied as a contact spray at
recommended field rates.
Yousten (1973) fed lethal quantities of Btk to larval cabbage
loopers (Trichoplusia ni) and just prior to death offered these
larvae to young Chinese praying mantids (Tenodera aridifolia
subspecies sinensis). The mantids were not susceptible to the
spore-crystal mixtures in the intact insect host.
Asquith (1975) found that black ladybird beetles (Stethorus
punctum) on apple trees were not affected by treatment with a
commercial Btk product. Buckner et al. (1974) monitored populations of
ground beetles following aerial spraying of spruce with two commercial
Btk products and found no effect on these predators. Harding et al.
(1972) detected no reduction in population levels of ladybird beetles
(coccinellids), rove beetles (staphyllinids), or checkered beetles
(clerids) in plots treated with a commercial Btk product. Johnson
(1974) evaluated several commercial Btk products as both sprays and
baits on tobacco. During the 2-year study, the populations of two
coccinellids (Hippodamia convergens and Colemegilla maculata)
were not affected by the microbial treatments. Wallner & Surgeoner
(1974) found no effects on coccinellids (Cycloneda munda,
Chilocorus bivulnerus and Adalia bipuncta) following forest
sprays with a commercial Btk product.
While evaluating a commercial Btk product for the control of
gypsy moth (Lymantria dispar) and elm spanworm ( Ennomos
subsignacius ), Dunbar et al. (1972) found no adverse effect on
Table 13. Effects of Btk on non-target arthropods
Arthropod order Type of study Resultsa References
Acarina Field - Weires & Smith, 1977;
Horsburgh & Cobb, 1981
Coleoptera Laboratory - Wilkinson et al., 1975; Obadofin & Finlayson, 1977
Field - Harding et al., 1972; Buckner et al., 1974;
Johnson, 1974; Wallner & Surgeoner, 1974;
Asquith, 1975
Dermaptera Laboratory - Workman, 1977
Diptera Laboratory - Hamed, 1978-1979
Laboratory - Horn, 1983
Field - Dunbar et al., 1972; Fusco, 1980
Heteroptera Laboratory - Hamed, 1978-1979
Field - Harding et al., 1972; Elsey, 1973;
Jensen, 1974; Wallner & Surgeoner, 1974
Hymenoptera Laboratory - Krieg, 1973
(Honey-bees) Laboratory - Krieg et al., 1980
Field - Buckner et al., 1974
Hymenoptera Laboratory - Wallner & Surgeoner, 1974;
(Parasitoids) Laboratory + Hassan & Krieg, 1975; Krieg et al., 1980
Dunbar & Johnson, 1975;
Mück et al., 1981; Weseloh &
Andreadis, 1982; Wallner et al., 1983;
Thomas & Watson, 1986
Field - Dunbar et al., 1972; Buckner et al., 1974;
Wanller & Surgeoner, 1974; Hamel, 1977;
Morris et al., 1977; Morris et al., 1980;
Fusco, 1980
Field + Weseloh et al., 1983
Table 13. (cont'd)
Arthropod order Type of study Resultsa References
Neuroptera Laboratory - Wilkinson et al., 1975; Hassan, 1983
Dictyoptera Laboratory - Yousten, 1973
(mantis)
a - = no effect reported; + an effect was reported, but does not imply that either
individual arthropods or populations of arthropods were adversely affected.
Table 14. Effects of different Bt strains on non-target arthropods
Arthropod order Type of study Resulta References
Btg
Hymenoptera Laboratory - Cantwell & Shieh, 1981
(Honey-bees) Field - Burges, 1977
Field - Burges & Bailey, 1968
Btt
Coleoptera Field + Kazakova & Dzhunusov, 1977
Hymenoptera Laboratory + Krieg & Herfs, 1963; Krieg, 1973
(Honey-bees) Laboratory - Martouret & Euverte, 1964;
Cantwell et al., 1966
Hymenoptera Laboratory + Hassan & Krieg, 1975;
(Parasitoids) Salama et al., 1982; Hassan, 1983;
Salama & Zaki, 1983
Laboratory - Krieg et al., 1980
Table 14. (cont'd)
Arthropod order Type of study Resulta References
Bte
Coleoptera Laboratory - Salama & Zaki, 1983
Laboratory + Salama et al., 1982
Hymenoptera Laboratory + Salama & Zaki, 1983
(Parasitoids)
Neuroptera Laboratory + Salama et al., 1982
a - = no effect reported; + = an effect was reported, but does not imply
that either individual arthropods or populations of arthropods were adversely affected.
two tachinids (Blepharipa scutellata and Parasitigena agilis).
Fusco (1980) reported an increased incidence of parasitism by two
tachinids (Blepharipa pratensis and Compsilura concinnata) when
Btk was applied in a field study.
Elsey (1973) reported no detrimental effect on spined stiltbug
nymphs or adults (Jalysus spinosus) during a 2-month field study
with a commercial Btk product. Harding et al. (1972) conducted a 2
year study to evaluate the effects of Btk on the natural enemies of
the bollworm (Helicoverpa zea) on cotton (Gossypium hirsutum).
Following applications of Btk against this pest, they reported no
detectable effects on Anthocoridae (minute pirate bugs, Orius
species), Lygaediae (bigeyed bugs, Geocoris species), Nabidae
(damsel bugs, Nabis species), or Reduviidae (assassin bugs).
Jensen (1974) used a commercial Btk product on soybeans (Glycine
canescens) to control the green cloverworm (Plathypena scabra) and
the velvetbean caterpillar (Anticarsis gemmitalis). No adverse
effect was observed on Lygaediae (bigeyed bugs, Geocoris species)
or Nabidae (damsel bugs, Nabis species).
Wallner & Surgeoner (1974) found no effect on the spined soldier
bug (Podisus maculiventris), following forest sprays of commercial
Btk products to control the oakleaf caterpillar (Heterocampa
manteo).
Although many data exist, in a review of the effects of the use
of Btk in Canada, Addison (1993) concluded that few studies on NTOs
had used soil invertebrate species and soil conditions relevant to
field conditions in Canadian forests.
Salama & Zaki (1983) reared cotton leafworm larvae (Spodoptera
littoralis) on a diet containing Bte and then fed these larvae to
adult staphylinid beetles (Paederus alferii). Predator longevity was
not significantly affected and no difference was seen in acceptability
to predators between untreated larvae and those exposed to Bte.
Salama et al. (1982) treated aphids with sprays of Bte and
provided these treated insects to newly hatched coccinellid larvae
(Coccinella undecimpunctata). The survival of larvae of predators
was not affected by feeding on the treated prey. However, the duration
of predator larval development was increased in the group treated with
Bte and there was a definite reduction in prey consumption.
Salama et al. (1982) evaluated the effect of Bte on the
development of lacewing larvae (Chrysopa carnea) by presenting them
with either sprayed aphids or treated cotton leafworm larvae
(Spodoptera littoralis). When fed either the sprayed aphids or the
treated cotton leafworms, the duration of larval development was
significantly extended and prey consumption was significantly reduced.
6.4.2.3 Honey-bees
Krieg (1973) observed mortality in adult honey-bees (Apis
mellifera) that were fed non-sporulated broth cultures of Btk. The
mortalities were attributed to the thermolabile alpha-toxin. Since
alpha-toxin is inactivated during sporulation, the toxin would not
present a problem in sporulated commercial Btk products. When Krieg et
al. (1980) fed fully sporulated cultures of Btk to adult honey-bees at
concentrations of 1 × 108 spores and crystal per bee over a 7-day
period, no harmful effects were observed.
Cantwell & Shieh (1981) fed a 1:20 solution of Btg in a sucrose
solution to newly emerged adult honey-bees. After 14 days, there was
no difference in mortality between treated and untreated groups.
Treatment of hives resulted in no adverse effect on the adult workers
or colony life as determined by egg laying, brood production, brood
capping, or honey production.
Buckner et al. (1974) observed no adverse effects on honey-bees
following aerial spraying of spruce (Picea species) with commercial
Btk products.
Cantwell et al. (1966) fed honey-bees sugar solutions containing
Btt spores, Btt culture supernatant with beta-exotoxin, and Btt
crystals. The crystals did not harm the bees, but the supernatant
caused nearly 100% mortality at day 7. Significant mortality was seen
in the spore-treated bees at 8 days and was attributed to bacterial
septicaemia. It should be noted that the dosages of each treatment
were many times higher than the bees would be exposed to in the course
of a lepidopteran control programme. Krieg (1973) reported mortality
in honey-bees fed whole nonsporulated cultures of Btt, which was
attributed to the presence of beta-exotoxin. Krieg & Herfs (1963)
reported that vegetative cells of Btt did not harm honey-bees;
however, they reported toxicity in Btt preparations containing the
beta-exotoxin. Martouret & Euverte (1964) fed worker honey-bees
cultures of Btt incorporated into mixtures of sugar, honey and clay.
Complete mortality was seen at 7 days for the spore-crystal-exotoxin
preparation and at 14 days for the spore-crystal complex.
6.4.2.4 Parasitoids
a) Btk
Dunbar & Johnson (1975) collected adult parasitoids
(Cardiochiles nigriceps) in the field and fed them suspensions of a
commercial Btk product. In the group fed Btk, shorter life spans were
reported. Since the investigators could not be sure whether feeding
actually took place, starvation may have been the cause of death.
Hassan & Krieg (1975) observed no adverse effects on adult
chalcid wasps (Trichogramma cacoeciae) that were fed suspensions of
a commercial Btk product. Krieg et al. (1980) fed washed spores and
crystals of Btk (5 × 107 spores and crystals) for 7 days to adult
chalcid wasps (Trichogramma cacoeciae) and observed no mortality or
reduced capacity to parasitize.
Mück et al. (1981) reported significant mortality in adult
braconids (Cotesia glomerata) that were fed a commercial Btk product
at rates of 108 and 109 spores per ml, but observed little effect on
the adult parasitoids (Pimpla turionellae). They reported midgut
epithelial damage in the Pimpla turionellae, which resulted from the
ICP.
Thomas & Watson (1986) found lower survival in adult ichneumonids
(Hyposoter exiguae) fed suspensions of a commercial Btk product.
They concluded the mortality was due to the spore-crystal complex.
Wallner & Surgeoner (1974) observed no effect on parasitoids
following treatments with commercial Btk products for control of the
notodontid moth (Heterocampa manteo).
Wallner et al. (1983) reported an indirect effect on the braconid
Rogas lymantriae when it parasitized gypsy moth (Lymantria dispar)
hosts fed Btk. The sex ratio of the parasitoid offspring was skewed
towards males in the treated larvae, as the female parasitoids lay
more fertilized eggs in larger, untreated host larvae.
Weseloh & Andreadis (1982) reported synergism in laboratory tests
with gypsy moth larvae (Lymantria dispar) fed a commercial Btk
product and exposed to the braconid (Cotesia melanoscelus). The
percentage of parasitism was increased in Btk-intoxicated larvae since
these grew more slowly and were at the approximate size suitable for
parasitism for a longer time.
Buckner et al. (1974) reported no detrimental effects on
parasitoid populations following field application of a commercial Btk
product.
Dunbar et al. (1972) reported an increase in the percentage of
parasitism of gypsy moth (Lymantria dispar) and elm spanworm
(Ennomos subsignarius) larvae in forestry plots treated with a
commercial Btk product.
Fusco (1980) reported an increase in the percentage of parasitism
of gypsy moth (Lymantria dispar) larvae by the braconids Cotesia
melanoscelus and Phobocampe unicincta following aerial sprays with
a commercial Btk product.
Hamel (1977) found that parasitoids attacking early instar
western spruce budworm larvae (Choristoneura occidentalis) increased
in number following aerial application of a commercial Btk product,
while older budworm larvae were reduced in number.
In two field studies, commercial Btk products showed no
detrimental effects on parasitoid populations (Morris et al., 1977,
1980).
Wallner & Surgeoner (1974) demonstrated 6- to 12-fold increases
in the percentage of parasitism in gypsy moth larvae (Lymantria
dispar) by the braconid Cotesia melanoscelus in forestry plots
treated with a commercial Btk product.
b) Btt
Hassan (1983) observed the chalcid Trichogramma cacoeciae was
not affected by exposure to dried surface films of Btt.
Hassan & Krieg (1975) fed a suspension of three different
commercial Bt products to adult chalcids (Trichogramma cacoeciae)
and reported a minor reduction in the capacity to parasitize with
Btt, but none with the other Bt products. The effect of the Btt
product may have been due to the beta-exotoxin.
Krieg et al. (1980) fed washed spores and crystals of Btt to
adult chalcids (Trichogramma cacoeciae) for 7 days and observed no
mortality or reduced capacity to parasitize.
Lowered reproductive potential was observed for both the
braconids Microplitis demolitor and Zele chlorophthalma
following exposure to Btt (Salama et al., 1982; Salama & Zaki, 1983).
Salama & Zaki (1983) reported increased development times for
Zele chlorophthalma parasitizing the cotton leafworm Spodoptera
littoralis treated with Bte.
7. EXPOSURE AND EFFECTS ON HUMANS
There are some case reports on the occurrence of Bt in patients
with different infectious diseases. However, none of these studies
demonstrate an actual risk to human health by the use of Bt. They
emphasize the need for further research on the production of toxins,
knowledge of factors causing the genes of the toxins to be expressed,
and knowledge on the natural occurrence of Bt and Bc. The medical
practice does not discriminate between Bt and Bc as causative agents
in infectious diseases. Therefore, the true proportion of Bt in Bc-
induced disease is not known.
7.1 Bacillus thuringiensis
For aeons, humans have been exposed to Bt in their natural
habitats, particularly from soil, water and the phylloplane. However
in the recorded scientific literature, only few adverse effects to
these environmental Bt levels have been documented.
The manufacture and field application of Bt products can result
in aerosol and dermal exposure of workers and the human population,
especially by spraying programmes in populated areas. Agricultural and
horticultural uses of Bt can also result in dietary exposure.
7.1.1 Experimental exposure of humans
Eight human volunteers ingested 1 gram of a Btk formulation
(3 × 109 spores/g of powder) daily for 5 days. Of the eight
volunteers, five also inhaled 100 mg of the Btk powder daily for five
days. Comprehensive medical examinations immediately before, after,
and 4 to 5 weeks later failed to demonstrate any adverse health
effects, and all the blood chemistry and urinalysis tests were
negative (Fisher & Rosner, 1959).
Pivovarov et al. (1977) reported that ingestion of foods
contaminated with Btg at concentrations of 105 to 109 cells/g
caused nausea, vomiting, diarrhoea and tenesmus, colic-like pains in
the abdomen, and fever in three of the four volunteers studied. The
toxicity of the Btg strain may have been due to beta-exotoxin (Ray,
1990).
7.1.2. Exposure of workers during manufacture
Many manufacturers of Bt products monitor the exposure and the
associated health risks of their workers. Over a period of 30 years of
production, there have been no reports of such workers having been
adversely affected (RJ Cibulsky, personal communication, 1997).
7.1.3 Exposure of workers in spraying operations
Noble et al. (1992) studied aerosol Btk exposure and subsequent
nose and throat carriage of Bt by workers during a major spray
programme for gypsy moth (Lymantria dispar) control. Spraying down
from high lifts, spraying low foliage or spraying with prevailing
breezes resulted in lower exposures of spray operators than did
spraying upwards into trees. The mean exposure values ranged from
3.0 × 103 to 5.9 × 106 Bt spores/m3 sampled air. Individuals working
most shifts during the spray period were exposed to 5.4 × 106 to
7.2 × 107 organisms. Nearly all the workers exposed to higher
concentrations for several shifts (5 to 20) were culture-positive for
Bt, and the majority of the workers remained culture-positive for 14
to 30 days. Of those who were culture-positive, eight workers reverted
to a culture-negative status during the project or within 30 days of
project completion. During the spray programme, some workers
experienced chapped lips, dry skin, eye irritation, and nasal drip and
stuffiness, but no serious health problems resulted. These symptoms
were transient and frequently occurred during the beginning of a spray
run and when Bt spray concentrations were increased. No significant
differences were found with respect to gender or smoking status.
In the same study, Noble et al. (1992) evaluated the health
records of the general population in the county where the Btk spray
programme was conducted. After examining the records of 3500 hospital
emergency room admissions, 1140 family practice patients, and over 400
bacterial cultures from 10 hospitals, no evidence for community
illness or infections attributed to Btk could be documented.
Laferrière et al. (1987) demonstrated antibody titres in 11 of
107 workers exposed to Btk during a 2-year spraying period. By the
middle of the spray operation, seven had developed titres to
spore-crystal complexes, six to vegetative cells, and one to spores.
Their titres tended to be low, but were higher in those exposed for a
second year. Two months after the exposure ended, nine workers were
retested. Of these workers, five had no detectable antibodies to the
spore-crystal complexes, and four who had been among those with the
highest titres against vegetative cells had significantly lower
titres.
Elliott et al. (1988) measured the exposure of individual workers
and other individuals within the spraying area on the day of
application during an aerial Btk spray programme for gypsy moth
(Lymantria dispar) control. Concentrations of spores were measured
using personal air sampling devices. The concentration of spores
ranged from 0 to 1.1 × 104 cfu/m3 for individual workers, the
highest concentration being incurred by a spray card checker who was
in brief contact with the material. For non-working individuals, the
average Bt exposure was 1.3 × 103 cfu/m3. In the spray area, a
general survey showed concentrations of 0 to 4.2 × 103 cfu/m3.
7.1.4 Exposure of human populations by spraying operations over
populated areas
Btk and Bti have been sprayed over populated areas in several
countries, including the USA, Canada and New Zealand. Some of these
applications have been followed by public health surveillance
programmes. In general, no (or very few) harmful effects have been
reported among residents of the sprayed communities.
7.1.5 Clinical case reports
Commercial Bt products have been used for over two decades, but
Bt has been isolated in only a few cases of human bacterial infection.
Samples & Buettner (1983) reported that a farm worker developed a
corneal ulcer in one eye. It had been accidentally splashed with a
commercial Btk product and Bt was subsequently isolated from the
affected eye. The eye was treated with a topical antibiotic and
corticosteroid and the corneal ulcer resolved 14 days after treatment.
The report attributed the corneal ulcer to Bt infection. However, the
possibility that Bt may have been a non-pathogenic contaminant of the
ulcer was not considered. There are no other reports of Bt being
associated with ocular infections in workers.
During the investigation of a gastroenteritis outbreak in a
chronic care institution, bacteria were isolated from four individuals
and were identified as B. thuringiensis. The Bt isolates showed
cytotoxic effects characteristic of B. cereus (Jackson et al.,
1995).
Damgaard et al. (1997a) isolated Bt in burn wounds in two
patients. None of the isolates showed any toxicity to Vero cells.
Hernandez et al. (1998) isolated Bt from a war wound; this strain (Bt
konkukian) could infect immunosuppressed mice after cutaneous
application.
Warren et al. (1984) reported that a research worker developed a
marked local reaction and lymphadenitis following a needle stick
injury when handling Bti. Acinetobacter calcoaceticus and Bt were
cultured from the exudate. The condition responded to penicillin.
Green et al. (1990) reported that Bt was isolated from body
fluids of 55 patients with different infectious diseases. In 52 of
them, it was considered a contaminant, while in three cases with
pre-existing medical problems, no firm conclusion was established
concerning a causal relationship between the infection and Bt.
Furthermore, Bt was isolated from the conjunctiva of a worker
presenting with conjunctivitis, and with a history of a splash with a
Bt product.
Despite the widespread use of Bt-based products, only two
incidents of possible allergic reactions have been reported to the US
EPA (McClintock et al., 1995). After detailed analysis, neither of
these was considered to be causally related to Bt.
7.1.6 Dietary exposure of the general population
In some Asian countries, Bti has been added to domestic
containers of drinking-water for mosquito control. From these high Bt
exposures in drinking-water, no adverse effects in humans have been
reported. In Africa, some rivers have been dosed with Bti at weekly
intervals for blackfly control. No adverse effects in the human
populations that drink the river water have been reported. Btk has
been reported to survive for 1 to 2 months in fresh water and in
seawater. However, viable Bt cultures have not been isolated from
drinking-water supplies (Menon & De Mestral, 1985).
There is little information on levels of Bt to be found in food,
but it is possible that, in view of the widespread prevalence of Bt,
its presence in food is common and is not always related to its use on
food products. Bt spores have been shown to be unable to germinate in
mammalian digestive systems; however, Bt has been isolated from faecal
and urinary samples in occupational studies.
Noble et al. (1992) reported that 5 out of 10 vegetable samples
were positive for Btk. The positive samples were obtained from both
supermarkets and from organically grown products. Such results may
account for the recovery of Bt from faecal and urinary samples during
the occupational studies and may reflect community exposure through
food.
7.2 Bacillus cereus
The close affiliation between Bt and Bc raises the question of
whether strains of Bt can cause human illness during vegetative
growth. During vegetative growth Bc can produce different kinds of
toxins; these toxins can cause gastrointestinal diseases in humans
after ingestion. The emetic toxin is an enzymatically synthesized
peptide that causes vomiting (Granum, 1997) a few hours after
ingestion. Most Bc strains producing this toxin seem to belong to the
same serotype (Mikami et al., 1995; Nishikawa et al., 1996). The
enterotoxins are a group of proteins causing abdominal pain and
diarrhoea after an incubation period of 8-16 h. The enterotoxins
causing gastrointestinal disease are most likely produced in the small
intestine. Characteristics of the two types of disease caused by Bc
are shown Table 15. Based on analysis of outbreaks the infective dose
is believed to vary between 105 and 108 vegetative cells or
activated spores per gram, but it may be so low as 104 (Granum,
1997). In addition to the two toxins, Bc can produce different lytic
enzymes, e.g., haemolysins, which most likely are involved in the
gastrointestinal diseases. In addition to gastrointestinal diseases Bc
can cause various diseases, notably in immunosuppressed individuals
(Drobniewski, 1993).
Analysis of reported foodborne diseases reveals that Bc is
frequently diagnosed as the cause of gastrointestinal disorders
(Notermans & Batt, 1998) in many countries. Several food-borne disease
outbreaks caused by Bc have been reported by Notermans & Batt (1998).
However, Bc gastrointestinal diseases are highly under-reported, as
both types of illness are relatively mild and usually last less than
24 h (Granum, 1997; Notermans & Batt, 1998). The incidence of Bc in
foods varies between 101 and 107, the highest concentrations being
found in herbs/spices and boiled rice (Notermans & Batt, 1998). The
degree of toxicity of enterotoxins varies from Bc strain to strain,
probably due to differences in toxin components (Lund & Granum, 1997).
Hassan & Nabbut (1996) found that clinical Bc isolates from human
diarrhoeal faeces were strong producers of diarrhoeal enterotoxin,
while isolates from blood, wounds, normal faeces, milk and rice were
weak producers of diarrhoeal enterotoxin (Hassan & Nabbut, 1996). This
variation is reflected in the variable numbers (105-108 viable cells
or spores per g) of Bc reported to cause symptoms in humans, and it
has been suggested that foods containing more than 104 Bc per g may
not be safe for consumption (Granum, 1997). Several European countries
have a critical level of 104-105 Bc per g for acceptance of food
products (Notermans & Batt, 1998). This critical level will include
Bt, as the methods used do not discriminate between Bc and Bt.
Table 15. Characteristics of the two types of disease caused by B. cereus
(from Granum, 1997)
Characteristic Emetic syndrome Diarrhoeal
Infective dose (cells/g) 105-108 104-107
Toxin produced Preformed in food In the small intestine
Type of toxin Cyclic peptide Protein
Incubation period (h) 0.5-5 8-16 (occasionally >24)
Duration of illness 6-24 12-24 (occasionally many days)
Symptoms Vomiting, nausea, malaise Abdominal pain, diarrhoea
8. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and from biologically active products other than
the ICPs. Bt products may be safely used for the control of insect
pests of agricultural and horticultural crops as well as forests. Bt
is also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt have the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
9. CONCLUSIONS AND RECOMMENDATIONS
* Bt may be safely used for the control of insect pests of
agricultural crops and forests.
* Bti is safe for use in aquatic environments, including
drinking-water reservoirs, for the control of mosquito, blackfly
and nuisance insect larvae.
* Bt products should contain the ICPs and be free from other
microorganisms and biologically active metabolites.
* New Bt products based on either new Bt strains and/or new ICPs
require appropriate assessment.
* FAO and WHO should develop standard specifications for Bt
preparations as is done for chemical pesticides.
* Good industrial large-scale practice (GILSP) standards should be
employed for the production of Bt products.
* Standardized valid methods for the assessment of gastrointestinal
consequences of vegetatively produced agents should be developed.
* The occurrence of resistant insect populations underscores the
need for research on the relationships between cry-toxins and
the ecology of Bt.
* More research on the fate of Bt spores and ICPs in the
environment is needed. This should cover the natural occurrence
of Bt and Bc in foods and its relationship to exposure to Bt from
its pesticide use.
* Research into dose-response analysis and the consequent
acceptable daily intake levels of Bt in the diet and beverages is
a high priority.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANISATIONS
WHO (1985) considered the safe use of MPCA at the Ninth Meeting
of the WHO Expert Committee on Vector Control in 1984. The report
considered that addition of live microorganisms to drinking-water is
undesirable and recommended that the use of Bt H-14 for the control of
Aedes aegypti in drinking-water should be restricted to the
asporogenic form. At the 1990 meeting, WHO (1991), after reviewing new
research data, stated that its previous recommendation was unduly
restrictive, provided that properly designed formulations were used.
REFERENCES
Addison JA (1993) Persistence and nontarget effects of Bacillus
thuringiensis in soil: a review. Can J Forensic Res, 23: 2329-2342.
Agata N, Ohta M, Arakawa Y, & Mori M (1995) The bceT gene of
Bacillus cereus encodes an enterotoxic protein. Microbiology, 141:
983-988.
Ahmed R, Sankar-Mistry P, Jackson S, Ackermann H-W, & Kasatiya SS
(1995) Bacillus cereus phage typing as an epidemiological tool in
outbreaks of food poisoning. J Clin Microbiol, 33: 636-640.
Akiba Y (1986) Microbial ecology of Bacillus thuringiensis: VI.
Germination of Bacillus thuringiensis spores in the soil. Appl
Entomol Zool, 21: 76-80.
Akiba Y (1991) Assessment of rainwater-mediated dispersion of
field-sprayed Bacillus thuringiensis in the soil. Appl Entomol Zool,
26: 477-483.
Ali A (1980) Nuisance chironomids and their control: a review. Bull
Entomol Soc Am, 26: 3-16.
Ali A (1981) Bacillus thuringiensis serovar. israelensis
(ABG-6108) against chironomids and some nontarget aquatic
invertebrates. J Invertebr Pathol, 38: 264-272.
Ali A & Baggs RD (1981) Susceptibility of some Florida chironomids and
mosquitoes to various formulations of Bacillus thuringiensis serovar
israelensis de Barjac. J Econ Entomol, 74: 672-677.
Aly C (1985) Germination of Bacillus thuringiensis var. israelensis
spores in the gut of Aedes larvae (Diptera: Culicidae). J Invertebr
Pathol, 45: 1-8.
Aly C & Mulla MS (1987) Effect of two microbial insecticides on
aquatic predators of mosquitoes. J Appl Entomol, 103: 113-118.
Aly C, Mulla MS, & Federici BA (1985) Sporulation and toxin production
by Bacillus thuringiensis var. israelensis in cadavers of mosquito
larvae (Diptera: Culicidae). J Invertebr Pathol, 46: 251-258.
Andersson MA, Mikkola R, Helin J, Andersson MC, & Salkinoja-Salonen M
(1998) A novel sensitive bioassay for detection of Bacillus cereus
emetic toxin and related depsipeptide ionophores. Appl Environ
Microbiol, 64: 1338-1343.
Andrews RE Jr, Faust RM, Wabiko H, Raymond KC, & Bulla LA Jr (1987)
The biotechnology of Bacillus thuringiensis. CRC Crit Rev
Biotechnol, 6: 163-232.
Angus TA (1954) A bacterial toxin paralysing silkworm larvae. Nature
(Lond), 173: 545-546.
Aronson AI, Han ES, McGaughey W, & Johnson D (1991) The solubility of
inclusion proteins from Bacillus thuringiensis is dependent upon
protoxin composition and is a factor in toxicity to insects. Appl
Environ Microbiol, 57: 981-986.
Asano S-I, Nukumizu Y, Bando H, Hzuka T, & Yamamoto T (1997) Cloning
of novel enterotoxin genes from Bacillus cereus and Bacillus
thuringiensis. Appl Environ Microbiol, 63: 1054-1057.
Ash C, Farrow JA, Dorsch M, Stackebrandt E, & Collins MD (1991)
Comparative analysis of Bacillus anthracis, Bacillus cereus, and
related species on the basis of reverse transcriptase sequencing of
16S rRNA. Int J System Bacteriol, 41: 343-346.
Asquith D (1975) Response of the predaceous black lady beetle
Stethorus punctum (DeConte) to apple orchard insecticide treatments
(Experiment No. D986-4007). Chicago, Illinois, Abbott Laboratories.
Baida GE & Kuzmin NP (1995) Cloning and primary structure of a new
hemolysin gene from Bacillus cereus. Biochim Biophys Acta, 1264:
151-154.
Baumann L, Okamoto K, Unterman BM, Lynch MJ, & Bauman P (1984)
Phenotypic characterization of Bacillus thuringiensis and Bacillus
cereus. J Invertebr Pathol, 44: 329-341.
Beach RM (1990) Application for an experimental use permit to ship and
use a pesticide for experimental purposes only -- Permit No.
58788-EUP-4 for InCideM 586. Hanover, Maryland, Crop Genetics
International.
Beattie SH, Holt C, Hirst D, & Williams AG (1998) Discrimination among
Bacillus cereus, B. mycoides and B. thuringiensis and some other
species of the genus Bacillus by Fourier transform infrared
spectroscopy. FEMS Microbiol Lett, 164: 201-206.
Beavers JB (1991a) ABG-6305: An avian oral pathogenicity and toxicity
study in the mallard (Project No. 161-118). Easton, Maryland, Wildlife
International Ltd, pp 1-20 (Unpublished Abbott document).
Beavers JB (1991b) ABG-6305: An avian oral pathogenicity and toxicity
study in the bobwhite (Project No. 161-117). Easton, Maryland,
Wildlife International Ltd, pp 1-21 (Unpublished Abbott document).
Beavers JB, Larsen AC, & Jaber M (1989a) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the bobwhite (Project No. 161-109). Eston, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Beavers JB, Clauss BS, & Jaber M (1989b) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the mallard (Project No. 161-108). Easton, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Becker N & Margalit J (1993) Use of Bacillus thuringiensis
israelensis against mosquitoes and blackflies. In: Entwistle PF, Cory
JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 147-170.
Beegle CC, Dulmage HT, Wolfenbarger DA, & Martinez E (1981)
Persistence of Bacillus thuringiensis Berliner insecticidal activity
on cotton foliage. Environ Entomol, 10: 400-401.
Benz G & Altwegg A (1975) Safety of Bacillus thuringiensis for
earthworms. J Invertebr Pathol, 26: 125-126.
Berliner E (1915) [About the sleep sickness of the Ephestia kühniella
Zell. and its vector Bacillus thuringiensis.] Z Angew Entomol, 2:
29-56 (in German).
Bernhard K & Utz R (1993) Production of Bacillus thuringiensis
insecticides for experimental and commercial uses. In: Entwistle PF,
Cory JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 255-267.
Bernier RL Jr, Gannon DJ, Moser GP, Mazzarello M, Griffiths MM, &
Guest PJ (1990) Development of a novel Bt strain for the control of
forestry pests. In: Brighton Crop Protection Conference -- Pests and
Diseases -- 1990: Proceedings. Farnham, Surrey, British Crop
Protection Council, pp 245-252.
Boeri RL (1991) Acute toxicity of ABG-6305 to the rainbow trout
(Oncorhynchus mykiss) (Project No. 9107-A). Hampton, New Hampshire,
Resource Analysts Inc, Enviro Systems Division, pp 1-26 (Unpublished
Abbott document).
Bonnefoi A & Béguin S (1959) Recherches sur l'action des cristaux de
Bacillus thuringiensis souche "anduze". Entomophaga, 4: 193-199.
Bourque SN, Valero JR, Lavoie MC, & Levesque RC (1995) Comparative
analysis of the 16S-23S ribosomal intergenic spacer sequences of
Bacillus thuringiensis strains and subspecies and of closely related
species. Appl Environ Microbiol, 61: 1623-1626.
Bravo A, Sarabia S, Lopez L, Ontiveros H, Abarca C, Ortiz M, Lina L,
Villalobos FJ, Pena G, Nunez-Valdez M-E, Soberón M, & Quintero R
(1998) Characterization of cry genes in a Mexican Bacillus
thuringienis strain collection. Appl Environ Microbiol, 64:
4965-4972.
Brousseau R, Saint-Onge A, Prefontaine G, Masson L, & Cabana J (1992)
Arbitrary primer polymerase chain reaction, a powerful method to
identify Bacillus thuringiensis serovars and strains. Appl Environ
Microbiol, 59: 114-119.
Buckner CH, Kingsbury PD, McLeod BB, Mortensen KL, & Ray DGH (1974)
Impact of aerial treatment on non-target organisms, Algonquin Park,
Ontario, and Spruce Woods, Manitoba, Section F. In: Evaluation of
commercial preparations of Bacillus thuringiensis with and without
chitinase against spruce budworm. Ottawa, Ontario, Canadian Forestry
Service, Chemical Control Research Institute, pp 1-72 (Information
report CC-X-59).
Bulla LA Jr, Kramer KJ, & Davidson LI (1977) Characterization of the
entomocidal parasporal crystal of Bacillus thuringiensis. J
Bacteriol, 130: 375-383.
Bulla LA Jr, Faust RM, Andrews R, & Goodman N (1985) Insecticidal
bacilli. In: Dubnau DA ed. The molecular biology of the bacilli. New
York, London, Academic Press Inc., vol 2, pp 185-209.
Burges HD (1977) Control of the wax moth Galleria mellonella on
beecomb by H-serotype V Bacillus thuringiensis and the effect of
chemical additives. Apidologie, 8: 155-168.
Burges HD (1980) Safety, safety testing and quality control of
microbial pesticides. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
738-767.
Burges HD & Bailey L (1968) Control of the greater and lesser wax
moths (Galleria mellonella and Achroia griesella) with Bacillus
thuringiensis. J Invertebr Pathol, 11: 184-195.
Burges HD & Hurst JA (1977) Ecology of Bacillus thuringiensis in
storage moths. J Invertebr Pathol, 30: 131-139.
Calamari D, Yameogo L, Hougard J-M, & Levêque C (1998) Environmental
assessment of larvicide use in the onchocerciasis control programme.
Parasitol Today, 14: 485-489.
Campbell DP, Dieball DE, & Brackett JM (1987) Rapid HPLC assay for the
ß-exotoxin of Bacillus thuringiensis. J Agric Food Chem, 35:
156-158.
Cannon RJC (1996) Bacillus thuringiensis use in agriculture: a
molecular perspective. Biol Rev Cambridge Philos Soc, 71: 561-636.
Cantwell GE & Shieh TR (1981) CertanTM: A new bacterial insecticide
against the greater wax moth. Am Bee J, 121: 424-431.
Cantwell GE, Heimpel AM, & Thompson MJ (1964) The production of an
exotoxin by various crystal-forming bacteria related to Bacillus
thuringiensis var. thuringiensis Berliner. J Insect Pathol, 6:
466-480.
Cantwell GE, Knox DA, Lehnert T, & Michael AS (1966) Mortality of the
honey bee, Apis mellifera, in colonies treated with certain
biological insecticides. J Invertebr Pathol, 8: 228-233.
Car M (1984) Laboratory and field trials with two Bacillus
thuringiensis var. israelensis products for Simulium (Diptera:
Nematocera) control in a small polluted river in South Africa.
Onderstepoort J Vet Res, 51: 141-144.
Car M & de Moor FC (1984) The response of Vaal River drift and benthos
to Simulium (Diptera: Nematocera) control using Bacillus
thuringiensis var. israelensis (H-14). Onderstepoort J Vet Res, 51:
155-160.
Carlberg G, Kihamia CM, & Minhar J (1985) Microbial control of flies
in latrines in Dar es Salaam with a Bacillus thuringiensis (serotype
1) preparation, Muscabac. J Appl Microbiol Biotechnol, 1: 33-44.
Carlson CR & Kolsto A-B (1993) A complete physical map of a Bacillus
thuringiensis chromosome. J Bacteriol, 175: 1053-1060.
Carlson CR, Caugant DA, & Kolstr A-B (1994) Genotypic diversity among
Bacillus cereus and Bacillus thuringiensis strains. Appl Environ
Microbiol, 60: 1719-1725.
Carlton BC, Gawron-Burke C, & Johnson TB (1990) Exploiting the genetic
diversity of Bacillus thuringiensis for the creation of new
bioinsecticides. In: Proceedings of the 5th International Colloquium
on Invertebrate Pathology and Microbial Control. Adelaide, Australia,
Society for Invertebrate Pathology, pp 18-22.
Chilcott CN, Pillai JS, & Kalmakoff J (1983) Efficacy of Bacillus
thuringiensis var. israelensis as a biocontrol agent against
larvae of Simuliidae (Diptera) in New Zealand. NZ J Zool, 10: 319-326.
Christensen KP (1990a) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 32-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-53 (Unpublished Abbott document No. 90-1-3211).
Christensen KP (1990b) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-57
(Unpublished Abbott document No. 90-2-3219).
Christensen KP (1990c) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc.,
vol 2, pp 253-308 (Unpublished Abbott document No. 90-5-3317).
Christensen KP (1990d) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to rainbow trout (Oncorhynchus mykiss)
during a 30-day static renewal test. Wareham, Massachusetts,
Springborn Laboratories Inc., pp 1-54 (Unpublished Abbott document No.
90-3-3263).
Christensen KP (1990e) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to sheepshead minnow (Cyprinodon
variegatus) during a 30-day static renewal test. Wareham,
Massachusetts, Springborn Laboratories Inc., pp 1-50 (Unpublished
Abbott document No. 90-6-3348).
Christensen KP (1990f) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-55 (Unpublished Abbott document No. 90-2-3228).
Christensen KP (1990g) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-55
(Unpublished Abbott document No. 90-2-3242).
Christensen KP (1990h) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-57 (Unpublished Abbott document No. 90-4-3288).
Cibulsky RJ & Fusco RA (1987) Recent experiences with Vectobac for
black fly control: An industrial perspective on future developments.
In: Kim KC & Merritt RW ed. Black flies: Ecology, population
management, and annotated world list. University Park, Pennsylvania,
Pennsylvania State University Press, pp 419-424.
Claus D & Berkeley RCW (1986) Genus Bacillus Cohn 1872. In: Sneath
PHA, Mair NS, Sharp ME, & Holt JG ed. Bergey's manual of systematic
bacteriology. Baltimore, Maryland, Williams & Wilkins, vol 2, pp
1105-1139.
Cooksey KE (1971) The protein crystal toxin of Bacillus
thuringiensis: Biochemistry and mode of action. In: Burges HD &
Hussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc., pp 247-274.
Crickmore N, Zeigler DR, Feitelson J, Schnepf E, van Rie J, Lereclus
D, Baum J, & Dean DH (1998) Revision of the nomenclature for the
Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol
Biol Rev, 62: 807-813.
Damgaard PH (1995) Diarrhoeal enterotoxin production by strains of
Bacillus thuringiensis isolated from commercial Bacillus
thuringiensis -- based insecticides. FEMS Immunol Med Microbiol, 12:
245-250.
Damgaard PH, Granum PE, Bresciani J, Torregrossa MV, Eilenberg J, &
Valentino L (1997a) Characterization of Bacillus thuringiensis
isolated from infections in burn wounds. FEMS Immunol Med Microbiol,
18: 47-53.
Damgaard PH, Hansen BM, Pedersen JC, & Eilenberg J (1997b) Natural
occurrence of B. thuringiensis on cabbage foliage and in insects
associated with cabbage crops. J Appl Bacteriol, 82: 253-258.
Damgaard PH, Larsen HD, Hansen BM, Bresciani J, & Jorgensen K (1996a)
Enterotoxin-producing strains of Bacillus thuringiensis isolated
from food. Lett Appl Microbiol, 23: 146-150.
Damgaard PH, Jacobsen CS, & Sorensen J (1996b) Development and
application of a primer set for specific detection of Bacillus
thuringiensis and Bacillus cereus in soil using magnetic
capture-hybridization and PCR amplification. System Appl Microbiol,
19: 436-441.
de Barjac H (1981a) Identification of H-serotypes of Bacillus
thuringiensis. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
35-43.
de Barjac H (1981b) Insect pathogens in the genus Bacillus. In:
Berkley RCW & Goodfellow M ed. The aerobic endospore-forming bacteria:
Classification and identification. New York, London, Academic Press
Inc., pp 241-250.
de Barjac H & Bonnefoi A (1962) Essai de classification biochimique et
sérologique de 24 souches de Bacillus de type thuringiensis.
Entomophaga, 7: 5-31.
de Barjac H & Larget-Thiery I (1984) Characteristics of IPS 82 as
standard for biological assays of Bacillus thuringiensis H-14
preparations. Geneva, World Health Organization (WHO/VBC/84.892).
de Barjac H, Larget I, Bénichou L, Cosmao V, Viviani G, Ripouteau H, &
Papion S (1980) Test d'innocuité sur mammifères avec du sérotype H 14
de Bacillus thuringiensis. Geneva, World Health Organization
(WHO/VBC/80.761).
Dean DH (1984) Biochemical genetics of the bacterial insect-control
agent Bacillus thuringiensis: Basic principles and prospects for
genetic engineering. Biotechnol Genet Eng Rev, 2: 341-363.
Dean DH, Rajamohan F, Lee MK, Wu SJ, Chen XJ, Alcantara E, & Hussain
SR (1996) Probing the mechanism of action of Bacillus thuringiensis
insecticidal proteins by site-directed mutagenesis - a minireview.
Gene, 179: 111-117.
DeLucca AJ II, Simonson JG, & Larson AD (1981) Bacillus thuringiensis
distribution in soils of the United States. Can J Microbiol, 27:
865-870.
Demezas DH & Bell J (1995) Evaluation of low molecular weight RNA
profiles and ribotyping to differentiate some Bacillus species.
System Appl Microbiol, 18: 582-589.
Denolf P, Hendrickx K, Vandamme J, Jansens S, Peferoen M, Degheele D,
& Vanrie J (1997) Cloning and characterization of Manduca sexta and
Plutella xylostella midgut aminopeptidase N enzymes related to
Bacillus thuringiensis toxin-binding proteins. Eur J Biochem, 248:
748-761.
Dent DR (1993) The use of Bacillus thuringiensis as an insecticide.
In: Jones DG ed. Exploitation of microorganisms. London, Chapman &
Hall, pp 19-44.
Drobniewski FA (1993) Bacillus cereus and related species. Clin
Microbiol Rev, 6: 324-338.
Dulmage HT & cooperators (1981) Insecticidal activity of isolates of
Bacillus thuringiensis and their potential for pest control. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 193-223.
Dunbar JP & Johnson AW (1975) Bacillus thuringiensis: Effects on the
survival of a tobacco budworm parasitoid and predator in the
laboratory. Environ Entomol, 4: 352-354.
Dunbar DM, Kaya HK, Doane CC, Anderson JF, & Weseloh RM (1972) Aerial
application of Bacillus thuringiensis against larvae of the elm
spanworm and gypsy moth and effects on parasitoids of the gypsy moth.
Connecticut Experiment Station (Bulletin No. 735).
Eilenberg J, Damgaard PH, Hansen BM, Pedersen JC, Bresciani J, &
Larsson R (in press) Natural interactions between Strongwellsea
castrans, Cystosporogenes deliaradicae and Bacillus thuringiensis
in the host, Delia radicum. J Invertebr Pathol.
Elliott LJ, Sokolow R, Heumann M, & Elefant SL (1988), An exposure
characterization of a large scale application of a biological
insecticide, Bacillus thuringiensis. Appl Ind Hyg, 3: 119-122.
Elsey KD (1973) Jalysus spinosus effect of insecticide treatments on
this predator of tobacco pests. Environ Entomol, 2: 240-243.
Entwistle PF, Cory JS, Bailey MJ, & Higgs S (1983) Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, John Wiley & Sons, 311 pp.
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, & Koziel MG
(1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal
protein with a wide spectrum of activities against lepidopteran
insects. Proc Natl Acad Sci (USA), 93: 5389-5394.
Farkas J, Sebesta K, Horská K, Samek Z, Dolejs L, & Sorm F (1977)
Structure of thuringiensin, the thermostable exotoxin from Bacillus
thuringiensis. Coll Czech Chem Commun, 42: 909-929.
Fast PG (1971) Isolation of a water-soluble toxin from a commercial
microbial insecticide based on Bacillus thuringiensis. J Invertebr
Pathol, 17: 301.
Fast PG (1981) The crystal toxin of Bacillus thuringiensis. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 223-248.
Faust RM (1973) The Bacillus thuringiensis ß-exotoxin: Current
status. Bull Entomol Soc Am, 19: 153-156.
Feitelson JS (1993) The Bacillus thuringiensis family tree. In: Kim
L ed. Advanced engineered pesticides. New York, Basel, Marcel Dekker
Inc., pp 63-71.
Fisher R & Rosner L (1959) Toxicology of the microbial insecticide,
Thuricide. J Agric Food Chem, 7: 686-688.
Frommer W, Ager B, Archer L, Brunius G, Collins CH, Donikian R,
Frontali C, Hamp S, Houwink EH, Kuenzi MT, Krämer P, Lagast H, Lund S,
Mahler JL, Normand-Plessier F, Sargeant K, Tuijnenburg-Muijs G, Vranch
SP, & Werner RG (1989) Safe biotechnology: III. Safety precautions for
handling microorganisms of different risk classes. Appl Microbiol
Biotechnol, 30: 541-552.
Fusco RA (1980) Field evaluation of a commercial preparation of
Bacillus thuringiensis, DIPEL 4L: Progress report. Pennsylvania
Bureau of Forestry, Gypsy Moth Pest Management Methods Development
Project.
Gaddis PK & Corkran CC (1986) Secondary effects of Bt spray on avian
predators: the reproductive success of chestnut-backed chickadees.
Northwest Ecological Research Institute (Unpublished Research Report
No. 86-03).
Garcia R, Des Rochers B, & Tozer W (1980) Studies on the toxicity of
Bacillus thuringiensis var. israelensis against organisms found in
association with mosquito larvae. Berkeley, California, University of
California, Division of Biological Control.
Gelernter WD (1990) Targeting insecticide-resistant markets: New
developments in microbial-based products. In: Green MB, Moberg WK, &
LeBaron H ed. Managing resistance to agrochemicals: From fundamental
research to practical strategies. Washington, DC, American Chemical
Society, pp 105-117 (ACS Series 421).
Giffel MC, Beumer RR, Klijn N, Wagendorp A, & Rombouts FM (1997)
Discrimination between Bacillus cereus and Bacillus thuringiensis
using specific DNA probes based on variable regions of 16S rRNA. FEMS
Microbiol Lett, 146: 47-51.
Gill SS, Hornung JM, Ibarra JE, Singh GJP, & Federici BA (1987)
Cytolytic activity and immunological similarity of Bacillus
thuringiensis subsp. israelensis and Bacillus thuringiensis
subsp. morrisoni isolate PG-14 toxins. Appl Environ Microbiol, 53:
1251-1256.
Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, & Goebel W
(1989) A Bacillus cereus cytolytic determinant, cereolysin AB, which
comprises the phospholipase C and sphingomyelinase genes: Nucleotide
sequence and genetic linkage. J Bacteriol, 171: 744-753.
Goldberg LJ & Margalit J (1977) A bacterial spore demonstrating rapid
larvicidal activity against Anopheles sergentii, Uranotaenia
unguiculata, Culex univitattus, Aedes aegypti and Culex
pipiens. Mosq News, 37: 355-358.
González JM Jr & Carlton BC (1980) Patterns of plasmid DNA in
crystalliferous and acrystalliferous strains of Bacillus
thuringiensis. Plasmid, 3: 92-98.
González JM Jr, Dulmage HT, & Carlton BC (1981) Correlation between
specific plasmids and delta-endotoxin production in Bacillus
thuringiensis. Plasmid, 5: 351-365.
González JM, Jr Brown BJ, & Carlton BC (1982) Transfer of Bacillus
thuringiensis plasmids coding for delta-endotoxin among strains of
B. thuringiensis and B. cereus. Proc Natl Acad Sci (USA), 79:
6951-6955.
Gordon RE (1977) Some taxonomic observations on the genus Bacillus.
In: Briggs JB ed. Biological regulations of vectors: The saprophytic
and aerobic bacteria and fungi. Washington, DC, US Department of
Health, Education and Welfare, pp 67-82 (Publication NIH 77-1180).
Granum PE (1997) Bacillus cereus. In: Doyle MP, Beuchat LR, &
Montville TJ ed. Food microbiology fundamentals and frontiers.
Washington, DC, ASM Press, pp 327-336.
Grassi S & Deseö KV (1984) [The natural occurrence of Bacillus
thuringiensis Berl. And its importance in the plant protection.] In:
[Proceedings of Seminar on Phytopathology, Sorrento, Italy, 26-29
March 1984.] Bologna, Italy, Clueb Publishing Co., vol 2, pp 424-433
(in Italian).
Green M, Heumann M, Sokolow R, Foster LR, Bryant R, & Skeels M (1990)
Public health implications of the microbial pesticide Bacillus
thuringiensis: An epidemiological study, Oregon, 1985-1986. Am J
Publ Health, 80: 848-852.
Griego VM & Spence KD (1978) Inactivation of Bacillus thuringiensis
spores by ultraviolet and visible light. Appl Environ Microbiol, 35:
906-910.
Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL,
Brousseau R, & Cygler M (1995) Bacillus thuringiensis CryIA(a)
insecticidal toxin: crystal structure and channel formation. J Mol
Biol, 254: 447-464.
Hadley WM, Burchiel SW, McDowell TD, Thilsted JP, Hibbs CM, Whorton
JA, Day PW, Friedman MB, & Stoll RE (1987) Five-month oral (diet)
toxicity/ infectivity study of Bacillus thuringiensis insecticides
in sheep. Fundam Appl Toxicol, 8: 236-242.
Hamed AR (1978-79) [Effects of Bacillus thuringiensis on parasites
and predators of Yponomeuta evenymellus (Lep., Yponomeutidae).] Z
Angew Entomol, 87: 294-311 (in German).
Hamel DR (1977) The effects of Bacillus thuringiensis on parasitoids
of the western spruce budworm, Choristoneura occidentalis
(Lepidoptera: Tortricidae), and the spruce coneworm, Dioryctria
reniculelloides (Lepidoptera: Pyralidae), in Montana. Can Entomol,
109: 1409-1415.
Hannay CL (1953) Crystalline inclusions in aerobic spore-forming
bacteria. Nature (Lond), 172: 1004.
Hansen BM & Hendriksen NB (1997a) Bacillus thuringiensis and B.
cereus enterotoxins: Proceedings of the 6th European Meeting of the
International Organization for Biological and Integrated Control of
Noxious Animals and Plants/West Paleaearctic Regional Section,
Copenhagen, 10-15 August 1997. Copenhagen, Denmark, Royal Veterinary
and Agricultural University, Department of Ecology and Molecular
Biology.
Hansen BM & Hendriksen NB (1997b) Comparative PCR analysis of B.
thuringiensis and B. cereus: Abstract for the International
Workshop on Molecular Biology of B. cereus, B. anthracis and
B. thuringiensis, Soria Moria, Oslo, 23-25 May 1997.
Hansen BM & Hendriksen NB (1999) Ecological aspects of the survival in
soil of spray released Bacillus thuringiensis subsp. kurstaki. In:
Abstract for the IOBC Symposium on Indirect Ecological Effects of
Biological Control, Montpelier, 17-20 October 1999. Montpelier,
France, Agropolis International.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1996) Bacillus
thuringiensis, ecology and environmental effects of its use for
microbial pest control (Environmental Project No. 316). Copenhagen,
Denmark, Ministry of Environment and Energy, Danish Environmental
Protection Agency.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1998) Molecular
and phenotypic characterization of Bacillus thuringiensis isolated
from leaves and insects. J Invertebr Pathol, 71: 106-114.
Harding J, Wolfenbarger D, Dupnik T, & Fuchs T (1972) Large scale
tests comparing Bacillus thuringiensis with methyl-parathion for
cotton insect control, field test report. Chicago, Illinois, Abbott
Laboratories (Unpublished document).
Hassan SA (1983) Results of laboratory testing of a series of
pesticides on egg parasites of the genus Trichogramma (Hymenoptera,
Trichogrammatidae). Nachr.bl Dtsch Pflanzenschutzdienstes
(Braunschweig), 35: 21.
Hassan S & Krieg A (1975) [ Bacillus thuringiensis preparations
harmless to the parasite Trichogramma cacoeciae (Hym.:
Trichogrammatidae).] Z Pflanzenkr Pflanzenchutz, 82: 515-521 (in
German).
Hassan G & Nabbut N (1996) Prevalence and characterization of
Bacillus cereus isolates from clinical and natural sources. J Food
Prot, 59: 193-196.
Heimpel AM (1954) Investigations of the mode of action of strains of
Bacillus cereus Frankland and Frankland pathogenic for the larch
sawfly, Pristiphora chrichsonii (Htg.). Kingston, Canada, Queen's
University, 155 pp (Doctoral dissertation).
Heimpel AM (1967) A critical review of Bacillus thuringiensis var.
thuringiensis Berliner and other crystalliferous bacteria. Annu Rev
Entomol, 12: 287-322.
Heimpel AM & Angus TA (1958) The taxonomy of insect pathogens related
to Bacillus cereus Frankland and Frankland. Can J Microbiol, 4:
531-541.
Helgason E, Caugant DA, Lecadet M-M, Chen Y, Mahillon J, Lövgren A,
Hegna I, Kvaloy K, & Kolsto A-B (1998) Genetic diversity of Bacillus
cereus/ B. thuringiensis isolates from natural sources. Curr
Microbiol, 37: 80-87.
Hendriksen NB & Hansen BM (1998) Phylogenetic relations of Bacillus
thuringiensis: Implications for risks associated to its use as a
microbiological pest control agent. IOBC Bull, 21: 5-8.
Hernandez E, Ramisse F, Ducoureau J-P, Cruel T, & Cavallo J-D (1998)
Bacillus thuringiensis subsp. konkukian (serotype H34)
superinfection: Case report and experimental evidence of pathogenicity
in immunosuppressed mice. J Clin Microbiol, 36: 2138-2139.
Höfte H & Whiteley HR (1989) Insecticidal crystal proteins of
Bacillus thuringiensis. Microbiol Rev, 53: 242-255.
Honée G & Visser B (1993) The mode of action of Bacillus
thuringiensis crystal proteins. Entomol Exp Appl, 69: 145-155.
Horn DJ (1983) Selective mortality of parasitoids and predators of
Myzus persicae on collards treated with malathion, carbaryl, or
Bacillus thuringiensis. Entomol Exp Appl, 34: 208-211.
Horsburgh R & Cobb L (1981) Effect of Dipel WP and Dipel + Guthion
tank mix combination for control of variegated leafroller, turfed
apple bud moth, and red-banded leafroller larvae on apples in a full
season and late season program in Virginia (Experiment No.
D-986-4240). Chicago, Illinois, Abbott Laboratories Inc.
Hostetter DL, Ignoffo CM, & Kearby WH (1975) Persistence of
formulations of Bacillus thuringiensis spores and crystals on
eastern red cedar foliage in Missouri. J Kansas Entomol Soc, 48:
189-193.
Hotha S & Banik RM (1997) Production of alkaline protease by Bacillus
thuringiensis H 14 in aqueous two-phase systems. J Chem Technol
Biotechnol, 69: 5-10.
Huber HE & Lüthy P (1981) Bacillus thuringiensis delta-endotoxin:
Composition and activation. In: Davidson EW ed. Pathogenesis of
invertebrate microbial diseases. Totowa, New Jersey, Allanheld-Osmun
Publishers, pp 209-234.
Huber HE, Lüthy P, Ebersold HR, & Cordier JL (1981) The subunits of
the parasporal crystal of Bacillus thuringiensis: size, linkage and
toxicity. Arch Microbiol, 129: 14-18.
Ignoffo CM (1973) Effects of entomopathogens on vertebrates. Ann NY
Acad Sci, 217: 141-164.
Ignoffo CM (1992) Environmental factors affecting persistence of
entomopathogens. Fla Entomol, 75: 516-525.
Innes DGL & Bendell JF (1989) The effects on small-mammal populations
of aerial applications of Bacillus thuringiensis, fenitrothion, and
Matacil(R) used against jack pine budworm in Ontario. Can J Zool,
67: 1318-1323.
Jackson SG, Goodbrand RB, Ahmed R, & Kasatiya S (1995) Bacillus
cereus and Bacillus thuringiensis isolated in a gastroenteritis
outbreak investigation. Lett Appl Microbiol, 21: 103-105.
Jaquet F, Hütter R, & Lüthy P (1987) Specificity of Bacillus
thuringiensis delta-endotoxin. Appl Environ Microbiol, 53: 500-504.
Jarrett P & Stephenson M (1990) Plasmid transfer between strains of
Bacillus thuringiensis infecting Galleria mellonella and
Spodoptera littoralis. Appl Environ Microbiol, 56: 1608-1614.
Jensen R (1974) Comparison of various insecticides including DIPEL WP
for control of the velvetbean caterpillar (Anticarsia gemmatalis)
and the green cloverworm (Plathypena scabra) on soybeans and the
effect on non-target species (Experiment No D911-1582). Chicago,
Illinois, Abbott Laboratories Inc.
Johnson AW (1974) Bacillus thuringiensis and tobacco budworm control
on flue-cured tobacco. J Econ Entomol, 67: 755-759.
Juarez-Perez VM, Ferrandis MD, & Frutos R (1997) PCR-based approach
for detection of novel Bacillus thuringiensis cry genes. Appl
Environ Microbiol, 63: 2997-3002.
Kaneko T, Nozaki R, & Aizawa K (1978) Deoxyribonucleic acid
relatedness between Bacillus anthracis, Bacillus cereus and
Bacillus thuringiensis. Microbiol Immunol, 22: 639-641.
Kazakova SB & Dzhunusov KK (1977) The effect of Bitoxibacillin-202 on
certain orchard insects in the Issyk-Kul' depression. Rev Appl
Entomol, A65: 5987.
Keller B & Langenbruch G-A (1993) Control of coleopteran pests by
Bacillus thuringiensis. In: Entwistle PF, Cory JS, Bailey MJ, &
Higgs S ed. Bacillus thuringiensis, an environmental biopesticide:
Theory and practice. Chichester, New York, Toronto, Wiley & Sons, pp
171-191.
Khawaled K, Ben-Dov E, Zaritsky A, & Barak Z (1990) The fate of
Bacillus thuringiensis var. israelensis in B. thuringiensis
var. israelensis-killed pupae of Aedes aegypti. J Invertebr
Pathol, 56: 312-316.
Kim HS, Lee DW, Woo SD, Yu YM, & Kang SK (1998) Seasonal distribution
and characterization of Bacillus thuringiensis isolated from
sericultural environments in Korea. J Gen Appl Microbiol, 44: 133-138.
Klier AF & Lecadet MM (1976) Arguments based on
hybridization-competition experiments in favor of the in vitro
synthesis of sporulation-specific mRNAs by the RNA polymerase of
Bacillus thuringiensis. Biochem Biophys Res Commun, 73: 263-270.
Knowles BH & Ellar DJ (1987) Colloid-osmotic lysis is a general
feature of the mechanisms of action of Bacillus thuringiensis
delta-endotoxins with different insect specificity. Biochim Biophys
Acta, 924: 509-518.
Krieg A (1971) Concerning alpha-exotoxin produced by vegetative cells
of Bacillus thuringiensis and Bacillus cereus. J Invertebr Pathol,
17: 134-135.
Krieg A (1973) About toxic effects of cultures of Bacillus cereus
and Bacillus thuringiensis on honey bees (Apis mellifera).
Z Pflanzenkr Pflanzenschutz, 80: 483-486.
Krieg A & Herfs W (1963) The effects of Bacillus thuringiensis on
honey bees. Entomol Exp Appl, 6: 1-9.
Krieg A, Hassan S, & Pinsdorf W (1980) Comparison of the effects of
the variety israelensis with other varieties of B. thuringiensis
on non-target organisms of the order Hymenoptera: Trichogramma
cacoeciae and Apis mellifera. Anz Schädlingsk Pflanz Umweltschutz,
53: 81-83.
Krieg A, Huger AM, Langenbruch GA, & Schnetter W (1983) Bacillus
thuringiensis var. tenebrionis, a new pathotype effective against
larvae of Coleoptera .. Z Angew Entomol, 96(5): 500-508.
Krywienczyk J, Dulmage HT, & Fast PG (1978) Occurrence of two
serologically distinct groups within Bacillus thuringiensis serotype
3ab variety kurstaki. J Invertebr Pathol, 37: 372-375.
Kumar PA, Sharma RP, & Malik VS (1996) The insecticidal protein of
Bacillus thuringiensis. Adv Appl Microbiol, 42: 1-43.
Lacey LA & Mulla MS (1990) Safety of Bacillus thuringiensis ssp.
israelensis and Bacillus sphaericus to nontarget organisms in the
aquatic environment. In: Laird M, Lacey LA, & Davidson EW ed. Safety
of microbial pesticides. Boca Raton, Florida, CRC Press, pp 169-188.
Lacey LA, Escaffre H, Philippon B, Sékétéli A, & Guillet P (1982)
Large river treatment with Bacillus thuringiensis (H-14) for the
control of Simulium damnosum s.l. in the Onchocerciasis Control
Programme. Tropenmed Parasitol, 33: 97-101.
Laferrière M, Bastille A, & Nadeau A (1987) Etude immunologique
impliquant les composantes de l'insecticide biologique Bacillus
thuringiensis var. kurstaki. St. Henri Rivière-du-Loup, Québec,
Department of Public Health, Grand-Portage Regional Hospital Center,
pp 1-19 (Unpublished report).
Lattin A, Grimes J, Hoxter KA, & Smith GJ (1990a) Dipel technical
material (Bacillus thuringiensis var. kurstaki): An avian oral
toxicity and pathogenicity study in the bobwhite (Project No.
161-112). Easton, Maryland, Wildlife International Ltd, pp 1-25
(Unpublished Abbott document).
Lattin A, Grimes J, Hoxter K, & Smith GJ (1990b) VectoBac technical
material (Bacillus thuringiensis var. israelensis): An avian oral
toxicity and pathogenicity study in the mallard (Project No. 161-115).
Easton, Maryland, Wildlife International Ltd, pp 1-24 (Unpublished
Abbott document).
Lattin A, Hoxter K, Driscoll C, Grimes J, & Jaber M (1990c) Dipel
technical material (Bacillus thuringiensis var. kurstaki): An
avian oral toxicity and pathogenicity study in the mallard (Project
No. 161-113). Easton, Maryland, Wildlife International Ltd, pp 1-28
(Unpublished Abbott document).
Lattin A, Hoxter K, & Smith GJ (1990d) VectoBac technical material
(Bacillus thuringiensis var. israelensis): An avian oral toxicity
and pathogenicity study in the bobwhite (Project No. 161-114). Easton,
Maryland, Wildlife International Ltd, pp 1-27 (Unpublished Abbott
document).
Lechner S, Mayr R, Francis KP, Prüß BM, Kaplan T, Wießner-Gunkel E,
Stewart GSAB, & Scherer S (1998) Bacillus weihenstephanensis sp.
nov. is a new psychrotolerant species of the Bacillus cereus group.
Int J System Bacteriol, 48: 1373-1378.
Leong KLH, Cano RJ, & Kubinski AM (1980) Factors affecting Bacillus
thuringiensis total field persistence. Environ Entomol, 9: 593-599.
Levêque C, Fairhurst CP, Abbau K, Pangy D, Curtis MS, & Traoré K
(1988) Onchocerciasis control programme in West Africa: ten years of
monitoring fish populations. Chemosphere, 17: 421-440.
Li J, Carroll J, & Ellar DJ (1991) Crystal structure of insecticidal
(delta)-endotoxin from Bacillus thuringiensis at 2.5 Å resolution.
Nature (Lond), 353: 815-821.
Logan NA & Berkeley CW (1984) Identification of Bacillus strains
using the API system. J Gen Microbiol, 130: 1871-1882.
Lund T & Granum PE (1997) Comparison of biological effect of the two
different enterotoxin complexes isolated from three different strains
of Bacillus cereus. Microbiology, 143: 3329-3336.
Lüthy P (1986) Insect pathogenic bacteria as pest control agents.
Fortschr Zool, 32: 201-216.
Lüthy P & Ebersold HR (1981) The entomocidal toxins of Bacillus
thuringiensis. Pharmacol Ther, 13: 257-283.
Lynch MJ & Baumann P (1985) Immunological comparisons of the crystal
protein from strains of Bacillus thuringiensis. J Invertebr Pathol,
46: 47-57.
Lynch RE, Lewis LC, & Brindley TA (1976) Bacteria associated with eggs
and first-instar larvae of the European corn borer: Isolation
techniques and pathogenicity. J Invertebr Pathol, 27: 325-331.
McClintock JT, Schaffer CR, & Sjoblad RD (1995) A comparative review
of the mammalian toxicity of Bacillus thuringiensis-based
pesticides. Pestic Sci, 45: 95-105.
Manasherob R, Ben-Dov E, Zaritsky A, & Barak Z (1998) Germination,
growth, and sporulation of Bacillus thuringiensis subsp.
israelensis in excreted food vacuoles of the protozoan Tetrahymena
pyriformis. Appl Environ Microbiol, 64: 1750-1758.
Margalit J & Dean D (1985) The story of Bacillus thuringiensis var.
israelensis. J Am Mosq Control Assoc, 1: 1-7.
Martin PAW & Travers RS (1989) Worldwide abundance and distribution of
Bacillus thuringiensis isolates. Appl Environ Microbiol, 55(10):
2437-2442.
Martin WF & Reichelderfer CF (1989) Bacillus thuringiensis:
Persistence and movement in field crops. In: Abstracts of the SIP
XXIInd Annual Meeting, Centre for Adult Education, University of
Maryland, College Park, Maryland, 20-24 August 1989. Society for
Invertebrate Pathology, p 25.
Martouret D & Euverte G (1964) The effect of Bacillus thuringiensis
Berliner preparations on the honey bee under conditions of forced
feeding. J Insect Pathol, 6: 198-203.
Meadows MP (1993) Bacillus thuringiensis in the environment: Ecology
and risk assessment. In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S
ed. Bacillus thuringiensis, an environmental biopesticide: Theory
and practice. Chichester, New York, Toronto, Wiley & Sons, pp 193-220.
Meadows MP, Ellis DJ, Butt J, Jarrett P, & Burges HD (1992)
Distribution, frequency and diversity of Bacillus thuringiensis in
an animal feed mill. Appl Environ Microbiol, 58(4): 1344-1350.
Melin BE & Cozzi EM (1990) Safety to nontarget invertebrates of
Lepidopteran strains of Bacillus thuringiensis and their
(ß)-exotoxins. In: Laird M, Lacey LA, & Davidson EW ed. Safety of
microbial insecticides. Boca Raton, Florida, CRC Press, pp 149-167.
Menon AS & De Mestral J (1985) Survival of Bacillus thuringiensis
var. kurstaki in waters. Water Air Soil Pollut, 25: 265-274.
Merritt RW, Walker ED, Wilzbach MA, Cummins KW, & Morgan WT (1989) A
broad evaluation of Bti for black fly (Diptera:Simuliidae) control in
a Michigan river: efficacy, carry and nontarget effects on
invertebrates and fish. J Am Mosq Control Assoc, 5: 397-415.
Mikami T, Horikawa T, Murakami T, Sato N, Ono Y, Matsumoto T, Yamakawa
A, Murayama S, Katagiri S, & Suzuki M (1995) Examination of toxin
production from environmental Bacillus cereus and Bacillus
thuringiensis. J Pharm Soc Jpn, 115: 742-748.
