
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Environmental Health Criteria 219
FUMONISIN B1
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Professor W.F.O. Marasas (Medical Research
Council, Tygerberg, South Africa), Professor J.D. Miller (Carlton
University, Ottawa, Canada), Dr R.T. Riley (US Department of
Agriculture, Athens, USA) and Dr A. Visconti (National Research
Council, Bari, Italy)
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organization
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer-review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Fumonisin B1.
(Environmental health criteria; 219)
1.Carboxylic acids - toxicity 2.Food contamination
3.Environmental exposure 4.Risk assessment I.Series
ISBN 92 4 157219 1 (NLM Classification: QD 341.P5)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
PREAMBLE
ABBREVIATIONS
INTRODUCTION
1. SUMMARY, EVALUATION AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, physical and chemical properties, and
analytical methods
1.1.2. Sources of human exposure
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in animals
1.1.6. Effects on animals and in vitro test systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory
1.2. Evaluation of human health risks
1.2.1. Exposure
1.2.2. Hazard identification
1.2.3. Dose-response assessment
1.2.4. Risk characterization
1.3. Recommendations for protection of human health
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties of the pure substance
2.3. Analytical methods
2.3.1. Sampling and preparation procedures
2.3.2. Extraction
2.3.3. Analysis
3. SOURCES OF HUMAN EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. KINETICS AND METABOLISM IN ANIMALS
6.1. Absorption
6.2. Distribution
6.3. Elimination, excretion and metabolic transformation
6.4. Retention and turnover
6.5. Reaction with body components
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Laboratory animals and in vitro test systems
7.1.1. Single exposure
7.1.2. Repeated exposure
7.1.2.1 Body weight loss
7.1.2.2 Hepatocarcinogenicity and nephrotoxicity
7.1.2.3 Immunotoxicity
7.1.3. Skin and eye irritation
7.1.4. Reproductive toxicity, embryotoxicity and
teratogenicity
7.1.5. Mutagenicity and related end-points
7.1.6. Carcinogenicity
7.1.6.1 Carcinogenicity bioassays
7.1.6.2 Short-term assays for carcinogenicity
7.2. Other mammals
7.2.1. Equine leukoencephalomalacia
7.2.2. Porcine pulmonary oedema syndrome
7.2.3. Poultry toxicity
7.2.4. Non-human primate toxicity
7.2.5. Other species
7.3. Mechanisms of toxicity - mode of action
7.3.1. Disruption of sphingolipid metabolism
7.3.1.1 Sphingolipids and their metabolism
7.3.1.2 Fumonisin-induced disruption of sphingolipid
metabolism in vitro
7.3.1.3 Fumonisin disruption of sphingolipid
metabolism in vivo
7.3.1.4 Tissue and species specificity
7.3.1.5 Fumonisin-induced sphingolipid alterations:
effects on growth, differentiation and cell
death
7.3.1.6 Sphingolipid-mediated cellular deregulation
and fumonisin diseases
7.3.2. Altered fatty acid metabolism in liver
7.3.3. Other biochemical changes
7.4. Factors modifying toxicity; toxicity of metabolites
8. EFFECTS ON HUMANS
8.1. Transkei, South Africa
8.2. China
8.3. Northern Italy
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
9.1. Microorganisms
9.2. Plants
9.2.1. Duckweed and jimsonweed
9.2.2. Tomato
9.2.3. Maize
10. FURTHER RESEARCH
11. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANIZATIONS
REFERENCES
APPENDIX 1. NATIONAL GUIDELINES FOR FUMONISINS
APPENDIX 2. NATURAL OCCURRENCE OF FUMONISIN B1 (FB1) IN MAIZE-BASED
PRODUCTS
RESUME, EVALUATION ET RECOMMANDATIONS
RESUMEN, EVALUACION Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g. for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are used only when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the
Preparation of Environmental Health Criteria Monographs. PCS/90.69,
Geneva, World Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g. IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart on p. xv. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared by
consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals, and reference
data bases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. Although
observers may provide a valuable contribution to the process, they can
only speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
Members
Dr R.V. Bhat, Food and Drug Toxicology Research Centre, National
Institute of Nutrition, Indian Council of Medical Research,
Hyderabad, India
Dr M. Hirose, Division of Pathology, Biological Research Centre,
National Institute of Health Sciences, Tokyo, Japan
Dr P.C. Howard, Division of Biochemical Toxicology, National Center
for Toxicology Research, US Food and Drug Administration,
Jefferson, Arkansas, USA
Dr S. Humphreys, Center for Food Safety and Applied Nutrition, US Food
and Drug Administration, Washington DC, USA
Professor M. Kirsch-Volders, Laboratory for Cellular Genetics,
Brussels, Belgium (Chairman)
Professor W.F.O. Marasas, Medical Research Council, Tygerberg, South
Africa
Professor J.D. Miller, Department of Chemistry, Carleton University,
Ottawa, Ontario, Canada
Dr J.H. Olsen, Institute of Cancer Epidemiology, Danish Cancer
Society, Copenhagen, Denmark
Dr R. Plestina, Toxicology Unit, Institute for Medical Research and
Occupational Health, Zagreb, Croatia
Dr R.T. Riley, Agricultural Research Service, US Department of
Agriculture, Athens, USA
Dr A. Visconti, Institute for Toxins and Mycotoxins of Plant
Parasites, National Research Council, Bari, Italy
(Vice-Chairman)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Joint Secretary)
Mr Y. Hayashi, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Joint Secretary)
Dr J.M. Rice, International Agency for Research on Cancer, Lyon,
France
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
A WHO Task Group on Environmental Health Criteria for Fumonisin
B1 met at the World Health Organization, Geneva, Switzerland from 10
to 14 May 1999. Dr M. Younes, Acting Coordinator, Programme for the
Promotion of Chemical Safety, opened the meeting and welcomed the
participants on behalf of the IPCS and its three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to fumonisin B1.
Professor W.F.O. Marasas, Professor J.D. Miller, Dr R.T. Riley
and Dr A. Visconti prepared the first draft of this monograph. The
second draft incorporated comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs.
Dr A. Aitio, Mr Y. Hayashi and Dr P. Jenkins of the IPCS Central
Unit were responsible for the overall scientific content and technical
editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the US Food
and Drug Administration as part of its contributions to the IPCS.
ABBREVIATIONS
2-AAF 2-acetylaminofluorene
AAL-toxin Alternaria alternata lycopersici toxin
AMP adenosine monophosphate
AP aminopentol
CV coefficient of variation
CZE capillary zone electrophoresis
DEN diethylnitrosamine
DNA deoxyribonucleic acid
EDL effective dose level
EGF epidermal growth factor
ELEM equine leukoencephalomalacia
ELISA enzyme-linked immunosorbent assay
FA, FAK fumonisin A, fumonisin AK
FB fumonisin B
FC fumonisin C
FP fumonisin P
GC gas chromatography
GGT gamma-glutamyltranspeptidase
HPLC high-performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
IFN-gamma interferon-gamma
LPS lipopolysaccharide
MAPK mitogen-activated protein kinase
MME monomethyl ester
MS mass spectrometry
NADH reduced nicotinamide adenine dinucleotide
NADPH reduced nicotinamide adenine dinucleotide phosphate
NCTR National Center for Toxicological Research (USA)
NMBA N-methylbenzylnitrosamine
NOEL no-observed-effect level
NTD neural tube defect
NTP National Toxicology Program (USA)
OPA o-phthaldialdehyde
PDI probable daily intake
PFC plaque-forming cell
PGST placental glutathione S-transferase
PIM pulmonary intravascular/interstitial macrophage
PKC protein kinase C
PPE porcine pulmonary oedema
PUFA polyunsaturated fatty acid
Sa/So sphinganine/sphingosine
TCA tricarbalyllic acid moiety
TLC thin-layer chromatography
TNF-alpha tumour necrosis factor-alpha
INTRODUCTION
In this document, the fungus previously referred to as
Fusarium moniliforme Sheldon, is referred to as Fusarium
verticillioides (Sacc.) Nirenberg in accordance with a decision
taken at the 8th International Fusarium Workshop held at CABI
BioScience, Egham, United Kingdom, 17-20 August 1998.
This monograph focuses on fumonisin B1, the most abundant
naturally occurring fumonisin. Some information is also given on
fumonisins B2 and B3, which frequently occur with FB1, both in
culture material and in naturally contaminated samples.
1. SUMMARY, EVALUATION AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Fumonisin B1 (FB1) has the empirical formula C34H59NO15 and
is the diester of propane-1,2,3-tricarboxylic acid and
2-amino-12,16-dimethyl-3,5,10,14,15-pentahydroxyeicosane (relative
molecular mass: 721). It is the most prevalent of fumonisins, a family
of toxins with at least 15 identified members. The pure substance is a
white hygroscopic powder, which is soluble in water,
acetonitrile-water or methanol, is stable in acetonitrile-water (1:1),
is unstable in methanol, and is stable at food processing temperature
and to light.
Several analytical methods have been reported, including
thin-layer chromatography (TLC) and liquid chromatographic (LC), mass
spectroscopic (MS), post-hydrolysis gas chromatographic and
immunochemical methods, although the majority of studies have been
performed using LC analysis of a fluorescent derivative.
1.1.2 Sources of human exposure
FB1 is produced by several Fusarium species, mainly by
Fusarium verticillioides (Sacc.) Nirenberg (= Fusarium
moniliforme Sheldon), which is one of the most common fungi
associated with maize worldwide. Significant accumulation of FB1 in
maize occurs when weather conditions favour Fusarium kernel rot.
1.1.3 Environmental transport, distribution and transformation
There is evidence that fumonisins can be metabolized by some soil
microorganisms. However, little is known about the environmental fate
of fumonisins after they are either excreted or processed.
1.1.4 Environmental levels and human exposure
FB1 has been detected in maize and maize-based products
worldwide at mg/kg levels, sometimes in combination with other
mycotoxins. Concentrations at mg/kg levels have also been reported in
food for human consumption. Dry milling of maize results in the
distribution of fumonisin into the bran, germ and flour. In
experimental wet milling, fumonisin was detected in steep water,
gluten, fibre and germ, but not in the starch. FB1 is stable in maize
and polenta, whereas it is hydrolysed in nixtamalized maize-based
foods, i.e. foods processed with hot alkali solutions.
FB1 is not present in milk, meat or eggs from animals fed grain
containing FB1 at levels that would not affect the health of the
animals. Human exposure estimates for the USA, Canada, Switzerland,
the Netherlands and the Transkei (South Africa) ranged from 0.017 to
440 µg/kg body weight per day. No data on occupational inhalation
exposure are available.
1.1.5 Kinetics and metabolism in animals
There have been no reports on the kinetics or metabolism of FB1
in humans. In experimental animals it is poorly absorbed when dosed
orally, is rapidly eliminated from circulation and is recovered
unmetabolized in faeces. Biliary excretion is important, and small
amounts are excreted in urine. It can be degraded to partially
hydrolysed FB1 in the gut of non-human primates and some ruminants. A
small amount is retained in the liver and kidney.
1.1.6 Effects on animals and in vitro test systems
FB1 is hepatotoxic in all animal species tested including mice,
rats, equids, rabbits, pigs and non-human primates. With the exception
of Syrian hamsters, embryotoxicity or teratogenicity is only observed
concurrent with or subsequent to maternal toxicity. Fumonisins are
nephrotoxic in pigs, rats, sheep, mice and rabbits. In rats and
rabbits, renal toxicity occurs at lower doses than hepatotoxicity.
Fumonisins are known to be the cause of equine leukoencephalomalacia
and porcine pulmonary oedema syndrome, both associated with the
consumption of maize-based feeds. Limited information on immunological
properties of FB1 is available. It was hepatocarcinogenic to male
rats in one strain and nephrocarcinogenic in another strain at the
same dose levels (50 mg/kg diet), and was hepatocarcinogenic at 50
mg/kg diet in female mice. There appears to be a correlation between
organ toxicity and cancer development. FB1 was the first specific
inhibitor of de novo sphingolipid metabolism to be discovered and is
currently widely used to study the role of sphingolipids in cellular
regulation. FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in animals, plants
and some yeasts. It did not induce gene mutations in bacteria or
unscheduled DNA synthesis in primary rat hepatocytes, but induced a
dose-dependent increase in chromosomal aberrations at low
concentration levels in one study on primary rat hepatocytes.
1.1.7 Effects on humans
There are no confirmed records of acute fumonisin toxicity in
humans. Available correlation studies from the Transkei, South Africa,
suggest a link between dietary fumonisin exposure and oesophageal
cancer. This was observed where relatively high fumonisin exposure has
been demonstrated and where environmental conditions promote fumonisin
accumulation in maize, which is the staple diet. Correlation studies
are also available from China. However, no clear picture on the
relationship between either fumonisin or F. verticillioides
contamination and oesophageal cancer emerged. Owing to the absence of
fumonisin exposure data, no conclusion can be drawn from a case
control study of males in Italy showing an association between maize
intake and upper gastrointestinal tract cancer among subjects with
high alcohol consumption.
There are no validated biomarkers for human exposure to FB1.
1.1.8 Effects on other organisms in the laboratory
FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in Saccharomyces
cerevisiae.
FB1 is phytotoxic, damages cell membranes and reduces
chlorophyll synthesis. It also disrupts the biosynthesis of
sphingolipids in plants and may play a role in the pathogenicity of
maize by fumonisin-producing Fusarium species.
1.2 Evaluation of human health risks
1.2.1 Exposure
Human exposure as demonstrated by the occurrence of FB1 in maize
intended for human consumption is common worldwide. There are
considerable differences in the extent of human exposure between
different maize-growing regions. This is most evident when comparing
fully developed and developing countries. For example, although FB1
can occur in maize products in the USA, Canada and western Europe,
human consumption of those products is modest. In parts of Africa,
South-Central America and Asia, some populations consume a high
percentage of their calories as maize meal where FB1 contamination
may be high (see Appendix 2). Maize contaminated naturally by FB1 can
be simultaneously contaminated with other F. verticillioides or
F. proliferatum toxins or with other agriculturally important toxins
including deoxynivalenol, zearalenone, aflatoxin and ochratoxin.
FB1 is stable to food processing methods used in North America
and western Europe. Treating maize with base and/or water washing
effectively lowers the FB1 concentrations. However, its
hepatotoxicity and/or nephrotoxicity in experimental animals are still
evident. Little is known about how food processing techniques used in
the developing world affect FB1 in maize products.
1.2.2 Hazard identification
The causal role of FB1 exposure in the disease equine
leukoencephalomalacia has been established. Large-scale outbreaks of
this fatal disease occurred in the USA during the 19th century and as
recently as 1989-1990. The causal role of FB1 exposure in the fatal
disease porcine pulmonary oedema has been established. As observed in
pregnant females, low exposures to FB1 are fatal to rabbits. Exposure
has been demonstrated to result in renal toxicity and causes
hepatotoxicity in all animal species studied, including non-human
primates. FB1 exposure causes hypercholesterolaemia in several animal
species, including non-human primates. There is good evidence for
altered lipid metabolism in the animal diseases associated with FB1
exposure. Disruption of sphingolipid metabolism is evident either
before or concurrent with in vitro and in vivo toxicity. The use
of fumonisins as tools to study the function of sphingolipids has
revealed that sphingolipids are required for cell growth and affect
signalling molecules in several pathways, leading to apoptotic and
necrotic cell death, cellular differentiation and altered immune
responses. Altered lipid metabolism and changes in the activity and/or
expression of key enzymes responsible for normal cell cycle progress
appear to be common factors following exposure to FB1. FB1 is not a
developmental toxin to rat, mouse or rabbit. It induces fetotoxicity
in Syrian hamster at high doses without maternal toxicity.
The carcinogenicity of FB1 in rodents varies between species,
strains and sex. The only study with B6C3F1 mice indicated that FB1
was hepatocarcinogenic to females at 50 mg/kg in the diet. Primary
hepatocellular carcinomas and cholangial carcinomas were induced in
male BD IX rats fed diets at 50 mg FB1/kg for up to 26 months. Renal
tubule adenomas and carcinomas were detected in male F344/N Nctr rats
fed 50 mg FB1/kg. There appears to be a correlation between organ
toxicity and cancer development.
A limited number of genotoxicity studies are available. FB1 was
not mutagenic in bacterial assays. In in vitro mammalian cells,
unscheduled DNA synthesis was not detected but FB1 caused chromosomal
breaks in rat hepatocytes in one study. Other studies have shown that
FB1 causes increased lipid peroxidation in vivo and in vitro. It
is possible that chromosome-breaking effects and lipid peroxidation
are causally related.
FB1 levels above 100 mg/kg, which have been reported in maize
consumed by humans in Africa and China, would probably cause
leukoencephalomalacia, pulmonary oedema syndrome or cancer if fed to
horses, pigs and rats or mice, respectively. Despite these cases of
very high human exposure, there are no confirmed records of acute
fumonisin toxicity in humans. Available correlation studies from the
Transkei, South Africa, suggest a link between dietary fumonisin
exposure and oesophageal cancer. Elevated rates of oesophageal cancer
have been observed where relatively high fumonisin exposure has been
demonstrated and where environmental conditions promote the
accumulation of fumonisin in maize, which is the staple diet.
One case-control study in males from Italy found an association
between maize intake and cancers of the upper digestive tract,
including oesophageal cancer, among subjects with high alcohol
consumption. There were no data on fumonisin exposure.
1.2.3 Dose-response assessment
The lowest dose of FB1 that induced hepatocarcinomas in
experimental animals was 50 mg/kg diet in male BD IX rats and female
B6C3F1/Nctr mice; no cancer induction was observed at 25 or 15 mg/kg
diet, respectively. In each case, indications of hepatotoxicity or
lipid alterations were noted at the same or lower doses in studies
with these same rat and mouse strains. The lowest dose of FB1 that
induced renal carcinomas in the male F344/N Nctr rats was 50 mg/kg
diet; no cancer induction was observed at 15 mg/kg diet. Renal tubular
apoptosis and cell proliferation, as well as tissue and urinary
sphingolipid changes, occurred at lower doses than those required for
the induction of cancer in these studies.
No data are available to assess quantitatively the relationship
between exposure to FB1 and possible effects in humans.
1.2.4 Risk characterization
FB1 is carcinogenic in mice and rats and induces fatal diseases
in pigs and horses at levels of exposure that humans encounter. The
Task Group was not in a position to perform a quantitative estimation
of the human health risks, but considered that such an estimation is
urgently needed.
1.3 Recommendations for protection of human health
a) Limits for human dietary exposure should be established.
Special consideration should be given to populations
consuming a high percentage of their calories as maize meal.
b) Measures should be taken to limit fumonisin exposure and
maize contamination by:
* planting alternative crops in areas where maize is not
well adapted;
* developing maize resistant to Fusarium kernel rot;
* practising better crop management;
* segregating mouldy kernels.
c) Early awareness of potential food contamination should be
increased by improving communication between veterinarians
and public health officials on outbreaks of mycotoxicoses in
domestic animals.
d) A robust, low-cost and simple screening method for the
detection of fumonisin contamination in maize should be
developed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Common name: Fumonisin B1 (FB1)
Chemical formula: C34H59NO15
Chemical structure:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
Members
Dr R.V. Bhat, Food and Drug Toxicology Research Centre, National
Institute of Nutrition, Indian Council of Medical Research,
Hyderabad, India
Dr M. Hirose, Division of Pathology, Biological Research Centre,
National Institute of Health Sciences, Tokyo, Japan
Dr P.C. Howard, Division of Biochemical Toxicology, National Center
for Toxicology Research, US Food and Drug Administration,
Jefferson, Arkansas, USA
Dr S. Humphreys, Center for Food Safety and Applied Nutrition, US Food
and Drug Administration, Washington DC, USA
Professor M. Kirsch-Volders, Laboratory for Cellular Genetics,
Brussels, Belgium (Chairman)
Professor W.F.O. Marasas, Medical Research Council, Tygerberg, South
Africa
Professor J.D. Miller, Department of Chemistry, Carleton University,
Ottawa, Ontario, Canada
Dr J.H. Olsen, Institute of Cancer Epidemiology, Danish Cancer
Society, Copenhagen, Denmark
Dr R. Plestina, Toxicology Unit, Institute for Medical Research and
Occupational Health, Zagreb, Croatia
Dr R.T. Riley, Agricultural Research Service, US Department of
Agriculture, Athens, USA
Dr A. Visconti, Institute for Toxins and Mycotoxins of Plant
Parasites, National Research Council, Bari, Italy
(Vice-Chairman)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Joint Secretary)
Mr Y. Hayashi, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Joint Secretary)
Dr J.M. Rice, International Agency for Research on Cancer, Lyon,
France
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
A WHO Task Group on Environmental Health Criteria for Fumonisin
B1 met at the World Health Organization, Geneva, Switzerland from 10
to 14 May 1999. Dr M. Younes, Acting Coordinator, Programme for the
Promotion of Chemical Safety, opened the meeting and welcomed the
participants on behalf of the IPCS and its three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to fumonisin B1.
Professor W.F.O. Marasas, Professor J.D. Miller, Dr R.T. Riley
and Dr A. Visconti prepared the first draft of this monograph. The
second draft incorporated comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs.
Dr A. Aitio, Mr Y. Hayashi and Dr P. Jenkins of the IPCS Central
Unit were responsible for the overall scientific content and technical
editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the US Food
and Drug Administration as part of its contributions to the IPCS.
ABBREVIATIONS
2-AAF 2-acetylaminofluorene
AAL-toxin Alternaria alternata lycopersici toxin
AMP adenosine monophosphate
AP aminopentol
CV coefficient of variation
CZE capillary zone electrophoresis
DEN diethylnitrosamine
DNA deoxyribonucleic acid
EDL effective dose level
EGF epidermal growth factor
ELEM equine leukoencephalomalacia
ELISA enzyme-linked immunosorbent assay
FA, FAK fumonisin A, fumonisin AK
FB fumonisin B
FC fumonisin C
FP fumonisin P
GC gas chromatography
GGT gamma-glutamyltranspeptidase
HPLC high-performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
IFN-gamma interferon-gamma
LPS lipopolysaccharide
MAPK mitogen-activated protein kinase
MME monomethyl ester
MS mass spectrometry
NADH reduced nicotinamide adenine dinucleotide
NADPH reduced nicotinamide adenine dinucleotide phosphate
NCTR National Center for Toxicological Research (USA)
NMBA N-methylbenzylnitrosamine
NOEL no-observed-effect level
NTD neural tube defect
NTP National Toxicology Program (USA)
OPA o-phthaldialdehyde
PDI probable daily intake
PFC plaque-forming cell
PGST placental glutathione S-transferase
PIM pulmonary intravascular/interstitial macrophage
PKC protein kinase C
PPE porcine pulmonary oedema
PUFA polyunsaturated fatty acid
Sa/So sphinganine/sphingosine
TCA tricarbalyllic acid moiety
TLC thin-layer chromatography
TNF-alpha tumour necrosis factor-alpha
INTRODUCTION
In this document, the fungus previously referred to as
Fusarium moniliforme Sheldon, is referred to as Fusarium
verticillioides (Sacc.) Nirenberg in accordance with a decision
taken at the 8th International Fusarium Workshop held at CABI
BioScience, Egham, United Kingdom, 17-20 August 1998.
This monograph focuses on fumonisin B1, the most abundant
naturally occurring fumonisin. Some information is also given on
fumonisins B2 and B3, which frequently occur with FB1, both in
culture material and in naturally contaminated samples.
1. SUMMARY, EVALUATION AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Fumonisin B1 (FB1) has the empirical formula C34H59NO15 and
is the diester of propane-1,2,3-tricarboxylic acid and
2-amino-12,16-dimethyl-3,5,10,14,15-pentahydroxyeicosane (relative
molecular mass: 721). It is the most prevalent of fumonisins, a family
of toxins with at least 15 identified members. The pure substance is a
white hygroscopic powder, which is soluble in water,
acetonitrile-water or methanol, is stable in acetonitrile-water (1:1),
is unstable in methanol, and is stable at food processing temperature
and to light.
Several analytical methods have been reported, including
thin-layer chromatography (TLC) and liquid chromatographic (LC), mass
spectroscopic (MS), post-hydrolysis gas chromatographic and
immunochemical methods, although the majority of studies have been
performed using LC analysis of a fluorescent derivative.
1.1.2 Sources of human exposure
FB1 is produced by several Fusarium species, mainly by
Fusarium verticillioides (Sacc.) Nirenberg (= Fusarium
moniliforme Sheldon), which is one of the most common fungi
associated with maize worldwide. Significant accumulation of FB1 in
maize occurs when weather conditions favour Fusarium kernel rot.
1.1.3 Environmental transport, distribution and transformation
There is evidence that fumonisins can be metabolized by some soil
microorganisms. However, little is known about the environmental fate
of fumonisins after they are either excreted or processed.
1.1.4 Environmental levels and human exposure
FB1 has been detected in maize and maize-based products
worldwide at mg/kg levels, sometimes in combination with other
mycotoxins. Concentrations at mg/kg levels have also been reported in
food for human consumption. Dry milling of maize results in the
distribution of fumonisin into the bran, germ and flour. In
experimental wet milling, fumonisin was detected in steep water,
gluten, fibre and germ, but not in the starch. FB1 is stable in maize
and polenta, whereas it is hydrolysed in nixtamalized maize-based
foods, i.e. foods processed with hot alkali solutions.
FB1 is not present in milk, meat or eggs from animals fed grain
containing FB1 at levels that would not affect the health of the
animals. Human exposure estimates for the USA, Canada, Switzerland,
the Netherlands and the Transkei (South Africa) ranged from 0.017 to
440 µg/kg body weight per day. No data on occupational inhalation
exposure are available.
1.1.5 Kinetics and metabolism in animals
There have been no reports on the kinetics or metabolism of FB1
in humans. In experimental animals it is poorly absorbed when dosed
orally, is rapidly eliminated from circulation and is recovered
unmetabolized in faeces. Biliary excretion is important, and small
amounts are excreted in urine. It can be degraded to partially
hydrolysed FB1 in the gut of non-human primates and some ruminants. A
small amount is retained in the liver and kidney.
1.1.6 Effects on animals and in vitro test systems
FB1 is hepatotoxic in all animal species tested including mice,
rats, equids, rabbits, pigs and non-human primates. With the exception
of Syrian hamsters, embryotoxicity or teratogenicity is only observed
concurrent with or subsequent to maternal toxicity. Fumonisins are
nephrotoxic in pigs, rats, sheep, mice and rabbits. In rats and
rabbits, renal toxicity occurs at lower doses than hepatotoxicity.
Fumonisins are known to be the cause of equine leukoencephalomalacia
and porcine pulmonary oedema syndrome, both associated with the
consumption of maize-based feeds. Limited information on immunological
properties of FB1 is available. It was hepatocarcinogenic to male
rats in one strain and nephrocarcinogenic in another strain at the
same dose levels (50 mg/kg diet), and was hepatocarcinogenic at 50
mg/kg diet in female mice. There appears to be a correlation between
organ toxicity and cancer development. FB1 was the first specific
inhibitor of de novo sphingolipid metabolism to be discovered and is
currently widely used to study the role of sphingolipids in cellular
regulation. FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in animals, plants
and some yeasts. It did not induce gene mutations in bacteria or
unscheduled DNA synthesis in primary rat hepatocytes, but induced a
dose-dependent increase in chromosomal aberrations at low
concentration levels in one study on primary rat hepatocytes.
1.1.7 Effects on humans
There are no confirmed records of acute fumonisin toxicity in
humans. Available correlation studies from the Transkei, South Africa,
suggest a link between dietary fumonisin exposure and oesophageal
cancer. This was observed where relatively high fumonisin exposure has
been demonstrated and where environmental conditions promote fumonisin
accumulation in maize, which is the staple diet. Correlation studies
are also available from China. However, no clear picture on the
relationship between either fumonisin or F. verticillioides
contamination and oesophageal cancer emerged. Owing to the absence of
fumonisin exposure data, no conclusion can be drawn from a case
control study of males in Italy showing an association between maize
intake and upper gastrointestinal tract cancer among subjects with
high alcohol consumption.
There are no validated biomarkers for human exposure to FB1.
1.1.8 Effects on other organisms in the laboratory
FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in Saccharomyces
cerevisiae.
FB1 is phytotoxic, damages cell membranes and reduces
chlorophyll synthesis. It also disrupts the biosynthesis of
sphingolipids in plants and may play a role in the pathogenicity of
maize by fumonisin-producing Fusarium species.
1.2 Evaluation of human health risks
1.2.1 Exposure
Human exposure as demonstrated by the occurrence of FB1 in maize
intended for human consumption is common worldwide. There are
considerable differences in the extent of human exposure between
different maize-growing regions. This is most evident when comparing
fully developed and developing countries. For example, although FB1
can occur in maize products in the USA, Canada and western Europe,
human consumption of those products is modest. In parts of Africa,
South-Central America and Asia, some populations consume a high
percentage of their calories as maize meal where FB1 contamination
may be high (see Appendix 2). Maize contaminated naturally by FB1 can
be simultaneously contaminated with other F. verticillioides or
F. proliferatum toxins or with other agriculturally important toxins
including deoxynivalenol, zearalenone, aflatoxin and ochratoxin.
FB1 is stable to food processing methods used in North America
and western Europe. Treating maize with base and/or water washing
effectively lowers the FB1 concentrations. However, its
hepatotoxicity and/or nephrotoxicity in experimental animals are still
evident. Little is known about how food processing techniques used in
the developing world affect FB1 in maize products.
1.2.2 Hazard identification
The causal role of FB1 exposure in the disease equine
leukoencephalomalacia has been established. Large-scale outbreaks of
this fatal disease occurred in the USA during the 19th century and as
recently as 1989-1990. The causal role of FB1 exposure in the fatal
disease porcine pulmonary oedema has been established. As observed in
pregnant females, low exposures to FB1 are fatal to rabbits. Exposure
has been demonstrated to result in renal toxicity and causes
hepatotoxicity in all animal species studied, including non-human
primates. FB1 exposure causes hypercholesterolaemia in several animal
species, including non-human primates. There is good evidence for
altered lipid metabolism in the animal diseases associated with FB1
exposure. Disruption of sphingolipid metabolism is evident either
before or concurrent with in vitro and in vivo toxicity. The use
of fumonisins as tools to study the function of sphingolipids has
revealed that sphingolipids are required for cell growth and affect
signalling molecules in several pathways, leading to apoptotic and
necrotic cell death, cellular differentiation and altered immune
responses. Altered lipid metabolism and changes in the activity and/or
expression of key enzymes responsible for normal cell cycle progress
appear to be common factors following exposure to FB1. FB1 is not a
developmental toxin to rat, mouse or rabbit. It induces fetotoxicity
in Syrian hamster at high doses without maternal toxicity.
The carcinogenicity of FB1 in rodents varies between species,
strains and sex. The only study with B6C3F1 mice indicated that FB1
was hepatocarcinogenic to females at 50 mg/kg in the diet. Primary
hepatocellular carcinomas and cholangial carcinomas were induced in
male BD IX rats fed diets at 50 mg FB1/kg for up to 26 months. Renal
tubule adenomas and carcinomas were detected in male F344/N Nctr rats
fed 50 mg FB1/kg. There appears to be a correlation between organ
toxicity and cancer development.
A limited number of genotoxicity studies are available. FB1 was
not mutagenic in bacterial assays. In in vitro mammalian cells,
unscheduled DNA synthesis was not detected but FB1 caused chromosomal
breaks in rat hepatocytes in one study. Other studies have shown that
FB1 causes increased lipid peroxidation in vivo and in vitro. It
is possible that chromosome-breaking effects and lipid peroxidation
are causally related.
FB1 levels above 100 mg/kg, which have been reported in maize
consumed by humans in Africa and China, would probably cause
leukoencephalomalacia, pulmonary oedema syndrome or cancer if fed to
horses, pigs and rats or mice, respectively. Despite these cases of
very high human exposure, there are no confirmed records of acute
fumonisin toxicity in humans. Available correlation studies from the
Transkei, South Africa, suggest a link between dietary fumonisin
exposure and oesophageal cancer. Elevated rates of oesophageal cancer
have been observed where relatively high fumonisin exposure has been
demonstrated and where environmental conditions promote the
accumulation of fumonisin in maize, which is the staple diet.
One case-control study in males from Italy found an association
between maize intake and cancers of the upper digestive tract,
including oesophageal cancer, among subjects with high alcohol
consumption. There were no data on fumonisin exposure.
1.2.3 Dose-response assessment
The lowest dose of FB1 that induced hepatocarcinomas in
experimental animals was 50 mg/kg diet in male BD IX rats and female
B6C3F1/Nctr mice; no cancer induction was observed at 25 or 15 mg/kg
diet, respectively. In each case, indications of hepatotoxicity or
lipid alterations were noted at the same or lower doses in studies
with these same rat and mouse strains. The lowest dose of FB1 that
induced renal carcinomas in the male F344/N Nctr rats was 50 mg/kg
diet; no cancer induction was observed at 15 mg/kg diet. Renal tubular
apoptosis and cell proliferation, as well as tissue and urinary
sphingolipid changes, occurred at lower doses than those required for
the induction of cancer in these studies.
No data are available to assess quantitatively the relationship
between exposure to FB1 and possible effects in humans.
1.2.4 Risk characterization
FB1 is carcinogenic in mice and rats and induces fatal diseases
in pigs and horses at levels of exposure that humans encounter. The
Task Group was not in a position to perform a quantitative estimation
of the human health risks, but considered that such an estimation is
urgently needed.
1.3 Recommendations for protection of human health
a) Limits for human dietary exposure should be established.
Special consideration should be given to populations
consuming a high percentage of their calories as maize meal.
b) Measures should be taken to limit fumonisin exposure and
maize contamination by:
* planting alternative crops in areas where maize is not
well adapted;
* developing maize resistant to Fusarium kernel rot;
* practising better crop management;
* segregating mouldy kernels.
c) Early awareness of potential food contamination should be
increased by improving communication between veterinarians
and public health officials on outbreaks of mycotoxicoses in
domestic animals.
d) A robust, low-cost and simple screening method for the
detection of fumonisin contamination in maize should be
developed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Common name: Fumonisin B1 (FB1)
Chemical formula: C34H59NO15
Chemical structure:
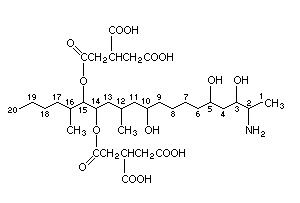 Relative molecular mass: 721
CAS Name: 1,2,3-Propanetricarboxylic acid,
1,1'-[1-(12-amino-4,9,11-trihydroxy-2-methyl-
tridecyl)-2-(1-methylpentyl)-1,2-ethane-diyl]
ester
IUPAC name: None
CAS registry number: 116355-83-0
RTECS No.: TZ 8350000
Synonym: Macrofusine
At least 15 different fumonisins have so far been reported and
other minor metabolites have been identified, although most of them
have not been shown to occur naturally. They have been grouped into
four main categories (Plattner, 1995; Abbas & Shier, 1997; Musser &
Plattner, 1997): FA1, FA2, FA3, FAK1; FB1, FB2, FB3, FB4;
FC1, FC2, FC3, FC4; FP1, FP2 and FP3. FB2, FB3 and FB4
differ from FB1 in that they lack hydroxyl groups present in FB1;
FA1, FA2 and FA3 are like FB1, FB2 and FB3, but are
N-acetylated; FAK1 is like FA1 but is 15-keto functionalized; FCs
are like FBs but lack the methyl group adjacent to the amino group;
FPs have a 3-hydroxypyridium group instead of the amine group in the
FBs. This monograph will focus mainly on FB1, the most abundant of
the naturally occurring fumonisins.
2.2 Physical and chemical properties of the pure substance
Physical state: White hygroscopic powder
Melting point: Not known (has not been crystallized)
Optical rotation: Not known
Spectroscopy: Mass spectral and nuclear magnetic resonance data
are given in Bezuidenhout et al. (1988), Laurent
et al. (1989a) and Savard & Blackwell (1994)
Solubility: Soluble in water to at least to 20 mg/ml (US NTP,
1999); soluble in methanol, acetonitrile-water.
n-Octanol/water 1.84 (Norred et al., 1997)
partition
coefficient
(log P):
Stability: Stable in acetonitrile-water (1:1) for up to 6
months at 25°C; unstable in methanol (25% or 35%
concentration decrease after 3 or 6 weeks at 25°C,
respectively), giving rise to monomethyl or
dimethyl esters (Gelderblom et al., 1992a;
Visconti et al., 1994); stable in methanol up to 6
weeks at -18°C (Visconti et al., 1994); stable at
78°C for 16 h in buffer solutions at pH between
3.5 and 9 (Howard et al., 1998)
2.3 Analytical methods
Six general analytical methods have been reported: thin-layer
chromatographic (TLC), liquid chromatographic (LC), mass spectrometric
(MS), post-hydrolysis gas chromatographic, immunochemical and
electrophoretic methods (Sydenham & Shephard, 1996; Shephard, 1998).
The majority of studies have been performed using LC analysis of a
fluorescent derivative.
2.3.1 Sampling and preparation procedures
In raw maize, FB1 is present in both visibly damaged and
undamaged kernels (Bullerman & Tsai, 1994). This means that the
problem that occurs with the mycotoxin aflatoxin, i.e., a few highly
contaminated kernels in otherwise aflatoxin-free kernels, is probably
less of an issue. However, it has been shown that higher levels of
fumonisins are concentrated in visibly damaged kernels (Pascale et
al., 1997). Studies to determine the minimum representative sample in
a lot of maize have not been reported. However, homogeneous material
(CV < 10%) for fumonisin analysis was obtained by grinding
contaminated maize to a particle size less than 2 mm with test portion
sizes of 25 and 10 g (Visconti & Boenke, 1995).
2.3.2 Extraction
Methanol-water (3:1) is the solvent of choice (e.g., Shephard et
al., 1990; Stack & Eppley, 1992; Doko & Visconti, 1994; Scott &
Lawrence, 1994) with a long shaking time or homogenization with a
blender (Sydenham et al., 1992; Bennett & Richard, 1994; Visconti &
Boenke, 1995; Visconti et al., 1995). The use of acetonitrile-water
has also been reported, with conflicting data on its performance
relative to methanol-water (Sydenham et al., 1992a; Bennett & Richard,
1994; Visconti & Boenke, 1995). Use of an acidic extraction procedure
may lead to higher extraction efficiencies (Zoller et al., 1994;
Meister, 1998). However, remarkable variability in extraction
efficiency has been reported by several authors, and more work needs
to be done to establish the best extraction solvents for various food
products.
Clean-up involves the use of solid-phase extraction with strong
anion exchange (Shephard et al., 1990) or C18 reversed-phase (Ross et
al., 1990) or a combination of both (Miller et al., 1993). Improved
recoveries can be achieved by using anion exchange instead of
reversed-phase material for sample clean-up (Stockenström et al.,
1994; Dawlatana et al., 1995). Immunoaffinity columns (Scott &
Trucksess, 1997) have also been shown to be useful for clean-up of
crude extracts of maize (Ware et al., 1994; Duncan et al., 1998),
sweet corn (Trucksess et al., 1995), beer (Scott & Lawrence, 1995) and
milk (Scott et al., 1994).
Fumonisins are relatively stable compounds (Alberts et al., 1990;
Dupuy et al., 1993a; Le Bars et al., 1994; Visconti et al., 1994;
Pascale et al., 1995; Jackson et al., 1996a,b, 1997). A number of
factors make them difficult to extract from processed food (Scott,
1993; Bullerman & Tsai, 1994). Binding of FB1 to maize bran flour
occurs at room temperature and above (Scott & Lawrence, 1994). Added
iron may also affect recoveries of fumonisin (Scott & Lawrence, 1994).
Unknown processing factors or ingredients can change the recovery of
fumonisin from cereal products (Scott & Lawrence, 1994). Only 45% of
FB1 present in spiked corn meal was recovered following baking at
175-200°C for 20 min (Jackson et al., 1997). Fumonisins have been
shown to react with reducing sugars at elevated temperatures (Murphy
et al., 1996; Lu et al., 1997). The product of the reaction of FB1
with reducing sugars was identified as N-carboxymethyl-FB1 (Howard
et al., 1998). This product was found in raw corn samples at 4% of the
FB1 levels (Howard et al., 1998). Ammoniation and treatment with base
reduces apparent fumonisin concentrations while increasing the
concentration of hydrolysed fumonisins without eliminating the
toxicity of the treated product, again suggesting analytical
difficulties (Norred et al., 1991; Hendrich et al., 1993).
Methods have been reported for the extraction of FB1 and FB2 in
plasma and urine (Shephard et al., 1992c, 1995c; Shetty & Bhat, 1998),
bile of rats and vervet monkeys (Shephard et al., 1994c, 1995a),
faeces of vervet monkeys (Shephard et al., 1994b), liver, kidney and
muscle of beef cattle (Smith & Thakur, 1996), and milk (Maragos &
Richard, 1994; Scott et al., 1994; Prelusky et al., 1996a).
2.3.3 Analysis
Normal phase silica TLC can be used for analysis, with fumonisins
being visualized by spraying with p-anisaldehyde (Plattner et al.,
1990; Sydenham et al., 1990a; Dupuy et al., 1993b). For C18 HPLC or
TLC, visualization has been accomplished with fluorescamine
(Rottinghaus et al., 1992; Miller et al., 1995) and vanillin (Pittet
et al., 1992). The detection limit for fumonisins in maize by these
methods is 1 mg/kg (Miller et al., 1995). Improved TLC methods with
adequate sensitivity are needed, particularly to control maize
contamination in developing countries.
A number of fluorescent derivatives have been used for HPLC
detection including fluorescamine (Ross et al., 1991a,b),
naphthalene-2,3-dicarboxaldehyde/potassium cyanide (Ware et al., 1993;
Bennett & Richard, 1994; Scott & Lawrence, 1994),
4-fluoro-7-nitrobenzo-2-oxa-1,3-diazole (Scott & Lawrence, 1992,
1994), 6-aminoquinolyl N-hydroxysuccinimidylcarbamate (Velázquez et
al., 1995), 9-fluorenylmethyl chloroformate (Holcomb et al., 1993) and
o-phthaldialdehyde (OPA) (Shephard et al., 1990; Sydenham et al.,
1992). In most laboratories, these methods have reported limits of
detection or limits of quantification ranging from 5 to 100 µg/kg. The
OPA method is widely used and methodology using this derivative has
been the subject of international collaborative trials (Thiel et al.,
1993; Visconti et al., 1993; Sydenham et al., 1996). Particularly
satisfactory results were achieved in the trial by Sydenham et al.
(1996) with FB1 concentrations ranging from 0.5 to 8.0 mg/kg.
Relative standard deviations for within-laboratory repeatability
ranged from 5.8% to 13.2% for FB1. Relative standard deviations for
between-laboratory reproducibility were 13.9% to 22.2% for FB1.
HORRAT ratios for 7 samples in the test varied from 0.75 to 1.73 for
FB1 (Sydenham et al., 1996). Ratios of less than 2 are considered
acceptable. This method has been adopted by the Association of
Official Analytical Chemists International as an official method for
the analysis of maize.
There are no standardized methodologies for fumonisin analysis in
different food products. A method for the extraction and analysis of
FB1 in beer has been reported (Scott & Lawrence, 1994; Scott et al.,
1997).
Hydrolysis of samples to the aminopentol chain followed by the GC
analysis of the trimethylsilyl or trifluoroacetate derivative by flame
ionization detection or mass spectrometry has been reported (Plattner
et al., 1990, 1992; Plattner & Branham, 1994). Determination of
hydrolysed FB1 in alkali-processed corn foods by HPLC with
fluorescent derivatives has also been reported (Scott & Lawrence,
1996).
Analyses of maize extracts with antibodies reactive with FB1
(and FB2 plus FB3) by direct and indirect assays have been reported
(Azcona-Olivera et al., 1992a,b; Usleber et al., 1994; Scott &
Trucksess, 1997; Mullett et al., 1998). Detection limits using these
methods have been reported to be 0.1-100 µg/litre. In one study, an
ELISA method gave higher estimates of fumonisin concentrations
compared to GC-MS and HPLC (Pestka et al., 1994).
To a very limited extent, fumonisins have also been determined by
capillary zone electrophoresis (CZE). In order to achieve resolution
of the FB1 and FB2 analogues, samples were derivatized with either
9-fluorenylmethyl chloroformate (Holcomb & Thompson, 1996) or
fluorescein isothiocyanate (Maragos, 1995) prior to separation.
As an analytical tool for the determination of fumonisins, MS was
initially used as a detector after gas chromatographic separation of
the hydrolysed fumonisins (Plattner et al., 1990). Although MS methods
using fast-atom bombardment (Plattner & Branham, 1994) and particle
beam interfaces (Young & Lafontaine, 1993) have been described, the
application of the electrospray interface has led to the greatest
advance in the use of MS for fumonisin determination. These methods
rely on the LD separation of the underivatized fumonisins and
detection of the different analogues as their protonated molecular
ions (Doerge et al., 1994; Plattner, 1995; Lukacs et al., 1996;
Churchwell et al., 1997). A combined on-line immunoaffinity capture,
HPLC/MS method has also been described, and this permits analysis of
non-derivatized fumonisins at sub µg/kg levels (Newkirk et al., 1998).
3. SOURCES OF HUMAN EXPOSURE
FB1 was isolated in 1988 by Gelderblom et al. (1988). It was
chemically characterized by Bezuidenhout et al. (1988), and shortly
thereafter as "macrofusine" by Laurent et al. (1989a), from cultures
of Fusarium verticillioides (Sacc.) Nirenberg (Fusarium
moniliforme Sheldon). A selection of FB1 occurrence data in maize
and food products is given in Table 1 and Appendix 2. A worldwide
survey of fumonisin contamination of maize and maize-based products
was reported by Shephard et al. (1996a).
FB1 is produced by isolates of Fusarium verticillioides,
F. proliferatum, F. anthophilum, F. beomiforme, F. dlamini,
F. globosum, F. napiforme, F. nygamai, F. oxysporum,
F. polyphialidicum, F. subglutinans and F. thapsinum isolated from
Africa, the Americas, Oceania, Asia and Europe (Gelderblom et al.,
1988; Ross et al., 1990; Thiel et al., 1991a; Nelson et al., 1991,
1992; Chelkowski & Lew, 1992; Leslie et al., 1992, 1996; Rapior et
al., 1993; Miller et al., 1993, 1995; Visconti & Doko, 1994;
Desjardins et al., 1994; Abbas et al., 1995; Abbas & Ocamb, 1995;
Logrieco et al., 1995; Klittich et al., 1997; Musser & Plattner, 1997;
Sydenham et al., 1997). A species of Alternaria (A. alternata f. sp.
lycopersici) has also been demonstrated to synthesize B fumonisins
(Abbas & Riley, 1996). Fumonisins can be produced by culturing strains
of the Fusarium species that produce these toxins on sterilized
maize (Cawood et al., 1991), and yields of up to 17.9 g/kg have been
obtained with F. verticillioides strain MRC 826 (Alberts et al.,
1990). Yields of 500-700 mg/litre for FB1 plus FB2 have been
obtained in liquid fermentations and high recoveries of the toxins are
possible (Miller et al., 1994). The most predominant toxin produced is
FB1. FB1 frequently occurs together with FB2, which may comprise
15-35% of FB1 (IARC, 1993; Diaz & Boermans, 1994; Visconti & Doko,
1994).
Fusarium verticillioides and F. proliferatum are amongst the
most common fungi associated with maize. These fungi can be recovered
from most maize kernels including those that appear healthy
(Hesseltine et al., 1981; Bacon & Williamson, 1992; Pitt el al., 1993;
Sanchis et al., 1995). The formation of fumonisins in maize in the
field is positively correlated with the occurrence of these two fungal
species, which are predominant during the late maturity stage (Chulze
et al., 1996). These species can cause Fusarium kernel rot of maize,
which is one of the most important ear diseases in hot maize-growing
areas (King & Scott, 1981; Ochor et al., 1987; De León & Pandey, 1989)
and is associated with warm, dry years and/or insect damage
(Shurtleff, 1980).
Table 1a Worldwide occurrence of fumonisin B1 (FB1) in maize-based products
Product Countries Detected / total FB1 (mg/kg)
North America
Maize Canada, USA 324/729 0.08-37.9
Maize flour, grits Canada, USA 73/87 0.05-6.32
Miscellaneous maize foodsb USA 66/162 0.004-1.21
Maize feed USA 586/684 0.1-330
Latin America
Maize Argentina, Uruguay, Brazil 126/138 0.17-27.05
Maize flour, alkali-treated
kernels, polenta Peru, Venezuela, Uruguay 5/17 0.07-0.66
Miscellaneous maize foodsb Uruguay, Texas-Mexico border 63/77 0.15-0.31
Maize feed Brazil, Uruguay 33/34 0.2-38.5
Europe
Maize Austria, Croatia, Germany, Hungary, Italy, Poland, 248/714 0.007-250
Portugal, Romania, Spain, United Kingdom
Maize flour, maize grits,
polenta, semolina Austria, Bulgaria, Czech Republic, France, Germany, 181/258 0.008-16
Italy, Netherlands, Spain, Switzerland, United Kingdom
Miscellaneous maize foodsb Czech Republic, France, Germany, Italy, Netherlands, 167/437 0.008-6.10
Spain, Sweden, Switzerland, United Kingdom
Imported maize, grits and
flour Germany, Netherlands, Switzerland 143/165 0.01-3.35
Maize feed France, Italy, Spain, Switzerland, United Kingdom 271/344 0.02-70
Table 1 (continued)
Product Countries Detected / total FB1 (mg/kg)
Africa
Maize Benin, Kenya, Malawi, Mozambique, South Africa, 199/260 0.02-117.5
Tanzania, Uganda, Zambia, Zimbabwe
Maize flour, grits Botswana, Egypt, Kenya, South Africa, Zambia, 73/90 0.05-3.63
Zimbabwe
Miscellaneous maize foodsb Botswana, South Africa 8/17 0.03-0.35
Maize feed South Africa 16/16 0.47-8.85
Asia
Maize China, Indonesia, Nepal, Philippines, Thailand, 361/614 0.01-155
Vietnam
Maize flour, grits, gluten China, India, Japan, Thailand, Vietnam 44/53 0.06-2.60
Miscellaneous maize foodsb Japan, Taiwan 52/199 0.07-2.39
Maize feed Korea, Thailand 10/34 0.05-1.59
Oceania
Maize Australia 67/70 0.3-40.6
Maize flour New Zealand 0/12 -
a This table is a summary of the information in Appendix 2
b Includes maize snacks, canned maize, frozen maize, extruded maize, bread, maize-extruded bread, biscuits, cereals, chips,
flakes, pastes, starch, sweet maize, infant foods, gruel, purée, noodles, popcorn, porridge, tortillas, tortilla chips,
masas, popped maize, soup, taco, tostada
There is a strong relationship between insect damage and Fusarium
kernel rot. A field survey demonstrated that the incidence of the
European corn borer increased F. verticillioides disease and
fumonisin concentrations (Lew et al., 1991). Disease incidence was
also shown to correlate to populations of thrips (Frankliniella
occidentalis) (Farrar & Davis, 1991). Hybrids with a thin kernel
pericarp were more susceptible to insect wounds, which allowed easier
access to the fungus (Hoenisch & Davis, 1994). Hybrids with an
increased propensity for kernel splitting had more disease (Odvody et
al., 1990). Kernel splitting is worse under drought conditions. Ears
infected by F. graminearum may be predisposed to
F. verticillioides infection and fumonisin accumulation (Schaafsma
et al., 1993). In maize ears inoculated one week after silk emergence
with F. verticillipodes fumonisins accumulated in the visibly
damaged (mouldy) kernels (Pascale et al., 1997; Desjardins et al.
1998). Sydenham et al. (1995) showed that in lightly contaminated
kernels FB1 was concentrated in the pericarp of the maize kernel.
A study of fumonisin occurrence in hybrids grown across the USA
maize belt indicated that hybrids grown outside their range of
adaptation had higher fumonisin concentrations (Shelby et al., 1994b),
again suggesting the important role of temperature stress. Data from
samples collected in Africa, Italy and Croatia also indicate fumonisin
accumulation in lines grown outside their area of adaptation (Doko et
al., 1995; Visconti, 1996). The occurrence of fumonisin in Ontario,
Canada (a cool maize-growing region) was limited to drought-stressed
fields (Miller et al., 1995).
Significant fumonisin accumulation in maize occurs when weather
conditions favour Fusarium kernel rot, and the severity of ear
infection has been found to be a good indicator of fumonisin
accumulation in maize ears artificially inoculated with
F. verticillioides (Pascale et al., 1997). Since monitoring began in
the USA, warm, dry years have greater concentrations than cooler years
(Murphy et al., 1993). The direct influence of low moisture and dry
weather on fumonisin accumulation could not be proven (Murphy et al.,
1996; Pascale et al., 1997), although maize grown under normal
conditions in cooler maize-growing areas is not significantly
contaminated by fumonisin (Doko et al., 1995; Miller et al., 1995).
Dry milling of maize results in the distribution of fumonisin
into the bran, germ and flour (Bullerman & Tsai, 1994). Fumonisin may
be present in beer where maize has been used as a wort additive (Scott
et al., 1995). Little degradation of fumonisin occurs during
fermentation and the fumonisins are found in the spent grain. No
toxins can be detected in the distilled ethanol (Bothast et al., 1992;
Scott et al., 1995; Bennett & Richard, 1996). Fumonisin is stable in
polenta (Pascale et al., 1995), whereas it is hydrolysed, and the
pericarp is removed, by nixtamalization, i.e. the treatment of
maize-based foods with calcium hydroxide and heat (Hendrich et al.,
1993). FB1 has been shown to form N-(carboxymethyl)-FB1 when
heated in the presence of reducing sugars (Howard et al. 1998), and
the latter substance has been detected in raw corn (Howard et al.,
1998).
FB1 is not significantly transferred into pork, chicken meat or
eggs (Prelusky et al., 1994, 1996a; Vudathala et al., 1994), but a
small amount accumulates in the liver and kidney of pigs as a function
of exposure (Prelusky et al., 1996b; see also section 6.2). Fumonisin
is not significantly transferred into milk from short-term dietary
exposure (Scott et al., 1994; Prelusky et al., 1996a), and FB1 was
found in only one of 165 samples of milk from Wisconsin, USA at a
level close to 5 ng/ml (Maragos & Richard, 1994).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Maize is the only commodity that contains significant amounts of
fumonisins. It is consumed either directly or processed into products
for human or animal consumption. Because fumonisins are known to be
heat stable (Dupuy et al., 1993a; Howard et al., 1998), light stable
(IARC, 1993), water soluble (US NTP, 1999), poorly absorbed, poorly
metabolized and rapidly excreted by animals (see sections 6.1 to 6.5),
most fumonisin will eventually end up being recycled into the
environment in a manner that will concentrate its spatial
distribution. The amount that enters the environment may be quite
large. For example, in the USA, maize production exceeds 200 million
tonnes per year. The concentration of FB1 and FB2 in field maize in
the USA often exceeds 1 g/tonne of maize (Murphy et al., 1993 and
Appendix 2). There is some evidence that fumonisins can be metabolized
by some microorganisms (Duvick et al., 1994, 1998). However, little is
known about the environmental fate of fumonisin after it is either
excreted or processed.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Table 1 summarizes the results of a number of surveys on the
natural occurrence of FB1 in maize and maize-based foods and feeds
(see Appendix 2 for more detail). The list is not exhaustive of the
surveys carried out worldwide as there is continual production of
similar data from every corner of the globe. Based on Table 1, 60% of
the 5211 samples analysed have been found to be contaminated with
FB1, the highest incidences of contamination being in Oceania (82% of
82 samples) and Africa (77% of 383 samples), followed by Latin America
(85% of 266 samples), North America (63% of 1662 samples), Europe (53%
of 1918 samples) and Asia (52% of 900 samples).
The data show that levels and incidence of contamination vary
considerably in relation to the commodities tested and the source. The
highest incidence was recorded in maize feeds (82% of 1112 samples),
followed by ground maize products, such as flour, grits, polenta,
semolina and gluten (73% of 517 samples), maize kernels (52% of 2525
samples) and miscellaneous maize foods (40% of 892 samples).
FB1 levels in animal feedstuffs can be exceptionally high, and
reached maximum values of 330, 70, 38, 9 and 2 mg/kg in North America
(USA), Europe (Italy), Latin America (Brazil), Africa (South Africa)
and Asia (Thailand), respectively. The majority of the highly
contaminated feeds were implicated in cases of equine
leukoencephalomalacia, porcine pulmonary oedema and other
mycotoxicoses.
In maize kernels available commercially or from experimental or
breeding stations, FB1 has been detected in 96% (of 70 samples), 91%
(of 138 samples), 76% (of 260 samples), 59% (of 614 samples), 44% (of
729 samples) and 35% (of 714 samples) of samples from Oceania, Latin
America, Africa, Asia, North America and Europe, respectively. Maximum
FB1 levels were 40.6 mg/kg (Australia), 27 mg/kg (Argentina),
117 mg/kg (South Africa), 155 mg/kg (China), 38 mg/kg (USA) and 250
mg/kg (Italy).
The list of commercial retail foods subject to fumonisin
contamination (Table 1) includes maize flour, grits, polenta,
semolina, maize snacks, cornflakes, sweet maize, canned maize, frozen
maize, extruded maize, bread, maize-extruded bread, biscuits, cereals,
chips, pastes, starch, infant foods, gruel, purée, noodles, popcorn,
porridge, tortillas, tortilla chips, masas, popped maize, soup, taco
and tostada.
Of these samples, the global incidence of contamination in
non-treated or minimally treated maize products (flour, grits,
polenta, semolina) was 73% out of 517 samples analysed. The highest
FB1 levels were recorded in Europe (16 mg/kg), followed by North
America (6.3 mg/kg), Africa (3.6 mg/kg), Asia (2.6 mg/kg) and Latin
America (0.7 mg/kg). In the remaining food products (892 samples) the
incidence of contamination was 40%, the highest level (6.1 mg/kg FB1)
being found in a sample of extruded maize from Italy. Generally
processed maize foods have lower levels and incidence of contamination
than non-treated maize. These differences might be the results of
dilution of maize in food commodities, or may depend on the
differences in maize cultivar or quality requirements for various
destinations.
Apart from maize and maize products, fumonisins have seldom been
found in other food products, such as rice (Abbas et al., 1998),
asparagus (Logrieco et al., 1998), beer (Torres et al., 1998) and
sorghum (Shetty & Bhat, 1997). Surveys on other cereals, such as
wheat, rye, barley and oats, did not show the occurrence of the toxin
(Meister et al., 1996).
Human exposure estimates have been made for fumonisins in several
countries, including Switzerland, Canada, South Africa, USA and the
Netherlands (Zoller et al., 1994; Contaminants Standards Monitoring
and Programs Branch, 1996a,b; Gelderblom et al., 1996b; Kuiper-Goodman
et al., 1996; Humphreys et al., 1997; Marasas, 1997; de Nijs, 1998).
Human exposure estimates of 0.017-0.089 µg/kg body weight per day have
been prepared for Canada for the period 1991 to early 1995
(Kuiper-Goodman et al., 1996). For the USA, a preliminary estimate of
human exposure to fumonisins for maize eaters was 0.08 µg/kg body
weight per day (Humphreys et al., 1997). The mean daily intake of
fumonisins in Switzerland is estimated to be 0.030 µg/kg body weight
per day (Zoller et al., 1994).
Based on the daily average intakes of maize and maize products of
3 g (general population average), 42 g (regular maize product eaters)
and 162 g (individuals with gluten intolerance) in the Netherlands,
the respective population groups had an estimated daily intake of 4,
57 and 220 µg FB1 per person, respectively, based on a mean FB1
content of 1.36 mg/kg maize produce. De Nijs et al. (1998a) estimated
conservatively that 97% of individuals with gluten intolerance had a
daily exposure of at least 1 µg FB1 and 37% at least 100 µg, while
the proportions of the general population exposed to these levels of
FB1 were 49% and 1%, respectively (de Nijs, 1998; de Nijs et al.,
1998a).
Thiel et al. (1992) estimated that human exposures in the
Transkei, South Africa, are 14 and 440 µg FB1/kg body weight per day
for healthy and mouldy corn, respectively. More recent estimates of
the probable daily intake (PDI) of South Africans are summarized in
Table 2. These vary from 1.2 to 355 µg/kg body weight per day in rural
blacks in Transkei consuming home-grown mouldy maize (Gelderblom et
al., 1996b; Marasas, 1997).
These exposure estimates will vary considerably according to the
source and extent of maize in the diet as well as the extent of
Fusarium kernel rot prevalent in the harvested crop.
Table 2. Probable daily intake of fumonisin in South Africaa
Product Country of No. of Mean FB1 + FB2 Probable daily intake
origin samples concentration (µg/kg body weight per day)
(µg/kg)
Rural population Urban population
Commercial maize South Africa 68 400 2.6 1.6
Commercial maize South Africa 209 300 2.0 1.2
Corn meal South Africa 52 200 1.3 0.8
Home-grown maize South Africab 18 7100 46.6 28.0
Home-grown maize South Africac 18 54 000 354.9 212.9
Imported maize USAd 1682 1100 7.2 4.3
Maize consumption
(g/70 kg body weight per day) 460 276
a From: Marasas (1997)
b Transkei, from individual farms in high oesophageal cancer area, healthy maize
c Transkei, from individual farms in high oesophageal cancer area, mouldy maize
d Imported in 1993
Occupational inhalation exposure could be a problem. In addition
to the presence of fumonisins in maize dust, FB1 is present in the
spores and mycelia of F. verticillioides (Tejada-Simon et al.,
1995). No data have been collected on airborne levels of fumonisin
during the harvesting, processing and handling of
fumonisin-contaminated maize.
6. KINETICS AND METABOLISM IN ANIMALS
There have been no reports on the kinetics and metabolism of
fumonisins in humans. Because fumonisins are known to be consumed by
farm animals and are the causative agent or a suspected contributing
factor in farm animal diseases, an effort has been made to understand
the kinetics and metabolism in cows, pigs and poultry. Thus, this
chapter will summarize results of studies on both laboratory and farm
animals.
To date, published studies with radiolabelled FB1 or FB2 have
been conducted with either [21,22-14C]fumonisins, biosynthesized
using L-[methyl-14C]methionine (Plattner & Shackelford, 1992; Alberts
et al., 1993), or [U-14C]FB1 labelled using [1,2-14C]acetate
(Blackwell et al., 1994). In these studies the final [14C]fumonisins
had a specific activity of < 1 mCi/mmol and radiochemical purity of
> 95%. Several studies have used unlabelled fumonisins with reported
purities ranging from 70% (Hopmans et al., 1997) to 98% (Prelusky et
al., 1996a).
Briefly, FB1 is: poorly absorbed when dosed orally; it is
rapidly eliminated from plasma or circulation and recovered in faeces;
biliary excretion is important; enterohepatic cycling is clearly
important in some animals; small amounts are excreted in urine; a
small but persistent (and biologically active) pool of [14C]label
appears to be retained in liver and kidney; and some is degraded to
partially hydrolysed FB1 in the gut of vervet monkeys. In a study
with FB2 in rats, the results were similar to those of FB1 (Shephard
et al., 1995b).
6.1 Absorption
There are no reports available of fumonisin absorption through
inhalation or dermal exposure. However, because fumonisins are present
in F. verticillioides cells (mycelia, spores and conidiophores)
(Tejada-Simon et al., 1995), there is a potential for absorption
through inhalation or buccal exposure. The risk from absorption due to
dermal exposure would seem slight, since fumonisins are very water
soluble and, typically, polar compounds do not easily penetrate the
undamaged skin (Flynn, 1985).
The quantity of FB1 detected in plasma after oral dosing in
pigs, laying hens, vervet monkeys, dairy cows and rats is very low. In
rats (BD IX, Sprague-Dawley or Wistar) administered [14C]FB1 orally,
accumulation of 14C-labelled compounds in tissues is also very low,
suggesting that absorption is very poor (negligible to < 4% of dose)
(Shephard et al., 1992a,b, 1994c; Norred et al., 1993). Similar
results indicating that fumonisins are poorly absorbed (2 to < 6% of
dose) have been reported in vervet monkeys, dairy cows and pigs
(Prelusky et al., 1994, 1995, 1996a,b; Shephard et al., 1994a,b). In
orally dosed laying hens and dairy cows, systemic absorption based on
plasma levels and accumulation of 14C-labelled compounds in tissues
has been estimated to be less than 1% of dose (Scott et al., 1994;
Vudathala et al., 1994; Prelusky et al., 1996a). A study using beef
cattle fed F. verticillioides culture material (corn grits)
containing FB1 plus FB2 (530 mg/kg) found that the majority of the
fumonisin dose was recovered unmetabolized in faeces, and only traces
were detected in blood and urine (Smith & Thakur, 1996). Following
single gavage doses of 1 or 5 mg/kg body weight to cows, no FB1 or
known metabolites could be found in the plasma, indicating no or very
limited bioavailability in ruminants (Prelusky et al., 1995). Rumen
metabolism may reduce the bioavailability of FB1 as the hydrolysed
form of FB1 comprised 60-90% of the total amount of FB1 found in
faeces. In non-ruminants the parent compound was the dominant species
present (Rice & Ross, 1994).
6.2 Distribution
In rats and pigs orally dosed with [14C]FB1, the 14C label is
distributed to most tissues, with the liver and kidney containing the
highest concentration of radiolabel (Shephard et. al., 1992b; Norred
et al., 1993; Prelusky et al., 1994, 1996a,b; Haschek et al., 1996).
Typically, the liver contains more 14C label than the kidney,
although in the study by Norred et al. (1993) the measured
radioactivity in the kidney was greater than in the liver. In chickens
and dairy cows the poor absorption of [14C]FB1 (< 1% of oral dose)
was reflected in the fact that only trace amounts of radioactivity
were recovered in tissues (Prelusky et al., 1996a), no residues were
recovered in eggs of laying hens (Vudathala et al., 1994) and no FB1
or aminopentol hydrolysis products were recovered in milk (Scott et
al., 1994; Prelusky et al., 1996a). In pregnant rats dosed
intravenously with [14C]fumonisin, approximately 14.5% and 4% of the
dose were recovered in the liver and kidney, respectively, after 1 h
(Voss et al., 1996a). Based on the known pharmacokinetics (Norred et
al., 1993) in the rat, 1-h exposure and intravenous injection were
chosen so as to optimize the presentation in blood of the [14C]FB1
to the placentae. In contrast to liver and kidney, the uteri contained
0.24 to 0.44%, individual placentae contained 0 to 0.04%, and total
fetal recovery was < 0.015% of dose/dam (Voss et al., 1996a).
Recent studies have confirmed the lack of placental transfer of FB1
in rats (Collins et al., 1998a,b) and rabbits (LaBorde et al., 1997).
FB1 inhibition of the enzyme sphinganine N-acyltransferase
results in a large increase in intercellular free sphinganine (Wang et
al., 1991; Yoo et al., 1992). In animal tissues the fumonisin-induced
increase in free sphinganine tends to parallel the distribution of
14C label reported in the studies cited above using [14C]FB1. For
example, relative to other tissues examined, liver and kidney in
rabbits, pigs and catfish showed the greatest increases in free
sphinganine following exposure of animals to fumonisins or consumption
of diets containing fumonisins (Goel et al., 1994; Gumprecht et al.,
1995). The free sphinganine concentration in tissues has been shown to
be an easily detectable biomarker for exposure to fumonisins (Riley et
al., 1994c), although it has not been validated as a biomarker in
humans.
6.3 Elimination, excretion and metabolic transformation
When [14C]FB1 is dosed by intraperitoneal or intravenous
injection in rats (BD IX, Sprague-Dawley or Wistar), initial
elimination (subsequent to the distribution phase) is rapid (half-life
of approximately 10-20 min) with little evidence of metabolism
(Shephard et al., 1992a,b, 1994c; Norred et al., 1993). In rats the
elimination kinetics based on intraperitoneal or intravenous dosing
are consistent with a one- (Shephard et al., 1992b) or two-compartment
model (Norred et al., 1993). Because FB1 is poorly absorbed from the
rat gastrointestinal tract and extensively distributed in rat tissues
(Norred et al., 1993), the tissue elimination kinetics following oral
dosing is not as easily described. In vervet monkeys, as in rats, the
14C label is widely distributed and rapidly eliminated (half-life of
40 min) after intravenous injection (Shephard et al., 1994a,b). The
elimination kinetics following oral dosing in a non-human primate has
not been determined. Following single intravenous injection of 0.05 or
0.20 mg FB1/kg body weight to cows, the toxin is cleared rapidly from
the blood. A two-compartment model (half-lives of < 2 and 15-18 min,
respectively) satisfactorily described the plasma kinetics. No toxin
could be detected 120 min after dosing. No known metabolites were
detected in the plasma (Prelusky et al., 1995).
In pigs, clearance of [14C]FB1 from blood following an
intravenous injection was best described by a 3-compartment model
(half-lives of 2.5, 10.5 and 183 min, respectively), and cannulation
of the bile duct (bile removed) resulted in a much more rapid
clearance (best described by a 2-compartment model). The effect of
bile removal was observed whether dosing was intravenous or
intragastric (Prelusky et al., 1994, 1996a). The half-life in pigs
dosed intragastrically without bile removal was determined to be 96
min (Prelusky et al., 1996a). The studies with pigs strongly support
the importance of enterohepatic circulation of FB1 in pigs. As in the
study with rats, the majority of 14C label dosed orally was recovered
in faeces (approximately 90%) with less than 1% recovered in urine
(Prelusky et al., 1994, 1996a). In the LLC-PK1 renal cell line,
uptake of [14C]FB1 reached an equilibrium concentration with the
extracellular [14C]FB1 concentration after 4 to 16 h, and kinetics
were indicative of a simple diffusion process (Riley & Yoo, 1995).
Efflux was rapid with a half-life of less than 5 min.
Following intravenous injection into rats, FB1 is excreted
unchanged in bile (Norred et al., 1993; Shephard et al., 1994c). In
vervet monkeys there is evidence of metabolism to partially hydrolysed
(one propane tricarboxylic acid residue removed) FB1, and to a much
lesser extent the fully hydrolysed (both propane tricarboxylic acid
residues removed) aminopentol backbone, in faeces while in urine 96%
of the 14C label was recovered as FB1 (Shephard et al., 1994a,b).
Metabolism was most likely occurring in the gut since partially
hydrolysed and fully hydrolysed FB1 were recovered in the faeces but
not in the bile of vervet monkeys (Shephard et al., 1995a). Because
hydrolysed FB1 and FB1-fructose adduct can be formed during
processing, Hopmans et al. (1997) evaluated the excretion of these
products and FB1 in Fischer-344 rats. Based on the amount of each
FB1-related compound recovered in urine and faeces, it was concluded
that hydrolysed FB1 and the FB1-fructose adduct were better absorbed
than FB1 (Hopmans et al., 1997).
Dairy cows dosed with pure FB1 either orally (1.0 and 5.0 mg
FB1/kg body weight) or by intravenous injection (0.05 and 0.20 mg
FB1/kg body weight) showed no detectable residues of FB1, AP1 (the
aminopentol hydrolysis product of FB1) or their conjugates in the
milk (Scott et al. 1994). FB1 does not react with monoamine or
diamine oxidase (Murphy et al., 1996). In vitro studies using rat
primary hepatocytes and microsomal preparations (Cawood et al., 1994)
or studies with the LLC-PK1 renal epithelial cell line (Riley & Yoo,
1995) indicated that there was no metabolism of FB1 in these systems.
Repeated intraperitoneal injection of FB1 resulted in induction
of cytochrome P-4501A1 and P-4504A1 activities (Martinez-Larrañaga et
al., 1996). However there is no evidence that fumonisin is metabolized
by P-450 enzymes. Whether or not the induction was due to a direct
interaction between fumonisins and the metabolizing systems could not
be determined. However, it has been shown that some of the same
sphingolipid metabolites that are altered in fumonisin-treated animals
also mediate the cytokine-induced alterations in P-4502C11 in rat
hepatocytes (Nikolova-Karakashian et al., 1997).
6.4 Retention and turnover
[14C]FB1 is widely distributed in tissues of the rat and pig.
However, only the liver and kidney retain small but persistent amounts
of 14C label based on measured radioactivity (Norred et al., 1993;
Prelusky et al., 1994, 1996b). In rats given three repeated oral
doses, once accumulated, the measured radioactivity in liver and
kidney remained unchanged for at least 72 h after the last
intragastric dose (Norred et al., 1993). In pigs, it was estimated
that exposure to dietary FB1 at 2-3 mg/kg in feed would require a
withdrawal period of at least 2 weeks for the 14C label to be
eliminated from the liver and kidney (Prelusky et al., 1996b). The
chemical nature of the 14C-labelled material retained in liver and
kidney was primarily FB1.
In vitro studies with rat primary hepatocytes and the cultured
kidney cell line LLC-PK1 also indicate that a low but persistent pool
of 14C-labelled material is retained inside cells long after the
rapidly diffusible pool of [14C]fumonisin has exited the cells
(Cawood et al., 1994; Riley et al., 1998). This retained pool appears
to be capable of maintaining the elevation of cellular (LLC-PK1
cells) and urinary (in rats) free sphingoid base concentration, a
biomarker of fumonisin exposure (Solfrizzo et al. 1997b; Riley et al.,
1998; Wang et al., 1999).
6.5 Reaction with body components
Fumonisins are potent inhibitors of the enzyme sphinganine
(sphingosine) N-acyltransferase in the de novo sphingolipid
biosynthesis and sphingolipid turnover pathways (Wang et al., 1991).
The consequences of this reaction will be discussed in sections 7.8
and 7.9. FB1 may also interact directly with protein kinase C (Yeung
et al., 1996) and/or with mitogen-activated protein kinases
(Wattenberg et al., 1996). The only other information concerning
reaction with body components is that FB1 does not bind strongly to
chicken plasma proteins (Vudathala et al., 1994).
Cytotoxicity studies in primary rat hepatocytes and binding
studies using subcellular fractions indicated that 14C-labelled FB1
binds tightly to hepatocytes and microsomal and plasma membrane
fractions (Cawood et al. 1994). FB1 has been shown to interact
directly with liposomes (Yin et al., 1996). Since fumonisins are water
soluble, are not accumulated and are rapidly eliminated, the
toxicological significance of this finding is unclear.
7. EFFECTS ON ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Laboratory animals and in vitro test systems
The studies described below used either purified FB1, naturally
contaminated corn or cultures of Fusarium. It is generally accepted
that the in vivo toxicity of Fusarium verticillioides MRC 826
culture material is the result of its high FB1 content. Culture
materials other than MRC 826 may contain several other products such
as other fumonisins, fusarins, moniliformin and beauvericin.
7.1.1 Single exposure
In the male Sprague-Dawley rat, intravenous injection of FB1
(95% purity) at 1.25 mg/kg body weight resulted in renal lesions
localized to the tubules in the outer medulla and consisted of both
proliferation and death of cells. An increased number of mitotic
figures, stained with 5-bromo-2'-deoxyuridine (not quantified), and
apoptosis followed by severe nephrosis were observed (Lim et al.,
1996). Cell proliferation was also detected in the liver 24 h after
dosing, but was not significantly different from control values at
later times (day 2 to day 5). In the oesophagus, increased cell
proliferation was measured on day 3, but this returned to the control
level on day 5. While kidney lesions were reported as severe, the
increased mitotic activity in the liver and oesophagus occurred in the
absence of morphological injury (Lim et al., 1996).
No information is available on the toxicological effects of
single exposure to FB1 by the inhalation or dermal route.
7.1.2 Repeated exposure
7.1.2.1 Body weight loss
In male BD IX rats consuming a diet containing 1 g FB1/kg during
a 4-week promotion treatment, the mean body weights were 50% lower
than those of non-treated rats ( P < 0.0001), both with and without
initiation with diethylnitrosamine (DEN) (Gelderblom et al., 1988).
Similarly, the body weight gains of male Fischer rats fed the same
concentration of FB1 over a 26-day initiation period were 80% lower
than those of the controls ( P < 0.0025) (Gelderblom et al., 1992b).
Male Fischer rats fed diets containing 1 g FB1 (and FB2 plus
FB3) per kg over a 21-day initiating period started to lose weight
within the first week, and the level of the FB compounds had to be
reduced by half (Gelderblom et al., 1993). Body weight losses were
first observed in rats fed FB2, where a significant ( P = 0.008)
reduction compared to the controls was recorded after 4-5 days. In the
case of FB1 and FB3, significant ( P = 0.01) reductions in body
weight occurred after 7-8 days. Body weight loss induced by FB1 and
FB2 was significantly ( P = 0.001) higher than that induced by FB3
(Gelderblom et al., 1993). In female Sprague-Dawley rats administered
purified FB1 at gavage doses of 0, 1, 5, 15, 35 or 75 mg FB1/kg body
weight per day for 11 consecutive days, significant depression of body
weight and food consumption was observed at 35 and 75 mg FB1/kg body
weight per day (Bondy et al., 1998).
The reduction in body weight gain of male Fischer rats induced by
FB1 is apparently due to a feed refusal effect (Gelderblom et al.,
1994). During a feeding study over 21 days, the body weight gains of
rats receiving 750, 500, 250 and 100 mg FB1/kg diet were
significantly (0.01 < P < 0.05) lower than those of the controls
as well as those of rats receiving 50 and 25 mg/kg. Based on the
weekly feed intake profiles, the reduction in body weight gain was
accompanied by a concomitant reduction in feed intake. The reduction
in feed intake was overcome after the second week, resulting in a feed
intake similar to that of the controls at the end of the 21-day
initiating treatment (Gelderblom et al., 1994).
In male Fischer-344/N Nctr BR rats, exposure to 234 and 484 mg
FB1/kg diet resulted in 10% and 17%, respectively, less gain in body
weight after 28 days of feeding in the range-finding study by the US
National Toxicology Program (US NTP, 1999). Female rats had decreased
body weight only at 484 mg FB1/kg diet.
In the NTP 2-year carcinogenicity study (US NTP, 1999) (see
section 7.1.6.1), there was no difference in body weight or feed
consumption in male or female Fischer-344/N Nctr BR rats or
B6C3F1/Nctr BR mice fed FB1 when compared to rats or mice on control
diets.
The characteristic reduction in the body weight of rats induced
by FB1, was also induced by FB2, FB3 and the monomethyl esters of
FB1 (MME, an artefact of the isolation procedure of FB1 and a minor
contaminant of FB1 preparations), and to a much lesser extent by the
N-acetylated analogue FA1, but not by the aminopolyol hydrolysis
products AP1 and AP2 or the tricarbalyllic acid moiety (TCA)
(Gelderblom et al., 1993).
7.1.2.2 Hepatotoxicity and nephrotoxicity
The acute toxicity of FB1 was tested by dosing four male BD IX
rats orally with 240 mg FB1/kg body weight per day (Gelderblom et
al., 1988). Three of the four rats died within 3 days and exhibited
toxic hepatosis characterized by scattered single-cell necrosis
accompanied by mild fatty changes, hydropic (i.e., the abnormal
accumulation of serous fluid in the cellular tissue or in a body
cavity) degeneration and hyaline droplet degeneration. Hepatocellular
nuclei varied in size and some were markedly enlarged. In addition to
the hepatotoxic changes, fatty changes and scant necrosis were present
in the proximal convoluted tubules of the kidney, prominent lymphoid
necrosis was observed in Peyer's patches, and severe disseminated
acute myocardial necrosis and severe pulmonary oedema were observed in
two of the rats (Gelderblom et al., 1988).
In a separate experiment, male BD IX rats were dosed orally with
48 mg FB1/kg body weight per day for 12 days, followed by 70 mg
FB1/kg body weight per day for the remaining 9 days of the experiment
(Gelderblom et al., 1988). In the rats killed after 21 days, chronic
toxic hepatosis was present and characterized by marked hydropic
degeneration, single-cell necrosis and a few hyaline droplets, early
bile duct proliferation and fibrosis, and enlargement of
hepatocellular nuclei (Gelderblom et al., 1988).
In the livers of rats killed after 33 days on a diet containing
1 g FB1/kg, the hepatic changes were similar to those described
above, but more advanced (Gelderblom et al., 1988). The proliferation
of bile ducts and fibrosis caused distortion of the lobular structure
of the liver and, together with the development of hyperplastic
nodules, gave the liver a distinctly nodular appearance. The authors
reported that many nuclei were enlarged in hepatic cells and numerous
mitotic figures, some of which were abnormal, were present. The
lesions in the kidneys were similar, but less severe, than those seen
in the rats that died within 3 days (Gelderblom et al., 1988).
In male Fischer rats fed a diet containing 1 g FB1 (90-95% pure)
per kg during an initiating period of 26 days, followed by partial
hepatectomy and a promoting regimen of 2-acetyl-aminofluorene (2-AAF)
and carbon tetrachloride, early pathological changes in the liver were
very similar to those described above (Gelderblom et al., 1992b).
Early hepatocyte nodules were evident as discrete focal changes in
hepatocytes characterized by somewhat bigger cells that displayed more
mitotic figures than the cells in the surrounding liver and also
showed vacuolization. Another prominent pathological feature was the
mild-to-moderate proliferation of bile ducts (Gelderblom et al.,
1992b). Similar hepatic changes have been described in male Fischer
rats fed diets containing, at a level of 0.5-1 g/kg, FB1, FB2, FB3
and MME during an initiating period of 21 days followed by a promoting
treatment of 2-AAF and partial hepatectomy (Gelderblom et al., 1993).
The short-term toxicological effects in rats of FB2 and FB3 are
similar to those of FB1 (Gelderblom et al., 1992a).
Changes including hydropic swelling, hyaline droplet
accumulation, single-cell necrosis, increased mitotic figures, lipid
accumulation, fibrosis, and bile duct proliferation were also observed
in the liver of male Fischer rats that died after gavage treatment
with 50 mg FB1/kg body weight in 6 dosages over 11 days (Gelderblom
et al., 1994).
A 4-week exposure of Sprague-Dawley rats to aqueous extracts of
Fusarium verticillioides (MRC 826) cultures (containing fumonisins)
resulted in decreased body weights, increased serum alanine and
aspartate aminotransferase and alkaline phosphatase activities,
decreased relative liver weights and microscopic liver lesions in rats
(Voss et al., 1990).
Male and female Sprague-Dawley rats (3 of each sex per group)
were fed diets containing 0, 15, 50 and 150 mg/kg of FB1 (> 99%
pure) for 4 weeks (Voss et al., 1993). No significant differences in
weight gain or food consumption were found, but significant increases
in serum triglycerides, cholesterol and alkaline phosphatase confirmed
that a dietary level of 150 mg/kg was hepatotoxic to both sexes.
Histopathological changes in the liver of these rats were
characterized by scattered single-cell hepatocellular necrosis,
variability in nuclear size and staining and hepatocellular
cytoplasmic vacuolation. Nephrosis, consisting of focal cortical
proximal tubular epithelial basophilia, hyperplasia and single cell
necrosis or pyknosis, was found in males fed > 15 mg/kg and in
females fed > 50 mg/kg (Voss et al., 1993). The incidence and
severity of ultrastructural alterations in kidney and liver were
closely correlated with increased sphinganine concentration in
tissues, serum and urine (Riley et al., 1994a).
The apparent no-observed-effect level (NOEL) for renal toxicity
in FB1-fed rats was less than the NOEL for hepatic effects (4.1
< NOEL < 13.6 mg/kg diet for 28 days), and renal toxicity was more
severe in males (NOEL < 1.4 mg/kg diet for 28 days) than females
(1.4 < NOEL < 4.1 mg/kg diet for 28 days). Furthermore, liver
lesions found in females appeared (subjectively) more advanced than
those found in males. The results of subacute toxicity studies (7.5
and 10 mg/kg body weight per day for 4 days) (Bondy et al., 1995;
Suzuki et al., 1995) and of an independent (Tolleson et al., 1996a)
4-week study in Fischer-344 rats fed 0, 99, 163, 234 or 484 mg FB1/kg
diet corroborated the findings of nephrotoxicity by Voss et al.
(1993). Hepatopathy of the same type was found in males fed > 234
mg/kg diet and females fed > 163 mg/kg diet. Nephropathy was found
in males from all FB1-fed groups and in females fed > 163 mg/kg
diet (Tolleson et al., 1996a). Apoptotic hepatocytes and renal
proximal tubule epithelial cells were accompanied by cell
proliferation in Fischer-344 rats, suggesting that fumonisin induces
or accelerates programmed cell death in both liver and kidney
(Tolleson et al. 1996a; US NTP, 1999).
In male and female B6C3F1 mice administered FB1 at gavage doses
ranging from 1 to 75 mg FB1/kg body weight per day for 14 days,
effects on liver, bone marrow, adrenals and kidneys were observed. In
general, however, the degree of change observed indicates that mice
are not as sensitive to FB1 toxicity as rats (Bondy et al., 1995,
1997).
In B6C3F1 mice fed 99 to 484 mg FB1/kg diet for 4 weeks, the
liver, not the kidney, was the target organ (US NTP, 1999). As for
rats, the NOEL was lower in females as liver lesions were found in the
females of all FB1-fed groups, while in males hepatopathy was
confined to the highest dose group. In male BALB/c mice dosed
subcutaneously (0.25 to 6.25 mg FB1/kg body weight per day), a
dose-dependent increase in apoptosis was observed in both liver and
kidney (Sharma et al., 1997).
To obtain dose-response data under longer-term exposure
conditions, Fischer-344 rats and B6C3F1 mice were fed diets
containing 0, 1, 3, 9, 27 or 81 mg FB1/kg diet for 13 weeks (Voss et
al., 1995). In rats, toxicity was confined to the kidneys. Lesions of
the proximal tubule located in the outer medulla (sometimes referred
to as the corticomedullary junction) were found in males fed > 9
mg/kg diet and in females fed 81 mg/kg diet. Qualitatively these
lesions were of the same type as those found in the 4-week study (Voss
et al., 1993). No differences in the incidence or severity of
nephropathy between rats examined after 4 (n = 5 rats/group) or 13
(n = 10/group) weeks were found.
Renal lesions were accompanied by decreased relative kidney
weight (as a percentage of body weight), which was found in males fed
> 27 mg/kg diet for 4 weeks and in both sexes fed > 9 mg/kg diet
for 13 weeks. Serum creatinine was increased after 13, but not 4,
weeks in males fed > 27 mg/kg diet and in females fed 81 mg/kg diet
(Voss et al. 1995).
In mice, hepatopathy and serum chemical evidence of liver
dysfunction were found after 13 weeks in females fed 81 mg FB1/kg
diet (Voss et al., 1995). Liver lesions in female mice were primarily
centrilobular, although some midzonal involvement and apparent
"bridging" between adjacent central areas were evident. Single cell
hepatocyte necrosis, cytomegaly, increased numbers of mitotic figures,
some mixed infiltration of neutrophils and macrophages were present
and, in more advanced lesions, the loss of hepatocytes caused an
apparent collapse around the central vein. Hepatopathy was not found
in male mice and FB1-related kidney lesions did not occur in either
sex. A few macrophages containing minimal to mild amounts of
cytoplasmic pigment, presumably ceroid, were also found in the adrenal
cortex of high-dose (81 mg/kg diet) females only.
Taken together, the findings from 4-week and 90-day toxicity
studies in rats and mice (Voss et al., 1993, 1995; Tolleson et al.,
1996a; US NTP, 1999) indicate that the liver is a target organ in both
species, and the data seem to indicate that females exhibit hepatic
effects at lower doses than males. In rats, however, the kidney is
also an important target organ and, in contrast to liver, the males
were affected at lower doses.
7.1.2.3 Immunotoxicity
There have been very few studies that address directly the
potential for fumonisins to modify immune response in vivo.
Nonetheless, there are many studies with fumonisins or
fumonisin-containing diets that show either altered function of blood
cells in vitro or changes in haematological parameters in vivo.
Fumonisins are inhibitors of ceramide synthase (see section 7.3) and
ceramide and glycosphingolipids are important signalling molecules and
recognition sites in the cellular immune response and attachment sites
for many infectious agents and microbial toxins (Ballou et al., 1996;
Merrill et al., 1997a).
In a study with pure FB1, changes in selected haematological
parameters in pigs were reported at dietary levels as low as 1 mg/kg
(Rotter et al., 1996). Consumption of culture-material diets (MRC 826)
containing fumonisins decreased the ability to clear Pseudomonas
aeruginosa and inhibited pulmonary interstitial macrophage function
(Smith et al., 1996c). It was hypothesized that pulmonary
intravascular macrophage (PIM) dysfunction could contribute to
increase susceptibility to microbial diseases (Smith et al., 1996c).
Cytokine production has been shown to be modified by exposure to
fumonisin. For example, serum tumour necrosis factor-alpha
(TNF-alpha)-like activity was increased in pigs fed culture material
(M 1325 = MRC 826) containing 150 mg/kg fumonisins (Guzman et al.,
1997). Fumonisin-induced changes in the TNF pathway have also been
seen in lipopolysaccharide (LPS)-stimulated macrophages collected from
BALB/c mice dosed with pure FB1 (Dugyala et al., 1998).
Immunosuppression in chickens was produced in birds fed maize
cultured with F. verticillioides (MRC 826) (Marijanovic et al.,
1991). Broiler chicks fed diets containing 10 mg pure FB1/kg diet, or
diets formulated from Fusarium verticillioides (MRC 826) culture
material to contain 30 to 300 mg FB1/kg diet, had reduced spleen
and/or bursa weights and altered haematological parameters (Espada et
al., 1994, 1997).
In male and female rats (10 rats/group) gavaged daily for 14 days
with doses of 0, 5, 15 or 25 mg FB1/kg body weight per day, a
significant dose-related linear trend toward decreased plaque-forming
cell number per 106 spleen mononuclear leukocytes (PFC per 106
splenocytes) ( P = 0.003) and PFC per spleen cells ( P = 0.001) was
observed in the male rats. However, the PFC numbers in female rats
were not affected significantly by treatment ( P > 0.05) (Tryphonas
et al., 1997).
7.1.3 Skin and eye irritation
No information is available on the effects of FB1 on skin and
eye irritation and/or sensitization.
7.1.4 Reproductive toxicity, embryotoxicity and teratogenicity
Concern about the reproductive and developmental effects of
fumonisins originated with: (a) the observation of abortions in
pregnant sows fed fumonisin-contaminated diets (Harrison et al.,
1990); (b) the suggestion that a cluster of birth defects among
residents in Brownsville, Texas, USA (Hendricks, 1999) might be
associated with consumption of maize from the 1989 maize crop; (c) the
association of "mystery swine disease" with fumonisin-contaminated
maize (Bane et al., 1992); and (d) the discovery that fumonisins are
inhibitors of sphingolipid biosynthesis (Wang et al., 1991). Currently
there are no data to support the conclusion that consumption of
fumonisins is a developmental or reproductive toxicant in farm animals
or humans. There are also no data demonstrating that fumonisin
consumption results in transfer to chicken eggs (Vudathala et al.,
1994; Prelusky et al., 1996a) or that it crosses the placenta in rats
(Voss et al., 1996a; Collins et al., 1998a,b), mice (Reddy et al.,
1996) or rabbits (LaBorde et al., 1997).
