
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 29
2,4-DICHLOROPHENOXYACETIC ACID (2,4-D)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154089 3
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2,4-DICHLOROPHENOXYACETIC ACID
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Analytical methods
1.1.1.1 2,4-D, 2,4-D alkali metal salts or 2,4-D
amine salts and 2,4-D esters
1.1.1.2 Contaminants in 2,4-D herbicides
1.1.2. Sources of environmental pollution
1.1.3. Environmental distribution and transformations
1.1.4. Environmental exposure levels
1.1.5. Uptake and fate of 2,4-D in the body
1.1.6. Effects on animals
1.1.6.1 Acute toxic effects
1.1.6.2 Chronic toxic effects
1.1.6.3 Teratogenic and reproductive effects
1.1.6.4 Mutagenic effects
1.1.6.5 Carcinogenic effects
1.1.7. Effects on human beings
1.1.7.1 Acute toxic effects
1.1.7.2 Chronic toxic effects
1.1.7.3 Teratogenic and reproductive effects
1.1.7.4 Mutagenic effects
1.1.7.5 Carcinogenic effects
1.2. Recommendations for further studies
1.2.1. Analytical methods
1.2.2. Environmental exposure levels
1.2.3. Studies on animals
1.2.4. Studies on human beings
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties of 2,4-D
2.1.1. Introduction
2.1.2. Synthesis of 2,4-D
2.1.3. Important chemical reactions of 2,4-D
2.1.4. Composition of technical 2,4-D materials
2.1.5. Volatility of 2,4-D derivatives
2.2. Determination of 2,4-D
2.2.1. General comments
2.2.2. Analysis of technical and formulated 2,4-D products
2.2.3. Determination of 2,4-D residues
2.2.3.1 Sampling, extraction, and clean-up
2.2.4. Derivatization and quantification
2.2.5. Confirmation
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D herbicides
3.2. Uses
3.3. Disposal of wastes
3.3.1. Industrial wastes
3.3.2. Agricultural wastes
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION OF
2,4-D
4.1. Drift and volatilization in the atmosphere
4.2. Movement within and from the soil
4.3. Contamination of water
4.4. Environmental transformation and degradation processes
4.4.1. Metabolism in plants
4.4.1.1 Side-chain degradation
4.4.1.2 Ring hydroxylation
4.4.1.3 Conjugation with plant constituents
4.4.2. Degradation of 2,4-D in the soil
4.4.3. Degradation in the aquatic ecosystem
4.4.4. Photochemical degradation
4.5. Bioconcentration
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1. Levels of 2,4-D residues in the environmment
5.1.1. In air
5.1.1.1 Field exposure
5.1.1.2 General environment exposure
5.1.2. In water
5.1.3. In soil
5.1.4. In food sources
5.1.4.1 Residues in retail food supplies
5.1.4.2 Residues in fish and shellfish
5.1.4.3 Residues in wild fruits and mushrooms
5.1.4.4 Residues in food derived from animals
5.2. Occupational exposure to 2,4-D during the production,
handling, and use of chlorophenoxy herbicides
5.2.1. Industrial exposure
5.2.2. Exposure related to herbicide use
5.3. Exposure of bystanders to 2,4-D
5.4. Estimated exposure by the general population in 2,4-D-use
areas
5.4.1. Intake of 2,4-D residues from air
5.4.2. Intake of 2,4-D residues from potable water
5.4.3. Intake of 2,4-D residues from soil
5.4.4. Intake of 2,4-D residues from food
5.4.5. Total exposure by the general population in a
2,4-D-use area
5.4.6. Total exposure of persons occupationally exposed in
agriculture
5.4.7. Total exposure of the general population outside
areas of 2,4-D use
6. CHEMOBIOKINETICS AND METABOLISM
6.1. Uptake via different routes of exposure
6.1.1. Uptake by inhalation
6.1.1.1 Animals
6.1.1.2 Human beings
6.1.2. Dermal uptake
6.1.2.1 Animals
6.1.2.2 Human beings
6.1.3. Oral uptake
6.1.3.1 Animals
6.1.3.2 Human beings
6.2. Distribution and transformation in the body
6.2.1. Animals
6.2.2. Human beings
6.3. 2.4-D levels in body tissues and fluids
6.3.1. Animals
6.3.2. Human beings
6.4. Elimination and biological half life
6.4.1. Animals
6.4.2. Human beings
6.5. Chlorinated dibenzo- p-dioxins (CDDs)
7. EFFECTS OF 2,4-D ON ANIMALS
7.1. General introduction
7.2. Acute effects
7.2.1. Skin and eye irritancy
7.2.2. Skin sensitization
7.2.3. Lethal doses and concentrations (LD50 and LC50)
7.2.3.1 Acute oral LD50
7.2.3.l.l Mammals
7.2.3.1.2 Birds
7.2.3.2 Acute dermal LD50
7.2.3.2.1 Mammals
7.2.3.3 Acute inhalation LC50
7.2.3.4 Parenteral LD50
7.2.4. Acute toxicity in aquatic organisms
7.3. Subchronic and chronic toxicity
7.3.1. Mammals
7.3.1.1 Clinical signs of poisoning
7.3.1.2 Effects on food and water consumption, and
on body weight
7.3.1.3 Effects on the central nervous system
(CNS)
7.3.1.4 Effects on the peripheral nervous system
7.3.1.5 Myotoxic effects
7.3.1.6 Cardiovascular effects
7.3.1.7 Haematological effects
7.3.1.8 Effects on blood chemistry
7.3.1.9 Other biochemical effects observed in vivo
or in vitro
7.3.1.10 Pulmonary effects
7.3.1.11 Hepatotoxic effects
7.3.1.12 Effects on the kidney
7.3.1.13 Effects on endocrine organs
7.3.1.14 Effects on the digestive tract
7.3.2. Birds
7.3.3. Cold-blooded animals
7.4. Fetotoxicity, teratogenicity, and reproductive effects
7.4.1. Rats
7.4.1.1 Effects on adult rats
7.4.1.2 Effects on offspring
7.4.2. Mice
7.4.3. Birds
7.4.4. Cold-blooded animals
7.4.4.1 Amphibians
7.4.4.2 Fish
7.5. Mutagenicity and related effects
7.5.1. 2,4-D and its derivatives
7.6. Carcinogenic effects on experimental animals
7.6.1. 2,4-D and its derivatives
7.6.2. Contaminants in 2,4-D
8. EFFECTS ON MAN, CLINICAL AND EPIDEMIOLOGICAL STUDIES
8.1. Acute poisoning and occupational overexposure
8.1.1. Neurotoxic effects of 2,4-D and related compounds
8.1.1.1 Effects on the central nervous system
8.1.1.2 Effects on the peripheral nervous system
8.1.2. Myotoxic effects of 2,4-D
8.1.3. Cardiopathies and cardiovascular effects
8.1.4. Haematological effects
8.1.5. Blood chemistry effects
8.1.6. Pulmonary effects
8.1.7. Hepatotoxic effects
8.1.8. Nephrotoxic effects
8.1.9. Effects on the digestive tract
8.1.10. Effects on endocrine organs
8.1.11. Irritative and allergenic effects
8.2. Epidemiological studies of the chronic effects of 2,4-D
8.2.1. Reproductive, fetotoxic, and teratogenic effects
8.3. Studies on mutagenic effects in workers exposed to 2,4-D
8.4. Carcinogenic effects
8.4.1. Epidemiological studies
8.4.2. Evidence on the carcinogenicity of 2,4-D
8.5. Treatment of poisoning in human beings
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO 2,4-D
9.1. General considerations
9.2. Estimated intake of 2,4-D by the population in a 2,4-D-use
area
9.2.1. Intake by bystanders
9.2.2. Occupational intake
9.3. Safety factors
9.3.1. Definitions
9.3.2. Determination of safety factors
9.3.2.1 Acute poisoning
9.3.2.2 Chronic toxicity
9.3.2.3 Embryonic, fetotoxic, and teratogenic
effects
9.3.2.4 Mutagenic effects
9.3.2.5 Carcinogenic effects
9.4. Evaluation of health risks from 2,4-D exposure
9.5. Recommendations on exposure
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealth with in the
criteria documents are kindly requested to make available to the
WHO Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conclusions contained in the
criteria documents.
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR
2,4-DICHLORPHENOXYACETIC ACID
Members
Dr. E. Astolfi, Faculty of Medicine of Buenos Aires, Buenos Aires,
Argentina (Chairman)
Dr. L.A. Dobrovolski, Kiev Institute of Labour, Hygiene and
Occupational Diseases, Kiev, USSR
Dr. B. Gilbert, Centre for Research of Natural Products, University
of Rio de Janeiro, Rio de Janeiro, Brazil
Dr. D. Grant, Foods Directorate, Health Protection Branch, Health &
Welfare Canada, Ottawa, Ontario, Canada
Dr. O. Hutzinger, University of Amsterdam, Amsterdam, The
Netherlands
Dr. R.N. Khanna, Industrial Toxicology Research Centre, Lucknow
(UP) India
Dr. R.D. Kimbrough, Center for Environmental Health, Center for
Disease Control, Department of Health and Human Services,
Public Health Service, Atlanta, Georgia, USA
Dr. D.G. Lindsay, Ministry of Agriculture, Fisheries & Food,
London, England, (Rapporteur)
Dr. P.J. Madati, Ministry of Health, Dar-es-Salam, Tanzania
Representatives of Other Organizations
Dr. M.L. Leng, International Group of National Associations of
Manufacturers of Agrochemical Products, c/o Dow Chemical
Company, Midland, Michigan, USA
Dr. T.F. McCarthy, Permanent Commission and International
Association on Occupational Health
Secretariat
Dr. D. Riedel, Environmental Health Directorate, Health & Welfare
Canada, Environmental Health Centre, Ottawa, Canda, (Temporary
Advisor)
Dr. F. Valic, World Health Organization, Geneva, Switzerland,
(Secretary)
Mr. J.D. Wilbourn, International Agency for Research on Cancer,
Lyons, France
Observers
Dr. H. Spencer, US Environmental Protection Agency, Washington, DC,
USA
ENVIRONMENTAL HEALTH CRITERIA FOR 2,4-DICHLOROPHENOXYACETIC ACID
(2,4-D)
Further to the recommendations of the Stockholm United Nations
Conference on the Human Environment in 1972, and in response to a
number of World Health Assembly resolutions (WHA23.60, WHA24.47,
WHA25.58, WHA26.68) and the recommendation of the Governing Council
of the United Nations Environment Programme, (UNEP/GC/10,
July 3 1973), a programme on the integrated assessment of the
health effects of environmental pollution was initiated in 1973.
The programme, known as the WHO Environmental Health Criteria
Programme, has been implemented with the support of the Environment
Fund of the United Nations Environment Programme. In 1980, the
Environmental Health Criteria Programme was incorporated into the
International Programme on Chemical Safety (IPCS). The result of
the Environmental Health Criteria Programme is a series of criteria
documents.
The Environmental Health Directorate, Health Protection Branch,
Department of National Health and Welfare, Canada (Director-General
Dr. E. Somers) was responsible, as a Lead Institution of the IPCS,
for the preparation of the first and second drafts of the
Environmental Health Criteria Document on 2,4-D. Dr. D. Riedel
co-ordinated the work.
The Task Group for the Environmental Health Criteria for 2.4-D
met in Ottawa from 4 to 11 July, 1983. The meeting was opened by
Dr. E. Somers. Dr. A.B. Morrison, Assistant Deputy Minister,
Department of National Health and Welfare, Canada welcomed the
participants on behalf of the host government and Dr F. Valic, on
behalf of the 3 co-sponsoring organizations of the IPCS
(UNEP/ILO/WHO). The Task Group reviewed and revised the second
draft criteria document and made an evaluation of the health risks
of exposure to 2,4-D.
The efforts of all who helped in the preparation and the
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Insitute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Analytical methods
1.1.1.1. 2,4-D, 2,4-D alkali metal salts or 2,4-D amine salts, and
2,4-D esters
The available analytical results concerning 2,4-dichloro-
phenoxyacetic acid (2,4-D) and its derivatives in herbicides and
biological and environmental matrices were collected over a span of
almost 40 years, by diverse and, until fairly recently, not
sufficiently specific or sensitive methods. This makes comparison
of most of the data reported in the literature difficult.
1.1.1.2. Contaminants in 2,4-D herbicides
Adequately specific and sensitive methods for the reliable
identification of such potentially hazardous contaminants as the
di-, tri-, and tetrachlorodibenzo- p-dioxin isomers and
N-nitrosamines have only recently been developed. Available
analytical data are limited to a few manufactured products.
1.1.2. Sources of environmental pollution
Most of the 2,4-D residues result from the production and use
of 2,4-D herbicides. Other possible minor sources of 2,4-D include
the use of 2,4-dichlorophenoxybutyric acid (2,4-DB).
Little information is available on the uses of 2,4-D products
and the amounts used in various parts of the world.
The drifting of vapours of the more volatile short-chain 2,4-D
esters may result in air pollution and crop damage, and these
products are being replaced by less volatile long-chain esters or
by amine salts.
The use of 2,4-D for aquatic weed control may lead to
contamination of sources of irrigation and drinking-water.
Environmental pollution also arises through inadequate disposal
practice.
1.1.3. Environmental distribution and transformations
Various amounts of 2,4-D products applied to a target area may
be distributed in the general environment, within a few hours or
days, by the movements of air, water, or soil, particularly during
periods of rain, high winds, or high temperature.
2,4-D and its derivatives are fairly rapidly broken down by
hydrolysis, photolysis, and by biological action.
Persistence or accumulation of 2,4-D residues from normal use
is occasionally possible, mainly under dry or cold conditions where
there is little biological activity.
Nothing is known about the environmental fate of the impurities
present in 2,4-D herbicides.
1.1.4. Environmental exposure levels
Available data indicate that residues of 2,4-D rarely exceed
1 mg/kg in soil, several µg/litre in water, several µg/m3 in air,
and a few tens of µg/kg in food sources. Exceptions may occur in
the vicinity of 2,4-D herbicide spills, in water treated with
aquatic 2,4-D herbicides, in berries and mushrooms grown in treated
right-of-way areas, or when the herbicide is used in quantities far
in excess of the rates applied in normal agricultural or forestry
practice. No information is available on the corresponding
exposure levels for the contaminants present in 2,4-D herbicides.
Exposure to 2,4-D, in the work environment, of persons
producing, handling, or using herbicides may result in absorption
of detectable amounts of 2,4-D.
1.1.5. Uptake and fate of 2,4-D in the body
2,4-D and its derivatives can be absorbed via the oral, dermal,
and inhalation routes. General population exposure is mainly by
the oral route, but under occupational and bystander exposure
conditions, the dermal route is by far the most important.
Distribution of 2,4-D occurs throughout the body, but there is
no evidence that it is accumulated. Transformation in mammals
appears to occur only to a slight extent and mainly involves the
production of 2,4-D conjugates with sugars or amino acids. A
single dose is excreted within a few days, mainly with the urine,
and to a much lesser extent in the bile and faeces.
Little is known about the uptake and subsequent fate of the
contaminants of 2,4-D other than 2,4-dichlorophenol.
1.1.6. Effects on animals
1.1.6.1. Acute toxic effects
Death may result in mammals and birds administered oral doses
of 2,4-D exceeding approximately 100 - 300 mg/kg body weight.
The most characteristic signs of severe 2,4-D poisoning are
those of myotonia, but various other physiological, haematological,
biochemical, and histological changes have been described.
The no-observed-adverse-effect level for a single dose of 2,4-D
in animals has not been clearly established for all species.
No adequately documented reports of acute accidental 2,4-D
poisoning of mammals or birds have been found.
1.1.6.2. Chronic toxic effects
The no-observed-adverse-effect level for some of the chronic
adverse effects of 2,4-D in mammals has not been established
firmly.
1.1.6.3. Teratogenic and reproductive effects
The no-observed-adverse-effect level for the teratogenic,
embryotoxic, or fetotoxic effects of 2,4-D in mammals and birds
appears to be about 10 mg/kg body weight per day.
1.1.6.4. Mutagenic effects
Studies available at present are not adequate for the
quantitive evaluation of the mutagenic effects of 2,4-D and its
derivatives in short-term tests. However, the evidence does not
suggest that 2,4-D derivatives are potent mutagens.
1.1.6.5. Carcinogenic effects
The carcinogenic potential of 2,4-D and its derivatives such as
the amine salts and esters has not been adequately tested. The
reports on animal bioassays carried out so far are either too brief
for proper evaluation, or have become the subject of scientific
controversy.
1.1.7. Effects on human beings
1.1.7.1. Acute toxic effects
2,4-D drug trials and studies on volunteers have shown that
doses of between 5 and about 30 mg/kg body weight do not cause any
acute toxic effects.
Accidental and intentional 2,4-D poisonings indicate that the
toxic effects of 2,4-D are the same in human beings as in other
mammals. The lethal single oral dose is uncertain.
1.1.7.2. Chronic toxic effects
It is uncertain whether the chronic toxic effects of 2,4-D
products reported in occupationally-exposed people are solely
attributable to 2,4-D.
1.1.7.3. Teratogenic and reproductive effects
Scientifically valid studies have not shown any adverse
reproductive effects in human beings accidentally or occupationally
exposed to 2,4-D.
1.1.7.4. Mutagenic effects
The results of studies suggesting that occupational exposure to
2,4-D may result in chromosome abnormalities are equivocal.
1.1.7.5. Carcinogenic effects
The results of some epidemiological studies have suggested an
association between exposure to phenoxy herbicides and increased
incidences of malignant tumours and tumour mortality. It is not
clear, at present, whether this represents a true association, and
if so, whether it is specifically related to 2,4-D.
1.2. Recommendations for Further Studies
1.2.1. Analytical methods
Methods not requiring highly sophisticated and expensive
equipment are available for the accurate, specific, and sensitive
determination of 2,4-D residues in a wide variety of environmental
and biological materials. However, it would be desirable to
develop simpler but specific methods for the detection and
quantification of dioxin contaminants.
1.2.2. Environmental exposure levels
Further studies should be undertaken to determine the total
2,4-D intake of various sub-populations in areas of 2,4-D use.
It would be desirable to monitor 2,4-D residues in aquatic
organisms taken from lakes or rivers receiving discharges or
treatment with 2,4-D.
Further work on the relationship between the factors
influencing the dermal absorption of various 2,4-D formulated
products in human beings and animals should be carried out.
1.2.3. Studies on animals
More animal studies are desirable to investigate the possible
interactions between 2,4-D and other herbicides commonly used in
conjunction with 2,4-D.
Further work is required to accurately define the no-observed-
adverse-effect level for 2,4-D in long-term exposures.
Where unknown, the chronic toxicity of the alcohols and amines
used in preparing 2,4-D derivatives, should be investigated.
More studies are needed to assess the mutagenic potential of
2,4-D derivatives.
1.2.4. Studies on human beings
In the case of occupationally-exposed workers further
consideration should be given to the chemobiokinetics of 2,4-D
under repeated exposure conditions.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties of 2,4-D
2.1.1. Introduction
The structures of 2,4-dichlorophenoxyacetic acid (2,4-D) and of
chemically-related phenoxy herbicides in common use are given in
Fig. 1.
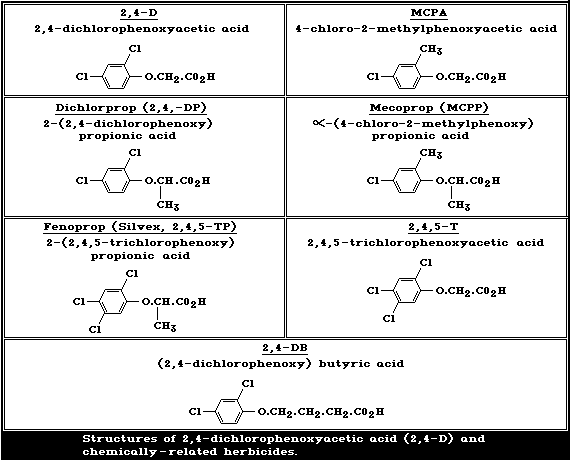 Some physical properties of 2,4-D and of the 2,4-D derivatives
that are used in agriculture are summarized in Table 1.
Table 1. Physical properties of 2,4-D
---------------------------------------------------
Molecular formula: C8H6Cl2O3
Relative molecular mass: 221.0
Melting point: 140-141 °C
Solubility in water: slightly soluble
Solubility in organic solvents: soluble
Vapour pressure: 52.3 Pa at 160 °C
pKa at 25 °C: 2.64-3.31
---------------------------------------------------
2,4-D has growth-regulating and herbicidal properties in
broad-leaved plants. Because of its solubility, 2,4-D is
rarely used in the form of the acid; commercial 2,4-D
herbicide formulations consist of the more soluble forms such
as alkali salts, amine salts, or esters. These are combined
with solvents, carriers, or surfactants and are marketed in
the form of dusts, granules, emulsions, or oil and water
solutions in a wide range of concentrations.
2.1.2. Synthesis of 2,4-D
2,4-D is commonly prepared by the condensation of 2,4-
dichlorophenol with monochloroacetic acid in a strongly alkaline
medium at moderate temperatures (Canada, NRC, 1978; Sittig 1980;
Que Hee & Sutherland, 1981), or by the chlorination of
phenoxyacetic acid, but this method leads to a product with a high
content of 2,4-dichlorophenol and other impurities (Melnikov,
l97l). Higher reaction temperatures and alkaline conditions during
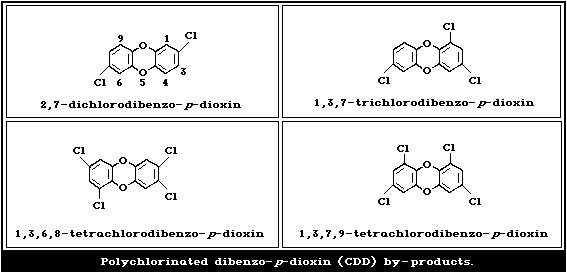
the manufacture of 2,4-D increase the formation of polychlorinated
dibenzo- p-dioxin (CDD) by-products (Fig. 2). The alkali metal
salts of 2,4-D are produced by the reaction of 2,4-D with the
appropriate metal base. Amine salts are obtained by reacting
stoichiometric quantities of amine and 2,4-D in a compatible
solvent (Que Hee & Sutherland, 1974, 1981). Esters are formed by
acid-catalysed esterification with azeotropic distillation of water
(Que Hee & Sutherland, 1981) or by a direct synthesis in which the
appropriate ester of monochloroacetic acid is reacted with
dichlorophenol to form the 2,4-D ester (Canada, NRC, 1978).
2.1.3. Important chemical reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides (Que Hee & Sutherland, 1975a). Pyrolysis of
2,4-D and its derivatives is likely to produce certain CDD isomers
(section 2.1.4). 2,4-D is readily photodegraded (section 4.4.4).
Some physical properties of 2,4-D and of the 2,4-D derivatives
that are used in agriculture are summarized in Table 1.
Table 1. Physical properties of 2,4-D
---------------------------------------------------
Molecular formula: C8H6Cl2O3
Relative molecular mass: 221.0
Melting point: 140-141 °C
Solubility in water: slightly soluble
Solubility in organic solvents: soluble
Vapour pressure: 52.3 Pa at 160 °C
pKa at 25 °C: 2.64-3.31
---------------------------------------------------
2,4-D has growth-regulating and herbicidal properties in
broad-leaved plants. Because of its solubility, 2,4-D is
rarely used in the form of the acid; commercial 2,4-D
herbicide formulations consist of the more soluble forms such
as alkali salts, amine salts, or esters. These are combined
with solvents, carriers, or surfactants and are marketed in
the form of dusts, granules, emulsions, or oil and water
solutions in a wide range of concentrations.
2.1.2. Synthesis of 2,4-D
2,4-D is commonly prepared by the condensation of 2,4-
dichlorophenol with monochloroacetic acid in a strongly alkaline
medium at moderate temperatures (Canada, NRC, 1978; Sittig 1980;
Que Hee & Sutherland, 1981), or by the chlorination of
phenoxyacetic acid, but this method leads to a product with a high
content of 2,4-dichlorophenol and other impurities (Melnikov,
l97l). Higher reaction temperatures and alkaline conditions during
the manufacture of 2,4-D increase the formation of polychlorinated
dibenzo- p-dioxin (CDD) by-products (Fig. 2). The alkali metal
salts of 2,4-D are produced by the reaction of 2,4-D with the
appropriate metal base. Amine salts are obtained by reacting
stoichiometric quantities of amine and 2,4-D in a compatible
solvent (Que Hee & Sutherland, 1974, 1981). Esters are formed by
acid-catalysed esterification with azeotropic distillation of water
(Que Hee & Sutherland, 1981) or by a direct synthesis in which the
appropriate ester of monochloroacetic acid is reacted with
dichlorophenol to form the 2,4-D ester (Canada, NRC, 1978).
2.1.3. Important chemical reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides (Que Hee & Sutherland, 1975a). Pyrolysis of
2,4-D and its derivatives is likely to produce certain CDD isomers
(section 2.1.4). 2,4-D is readily photodegraded (section 4.4.4).
 2.1.4. Composition of technical 2,4-D materials
Technical 2,4-D may range in purity from less than 90% to 99%.
Typical levels for impurities are listed in Table 2. Trace levels
of CDDs have been found in amine and ester formulations (Table 3).
It can be seen that the amine formulations tend to be less highly
contaminated with di- and tetra-CDD than the ester products. The
structures of these impurities are shown in Fig. 2.
Table 2. Typical levels of 2,4-D and major impurities
in technical 2,4-Da
------------------------------------------------------
Component % range
------------------------------------------------------
2,4-dichlorophenoxyacetic acid 94 - 99
2,6-dichlorophenoxyacetic acid 1.5 - 0.5
2-monochlorophenoxyacetic acid 0.5 - 0.1
4-monochlorophenoxyacetic acid 0.8 - 0.2
bis(2,4-dichlorophenoxy) acetic acid 2.0 - 0.1
phenoxyacetic acid trace - 0.2
2,4-dichlorophenol 0.6 - 0.1
2,6-dichlorophenol 0.048 - 0.001
2,4,6-trichlorophenol 0.14 - 0.001
2-chlorophenol 0.04 - 0.0004
4-chlorophenol 0.005 - 0.0004
water 0.8 - 0.1
------------------------------------------------------
a From: Cochrane (1981).
Table 3. Ranges of levels of chlorinated dibenzo- p-dioxins (CDD)
in 2,4-D amine and ester formulationsa
-------------------------------------------------------------------
CDD isomers found (µg/kg)b
Type of 2,7-di- 1,3,7-tri- 1,3,6,8/ 2,3,7,8-tetra
formulation 1,3,7,9-tetra
-------------------------------------------------------------------
2,4-D amines ndc- 409 nd - 587 nd - 278 nd
2,4-D esters nd - 23815 nd - 450 nd - 8730 nd
-------------------------------------------------------------------
a From: Cochrane et al. (1981).
b Expressed in terms of 2,4-D.
c nd (not detected < 1 µg/kg).
The composition of technical 2,4-D depends on the manufacturing
process and especially on the purity of 2,4-dichlorophenol when
this is the starting material. During 2,4-D synthesis from
monochloroacetic acid and 2,4-dichlorophenol, the latter compound
as well as other ortho-chlorinated by-products can give rise to a
wide variety of chorinated by-products at a high temperature and
high pH. Self condensation of 2,4-dichlorophenol may form 2,7-
dichlorodibenzo- p-dioxin, while trichlorophenols may give rise to
a mixture of 1,3,6,8- and 1,3,7,9-tetrachlorodibenzo- p-dioxins
(but not 2,3,7,8-TCDD) by self-condensation, or to 1,3,7-
trichlorodibenzo- p-dioxin by cross-condensation with 2,4-
dichlorophenol.
A different type of toxic trace impurity, namely N-
nitrosamines, can occur in amine formulations of 2,4-D, especially
when nitrite is added as a corrosion inhibitor for containers.
Dimethyl- N-nitrosamine has been found in some 2,4-D dimethylamine
products at levels of up to 0.3 mg/litre (Ross et al., 1977; Cohen,
et al., 1978).
2.1.5. Volatility of 2,4-D derivatives
2,4-D esters with short-chain alcohols are highly volatile
(Table 1). This influences the effectiveness of their application
to target crops, their effects on neighbouring crops, and the
degree of contamination of the atmosphere. 2,4-D alkali salts or
amine salts are much less volatile than esters (Carter, 1960;
Canada, NRC, 1978; Que Hee & Sutherland, 1981, and section 4.1),
and these products are to be preferred when the use of 2,4-D esters
might lead to evaporative 2,4-D losses and to crop damage.
2.2. Determination of 2,4-D
2.2.1. General comments
General comments on criteria for acceptable analytical methods
and on other pertinent aspects of 2,4-D determination can be found
in the publications of Gunther (1962), Currie (1968), Kaiser
(1973), Carl (1979), Kateman & Pijper (1981), Que Hee & Sutherland
(1981) and Chau et al. (1982).
2.2.2. Analysis of technical and formulated 2,4-D products
In the past, the quality of 2,4-D products was assessed by an
acid-base titration or by a total chlorine determination
(Collaborative International Pesticides Analytical Council, 1970).
These non-specific and thus inaccurate methods have been superseded
by specific gas-liquid chromatography (GLC) or high pressure liquid
chromatography (HPLC), making it possible to determine various by-
products (Henshaw et al., 1975; Bontoyan, 1977; Skelly et al.,
1977; Stevens et al., l978; Cochrane et al., 1982). The isomer-
specific HPLC method is now preferred by many 2,4-D producers and
regulatory agencies. The chlorinated dibenzo- p-dioxins (CDDs) are
usually produced only in trace amounts and are difficult to
separate and identify; highly specialised equipment and skills are
necessary (Crummett & Stehl, 1973; Huckins et al., 1978; Norström
et al., 1979; Baker et al., 1981; Cochrane et al., 1981; Hass et
al., 1981, and National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1981).
2.2.3. Determination of 2,4-D residues
All exposure determinations and risk assessments ultimately
depend on accurate chemical analyses, and therefore some critical
aspects of analysis for 2,4-D residues have been included in the
present document.
Before 2,4-D residues can be measured, they have to be
quantitatively extracted and purified to remove substances that
could interfere with the final residue determination. They must
then be converted to a stable product (derivative) suitable for
determination with a given type of detector.
When comparing analytical results, it should be kept in mind
that the older methods of extraction and clean-up contained
considerable sources of errors, and that the early methods
for measuring 2,4-D residues, such as colorimetry and
spectrophotometry, were not as sensitive or specific as those
developed in recent years.
2.2.3.1. Sampling, extraction, and clean-up
Methods for the sampling, extraction, and clean-up of 2,4-D
residues in water, air, soil, and biological materials have
recently been reviewed by National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978) and by Que Hee & Sutherland (1981). Problems caused
by the conjugate formation of 2,4-D with amino acids, proteins,
sugars, or lipids, or the absorption of 2,4-D onto container
surfaces, including those of glass vessels, have been solved by
Chow et al. (1971), Renberg (1974), Osadchuk et al. (1977), Lokke
(1979), Jensen & Glas (1981), and Bristol et al. (1982). For
sampling and extracting 2,4-D residues, the following references
should also be consulted:
Air: Van Dyk & Visweswariah (1975), Farwell et al. (1976a,b),
Grover et al. (1976), Johnson et al. (1977), Gluck & Melcher
(1980), and Grover & Kerr (1981); water: Suffet (1973a,b), Renberg
(1974), Mierzwa & Witek (1977), Chau & Thomson (1978); soil:
Woodham et al. (1971); Smith (1972, 1976a), Foster & McKercher
(1973); food: Que Hee & Sutherland (1981), Bjerk et al. (1972),
Jensen & Glas (1972), Lokke (1975); biological media: Smith
(1976b), (blood, urine); Senczuk & Pogorzelska (1981).
2.2.4. Derivatization and quantification
At present, gas-liquid chromatography with electron-capture
detection (GLC-EC) is the most commonly used and generally most
sensitive method (picogram level) for measuring 2,4-D residues.
To improve the sensitivity of detection, the 2,4-D has to be
transformed (derivatized), usually to a methyl ester by reacting
with BF3-methanol, diazomethane, or with concentrated sulfuric
acid-methanol; the first method may give the best results (Munro,
1972; Horner et al., 1974; Olson et al., 1978).
For a recent review of derivatization methods and GLC columns
for various substrates see Cochrane (1981).
Thin-layer chromatography (TLC) has been used for herbicide
residue determination (Guardigli et al., 1971, Yip 1975). It has
recently been recommended by Batora et al. (1981) as a simplified
method for determining pesticide residues that requires a minimum
of costly equipment. TLC is suitable for food inspection and could
be of use in the establishment of new residue laboratories in
developing countries.
High-pressure liquid chromatography (HPLC) is less sensitive
than GLC-EC i.e., nanogram (ng) versus picogram levels, but may be
advantageous under some circumstances (Tuinstra et al., 1976;
Arjmand et al., 1978; Connick & Simoneaux, 1982). Using mass
fragmentography with deuterated internal standards it is possible
to determine nanogram amounts of 2,4-D and related compounds in
urine and plasma (De Beer et al., 1981); it is also suitable for
chemobiokinetic studies on subtoxic doses of 2,4-D in blood.
2.2.5. Confirmation
The ultimate confirmatory technique is gas chromatography
coupled with mass spectrometry and specific ion monitoring, with a
sensitivity down to the femtogram level (Farwell et al., 1976a).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use
were not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United
Kingdom. World-wide use of herbicides and annual production, which
probably exceeds 5 x 107 kg per year, are increasing, National
Research Council of Canada, Associate Committee on Scientific
Criteria for Environmental Quality, 1978; Bovey & Young, 1980).
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaf weeds in cereal crops as well as on
pastures and lawns, in parks, and on golf courses at rates of about
0.2 - 2.0 kg active ingredient (acid equivalent) per hectare.
Esters are also used at rates of up to 6 kg (acid equivalent) per
ha to suppress weeds, brush, and deciduous trees along rights-of-
way and in conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides
in or along irrigation and other canals, in ponds, and lakes at
rates ranging from 1 to 122 kg/ha (Pal'mova & Galuzova, 1963; Smith
& Isom, 1967; National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Bovey & Young, 1980).
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays
containing 20 - 40 mg 2,4-D/litre on apple trees to reduce
premature fruit drop, on potato plants to increase the proportion
of medium-size tubers or to intensify the tuber skin colour of the
red varieties (Bristol et al., 1982), and in citrus culture to
reduce pre-harvest fruit drop and to increase fruit storage life.
The highly volatile ethyl, isopropyl, and butyl esters are
being replaced by low-volatile esters or by amine salts to reduce
crop damage resulting from 2,4-D vapour drift, and to decrease
atmospheric pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public,
has been reduced in some countries, because of increasing concern
about possible toxic effects, especially in relation to CDDs.
The ecological effects of using high rates of 2,4-D and
repeated treatments have been reviewed by Bovey & Young (1980).
3.3. Disposal of wastes
3.3.1. Industrial Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localised
to the production site and to areas of waste dumping, and it is
likely to be more dispersed if disposal or leaching has occurred
into water courses. Combustion of 2,4-D and its by-products at low
temperatures could lead to the formation of CDDs. A temperature
approaching 1000 °C, however, gives almost complete destruction of
2,4-D (Sittig, ed., 1980). The spread of 2,4-D from waste dumps
may be reduced by the use of properly enclosed impermeable clay-
lined pits, away from water sources.
3.3.2. Agricultural wastes
Disposal of unused 2,4-D and washing of equipment may result in
localised land pollution and also pollution of water supplies
through direct contamination or leaching from soil.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION OF 2,4-D
2,4-D does not persist or accumulate in the environment, as it
is readily degraded by physical, chemical, and biological action.
It is susceptible to photolysis in air and water, on soil, and on
plant surfaces. Thus, the question of environmental distribution
is limited to the immediate transfer of 2,4-D between compartments
of the environment.
4.1. Drift and Volatilization in the Atmosphere
The atmosphere can be contaminated with 2,4-D during both its
manufacture and use. The production of 2,4-D may result in the
emission into the air of dichlorophenol, chloroacetic acid, and
ammonia (Sittig, ed., 1980), in addition to 2,4-D vapours (Grover
et al., 1976).
According to the formulation of 2,4-D used, environmental
transfer into the atmosphere will occur by either drift (depending
on the particle size of the droplet, the spray technique, and
climatic conditions), or by volatilization, or by a combination of
both. It is very difficult to calculate the extent to which drift
or volatisation occurs, and this is illustrated by the range of
2,4-D concentrations observed in the air after 2,4-D use (Table 4).
The factors affecting the amount of herbicide spray that lands
on a target crop and the proportion that is lost by drifting or
volatilization have been described (Grover et al., 1972; Grover,
1976; Maybank et al., 1978; Que Hee & Sutherland, 1981). Unwanted
residues may be deposited on non-target crops (Akesson & Yates,
1961; Yates & Akesson, 1973). The National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) cited reports of drift damage
caused in susceptible crops by phenoxy herbicide applications,
particularly in cotton, tobacco, tomatoes, grapes, rapeseed,
clover, and a number of horticultural species.
Widespread damage in vineyards and in other crops due to 2,4-D
drift from sprayed wheat fields was reported by Robinson & Fox
(1978) with two different damage patterns, one localized and the
other widespread. The first was characterized by severe localized
damage with a very clear gradient of decreasing severity away from
the zone, following the drift of spray droplets in the immediate
treatment area. The more widespread damage was of greater concern.
It was characterized by more or less uniform symptoms and appeared
to be attributable to the passage of a large cloud of vapour that
may have extended for several km. Both problems could have been
avoided by the use of low-volatile preparations and proper
application methods.
Table 4. Concentrations of total 2,4-D residues in ambient air
------------------------------------------------------------------------------------------------------------------
Days when 2,4-D was 2,4-D residues
Site location µg/m3air Predominant
No. of stations, height ------------------------------------------- type of 2,4-D Reference
Regional characteristics found Sample Meana Max. residue
present time
mean max. h
------------------------------------------------------------------------------------------------------------------
Saskatchewan, Canada not
(1) 150 m (aircraft) stated 1 min 1.0c 2.5 butyl ester Elias (1975)
Saskatchewan, Canada 48 33 36 24 h 0.5c 13.5 butyl ester Grover et al.
(8) 2 m above ground 24 h 0-6d isooctyl ester (1976)e,f
level, wheat area 24 h - - amine salt
California, USA 41 (1973) 24 h 0.4 0.9 high volatile Farwell et al.
(7-8) near ground 73 (1974) 24 h 0.1 0.4 low volatile (1976)
level 24 h 0.1 0.2 non volatile
Washington State, USA 105-106 81 89 24 h 0.2 2.2 isopropyl ester Adams et al.
(2) near ground 65 69 24 h 0.09 2.2 butyl ester (1964)b
level, wheat area 8 11 24 h 0.003 3.1 isooctyl ester
Washington State, 99-102 34 39 24 h 0.08 2.0 isopropyl ester droplets Bamesberger &
(2) near ground 8 15 24 h 0.08 1.3 isopropyl ester vapour Adamsb
level, wheat area 18 22 24 h 0.07 1.0 butyl ester droplets
3 5 24 h 0.03 1.3 butyl ester vapour
1 1 24 h 0.005 0.5 isooctyl ester droplets
- - 24 h - - isooctyl ester vapour
4 5 24 h 0.01 0.5 acid, salts, droplets
5 5 24 h 0.04 5.1 acid, salts, vapour
------------------------------------------------------------------------------------------------------------------
a Values measured at different sites or at different times have been averaged and reduced to a single significant
figure for simplicity.
b At the time of these studies, GLC methods were less highly developed.
c Centre of principal range observed.
d Maximum values recorded in a previous study (Que Hee et al., 1975) were shown to be equivalent to concentrations
existing directly over an open pan of formulated butyl ester; the implication was made that accidental
laboratory contamination could have occurred.
f The results of Maybank & Yoshida (1969), Maybank et al. (1978), and Stanley et al. (1971) could not be adapted
to this table.
Volatilization of 2,4-D products in the air during the spraying
operation and from the surface of plants and the soil is difficult
to distinguish from the drift of spray droplets. Evaporation
occurs to a greater extent with the highly volatile ethyl,
isopropyl, or butyl esters; very little occurs with amine salt
formulations, and it is greatly reduced when 2,4-D is dissolved in
corn oil, cottonseed oil, or diesel oil (Marth & Mitchell, 1949).
In one experiment, no significant amounts of 2,4-D amine, but 20 -
40% of the initially deposited 2,4-D butyl ester, and 10 - 15% of
the octyl ester of 2,4-D vapourized within 2 h of spraying (Grover
et al., 1972); less volatilization occurs with the higher esters of
2,4-D. For this reason, the use of the more volatile esters has
been discontinued in some countries. Studies of 2,4-D aerial drift
following ground spray operations have shown that only 3 - 8% of
the applied herbicides drift as spray droplets when low volatile
preparations are applied as large droplets. However, ultra-low-
volume (ULV) applications by aircraft, or the use of highly
volatile esters may cause as much as 25 - 30% of the 2,4-D sprayed
to drift off the target (Grover et al., 1972; Maas & Kerssen, 1973;
Maybank et al., 1978).
4.2. Movement Within and From the Soil
The movement of pesticides within and from the soil can be
divided into three categories: diffusion, leaching, and surface
movement. Diffusion is a localized process and depends on the
concentration gradient of the pesticide in the soil medium, on the
soil mineral type, and on the organic matter content, temperature,
pH, and other factors. Leaching refers to the movement of
pesticides through the soil profile with percolating water.
Surface movement refers to wind erosion of dust particles and
surface run-off in flowing water.
Examination of the behaviour of 2,4-D in soils (Liu & Cibes-
Viade, 1973; Grover & Smith, 1974; Moreale & Van Bladel, 1980) has
shown that organic matter, soil pH (surface horizons), and
exchangeable aluminium (clay sub-horizons) are the key determinants
for the percentage of 2,4-D adsorbed. As the adsorption/desorption
process is the basic mechanism influencing herbicide availability,
mobility, and degradation in soil, 2,4-D is likely to be more
strongly bound in soils with a high content of organic matter than
in those with a low content.
4.3. Contamination of Water
Residues of 2,4-D in aqueous systems can result from the
deposition of spray drifts, the "washout" of 2,4-D in the vapour or
droplet phase from the atmosphere during rainfall, the run-off from
treated fields, or following the application of 2,4-D to water for
the control of aquatic weeds. Industrial discharges, either from
accidental spills or through sewage systems, may also contribute to
the contamination of water. The National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) has tabulated data that demonstrate
the influence of environmental factors on the clearance of 2,4-D
and its derivatives from water. The principal processes involved
are ester and amine hydrolysis, volatilization, microbial
degradation, photolysis, and sorption. There is little movement of
2,4-D into drainage water in organic soils, because it is strongly
bound to organic materials.
4.4. Environmental Transformation and Degradation Processes
4.4.1. Metabolism in plants
Plants hydrolyse 2,4-D esters to 2,4-D, which is the active
herbicide (Morton et al., 1967; Matsunaka, 1972). Further
metabolism in plants occurs through three mechanisms, namely, side-
chain degradation, hydroxylation of the aromatic ring, and
conjugation with plant constituents (Crafts, 1960; Morre & Rogers,
1960; Erickson et al., 1963).
4.4.1.1. Side-chain degradation
Degradation of the side-chain of 2,4-D has been observed in
many plants (Loos, 1969), but in only a few species or varieties
does it appear to play a major role in herbicide breakdown.
Luckwill & Lloyd Jones (1960a,b) suggested two degradation
pathways leading to the formation of 2,4-dichlorophenol.
4.4.1.2. Ring hydroxylation
Thomas et al. (1964a,b), and, more recently, Feung et al.
(1971, 1972, 1973b) identified 2,5-dichloro-4-hydroxyphenoxyacetic
acid and 2,3-dichloro-4-hydroxyphenoxyacetic acid as major and
minor phenolic acid metabolites, respectively. Evidence was found
by Fleeker & Stein (1971) indicating hydroxylation resulting in the
elimination of the 4-chloro substituent from the aromatic ring, in
addition to migration of the chlorine at the 4-position to an
adjacent carbon on the ring. A small amount of 2-chloro-4-hydroxy-
phenoxyacetic acid was produced from 2,4-D by wild buckwheat, wild
oats, leafy spurge, and yellow foxtail.
4.4.1.3. Conjugation with plant constituents
Studies indicate that resistant crops, i.e., grasses and
cereals, form water-soluble conjugates with sugars, whereas
sensitive broad-leaved crops (such as beans) form mainly water-
insoluble amino acid conjugates (Montgomery et al., 1971; Feung et
al., 1971, 1972, 1973b, 1975).
4.4.2. Degradation of 2,4-D in the soil
Deposition of 2,4-D esters on the soil is followed fairly
rapidly by hydrolysis. Burcar et al. (1966) observed that the
2,4-D isooctyl ester disappeared after 2 weeks, though free acid
could be detected up to 6 weeks after application. The breakdown
of the iso-propyl, n-butyl, and isooctyl esters of 2,4-D on three
Canadian prairie soils was studied by Smith (1972) who found that
after 24 h no iso-propyl or n-butyl esters remained, whereas
20 - 30% of the isooctyl ester was still intact. The author
concluded that an initial rapid phase of hydrolysis of the 2,4-D
esters to the anion in soil was the result of chemical and not
microbial action.
Microbial degradation of phenoxy herbicides does occur and has
been comprehensively reviewed by Loos (1975), Cripps & Roberts
(1978) and The National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, (1978).
Early studies of the persistence of 2,4-D in soil indicated that
warm moist conditions and the presence of organic matter favoured
the rapid disappearance of 2,4-D. Sterilization of the soil
inhibited breakdown, indicating that the degradation was microbial.
In addition, Pemberton (1979) reported the discovery of specific
2,4-D plasmids within some bacterial strains, transmitted from one
cell to another, and carrying with them a genetic capability
enabling the bacteria to degrade 2,4-D.
Two principal pathways have been proposed for the microbial
degradation of 2,4-D in soil. Firstly the side chain may be
removed to form 2,4-dichlorophenol, followed by orthohydroxylation
of the phenol to produce a catechol (Bollag et al., 1968). The
catechol may then be cleaved to yield a muconic acid and further
conversion products. The second possible pathway is via a
hydroxyphenoxyacetic acid intermediate (Evans et al., 1971).
4.4.3. Degradation in the aquatic ecosystem
A multitude of variables influence the partitioning and removal
of phenoxy herbicides within an aquatic ecosystem. Detectable
residues have been reported to persist for 4 weeks in some
situations and up to 4 months in others (Frank & Comes, 1967;
Wojtalik et al., 1971; Schultz & Harman, 1974). Photolysis is an
important means of degradation of 2,4-D in natural water and is
more rapid than that of 2,4,5-T (Crosby & Wong, 1973). The
partition of residues between water and sediment will have an
effect on the rate of breakdown, as will temperature and intensity
of light. Anaerobic conditions will favour microbial breakdown.
The effects of some of these factors have been tabulated by the
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (1978).
4.4.4. Photochemical degradation
Photodecomposition of 2,4-D was studied in detail by Crosby &
Tutass (1966), Boval & Smith (1973), and reviewed recently by Que
Hee & Sutherland (1981). It leads to the formation of a variety of
products but commonly involves reductive dechlorination of the
acid, esters, and salts in aqueous or in organic solutions, with
2,4-dichlorophenol acting as a catalyst for the breakdown of
2,4-D, which may involve rupture of the aromatic ring. Que Hee &
Sutherland (1987) studied the vapour and liquid phase photolysis of
the n-butyl ester of 2,4-D and observed dechlorination at the
second position with simultaneous reduction and re-arrangement to
produce a variety of photoproducts. According to Boval & Smith
(1973), carbon dioxide is the final oxidation product when aqueous
solutions of 2,4-D undergo photodecomposition.
4.5. Bioconcentration
There is no evidence that bioconcentration of 2,4-D occurs
through the food chain or in any compartment of the environment.
This has been demonstrated by large-scale monitoring for 2,4-D
residues in soils, foods, feedstuffs, wildlife, and human beings,
and from examinations of the many routes of metabolism and
degradation that exist in ecosystems (sections 5.1.3 and 5.1.4).
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1. Levels of 2,4-D Residues in the Environment
Most of the available information on 2,4-D levels in the
environment has been reviewed in detail (National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality, 1978; Ramel, 1978; Bovey & Young, 1980;
Canada, Health & Welfare, 1980; Shearer & Halter, 1980; US EPA,
1980a). In comparing early and recent results, it should be kept
in mind that the analytical procedures used before about 1965 were
often unreliable and may have resulted in under- or overestimation
of the actual levels of 2,4-D derivatives. No information is
available on the levels of 2,4-D-related dioxin by-products in the
environment.
5.1.1. In air
Some levels of 2,4-D in ambient air are shown in Table 4.
These 2,4-D residues consist mainly of esters, particularly the
highly volatile butyl esters (Bamesberger & Adams, 1966; Farwell et
al., 1976b; Grover et al., 1976). Total 2,4-D residues in the air
were found to decrease during periods of rain, suggesting a
"washout effect" (Grover et al., 1976). In the majority of cases,
the levels reported were those found shortly after spraying.
5.1.1.1. Field exposure
Concentrations of 2,4-D that occurred during and after
herbicide use in the air of the work zone of people engaged in
herbicide spray operations in various use situations, are given in
Table 5. Workers involved in these operations were exposed to
2,4-D levels of up to 0.2 mg/m3 air during the period of actual
application.
5.1.1.2. General environmental exposure
In large-scale studies in areas of intense 2,4-D use, about 40%
of all air samples were found to contain between 0.01 and 0.1 µg
2,4-D/m3 (Grover et al., 1975). In a similar study undertaken by
Que Hee et al. (1976), much higher levels were recorded in one
urban location, reaching an average of 339 µg/m3 air during 3 days.
However, Grover et al. (1976), in their subsequent work, showed
that such concentrations could only be produced under artificial
conditions that could not reflect environmental conditions. In a
general programme of air monitoring undertaken in citrus-growing
regions in the USA, only one out of 880 samples analysed was found
to contain 2,4-D, at a level of 0.004 mg/m3. The sites were not
chosen in relation to 2,4-D use (Stanley et al., 1971).
Table 5. Concentrations of total 2,4-D in air related to occupational exposure
---------------------------------------------------------------------------------------------------------
Days Mean of 2,4-D
Herbicide Circumstances Type of exposure after concentrations References
product monitoring spraying in air (mg/m3)
---------------------------------------------------------------------------------------------------------
2,4-dimethylamine agricultural spray Analyses of air 0 0.02 Thiele et al.
salt (0.9% aqueous operations with in tractor cabs (1981a,b)
solution) tractor-drawn
equipment
2,4-D butoxyethanol Exposure during Analyses of air 0 0.1-0.2 Kolmodin-Hedman
ester. 2% emulsion forest spray in breathing zone & Erne (1980),
in water operation with of workers Kolmodin-Hedman
tractor driven et al. (1979)
equipment
2,4-D isooctyl- 3-day aerial spray Analyses of air 0 0.002-01a Franklin et al.
ester in diesel oil operation with single in breathing zone (1982)
engine aircraft of pilot and
ground crew
2,4-D PGBE ester Two 1-day aerial Analyses of air 0 <0.00001b Lavy et al.
emulsion in water forest spray in breathing zone (1982)
operations by of ground crew
helicopter
---------------------------------------------------------------------------------------------------------
a Application using large spray droplets.
b One flagman was recorded as being exposed to 0.1 mg/m3.
5.1.2. In water
2,4-D, as well as chlorophenol residues resulting from the
microbial transformation of 2,4-D, may occur in raw and finished
supplies of drinking-water (Faust & Aly, 1963; US EPA, 1976, 1980a;
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality, 1978; Bovey & Young,
1980; Canada, Health & Welfare, 1980; Shearer & Halter, 1980).
Information on 2,4-D-related dioxins in water was not
available.
Drinking-water in the USA is routinely analysed by the FDA as
part of the beverage-food group in their "market basket" analysis
programme; 2,4-D has not been detected in these studies, where the
limit of detection is 0.005 mg/litre for beverages (Table 6). This
indicates that drinking-water is not a significant source of human
exposure outside directly sprayed areas.
The same conclusion can be drawn from the results of large-
scale surveys of pesticide residues, including 2,4-D in surface
waters in areas of 2,4-D use (Table 7).
Levels much higher than those found in these studies have been
observed, but only in relation to local spills or direct
contamination (Frank et al., 1979; Frank & Sirons, 1980). A very
wide fluctuation has been found in water samples following
treatment of bodies of water, shores, ditches, or stream banks with
herbicides (Averitt, 1967; Frank & Comes, 1967; Bartley & Hattrup,
1970; Frank et al., 1970; Wojtalik et al., 1970; Frank, 1972;
Whitney et al., 1973; Schultz & Harman, 1974; Schultz & Whitney,
1974; Paderova, 1975; Province of British Columbia, 1981).
Occasional high contamination levels in samples of potable water
have been reported following experimental treatments of reservoirs
with 2,4-D (Wojtalik et al., 1971). However, the mean levels
tended to remain below 2 µg/litre, even in samples of raw or
processed water from 2,4-D-treated reservoirs (Smith & Isom, 1967;
Wojtalik et al., 1971; Province of British Columbia, 1981).
Generally, 2,4-D residues were < 0.1 µg/litre in two large-scale
monitoring programmes of surface waters (Frank & Sirons, 1980;
Gummer, 1980). This is not unexpected in view of the moderately
rapid microbial degradation of 2,4-D in the environment (Robson,
1966; Averitt, 1967; Frank, 1972; Nesbitt & Watson, 1980a,b;
Province of British Columbia, 1981).
2,4-D and especially its transformation product,
dichlorophenol, at levels exceeding 20 µg/litre will impart an
objectionable odour and taste to contaminated water (Pal'mova &
Galuzova, 1963; Faust & Suffet, 1966). This organoleptic effect
may reduce the likelihood of highly contaminated water being
ingested. It is noteworthy that public water supplies containing
"traces" of 2,4-D, and wells contaminated with 2,4-D or other
herbicides have been shut down because of objectionable odours or
tastes (Gribanov, 1968; Kramer & Schmaland, 1974; Frank et al.,
1979).
Table 6. 2,4-D residues reported in market basket samples in the USA
---------------------------------------------------------------------------------------------------------
Types of samples Nature of samples % of samples Residue levels
Years analysed containing residues with residues (mg/kg) References
---------------------------------------------------------------------------------------------------------
1965-65 Total diets sugars and adjunctsa 4.2 < 0.02-0.16 Duggan & Corneliussen
1966-66 leafy vegetables (1) 3.0 < 0.02-0.03 (1972)
low fats
1967-67 leafy vegetables (2) 1.7 0.03
oil fats (1)
1968-68 dairy produce (1) 0.6 0.02-0.13
1969-69 fruits (1), sugars (2) 0.3 < 0.2
1970-70 leafy vegetables (1) 0.3 < 0.02
dairy produce (1)
1970-71 Total diets leafy vegetables (3) - 0.01-0.02 Manske & Corneliussen
(1975)
1971-72 Total diets dairy products (1) - 0.01 Manske & Johnson
(1975)
1973-80 Total diets 0 < 0.01 Manske & Johnson
(1976)
Johnson et al.
(1981a,b)
Johnson et al. (1977)
1972-73 Potatoes from raw, boiled or baked - < 0.02-0.12 Bristol et al. (1982)
fields treated
with herbicide
---------------------------------------------------------------------------------------------------------
a No. of positives not specified.
Table 7. Concentrations of 2,4-D residues in surface water samples following application of 2,4-D to
agricultural landsa
------------------------------------------------------------------------------------------------------
Site No. of samples in 2,4-D applied 2,4-D residues
Number of Stations which 2,4-D was: in watershed (µg/litre) References
Regional Characteristics ----------------- (kg/ha) ---------------
analysed found meanb max.
------------------------------------------------------------------------------------------------------
Ontario, Canada 949 66 0.8 <0.1 3.9e Frank & Sirons (1980)
11
streams
Saskatchewan, Canada 15 10 - 2 21.6 Choi et al. (1976)
5
river
Western Canada 186 10 - 0.5c 4.3d Gummer (1980)
14
diverse sites
------------------------------------------------------------------------------------------------------
a Studies in which the analytical procedures were not described or were considered unreliable have not
been included.
b Values measured at different sites or at different times have been averaged and reduced to a single
significant figure for simplicity.
c Reported data are very close to analytical detection limits.
d The maximum value, which raises the average value considerably, occurred in the effluent of an
industrial plant.
e Levels of 15.9 and 320 µg/litre were recorded at two sites but were related to spillage or actual
spraying at the sampling locality.
5.1.3. In soil
Most of the information available at present concerning 2,4-D
and other chlorophenoxy herbicide residues in soils has been
reviewed by the National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Bovey (1980a), and by Que Hee & Sutherland (1981). In highly
acidic soils, or in soils in cold or arid regions, 2,4-D
degradation is apparently slow (Lavy et al., 1973; Buslovich &
Milchina, 1976; Ou et al., 1978; Moreale & Van Bladel, 1980;
Nesbitt & Watson, 1980b). However, even at about 20 - 2000 times
the normal agricultural application rates, little or no detectable
2,4-D was left in soils under temperate climatic conditions with
prolonged winters, after intervals of 385 - 440 days (Young et al.,
1974; Stewart & Gaul, 1977; Bovey, 1980a). Furthermore, results of
a laboratory study on 2,4-D degradation in the soil showed a half-
life of 4 days (Altom & Stritzke, 1973). Several soil monitoring
studies in North America, in areas with regular 2,4-D use, have
shown residues in less than 10% of the samples, and at levels of
less than 1 mg/kg (Stevens et al., 1970; Wiersma et al., 1972;
Gowen et al., 1976).
The available data are inadequate for establishing regional and
seasonal profiles of 2,4-D soil residues and of direct population
exposure, but it is likely that direct exposure would be minor,
except during or soon after herbicide application. Indirect
exposure through the transfer of 2,4-D residues from soil to air,
or food sources is assessed separately.
5.1.4. In food sources
Although 2,4-D and its transformation products do not tend to
accumulate in plants and plant products, detectable residues of
2,4-D on food plants may be consumed by human beings or animals and
may thus contribute to the overall exposure of the human population
to this chemical.
The results of pertinent studies on 2,4-D residues on or in
foods, and in food sources for human beings and animals, are
summarized in Tables 8 - 11. Theoretically, some contribution to
the reported 2,4-D residues may have been partly derived from other
phenoxy herbicides, as 2,4-DB undergoes beta-oxidation to 2,4-D in
some plants and fish, and in cattle (Lisk et al., 1963; Gutenmann &
Lisk, 1965; Sundström et al., 1979; Bovey, 1980a).
5.1.4.1. Residues in retail food supplies
The frequency of occurrence and the levels of 2,4-D residues in
over 110 000 samples of a variety of different ready-to-eat foods,
beverages, and infant and young children's diets, have been studied
over the last 20 years in the USA (Lipscomb, 1968; Corneliussen,
1970, 1972; Duggan et al., 1971; Duggan & Corneliussen, 1972;
Johnson et al., 1979, 1981a,b). The 2,4-D residues found in such
samples are reported in Table 6. The theoretical daily intake
resulting from these residues was variously estimated to be < 1 -
5 µg/person per day (Duggan & Corneliusson, 1972).
Studies undertaken since 1970 have failed to detect residues of
2,4-D in any of the US diet samples analysed, except for a single
positive sample in the dairy product food group which was estimated
at 0.01 mg/kg (Manske & Johnson, 1975).
5.1.4.2. Residues in fish and shellfish
Fish and shellfish may be exposed to 2,4-D as a consequence of
aquatic herbicide use, or through the agricultural use of 2,4-D.
The residues in the edible portions of such fish rarely exceed 1
mg/kg wet weight (Erne, 1974, 1975 and Table 8). Residues of 2,4-D
have not been detected in retail samples of fish and shellfish
analysed as part of the US "market basket" studies (section
5.1.4.1).
There is some evidence that the organoleptic properties of the
2,4-D residues may reduce the likelihood of the consumption of fish
flesh contaminated with higher levels of 2,4-D (Gavrilova, 1965;
Folmar, 1979).
5.1.4.3. Residues in wild fruits and mushrooms
Uncultivated fruits and mushrooms taken from areas where 2,4-D
was used, or was likely to have been used, were examined for
residues of 2,4-D by Erne & von Haartman (1973), Erne, (1980),
Sietanen et al. (1981), and Frank et al. (1982). The results in
Table 9 show that residues of 2,4-D in berries in field-trial
studies have been as high as 30 mg/kg immediately after
application, but residues in berries and mushrooms taken from the
wild are generally < 1 mg/kg.
High residues of 2,4-D can produce disagreeable odours or
flavours in wild fruits and vegetables (Ingelög et al., 1977;
McArdle et al., 1961), and this may reduce the likelihood that
highly contaminated foods are ingested.
5.1.4.4. Residues in food derived from animals
Domestic meat-, milk-, and egg-producing animals, and game
animals may consume forage or feed containing 2,4-D residues, and
thus, their tissues and products may contain residues. Published
data on 2,4-D residues in feed and forage from the Northern
Hemisphere are summarized in Table 10. Immediately after
application of phenoxy herbicides, 2,4-D residues in or on grass,
generally average about 100 mg/kg for each kg of herbicide applied
per hectare. Such residues decline with a half-life of about 1 - 2
weeks, to about 20 mg/kg, within 4 weeks after an application of 1
kg/ha (Leng, 1972). Residues in 2,4-D-treated feed grains are
significantly lower than the levels reported above and no residues
would be expected in meat, milk, or eggs from such sources (Table
10).
Table 8. 2,4-D residues reported in field studies on fish and shellfish
-----------------------------------------------------------------------------------------------------
Country Year(s) 2,4-D application Types of samples 2,4-D residues in References
rate tissues (mg/kg)
-----------------------------------------------------------------------------------------------------
USA 1961 0.1 mg/litre oyster (1 species) 1.6-2.0 Butler (1965)
fish (1 species) 0.3-1.0 Cope et al. (1970)
USA 1966 44.8-112 kg/ha mussels < 0.14-1.12 Smith & Isom (1967)
clams < 0.14
fish (5 species) < 0.14
USA 1968 112 kg/ha fish (4 species) < 0.10-0.24 Whitney et al. (1973)
USA 1969 22.4-44.8 kg/ha mussels < 0.05-2.7 Wojtalik et al. (1971)
fish (8 species) < 0.10-0.34
USA 1971 2.24-8.96 kg/ha fish (3 species) < 0.005-1.075 Schultz & Harman (1974)
USA 1971 4.48 kg/ha fish (5 species) 0.000-0.162 Schultz & Whitney (1974)
-----------------------------------------------------------------------------------------------------
Table 9. 2,4-D residues in wild berries and mushrooms collected in fields or forests following application
of phenoxyalkanoic herbicides
--------------------------------------------------------------------------------------------------------------
Country Year(s) Sample 2,4-D application Days after No. samples 2,4-D residues References
rate (kg a.i./ha) treatment analysed (mg/kg)
--------------------------------------------------------------------------------------------------------------
Canadaa 1979-81 raspberries 1.1-3.9 2 124 2.6-31.0 Frank et
14-35 0.1-3.3 al. (1982)
Finlanda 1974-76 vaccinium berries 2.5 10-356 44 Mukula et
jam not known not known 1 2.2 al. (1978)
mushrooms 14-300 28 < 0.05-1.2
Finlanda 1975-76 vaccinium berries 0.25-2.25 365 not stated < 0.05 Siltanen et
al. (1981)
Swedena 1970 raspberries 1.5-2.2 2-32 9 < 0.03-0.9 Erne & Von
vaccinium berries 1.5-2.2 2-32 68 < 0.03-7.7 Haartman
blueberries 1.5-2.2 2-32 19 < 0.03-2.9 (1973)c
mushrooms 1.5-2.2 2-32 15 < 0.03
Swedena 1973-79 vaccinium berriesb 0.25-2.25 365 61 nd (< 0.05) Erne (1980)
raspberries not stated 14 not stated nd-2.5
blueberries not stated 2 not stated nd-10.0
cowberries not stated 1-28 not stated nd-6.0
mushrooms not stated 7 1 0.3
Swedena blueberries 0.4-1.5 1-35 not stated 0.2-5.3 Ingelög et
vaccinium berries 0.4-1.5 30-35 not stated 0.5-4.5 al. (1977)
raspberries 0.4-1.5 1-10 not stated 0.2-2.0
--------------------------------------------------------------------------------------------------------------
a Samples taken from areas treated with 2,4-D.
b Samples entering factory for processing.
c Data from authors' Table 1.
Table 10. 2,4-D residues reported in samples of herbicide-treated forage or feed
--------------------------------------------------------------------------------------------------------------------------
Country Year(s) Type of samples 2,4-D application Post- No. samples 2,4-D residues References
rate, (kg a.i./ha) treatment examined & (mg/kg)
interval, positive
(days)
--------------------------------------------------------------------------------------------------------------------------
Canada 1971 wheat plants 0.42 1-36 ? ? 8.35-0.011 Cochrane & Russell (1975)
Finland 1962-68 green forage 1-4 7.21 ? ? 600-3.7 Finnish State Institute of
(grass and 3.5 7-28 ? ? 13-0.4 Agriculture (1963-1969)
clover)
Finland 1974-76 aspen leaves 2.5 60-300 32 30-0.3 Mukula et al. (1978)
and twigs
birch leaves 60-300 16 31-0.1
and twigs
cowberry plants 365 8 < 26-0.05
Germany, wheat, barley, 0.375-0.735 64-101 ? ? < 0.015-0.01 Maier-Bode (1971)
Federal rye, oat grains
Republic wheat, barley, < 0.34-0.02
of rye, oat straw
Hungary 1971 silo corn 1.4-1.5 56-120 ? ? 0.8-0.075 Bodai et al. (1974)
Sweden 1972-76 barley, oats ? ? 3 2 0.7-0.4 Erne & Rutqvist (1979)
grass 7 1 0.4
lichens ? ? 2 2 0.4-0.2
USA 1949 pasture plants 4.48 1 8 8 14.6-1.65 Grigsby & Farwell (1950)
USA 1967 forage grasses 0.56-2.2 0-112 ? ? 100-1 Morton et al. (1967)
USA 1969 sorghum plants 1.4 2 ? ? 1.06 Ketchersid et al. (1970)
1.4-2.8 30-60 < 5.25-0.2
USA 1969 pasture plants 6.6-8.8 0-28 24 24 700-150 Leng (1972)
--------------------------------------------------------------------------------------------------------------------------
No residues of 2,4-D were detected (detection limit of 0.02 mg)
in the milk of dairy cows fed 2,4-D at a level of 300 mg/kg total
diet (Bjerke et al., 1972; Leng, 1972). A range of 0.06 - 0.08 mg
2,4-D/litre was found in the milk of cows fed for 3 weeks at a
level of 1000 mg 2,4-D/kg total diet.
When young beef cattle were fed 2,4-D at levels of 300, 1000,
and 2000 mg/kg total diet for 28 days, 2,4-D residue levels were
highest in the kidney and liver, but did not exceed 0.1 mg/kg in
muscle and fat, even at the highest dose level (Clark et al., 1975;
Leng, 1972, 1977). 2,4-D residues were not detected in more than
12 000 samples each of meat and dairy products analysed in the USA
between 1963 and 1969 (Duggan et al., 1971).
Results of feeding studies with hares and reindeer in
Scandinavia indicated that 2,4-D levels of 25 - 30 mg/kg forage
(equivalent to an intake of about 1 mg 2,4-D/kg body weight per
day) produce maximum 2,4-D residues of 1.1 mg/kg wet weight in
liver, and 8.9 mg/kg in kidney tissues (Erne, 1974). Residues of
2,4-D were detected in the liver and kidney of a few game animals
shot by hunters, or found dead in or near areas sprayed with
phenoxy herbicides (Table 11, Erne, 1974, 1975). The residues in
muscle tissue were not measured but would be lower than in the
liver and kidney, as indicated by the data summarized in Table 11.
On the whole, the available evidence indicates that 2,4-D is
rarely detected in commercial foods and that residues in food taken
from areas where 2,4-D has been sprayed will usually be < 1 mg/kg
food. The liver and kidney from range animals are possible
exceptions, but these contribute little to the total diet of the
general population.
5.2. Occupational Exposure to 2,4-D During the Production, Handling,
and Use of Chlorophenoxy Herbicides
During occupational exposure to 2,4-D, the chemical may be
absorbed via the inhalation, oral, and dermal routes, but more than
90% of the total amount of 2,4-D or other chlorophenoxy compounds
entering the body under these circumstances appears to be absorbed
through the skin and excreted relatively quantatively in the urine
as the phenoxy acid and readily-hydrolysed conjugates (Kolmodin-
Hedman et al., 1979, 1980; Libich et al., 1981; Draper & Street,
1982; Franklin et al., 1982; Leng et al., 1982; Nash et al., 1982)
(section 6).
Data from occupational exposure studies concerning the amounts
of 2,4-D found on the clothing or on cloth patches worn by workers
are not included in this review because the correlation between
these amounts and amounts absorbed into the body and then excreted
in urine is poor (Franklin et al., 1982; Lavy et al., 1982; Leng et
al., 1982).
Table 11. 2,4-D residues in game and domestic animals and animal products
------------------------------------------------------------------------------------------------------------------
Country Year(s) Species 2,4-D treatment Post- Type of 2,4-D residues References
rate (kg a.i./ha) treatment samples (mg/kg)
interval examined
(days)
------------------------------------------------------------------------------------------------------------------
Sweden 1968 moose (Alces game animals found ? liver and < 0.05-6 Erne (1974,
-1972 alces) dead, or shot by kidney from 1975)
deer (Capreolus hunters in herbicide- 250 animals
capreolus) treated areas found dead
hares (Lepus
lepus)
pheasants ? liver and < 0.05-4.5
grouse kidney from (2,4-D and
130 animals 2,4,5-T)
shot by
hunters
USA 1963 Jersey cow 50 ppm in diet for 4 0-2 milk < 0.1 Bache et al.
days (1964a)
USA 1974(?) adult beef 0, 9, 30 or 60 mg 0 muscle < 0.05-0.07 Clark et al.
cattle 2,4-D acid/kg bw/day 28 fat < 0.13-0.34 (1975)
for days (0, 300, liver < 0.05-0.23
1,000 2000 mg/kg feed) kidney 2.53-10.9
USA 1974(?) adult sheep 2000 mg/kg feed for 0-7 muscle < 0.05-0.06 Clark et al.
28 days fat 0.10-0.15 (1975)
liver 0.29-0.98
kidney 0.37-9.17
USA 1965(?) dairy cows animals grazing on 2 milk 0.01-0.09 Klingman et al.
pasture sprayed with 4 (1966)
herbicide at 2, 24 kg
a.i./ha
USA 1972 dairy cows 30, 300, 1000 mg/kg 0 milk < 0.05-0.16 Bjerke et al.
in feed for 2-3 weeks (1972)
1-3 < 0.05 Leng (1972)
USSR 1975 "livestock" ? ? muscle 0.04 Fyodorova et
liver 0.04 al. (1977)
kidney 0.03 (mean)
------------------------------------------------------------------------------------------------------------------
5.2.1. Industrial exposure
Several studies have been published on the levels of 2,4-D to
which workers producing or packaging 2,4-D herbicides are exposed
(Fetisov, 1966; Johnson, 1971; Juzwiak et al., 1973; Andreasik et
al., 1979). In every case the amount of 2,4-D absorbed by the
workers was uncertain and, therefore, the data are inadequate for
estimating industrial exposure to 2,4-D. Workers manufacturing
2,4-D were also exposed to other chemicals (Assouly, 1951; Bashirov
& Ter-Bagdasarova, 1970).
5.2.2. Exposure related to herbicide use
The available studies on the occupational exposure to 2,4-D of
workers during the use of 2,4-D herbicides are summarized in Table
12. Studies on the exposure of back-pack sprayers to 2,4-D have
not been published. However, comparable exposure data are
available for 2,4,5-T back-pack sprayers, and they have been
included in Table 12 for comparison. The levels of 2,4-D found in
the air of the working zone in these and other studies have already
been referred to in section 5.1.1.1 and Table 5.
In studies undertaken before 1980, only the amounts of 2,4-D in
the air, on the clothing, or on the skin were determined, except
for 2 urinary 2,4-D values reported by Shafik et al. (1971). Thus
the amounts of 2,4-D actually absorbed cannot be reliably estimated
from these early reports and are not included in Table 12.
After 1980, several detailed occupational exposure studies were
carried out to determine the amounts of 2,4-D or other chlorophenoxy
acids absorbed by various members of ground and aerial spray teams,
using a variety of equipment for dispersing aqueous or oil solutions
or emulsions (Kolmodin-Hedman et al., 1979; Kolmodin-Hedman & Erne,
1980; Libich et al., 1981; Draper & Street, 1982; Franklin et al.,
1982; Lavy et al., 1982; Leng et al., 1982; Nash et al., 1982).
The total 2,4-D urinary excretion levels reported in Table 12
reflect a wide variety of uses and show that the excretion does not
usually exceed 0.1 mg 2,4-D/kg body weight per day of exposure.
However, so far, a comprehensive comparison of the relative
exposures resulting from different methods of application and
different 2,4-D derivatives (amine salts and esters) or formulations
(aqueous, oil) cannot be carried out, because the available data are
still incomplete. The amount of 2,4-D absorbed depends on the type
of work performed, and on the degree of care taken to avoid direct
dermal contact with the herbicide concentrate, spray solution, or
spray. The most heavily-exposed workers tend to be the mixer-
loaders, who handle the herbicide concentrate, and the spray
personnel. However, if careful, they may be exposed to less 2,4-D
than, for example, a pilot of a spray plane who is not careful
(Franklin et al., 1982; Leng et al., 1982; Lavy et al., 1982; Nash
et al., 1982). The reports by Libich et al. (1981) and Leng et al.
(1982) on ground spray crews indicate that, even under unfavourable
working conditions, the amount of 2,4-D absorbed may be greatly
reduced simply by wearing clean gloves and overalls, and by making
the workers more aware of the importance of safe work habits.
Table 12. Exposure related to herbicide use
------------------------------------------------------------------------------------------------------------------------
Product No. of Type of Daily concentr- Duration of Total 2,4-D in urine References
people application ation of 2,4-D collection of excreted (mg/kg bw/
exposed in urine 24-h urine day of exposure)
(mg/litre)e samples (days)
------------------------------------------------------------------------------------------------------------------------
2,4-D and dicamba 2 boom spray 1 - 4 - - Draper & Street
dimethylene salts single use (1982)
in aqueous solution 2 repeated use 3 - 20 - -
2,4-D isooctyl 4 3 applications - 4 0.004 - 0.04 Franklin et al.
ester in diesel oil by single- (1982)
engine aircraft
2,4-D/2,4,5-T 4 tractor-drawn 1 - 14 7 - Kolmodin-Hedman
butoxyethyl sprayers, forestry (1979, 1980)
esters as 2% exposure daily,
emulsion in water for one week
2,4-D PGBE ester 26 helicopter in - 5 nd - 0.06a Lavy et al.
26 forestry use - 5 nd - 0.02b (1982)
2,4,5-T PGBE ester 7 Back-pack 5 0.01 - 0.09 Leng et al.
forestry use (2,4,5-T) (1982)
single exposures,
one week apart
2,4-D/2,4-DPc and 23 roadside and < 0.01 - 8 3 - Libich et al.
2,4-D/picloramc right-of-way (one usually (1981)
ground equipment high result
incl. mist blowers of 31)
2,4-Dc 17 aircraft repeated - 7 0.006 - 0.02d Nash et al.
exposure (mean values/day) (1982)
2,4-D amine salt 26 ground equipment - 7 nd - 0.08
and ester (single exposure)
------------------------------------------------------------------------------------------------------------------------
a No special precautions taken.
b Protective clothing worn.
c Preparation used not specified.
d Mean values per day recorded for different individuals.
e It is not possible to calculate the total 2,4-D excretion in urine from these data, because of individual variations
in urine concentrations from day to day from sample to sample.
As the chemobiokinetic profiles of urinary 2,4-D output are
reported in only a few of the studies, summarized in Table 12, it
is not possible to estimate the total 2,4-D intake in all cases.
The results of the studies by Libich et al. (1981) and by
Draper & Street (1982) suggest that using single-exposure studies
to estimate the peak exposure levels reached by workers exposed
several days in succession may give an underestimation.
No information is available on the amounts of chlorinated
dibenzodioxins, or other by-products or contaminants, absorbed as a
consequence of occupational exposure to 2,4-D herbicides.
In one extensive occupational monitoring programme undertaken
in 1979 - 82, about 3000 urine samples were analysed for herbicide
residues (Simpson, 1982). The subjects included pesticide factory
staff, pest control operators, farmers, park workers, and others
potentially exposed to 2,4-D. During the first year of the study,
no 2,4-D was detected (< 0.001 mg/litre) in 735 of 973 samples.
Most of the other samples contained less than 0.1 mg/litre and only
27 contained more than 1 mg/litre. The highest value was 31
mg/litre. The study is continuing.
5.3. Exposure of Bystanders to 2,4-D
Aerial drift and other forms of pesticide transport, as well as
the contamination of surfaces during or after herbicide production,
distribution, or use, may bring 2,4-D into contact with bystanders,
i.e., persons other than those who are occupationally exposed. Few
studies of bystander exposure to 2,4-D or other chlorophenoxy
herbicides have been published. Studies available for review
included that of Lavy et al. (1982) concerning 9 supervisors and
observers present at two helicopter forest spray operations using
2,4-D propyleneglycol butylether (PGBE) ester, respectively, for
unspecified durations. These people excreted a maximum of 1.3 µg
2,4-D/kg body weight. In a forest ground spray operation with
tractor-drawn equipment, 2,4-D was not detected (< 0.05 mg/litre)
in the urine of bystanders (Kolmodin-Hedman et al., 1980).
Additional bystander exposure studies for various 2,4-D use
patterns are desirable. However, the 2,4-D intake of bystanders is
unlikely to exceed the 2,4-D intake during occupational exposure.
5.4. Estimated Exposure of the General Population in 2,4-D-Use Areas
Data useful for estimating the intake by the general population
of 2,4-D residues in the environment including those in food
sources have been generated. The present calculations of the
intake of the general population in an area of 2,4-D use are based
on these data and on a series of stated assumptions aimed at
obtaining a moderate overestimation rather than underestimation of
the actual exposure.
5.4.1. Intake of 2,4-D residues from air
On the basis of available information, it can be assumed that
the general population in areas of 2,4-D herbicide use would rarely
be exposed to 2,4-D concentrations exceeding 0.1 µg/m3 air.
Assuming an air level of 0.1 µg 2,4-D/m3, a body weight of 60
kg, an air intake of 20 m3 per day, and a 100% retention of 2,4-D,
it can be calculated that the respiratory intake would be 0.03 µg
2,4-D/kg body weight per day.
5.4.2. Intake of 2,4-D residues from potable water
The larger surveys of potable water (Table 7) show mean 2,4-D
residues in surface water to be generally < 0.1 µg/litre, but for
the present estimate, it is assumed that potable water from surface
sources or from treatment plants, during a period of about 10 days
after reservoir treatment, can contain an average 2,4-D residue
level of 2 µg/litre (Wojtalik et al., 1971 and Table 7). Assuming
a 2,4-D concentration in water of 2 µg/litre, a body weight of 60
kg, a water intake of 2 litres per day (Canada, Health & Welfare,
1980), and a 100% absorption of the ingested 2,4-D, it can be
calculated that the 2,4-D intake of the general population in a
2,4-D use area resulting from water could approach 0.07 µg/kg body
weight per day, which could occur for about 10 days.
Insufficient data are available to give a reliable estimate of
2,4-D intake from ground water sources, but it is likely to be
lower than the above value.
5.4.3. Intake of 2,4-D residues from soil
2,4-D on soil particles ingested with food or water, or carried
into the air and inhaled, is considered to be part of the exposure
due to residues in air, water, or food and is therefore assumed to
be completely covered in these exposure estimates.
5.4.4. Intake of 2,4-D residues from food
The data in Tables 8 - 11 indicate that there is unlikely to be
any exposure of the general population to 2,4-D residues in retail
food supplies. The possibility that individuals are exposed to
contaminated local sources of food has been assessed in section
5.1.4. In the case of milk or muscle meat, it can be assumed that
no individual will be exposed to levels in excess of 0.02 mg/kg of
these foods, the limit of detection of the method of analysis used.
Assuming a concentration of 0.02 mg 2,4-D/litre in milk, and a
consumption of 1.5 litre per day, the maximum intake from this
source would be 0.0005 mg/kg body weight per day for a 60 kg adult.
Individuals who consume wild berries taken from 2,4-D-treated areas
could be exposed through this food source. Assuming consumption of
100 g of berries per serving and a maximum 2,4-D concentration of 1
mg/kg, the intake from this source would be 0.002 mg/kg body weight
per serving.
5.4.5. Total exposure of the general population in a 2,4-D-use area
The above considerations suggest that the total daily 2,4-D
intake of the population in use areas will not normally exceed
about 0.002 µg/kg body weight during the application period (Table
13).
Table 13. Components of estimasted exposure to 2,4-D
-------------------------------------------------------------------
Estimated amount of
Exposed Group intake (µg 2,4-D/kg Source of 2,4-D
bw/day)
-------------------------------------------------------------------
Occupational
i. Factory workers insufficient data mainly dermal contact
ii. Applicator crews 100a
iii. Bystanders - b
General population in
areas with 2,4-D use 0.03 air
0.07 water
0.5 milk
ND retail food
2.0 wild berries, mushrooms
etc.
-------------------------------------------------------------------
a Based on total urinary output after several days of exposure.
b Unlikely to exceed occupational exposure.
5.4.6. Total exposure of persons occupationally exposed in agriculture
An accurate maximum occupational intake of 2,4-D cannot be
determined on the basis of the limited studies undertaken.
However, the available data suggest that work performed in the
preparation of, and during, agricultural application of 2,4-D
herbicide will probably result in an exposure of not more than
about 0.1 mg 2,4-D/kg body weight per day, providing that minimum
precautions are taken against excessive exposure.
5.4.7. Total exposure of the general population outside areas of 2,4-D use
Monitoring of air, water, and food outside areas of known 2,4-D
use show that intake is below present detection limits.
6. CHEMOBIOKINETICS AND METABOLISM
With the exception of recent occupational exposure studies and
studies on animals published in 1979 or later, the available
information on the uptake, distribution, transformation, and
excretion of 2,4-D by human beings and other mammals has already
been reviewed by Leng (1977), National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Young et al. (1978), Bovey (1980a,b), Shearer
(1980), and United States Veterans' Administration (1981).
6.1. Uptake via Different Routes of Exposure
6.1.1. Uptake by inhalation
6.1.1.1. Animals
Burton et al. (1974) found that small amounts of 2,4-D
instilled into the rat lung were rapidly absorbed, apparently by a
non-saturable process following first-order kinetics, with an
absorption half time of 1.4 - 1.7 min. The kinetics of the
absorption of 2,4-D vapours or aerosols in the respiratory tract of
animals have not yet been studied.
6.1.1.2. Human beings
The uptake of 2,4-D and of 2,4-D derivatives via the human
respiratory tract does not appear to have been studied under
controlled conditions. However, the observations of Kolmodin-
Hedman & Erne (1980), Libich et al. (1981), Franklin et al. (1982),
and Lavy et al. (1982) on people occupationally exposed to 2,4-D
indicated that only a small percentage of the total amount of 2,4-D
absorbed via all routes of exposure was taken in through the
respiratory tract.