Miura T, Takahashi RM, & Mulligan FS III (1980) Effects of the
bacterial mosquito larvicide, Bacillus thuringiensis serotype H-14
on selected aquatic organisms. Mosq News, 40: 619-622.
Mohd-Salleh MB, Beegle CC, & Lewis LC (1980) Fermentation media and
production of exotoxin by three varieties of Bacillus thuringiensis.
J Invertebr Pathol, 35: 75-83.
Molloy DP (1992) Impact of the black fly (Diptera: Simuliidae) control
agent Bacillus thuringiensis var. israelensis on Chironomids
(Diptera: Chronomidae) and other non-target insects: results of ten
field trials. J Am Mosq Control Assoc, 8: 24-31.
Morris ON, Armstrong JA, & Hildebrand MJ (1977) Aerial field trials
with a new formulation of Bacillus thuringiensis against the spruce
budworm, Choristoneura fumiferana (Clem.). Ottawa, Canada, Chemical
Control Research Institute (Report CC-X-144).
Morris ON, Hildebrand MJ, & Armstrong JA (1980) Preliminary field
studies on the use of additives to improve deposition rate and
efficacy of commercial formulations of Bacillus thuringiensis
applied against the spruce budworm, Choristoneura fumiferana
(Lepidoptera: Tortricidae). Sault Ste. Marie, Ontario, Canada, Forest
Pest Management Institute, pp 1-23 (Report FPM-X-32).
Moulinier CL, Mas JP, Moulinier Y, De Barjac H, Giap G, & Couprie B
(1995) Étude de l'innocuité de Bacillus thuringiensis var.
israelensis pour les larves d'huitre. Bull Soc Pathol Exot, 74(4):
381-391.
Mück O, Hassan S, Huger AM, & Krieg A (1981) Effects of Bacillus
thuringiensis Berliner on the parasitic hymenoptera Apanteles
glomeratus L. (Braconidae) and Pimpla turionellae (L.)
(Ichneumonidiae). Z Angew Entomol, 92: 303-314.
Mulla MS (1988) Activity, field efficacy and use of Bacillus
thuringiensis (H-14) against mosquitoes. In: de Barjac H &
Sutherland DJ ed. Bacterial control of mosquitoes and blackflies. New
Brunswick, New Jersey, Rutgers University Press.
Mulla MS & Khasawinah AM (1969) Laboratory and field evaluation of
larvicides against chironomid midges. J Econ Entomol, 62: 37-41.
Mulla MS, Norland RL, Fanara DM, Darwazeh HA, & McKean DW (1971)
Control of chironomid midges in recreational lakes. J Econ Entomol
64(1): 300-307.
Mulla MS, Federici BA, & Darwazeh HA (1982) Larvicidal efficacy of
Bacillus thuringiensis serotype H-14 against stagnant-water
mosquitoes and its effects on nontarget organisms. Environ Entomol,
11: 788-795.
Mulligan FS III & Schaefer CH (1982) Integration of a selective
mosquito control agent Bacillus thuringiensis serotype H-14, with
natural predator populations in pesticide-sensitive habitats. Proc
Calif Mosq Vector Control Assoc, 49: 19-22.
Murakami T, Hiraoka T, Matsumoto T, Katagiri S, Shinagawa K, & Suzuki
M (1993) Analysis of common antigen of flagella in Bacillus cereus
and Bacillus thuringiensis. FEMS Microbiol Lett, 107: 179-184.
Narva KE, Payne JM, Schwab GE, Hickle LA, Galasan T, & Sick AJ (1991)
Novel Bacillus thuringiensis microbes active against nematodes, and
genes encoding novel nematode-active toxins cloned from Bacillus
thuringiensis isolates: European patent application EP0462 721A2.
Munich, Germany, European Patent Office.
Navon A (1993) Control of Lepidopteran pests with Bacillus
thuringiensis. In: Bacillus thuringiensis, An Environmental
Biopesticide: Theory and Practice. Eds, Entwistle PF, Cory JS, Bailey
MJ & Higgs S. Chichester, New York, Toronto, Wiley & Sons, pp 126-146.
Nielsen-LeRoux C, Hansen BM, Henriksen NB (1998) Safety of Bacillus
thuringiensis. IOBC Bull, 21: 269-272.
Nishikawa Y, Kramer JM, Hanaoka M, & Yasukawa A (1996) Evaluation of
serotyping, biotyping, plasmid banding pattern analysis, and HEp-2
vacuolation factor assay in the epidemiological investigation of
Bacillus cereus emetic-syndrome food poisoning. Int J Food
Microbiol, 31: 149-159.
Noble MA, Riben PD, & Cook GJ (1992) Microbiological and
epidemiological surveillance programme to monitor the health effects
of Foray 48B BTK spray. Vancouver, Canada, Ministry of Forests of the
Province of British Columbia, pp 1-63.
Norris JR (1971) The protein crystal toxin of Bacillus
thuringiensis: biosynthesis and physical structure. In: Burges HD &
Mussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc, pp 229-246.
Notermans S & Batt CA (1998) A risk assessment approach for food-borne
Bacillus cereus and its toxins. J Appl Environ Microbiol Symp,
84(suppl): 51S-61S.
Obadofin AA & Finlayson DG (1977) Interactions of several insecticides
and a carabid predator (Bembidion lampros (Hrbst)) and their effects
on Hylemya brassicae (Bouché). Can J Plant Sci, 57: 1121-1126.
Ohana B, Margalit J, & Barak Z (1987) Fate of Bacillus thuringiensis
subsp. israelensis under simulated field conditions. Appl Environ
Microbiol, 53: 828-831.
Ohba M & Aizawa K (1978) Serological identification of Bacillus
thuringiensis and related bacteria isolated in Japan. J Invertebr
Pathol, 32: 303-309.
Ohba M & Aizawa K (1986) Distribution of Bacillus thuringiensis in
soils of Japan. J Invertebr Pathol, 47: 277-282.
Ohba M, Yu YM, & Aizawa K (1988) Occurrence of noninsecticidal
Bacillus thuringiensis flagellar serotype 14 in the soil of Japan.
Appl Microbiol, 11: 85-89.
Olejnicek J & Maryskova B (1986), The influence of Bacillus
thuringiensis on the mosquito predator Notonecta glauca. Folia
Parasitol (Prague), 37: 279.
Otvos IS & Vanderveen S (1993) Environmental report and current status
of Bacillus thuringiensis var. kurstaki use for control of forest
and agricultural insect pests. Victoria, British Columbia, Ministry of
Forests, Forestry Canada, pp 1-81.
Pedersen JC, Damgaard PH, Eilenberg E, & Hansen BM (1995) Dispersal of
Bacillus thuringiensis var. kurstaki in an experimental cabbage
field. Can J Microbiol, 41: 118-125.
Petras SF & Casida LE Jr (1985) Survival of Bacillus thuringiensis
spores in soil. Appl Environ Microbiol, 50: 1496-1501.
Pinnock DE, Brand RJ, Jackson KL, & Milstead JE (1974) The field
persistence of Bacillus thuringiensis spores on Cercis occidentalis
leaves. J Invertebr Pathol, 23: 341-346.
Pinnock DE, Milstead JE, Kirby ME, & Nelson BJ (1977) Stability of
entomopathogenic bacteria. In: Environmental stability of microbial
insecticides: Miscellaneous publications. Lanham, Maryland,
Entomological Society of America, vol 10, pp 77-97.
Pivovarov Yu P, Ivashina SA, & Padalkin VP (1977) [Hygienic assessment
of investigation of insecticide preparations.] Gig i Sanit, 8: 45-48
(in Russian).
Prasertphon S, Areekul P, & Tanada Y (1973) Sporulation of Bacillus
thuringiensis in host cadavers. J Invertebr Pathol, 21: 205-207.
Prefontaine G, Fast P, Lau PCK, Hefford MA, Hanna Z, & Brousseau R
(1987) Use of oligonucleotide probes to study the relatedness of
delta-endotoxin genes among Bacillus thuringiensis subspecies and
strains. Appl Environ Microbiol, 53: 2808-2814.
Priest FG, Goodfellow M, & Todd C (1988) A numerical classification of
the genus Bacillus. J Gen Microbiol, 134: 1847-1882.
Priest FG, Kaji DA, Rosato YB, & Canhos VP (1994) Characterization of
Bacillus thuringiensis and related bacteria by ribosomal RNA gene
restriction fragment length polymorphisms. Microbiology, 140:
1015-1022.
Purcell BH (1981) Effects of Bacillus thuringiensis var.
israelensis on Aedes taeniorhynchus and some non-target organisms
in the salt marsh. Mosq News, 41(3): 476-484.
Pusztai M, Fast P, Gringorten L, Kaplan H, Lessard T, & Carey PR
(1991) The mechanism of sunlight-mediated inactivation of Bacillus
thuringiensis crystals. Biochem J, 273: 43-47.
Quinlan RJ (1990) Registration requirements and safety considerations
for microbial pest control agents in the European Economic Community.
In: Laird M, Lacey LA, & Davidson EW ed. Safety of microbial
pesticides. Boca Raton, Florida, CRC Press, pp 11-18.
Ray DE (1990) Pesticides derived from plants and other organisms. In:
Hayes WJ & Laws ER ed. Handbook of pesticide toxicology. New York,
London, Academic Press, pp 585-636.
Reardon RC & Haissig K (1983) Spruce budworm (Lepidoptera:
tortricidae) larval populations and field persistence of Bacillus
thuringiensis after treatment in Wisconsin. J Econ Entomol, 76:
1139-1143.
Reddy A, Battisti L, & Thorne CB (1987) Identification of
self-transmissible plasmids in four Bacillus thuringiensis
subspecies. J Bacteriol 169(11): 5263-5270.
Rogatin AB & Baizhanov M (1984) [Laboratory study of Bacillus
thuringiensis israelensis serotype H-14 on various groups of
hydrobionts.] Izv Akad Nauk Kaz SSR Ser Biol, D6: 22-25 (in Russian).
Salama HS & Zaki FN (1983) Interaction between Bacillus thuringiensis
Berliner and the parasites and predators of Spodoptera littoralis
in Egypt. Z Angew Entomol, 95: 425-429.
Salama HS, Zaki FN, & Sharaby AF (1982) Effect of Bacillus
thuringiensis Berl. on parasites and predators of the cotton
leafworm Spodoptera littoralis (Boisd). Z Angew Entomol, 94:
498-504.
Saleh SM, Harris RF, & Allen ON (1970) Fate of Bacillus thuringiensis
in soil: Effect of soil pH and organic amendment. Can J Microbiol,
16: 677-680.
Samples JR & Buettner H (1983) Corneal ulcer caused by a biologic
insecticide (Bacillus thuringiensis). Am J Ophthalmol, 95(2):
258-260.
Sampson MN & Gooday GW (1998) Involvement of chitinases of Bacillus
thuringiensis during pathogenesis in insects. Microbiology, 144:
2189-2194.
Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J,
Zeigler DR, & Dean DH (1998) Bacillus thuringiensis and its
pesticidal crystal proteins. Microbiol Mol Biol Rev, 62: 775-806.
Schnetter W, Engler S, Morawcsik J, & Becker N (1981) [Efficacy of
Bacillus thurigiensis var. israelensis against mosquito larvae
and non-target organisms.] Mitt Dtsch Ges Allg Angew Entomol, 2:
195-202 (in German).
Schwartz JL, Garneau L, Masson L, & Brousseau R (1991) Early response
of cultured lepidopteran cells to exposure to delta-endotoxin from
Bacillus thuringiensis: involvement of calcium and anionic channels.
Biochem Biophys Acta, 1065: 250-260.
Shadduck JA (1980) Bacillus thuringiensis serotype H-14 maximum
challenge and eye irritation safety tests in mammals. Geneva, World
Health Organization, pp 1-21 (WHO/VBC/80.763).
Shadduck JA (1983) Some considerations on the safety evaluation of
nonviral microbial insecticides. WHO Bull, 6(1): 117-128.
Sheeran W & Fisher SW (1992) The effects of agitation, sediment and
competition on the persistence and efficacy of Bacillus thuringiensis
var. israelensis (Bti). Ecotoxicol Environ Saf, 24: 338-346.
Shevelev AB, Lewitin E, Novikova SI, Wojciechowska YaA, Usacheva EA,
Chestukhina GG, & Stepanov (1998) A new PCR-based approach to a fast
search of a wide spectrum of cry genes from Bacillus thuringiensis
strains. Biochem Mol Biol Int, 45: 1265-1271.
Shinagawa K (1990) Purification and characterization of Bacillus
cereus enterotoxin and its application to diagnosis. In: Pohland AE,
Dowell VR Jr, & Richard JL ed. Microbial toxins in foods and feeds:
Cellular and molecular modes of action. New York, Plenum Press, pp
181-193.
Siegel JP & Shadduck JA (1990) Clearance of Bacillus sphaericus and
Bacillus thuringiensis ssp. israelensis from mammals. J Econ
Entomol, 83: 347-355.
Siegel JP, Shadduck JA, & Szabo J (1987) Safety of the entomopathogen
Bacillus thuringiensis var. israelensis for mammals. J Econ
Entomol, 80: 717-723.
Slatin SL, Abrams CK, & English L (1990) Delta-endotoxins form
cation-selective channels in planar lipid bilayers. Biochem Biophys
Res Commun, 169: 765-772.
Slepecky RA & Hemphill HE (1992) The genus Bacillus-nonmedical. In:
Balows A, Truper HG, Dworkin M, Harder W, & Schleifer K-H ed. The
prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol II, pp
1663-1696.
Smedley DP & Ellar DJ (1996) Mutagenesis of 3 surface-exposed loops of
a Bacillus thuringiensis insecticidal toxin reveals residues
important for toxicity, recognition and possibly membrane insertion.
Microbiology, 142: 1617-1624.
Smith RA (1987) Use of crystal serology to differentiate among
varieties of Bacillus thuringiensis. J Invertebr Pathol, 50: 1-8.
Smith RA & Couche GA (1991) The phylloplane as a source of Bacillus
thuringiensis variants. Appl Environ Microbiol, 57: 311-315.
Snarski VM (1990) Interactions between Bacillus thuringiensis subsp.
israelensis and fathead minnows, Pimephales promelas Rafinesque,
under laboratory conditions. Appl Environ Microbiol, 56: 2618-2622.
Sorenson AA & Falcon LA (1980) Comparison of microdroplet and high
volume application of Bacillus thuringiensis on pear suppression of
fruit tree leaf roller Acrhips argyrospilus and coverage on foliage
and fruit. Environ Entomol, 9: 350.
Stahly DP, Dingman DW, Bulla LA Jr, & Aronson AI (1978) Possible
origin and function of the parasporal crystals in Bacillus
thuringiensis. Biochem Biophys Res Commun, 84(3): 581-588.
Stahly DP, Andrews RE, & Yousten AA (1991) The genus Bacillus-insect
pathogens. In: Balows A, Truper HG, Dworkin M, Harder W, & Schleifer
K-H ed. The prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol
II, pp 1697-1745.
Surprenant DC (1989) Acute toxicity of Bacillus thuringiensis var.
tenebrionis technical material to rainbow trout (Salmo gairdneri)
under static renewal conditions. Wareham, Massachusetts, Springborn
Life Sciences Inc., pp 1-19 (Unpublished Abbott document).
Tayabali AF & Seligy VL (1997) Cell integrity markers for in vitro
evaluation of cytotoxic responses to bacteria-containing commercial
insecticides. Ecotoxicol Environ Saf, 37: 152-162.
Thomas WE & Ellar DJ (1983) Bacillus thuringiensis var. israelensis
crystal delta-endotoxin: effects on insect and mammalian cells in
vitro and in vivo. J Cell Sci, 60: 181-197.
Thomas EM & Watson TF (1986) Effect of Dipel (Bacillus thuringiensis)
on the survival of immature and adult Hyposoter exiguae
(Hymenoptera: Ichneumonidae). J Invertebr Pathol, 47: 178-183.
Tomlin CDS ed. (1997) The pesticide manual, 11th ed. Farnham, Surrey,
British Crop Protection Council, 1606 pp.
Travers RS, Martin PAW, & Reichelderfer CF (1987) Selective process
for efficient isolation of soil Bacillus spp. Appl Environ
Microbiol, 53: 1263-1266.
Väisänen OM, Mwaisumo NJ, & Salkinoja-Salonen MS (1991)
Differentiation of dairy strains of the Bacillus cereus group by
phage typing, minimum growth temperature, and fatty acid analysis. J
Appl Bacteriol, 70: 315-324.
Van Frankenhuyzen K (1993) The challenge of Bacillus thuringiensis.
In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 1-35.
Vaòková J & Purrini K (1979) Natural epizooties caused by bacilli of
the species Bacillus thuringiensis and Bacillus cereus. Z Angew
Entomol, 88: 216-221.
Van Rie J, Jansens S, Höfte H, Degheele D, & Van Mellaert H (1989),
Specificity of Bacillus thuringiensis delta-endotoxins: Importance
of specific receptors on the brush border membrane of the mid-gut of
target insects. Eur J Biochem, 186: 239-247.
Venkateswerlu G & Stotzky G (1992) Binding of the protoxin and toxin
proteins of Bacillus thuringiensis subsp. kurstaki on clay
minerals. Curr Microbiol, 25: 225-233.
Visser B, Bosch D, & Honée G (1993) Domain-function studies of
Bacillus thuringiensis crystal proteins: a genetic approach. In:
Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental piopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 71-88.
Wallner W & Surgeoner G (1974) Control of oakleaf caterpillar,
Heterocampa manteo, and the impact of controls on nontarget
organisms. Chicago, Illinois, Abbott Laboratories (Unpublished
document).
Wallner WE, Dubois NR, & Grinberg PS (1983) Alteration of parasitism
by Rogas lymantriae (Hymenoptera: Braconidae) in Bacillus
thuringiensis-stressed gypsy moth (Lepidoptera: Lymantriidae) hosts.
J Econ Entomol, 76: 275-277.
Warren RE, Rubenstein D, Ellar DJ, Kramer JM, & Gilbert RJ (1984)
Bacillus thuringiensis var. israelensis: Protoxin activation and
safety. Lancet: March 24: 678-679.
Weires RW & Smith GL (1977) Apple mite control. Hudson Valley, New
York, New York State Agricultural Experimental Station.
Weseloh RM & Andreadis TG (1982) Possible mechanism for synergism
between Bacillus thuringiensis and the gypsy moth (Lepidoptera:
Lymantriidae) parasitoid, Apanteles melanoscelus (Hymenoptera:
Braconidae). Ann Entomol Soc Am, 75: 435-438.
Weseloh RM, Andreadis TG, Moore REB, & Anderson JF (1983) Field
confirmation of a mechanism causing synergism between Bacillus
thuringiensis and the gypsy moth parasitoid, Apanteles
melanoscelus. J Invertebr Pathol, 41: 99-103.
West AW, Burges HD, & Wyborn CH (1984a) Effect of incubation in
natural and autoclaved soil upon potency and viability of Bacillus
thuringiensis. J Invertebr Pathol, 44: 121-127.
West AW, Burges HD, White JR, & Wyborn CH (1984b) Persistence of
Bacillus thuringiensis parasporal crystal insecticidal activity in
soil. J Invertebr Pathol, 44: 128-133.
WHO (1982) Data sheet on the biological control agent Bacillus
thuringiensis serotype H-14 (de Barjac 1978). Geneva, World Health
Organization (WHO/VBC/79.750 Rev. 1).
WHO (1985) Ninth report of the WHO Expert Committee on Vector Biology
and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 720).
WHO (1991) Fourteenth report of the WHO Expert Committee on Vector
Biology and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 813).
Wilkinson JD, Biever KD, & Ignoffo CM (1975) Contact toxicity of some
chemical and biological pesticides to several insect parasitoids and
predators. Entomophaga, 20: 113-120.
Workman RB (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc Fla State Hortic Soc, 90: 401-402.
Wunschel D, Fox KF, Black GE, & Fox A (1994) Discrimination among the
B. cereus group, in comparison to B. subtilis, by structural
carbohydrate profiles and ribosomal RNA spacer region PCR. System Appl
Microbiol, 17: 625-635.
Yameogo L, Levêque C, Traoré K, & Fairhurst CP (1988) Dix ans de
surveillance de la faune aquatique des rivières d'Afrique de l'Ouest
traitées contre les Simulides (Diptera: Simuliidae), agents vecteurs
de l'onchocercose humaine. Nat Can, 115: 287-298.
Yousten AA (1973) Effect of the Bacillus thuringiensis
delta-endotoxin on an insect predator which has consumed intoxicated
cabbage looper larvae. J Invertebr Pathol, 21: 312-314.
Yousten AA, Genthner FJ, & Benfield EF (1992) Fate of Bacillus
sphaericus and Bacillus thuringiensis serovar israelensis in the
aquatic environment. J Am Mosq Control Assoc, 8: 143-148.
Zahner V, Momen H, Salles CA, & Rabinovitch L (1989) A comparative
study of enzyme variation in Bacillus cereus and Bacillus
thuringiensis. J Appl Bacteriol, 67: 275-282.
Zukowski K (1995) [Laboratory examination of the effectiveness of new
biological preparations for reducing populations of cockroaches
(Blattella germanica L.)]. Rocz Panstw Zakl Hig, 46: 293-297 (in
Polish).
RÉSUMÉ
La présente monographie traite des agents microbiens de lutte
contre les nuisibles utilisant le bacille Bacillus thuringiensis
(Bt). Ce bacille, qui est aussi appelé bacille de Thuringe, est
également une source très importante de gènes utilisée pour conférer
aux plantes et aux microorganismes transgéniques (ou organismes
génétiquement modifiés, OGM) la faculté de résister aux ravageurs et
autres nuisibles. Les effets que pourraient avoir ces OGM sur la santé
humaine et l'environnement présentent divers aspects qui sont sans
rapport ou tout au plus en rapport lointain avec les produits à base
de Bt et n'entrent pas, par conséquent, dans le cadre de la présente
monographie.
1. Identité, caractéristiques biologiques et méthodes d'analyse
Bacillus thuringiensis est une bactérie anaérobie facultative,
gram-positif, qui forme des inclusions protéiques caractéristiques
adjacentes à l'endospore. Certaines sous-espèces de Bt peuvent
synthétiser plusieurs inclusions parasporales. Le Bt est génétiquement
indiscernable du Bc, exception faite de son aptitude à former des
inclusions cristallines parasporales qui sont toxiques pour certains
invertébrés, notamment les larves d'insectes appartenant aux ordres
suivants: coléoptères, diptères et lépidoptères. Les inclusions
parasporales sont constituées de diverses protéines cristallisées
insecticides (ICP). Ces cristaux sont de forme variable (bipyramidale,
cuboïdale, rhomboïdale plane, sphérique ou composite, c'est-à-dire
comportant deux types de cristaux) selon la nature des protéines qui
les composent. On a établi une corrélation partielle entre la
morphologie des cristaux, la composition en protéines cristallisées et
l'activité biologique vis-à-vis des insectes.
Le taxon phénotypique fondamental est la sous-espèce,
caractérisée par son sérotype flagellaire (H). En 1998, on avait déjà
décrit 67 sous-espèces. Les gènes qui codent pour les ICP sont pour la
plupart situés sur les plasmides. Chacune de ces protéines n'est le
produit que d'un seul gène. La plupart des plasmides porteurs de gènes
ICP se transmettent facilement d'une souche bactérienne à l'autre par
conjugaison et peuvent aussi passer à une espèce bactérienne voisine.
La classification phénotypique est maintenant complétée par une
caractérisation basée sur la biologie moléculaire et plus précisément
sur la séquence des gènes codant pour les cristaux (cry et cyt)
plutôt que sur la spécificité vis-à-vis des insectes cibles. Divers
domaines des ICP sont responsables de la sensibilité de l'hôte
(reconnaissance des récepteurs) et de la toxicité (formation de
pores).
Parmi les techniques couramment utilisées pour caractériser les
souches de Bt ou les inclusions protéiques elles-mêmes, on peut citer
l'analyse des acides gras pariétaux, les anticorps monoclonaux, les
sondes d'ADN oligonucléotidiques, les profils plasmidiques, l'analyse
par amplification génique (PCR), la technique des empreintes
génétiques et les profils électrophorétiques SDS-PAGE (électrophorèse
en gel de polyacrylamide en présence de dodécylsulfate de sodium).
Certaines sous-espèces de Bt produisent pendant leur croissance
une bêta-exotoxine constitué d'un nucléotide thermostable qui peut
contaminer les produits. Cette bêta-exotoxine est toxique pour presque
toutes les formes de vie, y compris l'Homme et les insectes cibles. Au
cours de leur croissance, les diverses souches de Bt produisent toutes
sortes d'antibiotiques, d'enzymes, de métabolites et de toxines, y
compris des toxines Bc, qui peuvent avoir des effets nocifs sur les
organismes visés ou non visés. La numération des spores n'est pas le
reflet fidèle de l'activité insecticide d'une souche de Bt ou d'un
produit qui en dérive. Pour mesurer l'activité (en unités
toxicologiques internationales (ITU) par mg), on procède à un test
biologique sur insecte au moyen d'un étalon international.
2. Mode d'action sur les insectes cibles
Les Bt sporulés ou les complexes spores-ICP doivent être ingérés
par les larves d'insectes appartenant aux espèces sensibles.
L'efficacité des ICP dépend de plusieurs facteurs: solubilisation dans
l'intestin moyen, conversion de la protoxine en toxine biologiquement
active sous l'action des enzymes protéolytiques, fixation de la toxine
active aux récepteurs membranaires spécifiques par sa région
C-terminale et formation de pores par la région N-terminale entraînant
la lyse des cellules épithéliales. La germination des spores et la
prolifération de cellules bactériennes végétatives dans l'hémocèle
peut entraîner une septicémie qui contribue également à la mort. La
spécificité d'hôte des différentes ICP est essentiellement déterminée
par leur fixation aux récepteurs.
3. Habitats
On a isolé de nombreuses sous-espèces de Bt sur des insectes
morts ou mourants appartenant principalement à l'ordre des
coléoptères, des diptères et des lépidoptères, mais nombreuses sont
également celles qui ont été isolées du sol, de la surface des
feuilles ou d'autres habitats. Les cadavres d'insectes contiennent
souvent de grandes quantités de spores et d'ICP susceptibles de
pénétrer dans l'environnement. Les sous-espèces actives contre les
coléoptères et les lépidoptères sont principalement associées au sol
et aux surfaces foliaires, alors que les sous-espèces actives contre
les diptères se rencontrent communément dans les milieux aquatiques.
Dans l'environnement, les spores sont capables de persister et de se
développer en présence de conditions favorables et de nutriments
appropriés.
4. Produits du commerce. Production et épandage
Les produits commerciaux classiques à base de bacille de
Thuringe, qui utilisent des souches naturelles, représentent environ
90% du marché mondial des agents microbiologiques de lutte contre les
nuisibles. La plupart de ces produits contiennent des spores viables
et des ICP, mais dans certains d'entre eux, les spores sont inactivées
(Bti). Chaque année, on en produit quelque 13 000 tonnes par la
technique de fermentation aérobie. Les produits classiques à base de
Bt sont principalement destinés à lutter contre les lépidoptères qui
ravagent les cultures et les plantations forestières; toutefois, ces
dernières années, on a également commercialisé des souches actives
contre les coléoptères. Les programmes de santé publique utilisent
également des souches de Bti actives contre les diptères vecteurs de
maladies virales ou parasitaires.
Les formulations commerciales de bacille de Thuringe peuvent être
épandues sur le feuillage, le sol, les étendues d'eaux ou dans les
entrepôts de denrées alimentaires pour combattre les insectes. Une
fois le produit épandu dans l'écosystème, les cellules bactériennes
végétatives et les spores peuvent persister à des concentrations
progressivement décroissantes pendant des semaines, des mois ou des
années en tant que constituants de la microflore naturelle. Par
contre, les ICP perdent leur activité biologique au bout de quelques
heures ou de quelques jours.
5. Effets du Bt sur les organismes non visés
Les études effectuées sur des mammifères et notamment celles qui
ont porté sur des animaux de laboratoire ont consisté a évaluer
l'infectiosité et la toxicité éventuelles de diverses préparations à
base de Bt contenant notamment des ICP, des spores et des cellules
bactériennes en phase végétative. Sous ces trois formes, les
différentes sous-espèces de Bt se sont révélées pour la plupart non
pathogènes et non toxiques pour les diverses espèces animales
utilisées. On a montré que les cellules bactériennes en phase
végétative et les spores persistaient pendant plusieurs semaines sans
causer d'effets nocifs. En particulier, on a constaté que le Bt
n'avait pas d'effets indésirables sur les oiseaux, les poissons et de
nombreux autres vertébrés aquatiques non visés, lors d'études en
laboratoire ou sur le terrain portant sur un grand nombre de
spécimens. Il n'y a que relativement peu d'invertébrés aquatiques qui
se révèlent sensibles au Bt en laboratoire ou sur le terrain. Par
ailleurs, le bacille de Thuringe n'exerce pas non plus d'effets nocifs
sur les lombrics.
L'activité insecticide des différentes sous-espèces de Bt
présente en général une spécificité d'hôte très marquée vis-à-vis des
coléoptères, des diptères et des lépidoptères et on a montré qu'elle
n'avait pratiquement aucun effet toxique direct sur les arthropodes
non visés. La plupart des données relatives à l'innocuité de ces
produits pour les arthropodes non visés concernent les sous-espèces de
Bt actives contre les diptères et les lépidoptères.
Les études consacrées aux formulations de Bti exemptes de
contaminants toxiques ont montré qu'elles étaient sans danger pour la
plupart des arthropodes non visés. Certains moucherons (chironomides
appartenant à l'ordre des diptères) très proches des moustiques se
sont révélés sensibles à de fortes doses de Bti mais ne sont nullement
affectés aux doses utilisées pour la destruction des larves de
moustiques. Des études sur le terrain ont mis en évidence des cas de
réduction ou au contraire d'augmentation de certaines populations
d'arthropodes non visés.
Les études toxicologiques auxquelles ont été soumis de nombreux
ordres d'insectes n'ont, pour la plupart d'entre eux, révélé aucun
effet toxique imputable au Btk.
On a observé une certaine mortalité chez des abeilles
(Apis mellifera) qui avaient été soumises à des bacilles des
sous-espèces Btt et Btk en phase végétative, mais il ne semble pas que
les spores ou les ICP soient capables de produire un tel effet. En
laboratoire et sur le terrain, le Btg n'a aucun effet toxique sur les
abeilles.
Les souches de Bte productrices de bêta-exotoxine se sont
révélées capables d'exercer des effets toxiques sur les arthropodes
non visés.
6. Exposition humaine et effets du bacille de Thuringe sur l'Homme
Les ouvriers qui épandent des produits à base de Bt peuvent être
fortement exposés à ces produits par contact cutané ou par inhalation
d'aérosols. L'usage du Bt en agriculture peut entraîner la
contamination de l'eau potable et des denrées alimentaires par le
bacille. Toutefois, à l'exception de quelques cas d'irritation des
yeux ou de la peau, on n'a pas connaissance d'effets nocifs attestés
qui résulteraient d'une exposition professionnelle à des produits à
base de Bt. Des volontaires qui avaient ingéré ou inhalé de grandes
quantités de diverses formulations de Btk, n'ont ressenti aucun effet
indésirable. On a mis en évidence des anticorps dirigés contre les
cellules bactériennes, les spores et les complexes spores-cristaux
chez des ouvriers chargés de l'épandage de produits à base de Bt;
aucun effet indésirable n'a cependant été observé. On connaît le cas
d'un certain nombre de patients atteints de maladies infectieuses chez
lesquels la présence de Bt a été mise en évidence. Toutefois, aucune
des études qui leur ont été consacrées n'a permis de conclure de façon
certaine que l'utilisation du Bt comporte effectivement un risque pour
la santé humaine. Il ne semble pas non plus que la présence de Bt dans
l'eau destinée à la consommation ou dans les denrées alimentaires soit
à l'origine d'effets indésirables chez l'Homme.
7. Conclusions
Compte tenu de la spécificité de leur mode d'action, il est
improbable que les produits à base de Bt constituent un danger
quelconque pour l'Homme et les vertébrés ni pour la très grande
majorité des invertébrés non visés, pour autant qu'ils ne contiennent
pas d'autres microorganismes ou de substances biologiquement actives
autres que les ICP. On peut utiliser ces produits en toute sécurité
pour détruire les insectes qui ravagent les domaines agricoles et
horticoles ainsi que les forêts. Ils sont également sans danger pour
le milieu aquatique et on peut notamment les épandre dans les
réservoirs d'eau potable pour lutter contre les moustiques, les
simulies et les larves d'insectes incommodants. Il convient cependant
de noter qu'en phase végétative, le Bt est capable de produire des
toxines de type Bc dont on ignore si elles sont susceptibles de
provoquer des maladies chez l'Homme.
1. RESUMEN
Esta monografía trata sobre los plaguicidas microbianos (PM)
basados en Bacillus thuringiensis (Bt). Esta bacteria es también una
fuente clave de genes cuya expresión transgénica confiere resistencia
frente a plagas a plantas y microorganismos, actuando como plaguicida
en los denominados organismos modificados genéticamente (OMG). Los
posibles efectos de los OMG sobre la salud humana y el medio están
poco o nada relacionados con los productos basados en Bt, por lo que
quedan fuera del ámbito de esta monografía.
1. Identidad, características biológicas y métodos de laboratorio
Bt es una bacteria gram-positiva y anaerobia facultativa que
forma inclusiones proteicas características junto a la endospora. Las
subespecies de Bt pueden sintetizar más de una inclusión parasporal.
Desde el punto de vista genético, Bt es indistinguible de Bc,
exceptuando la capacidad de Bt para producir inclusiones parasporales
cristalinas que son tóxicas para ciertos invertebrados, en particular
para las larvas de insectos pertenecientes a los órdenes Coleóptera,
Díptera y Lepidóptera. Dichas inclusiones parasporales están
formadas por distintas proteínas cristalinas insecticidas (PCI). Los
cristales tienen formas diversas (bipiramidales, cuboides, romboides
planos, esféricos o compuestos por dos tipos de cristales),
dependiendo de su composición en PCI. Se ha comprobado que existe una
correlación parcial entre la morfología del cristal, la composición en
PCI y la bioactividad frente a los insectos diana.
El taxón fenotípico básico es la subespecie, identificada por el
serotipo flagelar (H). Hasta 1998 se habían descrito 67 subespecies.
Los genes que codifican las PCI se encuentran fundamentalmente en los
plásmidos. Cada PCI es el producto de un solo gen. La mayoría de los
plásmidos con genes de PCI se transfieren fácilmente por conjugación
entre cepas de Bt y pueden transferirse a especies bacterianas
emparentadas. La clasificación fenotípica se ha complementado en la
actualidad con la caracterización biomolecular, basada en la secuencia
de los genes de las proteínas cristalinas (cry y cyt), no en la
especificidad para las especies diana. En las PCI, la susceptibilidad
del huésped (reconocimiento de receptores) y la toxicidad (formación
de poros) son responsabilidad de dominios distintos de la molécula.
Las técnicas utilizadas habitualmente para caracterizar las cepas
de Bt o la propia PCI consisten en análisis de los ácidos grasos de la
pared celular, anticuerpos monoclonales, sondas de oligonucleótidos de
ADN, perfiles de plásmidos, análisis por reacción en cadena de la
polimerasa (PCR), estudios del ADN (huella genética) y perfiles de
SDS-PAGE (dodecil sulfato sódico -- electroforesis en gel de
poliacrilamida).
La beta-exotoxina, un nucleótido termoestable, es sintetizada por
algunas subespecies de Bt durante el crecimiento vegetativo y puede
contaminar los productos. Es tóxica para casi todas las formas de
vida, incluidos los seres humanos y los órdenes de insectos diana.
Durante el crecimiento vegetativo, varias cepas de Bt producen una
gama de antibióticos, enzimas, metabolitos y toxinas, incluidas
toxinas de Bc, que pueden tener efectos nocivos tanto en las especies
que son objetivo del plaguicida como en las que no lo son. Los
recuentos de esporas no reflejan con exactitud la actividad
insecticida de una cepa o un preparado de Bt. Se mide la potencia
(UTI/mg) de cada producto mediante ensayos biológicos para los que se
utiliza un patrón internacional basado en un insecto concreto.
2. Modo de acción en los insectos diana
Es preciso que las larvas de los insectos susceptibles ingieran
Bt esporulado con PCI o con complejos espora-PCI. La eficacia de la
PCI depende de su solubilización en el intestino medio, de la
conversión de la protoxina en la toxina biológicamente activa por la
acción de enzimas proteolíticas, de la unión específica del dominio
C-terminal de la toxina activa al receptor de membrana y de la
formación de poros por parte del dominio N-terminal, con la
consiguiente lisis de las células epiteliales. La germinación de
esporas y la proliferación de células vegetativas en el hemocele puede
ocasionar una septicemia y contribuir a la muerte. La unión de la PCI
al receptor es el determinante principal de la especificidad de
huésped para las distintas PCI de Bt.
3. Hábitats
Se han aislado muchas subespecies de Bt a partir de insectos
muertos o moribundos, la mayoría pertenecientes a los órdenes
Coleóptera, Díptera y Lepidóptera, pero también del suelo, de
superficies foliares y de otros hábitats. Los exoesqueletos de
insectos muertos contienen a menudo grandes cantidades de esporas y
PCI que pueden incorporarse al medio. Las subespecies de Bt activas
frente a coleópteros y lepidópteros se asocian fundamentalmente con el
suelo y el filoplano (superficies foliares), mientras que las activas
frente a dípteros se hallan generalmente en medios acuáticos. En el
ambiente, las esporas persisten y pueden entrar en crecimiento
vegetativo cuando las condiciones son favorables y hay nutrientes
disponibles.
4. Productos comerciales, producción y aplicación
Los preparados convencionales de Bt, que utilizan cepas de Bt que
aparecen de forma espontánea en la naturaleza, representan
aproximadamente el 90% del mercado mundial de los PM. La mayoría de
los preparados de Bt contienen PCI y esporas viables, pero en algunos
productos de Bti las esporas están inactivadas. Cada año se producen
aproximadamente 13.000 toneladas utilizando tecnología de fermentación
aerobia. Los preparados convencionales de Bt tienen como objetivos
primarios las plagas de lepidópteros que afectan a los cultivos
agrícolas y forestales; sin embargo, en los últimos años también se
han comercializado cepas de Bt activas frente a plagas de coleópteros.
Se están utilizando en programas de salud pública cepas de Bti activas
frente a dípteros vectores de enfermedades parasitarias y víricas.
Las formulaciones comerciales de Bt pueden aplicarse como
insecticidas al follaje, el suelo, el medio acuático o instalaciones
de almacenamiento de alimentos. Tras aplicar una subespecie de Bt a un
ecosistema, las células vegetativas y las esporas pueden persistir en
concentraciones gradualmente decrecientes durante semanas, meses o
años como un componente de la microflora natural. Sin embargo, las PCI
pierden su actividad biológica en horas o días.
5. Efectos de Bt sobre especies que no son objetivo del plaguicida
En estudios con mamíferos, en particular con animales de
laboratorio, se ha evaluado la posible infecciosidad y toxicidad de
diversos preparados de Bt, que comprenden las PCI, células vegetativas
y esporas. Las PCI, las esporas y las células vegetativas de las
subespecies de Bt, que se administraron por distintas vías, carecían
en su mayoría de patogenicidad y toxicidad para las diversas especies
animales estudiadas. Se comprobó que las células vegetativas o las
esporas de Bt persistían durante semanas sin causar efectos adversos.
No se ha observado que Bt afecte a pájaros, peces o muchas otras
especies de vertebrados acuáticos que no son objetivo del plaguicida y
se han estudiado en gran número de trabajos de laboratorio y de campo.
Son relativamente pocas las especies de invertebrados acuáticos
susceptibles a Bt, tanto en condiciones de laboratorio como de campo.
Bt no afecta a las lombrices de tierra.
En general, las subespecies de Bt muestran gran especificidad en
su actividad insecticida frente a Coleóptera, Díptera y
Lepidóptera, así como una toxicidad directa escasa, si no nula,
frente a los artrópodos que no son su objetivo. La mayor parte de los
datos disponibles sobre inocuidad en éstos se han obtenido con las
subespecies de Bt activas frente a Díptera y Lepidóptera.
Los estudios sobre formulaciones de Bti sin contaminantes tóxicos
no han puesto de manifiesto efectos nocivos en la gran mayoría de los
artrópodos que no son objetivo del plaguicida. Se ha comprobado que
algunas moscas enanas (Díptera: Chironomidae), estrechamente
emparentadas con los mosquitos, son sensibles a dosis altas de Bti,
pero no se ven afectadas por dosis letales para larvas de mosquito. En
estudios de campo se han descrito disminuciones o aumentos
transitorios de las poblaciones de algunos artrópodos que no son
objetivo del plaguicida.
Se han estudiado muchos órdenes de insectos, tanto en el
laboratorio como en trabajos de campo, y se ha comprobado que en la
mayoría de ellos Btk no tiene efecto.
Se ha observado mortalidad en abejas melíferas (Apis mellifera)
tras la exposición a Btt y Btk en crecimiento vegetativo, pero el
efecto no parece guardar relación con las esporas o las PCI. En los
estudios de laboratorio y de campo, Btg no mostró efectos adversos
sobre las abejas melíferas.
Se ha comprobado que cepas de Bte productoras de beta-exotoxina
tienen efectos adversos sobre artrópodos que no son objetivo del
plaguicida.
6. Exposición a Bt y efectos sobre los seres humanos
La aplicación agrícola de preparados de Bt puede suponer una
considerable exposición de los trabajadores, tanto en aerosol como
dérmica. Puede, asimismo, causar la contaminación del agua potable y
los alimentos por la bacteria. Salvo casos notificados de irritación
ocular y dérmica, no se han documentado efectos adversos sobre la
salud tras la exposición laboral a preparados de Bt. Individuos
voluntarios ingirieron e inhalaron grandes cantidades de una
formulación de Btk sin sufrir efectos adversos. Se detectaron títulos
de anticuerpos frente a las células vegetativas, las esporas y los
complejos espora-cristal en trabajadores que pulverizaban preparados
de Bt, pero no se registraron efectos adversos. Se han descrito
algunos casos de presencia de Bt en pacientes con diversas
enfermedades infecciosas. Sin embargo, ninguno de estos estudios
demuestra de forma inequívoca que el uso de Bt entrañe un riesgo real
para la salud humana. No se ha demostrado que Bt tenga efectos
adversos en seres humanos cuando está presente en el agua potable o
los alimentos.
7. Conclusiones
Debido a la especificidad de su modo de acción, es improbable que
los preparados de Bt entrañen peligro alguno para los seres humanos u
otros vertebrados, o para la gran mayoría de los invertebrados que no
constituyen su objetivo, siempre y cuando no contengan microorganismos
distintos de Bt y productos biológicamente activos distintos de las
PCI. Los preparados de Bt pueden utilizarse con seguridad para
controlar las plagas de insectos de los cultivos agrícolas y
hortícolas, así como las forestales. También es seguro su uso en
medios acuáticos, incluidos los depósitos de agua potable, para
controlar el mosquito, la mosca negra y las larvas de insectos
dañinos. Sin embargo, es preciso señalar que las formas vegetativas de
Bt pueden sintetizar toxinas del tipo de las producidas por Bc, cuya
importancia como causa de enfermedades humanas se desconoce.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS
THURINGIENSIS
Members
Professor D. Calamari, Department of Biology, Structure and Function,
University of Milan, Varese, Italy
Dr C. Cummins, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr N. Gratz, Commugny, Switzerland (Rapporteur)
Dr A. Klier, Unité de Biochimie Microbienne, Département des
Biotechnologies, Institut Pasteur, Paris, France
Professor P. Lüthy, Microbiological Institute ETH, Zurich, Switzerland
Dr C.M. Scanlan, Texas A & M University, Department of Veterinary
Pathobiology, Texas Veterinary Medical Center, Texas, USA (Chairman)
Observers
Dr R.J. Cibulsky, Abbott Laboratories, Chemical and Agricultural
Products, North Chicago, Illinois, USA
Dr E. Cozzi, Clinical Research and Development, Animal Health
Products, Abbott Laboratories, North Chicago, Illinois, USA
Dr M. Ronchin, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Secretariat
Professor M. Maroni, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Dr R. Plestina, Zagreb, Croatia
ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS THURINGIENSIS
A WHO Task Group on Environmental Health Criteria for the
microbial pest control agent Bacillus thuringiensis (Bt) met at the
International Centre for Pesticide Safety in Busto Garolfo, Milan,
Italy, from 27 to 31 October 1997. Professor M. Maroni, Director of
the Centre, welcomed participants on behalf of the Centre, which was
responsible for organizing the meeting. Dr R. Plestina, IPCS temporary
adviser, opened the meeting and welcomed participants on behalf of Dr
M. Mercier, Director of IPCS. The Group reviewed and revised the draft
and made an evaluation of the risks for human health and the
environment from exposure to Bt products. Drs R. Plestina and A. Aitio
of the IPCS Central Unit were responsible for the scientific aspects
of the monograph, and Dr P.G. Jenkins for the editing. The assistance
of manufacturers, notably Abbott Laboratories, in providing
unpublished documentation for the review is greatly appreciated.
Microbial Pest Control Agents (MPCAs), notably products of
various Bt subspecies, are increasingly used in pest management
programmes against the larvae of several insect pests of major
agricultural crops and forests, and several insect vectors of human
diseases, and some nuisance pests. Bt products have been used
worldwide, and their commercial production is about 1% of that of
chemical pesticides. A number of reviews have recently been published
on various aspects of Bt (Entwistle et al., 1983; McClintock et al.,
1995; Cannon, 1996; Dean et al., 1996; Kumar et al., 1996; Schnepf et
al., 1998; Nielsen-LeRoux et al., 1998).
The activities in preparing this document were recommended by an
Informal Consultation on the Safety of Microbial Pest Control Agents
held in Geneva in June 1993. The first draft of the monograph was
prepared by a drafting group in 1994 and was subsequently amended by
Professor C.M. Scanlan (Texas A&M University, Department of Veterinary
Pathobiology, The Texas Veterinary Medical Center, Texas, USA). The
revised draft was circulated to the IPCS contact points. Based on the
comments received, it was amended and updated by Drs B.M. Hansen and
N.B. Hendriksen (National Environmental Research Institute, Roskilde,
Denmark). The document was finally approved by the Task Group members.
ABBREVIATIONS
Bc Bacillus cereus
Bt Bacillus thuringiensis
Bta Bacillus thuringiensis subspecies aizawai
Btd Bacillus thuringiensis subspecies darmstadiensis
Bte Bacillus thuringiensis subspecies entomocidus
Btg Bacillus thuringiensis subspecies galleriae
Bti Bacillus thuringiensis subspecies israelensis
Btk Bacillus thuringiensis subspecies kurstaki
Btko Bacillus thuringiensis subspecies konkukian
Btt Bacillus thuringiensis subspecies thuringiensis
Btte Bacillus thuringiensis subspecies tenebrionis
cfu colony forming unit
GILSP good industrial large-scale practice
GMO genetically modified organism
HPLC high-performance liquid chromatography
ICP insecticidal crystal protein
ITU international toxic unit
IUPAC International Union of Pure and Applied Chemistry
MPCA microbial pest control agent
NTO non-target organism
PCR polymerase chain reaction
SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
1. SUMMARY
This monograph deals with microbial pest control agents (MCPAs)
based on Bacillus thuringiensis (Bt). This bacterium is also a key
source of genes for transgenic expression to provide pest resistance
in plants and microorganisms as pest control agents in so-called
genetically modified organisms (GMOs). The potential effects on human
health and the environment of GMOs involve several aspects that are
only remotely or not at all related to Bt products, and they are
therefore outside the scope of this monograph.
1.1 Identity, biological characteristics and analytical methods
Bt is a facultative anaerobic, gram-positive bacterium that forms
characteristic protein inclusions adjacent to the endospore. Bt
subspecies can synthesize more than one parasporal inclusion. Bt is
genetically indistinguishable from Bc, except for the ability of Bt to
produce parasporal crystalline inclusions, which are toxic for certain
invertebrates, especially species of insect larvae belonging to the
insect orders Coleoptera, Diptera and Lepidoptera. The
parasporal inclusions are formed by different insecticidal crystal
proteins (ICP). The crystals have various shapes (bipyramidal,
cuboidal, flat rhomboid, spherical or composite with two crystal
types), depending on their ICP composition. A partial correlation
between crystal morphology, ICP composition, and bioactivity against
target insects has been established.
The basic phenotypic taxon is the subspecies, identified by the
flagellar (H) serotype. By 1998, 67 subspecies had been described. The
genes that encode the ICPs are mostly on plasmids. Each ICP is the
product of a single gene. Most plasmids with ICP genes are readily
transferred by conjugation between Bt strains and may be transferred
to related species of bacteria. The phenotypic classification has now
been complemented by molecular biological characterization, based on
the sequence of the crystal (cry and cyt) genes rather than target
organism specificity. Different domains of the ICP are responsible for
host susceptibility (receptor recognition) and toxicity (pore
formation).
Techniques commonly used to characterize Bt strains or the ICP
itself include cell wall fatty acid analysis, monoclonal antibodies,
oligonucleotide DNA probes, plasmid profiles, polymerase chain
reaction (PCR) analysis, DNA fingerprinting and SDS-PAGE profiles.
Beta-exotoxin, a heat-stable nucleotide, is produced by some Bt
subspecies during vegetative growth and may contaminate the products.
Beta-exotoxin is toxic for almost all forms of life including humans
and the target insect orders. During vegetative growth, various Bt
strains produce an assortment of antibiotics, enzymes, metabolites and
toxins, including Bc toxins, that may have detrimental effects on both
target organisms and non-target organisms (NTOs). Spore counts do not
accurately reflect the insecticidal activity of a Bt strain or Bt
product. The potency (ITU/mg) of each Bt product is bioassayed using
an international standard that uses a specific test insect.
1.2 Mode of action on target insects
The sporulated Bt with ICP or spore-ICP complexes must be
ingested by a susceptible insect larva. The efficacy of the ICP
depends on the solubilization in the midgut, the conversion of the
protoxin to the biologically active toxin by proteolytic enzymes,
specific membrane receptor binding by the C-terminal domain of the
active toxin, and pore formation by the N-terminal domain with
subsequent lysis of the epithelial cells. Spore germination and
proliferation of the vegetative cells into the haemocoel may result in
a septicaemia, contributing to the cause of death. Receptor binding by
the ICP is the major determinant of host specificity by the different
Bt ICPs.
1.3 Habitats
Many different Bt subspecies have been isolated from dead or
dying insects mostly from the orders Coleoptera, Diptera and
Lepidoptera, but many subspecies have also been isolated from soil,
leaf surfaces and other habitats. The carcasses of dead insects often
contain large quantities of spores and ICPs that may enter the
environment. The coleopteran-active and lepidopteran-active Bt
subspecies are primarily associated with the soil and phylloplane
(leaf surfaces), whereas the dipteran-active Bt subspecies are
commonly found in aquatic environments. In the environment, the spores
persist and vegetative growth may occur when conditions are favourable
and nutrients are available.
1.4 Commercial products, production and application
Conventional Bt products, which utilize naturally-occurring Bt
strains, account for approximately 90% of the world MPCA market. Most
Bt products contain ICP and viable spores, but in some Bti products
the spores are inactivated. Each year some 13 000 tonnes are produced
using aerobic fermentation technology. Conventional Bt products have
been targeted primarily against lepidopteran pests of agricultural and
forestry crops; however in recent years, Bt strains active against
coleopteran pests have also been marketed. Strains of Bti active
against dipteran vectors of parasitic and viral diseases are being
used in public health programmes.
Commercial Bt formulations may be applied as an insecticide to
foliage, soil, water environments or food storage facilities. After
the application of a Bt subspecies to an ecosystem, the vegetative
cells and spores may persist at gradually decreasing concentrations
for weeks, months or years as a component of the natural microflora.
The ICPs, however, are rendered biologically inactive within hours or
days.
1.5 Effects of Bt on non-target organisms
Studies on mammals, particularly those on laboratory animals,
have evaluated possible infectivity and toxicity of various Bt
preparations, which include the ICPs, vegetative cells and spores. The
ICPs, spores and vegetative cells of the Bt subspecies, which were
administered by different routes, were mostly non-pathogenic and
non-toxic to the various animal species tested. The vegetative cells
and/or spores of Bt were demonstrated to persist for weeks without
causing adverse effects. Bt has not been observed to adversely affect
birds, fish or many other non-target aquatic vertebrates tested in a
large number of laboratory and field studies. Relatively few species
of aquatic invertebrates are susceptible to Bt under either laboratory
or field conditions. Bt does not adversely affect earthworms.
The Bt subspecies have generally been shown to be highly specific
in their insecticidal activity for Coleoptera, Diptera and
Lepidoptera and have demonstrated little, if any, direct toxicity to
non-target arthropods. Most of the existing safety data on non-target
arthropods has been generated using the Bt subspecies with activity
against Diptera and Lepidoptera.
Studies of Bti formulations free of toxic contaminants have not
demonstrated deleterious effects on the vast majority of non-target
arthropods. Some midges (Diptera: Chironomidae), which are closely
related to mosquitos, have been shown to be susceptible to high
dosages of Bti, but are not affected by mosquito larvicidal dosages.
In field studies, transient decreases or increases in populations of
some non-target arthropods have been reported.
Many insect orders have been tested in either the laboratory or
field, most of which have shown no effect from Btk.
Mortality has been observed in honey-bees (Apis mellifera)
after exposure to vegetatively growing Btt and Btk, but the effect
does not seem to be related to spores or ICPs. In laboratory and field
studies Btg demonstrated no adverse effect on honey-bees.
Bte strains that produce beta-exotoxin have been shown to have
adverse effects on non-target arthropods.
1.6 Exposure and effects of Bt on humans
The field application of Bt products can result in considerable
aerosol and dermal exposure of workers. Agricultural uses of Bt can
result in Bt contamination of potable water and food. With the
exception of case reports on ocular and dermal irritation, no adverse
health effects have been documented after occupational exposure to Bt
products. Human volunteers ingested and inhaled large quantities of a
Btk formulation but experienced no adverse health effects. Antibody
titres to the vegetative cells, spores and spore-crystal complexes
have been demonstrated in workers who spray Bt products; however, no
adverse health effects were reported. There have been some case
reports on the occurrence of Bt in patients with different infectious
diseases. However, none of these studies unequivocally demonstrates an
actual risk to human health from the use of Bt. Bt has not been
reported to cause adverse effects on human health when present in
drinking-water or food.
1.7 Conclusions
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and biologically active products other than the
ICPs. Bt products may be safely used for the control of insect pests
of agricultural and horticultural crops as well as forests. They are
also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt has the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
2. IDENTITY, BIOLOGICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Commercial Bacillus thuringiensis (Bt) products are microbial
pest control agents (MPCAs) containing specific insecticidal
crystalline proteins (ICPs) and most often living spores as well as
formulating agents. They are processed fermentation products.
2.1.1 Bacillus thuringiensis
Bt is a facultative anaerobic, motile, gram-positive,
spore-forming bacterium. The formation of parasporal crystals adjacent
to the endospore during sporulation stages III to IV distinguishes Bt
from other Bacillus species.
Bt, like other Bacillus species, has been classified on the
basis of its cellular, cultural, biochemical and genetic
characteristics (Baumann et al., 1984; Claus & Berkley, 1986; Slepecky
& Hemphill, 1992; Carlson & Kolsto, 1993; Hansen et al., 1998). In
1958, Heimpel & Angus (1958) introduced a classification scheme to
identify these crystalliferous bacteria based on their morphological
and biochemical characteristics. However recent molecular analysis
shows that several variations can be found within serotypes, and that
specific biochemical characteristics do not always refer to a specific
serotype (Helgason et al., 1998; Hansen et al., 1998).
2.1.2 Relationship between Bacillus thuringiensis and Bacillus cereus
Bt is a member of the Bc group, which also contains Bacillus
cereus (Bc), B. mycoides and B. anthracis. Furthermore, the
psychrotolerant B. weihenstephanensis has recently been proposed as
a new member of the group (Lechner et al., 1998). Bt can only be
distinguished from Bc by the production during the sporulation process
of one or more inclusion bodies, which have been found to be toxic for
invertebrates, primarily insect species in the orders Coleoptera,
Diptera and Lepidoptera (de Barjac, 1981b; Andrews et al., 1987).
Several studies have been dedicated to a comparison of Bt and Bc on
the basis of characters not related to the production of ICPs
(Hendriksen & Hansen, 1998). Phenotypic differentiation of Bt and Bc
is not possible on the basis of morphology or utilization of organic
compounds (Baumann et al., 1984; Logan & Berkeley, 1984; Priest et
al., 1988), characterization of cell content of fatty acids (Väisänen
et al., 1991) or sugars (Wunschel et al., 1994), multilocus enzyme
electrophoresis (Zahner et al., 1989; Carlson et al., 1994),
enterotoxin production (Damgaard et al., 1996a; Hansen & Hendriksen,
1997a), or serological- and phage-typing techniques (Ohba & Aizawa,
1978; 1986; Väisänen et al., 1991; Murakami et al., 1993; Ahmed et
al., 1995). Likewise, genotypic differentiation of Bt and Bc is not
possible by DNA homology analysis (Kaneko et al., 1978), ribotyping
(Priest et al., 1994; Demezas & Bell, 1995), 16S rDNA sequencing (Ash
et al., 1991); analysis of the 16S-23S internal transcribed sequence
(Wunschel et al., 1994; Bourque et al., 1995), PCR analysis of genes
encoding Bc-like toxic products (Damgaard et al., 1996b; Asano et al.,
1997; Hansen & Hendriksen, 1997b) or pulsed field gel electrophoresis
(Carlson & Kolsto, 1993; Carlson et al., 1994). Giffel et al. (1997)
found differences in 16S rDNA sequences between a limited number Bt
and Bc. Beattie et al. (1998) were able to discriminate among members
of the Bc group by Fourier transform infrared spectroscopy, and
Brousseau et al. (1992) were able to distinguish Bt and Bc by random
amplified polymorphic DNA fingerprinting. However, the transfer of ICP
encoding plasmids from Bt to Bc makes the receptor Bc
indistinguishable from Bt, and vice versa (González et al., 1981,
1982).
2.1.3 Crystal composition and morphology
The existence of parasporal inclusions in Bt was first noted in
1915 (Berliner, 1915), but their protein composition was not
delineated until the 1950s (Angus, 1954). Hannay (1953) detected the
crystalline fine structure that is a property of most of the
parasporal inclusions. Bt subspecies can synthesize more than one
inclusion, which may contain different ICPs. ICPs have also been
called delta endotoxins; however, since the term endotoxin usually
refers to toxins associated with the outer membranes of gram-negative
bacteria, comprising a core lipopoplysaccharide, lipid A and somatic
(O) antigens, this term is not used in this monograph. Depending on
their ICP composition, the crystals have various forms (bipyramidal,
cuboidal, flat rhomboid, or a composite with two or more crystal
types). A partial correlation between crystal morphology, ICP
composition, and bioactivity against target insects has been
established (Bulla et al., 1977; Höfte & Whiteley, 1989; Lynch &
Baumann, 1985).
2.1.4 Classification of Bt subspecies
The classification of Bt subspecies based on the serological
analysis of the flagella (H) antigens was introduced in the early
1960s (de Barjac & Bonnefoi, 1962). This classification by serotype
has been supplemented by morphological and biochemical criteria (de
Barjac, 1981a). Until 1977, only 13 Bt subspecies had been described,
and at that time all subspecies were toxic to Lepidopteran larvae
only. The discovery of other subspecies toxic to Diptera (Goldberg &
Margalit, 1977), Coleoptera (Krieg et al., 1983) and apparently
Nematoda (Narva et al., 1991) enlarged the host range and markedly
increased the number of subspecies. Up to the end of 1998, over 67
subspecies based on flagellar H-serovars had been identified (Table
1). Updated lists of the serovarieties can be obtained from the
reference centre of the Pasteur Institute in Paris (Unité des
Bactéries Entomopathogènes, Institut Pasteur, Paris, France).
Table 1. Current classification of 67 Bacillus thuringiensis subspecies based
on their flagellar (H) antigensa
Flagellar antigens B. thuringiensis Flagellar antigens B. thuringiensis
subspecies subspecies
1 thuringiensis 28a, 28c jegathesan
2 finitimus 29 amagiensis
3a, 3c alesti 30 medellin
3a, 3b, 3c kurstaki 31 toguchini
3a, 3d sumiyoshiensis 32 cameroun
3a, 3d, 3e fukuokaensis 33 leesis
4a, 4b sotto 34 konkukian
4a, 4c kenyae 35 seoulensis
5a, 5c galleriae 36 malaysiensis
5a, 5c canadensis 37 anadalousiensis
6 entomocidus 38 oswaldocruzi
7 aizawai 39 brasiliensis
8a, 8b morrisoni 40 huazhongensis
8a, 8c ostriniae 41 sooncheon
8b, 8d nigeriensis 42 jinghongiensis
9 tolworthi 43 guiyanguebsus
10a, 10b darmstadiensis 44 higo
10a, 10c londrina 45 roskildiensis
11a, 11b toumanoffi 46 chanpaisis
11a, 11c kyushuensis 47 wratislaviensis
12 thompsoni 48 balearica
13 pakistani 49 muju
14 israelensis 50 navarrensis
15 dakota 51 xiaguangiensis
16 indiana 52 kim
17 tohokuensis 53 asturiensis
18a, 18b kumamotoensis 54 poloniensis
18a, 18c yosoo 55 palmanyolensis
19 tochigiensis 56 rongseni
20a, 20b yunnanensis 57 pirenaica
20a, 20c pondicheriensis 58 argentinensis
21 colmeri 59 iberica
22 shandongiensis 60 pingluonsis
23 japonensis 61 sylvestriensis
24a, 24b neoleonensis 62 zhaodongensis
24a, 24c novosibirsk 63 bolivia
25 coreanensis 64 azorensis
26 silo 65 pulsiensis
27 mexicanensis 66 gracioensis
28a, 28b monterrey 67 vazensis
a Data provided by the Unité des Bactéries Entomopathogènes, Institut Pasteur,
Paris, France
Crystal serology has shown that a particular crystal type may be
produced by more than one H-serovar (Krywienczyk et al., 1978; Smith,
1987).
2.1.5 Genetics of ICP
In the early 1980s, it was established that most genes coding for
the ICPs reside on large transmissible plasmids, of which most are
readily exchanged between strains by conjugation (González & Carlton,
1980; González et al., 1981). Since these initial studies, numerous
ICP genes have been cloned, sequenced and used to construct Bt strains
with novel insecticidal spectra (Höfte & Whiteley, 1989).
The currently known crystal (cry) gene types encode ICPs that
are specific to either Lepidoptera (cryI), Diptera and
Lepidoptera (cryII), Coleoptera (cryIII), Diptera (cryIV),
or Coleoptera and Lepidoptera (cryV) (Höfte & Whiteley, 1989).
A separate designation is used for the cytolytic (cyt) genes that
encode a nonspecific cytolytic factor, present in Bti ICP and some
other Bt subspecies. However, due to the increasing number of
characterized ICP genes and inconsistencies in the existing cry gene
nomenclature, which is based on insecticidal spectrum, Crickmore et
al. (1998) proposed a new nomenclature based on ICP gene sequences.
The new cry genes are listed in Tables 2 and 3, and Table 4 is a
conversion list from old to new cry gene names. Current
identification of Bt employs both the identity of the cry genes,
which define the host range, and the H-serovars, which define the
subspecies, and recently DNA fingerprinting has been used for further
characterization of subspecies (Hansen et al., 1998).
The ICP gene sequences provided the basis for the construction of
gene-specific probes to screen established Bt strains by hybridization
and PCR analysis for the presence of known nucleotide sequences, and
for characterizing the ICPs from new Bt isolates (Prefontaine et al.,
1987; Juarez-Perez et al., 1997; Bravo et al., 1998; Shevelev et al.,
1998). These studies have permitted the distinction of numerous
subclasses of ICP genes based on sequence homology and toxicity
spectra of the encoded proteins.
All ICPs described to date attack the insect gut upon ingestion
(see chapter 3). To date, each of the proteolytically activated ICP
molecules with insecticidal activity has a variable C-terminal domain,
which is responsible for receptor recognition (host susceptibility),
and a conserved N-terminal domain, which induces pore formation
(toxicity) (Li et al., 1991).