Injection of purified FB1 into fertile chicken eggs resulted in
time- and dose-dependent embryopathic and embryocidal effects (Javed
et al., 1993b). Embryonic changes included hydrocephalus, enlarged
beaks and elongated necks. Pathological changes were noted in most
organ systems. At the low FB1 dose (1 µM = 0.72 µg/ml), stimulation
of chick embryo development was observed. Stimulated embryo
development in vitro in pre-somite rat embryos exposed to
0.5-1 µg/ml of hydrolysed FB1 has been reported in an abstract (Flynn
et al., 1994). Higher concentrations of fully hydrolysed FB1 (Flynn
et al., 1997) and all concentrations of FB1 > 0.2 µg/ml inhibited
growth and development of pre-somite rat embryos in vitro (Flynn et
al., 1994, 1996). Johnson et al. (1993) reported that FB1 was a weak
developmental toxin to organogenesis stage rat embryos (day 10.5;
lowest-observed-effect level = 0.5 mM). FB1 (> 2.5 mM = 1.8 µg/ml)
inhibited reaggregation and growth of chicken embryo neural retina
cells, a commonly used in vitro assay for screening potential
developmental toxins (Bradlaw et al., 1994). Bacon et al. (1995) found
effects of FB1 in fertile chicken eggs similar to those reported by
Javed et al. (1993b). In addition it was found that co-injection of
fusaric acid and FB1 resulted in a synergistic toxic response (Bacon
et al., 1995). Zacharias et al. (1996) found that morphological
changes, due to direct administration of FB1 to chick embryos, were
correlated with inhibition of glycosphingolipid biosynthesis.
Syrian hamsters orally gavaged with aqueous extracts of
F. verticillioides (M 1325 = MRC 826) culture material containing
fumonisins (0.25-18 mg FB1/kg body weight) or pure FB1 (12 mg/kg and
18 mg/kg) did not exhibit maternal toxicity based on weight gain,
serum aspartate aminotransferase activity or total bilirubin.
Histological examination of liver, kidney and placenta did not reveal
important changes, although mild karyomegalic changes in liver were
observed in the hamsters dosed with either aqueous extracts or pure
FB1 at > 6 mg FB1/kg body weight (Floss et al., 1994a,b). When
aqueous extracts were given by oral gavage from day 8 to day 10 or 12
of gestation, there appeared to be an increase in the number of fetal
deaths, but statistical significance was not achieved (Floss et al.,
1994a). Relative to controls, statistically significant increases in
fetal deaths occurred only in the hamsters given 18 mg FB1/kg body
weight (aqueous culture extracts and pure material) (Floss et al.,
1994b). Prenatal exposure to aqueous culture extracts containing
fumonisins or to pure FB1 were detrimental to fetal hamster
survivability in the absence of maternal toxicity (Floss et al.,
1994a,b; Penner et al. 1998).
In Fischer-344/N rats dosed orally from day 8 to 12 of gestation
with 30 or 60 mg purified FB1/kg body weight, the high dose
significantly suppressed growth and fetal bone development while an
extract of F. proliferatum (M 5991) in corn culture did not
(Lebepe-Mazur et al., 1995a). Voss et al. (1996a) formulated diets
using F. verticillioides (MRC 826) culture material to provide 0, 1,
10 or 55 mg FB1/kg diet. Based on consumption, the diet containing
55 mg/kg provided about 3 to 4 mg FB1/kg body weight per day to the
dams. The diets were fed to male and female Sprague-Dawley rats prior
to and during the mating, gestational and lactational phases of the
study. Nephropathy was observed in males and females fed diets
containing > 10 mg/kg and 55 mg/kg, respectively. No statistically
significant reproductive effects were observed in any of the males or
females, and no developmental effects were found in fetuses during any
phase of the study. Litter weight gains in the 10 and 55 mg/kg diet
groups were slightly decreased. Increased levels of free sphinganine,
a biomarker for fumonisin exposure, were demonstrated in the livers of
dams in the 55 mg/kg diet group on gestation day 15. In contrast, no
increase in the sphinganine/sphingosine (Sa/So) ratio was observed in
fetuses at that time, suggesting that fetuses were not exposed
in utero to FB1. This finding was supported by the study in which
an intravenous injection of [14C]FB1 was given to dams on gestation
day 15. Radiolabel was easily detected in tissues of pregnant females
but was not detected in their fetuses. Culture material containing
fumonisins, and by inference FB1, did not have reproductive effects
at doses that were minimally toxic (Voss et al., 1996a). These
findings have been recently confirmed (Sa/So ratios in fetuses were
not affected and FB1 was not teratogenic at the doses tested) in a
Charles River CD rats (Collins et al., 1998a,b).
Gross et al. (1994) gavaged pregnant CD1 mice daily between
gestation days 7 and 15 with a diet containing partially purified FB1
extracted from F. verticillioides (M 1325 = MRC 826) culture
material. Maternal toxicity and fetal developmental abnormalities
(e.g., hydrocephalus, digital and sternal ossification) occurred at
FB1 dosages greater than 12.5 mg/kg body weight per day. Similar
results were obtained in a second study using purified FB1 (Reddy et
al., 1996). As in the study by Voss et al. (1996b), the Sa/So ratio
was significantly increased in maternal liver but not in fetal liver,
suggesting that developmental effects were mediated through maternal
toxicity (Reddy et al., 1996).
Unlike CD1 mice and Syrian hamsters, pregnant New Zealand white
rabbits are very sensitive to the toxic effects of FB1 (LaBorde et
al., 1997). Maternal toxicity was observed at daily gavage dosages (in
water) as low as 0.25 mg/kg body weight from gestational day 3 to
gestational day 19. Compared to controls there was no increase in
fetal loss or in gross visceral or skeletal abnormalities, and no
decrease in fetal weight or fetal organ weight at any dosage (0 to
1.75 mg/kg body weight) (LaBorde et al., 1997). The maternal kidney,
serum and urine Sa/So ratios were increased, but there were no
increases in these ratios in fetal liver, brain or kidney (LaBorde et
al., 1997). While FB1 is toxic in the pregnant dam, it is not a
developmental toxin but is maternally toxic in rabbits (LaBorde et
al., 1997). However, the lowest-observed-effect level for maternal
toxicity was 0.1 mg FB1/kg body weight, which is equivalent to a
calculated dietary fumonisin level of 2.3 mg/kg diet (LaBorde et al.,
1997). Thus, in sensitive species, maternal toxicity and consequent
fetal toxicity could occur at low dosages of FB1.
There is currently no evidence of neonatal toxicity. However,
average mean litter weights were reduced in litters from
Sprague-Dawley dams fed F. verticillioides (MRC 826) culture
material containing 10 or 55 mg FB1/kg (Voss et al., 1996a). The
Sa/So ratio was increased in litters at lactation day 21. However,
given the likelihood that offspring had consumed the contaminated
diets (Voss et al., 1996a), the authors could not ascertain the route
of exposure (via milk or diet). Reduced weights and several
alterations in haematological parameters were reported in mink kits
lactationally exposed to fumonisins (Powell et al., 1996).
No FB1 was detected in the milk of lactating sows fed diets
containing non-lethal levels of FB1 and there was no evidence of
toxicosis in their suckling pigs (Becker et al., 1995). However, in a
study with lactating cows administered FB1 intravenously, the
carry-over rate of FB1 into the milk reached a maximum of 0.11%
(Hammer et al., 1996), while in other studies no fumonisins were
detected in cow's milk (Scott et al., 1994; Richard et al., 1996). In
a reproductive study with mink, fumonisins were detected in the milk
at 0.7% of the dietary fumonisin concentrations (Powell et al., 1996).
The question of neonatal toxicity is of concern since neonates
may be more sensitive to fumonisins than adults. For example, a recent
report by Kwon et al. (1997b) indicated that subcutaneous injection of
FB1 in neonatal rats caused elevation in the Sa/So ratio in brain
tissue and reduced myelin deposition. The elevated sphinganine level
was determined to be the result of a direct effect on the neonate
brain, indicating that FB1 can cross the blood-brain barrier (Kwon et
al., 1997a). When maternal toxicity was minimal, there was little or
no evidence of neonatal toxicity in rats (Ferguson et al., 1997).
7.1.5 Mutagenicity and related end-points
The fumonisins FB1, FB2 and FB3 (98, 98, 90% pure,
respectively) were non-mutagenic in the Salmonella assay against the
tester strains TA97a, TA98, TA100 and TA102, in both the presence and
absence of the S-9 microsomal preparation (Gelderblom & Snyman, 1991).
The non-mutagenicity of FB1 (approximately 90% pure) to Salmonella
tester strain TA100 at concentrations up to 100 mg/plate was confirmed
by Park et al. (1992). Similarly negative results were reported with
FB1 in Salmonella TA98 and TA100, as well as in SOS chromotest in
E. coli PQ37 and differential DNA repair assays with E. coli K12
strains (343/753, uvrB/ recA and 343/765, uvr+ rec+)
(Knasmüller et al., 1997). In contrast, Sun & Stahr (1993), using a
commercial bioluminescent bacterial (Vibrio fischeri) genotoxicity
test, reported that FB1 showed in the concentration range 5-20 µg/ml
genotoxic activity without the metabolic activation of S-9 fraction.
FB1 (and FB2) were non-genotoxic in the in vitro rat
hepatocyte DNA repair assay at concentrations ranging from 0.04 to 80
µM (and FB2 from 0.04 to 40 µM) as well as in the in vivo assay at
a dose of 100 mg/kg body weight administered by gavage (FB1 or FB2)
(Gelderblom et al., 1989, 1992b). The finding that FB1 does not
induce unscheduled DNA synthesis was confirmed in the in vitro assay
in primary rat hepatocytes at concentrations ranging from 0.5 to 250
µM (Norred et al., 1992a).
FB1 induced DNA strand breaks in isolated rat liver nuclei (Sahu
et al., 1998).
FB1 induced a moderate increase in the micronucleus frequency in
primary rat hepatocytes at concentrations ranging from 0.01 to
1 µg/ml. No concentration-dependent increase of micronuclei occurred.
A significant concentration-dependent increase in chromosomal
aberrations was observed in isolated hepatocytes exposed to FB1 at
concentrations ranging from 1 to 100 µg/ml (Knasmüller et al., 1997).
FB1 induced lipid peroxidation in isolated rat liver nuclei at
concentrations ranging from 40 to 300 µM (Sahu et al., 1998). It also
increased significantly the level of thiobarbituric-acid-reactive
substances in vitro in primary rat hepatocytes at concentrations of
75 and 150 µM and in vivo in the liver of rats fed a dietary FB1
level of 250 or 500 mg/kg for 21 days (Abel & Gelderblom, 1998).
Single gavage doses of 50, 100 and 200 mg FB1/kg body weight
significantly ( P = 0.05) inhibited hepatocyte proliferation as
measured by the incorporation of radiolabelled thymidine into DNA in
partially hepatectomized male Fischer rats (Gelderblom et al., 1994).
Inhibition of hepatocyte proliferation was also observed after dietary
exposure to FB1 (> 50 mg/kg diet) (Gelderblom et al., 1996c). FB1
also inhibited DNA synthesis induced by epidermal growth factor in
primary rat hepatocytes (Gelderblom et al., 1995).
In BALB/3T3 A31-1-1 mouse embryo cells, FB1 (90% pure) treatment
produced transforming activity at 500 µg/ml but not at lower or higher
concentrations (Sheu et al., 1996).
7.1.6 Carcinogenicity
7.1.6.1 Carcinogenicity bioassays
When inbred BD IX rats were fed commercial diet containing
freeze-dried or oven-dried culture material inoculated with
F. verticillioides MRC 826 for 2 years, the incidence of liver
tumours (hepatocellular and cholangiocellular carcinomas combined) was
increased (control: 0/20, freeze-dried 13/20, oven-dried, 16/20)
(Marasas et al, 1984b). When 30 rats of the same inbred strain were
given F. verticillioides MR 826 (containing fusarin C and later
found to produce FB1 and FB2) for 23-27 months, two hepatocellular
and eight cholangiocellular cancers were observed. In addition,
neoplastic hepatic nodules were observed in all surviving 21 animals,
but none among the controls. However, in rats similarly administered
F. verticillioides MRC 1069 culture material containing 104 mg/kg
fusarin C (but suspected of being low in FB1), no increase in hepatic
carcinomas was observed (Jaskiewicz et al., 1987b).
Maize from a field outbreak of equine leukoencephalomalacia in
the USA, shown to be naturally contaminated with
F. erticillioides, was fed to 12 male Fischer-344 rats and
commercial rodent feed to 12 controls by Wilson et al. (1985). All
treated rats necropsied from 123 to 176 days had multiple hepatic
neoplastic nodules, adenofibrosis and cholangiocarcinoma, whereas no
such lesions were found in the controls. The authors considered these
lesions in the livers of male Fischer-344 rats to be similar to those
described in male BD IX rats by Marasas et al. (1984b). The fact that
the lesions observed by Wilson et al. (1985) developed more rapidly
(as early as 123 days) than those described by Marasas et al. (1984b)
(more than 450 days) was attributed by Wilson et al. (1985) to the
dietary deficiencies, which included choline and methionine, in the
maize-only diet used in their study.
Hendrich et al. (1993) reported that in Fischer-344/N rats fed
diets containing Fusarium proliferatum maize culture material (with
known amounts of FB1) there was an increased incidence of
hepatocellular adenomas, relative to rats fed the control diets. When
rats were fed nixtamalized Fusarium proliferatum (M 5991) maize
culture material diets (converting FB1 to hydrolysed FB1) the
incidence of hepatic adenomas and cholangiomas was reduced relative to
rats fed the diets containing FB1. The frequency of
hepatic/cholangiocellular adenomas in rats given the nixtamalized diet
was higher in rats receiving nutrient-supplemented diet (equivalent to
AIN-76) than in rats given a diet not supplemented with nutrients
(nutritionally deficient relative to AIN-76). In a 4-week feeding
study with male Sprague-Dawley rats, Voss et al. (1996c) found that
hydrolysed FB1 (58 mg/kg diet) from nixtamalized
F. verticillioides (MRC 826) was both hepatotoxic and nephrotoxic.
However, the extent and severity of the hepatotoxicity was
significantly less than that caused by FB1 (71 mg/kg diet) from
F. verticillioides (MRC 826), whereas the kidney toxicity was
similar (Voss et al., 1996c).
In male BD IV rats treated with the known oesophageal carcinogen
N-methylbenzylnitrosamine (NMBA) (2.5 mg/kg body weight) and FB1
(5 mg/kg body weight), there was no synergistic interaction between
NMBA and FB1 in the rat oesophagus when the two compounds were
administered together (Wild et al., 1997).
A semi-purified maize-based diet containing FB1 (not less than
90% pure) at 50 mg/kg diet was fed to 25 inbred male BD IX rats over a
period of 26 months (Gelderblom et al., 1991). A control group
received the same diet without FB1 (the FB1 content of the control
diet was approximately 0.5 mg/kg and no aflatoxin B1 could be
detected). Five rats from each group were killed at 6, 12, 20 and 26
months. All FB1-treated rats (50 mg/kg diet) that died or were killed
from 18 months onward suffered from a micro- and macronodular
cirrhosis and had large expansile nodules of cholangiofibrosis at the
hilus of the liver. The pathological changes terminating in cirrhosis
and cholangiofibrosis were already present in the liver of rats killed
6 months after the initiation of the experiment and included fibrosis,
bile duct hyperplasia and lobular distortion. The severity of the
hepatic lesions increased with time and the histological changes were
consistent with those of a chronic toxic hepatosis progressing to
cirrhosis. Ten out of 15 FB1-treated (50 mg/kg diet) rats (66%) --
but none in the controls -- that were killed or died between 18 and 26
months developed primary hepatocellular carcinoma. Metastases to the
heart, lungs or kidneys were present in four of the rats with
hepatocellular carcinoma. Apart from the hepatocellular carcinoma,
FB1 also induced cholangiofibrosis consistently from 6 months onward,
and toward the end of the experiment, cholangiocarcinoma. However, the
authors noted that the experiment was performed under nutritionally
compromised conditions, using diets deficient in vitamins, methionine
and choline, that may have had an enhancing effect on the action of
FB1 in the liver (Table 3).
The detailed results of a second long-term experiment in rats fed
diets containing 0, 1, 10 and 25 mg FB1/kg diet over a period of
24 months have not yet been published (Gelderblom et al., 1996b).
However, in preliminary reports, Gelderblom et al. (1996b, 1997) noted
that no cancers were observed in these rats, including those fed 25 mg
FB1/kg diet.
Male and female Fischer-344/N Nctr BR rats and B6C3F1/Nctr BR
mice were given diets containing FB1 for 2 years as part of the US
National Toxicology Program (NTP) tumorigenesis studies on FB1
(US NTP, 1999). The FB1 that was used in these studies was > 96%
pure. Dietary levels of FB1 were 0, 5, 15, 50 and 150 mg/kg diet for
the male rats, resulting in average daily FB1 doses of 0, 0.26, 0.76, 2.5
and 7.5 mg/kg body weight. The dietary levels for female rats were 0, 5, 15, 50
and 100 mg/kg diet, resulting in average daily FB1 doses of 0.31, 0.91, 3.0
and 6.1 mg/kg body weight.
There was no difference in body weight, survival or feed
consumption in male rats fed FB1 when compared to rats on control
diets. The only compound-related change in tumour incidence was the
induction of renal adenomas and carcinomas in male rats. The overall
incidence of renal tubule tumours in male rats receiving 0, 5, 15, 50
and 150 mg FB1/kg diet were 0/48, 0/40, 0/48, 2/48 and 5/48 for
adenomas and 0/48, 0/40, 0/48, 7/48 and 10/48 for carcinomas. Renal
tubule adenomas were characterized as an expansive proliferation of
Table 3. Summary of the induction of neoplasia in long-term feeding studiesa
Neoplasia Species and strain Sex Fumonisin concentration (mg/kg feed) Reference
0 5 15 50 80 100 150
Hepatocellular carcinoma BD IX rats male 0/15 10/15 Gelderblom
Cholangiofibrosisb BD IX rats male 0/15 15/15 et al. (1991)
Renal tubule adenoma F-344/N Nctr rats male 0/48 0/40 0/48 2/48 5/48 US NTP (1999)
Renal tubule carcinoma F-344/N Nctr rats male 0/48 0/40 0/48 7/48 10/48 US NTP (1999)
Renal tubule adenoma F-344/N Nctr rats female 0/48 0/40 1/48 0/48 0/48 US NTP (1999)
Renal tubule carcinoma F-344/N Nctr rats female 0/48 0/40 0/48 0/48 1/48 US NTP (1999)
Hepatocellular adenoma B6C3F1/Nctr mice female 5/47 3/48 1/48 16/47 31/45 US NTP (1999)
Hepatocellular carcinoma B6C3F1/Nctr mice female 0/47 0/48 0/48 10/47 9/45 US NTP (1999)
Hepatocellular adenoma B6C3F1/Nctr mice male 9/47 7/47 7/48 6/48 8/48 US NTP (1999)
Hepatocellular carcinoma B6C3F1/Nctr mice male 4/47 3/47 4/48 3/48 2/48 US NTP (1999)
a This summarizes the data for tissues where fumonisin dose-dependent induction of tumours was detected (US NTP, 1999)
b In Gelderblom et al. (1991), cholangiofibrosis was considered to have progressed to cholangiocarcinoma
renal tubule epithelial cells that tended to be separated into lobules
by a delicate fibrous stroma. The neoplastic cells had nuclei that
were slightly larger with increased cytoplasmic volume. The
cytoplasmic changes were uniform within individual lesions and varied
from clear to basophilic. Renal tubule carcinomas were characterized
by cellular atypia, necrosis within a lesion, invasion of the adjacent
normal renal parenchyma, or metastasis to distant organs.
Historically, renal tubule carcinomas have not occurred in
Fischer-344/N Nctr male rats on control diets. There was no apparent
involvement of alpha-2 microglobulin in the tumorigenicity in male rat
kidneys. The incidence in renal tumours was accompanied by an
increased incidence in renal tubule epithelial cell hyperplasia at 50
and 150 mg FB1/kg diet at 2 years (2/48, 1/40, 4/48, 14/48 and 8/48
of the male rats receiving 0, 5, 15, 50 and 150 mg FB1/kg diet).
Similarly, increased renal tubule epithelial cell apoptosis and
proliferation were detected at 50 and 150 mg FB1/kg diet in male rats
sacrificed following 6, 10, 14 and 26 weeks on FB1-containing diets.
As in the 28-day range-finding studies, the apoptosis was confined to
tubules of the inner cortex and was characterized by cellular
shrinkage from adjacent cells, cytoplasmic eosinophilia, and chromatin
condensation and margination in the nucleus. Apoptotic cells were
additionally detected using an in situ method for detection of DNA
fragmentation. In serum removed from male rats killed at 6, 10, 14 or
26 weeks, no FB1-dependent changes were noted (e.g., in cholesterol,
triglycerides or serum alanine aminotransferase levels). The urinary
Sa/So ratio was increased in the urine of male rats fed 5, 15, 50 or
150 mg FB1/kg diet, while kidney tissue Sa/So ratios were increased
at 15, 50 or 150 mg FB1/kg diet. Increased tissue sphingoid base
changes, and renal tubule tumour incidence and hyperplasia were
detected at 50 and 150 mg FB1/kg diet, while urinary sphingolipid
changes were detected at 15, 50 or 150 mg FB1/kg diet. In the livers
of the male rats, an increase in basophilic foci was detected at 150
mg FB1/kg diet, but liver tissue Sa/So ratios were not affected.
FB1 at 5, 15, 50 and 100 mg/kg diet did not affect body weight,
survival, feed consumption, serum analytes or tumour incidence in the
female rats. Significant increases in urinary and kidney tissue Sa/So
ratios were detected at 50 and 100 mg FB1/kg diet, but the extent of
induction was not as high as in the male rats.
In the female mice, dietary levels of FB1 were 0, 5, 15, 50 and
80 mg/kg diet, resulting in average daily FB1 consumption of 0.7,
2.1, 7.0 and 12.5 mg/kg body weight, respectively. In the male mice,
dose levels of 0, 5, 15, 80 and 150 mg FB1/kg diet resulted in
average daily FB1 doses of 0, 0.6, 1.7, 9.5 and 17 mg/kg body weight,
respectively.
In female B6C3F1/Nctr mice, there were essentially no
differences in the mean body weights and diet consumption. The body
weights of the mice on this study were less than those reported for
the NTP and control studies at the National Center for Toxicological
Research (NCTR). This was attributed to an unintended restriction of
the powdered feed in the individual feeders. The female mice consuming
diets containing 80 mg FB1/kg diet had a significantly reduced
survival compared to the female mice on control diets.
The only tissue that demonstrated FB1-dependent changes in
tumour incidence was the liver in the female mice. Hepatocellular
adenomas were present in 5/47, 3/48, 1/48, 16/47 and 31/45 female mice
and hepatocellular carcinomas were present in 0/47, 0/48, 0/48, 10/47
and 9/45 female mice consuming 0, 5, 15, 50 and 80 mg FB1/kg diet,
respectively. Hepatocellular adenomas were characterized as discrete
lesions with compression of adjacent normal tissues. The normal
hepatic lobular structure was absent with uneven growth patterns. The
cells in the adenoma appeared to be well differentiated and either
eosinophilic, basophilic or vacuolated. Hepatocellular carcinomas were
characterized as foci of cells with distinct trabecular or adenoid
structure. Histological evidence of local invasiveness or metastasis
was usually evident. The cells within the carcinoma were poorly
differentiated or anaplastic. Some of the carcinomas appeared to arise
within adenomas. The incidence of hepatocellular adenomas and
carcinomas in the female mice was within the range of historical
occurrence in B6C3F1/Nctr mice. The increased tumour incidence was
accompanied by increased hepatocellular hypertrophy (0/47, 0/48, 0/48,
27/47, 31/45) and hepatocellular apoptosis (0/47, 0/48, 0/48, 7/47,
14/45) in the female mice. The mean liver weights (relative to body
weight) of the mice after 2 years were increased at 50 and 80 mg
FB1/kg diet. There were no consistent increases in serum analytes in
mice killed at 3, 7, 9 and 24 weeks. Liver sphingoid bases were
increased at 80 mg FB1/kg diet at weeks 3, 7 and 9 but not week 24.
Levels of urinary sphingoid bases were not determined.
No FB1-dependent changes in tumour incidences in the male mice
receiving diets of 0, 5, 15, 80 and 150 mg FB1/kg diet were
identified. The body weights, organ weights, survival, feed
consumption and serum analytes were not affected by FB1 dose. The
lack of demonstration of statistically significant sphingoid base
increases in livers at intermediate sacrifices (3, 7 and 9 weeks) were
probably due to the low sample number (n = 4) (US NTP, 1999).
7.1.6.2 Short-term assays for carcinogenicity
The short-term assays have mainly used rat liver nodules as the
end-point, assessed either using traditional microscopy or
histochemical analysis of different enzyme activities such as
gamma-glutamyl transferase (GGT) or placental glutathione
S-transferase (PGST). In many of these assays, different stages
(initiation, promotion) of the multistage carcinogenesis model have
been investigated by combining the FB1 treatment with classical
promotion assays. In these studies FB1 (or a Fusarium extract) was
administered alone, or before a promoting treatment, or after an
initiating treatment.
a) Initiation studies
In male BD IX rats fed a diet containing 0.1% FB1 during 4
weeks, GGT-positive (GGT+) foci were induced in the liver
(Gelderblom et al., 1988).
GGT+ foci were induced in the liver of male Fischer rats fed a
diet containing 0.5-1 g/kg of FB1 (90-95% pure) for 21-26 days,
followed by partial hepatectomy and treatment with 2-AAF and carbon
tetrachloride. However, foci were not induced when single or multiple
doses (50-200 mg/kg) of FB1 (and FB2) were administered by gavage to
hepatectomized rats (Gelderblom et al., 1992b, 1993).
In subsequent dose-response studies in male Fischer rats using
the same experimental approach, Gelderblom et al. (1994) reported that
the lowest dietary level to produce cancer initiation (GGT+-foci)
over 21 days was 250 mg FB1/kg diet. The lowest levels to cause
cancer initiation over 14 and 7 days were 500 mg and 750 mg FB1/kg
diet, respectively. Based on the feed intake values, the effective
dosage level (EDL) for cancer initiation over a period of 21 days was
142 < EDL < 308 mg FB1/kg body weight and over 14 days the amount
required for cancer initiation was 233 < EDL < 335 mg FB1/kg body
weight. The dietary level of FB1 required for cancer initiation is
dependent on the duration of exposure since a dose of 293 mg FB1/kg
body weight over 7 days did not initiate cancer whereas a similar dose
(308 mg FB1/kg body weight) over 21 days did.
Lebepe-Mazur et al. (1995b) fed female Fischer-344/N rats for one
week with a semipurified diet with or without an aqueous extract of a
Fusarium verticillioides (M 1325 = MRC 826) culture, providing 20 mg
FB1/kg diet, and administered a single dose of 30 mg/kg body weight
diethylnitrosamine (DEN) thereafter. Rats fed the Fusarium culture
showed more PGST+ hepatocytes than those treated with DEN alone.
Continued dietary treatment with the Fusarium moniliforme culture
for 12 weeks after the DEN administration did not further increase the
number of PGST foci. When the FB1 diet (one week) was followed by the
DEN administration and a 7-day-non-treatment interval, no increase in
PGST foci by FB1 was observed.
In another study (Lebepe-Mazur et al., 1995c), female
Sprague-Dawley rats were fed a diet supplemented with corn
contaminated with Fusarium proliferatum (containing 20 or 50 mg/kg
FB1) for six months; GGT-foci were not observed but the number of
PGST+ altered hepatic foci was increased in treated rats in
comparison to rats fed a semipurified diet without supplementation. In
a similar study (Lebepe-Mazur et al., 1995b), feeding diet containing
20 mg/kg FB1 for one or 13 weeks failed to induce a statistically
significant increase in PGST-altered foci.
In male Sprague-Dawley rats administered purified FB1
intraperitoneally at 10 mg/kg body weight per day for 4 days, as well
as in male and female Sprague-Dawley rats given 35 and 75 mg/kg body
weight per day orally for 11 days, significant increases in PGST+
hepatocytes were observed (Mehta et al., 1998).
b) Promotion studies
In a study on the promotion activity of FB1, it was administered
to male Fischer-344 rats (10, 50, 100, 250 or 500 mg/kg diet for 21
days) after a dose of DEN (200 mg/kg body weight) (Gelderblom et al.,
1996c). Dietary levels of 50 mg/kg or more markedly increased the
number and size of the PGST+ foci in the liver. It was thus
concluded (Gelderblom et al., 1996b,c) that the dose of FB1 required
for cancer initiation was markedly higher than that required for
cancer promotion.
Female Sprague-Dawley rats were fed a semipurified diet with or
without an aqueous extract of a Fusarium verticillioides (M 1325 =
MRC 826) culture, providing 20 or 50 mg FB1/kg for 6 months, after a
single dose of 30 mg DEN/kg. The number of PGST-altered foci was
increased at 20 mg/kg but not in the high-dose group, as compared to
rats treated with DEN alone. GGT-altered foci were not observed
(Lebepe-Mazur et al., 1995c).
Gelderblom et al. (1996b) suggested that FB1-induced
hepatocarcinogenesis in male BD IX rats developed against a background
of chronic toxic hepatosis culminating in cirrhosis. Chronic
hepatotoxicity appears to be a prerequisite for the development of
liver cancer in the BD IX rat (Gelderblom et al., 1996b).
7.2 Other mammals
7.2.1 Equine leukoencephalomalacia
Equine leukoencephalomalacia (ELEM) syndrome is characterized by
the presence of liquefactive necrotic lesions in the white matter of
the cerebrum. The name is somewhat misleading since the gray matter
may also be involved (Marasas et al., 1988a). This fatal disease
apparently occurs only in equids, although there has been one
unconfirmed report of fumonisin-induced brain lesions and haemorrhage
in rabbits gavaged with FB1 (Bucci et al., 1996) and there is some
evidence that FB1 can cross the blood-brain barrier and disrupt brain
sphingolipid metabolism in neonatal rats (Kwon et al., 1997b). In
equids, the ELEM syndrome has been recognized since the 19th century
as a sporadically occurring condition. ELEM was experimentally
produced by feeding mouldy maize obtained from a field case in Kansas
by Butler (1902). The disease was known as "mouldy maize poisoning"
but attempts to identify the responsible fungus failed.
Wilson & Maronpot (1971) succeeded in establishing the causative
agent when they isolated F. verticillioides as the predominant
contaminant of mouldy maize that had caused cases of ELEM in Egypt and
reproduced ELEM by feeding culture material of the fungus on maize to
two donkeys. Subsequently investigators in South Africa confirmed the
ability of F. verticillioides (MRC 826) culture material to induce
the characteristic clinical signs and pathological changes of ELEM as
well as hepatosis in horses and donkeys (Kellerman et al., 1972;
Marasas et al., 1976, 1988a; Kriek et al., 1981).
The first symptoms of the syndrome are lethargy, head pressing
and inappetence, followed by convulsions and death after several days.
Elevated serum enzyme levels indicative of liver damage (Wilson et
al., 1992) are preceded by elevation in the serum Sa/So ratio (Wang et
al., 1992; Riley et al., 1997). Serum enzyme levels often return to
near normal concentrations (Wang et al., 1992; Wilson et al., 1992;
Ross et al., 1993; Riley et al., 1997) but usually increase markedly
immediately prior to or at the onset of behavioral changes (Kellerman
et al., 1990; Wang et al., 1992; Ross et al., 1993; Riley et al.,
1997).
In addition to the brain lesions, histopathological abnormalities
in liver and kidney have been reported in horses orally dosed with
pure fumonisins, maize screenings naturally contaminated with
fumonisins, or culture material containing known amounts of fumonisins
(Kellerman et al., 1990; Wilson et al., 1992; Ross et al., 1993;
Caramelli et al., 1993).
Shortly after the isolation and structure elucidation of
fumonisins in 1988 (Bezuidenhout et al., 1988; Gelderblom et al.,
1988), Marasas et al. (1988a) successfully produced ELEM in a horse by
the intravenous administration of pure FB1. This was done by avoiding
as much as possible hepatotoxicity using serum enzymes indicative of
it. ELEM has also been produced in horses given pure FB1 by stomach
tube, again monitoring for liver toxicity (Kellerman et al., 1990).
Fatal liver disease in the absence of any brain lesions has been
induced by intravenous injection of FB1 (Laurent et al., 1989b). ELEM
concurrent with significant liver disease has been observed in horses
and ponies fed feeds naturally contaminated with fumonisins at low
concentrations (Wilson et al., 1992; Ross et al., 1993). The
development of brain lesions in the absence of major liver lesions
does not preclude biochemical dysfunction in non-brain tissue from
contributing to the brain lesions. Ross et al. (1993) concluded that
length of exposure, level of contamination, individual animal
differences, previous exposure, or pre-existing liver impairment may
all contribute to the appearance of the clinical disease.
To date, the lowest FB1 dose that has resulted in ELEM, in a
controlled experiment, is 22 mg/kg in diets formulated with naturally
contaminated maize screenings (Wilson et al., 1992). Analysis of feeds
from confirmed cases of ELEM indicated that consumption of feed with a
FB1 concentration greater than 10 mg/kg diet is associated with
increased risk of development of ELEM, whereas, a concentration less
than 6 mg/kg diet is not (Ross, 1994).
A study by the National Veterinary Services Laboratory of the US
Animal and Plant Health Inspection Agency (National Veterinary
Services Laboratory, 1995) showed that horses fed 15 mg FB1/kg in
diets formulated from F. proliferatum (M 5991) culture material did
not exhibit any clinical signs or altered serum biochemical parameters
(including changes in the Sa/So ratio) after 150 days. A similar
result was found with a pony fed a diet containing maize screenings
naturally contaminated with 15 mg FB1/kg (Wang et al., 1992). Thus,
the minimum toxic dose in equids appears to be < 22 mg/kg > 15 mg/kg
based on studies with naturally contaminated maize screenings or
culture material (F. proliferatum) containing fumonisins. The
minimum toxic dose of pure fumonisins is unknown.
In a study using culture material containing primarily FB2 or
FB3, Ross et al. (1994) found that a diet formulated from
F. proliferatum (M 6290 and M 6104) culture material containing
primarily FB2 at 75 mg/kg was capable of inducing ELEM with hepatic
involvement in ponies after 150 days. In contrast, diets containing
primarily FB3 (75 mg/kg) were without any effect (serum enzymes,
clinical signs and histology were all normal relative to control
ponies) after 57 to 65 days. It was concluded that FB3 was less toxic
than FB2 or FB1 (Ross et al., 1994). However, analysis of serum and
tissues from ponies fed the FB3 diets revealed that the FB3 diets
significantly increased concentrations of free sphingoid bases
relative to controls and that serum enzymes were elevated but within
the normal range for ponies (Riley et al., 1997).
7.2.2 Porcine pulmonary oedema syndrome
The first report of the disease now known as porcine pulmonary
oedema (PPE) was by Kriek et al. (1981). In experimental trials,
culture material of F. verticillioides (MRC 826) was fed to horses,
pigs, sheep, rats and baboons (Kriek et al.,1981). Lung oedema
occurred only in pigs. Clinical signs of PPE typically occur soon
(2-7 days) after pigs consume diets (culture material or contaminated
maize screenings) containing large amounts of fumonisins over a short
period of time. Clinical signs usually include dyspnoea, weakness,
cyanosis and death (Osweiler et al., 1992). At necropsy, the animals
exhibit varying degrees of interstitial and interlobular oedema, with
pulmonary oedema and hydrothorax (Colvin & Harrison, 1992; Colvin et
al., 1993). Varying amounts of clear yellow fluid accumulate in the
pleural cavity.
Toxic hepatosis occurs concurrently with PPE (Osweiler et al.,
1992; Colvin et al., 1993) and is also observed in animals that
consume high levels of fumonisins but do not develop PPE (Haschek et
al., 1996). Typically, the liver contains multiple foci of coagulative
necrosis that do not show zonal distribution across the three zones of
the liver (Osweiler et al., 1992; Colvin et al., 1993). Two studies
have reported nodular hyperplasia in the pig liver (Casteel et al.,
1993, 1994).
The physiological alteration that results in the inability of the
lung to maintain fluid equilibrium is unknown. However, several
hypotheses have been proposed that are supported by experimentation.
Casteel et al. (1994) found that feeding culture material diets
(M 1325 = MRC 826) containing 150 to 170 mg FB1/kg for 210 days
resulted in right ventricular hypertrophy and medial hypertrophy of
the pulmonary arterioles. It was suggested that this cardiotoxic
effect was an indirect consequence of fumonisin-induced
hepatotoxicity. Cardiac failure is a well-known physiological
mechanism inducing altered pulmonary haemodynamics which can result in
pulmonary oedema (Colvin et al., 1993). Significant changes in oxygen
consumption and several haemodynamic parameters in pigs fed diets
containing fumonisins suggest that pulmonary hypertension caused by
hypoxic vasoconstriction may contribute to PPE (Smith et al.,
1996a,b). It has been hypothesized that the cardiovascular alterations
are a consequence of sphingoid-base-induced inhibition of L-type
calcium channels (Smith et al., 1996b).
Haschek et al. (1992) hypothesized that PPE might be induced by
dysfunction of pulmonary interstitial macrophages (PIM) resulting in
release of vasoactive mediators. The accumulation of membranous
materials in PIM, secondary to hepatotoxicity, was postulated as the
possible basis for PIM dysfunction (Haschek et al., 1992). A similar
phenomena has been observed in alveolar endothelial cells (Gumprecht
et al., 1998). It has been shown that consumption of culture material
diets (MRC 826) containing fumonisins does in fact alter PIM function
(Smith et al., 1996c). How this might contribute to pulmonary oedema
is not clear. However, it has been hypothesized that PIM dysfunction
could contribute to increased susceptibility to microbial diseases
(Smith et al., 1996c). It has been shown that serum tumour necrosis
factor-alpha (TNF-alpha)-like activity was increased in pigs fed
culture material (M 1325 = MRC 826) containing 150 mg FB1/kg (Guzman
et al., 1997). Fumonisin-induced changes in the TNF pathway have also
been seen in lipopolysaccharide-stimulated macrophages collected from
BALB/c mice dosed with pure FB1 (Dugyala et al., 1998).
In 1989-1990 outbreaks of this disease were reported in different
parts of the USA (Harrison et al., 1990; Osweiler et al., 1992; Ross
et al., 1992). Maize screenings obtained from farms (Harrison et al.,
1990; Osweiler et al., 1992) where pigs died of PPE were predominantly
contaminated with F. verticillioides. Feeding (Kriek et al., 1981;
Osweiler et al., 1992; Fazekas et al., 1998) or intubation (Colvin et
al., 1993) of F. verticillioides culture material (MRC 826) produces
PPE. Also, PPE and hepatotoxicity have been produced by feeding diets
containing maize screenings naturally contaminated with fumonisins
(Osweiler et al., 1992; Motelin et al., 1994). Purified FB1 has been
shown to produce the disease when administered intravenously (Harrison
et al., 1990; Haschek et al., 1992; Osweiler et al., 1992). However,
PPE has not yet been produced by oral administration of pure
fumonisins.
As with ELEM, there is a strong correlation between fumonisin
content of maize screenings obtained from different farms and
outbreaks of PPE (Osweiler et al., 1992; Ross et al., 1992; Ross,
1994). The highest concentration of FB1 ever reported was from maize
screenings (330 mg/kg) associated with an outbreak of PPE (Ross et
al., 1992). The minimum toxic dose has not been clearly established.
Osweiler et al. (1992) induced PPE by feeding F. verticillioides
(MRC-3033) maize culture material reportedly containing 17 mg FB1/kg
for 5 days. In the same study, maize screenings containing fumonisins
at 92 mg/kg induced PPE in several pigs after 5-7 days; similar
results were obtained in studies by Harrison et al. (1990), Haschek et
al. (1992) and Motelin et al. (1994). Based on feeding studies with
maize screenings naturally contaminated with fumonisins, FB1
concentrations of 92 to 166 mg/kg have induced PPE in 4-7 days.
Pigs fed diets containing fumonisins (formulated with culture
material or naturally contaminated maize screenings) often do not die
of PPE, even when fumonisins are reported to be present at very high
concentrations in the diet. Concentrations of FB1 as low as 17 mg/kg
in culture material diets (MRC-3033) induced PPE in 5 days (Osweiler
et al.,1992). In contrast, culture-material-formulated (M 1325 = MRC
826) diets containing as much as 190 mg/kg have been fed for 83 days
with no reported evidence of respiratory distress (Casteel et al.,
1993) and a dose of 150 to 170 mg/kg diet for up to 210 days caused
liver effects early on but no evidence of pulmonary oedema (Casteel et
al., 1994).
Colvin et al. (1993) concluded that the primary determinant of
whether pulmonary oedema or liver failure caused death was the
quantity of fumonisins fed or intubated per kg body weight per day.
They proposed that > 16 mg/kg body weight per day induced PPE and
< 16 mg/kg body weight per day induced liver failure. However, daily
oral intake levels of FB1 plus FB2 (maize screenings) from 4.5 to
6.3 mg/kg body weight have induced PPE (Haschek et al., 1992; Motelin
et al., 1994). The FB1 concentration in these diets was 166 mg/kg and
129 mg/kg, respectively. Liver lesions have been induced with maize
screenings at 1.1 mg/kg body weight per day (17 mg FB1/kg diet)
(Motelin et al., 1994).
In pigs, tissues other than liver and lung have been reported to
be targets for fumonisins, e.g., pancreas (Harrison et al., 1990),
heart (Casteel et al., 1994), kidney (Colvin et al., 1993; Harvey et
al., 1995, 1996), pulmonary intravascular macrophages (Haschek et al.,
1992), and oesophagus (Casteel et al., 1993). None of these studies
were conducted with pure fumonisins. In a recent study with pure FB1,
altered growth and changes in selected haematological parameters in
pigs were reported at dietary levels as low as 1 mg/kg (Rotter et al.,
1996).
7.2.3 Poultry toxicity
Several reports have been published implicating
F. verticillioides contamination of feed in diseases of poultry
(Marasas et al., 1984a; Bryden et al., 1987; Jeschke et al., 1987;
Prathapkumar et al., 1997). The clinical features of the disease often
include diarrhoea, weight loss, increased liver weight and poor
performance. Immunosuppression in chickens was also produced in birds
fed maize cultured with several different isolates of the fungus
(Marijanovic et al., 1991). Functional and morphological changes were
observed in chicken exposed to FB1 (Qureshi & Hagler, 1992). Several
studies have confirmed that F. verticillioides, F. proliferatum,
FB1 and moniliformin are toxic to poultry (broiler chicks, turkeys,
ducklings) (Ledoux et al., 1992, 1996; Brown et al., 1992;
Dombrink-Kurtzman et al., 1993; Javed et al., 1993a, 1995; Weibking et
al., 1993a,b, 1995; Kubena et al., 1995a,b; Hall et al., 1995;
Bermudez et al., 1996; Vesonder & Wu, 1998) and chicken embryos (Javed
et al., 1993b; Bacon et al., 1995). The levels of fumonisins used in
these studies were 75-644 mg/kg diet. Culture materials and naturally
contaminated maize containing F. proliferatum may contain, in
addition to fumonisins, moniliformin and beauvericin (Kriek et al.,
1977; Logrieco et al., 1993; Plattner & Nelson, 1994). Espada et al.
(1994) reported toxicity and altered haematological parameters (Espada
et al., 1997) in broiler chicks fed diets containing pure FB1
(10 mg/kg) and FB1 (30 mg/kg) from Fusarium verticillioides
(MRC 826) culture material.
7.2.4 Non-human primate toxicity
Kriek et al. (1981) fed three baboons F. verticillioides
culture material (MRC 826). Baboon 1 and baboon 2 died of acute
congestive heart failure after 248 and 143 days, respectively. The
remaining baboon continued on feed for 720 days, at which time it was
killed. Autopsy of baboon 3 revealed that the principle lesion was
cirrhosis of the liver. Vervet monkeys fed F. verticillioides
culture material (MRC 826) for 180 days exhibited various degrees of
toxic hepatosis (Jaskiewicz et al., 1987a). Subsequent long-term
studies (Fincham et al., 1992) with vervet monkeys fed MRC 826 culture
material shown to contain fumonisins revealed an increase in serum
cholesterol, plasma fibrinogen and blood coagulation factor VII
(factors known to promote atherosclerosis). These changes occurred
secondary to chronic hepatotoxicity at a dose calculated to average
0.3 mg total fumonisins/kg body weight per day (low-dose diet) based
on a retrospective analysis of the diets (Fincham et al., 1992) and a
high-dose diet averaging approximately 0.8 mg total fumonisins/kg body
weight per day (Shephard et al., 1996b). Analysis of the free
sphingoid bases in serum from some of the animals used in the study by
Fincham et al. (1992) showed that in serum the free sphinganine
concentration and Sa/So ratio were significantly elevated in both the
low-dose and high-dose animals (Shephard et al., 1996b). Free
sphinganine and the Sa/So ratio were also elevated in urine at both
dose levels, but not significantly (Shephard et al., 1996b).
7.2.5 Other species
Other species that have been studied using pure fumonisins,
contaminated maize screenings or maize culture material of
F. verticillioides include the following: catfish (Brown et al.,
1994; Goel et al., 1994); cattle (Osweiler et al., 1993); hamsters
(Floss et al., 1994a,b); lambs (Edrington et al., 1995); mink (Restum
et al., 1995); and rabbits (Gumprecht et al., 1995; Bucci et al.,
1996; LaBorde et al., 1997). In all cases where toxicity was evident
it involved liver and/or kidney or homologous organs.
7.3 Mechanisms of toxicity -- mode of action
Several biochemical modes of action have been proposed to explain
all or some of the fumonisin-induced animal diseases. Two of these
invoke disruption of lipid metabolism as initial site of action. There
are also several studies that hypothesize fumonisin-induced changes in
key enzymes involved in cell cycle regulation, differentiation and/or
apoptosis as initial or secondary sites of action.
7.3.1 Disruption of sphingolipid metabolism
The structural similarity between sphinganine and FB1 led Wang
et al. (1991) to hypothesize that the mechanism of action of this
mycotoxin might be via disruption of sphingolipid metabolism or a
function of sphingolipids. At the moment, there are considerable data
supporting the hypothesis that fumonisin-induced disruption of
sphingolipid metabolism is an important event in the cascade of events
leading to altered cell growth, differentiation and cell injury
observed both in vitro and in vivo.
7.3.1.1 Sphingolipids and their metabolism
The pathways of biosynthesis and turnover (Fig. 1) have not been
as well studied in sphingolipids as in other lipid classes. In order
to understand how disruption of sphingolipid metabolism might
contribute to the farm animal and laboratory animal diseases
associated with consumption of fumonisins, it is necessary to
understand how sphingolipids are biosynthesized. Eukaryotic cells
synthesize a diverse array (over 400 distinct molecules) of
sphingolipids which serve as important structural molecules in
membranes and as regulators of many cell functions (Bell et al.,
1993). While sphingolipids have also been found in procaryotes
(Karlsson, 1970), their biosynthesis and role in cellular regulation
is poorly understood.
Typically, de novo sphingolipid biosynthesis proceeds via the
reactions described below (Merrill & Jones, 1990; Sweeley, 1991; Bell
et al., 1993). The first is the condensation of serine with
palmitoyl-CoA by serine palmitoyltransferase, a pyridoxal
5'-phosphate-dependent enzyme, and the resulting 3-ketosphinganine is
reduced to sphinganine using NADPH. Sphinganine is acylated to
dihydroceramides (also called N-acylsphinganines) by ceramide
synthase using various fatty acyl-CoAs. Headgroups (e.g.,
phosphorylcholine, glucose, etc.) are subsequently added to the
1-hydroxyl group. The 4,5-trans-double bond of the sphingosine
backbone is added after acylation of the amino group of sphinganine by
the enzyme dihydroceramide desaturase (Michel et al., 1997). Both
dihydroceramide and dihydrosphingomyelin are substrates for the
enzyme. Thus, free sphingosine is not an intermediate of de novo
sphingolipid biosynthesis (Merrill, 1991; Rother et al., 1992).
Sphingolipid turnover is thought to involve the hydrolysis of complex
sphingolipids to ceramides, then to sphingosine. Sphingosine is either
reacylated or phosphorylated and cleaved to a fatty aldehyde and
ethanolamine phosphate. The fatty aldehyde and ethanolamine phosphate
can be redirected into the biosynthesis of glycerophospholipids and
other fats (Van Veldhoven & Mannaerts, 1993).
In animal cells the initial steps from the condensation of serine
and palmitoyl-CoA to the formation of ceramide take place in the
endoplasmic reticulum. Subsequent processing of ceramide into
glycosphingolipids and sphingomyelin takes place in the endoplasmic
reticulum and Golgi apparatus. Degradation of complex sphingolipids
occurs in the lysosomes, endosomes and the plasma membrane with
degradation of free sphingoid bases occurring in the cytosol. For
reviews of sphingolipid metabolism, see Merrill & Jones (1990),
Merrill (1991), Sweeley (1991) and the volumes edited by Bell et al.
(1993).
7.3.1.2 Fumonisin-induced disruption of sphingolipid metabolism in
vitro
The term "fumonisin disruption of sphingolipid metabolism"
includes inhibition of sphingosine and ceramide biosynthesis,
depletion of more complex sphingolipids, increase in free sphinganine,
decrease in reacylation of sphingosine derived from complex
sphingolipid turnover and degradation of dietary sphingolipids,
increase in sphingoid base degradation products (i.e. sphingosine
(sphinganine) 1-phosphate, ethanolamine phosphate and fatty
aldehydes), and increase in lipid products derived from the increase
in the sphingoid base degradation products. FB1 is now widely used to
reveal the function of sphingolipids and sphingolipid metabolism in
cells (Merrill et al., 1996a).
Fumonisins potently inhibit the acylation of sphinganine and
sphingosine (Wang et al., 1991; Yoo et al., 1992; Merrill et al.,
1993c). In primary rat hepatocytes, the IC50 for inhibition of serine
incorporation into sphingosine is approximately 0.1 µM for FB1 and
FB2 (Wang et al., 1991; Merrill et al., 1993c). In P388 murine
macrophages, the IC50 is less than 0.5 µM (Balsinde et al., 1997). In
cultured pig renal cells (LLC-PK1) the IC50 for inhibition of
de novo sphingosine biosynthesis is approximately 20 µM FB1 (Yoo et
al., 1992). The basis for this difference in sensitivity is unknown.
.FIGURE 1a and 1b;V219EH03.BMP
Fig. 1a. The pathway of de novo sphingolipid biosynthesis and
turnover in a mammalian cell. Large solid arrows indicate the
enzymatic steps leading to biosynthesis of ceramide, a known effector
of cell death, and large broken arrows show the enzymatic steps
leading to the production of sphingosine 1-phosphate, an effector of
cell survival. Also shown is the proposed role of mitochondrial
perturbations triggering a redirection of palmitate from
beta-oxidation into the de novo pathway, resulting in increased
biosynthesis of ceramide under conditions of oxidative stress.
Fig. 1b. The sites of action of serine palmitoyltransferase (SPTase)
inhibitors (SPTI) such as ISP-I = myriocin = thermozymozydin, and
ceramide synthase (CER synthase) inhibitors (FB) such as fumonisins.
The block on the ceramide synthase responsible for reacylation of
sphingosine results in an increase in free sphingosine and possibly
sphingosine 1-phosphate. However, it has been reported that unlike
sphingosine 1-phosphate, sphinganine 1-phosphate does not exert a
marked cytoprotective effect, but does bind to and signal via the G
protein-coupled receptor encoded by endothelial differentiation gene 1
(EDG1) (Spiegel, 1999).
In both Fig 1a and Fig 1b, the biochemicals shown in all capital
letters are those known or suspected to be lipid secondary messengers.
Also shown in Fig 1a is the generation of ceramide by ligand-induced
sphingomyelin hydrolysis.
Abbreviations: DHC-desaturase (dihydroceramide desaturase), A-SMase
(acidic sphingomyelinase), So-kinase and -lyase (sphingosine kinase
and lyase).
Inhibition of sphinganine (sphingosine) N-acyltransferase
(ceramide synthase) in cells leads to a concentration-dependent
reduction in total complex sphingolipids, including sphingomyelin and
glycosphingolipids (Wang et al., 1991; Merrill et al., 1993b; Yoo et
al., 1996). In LLC-PK1 cells, the decrease in complex sphingolipids
does not become apparent until 24 to 48 h after cells have been
exposed to FB1 but before inhibition of cell growth and increased
cell death (Yoo et al., 1996). In microsomal preparations from
cultured mouse cerebellar neurons, inhibition of ceramide synthase was
competitive with respect to both the long-chain (sphingoid) base and
fatty acyl-CoA (Merrill et al., 1993b). This observation suggests that
ceramide synthase recognizes both the amino group (sphingoid binding
domain) and the tricarballylic acid side-chains (fatty acyl-CoA
domain) of FB1 (Merrill et al., 1996b). The reduced inhibition by the
hydrolysed derivatives of the fumonisin B series (Norred et al., 1997;
van der Westhuizen et al., 1998) supports this hypothesis. Ceramide
synthase has recently been shown to acylate hydrolysed FB1, AP1, to
form N-palmitoyl-AP1. The product was also found to be an inhibitor
of ceramide synthase and to be 10 times more toxic than FB1 in a
human colonic cell line, HT29 (Humpf et al., 1998). Sphingomyelin
biosynthesis is approximately 10-fold more sensitive to inhibition by
FB1 than glycosphingolipids. This is true for other inhibitors of
ceramide synthesis, such as ß-fluoroalanine (Medlock & Merrill, 1988;
Merrill et al.,1993b).
The complete inhibition of ceramide synthase by fumonisins causes
the intracellular sphinganine concentration to increase rapidly (Wang
et al., 1991; Yoo et al., 1992). The amount of free sphinganine that
accumulates in cells is a function of several factors. These include
the extent of inhibition of ceramide synthase, the concentration of
essential precursors (serine, palmitoyl-CoA), the growth rate of the
cells, the rate of sphinganine degradation, and the rate of
elimination from the cells. For example, in rat primary hepatocytes
treated with a concentration of 1 µM FB1, which inhibits serine
incorporation into sphingosine by > 90%, there was a significant
increase in free sphinganine after 1 h, which increased to 110-fold
over controls after 4 days (Wang et al., 1991). In LLC-PK1 cells
approximately 50-fold and 128-fold increases were measured after
exposure to FB1 (35 µM, 50 to 60% inhibition) for 6 h and 24 h,
respectively (Yoo et al., 1992). Proliferating LLC-PK1 cells
accumulate much higher levels of free sphingoid bases than confluent
monolayers and cytotoxicity is only observed in proliferating cells
(Yoo et al., 1996).
While the level of free sphingosine does not increase in primary
rat hepatocytes (Wang et al., 1991; Gelderblom et al., 1995), it does
in LLC-PK1 cells, presumably due to inhibition of reacylation of
sphingosine derived from sphingolipid turnover or from the growth
medium. When cells begin to die, sphingosine levels will increase due
to the breakdown of membrane lipids. However, in LLC-PK1 cells the
increase in free sphingosine occurs before any evidence of increased
cell death or inhibition of cell proliferation (Yoo et al., 1992).
Nonetheless, approximately 95% of the increase in the levels of free
sphingoid bases in LLC-PK1 cells was found to be due to the increase
in free sphinganine level (Yoo et al., 1992). The fate of the
accumulated sphinganine is unclear. While sphingosine has little
difficulty in crossing cell membranes (Hannun et al., 1991), the
half-life of sphinganine inside LLC-PK1 cells is much longer than the
half-life of FB1 in LLC-PK1 cells (Riley et al., 1998), which
suggests either that the inhibition of sphinganine N-acyltransferase
is persistent, that sphinganine does not easily diffuse out of cells,
or that sphinganine degradation is slow relative to its biosynthesis.
In urine from rats fed FB1, > 95% of the free sphinganine is
recovered in dead cells which have apparently sloughed into the urine
(Riley et al., 1994a). Thus, in urine sphinganine is tightly
associated with the cells.
In rat hepatocytes, a portion of the accumulated sphinganine is
metabolized to sphinganine 1-phosphate and then cleaved into a fatty
aldehyde and ethanolamine phosphate (Merrill et al., 1993c), both of
which can be redirected into other biosynthetic pathways. The enzyme
responsible for hydrolysis of sphingosine (sphinganine) 1-phosphate is
sphingosine-phosphate lyase (Van Veldhoven & Mannaerts, 1993). In J774
cells exogenous sphinganine has been shown to be initially accumulated
and then rapidly metabolized (Smith & Merrill, 1995). About one-third
of the ethanolamine in phosphatidylethanolamine is derived from
long-chain base catabolism when fumonisin is added (Smith & Merrill,
1995). Similar findings have been reported using fumonisin-treated
Chinese hamster ovary cells (Badiani et al., 1996), confirming that,
in some cell types, accumulated free sphingoid bases are rapidly
metabolized into ethanolamine and fatty aldehydes. In vitro and
in vivo, rat liver lipid composition is markedly altered by FB1
(Wang et al., 1991; Gelderblom et al., 1997). In addition to changes
in free sphingoid bases, more complex sphingolipids (ceramides, etc.)
and phosphatidylethanolamine (Wang et al., 1991; Merrill et al.,
1993c), there are many changes in the fatty acid composition of liver
phospholipids (Gelderblom et al., 1997). The ability of cells to
rapidly metabolize bioactive sphingoid bases into other products may
protect cells from the toxicity associated with accumulation of free
sphingoid bases or ceramide (Spiegel, 1999). Chronically disrupted
sphingolipid metabolism leads to imbalances in phosphoglycerolipid and
fatty acid metabolism. Because the accumulation of specific
end-products and intermediates is dependent upon the balance between
anabolic and catabolic processes, it is conceivable that changes in
concentration of specific end-products could occur with no change in
the steady-state concentration of free sphingoid bases.
7.3.1.3 Fumonisin disruption of sphingolipid metabolism in vivo
a) Equids
Typically free sphingosine and sphinganine are present in normal
tissues and cells in trace amounts (Merrill et al., 1988; Riley et
al., 1994c). This is to be expected since free sphinganine is a
metabolic intermediate in the sphingolipid biosynthetic pathway, and
free sphingosine is generated primarily as a consequence of
sphingolipid turnover or degradation (Merrill, 1991; Rother et al.,
1992; Michel et al., 1997). The first in vivo study to test if
dietary fumonisins could change free sphingoid base concentration used
serum obtained from ponies fed diets containing maize screenings
naturally contaminated with fumonisins (primarily FB1) (Wang et al.,
1992). Upon consumption of diets containing fumonisin, all of the
ponies exhibited large increases in sphinganine, although the
magnitude of the changes varied among the animals. The elevation in
serum sphinganine is reversible and the increase in free sphinganine
and the Sa/So ratio occurred before increases in serum transaminase
activity and clinical signs of ELEM (Wang et al., 1992). For example,
a pony was given feed (corn screenings) containing 15 to 22 mg
FB1/kg. The Sa/So ratio increase by day 182 was followed by an
increase in serum biochemical indices of cellular injury by day 223
(Wang et al., 1992). The pony died of ELEM on day 241.
In another study, horses were fed diets containing
F. proliferatum (M 6290 and M 6104) culture material, which
contained primarily either FB2 (75 mg/kg) or FB3 (75 mg/kg) (Ross et
al., 1994). Analysis of serum and tissues from these horses showed a
qualitatively similar response to horses fed FB1 (Wang et al. 1992),
but the magnitude of the increase in free sphinganine in serum, liver
and kidney was much less in the horses fed FB3 diets (Riley et al.,
1997). While two of the three ponies fed FB2 diets developed ELEM,
the three ponies fed FB3 diets showed no clinical signs of ELEM or
evidence of liver damage after 60 days (Ross et al., 1994).
There was also a reduction in the amount of complex sphingolipids
in the serum, liver and kidney of ponies fed diets containing
fumonisins (Wang et al., 1992; Riley et al., 1997), as would be
expected if the elevation in sphinganine was due to inhibition of
de novo sphingolipid biosynthesis. In ponies fed diets containing
FB2 or FB3 (75 mg/kg), the levels of complex sphingolipids in the
liver were reduced by 88% and 72%, respectively, and the kidney was
similarly affected (Riley et al., 1997). In contrast to ponies fed
FB2 diets, ponies fed FB3 diets exhibited no liver or kidney
pathology (Ross et al., 1994). Thus, prolonged exposure to fumonisins
can result in a marked depletion of the complex sphingolipids in liver
with no evidence of liver pathology.
b) Pigs
Similar results were obtained for pigs, confirming that there was
a dose-response relationship between the ratio of free sphinganine to
free sphingosine in serum and tissues and the amount of
fumonisin-contaminated feed consumed (Riley et al., 1993). Pigs were
fed diets formulated from naturally contaminated maize screenings at 0
(< 1), 5, 23, 39, 101 and 175 mg/kg total fumonisins (FB1 plus
FB2). The results showed that the Sa/So ratio was significantly
elevated in the liver, lung and kidney from pigs consuming feeds
containing > 23 mg/kg fumonisins. Liver injury was observed at
fumonisin levels > 23 mg/kg. However, injury to the kidney was not
observed at any dose even though it contained equal or greater amounts
of free sphingoid bases. In lung tissue, free sphingoid base content
was elevated at doses > 23 mg/kg, but lung lesions were only
observed in pigs fed the diet containing 175 mg/kg. Smith et al.
(1996a) showed that in pigs fed fumonisins significant effects on
cardiovascular function were associated with significant increases in
free sphingoid bases in heart tissue. Subsequent studies found that
damage to pig alveolar endothelial cells, in vivo, was preceded by
accumulation of free sphingoid bases in lung tissue (Gumprecht et al.,
1998).
Elevation of the Sa/So ratio in pig serum paralleled the increase
in tissues (Riley et al., 1993). This finding supported the earlier
hypothesis (Wang et al., 1992) that the elevated ratio in serum was
due to the movement of free sphinganine (accumulating as a result of
inhibition of sphinganine N-acyltransferase) from tissues into the
blood. Statistically significant increases in the serum ratio were
observed at feed concentrations as low as 5 mg/kg total fumonisins
(after 14 days) and in pigs (at higher concentrations) in which other
serum biochemistry parameters were not changed and in which there were
no observable gross or microscopic lesions in liver, lung or kidney.
Thus, the increase in the Sa/So ratio was an earlier and more
sensitive indicator of fumonisin exposure than the development of
lesions in liver or lung in pigs detectable by light microscopy.
Nonetheless, the increases in free sphinganine in tissues and serum
closely paralleled the dose-dependent increases in other biochemical
parameters measured at 14 days (Motelin et al., 1994).
It has been proposed that the ratio of free sphinganine to free
sphingosine and the presence of elevated levels of free sphinganine in
serum, urine and tissue be used as indicators for consumption of
fumonisins by farm animals (Riley et al., 1994c). However, a
subsequent study with pigs found altered growth at doses of FB1 that
did not cause an increase in free sphinganine (Rotter et al., 1996).
Thus, in pigs, elevation of free sphinganine appears to occur at
dosages that are greater than those that cause subtle changes in
performance but lower than those that are toxic.
c) Poultry and other commercially important animals
Chickens fed diets supplemented with F. verticillioides culture
materials (Weibking et al., 1993a, 1995) or pure FB1 (Henry, 1993)
exhibited elevated sphinganine levels and elevated ratios in tissues
and serum. Similar findings have been made in the rabbit (Gumprecht,
et al., 1995; LaBorde et al., 1997), catfish (Goel et al., 1994), mink
(Restum et al., 1995; Morgan et al., 1997) and trout (Meredith et al.,
1998).
d) Laboratory animals
In short-term studies with rats, rabbits and mice, disruption of
sphingolipid metabolism, as shown by statistically significant
increases in free sphinganine concentration, occurs at or below the
fumonisin dosages than cause liver or kidney lesions (Riley et al.,
1994a; Martinova & Merrill, 1995; LaBorde et al., 1997; de Nijs, 1998;
Tsunoda et al., 1998; Voss et al., 1998). In rats (Sprague-Dawley,
RIVM:WU) and mice (BALB/c) dosed with fumonisins, the increase in free
sphinganine concentration in the kidney and/or liver is closely
correlated with the extent and severity of lesions (Riley et al.,
1994a; de Nijs, 1998; Tsunoda et al., 1998; Voss et al., 1998). In two
separate 21-day feeding studies (Fischer-344 rats), liver free
sphinganine level was increased, although not significantly, at the
lowest FB1 dose (50 mg/kg diet) that had liver cancer-promoting
potential (Gelderblom et al., 1996c, 1997). In rats and rabbits, the
concentration of fumonisin that causes nephrotoxicity and an increase
in kidney free sphinganine concentration is lower than the fumonisin
dose that causes hepatotoxicity (Voss et al., 1993, 1996b, 1998;
Gumprecht et al., 1995; LaBorde et al., 1997; de Nijs, 1998). For
example, in Sprague-Dawley rats significant elevation of free
sphinganine levels and hepatosis were observed at > 15 mg/kg
< 50 mg/kg dietary FB1 (Riley et al., 1994a), whereas the NOEL for
nephrosis in male Sprague-Dawley rats is 9 mg/kg (Voss et al., 1995)
and significant increases in kidney free sphinganine have been
detected in rats fed AIN-76 diets containing 1 mg FB1/kg (Wang et
al., 1999). In male RIVM:WU rats, the liver free sphinganine level was
significantly elevated at > 0.19 < 0.75 mg FB1/kg body weight
(equivalent to 1.9 and 7.5 mg/kg dietary FB1) in the absence of any
evidence of hepatosis (de Nijs, 1998) and the NOEL for tubular cell
death and significant increases in kidney free sphinganine was
< 0.19 mg FB1/kg body weight, which was equivalent to 1.9 mg/kg in
feed (de Nijs, 1998).
In the US National Toxicology Program, long-term feeding study
with Fischer-344/N Nctr BR rats, pure FB1 induced an increase in the
Sa/So ratio in kidney tissue, which correlated with increased
non-neoplastic and neoplastic lesions (US NTP, 1999). In B6C3F1/Nctr
BR female mouse liver, free sphinganine and the Sa/So ratio were
increased after 3 and 9 weeks at 50 and 80 mg FB1/kg diet, which were
the same doses that induced liver adenoma and carcinoma (US NTP,
1999). Livers taken from rats (BD IX) in a long-term study (2 years)
that were fed diets containing 10 and 25 mg/kg FB1 did not show
significant changes in liver free sphingoid bases, although the mean
concentration of free sphinganine and free sphingosine in the liver of
rats fed 25 mg FB1/kg diet was 8- and 3-fold, respectively, higher
than control values (Gelderblom et al., 1997).
In rats, rabbits and vervet monkeys, increases in free
sphinganine concentration have been detected in the urine of animals
fed fumonisin-containing diets (Riley et al., 1994a; Castegnaro et
al., 1996; Shephard et al., 1996b; LaBorde et al., 1997; Merrill et
al., 1997b; Solfrizzo et al., 1997a,b; Wang et al., 1999).
Accumulation of free sphinganine in rat urine (associated with
accumulation of dead cells) closely reflected the changes which
occurred in the kidney (Riley et al., 1994a). Analysis of urine from
rats fed commercially available chows showed a statistically
significant correlation between the free sphinganine to free
sphingosine ratio in urine and the fumonisin concentration in the
chows. The FB1 concentration in the chows ranged from undetected to
3.3 mg/kg (Merrill et al., 1997b). Feeding studies with pure FB1 in
AIN-76 diets indicate that the no-observed-effect level (NOEL) for
elevation of urinary free sphinganine level in Sprague-Dawley rats is
> 1 mg/kg diet < 5 mg/kg diet (Wang et al., 1999). In other
studies, rats fed a diet containing mixture of fumonisins (from
culture material) for 13 days showed a NOEL of between 1 and 2 mg/kg
diet (Solfrizzo et al., 1997b).
In rats fed an AIN-76 diet containing 10 mg FB1/kg for 10 days
and then put on a control diet, the urinary sphinganine concentration
returned to control levels in 10 days. However, if the diet contained
1 mg FB1/kg, the urinary sphinganine concentration remained markedly
elevated for at least 10 days after changing the feed (Wang et al.,
1999). Thus, in this study, once elevated by feeding toxic levels of
FB1, apparently non-toxic concentrations kept the free sphinganine
concentration significantly elevated to concentrations that were
equivalent to those of the nephrotoxic fumonisin dosage. This result
is, however, in contrast with the findings of Solfrizzo et al.
(1997b), showing that the elevated levels of sphingoid bases after
exposure to relatively high levels of fumonisins (7-15 mg/kg for 13
days) return to their original values when rats are exposed to a
low-fumonisin diet (1 mg/kg or less) for a period of time that is
directly dependent on the previous level of exposure, in terms of dose
and time (Solfrizzo et al., 1997b).
7.3.1.4 Tissue and species specificity
The tissue specificity and the severity of the pathology in rats
(Sprague-Dawley, Fischer-344, Wistar, RIVM:WV) and mice (BALB/c) seem
to correlate well with the disruption of sphingolipid biosynthesis
(Riley et al., 1994a; Tsunoda et al., 1998; Voss et al., 1998). This
is not the case in pigs and horses, where the kidney appears to be
equally or more sensitive than the liver with regards to the
fumonisin-induced increase in free sphinganine (Riley et al., 1993,
1997). There is significant liver pathology in horses and pigs (Ross
et al., 1994; Haschek et al., 1996), with little evidence of kidney
damage (Harvey et al., 1996).
It has been suggested that these differences in tissue and
species specificity may be due to differing susceptibility to the
adverse cellular effects of disrupted sphingolipid metabolism (Voss et
al., 1996b). For example, liver and kidney may have different
abilities to metabolize or eliminate free sphinganine or to compensate
for depletion of complex sphingolipids. In addition, fumonisin, free
sphingoid bases or their metabolites, in serum may affect the function
of the vasculature and thus indirectly affect tissues that are not
directly affected by fumonisin inhibition of ceramide synthase
(Ramasamy et al., 1995; Smith et al., 1996b). For example, the
correlation between the fumonisin-induced increase in serum
free-sphinganine (Wang et al., 1992) and the onset of ELEM could be
explained if the vascular function in horse brain was altered due to
elevated serum free sphinganine. In pigs, it has been hypothesized
that cardiovascular dysfunction, subsequent to increased free
sphingoid base concentration in the heart, is the cause of PPE (Smith
et al., 1996b).
Riley et al. (1996) have recommended that detection of high
concentrations of free sphinganine in urine, serum or tissues should
be viewed as a clinical tool to be developed and used in conjunction
with other clinical tools in situations where animal toxicity
resulting from exposure to fumonisins is suspected. Changes in
sphingolipid profiles in serum and urine in vervet monkeys fed
fumonisin-containing diets have been reported (Shephard et al.,
1996b). Whether human exposure to fumonisins in maize and maize
products will result in increased free sphinganine concentration in
tissues, urine or serum is not known. However, free sphingoid bases
can be detected in human urine (Castegnaro et al., 1996; Solfrizzo et
al., 1997a).
7.3.1.5 Fumonisin-induced sphingolipid alterations: effects on
growth, differentiation and cell death
There are many ways that disruption of sphingolipid metabolism
could account for the cell damage caused by fumonisins. In order to
fully understand the possibilities, it is necessary to consider the
multitude of functions of complex sphingolipids (Bell et al., 1993),
the potent bioactivity of sphinganine and its metabolites (Merrill et
al., 1993a), and the parallel or branch metabolic pathways that can be
affected by disruption of sphingolipid metabolism (Riley et al., 1996;
Merrill et al., 1997a,b). Since the steady-state concentration of many
biologically active lipid intermediates and end-products could be
altered, there are also many potential molecular sites that could be
affected by fumonisin-induced disruption of sphingolipid metabolism.
Thus, it can be expected that there will also be a diversity of
alterations in cellular regulation.
The earliest effect of fumonisin on sphingolipid metabolism
in vitro is the decrease in serine incorporation into ceramide,
followed by an increase in free sphinganine concentration (Yoo et al.,
1992). There is also a concentration-dependent decrease in more
complex sphingolipids (Yoo et al., 1996). Because long-chain
(sphingoid) bases are growth inhibitory, cytotoxic and induce
apoptosis under some conditions (Merrill, 1983; Stevens et al., 1990;
Nakamura et al., 1996; Sweeney et al., 1996; Yoo et al., 1996), and
are growth stimulating under certain conditions (Zhang et al., 1990,
1991; Schroeder et al., 1994), the accumulation of sphinganine (and
sometimes sphingosine) might account for these same effects of
fumonisins. Yoo et al. (1992) have shown that in the renal epithelial
cell line (LLC-PK1 cells) there is a concentration-dependent
association between the inhibition of sphingolipid biosynthesis by
FB1 and growth inhibition and cell death. After 24 h of exposure to
FB1 many cells began to develop a fibroblast-like appearance, with
loss of cell-cell contact and an elongated, spindle shape. If
fumonisin was removed, the cells that survived resumed growth and had
a normal epithelial morphology. Addition of exogenous sphinganine
induces cell death at intracellular concentrations that are similar to
those induced by FB1 (Yoo et al., 1996).
The two most likely explanations for the increased cell death
after inhibition of sphingolipid biosynthesis by fumonisins are: (1)
that the free sphinganine (or a sphinganine degradation product) is
growth inhibitory and cytotoxic for the cells, as has been seen in
many other systems (Stevens et al., 1990; Hannun et al., 1991; Sweeney
et al., 1996); and (2) that more complex sphingolipids are required
for cell survival and growth, as has been proven with mutants lacking
serine palmitoyltransferase (Hanada et al., 1990, 1992) and in studies
with specific inhibitors of glycosphingolipid biosynthesis (Radin,
1994; Nakamura et al., 1996). ß-Chloroalanine, a non-specific serine
palmitoyltransferase inhibitor, in the presence of FB1 reduced the
intracellular concentration of free sphinganine and also reduced the
inhibition of cell growth (50 to 60%) and the extent of cell death (50
to 60%) (Yoo et al., 1996). More recent studies with LLC-PK1 cells
indicate that fumonisin inhibition of cell proliferation and increased
cell death (apoptosis) are prevented by > 90% using the specific
serine palmitoyltransferase inhibitor, myriocin (ISP-1) (Riley et al.,
1999). Similar results have been obtained with HT29 cells, a human
colonic cell line (Schmelz et al., 1998). However, in the LLC-PK1
cells, the morphological changes, such as decreased cell-cell contact
and increased fibroblast-like appearance, are not reversed. In primary
human keratinocytes, both ß-chloroalanine and N-acetylsphingosine
partially protected against FB1-induced apoptosis (Tolleson et al.,
1999). However, both exogenous sphinganine and N-acetylsphingosine
alone induced apoptosis in these same cells (Tolleson et al., 1999).
Thus, in cultured cells sphingolipid-dependent mechanisms for inducing
apoptosis include accumulation of excess ceramide or sphingoid bases,
or depletion of ceramide, or more complex sphingolipids.
In addition to the cell types described above, apoptosis in
response to exposure to FB1 in vitro has been reported using turkey
lymphocytes (Dombrink-Kurtzman et al., 1994a,b), human fibroblasts,
oesophageal epithelial cells and hepatoma cells (Tolleson et al.,
1996b), and CV-1 monkey kidney cells (Wang et al., 1996).
The adverse effects of fumonisin-induced depletion of more
complex sphingolipids have been demonstrated in numerous other
studies. For example, in hippocampal neurons, FB1 inhibition of
complex sphingolipid biosynthesis was correlated with decreased axonal
growth (Harel & Futerman, 1993). The FB1 inhibition of axonal growth
could be reversed by addition of ceramide with FB1 (Harel & Futerman,
1993; Schwarz et al., 1995). The ability of growth factors to
stimulate axonal cell growth is dependent on sphingolipid biosynthesis
(Boldin & Futerman, 1997). In fibroblasts (Swiss 3T3 cells),
fumonisin-induced morphological changes could be reversed by
ganglioside GM1. However, GM1 did not prevent the inhibition of cell
proliferation (Meivar-Levy et al., 1997). FB1 and/or myriocin (ISP-1)
inhibition of glycosphingolipid biosynthesis disrupts cell substratum
adhesion in mouse melanoma cells (Hidari et al., 1996). FB1 has also
been shown to alter the manner in which glycosyl
phosphatidylinositol-anchored proteins, such as the folate receptor,
are organized and function in membranes (Hanada et al., 1993; Stevens
& Tang, 1997). FB1 inhibition of galactosylceramide biosynthesis has
been shown to disrupt the assembly and disassembly of cytoskeletal
proteins responsible for lipid transport and maintenance of the
subcellular architecture in SW13 cells (derived from a human adrenal
carcinoma) (Gillard et al., 1996). Thus, there is no doubt that the
loss of complex sphingolipids also plays a role in the abnormal
behavior and altered morphology of fumonisin-treated cells.
Currently Swiss 3T3 cells are the only type of cell that respond
to fumonisins with increased DNA synthesis (Schroeder et al., 1994).
It was proposed that this in vitro model would be useful for
understanding if, within the complex in vivo milieu of cells in the
liver, there might exist cells that could be inappropriately selected
to enter the cell cycle. Defects in cell cycle control have been shown
to promote genomic instability and progression to malignancy (Hartwell
& Kastan, 1994). Incubation of Swiss 3T3 cells with
DL-erythro-sphinganine caused an increase in [3H]thymidine
incorporation into DNA. Addition of FB1 to the cells elevated
sphinganine and induced a comparable increase in [3H]thymidine
incorporation into DNA. These findings associated an accumulation of
sphinganine with the induction of DNA synthesis by FB1 but did not
prove that they were causally linked. However, this was proven using
an inhibitor of serine palmitoyltransferase in combination with FB1.
Reduction in cellular sphinganine when ß-fluoro-L-alanine was added to
Swiss 3T3 cells, demonstrated that this reduction in sphinganine
completely removed the insulin-dependent stimulation of [3H]thymidine
incorporation into DNA by FB1. Therefore, the formation of
sphinganine is required for stimulation of DNA synthesis by fumonisins
in Swiss 3T3 cells (Schroeder et al., 1994).
Fumonisin inhibition of ceramide synthesis can deregulate many
normal cell functions including non-accidental programmed cell death.
Some of the processes have been shown to be modulated by fumonisin
inhibition of ceramide synthase in vitro (Table 4).
It is important to recognize that ceramide signaling is also
mediated by sphingomyelin hydrolysis (Perry & Hannun, 1998) via
enzymes that are not inhibited by fumonisin. When fumonisins are added
to cells for the purpose of inhibiting de novo ceramide generation,
there is also the potential for accumulation of free sphingoid bases
and their downstream sphingoid base 1-phosphates. Thus, there is the
Table 4. Examples of cell functions modulated by de novo ceramide
biosynthesis as shown by inhibition of the process by
fumonisin-treatment
* sphingosine-induced germinal vesicle breakdown and Xenopus oocyte
maturation (Strum et al., 1995)
* daunorubicin-activated apoptosis in P388, U937 and chicken granulosa
cells (Bose et al., 1995; Witty et al., 1996)
* chemotherapeutic agent (CPT-11)-induced interleukin 1-beta
converting enzyme (ICE) cascade-dependent apoptosis in 4B1 (L929)
mouse fibroblasts (Suzuki et al., 1997)
* carnitine palmitoyltransferase inhibition-induced apoptosis in LyD9
mouse haematopoietic precursor cells (Paumen et al., 1997)
* lipopolysaccharide (LPS)/platelet activating factor (PAF) induced
arachidonic acid release in murine P388D1 macrophages (Balsinde et
al., 1997)
* chemical hypoxia-induced cell death in LLC-PK1 cells (Ueda et al.,
1998)
* Fas-transduced-caspase-dependent T-cell proliferation (Sakata et
al., 1998)
* fenretinide-induced poly-(ADP-ribose) polymerase (PARP) cleavage and
apoptosis in HL-60 cells (DiPietrantonio et al., 1998)
* serum-stimulated retinoblastoma (Rb) protein dephosphorylation and
cell cycle progression (Lee et al., 1998)
* multidrug resistance modulator-dependent cytotoxicity (Cabot et al.,
1998, 1999)
* TNF-alpha/cycloheximide-induced endothelial cell death (Xu et al.,
1998)
* 12- O-tetradecanoylphorbol-13-acetate (TPA)-induced apoptosis in
prostate cancer cells (Garzotto et al., 1998)
* hexadecylphosphocholine-induced apoptosis in HaCaT cells (Wieder et
al., 1998)
* fatty acid-induced nitric oxide synthase-dependent apoptosis in
cultured rat prediabetic islets (Shimabukuro et al., 1998)
* ionizing radiation-induced DNA damaged and cell death in various
cell types (Liao et al., 1999)
potential for misinterpreting the results of experiments using
fumonisins as an inhibitor of ceramide biosynthesis as was recently
pointed out by Lemmer et al. (1998).
The ability of fumonisin inhibition of ceramide biosynthesis to
protect cells is of interest since primary rat hepatocyte necrotic
cell death has been shown to be mediated by ceramide-induced (but not
dihydroceramide) mitochondrial dysfunction (Arora et al., 1997). The
activity of the enzyme responsible for desaturation of the inactive
dihydroceramide (dihydroceramide desaturase) is regulated by the
intracellular redox state of the cell (Michel et al., 1997). Taken
together, these findings suggest that ceramide metabolism is sensitive
to oxidative stress and that fumonisin-inhibition of ceramide will
modify apoptosis induced by mitochondrial damage or oxidative stress.
In primary rat hepatocytes and rat liver slices, large increases
in free sphinganine occur at FB1 concentrations ranging from 0.1 to 1
µM (Wang et al., 1992; Gelderblom et al., 1996b; Norred et al., 1996)
that are 300- to 3000-fold less than those that are cytotoxic and 10-
to 100-fold less than those that cause inhibition of epidermal growth
factor-induced [3H]thymidine incorporation into DNA (Gelderblom et
al., 1996b). There appears to be no relationship between
fumonisin-induced increases in free sphinganine and fumonisin-induced
inhibition of [3H]thymidine incorporation and cell death in primary
rat hepatocytes (Gelderblom et al., 1996a,b) or the cytotoxicity in
rat liver slices (Norred et al., 1997). At the moment there is no
adequate explanation for the resistance of primary rat hepatocytes and
rat liver slices to fumonisin-induced cytotoxicity or inhibition of
cell proliferation. This is puzzling in light of the fact that other
primary cell cultures, such as rabbit renal epithelial cells, are very
sensitive to fumonisin-induced inhibition of cell growth (Counts et
al., 1996) and that in liver, in vivo, the intracellular
concentrations of FB1 that cause hepatotoxicity are relatively low
based on pharmacokinetic considerations (see previous sections).
7.3.1.6 Sphingolipid-mediated cellular deregulation and fumonisin
diseases
Sphingolipids have been associated with many facets of cellular
regulation (Merrill et al., 1993a; Bell et al., 1993; Ballou et al.,
1996; Merrill et al., 1997b; Kolesnick & Krönke, 1998; Perry & Hannun,
1998) that could contribute to or modify the expression of
fumonisin-associated diseases (Table 5).
Chronic fumonisin disruption of sphingolipid metabolism has been
hypothesized to be a contributing factor leading to cellular
deregulation and organ toxicity (Merrill et al., 1993c; Riley et al.,
1994b; Schroeder et al., 1994; Tolleson et al., 1996a,b, 1999). The
consequences of disrupted sphingolipid metabolism in vitro are
specific to cell type.
Table 5. Examples of cellular regulatory processes that have been
shown to be modulated by sphingolipids and are known to be
important in the control of normal cell growth,
differentiation, apoptosis and immune responsea
Sphingoid bases and their metabolites
* inhibition of protein kinase C
* activation of phospholipase D/inhibition of phosphatidic acid
phosphatase
* activation of the epidermal growth factor (EGF) receptor kinase
(probably via mitogen-activated protein kinase)
* control of intracellular calcium (seemingly via sphingosine 1-
phosphate)
* control of plasma membrane potassium permeability in myocytes
* inhibition of DNA primase and increases in transcription factor
AP-1, an early step in the growth of some cell types
Ceramide
* second messenger in cytokine signal transduction
* activates protein kinases, phosphatases and MAP kinases
* inhibits phospholipase D
More complex sphingolipids
* binding of cytoskeletal proteins
* participation in cell-cell communication and cell-substratum
interactions
* protein transport, sorting and targeting
a For additional processes regulated by ceramide generated de novo,
see Table 4 and the reviews cited in the text.
The evidence for fumonisin-induced disruption of sphingolipid
metabolism in target tissues has been demonstrated repeatedly in many
independent studies. Nonetheless, the precise mechanism by which
disrupted sphingolipid metabolism contributes to the increased organ
toxicity in rodents is unclear. The current understanding of the
sphingolipid signalling pathways (Merrill et al., 1997a,b; Kolesnick &
Krönke, 1998; Perry & Hannun, 1998; Spiegel, 1999) indicates that the
balance between the intracellular concentration of sphingolipid
effectors that protect cells from apoptosis (decreased ceramide,
increased sphingosine 1-phosphate) and the effectors that induce
apoptosis (increased ceramide, increased free sphingoid bases,
increased fatty acids) will determine the observed cellular response
(the critical set-points will be cell-type specific). Since the
balance between the rates of apoptosis and proliferation are critical
determinants in the process of tumorigenesis, in cells exposed to
fumonisins, those cells sensitive to the proliferative effect of
decreased ceramide and increased sphingosine 1-phosphate should be
selected to survive and proliferate when the conditions under which
the cells are exposed to fumonisins are such that increased
intracellular free sphingoid base concentration is not growth
inhibitory. Conversely, when the rate of increase in free sphingoid
bases exceeds a cell's ability to convert sphinganine/sphingosine to
dihydroceramide/ceramide or their sphingoid base 1-phosphate, then
free sphingoid bases will accumulate. In this case cells that are
sensitive to sphingoid base-induced growth arrest will cease growing
and insensitive cells will survive. Another condition that would
promote increased apoptosis would be if the block on ceramide synthase
was either reduced or ceramide synthesis was increased while free
sphinganine levels were still high.
7.3.2 Altered fatty acid metabolism in liver
Gelderblom et al. (1995) reported that there was no relationship
between the fumonisin-induced increase in free sphinganine and the
mitoinhibitory effect or the cytotoxicity of FB1 in primary rat
hepatocytes. In addition it was found that free sphinganine
concentration increased markedly even in primary rat hepatocytes that
had not been exposed to FB1. However, in cultured cells the simple
act of changing the culture medium can result in a transient increase
in free sphingoid bases (Smith & Merrill, 1995; Smith et al., 1997).
In fumonisin-treated primary rat hepatocytes, the Sa/So ratio was
maximally elevated at 1 µM FB1, whereas cytotoxicity was observed at
> 250 µM FB1 (Gelderblom et al., 1995, 1996b). Polyunsaturated
fatty acids (PUFAs) were shown to accumulate at the cytotoxic doses
(Gelderblom et al., 1996b).
In other studies, fumonisins were shown to create a multitude of
changes in liver cholesterol, phospholipids, sphingoid bases and free
fatty acid composition (Gelderblom et al., 1996a, 1997). In both the
short-term and the long-term feeding studies, changes in fatty acid
profiles indicated that FB1 treatment altered the n-6 fatty acid
metabolic pathway. In the long-term study (2 years), significant
changes were observed in livers from rats fed 10 and 25 mg FB1/kg
diet (Gelderblom et al., 1997). These data suggested that the increase
in the n-3 fatty acid content of liver could, through altered
eicosanoid biosynthesis, modulate hepatocyte proliferation (Gelderblom
et al., 1997). Recently, fumonisin treatment has been shown to
increase the extent of lipid peroxidation in rat (Fischer-344) primary
hepatocytes and liver in vivo in a concentration- and dose-dependent
manner (Abel & Gelderblom, 1998). The increased susceptibility to
lipid peroxidation may be a consequence of the other lipid changes
described above.
7.3.3 Other biochemical changes
Numerous studies using fumonisins have found changes in cellular
regulation and cell function (Table 6). Many of these effects could be
relevant to the organ toxicity of fumonisins.
In conclusion, fumonisin-induced disruption of sphingolipid
metabolism is observed both in vitro and in vivo. With the
exception of primary rat hepatocytes, disruption of sphingolipid
metabolism is closely correlated in both a time- and
concentration-dependent manner with alterations in cell proliferation
and increased cell death. In vivo, evidence for disruption of
sphingolipid metabolism is closely correlated with the onset and
progression of F. verticillioides-associated diseases in pigs,
horses, rabbits, mice and rats. However, disrupted sphingolipid
metabolism is also observed in tissues that are not considered target
organs (i.e., pig and horse kidney, pig heart, endothelial cells).
Thus, fumonisin-induced disruption of sphingolipid metabolism could
contribute both directly and indirectly to the diseases known to be
caused by consumption of fumonisins. Fumonisins also affect other
sites of cellular regulation that are apparently independent of the
disruption of sphingolipid metabolism. However, disruption of various
aspects of lipid metabolism and signal transduction pathways mediated
by lipid second messengers appears to be an important aspect of all
the various proposed mechanisms of action.
7.4 Factors modifying toxicity; toxicity of metabolites
Voss et al. (1996c) found that nixtamalization of
F. verticillioides (MRC 826) culture material effectively eliminated
FB1, but the resulting material (containing hydrolysed FB1) was less
hepatotoxic but equally nephrotoxic when fed to rats. In a recent
abstract, it was reported that pure FB1 at 50.5 and 101 mg/kg diet
(70 and 140 µmol/kg diet) was toxic to female B6C3F1/Nctr mice when
fed for 28 days, but FB2, FB3 and AP1 were not hepatotoxic (Howard
et al., 1999). These considerations are important in the evaluation of
the potential of calcium hydroxide treatment for the detoxification of
fumonisin-contaminated maize (Sydenham et al., 1995).