6.1.2. Dermal uptake
6.1.2.1. Animals
Mice whose tails had been immersed in 2,4-D butyl or crotyl
ester solutions, 4 h daily for 3-5 days, absorbed lethal amounts of
the chemicals (Fetisov, 1966). However, the actual doses absorbed
and other details were not given. In contrast, no major ill
effects were reported in studies in which rabbits were treated
percutaneously for 2 or 3 weeks with 130 - 180 mg/kg body
weight/day of a 50% aqueous solution of 2,4-D octyl ester, or with
unspecified amounts of solutions of 2,4-D dimethylamine salt in
water, or oil solutions of 2,4-D isooctyl or butyl ester
(Vinokurova, 1960; Kay et al., 1965).
6.1.2.2. Human beings
Only 5.8% of a dilute solution of 14C-labelled 2,4-D in acetone
applied at a dose of 4 µg a.i./cm2 to the ventral forearm of adults
was excreted in the urine compared with 100% of a small intravenous
dose (Feldmann & Maibach, 1974) (Table 14). The 2,4-D excretion in
urine is delayed and more prolonged after dermal application than
after intraveneous or oral administration (Feldmann & Maibach,
1974; Sauerhoff et al., 1977), and complete elimination may take
about one week (Levy et al., 1982; Leng et al., 1982). Cases of
acute occupational 2,4-D poisoning following combined dermal and
inhalation exposures (Monarca & Divito, 1961; Tsapko, 1966;
Paggiaro et al., 1974), as well as occupational exposure studies
(Table 12), suggest a fairly efficient dermal absorption of 2,4-D.
However, the importance of solvents, surfactants, and other
ingredients of the herbicides in the uptake of 2,4-D via the dermal
route still needs to be defined.
6.1.3. Oral uptake
6.1.3.1. Animals
The uptake of 2,4-D from the gut of rats, mice, guinea-pigs,
cattle, pigs, and sheep appears to be similar in both rapidity and
extent to that observed in human beings (Mitchell et al., 1946;
Lisk et al., 1963; Bache et al., 1964a; Erne, 1966a,b; Milhaud et
al., 1970; Shafik et al., 1971; Buslovich et al., 1973; Fedorova &
Belova, 1974; Clark et al., 1975; Senczuk & Pogorzelska, 1975,
1981; Van Peteghem & Heyndrickx, 1975). In some of the ungulates,
2,4-DB acid, and 2,4-D amine salts or esters are at least partially
converted to 2,4-D in the rumen, before being absorbed (Gutenmann
et al., 1963; Lisk et al., 1963). Some of the esters may be less
well absorbed from the gut than the acid or its alkali or amine
salts (Erne, 1966a; Buslovich et al., 1973), but the uptake
mechanisms for 2,4-D and its salts or esters is not known, and thus
deserves further study.
6.1.3.2. Human beings
Information on the uptake of 2,4-D by human beings via the
oral route has been gathered in studies on two groups of 5 - 6
volunteers each, who ingested single doses of 5 mg 2,4-D/kg body
weight (Table 14), and by chemobiokinetic studies on individuals
who, with suicidal intent, swallowed lethal or non-lethal amounts
of various 2,4-D herbicides (Geldmacher-Von Mallinckrodt &
Lautenbach, 1966; Rivers et al., 1970; Kohli et al., 1974;
Sauerhoff et al., 1976, 1977; Khanna & Kohli, 1977; Young & Haley,
1977; Prescott et al., 1979) (Table 15). These results show that
single doses of 2,4-D are fairly rapidly and completely absorbed
from the human digestive tract, unless the dose is so large that
toxic effects interfere with absorption. However, in the two
studies on volunteers, considerable individual variation in the
rate and extent of absorption from the digestive tract was
observed. The absorption mechanism appears to involve first-order
kinetics (Kohli et al., 1974; Khanna & Kohli, 1977) and may fit a
single- or multi-compartment chemobiokinetic model, depending on
individual characteristics (Sauerhoff et al., 1977).
Table 14. Chemobiokinetics of 2,4-D in human beings following administration under controlled conditions
--------------------------------------------------------------------------------------------------------------------------
Product Dose and dosing Subjects Observations Toxic effects References
schedule EL NOELa
(mg/kg bw)
single dose
--------------------------------------------------------------------------------------------------------------------------
14C-2,4-D 1) Intravenous injection: 6 (sex & Scintillation counting ? ? Feldmann &
(New England Dose (7 µCi) not stated as age not 1) 100% of dose excreted in urine Maibach
Co., and 2,4-D/weight unit stated) urine in 120 h Mean t0.5 = 13 h (1974)
Amersham 2) Dermal application: 6 (sex & 2) 5.8% of applied dose excreted ? ?
Searle Co.) 1 x 4 µg 2,4-D (in acetone)/ age not in 120 h
cm2 of skin of forearm. stated)
Application site was not
washed for 24 h
2,4-D, 99% Oral administration: 6 gas chromatography of blood & ? 5 Khanna &
pure (Dow 1 x 2, 3, or 5 mg/kg bw, in (adult M) urine samples; no ill effects; no Kohli (1977);
Chemical gelatin capsule, with water, changes in clinical parameters: Kohli et al.
Co.) following breakfast blood pressure, pulse rate, Hb, WBC (1974)
counts (total & differential); mean
plasma clearance t0.5 = 33 ± 3.1 h;
peak plasma conc. at 7-24 h = 40
mg/litre; ~75% of dose excreted
in urine in 96 h
"no metabolic transformation
at up to 5 mg/kg"
2,4-D, Oral administration: 6 gas chromatography - mass ? 5 Sauerhoff
analytical 1 x 5 mg/kg bw as a slurry (adult M, spectrometry of blood & urine et al.
grade in milk, or in powder form, 70-90 kg) samples; no ill effects; (1976, 1977)
with some water, following essentially all of the dose
breakfast absorbed; peak plasma conc. = 10-30
mg/litre within 24 h; mean plasma
clearance t0.5 = 11.6 h; mean
urinary excretion t0.5 = 17.7 h;
total excreted amount ~82% of dose
administered; 4.8-27.1% of
excreted compound was conjugated
--------------------------------------------------------------------------------------------------------------------------
a NOEL = No-observed-adverse-effect level.
Table 15. Chemobiokinetics of 2,4-D by human beings following accidental or intentional ingestion of herbicides
----------------------------------------------------------------------------------------------------------------
Products Circum- Subject Observations References
stances
----------------------------------------------------------------------------------------------------------------
2,4-D suicide; F, 33 death in about 30 h; post mortem 2,4-D Geldmacher-Von
ingestion years concentration: Mallinckrodt &
of unknown mg/litre mg/kg Lautenbach (1966)
amount of blood urine brain liver lung heart
herbicide 23 164 100 116 88 63
no metabolites were identified
Herbicide suicide; F, 51 death in about 96 h; concentration of 2,4-D
containing ingestion years, plus MCPA:
2,4-D plus of unknown 66 kg mg/litre mg/kg
MCPA amount of blood urine liver kidney muscle
("U46 COMBI") herbicide 42 420 100 trace 40
(BASF 2,4-dichlorophenol not detected; several
Ludwigshafen) other metabolites or herbicide by-products
found, but not identified
Herbicide suicide M, 39 severe toxic effects; unconsciousness; Prescott et al. (1979)
containing attempt; years recovery within 11 days following treatment by
2,4-D plus ingestion alkaline diuresis; initial plasma concentration:
mecoprop of 6.7 g 2,4-D = 400 mg/litre, mecoprop = 750 mg/litre;
(10%) + (20%) 2,4-D and no pretreatment change in 2,4-D level following
7.6 g alkaline diuresis; plasma clearance t0.5 = 3.7 h
mecoprop for 2,4-D, 11-28 h for mecoprop
----------------------------------------------------------------------------------------------------------------
6.2. Distribution and Transformation in the Body
6.2.1. Animals
The absorption and distribution kinetics and metabolism of pure
2,4-D and of a variety of pure or commercial 2,4-D or 2,4-DB amine
salts and esters have been repeatedly studied both in vivo and in
vitro in a wide variety of animals including rats, mice, rabbits,
guinea-pigs, cattle, sheep, goats, pigs, chickens, fish, and spiny
lobsters (Gutenmann & Lisk, 1965; Erne & Sperber, 1974; Guarino &
Arnold, 1979; James, 1979; Koschier & Pritchard, 1979; Pritchard
& James, 1979; Pritchard & Miller 1980). The considerable
differences observed in the relative amounts of residues found in
the cells and plasma of mouse, rat, and horse blood, after dosing
animals or after in vitro addition of 2,4-D (Erne, 1966a; Jenssen
& Renberg, 1976), in different tissues of rats, mice, and sheep
(Erne, 1966a,b; Lindquist & Ullberg, 1971; Milhaud et al., 1970;
Buslovich et al., 1973; Clark et al., 1975; Elo & Ylitalo, 1979),
and in the soluble and particulate fractions of rat tissues (Khanna
& Fang, 1974) support the idea that there is more than one
physiological compartment for 2,4-D storage. The distribution
volumes appear to be equivalent to the volume occupied by about
25 - 50% of the body mass (Erne, 1966a).
2,4-D is reversibly bound to blood plasma proteins,
particularly albumins, possibly at sites for which it competes with
related compounds. The same sites are apparently also binding
sites for palmitic acid and thyroxine. The extent of 2,4-D binding
depends, in part, on pH and 2,4-D concentration (Florsheim et al.,
1963; Erne, 1966b; Kolberg et al., 1973; Hacque et al., 1975;
Mason, 1975; Orberg, 1980a), and may affect the rate and extent of
renal 2,4-D excretion (Pritchard & James, 1979; Pritchard & Miller,
1980) and thus the toxicity of 2,4-D.
In pregnant mammals, up to about 17% of a single dose of 2,4-D
may rapidly cross the placenta to reach the embryos or fetuses
(Lindquist & Ullberg, 1971; Fedorova & Belova, 1974; Antonenko,
1977).
Pigs and rats hydrolyse 2,4-D esters both in the gut and after
absorption in the body (Erne, 1966a,b). Observations from several
studies indicate that 2,4-D is not significantly metabolized in
animals, except in ruminants. No 14CO2 was produced by rats given
C1- or C2-labelled 14C-2,4-D (Khanna & Fang, 1966). No 2,4-
dichlorophenol (2,4-DCP) was detected in the tissues of mice or
rats dosed by various routes with 2,4-D (Zielinski & Fishbein,
1967; Shafik et al., 1971; Federova & Belova, 1974; Grunow & Böhme,
1974). However, residues of 2,4-DCP were detected in the milk of
dairy cows fed 100 mg 2,4-D/kg diet for 3 weeks (Bjerke et al.,
1972; Leng, 1972), and in the livers and kidneys of cattle and
sheep fed up to 2000 mg/kg diet for 4 weeks (Clark, 1975; Leng,
1972, 1977). These 2,4-DCP residues probably resulted from the
bacterial degradation of 2,4-D in the rumen of the animals.
Bacterial degradation may also account for the 2,4-DCP reported by
Antonenko (1977) in pregnant or lactating rats and rat fetuses.
Other investigators did not detect 2,4-DCP in the tissues of
mice or rats dosed with 2,4-D by various routes (Zielinski &
Fishbein, 1967; Shafik et al., 1971; Fedorova & Belova, 1974;
Grunow & Böhme, 1974).
Results of studies on experimental animals have suggested that
2,4-D conjugates are formed in the kidney tubules (Erne, 1966a,b;
Erne & Sperber, 1974; Grunow & Böhme, 1974).
Taurine and glycine conjugates, as well as various other
unidentified conjugates of 2,4-D have been found in the urine of
rats, pigs, chickens, and the dogfish shark (Squalus acanthias)
(Erne, 1966b; Erne & Sperber, 1974; Grunow & Böhme, 1974; Koschier
& Pritchard, 1979). However, in rats and pigs, only about 10-20%,
and in the chicken, less than 5% of the total amount of 2,4-D
appeared to be excreted in this form. In the dogfish shark, the
taurine conjugate may be primarily formed in the tubular cells of
the kidney (Koschier & Pritchard, 1979). The site and mechanism of
2,4-D conjugation seem to be unknown in the other species.
6.2.2. Human beings
Studies on human volunteers who ingested pure 2,4-D, and on
cases of accidental or voluntary acute poisoning with various 2,4-D
herbicides have shown that 2,4-D is very rapidly absorbed from the
gut and carried in the blood to cells and tissues throughout the
body, but that is not extensively transformed (Tables 14, 15)
(Curry, 1962; Herbich & Machata, 1963; Nielsen et al., 1965; Dudley
& Thapar, 1972; Coutselinis et al., 1977). The kinetics following
ingestion suggest a 1- or 2-compartment distribution, depending on
individual characteristics (Sauerhoff et al., 1977; Young & Haley,
1977). Following absorption of purified 2,4-D, or of herbicides
containing only 2,4-D, no transformation products, including 2,4-
dichlorophenol, were found in blood or tissues. After ingestion of
herbicides containing 2,4-D and other compounds, some 2,4-D
metabolites or manufacturing by-products were detected in tissues,
but were not identified (Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Prescott et al., 1979). Unidentified 2,4-D conjugates were
also found in urine following ingestion of pure 2,4-D. These
conjugates represented up to 27% of the 2,4-D ingested (Sauerhoff
et al., 1976, 1977). Of the 5 North American volunteers studied by
these authors, only one did not produce a conjugate; in contrast,
apparently none of the 6 Indian subjects studied by Kohli et al.
(1974) and by Khanna & Kohli (1977) produced 2,4-D metabolites.
6.3. 2,4-D Levels in Body Tissues and Fluids
6.3.1. Animals
2,4-D levels in the blood and organs of mammals have been
determined, e.g., by Erne (1966a,b), Milhaud (1970), Buslovich et
al. (1973), Khanna & Fang (1974), Clark et al. (1975), Jenssen &
Renberg (1976), and Elo & Ylitalo (1979). The highest residue
levels were usually found in liver, kidney, lungs, spleen, and
heart. In a study by Fedorova & Belova (1974), 6 - 8% of the
amount of 2,4-D administered was found in all of the tissues
examined in rats dosed orally 26 - 35 days previously with this
chemical. However, the 2,4-D residue levels quoted were close to
the limit of detection for the analytical method used.
6.3.2. Human beings
In volunteers, each of whom ingested a single dose of 5 mg
2,4-D/kg body weight, the 2,4-D levels in blood plasma reached
peaks of about 10 - 45 mg/litre within about 7 - 24 h, and then
declined (Kohli et al., 1974; Khanna & Kohli, 1977; Sauerhoff et
al., 1977). In one group of workers occupationally exposed to
2,4-D for one week while using ground equipment for spraying
(Kolmodin-Hedman et al., 1979), plasma levels ranged from the
detection limit (0.02 mg/ml) to 0.2 mg/ml, while urinary levels
ranged from 1 to 14 mg/litre. Urinary 2,4-D levels reported in
other occupational exposure studies are summarized in Table 12.
However, it should be noted that analysis of single urine specimens
is not adequate for estimating the dose absorbed by individuals,
because excretion follows a diurnal pattern and continues for
several days after dermal exposure (Leng et al., 1977; Sauerhoff et
al., 1979; Lavy et al., 1982). Thus, levels found after several
days of spraying will probably be higher than first day levels, as
reported by Libich et al. (1981) and Draper & Street (1982).
However, excretion of 2,4-D should be completed within one week
following the last exposure (Feldmann & Maibach, 1974; Lavy et al.,
1982; Leng et al., 1982).
The toxic and lethal levels of 2,4-D in human blood and tissues
are still not well defined. A woman with reportedly 335 mg
2,4-D/litre plasma did not show any signs of poisoning; in general,
the acute lethal levels of 2,4-D appear to lie between 447 and 826
mg/litre plasma (Herbich & Machata, 1963; Nielsen et al.,
1965; Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Coutselinis
et al., 1977; Prescott et al., 1979). The lowest lethal 2,4-D
levels in blood or tissues were recorded several days after the
chemical was ingested, i.e., after most of the 2,4-D had probably
been eliminated. Among the different organs examined post mortem
in cases of fatal 2,4-D poisoning, liver and kidney tended to
contain the highest concentrations of 2,4-D, while brain and other
fatty organs, and muscle including the heart, usually had lower
2,4-D levels (Table 15) (Curry, 1962; Herbich & Machata, 1963;
Nielsen et al., 1965; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972; Coutselinis et al., 1977). As in the
case of blood, the values for the different tissues may vary
according to the proportion of 2,4-D eliminated by the time death
occurs.
6.4. Elimination and Biological Half-Life
The term "biological half-life" will be used to indicate the
time required to eliminate one half of a single dose of 2,4-D, or
to reduce 2,4-D residues in the body fluids or tissues to one-half
of the peak concentration. Biological half-life, as defined here,
is a useful concept with which it is possible to make rough
comparisons of the elimination rate of 2,4-D with that of other
toxic chemicals.
6.4.1. Animals
The half-life values recorded in mammals fall into the range
observed in the rat (Erne, 1966a,b; Federova & Belova, 1974; Khanna
& Fang, 1974), with the exception of the very low value for 2,4-D
butyl ester in whole mouse carcasses (0.85 - 1.11 h) observed by
Zielinski & Fishbein (1967). Results of the investigations
conducted by these authors also suggest that prior dosing with
2,4-D ("priming") may increase the rate of elimination of 2,4-D
butyl ester in mice, presumably through stimulation of the renal
excretory mechanism.
An important correlation between diet and 2,4-D elimination
rate was observed by Orberg (1980b) in the goat. A protein-poor
diet reduced the 2,4-D plasma clearance rate by about 20 - 50%,
possibly because of decreased renal size and renal blood flow.
The many species-related factors known to affect the rate of
2,4-D elimination make interspecies comparisons difficult, and
often the published reports do not include such details as diet,
age, sex, and body weight of the test animals, type and purity of
the test compounds, and important environmental factors such as the
ambient temperature, which are necessary for valid comparisons.
6.4.2. Human beings
The studies on volunteers and on cases of accidental or
voluntary 2,4-D poisoning summarized in Tables 14 and 15 show that
human beings excrete 2,4-D mainly in the urine, and that the
blood plasma clearance times depend on the dose, individual
characteristics, and the presence or absence of compounds that may
competitively inhibit 2,4-D excretion. For single oral doses of
2,4-D, the biological half-life in blood plasma is about one day,
depending on the circumstances. However, forced alkaline diuresis
may reduce this to as little as 3.7 h (Sauerhoff et al., 1977;
Prescott et al., 1979).
In occupationally-exposed people, absorption of successive
daily doses of 2,4-D makes its biological half-life difficult to
determine, but for single occupational exposures it has been
estimated to be 35-48 h (Nash et al., 1982).
6.5. Chlorinated Dibenzo- p-Dioxins (CDDs)
The metabolism in the rat of several dibenzo- p-dioxins,
including the 2,7-CDD found in 2,4-D, was described by Tulp &
Hutzinger (1978). The primary pathway of transformation appears
to involve hydroxylation at the 2-, 3-, 7-, or 8-position in the
molecule; some sulfur-containing conversion products have also been
identified.
7. EFFECTS OF 2,4-D ON ANIMALS
7.1. General Introduction
Many studies of the toxicity of 2,4-D were carried out before
the possible toxicological importance of manufacturing by-products,
such as 2,6-dichlorophenoxyacetic acid, 2,4,6-trichlorophenoxy-
acetic acid (2,6-D and 2,4,6-T) monochlorophenoxyacetic acid, or
N-nitroso compounds, was known or appreciated.
Furthermore, 2,4-D may be contaminated with several chlorinated
dibenzo- p-dioxins (section 2.1.4). The toxicity of a number of
these contaminants has not been examined in detail, but whether or
not their presence would affect the toxicity of 2,4-D and its
derivatives would depend on the amount of CDD present in the
product, and on the inherent toxicity of the particular CDD
isomers. The most toxic CDD isomer, namely 2,3,7,8-TCDD (Schwetz
et al., 1973; McConnell et al., 1978; Leng, 1979; Kociba & Schwetz,
1982), is not normally found in 2,4-D products (see also section
2.1.4). However, there have been instances in which the same
manufacturing equipment was used to produce both 2,4,5-T and 2,4-D,
resulting in cross-contamination of 2,4-D with 2,4,5-T and 2,3,7,8-
TCDD (US EPA, 1982).
Studies of structure-activity relationships using in vitro
systems have shown that the CDDs that may be present in 2,4-D and
its derivatives have a much lower biological activity than 2,3,7,8-
TCDD (Poland & Glover, 1973; Poland & Kende, 1974; Poland et al.,
1976; Bradlaw et al., 1980; Knutson & Poland, 1980). However,
except for a study of the carcinogenic potential of 2,7-
dichlorodibenzodioxin (2,7-DCDD) (US National Cancer Institute,
1979), the chronic toxicity of the CDD detected in 2,4-D and its
derivatives has not been studied.
2,4-D has been in use as a herbicide for nearly 40 years, and
during this time a great deal of literature on the toxicology of
this chemical has accumulated. The extent to which its toxicity to
various organisms has been tested, and the types of 2,4-D products
used for such testing have varied over the years. Earlier 2,4-D
products probably contained higher concentrations of trace
contaminants than the 2,4-D in use today, and therefore it may have
been found to be more toxic in earlier than in more recent studies.
In addition, the generally accepted standards and protocols for
pesticide toxicity tests have changed, making some of the older
tests inadequate by present day standards. For these reasons,
attention has been focused on the more recent studies. The older
studies are, in many instances, only cited for completeness and
should be used with caution, especially when ascribing specific
toxic effects to unspecified 2,4-D products and when establishing
an effect level or no-observed-adverse-effect level for adverse
effects of 2,4-D. The Task Group noted that a number of additional
studies on 2,4-D are at present in progress. As the additional
information becomes available, the present document will need to be
updated.
7.2. Acute Effects
The reports of experimental studies to define the toxic and
other effects of 2,4-D or its derivatives cover a wide range of
organisms commonly referred to as "animals", including worms,
molluscs, arthropods, lower vertebrates, birds, and mammals. Much
of this information, especially on acute toxic effects, was
recently tabulated by the National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Schneider (1979), Bovey & Young (1980), Shearer &
Halter (1980), and the Commission of the European Communities (CEC,
1981).
The usual mandatory acute and subacute safety tests for
pesticides include assays for eye and skin irritancy and for skin
sensitization, and determinations of the acute oral, percutaneous,
and parenteral lethal doses, or of the corresponding lethal
concentrations in the air, diet, or water.
7.2.1. Skin and eye irritancy
2,4-D does not appear to be an eye or skin irritant (Schneider,
l979). Adequate tests of the potential irritative properties of
2,4-D derivatives have not been reported in the literature.
7.2.2. Skin sensitization
No adequate published information is available on the dermal
sensitization potential of 2,4-D and its derivatives in mammals.
7.2.3. Lethal doses and concentrations (LD50 and L50)
The lethal potential of a chemical is usually measured as the
dose (mg/kg body weight), or as the concentration in the air, diet,
or water (mg/kg, mg/m3, mg/litre, respectively) that will kill 50%
of the test animals in a specified time interval. These amounts
are referred to as the LD50 or LC50. For 2,4-D and its derivatives,
and for 2,4-D herbicide formulations, these statistically estimated
values vary depending on the test product, the test species, and
the route and frequency of administration (Tables 16 and 17).
7.2.3.1. Acute oral LD50
7.2.3.1.1. Mammals
Published acute oral LD50 values vary for different 2,4-D
products and test species (Table 16). It appears that 2,4-D has a
moderate acute toxicity for mammals (WHO, 1976).
7.2.3.1.2. Birds
Table 17 shows published oral LD50 values for chickens.
Table 16. Acute oral toxicity of 2,4-D, esters, and salts
------------------------------------------------------------------------
Compound Species Sex LD50 Reference
(mg/kg bw)
------------------------------------------------------------------------
2,4-D mouse M 375 Hill & Carlisle (1947)
mouse M 368 Rowe & Hymas (1954)
rat M 375 Rowe & Hymas (1954)
rat 666 Hill & Carlisle (1947)
guinea-pig M & F 469 Rowe & Hymas (1954)
guinea-pig 1000 Hill & Carlisle (1947)
rabbit 800 Hill & Carlisle (1947)
dog 100 Drill & Hiratzka (1953)
butyl ester mouse 380 Konstantinova (1970)
rat 1500 Schillinger (1960)
rat 920 Konstantinova (1970)
cat 820 Konstantinova (1970)
esters of mono-, rat F 570 Rowe & Hymas (1954)
di-, and tripro-
pylene glycol
butyl ethers
isopropyl ester mouse M 541 Rowe & Hymas (1954)
rat M & F 700 Rowe & Hymas (1954)
guinea-pig M 550 Rowe & Hymas (1954)
mixed butyl mouse F 713 Rowe & Hymas (1954)
esters rat F 620 Rowe & Hymas (1954)
guinea-pig F 848 Rowe & Hymas (1954)
rabbit M & F 1420 Rowe & Hymas (1954)
sodium salt mouse 375 Rowe & Hymas (1954)
rat F 805 Rowe & Hymas (1954)
rat 2000 Schillinger (1960)
guinea-pig M 551 Rowe & Hymas (1954)
rabbit 800 Rowe & Hymas (1954)
------------------------------------------------------------------------
7.2.3.2. Acute dermal LD50
7.2.3.2.1. Mammals
Reports by Buslovich (1963) and Fetisov (1966) indicate that,
under extreme test conditions, mice and rats may absorb lethal
amounts of 2,4-D amine salts and esters through their skin, but no
acute dermal LD50 values were available for review.
7.2.3.3. Acute inhalation LC50
No accurate measurements of acute inhalation LC50 values
for 2,4-D products were available.
Table 17. Acute LD50 for various 2,4-D products in domestic chickens (Gallus domesticus)
------------------------------------------------------------------------------------------------------------------
Product Animals Procedure LD50 Acid References
(mg/kg bw) equivalent
------------------------------------------------------------------------------------------------------------------
2,4-D chick, M & F P.O., in olive oil 541 (358-817) 541 Rowe & Hymas (1954)
2,4-D Na salt adult hen P.O., in water 655 646 Loktionov et al. (1973)
2,4-D alkanolamine chick P.O., in water > 380 < 765 368 Bjorn & Northern (1948)
salts Rowe & Hymas (1954)
2,4-D amine salt 6-month chicken P.O., in water 1950 1238 Loktionov et al. (1973)
2,4-D butyl esters chick, M & F P.O., undiluted 2000 (1350-2960) 1503 Rowe & Hymas (1954)
2,4-D butoxyethyl chick, 330 g P.O., in food 900 588 Whitehead & Pettigrew
esters (1972)
2,4-D isopropyl esters chick, M & F P.O., in olive oil 1420 (1127-1789) 1145 Rowe & Hymas (1954)
2,4-D/2,4,5-T (1:1), chick, M & F P.O., undiluted 4000 (2700-5900) - Rowe & Hymas (1954)
butyl ester 3 weeks old
------------------------------------------------------------------------------------------------------------------
7.2.3.4. Parenteral LD50
The acute parenteral LD50 values reported for various
laboratory animals and 2,4-D products ranged from 220 to 666 mg
a.i./kg body weight (National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Young et al., 1978; Schneider, 1979).
7.2.4. Acute toxicity in aquatic organisms
The available reports indicate great differences in acute LC50
values obtained with different test species, and with different
2,4-D derivatives and test formulations. The esters appear to be
considerably more toxic than the water-soluble salts. For fish,
the LC50 for various 2,4-D isooctyl ester products may range from 5
to 68 mg/litre for bluegill sunfish (Lepomis spp.) and from 62 to
153 mg/litre for rainbow trout (Salmo gairdneri) (Schneider,
1979). This variability may, in part, be due to differences in
test animal species and strains, in test conditions (temperature,
pH, 02 tension, mineral content of water), and, in part, to the
effects of chemicals other than 2,4-D in the herbicide
formulations, or of 2,4-dichlorophenol, which may occur in water
as a decomposition product of 2,4-D (Holcombe et al., 1980).
Discussions on this variablility can be found in numerous reviews:
Way (1969), Katz et al. (1972), National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), and of Halter (1980), as well as in
the reports of studies by Harrisson & Rees (1946), King & Penfound
(1946), Lopez (1961), Hughes & Davis (1963), Butler (1965),
Hiltibran (1967), Mount & Stephan (1967), Alabaster (1969), Sanders
(1970a), Andrushaitis (1972), Cooke (1972), Shim & Self (1973),
Fabacher & Chambers (1974), Meehan et al. (1974), Nishiuchi &
Yoshida (1974), Rehwoldt et al. (1977), Vardia & Durve (1981), and
particularly in the extensive and methodical investigation of
Pravda (1973).
Similar information on aquatic invertebrates is available in
the review of Mackenthun & Keup (1972) and in the studies of Hooper
(1958), Sudak & Claff (1960), Beaven et al. (1962), Rawls (1965),
Sanders (1970b), Klekowski & Zvirgzds (1971), Wierzbicka (1974a,b),
and Caldwell et al. (1979).
The observations of Hansen et al. (1972, 1973) and Folmar
(1976) indicate that fish and aquatic invertebrates will try
to avoid water containing toxic amounts of 2,4-D products.
7.3. Subchronic and Chronic Toxicity
7.3.1. Mammals
Most of the published long-term studies with mammals have
already been reviewed by Bodyagin et al. (1969), Way (1969), IARC
(1977, 1982), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Young et al. (1978), Bovey & Young (1980), Shearer (1980), and US
Veterans' Administration (1981).
In the long-term as in the short-term studies on mammals, the
test products were mainly administered orally. The composition of
the test products was not adequately known by present standards,
but many of the tests were carried out with more or less purified
2,4-D or its alkali salts. It is difficult to decide to what
extent the available results reflect the toxicological properties
of the present 2,4-D products, in which the maximum contents of CDD
and other toxic by-products are kept at very low levels.
A long-term feeding study in the rat (Hansen, 1971) was
evaluated by FAO/WHO JMPR in 1971 (FAO/WHO, 1972). It was
concluded that a no-observed-adverse-effect level in the rat was
equivalent to 31 mg/kg body weight per day. However, new studies
on mice and rats are in progress.
7.3.1.1. Clinical signs of poisoning
Signs of toxic effects on the digestive tract, such as
diarrhoea, vomiting, dysphagia, decreased gut motility, irritation,
or necrotic changes (in dogs including necrosis of oral tissues)
are likely to appear in animals following the absorption of high
doses of 2,4-D or its derivatives by the oral, dermal, or
inhalation routes, and after parenteral injections (Bucher, 1946;
Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Thomssen, 1958; Björklund & Erne, 1966; Erne, 1966a; Palmer &
Radeleff, 1969). Blood-tinged discharges from the nose, and in
dogs also nasal and eye irritation and skin lesions may also occur
(Bucher, 1946; Hill & Carlisle, 1947; Kosyan et al., 1974). Cattle
may suffer from tympanitis, and may show signs of thirst (Björklund
& Erne, 1966). However, some of the signs of "2,4-D poisoning"
reported in herbivores pastured on 2,4-D-treated vegetation may
have been caused by the ingestion of inherently poisonous plants
(Maclean & Davidson, 1970). Pigs may refuse to eat feed containing
high amounts of 2,4-D (Strach & Bohosiewicz, 1964), but sheep have
been reported to feed avidly on 2,4-D-treated vegetation (Sadykov
et al., 1972).
Characteristic signs of severe 2,4-D poisoning in mammals
appear to be muscular weakness, stiffness, stilted gait, and muscle
spasms (myotonia), alleviated by exercise and exacerbated by rest.
There may also be muscular incoordination progressing to paralysis
especially in the hind limbs; in rodents, caudal rigidity has also
been observed. These clinical signs are the result of the myotoxic
action of 2,4-D discussed below, which, through its effects on the
heart, may also lead to hypo- or hypertension, cardiac
fibrillation, and death. Prolonged inactivity may also occur and
may lead to pulmonary congestion, emphysema, and pneumonia (Bucher,
1946; Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Vinokurova,
1960; Björklund & Erne, 1966; Gorshkov, 1972; Sadykov et al., 1972;
Kosyan et al., 1974).
At high doses, 2,4-D and its derivatives may act as a central
nervous system depressant and cause lethargy, slowed respiration,
stupor, coma, and death (Bucher, 1946; Hill & Carlisle, 1947; Drill
& Hiratzka, 1953; Vinokurova, 1960; Desi et Sos, 1962a,b; Desi et
al., 1962a,b; Kosyan et al., 1974; Elo & Ylitalo, 1977, 1979).
7.3.1.2. Effects on food and water consumption, and on body weight
Relatively high concentrations of 2,4-D or its derivatives in
the diet or drinking water, or given by capsule, gavage, or
parenterally, may cause a reduction in food and water consumption,
and weight loss or reduced body weight gain in rats (Rowe & Hymas,
1954; Thomssen, 1958; Björklund & Erne, 1966; Chang et al., 1974;
Chen et al., 1981; Gorshkov, 1972), as well as in dogs (Bucher,
1946; Drill & Hiratzka, 1953), in pigs (Strach & Bohosiewicz, 1964;
Björklund & Erne, l966), in rabbits (Loktionov et al., 1973), in
cattle (Rowe & Hymas, 1954; Björklund & Erne, 1966; Palmer &
Radeleff, 1969; McLennan, 1974), and sheep (Palmer & Radeleff,
1969). However, the same authors, as well as Guseva (1956) and
Hansen et al. (1971) did not observe these effects at low doses or
low dietary concentrations of 2,4-D. In fact, in the studies by
Raoul & Marnay (1948) and Strach & Bohosiewicz (1964) on rats and
piglets fed low dietary concentrations of 2,4-D, an unimpaired
appetite and an improved body weight gain was seen in some groups
of animals. In these studies, the dosages ranged between 15 and
119 mg 2,4-D/kg body weight.
Observations of Thomssen (1958), Strach & Bohosiewicz (1964),
Martynov (1970), Gorshkov (1972), Sadykov et al. (1972), and Erne
(1974), on hares (Lepus timidus), European elk, pigs, and rats
suggest that, given a choice, animals will refuse to eat food or to
drink water containing more than a certain amount of 2,4-D, and
that this may, in part, be because of the strong characteristic
odour and taste of this compound, or of organic solvents such as
diesel fuel that are used in herbicide formulations or as diluents.
7.3.1.3. Effects on the central nervous system (CNS)
It has been suggested that the signs of central nervous system
depression in animals severely poisoned with 2,4-D are related to a
partial breakdown of the blood-brain barrier, possibly as a result
of damage to capillary vessels, and a subsequent accumulation of
2,4-D in the CNS (Desi & Sos, 1962a,b; Elo & Ylitalo, 1977, 1979).
However, further studies are needed to clarify the mechanism(s)
by which 2,4-D acts on the central nervous system.
7.3.1.4. Effects on the peripheral nervous system
No peripheral neuropathy was attributed to 2,4-D in the
available reports on short-term and long-term studies with 2,4-D in
a variety of animals (Shillinger & Naumova, 1957; Stupnikov, 1959).
The partial or complete paralysis, especially of the hind legs, of
2,4-D poisoned animals reported already by Bucher (1946) and Hill &
Carlisle (1947) may be a myotoxic rather than a neurotoxic effect
of 2,4-D. Moreover, in severe 2,4-D poisoning, a general weakness
may lead to inactivity that might be interpreted as paralysis.
No signs of neuropathy were reported by Kay et al. (1965) in
rabbits given large percutaneous doses of 2,4-D dimethylamine salt,
2,4-D butyl ester, or 2,4-D isooctyl ester. In similar studies in
which rats or rabbits were exposed by the dermal route, Buslovich
(1963) reported myotonia and death in rats given unspecified doses
of 2,4-D amine salt or 2,4-D butyl ester, but no peripheral
neuropathy, while Vinokurova (1960) found 130 - 180 mg of a 50%
aqueous emulsion of 2,4-D octyl ester/kg body weight to have "no
systemic effect" on rabbits.
The neurotoxic effects in animals of 2,4-D and of other related
compounds have not been adequately studied. Further research is
required to elucidate the mechanism of the neurotoxic and myotoxic
action of 2,4-D in animals.
7.3.1.5. Myotoxic effects
Most of the studies of the effects of 2,4-D on vertebrate
muscles were carried out because the 2,4-D-induced abnormalities
resemble a heritable muscle disorder in human beings, namely
myotonia congenita (Hofmann et al., 1966; Laskowski & Dettbarn,
1977). As a rule, high doses of 2,4-D (1/4 to 1/2 LD50) are
required to obtain severe and prolonged myotonia. The effects of
2,4-D on muscle cells are complex, and include disturbances in:
the activity of various enzymes (leading, for example, to increased
lactate production); potassium levels; membrane resistance; and in
chloride conductance. There are also shifts in calcium-binding
sites, changes in muscle and nerve cell electrical potentials, and
mitochondrial structural and ultrastructural degenerative changes
in muscle (Eyzaguirre et al., 1948; Kuhn & Stein, 1964, 1965, 1966;
Heene 1966, 1968, 1975; Hofmann et al., 1966; Kenigsberg, 1968;
Stein & Kuhn, 1968; Bodem et al., 1971; Preiss & Rossner, 1971;
Seiler, 1971; Buslovich & Koldobskaya, 1972; Rüdiger et al., 1972;
Senges & Rüdel, 1972; Brody, 1973; Danon et al., 1976; Iyer et al.,
1976; Bretag & Caputo, 1978; Dux et al. 1978; De Reuck et al.,
1979; Eberstein & Goodgold, 1979; Mazarean et al., 1979a,b).
Similar effects are produced by the chlorophenoxy drug clofibrate
(atromid) (Pierides et al., 1975; Smals et al., 1977). None of the
available studies on 2,4-D-induced myotonia were designed to
establish no-observed-adverse-effect levels for the various
myotoxic effects in intact animals, and therefore additional
studies should be carried out for this purpose.
7.3.1.6. Cardiovascular effects
As part of its myotoxic action, 2,4-D and its derivatives may
in high doses cause biochemical, physiological, and structural
damage to the myocardium in vitro and in vivo (Bodem et al., 1971;
Preiss & Rossner, 1971; Rüdiger et al., 1972; Mazarean et al.,
1979a,b).
7.3.1.7. Haematological effects
Shifts have been reported in the number or types of
erythrocytes, leukocytes, or bone marrow cells, or changes in
haemoglobin levels, in a variety of laboratory and domestic mammals
given 2,4-D or 2,4-D derivatives (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Hansen et al., 1971;
Sadykov et al., 1972; Loktionov et al., 1973; Kosyan et al., 1974;
and Halliop et al., 1980).
7.3.1.8. Effects on blood chemistry
Rowe & Hymas (1954) were apparently the first investigators to
monitor blood chemistry in 2,4-D-treated animals. They did not
observe any adverse effects at 2,4-D dietary levels as high as 300
mg 2,4-D/kg in rats treated for 113 days.
Other investigators noted changes in various serum, plasma, or
erythrocyte enzyme activity levels, and shifts in the levels of
electrolytes, glucose, blood proteins, or other chemicals in
response to treatment with 2,4-D or 2,4-D derivatives in rats,
rabbits, cattle, pigs, or sheep, or in blood from such animals to
which 2,4-D was added in vitro (Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Shevchenko, 1966;
Dzhaparov & Tsilikov, 1969; Hunt et al., 1970; Szöcs et al., 1970;
Gorshkov, 1972; Kosyan et al., 1974; Kuzminskaya & Bersan, 1975;
Chen et al., 1981). Some of the changes in transaminase (SGOT,
SGPT), blood urea nitrogen (BUN), or glucose levels appeared to be
secondary to myotoxic, nephrotoxic, or hepatotoxic effects of high
doses of 2,4-D.
7.3.1.9. Other biochemical effects observed in vivo or in vitro
Sufficiently high doses of 2,4-D may induce changes in
mitochondrial oxygen consumption and oxidative phosphorylation, in
electrolyte, ascorbic acid, glycogen, lipid, and nucleic acid
contents of organs, tissues, cells, organelles, or cell fractions
from a variety of mammals (Brody, 1952, 1973; Guseva, 1956; Baker
et al., 1960; Kuhn & Stein, 1964; Heene, 1966, 1967, 1968;
Shevchenko, 1966; Philleo & Fang, 1967; Kenigsberg, 1968; Dzhaparov
& Tsilikov, 1969; Stanosz, 1969; Buslovich & Koldobskaya, 1972;
Graff et al., 1972; Buslovich et al., 1973; Abo-khatwa &
Hollingworth, 1974; Chang et al., 1974; Nikandrov, 1974; Venkaiah &
Patwardhan, 1978; Podolak, 1979).
7.3.1.10. Pulmonary effects
No pulmonary abnormalities were reported in studies with mice,
rabbits, rats, or dogs conducted by Bucher (1946), Drill & Hiratzka
(1953), Rowe & Hymas (1954), Shillinger & Naumova (1957),
Schillinger (1960), or Björklund & Erne (1966). In contrast, Hill
& Carlisle (1947) and Palmer (1972) noted congestion of pulmonary
vessels, pulmonary petechial haemorrhages, and pulmonary edema or
emphysema in mice, rats, dogs, cattle, or sheep that died of 2,4-D
poisoning.
7.3.1.11. Hepatotoxic effects
Rabbits, rats, mice, dogs, cattle, or sheep treated for a
prolonged period with toxic doses of 2,4-D were found to develop a
subacute toxic hepatitis with congestion of hepatic blood vessels,
and cloudy swelling, fatty infiltration, local necrosis,
degeneration, or atrophy of hepatocytes, especially of the
parenchyma in the centrolobular areas (Bucher, 1946; Hill &
Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Björklund & Erne, 1966; Szöcs et al., 1970; Palmer, 1972). High
doses of 2,4-D may induce a proliferation of peroxisomes and
increased levels of mixed function oxidases in liver cells of rats
and hamsters (Buslovich et al., 1982; Vainio et al., 1982, 1983).
Changes in the levels of certain liver enzymes such as maleate
or succinic dehydrogenase, and in ascorbic acid or glycogen content
or hippuric acid production have also been reported (Shillinger &
Naumova, 1957; Baker et al., 1960; Dzhaparov & Tsilikov, 1969;
Szöcs et al., 1970; Buslovich et al., 1973; Chang et al. 1974;
Nikandrov, 1974). Some of the results concerning liver glycogen
levels in rats suggest a reverse trend at high doses, as Dzhaparov
& Tsilikov (1969) found that a dose equivalent to 1/16 LD50 per day
lowered the liver glycogen content, while Chang et al. (1974)
observed the opposite effect at doses of about 100 mg/rat per day.
7.3.1.12. Effects on the kidney
In early studies with high doses of 2,4-D, signs of impaired
kidney function, increased relative kidney weight, and of gross and
histological abnormalities (parenchymatous degeneration,
hypertrophy, and hyperplasia, cloudy swelling especially in the
cells of the proximal convoluted tubules, and glomerular lesions)
were noted in mice, rats, and dogs (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954). That the kidney
is a target organ for the structural, physiological, and chemical
effects of 2,4-D was confirmed repeatedly by later and more
detailed studies on a wider range of test species including pigs,
goats, and sheep (Schillinger, 1960; Björklund & Erne, 1966; Erne,
1966a; Stanosz, 1969; Hunt et al., 1970; Milhaud et al., 1970;
Gorshkov, 1972; Palmer, 1972; Senczuk & Pogorzelska, 1975; Koschier
et al., 1978; Orberg, 1980a). In a recent 13-week study on rats,
the no-observed-adverse-effect level for histological changes
induced with pure 2,4-D in mammalian kidney appeared to be 15 mg/kg
body weight per day (Chen et al., 1981).
7.3.1.13. Effects on endocrine organs
Swelling and congestion of the thyroid were noted by Palmer
(1972) in cattle and sheep fatally poisoned with various 2,4-D
products. Effects of 2,4-D on iodide uptake by the thyroid gland
were first decribed by Sos & Kertai (1958) and studied further by
Florsheim & Velcoff (1962), Florsheim et al. (1963), Tsilikov
(1969), and Gorshkov (1972). Their results indicate that doses of
5 - 250 mg 2,4-D sodium salt or equivalent/kg body weight per day
have a stimulatory effect on thyroid function.
Effects of 2,4-D on adrenal function and its relationship to
muscle carbohydrate metabolism and 2,4-D-induced myotonia have been
studied by Kenigsberg (1968), and by Buslovich & Koldobskaya
(1972). 2,4-D-induced changes in adrenal or thyroid function may
also be implicated in the abnormal temperature regulation in 2,4-D-
treated rats described by Sudak et al. (1966). However, it is
noteworthy that Buslovich (1963) was unable to induce a change in
the body temperature of rats by giving 15 - 20 oral doses of 2,4-D
sodium salt, amine salt, or butyl ester at a level of 1/10 or 1/5
LD50/day.
7.3.1.14. Effects on the digestive tract
Vomiting, diarrhoea, hyperaemia, bloody exudates in the gut,
necrotic changes in the mucosa, and other non-acute toxic effects
on the digestive tract have been reported after administration of
high doses of 2,4-D by either the oral or parenteral route in mice,
rats, and dogs (Bucher, 1946; Hill & Carlisle, 1947; Drill &
Hiratzka, 1953; Kosyan et al., 1974). However, Hansen et al.
(1971) did not observe any such effects in a 2-year feeding study
at dietary levels of 2,4-D corresponding to about 37.5 and 67.5 mg
2,4-D/kg body weight per day, respectively, for rats and dogs.
Their observations on dogs contrast with those of Drill & Hiratzka
(1953) who found repeated doses of 20 mg 2,4-D/kg body weight per
day to be fatal in 3 out of 4 dogs studied by them.
7.3.2. Birds
The published reports on the toxic effects of 2,4-D and 2,4-D
products in birds deal mainly with chickens (Gallus domesticus)
and game birds. The available data on cumulative oral lethal doses
for game birds have been summarized in Table 18; studies on the
reproductive, embryotoxic, and teratogenic effects of 2,4-D are
dealt with in section 7.3.6.
Table 18. Cumulative oral lethal doses of 2,4-D herbicides for game birds
------------------------------------------------------------------------------------
Product Species Dietary LC50 Cumulative LD50 References
(mg a.i./kg (mg a.i./kg bw)a
diet)a
------------------------------------------------------------------------------------
2,4-D quail (young) 5000 28 000 Dewitt et al.
dimethylamine (1962)
salt
mallard duck 5000 8250
(young) 5000 8250
(adult) > 2500 > 34 000
2,4-D quail
butoxyethanol (young) 5000 38 000
ester (adult) 5000 40 700
pheasant (young) 5000 29 500
mallard duck (adult) 5000 > 33 000
------------------------------------------------------------------------------------
a a.i. = active ingredient
The clinical signs of 2,4-D poisoning in birds appear to be
similar to those in mammals, and the kidney seems to be the most
sensitive organ. No adverse effects were reported in chickens fed
dietary levels of 1000 mg/kg for 21 days (142 mg/kg body weight)
whereas kidney enlargement occurred in chickens fed 5000 mg/kg of
diet for 21 days (Whitehead & Pettigrew, 1972).
7.3.3. Cold-blooded animals
The literature on the chronic effects of 2,4-D and its
derivatives on cold-blooded animals (poikilotherms) was not
reviewed in detail. The available reports indicate that for
aquatic vertebrates in general, 2,4-D esters are more toxic than
2,4-D amines, and that the overall no-observed-adverse-effect level
for toxic effects on fish for the esters is at or near 1 mg/litre
(King & Penfound, 1946; Zhiteneva & Chesnokova, 1973; Fabacher &
Chambers, 1974; Meehan et al., 1974). 2,4-dichlorophenol, which
may occur in water as a by-product or transformation product of
2,4-D, is similarly toxic to fish (Holcombe et al., 1980).
7.4. Fetotoxicity, Teratogenicity, and Reproductive Effects
The scientific literature contains a fairly large number of
reports on the fetotoxic, teratogenic, and reproductive effects of
2,4-D in livestock and in laboratory animals. However, most of the
observations on livestock are either too sketchy to be useful or
neglect to take into account various confounding factors. Examples
of this are studies by Björklund & Erne (1966) on a single pregnant
pig, by Sadykov et al. (1972) on sheep grazing on pastures
apparently containing a high percentage of poisonous plants, and
the report by Bodai et al., (1974), on reproductive disturbances in
cattle feeding on either 2,4-D-treated vegetation possibly
including poisonous plants, or on contaminated silage.
Some of the studies carried out with rodents under laboratory
conditions also provide little useful information, either because
it is difficult or impossible to determine the dose levels used in
the studies (Weinmann, 1957; Schuphan, 1963, 1965, 1969; Schiller,
1964; Buslovich et al., 1976); or because the information provided
is insufficient and the studies cannot be properly evaluated
(Bucher, 1946; Hansen et al., 1971; King et al., 1971). The study
by Weinmann (1957) can be considered invalid, as a high mortality
occurred in both control and experimental animals, when they were
exposed to cold stress because of construction work affecting the
animal quarters during the winter months. Moreover, Weinmann
(1957) and several other authors, including Schuphan (1963, 1965,
1969) Schiller (1964), Schillinger (1960), and Shillinger & Naumova
(1957) did not in fact examine the effects of 2,4-D, but rather the
effects of foods or food extracts prepared from crops that had been
sprayed with 2,4-D, and, in some cases, also with other plant
growth substances. As none of these authors appears to have
carried out an analysis to demonstrate the presence of 2,4-D
residues in the test material, it is questionable whether the test
animals ingested any 2,4-D or 2,4-D residues, and these studies are
therefore not reviewed in detail. Buslovich et al. (1976) tested
only a single dose level (1/2 LD50) and this reduces the usefulness
of their study. Pertinent reports are discussed below.
7.4.1. Rats
7.4.1.1. Effects on adult rats
No deleterious effects on the health or fertility of rats
receiving the maximum tolerated dose of 87.5 mg/kg body weight in
terms of 2,4-D or its molar equivalent of the isooctyl ester or the
propylene glycol butyl ether ester per day, on days 6 - 15 of
pregnancy, were reported by Schwetz et al. (1971) and Unger et al.
(1980). Even higher amounts of 2,4-D or its equivalent in 2,4-D
derivatives were used by Björklund & Erne (1966), Hansen et al.,
(1971) and Khera & McKinley (1972) with similar results.
Reduced testis and prostate size, abnormal spermatogenesis (and
also liver and kidney damage) were reported by Schillinger (1960)
in some of the male rats given 375 mg/kg body weight per day (about
1/4 LD50) of a Soviet-made 2,4-D butyl ether formulation containing
polyethylene glycol alkyl phenyl ether surfactant. These effects
were not noted at 1/10 of this dose level, i.e., at 37.5 mg/kg body
weight per day. Some of the toxic effects reported by Schillinger
(1960) may have been caused by the surfactant, but as a surfactant
control group was not included in this study, this cannot be
confirmed. Thus, the available studies suggest that the no-
observed-adverse-effect level for reproducing adult rats lies
between 37.5 and 87.5 mg 2,4-D/kg body weight per day.
7.4.1.2. Effects on offspring
Björklund & Erne (1966) gave pregnant rats a 2,4-D
concentration in drinking-water of 1000 mg/litre during pregnancy,
and for the following 10 months. No effects on reproduction were
noted.
Hansen et al. (1971) fed male and female rats dietary levels of
technical 2,4-D of 100, 500, and 1500 mg/kg (ppm), and the rats
were bred through 3 successive generations. At dietary levels of
100 and 500 mg/kg, no effects were noted. However, at a dietary
level of 1500 mg/kg, survival of pups to weaning was reduced. The
number of pups surviving ranged from 70 - 97% in the control group
and from 60 - 93% at the 100 and 500 mg/kg (ppm) dietary levels;
survival at the highest dose ranged from 20 - 62%.
Resorptions, reduced fetal weight and size, enlarged ventricles
of the brain and haemoperitoneum were found by Buslovich et al.
(1976) in the offspring of rats treated with two different 2,4-D
derivatives at a dose of one-half LD50 (value unspecified).
Schwetz et al. (1971) dosed female rats from day 6 - 15 of
pregnancy by gavage at dose levels of 0, 12.5, 25, 50, and 87.5
mg/kg body weight per day with 2,4-D or equimolar dose levels of
the propylene glycol butyl ether (PGBE) and the isooctyl esters
(IO). At doses of 50 and 87.5 mg/kg, a decrease in fetal body
weight was noted for all three compounds.
Subcutaneous oedema, delayed ossification of sternebrae,
sternebrae with split centres of ossification, wavy ribs, and
lumbar ribs increased with increasing doses, for at least one of
the agents studied. However, these anomalies were not
significantly increased at the 12.5 or 25 mg/kg dose level for the
3 compounds. A small but significant increase in the incidence of
subcutaneous oedema was observed in fetuses from dams receiving
12.5 mg/kg molar equivalent of IO. The incidence of missing
sternebrae was significantly increased at dose levels of 87.5 mg/kg
for 2,4-D and 12.5 and 87.5 mg/kg for PGBE.
Unger et al. (1980), in a later study, using the same dosing
regimens for 2,4-D IO and 2,4-D PGBE did not observe the effects
reported by Schwetz et al., except for a statistically significant
increase in rib buds at the highest dose (87.5 mg/kg) tested for
both compounds ( P < 0.05).
Khera & McKinley (1972) dosed female rats from day 6 - 15 of
pregnancy by gavage with 2,4-D, 2,4-D IO, 2,4-D butyl ester, 2,4-D
butoxyethanol ester and 2,4-D dimethylamine salt. The butyl and
isooctyl ester depressed fetal weight and decreased fetal viability
at the highest dose of 150 mg/kg body weight. Wavy ribs,
additional ribs, retarded ossification and sternal defects, fused
ribs, small-sized distorted scapula and micromelia were observed as
anomalies among the treated groups. A statistically significant
increase in malformed fetuses was noted at 2,4-D levels of 25 mg/kg
body weight ( P < 0.05) and at levels of 150 mg/kg or more for the
other compounds.
Konstantinova et al. (1975) reported hemorrhage into internal
organs in the fetuses of rats treated with a 2,4-D level of 50
mg/kg body weight.
The results of all these studies suggest that dosage levels of
less than 12.5 mg/kg body weight for the various 2,4-D derivatives
do not cause fetotoxic or teratological effects in rats, and the
results of the more recent study by Unger et al. (1980) indicate
that higher doses may be without deleterious effects on the
fetuses.
Thus, at present, a daily dose level of 10 mg 2,4-D or 2,4-D
acid equivalent/kg body weight can be considered to be without
significant fetotoxic or teratogenic effects in rats.
7.4.2. Mice
The report by Courtney (1977) indicated that in CD-1 mice,
doses of 1 mM/kg body weight of 2,4-D and its n-butyl and PGBE
esters reduced fetal body weight, and increased fetal mortality.
The compounds were also teratogenic, inducing cleft palates, at
levels of 124 mg/kg body weight per day (2,4-D or acid equivalent)
or more. However, the 2,4-D isopropyl and isooctyl esters appeared
to be less teratogenic than 2,4-D, as they did not induce birth
defects at a 2,4-D equivalent level of 124 mg/kg body weight per
day. Furthermore, the 2 esters did not induce fetal death in CD-1
mice at doses of 2,4-D equivalent up to 221 mg/kg body weight per
day.
7.4.3. Birds
In the 1960s and 1970s, a number of tests of the embryotoxic,
teratogenic, and other reproductive effects of 2,4-D were carried
out on birds' eggs and embryos. These results may not apply to
mammals, because bird embryos develop in a closed environment
different from that of mammalian embryos, and because birds differ
anatomically from mammals.
Lutz-Ostertag & Lutz (1970, 1974) reported mortality and severe
deformities in wild bird embryos exposed to 2,4-D amine salt, but
they did not provide crucial experimental details, and other
investigators (Dunachie & Fletcher, 1967, 1970; Kopischke, 1972;
Grolleau et al., 1974; Gyrd-Hansen & Dalgaard-Mikkelsen, 1974;
Somers et al., 1974a,b,c; Hilbig et al., 1976a,b; Spittler, 1976)
were unable to duplicate their results either with 2,4-D amine
salts or esters. Some of the teratogenic effects attributed to
2,4-D by Lutz-Ostertag & Lutz (1970, 1974) resemble those induced
by excessively high incubation temperatures (Nielsen, 1968), and
may thus have been experimental artifacts.
On the whole, the available studies on bird embryos indicate
that the no-observed-adverse-effect level for 2,4-D-induced
embryotoxic and teratogenic effects lies near 0.5 mg active
ingredient/egg (equivalent to about 10 mg/kg) and is thus similar
to that in mammals.
7.4.4. Cold-blooded animals
The available literature contained little information
concerning the possible reproductive, embryotoxic, or teratogenic
effects of 2,4-D or 2,4-D derivatives on cold-blooded animals.
7.4.4.1. Amphibians
Lopez (1961) noted that the motility of frog spermatozoa was
not affected by low concentrations of 2,4-D or its sodium salt, and
that the inhibition of movement, or the lysis observed under some
conditions were caused by changes in the pH of the test solution.
Aqueous solutions of less than 0.05% 2,4-D sodium salt did not
induce any macroscopic abnormalities in developing frog eggs or
embryos (Lhoste & Roth, 1946), while Cooke (1972) did not find
either toxic effects or 2,4-D residues in frog tadpoles exposed for
1 or 2 days to up to 50 mg 2,4-D/litre. According to Sanders
(1970a) a commercial 2,4-D dimethylamine salt had an LC50 of 100
mg/litre for frog tadpoles.
7.4.4.2. Fish
Only three brief reports were available on the effects of 2,4-D
on developing fish eggs and embryos. Andrusaitis (1972) found that
2,4-D reduced the oxygen consumption of 32-cell blastomeres, and
increased the oxygen consumption in 64 - 128 cell blastomeres of
Misgurnus fossilis. In a study by Mount & Stephan (1967), 2,4-D
butoxyethanol ester (BEE) at a concentration of up to 0.31
mg/litre, or 2,4-D at 0.80 mg/litre did not reduce the reproduction
rate in fathead minnows (Pimephales promelas), whereas 2,4-D BEE
at 1.5 mg/litre killed minnow eggs in 48 h. Rehwoldt et al. (1977)
similarly found that 0.1 mg 2,4-D/litre did not have any adverse
effect on reproduction in guppies.
These reports suggest that the no-observed-adverse-effect level
of 2,4-D BEE for the reproductive or teratogenic effects of 2,4-D
in fish may be about 1 mg a.i./litre water.
7.5. Mutagenicity and Related Effects
7.5.1. 2,4-D and its derivatives
Studies on the mutagenicity of 2,4-D and its derivatives have
been reviewed by Andersen et al. (1972), National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), Ramel (1978), Seiler (1978),
Vachkova-Petrova (1978), Kas'yanenko & Koroleva (1979), Murthy
(1979), Shearer (1980), US Veterans' Administration (1981), Waters
et al. (1981), and Linainmaa (1983).
A recent IARC Working Group (IARC, 1982) evaluated the activity
of 2,4-D and derivatives in short-term tests. It was reported that
2,4-D induced unscheduled DNA synthesis in cultured human
fibroblasts (Ahmed et al., 1977a), but not in rat hepatocytes
(Probst et al., 1981). 2,4-D was not mutagenic in bacterial
systems (Andersen et al., 1972; Sherasen et al., Zetterberg, 1977;
Moriya et al., 1983). 2,4-D was mutagenic in yeast, when tested at
low pH (Zetterberg et al., 1977), but was not active under other
conditions (Zetterberg, 1977) or in a host-mediated assay
(Zetterberg et al., 1977). Results of four studies on Drosophila
melanogaster were reported to be positive (Rasmuson & Persson-
Svahalin, 1978): results of three other studies were negative
Berin & Buslovich, 1971; (Vogel & Chandler, 1974; Magnusson et al.,
1977; Rasmuson & Svahlin, 1978).
2,4-D was reportedly mutagenic in cultured Chinese hamster
ovary (CHO) cells (Ahmed et al., 1977b), but did not induce a
statistically significant increase in sister chromatid exchanges
(SCEs) in CHO cells in vitro (Linainmaa, 1983). Chromosomal
effects have been reported in plants (Khalatkar & Bhargava, 1982).
Chromosomal aberrations or SCEs were found in cultured human
lymphocytes (Pilinskaya, 1974; Korte & Jatal, 1982), but
chromosomal aberrations were not found in cultured embryonic bovine
kidney cells (Bongso & Basrur, 1973).
In mice, single oral doses of 100 - 300 mg 2,4-D/kg body weight
reportedly induced chromosomal aberrations (Pilinskaja, 1974), but
micronuclei were not found in mice after ip injection of single
doses of 100 mg 2,4-D/kg body weight (Jenssen & Renberg, 1976).
2,4-D was not active in a dominant lethal test in mice (Epstein et
al., 1972), and did not induce SCEs in rats after oral
administration of 2,4-D amine salt at daily doses of 100 mg/kg body
weight for two weeks (Linnainmaa et al., 1983). Recent evidence
(Buslovich et al., 1982; Vainio et al., 1982) suggests that 2,4-D
may have an indirect effect on genetic material via the production
of active oxygen radicals derived from peroxisome proliferation,
which has been demonstrated in in vivo and in vitro studies in
liver cells of rats and hamsters (Reddy et al., 1980, 1982; Vainio
et al., 1982; Gray et al., 1983).
At present, available studies are inadequate to evaluate the
genetic effects of 2,4-D and its derivatives in short-term tests
(IARC, 1982). No data on cell transformation are available.
7.6. Carcinogenic Effects on Experimental Animals
7.6.1. 2,4-D and its derivatives
A number of studies have been carried out on mice and rats to
assess the potential carcinogenic effects of 2,4-D and its
derivatives. Two IARC Working Groups have reviewed these studies
(IARC, 1977, 1982) and concluded that it was not possible to make
an evaluation on the basis of the available data. 2,4-D and
several of its esters were tested by oral administration and by a
single sc injection in the newborn of 2 strains of mice (Innes et
al., 1969); 2,4-D or its amine salts were also tested in rats by
oral administration (Arkhipov & Kozlova, 1974; Hansen et al.,
1971).
Nearly 20 years have passed since these studies were carried
out and because of basic flaws in their design and execution, it is
unlikely that further reviews of these studies will lead to
generally accepted conclusions. The Task Group was aware that
further long-term studies in mice and rats were in progress, and
these should prove useful in the future evaluation of the potential
carcinogenicity of 2,4-D.
7.6.2. Contaminants in 2,4-D
2,7-DCDD was tested for carcinogenicity in mice and rats fed
dietary levels of 5000 and 10 000 mg/kg for 90 weeks, followed by a
short observation period of about 1 - 10 weeks, at which time all
animals were killed. Sufficient numbers of animals survived to
evaluate the development of late-appearing tumours. Although an
increased incidence of hepatocellular adenomas and carcinomas was
observed in treated male mice (20/50 in the low dose and 17/42 in
the high dose compared with 8/49 in matched controls), it was noted
that the incidence of liver tumours in historical controls ranged
from 16 - 32%. No tumorigenic effects were found in rats (US
National Cancer Institute, 1979).
8. EFFECTS ON MAN, CLINICAL AND EPIDEMIOLOGICAL STUDIES
The available clinical and epidemiological studies fall into 4
groups: (a) studies on patients treated with 2,4-D as an
anticancer drug (Apffel, 1959a) or antibiotic (Seabury, 1963), (b)
reports on acute 2,4-D poisoning due to voluntary or accidental
ingestion of herbicides (Tables 19 and 20), (c) reports on workers
(mainly men) overexposed to 2,4-D during the manufacture,
processing, or use of 2,4-D herbicides, and (d) epidemiological
studies on groups of people who were actually or potentially
exposed as a result of herbicide spray programmes, or who lived in
areas in which herbicides were used. With the exception of the
case studies of Apffel (1959a) and Seabury (1963), almost all of
the reports deal with mixed exposures to 2,4-D and other chemicals,
and therefore it is often unclear to what extent 2,4-D, its alkali
or amine salts, or its esters contributed to the effects reported
by the authors of the studies.
Much of the literature on acute poisonings and on the health
effects of occupational overexposure to 2,4-D or other
chlorophenoxy compounds or their toxic by-products has been
recently reviewed by Pocchiari et al. (1979), Bovey & Young (1980),
Huff et al. (1980), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1981),
CEC (1981), Rappe & Buser (198l), US Veterans' Administraton
(1981), Coggon & Acheson (1982), Hay (1982), IARC (1982, 1983), and
Dobrovolski (unpublished data, 1983). However, most of these
reviews concentrated on 2,4,5-T, Agent Orange, and other herbicides
used in the Vietnam war, or on industrial accidents resulting in
massive exposures to largely undefined mixtures of chlorophenols,
chlorinated dibenzodioxins, and other reaction products. In
contrast, the present review focuses mainly on 2,4-D herbicides.
In evaluating human exposure to mixtures of chemicals that
include 2,4-D and various concentrations of contaminants of 2,4-D,
it is in many instances difficult or impossible to determine
whether any of the described effects can actually be attributed to
the exposure to 2,4-D or its derivatives.
8.1. Acute Poisoning and Occupational Overexposure
Pertinent reports on acute poisoning with 2,4-D, or of the
effects of occupational overexposure to 2,4-D herbicides are
summarized or cited in Tables 19 and 20.
Signs and symptoms of acute overexposure to 2,4-D or its
derivatives occurred after ingestion or absorption of large
amounts, or where poor occupational hygiene was practised leading
to pronounced dermal absorption of the material. It is unlikely
that, with good agricultural practice, good personal protection,
and occupational hygiene, resulting in exposures to low
concentrations of 2,4-D, any of the acute symptoms and signs
reported below would be expected to occur.
Table 19. Acute toxicity of 2,4-D, fatal poisonings with herbicides containing 2,4-D
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
a) Fatal poisonings
2,4-D diethyl not stated F 50.8 kg 60-90 g 40-400 coma; death in about Curry (1962)
ester (1180-1770 mg 2 days; degeneration
(DICOTEX EXTRA) DOCOTOX/kg bw) of convoluted kidney
tubules
"2,4-D" suicide M ? ~ 125 ml ? loss of consciousness, Delarrard &
400 g (< 400 bw) coma, generalized Barbaste (1969)
a.i./litre muscular hypotonia,
loss of all reflexes,
hypotension,
hyperglycaemia,
proteinuria; death in
12 h
"pure" 2,4-D suicide M 55 kg ? 57.6-407.9 coma, myotonia, fever, Dudley &
acid in pulmonary emphysema Thapar (1972)
kerosene and edema, liver
necrosis, degeneration
of kidney tubules;
death in 6 days
"2,4-D" suicide F ? ? 20-116 loss of consciousness, Geldmacher-Van
vomiting, uterine Mallinckrodt &
bleeding, tachycardia, Lautenbach
and circulatory (1966)
failure; death in
about 30 h; edema and
congestion of brain;
fatty liver cell
changes; fatty changes
in kidney tubules;
pulmonary hyperaemia &
edema with isolated
haemorrhages
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
"2,4-D" suicide M ? "at least" stiffness in legs, Herbich &
13.5 g vomiting, loss of Machata
consciousness; death (1963)
in 14 h; hyperaemia
and edema of brain;
pulmonary edema
2,4-D suicide M 75 kg 120 ml 12.5-7700 vomiting; congestion, Nielsen et al.
dimethylamine (80 mg/kg) pulmonary emphysema; (1965)
formulation CNS congestion &
perivascular
haemorrhages, severe
degeneration of
ganglion cells; death
within hours of
ingestion
Herbicide suicide M 26 360 ml 2,4-D plasma clinical and Osterloh et al.
containing years and mecoprop 321 (1.5 h) pharmacokinetic (1983)
2,4-D, mecoprop amine salt 540.9 (21 h) study coma with
(MCPP) and (10.6%, 11.6% 480.8 (30 h) pin-point pupils
chlorpyrifos a.i.) and 360 tachycardia,
ml chlorpyrifos urine (on hypertension,
in kerosene admission): myoclonus, diarrhoea,
(6.7% a.i.) 230.3 then hypotension,
plus few cardiac arrhythmias,
granules gastric asystole, and death
Warfarin (0.025 content (on after 30 h
% a.i.) (2,4-D admission):
= 600 mg/kg bw; 108.2
mecoprop = 600
mg/kg bw) tissues
(post mortem)
brain: 186.4
blood: 389.5
liver: 293.5
heart: 301.2
kidney: 315.0
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
b) Non-fatal poisonings
"2,4-D" accidental M ? (5- ? ? drowsiness, Duric et al.
"DEHERBAN A" ingestion year unsteadiness, (1979)
herbicide old difficulties with
formulation, child) speech; dilated pupils;
400 g on fourth day, toxic
a.i./litre myocarditis with
abnormal ECG; kidney
damage indicated by
increased blood urea
levels; no liver
damage; complete
recovery within about
one month
herbicide suicide F 58 ? ? no signs of toxicity Prescott et al.
containing attempt; years on admission to (1979)
2,4-D plus ingestion of hospital with plasma
dichlorprop unspecified concentration of
(16.1% + 21.4%) amount of 335 mg 2,4-D/litre &
herbicide 400 mg mecoprop/litre;
plasma clearance
t = 143 h for
2,4-D vs 95 h for
dichlorprop; 91% of
ingested 2,4-D and
70% of ingested
dichlorprop excreted
unchanged; metabolites
not identified
----------------------------------------------------------------------------------------------------------------------- not identified
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
herbicide suicide F 30.2 kg 100 ml ? pharmacokinetic study Rivers et al.
containing attempt herbicide of 2,4-D/dicamba (1970)
salts of 2,4-D (13.6 g 2,4-D) excretion; no Young & Haley
(20.1%) and information on toxic (1977)
dicamba (1.9%) effects; excretion of
2,4-D initially slowed
by competitive
excretion of dicamba;
small amounts of 2,4-D
still excreted 3 weeks
after ingestion; only
52% of ingested 2,4-D
excreted in urine;
rest assumed to have
been excreted by
faecal route; plasma
clearance t = 59.2 h
initially, and 16.7 h
after most of the
dicamba was excreted
-----------------------------------------------------------------------------------------------------------------------
Table 20. Acute or non-acute effects attributed to occupational or bystander overexposure to 2,4-D
herbicide
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Central i) unconsciousness Radionov et al. (1967); Paggiaro et al. (1974)
nervous ii) electroencephalograph changes Kontek et al. (1973); Andreasik et al. (1979)
system iii) subjective symptoms Assouly (1951); Goldstein et al. (1959); Monarco &
Divito (1961); Foissac-Gegoux et al. (1962);
Berkley & Magee (1963); Belomyttseva (1965);
Fetisov (1966); Radionov et al. (1967); Bashirov
(1969); SARE (1972); Andreasik et al. (1979);
Kuzyk (1979)
Peripheral i) polyneuritis Foissac-Gegoux et al. (1962); Todd (1962);
nervous Belomyttseva & Karimova (1963); Bashirov (1969)
ii) partial paralysis Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Todd (1962)
iii) functional changes Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Berkley & Magee (1963); Belomyttseva
(1967); Wallis et al. (1970); Andreasik et al.
(1979); Singer et al. (1982)
iv) subjective symptoms
Skeletal i) myotonia, myokymia, fibrillation Foissac-Gegoux et al. (1962) Berkley & Magee
muscles stiffness (1963); Wallis et al. (1970); Paggiaro et al.
(1974)
ii) muscle damage or atrophy Wallis et al. (1970)
iii) subjective symptoms Assouly (1951); Foissac-Gegoux et al. (1962); Todd
(1962); Fetisov (1966); Radionov et al. (1967);
Bashirov (1969); Wallis et al. (1970)
Digestive i) vomiting, diarrhoea Goldstein et al. (1959); Monarca & Divito (1961);
system Todd (1962); Belomyttseva (1967); Radionov et al.
(1967); Paggiaro et al. (1974); Dennis (1976)
ii) various functional disorders or Assouly (1951); Monarca & Divito (1961); Bashirov
subjective symptoms (1969); Wallis et al. (1970); Kuzyk (1979)
---------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Respiratory i) irritation coughing Assouly (1951); Belomyttseva & Karimova (1963);
system Belomyttseva (1965); Wallis et al. (1970);
Andreasik et al. (1979)
ii) functional disorders Belomyttseva & Karimova (1963); Bashirov (1969);
Wallis et al. (1970)
Circulatory i) functional changes: Belomyttseva & Karimova (1963); Belomyttseva
system - cardiac involvement (1967); Winkelmann (1960); Bashirov (1969);
- vascular involvement Paggiaro et al. (1974); Andreasik et al. (1979)
ii) haematological or chemical Monarca & Divito (1961); Todd (1962); Belomyttseva
changes (1967); Radionov et al. (1967); Long et al.
(1969); Paggiaro et al. (1974); Andreasik et al.
(1979)
iii) subjective symptoms Radionov et al. (1967); Bashirov (1969); Andreasik
et al. (1979)
Liver functional abnormalities Belomyttseva & Karimova (1963); Belomyttseva
(1967); Bashirov (1969); Andreasik et al. (1979)
Kidney functional abnormalities Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Belomyttseva (1967); Bashirov (1969);
Paggiaro et al. (1974); Andreasik et al. (1979)
Skin i) irritation or allergic reactions Belomyttseva (1967); Radionov et al. (1967);
Dennis (1976); Kuzyk (1979)
ii) desquamation Foissac-Gegoux et al. (1962)
iii) chloracne Londońo (1966)
Reproductive functional abnormalities (women) Elina et al. (1975)
system decreased libido (men) Andreasik et al. (1979)
impotence Espir et al. (1970)
---------------------------------------------------------------------------------------------------------
The observations of Apffel (1959a) and Seabury (1963) on
patients treated with 2,4-D suggest that the acute no-observed-
adverse-effect level for biological effects in human beings may be
as high as 36 mg of purified 2,4-D/kg body weight, or its
equivalent in alkali or amine salts or esters. The lack of
subjective or clinical effects in studies in which volunteers
ingested low doses (5 mg/kg body weight) of purified 2,4-D also
supports the idea that a dose of a few mg/kg body weight is
unlikely to be toxic (Khanna & Kohli, 1977; Sauerhoff et al.,
1977). However, it is less certain whether this is true also of
the less pure commercial 2,4-D products. From the point of view of
occupational and bystander safety, it is reassuring that no reports
were found of fatal poisonings following dermal exposure or
inhalation, though temporary unconsciousness and other severe acute
effects have been attributed to massive combined dermal and
inhalation exposures to 2,4-D herbicides.
Most poisonings with 2,4-D herbicides involved formulations
containing more than one toxic ingredient, including solvents,
surfactants, and other additives. The symptoms and clinical signs
therefore tended to vary with different products.
8.1.1. Neurotoxic effects of 2,4-D and related compounds
Some of the case reports cited below on poisonings with, and
occupational overexposures to, herbicides indicate that 2,4-D and
other chlorophenoxy compounds may affect both the central and
peripheral nervous systems.
8.1.1.1. Effects on the central nervous system
In addition to subjective symptoms of the central nervous
system, impaired coordination, impaired responses to external
stimuli, unconsciousness, coma, and death have been observed in
human beings mainly after absorption of lethal or nearly lethal
doses of 2,4-D. The acute effects on the central nervous system
seem to resemble those produced by alcohol, sedative drugs, or
aromatic chlorinated hydrocarbons rather than those produced by
organophosphate or carbamate neurological poisons (Geldmacher-Von
Mallinckrodt & Lautenbach, 1966; Prescott et al., 1979). Acute
cerebral demyelination occurred in a suicide who ingested a
solution of 2,4-D in kerosene (Dudley & Thapar, 1972). Severe
degeneration of brain ganglion cells was observed in another
suicide who drank a herbicide product containing 2,4-D in the form
of water-soluble dimethylamine salt (Nielsen et al., 1965). It is
therefore possible that lethal doses of chlorophenoxy compounds may
cause structural as well as functional damage to the brain.
Craniocerebral and peripheral functional nerve damage was noted
by Bezuglyi et al. (1979) in a group of women occupationally
overexposed to 2,4-D herbicides and other pesticides. Electro-
encephalographic (EEG) abnormalities were observed in tractor
drivers spraying herbicides containing 2,4-D, MCPA, or mecoprop
(Kontek et al., 1973), in "individuals exposed to" chlorophenoxy
herbicides, including 2,4-D and MCPA (Bielski & Madra, 1976) and in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979). On
the other hand, a case of neuropathy with EEG abnormalities and
flaccid quadriplegia, originally attributed to occupational
overexposure to MCPA, was diagnosed as being of viral origin
(Nayrac et al., 1958), and therefore some of the neurological
damage attributed to 2,4-D, may have been caused by viruses. There
is some evidence that 2,4-D herbicides may affect the sensory
system, as Andreasik et al. (1979) and Assouly (1951) reported
intolerance to certain odours, hypersensitivity to noise, and other
sensory abnormalities in workers producing or packaging 2,4-D
herbicides, while Fetisov (1966) reported hyposmia and other
sensory deficiencies in similarly-exposed workers.
8.1.1.2. Effects on the peripheral nervous system
"Peripheral neuropathy" and reduced peripheral nerve conduction
velocities have been reported in workers producing 2,4-D and
2,4,5-T (Poland et al., 1971; Singer et al., 1982). More than one
dozen other studies of persons overexposed to chlorophenoxy
herbicides also indicated detrimental effects of 2,4-D products on
the peripheral nervous system (Goldstein et al., 1959; Goldstein &
Brown, 1960; Foissac-Gegoux et al., 1962; Todd, 1962; Berkley &
Magee, 1963; Wallis et al., 1970; Bezuglyi et al., 1979). Long-
lasting flaccid paraparesis or quadriparesis following skin contact
with 2,4-D herbicides was reported by Goldstein et al. (1959) and
Goldstein & Brown (1960). Abnormal tendon reflexes in these or
similar cases were reported by the same authors and by Andreasik et
al. (1979), Berkley & Magee (1963), and Foissac-Gegoux et al.
(1962). Cases of sensory neuropathy attributed to the ingestion
of, or dermal exposure to, 2,4-D herbicides have also been reported
by Monarca & Divito (1961), Foissac-Gegoux et al. (1962), Todd
(1962), Wallis et al. (1970), Sare (1972), and Bezuglyi et al.
(1979). However, no signs of peripheral neuropathy were reported
by Apffel (1959a), Seabury (1963), Paggiaro et al. (1974), and
Prescott et al. (1979), in similar cases of massive exposure to
2,4-D herbicide, and in patients given relatively large amounts of
purified 2,4-D or 2,4,5-T salts or esters as drugs. One
explanation for this may be great individual differences in
susceptibility to poisoning with chlorophenoxy herbicides
(Rosenberg, 1980), as attested by the case of a 58-year-old woman
who "was fully conscious with no clinical evidence of toxicity"
after ingesting enough herbicide to allegedly attain 2,4-D and
mecoprop blood plasma concentrations of 335 and 400 mg/litre
respectively (Prescott et al., 1979). By comparison, Herbich &
Machata (1963) reported that a plasma concentration of 447 mg
2,4-D/litre caused death in a 46-year-old man.
Herbicide ingredients other than 2,4-D and related compounds
might be at least partly responsible for the observed neurotoxic
effects. In particular, organic solvents, emulsifiers, and
ethylene glycol present in herbicide formulations have been
mentioned in this connection (Goldstein & Brown, 1960; Goldwater,
1960). Solvents such as alcohols and trichloroethylene, used in
the manufacture of the active herbicide ingredients, might account
for some of the abnormalities observed in workers involved in the
production of chlorophenoxy compounds (Assouly, 1951; Bashirov,
1969; Bashirov & Ter-Bagdasarova, 1970).
Some of the reported cases of central nervous system
dysfunction or peripheral neuritis may have been merely
coincidental to the herbicide exposure, as there are many known
causes of neuropathy, such as nutritional and hereditary factors,
infectious diseases, and many toxic chemicals, including alcohol
(Freemon, 1975). Thus, alcoholism may have been a contributory
factor in one case of "2,4-D polyneuropathy" reported by Brandt
(1971).
Further studies of the possible effects of 2,4-D and other
chlorophenoxy compounds or their by-products on the human nervous
system are desirable, including studies of behavioural effects
measurable by recently-developed test batteries (Baker et al.,
1983).
8.1.2. Myotoxic effects of 2,4-D
Muscle fibrillations, myotonia, myoglobinuria, muscular
weakness and other indications of a myotoxic effect of 2,4-D have
been reported in patients treated with large doses of purified
2,4-D products by Apffel (1959a) and Seabury (1963), as well as in
cases of suicidal or accidental ingestion of 2,4-D herbicides or
following occupational overexposure (Herbich & Machata, 1963;
Berwick, 1970; Dudley & Thapar, 1972; Prescott et al., 1979). In
laboratory animals, myotonia and structural or biochemical muscle
lesions can be reliably induced by doses in excess of about 100 mg
2,4-D/kg body weight. In human beings, the threshold dose for
gross myotoxic effects certainly exceeds 5 mg/kg body weight per
day, and may be above 36 mg/kg body weight per day (Seabury, 1963;
Khanna & Kohli, 1977; Sauerhoff et al., 1977).
8.1.3. Cardiopathies and cardiovascular effects
Myocardial dystrophy, myocarditis, cardiac arrhythmias or
fibrillations, a slowed heart rate, or electrocardiographic (ECG)
changes were observed in human beings who ingested herbicides
containing 2,4-D (Duric et al., 1979) or 2,4-D and mecoprop
(Prescott et al., 1979), and also after occupational overexposure
to chlorophenoxy herbicides (Belomyttseva, 1965; Khibin et al.,
1968; Zakharov et al., 1968; Paggiaro et al., 1974; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979; Prescott et al., 1979).
However, in other cases of acute poisoning with 2,4-D herbicides,
the ECG was essentially normal (Berwick, 1970; Monarca & Divito,
1961).
Thus, further studies are desirable to determine the threshold
2,4-D doses at which electrophysiological cardiac abnormalities can
be observed, and to determine whether they result from a direct
effect on the nerve conducting system of the heart, or secondarily
from a toxic action on the myocardium.
It has been suggested that allergic reactions and an increased
sensitivity may be involved in 2,4-D-related cardiac arrhythmias
(Winkelmann, 1960; Rea, 1978).
Both hypertension and hypotension have been reported following
exposure to high doses of 2,4-D (Apffel, 1959a; Kaskevich &
Sobolieva, 1978; Bezugly et al., 1979), but no conclusions could be
drawn from these studies.
8.1.4. Haematological effects
Monarca & Divito (196l), Todd (1962), Radionov et al. (1967),
Bashirov (1969), Bashirov & Ter-Bagdarasova (1970), Brandt (1971),
Andreasik et al. (1979), and Bezuglyi et al. (1979) observed
haematological changes such as mild anaemia, bone marrow
depression, mono- or lymphocytosis or eosinophilia, changes in
erythrocyte volume or size, or methaemoglobinaemia following
ingestion of, or overexposure to 2,4-D. However, these changes may
have been due to other causes, as Apffel (1959a) did not observe
either haematological changes or effects on haematopoiesis in
patients given 0.1 - 0.3 g of purified 2,4-D per day as an
anticancer drug.
8.1.5. Blood chemistry effects
Hyperglycaemia, hypercholesterolaemia, elevated levels of blood
urea, transaminase (SGOT, SGPT), and creatine phosphokinase (CPK),
or altered blood albumin, globulin, or phospholipid levels
following acute poisoning with, or occupational overexposure to
2,4-D were reported by Bashirov (1969), Bashirov & Ter-Bagdarasova
(1970), Berwick (1970), Lukoshkina et al. (1970), Brandt (1971),
Bezuglyi et al. (1979), Duric et al. (1979) and Prescott et al.