Most naturally occurring Bt strains contain ICPs active against a
single order of insects. However, conjugative transfer between Bt
strains or related species can occur, resulting in new strains with
various plasmid contents. Thus the mobility of the cry genes and the
exchange of plasmids may explain the diverse and complex activity
Table 2. Bacillus thuringiensis crystal protein genes (Crickmore et al., 1998)
Name Acc No Reference Journal Coding
Cry1aa1 M11250 Schnepf et al., 1985 JBC 260 6264-6272 527-4054
Cry1aa2 M10917 Shibano et al., 1985 Gene 34 243-251 153->2955
Cry1aa3 D00348 Shimizu et al., 1988 ABC 52 1565-1573 73-3603
Cry1aa4 X13535 Masson et al., 1989 NAR 17 446-446 1-3528
Cry1aa5 D17518 Udayasuriyan et al., 1994 BBB 58 830-835 81-3611
Cry1aa6 U43605 Masson et al., 1994 Mol Micro 14 851-860 1->1860
Cry1aa7 AF081790 Osman, 1998 unpublished
Cry1aa8 I26149 Liu, 1996 USP 5556784 148-3675
Cry1ab1 M13898 Wabiko et al., 1986 DNA 5 305-314 142-3606
Cry1ab2 M12661 Thorne et al., 1986 J Bact 166 801-811 155-3625
Cry1ab3 M15271 Geiser et al., 1986 Gene 48 109-118 156-3623
Cry1ab4 D00117 Kondo et al., 1987 ABC 51 455-463 163-3630
Cry1ab5 X04698 Hofte et al., 1986 EJB 161 273-280 141-3605
Cry1ab6 M37263 Hefford et al., 1987 J Biotech 6 307-322 73-3540
Cry1ab7 X13233 Haider & Ellar, 1988 NAR 16 10927-10927 1-3465
Cry1ab8 M16463 Oeda et al., 1987 Gene 53 113-119 157-3624
Cry1ab9 X54939 Chak & Jen, 1993 PNSCRC 17 7-14 73-3540
Cry1ab10 A29125 Fischhoff et al., 1987 Bio/technology peptide seq
5 807-813
Cry1ab11 I12419 Ely & Tippett, 1995 USP 5424409 73-
Cry1ab12 AF057670 Silva-Werneck et al., 1998 unpublished 41-3505
Cry1ac1 M11068 Adang et al., 1985 Gene 36 289-300 388-3921
Cry1ac2 M35524 Von Tersch et al., 1991 AEM 57 349-358 239-3772
Cry1ac3 X54159 Dardenne et al., 1990 NAR 18 5546-5546 339->2192
Cry1ac4 M73249 Payne et al., 1991 USP 4990332 1-3537
Cry1ac5 M73248 Payne et al., 1992 USP 5135867 1-3534
Cry1ac6 U43606 Masson et al., 1994 Mol Micro 14 851-860 1->1821
Cry1ac7 U87793 Herrera et al., 1994 AEM 60 682-690 976-4512
Cry1ac8 U87397 Omolo et al., 1997 Curr Micro 34 118-121 153-3686
Cry1ac9 U89872 Gleave et al., 1992 NZJCHS 20 27-36 388-3921
Cry1ac10 AJ002514 Sun & Yu, 1997 unpublished 388-3921
Cry1ac11 AJ130970 Makhdoom & Riazuddin, 1998 unpublished 156-3689
Cry1ac12 I12418 Ely & Tippett, 1995 USP 5424409 81->2990
Cry1ad1 M73250 Payne & Sick, 1993 USP 5246852 1-3537
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1ad2 A27531 Payne & Sick, 1995 AUP 632335 1-3537
Cry1ae1 M65252 Lee & Aronson, 1991 J Bact 173 6635-6638 81-3623
Cry1af1 U82003 Kang et al., 1997 unpublished 172->2905
Cry1ag1 AF081248 Osman, 1998 unpublished
Cry1ba1 X06711 Brizzard & Whiteley, 1988 NAR 16 2723-2724 1-3684
Cry1ba2 X95704 Soetaert, 1996 unpublished 186-3869
Cry1bb1 L32020 Donovan et al., 1994 USP 5322687 67-3753
Cry1bc1 Z46442 Bishop et al., 1994 unpublished 141-3839
Cry1bd1 U70726 Kuo & Chak, 1999 unpublished 842-4534
Cry1be1 Payne et al., 1998 USP 5723758 1-3681
Cry1ca1 X07518 Honee et al., 1988 NAR 16 6240-6240 47-3613
Cry1ca2 X13620 Sanchis et al., 1989 Mol Micro 3 229-238 241->2711
Cry1ca3 M73251 Payne & Sick, 1993 USP 5246852 1-3570
Cry1ca4 A27642 Van Mellaert et al., 1990 EP 0400246 234-3800
Cry1ca5 X96682 Strizhov, 1996 unpublished 1->2286
Cry1cb1 M97880 Kalman et al., 1993 AEM 59 1131-1137 296-3823
Cry1da1 X54160 Hofte et al., 1990 NAR 18 5545-5545 264-3758
Cry1da2 I76415 Payne & Sick, 1997 USP 5691308 1-3495
Cry1db1 Z22511 Lambert, 1993 unpublished 241-3720
Cry1ea1 X53985 Visser et al., 1990 J Bact 172 6783-6788 130-3642
Cry1ea2 X56144 Bosse et al., 1990 NAR 18 7443-7443 1-3516
Cry1ea3 M73252 Payne & Sick, 1991 USP 5039523 1-3516
Cry1ea4 U94323 Barboza-Corona et al., 1998 WJMB 14 437-441 388-3900
Cry1ea5 A15535 Botterman et al., 1994 EP 0358557 54-3566
Cry1eb1 M73253 Payne & Sick, 1993 USP 5206166 1-3522
Cry1fa1 M63897 Chambers et al., 1991 J Bact 173 3966-3976 478-3999
Cry1fa2 M73254 Payne & Sick, 1993 USP 5188960 1-3525
Cry1fb1 Z22512 Lambert, 1993 unpublished 483-4004
Cry1fb2 AB012288 Masuda & Asano, 1998 unpublished 84-3587
Cry1fb3 AF062350 Song & Zhang, 1998 unpublished
Cry1fb4 I73895 Payne et al., 1997 USP 5686069 peptide seq
Cry1ga1 Z22510 Lambert, 1993 unpublished 67-3564
Cry1ga2 Y09326 Shevelev et al., 1997 Febs Lett 404 148-152 692-4210
Cry1gb1 U70725 Kuo & Chak, 1999 unpublished 532-4038
Cry1ha1 Z22513 Lambert, 1993 unpublished 530-4045
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1hb1 U35780 Koo et al., 1995 unpublished 728-4195
Cry1ia1 X62821 Tailor et al., 1992 Mol Micro 6 1211-1217 355-2511
Cry1ia2 M98544 Gleave et al., 1993 AEM 59 1683-1687 1-2160
Cry1ia3 L36338 Shin et al., 1995 AEM 61 2402-2407 279-2438
Cry1ia4 L49391 Kostichka et al., 1996 J Bact 178 2141-2144 61-2217
Cry1ia5 Y08920 Selvapandiyan, 1996 unpublished 524-2680
Cry1ia6 AF076953 Zhong et al., 1998 unpublished 1-2157
Cry1ib1 U07642 Shin et al., 1995 AEM 61 2402-2407 237-2393
Cry1ic1 AF056933 Osman et al., 1998 unpublished 1-2157
Cry1i-like I90732 Payne et al., 1998 See Table 3 peptide seq
Cry1ja1 L32019 Donovan et al., 1994 USP 5322687 99-3519
Cry1jb1 U31527 Von Tersch & Gonzalez, 1994 USP 5356623 177-3686
Cry1jc1 I90730 Payne et al., 1998 USP 5723758 peptide seq
Cry1ka1 U28801 Koo et al., 1995 FEMS 134 159-164 451-4098
Cry1-like I90729 Payne et al., 1998 See Table 3 peptide seq
Cry2aa1 M31738 Donovan et al., 1989 JBC 264 4740-4740 156-2054
Cry2aa2 M23723 Widner & Whiteley, 1989 J Bact 171 965-974 1840-3741
Cry2aa3 D86064 Sasaki et al., 1997 Curr Micro 35 1-8 2007-3911
Cry2aa4 AF047038 Misra et al., 1998 Unpublished 10-1908
Cry2aa5 AJ132464 Yu & Pang, 1999 Unpublished <1-1860
Cry2aa6 AJ132465 Yu & Pang 1999 Unpublished <1-1860
Cry2aa7 AJ132463 Yu & Pang, 1999 Unpublished <1->1611
Cry2ab1 M23724 Widner & Whiteley, 1989 J Bact 171 965-974 1-1899
Cry2ab2 X55416 Dankocsik et al., 1990 Mol Micro 4 2087-2094 874-2775
Cry2ac1 X57252 Wu et al., 1991 FEMS 81 31-36 2125-3990
Cry3aa1 M22472 Herrnstadt et al., 1987 Gene 57 37-46 25-1956
Cry3aa2 J02978 Sekar et al., 1987 PNAS 84 7036-7040 241-2175
Cry3aa3 Y00420 Hofte et al., 1987 NAR 15 7183-7183 566-2497
Cry3aa4 M30503 McPherson et al., 1988 Bio/technology 6 61-66 201-2135
Cry3aa5 M37207 Donovan et al., 1988 MGG 214 365-372 569-2503
Cry3aa6 U10985 Adams et al., 1994 Mol Micro 14 381-389 569-2503
Cry3ba1 X17123 Sick et al., 1990 NAR 18 1305-1305 25-1977
Cry3ba2 A07234 Peferoen et al., 1990 EP 0382990 342-2297
Cry3bb1 M89794 Donovan et al., 1992 AEM 58 3921-3927 202-2157
Cry3bb2 U31633 Donovan et al., 1995 USP 5378625 144-2099
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry3bb3 I15475 Peferoen et al., 1995 USP 5466597 <1->1291
Cry3ca1 X59797 Lambert et al., 1992 Gene 110 131-132 232-2178
Cry4aa1 Y00423 Ward & Ellar, 1987 NAR 15 7195-7195 1-3540
Cry4aa2 D00248 Sen et al., 1988 ABC 52 873-878 393-3935
Cry4ba1 X07423 Chungjatpornchai et al., 1988 EJB 173 9-16 157-3564
Cry4ba2 X07082 Tungpradubkul et al., 1988 NAR 16 1637-1638 151-3558
Cry4ba3 M20242 Yamamoto et al., 1988 Gene 66 107-120 526-3933
Cry4ba4 D00247 Sen et al., 1988 ABC 52 873-878 461-3868
Cry5aa1 L07025 Sick et al., 1994 USP 5281530 1-4155
Cry5ab1 L07026 Narva et al., 1991 EP 0462721 1-3867
Cry5ac1 I34543 Payne et al., 1997 USP 5596071 1-3660
Cry5ba1 U19725 Payne et al., 1997 USP 5596071 1-3735
Cry6aa1 L07022 Narva et al., 1993 USP 5236843 1-1425
Cry6ba1 L07024 Narva et al., 1991 EP 0462721 1-1185
Cry7aa1 M64478 Lambert et al., 1992 AEM 58 2536-2542 184-3597
Cry7ab1 U04367 Payne & Fu, 1994 USP 5286486 1-3414
Cry7ab2 U04368 Payne & Fu, 1994 USP 5286486 1-3414
Cry8aa1 U04364 Foncerrada et al., 1992 EP 0498537 1-3471
Cry8ba1 U04365 Michaels et al., 1993 WO 93/15206 1-3507
Cry8ca1 U04366 Ogiwara et al., 1995 Curr Micro 30 227-235 1-3447
Cry9aa1 X58120 Smulevitch et al., 1991 FEBS 293 25-28 5807-9274
Cry9aa2 X58534 Gleave et al., 1992 JGM 138 55-62 385->3837
Cry9ba1 X75019 Shevelev et al., 1993 FEBS 336 79-82 26-3488
Cry9ca1 Z37527 Lambert et al., 1996 AEM 62 80-86 2096-5569
Cry9da1 D85560 Asano et al., 1997 AEM 63 1054-1057 47-3553
Cry9Da2 AF042733 Wasano & Ohba, 1998 Unpublished <1->1937
Cry9Ea1 AB011496 Midoh & Oyama, 1998 Unpublished 211-3660
Cry9 like AF093107 Wasano & Ohba, 1998 See Table 3 <1->1917
Cry10Aa1 M12662 Thorne et al., 1986 J Bact 166 801-811 941-2965
Cry10Aa2 E00614 Uorufuiirudo, 1996 JP 1986005098 940-2968
Cry11Aa1 M31737 Donovan et al., 1988 J Bact 170 4732-4738 41-1969
Cry11Aa2 M22860 Adams et al., 1989 J Bact 171 521-530 <1-235
Cry11Ba1 X86902 Delecluse, 1995 AEM 61 4230-4235 64-2238
Cry11Bb1 AF017416 Orduz et al., 1998 BBA 1388 267-272 97-2346
Cry12Aa1 L07027 Narva et al., 1991 EP 0462721 1->3771
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry13Aa1 L07023 Narva et al., 1992 WO 92/19739 1-2409
Cry14Aa1 U13955 Narva et al., 1994 WO 94/16079 1-3558
Cry15Aa1 M76442 Brown & Whiteley, 1992 J Bact 174 549-557 1036-2055
Cry16Aa1 X94146 Barloy et al., 1996 J Bact 178 3099-3105 158-1996
Cry17Aa1 X99478 Barloy et al., 1998 Gene 211 293-299 12-1865
Cry18Aa1 X99049 Zhang et al., 1997 J Bact 179 4336-4341 1451-3571
Cry19Aa1 Y07603 Rosso & Delecluse, 1996 AEM 63 4449-4455 719-2662
Cry19Ba1 D88381 Hwang et al., 1998 SAB 21 179-184 626-2671
Cry20Aa1 U82518 Lee & Gill, 1997 AEM 63 4664-4670 60-2318
Cry21Aa1 I32932 Payne et al., 1996 USP 5589382 1-3501
Cry21Aa2 I66477 Feitelson, 1997 USP 5670365 1-3501
Cry22Aa1 I34547 Payne et al., 1997 USP 5596071 1-2169
Cry23Aa1 AF03048 Donovan & Slaney, 1998 WO 98/13498
Cry24Aa1 U88188 Kawalek & Gill, 1998 Unpublished 1-2022
Cry25Aa1 U88189 Kawalek & Gill, 1998 Unpublished 1-2028
Cyt1Aa1 X03182 Waalwijk et al., 1985 NAR 13 8207-8217 140-886
Cyt1Aa2 X04338 Ward & Ellar, 1986 JMB 191 1-11 509-1255
Cyt1Aa3 Y00135 Earp & Ellar, 1987 NAR 15 3619-3619 36-782
Cyt1Aa4 M35968 Galjart et al., 1987 Curr Micro 16 171-177 67-816
Cyt1Ab1 X98793 Thiery et al., 1997 AEM 63 468-473 28-777
Cyt1Ba1 U37196 Payne et al., 1995 USP 5436002 1-795
Cyt2Aa1 Z14147 Koni & Ellar, 1993 JMB 229 319-327 270-1046
Cyt2Ba1 U52043 Guerchicoff et al., 1997 AEM 63 2716-2721 287-655
Cyt2Ba2 AF020789 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba3 AF022884 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba4 AF022885 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba5 AF022886 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->471
Cyt2Ba6 AF034926 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->472
Cyt2Bb1 U82519 Cheong & Gill, 1997 AEM 63 3254-3260 416-1204
spectra observed in Bt (González & Carlton, 1980; González et al.,
1981; González et al., 1982; Reddy et al., 1987; Jarrett & Stephenson,
1990). New Bt strains have been developed by conjugation that are
toxic to two insect orders.
2.1.6 Beta-exotoxin
Beta-exotoxin is associated with certain Bt subspecies (Btd, Btg,
Btte and Btt), and products made from these Bt subspecies may contain
the toxin (Cantwell et al., 1964; Mohd-Salleh et al., 1980). This
heat-stable nucleotide, which is composed of adenine, glucose and
allaric acid, inhibits RNA polymerase enzymes by acting competitively
with ATP (Faust, 1973; Farkas et al., 1977). Since RNA synthesis is a
vital process in all life, beta-exotoxin exerts its toxicity for
almost all forms of life tested including numerous insect species in
the orders Coleoptera, Diptera and Lepidoptera. The presence of
beta-exotoxin can be assayed using houseflies (Musca domestica) or
high-performance liquid chromatography (HPLC) techniques (Campbell et
al., 1987).
Bt containing beta-exotoxin is used for the control of houseflies
in some countries, but regulatory agencies currently prohibit the use
of beta-exotoxin for other purposes.
2.1.7 Other Bt metabolites
Commercial Bt products do not contain metabolites that are
considered hazardous to humans and the environment. However, Bt, like
other bacteria, may produce during the vegetative growth and
sporulation stages an assortment of antibiotics, enzymes, metabolites
and toxins that are biologically active and may have effects on both
target and non-target organisms (NTOs).
Using a non-quantitative (Lund & Granum, 1997) commercial Bc
enterotoxin immunoassay (Tecra), Damgaard (1995) reported that
vegetative cells grown from spores of commercial Bt products excreted
a diarrhoeal enterotoxin. Damgaard et al. (1996a) found by Vero cell
assay that Bt isolated from food was enterotoxigenic. None of these
investigations estimated the quantity or activity of enterotoxins
produced by the Bt strains. However, Shinagawa (1990) investigated a
number of Bc and Bt isolates with an immunological assay and concluded
that 43% of the Bt isolates had the same level of enterotoxins as
enterotoxic Bc. Tayabali & Seligy (1997) found that vegetative Bt
obtained from commercial products caused extensive damage to
cultivated insect cells.
At least three enterotoxic activities have been described in Bc
(Agata et al., 1995; Lund & Granum, 1997), and some Bc isolates are
known to produce an emetic toxin (Andersson et al., 1998). The emetic
toxin is primarily associated with the Bc H-1 (Kramer & Gilbert, 1992;
Nishikawa et al., 1996) serotype, and has not so far been associated
with Bt isolates.
Table 3. Bt-associated toxins or toxin-like proteins that have not been assigned a name or entered
into the nomenclature for the reasons given
Name Accession Journal Coding region Reason Reference
Cry1i-like I90732 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry1-like I90729 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry9-like AF093107 unpublished <1->1917 Insufficient sequence data Wasano & Ohba, 1998
40kda M76442 J Bact 174 549-557 45-971 No reported toxicity Brown & Whiteley, 1992
Cryc35 X92691 unpublished 1-981 No reported toxicity Juarez-Perez et al., 1995
Crytdk D86346 unpublished 177-2645 No reported toxicity Hashimoto, 1996
Cryc53 X98616 unpublished 1-1005 No reported toxicity Juarez-Perez et al., 1996
p21med X98794 AEM 63 468-473 1-552 No reported toxicity Thiery et al., 1997
ET34 AF038049 WO 98/13498 No reported toxicity Donovan & Slaney, 1998
Vip3a(a) L48811 PNAS 93 5389-5394 739-3105 Not a crystal protein Estruch et al., 1996
Vip3a(b) L48812 PNAS 93 5389-5394 118-2484 Not a crystal protein Estruch et al., 1996
Table 4. Bacillus thuringiensis holotype toxins
Name Old name Name Old name
Cry1Aa CryIA(a) Cry5Ac
Cry1Ab CryIA(b) Cry5Ba
Cry1Ac CryIA(c) Cry6Aa CryVIA
Cry1Ad CryIA(d) Cry6Ba CryVIB
Cry1Ae CryIA(e) Cry7Aa CryIIIC
Cry1Af Cry7Ab CryIIICb
Cry1Ag Cry8Aa CryIIIE
Cry1Ba CryIB Cry8Ba CryIIIG
Cry1Bb ET5 Cry8Ca CryIIIF
Cry1Bc PEG5 Cry9Aa CryIG
Cry1Bd CryE1 Cry9Ba CryIX
Cry1Be Cry9Ca CryIH
Cry1Ca CryIC Cry9Da
Cry1Cb CryIC(b) Cry9Ea
Cry1Da CryID Cry10Aa CryIVC
Cry1Db PrtB Cry11Aa CryIVD
Cry1Ea CryIE Cry11Ba Jeg80
Cry1Eb CryIE(b) Cry11Bb
Cry1Fa CryIF Cry12Aa CryVB
Cry1Fb PrtD Cry13Aa CryVC
Cry1Ga PrtA Cry14Aa CryVD
Cry1Gb CryH2 Cry15Aa 34kDa
Cry1Ha PrtC Cry16Aa cbm71
Cry1Hb Cry17Aa cbm72
Cry1Ia CryV Cry18Aa CryBP1
Cry1Ib CryV Cry19Aa Jeg65
Cry1Ic Cry19Ba
Cry1Ja ET4 Cry20Aa
Cry1Jb ET1 Cry21Aa
Cry1Jc Cry22Aa
Cry1Ka Cry23Aa
Cry2Aa CryIIA Cry24Aa Jeg72
Cry2Ab CryIIB Cry25Aa Jeg74
Cry2Ac CryIIC Cry26Aa
Cry3Aa CryIIIA Cry27Aa
Cry3Ba CryIIIB Cry28Aa
Cry3Bb CryIIIBb Cyt1Aa CytA
Cry3Ca CryIIID Cyt1Ab CytM
Cry4Aa CryIVA Cyt1Ba
Cry4Ba CryIVB Cyt2Aa CytB
Cry5Aa CryVA(a) Cyt2Ba "CytB"
Cry5Ab CryVA(b) Cyt2Bb
Alpha-toxin is a phospholipase C, which primarily affects the
cell membrane phospholipids (Heimpel, 1954; Bonnefoi & Béguin, 1959).
Gamma-toxin is toxic to sawflies (Tenthredinidae), but the mode of
action of this heat-labile toxin has not been determined (Heimpel,
1967).
The so called "water-soluble toxin" paralyses Lepidoptera
(Fast, 1971), and the "mouse factor exotoxin" is toxic to mice as
well as to Lepidoptera (Krieg, 1971). The modes of action of these
toxins have not been delineated.
A novel Bt vegetative insecticidal protein (Vip3A) has been
identified from the culture media of some Bt strains (Estruch et al.,
1996).
Several Bt and Bc enzymes have been described which may play a
role in non-target activity: phospholipase (Damgaard et al., 1996b),
sphingomyelinase (Gilmore et al., 1989), protease (Hotha & Banik,
1997), chitinase (Sampson & Gooday, 1998), and haemolysin (Baida &
Kuzmin, 1995).
2.2 Bioassays
2.2.1 Spore counts
Bacterial spore counts do not necessarily reflect the
insecticidal activity of a Bt strain or Bt product because the number
and amount of ICPs produced per bacterial cell can vary.
2.2.2 International bioassay for ICPs
The final formulation of each Bt product is bioassayed against an
accepted international standard using a specific test insect (Dulmage
et al., 1981; de Barjac & Larget-Thiery, 1984). Its potency is defined
in ITU/mg product. The standardization allows comparison of different
formulations in the laboratory. Currently, the larvicidal activity is
expressed in terms of lethal doses (LD50) or lethal concentrations
(e.g., LC50, LC90) according to the bioassay method used. For
example, when susceptible mosquito larvae are exposed to Bti ICP, they
have an LC50 of approximately 10 ng/ml water. A Bti whole culture
gives an LC50 of approximately 103 cells/ml for susceptible mosquito
larvae while a 109 cells/ml culture does not affect any laboratory
mammals exposed by various routes.
3. MODE OF ACTION ON TARGET INSECTS
3.1 Bioactivity of field isolates
The mode of action of Bt has been reviewed by Schnepf et al.
(1998) and can be summarized in the following stages: 1) ingestion of
sporulated Bt and ICP by an insect larva; 2) solubilization of the
crystalline ICP in the midgut; 3) activation of the ICP by proteases;
4) binding of the activated ICP to specific receptors in the midgut
cell membrane; 5) insertion of the toxin in the cell membrane and
formation of pores and channels in the gut cell membrane, followed by
destruction of the epithelial cells (Cooksey, 1971; Norris 1971; Fast,
1981; Huber & Lüthy, 1981; Lüthy & Ebersold, 1981; Smedley & Ellar,
1996); and 6) subsequent Bt spore germination and septicaemia may
enhance mortality (Fig. 1).
The specific bioactivity of Bt is dominated by the ICPs that are
encoded by the cry genes and are active against susceptible species
in the insect orders Coleoptera, Diptera and Lepidoptera.
Specific Bt activities against other insect orders (Hymenoptera,
Homoptera, Dictyoptera, Mallophaga) and to nematodes
(Strongylida, Tylenchida), mites (Acari), flatworms (Digenea)
and protozoa (Diplomonadida) have been described (Feitelson, 1993;
Zukowski, 1995). The ICP must be ingested to be effective against the
target (Visser et al., 1993).
3.2 Mechanism of action of Bt formulations
The ICP-spore complexes of Bt are ingested by susceptible insect
larvae. In the midgut the parasporal crystalline ICP is dissociated to
the protoxin form, and the protoxin is then activated to a holotoxin
by gut proteases (Warren et al., 1984; Jaquet et al., 1987; Aronson et
al., 1991; Honée & Visser, 1993). Shortly afterwards, the gut becomes
paralysed and the larva ceases to feed.
The ICP structure and function have been reviewed in detail by
Schnepf et al. (1998). Binding of the ICP to putative receptors is a
major determinant of ICP specificity and the formation of pores in the
midgut epithelial cells is a major mechanism of toxicity (Van
Frankenhuyzen, 1993).
The active toxin consists of three distinct domains (Höfte &
Whiteley, 1989; Li et al., 1991; Grochulski et al., 1995). The three
domains interact in a complex manner, but experimental data suggest
that the C-terminal and middle domains of the toxin are involved in
epithelial cell receptor binding and structural functions, while the
N-terminal domain is primarily involved in ion channel and pore
formation (Huber et al., 1981; Schnepf et al., 1998; Dean et al.,
1996).
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS
THURINGIENSIS
Members
Professor D. Calamari, Department of Biology, Structure and Function,
University of Milan, Varese, Italy
Dr C. Cummins, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr N. Gratz, Commugny, Switzerland (Rapporteur)
Dr A. Klier, Unité de Biochimie Microbienne, Département des
Biotechnologies, Institut Pasteur, Paris, France
Professor P. Lüthy, Microbiological Institute ETH, Zurich, Switzerland
Dr C.M. Scanlan, Texas A & M University, Department of Veterinary
Pathobiology, Texas Veterinary Medical Center, Texas, USA (Chairman)
Observers
Dr R.J. Cibulsky, Abbott Laboratories, Chemical and Agricultural
Products, North Chicago, Illinois, USA
Dr E. Cozzi, Clinical Research and Development, Animal Health
Products, Abbott Laboratories, North Chicago, Illinois, USA
Dr M. Ronchin, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Secretariat
Professor M. Maroni, International Centre for Pesticide Safety, Busto
Garolfo, Milan, Italy
Dr R. Plestina, Zagreb, Croatia
ENVIRONMENTAL HEALTH CRITERIA FOR BACILLUS THURINGIENSIS
A WHO Task Group on Environmental Health Criteria for the
microbial pest control agent Bacillus thuringiensis (Bt) met at the
International Centre for Pesticide Safety in Busto Garolfo, Milan,
Italy, from 27 to 31 October 1997. Professor M. Maroni, Director of
the Centre, welcomed participants on behalf of the Centre, which was
responsible for organizing the meeting. Dr R. Plestina, IPCS temporary
adviser, opened the meeting and welcomed participants on behalf of Dr
M. Mercier, Director of IPCS. The Group reviewed and revised the draft
and made an evaluation of the risks for human health and the
environment from exposure to Bt products. Drs R. Plestina and A. Aitio
of the IPCS Central Unit were responsible for the scientific aspects
of the monograph, and Dr P.G. Jenkins for the editing. The assistance
of manufacturers, notably Abbott Laboratories, in providing
unpublished documentation for the review is greatly appreciated.
Microbial Pest Control Agents (MPCAs), notably products of
various Bt subspecies, are increasingly used in pest management
programmes against the larvae of several insect pests of major
agricultural crops and forests, and several insect vectors of human
diseases, and some nuisance pests. Bt products have been used
worldwide, and their commercial production is about 1% of that of
chemical pesticides. A number of reviews have recently been published
on various aspects of Bt (Entwistle et al., 1983; McClintock et al.,
1995; Cannon, 1996; Dean et al., 1996; Kumar et al., 1996; Schnepf et
al., 1998; Nielsen-LeRoux et al., 1998).
The activities in preparing this document were recommended by an
Informal Consultation on the Safety of Microbial Pest Control Agents
held in Geneva in June 1993. The first draft of the monograph was
prepared by a drafting group in 1994 and was subsequently amended by
Professor C.M. Scanlan (Texas A&M University, Department of Veterinary
Pathobiology, The Texas Veterinary Medical Center, Texas, USA). The
revised draft was circulated to the IPCS contact points. Based on the
comments received, it was amended and updated by Drs B.M. Hansen and
N.B. Hendriksen (National Environmental Research Institute, Roskilde,
Denmark). The document was finally approved by the Task Group members.
ABBREVIATIONS
Bc Bacillus cereus
Bt Bacillus thuringiensis
Bta Bacillus thuringiensis subspecies aizawai
Btd Bacillus thuringiensis subspecies darmstadiensis
Bte Bacillus thuringiensis subspecies entomocidus
Btg Bacillus thuringiensis subspecies galleriae
Bti Bacillus thuringiensis subspecies israelensis
Btk Bacillus thuringiensis subspecies kurstaki
Btko Bacillus thuringiensis subspecies konkukian
Btt Bacillus thuringiensis subspecies thuringiensis
Btte Bacillus thuringiensis subspecies tenebrionis
cfu colony forming unit
GILSP good industrial large-scale practice
GMO genetically modified organism
HPLC high-performance liquid chromatography
ICP insecticidal crystal protein
ITU international toxic unit
IUPAC International Union of Pure and Applied Chemistry
MPCA microbial pest control agent
NTO non-target organism
PCR polymerase chain reaction
SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
1. SUMMARY
This monograph deals with microbial pest control agents (MCPAs)
based on Bacillus thuringiensis (Bt). This bacterium is also a key
source of genes for transgenic expression to provide pest resistance
in plants and microorganisms as pest control agents in so-called
genetically modified organisms (GMOs). The potential effects on human
health and the environment of GMOs involve several aspects that are
only remotely or not at all related to Bt products, and they are
therefore outside the scope of this monograph.
1.1 Identity, biological characteristics and analytical methods
Bt is a facultative anaerobic, gram-positive bacterium that forms
characteristic protein inclusions adjacent to the endospore. Bt
subspecies can synthesize more than one parasporal inclusion. Bt is
genetically indistinguishable from Bc, except for the ability of Bt to
produce parasporal crystalline inclusions, which are toxic for certain
invertebrates, especially species of insect larvae belonging to the
insect orders Coleoptera, Diptera and Lepidoptera. The
parasporal inclusions are formed by different insecticidal crystal
proteins (ICP). The crystals have various shapes (bipyramidal,
cuboidal, flat rhomboid, spherical or composite with two crystal
types), depending on their ICP composition. A partial correlation
between crystal morphology, ICP composition, and bioactivity against
target insects has been established.
The basic phenotypic taxon is the subspecies, identified by the
flagellar (H) serotype. By 1998, 67 subspecies had been described. The
genes that encode the ICPs are mostly on plasmids. Each ICP is the
product of a single gene. Most plasmids with ICP genes are readily
transferred by conjugation between Bt strains and may be transferred
to related species of bacteria. The phenotypic classification has now
been complemented by molecular biological characterization, based on
the sequence of the crystal (cry and cyt) genes rather than target
organism specificity. Different domains of the ICP are responsible for
host susceptibility (receptor recognition) and toxicity (pore
formation).
Techniques commonly used to characterize Bt strains or the ICP
itself include cell wall fatty acid analysis, monoclonal antibodies,
oligonucleotide DNA probes, plasmid profiles, polymerase chain
reaction (PCR) analysis, DNA fingerprinting and SDS-PAGE profiles.
Beta-exotoxin, a heat-stable nucleotide, is produced by some Bt
subspecies during vegetative growth and may contaminate the products.
Beta-exotoxin is toxic for almost all forms of life including humans
and the target insect orders. During vegetative growth, various Bt
strains produce an assortment of antibiotics, enzymes, metabolites and
toxins, including Bc toxins, that may have detrimental effects on both
target organisms and non-target organisms (NTOs). Spore counts do not
accurately reflect the insecticidal activity of a Bt strain or Bt
product. The potency (ITU/mg) of each Bt product is bioassayed using
an international standard that uses a specific test insect.
1.2 Mode of action on target insects
The sporulated Bt with ICP or spore-ICP complexes must be
ingested by a susceptible insect larva. The efficacy of the ICP
depends on the solubilization in the midgut, the conversion of the
protoxin to the biologically active toxin by proteolytic enzymes,
specific membrane receptor binding by the C-terminal domain of the
active toxin, and pore formation by the N-terminal domain with
subsequent lysis of the epithelial cells. Spore germination and
proliferation of the vegetative cells into the haemocoel may result in
a septicaemia, contributing to the cause of death. Receptor binding by
the ICP is the major determinant of host specificity by the different
Bt ICPs.
1.3 Habitats
Many different Bt subspecies have been isolated from dead or
dying insects mostly from the orders Coleoptera, Diptera and
Lepidoptera, but many subspecies have also been isolated from soil,
leaf surfaces and other habitats. The carcasses of dead insects often
contain large quantities of spores and ICPs that may enter the
environment. The coleopteran-active and lepidopteran-active Bt
subspecies are primarily associated with the soil and phylloplane
(leaf surfaces), whereas the dipteran-active Bt subspecies are
commonly found in aquatic environments. In the environment, the spores
persist and vegetative growth may occur when conditions are favourable
and nutrients are available.
1.4 Commercial products, production and application
Conventional Bt products, which utilize naturally-occurring Bt
strains, account for approximately 90% of the world MPCA market. Most
Bt products contain ICP and viable spores, but in some Bti products
the spores are inactivated. Each year some 13 000 tonnes are produced
using aerobic fermentation technology. Conventional Bt products have
been targeted primarily against lepidopteran pests of agricultural and
forestry crops; however in recent years, Bt strains active against
coleopteran pests have also been marketed. Strains of Bti active
against dipteran vectors of parasitic and viral diseases are being
used in public health programmes.
Commercial Bt formulations may be applied as an insecticide to
foliage, soil, water environments or food storage facilities. After
the application of a Bt subspecies to an ecosystem, the vegetative
cells and spores may persist at gradually decreasing concentrations
for weeks, months or years as a component of the natural microflora.
The ICPs, however, are rendered biologically inactive within hours or
days.
1.5 Effects of Bt on non-target organisms
Studies on mammals, particularly those on laboratory animals,
have evaluated possible infectivity and toxicity of various Bt
preparations, which include the ICPs, vegetative cells and spores. The
ICPs, spores and vegetative cells of the Bt subspecies, which were
administered by different routes, were mostly non-pathogenic and
non-toxic to the various animal species tested. The vegetative cells
and/or spores of Bt were demonstrated to persist for weeks without
causing adverse effects. Bt has not been observed to adversely affect
birds, fish or many other non-target aquatic vertebrates tested in a
large number of laboratory and field studies. Relatively few species
of aquatic invertebrates are susceptible to Bt under either laboratory
or field conditions. Bt does not adversely affect earthworms.
The Bt subspecies have generally been shown to be highly specific
in their insecticidal activity for Coleoptera, Diptera and
Lepidoptera and have demonstrated little, if any, direct toxicity to
non-target arthropods. Most of the existing safety data on non-target
arthropods has been generated using the Bt subspecies with activity
against Diptera and Lepidoptera.
Studies of Bti formulations free of toxic contaminants have not
demonstrated deleterious effects on the vast majority of non-target
arthropods. Some midges (Diptera: Chironomidae), which are closely
related to mosquitos, have been shown to be susceptible to high
dosages of Bti, but are not affected by mosquito larvicidal dosages.
In field studies, transient decreases or increases in populations of
some non-target arthropods have been reported.
Many insect orders have been tested in either the laboratory or
field, most of which have shown no effect from Btk.
Mortality has been observed in honey-bees (Apis mellifera)
after exposure to vegetatively growing Btt and Btk, but the effect
does not seem to be related to spores or ICPs. In laboratory and field
studies Btg demonstrated no adverse effect on honey-bees.
Bte strains that produce beta-exotoxin have been shown to have
adverse effects on non-target arthropods.
1.6 Exposure and effects of Bt on humans
The field application of Bt products can result in considerable
aerosol and dermal exposure of workers. Agricultural uses of Bt can
result in Bt contamination of potable water and food. With the
exception of case reports on ocular and dermal irritation, no adverse
health effects have been documented after occupational exposure to Bt
products. Human volunteers ingested and inhaled large quantities of a
Btk formulation but experienced no adverse health effects. Antibody
titres to the vegetative cells, spores and spore-crystal complexes
have been demonstrated in workers who spray Bt products; however, no
adverse health effects were reported. There have been some case
reports on the occurrence of Bt in patients with different infectious
diseases. However, none of these studies unequivocally demonstrates an
actual risk to human health from the use of Bt. Bt has not been
reported to cause adverse effects on human health when present in
drinking-water or food.
1.7 Conclusions
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and biologically active products other than the
ICPs. Bt products may be safely used for the control of insect pests
of agricultural and horticultural crops as well as forests. They are
also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt has the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
2. IDENTITY, BIOLOGICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Commercial Bacillus thuringiensis (Bt) products are microbial
pest control agents (MPCAs) containing specific insecticidal
crystalline proteins (ICPs) and most often living spores as well as
formulating agents. They are processed fermentation products.
2.1.1 Bacillus thuringiensis
Bt is a facultative anaerobic, motile, gram-positive,
spore-forming bacterium. The formation of parasporal crystals adjacent
to the endospore during sporulation stages III to IV distinguishes Bt
from other Bacillus species.
Bt, like other Bacillus species, has been classified on the
basis of its cellular, cultural, biochemical and genetic
characteristics (Baumann et al., 1984; Claus & Berkley, 1986; Slepecky
& Hemphill, 1992; Carlson & Kolsto, 1993; Hansen et al., 1998). In
1958, Heimpel & Angus (1958) introduced a classification scheme to
identify these crystalliferous bacteria based on their morphological
and biochemical characteristics. However recent molecular analysis
shows that several variations can be found within serotypes, and that
specific biochemical characteristics do not always refer to a specific
serotype (Helgason et al., 1998; Hansen et al., 1998).
2.1.2 Relationship between Bacillus thuringiensis and Bacillus cereus
Bt is a member of the Bc group, which also contains Bacillus
cereus (Bc), B. mycoides and B. anthracis. Furthermore, the
psychrotolerant B. weihenstephanensis has recently been proposed as
a new member of the group (Lechner et al., 1998). Bt can only be
distinguished from Bc by the production during the sporulation process
of one or more inclusion bodies, which have been found to be toxic for
invertebrates, primarily insect species in the orders Coleoptera,
Diptera and Lepidoptera (de Barjac, 1981b; Andrews et al., 1987).
Several studies have been dedicated to a comparison of Bt and Bc on
the basis of characters not related to the production of ICPs
(Hendriksen & Hansen, 1998). Phenotypic differentiation of Bt and Bc
is not possible on the basis of morphology or utilization of organic
compounds (Baumann et al., 1984; Logan & Berkeley, 1984; Priest et
al., 1988), characterization of cell content of fatty acids (Väisänen
et al., 1991) or sugars (Wunschel et al., 1994), multilocus enzyme
electrophoresis (Zahner et al., 1989; Carlson et al., 1994),
enterotoxin production (Damgaard et al., 1996a; Hansen & Hendriksen,
1997a), or serological- and phage-typing techniques (Ohba & Aizawa,
1978; 1986; Väisänen et al., 1991; Murakami et al., 1993; Ahmed et
al., 1995). Likewise, genotypic differentiation of Bt and Bc is not
possible by DNA homology analysis (Kaneko et al., 1978), ribotyping
(Priest et al., 1994; Demezas & Bell, 1995), 16S rDNA sequencing (Ash
et al., 1991); analysis of the 16S-23S internal transcribed sequence
(Wunschel et al., 1994; Bourque et al., 1995), PCR analysis of genes
encoding Bc-like toxic products (Damgaard et al., 1996b; Asano et al.,
1997; Hansen & Hendriksen, 1997b) or pulsed field gel electrophoresis
(Carlson & Kolsto, 1993; Carlson et al., 1994). Giffel et al. (1997)
found differences in 16S rDNA sequences between a limited number Bt
and Bc. Beattie et al. (1998) were able to discriminate among members
of the Bc group by Fourier transform infrared spectroscopy, and
Brousseau et al. (1992) were able to distinguish Bt and Bc by random
amplified polymorphic DNA fingerprinting. However, the transfer of ICP
encoding plasmids from Bt to Bc makes the receptor Bc
indistinguishable from Bt, and vice versa (González et al., 1981,
1982).
2.1.3 Crystal composition and morphology
The existence of parasporal inclusions in Bt was first noted in
1915 (Berliner, 1915), but their protein composition was not
delineated until the 1950s (Angus, 1954). Hannay (1953) detected the
crystalline fine structure that is a property of most of the
parasporal inclusions. Bt subspecies can synthesize more than one
inclusion, which may contain different ICPs. ICPs have also been
called delta endotoxins; however, since the term endotoxin usually
refers to toxins associated with the outer membranes of gram-negative
bacteria, comprising a core lipopoplysaccharide, lipid A and somatic
(O) antigens, this term is not used in this monograph. Depending on
their ICP composition, the crystals have various forms (bipyramidal,
cuboidal, flat rhomboid, or a composite with two or more crystal
types). A partial correlation between crystal morphology, ICP
composition, and bioactivity against target insects has been
established (Bulla et al., 1977; Höfte & Whiteley, 1989; Lynch &
Baumann, 1985).
2.1.4 Classification of Bt subspecies
The classification of Bt subspecies based on the serological
analysis of the flagella (H) antigens was introduced in the early
1960s (de Barjac & Bonnefoi, 1962). This classification by serotype
has been supplemented by morphological and biochemical criteria (de
Barjac, 1981a). Until 1977, only 13 Bt subspecies had been described,
and at that time all subspecies were toxic to Lepidopteran larvae
only. The discovery of other subspecies toxic to Diptera (Goldberg &
Margalit, 1977), Coleoptera (Krieg et al., 1983) and apparently
Nematoda (Narva et al., 1991) enlarged the host range and markedly
increased the number of subspecies. Up to the end of 1998, over 67
subspecies based on flagellar H-serovars had been identified (Table
1). Updated lists of the serovarieties can be obtained from the
reference centre of the Pasteur Institute in Paris (Unité des
Bactéries Entomopathogènes, Institut Pasteur, Paris, France).
Table 1. Current classification of 67 Bacillus thuringiensis subspecies based
on their flagellar (H) antigensa
Flagellar antigens B. thuringiensis Flagellar antigens B. thuringiensis
subspecies subspecies
1 thuringiensis 28a, 28c jegathesan
2 finitimus 29 amagiensis
3a, 3c alesti 30 medellin
3a, 3b, 3c kurstaki 31 toguchini
3a, 3d sumiyoshiensis 32 cameroun
3a, 3d, 3e fukuokaensis 33 leesis
4a, 4b sotto 34 konkukian
4a, 4c kenyae 35 seoulensis
5a, 5c galleriae 36 malaysiensis
5a, 5c canadensis 37 anadalousiensis
6 entomocidus 38 oswaldocruzi
7 aizawai 39 brasiliensis
8a, 8b morrisoni 40 huazhongensis
8a, 8c ostriniae 41 sooncheon
8b, 8d nigeriensis 42 jinghongiensis
9 tolworthi 43 guiyanguebsus
10a, 10b darmstadiensis 44 higo
10a, 10c londrina 45 roskildiensis
11a, 11b toumanoffi 46 chanpaisis
11a, 11c kyushuensis 47 wratislaviensis
12 thompsoni 48 balearica
13 pakistani 49 muju
14 israelensis 50 navarrensis
15 dakota 51 xiaguangiensis
16 indiana 52 kim
17 tohokuensis 53 asturiensis
18a, 18b kumamotoensis 54 poloniensis
18a, 18c yosoo 55 palmanyolensis
19 tochigiensis 56 rongseni
20a, 20b yunnanensis 57 pirenaica
20a, 20c pondicheriensis 58 argentinensis
21 colmeri 59 iberica
22 shandongiensis 60 pingluonsis
23 japonensis 61 sylvestriensis
24a, 24b neoleonensis 62 zhaodongensis
24a, 24c novosibirsk 63 bolivia
25 coreanensis 64 azorensis
26 silo 65 pulsiensis
27 mexicanensis 66 gracioensis
28a, 28b monterrey 67 vazensis
a Data provided by the Unité des Bactéries Entomopathogènes, Institut Pasteur,
Paris, France
Crystal serology has shown that a particular crystal type may be
produced by more than one H-serovar (Krywienczyk et al., 1978; Smith,
1987).
2.1.5 Genetics of ICP
In the early 1980s, it was established that most genes coding for
the ICPs reside on large transmissible plasmids, of which most are
readily exchanged between strains by conjugation (González & Carlton,
1980; González et al., 1981). Since these initial studies, numerous
ICP genes have been cloned, sequenced and used to construct Bt strains
with novel insecticidal spectra (Höfte & Whiteley, 1989).
The currently known crystal (cry) gene types encode ICPs that
are specific to either Lepidoptera (cryI), Diptera and
Lepidoptera (cryII), Coleoptera (cryIII), Diptera (cryIV),
or Coleoptera and Lepidoptera (cryV) (Höfte & Whiteley, 1989).
A separate designation is used for the cytolytic (cyt) genes that
encode a nonspecific cytolytic factor, present in Bti ICP and some
other Bt subspecies. However, due to the increasing number of
characterized ICP genes and inconsistencies in the existing cry gene
nomenclature, which is based on insecticidal spectrum, Crickmore et
al. (1998) proposed a new nomenclature based on ICP gene sequences.
The new cry genes are listed in Tables 2 and 3, and Table 4 is a
conversion list from old to new cry gene names. Current
identification of Bt employs both the identity of the cry genes,
which define the host range, and the H-serovars, which define the
subspecies, and recently DNA fingerprinting has been used for further
characterization of subspecies (Hansen et al., 1998).
The ICP gene sequences provided the basis for the construction of
gene-specific probes to screen established Bt strains by hybridization
and PCR analysis for the presence of known nucleotide sequences, and
for characterizing the ICPs from new Bt isolates (Prefontaine et al.,
1987; Juarez-Perez et al., 1997; Bravo et al., 1998; Shevelev et al.,
1998). These studies have permitted the distinction of numerous
subclasses of ICP genes based on sequence homology and toxicity
spectra of the encoded proteins.
All ICPs described to date attack the insect gut upon ingestion
(see chapter 3). To date, each of the proteolytically activated ICP
molecules with insecticidal activity has a variable C-terminal domain,
which is responsible for receptor recognition (host susceptibility),
and a conserved N-terminal domain, which induces pore formation
(toxicity) (Li et al., 1991).
Most naturally occurring Bt strains contain ICPs active against a
single order of insects. However, conjugative transfer between Bt
strains or related species can occur, resulting in new strains with
various plasmid contents. Thus the mobility of the cry genes and the
exchange of plasmids may explain the diverse and complex activity
Table 2. Bacillus thuringiensis crystal protein genes (Crickmore et al., 1998)
Name Acc No Reference Journal Coding
Cry1aa1 M11250 Schnepf et al., 1985 JBC 260 6264-6272 527-4054
Cry1aa2 M10917 Shibano et al., 1985 Gene 34 243-251 153->2955
Cry1aa3 D00348 Shimizu et al., 1988 ABC 52 1565-1573 73-3603
Cry1aa4 X13535 Masson et al., 1989 NAR 17 446-446 1-3528
Cry1aa5 D17518 Udayasuriyan et al., 1994 BBB 58 830-835 81-3611
Cry1aa6 U43605 Masson et al., 1994 Mol Micro 14 851-860 1->1860
Cry1aa7 AF081790 Osman, 1998 unpublished
Cry1aa8 I26149 Liu, 1996 USP 5556784 148-3675
Cry1ab1 M13898 Wabiko et al., 1986 DNA 5 305-314 142-3606
Cry1ab2 M12661 Thorne et al., 1986 J Bact 166 801-811 155-3625
Cry1ab3 M15271 Geiser et al., 1986 Gene 48 109-118 156-3623
Cry1ab4 D00117 Kondo et al., 1987 ABC 51 455-463 163-3630
Cry1ab5 X04698 Hofte et al., 1986 EJB 161 273-280 141-3605
Cry1ab6 M37263 Hefford et al., 1987 J Biotech 6 307-322 73-3540
Cry1ab7 X13233 Haider & Ellar, 1988 NAR 16 10927-10927 1-3465
Cry1ab8 M16463 Oeda et al., 1987 Gene 53 113-119 157-3624
Cry1ab9 X54939 Chak & Jen, 1993 PNSCRC 17 7-14 73-3540
Cry1ab10 A29125 Fischhoff et al., 1987 Bio/technology peptide seq
5 807-813
Cry1ab11 I12419 Ely & Tippett, 1995 USP 5424409 73-
Cry1ab12 AF057670 Silva-Werneck et al., 1998 unpublished 41-3505
Cry1ac1 M11068 Adang et al., 1985 Gene 36 289-300 388-3921
Cry1ac2 M35524 Von Tersch et al., 1991 AEM 57 349-358 239-3772
Cry1ac3 X54159 Dardenne et al., 1990 NAR 18 5546-5546 339->2192
Cry1ac4 M73249 Payne et al., 1991 USP 4990332 1-3537
Cry1ac5 M73248 Payne et al., 1992 USP 5135867 1-3534
Cry1ac6 U43606 Masson et al., 1994 Mol Micro 14 851-860 1->1821
Cry1ac7 U87793 Herrera et al., 1994 AEM 60 682-690 976-4512
Cry1ac8 U87397 Omolo et al., 1997 Curr Micro 34 118-121 153-3686
Cry1ac9 U89872 Gleave et al., 1992 NZJCHS 20 27-36 388-3921
Cry1ac10 AJ002514 Sun & Yu, 1997 unpublished 388-3921
Cry1ac11 AJ130970 Makhdoom & Riazuddin, 1998 unpublished 156-3689
Cry1ac12 I12418 Ely & Tippett, 1995 USP 5424409 81->2990
Cry1ad1 M73250 Payne & Sick, 1993 USP 5246852 1-3537
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1ad2 A27531 Payne & Sick, 1995 AUP 632335 1-3537
Cry1ae1 M65252 Lee & Aronson, 1991 J Bact 173 6635-6638 81-3623
Cry1af1 U82003 Kang et al., 1997 unpublished 172->2905
Cry1ag1 AF081248 Osman, 1998 unpublished
Cry1ba1 X06711 Brizzard & Whiteley, 1988 NAR 16 2723-2724 1-3684
Cry1ba2 X95704 Soetaert, 1996 unpublished 186-3869
Cry1bb1 L32020 Donovan et al., 1994 USP 5322687 67-3753
Cry1bc1 Z46442 Bishop et al., 1994 unpublished 141-3839
Cry1bd1 U70726 Kuo & Chak, 1999 unpublished 842-4534
Cry1be1 Payne et al., 1998 USP 5723758 1-3681
Cry1ca1 X07518 Honee et al., 1988 NAR 16 6240-6240 47-3613
Cry1ca2 X13620 Sanchis et al., 1989 Mol Micro 3 229-238 241->2711
Cry1ca3 M73251 Payne & Sick, 1993 USP 5246852 1-3570
Cry1ca4 A27642 Van Mellaert et al., 1990 EP 0400246 234-3800
Cry1ca5 X96682 Strizhov, 1996 unpublished 1->2286
Cry1cb1 M97880 Kalman et al., 1993 AEM 59 1131-1137 296-3823
Cry1da1 X54160 Hofte et al., 1990 NAR 18 5545-5545 264-3758
Cry1da2 I76415 Payne & Sick, 1997 USP 5691308 1-3495
Cry1db1 Z22511 Lambert, 1993 unpublished 241-3720
Cry1ea1 X53985 Visser et al., 1990 J Bact 172 6783-6788 130-3642
Cry1ea2 X56144 Bosse et al., 1990 NAR 18 7443-7443 1-3516
Cry1ea3 M73252 Payne & Sick, 1991 USP 5039523 1-3516
Cry1ea4 U94323 Barboza-Corona et al., 1998 WJMB 14 437-441 388-3900
Cry1ea5 A15535 Botterman et al., 1994 EP 0358557 54-3566
Cry1eb1 M73253 Payne & Sick, 1993 USP 5206166 1-3522
Cry1fa1 M63897 Chambers et al., 1991 J Bact 173 3966-3976 478-3999
Cry1fa2 M73254 Payne & Sick, 1993 USP 5188960 1-3525
Cry1fb1 Z22512 Lambert, 1993 unpublished 483-4004
Cry1fb2 AB012288 Masuda & Asano, 1998 unpublished 84-3587
Cry1fb3 AF062350 Song & Zhang, 1998 unpublished
Cry1fb4 I73895 Payne et al., 1997 USP 5686069 peptide seq
Cry1ga1 Z22510 Lambert, 1993 unpublished 67-3564
Cry1ga2 Y09326 Shevelev et al., 1997 Febs Lett 404 148-152 692-4210
Cry1gb1 U70725 Kuo & Chak, 1999 unpublished 532-4038
Cry1ha1 Z22513 Lambert, 1993 unpublished 530-4045
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry1hb1 U35780 Koo et al., 1995 unpublished 728-4195
Cry1ia1 X62821 Tailor et al., 1992 Mol Micro 6 1211-1217 355-2511
Cry1ia2 M98544 Gleave et al., 1993 AEM 59 1683-1687 1-2160
Cry1ia3 L36338 Shin et al., 1995 AEM 61 2402-2407 279-2438
Cry1ia4 L49391 Kostichka et al., 1996 J Bact 178 2141-2144 61-2217
Cry1ia5 Y08920 Selvapandiyan, 1996 unpublished 524-2680
Cry1ia6 AF076953 Zhong et al., 1998 unpublished 1-2157
Cry1ib1 U07642 Shin et al., 1995 AEM 61 2402-2407 237-2393
Cry1ic1 AF056933 Osman et al., 1998 unpublished 1-2157
Cry1i-like I90732 Payne et al., 1998 See Table 3 peptide seq
Cry1ja1 L32019 Donovan et al., 1994 USP 5322687 99-3519
Cry1jb1 U31527 Von Tersch & Gonzalez, 1994 USP 5356623 177-3686
Cry1jc1 I90730 Payne et al., 1998 USP 5723758 peptide seq
Cry1ka1 U28801 Koo et al., 1995 FEMS 134 159-164 451-4098
Cry1-like I90729 Payne et al., 1998 See Table 3 peptide seq
Cry2aa1 M31738 Donovan et al., 1989 JBC 264 4740-4740 156-2054
Cry2aa2 M23723 Widner & Whiteley, 1989 J Bact 171 965-974 1840-3741
Cry2aa3 D86064 Sasaki et al., 1997 Curr Micro 35 1-8 2007-3911
Cry2aa4 AF047038 Misra et al., 1998 Unpublished 10-1908
Cry2aa5 AJ132464 Yu & Pang, 1999 Unpublished <1-1860
Cry2aa6 AJ132465 Yu & Pang 1999 Unpublished <1-1860
Cry2aa7 AJ132463 Yu & Pang, 1999 Unpublished <1->1611
Cry2ab1 M23724 Widner & Whiteley, 1989 J Bact 171 965-974 1-1899
Cry2ab2 X55416 Dankocsik et al., 1990 Mol Micro 4 2087-2094 874-2775
Cry2ac1 X57252 Wu et al., 1991 FEMS 81 31-36 2125-3990
Cry3aa1 M22472 Herrnstadt et al., 1987 Gene 57 37-46 25-1956
Cry3aa2 J02978 Sekar et al., 1987 PNAS 84 7036-7040 241-2175
Cry3aa3 Y00420 Hofte et al., 1987 NAR 15 7183-7183 566-2497
Cry3aa4 M30503 McPherson et al., 1988 Bio/technology 6 61-66 201-2135
Cry3aa5 M37207 Donovan et al., 1988 MGG 214 365-372 569-2503
Cry3aa6 U10985 Adams et al., 1994 Mol Micro 14 381-389 569-2503
Cry3ba1 X17123 Sick et al., 1990 NAR 18 1305-1305 25-1977
Cry3ba2 A07234 Peferoen et al., 1990 EP 0382990 342-2297
Cry3bb1 M89794 Donovan et al., 1992 AEM 58 3921-3927 202-2157
Cry3bb2 U31633 Donovan et al., 1995 USP 5378625 144-2099
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry3bb3 I15475 Peferoen et al., 1995 USP 5466597 <1->1291
Cry3ca1 X59797 Lambert et al., 1992 Gene 110 131-132 232-2178
Cry4aa1 Y00423 Ward & Ellar, 1987 NAR 15 7195-7195 1-3540
Cry4aa2 D00248 Sen et al., 1988 ABC 52 873-878 393-3935
Cry4ba1 X07423 Chungjatpornchai et al., 1988 EJB 173 9-16 157-3564
Cry4ba2 X07082 Tungpradubkul et al., 1988 NAR 16 1637-1638 151-3558
Cry4ba3 M20242 Yamamoto et al., 1988 Gene 66 107-120 526-3933
Cry4ba4 D00247 Sen et al., 1988 ABC 52 873-878 461-3868
Cry5aa1 L07025 Sick et al., 1994 USP 5281530 1-4155
Cry5ab1 L07026 Narva et al., 1991 EP 0462721 1-3867
Cry5ac1 I34543 Payne et al., 1997 USP 5596071 1-3660
Cry5ba1 U19725 Payne et al., 1997 USP 5596071 1-3735
Cry6aa1 L07022 Narva et al., 1993 USP 5236843 1-1425
Cry6ba1 L07024 Narva et al., 1991 EP 0462721 1-1185
Cry7aa1 M64478 Lambert et al., 1992 AEM 58 2536-2542 184-3597
Cry7ab1 U04367 Payne & Fu, 1994 USP 5286486 1-3414
Cry7ab2 U04368 Payne & Fu, 1994 USP 5286486 1-3414
Cry8aa1 U04364 Foncerrada et al., 1992 EP 0498537 1-3471
Cry8ba1 U04365 Michaels et al., 1993 WO 93/15206 1-3507
Cry8ca1 U04366 Ogiwara et al., 1995 Curr Micro 30 227-235 1-3447
Cry9aa1 X58120 Smulevitch et al., 1991 FEBS 293 25-28 5807-9274
Cry9aa2 X58534 Gleave et al., 1992 JGM 138 55-62 385->3837
Cry9ba1 X75019 Shevelev et al., 1993 FEBS 336 79-82 26-3488
Cry9ca1 Z37527 Lambert et al., 1996 AEM 62 80-86 2096-5569
Cry9da1 D85560 Asano et al., 1997 AEM 63 1054-1057 47-3553
Cry9Da2 AF042733 Wasano & Ohba, 1998 Unpublished <1->1937
Cry9Ea1 AB011496 Midoh & Oyama, 1998 Unpublished 211-3660
Cry9 like AF093107 Wasano & Ohba, 1998 See Table 3 <1->1917
Cry10Aa1 M12662 Thorne et al., 1986 J Bact 166 801-811 941-2965
Cry10Aa2 E00614 Uorufuiirudo, 1996 JP 1986005098 940-2968
Cry11Aa1 M31737 Donovan et al., 1988 J Bact 170 4732-4738 41-1969
Cry11Aa2 M22860 Adams et al., 1989 J Bact 171 521-530 <1-235
Cry11Ba1 X86902 Delecluse, 1995 AEM 61 4230-4235 64-2238
Cry11Bb1 AF017416 Orduz et al., 1998 BBA 1388 267-272 97-2346
Cry12Aa1 L07027 Narva et al., 1991 EP 0462721 1->3771
Table 2 (contd).
Name Acc No Reference Journal Coding
Cry13Aa1 L07023 Narva et al., 1992 WO 92/19739 1-2409
Cry14Aa1 U13955 Narva et al., 1994 WO 94/16079 1-3558
Cry15Aa1 M76442 Brown & Whiteley, 1992 J Bact 174 549-557 1036-2055
Cry16Aa1 X94146 Barloy et al., 1996 J Bact 178 3099-3105 158-1996
Cry17Aa1 X99478 Barloy et al., 1998 Gene 211 293-299 12-1865
Cry18Aa1 X99049 Zhang et al., 1997 J Bact 179 4336-4341 1451-3571
Cry19Aa1 Y07603 Rosso & Delecluse, 1996 AEM 63 4449-4455 719-2662
Cry19Ba1 D88381 Hwang et al., 1998 SAB 21 179-184 626-2671
Cry20Aa1 U82518 Lee & Gill, 1997 AEM 63 4664-4670 60-2318
Cry21Aa1 I32932 Payne et al., 1996 USP 5589382 1-3501
Cry21Aa2 I66477 Feitelson, 1997 USP 5670365 1-3501
Cry22Aa1 I34547 Payne et al., 1997 USP 5596071 1-2169
Cry23Aa1 AF03048 Donovan & Slaney, 1998 WO 98/13498
Cry24Aa1 U88188 Kawalek & Gill, 1998 Unpublished 1-2022
Cry25Aa1 U88189 Kawalek & Gill, 1998 Unpublished 1-2028
Cyt1Aa1 X03182 Waalwijk et al., 1985 NAR 13 8207-8217 140-886
Cyt1Aa2 X04338 Ward & Ellar, 1986 JMB 191 1-11 509-1255
Cyt1Aa3 Y00135 Earp & Ellar, 1987 NAR 15 3619-3619 36-782
Cyt1Aa4 M35968 Galjart et al., 1987 Curr Micro 16 171-177 67-816
Cyt1Ab1 X98793 Thiery et al., 1997 AEM 63 468-473 28-777
Cyt1Ba1 U37196 Payne et al., 1995 USP 5436002 1-795
Cyt2Aa1 Z14147 Koni & Ellar, 1993 JMB 229 319-327 270-1046
Cyt2Ba1 U52043 Guerchicoff et al., 1997 AEM 63 2716-2721 287-655
Cyt2Ba2 AF020789 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba3 AF022884 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba4 AF022885 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->469
Cyt2Ba5 AF022886 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->471
Cyt2Ba6 AF034926 Guerchicoff et al., 1997 AEM 63 2716-2721 <1->472
Cyt2Bb1 U82519 Cheong & Gill, 1997 AEM 63 3254-3260 416-1204
spectra observed in Bt (González & Carlton, 1980; González et al.,
1981; González et al., 1982; Reddy et al., 1987; Jarrett & Stephenson,
1990). New Bt strains have been developed by conjugation that are
toxic to two insect orders.
2.1.6 Beta-exotoxin
Beta-exotoxin is associated with certain Bt subspecies (Btd, Btg,
Btte and Btt), and products made from these Bt subspecies may contain
the toxin (Cantwell et al., 1964; Mohd-Salleh et al., 1980). This
heat-stable nucleotide, which is composed of adenine, glucose and
allaric acid, inhibits RNA polymerase enzymes by acting competitively
with ATP (Faust, 1973; Farkas et al., 1977). Since RNA synthesis is a
vital process in all life, beta-exotoxin exerts its toxicity for
almost all forms of life tested including numerous insect species in
the orders Coleoptera, Diptera and Lepidoptera. The presence of
beta-exotoxin can be assayed using houseflies (Musca domestica) or
high-performance liquid chromatography (HPLC) techniques (Campbell et
al., 1987).
Bt containing beta-exotoxin is used for the control of houseflies
in some countries, but regulatory agencies currently prohibit the use
of beta-exotoxin for other purposes.
2.1.7 Other Bt metabolites
Commercial Bt products do not contain metabolites that are
considered hazardous to humans and the environment. However, Bt, like
other bacteria, may produce during the vegetative growth and
sporulation stages an assortment of antibiotics, enzymes, metabolites
and toxins that are biologically active and may have effects on both
target and non-target organisms (NTOs).
Using a non-quantitative (Lund & Granum, 1997) commercial Bc
enterotoxin immunoassay (Tecra), Damgaard (1995) reported that
vegetative cells grown from spores of commercial Bt products excreted
a diarrhoeal enterotoxin. Damgaard et al. (1996a) found by Vero cell
assay that Bt isolated from food was enterotoxigenic. None of these
investigations estimated the quantity or activity of enterotoxins
produced by the Bt strains. However, Shinagawa (1990) investigated a
number of Bc and Bt isolates with an immunological assay and concluded
that 43% of the Bt isolates had the same level of enterotoxins as
enterotoxic Bc. Tayabali & Seligy (1997) found that vegetative Bt
obtained from commercial products caused extensive damage to
cultivated insect cells.
At least three enterotoxic activities have been described in Bc
(Agata et al., 1995; Lund & Granum, 1997), and some Bc isolates are
known to produce an emetic toxin (Andersson et al., 1998). The emetic
toxin is primarily associated with the Bc H-1 (Kramer & Gilbert, 1992;
Nishikawa et al., 1996) serotype, and has not so far been associated
with Bt isolates.
Table 3. Bt-associated toxins or toxin-like proteins that have not been assigned a name or entered
into the nomenclature for the reasons given
Name Accession Journal Coding region Reason Reference
Cry1i-like I90732 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry1-like I90729 USP 5723758 Peptide seq Insufficient sequence data Payne et al., 1998
Cry9-like AF093107 unpublished <1->1917 Insufficient sequence data Wasano & Ohba, 1998
40kda M76442 J Bact 174 549-557 45-971 No reported toxicity Brown & Whiteley, 1992
Cryc35 X92691 unpublished 1-981 No reported toxicity Juarez-Perez et al., 1995
Crytdk D86346 unpublished 177-2645 No reported toxicity Hashimoto, 1996
Cryc53 X98616 unpublished 1-1005 No reported toxicity Juarez-Perez et al., 1996
p21med X98794 AEM 63 468-473 1-552 No reported toxicity Thiery et al., 1997
ET34 AF038049 WO 98/13498 No reported toxicity Donovan & Slaney, 1998
Vip3a(a) L48811 PNAS 93 5389-5394 739-3105 Not a crystal protein Estruch et al., 1996
Vip3a(b) L48812 PNAS 93 5389-5394 118-2484 Not a crystal protein Estruch et al., 1996
Table 4. Bacillus thuringiensis holotype toxins
Name Old name Name Old name
Cry1Aa CryIA(a) Cry5Ac
Cry1Ab CryIA(b) Cry5Ba
Cry1Ac CryIA(c) Cry6Aa CryVIA
Cry1Ad CryIA(d) Cry6Ba CryVIB
Cry1Ae CryIA(e) Cry7Aa CryIIIC
Cry1Af Cry7Ab CryIIICb
Cry1Ag Cry8Aa CryIIIE
Cry1Ba CryIB Cry8Ba CryIIIG
Cry1Bb ET5 Cry8Ca CryIIIF
Cry1Bc PEG5 Cry9Aa CryIG
Cry1Bd CryE1 Cry9Ba CryIX
Cry1Be Cry9Ca CryIH
Cry1Ca CryIC Cry9Da
Cry1Cb CryIC(b) Cry9Ea
Cry1Da CryID Cry10Aa CryIVC
Cry1Db PrtB Cry11Aa CryIVD
Cry1Ea CryIE Cry11Ba Jeg80
Cry1Eb CryIE(b) Cry11Bb
Cry1Fa CryIF Cry12Aa CryVB
Cry1Fb PrtD Cry13Aa CryVC
Cry1Ga PrtA Cry14Aa CryVD
Cry1Gb CryH2 Cry15Aa 34kDa
Cry1Ha PrtC Cry16Aa cbm71
Cry1Hb Cry17Aa cbm72
Cry1Ia CryV Cry18Aa CryBP1
Cry1Ib CryV Cry19Aa Jeg65
Cry1Ic Cry19Ba
Cry1Ja ET4 Cry20Aa
Cry1Jb ET1 Cry21Aa
Cry1Jc Cry22Aa
Cry1Ka Cry23Aa
Cry2Aa CryIIA Cry24Aa Jeg72
Cry2Ab CryIIB Cry25Aa Jeg74
Cry2Ac CryIIC Cry26Aa
Cry3Aa CryIIIA Cry27Aa
Cry3Ba CryIIIB Cry28Aa
Cry3Bb CryIIIBb Cyt1Aa CytA
Cry3Ca CryIIID Cyt1Ab CytM
Cry4Aa CryIVA Cyt1Ba
Cry4Ba CryIVB Cyt2Aa CytB
Cry5Aa CryVA(a) Cyt2Ba "CytB"
Cry5Ab CryVA(b) Cyt2Bb
Alpha-toxin is a phospholipase C, which primarily affects the
cell membrane phospholipids (Heimpel, 1954; Bonnefoi & Béguin, 1959).
Gamma-toxin is toxic to sawflies (Tenthredinidae), but the mode of
action of this heat-labile toxin has not been determined (Heimpel,
1967).
The so called "water-soluble toxin" paralyses Lepidoptera
(Fast, 1971), and the "mouse factor exotoxin" is toxic to mice as
well as to Lepidoptera (Krieg, 1971). The modes of action of these
toxins have not been delineated.
A novel Bt vegetative insecticidal protein (Vip3A) has been
identified from the culture media of some Bt strains (Estruch et al.,
1996).
Several Bt and Bc enzymes have been described which may play a
role in non-target activity: phospholipase (Damgaard et al., 1996b),
sphingomyelinase (Gilmore et al., 1989), protease (Hotha & Banik,
1997), chitinase (Sampson & Gooday, 1998), and haemolysin (Baida &
Kuzmin, 1995).
2.2 Bioassays
2.2.1 Spore counts
Bacterial spore counts do not necessarily reflect the
insecticidal activity of a Bt strain or Bt product because the number
and amount of ICPs produced per bacterial cell can vary.
2.2.2 International bioassay for ICPs
The final formulation of each Bt product is bioassayed against an
accepted international standard using a specific test insect (Dulmage
et al., 1981; de Barjac & Larget-Thiery, 1984). Its potency is defined
in ITU/mg product. The standardization allows comparison of different
formulations in the laboratory. Currently, the larvicidal activity is
expressed in terms of lethal doses (LD50) or lethal concentrations
(e.g., LC50, LC90) according to the bioassay method used. For
example, when susceptible mosquito larvae are exposed to Bti ICP, they
have an LC50 of approximately 10 ng/ml water. A Bti whole culture
gives an LC50 of approximately 103 cells/ml for susceptible mosquito
larvae while a 109 cells/ml culture does not affect any laboratory
mammals exposed by various routes.
3. MODE OF ACTION ON TARGET INSECTS
3.1 Bioactivity of field isolates
The mode of action of Bt has been reviewed by Schnepf et al.
(1998) and can be summarized in the following stages: 1) ingestion of
sporulated Bt and ICP by an insect larva; 2) solubilization of the
crystalline ICP in the midgut; 3) activation of the ICP by proteases;
4) binding of the activated ICP to specific receptors in the midgut
cell membrane; 5) insertion of the toxin in the cell membrane and
formation of pores and channels in the gut cell membrane, followed by
destruction of the epithelial cells (Cooksey, 1971; Norris 1971; Fast,
1981; Huber & Lüthy, 1981; Lüthy & Ebersold, 1981; Smedley & Ellar,
1996); and 6) subsequent Bt spore germination and septicaemia may
enhance mortality (Fig. 1).
The specific bioactivity of Bt is dominated by the ICPs that are
encoded by the cry genes and are active against susceptible species
in the insect orders Coleoptera, Diptera and Lepidoptera.
Specific Bt activities against other insect orders (Hymenoptera,
Homoptera, Dictyoptera, Mallophaga) and to nematodes
(Strongylida, Tylenchida), mites (Acari), flatworms (Digenea)
and protozoa (Diplomonadida) have been described (Feitelson, 1993;
Zukowski, 1995). The ICP must be ingested to be effective against the
target (Visser et al., 1993).