Table 6. Summary of studies using fumonisins that have found
changes in cellular regulation and cell function
* repression of expression of protein kinase C (PKC), AP-1-dependent
transcription, stimulation of a cyclic AMP response element in CV-I
African green monkey kidney cells (1-10 µM FB1, 3 to 16 h) (Huang et
al., 1995)
* decreased phorbol dibutyrate binding, increased cytosolic PKC
activity, with both exogenous sphinganine and FB1 to J774A.1 cells
(Smith et al.,1997)
* inhibited phorbol dibutyrate binding in short-term incubations using
crude cerebrocortical membrane preparation and both FB1 and exogenous
sphingosine (Yeung et al., 1996)
* activation of the mitogen-activated protein kinase (MAPK) in Swiss
3T3 cells with FB1 (Wattenberg et al., 1996)
* inhibition of serine/threonine phosphatases (PP5, IC50 of 80 µM) in
isolated enzyme preparations (Fukuda et al., 1996)
* over-expression of nuclear cyclin D1 and increased cyclin-dependent
kinase 4 (CDK4) activity in rat livers obtained from a long-term
feeding study and a 21-day feeding study with FB1 (an abstract
Ramljak et al., 1996)
* dephosphorylation of the retinoblastoma protein, repression of CDK2,
and induction of two CDK inhibitors in CV-1 cells with FB1
(Ciacci-Zanella et al., 1998)
* apoptosis inhibitor and protease inhibitor protection of CV-1 cells
and primary human cells from FB1-induced apoptosis (Ciacci-Zanella
& Jones, 1999)
* increased TNF secretion in LPS-activated intraperitoneal macrophages
from FB1-treated mice (Dugyala et al., 1998)
* altered calcium homeostasis in frog (Rana esculenta) atrial muscle
in vitro (Sauviat et al., 1991)
* glutathione depletion and lipid peroxidation in cultured cells
(Azuka et al., 1993; Kang & Alexander, 1996; Sahu et al., 1998;
Abado-Becognee et al., 1998; Yin et al., 1998) and in vivo (Lim et
al., 1996; Abel & Gelderblom, 1998)
* stimulation of nitric oxide production (Rotter & Oh, 1996)
In naturally contaminated maize, the simultaneous occurrence of
multiple fumonisins is likely. Therefore, the relative toxicity of the
various fumonisins is of concern for hazard assessment. Gelderblom et
al. (1993) found that the aminopentols (AP1 and AP2) of FB1 and
FB2 did not act as cancer initiators in orally dosed male Fischer
rats, although they were more toxic than the parent compounds in
primary cultures of rat hepatocytes. AP1 is less toxic than FB1
(Flynn et al., 1994, 1997). The aminopentols of FB1, FB2 and FB3
are also less effective inhibitors of sphinganine N-acyltransferase
in rat primary hepatocytes and liver slices (Merrill et al., 1993c;
Norred et al., 1997). In gestation day 9.5 rat embryos exposed
in vitro to 0, 3, 10, 30, 100 or 300 µM AP1 throughout the entire
45-h cultured period, significant increase in the incidence of
abnormal embryos including neural tube defects (NTD) were observed at
concentrations of 100 µM and above (Flynn et al., 1997). A recent
study by Norred et al. (1997) found that the following mycotoxins had
no effect on sphinganine levels in rat liver slices: aflatoxin B1,
cyclopiazonic acid, beauvericin, T-2 toxin, sterigmatocystin,
luteoskyrin, verrucarin A, scirpentriol and zearalenone. Fumonisins
FB1, FB2, FB3, FB4, FC4, and hydrolysed FB1, FB2, FB3 and
Aal-toxin all caused significant elevation in free sphinganine (Norred
et al., 1997). Fumonisin B4, C4 and AAl-toxin are the most effective
inhibitors of sphinganine N-acyltransferase based on sphinganine
accumulation in rat liver slices (Norred et al., 1997). However, their
toxicity in vivo is unknown. Pure FB3 was less effective than FB1
or FB2 in causing reduced weight gain in rats (Gelderblom et al.,
1993) but FB2 and FB3 are equally effective inhibitors of
sphinganine N-acyltranferase (Norred et al., 1997). A diet
containing FB3 was less effective than a diet containing FB2 in
inducing ELEM in ponies (Ross et al., 1994). The ability of diets
containing FB3 to disrupt sphingolipid metabolism in vivo was less
than that of diets containing FB2 (Riley et al., 1997). However, in
primary rat hepatocytes, FB3 was found to be more cytotoxic
(Gelderblom et al., 1993). Acetylated FB1 had no effect (relative to
controls) on weight gain in rats nor did it have any cancer-initiating
activity (Gelderblom et al., 1993). It does not cause sphinganine
accumulation in liver slices (Norred et al., 1997) but did cause
sphinganine accumulation in a study with primary rat hepatocytes (van
der Westhuizen et al., 1998).
Because fumonisins occur naturally in combination with other
fungal toxins (Chu & Li, 1994; Bottalico et al., 1995; Logrieco et
al., 1995; Yamashita et al., 1995; Ueno et al., 1997), the possibility
of toxic synergisms exists. There are numerous reports of additive
effects but the dosages have been much greater than those known to
occur naturally (Kubena et al., 1995a,b). Toxic synergisms have been
reported in growing pigs fed diets containing culture material with
FB1 (50 mg/kg diet) and deoxynivalenol-contaminated wheat (4 mg/kg
diet) (Harvey et al., 1996) and in a similar study with aflatoxin (2.5
mg/kg diet) and FB1 (100 mg/kg diet) culture material (Harvey et al.,
1995).
Several reports have been published indicating that no toxic
synergism is observed in poultry. In turkeys fed a ration containing
200 mg FB1 and 100 mg moniliformin/kg diet from 1 to 21 days of age,
no additive or synergistic effects were observed (Bermudez et al.,
1997). Female turkey poults (Nicholas Large Whites) from day of hatch
to 3 week of age fed diets containing 300 mg FB1, as well as 4 mg
diacetoxyscirpenol or 3 mg ochratoxin A, exhibited additive or less
than additive toxicity, but not toxic synergy (Kubena et al., 1997a).
In male broiler chicks from day of hatch to 19 or 21 days of age fed
diets containing 300 mg FB1, as well as 5 mg T-2 toxin/kg diet or 15
mg deoxynivalenol/kg diet from naturally contaminated wheat, toxic
synergy was not observed for either of these toxin combinations
(Kubena et al., 1997b).
Fumonisins inhibit the in vitro biosynthesis of
glycosphingolipid receptors for cholera toxin and shiga-like toxins
(Sandvig et al., 1996) and inhibit the accumulation of
glycosphingolipids believed to be responsible for multidrug-resistance
in certain cancer cells (Lavie et al. 1996). Glycosphingolipids are
known to be receptors and adhesion sites for viruses, bacteria and
fungi.
8. EFFECTS ON HUMANS
There has been one report of a disease outbreak characterized by
abdominal pain, borborygmi and diarrhoea in India suspected to be
associated with foodborne FB1 (Bhat et al., 1997).
8.1 Transkei, South Africa
The only studies available were correlation studies, most of
which indicated some relationship between oesophageal cancer rates and
the occurrence of F. verticillioides (IARC, 1993).
A very high incidence of oesophageal cancer among the black
population of the Transkei, South Africa, has been reported in several
surveys (Jaskiewicz et al., 1987c; Makaula et al., 1996), some of
which have been reviewed by IARC (1993). The incidence was higher in
both sexes in the south (Butterworth and Kentani Districts) compared
to the northern parts of the region (Bizana and Lusikisiki Districts).
Based on the performance of hybrids in small experimental plots,
maize grows well in both areas (Rheeder et al., 1994). The sites are
about 200 km apart and the northern area is about 500 m higher.
Although some soil fertility factors were different between the high
and low oesophageal cancer areas, there is no evidence that any
nutrient was limiting at least for hybrid maize production (Rheeder et
al., 1994). Farmers grow open-pollinated maize of varying genotypes
passed on from farm to farm and season to season. Kernel types include
large flour-maize kernels as well as dent and flint-type white, yellow
and blue kernels. Both areas depend on home-grown maize for around
50-100% of the year's supply, the remaining being purchased from
commercial sources or imports.
Maize porridge is the staple diet (up to 100% of calories).
Adults also consume beer deliberately made from mouldy maize selected
by the housewife from the harvest. This maize has been found to
contain up to 118 mg/kg fumonisins (Rheeder et al., 1992). Based on
experiments conducted on beer made from wort containing added FB1
(Scott et al., 1995), such beers could contain fumonisin
concentrations of 30 mg/litre beer.
Contamination of home-grown maize in Transkei by a number of
toxigenic Fusarium species, particularly F. verticillioides, has
been observed since the early 1970s (Marasas et al., 1979a, 1981,
1988b, 1993; Marasas, 1993, 1994, 1995, 1996, 1997). Another
Fusarium species associated with maize in Transkei is
F. graminearum, and the mycotoxins deoxynivalenol and zearalenone
produced by this fungus occur in home-grown maize intended for human
consumption (Marasas et al., 1979b). However, the occurrence of
F. graminearum in maize kernels was found to be greater in low-risk
than in high-risk areas for oesophageal cancer in later studies
(Marasas et al., 1981, 1988b), and Sydenham et al. (1990b) confirmed
that deoxynivalenol and zearalenone levels were significantly higher
in home-grown maize from areas with low rates of oesophageal cancer
than from those with high rates.
In contrast, the occurrence of F. verticillioides in maize
kernels was significantly correlated to oesophageal cancer rates.
However, the rules for selection of families was different between the
two populations. The prevalence of F. verticillioides was greater in
home-grown maize collected in 12 households in the high-incidence area
compared to a similar collection in a low-incidence area. Households
in the high-incidence area were selected on the basis of cytological
examination of cells collected from the oesophagus (Marasas et al.,
1988b). Subsequent studies conducted after the chemical
characterization of the fumonisins in 1988 also found significantly
higher levels of F. verticillioides and fumonisins (20 times higher)
in areas with high rates of oesophageal cancer in Transkei than in
areas with low rates (Sydenham et al., 1990a,b; Rheeder et al., 1992).
F. verticillioides and F. graminearum cause maize ear disease
under quite different ecological conditions. The environmental
conditions that prevail in the areas in the Transkei with high rates
of oesophageal cancer clearly favour colonization of maize ears by
F. verticillioides. Significant fumonisin accumulation in maize
occurs periodically in all such environments examined so far,
primarily in relation to drought and other environmental stressors.
Taking this into account, the South African studies have shown that
the level of fumonisin in home-grown maize has been consistently high
in the areas in the Transkei with high rates of oesophageal cancer.
Cancer registry data have shown these areas to have consistently high
rates of oesophageal cancer since 1955 (Jaskiewicz et al., 1987c;
Makaula et al., 1996).
8.2 China
The only studies available were correlation studies where there
was no clear picture on the association of either fumonisin or
F. verticillioides contamination with oesophageal cancer.
Maize is consumed as a staple in a number of areas in China,
including Linxian and Cixian counties in Henan province (Zhen, 1984;
Chu & Li, 1994; Yoshizawa et al., 1994). Mortality rates for males in
the high-risk areas ranged from 26 to 36 per 100 000 in the low-risk
counties and from 76 to 161 per 100 000 in the high-risk counties. The
incidence of F. verticillioides has been reported to be higher in
maize in high- than low-risk areas, but the mycological data are
fragmentary (Zhen, 1984) and difficult to evaluate.
Maize samples from Linxian and Cixian Counties, both
high-incidence areas of oesophageal cancer in China, were analysed for
FB1 by Chu & Li (1994). All 31 samples contained FB1 at levels
ranging from 18 to 155 mg/kg. These results established that
home-grown maize in high incidence areas of oesophageal cancer in
China may be contaminated with very high levels of FB1. Another
investigation carried out on 246 maize samples showed that people
residing in an area with high incidence of human oesophageal cancer
are more exposed to fumonisins, although the exposure varied greatly
(Zhang et al., 1997). However, no relationship between fumonisin and
human oesophageal cancer incidence was evident from this study. In a
comparative study of FB1 levels in maize from high (Linxian) and low
(Shangqiu) oesophageal cancer areas, Yoshizawa et al. (1994) found no
significant differences between the areas, which was further confirmed
in a subsequent study (Gao & Yoshizawa, 1997). Levels of FB1 in 13/27
samples from the high incidence area were 0.18-2.9 (mean 0.87) mg/kg,
and in 5/20 samples from the low incidence areas, FB1 levels were
0.19-1.7 (mean 0.89) mg/kg (Yoshizawa et al., 1994).
In a comparative study of maize samples from high-risk (Haimen)
and low-risk (Penlai) areas for human primary liver cancer in China,
Ueno et al. (1997) reported significantly higher levels ( P < 0.01)
of total fumonisins in the high- than the low-risk area. Fumonisin
levels in 80/120 samples from the high-risk area for liver cancer were
0.14-34.9 mg/kg and from the low-risk area were 0.08-15.1 mg/kg in
54/120 samples for 2 of the 3 years under investigation (Ueno et al.,
1997).
8.3 Northern Italy
One analytical study was reported from Northern Italy.
Pordenone Province in the northeast of Italy has the highest
mortality rate for oral and pharyngeal cancers and oesophageal cancer
in Italy and amongst the highest in Europe (Franceschi et al., 1990).
Risk factors identified included alcohol and tobacco use, and
significant associations with maize consumption were found for oral
cancer (179 cases; odds ratios 3.3; confidence intervals 2.0-5.3),
pharyngeal cancer (170; 3.2; 2.0-5.3) and oesophageal cancer (68; 2.8;
1.5-5.1). There were 505 hospital controls. The elevated risk of upper
digestive tract cancer was, however, limited to persons consuming more
than 42 weekly drinks of alcohol (Franceschi et al., 1990). The
possibility of reporting bias can not be excluded and no measures of
fumonisin or F. verticillioides contamination were available. The
analysis was restricted to men.
In this region, most maize is locally produced and eaten as
cooked maize meal (polenta). Fumonisin-producing Fusarium species
were found on maize produced in Northern Italy (Logrieco et al.,
1995). One study showed that 20 samples of polenta produced in Italy
in 1993 and 1994 contained 0.15-3.76 mg FB1/kg (Pascale et al.,
1995).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
9.1 Microorganisms
In the only available study on the effects of fumonisins on
bacteria, Becker et al. (1997) reported that FB1 at concentrations
from 50 to 1000 µM (36-721 mg/litre) did not inhibit the growth of
various Gram-positive and Gram-negative bacteria. There was also no
indication that FB1 was metabolized by any of the bacteria tested.
Fumonisin was reported not to affect ethanol production
(presumably by Saccharomyces) in distillers wash made from maize
contaminated with 15 and 36 mg FB1/kg (Bothast et al., 1992).
Fumonisin concentrations of 25-100 mg/litre resulted in altered
sphingolipid precursors in Pichia ciferri (Kaneshiro et al., 1992).
This species accumulated some trihydro fatty acids in the presence of
50 mg FB1/litre. However, in Rhodotorula species, FB1 depressed
production of the same compounds (Kaneshiro et al., 1993). Pure FB1
inhibits cell growth of Saccharomyces cerevisiae and causes
accumulation of free sphingoid bases and disruption of lipid
metabolism (Wu et al., 1995).
9.2 Plants
9.2.1 Duckweed and jimsonweed
Because of their structural similarity to AAL toxins from
Alternaria alternata f.sp. lycopersici (also called TA toxins;
Bottini et al., 1981; Mirocha et al., 1992), fumonisins were suspected
of being phytotoxic and virulence factors by several investigators.
Fumonisin reduced chlorophyll synthesis by 59% in duckweed (Lemma
minor) fronds at 10-6 M (Vesonder et al., 1992). Photobleaching
occurred in excised jimsonweed (Datura stramonium) leaves, also in
the µM range, and at approximately 10-4 M damage to mesophyll cells
occurred after 6 h (Abbas et al., 1992). Fumonisin apparently causes
membrane damage, as shown by electrolyte loss in jimsonweed (Abbas et
al., 1991, 1993). Additionally, fumonisin disrupts the synthesis of
sphingolipids in these plants (Abbas et al., 1994).
9.2.2 Tomato
FB1 has similar toxicity to AAL-toxin-susceptible tomato
cultivars and is not active against AAL-resistant lines. Leaf necrosis
was reported at 0.4 µM by Mirocha et al. (1992) and at > 0.1 µM by
Lamprecht et al. (1994). Fumonisins were reported to cause a
dose-dependent reduction in shoot and root length and dry mass in
tomato seedlings (Lamprecht et al., 1994). As with duckweed, fumonisin
has been shown to disrupt sphingolipid metabolism in tomato (Abbas et
al., 1994). In contrast, FB1 added directly to excised shoots has
been reported to induce callus and roots at what appear to be high
doses (Bacon et al., 1994).
9.2.3 Maize
Despite reports to the contrary (Abbas & Boyette, 1992), FB1 is
toxic to maize cells. FB1 exposure did not reduce maize seed
germination but reduced radicle elongation when the solution
concentration was above 10-4 M, and seed amylase production was
inhibited (Doehlert et al., 1994). Fumonisin at concentrations in the
10 µM range decreased shoot length, shoot dry mass and root length
(Lamprecht et al., 1994). FB1 incorporated into plant tissue culture
media reduced the growth of maize callus at 10-6 M (Van Asch et al.,
1992). FB1 has been shown to disrupt sphingolipid metabolism in maize
seedlings (Riley et al., 1996). In crosses of high- and
low-fumonisin-producing strains of F. verticillioides, only progeny
that produced high concentrations of fumonisin in vitro caused
significant stem rot (Nelson et al., 1993). These data provide
indirect evidence that fumonisins play a role in the pathogenicity of
F. verticillioides to maize (Miller, 1995).
10. FURTHER RESEARCH
* There is urgent need for an internationally available standard of
pure fumonisin B1.
* An understanding of the fate of fumonisins in maize food
processing and cooking, particularly in developing countries, is
urgently required.
* There is urgent need to develop a validated biomarker for human
exposure to fumonisin.
* Epidemiological studies on the effects of fumonisins on human
health need to be conducted, based on sound intake estimates and
biomarkers.
* Valid methods for sampling for fumonisins in maize and for
sampling, extracting and quantifying fumonisins in foods need to
be developed.
* The influence of fumonisin on the carcinogenicity of other
agriculturally important mycotoxins (e.g., aflatoxin) and
carcinogenic infectious agents requires further study.
* The importance of other routes of exposure to fumonisins,
including occupational exposure through inhalation, needs to be
determined.
* There is urgent need for increased research on non-carcinogenic
end-points including hepatotoxicity, nephrotoxicity,
neurotoxicity, immunotoxicity, gastrointestinal toxicity,
cardiovascular toxicity, and the mechanistic basis for the
organ-selective toxicities.
* Research is needed to assess further the genotoxicity of
fumonisin in both germ and somatic cells in vitro and
in vivo.
* The basis for the sex differences in animals in the response to
fumonisin requires further investigation.
* The environmental fate of fumonisin in the ecosystem needs to be
established.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANIZATIONS
The International Agency for Research on Cancer evaluated FB1 in
1992 (IARC, 1993) and reached the following conclusions.
There is inadequate evidence in humans for the carcinogenicity
of toxins derived from F. verticillioides.
There is sufficient evidence in experimental animals for the
carcinogenicity of cultures of F. verticillioides that contain
significant amounts of fumonisins.
There is limited evidence in experimental animals for the
carcinogenicity of FB1.
Overall Evaluation: Toxins derived from Fusarium
verticillioides are possibly carcinogenic to humans (Group 2B).
REFERENCES
Abado-Becognee K, Mobio TA, Ennamany R, Fleurat-Lessard F, Shier WT,
Badria F, & Creppy EE (1998) Cytotoxicity of fumonisin B1:
implication of lipid peroxidation and inhibition of protein and DNA
syntheses. Arch Toxicol, 72: 233-236.
Abbas HK & Boyette CD (1992) Phytotoxicity of fumonisin B1 on weed
and crop species. Weed Technol, 6: 548-552.
Abbas HK & Ocamb CM (1995) First report of production of fumonisin B1
by Fusarium polyphialidicum collected from seeds of Pinus
strobus. Plant Dis, 79: 642.
Abbas HK & Riley RT (1996) The presence and phytotoxicity of
fumonisins and AAL-toxin in Alternaria alternata. Toxicon, 34:
133-136.
Abbas HK & Shier WT (1997) Phytotoxicity of australifungin and
fumonisins to weeds. In: Proceedings of the 1997 Brighton Crop
Protection Conference-Weeds. Croydon, United Kingdom, British Crop
Protection Council, pp 795-800.
Abbas HK, Boyette CD, Hoagland RE, & Vesonder RF (1991) Bioherbicidal
potential of Fusarium moniliforme and its phytotoxin, fumonisin.
Weed Sci, 39: 673-677.
Abbas HK, Paul RN, Boyette CD, Duke SO, & Vesonder RF (1992)
Physiological and ultrastructural effects of fumonisin on jimsonweed
leaves. Can J Bot, 70: 1824-1833.
Abbas HK, Gelderblom WCA, Cawood ME, & Shier WT (1993) Biological
activities of fumonisins, mycotoxins from Fusarium moniliforme in
jimsonweed (Datura stramonium L.) and mammalian cell cultures.
Toxicon, 31: 345-353.
Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions
AE, Wang E, Merrill AH, & Riley RT (1994) Fumonisin- and
AAL-toxin-induced disruption of sphingolipid metabolism with
accumulation of free sphingoid bases. Plant Physiol, 106: 1085-1093.
Abbas HK, Ocamb CM, Xie W, Mirocha CJ, & Shier WT (1995) First report
of fumonisin B1, B2, and B3 production by Fusarium oxysporum var.
redolens. Plant Dis, 79: 968.
Abbas HK, Cartwright RD, Shier WT, Abouzied MM, Bird CB, Rice LG, Ross
PF, Sciumbato GL, & Meredith FI (1998) Natural occurrence of
fumonisins in rice with Fusarium sheat rot disease. Plant Disease,
82: 22-25.
Abel S & Gelderblom WCA (1998) Oxidative damage and fumonisin
B1-induced toxicity in primary rat hepatocytes and rat liver
in vivo. Toxicology, 131(2-3): 121-131.
Alberts JF, Gelderblom WCA, Thiel PG, Marasas WFO, Van Schalkwyk DJ, &
Behrend Y (1990) Effects of temperature and incubation period on
production of fumonisin B1 by Fusarium moniliforme. Appl Environ
Microbiol, 56: 1729-1733.
Alberts JF, Gelderblom WCA, Vleggaar R, Marasas WFO, & Rheeder JP
(1993) Production of 14C-labelled fumonisin B1 by Fusarium
moniliforme MRC 826 in corn culture. Appl Environ Microbiol, 59:
2673-2677.
Ali NS, Yamashita A, & Yoshizawa T (1998) Natural co-occurrence of
aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol,
nivalenol and zearalenone) in corn from Indonesia. Food Addit Contam,
15: 377-384.
Arora A, Jones BJ, Patel TC, Bronk SF, & Gores GJ (1997) Ceramide
induces hepatocyte cell death through disruption of mitochondrial
function in the rat. Hepatology, 25: 958-963.
Azcona-Olivera JI, Abouzied MM, Plattner RD, Norred WP, & Pestka JJ
(1992a) Generation of antibodies reactive with fumonisins B1, B2 and
B3 by using cholera toxin as the carrier-adjuvant. Appl Environ
Microbiol, 58: 169-173.
Azcona-Olivera JI, Abouzied MM, Plattner RD, & Pestka JJ (1992b)
Production of monoclonal antibodies to the mycotoxins fumonisin B1,
B2, and B3. J Agric Food Chem, 40: 531-534.
Azuka CE, Osweiler GD, & Reynolds DL (1993) Effect of glutathione and
vitamin E on cytotoxicity of fumonisins. Toxicologist, 13: 233.
Bacon CW & Williamson JW (1992) Interactions of Fusarium
moniliforme, its metabolites and bacteria with corn. Mycopathologia,
117: 65-71.
Bacon CW, Hinton DM, Chamberlain WJ, & Norred WP (1994) De novo
induction of adventitious roots in excised shoots of tomatoes by
fumonisin B1, a metabolite of Fusarium moniliforme. J Plant Growth
Regul, 13: 53-57.
Bacon CW, Porter JK, & Norred WP (1995) Toxic interaction of fumonisin
B1 and fusaric acid measured by injection into fertile chicken egg.
Mycopathologia, 129: 29-35.
Badiani K, Byers DM, Cook HW, & Ridgway ND (1996) Effect of fumonisin
B1 on phosphatidylethanolamine biosynthesis in Chinese hamster ovary
cells. Biochim Biophys Acta, 1304: 190-196.
Ballou LR, Laulederkind SJF, Rosloniec EF, & Raghow R (1996) Ceramide
signalling and the immune response. Biochim Biophys Acta, 1301:
273-287.
Balsinde J, Balboa MA, & Dennis EA (1997) Inflammatory activation of
arachidonic acid signaling in murine P388D1 macrophages via
sphingomyelin synthesis. J Biol Chem, 272: 20373-20377.
Bane DP, Neumann EJ, Hall WF, Harlin KS, & Slife LN (1992)
Relationship between fumonisin contamination of feed and mystery swine
disease. Mycopathologia, 117: 121-124.
Becker BA, Pace L, Rottinghaus GE, Shelby R, Misfeldt M, & Ross MS
(1995) Effects of feeding fumonisin B1 in lactating sows and their
suckling pigs. Am J Vet Res, 56: 1253-1258.
Becker B, Bresch H, Schillinger U, & Thiel PG (1997) The effect of
fumonisin B1 on the growth of bacteria. World J Microbiol Biotechnol,
13: 539-543.
Bell RM, Hannun YA, & Merrill AH Jr ed. (1993) Advances in lipid
research: sphingolipids and their metabolites. Orlando, Florida,
Academic Press, vol 25-26.
Bennett GA & Richard JL (1994) Liquid chromatographic method for the
naphthalene dicarboxaldehyde derivate of fumonisins. J Assoc Off Anal
Chem Int, 77: 501-505.
Bennett GA & Richard JL (1996) Influence of processing on Fusarium
mycotoxins in contaminated grains. Food Technol, May: 235-238.
Bermudez AJ, Ledoux DR, Turk JR, & Rottinghaus GE (1996) The chronic
effects of Fusarium moniliforme culture material containing known
levels of fumonisin B1, in turkeys. Avian Dis, 40: 231-235.
Bermudez AJ, Ledoux DR, Rottinghaus GE, & Bennett GA (1997) The
individual and combined effects of the Fusarium mycotoxins
moniliformin and fumonisin B1 in turkeys. Avian Dis, 41: 304-311.
Bezuidenhout SC, Gelderblom WCA, Gorst-Allman CP, Horak RM, Marasas
WFO, Spiteller G, & Vleggaar R (1988) Structure elucidation of the
fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem
Commun, 1988: 743-745.
Bhat RV, Shetty PH, Amruth RP, & Sudershan RV (1997) A foodborne
disease outbreak due to the consumption of moldy sorghum and maize
containing fumonisin mycotoxins. Clin Toxicol, 35: 249-255.
Blackwell BA, Miller JD, & Savard ME (1994) Production of carbon
14-labeled fumonisin in liquid culture. J Assoc Off Anal Chem Int, 77:
506-511.
Boland MP, Foster SJ, & O'Neill LAJ (1997) Daunorubicin activates NFkB
and induces kB-dependent gene expression in HL-60 promyelocytic and
Jurkat T lymphoma cells. J Biol Chem, 272: 12952-12960.
Boldin S & Futerman AH (1997) Glucosylceramide synthesis is required
for basic fibroblast growth factor and laminin to stimulate axonal
growth. J Neurochem, 68: 882-885.
Bondy G, Suzuki C, Barker M, Armstrong C, Fernie S, Hierlihy L,
Rowsell P, & Mueller R (1995) Toxicity of fumonisin B1 administered
intraperitoneally to male Sprague-Dawley rats. Food Chem Toxicol, 33:
653-665.
Bondy GS, Suzuki CAM, Fernie SM, Armstrong CL, Hierlihy SL, Savard ME,
& Barker MG (1997) Toxicity of fumonisin B1 to B6C3F1 mice: a 14-day
gavage study. Food Chem Toxicol, 35: 981-989.
Bondy GS, Suzuki CAM, Mueller RW, Fernie SM, Armstrong CL, Hierlihy
SL, Savard ME, & Barker MG (1998) Gavage administration of the fungal
toxin fumonisin B1 to female Sprague-Dawley rats. J Toxicol Environ
Health, 53: 135-151.
Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, & Kolesnick
R (1995) Ceramide synthase mediates daunorubicin-induced apoptosis: An
alternative mechanism for generating death signals. Cell, 82: 404-414.
Bothast RJ, Bennett GA, Vancauwenberge JE, & Richard JL (1992) Fate of
fumonisin B1 in naturally contaminated corn during ethanol
fermentation. Appl Environ Microbiol, 58: 233-236.
Bottalico A, Logrieco A, Ritieni A, Moretti A, Randazzo G, & Corda P
(1995) Beauvericin and fumonisin B1 in preharvest Fusarium
moniliforme maize ear rot in Sardinia. Food Addit Contam, 12:
599-607.
Bottini AT, Bowen JR, & Gilchrist DG (1981) Phytotoxins: II.
Characterization of a phytotoxic fraction from Alternaria
alternata f. sp. lycopersici. Tetrahedron Lett, 22: 2723-2726.
Bradlaw J, Pritchard D, Flynn T, Eppley R, & Stack M (1994) In
vitro assessment of fumonisin B1 toxicity using reaggregate
cultures of chick embryo neural retina cells (CERC). In Vitro Cell Dev
Biol, 30A: 92.
Brown DW, McCoy CP, & Rottinghaus GE (1994) Experimental feeding of
Fusarium moniliforme culture material containing fumonisin B1 to
channel catfish, Ictalurus punctatus. J Vet Diagn Invest, 6:
123-124.
Brown TP, Rottinghaus GE, & Williams ME (1992) Fumonisin mycotoxicosis
in broilers: Performance and pathology. Avian Dis, 36: 450-454.
Bryden WL, Love RJ, & Burgess LW (1987) Feeding grain contaminated
with Fusarium graminearum and Fusarium moniliforme to pigs and
chickens. Aust Vet J, 64: 225-226.
Bryden WL, Ravindran G, Amba MT, Gill RJ, & Burgess LW (1996)
Mycotoxin contamination of maize grown in Australia, the Philippines
and Vietnam. In: Miraglia M, Brera C, & Onori R ed. Ninth
International IUPAC Symposium on Mycotoxins and Phycotoxins, Rome,
27-31 May 1996: Abstract book. Rome, Istituto Superiore di Sanità, p
41.
Bucci TJ, Hansen DK, & LaBorde JB (1996) Leukoencephalomalacia and
hemorrhage in the brain of rabbits gavaged with mycotoxin fumonisin
B1. Nat Toxins, 4: 51-52.
Bullerman LB & Tsai W-Y (1994) Incidence and levels of Fusarium
moniliforme, Fusarium proliferatum and fumonisins in corn and
corn-based foods and feeds. J Food Prot, 57: 541-546.
Burdaspal PA & Legarda TM (1996) Occurrence of fumonisins in corn and
processed corn-based commodities for human consumption in Spain. In:
Miraglia M, Brera C, & Onori R ed. IX International IUPAC Symposium on
Mycotoxins and Phycotoxins, Rome, 27-31 May 1996: Abstract book. Rome,
Istituto Superiore di Sanità, p 167.
Butler T (1902) Notes on a feeding experiment to produce
leucoencephalitis in a horse, with positive results. Am Vet Rev, 26:
748-751.
Cabot MC, Han T-Y, & Giuliano AE (1998) The multidrug resistance
modulator SDZ PSC 833 is a potent activator of cellular ceramide
formation. FEBS Lett, 431: 185-188.
Cabot MC, Giuliano AE, Han T-Y, & Liu Y-Y (1999) SDZ PSC 833, the
cyclosporine A analogue and multidrug resistance modulator, activates
ceramide synthesis and increases vinblastine sensitivity in
drug-sensitive and drug-resistant cancer cells. Cancer Res, 59:
880-885.
Caramelli M, Dondo A, Cantini Cortellazzi G, Visconti A, Minervini F,
Doko MB, & Guarda F (1993) [Equine leukoencephalomalacia from
fumonisin: First case in Italy.] Ippologia, 4: 49-56 (in Italian).
Casteel SW, Turk JR, Cowart RP, & Rottinghaus GE (1993) Chronic
toxicity of fumonisin in weanling pigs. J Vet Diagn Invest, 5:
413-417.
Casteel SW, Turk JR, & Rottinghaus GE (1994) Chronic effects of
dietary fumonisin on the heart and pulmonary vasculature of swine.
Fundam Appl Toxicol, 23: 518-524.
Castegnaro M, Garren L, Gaucher I, & Wild CP (1996) Development of a
new method for the analysis of sphinganine and sphingosine in urine
and tissues. Nat Toxins, 4: 284-290.
Castella G, Bragulat MR, & Cabañes FJ (1997) Occurrence of
Fusarium species and fumonisins in some animal feeds and raw
materials. Cereal Res Commun, 25: 355-356.
Cawood ME, Gelderblom WCA, Vleggaar R, Behrend Y, Thiel PG, & Marasas
WFO (1991) Isolation of the fumonisin mycotoxins: A quantitative
approach. J Agric Food Chem, 39: 1958-1962.
Cawood ME, Gelderblom WCA, Alberts JF, & Snyman SD (1994) Interaction
of 14C-labelled fumonisin B mycotoxins with primary rat hepatocyte
cultures. Food Chem Toxicol, 32: 627-632.
Chamberlain WJ, Voss KA, & Norred WP (1993) Analysis of commercial
laboratory rat rations for fumonisin B1, a mycotoxin produced on corn
by Fusarium moniliforme. Contemp Top, 32: 26-28.
Chelkowski J & Lew H (1992) Fusarium species of Liseola section -
occurrence in cereals and ability to produce fumonisins. Microbiol
Alim Nutr, 10: 49-53.
Chu FS & Li GY (1994) Simultaneous occurrence of fumonisin B1 and
other mycotoxins in moldy corn collected from the People's Republic of
China in regions with high incidences of esophageal cancer. Appl
Environ Microbiol, 60: 847-852.
Chulze SN, Ramirez ML, Farnochi MC, Pascale M, Visconti A, & March G
(1996) Fusarium and fumonisin occurrence in Argentinian corn at
different ear maturity stages. J Agric Food Chem, 44: 2797-2801.
Churchwell MI, Cooper WM, Howard PC, & Doerge DR (1997) Determination
of fumonisins in rodent feed using HPLC with electrospray mass
spectrometric detection. J Agric Food Chem, 45: 2573-2578.
Ciacci-Zanella JR & Jones C (1999) Analysis of apoptosis induced by
fumonisin B1, a novel mycotoxin frequently found in cereal grains.
Food Chem Toxicol, 37: 703-712.
Ciacci-Zanella JR, Merrill AH Jr, Wang E, & Jones C (1998)
Characterization of cell cycle arrest by fumonisin B1 in CV-1 cells.
Food Chem Toxicol, 36: 791-804.
Collins TF, Shacklelford ME, Sprando RL, Black TN, LaBorde JB, Hansen
DK, Eppley RM, Trucksess MW, Howard PC, Bryant MA, Ruggles DI, Olejnik
N, & Rorie JI (1998a) Effects of fumonisin B1 in pregnant rats. Food
Chem Toxicol, 36: 397-408.
Collins TF, Sprando RL, Black TN, Shacklelford ME, LaBorde JB, Hansen
DK, Eppley RM, Trucksess MW, Howard PC, Bryant MA, Ruggles DI, Olejnik
N, & Rorie JI (1998b) Effects of fumonisins in pregnant rats, part 2.
Food Chem Toxicol, 36: 673-685.
Colvin BM & Harrison LR (1992) Fumonisin-induced pulmonary edema and
hydrothorax in swine. Mycopathologia, 117: 79-82.
Colvin BM, Cooley AJ, & Beaver RW (1993) Fumonisin toxicosis in swine:
Clinical and pathologic findings. J Vet Diagn Invest, 5: 232-241.
Counts RS, Nowak G, Wyatt RD, & Schnellmann RG (1996) Nephrotoxicant
inhibition of renal proximal tubule cell regeneration. Am J Physiol,
269: F274-F281.
Dawlatana M, Coker RD, Nagler MJ, & Blunden G (1995) A normal phase
HPTLC method for the quantitative determination of fumonisin B1 in
rice. Chromatographia, 41: 187-190.
De León C & Pandey S (1989) Improvement of resistance to ear and stalk
rots and agronomic traits in tropical maize gene pools. Crop Sci, 29:
12-17.
de Nijs M (1998) Public health aspects of Fusarium mycotoxins in
food in The Netherlands - a risk assessment. Wageningen, The
Netherlands, Agricultural University, 140 pp (Thesis).
de Nijs M, van Egmond HP, Nauta M, Rombouts FM, & Notermans SHW
(1998a) Assessment of human exposure to fumonisin B1. J Food Prot,
61: 879-884.
de Nijs M, Sizoo EA, Rombouts FM, Notermans SHW, & van Egmond HP
(1998b) Fumonisin B1 in maize for food production imported in The
Netherlands. Food Addit Contam, 15: 389-392.
de Nijs M, Sizoo EA, Vermunt AEM, Notermans SHW, & van Egmond HP
(1998c) The occurrence of fumonisin B1 in maize-containing foods in
The Netherlands. Food Addit Contam, 15: 385-388.
Desjardins AE, Plattner RD, & Nelson PE (1994) Fumonisin production
and other traits of Fusarium moniliforme strains from maize in
northeast Mexico. Appl Environ Microbiol, 60: 1695-1697.
Desjardins AE, Plattner RD, Lu M, & Claflin LE (1998) Distribution of
fumonisins in maize ears infected with strains of Fusarium
moniliforme that differ in fumonisin production. Plant Dis, 82:
953-958.
Diaz GJ & Boermans HJ (1994) Fumonisin toxicosis in domestic animals:
a review. Vet Hum Toxicol, 36: 548-555.
DiPietrantonio AM, Hsieh T, Olson SC, & Wu JM (1998) Regulation of
G1/S transition and induction of apoptosis in HL-60 leukemia cells by
fenretinide (4HPR). Int J Cancer, 78: 53-61.
Doehlert DC, Knutson CA, & Vesonder RF (1994) Phytotoxic effects of
fumonisin B1 on maize seedling growth. Mycopathologia, 127: 117-121.
Doerge DR, Howard PC, Bajic S, & Preece S (1994) Determination of
fumonisins using on-line liquid chromatography coupled to electrospray
mass spectrometry. Rapid Commun Mass Spectrom, 8: 603-606.
Doko MB & Visconti A (1994) Occurrence of fumonisins B1 and B2 in
corn and corn-based human foodstuffs in Italy. Food Addit Contam, 11:
433-439.
Doko MB, Rapior S, & Visconti A (1994) Screening for fumonisins B1
and B2 in corn and corn-based foods and feeds from France. In:
Abstracts of the 7th International Congress of the IUMS Mycology
Division, Prague, Czech Republic, 3-8 July 1994, p 468.
Doko MB, Rapior S, Visconti A, & Schjth JE (1995) Incidence and
levels of fumonisin contamination in maize genotypes grown in Europe
and Africa. J Agric Food Chem, 43: 429-434.
Doko MB, Canet C, Brown N, Sydenham EW, Mpuchane S, & Siame BA (1996)
Natural co-occurrence of fumonisins and zearalenone in cereals and
cereal-based foods from Eastern and Southern Africa. J Agric Food
Chem, 44: 3240-3243.
Dombrink-Kurtzman MA, Javid T, Bennett GA, Richard JL, Cote LM, & Buck
WB (1993) Lymphocyte cytotoxicity and erythrocytic abnormalities
induced in broiler chicks by fumonisins B1 and B2 and moniliformin
from Fusarium proliferatum. Mycopathologia, 124: 47-54.
Dombrink-Kurtzman MA, Bennett GA, & Richard JL (1994a) An optimized
MTT bioassay for determination of cytotoxicity of fumonisins in turkey
lymphocytes. J Assoc Off Anal Chem Int, 77: 512-516.
Dombrink-Kurtzman MA, Bennett GA, & Richard JL (1994b) Induction of
apoptosis in turkey lymphocytes by fumonisin. FASEB J, 8: A488.
Dragoni I, Pascale M, Piantanida L, Tirilly Y, & Visconti A (1996)
[Presence of fumonisin in foodstuff destined for feeding to pigs in
Britanny (France).] Microbiol Alim Nutr, 14: 97-103 (in Italian).
Dugyala RR, Sharma RP, Tsunoda M, & Riley RT (1998) Tumor necrosis
factor- alpha as a contributor in fumonisin B1 toxicity. J Pharmacol
Exp Ther, 285: 317-324.
Duncan K, Kruger S, Zabe N, Kohn B, & Prioli R (1998) Improved
fluorometric and chromatographic methods for the quantification of
fumonisins B1, B2 and B3. J Chromatogr, A815: 41-47.
Dupuy J, Le Bars P, Boudra H, & Le Bars J (1993a) Thermostability of
fumonisin B1, a mycotoxin from Fusarium moniliforme, in corn. Appl
Environ Microbiol, 59: 2864-2867.
Dupuy J, Le Bars P, Le Bars J, & Boudra H (1993b) Determination of
fumonisin B1 in corn by instrumental thin layer chromatography. J
Planar Chromatogr, 6: 476-480.
Dutton MF (1996) Fumonisins, mycotoxins of increasing importance:
their nature and their effects. Pharmacol Ther, 70: 137-161.
Duvick J, Rood T, & Grant S (1994) Isolation of fumonisin-metabolizing
fungi from maize seed. In: Proceedings of the Fifth International
Mycological Congress, Vancouver, Canada, 14-21 August 1994, 56 pp.
Duvick J, Rood T, Maddox J, & Gilliam J (1998) Detoxification of
mycotoxins in plants as a strategy for improving grain quality and
disease resistance: Identification of fumonisin-degrading microbes
from maize. In: Kohmoto K & Yoder OC ed. Molecular genetics of host
specific toxins in plant diseases. Dordrecht, Kluwer Academic
Publishers, pp 369-381.
Edrington TS, Kamps-Holtzapple CA, Harvey RB, Kubena LF, Elissalde MH,
& Rottinghaus GE (1995) Acute hepatic and renal toxicity in lambs
dosed with fumonisin-containing culture material. J Anim Sci, 73:
508-515.
Espada Y, Ruiz de Gopegui R, Cuadradas C, & Cabañes FJ (1994)
Fumonisin mycotoxicosis in broilers: Weights and serum chemistry
modifications. Avian Dis, 38: 454-460.
Espada Y, Ruiz de Gopegui R, Cuadradas C, & Cabañes FJ (1997)
Fumonisin mycotoxicosis in broilers: Plasma proteins and coagulation
modifications. Avian Dis, 41: 73-79.
Farrar JJ & Davis RM (1991) Relationships among ear morphology,
western flower thrips and Fusarium ear rot of corn. Phytopathology,
81: 661-666.
Fazekas B, Bajmócy E, Glávits R, Fenyvesi A, & Tanyi J (1998)
Fumonisin B1 contamination of maize and experimental acute fumonisin
toxicosis in pigs. J Vet Med, B45: 171-181.
Ferguson SA, Omer VE, Kwon OS, Holson RR, Houston RJ, Rottinghaus GE,
& Slikker W Jr (1997) Prenatal fumonisin (FB1) treatment in rats
results in minimal maternal or offspring toxicity. Neurotoxicology,
18: 561-569.
Fincham JE, Marasas WFO, Taljaard JJF, Kriek NPJ, Badenhorst CJ,
Gelderblom WCA, Seier JV, Smuts CM, Faber M, Weight MJ, Slazus W,
Woodroof CW, van Wyk MJ, Kruger M, & Thiel PG (1992) Atherogenic
effects in a non-human primate of Fusarium moniliforme cultures
added to a carbohydrate diet. Atherosclerosis, 94: 13-25.
Floss JL, Casteel SW, Johnson GC, & Rottinghaus GE (1994a)
Developmental toxicity in hamsters of an aqueous extract of
Fusarium moniliforme culture material containing known quantities of
fumonisin B1. Vet Hum Toxicol, 36: 5-10.
Floss JL, Casteel SW, Johnson GC, Rottinghaus GE, & Krause GF (1994b)
Developmental toxicity of fumonisin in Syrian hamsters.
Mycopathologia, 128: 33-38.
Flynn GL (1985) Mechanism of percutaneous absorption from
physicochemical evidence. In: Bronaugh RL & Maibach HI ed.
Percutaneous absorption. New York, Marcel Dekker, pp 17-42.
Flynn TJ, Pritchard D, Bradlaw J, Eppley R, & Page S (1994) Effects of
the mycotoxin fumonisin B1 and its alkaline hydrolysis product on
pre-somite rat embryos in vitro. Teratology, 49: 404.
Flynn TJ, Pritchard D, Bradlaw JA, Eppley R, & Page S (1996) In
vitro embryotoxicity of fumonisin B1 evaluated with cultured
postimplantation staged rat embryos. Toxicol In Vitro, 9: 271-279.
Flynn TJ, Stack ME, Troy AL, & Chirtel SJ (1997) Assessment of the
embryotoxic potential of the total hydrolysis product of fumonisin B1
using cultured organogenesis-staged rat embryos. Food Chem Toxicol,
35: 1135-1141.
Franceschi S, Bidoli E, Barón AE, & La Vecchia C (1990) Maize and risk
of cancer of the oral cavity, pharynx and esophagus in northeastern
Italy. J Natl Cancer Inst, 82: 1407-1411.
Fukuda H, Shima H, Vesonder RF, Tokuda H, Nishino H, Katoh S, Tamura
S, Sugimura T, & Nagao M (1996) Inhibition of protein serine/threonine
phosphatases by fumonisin B1, a mycotoxin. Biochem Biophys Res
Commun, 220: 160-165.
Furuya S, Ono K, & Hirabayashi Y (1995) Sphingolipid biosynthesis is
necessary for dendrite growth and survival of cerebellar Purkinje
cells in culture. J Neurochem, 65: 1551-1561.
Gao H-P & Yoshizawa T (1997) Further study on Fusarium mycotoxins in
corn and wheat from a high-risk area for human esophageal cancer in
China. Mycotoxins, 45: 51-55.
Garzotto M, White-Jones M, Jiang Y, Ehleiter D, Liao W-C,
Haimovitz-Friedman A, Fuks Z, & Kolesnick R (1998)
12- O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP
cells is mediated through ceramide synthase. Cancer Res, 58:
2260-2264.
Gelderblom WCA & Snyman SD (1991) Mutagenicity of potentially
carcinogenic mycotoxins produced by Fusarium moniliforme. Mycotoxin
Res, 7: 46-52.
Gelderblom WCA, Jaskiewicz K, Marasas WFO, Thiel PG, Horak RM,
Vleggaar R, & Kriek NPJ (1988) Fumonisins - Novel mycotoxins with
cancer-promoting activity produced by Fusarium moniliforme. Appl
Environ Microbiol, 54: 1806-1811.
Gelderblom W, Marasas W, Thiel P, Semple E, & Farber E (1989) Possible
non-genotoxic nature of active carcinogenic components produced by
Fusarium moniliforme. Proc Am Assoc Cancer Res, 30: 144.
Gelderblom WCA, Kriek NPJ, Marasas WFO, & Thiel PG (1991) Toxicity and
carcinogenicity of the Fusarium moniliforme metabolite, fumonisin
B1, in rats. Carcinogenesis, 12: 1247-1251.
Gelderblom WCA, Marasas WFO, Vleggaar R, Thiel PG, & Cawood ME (1992a)
Fumonisins: Isolation, chemical characterization and biological
effects. Mycopathologia, 117: 11-16.
Gelderblom WCA, Semple E, Marasas WFO, & Farber E (1992b) The
cancer-initiating potential of the fumonisin B mycotoxins.
Carcinogenesis, 13: 433-437.
Gelderblom WCA, Cawood ME, Snyman SD, Vleggaar R, & Marasas WFO (1993)
Structure-activity relationships of fumonisins in short-term
carcinogenesis and cytotoxicity assays. Food Chem Toxicol, 31:
407-414.
Gelderblom WCA, Cawood ME, Snyman SD, & Marasas WFO (1994) Fumonisin
B1 dosimetry in relation to cancer initiation in rat liver.
Carcinogenesis, 15: 209-214.
Gelderblom WCA, Snyman SD, van der Westhuizen L, & Marasas WFO (1995)
Mitoinhibitory effect of fumonisin B1 on rat hepatocytes in primary
culture. Carcinogenesis, 16: 625-631.
Gelderblom WCA, Smuts CM, Abel S, Snyman SD, Cawood MA, van der
Westhuizen L, & Swanevelder S (1996a) Effect of fumonisin B1 on
protein and lipid synthesis in primary rat hepatocytes. Food Chem
Toxicol, 34: 361-369.
Gelderblom WCA, Snyman SD, Abel S, Lebepe-Mazur S, Smuts CM, van der
Westhuizen L, Marasas WFO, Victor TC, Knasmüller S, & Huber W (1996b)
Hepatotoxicity and carcinogenicity of the fumonisins in rats: A review
regarding mechanistic implications for establishing risk in humans.
Adv Exp Med Biol, 392: 279-296.
Gelderblom WCA, Snyman SD, Lebepe-Mazur S, van der Westhuizen L, Kriek
NPJ, & Marasas WFO (1996c) The cancer-promoting potential of fumonisin
B1 in rat liver using diethylnitrosamine as a cancer initiator.
Cancer Lett, 109: 101-108.
Gelderblom WCA, Smuts CM, Abel S, Snyman SD, van der Westhuizen L,
Huber WW, & Swanevelder S (1997) Effect of fumonisin B1 on the levels
and fatty acid composition of selected lipids in rat liver, in
vivo. Food Chem Toxicol, 35: 647-656.
Gillard BK, Harrell RG, & Marcus DM (1996) Pathways of
glycosphingolipid biosynthesis in SW13 cells in the presence and
absence of vimentin intermediate filaments. Glycobiology, 6: 33-42.
Goel S, Lenz SD, Lumlertdacha S, Lovell RT, Shelby RA, Li M, Riley RT,
& Kemppainen BW (1994) Sphingolipid levels in catfish consuming
Fusarium moniliforme corn culture material containing fumonisins.
Aquat Toxicol, 30: 285-294.
Gross SM, Reddy RV, Rottinghaus GE, Johnson G, & Reddy CS (1994)
Developmental effects of fumonisin B1-containing Fusarium
moniliforme culture extract in CD1 mice. Mycopathologia, 128:
111-118.
Gumprecht LA, Marcucci A, Weigel RM, Vesonder RF, Riley RT, Showker
JL, Beasley VR, & Haschek WM (1995) Effects of intravenous fumonisin
B1 in rabbits: nephrotoxicity and sphingolipid alterations. Nat
Toxins, 3: 395-403.
Gumprecht LA, Beasley VR, Weigel RM, Parker HM, Tumbleson ME, Bacon
CW, Meredith FI, & Haschek WM (1998) Development of fumonisin-induced
hepatotoxicity and pulmonary edema in orally dosed swine:
morphological and biochemical alterations. Toxicol Pathol, 26:
777-788.
Guzman RE, Bailey K, Casteel SW, Turk J, & Rottinghaus GE (1997)
Dietary Fusarium moniliforme culture material induces in vitro
tumor necrosis factor-alpha like activity in sera of swine.
Immunopharmacol Immuntoxicol, 19: 279-289.
Hall JO, Javed T, Bennett GA, Richard JL, Dombrink-Kurtzman MA, Côté
LM, & Buck WB (1995) Serum vitamin A (retinol) reduction in broiler
chicks on feed amended with Fusarium proliferatum culture material
or fumonisin B1 and moniliformin. J Vet Diagn Invest, 7: 416-418.
Hammer P, Blüthgen A, & Walte HG (1996) Carry-over of fumonisin B1
into the milk of lactating cows. Milchwissenschaft, 51: 691-695.
Hanada K, Nishijima M, & Akamatsu Y (1990) A temperature-sensitive
mammalian cell mutant with thermolabile serine palmitoyltransferase
for the sphingolipid biosynthesis. J Biol Chem, 265: 22137-22142.
Hanada K, Nishijima M, Kiso H Jr, Hasegawa A, Fujita S, Ogawa T, &
Akamatsu Y (1992) Sphingolipids are essential for the growth of
Chinese hamster ovary cells: Restoration of the growth of a mutant
defective in sphingoid base biosynthesis by exogenous sphingolipids. J
Biol Chem, 267: 23527-23533.
Hanada K, Izawa K, Nishijima M, & Akamatsu Y (1993) Sphingolipid
deficiency induces hypersensitivity of CD14, a glycosyl
phosphatidylinositol-anchored protein, to
phosphatidylinositol-specific phospholipase C. J Biol Chem, 268:
13820-13823.
Hannun YA, Merrill AH Jr, & Bell RM (1991) Use of sphingosine as an
inhibitor of protein kinase C. Meth Enzymol, 201: 316-328.
Harel R & Futerman AH (1993) Inhibition of sphingolipid synthesis
affects axonal outgrowth in cultured hippocampal neurons. J Biol Chem,
268: 14476-14481.
Harrison LR, Colvin BM, Greene JT, Newman LE, & Cole JR Jr (1990)
Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a
toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest, 2:
217-221.
Hartwell LH & Kastan MB (1994) Cell cycle control and cancer. Science,
266: 1821-1828.
Harvey RB, Edrington TS, Kubena LF, Elissalde MH, & Rottinghaus GE
(1995) Influence of aflatoxin and fumonisin B1-containing culture
material on growing barrows. Am J Vet Res, 56: 1668-1672.
Harvey RB, Edrington TS, Kubena LF, Elissalde MH, Casper HH,
Rottinghaus GE, & Turk JR (1996) Effects of dietary fumonisin
B1-containing culture material, deoxynivalenol-contaminated wheat, or
their combination on growing barrows. Am J Vet Res, 57: 1790-1794.
Haschek WM, Motelin G, Ness DK, Harlin KS, Hall WF, Vesonder R,
Peterson RE, & Beasley VR (1992) Characterization of fumonisin
toxicity in orally and intravenously dosed swine. Mycopathologia, 117:
83-96.
Haschek WM, Gumprecht LA, Smith GW, Parker HM, Beasley VR, & Tumbleson
ME (1996) Effects of fumonisins in swine. In: Advances in swine
biomedical research. New York, Plenum Press, pp 99-112.
Hendrich S, Miller KA, Wilson TM, & Murphy PA (1993) Toxicity of
Fusarium proliferatum-fermented nixtamalized corn-based diets fed to
rats: Effect of nutritional status. J Agric Food Chem, 41: 1649-1654.
Hendricks K (1999) Fumonisins and neural tube defects in South Texas.
Epidemiology, 10: 198-200.
Henry MH (1993) Bioproduction of fumonisin B1 in chicken embryos and
broiler chicks. University of Georgia, 93 pp (Masters of Science
Thesis).
Hesseltine CW, Rogers RF, & Shotwell OL (1981) Aflatoxin and mold
flora in North Carolina in 1977 corn crop. Mycologia, 73: 216-228.
Hidari KI-PJ, Ichikawa S, Fujita T, Sakiyama H, & Hirabayashi Y (1996)
Complete removal of sphingolipids from the plasma membrane disrupts
cell to substratum adhesion of mouse melanoma cells. J Biol Chem, 271:
14636-14641.
Hirooka EY, Yamaguchi MM, Aoyama S, Sugiura Y, & Ueno Y (1996) The
natural occurrence of fumonisins in Brazilian corn kernels. Food Addit
Contam, 13: 173-183.
Hoenisch RW & Davis RM (1994) Relationship between kernel pericarp
thickness and susceptibility to Fusarium ear rot in field corn.
Plant Dis, 78: 517-519.
Holcomb M & Thompson HC Jr (1996) Analysis of fumonisin B1 in rodent
feed by CE with fluorescence detection of the FMOC derivative. J Cap
Electrophor, 3: 205-208.
Holcomb M, Thompson HC Jr, & Hankins LJ (1993) Analysis of fumonisin
B1 in rodent feed by gradient elution HPLC using precolumn
derivatization with FMOC and fluorescence detection. J Agric Food
Chem, 41: 764-767.
Hopmans EC & Murphy PA (1993) Detection of fumonisins B1, B2, and
B3 and hydrolyzed fumonisin B1 in corn-containing foods. J Agric
Food Chem, 41: 1655-1658.
Hopmans EC, Hauck CC, Hendrich S, & Murphy PA (1997) Excretion of
fumonisin B1, hydrolyzed fumonisin B1, and the fumonisin
FB1-fructose adduct in rats. J Agric Food Chem, 45: 2618-2625.
Horvath A, Sutterlin C, Manning-Krieg U, Movva NR, & Riezman H (1994)
Ceramide synthesis enhances transport of GPI-anchored proteins to the
Golgi apparatus in yeast. EMBO J, 13:3687-3695.
Howard PC, Churchwell MI, Couch LH, Marques MM, & Doerge DR (1998)
Formation of N-(carboxymethyl)fumonisin B1, following the reaction
of fumonisin B1 with reducing sugars. J Agric Food Chem, 46:
3546-3557.
Howard PC, Couch LH, Muskhelishvili M, Eppley RM, Doerge DR, &
Okerberg C (1999) Comparative toxicity of fumonisin derivatives in
28-day feeding study using female B6C3F1 mice. Toxicologist, 48: 34.
Huang C, Dickman M, Henderson G, & Jones C (1995) Repression of
protein kinase C and stimulation of cyclic AMP response elements by
fumonisin, a fungal encoded toxin which is a carcinogen. Cancer Res,
55: 1655-1659.
Humpf H-U, Schmelz E-M, Meredith FI, Vesper H, Vales TR, Wang E,
Menaldino DS, Liotta DC, & Merrill AH Jr (1998) Acylation of naturally
occurring and synthetic 1-deoxysphinganines by ceramide synthase:
Formation of N-palmitoyl-aminopentol produces a toxic metabolite of
hydrolyzed fumonisin, AP1, and a new category of ceramide synthase
inhibitor. J Biol Chem, 273: 19060-19064.
Humphreys SH, Carrington C, & Bolger PM (1997) Fumonisin risk
scenarios. Toxicologist, 36: 170.
IARC (1993) Toxins derived from Fusarium moniliforme: Fumonisins B1
and B2 and fusarin C. In: Some naturally occurring substances: Food
items and constituents, heterocyclic aromatic amines and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 445-466 (IARC
Monographs on the Evaluation of Carcinogenic Risk to Humans, Volume
56).
Inooka S & Toyokuni T (1996) Sphingosine transfer in cell-to-cell
interaction. Biochem Biophys Res Commun, 218: 872-876.
Jackson LS, Hlywka JJ, Senthil KR, Bullerman LB, & Musser SM (1996a)
Effects of time, temperature, and pH on the stability of fumonisin B1
in an aqueous model system. J Agric Food Chem, 44: 906-912.
Jackson LS, Hlywka JJ, Senthil KR, & Bullerman LB (1996b) Effects of
thermal processing on the stability of fumonisin B2 in an aqueous
system. J Agric Food Chem, 44: 1984-1987.
Jackson LS, Katta SK, Fingerhut DD, DeVries JW, & Bullerman LB (1997)
Effects of baking and frying on the fumonisin B1 content of
corn-based foods. J Agric Food Chem, 45: 4800-4805.
Jaffrezou J-P, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N,
Vermeersch S, Rousse A, & Laurent G (1996) Daunorubicin-induced
apoptosis: Triggering of ceramide generation through sphingomyelin
hydrolysis. EMBO J, 15: 2417-2424.
Jaskiewicz K, Marasas WFO, & Taljaard JJF (1987a) Hepatitis in vervet
monkeys caused by Fusarium moniliforme. J Comp Pathol, 97: 281-291.
Jaskiewicz K, Van Rensburg SJ, Marasas WF, & Gelderblom WC (1987b)
Carcinogenicity of Fusarium moniliforme culture material in rats.
J Natl Cancer Inst, 78: 321-325.
Jaskiewicz K, Marasas WFO, & Van der Walt FE (1987c) Oesophageal and
other main cancer patterns in four districts of Transkei, 1981-1984.
S Afr Med J, 72: 27-30.
Javed T, Bennett GA, Richard JL, Dombrink-Kurtzman MA, Côté LM, & Buck
WB (1993a) Mortality in broiler chicks on feed amended with a
Fusarium proliferatum culture material or with purified fumonisin
B1 and moniliformin. Mycopathologia, 123: 171-184.
Javed T, Richard JL, Bennett GA, Dombrink-Kurtzman MA, Bunte RM,
Koelkebeck KW, Côté LM, Leeper RW, & Buck WB (1993b) Embryopathic and
embryocidal effects of purified fumonisin B1 or Fusarium
proliferatum culture material extract on chicken embryos.
Mycopathologia, 123: 185-193.
Javed T, Dombrink-Kurtzman MA, Richard JL, Bennett GA, Côté LM, & Buck
WB (1995) Sero-hematologic alterations in broiler chicks on feed
amended with Fusarium proliferatum culture material or fumonisin B1
and moniliformin. J Vet Diagn Invest, 7: 520-526.
Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva MY,
Obeid LM, & Hannun YA (1995) Role for ceramide in cell cycle arrest. J
Biol Chem, 270: 2047-2052.
Jeschke N, Nelson PE, & Marasas WFO (1987) Toxicity to ducklings of
Fusarium moniliforme isolated from corn intended for use in poultry
feed. Poult Sci, 66: 1619-1623.
Johnson P, Smith EE, Phillips TD, Gentles AB, Small M, & Duffus E
(1993) The effects of fumonisin B1 on rat embryos in culture.
Toxicologist, 13: 257.
Kaneshiro T, Vesonder RF, & Peterson RE (1992) Fumonisin-stimulated
N-acetyldihydro-sphingosine, N-acetylphytosphingosine, and
phytosphingosine products of Pichia (Hansenula) ciferri, NRRL
Y-1031. Curr Microbiol, 24: 319-324.
Kaneshiro T, Vesonder RF, Peterson RE, & Bagby MO (1993)
2-hydroyhexadecanoic and 8,9,13-trihydroxydocosanoic acid accumulation
by yeasts treated by fumonisins B1. Lipids, 28: 397-401.
Kang YK & Alexander JM (1996) Alterations of the glutathione redox
cycle status in fumonisin B1-treated pig kidney cells. J Biochem
Toxicol, 11: 121-126.
Kang HJ, Kim JC, Seo JA, Lee YW, & Son DH (1994) Contamination of
Fusarium mycotoxins in corn samples imported from China. Agric Chem
Biotechnol, 37: 385-391.
Karlsson K-A (1970) Sphingolipid long chain bases. Lipids, 5: 878-891.
Kellerman TS, Marasas WFO, Pienaar JG, & Naudé TW (1972) A
mycotoxicosis of Equidae caused by Fusarium moniliforme Sheldon.
Onderstepoort J Vet Res, 39: 205-208.
Kellerman TS, Marasas WFO, Thiel PG, Gelderblom WCA, Cawood M, &
Coetzer JAW (1990) Leukoencephalomalacia in two horses induced by oral
dosing of fumonisin B1. Onderstepoort J Vet Res, 57: 269-275.
King SB & Scott GE (1981) Genotypic differences in maize to kernel
infection by Fusarium moniliforme. Phytopathology, 71: 1245-1247.
Klittich CJR, Leslie JF, Nelson PE, & Marasas WFO (1997) Fusarium
thapsinum (Gibberella thapsina): A new species in section Liseola
from sorghum. Mycologia, 89: 643-652.
Knasmüller S, Bresgen N, Kassie F, Mersch-Sundermann V, Gelderblom W,
Zöhrer E, & Eckl PM (1997) Genotoxic effects of three Fusarium
mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and
in primary cultures of rat hepatocytes. Mutat Res, 391: 39-48.
Kolesnick RN & Krönke M (1998) Regulation of ceramide production and
apoptosis. Annu Rev Physiol, 60: 643-665.
Kriek NPJ, Marasas WFO, Steyn PS, van Rensburg SL, & Steyn M (1977)
Toxicity of a moniliformin-producing strain of Fusarium moniliforme
var. subglutinans isolated from maize. Food Cosmet Toxicol, 15:
579-587.
Kriek NPJ, Kellerman TS, & Marasas WFO (1981) A comparative study of
the toxicity of Fusarium verticillioides (= F. moniliforme) to
horses, primates, pigs, sheep and rats. Onderstepoort J Vet Res, 48:
129-131.
Kubena LF, Edrington TS, Kamps-Holtzapple C, Harvey RB, Elissalde MH,
& Rottinghaus GE (1995a) Influence of fumonisin B1, present in
Fusarium moniliforme culture material, and T-2 toxin on turkey
poults. Poult Sci, 74: 306-313.
Kubena LF, Edrington TS, Kamps-Holtzapple C, Harvey RB, Elissalde MH,
& Rottinghaus GE (1995b) Effects of feeding fumonisin B1 present in
Fusarium moniliforme culture material and aflatoxin singly and in
combination to turkey poults. Poult Sci, 74: 1295-1303.
Kubena LF, Edrington TS, Harvey RB, Phillips TD, Sarr AB, &
Rottinghaus GE (1997a) Individual and combined effects of fumonisin
B1 present in Fusarium moniliforme culture material and
diacetoxyscirpenol or ochratoxin A in turkey poults. Poult Sci, 76:
256-264.
Kubena LF, Edrington TS, Harvey RB, Buckley SA, Phillips TD,
Rottinghaus GE, & Casper HH (1997b) Individual and combined effects of
fumonisin B1 present in Fusarium moniliforme culture material and
T-2 toxin or deoxynivalenol in broiler chicks. Poult Sci, 76:
1239-1247.
Kuiper-Goodman T, Scott PM, McEwen NP, Lombaert GA, & Ng W (1996)
Approaches to the risk assessment of fumonisins in corn-based foods in
Canada. Adv Exp Med Biol, 392: 369-393.
Kwon OS, Sandberg JA, & Slikker W Jr (1997a) Effects of fumonisin B1
treatment on blood-brain barrier transfer in developing rats.
Neurotoxicol Teratol, 19: 151-155.
Kwon OS, Schmued LC, & Slikker W Jr (1997b) Fumonisin B1 in
developing rats alters brain sphinganine levels and myelination.
Neurotoxicology, 18: 571-579.
LaBorde JB, Terry KK, Howard PC, Chen JJ, Collins FX, Shackelford ME,
& Hansen DK (1997) Lack of embryotoxicity of fumonisin B1 in New
Zealand white rabbits. Fundam Appl Toxicol, 40: 120-128.
Lamprecht SC, Marasas WFO, Alberts JF, Cawood ME, Gelderblom WCA,
Shephard GS, Thiel PG, & Calitz FJ (1994) Phytotoxicity of fumonisins
and TA-toxin to corn and tomato. Phytopathology, 84: 383-391.
Laurent D, Platzer N, Kohler F, Sauviat MP, & Pellegrin F (1989a)
Macrofusine et micromoniline: Deux nouvelles mycotoxines isolées de
maïs infesté par Fusarium moniliforme Sheld. Microbiol Alim Nutr, 7:
9-16.
Laurent D, Pellegrin F, Kohler F, Costa R, Thevenon J, Lambert C, &
Huerre M (1989b) La fumonisine B1 dans la pathogénie de la
leucoencéphalomalacie équine. Microbiol Alim Nutr, 7: 285-291.
Lavie Y, Cao H-t, Bursten SL, Guiliano AE, & Cabot MC (1996)
Accumulation of glucosylceramides in multidrug-resistant cancer cells.
J Biol Chem, 271: 19530-19536.
Le Bars J, Le Bars P, Dupuy J, Boudra H, & Cassini R (1994) Biotic and
abiotic factors in fumonisin B1 production and stability. J Assoc Off
Anal Chem Int, 77: 517-521.
Lebepe-Mazur S, Bal H, Hopmans E, Murphy P, & Hendrich S (1995a)
Fumonisin B1 is fetotoxic in rats. Vet Hum Toxicol, 37: 126-130.
Lebepe-Mazur S, Hopmans E, Murphy PA, & Hendrich S (1995b) Fed before
diethylnitrosamine, Fusarium moniliforme and F. proliferatum
mycotoxins alter the persistence of placental glutathione
S-transferase-positive hepatocytes in rats. Vet Hum Toxicol, 37:
209-214.
Lebepe-Mazur S, Wilson T, & Hendrich S (1995c) Fusarium
proliferatum-fermented corn stimulates development of placental
glutathione S-transferase-positive altered hepatic foci in female
rats. Vet Hum Toxicol, 37: 55-59.
Ledoux DR, Brown TP, Weibking TS, & Rottinghaus GE (1992) Fumonisin
toxicity in broiler chicks. J Vet Diagn Invest, 4: 330-333.
Ledoux DR, Bermudez AJ, & Rottinghaus GE (1996) Effects of feeding
Fusarium moniliforme culture material, containing known levels of
fumonisin B1, in the young turkey poult. Poult Sci, 75: 1472-1478.
Lee U-S, Lee M-Y, Shin K-S, Min Y-S, Cho C-M, & Ueno Y (1994)
Production of fumonisin B1 and B2 by Fusarium moniliforme isolated
from Korean corn kernels for feed. Mycotoxin Res, 10: 67-72.
Lee JY, Leonhardt LG, & Obeid LM (1998) Cell-cycle-dependent changes
in ceramide levels preceding retinoblastoma protein dephosphorylation
in G2/M. Biochem J, 334: 457-461.
Lemmer ER, Hall P De La M, Gelderblom WCA, & Marasas WFO (1998) Poor
reporting of oocyte apoptosis. Nat Med, 4: 373.
Leslie JF, Plattner RD, Desjardins AE, & Klittich CJR (1992) Fumonisin
B1 production by strains from different mating populations of
Gibberella fujikuroi (Fusarium section Liseola). Phytopathology,
82: 341-345.
Leslie JF, Marasas WFO, Shephard GS, Sydenham EW, Stockenström S, &
Thiel PG (1996) Duckling toxicity and the production of fumonisin and
moniliformin by isolates in the A and F mating populations of
Gibberella fujikuroi (Fusarium moniliforme). Appl Environ Microbiol,
62: 1182-1187.
Lew H, Adler A, & Edinger W (1991) Moniliformin and the European corn
borer. Mycotoxin Res, 7: 71-76.
Liao W-C, Haimovitz-Friedman A, Persaud RS, McLoughlin M, Ehleiter D,
Zhang N, Gatei M, Lavin M, Kolesnick R, & Fuks Z (1999) Ataxia
telangiectasia-mutated gene product inhibits DNA damage-induced
apoptosis via ceramide synthase. J Biol Chem, 274: 17908-17917.
Lim CW, Parker HM, Vesonder RF, & Haschek WM (1996) Intravenous
fumonisin B1 induces cell proliferation and apoptosis in the rat. Nat
Toxins, 4: 34-41.
Logrieco A, Moretti A, Ritieni A, Chelkowski J, Altomare C, Bottalico
A, & Randazzo G (1993) Natural occurrence of beauvericin in preharvest
Fusarium subglutinans infected corn ears in Poland. J Agric Food
Chem, 41: 2149-2152.
Logrieco A, Moretti A, Ritieni A, Bottalico A, & Corda P (1995)
Occurrence and toxigenicity of Fusarium proliferatum from preharvest
maize ear rot, and associated mycotoxins, in Italy. Plant Dis, 79:
727-731.
Logrieco A, Doko MB, Moretti A, Frisullo S, & Visconti A (1998)
Occurrence of fumonisin B1 and B2 in Fusarium proliferatum
infected asparagus plants. J Agric Food Chem, 46: 5201-5204.
Lu Z, Dantzer WR, Hopmans EC, Prisk V, Cunnick JE, Murphy PA, &
Hendrich S (1997) Reaction with fructose detoxifies fumonisin B1
while stimulating liver-associated natural killer cell activity in
rats. J Agric Food Chem, 45: 803-809.
Lukacs Z, Schaper S, Herderich M, Schreier P, & Humpf H-U (1996)
Identification and determination of fumonisin B1 and FB2 in corn
products by high-performance liquid
chromatographic-electrospray-ionization tandem mass spectrometry
(HPLC-ESI-MS-MS). Chromatographia, 43: 124-128.
Makaula NA, Marasas WFO, Venter FS, Badenhorst CJ, Bradshaw D, &
Swanevelder S (1996) Oesophageal and other cancer patterns in four
selected districts of Transkei, Southern Africa: 1985-1990. Afr J
Health Sci, 3:11-15.
Maragos CM (1995) Capillary zone electrophoresis and HPLC for the
analysis of fluorescein isothiocyanate-labelled fumonisin B1. J Agric
Food Chem, 43: 390-394.
Maragos CM & Richard JL (1994) Quantitation and stability of
fumonisins B1 and B2 in milk. J Assoc Off Anal Chem Int, 77:
1162-1167.
Marasas WFO (1993) Occurrence of Fusarium moniliforme and fumonisins
in maize in relation to human health (Editorial). S Afr Med J, 83:
382-383.
Marasas WFO (1994) Fusarium. In: Hui YM, Gorham JR, Murrell KD, &
Cliver DO ed. Foodborne disease handbook. New York, Marcel Dekker, vol
2, pp 521-573.
Marasas WFO (1995) Fumonisins: Their implications for human and animal
health. Nat Toxins, 3: 193-198.
Marasas WFO (1996) Fumonisins: History, world-wide occurrence and
impact. Adv Exp Med Biol, 392: 1-17.
Marasas WFO (1997) Risk assessment of fumonisins produced by
Fusarium moniliforme in corn. Cereal Res Commun 25: 399-406.
Marasas WFO, Kellerman TS, Pienaar JG, & Naudé TW (1976)
Leukoencephalomalacia: A mycotoxicosis of Equidae caused by
Fusarium moniliforme Sheldon. Onderstepoort J Vet Res, 43: 113-122.
Marasas WFO, Kriek NPJ, Wiggins VM, Steyn PS, Towers DK, & Hastie TJ
(1979a) Incidence, geographic distribution and toxigenicity of
Fusarium species in South African corn. Phytopathology, 69:
1181-1185.
Marasas WFO, Van Rensburg SJ, & Mirocha CJ (1979b) Incidence of
Fusarium species and the mycotoxins, deoxynivalenol and zearalenone,
in corn produced in esophageal cancer areas in Transkei. J Agric Food
Chem, 27: 1108-1112.
Marasas WFO, Wehner FC, Van Rensburg SJ, & Van Schalkwyk DJ (1981)
Mycoflora of corn produced in human esophageal cancer areas in
Transkei, Southern Africa. Phytopathology, 71: 792-796.
Marasas WFO, Nelson PE, & Toussoun TA (1984a) Toxigenic Fusarium
species: Identity and mycotoxicology. University Park, Pennsylvania,
The Pennsylvania State University Press, 328 pp.
Marasas WFO, Kriek NPJ, Fincham JE, & Van Rensburg SJ (1984b) Primary
liver cancer and oesophageal basal cell hyperplasia in rats caused by
Fusarium moniliforme. Int J Cancer, 34: 383-387.
Marasas WFO, Kellerman TS, Gelderblom WCA, Coetzer JAW, Thiel PG, &
Van der Lugt JJ (1988a) Leukoencephalomalacia in a horse induced by
fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J
Vet Res, 55: 197-203.
Marasas WFO, Jaskiewicz K, Venter FS, & Van Schalkwyk DJ (1988b)
Fusarium moniliforme contamination of maize in oesophageal cancer
areas in Transkei. S Afr Med J, 74: 110-114.
Marasas WFO, Thiel PG, Gelderblom WCA, Shephard GS, Sydenham EW, &
Rheeder JP (1993) Fumonisins produced by Fusarium moniliforme in
maize: Foodborne carcinogens of Pan African importance. Afr Newslett
Occup Health Saf, 2/93(suppl): 11-18.
Marijanovic DR, Holt P, Norred WP, Bacon CW, Voss KA, Stancel PC, &
Ragland WL (1991) Immunosuppressive effects of Fusarium moniliforme
corn cultures in chickens. Poult Sci, 70: 1895-1901.
Martinez-Larrañaga MR, Anadón A, Diaz MJ, Fernandez R, Sevil B,
Fernandez-Cruz ML, Fernandez MC, Martinez MA, & Anton R (1996)
Induction of cytochrome P4501A1 and P4504A1 activities and peroxisomal
proliferation by fumonisin B1. Toxicol Appl Pharmacol, 141: 185-194.
Martinova EA & Merrill AH Jr (1995) Fumonisin B1 alters sphingolipid
metabolism and immune function in BALB/c mice: Immunological responses
to fumonisin B1. Mycopathologia, 130: 163-170.
Matteri RL, Becker BA, Pace L, & Rottinghaus GE (1994) Effects of
thermal environment and maternal exposure to fumonisin B1 on
luteinizing hormone secretion in preweaning gilts. Biol Reprod, 50
(suppl 1): 68.
Medlock KA & Merrill AH Jr (1988) Inhibition of serine
palmitoyltransferase in vitro and long-chain base biosynthesis in
intact Chinese hamster ovary cells by beta-chloroalanine.
Biochemistry, 27: 7079-7084.
Mehta R, Lok E, Rowsell PR, Miller JD, Suzuki CA, & Bondy GS (1998)
Glutathione S-transferase-placental form expression and proliferation
of hepatocytes in fumonisin B1-treated male and female Sprague-Dawley
rats. Cancer Lett, 128: 31-39.
Meister U, Symmank H, & Dahlke H (1996) [Investigation and evaluation
of the contamination of native and imported cereals with fumonisins.]
Z Lebensm Unters Forsch, 203: 528-533 (in German).
Meister U (1998) [Effect of extraction and extract clean-up on the
determinable levels of fumonisins in maize and maize products:
Investigations on the acid extraction and the use of immunoaffinity
columns.] In: Wolff J & Betsche T ed. [Proceedings 20. Workshop on
Mycotoxins, Detmold, 8-10 June 1998.] Institute of Biochemistry of
Grain and Potatoes, Federal Agency for Grain, Potatoes and Fat
Research, pp 241-245 (in German).
Meivar-Levy I, Sabanay H, Bershadsky AD, & Futerman AH (1997) The role
of sphingolipids in the maintenance of fibroblast morphology. J Biol
Chem, 272: 1558-1564.
Meredith FI, Riley RT, Bacon CW, Williams DE, & Carlson DB (1998)
Extraction, quantification, and biological availability of fumonisin
B1 incorporated into Oregon test diet and fed to rainbow trout. J
Food Prot, 61: 1034-1038.
Merrill AH Jr (1983) Characterization of serine palmitoyltransferase
activity in Chinese hamster ovary cells. Biochim Biophys Acta, 754:
284-291.
Merrill AH Jr (1991) Cell regulation by sphingosine and more complex
sphingolipids. J Bioenerget Biomembr, 23: 83-104.
Merrill AH Jr & Jones DD (1990) An update of the enzymology and
regulation of sphingomyelin metabolism. Biochim Biophys Acta, 1044:
1-12.
Merrill AH Jr, Wang E, Mullins RE, Jamison WCL, Nimkar S, & Liotta DC
(1988) Quantitation of free sphingosine in liver by high-performance
liquid chromatography. Anal Biochem, 171: 373-381.
Merrill AH Jr, Hannun YA, & Bell RM (1993a) Sphingolipids and their
metabolites in cell regulation. In: Bell RM, Hannun YA, & Merrill AH
Jr ed. Advances in lipid research: Sphingolipids and their
metabolites. Orlando, Florida, Academic Press, vol 25, pp 1-24.
Merrill AH Jr, van Echten G, Wang E, & Sandhoff K (1993b) Fumonisin
Bl inhibits sphingosine (sphinganine) N-acetyltransferase and
de novo sphingolipid biosynthesis in cultured neurons in situ.
J Biol Chem, 268: 27299-27306.
Merrill AH Jr, Wang E, Gilchrist DG, & Riley RT (1993c) Fumonisins and
other inhibitors of de novo sphingolipid biosynthesis. In: Bell RM,
Hannun YA, & Merrill AH Jr ed. Advances in lipid research:
Sphingolipids and their metabolites. Orlando, Florida, Academic Press,
vol 26, pp 215-234.
Merrill AH Jr, Wang E, Schroeder JJ, Smith ER, Yoo H-S, & Riley RT
(1995) Disruption of sphingolipid metabolism in the toxicity and
carcinogenicity of fumonisins. In: Eklund M, Richard J, & Mise K ed.
Molecular approaches to food safety issues involving toxic
microorganisms. Fort Collins, Colorado, Alaken Press, pp 429-443.
Merrill AH Jr, Liotta DC, & Riley RT (1996a) Fumonisins: fungal toxins
that shed light on sphingolipid function. Trends Cell Biol, 6:
218-224.
Merrill AH Jr, Wang E, Vales TR, Smith ER, Schroeder JJ, Menaldino DS,
Alexander C, Crane HM, Xia J, Liotta DC, Meredith FI, & Riley RT
(1996b) Fumonisin toxicity and sphingolipid biosynthesis. Adv Exp Med
Biol, 392: 297-306.
Merrill AH Jr, Schmelz E-M, Dillehay DL, Spiegel S, Shayman JA,
Schroeder JJ, Riley RT, Voss KA, & Wang E (1997a) Sphingolipids -- the
enigmatic lipid class: Biochemistry, physiology, and pathophysiology.
Toxicol Appl Pharmacol, 142: 208-225.
Merrill AH Jr, Schmelz E-M, Wang E, Dillehay DL, Rice LG, Meredith FI,
& Riley RT (1997b) Importance of sphingolipids and inhibitors of
sphingolipid metabolism as components of animal diets. J Nutr, 127:
830S-833S.
Michel C, Van Echten-Deckert G, Rother J, Sandoff K, Wang E, & Merrill
AH Jr (1997) Characterization of ceramide synthesis. J Biol Chem, 272:
22432-22437.
Miller JD (1995) Fungi and mycotoxins in grain: Implications for
stored product research. J Stored Prod Res, 31: 1-16.
Miller JD, Savard ME, Sibilia A, Rapior S, Hocking AD, & Pitt JI
(1993) Production of fumonisins and fusarins by Fusarium
moniliforme from southeast Asia. Mycologia, 85: 385-391.
Miller JD, Savard ME, & Rapior S (1994) Production and purification of
fumonisins from a stirred jar fermenter. Nat Toxins, 2: 354-359.
Miller JD, Savard ME, Schaafsma AW, Seifert KA, & Reid LM (1995)
Mycotoxin production by Fusarium moniliforme and Fusarium
proliferatum from Ontario and occurrence of fumonisin in the 1993
corn crop. Can J Plant Pathol, 17: 233-239.
Miller MA, Honstead JP, & Lovell RA (1996) Regulatory aspects of
fumonisins with respect to animal feed. Adv Exp Med Biol, 392:
363-368.
Minervini F, Bottalico C, Pestka J, & Visconti A (1992) [On the
occurrence of fumonisins in feeds in Italy.] In [Proceedings of the
46th National Congress of the Italian Society of Veterinary Science,
Venice, Italy, 30 September-3 October], pp 1365-1368 (in Italian).
Mirocha CJ, Gilchrist DG, Shier WT, Abbas HK, Wen Y, & Vesonder RF
(1992) AAL toxins, fumonisins (biology and chemistry) and
host-specificity concepts. Mycopathologia, 17: 47-56.
Morgan MK, Schroeder JJ, Rottinghaus GE, Powell DC, Bursian SJ, &
Aulerich RJ (1997) Dietary fumonisins disrupt sphingolipid metabolism
in mink and increase the free sphinganine to sphingosine ratio in
urine but not in hair. Vet Hum Toxicol, 39: 334-336.
Motelin GK, Haschek WM, Ness DK, Hall WF, Harlin KS, Schaeffer DJ, &
Beasley VR (1994) Temporal and dose-response features in swine fed
corn screenings contaminated with fumonisin mycotoxins.
Mycopathologia, 126: 27-40.
Mullett W, Lai EP, & Yeung JM (1998) Immunoassay of fumonisins by a
surface plasmon resonance biosensor. Anal Biochem, 258: 161-167.
Murphy PA, Rice LG, & Ross PF (1993) Fumonisin B1, B2, and B3
content of Iowa, Wisconsin and Illinois corn and corn screenings. J
Agric Food Chem, 41: 263-266.
Murphy PA, Hopmans EC, Miller K, & Hendrich S (1995) Can fumonisins in
foods be detoxified? In: Toxicants in food. Lancaster, Pennsylvania,
Technomic Publishing, vol 1, pp 105-117.
Murphy PA, Hendrich S, Hopmans EC, Hauck CC, Lu Z, Buseman G, &
Munkvold G (1996) Effect of processing on fumonisin content of corn.
Adv Exp Med Biol, 392: 323-334.
Musser SM & Plattner RD (1997) Fumonisin composition in cultures of
Fusarium moniliforme, Fusarium proliferatum, and Fusarium nygami.
J Agric Food Chem, 45: 1169-1173.
Nakamura S, Kozutsumi Y, Sun Y, Miyake Y, Fujita T, & Kawasaki T
(1996) Dual role of sphingolipids in signaling of the escape from and
onset of apoptosis in a mouse cytotoxic T-cell line, CTLL-2. J Biol
Chem, 271: 1255-1257.
National Veterinary Services Laboratory (1995) Toxicity of fumonisins
in horses (equine leukoencephalomalacia [ELEM]) - Developmental
project final report. US Animal and Plant Health Inspection Agency,
National Veterinary Services Laboratory (Report No. PL/TC/94-2).
Nelson PE, Plattner RD, Shackelford DD, & Desjardins AE (1991)
Production of fumonisins by Fusarium moniliforme strains from
various substrates and geographic areas. Appl Environ Microbiol, 57:
2410-2412.
Nelson PE, Plattner RD, Shackelford DD, & Desjardins AE (1992)
Fumonisin B1 production by Fusarium species other than
F. moniliforme in section Liseola and by some related species.
Appl Environ Microbiol, 58: 984-989.
Nelson PE, Desjardins AE, & Plattner RD (1993) Fumonisins, mycotoxins
produced by Fusarium species: Biology, chemistry, and significance.
Ann Rev Phytopathol, 31: 233-252.
Newkirk DK, Benson RW, Howard PC, Churchwell MI, Doerge DR, & Roberts
DW (1998) On-line immunoaffinity capture, coupled with HPLC and
electrospray ionization mass spectrometry, for automated determination
of fumonisins. J Agric Food Chem, 46: 1677-1688.
Nikolova-Karakashian M, Morgan ET, Alexander C, Liotta DC, & Merrill
AH Jr (1997) Bimodal regulation of ceramidase by interleukin-1ß.
J Biol Chem, 272: 18718-18724.
Norred WP, Voss KA, Bacon CW, & Riley RT (1991) Effectiveness of
ammonia treatment in detoxification of fumonisin-contaminated corn.
Food Chem Toxicol, 29: 815-819.
Norred WP, Plattner RD, Vesonder RF, Bacon CW, & Voss KA (1992a)
Effects of selected secondary metabolites of Fusarium moniliforme on
unscheduled synthesis of DNA by rat primary hepatocytes. Food Chem
Toxicol, 30: 233-237.
Norred WP, Wang E, Yoo H, Riley RT, & Merrill AH Jr (1992b) In
vitro toxicology of fumonisins and the mechanistic implications.
Mycopathologia, 117: 73-78.
Norred WP, Plattner RD, & Chamberlain WJ (1993) Distribution and
excretion of [14C]fumonisin B1 in male Sprague-Dawley rats. Nat
Toxins, 1: 341-346.
Norred WP, Riley RT, Meredith FI, Bacon CW, & Voss KA (1996) Time- and
dose-response effects of the mycotoxin, fumonisin B1 on sphingoid
base elevation in precision-cut rat liver and kidney slices. Toxicol
In Vitro, 10: 349-358.
Norred WP, Plattner RD, Dombrink-Kurtzman MA, Meredith FI, & Riley RT
(1997) Mycotoxin-induced elevation of free sphingoid bases in
precision-cut rat liver slices: specificity of the response and
structure-activity relationships. Toxicol Appl Pharmacol, 147: 63-70.
Ochor TE, Trevathan LE, & King SB (1987) Relationship of harvest date
and host genotype to infection of maize kernels by Fusarium
moniliforme. Plant Dis, 71: 311-313.
Odvody GN, Remmers JC, & Spencer NM (1990) Association of kernel
splitting with kernel and ear rots of corn in a commercial hybrid
grown in the coastal bend of Texas. Phytopathology, 80: 1045.
Ostr V & Ruprich J (1998) Determination of the mycotoxin fumonisins
in gluten-free diet (corn-based commodities) in the Czech Republic.
Cent Eur J Public Health, 6: 57-60.
Osweiler GD, Ross PF, Wilson TM, Nelson PE, Witte ST, Carson TL, Rice
LG, & Nelson HA (1992) Characterization of an epizootic of pulmonary
edema in swine associated with fumonisin in corn screenings. J Vet
Diagn Invest, 4: 53-59.
Osweiler GD, Kehrli ME, Stabel JR, Thruston JR, Ross PF, & Wilson TM
(1993) Effects of fumonisin-contaminated corn screenings on growth and
health of feeder calves. J Anim Sci, 71: 459-466.
Park DL, Rua SM Jr, Mirocha CJ, Abd-Alla EAM, & Weng CY (1992)
Mutagenic potentials of fumonisin contaminated corn following ammonia
decontamination procedure. Mycopathologia, 117: 105-108.
Pascale M, Doko MB, & Visconti A (1995) [Determination of fumonisins
in polenta by high performance liquid chromatography.] In:
[Proceedings of the 2nd National Congress on Food Chemistry,
Giardini-Naxos, 24-27 May 1995.] Messina, Italy, La Grafica
Editoriale, pp 1067-1071 (in Italian).
Pascale M, Visconti A, Proñczuk M, Winiewska H, & Chelkowki J (1997)
Accumulation of fumonisins in maize hybrids inoculated under field
conditions with Fusarium moniliforme Sheldon. J Sci Food Agric, 74:
1-6.
Patel S, Hazel CM, Winterton AGM, & Mortby E (1996) Survey of ethnic
foods for mycotoxins. Food Addit Contam, 13: 833-841.
Patel S, Hazel CM, Winterton AGM, & Gleadle AE (1997) Surveillance of
fumonisins in UK maize-based foods and other cereals. Food Addit
Contam, 14: 187-191.
Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, & Honjo T (1997)
Inhibition of carnitine palmitoyltransferase I augments sphingolipid
synthesis and palmitate-induced apoptosis. J Biol Chem, 272:
3324-3329.
Penner JD, Casteel SW, Pittman L Jr, Rottinghaus GE, & Wyatt RD (1998)
Developmental toxicity of purified fumonisin B1 in pregnant Syrian
hamsters. J Appl Toxicol, 18 :197-203.
Perez GI, Knudson CM, Leykin L, Korseyer SJ, & Tilley JL (1997)
Apoptosis-associated signaling pathways are required for
chemotherapy-mediated female germ cell destruction. Nat Med, 3:
1228-1232.
Perry DK & Hannun YA (1998) The role of ceramide in cell signaling.
Biochi Biophys Acta, 1436: 233-243.
Pestka JJ, Azcona-Olivera JI, Plattner RD, Minervini F, Doko MB, &
Visconti A (1994) Comparative assessment of fumonisin in grain-based
foods by ELISA, GC-MS and HPLC. J Food Prot, 57: 169-172.
Piñeiro MS, Silva GE, Scott PM, Lawrence GA, & Stack ME (1997)
Fumonisin levels in Uruguayan corn products. J Assoc Off Anal Chem
Int, 80: 825-828.