(1979). However, Bashirov (1969) reported hypoglycaemia (< 700
mg/litre) and abnormally slow return to normal values in glucose
tolerance tests in about one-third of a group of workers producing
2,4-D. Increased activity of erythrocytic glycolytic enzymes was
found in Polish workers packaging 2,4-D sodium salt (Andreasik et
al., 1979). In one case of intentional 2,4-D poisoning, there was
hyperglycaemia (De Larrard & Barbaste, 1969), while in some cases
of 2,4-D overexposure, blood glucose abnormalities were not
observed (Goldstein et al., 1959). Apffel (1959a) never observed
hyperglycaemia in his patients, on the contrary, daily doses of
1 - 1.25 g 2,4-D led to hypoglycaemia. Thus, under some
circumstances, high doses of 2,4-D apparently can affect glucose
metabolism, and produce hypo- or hyperglycaemia. Gamble (1975)
proposed that 2,4-D might inhibit certain APT-dependent enzymes and
thus affect lipid metabolism.
8.1.6. Pulmonary effects
Pulmonary emphysema, oedema, hyperaemia and haemorrhages were
found in cases of fatal poisonings due to 2,4-D herbicide ingestion
(Herbich & Machata, 1963; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972). It is not clear whether the acute
pulmonary effects were caused by the 2,4-D preparations or by the
solvents such as kerosene or fuel oil. However, it is unlikely
that the pulmonary emphysema was caused by acute exposure to 2,4-D.
Dyspnoea or respiratory tract irritation were occasionally
reported following occupational overexposure of 2,4-D production
workers or herbicide sprayers (Assouly, 1951; Belomyttseva, 1964;
Bashirov, 1969; Bezuglyi et al., 1979).
8.1.7. Hepatotoxic effects
Liver necrosis or fatty liver cell changes were observed in 2
fatal cases following 2,4-D herbicide ingestion (Geldmacher-Von
Mallindkrodt & Lautenbach, 1966; Dudley & Thapar, 1972). In
several non-fatal 2,4-D poisonings, no biochemical evidence of
liver damage was noted, and neither Apffel (1959a) nor Seabury
(1963) reported indications of liver damage in patients treated
with up to 2.5 g/day of purified 2,4-D, salts, or esters.
Hyperbilirubinaemia, elevated urobilinogen levels, or liver
enlargement were reported in workers occupationally exposed both to
2,4-D herbicides and to other chemicals (Belomyttesva, 1964, 1965;
Bashirov, 1969; Bashirov & Ter-Bagdasarova, 1970; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979).
8.1.8. Nephrotoxic effects
Degeneration of, or fatty changes in kidney tubules, or
proteinuria, increased blood urea levels, and other indications of
a nephrotoxic effects were observed in cases of fatal or nearly
fatal herbicide ingestion (Goldstein et al., 1959; Curry, 1962;
Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Brandt, 1971;
Dudley & Thapar, 1972; Duric et al., 1979). Impaired renal
function was reported in occupationally-exposed persons by Bashirov
(1969), Bashirov & Ter-Bagdasarova (1970), Paggiaro et al. (1974),
and by Andreasik et al. (1979). On the other hand, neither Apffel
(1959a) nor Seabury (1963) reported any evidence of kidney damage
in their patients, some of whom received in excess of 2 g of pure
2,4-D per day.
8.1.9. Effects on the digestive tract
Vomiting, diarrhoea, nausea, and other indications of toxic
effects on the digestive tract were observed by Apffel (1959a) in
patients injected intramuscularly with large doses (up to 2.5 g) of
purified 2,4-D products.
The same effects have also been noted after ingestion of large
doses of 2,4-D herbicides, or after combined inhalation and dermal
overexposure (Goldstein et al., 1959; Monarca & Divito, 1961;
Nielsen et al., 1965; Tsapko, 1966; Radionov et al., 1967; Paggiaro
et al., 1974; Dennis, 1976; Kuzyk, 1979; Prescott et al., 1979).
However, no gastrointestinal symptoms were reported by volunteers
who ingested a single dose of 5 mg pure 2,4-D/kg body weight
(Khanna & Kohli, 1977; Sauerhoff et al., 1977). Thus, an intake of
more than 300 mg 2,4-D per adult appears to be required to induce
acute toxic effects on the gastrointestinal tract.
8.1.10. Effects on endocrine organs
Andreasik et al. (1979) found an impaired iodine uptake by the
thyroid, and decreased thyroxine, thyroxine clearance, and
thyroxine iodine values in workers packaging 2,4-D sodium salt.
Since these workers were exposed to a variety of chemicals, these
results need confirmation.
8.1.11. Irritative and allergenic effects
Chronic tonsillitis and paranasal sinusitis were reported in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979).
Acute eye or skin irritation, as well as skin reactions of an
allergic type, including anaphylactoid purpura (allergic angiitis)
and contact eczema have been reported in agricultural and forestry
workers following occupational exposure to 2,4-D herbicides
(Winkelmann, 1960; Radionov et al., 1967; Balo-Banga et al., 1973;
Jung & Wolf, 1977; Kuzyk, 1979). Jung & Wolf (1977) found that
exposure to the vapour of a 2,4-D/2,4,5-T formulation in diesel oil
(SELEST 100) caused an acute allergic reaction in the skin of
sensitized herbicide applicators, and that the allergic reactions
were caused by the mixture of 2,4-D/2,4,5-T esters and not by the
diesel oil.
8.2. Epidemiological Studies of the Chronic Effects of 2,4-D
Much concern has been raised about the phenoxy herbicides,
including 2,4-D, especially in relation to birth defects and cancer
in human beings.
Several episodes have also been reported in which defined
populations were exposed to mixtures of 2,4-D and 2,4,5-T, in which
the 2,4,5-T was contaminated with various amounts of 2,3,7,8-
tetrachlorodibenzodioxin (2,3,7,8-TCDD) (Bleiberg et al., 1964;
Huff et al., 1980). It is now generally accepted that chloracne
and porphyria cutanea tarda observed in these studies were caused
by exposure to 2,3,7,8-TCDD and not by exposure to 2,4,5-T or 2,4-D
(Kimbrough, 1980).
The following sections concentrate on epidemiological studies
or other related studies on human beings in which actual or
potential exposures to 2,4-D products alone or to mixtures of 2,4-D
with other chlorphenoxy herbicides were demonstrated.
8.2.1. Reproductive, fetotoxic, and teratogenic effects
Although effects on reproduction have been demonstrated in
animals with 2,4,5-T, 2,4-D and 2,3,7,8-TCDD, all of the attempts
made to determine whether human beings suffer similar effects have
been frustrated by the poor design of the studies, inadequate
determination of exposure, or inadequate information about the
background incidence of spontaneous abortions and other abnormal
reproductive outcomes, by inadequate evaluation of confounding
variables, by inadequate assessment of exposure, and by mixed
exposures (Aldred et al., 1978; Lee, 1978; Field & Kerr, 1979;
Brogan et al., 1980; Hanify, 1980; Carmelli et al., 1981). For
these reasons they are not discussed in detail in this report.
Conclusive evidence of reproductive effects caused by 2,4-D in
populations that might be exposed to chlorophenoxy herbicides is
unlikely to be obtained from new epidemiological studies on
indirectly-exposed populations living in, or adjacent to, areas in
which phenoxy herbicides are used. Doses of 2,4-D absorbed by
bystanders are far below those expected to be toxic, as shown by
occupational exposure studies with 2,4-D and 2,4,5-T herbicides
(section 5). Any effects induced by such small amounts would
probably be obscured by more potent confounding factors (Janerich,
1973; Karkinen-Jääskelainen & Saxén, 1974; Saxén et al., 1974;
Elwood & Rogers, 1975; Granroth et al., 1977, 1978; James, 1977;
Holmberg, 1979; Lappe, 1979; Schacter et al., 1979).
Additional studies on female workers occupationally exposed to
significantly higher levels of 2,4-D than bystanders would be
useful to clarify some of the uncertainties raised by past studies,
if sufficiently large cohorts could be identified.
8.3. Studies on Mutagenic Effects in Workers Exposed to 2,4-D
Lymphocytes from ten workers exposed to 2,4-D esters during the
manufacture of 2,4-D herbicides, or from 15 workers packaging 2,4-D
sodium salt, did not show any chromosome abnormalities (Johnson,
1971; Andreasik et al., 1979). Chromosome or chromatid
abnormalities in lymphoctyes from some pesticide sprayers applying
a variety of agricultural chemicals, including in some cases 2,4-D,
were observed by Yoder et al. (1973) and Crossen et al. (1978).
Högstedt et al. (1980) did not observe any significant increases in
chromosome abnormalities in workers exposed to 2,4-D and other
pesticides.
The induction of SCEs among workers occupationally exposed to
the phenoxy herbicides 2,4-D and MCPA has been recently studied.
The subjects used only 2,4-D and MCPA or mixtures of the two for
spraying, and the exposure levels were estimated by determining the
urinary 2,4-D and MCPA excretion by the workers. No dose-related
differences in the frequencies of SCEs could be found either in
relation to the exposure level or to the length of the exposure
(Linnainmaa, 1983).
Although some studies suggest that occupational exposure to
2,4-D may result in chromosome abnormalities, the results are
conflicting. Moreover, the possiblity of mixed exposure and other
confounding variables cannot be excluded in the studies with
positive results.
8.4. Carcinogenic effects
8.4.1. Epidemiological studies
In two case-control studies of soft-tissue sarcoma (Hardell &
Sundström, 1979; Eriksson et al., 1981) and one of lymphoma
(Hardell et al., 1981), exposure to phenoxyacetic acids (mainly
2,4,5-T, 2,4-D, and MCPA) was associated with approximately 5-fold
increases in the risk of soft-tissue sarcomas. Exposure to 2,4-D,
either with or without MCPA exposure, also increased relative
risks. In the study of malignant lymphomas, 7 cases and 1 control
were apparently exposed to 2,4-D only (relative risk, 19.6; 95%
confidence interval, 4.3 - 89.8).
In a different case-control study with a small number of cases
and controls, no increased risk was observed (Smith et al., 1982).
A follow-up was also carried out on 348 railroad workers
exposed for less than 45 days during the period 1957 - 72 to the
herbicides 2,4-D, 2,4,5-T, atrazine, mecoprop, dichloropropionic
acid, and amitrole (Axelson & Sundell, 1974). The authors found a
significant increase in cancer mortality and morbidity among
workers exposed to amitrole.
Axelson et al. (1980) reported a further follow-up of these
workers, up to October, 1978, accumulating 5541 person-years. The
herbicide exposure of the workers was analysed in terms of exposure
to either amitrole, or phenoxy acids, or to a combination of the
two. A 10-year lapse period from the first day of exposure was
used as the induction latency. They found 15 cases of cancer
versus 6.87 expected (relative risk, 2.2). In the cohort with
combined exposure to amitrole and phenoxy acids, 6 cases were
observed versus 1.78 expected (relative risk, 3.4); in the group
exposed to amitrole alone, 3 tumours were observed versus 1.95
expected (relative risk, 1.5); and 6 cancers were observed versus
3.14 expected (relative risk, 1.9) in the phenoxy acid-exposed
group. All cancers, as well as cancers of the stomach, occurred in
statistically-significant excesses in the cohort as a whole. In
the groups exposed to amitrol plus phenoxy acids, there was a
significant excess of all cancers. In the group exposed only to
phenoxy acids, stomach cancer occurred in significant excess (2
observed, 0.33 expected; relative risk, 6.1). No soft-tissue
sarcomas were identified, but the statistical power of this study
to detect an excess of a rare cancer was limited.
Högstedt & Westerlund (1980) conducted a restrospective
mortality study on 142 forestry workers exposed to phenoxy
pesticides and 244 unexposed forestry workers, comparing their
mortality experience with national statistics. Work supervisors,
who were more highly exposed to phenoxy herbicides than the others,
had a significantly elevated tumour mortality (5 observed, 1.4
expected). No particular tumour type predominated, and no soft-
tissue sarcomas were observed, though the authors noted that the
study had limited statistical power and was inconclusive, because
of the relatively short follow-up period.
Riihimäki et al. (1982) reported on a prospective cohort study
of 2,4-D and 2,4,5-T spray personnel which was in progress.
Because of the small number of deaths and the brief follow-up
period, no conclusions can so far be drawn from this study.
Follow-up studies on cohorts of pesticide sprayers, farmers,
and agricultural workers occupationally exposed to a variety of
chemicals, in some cases including phenoxy herbicides, have been
reviewed by IARC (1983).
Follow-up studies on groups of industrial workers exposed to
chlorophenols, 2,4,5-T, or other chlorophenoxy compounds, and to
2,3,7,8-TCDD or other dioxins, during the manufacture of
chlorophenoxy herbicides, have recently been reviewed by Huff et
al. (1980) and IARC (1983).
8.4.2. Evidence on the carcinogenicity of 2,4-D
The available studies suggest that an association exists
between mixed exposure to phenoxy herbicides, chlorinated phenols,
and chlorinated dibenzodioxins, and an increased incidence of soft
tissue sarcomas and malignant lymphomas. It is not clear, at
present, whether these findings represent true associations, and
further studies are in progress (Muir & Wagner, 1981) to clarify
this point. Since many of the tumour cases had been exposed to
combinations of phenoxy herbicides and their contaminants as well
as other chemicals, it is not known whether exposure to 2,4-D is
specifically associated with the development of soft tissue
sarcomas.
8.5. Treatment of Poisoning in Human Beings
Successfully treated cases of 2,4-D poisoning indicate that
forced alkaline diuresis is helpful in reducing the level of 2,4-D
in the blood and tissues (Young & Haley, 1977; Prescott et al.,
1979). Heart and kidney damage should be anticipated and
counteracted in cases of severe poisoning (Duric et al., 1979).
In cases of acute poisoning due to 2,4-D, the nearest Poison
Control Centre should be contacted for additional information on
symptoms and recommended treatment.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO 2,4-D
9.1. General Considerations
In areas of 2,4-D herbicide production, handling, or use, the
highest exposure will be incurred by those who are directly
involved in these processes, followed by bystanders indirectly
exposed to 2,4-D vapour, dust, or droplets, or to contaminated
vegetation, soil, or water. In these two groups, exposure will
usually be via the skin. The general population in 2,4-D-use areas
would be exposed to a lesser extent, mainly through food containing
2,4-D residues and to a lesser extent through 2,4-D residues in
water. The contribution from air is negligible. As far as the
general population is concerned, 2,4-D intake from any source, is
negligible.
9.2. Estimated Intake of 2,4-D by the Population in a 2,4-D-use Area
The total contribution from air, food, and water is estimated
to be 0.03 - 2 µg/kg body weight per day (Table 13).
9.2.1. Intake by bystanders
Given the limited data available and the many uncertainties
involved, an adequate estimate of 2,4-D intake by bystanders is not
possible at this time, but it should generally be less than that
for occupationally-exposed persons.
9.2.2. Occupational intake
Workers using 2,4-D may, on average, absorb about 0.1 mg
2,4-D/kg body weight per day. However, this level may be exceeded
if good occupational hygiene is not practiced (section 5.2).
Simple precautions against excessive exposure can reduce the amount
of 2,4-D uptake.
9.3. Safety Factors
9.3.1. Definitions
For the present assessment, the safety factor is defined as the
integer obtained by dividing the overall no-observed-adverse-effect
level for a known adverse effect of 2,4-D (determined from all
available information on human beings or animals) by the daily
exposure value (absorbed dose of 2,4-D) for the various exposed
groups.
9.3.2. Determination of safety factors
9.3.2.1. Acute poisoning
Based on clinical studies in which 2,4-D was injected into
patients as a drug, the no-observed-adverse-effect level for signs
and symptoms of acute 2,4-D poisoning in children and adults
appears to be at or near 36 mg/kg body weight (section 8.1). Based
on the available studies of the amounts of 2,4-D absorbed by
occupationally-exposed person, bystanders, and populations in
2,4-D-use areas, the safety factors for acute 2,4-D poisoning are
likely to be:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) at least 360 for occupationally-exposed spraying crews.
The margin of safety for persons with excessive occupational
exposures would be smaller.
9.3.2.2. Chronic toxicity
Dose-effect relationships for the chronic toxic effects of
2,4-D or 2,4-D derivatives are available only from animal studies.
The no-observed-adverse effect levels for certain chronic toxic
effects of 2,4-D in animals have not been firmly established, and
for this reason safety factors cannot be established (section
7.2.1) for all of the chronic effects of 2,4-D.
9.3.2.3. Embryotoxic, fetotoxic, and teratogenic effects
The no-observed-adverse-effect level for embryotoxic,
fetotoxic, or teratogenic effects of 2,4-D in mammals appears to
lie at 10 mg/kg body weight per day (section 7.3.1.2). Assuming
that the same is true for human beings, then the corresponding
safety factors for the various exposed groups are:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) 100 for occupationally-exposed spraying crews using
precautions against excessive exposure.
9.3.2.4. Mutagenic effects
The available information was inadequate for an assessment of
the mutagenic potential of 2,4-D in mammals.
9.3.2.5. Carcinogenic effects
Available animal bioassays and epidemiological studies are
inadequate for an assessment of the carcinogenic potential of 2,4-D
or of its derivatives.
9.4. Evaluation of Health Risks from 2,4-D Exposure
From the data available at present, the Task Group assumes that
a possible health risk will exist, when the safety factor is less
than 100.
9.5. Recommendations on Exposure
Results of recent exposure and occupational health studies
suggest that excessive exposure to 2,4-D can be avoided by fairly
simple measures of occupational hygiene, such as those recommended
in two pertinent publications of the International Labour Office
(ILO, 1977, 1979). Laundering procedures for 2,4-D-contaminated
clothing have been published by Easley et al. (1983), and these
should be considered.
REFERENCES
ABO-KHATWA, N. & HOLLINGWORTH, R.M. (1974) Pesticidal chemicals
affecting some energy linked functions of rat mitochondria in
vitro. Bull. environ. Contam. Toxicol., 12(4): 446-454.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-induced
RNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide-induced
ouabain resistant mutants in Chinese hamster V79 cells. Chem.-biol.
Interact., 19: 369-374.
AKESSON, N.B. & YATES, W.E. (196l) Drift residues on alfalfa hay.
A progress report of investigations of pesticide drift from
aircraft applications. Calif. Agric., 15: 4-7.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous
substances. Int. Pest Control, 11(2): 29-35.
ALDRED, J.E., BELCHER, R.S., CHRISTOPHERS, A.J., CLEMENTS, A.,
DANKS, D.M., FRENCH, E.L., GALLOWAY, D.B., GARSON, O.M., PARSONS,
W., RAPER, C., ROUCH, G., & SINN, H.J. (1978) Report of the
Consultative Council on Congenital Abnormalities in the Yarram
District. Alleged relationship of congenital abnormalities to the
use of the herbicides 2,4-D and 2,4,5-T. State of Victoria,
Australia, Ministry of Health, viii + 55 pp (Document PB 338).
ALTOM, J.D. & STRITZKE, J.F. (1973) Degradation of dicamba,
picloram and familiar phenoxy herbicides in soils. Weed Sci., 21:
556-560.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20(3): 649-656.
ANDREASIK, Z., KOLON, S., & SMOLIK, R. (1979) Health status
evaluation in workers packaging the herbicide "Pielik"
(cholorophenol). Arh. Hig. Rad. Toksikol., 30 (Suppl.): 599-602.
ANDRUSHAITIS G.P. (1972) [The effect of pesticides on the
environment.] Latvi. PSR Zinatnu Akad. Vest., 4:(297) 44-49 (in
Russian, Health & Welfare Canada Translation No. 2441).
ANTONENKO, T.A. (1977) [Experimental data from a study of the
permeability of the placental barrier to the herbicide 2,4-D and
its passage with the mother's milk during feeding.] Gig. Aspekt.
Okhr. Zdor. Naseleniya (Saratov). In: Saratov, Saratov Institute
for Oral Hygiene, pp. 177-178 (in Russian, Health & Welfare Canada
Translation No. 2370).
APFFEL, C.A. (1959a) Action cytostatique de certaines auxines,
compte rendu préliminaire. Presse med., 67(6): 207-209.
ARJMAND, M., HAMILTON, R.H., & MUMMA, R.O. (1978) Separation of
amino acid conjugates of 2,4-dichlorophenoxyacetic acid by high-
pressure liquid chromatography employing ion-pair techniques.
J. agric. food Chem., 26: 971-973.
ARKHIPOV, G.N. & KOZLOVA, I.N. (1974) [A study of the
carcinogenic potential of a herbicide: 2,4-D amine salt.] Vopr.
Pit., 5: 83-84 (in Russian).
ASSOULY, M. (1951) Désherbants sélectifs et substances de
croissance aperēu technique. Effet pathologiques sur l'homme au
cours de la fabrication de l'ester du 2,4-D. Arch. Mal. prof. Hyg.
Toxicol. Ind., 12(1): 26-30.
AVERITT, W.K. (1967) An evaluation of the persistence of 2,4-D
amine in surface waters in the state of Louisiana. Proc. South.
Weed Sci. Soc., 20: 342-347.
AXELSON, O. & SUNDELL, L. (1974) Herbicide exposure, mortality
and tumor incidence. An epidemiological investigation on Swedish
railroad workers. Scand. J. Work Environ. Health, 11:21-28.
AXELSON, O., SUNDELL, L., ANDERSSON, K., EDLING, C., HOGSTEDT, C.,
& KLING, H. (1980) Herbicide exposure and tumor mortality: An
updated epidemiological investigation on Swedish railroad workers.
Scand. J. Work Environ. Health, 6: 73-79.
BACHE, C.A., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1964a)
Absence of phenoxyacid herbicide residues in the milk of dairy cows
at high feeding levels. J. dairy Sci., 47(3): 298-299.
BAKER, B.R., LEE, W.W., SKINNER, W.A., MARTINEZ, A.P., & TONG, E.
(1960) Potential anticancer agents. I. Non-classical
antimetabolites. II. Some factors in the design of exo-alkylating
enzyme inhibitors, particularly of lactic dehydrogenase. J. med.
pharm. Chem., 2(6): 633-657.
BAKER, E.L., FELDMAN, R.G., WHITE, R.F., HARLEY, J.P., DINSE, G.E.,
& BERKEY, C.S. (1983) Monitoring neurotoxins in industry:
Development of a neuro-behavioral test battery. J. occup. Med.,
25(2): 125-130.
BAKER, P.G., HOODLESS, R.A., & TYLER, J.F.C. (1981) A review of
methods for the determination of polychlorodibenzo-p-dioxins and
polychlorodibenzofurans in phenoxyalkanoic acid herbicides.
Pestic. Sci., 12: 294-304.
BALO-BANGA, M.J., ANTONI, F., ALTMANN, H., VINCZE, I., & KOCSIS, F.
(1973) Effect of 2,4-D on semiconservative and DNA repair
synthesis. (Case report: bullous dermatitis after exposure to
2,4-D). Ber. Oesterr. Studienges Atomenerg. (Forschungszentrum
Seibersdorf), 2218: 1-7.
BAMESBERGER, W.L. & ADAMS, D.F. (1966) An atmospheric survey for
aerosol and gaseous 2,4-D components. Adv. Chem. Ser., 60:
219-227.
BARTLEY, T.R. & HATTRUP, A.R. (1970) 2,4-D contamination and
persistence in irrigation water. Proc. West. Soc. Weed Sci.,
23: 10-33.
BASHIROV, A.A. (1969) [The health of workers involved in the
production of amine and butyl 2,4-D herbicides.] Vrach. Delo,
10: 92-95 (in Russian, Health & Welfare Canada Translation No.
2181).
BASHIROV, A.A. & TER-BAGDASAROVA, I.K. (1970) [The state of the
cardiovascular system in workers engaged in the production of amine
salt herbicides and 2,4-dichlorophenoxyacetic acid butyl ester.]
Azerb. Med. Zh., 47 (12): 44-48 (in Russian).
BATORA, V., VITOROUIC, S.L.J., THIER, H.P., & KLISENKO, N.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BEAVEN, F.G., RAWLS, C.K., & BECKET, G.E. (1962) Field
observations upon estuarine animals exposed to 2,4-D. Proc.
northeast. Weed Contr. Conf., 16: 449-458.
BELOMYTTSEVA, L.A. (1964) [Toxic effects of the herbicide 2,4-D
sodium on the lungs.] Sb. Nauchn. Tr. Bashk. Gos. Med. Inst.,
16(2): 135-141 (in Russian).
BELOMYTTSEVA, L.A. (1965) [In: Contributions to the 3rd All-Union
Scientific Conference on the Problems of Hygiene and Toxicology
related to the Use of Chemicals in the National Economy, Kiev,]
p. 380 (in Russian).
BELOMYTTSEVA, L.A. (1967) The toxic effect of the sodium salt of
2,4-dichlorophenoxyacetic acid. UFA 1967.
BELOMYTTSEVA, L.A. & KARIMOVA, A.K. (1963) In: [Proceedings of
the UFA Scientific Research Institute of Hygiene and Occupational
Diseases, Vol. 2.] (in Russian).
BERKLEY, M.C. & MAGEE, K.R. (1963) Neuropathy following exposure
to a dimethylamine salt of 2,4-D. Arch. internal. Med., 111:
351-352.
BERWICK, P. (1970) 2,4-dichlorophenoxyacetic acid poisoning in
man. J. Am. Med. Assoc., 214(6): 1114-1117.
BEZUGLYI, V.P., FOKINA, K.V., KOMAROVA, L.I., SIVITSKAJA, I.I.,
ILINA, V.I., & GORSKAJA, I.I. (1979) [Clinical manifestations of
long-term sequels of acute poisoning with 2,4-D.] Gig. Truda Prof.
Zabol., 3: 47-48 (in Russian).
BIELSKI, J. & MADRA, B. (1976) [Acute poisoning with pesticides.]
Pol. Tyg. Lek., 31(46): 1971-1973 (in Polish, Health & Welfare
Canada Translation No. 2245).
BJERKE, E.L., HERMAN, J.L., MILLER, P.W., & WETTERS, J.H. (1972)
Residue study of phenoxy herbicides in milk and cream. J. agric.
food Chem., 20: 963-967.
BJORKLUND, N.E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. scand., 7: 364-390.
BJORN, M.D. & NORTHEN, H.T. (1948) Effects of 2,4-dichloro-
phenoxyacetic acid on chicks. Science, 108: 479-480.
BLEIBERG, J., WALLEN, M., & BRODKIN, R. (1964) Industrially
acquired porphyria. Arch. Derm., 89: 793-797.
BODAI, J., TAMAS, L., & VEGH, A. (1974) [Reproductive
consequences of Dikonirt toxicosis in cattle.] Magy. Allatorv.
Lapja, 29(5): 319-322 (in Hungarian, Health & Welfare Canada
Translation No. 2238).
BODEM, R., PREISS, D., & KUHN, E. (1971) [On the delay of the
relaxation of the guinea pig papillary muscle by 2,4-dichloro-
phenoxy-acetic acid (2,4-D).] Klin. Wochenschr., 49(1): 227-228
(in German).
BODYAGIN, D.A., SYKIN, A.B., LUPINOSOV, Y.V., & ZAITSEVA, L.A.
(1969) [The effects of chlorophenoxyacetic acid derivatives
(herbicides) on animals and human beings. (A literature review).]
Farmakol. Toksikol., 32 (6): 747-751 (in Russian, Health & Welfare
Canada Translation No. 2031).
BOLLAG, J.M., HELLINGS, C.S., & ALEXANDER, M. (1968) 2,4-D
metabolism. Enzymatic hydroxylation of chlorinated phenols.
J. agric. food Chem., 16: 826-828.
BONGSO, T.A. & BASRUR, P.K. (1973) In vitro response of bovine
cells to 2,4-dichlorophenoxy acetic acid. In vitro, 8: 416-417.
BONTOYAN, W.R. (1977) Report on pesticide formulations. J. Assoc.
Off. Agric. Chem., 60: 324-327.
BOVAL, B. & SMITH, J.M. (1973) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. Chem. Eng. Sci., 28: 1661-1675.
BOVEY, R.W. (1980a) Residues and fate of phenoxy herbicides in
the environment. In: Bovey, R.W. & Young, A.L., ed. The science
of 2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 301-356.
BOVEY, R.W. (1980b) Human risk with phenoxy herbicides in the
environment. In: Bovey, R.W. & Young, A.L., ed. The science of
2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 425-433.
BOVEY, R.W. & YOUNG, A.L., ed. (1980) The science of 2,4,5-T and
associated phenoxy herbicides, New York, Chichester, Brisbane,
Toronto, John Willey & Sons, 462 pp.
BRADLAW, H.A., GARTHOFF, L.H., HURLEY, N.E., & FIRESTONE, D. (1980)
Comparative induction of aryl hydrocarbon hydroxylase activity
in vitro by analoques of dibenzo- p-dioxin. Food cosmet. Toxicol.,
18: 627-635.
BRANDT, M.R. (1971) [Herbatox poisoning. A short survey and a
report of a case.] Ugeskrift Laeger, 133(11): 500-503 (in Danish,
Health & Welfare Canada Translation No. 2147.
BRETAG, L.H. & CAPUTO, C. (1978) The nature of the antimyotonic
action of 2,4-D on the soleus muscle of the rat. Proc. Aust.
Physiol. Pharmacol. Soc., 9(2): 150 pp.
BRISTOL, D.W., COOK, L.W., KOTERBA, M.T., & NELSON, D.C. (1982)
Determination of free and hydrolyzable residues of 2,4-
dichlorophenoxyacetic acid and 2,4-dichlorophenol in potatoes.
J. agric. food Chem., 30: 137-144.
BRODY, I.A. (1973) Myotonia induced by monocarboxylic aromatic
acids: A possible mechanism. Arch. Neurol., 28: 243-246.
BRODY, T.M. (1952) Effect of certain plant growth substances on
oxidative phosphorylation in rat liver mitochondria. Proc. Soc.
Exp. Bio. Med., 80: 533-536.
BROGAN, W.F., BROGAN, C.E., & DADD, J.T. (1980) Herbicides and
cleft lip and palate. Lancet, 2: 597.
BUCHER, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid
on experimental animals. Proc. Soc. Exp. Biol. Med., 63: 204-205.
BURCAR, P.J., WERSHAW, R.L., GOLDBERG, M.C., & KHAN, L. (1966)
Gas chromatographic study of the behaviour of the isooctyl ester
of 2,4-D under field conditions in North Park, Colorado. Anal.
Instrum., 4: 215-224.
BURTON, J.A., GARDINER, T.H., & SCHANKER, L.S. (1974) Absorption
of herbicides from the rat lung. Arch. environ. Health, 29: 31-33.
BUSLOVICH, S.Y. (1963) [The effect of the chlorinated derivatives
of phenoxyacetic acid.] Zdrav. Beloruss., 9: 41-45 (in Russian,
Health & Welfare Canada Translation No. 2315).
BUSLOVICH, S.Y. & KOLDOBSKAYA, F.D. (1972) [Activity of
hexokinase in skeletal muscles of albino rats in experimental
myotonia.] Vopr. Med. Khim., 18(4): 403-406 (in Russian).
BUSLOVICH, S.Y & MILCHINA, M.G. (1976) [The dynamics of 2,4-D
amine decomposition in soil.] Gig. i Sanit., 5: 109-110
(in Russian).
BUSLOVICH, S.Y., VOINOVA, I.V., & MILCHINA, M.G. (1973) [The
distribution of chlorinated phenoxy acid herbicides in albino
rats.] Gig. Tr. Prof. Zabol, 17(3): 171-173 (in Russian, Health &
Welfare Canada Translation No. 2237).
BUSLOVICH, S.Y., ALEKSASHINA, Z.A., & KOLOSOVSKAYA, V.M. (1976)
[Embryotoxic effects of herbicides: Chlorinated phenoxy acids.]
Zdrav. Beloruss., 10: 83-84 (in Russian).
BUSLOVICH, S.Y., KOLDOBSKAYA, F.D., DAVYDENKO, L.I., & KRYSANOVA,
A.I. (1982) [The role of mixed-function oxidases in the
detoxication of some herbicides: Chlorinated derivatives of
phenoxyacids.] Gig. i Sanit., 10: 76-77 (in Russian).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna.
Proc. South. Weed Sci. Soc., 18: 576-580.
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON, M.H., &
MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D, DEF,
propanil, and trifluralin to the Dungeness crab, Cancer magister.
Arch. environ. contam. Toxicol., 8: 383-396.
CANADA, HEALTH & WELFARE (1980) Pesticides. In: Guidelines for
Canadian drinking water quality, 1978. Supporting documentation,
Ottawa, Supply and Services, Canada, pp. 457-488.
CARL, M. (1979) Internal laboratory quality control in the
routine determination of chlorinated pesticide residues. Adv.
pestic. Sci., 3:660-663.
CARMELLI, D., HOFHERR, L., TOMSIC, J., & MORGAN, R.W. (1981) A
case-control study of the relationship between exposure to 2,4-D
and spontaneous abortions in humans, Menlo Park, California, SRI
International (Final Report).
CARTER, E.P. (1960) Volatility of ester forms of hormone type
herbicides III. J. Assoc. Off. Anal. Chem., 43: 367-370.
CHANG, H., RIP, J.W., & CHERRY, J.H. (1974) Effects of
phenoxyacetic acids on rat liver tissues. J. agric. food Chem.,
22(1): 62-65.
CHAU, A.S.Y. & THOMSON, K. (1978) Investigation of the integrity
of seven herbicidal acids in water samples. J. Assoc. Off. Anal.
Chem., 61: 1481-1485.
CHAU, A.S.Y., AFGHAN, B.K., & ROBINSON, J.W. (1982) Analysis of
pesticides in water. Volume II. Chlorine- and phosphorus-
containing pesticides. Boca Raton (Florida), CRC Press Inc.,
pp. 238.
CHEN, W.L., GORZINSKI, S.J., KOCIBA, R.J., WADE, C.E., MORDEN,
D.C., KEYES, D.G., & SCHWETZ, B.A. (1981) 2,4-D (2,4-
dichlorophenoxy) acetic acid: results of a 13-week feeding study in
CDF Fisher 344 rats. Proc. Annu. Meet. Can. Fed. Biol. Soc.,
24: 233.
CHOI, K.L., QUE HEE, S.S., & SUTHERLAND, R.G. (1976) 2,4-D levels
in the South Saskatchewan River in 1973 as determined by a GLC
method. J. environ. Sci. Health, Part B, 11: 175-183.
CHOW, C., MONTGOMERY, M.L., & YU, T.C. (1971) Methodology and
analysis for residues of MCP and 2,4,5-T in wheat. Bull. environ.
Contam. Toxicol., 18: 361-365.
CLARK, D.E., PALMER, J.S., RADELEFF, R.D., CROOKSHANK, H.R., &
FARR, F.M. (1975) Residues of chlorophenoxy acid herbicides and
their phenolic metabolites in sheep and cattle. J. agric. food
Chem., 23(3): 573-578.
COCHRANE, W.P. (1981) Chemical derivatization in pesticide
analysis. In: Frei, R.W. & Lawrence, J.F., ed. Chemical
derivatization in analytical chemistry, I: Chromatography, New
York, Plenum Press, pp. 1-97.
COCHRANE, W.P. & RUSSELL, J.B. (1975) Residues in wheat and soil
treated with the mixed butyl esters of 2,4-D. Can. J. plant Sci.,
55: 323-325.
COCHRANE, W.P., SINGH, J., MILES, W., & WAKEFORD, B. (1981)
Determination of chlorinated dibenzo- p-dioxin contaminants in
2,4-D products by gas chromatography - mass spectrometric
techniques. J. Chromatogr., 217: 289-299.
COCHRANE, W.P., LANOUETTE, M., & SINGH, J. (1982) High pressure
liquid chromatographic determination of impurity phenols in
technical 2,4-D acid and 2,4-dichlorophenol. J. Assoc. Off. Anal.
Chem., in press.
COGGON, D. & ACHESON, E.D. (1982) Do phenoxy herbicides cause
cancer in man? Lancet, 1: 1057-1059.
COHEN, S.Z., ZWEIG, G., LAW, M., WRIGHT, D., & BONTOYAN, W.R.
(1978) Analytical determination of N-nitroso compounds in
pesticides by the United States Environmental Protection Agency.
In: Walker, E.A., Castegnaro, M., Criciute, L., & Lyle, R.E., ed.
Environmental Aspects of N-nitroso compounds, Lyons, IARC
Scientific Publications, 19: 333-342.
COLLABORATIVE INTERNATIONAL PESTICIDES ANALYTICAL COUNCIL LTD
(1970) CIPAC Handbook, Vol. I, Analysis of technical and
formulated pesticides, Harpenden, England, CIPAC, pp. 241-273.
COMMISSION OF EUROPEAN COMMUNITIES (1981) Criteria (dose/effect
relationships) for organochlorine pesticides. Oxford, New York,
Toronto, Sydney, Paris, Frankfurt, Pergamon Press, pp. 223-240.
CONNICK, W.J., Jr & SIMONEAUX, J.M. (1982) Determination of (2,4-
dichlorophenoxy) acetic acid and of 2,6-dichlorobenzo-nitrile in
water by high performance liquid chromatography. J. agric. food
Chem., 30: 258-260.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99(1): 1-12.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet
samples (V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet
samples (VI). Pestic. monit. J., 5(4): 313-330.
COURTNEY, K.D. (1977) Prenatal effects of herbicides: Evaluation
by the prenatal development index. Arch. environ. Contamin.
Toxicol., 6: 33-46.
COUTSELINIS, A., KENTARCHOV, R., & BOUKIS, D. (1977) Concentration
levels of 2,4-D and 2,4,5-T in forensic material. Forensic Sci.,
1: 203-204.
CRAFTS, A.S. (1960) Evidence for hydrolysis of esters of 2,4-D
during absorption by plants. Weeds, 8: 19-25.
CRIPPS, R.E. & ROBERTS, T. (1978) Microbial degradation of
herbicides. In: Hill, I.R. & Wright, S.J.L., ed.: Pesticide
microbiology - Microbial aspects of pesticide behaviour in the
environment, London, New York, San Francisco, Academic Press,
pp. 669-730.
CROSBY, D.G. & TUTASS, H.O. (1966) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. J. agric. Food Chem., 14: 596.
CROSBY, D.G. & WONG, A.S. (1973) Photodecomposition of 2,4,5-
trichlorophenoxyacetic acid (2,4,5-T) in water. J. agric. Food
Chem., 21(6): 1052-1054.
CROSSEN, P.E., MORGAN, W.F., HORAN, J.J., & STEWART, J. (1978)
Cytogenetic studies of pesticide and herbicide sprayers. New
Zealand med. J., 88(619): 192-195.
CRUMMET, W.P. & STEHL, R.H. (1973) Determination of chlorinated
dibenzo- p-dioxins and dibenzofurans in various materials. Environ.
Health Perspect., 5: 15-25.
CURRIE, L.A. (1968) Limits of qualitative detection and
quantitative determination. Anal. Chem., 40: 586-593.
CURRY, A.S. (1962) Twenty-one uncommon cases of poisoning. Br.
med. J., 1: 687-689.
DANON, J.M., KARPATI, G., CARPENTER, S., & WOLFE, L.S. (1976)
Experimental myotonic myopathy. Neurology, 26: 384.
DEBEER, J.O., VAN PETEGHEM, C.H., HEYNDRICKX, A.M., & VAN ZELE, W.
(1981) Mass fragmentographic determination of 2,4-D and 2,4,DP
trace levels in biosamples using intramolecular deuterated interval
standards. Med. Fac. Landbouww. Rijksuniv. Gent, 46(1): 305-316.
DELARRARD, J. & BARBASTE, M. (1969) Intoxication suicidaire
mortelle agro-chimique de l'hormone désherbante 2,4-D. Arch. Mal.
prof. Med. Trav. Secur. soc., 30(7-8): 434.
DENNIS, C.A.R. (1976) Health effects of 2,4-D. In: National
Research Council of Canada Associate Committee on Scientific
Criteria for Environmental Quality, ed. Phenoxy herbicides: their
effects on environmental quality, Ottawa, NRCC Publications
Service, p.333.
DEREUCK, J., DECOSTER, W., WILLEMS, J., & VANDER EECKEN, H. (1979)
Influence of 2,4-dichlorophenoxyacetate and of dantrolene sodium on
the target phenomenon in tenotomized rat gastrocnemius muscle.
Acta neuropathol., 46: 167-168.
DESI, I. & SOS, J. (1962a) Central nervous injury by a chemical
herbicide. Acta med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1962b) [The effect of 2,4-dichloro-
phenoxyacetic acid and triorthocresyl phosphate on the function of
the upper central nervous system (bioelectric activity and
conditioned reflexes).] Gig. i Sanit., 12: 38-46 (in Russian,
Health & Welfare Canada Translation No. 2183).
DESI, I., SOS, J., & NIKOLITS, I. (1962a) New evidence concerning
the nervous site of action of a chemical herbicide causing
professional intoxication. Pathophysiologica (Budapest), 22:
73-80.
DESI, I., SOS, J., OLASZ, J., SULE, F., & MARKUS, V. (1962b)
Nervous system effects of a chemical herbicide. Arch. environ.
Health, 4: 95-102.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B., & GEORGE, J.L. (1962)
Effects on wildlife. pp. 4-52. In: Effects of pesticides on fish
and wildlife: A review of investigations during 1960. Washington,
DC, US Dept. of the Interior (Fish and Wildlife Circ. No. 143).
DRAPER, W.M. & STREET, J.C. (1982) Applicator exposure to 2,4-D,
dicamba, and a dicamba isomer. J. environ. Sci. Health, B17(4):
321-339.
DRILL, V.A. & HIRATZKA, T. (1953) Toxicity of 2,4-D and 2,4,5-T,
a report on their acute and chronic toxicity in dogs. Arch. ind.
Hyg. occup. Med., 7: 61-67.
DUDLEY, A.W. & THAPAR, N.T. (1972) Fatal human ingestion of
2,4-D, a common herbicide. Arch. Pathol., 94: 270-275.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States (III), June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E., LIPSCOMB, G.Q., COX, E.L., HEATWOLE, R.E., & KLING,
R.C. (1971) Pesticide residue levels in foods in the United
States from July 1, 1963 to June 30, 1969. Pestic. monit. J.,
5(2): 73-80.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some herbicides
on the hatching rate of hens' eggs. Nature (Lond.), 215:
1406-1407.
DUNACHIE, J.F. & FLETCHER, W.W. (1970) The toxicity of certain
herbicides to hens' eggs assessed by the egg injection technique.
Ann. app. Biol., 66: 515-520.
DURIC, V., STOJANOVIC, V., & IGIC, B. (1979) [Presentation of
patients with pesticide poisoning.] Med. Pregl., 32(3/4): 129-133.
(In Serbo-Croatian, Health & Welfare Canada Translation No. 2382.)
DUX, E., TOTH, I., DUX, L., & JOO, F. (1978) The localization of
calcium by X-ray microanalysis in myopathic muscle fibers.
Histochemistry, 56: 239-244.
DZHAPAROV, I.R. & TSILIKOV, V.V. (1969) [The effect of the sodium
salt of 2,4-D and carbothione on the content of glycogen in the
liver and the blood sugar level in rats.] Farmakol. Tsentr.
Kholinolitikov Drugikh Neirotropnykh Sredstv., p. 180 (in
Russian).
EASLEY, C.B., LAUGHLIN, J.M., GOLD, R.E., & TUPY, D.R. (1983)
Laundering procedures for removal of 2,4-dichloro-phenoxyacetic
acid ester and amine herbicides from contaminated fabrics. Arch.
Environ. Contam. Toxicol., 12: 71-76.
EBERSTEIN, A. & GOODGOLD, J. (1979) Experimental myotonia induced
in denervated muscles by 2,4-D. Muscle Nerve, 2: 364-368.
ELO, H. & YLITALO, P. (1977) Substantial increase in the levels
of chlorophenoxyacetic acids in the CNS of rats as a result of
severe intoxication. Acta pharmacol. toxicol., 41: 280-284.
ELO, H. & YLITALO, P. (1979) Distribution of 2-methyl-4-
chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid in male
rats: evidence for the involvement of the central nervous system
in their toxicity. Toxicol. appl. Pharmacol., 51: 439-446.
ELWOOD, J.M. & ROGERS, J.R. (1975) The incidence of congenital
abnormalities in British Columbia, Alberta, Manitoba and New
Brunswick, 1966-1969. Can. J. public Health, 66: 471-475.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERICKSON, L.C., BRANNAMAN, B.L., & COGGINS, C.W. Jr. (1963)
Residues in stored lemons treated with various formulations of
2,4-D. J. agric. food Chem., 11: 437-440.
ERIKSSON, M., HARDELL, M., BERG, N.O., MOLLER, T., & AXELSON, O.
(1981) Soft-tissue sarcomas and exposure to chemical substances: a
case-referent study. Br. J. ind. Med., 38: 27-33.
ERNE, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta vet. Scand., 7: 240-256.
ERNE, K. (1966b) Studies on the animal metabolism of
phenoxyacetic herbicides. Acta vet. Scand., 7: 264-271.
ERNE, K. (1974) Weed-killers and wildlife. In: Proceedings of
the XI International Congress on Game Biology, Stockholm, 1973.
pp. 415-422 (National Swedish Environment Protection Board
Publications No. 13E).
ERNE, K. (1975) Phenoxy herbicide residues in Swedish fish and
wildlife. In: Coulston, F., Korte, F., Klein, W., & Rosenblum,
I., ed. Pesticides: Environmental quality and safety, Stuttgart,
George Thieme Publishers, Suppl. Vol. III., pp. 192-195.
ERNE, K. (1980) [Further studies of phenoxy herbicide residues in
woodland berries and mushrooms, 1973-1979.] Vr Föda, 32(5):
285-292 (in Swedish).
ERNE, K. & RUTQVIST, L. (1979) [Pesticide residues in feedstuffs
in Sweden.] Nord. Veterinaermed., 31: 263-274 (in Swedish).
ERNE, K. & SPERBER, I. (1974) Renal tubular transfer of
phenoxyacetic acids in the chicken. Acta pharmacol. toxicol., 35:
233-241.
ERNE, K. & VON HAARTMAN, U. (1973) [Phenoxy acid residues in
forest berries and mushrooms.] Vr Föda, 25: 146-153 (in Swedish).
EVANS, W.C., SMITH, B.S.W., FERNLEY, H.M., & DAVIES, J.I. (1971)
Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem. J.,
122: 543-551.
EYZAGUIRRE, C., FOLK, B.P., ZIERLER, K.L., & LILIENTHAL, J.L. Jr.
(1948) Experimental myotonia and repetitive phenomena: The
veratrinic effects of 2,4-D acetate in the rat. Am. J. Physiol.,
155: 69-77.
FABACHER, D.L. & CHAMBERS, H. (1974) Resistance to herbicides in
insecticide-resistant mosquitofish, Gambusia affinis. Environ.
Lett., 7(1): 15-20.
FAO (1981) Pesticide residues in food: 1980 evaluations. Rome,
FAO, pp. 109-110 (Plant Production and Protection Paper 26 Suppl.)
FAO/WHO (1971) 2,4-D. In: 1970 Evaluations of some pesticide
residues in food, Rome, FAO/WHO, pp. 59-86 (FAO/AGP/1970/M/ 12/1,
WHO Food Additive Series, 71, 42).
FAO/WHO (1972) 1971 evaluations of some pesticide residues in
food: 2,4-D. WHO Pesticide Residues Series, 1: 83-97.
FAO/WHO (1973) Pesticide residues in food. Report of the 1972
Joint FAO/WHO meeting, Geneva, (WHO Techn. Rep. Series No. 525).
FARWELL, S.O., BOWES, F.W., & ADAMS, D.F. (1976a) Determination
of chlorophenoxy herbicides in air by gas chromatography/mass
spectrometry: selective ion monitoring. Anal. Chem., 48: 420-426.
FARWELL, S.O., ROBINSON, E., POWELL, W.J., & ADAMS, D.F. (1976b)
Survey of airborne 2,4-D in south central Washington. J. Air
Pollut. Control Assoc., 26: 224-230.
FAUST, S.D. & ALY, O.M. (1963) Some effects of 2,4-D and 2,4-DCP
on drinking water quality. Proc. Northeast. Weed Contr. Conf., 17:
460-470.
FAUST, S.D. & SUFFET, I.H. (1966) Recovery, separation, and
identification of organic pesticides from natural and potable
waters. Residue Rev., 15: 44-116.
FEDOROVA, L.M. & BELOVA, R.S. (1974) [Inclusion of 2,4-
dichlorophenoxyacetic acid in organs of animals: Paths and
dynamics of its excretion.] Gig. i Sanit., 39(2): 105-107
(in Russian, US NTC Translation No. 76-10918-05J).
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FETISOV, M.I. (1966) Occupational hygiene in the application of
herbicides of the 2,4-D group. Hyg. Sanit., 31(7-9): 383-386.
FEUNG, C.S., HAMILTON, R.H., & WITHAM, F.H. (1971) Metabolism of
2,4-dichlorophenoxyacetic acid by soybean cotyledon callus tissue
cultures. J. agric. food Chem., 19: 474-479.
FEUNG, C.S., HAMILTON, R.H., WITHAM, F.H., & MUMMA, R.O. (1972)
The relative amounts and identification of some 2,4-
dichlorophenoxyacetic acid metabolites isolated from soybean
cotyledon callus cultures. J. agric. food Chem., 50: 80-86.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1973b) Metabolism of
2,4-dichlorophenoxyacetic acid. V. Identification of metabolites
in soybean callus tissue cultures. J. agric. food Chem., 21:
637-640.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1975) Metabolism of
2,4-dichlorophenoxyacetic acid. VII. Comparison of metabolites
from five species of plant callus tissue cultures. J. agric. food
Chem., 23: 373-376.
FEUNG, C.S., LOERCH, S.L., HAMILTON, R.H., & MUMMA, R.O. (1978)
Comparative metabolic fate of 2,4-dichlorophenoxy acetic acid in
plants and plant tissue culture. J. Agric. Food Chem., 26:
1064-1067.
FIELD, B. & KERR, C. (1979) Herbicide use and incidence of
neural tube defects. Lancet, 1: 1341-1342.
FINNISH STATE INSTITUTE OF AGRICULTURAL CHEMISTRY (Helsinki)
(1963-1969) Investigations on pesticide residues. pp. 83.
FLEEKER, J.R. & STEEN, R. (1971) Hydroxylation of 2,4-D in
several weed species. Weed Sci., 19: 507-510.
FLORSHEIM, W.H. & VELCOFF, S.M. (1962) Some effects of
2,4-dichlorophenoxyacetic acid on thyroid function in the
rat: Effects on iodine accumulation. Endocrinology, 71(1):
1-6.
FLORSHEIM, W.H., VELCOFF, S.M., & WILLIAMS, A.D. (1963) Some
effects of 2,4-D on thyroid function in the rat: Effects on
peripheral thyroxine. Endocrinology, 72: 327-333.
FOISSAC-GEGOUX, P., LELIEVRE, A., BASIN, B., & WAROT, P. (1962)
Polynevrite aprčs usage d'un desherbant: l'acide 2,4-D. Lille Med.,
7(10): 1049-1051.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry
to nine herbicides. Bull. environ. Contam. Toxicol., 15(5):
509-514.
FOLMAR, L.C. (1979) Effects of short term field applications of
acrolein and 2,4-D (DMA) on flavor of the flesh of rainbow trout,
124 pp.
FOSTER, R.K. & McKERCHER, R.B. (1973) Extraction of 14C-labelled
chlorophenoxyacetic acids from soil. Can. J. soil Sci., 53:
135-136.
FRANK, P.A. (1972) Herbicidal residues in aquatic environments.
In: Faust, S.D., ed. Fate of organic pesticides in the aquatic
environment. Washington, DC, American Chemical Society,
pp. 135-148 (Advances in Chemistry Series, No. III).
FRANK, P.A. & COMES, R.D. (1967) Herbicidal residues in pond
water and hydrosoil. Weeds, 15(3): 210-213.
FRANK, P.A., DEMINT, R.J., & COMES, R.D. (1970) Herbicides in
irrigation water following canal-bank treatment for weed control.
Weed Sci., 18(6): 687-692.
FRANK, R. & SIRONS, G.J. (1980) Chlorophenoxy and chlorobenzoic
acid herbicides; their use in eleven agricultural waterbeds and
their loss to stream waters in southern Ontario, Canada, 1975-1977.
Sci. total Environ., 15: 149-167.
FRANK, R., SIRONS, G.J., & RIPLEY, B.D. (1979) Herbicide
contamination and decontamination of well waters in Ontario,
Canada, 1969-1978. Pestic. monit. J., 13(3): 120-127.
FRANK, R., SIRONS, G.J., CAMPBELL, R.A., & MEWETT, D. (1982)
Residues of 2,4-D, dichlorprop, and picloram in wild berries from
treated rights-of-way and conifer release sites in Ontario,
1979-81. Can. J. Plant Sci., 63: 196-209.
FRANKLIN, C.A., GROVER, R., MARKHAM, J.W., SMITH, A.E., & YOSHIDA,
K. (1982) Effect of various factors on exposure of workers
involved in the aerial application of herbicides. Am. Conf. of
Govern. Ind. Hygienists, Transactions, 43: 97-117.
FREEMON, F.R. (1975) Causes of polyneuropathy. Acta neurol.
Scand., 51(Suppl. 59): 5-43.
FYODOROVA, L.M., BELOVA, R.S., KIRYUHINA, N.N., VOLCHONOK, M.G., &
KOVTUNOVA, N.Y. (1977) [Residual content of chlorophenoxyacetic
acids in the environment and human body.] Gig. i Sanit., 6: 101-103
(in Russian).
GAMBLE, W. (1975) Mechanism of action of hypolipidemic and
herbicidal aryloxy acids. J. theor. Biol., 54: 181-190.
GAVRILOVA, L.I. (1965) Experimental substantiation of the
permissible concentration of 2,4-dichlorophenoxy-gamma-butyric (DB)
acid in water bodies. Hyg. Sanit., 29(1): 11-16.
GELDMACHER- VON MALLINCKRODT, M., & LAUTENBACH, L. (1966) [Two
fatal poisonings (suicides) with chlorinated phenoxyacetic acids
(2,4-D and MCPA).] Arch. Toxicol., 21: 261-278 (in German).
GLUCK, S.J. & MELCHER, R.G. (1980) Concentration and
determination of the propylene glycol butyl ether esters of
2,4-dichlorophenoxyacetic acid (PGBE 2,4-D) in air. Am. Hyg. Ass.
J., 41(12): 932-934.
GOLDSTEIN, N.P. & BROWN, J.R. (1960) Peripheral neuropathy.
J. Am. Med. Assoc., 173: 171.
GOLDSTEIN, N.P., JONES, P.H., & BROWN, J.R. (1959) Peripheral
neuropathy after exposure to an ester of dichlorophenoxyacetic
acid. J. Am. Med. Assoc., 171: 1306-1309.
GOLDWATER, L.J. (1960) Peripheral neuropathy. J. Am. Med.
Assoc., 173(1): 171.
GORSHKOV, A.I. (1972) Hygienic evaluation of chlorocholine
chloride (Preparation CCC) and its combination with 2,4-D amine
salt. Hyg. Sanit., 36(10-12): 208-211.
GOWEN, J.A., WIERSMA, G.B., & TAI, H. (1976) Mercury and 2,4-D
levels in wheat and soils from sixteen states, 1969. Pestic. Monit.
J., 10(3): 111-113.
GRAFF, G.L.A., GUENING, C., & VANDERKELEN, B. (1972) Influence de
l'acide 2,4-dichlorophénoxyacéticque (2,4-D), un agent myotonisant,
sur le métabolisme des phosphates acidosolubles des muscle
squelettiques du rat. C. R. Soc. Biol. (Paris), 166(II):
1565-1568.
GRANROTH, G., HAKAMA, M., & SAXEN, L. (1977) Defects of the
central nervous system in Finland: I. Variations in time and
space, sex distribution, and parental age. Br. J. prev. soc.
Med., 31: 164-170.
GRANROTH, G., HAAPAKOSKI, J., & SAXEN, L. (1978) Defects of the
central nervous system in Finland: V. Multivariate analysis of
risk indicators. Int. J. Epidemiol., 7(4): 301-308.
GRANT, W. (1976) 2,4-D. In: Hart, R.W., Kraybill, H.F., &
DeSerres, F.J., ed.: A rational evaluation of pesticidal vs
mutagenic/carcinogenic action. Bethesda, Maryland, National
Institute of Health (Publication No. (NIH) 78-1306 U.S. Department
of Health, Education & Welfare, Public Health Service).
GRAY, T.J.B., LAKE, B.G., BEAMAND, J.A., FOSTER, J.R., & GANGOLLI,
S.D. (1983) Peroxisome proliferation in primary cultures of rat
hepatocytes. Toxicol. appl. Pharmacol., 67: 15-25
GREEN, S. & MORELAND, F.S. (1975) Cytogenic evaluation of
several dioxins in the rat. Toxicol. appl. Pharmacol., 33: 161.
GRIBANOV, O.I. (1968) Pollution of an open water source at
Shortandy Railroad Station by the herbicide 2,4-D butyl ester.
Hyg. Sanit., 32: 267-268.
GRIGSBY, B.H. & FARWELL, E.D. (1950) Some effects of herbicides
on pasture and on grazing livestock. Mich. Agric. Exp. St. Bull.,
32(3): 378-385.
GROLLEAU, G., DE LAVAUR, E., SIOU, G., & FROUX, Y. (1974) Effects
of 2,4-D on the reproduction of quail and partridge after the
application of the product by spraying on eggs. Ann. Zool. Ecol.
anim., 6(2): 313-331.
GROVER, R. (1976) Relative volatilities of ester and amine forms
of 2,4-D. Weed Sci., 24: 26-28.
GROVER, R. & KERR, L.A. (1981) Evaluation of polyurethane foam as
a trapping medium for herbicide vapour in air monitoring and worker
inhalation studies. J. environ. Sci. Health, B 16(1): 59-66.
GROVER, R. & SMITH, A.E. (1974) Adsorption studies with the acid
and dimethylamine forms of 2,4-D and dicamba. Can. J. Soil Sci.,
54: 179-186.
GROVER, R., MAYBANK, J., & YOSHIDA, K. (1972) Droplet and vapour
drift from butyl ester and dimethylamine salt of 2,4-D. Weed Sci.,
20: 320-324.
GROVER, R., KERR, L.A., WALLACE, K., YOSHIDA, K., & MAYBANK, J.
(1976) Residues of 2,4-D in air samples from Saskatchewan
1966-1975. J. environ. Sci. Health, B 11(4): 331-347.
GRUNOW, W. & BOHME, C. (1974) [On the metabolism of 2,4,5-T and
2,4-D in rats and mice.] Arch. Toxicol., 32: 217-225 (in German).
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1971) Determination of
some acidic herbicides by thin layer chromatography. J. agric.
food Chem., 19: 1181-1182.
GUARINO, A.M. & ARNOLD, S.T. (1979) Xenobiotic transport
mechanisms and pharmacokinetics in the dogfish shark. In: Khan,
M.A.Q., Lech, J.J., & Menn, J.J., ed. Pesticide and xenobiotic
metabolism in aquatic organisms. Washington, DC, American Chemical
Society, pp. 250-258.
GUMMER, W.D. (1980) Pesticide monitoring in the prairies of
western Canada. Environ. Sci. Res., 16: 345-372.
GUNTHER, F.A. (1962) Instrumentation in pesticide residue
determination. In: Metcalf, R.L., ed. Advances in Pest Control
Research, 5, New York, Interscience Publishers, pp. 191-319.
GUSEVA, E.N. (1956) [On the pharmacology of 2,4-dichloro-
phenoxyacetic acid.] Farmakol. Toksikol. (Mosc.), 19(4):
41-44 (in Russian).
GUTENMANN, W.H. & LISK, D.J. (1965) Conversion of 4-(2,4-DB)
herbicide to 2,4-D by bluegills. NY Fish Game J., 12(1): 108-111.
GUTENMANN, W.H., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1963)
Disappearance of 4(2,4-dichlorophenoxy-butyric) acid herbicide in
the dairy cow. J. dairy Sci., 46: 991-992.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The effects of
phenoxy herbicides on the hatchability of eggs and the viability of
the chicks. Acta pharmacol. toxicol., 35: 300-308.
HACQUE, R., DEAGEN, J., & SCHMEDDING, D. (1975) Binding of
2,4-dichloro- and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. A proton magnetic resonance study. J. agric. food Chem.,
23: 763-766.
HALLIOP, J., TOCHMAN, A., & LATALSKI, M. (1980) [Ultrastructural
investigations and myeloperoxidase determinations in rat
neutrophils in acute poisoning with 2,4-dichlorophenoxyacetic
acid.] Acta haematol. Pol., 11(4): 249-257 (in Polish).
HALTER, M. (1980) 2,4-D in the aquatic environment In: Shearer,
R., & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlobenil, diquat & endothall, Seattle
Municipality of Metropolital Seattle, January 1980, pp. 89-182.
HANIFY, J.A., METCALF, P., NOBBS, C.L., & WORSLEY, K.J. (1980)
Congenital malformations in the newborn in Northland: 1966-1977.
New Zealand med. J., 92: 245-248.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquito fish, Gambusa
affinis. Bull. environ. Contam. Toxicol., 8(1): 46-51.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973) Avoidance of
pesticides by grass shrimp (Palaeomonetes pugio). Bull. environ.
Contam. Toxicol., 9(3): 129-133.
HANSEN, W.H., QUAIFE, M.L., HABERMANN, R.T., & FITZHUGH, O.G,
(1971) Chronic toxicity of 2,4-dichlorophenoxyacetic acid in rats
and dogs. Toxicol. appl. Pharmacol., 20: 122-129.
HARDELL, L. & SANDSTRÖM, A. (1979) Case-control study: soft
tissue sarcomas and exposure to phenoxyacetic acids or
chlorophenols. Br. J. Cancer, 39: 711.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 43: 169-176.
HARRISON, J.W.E. & REES, E.W. (1946) 2,4-D toxicity - I.
Toxicity towards certain species of fish. Am. J. Pharm., 118:
422-425.
HASS, J.R., FRIESEN, M.D., & HOFFMAN, M.K. (1981) Recent mass
spectrometric techniques for the analysis of environmental
contaminants. In: McKinney, J. D., ed. Environ. Health Chem.,
Ann Arbor, Arbor Sciences, pp. 219-243.
HATCH, T.F. & GROSS, P. (1964) Pulmonary desposition and
retention of inhaled aerosols. New York, London, Academic Press,
XIV + 192 pp (American Industrial Hygiene Association Monograph
Series on Industrial Hygiene).
HAYES, W.J. (1982) 2,4-D. In: Pesticides studied in man.
Baltimore & London, Williams & Wilkins.
HEENE, R. (1966) [Histochemical proof of phosphorylase inhibition
by 2,4-D in the skeletal muscles and liver of the rat.]
Naturwissenschaften, 53: 308 (in German).
HEENE, R. (1967) [Inhibition of glycogen-forming enzymes by
2,4-D.] Histochemie, 8: 45-53 (in German).
HEENE, R. (1968) Histochemical and morphological findings in
experimental 2,4-D myopathy in warmblooded animals. Acta
neuropathol., 10: 166-169.
HEENE, R. (1975) Experimental myopathies and muscular dystrophy.
Studies in the formal pathogenesis of the myopathy of 2,4-D
acetate. Schriftenr. Neurol. Ser., 16: 1-97.
HENSHAW, B., QUE HEE, S.S., SUTHERLAND, R.F., & LEE, C.D. (1975)
Gas-liquid chromatography and gas-liquid chromatography combined
with mass spectrometry of a butyl ester formulation of 2,4-
dichlorophenoxy-acetic acid. J. Chromatogr., 106: 33-39.
HERBICH, J. & MACHATA, G. (1963). [Poisoning with
2,4-dichlorophenoxy-acetic acid (2,4-D).] Beitr. gerichtl. Med.,
22: 133-139 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies to determine
the toxic effects of derivatives of 2,4-dichloro- and 2,4,5-
trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated eggs
of pheasants, quails, and chickens. First Report.] Anz.
Schaedling-sk. D. Pflanzenschutz Umweltschutz, 49: 21-25 (in
German).
HILBIG, V., LUCAS, K., SEBEK, V., & MUNCHOW, H. (1976b) [Studies
to determine the toxic effects of derivatives of 2,4-dichloro- and
2,4,5-trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated
eggs of pheasants, quails, and chickens. Second report.] Anz.
Schaedlingsk. D. Pflanzenschutz Umweltschutz, 49: 65-68 (in
German).
HILL, E.V. & CARLISLE, H. (1947) Toxicity of 2,4-D for
experimental animals. J. ind. Hyg. Toxicol., 29(2): 85-95.
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized
fish eggs and fry. Trans. Am. Fish. Soc., 96(4): 414-416.
HOFMANN, W.W., ALSTON, W., & ROWE, G. (1966) A study of
individual neuro-muscular junctions in myotonia.
Electroencephalogr. clin. Neuro-physiol., 21: 521-537.
HOGSTEDT, C. & WESTERLUND, B. (1980) Cohort studies of cause of
death of forest workers with and without exposure to phenoxy
preparations. Läkartidninger, 77(19): 1828-1831.
HOGSTEDT, B., KOLNIG, A.M., MITELMAN, R., & SKERFVING, S. (1980)
Cytogenetic study of pesticides in agricultural work. Hered.,
92: 177-178.
HOLCOMBE, G.W., FIANDT, J.T., & PHIPPS, G.L. (1980) Effects of pH
increases and sodium chloride additions on the acute toxicity of
2,4-dichlorophenol to the fathead minnow. Water Res., 14(8):
1073-1077.
HOLMBERG, P.C. (1979) Central nervous system defects in children
born to mothers exposed to organic solvents during pregnancy.
Lancet, 2: 177-179.
HOOPER, F.N. (1958) The effect of applications of pelleted 2,4-D
upon the bottom fauna of Kemt Lake, Oakland County, Michigan. Proc.
Ann. Meet. North Centr. Weed Control Conf., 41.
HORNER, J., QUE HEE, S.S., & SUTHERLAND, R.G. (1974)
Esterification of (2,4-dichlorophenoxy) acetic acid - a
quantitative comparison of esterification techniques Anal. Chem.,
46: 110-112.
HUCKINS, J.N., STALLING, D.C., & SMITH, W.A. (1978) Foam-charcoal
chromatography for the analysis of polychlorinated dibenzodioxins
in Herbicide Orange. J. Assoc. Anal. Chem., 61: 32-38.
HUFF, J.E., MOORE, J.A., SARACCI, R., & TOMATIS, L. (1980) Long
term hazards of polychlorinated dibenzodioxins and polychlorinated
dibenzofurans. Environ. Health Persp., 36: 221-240.
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to
bluegill sunfish of phenoxy herbicides. Weeds, 11(1): 50-53.
HUNT, L.M., GILBERT, B.N., & PALMER, J.S. (1970) Effects of a
herbicide, 2-ethylhexyl ester of 2,4-D on magnesium: calcium ratios
and blood urea nitrogen levels in sheep and cattle. Bull. environ.
Contam. Toxicol., 5(1): 54-60.
IARC (1977) 2,4-D and esters. In: Some fumigants, the herbicides
2,4-D and 2,4,5-T, chlorinated dibenzodioxins, and miscellaneous
industrial chemicals. Lyons, International Agency for Research on
Cancer, pp. 111-138 (IARC monograph on the evaluation of the
carcinogenic risk of chemicals to man Vol. 15).
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. Lyons, International Agency for
Research on Cancer, p. 101 (IARC Monographs of 1-29, Supplement 4).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer, Vol. 30.
ILO (1977) Safe use of pesticides, Geneva, International
Labour Office (Occupational Safety and Health Series, No. 38).
ILO (1979) Guide to health and hygiene in agricultural work,
Geneva, International Labour Organisation, pp. 309.
INGELOG, T., ERNE, K., PAULSSON, A.M., JONASSON, H., & HOLMGREN, A.
(1977) [Taste of flavour changes in woodland berries after
herbicide spraying.] Vr Föda, 29(6): 1-16 (in Swedish).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, L.H.,
& GART, J.J. (1969) Bioassay of pesticides and industrial
chemicals for tumorigenicity in mice: A preliminary note. J. Natl
Cancer Inst., 42: 1101-1114.
IYER, V., WHITING, M., & FENICHEL, G. (1976) Neural influence in
experimental myotonia. Neurology, 26: 384.
JACKSON, J.W. & THOMAS, T.C. (1978) A simple and inexpensive
method for sampling 2,4-D and 2,4,5-T herbicides in air. J. Air
Pollution Control Assoc., 28(11): 1145-1147.
JAMES, M.O. (1979) Dispositon of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Palinurus argus).
Pharmacologist, 21(3): 251.
JAMES, W.H. (1977) Clomiphene, acencephaly, and spina bifida.
Lancet, 1: 603.
JANERICH, D.T. (1973) Epidemic waves in the prevalence of
anencephally and spina bifida in New York State. Teratology, 8:
253-256.
JENSEN, D.J. & GLAS, R.D. (1981) Analysis for residues of acidic
herbicides. In: Moye, H.A., ed. Analysis of pesticide residues,
New York, Chichester, Brisbane, Toronto, John Wiley & Sons, pp.
223-261.
JENSSEN, D. & RENBERG, L. (1976) Distribution and cytogenetic
test of 2,4-D and 2,4,5-T phenoxyacetic acids in mouse blood
tissues. Chem. biol. Interactions, 14: 291-299.
JOHNSON, J.E. (1971) The public health implications of widespread
use of the phenoxy herbicides and picloram. Bioscience, 27(17):
899-905.
JOHNSON, E.R., YU, T.C., & MONTGOMERY, M.L. (1977) Trapping and
analysis of atmospheric residues of 2,4-D. Bull. environ. Contam.
Toxicol., 17: 369-372.
JOHNSON, R.D. & MANSKE, D.D. (1976) Pesticide residues in total
diet samples (IX) Pestic. Monit. J., 9(4): 157-168.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples - (I) - August 1974 - July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1981a)
Pesticide, heavy metal and other chemical residues in infant and
total diet samples - (II) - August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-53.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b)
Pesticide, heavy metal, and other chemical residues in adult total
diet samples (XII) - August 1975 - July 1976. Pestic. monit. J.,
15(1): 54-69.
JUNG, H.D. & WOLF, F. (1977) [Contact eczemas due to forestry use
of the herbicide SELEST100.] Dtsch. Gesundheit., 32(31): 1464-1467
(in German).
JUZWIAK, I., JUZWIAK, J., SUCHOWIAK, J., & ORDA, E. (1973) [A
study of the extent of the pesticide threat to the health of
agricultural employees in Olesnicki Country.] Pol. Tyg. Lek.,
28(11): 419-422 (in Polish, Health & Welfare Canada Translation
No. 2157).
KAISER, H. (1973) Guiding concepts relating to trace analysis.
Pure appl. Chem., 34: 35-61.
KARKINEN-JAASKELAINEN, M. & SAXEN, L. (1974) Maternal influenza,
drug consumption, and congential defects of the central nervous
system. Am. J. Obstet. Gynecol., 118(6): 815-818.
KASKEVICH, L.M. & SOBOLOVA, L. P. (1978) [A case of acute
poisoning with herbicide 2,4-D (late after effects).] Gig. Tr.
Prof. Zabol., 22 (10): 49-50 (in Russian, US National
Translations Center, Translation No. 79-11916-06T).
KAS'JANENKO, A. G. & KOROLEVA, N. S. (1979) [An evaluation of the
genetic danger of pesticides.] Izv. Akad. Nauk SSSR Ser. Biol.,
3: 401-409 (in Russian).
KATEMAN, G. & PIJPERS, F.W. (1981) Quality Control in analytical
chemistry, New York, John Wiley & Sons, 276 pp.
KATZ, M., LEGORE, R.S., WEITKAMP, D., CUMMINS, J., ANDERSON D., &
MAY, D.R. (1972) Effects on fresh water fish. J. Water Pollut.
Control Fed., 44(6): 122-1250.
KAY, J.H., PALAZZOLO, B.S., & CALANDRA, J.C. (1965) Subacute
dermal toxicity of 2,4-D. Arch. environ. Health, 11: 648-651.
KENIGSBERG, Y.E. (1968) [Model myotonia in rats induced by
means of the diethylamine salt of 2,4-dichlorophenoxyacetic acid.]
Dok. Akad. Nauk Beloruss. SSR, 12(5): 473-475 (in Russian, Health &
Welfare Canada Translation No. 2440).
KETCHERSID, M.L., FLETCHALL, O.H., SANTELMANN, P.W., & MERKLE, M.G.
(1970) Residues in sorghum treated with the isooctyl ester of
2,4-D. Pestic. monit. J., 4(3): 111-113.
KHALATKAR, A.S. & BHARGAVA, Y.R. (1982) 2,4-dichlorophenoxy-
acetic acid. A new environmental mutagen. Mutat. Res., 103:
111-114.
KHANNA, S. & FANG, S. (1966) Metabolism of C14-labelled
2,4-dichlorophenoxyacetic acid in rats. J. agric. Food Chem.,
14: 500-503.
KHANNA, R.N. & KOHLI, J.D. (1977) Metabolism of chlorophenoxy
herbicides in man. In: Zaidi, S. H., ed. Proceedings of the
International Symposium on Industrial Toxicology, Lucknow, India,
November 1975, Lucknow, Industrial Toxicology Research Centre,
pp. 590-599.
KHERA, K.S. & McKINLEY, W.P. (1972) Pre- and postnatal studies on
2,4,5-T, 2,4-D and their derivatives in rats. Toxicol. appl.
Pharmacol., 22: 14-28.
KHERA, K.S. & RUDDICK, J.A. (1973) Polychlorodibenzo- p-dioxins.
Perinateal effects and the dominant lethal test in Wistar rats. In:
Blair, E.H., ed. Chlorodioxins - origin and fate. Washington, DC,
American Chemical Society, pp. 70-84 (Advances in Chemistry Series
No. 120).
KHIBIN, L.S., TAITSEL', L.A., & SLATOV, I.V. (1968) [On the
clinical aspects of acute poisoning with Dicotex 40.] Gig. Tr.
Prof. Zabol., 3: 52-53 (in Russian, Health & Welfare Canada
Translation No. 2240).
KIMBROUGH, R.D., ed. (1980) Halogenated biphenyls, terphenyls,
naphthalenes, debenzodioxins and related products. Amsterdam,
New York, Oxford, Elsevier, North Holland Biomedical Press.
KING, J.E. & PENFOUND, W.T. (1946) Effects of two of the new
formagenic herbicides on bream and largemouth bass. Ecology,
27(4): 327-374.
KING, C.T.G., HORIGAN, E.A., & WILK, A.L. (1971) Screening of the
herbicides 2,4,5-T and 2,4-D for cleft palate. Teratology, 4(2):
233.
KLEKOWSKI, R.Z. & ZVIRGZDS, J. (1971) [The influence of herbicide
2,4-D Na on respiration and survival of Simocephalus vetulus O.F.
Muller (Cladocera).] Pol. Arch. Hydrobiol., 18(4): 393-400 (in
Polish).
KLINGMAN, D.L., GORDON, C.H., YIP, G., & BURCHFIELD, H.P. (1966)
Residues in the forage and milk from cows grazing forage treated
with esters of 2,4-D. Weeds, 14: 164-167.
KNUTSON, J.C. & POLAND, A. (1980) Keratinization of mouse
teratoma cell line xB produced by 2,3,7,8-tetrachlorodibenzo- p-
dioxin: an in vitro model of toxicity. Cell, 22: 27-36.
KOCIBA, R.J. & SCHWETZ, B.A. (1982) A review of the toxicity of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) with a comparison to the
toxicity of other chlorinated dioxin isomers. Assoc. Food Drug
Offic. Q. Bull., 46(3): 168-188.
KOHLI, J.D., KHANNA, R.N., GUPTA, B.N., DHAR, M.M., TANDON, J.S., &
SIRCAR, K.P. (1974) Absorption and excretion of 2,4-
dichlorophenoxyacetic acid in man. Xenobiotica, 4(2): 97-100.
KOLBERG, J., HELCELAND, K., & JONSEN, J. (1973) Binding of
2,4-dichloro and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. Acta pharmacol. toxicol., 33(5-6): 470-475.
KOLMODIN-HEDMAN, B., ERNE, K., HÜKANSSON, M., & ENGQUIST, A. (1979)
[Control of occupational exposure to phenoxyacids (2,4-D and
2,4-5-T).] Arbete och Hälsa, 17: 3-26 (in Swedish).
KOLMODIN-HEDMAN, B. & ERNE, K. (1980) Estimation of occupational
exposure to phenoxy acids (2,4-D and 2,4,5-T). Arch. Toxicol.,
(Suppl. 4): 318-321.
KOLMODIN-HEDMAN, B., ERNE, K., & AKERBLOM, M. (1980) Field
application of phenoxy acid herbicides. In: Tordoir, W.F. &
Van Heemstra, E.A.H., ed. Field workers exposure during pesticide
application. New York, Elsevier Scientific Publishing Co., pp.73-77
(Studies in Environmental Science, Vol. 7).
KONSTANTINOVA, T.K. (1970) Toxicology and some problems of the
embryotropic action of the butyl ester of 2,4-D. In: Vygodchikov,
G.V., ed. Proceedings of a Conference on Problems on Hygiene and
Toxicology of Pesticides, USSR, 1967, Moscow, Meditsina, pp.
177-179.
KONSTANTINOVA, T.K., EFIMENKO, L.P., & ANTONEKO, T.A. (1975)
[Embryotropic effects of the products from the decomposition of
herbicides based on 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
11: 102-105 (in Russian, US National Translation Centre,
Translation No. 78-14230-06F.)
KONTEK, M., MARCINKOWSKA, B., & PIETRASZEK, Z. (1973) [Electro-
encephalographic investigations in farm workers exposed to
derivatives of arylakanocarboxylic acids.] Pol. Tyg. Lek.,
28(25): 937-939 (in Polish, Health & Welfare Canada
Translation No. 2215).
KOPISCHKE, E.D. (1972) The effect of 2,4-D and diesel fuel on egg
hatchability. J. Wildl. Manage., 36(4): 1353-1356.
KORTE, C. & JALAL, S.M. (1982) 2,4-D-induced clastogenicity and
elevated rates of sister chromatid exchanges in cultural human
lymphocytes. J. Hered., 73: 224-226.
KOSCHIER, F.J., GIRAD, P., & HONG, S.K. (1978) Transport of
2,4-dichlorophenoxyacetate by rat renal cortical slides. Toxicol.
appl. Pharmacol., 45: 883-894.
KOSCHIER, F.J. & PRITCHARD, J.B. (1979) Excretion of
2,4-dichlorophenoxyacetate (2,4-D) by the dogfish shark (Squalus
acanthias). Pharmacologist, 21(3):184.
KOSYAN, S.A., MARKOSYAN, V.E., SARKISYAN, L.S., & MANUKYAN, R.A.
(1974) [A toxicological evaluation of the new herbicide 3-nitro-4-
oxybenzyl ether of (2,4-dichlorophenoxy) acetic acid (NK-2).]
Zh. eksp. klin. Med., 14(3): 14-18 (in Russian, Health & Welfare
Canada Translation No. 2436).
KRAMER, D. & SCHMALAND, G. (1974) [Plant protection agent
residues in water: Problems in water management as a consequence of
biocide applications.] Wasserwirtsch. Wassertech., 24(5): 161-167
(in German).
KUHN, E. & STEIN, W. (1964) [Experimental myotonia with 2,4-D in
the rat.] Klin. Wochenschr., 42: 1215-1216 (in German).
KUHN, E. & STEIN, W. (1965) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D) in the rat. In vitro and in vivo
studies on the effects of 2,4-D on the energy metabolism of
muscle.] Klin. Wochenschr., 43: 673-677 (in German).
KUHN, E. & STEIN, W. (1966) [Model myotonia with 2,4-
dichlorophenoxyacetate (2,4-D).] Klin. Wochenschr., 44: 700-702
(in German).
KUZMINSKAJA, U.A. & BERSAN, L.V. (1975) [The effects of the
sodium salt of dichlorophenoxyacetic acid on the glycolysis,
ATPase, and transketolase activity of erythrocytes.] Farmakol.
Toksikol., 38(1): 102-104 (in Russian).
KUZYK, A. (1979) A report on a survey of the Alberta farming
community respecting the use of pesticides, Edmonton, Alberta
Department of the Environment, Pollution Control Division.
LAPPE, M. (1979) HLA homozygosity and neural-tube defects.
Lancet, 1: 1342.
LASKOWSKI, M.B. & DETTBARN, W.D. (1977) The pharmacology of
experimental myopathies. Ann. Rev. Pharmacol. Toxicol., 17:
387-409.
LAVY, T.L. ROETH, F.W., & FENSTER, C.R. (1973) Degradation of
2,4-D and atrazine at three soil depths in the field. J. Environ.
Qual., 2(1): 132-137.
LAVY, T.L., WALSTAD, J.D., FLYNN, R.R., & MATTICE, J.D. (1982)
(2,4-dichlorophenoxy) acetic acid exposure received by aerial
applications crew during forest spray operations. J. agric. food
Chem., 30: 373-381.
LEE, B. (1978) Herbicides under suspicion in Australia. New
Sci., 80(1125): 159.
LENG, M. (1972) Residues in milk and meat and safety to livestock
from the use of phenoxy herbicides in pasture and rangeland. Down
Earth, 28(1): 12-20.
LENG, M. (1977) Comparative metabolism of phenoxy herbicides in
animals. In: Ivie, G.W. & Dorough, H.W., ed. Fate of pesticides in
large animals, New York, San Francisco, London, Academic Press,
pp. 53-76.
LENG, M.L. (1979) Comparative toxicology of various chlorinated
dioxins as related to chemical structure. In: Bontoyan, W.R., ed.
Collaborative International Pesticides Analytical Council
Proceedings Symposium Series I, Cambridge, Heffers Printers Ltd.,
pp. 141-169.
LENG, M.L., LAVY, R.L., RAMSEY, J.C., & BRAUN, W.H. (1982)
Review of the studies with 2,4,5-T in humans including applicators
under field conditions, pp. 133-156. In: Plimmer, J.R., ed.:
Pesticide residues and exposure. Washington, DC, American Chemical
Society, (ACS Symposium Series 182).
LHOSTE, J. & ROTH, P. (1946) [The effect of aqueous sodium
2,4,-dichlorophenoxyacetate on the development of eggs of Rana
temporaria L.] C.R. Soc. Biol. Paris, 140: 272-274.
LIBICH, S., TO, J.C., WANG, K. Y., & WATSON, D. A. (1981) A
study to assess the occupational exposure to 2,4-D herbicides,
Toronto, Safety Services Department, Health and Safety Division.
pp. 16 (Ontario Hydro. Report SSD-81-1).
LINDQUIST, N.G. & ULLBERG, S. (1971) Distribution of the
herbicides 2,4,5-T and 2,4-D in pregnant mice. Accumulation in the
yolk sac epithelium. Experientia (Basel), 27: 1439-1441.
LINNAINMAA, K. (1983a) Non-mutagenicity of phenoxyacid herbicides
2,4-D and MCPA. In: Keith, L.H., Choudhary, G., & Rappe, C., ed.:
Chlorinated dioxins and dibenzofurans in the total environment.
Ann Arbor, Ann Arbor Sci. Publ. Inc., Vol. 1.
LINNAINMAA, K. (1983b) Induction of sister chromatid exchanges by
the peroxisome proliferators 2,4-D, MCPA, and clofibrate in vivo
and in vitro. Carcinogenesis, accepted for publication.