3.2 Mechanism of action of Bt formulations
The ICP-spore complexes of Bt are ingested by susceptible insect
larvae. In the midgut the parasporal crystalline ICP is dissociated to
the protoxin form, and the protoxin is then activated to a holotoxin
by gut proteases (Warren et al., 1984; Jaquet et al., 1987; Aronson et
al., 1991; Honée & Visser, 1993). Shortly afterwards, the gut becomes
paralysed and the larva ceases to feed.
The ICP structure and function have been reviewed in detail by
Schnepf et al. (1998). Binding of the ICP to putative receptors is a
major determinant of ICP specificity and the formation of pores in the
midgut epithelial cells is a major mechanism of toxicity (Van
Frankenhuyzen, 1993).
The active toxin consists of three distinct domains (Höfte &
Whiteley, 1989; Li et al., 1991; Grochulski et al., 1995). The three
domains interact in a complex manner, but experimental data suggest
that the C-terminal and middle domains of the toxin are involved in
epithelial cell receptor binding and structural functions, while the
N-terminal domain is primarily involved in ion channel and pore
formation (Huber et al., 1981; Schnepf et al., 1998; Dean et al.,
1996).
 Binding to specific receptors has been demonstrated to be closely
related to the insecticidal spectrum of the ICPs (Denolf et al.,
1997). Van Rie et al. (1989) found the affinity of these toxins
similar for the tobacco budworm (Heliothis virescens) and the tomato
hornworm (Manduca sexta) brush border membrane vesicles, but the
number of binding sites differed and reflected varied bioactivity.
However, the toxin affinity for binding sites does not appear constant
for all insects.
Pore or ion channel formation occurs after the binding to the
receptor and insertion of the N-terminal domain into the membrane,
whereby the regulation of the trans-membrane electric potential is
disturbed. This can result in colloid-osmotic lysis of the cells,
which is the main cytolytic mechanism that is common to all ICPs
(Knowles & Ellar, 1987; Slatin et al., 1990; Schwartz et al., 1991;
Schnepf et al., 1998). When the midgut epithelium of the larva is
damaged, the haemolymph and gut contents can mix. This results in
favourable conditions for the Bt spores to germinate. The resulting
vegetative cells of Bt and the pre-existing microorganisms in the gut
proliferate in the haemocoel causing septicaemia, and may thus
contribute to the mortality of the insect larva.
3.3 Resistance of insect populations
A number of insect populations of several different species with
different levels of resistance to Bt have been obtained by laboratory
selection experiments during the last 15 years (Schnepf et al., 1998).
The species include Plodia interpunctella, Cadra cautella,
Leptinotarsa decemlineata, Chrysomela scripta, Tricholplusia ni,
Spodoptera littoralis, Spodoptera exigua, Heliothis virescens,
Ostrinia nubilalis and Culex quinquefasciatus (Schnepf et al.,
1998) and resistance is shown to either Btk, Bti, Btte or other Bt
subspecies.
During the last few years populations of the diamondback moth,
Plutella xylostella, resistant to Btk and Bta have been found in
heavily treated areas in numerous geographically isolated regions in
the world, including Hawaii, Phillippines, Indonesia, Malaysia,
Central America and some USA states (Schnepf et al., 1998). It is
clear that this widespread appearance of resistance to Bt presents a
cautionary tale for the way of using Bt and Bt toxin genes in pest
management. Schnepf et al. (1998) have reviewed resistance management
of Bt.
4. NATURAL AND TREATED HABITATS
The Bt subspecies represents a group of organisms that occur
naturally and can be added to an ecosystem to achieve insect control
(Andrews et al., 1987; Stahly et al., 1991). In this monograph, a
natural habitat is considered to be one where Bt can be isolated when
there has been no previous history of application of the organism to
that ecosystem, whereas a treated habitat is one where Bt can be
isolated after a previous history of application of the organism for
insect control.
Insecticides formulated with Bt are being manufactured and used
worldwide. These commercial Bt products may be applied as an
insecticide to foliage, soil, water environments and food storage
facilities. After application of Bt to an ecosystem, the organism may
persist as a component of the natural microflora.
4.1 Natural occurrence of Bt
Members of the Bacillus cereus group can be found in most
ecological niches. Hansen et al. (1996) reviewed the occurrence of Bt
in the environment. Although the early Bt isolates were pathogenic for
insects, it is now apparent that several Bt isolates have no known
target (Ohba & Aizawa, 1986; Ohba et al., 1988; Hansen et al., 1996,
1998; Damgaard et al., 1997b). This lack of insecticidal activity may
be attributed to the loss of ability to produce ICPs (Gordon, 1977),
which may be due to a mutation in the ICP gene that could prevent
expression (Klier & Lecadet, 1976; Stahly et al., 1978; Dean, 1984) or
to the loss of ICP encoding sequences. Finally, the lack of known
activity of a Bt crystalline toxin might simply be explained by a
failure to test against the actual target organism. The list of Bt
targets is still increasing. Although our knowledge of the activity of
Bt populations in the environment is limited, a certain level of
turn-over and vegetative growth must occur, as annual and seasonal
variations in numbers and subspecies diversity of Bt populations have
been observed (Damgaard et al., 1997b; Kim et al., 1998).
4.1.1 Bt in insect hosts
Numerous Bt subspecies have been isolated from dead or dying
insect larvae and in most cases the isolate has toxic activity to the
insect from which it was isolated (Goldberg & Margalit, 1977; de
Barjac, 1981b; Hansen et al., 1996). These organisms have a narrow
host range in the orders Coleoptera, Diptera and Lepidoptera and
can proliferate within the bodies of their host insects. When the
infected insect larva dies, the dead insect carcass usually contains
relatively large quantities of spores and crystals that may be
released into the environment (Prasertphon et al., 1973; Grassi &
Deseö, 1984; Aly, 1985; Aly et al., 1985). Growth of Bt in non-target
organisms has also been described. Eilenberg et al. (in press) found
that Bt multiplication had occurred in non-target insects, which were
also infected by insect pathogenic fungi.
Akiba (1986) reported recycling of naturally occurring Bt in
insect cadavers when competitive microorganisms were at a low density.
Outbreaks of Bt in susceptible insect populations occur relatively
infrequently; most outbreaks have been limited to situations where the
insect density is relatively high, providing better opportunity for
establishing the disease within the insect population (Lynch et al.,
1976; Burges & Hurst, 1977; Vaòková & Purrini, 1979; Margalit & Dean,
1985).
4.1.2 Bt in soil
The spores of Bt persist in soil, and vegetative growth occurs
when nutrients are available (DeLucca et al., 1981; Akiba, 1986; Ohba
& Aizawa, 1986; Travers et al., 1987; Martin & Travers, 1989).
DeLucca et al. (1981) found that Bt represented between 0.5% and
0.005% of all Bacillus species isolated from soil samples in the
USA. Martin & Travers (1989) recovered Bt from soils globally. Meadows
(1993) isolated Bt from 785 of 1115 soil samples, and the percentage
of samples that contained Bt ranged from 56% in New Zealand to 94% in
samples from Asia and central and southern Africa. Ohba & Aizawa
(1986) isolated Bt from 136 out of 189 soil samples in Japan.
4.1.3 Bt on plant surfaces
Bt has been found extensively in the phylloplane. Numerous Bt
subspecies have been recovered from coniferous trees, deciduous trees
and vegetables, as well as from other herbs (Smith & Couche, 1991;
Damgaard et al., 1997b). The Bt isolates have demonstrated a broad
diversity both with specific activities to insects from the orders
Coleoptera and Lepidoptera and with unknown activities (Smith &
Couche, 1991; Damgaard et al., 1997b; Hansen et al., 1998). The
bacterium has also been isolated from stored grain products (Meadows
et al., 1992).
4.2 Treated habitats
Treated habitats are the locations where Bt insecticides (usually
a mixture of spores and crystals) have been applied.
In Canada, Meadows (1993) estimated that approximately 1015
viable Btk spores per ha were released in a typical spray operation to
control spruce budworm (Choristoneura fumiferana).
4.3 Environmental fate, distribution and movement
Bt, like other members of the genus Bacillus, has the ability
to form endospores that are resistant to inactivation by heat and
desiccation and that persist in the environment under adverse
conditions (Stahly et al., 1991). When considering the degradation of
Bt in the environment, it is important to distinguish between changes
in the numbers of viable spores and changes in biocidal activity. The
survival and activity in the environment has been reviewed by Hansen
et al. (1996).
The distribution and environmental transport of applied Bt
formulations are influenced by the type of application and various
environmental factors (Bulla et al., 1985; Andrews et al., 1987). Bt
formulations are used in agriculture and forestry against coleopteran
and lepidopteran pests and are usually directed towards the surface of
plants, while the Bt formulations for control of dipteran pests
(mosquitos and blackflies) are applied to their aquatic, larval
habitats. Many Bt insecticides exhibit poor stability under field
conditions, and so frequent reapplication is required (Griego &
Spence, 1978; Sorenson & Falcon, 1980; Beegle et al., 1981).
4.3.1 Distribution and fate of Bt in terrestrial habitats
4.3.1.1 Fate of Bt and ICP on plant surfaces
Solar radiation appears to be the environmental factor most
damaging to the stability of Bt ICP (Pinnock et al., 1974; Pinnock et
al., 1977; Griego & Spence, 1978; Pusztai et al., 1991).
Griego & Spence (1978) demonstrated that Bt spores are
inactivated rapidly when exposed to UV radiation, while Pusztai et al.
(1991) demonstrated that the tryptophan residues of the Bt protoxin
are damaged by solar radiation in the 300-380 nm range.
The combined effect of sunlight, leaf temperature and vapour
pressure deficit appeared to contribute more to the reduction of
bioactivity than any other single factor (Leong et al., 1980). Residue
bioactivity may be detected up to 2 weeks after treatment with
formulations containing UV protectants (Hostetter et al., 1975). Other
studies on the effect of environmental exposure to Bt spores revealed
that spore survival can be affected by the surface to which the
material is applied.
Pinnock et al. (1974) reported that the half-life of Bt spores on
leaves of California live oak (Quercus agrifolia) was 3.9 days, as
compared to a half-life of 0.63 days when applied to leaves of western
redbud (Cercis occidentalis).
Ignoffo (1992) summarized data for the reduction of spore
viability and ICP activity on leaves of various plants in sunlight: Bt
spore viability was reduced 80% in one day on red cedar leaves and 8%
on soy bean leaves, while the ICP activity declined by 20% on red
cedar leaves but 65% on soy bean leaves.
Dent (1993) reported that Bt formulations on foliage frequently
have half-lives of up to 10 days. However, unformulated Bt may have a
half-life of only a few hours. Pedersen et al. (1995) found that the
initial spore half-life was 16 h during the first week after spraying
cabbage with unformulated Btk.
There is also evidence that plant chemicals can inactivate Bt or
influence infectivity. Lüthy (1986) demonstrated that extracts
prepared from cotton leaves could inactivate ICPs.
Commercially applied Bt may persist at low levels for
considerable periods of time. Reardon & Haissig (1983) reported that
Btk was still present on balsam fir (Abies balsamea) one year after
applications to control spruce budworm. The proliferation of spores
through infection of susceptible insects should not be discounted as a
source of low levels of Bt in treated areas. Several studies have
demonstrated that Bt is able to grow and sporulate in insect cadavers
(Meadows, 1993). From dead Egyptian cotton leafworm (Spodoptera
littoralis), Jarrett & Stephenson (1990) isolated between 5.0 × 105
and 9.2 × 107 spores per larva.
Bt may be lost to the soil by overspray during application or by
the action of rain on plant surfaces. Further losses arise from in
situ degradation by environmental factors, such as ultraviolet (UV)
radiation and microbial activity (Griego & Spence, 1978; Sorenson &
Falcon, 1980; Beegle et al., 1981; West et al., 1984a,b). Pedersen et
al. (1995) found that Bt was dispersed by rain splash from the soil to
the lower leaves of cabbage.
4.3.1.2 Fate of Bt in soil
Petras & Casida (1985) reported that Bt spore counts in soil
declined by a factor of ten in the first 2 weeks after application and
then remained constant for 8 months. The response was similar in
spores from commercial and laboratory cultures. In contrast,
vegetative cells introduced into the soil persisted for only a short
time. Soil pH had little effect on their survival. Spore persistence
for more than 2 weeks apparently resulted from the inability of the
spores to germinate in the soil.
Pedersen et al. (1995) sprayed unformulated Btk (1.2 5 104 cfu
per g soil, spontaneous rifampicin-resistant mutant) on soil in 1993,
and 2.3 × 103 cfu per g soil remained after 336 days. The field was
left undisturbed, and 5´ years later spots with 1.5 × 103 Btk per g
soil were found (Hansen & Hendriksen, 1999), but spots with very low
Btk numbers were also recorded. These data indicate that the Btk had
multiplied.
West et al. (1984a,b) reported that vegetative cells in soil
disappeared at a rapid, exponential rate, whereas parasporal crystals
disappeared at a slower, non-exponential rate, and spore numbers
remained unaltered through 91 days of incubation at 25°C, with no
detectable germination. The proteinaceous crystalline protoxin of Bt
has been shown to be degraded by soil microorganisms at an exponential
rate with a half-life of about 3-6 days.
Saleh et al. (1970) reported that Bt spores could remain viable
for several months in the soil and germinate when soil conditions
favoured bacterial growth.
Bt spores do not appear to germinate readily in most soils and
the crystalline protoxins are metabolized by other microorganisms.
West et al. (1984a) reported that Bta in soil showed an exponential
loss of insecticidal activity. The rate of loss was greater in
non-sterile soil than in autoclaved soil. There was an initial rapid
decrease, which stabilized at approximately 10% of the original
inoculum level after 250 days incubation, until the cessation of
sampling after two years. No loss of insecticidal activity was
observed in autoclaved soil, which suggests that soil microorganisms
were responsible for the loss of insecticidal activity in the natural,
non-sterilized soil.
Several studies determined that Bt did not grow under most
natural soil conditions (West et al., 1984a,b; Akiba, 1986). The data
suggested that this was attributable to a failure of Bt spores to
germinate in soil under these conditions. The spore is the only state
in which Bt persists in natural soils.
An environmental fate study demonstrated no significant spore
accumulation in either the organic or the mineral layers of soil over
an 11-month period when Bt was applied aerially at 100 times the
concentration used for operational programmes (Bernier et al., 1990).
Studies have indicated that Bt is relatively immobile in soil.
Martin & Reichelderfer (1989) found no vertical movement beyond a 6-cm
deep zone in soil and less than 10 m lateral movement, even along
drainage courses.
Akiba (1991) reported a one-month irrigation study simulating the
summer rainy season in Japan. There was no translocation of Bt below a
depth of 10 cm. In soils receiving 45 cm simulated rainfall, Bt was
detected to a depth of 3-6 cm. In tests using soil columns, Bt did not
pass through a column of volcanic ash soil but a few spores were
detected in flow-through water from an alluvial sand column. Results
suggested that the major factor causing a decrease in the level of Bt
was not a physical dilution due to the rainwater, but possibly an
affinity of the spores for the soil particles.
Venkateswerlu & Stotsky (1992) reported that adsorption and
binding of Bt toxin proteins to soil particles were greater on
montmorillonite than on kaolinite clays. Maximum adsorption occurred
within 30 min, and adsorption was not significantly affected by
temperature between 7°C and 50°C.
4.3.2 Distribution and fate of Bt in aquatic habitats
Bti is often applied directly to water for the control of
mosquitos and blackflies. Rapid sedimentation in all but the fastest
flowing streams is regarded as an important limitation on the efficacy
of such applications.
Sheeran & Fisher (1992) demonstrated that the sedimentation of
Bti is facilitated by adsorption onto particulate material.
Ohana et al. (1987) found that spores may persist for at least
22 days in sediments, and the spores may be mobilized with such
sediments during floods.
Btk has been reported to survive in fresh water and in seawater
for more than 70 and 40 days, respectively, at 20°C (Menon & De
Mestral, 1985). A higher percentage of Btk was found to survive for
extended periods in lake water than in tap and distilled water,
presumably due to the presence of more nutrients in lake water. Bt has
not been isolated from any drinking-water supplies.
Spores of Bti remained viable for shorter periods when suspended
in moving water than when in static bottles, indicating that static
laboratory trials may overestimate the longevity of these spores in
the environment (Yousten et al., 1992).
Carcases of mosquito larvae killed by Bti have been shown to
allow for the complete growth cycle (germination, vegetative growth
and sporulation), thus becoming toxic themselves to scavenging yellow
fever mosquito (Aedes aegypti) larvae (Khawaled et al., 1990).
Contact of Bti with mud can result in an immediate disappearance
of larvicidal activity, but it has little influence on spore viability
(Ohana et al., 1987). The cessation of toxicity was found to be caused
by bacterial adsorption to soil particles, but the inactivation could
be reversed by washing the mud away.
Special Bti formulations have been developed to prolong residence
time of Bt at the surface or in the water column, where target insects
feed.
Manasherob et al. (1998) found germination, growth and
sporulation of Bti in excreted food vacuoles of a protozoan.
4.3.3 Transport of Bt by non-target organisms
In a field trial where Btk was sprayed on cabbage and soil,
Pedersen et al. (1995) found that the Btk could be transported by
non-target insects. Up to 103 cfu per g were found on surface-active
insects, and carabid beetles carrying Btk were found up to 135 m from
the Btk-treated area.
In a study of interactions between Bti and fathead minnows
(Pimephales promelas), ingestion of Bt resulted in a high number of
recoverable spores in the gastrointestinal tract and faeces (Snarski,
1990). Bti spore counts decreased rapidly after test fish were
transferred to clean water, but spores were detected in low numbers in
faeces for over 2 weeks. The data indicated that minnows could
disperse Bt spores in the freshwater environment.
Meadows (1993) reported that, after the application of Bt on
land, it may be dispersed by birds and mammals feeding on infected
target insects. Some Bt-infected insect larvae may contain 6.6 × 108
to 4.2 × 109 spores per gram dry mass (Burges & Hurst 1977).
5. COMMERCIAL PRODUCTION
5.1 History of Bt and its commercial applications
5.1.1 Production levels
Conventional Bt products, which utilize naturally occurring or
modified Bt strains, account for approximately 90% of the world MPCA
market (Bernhard & Utz, 1993). Current annual production of Bt has
been estimated at 3000 or more tonnes in developed countries. In
China, up to 10 000 tons are produced annually (personal communication
by Guan Xiong, Fujian Agricultural, University, 1997).
5.1.2 Production processes, formulations and quality standards
Bt products are produced using fermentation technology (Bernhard
& Utz, 1993). Most commercial products contain ICP and viable Bt
spores, but the spores are inactivated in some Bti products. During
commercial-scale production of Bt products, there is little loss of
bioactive components to the environment. The type of loss incurred is
a function of the recovery method involved. No significant amount of
bioactive component is lost from fermenter harvests if filtration is
used to separate the insoluble solids (active ingredients) from the
soluble liquid (inert) fraction of the harvest liquor, as shown by
complete lack of bioactivity in the resulting liquid fraction. The
liquid waste fractions may contain a low concentration of insoluble
active component, but this is typically inactivated by processing in
on-site waste treatment facilities. Although it is not a physical
loss, measurable bioactivity is diminished if the recovered active
material is processed through a dryer, due to the exposure of the
bioactive components to the high temperatures required for drying.
Guidelines for the handling of microorganisms during manufacture have
been reviewed by Frommer et al. (1989).
Commercial Bt formulations include wettable powders, suspension
concentrates, water dispersible granules, oil miscible suspensions,
capsule suspensions and granules (Tomlin, 1997).
Quality standards for Bt fermentation products have been accepted
by IUPAC (Quinlan, 1990). These standards include limits on the
concentration of microbial contaminants and metabolites (Table 5).
In most cases strains of Bt that produce beta-exotoxins are not
approved for commercial application, although some commercial use has
been approved for control of certain fly species that are not
susceptible to ICPs (Carlberg et al., 1985).
Table 5. Maximum allowable levels of microbial contamination in
bacterial insecticides (IUPAC Recommendation; Quinlan, 1990)
Types of microorganisms Maximum concentrations
Viable mesophiles <1 × 105/g
Viable yeasts and moulds <100/g
Coliforms <10/g
Staphylococcus aureus <1/g
Salmonella <1/10g
Lancefield Group D Streptococci <1 × 104/g
5.1.3 General patterns of use
Commercial applications of Bt have been directed mainly against
lepidopteran pests of agricultural and forest crops; however, in
recent years strains active against coleopteran pests have also been
marketed (Table 6).
Strains of Bti active against dipteran vectors of parasitic
disease organisms have been used in public health programmes.
5.1.3.1 Applications in agriculture and forestry
Commercial use of Bt on agricultural and forest crops dates back
nearly 30 years, when it became available in France. Use of Bt has
increased greatly in recent years and the number of companies with a
commercial interest in Bt products has increased from four in 1980 to
at least 18 (Van Frankenhuyzen, 1993). Several commercial Bt products
with Bta, Btk or Btte have been applied to crops using conventional
spraying technology (Table 6). Various formulations have been used on
major crops such as cotton, maize, soybeans, potatoes, tomatoes,
various crop trees and stored grains. Formulations have ranged from
ultralow-volume oil to high-volume, wettable powder and aqueous
suspensions. In the main, naturally occurring Bt strains have been
used, but transgenic microorganisms expressing Bt toxins have been
developed by conjugation and by genetic manipulation, and in some
cases, these have reached the commercial market. These modified
organisms have been developed in order to increase host range, prolong
field activity or improve delivery of toxins to target organisms. For
example, the coleopteran-active cryIIIA gene has been transferred to
a lepidopteran-active Btk (Carlton et al., 1990). A plasmid bearing an
ICP gene has been transferred from Bt to a non-pathogenic
leaf-colonizing isolate of Pseudomonas fluorescens; fixation of the
transgenic cells produces ICP contained within a membrane which
prolongs persistence (Gelernter, 1990). The gene expressing cryIA(c)
ICP has been inserted in Clavibacter xyli subspecies cynodontis, a
bacterium that colonizes plant vascular systems. This has been shown
to deliver the ICP effectively to European corn borer (Ostrinia
nubilalis) feeding within plant stems (Beach, 1990). Improvements in
performance arising from such modifications are such that transgenic
organisms and their products are likely to be used much more widely in
the future.
5.1.3.2 Applications in vector control
Bti has been used to control both mosquitos and blackflies in
large-scale programmes (Lacey et al., 1982; Chilcott et al., 1983;
Car, 1984; Car & de Moor, 1984; Cibulsky & Fusco, 1987; Becker &
Margalit, 1993; Bernhard & Utz, 1993). For example, in Germany 23
tonnes of Bti wettable powder and 19 000 litres of liquid concentrate
were used to control mosquitos (Anopheles and Culex species)
between 1981 and 1991 in the Upper Rhine Valley (Becker & Margalit,
1993). In China, approximately 10 tonnes of Bti have been used in
recent years to control the malarial vector, Anopheles sinensis.
The Onchocerciasis Control Programme of West Africa used more
than five million litres of Bti from 1982 to 1997 to control
blackflies (Simulium damnosum), the vector of the onchocerciasis
filarial worm (Onchocerca volvulus), on the Upper Volta River
System. The Programme was initially based solely on the control of the
vector, Simulium damnosum sensu lato, by spraying the insecticide
at breeding sites on river systems, where larval stages develop. At
the peak of larvicidal activities about 50 000 km of rivers were
treated in an area of over one million km2. The purpose was to
interrupt the transmission of the parasite Onchocerca volvulus. The
interruption of the cycle is achieved by destroying larval stages
through aerial application of insecticides to breeding sites.
Insecticide application was undertaken weekly (Moulinier et al.,
1995). In order to assess the environmental impact of such treatments
a network of sampling stations throughout the programme area were
established.
Formulations of Bti range from wettable powder and fluid
concentrates applied via conventional spray equipment from ground and
air to slow-release briquet and tablet formulations. Examples of
commercial Bti products are listed in Table 6.
Table 6. Examples of commercial Bt products used against agricultural, forestry and public health pests
(Tomlin, 1997; see also the Internet site http://www.sipweb.org/bacteria.htm)
Bt sub-species Commercial products Producer
Bt (not defined) Rijin Scientific & Technological Development
Bt (not defined) Bitayon Jewin-Joffe Industry Ltd
Bt (not defined) Delfin, Thuricide SDS Biotech KK
Btk Bactospeine, Biobit, Dipel, Foray Abbott (USA)
Bollgard Ecogen/Crop Care
Bactucide Caffaro
Baturad Cequisa
Condor, Crymax, Cutlass, Ecogen
Foil, Jackpot, Lepinox,
Rapax, Raven
Jackpot, Lepinox, Rapax Intrachem
Cordalene Agrichem
Larvo Troy
Costar, Delfin, Design, Novartis/Thermo Trilogy Co
Javelin, Steward, Thuricide,
Vault
Ecotech Bio, Ecotech Pro Ecogen/Roussel-Uclaf
Halt Wockhardt
Bta Xentari, Florbac Abbott
Certan Novartis
Btte Novodor Abbott
Bti Bactimos, Gnatrol, Vectobac Abbott
Acrobe Cyanamid
JieJueLing, MieJueLing Huazhong Agricultural University
Teknar Novartis/ThermoTrilogy Co
Bt Ybt-1520 Mianfeng pesticide Huazhong Agricultural University
Bt chinesensis Shuangdo preparation Huazhong Agricultural University
Btg Spicturin Tuticorin Alkali Chemicals and
Fertilisers Ltd
6. EFFECTS ON ANIMALS
6.1 Mammals
Microbial pest control agents (MPCA) can, in principle, cause
harmful effects via toxicity, inflammation, or a combination of these
effects. The presence of bacteria in a specimen derived from tissues
does not necessarily mean infection. Colonization refers to the
multiplication of MPCA either on the surface or within an animal/human
organism without causing any tissue damage. Persistence refers to
the ability to recover the inoculum of the MPCA over time. Persistence
and transient disturbances of the normal microbial flora are to be
expected after exposure of experimental animals to MPCA, since
clearance of the inoculum is not instantaneous. Persistence may not be
equated with infection (Siegel & Shadduck, 1990). Infection by a
MPCA means that there is evidence of the establishment and
proliferation of the MPCA in tissues, coupled with tissue damage.
Evidence of multiplication includes a measurable increase in the total
amount of MPCA, recovery of vegetative stages when spores were
administered, and failure of the inoculum to clear. It cannot be
determined solely on the basis of lesions since injection of foreign
material can elicit an inflammatory process (Siegel et al., 1987).
A classification of MPCA toxicity and infectivity has been
proposed, in which MPCA is classified as toxic if an oral dose
< 106 cfu per mouse causes mortality or clinical or pathological
changes (Burges, 1980). However, any classification is very difficult
because of the complexicity of the issue when dealing with living
organisms (Ignoffo, 1973; Shadduck, 1983).
Older reports do not discriminate between different strains of Bt
but modern molecular techniques have proven that variability exists
within strains with the same serotype (Helgason et al., 1998; Hansen &
Hendriksen, 1997a,b).
Mammalian toxicity studies on Bt-containing pesticides
demonstrate that the tested isolates are not toxic or pathogenic
(McClintock et al., 1995), as they occur in the products. Toxicity
studies submitted to the US Environmental Protection Agency to support
registration of Bt subspecies, and reviewed by McClintock et al.
(1995), failed to show any significant adverse effects on body weight
gain, clinical observations or upon necropsy.
Infectivity/pathogenicity studies have shown that the intact rodent
system responds as expected to eliminate Bt gradually from the body
after oral, pulmonary or intravenous challenge. However, clearance of
Bti and Btk is not instantaneous. An intact immune system is not a
prerequisite for clearance of Bti and Btk.
6.1.1 Oral exposure
In studies conducted with a single oral dose of laboratory grown
Bt and commercial Bt formulations, there was no mortality associated
with ingestion of Bti or Btk in mice and rats (Fisher & Rosner, 1959;
de Barjac et al., 1980; Shadduck, 1980; Siegel et al., 1987)
(Table 7).
Additionally, in data summarized by McClintock et al. (1995), no
toxicity or infectivity was observed following oral administration of
various Bt subspecies at doses of up to 4.7 × 1011 cfu/kg in rats.
In a study involving repeated oral exposure of mice and rats for
21 days with laboratory grown Bti, there was no mortality associated
with ingestion of Bti and normal weight gain was observed in all
treated rodents (de Barjac et al., 1980) (Table 8).
Hadley et al. (1987) conducted a study in which sheep were
repeatedly treated with two commercial Btk formulations for 60 days.
The only clinical sign was loose stools in sheep exposed to one Btk
formulation. There was also microscopic evidence of moderate to marked
lymphoid hyperplasia of the Peyer's patches in the caecum and colon of
two out of six sheep treated with one Btk formulation and in one out
of six sheep treated with the other Btk formulation. The authors did
not consider these findings clinically significant.
Other multiple dose studies with Bt were summarized by McClintock
et al. (1995). In rats, no toxicity or infectivity was associated with
dietary exposure to Bti (4 g/kg per day ) for 3 months. Administration
of 1.3 × 109 Btk spores/kg per day to rats by oral gavage was not
toxic or infectious. McClintock et al. (1995) also reported the
results of a 2-year study in which a commercial Btk preparation was
fed to rats at 8400 mg/kg per day in the diet. Despite this excessive
dose, the only effect observed was a decrease in body weight of
females during weeks 10-104 of the study.
6.1.2 Inhalation exposure
These tests primarily address the potential infectivity of a
MPCA. Inhalation is a likely route by which humans and animals may be
exposed to Bt during application.
De Barjac et al. (1980) exposed 10 female Swiss mice for 12 min
to 2 × 108 Bti spores (48-h laboratory grown whole culture). The mice
were monitored for clinical signs for 15 days, and then killed. The
lungs were removed aseptically and cultured for bacteria, but no Bti
was recovered.
Siegel et al. (1987) exposed 27 female Sprague-Dawley rats to
2 × 106 spores of a commercial Bti formulation for 30 min. Rats were
serially killed over a 27-day period and the lungs were cultured.
Table 7. Acute toxicity (single oral exposure) of Bt in experimental animals
Subspecies Material tested Test animal Dosea Mortalityb Reference
tested
Btk Washed cells, 24-h culturec Rat, female, Sprague-Dawley 1.4 × 107cfu 0/6 Shadduck, 1980
Btk Commercial product Ratd 2 × 1011cfu 0/10 Fisher & Rosner, 1959
Bti 48-h culturec Mouse, female, Swiss 1.7 × 108cfu 0/20 de Barjac et al., 1980
Bti 48-h culturec Rat, female, Wistar 3.4 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellsc, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rat, female, Sprague-Dawley 4 × 107cfu 0/10 Siegel et al., 1987
a All doses given are per animal
b Number dead/number treated
c Laboratory grown culture
d Breed and sex unknown
Table 8. Repeated dose (oral exposure) toxicity of Bt in mice, rats and sheep
Subspecies Material tested Test animal Dose Exposure Mortalitya Reference
tested time
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Bti 48-h cultureb Mouse, female, Swiss 4.7 × 1010cfu 21 days 0/20 de Barjac et al., 1980
Bti 48-h cultureb Rat, female, Wistar 1.2 × 1011cfu 21 days 0/10 de Barjac et al., 1980
a Number dead/number exposed
b Laboratory grown culture
Recovery of Bti declined from 5.92 × 103 cfu/g lung tissue at 3 h
after exposure to none detected at 7 days after exposure. No gross
lung lesions were observed.
Fisher & Rosner (1959) exposed 10 mice to 3 × 1010 spores of a
commercial Btk product 4 times in a 6-day period. The Btk-treated mice
exhibited no clinical signs during the treatment period and no gross
pathological changes at necropsy.
6.1.3 Dermal exposure
This test is similar to the dermal exposure tests used in
chemical toxicology. Bt does not have any external contact toxicity
due to its mode of action, as shown in the following studies.
De Barjac et al. (1980) applied 5.1 × 107 cfu Bti of a 48-h
laboratory grown culture to the skin of 20 female Swiss mice. No
mortality was observed and there was no evidence of skin inflammation.
Other studies, reviewed by McClintock et al. (1995), indicate
that Bt was not toxic or pathogenic to rabbits following dermal
exposure to various Bt subspecies at doses of up to 2500 mg/kg. In
some cases, mild irritation was observed.
6.1.4 Dermal scarification exposure
This test evaluates both the potential toxicity and infectivity
of a MPCA. In the case of Bt, toxicity is unlikely due to its mode of
action. However, this test also evaluates the importance of intact
skin in preventing infection by Bt.
Fisher & Rosner (1959) scarified the skin of 4 rabbits, then
applied 2.2 × 106 cfu of a commercial Btk formulation. No skin
inflammation or sign of infection was observed.
De Barjac et al. (1980) applied 3.3 × 1013 cfu of a commercial
Bti formulation to the skin of 6 male New Zealand White rabbits. No
skin inflammation or sign of infection was observed.
6.1.5 Subcutaneous inoculation
This route of exposure is considered a more challenging test of
potential infectivity than oral or dermal exposure, because the
barrier of the skin is breached. However, subcutaneous exposure may
take place only if the skin is damaged by the spraying or is already
otherwise damaged.
De Barjac et al. (1980) subcutaneously inoculated 20 female Swiss
mice and 10 tricolour guinea-pigs, respectively, with 8.5 × 107 cfu
and 1.7 × 108 cfu of a 48-h laboratory-grown Bti culture. There was
no evidence of infection and no mortality was observed.
Siegel et al. (1987) subcutaneously inoculated 15 female BALB/c
mice with 1 × 109 cfu of a commercial Bti formulation. Abscesses
occurred at the injection site but these were attributed to the
introduction of high concentrations of heat-stable foreign material,
since they also occurred when autoclaved Bti was injected. There was
no evidence of infection and no mortality was observed.
6.1.6 Ocular exposure
The primary purpose of this procedure is to test for the
irritancy of a MPCA, although this test also evaluates potential
infectivity as well. In these tests, Bt may persist for days in rabbit
eyes because of the depth of the conjunctival sac coupled with limited
tear production by rabbits.
De Barjac et al. (1980) inoculated the eyes of six male New
Zealand White rabbits with 3.7 × 107 cfu of a 48-h laboratory-grown
Bti culture. No conjunctivitis or ocular irritation was observed.
Siegel & Shadduck (1990) inoculated 12 female New Zealand White
rabbits with 5.4 × 106 cfu of a commercial Bti formulation. No ocular
irritation or conjunctivitis was observed and no Bti was recovered by
swabbing after one week.
In data reviewed by McClintock et al. (1995), only mild
irritation was observed following ocular administration of certain Bt
subspecies to rabbits.
Siegel et al. (1987) inoculated the eyes of 6 male New Zealand
White rabbits with 50 mg of a dry powder-commercial Bti formulation
for 9 days, and another 6 male New Zealand White rabbits were treated
with 50 mg of a laboratory-grown Bti culture for 9 days. No ocular
irritation or conjunctivitis was observed in the rabbits that received
the commercial powder. The rabbits that received the laboratory
culture experienced severe conjunctival congestion and discharge. This
was not attributed to Bti but rather to the nature of the inoculum.
The laboratory strain was a dry paste with hard clumps while the
commercial formulation was a soft powder.
6.1.7 Intraperitoneal exposure
The administration of a MPCA by this route is considered a highly
challenging route of exposure. Human and animal exposure to Bt by this
route is very unlikely to occur during the course of normal
application of Bt. This route evaluates the ability of Bt to cause
infection or produce toxic metabolites in the peritoneal cavity. Some
of the safety studies that utilized this route of exposure also
evaluated the clearance of Bt over time (Table 9).
Additional studies employing mice have been conducted using this
route of exposure, which evaluates the role played by an intact immune
system in preventing infection by Bt. These studies were deemed
necessary to assess the risk posed by Bt to humans undergoing
immunosuppressive chemotherapy and the risk posed by Bt to humans
infected with the human immunodeficiency viruses. Immune suppression
in mice was accomplished either by use of corticosteroids, which
inhibited helper T-cells and selectively damaged B-cell activity, or
through the use of athymic mice, which lack the functional T
lymphocyte component of their immune system.
6.1.7.1 Immune-intact animals
De Barjac et al. (1980) intraperitoneally injected 100 female
Swiss mice with 3.4 × 107 cfu of a 48-h laboratory-grown Bti culture
and killed groups of 10 mice daily (Table 9). Blood samples were taken
by cardiac puncture and Bt was recovered until day 8. No mortality was
observed.
Fisher & Rosner (1959) intraperitoneally injected 30 mice of
unspecified sex and strain with a laboratory-grown Btk culture and
withdrew cardiac blood samples 24, 48 and 72 h after injection. There
was no mortality and Btk was recovered as late as 48 h after injection
from heart blood.
Siegel & Shadduck (1990) conducted three clearance studies using
female CD-1 mice. In one experiment, 33 females were injected with
2.7 × 107 cfu of a washed commercial Bti formulation and serially
killed over 80 days. Bti did not clear and was recovered from the
heart blood on days 67 and 80. The investigators noted that the
initial inoculum was composed of approximately 95% vegetative cells
and that vegetative cells take longer to clear than spores. This was
confirmed in a follow-up experiment in which two groups of 16 females
each were injected with inocula containing 1.5 × 107 cfu of spores
only or a 25% vegetative cell and 75% spore mixture. Both inocula
cleared exponentially from the spleens of the mice but the 100% spore
inoculum cleared sooner than did the inoculum that contained
vegetative cells. These experiments demonstrated that Bti and Btk
persist for a variable length of time in mice following injection but
that they are cleared over time. These studies also suggest that the
nature of the inoculum may play a role in the speed by which it
is cleared.
Data summarized by McClintock et al. (1995) indicate that
toxicity (100% mortality in extreme cases) may be observed following
injection of >108 cfu of certain Bt subspecies intraperitoneally
in mice. Lower doses (< 107 cfu/mouse) were non-toxic. Death
generally occurred shortly after injection, indicating that an
infectious process had not occurred. Although the basis for the
toxicity observed at doses >108 cfu is not understood, these
findings are not considered as evidence of a hazard associated with Bt
products, since the route of administration is not relevant to human
and animal exposure conditions.
Table 9. Acute toxicity of Bt after intraperitoneal injection of guinea-pigs, mice and rats
Subspecies Material tested Test animal Dose Mortalitya Reference
tested
Btk Washed cell, 24-h cultureb Rat, female, Sprague-Dawley 1.4 × 109cfu 0/6 Shadduck, 1980
Btk Commercial product Mouse 3 × 109cfu 0/5 Fisher & Rosner, 1959
Bti 48-h cultureb Mouse, female, Swiss 6.8 × 107cfu 0/20 de Barjac et al., 1980
Bti 48-h cultureb Guinea-pig, female, tricolour 1.7 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellb, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rats, male and female Sprague-Dawley 4 × 107cfu 1/20 Siegel et al., 1987
a Number dead/number treated
b Laboratory grown culture
6.1.7.2 Immune-suppressed animals
Siegel et al. (1987) injected 42 female BALB/c mice with 1.25 mg
of a cortisone acetate twice weekly in order to suppress their immune
system and subsequently injected them with 3.4 × 107 cfu of a washed
commercial Bti formulation. Three cortisone-treated mice but none of
the non-cortisone-treated mice died but this mortality was attributed
to injury caused by injection. In the remaining 39 mice Bti was still
recovered in the spleen 49 days after injection. In a companion
experiment, 42 athymic mice were injected with the same dose of a
washed commercial Bti formulation. Twenty-six of the 42 died within 5
to 10 h after injection; autopsy did not reveal the cause of death. In
the surviving mice, Bti was recovered in the spleen 49 days after the
injection. In a follow-up experiment, 30 athymic mice were injected
with 2.6 × 107 cfu of another (washed) commercial Bti formulation and
serially killed over a 36-day period. No mortality occurred. Bti was
still recovered on day 36 after injection.
Siegel & Shadduck (1990) injected 24 athymic mice with 2.7 × 107
cfu of a washed commercial Bti formulation and evaluated clearance
over a 27-day period. No mortality was observed and clearance was
faster in the euthymic than athymic mice. Bti was still recovered 27
days after injection.
These experiments demonstrated that an intact immune system is
not essential to prevent infection by Bti and Btk, but the kinetics of
clearance differ between athymic and euthymic mice as well as between
corticosteroid-treated and untreated euthymic mice. Based on these
data, immune-suppressed individuals do not face any increased risk of
infection by Bt.
6.1.8 Effects of activated Bt ICP
It has been demonstrated that the alkali-solubilized ICP from Bti
is lethal when injected into mice (Thomas & Ellar, 1983).
Alkali-solubilized Bti ICP was also cytolytic to human erythrocytes,
mouse fibroblasts, and primary pig lymphocytes in vitro (Thomas &
Ellar, 1983; Gill et al., 1987). This activity is attributed to a
cytolytic factor encoded by Cyt A gene of Bti. Most other Bt
subspecies lack this gene. Human exposure to activated Bti ICP is most
unlikely.
6.1.9 Studies in wild animals
Numerous studies have been conducted on wild animals as part of
the registration process. Most of the data are proprietary and not
publicly available. No adverse effects have been reported.
In Canada, Innes & Bendell (1989) studied the effect of a
commercial Btk formulation on small mammal populations in woodland.
Populations of eight species of rodents (Clethrionomys gapperi,
Eutamius minimus, Microtus chrotorrhinus, Napaeozapus insignis,
Peromyscus maniculatus, Phenacomys intermedius, Tamias striatus
and Zapus hudsonius) and four species of shrew (Blarina
brevicauda, Sorex cinereus, Sorex fumeus and Sorex hoyii)
were studied by trapping over a 3-month period and shown to be
unaffected when compared to populations from untreated areas. This
suggests that the ingestion of infected insects by shrews had no
immediate effects on their populations.
6.2 Effects on birds
In a number of studies (Table 10), the acute toxicity and
pathogenicity of commercial Bta, Bti, Btk and Btte formulations for
young bobwhite quail (Colinus virginianus) and young mallards (Anas
platyrhynchus), when administered daily by oral gavage at high
dosages, were evaluated (Beavers et al., 1989a,b; Lattin et al.,
1990a,b,c,d; Beavers, 1991a,b). The Bt-treated birds showed no
apparent toxicity or pathogenicity. In those studies which also
evaluated feed consumption and weight gain, the Bt-treated birds
showed no effect when compared with the non-treated controls.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
breeding bird populations (13-14 families, 33-34 species) during a
field trial for spruce budworm control. The bird populations in 10-ha
control and treated plots were measured before and daily for 3 weeks
after application. No differences were detected between the
populations in the control and treated plots.
In the USA, Gaddis & Corkran (1986) evaluated the effect of a Bt
spray programme on the reproductive performance of the chestnut-backed
chickadee (Parus rufescens). This study was undertaken to determine
if secondary effects on the chickadees would result from the possible
reduction of lepidopteran species, which contribute to the diet of
this species. The data showed no treatment-related effect on the
number of eggs per nest, percentage of eggs hatched, percentage of
young fledged, percentage of nests fledging at least one young, or the
body weight of the nestlings.
6.3 Effects on aquatic vertebrates
The World Health Organization (WHO, 1982) reviewed laboratory and
field studies, performed by that time, that examined the impact of Bt
on frogs (Hyla regilla, Rana temporaria), goldfish (Carassius
auratus), mosquito fish (Gambusia affinis), newts (Taricha
torosa, Triturus vulgaris), rainwater killifish (Lucania parva)
and toads (Bufo species). No adverse effects were reported.
Under static renewal conditions, Boeri (1991) exposed rainbow
trout (Oncorhynchus mykiss) to high concentrations (100 mg/litre) of
a commercial Bta formulation for 96 h and observed no adverse effects
(Table 11).
Table 10. Effects of oral 5-day exposure of Bt on birds
Materials Species Dose Results Reference
testeda
Bta Colinus virginianus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991b
pathogenicity observed
Anas platyrhynchus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991a
pathogenicity observed
Bti Colinus virginianus 3077 mg (3.4 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990d
pathogenicity observed
Anas platyrhynchus 3077 mg (6.2 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990b
pathogenicity observed
Btk Colinus virginianus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990a
pathogenicity observed
Anas platyrhynchus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990c
pathogenicity observed
Btte Colinus virginianus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989a
pathogenicity observed
Anas platyrhynchus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989b
pathogenicity observed
a commercial preparation
Under static renewal conditions, Surprenant (1989) exposed
rainbow trout (Oncorhynchus mykiss) to high concentrations (100
mg/litre) of a commercial Btte formulation for 96 h and observed no
adverse effects (Table 11).
During 30- or 32-day static renewal tests, bluegill sunfish
(Lepomis macrochirus), sheepshead minnow (Cyprinodon variegatus)
and rainbow trout (Oncorhynchus mykiss) were exposed to commercial
Bti, Btk or Btte formulations at aqueous and dietary concentrations
from 100 to 500 times the expected environmental concentrations
(Table 11) (Christensen, 1990a,b,c,d,e,f,g,h). The results of these
studies indicated that exposure to very high concentrations of Bti,
Btk and Btte did not adversely affect the survival of these fish, nor
did it produce lesions. In the Btk study, the rainbow trout had a 20%
mortality during the last 4 days of the study (Christensen,1990b).
This effect was attributed to the excessive competition for food that
resulted from poor visibility due to the turbidity and the presence of
suspended solids encountered in the water.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
brook trout (Salvelinus fontinalis Mitchell), common white suckers
(Catostomus commersoni Lacepede) and smallmouth bass (Micropterus
dolomieui Lacepede) during a field trial for spruce budworm control.
The fish populations were assessed visually in underwater surveys
before and after the spray programme. No effect on their populations
was seen.
Two analyses of surveys of the impact of the larvicidal campaign
in the Onchocerciasis Control Programme of West Africa, which compared
fish populations during the programme with the normal yearly
fluctuation, observed little or no effects on the non-target
populations. However, few details were provided (Yameogo et al., 1988;
Levêque et al., 1988; Calamari et al., 1998).
6.4. Effects on invertebrates
6.4.1 Effects on invertebrates other than insects
The World Health Organization (WHO, 1982), reviewed laboratory
and field studies performed up to that time that examined the impact
of Bt on aquatic invertebrates, which included bivalve mollusks
(oyster larvae, Crassostrea gigas, Ostrea edulis), copepods,
decapods, flatworms, isopods, gastropods and ostracods. Of these
organisms, only a few demonstrated any adverse effects.
In Canada, Buckner et al. (1974) evaluated the impact of Btk on a
number of aquatic invertebrates during a field trial for control of
the spruce budworm. Populations of Amphipoda (amphipods), Decapoda
(crayfish), Hydracarina (water-mites), Hirudinea (leeches),
Table 11. Effects of Bt on fish
Material Species Concentration Duration Results Reference
testeda
Bta Oncorhynchus mykiss 100 mg/litre water 96 h No-observed-effect level Boeri, 1991
Btk Lepomis macrochirus 2.9 × 109cfu/litre waterb 32 days No significant toxicity Christensen, 1990a
1.2 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 2.9 × 109cfu/litre waterb 32 days 20% mortality but Christensen, 1990b
1.1 × 1010cfu/g dietc not infectivity
Cyprinodon variegatus 2.6 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990c
3.3 × 109cfu/g dietc or pathology
Bti Lepomis macrochirus 1.2 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990f
1.3 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 1.1 × 1010cfu/litre waterc 32 days No significant toxicity Christensen, 1990g
1.7 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 1.3 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990h
2.1 × 1010cfu/g dietc or pathology
Btte Salmo gairdneri 100 mg/litre water 96 h No-observed-effect level Surprenant, 1989
Oncorhynchus mykiss 1.6 × 1010 cfu/litre waterc 30 days No significant toxicity Christensen, 1990d
1.34 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 9.94 × 109cfu/g diet 30 days No significant toxicity Christensen, 1990e
or pathology
a commercial formulations
b nominal concentration
c measured average concentration
Hydrozoa (freshwater hydra), Nematoda (roundworms), Oligochaeta
(segmented worms), Porifera (freshwater sponges), Pulmonata
(freshwater snails) and Turbellaria (flatworms) were determined by
sampling 14 days prior to and up to 28 days after treatment. The
populations of these aquatic invertebrates were not affected by the
Btk treatment.
Benz & Altwegg (1975) studied the impact of Bt treatment at 100
times the recommended rate on populations of the earthworm Lumbricus
terrestris and found no effect.
Horsburgh & Cobb (1981) reported that populations of the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi
were not affected by biweekly sprays with a commercial Btk product.
Weires & Smith (1977) determined that sprays of a commercial Btk
product on apples during a 4-month season had no effect on the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi,
or on two predatory mites (Amblyseius fallacis and Zetzellia
mali).
6.4.2 Effects on non-target insects
An extensive literature exists on the consequences of exposure of
NTOs to Bt, including reports of several long-term field studies. The
data have been reviewed periodically (e.g., WHO, 1982; Lacey & Mulla,
1990; Melin & Cozzi, 1990; Molloy, 1992; Otvos & Vanderveen, 1993).
The range of non-target species that have been found to be susceptible
to direct toxic action of Bt has remained small. A list of non-target
species found to be insensitive to Btte was issued by Keller &
Langenbruch (1993). In more than 30 years of commercial use, no
serious, direct effects on NTOs have been reported as arising from
Bt-based MPCAs. Several studies which identified effects of Bt on
predators or parasitoids of susceptible insect species are listed by
Navon (1993), but the effects have been small. Mortality in bees has
been observed after exposure to vegetatively growing Bt but the effect
does not seem to be related to spores or ICPs.
6.4.2.1 Aquatic insects
Bti is specific in its toxicity to dipterans. Nevertheless, many
studies have tested the effect of Bti applications on a wide range of
aquatic insects.
Lacey & Mulla (1990) summarized a number of studies of the
effects of Bti on certain non-target arthropod species and arthropod
populations in the laboratory and field (Table 12). The results of
representative studies are summarized below.
Table 12. Effects of Bti on non-target arthropodsa
Arthropod order Type of study Resulta References
Coleoptera Laboratory - Schnetter et al., 1981
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982; Mulla, 1988
Diptera Laboratory + Garcia et al., 1980; Ali, 1981;
(Chironomidae) Ali & Baggs, 1981; Schnetter et al., 1981
Field + Mulla et al., 1971; Ali, 1981;
Mulligan & Schaefer, 1982;
Rogatin & Baizhanov, 1984
Field - Miura et al., 1980
Ephemeroptera Laboratory - Ali, 1980; Schnetter et al., 1981;
Mulligan & Schaefer, 1982
Field - Schnetter et al., 1981; Mulla et al., 1982,
Mulligan & Schaefer, 1982; Mulla, 1988
Heteroptera Field - Schnetter et al., 1981;
(Corixidae) Mulligan & Schaefer, 1982
Heteroptera Laboratory - Schnetter et al., 1981;
(Notonectidae) Olejnicek & Maryskova, 1986;
Aly & Mulla, 1987
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982;
Mulla, 1988
Field + Purcell, 1981
Odonata Laboratory - Mulla & Khasawinah, 1969;
Mulligan & Schaefer, 1982; Aly, 1985;
Aly & Mulla, 1987
Field - Mulla, 1988
a - = no effect reported; + = an effect was reported, but does not necessarily imply that either
individual arthropods or populations of arthropods were adversely affected.
Four species of chironomid larvae (Chironomus crassicaudatus,
Chironomus decorus, Glyptotendipes paripes, Tanytarsus species)
were tested with four Bti preparations. The chironomid larvae were
less susceptible to Bti, being 13- to 75-fold more tolerant than
mosquito larvae to the various Bti preparations (Ali, 1981; Ali &
Baggs 1981). Garcia et al. (1980) induced low to high levels of
mortality in some nematocerous Diptera, including a variety of taxa
in the families Ceratopogonidae, Chironomidae and Dixidae, using
dosages of Bti that were 50 to several hundredfold
higher than concentrations used for mosquito control. Schnetter et al.
(1981) reported complete mortality in chironomid larvae ( Chironomus
thummi ) exposed to high levels of Bti for 48 h without food.
Field-collected adult aquatic beetles exposed to Bti suffered little
or no mortality (Schnetter et al., 1981). Ali (1980) tested a Bti
formulation at 20 times the larvicidal dosage for mosquitos and
reported no adverse effects against larval mayflies (Baetis
species). Schnetter et al. (1981) reported that mayflies (Cloeon
species) suffered no mortality when fed Bti at high dosages.
Aly & Mulla (1987) fed Bti intoxicated mosquito larvae (Culex
quinquefasciatus) to field-collected fourth to fifth instar
backswimmers (Notonecta undulata). The predators were fed at the
rate of 10 larvae per predator per day for 4 days, then the predators
were fed unintoxicated mosquito larvae and observed for 15 to 17 days.
The nymph and adult notonectids exhibited no adverse effects.
Olejnicek & Maryskova (1986) observed no marked mortality in
backswimmers (Notonecta glauca) that were fed Bti intoxicated
mosquito larvae. Schnetter et al. (1981) found no mortality in
backswimmers (Notonecta glauca) exposed for 48 h to high levels of
Bti.
Mosquito larvae intoxicated with extremely high dosages of Bti
were fed to naiads of the dragonfly Tarnetrum corruptum and
damselfly Enallagma civile; the duration of development of the
dragonfly and damselfly naiads, from the time of exposure to
emergence, was not affected (Aly, 1985; Aly & Mulla, 1987)
Merritt et al. (1989) reported no evident of effects on the drift
of aquatic invertebrates, or on the numbers of these invertebrates in
benthic Surber samples, during a blackfly (Simulium species) control
programme. In the USA, Molloy (1992) reviewed ten field trials where
Bti was used against blackfly (Simulium species) larvae. He
concluded that, although there was a potential for adverse impact of
Bti on filter-feeding chironomids, the impact on stream insect
communities overall was very small.
Over a three-season period, Bti administered at mosquito
larvicidal rates had no adverse effects on the larvae of diving
beetles (Dytiscidae) or water scavengers (Hydrophylidae) (Mulla et
al., 1982; Mulla, 1988).
The application of a Bti formulation in a wildlife marsh showed
no adverse effects on beetle larvae (Mulligan & Schaefer, 1982).
Ali (1981) evaluated the efficacy of various levels of a Bti
formulation against chironomids in the families Chironominae and
Tanytarsinae and obtained mortality at dosages higher than those
employed to control mosquito larvae. Miura et al. (1980), using
mosquito larvicidal dosages of a commercial Bti product, showed no
reduction in the field populations of chironomids following treatment.
Mulla et al. (1971) reported marked reductions in some chironomid
populations, using a commercial Bti product at rates of 20 to 40 times
the mosquitocidal rates. Mulligan & Schaefer (1982) reported a 40 to
70% reduction in some chironomid species after application of a Bti
formulation to a wildlife marsh. Rogatin & Baizhanov (1984) noted a
significant reduction of chironomids after Bti exposure.
Extensive quantitative observations were made on mayfly nymphs,
mostly Callibaetis pacificus, but no notable effects were observed
when Bti was applied against mosquito larvae (Mulla et al., 1982;
Mulla, 1988). Mulligan & Schaefer (1982) found that Bti did not
adversely affect mayfly nymphs (Callibaetis species). Schnetter et
al. (1981) reported that mayflies (Cloeon species) were not affected
when Bti was used in floodwater mosquito (Aedes vexans) larval
habitats. Schnetter et al. (1981) collected water boatmen (Corisella
species) from mosquito larval habitats on the upper Rhine river in
Germany. The water boatman population was not affected after exposure
to Bti for 48 h. Adverse effects were noted on backswimmers (Buenoa
species, Notonecta undulata, and Notonecta unifasciata) during
field trials with Bti (Mulla et al., 1982; Mulla, 1988). Mulligan &
Schaefer (1982) reported that the backswimmer (Notonecta species)
populations in a wetland marsh were not adversely affected by the
application of a Bti formulation. Purcell (1981) noted reductions in
populations of backswimmers (Buenoa elegans, Notonecta indica)
after application of Bti, but attributed this to the flying activity
of these predators.
No adverse effects on naiads of the dragonfly (Tarnetrum
corruptum) and damselfly (Enallagma civile) were reported when Bti
was used against larval mosquito populations (Mulla & Khasawinah,
1969; Mulla, 1988).
No notable reduction in the number of nymphs of several species
of dragonfly (Anisoptera) and damselfly (Zygoptera) occurred when
Bti was applied in a wetland marsh (Mulligan & Schaefer, 1982).
In the follow-up to the Onchocerciasis Control Programme of West
Africa (section 5.1.3.2), little or no effect on the non-target
populations was observed. However, few details were provided (Yameogo
et al., 1988; Levêque et al., 1988; Calamari et al., 1998).
6.4.2.2 Terrestrial insects
Melin & Cozzi (1990) summarized a number of studies on the
effects of Btk, Btg, Btt and Bte on non-target arthropod species and
arthropod populations in the laboratory and field. Representative
studies on Btk, Btg, Btt and Bte are listed in Tables 13 and 14.
Obadofin & Finlayson (1977) determined that a commercial Btk
product had a minimal effect on the ground beetle ( Bembidion
lampros ). Wilkinson et al. (1975) evaluated the contact activity of
a commercial Btk product for 5 days at levels equivalent to field
rates on an adult ladybird beetle (Hippodamia convergens) and found
no adverse effects.
Workman (1977) exposed earwigs (Labidura riparia) to a
commercial Btk product at rates equivalent to 10 times the normal
field application rate. No mortality was observed in these predators.
Hamed (1978-1979) found that two tachinid species (Bessa fugax
and Zenilla dolosa) were not affected after being fed suspensions
of a commercial Btk product. Horn (1983) observed a reduction in the
number of syrphid larvae on collards sprayed with a commercial Btk
product. This effect was attributed to a repellent effect on the
syrphid adults.
Hamed (1978-1979) found that Picromerus bidens was not
adversely affected after feeding upon lepidopteran larvae (Yponomeuta
evonymellus) that had fed upon leaves treated with commercial Btk
products.
Hassan (1983) determined a commercial Btk product to be harmless
to adult lacewings (Chrysopa carnea) when they were exposed at
normal field rate concentrations. Wilkinson et al. (1975) found
negligible mortality in larval or adult lacewings (Chrysopa carnea)
when a commercial Btk product was applied as a contact spray at
recommended field rates.
Yousten (1973) fed lethal quantities of Btk to larval cabbage
loopers (Trichoplusia ni) and just prior to death offered these
larvae to young Chinese praying mantids (Tenodera aridifolia
subspecies sinensis). The mantids were not susceptible to the
spore-crystal mixtures in the intact insect host.
Asquith (1975) found that black ladybird beetles (Stethorus
punctum) on apple trees were not affected by treatment with a
commercial Btk product. Buckner et al. (1974) monitored populations of
ground beetles following aerial spraying of spruce with two commercial
Btk products and found no effect on these predators. Harding et al.
(1972) detected no reduction in population levels of ladybird beetles
(coccinellids), rove beetles (staphyllinids), or checkered beetles
(clerids) in plots treated with a commercial Btk product. Johnson
(1974) evaluated several commercial Btk products as both sprays and
baits on tobacco. During the 2-year study, the populations of two
coccinellids (Hippodamia convergens and Colemegilla maculata)
were not affected by the microbial treatments. Wallner & Surgeoner
(1974) found no effects on coccinellids (Cycloneda munda,
Chilocorus bivulnerus and Adalia bipuncta) following forest
sprays with a commercial Btk product.
While evaluating a commercial Btk product for the control of
gypsy moth (Lymantria dispar) and elm spanworm ( Ennomos
subsignacius ), Dunbar et al. (1972) found no adverse effect on
Table 13. Effects of Btk on non-target arthropods
Arthropod order Type of study Resultsa References
Acarina Field - Weires & Smith, 1977;
Horsburgh & Cobb, 1981
Coleoptera Laboratory - Wilkinson et al., 1975; Obadofin & Finlayson, 1977
Field - Harding et al., 1972; Buckner et al., 1974;
Johnson, 1974; Wallner & Surgeoner, 1974;
Asquith, 1975
Dermaptera Laboratory - Workman, 1977
Diptera Laboratory - Hamed, 1978-1979
Laboratory - Horn, 1983
Field - Dunbar et al., 1972; Fusco, 1980
Heteroptera Laboratory - Hamed, 1978-1979
Field - Harding et al., 1972; Elsey, 1973;
Jensen, 1974; Wallner & Surgeoner, 1974
Hymenoptera Laboratory - Krieg, 1973
(Honey-bees) Laboratory - Krieg et al., 1980
Field - Buckner et al., 1974
Hymenoptera Laboratory - Wallner & Surgeoner, 1974;
(Parasitoids) Laboratory + Hassan & Krieg, 1975; Krieg et al., 1980
Dunbar & Johnson, 1975;
Mück et al., 1981; Weseloh &
Andreadis, 1982; Wallner et al., 1983;
Thomas & Watson, 1986
Field - Dunbar et al., 1972; Buckner et al., 1974;
Wanller & Surgeoner, 1974; Hamel, 1977;
Morris et al., 1977; Morris et al., 1980;
Fusco, 1980
Field + Weseloh et al., 1983
Table 13. (cont'd)
Arthropod order Type of study Resultsa References
Neuroptera Laboratory - Wilkinson et al., 1975; Hassan, 1983
Dictyoptera Laboratory - Yousten, 1973
(mantis)
a - = no effect reported; + an effect was reported, but does not imply that either
individual arthropods or populations of arthropods were adversely affected.
Table 14. Effects of different Bt strains on non-target arthropods
Arthropod order Type of study Resulta References
Btg
Hymenoptera Laboratory - Cantwell & Shieh, 1981
(Honey-bees) Field - Burges, 1977
Field - Burges & Bailey, 1968
Btt
Coleoptera Field + Kazakova & Dzhunusov, 1977
Hymenoptera Laboratory + Krieg & Herfs, 1963; Krieg, 1973
(Honey-bees) Laboratory - Martouret & Euverte, 1964;
Cantwell et al., 1966
Hymenoptera Laboratory + Hassan & Krieg, 1975;
(Parasitoids) Salama et al., 1982; Hassan, 1983;
Salama & Zaki, 1983
Laboratory - Krieg et al., 1980
Table 14. (cont'd)
Arthropod order Type of study Resulta References
Bte
Coleoptera Laboratory - Salama & Zaki, 1983
Laboratory + Salama et al., 1982
Hymenoptera Laboratory + Salama & Zaki, 1983
(Parasitoids)
Neuroptera Laboratory + Salama et al., 1982
a - = no effect reported; + = an effect was reported, but does not imply
that either individual arthropods or populations of arthropods were adversely affected.
two tachinids (Blepharipa scutellata and Parasitigena agilis).
Fusco (1980) reported an increased incidence of parasitism by two
tachinids (Blepharipa pratensis and Compsilura concinnata) when
Btk was applied in a field study.
Elsey (1973) reported no detrimental effect on spined stiltbug
nymphs or adults (Jalysus spinosus) during a 2-month field study
with a commercial Btk product. Harding et al. (1972) conducted a 2
year study to evaluate the effects of Btk on the natural enemies of
the bollworm (Helicoverpa zea) on cotton (Gossypium hirsutum).
Following applications of Btk against this pest, they reported no
detectable effects on Anthocoridae (minute pirate bugs, Orius
species), Lygaediae (bigeyed bugs, Geocoris species), Nabidae
(damsel bugs, Nabis species), or Reduviidae (assassin bugs).
Jensen (1974) used a commercial Btk product on soybeans (Glycine
canescens) to control the green cloverworm (Plathypena scabra) and
the velvetbean caterpillar (Anticarsis gemmitalis). No adverse
effect was observed on Lygaediae (bigeyed bugs, Geocoris species)
or Nabidae (damsel bugs, Nabis species).
Wallner & Surgeoner (1974) found no effect on the spined soldier
bug (Podisus maculiventris), following forest sprays of commercial
Btk products to control the oakleaf caterpillar (Heterocampa
manteo).
Although many data exist, in a review of the effects of the use
of Btk in Canada, Addison (1993) concluded that few studies on NTOs
had used soil invertebrate species and soil conditions relevant to
field conditions in Canadian forests.
Salama & Zaki (1983) reared cotton leafworm larvae (Spodoptera
littoralis) on a diet containing Bte and then fed these larvae to
adult staphylinid beetles (Paederus alferii). Predator longevity was
not significantly affected and no difference was seen in acceptability
to predators between untreated larvae and those exposed to Bte.
Salama et al. (1982) treated aphids with sprays of Bte and
provided these treated insects to newly hatched coccinellid larvae
(Coccinella undecimpunctata). The survival of larvae of predators
was not affected by feeding on the treated prey. However, the duration
of predator larval development was increased in the group treated with
Bte and there was a definite reduction in prey consumption.
Salama et al. (1982) evaluated the effect of Bte on the
development of lacewing larvae (Chrysopa carnea) by presenting them
with either sprayed aphids or treated cotton leafworm larvae
(Spodoptera littoralis). When fed either the sprayed aphids or the
treated cotton leafworms, the duration of larval development was
significantly extended and prey consumption was significantly reduced.
6.4.2.3 Honey-bees
Krieg (1973) observed mortality in adult honey-bees (Apis
mellifera) that were fed non-sporulated broth cultures of Btk. The
mortalities were attributed to the thermolabile alpha-toxin. Since
alpha-toxin is inactivated during sporulation, the toxin would not
present a problem in sporulated commercial Btk products. When Krieg et
al. (1980) fed fully sporulated cultures of Btk to adult honey-bees at
concentrations of 1 × 108 spores and crystal per bee over a 7-day
period, no harmful effects were observed.
Cantwell & Shieh (1981) fed a 1:20 solution of Btg in a sucrose
solution to newly emerged adult honey-bees. After 14 days, there was
no difference in mortality between treated and untreated groups.
Treatment of hives resulted in no adverse effect on the adult workers
or colony life as determined by egg laying, brood production, brood
capping, or honey production.
Buckner et al. (1974) observed no adverse effects on honey-bees
following aerial spraying of spruce (Picea species) with commercial
Btk products.
Cantwell et al. (1966) fed honey-bees sugar solutions containing
Btt spores, Btt culture supernatant with beta-exotoxin, and Btt
crystals. The crystals did not harm the bees, but the supernatant
caused nearly 100% mortality at day 7. Significant mortality was seen
in the spore-treated bees at 8 days and was attributed to bacterial
septicaemia. It should be noted that the dosages of each treatment
were many times higher than the bees would be exposed to in the course
of a lepidopteran control programme. Krieg (1973) reported mortality
in honey-bees fed whole nonsporulated cultures of Btt, which was
attributed to the presence of beta-exotoxin. Krieg & Herfs (1963)
reported that vegetative cells of Btt did not harm honey-bees;
however, they reported toxicity in Btt preparations containing the
beta-exotoxin. Martouret & Euverte (1964) fed worker honey-bees
cultures of Btt incorporated into mixtures of sugar, honey and clay.
Complete mortality was seen at 7 days for the spore-crystal-exotoxin
preparation and at 14 days for the spore-crystal complex.
6.4.2.4 Parasitoids
a) Btk
Dunbar & Johnson (1975) collected adult parasitoids
(Cardiochiles nigriceps) in the field and fed them suspensions of a
commercial Btk product. In the group fed Btk, shorter life spans were
reported. Since the investigators could not be sure whether feeding
actually took place, starvation may have been the cause of death.
Hassan & Krieg (1975) observed no adverse effects on adult
chalcid wasps (Trichogramma cacoeciae) that were fed suspensions of
a commercial Btk product. Krieg et al. (1980) fed washed spores and
crystals of Btk (5 × 107 spores and crystals) for 7 days to adult
chalcid wasps (Trichogramma cacoeciae) and observed no mortality or
reduced capacity to parasitize.
Mück et al. (1981) reported significant mortality in adult
braconids (Cotesia glomerata) that were fed a commercial Btk product
at rates of 108 and 109 spores per ml, but observed little effect on
the adult parasitoids (Pimpla turionellae). They reported midgut
epithelial damage in the Pimpla turionellae, which resulted from the
ICP.
Thomas & Watson (1986) found lower survival in adult ichneumonids
(Hyposoter exiguae) fed suspensions of a commercial Btk product.
They concluded the mortality was due to the spore-crystal complex.
Wallner & Surgeoner (1974) observed no effect on parasitoids
following treatments with commercial Btk products for control of the
notodontid moth (Heterocampa manteo).