Pitt JI, Hocking AD, Bhudhasamai K, Miscambe BF, Wheeler KA, &
Tanboon-Ek P (1993) The normal mycoflora of commodities from Thailand:
1. Nuts and oilseeds. Int J Food Microbiol, 20: 211-226.
Pittet A, Parisod V, & Schellenberg M (1992) Occurrence of fumonisins
B1 and B2 in corn-based products from the Swiss market. J Agric Food
Chem, 40: 1352-1354.
Plattner RD (1995) Detection of fumonisins produced in Fusarium
moniliforme cultures by HPLC with electrospray MS and evaporative
light scattering detectors. Nat Toxins, 3: 294-298.
Plattner RD & Branham BE (1994) Labeled fumonisins: Production and use
of fumonisin B1 containing stable isotopes. J Assoc Off Anal Chem
Int, 77: 525-532.
Plattner RD & Nelson PE (1994) Production of beauvericin by a strain
of Fusarium proliferatum isolated from corn fodder for swine. Appl
Environ Microbiol, 60: 3894-3896.
Plattner RD & Shackelford DD (1992) Biosynthesis of labeled fumonisins
in liquid cultures of Fusarium moniliforme. Mycopathologia, 117:
17-22.
Plattner RD, Norred WP, Bacon CW, Voss KW, Peterspn R, Shackelford DD,
& Weisleder D (1990) A method of detection of fumonisins in corn
samples associated with field cases of equine leukoencephalomalacia.
Mycologia, 82: 698-702.
Plattner RD, Weisleder D, Shackelford DD, Peterson R, & Powell RG
(1992) A new fumonisin from solid cultures of Fusarium moniliforme.
Mycopathologia, 117: 23-28.
Powell DC, Bursian SJ, Bush CR, Render JA, Rottinghaus GE, & Aulerich
RJ (1996) Effects of dietary exposure to fumonisins from Fusarium
moniliforme culture material (M1325) on the reproductive performance
of female mink. Arch Environ Contam Toxicol, 31: 286-292.
Prathapkumar SH, Rao VS, Paramkishan RJ, & Bhat RV (1997) Disease
outbreak in laying hens arising from the consumption of
fumonisin-contaminated food. Br Poult Sci, 38: 475-479.
Prelusky DB, Trenholm, HL, & Savard ME (1994) Pharmacokinetic fate of
14C-labelled fumonisin B1 in swine. Nat Toxins, 2: 73-80.
Prelusky DB, Savard ME, & Trenholm HL (1995) Pilot study on the plasma
pharmacokinetics of fumonisin B1 in cows following a single dose by
oral gavage or intravenous administration. Nat Toxins, 3: 389-394.
Prelusky DB, Trenholm HL, Rotter BA, Miller JD, Savard ME, Yeung JM, &
Scott PM (1996a) Biological fate of fumonisin B1 in food-producing
animals. Adv Exp Med Biol, 392: 265-278.
Prelusky DB, Miller JD, & Trenholm HL (1996b) Disposition of
14C-derived residues in tissues of pigs fed radiolabelled fumonisin
B1. Food Addit Contam, 13: 155-162.
Price WD, Lovell RA, & McChesney DG (1993) Naturally occurring toxins
in feedstuffs: Center for Veterinary Medicine perspective. J Anim Sci,
71: 2556-2562.
Qureshi MA & Hagler WM Jr (1992) Effect of fumonisin-B1 exposure on
chicken macrophage function in vitro. Poult Sci, 71: 104-112.
Radin NS (1994) Glucosylceramide in the nervous system: a mini review.
Neurochem Res, 19: 533-540.
Ramasamy S, Wang E, Hennig B, & Merrill AH Jr (1995) Fumonisin B1
alters sphingolipid metabolism and disrupts the barrier function of
endothelial cells in culture. Toxicol Appl Pharmacol, 133: 343-348.
Ramirez ML, Pascale M, Chulze S, Reynoso MM, March G, & Visconti A
(1996) Natural occurrence of fumonisins and their correlation to
Fusarium contamination in commercial corn hybrids growth in
Argentina. Mycopathologia, 135: 29-34.
Ramljak D, Diwan BA, Ramakrishna G, Victor TC, Marasas WFO, &
Gelderblom WCA (1996) Overexpression of cyclin D1 is an early event
in, and possible mechanism responsible for, fumonisin B1 liver
tumorigenesis in rats. Proc Am Assoc Cancer Res, 38: 495.
Rapior S, Miller JD, Savard ME, & Apsimon JW (1993) Production
in vitro de fumonisines et de fusarines par des souches européennes
de Fusarium moniliforme. Microbiol Alim Nutr, 11: 327-333.
Reddy RV, Johnson G, Rottinghaus GE, Casteel SW, & Reddy CS (1996)
Developmental effects of fumonisin B1 in mice. Mycopathologia, 134:
161-166.
Restum JC, Bursian SJ, Millerick M, Render JA, Merrill AH Jr, Wang E,
Rottinghaus GE, & Aulerich RJ (1995) Chronic toxicity of fumonisins
from Fusarium moniliforme culture material (M-1325) to mink. Arch
Environ Contam Toxicol, 29: 545-550.
Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, Shephard GS, & van
Schalkwyk DJ (1992) Fusarium moniliforme and fumonisins in corn in
relation to human esophageal cancer in Transkei. Phytopathology, 82:
353-357.
Rheeder JP, Marasas WFO, Farina MPW, Thompson GR, & Nelson PE (1994)
Soil fertility factors in relation to oesophageal cancer risk areas in
Transkei, Southern Africa. Eur J Cancer Prev, 3: 49-56.
Rice LG & Ross PF (1994) Methods for detection and quantitation of
fumonisins in corn, cereal products and animal excreta. J Food Prot,
57: 536-540.
Richard JL, Meerdink G, Maragos CM, Tumbleson M, Bordson G, Rice LG, &
Ross PF (1996) Absence of detectable fumonisins in the milk of cows
fed Fusarium proliferatum (Matsushima) Nirenberg culture material.
Mycopathologia, 133: 123-126.
Riley RT & Yoo H-S (1995) Time and dose relationship between the
cellular effects of fumonisin B1 (FB1) and the uptake and
accumulation of [14C]FB1 in LLC-PK1 cells. Toxicologist, 15(1):
290.
Riley RT, An N-H, Showker JL, Yoo H-S, Norred WP, Chamberlain WJ, Wang
E, Merrill AH Jr, Motelin G, Beasley VR, & Haschek WM (1993)
Alteration of tissue and serum sphinganine to sphingosine ratio: An
early biomarker of exposure to fumonisin-containing feeds in pigs.
Toxicol Appl Pharmacol, 118: 105-112.
Riley RT, Hinton DM, Chamberlain WJ, Bacon CW, Wang E, Merrill AH Jr,
& Voss KA (1994a) Dietary fumonisin B1 induces disruption of
sphingolipid metabolism in Sprague-Dawley rats: A new mechanism of
nephrotoxicity. J Nutr, 124: 594-603.
Riley RT, Voss KA, Yoo H-S, Gelderblom WCA, & Merrill AH Jr (1994b)
Mechanism of fumonisin toxicity and carcinogenesis. J Food Prot, 57:
638-645.
Riley RT, Wang E, & Merrill AH Jr (1994c) Liquid chromatographic
determination of sphinganine and sphingosine: Use of the free
sphinganine-to-sphingosine ratio as a biomarker for consumption of
fumonisins. J Assoc Off Anal Chem Int, 77: 533-540.
Riley RT, Wang E, Schroeder JJ, Smith ER, Plattner RD, Abbas H, Yoo
H-S, & Merrill AH Jr (1996) Evidence for disruption of sphingolipid
metabolism as a contributing factor in the toxicity and
carcinogenicity of fumonisins. Nat Toxins, 4: 3-15.
Riley RT, Showker JL, Owens DL, & Ross PF (1997) Disruption of
sphingolipid metabolism and induction of equine leukoencephalomalacia
by Fusarium proliferatum culture material containing fumonisin B1
or B3. Environ Toxicol Pharmacol, 3: 221-228.
Riley RT, Voss KA, Norred WP, Wang E, & Merrill AH Jr (1998)
Fumonisins: mechanism of mycotoxicity. Rev Méd Vét, 149: 617-626.
Riley RT, Voss KA, Norred WP, Bacon CW, Meredith FI, & Sharma RP
(1999) Serine palmitoyltransferase inhibition reverses
anti-proliferative effects of ceramide synthase inhibition in cultured
renal cells and suppresses free sphingoid base accumulation in kidney
of BALBc mice. Environ Toxicol Pharmacol, 7: 109-118.
Ross PF (1994) What are we going to do with this dead horse? J Assoc
Off Anal Chem Int, 77: 491-494.
Ross PF, Nelson PE, Richard JL, Osweiler GD, Rice LG, Plattner RD, &
Wilson TM (1990) Production of fumonisins by Fusarium moniliforme
and Fusarium proliferatum isolates associated with equine
leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl
Environ Microbiol, 56: 3225-3226.
Ross PF, Rice LG, Plattner RD, Osweiler GD, Wilson TM, Owens DL,
Nelson HA, & Richard JL (1991a) Concentrations of fumonisin B1 in
feeds associated with animal health problems. Mycopathologia, 114:
129-135.
Ross PF, Rice LG, Reagor JC, Osweiler GD, Wilson TM, Nelson HA, Owens
DL, Plattner RD, Harlin KA, Richard JL, Colvin BM, & Banton MI (1991b)
Fumonisin B1 concentrations in feeds from 45 confirmed equine
leukoencephalomalacia cases. J Vet Diagn Invest, 3: 238-241.
Ross PF, Rice LG, Osweiler GD, Nelson PE, Richard JL, & Wilson TM
(1992) A review and update of animal toxicoses associated with
fumonisin-contaminated feeds and production of fumonisins by
Fusarium isolates. Mycopathologia, 117: 109-114.
Ross PF, Ledet AE, Owens DL, Rice LG, Nelson HA, Osweiler GD, & Wilson
TM (1993) Experimental equine leukoencephalomalacia, toxic hepatosis,
and encephalopathy caused by corn naturally contaminated with
fumonisins. J Vet Diagn Invest, 5: 69-74.
Ross PF, Nelson PE, Owens DL, Rice LG, Nelson HA, & Wilson TM (1994)
Fumonisin B2 in cultured Fusarium proliferatum, M-6104, causes
equine leukoencephalomalacia. J Vet Diagn Invest, 6: 263-265.
Rother J, Van Echten G, Schwarzmann G, & Sandhoff K (1992)
Biosynthesis of sphingolipids: Dihydroceramide and not sphinganine is
desaturated by cultured cells. Biochem Biophys Res Commun, 189: 14-20.
Rotter BA, & Oh Y-N (1996) Mycotoxin fumonisin B1 stimulates nitric
oxide production in a murine macrophage cell line. Nat Toxins, 4:
291-294.
Rotter BA, Thompson BK, Prelusky DB, Trenholm HL, Stewart B, Miller
JD, & Savard ME (1996) Response of growing swine to dietary exposure
pure fumonisin B1 during an eight-week period: Growth and clinical
parameters. Nat Toxins, 4: 42-50.
Rottinghaus GE, Coatney CE, & Minor HC (1992) A rapid, sensitive thin
layer chromatography procedure for the detection of fumonisin B1 and
B2. J Vet Diagn Invest, 4: 326-329.
Rumbeiha WK & Oehme FW (1997) Fumonisin exposure to Kansans through
consumption of corn-based market foods. Vet Hum Toxicol, 39: 220-225.
Sahu SC, Eppley RM, Page SW, Gray GC, Barton CN, & O'Donnell MW (1998)
Peroxidation of membrane lipids and oxidative DNA damage by fumonisin
B1 in isolated rat liver nuclei. Cancer Lett, 125: 117-121.
Sakata K, Sakata A, Vela-Roch N, Espinosa R, Escalante A, Kong L,
Nakabayashi T, Cheng J, Talal N, & Dang H (1998) Fas (CD95)-transduced
signal preferentially stimulates lupus peripheral T lymphocytes. Eur J
Immunol, 28: 2648-2660.
Sanchis V, Abadias M, Oncins L, Sala N, Viñas I, & Canela R (1994)
Occurrence of fumonisins B1 and B 2 in corn-based products from the
Spanish market. Appl Environ Microbiol, 60: 2147-2148.
Sanchis V, Abadias M, Oncins L, Sala N, Viñas I, & Canela R (1995)
Fumonisins B1 and B2 and toxigenic Fusarium strains in feeds from
the Spanish market. Intl J Food Microbiol, 27: 37-44.
Sandvig K, Garred O, Van Helvoort A, Van Meer G, & Van Deurs B (1996)
Importance of glycolipid synthesis for butryic acid-induced
sensitization to Shiga toxin and intracellular sorting of toxin in
A431 cells. Mol Biol Cell, 7: 1391-1404.
Sauviat MP, Laurent D, Kohler F, & Pellegrin F (1991) Fumonisin, a
toxin from the fungus Fusarium moniliforme Sheld, blocks both the
calcium current and the mechanical activity in frog atrial muscle.
Toxicon, 29: 1025-1031.
Savard ME & Blackwell BA (1994) Spectral characteristics of secondary
metabolites from Fusarium fungi. In: Miller JD & Trenholm HL ed.
Mycotoxins in grain: Compounds other than aflatoxin. St. Paul,
Minnesota, Eagan Press, pp 59-260.
Schaafsma AW, Miller JD, Savard ME, & Ewing RJ (1993) Ear rot
development and mycotoxin production in corn in relation to
inoculation method, corn hybrid, and species of Fusarium. Can J
Plant Pathol, 15: 185-192.
Schmelz EM, Dombrink-Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T,
& Merrill AH Jr (1998) Induction of apoptosis by fumonisin B1 in HT29
cells is mediated by the accumulation of endogenous free sphingoid
bases. Toxicol Appl Pharmacol, 148: 252-260.
Schroeder JJ, Crane HM, Xia J, Liotta DC, & Merrill AH Jr (1994)
Disruption of sphingolipid metabolism and stimulation of DNA synthesis
by fumonisin B1: A molecular mechanism for carcinogenesis associated
with Fusarium moniliforme. J Biol Chem, 269: 3475-3481.
Schwarz A, Rapaport E, Hirschberg K, & Futerman H (1995). A regulatory
role for sphingolipids in neuronal growth: Inhibition of sphingolipid
synthesis and degradation have opposite effects on axonal branching. J
Biol Chem, 270: 10990-10998.
Scott PM (1993) Fumonisins. Int J Food Microbiol, 18: 257-270.
Scott PM & Lawrence GA (1992) Liquid chromatographic determination of
fumonisins with 4-fluoro-7-nitrobenzofurazan. J Assoc Off Anal Chem
Int, 75: 829-834.
Scott PM & Lawrence GA (1994) Stability and problems in recovery of
fumonisins added to corn-based foods. J Assoc Off Anal Chem Int, 77:
541-545.
Scott PM & Lawrence GA (1995) Analysis of beer for fumonisins. J Food
Prot, 58: 1379-1382.
Scott PM & Lawrence GA (1996) Determination of hydrolysed fumonisin
B1 in alkali-processed corn foods. Food Addit Contam, 13: 823-832.
Scott PM & Trucksess MW (1997) Application of immunoaffinity columns
to mycotoxin analysis. J Assoc Off Anal Chem Int, 80: 941-949.
Scott PM, Delgado T, Prelusky DB, Trenholm HL, & Miller JD (1994)
Determination of fumonisins in milk. J Environ Sci Health, B29:
989-998.
Scott PM, Kanhere SR, Lawrence GA, Daley EF, & Farber JM (1995)
Fermentation of wort containing added ochratoxin A and fumonisins B1
and B2. Food Addit Contam, 12: 31-40.
Scott PM, Yeung JM, Lawrence GA, & Prelusky DB (1997) Evaluation of
enzyme-linked immunosorbent assay for analysis of beer for fumonisins.
Food Addit Contam, 14: 445-450.
Scudamore KA & Chan HK (1993) Occurrence of fumonisin mycotoxins in
maize and millet imported into the United Kingdom. In: Scudamore K ed.
Occurrence and significance of mycotoxins. Slough, United Kingdom,
Ministry of Agriculture, Fisheries and Food, pp 186-189.
Scudamore KA, Nawaz S, & Hetmanski MT (1998) Mycotoxins in ingredients
of animal feeding stuffs: II. Determination of mycotoxins in maize and
maize products. Food Addit Contam, 15: 30-55.
Sharma RP, Dugyala RR, & Voss KA (1997) Demonstration of in-situ
apoptosis in mouse liver and kidney after short-term repeated exposure
to fumonisin B1. J Comp Pathol, 117: 371-381.
Shelby RA, Rottinghaus GE, & Minor HC (1994a) Comparison of thin-layer
chromatography and competitive immunoassay methods for detecting
fumonisin on maize. J Agric Food Chem, 42: 2064-2067.
Shelby RA, White DG, & Bauske EM (1994b) Differential fumonisin
production in maize hybrids. Plant Dis, 78: 582-584.
Shephard GS (1998) Chromatographic determination of the fumonisin
mycotoxins. J Chromatogr, A815: 31-39.
Shephard GS, Sydenham EW, Thiel PG, & Gelderblom WCA (1990)
Quantitative determination of fumonisins B1 and B2 by
high-performance liquid chromatography with fluorescence detection.
J Liq Chromatogr, 13: 2077-2087.
Shephard GS, Thiel PG, & Sydenham EW (1992a) Initial studies on the
toxicokinetics of fumonisin B1 in rats. Food Chem Toxicol, 30:
277-279.
Shephard GS, Thiel PG, Sydenham EW, Alberts JF, & Gelderblom WCA
(1992b) Fate of a single dose of the 14C-labelled mycotoxin fumonisin
B1, in rats. Toxicon, 30: 768-770.
Shephard GS, Thiel PG, & Sydenham EW (1992c) Determination of
fumonisin B1 in plasma and urine by high-performance liquid
chromatography. J Chromatogr, 574: 299-304.
Shephard GS, Thiel PG, Sydenham EW, Alberts JF, & Cawood ME (1994a)
Distribution and excretion of a single dose of the mycotoxin fumonisin
B1 in a non-human primate. Toxicon, 32: 735-741.
Shephard GS, Thiel PG, Sydenham EW, Vleggaar R, & Alberts JF (1994b)
Determination of the mycotoxin fumonisin B1 and identification of its
partially hydrolysed metabolites in the faeces of non-human primates.
Food Chem Toxicol, 32: 23-29.
Shephard GS, Thiel PG, Sydenham EW, & Alberts JF (1994c) Biliary
excretion of the mycotoxin fumonisin B1 in rats. Food Chem Toxicol,
32: 489-491.
Shephard GS, Thiel PG, Sydenham EW, & Savard ME (1995a) Fate of a
single dose of 14C-labelled fumonisin B1 in vervet monkeys. Nat
Toxins, 3: 145-150.
Shephard GS, Thiel PG, Sydenham EW, & Snijman PW (1995b)
Toxicokinetics of the mycotoxin fumonisin B2 in rats. Food Chem
Toxicol, 33: 591-595.
Shephard GS, Thiel PG, & Sydenham EW (1995c) Liquid chromatographic
determination of the mycotoxin fumonisin B2 in physiological samples.
J Chromatogr, A692: 39-43.
Shephard GS, Thiel PG, Stockenström S, & Sydenham EW (1996a) Worldwide
survey of fumonisin contamination of corn and corn-based products.
J Assoc Off Anal Chem Int, 79: 671-687.
Shephard GS, van der Westhuizen L, Thiel PG, Gelderblom WCA, Marasas
WFO, & Van Schalkwyk DJ (1996b) Disruption of sphingolipid metabolism
in non-human primates consuming diets of fumonisin-containing
Fusarium moniliforme culture material. Toxicon, 34: 527-534.
Shetty PH & Bhat RV (1997) Natural occurrence of fumonisin B1 and its
co-occurrence with aflatoxin B1 in Indian sorghum, maize and poultry
feeds. J Agric Food Chem, 45: 2170-2173.
Shetty PH & Bhat RV (1998) Sensitive method for the detection of
fumonisin B1 in human urine. J Chromatogr, B705: 171-173.
Sheu CW, Rodriguez I, Eppley RM, & Lee JK (1996) Lack of transforming
activity of fumonisin B1 in BALB/3T3 A31-1-1 mouse embryo cells. Food
Chem Toxicol, 34: 751-753.
Shimabukuro M, Zhou Y-T, Levi M, & Unger RH (1998) Fatty acid-induced
ß cell apoptosis: a link between obesity and diabetes. Proc Natl Acad
Sci (USA), 95: 2498-2502.
Shurtleff MC (1980) Compendium of corn diseases. St. Paul, Minnesota,
American Phytopathological Society, p 105.
Smith ER & Merrill AH Jr (1995) Differential roles of de novo
sphingolipid biosynthesis and turnover in the "burst" of free
sphingosine and sphinganine, and their 1-phosphates and
N-acyl-derivatives, that occurs upon changing the medium of cells in
culture. J Biol Chem, 270: 18749-18758.
Smith JS & Thakur RA (1996) Occurrence and fate of fumonisins in beef.
Adv Exp Med Biol, 392: 39-55.
Smith GW, Constable PD, Bacon CW, Meredith FI, & Haschek WM (1996a)
Cardiovascular effects of fumonisins in swine. Fundam Appl Toxicol,
31: 169-172.
Smith GW, Constable PD, & Haschek WM (1996b) Cardiovascular responses
to short-term fumonisin exposure in swine. Fundam Appl Toxicol, 33:
140-148.
Smith GW, Constable PD, Smith AR, Bacon CW, Meredith FI, Wollenberg
GK, & Haschek WM (1996c) Effects of fumonisin-containing culture
material on pulmonary clearance in swine. Am J Vet Res, 57: 1233-1238.
Smith ER, Jones PL, Boss JM, & Merrill AH Jr (1997) Changing J774A.1
cells to new medium perturbs multiple signaling pathways, including
the modulation of protein kinase C by endogenous sphingoid bases.
J Biol Chem, 272: 5640-5646.
Solfrizzo M, Avantaggiato G, & Visconti A (1997a) Rapid method to
determine sphinganine/sphingosine in human and animal urine as a
biomarker for fumonisin exposure. J Chromatogr Biomed Appl, 692:
87-93.
Solfrizzo M, Avantaggiato G, & Visconti A (1997b) In vivo validation
of the sphinganine/sphingosine ratio as a biomarker to display
fumonisin ingestion. Cereal Res Commun, 25: 437-441.
Spiegel S (1999) Sphingosin 1-phosphate: a prototype of a new class of
second messengers. J Leukoc Biol, 65: 341-344.
Stack ME (1998) Analysis of fumonisin B1 and its hydrolysis product
in tortillas. J Assoc Off Anal Chem Int, 81: 737-740.
Stack ME & Eppley RM (1992) Liquid chromatographic determination of
fumonisins B1 and B2 in corn and corn products. J Assoc Off Anal
Chem Int, 75: 834-837.
Stevens VL & Tang J (1997) Fumonisin B1-induced sphingolipid
depletion inhibits vitamin uptake via the
glycosylphosphatidylinositol-anchored folate receptor. J Biol Chem,
272: 18020-18025.
Stevens VL, Nimkar S, Jamison WC, Liotta DC, & Merrill AH Jr (1990)
Characteristics of the growth inhibition and cytotoxicity of
long-chain (sphingoid) bases for Chinese hamster ovary cells: Evidence
for an involvement of protein kinase C. Biochim Biophys Acta, 1051:
37-45.
Stockenström S, Sydenham EW, & Thiel PG (1994) Determination of
fumonisins in corn: Evaluation of two purification procedures.
Mycotoxin Res, 10: 9-14.
Strum JC, Swenson KI, Turner JE, & Bell RM (1995) Ceramide triggers
meiotic cell cycle progression in Xenopus oocytes. A potential
mediator of progesterone-induced maturation. J Biol Chem, 270:
13541-13547.
Sun TSC & Stahr HM (1993) Evaluation and application of a
bioluminescent bacterial genotoxicity test. J Assoc Off Anal Chem Int,
76: 893-898.
Suzuki CAM, Hierlihy L, Barker M, Curran I, Mueller R, & Bondy GS
(1995) The effects of fumonisins on several markers of nephrotoxicity
in rats. Toxicol Appl Pharmacol, 133: 207-214.
Suzuki A, Iwasaki M, Kato M, & Wagai N (1997) Sequential operation of
ceramide synthesis and ICE cascade in CPT-11-initiated apoptotic death
signaling. Exp Cell Res, 233: 41-47.
Sweeley CC (1991) Sphingolipids. In: Vance DE & Vance JE ed.
Biochemistry of lipids, lipoproteins and membranes. Amsterdam,
Elsevier Science Publishers, pp 327-361.
Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S-I,
& Igarashi Y (1996) Sphingosine and its methylated derivative
N,N-methylsphingosine (DMS) induce apoptosis in a variety of human
cancer cell lines. Int J Cancer, 66: 358-366.
Swiss Federal Office of Public Health (1997a) Révision d'ordonnance
sur les substances étrangères et les composants OSEC (RS 817.021.23).
Bern, Swiss Federal Office of Public Health, 2 pp (Circular No. 11).
Swiss Federal Office of Public Health (1997b) Liquid chromatographic
determination of fumonisins B1, B2, and B3 in foods and feeds. J
Assoc Off Anal Chem Int, 75: 313-318.
Sydenham EW (1994) Fumonisins: Chromatographic methodology and their
role in human and animal health. Cape Town, South Africa, University
of Cape Town (Ph.D. Thesis).
Sydenham EW & Shephard GS (1996) Chromatographic and allied methods of
analysis for selected mycotoxins. In: Gilbert J ed. Progress in food
contaminant analysis. London, Blackie, pp 65-146.
Sydenham EW, Gelderblom WCA, Thiel PG, & Marasas WFO (1990a) Evidence
for the natural occurrence of fumonisin B1, a mycotoxin produced by
Fusarium moniliforme, in corn. J Agric Food Chem, 38: 285-290.
Sydenham EW, Thiel PG, Marasas WFO, Shephard GS, Van Schalkwyk DJ, &
Koch KR (1990b) Natural occurrence of some Fusarium mycotoxins in
corn from low and high esophageal cancer prevalence areas of the
Transkei, Southern Africa. J Agric Food Chem, 38: 1900-1903.
Sydenham EW, Shephard GS, Thiel PG, Marasas WFO, & Stockenström S
(1991) Fumonisin contamination of commercial corn-based human
foodstuffs. J Agric Food Chem, 39: 2014-2018.
Sydenham EW, Marasas WFO, Shephard GS, Thiel PG, & Hirooka EY (1992)
Fumonisin concentrations in Brazilian feeds associated with field
outbreaks of confirmed and suspected animal mycotoxicoses. J Agric
Food Chem, 40: 994-997.
Sydenham EW, Shepard GS, Gelderblom WCA, Thiel PG, & Marasas WFO
(1993a) Fumonisins: their implications for human and animal health.
In: Scudamore K ed. Proceedings of the UK Workshop on Occurrence and
Significance of Mycotoxins. Slough, United Kingdom, Ministry of
Agriculture, Fisheries and Food, Central Science Laboratory, pp 42-48.
Sydenham EW, Shephard GS, Thiel PG, Marasas WFO, Rheeder JP, Sanhueza
CEP, González HHL, & Resnick SL (1993b) Fumonisins in Argentinian
field-trial corn. J Agric Food Chem, 41: 891-895.
Sydenham EW, Stockenström S, Thiel PG, Shephard GS, Koch KR, & Marasas
WFO (1995) Potential of alkaline hydrolysis for the removal of
fumonisins from contaminated corn. J Agric Food Chem, 43: 1198-1201.
Sydenham EW, Shephard GS, Thiel PG, Stockenström S, Snijman PW, & Van
Schalkwyk DJ (1996) Liquid chromatographic determination of fumonisins
B1, B2, and B3 in corn: AOAC-IUPAC collaborative study. J Assoc Off
Anal Chem Int, 79: 688-696.
Sydenham EW, Shephard GS, Stockenström S, Rheeder JP, Marasas WFO, &
van der Merwe MJ (1997) Production of fumonisin B analogues and
related compounds by Fusarium globosum, a newly described species
from corn. J Agric Food Chem, 45: 4004-4010.
Tejada-Simon MV, Marovatsanga LT, & Pestka JJ (1995) Comparative
detection of fumonisin by HPLC, ELISA, and immunocytochemical
localization in Fusarium cultures. J Food Prot, 58: 666-672.
Thiel PG, Marasas WFO, Sydenham EW, Shephard GS, Gelderblom WCA, &
Nieuwenhuis JJ (1991a) Survey of fumonisin production by Fusarium
species. Appl Environ Microbiol, 57: 1089-1093.
Thiel PG, Shephard GS, Sydenham EW, Marasas WFO, Nelson PE, & Wilson
TM (1991b) Levels of fumonisins B1 and B2 in feeds associated with
confirmed cases of equine leukoencephalomalacia. J Agric Food Chem,
39: 109-111.
Thiel PG, Marasas WFO, Sydenham EW, Shephard GS, & Gelderblom WCA
(1992) The implications of naturally occurring levels of fumonisins in
corn for human and animal health. Mycopathologia, 117: 3-9.
Thiel PG, Sydenham EW, Shephard GS, & Van Schalkwyk DJ (1993) Study of
the reproducibility characteristics of a liquid chromatographic method
for the determination of fumonisins B1 and B2 in corn: IUPAC
collaborative study. J Assoc Off Anal Chem Int, 76: 361-366.
Tolleson WH, Dooley KL, Sheldon WG, Thurman JD, Bucci TJ, & Howard PC
(1996a) The mycotoxin fumonisin induces apoptosis in cultured human
cells and in livers and kidneys of rats. Adv Exp Med Biol, 392:
237-250.
Tolleson WH, Melchior WB Jr, Morris SM, McGarrity LJ, Domon OE,
Muskhelishvili L, James SJ, & Howard PC (1996b) Apoptotic and
antiproliferative effects of fumonisin B1 in human keratinocytes,
fibroblasts, esophageal epithelial cells and hepatoma cells.
Carcinogenesis, 17: 239-249.
Tolleson WH, Couch LH, Melchior WB Jr, Jenkins GR, Muskhelishvili M,
Muskhelishvili L, McGarrity LJ, Domon O, Morris SM, & Howard PC (1999)
Fumonisin B1 induces apoptosis in cultured human keratinocytes
through sphinganine accumulation and ceramide depletion. Int J Oncol,
14: 833-843.
Torres MR, Sanchis V, & Ramos AJ (1998) Occurrence of fumonisins in
Spanish beers analyzed by an enzyme-linked immunosorbent assay method.
Int J Food Microbiol, 39: 139-143.
Trucksess MW, Stack ME, Allen S, & Barrion N (1995) Immunoaffinity
column coupled with liquid chromatography for determination of
fumonisin B1 in canned and frozen sweet corn. J Assoc Off Anal Chem
Int, 78: 705-710.
Tryphonas H, Bondy G, Miller JD, Lacroix F, Hodgen M, McGuire P,
Fernie S, Miller D, & Hayward S (1997) Effects of fumonisin B1 on the
immune system of Sprague-Dawley rats following a 14-day oral (gavage)
exposure. Fundam Appl Toxicol, 39: 53-59.
Tseng T-C & Liu C-Y (1997) Natural occurrence of fumonisins in
corn-based foodstuffs in Taiwan. Cereal Res Commun, 25: 393-394.
Tsunoda M, Sharma RP, & Riley RT (1998) Early fumonisin B1 toxicity
in relation to disrupted sphingolipid metabolism in male BALB/c mice.
J Biochem Mol Toxicol, 12: 281-289.
Ueda N, Kaushal GP, Hong X, & Shah SV (1998) Role of enhanced ceramide
generation in DNA damage and cell death in chemical hypoxic injury to
LLC-PK1 cells. Kidney Int, 54: 399-406.
Ueno Y, Aoyama S, Sugiura Y, Wang D-S, Lee U-S, Hirooka EY, Hara S,
Karki T, Chen G, & Yu S-Z (1993) A limited survey of fumonisins in
corn and corn-based products in Asian countries. Mycotoxin Res, 9:
27-34.
Ueno Y, Umemori K, Niimi E, Tanuma S, Nagata S, Sugamata M, Ihara T,
Sekijima M, Kawai K, Ueno I, & Tashiro F (1995) Induction of apoptosis
by T-2 toxin and other natural toxins in HL-60 human promyelotic
leukemia cells. Nat Toxins, 3: 129-137.
Ueno Y, Iijima K, Wang S-D, Sugiura Y, Sekijima M, Tanaka T, Chen C, &
Yu S-Z (1997) Fumonisins as a possible contributory risk factor for
primary liver cancer: A 3-year study of corn harvested in Haimen,
China by HPLC and ELISA. Food Chem Toxicol, 35: 1143-1150.
Usleber E, Straka M, & Terplan G (1994a) Enzyme immunoassay for
fumonisin B1 applied to corn-based food. J Agric Food Chem, 42:
1392-1396.
Usleber E, Schlichtherle C, Märtlbauer E (1994b) DMZ. Mag Food Dairy
Ind, 115: 1220-1227.
US NTP (1999) NTP technical report on the toxicology and
carcinogenesis studies of fumonisin B1 (CAS No. 116355-83-0) in
F344/N rats and B6C3F1 mice (feed studies). Research Triangle Park,
North Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP TR 496; NIH Publication No. 99-3955).
Van Asch MAJ, Rijkenberg FHJ, & Coutinho TA (1992) Phytotoxicity of
fumonisin B1, moniliformin, and T-2 toxin to corn callus cultures.
Phytopathology, 82: 1330-1332.
Van der Westhuizen L, Shephard GS, Snyman SD, Abel S, Swanevelder S, &
Gelderblom WCA (1998) Inhibition of sphingolipid biosynthesis in rat
primary hepatocyte cultures by fumonisin B1 and other structurally
related compounds. Food Chem Toxicol, 36: 497-503.
Van Veldhoven PP & Mannaerts GP (1993) Sphingosine-phosphate lyase.
In: Bell RM, Hannun YA, & Merrill AH Jr ed. Advances in lipid
research: Sphingolipids and their metabolites. Orlando, Florida,
Academic Press, vol 26, pp 69-98.
Velázquez C, van Bloemendal C, Sanchis V, & Canela R (1995) Derivation
of fumonisins B1 and B2 with 6-aminoquinolyl
N-hydroxysuccinimidylcarbamate. J Agric Food Chem, 43: 1535-1537.
Vesonder RF & Wu W (1998) Correlation of moniliformin, but not
fumonisin B1 levels, in culture materials of Fusarium isolates to
acute death in ducklings. Poult Sci, 77: 67-72.
Vesonder RF, Labeda DP, & Peterson RE (1992) Phytotoxic activity of
selected water-soluble metabolites of Fusarium against Lemma
minor L. (Duckweed). Mycopathologia, 118: 185-189.
Viljoen JH, Marasas WFO, & Thiel PG (1994) Fungal infection and
mycotoxic contamination of commercial maize. In: Du Plessis JG, Van
Rensberg JBJ, McLaren NW, & Flett BC ed. Proceedings of the Tenth
South African Maize Breeding Symposium. Potchefstroom, South Africa,
Department of Agriculture, pp 26-37.
Visconti A (1996) Fumonisins in maize genotypes grown in various
geographic areas. Adv Exp Med Biol, 392: 193-204.
Visconti A & Boenke A (1995) Improvement of the determination of
fumonisins (FB1 and FB2) in maize and maize based feeds. Brussels,
European Commission, BCR Information, 101 pp (Report EUR 16617 EN).
Visconti A & Doko MB (1994) Survey of fumonisin production by
Fusarium isolated from cereals in Europe. J Assoc Off Anal Chem Int,
77: 546-550.
Visconti A, Doko MB, Schurer B, & Boenke A (1993) Intercomparison
study for the analysis of fumonisins B1 and B2 in an unknown
solution. In. Scudamore K ed. Proceedings of the UK Workshop on
Occurrence and Significance of Mycotoxins. Slough, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Central Science
Laboratory, pp 200-202.
Visconti A, Doko MB, Bottalico C, Schurer B, & Boenke A (1994)
Stability of fumonisins (FB1 and FB2) in solution. Food Addit
Contam, 11: 427-431.
Visconti A, Boenke A, Doko MB, Solfrizzo M, & Pascale M (1995)
Occurrence of fumonisins in Europe and the BCR-measurements and
testing projects. Nat Toxins, 3: 269-274.
Voss KA, Plattner RD, Bacon CW, & Norred WP (1990) Comparative studies
of hepatotoxicity and fumonisin B1 and B2 content of water and
chloroform/methanol extracts of Fusarium moniliforme strain MRC 826
culture material. Mycopathologia, 112: 81-92.
Voss KA, Chamberlain WJ, Bacon CW, & Norred WP (1993) A preliminary
investigation on renal and hepatic toxicity in rats fed purified
fumonisin B1. Nat Toxins, 1: 222-228.
Voss KA, Chamberlain WJ, Bacon CW, Herbert RA, Walters DB, & Norred WP
(1995) Subchronic feeding study of the mycotoxin fumonisin B1 in
B6C3F1 mice and Fischer 344 rats. Fundam Appl Toxicol, 24: 102-110.
Voss KA, Bacon CW, Norred WP, Chapin RE, Chamberlain WJ, Plattner RD,
& Meredith FI (1996a) Studies on the reproductive effects of
Fusarium moniliforme culture material in rats and the
biodistribution of [14C]fumonisin B1 in pregnant rats. Nat Toxins,
4: 24-33.
Voss KA, Riley RT, Bacon CW, Chamberlain WJ, & Norred WP (1996b)
Subchronic toxic effects of Fusarium moniliforme and fumonisin B1
in rats and mice. Nat Toxins, 4: 16-23.
Voss KA, Bacon CW, Meredith FI, & Norred WP (1996c) Comparative
subchronic toxicity studies of nixtamalized and water-extracted
Fusarium moniliforme culture material. Food Chem Toxicol, 34:
623-632.
Voss KA, Riley RT, Bacon CW, Meredith FI, & Norred WP (1998) Toxicity
and sphinganine levels are correlated in rats fed fumonisin B1 (FB1)
or hydrolyzed FB1. Environ Toxicol Pharmacol, 5: 101-104.
Vudathala DK, Prelusky DB, Ayroud M, Trenholm HL, & Miller JD (1994)
Pharmacokinetic fate and pathological effects of 14C-fumonisin B1 in
laying hens. Nat Toxins, 2: 81-88.
Wang D-S, Sugiura Y, Ueno Y, Buddhanont P, & Suttajit M (1993) A
limited survey for the natural occurrence of fumonisins in Thailand.
Thai J Toxicol, 9: 42-46.
Wang E, Norred WP, Bacon CW, Riley RT, & Merrill AH Jr (1991)
Inhibition of sphingolipid biosynthesis by fumonisins: implications
for diseases associated with Fusarium moniliforme. J Biol Chem, 266:
14486-14490.
Wang E, Ross PF, Wilson TM, Riley RT, & Merrill AH Jr (1992) Increases
in serum sphingosine and sphinganine and decreases in complex
sphingolipids in ponies given feed containing fumonisins, mycotoxins
produced by Fusarium moniliforme. J Nutr, 122: 1706-1716.
Wang D-S, Liang Y-X, Chau NT, Dien LD, Tanaka T, & Ueno Y (1995)
Natural co-occurrence of Fusarium toxins and aflatoxin B1 in corn
for feed in North Vietnam. Nat Toxins, 3: 445-449.
Wang H, Jones C, Ciacci-Zanella J, Holt T, Gilchrist DG, & Dickman MB
(1996) Fumonisins and Alternaria alternata lycopersici toxins:
Sphinganine analog mycotoxins induce apoptosis in monkey kidney cells.
Proc Natl Acad Sci (USA), 93: 3461-3465.
Wang E, Riley RT, Meredith FI, & Merrill AH Jr (1999) Fumonisin B1
consumption by rats causes reversible, dose-dependent increases in
urinary sphinganine and sphingosine. J Nutr, 129: 214-220.
Ware GM, Francis O, Kuan SS, Umrigar P, Carman A, Carter L, & Bennett
GA (1993) Determination of fumonisin B1 in corn by high performance
liquid chromatography with fluorescence detection. Anal Lett, 26:
1751-1770.
Ware GM, Umrigar PP, Carman AS Jr, & Kuan SS (1994) Evaluation of
fumonitest immunoaffinity columns. Anal Lett, 27: 693-715.
Wattenberg EV, Badria FA, & Shier WT (1996) Activation of
mitogen-activated protein kinase by the carcinogenic mycotoxin
fumonisin B1. Biochem Biophys Res Commun, 227: 622-627.
Weibking TS, Ledoux DR, Brown TP, & Rottinghaus GE (1993a) Fumonisin
toxicity in turkey poults. J Vet Diagn Invest, 5: 75-83.
Weibking TS, Ledoux DR, Bermudez AJ, Turk JR, Rottinghaus GE, Wang E,
& Merrill AH Jr (1993b) Effects of feeding Fusarium moniliforme
culture material, containing known levels of fumonisin B1, on the
young broiler chick. Poult Sci, 72: 456-466.
Weibking T, Ledoux DR, Bermudez AJ, Turk JR, & Rottinghaus GE (1995)
Effects on turkey poults of feeding Fusarium moniliforme M-1325
culture material grown under different environmental conditions. Avian
Dis, 39: 32-38.
Wieder T, Orfanos CE, & Geilen CC (1998) Induction of
ceramide-mediated apoptosis by the anticancer phospholipid analog,
hexadecylphosphocholine. J Biol Chem, 273: 11025-11031.
Wild CP, Castegnaro M, Ohgaki H, Garren L, Galendo D, & Miller JD
(1997) Absence of a synergistic effect between fumonisin B1 and
N-nitrosomethylbenzylamine in the induction of oesophageal papillomas
in the rat. Nat Toxins, 5: 126-131.
Wilson BJ & Maronpot RR (1971) Causative fungus agent of
leukoencephalomalacia in equine animals. Vet Rec, 88: 484-486.
Wilson TM, Nelson PE, & Knepp CR (1985) Hepatic neoplastic nodules,
adenofibrosis, and cholangiocarcinomas in male Fisher 344 rats fed
corn naturally contaminated with Fusarium moniliforme.
Carcinogenesis, 6: 1155-1160.
Wilson TM, Ross PF, Rice LG, Osweiler GD, Nelson HA, Owens DL,
Plattner RD, Reggiardo C, Noon TH, & Pickrell JW (1990) Fumonisin B1
levels associated with an epizootic of equine leukoencephalomalacia.
J Vet Diagn Invest, 2: 213-216.
Wilson TM, Ross PF, Owens DL, Rice LG, Green SA, Jenkins SJ, & Nelson
HA (1992) Experimental reproduction of ELEM-A study to determine the
minimum toxic dose in ponies. Mycopathologia, 117: 115-120.
Witty JP, Bridgham JT, & Johnson AL (1996) Induction of apoptotic cell
death in hen granulosa cells by ceramide. Endocrinology,
137: 5269-5277.
Wu W-I, McDonough VM, Nickels JT, Ko SJ, Fischl AS, Vales TR, Merrill
AH Jr, & Carman GM (1995) Regulation of lipid biosynthesis in
Saccharomyces cerevisiae by fumonisin B1. J Biol Chem,
270: 13171-13178.
Xu J, Yeh C-H, Chen S, He L, Sensi SL, Canzoniero LMT, Choi DW, & Hsu
CY (1998) Involvement of de novo ceramide biosynthesis in tumor
necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell
death. J Biol Chem, 273: 16521-16526.
Yamashita A, Yoshizawa T, Aiura Y, Sanchez P, Dizon EI, Arim RH, &
Sardjono (1995) Fusarium mycotoxins (fumonisins, nivalenol and
zearalenone) and aflatoxins in corn from southeast Asia. Biosci
Biotechnol Biochem, 59: 1804-1807.
Yeung JM, Wang H-Y, & Prelusky DB (1996) Fumonisin B1 induces protein
kinase C translocation via direct interaction with diacylglycerol
binding site. Toxicol Appl Pharmacol, 141: 178-184.
Yin J-J, Smith MJ, Eppley RM, Troy AL, Page SW, & Sphon JA (1996)
Effects of fumonisin B1 and (hydrolyzed) fumonisin backbone AP1 on
membranes: A spin-label study. Arch Biochem Biophys, 335: 13-22.
Yin J-J, Smith MJ, Eppley RM, Page SW, & Sphon JA (1998) Effects of
fumonisin B1 on lipid peroxidation in membranes. Biochim Biophys Acta,
1371: 134-142.
Yoo H-S, Norred WP, Wang E, Merrill AH Jr, & Riley RT (1992) Fumonisin
inhibition of de novo sphingolipid biosynthesis and cytotoxicity are
correlated in LLC-PK1 cells. Toxicol Appl Pharmacol, 114: 9-15.
Yoo H-S, Norred WP, Showker JL, & Riley RT (1996) Elevated sphingoid
bases and complex sphingolipid depletion as contributing factors in
fumonisin-induced cytotoxicity. Toxicol Appl Pharmacol, 138: 211-218.
Yoshizawa T, Yamashita A, & Luo Y (1994) Fumonisin occurrence in corn
from high- and low-risk areas for human esophageal cancer in China.
Appl Environ Microbiol, 60: 1626-1629.
Young JC & Lafontaine P (1993) Detection and characterization of
fumonisin mycotoxins as their methyl esters by liquid
chromatography/particle-beam mass spectrometry. Rapid Commun Mass
Spectrom, 7: 352-359.
Zacharias C, Van Echten-Deckert G, Wang E, Merrill AH Jr, & Sandoff K
(1996) The effect of fumonisin B1 on developing chick embryos:
Correlation between de novo sphingolipid biosynthesis and gross
morphological changes. Glycoconj J, 13: 167-175.
Zhang H, Buckley NE, Gibson K, & Spiegel S (1990) Sphingosine
stimulates cellular proliferation via a protein kinase C-independent
pathway. J Biol Chem, 265: 76-81.
Zhang H, Desai NN, Olivera A, Seki T, Booker G, & Spiegel S (1991)
Sphingosine-1-phosphate, a novel lipid, involved in cellular
proliferation. J Cell Biol, 114: 155-167.
Zhang H, Nagashima H, & Goto T (1997) Natural occurrence of mycotoxins
in corn, samples from high and low risk areas for human esophageal
cancer in China. Mycotoxins, 44: 29-35.
Zhen YZ (1984) [The culture and isolation of fungi from the cereals in
five high and three low incidence counties of esophageal cancer in
Henan Province.] J Chin Tumor, 6: 27-29 (in Chinese).
Zoller O, Sager F, & Zimmerli B (1994) [Occurrence of fumonisins in
food.] Mitt Gebiete Lebensm Hyg, 85: 81-99 (in German).
APPENDIX 1. NATIONAL GUIDELINES FOR FUMONISINS
The US Food and Drug Administration, Center for Veterinary
Medicine unofficial guidelines (Miller et al., 1996) recommend that
the non-roughage portion of feeds for equine species should be less
than 5 ppm FB1 (< 5 mg/kg), for porcine species the total diet
should contain less than 10 ppm FB1 (< 10 mg/kg), for beef cattle
the non-roughage portion should be less than 50 ppm FB1
(< 50 mg/kg), and for poultry the complete feed should contain less
than 50 ppm FB1 (< 50 mg/kg).
An official tolerance value for dry maize products (1 mg/kg FB1
plus FB2) has been issued in Switzerland (Swiss Federal Office of
Public Health, 1997).
APPENDIX 2. NATURAL OCCURRENCE OF FUMONISIN B1 (FB1)
IN MAIZE-BASED PRODUCTS
Product Country Positive/ FB1 Reference
total (mg/kg)
North America
maize Canada 1/1 0.08 Stack & Eppley
(1992)
maize Canada 9/98 <1-2.5 Miller et al. (1995)
maize flour Canada 1/2 0.05 Sydenham et al.
(1991)
maize feed USA 3/3 37-122 Wilson et al. (1990)
maize feed USA 2/2 12-130 Plattner et al. (1990)
maize feed USA 81/93 <1-126 Ross et al. (1991a)
maize feed USA 158/213 <1-330 Ross et al. (1991b)
maize feed USA 15/15 1.3-5.2 Thiel et al. (1991b)
maize feed USA 2/2 105-155 Colvin & Harrison
(1992)
maize feed USA 29/29 3-330 Osweiler et al. (1992)
maize feed USA 20/21 <1-73 a Bane et al. (1992)
maize USA 6/6 1.7-196.5 Stack & Eppley
screenings (1992)
(feed)
maize feed USA 1/1 86.0 Park et al. (1992)
maize feed USA 14/14 1.3-27.0 Sydenham et al.
(1992)
maize feed USA 160/160 0.1-239 Murphy et al. (1993)
maize feed USA 85/85 2.6-32 a Price et al. (1993)
maize feed USA 5/5 0.22-1.41 Hopmans & Murphy
(1993)
maize feed USA 0/29 - Chamberlain et al.
(1993)
maize feed USA 0/1 - Holcomb et al. (1993)
maize feed USA 5/5 0.77-6.2 Rumbeiha & Oehme
(1997)
maize USA 6/6 0.14-16.31 Stack & Eppley (1992)
maize USA 13/99 1.2-3.2 Price et al. (1993)
maize USA 155/175 < 1-37.9 Murphy et al. (1993)
maize USA 24/28 av. 0.87 Chamberlain et al.
(1993)
maize USA 116/322 1-> 10 Shelby et al. (1994a)
maize flour USA 13/25 0.05-0.35 Rumbeiha & Oehme
(1997)
maize flour USA 7/7 0.40-6.32 Pestka et al. (1994)
maize flour USA 15/16 0.05-2.79 Sydenham et al.
(1991)
maize flour USA 16/16 0.28-2.05 Stack & Eppley
(1992)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flour USA 6/6 0.21-0.84 Hopmans & Murphy
(1993)
maize grits USA 10/10 0.11-2.55 Sydenham et al.
(1991)
maize grits USA 5/5 0.14-0.27 Stack & Eppley
(1992)
maize flakes USA 0/2 - Sydenham et al.
(1991)
maize flakes USA 2/5 0.01 Stack & Eppley
(1992)
maize cereals USA 7/12 0.06-0.33 Stack & Eppley
(bran, fibre, (1992)
pops)
popcorn USA 1/18 0.07 Rumbeiha & Oehme
(1997)
sweet maize USA 1/1 0.026 Hopmans & Murphy
(1993)
sweet maize USA 37/97 0.004-0.35 Trucksess et al.
(1995)
tortillas USA 1/3 0.05-0.06 Sydenham et al.
(1991)
miscellaneous USA 4/4 0.09-0.70 Sydenham et al.
maize foods b (1991)
miscellaneous USA 6/11 0.01-0.12 Stack & Eppley
maize foods b (1992)
miscellaneous USA 4/4 0.02-0.32 Hopmans & Murphy
maize foods b (1993)
miscellaneous USA 3/5 0.05-1.21 Pestka et al. (1994)
maize foods b
Latin America
maize Argentina 17/17 1.11-6.70 Sydenham et al.
(1993b)
maize Argentina 51/51 0.18-27.05 Visconti et al.
(1995); Ramirez et
al. (1996)
maize feed Brazil 20/21 0.2-38.5 Sydenham et al.
(1992)
maize Brazil 47/48 0.6-18.5 Hirooka et al. (1996)
tortillas Texas-Mexico 50/52 av. 0.19 Stack (1998)
border
masas Texas-Mexico 8/8 av. 0.26 Stack (1998)
border
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flour Peru 1/2 0.66 Sydenham et al.
(1991)
alkali-treated Peru 0/2 - Sydenham et al.
kernels (1991)
maize feed Uruguay 13/13 0.26-6.3 Piñeiro et al. (1997)
maize Uruguay 11/22 0.17-3.7 Piñeiro et al. (1997)
maize snacks Uruguay 4/10 0.15-0.31 Piñeiro et al. (1997)
frozen maize Uruguay 1/7 0.16 Piñeiro et al. (1997)
polenta Uruguay 3/12 0.1-0.43 Piñeiro et al. (1997)
maize flour Venezuela 1/1 0.07 Stack & Eppley
(1992)
Europe
maize Austria 6/9 1-15 Lew et al. (1991)
maize flour Austria -/3 f 0.05-1.15 Sydenham et al.
(1993a)
maize flour Bulgaria -/15 f 0.05-0.21 Sydenham et al.
(1993a)
maize Croatia 11/19 0.01-0.06 Doko et al. (1995)
maize flour Czech 22/22 0.01-0.49 a Ostry & Ruprich
(1998)
maize pastes Czech 6/11 0.01-0.51 a Ostry & Ruprich
(1998)
maize-extruded Czech 30/35 0.01-1.8 a Ostry & Ruprich
bread (1998)
polenta Czech 6/7 0.01-1.2 a Ostry & Ruprich
(1998)
porridge Czech 18/19 0.01-0.79 a Ostry & Ruprich
(1998)
maize feed France 43/58 0.02-8.82 Doko et al. (1994)
maize feed France 35/35 0.02-2.17 Dragoni et al. (1996)
maize flour France 1/1 1.24 Sydenham et al.
(1993a)
miscellaneous France 10/22 0.02-1.50 Visconti et al. (1995)
maize foods b
maize Germany 49/458 0.007-4.83 Meister et al. (1996)
imported maize Germany 21/21 0.014-1.11 a Meister et al. (1996)
maize grits Germany 1/2 0.01 Usleber et al. (1994a)
grits flour, Germany 60/71 0.01-16.00 Usleber et al.
semolina (1994b) c
semolina Germany 10/11 0.01-1.23 Usleber et al. (1994a)
popcorn Germany 13/29 0.01-0.16 Usleber et al.
(1994b) c
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
popcorn Germany 4/6 0.01-0.11 Usleber et al. (1994a)
infant foods Germany 0/91 - Usleber et al.
(1994b) c
maize Hungary 56/92 0.05-75.10 Fazekas et al. (1998)
maize feed Italy 23/25 0.02-8.40 Minervini et al. (1992)
maize screen Italy 3/3 55.2-70.0 Caramelli et al. (1993)
(feed)
maize feed Italy 1/1 60 Doko & Visconti
(1994)
maize Italy 7/7 0.1-5.3 Doko & Visconti
(1994)
maize Italy 6/6 125-250 Bottalico et al. (1995)
maize Italy 26/26 0.01-2.33 Doko & Visconti
genotypes (1994)
commercial Italy 7/7 0.10-5.31 Doko & Visconti
maize kernels (1994)
maize flour Italy 7/7 0.42-3.73 Doko & Visconti
(1994)
maize grits Italy 1/1 3.76 Doko & Visconti
(1994)
polenta Italy 20/20 0.15-3.76 Pascale et al. (1995)
popcorn, Italy 6/10 0.01-0.06 Doko & Visconti
maize flakes, (1994)
tortilla chips
extruded maize Italy 6/6 0.79-6.10 Doko & Visconti
(1994)
sweet maize Italy 5/5 0.06-0.79 Doko & Visconti
(1994)
maize flour Netherlands 5/7 0.008-0.09 de Nijs et al. (1998c)
maize for bread Netherlands 8/19 0.008-0.38 de Nijs et al. (1998c)
maize for Netherlands 2/10 0.008-0.11 de Nijs et al. (1998c)
popcorn
maize foods Netherlands 12/42 0.008-1.43 de Nijs et al. (1998c)
imported maize Netherlands 61/62 0.03-3.35 de Nijs et al. (1998b)
maize Poland 2/7 0.01-0.02 Doko et al. (1995)
maize Portugal 9/9 0.09-2.30 Doko et al. (1995)
maize Romania 3/6 0.01-0.02 Doko et al. (1995)
maize feed Spain 136/171 av. 3.3 Castella et al. (1997)
maize kernels Spain 1/1 0.72 Visconti et al. (1995)
maize flour Spain 1/3 0.05-0.07 Sanchis et al. (1994)
maize flour Spain 16/17 < 0.50 a Burdaspal & Legarda
(1996)
maize grits Spain 3/15 0.05-0.09 Sanchis et al. (1994)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flakes Spain 2/12 0.05-0.10 Sanchis et al. (1994)
maize starch Spain 1/13 0.03 Burdaspal & Legarda
(1996)
sweet maize Spain 3/3 0.72 Visconti et al. (1995)
miscellaneous Spain 2/20 0.05-0.20 Sanchis et al. (1994)
maize foods b
maize flour Sweden 1/1 0.13 Visconti et al. (1995)
popcorn Sweden 1/1 0.13 Visconti et al. (1995)
maize feed Switzerland 6/22 av. 0.24 Pittet et al. (1992)
maize flour Switzerland 2/7 av. 0.09 Pittet et al. (1992)
maize grits d Switzerland 34/55 av. 0.26 Pittet et al. (1992)
maize grits, Switzerland 27/27 0.01-2.20 Zoller et al. (1994)
flour d
maize flakes Switzerland 1/12 0.06 Pittet et al. (1992)
popcorn Switzerland 8/13 0.005-0.25 Zoller et al. (1994)
sweet maize Switzerland 1/7 0.07 Pittet et al. (1992)
miscellaneous Switzerland 0/17 - Pittet et al. (1992)
maize foods b
maize feed UK 24/29 0.05-4.55 Scudamore & Chan
(1993)
maize UK 65/67 0.03-24 Scudamore et al.
(1998)
polenta UK 16/20 0.02-2.12 a Patel et al. (1997)
maize snacks UK 31/40 0.01-0.22 a Patel et al. (1997)
popcorn UK 6/22 0.01-0.78 a Patel et al. (1997)
Africa
maize Benin 9/11 0.02-2.63 Doko et al. (1995)
maize flour Botswana 5/5 0.18-0.45 Sydenham et al.
(1993a)
miscellaneous Botswana 6/6 0.03-0.35 Doko et al. (1996)
maize foods b
maize flour Egypt 2/2 1.78-2.98 Sydenham et al.
(1991)
maize kernels Kenya 1/1 0.78 Doko et al. (1996)
maize flour Kenya -/3 f 0.05-0.11 Sydenham et al.
(1993a)
maize kernels Malawi 7/8 0.02-0.11 Doko et al. (1996)
maize kernels Mozambique 3/3 0.24-0.29 Doko et al. (1996)
maize feed South Africa 15/15 0.47-4.34 Viljoen et al. (1994)
mixed feed South Africa 1/1 8.85 Shephard et al. (1990)
maize South Africa 3/3 10-83 Sydenham et al.
(1990a)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize South Africa 60/60 0.2-46.9 Sydenham et al.
(1990b)
maize South Africae 62/74 0.05-117.5 Rheeder et al. (1992)
maize South Africa 24/68 0.05-0.87 Sydenham (1994) c
maize flour South Africa 46/52 0.05-0.48 Sydenham et al.
(1991)
maize flour South Africa 2/2 0.06-0.07 Doko et al. (1996)
maize grits South Africa 10/18 0.05-0.19 Sydenham et al.
(1991)
maize flakes South Africa 0/3 - Sydenham et al.
(1991)
miscellaneous South Africa 2/8 0.05-0.09 Sydenham et al.
maize foods b (1991)
maize kernels Tanzania 8/9 0.02-0.16 Doko et al. (1996)
maize kernels Uganda 1/1 0.60 Doko et al. (1996)
maize Zambia 20/20 0.02-1.42 Doko et al. (1995)
maize flour Zambia 1/1 0.74 Doko et al. (1996)
maize kernels Zimbabwe 1/2 0.12 Doko et al. (1996)
maize flour Zimbabwe 3/3 1.06-3.63 Sydenham et al.
(1993a)
maize flour Zimbabwe 4/4 0.05-1.91 Doko et al. (1996)
Asia
maize China 2/5 5.3-8.4 Ueno et al. (1993)
maize China 18/47 0.18-2.9 Yoshizawa et al.
(1994)
maize China 27/68 0.01-1.4 Kang et al. (1994)
maize China e 34/34 18-155 Chu & Li (1994)
maize China e 134/240 0.08-34.87 Ueno et al. (1997)
maize China e 37/54 0.08-21 Gao & Yoshizawa
(1997)
maize flour China 0/3 - Sydenham et al.
(1993a)
maize flour China 3/4 0.06-0.2 Ueno et al. (1993)
gluten India 1/1 0.7 Scudamore & Chan
(1993)
maize Indonesia 7/12 0.05-1.8 Yamashita et al.
(1995)
maize Indonesia 16/16 0.05-2.44 Ali et al. (1998)
maize grits Japan 14/17 0.20-2.60 Ueno et al. (1993)
sweet maize Japan 0/8 - Ueno et al. (1993)
maize snack Japan 0/31 - Ueno et al. (1993)
maize soup Japan 0/7 - Ueno et al. (1993)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize feed Korea 5/12 0.05-1.33 Lee et al. (1994)
maize Nepal 12/24 0.05-4.6 Ueno et al. (1993)
maize Philippines 26/50 0.05-1.8 Yamashita et al.
(1995)
maize Philippines 9/10 0.3-10.0 Bryden et al. (1996)
miscellaneous Taiwan 52/153 0.07-2.39 Tseng & Liu (1997)
maize foods b
maize Thailand 19/27 0.06-18.8 Yamashita et al.
(1995)
maize feed Thailand 5/22 0.05-1.59 Wang et al. (1993)
maize flour Thailand 6/6 0.48-0.88 Wang et al. (1993)
maize grits Thailand 5/5 0.25-1.82 Wang et al. (1993)
maize Vietnam 12/12 0.3-9.1 Bryden et al. (1996)
maize Vietnam 8/15 0.27-3.45 Wang et al. (1995)
maize powder Vietnam 15/17 0.27-1.52 Wang et al. (1995)
Oceania
maize Australia 67/70 0.3-40.6 Bryden et al. (1996)
maize flour New Zealand 0/12 - Sydenham et al.
(1993a)
a Fumonisins B1 + B2 + B3
b Includes maize snacks, canned maize, frozen maize, extruded maize, bread,
maize-extruded bread, biscuit, cereals, chips, flakes, pastes, starch, sweet
maize, infant foods, gruel, purée, noodles popcorn, porridge, tortillas,
tortilla chips, masas, popped maize, soup, taco, tostada
c From: Shephard et al. (1996a)
d Maize grits and flour samples analysed were imported cereals (mainly from
Argentina)
e Fumonisin levels in low- and high-risk area for human oesophageal cancer
f The number of positive samples was not indicated in the original report
RESUME, EVALUATION ET RECOMMANDATIONS
1. Résumé
1.1 Identité, propriétés physique et chimiques et méthodes
d'analyse
La fumonisine B1 (FB1), de formule brute C34H59NO15, est le
diester de l'acide propane-1,2,3-tricarboxylique et du
2-amino-12,16-diméthyl-3,5,10,14,15-pentahydroxyeicosane (masse
moléculaire relative: 721). C'est la plus abondante des fumonisines,
qui constituent une famille de toxines dont on a identifié au moins 15
membres. A l'état pur, ce composé se présente sous la forme d'une
poudre hygroscopique de couleur blanche, soluble dans l'eau, le
mélange eau-acétonitrile et le méthanol. Elle est stable dans le
mélange eau-acétonitrile à 1:1 et instable dans le méthanol. Elle est
stable aux températures utilisées pour la transformation des denrées
alimentaires ainsi qu'à la lumière.
Plusieurs méthodes d'analyse ont été proposées, notamment la
chromatographie sur couche mince ou en phase liquide, la spectrométrie
de masse, la chromatographie en phase gazeuse après hydrolyse et un
certain nombre de méthodes immunochimiques. En fait, la plupart des
dosages se font par chromatographie en phase liquide d'un dérivé
fluorescent.
1.2 Sources d'exposition humaine
La FB1 est produite par plusieurs espèces de Fusarium, mais
essentiellement par Fusarium verticillioides (Sacc.) Nirenberg
(= Fusarium moniloforme Sheldon) qui est l'un des parasites
cryptogamiques les plus fréquents du maïs. La FB1 peut s'accumuler en
quantités importantes dans le maïs lorsque les conditions
météorologiques sont favorables à l'apparition de la fusariose.
1.3 Transport, distribution et transformation dans l'environnement
On est fondé à penser que les fumonisines peuvent être
métabolisées par certains microorganismes terricoles. On sait
toutefois peu de choses de leur devenir dans l'environnement une fois
qu'elles ont été excrétées ou que les produits qui en contiennent ont
été transformés.
1.4 Concentrations dans l'environnement et exposition humaine
On a mis en évidence de la FB1 dans le maïs et les produits qui
en dérivent partout dans le monde à des concentrations de l'ordre du
mg/kg, parfois en association avec d'autres mycotoxines. Des
concentrations de cet ordre ont également été observées dans des
denrées alimentaires destinées à la consommation humaine. Lors de la
mouture à sec du maïs, la fumonisine se répartit dans le son, le germe
et la farine. Lors d'essais de mouture par voie humide, on a mis en
évidence la toxine dans l'eau de macération, dans le gluten, dans les
fibres et les germes, à l'exclusion de l'amidon. La FB1 reste stable
dans le maïs et la polenta, mais elle s'hydrolyse dans les aliments à
base de maïs traités par des solutions alcalines à chaud.
La FB1 est absente du lait, de la viande et des oeufs provenant
d'animaux nourris avec du maïs dont la teneur en toxine ne représente
aucun danger pour eux. On estime que l'exposition humaine journalière
aux Etats-Unis, au Canada, en Suisse, aux Pays-Bas et au Transkei
(Afrique du Sud) varie entre 0,017 et 440 µg/kg de poids corporel. On
ne possède aucune donnée sur l'exposition professionnelle par
inhalation.
1.5 Cinétique et métabolisme chez l'animal
On ne dispose d'aucune donnée sur la cinétique ou le métabolisme
de la FB1 chez l'Homme. Chez les animaux de laboratoire, le composé
est peu résorbé après ingestion; il disparaît rapidement du courant
sanguin et se retrouve inchangé dans les matières fécales. Il est
excrété en quantité importante par la voie biliaire et en faible
proportion dans les urines. Chez les primates non humains et certains
ruminants, la fumonisine peut subir une dégradation hydrolytique
partielle dans l'intestin. Elle subsiste en petite quantité dans le
foie et les reins.
1.6 Effets sur l'animal et les systèmes d'épreuve in vitro
La FB1 s'est révélée hépatotoxique pour les espèces animales sur
lesquelles elle a été testée, notamment le rat, la souris, les
équidés, le lapin, le porc et les primates non humains. Sauf dans le
cas du hamster doré, on n'observe d'effets embryotoxiques ou
tératogènes que concurremment ou postérieurement aux manifestations
toxiques qui se produisent chez la mère. Les fumonisines sont
néphrotoxiques chez le porc, le rat, le mouton, la souris et le lapin.
Dans le cas du rat et du lapin, la néphrotoxicité se manifeste à des
doses plus faibles que l'hépatotoxicité. On sait que la
leucoencéphalomalacie équine et l'oedème pulmonaire porcin observés
après consommation de provendes à base de maïs sont dus à la présence
de fumonisines. Les données dont on dispose sur les propriétés
immunologiques de la FB1 sont limitées. A la dose de 50 mg/kg de
nourriture, le composé a provoqué des cancers du foie chez une souche
de rats et des cancers du rein chez une autre souche; dans les mêmes
conditions de dosage et d'administration, il a également provoqué des
cancers du foie chez des souris femelles. Il semble qu'il existe une
corrélation entre les effets toxiques sur les organes et l'apparition
de cancers. La FB1 a été le premier inhibiteur du métabolisme des
sphingolipides qui ait été découvert et on l'utilise beaucoup depuis
lors pour étudier le rôle des sphingolipides dans la régulation
cellulaire. La FB1 inhibe la croissance cellulaire; elle entraîne
l'accumulation de bases sphingoïdes libres et modifie le métabolisme
des lipides chez les animaux, les plantes et certaines levures. Elle
ne provoque pas de mutation génique chez les bactéries et mise en
présence d'une culture primaire d'hépatocytes de rat, elle n'entraîne
pas une synthèse non programmée de l'ADN. On a constaté par contre
qu'elle pouvait provoquer des aberrations chromosomiques à faible dose
dans ces mêmes cultures cellulaires.
1.7 Effets sur l'Homme
On ne dispose d'aucune donnée confirmée relative à la toxicité
aiguë des fumosinines pour l'Homme. Des études effectuées au Transkei
(Afrique du Sud) sur la corrélation entre divers effets toxiques et la
présence de fumonisines dans la ration alimentaire suggèrent
l'existence d'un lien entre ces composés et le cancer de l'oesophage.
Cette corrélation a été observée dans des circonstances où il y avait
une forte exposition aux fumonisines et où les conditions
environnementales étaient favorables à une importante accumulation de
toxines dans le maïs, qui constitue une denrée alimentaire de base
dans la région. Des études du même genre ont également été effectuées
en Chine. Ces dernières n'ont toutefois pas permis de dégager une
relation claire entre la contamination par les fumonisines ou
F. verticillioides et le cancer de l'oesophage. Fautes de données
concernant l'exposition aux fumonisines, il n'est pas possible non
plus de tirer de conclusions d'une étude cas-témoins effectuée en
Italie sur des sujets de sexe masculin et qui révèle l'existence d'une
association entre la consommation de maïs et les cancers des voies
digestives supérieures chez les gros buveurs.
Il n'existe pas de marqueurs biologiques valables de l'exposition
à la FB1.
1.8 Effets sur les autres organismes en laboratoire
La FB1 inhibe la croissance cellulaire et provoque
l'accumulation de bases sphingoïdes libres ainsi que la modification
du métabolisme des lipides chez Saccharomyces cerevisiae.
La FB1 est phytotoxique, elle provoque des lésions de la
membrane cellulaire et réduit la synthèse chlorophyllienne. Elle
perturbe également la biosynthèse des sphingolipides chez les végétaux
et pourrait jouer un rôle dans les maladies du maïs dues aux espèces
de Fusarium qui produisent des fumonisines
2. Evaluation des risques pour la santé humaine
2.1 Exposition
L'Homme est exposé partout dans le monde puisque la FB1 est
présente dans le maïs destiné à la consommation humaine. Toutefois,
des différences notables existent entre les régions du culture de
cette céréale. Cette constatation s'impose lorsque l'on compare pays
développés et pays en développement. Par exemple, même si aux
Etats-Unis, au Canada et en Europe occidentale la FB1 est présente
dans les produits tirés du maïs, la consommation de ces produits y
reste à un niveau modeste. Dans certaines régions d'Afrique,
d'Amérique centrale et d'Asie, il y a des populations dont l'apport
calorique alimentaire est constitué pour une large part de farine de
maïs et la contamination par la FB1 peut y être importante (voir
Appendice 2). Le maïs naturellement contaminé par la FB1 peut
également l'être par d'autres toxines de F. verticillioides ou
F. proliferatum ou encore par des toxines d'importance agricole
telles que le désoxynivalénol, la zéaralénone, l'aflatoxine ou
l'ochratoxine.
Les procédés de transformation des denrées alimentaires utilisés
en Amérique du Nord ou en Europe occidentale n'ont aucun effet sur la
stabilité de la FB1. Le traitement du maïs par une base ou son lavage
à l'eau réduisent sensiblement la teneur en toxine. Cependant on
constate toujours des effets hépatotoxiques et néphrotoxiques chez les
animaux de laboratoire. On sait peu de choses sur la manière dont les
techniques de préparation des aliments utilisées dans le monde en
développement peuvent agir sur la FB1 présente dans les produits
tirés du maïs.
2.2 Nature des dangers
Le rôle étiologique de la FB1 dans la leucoencéphalomalacie
équine est établi. D'importantes flambées de cette zoonose mortelle se
sont produites aux Etats-Unis au cours du 19ème siècle et plus
récemment en 1989-1990. De même, on a également montré que cette
toxine était à l'origine d'une zoonose tout aussi mortelle, l'oedème
pulmonaire porcin. Une faible dose de FB1 peut également être
mortelle pour le lapin, comme on a pu l'observer sur des lapines
gravides. Chez toutes les espèces animales étudiées, y compris les
primates non humains, on a constaté que cette toxine provoquait des
lésions rénales et hépatiques. La FB1 provoque une
hypercholestérolémie chez plusieurs espèces animales, dont les
primates non humains. On de bonnes raisons de penser que dans les
maladies animales dues à une exposition à la FB1, il y a modification
du métabolisme des lipides. Les effets toxiques observés in vivo ou
in vitro sont précédés ou accompagnés d'une perturbation du
métabolisme des sphingolipides. L'utilisation des fumonisines dans
l'étude de la fonction des sphingolipides a montré que ces composés
sont nécessaires à la croissance cellulaire et qu'il peuvent affecter
les molécules signal de différentes manières, avec pour conséquence la
mort cellulaire par apoptose ou nécrose, la différenciation cellulaire
ou encore la modification de la réponse immunitaire. Après exposition
à la FB1, on observe communément une modification du métabolisme des
lipides et de l'expression ou de l'activité des enzymes qui jouent un
rôle clé dans la progression du cycle cellulaire. La FB1 n'exerce pas
d'effets toxiques sur le développement chez le rat, la souris ou le
lapin. En revanche, elle est foetotoxique chez le hamster doré à forte
dose même quand elle n'a pas d'effet sur la mère.
Chez les rongeurs, la cancérogénicité de la FB1 varie selon
l'espèce, la souche et le sexe. La seule étude qui ait été effectuée
sur des souris B6C3F1 a montré que la toxine provoquait des cancers
du foie chez les femelles à la dose de 50 mg/kg de nourriture. Des
cancers primitifs du foie et des cholangiomes ont été observés chez
des rats mâles BD IX qui avaient reçu, pendant une durée allant
jusqu'à 26 mois, une alimentation contenant 50 mg/kg de FB1. Chez des
rats mâles F344/N alimentés dans les mêmes conditions de dosage, on a
mis en évidence des adénomes et des carcinomes au niveau des tubules
rénaux. Il semble qu'il y ait corrélation entre la toxicité pour tel
ou tel organe et l'apparition de cancers à ce niveau.
Les études de génotoxicité sont en nombre limité. Celles qui
portent sur des bactéries n'ont pas révélé d'effets mutagènes. Dans
des cultures de cellules mammaliennes on n'a pas non plus décelé de
synthèse non programmée de l'ADN mais selon une étude, la FB1 a
provoqué des cassures chromosomiques dans des hépatocytes de rat.
Selon d'autres travaux, la FB1 accroît la peroxydation des lipides
in vivo et in vitro. Il est possible qu'il existe une relation de
cause à effet entre la peroxydation des lipides et les cassures
chromosomiques.
Les teneurs en FB1 supérieures à 100 mg/kg constatées dans le
maïs consommé par l'Homme en Afrique et en Chine pourraient
probablement provoquer selon le cas, des leucoencéphalomalacies, des
oedèmes pulmonaires et des cancers si on donnait à manger ce maïs à
des chevaux, des porcs, des rats ou des souris. On connaît des cas où
l'exposition humaine à cette toxine est très importante, mais aucun
cas d'intoxication aiguë par une fumonisine n'a été décrit. Les études
de corrélation effectuées au Transkei (Afrique du Sud) incitent à
penser qu'il pourrait y avoir un lien entre une exposition à la
fumonisine par voie alimentaire et le cancer de l'oesophage. On a
effectivement constaté un taux élevé de cancers de l'oesophage là où
l'exposition à cette toxine était importante et où les conditions
environnementales étaient favorables à l'accumulation de fumonisines
dans le maïs, qui constitue un aliment de base dans ces régions.
Une étude cas-témoins réalisée en Italie a mis en évidence une
association entre la consommation de maïs et les cancers des voies
digestives supérieures, et notamment le cancer de l'oesophage chez les
gros buveurs. Aucune donnée sur l'exposition à la toxine n'a été
fournie.
2.3 Relation dose-réponse
La dose la plus faible capable de provoquer l'apparition de
cancers du foie chez l'animal de laboratoire est égale à 50 mg par kg
de nourriture dans le cas de rats mâles BD IX et de souris femelles
B6C3F1/Nctr; aucun cancer n'a été observé aux doses respectives de 25
ou 15 mg/kg de nourriture. Dans chaque cas et avec les mêmes souches
de rats et de souris, on a observé des signes d'hépatotoxicité ou de
modification du métabolisme des lipides à ces mêmes doses ou à des
doses inférieures. La dose la plus faible ayant provoqué des cancers
du rein chez des rats mâles de souche F344/Nctr était égale à 50 mg/kg
de nourriture; aucun effet cancérogène n'a été observé à la dose de 15
mg/kg de nourriture. Ces études ont également montré qu'à des doses
inférieures à celles qui provoquaient l'apparition de cancers, il y
avait apoptose et prolifération des cellules des tubules rénaux, avec
des modifications au niveau des sphingolipides tissulaires et
urinaires.
On ne possède aucune donnée qui puisse permettre d'établir une
relation quantitative entre l'exposition à la FB1 et d'éventuels
effets sur l'organisme humain.
2.4 Caractérisation du risque
La FB1 est cancérogène pour la souris et le rat et alle provoque
des maladies mortelles chez le porc et le cheval à des concentrations
auxquelles l'Homme pourrait être exposé. Le Groupe de travail n'a pas
été en mesure de formuler une estimation quantitative du risque pour
la santé humaine mais il a été d'avis qu'il y avait urgence à cet
égard.
3. Recommandations pour la protection de la santé humaine
a) Il faudrait établir les limites d'exposition par voie
alimentaire. On devra s'attacher en particulier aux populations
dont l'apport calorique provient pour une grande part de la
farine de maïs.
b) Des mesures devraient être prises pour limiter l'exposition aux
fumonisines et la contamination du maïs par ces toxines; elles
pourraient consister
* à changer de culture là où le maïs n'est pas vraiment adapté
* à mettre au point des variétés de maïs qui résistent à la
fusariose
* à mieux gérer les cultures
* à éliminer les grains parasités
c) On veillera à faire prendre conscience suffisamment tôt du risque
de contamination alimentaire en faisant en sorte qu'il y ait une
meilleure communication entre les vétérinaires et les
responsables de la santé publique en cas de flambées de
mycotoxicoses parmi les animaux domestiques.
d) Il faudrait mettre au point une méthode de recherche de la
contamination du maïs par les fumonisines qui soit bon marché,
simple et peu sensible aux conditions d'application.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
La fumonisina B1 (FB1) tiene la fórmula empírica C34H59NO15 y
es el diéster del ácido propano-1,2,3-tricarboxílico y el 2-amino-12,
16-dimetil-3, 5, 10, 14, 15-pentahidroxieicosano (masa molecular
relativa: 721). Es la más frecuente de las fumonisinas, familia de
toxinas con 15 miembros identificados por lo menos. La sustancia pura
es un polvo higroscópico blanco, soluble en agua, acetonitrilo-agua o
metanol, estable en acetonitrilo-agua (1:1), inestable en metanol y
estable a la temperatura de elaboración de los alimentos y la luz.
Se han notificado varios métodos analíticos, en particular la
cromatografía en capa fina y la cromatografía líquida, la
espectrometría de masas, la cromatografía de gases poshidrólisis y
métodos inmunoquímicos, aunque la mayoría de los estudios se han
realizado utilizando análisis por cromatografía líquida de un derivado
fluorescente.
1.2 Fuentes de exposición humana
La FB1 se produce en varias especies de Fusarium,
principalmente Fusarium verticillioides (Sacc.) Niremberg
(= Fusarium moniliforme Sheldon), que es uno de los hongos más
comunes asociados con el maíz en todo el mundo. En el maíz hay una
acumulación importante de FB1 cuando las condiciones climatológicas
favorecen la podredumbre del grano debida a Fusarium.
1.3 Transporte, distribución y transformación en el medio
ambiente
Hay pruebas de que algunos microorganismos del suelo pueden
metabolizar las fumonisinas. Sin embargo, se sabe poco acerca del
destino de las fumonisinas en el medio ambiente tras la excreción o la
transformación.
1.4 Niveles en el medio ambiente y exposición humana
Se ha detectado FB1 en el maíz y sus productos en todo el mundo
en concentraciones de varios mg/kg, a veces combinada con otras
micotoxinas. Se han notificado también concentraciones de mg/kg en
alimentos para consumo humano. Como consecuencia de la elaboración en
seco del maíz, la fumonisina se distribuye en el salvado, los gérmenes
y la harina. En la elaboración en húmedo experimental, se detectó
fumonisina en el agua de remojo, el glúten, la fibra y los gérmenes,
pero no en el almidón. La FB1 es estable en el maíz y la polenta,
mientras que se hidroliza en los alimentos nixtamalizados a base de
maíz, es decir, alimentos elaborados con soluciones alcalinas
calientes.
La FB1 no está presente en la leche, la carne o los huevos de
animales alimentados con grano que contiene FB1 en concentraciones
que no afectarían a la salud de los animales. Las estimaciones de la
exposición humana para los Estados Unidos, el Canadá, Suiza, los
Países Bajos y el Transkei (Sudáfrica) oscilaron entre 0,017 y
440 µg/kg de peso corporal al día. No se dispone de datos sobre la
exposición por inhalación en el puesto de trabajo.
1.5 Cinética y metabolismo en los animales
No hay informes sobre la cinética o el metabolismo de la FB1 en
el ser humano. En animales experimentales se absorbe muy poco cuando
se administra por vía oral, se elimina rápidamente de la circulación y
se recupera sin metabolizar en las heces. La excreción biliar es
importante y se excretan pequeñas cantidades en la orina. Se puede
degradar a FB1 parcialmente hidrolizada en el intestino de primates
no humanos y en algunos rumiantes. Se retiene una pequeña cantidad en
el hígado y el riñón.
1.6 Efectos en los animales y en los sistemas de prueba in vitro
La FB1 es hepatotóxica en todas las especies animales sometidas
a prueba, en particular ratones, ratas, équidos, conejos, cerdos y
primates no humanos. Con la excepción de los hámsteres sirios, sólo se
observa embriotoxicidad o teratogenicidad cuando se produce toxicidad
materna o después de ella. Las fumonisinas son nefrotóxicas en cerdos,
ratas, ovejas, ratones y conejos. En ratas y conejos se produce
toxicidad renal a dosis inferiores a las de la hepatotoxicidad. Se
sabe que las fumonisinas producen leucoencefalomalacia equina y
síndrome de edema pulmonar porcino, asociados ambos con el consumo de
piensos a base de maíz. Es limitada la información sobre las
propiedades inmunológicas de la FB1. Fue hepatocarcinogénica para las
ratas machos de una raza y nefrocarcinogénica en otra raza, utilizando
la misma dosificación (50 mg/kg de alimentos) y fue
hepatocarcinogénica con 50 mg/kg de alimentos en ratones hembra.
Parece haber una correlación entre la toxicidad en los órganos y la
aparición de cáncer. La FB1 fue el primer inhibidor específico del
metabolismo de los esfingolípidos de novo que se descubrió y se está
utilizando ampliamente en la actualidad para estudiar su función en la
regulación celular. La FB1 inhibe el crecimiento celular y produce la
acumulación de bases esfingoideas libres y la alteración del
metabolismo lipídico en animales, plantas y algunas levaduras. No
indujo mutaciones génicas en bacterias o síntesis no programada de ADN
en hepatocitos primarios de rata, pero sí un aumento de las
aberraciones cromosómicas dependiente de la dosis con concentraciones
bajas en un estudio sobre los hepatocitos primarios de rata.
1.7 Efectos en el ser humano
No hay registros confirmados de toxicidad aguda de la fumonisina
en el ser humano. Los estudios de correlación disponibles procedentes
de Transkei (Sudáfrica) parecen indicar una vinculación entre la
exposición a la fumonisina en los alimentos y el cáncer de esófago.
Esto se ha observado en lugares donde se ha demostrado una exposición
relativamente alta a la fumonisina y donde las condiciones ambientales
favorecen la acumulación de fumonisina en el maíz, que es el alimento
básico. También hay estudios de correlación de China. Sin embargo, no
se obtuvo una imagen clara de la relación entre la contaminación bien
por fumonisina o bien por F. verticillioides y el cáncer de esófago.
Debido a la ausencia de datos sobre la exposición a la fumonisina, no
se puede llegar a ninguna conclusión a partir de un estudio de casos y
testigos de varones en Italia que mostraba una asociación entre el
consumo de maíz y el cáncer en la parte superior del aparato
gastrointestinal en personas con un elevado consumo de alcohol.
No hay biomarcadores validados para la exposición humana a la
FB1
1.8 Efectos en otros organismos en el laboratorio
La FB1 inhibe el crecimiento celular y produce acumulación de
bases esfingoideas libres y la alteración del metabolismo lipídico en
Saccharomyces cerevisiae.
La FB1 es fitotóxica, provoca lesiones en las membranas
celulares y reduce la síntesis de clorofila. También altera la
biosíntesis de esfingolípidos en las plantas y puede desempeñar una
función en la patogenicidad del maíz por las especies de Fusarium
que producen fumonisina.
2. Evaluación de los riesgos para la salud humana
2.1 Exposición
La exposición humana, demostrada por la presencia de FB1 en el
maíz para consumo humano, es común en todo el mundo. Hay diferencias
considerables en el grado de exposición humana entre las diferentes
regiones de cultivo de maíz. Esto se pone de manifiesto sobre todo
cuando se establece una comparación entre países plenamente
desarrollados y en desarrollo. Por ejemplo, aunque puede haber FB1 en
productos de maíz en los Estados Unidos, el Canadá y Europa
occidental, el consumo humano de estos productos es pequeño. En
algunas partes de Africa, América del Sur y Central y Asia, algunas
poblaciones consumen un elevado porcentaje de sus calorías como harina
de maíz, cuya contaminación por FB1 puede ser alta (véase el apéndice
2). El maíz contaminado de forma natural por FB1 puede estar
contaminado simultáneamente por otras toxinas de F. verticillioides
o F. proliferatum o por otras toxinas importantes desde el punto de
vista agrícola, en particular el deoxinivalenol, la zearalenona, la
aflatoxina y la ocratoxina.
La FB1 es estable en los métodos de elaboración de alimentos que
se utilizan en América del Norte y Europa occidental. El tratamiento
del maíz con bases y/o el lavado con agua reduce de manera eficaz las
concentraciones de FB1. Sin embargo, en animales experimentales
siguen siendo evidentes su hepatotoxicidad y/o nefrotoxicidad. Se sabe
poco acerca de la influencia de las técnicas de elaboración de
alimentos utilizadas en el mundo en desarrollo en la FB1 en los
productos de maíz.
2.2 Identificación de peligros
Se ha demostrado la función causal de la exposición a la FB1 en
la leucoencefalomalacia equina. Durante el siglo XIX se produjeron en
los Estados Unidos brotes en gran escala de esta enfermedad letal, y
también en épocas tan recientes como 1989-1990. Se ha establecido
asimismo la función causal de la exposición a la FB1 en la enfermedad
mortal del edema pulmonar porcino. Tal como se observó en hembras
preñadas, una exposición baja a la FB1 es letal para los conejos. Se
ha demostrado que la exposición provoca toxicidad renal y
hepatotoxicidad en todas las especies animales estudiadas, incluidos
los primates no humanos. La exposición a la FB1 produce
hipercolesterolemia en varias especies animales, en particular en
primates no humanos. Hay pruebas convincentes de que en las
enfermedades animales asociadas con la exposición a la FB1 se altera
el metabolismo lipídico. Es manifiesta la perturbación del metabolismo
de los esfingolípidos antes de la toxicidad in vitro e in vivo o
coincidiendo con ella. El uso de fumonisinas como instrumento para
estudiar la función de los esfingolípidos ha puesto de manifiesto que
éstos se requieren para el crecimiento celular y afectan de varias
formas a la señalización de las moléculas, provocando muerte celular
apoptótica y necrótica, diferenciación celular y respuestas
inmunitarias alteradas. Parece que son factores comunes tras la
exposición a la FB1 la alteración del metabolismo lipídico y los
cambios en la actividad y/o la expresión de enzimas fundamentales
encargados del funcionamiento normal del ciclo celular. La FB1 no es
tóxica para el desarrollo en ratas, ratones o conejos. A dosis
elevadas sin toxicidad materna induce fetotoxicidad en el hámster
sirio.
La carcinogenicidad de la FB1 en roedores varía en función de
las especies, las razas y el sexo. El único estudio con ratones
B6C3F1 puso de manifiesto que la FB1 era hepatocarcinogénica para
las hembras a 50 mg/kg en los alimentos. En ratas BD IX macho que
recibieron alimentos con 50 mg de FB1/kg durante un período de hasta
26 meses se observó la inducción de carcinomas hepatocelulares
primarios y carcinomas colangiales. Se detectaron adenomas y
carcinomas de los túbulos renales en ratas F344/N Nctr macho a las que
se suministraron 50 mg de FB1/kg. Parece existir una correlación
entre la toxicidad en los órganos y la aparición de cáncer.
El número de estudios de genotoxicidad disponible es limitado. La
FB1 no fue mutagénica en valoraciones bacterianas. En un estudio
realizado con células de mamífero in vitro no se detectó síntesis de
ADN no programado, pero la FB1 provocó roturas cromosómicas en
hepatocitos de rata. En otros estudios se ha puesto de manifiesto que
la FB1 provoca un aumento de la peroxidación de los lípidos in
vivo e in vitro. Es posible que los efectos de la rotura de los
cromosomas y la peroxidación de los lípidos tengan una relación
causal.
Las concentraciones de FB1 superiores a 100 mg/kg, notificadas
en el maíz de consumo humano en Africa y China, probablemente
provoquen leucoencefalomalacia, síndrome de edema pulmonar o cáncer si
se administran a caballos, cerdos y ratas o ratones, respectivamente.
A pesar de estos casos de exposición humana muy alta, no hay datos
confirmados de intoxicación aguda por fumonisina en personas. Los
estudios de correlación disponibles del Transkei, Sudáfrica, parecen
indicar una relación entre la exposición a la fumonisina a través de
los alimentos y el cáncer de esófago. Se han observado índices
elevados de cáncer de esófago donde se ha demostrado una exposición
relativamente elevada a la fumonisina y donde las condiciones
ambientales favorecen la acumulación de fumonisina en el maíz, que es
el alimento básico.
En un estudio de casos y testigos realizado con varones en Italia
se observó una asociación entre el consumo de maíz y la aparición de
cáncer en la parte superior del aparato digestivo, incluido el cáncer
de esófago, entre personas habituadas a un consumo de alcohol alto. No
había datos sobre la exposición a la fumonisina.
2.3 Evaluación de la respuesta en función de la dosis
La dosis más baja de FB1 que indujo hepatocarcinomas en animales
experimentales fue de 50 mg/kg de alimentos en ratas BD IX macho y en
ratones B6C3F1/Nctr hembra; no se observó inducción de cáncer con 25
ó 15 mg/kg de alimentos, respectivamente. En cada caso, se detectaron
indicios de hepatotoxicidad o alteraciones lipídicas con dosis iguales
o inferiores en estudios con esas mismas razas de ratas y ratones. La
dosis más baja de FB1 que indujo carcinomas renales en ratas F344/N
Nctr macho fue de 50 mg/kg de alimentos; no se observó inducción de
cáncer con 15 mg/kg de alimentos. Se produjo apoptosis tubular renal y
proliferación celular, así como cambios en los esfingolípidos
tisulares y urinarios, con dosis inferiores a las normalmente
necesarias para la inducción de cáncer en esos estudios.