LIPSCOMB, G.Q. (1968) Pesticide residues in prepared baby foods
in the United States. Pestic. monit. J., 2(32):104-108.
LISK, D.J., GUTENMANN, W.H., BACHE, C.A., WARNER, R.G., & WAGNER,
D.G. (1963) Elimination of 2,4-D in the urine of steers fed 4
(2,4-DB) or 2,4-D. J. dairy Sci., 46: 1434-1435.
LIU, L.C. & CIBES-VIADE, H.R. (1973) Adsorption of fluometuron,
prometryne, Sencor and 2,4-D by soils. Univ. Puerto Rico, J.
Agric., 57: 286-293.
LOKKE, H. (1975) Analysis of free and bound chlorophenoxy-acids
in cereals. Bull. environ. Contam. Toxicol., 13: 730-736.
LOKTIONOV, V.N., BUDARKOV, V.A., KUZNETSOV, A.E., & KUZNETSOVA,
N.V. (1973) [Toxicological characteristics of 2,4-D derivatives.]
Veterinariya, 5: 107-109 (in Russian, Health & Welfare Canada
Translation No. 2173).
LONDONO, F. (1966) [Occupational acne. Five cases caused by
herbicide.] Med. cutanea, 3: 225-232 (in Spanish).
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMANN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. J. Am. Ind. Hyg.
Assoc., 20(3): 298-304.
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel
Dekker, pp. 1-49.
LOOS, M.A. (1975) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Herbicides: Chemistry, degradation and mode of
action, New York, Marcel Dekker, pp. 1-128.
LOPEZ, M.R. (1961) [Action of 2,4-dichlorophenoxyacetic acid and
its sodium salt on the spermatozoa of the frog.] Biol. R. Soc.
Esp. Hist. Nat., 59: 219-226 (in Spanish).
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960a) Metabolism of plant
growth-regulators. I. 2,4-dichlorophenoxyacetic acid in leaves of
red- and blackcurrant. Ann. appl. Biol., 48: 613-625.
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960b) Metabolism of plant
growth-regulators. II. Decarboxylation of 2,4-dichloro-
phenoxyacetic acid in leaves of apple and strawberry. Ann. appl.
Biol., 48: 626-636.
LUKOSHKINA, L.P., GUESEINOVA, R.S., & MELIK-ZADE, T.M. (1970)
Study of certain aspects of lipid-protein-carbohydrate metabolism
in workers engaged in 2,4-D production. Trud. Azerbaid. Nauchno-
Issled. Instituta Gig. prof. Zabol., No.5: 146-149.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de l'herbicide
2,4-D sur le développement embryonaire et la fécondité du gibier ą
plumes. C.R. Acad. Sci. Paris, Ser. D., 271(25): 2418-2421.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1974) Sexualité et les pesticides.
Ann. Biol. anim. (Paris), 13(3-4): 173-185.
MAAS, W. & KERSSEN, M.C. (1973) Drift studies in ULV and
conventional applications. Agric. Aviation, 15: 41-50.
MACKENTHUN, K.M. & KEUP L.E. (1972) Freshwater macroinvertebrates.
J. Water Pollut. Control Fed., 44(6): 1137-1150.
MACLEAN, G.J. & DAVIDSON, J.H. (1970) Diagnosis of disorders in
pastured livestock. Down Earth, 25(4): 12-16.
MAGNUSSON, J., RAMEL, C., & ERIKSSON, A. (1977) Mutagenic effects
of chlorinated phenoxyacetic acids in Drosophila melanogaster.
Hereditas, 85: 121-123.
MAIER-BODE, H. (1971) [Herbicides and their residues.], Stuttgart,
FRG, Verlag Eugen Ulmer, 479 pp. (in German).
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VII). Pesticide Mont. J., 8(2): 110-124.
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VIII). Pesticide Mont. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples VII. Pestic. Monit. J., 9(2): 94-105.
MARTH, P.C. & MITCHELL, J.W. (1949) Comparative volatility of
various forms of 2,4-D. Bot. Gaz., 110: 632-636.
MARTYNOV, Y.E. (1970) The effect of 2,4-d compounds on wild warm
blooded animals. Lesn. Khoz., 6: 57-59 (in Russian, Health &
Welfare Canada Translation No. 2186).
MASON, R.W. (1975) Binding of some phenoxyalkanoic acids to
bovine serum albumin in vitro. Pharmacology, 13: 177-186.
MATSUNAKA, S. (1972) Metabolism of pesticides in higher plants.
In: Matsumura, F., Boush, G.M., & Mitsato, T., ed. Environmental
toxicology of pesticides, New York, Academic Press, pp. 341-365.
MAYBANK, J. & YOSHIDA, K. (1969) Delineation of herbicide-drift
hazards on the Canadian prairies. Trans. Am. Soc. Agric. Eng.,
12(6): 759-762.
MAYBANK, J., YOSHIDA, K., & GROVER, R. (1978) Spray drift from
agricultural pesticide applications. J. Air Poll. Control Assoc.,
28: 1009-1014.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979a) Changes of metabolism
during experimentally induced myotonia of rats. I. Alterations in
lactate and malate dehydrogenase isoenzyme activities. Biochem.
Med., 22: 350-358.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979b) Changes of metabolism
during experimentally induced myotonia of rats. II. Alterations in
creatinine kinase and acid phosphatase activities: a possible
mechanism in the development of muscle damage. Biochem. Med.,
22: 359-364.
MCARDLE, F.J., MARETZKI, A.N., WILEY, R.C., & MODREY, M.G. (1961)
Influence of herbicides on flavor of processed fruits and
vegetables. J. agric. food Chem., 9: 228-230.
MCCONNELL, E.E., MOORE, J.A., HASEMAN, J.K., & HARRIS, M.W. (1978)
The comparative toxicity of chlorinated dibenzo- p-dioxins in mice
and guniea pigs. Toxicol. appl. Pharmacol., 44: 335-356.
MCLENNAN, M.W. (1974) 2,4-D toxicity in diary cattle. Aust.
vet. J., 50: 578.
MEEHAN, W.R., NORRIS, L.R., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in Southeast Alaska.
J. Fish. Res. Board Can., 31(4): 480-485.
MELNIKOV, M.N. (1971) Chemistry of pesticides. Res. Rev.,
36: 165-166.
MIERZWA, S. & WITEK, S. (1977) Gas-liquid chromatographic
method with electroncapture detection for the determination of
residues of some phenoxyacetic acid herbicides in water as their
2,2,2-trichloroethyl esters. J. Chromatogr., 136: 105-111.
MILHAUD, G., RIET ALVARIZA, F., CHARLES, E., & SABBACH, M. (1970)
Toxicologie des phytohormones. Rec. Méd. Vet., 146: 345-357.
MITCHELL, J.W., HODGSON, R.E., & GAETJENS, C.F. (1946) Tolerance
of farm animals to feed containing 2,4-dichloro-phenoxyacetic acid.
J. anim. Sci., 5(1): 226-232.
MONARCA, G. & DIVITO, G. (1961) [On acute poisoning by a herbicide
(2,4-dichlorophenoxyacetic acid).] Folia med. (Naples), 44(6):
480-485 (in Italian, Health & Welfare Canada Translation No. 2234).
MONTGOMERY, M.L., CHANG, U.L., & FREED, V.H. (1971) Comparative
metabolism of 2,4-D by bean and corn plants. J. agric. food Chem.,
19: 1219-1221.
MOREALE, A. & VAN BLADEL, R. (1980) Behaviour of 2,4-D in Belgian
soils. J. environ. Qual., 9: 627-633.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
microbial reversion assay systems. Mutat. Res., 116: 185-216.
MORRE, D.J. & ROGERS, B.J. (1960) The fate of long chain esters
of 2,4-D in plants. Weeds, 8: 436-447.
MORTON, H.L., ROBINSON, E.D., & MEYER, R.T. (1967) Persistence of
2,4-D, 2,4,5-T, and dicamba in range forage grasses. Weeds,
15: 268-271.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxy-
ethane ester of 2,4-D. Trans. Am. Fish. Soc., 96(2): 185-193.
MUIR, C.S. & WAGNER, G. (1982) Directory of on-going research in
cancer epidemiology 1982, Lyons, International Agency for Research
on Cancer, (IARC Scientific publications No. 46) pp. 86 (Abstract
No. 227), 432(1146) 460(1221).
MUKULA, J., SILTANEN, H., ROSENBERG, C., IANPAAN-HEIKKILA, J., &
LALLUKKA, R. (1978) Phenoxy herbicide residues in cowberries,
mushrooms and the twigs of forest plants. Ann. Agric. Fenn.,
17: 23-31.
MUNRO, H.E. (1972) Determination of 2,4-dichloro-phenoxyacetic
acid and 2,4,5-trichlorophenoxyacetic acid in tomato plants and
other commercial crops by microcoulometric gas chromatography.
Pestic. Sci., 3: 371-377.
MURTHY, M.S.S. (1979) Induction of gene conversion in diploid
yeast by chemicals: Correlation with mutagenic action and its
relevance in genotoxicity screening. Mutat. Res., 64: 1-17.
NASH, R.G., KEARNEY, P.C., MAITLEN, J.C., SELL, C.R., & FERTIG,
S.N. (1982) Agricultural applicators' exposure to 2,4-
dichlorophenoxyacetic acid. In: Plimmer, J.R., ed. Pesticide
residues and exposure, Washington, DC, American Chemical Society,
pp. 119-132 (ACS Symposium Series 182).
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1981)
Polychlorinated dibenzo- p-dioxins: Criteria for their effects on
man and his environment, Ottawa, NRCC Publications Service, NRCC
Publication No. 18574, 251 pp.
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1978) Phenoxy
herbicides. Their effects on environmental quality, Ottawa, NRCC
Publications Service, NRCC publication no. 16075, 437 pp.
NAYRAC, P., FONTAN, P., WAROT, P., LESCUT, J., & DELAHOUSSE, J.
(1958) Polyradiculonévrite subaciguė chez deux agriculteurs:
intoxication par des produits anti-parasitaires. Lille Med.,
3(3): 161-164.
NESBITT, H.J. & WATSON, J.R. (1980a) Degradation of the
herbicide 2,4-D in river water. I. Description of the study
area, and survey of rate determining factors. Water Res.,
14(12): 1683-1688.
NESBITT, H.J. & WATSON, J.R. (1980b) Degradation of the
herbicide 2,4-D in river water. II. The role of suspended
sediment, nutrients, and water temperature. Water Res.,
14(12): 1689-1694.
NIELSEN, N.O. (1968) Teratogenic effects of hyperthermia in
chicken embryo. Preliminary report. Teratology, 1: 102-107.
NIELSEN, K., KAEMPE, B., & JENSEN-HOLM, J. (1965) Fatal
poisoning in man by 2,4-D: Determination of the agent in
forensic materials. Acta pharmacol. toxicol., 22: 224-234.
NIKANDROV, V.N. (1974). [The activity of malate dehydrogenase in
some organs and tissues of albino rats after administration of the
amine salt of 2,4-D,] Vesti Akad. Navuk Belaruskai SSR (Ser.
Biol.) 1: 126-127 (in Belorussian).
NISHIUCHI, Y. & YOSHIDA, K. (1974) [Toxicity of new agricultural
chemicals to tadpoles.] Bull. agric. Chem. Insp. St., 14: 66-68
(in Japanese).
NORSTROM, A., RAPPE, C., LINDAHL, R., & BUSTER, H.R. (1979)
Analysis of some older Scandinavian formulations of 2,4-D and
2,4,5-T for contents of chlorinated dibenzo- p-dioxins and
dibenzofurans. Scand. J. Work Environ. Health, 5: 375-378.
OLSON, B.A., SNEATH, T.C., & JAIN, N.C. (1978) Rapid, simple
procedures for the simultaneous gas chromatographic analysis
of four chlorophenoxy herbicides in water and soil samples.
J. agric. food Chem., 26: 640-643.
ORBERG, J. (1980a) Observations on the 2,4-dichlorophenoxy-
acetic acid (2,4-D) excretion in the goat. Acta pharmacol.
toxicol., 46: 78-80.
ORBERG, J. (1980b) Effects of low protein consumption on the
renal clearance of 2,4-dichlorophenoxyacetic acid (2,4-D) in goats.
Acta pharmacol. toxicol., 46: 138-140.
OSADCHUK, M., SALAHUB, E., & ROBINSON, P. (1977) Isolation of
chlorophenoxy herbicides on columns of glass beads. J. Assoc. Off.
Anal. Chem., 60: 1327-1327.
OSTERLOH, J., LOTTI, M., & POND, S.M. (1983) Toxicologic studies
in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. anal.
Toxicol., 7: 125-129.
OU, L.T., ROTHWELL, D.F., WHEELER, W.B., & DAVIDSON, J.M. (1978)
The effect of high 2,4-D concentrations on degradation and carbon
dioxide evolution in soils. J. environmental Qual., 7(2): 241-246.
PADEROVA, V.P. (1975) [Some hygienic problems of using 2,4-D
herbicides.] Gig. i Sanit., 40(9): 96-97 (in Russian).
PAGGIARO, P.L., MARTINO, E., & MARIOTTI, S. (1974) [On a case of
poisoning by 2,4-dichlorophenoxyacetic acid.] Med. lav., 65(3-4):
128-135 (in Italian, Health & Welfare Canada Translation No. 2200).
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens. In: Production Research Report, Washington,
DC, Agricultural Research Service, pp. 1-38.
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some
organic herbicides to cattle, sheep, and chickens. In: Production
Research Report, Washington, DC, Agriculture Research Service,
pp. 1-26.
PAL'MOVA, I.V. & GALUZOVA, L.V. (1963) [Maximum permissible
concentration of sodium salt and butyl ester formulations of
2,4-D in water.] Gig. i Sanit., 28(7): 11-13 (in Russian,
Health & Welfare Canada Translation No. 2717).
PEMBERTON, J.M. (1979) Pesticide degrading plasmids: a
biological answer to environmental pollution by phenoxyherbicides.
Ambio, 8: 202-205.
PHILLEO, W.W. & FANG, S.C. (1967) Effects of 2,4-D on metabolism.
J. agric. food Chem., 15(2): 256-260.
PIERIDES, A.M., ALVAREZ-UDE, F., KERR, D.N.S., & SKILLEN, A.W.
(1975) Clofibrate-induced muscle damage in patients with chronic
renal failure. Lancet, 2: 1279-1282.
PILINSKAJA, M.A. (1974) [Cytogenetic effect of the herbicide
2,4-D on human and animal chromosomes.] Tsitol. Genet., 8(3):
202-206 (in Russian).
POCCHIARI, F., SILANO, V., & ZAMPIER, A. (1979) Human health
effects from accidental release of tetrachlorodibenzo- p-dioxin
(TCDD) at Seveso, Italy. Ann. NY Acad. Sci., 320: 311-320.
PODOLAK, M. (1979) [Effect of paraquat and 2,4-dichloro-
phenoxyacetic acid (2,4-D) on oxidative phosphorylation and
respiration of brain mitochondria in the rat.] Bromatol. Chem.
Toksykol., 12(2): 147-152 (In Polish).
POLAND, A. & GLOVER, E. (1973) 2,3,7,8-Tetrachlorodibenzo- p-
dioxin: a potent inducer of S-aminolevulinic acid synthetase.
Sci., 179: 476.
POLAND, A. & GLOVER, E. (1973) Chlorinated dibenzo- p-dioxins:
potent inducers of -aminolevulinic acid synthetase and aryl
hydrocarbon hydroxylase. Molec. Pharmacol., 9: 736-747.
POLAND, A. GLOVER, E. (1976) Sterospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo -p-dioxin by hepatic cytosol.
J. Biol. Chem., 251(16): 4936-4946.
POLAND, A. & KENDE, A. (1976) 2,3,7,8-tetra-chlorodibenzo- p-
dioxin environmental contaminant and molecular probe. Fed. Proc.,
35: 2404-2411.
POLAND, A., SMITH, D., METTER, G., & POSSICK, P. (1971) A health
survey of workers in a 2,4-D and 2,4,5-T plant. Arch. environ.
Health, 22: 316-327.
PRAVDA, O. (1973) [On the influence of herbicides on some fresh-
water animals.] Hydrobiologia, 42(1): 97-142 (in German).
PREISS, D. & ROSSNER, J.A. (1971) [Effect of 2,4-
dichlorophenoxyacetic on the transverse tubular system of the
mycardium.] Naturwissenschaften, 11: 576-577 (in German).
PRESCOTT, I.F., PARK, J., & DARRIEN, I. (1979) Treatment of
severe 2,4-D and mecoprop intoxication with alkaline diuresis.
Br. J. clin. Pharmacol., 7: 111-116.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder.
J. Pharmacol. exp. Therap., 208(2): 280-286.
PRITCHARD, J.B. & MILLER, D.S. (1980) Teleost kidney in
evaluation of xenobiotic toxicity and elimination. Fed. Proc.,
39(14): 3207-3212.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis
in primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PROVINCE OF BRITISH COLUMBIA, MINISTRY OF ENVIRONMENT (1981)
A review of the use of 2,4-D for Eurasian water milfoil control in
the Okanagan Lakes, 1976-1980. Inf. Bull., aquatic Plant Manage.
Program, 10: 1-10.
QUE HEE, S.S. & SUTHERLAND, R.G. (1974) Purity of reagent grade
p- and o-chlorophenoxyacetic acids and its biological implications.
J. agric. food Chem., 22: 726-727.
QUE HEE, S.S. & SUTHERLAND, R.G. (1975) A specific gas-liquid
chromatographic method for analysis of some amine salts of 2,4-
dichlorophenoxyacetic acid. J. agric. food hem., 23: 1007-1008.
QUE HEE, S.S. & SUTHERLAND, R.G. (1981) The phenoxyalkanoic
herbicides, Vol. I. Chemistry, analysis and environmental
pollution, Boca Raton, CRC Press, Inc., 321 pp.
RADIONOV, A.D., CHUMACHENKO, A.N., & KIRILENKO, I.I. (1967)
The toxic properties of the herbicide 2,4-D. Hyg. Sanit.,
32(4-6): 116-118.
RAMEL, C. (1978) Chlorinated phenoxy acids and their dioxins.
Mode of action, health risks, and environmental effects. Ecol.
Bull. (Stockholm), 27: 302.
RAOUL, Y. & MARNAY, O. (1948) [Effect of 3-indolacetic acid
2,4-dichlorophenoxyacetic acid on the growing rat.] C. R. Acad.
Sci. (Paris), 226: 1043-1045.
RAPPE, C. & BUSER, H.R. (1981) Occupational exposure to
polychlorinated dioxins and dibenzofurans. In: Choudhary, G.,
Chemical hazards in the workplace: Measurement and control,
Washington, DC, American Chemical Society, pp. 319-342 (ACS
Symposium Series No. 149).
RASMUSON, B. & SVAHLIN, H. (1978) Mutagenicity tests of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid in genetically stable and unstable strains of Drosophila
melanogaster. Ecol. Bull. (Stockholm), 27: 190-192.
RAWLS, C.K. (1965) Field tests of herbicide toxicity to
certain estuarine animals. Chesapeake Sci., 6(3): 150-161.
REA, W.J. (1978) Environmentally triggered cardiac disease.
Ann. Allergy, 40(4): 243-251.
REDDY, J.K., AZARNOFF, D.L., & HIGNITE, E.E. (1980)
Hypolipidaemic hepatic peroxisome proliferators form a novel
class of chemical carcinogens. Nature (Lond.), 283: 397-398.
REDDY, J.K., QURESHI, S.A., LALWANI, N.D., REDDY, M.K., & MOEHLE,
C.M. (1982a) Induction by ciprofibrate of hepatic peroxisome
proliferation in rats, pigeons, chickens, cats and rhesus monkeys.
Fed. Proc., 41: 1741.
REDDY, J.K., LALWANI, N.D., REDDY, M.K., & QURESHI, S.A. (1982b)
Excessive accumulation of autofluorescent lipofuscin in the liver
during hepatocarcinogenesis by methyl clofenapate and other
hypolipidaemic peroxisome proliferators. Cancer Res., 42: 259-266.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977) Investigations
into the acute toxicity and some chronic effects of selected
herbicides and pesticides on several fresh water fish species.
Bull. environ. Contam. Toxicol., 18(3): 361-365.
RENBERG, L. (1974) Ion exchange technique for the determination
of chlorinated phenols and phenoxy acids in organic tissue, soil
and water. Anal. Chem., 46: 459-461.
RIIHIMAKI, V., ASP, S., & HERNBERG, S. (1982) Mortality of
chlorinated phenoxyacid herbicide 2,4-D and 2,4,5-T applicators in
Finland. First report of an ongoing prospective follow-up study.
Scand. J. Work Environ. Health, 8: 37-42.
RIVERS, J.B., YAUGER, W.L., Jr., & KLEMMER, H.W. (1970)
Simultaneous gas chromatographic determination of 2,4-D and
dicamba in human blood and urine. J. Chromatogr., 50: 334-337.
ROBINSON, E. & FOX, L.L. (1978) 2,4-D herbicides in central
Washington. J. Air Poll. Control Assoc., 28: 1015-1020.
ROBSON, T.O. (1966) Some studies of the persistence of 2,4-D in
natural surface waters in Britain. Proc. Br. Weed Control Conf.,
2: 594-597.
ROSENBERG, J. (1980) 2,4-Dichlorophenoxyacetic acid (2,4-D)
- Evaluation of human health hazards, Berkeley, California,
Hazard Alert System, Epidemiological Studies Laboratory, 64 pp.
ROSS, R.D. MORRISON, J., ROUNBEHLER, D.P., FAN, S., & FINE, D.H.
(1977) N-nitroso compound impurities in herbicide formulations.
J. agric. food Chem., 25: 1416-1418.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation
of the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUDIGER, K.D., MEERBACH, W., OSSKE, G., & FISCHER, W. (1972) [On
the experimental 2,4-dichlorophenoxyacetate myopathy.] Zentralbl.
allg. Pathol., 115: 145-152 (in German).
SADYKOV, R.E., RABOCHEV, V.K., & STROKOV, Y.N. (1972) [The
effect of butyl 2,4-D treatment of pastures on the reproductive
functions of sheep.] Zhivodnovodstvo, 34(1): 73-74 (in Russian).
SANDERS, H.O. (1970a) Pesticide toxicities to tadpoles of the
western chorus frog, Pseudacris triseriata and Fowler's toad, Burfo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. (1970b) Toxicities of some herbicides to six
species of freshwater crustaceans. J. Water Pollut. Control Fed.,
42(8): 1554-1550.
SARE, W.M. (1972) The weedicide 2,4-D as a cause of headaches and
diplopia. New Z. med. J., 75(478): 173-174.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & LEBEAU, J.E. (1976)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicol. appl. Pharmacol., 37(1): 136-137.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & GEHRING, P.J. (1977)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicology, 8: 3-11.
SAXEN, L., KLEMETTI, A., & HARO, A.S. (1974) A matched-pair
register for studies of selected congenital defects. Am. J.
Epidemiol., 100(3): 297-306.
SCHACTER, B., GYVES, M., MUIR, A., & TASIN, M. (1979) HLA-A, B
compatibility in parents of offspring with neural-tube defects or
couples experiencing involuntary fetal wastage. Lancet, 1: 796-797.
SCHILLER, K. (1964) [Effects on the fertility of rats produced by
substances with special effects in potato cultivation. 1st
communication: Growth hormones and weed control agents 2,4-D and
MCPA.] Landbauforsch. Völkenrode, 14(2): 111-114 (in German).
SCHILLINGER, J.E. (1960) [Hygienic evaluation of agricultural
products cultivated with the use of herbicides.] J. Hyg.
Epidemiol. Microbiol. Immunol., 4: 243-252 (in German).
SCHNEIDER, B.A. (1979) Toxicological evaluation of pesticides by
the EPA Chemical and Biological Investigations Branch Laboratories
(1966-77). In: Toxicology handbook, mammalian and aquatic data.
Book 1: Toxicology data. Report for 1966-67, Beltsville, Md., US,
Environmental Protection Agency.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8(3): 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues at
Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7(3/4):
146-152.
SCHUPHAN, W. (1963) [Impaired health due to plant protection
agents.] Der Landarzt, 39: 1217-1222 (in German).
SCHUPHAN, W. (1965) [Plant protection problems reflected in
quality testing.] Anz. Schädlingsk. D. Pflanzenschutz.
Umweltschutz, 38(7): 97-104 (in German).
SCHUPHAN, W. (1969) [Pesticides: Usefulness and possible harm.]
Zentralbl. Bakteriol., 210: 240-258 (in German).
SCHWETZ, B.A., SPARSCHU, G.L., & GEHRING, P.J. (1971) The effect
of 2,4-D and esters of 2,4-D on rat embryonal, foetal and neonatal
growth and development. Food cosmet. Toxicol., 9: 801-817.
SCHWETZ, B.A., NORRIS, J.M., SPARSHU, G.L., ROWE, V.K., GEHRING,
P.J., EMERSON, J.L., & GERBIG, C.G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect., Exp.
Issue 5: 87-99.
SEABURY, J.H. (1963) Toxicity of 2,4-dichlorophenoxyacetic acid
for man and dog. Arch. environ. Health, 7: 202-209.
SEILER, D. (1971) The ATPase of the sarcolemma of skeletal
muscle in experimental myotonia. Experientia (Basel), 27:
1170-1171.
SEILER, J.P. (1978) The genetic toxicology of phenoxy acids
other than 2,4,5-T. Mutat. Res., 55: 197-226.
SENCZUK, W. & POGORZELSKA, H. (1975) [Urinary excretion of
2,4-dichlorophenoxyacetic acid.] Rocz. Panstw. Zakl. Hig.,
26(2): 217-222 (in Polish, Health & Welfare Canada Translation
No. 2451).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxodynamic properties of phenoxycarboxylic acid derivatives, II.
Methods of determining derivatives of phenoxyacetic and
phenoxypropionic acids in blood and urine.] Rocz. Panstw. Zakl.
Hig., 32: 339-344 (in Polish).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxicodynamic properties of derivatives of phenoxyacetic and
phenoxypropionic acid, III. The pattern of absorption into the
bloodstream and measurements of urinary excretion of phenoxyacetic
and phenoxypropionic acid.] Roczn. Panstw. Zak. Hig., 32(5-6):
419-426 (in Polish).
SENCZUK, W., POGORZELSKA, H., & DEBSKA, M. (1980) [Absorption of
2,4-dichlorophenoxyacetic acid through the skin.] Roczn. Panstw.
Zak. Hig., 31(6): 611-614 (in Polish).
SENGES, J. & RDEL, R. (1972) Experimental myotonia in mammalian
skeletal muscle: changes in contractile properties. Pflueger.
Arch., 331: 315-323.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method for
determination of low levels of exposure to 2,4-D and 2,4,5-T.
Int. J. environ. anal. Chem., 1: 23-33.
SHEARER, R.W. (1980) Public health effects of the aquatic use of
herbicides: 2,4-D dichlobenil, endothall and diquat. In: Shearer,
R.W. & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlorbenil, diquat & endothall, Municipality of
Washington, US, Metropolitan Seattle, pp. 1-76.
SHEARER, R. & HALTER, M. (1980) Literature reviews of four
selected herbicides: 2,4-D, dichlobenil, diquat, and endothall.
Municipality of Washington, US, Metropolitan Seattle.
SHILLINGER, U.Y.I. & NAUMOVA, L.P. (1957) [Hygienic evaluation of
a harvest in connection with the treatment of crops with the
herbicide 2,4-D.] Gig. i Sanit., 7: 33-38 (in Russian).
SHIM, J.C. & SELF, L.S. (1973). Toxicity of agricultural
chemicals to larvivorous fish in Korean rice fields. Trop. Med.,
15(3): 123-130.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., & RAATIKAINEN, M.
(1981). Triclopyr, glyphosate and phenoxyherbicide residues in
cowberries, bilberries and lichen. Bull. Environ. Contam Toxicol.,
27: 731-737.
SIMPSON, G.R. (1982a) Pesticide exposure - New South Wales
Services. In: Education and safe handling in pesticide application,
studies in environmental science, 18: 209-214. Amsterdam,
Oxford, New York, Elsevier Scientific Publishing Co.
SITTIG, M. ed., (1980) Pesticide manufacturing and toxic
materials control encyclopedia. Park Ridge, NJ, USA, Noyes Data
Corp., pp. 229-234.
SKELLY, N.E., STEVENS, T.S., & MAPES, D.A. (1977) Isomer-specific
assay of ester and salt formulations of 2,4-dichlorophenoxyacetic
acid by automated high pressure liquid chromatography. J. Assoc.
Off. Anal. Chem., 60: 868-872.
SMALS, A.G.H., BEEX, L.A.V.M., & KLOPPENBORG, P.W.C. (1977)
Clofibrate-induced muscle damage with myoglobinuria and
cardiomyopathy. N. Engl. J. Med., 296: 942.
SMITH, A.E. (1972) The hydrolysis of 2,4-dichlorophenoxy-acetate
esters to 2,4-dichlorophenoxyacetic acid in Saskatchewan soils.
Weed Res., 12: 364-372.
SMITH, A.E. (1976a) The hydrolysis of herbicidal phenoxy-alkanoic
esters to phenoxyalkanoic acids in Saskatchewan soils. Weed Res.,
16: 19-22.
SMITH, A.E. (1976b) Method for the determination of
2,4-dichlorophenoxyacetic acid in urine. J. Chromatogr, 171:
482-485.
SMITH, A.H., FISHER, D.O., PEARCE, N., & CHAPMAN, C.J. (1982)
Congenital defects and miscarriages among New Zealand 2,4,5-T
sprayers. Arch. environ. Health, 37(4): 197-200.
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of
large-scale applications of 2,4-D on aquatic fauna and water
quality. Pestic. monitoring J., 1(3): 16-21.
SOMERS, J., MORAN, E.T., REINHART, B.S., & STEPHENSON, G.R. (1974a)
Effect of external application of pesticides to the fertile egg on
hatching success and early chick performance. 1. Pre-incubation
spraying with DDT and commercial mixtures of 2,4-D: picloram, and
2,4-D: 2,4,5-T. Bull. environ. Contam. Toxicol., 11(1) 33-38.
SOMERS, J., MORAN, E.T., & REINHART, B.S. (1974b) Effect of
external application of pesticides to the fertile egg on
hatching success and early chick performance. 2. Commercial
herbicide mixtures of 2,4-D with pioloram or 2,4,5-T using the
pheasant. Bull. environ. Contam. Toxicol., 11(4): 339-342.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974c) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11(4): 511-516.
SOS, J. & KERTAI, P. (1958) Effect of dichlorophenoxyacetic acid
upon the I131-uptake of the thyroid. Acta physiol. Acad. Sci.
Hung., 14: 367-369.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on
the embryonic development of Phasianus colchicus (L.) and Coturni
coturnix japonica (L.).] Z. Jagdwiss., 22: 197-201 (in German).
STANLEY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANOSZ, S. (1969) [The effects of sodium salt of 2,4-
dichlorophenoxy-acetic acid on cell respiration in the parenchymal
organs of rats.] Med. Prac., 20(6): 600-605 (in Polish).
STEIN, W. & KUHN, E. (1968) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D). Isolated rat diaphragm preparation
as simple experimental object.] Klin. Wochenschr., 46: 328-330
(in German).
STEVENS, L. J., COLLIER, C. W., & WOODHAM, D. W. (1970)
Monitoring pesticides in soils from areas of regular, limited, and
no pesticide use. Pestic. Monit. J., 4(3): 145-164.
STEVENS, T.S.A., SKELLY, N.E., & GRORUD, R.B. (1978) Isomer-
specific assay of ester and salt formulations of 2,4-
dichlorophenoxyacetic acid by high pressure liquid chromatography:
Collaborative study. J. Assoc. Off. Anal. Chem., 61: 1163-1165.
STEWART, D.K.R. & GAUL, S.O. (1977) Persistence of 2,4-D,
2,4,5-T, and dicamba in a dykeland soil. Bull. environ. Contam.
Toxicol., 18(2): 210-218.
STRACH, S. & BOHOSIEWICZ, M. (1964) [Studies of the toxicity for
pigs of the weed-killer "Pielik."] Med. weter, 20(11): 662-666
(in Polish, Health & Welfare Canada Translation No. 2467).
STUPNIKOV, A.A. (1959) In: [Collected works of the Leningrad
Veterinary Sciences Research Institute.] No. 8: 289 (in Russian).
SUDAK, F.N. & CLAFF, C.L. (1960) Survival of Uca pugnax in sand,
water, and vegetation contaminated with 2,4-dichloro-phenoxyacetic
acid. Proc. Northeast. Weed Control Conf., 14: 508-510.
SUDAK, F.N., CLAFF, C.L., & CANTOR, M. (1966) Body temperature
regulation in rats treated with 2,4-dichloro-phenoxyacetic acid.
Arch. int. Pharmacodyn. Ther., 160(2): 253-264.
SUFFET, I.H. (1973a) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 2.
Selection of water: solvent ratios and number of extractions.
J. agric. food Chem., 21: 288-294.
SUFFET, I.H. (1973b) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 3.
Liquid-liquid extraction of phenoxy acid herbicides from water.
J. agric. food Chem., 21: 591-598.
SUNDSTROM, G., JENSEN, S., JANSSON, B., & ERNE, K. (1979)
Chlorinated phenoxyacetic acid derivatives and tetrachlorodibenzo- p-
dioxin in foliage after application of 2,4,5-trichlorophenoxyacetic
acid esters. Arch. environ. Contam. Toxicol., 8: 441-448.
SZOCS, J., MOLNAR, V., BALOGH, E., AJTAY, M., & FULOP, I. (1970)
[Experimental data on the toxic effects of 2,4-D (Diclordon)
herbicide.] Rev. Med. (Tirgu-Mures, Rom.), 16: 91-93 (in
Hungarian).
THIELE, E., BAUFELD, W., GRIMM, U., LEHNIGK, B., & THIELE, W.
(1981a) [Results of analysis for deriving exposure time limits in
the industrial hygiene monitoring of people working at agrochemical
centres who are exposed to pesticides.] Z. gesamte Hyg. Grenzgeb.,
27(3): 173-177 (in German).
THIELE, E., FIEBER, C., JASTROCH, S., KNOLL, W., & LANGE, B.
(1981b) [Determination of toxic concentrations at places of work
and patterns of distribution of pesticide exposure of people
working at agrochemical centres subdivided by substance groups.]
Z. gesamte Hyg. Grenzgeb., 27(3): 177-180 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & LINK, R. (1982) Mortality
study of persons exposed to dioxin in a trichlorophenol-process
accident that occurred in the BASF AG on November 17, 1953.
Am. J.ind. Med., 3: 179-189.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964a) Metabolic
fate of some chlorinated phenoxyacetic acids in the stem tissue of
Avena sativa. Nature (Lond.), 204: 286.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964b) Metabolic
fate of 2,4-dichlorophenoxyacetic acid in the stem tissue of
Phaseolus vulgaris. Nature (Lond.), 204: 884-885.
THOMSSEN, C. (1958) [Contribution to the question of possible
poisoning of domestic animals through growth hormone-based weed
control agents.] Arch. exp. Veterinaermed., 12: 216-221 (in
German).
TODD, R.L. (1962) A case of 2,4-D intoxication. J. Iowa Med.
Soc., 52: 663-664.
TSAPKO, V.P. (1966) The herbicide 2,4-D as a health hazard in
agriculture. Hyg. Sanit., 9: 449-450.
TSILIKOV, V.V. (1969) [Effect of the sodium salt of 2,4-D,
trichlorometaphos, nicotine, and arecoline on thyroid function.]
In: Denisenko, P.P., ed. Farmakol. Tsent. Kholinolitikov Drugikh
Neirootropnykh Sredstv., pp. 181-185 (in Russian, Health & Welfare
Canada Translation No. 2447).
TUINSTRA, L.G.M., ROOS, A.H., & BRONSGEEST, J.M. (1976)
Suggestions for a sensitive multi-detection high-performance
liquid chromatography method for several phenoxy-acid herbicides.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 41: 1443-1448.
TULP, M.T.M. & HUTZINGER, O. (1978) Rat metabolism of
polychlorinated dibenzo- p-dioxins. Chemosphere, 7(9): 761-768.
UNGER, T.M., KLIETHERMES, J., VAN GOEHAM, D., & SHORT, R.D.
(1980) Teratology and postnatal studies in rats on propylene
glycol butyl ether and isooctyl esters of 2,4-dichloro-
phenoxyacetic acid, Washington, US Environmental Protection
Agency (60/12-80).
US EPA (1976) National interim primary drinking water
regulations, Washington, DC, US Environmental Protection Agency.
US EPA (1980a) Environmental Protection Agency Review and
Conclusions Concerning Potential Health Effects of the Herbicide
2,4-D, April 1980, Chem. Regul. Rep., 4(5): 134-136.
US EPA (1982) Tetrachlorodibenzo- p-dioxin; re-evaluation of
disposal prohibition. Fed. Reg., 47(2): 103.
US NATIONAL CANCER INSTITUTE (1979) Bioassay of 2,7-
dichlorodibenzo-p-dioxin (DCDD) for possible carcinogenicity.
Springfield, Virginia, National Technical Information Service
Document PB 290570 (Report No. NCI-CG-TR 123).
US VETERANS' ADMINISTRATION. DEPARTMENT OF MEDICINE AND SURGERY
(1981) Review of literature on herbicide, including phenoxy
herbicides and associated dioxins. Volume I. Analysis of
literature. Volume II. Annotated bibliography, Washington, DC,
US Veterans' Administration, 397 pp.
VACHKOVA-PETROVA, R. (1978) [Mutagenic activity of pesticides.]
Hig. Zdraveopaz., 21(5): 496-506 (in Bulgarian).
VAINIO, H., NICKELS, J., & LINNAINMAA, K. (1982) Phenoxy acid
herbicides cause peroxisome proliferation in Chinese hamsters.
Scand. J. Work. Environ. Health, 8: 70-73.
VAINIO, H., LINNAINMAA, K., KAHONEN, M., NICKELS, J., HIETANEN, E.,
MARNIEMI, J., & PELTONEN, P. (1983) Hypolipidemia and peroxisome
proliferation induced by phenoxyacetic acid herbicides in rats.
Biochem. Pharmacol., (in press).
VAN DYK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air:
sampling methods. Res. Rev., 55: 91-134.
VAN PETECHEM, C.R. & HEYNDRICKX, A.M. (1975) Beta oxidation of
chlorophenoxybutyric acid herbicides in guinea pigs. Bull.
environ. Contam. Toxicol., 14(5): 632-640.
VARDIA, H.K. & DURVE, V.S. (1981) Bioassay study on some fresh
water fishes exposed to 2,4-dichlorophenoxyacetic acid. Acta
hydrochim. hydrobiol., 9(2): 219-223.
VENKAIAH, B. & PATWARDHAN, M.V. (1978) Inhibition of
mitochondrial energy transfer reactions by phenoxy acids. Indian
J. Biochem. Biophys., 15(3): 219-221.
VINOKUROVA, M.K. (1960) [On the toxicity of a herbicide: The
octyl ester of 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
12: 31-34 (in Russian, Health & Welfare Canada Translation No.
2324).
VOGEL, E. & CHANDLER, J.L.R. (1974) Mutagenicity testing of
cyclamate and some pesticides in Drosophila melanogaster.
Experientia (Basel), 30(6): 621-623.
WALLIS, W.E., VAN POZNAK, A., & PLUM, F. (1970) Generalized
muscular stiffness, fasciculations, and myokymia of peripheral
nerve origin. Arch. Neurol., 22: 430-439.
WATERS, M.D., MITCHELL, A.D., JORGENSON, T.A., & VALENCIA, R.
(1981) Pesticides: Mutagenic and carcinogenic potential.
In: Bandal, S.K., Marco, G.J., Golberg, L., & Leng, M.L.,
ed., The pesticide chemist and modern toxicology. Washington,
DC, American Chemical Society (ACS Symposium Series 160).
WAY, J.M. (1969) Toxicity of hazards to man, domestic
animals and wildlife from some commonly used auxin herbicides.
Res. Rev., 26: 37-62.
WEINMANN, W. (1957) [Investigations of the influence of a
treatment of tomato plants with growth substances on the
nutritional and physiological value of the fruits. Offspring
mortality in albino rats.] Qual. Plant. Mater. Veg., 2(4):
342-349 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972) The subacute
toxicity of 2,4-dichlorophenoxyacetic acid and 2,4,5-
trichlorophenoxyacetic acid to chicks. Toxicol. appl.
Pharmacol., 21:348.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., & GANGSTAD, E.O.
(1973) The effects of a 2,4-D application on the biota and water
quality in Currituck Sound, North Carolina. Hyacinth Control J.,
11: 13-17.
WHO (1976) Pesticide residues in food. Report of the 1975
Joint FAO/WHO meeting (WHO Tech. Report Series, 592).
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residue
Series No. 1).
WHO/FAO (1976) 1975 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residues
Series No. 5).
WHO/IARC (1980) Walker, E.A., Castegnaro, M., Griciute, L.,
Börzsönyi, M., & Davis, W., ed.: N-Nitroso compounds: Analysis,
formation and occurrence. Lyons, International Agency for
Research on Cancer, 841 pp (IARC Scientific Publications No. 31).
WHO/IARC (1982) Bartsch, H., Castegnaro, M., O'Neill, I.K.,
Okada, M., & Davis, W., ed.: N-Nitroso compounds: Occurrence
and biological effects. Lyons, International Agency for Research
on Cancer, 755 pp (IARC Scientific Publications No. 41).
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972) Pesticide residue
levels in soils, F.Y. 1969 - National soils monitoring program.
Pestic. monit. J., 6(3): 194-228.
WIERZBICKA, M. (1974a) Haemolymph concentration in Cyclopoida
copepodids during active and resting stage and the effect of
2,4-D sodium salt. Pol. Arch. Hydrobiol., 21(2): 269-273.
WIERZBICKA, M. (1974b) Influence of 2,4-D sodium salt on the
survival of some Copepodia species. Pol. Arch. Hydrobiol.,
21(2): 275-282.
WINKELMANN, R.K. (1960) Diagnosis and treatment of allergic
angiitis (anaphylactoid purpura). Postgrad. Med., 27: 437-444.
WOJTALIK, T.A., HALL, T.F., & HILL, L.O. (1971). Monitoring
ecological conditions associated with wide-scale applications
of DMA-2,4-D to aquatic environmental. Pestic. monit. J.,
4(4): 184-203.
WOODHAM, D.W., MITCHELL, W.G., LOFTIS, C.D., & COLLIER, C.W.
(1971) An improved gas chromatographic method for the analysis of
2,4-D free acid in soil. J. agric. food Chem., 19: 186-188.
YIP, G. (1975) Analysis for herbicides and metabolites. J.
Chromatogr. Sci., 13: 225-230.
YODER, J., WATSON, M., & BENSON, W. (1973) Lymphocyte chromosome
analysis of agriculture workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUNG, A.L., ARNOLD, E.L., & WACHINSKI, A.M. (1974) Field
studies in soil persistence and movement of 2,4-D, 2,4,5-T and
TCDD. Weed Sci. Soc. Am., (Abstract No. 226).
YOUNG, A.L., CALCAGNI, J.A., THALKEN, C.E., & TREMBLAY, J.W.
(1978) The toxicology, environmental fate, and human risk of
Herbicide Orange and its associated dioxin. Springfield, Va.,
USA, US Air Force Occupational & Environmental Health
Laboratory (Technical Report No. TR-78-92, US National
Technical Information Service.)
YOUNG, F. & HALEY, T.J. (1977) Pharmacokinetic study of a
patient introduced with 2,4-dichlorophenoxyacetic acid and
2-methoxy-3,6-di-chlorobenzoic acid. Clin. Toxicol., 11(5)
489-500.
ZAKHAROV, G.G., SHEVCHENKO, A.M., ZUB, G.A., & PERSHAI, L.K.
(1968) [The clinical picture and prophylaxis of "Dikotex"
poisoning.] Zdrav. Beloruss., 9: 7-21 (in Russian, Health &
Welfare Canada Translation No. 2367).
ZETTERBERG, G. (1977) Experimental results of phenoxy acids
on microorganisms. In: Ramel, C., ed. Chlorinated phenoxy acids
and their dioxins: Mode of action, health risks and environmental
effects. Ecol. Bull. (Stockholm), 27.
ZETTERBERG. G., BUSK, L., ELOVSON, R., STAREC-NORDENHAMMAR, I., &
RYTTMAN, H. (1977) The influence of pH on the effects of 2,4-D on
Saccharomyces cerevisiae and Salmonella typhimurium. Mutat. Res.,
42(1): 3-18.
ZHITENEVA, L.D. & CHESNOKOVA, T.V. (1973) [Alteration of the
morphological composition of the blood in white amur larvae under
the effect of herbicides.] Eksp. vod. toksikol., 4: 56-67
(in Russian).
ZIELENSKI, W.L., Jr & FISHBEIN, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4-D and 2,4,5-T acids and
2,4-D esters in mice. J. agric food Chem., 15(5): 841-844.
ZIEM, G. & MAY, G. (1983) Tetrachlorodibenzodioxin. Br. J. ind.
Med., 40: 116.
2.1.4. Composition of technical 2,4-D materials
Technical 2,4-D may range in purity from less than 90% to 99%.
Typical levels for impurities are listed in Table 2. Trace levels
of CDDs have been found in amine and ester formulations (Table 3).
It can be seen that the amine formulations tend to be less highly
contaminated with di- and tetra-CDD than the ester products. The
structures of these impurities are shown in Fig. 2.
Table 2. Typical levels of 2,4-D and major impurities
in technical 2,4-Da
------------------------------------------------------
Component % range
------------------------------------------------------
2,4-dichlorophenoxyacetic acid 94 - 99
2,6-dichlorophenoxyacetic acid 1.5 - 0.5
2-monochlorophenoxyacetic acid 0.5 - 0.1
4-monochlorophenoxyacetic acid 0.8 - 0.2
bis(2,4-dichlorophenoxy) acetic acid 2.0 - 0.1
phenoxyacetic acid trace - 0.2
2,4-dichlorophenol 0.6 - 0.1
2,6-dichlorophenol 0.048 - 0.001
2,4,6-trichlorophenol 0.14 - 0.001
2-chlorophenol 0.04 - 0.0004
4-chlorophenol 0.005 - 0.0004
water 0.8 - 0.1
------------------------------------------------------
a From: Cochrane (1981).
Table 3. Ranges of levels of chlorinated dibenzo- p-dioxins (CDD)
in 2,4-D amine and ester formulationsa
-------------------------------------------------------------------
CDD isomers found (µg/kg)b
Type of 2,7-di- 1,3,7-tri- 1,3,6,8/ 2,3,7,8-tetra
formulation 1,3,7,9-tetra
-------------------------------------------------------------------
2,4-D amines ndc- 409 nd - 587 nd - 278 nd
2,4-D esters nd - 23815 nd - 450 nd - 8730 nd
-------------------------------------------------------------------
a From: Cochrane et al. (1981).
b Expressed in terms of 2,4-D.
c nd (not detected < 1 µg/kg).
The composition of technical 2,4-D depends on the manufacturing
process and especially on the purity of 2,4-dichlorophenol when
this is the starting material. During 2,4-D synthesis from
monochloroacetic acid and 2,4-dichlorophenol, the latter compound
as well as other ortho-chlorinated by-products can give rise to a
wide variety of chorinated by-products at a high temperature and
high pH. Self condensation of 2,4-dichlorophenol may form 2,7-
dichlorodibenzo- p-dioxin, while trichlorophenols may give rise to
a mixture of 1,3,6,8- and 1,3,7,9-tetrachlorodibenzo- p-dioxins
(but not 2,3,7,8-TCDD) by self-condensation, or to 1,3,7-
trichlorodibenzo- p-dioxin by cross-condensation with 2,4-
dichlorophenol.
A different type of toxic trace impurity, namely N-
nitrosamines, can occur in amine formulations of 2,4-D, especially
when nitrite is added as a corrosion inhibitor for containers.
Dimethyl- N-nitrosamine has been found in some 2,4-D dimethylamine
products at levels of up to 0.3 mg/litre (Ross et al., 1977; Cohen,
et al., 1978).
2.1.5. Volatility of 2,4-D derivatives
2,4-D esters with short-chain alcohols are highly volatile
(Table 1). This influences the effectiveness of their application
to target crops, their effects on neighbouring crops, and the
degree of contamination of the atmosphere. 2,4-D alkali salts or
amine salts are much less volatile than esters (Carter, 1960;
Canada, NRC, 1978; Que Hee & Sutherland, 1981, and section 4.1),
and these products are to be preferred when the use of 2,4-D esters
might lead to evaporative 2,4-D losses and to crop damage.
2.2. Determination of 2,4-D
2.2.1. General comments
General comments on criteria for acceptable analytical methods
and on other pertinent aspects of 2,4-D determination can be found
in the publications of Gunther (1962), Currie (1968), Kaiser
(1973), Carl (1979), Kateman & Pijper (1981), Que Hee & Sutherland
(1981) and Chau et al. (1982).
2.2.2. Analysis of technical and formulated 2,4-D products
In the past, the quality of 2,4-D products was assessed by an
acid-base titration or by a total chlorine determination
(Collaborative International Pesticides Analytical Council, 1970).
These non-specific and thus inaccurate methods have been superseded
by specific gas-liquid chromatography (GLC) or high pressure liquid
chromatography (HPLC), making it possible to determine various by-
products (Henshaw et al., 1975; Bontoyan, 1977; Skelly et al.,
1977; Stevens et al., l978; Cochrane et al., 1982). The isomer-
specific HPLC method is now preferred by many 2,4-D producers and
regulatory agencies. The chlorinated dibenzo- p-dioxins (CDDs) are
usually produced only in trace amounts and are difficult to
separate and identify; highly specialised equipment and skills are
necessary (Crummett & Stehl, 1973; Huckins et al., 1978; Norström
et al., 1979; Baker et al., 1981; Cochrane et al., 1981; Hass et
al., 1981, and National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1981).
2.2.3. Determination of 2,4-D residues
All exposure determinations and risk assessments ultimately
depend on accurate chemical analyses, and therefore some critical
aspects of analysis for 2,4-D residues have been included in the
present document.
Before 2,4-D residues can be measured, they have to be
quantitatively extracted and purified to remove substances that
could interfere with the final residue determination. They must
then be converted to a stable product (derivative) suitable for
determination with a given type of detector.
When comparing analytical results, it should be kept in mind
that the older methods of extraction and clean-up contained
considerable sources of errors, and that the early methods
for measuring 2,4-D residues, such as colorimetry and
spectrophotometry, were not as sensitive or specific as those
developed in recent years.
2.2.3.1. Sampling, extraction, and clean-up
Methods for the sampling, extraction, and clean-up of 2,4-D
residues in water, air, soil, and biological materials have
recently been reviewed by National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978) and by Que Hee & Sutherland (1981). Problems caused
by the conjugate formation of 2,4-D with amino acids, proteins,
sugars, or lipids, or the absorption of 2,4-D onto container
surfaces, including those of glass vessels, have been solved by
Chow et al. (1971), Renberg (1974), Osadchuk et al. (1977), Lokke
(1979), Jensen & Glas (1981), and Bristol et al. (1982). For
sampling and extracting 2,4-D residues, the following references
should also be consulted:
Air: Van Dyk & Visweswariah (1975), Farwell et al. (1976a,b),
Grover et al. (1976), Johnson et al. (1977), Gluck & Melcher
(1980), and Grover & Kerr (1981); water: Suffet (1973a,b), Renberg
(1974), Mierzwa & Witek (1977), Chau & Thomson (1978); soil:
Woodham et al. (1971); Smith (1972, 1976a), Foster & McKercher
(1973); food: Que Hee & Sutherland (1981), Bjerk et al. (1972),
Jensen & Glas (1972), Lokke (1975); biological media: Smith
(1976b), (blood, urine); Senczuk & Pogorzelska (1981).
2.2.4. Derivatization and quantification
At present, gas-liquid chromatography with electron-capture
detection (GLC-EC) is the most commonly used and generally most
sensitive method (picogram level) for measuring 2,4-D residues.
To improve the sensitivity of detection, the 2,4-D has to be
transformed (derivatized), usually to a methyl ester by reacting
with BF3-methanol, diazomethane, or with concentrated sulfuric
acid-methanol; the first method may give the best results (Munro,
1972; Horner et al., 1974; Olson et al., 1978).
For a recent review of derivatization methods and GLC columns
for various substrates see Cochrane (1981).
Thin-layer chromatography (TLC) has been used for herbicide
residue determination (Guardigli et al., 1971, Yip 1975). It has
recently been recommended by Batora et al. (1981) as a simplified
method for determining pesticide residues that requires a minimum
of costly equipment. TLC is suitable for food inspection and could
be of use in the establishment of new residue laboratories in
developing countries.
High-pressure liquid chromatography (HPLC) is less sensitive
than GLC-EC i.e., nanogram (ng) versus picogram levels, but may be
advantageous under some circumstances (Tuinstra et al., 1976;
Arjmand et al., 1978; Connick & Simoneaux, 1982). Using mass
fragmentography with deuterated internal standards it is possible
to determine nanogram amounts of 2,4-D and related compounds in
urine and plasma (De Beer et al., 1981); it is also suitable for
chemobiokinetic studies on subtoxic doses of 2,4-D in blood.
2.2.5. Confirmation
The ultimate confirmatory technique is gas chromatography
coupled with mass spectrometry and specific ion monitoring, with a
sensitivity down to the femtogram level (Farwell et al., 1976a).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use
were not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United
Kingdom. World-wide use of herbicides and annual production, which
probably exceeds 5 x 107 kg per year, are increasing, National
Research Council of Canada, Associate Committee on Scientific
Criteria for Environmental Quality, 1978; Bovey & Young, 1980).
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaf weeds in cereal crops as well as on
pastures and lawns, in parks, and on golf courses at rates of about
0.2 - 2.0 kg active ingredient (acid equivalent) per hectare.
Esters are also used at rates of up to 6 kg (acid equivalent) per
ha to suppress weeds, brush, and deciduous trees along rights-of-
way and in conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides
in or along irrigation and other canals, in ponds, and lakes at
rates ranging from 1 to 122 kg/ha (Pal'mova & Galuzova, 1963; Smith
& Isom, 1967; National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Bovey & Young, 1980).
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays
containing 20 - 40 mg 2,4-D/litre on apple trees to reduce
premature fruit drop, on potato plants to increase the proportion
of medium-size tubers or to intensify the tuber skin colour of the
red varieties (Bristol et al., 1982), and in citrus culture to
reduce pre-harvest fruit drop and to increase fruit storage life.
The highly volatile ethyl, isopropyl, and butyl esters are
being replaced by low-volatile esters or by amine salts to reduce
crop damage resulting from 2,4-D vapour drift, and to decrease
atmospheric pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public,
has been reduced in some countries, because of increasing concern
about possible toxic effects, especially in relation to CDDs.
The ecological effects of using high rates of 2,4-D and
repeated treatments have been reviewed by Bovey & Young (1980).
3.3. Disposal of wastes
3.3.1. Industrial Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localised
to the production site and to areas of waste dumping, and it is
likely to be more dispersed if disposal or leaching has occurred
into water courses. Combustion of 2,4-D and its by-products at low
temperatures could lead to the formation of CDDs. A temperature
approaching 1000 °C, however, gives almost complete destruction of
2,4-D (Sittig, ed., 1980). The spread of 2,4-D from waste dumps
may be reduced by the use of properly enclosed impermeable clay-
lined pits, away from water sources.
3.3.2. Agricultural wastes
Disposal of unused 2,4-D and washing of equipment may result in
localised land pollution and also pollution of water supplies
through direct contamination or leaching from soil.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION OF 2,4-D
2,4-D does not persist or accumulate in the environment, as it
is readily degraded by physical, chemical, and biological action.
It is susceptible to photolysis in air and water, on soil, and on
plant surfaces. Thus, the question of environmental distribution
is limited to the immediate transfer of 2,4-D between compartments
of the environment.
4.1. Drift and Volatilization in the Atmosphere
The atmosphere can be contaminated with 2,4-D during both its
manufacture and use. The production of 2,4-D may result in the
emission into the air of dichlorophenol, chloroacetic acid, and
ammonia (Sittig, ed., 1980), in addition to 2,4-D vapours (Grover
et al., 1976).
According to the formulation of 2,4-D used, environmental
transfer into the atmosphere will occur by either drift (depending
on the particle size of the droplet, the spray technique, and
climatic conditions), or by volatilization, or by a combination of
both. It is very difficult to calculate the extent to which drift
or volatisation occurs, and this is illustrated by the range of
2,4-D concentrations observed in the air after 2,4-D use (Table 4).
The factors affecting the amount of herbicide spray that lands
on a target crop and the proportion that is lost by drifting or
volatilization have been described (Grover et al., 1972; Grover,
1976; Maybank et al., 1978; Que Hee & Sutherland, 1981). Unwanted
residues may be deposited on non-target crops (Akesson & Yates,
1961; Yates & Akesson, 1973). The National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) cited reports of drift damage
caused in susceptible crops by phenoxy herbicide applications,
particularly in cotton, tobacco, tomatoes, grapes, rapeseed,
clover, and a number of horticultural species.
Widespread damage in vineyards and in other crops due to 2,4-D
drift from sprayed wheat fields was reported by Robinson & Fox
(1978) with two different damage patterns, one localized and the
other widespread. The first was characterized by severe localized
damage with a very clear gradient of decreasing severity away from
the zone, following the drift of spray droplets in the immediate
treatment area. The more widespread damage was of greater concern.
It was characterized by more or less uniform symptoms and appeared
to be attributable to the passage of a large cloud of vapour that
may have extended for several km. Both problems could have been
avoided by the use of low-volatile preparations and proper
application methods.
Table 4. Concentrations of total 2,4-D residues in ambient air
------------------------------------------------------------------------------------------------------------------
Days when 2,4-D was 2,4-D residues
Site location µg/m3air Predominant
No. of stations, height ------------------------------------------- type of 2,4-D Reference
Regional characteristics found Sample Meana Max. residue
present time
mean max. h
------------------------------------------------------------------------------------------------------------------
Saskatchewan, Canada not
(1) 150 m (aircraft) stated 1 min 1.0c 2.5 butyl ester Elias (1975)
Saskatchewan, Canada 48 33 36 24 h 0.5c 13.5 butyl ester Grover et al.
(8) 2 m above ground 24 h 0-6d isooctyl ester (1976)e,f
level, wheat area 24 h - - amine salt
California, USA 41 (1973) 24 h 0.4 0.9 high volatile Farwell et al.
(7-8) near ground 73 (1974) 24 h 0.1 0.4 low volatile (1976)
level 24 h 0.1 0.2 non volatile
Washington State, USA 105-106 81 89 24 h 0.2 2.2 isopropyl ester Adams et al.
(2) near ground 65 69 24 h 0.09 2.2 butyl ester (1964)b
level, wheat area 8 11 24 h 0.003 3.1 isooctyl ester
Washington State, 99-102 34 39 24 h 0.08 2.0 isopropyl ester droplets Bamesberger &
(2) near ground 8 15 24 h 0.08 1.3 isopropyl ester vapour Adamsb
level, wheat area 18 22 24 h 0.07 1.0 butyl ester droplets
3 5 24 h 0.03 1.3 butyl ester vapour
1 1 24 h 0.005 0.5 isooctyl ester droplets
- - 24 h - - isooctyl ester vapour
4 5 24 h 0.01 0.5 acid, salts, droplets
5 5 24 h 0.04 5.1 acid, salts, vapour
------------------------------------------------------------------------------------------------------------------
a Values measured at different sites or at different times have been averaged and reduced to a single significant
figure for simplicity.
b At the time of these studies, GLC methods were less highly developed.
c Centre of principal range observed.
d Maximum values recorded in a previous study (Que Hee et al., 1975) were shown to be equivalent to concentrations
existing directly over an open pan of formulated butyl ester; the implication was made that accidental
laboratory contamination could have occurred.
f The results of Maybank & Yoshida (1969), Maybank et al. (1978), and Stanley et al. (1971) could not be adapted
to this table.
Volatilization of 2,4-D products in the air during the spraying
operation and from the surface of plants and the soil is difficult
to distinguish from the drift of spray droplets. Evaporation
occurs to a greater extent with the highly volatile ethyl,
isopropyl, or butyl esters; very little occurs with amine salt
formulations, and it is greatly reduced when 2,4-D is dissolved in
corn oil, cottonseed oil, or diesel oil (Marth & Mitchell, 1949).
In one experiment, no significant amounts of 2,4-D amine, but 20 -
40% of the initially deposited 2,4-D butyl ester, and 10 - 15% of
the octyl ester of 2,4-D vapourized within 2 h of spraying (Grover
et al., 1972); less volatilization occurs with the higher esters of
2,4-D. For this reason, the use of the more volatile esters has
been discontinued in some countries. Studies of 2,4-D aerial drift
following ground spray operations have shown that only 3 - 8% of
the applied herbicides drift as spray droplets when low volatile
preparations are applied as large droplets. However, ultra-low-
volume (ULV) applications by aircraft, or the use of highly
volatile esters may cause as much as 25 - 30% of the 2,4-D sprayed
to drift off the target (Grover et al., 1972; Maas & Kerssen, 1973;
Maybank et al., 1978).
4.2. Movement Within and From the Soil
The movement of pesticides within and from the soil can be
divided into three categories: diffusion, leaching, and surface
movement. Diffusion is a localized process and depends on the
concentration gradient of the pesticide in the soil medium, on the
soil mineral type, and on the organic matter content, temperature,
pH, and other factors. Leaching refers to the movement of
pesticides through the soil profile with percolating water.
Surface movement refers to wind erosion of dust particles and
surface run-off in flowing water.
Examination of the behaviour of 2,4-D in soils (Liu & Cibes-
Viade, 1973; Grover & Smith, 1974; Moreale & Van Bladel, 1980) has
shown that organic matter, soil pH (surface horizons), and
exchangeable aluminium (clay sub-horizons) are the key determinants
for the percentage of 2,4-D adsorbed. As the adsorption/desorption
process is the basic mechanism influencing herbicide availability,
mobility, and degradation in soil, 2,4-D is likely to be more
strongly bound in soils with a high content of organic matter than
in those with a low content.
4.3. Contamination of Water
Residues of 2,4-D in aqueous systems can result from the
deposition of spray drifts, the "washout" of 2,4-D in the vapour or
droplet phase from the atmosphere during rainfall, the run-off from
treated fields, or following the application of 2,4-D to water for
the control of aquatic weeds. Industrial discharges, either from
accidental spills or through sewage systems, may also contribute to
the contamination of water. The National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) has tabulated data that demonstrate
the influence of environmental factors on the clearance of 2,4-D
and its derivatives from water. The principal processes involved
are ester and amine hydrolysis, volatilization, microbial
degradation, photolysis, and sorption. There is little movement of
2,4-D into drainage water in organic soils, because it is strongly
bound to organic materials.
4.4. Environmental Transformation and Degradation Processes
4.4.1. Metabolism in plants
Plants hydrolyse 2,4-D esters to 2,4-D, which is the active
herbicide (Morton et al., 1967; Matsunaka, 1972). Further
metabolism in plants occurs through three mechanisms, namely, side-
chain degradation, hydroxylation of the aromatic ring, and
conjugation with plant constituents (Crafts, 1960; Morre & Rogers,
1960; Erickson et al., 1963).
4.4.1.1. Side-chain degradation
Degradation of the side-chain of 2,4-D has been observed in
many plants (Loos, 1969), but in only a few species or varieties
does it appear to play a major role in herbicide breakdown.
Luckwill & Lloyd Jones (1960a,b) suggested two degradation
pathways leading to the formation of 2,4-dichlorophenol.
4.4.1.2. Ring hydroxylation
Thomas et al. (1964a,b), and, more recently, Feung et al.
(1971, 1972, 1973b) identified 2,5-dichloro-4-hydroxyphenoxyacetic
acid and 2,3-dichloro-4-hydroxyphenoxyacetic acid as major and
minor phenolic acid metabolites, respectively. Evidence was found
by Fleeker & Stein (1971) indicating hydroxylation resulting in the
elimination of the 4-chloro substituent from the aromatic ring, in
addition to migration of the chlorine at the 4-position to an
adjacent carbon on the ring. A small amount of 2-chloro-4-hydroxy-
phenoxyacetic acid was produced from 2,4-D by wild buckwheat, wild
oats, leafy spurge, and yellow foxtail.
4.4.1.3. Conjugation with plant constituents
Studies indicate that resistant crops, i.e., grasses and
cereals, form water-soluble conjugates with sugars, whereas
sensitive broad-leaved crops (such as beans) form mainly water-
insoluble amino acid conjugates (Montgomery et al., 1971; Feung et
al., 1971, 1972, 1973b, 1975).
4.4.2. Degradation of 2,4-D in the soil
Deposition of 2,4-D esters on the soil is followed fairly
rapidly by hydrolysis. Burcar et al. (1966) observed that the
2,4-D isooctyl ester disappeared after 2 weeks, though free acid
could be detected up to 6 weeks after application. The breakdown
of the iso-propyl, n-butyl, and isooctyl esters of 2,4-D on three
Canadian prairie soils was studied by Smith (1972) who found that
after 24 h no iso-propyl or n-butyl esters remained, whereas
20 - 30% of the isooctyl ester was still intact. The author
concluded that an initial rapid phase of hydrolysis of the 2,4-D
esters to the anion in soil was the result of chemical and not
microbial action.
Microbial degradation of phenoxy herbicides does occur and has
been comprehensively reviewed by Loos (1975), Cripps & Roberts
(1978) and The National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, (1978).
Early studies of the persistence of 2,4-D in soil indicated that
warm moist conditions and the presence of organic matter favoured
the rapid disappearance of 2,4-D. Sterilization of the soil
inhibited breakdown, indicating that the degradation was microbial.
In addition, Pemberton (1979) reported the discovery of specific
2,4-D plasmids within some bacterial strains, transmitted from one
cell to another, and carrying with them a genetic capability
enabling the bacteria to degrade 2,4-D.
Two principal pathways have been proposed for the microbial
degradation of 2,4-D in soil. Firstly the side chain may be
removed to form 2,4-dichlorophenol, followed by orthohydroxylation
of the phenol to produce a catechol (Bollag et al., 1968). The
catechol may then be cleaved to yield a muconic acid and further
conversion products. The second possible pathway is via a
hydroxyphenoxyacetic acid intermediate (Evans et al., 1971).
4.4.3. Degradation in the aquatic ecosystem
A multitude of variables influence the partitioning and removal
of phenoxy herbicides within an aquatic ecosystem. Detectable
residues have been reported to persist for 4 weeks in some
situations and up to 4 months in others (Frank & Comes, 1967;
Wojtalik et al., 1971; Schultz & Harman, 1974). Photolysis is an
important means of degradation of 2,4-D in natural water and is
more rapid than that of 2,4,5-T (Crosby & Wong, 1973). The
partition of residues between water and sediment will have an
effect on the rate of breakdown, as will temperature and intensity
of light. Anaerobic conditions will favour microbial breakdown.
The effects of some of these factors have been tabulated by the
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (1978).
4.4.4. Photochemical degradation
Photodecomposition of 2,4-D was studied in detail by Crosby &
Tutass (1966), Boval & Smith (1973), and reviewed recently by Que
Hee & Sutherland (1981). It leads to the formation of a variety of
products but commonly involves reductive dechlorination of the
acid, esters, and salts in aqueous or in organic solutions, with
2,4-dichlorophenol acting as a catalyst for the breakdown of
2,4-D, which may involve rupture of the aromatic ring. Que Hee &
Sutherland (1987) studied the vapour and liquid phase photolysis of
the n-butyl ester of 2,4-D and observed dechlorination at the
second position with simultaneous reduction and re-arrangement to
produce a variety of photoproducts. According to Boval & Smith
(1973), carbon dioxide is the final oxidation product when aqueous
solutions of 2,4-D undergo photodecomposition.
4.5. Bioconcentration
There is no evidence that bioconcentration of 2,4-D occurs
through the food chain or in any compartment of the environment.
This has been demonstrated by large-scale monitoring for 2,4-D
residues in soils, foods, feedstuffs, wildlife, and human beings,
and from examinations of the many routes of metabolism and
degradation that exist in ecosystems (sections 5.1.3 and 5.1.4).
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1. Levels of 2,4-D Residues in the Environment
Most of the available information on 2,4-D levels in the
environment has been reviewed in detail (National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality, 1978; Ramel, 1978; Bovey & Young, 1980;
Canada, Health & Welfare, 1980; Shearer & Halter, 1980; US EPA,
1980a). In comparing early and recent results, it should be kept
in mind that the analytical procedures used before about 1965 were
often unreliable and may have resulted in under- or overestimation
of the actual levels of 2,4-D derivatives. No information is
available on the levels of 2,4-D-related dioxin by-products in the
environment.
5.1.1. In air
Some levels of 2,4-D in ambient air are shown in Table 4.
These 2,4-D residues consist mainly of esters, particularly the
highly volatile butyl esters (Bamesberger & Adams, 1966; Farwell et
al., 1976b; Grover et al., 1976). Total 2,4-D residues in the air
were found to decrease during periods of rain, suggesting a
"washout effect" (Grover et al., 1976). In the majority of cases,
the levels reported were those found shortly after spraying.
5.1.1.1. Field exposure
Concentrations of 2,4-D that occurred during and after
herbicide use in the air of the work zone of people engaged in
herbicide spray operations in various use situations, are given in
Table 5. Workers involved in these operations were exposed to
2,4-D levels of up to 0.2 mg/m3 air during the period of actual
application.
5.1.1.2. General environmental exposure
In large-scale studies in areas of intense 2,4-D use, about 40%
of all air samples were found to contain between 0.01 and 0.1 µg
2,4-D/m3 (Grover et al., 1975). In a similar study undertaken by
Que Hee et al. (1976), much higher levels were recorded in one
urban location, reaching an average of 339 µg/m3 air during 3 days.
However, Grover et al. (1976), in their subsequent work, showed
that such concentrations could only be produced under artificial
conditions that could not reflect environmental conditions. In a
general programme of air monitoring undertaken in citrus-growing
regions in the USA, only one out of 880 samples analysed was found
to contain 2,4-D, at a level of 0.004 mg/m3. The sites were not
chosen in relation to 2,4-D use (Stanley et al., 1971).
Table 5. Concentrations of total 2,4-D in air related to occupational exposure
---------------------------------------------------------------------------------------------------------
Days Mean of 2,4-D
Herbicide Circumstances Type of exposure after concentrations References
product monitoring spraying in air (mg/m3)
---------------------------------------------------------------------------------------------------------
2,4-dimethylamine agricultural spray Analyses of air 0 0.02 Thiele et al.
salt (0.9% aqueous operations with in tractor cabs (1981a,b)
solution) tractor-drawn
equipment
2,4-D butoxyethanol Exposure during Analyses of air 0 0.1-0.2 Kolmodin-Hedman
ester. 2% emulsion forest spray in breathing zone & Erne (1980),
in water operation with of workers Kolmodin-Hedman
tractor driven et al. (1979)
equipment
2,4-D isooctyl- 3-day aerial spray Analyses of air 0 0.002-01a Franklin et al.
ester in diesel oil operation with single in breathing zone (1982)
engine aircraft of pilot and
ground crew
2,4-D PGBE ester Two 1-day aerial Analyses of air 0 <0.00001b Lavy et al.
emulsion in water forest spray in breathing zone (1982)
operations by of ground crew
helicopter
---------------------------------------------------------------------------------------------------------
a Application using large spray droplets.
b One flagman was recorded as being exposed to 0.1 mg/m3.
5.1.2. In water
2,4-D, as well as chlorophenol residues resulting from the
microbial transformation of 2,4-D, may occur in raw and finished
supplies of drinking-water (Faust & Aly, 1963; US EPA, 1976, 1980a;
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality, 1978; Bovey & Young,
1980; Canada, Health & Welfare, 1980; Shearer & Halter, 1980).
Information on 2,4-D-related dioxins in water was not
available.
Drinking-water in the USA is routinely analysed by the FDA as
part of the beverage-food group in their "market basket" analysis
programme; 2,4-D has not been detected in these studies, where the
limit of detection is 0.005 mg/litre for beverages (Table 6). This
indicates that drinking-water is not a significant source of human
exposure outside directly sprayed areas.
The same conclusion can be drawn from the results of large-
scale surveys of pesticide residues, including 2,4-D in surface
waters in areas of 2,4-D use (Table 7).
Levels much higher than those found in these studies have been
observed, but only in relation to local spills or direct
contamination (Frank et al., 1979; Frank & Sirons, 1980). A very
wide fluctuation has been found in water samples following
treatment of bodies of water, shores, ditches, or stream banks with
herbicides (Averitt, 1967; Frank & Comes, 1967; Bartley & Hattrup,
1970; Frank et al., 1970; Wojtalik et al., 1970; Frank, 1972;
Whitney et al., 1973; Schultz & Harman, 1974; Schultz & Whitney,
1974; Paderova, 1975; Province of British Columbia, 1981).
Occasional high contamination levels in samples of potable water
have been reported following experimental treatments of reservoirs
with 2,4-D (Wojtalik et al., 1971). However, the mean levels
tended to remain below 2 µg/litre, even in samples of raw or
processed water from 2,4-D-treated reservoirs (Smith & Isom, 1967;
Wojtalik et al., 1971; Province of British Columbia, 1981).
Generally, 2,4-D residues were < 0.1 µg/litre in two large-scale
monitoring programmes of surface waters (Frank & Sirons, 1980;
Gummer, 1980). This is not unexpected in view of the moderately
rapid microbial degradation of 2,4-D in the environment (Robson,
1966; Averitt, 1967; Frank, 1972; Nesbitt & Watson, 1980a,b;
Province of British Columbia, 1981).
2,4-D and especially its transformation product,
dichlorophenol, at levels exceeding 20 µg/litre will impart an
objectionable odour and taste to contaminated water (Pal'mova &
Galuzova, 1963; Faust & Suffet, 1966). This organoleptic effect
may reduce the likelihood of highly contaminated water being
ingested. It is noteworthy that public water supplies containing
"traces" of 2,4-D, and wells contaminated with 2,4-D or other
herbicides have been shut down because of objectionable odours or
tastes (Gribanov, 1968; Kramer & Schmaland, 1974; Frank et al.,
1979).
Table 6. 2,4-D residues reported in market basket samples in the USA
---------------------------------------------------------------------------------------------------------
Types of samples Nature of samples % of samples Residue levels
Years analysed containing residues with residues (mg/kg) References
---------------------------------------------------------------------------------------------------------
1965-65 Total diets sugars and adjunctsa 4.2 < 0.02-0.16 Duggan & Corneliussen
1966-66 leafy vegetables (1) 3.0 < 0.02-0.03 (1972)
low fats
1967-67 leafy vegetables (2) 1.7 0.03
oil fats (1)
1968-68 dairy produce (1) 0.6 0.02-0.13
1969-69 fruits (1), sugars (2) 0.3 < 0.2
1970-70 leafy vegetables (1) 0.3 < 0.02
dairy produce (1)
1970-71 Total diets leafy vegetables (3) - 0.01-0.02 Manske & Corneliussen
(1975)
1971-72 Total diets dairy products (1) - 0.01 Manske & Johnson
(1975)
1973-80 Total diets 0 < 0.01 Manske & Johnson
(1976)
Johnson et al.
(1981a,b)
Johnson et al. (1977)
1972-73 Potatoes from raw, boiled or baked - < 0.02-0.12 Bristol et al. (1982)
fields treated
with herbicide
---------------------------------------------------------------------------------------------------------
a No. of positives not specified.
Table 7. Concentrations of 2,4-D residues in surface water samples following application of 2,4-D to
agricultural landsa
------------------------------------------------------------------------------------------------------
Site No. of samples in 2,4-D applied 2,4-D residues
Number of Stations which 2,4-D was: in watershed (µg/litre) References
Regional Characteristics ----------------- (kg/ha) ---------------
analysed found meanb max.
------------------------------------------------------------------------------------------------------
Ontario, Canada 949 66 0.8 <0.1 3.9e Frank & Sirons (1980)
11
streams
Saskatchewan, Canada 15 10 - 2 21.6 Choi et al. (1976)
5
river
Western Canada 186 10 - 0.5c 4.3d Gummer (1980)
14
diverse sites
------------------------------------------------------------------------------------------------------
a Studies in which the analytical procedures were not described or were considered unreliable have not
been included.
b Values measured at different sites or at different times have been averaged and reduced to a single
significant figure for simplicity.
c Reported data are very close to analytical detection limits.
d The maximum value, which raises the average value considerably, occurred in the effluent of an
industrial plant.
e Levels of 15.9 and 320 µg/litre were recorded at two sites but were related to spillage or actual
spraying at the sampling locality.
5.1.3. In soil
Most of the information available at present concerning 2,4-D
and other chlorophenoxy herbicide residues in soils has been
reviewed by the National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Bovey (1980a), and by Que Hee & Sutherland (1981). In highly
acidic soils, or in soils in cold or arid regions, 2,4-D
degradation is apparently slow (Lavy et al., 1973; Buslovich &
Milchina, 1976; Ou et al., 1978; Moreale & Van Bladel, 1980;
Nesbitt & Watson, 1980b). However, even at about 20 - 2000 times
the normal agricultural application rates, little or no detectable
2,4-D was left in soils under temperate climatic conditions with
prolonged winters, after intervals of 385 - 440 days (Young et al.,
1974; Stewart & Gaul, 1977; Bovey, 1980a). Furthermore, results of
a laboratory study on 2,4-D degradation in the soil showed a half-
life of 4 days (Altom & Stritzke, 1973). Several soil monitoring
studies in North America, in areas with regular 2,4-D use, have
shown residues in less than 10% of the samples, and at levels of
less than 1 mg/kg (Stevens et al., 1970; Wiersma et al., 1972;
Gowen et al., 1976).
The available data are inadequate for establishing regional and
seasonal profiles of 2,4-D soil residues and of direct population
exposure, but it is likely that direct exposure would be minor,
except during or soon after herbicide application. Indirect
exposure through the transfer of 2,4-D residues from soil to air,
or food sources is assessed separately.
5.1.4. In food sources
Although 2,4-D and its transformation products do not tend to
accumulate in plants and plant products, detectable residues of
2,4-D on food plants may be consumed by human beings or animals and
may thus contribute to the overall exposure of the human population
to this chemical.
The results of pertinent studies on 2,4-D residues on or in
foods, and in food sources for human beings and animals, are
summarized in Tables 8 - 11. Theoretically, some contribution to
the reported 2,4-D residues may have been partly derived from other
phenoxy herbicides, as 2,4-DB undergoes beta-oxidation to 2,4-D in
some plants and fish, and in cattle (Lisk et al., 1963; Gutenmann &
Lisk, 1965; Sundström et al., 1979; Bovey, 1980a).
5.1.4.1. Residues in retail food supplies
The frequency of occurrence and the levels of 2,4-D residues in
over 110 000 samples of a variety of different ready-to-eat foods,
beverages, and infant and young children's diets, have been studied
over the last 20 years in the USA (Lipscomb, 1968; Corneliussen,
1970, 1972; Duggan et al., 1971; Duggan & Corneliussen, 1972;
Johnson et al., 1979, 1981a,b). The 2,4-D residues found in such
samples are reported in Table 6. The theoretical daily intake
resulting from these residues was variously estimated to be < 1 -
5 µg/person per day (Duggan & Corneliusson, 1972).
Studies undertaken since 1970 have failed to detect residues of
2,4-D in any of the US diet samples analysed, except for a single
positive sample in the dairy product food group which was estimated
at 0.01 mg/kg (Manske & Johnson, 1975).
5.1.4.2. Residues in fish and shellfish
Fish and shellfish may be exposed to 2,4-D as a consequence of
aquatic herbicide use, or through the agricultural use of 2,4-D.
The residues in the edible portions of such fish rarely exceed 1
mg/kg wet weight (Erne, 1974, 1975 and Table 8). Residues of 2,4-D
have not been detected in retail samples of fish and shellfish
analysed as part of the US "market basket" studies (section
5.1.4.1).
There is some evidence that the organoleptic properties of the
2,4-D residues may reduce the likelihood of the consumption of fish
flesh contaminated with higher levels of 2,4-D (Gavrilova, 1965;
Folmar, 1979).
5.1.4.3. Residues in wild fruits and mushrooms
Uncultivated fruits and mushrooms taken from areas where 2,4-D
was used, or was likely to have been used, were examined for
residues of 2,4-D by Erne & von Haartman (1973), Erne, (1980),
Sietanen et al. (1981), and Frank et al. (1982). The results in
Table 9 show that residues of 2,4-D in berries in field-trial
studies have been as high as 30 mg/kg immediately after
application, but residues in berries and mushrooms taken from the
wild are generally < 1 mg/kg.
High residues of 2,4-D can produce disagreeable odours or
flavours in wild fruits and vegetables (Ingelög et al., 1977;
McArdle et al., 1961), and this may reduce the likelihood that
highly contaminated foods are ingested.
5.1.4.4. Residues in food derived from animals
Domestic meat-, milk-, and egg-producing animals, and game
animals may consume forage or feed containing 2,4-D residues, and
thus, their tissues and products may contain residues. Published
data on 2,4-D residues in feed and forage from the Northern
Hemisphere are summarized in Table 10. Immediately after
application of phenoxy herbicides, 2,4-D residues in or on grass,
generally average about 100 mg/kg for each kg of herbicide applied
per hectare. Such residues decline with a half-life of about 1 - 2
weeks, to about 20 mg/kg, within 4 weeks after an application of 1
kg/ha (Leng, 1972). Residues in 2,4-D-treated feed grains are
significantly lower than the levels reported above and no residues
would be expected in meat, milk, or eggs from such sources (Table
10).
Table 8. 2,4-D residues reported in field studies on fish and shellfish
-----------------------------------------------------------------------------------------------------
Country Year(s) 2,4-D application Types of samples 2,4-D residues in References
rate tissues (mg/kg)
-----------------------------------------------------------------------------------------------------
USA 1961 0.1 mg/litre oyster (1 species) 1.6-2.0 Butler (1965)
fish (1 species) 0.3-1.0 Cope et al. (1970)
USA 1966 44.8-112 kg/ha mussels < 0.14-1.12 Smith & Isom (1967)
clams < 0.14
fish (5 species) < 0.14
USA 1968 112 kg/ha fish (4 species) < 0.10-0.24 Whitney et al. (1973)
USA 1969 22.4-44.8 kg/ha mussels < 0.05-2.7 Wojtalik et al. (1971)
fish (8 species) < 0.10-0.34
USA 1971 2.24-8.96 kg/ha fish (3 species) < 0.005-1.075 Schultz & Harman (1974)
USA 1971 4.48 kg/ha fish (5 species) 0.000-0.162 Schultz & Whitney (1974)
-----------------------------------------------------------------------------------------------------
Table 9. 2,4-D residues in wild berries and mushrooms collected in fields or forests following application
of phenoxyalkanoic herbicides
--------------------------------------------------------------------------------------------------------------
Country Year(s) Sample 2,4-D application Days after No. samples 2,4-D residues References
rate (kg a.i./ha) treatment analysed (mg/kg)
--------------------------------------------------------------------------------------------------------------
Canadaa 1979-81 raspberries 1.1-3.9 2 124 2.6-31.0 Frank et
14-35 0.1-3.3 al. (1982)
Finlanda 1974-76 vaccinium berries 2.5 10-356 44 Mukula et
jam not known not known 1 2.2 al. (1978)
mushrooms 14-300 28 < 0.05-1.2
Finlanda 1975-76 vaccinium berries 0.25-2.25 365 not stated < 0.05 Siltanen et
al. (1981)
Swedena 1970 raspberries 1.5-2.2 2-32 9 < 0.03-0.9 Erne & Von
vaccinium berries 1.5-2.2 2-32 68 < 0.03-7.7 Haartman
blueberries 1.5-2.2 2-32 19 < 0.03-2.9 (1973)c
mushrooms 1.5-2.2 2-32 15 < 0.03
Swedena 1973-79 vaccinium berriesb 0.25-2.25 365 61 nd (< 0.05) Erne (1980)
raspberries not stated 14 not stated nd-2.5
blueberries not stated 2 not stated nd-10.0
cowberries not stated 1-28 not stated nd-6.0
mushrooms not stated 7 1 0.3
Swedena blueberries 0.4-1.5 1-35 not stated 0.2-5.3 Ingelög et
vaccinium berries 0.4-1.5 30-35 not stated 0.5-4.5 al. (1977)
raspberries 0.4-1.5 1-10 not stated 0.2-2.0
--------------------------------------------------------------------------------------------------------------
a Samples taken from areas treated with 2,4-D.
b Samples entering factory for processing.
c Data from authors' Table 1.