Wallner et al. (1983) reported an indirect effect on the braconid
Rogas lymantriae when it parasitized gypsy moth (Lymantria dispar)
hosts fed Btk. The sex ratio of the parasitoid offspring was skewed
towards males in the treated larvae, as the female parasitoids lay
more fertilized eggs in larger, untreated host larvae.
Weseloh & Andreadis (1982) reported synergism in laboratory tests
with gypsy moth larvae (Lymantria dispar) fed a commercial Btk
product and exposed to the braconid (Cotesia melanoscelus). The
percentage of parasitism was increased in Btk-intoxicated larvae since
these grew more slowly and were at the approximate size suitable for
parasitism for a longer time.
Buckner et al. (1974) reported no detrimental effects on
parasitoid populations following field application of a commercial Btk
product.
Dunbar et al. (1972) reported an increase in the percentage of
parasitism of gypsy moth (Lymantria dispar) and elm spanworm
(Ennomos subsignarius) larvae in forestry plots treated with a
commercial Btk product.
Fusco (1980) reported an increase in the percentage of parasitism
of gypsy moth (Lymantria dispar) larvae by the braconids Cotesia
melanoscelus and Phobocampe unicincta following aerial sprays with
a commercial Btk product.
Hamel (1977) found that parasitoids attacking early instar
western spruce budworm larvae (Choristoneura occidentalis) increased
in number following aerial application of a commercial Btk product,
while older budworm larvae were reduced in number.
In two field studies, commercial Btk products showed no
detrimental effects on parasitoid populations (Morris et al., 1977,
1980).
Wallner & Surgeoner (1974) demonstrated 6- to 12-fold increases
in the percentage of parasitism in gypsy moth larvae (Lymantria
dispar) by the braconid Cotesia melanoscelus in forestry plots
treated with a commercial Btk product.
b) Btt
Hassan (1983) observed the chalcid Trichogramma cacoeciae was
not affected by exposure to dried surface films of Btt.
Hassan & Krieg (1975) fed a suspension of three different
commercial Bt products to adult chalcids (Trichogramma cacoeciae)
and reported a minor reduction in the capacity to parasitize with
Btt, but none with the other Bt products. The effect of the Btt
product may have been due to the beta-exotoxin.
Krieg et al. (1980) fed washed spores and crystals of Btt to
adult chalcids (Trichogramma cacoeciae) for 7 days and observed no
mortality or reduced capacity to parasitize.
Lowered reproductive potential was observed for both the
braconids Microplitis demolitor and Zele chlorophthalma
following exposure to Btt (Salama et al., 1982; Salama & Zaki, 1983).
Salama & Zaki (1983) reported increased development times for
Zele chlorophthalma parasitizing the cotton leafworm Spodoptera
littoralis treated with Bte.
7. EXPOSURE AND EFFECTS ON HUMANS
There are some case reports on the occurrence of Bt in patients
with different infectious diseases. However, none of these studies
demonstrate an actual risk to human health by the use of Bt. They
emphasize the need for further research on the production of toxins,
knowledge of factors causing the genes of the toxins to be expressed,
and knowledge on the natural occurrence of Bt and Bc. The medical
practice does not discriminate between Bt and Bc as causative agents
in infectious diseases. Therefore, the true proportion of Bt in Bc-
induced disease is not known.
7.1 Bacillus thuringiensis
For aeons, humans have been exposed to Bt in their natural
habitats, particularly from soil, water and the phylloplane. However
in the recorded scientific literature, only few adverse effects to
these environmental Bt levels have been documented.
The manufacture and field application of Bt products can result
in aerosol and dermal exposure of workers and the human population,
especially by spraying programmes in populated areas. Agricultural and
horticultural uses of Bt can also result in dietary exposure.
7.1.1 Experimental exposure of humans
Eight human volunteers ingested 1 gram of a Btk formulation
(3 × 109 spores/g of powder) daily for 5 days. Of the eight
volunteers, five also inhaled 100 mg of the Btk powder daily for five
days. Comprehensive medical examinations immediately before, after,
and 4 to 5 weeks later failed to demonstrate any adverse health
effects, and all the blood chemistry and urinalysis tests were
negative (Fisher & Rosner, 1959).
Pivovarov et al. (1977) reported that ingestion of foods
contaminated with Btg at concentrations of 105 to 109 cells/g
caused nausea, vomiting, diarrhoea and tenesmus, colic-like pains in
the abdomen, and fever in three of the four volunteers studied. The
toxicity of the Btg strain may have been due to beta-exotoxin (Ray,
1990).
7.1.2. Exposure of workers during manufacture
Many manufacturers of Bt products monitor the exposure and the
associated health risks of their workers. Over a period of 30 years of
production, there have been no reports of such workers having been
adversely affected (RJ Cibulsky, personal communication, 1997).
7.1.3 Exposure of workers in spraying operations
Noble et al. (1992) studied aerosol Btk exposure and subsequent
nose and throat carriage of Bt by workers during a major spray
programme for gypsy moth (Lymantria dispar) control. Spraying down
from high lifts, spraying low foliage or spraying with prevailing
breezes resulted in lower exposures of spray operators than did
spraying upwards into trees. The mean exposure values ranged from
3.0 × 103 to 5.9 × 106 Bt spores/m3 sampled air. Individuals working
most shifts during the spray period were exposed to 5.4 × 106 to
7.2 × 107 organisms. Nearly all the workers exposed to higher
concentrations for several shifts (5 to 20) were culture-positive for
Bt, and the majority of the workers remained culture-positive for 14
to 30 days. Of those who were culture-positive, eight workers reverted
to a culture-negative status during the project or within 30 days of
project completion. During the spray programme, some workers
experienced chapped lips, dry skin, eye irritation, and nasal drip and
stuffiness, but no serious health problems resulted. These symptoms
were transient and frequently occurred during the beginning of a spray
run and when Bt spray concentrations were increased. No significant
differences were found with respect to gender or smoking status.
In the same study, Noble et al. (1992) evaluated the health
records of the general population in the county where the Btk spray
programme was conducted. After examining the records of 3500 hospital
emergency room admissions, 1140 family practice patients, and over 400
bacterial cultures from 10 hospitals, no evidence for community
illness or infections attributed to Btk could be documented.
Laferrière et al. (1987) demonstrated antibody titres in 11 of
107 workers exposed to Btk during a 2-year spraying period. By the
middle of the spray operation, seven had developed titres to
spore-crystal complexes, six to vegetative cells, and one to spores.
Their titres tended to be low, but were higher in those exposed for a
second year. Two months after the exposure ended, nine workers were
retested. Of these workers, five had no detectable antibodies to the
spore-crystal complexes, and four who had been among those with the
highest titres against vegetative cells had significantly lower
titres.
Elliott et al. (1988) measured the exposure of individual workers
and other individuals within the spraying area on the day of
application during an aerial Btk spray programme for gypsy moth
(Lymantria dispar) control. Concentrations of spores were measured
using personal air sampling devices. The concentration of spores
ranged from 0 to 1.1 × 104 cfu/m3 for individual workers, the
highest concentration being incurred by a spray card checker who was
in brief contact with the material. For non-working individuals, the
average Bt exposure was 1.3 × 103 cfu/m3. In the spray area, a
general survey showed concentrations of 0 to 4.2 × 103 cfu/m3.
7.1.4 Exposure of human populations by spraying operations over
populated areas
Btk and Bti have been sprayed over populated areas in several
countries, including the USA, Canada and New Zealand. Some of these
applications have been followed by public health surveillance
programmes. In general, no (or very few) harmful effects have been
reported among residents of the sprayed communities.
7.1.5 Clinical case reports
Commercial Bt products have been used for over two decades, but
Bt has been isolated in only a few cases of human bacterial infection.
Samples & Buettner (1983) reported that a farm worker developed a
corneal ulcer in one eye. It had been accidentally splashed with a
commercial Btk product and Bt was subsequently isolated from the
affected eye. The eye was treated with a topical antibiotic and
corticosteroid and the corneal ulcer resolved 14 days after treatment.
The report attributed the corneal ulcer to Bt infection. However, the
possibility that Bt may have been a non-pathogenic contaminant of the
ulcer was not considered. There are no other reports of Bt being
associated with ocular infections in workers.
During the investigation of a gastroenteritis outbreak in a
chronic care institution, bacteria were isolated from four individuals
and were identified as B. thuringiensis. The Bt isolates showed
cytotoxic effects characteristic of B. cereus (Jackson et al.,
1995).
Damgaard et al. (1997a) isolated Bt in burn wounds in two
patients. None of the isolates showed any toxicity to Vero cells.
Hernandez et al. (1998) isolated Bt from a war wound; this strain (Bt
konkukian) could infect immunosuppressed mice after cutaneous
application.
Warren et al. (1984) reported that a research worker developed a
marked local reaction and lymphadenitis following a needle stick
injury when handling Bti. Acinetobacter calcoaceticus and Bt were
cultured from the exudate. The condition responded to penicillin.
Green et al. (1990) reported that Bt was isolated from body
fluids of 55 patients with different infectious diseases. In 52 of
them, it was considered a contaminant, while in three cases with
pre-existing medical problems, no firm conclusion was established
concerning a causal relationship between the infection and Bt.
Furthermore, Bt was isolated from the conjunctiva of a worker
presenting with conjunctivitis, and with a history of a splash with a
Bt product.
Despite the widespread use of Bt-based products, only two
incidents of possible allergic reactions have been reported to the US
EPA (McClintock et al., 1995). After detailed analysis, neither of
these was considered to be causally related to Bt.
7.1.6 Dietary exposure of the general population
In some Asian countries, Bti has been added to domestic
containers of drinking-water for mosquito control. From these high Bt
exposures in drinking-water, no adverse effects in humans have been
reported. In Africa, some rivers have been dosed with Bti at weekly
intervals for blackfly control. No adverse effects in the human
populations that drink the river water have been reported. Btk has
been reported to survive for 1 to 2 months in fresh water and in
seawater. However, viable Bt cultures have not been isolated from
drinking-water supplies (Menon & De Mestral, 1985).
There is little information on levels of Bt to be found in food,
but it is possible that, in view of the widespread prevalence of Bt,
its presence in food is common and is not always related to its use on
food products. Bt spores have been shown to be unable to germinate in
mammalian digestive systems; however, Bt has been isolated from faecal
and urinary samples in occupational studies.
Noble et al. (1992) reported that 5 out of 10 vegetable samples
were positive for Btk. The positive samples were obtained from both
supermarkets and from organically grown products. Such results may
account for the recovery of Bt from faecal and urinary samples during
the occupational studies and may reflect community exposure through
food.
7.2 Bacillus cereus
The close affiliation between Bt and Bc raises the question of
whether strains of Bt can cause human illness during vegetative
growth. During vegetative growth Bc can produce different kinds of
toxins; these toxins can cause gastrointestinal diseases in humans
after ingestion. The emetic toxin is an enzymatically synthesized
peptide that causes vomiting (Granum, 1997) a few hours after
ingestion. Most Bc strains producing this toxin seem to belong to the
same serotype (Mikami et al., 1995; Nishikawa et al., 1996). The
enterotoxins are a group of proteins causing abdominal pain and
diarrhoea after an incubation period of 8-16 h. The enterotoxins
causing gastrointestinal disease are most likely produced in the small
intestine. Characteristics of the two types of disease caused by Bc
are shown Table 15. Based on analysis of outbreaks the infective dose
is believed to vary between 105 and 108 vegetative cells or
activated spores per gram, but it may be so low as 104 (Granum,
1997). In addition to the two toxins, Bc can produce different lytic
enzymes, e.g., haemolysins, which most likely are involved in the
gastrointestinal diseases. In addition to gastrointestinal diseases Bc
can cause various diseases, notably in immunosuppressed individuals
(Drobniewski, 1993).
Analysis of reported foodborne diseases reveals that Bc is
frequently diagnosed as the cause of gastrointestinal disorders
(Notermans & Batt, 1998) in many countries. Several food-borne disease
outbreaks caused by Bc have been reported by Notermans & Batt (1998).
However, Bc gastrointestinal diseases are highly under-reported, as
both types of illness are relatively mild and usually last less than
24 h (Granum, 1997; Notermans & Batt, 1998). The incidence of Bc in
foods varies between 101 and 107, the highest concentrations being
found in herbs/spices and boiled rice (Notermans & Batt, 1998). The
degree of toxicity of enterotoxins varies from Bc strain to strain,
probably due to differences in toxin components (Lund & Granum, 1997).
Hassan & Nabbut (1996) found that clinical Bc isolates from human
diarrhoeal faeces were strong producers of diarrhoeal enterotoxin,
while isolates from blood, wounds, normal faeces, milk and rice were
weak producers of diarrhoeal enterotoxin (Hassan & Nabbut, 1996). This
variation is reflected in the variable numbers (105-108 viable cells
or spores per g) of Bc reported to cause symptoms in humans, and it
has been suggested that foods containing more than 104 Bc per g may
not be safe for consumption (Granum, 1997). Several European countries
have a critical level of 104-105 Bc per g for acceptance of food
products (Notermans & Batt, 1998). This critical level will include
Bt, as the methods used do not discriminate between Bc and Bt.
Table 15. Characteristics of the two types of disease caused by B. cereus
(from Granum, 1997)
Characteristic Emetic syndrome Diarrhoeal
Infective dose (cells/g) 105-108 104-107
Toxin produced Preformed in food In the small intestine
Type of toxin Cyclic peptide Protein
Incubation period (h) 0.5-5 8-16 (occasionally >24)
Duration of illness 6-24 12-24 (occasionally many days)
Symptoms Vomiting, nausea, malaise Abdominal pain, diarrhoea
8. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and from biologically active products other than
the ICPs. Bt products may be safely used for the control of insect
pests of agricultural and horticultural crops as well as forests. Bt
is also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt have the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
9. CONCLUSIONS AND RECOMMENDATIONS
* Bt may be safely used for the control of insect pests of
agricultural crops and forests.
* Bti is safe for use in aquatic environments, including
drinking-water reservoirs, for the control of mosquito, blackfly
and nuisance insect larvae.
* Bt products should contain the ICPs and be free from other
microorganisms and biologically active metabolites.
* New Bt products based on either new Bt strains and/or new ICPs
require appropriate assessment.
* FAO and WHO should develop standard specifications for Bt
preparations as is done for chemical pesticides.
* Good industrial large-scale practice (GILSP) standards should be
employed for the production of Bt products.
* Standardized valid methods for the assessment of gastrointestinal
consequences of vegetatively produced agents should be developed.
* The occurrence of resistant insect populations underscores the
need for research on the relationships between cry-toxins and
the ecology of Bt.
* More research on the fate of Bt spores and ICPs in the
environment is needed. This should cover the natural occurrence
of Bt and Bc in foods and its relationship to exposure to Bt from
its pesticide use.
* Research into dose-response analysis and the consequent
acceptable daily intake levels of Bt in the diet and beverages is
a high priority.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANISATIONS
WHO (1985) considered the safe use of MPCA at the Ninth Meeting
of the WHO Expert Committee on Vector Control in 1984. The report
considered that addition of live microorganisms to drinking-water is
undesirable and recommended that the use of Bt H-14 for the control of
Aedes aegypti in drinking-water should be restricted to the
asporogenic form. At the 1990 meeting, WHO (1991), after reviewing new
research data, stated that its previous recommendation was unduly
restrictive, provided that properly designed formulations were used.
REFERENCES
Addison JA (1993) Persistence and nontarget effects of Bacillus
thuringiensis in soil: a review. Can J Forensic Res, 23: 2329-2342.
Agata N, Ohta M, Arakawa Y, & Mori M (1995) The bceT gene of
Bacillus cereus encodes an enterotoxic protein. Microbiology, 141:
983-988.
Ahmed R, Sankar-Mistry P, Jackson S, Ackermann H-W, & Kasatiya SS
(1995) Bacillus cereus phage typing as an epidemiological tool in
outbreaks of food poisoning. J Clin Microbiol, 33: 636-640.
Akiba Y (1986) Microbial ecology of Bacillus thuringiensis: VI.
Germination of Bacillus thuringiensis spores in the soil. Appl
Entomol Zool, 21: 76-80.
Akiba Y (1991) Assessment of rainwater-mediated dispersion of
field-sprayed Bacillus thuringiensis in the soil. Appl Entomol Zool,
26: 477-483.
Ali A (1980) Nuisance chironomids and their control: a review. Bull
Entomol Soc Am, 26: 3-16.
Ali A (1981) Bacillus thuringiensis serovar. israelensis
(ABG-6108) against chironomids and some nontarget aquatic
invertebrates. J Invertebr Pathol, 38: 264-272.
Ali A & Baggs RD (1981) Susceptibility of some Florida chironomids and
mosquitoes to various formulations of Bacillus thuringiensis serovar
israelensis de Barjac. J Econ Entomol, 74: 672-677.
Aly C (1985) Germination of Bacillus thuringiensis var. israelensis
spores in the gut of Aedes larvae (Diptera: Culicidae). J Invertebr
Pathol, 45: 1-8.
Aly C & Mulla MS (1987) Effect of two microbial insecticides on
aquatic predators of mosquitoes. J Appl Entomol, 103: 113-118.
Aly C, Mulla MS, & Federici BA (1985) Sporulation and toxin production
by Bacillus thuringiensis var. israelensis in cadavers of mosquito
larvae (Diptera: Culicidae). J Invertebr Pathol, 46: 251-258.
Andersson MA, Mikkola R, Helin J, Andersson MC, & Salkinoja-Salonen M
(1998) A novel sensitive bioassay for detection of Bacillus cereus
emetic toxin and related depsipeptide ionophores. Appl Environ
Microbiol, 64: 1338-1343.
Andrews RE Jr, Faust RM, Wabiko H, Raymond KC, & Bulla LA Jr (1987)
The biotechnology of Bacillus thuringiensis. CRC Crit Rev
Biotechnol, 6: 163-232.
Angus TA (1954) A bacterial toxin paralysing silkworm larvae. Nature
(Lond), 173: 545-546.
Aronson AI, Han ES, McGaughey W, & Johnson D (1991) The solubility of
inclusion proteins from Bacillus thuringiensis is dependent upon
protoxin composition and is a factor in toxicity to insects. Appl
Environ Microbiol, 57: 981-986.
Asano S-I, Nukumizu Y, Bando H, Hzuka T, & Yamamoto T (1997) Cloning
of novel enterotoxin genes from Bacillus cereus and Bacillus
thuringiensis. Appl Environ Microbiol, 63: 1054-1057.
Ash C, Farrow JA, Dorsch M, Stackebrandt E, & Collins MD (1991)
Comparative analysis of Bacillus anthracis, Bacillus cereus, and
related species on the basis of reverse transcriptase sequencing of
16S rRNA. Int J System Bacteriol, 41: 343-346.
Asquith D (1975) Response of the predaceous black lady beetle
Stethorus punctum (DeConte) to apple orchard insecticide treatments
(Experiment No. D986-4007). Chicago, Illinois, Abbott Laboratories.
Baida GE & Kuzmin NP (1995) Cloning and primary structure of a new
hemolysin gene from Bacillus cereus. Biochim Biophys Acta, 1264:
151-154.
Baumann L, Okamoto K, Unterman BM, Lynch MJ, & Bauman P (1984)
Phenotypic characterization of Bacillus thuringiensis and Bacillus
cereus. J Invertebr Pathol, 44: 329-341.
Beach RM (1990) Application for an experimental use permit to ship and
use a pesticide for experimental purposes only -- Permit No.
58788-EUP-4 for InCideM 586. Hanover, Maryland, Crop Genetics
International.
Beattie SH, Holt C, Hirst D, & Williams AG (1998) Discrimination among
Bacillus cereus, B. mycoides and B. thuringiensis and some other
species of the genus Bacillus by Fourier transform infrared
spectroscopy. FEMS Microbiol Lett, 164: 201-206.
Beavers JB (1991a) ABG-6305: An avian oral pathogenicity and toxicity
study in the mallard (Project No. 161-118). Easton, Maryland, Wildlife
International Ltd, pp 1-20 (Unpublished Abbott document).
Beavers JB (1991b) ABG-6305: An avian oral pathogenicity and toxicity
study in the bobwhite (Project No. 161-117). Easton, Maryland,
Wildlife International Ltd, pp 1-21 (Unpublished Abbott document).
Beavers JB, Larsen AC, & Jaber M (1989a) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the bobwhite (Project No. 161-109). Eston, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Beavers JB, Clauss BS, & Jaber M (1989b) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the mallard (Project No. 161-108). Easton, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Becker N & Margalit J (1993) Use of Bacillus thuringiensis
israelensis against mosquitoes and blackflies. In: Entwistle PF, Cory
JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 147-170.
Beegle CC, Dulmage HT, Wolfenbarger DA, & Martinez E (1981)
Persistence of Bacillus thuringiensis Berliner insecticidal activity
on cotton foliage. Environ Entomol, 10: 400-401.
Benz G & Altwegg A (1975) Safety of Bacillus thuringiensis for
earthworms. J Invertebr Pathol, 26: 125-126.
Berliner E (1915) [About the sleep sickness of the Ephestia kühniella
Zell. and its vector Bacillus thuringiensis.] Z Angew Entomol, 2:
29-56 (in German).
Bernhard K & Utz R (1993) Production of Bacillus thuringiensis
insecticides for experimental and commercial uses. In: Entwistle PF,
Cory JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 255-267.
Bernier RL Jr, Gannon DJ, Moser GP, Mazzarello M, Griffiths MM, &
Guest PJ (1990) Development of a novel Bt strain for the control of
forestry pests. In: Brighton Crop Protection Conference -- Pests and
Diseases -- 1990: Proceedings. Farnham, Surrey, British Crop
Protection Council, pp 245-252.
Boeri RL (1991) Acute toxicity of ABG-6305 to the rainbow trout
(Oncorhynchus mykiss) (Project No. 9107-A). Hampton, New Hampshire,
Resource Analysts Inc, Enviro Systems Division, pp 1-26 (Unpublished
Abbott document).
Bonnefoi A & Béguin S (1959) Recherches sur l'action des cristaux de
Bacillus thuringiensis souche "anduze". Entomophaga, 4: 193-199.
Bourque SN, Valero JR, Lavoie MC, & Levesque RC (1995) Comparative
analysis of the 16S-23S ribosomal intergenic spacer sequences of
Bacillus thuringiensis strains and subspecies and of closely related
species. Appl Environ Microbiol, 61: 1623-1626.
Bravo A, Sarabia S, Lopez L, Ontiveros H, Abarca C, Ortiz M, Lina L,
Villalobos FJ, Pena G, Nunez-Valdez M-E, Soberón M, & Quintero R
(1998) Characterization of cry genes in a Mexican Bacillus
thuringienis strain collection. Appl Environ Microbiol, 64:
4965-4972.
Brousseau R, Saint-Onge A, Prefontaine G, Masson L, & Cabana J (1992)
Arbitrary primer polymerase chain reaction, a powerful method to
identify Bacillus thuringiensis serovars and strains. Appl Environ
Microbiol, 59: 114-119.
Buckner CH, Kingsbury PD, McLeod BB, Mortensen KL, & Ray DGH (1974)
Impact of aerial treatment on non-target organisms, Algonquin Park,
Ontario, and Spruce Woods, Manitoba, Section F. In: Evaluation of
commercial preparations of Bacillus thuringiensis with and without
chitinase against spruce budworm. Ottawa, Ontario, Canadian Forestry
Service, Chemical Control Research Institute, pp 1-72 (Information
report CC-X-59).
Bulla LA Jr, Kramer KJ, & Davidson LI (1977) Characterization of the
entomocidal parasporal crystal of Bacillus thuringiensis. J
Bacteriol, 130: 375-383.
Bulla LA Jr, Faust RM, Andrews R, & Goodman N (1985) Insecticidal
bacilli. In: Dubnau DA ed. The molecular biology of the bacilli. New
York, London, Academic Press Inc., vol 2, pp 185-209.
Burges HD (1977) Control of the wax moth Galleria mellonella on
beecomb by H-serotype V Bacillus thuringiensis and the effect of
chemical additives. Apidologie, 8: 155-168.
Burges HD (1980) Safety, safety testing and quality control of
microbial pesticides. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
738-767.
Burges HD & Bailey L (1968) Control of the greater and lesser wax
moths (Galleria mellonella and Achroia griesella) with Bacillus
thuringiensis. J Invertebr Pathol, 11: 184-195.
Burges HD & Hurst JA (1977) Ecology of Bacillus thuringiensis in
storage moths. J Invertebr Pathol, 30: 131-139.
Calamari D, Yameogo L, Hougard J-M, & Levêque C (1998) Environmental
assessment of larvicide use in the onchocerciasis control programme.
Parasitol Today, 14: 485-489.
Campbell DP, Dieball DE, & Brackett JM (1987) Rapid HPLC assay for the
ß-exotoxin of Bacillus thuringiensis. J Agric Food Chem, 35:
156-158.
Cannon RJC (1996) Bacillus thuringiensis use in agriculture: a
molecular perspective. Biol Rev Cambridge Philos Soc, 71: 561-636.
Cantwell GE & Shieh TR (1981) CertanTM: A new bacterial insecticide
against the greater wax moth. Am Bee J, 121: 424-431.
Cantwell GE, Heimpel AM, & Thompson MJ (1964) The production of an
exotoxin by various crystal-forming bacteria related to Bacillus
thuringiensis var. thuringiensis Berliner. J Insect Pathol, 6:
466-480.
Cantwell GE, Knox DA, Lehnert T, & Michael AS (1966) Mortality of the
honey bee, Apis mellifera, in colonies treated with certain
biological insecticides. J Invertebr Pathol, 8: 228-233.
Car M (1984) Laboratory and field trials with two Bacillus
thuringiensis var. israelensis products for Simulium (Diptera:
Nematocera) control in a small polluted river in South Africa.
Onderstepoort J Vet Res, 51: 141-144.
Car M & de Moor FC (1984) The response of Vaal River drift and benthos
to Simulium (Diptera: Nematocera) control using Bacillus
thuringiensis var. israelensis (H-14). Onderstepoort J Vet Res, 51:
155-160.
Carlberg G, Kihamia CM, & Minhar J (1985) Microbial control of flies
in latrines in Dar es Salaam with a Bacillus thuringiensis (serotype
1) preparation, Muscabac. J Appl Microbiol Biotechnol, 1: 33-44.
Carlson CR & Kolsto A-B (1993) A complete physical map of a Bacillus
thuringiensis chromosome. J Bacteriol, 175: 1053-1060.
Carlson CR, Caugant DA, & Kolstr A-B (1994) Genotypic diversity among
Bacillus cereus and Bacillus thuringiensis strains. Appl Environ
Microbiol, 60: 1719-1725.
Carlton BC, Gawron-Burke C, & Johnson TB (1990) Exploiting the genetic
diversity of Bacillus thuringiensis for the creation of new
bioinsecticides. In: Proceedings of the 5th International Colloquium
on Invertebrate Pathology and Microbial Control. Adelaide, Australia,
Society for Invertebrate Pathology, pp 18-22.
Chilcott CN, Pillai JS, & Kalmakoff J (1983) Efficacy of Bacillus
thuringiensis var. israelensis as a biocontrol agent against
larvae of Simuliidae (Diptera) in New Zealand. NZ J Zool, 10: 319-326.
Christensen KP (1990a) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 32-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-53 (Unpublished Abbott document No. 90-1-3211).
Christensen KP (1990b) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-57
(Unpublished Abbott document No. 90-2-3219).
Christensen KP (1990c) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc.,
vol 2, pp 253-308 (Unpublished Abbott document No. 90-5-3317).
Christensen KP (1990d) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to rainbow trout (Oncorhynchus mykiss)
during a 30-day static renewal test. Wareham, Massachusetts,
Springborn Laboratories Inc., pp 1-54 (Unpublished Abbott document No.
90-3-3263).
Christensen KP (1990e) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to sheepshead minnow (Cyprinodon
variegatus) during a 30-day static renewal test. Wareham,
Massachusetts, Springborn Laboratories Inc., pp 1-50 (Unpublished
Abbott document No. 90-6-3348).
Christensen KP (1990f) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-55 (Unpublished Abbott document No. 90-2-3228).
Christensen KP (1990g) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-55
(Unpublished Abbott document No. 90-2-3242).
Christensen KP (1990h) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-57 (Unpublished Abbott document No. 90-4-3288).
Cibulsky RJ & Fusco RA (1987) Recent experiences with Vectobac for
black fly control: An industrial perspective on future developments.
In: Kim KC & Merritt RW ed. Black flies: Ecology, population
management, and annotated world list. University Park, Pennsylvania,
Pennsylvania State University Press, pp 419-424.
Claus D & Berkeley RCW (1986) Genus Bacillus Cohn 1872. In: Sneath
PHA, Mair NS, Sharp ME, & Holt JG ed. Bergey's manual of systematic
bacteriology. Baltimore, Maryland, Williams & Wilkins, vol 2, pp
1105-1139.
Cooksey KE (1971) The protein crystal toxin of Bacillus
thuringiensis: Biochemistry and mode of action. In: Burges HD &
Hussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc., pp 247-274.
Crickmore N, Zeigler DR, Feitelson J, Schnepf E, van Rie J, Lereclus
D, Baum J, & Dean DH (1998) Revision of the nomenclature for the
Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol
Biol Rev, 62: 807-813.
Damgaard PH (1995) Diarrhoeal enterotoxin production by strains of
Bacillus thuringiensis isolated from commercial Bacillus
thuringiensis -- based insecticides. FEMS Immunol Med Microbiol, 12:
245-250.
Damgaard PH, Granum PE, Bresciani J, Torregrossa MV, Eilenberg J, &
Valentino L (1997a) Characterization of Bacillus thuringiensis
isolated from infections in burn wounds. FEMS Immunol Med Microbiol,
18: 47-53.
Damgaard PH, Hansen BM, Pedersen JC, & Eilenberg J (1997b) Natural
occurrence of B. thuringiensis on cabbage foliage and in insects
associated with cabbage crops. J Appl Bacteriol, 82: 253-258.
Damgaard PH, Larsen HD, Hansen BM, Bresciani J, & Jorgensen K (1996a)
Enterotoxin-producing strains of Bacillus thuringiensis isolated
from food. Lett Appl Microbiol, 23: 146-150.
Damgaard PH, Jacobsen CS, & Sorensen J (1996b) Development and
application of a primer set for specific detection of Bacillus
thuringiensis and Bacillus cereus in soil using magnetic
capture-hybridization and PCR amplification. System Appl Microbiol,
19: 436-441.
de Barjac H (1981a) Identification of H-serotypes of Bacillus
thuringiensis. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
35-43.
de Barjac H (1981b) Insect pathogens in the genus Bacillus. In:
Berkley RCW & Goodfellow M ed. The aerobic endospore-forming bacteria:
Classification and identification. New York, London, Academic Press
Inc., pp 241-250.
de Barjac H & Bonnefoi A (1962) Essai de classification biochimique et
sérologique de 24 souches de Bacillus de type thuringiensis.
Entomophaga, 7: 5-31.
de Barjac H & Larget-Thiery I (1984) Characteristics of IPS 82 as
standard for biological assays of Bacillus thuringiensis H-14
preparations. Geneva, World Health Organization (WHO/VBC/84.892).
de Barjac H, Larget I, Bénichou L, Cosmao V, Viviani G, Ripouteau H, &
Papion S (1980) Test d'innocuité sur mammifères avec du sérotype H 14
de Bacillus thuringiensis. Geneva, World Health Organization
(WHO/VBC/80.761).
Dean DH (1984) Biochemical genetics of the bacterial insect-control
agent Bacillus thuringiensis: Basic principles and prospects for
genetic engineering. Biotechnol Genet Eng Rev, 2: 341-363.
Dean DH, Rajamohan F, Lee MK, Wu SJ, Chen XJ, Alcantara E, & Hussain
SR (1996) Probing the mechanism of action of Bacillus thuringiensis
insecticidal proteins by site-directed mutagenesis - a minireview.
Gene, 179: 111-117.
DeLucca AJ II, Simonson JG, & Larson AD (1981) Bacillus thuringiensis
distribution in soils of the United States. Can J Microbiol, 27:
865-870.
Demezas DH & Bell J (1995) Evaluation of low molecular weight RNA
profiles and ribotyping to differentiate some Bacillus species.
System Appl Microbiol, 18: 582-589.
Denolf P, Hendrickx K, Vandamme J, Jansens S, Peferoen M, Degheele D,
& Vanrie J (1997) Cloning and characterization of Manduca sexta and
Plutella xylostella midgut aminopeptidase N enzymes related to
Bacillus thuringiensis toxin-binding proteins. Eur J Biochem, 248:
748-761.
Dent DR (1993) The use of Bacillus thuringiensis as an insecticide.
In: Jones DG ed. Exploitation of microorganisms. London, Chapman &
Hall, pp 19-44.
Drobniewski FA (1993) Bacillus cereus and related species. Clin
Microbiol Rev, 6: 324-338.
Dulmage HT & cooperators (1981) Insecticidal activity of isolates of
Bacillus thuringiensis and their potential for pest control. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 193-223.
Dunbar JP & Johnson AW (1975) Bacillus thuringiensis: Effects on the
survival of a tobacco budworm parasitoid and predator in the
laboratory. Environ Entomol, 4: 352-354.
Dunbar DM, Kaya HK, Doane CC, Anderson JF, & Weseloh RM (1972) Aerial
application of Bacillus thuringiensis against larvae of the elm
spanworm and gypsy moth and effects on parasitoids of the gypsy moth.
Connecticut Experiment Station (Bulletin No. 735).
Eilenberg J, Damgaard PH, Hansen BM, Pedersen JC, Bresciani J, &
Larsson R (in press) Natural interactions between Strongwellsea
castrans, Cystosporogenes deliaradicae and Bacillus thuringiensis
in the host, Delia radicum. J Invertebr Pathol.
Elliott LJ, Sokolow R, Heumann M, & Elefant SL (1988), An exposure
characterization of a large scale application of a biological
insecticide, Bacillus thuringiensis. Appl Ind Hyg, 3: 119-122.
Elsey KD (1973) Jalysus spinosus effect of insecticide treatments on
this predator of tobacco pests. Environ Entomol, 2: 240-243.
Entwistle PF, Cory JS, Bailey MJ, & Higgs S (1983) Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, John Wiley & Sons, 311 pp.
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, & Koziel MG
(1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal
protein with a wide spectrum of activities against lepidopteran
insects. Proc Natl Acad Sci (USA), 93: 5389-5394.
Farkas J, Sebesta K, Horská K, Samek Z, Dolejs L, & Sorm F (1977)
Structure of thuringiensin, the thermostable exotoxin from Bacillus
thuringiensis. Coll Czech Chem Commun, 42: 909-929.
Fast PG (1971) Isolation of a water-soluble toxin from a commercial
microbial insecticide based on Bacillus thuringiensis. J Invertebr
Pathol, 17: 301.
Fast PG (1981) The crystal toxin of Bacillus thuringiensis. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 223-248.
Faust RM (1973) The Bacillus thuringiensis ß-exotoxin: Current
status. Bull Entomol Soc Am, 19: 153-156.
Feitelson JS (1993) The Bacillus thuringiensis family tree. In: Kim
L ed. Advanced engineered pesticides. New York, Basel, Marcel Dekker
Inc., pp 63-71.
Fisher R & Rosner L (1959) Toxicology of the microbial insecticide,
Thuricide. J Agric Food Chem, 7: 686-688.
Frommer W, Ager B, Archer L, Brunius G, Collins CH, Donikian R,
Frontali C, Hamp S, Houwink EH, Kuenzi MT, Krämer P, Lagast H, Lund S,
Mahler JL, Normand-Plessier F, Sargeant K, Tuijnenburg-Muijs G, Vranch
SP, & Werner RG (1989) Safe biotechnology: III. Safety precautions for
handling microorganisms of different risk classes. Appl Microbiol
Biotechnol, 30: 541-552.
Fusco RA (1980) Field evaluation of a commercial preparation of
Bacillus thuringiensis, DIPEL 4L: Progress report. Pennsylvania
Bureau of Forestry, Gypsy Moth Pest Management Methods Development
Project.
Gaddis PK & Corkran CC (1986) Secondary effects of Bt spray on avian
predators: the reproductive success of chestnut-backed chickadees.
Northwest Ecological Research Institute (Unpublished Research Report
No. 86-03).
Garcia R, Des Rochers B, & Tozer W (1980) Studies on the toxicity of
Bacillus thuringiensis var. israelensis against organisms found in
association with mosquito larvae. Berkeley, California, University of
California, Division of Biological Control.
Gelernter WD (1990) Targeting insecticide-resistant markets: New
developments in microbial-based products. In: Green MB, Moberg WK, &
LeBaron H ed. Managing resistance to agrochemicals: From fundamental
research to practical strategies. Washington, DC, American Chemical
Society, pp 105-117 (ACS Series 421).
Giffel MC, Beumer RR, Klijn N, Wagendorp A, & Rombouts FM (1997)
Discrimination between Bacillus cereus and Bacillus thuringiensis
using specific DNA probes based on variable regions of 16S rRNA. FEMS
Microbiol Lett, 146: 47-51.
Gill SS, Hornung JM, Ibarra JE, Singh GJP, & Federici BA (1987)
Cytolytic activity and immunological similarity of Bacillus
thuringiensis subsp. israelensis and Bacillus thuringiensis
subsp. morrisoni isolate PG-14 toxins. Appl Environ Microbiol, 53:
1251-1256.
Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, & Goebel W
(1989) A Bacillus cereus cytolytic determinant, cereolysin AB, which
comprises the phospholipase C and sphingomyelinase genes: Nucleotide
sequence and genetic linkage. J Bacteriol, 171: 744-753.
Goldberg LJ & Margalit J (1977) A bacterial spore demonstrating rapid
larvicidal activity against Anopheles sergentii, Uranotaenia
unguiculata, Culex univitattus, Aedes aegypti and Culex
pipiens. Mosq News, 37: 355-358.
González JM Jr & Carlton BC (1980) Patterns of plasmid DNA in
crystalliferous and acrystalliferous strains of Bacillus
thuringiensis. Plasmid, 3: 92-98.
González JM Jr, Dulmage HT, & Carlton BC (1981) Correlation between
specific plasmids and delta-endotoxin production in Bacillus
thuringiensis. Plasmid, 5: 351-365.
González JM, Jr Brown BJ, & Carlton BC (1982) Transfer of Bacillus
thuringiensis plasmids coding for delta-endotoxin among strains of
B. thuringiensis and B. cereus. Proc Natl Acad Sci (USA), 79:
6951-6955.
Gordon RE (1977) Some taxonomic observations on the genus Bacillus.
In: Briggs JB ed. Biological regulations of vectors: The saprophytic
and aerobic bacteria and fungi. Washington, DC, US Department of
Health, Education and Welfare, pp 67-82 (Publication NIH 77-1180).
Granum PE (1997) Bacillus cereus. In: Doyle MP, Beuchat LR, &
Montville TJ ed. Food microbiology fundamentals and frontiers.
Washington, DC, ASM Press, pp 327-336.
Grassi S & Deseö KV (1984) [The natural occurrence of Bacillus
thuringiensis Berl. And its importance in the plant protection.] In:
[Proceedings of Seminar on Phytopathology, Sorrento, Italy, 26-29
March 1984.] Bologna, Italy, Clueb Publishing Co., vol 2, pp 424-433
(in Italian).
Green M, Heumann M, Sokolow R, Foster LR, Bryant R, & Skeels M (1990)
Public health implications of the microbial pesticide Bacillus
thuringiensis: An epidemiological study, Oregon, 1985-1986. Am J
Publ Health, 80: 848-852.
Griego VM & Spence KD (1978) Inactivation of Bacillus thuringiensis
spores by ultraviolet and visible light. Appl Environ Microbiol, 35:
906-910.
Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL,
Brousseau R, & Cygler M (1995) Bacillus thuringiensis CryIA(a)
insecticidal toxin: crystal structure and channel formation. J Mol
Biol, 254: 447-464.
Hadley WM, Burchiel SW, McDowell TD, Thilsted JP, Hibbs CM, Whorton
JA, Day PW, Friedman MB, & Stoll RE (1987) Five-month oral (diet)
toxicity/ infectivity study of Bacillus thuringiensis insecticides
in sheep. Fundam Appl Toxicol, 8: 236-242.
Hamed AR (1978-79) [Effects of Bacillus thuringiensis on parasites
and predators of Yponomeuta evenymellus (Lep., Yponomeutidae).] Z
Angew Entomol, 87: 294-311 (in German).
Hamel DR (1977) The effects of Bacillus thuringiensis on parasitoids
of the western spruce budworm, Choristoneura occidentalis
(Lepidoptera: Tortricidae), and the spruce coneworm, Dioryctria
reniculelloides (Lepidoptera: Pyralidae), in Montana. Can Entomol,
109: 1409-1415.
Hannay CL (1953) Crystalline inclusions in aerobic spore-forming
bacteria. Nature (Lond), 172: 1004.
Hansen BM & Hendriksen NB (1997a) Bacillus thuringiensis and B.
cereus enterotoxins: Proceedings of the 6th European Meeting of the
International Organization for Biological and Integrated Control of
Noxious Animals and Plants/West Paleaearctic Regional Section,
Copenhagen, 10-15 August 1997. Copenhagen, Denmark, Royal Veterinary
and Agricultural University, Department of Ecology and Molecular
Biology.
Hansen BM & Hendriksen NB (1997b) Comparative PCR analysis of B.
thuringiensis and B. cereus: Abstract for the International
Workshop on Molecular Biology of B. cereus, B. anthracis and
B. thuringiensis, Soria Moria, Oslo, 23-25 May 1997.
Hansen BM & Hendriksen NB (1999) Ecological aspects of the survival in
soil of spray released Bacillus thuringiensis subsp. kurstaki. In:
Abstract for the IOBC Symposium on Indirect Ecological Effects of
Biological Control, Montpelier, 17-20 October 1999. Montpelier,
France, Agropolis International.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1996) Bacillus
thuringiensis, ecology and environmental effects of its use for
microbial pest control (Environmental Project No. 316). Copenhagen,
Denmark, Ministry of Environment and Energy, Danish Environmental
Protection Agency.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1998) Molecular
and phenotypic characterization of Bacillus thuringiensis isolated
from leaves and insects. J Invertebr Pathol, 71: 106-114.
Harding J, Wolfenbarger D, Dupnik T, & Fuchs T (1972) Large scale
tests comparing Bacillus thuringiensis with methyl-parathion for
cotton insect control, field test report. Chicago, Illinois, Abbott
Laboratories (Unpublished document).
Hassan SA (1983) Results of laboratory testing of a series of
pesticides on egg parasites of the genus Trichogramma (Hymenoptera,
Trichogrammatidae). Nachr.bl Dtsch Pflanzenschutzdienstes
(Braunschweig), 35: 21.
Hassan S & Krieg A (1975) [ Bacillus thuringiensis preparations
harmless to the parasite Trichogramma cacoeciae (Hym.:
Trichogrammatidae).] Z Pflanzenkr Pflanzenchutz, 82: 515-521 (in
German).
Hassan G & Nabbut N (1996) Prevalence and characterization of
Bacillus cereus isolates from clinical and natural sources. J Food
Prot, 59: 193-196.
Heimpel AM (1954) Investigations of the mode of action of strains of
Bacillus cereus Frankland and Frankland pathogenic for the larch
sawfly, Pristiphora chrichsonii (Htg.). Kingston, Canada, Queen's
University, 155 pp (Doctoral dissertation).
Heimpel AM (1967) A critical review of Bacillus thuringiensis var.
thuringiensis Berliner and other crystalliferous bacteria. Annu Rev
Entomol, 12: 287-322.
Heimpel AM & Angus TA (1958) The taxonomy of insect pathogens related
to Bacillus cereus Frankland and Frankland. Can J Microbiol, 4:
531-541.
Helgason E, Caugant DA, Lecadet M-M, Chen Y, Mahillon J, Lövgren A,
Hegna I, Kvaloy K, & Kolsto A-B (1998) Genetic diversity of Bacillus
cereus/ B. thuringiensis isolates from natural sources. Curr
Microbiol, 37: 80-87.
Hendriksen NB & Hansen BM (1998) Phylogenetic relations of Bacillus
thuringiensis: Implications for risks associated to its use as a
microbiological pest control agent. IOBC Bull, 21: 5-8.
Hernandez E, Ramisse F, Ducoureau J-P, Cruel T, & Cavallo J-D (1998)
Bacillus thuringiensis subsp. konkukian (serotype H34)
superinfection: Case report and experimental evidence of pathogenicity
in immunosuppressed mice. J Clin Microbiol, 36: 2138-2139.
Höfte H & Whiteley HR (1989) Insecticidal crystal proteins of
Bacillus thuringiensis. Microbiol Rev, 53: 242-255.
Honée G & Visser B (1993) The mode of action of Bacillus
thuringiensis crystal proteins. Entomol Exp Appl, 69: 145-155.
Horn DJ (1983) Selective mortality of parasitoids and predators of
Myzus persicae on collards treated with malathion, carbaryl, or
Bacillus thuringiensis. Entomol Exp Appl, 34: 208-211.
Horsburgh R & Cobb L (1981) Effect of Dipel WP and Dipel + Guthion
tank mix combination for control of variegated leafroller, turfed
apple bud moth, and red-banded leafroller larvae on apples in a full
season and late season program in Virginia (Experiment No.
D-986-4240). Chicago, Illinois, Abbott Laboratories Inc.
Hostetter DL, Ignoffo CM, & Kearby WH (1975) Persistence of
formulations of Bacillus thuringiensis spores and crystals on
eastern red cedar foliage in Missouri. J Kansas Entomol Soc, 48:
189-193.
Hotha S & Banik RM (1997) Production of alkaline protease by Bacillus
thuringiensis H 14 in aqueous two-phase systems. J Chem Technol
Biotechnol, 69: 5-10.
Huber HE & Lüthy P (1981) Bacillus thuringiensis delta-endotoxin:
Composition and activation. In: Davidson EW ed. Pathogenesis of
invertebrate microbial diseases. Totowa, New Jersey, Allanheld-Osmun
Publishers, pp 209-234.
Huber HE, Lüthy P, Ebersold HR, & Cordier JL (1981) The subunits of
the parasporal crystal of Bacillus thuringiensis: size, linkage and
toxicity. Arch Microbiol, 129: 14-18.
Ignoffo CM (1973) Effects of entomopathogens on vertebrates. Ann NY
Acad Sci, 217: 141-164.
Ignoffo CM (1992) Environmental factors affecting persistence of
entomopathogens. Fla Entomol, 75: 516-525.
Innes DGL & Bendell JF (1989) The effects on small-mammal populations
of aerial applications of Bacillus thuringiensis, fenitrothion, and
Matacil(R) used against jack pine budworm in Ontario. Can J Zool,
67: 1318-1323.
Jackson SG, Goodbrand RB, Ahmed R, & Kasatiya S (1995) Bacillus
cereus and Bacillus thuringiensis isolated in a gastroenteritis
outbreak investigation. Lett Appl Microbiol, 21: 103-105.
Jaquet F, Hütter R, & Lüthy P (1987) Specificity of Bacillus
thuringiensis delta-endotoxin. Appl Environ Microbiol, 53: 500-504.
Jarrett P & Stephenson M (1990) Plasmid transfer between strains of
Bacillus thuringiensis infecting Galleria mellonella and
Spodoptera littoralis. Appl Environ Microbiol, 56: 1608-1614.
Jensen R (1974) Comparison of various insecticides including DIPEL WP
for control of the velvetbean caterpillar (Anticarsia gemmatalis)
and the green cloverworm (Plathypena scabra) on soybeans and the
effect on non-target species (Experiment No D911-1582). Chicago,
Illinois, Abbott Laboratories Inc.
Johnson AW (1974) Bacillus thuringiensis and tobacco budworm control
on flue-cured tobacco. J Econ Entomol, 67: 755-759.
Juarez-Perez VM, Ferrandis MD, & Frutos R (1997) PCR-based approach
for detection of novel Bacillus thuringiensis cry genes. Appl
Environ Microbiol, 63: 2997-3002.
Kaneko T, Nozaki R, & Aizawa K (1978) Deoxyribonucleic acid
relatedness between Bacillus anthracis, Bacillus cereus and
Bacillus thuringiensis. Microbiol Immunol, 22: 639-641.
Kazakova SB & Dzhunusov KK (1977) The effect of Bitoxibacillin-202 on
certain orchard insects in the Issyk-Kul' depression. Rev Appl
Entomol, A65: 5987.
Keller B & Langenbruch G-A (1993) Control of coleopteran pests by
Bacillus thuringiensis. In: Entwistle PF, Cory JS, Bailey MJ, &
Higgs S ed. Bacillus thuringiensis, an environmental biopesticide:
Theory and practice. Chichester, New York, Toronto, Wiley & Sons, pp
171-191.
Khawaled K, Ben-Dov E, Zaritsky A, & Barak Z (1990) The fate of
Bacillus thuringiensis var. israelensis in B. thuringiensis
var. israelensis-killed pupae of Aedes aegypti. J Invertebr
Pathol, 56: 312-316.
Kim HS, Lee DW, Woo SD, Yu YM, & Kang SK (1998) Seasonal distribution
and characterization of Bacillus thuringiensis isolated from
sericultural environments in Korea. J Gen Appl Microbiol, 44: 133-138.
Klier AF & Lecadet MM (1976) Arguments based on
hybridization-competition experiments in favor of the in vitro
synthesis of sporulation-specific mRNAs by the RNA polymerase of
Bacillus thuringiensis. Biochem Biophys Res Commun, 73: 263-270.
Knowles BH & Ellar DJ (1987) Colloid-osmotic lysis is a general
feature of the mechanisms of action of Bacillus thuringiensis
delta-endotoxins with different insect specificity. Biochim Biophys
Acta, 924: 509-518.
Krieg A (1971) Concerning alpha-exotoxin produced by vegetative cells
of Bacillus thuringiensis and Bacillus cereus. J Invertebr Pathol,
17: 134-135.
Krieg A (1973) About toxic effects of cultures of Bacillus cereus
and Bacillus thuringiensis on honey bees (Apis mellifera).
Z Pflanzenkr Pflanzenschutz, 80: 483-486.
Krieg A & Herfs W (1963) The effects of Bacillus thuringiensis on
honey bees. Entomol Exp Appl, 6: 1-9.
Krieg A, Hassan S, & Pinsdorf W (1980) Comparison of the effects of
the variety israelensis with other varieties of B. thuringiensis
on non-target organisms of the order Hymenoptera: Trichogramma
cacoeciae and Apis mellifera. Anz Schädlingsk Pflanz Umweltschutz,
53: 81-83.
Krieg A, Huger AM, Langenbruch GA, & Schnetter W (1983) Bacillus
thuringiensis var. tenebrionis, a new pathotype effective against
larvae of Coleoptera .. Z Angew Entomol, 96(5): 500-508.
Krywienczyk J, Dulmage HT, & Fast PG (1978) Occurrence of two
serologically distinct groups within Bacillus thuringiensis serotype
3ab variety kurstaki. J Invertebr Pathol, 37: 372-375.
Kumar PA, Sharma RP, & Malik VS (1996) The insecticidal protein of
Bacillus thuringiensis. Adv Appl Microbiol, 42: 1-43.
Lacey LA & Mulla MS (1990) Safety of Bacillus thuringiensis ssp.
israelensis and Bacillus sphaericus to nontarget organisms in the
aquatic environment. In: Laird M, Lacey LA, & Davidson EW ed. Safety
of microbial pesticides. Boca Raton, Florida, CRC Press, pp 169-188.
Lacey LA, Escaffre H, Philippon B, Sékétéli A, & Guillet P (1982)
Large river treatment with Bacillus thuringiensis (H-14) for the
control of Simulium damnosum s.l. in the Onchocerciasis Control
Programme. Tropenmed Parasitol, 33: 97-101.
Laferrière M, Bastille A, & Nadeau A (1987) Etude immunologique
impliquant les composantes de l'insecticide biologique Bacillus
thuringiensis var. kurstaki. St. Henri Rivière-du-Loup, Québec,
Department of Public Health, Grand-Portage Regional Hospital Center,
pp 1-19 (Unpublished report).
Lattin A, Grimes J, Hoxter KA, & Smith GJ (1990a) Dipel technical
material (Bacillus thuringiensis var. kurstaki): An avian oral
toxicity and pathogenicity study in the bobwhite (Project No.
161-112). Easton, Maryland, Wildlife International Ltd, pp 1-25
(Unpublished Abbott document).
Lattin A, Grimes J, Hoxter K, & Smith GJ (1990b) VectoBac technical
material (Bacillus thuringiensis var. israelensis): An avian oral
toxicity and pathogenicity study in the mallard (Project No. 161-115).
Easton, Maryland, Wildlife International Ltd, pp 1-24 (Unpublished
Abbott document).
Lattin A, Hoxter K, Driscoll C, Grimes J, & Jaber M (1990c) Dipel
technical material (Bacillus thuringiensis var. kurstaki): An
avian oral toxicity and pathogenicity study in the mallard (Project
No. 161-113). Easton, Maryland, Wildlife International Ltd, pp 1-28
(Unpublished Abbott document).
Lattin A, Hoxter K, & Smith GJ (1990d) VectoBac technical material
(Bacillus thuringiensis var. israelensis): An avian oral toxicity
and pathogenicity study in the bobwhite (Project No. 161-114). Easton,
Maryland, Wildlife International Ltd, pp 1-27 (Unpublished Abbott
document).
Lechner S, Mayr R, Francis KP, Prüß BM, Kaplan T, Wießner-Gunkel E,
Stewart GSAB, & Scherer S (1998) Bacillus weihenstephanensis sp.
nov. is a new psychrotolerant species of the Bacillus cereus group.
Int J System Bacteriol, 48: 1373-1378.
Leong KLH, Cano RJ, & Kubinski AM (1980) Factors affecting Bacillus
thuringiensis total field persistence. Environ Entomol, 9: 593-599.
Levêque C, Fairhurst CP, Abbau K, Pangy D, Curtis MS, & Traoré K
(1988) Onchocerciasis control programme in West Africa: ten years of
monitoring fish populations. Chemosphere, 17: 421-440.
Li J, Carroll J, & Ellar DJ (1991) Crystal structure of insecticidal
(delta)-endotoxin from Bacillus thuringiensis at 2.5 Å resolution.
Nature (Lond), 353: 815-821.
Logan NA & Berkeley CW (1984) Identification of Bacillus strains
using the API system. J Gen Microbiol, 130: 1871-1882.
Lund T & Granum PE (1997) Comparison of biological effect of the two
different enterotoxin complexes isolated from three different strains
of Bacillus cereus. Microbiology, 143: 3329-3336.
Lüthy P (1986) Insect pathogenic bacteria as pest control agents.
Fortschr Zool, 32: 201-216.
Lüthy P & Ebersold HR (1981) The entomocidal toxins of Bacillus
thuringiensis. Pharmacol Ther, 13: 257-283.
Lynch MJ & Baumann P (1985) Immunological comparisons of the crystal
protein from strains of Bacillus thuringiensis. J Invertebr Pathol,
46: 47-57.
Lynch RE, Lewis LC, & Brindley TA (1976) Bacteria associated with eggs
and first-instar larvae of the European corn borer: Isolation
techniques and pathogenicity. J Invertebr Pathol, 27: 325-331.
McClintock JT, Schaffer CR, & Sjoblad RD (1995) A comparative review
of the mammalian toxicity of Bacillus thuringiensis-based
pesticides. Pestic Sci, 45: 95-105.
Manasherob R, Ben-Dov E, Zaritsky A, & Barak Z (1998) Germination,
growth, and sporulation of Bacillus thuringiensis subsp.
israelensis in excreted food vacuoles of the protozoan Tetrahymena
pyriformis. Appl Environ Microbiol, 64: 1750-1758.
Margalit J & Dean D (1985) The story of Bacillus thuringiensis var.
israelensis. J Am Mosq Control Assoc, 1: 1-7.
Martin PAW & Travers RS (1989) Worldwide abundance and distribution of
Bacillus thuringiensis isolates. Appl Environ Microbiol, 55(10):
2437-2442.
Martin WF & Reichelderfer CF (1989) Bacillus thuringiensis:
Persistence and movement in field crops. In: Abstracts of the SIP
XXIInd Annual Meeting, Centre for Adult Education, University of
Maryland, College Park, Maryland, 20-24 August 1989. Society for
Invertebrate Pathology, p 25.
Martouret D & Euverte G (1964) The effect of Bacillus thuringiensis
Berliner preparations on the honey bee under conditions of forced
feeding. J Insect Pathol, 6: 198-203.
Meadows MP (1993) Bacillus thuringiensis in the environment: Ecology
and risk assessment. In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S
ed. Bacillus thuringiensis, an environmental biopesticide: Theory
and practice. Chichester, New York, Toronto, Wiley & Sons, pp 193-220.
Meadows MP, Ellis DJ, Butt J, Jarrett P, & Burges HD (1992)
Distribution, frequency and diversity of Bacillus thuringiensis in
an animal feed mill. Appl Environ Microbiol, 58(4): 1344-1350.
Melin BE & Cozzi EM (1990) Safety to nontarget invertebrates of
Lepidopteran strains of Bacillus thuringiensis and their
(ß)-exotoxins. In: Laird M, Lacey LA, & Davidson EW ed. Safety of
microbial insecticides. Boca Raton, Florida, CRC Press, pp 149-167.
Menon AS & De Mestral J (1985) Survival of Bacillus thuringiensis
var. kurstaki in waters. Water Air Soil Pollut, 25: 265-274.
Merritt RW, Walker ED, Wilzbach MA, Cummins KW, & Morgan WT (1989) A
broad evaluation of Bti for black fly (Diptera:Simuliidae) control in
a Michigan river: efficacy, carry and nontarget effects on
invertebrates and fish. J Am Mosq Control Assoc, 5: 397-415.
Mikami T, Horikawa T, Murakami T, Sato N, Ono Y, Matsumoto T, Yamakawa
A, Murayama S, Katagiri S, & Suzuki M (1995) Examination of toxin
production from environmental Bacillus cereus and Bacillus
thuringiensis. J Pharm Soc Jpn, 115: 742-748.
Miura T, Takahashi RM, & Mulligan FS III (1980) Effects of the
bacterial mosquito larvicide, Bacillus thuringiensis serotype H-14
on selected aquatic organisms. Mosq News, 40: 619-622.
Mohd-Salleh MB, Beegle CC, & Lewis LC (1980) Fermentation media and
production of exotoxin by three varieties of Bacillus thuringiensis.
J Invertebr Pathol, 35: 75-83.
Molloy DP (1992) Impact of the black fly (Diptera: Simuliidae) control
agent Bacillus thuringiensis var. israelensis on Chironomids
(Diptera: Chronomidae) and other non-target insects: results of ten
field trials. J Am Mosq Control Assoc, 8: 24-31.
Morris ON, Armstrong JA, & Hildebrand MJ (1977) Aerial field trials
with a new formulation of Bacillus thuringiensis against the spruce
budworm, Choristoneura fumiferana (Clem.). Ottawa, Canada, Chemical
Control Research Institute (Report CC-X-144).
Morris ON, Hildebrand MJ, & Armstrong JA (1980) Preliminary field
studies on the use of additives to improve deposition rate and
efficacy of commercial formulations of Bacillus thuringiensis
applied against the spruce budworm, Choristoneura fumiferana
(Lepidoptera: Tortricidae). Sault Ste. Marie, Ontario, Canada, Forest
Pest Management Institute, pp 1-23 (Report FPM-X-32).
Moulinier CL, Mas JP, Moulinier Y, De Barjac H, Giap G, & Couprie B
(1995) Étude de l'innocuité de Bacillus thuringiensis var.
israelensis pour les larves d'huitre. Bull Soc Pathol Exot, 74(4):
381-391.
Mück O, Hassan S, Huger AM, & Krieg A (1981) Effects of Bacillus
thuringiensis Berliner on the parasitic hymenoptera Apanteles
glomeratus L. (Braconidae) and Pimpla turionellae (L.)
(Ichneumonidiae). Z Angew Entomol, 92: 303-314.
Mulla MS (1988) Activity, field efficacy and use of Bacillus
thuringiensis (H-14) against mosquitoes. In: de Barjac H &
Sutherland DJ ed. Bacterial control of mosquitoes and blackflies. New
Brunswick, New Jersey, Rutgers University Press.
Mulla MS & Khasawinah AM (1969) Laboratory and field evaluation of
larvicides against chironomid midges. J Econ Entomol, 62: 37-41.
Mulla MS, Norland RL, Fanara DM, Darwazeh HA, & McKean DW (1971)
Control of chironomid midges in recreational lakes. J Econ Entomol
64(1): 300-307.
Mulla MS, Federici BA, & Darwazeh HA (1982) Larvicidal efficacy of
Bacillus thuringiensis serotype H-14 against stagnant-water
mosquitoes and its effects on nontarget organisms. Environ Entomol,
11: 788-795.
Mulligan FS III & Schaefer CH (1982) Integration of a selective
mosquito control agent Bacillus thuringiensis serotype H-14, with
natural predator populations in pesticide-sensitive habitats. Proc
Calif Mosq Vector Control Assoc, 49: 19-22.
Murakami T, Hiraoka T, Matsumoto T, Katagiri S, Shinagawa K, & Suzuki
M (1993) Analysis of common antigen of flagella in Bacillus cereus
and Bacillus thuringiensis. FEMS Microbiol Lett, 107: 179-184.
Narva KE, Payne JM, Schwab GE, Hickle LA, Galasan T, & Sick AJ (1991)
Novel Bacillus thuringiensis microbes active against nematodes, and
genes encoding novel nematode-active toxins cloned from Bacillus
thuringiensis isolates: European patent application EP0462 721A2.
Munich, Germany, European Patent Office.
Navon A (1993) Control of Lepidopteran pests with Bacillus
thuringiensis. In: Bacillus thuringiensis, An Environmental
Biopesticide: Theory and Practice. Eds, Entwistle PF, Cory JS, Bailey
MJ & Higgs S. Chichester, New York, Toronto, Wiley & Sons, pp 126-146.
Nielsen-LeRoux C, Hansen BM, Henriksen NB (1998) Safety of Bacillus
thuringiensis. IOBC Bull, 21: 269-272.
Nishikawa Y, Kramer JM, Hanaoka M, & Yasukawa A (1996) Evaluation of
serotyping, biotyping, plasmid banding pattern analysis, and HEp-2
vacuolation factor assay in the epidemiological investigation of
Bacillus cereus emetic-syndrome food poisoning. Int J Food
Microbiol, 31: 149-159.
Noble MA, Riben PD, & Cook GJ (1992) Microbiological and
epidemiological surveillance programme to monitor the health effects
of Foray 48B BTK spray. Vancouver, Canada, Ministry of Forests of the
Province of British Columbia, pp 1-63.
Norris JR (1971) The protein crystal toxin of Bacillus
thuringiensis: biosynthesis and physical structure. In: Burges HD &
Mussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc, pp 229-246.
Notermans S & Batt CA (1998) A risk assessment approach for food-borne
Bacillus cereus and its toxins. J Appl Environ Microbiol Symp,
84(suppl): 51S-61S.
Obadofin AA & Finlayson DG (1977) Interactions of several insecticides
and a carabid predator (Bembidion lampros (Hrbst)) and their effects
on Hylemya brassicae (Bouché). Can J Plant Sci, 57: 1121-1126.
Ohana B, Margalit J, & Barak Z (1987) Fate of Bacillus thuringiensis
subsp. israelensis under simulated field conditions. Appl Environ
Microbiol, 53: 828-831.
Ohba M & Aizawa K (1978) Serological identification of Bacillus
thuringiensis and related bacteria isolated in Japan. J Invertebr
Pathol, 32: 303-309.
Ohba M & Aizawa K (1986) Distribution of Bacillus thuringiensis in
soils of Japan. J Invertebr Pathol, 47: 277-282.
Ohba M, Yu YM, & Aizawa K (1988) Occurrence of noninsecticidal
Bacillus thuringiensis flagellar serotype 14 in the soil of Japan.
Appl Microbiol, 11: 85-89.
Olejnicek J & Maryskova B (1986), The influence of Bacillus
thuringiensis on the mosquito predator Notonecta glauca. Folia
Parasitol (Prague), 37: 279.
Otvos IS & Vanderveen S (1993) Environmental report and current status
of Bacillus thuringiensis var. kurstaki use for control of forest
and agricultural insect pests. Victoria, British Columbia, Ministry of
Forests, Forestry Canada, pp 1-81.
Pedersen JC, Damgaard PH, Eilenberg E, & Hansen BM (1995) Dispersal of
Bacillus thuringiensis var. kurstaki in an experimental cabbage
field. Can J Microbiol, 41: 118-125.
Petras SF & Casida LE Jr (1985) Survival of Bacillus thuringiensis
spores in soil. Appl Environ Microbiol, 50: 1496-1501.
Pinnock DE, Brand RJ, Jackson KL, & Milstead JE (1974) The field
persistence of Bacillus thuringiensis spores on Cercis occidentalis
leaves. J Invertebr Pathol, 23: 341-346.
Pinnock DE, Milstead JE, Kirby ME, & Nelson BJ (1977) Stability of
entomopathogenic bacteria. In: Environmental stability of microbial
insecticides: Miscellaneous publications. Lanham, Maryland,
Entomological Society of America, vol 10, pp 77-97.
Pivovarov Yu P, Ivashina SA, & Padalkin VP (1977) [Hygienic assessment
of investigation of insecticide preparations.] Gig i Sanit, 8: 45-48
(in Russian).
Prasertphon S, Areekul P, & Tanada Y (1973) Sporulation of Bacillus
thuringiensis in host cadavers. J Invertebr Pathol, 21: 205-207.
Prefontaine G, Fast P, Lau PCK, Hefford MA, Hanna Z, & Brousseau R
(1987) Use of oligonucleotide probes to study the relatedness of
delta-endotoxin genes among Bacillus thuringiensis subspecies and
strains. Appl Environ Microbiol, 53: 2808-2814.
Priest FG, Goodfellow M, & Todd C (1988) A numerical classification of
the genus Bacillus. J Gen Microbiol, 134: 1847-1882.
Priest FG, Kaji DA, Rosato YB, & Canhos VP (1994) Characterization of
Bacillus thuringiensis and related bacteria by ribosomal RNA gene
restriction fragment length polymorphisms. Microbiology, 140:
1015-1022.
Purcell BH (1981) Effects of Bacillus thuringiensis var.
israelensis on Aedes taeniorhynchus and some non-target organisms
in the salt marsh. Mosq News, 41(3): 476-484.
Pusztai M, Fast P, Gringorten L, Kaplan H, Lessard T, & Carey PR
(1991) The mechanism of sunlight-mediated inactivation of Bacillus
thuringiensis crystals. Biochem J, 273: 43-47.
Quinlan RJ (1990) Registration requirements and safety considerations
for microbial pest control agents in the European Economic Community.
In: Laird M, Lacey LA, & Davidson EW ed. Safety of microbial
pesticides. Boca Raton, Florida, CRC Press, pp 11-18.
Ray DE (1990) Pesticides derived from plants and other organisms. In:
Hayes WJ & Laws ER ed. Handbook of pesticide toxicology. New York,
London, Academic Press, pp 585-636.
Reardon RC & Haissig K (1983) Spruce budworm (Lepidoptera:
tortricidae) larval populations and field persistence of Bacillus
thuringiensis after treatment in Wisconsin. J Econ Entomol, 76:
1139-1143.
Reddy A, Battisti L, & Thorne CB (1987) Identification of
self-transmissible plasmids in four Bacillus thuringiensis
subspecies. J Bacteriol 169(11): 5263-5270.
Rogatin AB & Baizhanov M (1984) [Laboratory study of Bacillus
thuringiensis israelensis serotype H-14 on various groups of
hydrobionts.] Izv Akad Nauk Kaz SSR Ser Biol, D6: 22-25 (in Russian).
Salama HS & Zaki FN (1983) Interaction between Bacillus thuringiensis
Berliner and the parasites and predators of Spodoptera littoralis
in Egypt. Z Angew Entomol, 95: 425-429.
Salama HS, Zaki FN, & Sharaby AF (1982) Effect of Bacillus
thuringiensis Berl. on parasites and predators of the cotton
leafworm Spodoptera littoralis (Boisd). Z Angew Entomol, 94:
498-504.