No se dispone de datos para evaluar cuantitativamente la relación
entre la exposición a la FB1 y los posibles efectos en el ser humano.
2.4 Caracterización del riesgo
La FB1 es carcinogénica en ratones y ratas e induce enfermedades
letales en cerdos y caballos en concentraciones de exposición a las
que están sometidas los seres humanos. El Grupo Especial no estuvo en
condiciones de realizar una estimación cuantitativa de los riesgos
para la salud humana, pero consideró que se necesitaba con urgencia
dicha estimación.
3. Recomendaciones para la protección de la salud humana
a) Se deben establecer límites para la exposición de las personas a
través de los alimentos. Se debe prestar particular atención a
las poblaciones que consumen un porcentaje elevado de sus
calorías como harina de maíz.
b) Se deben adoptar medidas para limitar la exposición a la
fumonisina y la contaminación del maíz mediante:
* plantación de cultivos alternativos en zonas donde el maíz
no esté bien adaptado;
* obtención de maíz resistente a la podredumbre del grano por
Fusarium;
* aplicación de mejores prácticas de cultivo;
* separación de los granos mohosos.
c) Se debe aumentar la sensibilización temprana acerca de la
posibilidad de contaminación de los alimentos, mejorando la
comunicación entre los veterinarios y los funcionarios de salud
pública sobre los brotes de micotoxicosis en animales domésticos.
d) Se debe elaborar un método sólido, de bajo costo y sencillo para
la detección de la contaminación por fumonisina en el maíz.
Relative molecular mass: 721
CAS Name: 1,2,3-Propanetricarboxylic acid,
1,1'-[1-(12-amino-4,9,11-trihydroxy-2-methyl-
tridecyl)-2-(1-methylpentyl)-1,2-ethane-diyl]
ester
IUPAC name: None
CAS registry number: 116355-83-0
RTECS No.: TZ 8350000
Synonym: Macrofusine
At least 15 different fumonisins have so far been reported and
other minor metabolites have been identified, although most of them
have not been shown to occur naturally. They have been grouped into
four main categories (Plattner, 1995; Abbas & Shier, 1997; Musser &
Plattner, 1997): FA1, FA2, FA3, FAK1; FB1, FB2, FB3, FB4;
FC1, FC2, FC3, FC4; FP1, FP2 and FP3. FB2, FB3 and FB4
differ from FB1 in that they lack hydroxyl groups present in FB1;
FA1, FA2 and FA3 are like FB1, FB2 and FB3, but are
N-acetylated; FAK1 is like FA1 but is 15-keto functionalized; FCs
are like FBs but lack the methyl group adjacent to the amino group;
FPs have a 3-hydroxypyridium group instead of the amine group in the
FBs. This monograph will focus mainly on FB1, the most abundant of
the naturally occurring fumonisins.
2.2 Physical and chemical properties of the pure substance
Physical state: White hygroscopic powder
Melting point: Not known (has not been crystallized)
Optical rotation: Not known
Spectroscopy: Mass spectral and nuclear magnetic resonance data
are given in Bezuidenhout et al. (1988), Laurent
et al. (1989a) and Savard & Blackwell (1994)
Solubility: Soluble in water to at least to 20 mg/ml (US NTP,
1999); soluble in methanol, acetonitrile-water.
n-Octanol/water 1.84 (Norred et al., 1997)
partition
coefficient
(log P):
Stability: Stable in acetonitrile-water (1:1) for up to 6
months at 25°C; unstable in methanol (25% or 35%
concentration decrease after 3 or 6 weeks at 25°C,
respectively), giving rise to monomethyl or
dimethyl esters (Gelderblom et al., 1992a;
Visconti et al., 1994); stable in methanol up to 6
weeks at -18°C (Visconti et al., 1994); stable at
78°C for 16 h in buffer solutions at pH between
3.5 and 9 (Howard et al., 1998)
2.3 Analytical methods
Six general analytical methods have been reported: thin-layer
chromatographic (TLC), liquid chromatographic (LC), mass spectrometric
(MS), post-hydrolysis gas chromatographic, immunochemical and
electrophoretic methods (Sydenham & Shephard, 1996; Shephard, 1998).
The majority of studies have been performed using LC analysis of a
fluorescent derivative.
2.3.1 Sampling and preparation procedures
In raw maize, FB1 is present in both visibly damaged and
undamaged kernels (Bullerman & Tsai, 1994). This means that the
problem that occurs with the mycotoxin aflatoxin, i.e., a few highly
contaminated kernels in otherwise aflatoxin-free kernels, is probably
less of an issue. However, it has been shown that higher levels of
fumonisins are concentrated in visibly damaged kernels (Pascale et
al., 1997). Studies to determine the minimum representative sample in
a lot of maize have not been reported. However, homogeneous material
(CV < 10%) for fumonisin analysis was obtained by grinding
contaminated maize to a particle size less than 2 mm with test portion
sizes of 25 and 10 g (Visconti & Boenke, 1995).
2.3.2 Extraction
Methanol-water (3:1) is the solvent of choice (e.g., Shephard et
al., 1990; Stack & Eppley, 1992; Doko & Visconti, 1994; Scott &
Lawrence, 1994) with a long shaking time or homogenization with a
blender (Sydenham et al., 1992; Bennett & Richard, 1994; Visconti &
Boenke, 1995; Visconti et al., 1995). The use of acetonitrile-water
has also been reported, with conflicting data on its performance
relative to methanol-water (Sydenham et al., 1992a; Bennett & Richard,
1994; Visconti & Boenke, 1995). Use of an acidic extraction procedure
may lead to higher extraction efficiencies (Zoller et al., 1994;
Meister, 1998). However, remarkable variability in extraction
efficiency has been reported by several authors, and more work needs
to be done to establish the best extraction solvents for various food
products.
Clean-up involves the use of solid-phase extraction with strong
anion exchange (Shephard et al., 1990) or C18 reversed-phase (Ross et
al., 1990) or a combination of both (Miller et al., 1993). Improved
recoveries can be achieved by using anion exchange instead of
reversed-phase material for sample clean-up (Stockenström et al.,
1994; Dawlatana et al., 1995). Immunoaffinity columns (Scott &
Trucksess, 1997) have also been shown to be useful for clean-up of
crude extracts of maize (Ware et al., 1994; Duncan et al., 1998),
sweet corn (Trucksess et al., 1995), beer (Scott & Lawrence, 1995) and
milk (Scott et al., 1994).
Fumonisins are relatively stable compounds (Alberts et al., 1990;
Dupuy et al., 1993a; Le Bars et al., 1994; Visconti et al., 1994;
Pascale et al., 1995; Jackson et al., 1996a,b, 1997). A number of
factors make them difficult to extract from processed food (Scott,
1993; Bullerman & Tsai, 1994). Binding of FB1 to maize bran flour
occurs at room temperature and above (Scott & Lawrence, 1994). Added
iron may also affect recoveries of fumonisin (Scott & Lawrence, 1994).
Unknown processing factors or ingredients can change the recovery of
fumonisin from cereal products (Scott & Lawrence, 1994). Only 45% of
FB1 present in spiked corn meal was recovered following baking at
175-200°C for 20 min (Jackson et al., 1997). Fumonisins have been
shown to react with reducing sugars at elevated temperatures (Murphy
et al., 1996; Lu et al., 1997). The product of the reaction of FB1
with reducing sugars was identified as N-carboxymethyl-FB1 (Howard
et al., 1998). This product was found in raw corn samples at 4% of the
FB1 levels (Howard et al., 1998). Ammoniation and treatment with base
reduces apparent fumonisin concentrations while increasing the
concentration of hydrolysed fumonisins without eliminating the
toxicity of the treated product, again suggesting analytical
difficulties (Norred et al., 1991; Hendrich et al., 1993).
Methods have been reported for the extraction of FB1 and FB2 in
plasma and urine (Shephard et al., 1992c, 1995c; Shetty & Bhat, 1998),
bile of rats and vervet monkeys (Shephard et al., 1994c, 1995a),
faeces of vervet monkeys (Shephard et al., 1994b), liver, kidney and
muscle of beef cattle (Smith & Thakur, 1996), and milk (Maragos &
Richard, 1994; Scott et al., 1994; Prelusky et al., 1996a).
2.3.3 Analysis
Normal phase silica TLC can be used for analysis, with fumonisins
being visualized by spraying with p-anisaldehyde (Plattner et al.,
1990; Sydenham et al., 1990a; Dupuy et al., 1993b). For C18 HPLC or
TLC, visualization has been accomplished with fluorescamine
(Rottinghaus et al., 1992; Miller et al., 1995) and vanillin (Pittet
et al., 1992). The detection limit for fumonisins in maize by these
methods is 1 mg/kg (Miller et al., 1995). Improved TLC methods with
adequate sensitivity are needed, particularly to control maize
contamination in developing countries.
A number of fluorescent derivatives have been used for HPLC
detection including fluorescamine (Ross et al., 1991a,b),
naphthalene-2,3-dicarboxaldehyde/potassium cyanide (Ware et al., 1993;
Bennett & Richard, 1994; Scott & Lawrence, 1994),
4-fluoro-7-nitrobenzo-2-oxa-1,3-diazole (Scott & Lawrence, 1992,
1994), 6-aminoquinolyl N-hydroxysuccinimidylcarbamate (Velázquez et
al., 1995), 9-fluorenylmethyl chloroformate (Holcomb et al., 1993) and
o-phthaldialdehyde (OPA) (Shephard et al., 1990; Sydenham et al.,
1992). In most laboratories, these methods have reported limits of
detection or limits of quantification ranging from 5 to 100 µg/kg. The
OPA method is widely used and methodology using this derivative has
been the subject of international collaborative trials (Thiel et al.,
1993; Visconti et al., 1993; Sydenham et al., 1996). Particularly
satisfactory results were achieved in the trial by Sydenham et al.
(1996) with FB1 concentrations ranging from 0.5 to 8.0 mg/kg.
Relative standard deviations for within-laboratory repeatability
ranged from 5.8% to 13.2% for FB1. Relative standard deviations for
between-laboratory reproducibility were 13.9% to 22.2% for FB1.
HORRAT ratios for 7 samples in the test varied from 0.75 to 1.73 for
FB1 (Sydenham et al., 1996). Ratios of less than 2 are considered
acceptable. This method has been adopted by the Association of
Official Analytical Chemists International as an official method for
the analysis of maize.
There are no standardized methodologies for fumonisin analysis in
different food products. A method for the extraction and analysis of
FB1 in beer has been reported (Scott & Lawrence, 1994; Scott et al.,
1997).
Hydrolysis of samples to the aminopentol chain followed by the GC
analysis of the trimethylsilyl or trifluoroacetate derivative by flame
ionization detection or mass spectrometry has been reported (Plattner
et al., 1990, 1992; Plattner & Branham, 1994). Determination of
hydrolysed FB1 in alkali-processed corn foods by HPLC with
fluorescent derivatives has also been reported (Scott & Lawrence,
1996).
Analyses of maize extracts with antibodies reactive with FB1
(and FB2 plus FB3) by direct and indirect assays have been reported
(Azcona-Olivera et al., 1992a,b; Usleber et al., 1994; Scott &
Trucksess, 1997; Mullett et al., 1998). Detection limits using these
methods have been reported to be 0.1-100 µg/litre. In one study, an
ELISA method gave higher estimates of fumonisin concentrations
compared to GC-MS and HPLC (Pestka et al., 1994).
To a very limited extent, fumonisins have also been determined by
capillary zone electrophoresis (CZE). In order to achieve resolution
of the FB1 and FB2 analogues, samples were derivatized with either
9-fluorenylmethyl chloroformate (Holcomb & Thompson, 1996) or
fluorescein isothiocyanate (Maragos, 1995) prior to separation.
As an analytical tool for the determination of fumonisins, MS was
initially used as a detector after gas chromatographic separation of
the hydrolysed fumonisins (Plattner et al., 1990). Although MS methods
using fast-atom bombardment (Plattner & Branham, 1994) and particle
beam interfaces (Young & Lafontaine, 1993) have been described, the
application of the electrospray interface has led to the greatest
advance in the use of MS for fumonisin determination. These methods
rely on the LD separation of the underivatized fumonisins and
detection of the different analogues as their protonated molecular
ions (Doerge et al., 1994; Plattner, 1995; Lukacs et al., 1996;
Churchwell et al., 1997). A combined on-line immunoaffinity capture,
HPLC/MS method has also been described, and this permits analysis of
non-derivatized fumonisins at sub µg/kg levels (Newkirk et al., 1998).
3. SOURCES OF HUMAN EXPOSURE
FB1 was isolated in 1988 by Gelderblom et al. (1988). It was
chemically characterized by Bezuidenhout et al. (1988), and shortly
thereafter as "macrofusine" by Laurent et al. (1989a), from cultures
of Fusarium verticillioides (Sacc.) Nirenberg (Fusarium
moniliforme Sheldon). A selection of FB1 occurrence data in maize
and food products is given in Table 1 and Appendix 2. A worldwide
survey of fumonisin contamination of maize and maize-based products
was reported by Shephard et al. (1996a).
FB1 is produced by isolates of Fusarium verticillioides,
F. proliferatum, F. anthophilum, F. beomiforme, F. dlamini,
F. globosum, F. napiforme, F. nygamai, F. oxysporum,
F. polyphialidicum, F. subglutinans and F. thapsinum isolated from
Africa, the Americas, Oceania, Asia and Europe (Gelderblom et al.,
1988; Ross et al., 1990; Thiel et al., 1991a; Nelson et al., 1991,
1992; Chelkowski & Lew, 1992; Leslie et al., 1992, 1996; Rapior et
al., 1993; Miller et al., 1993, 1995; Visconti & Doko, 1994;
Desjardins et al., 1994; Abbas et al., 1995; Abbas & Ocamb, 1995;
Logrieco et al., 1995; Klittich et al., 1997; Musser & Plattner, 1997;
Sydenham et al., 1997). A species of Alternaria (A. alternata f. sp.
lycopersici) has also been demonstrated to synthesize B fumonisins
(Abbas & Riley, 1996). Fumonisins can be produced by culturing strains
of the Fusarium species that produce these toxins on sterilized
maize (Cawood et al., 1991), and yields of up to 17.9 g/kg have been
obtained with F. verticillioides strain MRC 826 (Alberts et al.,
1990). Yields of 500-700 mg/litre for FB1 plus FB2 have been
obtained in liquid fermentations and high recoveries of the toxins are
possible (Miller et al., 1994). The most predominant toxin produced is
FB1. FB1 frequently occurs together with FB2, which may comprise
15-35% of FB1 (IARC, 1993; Diaz & Boermans, 1994; Visconti & Doko,
1994).
Fusarium verticillioides and F. proliferatum are amongst the
most common fungi associated with maize. These fungi can be recovered
from most maize kernels including those that appear healthy
(Hesseltine et al., 1981; Bacon & Williamson, 1992; Pitt el al., 1993;
Sanchis et al., 1995). The formation of fumonisins in maize in the
field is positively correlated with the occurrence of these two fungal
species, which are predominant during the late maturity stage (Chulze
et al., 1996). These species can cause Fusarium kernel rot of maize,
which is one of the most important ear diseases in hot maize-growing
areas (King & Scott, 1981; Ochor et al., 1987; De León & Pandey, 1989)
and is associated with warm, dry years and/or insect damage
(Shurtleff, 1980).
Table 1a Worldwide occurrence of fumonisin B1 (FB1) in maize-based products
Product Countries Detected / total FB1 (mg/kg)
North America
Maize Canada, USA 324/729 0.08-37.9
Maize flour, grits Canada, USA 73/87 0.05-6.32
Miscellaneous maize foodsb USA 66/162 0.004-1.21
Maize feed USA 586/684 0.1-330
Latin America
Maize Argentina, Uruguay, Brazil 126/138 0.17-27.05
Maize flour, alkali-treated
kernels, polenta Peru, Venezuela, Uruguay 5/17 0.07-0.66
Miscellaneous maize foodsb Uruguay, Texas-Mexico border 63/77 0.15-0.31
Maize feed Brazil, Uruguay 33/34 0.2-38.5
Europe
Maize Austria, Croatia, Germany, Hungary, Italy, Poland, 248/714 0.007-250
Portugal, Romania, Spain, United Kingdom
Maize flour, maize grits,
polenta, semolina Austria, Bulgaria, Czech Republic, France, Germany, 181/258 0.008-16
Italy, Netherlands, Spain, Switzerland, United Kingdom
Miscellaneous maize foodsb Czech Republic, France, Germany, Italy, Netherlands, 167/437 0.008-6.10
Spain, Sweden, Switzerland, United Kingdom
Imported maize, grits and
flour Germany, Netherlands, Switzerland 143/165 0.01-3.35
Maize feed France, Italy, Spain, Switzerland, United Kingdom 271/344 0.02-70
Table 1 (continued)
Product Countries Detected / total FB1 (mg/kg)
Africa
Maize Benin, Kenya, Malawi, Mozambique, South Africa, 199/260 0.02-117.5
Tanzania, Uganda, Zambia, Zimbabwe
Maize flour, grits Botswana, Egypt, Kenya, South Africa, Zambia, 73/90 0.05-3.63
Zimbabwe
Miscellaneous maize foodsb Botswana, South Africa 8/17 0.03-0.35
Maize feed South Africa 16/16 0.47-8.85
Asia
Maize China, Indonesia, Nepal, Philippines, Thailand, 361/614 0.01-155
Vietnam
Maize flour, grits, gluten China, India, Japan, Thailand, Vietnam 44/53 0.06-2.60
Miscellaneous maize foodsb Japan, Taiwan 52/199 0.07-2.39
Maize feed Korea, Thailand 10/34 0.05-1.59
Oceania
Maize Australia 67/70 0.3-40.6
Maize flour New Zealand 0/12 -
a This table is a summary of the information in Appendix 2
b Includes maize snacks, canned maize, frozen maize, extruded maize, bread, maize-extruded bread, biscuits, cereals, chips,
flakes, pastes, starch, sweet maize, infant foods, gruel, purée, noodles, popcorn, porridge, tortillas, tortilla chips,
masas, popped maize, soup, taco, tostada
There is a strong relationship between insect damage and Fusarium
kernel rot. A field survey demonstrated that the incidence of the
European corn borer increased F. verticillioides disease and
fumonisin concentrations (Lew et al., 1991). Disease incidence was
also shown to correlate to populations of thrips (Frankliniella
occidentalis) (Farrar & Davis, 1991). Hybrids with a thin kernel
pericarp were more susceptible to insect wounds, which allowed easier
access to the fungus (Hoenisch & Davis, 1994). Hybrids with an
increased propensity for kernel splitting had more disease (Odvody et
al., 1990). Kernel splitting is worse under drought conditions. Ears
infected by F. graminearum may be predisposed to
F. verticillioides infection and fumonisin accumulation (Schaafsma
et al., 1993). In maize ears inoculated one week after silk emergence
with F. verticillipodes fumonisins accumulated in the visibly
damaged (mouldy) kernels (Pascale et al., 1997; Desjardins et al.
1998). Sydenham et al. (1995) showed that in lightly contaminated
kernels FB1 was concentrated in the pericarp of the maize kernel.
A study of fumonisin occurrence in hybrids grown across the USA
maize belt indicated that hybrids grown outside their range of
adaptation had higher fumonisin concentrations (Shelby et al., 1994b),
again suggesting the important role of temperature stress. Data from
samples collected in Africa, Italy and Croatia also indicate fumonisin
accumulation in lines grown outside their area of adaptation (Doko et
al., 1995; Visconti, 1996). The occurrence of fumonisin in Ontario,
Canada (a cool maize-growing region) was limited to drought-stressed
fields (Miller et al., 1995).
Significant fumonisin accumulation in maize occurs when weather
conditions favour Fusarium kernel rot, and the severity of ear
infection has been found to be a good indicator of fumonisin
accumulation in maize ears artificially inoculated with
F. verticillioides (Pascale et al., 1997). Since monitoring began in
the USA, warm, dry years have greater concentrations than cooler years
(Murphy et al., 1993). The direct influence of low moisture and dry
weather on fumonisin accumulation could not be proven (Murphy et al.,
1996; Pascale et al., 1997), although maize grown under normal
conditions in cooler maize-growing areas is not significantly
contaminated by fumonisin (Doko et al., 1995; Miller et al., 1995).
Dry milling of maize results in the distribution of fumonisin
into the bran, germ and flour (Bullerman & Tsai, 1994). Fumonisin may
be present in beer where maize has been used as a wort additive (Scott
et al., 1995). Little degradation of fumonisin occurs during
fermentation and the fumonisins are found in the spent grain. No
toxins can be detected in the distilled ethanol (Bothast et al., 1992;
Scott et al., 1995; Bennett & Richard, 1996). Fumonisin is stable in
polenta (Pascale et al., 1995), whereas it is hydrolysed, and the
pericarp is removed, by nixtamalization, i.e. the treatment of
maize-based foods with calcium hydroxide and heat (Hendrich et al.,
1993). FB1 has been shown to form N-(carboxymethyl)-FB1 when
heated in the presence of reducing sugars (Howard et al. 1998), and
the latter substance has been detected in raw corn (Howard et al.,
1998).
FB1 is not significantly transferred into pork, chicken meat or
eggs (Prelusky et al., 1994, 1996a; Vudathala et al., 1994), but a
small amount accumulates in the liver and kidney of pigs as a function
of exposure (Prelusky et al., 1996b; see also section 6.2). Fumonisin
is not significantly transferred into milk from short-term dietary
exposure (Scott et al., 1994; Prelusky et al., 1996a), and FB1 was
found in only one of 165 samples of milk from Wisconsin, USA at a
level close to 5 ng/ml (Maragos & Richard, 1994).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Maize is the only commodity that contains significant amounts of
fumonisins. It is consumed either directly or processed into products
for human or animal consumption. Because fumonisins are known to be
heat stable (Dupuy et al., 1993a; Howard et al., 1998), light stable
(IARC, 1993), water soluble (US NTP, 1999), poorly absorbed, poorly
metabolized and rapidly excreted by animals (see sections 6.1 to 6.5),
most fumonisin will eventually end up being recycled into the
environment in a manner that will concentrate its spatial
distribution. The amount that enters the environment may be quite
large. For example, in the USA, maize production exceeds 200 million
tonnes per year. The concentration of FB1 and FB2 in field maize in
the USA often exceeds 1 g/tonne of maize (Murphy et al., 1993 and
Appendix 2). There is some evidence that fumonisins can be metabolized
by some microorganisms (Duvick et al., 1994, 1998). However, little is
known about the environmental fate of fumonisin after it is either
excreted or processed.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Table 1 summarizes the results of a number of surveys on the
natural occurrence of FB1 in maize and maize-based foods and feeds
(see Appendix 2 for more detail). The list is not exhaustive of the
surveys carried out worldwide as there is continual production of
similar data from every corner of the globe. Based on Table 1, 60% of
the 5211 samples analysed have been found to be contaminated with
FB1, the highest incidences of contamination being in Oceania (82% of
82 samples) and Africa (77% of 383 samples), followed by Latin America
(85% of 266 samples), North America (63% of 1662 samples), Europe (53%
of 1918 samples) and Asia (52% of 900 samples).
The data show that levels and incidence of contamination vary
considerably in relation to the commodities tested and the source. The
highest incidence was recorded in maize feeds (82% of 1112 samples),
followed by ground maize products, such as flour, grits, polenta,
semolina and gluten (73% of 517 samples), maize kernels (52% of 2525
samples) and miscellaneous maize foods (40% of 892 samples).
FB1 levels in animal feedstuffs can be exceptionally high, and
reached maximum values of 330, 70, 38, 9 and 2 mg/kg in North America
(USA), Europe (Italy), Latin America (Brazil), Africa (South Africa)
and Asia (Thailand), respectively. The majority of the highly
contaminated feeds were implicated in cases of equine
leukoencephalomalacia, porcine pulmonary oedema and other
mycotoxicoses.
In maize kernels available commercially or from experimental or
breeding stations, FB1 has been detected in 96% (of 70 samples), 91%
(of 138 samples), 76% (of 260 samples), 59% (of 614 samples), 44% (of
729 samples) and 35% (of 714 samples) of samples from Oceania, Latin
America, Africa, Asia, North America and Europe, respectively. Maximum
FB1 levels were 40.6 mg/kg (Australia), 27 mg/kg (Argentina),
117 mg/kg (South Africa), 155 mg/kg (China), 38 mg/kg (USA) and 250
mg/kg (Italy).
The list of commercial retail foods subject to fumonisin
contamination (Table 1) includes maize flour, grits, polenta,
semolina, maize snacks, cornflakes, sweet maize, canned maize, frozen
maize, extruded maize, bread, maize-extruded bread, biscuits, cereals,
chips, pastes, starch, infant foods, gruel, purée, noodles, popcorn,
porridge, tortillas, tortilla chips, masas, popped maize, soup, taco
and tostada.
Of these samples, the global incidence of contamination in
non-treated or minimally treated maize products (flour, grits,
polenta, semolina) was 73% out of 517 samples analysed. The highest
FB1 levels were recorded in Europe (16 mg/kg), followed by North
America (6.3 mg/kg), Africa (3.6 mg/kg), Asia (2.6 mg/kg) and Latin
America (0.7 mg/kg). In the remaining food products (892 samples) the
incidence of contamination was 40%, the highest level (6.1 mg/kg FB1)
being found in a sample of extruded maize from Italy. Generally
processed maize foods have lower levels and incidence of contamination
than non-treated maize. These differences might be the results of
dilution of maize in food commodities, or may depend on the
differences in maize cultivar or quality requirements for various
destinations.
Apart from maize and maize products, fumonisins have seldom been
found in other food products, such as rice (Abbas et al., 1998),
asparagus (Logrieco et al., 1998), beer (Torres et al., 1998) and
sorghum (Shetty & Bhat, 1997). Surveys on other cereals, such as
wheat, rye, barley and oats, did not show the occurrence of the toxin
(Meister et al., 1996).
Human exposure estimates have been made for fumonisins in several
countries, including Switzerland, Canada, South Africa, USA and the
Netherlands (Zoller et al., 1994; Contaminants Standards Monitoring
and Programs Branch, 1996a,b; Gelderblom et al., 1996b; Kuiper-Goodman
et al., 1996; Humphreys et al., 1997; Marasas, 1997; de Nijs, 1998).
Human exposure estimates of 0.017-0.089 µg/kg body weight per day have
been prepared for Canada for the period 1991 to early 1995
(Kuiper-Goodman et al., 1996). For the USA, a preliminary estimate of
human exposure to fumonisins for maize eaters was 0.08 µg/kg body
weight per day (Humphreys et al., 1997). The mean daily intake of
fumonisins in Switzerland is estimated to be 0.030 µg/kg body weight
per day (Zoller et al., 1994).
Based on the daily average intakes of maize and maize products of
3 g (general population average), 42 g (regular maize product eaters)
and 162 g (individuals with gluten intolerance) in the Netherlands,
the respective population groups had an estimated daily intake of 4,
57 and 220 µg FB1 per person, respectively, based on a mean FB1
content of 1.36 mg/kg maize produce. De Nijs et al. (1998a) estimated
conservatively that 97% of individuals with gluten intolerance had a
daily exposure of at least 1 µg FB1 and 37% at least 100 µg, while
the proportions of the general population exposed to these levels of
FB1 were 49% and 1%, respectively (de Nijs, 1998; de Nijs et al.,
1998a).
Thiel et al. (1992) estimated that human exposures in the
Transkei, South Africa, are 14 and 440 µg FB1/kg body weight per day
for healthy and mouldy corn, respectively. More recent estimates of
the probable daily intake (PDI) of South Africans are summarized in
Table 2. These vary from 1.2 to 355 µg/kg body weight per day in rural
blacks in Transkei consuming home-grown mouldy maize (Gelderblom et
al., 1996b; Marasas, 1997).
These exposure estimates will vary considerably according to the
source and extent of maize in the diet as well as the extent of
Fusarium kernel rot prevalent in the harvested crop.
Table 2. Probable daily intake of fumonisin in South Africaa
Product Country of No. of Mean FB1 + FB2 Probable daily intake
origin samples concentration (µg/kg body weight per day)
(µg/kg)
Rural population Urban population
Commercial maize South Africa 68 400 2.6 1.6
Commercial maize South Africa 209 300 2.0 1.2
Corn meal South Africa 52 200 1.3 0.8
Home-grown maize South Africab 18 7100 46.6 28.0
Home-grown maize South Africac 18 54 000 354.9 212.9
Imported maize USAd 1682 1100 7.2 4.3
Maize consumption
(g/70 kg body weight per day) 460 276
a From: Marasas (1997)
b Transkei, from individual farms in high oesophageal cancer area, healthy maize
c Transkei, from individual farms in high oesophageal cancer area, mouldy maize
d Imported in 1993
Occupational inhalation exposure could be a problem. In addition
to the presence of fumonisins in maize dust, FB1 is present in the
spores and mycelia of F. verticillioides (Tejada-Simon et al.,
1995). No data have been collected on airborne levels of fumonisin
during the harvesting, processing and handling of
fumonisin-contaminated maize.
6. KINETICS AND METABOLISM IN ANIMALS
There have been no reports on the kinetics and metabolism of
fumonisins in humans. Because fumonisins are known to be consumed by
farm animals and are the causative agent or a suspected contributing
factor in farm animal diseases, an effort has been made to understand
the kinetics and metabolism in cows, pigs and poultry. Thus, this
chapter will summarize results of studies on both laboratory and farm
animals.
To date, published studies with radiolabelled FB1 or FB2 have
been conducted with either [21,22-14C]fumonisins, biosynthesized
using L-[methyl-14C]methionine (Plattner & Shackelford, 1992; Alberts
et al., 1993), or [U-14C]FB1 labelled using [1,2-14C]acetate
(Blackwell et al., 1994). In these studies the final [14C]fumonisins
had a specific activity of < 1 mCi/mmol and radiochemical purity of
> 95%. Several studies have used unlabelled fumonisins with reported
purities ranging from 70% (Hopmans et al., 1997) to 98% (Prelusky et
al., 1996a).
Briefly, FB1 is: poorly absorbed when dosed orally; it is
rapidly eliminated from plasma or circulation and recovered in faeces;
biliary excretion is important; enterohepatic cycling is clearly
important in some animals; small amounts are excreted in urine; a
small but persistent (and biologically active) pool of [14C]label
appears to be retained in liver and kidney; and some is degraded to
partially hydrolysed FB1 in the gut of vervet monkeys. In a study
with FB2 in rats, the results were similar to those of FB1 (Shephard
et al., 1995b).
6.1 Absorption
There are no reports available of fumonisin absorption through
inhalation or dermal exposure. However, because fumonisins are present
in F. verticillioides cells (mycelia, spores and conidiophores)
(Tejada-Simon et al., 1995), there is a potential for absorption
through inhalation or buccal exposure. The risk from absorption due to
dermal exposure would seem slight, since fumonisins are very water
soluble and, typically, polar compounds do not easily penetrate the
undamaged skin (Flynn, 1985).
The quantity of FB1 detected in plasma after oral dosing in
pigs, laying hens, vervet monkeys, dairy cows and rats is very low. In
rats (BD IX, Sprague-Dawley or Wistar) administered [14C]FB1 orally,
accumulation of 14C-labelled compounds in tissues is also very low,
suggesting that absorption is very poor (negligible to < 4% of dose)
(Shephard et al., 1992a,b, 1994c; Norred et al., 1993). Similar
results indicating that fumonisins are poorly absorbed (2 to < 6% of
dose) have been reported in vervet monkeys, dairy cows and pigs
(Prelusky et al., 1994, 1995, 1996a,b; Shephard et al., 1994a,b). In
orally dosed laying hens and dairy cows, systemic absorption based on
plasma levels and accumulation of 14C-labelled compounds in tissues
has been estimated to be less than 1% of dose (Scott et al., 1994;
Vudathala et al., 1994; Prelusky et al., 1996a). A study using beef
cattle fed F. verticillioides culture material (corn grits)
containing FB1 plus FB2 (530 mg/kg) found that the majority of the
fumonisin dose was recovered unmetabolized in faeces, and only traces
were detected in blood and urine (Smith & Thakur, 1996). Following
single gavage doses of 1 or 5 mg/kg body weight to cows, no FB1 or
known metabolites could be found in the plasma, indicating no or very
limited bioavailability in ruminants (Prelusky et al., 1995). Rumen
metabolism may reduce the bioavailability of FB1 as the hydrolysed
form of FB1 comprised 60-90% of the total amount of FB1 found in
faeces. In non-ruminants the parent compound was the dominant species
present (Rice & Ross, 1994).
6.2 Distribution
In rats and pigs orally dosed with [14C]FB1, the 14C label is
distributed to most tissues, with the liver and kidney containing the
highest concentration of radiolabel (Shephard et. al., 1992b; Norred
et al., 1993; Prelusky et al., 1994, 1996a,b; Haschek et al., 1996).
Typically, the liver contains more 14C label than the kidney,
although in the study by Norred et al. (1993) the measured
radioactivity in the kidney was greater than in the liver. In chickens
and dairy cows the poor absorption of [14C]FB1 (< 1% of oral dose)
was reflected in the fact that only trace amounts of radioactivity
were recovered in tissues (Prelusky et al., 1996a), no residues were
recovered in eggs of laying hens (Vudathala et al., 1994) and no FB1
or aminopentol hydrolysis products were recovered in milk (Scott et
al., 1994; Prelusky et al., 1996a). In pregnant rats dosed
intravenously with [14C]fumonisin, approximately 14.5% and 4% of the
dose were recovered in the liver and kidney, respectively, after 1 h
(Voss et al., 1996a). Based on the known pharmacokinetics (Norred et
al., 1993) in the rat, 1-h exposure and intravenous injection were
chosen so as to optimize the presentation in blood of the [14C]FB1
to the placentae. In contrast to liver and kidney, the uteri contained
0.24 to 0.44%, individual placentae contained 0 to 0.04%, and total
fetal recovery was < 0.015% of dose/dam (Voss et al., 1996a).
Recent studies have confirmed the lack of placental transfer of FB1
in rats (Collins et al., 1998a,b) and rabbits (LaBorde et al., 1997).
FB1 inhibition of the enzyme sphinganine N-acyltransferase
results in a large increase in intercellular free sphinganine (Wang et
al., 1991; Yoo et al., 1992). In animal tissues the fumonisin-induced
increase in free sphinganine tends to parallel the distribution of
14C label reported in the studies cited above using [14C]FB1. For
example, relative to other tissues examined, liver and kidney in
rabbits, pigs and catfish showed the greatest increases in free
sphinganine following exposure of animals to fumonisins or consumption
of diets containing fumonisins (Goel et al., 1994; Gumprecht et al.,
1995). The free sphinganine concentration in tissues has been shown to
be an easily detectable biomarker for exposure to fumonisins (Riley et
al., 1994c), although it has not been validated as a biomarker in
humans.
6.3 Elimination, excretion and metabolic transformation
When [14C]FB1 is dosed by intraperitoneal or intravenous
injection in rats (BD IX, Sprague-Dawley or Wistar), initial
elimination (subsequent to the distribution phase) is rapid (half-life
of approximately 10-20 min) with little evidence of metabolism
(Shephard et al., 1992a,b, 1994c; Norred et al., 1993). In rats the
elimination kinetics based on intraperitoneal or intravenous dosing
are consistent with a one- (Shephard et al., 1992b) or two-compartment
model (Norred et al., 1993). Because FB1 is poorly absorbed from the
rat gastrointestinal tract and extensively distributed in rat tissues
(Norred et al., 1993), the tissue elimination kinetics following oral
dosing is not as easily described. In vervet monkeys, as in rats, the
14C label is widely distributed and rapidly eliminated (half-life of
40 min) after intravenous injection (Shephard et al., 1994a,b). The
elimination kinetics following oral dosing in a non-human primate has
not been determined. Following single intravenous injection of 0.05 or
0.20 mg FB1/kg body weight to cows, the toxin is cleared rapidly from
the blood. A two-compartment model (half-lives of < 2 and 15-18 min,
respectively) satisfactorily described the plasma kinetics. No toxin
could be detected 120 min after dosing. No known metabolites were
detected in the plasma (Prelusky et al., 1995).
In pigs, clearance of [14C]FB1 from blood following an
intravenous injection was best described by a 3-compartment model
(half-lives of 2.5, 10.5 and 183 min, respectively), and cannulation
of the bile duct (bile removed) resulted in a much more rapid
clearance (best described by a 2-compartment model). The effect of
bile removal was observed whether dosing was intravenous or
intragastric (Prelusky et al., 1994, 1996a). The half-life in pigs
dosed intragastrically without bile removal was determined to be 96
min (Prelusky et al., 1996a). The studies with pigs strongly support
the importance of enterohepatic circulation of FB1 in pigs. As in the
study with rats, the majority of 14C label dosed orally was recovered
in faeces (approximately 90%) with less than 1% recovered in urine
(Prelusky et al., 1994, 1996a). In the LLC-PK1 renal cell line,
uptake of [14C]FB1 reached an equilibrium concentration with the
extracellular [14C]FB1 concentration after 4 to 16 h, and kinetics
were indicative of a simple diffusion process (Riley & Yoo, 1995).
Efflux was rapid with a half-life of less than 5 min.
Following intravenous injection into rats, FB1 is excreted
unchanged in bile (Norred et al., 1993; Shephard et al., 1994c). In
vervet monkeys there is evidence of metabolism to partially hydrolysed
(one propane tricarboxylic acid residue removed) FB1, and to a much
lesser extent the fully hydrolysed (both propane tricarboxylic acid
residues removed) aminopentol backbone, in faeces while in urine 96%
of the 14C label was recovered as FB1 (Shephard et al., 1994a,b).
Metabolism was most likely occurring in the gut since partially
hydrolysed and fully hydrolysed FB1 were recovered in the faeces but
not in the bile of vervet monkeys (Shephard et al., 1995a). Because
hydrolysed FB1 and FB1-fructose adduct can be formed during
processing, Hopmans et al. (1997) evaluated the excretion of these
products and FB1 in Fischer-344 rats. Based on the amount of each
FB1-related compound recovered in urine and faeces, it was concluded
that hydrolysed FB1 and the FB1-fructose adduct were better absorbed
than FB1 (Hopmans et al., 1997).
Dairy cows dosed with pure FB1 either orally (1.0 and 5.0 mg
FB1/kg body weight) or by intravenous injection (0.05 and 0.20 mg
FB1/kg body weight) showed no detectable residues of FB1, AP1 (the
aminopentol hydrolysis product of FB1) or their conjugates in the
milk (Scott et al. 1994). FB1 does not react with monoamine or
diamine oxidase (Murphy et al., 1996). In vitro studies using rat
primary hepatocytes and microsomal preparations (Cawood et al., 1994)
or studies with the LLC-PK1 renal epithelial cell line (Riley & Yoo,
1995) indicated that there was no metabolism of FB1 in these systems.
Repeated intraperitoneal injection of FB1 resulted in induction
of cytochrome P-4501A1 and P-4504A1 activities (Martinez-Larrañaga et
al., 1996). However there is no evidence that fumonisin is metabolized
by P-450 enzymes. Whether or not the induction was due to a direct
interaction between fumonisins and the metabolizing systems could not
be determined. However, it has been shown that some of the same
sphingolipid metabolites that are altered in fumonisin-treated animals
also mediate the cytokine-induced alterations in P-4502C11 in rat
hepatocytes (Nikolova-Karakashian et al., 1997).
6.4 Retention and turnover
[14C]FB1 is widely distributed in tissues of the rat and pig.
However, only the liver and kidney retain small but persistent amounts
of 14C label based on measured radioactivity (Norred et al., 1993;
Prelusky et al., 1994, 1996b). In rats given three repeated oral
doses, once accumulated, the measured radioactivity in liver and
kidney remained unchanged for at least 72 h after the last
intragastric dose (Norred et al., 1993). In pigs, it was estimated
that exposure to dietary FB1 at 2-3 mg/kg in feed would require a
withdrawal period of at least 2 weeks for the 14C label to be
eliminated from the liver and kidney (Prelusky et al., 1996b). The
chemical nature of the 14C-labelled material retained in liver and
kidney was primarily FB1.
In vitro studies with rat primary hepatocytes and the cultured
kidney cell line LLC-PK1 also indicate that a low but persistent pool
of 14C-labelled material is retained inside cells long after the
rapidly diffusible pool of [14C]fumonisin has exited the cells
(Cawood et al., 1994; Riley et al., 1998). This retained pool appears
to be capable of maintaining the elevation of cellular (LLC-PK1
cells) and urinary (in rats) free sphingoid base concentration, a
biomarker of fumonisin exposure (Solfrizzo et al. 1997b; Riley et al.,
1998; Wang et al., 1999).
6.5 Reaction with body components
Fumonisins are potent inhibitors of the enzyme sphinganine
(sphingosine) N-acyltransferase in the de novo sphingolipid
biosynthesis and sphingolipid turnover pathways (Wang et al., 1991).
The consequences of this reaction will be discussed in sections 7.8
and 7.9. FB1 may also interact directly with protein kinase C (Yeung
et al., 1996) and/or with mitogen-activated protein kinases
(Wattenberg et al., 1996). The only other information concerning
reaction with body components is that FB1 does not bind strongly to
chicken plasma proteins (Vudathala et al., 1994).
Cytotoxicity studies in primary rat hepatocytes and binding
studies using subcellular fractions indicated that 14C-labelled FB1
binds tightly to hepatocytes and microsomal and plasma membrane
fractions (Cawood et al. 1994). FB1 has been shown to interact
directly with liposomes (Yin et al., 1996). Since fumonisins are water
soluble, are not accumulated and are rapidly eliminated, the
toxicological significance of this finding is unclear.
7. EFFECTS ON ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Laboratory animals and in vitro test systems
The studies described below used either purified FB1, naturally
contaminated corn or cultures of Fusarium. It is generally accepted
that the in vivo toxicity of Fusarium verticillioides MRC 826
culture material is the result of its high FB1 content. Culture
materials other than MRC 826 may contain several other products such
as other fumonisins, fusarins, moniliformin and beauvericin.
7.1.1 Single exposure
In the male Sprague-Dawley rat, intravenous injection of FB1
(95% purity) at 1.25 mg/kg body weight resulted in renal lesions
localized to the tubules in the outer medulla and consisted of both
proliferation and death of cells. An increased number of mitotic
figures, stained with 5-bromo-2'-deoxyuridine (not quantified), and
apoptosis followed by severe nephrosis were observed (Lim et al.,
1996). Cell proliferation was also detected in the liver 24 h after
dosing, but was not significantly different from control values at
later times (day 2 to day 5). In the oesophagus, increased cell
proliferation was measured on day 3, but this returned to the control
level on day 5. While kidney lesions were reported as severe, the
increased mitotic activity in the liver and oesophagus occurred in the
absence of morphological injury (Lim et al., 1996).
No information is available on the toxicological effects of
single exposure to FB1 by the inhalation or dermal route.
7.1.2 Repeated exposure
7.1.2.1 Body weight loss
In male BD IX rats consuming a diet containing 1 g FB1/kg during
a 4-week promotion treatment, the mean body weights were 50% lower
than those of non-treated rats ( P < 0.0001), both with and without
initiation with diethylnitrosamine (DEN) (Gelderblom et al., 1988).
Similarly, the body weight gains of male Fischer rats fed the same
concentration of FB1 over a 26-day initiation period were 80% lower
than those of the controls ( P < 0.0025) (Gelderblom et al., 1992b).
Male Fischer rats fed diets containing 1 g FB1 (and FB2 plus
FB3) per kg over a 21-day initiating period started to lose weight
within the first week, and the level of the FB compounds had to be
reduced by half (Gelderblom et al., 1993). Body weight losses were
first observed in rats fed FB2, where a significant ( P = 0.008)
reduction compared to the controls was recorded after 4-5 days. In the
case of FB1 and FB3, significant ( P = 0.01) reductions in body
weight occurred after 7-8 days. Body weight loss induced by FB1 and
FB2 was significantly ( P = 0.001) higher than that induced by FB3
(Gelderblom et al., 1993). In female Sprague-Dawley rats administered
purified FB1 at gavage doses of 0, 1, 5, 15, 35 or 75 mg FB1/kg body
weight per day for 11 consecutive days, significant depression of body
weight and food consumption was observed at 35 and 75 mg FB1/kg body
weight per day (Bondy et al., 1998).
The reduction in body weight gain of male Fischer rats induced by
FB1 is apparently due to a feed refusal effect (Gelderblom et al.,
1994). During a feeding study over 21 days, the body weight gains of
rats receiving 750, 500, 250 and 100 mg FB1/kg diet were
significantly (0.01 < P < 0.05) lower than those of the controls
as well as those of rats receiving 50 and 25 mg/kg. Based on the
weekly feed intake profiles, the reduction in body weight gain was
accompanied by a concomitant reduction in feed intake. The reduction
in feed intake was overcome after the second week, resulting in a feed
intake similar to that of the controls at the end of the 21-day
initiating treatment (Gelderblom et al., 1994).
In male Fischer-344/N Nctr BR rats, exposure to 234 and 484 mg
FB1/kg diet resulted in 10% and 17%, respectively, less gain in body
weight after 28 days of feeding in the range-finding study by the US
National Toxicology Program (US NTP, 1999). Female rats had decreased
body weight only at 484 mg FB1/kg diet.
In the NTP 2-year carcinogenicity study (US NTP, 1999) (see
section 7.1.6.1), there was no difference in body weight or feed
consumption in male or female Fischer-344/N Nctr BR rats or
B6C3F1/Nctr BR mice fed FB1 when compared to rats or mice on control
diets.
The characteristic reduction in the body weight of rats induced
by FB1, was also induced by FB2, FB3 and the monomethyl esters of
FB1 (MME, an artefact of the isolation procedure of FB1 and a minor
contaminant of FB1 preparations), and to a much lesser extent by the
N-acetylated analogue FA1, but not by the aminopolyol hydrolysis
products AP1 and AP2 or the tricarbalyllic acid moiety (TCA)
(Gelderblom et al., 1993).
7.1.2.2 Hepatotoxicity and nephrotoxicity
The acute toxicity of FB1 was tested by dosing four male BD IX
rats orally with 240 mg FB1/kg body weight per day (Gelderblom et
al., 1988). Three of the four rats died within 3 days and exhibited
toxic hepatosis characterized by scattered single-cell necrosis
accompanied by mild fatty changes, hydropic (i.e., the abnormal
accumulation of serous fluid in the cellular tissue or in a body
cavity) degeneration and hyaline droplet degeneration. Hepatocellular
nuclei varied in size and some were markedly enlarged. In addition to
the hepatotoxic changes, fatty changes and scant necrosis were present
in the proximal convoluted tubules of the kidney, prominent lymphoid
necrosis was observed in Peyer's patches, and severe disseminated
acute myocardial necrosis and severe pulmonary oedema were observed in
two of the rats (Gelderblom et al., 1988).
In a separate experiment, male BD IX rats were dosed orally with
48 mg FB1/kg body weight per day for 12 days, followed by 70 mg
FB1/kg body weight per day for the remaining 9 days of the experiment
(Gelderblom et al., 1988). In the rats killed after 21 days, chronic
toxic hepatosis was present and characterized by marked hydropic
degeneration, single-cell necrosis and a few hyaline droplets, early
bile duct proliferation and fibrosis, and enlargement of
hepatocellular nuclei (Gelderblom et al., 1988).
In the livers of rats killed after 33 days on a diet containing
1 g FB1/kg, the hepatic changes were similar to those described
above, but more advanced (Gelderblom et al., 1988). The proliferation
of bile ducts and fibrosis caused distortion of the lobular structure
of the liver and, together with the development of hyperplastic
nodules, gave the liver a distinctly nodular appearance. The authors
reported that many nuclei were enlarged in hepatic cells and numerous
mitotic figures, some of which were abnormal, were present. The
lesions in the kidneys were similar, but less severe, than those seen
in the rats that died within 3 days (Gelderblom et al., 1988).
In male Fischer rats fed a diet containing 1 g FB1 (90-95% pure)
per kg during an initiating period of 26 days, followed by partial
hepatectomy and a promoting regimen of 2-acetyl-aminofluorene (2-AAF)
and carbon tetrachloride, early pathological changes in the liver were
very similar to those described above (Gelderblom et al., 1992b).
Early hepatocyte nodules were evident as discrete focal changes in
hepatocytes characterized by somewhat bigger cells that displayed more
mitotic figures than the cells in the surrounding liver and also
showed vacuolization. Another prominent pathological feature was the
mild-to-moderate proliferation of bile ducts (Gelderblom et al.,
1992b). Similar hepatic changes have been described in male Fischer
rats fed diets containing, at a level of 0.5-1 g/kg, FB1, FB2, FB3
and MME during an initiating period of 21 days followed by a promoting
treatment of 2-AAF and partial hepatectomy (Gelderblom et al., 1993).
The short-term toxicological effects in rats of FB2 and FB3 are
similar to those of FB1 (Gelderblom et al., 1992a).
Changes including hydropic swelling, hyaline droplet
accumulation, single-cell necrosis, increased mitotic figures, lipid
accumulation, fibrosis, and bile duct proliferation were also observed
in the liver of male Fischer rats that died after gavage treatment
with 50 mg FB1/kg body weight in 6 dosages over 11 days (Gelderblom
et al., 1994).
A 4-week exposure of Sprague-Dawley rats to aqueous extracts of
Fusarium verticillioides (MRC 826) cultures (containing fumonisins)
resulted in decreased body weights, increased serum alanine and
aspartate aminotransferase and alkaline phosphatase activities,
decreased relative liver weights and microscopic liver lesions in rats
(Voss et al., 1990).
Male and female Sprague-Dawley rats (3 of each sex per group)
were fed diets containing 0, 15, 50 and 150 mg/kg of FB1 (> 99%
pure) for 4 weeks (Voss et al., 1993). No significant differences in
weight gain or food consumption were found, but significant increases
in serum triglycerides, cholesterol and alkaline phosphatase confirmed
that a dietary level of 150 mg/kg was hepatotoxic to both sexes.
Histopathological changes in the liver of these rats were
characterized by scattered single-cell hepatocellular necrosis,
variability in nuclear size and staining and hepatocellular
cytoplasmic vacuolation. Nephrosis, consisting of focal cortical
proximal tubular epithelial basophilia, hyperplasia and single cell
necrosis or pyknosis, was found in males fed > 15 mg/kg and in
females fed > 50 mg/kg (Voss et al., 1993). The incidence and
severity of ultrastructural alterations in kidney and liver were
closely correlated with increased sphinganine concentration in
tissues, serum and urine (Riley et al., 1994a).
The apparent no-observed-effect level (NOEL) for renal toxicity
in FB1-fed rats was less than the NOEL for hepatic effects (4.1
< NOEL < 13.6 mg/kg diet for 28 days), and renal toxicity was more
severe in males (NOEL < 1.4 mg/kg diet for 28 days) than females
(1.4 < NOEL < 4.1 mg/kg diet for 28 days). Furthermore, liver
lesions found in females appeared (subjectively) more advanced than
those found in males. The results of subacute toxicity studies (7.5
and 10 mg/kg body weight per day for 4 days) (Bondy et al., 1995;
Suzuki et al., 1995) and of an independent (Tolleson et al., 1996a)
4-week study in Fischer-344 rats fed 0, 99, 163, 234 or 484 mg FB1/kg
diet corroborated the findings of nephrotoxicity by Voss et al.
(1993). Hepatopathy of the same type was found in males fed > 234
mg/kg diet and females fed > 163 mg/kg diet. Nephropathy was found
in males from all FB1-fed groups and in females fed > 163 mg/kg
diet (Tolleson et al., 1996a). Apoptotic hepatocytes and renal
proximal tubule epithelial cells were accompanied by cell
proliferation in Fischer-344 rats, suggesting that fumonisin induces
or accelerates programmed cell death in both liver and kidney
(Tolleson et al. 1996a; US NTP, 1999).
In male and female B6C3F1 mice administered FB1 at gavage doses
ranging from 1 to 75 mg FB1/kg body weight per day for 14 days,
effects on liver, bone marrow, adrenals and kidneys were observed. In
general, however, the degree of change observed indicates that mice
are not as sensitive to FB1 toxicity as rats (Bondy et al., 1995,
1997).
In B6C3F1 mice fed 99 to 484 mg FB1/kg diet for 4 weeks, the
liver, not the kidney, was the target organ (US NTP, 1999). As for
rats, the NOEL was lower in females as liver lesions were found in the
females of all FB1-fed groups, while in males hepatopathy was
confined to the highest dose group. In male BALB/c mice dosed
subcutaneously (0.25 to 6.25 mg FB1/kg body weight per day), a
dose-dependent increase in apoptosis was observed in both liver and
kidney (Sharma et al., 1997).
To obtain dose-response data under longer-term exposure
conditions, Fischer-344 rats and B6C3F1 mice were fed diets
containing 0, 1, 3, 9, 27 or 81 mg FB1/kg diet for 13 weeks (Voss et
al., 1995). In rats, toxicity was confined to the kidneys. Lesions of
the proximal tubule located in the outer medulla (sometimes referred
to as the corticomedullary junction) were found in males fed > 9
mg/kg diet and in females fed 81 mg/kg diet. Qualitatively these
lesions were of the same type as those found in the 4-week study (Voss
et al., 1993). No differences in the incidence or severity of
nephropathy between rats examined after 4 (n = 5 rats/group) or 13
(n = 10/group) weeks were found.
Renal lesions were accompanied by decreased relative kidney
weight (as a percentage of body weight), which was found in males fed
> 27 mg/kg diet for 4 weeks and in both sexes fed > 9 mg/kg diet
for 13 weeks. Serum creatinine was increased after 13, but not 4,
weeks in males fed > 27 mg/kg diet and in females fed 81 mg/kg diet
(Voss et al. 1995).
In mice, hepatopathy and serum chemical evidence of liver
dysfunction were found after 13 weeks in females fed 81 mg FB1/kg
diet (Voss et al., 1995). Liver lesions in female mice were primarily
centrilobular, although some midzonal involvement and apparent
"bridging" between adjacent central areas were evident. Single cell
hepatocyte necrosis, cytomegaly, increased numbers of mitotic figures,
some mixed infiltration of neutrophils and macrophages were present
and, in more advanced lesions, the loss of hepatocytes caused an
apparent collapse around the central vein. Hepatopathy was not found
in male mice and FB1-related kidney lesions did not occur in either
sex. A few macrophages containing minimal to mild amounts of
cytoplasmic pigment, presumably ceroid, were also found in the adrenal
cortex of high-dose (81 mg/kg diet) females only.
Taken together, the findings from 4-week and 90-day toxicity
studies in rats and mice (Voss et al., 1993, 1995; Tolleson et al.,
1996a; US NTP, 1999) indicate that the liver is a target organ in both
species, and the data seem to indicate that females exhibit hepatic
effects at lower doses than males. In rats, however, the kidney is
also an important target organ and, in contrast to liver, the males
were affected at lower doses.
7.1.2.3 Immunotoxicity
There have been very few studies that address directly the
potential for fumonisins to modify immune response in vivo.
Nonetheless, there are many studies with fumonisins or
fumonisin-containing diets that show either altered function of blood
cells in vitro or changes in haematological parameters in vivo.
Fumonisins are inhibitors of ceramide synthase (see section 7.3) and
ceramide and glycosphingolipids are important signalling molecules and
recognition sites in the cellular immune response and attachment sites
for many infectious agents and microbial toxins (Ballou et al., 1996;
Merrill et al., 1997a).
In a study with pure FB1, changes in selected haematological
parameters in pigs were reported at dietary levels as low as 1 mg/kg
(Rotter et al., 1996). Consumption of culture-material diets (MRC 826)
containing fumonisins decreased the ability to clear Pseudomonas
aeruginosa and inhibited pulmonary interstitial macrophage function
(Smith et al., 1996c). It was hypothesized that pulmonary
intravascular macrophage (PIM) dysfunction could contribute to
increase susceptibility to microbial diseases (Smith et al., 1996c).
Cytokine production has been shown to be modified by exposure to
fumonisin. For example, serum tumour necrosis factor-alpha
(TNF-alpha)-like activity was increased in pigs fed culture material
(M 1325 = MRC 826) containing 150 mg/kg fumonisins (Guzman et al.,
1997). Fumonisin-induced changes in the TNF pathway have also been
seen in lipopolysaccharide (LPS)-stimulated macrophages collected from
BALB/c mice dosed with pure FB1 (Dugyala et al., 1998).
Immunosuppression in chickens was produced in birds fed maize
cultured with F. verticillioides (MRC 826) (Marijanovic et al.,
1991). Broiler chicks fed diets containing 10 mg pure FB1/kg diet, or
diets formulated from Fusarium verticillioides (MRC 826) culture
material to contain 30 to 300 mg FB1/kg diet, had reduced spleen
and/or bursa weights and altered haematological parameters (Espada et
al., 1994, 1997).
In male and female rats (10 rats/group) gavaged daily for 14 days
with doses of 0, 5, 15 or 25 mg FB1/kg body weight per day, a
significant dose-related linear trend toward decreased plaque-forming
cell number per 106 spleen mononuclear leukocytes (PFC per 106
splenocytes) ( P = 0.003) and PFC per spleen cells ( P = 0.001) was
observed in the male rats. However, the PFC numbers in female rats
were not affected significantly by treatment ( P > 0.05) (Tryphonas
et al., 1997).
7.1.3 Skin and eye irritation
No information is available on the effects of FB1 on skin and
eye irritation and/or sensitization.
7.1.4 Reproductive toxicity, embryotoxicity and teratogenicity
Concern about the reproductive and developmental effects of
fumonisins originated with: (a) the observation of abortions in
pregnant sows fed fumonisin-contaminated diets (Harrison et al.,
1990); (b) the suggestion that a cluster of birth defects among
residents in Brownsville, Texas, USA (Hendricks, 1999) might be
associated with consumption of maize from the 1989 maize crop; (c) the
association of "mystery swine disease" with fumonisin-contaminated
maize (Bane et al., 1992); and (d) the discovery that fumonisins are
inhibitors of sphingolipid biosynthesis (Wang et al., 1991). Currently
there are no data to support the conclusion that consumption of
fumonisins is a developmental or reproductive toxicant in farm animals
or humans. There are also no data demonstrating that fumonisin
consumption results in transfer to chicken eggs (Vudathala et al.,
1994; Prelusky et al., 1996a) or that it crosses the placenta in rats
(Voss et al., 1996a; Collins et al., 1998a,b), mice (Reddy et al.,
1996) or rabbits (LaBorde et al., 1997).
Injection of purified FB1 into fertile chicken eggs resulted in
time- and dose-dependent embryopathic and embryocidal effects (Javed
et al., 1993b). Embryonic changes included hydrocephalus, enlarged
beaks and elongated necks. Pathological changes were noted in most
organ systems. At the low FB1 dose (1 µM = 0.72 µg/ml), stimulation
of chick embryo development was observed. Stimulated embryo
development in vitro in pre-somite rat embryos exposed to
0.5-1 µg/ml of hydrolysed FB1 has been reported in an abstract (Flynn
et al., 1994). Higher concentrations of fully hydrolysed FB1 (Flynn
et al., 1997) and all concentrations of FB1 > 0.2 µg/ml inhibited
growth and development of pre-somite rat embryos in vitro (Flynn et
al., 1994, 1996). Johnson et al. (1993) reported that FB1 was a weak
developmental toxin to organogenesis stage rat embryos (day 10.5;
lowest-observed-effect level = 0.5 mM). FB1 (> 2.5 mM = 1.8 µg/ml)
inhibited reaggregation and growth of chicken embryo neural retina
cells, a commonly used in vitro assay for screening potential
developmental toxins (Bradlaw et al., 1994). Bacon et al. (1995) found
effects of FB1 in fertile chicken eggs similar to those reported by
Javed et al. (1993b). In addition it was found that co-injection of
fusaric acid and FB1 resulted in a synergistic toxic response (Bacon
et al., 1995). Zacharias et al. (1996) found that morphological
changes, due to direct administration of FB1 to chick embryos, were
correlated with inhibition of glycosphingolipid biosynthesis.
Syrian hamsters orally gavaged with aqueous extracts of
F. verticillioides (M 1325 = MRC 826) culture material containing
fumonisins (0.25-18 mg FB1/kg body weight) or pure FB1 (12 mg/kg and
18 mg/kg) did not exhibit maternal toxicity based on weight gain,
serum aspartate aminotransferase activity or total bilirubin.
Histological examination of liver, kidney and placenta did not reveal
important changes, although mild karyomegalic changes in liver were
observed in the hamsters dosed with either aqueous extracts or pure
FB1 at > 6 mg FB1/kg body weight (Floss et al., 1994a,b). When
aqueous extracts were given by oral gavage from day 8 to day 10 or 12
of gestation, there appeared to be an increase in the number of fetal
deaths, but statistical significance was not achieved (Floss et al.,
1994a). Relative to controls, statistically significant increases in
fetal deaths occurred only in the hamsters given 18 mg FB1/kg body
weight (aqueous culture extracts and pure material) (Floss et al.,
1994b). Prenatal exposure to aqueous culture extracts containing
fumonisins or to pure FB1 were detrimental to fetal hamster
survivability in the absence of maternal toxicity (Floss et al.,
1994a,b; Penner et al. 1998).
In Fischer-344/N rats dosed orally from day 8 to 12 of gestation
with 30 or 60 mg purified FB1/kg body weight, the high dose
significantly suppressed growth and fetal bone development while an
extract of F. proliferatum (M 5991) in corn culture did not
(Lebepe-Mazur et al., 1995a). Voss et al. (1996a) formulated diets
using F. verticillioides (MRC 826) culture material to provide 0, 1,
10 or 55 mg FB1/kg diet. Based on consumption, the diet containing
55 mg/kg provided about 3 to 4 mg FB1/kg body weight per day to the
dams. The diets were fed to male and female Sprague-Dawley rats prior
to and during the mating, gestational and lactational phases of the
study. Nephropathy was observed in males and females fed diets
containing > 10 mg/kg and 55 mg/kg, respectively. No statistically
significant reproductive effects were observed in any of the males or
females, and no developmental effects were found in fetuses during any
phase of the study. Litter weight gains in the 10 and 55 mg/kg diet
groups were slightly decreased. Increased levels of free sphinganine,
a biomarker for fumonisin exposure, were demonstrated in the livers of
dams in the 55 mg/kg diet group on gestation day 15. In contrast, no
increase in the sphinganine/sphingosine (Sa/So) ratio was observed in
fetuses at that time, suggesting that fetuses were not exposed
in utero to FB1. This finding was supported by the study in which
an intravenous injection of [14C]FB1 was given to dams on gestation
day 15. Radiolabel was easily detected in tissues of pregnant females
but was not detected in their fetuses. Culture material containing
fumonisins, and by inference FB1, did not have reproductive effects
at doses that were minimally toxic (Voss et al., 1996a). These
findings have been recently confirmed (Sa/So ratios in fetuses were
not affected and FB1 was not teratogenic at the doses tested) in a
Charles River CD rats (Collins et al., 1998a,b).
Gross et al. (1994) gavaged pregnant CD1 mice daily between
gestation days 7 and 15 with a diet containing partially purified FB1
extracted from F. verticillioides (M 1325 = MRC 826) culture
material. Maternal toxicity and fetal developmental abnormalities
(e.g., hydrocephalus, digital and sternal ossification) occurred at
FB1 dosages greater than 12.5 mg/kg body weight per day. Similar
results were obtained in a second study using purified FB1 (Reddy et
al., 1996). As in the study by Voss et al. (1996b), the Sa/So ratio
was significantly increased in maternal liver but not in fetal liver,
suggesting that developmental effects were mediated through maternal
toxicity (Reddy et al., 1996).
Unlike CD1 mice and Syrian hamsters, pregnant New Zealand white
rabbits are very sensitive to the toxic effects of FB1 (LaBorde et
al., 1997). Maternal toxicity was observed at daily gavage dosages (in
water) as low as 0.25 mg/kg body weight from gestational day 3 to
gestational day 19. Compared to controls there was no increase in
fetal loss or in gross visceral or skeletal abnormalities, and no
decrease in fetal weight or fetal organ weight at any dosage (0 to
1.75 mg/kg body weight) (LaBorde et al., 1997). The maternal kidney,
serum and urine Sa/So ratios were increased, but there were no
increases in these ratios in fetal liver, brain or kidney (LaBorde et
al., 1997). While FB1 is toxic in the pregnant dam, it is not a
developmental toxin but is maternally toxic in rabbits (LaBorde et
al., 1997). However, the lowest-observed-effect level for maternal
toxicity was 0.1 mg FB1/kg body weight, which is equivalent to a
calculated dietary fumonisin level of 2.3 mg/kg diet (LaBorde et al.,
1997). Thus, in sensitive species, maternal toxicity and consequent
fetal toxicity could occur at low dosages of FB1.
There is currently no evidence of neonatal toxicity. However,
average mean litter weights were reduced in litters from
Sprague-Dawley dams fed F. verticillioides (MRC 826) culture
material containing 10 or 55 mg FB1/kg (Voss et al., 1996a). The
Sa/So ratio was increased in litters at lactation day 21. However,
given the likelihood that offspring had consumed the contaminated
diets (Voss et al., 1996a), the authors could not ascertain the route
of exposure (via milk or diet). Reduced weights and several
alterations in haematological parameters were reported in mink kits
lactationally exposed to fumonisins (Powell et al., 1996).
No FB1 was detected in the milk of lactating sows fed diets
containing non-lethal levels of FB1 and there was no evidence of
toxicosis in their suckling pigs (Becker et al., 1995). However, in a
study with lactating cows administered FB1 intravenously, the
carry-over rate of FB1 into the milk reached a maximum of 0.11%
(Hammer et al., 1996), while in other studies no fumonisins were
detected in cow's milk (Scott et al., 1994; Richard et al., 1996). In
a reproductive study with mink, fumonisins were detected in the milk
at 0.7% of the dietary fumonisin concentrations (Powell et al., 1996).
The question of neonatal toxicity is of concern since neonates
may be more sensitive to fumonisins than adults. For example, a recent
report by Kwon et al. (1997b) indicated that subcutaneous injection of
FB1 in neonatal rats caused elevation in the Sa/So ratio in brain
tissue and reduced myelin deposition. The elevated sphinganine level
was determined to be the result of a direct effect on the neonate
brain, indicating that FB1 can cross the blood-brain barrier (Kwon et
al., 1997a). When maternal toxicity was minimal, there was little or
no evidence of neonatal toxicity in rats (Ferguson et al., 1997).
7.1.5 Mutagenicity and related end-points
The fumonisins FB1, FB2 and FB3 (98, 98, 90% pure,
respectively) were non-mutagenic in the Salmonella assay against the
tester strains TA97a, TA98, TA100 and TA102, in both the presence and
absence of the S-9 microsomal preparation (Gelderblom & Snyman, 1991).
The non-mutagenicity of FB1 (approximately 90% pure) to Salmonella
tester strain TA100 at concentrations up to 100 mg/plate was confirmed
by Park et al. (1992). Similarly negative results were reported with
FB1 in Salmonella TA98 and TA100, as well as in SOS chromotest in
E. coli PQ37 and differential DNA repair assays with E. coli K12
strains (343/753, uvrB/ recA and 343/765, uvr+ rec+)
(Knasmüller et al., 1997). In contrast, Sun & Stahr (1993), using a
commercial bioluminescent bacterial (Vibrio fischeri) genotoxicity
test, reported that FB1 showed in the concentration range 5-20 µg/ml
genotoxic activity without the metabolic activation of S-9 fraction.
FB1 (and FB2) were non-genotoxic in the in vitro rat
hepatocyte DNA repair assay at concentrations ranging from 0.04 to 80
µM (and FB2 from 0.04 to 40 µM) as well as in the in vivo assay at
a dose of 100 mg/kg body weight administered by gavage (FB1 or FB2)
(Gelderblom et al., 1989, 1992b). The finding that FB1 does not
induce unscheduled DNA synthesis was confirmed in the in vitro assay
in primary rat hepatocytes at concentrations ranging from 0.5 to 250
µM (Norred et al., 1992a).
FB1 induced DNA strand breaks in isolated rat liver nuclei (Sahu
et al., 1998).
FB1 induced a moderate increase in the micronucleus frequency in
primary rat hepatocytes at concentrations ranging from 0.01 to
1 µg/ml. No concentration-dependent increase of micronuclei occurred.
A significant concentration-dependent increase in chromosomal
aberrations was observed in isolated hepatocytes exposed to FB1 at
concentrations ranging from 1 to 100 µg/ml (Knasmüller et al., 1997).
FB1 induced lipid peroxidation in isolated rat liver nuclei at
concentrations ranging from 40 to 300 µM (Sahu et al., 1998). It also
increased significantly the level of thiobarbituric-acid-reactive
substances in vitro in primary rat hepatocytes at concentrations of
75 and 150 µM and in vivo in the liver of rats fed a dietary FB1
level of 250 or 500 mg/kg for 21 days (Abel & Gelderblom, 1998).
Single gavage doses of 50, 100 and 200 mg FB1/kg body weight
significantly ( P = 0.05) inhibited hepatocyte proliferation as
measured by the incorporation of radiolabelled thymidine into DNA in
partially hepatectomized male Fischer rats (Gelderblom et al., 1994).
Inhibition of hepatocyte proliferation was also observed after dietary
exposure to FB1 (> 50 mg/kg diet) (Gelderblom et al., 1996c). FB1
also inhibited DNA synthesis induced by epidermal growth factor in
primary rat hepatocytes (Gelderblom et al., 1995).
In BALB/3T3 A31-1-1 mouse embryo cells, FB1 (90% pure) treatment
produced transforming activity at 500 µg/ml but not at lower or higher
concentrations (Sheu et al., 1996).
7.1.6 Carcinogenicity
7.1.6.1 Carcinogenicity bioassays
When inbred BD IX rats were fed commercial diet containing
freeze-dried or oven-dried culture material inoculated with
F. verticillioides MRC 826 for 2 years, the incidence of liver
tumours (hepatocellular and cholangiocellular carcinomas combined) was
increased (control: 0/20, freeze-dried 13/20, oven-dried, 16/20)
(Marasas et al, 1984b). When 30 rats of the same inbred strain were
given F. verticillioides MR 826 (containing fusarin C and later
found to produce FB1 and FB2) for 23-27 months, two hepatocellular
and eight cholangiocellular cancers were observed. In addition,
neoplastic hepatic nodules were observed in all surviving 21 animals,
but none among the controls. However, in rats similarly administered
F. verticillioides MRC 1069 culture material containing 104 mg/kg
fusarin C (but suspected of being low in FB1), no increase in hepatic
carcinomas was observed (Jaskiewicz et al., 1987b).
Maize from a field outbreak of equine leukoencephalomalacia in
the USA, shown to be naturally contaminated with
F. erticillioides, was fed to 12 male Fischer-344 rats and
commercial rodent feed to 12 controls by Wilson et al. (1985). All
treated rats necropsied from 123 to 176 days had multiple hepatic
neoplastic nodules, adenofibrosis and cholangiocarcinoma, whereas no
such lesions were found in the controls. The authors considered these
lesions in the livers of male Fischer-344 rats to be similar to those
described in male BD IX rats by Marasas et al. (1984b). The fact that
the lesions observed by Wilson et al. (1985) developed more rapidly
(as early as 123 days) than those described by Marasas et al. (1984b)
(more than 450 days) was attributed by Wilson et al. (1985) to the
dietary deficiencies, which included choline and methionine, in the
maize-only diet used in their study.
Hendrich et al. (1993) reported that in Fischer-344/N rats fed
diets containing Fusarium proliferatum maize culture material (with
known amounts of FB1) there was an increased incidence of
hepatocellular adenomas, relative to rats fed the control diets. When
rats were fed nixtamalized Fusarium proliferatum (M 5991) maize
culture material diets (converting FB1 to hydrolysed FB1) the
incidence of hepatic adenomas and cholangiomas was reduced relative to
rats fed the diets containing FB1. The frequency of
hepatic/cholangiocellular adenomas in rats given the nixtamalized diet
was higher in rats receiving nutrient-supplemented diet (equivalent to
AIN-76) than in rats given a diet not supplemented with nutrients
(nutritionally deficient relative to AIN-76). In a 4-week feeding
study with male Sprague-Dawley rats, Voss et al. (1996c) found that
hydrolysed FB1 (58 mg/kg diet) from nixtamalized
F. verticillioides (MRC 826) was both hepatotoxic and nephrotoxic.
However, the extent and severity of the hepatotoxicity was
significantly less than that caused by FB1 (71 mg/kg diet) from
F. verticillioides (MRC 826), whereas the kidney toxicity was
similar (Voss et al., 1996c).
In male BD IV rats treated with the known oesophageal carcinogen
N-methylbenzylnitrosamine (NMBA) (2.5 mg/kg body weight) and FB1
(5 mg/kg body weight), there was no synergistic interaction between
NMBA and FB1 in the rat oesophagus when the two compounds were
administered together (Wild et al., 1997).
A semi-purified maize-based diet containing FB1 (not less than
90% pure) at 50 mg/kg diet was fed to 25 inbred male BD IX rats over a
period of 26 months (Gelderblom et al., 1991). A control group
received the same diet without FB1 (the FB1 content of the control
diet was approximately 0.5 mg/kg and no aflatoxin B1 could be
detected). Five rats from each group were killed at 6, 12, 20 and 26
months. All FB1-treated rats (50 mg/kg diet) that died or were killed
from 18 months onward suffered from a micro- and macronodular
cirrhosis and had large expansile nodules of cholangiofibrosis at the
hilus of the liver. The pathological changes terminating in cirrhosis
and cholangiofibrosis were already present in the liver of rats killed
6 months after the initiation of the experiment and included fibrosis,
bile duct hyperplasia and lobular distortion. The severity of the
hepatic lesions increased with time and the histological changes were
consistent with those of a chronic toxic hepatosis progressing to
cirrhosis. Ten out of 15 FB1-treated (50 mg/kg diet) rats (66%) --
but none in the controls -- that were killed or died between 18 and 26
months developed primary hepatocellular carcinoma. Metastases to the
heart, lungs or kidneys were present in four of the rats with
hepatocellular carcinoma. Apart from the hepatocellular carcinoma,
FB1 also induced cholangiofibrosis consistently from 6 months onward,
and toward the end of the experiment, cholangiocarcinoma. However, the
authors noted that the experiment was performed under nutritionally
compromised conditions, using diets deficient in vitamins, methionine
and choline, that may have had an enhancing effect on the action of
FB1 in the liver (Table 3).
The detailed results of a second long-term experiment in rats fed
diets containing 0, 1, 10 and 25 mg FB1/kg diet over a period of
24 months have not yet been published (Gelderblom et al., 1996b).
However, in preliminary reports, Gelderblom et al. (1996b, 1997) noted
that no cancers were observed in these rats, including those fed 25 mg
FB1/kg diet.
Male and female Fischer-344/N Nctr BR rats and B6C3F1/Nctr BR
mice were given diets containing FB1 for 2 years as part of the US
National Toxicology Program (NTP) tumorigenesis studies on FB1
(US NTP, 1999). The FB1 that was used in these studies was > 96%
pure. Dietary levels of FB1 were 0, 5, 15, 50 and 150 mg/kg diet for
the male rats, resulting in average daily FB1 doses of 0, 0.26, 0.76, 2.5
and 7.5 mg/kg body weight. The dietary levels for female rats were 0, 5, 15, 50
and 100 mg/kg diet, resulting in average daily FB1 doses of 0.31, 0.91, 3.0
and 6.1 mg/kg body weight.
There was no difference in body weight, survival or feed
consumption in male rats fed FB1 when compared to rats on control
diets. The only compound-related change in tumour incidence was the
induction of renal adenomas and carcinomas in male rats. The overall
incidence of renal tubule tumours in male rats receiving 0, 5, 15, 50
and 150 mg FB1/kg diet were 0/48, 0/40, 0/48, 2/48 and 5/48 for
adenomas and 0/48, 0/40, 0/48, 7/48 and 10/48 for carcinomas. Renal
tubule adenomas were characterized as an expansive proliferation of
Table 3. Summary of the induction of neoplasia in long-term feeding studiesa
Neoplasia Species and strain Sex Fumonisin concentration (mg/kg feed) Reference
0 5 15 50 80 100 150
Hepatocellular carcinoma BD IX rats male 0/15 10/15 Gelderblom
Cholangiofibrosisb BD IX rats male 0/15 15/15 et al. (1991)
Renal tubule adenoma F-344/N Nctr rats male 0/48 0/40 0/48 2/48 5/48 US NTP (1999)
Renal tubule carcinoma F-344/N Nctr rats male 0/48 0/40 0/48 7/48 10/48 US NTP (1999)
Renal tubule adenoma F-344/N Nctr rats female 0/48 0/40 1/48 0/48 0/48 US NTP (1999)
Renal tubule carcinoma F-344/N Nctr rats female 0/48 0/40 0/48 0/48 1/48 US NTP (1999)
Hepatocellular adenoma B6C3F1/Nctr mice female 5/47 3/48 1/48 16/47 31/45 US NTP (1999)
Hepatocellular carcinoma B6C3F1/Nctr mice female 0/47 0/48 0/48 10/47 9/45 US NTP (1999)
Hepatocellular adenoma B6C3F1/Nctr mice male 9/47 7/47 7/48 6/48 8/48 US NTP (1999)
Hepatocellular carcinoma B6C3F1/Nctr mice male 4/47 3/47 4/48 3/48 2/48 US NTP (1999)
a This summarizes the data for tissues where fumonisin dose-dependent induction of tumours was detected (US NTP, 1999)
b In Gelderblom et al. (1991), cholangiofibrosis was considered to have progressed to cholangiocarcinoma
renal tubule epithelial cells that tended to be separated into lobules
by a delicate fibrous stroma. The neoplastic cells had nuclei that
were slightly larger with increased cytoplasmic volume. The
cytoplasmic changes were uniform within individual lesions and varied
from clear to basophilic. Renal tubule carcinomas were characterized
by cellular atypia, necrosis within a lesion, invasion of the adjacent
normal renal parenchyma, or metastasis to distant organs.
Historically, renal tubule carcinomas have not occurred in
Fischer-344/N Nctr male rats on control diets. There was no apparent
involvement of alpha-2 microglobulin in the tumorigenicity in male rat
kidneys. The incidence in renal tumours was accompanied by an
increased incidence in renal tubule epithelial cell hyperplasia at 50
and 150 mg FB1/kg diet at 2 years (2/48, 1/40, 4/48, 14/48 and 8/48
of the male rats receiving 0, 5, 15, 50 and 150 mg FB1/kg diet).
Similarly, increased renal tubule epithelial cell apoptosis and
proliferation were detected at 50 and 150 mg FB1/kg diet in male rats
sacrificed following 6, 10, 14 and 26 weeks on FB1-containing diets.
As in the 28-day range-finding studies, the apoptosis was confined to
tubules of the inner cortex and was characterized by cellular
shrinkage from adjacent cells, cytoplasmic eosinophilia, and chromatin
condensation and margination in the nucleus. Apoptotic cells were
additionally detected using an in situ method for detection of DNA
fragmentation. In serum removed from male rats killed at 6, 10, 14 or
26 weeks, no FB1-dependent changes were noted (e.g., in cholesterol,
triglycerides or serum alanine aminotransferase levels). The urinary
Sa/So ratio was increased in the urine of male rats fed 5, 15, 50 or
150 mg FB1/kg diet, while kidney tissue Sa/So ratios were increased
at 15, 50 or 150 mg FB1/kg diet. Increased tissue sphingoid base
changes, and renal tubule tumour incidence and hyperplasia were
detected at 50 and 150 mg FB1/kg diet, while urinary sphingolipid
changes were detected at 15, 50 or 150 mg FB1/kg diet. In the livers
of the male rats, an increase in basophilic foci was detected at 150
mg FB1/kg diet, but liver tissue Sa/So ratios were not affected.
FB1 at 5, 15, 50 and 100 mg/kg diet did not affect body weight,
survival, feed consumption, serum analytes or tumour incidence in the
female rats. Significant increases in urinary and kidney tissue Sa/So
ratios were detected at 50 and 100 mg FB1/kg diet, but the extent of
induction was not as high as in the male rats.
In the female mice, dietary levels of FB1 were 0, 5, 15, 50 and
80 mg/kg diet, resulting in average daily FB1 consumption of 0.7,
2.1, 7.0 and 12.5 mg/kg body weight, respectively. In the male mice,
dose levels of 0, 5, 15, 80 and 150 mg FB1/kg diet resulted in
average daily FB1 doses of 0, 0.6, 1.7, 9.5 and 17 mg/kg body weight,
respectively.
In female B6C3F1/Nctr mice, there were essentially no
differences in the mean body weights and diet consumption. The body
weights of the mice on this study were less than those reported for
the NTP and control studies at the National Center for Toxicological
Research (NCTR). This was attributed to an unintended restriction of
the powdered feed in the individual feeders. The female mice consuming
diets containing 80 mg FB1/kg diet had a significantly reduced
survival compared to the female mice on control diets.
The only tissue that demonstrated FB1-dependent changes in
tumour incidence was the liver in the female mice. Hepatocellular
adenomas were present in 5/47, 3/48, 1/48, 16/47 and 31/45 female mice
and hepatocellular carcinomas were present in 0/47, 0/48, 0/48, 10/47
and 9/45 female mice consuming 0, 5, 15, 50 and 80 mg FB1/kg diet,
respectively. Hepatocellular adenomas were characterized as discrete
lesions with compression of adjacent normal tissues. The normal
hepatic lobular structure was absent with uneven growth patterns. The
cells in the adenoma appeared to be well differentiated and either
eosinophilic, basophilic or vacuolated. Hepatocellular carcinomas were
characterized as foci of cells with distinct trabecular or adenoid
structure. Histological evidence of local invasiveness or metastasis
was usually evident. The cells within the carcinoma were poorly
differentiated or anaplastic. Some of the carcinomas appeared to arise
within adenomas. The incidence of hepatocellular adenomas and
carcinomas in the female mice was within the range of historical
occurrence in B6C3F1/Nctr mice. The increased tumour incidence was
accompanied by increased hepatocellular hypertrophy (0/47, 0/48, 0/48,
27/47, 31/45) and hepatocellular apoptosis (0/47, 0/48, 0/48, 7/47,
14/45) in the female mice. The mean liver weights (relative to body
weight) of the mice after 2 years were increased at 50 and 80 mg
FB1/kg diet. There were no consistent increases in serum analytes in
mice killed at 3, 7, 9 and 24 weeks. Liver sphingoid bases were
increased at 80 mg FB1/kg diet at weeks 3, 7 and 9 but not week 24.
Levels of urinary sphingoid bases were not determined.
No FB1-dependent changes in tumour incidences in the male mice
receiving diets of 0, 5, 15, 80 and 150 mg FB1/kg diet were
identified. The body weights, organ weights, survival, feed
consumption and serum analytes were not affected by FB1 dose. The
lack of demonstration of statistically significant sphingoid base
increases in livers at intermediate sacrifices (3, 7 and 9 weeks) were
probably due to the low sample number (n = 4) (US NTP, 1999).
7.1.6.2 Short-term assays for carcinogenicity
The short-term assays have mainly used rat liver nodules as the
end-point, assessed either using traditional microscopy or
histochemical analysis of different enzyme activities such as
gamma-glutamyl transferase (GGT) or placental glutathione
S-transferase (PGST). In many of these assays, different stages
(initiation, promotion) of the multistage carcinogenesis model have
been investigated by combining the FB1 treatment with classical
promotion assays. In these studies FB1 (or a Fusarium extract) was
administered alone, or before a promoting treatment, or after an
initiating treatment.
a) Initiation studies
In male BD IX rats fed a diet containing 0.1% FB1 during 4
weeks, GGT-positive (GGT+) foci were induced in the liver
(Gelderblom et al., 1988).
GGT+ foci were induced in the liver of male Fischer rats fed a
diet containing 0.5-1 g/kg of FB1 (90-95% pure) for 21-26 days,
followed by partial hepatectomy and treatment with 2-AAF and carbon
tetrachloride. However, foci were not induced when single or multiple
doses (50-200 mg/kg) of FB1 (and FB2) were administered by gavage to
hepatectomized rats (Gelderblom et al., 1992b, 1993).
In subsequent dose-response studies in male Fischer rats using
the same experimental approach, Gelderblom et al. (1994) reported that
the lowest dietary level to produce cancer initiation (GGT+-foci)
over 21 days was 250 mg FB1/kg diet. The lowest levels to cause
cancer initiation over 14 and 7 days were 500 mg and 750 mg FB1/kg
diet, respectively. Based on the feed intake values, the effective
dosage level (EDL) for cancer initiation over a period of 21 days was
142 < EDL < 308 mg FB1/kg body weight and over 14 days the amount
required for cancer initiation was 233 < EDL < 335 mg FB1/kg body
weight. The dietary level of FB1 required for cancer initiation is
dependent on the duration of exposure since a dose of 293 mg FB1/kg
body weight over 7 days did not initiate cancer whereas a similar dose
(308 mg FB1/kg body weight) over 21 days did.
Lebepe-Mazur et al. (1995b) fed female Fischer-344/N rats for one
week with a semipurified diet with or without an aqueous extract of a
Fusarium verticillioides (M 1325 = MRC 826) culture, providing 20 mg
FB1/kg diet, and administered a single dose of 30 mg/kg body weight
diethylnitrosamine (DEN) thereafter. Rats fed the Fusarium culture
showed more PGST+ hepatocytes than those treated with DEN alone.
Continued dietary treatment with the Fusarium moniliforme culture
for 12 weeks after the DEN administration did not further increase the
number of PGST foci. When the FB1 diet (one week) was followed by the
DEN administration and a 7-day-non-treatment interval, no increase in
PGST foci by FB1 was observed.
In another study (Lebepe-Mazur et al., 1995c), female
Sprague-Dawley rats were fed a diet supplemented with corn
contaminated with Fusarium proliferatum (containing 20 or 50 mg/kg
FB1) for six months; GGT-foci were not observed but the number of
PGST+ altered hepatic foci was increased in treated rats in
comparison to rats fed a semipurified diet without supplementation. In
a similar study (Lebepe-Mazur et al., 1995b), feeding diet containing
20 mg/kg FB1 for one or 13 weeks failed to induce a statistically
significant increase in PGST-altered foci.
In male Sprague-Dawley rats administered purified FB1
intraperitoneally at 10 mg/kg body weight per day for 4 days, as well
as in male and female Sprague-Dawley rats given 35 and 75 mg/kg body
weight per day orally for 11 days, significant increases in PGST+
hepatocytes were observed (Mehta et al., 1998).
b) Promotion studies
In a study on the promotion activity of FB1, it was administered
to male Fischer-344 rats (10, 50, 100, 250 or 500 mg/kg diet for 21
days) after a dose of DEN (200 mg/kg body weight) (Gelderblom et al.,
1996c). Dietary levels of 50 mg/kg or more markedly increased the
number and size of the PGST+ foci in the liver. It was thus
concluded (Gelderblom et al., 1996b,c) that the dose of FB1 required
for cancer initiation was markedly higher than that required for
cancer promotion.
Female Sprague-Dawley rats were fed a semipurified diet with or
without an aqueous extract of a Fusarium verticillioides (M 1325 =
MRC 826) culture, providing 20 or 50 mg FB1/kg for 6 months, after a
single dose of 30 mg DEN/kg. The number of PGST-altered foci was
increased at 20 mg/kg but not in the high-dose group, as compared to
rats treated with DEN alone. GGT-altered foci were not observed
(Lebepe-Mazur et al., 1995c).
Gelderblom et al. (1996b) suggested that FB1-induced
hepatocarcinogenesis in male BD IX rats developed against a background
of chronic toxic hepatosis culminating in cirrhosis. Chronic
hepatotoxicity appears to be a prerequisite for the development of
liver cancer in the BD IX rat (Gelderblom et al., 1996b).
7.2 Other mammals
7.2.1 Equine leukoencephalomalacia
Equine leukoencephalomalacia (ELEM) syndrome is characterized by
the presence of liquefactive necrotic lesions in the white matter of
the cerebrum. The name is somewhat misleading since the gray matter
may also be involved (Marasas et al., 1988a). This fatal disease
apparently occurs only in equids, although there has been one
unconfirmed report of fumonisin-induced brain lesions and haemorrhage
in rabbits gavaged with FB1 (Bucci et al., 1996) and there is some
evidence that FB1 can cross the blood-brain barrier and disrupt brain
sphingolipid metabolism in neonatal rats (Kwon et al., 1997b). In
equids, the ELEM syndrome has been recognized since the 19th century
as a sporadically occurring condition. ELEM was experimentally
produced by feeding mouldy maize obtained from a field case in Kansas
by Butler (1902). The disease was known as "mouldy maize poisoning"
but attempts to identify the responsible fungus failed.
Wilson & Maronpot (1971) succeeded in establishing the causative
agent when they isolated F. verticillioides as the predominant
contaminant of mouldy maize that had caused cases of ELEM in Egypt and
reproduced ELEM by feeding culture material of the fungus on maize to
two donkeys. Subsequently investigators in South Africa confirmed the
ability of F. verticillioides (MRC 826) culture material to induce
the characteristic clinical signs and pathological changes of ELEM as
well as hepatosis in horses and donkeys (Kellerman et al., 1972;
Marasas et al., 1976, 1988a; Kriek et al., 1981).
The first symptoms of the syndrome are lethargy, head pressing
and inappetence, followed by convulsions and death after several days.
Elevated serum enzyme levels indicative of liver damage (Wilson et
al., 1992) are preceded by elevation in the serum Sa/So ratio (Wang et
al., 1992; Riley et al., 1997). Serum enzyme levels often return to
near normal concentrations (Wang et al., 1992; Wilson et al., 1992;
Ross et al., 1993; Riley et al., 1997) but usually increase markedly
immediately prior to or at the onset of behavioral changes (Kellerman
et al., 1990; Wang et al., 1992; Ross et al., 1993; Riley et al.,
1997).
In addition to the brain lesions, histopathological abnormalities
in liver and kidney have been reported in horses orally dosed with
pure fumonisins, maize screenings naturally contaminated with
fumonisins, or culture material containing known amounts of fumonisins
(Kellerman et al., 1990; Wilson et al., 1992; Ross et al., 1993;
Caramelli et al., 1993).
Shortly after the isolation and structure elucidation of
fumonisins in 1988 (Bezuidenhout et al., 1988; Gelderblom et al.,
1988), Marasas et al. (1988a) successfully produced ELEM in a horse by
the intravenous administration of pure FB1. This was done by avoiding
as much as possible hepatotoxicity using serum enzymes indicative of
it. ELEM has also been produced in horses given pure FB1 by stomach
tube, again monitoring for liver toxicity (Kellerman et al., 1990).