Table 10. 2,4-D residues reported in samples of herbicide-treated forage or feed
--------------------------------------------------------------------------------------------------------------------------
Country Year(s) Type of samples 2,4-D application Post- No. samples 2,4-D residues References
rate, (kg a.i./ha) treatment examined & (mg/kg)
interval, positive
(days)
--------------------------------------------------------------------------------------------------------------------------
Canada 1971 wheat plants 0.42 1-36 ? ? 8.35-0.011 Cochrane & Russell (1975)
Finland 1962-68 green forage 1-4 7.21 ? ? 600-3.7 Finnish State Institute of
(grass and 3.5 7-28 ? ? 13-0.4 Agriculture (1963-1969)
clover)
Finland 1974-76 aspen leaves 2.5 60-300 32 30-0.3 Mukula et al. (1978)
and twigs
birch leaves 60-300 16 31-0.1
and twigs
cowberry plants 365 8 < 26-0.05
Germany, wheat, barley, 0.375-0.735 64-101 ? ? < 0.015-0.01 Maier-Bode (1971)
Federal rye, oat grains
Republic wheat, barley, < 0.34-0.02
of rye, oat straw
Hungary 1971 silo corn 1.4-1.5 56-120 ? ? 0.8-0.075 Bodai et al. (1974)
Sweden 1972-76 barley, oats ? ? 3 2 0.7-0.4 Erne & Rutqvist (1979)
grass 7 1 0.4
lichens ? ? 2 2 0.4-0.2
USA 1949 pasture plants 4.48 1 8 8 14.6-1.65 Grigsby & Farwell (1950)
USA 1967 forage grasses 0.56-2.2 0-112 ? ? 100-1 Morton et al. (1967)
USA 1969 sorghum plants 1.4 2 ? ? 1.06 Ketchersid et al. (1970)
1.4-2.8 30-60 < 5.25-0.2
USA 1969 pasture plants 6.6-8.8 0-28 24 24 700-150 Leng (1972)
--------------------------------------------------------------------------------------------------------------------------
No residues of 2,4-D were detected (detection limit of 0.02 mg)
in the milk of dairy cows fed 2,4-D at a level of 300 mg/kg total
diet (Bjerke et al., 1972; Leng, 1972). A range of 0.06 - 0.08 mg
2,4-D/litre was found in the milk of cows fed for 3 weeks at a
level of 1000 mg 2,4-D/kg total diet.
When young beef cattle were fed 2,4-D at levels of 300, 1000,
and 2000 mg/kg total diet for 28 days, 2,4-D residue levels were
highest in the kidney and liver, but did not exceed 0.1 mg/kg in
muscle and fat, even at the highest dose level (Clark et al., 1975;
Leng, 1972, 1977). 2,4-D residues were not detected in more than
12 000 samples each of meat and dairy products analysed in the USA
between 1963 and 1969 (Duggan et al., 1971).
Results of feeding studies with hares and reindeer in
Scandinavia indicated that 2,4-D levels of 25 - 30 mg/kg forage
(equivalent to an intake of about 1 mg 2,4-D/kg body weight per
day) produce maximum 2,4-D residues of 1.1 mg/kg wet weight in
liver, and 8.9 mg/kg in kidney tissues (Erne, 1974). Residues of
2,4-D were detected in the liver and kidney of a few game animals
shot by hunters, or found dead in or near areas sprayed with
phenoxy herbicides (Table 11, Erne, 1974, 1975). The residues in
muscle tissue were not measured but would be lower than in the
liver and kidney, as indicated by the data summarized in Table 11.
On the whole, the available evidence indicates that 2,4-D is
rarely detected in commercial foods and that residues in food taken
from areas where 2,4-D has been sprayed will usually be < 1 mg/kg
food. The liver and kidney from range animals are possible
exceptions, but these contribute little to the total diet of the
general population.
5.2. Occupational Exposure to 2,4-D During the Production, Handling,
and Use of Chlorophenoxy Herbicides
During occupational exposure to 2,4-D, the chemical may be
absorbed via the inhalation, oral, and dermal routes, but more than
90% of the total amount of 2,4-D or other chlorophenoxy compounds
entering the body under these circumstances appears to be absorbed
through the skin and excreted relatively quantatively in the urine
as the phenoxy acid and readily-hydrolysed conjugates (Kolmodin-
Hedman et al., 1979, 1980; Libich et al., 1981; Draper & Street,
1982; Franklin et al., 1982; Leng et al., 1982; Nash et al., 1982)
(section 6).
Data from occupational exposure studies concerning the amounts
of 2,4-D found on the clothing or on cloth patches worn by workers
are not included in this review because the correlation between
these amounts and amounts absorbed into the body and then excreted
in urine is poor (Franklin et al., 1982; Lavy et al., 1982; Leng et
al., 1982).
Table 11. 2,4-D residues in game and domestic animals and animal products
------------------------------------------------------------------------------------------------------------------
Country Year(s) Species 2,4-D treatment Post- Type of 2,4-D residues References
rate (kg a.i./ha) treatment samples (mg/kg)
interval examined
(days)
------------------------------------------------------------------------------------------------------------------
Sweden 1968 moose (Alces game animals found ? liver and < 0.05-6 Erne (1974,
-1972 alces) dead, or shot by kidney from 1975)
deer (Capreolus hunters in herbicide- 250 animals
capreolus) treated areas found dead
hares (Lepus
lepus)
pheasants ? liver and < 0.05-4.5
grouse kidney from (2,4-D and
130 animals 2,4,5-T)
shot by
hunters
USA 1963 Jersey cow 50 ppm in diet for 4 0-2 milk < 0.1 Bache et al.
days (1964a)
USA 1974(?) adult beef 0, 9, 30 or 60 mg 0 muscle < 0.05-0.07 Clark et al.
cattle 2,4-D acid/kg bw/day 28 fat < 0.13-0.34 (1975)
for days (0, 300, liver < 0.05-0.23
1,000 2000 mg/kg feed) kidney 2.53-10.9
USA 1974(?) adult sheep 2000 mg/kg feed for 0-7 muscle < 0.05-0.06 Clark et al.
28 days fat 0.10-0.15 (1975)
liver 0.29-0.98
kidney 0.37-9.17
USA 1965(?) dairy cows animals grazing on 2 milk 0.01-0.09 Klingman et al.
pasture sprayed with 4 (1966)
herbicide at 2, 24 kg
a.i./ha
USA 1972 dairy cows 30, 300, 1000 mg/kg 0 milk < 0.05-0.16 Bjerke et al.
in feed for 2-3 weeks (1972)
1-3 < 0.05 Leng (1972)
USSR 1975 "livestock" ? ? muscle 0.04 Fyodorova et
liver 0.04 al. (1977)
kidney 0.03 (mean)
------------------------------------------------------------------------------------------------------------------
5.2.1. Industrial exposure
Several studies have been published on the levels of 2,4-D to
which workers producing or packaging 2,4-D herbicides are exposed
(Fetisov, 1966; Johnson, 1971; Juzwiak et al., 1973; Andreasik et
al., 1979). In every case the amount of 2,4-D absorbed by the
workers was uncertain and, therefore, the data are inadequate for
estimating industrial exposure to 2,4-D. Workers manufacturing
2,4-D were also exposed to other chemicals (Assouly, 1951; Bashirov
& Ter-Bagdasarova, 1970).
5.2.2. Exposure related to herbicide use
The available studies on the occupational exposure to 2,4-D of
workers during the use of 2,4-D herbicides are summarized in Table
12. Studies on the exposure of back-pack sprayers to 2,4-D have
not been published. However, comparable exposure data are
available for 2,4,5-T back-pack sprayers, and they have been
included in Table 12 for comparison. The levels of 2,4-D found in
the air of the working zone in these and other studies have already
been referred to in section 5.1.1.1 and Table 5.
In studies undertaken before 1980, only the amounts of 2,4-D in
the air, on the clothing, or on the skin were determined, except
for 2 urinary 2,4-D values reported by Shafik et al. (1971). Thus
the amounts of 2,4-D actually absorbed cannot be reliably estimated
from these early reports and are not included in Table 12.
After 1980, several detailed occupational exposure studies were
carried out to determine the amounts of 2,4-D or other chlorophenoxy
acids absorbed by various members of ground and aerial spray teams,
using a variety of equipment for dispersing aqueous or oil solutions
or emulsions (Kolmodin-Hedman et al., 1979; Kolmodin-Hedman & Erne,
1980; Libich et al., 1981; Draper & Street, 1982; Franklin et al.,
1982; Lavy et al., 1982; Leng et al., 1982; Nash et al., 1982).
The total 2,4-D urinary excretion levels reported in Table 12
reflect a wide variety of uses and show that the excretion does not
usually exceed 0.1 mg 2,4-D/kg body weight per day of exposure.
However, so far, a comprehensive comparison of the relative
exposures resulting from different methods of application and
different 2,4-D derivatives (amine salts and esters) or formulations
(aqueous, oil) cannot be carried out, because the available data are
still incomplete. The amount of 2,4-D absorbed depends on the type
of work performed, and on the degree of care taken to avoid direct
dermal contact with the herbicide concentrate, spray solution, or
spray. The most heavily-exposed workers tend to be the mixer-
loaders, who handle the herbicide concentrate, and the spray
personnel. However, if careful, they may be exposed to less 2,4-D
than, for example, a pilot of a spray plane who is not careful
(Franklin et al., 1982; Leng et al., 1982; Lavy et al., 1982; Nash
et al., 1982). The reports by Libich et al. (1981) and Leng et al.
(1982) on ground spray crews indicate that, even under unfavourable
working conditions, the amount of 2,4-D absorbed may be greatly
reduced simply by wearing clean gloves and overalls, and by making
the workers more aware of the importance of safe work habits.
Table 12. Exposure related to herbicide use
------------------------------------------------------------------------------------------------------------------------
Product No. of Type of Daily concentr- Duration of Total 2,4-D in urine References
people application ation of 2,4-D collection of excreted (mg/kg bw/
exposed in urine 24-h urine day of exposure)
(mg/litre)e samples (days)
------------------------------------------------------------------------------------------------------------------------
2,4-D and dicamba 2 boom spray 1 - 4 - - Draper & Street
dimethylene salts single use (1982)
in aqueous solution 2 repeated use 3 - 20 - -
2,4-D isooctyl 4 3 applications - 4 0.004 - 0.04 Franklin et al.
ester in diesel oil by single- (1982)
engine aircraft
2,4-D/2,4,5-T 4 tractor-drawn 1 - 14 7 - Kolmodin-Hedman
butoxyethyl sprayers, forestry (1979, 1980)
esters as 2% exposure daily,
emulsion in water for one week
2,4-D PGBE ester 26 helicopter in - 5 nd - 0.06a Lavy et al.
26 forestry use - 5 nd - 0.02b (1982)
2,4,5-T PGBE ester 7 Back-pack 5 0.01 - 0.09 Leng et al.
forestry use (2,4,5-T) (1982)
single exposures,
one week apart
2,4-D/2,4-DPc and 23 roadside and < 0.01 - 8 3 - Libich et al.
2,4-D/picloramc right-of-way (one usually (1981)
ground equipment high result
incl. mist blowers of 31)
2,4-Dc 17 aircraft repeated - 7 0.006 - 0.02d Nash et al.
exposure (mean values/day) (1982)
2,4-D amine salt 26 ground equipment - 7 nd - 0.08
and ester (single exposure)
------------------------------------------------------------------------------------------------------------------------
a No special precautions taken.
b Protective clothing worn.
c Preparation used not specified.
d Mean values per day recorded for different individuals.
e It is not possible to calculate the total 2,4-D excretion in urine from these data, because of individual variations
in urine concentrations from day to day from sample to sample.
As the chemobiokinetic profiles of urinary 2,4-D output are
reported in only a few of the studies, summarized in Table 12, it
is not possible to estimate the total 2,4-D intake in all cases.
The results of the studies by Libich et al. (1981) and by
Draper & Street (1982) suggest that using single-exposure studies
to estimate the peak exposure levels reached by workers exposed
several days in succession may give an underestimation.
No information is available on the amounts of chlorinated
dibenzodioxins, or other by-products or contaminants, absorbed as a
consequence of occupational exposure to 2,4-D herbicides.
In one extensive occupational monitoring programme undertaken
in 1979 - 82, about 3000 urine samples were analysed for herbicide
residues (Simpson, 1982). The subjects included pesticide factory
staff, pest control operators, farmers, park workers, and others
potentially exposed to 2,4-D. During the first year of the study,
no 2,4-D was detected (< 0.001 mg/litre) in 735 of 973 samples.
Most of the other samples contained less than 0.1 mg/litre and only
27 contained more than 1 mg/litre. The highest value was 31
mg/litre. The study is continuing.
5.3. Exposure of Bystanders to 2,4-D
Aerial drift and other forms of pesticide transport, as well as
the contamination of surfaces during or after herbicide production,
distribution, or use, may bring 2,4-D into contact with bystanders,
i.e., persons other than those who are occupationally exposed. Few
studies of bystander exposure to 2,4-D or other chlorophenoxy
herbicides have been published. Studies available for review
included that of Lavy et al. (1982) concerning 9 supervisors and
observers present at two helicopter forest spray operations using
2,4-D propyleneglycol butylether (PGBE) ester, respectively, for
unspecified durations. These people excreted a maximum of 1.3 µg
2,4-D/kg body weight. In a forest ground spray operation with
tractor-drawn equipment, 2,4-D was not detected (< 0.05 mg/litre)
in the urine of bystanders (Kolmodin-Hedman et al., 1980).
Additional bystander exposure studies for various 2,4-D use
patterns are desirable. However, the 2,4-D intake of bystanders is
unlikely to exceed the 2,4-D intake during occupational exposure.
5.4. Estimated Exposure of the General Population in 2,4-D-Use Areas
Data useful for estimating the intake by the general population
of 2,4-D residues in the environment including those in food
sources have been generated. The present calculations of the
intake of the general population in an area of 2,4-D use are based
on these data and on a series of stated assumptions aimed at
obtaining a moderate overestimation rather than underestimation of
the actual exposure.
5.4.1. Intake of 2,4-D residues from air
On the basis of available information, it can be assumed that
the general population in areas of 2,4-D herbicide use would rarely
be exposed to 2,4-D concentrations exceeding 0.1 µg/m3 air.
Assuming an air level of 0.1 µg 2,4-D/m3, a body weight of 60
kg, an air intake of 20 m3 per day, and a 100% retention of 2,4-D,
it can be calculated that the respiratory intake would be 0.03 µg
2,4-D/kg body weight per day.
5.4.2. Intake of 2,4-D residues from potable water
The larger surveys of potable water (Table 7) show mean 2,4-D
residues in surface water to be generally < 0.1 µg/litre, but for
the present estimate, it is assumed that potable water from surface
sources or from treatment plants, during a period of about 10 days
after reservoir treatment, can contain an average 2,4-D residue
level of 2 µg/litre (Wojtalik et al., 1971 and Table 7). Assuming
a 2,4-D concentration in water of 2 µg/litre, a body weight of 60
kg, a water intake of 2 litres per day (Canada, Health & Welfare,
1980), and a 100% absorption of the ingested 2,4-D, it can be
calculated that the 2,4-D intake of the general population in a
2,4-D use area resulting from water could approach 0.07 µg/kg body
weight per day, which could occur for about 10 days.
Insufficient data are available to give a reliable estimate of
2,4-D intake from ground water sources, but it is likely to be
lower than the above value.
5.4.3. Intake of 2,4-D residues from soil
2,4-D on soil particles ingested with food or water, or carried
into the air and inhaled, is considered to be part of the exposure
due to residues in air, water, or food and is therefore assumed to
be completely covered in these exposure estimates.
5.4.4. Intake of 2,4-D residues from food
The data in Tables 8 - 11 indicate that there is unlikely to be
any exposure of the general population to 2,4-D residues in retail
food supplies. The possibility that individuals are exposed to
contaminated local sources of food has been assessed in section
5.1.4. In the case of milk or muscle meat, it can be assumed that
no individual will be exposed to levels in excess of 0.02 mg/kg of
these foods, the limit of detection of the method of analysis used.
Assuming a concentration of 0.02 mg 2,4-D/litre in milk, and a
consumption of 1.5 litre per day, the maximum intake from this
source would be 0.0005 mg/kg body weight per day for a 60 kg adult.
Individuals who consume wild berries taken from 2,4-D-treated areas
could be exposed through this food source. Assuming consumption of
100 g of berries per serving and a maximum 2,4-D concentration of 1
mg/kg, the intake from this source would be 0.002 mg/kg body weight
per serving.
5.4.5. Total exposure of the general population in a 2,4-D-use area
The above considerations suggest that the total daily 2,4-D
intake of the population in use areas will not normally exceed
about 0.002 µg/kg body weight during the application period (Table
13).
Table 13. Components of estimasted exposure to 2,4-D
-------------------------------------------------------------------
Estimated amount of
Exposed Group intake (µg 2,4-D/kg Source of 2,4-D
bw/day)
-------------------------------------------------------------------
Occupational
i. Factory workers insufficient data mainly dermal contact
ii. Applicator crews 100a
iii. Bystanders - b
General population in
areas with 2,4-D use 0.03 air
0.07 water
0.5 milk
ND retail food
2.0 wild berries, mushrooms
etc.
-------------------------------------------------------------------
a Based on total urinary output after several days of exposure.
b Unlikely to exceed occupational exposure.
5.4.6. Total exposure of persons occupationally exposed in agriculture
An accurate maximum occupational intake of 2,4-D cannot be
determined on the basis of the limited studies undertaken.
However, the available data suggest that work performed in the
preparation of, and during, agricultural application of 2,4-D
herbicide will probably result in an exposure of not more than
about 0.1 mg 2,4-D/kg body weight per day, providing that minimum
precautions are taken against excessive exposure.
5.4.7. Total exposure of the general population outside areas of 2,4-D use
Monitoring of air, water, and food outside areas of known 2,4-D
use show that intake is below present detection limits.
6. CHEMOBIOKINETICS AND METABOLISM
With the exception of recent occupational exposure studies and
studies on animals published in 1979 or later, the available
information on the uptake, distribution, transformation, and
excretion of 2,4-D by human beings and other mammals has already
been reviewed by Leng (1977), National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Young et al. (1978), Bovey (1980a,b), Shearer
(1980), and United States Veterans' Administration (1981).
6.1. Uptake via Different Routes of Exposure
6.1.1. Uptake by inhalation
6.1.1.1. Animals
Burton et al. (1974) found that small amounts of 2,4-D
instilled into the rat lung were rapidly absorbed, apparently by a
non-saturable process following first-order kinetics, with an
absorption half time of 1.4 - 1.7 min. The kinetics of the
absorption of 2,4-D vapours or aerosols in the respiratory tract of
animals have not yet been studied.
6.1.1.2. Human beings
The uptake of 2,4-D and of 2,4-D derivatives via the human
respiratory tract does not appear to have been studied under
controlled conditions. However, the observations of Kolmodin-
Hedman & Erne (1980), Libich et al. (1981), Franklin et al. (1982),
and Lavy et al. (1982) on people occupationally exposed to 2,4-D
indicated that only a small percentage of the total amount of 2,4-D
absorbed via all routes of exposure was taken in through the
respiratory tract.
6.1.2. Dermal uptake
6.1.2.1. Animals
Mice whose tails had been immersed in 2,4-D butyl or crotyl
ester solutions, 4 h daily for 3-5 days, absorbed lethal amounts of
the chemicals (Fetisov, 1966). However, the actual doses absorbed
and other details were not given. In contrast, no major ill
effects were reported in studies in which rabbits were treated
percutaneously for 2 or 3 weeks with 130 - 180 mg/kg body
weight/day of a 50% aqueous solution of 2,4-D octyl ester, or with
unspecified amounts of solutions of 2,4-D dimethylamine salt in
water, or oil solutions of 2,4-D isooctyl or butyl ester
(Vinokurova, 1960; Kay et al., 1965).
6.1.2.2. Human beings
Only 5.8% of a dilute solution of 14C-labelled 2,4-D in acetone
applied at a dose of 4 µg a.i./cm2 to the ventral forearm of adults
was excreted in the urine compared with 100% of a small intravenous
dose (Feldmann & Maibach, 1974) (Table 14). The 2,4-D excretion in
urine is delayed and more prolonged after dermal application than
after intraveneous or oral administration (Feldmann & Maibach,
1974; Sauerhoff et al., 1977), and complete elimination may take
about one week (Levy et al., 1982; Leng et al., 1982). Cases of
acute occupational 2,4-D poisoning following combined dermal and
inhalation exposures (Monarca & Divito, 1961; Tsapko, 1966;
Paggiaro et al., 1974), as well as occupational exposure studies
(Table 12), suggest a fairly efficient dermal absorption of 2,4-D.
However, the importance of solvents, surfactants, and other
ingredients of the herbicides in the uptake of 2,4-D via the dermal
route still needs to be defined.
6.1.3. Oral uptake
6.1.3.1. Animals
The uptake of 2,4-D from the gut of rats, mice, guinea-pigs,
cattle, pigs, and sheep appears to be similar in both rapidity and
extent to that observed in human beings (Mitchell et al., 1946;
Lisk et al., 1963; Bache et al., 1964a; Erne, 1966a,b; Milhaud et
al., 1970; Shafik et al., 1971; Buslovich et al., 1973; Fedorova &
Belova, 1974; Clark et al., 1975; Senczuk & Pogorzelska, 1975,
1981; Van Peteghem & Heyndrickx, 1975). In some of the ungulates,
2,4-DB acid, and 2,4-D amine salts or esters are at least partially
converted to 2,4-D in the rumen, before being absorbed (Gutenmann
et al., 1963; Lisk et al., 1963). Some of the esters may be less
well absorbed from the gut than the acid or its alkali or amine
salts (Erne, 1966a; Buslovich et al., 1973), but the uptake
mechanisms for 2,4-D and its salts or esters is not known, and thus
deserves further study.
6.1.3.2. Human beings
Information on the uptake of 2,4-D by human beings via the
oral route has been gathered in studies on two groups of 5 - 6
volunteers each, who ingested single doses of 5 mg 2,4-D/kg body
weight (Table 14), and by chemobiokinetic studies on individuals
who, with suicidal intent, swallowed lethal or non-lethal amounts
of various 2,4-D herbicides (Geldmacher-Von Mallinckrodt &
Lautenbach, 1966; Rivers et al., 1970; Kohli et al., 1974;
Sauerhoff et al., 1976, 1977; Khanna & Kohli, 1977; Young & Haley,
1977; Prescott et al., 1979) (Table 15). These results show that
single doses of 2,4-D are fairly rapidly and completely absorbed
from the human digestive tract, unless the dose is so large that
toxic effects interfere with absorption. However, in the two
studies on volunteers, considerable individual variation in the
rate and extent of absorption from the digestive tract was
observed. The absorption mechanism appears to involve first-order
kinetics (Kohli et al., 1974; Khanna & Kohli, 1977) and may fit a
single- or multi-compartment chemobiokinetic model, depending on
individual characteristics (Sauerhoff et al., 1977).
Table 14. Chemobiokinetics of 2,4-D in human beings following administration under controlled conditions
--------------------------------------------------------------------------------------------------------------------------
Product Dose and dosing Subjects Observations Toxic effects References
schedule EL NOELa
(mg/kg bw)
single dose
--------------------------------------------------------------------------------------------------------------------------
14C-2,4-D 1) Intravenous injection: 6 (sex & Scintillation counting ? ? Feldmann &
(New England Dose (7 µCi) not stated as age not 1) 100% of dose excreted in urine Maibach
Co., and 2,4-D/weight unit stated) urine in 120 h Mean t0.5 = 13 h (1974)
Amersham 2) Dermal application: 6 (sex & 2) 5.8% of applied dose excreted ? ?
Searle Co.) 1 x 4 µg 2,4-D (in acetone)/ age not in 120 h
cm2 of skin of forearm. stated)
Application site was not
washed for 24 h
2,4-D, 99% Oral administration: 6 gas chromatography of blood & ? 5 Khanna &
pure (Dow 1 x 2, 3, or 5 mg/kg bw, in (adult M) urine samples; no ill effects; no Kohli (1977);
Chemical gelatin capsule, with water, changes in clinical parameters: Kohli et al.
Co.) following breakfast blood pressure, pulse rate, Hb, WBC (1974)
counts (total & differential); mean
plasma clearance t0.5 = 33 ± 3.1 h;
peak plasma conc. at 7-24 h = 40
mg/litre; ~75% of dose excreted
in urine in 96 h
"no metabolic transformation
at up to 5 mg/kg"
2,4-D, Oral administration: 6 gas chromatography - mass ? 5 Sauerhoff
analytical 1 x 5 mg/kg bw as a slurry (adult M, spectrometry of blood & urine et al.
grade in milk, or in powder form, 70-90 kg) samples; no ill effects; (1976, 1977)
with some water, following essentially all of the dose
breakfast absorbed; peak plasma conc. = 10-30
mg/litre within 24 h; mean plasma
clearance t0.5 = 11.6 h; mean
urinary excretion t0.5 = 17.7 h;
total excreted amount ~82% of dose
administered; 4.8-27.1% of
excreted compound was conjugated
--------------------------------------------------------------------------------------------------------------------------
a NOEL = No-observed-adverse-effect level.
Table 15. Chemobiokinetics of 2,4-D by human beings following accidental or intentional ingestion of herbicides
----------------------------------------------------------------------------------------------------------------
Products Circum- Subject Observations References
stances
----------------------------------------------------------------------------------------------------------------
2,4-D suicide; F, 33 death in about 30 h; post mortem 2,4-D Geldmacher-Von
ingestion years concentration: Mallinckrodt &
of unknown mg/litre mg/kg Lautenbach (1966)
amount of blood urine brain liver lung heart
herbicide 23 164 100 116 88 63
no metabolites were identified
Herbicide suicide; F, 51 death in about 96 h; concentration of 2,4-D
containing ingestion years, plus MCPA:
2,4-D plus of unknown 66 kg mg/litre mg/kg
MCPA amount of blood urine liver kidney muscle
("U46 COMBI") herbicide 42 420 100 trace 40
(BASF 2,4-dichlorophenol not detected; several
Ludwigshafen) other metabolites or herbicide by-products
found, but not identified
Herbicide suicide M, 39 severe toxic effects; unconsciousness; Prescott et al. (1979)
containing attempt; years recovery within 11 days following treatment by
2,4-D plus ingestion alkaline diuresis; initial plasma concentration:
mecoprop of 6.7 g 2,4-D = 400 mg/litre, mecoprop = 750 mg/litre;
(10%) + (20%) 2,4-D and no pretreatment change in 2,4-D level following
7.6 g alkaline diuresis; plasma clearance t0.5 = 3.7 h
mecoprop for 2,4-D, 11-28 h for mecoprop
----------------------------------------------------------------------------------------------------------------
6.2. Distribution and Transformation in the Body
6.2.1. Animals
The absorption and distribution kinetics and metabolism of pure
2,4-D and of a variety of pure or commercial 2,4-D or 2,4-DB amine
salts and esters have been repeatedly studied both in vivo and in
vitro in a wide variety of animals including rats, mice, rabbits,
guinea-pigs, cattle, sheep, goats, pigs, chickens, fish, and spiny
lobsters (Gutenmann & Lisk, 1965; Erne & Sperber, 1974; Guarino &
Arnold, 1979; James, 1979; Koschier & Pritchard, 1979; Pritchard
& James, 1979; Pritchard & Miller 1980). The considerable
differences observed in the relative amounts of residues found in
the cells and plasma of mouse, rat, and horse blood, after dosing
animals or after in vitro addition of 2,4-D (Erne, 1966a; Jenssen
& Renberg, 1976), in different tissues of rats, mice, and sheep
(Erne, 1966a,b; Lindquist & Ullberg, 1971; Milhaud et al., 1970;
Buslovich et al., 1973; Clark et al., 1975; Elo & Ylitalo, 1979),
and in the soluble and particulate fractions of rat tissues (Khanna
& Fang, 1974) support the idea that there is more than one
physiological compartment for 2,4-D storage. The distribution
volumes appear to be equivalent to the volume occupied by about
25 - 50% of the body mass (Erne, 1966a).
2,4-D is reversibly bound to blood plasma proteins,
particularly albumins, possibly at sites for which it competes with
related compounds. The same sites are apparently also binding
sites for palmitic acid and thyroxine. The extent of 2,4-D binding
depends, in part, on pH and 2,4-D concentration (Florsheim et al.,
1963; Erne, 1966b; Kolberg et al., 1973; Hacque et al., 1975;
Mason, 1975; Orberg, 1980a), and may affect the rate and extent of
renal 2,4-D excretion (Pritchard & James, 1979; Pritchard & Miller,
1980) and thus the toxicity of 2,4-D.
In pregnant mammals, up to about 17% of a single dose of 2,4-D
may rapidly cross the placenta to reach the embryos or fetuses
(Lindquist & Ullberg, 1971; Fedorova & Belova, 1974; Antonenko,
1977).
Pigs and rats hydrolyse 2,4-D esters both in the gut and after
absorption in the body (Erne, 1966a,b). Observations from several
studies indicate that 2,4-D is not significantly metabolized in
animals, except in ruminants. No 14CO2 was produced by rats given
C1- or C2-labelled 14C-2,4-D (Khanna & Fang, 1966). No 2,4-
dichlorophenol (2,4-DCP) was detected in the tissues of mice or
rats dosed by various routes with 2,4-D (Zielinski & Fishbein,
1967; Shafik et al., 1971; Federova & Belova, 1974; Grunow & Böhme,
1974). However, residues of 2,4-DCP were detected in the milk of
dairy cows fed 100 mg 2,4-D/kg diet for 3 weeks (Bjerke et al.,
1972; Leng, 1972), and in the livers and kidneys of cattle and
sheep fed up to 2000 mg/kg diet for 4 weeks (Clark, 1975; Leng,
1972, 1977). These 2,4-DCP residues probably resulted from the
bacterial degradation of 2,4-D in the rumen of the animals.
Bacterial degradation may also account for the 2,4-DCP reported by
Antonenko (1977) in pregnant or lactating rats and rat fetuses.
Other investigators did not detect 2,4-DCP in the tissues of
mice or rats dosed with 2,4-D by various routes (Zielinski &
Fishbein, 1967; Shafik et al., 1971; Fedorova & Belova, 1974;
Grunow & Böhme, 1974).
Results of studies on experimental animals have suggested that
2,4-D conjugates are formed in the kidney tubules (Erne, 1966a,b;
Erne & Sperber, 1974; Grunow & Böhme, 1974).
Taurine and glycine conjugates, as well as various other
unidentified conjugates of 2,4-D have been found in the urine of
rats, pigs, chickens, and the dogfish shark (Squalus acanthias)
(Erne, 1966b; Erne & Sperber, 1974; Grunow & Böhme, 1974; Koschier
& Pritchard, 1979). However, in rats and pigs, only about 10-20%,
and in the chicken, less than 5% of the total amount of 2,4-D
appeared to be excreted in this form. In the dogfish shark, the
taurine conjugate may be primarily formed in the tubular cells of
the kidney (Koschier & Pritchard, 1979). The site and mechanism of
2,4-D conjugation seem to be unknown in the other species.
6.2.2. Human beings
Studies on human volunteers who ingested pure 2,4-D, and on
cases of accidental or voluntary acute poisoning with various 2,4-D
herbicides have shown that 2,4-D is very rapidly absorbed from the
gut and carried in the blood to cells and tissues throughout the
body, but that is not extensively transformed (Tables 14, 15)
(Curry, 1962; Herbich & Machata, 1963; Nielsen et al., 1965; Dudley
& Thapar, 1972; Coutselinis et al., 1977). The kinetics following
ingestion suggest a 1- or 2-compartment distribution, depending on
individual characteristics (Sauerhoff et al., 1977; Young & Haley,
1977). Following absorption of purified 2,4-D, or of herbicides
containing only 2,4-D, no transformation products, including 2,4-
dichlorophenol, were found in blood or tissues. After ingestion of
herbicides containing 2,4-D and other compounds, some 2,4-D
metabolites or manufacturing by-products were detected in tissues,
but were not identified (Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Prescott et al., 1979). Unidentified 2,4-D conjugates were
also found in urine following ingestion of pure 2,4-D. These
conjugates represented up to 27% of the 2,4-D ingested (Sauerhoff
et al., 1976, 1977). Of the 5 North American volunteers studied by
these authors, only one did not produce a conjugate; in contrast,
apparently none of the 6 Indian subjects studied by Kohli et al.
(1974) and by Khanna & Kohli (1977) produced 2,4-D metabolites.
6.3. 2,4-D Levels in Body Tissues and Fluids
6.3.1. Animals
2,4-D levels in the blood and organs of mammals have been
determined, e.g., by Erne (1966a,b), Milhaud (1970), Buslovich et
al. (1973), Khanna & Fang (1974), Clark et al. (1975), Jenssen &
Renberg (1976), and Elo & Ylitalo (1979). The highest residue
levels were usually found in liver, kidney, lungs, spleen, and
heart. In a study by Fedorova & Belova (1974), 6 - 8% of the
amount of 2,4-D administered was found in all of the tissues
examined in rats dosed orally 26 - 35 days previously with this
chemical. However, the 2,4-D residue levels quoted were close to
the limit of detection for the analytical method used.
6.3.2. Human beings
In volunteers, each of whom ingested a single dose of 5 mg
2,4-D/kg body weight, the 2,4-D levels in blood plasma reached
peaks of about 10 - 45 mg/litre within about 7 - 24 h, and then
declined (Kohli et al., 1974; Khanna & Kohli, 1977; Sauerhoff et
al., 1977). In one group of workers occupationally exposed to
2,4-D for one week while using ground equipment for spraying
(Kolmodin-Hedman et al., 1979), plasma levels ranged from the
detection limit (0.02 mg/ml) to 0.2 mg/ml, while urinary levels
ranged from 1 to 14 mg/litre. Urinary 2,4-D levels reported in
other occupational exposure studies are summarized in Table 12.
However, it should be noted that analysis of single urine specimens
is not adequate for estimating the dose absorbed by individuals,
because excretion follows a diurnal pattern and continues for
several days after dermal exposure (Leng et al., 1977; Sauerhoff et
al., 1979; Lavy et al., 1982). Thus, levels found after several
days of spraying will probably be higher than first day levels, as
reported by Libich et al. (1981) and Draper & Street (1982).
However, excretion of 2,4-D should be completed within one week
following the last exposure (Feldmann & Maibach, 1974; Lavy et al.,
1982; Leng et al., 1982).
The toxic and lethal levels of 2,4-D in human blood and tissues
are still not well defined. A woman with reportedly 335 mg
2,4-D/litre plasma did not show any signs of poisoning; in general,
the acute lethal levels of 2,4-D appear to lie between 447 and 826
mg/litre plasma (Herbich & Machata, 1963; Nielsen et al.,
1965; Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Coutselinis
et al., 1977; Prescott et al., 1979). The lowest lethal 2,4-D
levels in blood or tissues were recorded several days after the
chemical was ingested, i.e., after most of the 2,4-D had probably
been eliminated. Among the different organs examined post mortem
in cases of fatal 2,4-D poisoning, liver and kidney tended to
contain the highest concentrations of 2,4-D, while brain and other
fatty organs, and muscle including the heart, usually had lower
2,4-D levels (Table 15) (Curry, 1962; Herbich & Machata, 1963;
Nielsen et al., 1965; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972; Coutselinis et al., 1977). As in the
case of blood, the values for the different tissues may vary
according to the proportion of 2,4-D eliminated by the time death
occurs.
6.4. Elimination and Biological Half-Life
The term "biological half-life" will be used to indicate the
time required to eliminate one half of a single dose of 2,4-D, or
to reduce 2,4-D residues in the body fluids or tissues to one-half
of the peak concentration. Biological half-life, as defined here,
is a useful concept with which it is possible to make rough
comparisons of the elimination rate of 2,4-D with that of other
toxic chemicals.
6.4.1. Animals
The half-life values recorded in mammals fall into the range
observed in the rat (Erne, 1966a,b; Federova & Belova, 1974; Khanna
& Fang, 1974), with the exception of the very low value for 2,4-D
butyl ester in whole mouse carcasses (0.85 - 1.11 h) observed by
Zielinski & Fishbein (1967). Results of the investigations
conducted by these authors also suggest that prior dosing with
2,4-D ("priming") may increase the rate of elimination of 2,4-D
butyl ester in mice, presumably through stimulation of the renal
excretory mechanism.
An important correlation between diet and 2,4-D elimination
rate was observed by Orberg (1980b) in the goat. A protein-poor
diet reduced the 2,4-D plasma clearance rate by about 20 - 50%,
possibly because of decreased renal size and renal blood flow.
The many species-related factors known to affect the rate of
2,4-D elimination make interspecies comparisons difficult, and
often the published reports do not include such details as diet,
age, sex, and body weight of the test animals, type and purity of
the test compounds, and important environmental factors such as the
ambient temperature, which are necessary for valid comparisons.
6.4.2. Human beings
The studies on volunteers and on cases of accidental or
voluntary 2,4-D poisoning summarized in Tables 14 and 15 show that
human beings excrete 2,4-D mainly in the urine, and that the
blood plasma clearance times depend on the dose, individual
characteristics, and the presence or absence of compounds that may
competitively inhibit 2,4-D excretion. For single oral doses of
2,4-D, the biological half-life in blood plasma is about one day,
depending on the circumstances. However, forced alkaline diuresis
may reduce this to as little as 3.7 h (Sauerhoff et al., 1977;
Prescott et al., 1979).
In occupationally-exposed people, absorption of successive
daily doses of 2,4-D makes its biological half-life difficult to
determine, but for single occupational exposures it has been
estimated to be 35-48 h (Nash et al., 1982).
6.5. Chlorinated Dibenzo- p-Dioxins (CDDs)
The metabolism in the rat of several dibenzo- p-dioxins,
including the 2,7-CDD found in 2,4-D, was described by Tulp &
Hutzinger (1978). The primary pathway of transformation appears
to involve hydroxylation at the 2-, 3-, 7-, or 8-position in the
molecule; some sulfur-containing conversion products have also been
identified.
7. EFFECTS OF 2,4-D ON ANIMALS
7.1. General Introduction
Many studies of the toxicity of 2,4-D were carried out before
the possible toxicological importance of manufacturing by-products,
such as 2,6-dichlorophenoxyacetic acid, 2,4,6-trichlorophenoxy-
acetic acid (2,6-D and 2,4,6-T) monochlorophenoxyacetic acid, or
N-nitroso compounds, was known or appreciated.
Furthermore, 2,4-D may be contaminated with several chlorinated
dibenzo- p-dioxins (section 2.1.4). The toxicity of a number of
these contaminants has not been examined in detail, but whether or
not their presence would affect the toxicity of 2,4-D and its
derivatives would depend on the amount of CDD present in the
product, and on the inherent toxicity of the particular CDD
isomers. The most toxic CDD isomer, namely 2,3,7,8-TCDD (Schwetz
et al., 1973; McConnell et al., 1978; Leng, 1979; Kociba & Schwetz,
1982), is not normally found in 2,4-D products (see also section
2.1.4). However, there have been instances in which the same
manufacturing equipment was used to produce both 2,4,5-T and 2,4-D,
resulting in cross-contamination of 2,4-D with 2,4,5-T and 2,3,7,8-
TCDD (US EPA, 1982).
Studies of structure-activity relationships using in vitro
systems have shown that the CDDs that may be present in 2,4-D and
its derivatives have a much lower biological activity than 2,3,7,8-
TCDD (Poland & Glover, 1973; Poland & Kende, 1974; Poland et al.,
1976; Bradlaw et al., 1980; Knutson & Poland, 1980). However,
except for a study of the carcinogenic potential of 2,7-
dichlorodibenzodioxin (2,7-DCDD) (US National Cancer Institute,
1979), the chronic toxicity of the CDD detected in 2,4-D and its
derivatives has not been studied.
2,4-D has been in use as a herbicide for nearly 40 years, and
during this time a great deal of literature on the toxicology of
this chemical has accumulated. The extent to which its toxicity to
various organisms has been tested, and the types of 2,4-D products
used for such testing have varied over the years. Earlier 2,4-D
products probably contained higher concentrations of trace
contaminants than the 2,4-D in use today, and therefore it may have
been found to be more toxic in earlier than in more recent studies.
In addition, the generally accepted standards and protocols for
pesticide toxicity tests have changed, making some of the older
tests inadequate by present day standards. For these reasons,
attention has been focused on the more recent studies. The older
studies are, in many instances, only cited for completeness and
should be used with caution, especially when ascribing specific
toxic effects to unspecified 2,4-D products and when establishing
an effect level or no-observed-adverse-effect level for adverse
effects of 2,4-D. The Task Group noted that a number of additional
studies on 2,4-D are at present in progress. As the additional
information becomes available, the present document will need to be
updated.
7.2. Acute Effects
The reports of experimental studies to define the toxic and
other effects of 2,4-D or its derivatives cover a wide range of
organisms commonly referred to as "animals", including worms,
molluscs, arthropods, lower vertebrates, birds, and mammals. Much
of this information, especially on acute toxic effects, was
recently tabulated by the National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Schneider (1979), Bovey & Young (1980), Shearer &
Halter (1980), and the Commission of the European Communities (CEC,
1981).
The usual mandatory acute and subacute safety tests for
pesticides include assays for eye and skin irritancy and for skin
sensitization, and determinations of the acute oral, percutaneous,
and parenteral lethal doses, or of the corresponding lethal
concentrations in the air, diet, or water.
7.2.1. Skin and eye irritancy
2,4-D does not appear to be an eye or skin irritant (Schneider,
l979). Adequate tests of the potential irritative properties of
2,4-D derivatives have not been reported in the literature.
7.2.2. Skin sensitization
No adequate published information is available on the dermal
sensitization potential of 2,4-D and its derivatives in mammals.
7.2.3. Lethal doses and concentrations (LD50 and L50)
The lethal potential of a chemical is usually measured as the
dose (mg/kg body weight), or as the concentration in the air, diet,
or water (mg/kg, mg/m3, mg/litre, respectively) that will kill 50%
of the test animals in a specified time interval. These amounts
are referred to as the LD50 or LC50. For 2,4-D and its derivatives,
and for 2,4-D herbicide formulations, these statistically estimated
values vary depending on the test product, the test species, and
the route and frequency of administration (Tables 16 and 17).
7.2.3.1. Acute oral LD50
7.2.3.1.1. Mammals
Published acute oral LD50 values vary for different 2,4-D
products and test species (Table 16). It appears that 2,4-D has a
moderate acute toxicity for mammals (WHO, 1976).
7.2.3.1.2. Birds
Table 17 shows published oral LD50 values for chickens.
Table 16. Acute oral toxicity of 2,4-D, esters, and salts
------------------------------------------------------------------------
Compound Species Sex LD50 Reference
(mg/kg bw)
------------------------------------------------------------------------
2,4-D mouse M 375 Hill & Carlisle (1947)
mouse M 368 Rowe & Hymas (1954)
rat M 375 Rowe & Hymas (1954)
rat 666 Hill & Carlisle (1947)
guinea-pig M & F 469 Rowe & Hymas (1954)
guinea-pig 1000 Hill & Carlisle (1947)
rabbit 800 Hill & Carlisle (1947)
dog 100 Drill & Hiratzka (1953)
butyl ester mouse 380 Konstantinova (1970)
rat 1500 Schillinger (1960)
rat 920 Konstantinova (1970)
cat 820 Konstantinova (1970)
esters of mono-, rat F 570 Rowe & Hymas (1954)
di-, and tripro-
pylene glycol
butyl ethers
isopropyl ester mouse M 541 Rowe & Hymas (1954)
rat M & F 700 Rowe & Hymas (1954)
guinea-pig M 550 Rowe & Hymas (1954)
mixed butyl mouse F 713 Rowe & Hymas (1954)
esters rat F 620 Rowe & Hymas (1954)
guinea-pig F 848 Rowe & Hymas (1954)
rabbit M & F 1420 Rowe & Hymas (1954)
sodium salt mouse 375 Rowe & Hymas (1954)
rat F 805 Rowe & Hymas (1954)
rat 2000 Schillinger (1960)
guinea-pig M 551 Rowe & Hymas (1954)
rabbit 800 Rowe & Hymas (1954)
------------------------------------------------------------------------
7.2.3.2. Acute dermal LD50
7.2.3.2.1. Mammals
Reports by Buslovich (1963) and Fetisov (1966) indicate that,
under extreme test conditions, mice and rats may absorb lethal
amounts of 2,4-D amine salts and esters through their skin, but no
acute dermal LD50 values were available for review.
7.2.3.3. Acute inhalation LC50
No accurate measurements of acute inhalation LC50 values
for 2,4-D products were available.
Table 17. Acute LD50 for various 2,4-D products in domestic chickens (Gallus domesticus)
------------------------------------------------------------------------------------------------------------------
Product Animals Procedure LD50 Acid References
(mg/kg bw) equivalent
------------------------------------------------------------------------------------------------------------------
2,4-D chick, M & F P.O., in olive oil 541 (358-817) 541 Rowe & Hymas (1954)
2,4-D Na salt adult hen P.O., in water 655 646 Loktionov et al. (1973)
2,4-D alkanolamine chick P.O., in water > 380 < 765 368 Bjorn & Northern (1948)
salts Rowe & Hymas (1954)
2,4-D amine salt 6-month chicken P.O., in water 1950 1238 Loktionov et al. (1973)
2,4-D butyl esters chick, M & F P.O., undiluted 2000 (1350-2960) 1503 Rowe & Hymas (1954)
2,4-D butoxyethyl chick, 330 g P.O., in food 900 588 Whitehead & Pettigrew
esters (1972)
2,4-D isopropyl esters chick, M & F P.O., in olive oil 1420 (1127-1789) 1145 Rowe & Hymas (1954)
2,4-D/2,4,5-T (1:1), chick, M & F P.O., undiluted 4000 (2700-5900) - Rowe & Hymas (1954)
butyl ester 3 weeks old
------------------------------------------------------------------------------------------------------------------
7.2.3.4. Parenteral LD50
The acute parenteral LD50 values reported for various
laboratory animals and 2,4-D products ranged from 220 to 666 mg
a.i./kg body weight (National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Young et al., 1978; Schneider, 1979).
7.2.4. Acute toxicity in aquatic organisms
The available reports indicate great differences in acute LC50
values obtained with different test species, and with different
2,4-D derivatives and test formulations. The esters appear to be
considerably more toxic than the water-soluble salts. For fish,
the LC50 for various 2,4-D isooctyl ester products may range from 5
to 68 mg/litre for bluegill sunfish (Lepomis spp.) and from 62 to
153 mg/litre for rainbow trout (Salmo gairdneri) (Schneider,
1979). This variability may, in part, be due to differences in
test animal species and strains, in test conditions (temperature,
pH, 02 tension, mineral content of water), and, in part, to the
effects of chemicals other than 2,4-D in the herbicide
formulations, or of 2,4-dichlorophenol, which may occur in water
as a decomposition product of 2,4-D (Holcombe et al., 1980).
Discussions on this variablility can be found in numerous reviews:
Way (1969), Katz et al. (1972), National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), and of Halter (1980), as well as in
the reports of studies by Harrisson & Rees (1946), King & Penfound
(1946), Lopez (1961), Hughes & Davis (1963), Butler (1965),
Hiltibran (1967), Mount & Stephan (1967), Alabaster (1969), Sanders
(1970a), Andrushaitis (1972), Cooke (1972), Shim & Self (1973),
Fabacher & Chambers (1974), Meehan et al. (1974), Nishiuchi &
Yoshida (1974), Rehwoldt et al. (1977), Vardia & Durve (1981), and
particularly in the extensive and methodical investigation of
Pravda (1973).
Similar information on aquatic invertebrates is available in
the review of Mackenthun & Keup (1972) and in the studies of Hooper
(1958), Sudak & Claff (1960), Beaven et al. (1962), Rawls (1965),
Sanders (1970b), Klekowski & Zvirgzds (1971), Wierzbicka (1974a,b),
and Caldwell et al. (1979).
The observations of Hansen et al. (1972, 1973) and Folmar
(1976) indicate that fish and aquatic invertebrates will try
to avoid water containing toxic amounts of 2,4-D products.
7.3. Subchronic and Chronic Toxicity
7.3.1. Mammals
Most of the published long-term studies with mammals have
already been reviewed by Bodyagin et al. (1969), Way (1969), IARC
(1977, 1982), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Young et al. (1978), Bovey & Young (1980), Shearer (1980), and US
Veterans' Administration (1981).
In the long-term as in the short-term studies on mammals, the
test products were mainly administered orally. The composition of
the test products was not adequately known by present standards,
but many of the tests were carried out with more or less purified
2,4-D or its alkali salts. It is difficult to decide to what
extent the available results reflect the toxicological properties
of the present 2,4-D products, in which the maximum contents of CDD
and other toxic by-products are kept at very low levels.
A long-term feeding study in the rat (Hansen, 1971) was
evaluated by FAO/WHO JMPR in 1971 (FAO/WHO, 1972). It was
concluded that a no-observed-adverse-effect level in the rat was
equivalent to 31 mg/kg body weight per day. However, new studies
on mice and rats are in progress.
7.3.1.1. Clinical signs of poisoning
Signs of toxic effects on the digestive tract, such as
diarrhoea, vomiting, dysphagia, decreased gut motility, irritation,
or necrotic changes (in dogs including necrosis of oral tissues)
are likely to appear in animals following the absorption of high
doses of 2,4-D or its derivatives by the oral, dermal, or
inhalation routes, and after parenteral injections (Bucher, 1946;
Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Thomssen, 1958; Björklund & Erne, 1966; Erne, 1966a; Palmer &
Radeleff, 1969). Blood-tinged discharges from the nose, and in
dogs also nasal and eye irritation and skin lesions may also occur
(Bucher, 1946; Hill & Carlisle, 1947; Kosyan et al., 1974). Cattle
may suffer from tympanitis, and may show signs of thirst (Björklund
& Erne, 1966). However, some of the signs of "2,4-D poisoning"
reported in herbivores pastured on 2,4-D-treated vegetation may
have been caused by the ingestion of inherently poisonous plants
(Maclean & Davidson, 1970). Pigs may refuse to eat feed containing
high amounts of 2,4-D (Strach & Bohosiewicz, 1964), but sheep have
been reported to feed avidly on 2,4-D-treated vegetation (Sadykov
et al., 1972).
Characteristic signs of severe 2,4-D poisoning in mammals
appear to be muscular weakness, stiffness, stilted gait, and muscle
spasms (myotonia), alleviated by exercise and exacerbated by rest.
There may also be muscular incoordination progressing to paralysis
especially in the hind limbs; in rodents, caudal rigidity has also
been observed. These clinical signs are the result of the myotoxic
action of 2,4-D discussed below, which, through its effects on the
heart, may also lead to hypo- or hypertension, cardiac
fibrillation, and death. Prolonged inactivity may also occur and
may lead to pulmonary congestion, emphysema, and pneumonia (Bucher,
1946; Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Vinokurova,
1960; Björklund & Erne, 1966; Gorshkov, 1972; Sadykov et al., 1972;
Kosyan et al., 1974).
At high doses, 2,4-D and its derivatives may act as a central
nervous system depressant and cause lethargy, slowed respiration,
stupor, coma, and death (Bucher, 1946; Hill & Carlisle, 1947; Drill
& Hiratzka, 1953; Vinokurova, 1960; Desi et Sos, 1962a,b; Desi et
al., 1962a,b; Kosyan et al., 1974; Elo & Ylitalo, 1977, 1979).
7.3.1.2. Effects on food and water consumption, and on body weight
Relatively high concentrations of 2,4-D or its derivatives in
the diet or drinking water, or given by capsule, gavage, or
parenterally, may cause a reduction in food and water consumption,
and weight loss or reduced body weight gain in rats (Rowe & Hymas,
1954; Thomssen, 1958; Björklund & Erne, 1966; Chang et al., 1974;
Chen et al., 1981; Gorshkov, 1972), as well as in dogs (Bucher,
1946; Drill & Hiratzka, 1953), in pigs (Strach & Bohosiewicz, 1964;
Björklund & Erne, l966), in rabbits (Loktionov et al., 1973), in
cattle (Rowe & Hymas, 1954; Björklund & Erne, 1966; Palmer &
Radeleff, 1969; McLennan, 1974), and sheep (Palmer & Radeleff,
1969). However, the same authors, as well as Guseva (1956) and
Hansen et al. (1971) did not observe these effects at low doses or
low dietary concentrations of 2,4-D. In fact, in the studies by
Raoul & Marnay (1948) and Strach & Bohosiewicz (1964) on rats and
piglets fed low dietary concentrations of 2,4-D, an unimpaired
appetite and an improved body weight gain was seen in some groups
of animals. In these studies, the dosages ranged between 15 and
119 mg 2,4-D/kg body weight.
Observations of Thomssen (1958), Strach & Bohosiewicz (1964),
Martynov (1970), Gorshkov (1972), Sadykov et al. (1972), and Erne
(1974), on hares (Lepus timidus), European elk, pigs, and rats
suggest that, given a choice, animals will refuse to eat food or to
drink water containing more than a certain amount of 2,4-D, and
that this may, in part, be because of the strong characteristic
odour and taste of this compound, or of organic solvents such as
diesel fuel that are used in herbicide formulations or as diluents.
7.3.1.3. Effects on the central nervous system (CNS)
It has been suggested that the signs of central nervous system
depression in animals severely poisoned with 2,4-D are related to a
partial breakdown of the blood-brain barrier, possibly as a result
of damage to capillary vessels, and a subsequent accumulation of
2,4-D in the CNS (Desi & Sos, 1962a,b; Elo & Ylitalo, 1977, 1979).
However, further studies are needed to clarify the mechanism(s)
by which 2,4-D acts on the central nervous system.
7.3.1.4. Effects on the peripheral nervous system
No peripheral neuropathy was attributed to 2,4-D in the
available reports on short-term and long-term studies with 2,4-D in
a variety of animals (Shillinger & Naumova, 1957; Stupnikov, 1959).
The partial or complete paralysis, especially of the hind legs, of
2,4-D poisoned animals reported already by Bucher (1946) and Hill &
Carlisle (1947) may be a myotoxic rather than a neurotoxic effect
of 2,4-D. Moreover, in severe 2,4-D poisoning, a general weakness
may lead to inactivity that might be interpreted as paralysis.
No signs of neuropathy were reported by Kay et al. (1965) in
rabbits given large percutaneous doses of 2,4-D dimethylamine salt,
2,4-D butyl ester, or 2,4-D isooctyl ester. In similar studies in
which rats or rabbits were exposed by the dermal route, Buslovich
(1963) reported myotonia and death in rats given unspecified doses
of 2,4-D amine salt or 2,4-D butyl ester, but no peripheral
neuropathy, while Vinokurova (1960) found 130 - 180 mg of a 50%
aqueous emulsion of 2,4-D octyl ester/kg body weight to have "no
systemic effect" on rabbits.
The neurotoxic effects in animals of 2,4-D and of other related
compounds have not been adequately studied. Further research is
required to elucidate the mechanism of the neurotoxic and myotoxic
action of 2,4-D in animals.
7.3.1.5. Myotoxic effects
Most of the studies of the effects of 2,4-D on vertebrate
muscles were carried out because the 2,4-D-induced abnormalities
resemble a heritable muscle disorder in human beings, namely
myotonia congenita (Hofmann et al., 1966; Laskowski & Dettbarn,
1977). As a rule, high doses of 2,4-D (1/4 to 1/2 LD50) are
required to obtain severe and prolonged myotonia. The effects of
2,4-D on muscle cells are complex, and include disturbances in:
the activity of various enzymes (leading, for example, to increased
lactate production); potassium levels; membrane resistance; and in
chloride conductance. There are also shifts in calcium-binding
sites, changes in muscle and nerve cell electrical potentials, and
mitochondrial structural and ultrastructural degenerative changes
in muscle (Eyzaguirre et al., 1948; Kuhn & Stein, 1964, 1965, 1966;
Heene 1966, 1968, 1975; Hofmann et al., 1966; Kenigsberg, 1968;
Stein & Kuhn, 1968; Bodem et al., 1971; Preiss & Rossner, 1971;
Seiler, 1971; Buslovich & Koldobskaya, 1972; Rüdiger et al., 1972;
Senges & Rüdel, 1972; Brody, 1973; Danon et al., 1976; Iyer et al.,
1976; Bretag & Caputo, 1978; Dux et al. 1978; De Reuck et al.,
1979; Eberstein & Goodgold, 1979; Mazarean et al., 1979a,b).
Similar effects are produced by the chlorophenoxy drug clofibrate
(atromid) (Pierides et al., 1975; Smals et al., 1977). None of the
available studies on 2,4-D-induced myotonia were designed to
establish no-observed-adverse-effect levels for the various
myotoxic effects in intact animals, and therefore additional
studies should be carried out for this purpose.
7.3.1.6. Cardiovascular effects
As part of its myotoxic action, 2,4-D and its derivatives may
in high doses cause biochemical, physiological, and structural
damage to the myocardium in vitro and in vivo (Bodem et al., 1971;
Preiss & Rossner, 1971; Rüdiger et al., 1972; Mazarean et al.,
1979a,b).
7.3.1.7. Haematological effects
Shifts have been reported in the number or types of
erythrocytes, leukocytes, or bone marrow cells, or changes in
haemoglobin levels, in a variety of laboratory and domestic mammals
given 2,4-D or 2,4-D derivatives (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Hansen et al., 1971;
Sadykov et al., 1972; Loktionov et al., 1973; Kosyan et al., 1974;
and Halliop et al., 1980).
7.3.1.8. Effects on blood chemistry
Rowe & Hymas (1954) were apparently the first investigators to
monitor blood chemistry in 2,4-D-treated animals. They did not
observe any adverse effects at 2,4-D dietary levels as high as 300
mg 2,4-D/kg in rats treated for 113 days.
Other investigators noted changes in various serum, plasma, or
erythrocyte enzyme activity levels, and shifts in the levels of
electrolytes, glucose, blood proteins, or other chemicals in
response to treatment with 2,4-D or 2,4-D derivatives in rats,
rabbits, cattle, pigs, or sheep, or in blood from such animals to
which 2,4-D was added in vitro (Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Shevchenko, 1966;
Dzhaparov & Tsilikov, 1969; Hunt et al., 1970; Szöcs et al., 1970;
Gorshkov, 1972; Kosyan et al., 1974; Kuzminskaya & Bersan, 1975;
Chen et al., 1981). Some of the changes in transaminase (SGOT,
SGPT), blood urea nitrogen (BUN), or glucose levels appeared to be
secondary to myotoxic, nephrotoxic, or hepatotoxic effects of high
doses of 2,4-D.
7.3.1.9. Other biochemical effects observed in vivo or in vitro
Sufficiently high doses of 2,4-D may induce changes in
mitochondrial oxygen consumption and oxidative phosphorylation, in
electrolyte, ascorbic acid, glycogen, lipid, and nucleic acid
contents of organs, tissues, cells, organelles, or cell fractions
from a variety of mammals (Brody, 1952, 1973; Guseva, 1956; Baker
et al., 1960; Kuhn & Stein, 1964; Heene, 1966, 1967, 1968;
Shevchenko, 1966; Philleo & Fang, 1967; Kenigsberg, 1968; Dzhaparov
& Tsilikov, 1969; Stanosz, 1969; Buslovich & Koldobskaya, 1972;
Graff et al., 1972; Buslovich et al., 1973; Abo-khatwa &
Hollingworth, 1974; Chang et al., 1974; Nikandrov, 1974; Venkaiah &
Patwardhan, 1978; Podolak, 1979).
7.3.1.10. Pulmonary effects
No pulmonary abnormalities were reported in studies with mice,
rabbits, rats, or dogs conducted by Bucher (1946), Drill & Hiratzka
(1953), Rowe & Hymas (1954), Shillinger & Naumova (1957),
Schillinger (1960), or Björklund & Erne (1966). In contrast, Hill
& Carlisle (1947) and Palmer (1972) noted congestion of pulmonary
vessels, pulmonary petechial haemorrhages, and pulmonary edema or
emphysema in mice, rats, dogs, cattle, or sheep that died of 2,4-D
poisoning.
7.3.1.11. Hepatotoxic effects
Rabbits, rats, mice, dogs, cattle, or sheep treated for a
prolonged period with toxic doses of 2,4-D were found to develop a
subacute toxic hepatitis with congestion of hepatic blood vessels,
and cloudy swelling, fatty infiltration, local necrosis,
degeneration, or atrophy of hepatocytes, especially of the
parenchyma in the centrolobular areas (Bucher, 1946; Hill &
Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Björklund & Erne, 1966; Szöcs et al., 1970; Palmer, 1972). High
doses of 2,4-D may induce a proliferation of peroxisomes and
increased levels of mixed function oxidases in liver cells of rats
and hamsters (Buslovich et al., 1982; Vainio et al., 1982, 1983).
Changes in the levels of certain liver enzymes such as maleate
or succinic dehydrogenase, and in ascorbic acid or glycogen content
or hippuric acid production have also been reported (Shillinger &
Naumova, 1957; Baker et al., 1960; Dzhaparov & Tsilikov, 1969;
Szöcs et al., 1970; Buslovich et al., 1973; Chang et al. 1974;
Nikandrov, 1974). Some of the results concerning liver glycogen
levels in rats suggest a reverse trend at high doses, as Dzhaparov
& Tsilikov (1969) found that a dose equivalent to 1/16 LD50 per day
lowered the liver glycogen content, while Chang et al. (1974)
observed the opposite effect at doses of about 100 mg/rat per day.
7.3.1.12. Effects on the kidney
In early studies with high doses of 2,4-D, signs of impaired
kidney function, increased relative kidney weight, and of gross and
histological abnormalities (parenchymatous degeneration,
hypertrophy, and hyperplasia, cloudy swelling especially in the
cells of the proximal convoluted tubules, and glomerular lesions)
were noted in mice, rats, and dogs (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954). That the kidney
is a target organ for the structural, physiological, and chemical
effects of 2,4-D was confirmed repeatedly by later and more
detailed studies on a wider range of test species including pigs,
goats, and sheep (Schillinger, 1960; Björklund & Erne, 1966; Erne,
1966a; Stanosz, 1969; Hunt et al., 1970; Milhaud et al., 1970;
Gorshkov, 1972; Palmer, 1972; Senczuk & Pogorzelska, 1975; Koschier
et al., 1978; Orberg, 1980a). In a recent 13-week study on rats,
the no-observed-adverse-effect level for histological changes
induced with pure 2,4-D in mammalian kidney appeared to be 15 mg/kg
body weight per day (Chen et al., 1981).
7.3.1.13. Effects on endocrine organs
Swelling and congestion of the thyroid were noted by Palmer
(1972) in cattle and sheep fatally poisoned with various 2,4-D
products. Effects of 2,4-D on iodide uptake by the thyroid gland
were first decribed by Sos & Kertai (1958) and studied further by
Florsheim & Velcoff (1962), Florsheim et al. (1963), Tsilikov
(1969), and Gorshkov (1972). Their results indicate that doses of
5 - 250 mg 2,4-D sodium salt or equivalent/kg body weight per day
have a stimulatory effect on thyroid function.
Effects of 2,4-D on adrenal function and its relationship to
muscle carbohydrate metabolism and 2,4-D-induced myotonia have been
studied by Kenigsberg (1968), and by Buslovich & Koldobskaya
(1972). 2,4-D-induced changes in adrenal or thyroid function may
also be implicated in the abnormal temperature regulation in 2,4-D-
treated rats described by Sudak et al. (1966). However, it is
noteworthy that Buslovich (1963) was unable to induce a change in
the body temperature of rats by giving 15 - 20 oral doses of 2,4-D
sodium salt, amine salt, or butyl ester at a level of 1/10 or 1/5
LD50/day.
7.3.1.14. Effects on the digestive tract
Vomiting, diarrhoea, hyperaemia, bloody exudates in the gut,
necrotic changes in the mucosa, and other non-acute toxic effects
on the digestive tract have been reported after administration of
high doses of 2,4-D by either the oral or parenteral route in mice,
rats, and dogs (Bucher, 1946; Hill & Carlisle, 1947; Drill &
Hiratzka, 1953; Kosyan et al., 1974). However, Hansen et al.
(1971) did not observe any such effects in a 2-year feeding study
at dietary levels of 2,4-D corresponding to about 37.5 and 67.5 mg
2,4-D/kg body weight per day, respectively, for rats and dogs.
Their observations on dogs contrast with those of Drill & Hiratzka
(1953) who found repeated doses of 20 mg 2,4-D/kg body weight per
day to be fatal in 3 out of 4 dogs studied by them.
7.3.2. Birds
The published reports on the toxic effects of 2,4-D and 2,4-D
products in birds deal mainly with chickens (Gallus domesticus)
and game birds. The available data on cumulative oral lethal doses
for game birds have been summarized in Table 18; studies on the
reproductive, embryotoxic, and teratogenic effects of 2,4-D are
dealt with in section 7.3.6.
Table 18. Cumulative oral lethal doses of 2,4-D herbicides for game birds
------------------------------------------------------------------------------------
Product Species Dietary LC50 Cumulative LD50 References
(mg a.i./kg (mg a.i./kg bw)a
diet)a
------------------------------------------------------------------------------------
2,4-D quail (young) 5000 28 000 Dewitt et al.
dimethylamine (1962)
salt
mallard duck 5000 8250
(young) 5000 8250
(adult) > 2500 > 34 000
2,4-D quail
butoxyethanol (young) 5000 38 000
ester (adult) 5000 40 700
pheasant (young) 5000 29 500
mallard duck (adult) 5000 > 33 000
------------------------------------------------------------------------------------
a a.i. = active ingredient
The clinical signs of 2,4-D poisoning in birds appear to be
similar to those in mammals, and the kidney seems to be the most
sensitive organ. No adverse effects were reported in chickens fed
dietary levels of 1000 mg/kg for 21 days (142 mg/kg body weight)
whereas kidney enlargement occurred in chickens fed 5000 mg/kg of
diet for 21 days (Whitehead & Pettigrew, 1972).
7.3.3. Cold-blooded animals
The literature on the chronic effects of 2,4-D and its
derivatives on cold-blooded animals (poikilotherms) was not
reviewed in detail. The available reports indicate that for
aquatic vertebrates in general, 2,4-D esters are more toxic than
2,4-D amines, and that the overall no-observed-adverse-effect level
for toxic effects on fish for the esters is at or near 1 mg/litre
(King & Penfound, 1946; Zhiteneva & Chesnokova, 1973; Fabacher &
Chambers, 1974; Meehan et al., 1974). 2,4-dichlorophenol, which
may occur in water as a by-product or transformation product of
2,4-D, is similarly toxic to fish (Holcombe et al., 1980).
7.4. Fetotoxicity, Teratogenicity, and Reproductive Effects
The scientific literature contains a fairly large number of
reports on the fetotoxic, teratogenic, and reproductive effects of
2,4-D in livestock and in laboratory animals. However, most of the
observations on livestock are either too sketchy to be useful or
neglect to take into account various confounding factors. Examples
of this are studies by Björklund & Erne (1966) on a single pregnant
pig, by Sadykov et al. (1972) on sheep grazing on pastures
apparently containing a high percentage of poisonous plants, and
the report by Bodai et al., (1974), on reproductive disturbances in
cattle feeding on either 2,4-D-treated vegetation possibly
including poisonous plants, or on contaminated silage.
Some of the studies carried out with rodents under laboratory
conditions also provide little useful information, either because
it is difficult or impossible to determine the dose levels used in
the studies (Weinmann, 1957; Schuphan, 1963, 1965, 1969; Schiller,
1964; Buslovich et al., 1976); or because the information provided
is insufficient and the studies cannot be properly evaluated
(Bucher, 1946; Hansen et al., 1971; King et al., 1971). The study
by Weinmann (1957) can be considered invalid, as a high mortality
occurred in both control and experimental animals, when they were
exposed to cold stress because of construction work affecting the
animal quarters during the winter months. Moreover, Weinmann
(1957) and several other authors, including Schuphan (1963, 1965,
1969) Schiller (1964), Schillinger (1960), and Shillinger & Naumova
(1957) did not in fact examine the effects of 2,4-D, but rather the
effects of foods or food extracts prepared from crops that had been
sprayed with 2,4-D, and, in some cases, also with other plant
growth substances. As none of these authors appears to have
carried out an analysis to demonstrate the presence of 2,4-D
residues in the test material, it is questionable whether the test
animals ingested any 2,4-D or 2,4-D residues, and these studies are
therefore not reviewed in detail. Buslovich et al. (1976) tested
only a single dose level (1/2 LD50) and this reduces the usefulness
of their study. Pertinent reports are discussed below.
7.4.1. Rats
7.4.1.1. Effects on adult rats
No deleterious effects on the health or fertility of rats
receiving the maximum tolerated dose of 87.5 mg/kg body weight in
terms of 2,4-D or its molar equivalent of the isooctyl ester or the
propylene glycol butyl ether ester per day, on days 6 - 15 of
pregnancy, were reported by Schwetz et al. (1971) and Unger et al.
(1980). Even higher amounts of 2,4-D or its equivalent in 2,4-D
derivatives were used by Björklund & Erne (1966), Hansen et al.,
(1971) and Khera & McKinley (1972) with similar results.
Reduced testis and prostate size, abnormal spermatogenesis (and
also liver and kidney damage) were reported by Schillinger (1960)
in some of the male rats given 375 mg/kg body weight per day (about
1/4 LD50) of a Soviet-made 2,4-D butyl ether formulation containing
polyethylene glycol alkyl phenyl ether surfactant. These effects
were not noted at 1/10 of this dose level, i.e., at 37.5 mg/kg body
weight per day. Some of the toxic effects reported by Schillinger
(1960) may have been caused by the surfactant, but as a surfactant
control group was not included in this study, this cannot be
confirmed. Thus, the available studies suggest that the no-
observed-adverse-effect level for reproducing adult rats lies
between 37.5 and 87.5 mg 2,4-D/kg body weight per day.
7.4.1.2. Effects on offspring
Björklund & Erne (1966) gave pregnant rats a 2,4-D
concentration in drinking-water of 1000 mg/litre during pregnancy,
and for the following 10 months. No effects on reproduction were
noted.
Hansen et al. (1971) fed male and female rats dietary levels of
technical 2,4-D of 100, 500, and 1500 mg/kg (ppm), and the rats
were bred through 3 successive generations. At dietary levels of
100 and 500 mg/kg, no effects were noted. However, at a dietary
level of 1500 mg/kg, survival of pups to weaning was reduced. The
number of pups surviving ranged from 70 - 97% in the control group
and from 60 - 93% at the 100 and 500 mg/kg (ppm) dietary levels;
survival at the highest dose ranged from 20 - 62%.
Resorptions, reduced fetal weight and size, enlarged ventricles
of the brain and haemoperitoneum were found by Buslovich et al.
(1976) in the offspring of rats treated with two different 2,4-D
derivatives at a dose of one-half LD50 (value unspecified).
Schwetz et al. (1971) dosed female rats from day 6 - 15 of
pregnancy by gavage at dose levels of 0, 12.5, 25, 50, and 87.5
mg/kg body weight per day with 2,4-D or equimolar dose levels of
the propylene glycol butyl ether (PGBE) and the isooctyl esters
(IO). At doses of 50 and 87.5 mg/kg, a decrease in fetal body
weight was noted for all three compounds.
Subcutaneous oedema, delayed ossification of sternebrae,
sternebrae with split centres of ossification, wavy ribs, and
lumbar ribs increased with increasing doses, for at least one of
the agents studied. However, these anomalies were not
significantly increased at the 12.5 or 25 mg/kg dose level for the
3 compounds. A small but significant increase in the incidence of
subcutaneous oedema was observed in fetuses from dams receiving
12.5 mg/kg molar equivalent of IO. The incidence of missing
sternebrae was significantly increased at dose levels of 87.5 mg/kg
for 2,4-D and 12.5 and 87.5 mg/kg for PGBE.
Unger et al. (1980), in a later study, using the same dosing
regimens for 2,4-D IO and 2,4-D PGBE did not observe the effects
reported by Schwetz et al., except for a statistically significant
increase in rib buds at the highest dose (87.5 mg/kg) tested for
both compounds ( P < 0.05).
Khera & McKinley (1972) dosed female rats from day 6 - 15 of
pregnancy by gavage with 2,4-D, 2,4-D IO, 2,4-D butyl ester, 2,4-D
butoxyethanol ester and 2,4-D dimethylamine salt. The butyl and
isooctyl ester depressed fetal weight and decreased fetal viability
at the highest dose of 150 mg/kg body weight. Wavy ribs,
additional ribs, retarded ossification and sternal defects, fused
ribs, small-sized distorted scapula and micromelia were observed as
anomalies among the treated groups. A statistically significant
increase in malformed fetuses was noted at 2,4-D levels of 25 mg/kg
body weight ( P < 0.05) and at levels of 150 mg/kg or more for the
other compounds.
Konstantinova et al. (1975) reported hemorrhage into internal
organs in the fetuses of rats treated with a 2,4-D level of 50
mg/kg body weight.
The results of all these studies suggest that dosage levels of
less than 12.5 mg/kg body weight for the various 2,4-D derivatives
do not cause fetotoxic or teratological effects in rats, and the
results of the more recent study by Unger et al. (1980) indicate
that higher doses may be without deleterious effects on the
fetuses.
Thus, at present, a daily dose level of 10 mg 2,4-D or 2,4-D
acid equivalent/kg body weight can be considered to be without
significant fetotoxic or teratogenic effects in rats.
7.4.2. Mice
The report by Courtney (1977) indicated that in CD-1 mice,
doses of 1 mM/kg body weight of 2,4-D and its n-butyl and PGBE
esters reduced fetal body weight, and increased fetal mortality.
The compounds were also teratogenic, inducing cleft palates, at
levels of 124 mg/kg body weight per day (2,4-D or acid equivalent)
or more. However, the 2,4-D isopropyl and isooctyl esters appeared
to be less teratogenic than 2,4-D, as they did not induce birth
defects at a 2,4-D equivalent level of 124 mg/kg body weight per
day. Furthermore, the 2 esters did not induce fetal death in CD-1
mice at doses of 2,4-D equivalent up to 221 mg/kg body weight per
day.
7.4.3. Birds
In the 1960s and 1970s, a number of tests of the embryotoxic,
teratogenic, and other reproductive effects of 2,4-D were carried
out on birds' eggs and embryos. These results may not apply to
mammals, because bird embryos develop in a closed environment
different from that of mammalian embryos, and because birds differ
anatomically from mammals.
Lutz-Ostertag & Lutz (1970, 1974) reported mortality and severe
deformities in wild bird embryos exposed to 2,4-D amine salt, but
they did not provide crucial experimental details, and other
investigators (Dunachie & Fletcher, 1967, 1970; Kopischke, 1972;
Grolleau et al., 1974; Gyrd-Hansen & Dalgaard-Mikkelsen, 1974;
Somers et al., 1974a,b,c; Hilbig et al., 1976a,b; Spittler, 1976)
were unable to duplicate their results either with 2,4-D amine
salts or esters. Some of the teratogenic effects attributed to
2,4-D by Lutz-Ostertag & Lutz (1970, 1974) resemble those induced
by excessively high incubation temperatures (Nielsen, 1968), and
may thus have been experimental artifacts.
On the whole, the available studies on bird embryos indicate
that the no-observed-adverse-effect level for 2,4-D-induced
embryotoxic and teratogenic effects lies near 0.5 mg active
ingredient/egg (equivalent to about 10 mg/kg) and is thus similar
to that in mammals.
7.4.4. Cold-blooded animals
The available literature contained little information
concerning the possible reproductive, embryotoxic, or teratogenic
effects of 2,4-D or 2,4-D derivatives on cold-blooded animals.
7.4.4.1. Amphibians
Lopez (1961) noted that the motility of frog spermatozoa was
not affected by low concentrations of 2,4-D or its sodium salt, and
that the inhibition of movement, or the lysis observed under some
conditions were caused by changes in the pH of the test solution.
Aqueous solutions of less than 0.05% 2,4-D sodium salt did not
induce any macroscopic abnormalities in developing frog eggs or
embryos (Lhoste & Roth, 1946), while Cooke (1972) did not find
either toxic effects or 2,4-D residues in frog tadpoles exposed for
1 or 2 days to up to 50 mg 2,4-D/litre. According to Sanders
(1970a) a commercial 2,4-D dimethylamine salt had an LC50 of 100
mg/litre for frog tadpoles.
7.4.4.2. Fish
Only three brief reports were available on the effects of 2,4-D
on developing fish eggs and embryos. Andrusaitis (1972) found that
2,4-D reduced the oxygen consumption of 32-cell blastomeres, and
increased the oxygen consumption in 64 - 128 cell blastomeres of
Misgurnus fossilis. In a study by Mount & Stephan (1967), 2,4-D
butoxyethanol ester (BEE) at a concentration of up to 0.31
mg/litre, or 2,4-D at 0.80 mg/litre did not reduce the reproduction
rate in fathead minnows (Pimephales promelas), whereas 2,4-D BEE
at 1.5 mg/litre killed minnow eggs in 48 h. Rehwoldt et al. (1977)
similarly found that 0.1 mg 2,4-D/litre did not have any adverse
effect on reproduction in guppies.
These reports suggest that the no-observed-adverse-effect level
of 2,4-D BEE for the reproductive or teratogenic effects of 2,4-D
in fish may be about 1 mg a.i./litre water.
7.5. Mutagenicity and Related Effects
7.5.1. 2,4-D and its derivatives
Studies on the mutagenicity of 2,4-D and its derivatives have
been reviewed by Andersen et al. (1972), National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), Ramel (1978), Seiler (1978),
Vachkova-Petrova (1978), Kas'yanenko & Koroleva (1979), Murthy
(1979), Shearer (1980), US Veterans' Administration (1981), Waters
et al. (1981), and Linainmaa (1983).
A recent IARC Working Group (IARC, 1982) evaluated the activity
of 2,4-D and derivatives in short-term tests. It was reported that
2,4-D induced unscheduled DNA synthesis in cultured human
fibroblasts (Ahmed et al., 1977a), but not in rat hepatocytes
(Probst et al., 1981). 2,4-D was not mutagenic in bacterial
systems (Andersen et al., 1972; Sherasen et al., Zetterberg, 1977;
Moriya et al., 1983). 2,4-D was mutagenic in yeast, when tested at
low pH (Zetterberg et al., 1977), but was not active under other
conditions (Zetterberg, 1977) or in a host-mediated assay
(Zetterberg et al., 1977). Results of four studies on Drosophila
melanogaster were reported to be positive (Rasmuson & Persson-
Svahalin, 1978): results of three other studies were negative
Berin & Buslovich, 1971; (Vogel & Chandler, 1974; Magnusson et al.,
1977; Rasmuson & Svahlin, 1978).
2,4-D was reportedly mutagenic in cultured Chinese hamster
ovary (CHO) cells (Ahmed et al., 1977b), but did not induce a
statistically significant increase in sister chromatid exchanges
(SCEs) in CHO cells in vitro (Linainmaa, 1983). Chromosomal
effects have been reported in plants (Khalatkar & Bhargava, 1982).
Chromosomal aberrations or SCEs were found in cultured human
lymphocytes (Pilinskaya, 1974; Korte & Jatal, 1982), but
chromosomal aberrations were not found in cultured embryonic bovine
kidney cells (Bongso & Basrur, 1973).
In mice, single oral doses of 100 - 300 mg 2,4-D/kg body weight
reportedly induced chromosomal aberrations (Pilinskaja, 1974), but
micronuclei were not found in mice after ip injection of single
doses of 100 mg 2,4-D/kg body weight (Jenssen & Renberg, 1976).
2,4-D was not active in a dominant lethal test in mice (Epstein et
al., 1972), and did not induce SCEs in rats after oral
administration of 2,4-D amine salt at daily doses of 100 mg/kg body
weight for two weeks (Linnainmaa et al., 1983). Recent evidence
(Buslovich et al., 1982; Vainio et al., 1982) suggests that 2,4-D
may have an indirect effect on genetic material via the production
of active oxygen radicals derived from peroxisome proliferation,
which has been demonstrated in in vivo and in vitro studies in
liver cells of rats and hamsters (Reddy et al., 1980, 1982; Vainio
et al., 1982; Gray et al., 1983).
At present, available studies are inadequate to evaluate the
genetic effects of 2,4-D and its derivatives in short-term tests
(IARC, 1982). No data on cell transformation are available.
7.6. Carcinogenic Effects on Experimental Animals
7.6.1. 2,4-D and its derivatives
A number of studies have been carried out on mice and rats to
assess the potential carcinogenic effects of 2,4-D and its
derivatives. Two IARC Working Groups have reviewed these studies
(IARC, 1977, 1982) and concluded that it was not possible to make
an evaluation on the basis of the available data. 2,4-D and
several of its esters were tested by oral administration and by a
single sc injection in the newborn of 2 strains of mice (Innes et
al., 1969); 2,4-D or its amine salts were also tested in rats by
oral administration (Arkhipov & Kozlova, 1974; Hansen et al.,
1971).
Nearly 20 years have passed since these studies were carried
out and because of basic flaws in their design and execution, it is
unlikely that further reviews of these studies will lead to
generally accepted conclusions. The Task Group was aware that
further long-term studies in mice and rats were in progress, and
these should prove useful in the future evaluation of the potential
carcinogenicity of 2,4-D.
7.6.2. Contaminants in 2,4-D
2,7-DCDD was tested for carcinogenicity in mice and rats fed
dietary levels of 5000 and 10 000 mg/kg for 90 weeks, followed by a
short observation period of about 1 - 10 weeks, at which time all
animals were killed. Sufficient numbers of animals survived to
evaluate the development of late-appearing tumours. Although an
increased incidence of hepatocellular adenomas and carcinomas was
observed in treated male mice (20/50 in the low dose and 17/42 in
the high dose compared with 8/49 in matched controls), it was noted
that the incidence of liver tumours in historical controls ranged
from 16 - 32%. No tumorigenic effects were found in rats (US
National Cancer Institute, 1979).
8. EFFECTS ON MAN, CLINICAL AND EPIDEMIOLOGICAL STUDIES
The available clinical and epidemiological studies fall into 4
groups: (a) studies on patients treated with 2,4-D as an
anticancer drug (Apffel, 1959a) or antibiotic (Seabury, 1963), (b)
reports on acute 2,4-D poisoning due to voluntary or accidental
ingestion of herbicides (Tables 19 and 20), (c) reports on workers
(mainly men) overexposed to 2,4-D during the manufacture,
processing, or use of 2,4-D herbicides, and (d) epidemiological
studies on groups of people who were actually or potentially
exposed as a result of herbicide spray programmes, or who lived in
areas in which herbicides were used. With the exception of the
case studies of Apffel (1959a) and Seabury (1963), almost all of
the reports deal with mixed exposures to 2,4-D and other chemicals,
and therefore it is often unclear to what extent 2,4-D, its alkali
or amine salts, or its esters contributed to the effects reported
by the authors of the studies.