Saleh SM, Harris RF, & Allen ON (1970) Fate of Bacillus thuringiensis
in soil: Effect of soil pH and organic amendment. Can J Microbiol,
16: 677-680.
Samples JR & Buettner H (1983) Corneal ulcer caused by a biologic
insecticide (Bacillus thuringiensis). Am J Ophthalmol, 95(2):
258-260.
Sampson MN & Gooday GW (1998) Involvement of chitinases of Bacillus
thuringiensis during pathogenesis in insects. Microbiology, 144:
2189-2194.
Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J,
Zeigler DR, & Dean DH (1998) Bacillus thuringiensis and its
pesticidal crystal proteins. Microbiol Mol Biol Rev, 62: 775-806.
Schnetter W, Engler S, Morawcsik J, & Becker N (1981) [Efficacy of
Bacillus thurigiensis var. israelensis against mosquito larvae
and non-target organisms.] Mitt Dtsch Ges Allg Angew Entomol, 2:
195-202 (in German).
Schwartz JL, Garneau L, Masson L, & Brousseau R (1991) Early response
of cultured lepidopteran cells to exposure to delta-endotoxin from
Bacillus thuringiensis: involvement of calcium and anionic channels.
Biochem Biophys Acta, 1065: 250-260.
Shadduck JA (1980) Bacillus thuringiensis serotype H-14 maximum
challenge and eye irritation safety tests in mammals. Geneva, World
Health Organization, pp 1-21 (WHO/VBC/80.763).
Shadduck JA (1983) Some considerations on the safety evaluation of
nonviral microbial insecticides. WHO Bull, 6(1): 117-128.
Sheeran W & Fisher SW (1992) The effects of agitation, sediment and
competition on the persistence and efficacy of Bacillus thuringiensis
var. israelensis (Bti). Ecotoxicol Environ Saf, 24: 338-346.
Shevelev AB, Lewitin E, Novikova SI, Wojciechowska YaA, Usacheva EA,
Chestukhina GG, & Stepanov (1998) A new PCR-based approach to a fast
search of a wide spectrum of cry genes from Bacillus thuringiensis
strains. Biochem Mol Biol Int, 45: 1265-1271.
Shinagawa K (1990) Purification and characterization of Bacillus
cereus enterotoxin and its application to diagnosis. In: Pohland AE,
Dowell VR Jr, & Richard JL ed. Microbial toxins in foods and feeds:
Cellular and molecular modes of action. New York, Plenum Press, pp
181-193.
Siegel JP & Shadduck JA (1990) Clearance of Bacillus sphaericus and
Bacillus thuringiensis ssp. israelensis from mammals. J Econ
Entomol, 83: 347-355.
Siegel JP, Shadduck JA, & Szabo J (1987) Safety of the entomopathogen
Bacillus thuringiensis var. israelensis for mammals. J Econ
Entomol, 80: 717-723.
Slatin SL, Abrams CK, & English L (1990) Delta-endotoxins form
cation-selective channels in planar lipid bilayers. Biochem Biophys
Res Commun, 169: 765-772.
Slepecky RA & Hemphill HE (1992) The genus Bacillus-nonmedical. In:
Balows A, Truper HG, Dworkin M, Harder W, & Schleifer K-H ed. The
prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol II, pp
1663-1696.
Smedley DP & Ellar DJ (1996) Mutagenesis of 3 surface-exposed loops of
a Bacillus thuringiensis insecticidal toxin reveals residues
important for toxicity, recognition and possibly membrane insertion.
Microbiology, 142: 1617-1624.
Smith RA (1987) Use of crystal serology to differentiate among
varieties of Bacillus thuringiensis. J Invertebr Pathol, 50: 1-8.
Smith RA & Couche GA (1991) The phylloplane as a source of Bacillus
thuringiensis variants. Appl Environ Microbiol, 57: 311-315.
Snarski VM (1990) Interactions between Bacillus thuringiensis subsp.
israelensis and fathead minnows, Pimephales promelas Rafinesque,
under laboratory conditions. Appl Environ Microbiol, 56: 2618-2622.
Sorenson AA & Falcon LA (1980) Comparison of microdroplet and high
volume application of Bacillus thuringiensis on pear suppression of
fruit tree leaf roller Acrhips argyrospilus and coverage on foliage
and fruit. Environ Entomol, 9: 350.
Stahly DP, Dingman DW, Bulla LA Jr, & Aronson AI (1978) Possible
origin and function of the parasporal crystals in Bacillus
thuringiensis. Biochem Biophys Res Commun, 84(3): 581-588.
Stahly DP, Andrews RE, & Yousten AA (1991) The genus Bacillus-insect
pathogens. In: Balows A, Truper HG, Dworkin M, Harder W, & Schleifer
K-H ed. The prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol
II, pp 1697-1745.
Surprenant DC (1989) Acute toxicity of Bacillus thuringiensis var.
tenebrionis technical material to rainbow trout (Salmo gairdneri)
under static renewal conditions. Wareham, Massachusetts, Springborn
Life Sciences Inc., pp 1-19 (Unpublished Abbott document).
Tayabali AF & Seligy VL (1997) Cell integrity markers for in vitro
evaluation of cytotoxic responses to bacteria-containing commercial
insecticides. Ecotoxicol Environ Saf, 37: 152-162.
Thomas WE & Ellar DJ (1983) Bacillus thuringiensis var. israelensis
crystal delta-endotoxin: effects on insect and mammalian cells in
vitro and in vivo. J Cell Sci, 60: 181-197.
Thomas EM & Watson TF (1986) Effect of Dipel (Bacillus thuringiensis)
on the survival of immature and adult Hyposoter exiguae
(Hymenoptera: Ichneumonidae). J Invertebr Pathol, 47: 178-183.
Tomlin CDS ed. (1997) The pesticide manual, 11th ed. Farnham, Surrey,
British Crop Protection Council, 1606 pp.
Travers RS, Martin PAW, & Reichelderfer CF (1987) Selective process
for efficient isolation of soil Bacillus spp. Appl Environ
Microbiol, 53: 1263-1266.
Väisänen OM, Mwaisumo NJ, & Salkinoja-Salonen MS (1991)
Differentiation of dairy strains of the Bacillus cereus group by
phage typing, minimum growth temperature, and fatty acid analysis. J
Appl Bacteriol, 70: 315-324.
Van Frankenhuyzen K (1993) The challenge of Bacillus thuringiensis.
In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 1-35.
Vaòková J & Purrini K (1979) Natural epizooties caused by bacilli of
the species Bacillus thuringiensis and Bacillus cereus. Z Angew
Entomol, 88: 216-221.
Van Rie J, Jansens S, Höfte H, Degheele D, & Van Mellaert H (1989),
Specificity of Bacillus thuringiensis delta-endotoxins: Importance
of specific receptors on the brush border membrane of the mid-gut of
target insects. Eur J Biochem, 186: 239-247.
Venkateswerlu G & Stotzky G (1992) Binding of the protoxin and toxin
proteins of Bacillus thuringiensis subsp. kurstaki on clay
minerals. Curr Microbiol, 25: 225-233.
Visser B, Bosch D, & Honée G (1993) Domain-function studies of
Bacillus thuringiensis crystal proteins: a genetic approach. In:
Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental piopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 71-88.
Wallner W & Surgeoner G (1974) Control of oakleaf caterpillar,
Heterocampa manteo, and the impact of controls on nontarget
organisms. Chicago, Illinois, Abbott Laboratories (Unpublished
document).
Wallner WE, Dubois NR, & Grinberg PS (1983) Alteration of parasitism
by Rogas lymantriae (Hymenoptera: Braconidae) in Bacillus
thuringiensis-stressed gypsy moth (Lepidoptera: Lymantriidae) hosts.
J Econ Entomol, 76: 275-277.
Warren RE, Rubenstein D, Ellar DJ, Kramer JM, & Gilbert RJ (1984)
Bacillus thuringiensis var. israelensis: Protoxin activation and
safety. Lancet: March 24: 678-679.
Weires RW & Smith GL (1977) Apple mite control. Hudson Valley, New
York, New York State Agricultural Experimental Station.
Weseloh RM & Andreadis TG (1982) Possible mechanism for synergism
between Bacillus thuringiensis and the gypsy moth (Lepidoptera:
Lymantriidae) parasitoid, Apanteles melanoscelus (Hymenoptera:
Braconidae). Ann Entomol Soc Am, 75: 435-438.
Weseloh RM, Andreadis TG, Moore REB, & Anderson JF (1983) Field
confirmation of a mechanism causing synergism between Bacillus
thuringiensis and the gypsy moth parasitoid, Apanteles
melanoscelus. J Invertebr Pathol, 41: 99-103.
West AW, Burges HD, & Wyborn CH (1984a) Effect of incubation in
natural and autoclaved soil upon potency and viability of Bacillus
thuringiensis. J Invertebr Pathol, 44: 121-127.
West AW, Burges HD, White JR, & Wyborn CH (1984b) Persistence of
Bacillus thuringiensis parasporal crystal insecticidal activity in
soil. J Invertebr Pathol, 44: 128-133.
WHO (1982) Data sheet on the biological control agent Bacillus
thuringiensis serotype H-14 (de Barjac 1978). Geneva, World Health
Organization (WHO/VBC/79.750 Rev. 1).
WHO (1985) Ninth report of the WHO Expert Committee on Vector Biology
and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 720).
WHO (1991) Fourteenth report of the WHO Expert Committee on Vector
Biology and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 813).
Wilkinson JD, Biever KD, & Ignoffo CM (1975) Contact toxicity of some
chemical and biological pesticides to several insect parasitoids and
predators. Entomophaga, 20: 113-120.
Workman RB (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc Fla State Hortic Soc, 90: 401-402.
Wunschel D, Fox KF, Black GE, & Fox A (1994) Discrimination among the
B. cereus group, in comparison to B. subtilis, by structural
carbohydrate profiles and ribosomal RNA spacer region PCR. System Appl
Microbiol, 17: 625-635.
Yameogo L, Levêque C, Traoré K, & Fairhurst CP (1988) Dix ans de
surveillance de la faune aquatique des rivières d'Afrique de l'Ouest
traitées contre les Simulides (Diptera: Simuliidae), agents vecteurs
de l'onchocercose humaine. Nat Can, 115: 287-298.
Yousten AA (1973) Effect of the Bacillus thuringiensis
delta-endotoxin on an insect predator which has consumed intoxicated
cabbage looper larvae. J Invertebr Pathol, 21: 312-314.
Yousten AA, Genthner FJ, & Benfield EF (1992) Fate of Bacillus
sphaericus and Bacillus thuringiensis serovar israelensis in the
aquatic environment. J Am Mosq Control Assoc, 8: 143-148.
Zahner V, Momen H, Salles CA, & Rabinovitch L (1989) A comparative
study of enzyme variation in Bacillus cereus and Bacillus
thuringiensis. J Appl Bacteriol, 67: 275-282.
Zukowski K (1995) [Laboratory examination of the effectiveness of new
biological preparations for reducing populations of cockroaches
(Blattella germanica L.)]. Rocz Panstw Zakl Hig, 46: 293-297 (in
Polish).
RÉSUMÉ
La présente monographie traite des agents microbiens de lutte
contre les nuisibles utilisant le bacille Bacillus thuringiensis
(Bt). Ce bacille, qui est aussi appelé bacille de Thuringe, est
également une source très importante de gènes utilisée pour conférer
aux plantes et aux microorganismes transgéniques (ou organismes
génétiquement modifiés, OGM) la faculté de résister aux ravageurs et
autres nuisibles. Les effets que pourraient avoir ces OGM sur la santé
humaine et l'environnement présentent divers aspects qui sont sans
rapport ou tout au plus en rapport lointain avec les produits à base
de Bt et n'entrent pas, par conséquent, dans le cadre de la présente
monographie.
1. Identité, caractéristiques biologiques et méthodes d'analyse
Bacillus thuringiensis est une bactérie anaérobie facultative,
gram-positif, qui forme des inclusions protéiques caractéristiques
adjacentes à l'endospore. Certaines sous-espèces de Bt peuvent
synthétiser plusieurs inclusions parasporales. Le Bt est génétiquement
indiscernable du Bc, exception faite de son aptitude à former des
inclusions cristallines parasporales qui sont toxiques pour certains
invertébrés, notamment les larves d'insectes appartenant aux ordres
suivants: coléoptères, diptères et lépidoptères. Les inclusions
parasporales sont constituées de diverses protéines cristallisées
insecticides (ICP). Ces cristaux sont de forme variable (bipyramidale,
cuboïdale, rhomboïdale plane, sphérique ou composite, c'est-à-dire
comportant deux types de cristaux) selon la nature des protéines qui
les composent. On a établi une corrélation partielle entre la
morphologie des cristaux, la composition en protéines cristallisées et
l'activité biologique vis-à-vis des insectes.
Le taxon phénotypique fondamental est la sous-espèce,
caractérisée par son sérotype flagellaire (H). En 1998, on avait déjà
décrit 67 sous-espèces. Les gènes qui codent pour les ICP sont pour la
plupart situés sur les plasmides. Chacune de ces protéines n'est le
produit que d'un seul gène. La plupart des plasmides porteurs de gènes
ICP se transmettent facilement d'une souche bactérienne à l'autre par
conjugaison et peuvent aussi passer à une espèce bactérienne voisine.
La classification phénotypique est maintenant complétée par une
caractérisation basée sur la biologie moléculaire et plus précisément
sur la séquence des gènes codant pour les cristaux (cry et cyt)
plutôt que sur la spécificité vis-à-vis des insectes cibles. Divers
domaines des ICP sont responsables de la sensibilité de l'hôte
(reconnaissance des récepteurs) et de la toxicité (formation de
pores).
Parmi les techniques couramment utilisées pour caractériser les
souches de Bt ou les inclusions protéiques elles-mêmes, on peut citer
l'analyse des acides gras pariétaux, les anticorps monoclonaux, les
sondes d'ADN oligonucléotidiques, les profils plasmidiques, l'analyse
par amplification génique (PCR), la technique des empreintes
génétiques et les profils électrophorétiques SDS-PAGE (électrophorèse
en gel de polyacrylamide en présence de dodécylsulfate de sodium).
Certaines sous-espèces de Bt produisent pendant leur croissance
une bêta-exotoxine constitué d'un nucléotide thermostable qui peut
contaminer les produits. Cette bêta-exotoxine est toxique pour presque
toutes les formes de vie, y compris l'Homme et les insectes cibles. Au
cours de leur croissance, les diverses souches de Bt produisent toutes
sortes d'antibiotiques, d'enzymes, de métabolites et de toxines, y
compris des toxines Bc, qui peuvent avoir des effets nocifs sur les
organismes visés ou non visés. La numération des spores n'est pas le
reflet fidèle de l'activité insecticide d'une souche de Bt ou d'un
produit qui en dérive. Pour mesurer l'activité (en unités
toxicologiques internationales (ITU) par mg), on procède à un test
biologique sur insecte au moyen d'un étalon international.
2. Mode d'action sur les insectes cibles
Les Bt sporulés ou les complexes spores-ICP doivent être ingérés
par les larves d'insectes appartenant aux espèces sensibles.
L'efficacité des ICP dépend de plusieurs facteurs: solubilisation dans
l'intestin moyen, conversion de la protoxine en toxine biologiquement
active sous l'action des enzymes protéolytiques, fixation de la toxine
active aux récepteurs membranaires spécifiques par sa région
C-terminale et formation de pores par la région N-terminale entraînant
la lyse des cellules épithéliales. La germination des spores et la
prolifération de cellules bactériennes végétatives dans l'hémocèle
peut entraîner une septicémie qui contribue également à la mort. La
spécificité d'hôte des différentes ICP est essentiellement déterminée
par leur fixation aux récepteurs.
3. Habitats
On a isolé de nombreuses sous-espèces de Bt sur des insectes
morts ou mourants appartenant principalement à l'ordre des
coléoptères, des diptères et des lépidoptères, mais nombreuses sont
également celles qui ont été isolées du sol, de la surface des
feuilles ou d'autres habitats. Les cadavres d'insectes contiennent
souvent de grandes quantités de spores et d'ICP susceptibles de
pénétrer dans l'environnement. Les sous-espèces actives contre les
coléoptères et les lépidoptères sont principalement associées au sol
et aux surfaces foliaires, alors que les sous-espèces actives contre
les diptères se rencontrent communément dans les milieux aquatiques.
Dans l'environnement, les spores sont capables de persister et de se
développer en présence de conditions favorables et de nutriments
appropriés.
4. Produits du commerce. Production et épandage
Les produits commerciaux classiques à base de bacille de
Thuringe, qui utilisent des souches naturelles, représentent environ
90% du marché mondial des agents microbiologiques de lutte contre les
nuisibles. La plupart de ces produits contiennent des spores viables
et des ICP, mais dans certains d'entre eux, les spores sont inactivées
(Bti). Chaque année, on en produit quelque 13 000 tonnes par la
technique de fermentation aérobie. Les produits classiques à base de
Bt sont principalement destinés à lutter contre les lépidoptères qui
ravagent les cultures et les plantations forestières; toutefois, ces
dernières années, on a également commercialisé des souches actives
contre les coléoptères. Les programmes de santé publique utilisent
également des souches de Bti actives contre les diptères vecteurs de
maladies virales ou parasitaires.
Les formulations commerciales de bacille de Thuringe peuvent être
épandues sur le feuillage, le sol, les étendues d'eaux ou dans les
entrepôts de denrées alimentaires pour combattre les insectes. Une
fois le produit épandu dans l'écosystème, les cellules bactériennes
végétatives et les spores peuvent persister à des concentrations
progressivement décroissantes pendant des semaines, des mois ou des
années en tant que constituants de la microflore naturelle. Par
contre, les ICP perdent leur activité biologique au bout de quelques
heures ou de quelques jours.
5. Effets du Bt sur les organismes non visés
Les études effectuées sur des mammifères et notamment celles qui
ont porté sur des animaux de laboratoire ont consisté a évaluer
l'infectiosité et la toxicité éventuelles de diverses préparations à
base de Bt contenant notamment des ICP, des spores et des cellules
bactériennes en phase végétative. Sous ces trois formes, les
différentes sous-espèces de Bt se sont révélées pour la plupart non
pathogènes et non toxiques pour les diverses espèces animales
utilisées. On a montré que les cellules bactériennes en phase
végétative et les spores persistaient pendant plusieurs semaines sans
causer d'effets nocifs. En particulier, on a constaté que le Bt
n'avait pas d'effets indésirables sur les oiseaux, les poissons et de
nombreux autres vertébrés aquatiques non visés, lors d'études en
laboratoire ou sur le terrain portant sur un grand nombre de
spécimens. Il n'y a que relativement peu d'invertébrés aquatiques qui
se révèlent sensibles au Bt en laboratoire ou sur le terrain. Par
ailleurs, le bacille de Thuringe n'exerce pas non plus d'effets nocifs
sur les lombrics.
L'activité insecticide des différentes sous-espèces de Bt
présente en général une spécificité d'hôte très marquée vis-à-vis des
coléoptères, des diptères et des lépidoptères et on a montré qu'elle
n'avait pratiquement aucun effet toxique direct sur les arthropodes
non visés. La plupart des données relatives à l'innocuité de ces
produits pour les arthropodes non visés concernent les sous-espèces de
Bt actives contre les diptères et les lépidoptères.
Les études consacrées aux formulations de Bti exemptes de
contaminants toxiques ont montré qu'elles étaient sans danger pour la
plupart des arthropodes non visés. Certains moucherons (chironomides
appartenant à l'ordre des diptères) très proches des moustiques se
sont révélés sensibles à de fortes doses de Bti mais ne sont nullement
affectés aux doses utilisées pour la destruction des larves de
moustiques. Des études sur le terrain ont mis en évidence des cas de
réduction ou au contraire d'augmentation de certaines populations
d'arthropodes non visés.
Les études toxicologiques auxquelles ont été soumis de nombreux
ordres d'insectes n'ont, pour la plupart d'entre eux, révélé aucun
effet toxique imputable au Btk.
On a observé une certaine mortalité chez des abeilles
(Apis mellifera) qui avaient été soumises à des bacilles des
sous-espèces Btt et Btk en phase végétative, mais il ne semble pas que
les spores ou les ICP soient capables de produire un tel effet. En
laboratoire et sur le terrain, le Btg n'a aucun effet toxique sur les
abeilles.
Les souches de Bte productrices de bêta-exotoxine se sont
révélées capables d'exercer des effets toxiques sur les arthropodes
non visés.
6. Exposition humaine et effets du bacille de Thuringe sur l'Homme
Les ouvriers qui épandent des produits à base de Bt peuvent être
fortement exposés à ces produits par contact cutané ou par inhalation
d'aérosols. L'usage du Bt en agriculture peut entraîner la
contamination de l'eau potable et des denrées alimentaires par le
bacille. Toutefois, à l'exception de quelques cas d'irritation des
yeux ou de la peau, on n'a pas connaissance d'effets nocifs attestés
qui résulteraient d'une exposition professionnelle à des produits à
base de Bt. Des volontaires qui avaient ingéré ou inhalé de grandes
quantités de diverses formulations de Btk, n'ont ressenti aucun effet
indésirable. On a mis en évidence des anticorps dirigés contre les
cellules bactériennes, les spores et les complexes spores-cristaux
chez des ouvriers chargés de l'épandage de produits à base de Bt;
aucun effet indésirable n'a cependant été observé. On connaît le cas
d'un certain nombre de patients atteints de maladies infectieuses chez
lesquels la présence de Bt a été mise en évidence. Toutefois, aucune
des études qui leur ont été consacrées n'a permis de conclure de façon
certaine que l'utilisation du Bt comporte effectivement un risque pour
la santé humaine. Il ne semble pas non plus que la présence de Bt dans
l'eau destinée à la consommation ou dans les denrées alimentaires soit
à l'origine d'effets indésirables chez l'Homme.
7. Conclusions
Compte tenu de la spécificité de leur mode d'action, il est
improbable que les produits à base de Bt constituent un danger
quelconque pour l'Homme et les vertébrés ni pour la très grande
majorité des invertébrés non visés, pour autant qu'ils ne contiennent
pas d'autres microorganismes ou de substances biologiquement actives
autres que les ICP. On peut utiliser ces produits en toute sécurité
pour détruire les insectes qui ravagent les domaines agricoles et
horticoles ainsi que les forêts. Ils sont également sans danger pour
le milieu aquatique et on peut notamment les épandre dans les
réservoirs d'eau potable pour lutter contre les moustiques, les
simulies et les larves d'insectes incommodants. Il convient cependant
de noter qu'en phase végétative, le Bt est capable de produire des
toxines de type Bc dont on ignore si elles sont susceptibles de
provoquer des maladies chez l'Homme.
1. RESUMEN
Esta monografía trata sobre los plaguicidas microbianos (PM)
basados en Bacillus thuringiensis (Bt). Esta bacteria es también una
fuente clave de genes cuya expresión transgénica confiere resistencia
frente a plagas a plantas y microorganismos, actuando como plaguicida
en los denominados organismos modificados genéticamente (OMG). Los
posibles efectos de los OMG sobre la salud humana y el medio están
poco o nada relacionados con los productos basados en Bt, por lo que
quedan fuera del ámbito de esta monografía.
1. Identidad, características biológicas y métodos de laboratorio
Bt es una bacteria gram-positiva y anaerobia facultativa que
forma inclusiones proteicas características junto a la endospora. Las
subespecies de Bt pueden sintetizar más de una inclusión parasporal.
Desde el punto de vista genético, Bt es indistinguible de Bc,
exceptuando la capacidad de Bt para producir inclusiones parasporales
cristalinas que son tóxicas para ciertos invertebrados, en particular
para las larvas de insectos pertenecientes a los órdenes Coleóptera,
Díptera y Lepidóptera. Dichas inclusiones parasporales están
formadas por distintas proteínas cristalinas insecticidas (PCI). Los
cristales tienen formas diversas (bipiramidales, cuboides, romboides
planos, esféricos o compuestos por dos tipos de cristales),
dependiendo de su composición en PCI. Se ha comprobado que existe una
correlación parcial entre la morfología del cristal, la composición en
PCI y la bioactividad frente a los insectos diana.
El taxón fenotípico básico es la subespecie, identificada por el
serotipo flagelar (H). Hasta 1998 se habían descrito 67 subespecies.
Los genes que codifican las PCI se encuentran fundamentalmente en los
plásmidos. Cada PCI es el producto de un solo gen. La mayoría de los
plásmidos con genes de PCI se transfieren fácilmente por conjugación
entre cepas de Bt y pueden transferirse a especies bacterianas
emparentadas. La clasificación fenotípica se ha complementado en la
actualidad con la caracterización biomolecular, basada en la secuencia
de los genes de las proteínas cristalinas (cry y cyt), no en la
especificidad para las especies diana. En las PCI, la susceptibilidad
del huésped (reconocimiento de receptores) y la toxicidad (formación
de poros) son responsabilidad de dominios distintos de la molécula.
Las técnicas utilizadas habitualmente para caracterizar las cepas
de Bt o la propia PCI consisten en análisis de los ácidos grasos de la
pared celular, anticuerpos monoclonales, sondas de oligonucleótidos de
ADN, perfiles de plásmidos, análisis por reacción en cadena de la
polimerasa (PCR), estudios del ADN (huella genética) y perfiles de
SDS-PAGE (dodecil sulfato sódico -- electroforesis en gel de
poliacrilamida).
La beta-exotoxina, un nucleótido termoestable, es sintetizada por
algunas subespecies de Bt durante el crecimiento vegetativo y puede
contaminar los productos. Es tóxica para casi todas las formas de
vida, incluidos los seres humanos y los órdenes de insectos diana.
Durante el crecimiento vegetativo, varias cepas de Bt producen una
gama de antibióticos, enzimas, metabolitos y toxinas, incluidas
toxinas de Bc, que pueden tener efectos nocivos tanto en las especies
que son objetivo del plaguicida como en las que no lo son. Los
recuentos de esporas no reflejan con exactitud la actividad
insecticida de una cepa o un preparado de Bt. Se mide la potencia
(UTI/mg) de cada producto mediante ensayos biológicos para los que se
utiliza un patrón internacional basado en un insecto concreto.
2. Modo de acción en los insectos diana
Es preciso que las larvas de los insectos susceptibles ingieran
Bt esporulado con PCI o con complejos espora-PCI. La eficacia de la
PCI depende de su solubilización en el intestino medio, de la
conversión de la protoxina en la toxina biológicamente activa por la
acción de enzimas proteolíticas, de la unión específica del dominio
C-terminal de la toxina activa al receptor de membrana y de la
formación de poros por parte del dominio N-terminal, con la
consiguiente lisis de las células epiteliales. La germinación de
esporas y la proliferación de células vegetativas en el hemocele puede
ocasionar una septicemia y contribuir a la muerte. La unión de la PCI
al receptor es el determinante principal de la especificidad de
huésped para las distintas PCI de Bt.
3. Hábitats
Se han aislado muchas subespecies de Bt a partir de insectos
muertos o moribundos, la mayoría pertenecientes a los órdenes
Coleóptera, Díptera y Lepidóptera, pero también del suelo, de
superficies foliares y de otros hábitats. Los exoesqueletos de
insectos muertos contienen a menudo grandes cantidades de esporas y
PCI que pueden incorporarse al medio. Las subespecies de Bt activas
frente a coleópteros y lepidópteros se asocian fundamentalmente con el
suelo y el filoplano (superficies foliares), mientras que las activas
frente a dípteros se hallan generalmente en medios acuáticos. En el
ambiente, las esporas persisten y pueden entrar en crecimiento
vegetativo cuando las condiciones son favorables y hay nutrientes
disponibles.
4. Productos comerciales, producción y aplicación
Los preparados convencionales de Bt, que utilizan cepas de Bt que
aparecen de forma espontánea en la naturaleza, representan
aproximadamente el 90% del mercado mundial de los PM. La mayoría de
los preparados de Bt contienen PCI y esporas viables, pero en algunos
productos de Bti las esporas están inactivadas. Cada año se producen
aproximadamente 13.000 toneladas utilizando tecnología de fermentación
aerobia. Los preparados convencionales de Bt tienen como objetivos
primarios las plagas de lepidópteros que afectan a los cultivos
agrícolas y forestales; sin embargo, en los últimos años también se
han comercializado cepas de Bt activas frente a plagas de coleópteros.
Se están utilizando en programas de salud pública cepas de Bti activas
frente a dípteros vectores de enfermedades parasitarias y víricas.
Las formulaciones comerciales de Bt pueden aplicarse como
insecticidas al follaje, el suelo, el medio acuático o instalaciones
de almacenamiento de alimentos. Tras aplicar una subespecie de Bt a un
ecosistema, las células vegetativas y las esporas pueden persistir en
concentraciones gradualmente decrecientes durante semanas, meses o
años como un componente de la microflora natural. Sin embargo, las PCI
pierden su actividad biológica en horas o días.
5. Efectos de Bt sobre especies que no son objetivo del plaguicida
En estudios con mamíferos, en particular con animales de
laboratorio, se ha evaluado la posible infecciosidad y toxicidad de
diversos preparados de Bt, que comprenden las PCI, células vegetativas
y esporas. Las PCI, las esporas y las células vegetativas de las
subespecies de Bt, que se administraron por distintas vías, carecían
en su mayoría de patogenicidad y toxicidad para las diversas especies
animales estudiadas. Se comprobó que las células vegetativas o las
esporas de Bt persistían durante semanas sin causar efectos adversos.
No se ha observado que Bt afecte a pájaros, peces o muchas otras
especies de vertebrados acuáticos que no son objetivo del plaguicida y
se han estudiado en gran número de trabajos de laboratorio y de campo.
Son relativamente pocas las especies de invertebrados acuáticos
susceptibles a Bt, tanto en condiciones de laboratorio como de campo.
Bt no afecta a las lombrices de tierra.
En general, las subespecies de Bt muestran gran especificidad en
su actividad insecticida frente a Coleóptera, Díptera y
Lepidóptera, así como una toxicidad directa escasa, si no nula,
frente a los artrópodos que no son su objetivo. La mayor parte de los
datos disponibles sobre inocuidad en éstos se han obtenido con las
subespecies de Bt activas frente a Díptera y Lepidóptera.
Los estudios sobre formulaciones de Bti sin contaminantes tóxicos
no han puesto de manifiesto efectos nocivos en la gran mayoría de los
artrópodos que no son objetivo del plaguicida. Se ha comprobado que
algunas moscas enanas (Díptera: Chironomidae), estrechamente
emparentadas con los mosquitos, son sensibles a dosis altas de Bti,
pero no se ven afectadas por dosis letales para larvas de mosquito. En
estudios de campo se han descrito disminuciones o aumentos
transitorios de las poblaciones de algunos artrópodos que no son
objetivo del plaguicida.
Se han estudiado muchos órdenes de insectos, tanto en el
laboratorio como en trabajos de campo, y se ha comprobado que en la
mayoría de ellos Btk no tiene efecto.
Se ha observado mortalidad en abejas melíferas (Apis mellifera)
tras la exposición a Btt y Btk en crecimiento vegetativo, pero el
efecto no parece guardar relación con las esporas o las PCI. En los
estudios de laboratorio y de campo, Btg no mostró efectos adversos
sobre las abejas melíferas.
Se ha comprobado que cepas de Bte productoras de beta-exotoxina
tienen efectos adversos sobre artrópodos que no son objetivo del
plaguicida.
6. Exposición a Bt y efectos sobre los seres humanos
La aplicación agrícola de preparados de Bt puede suponer una
considerable exposición de los trabajadores, tanto en aerosol como
dérmica. Puede, asimismo, causar la contaminación del agua potable y
los alimentos por la bacteria. Salvo casos notificados de irritación
ocular y dérmica, no se han documentado efectos adversos sobre la
salud tras la exposición laboral a preparados de Bt. Individuos
voluntarios ingirieron e inhalaron grandes cantidades de una
formulación de Btk sin sufrir efectos adversos. Se detectaron títulos
de anticuerpos frente a las células vegetativas, las esporas y los
complejos espora-cristal en trabajadores que pulverizaban preparados
de Bt, pero no se registraron efectos adversos. Se han descrito
algunos casos de presencia de Bt en pacientes con diversas
enfermedades infecciosas. Sin embargo, ninguno de estos estudios
demuestra de forma inequívoca que el uso de Bt entrañe un riesgo real
para la salud humana. No se ha demostrado que Bt tenga efectos
adversos en seres humanos cuando está presente en el agua potable o
los alimentos.
7. Conclusiones
Debido a la especificidad de su modo de acción, es improbable que
los preparados de Bt entrañen peligro alguno para los seres humanos u
otros vertebrados, o para la gran mayoría de los invertebrados que no
constituyen su objetivo, siempre y cuando no contengan microorganismos
distintos de Bt y productos biológicamente activos distintos de las
PCI. Los preparados de Bt pueden utilizarse con seguridad para
controlar las plagas de insectos de los cultivos agrícolas y
hortícolas, así como las forestales. También es seguro su uso en
medios acuáticos, incluidos los depósitos de agua potable, para
controlar el mosquito, la mosca negra y las larvas de insectos
dañinos. Sin embargo, es preciso señalar que las formas vegetativas de
Bt pueden sintetizar toxinas del tipo de las producidas por Bc, cuya
importancia como causa de enfermedades humanas se desconoce.
Binding to specific receptors has been demonstrated to be closely
related to the insecticidal spectrum of the ICPs (Denolf et al.,
1997). Van Rie et al. (1989) found the affinity of these toxins
similar for the tobacco budworm (Heliothis virescens) and the tomato
hornworm (Manduca sexta) brush border membrane vesicles, but the
number of binding sites differed and reflected varied bioactivity.
However, the toxin affinity for binding sites does not appear constant
for all insects.
Pore or ion channel formation occurs after the binding to the
receptor and insertion of the N-terminal domain into the membrane,
whereby the regulation of the trans-membrane electric potential is
disturbed. This can result in colloid-osmotic lysis of the cells,
which is the main cytolytic mechanism that is common to all ICPs
(Knowles & Ellar, 1987; Slatin et al., 1990; Schwartz et al., 1991;
Schnepf et al., 1998). When the midgut epithelium of the larva is
damaged, the haemolymph and gut contents can mix. This results in
favourable conditions for the Bt spores to germinate. The resulting
vegetative cells of Bt and the pre-existing microorganisms in the gut
proliferate in the haemocoel causing septicaemia, and may thus
contribute to the mortality of the insect larva.
3.3 Resistance of insect populations
A number of insect populations of several different species with
different levels of resistance to Bt have been obtained by laboratory
selection experiments during the last 15 years (Schnepf et al., 1998).
The species include Plodia interpunctella, Cadra cautella,
Leptinotarsa decemlineata, Chrysomela scripta, Tricholplusia ni,
Spodoptera littoralis, Spodoptera exigua, Heliothis virescens,
Ostrinia nubilalis and Culex quinquefasciatus (Schnepf et al.,
1998) and resistance is shown to either Btk, Bti, Btte or other Bt
subspecies.
During the last few years populations of the diamondback moth,
Plutella xylostella, resistant to Btk and Bta have been found in
heavily treated areas in numerous geographically isolated regions in
the world, including Hawaii, Phillippines, Indonesia, Malaysia,
Central America and some USA states (Schnepf et al., 1998). It is
clear that this widespread appearance of resistance to Bt presents a
cautionary tale for the way of using Bt and Bt toxin genes in pest
management. Schnepf et al. (1998) have reviewed resistance management
of Bt.
4. NATURAL AND TREATED HABITATS
The Bt subspecies represents a group of organisms that occur
naturally and can be added to an ecosystem to achieve insect control
(Andrews et al., 1987; Stahly et al., 1991). In this monograph, a
natural habitat is considered to be one where Bt can be isolated when
there has been no previous history of application of the organism to
that ecosystem, whereas a treated habitat is one where Bt can be
isolated after a previous history of application of the organism for
insect control.
Insecticides formulated with Bt are being manufactured and used
worldwide. These commercial Bt products may be applied as an
insecticide to foliage, soil, water environments and food storage
facilities. After application of Bt to an ecosystem, the organism may
persist as a component of the natural microflora.
4.1 Natural occurrence of Bt
Members of the Bacillus cereus group can be found in most
ecological niches. Hansen et al. (1996) reviewed the occurrence of Bt
in the environment. Although the early Bt isolates were pathogenic for
insects, it is now apparent that several Bt isolates have no known
target (Ohba & Aizawa, 1986; Ohba et al., 1988; Hansen et al., 1996,
1998; Damgaard et al., 1997b). This lack of insecticidal activity may
be attributed to the loss of ability to produce ICPs (Gordon, 1977),
which may be due to a mutation in the ICP gene that could prevent
expression (Klier & Lecadet, 1976; Stahly et al., 1978; Dean, 1984) or
to the loss of ICP encoding sequences. Finally, the lack of known
activity of a Bt crystalline toxin might simply be explained by a
failure to test against the actual target organism. The list of Bt
targets is still increasing. Although our knowledge of the activity of
Bt populations in the environment is limited, a certain level of
turn-over and vegetative growth must occur, as annual and seasonal
variations in numbers and subspecies diversity of Bt populations have
been observed (Damgaard et al., 1997b; Kim et al., 1998).
4.1.1 Bt in insect hosts
Numerous Bt subspecies have been isolated from dead or dying
insect larvae and in most cases the isolate has toxic activity to the
insect from which it was isolated (Goldberg & Margalit, 1977; de
Barjac, 1981b; Hansen et al., 1996). These organisms have a narrow
host range in the orders Coleoptera, Diptera and Lepidoptera and
can proliferate within the bodies of their host insects. When the
infected insect larva dies, the dead insect carcass usually contains
relatively large quantities of spores and crystals that may be
released into the environment (Prasertphon et al., 1973; Grassi &
Deseö, 1984; Aly, 1985; Aly et al., 1985). Growth of Bt in non-target
organisms has also been described. Eilenberg et al. (in press) found
that Bt multiplication had occurred in non-target insects, which were
also infected by insect pathogenic fungi.
Akiba (1986) reported recycling of naturally occurring Bt in
insect cadavers when competitive microorganisms were at a low density.
Outbreaks of Bt in susceptible insect populations occur relatively
infrequently; most outbreaks have been limited to situations where the
insect density is relatively high, providing better opportunity for
establishing the disease within the insect population (Lynch et al.,
1976; Burges & Hurst, 1977; Vaòková & Purrini, 1979; Margalit & Dean,
1985).
4.1.2 Bt in soil
The spores of Bt persist in soil, and vegetative growth occurs
when nutrients are available (DeLucca et al., 1981; Akiba, 1986; Ohba
& Aizawa, 1986; Travers et al., 1987; Martin & Travers, 1989).
DeLucca et al. (1981) found that Bt represented between 0.5% and
0.005% of all Bacillus species isolated from soil samples in the
USA. Martin & Travers (1989) recovered Bt from soils globally. Meadows
(1993) isolated Bt from 785 of 1115 soil samples, and the percentage
of samples that contained Bt ranged from 56% in New Zealand to 94% in
samples from Asia and central and southern Africa. Ohba & Aizawa
(1986) isolated Bt from 136 out of 189 soil samples in Japan.
4.1.3 Bt on plant surfaces
Bt has been found extensively in the phylloplane. Numerous Bt
subspecies have been recovered from coniferous trees, deciduous trees
and vegetables, as well as from other herbs (Smith & Couche, 1991;
Damgaard et al., 1997b). The Bt isolates have demonstrated a broad
diversity both with specific activities to insects from the orders
Coleoptera and Lepidoptera and with unknown activities (Smith &
Couche, 1991; Damgaard et al., 1997b; Hansen et al., 1998). The
bacterium has also been isolated from stored grain products (Meadows
et al., 1992).
4.2 Treated habitats
Treated habitats are the locations where Bt insecticides (usually
a mixture of spores and crystals) have been applied.
In Canada, Meadows (1993) estimated that approximately 1015
viable Btk spores per ha were released in a typical spray operation to
control spruce budworm (Choristoneura fumiferana).
4.3 Environmental fate, distribution and movement
Bt, like other members of the genus Bacillus, has the ability
to form endospores that are resistant to inactivation by heat and
desiccation and that persist in the environment under adverse
conditions (Stahly et al., 1991). When considering the degradation of
Bt in the environment, it is important to distinguish between changes
in the numbers of viable spores and changes in biocidal activity. The
survival and activity in the environment has been reviewed by Hansen
et al. (1996).
The distribution and environmental transport of applied Bt
formulations are influenced by the type of application and various
environmental factors (Bulla et al., 1985; Andrews et al., 1987). Bt
formulations are used in agriculture and forestry against coleopteran
and lepidopteran pests and are usually directed towards the surface of
plants, while the Bt formulations for control of dipteran pests
(mosquitos and blackflies) are applied to their aquatic, larval
habitats. Many Bt insecticides exhibit poor stability under field
conditions, and so frequent reapplication is required (Griego &
Spence, 1978; Sorenson & Falcon, 1980; Beegle et al., 1981).
4.3.1 Distribution and fate of Bt in terrestrial habitats
4.3.1.1 Fate of Bt and ICP on plant surfaces
Solar radiation appears to be the environmental factor most
damaging to the stability of Bt ICP (Pinnock et al., 1974; Pinnock et
al., 1977; Griego & Spence, 1978; Pusztai et al., 1991).
Griego & Spence (1978) demonstrated that Bt spores are
inactivated rapidly when exposed to UV radiation, while Pusztai et al.
(1991) demonstrated that the tryptophan residues of the Bt protoxin
are damaged by solar radiation in the 300-380 nm range.
The combined effect of sunlight, leaf temperature and vapour
pressure deficit appeared to contribute more to the reduction of
bioactivity than any other single factor (Leong et al., 1980). Residue
bioactivity may be detected up to 2 weeks after treatment with
formulations containing UV protectants (Hostetter et al., 1975). Other
studies on the effect of environmental exposure to Bt spores revealed
that spore survival can be affected by the surface to which the
material is applied.
Pinnock et al. (1974) reported that the half-life of Bt spores on
leaves of California live oak (Quercus agrifolia) was 3.9 days, as
compared to a half-life of 0.63 days when applied to leaves of western
redbud (Cercis occidentalis).
Ignoffo (1992) summarized data for the reduction of spore
viability and ICP activity on leaves of various plants in sunlight: Bt
spore viability was reduced 80% in one day on red cedar leaves and 8%
on soy bean leaves, while the ICP activity declined by 20% on red
cedar leaves but 65% on soy bean leaves.
Dent (1993) reported that Bt formulations on foliage frequently
have half-lives of up to 10 days. However, unformulated Bt may have a
half-life of only a few hours. Pedersen et al. (1995) found that the
initial spore half-life was 16 h during the first week after spraying
cabbage with unformulated Btk.
There is also evidence that plant chemicals can inactivate Bt or
influence infectivity. Lüthy (1986) demonstrated that extracts
prepared from cotton leaves could inactivate ICPs.
Commercially applied Bt may persist at low levels for
considerable periods of time. Reardon & Haissig (1983) reported that
Btk was still present on balsam fir (Abies balsamea) one year after
applications to control spruce budworm. The proliferation of spores
through infection of susceptible insects should not be discounted as a
source of low levels of Bt in treated areas. Several studies have
demonstrated that Bt is able to grow and sporulate in insect cadavers
(Meadows, 1993). From dead Egyptian cotton leafworm (Spodoptera
littoralis), Jarrett & Stephenson (1990) isolated between 5.0 × 105
and 9.2 × 107 spores per larva.
Bt may be lost to the soil by overspray during application or by
the action of rain on plant surfaces. Further losses arise from in
situ degradation by environmental factors, such as ultraviolet (UV)
radiation and microbial activity (Griego & Spence, 1978; Sorenson &
Falcon, 1980; Beegle et al., 1981; West et al., 1984a,b). Pedersen et
al. (1995) found that Bt was dispersed by rain splash from the soil to
the lower leaves of cabbage.
4.3.1.2 Fate of Bt in soil
Petras & Casida (1985) reported that Bt spore counts in soil
declined by a factor of ten in the first 2 weeks after application and
then remained constant for 8 months. The response was similar in
spores from commercial and laboratory cultures. In contrast,
vegetative cells introduced into the soil persisted for only a short
time. Soil pH had little effect on their survival. Spore persistence
for more than 2 weeks apparently resulted from the inability of the
spores to germinate in the soil.
Pedersen et al. (1995) sprayed unformulated Btk (1.2 5 104 cfu
per g soil, spontaneous rifampicin-resistant mutant) on soil in 1993,
and 2.3 × 103 cfu per g soil remained after 336 days. The field was
left undisturbed, and 5´ years later spots with 1.5 × 103 Btk per g
soil were found (Hansen & Hendriksen, 1999), but spots with very low
Btk numbers were also recorded. These data indicate that the Btk had
multiplied.
West et al. (1984a,b) reported that vegetative cells in soil
disappeared at a rapid, exponential rate, whereas parasporal crystals
disappeared at a slower, non-exponential rate, and spore numbers
remained unaltered through 91 days of incubation at 25°C, with no
detectable germination. The proteinaceous crystalline protoxin of Bt
has been shown to be degraded by soil microorganisms at an exponential
rate with a half-life of about 3-6 days.
Saleh et al. (1970) reported that Bt spores could remain viable
for several months in the soil and germinate when soil conditions
favoured bacterial growth.
Bt spores do not appear to germinate readily in most soils and
the crystalline protoxins are metabolized by other microorganisms.
West et al. (1984a) reported that Bta in soil showed an exponential
loss of insecticidal activity. The rate of loss was greater in
non-sterile soil than in autoclaved soil. There was an initial rapid
decrease, which stabilized at approximately 10% of the original
inoculum level after 250 days incubation, until the cessation of
sampling after two years. No loss of insecticidal activity was
observed in autoclaved soil, which suggests that soil microorganisms
were responsible for the loss of insecticidal activity in the natural,
non-sterilized soil.
Several studies determined that Bt did not grow under most
natural soil conditions (West et al., 1984a,b; Akiba, 1986). The data
suggested that this was attributable to a failure of Bt spores to
germinate in soil under these conditions. The spore is the only state
in which Bt persists in natural soils.
An environmental fate study demonstrated no significant spore
accumulation in either the organic or the mineral layers of soil over
an 11-month period when Bt was applied aerially at 100 times the
concentration used for operational programmes (Bernier et al., 1990).
Studies have indicated that Bt is relatively immobile in soil.
Martin & Reichelderfer (1989) found no vertical movement beyond a 6-cm
deep zone in soil and less than 10 m lateral movement, even along
drainage courses.
Akiba (1991) reported a one-month irrigation study simulating the
summer rainy season in Japan. There was no translocation of Bt below a
depth of 10 cm. In soils receiving 45 cm simulated rainfall, Bt was
detected to a depth of 3-6 cm. In tests using soil columns, Bt did not
pass through a column of volcanic ash soil but a few spores were
detected in flow-through water from an alluvial sand column. Results
suggested that the major factor causing a decrease in the level of Bt
was not a physical dilution due to the rainwater, but possibly an
affinity of the spores for the soil particles.
Venkateswerlu & Stotsky (1992) reported that adsorption and
binding of Bt toxin proteins to soil particles were greater on
montmorillonite than on kaolinite clays. Maximum adsorption occurred
within 30 min, and adsorption was not significantly affected by
temperature between 7°C and 50°C.
4.3.2 Distribution and fate of Bt in aquatic habitats
Bti is often applied directly to water for the control of
mosquitos and blackflies. Rapid sedimentation in all but the fastest
flowing streams is regarded as an important limitation on the efficacy
of such applications.
Sheeran & Fisher (1992) demonstrated that the sedimentation of
Bti is facilitated by adsorption onto particulate material.
Ohana et al. (1987) found that spores may persist for at least
22 days in sediments, and the spores may be mobilized with such
sediments during floods.
Btk has been reported to survive in fresh water and in seawater
for more than 70 and 40 days, respectively, at 20°C (Menon & De
Mestral, 1985). A higher percentage of Btk was found to survive for
extended periods in lake water than in tap and distilled water,
presumably due to the presence of more nutrients in lake water. Bt has
not been isolated from any drinking-water supplies.
Spores of Bti remained viable for shorter periods when suspended
in moving water than when in static bottles, indicating that static
laboratory trials may overestimate the longevity of these spores in
the environment (Yousten et al., 1992).
Carcases of mosquito larvae killed by Bti have been shown to
allow for the complete growth cycle (germination, vegetative growth
and sporulation), thus becoming toxic themselves to scavenging yellow
fever mosquito (Aedes aegypti) larvae (Khawaled et al., 1990).
Contact of Bti with mud can result in an immediate disappearance
of larvicidal activity, but it has little influence on spore viability
(Ohana et al., 1987). The cessation of toxicity was found to be caused
by bacterial adsorption to soil particles, but the inactivation could
be reversed by washing the mud away.
Special Bti formulations have been developed to prolong residence
time of Bt at the surface or in the water column, where target insects
feed.
Manasherob et al. (1998) found germination, growth and
sporulation of Bti in excreted food vacuoles of a protozoan.
4.3.3 Transport of Bt by non-target organisms
In a field trial where Btk was sprayed on cabbage and soil,
Pedersen et al. (1995) found that the Btk could be transported by
non-target insects. Up to 103 cfu per g were found on surface-active
insects, and carabid beetles carrying Btk were found up to 135 m from
the Btk-treated area.
In a study of interactions between Bti and fathead minnows
(Pimephales promelas), ingestion of Bt resulted in a high number of
recoverable spores in the gastrointestinal tract and faeces (Snarski,
1990). Bti spore counts decreased rapidly after test fish were
transferred to clean water, but spores were detected in low numbers in
faeces for over 2 weeks. The data indicated that minnows could
disperse Bt spores in the freshwater environment.
Meadows (1993) reported that, after the application of Bt on
land, it may be dispersed by birds and mammals feeding on infected
target insects. Some Bt-infected insect larvae may contain 6.6 × 108
to 4.2 × 109 spores per gram dry mass (Burges & Hurst 1977).
5. COMMERCIAL PRODUCTION
5.1 History of Bt and its commercial applications
5.1.1 Production levels
Conventional Bt products, which utilize naturally occurring or
modified Bt strains, account for approximately 90% of the world MPCA
market (Bernhard & Utz, 1993). Current annual production of Bt has
been estimated at 3000 or more tonnes in developed countries. In
China, up to 10 000 tons are produced annually (personal communication
by Guan Xiong, Fujian Agricultural, University, 1997).
5.1.2 Production processes, formulations and quality standards
Bt products are produced using fermentation technology (Bernhard
& Utz, 1993). Most commercial products contain ICP and viable Bt
spores, but the spores are inactivated in some Bti products. During
commercial-scale production of Bt products, there is little loss of
bioactive components to the environment. The type of loss incurred is
a function of the recovery method involved. No significant amount of
bioactive component is lost from fermenter harvests if filtration is
used to separate the insoluble solids (active ingredients) from the
soluble liquid (inert) fraction of the harvest liquor, as shown by
complete lack of bioactivity in the resulting liquid fraction. The
liquid waste fractions may contain a low concentration of insoluble
active component, but this is typically inactivated by processing in
on-site waste treatment facilities. Although it is not a physical
loss, measurable bioactivity is diminished if the recovered active
material is processed through a dryer, due to the exposure of the
bioactive components to the high temperatures required for drying.
Guidelines for the handling of microorganisms during manufacture have
been reviewed by Frommer et al. (1989).
Commercial Bt formulations include wettable powders, suspension
concentrates, water dispersible granules, oil miscible suspensions,
capsule suspensions and granules (Tomlin, 1997).
Quality standards for Bt fermentation products have been accepted
by IUPAC (Quinlan, 1990). These standards include limits on the
concentration of microbial contaminants and metabolites (Table 5).
In most cases strains of Bt that produce beta-exotoxins are not
approved for commercial application, although some commercial use has
been approved for control of certain fly species that are not
susceptible to ICPs (Carlberg et al., 1985).
Table 5. Maximum allowable levels of microbial contamination in
bacterial insecticides (IUPAC Recommendation; Quinlan, 1990)
Types of microorganisms Maximum concentrations
Viable mesophiles <1 × 105/g
Viable yeasts and moulds <100/g
Coliforms <10/g
Staphylococcus aureus <1/g
Salmonella <1/10g
Lancefield Group D Streptococci <1 × 104/g
5.1.3 General patterns of use
Commercial applications of Bt have been directed mainly against
lepidopteran pests of agricultural and forest crops; however, in
recent years strains active against coleopteran pests have also been
marketed (Table 6).
Strains of Bti active against dipteran vectors of parasitic
disease organisms have been used in public health programmes.
5.1.3.1 Applications in agriculture and forestry
Commercial use of Bt on agricultural and forest crops dates back
nearly 30 years, when it became available in France. Use of Bt has
increased greatly in recent years and the number of companies with a
commercial interest in Bt products has increased from four in 1980 to
at least 18 (Van Frankenhuyzen, 1993). Several commercial Bt products
with Bta, Btk or Btte have been applied to crops using conventional
spraying technology (Table 6). Various formulations have been used on
major crops such as cotton, maize, soybeans, potatoes, tomatoes,
various crop trees and stored grains. Formulations have ranged from
ultralow-volume oil to high-volume, wettable powder and aqueous
suspensions. In the main, naturally occurring Bt strains have been
used, but transgenic microorganisms expressing Bt toxins have been
developed by conjugation and by genetic manipulation, and in some
cases, these have reached the commercial market. These modified
organisms have been developed in order to increase host range, prolong
field activity or improve delivery of toxins to target organisms. For
example, the coleopteran-active cryIIIA gene has been transferred to
a lepidopteran-active Btk (Carlton et al., 1990). A plasmid bearing an
ICP gene has been transferred from Bt to a non-pathogenic
leaf-colonizing isolate of Pseudomonas fluorescens; fixation of the
transgenic cells produces ICP contained within a membrane which
prolongs persistence (Gelernter, 1990). The gene expressing cryIA(c)
ICP has been inserted in Clavibacter xyli subspecies cynodontis, a
bacterium that colonizes plant vascular systems. This has been shown
to deliver the ICP effectively to European corn borer (Ostrinia
nubilalis) feeding within plant stems (Beach, 1990). Improvements in
performance arising from such modifications are such that transgenic
organisms and their products are likely to be used much more widely in
the future.
5.1.3.2 Applications in vector control
Bti has been used to control both mosquitos and blackflies in
large-scale programmes (Lacey et al., 1982; Chilcott et al., 1983;
Car, 1984; Car & de Moor, 1984; Cibulsky & Fusco, 1987; Becker &
Margalit, 1993; Bernhard & Utz, 1993). For example, in Germany 23
tonnes of Bti wettable powder and 19 000 litres of liquid concentrate
were used to control mosquitos (Anopheles and Culex species)
between 1981 and 1991 in the Upper Rhine Valley (Becker & Margalit,
1993). In China, approximately 10 tonnes of Bti have been used in
recent years to control the malarial vector, Anopheles sinensis.
The Onchocerciasis Control Programme of West Africa used more
than five million litres of Bti from 1982 to 1997 to control
blackflies (Simulium damnosum), the vector of the onchocerciasis
filarial worm (Onchocerca volvulus), on the Upper Volta River
System. The Programme was initially based solely on the control of the
vector, Simulium damnosum sensu lato, by spraying the insecticide
at breeding sites on river systems, where larval stages develop. At
the peak of larvicidal activities about 50 000 km of rivers were
treated in an area of over one million km2. The purpose was to
interrupt the transmission of the parasite Onchocerca volvulus. The
interruption of the cycle is achieved by destroying larval stages
through aerial application of insecticides to breeding sites.
Insecticide application was undertaken weekly (Moulinier et al.,
1995). In order to assess the environmental impact of such treatments
a network of sampling stations throughout the programme area were
established.
Formulations of Bti range from wettable powder and fluid
concentrates applied via conventional spray equipment from ground and
air to slow-release briquet and tablet formulations. Examples of
commercial Bti products are listed in Table 6.
Table 6. Examples of commercial Bt products used against agricultural, forestry and public health pests
(Tomlin, 1997; see also the Internet site http://www.sipweb.org/bacteria.htm)
Bt sub-species Commercial products Producer
Bt (not defined) Rijin Scientific & Technological Development
Bt (not defined) Bitayon Jewin-Joffe Industry Ltd
Bt (not defined) Delfin, Thuricide SDS Biotech KK
Btk Bactospeine, Biobit, Dipel, Foray Abbott (USA)
Bollgard Ecogen/Crop Care
Bactucide Caffaro
Baturad Cequisa
Condor, Crymax, Cutlass, Ecogen
Foil, Jackpot, Lepinox,
Rapax, Raven
Jackpot, Lepinox, Rapax Intrachem
Cordalene Agrichem
Larvo Troy
Costar, Delfin, Design, Novartis/Thermo Trilogy Co
Javelin, Steward, Thuricide,
Vault
Ecotech Bio, Ecotech Pro Ecogen/Roussel-Uclaf
Halt Wockhardt
Bta Xentari, Florbac Abbott
Certan Novartis
Btte Novodor Abbott
Bti Bactimos, Gnatrol, Vectobac Abbott
Acrobe Cyanamid
JieJueLing, MieJueLing Huazhong Agricultural University
Teknar Novartis/ThermoTrilogy Co
Bt Ybt-1520 Mianfeng pesticide Huazhong Agricultural University
Bt chinesensis Shuangdo preparation Huazhong Agricultural University
Btg Spicturin Tuticorin Alkali Chemicals and
Fertilisers Ltd
6. EFFECTS ON ANIMALS
6.1 Mammals
Microbial pest control agents (MPCA) can, in principle, cause
harmful effects via toxicity, inflammation, or a combination of these
effects. The presence of bacteria in a specimen derived from tissues
does not necessarily mean infection. Colonization refers to the
multiplication of MPCA either on the surface or within an animal/human
organism without causing any tissue damage. Persistence refers to
the ability to recover the inoculum of the MPCA over time. Persistence
and transient disturbances of the normal microbial flora are to be
expected after exposure of experimental animals to MPCA, since
clearance of the inoculum is not instantaneous. Persistence may not be
equated with infection (Siegel & Shadduck, 1990). Infection by a
MPCA means that there is evidence of the establishment and
proliferation of the MPCA in tissues, coupled with tissue damage.
Evidence of multiplication includes a measurable increase in the total
amount of MPCA, recovery of vegetative stages when spores were
administered, and failure of the inoculum to clear. It cannot be
determined solely on the basis of lesions since injection of foreign
material can elicit an inflammatory process (Siegel et al., 1987).
A classification of MPCA toxicity and infectivity has been
proposed, in which MPCA is classified as toxic if an oral dose
< 106 cfu per mouse causes mortality or clinical or pathological
changes (Burges, 1980). However, any classification is very difficult
because of the complexicity of the issue when dealing with living
organisms (Ignoffo, 1973; Shadduck, 1983).
Older reports do not discriminate between different strains of Bt
but modern molecular techniques have proven that variability exists
within strains with the same serotype (Helgason et al., 1998; Hansen &
Hendriksen, 1997a,b).
Mammalian toxicity studies on Bt-containing pesticides
demonstrate that the tested isolates are not toxic or pathogenic
(McClintock et al., 1995), as they occur in the products. Toxicity
studies submitted to the US Environmental Protection Agency to support
registration of Bt subspecies, and reviewed by McClintock et al.
(1995), failed to show any significant adverse effects on body weight
gain, clinical observations or upon necropsy.
Infectivity/pathogenicity studies have shown that the intact rodent
system responds as expected to eliminate Bt gradually from the body
after oral, pulmonary or intravenous challenge. However, clearance of
Bti and Btk is not instantaneous. An intact immune system is not a
prerequisite for clearance of Bti and Btk.
6.1.1 Oral exposure
In studies conducted with a single oral dose of laboratory grown
Bt and commercial Bt formulations, there was no mortality associated
with ingestion of Bti or Btk in mice and rats (Fisher & Rosner, 1959;
de Barjac et al., 1980; Shadduck, 1980; Siegel et al., 1987)
(Table 7).
Additionally, in data summarized by McClintock et al. (1995), no
toxicity or infectivity was observed following oral administration of
various Bt subspecies at doses of up to 4.7 × 1011 cfu/kg in rats.
In a study involving repeated oral exposure of mice and rats for
21 days with laboratory grown Bti, there was no mortality associated
with ingestion of Bti and normal weight gain was observed in all
treated rodents (de Barjac et al., 1980) (Table 8).
Hadley et al. (1987) conducted a study in which sheep were
repeatedly treated with two commercial Btk formulations for 60 days.
The only clinical sign was loose stools in sheep exposed to one Btk
formulation. There was also microscopic evidence of moderate to marked
lymphoid hyperplasia of the Peyer's patches in the caecum and colon of
two out of six sheep treated with one Btk formulation and in one out
of six sheep treated with the other Btk formulation. The authors did
not consider these findings clinically significant.
Other multiple dose studies with Bt were summarized by McClintock
et al. (1995). In rats, no toxicity or infectivity was associated with
dietary exposure to Bti (4 g/kg per day ) for 3 months. Administration
of 1.3 × 109 Btk spores/kg per day to rats by oral gavage was not
toxic or infectious. McClintock et al. (1995) also reported the
results of a 2-year study in which a commercial Btk preparation was
fed to rats at 8400 mg/kg per day in the diet. Despite this excessive
dose, the only effect observed was a decrease in body weight of
females during weeks 10-104 of the study.
6.1.2 Inhalation exposure
These tests primarily address the potential infectivity of a
MPCA. Inhalation is a likely route by which humans and animals may be
exposed to Bt during application.
De Barjac et al. (1980) exposed 10 female Swiss mice for 12 min
to 2 × 108 Bti spores (48-h laboratory grown whole culture). The mice
were monitored for clinical signs for 15 days, and then killed. The
lungs were removed aseptically and cultured for bacteria, but no Bti
was recovered.
Siegel et al. (1987) exposed 27 female Sprague-Dawley rats to
2 × 106 spores of a commercial Bti formulation for 30 min. Rats were
serially killed over a 27-day period and the lungs were cultured.
Table 7. Acute toxicity (single oral exposure) of Bt in experimental animals
Subspecies Material tested Test animal Dosea Mortalityb Reference
tested
Btk Washed cells, 24-h culturec Rat, female, Sprague-Dawley 1.4 × 107cfu 0/6 Shadduck, 1980
Btk Commercial product Ratd 2 × 1011cfu 0/10 Fisher & Rosner, 1959
Bti 48-h culturec Mouse, female, Swiss 1.7 × 108cfu 0/20 de Barjac et al., 1980
Bti 48-h culturec Rat, female, Wistar 3.4 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellsc, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rat, female, Sprague-Dawley 4 × 107cfu 0/10 Siegel et al., 1987
a All doses given are per animal
b Number dead/number treated
c Laboratory grown culture
d Breed and sex unknown
Table 8. Repeated dose (oral exposure) toxicity of Bt in mice, rats and sheep
Subspecies Material tested Test animal Dose Exposure Mortalitya Reference
tested time
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Btk Commercial product Sheep, male, Rambouillet/Merino 1 × 1012cfu 5 months 0/6 Hadley et al., 1987
Bti 48-h cultureb Mouse, female, Swiss 4.7 × 1010cfu 21 days 0/20 de Barjac et al., 1980
Bti 48-h cultureb Rat, female, Wistar 1.2 × 1011cfu 21 days 0/10 de Barjac et al., 1980
a Number dead/number exposed
b Laboratory grown culture
Recovery of Bti declined from 5.92 × 103 cfu/g lung tissue at 3 h
after exposure to none detected at 7 days after exposure. No gross
lung lesions were observed.
Fisher & Rosner (1959) exposed 10 mice to 3 × 1010 spores of a
commercial Btk product 4 times in a 6-day period. The Btk-treated mice
exhibited no clinical signs during the treatment period and no gross
pathological changes at necropsy.
6.1.3 Dermal exposure
This test is similar to the dermal exposure tests used in
chemical toxicology. Bt does not have any external contact toxicity
due to its mode of action, as shown in the following studies.
De Barjac et al. (1980) applied 5.1 × 107 cfu Bti of a 48-h
laboratory grown culture to the skin of 20 female Swiss mice. No
mortality was observed and there was no evidence of skin inflammation.
Other studies, reviewed by McClintock et al. (1995), indicate
that Bt was not toxic or pathogenic to rabbits following dermal
exposure to various Bt subspecies at doses of up to 2500 mg/kg. In
some cases, mild irritation was observed.
6.1.4 Dermal scarification exposure
This test evaluates both the potential toxicity and infectivity
of a MPCA. In the case of Bt, toxicity is unlikely due to its mode of
action. However, this test also evaluates the importance of intact
skin in preventing infection by Bt.
Fisher & Rosner (1959) scarified the skin of 4 rabbits, then
applied 2.2 × 106 cfu of a commercial Btk formulation. No skin
inflammation or sign of infection was observed.
De Barjac et al. (1980) applied 3.3 × 1013 cfu of a commercial
Bti formulation to the skin of 6 male New Zealand White rabbits. No
skin inflammation or sign of infection was observed.
6.1.5 Subcutaneous inoculation
This route of exposure is considered a more challenging test of
potential infectivity than oral or dermal exposure, because the
barrier of the skin is breached. However, subcutaneous exposure may
take place only if the skin is damaged by the spraying or is already
otherwise damaged.
De Barjac et al. (1980) subcutaneously inoculated 20 female Swiss
mice and 10 tricolour guinea-pigs, respectively, with 8.5 × 107 cfu
and 1.7 × 108 cfu of a 48-h laboratory-grown Bti culture. There was
no evidence of infection and no mortality was observed.
Siegel et al. (1987) subcutaneously inoculated 15 female BALB/c
mice with 1 × 109 cfu of a commercial Bti formulation. Abscesses
occurred at the injection site but these were attributed to the
introduction of high concentrations of heat-stable foreign material,
since they also occurred when autoclaved Bti was injected. There was
no evidence of infection and no mortality was observed.
6.1.6 Ocular exposure
The primary purpose of this procedure is to test for the
irritancy of a MPCA, although this test also evaluates potential
infectivity as well. In these tests, Bt may persist for days in rabbit
eyes because of the depth of the conjunctival sac coupled with limited
tear production by rabbits.
De Barjac et al. (1980) inoculated the eyes of six male New
Zealand White rabbits with 3.7 × 107 cfu of a 48-h laboratory-grown
Bti culture. No conjunctivitis or ocular irritation was observed.
Siegel & Shadduck (1990) inoculated 12 female New Zealand White
rabbits with 5.4 × 106 cfu of a commercial Bti formulation. No ocular
irritation or conjunctivitis was observed and no Bti was recovered by
swabbing after one week.
In data reviewed by McClintock et al. (1995), only mild
irritation was observed following ocular administration of certain Bt
subspecies to rabbits.
Siegel et al. (1987) inoculated the eyes of 6 male New Zealand
White rabbits with 50 mg of a dry powder-commercial Bti formulation
for 9 days, and another 6 male New Zealand White rabbits were treated
with 50 mg of a laboratory-grown Bti culture for 9 days. No ocular
irritation or conjunctivitis was observed in the rabbits that received
the commercial powder. The rabbits that received the laboratory
culture experienced severe conjunctival congestion and discharge. This
was not attributed to Bti but rather to the nature of the inoculum.
The laboratory strain was a dry paste with hard clumps while the
commercial formulation was a soft powder.
6.1.7 Intraperitoneal exposure
The administration of a MPCA by this route is considered a highly
challenging route of exposure. Human and animal exposure to Bt by this
route is very unlikely to occur during the course of normal
application of Bt. This route evaluates the ability of Bt to cause
infection or produce toxic metabolites in the peritoneal cavity. Some
of the safety studies that utilized this route of exposure also
evaluated the clearance of Bt over time (Table 9).
Additional studies employing mice have been conducted using this
route of exposure, which evaluates the role played by an intact immune
system in preventing infection by Bt. These studies were deemed
necessary to assess the risk posed by Bt to humans undergoing
immunosuppressive chemotherapy and the risk posed by Bt to humans
infected with the human immunodeficiency viruses. Immune suppression
in mice was accomplished either by use of corticosteroids, which
inhibited helper T-cells and selectively damaged B-cell activity, or
through the use of athymic mice, which lack the functional T
lymphocyte component of their immune system.