Fatal liver disease in the absence of any brain lesions has been
induced by intravenous injection of FB1 (Laurent et al., 1989b). ELEM
concurrent with significant liver disease has been observed in horses
and ponies fed feeds naturally contaminated with fumonisins at low
concentrations (Wilson et al., 1992; Ross et al., 1993). The
development of brain lesions in the absence of major liver lesions
does not preclude biochemical dysfunction in non-brain tissue from
contributing to the brain lesions. Ross et al. (1993) concluded that
length of exposure, level of contamination, individual animal
differences, previous exposure, or pre-existing liver impairment may
all contribute to the appearance of the clinical disease.
To date, the lowest FB1 dose that has resulted in ELEM, in a
controlled experiment, is 22 mg/kg in diets formulated with naturally
contaminated maize screenings (Wilson et al., 1992). Analysis of feeds
from confirmed cases of ELEM indicated that consumption of feed with a
FB1 concentration greater than 10 mg/kg diet is associated with
increased risk of development of ELEM, whereas, a concentration less
than 6 mg/kg diet is not (Ross, 1994).
A study by the National Veterinary Services Laboratory of the US
Animal and Plant Health Inspection Agency (National Veterinary
Services Laboratory, 1995) showed that horses fed 15 mg FB1/kg in
diets formulated from F. proliferatum (M 5991) culture material did
not exhibit any clinical signs or altered serum biochemical parameters
(including changes in the Sa/So ratio) after 150 days. A similar
result was found with a pony fed a diet containing maize screenings
naturally contaminated with 15 mg FB1/kg (Wang et al., 1992). Thus,
the minimum toxic dose in equids appears to be < 22 mg/kg > 15 mg/kg
based on studies with naturally contaminated maize screenings or
culture material (F. proliferatum) containing fumonisins. The
minimum toxic dose of pure fumonisins is unknown.
In a study using culture material containing primarily FB2 or
FB3, Ross et al. (1994) found that a diet formulated from
F. proliferatum (M 6290 and M 6104) culture material containing
primarily FB2 at 75 mg/kg was capable of inducing ELEM with hepatic
involvement in ponies after 150 days. In contrast, diets containing
primarily FB3 (75 mg/kg) were without any effect (serum enzymes,
clinical signs and histology were all normal relative to control
ponies) after 57 to 65 days. It was concluded that FB3 was less toxic
than FB2 or FB1 (Ross et al., 1994). However, analysis of serum and
tissues from ponies fed the FB3 diets revealed that the FB3 diets
significantly increased concentrations of free sphingoid bases
relative to controls and that serum enzymes were elevated but within
the normal range for ponies (Riley et al., 1997).
7.2.2 Porcine pulmonary oedema syndrome
The first report of the disease now known as porcine pulmonary
oedema (PPE) was by Kriek et al. (1981). In experimental trials,
culture material of F. verticillioides (MRC 826) was fed to horses,
pigs, sheep, rats and baboons (Kriek et al.,1981). Lung oedema
occurred only in pigs. Clinical signs of PPE typically occur soon
(2-7 days) after pigs consume diets (culture material or contaminated
maize screenings) containing large amounts of fumonisins over a short
period of time. Clinical signs usually include dyspnoea, weakness,
cyanosis and death (Osweiler et al., 1992). At necropsy, the animals
exhibit varying degrees of interstitial and interlobular oedema, with
pulmonary oedema and hydrothorax (Colvin & Harrison, 1992; Colvin et
al., 1993). Varying amounts of clear yellow fluid accumulate in the
pleural cavity.
Toxic hepatosis occurs concurrently with PPE (Osweiler et al.,
1992; Colvin et al., 1993) and is also observed in animals that
consume high levels of fumonisins but do not develop PPE (Haschek et
al., 1996). Typically, the liver contains multiple foci of coagulative
necrosis that do not show zonal distribution across the three zones of
the liver (Osweiler et al., 1992; Colvin et al., 1993). Two studies
have reported nodular hyperplasia in the pig liver (Casteel et al.,
1993, 1994).
The physiological alteration that results in the inability of the
lung to maintain fluid equilibrium is unknown. However, several
hypotheses have been proposed that are supported by experimentation.
Casteel et al. (1994) found that feeding culture material diets
(M 1325 = MRC 826) containing 150 to 170 mg FB1/kg for 210 days
resulted in right ventricular hypertrophy and medial hypertrophy of
the pulmonary arterioles. It was suggested that this cardiotoxic
effect was an indirect consequence of fumonisin-induced
hepatotoxicity. Cardiac failure is a well-known physiological
mechanism inducing altered pulmonary haemodynamics which can result in
pulmonary oedema (Colvin et al., 1993). Significant changes in oxygen
consumption and several haemodynamic parameters in pigs fed diets
containing fumonisins suggest that pulmonary hypertension caused by
hypoxic vasoconstriction may contribute to PPE (Smith et al.,
1996a,b). It has been hypothesized that the cardiovascular alterations
are a consequence of sphingoid-base-induced inhibition of L-type
calcium channels (Smith et al., 1996b).
Haschek et al. (1992) hypothesized that PPE might be induced by
dysfunction of pulmonary interstitial macrophages (PIM) resulting in
release of vasoactive mediators. The accumulation of membranous
materials in PIM, secondary to hepatotoxicity, was postulated as the
possible basis for PIM dysfunction (Haschek et al., 1992). A similar
phenomena has been observed in alveolar endothelial cells (Gumprecht
et al., 1998). It has been shown that consumption of culture material
diets (MRC 826) containing fumonisins does in fact alter PIM function
(Smith et al., 1996c). How this might contribute to pulmonary oedema
is not clear. However, it has been hypothesized that PIM dysfunction
could contribute to increased susceptibility to microbial diseases
(Smith et al., 1996c). It has been shown that serum tumour necrosis
factor-alpha (TNF-alpha)-like activity was increased in pigs fed
culture material (M 1325 = MRC 826) containing 150 mg FB1/kg (Guzman
et al., 1997). Fumonisin-induced changes in the TNF pathway have also
been seen in lipopolysaccharide-stimulated macrophages collected from
BALB/c mice dosed with pure FB1 (Dugyala et al., 1998).
In 1989-1990 outbreaks of this disease were reported in different
parts of the USA (Harrison et al., 1990; Osweiler et al., 1992; Ross
et al., 1992). Maize screenings obtained from farms (Harrison et al.,
1990; Osweiler et al., 1992) where pigs died of PPE were predominantly
contaminated with F. verticillioides. Feeding (Kriek et al., 1981;
Osweiler et al., 1992; Fazekas et al., 1998) or intubation (Colvin et
al., 1993) of F. verticillioides culture material (MRC 826) produces
PPE. Also, PPE and hepatotoxicity have been produced by feeding diets
containing maize screenings naturally contaminated with fumonisins
(Osweiler et al., 1992; Motelin et al., 1994). Purified FB1 has been
shown to produce the disease when administered intravenously (Harrison
et al., 1990; Haschek et al., 1992; Osweiler et al., 1992). However,
PPE has not yet been produced by oral administration of pure
fumonisins.
As with ELEM, there is a strong correlation between fumonisin
content of maize screenings obtained from different farms and
outbreaks of PPE (Osweiler et al., 1992; Ross et al., 1992; Ross,
1994). The highest concentration of FB1 ever reported was from maize
screenings (330 mg/kg) associated with an outbreak of PPE (Ross et
al., 1992). The minimum toxic dose has not been clearly established.
Osweiler et al. (1992) induced PPE by feeding F. verticillioides
(MRC-3033) maize culture material reportedly containing 17 mg FB1/kg
for 5 days. In the same study, maize screenings containing fumonisins
at 92 mg/kg induced PPE in several pigs after 5-7 days; similar
results were obtained in studies by Harrison et al. (1990), Haschek et
al. (1992) and Motelin et al. (1994). Based on feeding studies with
maize screenings naturally contaminated with fumonisins, FB1
concentrations of 92 to 166 mg/kg have induced PPE in 4-7 days.
Pigs fed diets containing fumonisins (formulated with culture
material or naturally contaminated maize screenings) often do not die
of PPE, even when fumonisins are reported to be present at very high
concentrations in the diet. Concentrations of FB1 as low as 17 mg/kg
in culture material diets (MRC-3033) induced PPE in 5 days (Osweiler
et al.,1992). In contrast, culture-material-formulated (M 1325 = MRC
826) diets containing as much as 190 mg/kg have been fed for 83 days
with no reported evidence of respiratory distress (Casteel et al.,
1993) and a dose of 150 to 170 mg/kg diet for up to 210 days caused
liver effects early on but no evidence of pulmonary oedema (Casteel et
al., 1994).
Colvin et al. (1993) concluded that the primary determinant of
whether pulmonary oedema or liver failure caused death was the
quantity of fumonisins fed or intubated per kg body weight per day.
They proposed that > 16 mg/kg body weight per day induced PPE and
< 16 mg/kg body weight per day induced liver failure. However, daily
oral intake levels of FB1 plus FB2 (maize screenings) from 4.5 to
6.3 mg/kg body weight have induced PPE (Haschek et al., 1992; Motelin
et al., 1994). The FB1 concentration in these diets was 166 mg/kg and
129 mg/kg, respectively. Liver lesions have been induced with maize
screenings at 1.1 mg/kg body weight per day (17 mg FB1/kg diet)
(Motelin et al., 1994).
In pigs, tissues other than liver and lung have been reported to
be targets for fumonisins, e.g., pancreas (Harrison et al., 1990),
heart (Casteel et al., 1994), kidney (Colvin et al., 1993; Harvey et
al., 1995, 1996), pulmonary intravascular macrophages (Haschek et al.,
1992), and oesophagus (Casteel et al., 1993). None of these studies
were conducted with pure fumonisins. In a recent study with pure FB1,
altered growth and changes in selected haematological parameters in
pigs were reported at dietary levels as low as 1 mg/kg (Rotter et al.,
1996).
7.2.3 Poultry toxicity
Several reports have been published implicating
F. verticillioides contamination of feed in diseases of poultry
(Marasas et al., 1984a; Bryden et al., 1987; Jeschke et al., 1987;
Prathapkumar et al., 1997). The clinical features of the disease often
include diarrhoea, weight loss, increased liver weight and poor
performance. Immunosuppression in chickens was also produced in birds
fed maize cultured with several different isolates of the fungus
(Marijanovic et al., 1991). Functional and morphological changes were
observed in chicken exposed to FB1 (Qureshi & Hagler, 1992). Several
studies have confirmed that F. verticillioides, F. proliferatum,
FB1 and moniliformin are toxic to poultry (broiler chicks, turkeys,
ducklings) (Ledoux et al., 1992, 1996; Brown et al., 1992;
Dombrink-Kurtzman et al., 1993; Javed et al., 1993a, 1995; Weibking et
al., 1993a,b, 1995; Kubena et al., 1995a,b; Hall et al., 1995;
Bermudez et al., 1996; Vesonder & Wu, 1998) and chicken embryos (Javed
et al., 1993b; Bacon et al., 1995). The levels of fumonisins used in
these studies were 75-644 mg/kg diet. Culture materials and naturally
contaminated maize containing F. proliferatum may contain, in
addition to fumonisins, moniliformin and beauvericin (Kriek et al.,
1977; Logrieco et al., 1993; Plattner & Nelson, 1994). Espada et al.
(1994) reported toxicity and altered haematological parameters (Espada
et al., 1997) in broiler chicks fed diets containing pure FB1
(10 mg/kg) and FB1 (30 mg/kg) from Fusarium verticillioides
(MRC 826) culture material.
7.2.4 Non-human primate toxicity
Kriek et al. (1981) fed three baboons F. verticillioides
culture material (MRC 826). Baboon 1 and baboon 2 died of acute
congestive heart failure after 248 and 143 days, respectively. The
remaining baboon continued on feed for 720 days, at which time it was
killed. Autopsy of baboon 3 revealed that the principle lesion was
cirrhosis of the liver. Vervet monkeys fed F. verticillioides
culture material (MRC 826) for 180 days exhibited various degrees of
toxic hepatosis (Jaskiewicz et al., 1987a). Subsequent long-term
studies (Fincham et al., 1992) with vervet monkeys fed MRC 826 culture
material shown to contain fumonisins revealed an increase in serum
cholesterol, plasma fibrinogen and blood coagulation factor VII
(factors known to promote atherosclerosis). These changes occurred
secondary to chronic hepatotoxicity at a dose calculated to average
0.3 mg total fumonisins/kg body weight per day (low-dose diet) based
on a retrospective analysis of the diets (Fincham et al., 1992) and a
high-dose diet averaging approximately 0.8 mg total fumonisins/kg body
weight per day (Shephard et al., 1996b). Analysis of the free
sphingoid bases in serum from some of the animals used in the study by
Fincham et al. (1992) showed that in serum the free sphinganine
concentration and Sa/So ratio were significantly elevated in both the
low-dose and high-dose animals (Shephard et al., 1996b). Free
sphinganine and the Sa/So ratio were also elevated in urine at both
dose levels, but not significantly (Shephard et al., 1996b).
7.2.5 Other species
Other species that have been studied using pure fumonisins,
contaminated maize screenings or maize culture material of
F. verticillioides include the following: catfish (Brown et al.,
1994; Goel et al., 1994); cattle (Osweiler et al., 1993); hamsters
(Floss et al., 1994a,b); lambs (Edrington et al., 1995); mink (Restum
et al., 1995); and rabbits (Gumprecht et al., 1995; Bucci et al.,
1996; LaBorde et al., 1997). In all cases where toxicity was evident
it involved liver and/or kidney or homologous organs.
7.3 Mechanisms of toxicity -- mode of action
Several biochemical modes of action have been proposed to explain
all or some of the fumonisin-induced animal diseases. Two of these
invoke disruption of lipid metabolism as initial site of action. There
are also several studies that hypothesize fumonisin-induced changes in
key enzymes involved in cell cycle regulation, differentiation and/or
apoptosis as initial or secondary sites of action.
7.3.1 Disruption of sphingolipid metabolism
The structural similarity between sphinganine and FB1 led Wang
et al. (1991) to hypothesize that the mechanism of action of this
mycotoxin might be via disruption of sphingolipid metabolism or a
function of sphingolipids. At the moment, there are considerable data
supporting the hypothesis that fumonisin-induced disruption of
sphingolipid metabolism is an important event in the cascade of events
leading to altered cell growth, differentiation and cell injury
observed both in vitro and in vivo.
7.3.1.1 Sphingolipids and their metabolism
The pathways of biosynthesis and turnover (Fig. 1) have not been
as well studied in sphingolipids as in other lipid classes. In order
to understand how disruption of sphingolipid metabolism might
contribute to the farm animal and laboratory animal diseases
associated with consumption of fumonisins, it is necessary to
understand how sphingolipids are biosynthesized. Eukaryotic cells
synthesize a diverse array (over 400 distinct molecules) of
sphingolipids which serve as important structural molecules in
membranes and as regulators of many cell functions (Bell et al.,
1993). While sphingolipids have also been found in procaryotes
(Karlsson, 1970), their biosynthesis and role in cellular regulation
is poorly understood.
Typically, de novo sphingolipid biosynthesis proceeds via the
reactions described below (Merrill & Jones, 1990; Sweeley, 1991; Bell
et al., 1993). The first is the condensation of serine with
palmitoyl-CoA by serine palmitoyltransferase, a pyridoxal
5'-phosphate-dependent enzyme, and the resulting 3-ketosphinganine is
reduced to sphinganine using NADPH. Sphinganine is acylated to
dihydroceramides (also called N-acylsphinganines) by ceramide
synthase using various fatty acyl-CoAs. Headgroups (e.g.,
phosphorylcholine, glucose, etc.) are subsequently added to the
1-hydroxyl group. The 4,5-trans-double bond of the sphingosine
backbone is added after acylation of the amino group of sphinganine by
the enzyme dihydroceramide desaturase (Michel et al., 1997). Both
dihydroceramide and dihydrosphingomyelin are substrates for the
enzyme. Thus, free sphingosine is not an intermediate of de novo
sphingolipid biosynthesis (Merrill, 1991; Rother et al., 1992).
Sphingolipid turnover is thought to involve the hydrolysis of complex
sphingolipids to ceramides, then to sphingosine. Sphingosine is either
reacylated or phosphorylated and cleaved to a fatty aldehyde and
ethanolamine phosphate. The fatty aldehyde and ethanolamine phosphate
can be redirected into the biosynthesis of glycerophospholipids and
other fats (Van Veldhoven & Mannaerts, 1993).
In animal cells the initial steps from the condensation of serine
and palmitoyl-CoA to the formation of ceramide take place in the
endoplasmic reticulum. Subsequent processing of ceramide into
glycosphingolipids and sphingomyelin takes place in the endoplasmic
reticulum and Golgi apparatus. Degradation of complex sphingolipids
occurs in the lysosomes, endosomes and the plasma membrane with
degradation of free sphingoid bases occurring in the cytosol. For
reviews of sphingolipid metabolism, see Merrill & Jones (1990),
Merrill (1991), Sweeley (1991) and the volumes edited by Bell et al.
(1993).
7.3.1.2 Fumonisin-induced disruption of sphingolipid metabolism in
vitro
The term "fumonisin disruption of sphingolipid metabolism"
includes inhibition of sphingosine and ceramide biosynthesis,
depletion of more complex sphingolipids, increase in free sphinganine,
decrease in reacylation of sphingosine derived from complex
sphingolipid turnover and degradation of dietary sphingolipids,
increase in sphingoid base degradation products (i.e. sphingosine
(sphinganine) 1-phosphate, ethanolamine phosphate and fatty
aldehydes), and increase in lipid products derived from the increase
in the sphingoid base degradation products. FB1 is now widely used to
reveal the function of sphingolipids and sphingolipid metabolism in
cells (Merrill et al., 1996a).
Fumonisins potently inhibit the acylation of sphinganine and
sphingosine (Wang et al., 1991; Yoo et al., 1992; Merrill et al.,
1993c). In primary rat hepatocytes, the IC50 for inhibition of serine
incorporation into sphingosine is approximately 0.1 µM for FB1 and
FB2 (Wang et al., 1991; Merrill et al., 1993c). In P388 murine
macrophages, the IC50 is less than 0.5 µM (Balsinde et al., 1997). In
cultured pig renal cells (LLC-PK1) the IC50 for inhibition of
de novo sphingosine biosynthesis is approximately 20 µM FB1 (Yoo et
al., 1992). The basis for this difference in sensitivity is unknown.
.FIGURE 1a and 1b;V219EH03.BMP
Fig. 1a. The pathway of de novo sphingolipid biosynthesis and
turnover in a mammalian cell. Large solid arrows indicate the
enzymatic steps leading to biosynthesis of ceramide, a known effector
of cell death, and large broken arrows show the enzymatic steps
leading to the production of sphingosine 1-phosphate, an effector of
cell survival. Also shown is the proposed role of mitochondrial
perturbations triggering a redirection of palmitate from
beta-oxidation into the de novo pathway, resulting in increased
biosynthesis of ceramide under conditions of oxidative stress.
Fig. 1b. The sites of action of serine palmitoyltransferase (SPTase)
inhibitors (SPTI) such as ISP-I = myriocin = thermozymozydin, and
ceramide synthase (CER synthase) inhibitors (FB) such as fumonisins.
The block on the ceramide synthase responsible for reacylation of
sphingosine results in an increase in free sphingosine and possibly
sphingosine 1-phosphate. However, it has been reported that unlike
sphingosine 1-phosphate, sphinganine 1-phosphate does not exert a
marked cytoprotective effect, but does bind to and signal via the G
protein-coupled receptor encoded by endothelial differentiation gene 1
(EDG1) (Spiegel, 1999).
In both Fig 1a and Fig 1b, the biochemicals shown in all capital
letters are those known or suspected to be lipid secondary messengers.
Also shown in Fig 1a is the generation of ceramide by ligand-induced
sphingomyelin hydrolysis.
Abbreviations: DHC-desaturase (dihydroceramide desaturase), A-SMase
(acidic sphingomyelinase), So-kinase and -lyase (sphingosine kinase
and lyase).
Inhibition of sphinganine (sphingosine) N-acyltransferase
(ceramide synthase) in cells leads to a concentration-dependent
reduction in total complex sphingolipids, including sphingomyelin and
glycosphingolipids (Wang et al., 1991; Merrill et al., 1993b; Yoo et
al., 1996). In LLC-PK1 cells, the decrease in complex sphingolipids
does not become apparent until 24 to 48 h after cells have been
exposed to FB1 but before inhibition of cell growth and increased
cell death (Yoo et al., 1996). In microsomal preparations from
cultured mouse cerebellar neurons, inhibition of ceramide synthase was
competitive with respect to both the long-chain (sphingoid) base and
fatty acyl-CoA (Merrill et al., 1993b). This observation suggests that
ceramide synthase recognizes both the amino group (sphingoid binding
domain) and the tricarballylic acid side-chains (fatty acyl-CoA
domain) of FB1 (Merrill et al., 1996b). The reduced inhibition by the
hydrolysed derivatives of the fumonisin B series (Norred et al., 1997;
van der Westhuizen et al., 1998) supports this hypothesis. Ceramide
synthase has recently been shown to acylate hydrolysed FB1, AP1, to
form N-palmitoyl-AP1. The product was also found to be an inhibitor
of ceramide synthase and to be 10 times more toxic than FB1 in a
human colonic cell line, HT29 (Humpf et al., 1998). Sphingomyelin
biosynthesis is approximately 10-fold more sensitive to inhibition by
FB1 than glycosphingolipids. This is true for other inhibitors of
ceramide synthesis, such as ß-fluoroalanine (Medlock & Merrill, 1988;
Merrill et al.,1993b).
The complete inhibition of ceramide synthase by fumonisins causes
the intracellular sphinganine concentration to increase rapidly (Wang
et al., 1991; Yoo et al., 1992). The amount of free sphinganine that
accumulates in cells is a function of several factors. These include
the extent of inhibition of ceramide synthase, the concentration of
essential precursors (serine, palmitoyl-CoA), the growth rate of the
cells, the rate of sphinganine degradation, and the rate of
elimination from the cells. For example, in rat primary hepatocytes
treated with a concentration of 1 µM FB1, which inhibits serine
incorporation into sphingosine by > 90%, there was a significant
increase in free sphinganine after 1 h, which increased to 110-fold
over controls after 4 days (Wang et al., 1991). In LLC-PK1 cells
approximately 50-fold and 128-fold increases were measured after
exposure to FB1 (35 µM, 50 to 60% inhibition) for 6 h and 24 h,
respectively (Yoo et al., 1992). Proliferating LLC-PK1 cells
accumulate much higher levels of free sphingoid bases than confluent
monolayers and cytotoxicity is only observed in proliferating cells
(Yoo et al., 1996).
While the level of free sphingosine does not increase in primary
rat hepatocytes (Wang et al., 1991; Gelderblom et al., 1995), it does
in LLC-PK1 cells, presumably due to inhibition of reacylation of
sphingosine derived from sphingolipid turnover or from the growth
medium. When cells begin to die, sphingosine levels will increase due
to the breakdown of membrane lipids. However, in LLC-PK1 cells the
increase in free sphingosine occurs before any evidence of increased
cell death or inhibition of cell proliferation (Yoo et al., 1992).
Nonetheless, approximately 95% of the increase in the levels of free
sphingoid bases in LLC-PK1 cells was found to be due to the increase
in free sphinganine level (Yoo et al., 1992). The fate of the
accumulated sphinganine is unclear. While sphingosine has little
difficulty in crossing cell membranes (Hannun et al., 1991), the
half-life of sphinganine inside LLC-PK1 cells is much longer than the
half-life of FB1 in LLC-PK1 cells (Riley et al., 1998), which
suggests either that the inhibition of sphinganine N-acyltransferase
is persistent, that sphinganine does not easily diffuse out of cells,
or that sphinganine degradation is slow relative to its biosynthesis.
In urine from rats fed FB1, > 95% of the free sphinganine is
recovered in dead cells which have apparently sloughed into the urine
(Riley et al., 1994a). Thus, in urine sphinganine is tightly
associated with the cells.
In rat hepatocytes, a portion of the accumulated sphinganine is
metabolized to sphinganine 1-phosphate and then cleaved into a fatty
aldehyde and ethanolamine phosphate (Merrill et al., 1993c), both of
which can be redirected into other biosynthetic pathways. The enzyme
responsible for hydrolysis of sphingosine (sphinganine) 1-phosphate is
sphingosine-phosphate lyase (Van Veldhoven & Mannaerts, 1993). In J774
cells exogenous sphinganine has been shown to be initially accumulated
and then rapidly metabolized (Smith & Merrill, 1995). About one-third
of the ethanolamine in phosphatidylethanolamine is derived from
long-chain base catabolism when fumonisin is added (Smith & Merrill,
1995). Similar findings have been reported using fumonisin-treated
Chinese hamster ovary cells (Badiani et al., 1996), confirming that,
in some cell types, accumulated free sphingoid bases are rapidly
metabolized into ethanolamine and fatty aldehydes. In vitro and
in vivo, rat liver lipid composition is markedly altered by FB1
(Wang et al., 1991; Gelderblom et al., 1997). In addition to changes
in free sphingoid bases, more complex sphingolipids (ceramides, etc.)
and phosphatidylethanolamine (Wang et al., 1991; Merrill et al.,
1993c), there are many changes in the fatty acid composition of liver
phospholipids (Gelderblom et al., 1997). The ability of cells to
rapidly metabolize bioactive sphingoid bases into other products may
protect cells from the toxicity associated with accumulation of free
sphingoid bases or ceramide (Spiegel, 1999). Chronically disrupted
sphingolipid metabolism leads to imbalances in phosphoglycerolipid and
fatty acid metabolism. Because the accumulation of specific
end-products and intermediates is dependent upon the balance between
anabolic and catabolic processes, it is conceivable that changes in
concentration of specific end-products could occur with no change in
the steady-state concentration of free sphingoid bases.
7.3.1.3 Fumonisin disruption of sphingolipid metabolism in vivo
a) Equids
Typically free sphingosine and sphinganine are present in normal
tissues and cells in trace amounts (Merrill et al., 1988; Riley et
al., 1994c). This is to be expected since free sphinganine is a
metabolic intermediate in the sphingolipid biosynthetic pathway, and
free sphingosine is generated primarily as a consequence of
sphingolipid turnover or degradation (Merrill, 1991; Rother et al.,
1992; Michel et al., 1997). The first in vivo study to test if
dietary fumonisins could change free sphingoid base concentration used
serum obtained from ponies fed diets containing maize screenings
naturally contaminated with fumonisins (primarily FB1) (Wang et al.,
1992). Upon consumption of diets containing fumonisin, all of the
ponies exhibited large increases in sphinganine, although the
magnitude of the changes varied among the animals. The elevation in
serum sphinganine is reversible and the increase in free sphinganine
and the Sa/So ratio occurred before increases in serum transaminase
activity and clinical signs of ELEM (Wang et al., 1992). For example,
a pony was given feed (corn screenings) containing 15 to 22 mg
FB1/kg. The Sa/So ratio increase by day 182 was followed by an
increase in serum biochemical indices of cellular injury by day 223
(Wang et al., 1992). The pony died of ELEM on day 241.
In another study, horses were fed diets containing
F. proliferatum (M 6290 and M 6104) culture material, which
contained primarily either FB2 (75 mg/kg) or FB3 (75 mg/kg) (Ross et
al., 1994). Analysis of serum and tissues from these horses showed a
qualitatively similar response to horses fed FB1 (Wang et al. 1992),
but the magnitude of the increase in free sphinganine in serum, liver
and kidney was much less in the horses fed FB3 diets (Riley et al.,
1997). While two of the three ponies fed FB2 diets developed ELEM,
the three ponies fed FB3 diets showed no clinical signs of ELEM or
evidence of liver damage after 60 days (Ross et al., 1994).
There was also a reduction in the amount of complex sphingolipids
in the serum, liver and kidney of ponies fed diets containing
fumonisins (Wang et al., 1992; Riley et al., 1997), as would be
expected if the elevation in sphinganine was due to inhibition of
de novo sphingolipid biosynthesis. In ponies fed diets containing
FB2 or FB3 (75 mg/kg), the levels of complex sphingolipids in the
liver were reduced by 88% and 72%, respectively, and the kidney was
similarly affected (Riley et al., 1997). In contrast to ponies fed
FB2 diets, ponies fed FB3 diets exhibited no liver or kidney
pathology (Ross et al., 1994). Thus, prolonged exposure to fumonisins
can result in a marked depletion of the complex sphingolipids in liver
with no evidence of liver pathology.
b) Pigs
Similar results were obtained for pigs, confirming that there was
a dose-response relationship between the ratio of free sphinganine to
free sphingosine in serum and tissues and the amount of
fumonisin-contaminated feed consumed (Riley et al., 1993). Pigs were
fed diets formulated from naturally contaminated maize screenings at 0
(< 1), 5, 23, 39, 101 and 175 mg/kg total fumonisins (FB1 plus
FB2). The results showed that the Sa/So ratio was significantly
elevated in the liver, lung and kidney from pigs consuming feeds
containing > 23 mg/kg fumonisins. Liver injury was observed at
fumonisin levels > 23 mg/kg. However, injury to the kidney was not
observed at any dose even though it contained equal or greater amounts
of free sphingoid bases. In lung tissue, free sphingoid base content
was elevated at doses > 23 mg/kg, but lung lesions were only
observed in pigs fed the diet containing 175 mg/kg. Smith et al.
(1996a) showed that in pigs fed fumonisins significant effects on
cardiovascular function were associated with significant increases in
free sphingoid bases in heart tissue. Subsequent studies found that
damage to pig alveolar endothelial cells, in vivo, was preceded by
accumulation of free sphingoid bases in lung tissue (Gumprecht et al.,
1998).
Elevation of the Sa/So ratio in pig serum paralleled the increase
in tissues (Riley et al., 1993). This finding supported the earlier
hypothesis (Wang et al., 1992) that the elevated ratio in serum was
due to the movement of free sphinganine (accumulating as a result of
inhibition of sphinganine N-acyltransferase) from tissues into the
blood. Statistically significant increases in the serum ratio were
observed at feed concentrations as low as 5 mg/kg total fumonisins
(after 14 days) and in pigs (at higher concentrations) in which other
serum biochemistry parameters were not changed and in which there were
no observable gross or microscopic lesions in liver, lung or kidney.
Thus, the increase in the Sa/So ratio was an earlier and more
sensitive indicator of fumonisin exposure than the development of
lesions in liver or lung in pigs detectable by light microscopy.
Nonetheless, the increases in free sphinganine in tissues and serum
closely paralleled the dose-dependent increases in other biochemical
parameters measured at 14 days (Motelin et al., 1994).
It has been proposed that the ratio of free sphinganine to free
sphingosine and the presence of elevated levels of free sphinganine in
serum, urine and tissue be used as indicators for consumption of
fumonisins by farm animals (Riley et al., 1994c). However, a
subsequent study with pigs found altered growth at doses of FB1 that
did not cause an increase in free sphinganine (Rotter et al., 1996).
Thus, in pigs, elevation of free sphinganine appears to occur at
dosages that are greater than those that cause subtle changes in
performance but lower than those that are toxic.
c) Poultry and other commercially important animals
Chickens fed diets supplemented with F. verticillioides culture
materials (Weibking et al., 1993a, 1995) or pure FB1 (Henry, 1993)
exhibited elevated sphinganine levels and elevated ratios in tissues
and serum. Similar findings have been made in the rabbit (Gumprecht,
et al., 1995; LaBorde et al., 1997), catfish (Goel et al., 1994), mink
(Restum et al., 1995; Morgan et al., 1997) and trout (Meredith et al.,
1998).
d) Laboratory animals
In short-term studies with rats, rabbits and mice, disruption of
sphingolipid metabolism, as shown by statistically significant
increases in free sphinganine concentration, occurs at or below the
fumonisin dosages than cause liver or kidney lesions (Riley et al.,
1994a; Martinova & Merrill, 1995; LaBorde et al., 1997; de Nijs, 1998;
Tsunoda et al., 1998; Voss et al., 1998). In rats (Sprague-Dawley,
RIVM:WU) and mice (BALB/c) dosed with fumonisins, the increase in free
sphinganine concentration in the kidney and/or liver is closely
correlated with the extent and severity of lesions (Riley et al.,
1994a; de Nijs, 1998; Tsunoda et al., 1998; Voss et al., 1998). In two
separate 21-day feeding studies (Fischer-344 rats), liver free
sphinganine level was increased, although not significantly, at the
lowest FB1 dose (50 mg/kg diet) that had liver cancer-promoting
potential (Gelderblom et al., 1996c, 1997). In rats and rabbits, the
concentration of fumonisin that causes nephrotoxicity and an increase
in kidney free sphinganine concentration is lower than the fumonisin
dose that causes hepatotoxicity (Voss et al., 1993, 1996b, 1998;
Gumprecht et al., 1995; LaBorde et al., 1997; de Nijs, 1998). For
example, in Sprague-Dawley rats significant elevation of free
sphinganine levels and hepatosis were observed at > 15 mg/kg
< 50 mg/kg dietary FB1 (Riley et al., 1994a), whereas the NOEL for
nephrosis in male Sprague-Dawley rats is 9 mg/kg (Voss et al., 1995)
and significant increases in kidney free sphinganine have been
detected in rats fed AIN-76 diets containing 1 mg FB1/kg (Wang et
al., 1999). In male RIVM:WU rats, the liver free sphinganine level was
significantly elevated at > 0.19 < 0.75 mg FB1/kg body weight
(equivalent to 1.9 and 7.5 mg/kg dietary FB1) in the absence of any
evidence of hepatosis (de Nijs, 1998) and the NOEL for tubular cell
death and significant increases in kidney free sphinganine was
< 0.19 mg FB1/kg body weight, which was equivalent to 1.9 mg/kg in
feed (de Nijs, 1998).
In the US National Toxicology Program, long-term feeding study
with Fischer-344/N Nctr BR rats, pure FB1 induced an increase in the
Sa/So ratio in kidney tissue, which correlated with increased
non-neoplastic and neoplastic lesions (US NTP, 1999). In B6C3F1/Nctr
BR female mouse liver, free sphinganine and the Sa/So ratio were
increased after 3 and 9 weeks at 50 and 80 mg FB1/kg diet, which were
the same doses that induced liver adenoma and carcinoma (US NTP,
1999). Livers taken from rats (BD IX) in a long-term study (2 years)
that were fed diets containing 10 and 25 mg/kg FB1 did not show
significant changes in liver free sphingoid bases, although the mean
concentration of free sphinganine and free sphingosine in the liver of
rats fed 25 mg FB1/kg diet was 8- and 3-fold, respectively, higher
than control values (Gelderblom et al., 1997).
In rats, rabbits and vervet monkeys, increases in free
sphinganine concentration have been detected in the urine of animals
fed fumonisin-containing diets (Riley et al., 1994a; Castegnaro et
al., 1996; Shephard et al., 1996b; LaBorde et al., 1997; Merrill et
al., 1997b; Solfrizzo et al., 1997a,b; Wang et al., 1999).
Accumulation of free sphinganine in rat urine (associated with
accumulation of dead cells) closely reflected the changes which
occurred in the kidney (Riley et al., 1994a). Analysis of urine from
rats fed commercially available chows showed a statistically
significant correlation between the free sphinganine to free
sphingosine ratio in urine and the fumonisin concentration in the
chows. The FB1 concentration in the chows ranged from undetected to
3.3 mg/kg (Merrill et al., 1997b). Feeding studies with pure FB1 in
AIN-76 diets indicate that the no-observed-effect level (NOEL) for
elevation of urinary free sphinganine level in Sprague-Dawley rats is
> 1 mg/kg diet < 5 mg/kg diet (Wang et al., 1999). In other
studies, rats fed a diet containing mixture of fumonisins (from
culture material) for 13 days showed a NOEL of between 1 and 2 mg/kg
diet (Solfrizzo et al., 1997b).
In rats fed an AIN-76 diet containing 10 mg FB1/kg for 10 days
and then put on a control diet, the urinary sphinganine concentration
returned to control levels in 10 days. However, if the diet contained
1 mg FB1/kg, the urinary sphinganine concentration remained markedly
elevated for at least 10 days after changing the feed (Wang et al.,
1999). Thus, in this study, once elevated by feeding toxic levels of
FB1, apparently non-toxic concentrations kept the free sphinganine
concentration significantly elevated to concentrations that were
equivalent to those of the nephrotoxic fumonisin dosage. This result
is, however, in contrast with the findings of Solfrizzo et al.
(1997b), showing that the elevated levels of sphingoid bases after
exposure to relatively high levels of fumonisins (7-15 mg/kg for 13
days) return to their original values when rats are exposed to a
low-fumonisin diet (1 mg/kg or less) for a period of time that is
directly dependent on the previous level of exposure, in terms of dose
and time (Solfrizzo et al., 1997b).
7.3.1.4 Tissue and species specificity
The tissue specificity and the severity of the pathology in rats
(Sprague-Dawley, Fischer-344, Wistar, RIVM:WV) and mice (BALB/c) seem
to correlate well with the disruption of sphingolipid biosynthesis
(Riley et al., 1994a; Tsunoda et al., 1998; Voss et al., 1998). This
is not the case in pigs and horses, where the kidney appears to be
equally or more sensitive than the liver with regards to the
fumonisin-induced increase in free sphinganine (Riley et al., 1993,
1997). There is significant liver pathology in horses and pigs (Ross
et al., 1994; Haschek et al., 1996), with little evidence of kidney
damage (Harvey et al., 1996).
It has been suggested that these differences in tissue and
species specificity may be due to differing susceptibility to the
adverse cellular effects of disrupted sphingolipid metabolism (Voss et
al., 1996b). For example, liver and kidney may have different
abilities to metabolize or eliminate free sphinganine or to compensate
for depletion of complex sphingolipids. In addition, fumonisin, free
sphingoid bases or their metabolites, in serum may affect the function
of the vasculature and thus indirectly affect tissues that are not
directly affected by fumonisin inhibition of ceramide synthase
(Ramasamy et al., 1995; Smith et al., 1996b). For example, the
correlation between the fumonisin-induced increase in serum
free-sphinganine (Wang et al., 1992) and the onset of ELEM could be
explained if the vascular function in horse brain was altered due to
elevated serum free sphinganine. In pigs, it has been hypothesized
that cardiovascular dysfunction, subsequent to increased free
sphingoid base concentration in the heart, is the cause of PPE (Smith
et al., 1996b).
Riley et al. (1996) have recommended that detection of high
concentrations of free sphinganine in urine, serum or tissues should
be viewed as a clinical tool to be developed and used in conjunction
with other clinical tools in situations where animal toxicity
resulting from exposure to fumonisins is suspected. Changes in
sphingolipid profiles in serum and urine in vervet monkeys fed
fumonisin-containing diets have been reported (Shephard et al.,
1996b). Whether human exposure to fumonisins in maize and maize
products will result in increased free sphinganine concentration in
tissues, urine or serum is not known. However, free sphingoid bases
can be detected in human urine (Castegnaro et al., 1996; Solfrizzo et
al., 1997a).
7.3.1.5 Fumonisin-induced sphingolipid alterations: effects on
growth, differentiation and cell death
There are many ways that disruption of sphingolipid metabolism
could account for the cell damage caused by fumonisins. In order to
fully understand the possibilities, it is necessary to consider the
multitude of functions of complex sphingolipids (Bell et al., 1993),
the potent bioactivity of sphinganine and its metabolites (Merrill et
al., 1993a), and the parallel or branch metabolic pathways that can be
affected by disruption of sphingolipid metabolism (Riley et al., 1996;
Merrill et al., 1997a,b). Since the steady-state concentration of many
biologically active lipid intermediates and end-products could be
altered, there are also many potential molecular sites that could be
affected by fumonisin-induced disruption of sphingolipid metabolism.
Thus, it can be expected that there will also be a diversity of
alterations in cellular regulation.
The earliest effect of fumonisin on sphingolipid metabolism
in vitro is the decrease in serine incorporation into ceramide,
followed by an increase in free sphinganine concentration (Yoo et al.,
1992). There is also a concentration-dependent decrease in more
complex sphingolipids (Yoo et al., 1996). Because long-chain
(sphingoid) bases are growth inhibitory, cytotoxic and induce
apoptosis under some conditions (Merrill, 1983; Stevens et al., 1990;
Nakamura et al., 1996; Sweeney et al., 1996; Yoo et al., 1996), and
are growth stimulating under certain conditions (Zhang et al., 1990,
1991; Schroeder et al., 1994), the accumulation of sphinganine (and
sometimes sphingosine) might account for these same effects of
fumonisins. Yoo et al. (1992) have shown that in the renal epithelial
cell line (LLC-PK1 cells) there is a concentration-dependent
association between the inhibition of sphingolipid biosynthesis by
FB1 and growth inhibition and cell death. After 24 h of exposure to
FB1 many cells began to develop a fibroblast-like appearance, with
loss of cell-cell contact and an elongated, spindle shape. If
fumonisin was removed, the cells that survived resumed growth and had
a normal epithelial morphology. Addition of exogenous sphinganine
induces cell death at intracellular concentrations that are similar to
those induced by FB1 (Yoo et al., 1996).
The two most likely explanations for the increased cell death
after inhibition of sphingolipid biosynthesis by fumonisins are: (1)
that the free sphinganine (or a sphinganine degradation product) is
growth inhibitory and cytotoxic for the cells, as has been seen in
many other systems (Stevens et al., 1990; Hannun et al., 1991; Sweeney
et al., 1996); and (2) that more complex sphingolipids are required
for cell survival and growth, as has been proven with mutants lacking
serine palmitoyltransferase (Hanada et al., 1990, 1992) and in studies
with specific inhibitors of glycosphingolipid biosynthesis (Radin,
1994; Nakamura et al., 1996). ß-Chloroalanine, a non-specific serine
palmitoyltransferase inhibitor, in the presence of FB1 reduced the
intracellular concentration of free sphinganine and also reduced the
inhibition of cell growth (50 to 60%) and the extent of cell death (50
to 60%) (Yoo et al., 1996). More recent studies with LLC-PK1 cells
indicate that fumonisin inhibition of cell proliferation and increased
cell death (apoptosis) are prevented by > 90% using the specific
serine palmitoyltransferase inhibitor, myriocin (ISP-1) (Riley et al.,
1999). Similar results have been obtained with HT29 cells, a human
colonic cell line (Schmelz et al., 1998). However, in the LLC-PK1
cells, the morphological changes, such as decreased cell-cell contact
and increased fibroblast-like appearance, are not reversed. In primary
human keratinocytes, both ß-chloroalanine and N-acetylsphingosine
partially protected against FB1-induced apoptosis (Tolleson et al.,
1999). However, both exogenous sphinganine and N-acetylsphingosine
alone induced apoptosis in these same cells (Tolleson et al., 1999).
Thus, in cultured cells sphingolipid-dependent mechanisms for inducing
apoptosis include accumulation of excess ceramide or sphingoid bases,
or depletion of ceramide, or more complex sphingolipids.
In addition to the cell types described above, apoptosis in
response to exposure to FB1 in vitro has been reported using turkey
lymphocytes (Dombrink-Kurtzman et al., 1994a,b), human fibroblasts,
oesophageal epithelial cells and hepatoma cells (Tolleson et al.,
1996b), and CV-1 monkey kidney cells (Wang et al., 1996).
The adverse effects of fumonisin-induced depletion of more
complex sphingolipids have been demonstrated in numerous other
studies. For example, in hippocampal neurons, FB1 inhibition of
complex sphingolipid biosynthesis was correlated with decreased axonal
growth (Harel & Futerman, 1993). The FB1 inhibition of axonal growth
could be reversed by addition of ceramide with FB1 (Harel & Futerman,
1993; Schwarz et al., 1995). The ability of growth factors to
stimulate axonal cell growth is dependent on sphingolipid biosynthesis
(Boldin & Futerman, 1997). In fibroblasts (Swiss 3T3 cells),
fumonisin-induced morphological changes could be reversed by
ganglioside GM1. However, GM1 did not prevent the inhibition of cell
proliferation (Meivar-Levy et al., 1997). FB1 and/or myriocin (ISP-1)
inhibition of glycosphingolipid biosynthesis disrupts cell substratum
adhesion in mouse melanoma cells (Hidari et al., 1996). FB1 has also
been shown to alter the manner in which glycosyl
phosphatidylinositol-anchored proteins, such as the folate receptor,
are organized and function in membranes (Hanada et al., 1993; Stevens
& Tang, 1997). FB1 inhibition of galactosylceramide biosynthesis has
been shown to disrupt the assembly and disassembly of cytoskeletal
proteins responsible for lipid transport and maintenance of the
subcellular architecture in SW13 cells (derived from a human adrenal
carcinoma) (Gillard et al., 1996). Thus, there is no doubt that the
loss of complex sphingolipids also plays a role in the abnormal
behavior and altered morphology of fumonisin-treated cells.
Currently Swiss 3T3 cells are the only type of cell that respond
to fumonisins with increased DNA synthesis (Schroeder et al., 1994).
It was proposed that this in vitro model would be useful for
understanding if, within the complex in vivo milieu of cells in the
liver, there might exist cells that could be inappropriately selected
to enter the cell cycle. Defects in cell cycle control have been shown
to promote genomic instability and progression to malignancy (Hartwell
& Kastan, 1994). Incubation of Swiss 3T3 cells with
DL-erythro-sphinganine caused an increase in [3H]thymidine
incorporation into DNA. Addition of FB1 to the cells elevated
sphinganine and induced a comparable increase in [3H]thymidine
incorporation into DNA. These findings associated an accumulation of
sphinganine with the induction of DNA synthesis by FB1 but did not
prove that they were causally linked. However, this was proven using
an inhibitor of serine palmitoyltransferase in combination with FB1.
Reduction in cellular sphinganine when ß-fluoro-L-alanine was added to
Swiss 3T3 cells, demonstrated that this reduction in sphinganine
completely removed the insulin-dependent stimulation of [3H]thymidine
incorporation into DNA by FB1. Therefore, the formation of
sphinganine is required for stimulation of DNA synthesis by fumonisins
in Swiss 3T3 cells (Schroeder et al., 1994).
Fumonisin inhibition of ceramide synthesis can deregulate many
normal cell functions including non-accidental programmed cell death.
Some of the processes have been shown to be modulated by fumonisin
inhibition of ceramide synthase in vitro (Table 4).
It is important to recognize that ceramide signaling is also
mediated by sphingomyelin hydrolysis (Perry & Hannun, 1998) via
enzymes that are not inhibited by fumonisin. When fumonisins are added
to cells for the purpose of inhibiting de novo ceramide generation,
there is also the potential for accumulation of free sphingoid bases
and their downstream sphingoid base 1-phosphates. Thus, there is the
Table 4. Examples of cell functions modulated by de novo ceramide
biosynthesis as shown by inhibition of the process by
fumonisin-treatment
* sphingosine-induced germinal vesicle breakdown and Xenopus oocyte
maturation (Strum et al., 1995)
* daunorubicin-activated apoptosis in P388, U937 and chicken granulosa
cells (Bose et al., 1995; Witty et al., 1996)
* chemotherapeutic agent (CPT-11)-induced interleukin 1-beta
converting enzyme (ICE) cascade-dependent apoptosis in 4B1 (L929)
mouse fibroblasts (Suzuki et al., 1997)
* carnitine palmitoyltransferase inhibition-induced apoptosis in LyD9
mouse haematopoietic precursor cells (Paumen et al., 1997)
* lipopolysaccharide (LPS)/platelet activating factor (PAF) induced
arachidonic acid release in murine P388D1 macrophages (Balsinde et
al., 1997)
* chemical hypoxia-induced cell death in LLC-PK1 cells (Ueda et al.,
1998)
* Fas-transduced-caspase-dependent T-cell proliferation (Sakata et
al., 1998)
* fenretinide-induced poly-(ADP-ribose) polymerase (PARP) cleavage and
apoptosis in HL-60 cells (DiPietrantonio et al., 1998)
* serum-stimulated retinoblastoma (Rb) protein dephosphorylation and
cell cycle progression (Lee et al., 1998)
* multidrug resistance modulator-dependent cytotoxicity (Cabot et al.,
1998, 1999)
* TNF-alpha/cycloheximide-induced endothelial cell death (Xu et al.,
1998)
* 12- O-tetradecanoylphorbol-13-acetate (TPA)-induced apoptosis in
prostate cancer cells (Garzotto et al., 1998)
* hexadecylphosphocholine-induced apoptosis in HaCaT cells (Wieder et
al., 1998)
* fatty acid-induced nitric oxide synthase-dependent apoptosis in
cultured rat prediabetic islets (Shimabukuro et al., 1998)
* ionizing radiation-induced DNA damaged and cell death in various
cell types (Liao et al., 1999)
potential for misinterpreting the results of experiments using
fumonisins as an inhibitor of ceramide biosynthesis as was recently
pointed out by Lemmer et al. (1998).
The ability of fumonisin inhibition of ceramide biosynthesis to
protect cells is of interest since primary rat hepatocyte necrotic
cell death has been shown to be mediated by ceramide-induced (but not
dihydroceramide) mitochondrial dysfunction (Arora et al., 1997). The
activity of the enzyme responsible for desaturation of the inactive
dihydroceramide (dihydroceramide desaturase) is regulated by the
intracellular redox state of the cell (Michel et al., 1997). Taken
together, these findings suggest that ceramide metabolism is sensitive
to oxidative stress and that fumonisin-inhibition of ceramide will
modify apoptosis induced by mitochondrial damage or oxidative stress.
In primary rat hepatocytes and rat liver slices, large increases
in free sphinganine occur at FB1 concentrations ranging from 0.1 to 1
µM (Wang et al., 1992; Gelderblom et al., 1996b; Norred et al., 1996)
that are 300- to 3000-fold less than those that are cytotoxic and 10-
to 100-fold less than those that cause inhibition of epidermal growth
factor-induced [3H]thymidine incorporation into DNA (Gelderblom et
al., 1996b). There appears to be no relationship between
fumonisin-induced increases in free sphinganine and fumonisin-induced
inhibition of [3H]thymidine incorporation and cell death in primary
rat hepatocytes (Gelderblom et al., 1996a,b) or the cytotoxicity in
rat liver slices (Norred et al., 1997). At the moment there is no
adequate explanation for the resistance of primary rat hepatocytes and
rat liver slices to fumonisin-induced cytotoxicity or inhibition of
cell proliferation. This is puzzling in light of the fact that other
primary cell cultures, such as rabbit renal epithelial cells, are very
sensitive to fumonisin-induced inhibition of cell growth (Counts et
al., 1996) and that in liver, in vivo, the intracellular
concentrations of FB1 that cause hepatotoxicity are relatively low
based on pharmacokinetic considerations (see previous sections).
7.3.1.6 Sphingolipid-mediated cellular deregulation and fumonisin
diseases
Sphingolipids have been associated with many facets of cellular
regulation (Merrill et al., 1993a; Bell et al., 1993; Ballou et al.,
1996; Merrill et al., 1997b; Kolesnick & Krönke, 1998; Perry & Hannun,
1998) that could contribute to or modify the expression of
fumonisin-associated diseases (Table 5).
Chronic fumonisin disruption of sphingolipid metabolism has been
hypothesized to be a contributing factor leading to cellular
deregulation and organ toxicity (Merrill et al., 1993c; Riley et al.,
1994b; Schroeder et al., 1994; Tolleson et al., 1996a,b, 1999). The
consequences of disrupted sphingolipid metabolism in vitro are
specific to cell type.
Table 5. Examples of cellular regulatory processes that have been
shown to be modulated by sphingolipids and are known to be
important in the control of normal cell growth,
differentiation, apoptosis and immune responsea
Sphingoid bases and their metabolites
* inhibition of protein kinase C
* activation of phospholipase D/inhibition of phosphatidic acid
phosphatase
* activation of the epidermal growth factor (EGF) receptor kinase
(probably via mitogen-activated protein kinase)
* control of intracellular calcium (seemingly via sphingosine 1-
phosphate)
* control of plasma membrane potassium permeability in myocytes
* inhibition of DNA primase and increases in transcription factor
AP-1, an early step in the growth of some cell types
Ceramide
* second messenger in cytokine signal transduction
* activates protein kinases, phosphatases and MAP kinases
* inhibits phospholipase D
More complex sphingolipids
* binding of cytoskeletal proteins
* participation in cell-cell communication and cell-substratum
interactions
* protein transport, sorting and targeting
a For additional processes regulated by ceramide generated de novo,
see Table 4 and the reviews cited in the text.
The evidence for fumonisin-induced disruption of sphingolipid
metabolism in target tissues has been demonstrated repeatedly in many
independent studies. Nonetheless, the precise mechanism by which
disrupted sphingolipid metabolism contributes to the increased organ
toxicity in rodents is unclear. The current understanding of the
sphingolipid signalling pathways (Merrill et al., 1997a,b; Kolesnick &
Krönke, 1998; Perry & Hannun, 1998; Spiegel, 1999) indicates that the
balance between the intracellular concentration of sphingolipid
effectors that protect cells from apoptosis (decreased ceramide,
increased sphingosine 1-phosphate) and the effectors that induce
apoptosis (increased ceramide, increased free sphingoid bases,
increased fatty acids) will determine the observed cellular response
(the critical set-points will be cell-type specific). Since the
balance between the rates of apoptosis and proliferation are critical
determinants in the process of tumorigenesis, in cells exposed to
fumonisins, those cells sensitive to the proliferative effect of
decreased ceramide and increased sphingosine 1-phosphate should be
selected to survive and proliferate when the conditions under which
the cells are exposed to fumonisins are such that increased
intracellular free sphingoid base concentration is not growth
inhibitory. Conversely, when the rate of increase in free sphingoid
bases exceeds a cell's ability to convert sphinganine/sphingosine to
dihydroceramide/ceramide or their sphingoid base 1-phosphate, then
free sphingoid bases will accumulate. In this case cells that are
sensitive to sphingoid base-induced growth arrest will cease growing
and insensitive cells will survive. Another condition that would
promote increased apoptosis would be if the block on ceramide synthase
was either reduced or ceramide synthesis was increased while free
sphinganine levels were still high.
7.3.2 Altered fatty acid metabolism in liver
Gelderblom et al. (1995) reported that there was no relationship
between the fumonisin-induced increase in free sphinganine and the
mitoinhibitory effect or the cytotoxicity of FB1 in primary rat
hepatocytes. In addition it was found that free sphinganine
concentration increased markedly even in primary rat hepatocytes that
had not been exposed to FB1. However, in cultured cells the simple
act of changing the culture medium can result in a transient increase
in free sphingoid bases (Smith & Merrill, 1995; Smith et al., 1997).
In fumonisin-treated primary rat hepatocytes, the Sa/So ratio was
maximally elevated at 1 µM FB1, whereas cytotoxicity was observed at
> 250 µM FB1 (Gelderblom et al., 1995, 1996b). Polyunsaturated
fatty acids (PUFAs) were shown to accumulate at the cytotoxic doses
(Gelderblom et al., 1996b).
In other studies, fumonisins were shown to create a multitude of
changes in liver cholesterol, phospholipids, sphingoid bases and free
fatty acid composition (Gelderblom et al., 1996a, 1997). In both the
short-term and the long-term feeding studies, changes in fatty acid
profiles indicated that FB1 treatment altered the n-6 fatty acid
metabolic pathway. In the long-term study (2 years), significant
changes were observed in livers from rats fed 10 and 25 mg FB1/kg
diet (Gelderblom et al., 1997). These data suggested that the increase
in the n-3 fatty acid content of liver could, through altered
eicosanoid biosynthesis, modulate hepatocyte proliferation (Gelderblom
et al., 1997). Recently, fumonisin treatment has been shown to
increase the extent of lipid peroxidation in rat (Fischer-344) primary
hepatocytes and liver in vivo in a concentration- and dose-dependent
manner (Abel & Gelderblom, 1998). The increased susceptibility to
lipid peroxidation may be a consequence of the other lipid changes
described above.
7.3.3 Other biochemical changes
Numerous studies using fumonisins have found changes in cellular
regulation and cell function (Table 6). Many of these effects could be
relevant to the organ toxicity of fumonisins.
In conclusion, fumonisin-induced disruption of sphingolipid
metabolism is observed both in vitro and in vivo. With the
exception of primary rat hepatocytes, disruption of sphingolipid
metabolism is closely correlated in both a time- and
concentration-dependent manner with alterations in cell proliferation
and increased cell death. In vivo, evidence for disruption of
sphingolipid metabolism is closely correlated with the onset and
progression of F. verticillioides-associated diseases in pigs,
horses, rabbits, mice and rats. However, disrupted sphingolipid
metabolism is also observed in tissues that are not considered target
organs (i.e., pig and horse kidney, pig heart, endothelial cells).
Thus, fumonisin-induced disruption of sphingolipid metabolism could
contribute both directly and indirectly to the diseases known to be
caused by consumption of fumonisins. Fumonisins also affect other
sites of cellular regulation that are apparently independent of the
disruption of sphingolipid metabolism. However, disruption of various
aspects of lipid metabolism and signal transduction pathways mediated
by lipid second messengers appears to be an important aspect of all
the various proposed mechanisms of action.
7.4 Factors modifying toxicity; toxicity of metabolites
Voss et al. (1996c) found that nixtamalization of
F. verticillioides (MRC 826) culture material effectively eliminated
FB1, but the resulting material (containing hydrolysed FB1) was less
hepatotoxic but equally nephrotoxic when fed to rats. In a recent
abstract, it was reported that pure FB1 at 50.5 and 101 mg/kg diet
(70 and 140 µmol/kg diet) was toxic to female B6C3F1/Nctr mice when
fed for 28 days, but FB2, FB3 and AP1 were not hepatotoxic (Howard
et al., 1999). These considerations are important in the evaluation of
the potential of calcium hydroxide treatment for the detoxification of
fumonisin-contaminated maize (Sydenham et al., 1995).
Table 6. Summary of studies using fumonisins that have found
changes in cellular regulation and cell function
* repression of expression of protein kinase C (PKC), AP-1-dependent
transcription, stimulation of a cyclic AMP response element in CV-I
African green monkey kidney cells (1-10 µM FB1, 3 to 16 h) (Huang et
al., 1995)
* decreased phorbol dibutyrate binding, increased cytosolic PKC
activity, with both exogenous sphinganine and FB1 to J774A.1 cells
(Smith et al.,1997)
* inhibited phorbol dibutyrate binding in short-term incubations using
crude cerebrocortical membrane preparation and both FB1 and exogenous
sphingosine (Yeung et al., 1996)
* activation of the mitogen-activated protein kinase (MAPK) in Swiss
3T3 cells with FB1 (Wattenberg et al., 1996)
* inhibition of serine/threonine phosphatases (PP5, IC50 of 80 µM) in
isolated enzyme preparations (Fukuda et al., 1996)
* over-expression of nuclear cyclin D1 and increased cyclin-dependent
kinase 4 (CDK4) activity in rat livers obtained from a long-term
feeding study and a 21-day feeding study with FB1 (an abstract
Ramljak et al., 1996)
* dephosphorylation of the retinoblastoma protein, repression of CDK2,
and induction of two CDK inhibitors in CV-1 cells with FB1
(Ciacci-Zanella et al., 1998)
* apoptosis inhibitor and protease inhibitor protection of CV-1 cells
and primary human cells from FB1-induced apoptosis (Ciacci-Zanella
& Jones, 1999)
* increased TNF secretion in LPS-activated intraperitoneal macrophages
from FB1-treated mice (Dugyala et al., 1998)
* altered calcium homeostasis in frog (Rana esculenta) atrial muscle
in vitro (Sauviat et al., 1991)
* glutathione depletion and lipid peroxidation in cultured cells
(Azuka et al., 1993; Kang & Alexander, 1996; Sahu et al., 1998;
Abado-Becognee et al., 1998; Yin et al., 1998) and in vivo (Lim et
al., 1996; Abel & Gelderblom, 1998)
* stimulation of nitric oxide production (Rotter & Oh, 1996)
In naturally contaminated maize, the simultaneous occurrence of
multiple fumonisins is likely. Therefore, the relative toxicity of the
various fumonisins is of concern for hazard assessment. Gelderblom et
al. (1993) found that the aminopentols (AP1 and AP2) of FB1 and
FB2 did not act as cancer initiators in orally dosed male Fischer
rats, although they were more toxic than the parent compounds in
primary cultures of rat hepatocytes. AP1 is less toxic than FB1
(Flynn et al., 1994, 1997). The aminopentols of FB1, FB2 and FB3
are also less effective inhibitors of sphinganine N-acyltransferase
in rat primary hepatocytes and liver slices (Merrill et al., 1993c;
Norred et al., 1997). In gestation day 9.5 rat embryos exposed
in vitro to 0, 3, 10, 30, 100 or 300 µM AP1 throughout the entire
45-h cultured period, significant increase in the incidence of
abnormal embryos including neural tube defects (NTD) were observed at
concentrations of 100 µM and above (Flynn et al., 1997). A recent
study by Norred et al. (1997) found that the following mycotoxins had
no effect on sphinganine levels in rat liver slices: aflatoxin B1,
cyclopiazonic acid, beauvericin, T-2 toxin, sterigmatocystin,
luteoskyrin, verrucarin A, scirpentriol and zearalenone. Fumonisins
FB1, FB2, FB3, FB4, FC4, and hydrolysed FB1, FB2, FB3 and
Aal-toxin all caused significant elevation in free sphinganine (Norred
et al., 1997). Fumonisin B4, C4 and AAl-toxin are the most effective
inhibitors of sphinganine N-acyltransferase based on sphinganine
accumulation in rat liver slices (Norred et al., 1997). However, their
toxicity in vivo is unknown. Pure FB3 was less effective than FB1
or FB2 in causing reduced weight gain in rats (Gelderblom et al.,
1993) but FB2 and FB3 are equally effective inhibitors of
sphinganine N-acyltranferase (Norred et al., 1997). A diet
containing FB3 was less effective than a diet containing FB2 in
inducing ELEM in ponies (Ross et al., 1994). The ability of diets
containing FB3 to disrupt sphingolipid metabolism in vivo was less
than that of diets containing FB2 (Riley et al., 1997). However, in
primary rat hepatocytes, FB3 was found to be more cytotoxic
(Gelderblom et al., 1993). Acetylated FB1 had no effect (relative to
controls) on weight gain in rats nor did it have any cancer-initiating
activity (Gelderblom et al., 1993). It does not cause sphinganine
accumulation in liver slices (Norred et al., 1997) but did cause
sphinganine accumulation in a study with primary rat hepatocytes (van
der Westhuizen et al., 1998).
Because fumonisins occur naturally in combination with other
fungal toxins (Chu & Li, 1994; Bottalico et al., 1995; Logrieco et
al., 1995; Yamashita et al., 1995; Ueno et al., 1997), the possibility
of toxic synergisms exists. There are numerous reports of additive
effects but the dosages have been much greater than those known to
occur naturally (Kubena et al., 1995a,b). Toxic synergisms have been
reported in growing pigs fed diets containing culture material with
FB1 (50 mg/kg diet) and deoxynivalenol-contaminated wheat (4 mg/kg
diet) (Harvey et al., 1996) and in a similar study with aflatoxin (2.5
mg/kg diet) and FB1 (100 mg/kg diet) culture material (Harvey et al.,
1995).
Several reports have been published indicating that no toxic
synergism is observed in poultry. In turkeys fed a ration containing
200 mg FB1 and 100 mg moniliformin/kg diet from 1 to 21 days of age,
no additive or synergistic effects were observed (Bermudez et al.,
1997). Female turkey poults (Nicholas Large Whites) from day of hatch
to 3 week of age fed diets containing 300 mg FB1, as well as 4 mg
diacetoxyscirpenol or 3 mg ochratoxin A, exhibited additive or less
than additive toxicity, but not toxic synergy (Kubena et al., 1997a).
In male broiler chicks from day of hatch to 19 or 21 days of age fed
diets containing 300 mg FB1, as well as 5 mg T-2 toxin/kg diet or 15
mg deoxynivalenol/kg diet from naturally contaminated wheat, toxic
synergy was not observed for either of these toxin combinations
(Kubena et al., 1997b).
Fumonisins inhibit the in vitro biosynthesis of
glycosphingolipid receptors for cholera toxin and shiga-like toxins
(Sandvig et al., 1996) and inhibit the accumulation of
glycosphingolipids believed to be responsible for multidrug-resistance
in certain cancer cells (Lavie et al. 1996). Glycosphingolipids are
known to be receptors and adhesion sites for viruses, bacteria and
fungi.
8. EFFECTS ON HUMANS
There has been one report of a disease outbreak characterized by
abdominal pain, borborygmi and diarrhoea in India suspected to be
associated with foodborne FB1 (Bhat et al., 1997).
8.1 Transkei, South Africa
The only studies available were correlation studies, most of
which indicated some relationship between oesophageal cancer rates and
the occurrence of F. verticillioides (IARC, 1993).
A very high incidence of oesophageal cancer among the black
population of the Transkei, South Africa, has been reported in several
surveys (Jaskiewicz et al., 1987c; Makaula et al., 1996), some of
which have been reviewed by IARC (1993). The incidence was higher in
both sexes in the south (Butterworth and Kentani Districts) compared
to the northern parts of the region (Bizana and Lusikisiki Districts).
Based on the performance of hybrids in small experimental plots,
maize grows well in both areas (Rheeder et al., 1994). The sites are
about 200 km apart and the northern area is about 500 m higher.
Although some soil fertility factors were different between the high
and low oesophageal cancer areas, there is no evidence that any
nutrient was limiting at least for hybrid maize production (Rheeder et
al., 1994). Farmers grow open-pollinated maize of varying genotypes
passed on from farm to farm and season to season. Kernel types include
large flour-maize kernels as well as dent and flint-type white, yellow
and blue kernels. Both areas depend on home-grown maize for around
50-100% of the year's supply, the remaining being purchased from
commercial sources or imports.
Maize porridge is the staple diet (up to 100% of calories).
Adults also consume beer deliberately made from mouldy maize selected
by the housewife from the harvest. This maize has been found to
contain up to 118 mg/kg fumonisins (Rheeder et al., 1992). Based on
experiments conducted on beer made from wort containing added FB1
(Scott et al., 1995), such beers could contain fumonisin
concentrations of 30 mg/litre beer.
Contamination of home-grown maize in Transkei by a number of
toxigenic Fusarium species, particularly F. verticillioides, has
been observed since the early 1970s (Marasas et al., 1979a, 1981,
1988b, 1993; Marasas, 1993, 1994, 1995, 1996, 1997). Another
Fusarium species associated with maize in Transkei is
F. graminearum, and the mycotoxins deoxynivalenol and zearalenone
produced by this fungus occur in home-grown maize intended for human
consumption (Marasas et al., 1979b). However, the occurrence of
F. graminearum in maize kernels was found to be greater in low-risk
than in high-risk areas for oesophageal cancer in later studies
(Marasas et al., 1981, 1988b), and Sydenham et al. (1990b) confirmed
that deoxynivalenol and zearalenone levels were significantly higher
in home-grown maize from areas with low rates of oesophageal cancer
than from those with high rates.
In contrast, the occurrence of F. verticillioides in maize
kernels was significantly correlated to oesophageal cancer rates.
However, the rules for selection of families was different between the
two populations. The prevalence of F. verticillioides was greater in
home-grown maize collected in 12 households in the high-incidence area
compared to a similar collection in a low-incidence area. Households
in the high-incidence area were selected on the basis of cytological
examination of cells collected from the oesophagus (Marasas et al.,
1988b). Subsequent studies conducted after the chemical
characterization of the fumonisins in 1988 also found significantly
higher levels of F. verticillioides and fumonisins (20 times higher)
in areas with high rates of oesophageal cancer in Transkei than in
areas with low rates (Sydenham et al., 1990a,b; Rheeder et al., 1992).
F. verticillioides and F. graminearum cause maize ear disease
under quite different ecological conditions. The environmental
conditions that prevail in the areas in the Transkei with high rates
of oesophageal cancer clearly favour colonization of maize ears by
F. verticillioides. Significant fumonisin accumulation in maize
occurs periodically in all such environments examined so far,
primarily in relation to drought and other environmental stressors.
Taking this into account, the South African studies have shown that
the level of fumonisin in home-grown maize has been consistently high
in the areas in the Transkei with high rates of oesophageal cancer.
Cancer registry data have shown these areas to have consistently high
rates of oesophageal cancer since 1955 (Jaskiewicz et al., 1987c;
Makaula et al., 1996).
8.2 China
The only studies available were correlation studies where there
was no clear picture on the association of either fumonisin or
F. verticillioides contamination with oesophageal cancer.
Maize is consumed as a staple in a number of areas in China,
including Linxian and Cixian counties in Henan province (Zhen, 1984;
Chu & Li, 1994; Yoshizawa et al., 1994). Mortality rates for males in
the high-risk areas ranged from 26 to 36 per 100 000 in the low-risk
counties and from 76 to 161 per 100 000 in the high-risk counties. The
incidence of F. verticillioides has been reported to be higher in
maize in high- than low-risk areas, but the mycological data are
fragmentary (Zhen, 1984) and difficult to evaluate.
Maize samples from Linxian and Cixian Counties, both
high-incidence areas of oesophageal cancer in China, were analysed for
FB1 by Chu & Li (1994). All 31 samples contained FB1 at levels
ranging from 18 to 155 mg/kg. These results established that
home-grown maize in high incidence areas of oesophageal cancer in
China may be contaminated with very high levels of FB1. Another
investigation carried out on 246 maize samples showed that people
residing in an area with high incidence of human oesophageal cancer
are more exposed to fumonisins, although the exposure varied greatly
(Zhang et al., 1997). However, no relationship between fumonisin and
human oesophageal cancer incidence was evident from this study. In a
comparative study of FB1 levels in maize from high (Linxian) and low
(Shangqiu) oesophageal cancer areas, Yoshizawa et al. (1994) found no
significant differences between the areas, which was further confirmed
in a subsequent study (Gao & Yoshizawa, 1997). Levels of FB1 in 13/27
samples from the high incidence area were 0.18-2.9 (mean 0.87) mg/kg,
and in 5/20 samples from the low incidence areas, FB1 levels were
0.19-1.7 (mean 0.89) mg/kg (Yoshizawa et al., 1994).
In a comparative study of maize samples from high-risk (Haimen)
and low-risk (Penlai) areas for human primary liver cancer in China,
Ueno et al. (1997) reported significantly higher levels ( P < 0.01)
of total fumonisins in the high- than the low-risk area. Fumonisin
levels in 80/120 samples from the high-risk area for liver cancer were
0.14-34.9 mg/kg and from the low-risk area were 0.08-15.1 mg/kg in
54/120 samples for 2 of the 3 years under investigation (Ueno et al.,
1997).
8.3 Northern Italy
One analytical study was reported from Northern Italy.
Pordenone Province in the northeast of Italy has the highest
mortality rate for oral and pharyngeal cancers and oesophageal cancer
in Italy and amongst the highest in Europe (Franceschi et al., 1990).
Risk factors identified included alcohol and tobacco use, and
significant associations with maize consumption were found for oral
cancer (179 cases; odds ratios 3.3; confidence intervals 2.0-5.3),
pharyngeal cancer (170; 3.2; 2.0-5.3) and oesophageal cancer (68; 2.8;
1.5-5.1). There were 505 hospital controls. The elevated risk of upper
digestive tract cancer was, however, limited to persons consuming more
than 42 weekly drinks of alcohol (Franceschi et al., 1990). The
possibility of reporting bias can not be excluded and no measures of
fumonisin or F. verticillioides contamination were available. The
analysis was restricted to men.
In this region, most maize is locally produced and eaten as
cooked maize meal (polenta). Fumonisin-producing Fusarium species
were found on maize produced in Northern Italy (Logrieco et al.,
1995). One study showed that 20 samples of polenta produced in Italy
in 1993 and 1994 contained 0.15-3.76 mg FB1/kg (Pascale et al.,
1995).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
9.1 Microorganisms
In the only available study on the effects of fumonisins on
bacteria, Becker et al. (1997) reported that FB1 at concentrations
from 50 to 1000 µM (36-721 mg/litre) did not inhibit the growth of
various Gram-positive and Gram-negative bacteria. There was also no
indication that FB1 was metabolized by any of the bacteria tested.
Fumonisin was reported not to affect ethanol production
(presumably by Saccharomyces) in distillers wash made from maize
contaminated with 15 and 36 mg FB1/kg (Bothast et al., 1992).
Fumonisin concentrations of 25-100 mg/litre resulted in altered
sphingolipid precursors in Pichia ciferri (Kaneshiro et al., 1992).
This species accumulated some trihydro fatty acids in the presence of
50 mg FB1/litre. However, in Rhodotorula species, FB1 depressed
production of the same compounds (Kaneshiro et al., 1993). Pure FB1
inhibits cell growth of Saccharomyces cerevisiae and causes
accumulation of free sphingoid bases and disruption of lipid
metabolism (Wu et al., 1995).
9.2 Plants
9.2.1 Duckweed and jimsonweed
Because of their structural similarity to AAL toxins from
Alternaria alternata f.sp. lycopersici (also called TA toxins;
Bottini et al., 1981; Mirocha et al., 1992), fumonisins were suspected
of being phytotoxic and virulence factors by several investigators.
Fumonisin reduced chlorophyll synthesis by 59% in duckweed (Lemma
minor) fronds at 10-6 M (Vesonder et al., 1992). Photobleaching
occurred in excised jimsonweed (Datura stramonium) leaves, also in
the µM range, and at approximately 10-4 M damage to mesophyll cells
occurred after 6 h (Abbas et al., 1992). Fumonisin apparently causes
membrane damage, as shown by electrolyte loss in jimsonweed (Abbas et
al., 1991, 1993). Additionally, fumonisin disrupts the synthesis of
sphingolipids in these plants (Abbas et al., 1994).
9.2.2 Tomato
FB1 has similar toxicity to AAL-toxin-susceptible tomato
cultivars and is not active against AAL-resistant lines. Leaf necrosis
was reported at 0.4 µM by Mirocha et al. (1992) and at > 0.1 µM by
Lamprecht et al. (1994). Fumonisins were reported to cause a
dose-dependent reduction in shoot and root length and dry mass in
tomato seedlings (Lamprecht et al., 1994). As with duckweed, fumonisin
has been shown to disrupt sphingolipid metabolism in tomato (Abbas et
al., 1994). In contrast, FB1 added directly to excised shoots has
been reported to induce callus and roots at what appear to be high
doses (Bacon et al., 1994).
9.2.3 Maize
Despite reports to the contrary (Abbas & Boyette, 1992), FB1 is
toxic to maize cells. FB1 exposure did not reduce maize seed
germination but reduced radicle elongation when the solution
concentration was above 10-4 M, and seed amylase production was
inhibited (Doehlert et al., 1994). Fumonisin at concentrations in the
10 µM range decreased shoot length, shoot dry mass and root length
(Lamprecht et al., 1994). FB1 incorporated into plant tissue culture
media reduced the growth of maize callus at 10-6 M (Van Asch et al.,
1992). FB1 has been shown to disrupt sphingolipid metabolism in maize
seedlings (Riley et al., 1996). In crosses of high- and
low-fumonisin-producing strains of F. verticillioides, only progeny
that produced high concentrations of fumonisin in vitro caused
significant stem rot (Nelson et al., 1993). These data provide
indirect evidence that fumonisins play a role in the pathogenicity of
F. verticillioides to maize (Miller, 1995).
10. FURTHER RESEARCH
* There is urgent need for an internationally available standard of
pure fumonisin B1.
* An understanding of the fate of fumonisins in maize food
processing and cooking, particularly in developing countries, is
urgently required.
* There is urgent need to develop a validated biomarker for human
exposure to fumonisin.
* Epidemiological studies on the effects of fumonisins on human
health need to be conducted, based on sound intake estimates and
biomarkers.
* Valid methods for sampling for fumonisins in maize and for
sampling, extracting and quantifying fumonisins in foods need to
be developed.
* The influence of fumonisin on the carcinogenicity of other
agriculturally important mycotoxins (e.g., aflatoxin) and
carcinogenic infectious agents requires further study.
* The importance of other routes of exposure to fumonisins,
including occupational exposure through inhalation, needs to be
determined.
* There is urgent need for increased research on non-carcinogenic
end-points including hepatotoxicity, nephrotoxicity,
neurotoxicity, immunotoxicity, gastrointestinal toxicity,
cardiovascular toxicity, and the mechanistic basis for the
organ-selective toxicities.
* Research is needed to assess further the genotoxicity of
fumonisin in both germ and somatic cells in vitro and
in vivo.
* The basis for the sex differences in animals in the response to
fumonisin requires further investigation.
* The environmental fate of fumonisin in the ecosystem needs to be
established.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL ORGANIZATIONS
The International Agency for Research on Cancer evaluated FB1 in
1992 (IARC, 1993) and reached the following conclusions.
There is inadequate evidence in humans for the carcinogenicity
of toxins derived from F. verticillioides.
There is sufficient evidence in experimental animals for the
carcinogenicity of cultures of F. verticillioides that contain
significant amounts of fumonisins.
There is limited evidence in experimental animals for the
carcinogenicity of FB1.
Overall Evaluation: Toxins derived from Fusarium
verticillioides are possibly carcinogenic to humans (Group 2B).
REFERENCES
Abado-Becognee K, Mobio TA, Ennamany R, Fleurat-Lessard F, Shier WT,
Badria F, & Creppy EE (1998) Cytotoxicity of fumonisin B1:
implication of lipid peroxidation and inhibition of protein and DNA
syntheses. Arch Toxicol, 72: 233-236.
Abbas HK & Boyette CD (1992) Phytotoxicity of fumonisin B1 on weed
and crop species. Weed Technol, 6: 548-552.
Abbas HK & Ocamb CM (1995) First report of production of fumonisin B1
by Fusarium polyphialidicum collected from seeds of Pinus
strobus. Plant Dis, 79: 642.
Abbas HK & Riley RT (1996) The presence and phytotoxicity of
fumonisins and AAL-toxin in Alternaria alternata. Toxicon, 34:
133-136.
Abbas HK & Shier WT (1997) Phytotoxicity of australifungin and
fumonisins to weeds. In: Proceedings of the 1997 Brighton Crop
Protection Conference-Weeds. Croydon, United Kingdom, British Crop
Protection Council, pp 795-800.
Abbas HK, Boyette CD, Hoagland RE, & Vesonder RF (1991) Bioherbicidal
potential of Fusarium moniliforme and its phytotoxin, fumonisin.
Weed Sci, 39: 673-677.
Abbas HK, Paul RN, Boyette CD, Duke SO, & Vesonder RF (1992)
Physiological and ultrastructural effects of fumonisin on jimsonweed
leaves. Can J Bot, 70: 1824-1833.
Abbas HK, Gelderblom WCA, Cawood ME, & Shier WT (1993) Biological
activities of fumonisins, mycotoxins from Fusarium moniliforme in
jimsonweed (Datura stramonium L.) and mammalian cell cultures.
Toxicon, 31: 345-353.
Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions
AE, Wang E, Merrill AH, & Riley RT (1994) Fumonisin- and
AAL-toxin-induced disruption of sphingolipid metabolism with
accumulation of free sphingoid bases. Plant Physiol, 106: 1085-1093.
Abbas HK, Ocamb CM, Xie W, Mirocha CJ, & Shier WT (1995) First report
of fumonisin B1, B2, and B3 production by Fusarium oxysporum var.
redolens. Plant Dis, 79: 968.
Abbas HK, Cartwright RD, Shier WT, Abouzied MM, Bird CB, Rice LG, Ross
PF, Sciumbato GL, & Meredith FI (1998) Natural occurrence of
fumonisins in rice with Fusarium sheat rot disease. Plant Disease,
82: 22-25.
Abel S & Gelderblom WCA (1998) Oxidative damage and fumonisin
B1-induced toxicity in primary rat hepatocytes and rat liver
in vivo. Toxicology, 131(2-3): 121-131.
Alberts JF, Gelderblom WCA, Thiel PG, Marasas WFO, Van Schalkwyk DJ, &
Behrend Y (1990) Effects of temperature and incubation period on
production of fumonisin B1 by Fusarium moniliforme. Appl Environ
Microbiol, 56: 1729-1733.
Alberts JF, Gelderblom WCA, Vleggaar R, Marasas WFO, & Rheeder JP
(1993) Production of 14C-labelled fumonisin B1 by Fusarium
moniliforme MRC 826 in corn culture. Appl Environ Microbiol, 59:
2673-2677.
Ali NS, Yamashita A, & Yoshizawa T (1998) Natural co-occurrence of
aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol,
nivalenol and zearalenone) in corn from Indonesia. Food Addit Contam,
15: 377-384.
Arora A, Jones BJ, Patel TC, Bronk SF, & Gores GJ (1997) Ceramide
induces hepatocyte cell death through disruption of mitochondrial
function in the rat. Hepatology, 25: 958-963.
Azcona-Olivera JI, Abouzied MM, Plattner RD, Norred WP, & Pestka JJ
(1992a) Generation of antibodies reactive with fumonisins B1, B2 and
B3 by using cholera toxin as the carrier-adjuvant. Appl Environ
Microbiol, 58: 169-173.
Azcona-Olivera JI, Abouzied MM, Plattner RD, & Pestka JJ (1992b)
Production of monoclonal antibodies to the mycotoxins fumonisin B1,
B2, and B3. J Agric Food Chem, 40: 531-534.
Azuka CE, Osweiler GD, & Reynolds DL (1993) Effect of glutathione and
vitamin E on cytotoxicity of fumonisins. Toxicologist, 13: 233.
Bacon CW & Williamson JW (1992) Interactions of Fusarium
moniliforme, its metabolites and bacteria with corn. Mycopathologia,
117: 65-71.
Bacon CW, Hinton DM, Chamberlain WJ, & Norred WP (1994) De novo
induction of adventitious roots in excised shoots of tomatoes by
fumonisin B1, a metabolite of Fusarium moniliforme. J Plant Growth
Regul, 13: 53-57.
Bacon CW, Porter JK, & Norred WP (1995) Toxic interaction of fumonisin
B1 and fusaric acid measured by injection into fertile chicken egg.
Mycopathologia, 129: 29-35.
Badiani K, Byers DM, Cook HW, & Ridgway ND (1996) Effect of fumonisin
B1 on phosphatidylethanolamine biosynthesis in Chinese hamster ovary
cells. Biochim Biophys Acta, 1304: 190-196.
Ballou LR, Laulederkind SJF, Rosloniec EF, & Raghow R (1996) Ceramide
signalling and the immune response. Biochim Biophys Acta, 1301:
273-287.
Balsinde J, Balboa MA, & Dennis EA (1997) Inflammatory activation of
arachidonic acid signaling in murine P388D1 macrophages via
sphingomyelin synthesis. J Biol Chem, 272: 20373-20377.
Bane DP, Neumann EJ, Hall WF, Harlin KS, & Slife LN (1992)
Relationship between fumonisin contamination of feed and mystery swine
disease. Mycopathologia, 117: 121-124.
Becker BA, Pace L, Rottinghaus GE, Shelby R, Misfeldt M, & Ross MS
(1995) Effects of feeding fumonisin B1 in lactating sows and their
suckling pigs. Am J Vet Res, 56: 1253-1258.
Becker B, Bresch H, Schillinger U, & Thiel PG (1997) The effect of
fumonisin B1 on the growth of bacteria. World J Microbiol Biotechnol,
13: 539-543.
Bell RM, Hannun YA, & Merrill AH Jr ed. (1993) Advances in lipid
research: sphingolipids and their metabolites. Orlando, Florida,
Academic Press, vol 25-26.
Bennett GA & Richard JL (1994) Liquid chromatographic method for the
naphthalene dicarboxaldehyde derivate of fumonisins. J Assoc Off Anal
Chem Int, 77: 501-505.
Bennett GA & Richard JL (1996) Influence of processing on Fusarium
mycotoxins in contaminated grains. Food Technol, May: 235-238.
Bermudez AJ, Ledoux DR, Turk JR, & Rottinghaus GE (1996) The chronic
effects of Fusarium moniliforme culture material containing known
levels of fumonisin B1, in turkeys. Avian Dis, 40: 231-235.
Bermudez AJ, Ledoux DR, Rottinghaus GE, & Bennett GA (1997) The
individual and combined effects of the Fusarium mycotoxins
moniliformin and fumonisin B1 in turkeys. Avian Dis, 41: 304-311.
Bezuidenhout SC, Gelderblom WCA, Gorst-Allman CP, Horak RM, Marasas
WFO, Spiteller G, & Vleggaar R (1988) Structure elucidation of the
fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem
Commun, 1988: 743-745.
Bhat RV, Shetty PH, Amruth RP, & Sudershan RV (1997) A foodborne
disease outbreak due to the consumption of moldy sorghum and maize
containing fumonisin mycotoxins. Clin Toxicol, 35: 249-255.
Blackwell BA, Miller JD, & Savard ME (1994) Production of carbon
14-labeled fumonisin in liquid culture. J Assoc Off Anal Chem Int, 77:
506-511.
Boland MP, Foster SJ, & O'Neill LAJ (1997) Daunorubicin activates NFkB
and induces kB-dependent gene expression in HL-60 promyelocytic and
Jurkat T lymphoma cells. J Biol Chem, 272: 12952-12960.
Boldin S & Futerman AH (1997) Glucosylceramide synthesis is required
for basic fibroblast growth factor and laminin to stimulate axonal
growth. J Neurochem, 68: 882-885.
Bondy G, Suzuki C, Barker M, Armstrong C, Fernie S, Hierlihy L,
Rowsell P, & Mueller R (1995) Toxicity of fumonisin B1 administered
intraperitoneally to male Sprague-Dawley rats. Food Chem Toxicol, 33:
653-665.
Bondy GS, Suzuki CAM, Fernie SM, Armstrong CL, Hierlihy SL, Savard ME,
& Barker MG (1997) Toxicity of fumonisin B1 to B6C3F1 mice: a 14-day
gavage study. Food Chem Toxicol, 35: 981-989.
Bondy GS, Suzuki CAM, Mueller RW, Fernie SM, Armstrong CL, Hierlihy
SL, Savard ME, & Barker MG (1998) Gavage administration of the fungal
toxin fumonisin B1 to female Sprague-Dawley rats. J Toxicol Environ
Health, 53: 135-151.
Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, & Kolesnick
R (1995) Ceramide synthase mediates daunorubicin-induced apoptosis: An
alternative mechanism for generating death signals. Cell, 82: 404-414.
Bothast RJ, Bennett GA, Vancauwenberge JE, & Richard JL (1992) Fate of
fumonisin B1 in naturally contaminated corn during ethanol
fermentation. Appl Environ Microbiol, 58: 233-236.
Bottalico A, Logrieco A, Ritieni A, Moretti A, Randazzo G, & Corda P
(1995) Beauvericin and fumonisin B1 in preharvest Fusarium
moniliforme maize ear rot in Sardinia. Food Addit Contam, 12:
599-607.
Bottini AT, Bowen JR, & Gilchrist DG (1981) Phytotoxins: II.
Characterization of a phytotoxic fraction from Alternaria
alternata f. sp. lycopersici. Tetrahedron Lett, 22: 2723-2726.
Bradlaw J, Pritchard D, Flynn T, Eppley R, & Stack M (1994) In
vitro assessment of fumonisin B1 toxicity using reaggregate
cultures of chick embryo neural retina cells (CERC). In Vitro Cell Dev
Biol, 30A: 92.
Brown DW, McCoy CP, & Rottinghaus GE (1994) Experimental feeding of
Fusarium moniliforme culture material containing fumonisin B1 to
channel catfish, Ictalurus punctatus. J Vet Diagn Invest, 6:
123-124.
Brown TP, Rottinghaus GE, & Williams ME (1992) Fumonisin mycotoxicosis
in broilers: Performance and pathology. Avian Dis, 36: 450-454.
Bryden WL, Love RJ, & Burgess LW (1987) Feeding grain contaminated
with Fusarium graminearum and Fusarium moniliforme to pigs and
chickens. Aust Vet J, 64: 225-226.
Bryden WL, Ravindran G, Amba MT, Gill RJ, & Burgess LW (1996)
Mycotoxin contamination of maize grown in Australia, the Philippines
and Vietnam. In: Miraglia M, Brera C, & Onori R ed. Ninth
International IUPAC Symposium on Mycotoxins and Phycotoxins, Rome,
27-31 May 1996: Abstract book. Rome, Istituto Superiore di Sanità, p
41.
Bucci TJ, Hansen DK, & LaBorde JB (1996) Leukoencephalomalacia and
hemorrhage in the brain of rabbits gavaged with mycotoxin fumonisin
B1. Nat Toxins, 4: 51-52.
Bullerman LB & Tsai W-Y (1994) Incidence and levels of Fusarium
moniliforme, Fusarium proliferatum and fumonisins in corn and
corn-based foods and feeds. J Food Prot, 57: 541-546.
Burdaspal PA & Legarda TM (1996) Occurrence of fumonisins in corn and
processed corn-based commodities for human consumption in Spain. In:
Miraglia M, Brera C, & Onori R ed. IX International IUPAC Symposium on
Mycotoxins and Phycotoxins, Rome, 27-31 May 1996: Abstract book. Rome,
Istituto Superiore di Sanità, p 167.
Butler T (1902) Notes on a feeding experiment to produce
leucoencephalitis in a horse, with positive results. Am Vet Rev, 26:
748-751.
Cabot MC, Han T-Y, & Giuliano AE (1998) The multidrug resistance
modulator SDZ PSC 833 is a potent activator of cellular ceramide
formation. FEBS Lett, 431: 185-188.
Cabot MC, Giuliano AE, Han T-Y, & Liu Y-Y (1999) SDZ PSC 833, the
cyclosporine A analogue and multidrug resistance modulator, activates
ceramide synthesis and increases vinblastine sensitivity in
drug-sensitive and drug-resistant cancer cells. Cancer Res, 59:
880-885.
Caramelli M, Dondo A, Cantini Cortellazzi G, Visconti A, Minervini F,
Doko MB, & Guarda F (1993) [Equine leukoencephalomalacia from
fumonisin: First case in Italy.] Ippologia, 4: 49-56 (in Italian).
Casteel SW, Turk JR, Cowart RP, & Rottinghaus GE (1993) Chronic
toxicity of fumonisin in weanling pigs. J Vet Diagn Invest, 5:
413-417.
Casteel SW, Turk JR, & Rottinghaus GE (1994) Chronic effects of
dietary fumonisin on the heart and pulmonary vasculature of swine.
Fundam Appl Toxicol, 23: 518-524.
Castegnaro M, Garren L, Gaucher I, & Wild CP (1996) Development of a
new method for the analysis of sphinganine and sphingosine in urine
and tissues. Nat Toxins, 4: 284-290.
Castella G, Bragulat MR, & Cabañes FJ (1997) Occurrence of
Fusarium species and fumonisins in some animal feeds and raw
materials. Cereal Res Commun, 25: 355-356.
Cawood ME, Gelderblom WCA, Vleggaar R, Behrend Y, Thiel PG, & Marasas
WFO (1991) Isolation of the fumonisin mycotoxins: A quantitative
approach. J Agric Food Chem, 39: 1958-1962.
Cawood ME, Gelderblom WCA, Alberts JF, & Snyman SD (1994) Interaction
of 14C-labelled fumonisin B mycotoxins with primary rat hepatocyte
cultures. Food Chem Toxicol, 32: 627-632.