Much of the literature on acute poisonings and on the health
effects of occupational overexposure to 2,4-D or other
chlorophenoxy compounds or their toxic by-products has been
recently reviewed by Pocchiari et al. (1979), Bovey & Young (1980),
Huff et al. (1980), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1981),
CEC (1981), Rappe & Buser (198l), US Veterans' Administraton
(1981), Coggon & Acheson (1982), Hay (1982), IARC (1982, 1983), and
Dobrovolski (unpublished data, 1983). However, most of these
reviews concentrated on 2,4,5-T, Agent Orange, and other herbicides
used in the Vietnam war, or on industrial accidents resulting in
massive exposures to largely undefined mixtures of chlorophenols,
chlorinated dibenzodioxins, and other reaction products. In
contrast, the present review focuses mainly on 2,4-D herbicides.
In evaluating human exposure to mixtures of chemicals that
include 2,4-D and various concentrations of contaminants of 2,4-D,
it is in many instances difficult or impossible to determine
whether any of the described effects can actually be attributed to
the exposure to 2,4-D or its derivatives.
8.1. Acute Poisoning and Occupational Overexposure
Pertinent reports on acute poisoning with 2,4-D, or of the
effects of occupational overexposure to 2,4-D herbicides are
summarized or cited in Tables 19 and 20.
Signs and symptoms of acute overexposure to 2,4-D or its
derivatives occurred after ingestion or absorption of large
amounts, or where poor occupational hygiene was practised leading
to pronounced dermal absorption of the material. It is unlikely
that, with good agricultural practice, good personal protection,
and occupational hygiene, resulting in exposures to low
concentrations of 2,4-D, any of the acute symptoms and signs
reported below would be expected to occur.
Table 19. Acute toxicity of 2,4-D, fatal poisonings with herbicides containing 2,4-D
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
a) Fatal poisonings
2,4-D diethyl not stated F 50.8 kg 60-90 g 40-400 coma; death in about Curry (1962)
ester (1180-1770 mg 2 days; degeneration
(DICOTEX EXTRA) DOCOTOX/kg bw) of convoluted kidney
tubules
"2,4-D" suicide M ? ~ 125 ml ? loss of consciousness, Delarrard &
400 g (< 400 bw) coma, generalized Barbaste (1969)
a.i./litre muscular hypotonia,
loss of all reflexes,
hypotension,
hyperglycaemia,
proteinuria; death in
12 h
"pure" 2,4-D suicide M 55 kg ? 57.6-407.9 coma, myotonia, fever, Dudley &
acid in pulmonary emphysema Thapar (1972)
kerosene and edema, liver
necrosis, degeneration
of kidney tubules;
death in 6 days
"2,4-D" suicide F ? ? 20-116 loss of consciousness, Geldmacher-Van
vomiting, uterine Mallinckrodt &
bleeding, tachycardia, Lautenbach
and circulatory (1966)
failure; death in
about 30 h; edema and
congestion of brain;
fatty liver cell
changes; fatty changes
in kidney tubules;
pulmonary hyperaemia &
edema with isolated
haemorrhages
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
"2,4-D" suicide M ? "at least" stiffness in legs, Herbich &
13.5 g vomiting, loss of Machata
consciousness; death (1963)
in 14 h; hyperaemia
and edema of brain;
pulmonary edema
2,4-D suicide M 75 kg 120 ml 12.5-7700 vomiting; congestion, Nielsen et al.
dimethylamine (80 mg/kg) pulmonary emphysema; (1965)
formulation CNS congestion &
perivascular
haemorrhages, severe
degeneration of
ganglion cells; death
within hours of
ingestion
Herbicide suicide M 26 360 ml 2,4-D plasma clinical and Osterloh et al.
containing years and mecoprop 321 (1.5 h) pharmacokinetic (1983)
2,4-D, mecoprop amine salt 540.9 (21 h) study coma with
(MCPP) and (10.6%, 11.6% 480.8 (30 h) pin-point pupils
chlorpyrifos a.i.) and 360 tachycardia,
ml chlorpyrifos urine (on hypertension,
in kerosene admission): myoclonus, diarrhoea,
(6.7% a.i.) 230.3 then hypotension,
plus few cardiac arrhythmias,
granules gastric asystole, and death
Warfarin (0.025 content (on after 30 h
% a.i.) (2,4-D admission):
= 600 mg/kg bw; 108.2
mecoprop = 600
mg/kg bw) tissues
(post mortem)
brain: 186.4
blood: 389.5
liver: 293.5
heart: 301.2
kidney: 315.0
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
b) Non-fatal poisonings
"2,4-D" accidental M ? (5- ? ? drowsiness, Duric et al.
"DEHERBAN A" ingestion year unsteadiness, (1979)
herbicide old difficulties with
formulation, child) speech; dilated pupils;
400 g on fourth day, toxic
a.i./litre myocarditis with
abnormal ECG; kidney
damage indicated by
increased blood urea
levels; no liver
damage; complete
recovery within about
one month
herbicide suicide F 58 ? ? no signs of toxicity Prescott et al.
containing attempt; years on admission to (1979)
2,4-D plus ingestion of hospital with plasma
dichlorprop unspecified concentration of
(16.1% + 21.4%) amount of 335 mg 2,4-D/litre &
herbicide 400 mg mecoprop/litre;
plasma clearance
t = 143 h for
2,4-D vs 95 h for
dichlorprop; 91% of
ingested 2,4-D and
70% of ingested
dichlorprop excreted
unchanged; metabolites
not identified
----------------------------------------------------------------------------------------------------------------------- not identified
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
herbicide suicide F 30.2 kg 100 ml ? pharmacokinetic study Rivers et al.
containing attempt herbicide of 2,4-D/dicamba (1970)
salts of 2,4-D (13.6 g 2,4-D) excretion; no Young & Haley
(20.1%) and information on toxic (1977)
dicamba (1.9%) effects; excretion of
2,4-D initially slowed
by competitive
excretion of dicamba;
small amounts of 2,4-D
still excreted 3 weeks
after ingestion; only
52% of ingested 2,4-D
excreted in urine;
rest assumed to have
been excreted by
faecal route; plasma
clearance t = 59.2 h
initially, and 16.7 h
after most of the
dicamba was excreted
-----------------------------------------------------------------------------------------------------------------------
Table 20. Acute or non-acute effects attributed to occupational or bystander overexposure to 2,4-D
herbicide
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Central i) unconsciousness Radionov et al. (1967); Paggiaro et al. (1974)
nervous ii) electroencephalograph changes Kontek et al. (1973); Andreasik et al. (1979)
system iii) subjective symptoms Assouly (1951); Goldstein et al. (1959); Monarco &
Divito (1961); Foissac-Gegoux et al. (1962);
Berkley & Magee (1963); Belomyttseva (1965);
Fetisov (1966); Radionov et al. (1967); Bashirov
(1969); SARE (1972); Andreasik et al. (1979);
Kuzyk (1979)
Peripheral i) polyneuritis Foissac-Gegoux et al. (1962); Todd (1962);
nervous Belomyttseva & Karimova (1963); Bashirov (1969)
ii) partial paralysis Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Todd (1962)
iii) functional changes Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Berkley & Magee (1963); Belomyttseva
(1967); Wallis et al. (1970); Andreasik et al.
(1979); Singer et al. (1982)
iv) subjective symptoms
Skeletal i) myotonia, myokymia, fibrillation Foissac-Gegoux et al. (1962) Berkley & Magee
muscles stiffness (1963); Wallis et al. (1970); Paggiaro et al.
(1974)
ii) muscle damage or atrophy Wallis et al. (1970)
iii) subjective symptoms Assouly (1951); Foissac-Gegoux et al. (1962); Todd
(1962); Fetisov (1966); Radionov et al. (1967);
Bashirov (1969); Wallis et al. (1970)
Digestive i) vomiting, diarrhoea Goldstein et al. (1959); Monarca & Divito (1961);
system Todd (1962); Belomyttseva (1967); Radionov et al.
(1967); Paggiaro et al. (1974); Dennis (1976)
ii) various functional disorders or Assouly (1951); Monarca & Divito (1961); Bashirov
subjective symptoms (1969); Wallis et al. (1970); Kuzyk (1979)
---------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Respiratory i) irritation coughing Assouly (1951); Belomyttseva & Karimova (1963);
system Belomyttseva (1965); Wallis et al. (1970);
Andreasik et al. (1979)
ii) functional disorders Belomyttseva & Karimova (1963); Bashirov (1969);
Wallis et al. (1970)
Circulatory i) functional changes: Belomyttseva & Karimova (1963); Belomyttseva
system - cardiac involvement (1967); Winkelmann (1960); Bashirov (1969);
- vascular involvement Paggiaro et al. (1974); Andreasik et al. (1979)
ii) haematological or chemical Monarca & Divito (1961); Todd (1962); Belomyttseva
changes (1967); Radionov et al. (1967); Long et al.
(1969); Paggiaro et al. (1974); Andreasik et al.
(1979)
iii) subjective symptoms Radionov et al. (1967); Bashirov (1969); Andreasik
et al. (1979)
Liver functional abnormalities Belomyttseva & Karimova (1963); Belomyttseva
(1967); Bashirov (1969); Andreasik et al. (1979)
Kidney functional abnormalities Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Belomyttseva (1967); Bashirov (1969);
Paggiaro et al. (1974); Andreasik et al. (1979)
Skin i) irritation or allergic reactions Belomyttseva (1967); Radionov et al. (1967);
Dennis (1976); Kuzyk (1979)
ii) desquamation Foissac-Gegoux et al. (1962)
iii) chloracne Londońo (1966)
Reproductive functional abnormalities (women) Elina et al. (1975)
system decreased libido (men) Andreasik et al. (1979)
impotence Espir et al. (1970)
---------------------------------------------------------------------------------------------------------
The observations of Apffel (1959a) and Seabury (1963) on
patients treated with 2,4-D suggest that the acute no-observed-
adverse-effect level for biological effects in human beings may be
as high as 36 mg of purified 2,4-D/kg body weight, or its
equivalent in alkali or amine salts or esters. The lack of
subjective or clinical effects in studies in which volunteers
ingested low doses (5 mg/kg body weight) of purified 2,4-D also
supports the idea that a dose of a few mg/kg body weight is
unlikely to be toxic (Khanna & Kohli, 1977; Sauerhoff et al.,
1977). However, it is less certain whether this is true also of
the less pure commercial 2,4-D products. From the point of view of
occupational and bystander safety, it is reassuring that no reports
were found of fatal poisonings following dermal exposure or
inhalation, though temporary unconsciousness and other severe acute
effects have been attributed to massive combined dermal and
inhalation exposures to 2,4-D herbicides.
Most poisonings with 2,4-D herbicides involved formulations
containing more than one toxic ingredient, including solvents,
surfactants, and other additives. The symptoms and clinical signs
therefore tended to vary with different products.
8.1.1. Neurotoxic effects of 2,4-D and related compounds
Some of the case reports cited below on poisonings with, and
occupational overexposures to, herbicides indicate that 2,4-D and
other chlorophenoxy compounds may affect both the central and
peripheral nervous systems.
8.1.1.1. Effects on the central nervous system
In addition to subjective symptoms of the central nervous
system, impaired coordination, impaired responses to external
stimuli, unconsciousness, coma, and death have been observed in
human beings mainly after absorption of lethal or nearly lethal
doses of 2,4-D. The acute effects on the central nervous system
seem to resemble those produced by alcohol, sedative drugs, or
aromatic chlorinated hydrocarbons rather than those produced by
organophosphate or carbamate neurological poisons (Geldmacher-Von
Mallinckrodt & Lautenbach, 1966; Prescott et al., 1979). Acute
cerebral demyelination occurred in a suicide who ingested a
solution of 2,4-D in kerosene (Dudley & Thapar, 1972). Severe
degeneration of brain ganglion cells was observed in another
suicide who drank a herbicide product containing 2,4-D in the form
of water-soluble dimethylamine salt (Nielsen et al., 1965). It is
therefore possible that lethal doses of chlorophenoxy compounds may
cause structural as well as functional damage to the brain.
Craniocerebral and peripheral functional nerve damage was noted
by Bezuglyi et al. (1979) in a group of women occupationally
overexposed to 2,4-D herbicides and other pesticides. Electro-
encephalographic (EEG) abnormalities were observed in tractor
drivers spraying herbicides containing 2,4-D, MCPA, or mecoprop
(Kontek et al., 1973), in "individuals exposed to" chlorophenoxy
herbicides, including 2,4-D and MCPA (Bielski & Madra, 1976) and in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979). On
the other hand, a case of neuropathy with EEG abnormalities and
flaccid quadriplegia, originally attributed to occupational
overexposure to MCPA, was diagnosed as being of viral origin
(Nayrac et al., 1958), and therefore some of the neurological
damage attributed to 2,4-D, may have been caused by viruses. There
is some evidence that 2,4-D herbicides may affect the sensory
system, as Andreasik et al. (1979) and Assouly (1951) reported
intolerance to certain odours, hypersensitivity to noise, and other
sensory abnormalities in workers producing or packaging 2,4-D
herbicides, while Fetisov (1966) reported hyposmia and other
sensory deficiencies in similarly-exposed workers.
8.1.1.2. Effects on the peripheral nervous system
"Peripheral neuropathy" and reduced peripheral nerve conduction
velocities have been reported in workers producing 2,4-D and
2,4,5-T (Poland et al., 1971; Singer et al., 1982). More than one
dozen other studies of persons overexposed to chlorophenoxy
herbicides also indicated detrimental effects of 2,4-D products on
the peripheral nervous system (Goldstein et al., 1959; Goldstein &
Brown, 1960; Foissac-Gegoux et al., 1962; Todd, 1962; Berkley &
Magee, 1963; Wallis et al., 1970; Bezuglyi et al., 1979). Long-
lasting flaccid paraparesis or quadriparesis following skin contact
with 2,4-D herbicides was reported by Goldstein et al. (1959) and
Goldstein & Brown (1960). Abnormal tendon reflexes in these or
similar cases were reported by the same authors and by Andreasik et
al. (1979), Berkley & Magee (1963), and Foissac-Gegoux et al.
(1962). Cases of sensory neuropathy attributed to the ingestion
of, or dermal exposure to, 2,4-D herbicides have also been reported
by Monarca & Divito (1961), Foissac-Gegoux et al. (1962), Todd
(1962), Wallis et al. (1970), Sare (1972), and Bezuglyi et al.
(1979). However, no signs of peripheral neuropathy were reported
by Apffel (1959a), Seabury (1963), Paggiaro et al. (1974), and
Prescott et al. (1979), in similar cases of massive exposure to
2,4-D herbicide, and in patients given relatively large amounts of
purified 2,4-D or 2,4,5-T salts or esters as drugs. One
explanation for this may be great individual differences in
susceptibility to poisoning with chlorophenoxy herbicides
(Rosenberg, 1980), as attested by the case of a 58-year-old woman
who "was fully conscious with no clinical evidence of toxicity"
after ingesting enough herbicide to allegedly attain 2,4-D and
mecoprop blood plasma concentrations of 335 and 400 mg/litre
respectively (Prescott et al., 1979). By comparison, Herbich &
Machata (1963) reported that a plasma concentration of 447 mg
2,4-D/litre caused death in a 46-year-old man.
Herbicide ingredients other than 2,4-D and related compounds
might be at least partly responsible for the observed neurotoxic
effects. In particular, organic solvents, emulsifiers, and
ethylene glycol present in herbicide formulations have been
mentioned in this connection (Goldstein & Brown, 1960; Goldwater,
1960). Solvents such as alcohols and trichloroethylene, used in
the manufacture of the active herbicide ingredients, might account
for some of the abnormalities observed in workers involved in the
production of chlorophenoxy compounds (Assouly, 1951; Bashirov,
1969; Bashirov & Ter-Bagdasarova, 1970).
Some of the reported cases of central nervous system
dysfunction or peripheral neuritis may have been merely
coincidental to the herbicide exposure, as there are many known
causes of neuropathy, such as nutritional and hereditary factors,
infectious diseases, and many toxic chemicals, including alcohol
(Freemon, 1975). Thus, alcoholism may have been a contributory
factor in one case of "2,4-D polyneuropathy" reported by Brandt
(1971).
Further studies of the possible effects of 2,4-D and other
chlorophenoxy compounds or their by-products on the human nervous
system are desirable, including studies of behavioural effects
measurable by recently-developed test batteries (Baker et al.,
1983).
8.1.2. Myotoxic effects of 2,4-D
Muscle fibrillations, myotonia, myoglobinuria, muscular
weakness and other indications of a myotoxic effect of 2,4-D have
been reported in patients treated with large doses of purified
2,4-D products by Apffel (1959a) and Seabury (1963), as well as in
cases of suicidal or accidental ingestion of 2,4-D herbicides or
following occupational overexposure (Herbich & Machata, 1963;
Berwick, 1970; Dudley & Thapar, 1972; Prescott et al., 1979). In
laboratory animals, myotonia and structural or biochemical muscle
lesions can be reliably induced by doses in excess of about 100 mg
2,4-D/kg body weight. In human beings, the threshold dose for
gross myotoxic effects certainly exceeds 5 mg/kg body weight per
day, and may be above 36 mg/kg body weight per day (Seabury, 1963;
Khanna & Kohli, 1977; Sauerhoff et al., 1977).
8.1.3. Cardiopathies and cardiovascular effects
Myocardial dystrophy, myocarditis, cardiac arrhythmias or
fibrillations, a slowed heart rate, or electrocardiographic (ECG)
changes were observed in human beings who ingested herbicides
containing 2,4-D (Duric et al., 1979) or 2,4-D and mecoprop
(Prescott et al., 1979), and also after occupational overexposure
to chlorophenoxy herbicides (Belomyttseva, 1965; Khibin et al.,
1968; Zakharov et al., 1968; Paggiaro et al., 1974; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979; Prescott et al., 1979).
However, in other cases of acute poisoning with 2,4-D herbicides,
the ECG was essentially normal (Berwick, 1970; Monarca & Divito,
1961).
Thus, further studies are desirable to determine the threshold
2,4-D doses at which electrophysiological cardiac abnormalities can
be observed, and to determine whether they result from a direct
effect on the nerve conducting system of the heart, or secondarily
from a toxic action on the myocardium.
It has been suggested that allergic reactions and an increased
sensitivity may be involved in 2,4-D-related cardiac arrhythmias
(Winkelmann, 1960; Rea, 1978).
Both hypertension and hypotension have been reported following
exposure to high doses of 2,4-D (Apffel, 1959a; Kaskevich &
Sobolieva, 1978; Bezugly et al., 1979), but no conclusions could be
drawn from these studies.
8.1.4. Haematological effects
Monarca & Divito (196l), Todd (1962), Radionov et al. (1967),
Bashirov (1969), Bashirov & Ter-Bagdarasova (1970), Brandt (1971),
Andreasik et al. (1979), and Bezuglyi et al. (1979) observed
haematological changes such as mild anaemia, bone marrow
depression, mono- or lymphocytosis or eosinophilia, changes in
erythrocyte volume or size, or methaemoglobinaemia following
ingestion of, or overexposure to 2,4-D. However, these changes may
have been due to other causes, as Apffel (1959a) did not observe
either haematological changes or effects on haematopoiesis in
patients given 0.1 - 0.3 g of purified 2,4-D per day as an
anticancer drug.
8.1.5. Blood chemistry effects
Hyperglycaemia, hypercholesterolaemia, elevated levels of blood
urea, transaminase (SGOT, SGPT), and creatine phosphokinase (CPK),
or altered blood albumin, globulin, or phospholipid levels
following acute poisoning with, or occupational overexposure to
2,4-D were reported by Bashirov (1969), Bashirov & Ter-Bagdarasova
(1970), Berwick (1970), Lukoshkina et al. (1970), Brandt (1971),
Bezuglyi et al. (1979), Duric et al. (1979) and Prescott et al.
(1979). However, Bashirov (1969) reported hypoglycaemia (< 700
mg/litre) and abnormally slow return to normal values in glucose
tolerance tests in about one-third of a group of workers producing
2,4-D. Increased activity of erythrocytic glycolytic enzymes was
found in Polish workers packaging 2,4-D sodium salt (Andreasik et
al., 1979). In one case of intentional 2,4-D poisoning, there was
hyperglycaemia (De Larrard & Barbaste, 1969), while in some cases
of 2,4-D overexposure, blood glucose abnormalities were not
observed (Goldstein et al., 1959). Apffel (1959a) never observed
hyperglycaemia in his patients, on the contrary, daily doses of
1 - 1.25 g 2,4-D led to hypoglycaemia. Thus, under some
circumstances, high doses of 2,4-D apparently can affect glucose
metabolism, and produce hypo- or hyperglycaemia. Gamble (1975)
proposed that 2,4-D might inhibit certain APT-dependent enzymes and
thus affect lipid metabolism.
8.1.6. Pulmonary effects
Pulmonary emphysema, oedema, hyperaemia and haemorrhages were
found in cases of fatal poisonings due to 2,4-D herbicide ingestion
(Herbich & Machata, 1963; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972). It is not clear whether the acute
pulmonary effects were caused by the 2,4-D preparations or by the
solvents such as kerosene or fuel oil. However, it is unlikely
that the pulmonary emphysema was caused by acute exposure to 2,4-D.
Dyspnoea or respiratory tract irritation were occasionally
reported following occupational overexposure of 2,4-D production
workers or herbicide sprayers (Assouly, 1951; Belomyttseva, 1964;
Bashirov, 1969; Bezuglyi et al., 1979).
8.1.7. Hepatotoxic effects
Liver necrosis or fatty liver cell changes were observed in 2
fatal cases following 2,4-D herbicide ingestion (Geldmacher-Von
Mallindkrodt & Lautenbach, 1966; Dudley & Thapar, 1972). In
several non-fatal 2,4-D poisonings, no biochemical evidence of
liver damage was noted, and neither Apffel (1959a) nor Seabury
(1963) reported indications of liver damage in patients treated
with up to 2.5 g/day of purified 2,4-D, salts, or esters.
Hyperbilirubinaemia, elevated urobilinogen levels, or liver
enlargement were reported in workers occupationally exposed both to
2,4-D herbicides and to other chemicals (Belomyttesva, 1964, 1965;
Bashirov, 1969; Bashirov & Ter-Bagdasarova, 1970; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979).
8.1.8. Nephrotoxic effects
Degeneration of, or fatty changes in kidney tubules, or
proteinuria, increased blood urea levels, and other indications of
a nephrotoxic effects were observed in cases of fatal or nearly
fatal herbicide ingestion (Goldstein et al., 1959; Curry, 1962;
Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Brandt, 1971;
Dudley & Thapar, 1972; Duric et al., 1979). Impaired renal
function was reported in occupationally-exposed persons by Bashirov
(1969), Bashirov & Ter-Bagdasarova (1970), Paggiaro et al. (1974),
and by Andreasik et al. (1979). On the other hand, neither Apffel
(1959a) nor Seabury (1963) reported any evidence of kidney damage
in their patients, some of whom received in excess of 2 g of pure
2,4-D per day.
8.1.9. Effects on the digestive tract
Vomiting, diarrhoea, nausea, and other indications of toxic
effects on the digestive tract were observed by Apffel (1959a) in
patients injected intramuscularly with large doses (up to 2.5 g) of
purified 2,4-D products.
The same effects have also been noted after ingestion of large
doses of 2,4-D herbicides, or after combined inhalation and dermal
overexposure (Goldstein et al., 1959; Monarca & Divito, 1961;
Nielsen et al., 1965; Tsapko, 1966; Radionov et al., 1967; Paggiaro
et al., 1974; Dennis, 1976; Kuzyk, 1979; Prescott et al., 1979).
However, no gastrointestinal symptoms were reported by volunteers
who ingested a single dose of 5 mg pure 2,4-D/kg body weight
(Khanna & Kohli, 1977; Sauerhoff et al., 1977). Thus, an intake of
more than 300 mg 2,4-D per adult appears to be required to induce
acute toxic effects on the gastrointestinal tract.
8.1.10. Effects on endocrine organs
Andreasik et al. (1979) found an impaired iodine uptake by the
thyroid, and decreased thyroxine, thyroxine clearance, and
thyroxine iodine values in workers packaging 2,4-D sodium salt.
Since these workers were exposed to a variety of chemicals, these
results need confirmation.
8.1.11. Irritative and allergenic effects
Chronic tonsillitis and paranasal sinusitis were reported in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979).
Acute eye or skin irritation, as well as skin reactions of an
allergic type, including anaphylactoid purpura (allergic angiitis)
and contact eczema have been reported in agricultural and forestry
workers following occupational exposure to 2,4-D herbicides
(Winkelmann, 1960; Radionov et al., 1967; Balo-Banga et al., 1973;
Jung & Wolf, 1977; Kuzyk, 1979). Jung & Wolf (1977) found that
exposure to the vapour of a 2,4-D/2,4,5-T formulation in diesel oil
(SELEST 100) caused an acute allergic reaction in the skin of
sensitized herbicide applicators, and that the allergic reactions
were caused by the mixture of 2,4-D/2,4,5-T esters and not by the
diesel oil.
8.2. Epidemiological Studies of the Chronic Effects of 2,4-D
Much concern has been raised about the phenoxy herbicides,
including 2,4-D, especially in relation to birth defects and cancer
in human beings.
Several episodes have also been reported in which defined
populations were exposed to mixtures of 2,4-D and 2,4,5-T, in which
the 2,4,5-T was contaminated with various amounts of 2,3,7,8-
tetrachlorodibenzodioxin (2,3,7,8-TCDD) (Bleiberg et al., 1964;
Huff et al., 1980). It is now generally accepted that chloracne
and porphyria cutanea tarda observed in these studies were caused
by exposure to 2,3,7,8-TCDD and not by exposure to 2,4,5-T or 2,4-D
(Kimbrough, 1980).
The following sections concentrate on epidemiological studies
or other related studies on human beings in which actual or
potential exposures to 2,4-D products alone or to mixtures of 2,4-D
with other chlorphenoxy herbicides were demonstrated.
8.2.1. Reproductive, fetotoxic, and teratogenic effects
Although effects on reproduction have been demonstrated in
animals with 2,4,5-T, 2,4-D and 2,3,7,8-TCDD, all of the attempts
made to determine whether human beings suffer similar effects have
been frustrated by the poor design of the studies, inadequate
determination of exposure, or inadequate information about the
background incidence of spontaneous abortions and other abnormal
reproductive outcomes, by inadequate evaluation of confounding
variables, by inadequate assessment of exposure, and by mixed
exposures (Aldred et al., 1978; Lee, 1978; Field & Kerr, 1979;
Brogan et al., 1980; Hanify, 1980; Carmelli et al., 1981). For
these reasons they are not discussed in detail in this report.
Conclusive evidence of reproductive effects caused by 2,4-D in
populations that might be exposed to chlorophenoxy herbicides is
unlikely to be obtained from new epidemiological studies on
indirectly-exposed populations living in, or adjacent to, areas in
which phenoxy herbicides are used. Doses of 2,4-D absorbed by
bystanders are far below those expected to be toxic, as shown by
occupational exposure studies with 2,4-D and 2,4,5-T herbicides
(section 5). Any effects induced by such small amounts would
probably be obscured by more potent confounding factors (Janerich,
1973; Karkinen-Jääskelainen & Saxén, 1974; Saxén et al., 1974;
Elwood & Rogers, 1975; Granroth et al., 1977, 1978; James, 1977;
Holmberg, 1979; Lappe, 1979; Schacter et al., 1979).
Additional studies on female workers occupationally exposed to
significantly higher levels of 2,4-D than bystanders would be
useful to clarify some of the uncertainties raised by past studies,
if sufficiently large cohorts could be identified.
8.3. Studies on Mutagenic Effects in Workers Exposed to 2,4-D
Lymphocytes from ten workers exposed to 2,4-D esters during the
manufacture of 2,4-D herbicides, or from 15 workers packaging 2,4-D
sodium salt, did not show any chromosome abnormalities (Johnson,
1971; Andreasik et al., 1979). Chromosome or chromatid
abnormalities in lymphoctyes from some pesticide sprayers applying
a variety of agricultural chemicals, including in some cases 2,4-D,
were observed by Yoder et al. (1973) and Crossen et al. (1978).
Högstedt et al. (1980) did not observe any significant increases in
chromosome abnormalities in workers exposed to 2,4-D and other
pesticides.
The induction of SCEs among workers occupationally exposed to
the phenoxy herbicides 2,4-D and MCPA has been recently studied.
The subjects used only 2,4-D and MCPA or mixtures of the two for
spraying, and the exposure levels were estimated by determining the
urinary 2,4-D and MCPA excretion by the workers. No dose-related
differences in the frequencies of SCEs could be found either in
relation to the exposure level or to the length of the exposure
(Linnainmaa, 1983).
Although some studies suggest that occupational exposure to
2,4-D may result in chromosome abnormalities, the results are
conflicting. Moreover, the possiblity of mixed exposure and other
confounding variables cannot be excluded in the studies with
positive results.
8.4. Carcinogenic effects
8.4.1. Epidemiological studies
In two case-control studies of soft-tissue sarcoma (Hardell &
Sundström, 1979; Eriksson et al., 1981) and one of lymphoma
(Hardell et al., 1981), exposure to phenoxyacetic acids (mainly
2,4,5-T, 2,4-D, and MCPA) was associated with approximately 5-fold
increases in the risk of soft-tissue sarcomas. Exposure to 2,4-D,
either with or without MCPA exposure, also increased relative
risks. In the study of malignant lymphomas, 7 cases and 1 control
were apparently exposed to 2,4-D only (relative risk, 19.6; 95%
confidence interval, 4.3 - 89.8).
In a different case-control study with a small number of cases
and controls, no increased risk was observed (Smith et al., 1982).
A follow-up was also carried out on 348 railroad workers
exposed for less than 45 days during the period 1957 - 72 to the
herbicides 2,4-D, 2,4,5-T, atrazine, mecoprop, dichloropropionic
acid, and amitrole (Axelson & Sundell, 1974). The authors found a
significant increase in cancer mortality and morbidity among
workers exposed to amitrole.
Axelson et al. (1980) reported a further follow-up of these
workers, up to October, 1978, accumulating 5541 person-years. The
herbicide exposure of the workers was analysed in terms of exposure
to either amitrole, or phenoxy acids, or to a combination of the
two. A 10-year lapse period from the first day of exposure was
used as the induction latency. They found 15 cases of cancer
versus 6.87 expected (relative risk, 2.2). In the cohort with
combined exposure to amitrole and phenoxy acids, 6 cases were
observed versus 1.78 expected (relative risk, 3.4); in the group
exposed to amitrole alone, 3 tumours were observed versus 1.95
expected (relative risk, 1.5); and 6 cancers were observed versus
3.14 expected (relative risk, 1.9) in the phenoxy acid-exposed
group. All cancers, as well as cancers of the stomach, occurred in
statistically-significant excesses in the cohort as a whole. In
the groups exposed to amitrol plus phenoxy acids, there was a
significant excess of all cancers. In the group exposed only to
phenoxy acids, stomach cancer occurred in significant excess (2
observed, 0.33 expected; relative risk, 6.1). No soft-tissue
sarcomas were identified, but the statistical power of this study
to detect an excess of a rare cancer was limited.
Högstedt & Westerlund (1980) conducted a restrospective
mortality study on 142 forestry workers exposed to phenoxy
pesticides and 244 unexposed forestry workers, comparing their
mortality experience with national statistics. Work supervisors,
who were more highly exposed to phenoxy herbicides than the others,
had a significantly elevated tumour mortality (5 observed, 1.4
expected). No particular tumour type predominated, and no soft-
tissue sarcomas were observed, though the authors noted that the
study had limited statistical power and was inconclusive, because
of the relatively short follow-up period.
Riihimäki et al. (1982) reported on a prospective cohort study
of 2,4-D and 2,4,5-T spray personnel which was in progress.
Because of the small number of deaths and the brief follow-up
period, no conclusions can so far be drawn from this study.
Follow-up studies on cohorts of pesticide sprayers, farmers,
and agricultural workers occupationally exposed to a variety of
chemicals, in some cases including phenoxy herbicides, have been
reviewed by IARC (1983).
Follow-up studies on groups of industrial workers exposed to
chlorophenols, 2,4,5-T, or other chlorophenoxy compounds, and to
2,3,7,8-TCDD or other dioxins, during the manufacture of
chlorophenoxy herbicides, have recently been reviewed by Huff et
al. (1980) and IARC (1983).
8.4.2. Evidence on the carcinogenicity of 2,4-D
The available studies suggest that an association exists
between mixed exposure to phenoxy herbicides, chlorinated phenols,
and chlorinated dibenzodioxins, and an increased incidence of soft
tissue sarcomas and malignant lymphomas. It is not clear, at
present, whether these findings represent true associations, and
further studies are in progress (Muir & Wagner, 1981) to clarify
this point. Since many of the tumour cases had been exposed to
combinations of phenoxy herbicides and their contaminants as well
as other chemicals, it is not known whether exposure to 2,4-D is
specifically associated with the development of soft tissue
sarcomas.
8.5. Treatment of Poisoning in Human Beings
Successfully treated cases of 2,4-D poisoning indicate that
forced alkaline diuresis is helpful in reducing the level of 2,4-D
in the blood and tissues (Young & Haley, 1977; Prescott et al.,
1979). Heart and kidney damage should be anticipated and
counteracted in cases of severe poisoning (Duric et al., 1979).
In cases of acute poisoning due to 2,4-D, the nearest Poison
Control Centre should be contacted for additional information on
symptoms and recommended treatment.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO 2,4-D
9.1. General Considerations
In areas of 2,4-D herbicide production, handling, or use, the
highest exposure will be incurred by those who are directly
involved in these processes, followed by bystanders indirectly
exposed to 2,4-D vapour, dust, or droplets, or to contaminated
vegetation, soil, or water. In these two groups, exposure will
usually be via the skin. The general population in 2,4-D-use areas
would be exposed to a lesser extent, mainly through food containing
2,4-D residues and to a lesser extent through 2,4-D residues in
water. The contribution from air is negligible. As far as the
general population is concerned, 2,4-D intake from any source, is
negligible.
9.2. Estimated Intake of 2,4-D by the Population in a 2,4-D-use Area
The total contribution from air, food, and water is estimated
to be 0.03 - 2 µg/kg body weight per day (Table 13).
9.2.1. Intake by bystanders
Given the limited data available and the many uncertainties
involved, an adequate estimate of 2,4-D intake by bystanders is not
possible at this time, but it should generally be less than that
for occupationally-exposed persons.
9.2.2. Occupational intake
Workers using 2,4-D may, on average, absorb about 0.1 mg
2,4-D/kg body weight per day. However, this level may be exceeded
if good occupational hygiene is not practiced (section 5.2).
Simple precautions against excessive exposure can reduce the amount
of 2,4-D uptake.
9.3. Safety Factors
9.3.1. Definitions
For the present assessment, the safety factor is defined as the
integer obtained by dividing the overall no-observed-adverse-effect
level for a known adverse effect of 2,4-D (determined from all
available information on human beings or animals) by the daily
exposure value (absorbed dose of 2,4-D) for the various exposed
groups.
9.3.2. Determination of safety factors
9.3.2.1. Acute poisoning
Based on clinical studies in which 2,4-D was injected into
patients as a drug, the no-observed-adverse-effect level for signs
and symptoms of acute 2,4-D poisoning in children and adults
appears to be at or near 36 mg/kg body weight (section 8.1). Based
on the available studies of the amounts of 2,4-D absorbed by
occupationally-exposed person, bystanders, and populations in
2,4-D-use areas, the safety factors for acute 2,4-D poisoning are
likely to be:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) at least 360 for occupationally-exposed spraying crews.
The margin of safety for persons with excessive occupational
exposures would be smaller.
9.3.2.2. Chronic toxicity
Dose-effect relationships for the chronic toxic effects of
2,4-D or 2,4-D derivatives are available only from animal studies.
The no-observed-adverse effect levels for certain chronic toxic
effects of 2,4-D in animals have not been firmly established, and
for this reason safety factors cannot be established (section
7.2.1) for all of the chronic effects of 2,4-D.
9.3.2.3. Embryotoxic, fetotoxic, and teratogenic effects
The no-observed-adverse-effect level for embryotoxic,
fetotoxic, or teratogenic effects of 2,4-D in mammals appears to
lie at 10 mg/kg body weight per day (section 7.3.1.2). Assuming
that the same is true for human beings, then the corresponding
safety factors for the various exposed groups are:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) 100 for occupationally-exposed spraying crews using
precautions against excessive exposure.
9.3.2.4. Mutagenic effects
The available information was inadequate for an assessment of
the mutagenic potential of 2,4-D in mammals.
9.3.2.5. Carcinogenic effects
Available animal bioassays and epidemiological studies are
inadequate for an assessment of the carcinogenic potential of 2,4-D
or of its derivatives.
9.4. Evaluation of Health Risks from 2,4-D Exposure
From the data available at present, the Task Group assumes that
a possible health risk will exist, when the safety factor is less
than 100.
9.5. Recommendations on Exposure
Results of recent exposure and occupational health studies
suggest that excessive exposure to 2,4-D can be avoided by fairly
simple measures of occupational hygiene, such as those recommended
in two pertinent publications of the International Labour Office
(ILO, 1977, 1979). Laundering procedures for 2,4-D-contaminated
clothing have been published by Easley et al. (1983), and these
should be considered.
REFERENCES
ABO-KHATWA, N. & HOLLINGWORTH, R.M. (1974) Pesticidal chemicals
affecting some energy linked functions of rat mitochondria in
vitro. Bull. environ. Contam. Toxicol., 12(4): 446-454.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-induced
RNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide-induced
ouabain resistant mutants in Chinese hamster V79 cells. Chem.-biol.
Interact., 19: 369-374.
AKESSON, N.B. & YATES, W.E. (196l) Drift residues on alfalfa hay.
A progress report of investigations of pesticide drift from
aircraft applications. Calif. Agric., 15: 4-7.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous
substances. Int. Pest Control, 11(2): 29-35.
ALDRED, J.E., BELCHER, R.S., CHRISTOPHERS, A.J., CLEMENTS, A.,
DANKS, D.M., FRENCH, E.L., GALLOWAY, D.B., GARSON, O.M., PARSONS,
W., RAPER, C., ROUCH, G., & SINN, H.J. (1978) Report of the
Consultative Council on Congenital Abnormalities in the Yarram
District. Alleged relationship of congenital abnormalities to the
use of the herbicides 2,4-D and 2,4,5-T. State of Victoria,
Australia, Ministry of Health, viii + 55 pp (Document PB 338).
ALTOM, J.D. & STRITZKE, J.F. (1973) Degradation of dicamba,
picloram and familiar phenoxy herbicides in soils. Weed Sci., 21:
556-560.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20(3): 649-656.
ANDREASIK, Z., KOLON, S., & SMOLIK, R. (1979) Health status
evaluation in workers packaging the herbicide "Pielik"
(cholorophenol). Arh. Hig. Rad. Toksikol., 30 (Suppl.): 599-602.
ANDRUSHAITIS G.P. (1972) [The effect of pesticides on the
environment.] Latvi. PSR Zinatnu Akad. Vest., 4:(297) 44-49 (in
Russian, Health & Welfare Canada Translation No. 2441).
ANTONENKO, T.A. (1977) [Experimental data from a study of the
permeability of the placental barrier to the herbicide 2,4-D and
its passage with the mother's milk during feeding.] Gig. Aspekt.
Okhr. Zdor. Naseleniya (Saratov). In: Saratov, Saratov Institute
for Oral Hygiene, pp. 177-178 (in Russian, Health & Welfare Canada
Translation No. 2370).
APFFEL, C.A. (1959a) Action cytostatique de certaines auxines,
compte rendu préliminaire. Presse med., 67(6): 207-209.
ARJMAND, M., HAMILTON, R.H., & MUMMA, R.O. (1978) Separation of
amino acid conjugates of 2,4-dichlorophenoxyacetic acid by high-
pressure liquid chromatography employing ion-pair techniques.
J. agric. food Chem., 26: 971-973.
ARKHIPOV, G.N. & KOZLOVA, I.N. (1974) [A study of the
carcinogenic potential of a herbicide: 2,4-D amine salt.] Vopr.
Pit., 5: 83-84 (in Russian).
ASSOULY, M. (1951) Désherbants sélectifs et substances de
croissance aperēu technique. Effet pathologiques sur l'homme au
cours de la fabrication de l'ester du 2,4-D. Arch. Mal. prof. Hyg.
Toxicol. Ind., 12(1): 26-30.
AVERITT, W.K. (1967) An evaluation of the persistence of 2,4-D
amine in surface waters in the state of Louisiana. Proc. South.
Weed Sci. Soc., 20: 342-347.
AXELSON, O. & SUNDELL, L. (1974) Herbicide exposure, mortality
and tumor incidence. An epidemiological investigation on Swedish
railroad workers. Scand. J. Work Environ. Health, 11:21-28.
AXELSON, O., SUNDELL, L., ANDERSSON, K., EDLING, C., HOGSTEDT, C.,
& KLING, H. (1980) Herbicide exposure and tumor mortality: An
updated epidemiological investigation on Swedish railroad workers.
Scand. J. Work Environ. Health, 6: 73-79.
BACHE, C.A., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1964a)
Absence of phenoxyacid herbicide residues in the milk of dairy cows
at high feeding levels. J. dairy Sci., 47(3): 298-299.
BAKER, B.R., LEE, W.W., SKINNER, W.A., MARTINEZ, A.P., & TONG, E.
(1960) Potential anticancer agents. I. Non-classical
antimetabolites. II. Some factors in the design of exo-alkylating
enzyme inhibitors, particularly of lactic dehydrogenase. J. med.
pharm. Chem., 2(6): 633-657.
BAKER, E.L., FELDMAN, R.G., WHITE, R.F., HARLEY, J.P., DINSE, G.E.,
& BERKEY, C.S. (1983) Monitoring neurotoxins in industry:
Development of a neuro-behavioral test battery. J. occup. Med.,
25(2): 125-130.
BAKER, P.G., HOODLESS, R.A., & TYLER, J.F.C. (1981) A review of
methods for the determination of polychlorodibenzo-p-dioxins and
polychlorodibenzofurans in phenoxyalkanoic acid herbicides.
Pestic. Sci., 12: 294-304.
BALO-BANGA, M.J., ANTONI, F., ALTMANN, H., VINCZE, I., & KOCSIS, F.
(1973) Effect of 2,4-D on semiconservative and DNA repair
synthesis. (Case report: bullous dermatitis after exposure to
2,4-D). Ber. Oesterr. Studienges Atomenerg. (Forschungszentrum
Seibersdorf), 2218: 1-7.
BAMESBERGER, W.L. & ADAMS, D.F. (1966) An atmospheric survey for
aerosol and gaseous 2,4-D components. Adv. Chem. Ser., 60:
219-227.
BARTLEY, T.R. & HATTRUP, A.R. (1970) 2,4-D contamination and
persistence in irrigation water. Proc. West. Soc. Weed Sci.,
23: 10-33.
BASHIROV, A.A. (1969) [The health of workers involved in the
production of amine and butyl 2,4-D herbicides.] Vrach. Delo,
10: 92-95 (in Russian, Health & Welfare Canada Translation No.
2181).
BASHIROV, A.A. & TER-BAGDASAROVA, I.K. (1970) [The state of the
cardiovascular system in workers engaged in the production of amine
salt herbicides and 2,4-dichlorophenoxyacetic acid butyl ester.]
Azerb. Med. Zh., 47 (12): 44-48 (in Russian).
BATORA, V., VITOROUIC, S.L.J., THIER, H.P., & KLISENKO, N.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BEAVEN, F.G., RAWLS, C.K., & BECKET, G.E. (1962) Field
observations upon estuarine animals exposed to 2,4-D. Proc.
northeast. Weed Contr. Conf., 16: 449-458.
BELOMYTTSEVA, L.A. (1964) [Toxic effects of the herbicide 2,4-D
sodium on the lungs.] Sb. Nauchn. Tr. Bashk. Gos. Med. Inst.,
16(2): 135-141 (in Russian).
BELOMYTTSEVA, L.A. (1965) [In: Contributions to the 3rd All-Union
Scientific Conference on the Problems of Hygiene and Toxicology
related to the Use of Chemicals in the National Economy, Kiev,]
p. 380 (in Russian).
BELOMYTTSEVA, L.A. (1967) The toxic effect of the sodium salt of
2,4-dichlorophenoxyacetic acid. UFA 1967.
BELOMYTTSEVA, L.A. & KARIMOVA, A.K. (1963) In: [Proceedings of
the UFA Scientific Research Institute of Hygiene and Occupational
Diseases, Vol. 2.] (in Russian).
BERKLEY, M.C. & MAGEE, K.R. (1963) Neuropathy following exposure
to a dimethylamine salt of 2,4-D. Arch. internal. Med., 111:
351-352.
BERWICK, P. (1970) 2,4-dichlorophenoxyacetic acid poisoning in
man. J. Am. Med. Assoc., 214(6): 1114-1117.
BEZUGLYI, V.P., FOKINA, K.V., KOMAROVA, L.I., SIVITSKAJA, I.I.,
ILINA, V.I., & GORSKAJA, I.I. (1979) [Clinical manifestations of
long-term sequels of acute poisoning with 2,4-D.] Gig. Truda Prof.
Zabol., 3: 47-48 (in Russian).
BIELSKI, J. & MADRA, B. (1976) [Acute poisoning with pesticides.]
Pol. Tyg. Lek., 31(46): 1971-1973 (in Polish, Health & Welfare
Canada Translation No. 2245).
BJERKE, E.L., HERMAN, J.L., MILLER, P.W., & WETTERS, J.H. (1972)
Residue study of phenoxy herbicides in milk and cream. J. agric.
food Chem., 20: 963-967.
BJORKLUND, N.E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. scand., 7: 364-390.
BJORN, M.D. & NORTHEN, H.T. (1948) Effects of 2,4-dichloro-
phenoxyacetic acid on chicks. Science, 108: 479-480.
BLEIBERG, J., WALLEN, M., & BRODKIN, R. (1964) Industrially
acquired porphyria. Arch. Derm., 89: 793-797.
BODAI, J., TAMAS, L., & VEGH, A. (1974) [Reproductive
consequences of Dikonirt toxicosis in cattle.] Magy. Allatorv.
Lapja, 29(5): 319-322 (in Hungarian, Health & Welfare Canada
Translation No. 2238).
BODEM, R., PREISS, D., & KUHN, E. (1971) [On the delay of the
relaxation of the guinea pig papillary muscle by 2,4-dichloro-
phenoxy-acetic acid (2,4-D).] Klin. Wochenschr., 49(1): 227-228
(in German).
BODYAGIN, D.A., SYKIN, A.B., LUPINOSOV, Y.V., & ZAITSEVA, L.A.
(1969) [The effects of chlorophenoxyacetic acid derivatives
(herbicides) on animals and human beings. (A literature review).]
Farmakol. Toksikol., 32 (6): 747-751 (in Russian, Health & Welfare
Canada Translation No. 2031).
BOLLAG, J.M., HELLINGS, C.S., & ALEXANDER, M. (1968) 2,4-D
metabolism. Enzymatic hydroxylation of chlorinated phenols.
J. agric. food Chem., 16: 826-828.
BONGSO, T.A. & BASRUR, P.K. (1973) In vitro response of bovine
cells to 2,4-dichlorophenoxy acetic acid. In vitro, 8: 416-417.
BONTOYAN, W.R. (1977) Report on pesticide formulations. J. Assoc.
Off. Agric. Chem., 60: 324-327.
BOVAL, B. & SMITH, J.M. (1973) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. Chem. Eng. Sci., 28: 1661-1675.
BOVEY, R.W. (1980a) Residues and fate of phenoxy herbicides in
the environment. In: Bovey, R.W. & Young, A.L., ed. The science
of 2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 301-356.
BOVEY, R.W. (1980b) Human risk with phenoxy herbicides in the
environment. In: Bovey, R.W. & Young, A.L., ed. The science of
2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 425-433.
BOVEY, R.W. & YOUNG, A.L., ed. (1980) The science of 2,4,5-T and
associated phenoxy herbicides, New York, Chichester, Brisbane,
Toronto, John Willey & Sons, 462 pp.
BRADLAW, H.A., GARTHOFF, L.H., HURLEY, N.E., & FIRESTONE, D. (1980)
Comparative induction of aryl hydrocarbon hydroxylase activity
in vitro by analoques of dibenzo- p-dioxin. Food cosmet. Toxicol.,
18: 627-635.
BRANDT, M.R. (1971) [Herbatox poisoning. A short survey and a
report of a case.] Ugeskrift Laeger, 133(11): 500-503 (in Danish,
Health & Welfare Canada Translation No. 2147.
BRETAG, L.H. & CAPUTO, C. (1978) The nature of the antimyotonic
action of 2,4-D on the soleus muscle of the rat. Proc. Aust.
Physiol. Pharmacol. Soc., 9(2): 150 pp.
BRISTOL, D.W., COOK, L.W., KOTERBA, M.T., & NELSON, D.C. (1982)
Determination of free and hydrolyzable residues of 2,4-
dichlorophenoxyacetic acid and 2,4-dichlorophenol in potatoes.
J. agric. food Chem., 30: 137-144.
BRODY, I.A. (1973) Myotonia induced by monocarboxylic aromatic
acids: A possible mechanism. Arch. Neurol., 28: 243-246.
BRODY, T.M. (1952) Effect of certain plant growth substances on
oxidative phosphorylation in rat liver mitochondria. Proc. Soc.
Exp. Bio. Med., 80: 533-536.
BROGAN, W.F., BROGAN, C.E., & DADD, J.T. (1980) Herbicides and
cleft lip and palate. Lancet, 2: 597.
BUCHER, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid
on experimental animals. Proc. Soc. Exp. Biol. Med., 63: 204-205.
BURCAR, P.J., WERSHAW, R.L., GOLDBERG, M.C., & KHAN, L. (1966)
Gas chromatographic study of the behaviour of the isooctyl ester
of 2,4-D under field conditions in North Park, Colorado. Anal.
Instrum., 4: 215-224.
BURTON, J.A., GARDINER, T.H., & SCHANKER, L.S. (1974) Absorption
of herbicides from the rat lung. Arch. environ. Health, 29: 31-33.
BUSLOVICH, S.Y. (1963) [The effect of the chlorinated derivatives
of phenoxyacetic acid.] Zdrav. Beloruss., 9: 41-45 (in Russian,
Health & Welfare Canada Translation No. 2315).
BUSLOVICH, S.Y. & KOLDOBSKAYA, F.D. (1972) [Activity of
hexokinase in skeletal muscles of albino rats in experimental
myotonia.] Vopr. Med. Khim., 18(4): 403-406 (in Russian).
BUSLOVICH, S.Y & MILCHINA, M.G. (1976) [The dynamics of 2,4-D
amine decomposition in soil.] Gig. i Sanit., 5: 109-110
(in Russian).
BUSLOVICH, S.Y., VOINOVA, I.V., & MILCHINA, M.G. (1973) [The
distribution of chlorinated phenoxy acid herbicides in albino
rats.] Gig. Tr. Prof. Zabol, 17(3): 171-173 (in Russian, Health &
Welfare Canada Translation No. 2237).
BUSLOVICH, S.Y., ALEKSASHINA, Z.A., & KOLOSOVSKAYA, V.M. (1976)
[Embryotoxic effects of herbicides: Chlorinated phenoxy acids.]
Zdrav. Beloruss., 10: 83-84 (in Russian).
BUSLOVICH, S.Y., KOLDOBSKAYA, F.D., DAVYDENKO, L.I., & KRYSANOVA,
A.I. (1982) [The role of mixed-function oxidases in the
detoxication of some herbicides: Chlorinated derivatives of
phenoxyacids.] Gig. i Sanit., 10: 76-77 (in Russian).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna.
Proc. South. Weed Sci. Soc., 18: 576-580.
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON, M.H., &
MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D, DEF,
propanil, and trifluralin to the Dungeness crab, Cancer magister.
Arch. environ. contam. Toxicol., 8: 383-396.
CANADA, HEALTH & WELFARE (1980) Pesticides. In: Guidelines for
Canadian drinking water quality, 1978. Supporting documentation,
Ottawa, Supply and Services, Canada, pp. 457-488.
CARL, M. (1979) Internal laboratory quality control in the
routine determination of chlorinated pesticide residues. Adv.
pestic. Sci., 3:660-663.
CARMELLI, D., HOFHERR, L., TOMSIC, J., & MORGAN, R.W. (1981) A
case-control study of the relationship between exposure to 2,4-D
and spontaneous abortions in humans, Menlo Park, California, SRI
International (Final Report).
CARTER, E.P. (1960) Volatility of ester forms of hormone type
herbicides III. J. Assoc. Off. Anal. Chem., 43: 367-370.
CHANG, H., RIP, J.W., & CHERRY, J.H. (1974) Effects of
phenoxyacetic acids on rat liver tissues. J. agric. food Chem.,
22(1): 62-65.
CHAU, A.S.Y. & THOMSON, K. (1978) Investigation of the integrity
of seven herbicidal acids in water samples. J. Assoc. Off. Anal.
Chem., 61: 1481-1485.
CHAU, A.S.Y., AFGHAN, B.K., & ROBINSON, J.W. (1982) Analysis of
pesticides in water. Volume II. Chlorine- and phosphorus-
containing pesticides. Boca Raton (Florida), CRC Press Inc.,
pp. 238.
CHEN, W.L., GORZINSKI, S.J., KOCIBA, R.J., WADE, C.E., MORDEN,
D.C., KEYES, D.G., & SCHWETZ, B.A. (1981) 2,4-D (2,4-
dichlorophenoxy) acetic acid: results of a 13-week feeding study in
CDF Fisher 344 rats. Proc. Annu. Meet. Can. Fed. Biol. Soc.,
24: 233.
CHOI, K.L., QUE HEE, S.S., & SUTHERLAND, R.G. (1976) 2,4-D levels
in the South Saskatchewan River in 1973 as determined by a GLC
method. J. environ. Sci. Health, Part B, 11: 175-183.
CHOW, C., MONTGOMERY, M.L., & YU, T.C. (1971) Methodology and
analysis for residues of MCP and 2,4,5-T in wheat. Bull. environ.
Contam. Toxicol., 18: 361-365.
CLARK, D.E., PALMER, J.S., RADELEFF, R.D., CROOKSHANK, H.R., &
FARR, F.M. (1975) Residues of chlorophenoxy acid herbicides and
their phenolic metabolites in sheep and cattle. J. agric. food
Chem., 23(3): 573-578.
COCHRANE, W.P. (1981) Chemical derivatization in pesticide
analysis. In: Frei, R.W. & Lawrence, J.F., ed. Chemical
derivatization in analytical chemistry, I: Chromatography, New
York, Plenum Press, pp. 1-97.
COCHRANE, W.P. & RUSSELL, J.B. (1975) Residues in wheat and soil
treated with the mixed butyl esters of 2,4-D. Can. J. plant Sci.,
55: 323-325.
COCHRANE, W.P., SINGH, J., MILES, W., & WAKEFORD, B. (1981)
Determination of chlorinated dibenzo- p-dioxin contaminants in
2,4-D products by gas chromatography - mass spectrometric
techniques. J. Chromatogr., 217: 289-299.
COCHRANE, W.P., LANOUETTE, M., & SINGH, J. (1982) High pressure
liquid chromatographic determination of impurity phenols in
technical 2,4-D acid and 2,4-dichlorophenol. J. Assoc. Off. Anal.
Chem., in press.
COGGON, D. & ACHESON, E.D. (1982) Do phenoxy herbicides cause
cancer in man? Lancet, 1: 1057-1059.
COHEN, S.Z., ZWEIG, G., LAW, M., WRIGHT, D., & BONTOYAN, W.R.
(1978) Analytical determination of N-nitroso compounds in
pesticides by the United States Environmental Protection Agency.
In: Walker, E.A., Castegnaro, M., Criciute, L., & Lyle, R.E., ed.
Environmental Aspects of N-nitroso compounds, Lyons, IARC
Scientific Publications, 19: 333-342.
COLLABORATIVE INTERNATIONAL PESTICIDES ANALYTICAL COUNCIL LTD
(1970) CIPAC Handbook, Vol. I, Analysis of technical and
formulated pesticides, Harpenden, England, CIPAC, pp. 241-273.
COMMISSION OF EUROPEAN COMMUNITIES (1981) Criteria (dose/effect
relationships) for organochlorine pesticides. Oxford, New York,
Toronto, Sydney, Paris, Frankfurt, Pergamon Press, pp. 223-240.
CONNICK, W.J., Jr & SIMONEAUX, J.M. (1982) Determination of (2,4-
dichlorophenoxy) acetic acid and of 2,6-dichlorobenzo-nitrile in
water by high performance liquid chromatography. J. agric. food
Chem., 30: 258-260.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99(1): 1-12.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet
samples (V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet
samples (VI). Pestic. monit. J., 5(4): 313-330.
COURTNEY, K.D. (1977) Prenatal effects of herbicides: Evaluation
by the prenatal development index. Arch. environ. Contamin.
Toxicol., 6: 33-46.
COUTSELINIS, A., KENTARCHOV, R., & BOUKIS, D. (1977) Concentration
levels of 2,4-D and 2,4,5-T in forensic material. Forensic Sci.,
1: 203-204.
CRAFTS, A.S. (1960) Evidence for hydrolysis of esters of 2,4-D
during absorption by plants. Weeds, 8: 19-25.
CRIPPS, R.E. & ROBERTS, T. (1978) Microbial degradation of
herbicides. In: Hill, I.R. & Wright, S.J.L., ed.: Pesticide
microbiology - Microbial aspects of pesticide behaviour in the
environment, London, New York, San Francisco, Academic Press,
pp. 669-730.
CROSBY, D.G. & TUTASS, H.O. (1966) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. J. agric. Food Chem., 14: 596.
CROSBY, D.G. & WONG, A.S. (1973) Photodecomposition of 2,4,5-
trichlorophenoxyacetic acid (2,4,5-T) in water. J. agric. Food
Chem., 21(6): 1052-1054.
CROSSEN, P.E., MORGAN, W.F., HORAN, J.J., & STEWART, J. (1978)
Cytogenetic studies of pesticide and herbicide sprayers. New
Zealand med. J., 88(619): 192-195.
CRUMMET, W.P. & STEHL, R.H. (1973) Determination of chlorinated
dibenzo- p-dioxins and dibenzofurans in various materials. Environ.
Health Perspect., 5: 15-25.
CURRIE, L.A. (1968) Limits of qualitative detection and
quantitative determination. Anal. Chem., 40: 586-593.
CURRY, A.S. (1962) Twenty-one uncommon cases of poisoning. Br.
med. J., 1: 687-689.
DANON, J.M., KARPATI, G., CARPENTER, S., & WOLFE, L.S. (1976)
Experimental myotonic myopathy. Neurology, 26: 384.
DEBEER, J.O., VAN PETEGHEM, C.H., HEYNDRICKX, A.M., & VAN ZELE, W.
(1981) Mass fragmentographic determination of 2,4-D and 2,4,DP
trace levels in biosamples using intramolecular deuterated interval
standards. Med. Fac. Landbouww. Rijksuniv. Gent, 46(1): 305-316.
DELARRARD, J. & BARBASTE, M. (1969) Intoxication suicidaire
mortelle agro-chimique de l'hormone désherbante 2,4-D. Arch. Mal.
prof. Med. Trav. Secur. soc., 30(7-8): 434.
DENNIS, C.A.R. (1976) Health effects of 2,4-D. In: National
Research Council of Canada Associate Committee on Scientific
Criteria for Environmental Quality, ed. Phenoxy herbicides: their
effects on environmental quality, Ottawa, NRCC Publications
Service, p.333.
DEREUCK, J., DECOSTER, W., WILLEMS, J., & VANDER EECKEN, H. (1979)
Influence of 2,4-dichlorophenoxyacetate and of dantrolene sodium on
the target phenomenon in tenotomized rat gastrocnemius muscle.
Acta neuropathol., 46: 167-168.
DESI, I. & SOS, J. (1962a) Central nervous injury by a chemical
herbicide. Acta med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1962b) [The effect of 2,4-dichloro-
phenoxyacetic acid and triorthocresyl phosphate on the function of
the upper central nervous system (bioelectric activity and
conditioned reflexes).] Gig. i Sanit., 12: 38-46 (in Russian,
Health & Welfare Canada Translation No. 2183).
DESI, I., SOS, J., & NIKOLITS, I. (1962a) New evidence concerning
the nervous site of action of a chemical herbicide causing
professional intoxication. Pathophysiologica (Budapest), 22:
73-80.
DESI, I., SOS, J., OLASZ, J., SULE, F., & MARKUS, V. (1962b)
Nervous system effects of a chemical herbicide. Arch. environ.
Health, 4: 95-102.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B., & GEORGE, J.L. (1962)
Effects on wildlife. pp. 4-52. In: Effects of pesticides on fish
and wildlife: A review of investigations during 1960. Washington,
DC, US Dept. of the Interior (Fish and Wildlife Circ. No. 143).
DRAPER, W.M. & STREET, J.C. (1982) Applicator exposure to 2,4-D,
dicamba, and a dicamba isomer. J. environ. Sci. Health, B17(4):
321-339.
DRILL, V.A. & HIRATZKA, T. (1953) Toxicity of 2,4-D and 2,4,5-T,
a report on their acute and chronic toxicity in dogs. Arch. ind.
Hyg. occup. Med., 7: 61-67.
DUDLEY, A.W. & THAPAR, N.T. (1972) Fatal human ingestion of
2,4-D, a common herbicide. Arch. Pathol., 94: 270-275.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States (III), June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E., LIPSCOMB, G.Q., COX, E.L., HEATWOLE, R.E., & KLING,
R.C. (1971) Pesticide residue levels in foods in the United
States from July 1, 1963 to June 30, 1969. Pestic. monit. J.,
5(2): 73-80.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some herbicides
on the hatching rate of hens' eggs. Nature (Lond.), 215:
1406-1407.
DUNACHIE, J.F. & FLETCHER, W.W. (1970) The toxicity of certain
herbicides to hens' eggs assessed by the egg injection technique.
Ann. app. Biol., 66: 515-520.
DURIC, V., STOJANOVIC, V., & IGIC, B. (1979) [Presentation of
patients with pesticide poisoning.] Med. Pregl., 32(3/4): 129-133.
(In Serbo-Croatian, Health & Welfare Canada Translation No. 2382.)
DUX, E., TOTH, I., DUX, L., & JOO, F. (1978) The localization of
calcium by X-ray microanalysis in myopathic muscle fibers.
Histochemistry, 56: 239-244.
DZHAPAROV, I.R. & TSILIKOV, V.V. (1969) [The effect of the sodium
salt of 2,4-D and carbothione on the content of glycogen in the
liver and the blood sugar level in rats.] Farmakol. Tsentr.
Kholinolitikov Drugikh Neirotropnykh Sredstv., p. 180 (in
Russian).
EASLEY, C.B., LAUGHLIN, J.M., GOLD, R.E., & TUPY, D.R. (1983)
Laundering procedures for removal of 2,4-dichloro-phenoxyacetic
acid ester and amine herbicides from contaminated fabrics. Arch.
Environ. Contam. Toxicol., 12: 71-76.
EBERSTEIN, A. & GOODGOLD, J. (1979) Experimental myotonia induced
in denervated muscles by 2,4-D. Muscle Nerve, 2: 364-368.
ELO, H. & YLITALO, P. (1977) Substantial increase in the levels
of chlorophenoxyacetic acids in the CNS of rats as a result of
severe intoxication. Acta pharmacol. toxicol., 41: 280-284.
ELO, H. & YLITALO, P. (1979) Distribution of 2-methyl-4-
chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid in male
rats: evidence for the involvement of the central nervous system
in their toxicity. Toxicol. appl. Pharmacol., 51: 439-446.
ELWOOD, J.M. & ROGERS, J.R. (1975) The incidence of congenital
abnormalities in British Columbia, Alberta, Manitoba and New
Brunswick, 1966-1969. Can. J. public Health, 66: 471-475.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERICKSON, L.C., BRANNAMAN, B.L., & COGGINS, C.W. Jr. (1963)
Residues in stored lemons treated with various formulations of
2,4-D. J. agric. food Chem., 11: 437-440.
ERIKSSON, M., HARDELL, M., BERG, N.O., MOLLER, T., & AXELSON, O.
(1981) Soft-tissue sarcomas and exposure to chemical substances: a
case-referent study. Br. J. ind. Med., 38: 27-33.
ERNE, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta vet. Scand., 7: 240-256.
ERNE, K. (1966b) Studies on the animal metabolism of
phenoxyacetic herbicides. Acta vet. Scand., 7: 264-271.
ERNE, K. (1974) Weed-killers and wildlife. In: Proceedings of
the XI International Congress on Game Biology, Stockholm, 1973.
pp. 415-422 (National Swedish Environment Protection Board
Publications No. 13E).
ERNE, K. (1975) Phenoxy herbicide residues in Swedish fish and
wildlife. In: Coulston, F., Korte, F., Klein, W., & Rosenblum,
I., ed. Pesticides: Environmental quality and safety, Stuttgart,
George Thieme Publishers, Suppl. Vol. III., pp. 192-195.
ERNE, K. (1980) [Further studies of phenoxy herbicide residues in
woodland berries and mushrooms, 1973-1979.] Vr Föda, 32(5):
285-292 (in Swedish).
ERNE, K. & RUTQVIST, L. (1979) [Pesticide residues in feedstuffs
in Sweden.] Nord. Veterinaermed., 31: 263-274 (in Swedish).
ERNE, K. & SPERBER, I. (1974) Renal tubular transfer of
phenoxyacetic acids in the chicken. Acta pharmacol. toxicol., 35:
233-241.
ERNE, K. & VON HAARTMAN, U. (1973) [Phenoxy acid residues in
forest berries and mushrooms.] Vr Föda, 25: 146-153 (in Swedish).
EVANS, W.C., SMITH, B.S.W., FERNLEY, H.M., & DAVIES, J.I. (1971)
Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem. J.,
122: 543-551.
EYZAGUIRRE, C., FOLK, B.P., ZIERLER, K.L., & LILIENTHAL, J.L. Jr.
(1948) Experimental myotonia and repetitive phenomena: The
veratrinic effects of 2,4-D acetate in the rat. Am. J. Physiol.,
155: 69-77.
FABACHER, D.L. & CHAMBERS, H. (1974) Resistance to herbicides in
insecticide-resistant mosquitofish, Gambusia affinis. Environ.
Lett., 7(1): 15-20.
FAO (1981) Pesticide residues in food: 1980 evaluations. Rome,
FAO, pp. 109-110 (Plant Production and Protection Paper 26 Suppl.)
FAO/WHO (1971) 2,4-D. In: 1970 Evaluations of some pesticide
residues in food, Rome, FAO/WHO, pp. 59-86 (FAO/AGP/1970/M/ 12/1,
WHO Food Additive Series, 71, 42).
FAO/WHO (1972) 1971 evaluations of some pesticide residues in
food: 2,4-D. WHO Pesticide Residues Series, 1: 83-97.
FAO/WHO (1973) Pesticide residues in food. Report of the 1972
Joint FAO/WHO meeting, Geneva, (WHO Techn. Rep. Series No. 525).
FARWELL, S.O., BOWES, F.W., & ADAMS, D.F. (1976a) Determination
of chlorophenoxy herbicides in air by gas chromatography/mass
spectrometry: selective ion monitoring. Anal. Chem., 48: 420-426.
FARWELL, S.O., ROBINSON, E., POWELL, W.J., & ADAMS, D.F. (1976b)
Survey of airborne 2,4-D in south central Washington. J. Air
Pollut. Control Assoc., 26: 224-230.
FAUST, S.D. & ALY, O.M. (1963) Some effects of 2,4-D and 2,4-DCP
on drinking water quality. Proc. Northeast. Weed Contr. Conf., 17:
460-470.
FAUST, S.D. & SUFFET, I.H. (1966) Recovery, separation, and
identification of organic pesticides from natural and potable
waters. Residue Rev., 15: 44-116.
FEDOROVA, L.M. & BELOVA, R.S. (1974) [Inclusion of 2,4-
dichlorophenoxyacetic acid in organs of animals: Paths and
dynamics of its excretion.] Gig. i Sanit., 39(2): 105-107
(in Russian, US NTC Translation No. 76-10918-05J).
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FETISOV, M.I. (1966) Occupational hygiene in the application of
herbicides of the 2,4-D group. Hyg. Sanit., 31(7-9): 383-386.
FEUNG, C.S., HAMILTON, R.H., & WITHAM, F.H. (1971) Metabolism of
2,4-dichlorophenoxyacetic acid by soybean cotyledon callus tissue
cultures. J. agric. food Chem., 19: 474-479.
FEUNG, C.S., HAMILTON, R.H., WITHAM, F.H., & MUMMA, R.O. (1972)
The relative amounts and identification of some 2,4-
dichlorophenoxyacetic acid metabolites isolated from soybean
cotyledon callus cultures. J. agric. food Chem., 50: 80-86.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1973b) Metabolism of
2,4-dichlorophenoxyacetic acid. V. Identification of metabolites
in soybean callus tissue cultures. J. agric. food Chem., 21:
637-640.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1975) Metabolism of
2,4-dichlorophenoxyacetic acid. VII. Comparison of metabolites
from five species of plant callus tissue cultures. J. agric. food
Chem., 23: 373-376.
FEUNG, C.S., LOERCH, S.L., HAMILTON, R.H., & MUMMA, R.O. (1978)
Comparative metabolic fate of 2,4-dichlorophenoxy acetic acid in
plants and plant tissue culture. J. Agric. Food Chem., 26:
1064-1067.
FIELD, B. & KERR, C. (1979) Herbicide use and incidence of
neural tube defects. Lancet, 1: 1341-1342.
FINNISH STATE INSTITUTE OF AGRICULTURAL CHEMISTRY (Helsinki)
(1963-1969) Investigations on pesticide residues. pp. 83.
FLEEKER, J.R. & STEEN, R. (1971) Hydroxylation of 2,4-D in
several weed species. Weed Sci., 19: 507-510.
FLORSHEIM, W.H. & VELCOFF, S.M. (1962) Some effects of
2,4-dichlorophenoxyacetic acid on thyroid function in the
rat: Effects on iodine accumulation. Endocrinology, 71(1):
1-6.
FLORSHEIM, W.H., VELCOFF, S.M., & WILLIAMS, A.D. (1963) Some
effects of 2,4-D on thyroid function in the rat: Effects on
peripheral thyroxine. Endocrinology, 72: 327-333.
FOISSAC-GEGOUX, P., LELIEVRE, A., BASIN, B., & WAROT, P. (1962)
Polynevrite aprčs usage d'un desherbant: l'acide 2,4-D. Lille Med.,
7(10): 1049-1051.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry
to nine herbicides. Bull. environ. Contam. Toxicol., 15(5):
509-514.
FOLMAR, L.C. (1979) Effects of short term field applications of
acrolein and 2,4-D (DMA) on flavor of the flesh of rainbow trout,
124 pp.
FOSTER, R.K. & McKERCHER, R.B. (1973) Extraction of 14C-labelled
chlorophenoxyacetic acids from soil. Can. J. soil Sci., 53:
135-136.
FRANK, P.A. (1972) Herbicidal residues in aquatic environments.
In: Faust, S.D., ed. Fate of organic pesticides in the aquatic
environment. Washington, DC, American Chemical Society,
pp. 135-148 (Advances in Chemistry Series, No. III).
FRANK, P.A. & COMES, R.D. (1967) Herbicidal residues in pond
water and hydrosoil. Weeds, 15(3): 210-213.
FRANK, P.A., DEMINT, R.J., & COMES, R.D. (1970) Herbicides in
irrigation water following canal-bank treatment for weed control.
Weed Sci., 18(6): 687-692.
FRANK, R. & SIRONS, G.J. (1980) Chlorophenoxy and chlorobenzoic
acid herbicides; their use in eleven agricultural waterbeds and
their loss to stream waters in southern Ontario, Canada, 1975-1977.
Sci. total Environ., 15: 149-167.
FRANK, R., SIRONS, G.J., & RIPLEY, B.D. (1979) Herbicide
contamination and decontamination of well waters in Ontario,
Canada, 1969-1978. Pestic. monit. J., 13(3): 120-127.
FRANK, R., SIRONS, G.J., CAMPBELL, R.A., & MEWETT, D. (1982)
Residues of 2,4-D, dichlorprop, and picloram in wild berries from
treated rights-of-way and conifer release sites in Ontario,
1979-81. Can. J. Plant Sci., 63: 196-209.
FRANKLIN, C.A., GROVER, R., MARKHAM, J.W., SMITH, A.E., & YOSHIDA,
K. (1982) Effect of various factors on exposure of workers
involved in the aerial application of herbicides. Am. Conf. of
Govern. Ind. Hygienists, Transactions, 43: 97-117.
FREEMON, F.R. (1975) Causes of polyneuropathy. Acta neurol.
Scand., 51(Suppl. 59): 5-43.
FYODOROVA, L.M., BELOVA, R.S., KIRYUHINA, N.N., VOLCHONOK, M.G., &
KOVTUNOVA, N.Y. (1977) [Residual content of chlorophenoxyacetic
acids in the environment and human body.] Gig. i Sanit., 6: 101-103
(in Russian).
GAMBLE, W. (1975) Mechanism of action of hypolipidemic and
herbicidal aryloxy acids. J. theor. Biol., 54: 181-190.
GAVRILOVA, L.I. (1965) Experimental substantiation of the
permissible concentration of 2,4-dichlorophenoxy-gamma-butyric (DB)
acid in water bodies. Hyg. Sanit., 29(1): 11-16.
GELDMACHER- VON MALLINCKRODT, M., & LAUTENBACH, L. (1966) [Two
fatal poisonings (suicides) with chlorinated phenoxyacetic acids
(2,4-D and MCPA).] Arch. Toxicol., 21: 261-278 (in German).
GLUCK, S.J. & MELCHER, R.G. (1980) Concentration and
determination of the propylene glycol butyl ether esters of
2,4-dichlorophenoxyacetic acid (PGBE 2,4-D) in air. Am. Hyg. Ass.
J., 41(12): 932-934.
GOLDSTEIN, N.P. & BROWN, J.R. (1960) Peripheral neuropathy.
J. Am. Med. Assoc., 173: 171.
GOLDSTEIN, N.P., JONES, P.H., & BROWN, J.R. (1959) Peripheral
neuropathy after exposure to an ester of dichlorophenoxyacetic
acid. J. Am. Med. Assoc., 171: 1306-1309.
GOLDWATER, L.J. (1960) Peripheral neuropathy. J. Am. Med.
Assoc., 173(1): 171.
GORSHKOV, A.I. (1972) Hygienic evaluation of chlorocholine
chloride (Preparation CCC) and its combination with 2,4-D amine
salt. Hyg. Sanit., 36(10-12): 208-211.
GOWEN, J.A., WIERSMA, G.B., & TAI, H. (1976) Mercury and 2,4-D
levels in wheat and soils from sixteen states, 1969. Pestic. Monit.
J., 10(3): 111-113.
GRAFF, G.L.A., GUENING, C., & VANDERKELEN, B. (1972) Influence de
l'acide 2,4-dichlorophénoxyacéticque (2,4-D), un agent myotonisant,
sur le métabolisme des phosphates acidosolubles des muscle
squelettiques du rat. C. R. Soc. Biol. (Paris), 166(II):
1565-1568.
GRANROTH, G., HAKAMA, M., & SAXEN, L. (1977) Defects of the
central nervous system in Finland: I. Variations in time and
space, sex distribution, and parental age. Br. J. prev. soc.
Med., 31: 164-170.
GRANROTH, G., HAAPAKOSKI, J., & SAXEN, L. (1978) Defects of the
central nervous system in Finland: V. Multivariate analysis of
risk indicators. Int. J. Epidemiol., 7(4): 301-308.
GRANT, W. (1976) 2,4-D. In: Hart, R.W., Kraybill, H.F., &
DeSerres, F.J., ed.: A rational evaluation of pesticidal vs
mutagenic/carcinogenic action. Bethesda, Maryland, National
Institute of Health (Publication No. (NIH) 78-1306 U.S. Department
of Health, Education & Welfare, Public Health Service).
GRAY, T.J.B., LAKE, B.G., BEAMAND, J.A., FOSTER, J.R., & GANGOLLI,
S.D. (1983) Peroxisome proliferation in primary cultures of rat
hepatocytes. Toxicol. appl. Pharmacol., 67: 15-25
GREEN, S. & MORELAND, F.S. (1975) Cytogenic evaluation of
several dioxins in the rat. Toxicol. appl. Pharmacol., 33: 161.
GRIBANOV, O.I. (1968) Pollution of an open water source at
Shortandy Railroad Station by the herbicide 2,4-D butyl ester.
Hyg. Sanit., 32: 267-268.
GRIGSBY, B.H. & FARWELL, E.D. (1950) Some effects of herbicides
on pasture and on grazing livestock. Mich. Agric. Exp. St. Bull.,
32(3): 378-385.
GROLLEAU, G., DE LAVAUR, E., SIOU, G., & FROUX, Y. (1974) Effects
of 2,4-D on the reproduction of quail and partridge after the
application of the product by spraying on eggs. Ann. Zool. Ecol.
anim., 6(2): 313-331.
GROVER, R. (1976) Relative volatilities of ester and amine forms
of 2,4-D. Weed Sci., 24: 26-28.
GROVER, R. & KERR, L.A. (1981) Evaluation of polyurethane foam as
a trapping medium for herbicide vapour in air monitoring and worker
inhalation studies. J. environ. Sci. Health, B 16(1): 59-66.
GROVER, R. & SMITH, A.E. (1974) Adsorption studies with the acid
and dimethylamine forms of 2,4-D and dicamba. Can. J. Soil Sci.,
54: 179-186.
GROVER, R., MAYBANK, J., & YOSHIDA, K. (1972) Droplet and vapour
drift from butyl ester and dimethylamine salt of 2,4-D. Weed Sci.,
20: 320-324.
GROVER, R., KERR, L.A., WALLACE, K., YOSHIDA, K., & MAYBANK, J.
(1976) Residues of 2,4-D in air samples from Saskatchewan
1966-1975. J. environ. Sci. Health, B 11(4): 331-347.
GRUNOW, W. & BOHME, C. (1974) [On the metabolism of 2,4,5-T and
2,4-D in rats and mice.] Arch. Toxicol., 32: 217-225 (in German).
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1971) Determination of
some acidic herbicides by thin layer chromatography. J. agric.
food Chem., 19: 1181-1182.
GUARINO, A.M. & ARNOLD, S.T. (1979) Xenobiotic transport
mechanisms and pharmacokinetics in the dogfish shark. In: Khan,
M.A.Q., Lech, J.J., & Menn, J.J., ed. Pesticide and xenobiotic
metabolism in aquatic organisms. Washington, DC, American Chemical
Society, pp. 250-258.
GUMMER, W.D. (1980) Pesticide monitoring in the prairies of
western Canada. Environ. Sci. Res., 16: 345-372.
GUNTHER, F.A. (1962) Instrumentation in pesticide residue
determination. In: Metcalf, R.L., ed. Advances in Pest Control
Research, 5, New York, Interscience Publishers, pp. 191-319.
GUSEVA, E.N. (1956) [On the pharmacology of 2,4-dichloro-
phenoxyacetic acid.] Farmakol. Toksikol. (Mosc.), 19(4):
41-44 (in Russian).
GUTENMANN, W.H. & LISK, D.J. (1965) Conversion of 4-(2,4-DB)
herbicide to 2,4-D by bluegills. NY Fish Game J., 12(1): 108-111.
GUTENMANN, W.H., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1963)
Disappearance of 4(2,4-dichlorophenoxy-butyric) acid herbicide in
the dairy cow. J. dairy Sci., 46: 991-992.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The effects of
phenoxy herbicides on the hatchability of eggs and the viability of
the chicks. Acta pharmacol. toxicol., 35: 300-308.
HACQUE, R., DEAGEN, J., & SCHMEDDING, D. (1975) Binding of
2,4-dichloro- and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. A proton magnetic resonance study. J. agric. food Chem.,
23: 763-766.
HALLIOP, J., TOCHMAN, A., & LATALSKI, M. (1980) [Ultrastructural
investigations and myeloperoxidase determinations in rat
neutrophils in acute poisoning with 2,4-dichlorophenoxyacetic
acid.] Acta haematol. Pol., 11(4): 249-257 (in Polish).
HALTER, M. (1980) 2,4-D in the aquatic environment In: Shearer,
R., & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlobenil, diquat & endothall, Seattle
Municipality of Metropolital Seattle, January 1980, pp. 89-182.
HANIFY, J.A., METCALF, P., NOBBS, C.L., & WORSLEY, K.J. (1980)
Congenital malformations in the newborn in Northland: 1966-1977.
New Zealand med. J., 92: 245-248.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquito fish, Gambusa
affinis. Bull. environ. Contam. Toxicol., 8(1): 46-51.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973) Avoidance of
pesticides by grass shrimp (Palaeomonetes pugio). Bull. environ.
Contam. Toxicol., 9(3): 129-133.
HANSEN, W.H., QUAIFE, M.L., HABERMANN, R.T., & FITZHUGH, O.G,
(1971) Chronic toxicity of 2,4-dichlorophenoxyacetic acid in rats
and dogs. Toxicol. appl. Pharmacol., 20: 122-129.
HARDELL, L. & SANDSTRÖM, A. (1979) Case-control study: soft
tissue sarcomas and exposure to phenoxyacetic acids or
chlorophenols. Br. J. Cancer, 39: 711.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 43: 169-176.
HARRISON, J.W.E. & REES, E.W. (1946) 2,4-D toxicity - I.
Toxicity towards certain species of fish. Am. J. Pharm., 118:
422-425.
HASS, J.R., FRIESEN, M.D., & HOFFMAN, M.K. (1981) Recent mass
spectrometric techniques for the analysis of environmental
contaminants. In: McKinney, J. D., ed. Environ. Health Chem.,
Ann Arbor, Arbor Sciences, pp. 219-243.
HATCH, T.F. & GROSS, P. (1964) Pulmonary desposition and
retention of inhaled aerosols. New York, London, Academic Press,
XIV + 192 pp (American Industrial Hygiene Association Monograph
Series on Industrial Hygiene).
HAYES, W.J. (1982) 2,4-D. In: Pesticides studied in man.
Baltimore & London, Williams & Wilkins.
HEENE, R. (1966) [Histochemical proof of phosphorylase inhibition
by 2,4-D in the skeletal muscles and liver of the rat.]
Naturwissenschaften, 53: 308 (in German).
HEENE, R. (1967) [Inhibition of glycogen-forming enzymes by
2,4-D.] Histochemie, 8: 45-53 (in German).
HEENE, R. (1968) Histochemical and morphological findings in
experimental 2,4-D myopathy in warmblooded animals. Acta
neuropathol., 10: 166-169.
HEENE, R. (1975) Experimental myopathies and muscular dystrophy.
Studies in the formal pathogenesis of the myopathy of 2,4-D
acetate. Schriftenr. Neurol. Ser., 16: 1-97.