6.1.7.1 Immune-intact animals
De Barjac et al. (1980) intraperitoneally injected 100 female
Swiss mice with 3.4 × 107 cfu of a 48-h laboratory-grown Bti culture
and killed groups of 10 mice daily (Table 9). Blood samples were taken
by cardiac puncture and Bt was recovered until day 8. No mortality was
observed.
Fisher & Rosner (1959) intraperitoneally injected 30 mice of
unspecified sex and strain with a laboratory-grown Btk culture and
withdrew cardiac blood samples 24, 48 and 72 h after injection. There
was no mortality and Btk was recovered as late as 48 h after injection
from heart blood.
Siegel & Shadduck (1990) conducted three clearance studies using
female CD-1 mice. In one experiment, 33 females were injected with
2.7 × 107 cfu of a washed commercial Bti formulation and serially
killed over 80 days. Bti did not clear and was recovered from the
heart blood on days 67 and 80. The investigators noted that the
initial inoculum was composed of approximately 95% vegetative cells
and that vegetative cells take longer to clear than spores. This was
confirmed in a follow-up experiment in which two groups of 16 females
each were injected with inocula containing 1.5 × 107 cfu of spores
only or a 25% vegetative cell and 75% spore mixture. Both inocula
cleared exponentially from the spleens of the mice but the 100% spore
inoculum cleared sooner than did the inoculum that contained
vegetative cells. These experiments demonstrated that Bti and Btk
persist for a variable length of time in mice following injection but
that they are cleared over time. These studies also suggest that the
nature of the inoculum may play a role in the speed by which it
is cleared.
Data summarized by McClintock et al. (1995) indicate that
toxicity (100% mortality in extreme cases) may be observed following
injection of >108 cfu of certain Bt subspecies intraperitoneally
in mice. Lower doses (< 107 cfu/mouse) were non-toxic. Death
generally occurred shortly after injection, indicating that an
infectious process had not occurred. Although the basis for the
toxicity observed at doses >108 cfu is not understood, these
findings are not considered as evidence of a hazard associated with Bt
products, since the route of administration is not relevant to human
and animal exposure conditions.
Table 9. Acute toxicity of Bt after intraperitoneal injection of guinea-pigs, mice and rats
Subspecies Material tested Test animal Dose Mortalitya Reference
tested
Btk Washed cell, 24-h cultureb Rat, female, Sprague-Dawley 1.4 × 109cfu 0/6 Shadduck, 1980
Btk Commercial product Mouse 3 × 109cfu 0/5 Fisher & Rosner, 1959
Bti 48-h cultureb Mouse, female, Swiss 6.8 × 107cfu 0/20 de Barjac et al., 1980
Bti 48-h cultureb Guinea-pig, female, tricolour 1.7 × 107cfu 0/10 de Barjac et al., 1980
Bti Washed cellb, 24-h culture Rat, female, Sprague-Dawley 6.9 × 107cfu 0/6 Shadduck, 1980
Bti Washed commercial product Rats, male and female Sprague-Dawley 4 × 107cfu 1/20 Siegel et al., 1987
a Number dead/number treated
b Laboratory grown culture
6.1.7.2 Immune-suppressed animals
Siegel et al. (1987) injected 42 female BALB/c mice with 1.25 mg
of a cortisone acetate twice weekly in order to suppress their immune
system and subsequently injected them with 3.4 × 107 cfu of a washed
commercial Bti formulation. Three cortisone-treated mice but none of
the non-cortisone-treated mice died but this mortality was attributed
to injury caused by injection. In the remaining 39 mice Bti was still
recovered in the spleen 49 days after injection. In a companion
experiment, 42 athymic mice were injected with the same dose of a
washed commercial Bti formulation. Twenty-six of the 42 died within 5
to 10 h after injection; autopsy did not reveal the cause of death. In
the surviving mice, Bti was recovered in the spleen 49 days after the
injection. In a follow-up experiment, 30 athymic mice were injected
with 2.6 × 107 cfu of another (washed) commercial Bti formulation and
serially killed over a 36-day period. No mortality occurred. Bti was
still recovered on day 36 after injection.
Siegel & Shadduck (1990) injected 24 athymic mice with 2.7 × 107
cfu of a washed commercial Bti formulation and evaluated clearance
over a 27-day period. No mortality was observed and clearance was
faster in the euthymic than athymic mice. Bti was still recovered 27
days after injection.
These experiments demonstrated that an intact immune system is
not essential to prevent infection by Bti and Btk, but the kinetics of
clearance differ between athymic and euthymic mice as well as between
corticosteroid-treated and untreated euthymic mice. Based on these
data, immune-suppressed individuals do not face any increased risk of
infection by Bt.
6.1.8 Effects of activated Bt ICP
It has been demonstrated that the alkali-solubilized ICP from Bti
is lethal when injected into mice (Thomas & Ellar, 1983).
Alkali-solubilized Bti ICP was also cytolytic to human erythrocytes,
mouse fibroblasts, and primary pig lymphocytes in vitro (Thomas &
Ellar, 1983; Gill et al., 1987). This activity is attributed to a
cytolytic factor encoded by Cyt A gene of Bti. Most other Bt
subspecies lack this gene. Human exposure to activated Bti ICP is most
unlikely.
6.1.9 Studies in wild animals
Numerous studies have been conducted on wild animals as part of
the registration process. Most of the data are proprietary and not
publicly available. No adverse effects have been reported.
In Canada, Innes & Bendell (1989) studied the effect of a
commercial Btk formulation on small mammal populations in woodland.
Populations of eight species of rodents (Clethrionomys gapperi,
Eutamius minimus, Microtus chrotorrhinus, Napaeozapus insignis,
Peromyscus maniculatus, Phenacomys intermedius, Tamias striatus
and Zapus hudsonius) and four species of shrew (Blarina
brevicauda, Sorex cinereus, Sorex fumeus and Sorex hoyii)
were studied by trapping over a 3-month period and shown to be
unaffected when compared to populations from untreated areas. This
suggests that the ingestion of infected insects by shrews had no
immediate effects on their populations.
6.2 Effects on birds
In a number of studies (Table 10), the acute toxicity and
pathogenicity of commercial Bta, Bti, Btk and Btte formulations for
young bobwhite quail (Colinus virginianus) and young mallards (Anas
platyrhynchus), when administered daily by oral gavage at high
dosages, were evaluated (Beavers et al., 1989a,b; Lattin et al.,
1990a,b,c,d; Beavers, 1991a,b). The Bt-treated birds showed no
apparent toxicity or pathogenicity. In those studies which also
evaluated feed consumption and weight gain, the Bt-treated birds
showed no effect when compared with the non-treated controls.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
breeding bird populations (13-14 families, 33-34 species) during a
field trial for spruce budworm control. The bird populations in 10-ha
control and treated plots were measured before and daily for 3 weeks
after application. No differences were detected between the
populations in the control and treated plots.
In the USA, Gaddis & Corkran (1986) evaluated the effect of a Bt
spray programme on the reproductive performance of the chestnut-backed
chickadee (Parus rufescens). This study was undertaken to determine
if secondary effects on the chickadees would result from the possible
reduction of lepidopteran species, which contribute to the diet of
this species. The data showed no treatment-related effect on the
number of eggs per nest, percentage of eggs hatched, percentage of
young fledged, percentage of nests fledging at least one young, or the
body weight of the nestlings.
6.3 Effects on aquatic vertebrates
The World Health Organization (WHO, 1982) reviewed laboratory and
field studies, performed by that time, that examined the impact of Bt
on frogs (Hyla regilla, Rana temporaria), goldfish (Carassius
auratus), mosquito fish (Gambusia affinis), newts (Taricha
torosa, Triturus vulgaris), rainwater killifish (Lucania parva)
and toads (Bufo species). No adverse effects were reported.
Under static renewal conditions, Boeri (1991) exposed rainbow
trout (Oncorhynchus mykiss) to high concentrations (100 mg/litre) of
a commercial Bta formulation for 96 h and observed no adverse effects
(Table 11).
Table 10. Effects of oral 5-day exposure of Bt on birds
Materials Species Dose Results Reference
testeda
Bta Colinus virginianus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991b
pathogenicity observed
Anas platyrhynchus 1714 mg (3.4 × 1011 cfu)/kg/day no toxicity or Beavers, 1991a
pathogenicity observed
Bti Colinus virginianus 3077 mg (3.4 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990d
pathogenicity observed
Anas platyrhynchus 3077 mg (6.2 × 1011 cfu)/kg/day no toxicity or Lattin et al., 1990b
pathogenicity observed
Btk Colinus virginianus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990a
pathogenicity observed
Anas platyrhynchus 2857 mg (5.7 × 1010 cfu)/kg/day no toxicity or Lattin et al., 1990c
pathogenicity observed
Btte Colinus virginianus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989a
pathogenicity observed
Anas platyrhynchus 740 mg (4 × 109 spores)/kg/day no toxicity or Beavers et al., 1989b
pathogenicity observed
a commercial preparation
Under static renewal conditions, Surprenant (1989) exposed
rainbow trout (Oncorhynchus mykiss) to high concentrations (100
mg/litre) of a commercial Btte formulation for 96 h and observed no
adverse effects (Table 11).
During 30- or 32-day static renewal tests, bluegill sunfish
(Lepomis macrochirus), sheepshead minnow (Cyprinodon variegatus)
and rainbow trout (Oncorhynchus mykiss) were exposed to commercial
Bti, Btk or Btte formulations at aqueous and dietary concentrations
from 100 to 500 times the expected environmental concentrations
(Table 11) (Christensen, 1990a,b,c,d,e,f,g,h). The results of these
studies indicated that exposure to very high concentrations of Bti,
Btk and Btte did not adversely affect the survival of these fish, nor
did it produce lesions. In the Btk study, the rainbow trout had a 20%
mortality during the last 4 days of the study (Christensen,1990b).
This effect was attributed to the excessive competition for food that
resulted from poor visibility due to the turbidity and the presence of
suspended solids encountered in the water.
In Canada, Buckner et al. (1974) assessed the impact of Btk on
brook trout (Salvelinus fontinalis Mitchell), common white suckers
(Catostomus commersoni Lacepede) and smallmouth bass (Micropterus
dolomieui Lacepede) during a field trial for spruce budworm control.
The fish populations were assessed visually in underwater surveys
before and after the spray programme. No effect on their populations
was seen.
Two analyses of surveys of the impact of the larvicidal campaign
in the Onchocerciasis Control Programme of West Africa, which compared
fish populations during the programme with the normal yearly
fluctuation, observed little or no effects on the non-target
populations. However, few details were provided (Yameogo et al., 1988;
Levêque et al., 1988; Calamari et al., 1998).
6.4. Effects on invertebrates
6.4.1 Effects on invertebrates other than insects
The World Health Organization (WHO, 1982), reviewed laboratory
and field studies performed up to that time that examined the impact
of Bt on aquatic invertebrates, which included bivalve mollusks
(oyster larvae, Crassostrea gigas, Ostrea edulis), copepods,
decapods, flatworms, isopods, gastropods and ostracods. Of these
organisms, only a few demonstrated any adverse effects.
In Canada, Buckner et al. (1974) evaluated the impact of Btk on a
number of aquatic invertebrates during a field trial for control of
the spruce budworm. Populations of Amphipoda (amphipods), Decapoda
(crayfish), Hydracarina (water-mites), Hirudinea (leeches),
Table 11. Effects of Bt on fish
Material Species Concentration Duration Results Reference
testeda
Bta Oncorhynchus mykiss 100 mg/litre water 96 h No-observed-effect level Boeri, 1991
Btk Lepomis macrochirus 2.9 × 109cfu/litre waterb 32 days No significant toxicity Christensen, 1990a
1.2 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 2.9 × 109cfu/litre waterb 32 days 20% mortality but Christensen, 1990b
1.1 × 1010cfu/g dietc not infectivity
Cyprinodon variegatus 2.6 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990c
3.3 × 109cfu/g dietc or pathology
Bti Lepomis macrochirus 1.2 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990f
1.3 × 1010cfu/g dietc or pathology
Oncorhynchus mykiss 1.1 × 1010cfu/litre waterc 32 days No significant toxicity Christensen, 1990g
1.7 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 1.3 × 1010cfu/litre waterc 30 days No significant toxicity Christensen, 1990h
2.1 × 1010cfu/g dietc or pathology
Btte Salmo gairdneri 100 mg/litre water 96 h No-observed-effect level Surprenant, 1989
Oncorhynchus mykiss 1.6 × 1010 cfu/litre waterc 30 days No significant toxicity Christensen, 1990d
1.34 × 1010cfu/g dietc or pathology
Cyprinodon variegatus 9.94 × 109cfu/g diet 30 days No significant toxicity Christensen, 1990e
or pathology
a commercial formulations
b nominal concentration
c measured average concentration
Hydrozoa (freshwater hydra), Nematoda (roundworms), Oligochaeta
(segmented worms), Porifera (freshwater sponges), Pulmonata
(freshwater snails) and Turbellaria (flatworms) were determined by
sampling 14 days prior to and up to 28 days after treatment. The
populations of these aquatic invertebrates were not affected by the
Btk treatment.
Benz & Altwegg (1975) studied the impact of Bt treatment at 100
times the recommended rate on populations of the earthworm Lumbricus
terrestris and found no effect.
Horsburgh & Cobb (1981) reported that populations of the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi
were not affected by biweekly sprays with a commercial Btk product.
Weires & Smith (1977) determined that sprays of a commercial Btk
product on apples during a 4-month season had no effect on the
two-spotted spider mite (Tetranchus urticae) and Panonychus ulmi,
or on two predatory mites (Amblyseius fallacis and Zetzellia
mali).
6.4.2 Effects on non-target insects
An extensive literature exists on the consequences of exposure of
NTOs to Bt, including reports of several long-term field studies. The
data have been reviewed periodically (e.g., WHO, 1982; Lacey & Mulla,
1990; Melin & Cozzi, 1990; Molloy, 1992; Otvos & Vanderveen, 1993).
The range of non-target species that have been found to be susceptible
to direct toxic action of Bt has remained small. A list of non-target
species found to be insensitive to Btte was issued by Keller &
Langenbruch (1993). In more than 30 years of commercial use, no
serious, direct effects on NTOs have been reported as arising from
Bt-based MPCAs. Several studies which identified effects of Bt on
predators or parasitoids of susceptible insect species are listed by
Navon (1993), but the effects have been small. Mortality in bees has
been observed after exposure to vegetatively growing Bt but the effect
does not seem to be related to spores or ICPs.
6.4.2.1 Aquatic insects
Bti is specific in its toxicity to dipterans. Nevertheless, many
studies have tested the effect of Bti applications on a wide range of
aquatic insects.
Lacey & Mulla (1990) summarized a number of studies of the
effects of Bti on certain non-target arthropod species and arthropod
populations in the laboratory and field (Table 12). The results of
representative studies are summarized below.
Table 12. Effects of Bti on non-target arthropodsa
Arthropod order Type of study Resulta References
Coleoptera Laboratory - Schnetter et al., 1981
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982; Mulla, 1988
Diptera Laboratory + Garcia et al., 1980; Ali, 1981;
(Chironomidae) Ali & Baggs, 1981; Schnetter et al., 1981
Field + Mulla et al., 1971; Ali, 1981;
Mulligan & Schaefer, 1982;
Rogatin & Baizhanov, 1984
Field - Miura et al., 1980
Ephemeroptera Laboratory - Ali, 1980; Schnetter et al., 1981;
Mulligan & Schaefer, 1982
Field - Schnetter et al., 1981; Mulla et al., 1982,
Mulligan & Schaefer, 1982; Mulla, 1988
Heteroptera Field - Schnetter et al., 1981;
(Corixidae) Mulligan & Schaefer, 1982
Heteroptera Laboratory - Schnetter et al., 1981;
(Notonectidae) Olejnicek & Maryskova, 1986;
Aly & Mulla, 1987
Field - Mulla et al., 1982;
Mulligan & Schaefer, 1982;
Mulla, 1988
Field + Purcell, 1981
Odonata Laboratory - Mulla & Khasawinah, 1969;
Mulligan & Schaefer, 1982; Aly, 1985;
Aly & Mulla, 1987
Field - Mulla, 1988
a - = no effect reported; + = an effect was reported, but does not necessarily imply that either
individual arthropods or populations of arthropods were adversely affected.
Four species of chironomid larvae (Chironomus crassicaudatus,
Chironomus decorus, Glyptotendipes paripes, Tanytarsus species)
were tested with four Bti preparations. The chironomid larvae were
less susceptible to Bti, being 13- to 75-fold more tolerant than
mosquito larvae to the various Bti preparations (Ali, 1981; Ali &
Baggs 1981). Garcia et al. (1980) induced low to high levels of
mortality in some nematocerous Diptera, including a variety of taxa
in the families Ceratopogonidae, Chironomidae and Dixidae, using
dosages of Bti that were 50 to several hundredfold
higher than concentrations used for mosquito control. Schnetter et al.
(1981) reported complete mortality in chironomid larvae ( Chironomus
thummi ) exposed to high levels of Bti for 48 h without food.
Field-collected adult aquatic beetles exposed to Bti suffered little
or no mortality (Schnetter et al., 1981). Ali (1980) tested a Bti
formulation at 20 times the larvicidal dosage for mosquitos and
reported no adverse effects against larval mayflies (Baetis
species). Schnetter et al. (1981) reported that mayflies (Cloeon
species) suffered no mortality when fed Bti at high dosages.
Aly & Mulla (1987) fed Bti intoxicated mosquito larvae (Culex
quinquefasciatus) to field-collected fourth to fifth instar
backswimmers (Notonecta undulata). The predators were fed at the
rate of 10 larvae per predator per day for 4 days, then the predators
were fed unintoxicated mosquito larvae and observed for 15 to 17 days.
The nymph and adult notonectids exhibited no adverse effects.
Olejnicek & Maryskova (1986) observed no marked mortality in
backswimmers (Notonecta glauca) that were fed Bti intoxicated
mosquito larvae. Schnetter et al. (1981) found no mortality in
backswimmers (Notonecta glauca) exposed for 48 h to high levels of
Bti.
Mosquito larvae intoxicated with extremely high dosages of Bti
were fed to naiads of the dragonfly Tarnetrum corruptum and
damselfly Enallagma civile; the duration of development of the
dragonfly and damselfly naiads, from the time of exposure to
emergence, was not affected (Aly, 1985; Aly & Mulla, 1987)
Merritt et al. (1989) reported no evident of effects on the drift
of aquatic invertebrates, or on the numbers of these invertebrates in
benthic Surber samples, during a blackfly (Simulium species) control
programme. In the USA, Molloy (1992) reviewed ten field trials where
Bti was used against blackfly (Simulium species) larvae. He
concluded that, although there was a potential for adverse impact of
Bti on filter-feeding chironomids, the impact on stream insect
communities overall was very small.
Over a three-season period, Bti administered at mosquito
larvicidal rates had no adverse effects on the larvae of diving
beetles (Dytiscidae) or water scavengers (Hydrophylidae) (Mulla et
al., 1982; Mulla, 1988).
The application of a Bti formulation in a wildlife marsh showed
no adverse effects on beetle larvae (Mulligan & Schaefer, 1982).
Ali (1981) evaluated the efficacy of various levels of a Bti
formulation against chironomids in the families Chironominae and
Tanytarsinae and obtained mortality at dosages higher than those
employed to control mosquito larvae. Miura et al. (1980), using
mosquito larvicidal dosages of a commercial Bti product, showed no
reduction in the field populations of chironomids following treatment.
Mulla et al. (1971) reported marked reductions in some chironomid
populations, using a commercial Bti product at rates of 20 to 40 times
the mosquitocidal rates. Mulligan & Schaefer (1982) reported a 40 to
70% reduction in some chironomid species after application of a Bti
formulation to a wildlife marsh. Rogatin & Baizhanov (1984) noted a
significant reduction of chironomids after Bti exposure.
Extensive quantitative observations were made on mayfly nymphs,
mostly Callibaetis pacificus, but no notable effects were observed
when Bti was applied against mosquito larvae (Mulla et al., 1982;
Mulla, 1988). Mulligan & Schaefer (1982) found that Bti did not
adversely affect mayfly nymphs (Callibaetis species). Schnetter et
al. (1981) reported that mayflies (Cloeon species) were not affected
when Bti was used in floodwater mosquito (Aedes vexans) larval
habitats. Schnetter et al. (1981) collected water boatmen (Corisella
species) from mosquito larval habitats on the upper Rhine river in
Germany. The water boatman population was not affected after exposure
to Bti for 48 h. Adverse effects were noted on backswimmers (Buenoa
species, Notonecta undulata, and Notonecta unifasciata) during
field trials with Bti (Mulla et al., 1982; Mulla, 1988). Mulligan &
Schaefer (1982) reported that the backswimmer (Notonecta species)
populations in a wetland marsh were not adversely affected by the
application of a Bti formulation. Purcell (1981) noted reductions in
populations of backswimmers (Buenoa elegans, Notonecta indica)
after application of Bti, but attributed this to the flying activity
of these predators.
No adverse effects on naiads of the dragonfly (Tarnetrum
corruptum) and damselfly (Enallagma civile) were reported when Bti
was used against larval mosquito populations (Mulla & Khasawinah,
1969; Mulla, 1988).
No notable reduction in the number of nymphs of several species
of dragonfly (Anisoptera) and damselfly (Zygoptera) occurred when
Bti was applied in a wetland marsh (Mulligan & Schaefer, 1982).
In the follow-up to the Onchocerciasis Control Programme of West
Africa (section 5.1.3.2), little or no effect on the non-target
populations was observed. However, few details were provided (Yameogo
et al., 1988; Levêque et al., 1988; Calamari et al., 1998).
6.4.2.2 Terrestrial insects
Melin & Cozzi (1990) summarized a number of studies on the
effects of Btk, Btg, Btt and Bte on non-target arthropod species and
arthropod populations in the laboratory and field. Representative
studies on Btk, Btg, Btt and Bte are listed in Tables 13 and 14.
Obadofin & Finlayson (1977) determined that a commercial Btk
product had a minimal effect on the ground beetle ( Bembidion
lampros ). Wilkinson et al. (1975) evaluated the contact activity of
a commercial Btk product for 5 days at levels equivalent to field
rates on an adult ladybird beetle (Hippodamia convergens) and found
no adverse effects.
Workman (1977) exposed earwigs (Labidura riparia) to a
commercial Btk product at rates equivalent to 10 times the normal
field application rate. No mortality was observed in these predators.
Hamed (1978-1979) found that two tachinid species (Bessa fugax
and Zenilla dolosa) were not affected after being fed suspensions
of a commercial Btk product. Horn (1983) observed a reduction in the
number of syrphid larvae on collards sprayed with a commercial Btk
product. This effect was attributed to a repellent effect on the
syrphid adults.
Hamed (1978-1979) found that Picromerus bidens was not
adversely affected after feeding upon lepidopteran larvae (Yponomeuta
evonymellus) that had fed upon leaves treated with commercial Btk
products.
Hassan (1983) determined a commercial Btk product to be harmless
to adult lacewings (Chrysopa carnea) when they were exposed at
normal field rate concentrations. Wilkinson et al. (1975) found
negligible mortality in larval or adult lacewings (Chrysopa carnea)
when a commercial Btk product was applied as a contact spray at
recommended field rates.
Yousten (1973) fed lethal quantities of Btk to larval cabbage
loopers (Trichoplusia ni) and just prior to death offered these
larvae to young Chinese praying mantids (Tenodera aridifolia
subspecies sinensis). The mantids were not susceptible to the
spore-crystal mixtures in the intact insect host.
Asquith (1975) found that black ladybird beetles (Stethorus
punctum) on apple trees were not affected by treatment with a
commercial Btk product. Buckner et al. (1974) monitored populations of
ground beetles following aerial spraying of spruce with two commercial
Btk products and found no effect on these predators. Harding et al.
(1972) detected no reduction in population levels of ladybird beetles
(coccinellids), rove beetles (staphyllinids), or checkered beetles
(clerids) in plots treated with a commercial Btk product. Johnson
(1974) evaluated several commercial Btk products as both sprays and
baits on tobacco. During the 2-year study, the populations of two
coccinellids (Hippodamia convergens and Colemegilla maculata)
were not affected by the microbial treatments. Wallner & Surgeoner
(1974) found no effects on coccinellids (Cycloneda munda,
Chilocorus bivulnerus and Adalia bipuncta) following forest
sprays with a commercial Btk product.
While evaluating a commercial Btk product for the control of
gypsy moth (Lymantria dispar) and elm spanworm ( Ennomos
subsignacius ), Dunbar et al. (1972) found no adverse effect on
Table 13. Effects of Btk on non-target arthropods
Arthropod order Type of study Resultsa References
Acarina Field - Weires & Smith, 1977;
Horsburgh & Cobb, 1981
Coleoptera Laboratory - Wilkinson et al., 1975; Obadofin & Finlayson, 1977
Field - Harding et al., 1972; Buckner et al., 1974;
Johnson, 1974; Wallner & Surgeoner, 1974;
Asquith, 1975
Dermaptera Laboratory - Workman, 1977
Diptera Laboratory - Hamed, 1978-1979
Laboratory - Horn, 1983
Field - Dunbar et al., 1972; Fusco, 1980
Heteroptera Laboratory - Hamed, 1978-1979
Field - Harding et al., 1972; Elsey, 1973;
Jensen, 1974; Wallner & Surgeoner, 1974
Hymenoptera Laboratory - Krieg, 1973
(Honey-bees) Laboratory - Krieg et al., 1980
Field - Buckner et al., 1974
Hymenoptera Laboratory - Wallner & Surgeoner, 1974;
(Parasitoids) Laboratory + Hassan & Krieg, 1975; Krieg et al., 1980
Dunbar & Johnson, 1975;
Mück et al., 1981; Weseloh &
Andreadis, 1982; Wallner et al., 1983;
Thomas & Watson, 1986
Field - Dunbar et al., 1972; Buckner et al., 1974;
Wanller & Surgeoner, 1974; Hamel, 1977;
Morris et al., 1977; Morris et al., 1980;
Fusco, 1980
Field + Weseloh et al., 1983
Table 13. (cont'd)
Arthropod order Type of study Resultsa References
Neuroptera Laboratory - Wilkinson et al., 1975; Hassan, 1983
Dictyoptera Laboratory - Yousten, 1973
(mantis)
a - = no effect reported; + an effect was reported, but does not imply that either
individual arthropods or populations of arthropods were adversely affected.
Table 14. Effects of different Bt strains on non-target arthropods
Arthropod order Type of study Resulta References
Btg
Hymenoptera Laboratory - Cantwell & Shieh, 1981
(Honey-bees) Field - Burges, 1977
Field - Burges & Bailey, 1968
Btt
Coleoptera Field + Kazakova & Dzhunusov, 1977
Hymenoptera Laboratory + Krieg & Herfs, 1963; Krieg, 1973
(Honey-bees) Laboratory - Martouret & Euverte, 1964;
Cantwell et al., 1966
Hymenoptera Laboratory + Hassan & Krieg, 1975;
(Parasitoids) Salama et al., 1982; Hassan, 1983;
Salama & Zaki, 1983
Laboratory - Krieg et al., 1980
Table 14. (cont'd)
Arthropod order Type of study Resulta References
Bte
Coleoptera Laboratory - Salama & Zaki, 1983
Laboratory + Salama et al., 1982
Hymenoptera Laboratory + Salama & Zaki, 1983
(Parasitoids)
Neuroptera Laboratory + Salama et al., 1982
a - = no effect reported; + = an effect was reported, but does not imply
that either individual arthropods or populations of arthropods were adversely affected.
two tachinids (Blepharipa scutellata and Parasitigena agilis).
Fusco (1980) reported an increased incidence of parasitism by two
tachinids (Blepharipa pratensis and Compsilura concinnata) when
Btk was applied in a field study.
Elsey (1973) reported no detrimental effect on spined stiltbug
nymphs or adults (Jalysus spinosus) during a 2-month field study
with a commercial Btk product. Harding et al. (1972) conducted a 2
year study to evaluate the effects of Btk on the natural enemies of
the bollworm (Helicoverpa zea) on cotton (Gossypium hirsutum).
Following applications of Btk against this pest, they reported no
detectable effects on Anthocoridae (minute pirate bugs, Orius
species), Lygaediae (bigeyed bugs, Geocoris species), Nabidae
(damsel bugs, Nabis species), or Reduviidae (assassin bugs).
Jensen (1974) used a commercial Btk product on soybeans (Glycine
canescens) to control the green cloverworm (Plathypena scabra) and
the velvetbean caterpillar (Anticarsis gemmitalis). No adverse
effect was observed on Lygaediae (bigeyed bugs, Geocoris species)
or Nabidae (damsel bugs, Nabis species).
Wallner & Surgeoner (1974) found no effect on the spined soldier
bug (Podisus maculiventris), following forest sprays of commercial
Btk products to control the oakleaf caterpillar (Heterocampa
manteo).
Although many data exist, in a review of the effects of the use
of Btk in Canada, Addison (1993) concluded that few studies on NTOs
had used soil invertebrate species and soil conditions relevant to
field conditions in Canadian forests.
Salama & Zaki (1983) reared cotton leafworm larvae (Spodoptera
littoralis) on a diet containing Bte and then fed these larvae to
adult staphylinid beetles (Paederus alferii). Predator longevity was
not significantly affected and no difference was seen in acceptability
to predators between untreated larvae and those exposed to Bte.
Salama et al. (1982) treated aphids with sprays of Bte and
provided these treated insects to newly hatched coccinellid larvae
(Coccinella undecimpunctata). The survival of larvae of predators
was not affected by feeding on the treated prey. However, the duration
of predator larval development was increased in the group treated with
Bte and there was a definite reduction in prey consumption.
Salama et al. (1982) evaluated the effect of Bte on the
development of lacewing larvae (Chrysopa carnea) by presenting them
with either sprayed aphids or treated cotton leafworm larvae
(Spodoptera littoralis). When fed either the sprayed aphids or the
treated cotton leafworms, the duration of larval development was
significantly extended and prey consumption was significantly reduced.
6.4.2.3 Honey-bees
Krieg (1973) observed mortality in adult honey-bees (Apis
mellifera) that were fed non-sporulated broth cultures of Btk. The
mortalities were attributed to the thermolabile alpha-toxin. Since
alpha-toxin is inactivated during sporulation, the toxin would not
present a problem in sporulated commercial Btk products. When Krieg et
al. (1980) fed fully sporulated cultures of Btk to adult honey-bees at
concentrations of 1 × 108 spores and crystal per bee over a 7-day
period, no harmful effects were observed.
Cantwell & Shieh (1981) fed a 1:20 solution of Btg in a sucrose
solution to newly emerged adult honey-bees. After 14 days, there was
no difference in mortality between treated and untreated groups.
Treatment of hives resulted in no adverse effect on the adult workers
or colony life as determined by egg laying, brood production, brood
capping, or honey production.
Buckner et al. (1974) observed no adverse effects on honey-bees
following aerial spraying of spruce (Picea species) with commercial
Btk products.
Cantwell et al. (1966) fed honey-bees sugar solutions containing
Btt spores, Btt culture supernatant with beta-exotoxin, and Btt
crystals. The crystals did not harm the bees, but the supernatant
caused nearly 100% mortality at day 7. Significant mortality was seen
in the spore-treated bees at 8 days and was attributed to bacterial
septicaemia. It should be noted that the dosages of each treatment
were many times higher than the bees would be exposed to in the course
of a lepidopteran control programme. Krieg (1973) reported mortality
in honey-bees fed whole nonsporulated cultures of Btt, which was
attributed to the presence of beta-exotoxin. Krieg & Herfs (1963)
reported that vegetative cells of Btt did not harm honey-bees;
however, they reported toxicity in Btt preparations containing the
beta-exotoxin. Martouret & Euverte (1964) fed worker honey-bees
cultures of Btt incorporated into mixtures of sugar, honey and clay.
Complete mortality was seen at 7 days for the spore-crystal-exotoxin
preparation and at 14 days for the spore-crystal complex.
6.4.2.4 Parasitoids
a) Btk
Dunbar & Johnson (1975) collected adult parasitoids
(Cardiochiles nigriceps) in the field and fed them suspensions of a
commercial Btk product. In the group fed Btk, shorter life spans were
reported. Since the investigators could not be sure whether feeding
actually took place, starvation may have been the cause of death.
Hassan & Krieg (1975) observed no adverse effects on adult
chalcid wasps (Trichogramma cacoeciae) that were fed suspensions of
a commercial Btk product. Krieg et al. (1980) fed washed spores and
crystals of Btk (5 × 107 spores and crystals) for 7 days to adult
chalcid wasps (Trichogramma cacoeciae) and observed no mortality or
reduced capacity to parasitize.
Mück et al. (1981) reported significant mortality in adult
braconids (Cotesia glomerata) that were fed a commercial Btk product
at rates of 108 and 109 spores per ml, but observed little effect on
the adult parasitoids (Pimpla turionellae). They reported midgut
epithelial damage in the Pimpla turionellae, which resulted from the
ICP.
Thomas & Watson (1986) found lower survival in adult ichneumonids
(Hyposoter exiguae) fed suspensions of a commercial Btk product.
They concluded the mortality was due to the spore-crystal complex.
Wallner & Surgeoner (1974) observed no effect on parasitoids
following treatments with commercial Btk products for control of the
notodontid moth (Heterocampa manteo).
Wallner et al. (1983) reported an indirect effect on the braconid
Rogas lymantriae when it parasitized gypsy moth (Lymantria dispar)
hosts fed Btk. The sex ratio of the parasitoid offspring was skewed
towards males in the treated larvae, as the female parasitoids lay
more fertilized eggs in larger, untreated host larvae.
Weseloh & Andreadis (1982) reported synergism in laboratory tests
with gypsy moth larvae (Lymantria dispar) fed a commercial Btk
product and exposed to the braconid (Cotesia melanoscelus). The
percentage of parasitism was increased in Btk-intoxicated larvae since
these grew more slowly and were at the approximate size suitable for
parasitism for a longer time.
Buckner et al. (1974) reported no detrimental effects on
parasitoid populations following field application of a commercial Btk
product.
Dunbar et al. (1972) reported an increase in the percentage of
parasitism of gypsy moth (Lymantria dispar) and elm spanworm
(Ennomos subsignarius) larvae in forestry plots treated with a
commercial Btk product.
Fusco (1980) reported an increase in the percentage of parasitism
of gypsy moth (Lymantria dispar) larvae by the braconids Cotesia
melanoscelus and Phobocampe unicincta following aerial sprays with
a commercial Btk product.
Hamel (1977) found that parasitoids attacking early instar
western spruce budworm larvae (Choristoneura occidentalis) increased
in number following aerial application of a commercial Btk product,
while older budworm larvae were reduced in number.
In two field studies, commercial Btk products showed no
detrimental effects on parasitoid populations (Morris et al., 1977,
1980).
Wallner & Surgeoner (1974) demonstrated 6- to 12-fold increases
in the percentage of parasitism in gypsy moth larvae (Lymantria
dispar) by the braconid Cotesia melanoscelus in forestry plots
treated with a commercial Btk product.
b) Btt
Hassan (1983) observed the chalcid Trichogramma cacoeciae was
not affected by exposure to dried surface films of Btt.
Hassan & Krieg (1975) fed a suspension of three different
commercial Bt products to adult chalcids (Trichogramma cacoeciae)
and reported a minor reduction in the capacity to parasitize with
Btt, but none with the other Bt products. The effect of the Btt
product may have been due to the beta-exotoxin.
Krieg et al. (1980) fed washed spores and crystals of Btt to
adult chalcids (Trichogramma cacoeciae) for 7 days and observed no
mortality or reduced capacity to parasitize.
Lowered reproductive potential was observed for both the
braconids Microplitis demolitor and Zele chlorophthalma
following exposure to Btt (Salama et al., 1982; Salama & Zaki, 1983).
Salama & Zaki (1983) reported increased development times for
Zele chlorophthalma parasitizing the cotton leafworm Spodoptera
littoralis treated with Bte.
7. EXPOSURE AND EFFECTS ON HUMANS
There are some case reports on the occurrence of Bt in patients
with different infectious diseases. However, none of these studies
demonstrate an actual risk to human health by the use of Bt. They
emphasize the need for further research on the production of toxins,
knowledge of factors causing the genes of the toxins to be expressed,
and knowledge on the natural occurrence of Bt and Bc. The medical
practice does not discriminate between Bt and Bc as causative agents
in infectious diseases. Therefore, the true proportion of Bt in Bc-
induced disease is not known.
7.1 Bacillus thuringiensis
For aeons, humans have been exposed to Bt in their natural
habitats, particularly from soil, water and the phylloplane. However
in the recorded scientific literature, only few adverse effects to
these environmental Bt levels have been documented.
The manufacture and field application of Bt products can result
in aerosol and dermal exposure of workers and the human population,
especially by spraying programmes in populated areas. Agricultural and
horticultural uses of Bt can also result in dietary exposure.
7.1.1 Experimental exposure of humans
Eight human volunteers ingested 1 gram of a Btk formulation
(3 × 109 spores/g of powder) daily for 5 days. Of the eight
volunteers, five also inhaled 100 mg of the Btk powder daily for five
days. Comprehensive medical examinations immediately before, after,
and 4 to 5 weeks later failed to demonstrate any adverse health
effects, and all the blood chemistry and urinalysis tests were
negative (Fisher & Rosner, 1959).
Pivovarov et al. (1977) reported that ingestion of foods
contaminated with Btg at concentrations of 105 to 109 cells/g
caused nausea, vomiting, diarrhoea and tenesmus, colic-like pains in
the abdomen, and fever in three of the four volunteers studied. The
toxicity of the Btg strain may have been due to beta-exotoxin (Ray,
1990).
7.1.2. Exposure of workers during manufacture
Many manufacturers of Bt products monitor the exposure and the
associated health risks of their workers. Over a period of 30 years of
production, there have been no reports of such workers having been
adversely affected (RJ Cibulsky, personal communication, 1997).
7.1.3 Exposure of workers in spraying operations
Noble et al. (1992) studied aerosol Btk exposure and subsequent
nose and throat carriage of Bt by workers during a major spray
programme for gypsy moth (Lymantria dispar) control. Spraying down
from high lifts, spraying low foliage or spraying with prevailing
breezes resulted in lower exposures of spray operators than did
spraying upwards into trees. The mean exposure values ranged from
3.0 × 103 to 5.9 × 106 Bt spores/m3 sampled air. Individuals working
most shifts during the spray period were exposed to 5.4 × 106 to
7.2 × 107 organisms. Nearly all the workers exposed to higher
concentrations for several shifts (5 to 20) were culture-positive for
Bt, and the majority of the workers remained culture-positive for 14
to 30 days. Of those who were culture-positive, eight workers reverted
to a culture-negative status during the project or within 30 days of
project completion. During the spray programme, some workers
experienced chapped lips, dry skin, eye irritation, and nasal drip and
stuffiness, but no serious health problems resulted. These symptoms
were transient and frequently occurred during the beginning of a spray
run and when Bt spray concentrations were increased. No significant
differences were found with respect to gender or smoking status.
In the same study, Noble et al. (1992) evaluated the health
records of the general population in the county where the Btk spray
programme was conducted. After examining the records of 3500 hospital
emergency room admissions, 1140 family practice patients, and over 400
bacterial cultures from 10 hospitals, no evidence for community
illness or infections attributed to Btk could be documented.
Laferrière et al. (1987) demonstrated antibody titres in 11 of
107 workers exposed to Btk during a 2-year spraying period. By the
middle of the spray operation, seven had developed titres to
spore-crystal complexes, six to vegetative cells, and one to spores.
Their titres tended to be low, but were higher in those exposed for a
second year. Two months after the exposure ended, nine workers were
retested. Of these workers, five had no detectable antibodies to the
spore-crystal complexes, and four who had been among those with the
highest titres against vegetative cells had significantly lower
titres.
Elliott et al. (1988) measured the exposure of individual workers
and other individuals within the spraying area on the day of
application during an aerial Btk spray programme for gypsy moth
(Lymantria dispar) control. Concentrations of spores were measured
using personal air sampling devices. The concentration of spores
ranged from 0 to 1.1 × 104 cfu/m3 for individual workers, the
highest concentration being incurred by a spray card checker who was
in brief contact with the material. For non-working individuals, the
average Bt exposure was 1.3 × 103 cfu/m3. In the spray area, a
general survey showed concentrations of 0 to 4.2 × 103 cfu/m3.
7.1.4 Exposure of human populations by spraying operations over
populated areas
Btk and Bti have been sprayed over populated areas in several
countries, including the USA, Canada and New Zealand. Some of these
applications have been followed by public health surveillance
programmes. In general, no (or very few) harmful effects have been
reported among residents of the sprayed communities.
7.1.5 Clinical case reports
Commercial Bt products have been used for over two decades, but
Bt has been isolated in only a few cases of human bacterial infection.
Samples & Buettner (1983) reported that a farm worker developed a
corneal ulcer in one eye. It had been accidentally splashed with a
commercial Btk product and Bt was subsequently isolated from the
affected eye. The eye was treated with a topical antibiotic and
corticosteroid and the corneal ulcer resolved 14 days after treatment.
The report attributed the corneal ulcer to Bt infection. However, the
possibility that Bt may have been a non-pathogenic contaminant of the
ulcer was not considered. There are no other reports of Bt being
associated with ocular infections in workers.
During the investigation of a gastroenteritis outbreak in a
chronic care institution, bacteria were isolated from four individuals
and were identified as B. thuringiensis. The Bt isolates showed
cytotoxic effects characteristic of B. cereus (Jackson et al.,
1995).
Damgaard et al. (1997a) isolated Bt in burn wounds in two
patients. None of the isolates showed any toxicity to Vero cells.
Hernandez et al. (1998) isolated Bt from a war wound; this strain (Bt
konkukian) could infect immunosuppressed mice after cutaneous
application.
Warren et al. (1984) reported that a research worker developed a
marked local reaction and lymphadenitis following a needle stick
injury when handling Bti. Acinetobacter calcoaceticus and Bt were
cultured from the exudate. The condition responded to penicillin.
Green et al. (1990) reported that Bt was isolated from body
fluids of 55 patients with different infectious diseases. In 52 of
them, it was considered a contaminant, while in three cases with
pre-existing medical problems, no firm conclusion was established
concerning a causal relationship between the infection and Bt.
Furthermore, Bt was isolated from the conjunctiva of a worker
presenting with conjunctivitis, and with a history of a splash with a
Bt product.
Despite the widespread use of Bt-based products, only two
incidents of possible allergic reactions have been reported to the US
EPA (McClintock et al., 1995). After detailed analysis, neither of
these was considered to be causally related to Bt.
7.1.6 Dietary exposure of the general population
In some Asian countries, Bti has been added to domestic
containers of drinking-water for mosquito control. From these high Bt
exposures in drinking-water, no adverse effects in humans have been
reported. In Africa, some rivers have been dosed with Bti at weekly
intervals for blackfly control. No adverse effects in the human
populations that drink the river water have been reported. Btk has
been reported to survive for 1 to 2 months in fresh water and in
seawater. However, viable Bt cultures have not been isolated from
drinking-water supplies (Menon & De Mestral, 1985).
There is little information on levels of Bt to be found in food,
but it is possible that, in view of the widespread prevalence of Bt,
its presence in food is common and is not always related to its use on
food products. Bt spores have been shown to be unable to germinate in
mammalian digestive systems; however, Bt has been isolated from faecal
and urinary samples in occupational studies.
Noble et al. (1992) reported that 5 out of 10 vegetable samples
were positive for Btk. The positive samples were obtained from both
supermarkets and from organically grown products. Such results may
account for the recovery of Bt from faecal and urinary samples during
the occupational studies and may reflect community exposure through
food.
7.2 Bacillus cereus
The close affiliation between Bt and Bc raises the question of
whether strains of Bt can cause human illness during vegetative
growth. During vegetative growth Bc can produce different kinds of
toxins; these toxins can cause gastrointestinal diseases in humans
after ingestion. The emetic toxin is an enzymatically synthesized
peptide that causes vomiting (Granum, 1997) a few hours after
ingestion. Most Bc strains producing this toxin seem to belong to the
same serotype (Mikami et al., 1995; Nishikawa et al., 1996). The
enterotoxins are a group of proteins causing abdominal pain and
diarrhoea after an incubation period of 8-16 h. The enterotoxins
causing gastrointestinal disease are most likely produced in the small
intestine. Characteristics of the two types of disease caused by Bc
are shown Table 15. Based on analysis of outbreaks the infective dose
is believed to vary between 105 and 108 vegetative cells or
activated spores per gram, but it may be so low as 104 (Granum,
1997). In addition to the two toxins, Bc can produce different lytic
enzymes, e.g., haemolysins, which most likely are involved in the
gastrointestinal diseases. In addition to gastrointestinal diseases Bc
can cause various diseases, notably in immunosuppressed individuals
(Drobniewski, 1993).
Analysis of reported foodborne diseases reveals that Bc is
frequently diagnosed as the cause of gastrointestinal disorders
(Notermans & Batt, 1998) in many countries. Several food-borne disease
outbreaks caused by Bc have been reported by Notermans & Batt (1998).
However, Bc gastrointestinal diseases are highly under-reported, as
both types of illness are relatively mild and usually last less than
24 h (Granum, 1997; Notermans & Batt, 1998). The incidence of Bc in
foods varies between 101 and 107, the highest concentrations being
found in herbs/spices and boiled rice (Notermans & Batt, 1998). The
degree of toxicity of enterotoxins varies from Bc strain to strain,
probably due to differences in toxin components (Lund & Granum, 1997).
Hassan & Nabbut (1996) found that clinical Bc isolates from human
diarrhoeal faeces were strong producers of diarrhoeal enterotoxin,
while isolates from blood, wounds, normal faeces, milk and rice were
weak producers of diarrhoeal enterotoxin (Hassan & Nabbut, 1996). This
variation is reflected in the variable numbers (105-108 viable cells
or spores per g) of Bc reported to cause symptoms in humans, and it
has been suggested that foods containing more than 104 Bc per g may
not be safe for consumption (Granum, 1997). Several European countries
have a critical level of 104-105 Bc per g for acceptance of food
products (Notermans & Batt, 1998). This critical level will include
Bt, as the methods used do not discriminate between Bc and Bt.
Table 15. Characteristics of the two types of disease caused by B. cereus
(from Granum, 1997)
Characteristic Emetic syndrome Diarrhoeal
Infective dose (cells/g) 105-108 104-107
Toxin produced Preformed in food In the small intestine
Type of toxin Cyclic peptide Protein
Incubation period (h) 0.5-5 8-16 (occasionally >24)
Duration of illness 6-24 12-24 (occasionally many days)
Symptoms Vomiting, nausea, malaise Abdominal pain, diarrhoea
8. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Owing to their specific mode of action, Bt products are unlikely
to pose any hazard to humans or other vertebrates or to the great
majority of non-target invertebrates provided that they are free from
non-Bt microorganisms and from biologically active products other than
the ICPs. Bt products may be safely used for the control of insect
pests of agricultural and horticultural crops as well as forests. Bt
is also safe for use in aquatic environments including drinking-water
reservoirs for the control of mosquito, black fly and nuisance insect
larvae. However, it should be noted that vegetative Bt have the
potential for the production of Bc-like toxins, the significance of
which as a cause of human disease is not known.
9. CONCLUSIONS AND RECOMMENDATIONS
* Bt may be safely used for the control of insect pests of
agricultural crops and forests.
* Bti is safe for use in aquatic environments, including
drinking-water reservoirs, for the control of mosquito, blackfly
and nuisance insect larvae.
* Bt products should contain the ICPs and be free from other
microorganisms and biologically active metabolites.
* New Bt products based on either new Bt strains and/or new ICPs
require appropriate assessment.
* FAO and WHO should develop standard specifications for Bt
preparations as is done for chemical pesticides.
* Good industrial large-scale practice (GILSP) standards should be
employed for the production of Bt products.
* Standardized valid methods for the assessment of gastrointestinal
consequences of vegetatively produced agents should be developed.
* The occurrence of resistant insect populations underscores the
need for research on the relationships between cry-toxins and
the ecology of Bt.
* More research on the fate of Bt spores and ICPs in the
environment is needed. This should cover the natural occurrence
of Bt and Bc in foods and its relationship to exposure to Bt from
its pesticide use.
* Research into dose-response analysis and the consequent
acceptable daily intake levels of Bt in the diet and beverages is
a high priority.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANISATIONS
WHO (1985) considered the safe use of MPCA at the Ninth Meeting
of the WHO Expert Committee on Vector Control in 1984. The report
considered that addition of live microorganisms to drinking-water is
undesirable and recommended that the use of Bt H-14 for the control of
Aedes aegypti in drinking-water should be restricted to the
asporogenic form. At the 1990 meeting, WHO (1991), after reviewing new
research data, stated that its previous recommendation was unduly
restrictive, provided that properly designed formulations were used.
REFERENCES
Addison JA (1993) Persistence and nontarget effects of Bacillus
thuringiensis in soil: a review. Can J Forensic Res, 23: 2329-2342.
Agata N, Ohta M, Arakawa Y, & Mori M (1995) The bceT gene of
Bacillus cereus encodes an enterotoxic protein. Microbiology, 141:
983-988.
Ahmed R, Sankar-Mistry P, Jackson S, Ackermann H-W, & Kasatiya SS
(1995) Bacillus cereus phage typing as an epidemiological tool in
outbreaks of food poisoning. J Clin Microbiol, 33: 636-640.
Akiba Y (1986) Microbial ecology of Bacillus thuringiensis: VI.
Germination of Bacillus thuringiensis spores in the soil. Appl
Entomol Zool, 21: 76-80.
Akiba Y (1991) Assessment of rainwater-mediated dispersion of
field-sprayed Bacillus thuringiensis in the soil. Appl Entomol Zool,
26: 477-483.
Ali A (1980) Nuisance chironomids and their control: a review. Bull
Entomol Soc Am, 26: 3-16.
Ali A (1981) Bacillus thuringiensis serovar. israelensis
(ABG-6108) against chironomids and some nontarget aquatic
invertebrates. J Invertebr Pathol, 38: 264-272.
Ali A & Baggs RD (1981) Susceptibility of some Florida chironomids and
mosquitoes to various formulations of Bacillus thuringiensis serovar
israelensis de Barjac. J Econ Entomol, 74: 672-677.
Aly C (1985) Germination of Bacillus thuringiensis var. israelensis
spores in the gut of Aedes larvae (Diptera: Culicidae). J Invertebr
Pathol, 45: 1-8.
Aly C & Mulla MS (1987) Effect of two microbial insecticides on
aquatic predators of mosquitoes. J Appl Entomol, 103: 113-118.
Aly C, Mulla MS, & Federici BA (1985) Sporulation and toxin production
by Bacillus thuringiensis var. israelensis in cadavers of mosquito
larvae (Diptera: Culicidae). J Invertebr Pathol, 46: 251-258.
Andersson MA, Mikkola R, Helin J, Andersson MC, & Salkinoja-Salonen M
(1998) A novel sensitive bioassay for detection of Bacillus cereus
emetic toxin and related depsipeptide ionophores. Appl Environ
Microbiol, 64: 1338-1343.
Andrews RE Jr, Faust RM, Wabiko H, Raymond KC, & Bulla LA Jr (1987)
The biotechnology of Bacillus thuringiensis. CRC Crit Rev
Biotechnol, 6: 163-232.
Angus TA (1954) A bacterial toxin paralysing silkworm larvae. Nature
(Lond), 173: 545-546.
Aronson AI, Han ES, McGaughey W, & Johnson D (1991) The solubility of
inclusion proteins from Bacillus thuringiensis is dependent upon
protoxin composition and is a factor in toxicity to insects. Appl
Environ Microbiol, 57: 981-986.
Asano S-I, Nukumizu Y, Bando H, Hzuka T, & Yamamoto T (1997) Cloning
of novel enterotoxin genes from Bacillus cereus and Bacillus
thuringiensis. Appl Environ Microbiol, 63: 1054-1057.
Ash C, Farrow JA, Dorsch M, Stackebrandt E, & Collins MD (1991)
Comparative analysis of Bacillus anthracis, Bacillus cereus, and
related species on the basis of reverse transcriptase sequencing of
16S rRNA. Int J System Bacteriol, 41: 343-346.
Asquith D (1975) Response of the predaceous black lady beetle
Stethorus punctum (DeConte) to apple orchard insecticide treatments
(Experiment No. D986-4007). Chicago, Illinois, Abbott Laboratories.
Baida GE & Kuzmin NP (1995) Cloning and primary structure of a new
hemolysin gene from Bacillus cereus. Biochim Biophys Acta, 1264:
151-154.
Baumann L, Okamoto K, Unterman BM, Lynch MJ, & Bauman P (1984)
Phenotypic characterization of Bacillus thuringiensis and Bacillus
cereus. J Invertebr Pathol, 44: 329-341.
Beach RM (1990) Application for an experimental use permit to ship and
use a pesticide for experimental purposes only -- Permit No.
58788-EUP-4 for InCideM 586. Hanover, Maryland, Crop Genetics
International.
Beattie SH, Holt C, Hirst D, & Williams AG (1998) Discrimination among
Bacillus cereus, B. mycoides and B. thuringiensis and some other
species of the genus Bacillus by Fourier transform infrared
spectroscopy. FEMS Microbiol Lett, 164: 201-206.
Beavers JB (1991a) ABG-6305: An avian oral pathogenicity and toxicity
study in the mallard (Project No. 161-118). Easton, Maryland, Wildlife
International Ltd, pp 1-20 (Unpublished Abbott document).
Beavers JB (1991b) ABG-6305: An avian oral pathogenicity and toxicity
study in the bobwhite (Project No. 161-117). Easton, Maryland,
Wildlife International Ltd, pp 1-21 (Unpublished Abbott document).
Beavers JB, Larsen AC, & Jaber M (1989a) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the bobwhite (Project No. 161-109). Eston, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Beavers JB, Clauss BS, & Jaber M (1989b) Bacillus thuringiensis var.
tenebrionis: An avian single dose oral toxicity and pathogenicity
study in the mallard (Project No. 161-108). Easton, Maryland, Wildlife
International Ltd, pp 1-19 (Unpublished Abbott document).
Becker N & Margalit J (1993) Use of Bacillus thuringiensis
israelensis against mosquitoes and blackflies. In: Entwistle PF, Cory
JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 147-170.
Beegle CC, Dulmage HT, Wolfenbarger DA, & Martinez E (1981)
Persistence of Bacillus thuringiensis Berliner insecticidal activity
on cotton foliage. Environ Entomol, 10: 400-401.
Benz G & Altwegg A (1975) Safety of Bacillus thuringiensis for
earthworms. J Invertebr Pathol, 26: 125-126.
Berliner E (1915) [About the sleep sickness of the Ephestia kühniella
Zell. and its vector Bacillus thuringiensis.] Z Angew Entomol, 2:
29-56 (in German).
Bernhard K & Utz R (1993) Production of Bacillus thuringiensis
insecticides for experimental and commercial uses. In: Entwistle PF,
Cory JS, Bailey MJ, & Higgs S ed. Bacillus thuringiensis, an
environmental biopesticide: Theory and practice. Chichester, New York,
Toronto, Wiley & Sons, pp 255-267.
Bernier RL Jr, Gannon DJ, Moser GP, Mazzarello M, Griffiths MM, &
Guest PJ (1990) Development of a novel Bt strain for the control of
forestry pests. In: Brighton Crop Protection Conference -- Pests and
Diseases -- 1990: Proceedings. Farnham, Surrey, British Crop
Protection Council, pp 245-252.
Boeri RL (1991) Acute toxicity of ABG-6305 to the rainbow trout
(Oncorhynchus mykiss) (Project No. 9107-A). Hampton, New Hampshire,
Resource Analysts Inc, Enviro Systems Division, pp 1-26 (Unpublished
Abbott document).
Bonnefoi A & Béguin S (1959) Recherches sur l'action des cristaux de
Bacillus thuringiensis souche "anduze". Entomophaga, 4: 193-199.
Bourque SN, Valero JR, Lavoie MC, & Levesque RC (1995) Comparative
analysis of the 16S-23S ribosomal intergenic spacer sequences of
Bacillus thuringiensis strains and subspecies and of closely related
species. Appl Environ Microbiol, 61: 1623-1626.
Bravo A, Sarabia S, Lopez L, Ontiveros H, Abarca C, Ortiz M, Lina L,
Villalobos FJ, Pena G, Nunez-Valdez M-E, Soberón M, & Quintero R
(1998) Characterization of cry genes in a Mexican Bacillus
thuringienis strain collection. Appl Environ Microbiol, 64:
4965-4972.
Brousseau R, Saint-Onge A, Prefontaine G, Masson L, & Cabana J (1992)
Arbitrary primer polymerase chain reaction, a powerful method to
identify Bacillus thuringiensis serovars and strains. Appl Environ
Microbiol, 59: 114-119.
Buckner CH, Kingsbury PD, McLeod BB, Mortensen KL, & Ray DGH (1974)
Impact of aerial treatment on non-target organisms, Algonquin Park,
Ontario, and Spruce Woods, Manitoba, Section F. In: Evaluation of
commercial preparations of Bacillus thuringiensis with and without
chitinase against spruce budworm. Ottawa, Ontario, Canadian Forestry
Service, Chemical Control Research Institute, pp 1-72 (Information
report CC-X-59).
Bulla LA Jr, Kramer KJ, & Davidson LI (1977) Characterization of the
entomocidal parasporal crystal of Bacillus thuringiensis. J
Bacteriol, 130: 375-383.
Bulla LA Jr, Faust RM, Andrews R, & Goodman N (1985) Insecticidal
bacilli. In: Dubnau DA ed. The molecular biology of the bacilli. New
York, London, Academic Press Inc., vol 2, pp 185-209.
Burges HD (1977) Control of the wax moth Galleria mellonella on
beecomb by H-serotype V Bacillus thuringiensis and the effect of
chemical additives. Apidologie, 8: 155-168.
Burges HD (1980) Safety, safety testing and quality control of
microbial pesticides. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
738-767.
Burges HD & Bailey L (1968) Control of the greater and lesser wax
moths (Galleria mellonella and Achroia griesella) with Bacillus
thuringiensis. J Invertebr Pathol, 11: 184-195.
Burges HD & Hurst JA (1977) Ecology of Bacillus thuringiensis in
storage moths. J Invertebr Pathol, 30: 131-139.
Calamari D, Yameogo L, Hougard J-M, & Levêque C (1998) Environmental
assessment of larvicide use in the onchocerciasis control programme.
Parasitol Today, 14: 485-489.
Campbell DP, Dieball DE, & Brackett JM (1987) Rapid HPLC assay for the
ß-exotoxin of Bacillus thuringiensis. J Agric Food Chem, 35:
156-158.
Cannon RJC (1996) Bacillus thuringiensis use in agriculture: a
molecular perspective. Biol Rev Cambridge Philos Soc, 71: 561-636.
Cantwell GE & Shieh TR (1981) CertanTM: A new bacterial insecticide
against the greater wax moth. Am Bee J, 121: 424-431.
Cantwell GE, Heimpel AM, & Thompson MJ (1964) The production of an
exotoxin by various crystal-forming bacteria related to Bacillus
thuringiensis var. thuringiensis Berliner. J Insect Pathol, 6:
466-480.
Cantwell GE, Knox DA, Lehnert T, & Michael AS (1966) Mortality of the
honey bee, Apis mellifera, in colonies treated with certain
biological insecticides. J Invertebr Pathol, 8: 228-233.
Car M (1984) Laboratory and field trials with two Bacillus
thuringiensis var. israelensis products for Simulium (Diptera:
Nematocera) control in a small polluted river in South Africa.
Onderstepoort J Vet Res, 51: 141-144.
Car M & de Moor FC (1984) The response of Vaal River drift and benthos
to Simulium (Diptera: Nematocera) control using Bacillus
thuringiensis var. israelensis (H-14). Onderstepoort J Vet Res, 51:
155-160.
Carlberg G, Kihamia CM, & Minhar J (1985) Microbial control of flies
in latrines in Dar es Salaam with a Bacillus thuringiensis (serotype
1) preparation, Muscabac. J Appl Microbiol Biotechnol, 1: 33-44.
Carlson CR & Kolsto A-B (1993) A complete physical map of a Bacillus
thuringiensis chromosome. J Bacteriol, 175: 1053-1060.
Carlson CR, Caugant DA, & Kolstr A-B (1994) Genotypic diversity among
Bacillus cereus and Bacillus thuringiensis strains. Appl Environ
Microbiol, 60: 1719-1725.
Carlton BC, Gawron-Burke C, & Johnson TB (1990) Exploiting the genetic
diversity of Bacillus thuringiensis for the creation of new
bioinsecticides. In: Proceedings of the 5th International Colloquium
on Invertebrate Pathology and Microbial Control. Adelaide, Australia,
Society for Invertebrate Pathology, pp 18-22.
Chilcott CN, Pillai JS, & Kalmakoff J (1983) Efficacy of Bacillus
thuringiensis var. israelensis as a biocontrol agent against
larvae of Simuliidae (Diptera) in New Zealand. NZ J Zool, 10: 319-326.
Christensen KP (1990a) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 32-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-53 (Unpublished Abbott document No. 90-1-3211).
Christensen KP (1990b) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-57
(Unpublished Abbott document No. 90-2-3219).
Christensen KP (1990c) Dipel technical material (Bacillus
thuringiensis var. kurstaki): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc.,
vol 2, pp 253-308 (Unpublished Abbott document No. 90-5-3317).
Christensen KP (1990d) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to rainbow trout (Oncorhynchus mykiss)
during a 30-day static renewal test. Wareham, Massachusetts,
Springborn Laboratories Inc., pp 1-54 (Unpublished Abbott document No.
90-3-3263).
Christensen KP (1990e) Bacillus thuringiensis var. tenebrionis:
Infectivity and pathogenicity to sheepshead minnow (Cyprinodon
variegatus) during a 30-day static renewal test. Wareham,
Massachusetts, Springborn Laboratories Inc., pp 1-50 (Unpublished
Abbott document No. 90-6-3348).
Christensen KP (1990f) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
bluegill sunfish (Lepomis macrochirus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-55 (Unpublished Abbott document No. 90-2-3228).
Christensen KP (1990g) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
rainbow trout (Oncorhynchus mykiss) during a 32-day static renewal
test. Wareham, Massachusetts, Springborn Laboratories Inc., pp 1-55
(Unpublished Abbott document No. 90-2-3242).
Christensen KP (1990h) Vectobac technical material (Bacillus
thuringiensis var. israelensis): Infectivity and pathogenicity to
sheepshead minnow (Cyprinodon variegatus) during a 30-day static
renewal test. Wareham, Massachusetts, Springborn Laboratories Inc., pp
1-57 (Unpublished Abbott document No. 90-4-3288).
Cibulsky RJ & Fusco RA (1987) Recent experiences with Vectobac for
black fly control: An industrial perspective on future developments.
In: Kim KC & Merritt RW ed. Black flies: Ecology, population
management, and annotated world list. University Park, Pennsylvania,
Pennsylvania State University Press, pp 419-424.
Claus D & Berkeley RCW (1986) Genus Bacillus Cohn 1872. In: Sneath
PHA, Mair NS, Sharp ME, & Holt JG ed. Bergey's manual of systematic
bacteriology. Baltimore, Maryland, Williams & Wilkins, vol 2, pp
1105-1139.
Cooksey KE (1971) The protein crystal toxin of Bacillus
thuringiensis: Biochemistry and mode of action. In: Burges HD &
Hussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc., pp 247-274.
Crickmore N, Zeigler DR, Feitelson J, Schnepf E, van Rie J, Lereclus
D, Baum J, & Dean DH (1998) Revision of the nomenclature for the
Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol
Biol Rev, 62: 807-813.
Damgaard PH (1995) Diarrhoeal enterotoxin production by strains of
Bacillus thuringiensis isolated from commercial Bacillus
thuringiensis -- based insecticides. FEMS Immunol Med Microbiol, 12:
245-250.
Damgaard PH, Granum PE, Bresciani J, Torregrossa MV, Eilenberg J, &
Valentino L (1997a) Characterization of Bacillus thuringiensis
isolated from infections in burn wounds. FEMS Immunol Med Microbiol,
18: 47-53.
Damgaard PH, Hansen BM, Pedersen JC, & Eilenberg J (1997b) Natural
occurrence of B. thuringiensis on cabbage foliage and in insects
associated with cabbage crops. J Appl Bacteriol, 82: 253-258.
Damgaard PH, Larsen HD, Hansen BM, Bresciani J, & Jorgensen K (1996a)
Enterotoxin-producing strains of Bacillus thuringiensis isolated
from food. Lett Appl Microbiol, 23: 146-150.
Damgaard PH, Jacobsen CS, & Sorensen J (1996b) Development and
application of a primer set for specific detection of Bacillus
thuringiensis and Bacillus cereus in soil using magnetic
capture-hybridization and PCR amplification. System Appl Microbiol,
19: 436-441.
de Barjac H (1981a) Identification of H-serotypes of Bacillus
thuringiensis. In: Burges HD ed. Microbial control of pests and
plant diseases 1970-1980. New York, London, Academic Press Inc., pp
35-43.
de Barjac H (1981b) Insect pathogens in the genus Bacillus. In:
Berkley RCW & Goodfellow M ed. The aerobic endospore-forming bacteria:
Classification and identification. New York, London, Academic Press
Inc., pp 241-250.
de Barjac H & Bonnefoi A (1962) Essai de classification biochimique et
sérologique de 24 souches de Bacillus de type thuringiensis.
Entomophaga, 7: 5-31.
de Barjac H & Larget-Thiery I (1984) Characteristics of IPS 82 as
standard for biological assays of Bacillus thuringiensis H-14
preparations. Geneva, World Health Organization (WHO/VBC/84.892).
de Barjac H, Larget I, Bénichou L, Cosmao V, Viviani G, Ripouteau H, &
Papion S (1980) Test d'innocuité sur mammifères avec du sérotype H 14
de Bacillus thuringiensis. Geneva, World Health Organization
(WHO/VBC/80.761).
Dean DH (1984) Biochemical genetics of the bacterial insect-control
agent Bacillus thuringiensis: Basic principles and prospects for
genetic engineering. Biotechnol Genet Eng Rev, 2: 341-363.
Dean DH, Rajamohan F, Lee MK, Wu SJ, Chen XJ, Alcantara E, & Hussain
SR (1996) Probing the mechanism of action of Bacillus thuringiensis
insecticidal proteins by site-directed mutagenesis - a minireview.
Gene, 179: 111-117.
DeLucca AJ II, Simonson JG, & Larson AD (1981) Bacillus thuringiensis
distribution in soils of the United States. Can J Microbiol, 27:
865-870.
Demezas DH & Bell J (1995) Evaluation of low molecular weight RNA
profiles and ribotyping to differentiate some Bacillus species.
System Appl Microbiol, 18: 582-589.
Denolf P, Hendrickx K, Vandamme J, Jansens S, Peferoen M, Degheele D,
& Vanrie J (1997) Cloning and characterization of Manduca sexta and
Plutella xylostella midgut aminopeptidase N enzymes related to
Bacillus thuringiensis toxin-binding proteins. Eur J Biochem, 248:
748-761.
Dent DR (1993) The use of Bacillus thuringiensis as an insecticide.
In: Jones DG ed. Exploitation of microorganisms. London, Chapman &
Hall, pp 19-44.
Drobniewski FA (1993) Bacillus cereus and related species. Clin
Microbiol Rev, 6: 324-338.
Dulmage HT & cooperators (1981) Insecticidal activity of isolates of
Bacillus thuringiensis and their potential for pest control. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 193-223.
Dunbar JP & Johnson AW (1975) Bacillus thuringiensis: Effects on the
survival of a tobacco budworm parasitoid and predator in the
laboratory. Environ Entomol, 4: 352-354.
Dunbar DM, Kaya HK, Doane CC, Anderson JF, & Weseloh RM (1972) Aerial
application of Bacillus thuringiensis against larvae of the elm
spanworm and gypsy moth and effects on parasitoids of the gypsy moth.
Connecticut Experiment Station (Bulletin No. 735).
Eilenberg J, Damgaard PH, Hansen BM, Pedersen JC, Bresciani J, &
Larsson R (in press) Natural interactions between Strongwellsea
castrans, Cystosporogenes deliaradicae and Bacillus thuringiensis
in the host, Delia radicum. J Invertebr Pathol.