Chamberlain WJ, Voss KA, & Norred WP (1993) Analysis of commercial
laboratory rat rations for fumonisin B1, a mycotoxin produced on corn
by Fusarium moniliforme. Contemp Top, 32: 26-28.
Chelkowski J & Lew H (1992) Fusarium species of Liseola section -
occurrence in cereals and ability to produce fumonisins. Microbiol
Alim Nutr, 10: 49-53.
Chu FS & Li GY (1994) Simultaneous occurrence of fumonisin B1 and
other mycotoxins in moldy corn collected from the People's Republic of
China in regions with high incidences of esophageal cancer. Appl
Environ Microbiol, 60: 847-852.
Chulze SN, Ramirez ML, Farnochi MC, Pascale M, Visconti A, & March G
(1996) Fusarium and fumonisin occurrence in Argentinian corn at
different ear maturity stages. J Agric Food Chem, 44: 2797-2801.
Churchwell MI, Cooper WM, Howard PC, & Doerge DR (1997) Determination
of fumonisins in rodent feed using HPLC with electrospray mass
spectrometric detection. J Agric Food Chem, 45: 2573-2578.
Ciacci-Zanella JR & Jones C (1999) Analysis of apoptosis induced by
fumonisin B1, a novel mycotoxin frequently found in cereal grains.
Food Chem Toxicol, 37: 703-712.
Ciacci-Zanella JR, Merrill AH Jr, Wang E, & Jones C (1998)
Characterization of cell cycle arrest by fumonisin B1 in CV-1 cells.
Food Chem Toxicol, 36: 791-804.
Collins TF, Shacklelford ME, Sprando RL, Black TN, LaBorde JB, Hansen
DK, Eppley RM, Trucksess MW, Howard PC, Bryant MA, Ruggles DI, Olejnik
N, & Rorie JI (1998a) Effects of fumonisin B1 in pregnant rats. Food
Chem Toxicol, 36: 397-408.
Collins TF, Sprando RL, Black TN, Shacklelford ME, LaBorde JB, Hansen
DK, Eppley RM, Trucksess MW, Howard PC, Bryant MA, Ruggles DI, Olejnik
N, & Rorie JI (1998b) Effects of fumonisins in pregnant rats, part 2.
Food Chem Toxicol, 36: 673-685.
Colvin BM & Harrison LR (1992) Fumonisin-induced pulmonary edema and
hydrothorax in swine. Mycopathologia, 117: 79-82.
Colvin BM, Cooley AJ, & Beaver RW (1993) Fumonisin toxicosis in swine:
Clinical and pathologic findings. J Vet Diagn Invest, 5: 232-241.
Counts RS, Nowak G, Wyatt RD, & Schnellmann RG (1996) Nephrotoxicant
inhibition of renal proximal tubule cell regeneration. Am J Physiol,
269: F274-F281.
Dawlatana M, Coker RD, Nagler MJ, & Blunden G (1995) A normal phase
HPTLC method for the quantitative determination of fumonisin B1 in
rice. Chromatographia, 41: 187-190.
De León C & Pandey S (1989) Improvement of resistance to ear and stalk
rots and agronomic traits in tropical maize gene pools. Crop Sci, 29:
12-17.
de Nijs M (1998) Public health aspects of Fusarium mycotoxins in
food in The Netherlands - a risk assessment. Wageningen, The
Netherlands, Agricultural University, 140 pp (Thesis).
de Nijs M, van Egmond HP, Nauta M, Rombouts FM, & Notermans SHW
(1998a) Assessment of human exposure to fumonisin B1. J Food Prot,
61: 879-884.
de Nijs M, Sizoo EA, Rombouts FM, Notermans SHW, & van Egmond HP
(1998b) Fumonisin B1 in maize for food production imported in The
Netherlands. Food Addit Contam, 15: 389-392.
de Nijs M, Sizoo EA, Vermunt AEM, Notermans SHW, & van Egmond HP
(1998c) The occurrence of fumonisin B1 in maize-containing foods in
The Netherlands. Food Addit Contam, 15: 385-388.
Desjardins AE, Plattner RD, & Nelson PE (1994) Fumonisin production
and other traits of Fusarium moniliforme strains from maize in
northeast Mexico. Appl Environ Microbiol, 60: 1695-1697.
Desjardins AE, Plattner RD, Lu M, & Claflin LE (1998) Distribution of
fumonisins in maize ears infected with strains of Fusarium
moniliforme that differ in fumonisin production. Plant Dis, 82:
953-958.
Diaz GJ & Boermans HJ (1994) Fumonisin toxicosis in domestic animals:
a review. Vet Hum Toxicol, 36: 548-555.
DiPietrantonio AM, Hsieh T, Olson SC, & Wu JM (1998) Regulation of
G1/S transition and induction of apoptosis in HL-60 leukemia cells by
fenretinide (4HPR). Int J Cancer, 78: 53-61.
Doehlert DC, Knutson CA, & Vesonder RF (1994) Phytotoxic effects of
fumonisin B1 on maize seedling growth. Mycopathologia, 127: 117-121.
Doerge DR, Howard PC, Bajic S, & Preece S (1994) Determination of
fumonisins using on-line liquid chromatography coupled to electrospray
mass spectrometry. Rapid Commun Mass Spectrom, 8: 603-606.
Doko MB & Visconti A (1994) Occurrence of fumonisins B1 and B2 in
corn and corn-based human foodstuffs in Italy. Food Addit Contam, 11:
433-439.
Doko MB, Rapior S, & Visconti A (1994) Screening for fumonisins B1
and B2 in corn and corn-based foods and feeds from France. In:
Abstracts of the 7th International Congress of the IUMS Mycology
Division, Prague, Czech Republic, 3-8 July 1994, p 468.
Doko MB, Rapior S, Visconti A, & Schjth JE (1995) Incidence and
levels of fumonisin contamination in maize genotypes grown in Europe
and Africa. J Agric Food Chem, 43: 429-434.
Doko MB, Canet C, Brown N, Sydenham EW, Mpuchane S, & Siame BA (1996)
Natural co-occurrence of fumonisins and zearalenone in cereals and
cereal-based foods from Eastern and Southern Africa. J Agric Food
Chem, 44: 3240-3243.
Dombrink-Kurtzman MA, Javid T, Bennett GA, Richard JL, Cote LM, & Buck
WB (1993) Lymphocyte cytotoxicity and erythrocytic abnormalities
induced in broiler chicks by fumonisins B1 and B2 and moniliformin
from Fusarium proliferatum. Mycopathologia, 124: 47-54.
Dombrink-Kurtzman MA, Bennett GA, & Richard JL (1994a) An optimized
MTT bioassay for determination of cytotoxicity of fumonisins in turkey
lymphocytes. J Assoc Off Anal Chem Int, 77: 512-516.
Dombrink-Kurtzman MA, Bennett GA, & Richard JL (1994b) Induction of
apoptosis in turkey lymphocytes by fumonisin. FASEB J, 8: A488.
Dragoni I, Pascale M, Piantanida L, Tirilly Y, & Visconti A (1996)
[Presence of fumonisin in foodstuff destined for feeding to pigs in
Britanny (France).] Microbiol Alim Nutr, 14: 97-103 (in Italian).
Dugyala RR, Sharma RP, Tsunoda M, & Riley RT (1998) Tumor necrosis
factor- alpha as a contributor in fumonisin B1 toxicity. J Pharmacol
Exp Ther, 285: 317-324.
Duncan K, Kruger S, Zabe N, Kohn B, & Prioli R (1998) Improved
fluorometric and chromatographic methods for the quantification of
fumonisins B1, B2 and B3. J Chromatogr, A815: 41-47.
Dupuy J, Le Bars P, Boudra H, & Le Bars J (1993a) Thermostability of
fumonisin B1, a mycotoxin from Fusarium moniliforme, in corn. Appl
Environ Microbiol, 59: 2864-2867.
Dupuy J, Le Bars P, Le Bars J, & Boudra H (1993b) Determination of
fumonisin B1 in corn by instrumental thin layer chromatography. J
Planar Chromatogr, 6: 476-480.
Dutton MF (1996) Fumonisins, mycotoxins of increasing importance:
their nature and their effects. Pharmacol Ther, 70: 137-161.
Duvick J, Rood T, & Grant S (1994) Isolation of fumonisin-metabolizing
fungi from maize seed. In: Proceedings of the Fifth International
Mycological Congress, Vancouver, Canada, 14-21 August 1994, 56 pp.
Duvick J, Rood T, Maddox J, & Gilliam J (1998) Detoxification of
mycotoxins in plants as a strategy for improving grain quality and
disease resistance: Identification of fumonisin-degrading microbes
from maize. In: Kohmoto K & Yoder OC ed. Molecular genetics of host
specific toxins in plant diseases. Dordrecht, Kluwer Academic
Publishers, pp 369-381.
Edrington TS, Kamps-Holtzapple CA, Harvey RB, Kubena LF, Elissalde MH,
& Rottinghaus GE (1995) Acute hepatic and renal toxicity in lambs
dosed with fumonisin-containing culture material. J Anim Sci, 73:
508-515.
Espada Y, Ruiz de Gopegui R, Cuadradas C, & Cabañes FJ (1994)
Fumonisin mycotoxicosis in broilers: Weights and serum chemistry
modifications. Avian Dis, 38: 454-460.
Espada Y, Ruiz de Gopegui R, Cuadradas C, & Cabañes FJ (1997)
Fumonisin mycotoxicosis in broilers: Plasma proteins and coagulation
modifications. Avian Dis, 41: 73-79.
Farrar JJ & Davis RM (1991) Relationships among ear morphology,
western flower thrips and Fusarium ear rot of corn. Phytopathology,
81: 661-666.
Fazekas B, Bajmócy E, Glávits R, Fenyvesi A, & Tanyi J (1998)
Fumonisin B1 contamination of maize and experimental acute fumonisin
toxicosis in pigs. J Vet Med, B45: 171-181.
Ferguson SA, Omer VE, Kwon OS, Holson RR, Houston RJ, Rottinghaus GE,
& Slikker W Jr (1997) Prenatal fumonisin (FB1) treatment in rats
results in minimal maternal or offspring toxicity. Neurotoxicology,
18: 561-569.
Fincham JE, Marasas WFO, Taljaard JJF, Kriek NPJ, Badenhorst CJ,
Gelderblom WCA, Seier JV, Smuts CM, Faber M, Weight MJ, Slazus W,
Woodroof CW, van Wyk MJ, Kruger M, & Thiel PG (1992) Atherogenic
effects in a non-human primate of Fusarium moniliforme cultures
added to a carbohydrate diet. Atherosclerosis, 94: 13-25.
Floss JL, Casteel SW, Johnson GC, & Rottinghaus GE (1994a)
Developmental toxicity in hamsters of an aqueous extract of
Fusarium moniliforme culture material containing known quantities of
fumonisin B1. Vet Hum Toxicol, 36: 5-10.
Floss JL, Casteel SW, Johnson GC, Rottinghaus GE, & Krause GF (1994b)
Developmental toxicity of fumonisin in Syrian hamsters.
Mycopathologia, 128: 33-38.
Flynn GL (1985) Mechanism of percutaneous absorption from
physicochemical evidence. In: Bronaugh RL & Maibach HI ed.
Percutaneous absorption. New York, Marcel Dekker, pp 17-42.
Flynn TJ, Pritchard D, Bradlaw J, Eppley R, & Page S (1994) Effects of
the mycotoxin fumonisin B1 and its alkaline hydrolysis product on
pre-somite rat embryos in vitro. Teratology, 49: 404.
Flynn TJ, Pritchard D, Bradlaw JA, Eppley R, & Page S (1996) In
vitro embryotoxicity of fumonisin B1 evaluated with cultured
postimplantation staged rat embryos. Toxicol In Vitro, 9: 271-279.
Flynn TJ, Stack ME, Troy AL, & Chirtel SJ (1997) Assessment of the
embryotoxic potential of the total hydrolysis product of fumonisin B1
using cultured organogenesis-staged rat embryos. Food Chem Toxicol,
35: 1135-1141.
Franceschi S, Bidoli E, Barón AE, & La Vecchia C (1990) Maize and risk
of cancer of the oral cavity, pharynx and esophagus in northeastern
Italy. J Natl Cancer Inst, 82: 1407-1411.
Fukuda H, Shima H, Vesonder RF, Tokuda H, Nishino H, Katoh S, Tamura
S, Sugimura T, & Nagao M (1996) Inhibition of protein serine/threonine
phosphatases by fumonisin B1, a mycotoxin. Biochem Biophys Res
Commun, 220: 160-165.
Furuya S, Ono K, & Hirabayashi Y (1995) Sphingolipid biosynthesis is
necessary for dendrite growth and survival of cerebellar Purkinje
cells in culture. J Neurochem, 65: 1551-1561.
Gao H-P & Yoshizawa T (1997) Further study on Fusarium mycotoxins in
corn and wheat from a high-risk area for human esophageal cancer in
China. Mycotoxins, 45: 51-55.
Garzotto M, White-Jones M, Jiang Y, Ehleiter D, Liao W-C,
Haimovitz-Friedman A, Fuks Z, & Kolesnick R (1998)
12- O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP
cells is mediated through ceramide synthase. Cancer Res, 58:
2260-2264.
Gelderblom WCA & Snyman SD (1991) Mutagenicity of potentially
carcinogenic mycotoxins produced by Fusarium moniliforme. Mycotoxin
Res, 7: 46-52.
Gelderblom WCA, Jaskiewicz K, Marasas WFO, Thiel PG, Horak RM,
Vleggaar R, & Kriek NPJ (1988) Fumonisins - Novel mycotoxins with
cancer-promoting activity produced by Fusarium moniliforme. Appl
Environ Microbiol, 54: 1806-1811.
Gelderblom W, Marasas W, Thiel P, Semple E, & Farber E (1989) Possible
non-genotoxic nature of active carcinogenic components produced by
Fusarium moniliforme. Proc Am Assoc Cancer Res, 30: 144.
Gelderblom WCA, Kriek NPJ, Marasas WFO, & Thiel PG (1991) Toxicity and
carcinogenicity of the Fusarium moniliforme metabolite, fumonisin
B1, in rats. Carcinogenesis, 12: 1247-1251.
Gelderblom WCA, Marasas WFO, Vleggaar R, Thiel PG, & Cawood ME (1992a)
Fumonisins: Isolation, chemical characterization and biological
effects. Mycopathologia, 117: 11-16.
Gelderblom WCA, Semple E, Marasas WFO, & Farber E (1992b) The
cancer-initiating potential of the fumonisin B mycotoxins.
Carcinogenesis, 13: 433-437.
Gelderblom WCA, Cawood ME, Snyman SD, Vleggaar R, & Marasas WFO (1993)
Structure-activity relationships of fumonisins in short-term
carcinogenesis and cytotoxicity assays. Food Chem Toxicol, 31:
407-414.
Gelderblom WCA, Cawood ME, Snyman SD, & Marasas WFO (1994) Fumonisin
B1 dosimetry in relation to cancer initiation in rat liver.
Carcinogenesis, 15: 209-214.
Gelderblom WCA, Snyman SD, van der Westhuizen L, & Marasas WFO (1995)
Mitoinhibitory effect of fumonisin B1 on rat hepatocytes in primary
culture. Carcinogenesis, 16: 625-631.
Gelderblom WCA, Smuts CM, Abel S, Snyman SD, Cawood MA, van der
Westhuizen L, & Swanevelder S (1996a) Effect of fumonisin B1 on
protein and lipid synthesis in primary rat hepatocytes. Food Chem
Toxicol, 34: 361-369.
Gelderblom WCA, Snyman SD, Abel S, Lebepe-Mazur S, Smuts CM, van der
Westhuizen L, Marasas WFO, Victor TC, Knasmüller S, & Huber W (1996b)
Hepatotoxicity and carcinogenicity of the fumonisins in rats: A review
regarding mechanistic implications for establishing risk in humans.
Adv Exp Med Biol, 392: 279-296.
Gelderblom WCA, Snyman SD, Lebepe-Mazur S, van der Westhuizen L, Kriek
NPJ, & Marasas WFO (1996c) The cancer-promoting potential of fumonisin
B1 in rat liver using diethylnitrosamine as a cancer initiator.
Cancer Lett, 109: 101-108.
Gelderblom WCA, Smuts CM, Abel S, Snyman SD, van der Westhuizen L,
Huber WW, & Swanevelder S (1997) Effect of fumonisin B1 on the levels
and fatty acid composition of selected lipids in rat liver, in
vivo. Food Chem Toxicol, 35: 647-656.
Gillard BK, Harrell RG, & Marcus DM (1996) Pathways of
glycosphingolipid biosynthesis in SW13 cells in the presence and
absence of vimentin intermediate filaments. Glycobiology, 6: 33-42.
Goel S, Lenz SD, Lumlertdacha S, Lovell RT, Shelby RA, Li M, Riley RT,
& Kemppainen BW (1994) Sphingolipid levels in catfish consuming
Fusarium moniliforme corn culture material containing fumonisins.
Aquat Toxicol, 30: 285-294.
Gross SM, Reddy RV, Rottinghaus GE, Johnson G, & Reddy CS (1994)
Developmental effects of fumonisin B1-containing Fusarium
moniliforme culture extract in CD1 mice. Mycopathologia, 128:
111-118.
Gumprecht LA, Marcucci A, Weigel RM, Vesonder RF, Riley RT, Showker
JL, Beasley VR, & Haschek WM (1995) Effects of intravenous fumonisin
B1 in rabbits: nephrotoxicity and sphingolipid alterations. Nat
Toxins, 3: 395-403.
Gumprecht LA, Beasley VR, Weigel RM, Parker HM, Tumbleson ME, Bacon
CW, Meredith FI, & Haschek WM (1998) Development of fumonisin-induced
hepatotoxicity and pulmonary edema in orally dosed swine:
morphological and biochemical alterations. Toxicol Pathol, 26:
777-788.
Guzman RE, Bailey K, Casteel SW, Turk J, & Rottinghaus GE (1997)
Dietary Fusarium moniliforme culture material induces in vitro
tumor necrosis factor-alpha like activity in sera of swine.
Immunopharmacol Immuntoxicol, 19: 279-289.
Hall JO, Javed T, Bennett GA, Richard JL, Dombrink-Kurtzman MA, Côté
LM, & Buck WB (1995) Serum vitamin A (retinol) reduction in broiler
chicks on feed amended with Fusarium proliferatum culture material
or fumonisin B1 and moniliformin. J Vet Diagn Invest, 7: 416-418.
Hammer P, Blüthgen A, & Walte HG (1996) Carry-over of fumonisin B1
into the milk of lactating cows. Milchwissenschaft, 51: 691-695.
Hanada K, Nishijima M, & Akamatsu Y (1990) A temperature-sensitive
mammalian cell mutant with thermolabile serine palmitoyltransferase
for the sphingolipid biosynthesis. J Biol Chem, 265: 22137-22142.
Hanada K, Nishijima M, Kiso H Jr, Hasegawa A, Fujita S, Ogawa T, &
Akamatsu Y (1992) Sphingolipids are essential for the growth of
Chinese hamster ovary cells: Restoration of the growth of a mutant
defective in sphingoid base biosynthesis by exogenous sphingolipids. J
Biol Chem, 267: 23527-23533.
Hanada K, Izawa K, Nishijima M, & Akamatsu Y (1993) Sphingolipid
deficiency induces hypersensitivity of CD14, a glycosyl
phosphatidylinositol-anchored protein, to
phosphatidylinositol-specific phospholipase C. J Biol Chem, 268:
13820-13823.
Hannun YA, Merrill AH Jr, & Bell RM (1991) Use of sphingosine as an
inhibitor of protein kinase C. Meth Enzymol, 201: 316-328.
Harel R & Futerman AH (1993) Inhibition of sphingolipid synthesis
affects axonal outgrowth in cultured hippocampal neurons. J Biol Chem,
268: 14476-14481.
Harrison LR, Colvin BM, Greene JT, Newman LE, & Cole JR Jr (1990)
Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a
toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest, 2:
217-221.
Hartwell LH & Kastan MB (1994) Cell cycle control and cancer. Science,
266: 1821-1828.
Harvey RB, Edrington TS, Kubena LF, Elissalde MH, & Rottinghaus GE
(1995) Influence of aflatoxin and fumonisin B1-containing culture
material on growing barrows. Am J Vet Res, 56: 1668-1672.
Harvey RB, Edrington TS, Kubena LF, Elissalde MH, Casper HH,
Rottinghaus GE, & Turk JR (1996) Effects of dietary fumonisin
B1-containing culture material, deoxynivalenol-contaminated wheat, or
their combination on growing barrows. Am J Vet Res, 57: 1790-1794.
Haschek WM, Motelin G, Ness DK, Harlin KS, Hall WF, Vesonder R,
Peterson RE, & Beasley VR (1992) Characterization of fumonisin
toxicity in orally and intravenously dosed swine. Mycopathologia, 117:
83-96.
Haschek WM, Gumprecht LA, Smith GW, Parker HM, Beasley VR, & Tumbleson
ME (1996) Effects of fumonisins in swine. In: Advances in swine
biomedical research. New York, Plenum Press, pp 99-112.
Hendrich S, Miller KA, Wilson TM, & Murphy PA (1993) Toxicity of
Fusarium proliferatum-fermented nixtamalized corn-based diets fed to
rats: Effect of nutritional status. J Agric Food Chem, 41: 1649-1654.
Hendricks K (1999) Fumonisins and neural tube defects in South Texas.
Epidemiology, 10: 198-200.
Henry MH (1993) Bioproduction of fumonisin B1 in chicken embryos and
broiler chicks. University of Georgia, 93 pp (Masters of Science
Thesis).
Hesseltine CW, Rogers RF, & Shotwell OL (1981) Aflatoxin and mold
flora in North Carolina in 1977 corn crop. Mycologia, 73: 216-228.
Hidari KI-PJ, Ichikawa S, Fujita T, Sakiyama H, & Hirabayashi Y (1996)
Complete removal of sphingolipids from the plasma membrane disrupts
cell to substratum adhesion of mouse melanoma cells. J Biol Chem, 271:
14636-14641.
Hirooka EY, Yamaguchi MM, Aoyama S, Sugiura Y, & Ueno Y (1996) The
natural occurrence of fumonisins in Brazilian corn kernels. Food Addit
Contam, 13: 173-183.
Hoenisch RW & Davis RM (1994) Relationship between kernel pericarp
thickness and susceptibility to Fusarium ear rot in field corn.
Plant Dis, 78: 517-519.
Holcomb M & Thompson HC Jr (1996) Analysis of fumonisin B1 in rodent
feed by CE with fluorescence detection of the FMOC derivative. J Cap
Electrophor, 3: 205-208.
Holcomb M, Thompson HC Jr, & Hankins LJ (1993) Analysis of fumonisin
B1 in rodent feed by gradient elution HPLC using precolumn
derivatization with FMOC and fluorescence detection. J Agric Food
Chem, 41: 764-767.
Hopmans EC & Murphy PA (1993) Detection of fumonisins B1, B2, and
B3 and hydrolyzed fumonisin B1 in corn-containing foods. J Agric
Food Chem, 41: 1655-1658.
Hopmans EC, Hauck CC, Hendrich S, & Murphy PA (1997) Excretion of
fumonisin B1, hydrolyzed fumonisin B1, and the fumonisin
FB1-fructose adduct in rats. J Agric Food Chem, 45: 2618-2625.
Horvath A, Sutterlin C, Manning-Krieg U, Movva NR, & Riezman H (1994)
Ceramide synthesis enhances transport of GPI-anchored proteins to the
Golgi apparatus in yeast. EMBO J, 13:3687-3695.
Howard PC, Churchwell MI, Couch LH, Marques MM, & Doerge DR (1998)
Formation of N-(carboxymethyl)fumonisin B1, following the reaction
of fumonisin B1 with reducing sugars. J Agric Food Chem, 46:
3546-3557.
Howard PC, Couch LH, Muskhelishvili M, Eppley RM, Doerge DR, &
Okerberg C (1999) Comparative toxicity of fumonisin derivatives in
28-day feeding study using female B6C3F1 mice. Toxicologist, 48: 34.
Huang C, Dickman M, Henderson G, & Jones C (1995) Repression of
protein kinase C and stimulation of cyclic AMP response elements by
fumonisin, a fungal encoded toxin which is a carcinogen. Cancer Res,
55: 1655-1659.
Humpf H-U, Schmelz E-M, Meredith FI, Vesper H, Vales TR, Wang E,
Menaldino DS, Liotta DC, & Merrill AH Jr (1998) Acylation of naturally
occurring and synthetic 1-deoxysphinganines by ceramide synthase:
Formation of N-palmitoyl-aminopentol produces a toxic metabolite of
hydrolyzed fumonisin, AP1, and a new category of ceramide synthase
inhibitor. J Biol Chem, 273: 19060-19064.
Humphreys SH, Carrington C, & Bolger PM (1997) Fumonisin risk
scenarios. Toxicologist, 36: 170.
IARC (1993) Toxins derived from Fusarium moniliforme: Fumonisins B1
and B2 and fusarin C. In: Some naturally occurring substances: Food
items and constituents, heterocyclic aromatic amines and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 445-466 (IARC
Monographs on the Evaluation of Carcinogenic Risk to Humans, Volume
56).
Inooka S & Toyokuni T (1996) Sphingosine transfer in cell-to-cell
interaction. Biochem Biophys Res Commun, 218: 872-876.
Jackson LS, Hlywka JJ, Senthil KR, Bullerman LB, & Musser SM (1996a)
Effects of time, temperature, and pH on the stability of fumonisin B1
in an aqueous model system. J Agric Food Chem, 44: 906-912.
Jackson LS, Hlywka JJ, Senthil KR, & Bullerman LB (1996b) Effects of
thermal processing on the stability of fumonisin B2 in an aqueous
system. J Agric Food Chem, 44: 1984-1987.
Jackson LS, Katta SK, Fingerhut DD, DeVries JW, & Bullerman LB (1997)
Effects of baking and frying on the fumonisin B1 content of
corn-based foods. J Agric Food Chem, 45: 4800-4805.
Jaffrezou J-P, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N,
Vermeersch S, Rousse A, & Laurent G (1996) Daunorubicin-induced
apoptosis: Triggering of ceramide generation through sphingomyelin
hydrolysis. EMBO J, 15: 2417-2424.
Jaskiewicz K, Marasas WFO, & Taljaard JJF (1987a) Hepatitis in vervet
monkeys caused by Fusarium moniliforme. J Comp Pathol, 97: 281-291.
Jaskiewicz K, Van Rensburg SJ, Marasas WF, & Gelderblom WC (1987b)
Carcinogenicity of Fusarium moniliforme culture material in rats.
J Natl Cancer Inst, 78: 321-325.
Jaskiewicz K, Marasas WFO, & Van der Walt FE (1987c) Oesophageal and
other main cancer patterns in four districts of Transkei, 1981-1984.
S Afr Med J, 72: 27-30.
Javed T, Bennett GA, Richard JL, Dombrink-Kurtzman MA, Côté LM, & Buck
WB (1993a) Mortality in broiler chicks on feed amended with a
Fusarium proliferatum culture material or with purified fumonisin
B1 and moniliformin. Mycopathologia, 123: 171-184.
Javed T, Richard JL, Bennett GA, Dombrink-Kurtzman MA, Bunte RM,
Koelkebeck KW, Côté LM, Leeper RW, & Buck WB (1993b) Embryopathic and
embryocidal effects of purified fumonisin B1 or Fusarium
proliferatum culture material extract on chicken embryos.
Mycopathologia, 123: 185-193.
Javed T, Dombrink-Kurtzman MA, Richard JL, Bennett GA, Côté LM, & Buck
WB (1995) Sero-hematologic alterations in broiler chicks on feed
amended with Fusarium proliferatum culture material or fumonisin B1
and moniliformin. J Vet Diagn Invest, 7: 520-526.
Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva MY,
Obeid LM, & Hannun YA (1995) Role for ceramide in cell cycle arrest. J
Biol Chem, 270: 2047-2052.
Jeschke N, Nelson PE, & Marasas WFO (1987) Toxicity to ducklings of
Fusarium moniliforme isolated from corn intended for use in poultry
feed. Poult Sci, 66: 1619-1623.
Johnson P, Smith EE, Phillips TD, Gentles AB, Small M, & Duffus E
(1993) The effects of fumonisin B1 on rat embryos in culture.
Toxicologist, 13: 257.
Kaneshiro T, Vesonder RF, & Peterson RE (1992) Fumonisin-stimulated
N-acetyldihydro-sphingosine, N-acetylphytosphingosine, and
phytosphingosine products of Pichia (Hansenula) ciferri, NRRL
Y-1031. Curr Microbiol, 24: 319-324.
Kaneshiro T, Vesonder RF, Peterson RE, & Bagby MO (1993)
2-hydroyhexadecanoic and 8,9,13-trihydroxydocosanoic acid accumulation
by yeasts treated by fumonisins B1. Lipids, 28: 397-401.
Kang YK & Alexander JM (1996) Alterations of the glutathione redox
cycle status in fumonisin B1-treated pig kidney cells. J Biochem
Toxicol, 11: 121-126.
Kang HJ, Kim JC, Seo JA, Lee YW, & Son DH (1994) Contamination of
Fusarium mycotoxins in corn samples imported from China. Agric Chem
Biotechnol, 37: 385-391.
Karlsson K-A (1970) Sphingolipid long chain bases. Lipids, 5: 878-891.
Kellerman TS, Marasas WFO, Pienaar JG, & Naudé TW (1972) A
mycotoxicosis of Equidae caused by Fusarium moniliforme Sheldon.
Onderstepoort J Vet Res, 39: 205-208.
Kellerman TS, Marasas WFO, Thiel PG, Gelderblom WCA, Cawood M, &
Coetzer JAW (1990) Leukoencephalomalacia in two horses induced by oral
dosing of fumonisin B1. Onderstepoort J Vet Res, 57: 269-275.
King SB & Scott GE (1981) Genotypic differences in maize to kernel
infection by Fusarium moniliforme. Phytopathology, 71: 1245-1247.
Klittich CJR, Leslie JF, Nelson PE, & Marasas WFO (1997) Fusarium
thapsinum (Gibberella thapsina): A new species in section Liseola
from sorghum. Mycologia, 89: 643-652.
Knasmüller S, Bresgen N, Kassie F, Mersch-Sundermann V, Gelderblom W,
Zöhrer E, & Eckl PM (1997) Genotoxic effects of three Fusarium
mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and
in primary cultures of rat hepatocytes. Mutat Res, 391: 39-48.
Kolesnick RN & Krönke M (1998) Regulation of ceramide production and
apoptosis. Annu Rev Physiol, 60: 643-665.
Kriek NPJ, Marasas WFO, Steyn PS, van Rensburg SL, & Steyn M (1977)
Toxicity of a moniliformin-producing strain of Fusarium moniliforme
var. subglutinans isolated from maize. Food Cosmet Toxicol, 15:
579-587.
Kriek NPJ, Kellerman TS, & Marasas WFO (1981) A comparative study of
the toxicity of Fusarium verticillioides (= F. moniliforme) to
horses, primates, pigs, sheep and rats. Onderstepoort J Vet Res, 48:
129-131.
Kubena LF, Edrington TS, Kamps-Holtzapple C, Harvey RB, Elissalde MH,
& Rottinghaus GE (1995a) Influence of fumonisin B1, present in
Fusarium moniliforme culture material, and T-2 toxin on turkey
poults. Poult Sci, 74: 306-313.
Kubena LF, Edrington TS, Kamps-Holtzapple C, Harvey RB, Elissalde MH,
& Rottinghaus GE (1995b) Effects of feeding fumonisin B1 present in
Fusarium moniliforme culture material and aflatoxin singly and in
combination to turkey poults. Poult Sci, 74: 1295-1303.
Kubena LF, Edrington TS, Harvey RB, Phillips TD, Sarr AB, &
Rottinghaus GE (1997a) Individual and combined effects of fumonisin
B1 present in Fusarium moniliforme culture material and
diacetoxyscirpenol or ochratoxin A in turkey poults. Poult Sci, 76:
256-264.
Kubena LF, Edrington TS, Harvey RB, Buckley SA, Phillips TD,
Rottinghaus GE, & Casper HH (1997b) Individual and combined effects of
fumonisin B1 present in Fusarium moniliforme culture material and
T-2 toxin or deoxynivalenol in broiler chicks. Poult Sci, 76:
1239-1247.
Kuiper-Goodman T, Scott PM, McEwen NP, Lombaert GA, & Ng W (1996)
Approaches to the risk assessment of fumonisins in corn-based foods in
Canada. Adv Exp Med Biol, 392: 369-393.
Kwon OS, Sandberg JA, & Slikker W Jr (1997a) Effects of fumonisin B1
treatment on blood-brain barrier transfer in developing rats.
Neurotoxicol Teratol, 19: 151-155.
Kwon OS, Schmued LC, & Slikker W Jr (1997b) Fumonisin B1 in
developing rats alters brain sphinganine levels and myelination.
Neurotoxicology, 18: 571-579.
LaBorde JB, Terry KK, Howard PC, Chen JJ, Collins FX, Shackelford ME,
& Hansen DK (1997) Lack of embryotoxicity of fumonisin B1 in New
Zealand white rabbits. Fundam Appl Toxicol, 40: 120-128.
Lamprecht SC, Marasas WFO, Alberts JF, Cawood ME, Gelderblom WCA,
Shephard GS, Thiel PG, & Calitz FJ (1994) Phytotoxicity of fumonisins
and TA-toxin to corn and tomato. Phytopathology, 84: 383-391.
Laurent D, Platzer N, Kohler F, Sauviat MP, & Pellegrin F (1989a)
Macrofusine et micromoniline: Deux nouvelles mycotoxines isolées de
maïs infesté par Fusarium moniliforme Sheld. Microbiol Alim Nutr, 7:
9-16.
Laurent D, Pellegrin F, Kohler F, Costa R, Thevenon J, Lambert C, &
Huerre M (1989b) La fumonisine B1 dans la pathogénie de la
leucoencéphalomalacie équine. Microbiol Alim Nutr, 7: 285-291.
Lavie Y, Cao H-t, Bursten SL, Guiliano AE, & Cabot MC (1996)
Accumulation of glucosylceramides in multidrug-resistant cancer cells.
J Biol Chem, 271: 19530-19536.
Le Bars J, Le Bars P, Dupuy J, Boudra H, & Cassini R (1994) Biotic and
abiotic factors in fumonisin B1 production and stability. J Assoc Off
Anal Chem Int, 77: 517-521.
Lebepe-Mazur S, Bal H, Hopmans E, Murphy P, & Hendrich S (1995a)
Fumonisin B1 is fetotoxic in rats. Vet Hum Toxicol, 37: 126-130.
Lebepe-Mazur S, Hopmans E, Murphy PA, & Hendrich S (1995b) Fed before
diethylnitrosamine, Fusarium moniliforme and F. proliferatum
mycotoxins alter the persistence of placental glutathione
S-transferase-positive hepatocytes in rats. Vet Hum Toxicol, 37:
209-214.
Lebepe-Mazur S, Wilson T, & Hendrich S (1995c) Fusarium
proliferatum-fermented corn stimulates development of placental
glutathione S-transferase-positive altered hepatic foci in female
rats. Vet Hum Toxicol, 37: 55-59.
Ledoux DR, Brown TP, Weibking TS, & Rottinghaus GE (1992) Fumonisin
toxicity in broiler chicks. J Vet Diagn Invest, 4: 330-333.
Ledoux DR, Bermudez AJ, & Rottinghaus GE (1996) Effects of feeding
Fusarium moniliforme culture material, containing known levels of
fumonisin B1, in the young turkey poult. Poult Sci, 75: 1472-1478.
Lee U-S, Lee M-Y, Shin K-S, Min Y-S, Cho C-M, & Ueno Y (1994)
Production of fumonisin B1 and B2 by Fusarium moniliforme isolated
from Korean corn kernels for feed. Mycotoxin Res, 10: 67-72.
Lee JY, Leonhardt LG, & Obeid LM (1998) Cell-cycle-dependent changes
in ceramide levels preceding retinoblastoma protein dephosphorylation
in G2/M. Biochem J, 334: 457-461.
Lemmer ER, Hall P De La M, Gelderblom WCA, & Marasas WFO (1998) Poor
reporting of oocyte apoptosis. Nat Med, 4: 373.
Leslie JF, Plattner RD, Desjardins AE, & Klittich CJR (1992) Fumonisin
B1 production by strains from different mating populations of
Gibberella fujikuroi (Fusarium section Liseola). Phytopathology,
82: 341-345.
Leslie JF, Marasas WFO, Shephard GS, Sydenham EW, Stockenström S, &
Thiel PG (1996) Duckling toxicity and the production of fumonisin and
moniliformin by isolates in the A and F mating populations of
Gibberella fujikuroi (Fusarium moniliforme). Appl Environ Microbiol,
62: 1182-1187.
Lew H, Adler A, & Edinger W (1991) Moniliformin and the European corn
borer. Mycotoxin Res, 7: 71-76.
Liao W-C, Haimovitz-Friedman A, Persaud RS, McLoughlin M, Ehleiter D,
Zhang N, Gatei M, Lavin M, Kolesnick R, & Fuks Z (1999) Ataxia
telangiectasia-mutated gene product inhibits DNA damage-induced
apoptosis via ceramide synthase. J Biol Chem, 274: 17908-17917.
Lim CW, Parker HM, Vesonder RF, & Haschek WM (1996) Intravenous
fumonisin B1 induces cell proliferation and apoptosis in the rat. Nat
Toxins, 4: 34-41.
Logrieco A, Moretti A, Ritieni A, Chelkowski J, Altomare C, Bottalico
A, & Randazzo G (1993) Natural occurrence of beauvericin in preharvest
Fusarium subglutinans infected corn ears in Poland. J Agric Food
Chem, 41: 2149-2152.
Logrieco A, Moretti A, Ritieni A, Bottalico A, & Corda P (1995)
Occurrence and toxigenicity of Fusarium proliferatum from preharvest
maize ear rot, and associated mycotoxins, in Italy. Plant Dis, 79:
727-731.
Logrieco A, Doko MB, Moretti A, Frisullo S, & Visconti A (1998)
Occurrence of fumonisin B1 and B2 in Fusarium proliferatum
infected asparagus plants. J Agric Food Chem, 46: 5201-5204.
Lu Z, Dantzer WR, Hopmans EC, Prisk V, Cunnick JE, Murphy PA, &
Hendrich S (1997) Reaction with fructose detoxifies fumonisin B1
while stimulating liver-associated natural killer cell activity in
rats. J Agric Food Chem, 45: 803-809.
Lukacs Z, Schaper S, Herderich M, Schreier P, & Humpf H-U (1996)
Identification and determination of fumonisin B1 and FB2 in corn
products by high-performance liquid
chromatographic-electrospray-ionization tandem mass spectrometry
(HPLC-ESI-MS-MS). Chromatographia, 43: 124-128.
Makaula NA, Marasas WFO, Venter FS, Badenhorst CJ, Bradshaw D, &
Swanevelder S (1996) Oesophageal and other cancer patterns in four
selected districts of Transkei, Southern Africa: 1985-1990. Afr J
Health Sci, 3:11-15.
Maragos CM (1995) Capillary zone electrophoresis and HPLC for the
analysis of fluorescein isothiocyanate-labelled fumonisin B1. J Agric
Food Chem, 43: 390-394.
Maragos CM & Richard JL (1994) Quantitation and stability of
fumonisins B1 and B2 in milk. J Assoc Off Anal Chem Int, 77:
1162-1167.
Marasas WFO (1993) Occurrence of Fusarium moniliforme and fumonisins
in maize in relation to human health (Editorial). S Afr Med J, 83:
382-383.
Marasas WFO (1994) Fusarium. In: Hui YM, Gorham JR, Murrell KD, &
Cliver DO ed. Foodborne disease handbook. New York, Marcel Dekker, vol
2, pp 521-573.
Marasas WFO (1995) Fumonisins: Their implications for human and animal
health. Nat Toxins, 3: 193-198.
Marasas WFO (1996) Fumonisins: History, world-wide occurrence and
impact. Adv Exp Med Biol, 392: 1-17.
Marasas WFO (1997) Risk assessment of fumonisins produced by
Fusarium moniliforme in corn. Cereal Res Commun 25: 399-406.
Marasas WFO, Kellerman TS, Pienaar JG, & Naudé TW (1976)
Leukoencephalomalacia: A mycotoxicosis of Equidae caused by
Fusarium moniliforme Sheldon. Onderstepoort J Vet Res, 43: 113-122.
Marasas WFO, Kriek NPJ, Wiggins VM, Steyn PS, Towers DK, & Hastie TJ
(1979a) Incidence, geographic distribution and toxigenicity of
Fusarium species in South African corn. Phytopathology, 69:
1181-1185.
Marasas WFO, Van Rensburg SJ, & Mirocha CJ (1979b) Incidence of
Fusarium species and the mycotoxins, deoxynivalenol and zearalenone,
in corn produced in esophageal cancer areas in Transkei. J Agric Food
Chem, 27: 1108-1112.
Marasas WFO, Wehner FC, Van Rensburg SJ, & Van Schalkwyk DJ (1981)
Mycoflora of corn produced in human esophageal cancer areas in
Transkei, Southern Africa. Phytopathology, 71: 792-796.
Marasas WFO, Nelson PE, & Toussoun TA (1984a) Toxigenic Fusarium
species: Identity and mycotoxicology. University Park, Pennsylvania,
The Pennsylvania State University Press, 328 pp.
Marasas WFO, Kriek NPJ, Fincham JE, & Van Rensburg SJ (1984b) Primary
liver cancer and oesophageal basal cell hyperplasia in rats caused by
Fusarium moniliforme. Int J Cancer, 34: 383-387.
Marasas WFO, Kellerman TS, Gelderblom WCA, Coetzer JAW, Thiel PG, &
Van der Lugt JJ (1988a) Leukoencephalomalacia in a horse induced by
fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J
Vet Res, 55: 197-203.
Marasas WFO, Jaskiewicz K, Venter FS, & Van Schalkwyk DJ (1988b)
Fusarium moniliforme contamination of maize in oesophageal cancer
areas in Transkei. S Afr Med J, 74: 110-114.
Marasas WFO, Thiel PG, Gelderblom WCA, Shephard GS, Sydenham EW, &
Rheeder JP (1993) Fumonisins produced by Fusarium moniliforme in
maize: Foodborne carcinogens of Pan African importance. Afr Newslett
Occup Health Saf, 2/93(suppl): 11-18.
Marijanovic DR, Holt P, Norred WP, Bacon CW, Voss KA, Stancel PC, &
Ragland WL (1991) Immunosuppressive effects of Fusarium moniliforme
corn cultures in chickens. Poult Sci, 70: 1895-1901.
Martinez-Larrañaga MR, Anadón A, Diaz MJ, Fernandez R, Sevil B,
Fernandez-Cruz ML, Fernandez MC, Martinez MA, & Anton R (1996)
Induction of cytochrome P4501A1 and P4504A1 activities and peroxisomal
proliferation by fumonisin B1. Toxicol Appl Pharmacol, 141: 185-194.
Martinova EA & Merrill AH Jr (1995) Fumonisin B1 alters sphingolipid
metabolism and immune function in BALB/c mice: Immunological responses
to fumonisin B1. Mycopathologia, 130: 163-170.
Matteri RL, Becker BA, Pace L, & Rottinghaus GE (1994) Effects of
thermal environment and maternal exposure to fumonisin B1 on
luteinizing hormone secretion in preweaning gilts. Biol Reprod, 50
(suppl 1): 68.
Medlock KA & Merrill AH Jr (1988) Inhibition of serine
palmitoyltransferase in vitro and long-chain base biosynthesis in
intact Chinese hamster ovary cells by beta-chloroalanine.
Biochemistry, 27: 7079-7084.
Mehta R, Lok E, Rowsell PR, Miller JD, Suzuki CA, & Bondy GS (1998)
Glutathione S-transferase-placental form expression and proliferation
of hepatocytes in fumonisin B1-treated male and female Sprague-Dawley
rats. Cancer Lett, 128: 31-39.
Meister U, Symmank H, & Dahlke H (1996) [Investigation and evaluation
of the contamination of native and imported cereals with fumonisins.]
Z Lebensm Unters Forsch, 203: 528-533 (in German).
Meister U (1998) [Effect of extraction and extract clean-up on the
determinable levels of fumonisins in maize and maize products:
Investigations on the acid extraction and the use of immunoaffinity
columns.] In: Wolff J & Betsche T ed. [Proceedings 20. Workshop on
Mycotoxins, Detmold, 8-10 June 1998.] Institute of Biochemistry of
Grain and Potatoes, Federal Agency for Grain, Potatoes and Fat
Research, pp 241-245 (in German).
Meivar-Levy I, Sabanay H, Bershadsky AD, & Futerman AH (1997) The role
of sphingolipids in the maintenance of fibroblast morphology. J Biol
Chem, 272: 1558-1564.
Meredith FI, Riley RT, Bacon CW, Williams DE, & Carlson DB (1998)
Extraction, quantification, and biological availability of fumonisin
B1 incorporated into Oregon test diet and fed to rainbow trout. J
Food Prot, 61: 1034-1038.
Merrill AH Jr (1983) Characterization of serine palmitoyltransferase
activity in Chinese hamster ovary cells. Biochim Biophys Acta, 754:
284-291.
Merrill AH Jr (1991) Cell regulation by sphingosine and more complex
sphingolipids. J Bioenerget Biomembr, 23: 83-104.
Merrill AH Jr & Jones DD (1990) An update of the enzymology and
regulation of sphingomyelin metabolism. Biochim Biophys Acta, 1044:
1-12.
Merrill AH Jr, Wang E, Mullins RE, Jamison WCL, Nimkar S, & Liotta DC
(1988) Quantitation of free sphingosine in liver by high-performance
liquid chromatography. Anal Biochem, 171: 373-381.
Merrill AH Jr, Hannun YA, & Bell RM (1993a) Sphingolipids and their
metabolites in cell regulation. In: Bell RM, Hannun YA, & Merrill AH
Jr ed. Advances in lipid research: Sphingolipids and their
metabolites. Orlando, Florida, Academic Press, vol 25, pp 1-24.
Merrill AH Jr, van Echten G, Wang E, & Sandhoff K (1993b) Fumonisin
Bl inhibits sphingosine (sphinganine) N-acetyltransferase and
de novo sphingolipid biosynthesis in cultured neurons in situ.
J Biol Chem, 268: 27299-27306.
Merrill AH Jr, Wang E, Gilchrist DG, & Riley RT (1993c) Fumonisins and
other inhibitors of de novo sphingolipid biosynthesis. In: Bell RM,
Hannun YA, & Merrill AH Jr ed. Advances in lipid research:
Sphingolipids and their metabolites. Orlando, Florida, Academic Press,
vol 26, pp 215-234.
Merrill AH Jr, Wang E, Schroeder JJ, Smith ER, Yoo H-S, & Riley RT
(1995) Disruption of sphingolipid metabolism in the toxicity and
carcinogenicity of fumonisins. In: Eklund M, Richard J, & Mise K ed.
Molecular approaches to food safety issues involving toxic
microorganisms. Fort Collins, Colorado, Alaken Press, pp 429-443.
Merrill AH Jr, Liotta DC, & Riley RT (1996a) Fumonisins: fungal toxins
that shed light on sphingolipid function. Trends Cell Biol, 6:
218-224.
Merrill AH Jr, Wang E, Vales TR, Smith ER, Schroeder JJ, Menaldino DS,
Alexander C, Crane HM, Xia J, Liotta DC, Meredith FI, & Riley RT
(1996b) Fumonisin toxicity and sphingolipid biosynthesis. Adv Exp Med
Biol, 392: 297-306.
Merrill AH Jr, Schmelz E-M, Dillehay DL, Spiegel S, Shayman JA,
Schroeder JJ, Riley RT, Voss KA, & Wang E (1997a) Sphingolipids -- the
enigmatic lipid class: Biochemistry, physiology, and pathophysiology.
Toxicol Appl Pharmacol, 142: 208-225.
Merrill AH Jr, Schmelz E-M, Wang E, Dillehay DL, Rice LG, Meredith FI,
& Riley RT (1997b) Importance of sphingolipids and inhibitors of
sphingolipid metabolism as components of animal diets. J Nutr, 127:
830S-833S.
Michel C, Van Echten-Deckert G, Rother J, Sandoff K, Wang E, & Merrill
AH Jr (1997) Characterization of ceramide synthesis. J Biol Chem, 272:
22432-22437.
Miller JD (1995) Fungi and mycotoxins in grain: Implications for
stored product research. J Stored Prod Res, 31: 1-16.
Miller JD, Savard ME, Sibilia A, Rapior S, Hocking AD, & Pitt JI
(1993) Production of fumonisins and fusarins by Fusarium
moniliforme from southeast Asia. Mycologia, 85: 385-391.
Miller JD, Savard ME, & Rapior S (1994) Production and purification of
fumonisins from a stirred jar fermenter. Nat Toxins, 2: 354-359.
Miller JD, Savard ME, Schaafsma AW, Seifert KA, & Reid LM (1995)
Mycotoxin production by Fusarium moniliforme and Fusarium
proliferatum from Ontario and occurrence of fumonisin in the 1993
corn crop. Can J Plant Pathol, 17: 233-239.
Miller MA, Honstead JP, & Lovell RA (1996) Regulatory aspects of
fumonisins with respect to animal feed. Adv Exp Med Biol, 392:
363-368.
Minervini F, Bottalico C, Pestka J, & Visconti A (1992) [On the
occurrence of fumonisins in feeds in Italy.] In [Proceedings of the
46th National Congress of the Italian Society of Veterinary Science,
Venice, Italy, 30 September-3 October], pp 1365-1368 (in Italian).
Mirocha CJ, Gilchrist DG, Shier WT, Abbas HK, Wen Y, & Vesonder RF
(1992) AAL toxins, fumonisins (biology and chemistry) and
host-specificity concepts. Mycopathologia, 17: 47-56.
Morgan MK, Schroeder JJ, Rottinghaus GE, Powell DC, Bursian SJ, &
Aulerich RJ (1997) Dietary fumonisins disrupt sphingolipid metabolism
in mink and increase the free sphinganine to sphingosine ratio in
urine but not in hair. Vet Hum Toxicol, 39: 334-336.
Motelin GK, Haschek WM, Ness DK, Hall WF, Harlin KS, Schaeffer DJ, &
Beasley VR (1994) Temporal and dose-response features in swine fed
corn screenings contaminated with fumonisin mycotoxins.
Mycopathologia, 126: 27-40.
Mullett W, Lai EP, & Yeung JM (1998) Immunoassay of fumonisins by a
surface plasmon resonance biosensor. Anal Biochem, 258: 161-167.
Murphy PA, Rice LG, & Ross PF (1993) Fumonisin B1, B2, and B3
content of Iowa, Wisconsin and Illinois corn and corn screenings. J
Agric Food Chem, 41: 263-266.
Murphy PA, Hopmans EC, Miller K, & Hendrich S (1995) Can fumonisins in
foods be detoxified? In: Toxicants in food. Lancaster, Pennsylvania,
Technomic Publishing, vol 1, pp 105-117.
Murphy PA, Hendrich S, Hopmans EC, Hauck CC, Lu Z, Buseman G, &
Munkvold G (1996) Effect of processing on fumonisin content of corn.
Adv Exp Med Biol, 392: 323-334.
Musser SM & Plattner RD (1997) Fumonisin composition in cultures of
Fusarium moniliforme, Fusarium proliferatum, and Fusarium nygami.
J Agric Food Chem, 45: 1169-1173.
Nakamura S, Kozutsumi Y, Sun Y, Miyake Y, Fujita T, & Kawasaki T
(1996) Dual role of sphingolipids in signaling of the escape from and
onset of apoptosis in a mouse cytotoxic T-cell line, CTLL-2. J Biol
Chem, 271: 1255-1257.
National Veterinary Services Laboratory (1995) Toxicity of fumonisins
in horses (equine leukoencephalomalacia [ELEM]) - Developmental
project final report. US Animal and Plant Health Inspection Agency,
National Veterinary Services Laboratory (Report No. PL/TC/94-2).
Nelson PE, Plattner RD, Shackelford DD, & Desjardins AE (1991)
Production of fumonisins by Fusarium moniliforme strains from
various substrates and geographic areas. Appl Environ Microbiol, 57:
2410-2412.
Nelson PE, Plattner RD, Shackelford DD, & Desjardins AE (1992)
Fumonisin B1 production by Fusarium species other than
F. moniliforme in section Liseola and by some related species.
Appl Environ Microbiol, 58: 984-989.
Nelson PE, Desjardins AE, & Plattner RD (1993) Fumonisins, mycotoxins
produced by Fusarium species: Biology, chemistry, and significance.
Ann Rev Phytopathol, 31: 233-252.
Newkirk DK, Benson RW, Howard PC, Churchwell MI, Doerge DR, & Roberts
DW (1998) On-line immunoaffinity capture, coupled with HPLC and
electrospray ionization mass spectrometry, for automated determination
of fumonisins. J Agric Food Chem, 46: 1677-1688.
Nikolova-Karakashian M, Morgan ET, Alexander C, Liotta DC, & Merrill
AH Jr (1997) Bimodal regulation of ceramidase by interleukin-1ß.
J Biol Chem, 272: 18718-18724.
Norred WP, Voss KA, Bacon CW, & Riley RT (1991) Effectiveness of
ammonia treatment in detoxification of fumonisin-contaminated corn.
Food Chem Toxicol, 29: 815-819.
Norred WP, Plattner RD, Vesonder RF, Bacon CW, & Voss KA (1992a)
Effects of selected secondary metabolites of Fusarium moniliforme on
unscheduled synthesis of DNA by rat primary hepatocytes. Food Chem
Toxicol, 30: 233-237.
Norred WP, Wang E, Yoo H, Riley RT, & Merrill AH Jr (1992b) In
vitro toxicology of fumonisins and the mechanistic implications.
Mycopathologia, 117: 73-78.
Norred WP, Plattner RD, & Chamberlain WJ (1993) Distribution and
excretion of [14C]fumonisin B1 in male Sprague-Dawley rats. Nat
Toxins, 1: 341-346.
Norred WP, Riley RT, Meredith FI, Bacon CW, & Voss KA (1996) Time- and
dose-response effects of the mycotoxin, fumonisin B1 on sphingoid
base elevation in precision-cut rat liver and kidney slices. Toxicol
In Vitro, 10: 349-358.
Norred WP, Plattner RD, Dombrink-Kurtzman MA, Meredith FI, & Riley RT
(1997) Mycotoxin-induced elevation of free sphingoid bases in
precision-cut rat liver slices: specificity of the response and
structure-activity relationships. Toxicol Appl Pharmacol, 147: 63-70.
Ochor TE, Trevathan LE, & King SB (1987) Relationship of harvest date
and host genotype to infection of maize kernels by Fusarium
moniliforme. Plant Dis, 71: 311-313.
Odvody GN, Remmers JC, & Spencer NM (1990) Association of kernel
splitting with kernel and ear rots of corn in a commercial hybrid
grown in the coastal bend of Texas. Phytopathology, 80: 1045.
Ostr V & Ruprich J (1998) Determination of the mycotoxin fumonisins
in gluten-free diet (corn-based commodities) in the Czech Republic.
Cent Eur J Public Health, 6: 57-60.
Osweiler GD, Ross PF, Wilson TM, Nelson PE, Witte ST, Carson TL, Rice
LG, & Nelson HA (1992) Characterization of an epizootic of pulmonary
edema in swine associated with fumonisin in corn screenings. J Vet
Diagn Invest, 4: 53-59.
Osweiler GD, Kehrli ME, Stabel JR, Thruston JR, Ross PF, & Wilson TM
(1993) Effects of fumonisin-contaminated corn screenings on growth and
health of feeder calves. J Anim Sci, 71: 459-466.
Park DL, Rua SM Jr, Mirocha CJ, Abd-Alla EAM, & Weng CY (1992)
Mutagenic potentials of fumonisin contaminated corn following ammonia
decontamination procedure. Mycopathologia, 117: 105-108.
Pascale M, Doko MB, & Visconti A (1995) [Determination of fumonisins
in polenta by high performance liquid chromatography.] In:
[Proceedings of the 2nd National Congress on Food Chemistry,
Giardini-Naxos, 24-27 May 1995.] Messina, Italy, La Grafica
Editoriale, pp 1067-1071 (in Italian).
Pascale M, Visconti A, Proñczuk M, Winiewska H, & Chelkowki J (1997)
Accumulation of fumonisins in maize hybrids inoculated under field
conditions with Fusarium moniliforme Sheldon. J Sci Food Agric, 74:
1-6.
Patel S, Hazel CM, Winterton AGM, & Mortby E (1996) Survey of ethnic
foods for mycotoxins. Food Addit Contam, 13: 833-841.
Patel S, Hazel CM, Winterton AGM, & Gleadle AE (1997) Surveillance of
fumonisins in UK maize-based foods and other cereals. Food Addit
Contam, 14: 187-191.
Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, & Honjo T (1997)
Inhibition of carnitine palmitoyltransferase I augments sphingolipid
synthesis and palmitate-induced apoptosis. J Biol Chem, 272:
3324-3329.
Penner JD, Casteel SW, Pittman L Jr, Rottinghaus GE, & Wyatt RD (1998)
Developmental toxicity of purified fumonisin B1 in pregnant Syrian
hamsters. J Appl Toxicol, 18 :197-203.
Perez GI, Knudson CM, Leykin L, Korseyer SJ, & Tilley JL (1997)
Apoptosis-associated signaling pathways are required for
chemotherapy-mediated female germ cell destruction. Nat Med, 3:
1228-1232.
Perry DK & Hannun YA (1998) The role of ceramide in cell signaling.
Biochi Biophys Acta, 1436: 233-243.
Pestka JJ, Azcona-Olivera JI, Plattner RD, Minervini F, Doko MB, &
Visconti A (1994) Comparative assessment of fumonisin in grain-based
foods by ELISA, GC-MS and HPLC. J Food Prot, 57: 169-172.
Piñeiro MS, Silva GE, Scott PM, Lawrence GA, & Stack ME (1997)
Fumonisin levels in Uruguayan corn products. J Assoc Off Anal Chem
Int, 80: 825-828.
Pitt JI, Hocking AD, Bhudhasamai K, Miscambe BF, Wheeler KA, &
Tanboon-Ek P (1993) The normal mycoflora of commodities from Thailand:
1. Nuts and oilseeds. Int J Food Microbiol, 20: 211-226.
Pittet A, Parisod V, & Schellenberg M (1992) Occurrence of fumonisins
B1 and B2 in corn-based products from the Swiss market. J Agric Food
Chem, 40: 1352-1354.
Plattner RD (1995) Detection of fumonisins produced in Fusarium
moniliforme cultures by HPLC with electrospray MS and evaporative
light scattering detectors. Nat Toxins, 3: 294-298.
Plattner RD & Branham BE (1994) Labeled fumonisins: Production and use
of fumonisin B1 containing stable isotopes. J Assoc Off Anal Chem
Int, 77: 525-532.
Plattner RD & Nelson PE (1994) Production of beauvericin by a strain
of Fusarium proliferatum isolated from corn fodder for swine. Appl
Environ Microbiol, 60: 3894-3896.
Plattner RD & Shackelford DD (1992) Biosynthesis of labeled fumonisins
in liquid cultures of Fusarium moniliforme. Mycopathologia, 117:
17-22.
Plattner RD, Norred WP, Bacon CW, Voss KW, Peterspn R, Shackelford DD,
& Weisleder D (1990) A method of detection of fumonisins in corn
samples associated with field cases of equine leukoencephalomalacia.
Mycologia, 82: 698-702.
Plattner RD, Weisleder D, Shackelford DD, Peterson R, & Powell RG
(1992) A new fumonisin from solid cultures of Fusarium moniliforme.
Mycopathologia, 117: 23-28.
Powell DC, Bursian SJ, Bush CR, Render JA, Rottinghaus GE, & Aulerich
RJ (1996) Effects of dietary exposure to fumonisins from Fusarium
moniliforme culture material (M1325) on the reproductive performance
of female mink. Arch Environ Contam Toxicol, 31: 286-292.
Prathapkumar SH, Rao VS, Paramkishan RJ, & Bhat RV (1997) Disease
outbreak in laying hens arising from the consumption of
fumonisin-contaminated food. Br Poult Sci, 38: 475-479.
Prelusky DB, Trenholm, HL, & Savard ME (1994) Pharmacokinetic fate of
14C-labelled fumonisin B1 in swine. Nat Toxins, 2: 73-80.
Prelusky DB, Savard ME, & Trenholm HL (1995) Pilot study on the plasma
pharmacokinetics of fumonisin B1 in cows following a single dose by
oral gavage or intravenous administration. Nat Toxins, 3: 389-394.
Prelusky DB, Trenholm HL, Rotter BA, Miller JD, Savard ME, Yeung JM, &
Scott PM (1996a) Biological fate of fumonisin B1 in food-producing
animals. Adv Exp Med Biol, 392: 265-278.
Prelusky DB, Miller JD, & Trenholm HL (1996b) Disposition of
14C-derived residues in tissues of pigs fed radiolabelled fumonisin
B1. Food Addit Contam, 13: 155-162.
Price WD, Lovell RA, & McChesney DG (1993) Naturally occurring toxins
in feedstuffs: Center for Veterinary Medicine perspective. J Anim Sci,
71: 2556-2562.
Qureshi MA & Hagler WM Jr (1992) Effect of fumonisin-B1 exposure on
chicken macrophage function in vitro. Poult Sci, 71: 104-112.
Radin NS (1994) Glucosylceramide in the nervous system: a mini review.
Neurochem Res, 19: 533-540.
Ramasamy S, Wang E, Hennig B, & Merrill AH Jr (1995) Fumonisin B1
alters sphingolipid metabolism and disrupts the barrier function of
endothelial cells in culture. Toxicol Appl Pharmacol, 133: 343-348.
Ramirez ML, Pascale M, Chulze S, Reynoso MM, March G, & Visconti A
(1996) Natural occurrence of fumonisins and their correlation to
Fusarium contamination in commercial corn hybrids growth in
Argentina. Mycopathologia, 135: 29-34.
Ramljak D, Diwan BA, Ramakrishna G, Victor TC, Marasas WFO, &
Gelderblom WCA (1996) Overexpression of cyclin D1 is an early event
in, and possible mechanism responsible for, fumonisin B1 liver
tumorigenesis in rats. Proc Am Assoc Cancer Res, 38: 495.
Rapior S, Miller JD, Savard ME, & Apsimon JW (1993) Production
in vitro de fumonisines et de fusarines par des souches européennes
de Fusarium moniliforme. Microbiol Alim Nutr, 11: 327-333.
Reddy RV, Johnson G, Rottinghaus GE, Casteel SW, & Reddy CS (1996)
Developmental effects of fumonisin B1 in mice. Mycopathologia, 134:
161-166.
Restum JC, Bursian SJ, Millerick M, Render JA, Merrill AH Jr, Wang E,
Rottinghaus GE, & Aulerich RJ (1995) Chronic toxicity of fumonisins
from Fusarium moniliforme culture material (M-1325) to mink. Arch
Environ Contam Toxicol, 29: 545-550.
Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, Shephard GS, & van
Schalkwyk DJ (1992) Fusarium moniliforme and fumonisins in corn in
relation to human esophageal cancer in Transkei. Phytopathology, 82:
353-357.
Rheeder JP, Marasas WFO, Farina MPW, Thompson GR, & Nelson PE (1994)
Soil fertility factors in relation to oesophageal cancer risk areas in
Transkei, Southern Africa. Eur J Cancer Prev, 3: 49-56.
Rice LG & Ross PF (1994) Methods for detection and quantitation of
fumonisins in corn, cereal products and animal excreta. J Food Prot,
57: 536-540.
Richard JL, Meerdink G, Maragos CM, Tumbleson M, Bordson G, Rice LG, &
Ross PF (1996) Absence of detectable fumonisins in the milk of cows
fed Fusarium proliferatum (Matsushima) Nirenberg culture material.
Mycopathologia, 133: 123-126.
Riley RT & Yoo H-S (1995) Time and dose relationship between the
cellular effects of fumonisin B1 (FB1) and the uptake and
accumulation of [14C]FB1 in LLC-PK1 cells. Toxicologist, 15(1):
290.
Riley RT, An N-H, Showker JL, Yoo H-S, Norred WP, Chamberlain WJ, Wang
E, Merrill AH Jr, Motelin G, Beasley VR, & Haschek WM (1993)
Alteration of tissue and serum sphinganine to sphingosine ratio: An
early biomarker of exposure to fumonisin-containing feeds in pigs.
Toxicol Appl Pharmacol, 118: 105-112.
Riley RT, Hinton DM, Chamberlain WJ, Bacon CW, Wang E, Merrill AH Jr,
& Voss KA (1994a) Dietary fumonisin B1 induces disruption of
sphingolipid metabolism in Sprague-Dawley rats: A new mechanism of
nephrotoxicity. J Nutr, 124: 594-603.
Riley RT, Voss KA, Yoo H-S, Gelderblom WCA, & Merrill AH Jr (1994b)
Mechanism of fumonisin toxicity and carcinogenesis. J Food Prot, 57:
638-645.
Riley RT, Wang E, & Merrill AH Jr (1994c) Liquid chromatographic
determination of sphinganine and sphingosine: Use of the free
sphinganine-to-sphingosine ratio as a biomarker for consumption of
fumonisins. J Assoc Off Anal Chem Int, 77: 533-540.
Riley RT, Wang E, Schroeder JJ, Smith ER, Plattner RD, Abbas H, Yoo
H-S, & Merrill AH Jr (1996) Evidence for disruption of sphingolipid
metabolism as a contributing factor in the toxicity and
carcinogenicity of fumonisins. Nat Toxins, 4: 3-15.
Riley RT, Showker JL, Owens DL, & Ross PF (1997) Disruption of
sphingolipid metabolism and induction of equine leukoencephalomalacia
by Fusarium proliferatum culture material containing fumonisin B1
or B3. Environ Toxicol Pharmacol, 3: 221-228.
Riley RT, Voss KA, Norred WP, Wang E, & Merrill AH Jr (1998)
Fumonisins: mechanism of mycotoxicity. Rev Méd Vét, 149: 617-626.
Riley RT, Voss KA, Norred WP, Bacon CW, Meredith FI, & Sharma RP
(1999) Serine palmitoyltransferase inhibition reverses
anti-proliferative effects of ceramide synthase inhibition in cultured
renal cells and suppresses free sphingoid base accumulation in kidney
of BALBc mice. Environ Toxicol Pharmacol, 7: 109-118.
Ross PF (1994) What are we going to do with this dead horse? J Assoc
Off Anal Chem Int, 77: 491-494.
Ross PF, Nelson PE, Richard JL, Osweiler GD, Rice LG, Plattner RD, &
Wilson TM (1990) Production of fumonisins by Fusarium moniliforme
and Fusarium proliferatum isolates associated with equine
leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl
Environ Microbiol, 56: 3225-3226.
Ross PF, Rice LG, Plattner RD, Osweiler GD, Wilson TM, Owens DL,
Nelson HA, & Richard JL (1991a) Concentrations of fumonisin B1 in
feeds associated with animal health problems. Mycopathologia, 114:
129-135.
Ross PF, Rice LG, Reagor JC, Osweiler GD, Wilson TM, Nelson HA, Owens
DL, Plattner RD, Harlin KA, Richard JL, Colvin BM, & Banton MI (1991b)
Fumonisin B1 concentrations in feeds from 45 confirmed equine
leukoencephalomalacia cases. J Vet Diagn Invest, 3: 238-241.
Ross PF, Rice LG, Osweiler GD, Nelson PE, Richard JL, & Wilson TM
(1992) A review and update of animal toxicoses associated with
fumonisin-contaminated feeds and production of fumonisins by
Fusarium isolates. Mycopathologia, 117: 109-114.
Ross PF, Ledet AE, Owens DL, Rice LG, Nelson HA, Osweiler GD, & Wilson
TM (1993) Experimental equine leukoencephalomalacia, toxic hepatosis,
and encephalopathy caused by corn naturally contaminated with
fumonisins. J Vet Diagn Invest, 5: 69-74.
Ross PF, Nelson PE, Owens DL, Rice LG, Nelson HA, & Wilson TM (1994)
Fumonisin B2 in cultured Fusarium proliferatum, M-6104, causes
equine leukoencephalomalacia. J Vet Diagn Invest, 6: 263-265.
Rother J, Van Echten G, Schwarzmann G, & Sandhoff K (1992)
Biosynthesis of sphingolipids: Dihydroceramide and not sphinganine is
desaturated by cultured cells. Biochem Biophys Res Commun, 189: 14-20.
Rotter BA, & Oh Y-N (1996) Mycotoxin fumonisin B1 stimulates nitric
oxide production in a murine macrophage cell line. Nat Toxins, 4:
291-294.
Rotter BA, Thompson BK, Prelusky DB, Trenholm HL, Stewart B, Miller
JD, & Savard ME (1996) Response of growing swine to dietary exposure
pure fumonisin B1 during an eight-week period: Growth and clinical
parameters. Nat Toxins, 4: 42-50.
Rottinghaus GE, Coatney CE, & Minor HC (1992) A rapid, sensitive thin
layer chromatography procedure for the detection of fumonisin B1 and
B2. J Vet Diagn Invest, 4: 326-329.
Rumbeiha WK & Oehme FW (1997) Fumonisin exposure to Kansans through
consumption of corn-based market foods. Vet Hum Toxicol, 39: 220-225.
Sahu SC, Eppley RM, Page SW, Gray GC, Barton CN, & O'Donnell MW (1998)
Peroxidation of membrane lipids and oxidative DNA damage by fumonisin
B1 in isolated rat liver nuclei. Cancer Lett, 125: 117-121.
Sakata K, Sakata A, Vela-Roch N, Espinosa R, Escalante A, Kong L,
Nakabayashi T, Cheng J, Talal N, & Dang H (1998) Fas (CD95)-transduced
signal preferentially stimulates lupus peripheral T lymphocytes. Eur J
Immunol, 28: 2648-2660.
Sanchis V, Abadias M, Oncins L, Sala N, Viñas I, & Canela R (1994)
Occurrence of fumonisins B1 and B 2 in corn-based products from the
Spanish market. Appl Environ Microbiol, 60: 2147-2148.
Sanchis V, Abadias M, Oncins L, Sala N, Viñas I, & Canela R (1995)
Fumonisins B1 and B2 and toxigenic Fusarium strains in feeds from
the Spanish market. Intl J Food Microbiol, 27: 37-44.
Sandvig K, Garred O, Van Helvoort A, Van Meer G, & Van Deurs B (1996)
Importance of glycolipid synthesis for butryic acid-induced
sensitization to Shiga toxin and intracellular sorting of toxin in
A431 cells. Mol Biol Cell, 7: 1391-1404.
Sauviat MP, Laurent D, Kohler F, & Pellegrin F (1991) Fumonisin, a
toxin from the fungus Fusarium moniliforme Sheld, blocks both the
calcium current and the mechanical activity in frog atrial muscle.
Toxicon, 29: 1025-1031.
Savard ME & Blackwell BA (1994) Spectral characteristics of secondary
metabolites from Fusarium fungi. In: Miller JD & Trenholm HL ed.
Mycotoxins in grain: Compounds other than aflatoxin. St. Paul,
Minnesota, Eagan Press, pp 59-260.
Schaafsma AW, Miller JD, Savard ME, & Ewing RJ (1993) Ear rot
development and mycotoxin production in corn in relation to
inoculation method, corn hybrid, and species of Fusarium. Can J
Plant Pathol, 15: 185-192.
Schmelz EM, Dombrink-Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T,
& Merrill AH Jr (1998) Induction of apoptosis by fumonisin B1 in HT29
cells is mediated by the accumulation of endogenous free sphingoid
bases. Toxicol Appl Pharmacol, 148: 252-260.
Schroeder JJ, Crane HM, Xia J, Liotta DC, & Merrill AH Jr (1994)
Disruption of sphingolipid metabolism and stimulation of DNA synthesis
by fumonisin B1: A molecular mechanism for carcinogenesis associated
with Fusarium moniliforme. J Biol Chem, 269: 3475-3481.
Schwarz A, Rapaport E, Hirschberg K, & Futerman H (1995). A regulatory
role for sphingolipids in neuronal growth: Inhibition of sphingolipid
synthesis and degradation have opposite effects on axonal branching. J
Biol Chem, 270: 10990-10998.
Scott PM (1993) Fumonisins. Int J Food Microbiol, 18: 257-270.
Scott PM & Lawrence GA (1992) Liquid chromatographic determination of
fumonisins with 4-fluoro-7-nitrobenzofurazan. J Assoc Off Anal Chem
Int, 75: 829-834.
Scott PM & Lawrence GA (1994) Stability and problems in recovery of
fumonisins added to corn-based foods. J Assoc Off Anal Chem Int, 77:
541-545.
Scott PM & Lawrence GA (1995) Analysis of beer for fumonisins. J Food
Prot, 58: 1379-1382.
Scott PM & Lawrence GA (1996) Determination of hydrolysed fumonisin
B1 in alkali-processed corn foods. Food Addit Contam, 13: 823-832.
Scott PM & Trucksess MW (1997) Application of immunoaffinity columns
to mycotoxin analysis. J Assoc Off Anal Chem Int, 80: 941-949.
Scott PM, Delgado T, Prelusky DB, Trenholm HL, & Miller JD (1994)
Determination of fumonisins in milk. J Environ Sci Health, B29:
989-998.
Scott PM, Kanhere SR, Lawrence GA, Daley EF, & Farber JM (1995)
Fermentation of wort containing added ochratoxin A and fumonisins B1
and B2. Food Addit Contam, 12: 31-40.
Scott PM, Yeung JM, Lawrence GA, & Prelusky DB (1997) Evaluation of
enzyme-linked immunosorbent assay for analysis of beer for fumonisins.
Food Addit Contam, 14: 445-450.
Scudamore KA & Chan HK (1993) Occurrence of fumonisin mycotoxins in
maize and millet imported into the United Kingdom. In: Scudamore K ed.
Occurrence and significance of mycotoxins. Slough, United Kingdom,
Ministry of Agriculture, Fisheries and Food, pp 186-189.
Scudamore KA, Nawaz S, & Hetmanski MT (1998) Mycotoxins in ingredients
of animal feeding stuffs: II. Determination of mycotoxins in maize and
maize products. Food Addit Contam, 15: 30-55.
Sharma RP, Dugyala RR, & Voss KA (1997) Demonstration of in-situ
apoptosis in mouse liver and kidney after short-term repeated exposure
to fumonisin B1. J Comp Pathol, 117: 371-381.
Shelby RA, Rottinghaus GE, & Minor HC (1994a) Comparison of thin-layer
chromatography and competitive immunoassay methods for detecting
fumonisin on maize. J Agric Food Chem, 42: 2064-2067.
Shelby RA, White DG, & Bauske EM (1994b) Differential fumonisin
production in maize hybrids. Plant Dis, 78: 582-584.
Shephard GS (1998) Chromatographic determination of the fumonisin
mycotoxins. J Chromatogr, A815: 31-39.
Shephard GS, Sydenham EW, Thiel PG, & Gelderblom WCA (1990)
Quantitative determination of fumonisins B1 and B2 by
high-performance liquid chromatography with fluorescence detection.
J Liq Chromatogr, 13: 2077-2087.
Shephard GS, Thiel PG, & Sydenham EW (1992a) Initial studies on the
toxicokinetics of fumonisin B1 in rats. Food Chem Toxicol, 30:
277-279.
Shephard GS, Thiel PG, Sydenham EW, Alberts JF, & Gelderblom WCA
(1992b) Fate of a single dose of the 14C-labelled mycotoxin fumonisin
B1, in rats. Toxicon, 30: 768-770.
Shephard GS, Thiel PG, & Sydenham EW (1992c) Determination of
fumonisin B1 in plasma and urine by high-performance liquid
chromatography. J Chromatogr, 574: 299-304.
Shephard GS, Thiel PG, Sydenham EW, Alberts JF, & Cawood ME (1994a)
Distribution and excretion of a single dose of the mycotoxin fumonisin
B1 in a non-human primate. Toxicon, 32: 735-741.
Shephard GS, Thiel PG, Sydenham EW, Vleggaar R, & Alberts JF (1994b)
Determination of the mycotoxin fumonisin B1 and identification of its
partially hydrolysed metabolites in the faeces of non-human primates.
Food Chem Toxicol, 32: 23-29.
Shephard GS, Thiel PG, Sydenham EW, & Alberts JF (1994c) Biliary
excretion of the mycotoxin fumonisin B1 in rats. Food Chem Toxicol,
32: 489-491.
Shephard GS, Thiel PG, Sydenham EW, & Savard ME (1995a) Fate of a
single dose of 14C-labelled fumonisin B1 in vervet monkeys. Nat
Toxins, 3: 145-150.
Shephard GS, Thiel PG, Sydenham EW, & Snijman PW (1995b)
Toxicokinetics of the mycotoxin fumonisin B2 in rats. Food Chem
Toxicol, 33: 591-595.
Shephard GS, Thiel PG, & Sydenham EW (1995c) Liquid chromatographic
determination of the mycotoxin fumonisin B2 in physiological samples.
J Chromatogr, A692: 39-43.
Shephard GS, Thiel PG, Stockenström S, & Sydenham EW (1996a) Worldwide
survey of fumonisin contamination of corn and corn-based products.
J Assoc Off Anal Chem Int, 79: 671-687.
Shephard GS, van der Westhuizen L, Thiel PG, Gelderblom WCA, Marasas
WFO, & Van Schalkwyk DJ (1996b) Disruption of sphingolipid metabolism
in non-human primates consuming diets of fumonisin-containing
Fusarium moniliforme culture material. Toxicon, 34: 527-534.
Shetty PH & Bhat RV (1997) Natural occurrence of fumonisin B1 and its
co-occurrence with aflatoxin B1 in Indian sorghum, maize and poultry
feeds. J Agric Food Chem, 45: 2170-2173.
Shetty PH & Bhat RV (1998) Sensitive method for the detection of
fumonisin B1 in human urine. J Chromatogr, B705: 171-173.
Sheu CW, Rodriguez I, Eppley RM, & Lee JK (1996) Lack of transforming
activity of fumonisin B1 in BALB/3T3 A31-1-1 mouse embryo cells. Food
Chem Toxicol, 34: 751-753.
Shimabukuro M, Zhou Y-T, Levi M, & Unger RH (1998) Fatty acid-induced
ß cell apoptosis: a link between obesity and diabetes. Proc Natl Acad
Sci (USA), 95: 2498-2502.
Shurtleff MC (1980) Compendium of corn diseases. St. Paul, Minnesota,
American Phytopathological Society, p 105.
Smith ER & Merrill AH Jr (1995) Differential roles of de novo
sphingolipid biosynthesis and turnover in the "burst" of free
sphingosine and sphinganine, and their 1-phosphates and
N-acyl-derivatives, that occurs upon changing the medium of cells in
culture. J Biol Chem, 270: 18749-18758.
Smith JS & Thakur RA (1996) Occurrence and fate of fumonisins in beef.
Adv Exp Med Biol, 392: 39-55.
Smith GW, Constable PD, Bacon CW, Meredith FI, & Haschek WM (1996a)
Cardiovascular effects of fumonisins in swine. Fundam Appl Toxicol,
31: 169-172.
Smith GW, Constable PD, & Haschek WM (1996b) Cardiovascular responses
to short-term fumonisin exposure in swine. Fundam Appl Toxicol, 33:
140-148.
Smith GW, Constable PD, Smith AR, Bacon CW, Meredith FI, Wollenberg
GK, & Haschek WM (1996c) Effects of fumonisin-containing culture
material on pulmonary clearance in swine. Am J Vet Res, 57: 1233-1238.
Smith ER, Jones PL, Boss JM, & Merrill AH Jr (1997) Changing J774A.1
cells to new medium perturbs multiple signaling pathways, including
the modulation of protein kinase C by endogenous sphingoid bases.
J Biol Chem, 272: 5640-5646.
Solfrizzo M, Avantaggiato G, & Visconti A (1997a) Rapid method to
determine sphinganine/sphingosine in human and animal urine as a
biomarker for fumonisin exposure. J Chromatogr Biomed Appl, 692:
87-93.
Solfrizzo M, Avantaggiato G, & Visconti A (1997b) In vivo validation
of the sphinganine/sphingosine ratio as a biomarker to display
fumonisin ingestion. Cereal Res Commun, 25: 437-441.
Spiegel S (1999) Sphingosin 1-phosphate: a prototype of a new class of
second messengers. J Leukoc Biol, 65: 341-344.
Stack ME (1998) Analysis of fumonisin B1 and its hydrolysis product
in tortillas. J Assoc Off Anal Chem Int, 81: 737-740.
Stack ME & Eppley RM (1992) Liquid chromatographic determination of
fumonisins B1 and B2 in corn and corn products. J Assoc Off Anal
Chem Int, 75: 834-837.
Stevens VL & Tang J (1997) Fumonisin B1-induced sphingolipid
depletion inhibits vitamin uptake via the
glycosylphosphatidylinositol-anchored folate receptor. J Biol Chem,
272: 18020-18025.
Stevens VL, Nimkar S, Jamison WC, Liotta DC, & Merrill AH Jr (1990)
Characteristics of the growth inhibition and cytotoxicity of
long-chain (sphingoid) bases for Chinese hamster ovary cells: Evidence
for an involvement of protein kinase C. Biochim Biophys Acta, 1051:
37-45.
Stockenström S, Sydenham EW, & Thiel PG (1994) Determination of
fumonisins in corn: Evaluation of two purification procedures.
Mycotoxin Res, 10: 9-14.
Strum JC, Swenson KI, Turner JE, & Bell RM (1995) Ceramide triggers
meiotic cell cycle progression in Xenopus oocytes. A potential
mediator of progesterone-induced maturation. J Biol Chem, 270:
13541-13547.
Sun TSC & Stahr HM (1993) Evaluation and application of a
bioluminescent bacterial genotoxicity test. J Assoc Off Anal Chem Int,
76: 893-898.
Suzuki CAM, Hierlihy L, Barker M, Curran I, Mueller R, & Bondy GS
(1995) The effects of fumonisins on several markers of nephrotoxicity
in rats. Toxicol Appl Pharmacol, 133: 207-214.
Suzuki A, Iwasaki M, Kato M, & Wagai N (1997) Sequential operation of
ceramide synthesis and ICE cascade in CPT-11-initiated apoptotic death
signaling. Exp Cell Res, 233: 41-47.
Sweeley CC (1991) Sphingolipids. In: Vance DE & Vance JE ed.
Biochemistry of lipids, lipoproteins and membranes. Amsterdam,
Elsevier Science Publishers, pp 327-361.
Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S-I,
& Igarashi Y (1996) Sphingosine and its methylated derivative
N,N-methylsphingosine (DMS) induce apoptosis in a variety of human
cancer cell lines. Int J Cancer, 66: 358-366.
Swiss Federal Office of Public Health (1997a) Révision d'ordonnance
sur les substances étrangères et les composants OSEC (RS 817.021.23).
Bern, Swiss Federal Office of Public Health, 2 pp (Circular No. 11).
Swiss Federal Office of Public Health (1997b) Liquid chromatographic
determination of fumonisins B1, B2, and B3 in foods and feeds. J
Assoc Off Anal Chem Int, 75: 313-318.
Sydenham EW (1994) Fumonisins: Chromatographic methodology and their
role in human and animal health. Cape Town, South Africa, University
of Cape Town (Ph.D. Thesis).
Sydenham EW & Shephard GS (1996) Chromatographic and allied methods of
analysis for selected mycotoxins. In: Gilbert J ed. Progress in food
contaminant analysis. London, Blackie, pp 65-146.
Sydenham EW, Gelderblom WCA, Thiel PG, & Marasas WFO (1990a) Evidence
for the natural occurrence of fumonisin B1, a mycotoxin produced by
Fusarium moniliforme, in corn. J Agric Food Chem, 38: 285-290.
Sydenham EW, Thiel PG, Marasas WFO, Shephard GS, Van Schalkwyk DJ, &
Koch KR (1990b) Natural occurrence of some Fusarium mycotoxins in
corn from low and high esophageal cancer prevalence areas of the
Transkei, Southern Africa. J Agric Food Chem, 38: 1900-1903.
Sydenham EW, Shephard GS, Thiel PG, Marasas WFO, & Stockenström S
(1991) Fumonisin contamination of commercial corn-based human
foodstuffs. J Agric Food Chem, 39: 2014-2018.
Sydenham EW, Marasas WFO, Shephard GS, Thiel PG, & Hirooka EY (1992)
Fumonisin concentrations in Brazilian feeds associated with field
outbreaks of confirmed and suspected animal mycotoxicoses. J Agric
Food Chem, 40: 994-997.
Sydenham EW, Shepard GS, Gelderblom WCA, Thiel PG, & Marasas WFO
(1993a) Fumonisins: their implications for human and animal health.
In: Scudamore K ed. Proceedings of the UK Workshop on Occurrence and
Significance of Mycotoxins. Slough, United Kingdom, Ministry of
Agriculture, Fisheries and Food, Central Science Laboratory, pp 42-48.
Sydenham EW, Shephard GS, Thiel PG, Marasas WFO, Rheeder JP, Sanhueza
CEP, González HHL, & Resnick SL (1993b) Fumonisins in Argentinian
field-trial corn. J Agric Food Chem, 41: 891-895.
Sydenham EW, Stockenström S, Thiel PG, Shephard GS, Koch KR, & Marasas
WFO (1995) Potential of alkaline hydrolysis for the removal of
fumonisins from contaminated corn. J Agric Food Chem, 43: 1198-1201.
Sydenham EW, Shephard GS, Thiel PG, Stockenström S, Snijman PW, & Van
Schalkwyk DJ (1996) Liquid chromatographic determination of fumonisins
B1, B2, and B3 in corn: AOAC-IUPAC collaborative study. J Assoc Off
Anal Chem Int, 79: 688-696.
Sydenham EW, Shephard GS, Stockenström S, Rheeder JP, Marasas WFO, &
van der Merwe MJ (1997) Production of fumonisin B analogues and
related compounds by Fusarium globosum, a newly described species
from corn. J Agric Food Chem, 45: 4004-4010.
Tejada-Simon MV, Marovatsanga LT, & Pestka JJ (1995) Comparative
detection of fumonisin by HPLC, ELISA, and immunocytochemical
localization in Fusarium cultures. J Food Prot, 58: 666-672.
Thiel PG, Marasas WFO, Sydenham EW, Shephard GS, Gelderblom WCA, &
Nieuwenhuis JJ (1991a) Survey of fumonisin production by Fusarium
species. Appl Environ Microbiol, 57: 1089-1093.
Thiel PG, Shephard GS, Sydenham EW, Marasas WFO, Nelson PE, & Wilson
TM (1991b) Levels of fumonisins B1 and B2 in feeds associated with
confirmed cases of equine leukoencephalomalacia. J Agric Food Chem,
39: 109-111.
Thiel PG, Marasas WFO, Sydenham EW, Shephard GS, & Gelderblom WCA
(1992) The implications of naturally occurring levels of fumonisins in
corn for human and animal health. Mycopathologia, 117: 3-9.
Thiel PG, Sydenham EW, Shephard GS, & Van Schalkwyk DJ (1993) Study of
the reproducibility characteristics of a liquid chromatographic method
for the determination of fumonisins B1 and B2 in corn: IUPAC
collaborative study. J Assoc Off Anal Chem Int, 76: 361-366.
Tolleson WH, Dooley KL, Sheldon WG, Thurman JD, Bucci TJ, & Howard PC
(1996a) The mycotoxin fumonisin induces apoptosis in cultured human
cells and in livers and kidneys of rats. Adv Exp Med Biol, 392:
237-250.
Tolleson WH, Melchior WB Jr, Morris SM, McGarrity LJ, Domon OE,
Muskhelishvili L, James SJ, & Howard PC (1996b) Apoptotic and
antiproliferative effects of fumonisin B1 in human keratinocytes,
fibroblasts, esophageal epithelial cells and hepatoma cells.
Carcinogenesis, 17: 239-249.
Tolleson WH, Couch LH, Melchior WB Jr, Jenkins GR, Muskhelishvili M,
Muskhelishvili L, McGarrity LJ, Domon O, Morris SM, & Howard PC (1999)
Fumonisin B1 induces apoptosis in cultured human keratinocytes
through sphinganine accumulation and ceramide depletion. Int J Oncol,
14: 833-843.
Torres MR, Sanchis V, & Ramos AJ (1998) Occurrence of fumonisins in
Spanish beers analyzed by an enzyme-linked immunosorbent assay method.
Int J Food Microbiol, 39: 139-143.
Trucksess MW, Stack ME, Allen S, & Barrion N (1995) Immunoaffinity
column coupled with liquid chromatography for determination of
fumonisin B1 in canned and frozen sweet corn. J Assoc Off Anal Chem
Int, 78: 705-710.
Tryphonas H, Bondy G, Miller JD, Lacroix F, Hodgen M, McGuire P,
Fernie S, Miller D, & Hayward S (1997) Effects of fumonisin B1 on the
immune system of Sprague-Dawley rats following a 14-day oral (gavage)
exposure. Fundam Appl Toxicol, 39: 53-59.
Tseng T-C & Liu C-Y (1997) Natural occurrence of fumonisins in
corn-based foodstuffs in Taiwan. Cereal Res Commun, 25: 393-394.
Tsunoda M, Sharma RP, & Riley RT (1998) Early fumonisin B1 toxicity
in relation to disrupted sphingolipid metabolism in male BALB/c mice.
J Biochem Mol Toxicol, 12: 281-289.
Ueda N, Kaushal GP, Hong X, & Shah SV (1998) Role of enhanced ceramide
generation in DNA damage and cell death in chemical hypoxic injury to
LLC-PK1 cells. Kidney Int, 54: 399-406.
Ueno Y, Aoyama S, Sugiura Y, Wang D-S, Lee U-S, Hirooka EY, Hara S,
Karki T, Chen G, & Yu S-Z (1993) A limited survey of fumonisins in
corn and corn-based products in Asian countries. Mycotoxin Res, 9:
27-34.
Ueno Y, Umemori K, Niimi E, Tanuma S, Nagata S, Sugamata M, Ihara T,
Sekijima M, Kawai K, Ueno I, & Tashiro F (1995) Induction of apoptosis
by T-2 toxin and other natural toxins in HL-60 human promyelotic
leukemia cells. Nat Toxins, 3: 129-137.
Ueno Y, Iijima K, Wang S-D, Sugiura Y, Sekijima M, Tanaka T, Chen C, &
Yu S-Z (1997) Fumonisins as a possible contributory risk factor for
primary liver cancer: A 3-year study of corn harvested in Haimen,
China by HPLC and ELISA. Food Chem Toxicol, 35: 1143-1150.
Usleber E, Straka M, & Terplan G (1994a) Enzyme immunoassay for
fumonisin B1 applied to corn-based food. J Agric Food Chem, 42:
1392-1396.