HENSHAW, B., QUE HEE, S.S., SUTHERLAND, R.F., & LEE, C.D. (1975)
Gas-liquid chromatography and gas-liquid chromatography combined
with mass spectrometry of a butyl ester formulation of 2,4-
dichlorophenoxy-acetic acid. J. Chromatogr., 106: 33-39.
HERBICH, J. & MACHATA, G. (1963). [Poisoning with
2,4-dichlorophenoxy-acetic acid (2,4-D).] Beitr. gerichtl. Med.,
22: 133-139 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies to determine
the toxic effects of derivatives of 2,4-dichloro- and 2,4,5-
trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated eggs
of pheasants, quails, and chickens. First Report.] Anz.
Schaedling-sk. D. Pflanzenschutz Umweltschutz, 49: 21-25 (in
German).
HILBIG, V., LUCAS, K., SEBEK, V., & MUNCHOW, H. (1976b) [Studies
to determine the toxic effects of derivatives of 2,4-dichloro- and
2,4,5-trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated
eggs of pheasants, quails, and chickens. Second report.] Anz.
Schaedlingsk. D. Pflanzenschutz Umweltschutz, 49: 65-68 (in
German).
HILL, E.V. & CARLISLE, H. (1947) Toxicity of 2,4-D for
experimental animals. J. ind. Hyg. Toxicol., 29(2): 85-95.
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized
fish eggs and fry. Trans. Am. Fish. Soc., 96(4): 414-416.
HOFMANN, W.W., ALSTON, W., & ROWE, G. (1966) A study of
individual neuro-muscular junctions in myotonia.
Electroencephalogr. clin. Neuro-physiol., 21: 521-537.
HOGSTEDT, C. & WESTERLUND, B. (1980) Cohort studies of cause of
death of forest workers with and without exposure to phenoxy
preparations. Läkartidninger, 77(19): 1828-1831.
HOGSTEDT, B., KOLNIG, A.M., MITELMAN, R., & SKERFVING, S. (1980)
Cytogenetic study of pesticides in agricultural work. Hered.,
92: 177-178.
HOLCOMBE, G.W., FIANDT, J.T., & PHIPPS, G.L. (1980) Effects of pH
increases and sodium chloride additions on the acute toxicity of
2,4-dichlorophenol to the fathead minnow. Water Res., 14(8):
1073-1077.
HOLMBERG, P.C. (1979) Central nervous system defects in children
born to mothers exposed to organic solvents during pregnancy.
Lancet, 2: 177-179.
HOOPER, F.N. (1958) The effect of applications of pelleted 2,4-D
upon the bottom fauna of Kemt Lake, Oakland County, Michigan. Proc.
Ann. Meet. North Centr. Weed Control Conf., 41.
HORNER, J., QUE HEE, S.S., & SUTHERLAND, R.G. (1974)
Esterification of (2,4-dichlorophenoxy) acetic acid - a
quantitative comparison of esterification techniques Anal. Chem.,
46: 110-112.
HUCKINS, J.N., STALLING, D.C., & SMITH, W.A. (1978) Foam-charcoal
chromatography for the analysis of polychlorinated dibenzodioxins
in Herbicide Orange. J. Assoc. Anal. Chem., 61: 32-38.
HUFF, J.E., MOORE, J.A., SARACCI, R., & TOMATIS, L. (1980) Long
term hazards of polychlorinated dibenzodioxins and polychlorinated
dibenzofurans. Environ. Health Persp., 36: 221-240.
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to
bluegill sunfish of phenoxy herbicides. Weeds, 11(1): 50-53.
HUNT, L.M., GILBERT, B.N., & PALMER, J.S. (1970) Effects of a
herbicide, 2-ethylhexyl ester of 2,4-D on magnesium: calcium ratios
and blood urea nitrogen levels in sheep and cattle. Bull. environ.
Contam. Toxicol., 5(1): 54-60.
IARC (1977) 2,4-D and esters. In: Some fumigants, the herbicides
2,4-D and 2,4,5-T, chlorinated dibenzodioxins, and miscellaneous
industrial chemicals. Lyons, International Agency for Research on
Cancer, pp. 111-138 (IARC monograph on the evaluation of the
carcinogenic risk of chemicals to man Vol. 15).
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. Lyons, International Agency for
Research on Cancer, p. 101 (IARC Monographs of 1-29, Supplement 4).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer, Vol. 30.
ILO (1977) Safe use of pesticides, Geneva, International
Labour Office (Occupational Safety and Health Series, No. 38).
ILO (1979) Guide to health and hygiene in agricultural work,
Geneva, International Labour Organisation, pp. 309.
INGELOG, T., ERNE, K., PAULSSON, A.M., JONASSON, H., & HOLMGREN, A.
(1977) [Taste of flavour changes in woodland berries after
herbicide spraying.] Vr Föda, 29(6): 1-16 (in Swedish).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, L.H.,
& GART, J.J. (1969) Bioassay of pesticides and industrial
chemicals for tumorigenicity in mice: A preliminary note. J. Natl
Cancer Inst., 42: 1101-1114.
IYER, V., WHITING, M., & FENICHEL, G. (1976) Neural influence in
experimental myotonia. Neurology, 26: 384.
JACKSON, J.W. & THOMAS, T.C. (1978) A simple and inexpensive
method for sampling 2,4-D and 2,4,5-T herbicides in air. J. Air
Pollution Control Assoc., 28(11): 1145-1147.
JAMES, M.O. (1979) Dispositon of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Palinurus argus).
Pharmacologist, 21(3): 251.
JAMES, W.H. (1977) Clomiphene, acencephaly, and spina bifida.
Lancet, 1: 603.
JANERICH, D.T. (1973) Epidemic waves in the prevalence of
anencephally and spina bifida in New York State. Teratology, 8:
253-256.
JENSEN, D.J. & GLAS, R.D. (1981) Analysis for residues of acidic
herbicides. In: Moye, H.A., ed. Analysis of pesticide residues,
New York, Chichester, Brisbane, Toronto, John Wiley & Sons, pp.
223-261.
JENSSEN, D. & RENBERG, L. (1976) Distribution and cytogenetic
test of 2,4-D and 2,4,5-T phenoxyacetic acids in mouse blood
tissues. Chem. biol. Interactions, 14: 291-299.
JOHNSON, J.E. (1971) The public health implications of widespread
use of the phenoxy herbicides and picloram. Bioscience, 27(17):
899-905.
JOHNSON, E.R., YU, T.C., & MONTGOMERY, M.L. (1977) Trapping and
analysis of atmospheric residues of 2,4-D. Bull. environ. Contam.
Toxicol., 17: 369-372.
JOHNSON, R.D. & MANSKE, D.D. (1976) Pesticide residues in total
diet samples (IX) Pestic. Monit. J., 9(4): 157-168.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples - (I) - August 1974 - July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1981a)
Pesticide, heavy metal and other chemical residues in infant and
total diet samples - (II) - August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-53.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b)
Pesticide, heavy metal, and other chemical residues in adult total
diet samples (XII) - August 1975 - July 1976. Pestic. monit. J.,
15(1): 54-69.
JUNG, H.D. & WOLF, F. (1977) [Contact eczemas due to forestry use
of the herbicide SELEST100.] Dtsch. Gesundheit., 32(31): 1464-1467
(in German).
JUZWIAK, I., JUZWIAK, J., SUCHOWIAK, J., & ORDA, E. (1973) [A
study of the extent of the pesticide threat to the health of
agricultural employees in Olesnicki Country.] Pol. Tyg. Lek.,
28(11): 419-422 (in Polish, Health & Welfare Canada Translation
No. 2157).
KAISER, H. (1973) Guiding concepts relating to trace analysis.
Pure appl. Chem., 34: 35-61.
KARKINEN-JAASKELAINEN, M. & SAXEN, L. (1974) Maternal influenza,
drug consumption, and congential defects of the central nervous
system. Am. J. Obstet. Gynecol., 118(6): 815-818.
KASKEVICH, L.M. & SOBOLOVA, L. P. (1978) [A case of acute
poisoning with herbicide 2,4-D (late after effects).] Gig. Tr.
Prof. Zabol., 22 (10): 49-50 (in Russian, US National
Translations Center, Translation No. 79-11916-06T).
KAS'JANENKO, A. G. & KOROLEVA, N. S. (1979) [An evaluation of the
genetic danger of pesticides.] Izv. Akad. Nauk SSSR Ser. Biol.,
3: 401-409 (in Russian).
KATEMAN, G. & PIJPERS, F.W. (1981) Quality Control in analytical
chemistry, New York, John Wiley & Sons, 276 pp.
KATZ, M., LEGORE, R.S., WEITKAMP, D., CUMMINS, J., ANDERSON D., &
MAY, D.R. (1972) Effects on fresh water fish. J. Water Pollut.
Control Fed., 44(6): 122-1250.
KAY, J.H., PALAZZOLO, B.S., & CALANDRA, J.C. (1965) Subacute
dermal toxicity of 2,4-D. Arch. environ. Health, 11: 648-651.
KENIGSBERG, Y.E. (1968) [Model myotonia in rats induced by
means of the diethylamine salt of 2,4-dichlorophenoxyacetic acid.]
Dok. Akad. Nauk Beloruss. SSR, 12(5): 473-475 (in Russian, Health &
Welfare Canada Translation No. 2440).
KETCHERSID, M.L., FLETCHALL, O.H., SANTELMANN, P.W., & MERKLE, M.G.
(1970) Residues in sorghum treated with the isooctyl ester of
2,4-D. Pestic. monit. J., 4(3): 111-113.
KHALATKAR, A.S. & BHARGAVA, Y.R. (1982) 2,4-dichlorophenoxy-
acetic acid. A new environmental mutagen. Mutat. Res., 103:
111-114.
KHANNA, S. & FANG, S. (1966) Metabolism of C14-labelled
2,4-dichlorophenoxyacetic acid in rats. J. agric. Food Chem.,
14: 500-503.
KHANNA, R.N. & KOHLI, J.D. (1977) Metabolism of chlorophenoxy
herbicides in man. In: Zaidi, S. H., ed. Proceedings of the
International Symposium on Industrial Toxicology, Lucknow, India,
November 1975, Lucknow, Industrial Toxicology Research Centre,
pp. 590-599.
KHERA, K.S. & McKINLEY, W.P. (1972) Pre- and postnatal studies on
2,4,5-T, 2,4-D and their derivatives in rats. Toxicol. appl.
Pharmacol., 22: 14-28.
KHERA, K.S. & RUDDICK, J.A. (1973) Polychlorodibenzo- p-dioxins.
Perinateal effects and the dominant lethal test in Wistar rats. In:
Blair, E.H., ed. Chlorodioxins - origin and fate. Washington, DC,
American Chemical Society, pp. 70-84 (Advances in Chemistry Series
No. 120).
KHIBIN, L.S., TAITSEL', L.A., & SLATOV, I.V. (1968) [On the
clinical aspects of acute poisoning with Dicotex 40.] Gig. Tr.
Prof. Zabol., 3: 52-53 (in Russian, Health & Welfare Canada
Translation No. 2240).
KIMBROUGH, R.D., ed. (1980) Halogenated biphenyls, terphenyls,
naphthalenes, debenzodioxins and related products. Amsterdam,
New York, Oxford, Elsevier, North Holland Biomedical Press.
KING, J.E. & PENFOUND, W.T. (1946) Effects of two of the new
formagenic herbicides on bream and largemouth bass. Ecology,
27(4): 327-374.
KING, C.T.G., HORIGAN, E.A., & WILK, A.L. (1971) Screening of the
herbicides 2,4,5-T and 2,4-D for cleft palate. Teratology, 4(2):
233.
KLEKOWSKI, R.Z. & ZVIRGZDS, J. (1971) [The influence of herbicide
2,4-D Na on respiration and survival of Simocephalus vetulus O.F.
Muller (Cladocera).] Pol. Arch. Hydrobiol., 18(4): 393-400 (in
Polish).
KLINGMAN, D.L., GORDON, C.H., YIP, G., & BURCHFIELD, H.P. (1966)
Residues in the forage and milk from cows grazing forage treated
with esters of 2,4-D. Weeds, 14: 164-167.
KNUTSON, J.C. & POLAND, A. (1980) Keratinization of mouse
teratoma cell line xB produced by 2,3,7,8-tetrachlorodibenzo- p-
dioxin: an in vitro model of toxicity. Cell, 22: 27-36.
KOCIBA, R.J. & SCHWETZ, B.A. (1982) A review of the toxicity of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) with a comparison to the
toxicity of other chlorinated dioxin isomers. Assoc. Food Drug
Offic. Q. Bull., 46(3): 168-188.
KOHLI, J.D., KHANNA, R.N., GUPTA, B.N., DHAR, M.M., TANDON, J.S., &
SIRCAR, K.P. (1974) Absorption and excretion of 2,4-
dichlorophenoxyacetic acid in man. Xenobiotica, 4(2): 97-100.
KOLBERG, J., HELCELAND, K., & JONSEN, J. (1973) Binding of
2,4-dichloro and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. Acta pharmacol. toxicol., 33(5-6): 470-475.
KOLMODIN-HEDMAN, B., ERNE, K., HÜKANSSON, M., & ENGQUIST, A. (1979)
[Control of occupational exposure to phenoxyacids (2,4-D and
2,4-5-T).] Arbete och Hälsa, 17: 3-26 (in Swedish).
KOLMODIN-HEDMAN, B. & ERNE, K. (1980) Estimation of occupational
exposure to phenoxy acids (2,4-D and 2,4,5-T). Arch. Toxicol.,
(Suppl. 4): 318-321.
KOLMODIN-HEDMAN, B., ERNE, K., & AKERBLOM, M. (1980) Field
application of phenoxy acid herbicides. In: Tordoir, W.F. &
Van Heemstra, E.A.H., ed. Field workers exposure during pesticide
application. New York, Elsevier Scientific Publishing Co., pp.73-77
(Studies in Environmental Science, Vol. 7).
KONSTANTINOVA, T.K. (1970) Toxicology and some problems of the
embryotropic action of the butyl ester of 2,4-D. In: Vygodchikov,
G.V., ed. Proceedings of a Conference on Problems on Hygiene and
Toxicology of Pesticides, USSR, 1967, Moscow, Meditsina, pp.
177-179.
KONSTANTINOVA, T.K., EFIMENKO, L.P., & ANTONEKO, T.A. (1975)
[Embryotropic effects of the products from the decomposition of
herbicides based on 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
11: 102-105 (in Russian, US National Translation Centre,
Translation No. 78-14230-06F.)
KONTEK, M., MARCINKOWSKA, B., & PIETRASZEK, Z. (1973) [Electro-
encephalographic investigations in farm workers exposed to
derivatives of arylakanocarboxylic acids.] Pol. Tyg. Lek.,
28(25): 937-939 (in Polish, Health & Welfare Canada
Translation No. 2215).
KOPISCHKE, E.D. (1972) The effect of 2,4-D and diesel fuel on egg
hatchability. J. Wildl. Manage., 36(4): 1353-1356.
KORTE, C. & JALAL, S.M. (1982) 2,4-D-induced clastogenicity and
elevated rates of sister chromatid exchanges in cultural human
lymphocytes. J. Hered., 73: 224-226.
KOSCHIER, F.J., GIRAD, P., & HONG, S.K. (1978) Transport of
2,4-dichlorophenoxyacetate by rat renal cortical slides. Toxicol.
appl. Pharmacol., 45: 883-894.
KOSCHIER, F.J. & PRITCHARD, J.B. (1979) Excretion of
2,4-dichlorophenoxyacetate (2,4-D) by the dogfish shark (Squalus
acanthias). Pharmacologist, 21(3):184.
KOSYAN, S.A., MARKOSYAN, V.E., SARKISYAN, L.S., & MANUKYAN, R.A.
(1974) [A toxicological evaluation of the new herbicide 3-nitro-4-
oxybenzyl ether of (2,4-dichlorophenoxy) acetic acid (NK-2).]
Zh. eksp. klin. Med., 14(3): 14-18 (in Russian, Health & Welfare
Canada Translation No. 2436).
KRAMER, D. & SCHMALAND, G. (1974) [Plant protection agent
residues in water: Problems in water management as a consequence of
biocide applications.] Wasserwirtsch. Wassertech., 24(5): 161-167
(in German).
KUHN, E. & STEIN, W. (1964) [Experimental myotonia with 2,4-D in
the rat.] Klin. Wochenschr., 42: 1215-1216 (in German).
KUHN, E. & STEIN, W. (1965) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D) in the rat. In vitro and in vivo
studies on the effects of 2,4-D on the energy metabolism of
muscle.] Klin. Wochenschr., 43: 673-677 (in German).
KUHN, E. & STEIN, W. (1966) [Model myotonia with 2,4-
dichlorophenoxyacetate (2,4-D).] Klin. Wochenschr., 44: 700-702
(in German).
KUZMINSKAJA, U.A. & BERSAN, L.V. (1975) [The effects of the
sodium salt of dichlorophenoxyacetic acid on the glycolysis,
ATPase, and transketolase activity of erythrocytes.] Farmakol.
Toksikol., 38(1): 102-104 (in Russian).
KUZYK, A. (1979) A report on a survey of the Alberta farming
community respecting the use of pesticides, Edmonton, Alberta
Department of the Environment, Pollution Control Division.
LAPPE, M. (1979) HLA homozygosity and neural-tube defects.
Lancet, 1: 1342.
LASKOWSKI, M.B. & DETTBARN, W.D. (1977) The pharmacology of
experimental myopathies. Ann. Rev. Pharmacol. Toxicol., 17:
387-409.
LAVY, T.L. ROETH, F.W., & FENSTER, C.R. (1973) Degradation of
2,4-D and atrazine at three soil depths in the field. J. Environ.
Qual., 2(1): 132-137.
LAVY, T.L., WALSTAD, J.D., FLYNN, R.R., & MATTICE, J.D. (1982)
(2,4-dichlorophenoxy) acetic acid exposure received by aerial
applications crew during forest spray operations. J. agric. food
Chem., 30: 373-381.
LEE, B. (1978) Herbicides under suspicion in Australia. New
Sci., 80(1125): 159.
LENG, M. (1972) Residues in milk and meat and safety to livestock
from the use of phenoxy herbicides in pasture and rangeland. Down
Earth, 28(1): 12-20.
LENG, M. (1977) Comparative metabolism of phenoxy herbicides in
animals. In: Ivie, G.W. & Dorough, H.W., ed. Fate of pesticides in
large animals, New York, San Francisco, London, Academic Press,
pp. 53-76.
LENG, M.L. (1979) Comparative toxicology of various chlorinated
dioxins as related to chemical structure. In: Bontoyan, W.R., ed.
Collaborative International Pesticides Analytical Council
Proceedings Symposium Series I, Cambridge, Heffers Printers Ltd.,
pp. 141-169.
LENG, M.L., LAVY, R.L., RAMSEY, J.C., & BRAUN, W.H. (1982)
Review of the studies with 2,4,5-T in humans including applicators
under field conditions, pp. 133-156. In: Plimmer, J.R., ed.:
Pesticide residues and exposure. Washington, DC, American Chemical
Society, (ACS Symposium Series 182).
LHOSTE, J. & ROTH, P. (1946) [The effect of aqueous sodium
2,4,-dichlorophenoxyacetate on the development of eggs of Rana
temporaria L.] C.R. Soc. Biol. Paris, 140: 272-274.
LIBICH, S., TO, J.C., WANG, K. Y., & WATSON, D. A. (1981) A
study to assess the occupational exposure to 2,4-D herbicides,
Toronto, Safety Services Department, Health and Safety Division.
pp. 16 (Ontario Hydro. Report SSD-81-1).
LINDQUIST, N.G. & ULLBERG, S. (1971) Distribution of the
herbicides 2,4,5-T and 2,4-D in pregnant mice. Accumulation in the
yolk sac epithelium. Experientia (Basel), 27: 1439-1441.
LINNAINMAA, K. (1983a) Non-mutagenicity of phenoxyacid herbicides
2,4-D and MCPA. In: Keith, L.H., Choudhary, G., & Rappe, C., ed.:
Chlorinated dioxins and dibenzofurans in the total environment.
Ann Arbor, Ann Arbor Sci. Publ. Inc., Vol. 1.
LINNAINMAA, K. (1983b) Induction of sister chromatid exchanges by
the peroxisome proliferators 2,4-D, MCPA, and clofibrate in vivo
and in vitro. Carcinogenesis, accepted for publication.
LIPSCOMB, G.Q. (1968) Pesticide residues in prepared baby foods
in the United States. Pestic. monit. J., 2(32):104-108.
LISK, D.J., GUTENMANN, W.H., BACHE, C.A., WARNER, R.G., & WAGNER,
D.G. (1963) Elimination of 2,4-D in the urine of steers fed 4
(2,4-DB) or 2,4-D. J. dairy Sci., 46: 1434-1435.
LIU, L.C. & CIBES-VIADE, H.R. (1973) Adsorption of fluometuron,
prometryne, Sencor and 2,4-D by soils. Univ. Puerto Rico, J.
Agric., 57: 286-293.
LOKKE, H. (1975) Analysis of free and bound chlorophenoxy-acids
in cereals. Bull. environ. Contam. Toxicol., 13: 730-736.
LOKTIONOV, V.N., BUDARKOV, V.A., KUZNETSOV, A.E., & KUZNETSOVA,
N.V. (1973) [Toxicological characteristics of 2,4-D derivatives.]
Veterinariya, 5: 107-109 (in Russian, Health & Welfare Canada
Translation No. 2173).
LONDONO, F. (1966) [Occupational acne. Five cases caused by
herbicide.] Med. cutanea, 3: 225-232 (in Spanish).
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMANN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. J. Am. Ind. Hyg.
Assoc., 20(3): 298-304.
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel
Dekker, pp. 1-49.
LOOS, M.A. (1975) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Herbicides: Chemistry, degradation and mode of
action, New York, Marcel Dekker, pp. 1-128.
LOPEZ, M.R. (1961) [Action of 2,4-dichlorophenoxyacetic acid and
its sodium salt on the spermatozoa of the frog.] Biol. R. Soc.
Esp. Hist. Nat., 59: 219-226 (in Spanish).
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960a) Metabolism of plant
growth-regulators. I. 2,4-dichlorophenoxyacetic acid in leaves of
red- and blackcurrant. Ann. appl. Biol., 48: 613-625.
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960b) Metabolism of plant
growth-regulators. II. Decarboxylation of 2,4-dichloro-
phenoxyacetic acid in leaves of apple and strawberry. Ann. appl.
Biol., 48: 626-636.
LUKOSHKINA, L.P., GUESEINOVA, R.S., & MELIK-ZADE, T.M. (1970)
Study of certain aspects of lipid-protein-carbohydrate metabolism
in workers engaged in 2,4-D production. Trud. Azerbaid. Nauchno-
Issled. Instituta Gig. prof. Zabol., No.5: 146-149.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de l'herbicide
2,4-D sur le développement embryonaire et la fécondité du gibier ą
plumes. C.R. Acad. Sci. Paris, Ser. D., 271(25): 2418-2421.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1974) Sexualité et les pesticides.
Ann. Biol. anim. (Paris), 13(3-4): 173-185.
MAAS, W. & KERSSEN, M.C. (1973) Drift studies in ULV and
conventional applications. Agric. Aviation, 15: 41-50.
MACKENTHUN, K.M. & KEUP L.E. (1972) Freshwater macroinvertebrates.
J. Water Pollut. Control Fed., 44(6): 1137-1150.
MACLEAN, G.J. & DAVIDSON, J.H. (1970) Diagnosis of disorders in
pastured livestock. Down Earth, 25(4): 12-16.
MAGNUSSON, J., RAMEL, C., & ERIKSSON, A. (1977) Mutagenic effects
of chlorinated phenoxyacetic acids in Drosophila melanogaster.
Hereditas, 85: 121-123.
MAIER-BODE, H. (1971) [Herbicides and their residues.], Stuttgart,
FRG, Verlag Eugen Ulmer, 479 pp. (in German).
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VII). Pesticide Mont. J., 8(2): 110-124.
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VIII). Pesticide Mont. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples VII. Pestic. Monit. J., 9(2): 94-105.
MARTH, P.C. & MITCHELL, J.W. (1949) Comparative volatility of
various forms of 2,4-D. Bot. Gaz., 110: 632-636.
MARTYNOV, Y.E. (1970) The effect of 2,4-d compounds on wild warm
blooded animals. Lesn. Khoz., 6: 57-59 (in Russian, Health &
Welfare Canada Translation No. 2186).
MASON, R.W. (1975) Binding of some phenoxyalkanoic acids to
bovine serum albumin in vitro. Pharmacology, 13: 177-186.
MATSUNAKA, S. (1972) Metabolism of pesticides in higher plants.
In: Matsumura, F., Boush, G.M., & Mitsato, T., ed. Environmental
toxicology of pesticides, New York, Academic Press, pp. 341-365.
MAYBANK, J. & YOSHIDA, K. (1969) Delineation of herbicide-drift
hazards on the Canadian prairies. Trans. Am. Soc. Agric. Eng.,
12(6): 759-762.
MAYBANK, J., YOSHIDA, K., & GROVER, R. (1978) Spray drift from
agricultural pesticide applications. J. Air Poll. Control Assoc.,
28: 1009-1014.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979a) Changes of metabolism
during experimentally induced myotonia of rats. I. Alterations in
lactate and malate dehydrogenase isoenzyme activities. Biochem.
Med., 22: 350-358.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979b) Changes of metabolism
during experimentally induced myotonia of rats. II. Alterations in
creatinine kinase and acid phosphatase activities: a possible
mechanism in the development of muscle damage. Biochem. Med.,
22: 359-364.
MCARDLE, F.J., MARETZKI, A.N., WILEY, R.C., & MODREY, M.G. (1961)
Influence of herbicides on flavor of processed fruits and
vegetables. J. agric. food Chem., 9: 228-230.
MCCONNELL, E.E., MOORE, J.A., HASEMAN, J.K., & HARRIS, M.W. (1978)
The comparative toxicity of chlorinated dibenzo- p-dioxins in mice
and guniea pigs. Toxicol. appl. Pharmacol., 44: 335-356.
MCLENNAN, M.W. (1974) 2,4-D toxicity in diary cattle. Aust.
vet. J., 50: 578.
MEEHAN, W.R., NORRIS, L.R., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in Southeast Alaska.
J. Fish. Res. Board Can., 31(4): 480-485.
MELNIKOV, M.N. (1971) Chemistry of pesticides. Res. Rev.,
36: 165-166.
MIERZWA, S. & WITEK, S. (1977) Gas-liquid chromatographic
method with electroncapture detection for the determination of
residues of some phenoxyacetic acid herbicides in water as their
2,2,2-trichloroethyl esters. J. Chromatogr., 136: 105-111.
MILHAUD, G., RIET ALVARIZA, F., CHARLES, E., & SABBACH, M. (1970)
Toxicologie des phytohormones. Rec. Méd. Vet., 146: 345-357.
MITCHELL, J.W., HODGSON, R.E., & GAETJENS, C.F. (1946) Tolerance
of farm animals to feed containing 2,4-dichloro-phenoxyacetic acid.
J. anim. Sci., 5(1): 226-232.
MONARCA, G. & DIVITO, G. (1961) [On acute poisoning by a herbicide
(2,4-dichlorophenoxyacetic acid).] Folia med. (Naples), 44(6):
480-485 (in Italian, Health & Welfare Canada Translation No. 2234).
MONTGOMERY, M.L., CHANG, U.L., & FREED, V.H. (1971) Comparative
metabolism of 2,4-D by bean and corn plants. J. agric. food Chem.,
19: 1219-1221.
MOREALE, A. & VAN BLADEL, R. (1980) Behaviour of 2,4-D in Belgian
soils. J. environ. Qual., 9: 627-633.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
microbial reversion assay systems. Mutat. Res., 116: 185-216.
MORRE, D.J. & ROGERS, B.J. (1960) The fate of long chain esters
of 2,4-D in plants. Weeds, 8: 436-447.
MORTON, H.L., ROBINSON, E.D., & MEYER, R.T. (1967) Persistence of
2,4-D, 2,4,5-T, and dicamba in range forage grasses. Weeds,
15: 268-271.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxy-
ethane ester of 2,4-D. Trans. Am. Fish. Soc., 96(2): 185-193.
MUIR, C.S. & WAGNER, G. (1982) Directory of on-going research in
cancer epidemiology 1982, Lyons, International Agency for Research
on Cancer, (IARC Scientific publications No. 46) pp. 86 (Abstract
No. 227), 432(1146) 460(1221).
MUKULA, J., SILTANEN, H., ROSENBERG, C., IANPAAN-HEIKKILA, J., &
LALLUKKA, R. (1978) Phenoxy herbicide residues in cowberries,
mushrooms and the twigs of forest plants. Ann. Agric. Fenn.,
17: 23-31.
MUNRO, H.E. (1972) Determination of 2,4-dichloro-phenoxyacetic
acid and 2,4,5-trichlorophenoxyacetic acid in tomato plants and
other commercial crops by microcoulometric gas chromatography.
Pestic. Sci., 3: 371-377.
MURTHY, M.S.S. (1979) Induction of gene conversion in diploid
yeast by chemicals: Correlation with mutagenic action and its
relevance in genotoxicity screening. Mutat. Res., 64: 1-17.
NASH, R.G., KEARNEY, P.C., MAITLEN, J.C., SELL, C.R., & FERTIG,
S.N. (1982) Agricultural applicators' exposure to 2,4-
dichlorophenoxyacetic acid. In: Plimmer, J.R., ed. Pesticide
residues and exposure, Washington, DC, American Chemical Society,
pp. 119-132 (ACS Symposium Series 182).
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1981)
Polychlorinated dibenzo- p-dioxins: Criteria for their effects on
man and his environment, Ottawa, NRCC Publications Service, NRCC
Publication No. 18574, 251 pp.
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1978) Phenoxy
herbicides. Their effects on environmental quality, Ottawa, NRCC
Publications Service, NRCC publication no. 16075, 437 pp.
NAYRAC, P., FONTAN, P., WAROT, P., LESCUT, J., & DELAHOUSSE, J.
(1958) Polyradiculonévrite subaciguė chez deux agriculteurs:
intoxication par des produits anti-parasitaires. Lille Med.,
3(3): 161-164.
NESBITT, H.J. & WATSON, J.R. (1980a) Degradation of the
herbicide 2,4-D in river water. I. Description of the study
area, and survey of rate determining factors. Water Res.,
14(12): 1683-1688.
NESBITT, H.J. & WATSON, J.R. (1980b) Degradation of the
herbicide 2,4-D in river water. II. The role of suspended
sediment, nutrients, and water temperature. Water Res.,
14(12): 1689-1694.
NIELSEN, N.O. (1968) Teratogenic effects of hyperthermia in
chicken embryo. Preliminary report. Teratology, 1: 102-107.
NIELSEN, K., KAEMPE, B., & JENSEN-HOLM, J. (1965) Fatal
poisoning in man by 2,4-D: Determination of the agent in
forensic materials. Acta pharmacol. toxicol., 22: 224-234.
NIKANDROV, V.N. (1974). [The activity of malate dehydrogenase in
some organs and tissues of albino rats after administration of the
amine salt of 2,4-D,] Vesti Akad. Navuk Belaruskai SSR (Ser.
Biol.) 1: 126-127 (in Belorussian).
NISHIUCHI, Y. & YOSHIDA, K. (1974) [Toxicity of new agricultural
chemicals to tadpoles.] Bull. agric. Chem. Insp. St., 14: 66-68
(in Japanese).
NORSTROM, A., RAPPE, C., LINDAHL, R., & BUSTER, H.R. (1979)
Analysis of some older Scandinavian formulations of 2,4-D and
2,4,5-T for contents of chlorinated dibenzo- p-dioxins and
dibenzofurans. Scand. J. Work Environ. Health, 5: 375-378.
OLSON, B.A., SNEATH, T.C., & JAIN, N.C. (1978) Rapid, simple
procedures for the simultaneous gas chromatographic analysis
of four chlorophenoxy herbicides in water and soil samples.
J. agric. food Chem., 26: 640-643.
ORBERG, J. (1980a) Observations on the 2,4-dichlorophenoxy-
acetic acid (2,4-D) excretion in the goat. Acta pharmacol.
toxicol., 46: 78-80.
ORBERG, J. (1980b) Effects of low protein consumption on the
renal clearance of 2,4-dichlorophenoxyacetic acid (2,4-D) in goats.
Acta pharmacol. toxicol., 46: 138-140.
OSADCHUK, M., SALAHUB, E., & ROBINSON, P. (1977) Isolation of
chlorophenoxy herbicides on columns of glass beads. J. Assoc. Off.
Anal. Chem., 60: 1327-1327.
OSTERLOH, J., LOTTI, M., & POND, S.M. (1983) Toxicologic studies
in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. anal.
Toxicol., 7: 125-129.
OU, L.T., ROTHWELL, D.F., WHEELER, W.B., & DAVIDSON, J.M. (1978)
The effect of high 2,4-D concentrations on degradation and carbon
dioxide evolution in soils. J. environmental Qual., 7(2): 241-246.
PADEROVA, V.P. (1975) [Some hygienic problems of using 2,4-D
herbicides.] Gig. i Sanit., 40(9): 96-97 (in Russian).
PAGGIARO, P.L., MARTINO, E., & MARIOTTI, S. (1974) [On a case of
poisoning by 2,4-dichlorophenoxyacetic acid.] Med. lav., 65(3-4):
128-135 (in Italian, Health & Welfare Canada Translation No. 2200).
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens. In: Production Research Report, Washington,
DC, Agricultural Research Service, pp. 1-38.
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some
organic herbicides to cattle, sheep, and chickens. In: Production
Research Report, Washington, DC, Agriculture Research Service,
pp. 1-26.
PAL'MOVA, I.V. & GALUZOVA, L.V. (1963) [Maximum permissible
concentration of sodium salt and butyl ester formulations of
2,4-D in water.] Gig. i Sanit., 28(7): 11-13 (in Russian,
Health & Welfare Canada Translation No. 2717).
PEMBERTON, J.M. (1979) Pesticide degrading plasmids: a
biological answer to environmental pollution by phenoxyherbicides.
Ambio, 8: 202-205.
PHILLEO, W.W. & FANG, S.C. (1967) Effects of 2,4-D on metabolism.
J. agric. food Chem., 15(2): 256-260.
PIERIDES, A.M., ALVAREZ-UDE, F., KERR, D.N.S., & SKILLEN, A.W.
(1975) Clofibrate-induced muscle damage in patients with chronic
renal failure. Lancet, 2: 1279-1282.
PILINSKAJA, M.A. (1974) [Cytogenetic effect of the herbicide
2,4-D on human and animal chromosomes.] Tsitol. Genet., 8(3):
202-206 (in Russian).
POCCHIARI, F., SILANO, V., & ZAMPIER, A. (1979) Human health
effects from accidental release of tetrachlorodibenzo- p-dioxin
(TCDD) at Seveso, Italy. Ann. NY Acad. Sci., 320: 311-320.
PODOLAK, M. (1979) [Effect of paraquat and 2,4-dichloro-
phenoxyacetic acid (2,4-D) on oxidative phosphorylation and
respiration of brain mitochondria in the rat.] Bromatol. Chem.
Toksykol., 12(2): 147-152 (In Polish).
POLAND, A. & GLOVER, E. (1973) 2,3,7,8-Tetrachlorodibenzo- p-
dioxin: a potent inducer of S-aminolevulinic acid synthetase.
Sci., 179: 476.
POLAND, A. & GLOVER, E. (1973) Chlorinated dibenzo- p-dioxins:
potent inducers of -aminolevulinic acid synthetase and aryl
hydrocarbon hydroxylase. Molec. Pharmacol., 9: 736-747.
POLAND, A. GLOVER, E. (1976) Sterospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo -p-dioxin by hepatic cytosol.
J. Biol. Chem., 251(16): 4936-4946.
POLAND, A. & KENDE, A. (1976) 2,3,7,8-tetra-chlorodibenzo- p-
dioxin environmental contaminant and molecular probe. Fed. Proc.,
35: 2404-2411.
POLAND, A., SMITH, D., METTER, G., & POSSICK, P. (1971) A health
survey of workers in a 2,4-D and 2,4,5-T plant. Arch. environ.
Health, 22: 316-327.
PRAVDA, O. (1973) [On the influence of herbicides on some fresh-
water animals.] Hydrobiologia, 42(1): 97-142 (in German).
PREISS, D. & ROSSNER, J.A. (1971) [Effect of 2,4-
dichlorophenoxyacetic on the transverse tubular system of the
mycardium.] Naturwissenschaften, 11: 576-577 (in German).
PRESCOTT, I.F., PARK, J., & DARRIEN, I. (1979) Treatment of
severe 2,4-D and mecoprop intoxication with alkaline diuresis.
Br. J. clin. Pharmacol., 7: 111-116.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder.
J. Pharmacol. exp. Therap., 208(2): 280-286.
PRITCHARD, J.B. & MILLER, D.S. (1980) Teleost kidney in
evaluation of xenobiotic toxicity and elimination. Fed. Proc.,
39(14): 3207-3212.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis
in primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PROVINCE OF BRITISH COLUMBIA, MINISTRY OF ENVIRONMENT (1981)
A review of the use of 2,4-D for Eurasian water milfoil control in
the Okanagan Lakes, 1976-1980. Inf. Bull., aquatic Plant Manage.
Program, 10: 1-10.
QUE HEE, S.S. & SUTHERLAND, R.G. (1974) Purity of reagent grade
p- and o-chlorophenoxyacetic acids and its biological implications.
J. agric. food Chem., 22: 726-727.
QUE HEE, S.S. & SUTHERLAND, R.G. (1975) A specific gas-liquid
chromatographic method for analysis of some amine salts of 2,4-
dichlorophenoxyacetic acid. J. agric. food hem., 23: 1007-1008.
QUE HEE, S.S. & SUTHERLAND, R.G. (1981) The phenoxyalkanoic
herbicides, Vol. I. Chemistry, analysis and environmental
pollution, Boca Raton, CRC Press, Inc., 321 pp.
RADIONOV, A.D., CHUMACHENKO, A.N., & KIRILENKO, I.I. (1967)
The toxic properties of the herbicide 2,4-D. Hyg. Sanit.,
32(4-6): 116-118.
RAMEL, C. (1978) Chlorinated phenoxy acids and their dioxins.
Mode of action, health risks, and environmental effects. Ecol.
Bull. (Stockholm), 27: 302.
RAOUL, Y. & MARNAY, O. (1948) [Effect of 3-indolacetic acid
2,4-dichlorophenoxyacetic acid on the growing rat.] C. R. Acad.
Sci. (Paris), 226: 1043-1045.
RAPPE, C. & BUSER, H.R. (1981) Occupational exposure to
polychlorinated dioxins and dibenzofurans. In: Choudhary, G.,
Chemical hazards in the workplace: Measurement and control,
Washington, DC, American Chemical Society, pp. 319-342 (ACS
Symposium Series No. 149).
RASMUSON, B. & SVAHLIN, H. (1978) Mutagenicity tests of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid in genetically stable and unstable strains of Drosophila
melanogaster. Ecol. Bull. (Stockholm), 27: 190-192.
RAWLS, C.K. (1965) Field tests of herbicide toxicity to
certain estuarine animals. Chesapeake Sci., 6(3): 150-161.
REA, W.J. (1978) Environmentally triggered cardiac disease.
Ann. Allergy, 40(4): 243-251.
REDDY, J.K., AZARNOFF, D.L., & HIGNITE, E.E. (1980)
Hypolipidaemic hepatic peroxisome proliferators form a novel
class of chemical carcinogens. Nature (Lond.), 283: 397-398.
REDDY, J.K., QURESHI, S.A., LALWANI, N.D., REDDY, M.K., & MOEHLE,
C.M. (1982a) Induction by ciprofibrate of hepatic peroxisome
proliferation in rats, pigeons, chickens, cats and rhesus monkeys.
Fed. Proc., 41: 1741.
REDDY, J.K., LALWANI, N.D., REDDY, M.K., & QURESHI, S.A. (1982b)
Excessive accumulation of autofluorescent lipofuscin in the liver
during hepatocarcinogenesis by methyl clofenapate and other
hypolipidaemic peroxisome proliferators. Cancer Res., 42: 259-266.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977) Investigations
into the acute toxicity and some chronic effects of selected
herbicides and pesticides on several fresh water fish species.
Bull. environ. Contam. Toxicol., 18(3): 361-365.
RENBERG, L. (1974) Ion exchange technique for the determination
of chlorinated phenols and phenoxy acids in organic tissue, soil
and water. Anal. Chem., 46: 459-461.
RIIHIMAKI, V., ASP, S., & HERNBERG, S. (1982) Mortality of
chlorinated phenoxyacid herbicide 2,4-D and 2,4,5-T applicators in
Finland. First report of an ongoing prospective follow-up study.
Scand. J. Work Environ. Health, 8: 37-42.
RIVERS, J.B., YAUGER, W.L., Jr., & KLEMMER, H.W. (1970)
Simultaneous gas chromatographic determination of 2,4-D and
dicamba in human blood and urine. J. Chromatogr., 50: 334-337.
ROBINSON, E. & FOX, L.L. (1978) 2,4-D herbicides in central
Washington. J. Air Poll. Control Assoc., 28: 1015-1020.
ROBSON, T.O. (1966) Some studies of the persistence of 2,4-D in
natural surface waters in Britain. Proc. Br. Weed Control Conf.,
2: 594-597.
ROSENBERG, J. (1980) 2,4-Dichlorophenoxyacetic acid (2,4-D)
- Evaluation of human health hazards, Berkeley, California,
Hazard Alert System, Epidemiological Studies Laboratory, 64 pp.
ROSS, R.D. MORRISON, J., ROUNBEHLER, D.P., FAN, S., & FINE, D.H.
(1977) N-nitroso compound impurities in herbicide formulations.
J. agric. food Chem., 25: 1416-1418.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation
of the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUDIGER, K.D., MEERBACH, W., OSSKE, G., & FISCHER, W. (1972) [On
the experimental 2,4-dichlorophenoxyacetate myopathy.] Zentralbl.
allg. Pathol., 115: 145-152 (in German).
SADYKOV, R.E., RABOCHEV, V.K., & STROKOV, Y.N. (1972) [The
effect of butyl 2,4-D treatment of pastures on the reproductive
functions of sheep.] Zhivodnovodstvo, 34(1): 73-74 (in Russian).
SANDERS, H.O. (1970a) Pesticide toxicities to tadpoles of the
western chorus frog, Pseudacris triseriata and Fowler's toad, Burfo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. (1970b) Toxicities of some herbicides to six
species of freshwater crustaceans. J. Water Pollut. Control Fed.,
42(8): 1554-1550.
SARE, W.M. (1972) The weedicide 2,4-D as a cause of headaches and
diplopia. New Z. med. J., 75(478): 173-174.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & LEBEAU, J.E. (1976)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicol. appl. Pharmacol., 37(1): 136-137.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & GEHRING, P.J. (1977)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicology, 8: 3-11.
SAXEN, L., KLEMETTI, A., & HARO, A.S. (1974) A matched-pair
register for studies of selected congenital defects. Am. J.
Epidemiol., 100(3): 297-306.
SCHACTER, B., GYVES, M., MUIR, A., & TASIN, M. (1979) HLA-A, B
compatibility in parents of offspring with neural-tube defects or
couples experiencing involuntary fetal wastage. Lancet, 1: 796-797.
SCHILLER, K. (1964) [Effects on the fertility of rats produced by
substances with special effects in potato cultivation. 1st
communication: Growth hormones and weed control agents 2,4-D and
MCPA.] Landbauforsch. Völkenrode, 14(2): 111-114 (in German).
SCHILLINGER, J.E. (1960) [Hygienic evaluation of agricultural
products cultivated with the use of herbicides.] J. Hyg.
Epidemiol. Microbiol. Immunol., 4: 243-252 (in German).
SCHNEIDER, B.A. (1979) Toxicological evaluation of pesticides by
the EPA Chemical and Biological Investigations Branch Laboratories
(1966-77). In: Toxicology handbook, mammalian and aquatic data.
Book 1: Toxicology data. Report for 1966-67, Beltsville, Md., US,
Environmental Protection Agency.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8(3): 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues at
Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7(3/4):
146-152.
SCHUPHAN, W. (1963) [Impaired health due to plant protection
agents.] Der Landarzt, 39: 1217-1222 (in German).
SCHUPHAN, W. (1965) [Plant protection problems reflected in
quality testing.] Anz. Schädlingsk. D. Pflanzenschutz.
Umweltschutz, 38(7): 97-104 (in German).
SCHUPHAN, W. (1969) [Pesticides: Usefulness and possible harm.]
Zentralbl. Bakteriol., 210: 240-258 (in German).
SCHWETZ, B.A., SPARSCHU, G.L., & GEHRING, P.J. (1971) The effect
of 2,4-D and esters of 2,4-D on rat embryonal, foetal and neonatal
growth and development. Food cosmet. Toxicol., 9: 801-817.
SCHWETZ, B.A., NORRIS, J.M., SPARSHU, G.L., ROWE, V.K., GEHRING,
P.J., EMERSON, J.L., & GERBIG, C.G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect., Exp.
Issue 5: 87-99.
SEABURY, J.H. (1963) Toxicity of 2,4-dichlorophenoxyacetic acid
for man and dog. Arch. environ. Health, 7: 202-209.
SEILER, D. (1971) The ATPase of the sarcolemma of skeletal
muscle in experimental myotonia. Experientia (Basel), 27:
1170-1171.
SEILER, J.P. (1978) The genetic toxicology of phenoxy acids
other than 2,4,5-T. Mutat. Res., 55: 197-226.
SENCZUK, W. & POGORZELSKA, H. (1975) [Urinary excretion of
2,4-dichlorophenoxyacetic acid.] Rocz. Panstw. Zakl. Hig.,
26(2): 217-222 (in Polish, Health & Welfare Canada Translation
No. 2451).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxodynamic properties of phenoxycarboxylic acid derivatives, II.
Methods of determining derivatives of phenoxyacetic and
phenoxypropionic acids in blood and urine.] Rocz. Panstw. Zakl.
Hig., 32: 339-344 (in Polish).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxicodynamic properties of derivatives of phenoxyacetic and
phenoxypropionic acid, III. The pattern of absorption into the
bloodstream and measurements of urinary excretion of phenoxyacetic
and phenoxypropionic acid.] Roczn. Panstw. Zak. Hig., 32(5-6):
419-426 (in Polish).
SENCZUK, W., POGORZELSKA, H., & DEBSKA, M. (1980) [Absorption of
2,4-dichlorophenoxyacetic acid through the skin.] Roczn. Panstw.
Zak. Hig., 31(6): 611-614 (in Polish).
SENGES, J. & RDEL, R. (1972) Experimental myotonia in mammalian
skeletal muscle: changes in contractile properties. Pflueger.
Arch., 331: 315-323.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method for
determination of low levels of exposure to 2,4-D and 2,4,5-T.
Int. J. environ. anal. Chem., 1: 23-33.
SHEARER, R.W. (1980) Public health effects of the aquatic use of
herbicides: 2,4-D dichlobenil, endothall and diquat. In: Shearer,
R.W. & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlorbenil, diquat & endothall, Municipality of
Washington, US, Metropolitan Seattle, pp. 1-76.
SHEARER, R. & HALTER, M. (1980) Literature reviews of four
selected herbicides: 2,4-D, dichlobenil, diquat, and endothall.
Municipality of Washington, US, Metropolitan Seattle.
SHILLINGER, U.Y.I. & NAUMOVA, L.P. (1957) [Hygienic evaluation of
a harvest in connection with the treatment of crops with the
herbicide 2,4-D.] Gig. i Sanit., 7: 33-38 (in Russian).
SHIM, J.C. & SELF, L.S. (1973). Toxicity of agricultural
chemicals to larvivorous fish in Korean rice fields. Trop. Med.,
15(3): 123-130.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., & RAATIKAINEN, M.
(1981). Triclopyr, glyphosate and phenoxyherbicide residues in
cowberries, bilberries and lichen. Bull. Environ. Contam Toxicol.,
27: 731-737.
SIMPSON, G.R. (1982a) Pesticide exposure - New South Wales
Services. In: Education and safe handling in pesticide application,
studies in environmental science, 18: 209-214. Amsterdam,
Oxford, New York, Elsevier Scientific Publishing Co.
SITTIG, M. ed., (1980) Pesticide manufacturing and toxic
materials control encyclopedia. Park Ridge, NJ, USA, Noyes Data
Corp., pp. 229-234.
SKELLY, N.E., STEVENS, T.S., & MAPES, D.A. (1977) Isomer-specific
assay of ester and salt formulations of 2,4-dichlorophenoxyacetic
acid by automated high pressure liquid chromatography. J. Assoc.
Off. Anal. Chem., 60: 868-872.
SMALS, A.G.H., BEEX, L.A.V.M., & KLOPPENBORG, P.W.C. (1977)
Clofibrate-induced muscle damage with myoglobinuria and
cardiomyopathy. N. Engl. J. Med., 296: 942.
SMITH, A.E. (1972) The hydrolysis of 2,4-dichlorophenoxy-acetate
esters to 2,4-dichlorophenoxyacetic acid in Saskatchewan soils.
Weed Res., 12: 364-372.
SMITH, A.E. (1976a) The hydrolysis of herbicidal phenoxy-alkanoic
esters to phenoxyalkanoic acids in Saskatchewan soils. Weed Res.,
16: 19-22.
SMITH, A.E. (1976b) Method for the determination of
2,4-dichlorophenoxyacetic acid in urine. J. Chromatogr, 171:
482-485.
SMITH, A.H., FISHER, D.O., PEARCE, N., & CHAPMAN, C.J. (1982)
Congenital defects and miscarriages among New Zealand 2,4,5-T
sprayers. Arch. environ. Health, 37(4): 197-200.
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of
large-scale applications of 2,4-D on aquatic fauna and water
quality. Pestic. monitoring J., 1(3): 16-21.
SOMERS, J., MORAN, E.T., REINHART, B.S., & STEPHENSON, G.R. (1974a)
Effect of external application of pesticides to the fertile egg on
hatching success and early chick performance. 1. Pre-incubation
spraying with DDT and commercial mixtures of 2,4-D: picloram, and
2,4-D: 2,4,5-T. Bull. environ. Contam. Toxicol., 11(1) 33-38.
SOMERS, J., MORAN, E.T., & REINHART, B.S. (1974b) Effect of
external application of pesticides to the fertile egg on
hatching success and early chick performance. 2. Commercial
herbicide mixtures of 2,4-D with pioloram or 2,4,5-T using the
pheasant. Bull. environ. Contam. Toxicol., 11(4): 339-342.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974c) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11(4): 511-516.
SOS, J. & KERTAI, P. (1958) Effect of dichlorophenoxyacetic acid
upon the I131-uptake of the thyroid. Acta physiol. Acad. Sci.
Hung., 14: 367-369.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on
the embryonic development of Phasianus colchicus (L.) and Coturni
coturnix japonica (L.).] Z. Jagdwiss., 22: 197-201 (in German).
STANLEY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANOSZ, S. (1969) [The effects of sodium salt of 2,4-
dichlorophenoxy-acetic acid on cell respiration in the parenchymal
organs of rats.] Med. Prac., 20(6): 600-605 (in Polish).
STEIN, W. & KUHN, E. (1968) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D). Isolated rat diaphragm preparation
as simple experimental object.] Klin. Wochenschr., 46: 328-330
(in German).
STEVENS, L. J., COLLIER, C. W., & WOODHAM, D. W. (1970)
Monitoring pesticides in soils from areas of regular, limited, and
no pesticide use. Pestic. Monit. J., 4(3): 145-164.
STEVENS, T.S.A., SKELLY, N.E., & GRORUD, R.B. (1978) Isomer-
specific assay of ester and salt formulations of 2,4-
dichlorophenoxyacetic acid by high pressure liquid chromatography:
Collaborative study. J. Assoc. Off. Anal. Chem., 61: 1163-1165.
STEWART, D.K.R. & GAUL, S.O. (1977) Persistence of 2,4-D,
2,4,5-T, and dicamba in a dykeland soil. Bull. environ. Contam.
Toxicol., 18(2): 210-218.
STRACH, S. & BOHOSIEWICZ, M. (1964) [Studies of the toxicity for
pigs of the weed-killer "Pielik."] Med. weter, 20(11): 662-666
(in Polish, Health & Welfare Canada Translation No. 2467).
STUPNIKOV, A.A. (1959) In: [Collected works of the Leningrad
Veterinary Sciences Research Institute.] No. 8: 289 (in Russian).
SUDAK, F.N. & CLAFF, C.L. (1960) Survival of Uca pugnax in sand,
water, and vegetation contaminated with 2,4-dichloro-phenoxyacetic
acid. Proc. Northeast. Weed Control Conf., 14: 508-510.
SUDAK, F.N., CLAFF, C.L., & CANTOR, M. (1966) Body temperature
regulation in rats treated with 2,4-dichloro-phenoxyacetic acid.
Arch. int. Pharmacodyn. Ther., 160(2): 253-264.
SUFFET, I.H. (1973a) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 2.
Selection of water: solvent ratios and number of extractions.
J. agric. food Chem., 21: 288-294.
SUFFET, I.H. (1973b) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 3.
Liquid-liquid extraction of phenoxy acid herbicides from water.
J. agric. food Chem., 21: 591-598.
SUNDSTROM, G., JENSEN, S., JANSSON, B., & ERNE, K. (1979)
Chlorinated phenoxyacetic acid derivatives and tetrachlorodibenzo- p-
dioxin in foliage after application of 2,4,5-trichlorophenoxyacetic
acid esters. Arch. environ. Contam. Toxicol., 8: 441-448.
SZOCS, J., MOLNAR, V., BALOGH, E., AJTAY, M., & FULOP, I. (1970)
[Experimental data on the toxic effects of 2,4-D (Diclordon)
herbicide.] Rev. Med. (Tirgu-Mures, Rom.), 16: 91-93 (in
Hungarian).
THIELE, E., BAUFELD, W., GRIMM, U., LEHNIGK, B., & THIELE, W.
(1981a) [Results of analysis for deriving exposure time limits in
the industrial hygiene monitoring of people working at agrochemical
centres who are exposed to pesticides.] Z. gesamte Hyg. Grenzgeb.,
27(3): 173-177 (in German).
THIELE, E., FIEBER, C., JASTROCH, S., KNOLL, W., & LANGE, B.
(1981b) [Determination of toxic concentrations at places of work
and patterns of distribution of pesticide exposure of people
working at agrochemical centres subdivided by substance groups.]
Z. gesamte Hyg. Grenzgeb., 27(3): 177-180 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & LINK, R. (1982) Mortality
study of persons exposed to dioxin in a trichlorophenol-process
accident that occurred in the BASF AG on November 17, 1953.
Am. J.ind. Med., 3: 179-189.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964a) Metabolic
fate of some chlorinated phenoxyacetic acids in the stem tissue of
Avena sativa. Nature (Lond.), 204: 286.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964b) Metabolic
fate of 2,4-dichlorophenoxyacetic acid in the stem tissue of
Phaseolus vulgaris. Nature (Lond.), 204: 884-885.
THOMSSEN, C. (1958) [Contribution to the question of possible
poisoning of domestic animals through growth hormone-based weed
control agents.] Arch. exp. Veterinaermed., 12: 216-221 (in
German).
TODD, R.L. (1962) A case of 2,4-D intoxication. J. Iowa Med.
Soc., 52: 663-664.
TSAPKO, V.P. (1966) The herbicide 2,4-D as a health hazard in
agriculture. Hyg. Sanit., 9: 449-450.
TSILIKOV, V.V. (1969) [Effect of the sodium salt of 2,4-D,
trichlorometaphos, nicotine, and arecoline on thyroid function.]
In: Denisenko, P.P., ed. Farmakol. Tsent. Kholinolitikov Drugikh
Neirootropnykh Sredstv., pp. 181-185 (in Russian, Health & Welfare
Canada Translation No. 2447).
TUINSTRA, L.G.M., ROOS, A.H., & BRONSGEEST, J.M. (1976)
Suggestions for a sensitive multi-detection high-performance
liquid chromatography method for several phenoxy-acid herbicides.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 41: 1443-1448.
TULP, M.T.M. & HUTZINGER, O. (1978) Rat metabolism of
polychlorinated dibenzo- p-dioxins. Chemosphere, 7(9): 761-768.
UNGER, T.M., KLIETHERMES, J., VAN GOEHAM, D., & SHORT, R.D.
(1980) Teratology and postnatal studies in rats on propylene
glycol butyl ether and isooctyl esters of 2,4-dichloro-
phenoxyacetic acid, Washington, US Environmental Protection
Agency (60/12-80).
US EPA (1976) National interim primary drinking water
regulations, Washington, DC, US Environmental Protection Agency.
US EPA (1980a) Environmental Protection Agency Review and
Conclusions Concerning Potential Health Effects of the Herbicide
2,4-D, April 1980, Chem. Regul. Rep., 4(5): 134-136.
US EPA (1982) Tetrachlorodibenzo- p-dioxin; re-evaluation of
disposal prohibition. Fed. Reg., 47(2): 103.
US NATIONAL CANCER INSTITUTE (1979) Bioassay of 2,7-
dichlorodibenzo-p-dioxin (DCDD) for possible carcinogenicity.
Springfield, Virginia, National Technical Information Service
Document PB 290570 (Report No. NCI-CG-TR 123).
US VETERANS' ADMINISTRATION. DEPARTMENT OF MEDICINE AND SURGERY
(1981) Review of literature on herbicide, including phenoxy
herbicides and associated dioxins. Volume I. Analysis of
literature. Volume II. Annotated bibliography, Washington, DC,
US Veterans' Administration, 397 pp.
VACHKOVA-PETROVA, R. (1978) [Mutagenic activity of pesticides.]
Hig. Zdraveopaz., 21(5): 496-506 (in Bulgarian).
VAINIO, H., NICKELS, J., & LINNAINMAA, K. (1982) Phenoxy acid
herbicides cause peroxisome proliferation in Chinese hamsters.
Scand. J. Work. Environ. Health, 8: 70-73.
VAINIO, H., LINNAINMAA, K., KAHONEN, M., NICKELS, J., HIETANEN, E.,
MARNIEMI, J., & PELTONEN, P. (1983) Hypolipidemia and peroxisome
proliferation induced by phenoxyacetic acid herbicides in rats.
Biochem. Pharmacol., (in press).
VAN DYK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air:
sampling methods. Res. Rev., 55: 91-134.
VAN PETECHEM, C.R. & HEYNDRICKX, A.M. (1975) Beta oxidation of
chlorophenoxybutyric acid herbicides in guinea pigs. Bull.
environ. Contam. Toxicol., 14(5): 632-640.
VARDIA, H.K. & DURVE, V.S. (1981) Bioassay study on some fresh
water fishes exposed to 2,4-dichlorophenoxyacetic acid. Acta
hydrochim. hydrobiol., 9(2): 219-223.
VENKAIAH, B. & PATWARDHAN, M.V. (1978) Inhibition of
mitochondrial energy transfer reactions by phenoxy acids. Indian
J. Biochem. Biophys., 15(3): 219-221.
VINOKUROVA, M.K. (1960) [On the toxicity of a herbicide: The
octyl ester of 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
12: 31-34 (in Russian, Health & Welfare Canada Translation No.
2324).
VOGEL, E. & CHANDLER, J.L.R. (1974) Mutagenicity testing of
cyclamate and some pesticides in Drosophila melanogaster.
Experientia (Basel), 30(6): 621-623.
WALLIS, W.E., VAN POZNAK, A., & PLUM, F. (1970) Generalized
muscular stiffness, fasciculations, and myokymia of peripheral
nerve origin. Arch. Neurol., 22: 430-439.
WATERS, M.D., MITCHELL, A.D., JORGENSON, T.A., & VALENCIA, R.
(1981) Pesticides: Mutagenic and carcinogenic potential.
In: Bandal, S.K., Marco, G.J., Golberg, L., & Leng, M.L.,
ed., The pesticide chemist and modern toxicology. Washington,
DC, American Chemical Society (ACS Symposium Series 160).
WAY, J.M. (1969) Toxicity of hazards to man, domestic
animals and wildlife from some commonly used auxin herbicides.
Res. Rev., 26: 37-62.
WEINMANN, W. (1957) [Investigations of the influence of a
treatment of tomato plants with growth substances on the
nutritional and physiological value of the fruits. Offspring
mortality in albino rats.] Qual. Plant. Mater. Veg., 2(4):
342-349 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972) The subacute
toxicity of 2,4-dichlorophenoxyacetic acid and 2,4,5-
trichlorophenoxyacetic acid to chicks. Toxicol. appl.
Pharmacol., 21:348.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., & GANGSTAD, E.O.
(1973) The effects of a 2,4-D application on the biota and water
quality in Currituck Sound, North Carolina. Hyacinth Control J.,
11: 13-17.
WHO (1976) Pesticide residues in food. Report of the 1975
Joint FAO/WHO meeting (WHO Tech. Report Series, 592).
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residue
Series No. 1).
WHO/FAO (1976) 1975 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residues
Series No. 5).
WHO/IARC (1980) Walker, E.A., Castegnaro, M., Griciute, L.,
Börzsönyi, M., & Davis, W., ed.: N-Nitroso compounds: Analysis,
formation and occurrence. Lyons, International Agency for
Research on Cancer, 841 pp (IARC Scientific Publications No. 31).
WHO/IARC (1982) Bartsch, H., Castegnaro, M., O'Neill, I.K.,
Okada, M., & Davis, W., ed.: N-Nitroso compounds: Occurrence
and biological effects. Lyons, International Agency for Research
on Cancer, 755 pp (IARC Scientific Publications No. 41).
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972) Pesticide residue
levels in soils, F.Y. 1969 - National soils monitoring program.
Pestic. monit. J., 6(3): 194-228.
WIERZBICKA, M. (1974a) Haemolymph concentration in Cyclopoida
copepodids during active and resting stage and the effect of
2,4-D sodium salt. Pol. Arch. Hydrobiol., 21(2): 269-273.
WIERZBICKA, M. (1974b) Influence of 2,4-D sodium salt on the
survival of some Copepodia species. Pol. Arch. Hydrobiol.,
21(2): 275-282.
WINKELMANN, R.K. (1960) Diagnosis and treatment of allergic
angiitis (anaphylactoid purpura). Postgrad. Med., 27: 437-444.
WOJTALIK, T.A., HALL, T.F., & HILL, L.O. (1971). Monitoring
ecological conditions associated with wide-scale applications
of DMA-2,4-D to aquatic environmental. Pestic. monit. J.,
4(4): 184-203.
WOODHAM, D.W., MITCHELL, W.G., LOFTIS, C.D., & COLLIER, C.W.
(1971) An improved gas chromatographic method for the analysis of
2,4-D free acid in soil. J. agric. food Chem., 19: 186-188.
YIP, G. (1975) Analysis for herbicides and metabolites. J.
Chromatogr. Sci., 13: 225-230.
YODER, J., WATSON, M., & BENSON, W. (1973) Lymphocyte chromosome
analysis of agriculture workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUNG, A.L., ARNOLD, E.L., & WACHINSKI, A.M. (1974) Field
studies in soil persistence and movement of 2,4-D, 2,4,5-T and
TCDD. Weed Sci. Soc. Am., (Abstract No. 226).
YOUNG, A.L., CALCAGNI, J.A., THALKEN, C.E., & TREMBLAY, J.W.
(1978) The toxicology, environmental fate, and human risk of
Herbicide Orange and its associated dioxin. Springfield, Va.,
USA, US Air Force Occupational & Environmental Health
Laboratory (Technical Report No. TR-78-92, US National
Technical Information Service.)
YOUNG, F. & HALEY, T.J. (1977) Pharmacokinetic study of a
patient introduced with 2,4-dichlorophenoxyacetic acid and
2-methoxy-3,6-di-chlorobenzoic acid. Clin. Toxicol., 11(5)
489-500.
ZAKHAROV, G.G., SHEVCHENKO, A.M., ZUB, G.A., & PERSHAI, L.K.
(1968) [The clinical picture and prophylaxis of "Dikotex"
poisoning.] Zdrav. Beloruss., 9: 7-21 (in Russian, Health &
Welfare Canada Translation No. 2367).
ZETTERBERG, G. (1977) Experimental results of phenoxy acids
on microorganisms. In: Ramel, C., ed. Chlorinated phenoxy acids
and their dioxins: Mode of action, health risks and environmental
effects. Ecol. Bull. (Stockholm), 27.
ZETTERBERG. G., BUSK, L., ELOVSON, R., STAREC-NORDENHAMMAR, I., &
RYTTMAN, H. (1977) The influence of pH on the effects of 2,4-D on
Saccharomyces cerevisiae and Salmonella typhimurium. Mutat. Res.,
42(1): 3-18.
ZHITENEVA, L.D. & CHESNOKOVA, T.V. (1973) [Alteration of the
morphological composition of the blood in white amur larvae under
the effect of herbicides.] Eksp. vod. toksikol., 4: 56-67
(in Russian).
ZIELENSKI, W.L., Jr & FISHBEIN, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4-D and 2,4,5-T acids and
2,4-D esters in mice. J. agric food Chem., 15(5): 841-844.
ZIEM, G. & MAY, G. (1983) Tetrachlorodibenzodioxin. Br. J. ind.
Med., 40: 116.
 Some physical properties of 2,4-D and of the 2,4-D derivatives
that are used in agriculture are summarized in Table 1.
Table 1. Physical properties of 2,4-D
---------------------------------------------------
Molecular formula: C8H6Cl2O3
Relative molecular mass: 221.0
Melting point: 140-141 °C
Solubility in water: slightly soluble
Solubility in organic solvents: soluble
Vapour pressure: 52.3 Pa at 160 °C
pKa at 25 °C: 2.64-3.31
---------------------------------------------------
2,4-D has growth-regulating and herbicidal properties in
broad-leaved plants. Because of its solubility, 2,4-D is
rarely used in the form of the acid; commercial 2,4-D
herbicide formulations consist of the more soluble forms such
as alkali salts, amine salts, or esters. These are combined
with solvents, carriers, or surfactants and are marketed in
the form of dusts, granules, emulsions, or oil and water
solutions in a wide range of concentrations.
2.1.2. Synthesis of 2,4-D
2,4-D is commonly prepared by the condensation of 2,4-
dichlorophenol with monochloroacetic acid in a strongly alkaline
medium at moderate temperatures (Canada, NRC, 1978; Sittig 1980;
Que Hee & Sutherland, 1981), or by the chlorination of
phenoxyacetic acid, but this method leads to a product with a high
content of 2,4-dichlorophenol and other impurities (Melnikov,
l97l). Higher reaction temperatures and alkaline conditions during
the manufacture of 2,4-D increase the formation of polychlorinated
dibenzo- p-dioxin (CDD) by-products (Fig. 2). The alkali metal
salts of 2,4-D are produced by the reaction of 2,4-D with the
appropriate metal base. Amine salts are obtained by reacting
stoichiometric quantities of amine and 2,4-D in a compatible
solvent (Que Hee & Sutherland, 1974, 1981). Esters are formed by
acid-catalysed esterification with azeotropic distillation of water
(Que Hee & Sutherland, 1981) or by a direct synthesis in which the
appropriate ester of monochloroacetic acid is reacted with
dichlorophenol to form the 2,4-D ester (Canada, NRC, 1978).
2.1.3. Important chemical reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides (Que Hee & Sutherland, 1975a). Pyrolysis of
2,4-D and its derivatives is likely to produce certain CDD isomers
(section 2.1.4). 2,4-D is readily photodegraded (section 4.4.4).
Some physical properties of 2,4-D and of the 2,4-D derivatives
that are used in agriculture are summarized in Table 1.
Table 1. Physical properties of 2,4-D
---------------------------------------------------
Molecular formula: C8H6Cl2O3
Relative molecular mass: 221.0
Melting point: 140-141 °C
Solubility in water: slightly soluble
Solubility in organic solvents: soluble
Vapour pressure: 52.3 Pa at 160 °C
pKa at 25 °C: 2.64-3.31
---------------------------------------------------
2,4-D has growth-regulating and herbicidal properties in
broad-leaved plants. Because of its solubility, 2,4-D is
rarely used in the form of the acid; commercial 2,4-D
herbicide formulations consist of the more soluble forms such
as alkali salts, amine salts, or esters. These are combined
with solvents, carriers, or surfactants and are marketed in
the form of dusts, granules, emulsions, or oil and water
solutions in a wide range of concentrations.
2.1.2. Synthesis of 2,4-D
2,4-D is commonly prepared by the condensation of 2,4-
dichlorophenol with monochloroacetic acid in a strongly alkaline
medium at moderate temperatures (Canada, NRC, 1978; Sittig 1980;
Que Hee & Sutherland, 1981), or by the chlorination of
phenoxyacetic acid, but this method leads to a product with a high
content of 2,4-dichlorophenol and other impurities (Melnikov,
l97l). Higher reaction temperatures and alkaline conditions during
the manufacture of 2,4-D increase the formation of polychlorinated
dibenzo- p-dioxin (CDD) by-products (Fig. 2). The alkali metal
salts of 2,4-D are produced by the reaction of 2,4-D with the
appropriate metal base. Amine salts are obtained by reacting
stoichiometric quantities of amine and 2,4-D in a compatible
solvent (Que Hee & Sutherland, 1974, 1981). Esters are formed by
acid-catalysed esterification with azeotropic distillation of water
(Que Hee & Sutherland, 1981) or by a direct synthesis in which the
appropriate ester of monochloroacetic acid is reacted with
dichlorophenol to form the 2,4-D ester (Canada, NRC, 1978).
2.1.3. Important chemical reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides (Que Hee & Sutherland, 1975a). Pyrolysis of
2,4-D and its derivatives is likely to produce certain CDD isomers
(section 2.1.4). 2,4-D is readily photodegraded (section 4.4.4).
 2.1.4. Composition of technical 2,4-D materials
Technical 2,4-D may range in purity from less than 90% to 99%.
Typical levels for impurities are listed in Table 2. Trace levels
of CDDs have been found in amine and ester formulations (Table 3).
It can be seen that the amine formulations tend to be less highly
contaminated with di- and tetra-CDD than the ester products. The
structures of these impurities are shown in Fig. 2.
Table 2. Typical levels of 2,4-D and major impurities
in technical 2,4-Da
------------------------------------------------------
Component % range
------------------------------------------------------
2,4-dichlorophenoxyacetic acid 94 - 99
2,6-dichlorophenoxyacetic acid 1.5 - 0.5
2-monochlorophenoxyacetic acid 0.5 - 0.1
4-monochlorophenoxyacetic acid 0.8 - 0.2
bis(2,4-dichlorophenoxy) acetic acid 2.0 - 0.1
phenoxyacetic acid trace - 0.2
2,4-dichlorophenol 0.6 - 0.1
2,6-dichlorophenol 0.048 - 0.001
2,4,6-trichlorophenol 0.14 - 0.001
2-chlorophenol 0.04 - 0.0004
4-chlorophenol 0.005 - 0.0004
water 0.8 - 0.1
------------------------------------------------------
a From: Cochrane (1981).
Table 3. Ranges of levels of chlorinated dibenzo- p-dioxins (CDD)
in 2,4-D amine and ester formulationsa
-------------------------------------------------------------------
CDD isomers found (µg/kg)b
Type of 2,7-di- 1,3,7-tri- 1,3,6,8/ 2,3,7,8-tetra
formulation 1,3,7,9-tetra
-------------------------------------------------------------------
2,4-D amines ndc- 409 nd - 587 nd - 278 nd
2,4-D esters nd - 23815 nd - 450 nd - 8730 nd
-------------------------------------------------------------------
a From: Cochrane et al. (1981).
b Expressed in terms of 2,4-D.
c nd (not detected < 1 µg/kg).
The composition of technical 2,4-D depends on the manufacturing
process and especially on the purity of 2,4-dichlorophenol when
this is the starting material. During 2,4-D synthesis from
monochloroacetic acid and 2,4-dichlorophenol, the latter compound
as well as other ortho-chlorinated by-products can give rise to a
wide variety of chorinated by-products at a high temperature and
high pH. Self condensation of 2,4-dichlorophenol may form 2,7-
dichlorodibenzo- p-dioxin, while trichlorophenols may give rise to
a mixture of 1,3,6,8- and 1,3,7,9-tetrachlorodibenzo- p-dioxins
(but not 2,3,7,8-TCDD) by self-condensation, or to 1,3,7-
trichlorodibenzo- p-dioxin by cross-condensation with 2,4-
dichlorophenol.
A different type of toxic trace impurity, namely N-
nitrosamines, can occur in amine formulations of 2,4-D, especially
when nitrite is added as a corrosion inhibitor for containers.
Dimethyl- N-nitrosamine has been found in some 2,4-D dimethylamine
products at levels of up to 0.3 mg/litre (Ross et al., 1977; Cohen,
et al., 1978).
2.1.5. Volatility of 2,4-D derivatives
2,4-D esters with short-chain alcohols are highly volatile
(Table 1). This influences the effectiveness of their application
to target crops, their effects on neighbouring crops, and the
degree of contamination of the atmosphere. 2,4-D alkali salts or
amine salts are much less volatile than esters (Carter, 1960;
Canada, NRC, 1978; Que Hee & Sutherland, 1981, and section 4.1),
and these products are to be preferred when the use of 2,4-D esters
might lead to evaporative 2,4-D losses and to crop damage.
2.2. Determination of 2,4-D
2.2.1. General comments
General comments on criteria for acceptable analytical methods
and on other pertinent aspects of 2,4-D determination can be found
in the publications of Gunther (1962), Currie (1968), Kaiser
(1973), Carl (1979), Kateman & Pijper (1981), Que Hee & Sutherland
(1981) and Chau et al. (1982).
2.2.2. Analysis of technical and formulated 2,4-D products
In the past, the quality of 2,4-D products was assessed by an
acid-base titration or by a total chlorine determination
(Collaborative International Pesticides Analytical Council, 1970).
These non-specific and thus inaccurate methods have been superseded
by specific gas-liquid chromatography (GLC) or high pressure liquid
chromatography (HPLC), making it possible to determine various by-
products (Henshaw et al., 1975; Bontoyan, 1977; Skelly et al.,
1977; Stevens et al., l978; Cochrane et al., 1982). The isomer-
specific HPLC method is now preferred by many 2,4-D producers and
regulatory agencies. The chlorinated dibenzo- p-dioxins (CDDs) are
usually produced only in trace amounts and are difficult to
separate and identify; highly specialised equipment and skills are
necessary (Crummett & Stehl, 1973; Huckins et al., 1978; Norström
et al., 1979; Baker et al., 1981; Cochrane et al., 1981; Hass et
al., 1981, and National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1981).
2.2.3. Determination of 2,4-D residues
All exposure determinations and risk assessments ultimately
depend on accurate chemical analyses, and therefore some critical
aspects of analysis for 2,4-D residues have been included in the
present document.
Before 2,4-D residues can be measured, they have to be
quantitatively extracted and purified to remove substances that
could interfere with the final residue determination. They must
then be converted to a stable product (derivative) suitable for
determination with a given type of detector.
When comparing analytical results, it should be kept in mind
that the older methods of extraction and clean-up contained
considerable sources of errors, and that the early methods
for measuring 2,4-D residues, such as colorimetry and
spectrophotometry, were not as sensitive or specific as those
developed in recent years.
2.2.3.1. Sampling, extraction, and clean-up
Methods for the sampling, extraction, and clean-up of 2,4-D
residues in water, air, soil, and biological materials have
recently been reviewed by National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978) and by Que Hee & Sutherland (1981). Problems caused
by the conjugate formation of 2,4-D with amino acids, proteins,
sugars, or lipids, or the absorption of 2,4-D onto container
surfaces, including those of glass vessels, have been solved by
Chow et al. (1971), Renberg (1974), Osadchuk et al. (1977), Lokke
(1979), Jensen & Glas (1981), and Bristol et al. (1982). For
sampling and extracting 2,4-D residues, the following references
should also be consulted:
Air: Van Dyk & Visweswariah (1975), Farwell et al. (1976a,b),
Grover et al. (1976), Johnson et al. (1977), Gluck & Melcher
(1980), and Grover & Kerr (1981); water: Suffet (1973a,b), Renberg
(1974), Mierzwa & Witek (1977), Chau & Thomson (1978); soil:
Woodham et al. (1971); Smith (1972, 1976a), Foster & McKercher
(1973); food: Que Hee & Sutherland (1981), Bjerk et al. (1972),
Jensen & Glas (1972), Lokke (1975); biological media: Smith
(1976b), (blood, urine); Senczuk & Pogorzelska (1981).
2.2.4. Derivatization and quantification
At present, gas-liquid chromatography with electron-capture
detection (GLC-EC) is the most commonly used and generally most
sensitive method (picogram level) for measuring 2,4-D residues.
To improve the sensitivity of detection, the 2,4-D has to be
transformed (derivatized), usually to a methyl ester by reacting
with BF3-methanol, diazomethane, or with concentrated sulfuric
acid-methanol; the first method may give the best results (Munro,
1972; Horner et al., 1974; Olson et al., 1978).
For a recent review of derivatization methods and GLC columns
for various substrates see Cochrane (1981).
Thin-layer chromatography (TLC) has been used for herbicide
residue determination (Guardigli et al., 1971, Yip 1975). It has
recently been recommended by Batora et al. (1981) as a simplified
method for determining pesticide residues that requires a minimum
of costly equipment. TLC is suitable for food inspection and could
be of use in the establishment of new residue laboratories in
developing countries.
High-pressure liquid chromatography (HPLC) is less sensitive
than GLC-EC i.e., nanogram (ng) versus picogram levels, but may be
advantageous under some circumstances (Tuinstra et al., 1976;
Arjmand et al., 1978; Connick & Simoneaux, 1982). Using mass
fragmentography with deuterated internal standards it is possible
to determine nanogram amounts of 2,4-D and related compounds in
urine and plasma (De Beer et al., 1981); it is also suitable for
chemobiokinetic studies on subtoxic doses of 2,4-D in blood.
2.2.5. Confirmation
The ultimate confirmatory technique is gas chromatography
coupled with mass spectrometry and specific ion monitoring, with a
sensitivity down to the femtogram level (Farwell et al., 1976a).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use
were not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United
Kingdom. World-wide use of herbicides and annual production, which
probably exceeds 5 x 107 kg per year, are increasing, National
Research Council of Canada, Associate Committee on Scientific
Criteria for Environmental Quality, 1978; Bovey & Young, 1980).
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaf weeds in cereal crops as well as on
pastures and lawns, in parks, and on golf courses at rates of about
0.2 - 2.0 kg active ingredient (acid equivalent) per hectare.
Esters are also used at rates of up to 6 kg (acid equivalent) per
ha to suppress weeds, brush, and deciduous trees along rights-of-
way and in conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides
in or along irrigation and other canals, in ponds, and lakes at
rates ranging from 1 to 122 kg/ha (Pal'mova & Galuzova, 1963; Smith
& Isom, 1967; National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Bovey & Young, 1980).
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays
containing 20 - 40 mg 2,4-D/litre on apple trees to reduce
premature fruit drop, on potato plants to increase the proportion
of medium-size tubers or to intensify the tuber skin colour of the
red varieties (Bristol et al., 1982), and in citrus culture to
reduce pre-harvest fruit drop and to increase fruit storage life.
The highly volatile ethyl, isopropyl, and butyl esters are
being replaced by low-volatile esters or by amine salts to reduce
crop damage resulting from 2,4-D vapour drift, and to decrease
atmospheric pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public,
has been reduced in some countries, because of increasing concern
about possible toxic effects, especially in relation to CDDs.
The ecological effects of using high rates of 2,4-D and
repeated treatments have been reviewed by Bovey & Young (1980).
3.3. Disposal of wastes
3.3.1. Industrial Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localised
to the production site and to areas of waste dumping, and it is
likely to be more dispersed if disposal or leaching has occurred
into water courses. Combustion of 2,4-D and its by-products at low
temperatures could lead to the formation of CDDs. A temperature
approaching 1000 °C, however, gives almost complete destruction of
2,4-D (Sittig, ed., 1980). The spread of 2,4-D from waste dumps
may be reduced by the use of properly enclosed impermeable clay-
lined pits, away from water sources.
3.3.2. Agricultural wastes
Disposal of unused 2,4-D and washing of equipment may result in
localised land pollution and also pollution of water supplies
through direct contamination or leaching from soil.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION OF 2,4-D
2,4-D does not persist or accumulate in the environment, as it
is readily degraded by physical, chemical, and biological action.
It is susceptible to photolysis in air and water, on soil, and on
plant surfaces. Thus, the question of environmental distribution
is limited to the immediate transfer of 2,4-D between compartments
of the environment.
4.1. Drift and Volatilization in the Atmosphere
The atmosphere can be contaminated with 2,4-D during both its
manufacture and use. The production of 2,4-D may result in the
emission into the air of dichlorophenol, chloroacetic acid, and
ammonia (Sittig, ed., 1980), in addition to 2,4-D vapours (Grover
et al., 1976).
According to the formulation of 2,4-D used, environmental
transfer into the atmosphere will occur by either drift (depending
on the particle size of the droplet, the spray technique, and
climatic conditions), or by volatilization, or by a combination of
both. It is very difficult to calculate the extent to which drift
or volatisation occurs, and this is illustrated by the range of
2,4-D concentrations observed in the air after 2,4-D use (Table 4).
The factors affecting the amount of herbicide spray that lands
on a target crop and the proportion that is lost by drifting or
volatilization have been described (Grover et al., 1972; Grover,
1976; Maybank et al., 1978; Que Hee & Sutherland, 1981). Unwanted
residues may be deposited on non-target crops (Akesson & Yates,
1961; Yates & Akesson, 1973). The National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) cited reports of drift damage
caused in susceptible crops by phenoxy herbicide applications,
particularly in cotton, tobacco, tomatoes, grapes, rapeseed,
clover, and a number of horticultural species.
Widespread damage in vineyards and in other crops due to 2,4-D
drift from sprayed wheat fields was reported by Robinson & Fox
(1978) with two different damage patterns, one localized and the
other widespread. The first was characterized by severe localized
damage with a very clear gradient of decreasing severity away from
the zone, following the drift of spray droplets in the immediate
treatment area. The more widespread damage was of greater concern.
It was characterized by more or less uniform symptoms and appeared
to be attributable to the passage of a large cloud of vapour that
may have extended for several km. Both problems could have been
avoided by the use of low-volatile preparations and proper
application methods.
Table 4. Concentrations of total 2,4-D residues in ambient air
------------------------------------------------------------------------------------------------------------------
Days when 2,4-D was 2,4-D residues
Site location µg/m3air Predominant
No. of stations, height ------------------------------------------- type of 2,4-D Reference
Regional characteristics found Sample Meana Max. residue
present time
mean max. h
------------------------------------------------------------------------------------------------------------------
Saskatchewan, Canada not
(1) 150 m (aircraft) stated 1 min 1.0c 2.5 butyl ester Elias (1975)
Saskatchewan, Canada 48 33 36 24 h 0.5c 13.5 butyl ester Grover et al.
(8) 2 m above ground 24 h 0-6d isooctyl ester (1976)e,f
level, wheat area 24 h - - amine salt
California, USA 41 (1973) 24 h 0.4 0.9 high volatile Farwell et al.
(7-8) near ground 73 (1974) 24 h 0.1 0.4 low volatile (1976)
level 24 h 0.1 0.2 non volatile
Washington State, USA 105-106 81 89 24 h 0.2 2.2 isopropyl ester Adams et al.