Elliott LJ, Sokolow R, Heumann M, & Elefant SL (1988), An exposure
characterization of a large scale application of a biological
insecticide, Bacillus thuringiensis. Appl Ind Hyg, 3: 119-122.
Elsey KD (1973) Jalysus spinosus effect of insecticide treatments on
this predator of tobacco pests. Environ Entomol, 2: 240-243.
Entwistle PF, Cory JS, Bailey MJ, & Higgs S (1983) Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, John Wiley & Sons, 311 pp.
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, & Koziel MG
(1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal
protein with a wide spectrum of activities against lepidopteran
insects. Proc Natl Acad Sci (USA), 93: 5389-5394.
Farkas J, Sebesta K, Horská K, Samek Z, Dolejs L, & Sorm F (1977)
Structure of thuringiensin, the thermostable exotoxin from Bacillus
thuringiensis. Coll Czech Chem Commun, 42: 909-929.
Fast PG (1971) Isolation of a water-soluble toxin from a commercial
microbial insecticide based on Bacillus thuringiensis. J Invertebr
Pathol, 17: 301.
Fast PG (1981) The crystal toxin of Bacillus thuringiensis. In:
Burges HD ed. Microbial control of pests and plant diseases 1970-1980.
New York, London, Academic Press Inc., pp 223-248.
Faust RM (1973) The Bacillus thuringiensis ß-exotoxin: Current
status. Bull Entomol Soc Am, 19: 153-156.
Feitelson JS (1993) The Bacillus thuringiensis family tree. In: Kim
L ed. Advanced engineered pesticides. New York, Basel, Marcel Dekker
Inc., pp 63-71.
Fisher R & Rosner L (1959) Toxicology of the microbial insecticide,
Thuricide. J Agric Food Chem, 7: 686-688.
Frommer W, Ager B, Archer L, Brunius G, Collins CH, Donikian R,
Frontali C, Hamp S, Houwink EH, Kuenzi MT, Krämer P, Lagast H, Lund S,
Mahler JL, Normand-Plessier F, Sargeant K, Tuijnenburg-Muijs G, Vranch
SP, & Werner RG (1989) Safe biotechnology: III. Safety precautions for
handling microorganisms of different risk classes. Appl Microbiol
Biotechnol, 30: 541-552.
Fusco RA (1980) Field evaluation of a commercial preparation of
Bacillus thuringiensis, DIPEL 4L: Progress report. Pennsylvania
Bureau of Forestry, Gypsy Moth Pest Management Methods Development
Project.
Gaddis PK & Corkran CC (1986) Secondary effects of Bt spray on avian
predators: the reproductive success of chestnut-backed chickadees.
Northwest Ecological Research Institute (Unpublished Research Report
No. 86-03).
Garcia R, Des Rochers B, & Tozer W (1980) Studies on the toxicity of
Bacillus thuringiensis var. israelensis against organisms found in
association with mosquito larvae. Berkeley, California, University of
California, Division of Biological Control.
Gelernter WD (1990) Targeting insecticide-resistant markets: New
developments in microbial-based products. In: Green MB, Moberg WK, &
LeBaron H ed. Managing resistance to agrochemicals: From fundamental
research to practical strategies. Washington, DC, American Chemical
Society, pp 105-117 (ACS Series 421).
Giffel MC, Beumer RR, Klijn N, Wagendorp A, & Rombouts FM (1997)
Discrimination between Bacillus cereus and Bacillus thuringiensis
using specific DNA probes based on variable regions of 16S rRNA. FEMS
Microbiol Lett, 146: 47-51.
Gill SS, Hornung JM, Ibarra JE, Singh GJP, & Federici BA (1987)
Cytolytic activity and immunological similarity of Bacillus
thuringiensis subsp. israelensis and Bacillus thuringiensis
subsp. morrisoni isolate PG-14 toxins. Appl Environ Microbiol, 53:
1251-1256.
Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, & Goebel W
(1989) A Bacillus cereus cytolytic determinant, cereolysin AB, which
comprises the phospholipase C and sphingomyelinase genes: Nucleotide
sequence and genetic linkage. J Bacteriol, 171: 744-753.
Goldberg LJ & Margalit J (1977) A bacterial spore demonstrating rapid
larvicidal activity against Anopheles sergentii, Uranotaenia
unguiculata, Culex univitattus, Aedes aegypti and Culex
pipiens. Mosq News, 37: 355-358.
González JM Jr & Carlton BC (1980) Patterns of plasmid DNA in
crystalliferous and acrystalliferous strains of Bacillus
thuringiensis. Plasmid, 3: 92-98.
González JM Jr, Dulmage HT, & Carlton BC (1981) Correlation between
specific plasmids and delta-endotoxin production in Bacillus
thuringiensis. Plasmid, 5: 351-365.
González JM, Jr Brown BJ, & Carlton BC (1982) Transfer of Bacillus
thuringiensis plasmids coding for delta-endotoxin among strains of
B. thuringiensis and B. cereus. Proc Natl Acad Sci (USA), 79:
6951-6955.
Gordon RE (1977) Some taxonomic observations on the genus Bacillus.
In: Briggs JB ed. Biological regulations of vectors: The saprophytic
and aerobic bacteria and fungi. Washington, DC, US Department of
Health, Education and Welfare, pp 67-82 (Publication NIH 77-1180).
Granum PE (1997) Bacillus cereus. In: Doyle MP, Beuchat LR, &
Montville TJ ed. Food microbiology fundamentals and frontiers.
Washington, DC, ASM Press, pp 327-336.
Grassi S & Deseö KV (1984) [The natural occurrence of Bacillus
thuringiensis Berl. And its importance in the plant protection.] In:
[Proceedings of Seminar on Phytopathology, Sorrento, Italy, 26-29
March 1984.] Bologna, Italy, Clueb Publishing Co., vol 2, pp 424-433
(in Italian).
Green M, Heumann M, Sokolow R, Foster LR, Bryant R, & Skeels M (1990)
Public health implications of the microbial pesticide Bacillus
thuringiensis: An epidemiological study, Oregon, 1985-1986. Am J
Publ Health, 80: 848-852.
Griego VM & Spence KD (1978) Inactivation of Bacillus thuringiensis
spores by ultraviolet and visible light. Appl Environ Microbiol, 35:
906-910.
Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL,
Brousseau R, & Cygler M (1995) Bacillus thuringiensis CryIA(a)
insecticidal toxin: crystal structure and channel formation. J Mol
Biol, 254: 447-464.
Hadley WM, Burchiel SW, McDowell TD, Thilsted JP, Hibbs CM, Whorton
JA, Day PW, Friedman MB, & Stoll RE (1987) Five-month oral (diet)
toxicity/ infectivity study of Bacillus thuringiensis insecticides
in sheep. Fundam Appl Toxicol, 8: 236-242.
Hamed AR (1978-79) [Effects of Bacillus thuringiensis on parasites
and predators of Yponomeuta evenymellus (Lep., Yponomeutidae).] Z
Angew Entomol, 87: 294-311 (in German).
Hamel DR (1977) The effects of Bacillus thuringiensis on parasitoids
of the western spruce budworm, Choristoneura occidentalis
(Lepidoptera: Tortricidae), and the spruce coneworm, Dioryctria
reniculelloides (Lepidoptera: Pyralidae), in Montana. Can Entomol,
109: 1409-1415.
Hannay CL (1953) Crystalline inclusions in aerobic spore-forming
bacteria. Nature (Lond), 172: 1004.
Hansen BM & Hendriksen NB (1997a) Bacillus thuringiensis and B.
cereus enterotoxins: Proceedings of the 6th European Meeting of the
International Organization for Biological and Integrated Control of
Noxious Animals and Plants/West Paleaearctic Regional Section,
Copenhagen, 10-15 August 1997. Copenhagen, Denmark, Royal Veterinary
and Agricultural University, Department of Ecology and Molecular
Biology.
Hansen BM & Hendriksen NB (1997b) Comparative PCR analysis of B.
thuringiensis and B. cereus: Abstract for the International
Workshop on Molecular Biology of B. cereus, B. anthracis and
B. thuringiensis, Soria Moria, Oslo, 23-25 May 1997.
Hansen BM & Hendriksen NB (1999) Ecological aspects of the survival in
soil of spray released Bacillus thuringiensis subsp. kurstaki. In:
Abstract for the IOBC Symposium on Indirect Ecological Effects of
Biological Control, Montpelier, 17-20 October 1999. Montpelier,
France, Agropolis International.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1996) Bacillus
thuringiensis, ecology and environmental effects of its use for
microbial pest control (Environmental Project No. 316). Copenhagen,
Denmark, Ministry of Environment and Energy, Danish Environmental
Protection Agency.
Hansen BM, Damgaard PH, Eilenberg J, & Pedersen JC (1998) Molecular
and phenotypic characterization of Bacillus thuringiensis isolated
from leaves and insects. J Invertebr Pathol, 71: 106-114.
Harding J, Wolfenbarger D, Dupnik T, & Fuchs T (1972) Large scale
tests comparing Bacillus thuringiensis with methyl-parathion for
cotton insect control, field test report. Chicago, Illinois, Abbott
Laboratories (Unpublished document).
Hassan SA (1983) Results of laboratory testing of a series of
pesticides on egg parasites of the genus Trichogramma (Hymenoptera,
Trichogrammatidae). Nachr.bl Dtsch Pflanzenschutzdienstes
(Braunschweig), 35: 21.
Hassan S & Krieg A (1975) [ Bacillus thuringiensis preparations
harmless to the parasite Trichogramma cacoeciae (Hym.:
Trichogrammatidae).] Z Pflanzenkr Pflanzenchutz, 82: 515-521 (in
German).
Hassan G & Nabbut N (1996) Prevalence and characterization of
Bacillus cereus isolates from clinical and natural sources. J Food
Prot, 59: 193-196.
Heimpel AM (1954) Investigations of the mode of action of strains of
Bacillus cereus Frankland and Frankland pathogenic for the larch
sawfly, Pristiphora chrichsonii (Htg.). Kingston, Canada, Queen's
University, 155 pp (Doctoral dissertation).
Heimpel AM (1967) A critical review of Bacillus thuringiensis var.
thuringiensis Berliner and other crystalliferous bacteria. Annu Rev
Entomol, 12: 287-322.
Heimpel AM & Angus TA (1958) The taxonomy of insect pathogens related
to Bacillus cereus Frankland and Frankland. Can J Microbiol, 4:
531-541.
Helgason E, Caugant DA, Lecadet M-M, Chen Y, Mahillon J, Lövgren A,
Hegna I, Kvaloy K, & Kolsto A-B (1998) Genetic diversity of Bacillus
cereus/ B. thuringiensis isolates from natural sources. Curr
Microbiol, 37: 80-87.
Hendriksen NB & Hansen BM (1998) Phylogenetic relations of Bacillus
thuringiensis: Implications for risks associated to its use as a
microbiological pest control agent. IOBC Bull, 21: 5-8.
Hernandez E, Ramisse F, Ducoureau J-P, Cruel T, & Cavallo J-D (1998)
Bacillus thuringiensis subsp. konkukian (serotype H34)
superinfection: Case report and experimental evidence of pathogenicity
in immunosuppressed mice. J Clin Microbiol, 36: 2138-2139.
Höfte H & Whiteley HR (1989) Insecticidal crystal proteins of
Bacillus thuringiensis. Microbiol Rev, 53: 242-255.
Honée G & Visser B (1993) The mode of action of Bacillus
thuringiensis crystal proteins. Entomol Exp Appl, 69: 145-155.
Horn DJ (1983) Selective mortality of parasitoids and predators of
Myzus persicae on collards treated with malathion, carbaryl, or
Bacillus thuringiensis. Entomol Exp Appl, 34: 208-211.
Horsburgh R & Cobb L (1981) Effect of Dipel WP and Dipel + Guthion
tank mix combination for control of variegated leafroller, turfed
apple bud moth, and red-banded leafroller larvae on apples in a full
season and late season program in Virginia (Experiment No.
D-986-4240). Chicago, Illinois, Abbott Laboratories Inc.
Hostetter DL, Ignoffo CM, & Kearby WH (1975) Persistence of
formulations of Bacillus thuringiensis spores and crystals on
eastern red cedar foliage in Missouri. J Kansas Entomol Soc, 48:
189-193.
Hotha S & Banik RM (1997) Production of alkaline protease by Bacillus
thuringiensis H 14 in aqueous two-phase systems. J Chem Technol
Biotechnol, 69: 5-10.
Huber HE & Lüthy P (1981) Bacillus thuringiensis delta-endotoxin:
Composition and activation. In: Davidson EW ed. Pathogenesis of
invertebrate microbial diseases. Totowa, New Jersey, Allanheld-Osmun
Publishers, pp 209-234.
Huber HE, Lüthy P, Ebersold HR, & Cordier JL (1981) The subunits of
the parasporal crystal of Bacillus thuringiensis: size, linkage and
toxicity. Arch Microbiol, 129: 14-18.
Ignoffo CM (1973) Effects of entomopathogens on vertebrates. Ann NY
Acad Sci, 217: 141-164.
Ignoffo CM (1992) Environmental factors affecting persistence of
entomopathogens. Fla Entomol, 75: 516-525.
Innes DGL & Bendell JF (1989) The effects on small-mammal populations
of aerial applications of Bacillus thuringiensis, fenitrothion, and
Matacil(R) used against jack pine budworm in Ontario. Can J Zool,
67: 1318-1323.
Jackson SG, Goodbrand RB, Ahmed R, & Kasatiya S (1995) Bacillus
cereus and Bacillus thuringiensis isolated in a gastroenteritis
outbreak investigation. Lett Appl Microbiol, 21: 103-105.
Jaquet F, Hütter R, & Lüthy P (1987) Specificity of Bacillus
thuringiensis delta-endotoxin. Appl Environ Microbiol, 53: 500-504.
Jarrett P & Stephenson M (1990) Plasmid transfer between strains of
Bacillus thuringiensis infecting Galleria mellonella and
Spodoptera littoralis. Appl Environ Microbiol, 56: 1608-1614.
Jensen R (1974) Comparison of various insecticides including DIPEL WP
for control of the velvetbean caterpillar (Anticarsia gemmatalis)
and the green cloverworm (Plathypena scabra) on soybeans and the
effect on non-target species (Experiment No D911-1582). Chicago,
Illinois, Abbott Laboratories Inc.
Johnson AW (1974) Bacillus thuringiensis and tobacco budworm control
on flue-cured tobacco. J Econ Entomol, 67: 755-759.
Juarez-Perez VM, Ferrandis MD, & Frutos R (1997) PCR-based approach
for detection of novel Bacillus thuringiensis cry genes. Appl
Environ Microbiol, 63: 2997-3002.
Kaneko T, Nozaki R, & Aizawa K (1978) Deoxyribonucleic acid
relatedness between Bacillus anthracis, Bacillus cereus and
Bacillus thuringiensis. Microbiol Immunol, 22: 639-641.
Kazakova SB & Dzhunusov KK (1977) The effect of Bitoxibacillin-202 on
certain orchard insects in the Issyk-Kul' depression. Rev Appl
Entomol, A65: 5987.
Keller B & Langenbruch G-A (1993) Control of coleopteran pests by
Bacillus thuringiensis. In: Entwistle PF, Cory JS, Bailey MJ, &
Higgs S ed. Bacillus thuringiensis, an environmental biopesticide:
Theory and practice. Chichester, New York, Toronto, Wiley & Sons, pp
171-191.
Khawaled K, Ben-Dov E, Zaritsky A, & Barak Z (1990) The fate of
Bacillus thuringiensis var. israelensis in B. thuringiensis
var. israelensis-killed pupae of Aedes aegypti. J Invertebr
Pathol, 56: 312-316.
Kim HS, Lee DW, Woo SD, Yu YM, & Kang SK (1998) Seasonal distribution
and characterization of Bacillus thuringiensis isolated from
sericultural environments in Korea. J Gen Appl Microbiol, 44: 133-138.
Klier AF & Lecadet MM (1976) Arguments based on
hybridization-competition experiments in favor of the in vitro
synthesis of sporulation-specific mRNAs by the RNA polymerase of
Bacillus thuringiensis. Biochem Biophys Res Commun, 73: 263-270.
Knowles BH & Ellar DJ (1987) Colloid-osmotic lysis is a general
feature of the mechanisms of action of Bacillus thuringiensis
delta-endotoxins with different insect specificity. Biochim Biophys
Acta, 924: 509-518.
Krieg A (1971) Concerning alpha-exotoxin produced by vegetative cells
of Bacillus thuringiensis and Bacillus cereus. J Invertebr Pathol,
17: 134-135.
Krieg A (1973) About toxic effects of cultures of Bacillus cereus
and Bacillus thuringiensis on honey bees (Apis mellifera).
Z Pflanzenkr Pflanzenschutz, 80: 483-486.
Krieg A & Herfs W (1963) The effects of Bacillus thuringiensis on
honey bees. Entomol Exp Appl, 6: 1-9.
Krieg A, Hassan S, & Pinsdorf W (1980) Comparison of the effects of
the variety israelensis with other varieties of B. thuringiensis
on non-target organisms of the order Hymenoptera: Trichogramma
cacoeciae and Apis mellifera. Anz Schädlingsk Pflanz Umweltschutz,
53: 81-83.
Krieg A, Huger AM, Langenbruch GA, & Schnetter W (1983) Bacillus
thuringiensis var. tenebrionis, a new pathotype effective against
larvae of Coleoptera .. Z Angew Entomol, 96(5): 500-508.
Krywienczyk J, Dulmage HT, & Fast PG (1978) Occurrence of two
serologically distinct groups within Bacillus thuringiensis serotype
3ab variety kurstaki. J Invertebr Pathol, 37: 372-375.
Kumar PA, Sharma RP, & Malik VS (1996) The insecticidal protein of
Bacillus thuringiensis. Adv Appl Microbiol, 42: 1-43.
Lacey LA & Mulla MS (1990) Safety of Bacillus thuringiensis ssp.
israelensis and Bacillus sphaericus to nontarget organisms in the
aquatic environment. In: Laird M, Lacey LA, & Davidson EW ed. Safety
of microbial pesticides. Boca Raton, Florida, CRC Press, pp 169-188.
Lacey LA, Escaffre H, Philippon B, Sékétéli A, & Guillet P (1982)
Large river treatment with Bacillus thuringiensis (H-14) for the
control of Simulium damnosum s.l. in the Onchocerciasis Control
Programme. Tropenmed Parasitol, 33: 97-101.
Laferrière M, Bastille A, & Nadeau A (1987) Etude immunologique
impliquant les composantes de l'insecticide biologique Bacillus
thuringiensis var. kurstaki. St. Henri Rivière-du-Loup, Québec,
Department of Public Health, Grand-Portage Regional Hospital Center,
pp 1-19 (Unpublished report).
Lattin A, Grimes J, Hoxter KA, & Smith GJ (1990a) Dipel technical
material (Bacillus thuringiensis var. kurstaki): An avian oral
toxicity and pathogenicity study in the bobwhite (Project No.
161-112). Easton, Maryland, Wildlife International Ltd, pp 1-25
(Unpublished Abbott document).
Lattin A, Grimes J, Hoxter K, & Smith GJ (1990b) VectoBac technical
material (Bacillus thuringiensis var. israelensis): An avian oral
toxicity and pathogenicity study in the mallard (Project No. 161-115).
Easton, Maryland, Wildlife International Ltd, pp 1-24 (Unpublished
Abbott document).
Lattin A, Hoxter K, Driscoll C, Grimes J, & Jaber M (1990c) Dipel
technical material (Bacillus thuringiensis var. kurstaki): An
avian oral toxicity and pathogenicity study in the mallard (Project
No. 161-113). Easton, Maryland, Wildlife International Ltd, pp 1-28
(Unpublished Abbott document).
Lattin A, Hoxter K, & Smith GJ (1990d) VectoBac technical material
(Bacillus thuringiensis var. israelensis): An avian oral toxicity
and pathogenicity study in the bobwhite (Project No. 161-114). Easton,
Maryland, Wildlife International Ltd, pp 1-27 (Unpublished Abbott
document).
Lechner S, Mayr R, Francis KP, Prüß BM, Kaplan T, Wießner-Gunkel E,
Stewart GSAB, & Scherer S (1998) Bacillus weihenstephanensis sp.
nov. is a new psychrotolerant species of the Bacillus cereus group.
Int J System Bacteriol, 48: 1373-1378.
Leong KLH, Cano RJ, & Kubinski AM (1980) Factors affecting Bacillus
thuringiensis total field persistence. Environ Entomol, 9: 593-599.
Levêque C, Fairhurst CP, Abbau K, Pangy D, Curtis MS, & Traoré K
(1988) Onchocerciasis control programme in West Africa: ten years of
monitoring fish populations. Chemosphere, 17: 421-440.
Li J, Carroll J, & Ellar DJ (1991) Crystal structure of insecticidal
(delta)-endotoxin from Bacillus thuringiensis at 2.5 Å resolution.
Nature (Lond), 353: 815-821.
Logan NA & Berkeley CW (1984) Identification of Bacillus strains
using the API system. J Gen Microbiol, 130: 1871-1882.
Lund T & Granum PE (1997) Comparison of biological effect of the two
different enterotoxin complexes isolated from three different strains
of Bacillus cereus. Microbiology, 143: 3329-3336.
Lüthy P (1986) Insect pathogenic bacteria as pest control agents.
Fortschr Zool, 32: 201-216.
Lüthy P & Ebersold HR (1981) The entomocidal toxins of Bacillus
thuringiensis. Pharmacol Ther, 13: 257-283.
Lynch MJ & Baumann P (1985) Immunological comparisons of the crystal
protein from strains of Bacillus thuringiensis. J Invertebr Pathol,
46: 47-57.
Lynch RE, Lewis LC, & Brindley TA (1976) Bacteria associated with eggs
and first-instar larvae of the European corn borer: Isolation
techniques and pathogenicity. J Invertebr Pathol, 27: 325-331.
McClintock JT, Schaffer CR, & Sjoblad RD (1995) A comparative review
of the mammalian toxicity of Bacillus thuringiensis-based
pesticides. Pestic Sci, 45: 95-105.
Manasherob R, Ben-Dov E, Zaritsky A, & Barak Z (1998) Germination,
growth, and sporulation of Bacillus thuringiensis subsp.
israelensis in excreted food vacuoles of the protozoan Tetrahymena
pyriformis. Appl Environ Microbiol, 64: 1750-1758.
Margalit J & Dean D (1985) The story of Bacillus thuringiensis var.
israelensis. J Am Mosq Control Assoc, 1: 1-7.
Martin PAW & Travers RS (1989) Worldwide abundance and distribution of
Bacillus thuringiensis isolates. Appl Environ Microbiol, 55(10):
2437-2442.
Martin WF & Reichelderfer CF (1989) Bacillus thuringiensis:
Persistence and movement in field crops. In: Abstracts of the SIP
XXIInd Annual Meeting, Centre for Adult Education, University of
Maryland, College Park, Maryland, 20-24 August 1989. Society for
Invertebrate Pathology, p 25.
Martouret D & Euverte G (1964) The effect of Bacillus thuringiensis
Berliner preparations on the honey bee under conditions of forced
feeding. J Insect Pathol, 6: 198-203.
Meadows MP (1993) Bacillus thuringiensis in the environment: Ecology
and risk assessment. In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S
ed. Bacillus thuringiensis, an environmental biopesticide: Theory
and practice. Chichester, New York, Toronto, Wiley & Sons, pp 193-220.
Meadows MP, Ellis DJ, Butt J, Jarrett P, & Burges HD (1992)
Distribution, frequency and diversity of Bacillus thuringiensis in
an animal feed mill. Appl Environ Microbiol, 58(4): 1344-1350.
Melin BE & Cozzi EM (1990) Safety to nontarget invertebrates of
Lepidopteran strains of Bacillus thuringiensis and their
(ß)-exotoxins. In: Laird M, Lacey LA, & Davidson EW ed. Safety of
microbial insecticides. Boca Raton, Florida, CRC Press, pp 149-167.
Menon AS & De Mestral J (1985) Survival of Bacillus thuringiensis
var. kurstaki in waters. Water Air Soil Pollut, 25: 265-274.
Merritt RW, Walker ED, Wilzbach MA, Cummins KW, & Morgan WT (1989) A
broad evaluation of Bti for black fly (Diptera:Simuliidae) control in
a Michigan river: efficacy, carry and nontarget effects on
invertebrates and fish. J Am Mosq Control Assoc, 5: 397-415.
Mikami T, Horikawa T, Murakami T, Sato N, Ono Y, Matsumoto T, Yamakawa
A, Murayama S, Katagiri S, & Suzuki M (1995) Examination of toxin
production from environmental Bacillus cereus and Bacillus
thuringiensis. J Pharm Soc Jpn, 115: 742-748.
Miura T, Takahashi RM, & Mulligan FS III (1980) Effects of the
bacterial mosquito larvicide, Bacillus thuringiensis serotype H-14
on selected aquatic organisms. Mosq News, 40: 619-622.
Mohd-Salleh MB, Beegle CC, & Lewis LC (1980) Fermentation media and
production of exotoxin by three varieties of Bacillus thuringiensis.
J Invertebr Pathol, 35: 75-83.
Molloy DP (1992) Impact of the black fly (Diptera: Simuliidae) control
agent Bacillus thuringiensis var. israelensis on Chironomids
(Diptera: Chronomidae) and other non-target insects: results of ten
field trials. J Am Mosq Control Assoc, 8: 24-31.
Morris ON, Armstrong JA, & Hildebrand MJ (1977) Aerial field trials
with a new formulation of Bacillus thuringiensis against the spruce
budworm, Choristoneura fumiferana (Clem.). Ottawa, Canada, Chemical
Control Research Institute (Report CC-X-144).
Morris ON, Hildebrand MJ, & Armstrong JA (1980) Preliminary field
studies on the use of additives to improve deposition rate and
efficacy of commercial formulations of Bacillus thuringiensis
applied against the spruce budworm, Choristoneura fumiferana
(Lepidoptera: Tortricidae). Sault Ste. Marie, Ontario, Canada, Forest
Pest Management Institute, pp 1-23 (Report FPM-X-32).
Moulinier CL, Mas JP, Moulinier Y, De Barjac H, Giap G, & Couprie B
(1995) Étude de l'innocuité de Bacillus thuringiensis var.
israelensis pour les larves d'huitre. Bull Soc Pathol Exot, 74(4):
381-391.
Mück O, Hassan S, Huger AM, & Krieg A (1981) Effects of Bacillus
thuringiensis Berliner on the parasitic hymenoptera Apanteles
glomeratus L. (Braconidae) and Pimpla turionellae (L.)
(Ichneumonidiae). Z Angew Entomol, 92: 303-314.
Mulla MS (1988) Activity, field efficacy and use of Bacillus
thuringiensis (H-14) against mosquitoes. In: de Barjac H &
Sutherland DJ ed. Bacterial control of mosquitoes and blackflies. New
Brunswick, New Jersey, Rutgers University Press.
Mulla MS & Khasawinah AM (1969) Laboratory and field evaluation of
larvicides against chironomid midges. J Econ Entomol, 62: 37-41.
Mulla MS, Norland RL, Fanara DM, Darwazeh HA, & McKean DW (1971)
Control of chironomid midges in recreational lakes. J Econ Entomol
64(1): 300-307.
Mulla MS, Federici BA, & Darwazeh HA (1982) Larvicidal efficacy of
Bacillus thuringiensis serotype H-14 against stagnant-water
mosquitoes and its effects on nontarget organisms. Environ Entomol,
11: 788-795.
Mulligan FS III & Schaefer CH (1982) Integration of a selective
mosquito control agent Bacillus thuringiensis serotype H-14, with
natural predator populations in pesticide-sensitive habitats. Proc
Calif Mosq Vector Control Assoc, 49: 19-22.
Murakami T, Hiraoka T, Matsumoto T, Katagiri S, Shinagawa K, & Suzuki
M (1993) Analysis of common antigen of flagella in Bacillus cereus
and Bacillus thuringiensis. FEMS Microbiol Lett, 107: 179-184.
Narva KE, Payne JM, Schwab GE, Hickle LA, Galasan T, & Sick AJ (1991)
Novel Bacillus thuringiensis microbes active against nematodes, and
genes encoding novel nematode-active toxins cloned from Bacillus
thuringiensis isolates: European patent application EP0462 721A2.
Munich, Germany, European Patent Office.
Navon A (1993) Control of Lepidopteran pests with Bacillus
thuringiensis. In: Bacillus thuringiensis, An Environmental
Biopesticide: Theory and Practice. Eds, Entwistle PF, Cory JS, Bailey
MJ & Higgs S. Chichester, New York, Toronto, Wiley & Sons, pp 126-146.
Nielsen-LeRoux C, Hansen BM, Henriksen NB (1998) Safety of Bacillus
thuringiensis. IOBC Bull, 21: 269-272.
Nishikawa Y, Kramer JM, Hanaoka M, & Yasukawa A (1996) Evaluation of
serotyping, biotyping, plasmid banding pattern analysis, and HEp-2
vacuolation factor assay in the epidemiological investigation of
Bacillus cereus emetic-syndrome food poisoning. Int J Food
Microbiol, 31: 149-159.
Noble MA, Riben PD, & Cook GJ (1992) Microbiological and
epidemiological surveillance programme to monitor the health effects
of Foray 48B BTK spray. Vancouver, Canada, Ministry of Forests of the
Province of British Columbia, pp 1-63.
Norris JR (1971) The protein crystal toxin of Bacillus
thuringiensis: biosynthesis and physical structure. In: Burges HD &
Mussey NW ed. Microbial control of insects and mites. New York,
London, Academic Press Inc, pp 229-246.
Notermans S & Batt CA (1998) A risk assessment approach for food-borne
Bacillus cereus and its toxins. J Appl Environ Microbiol Symp,
84(suppl): 51S-61S.
Obadofin AA & Finlayson DG (1977) Interactions of several insecticides
and a carabid predator (Bembidion lampros (Hrbst)) and their effects
on Hylemya brassicae (Bouché). Can J Plant Sci, 57: 1121-1126.
Ohana B, Margalit J, & Barak Z (1987) Fate of Bacillus thuringiensis
subsp. israelensis under simulated field conditions. Appl Environ
Microbiol, 53: 828-831.
Ohba M & Aizawa K (1978) Serological identification of Bacillus
thuringiensis and related bacteria isolated in Japan. J Invertebr
Pathol, 32: 303-309.
Ohba M & Aizawa K (1986) Distribution of Bacillus thuringiensis in
soils of Japan. J Invertebr Pathol, 47: 277-282.
Ohba M, Yu YM, & Aizawa K (1988) Occurrence of noninsecticidal
Bacillus thuringiensis flagellar serotype 14 in the soil of Japan.
Appl Microbiol, 11: 85-89.
Olejnicek J & Maryskova B (1986), The influence of Bacillus
thuringiensis on the mosquito predator Notonecta glauca. Folia
Parasitol (Prague), 37: 279.
Otvos IS & Vanderveen S (1993) Environmental report and current status
of Bacillus thuringiensis var. kurstaki use for control of forest
and agricultural insect pests. Victoria, British Columbia, Ministry of
Forests, Forestry Canada, pp 1-81.
Pedersen JC, Damgaard PH, Eilenberg E, & Hansen BM (1995) Dispersal of
Bacillus thuringiensis var. kurstaki in an experimental cabbage
field. Can J Microbiol, 41: 118-125.
Petras SF & Casida LE Jr (1985) Survival of Bacillus thuringiensis
spores in soil. Appl Environ Microbiol, 50: 1496-1501.
Pinnock DE, Brand RJ, Jackson KL, & Milstead JE (1974) The field
persistence of Bacillus thuringiensis spores on Cercis occidentalis
leaves. J Invertebr Pathol, 23: 341-346.
Pinnock DE, Milstead JE, Kirby ME, & Nelson BJ (1977) Stability of
entomopathogenic bacteria. In: Environmental stability of microbial
insecticides: Miscellaneous publications. Lanham, Maryland,
Entomological Society of America, vol 10, pp 77-97.
Pivovarov Yu P, Ivashina SA, & Padalkin VP (1977) [Hygienic assessment
of investigation of insecticide preparations.] Gig i Sanit, 8: 45-48
(in Russian).
Prasertphon S, Areekul P, & Tanada Y (1973) Sporulation of Bacillus
thuringiensis in host cadavers. J Invertebr Pathol, 21: 205-207.
Prefontaine G, Fast P, Lau PCK, Hefford MA, Hanna Z, & Brousseau R
(1987) Use of oligonucleotide probes to study the relatedness of
delta-endotoxin genes among Bacillus thuringiensis subspecies and
strains. Appl Environ Microbiol, 53: 2808-2814.
Priest FG, Goodfellow M, & Todd C (1988) A numerical classification of
the genus Bacillus. J Gen Microbiol, 134: 1847-1882.
Priest FG, Kaji DA, Rosato YB, & Canhos VP (1994) Characterization of
Bacillus thuringiensis and related bacteria by ribosomal RNA gene
restriction fragment length polymorphisms. Microbiology, 140:
1015-1022.
Purcell BH (1981) Effects of Bacillus thuringiensis var.
israelensis on Aedes taeniorhynchus and some non-target organisms
in the salt marsh. Mosq News, 41(3): 476-484.
Pusztai M, Fast P, Gringorten L, Kaplan H, Lessard T, & Carey PR
(1991) The mechanism of sunlight-mediated inactivation of Bacillus
thuringiensis crystals. Biochem J, 273: 43-47.
Quinlan RJ (1990) Registration requirements and safety considerations
for microbial pest control agents in the European Economic Community.
In: Laird M, Lacey LA, & Davidson EW ed. Safety of microbial
pesticides. Boca Raton, Florida, CRC Press, pp 11-18.
Ray DE (1990) Pesticides derived from plants and other organisms. In:
Hayes WJ & Laws ER ed. Handbook of pesticide toxicology. New York,
London, Academic Press, pp 585-636.
Reardon RC & Haissig K (1983) Spruce budworm (Lepidoptera:
tortricidae) larval populations and field persistence of Bacillus
thuringiensis after treatment in Wisconsin. J Econ Entomol, 76:
1139-1143.
Reddy A, Battisti L, & Thorne CB (1987) Identification of
self-transmissible plasmids in four Bacillus thuringiensis
subspecies. J Bacteriol 169(11): 5263-5270.
Rogatin AB & Baizhanov M (1984) [Laboratory study of Bacillus
thuringiensis israelensis serotype H-14 on various groups of
hydrobionts.] Izv Akad Nauk Kaz SSR Ser Biol, D6: 22-25 (in Russian).
Salama HS & Zaki FN (1983) Interaction between Bacillus thuringiensis
Berliner and the parasites and predators of Spodoptera littoralis
in Egypt. Z Angew Entomol, 95: 425-429.
Salama HS, Zaki FN, & Sharaby AF (1982) Effect of Bacillus
thuringiensis Berl. on parasites and predators of the cotton
leafworm Spodoptera littoralis (Boisd). Z Angew Entomol, 94:
498-504.
Saleh SM, Harris RF, & Allen ON (1970) Fate of Bacillus thuringiensis
in soil: Effect of soil pH and organic amendment. Can J Microbiol,
16: 677-680.
Samples JR & Buettner H (1983) Corneal ulcer caused by a biologic
insecticide (Bacillus thuringiensis). Am J Ophthalmol, 95(2):
258-260.
Sampson MN & Gooday GW (1998) Involvement of chitinases of Bacillus
thuringiensis during pathogenesis in insects. Microbiology, 144:
2189-2194.
Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J,
Zeigler DR, & Dean DH (1998) Bacillus thuringiensis and its
pesticidal crystal proteins. Microbiol Mol Biol Rev, 62: 775-806.
Schnetter W, Engler S, Morawcsik J, & Becker N (1981) [Efficacy of
Bacillus thurigiensis var. israelensis against mosquito larvae
and non-target organisms.] Mitt Dtsch Ges Allg Angew Entomol, 2:
195-202 (in German).
Schwartz JL, Garneau L, Masson L, & Brousseau R (1991) Early response
of cultured lepidopteran cells to exposure to delta-endotoxin from
Bacillus thuringiensis: involvement of calcium and anionic channels.
Biochem Biophys Acta, 1065: 250-260.
Shadduck JA (1980) Bacillus thuringiensis serotype H-14 maximum
challenge and eye irritation safety tests in mammals. Geneva, World
Health Organization, pp 1-21 (WHO/VBC/80.763).
Shadduck JA (1983) Some considerations on the safety evaluation of
nonviral microbial insecticides. WHO Bull, 6(1): 117-128.
Sheeran W & Fisher SW (1992) The effects of agitation, sediment and
competition on the persistence and efficacy of Bacillus thuringiensis
var. israelensis (Bti). Ecotoxicol Environ Saf, 24: 338-346.
Shevelev AB, Lewitin E, Novikova SI, Wojciechowska YaA, Usacheva EA,
Chestukhina GG, & Stepanov (1998) A new PCR-based approach to a fast
search of a wide spectrum of cry genes from Bacillus thuringiensis
strains. Biochem Mol Biol Int, 45: 1265-1271.
Shinagawa K (1990) Purification and characterization of Bacillus
cereus enterotoxin and its application to diagnosis. In: Pohland AE,
Dowell VR Jr, & Richard JL ed. Microbial toxins in foods and feeds:
Cellular and molecular modes of action. New York, Plenum Press, pp
181-193.
Siegel JP & Shadduck JA (1990) Clearance of Bacillus sphaericus and
Bacillus thuringiensis ssp. israelensis from mammals. J Econ
Entomol, 83: 347-355.
Siegel JP, Shadduck JA, & Szabo J (1987) Safety of the entomopathogen
Bacillus thuringiensis var. israelensis for mammals. J Econ
Entomol, 80: 717-723.
Slatin SL, Abrams CK, & English L (1990) Delta-endotoxins form
cation-selective channels in planar lipid bilayers. Biochem Biophys
Res Commun, 169: 765-772.
Slepecky RA & Hemphill HE (1992) The genus Bacillus-nonmedical. In:
Balows A, Truper HG, Dworkin M, Harder W, & Schleifer K-H ed. The
prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol II, pp
1663-1696.
Smedley DP & Ellar DJ (1996) Mutagenesis of 3 surface-exposed loops of
a Bacillus thuringiensis insecticidal toxin reveals residues
important for toxicity, recognition and possibly membrane insertion.
Microbiology, 142: 1617-1624.
Smith RA (1987) Use of crystal serology to differentiate among
varieties of Bacillus thuringiensis. J Invertebr Pathol, 50: 1-8.
Smith RA & Couche GA (1991) The phylloplane as a source of Bacillus
thuringiensis variants. Appl Environ Microbiol, 57: 311-315.
Snarski VM (1990) Interactions between Bacillus thuringiensis subsp.
israelensis and fathead minnows, Pimephales promelas Rafinesque,
under laboratory conditions. Appl Environ Microbiol, 56: 2618-2622.
Sorenson AA & Falcon LA (1980) Comparison of microdroplet and high
volume application of Bacillus thuringiensis on pear suppression of
fruit tree leaf roller Acrhips argyrospilus and coverage on foliage
and fruit. Environ Entomol, 9: 350.
Stahly DP, Dingman DW, Bulla LA Jr, & Aronson AI (1978) Possible
origin and function of the parasporal crystals in Bacillus
thuringiensis. Biochem Biophys Res Commun, 84(3): 581-588.
Stahly DP, Andrews RE, & Yousten AA (1991) The genus Bacillus-insect
pathogens. In: Balows A, Truper HG, Dworkin M, Harder W, & Schleifer
K-H ed. The prokaryotes, 2nd ed. New York, Basel, Springer-Verlag, vol
II, pp 1697-1745.
Surprenant DC (1989) Acute toxicity of Bacillus thuringiensis var.
tenebrionis technical material to rainbow trout (Salmo gairdneri)
under static renewal conditions. Wareham, Massachusetts, Springborn
Life Sciences Inc., pp 1-19 (Unpublished Abbott document).
Tayabali AF & Seligy VL (1997) Cell integrity markers for in vitro
evaluation of cytotoxic responses to bacteria-containing commercial
insecticides. Ecotoxicol Environ Saf, 37: 152-162.
Thomas WE & Ellar DJ (1983) Bacillus thuringiensis var. israelensis
crystal delta-endotoxin: effects on insect and mammalian cells in
vitro and in vivo. J Cell Sci, 60: 181-197.
Thomas EM & Watson TF (1986) Effect of Dipel (Bacillus thuringiensis)
on the survival of immature and adult Hyposoter exiguae
(Hymenoptera: Ichneumonidae). J Invertebr Pathol, 47: 178-183.
Tomlin CDS ed. (1997) The pesticide manual, 11th ed. Farnham, Surrey,
British Crop Protection Council, 1606 pp.
Travers RS, Martin PAW, & Reichelderfer CF (1987) Selective process
for efficient isolation of soil Bacillus spp. Appl Environ
Microbiol, 53: 1263-1266.
Väisänen OM, Mwaisumo NJ, & Salkinoja-Salonen MS (1991)
Differentiation of dairy strains of the Bacillus cereus group by
phage typing, minimum growth temperature, and fatty acid analysis. J
Appl Bacteriol, 70: 315-324.
Van Frankenhuyzen K (1993) The challenge of Bacillus thuringiensis.
In: Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental biopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 1-35.
Vaòková J & Purrini K (1979) Natural epizooties caused by bacilli of
the species Bacillus thuringiensis and Bacillus cereus. Z Angew
Entomol, 88: 216-221.
Van Rie J, Jansens S, Höfte H, Degheele D, & Van Mellaert H (1989),
Specificity of Bacillus thuringiensis delta-endotoxins: Importance
of specific receptors on the brush border membrane of the mid-gut of
target insects. Eur J Biochem, 186: 239-247.
Venkateswerlu G & Stotzky G (1992) Binding of the protoxin and toxin
proteins of Bacillus thuringiensis subsp. kurstaki on clay
minerals. Curr Microbiol, 25: 225-233.
Visser B, Bosch D, & Honée G (1993) Domain-function studies of
Bacillus thuringiensis crystal proteins: a genetic approach. In:
Entwistle PF, Cory JS, Bailey MJ, & Higgs S ed. Bacillus
thuringiensis, an environmental piopesticide: Theory and practice.
Chichester, New York, Toronto, Wiley & Sons, pp 71-88.
Wallner W & Surgeoner G (1974) Control of oakleaf caterpillar,
Heterocampa manteo, and the impact of controls on nontarget
organisms. Chicago, Illinois, Abbott Laboratories (Unpublished
document).
Wallner WE, Dubois NR, & Grinberg PS (1983) Alteration of parasitism
by Rogas lymantriae (Hymenoptera: Braconidae) in Bacillus
thuringiensis-stressed gypsy moth (Lepidoptera: Lymantriidae) hosts.
J Econ Entomol, 76: 275-277.
Warren RE, Rubenstein D, Ellar DJ, Kramer JM, & Gilbert RJ (1984)
Bacillus thuringiensis var. israelensis: Protoxin activation and
safety. Lancet: March 24: 678-679.
Weires RW & Smith GL (1977) Apple mite control. Hudson Valley, New
York, New York State Agricultural Experimental Station.
Weseloh RM & Andreadis TG (1982) Possible mechanism for synergism
between Bacillus thuringiensis and the gypsy moth (Lepidoptera:
Lymantriidae) parasitoid, Apanteles melanoscelus (Hymenoptera:
Braconidae). Ann Entomol Soc Am, 75: 435-438.
Weseloh RM, Andreadis TG, Moore REB, & Anderson JF (1983) Field
confirmation of a mechanism causing synergism between Bacillus
thuringiensis and the gypsy moth parasitoid, Apanteles
melanoscelus. J Invertebr Pathol, 41: 99-103.
West AW, Burges HD, & Wyborn CH (1984a) Effect of incubation in
natural and autoclaved soil upon potency and viability of Bacillus
thuringiensis. J Invertebr Pathol, 44: 121-127.
West AW, Burges HD, White JR, & Wyborn CH (1984b) Persistence of
Bacillus thuringiensis parasporal crystal insecticidal activity in
soil. J Invertebr Pathol, 44: 128-133.
WHO (1982) Data sheet on the biological control agent Bacillus
thuringiensis serotype H-14 (de Barjac 1978). Geneva, World Health
Organization (WHO/VBC/79.750 Rev. 1).
WHO (1985) Ninth report of the WHO Expert Committee on Vector Biology
and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 720).
WHO (1991) Fourteenth report of the WHO Expert Committee on Vector
Biology and Control -- Safe Use of Pesticides. Geneva, World Health
Organization (WHO Technical Report Series No. 813).
Wilkinson JD, Biever KD, & Ignoffo CM (1975) Contact toxicity of some
chemical and biological pesticides to several insect parasitoids and
predators. Entomophaga, 20: 113-120.
Workman RB (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc Fla State Hortic Soc, 90: 401-402.
Wunschel D, Fox KF, Black GE, & Fox A (1994) Discrimination among the
B. cereus group, in comparison to B. subtilis, by structural
carbohydrate profiles and ribosomal RNA spacer region PCR. System Appl
Microbiol, 17: 625-635.
Yameogo L, Levêque C, Traoré K, & Fairhurst CP (1988) Dix ans de
surveillance de la faune aquatique des rivières d'Afrique de l'Ouest
traitées contre les Simulides (Diptera: Simuliidae), agents vecteurs
de l'onchocercose humaine. Nat Can, 115: 287-298.
Yousten AA (1973) Effect of the Bacillus thuringiensis
delta-endotoxin on an insect predator which has consumed intoxicated
cabbage looper larvae. J Invertebr Pathol, 21: 312-314.
Yousten AA, Genthner FJ, & Benfield EF (1992) Fate of Bacillus
sphaericus and Bacillus thuringiensis serovar israelensis in the
aquatic environment. J Am Mosq Control Assoc, 8: 143-148.
Zahner V, Momen H, Salles CA, & Rabinovitch L (1989) A comparative
study of enzyme variation in Bacillus cereus and Bacillus
thuringiensis. J Appl Bacteriol, 67: 275-282.
Zukowski K (1995) [Laboratory examination of the effectiveness of new
biological preparations for reducing populations of cockroaches
(Blattella germanica L.)]. Rocz Panstw Zakl Hig, 46: 293-297 (in
Polish).
RÉSUMÉ
La présente monographie traite des agents microbiens de lutte
contre les nuisibles utilisant le bacille Bacillus thuringiensis
(Bt). Ce bacille, qui est aussi appelé bacille de Thuringe, est
également une source très importante de gènes utilisée pour conférer
aux plantes et aux microorganismes transgéniques (ou organismes
génétiquement modifiés, OGM) la faculté de résister aux ravageurs et
autres nuisibles. Les effets que pourraient avoir ces OGM sur la santé
humaine et l'environnement présentent divers aspects qui sont sans
rapport ou tout au plus en rapport lointain avec les produits à base
de Bt et n'entrent pas, par conséquent, dans le cadre de la présente
monographie.
1. Identité, caractéristiques biologiques et méthodes d'analyse
Bacillus thuringiensis est une bactérie anaérobie facultative,
gram-positif, qui forme des inclusions protéiques caractéristiques
adjacentes à l'endospore. Certaines sous-espèces de Bt peuvent
synthétiser plusieurs inclusions parasporales. Le Bt est génétiquement
indiscernable du Bc, exception faite de son aptitude à former des
inclusions cristallines parasporales qui sont toxiques pour certains
invertébrés, notamment les larves d'insectes appartenant aux ordres
suivants: coléoptères, diptères et lépidoptères. Les inclusions
parasporales sont constituées de diverses protéines cristallisées
insecticides (ICP). Ces cristaux sont de forme variable (bipyramidale,
cuboïdale, rhomboïdale plane, sphérique ou composite, c'est-à-dire
comportant deux types de cristaux) selon la nature des protéines qui
les composent. On a établi une corrélation partielle entre la
morphologie des cristaux, la composition en protéines cristallisées et
l'activité biologique vis-à-vis des insectes.
Le taxon phénotypique fondamental est la sous-espèce,
caractérisée par son sérotype flagellaire (H). En 1998, on avait déjà
décrit 67 sous-espèces. Les gènes qui codent pour les ICP sont pour la
plupart situés sur les plasmides. Chacune de ces protéines n'est le
produit que d'un seul gène. La plupart des plasmides porteurs de gènes
ICP se transmettent facilement d'une souche bactérienne à l'autre par
conjugaison et peuvent aussi passer à une espèce bactérienne voisine.
La classification phénotypique est maintenant complétée par une
caractérisation basée sur la biologie moléculaire et plus précisément
sur la séquence des gènes codant pour les cristaux (cry et cyt)
plutôt que sur la spécificité vis-à-vis des insectes cibles. Divers
domaines des ICP sont responsables de la sensibilité de l'hôte
(reconnaissance des récepteurs) et de la toxicité (formation de
pores).
Parmi les techniques couramment utilisées pour caractériser les
souches de Bt ou les inclusions protéiques elles-mêmes, on peut citer
l'analyse des acides gras pariétaux, les anticorps monoclonaux, les
sondes d'ADN oligonucléotidiques, les profils plasmidiques, l'analyse
par amplification génique (PCR), la technique des empreintes
génétiques et les profils électrophorétiques SDS-PAGE (électrophorèse
en gel de polyacrylamide en présence de dodécylsulfate de sodium).
Certaines sous-espèces de Bt produisent pendant leur croissance
une bêta-exotoxine constitué d'un nucléotide thermostable qui peut
contaminer les produits. Cette bêta-exotoxine est toxique pour presque
toutes les formes de vie, y compris l'Homme et les insectes cibles. Au
cours de leur croissance, les diverses souches de Bt produisent toutes
sortes d'antibiotiques, d'enzymes, de métabolites et de toxines, y
compris des toxines Bc, qui peuvent avoir des effets nocifs sur les
organismes visés ou non visés. La numération des spores n'est pas le
reflet fidèle de l'activité insecticide d'une souche de Bt ou d'un
produit qui en dérive. Pour mesurer l'activité (en unités
toxicologiques internationales (ITU) par mg), on procède à un test
biologique sur insecte au moyen d'un étalon international.
2. Mode d'action sur les insectes cibles
Les Bt sporulés ou les complexes spores-ICP doivent être ingérés
par les larves d'insectes appartenant aux espèces sensibles.
L'efficacité des ICP dépend de plusieurs facteurs: solubilisation dans
l'intestin moyen, conversion de la protoxine en toxine biologiquement
active sous l'action des enzymes protéolytiques, fixation de la toxine
active aux récepteurs membranaires spécifiques par sa région
C-terminale et formation de pores par la région N-terminale entraînant
la lyse des cellules épithéliales. La germination des spores et la
prolifération de cellules bactériennes végétatives dans l'hémocèle
peut entraîner une septicémie qui contribue également à la mort. La
spécificité d'hôte des différentes ICP est essentiellement déterminée
par leur fixation aux récepteurs.
3. Habitats
On a isolé de nombreuses sous-espèces de Bt sur des insectes
morts ou mourants appartenant principalement à l'ordre des
coléoptères, des diptères et des lépidoptères, mais nombreuses sont
également celles qui ont été isolées du sol, de la surface des
feuilles ou d'autres habitats. Les cadavres d'insectes contiennent
souvent de grandes quantités de spores et d'ICP susceptibles de
pénétrer dans l'environnement. Les sous-espèces actives contre les
coléoptères et les lépidoptères sont principalement associées au sol
et aux surfaces foliaires, alors que les sous-espèces actives contre
les diptères se rencontrent communément dans les milieux aquatiques.
Dans l'environnement, les spores sont capables de persister et de se
développer en présence de conditions favorables et de nutriments
appropriés.
4. Produits du commerce. Production et épandage
Les produits commerciaux classiques à base de bacille de
Thuringe, qui utilisent des souches naturelles, représentent environ
90% du marché mondial des agents microbiologiques de lutte contre les
nuisibles. La plupart de ces produits contiennent des spores viables
et des ICP, mais dans certains d'entre eux, les spores sont inactivées
(Bti). Chaque année, on en produit quelque 13 000 tonnes par la
technique de fermentation aérobie. Les produits classiques à base de
Bt sont principalement destinés à lutter contre les lépidoptères qui
ravagent les cultures et les plantations forestières; toutefois, ces
dernières années, on a également commercialisé des souches actives
contre les coléoptères. Les programmes de santé publique utilisent
également des souches de Bti actives contre les diptères vecteurs de
maladies virales ou parasitaires.
Les formulations commerciales de bacille de Thuringe peuvent être
épandues sur le feuillage, le sol, les étendues d'eaux ou dans les
entrepôts de denrées alimentaires pour combattre les insectes. Une
fois le produit épandu dans l'écosystème, les cellules bactériennes
végétatives et les spores peuvent persister à des concentrations
progressivement décroissantes pendant des semaines, des mois ou des
années en tant que constituants de la microflore naturelle. Par
contre, les ICP perdent leur activité biologique au bout de quelques
heures ou de quelques jours.
5. Effets du Bt sur les organismes non visés
Les études effectuées sur des mammifères et notamment celles qui
ont porté sur des animaux de laboratoire ont consisté a évaluer
l'infectiosité et la toxicité éventuelles de diverses préparations à
base de Bt contenant notamment des ICP, des spores et des cellules
bactériennes en phase végétative. Sous ces trois formes, les
différentes sous-espèces de Bt se sont révélées pour la plupart non
pathogènes et non toxiques pour les diverses espèces animales
utilisées. On a montré que les cellules bactériennes en phase
végétative et les spores persistaient pendant plusieurs semaines sans
causer d'effets nocifs. En particulier, on a constaté que le Bt
n'avait pas d'effets indésirables sur les oiseaux, les poissons et de
nombreux autres vertébrés aquatiques non visés, lors d'études en
laboratoire ou sur le terrain portant sur un grand nombre de
spécimens. Il n'y a que relativement peu d'invertébrés aquatiques qui
se révèlent sensibles au Bt en laboratoire ou sur le terrain. Par
ailleurs, le bacille de Thuringe n'exerce pas non plus d'effets nocifs
sur les lombrics.
L'activité insecticide des différentes sous-espèces de Bt
présente en général une spécificité d'hôte très marquée vis-à-vis des
coléoptères, des diptères et des lépidoptères et on a montré qu'elle
n'avait pratiquement aucun effet toxique direct sur les arthropodes
non visés. La plupart des données relatives à l'innocuité de ces
produits pour les arthropodes non visés concernent les sous-espèces de
Bt actives contre les diptères et les lépidoptères.
Les études consacrées aux formulations de Bti exemptes de
contaminants toxiques ont montré qu'elles étaient sans danger pour la
plupart des arthropodes non visés. Certains moucherons (chironomides
appartenant à l'ordre des diptères) très proches des moustiques se
sont révélés sensibles à de fortes doses de Bti mais ne sont nullement
affectés aux doses utilisées pour la destruction des larves de
moustiques. Des études sur le terrain ont mis en évidence des cas de
réduction ou au contraire d'augmentation de certaines populations
d'arthropodes non visés.
Les études toxicologiques auxquelles ont été soumis de nombreux
ordres d'insectes n'ont, pour la plupart d'entre eux, révélé aucun
effet toxique imputable au Btk.
On a observé une certaine mortalité chez des abeilles
(Apis mellifera) qui avaient été soumises à des bacilles des
sous-espèces Btt et Btk en phase végétative, mais il ne semble pas que
les spores ou les ICP soient capables de produire un tel effet. En
laboratoire et sur le terrain, le Btg n'a aucun effet toxique sur les
abeilles.
Les souches de Bte productrices de bêta-exotoxine se sont
révélées capables d'exercer des effets toxiques sur les arthropodes
non visés.
6. Exposition humaine et effets du bacille de Thuringe sur l'Homme
Les ouvriers qui épandent des produits à base de Bt peuvent être
fortement exposés à ces produits par contact cutané ou par inhalation
d'aérosols. L'usage du Bt en agriculture peut entraîner la
contamination de l'eau potable et des denrées alimentaires par le
bacille. Toutefois, à l'exception de quelques cas d'irritation des
yeux ou de la peau, on n'a pas connaissance d'effets nocifs attestés
qui résulteraient d'une exposition professionnelle à des produits à
base de Bt. Des volontaires qui avaient ingéré ou inhalé de grandes
quantités de diverses formulations de Btk, n'ont ressenti aucun effet
indésirable. On a mis en évidence des anticorps dirigés contre les
cellules bactériennes, les spores et les complexes spores-cristaux
chez des ouvriers chargés de l'épandage de produits à base de Bt;
aucun effet indésirable n'a cependant été observé. On connaît le cas
d'un certain nombre de patients atteints de maladies infectieuses chez
lesquels la présence de Bt a été mise en évidence. Toutefois, aucune
des études qui leur ont été consacrées n'a permis de conclure de façon
certaine que l'utilisation du Bt comporte effectivement un risque pour
la santé humaine. Il ne semble pas non plus que la présence de Bt dans
l'eau destinée à la consommation ou dans les denrées alimentaires soit
à l'origine d'effets indésirables chez l'Homme.
7. Conclusions
Compte tenu de la spécificité de leur mode d'action, il est
improbable que les produits à base de Bt constituent un danger
quelconque pour l'Homme et les vertébrés ni pour la très grande
majorité des invertébrés non visés, pour autant qu'ils ne contiennent
pas d'autres microorganismes ou de substances biologiquement actives
autres que les ICP. On peut utiliser ces produits en toute sécurité
pour détruire les insectes qui ravagent les domaines agricoles et
horticoles ainsi que les forêts. Ils sont également sans danger pour
le milieu aquatique et on peut notamment les épandre dans les
réservoirs d'eau potable pour lutter contre les moustiques, les
simulies et les larves d'insectes incommodants. Il convient cependant
de noter qu'en phase végétative, le Bt est capable de produire des
toxines de type Bc dont on ignore si elles sont susceptibles de
provoquer des maladies chez l'Homme.
1. RESUMEN
Esta monografía trata sobre los plaguicidas microbianos (PM)
basados en Bacillus thuringiensis (Bt). Esta bacteria es también una
fuente clave de genes cuya expresión transgénica confiere resistencia
frente a plagas a plantas y microorganismos, actuando como plaguicida
en los denominados organismos modificados genéticamente (OMG). Los
posibles efectos de los OMG sobre la salud humana y el medio están
poco o nada relacionados con los productos basados en Bt, por lo que
quedan fuera del ámbito de esta monografía.
1. Identidad, características biológicas y métodos de laboratorio
Bt es una bacteria gram-positiva y anaerobia facultativa que
forma inclusiones proteicas características junto a la endospora. Las
subespecies de Bt pueden sintetizar más de una inclusión parasporal.
Desde el punto de vista genético, Bt es indistinguible de Bc,
exceptuando la capacidad de Bt para producir inclusiones parasporales
cristalinas que son tóxicas para ciertos invertebrados, en particular
para las larvas de insectos pertenecientes a los órdenes Coleóptera,
Díptera y Lepidóptera. Dichas inclusiones parasporales están
formadas por distintas proteínas cristalinas insecticidas (PCI). Los
cristales tienen formas diversas (bipiramidales, cuboides, romboides
planos, esféricos o compuestos por dos tipos de cristales),
dependiendo de su composición en PCI. Se ha comprobado que existe una
correlación parcial entre la morfología del cristal, la composición en
PCI y la bioactividad frente a los insectos diana.
El taxón fenotípico básico es la subespecie, identificada por el
serotipo flagelar (H). Hasta 1998 se habían descrito 67 subespecies.
Los genes que codifican las PCI se encuentran fundamentalmente en los
plásmidos. Cada PCI es el producto de un solo gen. La mayoría de los
plásmidos con genes de PCI se transfieren fácilmente por conjugación
entre cepas de Bt y pueden transferirse a especies bacterianas
emparentadas. La clasificación fenotípica se ha complementado en la
actualidad con la caracterización biomolecular, basada en la secuencia
de los genes de las proteínas cristalinas (cry y cyt), no en la
especificidad para las especies diana. En las PCI, la susceptibilidad
del huésped (reconocimiento de receptores) y la toxicidad (formación
de poros) son responsabilidad de dominios distintos de la molécula.
Las técnicas utilizadas habitualmente para caracterizar las cepas
de Bt o la propia PCI consisten en análisis de los ácidos grasos de la
pared celular, anticuerpos monoclonales, sondas de oligonucleótidos de
ADN, perfiles de plásmidos, análisis por reacción en cadena de la
polimerasa (PCR), estudios del ADN (huella genética) y perfiles de
SDS-PAGE (dodecil sulfato sódico -- electroforesis en gel de
poliacrilamida).
La beta-exotoxina, un nucleótido termoestable, es sintetizada por
algunas subespecies de Bt durante el crecimiento vegetativo y puede
contaminar los productos. Es tóxica para casi todas las formas de
vida, incluidos los seres humanos y los órdenes de insectos diana.
Durante el crecimiento vegetativo, varias cepas de Bt producen una
gama de antibióticos, enzimas, metabolitos y toxinas, incluidas
toxinas de Bc, que pueden tener efectos nocivos tanto en las especies
que son objetivo del plaguicida como en las que no lo son. Los
recuentos de esporas no reflejan con exactitud la actividad
insecticida de una cepa o un preparado de Bt. Se mide la potencia
(UTI/mg) de cada producto mediante ensayos biológicos para los que se
utiliza un patrón internacional basado en un insecto concreto.
2. Modo de acción en los insectos diana
Es preciso que las larvas de los insectos susceptibles ingieran
Bt esporulado con PCI o con complejos espora-PCI. La eficacia de la
PCI depende de su solubilización en el intestino medio, de la
conversión de la protoxina en la toxina biológicamente activa por la
acción de enzimas proteolíticas, de la unión específica del dominio
C-terminal de la toxina activa al receptor de membrana y de la
formación de poros por parte del dominio N-terminal, con la
consiguiente lisis de las células epiteliales. La germinación de
esporas y la proliferación de células vegetativas en el hemocele puede
ocasionar una septicemia y contribuir a la muerte. La unión de la PCI
al receptor es el determinante principal de la especificidad de
huésped para las distintas PCI de Bt.
3. Hábitats
Se han aislado muchas subespecies de Bt a partir de insectos
muertos o moribundos, la mayoría pertenecientes a los órdenes
Coleóptera, Díptera y Lepidóptera, pero también del suelo, de
superficies foliares y de otros hábitats. Los exoesqueletos de
insectos muertos contienen a menudo grandes cantidades de esporas y
PCI que pueden incorporarse al medio. Las subespecies de Bt activas
frente a coleópteros y lepidópteros se asocian fundamentalmente con el
suelo y el filoplano (superficies foliares), mientras que las activas
frente a dípteros se hallan generalmente en medios acuáticos. En el
ambiente, las esporas persisten y pueden entrar en crecimiento
vegetativo cuando las condiciones son favorables y hay nutrientes
disponibles.
4. Productos comerciales, producción y aplicación
Los preparados convencionales de Bt, que utilizan cepas de Bt que
aparecen de forma espontánea en la naturaleza, representan
aproximadamente el 90% del mercado mundial de los PM. La mayoría de
los preparados de Bt contienen PCI y esporas viables, pero en algunos
productos de Bti las esporas están inactivadas. Cada año se producen
aproximadamente 13.000 toneladas utilizando tecnología de fermentación
aerobia. Los preparados convencionales de Bt tienen como objetivos
primarios las plagas de lepidópteros que afectan a los cultivos
agrícolas y forestales; sin embargo, en los últimos años también se
han comercializado cepas de Bt activas frente a plagas de coleópteros.
Se están utilizando en programas de salud pública cepas de Bti activas
frente a dípteros vectores de enfermedades parasitarias y víricas.
Las formulaciones comerciales de Bt pueden aplicarse como
insecticidas al follaje, el suelo, el medio acuático o instalaciones
de almacenamiento de alimentos. Tras aplicar una subespecie de Bt a un
ecosistema, las células vegetativas y las esporas pueden persistir en
concentraciones gradualmente decrecientes durante semanas, meses o
años como un componente de la microflora natural. Sin embargo, las PCI
pierden su actividad biológica en horas o días.
5. Efectos de Bt sobre especies que no son objetivo del plaguicida
En estudios con mamíferos, en particular con animales de
laboratorio, se ha evaluado la posible infecciosidad y toxicidad de
diversos preparados de Bt, que comprenden las PCI, células vegetativas
y esporas. Las PCI, las esporas y las células vegetativas de las
subespecies de Bt, que se administraron por distintas vías, carecían
en su mayoría de patogenicidad y toxicidad para las diversas especies
animales estudiadas. Se comprobó que las células vegetativas o las
esporas de Bt persistían durante semanas sin causar efectos adversos.
No se ha observado que Bt afecte a pájaros, peces o muchas otras
especies de vertebrados acuáticos que no son objetivo del plaguicida y
se han estudiado en gran número de trabajos de laboratorio y de campo.
Son relativamente pocas las especies de invertebrados acuáticos
susceptibles a Bt, tanto en condiciones de laboratorio como de campo.
Bt no afecta a las lombrices de tierra.
En general, las subespecies de Bt muestran gran especificidad en
su actividad insecticida frente a Coleóptera, Díptera y
Lepidóptera, así como una toxicidad directa escasa, si no nula,
frente a los artrópodos que no son su objetivo. La mayor parte de los
datos disponibles sobre inocuidad en éstos se han obtenido con las
subespecies de Bt activas frente a Díptera y Lepidóptera.
Los estudios sobre formulaciones de Bti sin contaminantes tóxicos
no han puesto de manifiesto efectos nocivos en la gran mayoría de los
artrópodos que no son objetivo del plaguicida. Se ha comprobado que
algunas moscas enanas (Díptera: Chironomidae), estrechamente
emparentadas con los mosquitos, son sensibles a dosis altas de Bti,
pero no se ven afectadas por dosis letales para larvas de mosquito. En
estudios de campo se han descrito disminuciones o aumentos
transitorios de las poblaciones de algunos artrópodos que no son
objetivo del plaguicida.
Se han estudiado muchos órdenes de insectos, tanto en el
laboratorio como en trabajos de campo, y se ha comprobado que en la
mayoría de ellos Btk no tiene efecto.
Se ha observado mortalidad en abejas melíferas (Apis mellifera)
tras la exposición a Btt y Btk en crecimiento vegetativo, pero el
efecto no parece guardar relación con las esporas o las PCI. En los
estudios de laboratorio y de campo, Btg no mostró efectos adversos
sobre las abejas melíferas.
Se ha comprobado que cepas de Bte productoras de beta-exotoxina
tienen efectos adversos sobre artrópodos que no son objetivo del
plaguicida.
6. Exposición a Bt y efectos sobre los seres humanos
La aplicación agrícola de preparados de Bt puede suponer una
considerable exposición de los trabajadores, tanto en aerosol como
dérmica. Puede, asimismo, causar la contaminación del agua potable y
los alimentos por la bacteria. Salvo casos notificados de irritación
ocular y dérmica, no se han documentado efectos adversos sobre la
salud tras la exposición laboral a preparados de Bt. Individuos
voluntarios ingirieron e inhalaron grandes cantidades de una
formulación de Btk sin sufrir efectos adversos. Se detectaron títulos
de anticuerpos frente a las células vegetativas, las esporas y los
complejos espora-cristal en trabajadores que pulverizaban preparados
de Bt, pero no se registraron efectos adversos. Se han descrito
algunos casos de presencia de Bt en pacientes con diversas
enfermedades infecciosas. Sin embargo, ninguno de estos estudios
demuestra de forma inequívoca que el uso de Bt entrañe un riesgo real
para la salud humana. No se ha demostrado que Bt tenga efectos
adversos en seres humanos cuando está presente en el agua potable o
los alimentos.
7. Conclusiones
Debido a la especificidad de su modo de acción, es improbable que
los preparados de Bt entrañen peligro alguno para los seres humanos u
otros vertebrados, o para la gran mayoría de los invertebrados que no
constituyen su objetivo, siempre y cuando no contengan microorganismos
distintos de Bt y productos biológicamente activos distintos de las
PCI. Los preparados de Bt pueden utilizarse con seguridad para
controlar las plagas de insectos de los cultivos agrícolas y
hortícolas, así como las forestales. También es seguro su uso en
medios acuáticos, incluidos los depósitos de agua potable, para
controlar el mosquito, la mosca negra y las larvas de insectos
dañinos. Sin embargo, es preciso señalar que las formas vegetativas de
Bt pueden sintetizar toxinas del tipo de las producidas por Bc, cuya
importancia como causa de enfermedades humanas se desconoce.