Usleber E, Schlichtherle C, Märtlbauer E (1994b) DMZ. Mag Food Dairy
Ind, 115: 1220-1227.
US NTP (1999) NTP technical report on the toxicology and
carcinogenesis studies of fumonisin B1 (CAS No. 116355-83-0) in
F344/N rats and B6C3F1 mice (feed studies). Research Triangle Park,
North Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP TR 496; NIH Publication No. 99-3955).
Van Asch MAJ, Rijkenberg FHJ, & Coutinho TA (1992) Phytotoxicity of
fumonisin B1, moniliformin, and T-2 toxin to corn callus cultures.
Phytopathology, 82: 1330-1332.
Van der Westhuizen L, Shephard GS, Snyman SD, Abel S, Swanevelder S, &
Gelderblom WCA (1998) Inhibition of sphingolipid biosynthesis in rat
primary hepatocyte cultures by fumonisin B1 and other structurally
related compounds. Food Chem Toxicol, 36: 497-503.
Van Veldhoven PP & Mannaerts GP (1993) Sphingosine-phosphate lyase.
In: Bell RM, Hannun YA, & Merrill AH Jr ed. Advances in lipid
research: Sphingolipids and their metabolites. Orlando, Florida,
Academic Press, vol 26, pp 69-98.
Velázquez C, van Bloemendal C, Sanchis V, & Canela R (1995) Derivation
of fumonisins B1 and B2 with 6-aminoquinolyl
N-hydroxysuccinimidylcarbamate. J Agric Food Chem, 43: 1535-1537.
Vesonder RF & Wu W (1998) Correlation of moniliformin, but not
fumonisin B1 levels, in culture materials of Fusarium isolates to
acute death in ducklings. Poult Sci, 77: 67-72.
Vesonder RF, Labeda DP, & Peterson RE (1992) Phytotoxic activity of
selected water-soluble metabolites of Fusarium against Lemma
minor L. (Duckweed). Mycopathologia, 118: 185-189.
Viljoen JH, Marasas WFO, & Thiel PG (1994) Fungal infection and
mycotoxic contamination of commercial maize. In: Du Plessis JG, Van
Rensberg JBJ, McLaren NW, & Flett BC ed. Proceedings of the Tenth
South African Maize Breeding Symposium. Potchefstroom, South Africa,
Department of Agriculture, pp 26-37.
Visconti A (1996) Fumonisins in maize genotypes grown in various
geographic areas. Adv Exp Med Biol, 392: 193-204.
Visconti A & Boenke A (1995) Improvement of the determination of
fumonisins (FB1 and FB2) in maize and maize based feeds. Brussels,
European Commission, BCR Information, 101 pp (Report EUR 16617 EN).
Visconti A & Doko MB (1994) Survey of fumonisin production by
Fusarium isolated from cereals in Europe. J Assoc Off Anal Chem Int,
77: 546-550.
Visconti A, Doko MB, Schurer B, & Boenke A (1993) Intercomparison
study for the analysis of fumonisins B1 and B2 in an unknown
solution. In. Scudamore K ed. Proceedings of the UK Workshop on
Occurrence and Significance of Mycotoxins. Slough, United Kingdom,
Ministry of Agriculture, Fisheries and Food, Central Science
Laboratory, pp 200-202.
Visconti A, Doko MB, Bottalico C, Schurer B, & Boenke A (1994)
Stability of fumonisins (FB1 and FB2) in solution. Food Addit
Contam, 11: 427-431.
Visconti A, Boenke A, Doko MB, Solfrizzo M, & Pascale M (1995)
Occurrence of fumonisins in Europe and the BCR-measurements and
testing projects. Nat Toxins, 3: 269-274.
Voss KA, Plattner RD, Bacon CW, & Norred WP (1990) Comparative studies
of hepatotoxicity and fumonisin B1 and B2 content of water and
chloroform/methanol extracts of Fusarium moniliforme strain MRC 826
culture material. Mycopathologia, 112: 81-92.
Voss KA, Chamberlain WJ, Bacon CW, & Norred WP (1993) A preliminary
investigation on renal and hepatic toxicity in rats fed purified
fumonisin B1. Nat Toxins, 1: 222-228.
Voss KA, Chamberlain WJ, Bacon CW, Herbert RA, Walters DB, & Norred WP
(1995) Subchronic feeding study of the mycotoxin fumonisin B1 in
B6C3F1 mice and Fischer 344 rats. Fundam Appl Toxicol, 24: 102-110.
Voss KA, Bacon CW, Norred WP, Chapin RE, Chamberlain WJ, Plattner RD,
& Meredith FI (1996a) Studies on the reproductive effects of
Fusarium moniliforme culture material in rats and the
biodistribution of [14C]fumonisin B1 in pregnant rats. Nat Toxins,
4: 24-33.
Voss KA, Riley RT, Bacon CW, Chamberlain WJ, & Norred WP (1996b)
Subchronic toxic effects of Fusarium moniliforme and fumonisin B1
in rats and mice. Nat Toxins, 4: 16-23.
Voss KA, Bacon CW, Meredith FI, & Norred WP (1996c) Comparative
subchronic toxicity studies of nixtamalized and water-extracted
Fusarium moniliforme culture material. Food Chem Toxicol, 34:
623-632.
Voss KA, Riley RT, Bacon CW, Meredith FI, & Norred WP (1998) Toxicity
and sphinganine levels are correlated in rats fed fumonisin B1 (FB1)
or hydrolyzed FB1. Environ Toxicol Pharmacol, 5: 101-104.
Vudathala DK, Prelusky DB, Ayroud M, Trenholm HL, & Miller JD (1994)
Pharmacokinetic fate and pathological effects of 14C-fumonisin B1 in
laying hens. Nat Toxins, 2: 81-88.
Wang D-S, Sugiura Y, Ueno Y, Buddhanont P, & Suttajit M (1993) A
limited survey for the natural occurrence of fumonisins in Thailand.
Thai J Toxicol, 9: 42-46.
Wang E, Norred WP, Bacon CW, Riley RT, & Merrill AH Jr (1991)
Inhibition of sphingolipid biosynthesis by fumonisins: implications
for diseases associated with Fusarium moniliforme. J Biol Chem, 266:
14486-14490.
Wang E, Ross PF, Wilson TM, Riley RT, & Merrill AH Jr (1992) Increases
in serum sphingosine and sphinganine and decreases in complex
sphingolipids in ponies given feed containing fumonisins, mycotoxins
produced by Fusarium moniliforme. J Nutr, 122: 1706-1716.
Wang D-S, Liang Y-X, Chau NT, Dien LD, Tanaka T, & Ueno Y (1995)
Natural co-occurrence of Fusarium toxins and aflatoxin B1 in corn
for feed in North Vietnam. Nat Toxins, 3: 445-449.
Wang H, Jones C, Ciacci-Zanella J, Holt T, Gilchrist DG, & Dickman MB
(1996) Fumonisins and Alternaria alternata lycopersici toxins:
Sphinganine analog mycotoxins induce apoptosis in monkey kidney cells.
Proc Natl Acad Sci (USA), 93: 3461-3465.
Wang E, Riley RT, Meredith FI, & Merrill AH Jr (1999) Fumonisin B1
consumption by rats causes reversible, dose-dependent increases in
urinary sphinganine and sphingosine. J Nutr, 129: 214-220.
Ware GM, Francis O, Kuan SS, Umrigar P, Carman A, Carter L, & Bennett
GA (1993) Determination of fumonisin B1 in corn by high performance
liquid chromatography with fluorescence detection. Anal Lett, 26:
1751-1770.
Ware GM, Umrigar PP, Carman AS Jr, & Kuan SS (1994) Evaluation of
fumonitest immunoaffinity columns. Anal Lett, 27: 693-715.
Wattenberg EV, Badria FA, & Shier WT (1996) Activation of
mitogen-activated protein kinase by the carcinogenic mycotoxin
fumonisin B1. Biochem Biophys Res Commun, 227: 622-627.
Weibking TS, Ledoux DR, Brown TP, & Rottinghaus GE (1993a) Fumonisin
toxicity in turkey poults. J Vet Diagn Invest, 5: 75-83.
Weibking TS, Ledoux DR, Bermudez AJ, Turk JR, Rottinghaus GE, Wang E,
& Merrill AH Jr (1993b) Effects of feeding Fusarium moniliforme
culture material, containing known levels of fumonisin B1, on the
young broiler chick. Poult Sci, 72: 456-466.
Weibking T, Ledoux DR, Bermudez AJ, Turk JR, & Rottinghaus GE (1995)
Effects on turkey poults of feeding Fusarium moniliforme M-1325
culture material grown under different environmental conditions. Avian
Dis, 39: 32-38.
Wieder T, Orfanos CE, & Geilen CC (1998) Induction of
ceramide-mediated apoptosis by the anticancer phospholipid analog,
hexadecylphosphocholine. J Biol Chem, 273: 11025-11031.
Wild CP, Castegnaro M, Ohgaki H, Garren L, Galendo D, & Miller JD
(1997) Absence of a synergistic effect between fumonisin B1 and
N-nitrosomethylbenzylamine in the induction of oesophageal papillomas
in the rat. Nat Toxins, 5: 126-131.
Wilson BJ & Maronpot RR (1971) Causative fungus agent of
leukoencephalomalacia in equine animals. Vet Rec, 88: 484-486.
Wilson TM, Nelson PE, & Knepp CR (1985) Hepatic neoplastic nodules,
adenofibrosis, and cholangiocarcinomas in male Fisher 344 rats fed
corn naturally contaminated with Fusarium moniliforme.
Carcinogenesis, 6: 1155-1160.
Wilson TM, Ross PF, Rice LG, Osweiler GD, Nelson HA, Owens DL,
Plattner RD, Reggiardo C, Noon TH, & Pickrell JW (1990) Fumonisin B1
levels associated with an epizootic of equine leukoencephalomalacia.
J Vet Diagn Invest, 2: 213-216.
Wilson TM, Ross PF, Owens DL, Rice LG, Green SA, Jenkins SJ, & Nelson
HA (1992) Experimental reproduction of ELEM-A study to determine the
minimum toxic dose in ponies. Mycopathologia, 117: 115-120.
Witty JP, Bridgham JT, & Johnson AL (1996) Induction of apoptotic cell
death in hen granulosa cells by ceramide. Endocrinology,
137: 5269-5277.
Wu W-I, McDonough VM, Nickels JT, Ko SJ, Fischl AS, Vales TR, Merrill
AH Jr, & Carman GM (1995) Regulation of lipid biosynthesis in
Saccharomyces cerevisiae by fumonisin B1. J Biol Chem,
270: 13171-13178.
Xu J, Yeh C-H, Chen S, He L, Sensi SL, Canzoniero LMT, Choi DW, & Hsu
CY (1998) Involvement of de novo ceramide biosynthesis in tumor
necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell
death. J Biol Chem, 273: 16521-16526.
Yamashita A, Yoshizawa T, Aiura Y, Sanchez P, Dizon EI, Arim RH, &
Sardjono (1995) Fusarium mycotoxins (fumonisins, nivalenol and
zearalenone) and aflatoxins in corn from southeast Asia. Biosci
Biotechnol Biochem, 59: 1804-1807.
Yeung JM, Wang H-Y, & Prelusky DB (1996) Fumonisin B1 induces protein
kinase C translocation via direct interaction with diacylglycerol
binding site. Toxicol Appl Pharmacol, 141: 178-184.
Yin J-J, Smith MJ, Eppley RM, Troy AL, Page SW, & Sphon JA (1996)
Effects of fumonisin B1 and (hydrolyzed) fumonisin backbone AP1 on
membranes: A spin-label study. Arch Biochem Biophys, 335: 13-22.
Yin J-J, Smith MJ, Eppley RM, Page SW, & Sphon JA (1998) Effects of
fumonisin B1 on lipid peroxidation in membranes. Biochim Biophys Acta,
1371: 134-142.
Yoo H-S, Norred WP, Wang E, Merrill AH Jr, & Riley RT (1992) Fumonisin
inhibition of de novo sphingolipid biosynthesis and cytotoxicity are
correlated in LLC-PK1 cells. Toxicol Appl Pharmacol, 114: 9-15.
Yoo H-S, Norred WP, Showker JL, & Riley RT (1996) Elevated sphingoid
bases and complex sphingolipid depletion as contributing factors in
fumonisin-induced cytotoxicity. Toxicol Appl Pharmacol, 138: 211-218.
Yoshizawa T, Yamashita A, & Luo Y (1994) Fumonisin occurrence in corn
from high- and low-risk areas for human esophageal cancer in China.
Appl Environ Microbiol, 60: 1626-1629.
Young JC & Lafontaine P (1993) Detection and characterization of
fumonisin mycotoxins as their methyl esters by liquid
chromatography/particle-beam mass spectrometry. Rapid Commun Mass
Spectrom, 7: 352-359.
Zacharias C, Van Echten-Deckert G, Wang E, Merrill AH Jr, & Sandoff K
(1996) The effect of fumonisin B1 on developing chick embryos:
Correlation between de novo sphingolipid biosynthesis and gross
morphological changes. Glycoconj J, 13: 167-175.
Zhang H, Buckley NE, Gibson K, & Spiegel S (1990) Sphingosine
stimulates cellular proliferation via a protein kinase C-independent
pathway. J Biol Chem, 265: 76-81.
Zhang H, Desai NN, Olivera A, Seki T, Booker G, & Spiegel S (1991)
Sphingosine-1-phosphate, a novel lipid, involved in cellular
proliferation. J Cell Biol, 114: 155-167.
Zhang H, Nagashima H, & Goto T (1997) Natural occurrence of mycotoxins
in corn, samples from high and low risk areas for human esophageal
cancer in China. Mycotoxins, 44: 29-35.
Zhen YZ (1984) [The culture and isolation of fungi from the cereals in
five high and three low incidence counties of esophageal cancer in
Henan Province.] J Chin Tumor, 6: 27-29 (in Chinese).
Zoller O, Sager F, & Zimmerli B (1994) [Occurrence of fumonisins in
food.] Mitt Gebiete Lebensm Hyg, 85: 81-99 (in German).
APPENDIX 1. NATIONAL GUIDELINES FOR FUMONISINS
The US Food and Drug Administration, Center for Veterinary
Medicine unofficial guidelines (Miller et al., 1996) recommend that
the non-roughage portion of feeds for equine species should be less
than 5 ppm FB1 (< 5 mg/kg), for porcine species the total diet
should contain less than 10 ppm FB1 (< 10 mg/kg), for beef cattle
the non-roughage portion should be less than 50 ppm FB1
(< 50 mg/kg), and for poultry the complete feed should contain less
than 50 ppm FB1 (< 50 mg/kg).
An official tolerance value for dry maize products (1 mg/kg FB1
plus FB2) has been issued in Switzerland (Swiss Federal Office of
Public Health, 1997).
APPENDIX 2. NATURAL OCCURRENCE OF FUMONISIN B1 (FB1)
IN MAIZE-BASED PRODUCTS
Product Country Positive/ FB1 Reference
total (mg/kg)
North America
maize Canada 1/1 0.08 Stack & Eppley
(1992)
maize Canada 9/98 <1-2.5 Miller et al. (1995)
maize flour Canada 1/2 0.05 Sydenham et al.
(1991)
maize feed USA 3/3 37-122 Wilson et al. (1990)
maize feed USA 2/2 12-130 Plattner et al. (1990)
maize feed USA 81/93 <1-126 Ross et al. (1991a)
maize feed USA 158/213 <1-330 Ross et al. (1991b)
maize feed USA 15/15 1.3-5.2 Thiel et al. (1991b)
maize feed USA 2/2 105-155 Colvin & Harrison
(1992)
maize feed USA 29/29 3-330 Osweiler et al. (1992)
maize feed USA 20/21 <1-73 a Bane et al. (1992)
maize USA 6/6 1.7-196.5 Stack & Eppley
screenings (1992)
(feed)
maize feed USA 1/1 86.0 Park et al. (1992)
maize feed USA 14/14 1.3-27.0 Sydenham et al.
(1992)
maize feed USA 160/160 0.1-239 Murphy et al. (1993)
maize feed USA 85/85 2.6-32 a Price et al. (1993)
maize feed USA 5/5 0.22-1.41 Hopmans & Murphy
(1993)
maize feed USA 0/29 - Chamberlain et al.
(1993)
maize feed USA 0/1 - Holcomb et al. (1993)
maize feed USA 5/5 0.77-6.2 Rumbeiha & Oehme
(1997)
maize USA 6/6 0.14-16.31 Stack & Eppley (1992)
maize USA 13/99 1.2-3.2 Price et al. (1993)
maize USA 155/175 < 1-37.9 Murphy et al. (1993)
maize USA 24/28 av. 0.87 Chamberlain et al.
(1993)
maize USA 116/322 1-> 10 Shelby et al. (1994a)
maize flour USA 13/25 0.05-0.35 Rumbeiha & Oehme
(1997)
maize flour USA 7/7 0.40-6.32 Pestka et al. (1994)
maize flour USA 15/16 0.05-2.79 Sydenham et al.
(1991)
maize flour USA 16/16 0.28-2.05 Stack & Eppley
(1992)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flour USA 6/6 0.21-0.84 Hopmans & Murphy
(1993)
maize grits USA 10/10 0.11-2.55 Sydenham et al.
(1991)
maize grits USA 5/5 0.14-0.27 Stack & Eppley
(1992)
maize flakes USA 0/2 - Sydenham et al.
(1991)
maize flakes USA 2/5 0.01 Stack & Eppley
(1992)
maize cereals USA 7/12 0.06-0.33 Stack & Eppley
(bran, fibre, (1992)
pops)
popcorn USA 1/18 0.07 Rumbeiha & Oehme
(1997)
sweet maize USA 1/1 0.026 Hopmans & Murphy
(1993)
sweet maize USA 37/97 0.004-0.35 Trucksess et al.
(1995)
tortillas USA 1/3 0.05-0.06 Sydenham et al.
(1991)
miscellaneous USA 4/4 0.09-0.70 Sydenham et al.
maize foods b (1991)
miscellaneous USA 6/11 0.01-0.12 Stack & Eppley
maize foods b (1992)
miscellaneous USA 4/4 0.02-0.32 Hopmans & Murphy
maize foods b (1993)
miscellaneous USA 3/5 0.05-1.21 Pestka et al. (1994)
maize foods b
Latin America
maize Argentina 17/17 1.11-6.70 Sydenham et al.
(1993b)
maize Argentina 51/51 0.18-27.05 Visconti et al.
(1995); Ramirez et
al. (1996)
maize feed Brazil 20/21 0.2-38.5 Sydenham et al.
(1992)
maize Brazil 47/48 0.6-18.5 Hirooka et al. (1996)
tortillas Texas-Mexico 50/52 av. 0.19 Stack (1998)
border
masas Texas-Mexico 8/8 av. 0.26 Stack (1998)
border
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flour Peru 1/2 0.66 Sydenham et al.
(1991)
alkali-treated Peru 0/2 - Sydenham et al.
kernels (1991)
maize feed Uruguay 13/13 0.26-6.3 Piñeiro et al. (1997)
maize Uruguay 11/22 0.17-3.7 Piñeiro et al. (1997)
maize snacks Uruguay 4/10 0.15-0.31 Piñeiro et al. (1997)
frozen maize Uruguay 1/7 0.16 Piñeiro et al. (1997)
polenta Uruguay 3/12 0.1-0.43 Piñeiro et al. (1997)
maize flour Venezuela 1/1 0.07 Stack & Eppley
(1992)
Europe
maize Austria 6/9 1-15 Lew et al. (1991)
maize flour Austria -/3 f 0.05-1.15 Sydenham et al.
(1993a)
maize flour Bulgaria -/15 f 0.05-0.21 Sydenham et al.
(1993a)
maize Croatia 11/19 0.01-0.06 Doko et al. (1995)
maize flour Czech 22/22 0.01-0.49 a Ostry & Ruprich
(1998)
maize pastes Czech 6/11 0.01-0.51 a Ostry & Ruprich
(1998)
maize-extruded Czech 30/35 0.01-1.8 a Ostry & Ruprich
bread (1998)
polenta Czech 6/7 0.01-1.2 a Ostry & Ruprich
(1998)
porridge Czech 18/19 0.01-0.79 a Ostry & Ruprich
(1998)
maize feed France 43/58 0.02-8.82 Doko et al. (1994)
maize feed France 35/35 0.02-2.17 Dragoni et al. (1996)
maize flour France 1/1 1.24 Sydenham et al.
(1993a)
miscellaneous France 10/22 0.02-1.50 Visconti et al. (1995)
maize foods b
maize Germany 49/458 0.007-4.83 Meister et al. (1996)
imported maize Germany 21/21 0.014-1.11 a Meister et al. (1996)
maize grits Germany 1/2 0.01 Usleber et al. (1994a)
grits flour, Germany 60/71 0.01-16.00 Usleber et al.
semolina (1994b) c
semolina Germany 10/11 0.01-1.23 Usleber et al. (1994a)
popcorn Germany 13/29 0.01-0.16 Usleber et al.
(1994b) c
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
popcorn Germany 4/6 0.01-0.11 Usleber et al. (1994a)
infant foods Germany 0/91 - Usleber et al.
(1994b) c
maize Hungary 56/92 0.05-75.10 Fazekas et al. (1998)
maize feed Italy 23/25 0.02-8.40 Minervini et al. (1992)
maize screen Italy 3/3 55.2-70.0 Caramelli et al. (1993)
(feed)
maize feed Italy 1/1 60 Doko & Visconti
(1994)
maize Italy 7/7 0.1-5.3 Doko & Visconti
(1994)
maize Italy 6/6 125-250 Bottalico et al. (1995)
maize Italy 26/26 0.01-2.33 Doko & Visconti
genotypes (1994)
commercial Italy 7/7 0.10-5.31 Doko & Visconti
maize kernels (1994)
maize flour Italy 7/7 0.42-3.73 Doko & Visconti
(1994)
maize grits Italy 1/1 3.76 Doko & Visconti
(1994)
polenta Italy 20/20 0.15-3.76 Pascale et al. (1995)
popcorn, Italy 6/10 0.01-0.06 Doko & Visconti
maize flakes, (1994)
tortilla chips
extruded maize Italy 6/6 0.79-6.10 Doko & Visconti
(1994)
sweet maize Italy 5/5 0.06-0.79 Doko & Visconti
(1994)
maize flour Netherlands 5/7 0.008-0.09 de Nijs et al. (1998c)
maize for bread Netherlands 8/19 0.008-0.38 de Nijs et al. (1998c)
maize for Netherlands 2/10 0.008-0.11 de Nijs et al. (1998c)
popcorn
maize foods Netherlands 12/42 0.008-1.43 de Nijs et al. (1998c)
imported maize Netherlands 61/62 0.03-3.35 de Nijs et al. (1998b)
maize Poland 2/7 0.01-0.02 Doko et al. (1995)
maize Portugal 9/9 0.09-2.30 Doko et al. (1995)
maize Romania 3/6 0.01-0.02 Doko et al. (1995)
maize feed Spain 136/171 av. 3.3 Castella et al. (1997)
maize kernels Spain 1/1 0.72 Visconti et al. (1995)
maize flour Spain 1/3 0.05-0.07 Sanchis et al. (1994)
maize flour Spain 16/17 < 0.50 a Burdaspal & Legarda
(1996)
maize grits Spain 3/15 0.05-0.09 Sanchis et al. (1994)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize flakes Spain 2/12 0.05-0.10 Sanchis et al. (1994)
maize starch Spain 1/13 0.03 Burdaspal & Legarda
(1996)
sweet maize Spain 3/3 0.72 Visconti et al. (1995)
miscellaneous Spain 2/20 0.05-0.20 Sanchis et al. (1994)
maize foods b
maize flour Sweden 1/1 0.13 Visconti et al. (1995)
popcorn Sweden 1/1 0.13 Visconti et al. (1995)
maize feed Switzerland 6/22 av. 0.24 Pittet et al. (1992)
maize flour Switzerland 2/7 av. 0.09 Pittet et al. (1992)
maize grits d Switzerland 34/55 av. 0.26 Pittet et al. (1992)
maize grits, Switzerland 27/27 0.01-2.20 Zoller et al. (1994)
flour d
maize flakes Switzerland 1/12 0.06 Pittet et al. (1992)
popcorn Switzerland 8/13 0.005-0.25 Zoller et al. (1994)
sweet maize Switzerland 1/7 0.07 Pittet et al. (1992)
miscellaneous Switzerland 0/17 - Pittet et al. (1992)
maize foods b
maize feed UK 24/29 0.05-4.55 Scudamore & Chan
(1993)
maize UK 65/67 0.03-24 Scudamore et al.
(1998)
polenta UK 16/20 0.02-2.12 a Patel et al. (1997)
maize snacks UK 31/40 0.01-0.22 a Patel et al. (1997)
popcorn UK 6/22 0.01-0.78 a Patel et al. (1997)
Africa
maize Benin 9/11 0.02-2.63 Doko et al. (1995)
maize flour Botswana 5/5 0.18-0.45 Sydenham et al.
(1993a)
miscellaneous Botswana 6/6 0.03-0.35 Doko et al. (1996)
maize foods b
maize flour Egypt 2/2 1.78-2.98 Sydenham et al.
(1991)
maize kernels Kenya 1/1 0.78 Doko et al. (1996)
maize flour Kenya -/3 f 0.05-0.11 Sydenham et al.
(1993a)
maize kernels Malawi 7/8 0.02-0.11 Doko et al. (1996)
maize kernels Mozambique 3/3 0.24-0.29 Doko et al. (1996)
maize feed South Africa 15/15 0.47-4.34 Viljoen et al. (1994)
mixed feed South Africa 1/1 8.85 Shephard et al. (1990)
maize South Africa 3/3 10-83 Sydenham et al.
(1990a)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize South Africa 60/60 0.2-46.9 Sydenham et al.
(1990b)
maize South Africae 62/74 0.05-117.5 Rheeder et al. (1992)
maize South Africa 24/68 0.05-0.87 Sydenham (1994) c
maize flour South Africa 46/52 0.05-0.48 Sydenham et al.
(1991)
maize flour South Africa 2/2 0.06-0.07 Doko et al. (1996)
maize grits South Africa 10/18 0.05-0.19 Sydenham et al.
(1991)
maize flakes South Africa 0/3 - Sydenham et al.
(1991)
miscellaneous South Africa 2/8 0.05-0.09 Sydenham et al.
maize foods b (1991)
maize kernels Tanzania 8/9 0.02-0.16 Doko et al. (1996)
maize kernels Uganda 1/1 0.60 Doko et al. (1996)
maize Zambia 20/20 0.02-1.42 Doko et al. (1995)
maize flour Zambia 1/1 0.74 Doko et al. (1996)
maize kernels Zimbabwe 1/2 0.12 Doko et al. (1996)
maize flour Zimbabwe 3/3 1.06-3.63 Sydenham et al.
(1993a)
maize flour Zimbabwe 4/4 0.05-1.91 Doko et al. (1996)
Asia
maize China 2/5 5.3-8.4 Ueno et al. (1993)
maize China 18/47 0.18-2.9 Yoshizawa et al.
(1994)
maize China 27/68 0.01-1.4 Kang et al. (1994)
maize China e 34/34 18-155 Chu & Li (1994)
maize China e 134/240 0.08-34.87 Ueno et al. (1997)
maize China e 37/54 0.08-21 Gao & Yoshizawa
(1997)
maize flour China 0/3 - Sydenham et al.
(1993a)
maize flour China 3/4 0.06-0.2 Ueno et al. (1993)
gluten India 1/1 0.7 Scudamore & Chan
(1993)
maize Indonesia 7/12 0.05-1.8 Yamashita et al.
(1995)
maize Indonesia 16/16 0.05-2.44 Ali et al. (1998)
maize grits Japan 14/17 0.20-2.60 Ueno et al. (1993)
sweet maize Japan 0/8 - Ueno et al. (1993)
maize snack Japan 0/31 - Ueno et al. (1993)
maize soup Japan 0/7 - Ueno et al. (1993)
APPENDIX 2. (contiued)
Product Country Positive/ FB1 Reference
total (mg/kg)
maize feed Korea 5/12 0.05-1.33 Lee et al. (1994)
maize Nepal 12/24 0.05-4.6 Ueno et al. (1993)
maize Philippines 26/50 0.05-1.8 Yamashita et al.
(1995)
maize Philippines 9/10 0.3-10.0 Bryden et al. (1996)
miscellaneous Taiwan 52/153 0.07-2.39 Tseng & Liu (1997)
maize foods b
maize Thailand 19/27 0.06-18.8 Yamashita et al.
(1995)
maize feed Thailand 5/22 0.05-1.59 Wang et al. (1993)
maize flour Thailand 6/6 0.48-0.88 Wang et al. (1993)
maize grits Thailand 5/5 0.25-1.82 Wang et al. (1993)
maize Vietnam 12/12 0.3-9.1 Bryden et al. (1996)
maize Vietnam 8/15 0.27-3.45 Wang et al. (1995)
maize powder Vietnam 15/17 0.27-1.52 Wang et al. (1995)
Oceania
maize Australia 67/70 0.3-40.6 Bryden et al. (1996)
maize flour New Zealand 0/12 - Sydenham et al.
(1993a)
a Fumonisins B1 + B2 + B3
b Includes maize snacks, canned maize, frozen maize, extruded maize, bread,
maize-extruded bread, biscuit, cereals, chips, flakes, pastes, starch, sweet
maize, infant foods, gruel, purée, noodles popcorn, porridge, tortillas,
tortilla chips, masas, popped maize, soup, taco, tostada
c From: Shephard et al. (1996a)
d Maize grits and flour samples analysed were imported cereals (mainly from
Argentina)
e Fumonisin levels in low- and high-risk area for human oesophageal cancer
f The number of positive samples was not indicated in the original report
RESUME, EVALUATION ET RECOMMANDATIONS
1. Résumé
1.1 Identité, propriétés physique et chimiques et méthodes
d'analyse
La fumonisine B1 (FB1), de formule brute C34H59NO15, est le
diester de l'acide propane-1,2,3-tricarboxylique et du
2-amino-12,16-diméthyl-3,5,10,14,15-pentahydroxyeicosane (masse
moléculaire relative: 721). C'est la plus abondante des fumonisines,
qui constituent une famille de toxines dont on a identifié au moins 15
membres. A l'état pur, ce composé se présente sous la forme d'une
poudre hygroscopique de couleur blanche, soluble dans l'eau, le
mélange eau-acétonitrile et le méthanol. Elle est stable dans le
mélange eau-acétonitrile à 1:1 et instable dans le méthanol. Elle est
stable aux températures utilisées pour la transformation des denrées
alimentaires ainsi qu'à la lumière.
Plusieurs méthodes d'analyse ont été proposées, notamment la
chromatographie sur couche mince ou en phase liquide, la spectrométrie
de masse, la chromatographie en phase gazeuse après hydrolyse et un
certain nombre de méthodes immunochimiques. En fait, la plupart des
dosages se font par chromatographie en phase liquide d'un dérivé
fluorescent.
1.2 Sources d'exposition humaine
La FB1 est produite par plusieurs espèces de Fusarium, mais
essentiellement par Fusarium verticillioides (Sacc.) Nirenberg
(= Fusarium moniloforme Sheldon) qui est l'un des parasites
cryptogamiques les plus fréquents du maïs. La FB1 peut s'accumuler en
quantités importantes dans le maïs lorsque les conditions
météorologiques sont favorables à l'apparition de la fusariose.
1.3 Transport, distribution et transformation dans l'environnement
On est fondé à penser que les fumonisines peuvent être
métabolisées par certains microorganismes terricoles. On sait
toutefois peu de choses de leur devenir dans l'environnement une fois
qu'elles ont été excrétées ou que les produits qui en contiennent ont
été transformés.
1.4 Concentrations dans l'environnement et exposition humaine
On a mis en évidence de la FB1 dans le maïs et les produits qui
en dérivent partout dans le monde à des concentrations de l'ordre du
mg/kg, parfois en association avec d'autres mycotoxines. Des
concentrations de cet ordre ont également été observées dans des
denrées alimentaires destinées à la consommation humaine. Lors de la
mouture à sec du maïs, la fumonisine se répartit dans le son, le germe
et la farine. Lors d'essais de mouture par voie humide, on a mis en
évidence la toxine dans l'eau de macération, dans le gluten, dans les
fibres et les germes, à l'exclusion de l'amidon. La FB1 reste stable
dans le maïs et la polenta, mais elle s'hydrolyse dans les aliments à
base de maïs traités par des solutions alcalines à chaud.
La FB1 est absente du lait, de la viande et des oeufs provenant
d'animaux nourris avec du maïs dont la teneur en toxine ne représente
aucun danger pour eux. On estime que l'exposition humaine journalière
aux Etats-Unis, au Canada, en Suisse, aux Pays-Bas et au Transkei
(Afrique du Sud) varie entre 0,017 et 440 µg/kg de poids corporel. On
ne possède aucune donnée sur l'exposition professionnelle par
inhalation.
1.5 Cinétique et métabolisme chez l'animal
On ne dispose d'aucune donnée sur la cinétique ou le métabolisme
de la FB1 chez l'Homme. Chez les animaux de laboratoire, le composé
est peu résorbé après ingestion; il disparaît rapidement du courant
sanguin et se retrouve inchangé dans les matières fécales. Il est
excrété en quantité importante par la voie biliaire et en faible
proportion dans les urines. Chez les primates non humains et certains
ruminants, la fumonisine peut subir une dégradation hydrolytique
partielle dans l'intestin. Elle subsiste en petite quantité dans le
foie et les reins.
1.6 Effets sur l'animal et les systèmes d'épreuve in vitro
La FB1 s'est révélée hépatotoxique pour les espèces animales sur
lesquelles elle a été testée, notamment le rat, la souris, les
équidés, le lapin, le porc et les primates non humains. Sauf dans le
cas du hamster doré, on n'observe d'effets embryotoxiques ou
tératogènes que concurremment ou postérieurement aux manifestations
toxiques qui se produisent chez la mère. Les fumonisines sont
néphrotoxiques chez le porc, le rat, le mouton, la souris et le lapin.
Dans le cas du rat et du lapin, la néphrotoxicité se manifeste à des
doses plus faibles que l'hépatotoxicité. On sait que la
leucoencéphalomalacie équine et l'oedème pulmonaire porcin observés
après consommation de provendes à base de maïs sont dus à la présence
de fumonisines. Les données dont on dispose sur les propriétés
immunologiques de la FB1 sont limitées. A la dose de 50 mg/kg de
nourriture, le composé a provoqué des cancers du foie chez une souche
de rats et des cancers du rein chez une autre souche; dans les mêmes
conditions de dosage et d'administration, il a également provoqué des
cancers du foie chez des souris femelles. Il semble qu'il existe une
corrélation entre les effets toxiques sur les organes et l'apparition
de cancers. La FB1 a été le premier inhibiteur du métabolisme des
sphingolipides qui ait été découvert et on l'utilise beaucoup depuis
lors pour étudier le rôle des sphingolipides dans la régulation
cellulaire. La FB1 inhibe la croissance cellulaire; elle entraîne
l'accumulation de bases sphingoïdes libres et modifie le métabolisme
des lipides chez les animaux, les plantes et certaines levures. Elle
ne provoque pas de mutation génique chez les bactéries et mise en
présence d'une culture primaire d'hépatocytes de rat, elle n'entraîne
pas une synthèse non programmée de l'ADN. On a constaté par contre
qu'elle pouvait provoquer des aberrations chromosomiques à faible dose
dans ces mêmes cultures cellulaires.
1.7 Effets sur l'Homme
On ne dispose d'aucune donnée confirmée relative à la toxicité
aiguë des fumosinines pour l'Homme. Des études effectuées au Transkei
(Afrique du Sud) sur la corrélation entre divers effets toxiques et la
présence de fumonisines dans la ration alimentaire suggèrent
l'existence d'un lien entre ces composés et le cancer de l'oesophage.
Cette corrélation a été observée dans des circonstances où il y avait
une forte exposition aux fumonisines et où les conditions
environnementales étaient favorables à une importante accumulation de
toxines dans le maïs, qui constitue une denrée alimentaire de base
dans la région. Des études du même genre ont également été effectuées
en Chine. Ces dernières n'ont toutefois pas permis de dégager une
relation claire entre la contamination par les fumonisines ou
F. verticillioides et le cancer de l'oesophage. Fautes de données
concernant l'exposition aux fumonisines, il n'est pas possible non
plus de tirer de conclusions d'une étude cas-témoins effectuée en
Italie sur des sujets de sexe masculin et qui révèle l'existence d'une
association entre la consommation de maïs et les cancers des voies
digestives supérieures chez les gros buveurs.
Il n'existe pas de marqueurs biologiques valables de l'exposition
à la FB1.
1.8 Effets sur les autres organismes en laboratoire
La FB1 inhibe la croissance cellulaire et provoque
l'accumulation de bases sphingoïdes libres ainsi que la modification
du métabolisme des lipides chez Saccharomyces cerevisiae.
La FB1 est phytotoxique, elle provoque des lésions de la
membrane cellulaire et réduit la synthèse chlorophyllienne. Elle
perturbe également la biosynthèse des sphingolipides chez les végétaux
et pourrait jouer un rôle dans les maladies du maïs dues aux espèces
de Fusarium qui produisent des fumonisines
2. Evaluation des risques pour la santé humaine
2.1 Exposition
L'Homme est exposé partout dans le monde puisque la FB1 est
présente dans le maïs destiné à la consommation humaine. Toutefois,
des différences notables existent entre les régions du culture de
cette céréale. Cette constatation s'impose lorsque l'on compare pays
développés et pays en développement. Par exemple, même si aux
Etats-Unis, au Canada et en Europe occidentale la FB1 est présente
dans les produits tirés du maïs, la consommation de ces produits y
reste à un niveau modeste. Dans certaines régions d'Afrique,
d'Amérique centrale et d'Asie, il y a des populations dont l'apport
calorique alimentaire est constitué pour une large part de farine de
maïs et la contamination par la FB1 peut y être importante (voir
Appendice 2). Le maïs naturellement contaminé par la FB1 peut
également l'être par d'autres toxines de F. verticillioides ou
F. proliferatum ou encore par des toxines d'importance agricole
telles que le désoxynivalénol, la zéaralénone, l'aflatoxine ou
l'ochratoxine.
Les procédés de transformation des denrées alimentaires utilisés
en Amérique du Nord ou en Europe occidentale n'ont aucun effet sur la
stabilité de la FB1. Le traitement du maïs par une base ou son lavage
à l'eau réduisent sensiblement la teneur en toxine. Cependant on
constate toujours des effets hépatotoxiques et néphrotoxiques chez les
animaux de laboratoire. On sait peu de choses sur la manière dont les
techniques de préparation des aliments utilisées dans le monde en
développement peuvent agir sur la FB1 présente dans les produits
tirés du maïs.
2.2 Nature des dangers
Le rôle étiologique de la FB1 dans la leucoencéphalomalacie
équine est établi. D'importantes flambées de cette zoonose mortelle se
sont produites aux Etats-Unis au cours du 19ème siècle et plus
récemment en 1989-1990. De même, on a également montré que cette
toxine était à l'origine d'une zoonose tout aussi mortelle, l'oedème
pulmonaire porcin. Une faible dose de FB1 peut également être
mortelle pour le lapin, comme on a pu l'observer sur des lapines
gravides. Chez toutes les espèces animales étudiées, y compris les
primates non humains, on a constaté que cette toxine provoquait des
lésions rénales et hépatiques. La FB1 provoque une
hypercholestérolémie chez plusieurs espèces animales, dont les
primates non humains. On de bonnes raisons de penser que dans les
maladies animales dues à une exposition à la FB1, il y a modification
du métabolisme des lipides. Les effets toxiques observés in vivo ou
in vitro sont précédés ou accompagnés d'une perturbation du
métabolisme des sphingolipides. L'utilisation des fumonisines dans
l'étude de la fonction des sphingolipides a montré que ces composés
sont nécessaires à la croissance cellulaire et qu'il peuvent affecter
les molécules signal de différentes manières, avec pour conséquence la
mort cellulaire par apoptose ou nécrose, la différenciation cellulaire
ou encore la modification de la réponse immunitaire. Après exposition
à la FB1, on observe communément une modification du métabolisme des
lipides et de l'expression ou de l'activité des enzymes qui jouent un
rôle clé dans la progression du cycle cellulaire. La FB1 n'exerce pas
d'effets toxiques sur le développement chez le rat, la souris ou le
lapin. En revanche, elle est foetotoxique chez le hamster doré à forte
dose même quand elle n'a pas d'effet sur la mère.
Chez les rongeurs, la cancérogénicité de la FB1 varie selon
l'espèce, la souche et le sexe. La seule étude qui ait été effectuée
sur des souris B6C3F1 a montré que la toxine provoquait des cancers
du foie chez les femelles à la dose de 50 mg/kg de nourriture. Des
cancers primitifs du foie et des cholangiomes ont été observés chez
des rats mâles BD IX qui avaient reçu, pendant une durée allant
jusqu'à 26 mois, une alimentation contenant 50 mg/kg de FB1. Chez des
rats mâles F344/N alimentés dans les mêmes conditions de dosage, on a
mis en évidence des adénomes et des carcinomes au niveau des tubules
rénaux. Il semble qu'il y ait corrélation entre la toxicité pour tel
ou tel organe et l'apparition de cancers à ce niveau.
Les études de génotoxicité sont en nombre limité. Celles qui
portent sur des bactéries n'ont pas révélé d'effets mutagènes. Dans
des cultures de cellules mammaliennes on n'a pas non plus décelé de
synthèse non programmée de l'ADN mais selon une étude, la FB1 a
provoqué des cassures chromosomiques dans des hépatocytes de rat.
Selon d'autres travaux, la FB1 accroît la peroxydation des lipides
in vivo et in vitro. Il est possible qu'il existe une relation de
cause à effet entre la peroxydation des lipides et les cassures
chromosomiques.
Les teneurs en FB1 supérieures à 100 mg/kg constatées dans le
maïs consommé par l'Homme en Afrique et en Chine pourraient
probablement provoquer selon le cas, des leucoencéphalomalacies, des
oedèmes pulmonaires et des cancers si on donnait à manger ce maïs à
des chevaux, des porcs, des rats ou des souris. On connaît des cas où
l'exposition humaine à cette toxine est très importante, mais aucun
cas d'intoxication aiguë par une fumonisine n'a été décrit. Les études
de corrélation effectuées au Transkei (Afrique du Sud) incitent à
penser qu'il pourrait y avoir un lien entre une exposition à la
fumonisine par voie alimentaire et le cancer de l'oesophage. On a
effectivement constaté un taux élevé de cancers de l'oesophage là où
l'exposition à cette toxine était importante et où les conditions
environnementales étaient favorables à l'accumulation de fumonisines
dans le maïs, qui constitue un aliment de base dans ces régions.
Une étude cas-témoins réalisée en Italie a mis en évidence une
association entre la consommation de maïs et les cancers des voies
digestives supérieures, et notamment le cancer de l'oesophage chez les
gros buveurs. Aucune donnée sur l'exposition à la toxine n'a été
fournie.
2.3 Relation dose-réponse
La dose la plus faible capable de provoquer l'apparition de
cancers du foie chez l'animal de laboratoire est égale à 50 mg par kg
de nourriture dans le cas de rats mâles BD IX et de souris femelles
B6C3F1/Nctr; aucun cancer n'a été observé aux doses respectives de 25
ou 15 mg/kg de nourriture. Dans chaque cas et avec les mêmes souches
de rats et de souris, on a observé des signes d'hépatotoxicité ou de
modification du métabolisme des lipides à ces mêmes doses ou à des
doses inférieures. La dose la plus faible ayant provoqué des cancers
du rein chez des rats mâles de souche F344/Nctr était égale à 50 mg/kg
de nourriture; aucun effet cancérogène n'a été observé à la dose de 15
mg/kg de nourriture. Ces études ont également montré qu'à des doses
inférieures à celles qui provoquaient l'apparition de cancers, il y
avait apoptose et prolifération des cellules des tubules rénaux, avec
des modifications au niveau des sphingolipides tissulaires et
urinaires.
On ne possède aucune donnée qui puisse permettre d'établir une
relation quantitative entre l'exposition à la FB1 et d'éventuels
effets sur l'organisme humain.
2.4 Caractérisation du risque
La FB1 est cancérogène pour la souris et le rat et alle provoque
des maladies mortelles chez le porc et le cheval à des concentrations
auxquelles l'Homme pourrait être exposé. Le Groupe de travail n'a pas
été en mesure de formuler une estimation quantitative du risque pour
la santé humaine mais il a été d'avis qu'il y avait urgence à cet
égard.
3. Recommandations pour la protection de la santé humaine
a) Il faudrait établir les limites d'exposition par voie
alimentaire. On devra s'attacher en particulier aux populations
dont l'apport calorique provient pour une grande part de la
farine de maïs.
b) Des mesures devraient être prises pour limiter l'exposition aux
fumonisines et la contamination du maïs par ces toxines; elles
pourraient consister
* à changer de culture là où le maïs n'est pas vraiment adapté
* à mettre au point des variétés de maïs qui résistent à la
fusariose
* à mieux gérer les cultures
* à éliminer les grains parasités
c) On veillera à faire prendre conscience suffisamment tôt du risque
de contamination alimentaire en faisant en sorte qu'il y ait une
meilleure communication entre les vétérinaires et les
responsables de la santé publique en cas de flambées de
mycotoxicoses parmi les animaux domestiques.
d) Il faudrait mettre au point une méthode de recherche de la
contamination du maïs par les fumonisines qui soit bon marché,
simple et peu sensible aux conditions d'application.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
La fumonisina B1 (FB1) tiene la fórmula empírica C34H59NO15 y
es el diéster del ácido propano-1,2,3-tricarboxílico y el 2-amino-12,
16-dimetil-3, 5, 10, 14, 15-pentahidroxieicosano (masa molecular
relativa: 721). Es la más frecuente de las fumonisinas, familia de
toxinas con 15 miembros identificados por lo menos. La sustancia pura
es un polvo higroscópico blanco, soluble en agua, acetonitrilo-agua o
metanol, estable en acetonitrilo-agua (1:1), inestable en metanol y
estable a la temperatura de elaboración de los alimentos y la luz.
Se han notificado varios métodos analíticos, en particular la
cromatografía en capa fina y la cromatografía líquida, la
espectrometría de masas, la cromatografía de gases poshidrólisis y
métodos inmunoquímicos, aunque la mayoría de los estudios se han
realizado utilizando análisis por cromatografía líquida de un derivado
fluorescente.
1.2 Fuentes de exposición humana
La FB1 se produce en varias especies de Fusarium,
principalmente Fusarium verticillioides (Sacc.) Niremberg
(= Fusarium moniliforme Sheldon), que es uno de los hongos más
comunes asociados con el maíz en todo el mundo. En el maíz hay una
acumulación importante de FB1 cuando las condiciones climatológicas
favorecen la podredumbre del grano debida a Fusarium.
1.3 Transporte, distribución y transformación en el medio
ambiente
Hay pruebas de que algunos microorganismos del suelo pueden
metabolizar las fumonisinas. Sin embargo, se sabe poco acerca del
destino de las fumonisinas en el medio ambiente tras la excreción o la
transformación.
1.4 Niveles en el medio ambiente y exposición humana
Se ha detectado FB1 en el maíz y sus productos en todo el mundo
en concentraciones de varios mg/kg, a veces combinada con otras
micotoxinas. Se han notificado también concentraciones de mg/kg en
alimentos para consumo humano. Como consecuencia de la elaboración en
seco del maíz, la fumonisina se distribuye en el salvado, los gérmenes
y la harina. En la elaboración en húmedo experimental, se detectó
fumonisina en el agua de remojo, el glúten, la fibra y los gérmenes,
pero no en el almidón. La FB1 es estable en el maíz y la polenta,
mientras que se hidroliza en los alimentos nixtamalizados a base de
maíz, es decir, alimentos elaborados con soluciones alcalinas
calientes.
La FB1 no está presente en la leche, la carne o los huevos de
animales alimentados con grano que contiene FB1 en concentraciones
que no afectarían a la salud de los animales. Las estimaciones de la
exposición humana para los Estados Unidos, el Canadá, Suiza, los
Países Bajos y el Transkei (Sudáfrica) oscilaron entre 0,017 y
440 µg/kg de peso corporal al día. No se dispone de datos sobre la
exposición por inhalación en el puesto de trabajo.
1.5 Cinética y metabolismo en los animales
No hay informes sobre la cinética o el metabolismo de la FB1 en
el ser humano. En animales experimentales se absorbe muy poco cuando
se administra por vía oral, se elimina rápidamente de la circulación y
se recupera sin metabolizar en las heces. La excreción biliar es
importante y se excretan pequeñas cantidades en la orina. Se puede
degradar a FB1 parcialmente hidrolizada en el intestino de primates
no humanos y en algunos rumiantes. Se retiene una pequeña cantidad en
el hígado y el riñón.
1.6 Efectos en los animales y en los sistemas de prueba in vitro
La FB1 es hepatotóxica en todas las especies animales sometidas
a prueba, en particular ratones, ratas, équidos, conejos, cerdos y
primates no humanos. Con la excepción de los hámsteres sirios, sólo se
observa embriotoxicidad o teratogenicidad cuando se produce toxicidad
materna o después de ella. Las fumonisinas son nefrotóxicas en cerdos,
ratas, ovejas, ratones y conejos. En ratas y conejos se produce
toxicidad renal a dosis inferiores a las de la hepatotoxicidad. Se
sabe que las fumonisinas producen leucoencefalomalacia equina y
síndrome de edema pulmonar porcino, asociados ambos con el consumo de
piensos a base de maíz. Es limitada la información sobre las
propiedades inmunológicas de la FB1. Fue hepatocarcinogénica para las
ratas machos de una raza y nefrocarcinogénica en otra raza, utilizando
la misma dosificación (50 mg/kg de alimentos) y fue
hepatocarcinogénica con 50 mg/kg de alimentos en ratones hembra.
Parece haber una correlación entre la toxicidad en los órganos y la
aparición de cáncer. La FB1 fue el primer inhibidor específico del
metabolismo de los esfingolípidos de novo que se descubrió y se está
utilizando ampliamente en la actualidad para estudiar su función en la
regulación celular. La FB1 inhibe el crecimiento celular y produce la
acumulación de bases esfingoideas libres y la alteración del
metabolismo lipídico en animales, plantas y algunas levaduras. No
indujo mutaciones génicas en bacterias o síntesis no programada de ADN
en hepatocitos primarios de rata, pero sí un aumento de las
aberraciones cromosómicas dependiente de la dosis con concentraciones
bajas en un estudio sobre los hepatocitos primarios de rata.
1.7 Efectos en el ser humano
No hay registros confirmados de toxicidad aguda de la fumonisina
en el ser humano. Los estudios de correlación disponibles procedentes
de Transkei (Sudáfrica) parecen indicar una vinculación entre la
exposición a la fumonisina en los alimentos y el cáncer de esófago.
Esto se ha observado en lugares donde se ha demostrado una exposición
relativamente alta a la fumonisina y donde las condiciones ambientales
favorecen la acumulación de fumonisina en el maíz, que es el alimento
básico. También hay estudios de correlación de China. Sin embargo, no
se obtuvo una imagen clara de la relación entre la contaminación bien
por fumonisina o bien por F. verticillioides y el cáncer de esófago.
Debido a la ausencia de datos sobre la exposición a la fumonisina, no
se puede llegar a ninguna conclusión a partir de un estudio de casos y
testigos de varones en Italia que mostraba una asociación entre el
consumo de maíz y el cáncer en la parte superior del aparato
gastrointestinal en personas con un elevado consumo de alcohol.
No hay biomarcadores validados para la exposición humana a la
FB1
1.8 Efectos en otros organismos en el laboratorio
La FB1 inhibe el crecimiento celular y produce acumulación de
bases esfingoideas libres y la alteración del metabolismo lipídico en
Saccharomyces cerevisiae.
La FB1 es fitotóxica, provoca lesiones en las membranas
celulares y reduce la síntesis de clorofila. También altera la
biosíntesis de esfingolípidos en las plantas y puede desempeñar una
función en la patogenicidad del maíz por las especies de Fusarium
que producen fumonisina.
2. Evaluación de los riesgos para la salud humana
2.1 Exposición
La exposición humana, demostrada por la presencia de FB1 en el
maíz para consumo humano, es común en todo el mundo. Hay diferencias
considerables en el grado de exposición humana entre las diferentes
regiones de cultivo de maíz. Esto se pone de manifiesto sobre todo
cuando se establece una comparación entre países plenamente
desarrollados y en desarrollo. Por ejemplo, aunque puede haber FB1 en
productos de maíz en los Estados Unidos, el Canadá y Europa
occidental, el consumo humano de estos productos es pequeño. En
algunas partes de Africa, América del Sur y Central y Asia, algunas
poblaciones consumen un elevado porcentaje de sus calorías como harina
de maíz, cuya contaminación por FB1 puede ser alta (véase el apéndice
2). El maíz contaminado de forma natural por FB1 puede estar
contaminado simultáneamente por otras toxinas de F. verticillioides
o F. proliferatum o por otras toxinas importantes desde el punto de
vista agrícola, en particular el deoxinivalenol, la zearalenona, la
aflatoxina y la ocratoxina.
La FB1 es estable en los métodos de elaboración de alimentos que
se utilizan en América del Norte y Europa occidental. El tratamiento
del maíz con bases y/o el lavado con agua reduce de manera eficaz las
concentraciones de FB1. Sin embargo, en animales experimentales
siguen siendo evidentes su hepatotoxicidad y/o nefrotoxicidad. Se sabe
poco acerca de la influencia de las técnicas de elaboración de
alimentos utilizadas en el mundo en desarrollo en la FB1 en los
productos de maíz.
2.2 Identificación de peligros
Se ha demostrado la función causal de la exposición a la FB1 en
la leucoencefalomalacia equina. Durante el siglo XIX se produjeron en
los Estados Unidos brotes en gran escala de esta enfermedad letal, y
también en épocas tan recientes como 1989-1990. Se ha establecido
asimismo la función causal de la exposición a la FB1 en la enfermedad
mortal del edema pulmonar porcino. Tal como se observó en hembras
preñadas, una exposición baja a la FB1 es letal para los conejos. Se
ha demostrado que la exposición provoca toxicidad renal y
hepatotoxicidad en todas las especies animales estudiadas, incluidos
los primates no humanos. La exposición a la FB1 produce
hipercolesterolemia en varias especies animales, en particular en
primates no humanos. Hay pruebas convincentes de que en las
enfermedades animales asociadas con la exposición a la FB1 se altera
el metabolismo lipídico. Es manifiesta la perturbación del metabolismo
de los esfingolípidos antes de la toxicidad in vitro e in vivo o
coincidiendo con ella. El uso de fumonisinas como instrumento para
estudiar la función de los esfingolípidos ha puesto de manifiesto que
éstos se requieren para el crecimiento celular y afectan de varias
formas a la señalización de las moléculas, provocando muerte celular
apoptótica y necrótica, diferenciación celular y respuestas
inmunitarias alteradas. Parece que son factores comunes tras la
exposición a la FB1 la alteración del metabolismo lipídico y los
cambios en la actividad y/o la expresión de enzimas fundamentales
encargados del funcionamiento normal del ciclo celular. La FB1 no es
tóxica para el desarrollo en ratas, ratones o conejos. A dosis
elevadas sin toxicidad materna induce fetotoxicidad en el hámster
sirio.
La carcinogenicidad de la FB1 en roedores varía en función de
las especies, las razas y el sexo. El único estudio con ratones
B6C3F1 puso de manifiesto que la FB1 era hepatocarcinogénica para
las hembras a 50 mg/kg en los alimentos. En ratas BD IX macho que
recibieron alimentos con 50 mg de FB1/kg durante un período de hasta
26 meses se observó la inducción de carcinomas hepatocelulares
primarios y carcinomas colangiales. Se detectaron adenomas y
carcinomas de los túbulos renales en ratas F344/N Nctr macho a las que
se suministraron 50 mg de FB1/kg. Parece existir una correlación
entre la toxicidad en los órganos y la aparición de cáncer.
El número de estudios de genotoxicidad disponible es limitado. La
FB1 no fue mutagénica en valoraciones bacterianas. En un estudio
realizado con células de mamífero in vitro no se detectó síntesis de
ADN no programado, pero la FB1 provocó roturas cromosómicas en
hepatocitos de rata. En otros estudios se ha puesto de manifiesto que
la FB1 provoca un aumento de la peroxidación de los lípidos in
vivo e in vitro. Es posible que los efectos de la rotura de los
cromosomas y la peroxidación de los lípidos tengan una relación
causal.
Las concentraciones de FB1 superiores a 100 mg/kg, notificadas
en el maíz de consumo humano en Africa y China, probablemente
provoquen leucoencefalomalacia, síndrome de edema pulmonar o cáncer si
se administran a caballos, cerdos y ratas o ratones, respectivamente.
A pesar de estos casos de exposición humana muy alta, no hay datos
confirmados de intoxicación aguda por fumonisina en personas. Los
estudios de correlación disponibles del Transkei, Sudáfrica, parecen
indicar una relación entre la exposición a la fumonisina a través de
los alimentos y el cáncer de esófago. Se han observado índices
elevados de cáncer de esófago donde se ha demostrado una exposición
relativamente elevada a la fumonisina y donde las condiciones
ambientales favorecen la acumulación de fumonisina en el maíz, que es
el alimento básico.
En un estudio de casos y testigos realizado con varones en Italia
se observó una asociación entre el consumo de maíz y la aparición de
cáncer en la parte superior del aparato digestivo, incluido el cáncer
de esófago, entre personas habituadas a un consumo de alcohol alto. No
había datos sobre la exposición a la fumonisina.
2.3 Evaluación de la respuesta en función de la dosis
La dosis más baja de FB1 que indujo hepatocarcinomas en animales
experimentales fue de 50 mg/kg de alimentos en ratas BD IX macho y en
ratones B6C3F1/Nctr hembra; no se observó inducción de cáncer con 25
ó 15 mg/kg de alimentos, respectivamente. En cada caso, se detectaron
indicios de hepatotoxicidad o alteraciones lipídicas con dosis iguales
o inferiores en estudios con esas mismas razas de ratas y ratones. La
dosis más baja de FB1 que indujo carcinomas renales en ratas F344/N
Nctr macho fue de 50 mg/kg de alimentos; no se observó inducción de
cáncer con 15 mg/kg de alimentos. Se produjo apoptosis tubular renal y
proliferación celular, así como cambios en los esfingolípidos
tisulares y urinarios, con dosis inferiores a las normalmente
necesarias para la inducción de cáncer en esos estudios.
No se dispone de datos para evaluar cuantitativamente la relación
entre la exposición a la FB1 y los posibles efectos en el ser humano.
2.4 Caracterización del riesgo
La FB1 es carcinogénica en ratones y ratas e induce enfermedades
letales en cerdos y caballos en concentraciones de exposición a las
que están sometidas los seres humanos. El Grupo Especial no estuvo en
condiciones de realizar una estimación cuantitativa de los riesgos
para la salud humana, pero consideró que se necesitaba con urgencia
dicha estimación.
3. Recomendaciones para la protección de la salud humana
a) Se deben establecer límites para la exposición de las personas a
través de los alimentos. Se debe prestar particular atención a
las poblaciones que consumen un porcentaje elevado de sus
calorías como harina de maíz.
b) Se deben adoptar medidas para limitar la exposición a la
fumonisina y la contaminación del maíz mediante:
* plantación de cultivos alternativos en zonas donde el maíz
no esté bien adaptado;
* obtención de maíz resistente a la podredumbre del grano por
Fusarium;
* aplicación de mejores prácticas de cultivo;
* separación de los granos mohosos.
c) Se debe aumentar la sensibilización temprana acerca de la
posibilidad de contaminación de los alimentos, mejorando la
comunicación entre los veterinarios y los funcionarios de salud
pública sobre los brotes de micotoxicosis en animales domésticos.
d) Se debe elaborar un método sólido, de bajo costo y sencillo para
la detección de la contaminación por fumonisina en el maíz.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
Members
Dr R.V. Bhat, Food and Drug Toxicology Research Centre, National
Institute of Nutrition, Indian Council of Medical Research,
Hyderabad, India
Dr M. Hirose, Division of Pathology, Biological Research Centre,
National Institute of Health Sciences, Tokyo, Japan
Dr P.C. Howard, Division of Biochemical Toxicology, National Center
for Toxicology Research, US Food and Drug Administration,
Jefferson, Arkansas, USA
Dr S. Humphreys, Center for Food Safety and Applied Nutrition, US Food
and Drug Administration, Washington DC, USA
Professor M. Kirsch-Volders, Laboratory for Cellular Genetics,
Brussels, Belgium (Chairman)
Professor W.F.O. Marasas, Medical Research Council, Tygerberg, South
Africa
Professor J.D. Miller, Department of Chemistry, Carleton University,
Ottawa, Ontario, Canada
Dr J.H. Olsen, Institute of Cancer Epidemiology, Danish Cancer
Society, Copenhagen, Denmark
Dr R. Plestina, Toxicology Unit, Institute for Medical Research and
Occupational Health, Zagreb, Croatia
Dr R.T. Riley, Agricultural Research Service, US Department of
Agriculture, Athens, USA
Dr A. Visconti, Institute for Toxins and Mycotoxins of Plant
Parasites, National Research Council, Bari, Italy
(Vice-Chairman)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Joint Secretary)
Mr Y. Hayashi, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Joint Secretary)
Dr J.M. Rice, International Agency for Research on Cancer, Lyon,
France
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
A WHO Task Group on Environmental Health Criteria for Fumonisin
B1 met at the World Health Organization, Geneva, Switzerland from 10
to 14 May 1999. Dr M. Younes, Acting Coordinator, Programme for the
Promotion of Chemical Safety, opened the meeting and welcomed the
participants on behalf of the IPCS and its three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to fumonisin B1.
Professor W.F.O. Marasas, Professor J.D. Miller, Dr R.T. Riley
and Dr A. Visconti prepared the first draft of this monograph. The
second draft incorporated comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs.
Dr A. Aitio, Mr Y. Hayashi and Dr P. Jenkins of the IPCS Central
Unit were responsible for the overall scientific content and technical
editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the US Food
and Drug Administration as part of its contributions to the IPCS.
ABBREVIATIONS
2-AAF 2-acetylaminofluorene
AAL-toxin Alternaria alternata lycopersici toxin
AMP adenosine monophosphate
AP aminopentol
CV coefficient of variation
CZE capillary zone electrophoresis
DEN diethylnitrosamine
DNA deoxyribonucleic acid
EDL effective dose level
EGF epidermal growth factor
ELEM equine leukoencephalomalacia
ELISA enzyme-linked immunosorbent assay
FA, FAK fumonisin A, fumonisin AK
FB fumonisin B
FC fumonisin C
FP fumonisin P
GC gas chromatography
GGT gamma-glutamyltranspeptidase
HPLC high-performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
IFN-gamma interferon-gamma
LPS lipopolysaccharide
MAPK mitogen-activated protein kinase
MME monomethyl ester
MS mass spectrometry
NADH reduced nicotinamide adenine dinucleotide
NADPH reduced nicotinamide adenine dinucleotide phosphate
NCTR National Center for Toxicological Research (USA)
NMBA N-methylbenzylnitrosamine
NOEL no-observed-effect level
NTD neural tube defect
NTP National Toxicology Program (USA)
OPA o-phthaldialdehyde
PDI probable daily intake
PFC plaque-forming cell
PGST placental glutathione S-transferase
PIM pulmonary intravascular/interstitial macrophage
PKC protein kinase C
PPE porcine pulmonary oedema
PUFA polyunsaturated fatty acid
Sa/So sphinganine/sphingosine
TCA tricarbalyllic acid moiety
TLC thin-layer chromatography
TNF-alpha tumour necrosis factor-alpha
INTRODUCTION
In this document, the fungus previously referred to as
Fusarium moniliforme Sheldon, is referred to as Fusarium
verticillioides (Sacc.) Nirenberg in accordance with a decision
taken at the 8th International Fusarium Workshop held at CABI
BioScience, Egham, United Kingdom, 17-20 August 1998.
This monograph focuses on fumonisin B1, the most abundant
naturally occurring fumonisin. Some information is also given on
fumonisins B2 and B3, which frequently occur with FB1, both in
culture material and in naturally contaminated samples.
1. SUMMARY, EVALUATION AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Fumonisin B1 (FB1) has the empirical formula C34H59NO15 and
is the diester of propane-1,2,3-tricarboxylic acid and
2-amino-12,16-dimethyl-3,5,10,14,15-pentahydroxyeicosane (relative
molecular mass: 721). It is the most prevalent of fumonisins, a family
of toxins with at least 15 identified members. The pure substance is a
white hygroscopic powder, which is soluble in water,
acetonitrile-water or methanol, is stable in acetonitrile-water (1:1),
is unstable in methanol, and is stable at food processing temperature
and to light.
Several analytical methods have been reported, including
thin-layer chromatography (TLC) and liquid chromatographic (LC), mass
spectroscopic (MS), post-hydrolysis gas chromatographic and
immunochemical methods, although the majority of studies have been
performed using LC analysis of a fluorescent derivative.
1.1.2 Sources of human exposure
FB1 is produced by several Fusarium species, mainly by
Fusarium verticillioides (Sacc.) Nirenberg (= Fusarium
moniliforme Sheldon), which is one of the most common fungi
associated with maize worldwide. Significant accumulation of FB1 in
maize occurs when weather conditions favour Fusarium kernel rot.
1.1.3 Environmental transport, distribution and transformation
There is evidence that fumonisins can be metabolized by some soil
microorganisms. However, little is known about the environmental fate
of fumonisins after they are either excreted or processed.
1.1.4 Environmental levels and human exposure
FB1 has been detected in maize and maize-based products
worldwide at mg/kg levels, sometimes in combination with other
mycotoxins. Concentrations at mg/kg levels have also been reported in
food for human consumption. Dry milling of maize results in the
distribution of fumonisin into the bran, germ and flour. In
experimental wet milling, fumonisin was detected in steep water,
gluten, fibre and germ, but not in the starch. FB1 is stable in maize
and polenta, whereas it is hydrolysed in nixtamalized maize-based
foods, i.e. foods processed with hot alkali solutions.
FB1 is not present in milk, meat or eggs from animals fed grain
containing FB1 at levels that would not affect the health of the
animals. Human exposure estimates for the USA, Canada, Switzerland,
the Netherlands and the Transkei (South Africa) ranged from 0.017 to
440 µg/kg body weight per day. No data on occupational inhalation
exposure are available.
1.1.5 Kinetics and metabolism in animals
There have been no reports on the kinetics or metabolism of FB1
in humans. In experimental animals it is poorly absorbed when dosed
orally, is rapidly eliminated from circulation and is recovered
unmetabolized in faeces. Biliary excretion is important, and small
amounts are excreted in urine. It can be degraded to partially
hydrolysed FB1 in the gut of non-human primates and some ruminants. A
small amount is retained in the liver and kidney.
1.1.6 Effects on animals and in vitro test systems
FB1 is hepatotoxic in all animal species tested including mice,
rats, equids, rabbits, pigs and non-human primates. With the exception
of Syrian hamsters, embryotoxicity or teratogenicity is only observed
concurrent with or subsequent to maternal toxicity. Fumonisins are
nephrotoxic in pigs, rats, sheep, mice and rabbits. In rats and
rabbits, renal toxicity occurs at lower doses than hepatotoxicity.
Fumonisins are known to be the cause of equine leukoencephalomalacia
and porcine pulmonary oedema syndrome, both associated with the
consumption of maize-based feeds. Limited information on immunological
properties of FB1 is available. It was hepatocarcinogenic to male
rats in one strain and nephrocarcinogenic in another strain at the
same dose levels (50 mg/kg diet), and was hepatocarcinogenic at 50
mg/kg diet in female mice. There appears to be a correlation between
organ toxicity and cancer development. FB1 was the first specific
inhibitor of de novo sphingolipid metabolism to be discovered and is
currently widely used to study the role of sphingolipids in cellular
regulation. FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in animals, plants
and some yeasts. It did not induce gene mutations in bacteria or
unscheduled DNA synthesis in primary rat hepatocytes, but induced a
dose-dependent increase in chromosomal aberrations at low
concentration levels in one study on primary rat hepatocytes.
1.1.7 Effects on humans
There are no confirmed records of acute fumonisin toxicity in
humans. Available correlation studies from the Transkei, South Africa,
suggest a link between dietary fumonisin exposure and oesophageal
cancer. This was observed where relatively high fumonisin exposure has
been demonstrated and where environmental conditions promote fumonisin
accumulation in maize, which is the staple diet. Correlation studies
are also available from China. However, no clear picture on the
relationship between either fumonisin or F. verticillioides
contamination and oesophageal cancer emerged. Owing to the absence of
fumonisin exposure data, no conclusion can be drawn from a case
control study of males in Italy showing an association between maize
intake and upper gastrointestinal tract cancer among subjects with
high alcohol consumption.
There are no validated biomarkers for human exposure to FB1.
1.1.8 Effects on other organisms in the laboratory
FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in Saccharomyces
cerevisiae.
FB1 is phytotoxic, damages cell membranes and reduces
chlorophyll synthesis. It also disrupts the biosynthesis of
sphingolipids in plants and may play a role in the pathogenicity of
maize by fumonisin-producing Fusarium species.
1.2 Evaluation of human health risks
1.2.1 Exposure
Human exposure as demonstrated by the occurrence of FB1 in maize
intended for human consumption is common worldwide. There are
considerable differences in the extent of human exposure between
different maize-growing regions. This is most evident when comparing
fully developed and developing countries. For example, although FB1
can occur in maize products in the USA, Canada and western Europe,
human consumption of those products is modest. In parts of Africa,
South-Central America and Asia, some populations consume a high
percentage of their calories as maize meal where FB1 contamination
may be high (see Appendix 2). Maize contaminated naturally by FB1 can
be simultaneously contaminated with other F. verticillioides or
F. proliferatum toxins or with other agriculturally important toxins
including deoxynivalenol, zearalenone, aflatoxin and ochratoxin.
FB1 is stable to food processing methods used in North America
and western Europe. Treating maize with base and/or water washing
effectively lowers the FB1 concentrations. However, its
hepatotoxicity and/or nephrotoxicity in experimental animals are still
evident. Little is known about how food processing techniques used in
the developing world affect FB1 in maize products.
1.2.2 Hazard identification
The causal role of FB1 exposure in the disease equine
leukoencephalomalacia has been established. Large-scale outbreaks of
this fatal disease occurred in the USA during the 19th century and as
recently as 1989-1990. The causal role of FB1 exposure in the fatal
disease porcine pulmonary oedema has been established. As observed in
pregnant females, low exposures to FB1 are fatal to rabbits. Exposure
has been demonstrated to result in renal toxicity and causes
hepatotoxicity in all animal species studied, including non-human
primates. FB1 exposure causes hypercholesterolaemia in several animal
species, including non-human primates. There is good evidence for
altered lipid metabolism in the animal diseases associated with FB1
exposure. Disruption of sphingolipid metabolism is evident either
before or concurrent with in vitro and in vivo toxicity. The use
of fumonisins as tools to study the function of sphingolipids has
revealed that sphingolipids are required for cell growth and affect
signalling molecules in several pathways, leading to apoptotic and
necrotic cell death, cellular differentiation and altered immune
responses. Altered lipid metabolism and changes in the activity and/or
expression of key enzymes responsible for normal cell cycle progress
appear to be common factors following exposure to FB1. FB1 is not a
developmental toxin to rat, mouse or rabbit. It induces fetotoxicity
in Syrian hamster at high doses without maternal toxicity.
The carcinogenicity of FB1 in rodents varies between species,
strains and sex. The only study with B6C3F1 mice indicated that FB1
was hepatocarcinogenic to females at 50 mg/kg in the diet. Primary
hepatocellular carcinomas and cholangial carcinomas were induced in
male BD IX rats fed diets at 50 mg FB1/kg for up to 26 months. Renal
tubule adenomas and carcinomas were detected in male F344/N Nctr rats
fed 50 mg FB1/kg. There appears to be a correlation between organ
toxicity and cancer development.
A limited number of genotoxicity studies are available. FB1 was
not mutagenic in bacterial assays. In in vitro mammalian cells,
unscheduled DNA synthesis was not detected but FB1 caused chromosomal
breaks in rat hepatocytes in one study. Other studies have shown that
FB1 causes increased lipid peroxidation in vivo and in vitro. It
is possible that chromosome-breaking effects and lipid peroxidation
are causally related.
FB1 levels above 100 mg/kg, which have been reported in maize
consumed by humans in Africa and China, would probably cause
leukoencephalomalacia, pulmonary oedema syndrome or cancer if fed to
horses, pigs and rats or mice, respectively. Despite these cases of
very high human exposure, there are no confirmed records of acute
fumonisin toxicity in humans. Available correlation studies from the
Transkei, South Africa, suggest a link between dietary fumonisin
exposure and oesophageal cancer. Elevated rates of oesophageal cancer
have been observed where relatively high fumonisin exposure has been
demonstrated and where environmental conditions promote the
accumulation of fumonisin in maize, which is the staple diet.
One case-control study in males from Italy found an association
between maize intake and cancers of the upper digestive tract,
including oesophageal cancer, among subjects with high alcohol
consumption. There were no data on fumonisin exposure.
1.2.3 Dose-response assessment
The lowest dose of FB1 that induced hepatocarcinomas in
experimental animals was 50 mg/kg diet in male BD IX rats and female
B6C3F1/Nctr mice; no cancer induction was observed at 25 or 15 mg/kg
diet, respectively. In each case, indications of hepatotoxicity or
lipid alterations were noted at the same or lower doses in studies
with these same rat and mouse strains. The lowest dose of FB1 that
induced renal carcinomas in the male F344/N Nctr rats was 50 mg/kg
diet; no cancer induction was observed at 15 mg/kg diet. Renal tubular
apoptosis and cell proliferation, as well as tissue and urinary
sphingolipid changes, occurred at lower doses than those required for
the induction of cancer in these studies.
No data are available to assess quantitatively the relationship
between exposure to FB1 and possible effects in humans.
1.2.4 Risk characterization
FB1 is carcinogenic in mice and rats and induces fatal diseases
in pigs and horses at levels of exposure that humans encounter. The
Task Group was not in a position to perform a quantitative estimation
of the human health risks, but considered that such an estimation is
urgently needed.
1.3 Recommendations for protection of human health
a) Limits for human dietary exposure should be established.
Special consideration should be given to populations
consuming a high percentage of their calories as maize meal.
b) Measures should be taken to limit fumonisin exposure and
maize contamination by:
* planting alternative crops in areas where maize is not
well adapted;
* developing maize resistant to Fusarium kernel rot;
* practising better crop management;
* segregating mouldy kernels.
c) Early awareness of potential food contamination should be
increased by improving communication between veterinarians
and public health officials on outbreaks of mycotoxicoses in
domestic animals.
d) A robust, low-cost and simple screening method for the
detection of fumonisin contamination in maize should be
developed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Common name: Fumonisin B1 (FB1)
Chemical formula: C34H59NO15
Chemical structure:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
Members
Dr R.V. Bhat, Food and Drug Toxicology Research Centre, National
Institute of Nutrition, Indian Council of Medical Research,
Hyderabad, India
Dr M. Hirose, Division of Pathology, Biological Research Centre,
National Institute of Health Sciences, Tokyo, Japan
Dr P.C. Howard, Division of Biochemical Toxicology, National Center
for Toxicology Research, US Food and Drug Administration,
Jefferson, Arkansas, USA
Dr S. Humphreys, Center for Food Safety and Applied Nutrition, US Food
and Drug Administration, Washington DC, USA
Professor M. Kirsch-Volders, Laboratory for Cellular Genetics,
Brussels, Belgium (Chairman)
Professor W.F.O. Marasas, Medical Research Council, Tygerberg, South
Africa
Professor J.D. Miller, Department of Chemistry, Carleton University,
Ottawa, Ontario, Canada
Dr J.H. Olsen, Institute of Cancer Epidemiology, Danish Cancer
Society, Copenhagen, Denmark
Dr R. Plestina, Toxicology Unit, Institute for Medical Research and
Occupational Health, Zagreb, Croatia
Dr R.T. Riley, Agricultural Research Service, US Department of
Agriculture, Athens, USA
Dr A. Visconti, Institute for Toxins and Mycotoxins of Plant
Parasites, National Research Council, Bari, Italy
(Vice-Chairman)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Joint Secretary)
Mr Y. Hayashi, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Joint Secretary)
Dr J.M. Rice, International Agency for Research on Cancer, Lyon,
France
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FUMONISIN B1
A WHO Task Group on Environmental Health Criteria for Fumonisin
B1 met at the World Health Organization, Geneva, Switzerland from 10
to 14 May 1999. Dr M. Younes, Acting Coordinator, Programme for the
Promotion of Chemical Safety, opened the meeting and welcomed the
participants on behalf of the IPCS and its three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to fumonisin B1.
Professor W.F.O. Marasas, Professor J.D. Miller, Dr R.T. Riley
and Dr A. Visconti prepared the first draft of this monograph. The
second draft incorporated comments received following the circulation
of the first draft to the IPCS Contact Points for Environmental Health
Criteria monographs.
Dr A. Aitio, Mr Y. Hayashi and Dr P. Jenkins of the IPCS Central
Unit were responsible for the overall scientific content and technical
editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the US Food
and Drug Administration as part of its contributions to the IPCS.
ABBREVIATIONS
2-AAF 2-acetylaminofluorene
AAL-toxin Alternaria alternata lycopersici toxin
AMP adenosine monophosphate
AP aminopentol
CV coefficient of variation
CZE capillary zone electrophoresis
DEN diethylnitrosamine
DNA deoxyribonucleic acid
EDL effective dose level
EGF epidermal growth factor
ELEM equine leukoencephalomalacia
ELISA enzyme-linked immunosorbent assay
FA, FAK fumonisin A, fumonisin AK
FB fumonisin B
FC fumonisin C
FP fumonisin P
GC gas chromatography
GGT gamma-glutamyltranspeptidase
HPLC high-performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
IFN-gamma interferon-gamma
LPS lipopolysaccharide
MAPK mitogen-activated protein kinase
MME monomethyl ester
MS mass spectrometry
NADH reduced nicotinamide adenine dinucleotide
NADPH reduced nicotinamide adenine dinucleotide phosphate
NCTR National Center for Toxicological Research (USA)
NMBA N-methylbenzylnitrosamine
NOEL no-observed-effect level
NTD neural tube defect
NTP National Toxicology Program (USA)
OPA o-phthaldialdehyde
PDI probable daily intake
PFC plaque-forming cell
PGST placental glutathione S-transferase
PIM pulmonary intravascular/interstitial macrophage
PKC protein kinase C
PPE porcine pulmonary oedema
PUFA polyunsaturated fatty acid
Sa/So sphinganine/sphingosine
TCA tricarbalyllic acid moiety
TLC thin-layer chromatography
TNF-alpha tumour necrosis factor-alpha
INTRODUCTION
In this document, the fungus previously referred to as
Fusarium moniliforme Sheldon, is referred to as Fusarium
verticillioides (Sacc.) Nirenberg in accordance with a decision
taken at the 8th International Fusarium Workshop held at CABI
BioScience, Egham, United Kingdom, 17-20 August 1998.
This monograph focuses on fumonisin B1, the most abundant
naturally occurring fumonisin. Some information is also given on
fumonisins B2 and B3, which frequently occur with FB1, both in
culture material and in naturally contaminated samples.
1. SUMMARY, EVALUATION AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Fumonisin B1 (FB1) has the empirical formula C34H59NO15 and
is the diester of propane-1,2,3-tricarboxylic acid and
2-amino-12,16-dimethyl-3,5,10,14,15-pentahydroxyeicosane (relative
molecular mass: 721). It is the most prevalent of fumonisins, a family
of toxins with at least 15 identified members. The pure substance is a
white hygroscopic powder, which is soluble in water,
acetonitrile-water or methanol, is stable in acetonitrile-water (1:1),
is unstable in methanol, and is stable at food processing temperature
and to light.
Several analytical methods have been reported, including
thin-layer chromatography (TLC) and liquid chromatographic (LC), mass
spectroscopic (MS), post-hydrolysis gas chromatographic and
immunochemical methods, although the majority of studies have been
performed using LC analysis of a fluorescent derivative.
1.1.2 Sources of human exposure
FB1 is produced by several Fusarium species, mainly by
Fusarium verticillioides (Sacc.) Nirenberg (= Fusarium
moniliforme Sheldon), which is one of the most common fungi
associated with maize worldwide. Significant accumulation of FB1 in
maize occurs when weather conditions favour Fusarium kernel rot.
1.1.3 Environmental transport, distribution and transformation
There is evidence that fumonisins can be metabolized by some soil
microorganisms. However, little is known about the environmental fate
of fumonisins after they are either excreted or processed.
1.1.4 Environmental levels and human exposure
FB1 has been detected in maize and maize-based products
worldwide at mg/kg levels, sometimes in combination with other
mycotoxins. Concentrations at mg/kg levels have also been reported in
food for human consumption. Dry milling of maize results in the
distribution of fumonisin into the bran, germ and flour. In
experimental wet milling, fumonisin was detected in steep water,
gluten, fibre and germ, but not in the starch. FB1 is stable in maize
and polenta, whereas it is hydrolysed in nixtamalized maize-based
foods, i.e. foods processed with hot alkali solutions.
FB1 is not present in milk, meat or eggs from animals fed grain
containing FB1 at levels that would not affect the health of the
animals. Human exposure estimates for the USA, Canada, Switzerland,
the Netherlands and the Transkei (South Africa) ranged from 0.017 to
440 µg/kg body weight per day. No data on occupational inhalation
exposure are available.
1.1.5 Kinetics and metabolism in animals
There have been no reports on the kinetics or metabolism of FB1
in humans. In experimental animals it is poorly absorbed when dosed
orally, is rapidly eliminated from circulation and is recovered
unmetabolized in faeces. Biliary excretion is important, and small
amounts are excreted in urine. It can be degraded to partially
hydrolysed FB1 in the gut of non-human primates and some ruminants. A
small amount is retained in the liver and kidney.
1.1.6 Effects on animals and in vitro test systems
FB1 is hepatotoxic in all animal species tested including mice,
rats, equids, rabbits, pigs and non-human primates. With the exception
of Syrian hamsters, embryotoxicity or teratogenicity is only observed
concurrent with or subsequent to maternal toxicity. Fumonisins are
nephrotoxic in pigs, rats, sheep, mice and rabbits. In rats and
rabbits, renal toxicity occurs at lower doses than hepatotoxicity.
Fumonisins are known to be the cause of equine leukoencephalomalacia
and porcine pulmonary oedema syndrome, both associated with the
consumption of maize-based feeds. Limited information on immunological
properties of FB1 is available. It was hepatocarcinogenic to male
rats in one strain and nephrocarcinogenic in another strain at the
same dose levels (50 mg/kg diet), and was hepatocarcinogenic at 50
mg/kg diet in female mice. There appears to be a correlation between
organ toxicity and cancer development. FB1 was the first specific
inhibitor of de novo sphingolipid metabolism to be discovered and is
currently widely used to study the role of sphingolipids in cellular
regulation. FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in animals, plants
and some yeasts. It did not induce gene mutations in bacteria or
unscheduled DNA synthesis in primary rat hepatocytes, but induced a
dose-dependent increase in chromosomal aberrations at low
concentration levels in one study on primary rat hepatocytes.
1.1.7 Effects on humans
There are no confirmed records of acute fumonisin toxicity in
humans. Available correlation studies from the Transkei, South Africa,
suggest a link between dietary fumonisin exposure and oesophageal
cancer. This was observed where relatively high fumonisin exposure has
been demonstrated and where environmental conditions promote fumonisin
accumulation in maize, which is the staple diet. Correlation studies
are also available from China. However, no clear picture on the
relationship between either fumonisin or F. verticillioides
contamination and oesophageal cancer emerged. Owing to the absence of
fumonisin exposure data, no conclusion can be drawn from a case
control study of males in Italy showing an association between maize
intake and upper gastrointestinal tract cancer among subjects with
high alcohol consumption.
There are no validated biomarkers for human exposure to FB1.
1.1.8 Effects on other organisms in the laboratory
FB1 inhibits cell growth and causes accumulation of free
sphingoid bases and alteration of lipid metabolism in Saccharomyces
cerevisiae.
FB1 is phytotoxic, damages cell membranes and reduces
chlorophyll synthesis. It also disrupts the biosynthesis of
sphingolipids in plants and may play a role in the pathogenicity of
maize by fumonisin-producing Fusarium species.
1.2 Evaluation of human health risks
1.2.1 Exposure
Human exposure as demonstrated by the occurrence of FB1 in maize
intended for human consumption is common worldwide. There are
considerable differences in the extent of human exposure between
different maize-growing regions. This is most evident when comparing
fully developed and developing countries. For example, although FB1
can occur in maize products in the USA, Canada and western Europe,
human consumption of those products is modest. In parts of Africa,
South-Central America and Asia, some populations consume a high
percentage of their calories as maize meal where FB1 contamination
may be high (see Appendix 2). Maize contaminated naturally by FB1 can
be simultaneously contaminated with other F. verticillioides or
F. proliferatum toxins or with other agriculturally important toxins
including deoxynivalenol, zearalenone, aflatoxin and ochratoxin.
FB1 is stable to food processing methods used in North America
and western Europe. Treating maize with base and/or water washing
effectively lowers the FB1 concentrations. However, its
hepatotoxicity and/or nephrotoxicity in experimental animals are still
evident. Little is known about how food processing techniques used in
the developing world affect FB1 in maize products.
1.2.2 Hazard identification
The causal role of FB1 exposure in the disease equine
leukoencephalomalacia has been established. Large-scale outbreaks of
this fatal disease occurred in the USA during the 19th century and as
recently as 1989-1990. The causal role of FB1 exposure in the fatal
disease porcine pulmonary oedema has been established. As observed in
pregnant females, low exposures to FB1 are fatal to rabbits. Exposure
has been demonstrated to result in renal toxicity and causes
hepatotoxicity in all animal species studied, including non-human
primates. FB1 exposure causes hypercholesterolaemia in several animal
species, including non-human primates. There is good evidence for
altered lipid metabolism in the animal diseases associated with FB1
exposure. Disruption of sphingolipid metabolism is evident either
before or concurrent with in vitro and in vivo toxicity. The use
of fumonisins as tools to study the function of sphingolipids has
revealed that sphingolipids are required for cell growth and affect
signalling molecules in several pathways, leading to apoptotic and
necrotic cell death, cellular differentiation and altered immune
responses. Altered lipid metabolism and changes in the activity and/or
expression of key enzymes responsible for normal cell cycle progress
appear to be common factors following exposure to FB1. FB1 is not a
developmental toxin to rat, mouse or rabbit. It induces fetotoxicity
in Syrian hamster at high doses without maternal toxicity.
The carcinogenicity of FB1 in rodents varies between species,
strains and sex. The only study with B6C3F1 mice indicated that FB1
was hepatocarcinogenic to females at 50 mg/kg in the diet. Primary
hepatocellular carcinomas and cholangial carcinomas were induced in
male BD IX rats fed diets at 50 mg FB1/kg for up to 26 months. Renal
tubule adenomas and carcinomas were detected in male F344/N Nctr rats
fed 50 mg FB1/kg. There appears to be a correlation between organ
toxicity and cancer development.
A limited number of genotoxicity studies are available. FB1 was
not mutagenic in bacterial assays. In in vitro mammalian cells,
unscheduled DNA synthesis was not detected but FB1 caused chromosomal
breaks in rat hepatocytes in one study. Other studies have shown that
FB1 causes increased lipid peroxidation in vivo and in vitro. It
is possible that chromosome-breaking effects and lipid peroxidation
are causally related.
FB1 levels above 100 mg/kg, which have been reported in maize
consumed by humans in Africa and China, would probably cause
leukoencephalomalacia, pulmonary oedema syndrome or cancer if fed to
horses, pigs and rats or mice, respectively. Despite these cases of
very high human exposure, there are no confirmed records of acute
fumonisin toxicity in humans. Available correlation studies from the
Transkei, South Africa, suggest a link between dietary fumonisin
exposure and oesophageal cancer. Elevated rates of oesophageal cancer
have been observed where relatively high fumonisin exposure has been
demonstrated and where environmental conditions promote the
accumulation of fumonisin in maize, which is the staple diet.
One case-control study in males from Italy found an association
between maize intake and cancers of the upper digestive tract,
including oesophageal cancer, among subjects with high alcohol
consumption. There were no data on fumonisin exposure.
1.2.3 Dose-response assessment
The lowest dose of FB1 that induced hepatocarcinomas in
experimental animals was 50 mg/kg diet in male BD IX rats and female
B6C3F1/Nctr mice; no cancer induction was observed at 25 or 15 mg/kg
diet, respectively. In each case, indications of hepatotoxicity or
lipid alterations were noted at the same or lower doses in studies
with these same rat and mouse strains. The lowest dose of FB1 that
induced renal carcinomas in the male F344/N Nctr rats was 50 mg/kg
diet; no cancer induction was observed at 15 mg/kg diet. Renal tubular
apoptosis and cell proliferation, as well as tissue and urinary
sphingolipid changes, occurred at lower doses than those required for
the induction of cancer in these studies.
No data are available to assess quantitatively the relationship
between exposure to FB1 and possible effects in humans.
1.2.4 Risk characterization
FB1 is carcinogenic in mice and rats and induces fatal diseases
in pigs and horses at levels of exposure that humans encounter. The
Task Group was not in a position to perform a quantitative estimation
of the human health risks, but considered that such an estimation is
urgently needed.
1.3 Recommendations for protection of human health
a) Limits for human dietary exposure should be established.
Special consideration should be given to populations
consuming a high percentage of their calories as maize meal.
b) Measures should be taken to limit fumonisin exposure and
maize contamination by:
* planting alternative crops in areas where maize is not
well adapted;
* developing maize resistant to Fusarium kernel rot;
* practising better crop management;
* segregating mouldy kernels.
c) Early awareness of potential food contamination should be
increased by improving communication between veterinarians
and public health officials on outbreaks of mycotoxicoses in
domestic animals.
d) A robust, low-cost and simple screening method for the
detection of fumonisin contamination in maize should be
developed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Common name: Fumonisin B1 (FB1)
Chemical formula: C34H59NO15
Chemical structure:
 Relative molecular mass: 721
CAS Name: 1,2,3-Propanetricarboxylic acid,
1,1'-[1-(12-amino-4,9,11-trihydroxy-2-methyl-
tridecyl)-2-(1-methylpentyl)-1,2-ethane-diyl]
ester
IUPAC name: None
CAS registry number:
Relative molecular mass: 721
CAS Name: 1,2,3-Propanetricarboxylic acid,
1,1'-[1-(12-amino-4,9,11-trihydroxy-2-methyl-
tridecyl)-2-(1-methylpentyl)-1,2-ethane-diyl]
ester
IUPAC name: None
CAS registry number: 