(2) near ground 65 69 24 h 0.09 2.2 butyl ester (1964)b
level, wheat area 8 11 24 h 0.003 3.1 isooctyl ester
Washington State, 99-102 34 39 24 h 0.08 2.0 isopropyl ester droplets Bamesberger &
(2) near ground 8 15 24 h 0.08 1.3 isopropyl ester vapour Adamsb
level, wheat area 18 22 24 h 0.07 1.0 butyl ester droplets
3 5 24 h 0.03 1.3 butyl ester vapour
1 1 24 h 0.005 0.5 isooctyl ester droplets
- - 24 h - - isooctyl ester vapour
4 5 24 h 0.01 0.5 acid, salts, droplets
5 5 24 h 0.04 5.1 acid, salts, vapour
------------------------------------------------------------------------------------------------------------------
a Values measured at different sites or at different times have been averaged and reduced to a single significant
figure for simplicity.
b At the time of these studies, GLC methods were less highly developed.
c Centre of principal range observed.
d Maximum values recorded in a previous study (Que Hee et al., 1975) were shown to be equivalent to concentrations
existing directly over an open pan of formulated butyl ester; the implication was made that accidental
laboratory contamination could have occurred.
f The results of Maybank & Yoshida (1969), Maybank et al. (1978), and Stanley et al. (1971) could not be adapted
to this table.
Volatilization of 2,4-D products in the air during the spraying
operation and from the surface of plants and the soil is difficult
to distinguish from the drift of spray droplets. Evaporation
occurs to a greater extent with the highly volatile ethyl,
isopropyl, or butyl esters; very little occurs with amine salt
formulations, and it is greatly reduced when 2,4-D is dissolved in
corn oil, cottonseed oil, or diesel oil (Marth & Mitchell, 1949).
In one experiment, no significant amounts of 2,4-D amine, but 20 -
40% of the initially deposited 2,4-D butyl ester, and 10 - 15% of
the octyl ester of 2,4-D vapourized within 2 h of spraying (Grover
et al., 1972); less volatilization occurs with the higher esters of
2,4-D. For this reason, the use of the more volatile esters has
been discontinued in some countries. Studies of 2,4-D aerial drift
following ground spray operations have shown that only 3 - 8% of
the applied herbicides drift as spray droplets when low volatile
preparations are applied as large droplets. However, ultra-low-
volume (ULV) applications by aircraft, or the use of highly
volatile esters may cause as much as 25 - 30% of the 2,4-D sprayed
to drift off the target (Grover et al., 1972; Maas & Kerssen, 1973;
Maybank et al., 1978).
4.2. Movement Within and From the Soil
The movement of pesticides within and from the soil can be
divided into three categories: diffusion, leaching, and surface
movement. Diffusion is a localized process and depends on the
concentration gradient of the pesticide in the soil medium, on the
soil mineral type, and on the organic matter content, temperature,
pH, and other factors. Leaching refers to the movement of
pesticides through the soil profile with percolating water.
Surface movement refers to wind erosion of dust particles and
surface run-off in flowing water.
Examination of the behaviour of 2,4-D in soils (Liu & Cibes-
Viade, 1973; Grover & Smith, 1974; Moreale & Van Bladel, 1980) has
shown that organic matter, soil pH (surface horizons), and
exchangeable aluminium (clay sub-horizons) are the key determinants
for the percentage of 2,4-D adsorbed. As the adsorption/desorption
process is the basic mechanism influencing herbicide availability,
mobility, and degradation in soil, 2,4-D is likely to be more
strongly bound in soils with a high content of organic matter than
in those with a low content.
4.3. Contamination of Water
Residues of 2,4-D in aqueous systems can result from the
deposition of spray drifts, the "washout" of 2,4-D in the vapour or
droplet phase from the atmosphere during rainfall, the run-off from
treated fields, or following the application of 2,4-D to water for
the control of aquatic weeds. Industrial discharges, either from
accidental spills or through sewage systems, may also contribute to
the contamination of water. The National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) has tabulated data that demonstrate
the influence of environmental factors on the clearance of 2,4-D
and its derivatives from water. The principal processes involved
are ester and amine hydrolysis, volatilization, microbial
degradation, photolysis, and sorption. There is little movement of
2,4-D into drainage water in organic soils, because it is strongly
bound to organic materials.
4.4. Environmental Transformation and Degradation Processes
4.4.1. Metabolism in plants
Plants hydrolyse 2,4-D esters to 2,4-D, which is the active
herbicide (Morton et al., 1967; Matsunaka, 1972). Further
metabolism in plants occurs through three mechanisms, namely, side-
chain degradation, hydroxylation of the aromatic ring, and
conjugation with plant constituents (Crafts, 1960; Morre & Rogers,
1960; Erickson et al., 1963).
4.4.1.1. Side-chain degradation
Degradation of the side-chain of 2,4-D has been observed in
many plants (Loos, 1969), but in only a few species or varieties
does it appear to play a major role in herbicide breakdown.
Luckwill & Lloyd Jones (1960a,b) suggested two degradation
pathways leading to the formation of 2,4-dichlorophenol.
4.4.1.2. Ring hydroxylation
Thomas et al. (1964a,b), and, more recently, Feung et al.
(1971, 1972, 1973b) identified 2,5-dichloro-4-hydroxyphenoxyacetic
acid and 2,3-dichloro-4-hydroxyphenoxyacetic acid as major and
minor phenolic acid metabolites, respectively. Evidence was found
by Fleeker & Stein (1971) indicating hydroxylation resulting in the
elimination of the 4-chloro substituent from the aromatic ring, in
addition to migration of the chlorine at the 4-position to an
adjacent carbon on the ring. A small amount of 2-chloro-4-hydroxy-
phenoxyacetic acid was produced from 2,4-D by wild buckwheat, wild
oats, leafy spurge, and yellow foxtail.
4.4.1.3. Conjugation with plant constituents
Studies indicate that resistant crops, i.e., grasses and
cereals, form water-soluble conjugates with sugars, whereas
sensitive broad-leaved crops (such as beans) form mainly water-
insoluble amino acid conjugates (Montgomery et al., 1971; Feung et
al., 1971, 1972, 1973b, 1975).
4.4.2. Degradation of 2,4-D in the soil
Deposition of 2,4-D esters on the soil is followed fairly
rapidly by hydrolysis. Burcar et al. (1966) observed that the
2,4-D isooctyl ester disappeared after 2 weeks, though free acid
could be detected up to 6 weeks after application. The breakdown
of the iso-propyl, n-butyl, and isooctyl esters of 2,4-D on three
Canadian prairie soils was studied by Smith (1972) who found that
after 24 h no iso-propyl or n-butyl esters remained, whereas
20 - 30% of the isooctyl ester was still intact. The author
concluded that an initial rapid phase of hydrolysis of the 2,4-D
esters to the anion in soil was the result of chemical and not
microbial action.
Microbial degradation of phenoxy herbicides does occur and has
been comprehensively reviewed by Loos (1975), Cripps & Roberts
(1978) and The National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, (1978).
Early studies of the persistence of 2,4-D in soil indicated that
warm moist conditions and the presence of organic matter favoured
the rapid disappearance of 2,4-D. Sterilization of the soil
inhibited breakdown, indicating that the degradation was microbial.
In addition, Pemberton (1979) reported the discovery of specific
2,4-D plasmids within some bacterial strains, transmitted from one
cell to another, and carrying with them a genetic capability
enabling the bacteria to degrade 2,4-D.
Two principal pathways have been proposed for the microbial
degradation of 2,4-D in soil. Firstly the side chain may be
removed to form 2,4-dichlorophenol, followed by orthohydroxylation
of the phenol to produce a catechol (Bollag et al., 1968). The
catechol may then be cleaved to yield a muconic acid and further
conversion products. The second possible pathway is via a
hydroxyphenoxyacetic acid intermediate (Evans et al., 1971).
4.4.3. Degradation in the aquatic ecosystem
A multitude of variables influence the partitioning and removal
of phenoxy herbicides within an aquatic ecosystem. Detectable
residues have been reported to persist for 4 weeks in some
situations and up to 4 months in others (Frank & Comes, 1967;
Wojtalik et al., 1971; Schultz & Harman, 1974). Photolysis is an
important means of degradation of 2,4-D in natural water and is
more rapid than that of 2,4,5-T (Crosby & Wong, 1973). The
partition of residues between water and sediment will have an
effect on the rate of breakdown, as will temperature and intensity
of light. Anaerobic conditions will favour microbial breakdown.
The effects of some of these factors have been tabulated by the
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (1978).
4.4.4. Photochemical degradation
Photodecomposition of 2,4-D was studied in detail by Crosby &
Tutass (1966), Boval & Smith (1973), and reviewed recently by Que
Hee & Sutherland (1981). It leads to the formation of a variety of
products but commonly involves reductive dechlorination of the
acid, esters, and salts in aqueous or in organic solutions, with
2,4-dichlorophenol acting as a catalyst for the breakdown of
2,4-D, which may involve rupture of the aromatic ring. Que Hee &
Sutherland (1987) studied the vapour and liquid phase photolysis of
the n-butyl ester of 2,4-D and observed dechlorination at the
second position with simultaneous reduction and re-arrangement to
produce a variety of photoproducts. According to Boval & Smith
(1973), carbon dioxide is the final oxidation product when aqueous
solutions of 2,4-D undergo photodecomposition.
4.5. Bioconcentration
There is no evidence that bioconcentration of 2,4-D occurs
through the food chain or in any compartment of the environment.
This has been demonstrated by large-scale monitoring for 2,4-D
residues in soils, foods, feedstuffs, wildlife, and human beings,
and from examinations of the many routes of metabolism and
degradation that exist in ecosystems (sections 5.1.3 and 5.1.4).
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1. Levels of 2,4-D Residues in the Environment
Most of the available information on 2,4-D levels in the
environment has been reviewed in detail (National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality, 1978; Ramel, 1978; Bovey & Young, 1980;
Canada, Health & Welfare, 1980; Shearer & Halter, 1980; US EPA,
1980a). In comparing early and recent results, it should be kept
in mind that the analytical procedures used before about 1965 were
often unreliable and may have resulted in under- or overestimation
of the actual levels of 2,4-D derivatives. No information is
available on the levels of 2,4-D-related dioxin by-products in the
environment.
5.1.1. In air
Some levels of 2,4-D in ambient air are shown in Table 4.
These 2,4-D residues consist mainly of esters, particularly the
highly volatile butyl esters (Bamesberger & Adams, 1966; Farwell et
al., 1976b; Grover et al., 1976). Total 2,4-D residues in the air
were found to decrease during periods of rain, suggesting a
"washout effect" (Grover et al., 1976). In the majority of cases,
the levels reported were those found shortly after spraying.
5.1.1.1. Field exposure
Concentrations of 2,4-D that occurred during and after
herbicide use in the air of the work zone of people engaged in
herbicide spray operations in various use situations, are given in
Table 5. Workers involved in these operations were exposed to
2,4-D levels of up to 0.2 mg/m3 air during the period of actual
application.
5.1.1.2. General environmental exposure
In large-scale studies in areas of intense 2,4-D use, about 40%
of all air samples were found to contain between 0.01 and 0.1 µg
2,4-D/m3 (Grover et al., 1975). In a similar study undertaken by
Que Hee et al. (1976), much higher levels were recorded in one
urban location, reaching an average of 339 µg/m3 air during 3 days.
However, Grover et al. (1976), in their subsequent work, showed
that such concentrations could only be produced under artificial
conditions that could not reflect environmental conditions. In a
general programme of air monitoring undertaken in citrus-growing
regions in the USA, only one out of 880 samples analysed was found
to contain 2,4-D, at a level of 0.004 mg/m3. The sites were not
chosen in relation to 2,4-D use (Stanley et al., 1971).
Table 5. Concentrations of total 2,4-D in air related to occupational exposure
---------------------------------------------------------------------------------------------------------
Days Mean of 2,4-D
Herbicide Circumstances Type of exposure after concentrations References
product monitoring spraying in air (mg/m3)
---------------------------------------------------------------------------------------------------------
2,4-dimethylamine agricultural spray Analyses of air 0 0.02 Thiele et al.
salt (0.9% aqueous operations with in tractor cabs (1981a,b)
solution) tractor-drawn
equipment
2,4-D butoxyethanol Exposure during Analyses of air 0 0.1-0.2 Kolmodin-Hedman
ester. 2% emulsion forest spray in breathing zone & Erne (1980),
in water operation with of workers Kolmodin-Hedman
tractor driven et al. (1979)
equipment
2,4-D isooctyl- 3-day aerial spray Analyses of air 0 0.002-01a Franklin et al.
ester in diesel oil operation with single in breathing zone (1982)
engine aircraft of pilot and
ground crew
2,4-D PGBE ester Two 1-day aerial Analyses of air 0 <0.00001b Lavy et al.
emulsion in water forest spray in breathing zone (1982)
operations by of ground crew
helicopter
---------------------------------------------------------------------------------------------------------
a Application using large spray droplets.
b One flagman was recorded as being exposed to 0.1 mg/m3.
5.1.2. In water
2,4-D, as well as chlorophenol residues resulting from the
microbial transformation of 2,4-D, may occur in raw and finished
supplies of drinking-water (Faust & Aly, 1963; US EPA, 1976, 1980a;
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality, 1978; Bovey & Young,
1980; Canada, Health & Welfare, 1980; Shearer & Halter, 1980).
Information on 2,4-D-related dioxins in water was not
available.
Drinking-water in the USA is routinely analysed by the FDA as
part of the beverage-food group in their "market basket" analysis
programme; 2,4-D has not been detected in these studies, where the
limit of detection is 0.005 mg/litre for beverages (Table 6). This
indicates that drinking-water is not a significant source of human
exposure outside directly sprayed areas.
The same conclusion can be drawn from the results of large-
scale surveys of pesticide residues, including 2,4-D in surface
waters in areas of 2,4-D use (Table 7).
Levels much higher than those found in these studies have been
observed, but only in relation to local spills or direct
contamination (Frank et al., 1979; Frank & Sirons, 1980). A very
wide fluctuation has been found in water samples following
treatment of bodies of water, shores, ditches, or stream banks with
herbicides (Averitt, 1967; Frank & Comes, 1967; Bartley & Hattrup,
1970; Frank et al., 1970; Wojtalik et al., 1970; Frank, 1972;
Whitney et al., 1973; Schultz & Harman, 1974; Schultz & Whitney,
1974; Paderova, 1975; Province of British Columbia, 1981).
Occasional high contamination levels in samples of potable water
have been reported following experimental treatments of reservoirs
with 2,4-D (Wojtalik et al., 1971). However, the mean levels
tended to remain below 2 µg/litre, even in samples of raw or
processed water from 2,4-D-treated reservoirs (Smith & Isom, 1967;
Wojtalik et al., 1971; Province of British Columbia, 1981).
Generally, 2,4-D residues were < 0.1 µg/litre in two large-scale
monitoring programmes of surface waters (Frank & Sirons, 1980;
Gummer, 1980). This is not unexpected in view of the moderately
rapid microbial degradation of 2,4-D in the environment (Robson,
1966; Averitt, 1967; Frank, 1972; Nesbitt & Watson, 1980a,b;
Province of British Columbia, 1981).
2,4-D and especially its transformation product,
dichlorophenol, at levels exceeding 20 µg/litre will impart an
objectionable odour and taste to contaminated water (Pal'mova &
Galuzova, 1963; Faust & Suffet, 1966). This organoleptic effect
may reduce the likelihood of highly contaminated water being
ingested. It is noteworthy that public water supplies containing
"traces" of 2,4-D, and wells contaminated with 2,4-D or other
herbicides have been shut down because of objectionable odours or
tastes (Gribanov, 1968; Kramer & Schmaland, 1974; Frank et al.,
1979).
Table 6. 2,4-D residues reported in market basket samples in the USA
---------------------------------------------------------------------------------------------------------
Types of samples Nature of samples % of samples Residue levels
Years analysed containing residues with residues (mg/kg) References
---------------------------------------------------------------------------------------------------------
1965-65 Total diets sugars and adjunctsa 4.2 < 0.02-0.16 Duggan & Corneliussen
1966-66 leafy vegetables (1) 3.0 < 0.02-0.03 (1972)
low fats
1967-67 leafy vegetables (2) 1.7 0.03
oil fats (1)
1968-68 dairy produce (1) 0.6 0.02-0.13
1969-69 fruits (1), sugars (2) 0.3 < 0.2
1970-70 leafy vegetables (1) 0.3 < 0.02
dairy produce (1)
1970-71 Total diets leafy vegetables (3) - 0.01-0.02 Manske & Corneliussen
(1975)
1971-72 Total diets dairy products (1) - 0.01 Manske & Johnson
(1975)
1973-80 Total diets 0 < 0.01 Manske & Johnson
(1976)
Johnson et al.
(1981a,b)
Johnson et al. (1977)
1972-73 Potatoes from raw, boiled or baked - < 0.02-0.12 Bristol et al. (1982)
fields treated
with herbicide
---------------------------------------------------------------------------------------------------------
a No. of positives not specified.
Table 7. Concentrations of 2,4-D residues in surface water samples following application of 2,4-D to
agricultural landsa
------------------------------------------------------------------------------------------------------
Site No. of samples in 2,4-D applied 2,4-D residues
Number of Stations which 2,4-D was: in watershed (µg/litre) References
Regional Characteristics ----------------- (kg/ha) ---------------
analysed found meanb max.
------------------------------------------------------------------------------------------------------
Ontario, Canada 949 66 0.8 <0.1 3.9e Frank & Sirons (1980)
11
streams
Saskatchewan, Canada 15 10 - 2 21.6 Choi et al. (1976)
5
river
Western Canada 186 10 - 0.5c 4.3d Gummer (1980)
14
diverse sites
------------------------------------------------------------------------------------------------------
a Studies in which the analytical procedures were not described or were considered unreliable have not
been included.
b Values measured at different sites or at different times have been averaged and reduced to a single
significant figure for simplicity.
c Reported data are very close to analytical detection limits.
d The maximum value, which raises the average value considerably, occurred in the effluent of an
industrial plant.
e Levels of 15.9 and 320 µg/litre were recorded at two sites but were related to spillage or actual
spraying at the sampling locality.
5.1.3. In soil
Most of the information available at present concerning 2,4-D
and other chlorophenoxy herbicide residues in soils has been
reviewed by the National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Bovey (1980a), and by Que Hee & Sutherland (1981). In highly
acidic soils, or in soils in cold or arid regions, 2,4-D
degradation is apparently slow (Lavy et al., 1973; Buslovich &
Milchina, 1976; Ou et al., 1978; Moreale & Van Bladel, 1980;
Nesbitt & Watson, 1980b). However, even at about 20 - 2000 times
the normal agricultural application rates, little or no detectable
2,4-D was left in soils under temperate climatic conditions with
prolonged winters, after intervals of 385 - 440 days (Young et al.,
1974; Stewart & Gaul, 1977; Bovey, 1980a). Furthermore, results of
a laboratory study on 2,4-D degradation in the soil showed a half-
life of 4 days (Altom & Stritzke, 1973). Several soil monitoring
studies in North America, in areas with regular 2,4-D use, have
shown residues in less than 10% of the samples, and at levels of
less than 1 mg/kg (Stevens et al., 1970; Wiersma et al., 1972;
Gowen et al., 1976).
The available data are inadequate for establishing regional and
seasonal profiles of 2,4-D soil residues and of direct population
exposure, but it is likely that direct exposure would be minor,
except during or soon after herbicide application. Indirect
exposure through the transfer of 2,4-D residues from soil to air,
or food sources is assessed separately.
5.1.4. In food sources
Although 2,4-D and its transformation products do not tend to
accumulate in plants and plant products, detectable residues of
2,4-D on food plants may be consumed by human beings or animals and
may thus contribute to the overall exposure of the human population
to this chemical.
The results of pertinent studies on 2,4-D residues on or in
foods, and in food sources for human beings and animals, are
summarized in Tables 8 - 11. Theoretically, some contribution to
the reported 2,4-D residues may have been partly derived from other
phenoxy herbicides, as 2,4-DB undergoes beta-oxidation to 2,4-D in
some plants and fish, and in cattle (Lisk et al., 1963; Gutenmann &
Lisk, 1965; Sundström et al., 1979; Bovey, 1980a).
5.1.4.1. Residues in retail food supplies
The frequency of occurrence and the levels of 2,4-D residues in
over 110 000 samples of a variety of different ready-to-eat foods,
beverages, and infant and young children's diets, have been studied
over the last 20 years in the USA (Lipscomb, 1968; Corneliussen,
1970, 1972; Duggan et al., 1971; Duggan & Corneliussen, 1972;
Johnson et al., 1979, 1981a,b). The 2,4-D residues found in such
samples are reported in Table 6. The theoretical daily intake
resulting from these residues was variously estimated to be < 1 -
5 µg/person per day (Duggan & Corneliusson, 1972).
Studies undertaken since 1970 have failed to detect residues of
2,4-D in any of the US diet samples analysed, except for a single
positive sample in the dairy product food group which was estimated
at 0.01 mg/kg (Manske & Johnson, 1975).
5.1.4.2. Residues in fish and shellfish
Fish and shellfish may be exposed to 2,4-D as a consequence of
aquatic herbicide use, or through the agricultural use of 2,4-D.
The residues in the edible portions of such fish rarely exceed 1
mg/kg wet weight (Erne, 1974, 1975 and Table 8). Residues of 2,4-D
have not been detected in retail samples of fish and shellfish
analysed as part of the US "market basket" studies (section
5.1.4.1).
There is some evidence that the organoleptic properties of the
2,4-D residues may reduce the likelihood of the consumption of fish
flesh contaminated with higher levels of 2,4-D (Gavrilova, 1965;
Folmar, 1979).
5.1.4.3. Residues in wild fruits and mushrooms
Uncultivated fruits and mushrooms taken from areas where 2,4-D
was used, or was likely to have been used, were examined for
residues of 2,4-D by Erne & von Haartman (1973), Erne, (1980),
Sietanen et al. (1981), and Frank et al. (1982). The results in
Table 9 show that residues of 2,4-D in berries in field-trial
studies have been as high as 30 mg/kg immediately after
application, but residues in berries and mushrooms taken from the
wild are generally < 1 mg/kg.
High residues of 2,4-D can produce disagreeable odours or
flavours in wild fruits and vegetables (Ingelög et al., 1977;
McArdle et al., 1961), and this may reduce the likelihood that
highly contaminated foods are ingested.
5.1.4.4. Residues in food derived from animals
Domestic meat-, milk-, and egg-producing animals, and game
animals may consume forage or feed containing 2,4-D residues, and
thus, their tissues and products may contain residues. Published
data on 2,4-D residues in feed and forage from the Northern
Hemisphere are summarized in Table 10. Immediately after
application of phenoxy herbicides, 2,4-D residues in or on grass,
generally average about 100 mg/kg for each kg of herbicide applied
per hectare. Such residues decline with a half-life of about 1 - 2
weeks, to about 20 mg/kg, within 4 weeks after an application of 1
kg/ha (Leng, 1972). Residues in 2,4-D-treated feed grains are
significantly lower than the levels reported above and no residues
would be expected in meat, milk, or eggs from such sources (Table
10).
Table 8. 2,4-D residues reported in field studies on fish and shellfish
-----------------------------------------------------------------------------------------------------
Country Year(s) 2,4-D application Types of samples 2,4-D residues in References
rate tissues (mg/kg)
-----------------------------------------------------------------------------------------------------
USA 1961 0.1 mg/litre oyster (1 species) 1.6-2.0 Butler (1965)
fish (1 species) 0.3-1.0 Cope et al. (1970)
USA 1966 44.8-112 kg/ha mussels < 0.14-1.12 Smith & Isom (1967)
clams < 0.14
fish (5 species) < 0.14
USA 1968 112 kg/ha fish (4 species) < 0.10-0.24 Whitney et al. (1973)
USA 1969 22.4-44.8 kg/ha mussels < 0.05-2.7 Wojtalik et al. (1971)
fish (8 species) < 0.10-0.34
USA 1971 2.24-8.96 kg/ha fish (3 species) < 0.005-1.075 Schultz & Harman (1974)
USA 1971 4.48 kg/ha fish (5 species) 0.000-0.162 Schultz & Whitney (1974)
-----------------------------------------------------------------------------------------------------
Table 9. 2,4-D residues in wild berries and mushrooms collected in fields or forests following application
of phenoxyalkanoic herbicides
--------------------------------------------------------------------------------------------------------------
Country Year(s) Sample 2,4-D application Days after No. samples 2,4-D residues References
rate (kg a.i./ha) treatment analysed (mg/kg)
--------------------------------------------------------------------------------------------------------------
Canadaa 1979-81 raspberries 1.1-3.9 2 124 2.6-31.0 Frank et
14-35 0.1-3.3 al. (1982)
Finlanda 1974-76 vaccinium berries 2.5 10-356 44 Mukula et
jam not known not known 1 2.2 al. (1978)
mushrooms 14-300 28 < 0.05-1.2
Finlanda 1975-76 vaccinium berries 0.25-2.25 365 not stated < 0.05 Siltanen et
al. (1981)
Swedena 1970 raspberries 1.5-2.2 2-32 9 < 0.03-0.9 Erne & Von
vaccinium berries 1.5-2.2 2-32 68 < 0.03-7.7 Haartman
blueberries 1.5-2.2 2-32 19 < 0.03-2.9 (1973)c
mushrooms 1.5-2.2 2-32 15 < 0.03
Swedena 1973-79 vaccinium berriesb 0.25-2.25 365 61 nd (< 0.05) Erne (1980)
raspberries not stated 14 not stated nd-2.5
blueberries not stated 2 not stated nd-10.0
cowberries not stated 1-28 not stated nd-6.0
mushrooms not stated 7 1 0.3
Swedena blueberries 0.4-1.5 1-35 not stated 0.2-5.3 Ingelög et
vaccinium berries 0.4-1.5 30-35 not stated 0.5-4.5 al. (1977)
raspberries 0.4-1.5 1-10 not stated 0.2-2.0
--------------------------------------------------------------------------------------------------------------
a Samples taken from areas treated with 2,4-D.
b Samples entering factory for processing.
c Data from authors' Table 1.
Table 10. 2,4-D residues reported in samples of herbicide-treated forage or feed
--------------------------------------------------------------------------------------------------------------------------
Country Year(s) Type of samples 2,4-D application Post- No. samples 2,4-D residues References
rate, (kg a.i./ha) treatment examined & (mg/kg)
interval, positive
(days)
--------------------------------------------------------------------------------------------------------------------------
Canada 1971 wheat plants 0.42 1-36 ? ? 8.35-0.011 Cochrane & Russell (1975)
Finland 1962-68 green forage 1-4 7.21 ? ? 600-3.7 Finnish State Institute of
(grass and 3.5 7-28 ? ? 13-0.4 Agriculture (1963-1969)
clover)
Finland 1974-76 aspen leaves 2.5 60-300 32 30-0.3 Mukula et al. (1978)
and twigs
birch leaves 60-300 16 31-0.1
and twigs
cowberry plants 365 8 < 26-0.05
Germany, wheat, barley, 0.375-0.735 64-101 ? ? < 0.015-0.01 Maier-Bode (1971)
Federal rye, oat grains
Republic wheat, barley, < 0.34-0.02
of rye, oat straw
Hungary 1971 silo corn 1.4-1.5 56-120 ? ? 0.8-0.075 Bodai et al. (1974)
Sweden 1972-76 barley, oats ? ? 3 2 0.7-0.4 Erne & Rutqvist (1979)
grass 7 1 0.4
lichens ? ? 2 2 0.4-0.2
USA 1949 pasture plants 4.48 1 8 8 14.6-1.65 Grigsby & Farwell (1950)
USA 1967 forage grasses 0.56-2.2 0-112 ? ? 100-1 Morton et al. (1967)
USA 1969 sorghum plants 1.4 2 ? ? 1.06 Ketchersid et al. (1970)
1.4-2.8 30-60 < 5.25-0.2
USA 1969 pasture plants 6.6-8.8 0-28 24 24 700-150 Leng (1972)
--------------------------------------------------------------------------------------------------------------------------
No residues of 2,4-D were detected (detection limit of 0.02 mg)
in the milk of dairy cows fed 2,4-D at a level of 300 mg/kg total
diet (Bjerke et al., 1972; Leng, 1972). A range of 0.06 - 0.08 mg
2,4-D/litre was found in the milk of cows fed for 3 weeks at a
level of 1000 mg 2,4-D/kg total diet.
When young beef cattle were fed 2,4-D at levels of 300, 1000,
and 2000 mg/kg total diet for 28 days, 2,4-D residue levels were
highest in the kidney and liver, but did not exceed 0.1 mg/kg in
muscle and fat, even at the highest dose level (Clark et al., 1975;
Leng, 1972, 1977). 2,4-D residues were not detected in more than
12 000 samples each of meat and dairy products analysed in the USA
between 1963 and 1969 (Duggan et al., 1971).
Results of feeding studies with hares and reindeer in
Scandinavia indicated that 2,4-D levels of 25 - 30 mg/kg forage
(equivalent to an intake of about 1 mg 2,4-D/kg body weight per
day) produce maximum 2,4-D residues of 1.1 mg/kg wet weight in
liver, and 8.9 mg/kg in kidney tissues (Erne, 1974). Residues of
2,4-D were detected in the liver and kidney of a few game animals
shot by hunters, or found dead in or near areas sprayed with
phenoxy herbicides (Table 11, Erne, 1974, 1975). The residues in
muscle tissue were not measured but would be lower than in the
liver and kidney, as indicated by the data summarized in Table 11.
On the whole, the available evidence indicates that 2,4-D is
rarely detected in commercial foods and that residues in food taken
from areas where 2,4-D has been sprayed will usually be < 1 mg/kg
food. The liver and kidney from range animals are possible
exceptions, but these contribute little to the total diet of the
general population.
5.2. Occupational Exposure to 2,4-D During the Production, Handling,
and Use of Chlorophenoxy Herbicides
During occupational exposure to 2,4-D, the chemical may be
absorbed via the inhalation, oral, and dermal routes, but more than
90% of the total amount of 2,4-D or other chlorophenoxy compounds
entering the body under these circumstances appears to be absorbed
through the skin and excreted relatively quantatively in the urine
as the phenoxy acid and readily-hydrolysed conjugates (Kolmodin-
Hedman et al., 1979, 1980; Libich et al., 1981; Draper & Street,
1982; Franklin et al., 1982; Leng et al., 1982; Nash et al., 1982)
(section 6).
Data from occupational exposure studies concerning the amounts
of 2,4-D found on the clothing or on cloth patches worn by workers
are not included in this review because the correlation between
these amounts and amounts absorbed into the body and then excreted
in urine is poor (Franklin et al., 1982; Lavy et al., 1982; Leng et
al., 1982).
Table 11. 2,4-D residues in game and domestic animals and animal products
------------------------------------------------------------------------------------------------------------------
Country Year(s) Species 2,4-D treatment Post- Type of 2,4-D residues References
rate (kg a.i./ha) treatment samples (mg/kg)
interval examined
(days)
------------------------------------------------------------------------------------------------------------------
Sweden 1968 moose (Alces game animals found ? liver and < 0.05-6 Erne (1974,
-1972 alces) dead, or shot by kidney from 1975)
deer (Capreolus hunters in herbicide- 250 animals
capreolus) treated areas found dead
hares (Lepus
lepus)
pheasants ? liver and < 0.05-4.5
grouse kidney from (2,4-D and
130 animals 2,4,5-T)
shot by
hunters
USA 1963 Jersey cow 50 ppm in diet for 4 0-2 milk < 0.1 Bache et al.
days (1964a)
USA 1974(?) adult beef 0, 9, 30 or 60 mg 0 muscle < 0.05-0.07 Clark et al.
cattle 2,4-D acid/kg bw/day 28 fat < 0.13-0.34 (1975)
for days (0, 300, liver < 0.05-0.23
1,000 2000 mg/kg feed) kidney 2.53-10.9
USA 1974(?) adult sheep 2000 mg/kg feed for 0-7 muscle < 0.05-0.06 Clark et al.
28 days fat 0.10-0.15 (1975)
liver 0.29-0.98
kidney 0.37-9.17
USA 1965(?) dairy cows animals grazing on 2 milk 0.01-0.09 Klingman et al.
pasture sprayed with 4 (1966)
herbicide at 2, 24 kg
a.i./ha
USA 1972 dairy cows 30, 300, 1000 mg/kg 0 milk < 0.05-0.16 Bjerke et al.
in feed for 2-3 weeks (1972)
1-3 < 0.05 Leng (1972)
USSR 1975 "livestock" ? ? muscle 0.04 Fyodorova et
liver 0.04 al. (1977)
kidney 0.03 (mean)
------------------------------------------------------------------------------------------------------------------
5.2.1. Industrial exposure
Several studies have been published on the levels of 2,4-D to
which workers producing or packaging 2,4-D herbicides are exposed
(Fetisov, 1966; Johnson, 1971; Juzwiak et al., 1973; Andreasik et
al., 1979). In every case the amount of 2,4-D absorbed by the
workers was uncertain and, therefore, the data are inadequate for
estimating industrial exposure to 2,4-D. Workers manufacturing
2,4-D were also exposed to other chemicals (Assouly, 1951; Bashirov
& Ter-Bagdasarova, 1970).
5.2.2. Exposure related to herbicide use
The available studies on the occupational exposure to 2,4-D of
workers during the use of 2,4-D herbicides are summarized in Table
12. Studies on the exposure of back-pack sprayers to 2,4-D have
not been published. However, comparable exposure data are
available for 2,4,5-T back-pack sprayers, and they have been
included in Table 12 for comparison. The levels of 2,4-D found in
the air of the working zone in these and other studies have already
been referred to in section 5.1.1.1 and Table 5.
In studies undertaken before 1980, only the amounts of 2,4-D in
the air, on the clothing, or on the skin were determined, except
for 2 urinary 2,4-D values reported by Shafik et al. (1971). Thus
the amounts of 2,4-D actually absorbed cannot be reliably estimated
from these early reports and are not included in Table 12.
After 1980, several detailed occupational exposure studies were
carried out to determine the amounts of 2,4-D or other chlorophenoxy
acids absorbed by various members of ground and aerial spray teams,
using a variety of equipment for dispersing aqueous or oil solutions
or emulsions (Kolmodin-Hedman et al., 1979; Kolmodin-Hedman & Erne,
1980; Libich et al., 1981; Draper & Street, 1982; Franklin et al.,
1982; Lavy et al., 1982; Leng et al., 1982; Nash et al., 1982).
The total 2,4-D urinary excretion levels reported in Table 12
reflect a wide variety of uses and show that the excretion does not
usually exceed 0.1 mg 2,4-D/kg body weight per day of exposure.
However, so far, a comprehensive comparison of the relative
exposures resulting from different methods of application and
different 2,4-D derivatives (amine salts and esters) or formulations
(aqueous, oil) cannot be carried out, because the available data are
still incomplete. The amount of 2,4-D absorbed depends on the type
of work performed, and on the degree of care taken to avoid direct
dermal contact with the herbicide concentrate, spray solution, or
spray. The most heavily-exposed workers tend to be the mixer-
loaders, who handle the herbicide concentrate, and the spray
personnel. However, if careful, they may be exposed to less 2,4-D
than, for example, a pilot of a spray plane who is not careful
(Franklin et al., 1982; Leng et al., 1982; Lavy et al., 1982; Nash
et al., 1982). The reports by Libich et al. (1981) and Leng et al.
(1982) on ground spray crews indicate that, even under unfavourable
working conditions, the amount of 2,4-D absorbed may be greatly
reduced simply by wearing clean gloves and overalls, and by making
the workers more aware of the importance of safe work habits.
Table 12. Exposure related to herbicide use
------------------------------------------------------------------------------------------------------------------------
Product No. of Type of Daily concentr- Duration of Total 2,4-D in urine References
people application ation of 2,4-D collection of excreted (mg/kg bw/
exposed in urine 24-h urine day of exposure)
(mg/litre)e samples (days)
------------------------------------------------------------------------------------------------------------------------
2,4-D and dicamba 2 boom spray 1 - 4 - - Draper & Street
dimethylene salts single use (1982)
in aqueous solution 2 repeated use 3 - 20 - -
2,4-D isooctyl 4 3 applications - 4 0.004 - 0.04 Franklin et al.
ester in diesel oil by single- (1982)
engine aircraft
2,4-D/2,4,5-T 4 tractor-drawn 1 - 14 7 - Kolmodin-Hedman
butoxyethyl sprayers, forestry (1979, 1980)
esters as 2% exposure daily,
emulsion in water for one week
2,4-D PGBE ester 26 helicopter in - 5 nd - 0.06a Lavy et al.
26 forestry use - 5 nd - 0.02b (1982)
2,4,5-T PGBE ester 7 Back-pack 5 0.01 - 0.09 Leng et al.
forestry use (2,4,5-T) (1982)
single exposures,
one week apart
2,4-D/2,4-DPc and 23 roadside and < 0.01 - 8 3 - Libich et al.
2,4-D/picloramc right-of-way (one usually (1981)
ground equipment high result
incl. mist blowers of 31)
2,4-Dc 17 aircraft repeated - 7 0.006 - 0.02d Nash et al.
exposure (mean values/day) (1982)
2,4-D amine salt 26 ground equipment - 7 nd - 0.08
and ester (single exposure)
------------------------------------------------------------------------------------------------------------------------
a No special precautions taken.
b Protective clothing worn.
c Preparation used not specified.
d Mean values per day recorded for different individuals.
e It is not possible to calculate the total 2,4-D excretion in urine from these data, because of individual variations
in urine concentrations from day to day from sample to sample.
As the chemobiokinetic profiles of urinary 2,4-D output are
reported in only a few of the studies, summarized in Table 12, it
is not possible to estimate the total 2,4-D intake in all cases.
The results of the studies by Libich et al. (1981) and by
Draper & Street (1982) suggest that using single-exposure studies
to estimate the peak exposure levels reached by workers exposed
several days in succession may give an underestimation.
No information is available on the amounts of chlorinated
dibenzodioxins, or other by-products or contaminants, absorbed as a
consequence of occupational exposure to 2,4-D herbicides.
In one extensive occupational monitoring programme undertaken
in 1979 - 82, about 3000 urine samples were analysed for herbicide
residues (Simpson, 1982). The subjects included pesticide factory
staff, pest control operators, farmers, park workers, and others
potentially exposed to 2,4-D. During the first year of the study,
no 2,4-D was detected (< 0.001 mg/litre) in 735 of 973 samples.
Most of the other samples contained less than 0.1 mg/litre and only
27 contained more than 1 mg/litre. The highest value was 31
mg/litre. The study is continuing.
5.3. Exposure of Bystanders to 2,4-D
Aerial drift and other forms of pesticide transport, as well as
the contamination of surfaces during or after herbicide production,
distribution, or use, may bring 2,4-D into contact with bystanders,
i.e., persons other than those who are occupationally exposed. Few
studies of bystander exposure to 2,4-D or other chlorophenoxy
herbicides have been published. Studies available for review
included that of Lavy et al. (1982) concerning 9 supervisors and
observers present at two helicopter forest spray operations using
2,4-D propyleneglycol butylether (PGBE) ester, respectively, for
unspecified durations. These people excreted a maximum of 1.3 µg
2,4-D/kg body weight. In a forest ground spray operation with
tractor-drawn equipment, 2,4-D was not detected (< 0.05 mg/litre)
in the urine of bystanders (Kolmodin-Hedman et al., 1980).
Additional bystander exposure studies for various 2,4-D use
patterns are desirable. However, the 2,4-D intake of bystanders is
unlikely to exceed the 2,4-D intake during occupational exposure.
5.4. Estimated Exposure of the General Population in 2,4-D-Use Areas
Data useful for estimating the intake by the general population
of 2,4-D residues in the environment including those in food
sources have been generated. The present calculations of the
intake of the general population in an area of 2,4-D use are based
on these data and on a series of stated assumptions aimed at
obtaining a moderate overestimation rather than underestimation of
the actual exposure.
5.4.1. Intake of 2,4-D residues from air
On the basis of available information, it can be assumed that
the general population in areas of 2,4-D herbicide use would rarely
be exposed to 2,4-D concentrations exceeding 0.1 µg/m3 air.
Assuming an air level of 0.1 µg 2,4-D/m3, a body weight of 60
kg, an air intake of 20 m3 per day, and a 100% retention of 2,4-D,
it can be calculated that the respiratory intake would be 0.03 µg
2,4-D/kg body weight per day.
5.4.2. Intake of 2,4-D residues from potable water
The larger surveys of potable water (Table 7) show mean 2,4-D
residues in surface water to be generally < 0.1 µg/litre, but for
the present estimate, it is assumed that potable water from surface
sources or from treatment plants, during a period of about 10 days
after reservoir treatment, can contain an average 2,4-D residue
level of 2 µg/litre (Wojtalik et al., 1971 and Table 7). Assuming
a 2,4-D concentration in water of 2 µg/litre, a body weight of 60
kg, a water intake of 2 litres per day (Canada, Health & Welfare,
1980), and a 100% absorption of the ingested 2,4-D, it can be
calculated that the 2,4-D intake of the general population in a
2,4-D use area resulting from water could approach 0.07 µg/kg body
weight per day, which could occur for about 10 days.
Insufficient data are available to give a reliable estimate of
2,4-D intake from ground water sources, but it is likely to be
lower than the above value.
5.4.3. Intake of 2,4-D residues from soil
2,4-D on soil particles ingested with food or water, or carried
into the air and inhaled, is considered to be part of the exposure
due to residues in air, water, or food and is therefore assumed to
be completely covered in these exposure estimates.
5.4.4. Intake of 2,4-D residues from food
The data in Tables 8 - 11 indicate that there is unlikely to be
any exposure of the general population to 2,4-D residues in retail
food supplies. The possibility that individuals are exposed to
contaminated local sources of food has been assessed in section
5.1.4. In the case of milk or muscle meat, it can be assumed that
no individual will be exposed to levels in excess of 0.02 mg/kg of
these foods, the limit of detection of the method of analysis used.
Assuming a concentration of 0.02 mg 2,4-D/litre in milk, and a
consumption of 1.5 litre per day, the maximum intake from this
source would be 0.0005 mg/kg body weight per day for a 60 kg adult.
Individuals who consume wild berries taken from 2,4-D-treated areas
could be exposed through this food source. Assuming consumption of
100 g of berries per serving and a maximum 2,4-D concentration of 1
mg/kg, the intake from this source would be 0.002 mg/kg body weight
per serving.
5.4.5. Total exposure of the general population in a 2,4-D-use area
The above considerations suggest that the total daily 2,4-D
intake of the population in use areas will not normally exceed
about 0.002 µg/kg body weight during the application period (Table
13).
Table 13. Components of estimasted exposure to 2,4-D
-------------------------------------------------------------------
Estimated amount of
Exposed Group intake (µg 2,4-D/kg Source of 2,4-D
bw/day)
-------------------------------------------------------------------
Occupational
i. Factory workers insufficient data mainly dermal contact
ii. Applicator crews 100a
iii. Bystanders - b
General population in
areas with 2,4-D use 0.03 air
0.07 water
0.5 milk
ND retail food
2.0 wild berries, mushrooms
etc.
-------------------------------------------------------------------
a Based on total urinary output after several days of exposure.
b Unlikely to exceed occupational exposure.
5.4.6. Total exposure of persons occupationally exposed in agriculture
An accurate maximum occupational intake of 2,4-D cannot be
determined on the basis of the limited studies undertaken.
However, the available data suggest that work performed in the
preparation of, and during, agricultural application of 2,4-D
herbicide will probably result in an exposure of not more than
about 0.1 mg 2,4-D/kg body weight per day, providing that minimum
precautions are taken against excessive exposure.
5.4.7. Total exposure of the general population outside areas of 2,4-D use
Monitoring of air, water, and food outside areas of known 2,4-D
use show that intake is below present detection limits.
6. CHEMOBIOKINETICS AND METABOLISM
With the exception of recent occupational exposure studies and
studies on animals published in 1979 or later, the available
information on the uptake, distribution, transformation, and
excretion of 2,4-D by human beings and other mammals has already
been reviewed by Leng (1977), National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Young et al. (1978), Bovey (1980a,b), Shearer
(1980), and United States Veterans' Administration (1981).
6.1. Uptake via Different Routes of Exposure
6.1.1. Uptake by inhalation
6.1.1.1. Animals
Burton et al. (1974) found that small amounts of 2,4-D
instilled into the rat lung were rapidly absorbed, apparently by a
non-saturable process following first-order kinetics, with an
absorption half time of 1.4 - 1.7 min. The kinetics of the
absorption of 2,4-D vapours or aerosols in the respiratory tract of
animals have not yet been studied.
6.1.1.2. Human beings
The uptake of 2,4-D and of 2,4-D derivatives via the human
respiratory tract does not appear to have been studied under
controlled conditions. However, the observations of Kolmodin-
Hedman & Erne (1980), Libich et al. (1981), Franklin et al. (1982),
and Lavy et al. (1982) on people occupationally exposed to 2,4-D
indicated that only a small percentage of the total amount of 2,4-D
absorbed via all routes of exposure was taken in through the
respiratory tract.
6.1.2. Dermal uptake
6.1.2.1. Animals
Mice whose tails had been immersed in 2,4-D butyl or crotyl
ester solutions, 4 h daily for 3-5 days, absorbed lethal amounts of
the chemicals (Fetisov, 1966). However, the actual doses absorbed
and other details were not given. In contrast, no major ill
effects were reported in studies in which rabbits were treated
percutaneously for 2 or 3 weeks with 130 - 180 mg/kg body
weight/day of a 50% aqueous solution of 2,4-D octyl ester, or with
unspecified amounts of solutions of 2,4-D dimethylamine salt in
water, or oil solutions of 2,4-D isooctyl or butyl ester
(Vinokurova, 1960; Kay et al., 1965).
6.1.2.2. Human beings
Only 5.8% of a dilute solution of 14C-labelled 2,4-D in acetone
applied at a dose of 4 µg a.i./cm2 to the ventral forearm of adults
was excreted in the urine compared with 100% of a small intravenous
dose (Feldmann & Maibach, 1974) (Table 14). The 2,4-D excretion in
urine is delayed and more prolonged after dermal application than
after intraveneous or oral administration (Feldmann & Maibach,
1974; Sauerhoff et al., 1977), and complete elimination may take
about one week (Levy et al., 1982; Leng et al., 1982). Cases of
acute occupational 2,4-D poisoning following combined dermal and
inhalation exposures (Monarca & Divito, 1961; Tsapko, 1966;
Paggiaro et al., 1974), as well as occupational exposure studies
(Table 12), suggest a fairly efficient dermal absorption of 2,4-D.
However, the importance of solvents, surfactants, and other
ingredients of the herbicides in the uptake of 2,4-D via the dermal
route still needs to be defined.
6.1.3. Oral uptake
6.1.3.1. Animals
The uptake of 2,4-D from the gut of rats, mice, guinea-pigs,
cattle, pigs, and sheep appears to be similar in both rapidity and
extent to that observed in human beings (Mitchell et al., 1946;
Lisk et al., 1963; Bache et al., 1964a; Erne, 1966a,b; Milhaud et
al., 1970; Shafik et al., 1971; Buslovich et al., 1973; Fedorova &
Belova, 1974; Clark et al., 1975; Senczuk & Pogorzelska, 1975,
1981; Van Peteghem & Heyndrickx, 1975). In some of the ungulates,
2,4-DB acid, and 2,4-D amine salts or esters are at least partially
converted to 2,4-D in the rumen, before being absorbed (Gutenmann
et al., 1963; Lisk et al., 1963). Some of the esters may be less
well absorbed from the gut than the acid or its alkali or amine
salts (Erne, 1966a; Buslovich et al., 1973), but the uptake
mechanisms for 2,4-D and its salts or esters is not known, and thus
deserves further study.
6.1.3.2. Human beings
Information on the uptake of 2,4-D by human beings via the
oral route has been gathered in studies on two groups of 5 - 6
volunteers each, who ingested single doses of 5 mg 2,4-D/kg body
weight (Table 14), and by chemobiokinetic studies on individuals
who, with suicidal intent, swallowed lethal or non-lethal amounts
of various 2,4-D herbicides (Geldmacher-Von Mallinckrodt &
Lautenbach, 1966; Rivers et al., 1970; Kohli et al., 1974;
Sauerhoff et al., 1976, 1977; Khanna & Kohli, 1977; Young & Haley,
1977; Prescott et al., 1979) (Table 15). These results show that
single doses of 2,4-D are fairly rapidly and completely absorbed
from the human digestive tract, unless the dose is so large that
toxic effects interfere with absorption. However, in the two
studies on volunteers, considerable individual variation in the
rate and extent of absorption from the digestive tract was
observed. The absorption mechanism appears to involve first-order
kinetics (Kohli et al., 1974; Khanna & Kohli, 1977) and may fit a
single- or multi-compartment chemobiokinetic model, depending on
individual characteristics (Sauerhoff et al., 1977).
Table 14. Chemobiokinetics of 2,4-D in human beings following administration under controlled conditions
--------------------------------------------------------------------------------------------------------------------------
Product Dose and dosing Subjects Observations Toxic effects References
schedule EL NOELa
(mg/kg bw)
single dose
--------------------------------------------------------------------------------------------------------------------------
14C-2,4-D 1) Intravenous injection: 6 (sex & Scintillation counting ? ? Feldmann &
(New England Dose (7 µCi) not stated as age not 1) 100% of dose excreted in urine Maibach
Co., and 2,4-D/weight unit stated) urine in 120 h Mean t0.5 = 13 h (1974)
Amersham 2) Dermal application: 6 (sex & 2) 5.8% of applied dose excreted ? ?
Searle Co.) 1 x 4 µg 2,4-D (in acetone)/ age not in 120 h
cm2 of skin of forearm. stated)
Application site was not
washed for 24 h
2,4-D, 99% Oral administration: 6 gas chromatography of blood & ? 5 Khanna &
pure (Dow 1 x 2, 3, or 5 mg/kg bw, in (adult M) urine samples; no ill effects; no Kohli (1977);
Chemical gelatin capsule, with water, changes in clinical parameters: Kohli et al.
Co.) following breakfast blood pressure, pulse rate, Hb, WBC (1974)
counts (total & differential); mean
plasma clearance t0.5 = 33 ± 3.1 h;
peak plasma conc. at 7-24 h = 40
mg/litre; ~75% of dose excreted
in urine in 96 h
"no metabolic transformation
at up to 5 mg/kg"
2,4-D, Oral administration: 6 gas chromatography - mass ? 5 Sauerhoff
analytical 1 x 5 mg/kg bw as a slurry (adult M, spectrometry of blood & urine et al.
grade in milk, or in powder form, 70-90 kg) samples; no ill effects; (1976, 1977)
with some water, following essentially all of the dose
breakfast absorbed; peak plasma conc. = 10-30
mg/litre within 24 h; mean plasma
clearance t0.5 = 11.6 h; mean
urinary excretion t0.5 = 17.7 h;
total excreted amount ~82% of dose
administered; 4.8-27.1% of
excreted compound was conjugated
--------------------------------------------------------------------------------------------------------------------------
a NOEL = No-observed-adverse-effect level.
Table 15. Chemobiokinetics of 2,4-D by human beings following accidental or intentional ingestion of herbicides
----------------------------------------------------------------------------------------------------------------
Products Circum- Subject Observations References
stances
----------------------------------------------------------------------------------------------------------------
2,4-D suicide; F, 33 death in about 30 h; post mortem 2,4-D Geldmacher-Von
ingestion years concentration: Mallinckrodt &
of unknown mg/litre mg/kg Lautenbach (1966)
amount of blood urine brain liver lung heart
herbicide 23 164 100 116 88 63
no metabolites were identified
Herbicide suicide; F, 51 death in about 96 h; concentration of 2,4-D
containing ingestion years, plus MCPA:
2,4-D plus of unknown 66 kg mg/litre mg/kg
MCPA amount of blood urine liver kidney muscle
("U46 COMBI") herbicide 42 420 100 trace 40
(BASF 2,4-dichlorophenol not detected; several
Ludwigshafen) other metabolites or herbicide by-products
found, but not identified
Herbicide suicide M, 39 severe toxic effects; unconsciousness; Prescott et al. (1979)
containing attempt; years recovery within 11 days following treatment by
2,4-D plus ingestion alkaline diuresis; initial plasma concentration:
mecoprop of 6.7 g 2,4-D = 400 mg/litre, mecoprop = 750 mg/litre;
(10%) + (20%) 2,4-D and no pretreatment change in 2,4-D level following
7.6 g alkaline diuresis; plasma clearance t0.5 = 3.7 h
mecoprop for 2,4-D, 11-28 h for mecoprop
----------------------------------------------------------------------------------------------------------------
6.2. Distribution and Transformation in the Body
6.2.1. Animals
The absorption and distribution kinetics and metabolism of pure
2,4-D and of a variety of pure or commercial 2,4-D or 2,4-DB amine
salts and esters have been repeatedly studied both in vivo and in
vitro in a wide variety of animals including rats, mice, rabbits,
guinea-pigs, cattle, sheep, goats, pigs, chickens, fish, and spiny
lobsters (Gutenmann & Lisk, 1965; Erne & Sperber, 1974; Guarino &
Arnold, 1979; James, 1979; Koschier & Pritchard, 1979; Pritchard
& James, 1979; Pritchard & Miller 1980). The considerable
differences observed in the relative amounts of residues found in
the cells and plasma of mouse, rat, and horse blood, after dosing
animals or after in vitro addition of 2,4-D (Erne, 1966a; Jenssen
& Renberg, 1976), in different tissues of rats, mice, and sheep
(Erne, 1966a,b; Lindquist & Ullberg, 1971; Milhaud et al., 1970;
Buslovich et al., 1973; Clark et al., 1975; Elo & Ylitalo, 1979),
and in the soluble and particulate fractions of rat tissues (Khanna
& Fang, 1974) support the idea that there is more than one
physiological compartment for 2,4-D storage. The distribution
volumes appear to be equivalent to the volume occupied by about
25 - 50% of the body mass (Erne, 1966a).
2,4-D is reversibly bound to blood plasma proteins,
particularly albumins, possibly at sites for which it competes with
related compounds. The same sites are apparently also binding
sites for palmitic acid and thyroxine. The extent of 2,4-D binding
depends, in part, on pH and 2,4-D concentration (Florsheim et al.,
1963; Erne, 1966b; Kolberg et al., 1973; Hacque et al., 1975;
Mason, 1975; Orberg, 1980a), and may affect the rate and extent of
renal 2,4-D excretion (Pritchard & James, 1979; Pritchard & Miller,
1980) and thus the toxicity of 2,4-D.
In pregnant mammals, up to about 17% of a single dose of 2,4-D
may rapidly cross the placenta to reach the embryos or fetuses
(Lindquist & Ullberg, 1971; Fedorova & Belova, 1974; Antonenko,
1977).
Pigs and rats hydrolyse 2,4-D esters both in the gut and after
absorption in the body (Erne, 1966a,b). Observations from several
studies indicate that 2,4-D is not significantly metabolized in
animals, except in ruminants. No 14CO2 was produced by rats given
C1- or C2-labelled 14C-2,4-D (Khanna & Fang, 1966). No 2,4-
dichlorophenol (2,4-DCP) was detected in the tissues of mice or
rats dosed by various routes with 2,4-D (Zielinski & Fishbein,
1967; Shafik et al., 1971; Federova & Belova, 1974; Grunow & Böhme,
1974). However, residues of 2,4-DCP were detected in the milk of
dairy cows fed 100 mg 2,4-D/kg diet for 3 weeks (Bjerke et al.,
1972; Leng, 1972), and in the livers and kidneys of cattle and
sheep fed up to 2000 mg/kg diet for 4 weeks (Clark, 1975; Leng,
1972, 1977). These 2,4-DCP residues probably resulted from the
bacterial degradation of 2,4-D in the rumen of the animals.
Bacterial degradation may also account for the 2,4-DCP reported by
Antonenko (1977) in pregnant or lactating rats and rat fetuses.
Other investigators did not detect 2,4-DCP in the tissues of
mice or rats dosed with 2,4-D by various routes (Zielinski &
Fishbein, 1967; Shafik et al., 1971; Fedorova & Belova, 1974;
Grunow & Böhme, 1974).
Results of studies on experimental animals have suggested that
2,4-D conjugates are formed in the kidney tubules (Erne, 1966a,b;
Erne & Sperber, 1974; Grunow & Böhme, 1974).
Taurine and glycine conjugates, as well as various other
unidentified conjugates of 2,4-D have been found in the urine of
rats, pigs, chickens, and the dogfish shark (Squalus acanthias)
(Erne, 1966b; Erne & Sperber, 1974; Grunow & Böhme, 1974; Koschier
& Pritchard, 1979). However, in rats and pigs, only about 10-20%,
and in the chicken, less than 5% of the total amount of 2,4-D
appeared to be excreted in this form. In the dogfish shark, the
taurine conjugate may be primarily formed in the tubular cells of
the kidney (Koschier & Pritchard, 1979). The site and mechanism of
2,4-D conjugation seem to be unknown in the other species.
6.2.2. Human beings
Studies on human volunteers who ingested pure 2,4-D, and on
cases of accidental or voluntary acute poisoning with various 2,4-D
herbicides have shown that 2,4-D is very rapidly absorbed from the
gut and carried in the blood to cells and tissues throughout the
body, but that is not extensively transformed (Tables 14, 15)
(Curry, 1962; Herbich & Machata, 1963; Nielsen et al., 1965; Dudley
& Thapar, 1972; Coutselinis et al., 1977). The kinetics following
ingestion suggest a 1- or 2-compartment distribution, depending on
individual characteristics (Sauerhoff et al., 1977; Young & Haley,
1977). Following absorption of purified 2,4-D, or of herbicides
containing only 2,4-D, no transformation products, including 2,4-
dichlorophenol, were found in blood or tissues. After ingestion of
herbicides containing 2,4-D and other compounds, some 2,4-D
metabolites or manufacturing by-products were detected in tissues,
but were not identified (Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Prescott et al., 1979). Unidentified 2,4-D conjugates were
also found in urine following ingestion of pure 2,4-D. These
conjugates represented up to 27% of the 2,4-D ingested (Sauerhoff
et al., 1976, 1977). Of the 5 North American volunteers studied by
these authors, only one did not produce a conjugate; in contrast,
apparently none of the 6 Indian subjects studied by Kohli et al.
(1974) and by Khanna & Kohli (1977) produced 2,4-D metabolites.
6.3. 2,4-D Levels in Body Tissues and Fluids
6.3.1. Animals
2,4-D levels in the blood and organs of mammals have been
determined, e.g., by Erne (1966a,b), Milhaud (1970), Buslovich et
al. (1973), Khanna & Fang (1974), Clark et al. (1975), Jenssen &
Renberg (1976), and Elo & Ylitalo (1979). The highest residue
levels were usually found in liver, kidney, lungs, spleen, and
heart. In a study by Fedorova & Belova (1974), 6 - 8% of the
amount of 2,4-D administered was found in all of the tissues
examined in rats dosed orally 26 - 35 days previously with this
chemical. However, the 2,4-D residue levels quoted were close to
the limit of detection for the analytical method used.
6.3.2. Human beings
In volunteers, each of whom ingested a single dose of 5 mg
2,4-D/kg body weight, the 2,4-D levels in blood plasma reached
peaks of about 10 - 45 mg/litre within about 7 - 24 h, and then
declined (Kohli et al., 1974; Khanna & Kohli, 1977; Sauerhoff et
al., 1977). In one group of workers occupationally exposed to
2,4-D for one week while using ground equipment for spraying
(Kolmodin-Hedman et al., 1979), plasma levels ranged from the
detection limit (0.02 mg/ml) to 0.2 mg/ml, while urinary levels
ranged from 1 to 14 mg/litre. Urinary 2,4-D levels reported in
other occupational exposure studies are summarized in Table 12.
However, it should be noted that analysis of single urine specimens
is not adequate for estimating the dose absorbed by individuals,
because excretion follows a diurnal pattern and continues for
several days after dermal exposure (Leng et al., 1977; Sauerhoff et
al., 1979; Lavy et al., 1982). Thus, levels found after several
days of spraying will probably be higher than first day levels, as
reported by Libich et al. (1981) and Draper & Street (1982).
However, excretion of 2,4-D should be completed within one week
following the last exposure (Feldmann & Maibach, 1974; Lavy et al.,
1982; Leng et al., 1982).
The toxic and lethal levels of 2,4-D in human blood and tissues
are still not well defined. A woman with reportedly 335 mg
2,4-D/litre plasma did not show any signs of poisoning; in general,
the acute lethal levels of 2,4-D appear to lie between 447 and 826
mg/litre plasma (Herbich & Machata, 1963; Nielsen et al.,
1965; Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Coutselinis
et al., 1977; Prescott et al., 1979). The lowest lethal 2,4-D
levels in blood or tissues were recorded several days after the
chemical was ingested, i.e., after most of the 2,4-D had probably
been eliminated. Among the different organs examined post mortem
in cases of fatal 2,4-D poisoning, liver and kidney tended to
contain the highest concentrations of 2,4-D, while brain and other
fatty organs, and muscle including the heart, usually had lower
2,4-D levels (Table 15) (Curry, 1962; Herbich & Machata, 1963;
Nielsen et al., 1965; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972; Coutselinis et al., 1977). As in the
case of blood, the values for the different tissues may vary
according to the proportion of 2,4-D eliminated by the time death
occurs.
6.4. Elimination and Biological Half-Life
The term "biological half-life" will be used to indicate the
time required to eliminate one half of a single dose of 2,4-D, or
to reduce 2,4-D residues in the body fluids or tissues to one-half
of the peak concentration. Biological half-life, as defined here,
is a useful concept with which it is possible to make rough
comparisons of the elimination rate of 2,4-D with that of other
toxic chemicals.
6.4.1. Animals
The half-life values recorded in mammals fall into the range
observed in the rat (Erne, 1966a,b; Federova & Belova, 1974; Khanna
& Fang, 1974), with the exception of the very low value for 2,4-D
butyl ester in whole mouse carcasses (0.85 - 1.11 h) observed by
Zielinski & Fishbein (1967). Results of the investigations
conducted by these authors also suggest that prior dosing with
2,4-D ("priming") may increase the rate of elimination of 2,4-D
butyl ester in mice, presumably through stimulation of the renal
excretory mechanism.
An important correlation between diet and 2,4-D elimination
rate was observed by Orberg (1980b) in the goat. A protein-poor
diet reduced the 2,4-D plasma clearance rate by about 20 - 50%,
possibly because of decreased renal size and renal blood flow.
The many species-related factors known to affect the rate of
2,4-D elimination make interspecies comparisons difficult, and
often the published reports do not include such details as diet,
age, sex, and body weight of the test animals, type and purity of
the test compounds, and important environmental factors such as the
ambient temperature, which are necessary for valid comparisons.
6.4.2. Human beings
The studies on volunteers and on cases of accidental or
voluntary 2,4-D poisoning summarized in Tables 14 and 15 show that
human beings excrete 2,4-D mainly in the urine, and that the
blood plasma clearance times depend on the dose, individual
characteristics, and the presence or absence of compounds that may
competitively inhibit 2,4-D excretion. For single oral doses of
2,4-D, the biological half-life in blood plasma is about one day,
depending on the circumstances. However, forced alkaline diuresis
may reduce this to as little as 3.7 h (Sauerhoff et al., 1977;
Prescott et al., 1979).
In occupationally-exposed people, absorption of successive
daily doses of 2,4-D makes its biological half-life difficult to
determine, but for single occupational exposures it has been
estimated to be 35-48 h (Nash et al., 1982).
6.5. Chlorinated Dibenzo- p-Dioxins (CDDs)
The metabolism in the rat of several dibenzo- p-dioxins,
including the 2,7-CDD found in 2,4-D, was described by Tulp &
Hutzinger (1978). The primary pathway of transformation appears
to involve hydroxylation at the 2-, 3-, 7-, or 8-position in the
molecule; some sulfur-containing conversion products have also been
identified.
7. EFFECTS OF 2,4-D ON ANIMALS
7.1. General Introduction
Many studies of the toxicity of 2,4-D were carried out before
the possible toxicological importance of manufacturing by-products,
such as 2,6-dichlorophenoxyacetic acid, 2,4,6-trichlorophenoxy-
acetic acid (2,6-D and 2,4,6-T) monochlorophenoxyacetic acid, or
N-nitroso compounds, was known or appreciated.
Furthermore, 2,4-D may be contaminated with several chlorinated
dibenzo- p-dioxins (section 2.1.4). The toxicity of a number of
these contaminants has not been examined in detail, but whether or
not their presence would affect the toxicity of 2,4-D and its
derivatives would depend on the amount of CDD present in the
product, and on the inherent toxicity of the particular CDD
isomers. The most toxic CDD isomer, namely 2,3,7,8-TCDD (Schwetz
et al., 1973; McConnell et al., 1978; Leng, 1979; Kociba & Schwetz,
1982), is not normally found in 2,4-D products (see also section
2.1.4). However, there have been instances in which the same
manufacturing equipment was used to produce both 2,4,5-T and 2,4-D,
resulting in cross-contamination of 2,4-D with 2,4,5-T and 2,3,7,8-
TCDD (US EPA, 1982).
Studies of structure-activity relationships using in vitro
systems have shown that the CDDs that may be present in 2,4-D and
its derivatives have a much lower biological activity than 2,3,7,8-
TCDD (Poland & Glover, 1973; Poland & Kende, 1974; Poland et al.,
1976; Bradlaw et al., 1980; Knutson & Poland, 1980). However,
except for a study of the carcinogenic potential of 2,7-
dichlorodibenzodioxin (2,7-DCDD) (US National Cancer Institute,
1979), the chronic toxicity of the CDD detected in 2,4-D and its
derivatives has not been studied.
2,4-D has been in use as a herbicide for nearly 40 years, and
during this time a great deal of literature on the toxicology of
this chemical has accumulated. The extent to which its toxicity to
various organisms has been tested, and the types of 2,4-D products
used for such testing have varied over the years. Earlier 2,4-D
products probably contained higher concentrations of trace
contaminants than the 2,4-D in use today, and therefore it may have
been found to be more toxic in earlier than in more recent studies.
In addition, the generally accepted standards and protocols for
pesticide toxicity tests have changed, making some of the older
tests inadequate by present day standards. For these reasons,
attention has been focused on the more recent studies. The older
studies are, in many instances, only cited for completeness and
should be used with caution, especially when ascribing specific
toxic effects to unspecified 2,4-D products and when establishing
an effect level or no-observed-adverse-effect level for adverse
effects of 2,4-D. The Task Group noted that a number of additional
studies on 2,4-D are at present in progress. As the additional
information becomes available, the present document will need to be
updated.
7.2. Acute Effects
The reports of experimental studies to define the toxic and
other effects of 2,4-D or its derivatives cover a wide range of
organisms commonly referred to as "animals", including worms,
molluscs, arthropods, lower vertebrates, birds, and mammals. Much
of this information, especially on acute toxic effects, was
recently tabulated by the National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Schneider (1979), Bovey & Young (1980), Shearer &
Halter (1980), and the Commission of the European Communities (CEC,
1981).
The usual mandatory acute and subacute safety tests for
pesticides include assays for eye and skin irritancy and for skin
sensitization, and determinations of the acute oral, percutaneous,
and parenteral lethal doses, or of the corresponding lethal
concentrations in the air, diet, or water.
7.2.1. Skin and eye irritancy
2,4-D does not appear to be an eye or skin irritant (Schneider,
l979). Adequate tests of the potential irritative properties of
2,4-D derivatives have not been reported in the literature.
7.2.2. Skin sensitization
No adequate published information is available on the dermal
sensitization potential of 2,4-D and its derivatives in mammals.
7.2.3. Lethal doses and concentrations (LD50 and L50)
The lethal potential of a chemical is usually measured as the
dose (mg/kg body weight), or as the concentration in the air, diet,
or water (mg/kg, mg/m3, mg/litre, respectively) that will kill 50%
of the test animals in a specified time interval. These amounts
are referred to as the LD50 or LC50. For 2,4-D and its derivatives,
and for 2,4-D herbicide formulations, these statistically estimated
values vary depending on the test product, the test species, and
the route and frequency of administration (Tables 16 and 17).
7.2.3.1. Acute oral LD50
7.2.3.1.1. Mammals
Published acute oral LD50 values vary for different 2,4-D
products and test species (Table 16). It appears that 2,4-D has a
moderate acute toxicity for mammals (WHO, 1976).
7.2.3.1.2. Birds
Table 17 shows published oral LD50 values for chickens.
Table 16. Acute oral toxicity of 2,4-D, esters, and salts
------------------------------------------------------------------------
Compound Species Sex LD50 Reference
(mg/kg bw)
------------------------------------------------------------------------
2,4-D mouse M 375 Hill & Carlisle (1947)
mouse M 368 Rowe & Hymas (1954)
rat M 375 Rowe & Hymas (1954)
rat 666 Hill & Carlisle (1947)
guinea-pig M & F 469 Rowe & Hymas (1954)
guinea-pig 1000 Hill & Carlisle (1947)
rabbit 800 Hill & Carlisle (1947)
dog 100 Drill & Hiratzka (1953)
butyl ester mouse 380 Konstantinova (1970)
rat 1500 Schillinger (1960)
rat 920 Konstantinova (1970)
cat 820 Konstantinova (1970)
esters of mono-, rat F 570 Rowe & Hymas (1954)
di-, and tripro-
pylene glycol
butyl ethers
isopropyl ester mouse M 541 Rowe & Hymas (1954)
rat M & F 700 Rowe & Hymas (1954)
guinea-pig M 550 Rowe & Hymas (1954)
mixed butyl mouse F 713 Rowe & Hymas (1954)
esters rat F 620 Rowe & Hymas (1954)
guinea-pig F 848 Rowe & Hymas (1954)
rabbit M & F 1420 Rowe & Hymas (1954)
sodium salt mouse 375 Rowe & Hymas (1954)
rat F 805 Rowe & Hymas (1954)
rat 2000 Schillinger (1960)
guinea-pig M 551 Rowe & Hymas (1954)
rabbit 800 Rowe & Hymas (1954)
------------------------------------------------------------------------
7.2.3.2. Acute dermal LD50
7.2.3.2.1. Mammals
Reports by Buslovich (1963) and Fetisov (1966) indicate that,
under extreme test conditions, mice and rats may absorb lethal
amounts of 2,4-D amine salts and esters through their skin, but no
acute dermal LD50 values were available for review.
7.2.3.3. Acute inhalation LC50
No accurate measurements of acute inhalation LC50 values
for 2,4-D products were available.
Table 17. Acute LD50 for various 2,4-D products in domestic chickens (Gallus domesticus)
------------------------------------------------------------------------------------------------------------------
Product Animals Procedure LD50 Acid References
(mg/kg bw) equivalent
------------------------------------------------------------------------------------------------------------------
2,4-D chick, M & F P.O., in olive oil 541 (358-817) 541 Rowe & Hymas (1954)
2,4-D Na salt adult hen P.O., in water 655 646 Loktionov et al. (1973)
2,4-D alkanolamine chick P.O., in water > 380 < 765 368 Bjorn & Northern (1948)
salts Rowe & Hymas (1954)
2,4-D amine salt 6-month chicken P.O., in water 1950 1238 Loktionov et al. (1973)
2,4-D butyl esters chick, M & F P.O., undiluted 2000 (1350-2960) 1503 Rowe & Hymas (1954)
2,4-D butoxyethyl chick, 330 g P.O., in food 900 588 Whitehead & Pettigrew
esters (1972)
2,4-D isopropyl esters chick, M & F P.O., in olive oil 1420 (1127-1789) 1145 Rowe & Hymas (1954)
2,4-D/2,4,5-T (1:1), chick, M & F P.O., undiluted 4000 (2700-5900) - Rowe & Hymas (1954)
butyl ester 3 weeks old
------------------------------------------------------------------------------------------------------------------
7.2.3.4. Parenteral LD50
The acute parenteral LD50 values reported for various
laboratory animals and 2,4-D products ranged from 220 to 666 mg
a.i./kg body weight (National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Young et al., 1978; Schneider, 1979).
7.2.4. Acute toxicity in aquatic organisms
The available reports indicate great differences in acute LC50
values obtained with different test species, and with different
2,4-D derivatives and test formulations. The esters appear to be
considerably more toxic than the water-soluble salts. For fish,
the LC50 for various 2,4-D isooctyl ester products may range from 5
to 68 mg/litre for bluegill sunfish (Lepomis spp.) and from 62 to
153 mg/litre for rainbow trout (Salmo gairdneri) (Schneider,
1979). This variability may, in part, be due to differences in
test animal species and strains, in test conditions (temperature,
pH, 02 tension, mineral content of water), and, in part, to the
effects of chemicals other than 2,4-D in the herbicide
formulations, or of 2,4-dichlorophenol, which may occur in water
as a decomposition product of 2,4-D (Holcombe et al., 1980).
Discussions on this variablility can be found in numerous reviews:
Way (1969), Katz et al. (1972), National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), and of Halter (1980), as well as in
the reports of studies by Harrisson & Rees (1946), King & Penfound
(1946), Lopez (1961), Hughes & Davis (1963), Butler (1965),
Hiltibran (1967), Mount & Stephan (1967), Alabaster (1969), Sanders
(1970a), Andrushaitis (1972), Cooke (1972), Shim & Self (1973),
Fabacher & Chambers (1974), Meehan et al. (1974), Nishiuchi &
Yoshida (1974), Rehwoldt et al. (1977), Vardia & Durve (1981), and
particularly in the extensive and methodical investigation of
Pravda (1973).
Similar information on aquatic invertebrates is available in
the review of Mackenthun & Keup (1972) and in the studies of Hooper
(1958), Sudak & Claff (1960), Beaven et al. (1962), Rawls (1965),
Sanders (1970b), Klekowski & Zvirgzds (1971), Wierzbicka (1974a,b),
and Caldwell et al. (1979).
The observations of Hansen et al. (1972, 1973) and Folmar
(1976) indicate that fish and aquatic invertebrates will try
to avoid water containing toxic amounts of 2,4-D products.
7.3. Subchronic and Chronic Toxicity
7.3.1. Mammals
Most of the published long-term studies with mammals have
already been reviewed by Bodyagin et al. (1969), Way (1969), IARC
(1977, 1982), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Young et al. (1978), Bovey & Young (1980), Shearer (1980), and US
Veterans' Administration (1981).
In the long-term as in the short-term studies on mammals, the
test products were mainly administered orally. The composition of
the test products was not adequately known by present standards,
but many of the tests were carried out with more or less purified
2,4-D or its alkali salts. It is difficult to decide to what
extent the available results reflect the toxicological properties
of the present 2,4-D products, in which the maximum contents of CDD
and other toxic by-products are kept at very low levels.
A long-term feeding study in the rat (Hansen, 1971) was
evaluated by FAO/WHO JMPR in 1971 (FAO/WHO, 1972). It was
concluded that a no-observed-adverse-effect level in the rat was
equivalent to 31 mg/kg body weight per day. However, new studies
on mice and rats are in progress.
7.3.1.1. Clinical signs of poisoning
Signs of toxic effects on the digestive tract, such as
diarrhoea, vomiting, dysphagia, decreased gut motility, irritation,
or necrotic changes (in dogs including necrosis of oral tissues)
are likely to appear in animals following the absorption of high
doses of 2,4-D or its derivatives by the oral, dermal, or
inhalation routes, and after parenteral injections (Bucher, 1946;
Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Thomssen, 1958; Björklund & Erne, 1966; Erne, 1966a; Palmer &
Radeleff, 1969). Blood-tinged discharges from the nose, and in
dogs also nasal and eye irritation and skin lesions may also occur
(Bucher, 1946; Hill & Carlisle, 1947; Kosyan et al., 1974). Cattle
may suffer from tympanitis, and may show signs of thirst (Björklund
& Erne, 1966). However, some of the signs of "2,4-D poisoning"
reported in herbivores pastured on 2,4-D-treated vegetation may
have been caused by the ingestion of inherently poisonous plants
(Maclean & Davidson, 1970). Pigs may refuse to eat feed containing
high amounts of 2,4-D (Strach & Bohosiewicz, 1964), but sheep have
been reported to feed avidly on 2,4-D-treated vegetation (Sadykov
et al., 1972).
Characteristic signs of severe 2,4-D poisoning in mammals
appear to be muscular weakness, stiffness, stilted gait, and muscle
spasms (myotonia), alleviated by exercise and exacerbated by rest.
There may also be muscular incoordination progressing to paralysis
especially in the hind limbs; in rodents, caudal rigidity has also
been observed. These clinical signs are the result of the myotoxic
action of 2,4-D discussed below, which, through its effects on the
heart, may also lead to hypo- or hypertension, cardiac
fibrillation, and death. Prolonged inactivity may also occur and
may lead to pulmonary congestion, emphysema, and pneumonia (Bucher,
1946; Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Vinokurova,
1960; Björklund & Erne, 1966; Gorshkov, 1972; Sadykov et al., 1972;
Kosyan et al., 1974).
At high doses, 2,4-D and its derivatives may act as a central
nervous system depressant and cause lethargy, slowed respiration,
stupor, coma, and death (Bucher, 1946; Hill & Carlisle, 1947; Drill
& Hiratzka, 1953; Vinokurova, 1960; Desi et Sos, 1962a,b; Desi et
al., 1962a,b; Kosyan et al., 1974; Elo & Ylitalo, 1977, 1979).
7.3.1.2. Effects on food and water consumption, and on body weight
Relatively high concentrations of 2,4-D or its derivatives in
the diet or drinking water, or given by capsule, gavage, or
parenterally, may cause a reduction in food and water consumption,
and weight loss or reduced body weight gain in rats (Rowe & Hymas,
1954; Thomssen, 1958; Björklund & Erne, 1966; Chang et al., 1974;
Chen et al., 1981; Gorshkov, 1972), as well as in dogs (Bucher,
1946; Drill & Hiratzka, 1953), in pigs (Strach & Bohosiewicz, 1964;
Björklund & Erne, l966), in rabbits (Loktionov et al., 1973), in
cattle (Rowe & Hymas, 1954; Björklund & Erne, 1966; Palmer &
Radeleff, 1969; McLennan, 1974), and sheep (Palmer & Radeleff,
1969). However, the same authors, as well as Guseva (1956) and
Hansen et al. (1971) did not observe these effects at low doses or
low dietary concentrations of 2,4-D. In fact, in the studies by
Raoul & Marnay (1948) and Strach & Bohosiewicz (1964) on rats and
piglets fed low dietary concentrations of 2,4-D, an unimpaired
appetite and an improved body weight gain was seen in some groups
of animals. In these studies, the dosages ranged between 15 and
119 mg 2,4-D/kg body weight.
Observations of Thomssen (1958), Strach & Bohosiewicz (1964),
Martynov (1970), Gorshkov (1972), Sadykov et al. (1972), and Erne
(1974), on hares (Lepus timidus), European elk, pigs, and rats
suggest that, given a choice, animals will refuse to eat food or to
drink water containing more than a certain amount of 2,4-D, and
that this may, in part, be because of the strong characteristic
odour and taste of this compound, or of organic solvents such as
diesel fuel that are used in herbicide formulations or as diluents.
7.3.1.3. Effects on the central nervous system (CNS)
It has been suggested that the signs of central nervous system
depression in animals severely poisoned with 2,4-D are related to a
partial breakdown of the blood-brain barrier, possibly as a result
of damage to capillary vessels, and a subsequent accumulation of
2,4-D in the CNS (Desi & Sos, 1962a,b; Elo & Ylitalo, 1977, 1979).
However, further studies are needed to clarify the mechanism(s)
by which 2,4-D acts on the central nervous system.
7.3.1.4. Effects on the peripheral nervous system
No peripheral neuropathy was attributed to 2,4-D in the
available reports on short-term and long-term studies with 2,4-D in
a variety of animals (Shillinger & Naumova, 1957; Stupnikov, 1959).
The partial or complete paralysis, especially of the hind legs, of
2,4-D poisoned animals reported already by Bucher (1946) and Hill &
Carlisle (1947) may be a myotoxic rather than a neurotoxic effect
of 2,4-D. Moreover, in severe 2,4-D poisoning, a general weakness
may lead to inactivity that might be interpreted as paralysis.
No signs of neuropathy were reported by Kay et al. (1965) in
rabbits given large percutaneous doses of 2,4-D dimethylamine salt,
2,4-D butyl ester, or 2,4-D isooctyl ester. In similar studies in
which rats or rabbits were exposed by the dermal route, Buslovich
(1963) reported myotonia and death in rats given unspecified doses
of 2,4-D amine salt or 2,4-D butyl ester, but no peripheral
neuropathy, while Vinokurova (1960) found 130 - 180 mg of a 50%
aqueous emulsion of 2,4-D octyl ester/kg body weight to have "no
systemic effect" on rabbits.
The neurotoxic effects in animals of 2,4-D and of other related
compounds have not been adequately studied. Further research is
required to elucidate the mechanism of the neurotoxic and myotoxic
action of 2,4-D in animals.
7.3.1.5. Myotoxic effects
Most of the studies of the effects of 2,4-D on vertebrate
muscles were carried out because the 2,4-D-induced abnormalities
resemble a heritable muscle disorder in human beings, namely
myotonia congenita (Hofmann et al., 1966; Laskowski & Dettbarn,
1977). As a rule, high doses of 2,4-D (1/4 to 1/2 LD50) are
required to obtain severe and prolonged myotonia. The effects of
2,4-D on muscle cells are complex, and include disturbances in:
the activity of various enzymes (leading, for example, to increased
lactate production); potassium levels; membrane resistance; and in
chloride conductance. There are also shifts in calcium-binding
sites, changes in muscle and nerve cell electrical potentials, and
mitochondrial structural and ultrastructural degenerative changes
in muscle (Eyzaguirre et al., 1948; Kuhn & Stein, 1964, 1965, 1966;
Heene 1966, 1968, 1975; Hofmann et al., 1966; Kenigsberg, 1968;
Stein & Kuhn, 1968; Bodem et al., 1971; Preiss & Rossner, 1971;
Seiler, 1971; Buslovich & Koldobskaya, 1972; Rüdiger et al., 1972;
Senges & Rüdel, 1972; Brody, 1973; Danon et al., 1976; Iyer et al.,
1976; Bretag & Caputo, 1978; Dux et al. 1978; De Reuck et al.,
1979; Eberstein & Goodgold, 1979; Mazarean et al., 1979a,b).
Similar effects are produced by the chlorophenoxy drug clofibrate
(atromid) (Pierides et al., 1975; Smals et al., 1977). None of the
available studies on 2,4-D-induced myotonia were designed to
establish no-observed-adverse-effect levels for the various
myotoxic effects in intact animals, and therefore additional
studies should be carried out for this purpose.
7.3.1.6. Cardiovascular effects
As part of its myotoxic action, 2,4-D and its derivatives may
in high doses cause biochemical, physiological, and structural
damage to the myocardium in vitro and in vivo (Bodem et al., 1971;
Preiss & Rossner, 1971; Rüdiger et al., 1972; Mazarean et al.,
1979a,b).
7.3.1.7. Haematological effects
Shifts have been reported in the number or types of
erythrocytes, leukocytes, or bone marrow cells, or changes in
haemoglobin levels, in a variety of laboratory and domestic mammals
given 2,4-D or 2,4-D derivatives (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Hansen et al., 1971;
Sadykov et al., 1972; Loktionov et al., 1973; Kosyan et al., 1974;
and Halliop et al., 1980).
7.3.1.8. Effects on blood chemistry
Rowe & Hymas (1954) were apparently the first investigators to
monitor blood chemistry in 2,4-D-treated animals. They did not
observe any adverse effects at 2,4-D dietary levels as high as 300
mg 2,4-D/kg in rats treated for 113 days.
Other investigators noted changes in various serum, plasma, or
erythrocyte enzyme activity levels, and shifts in the levels of
electrolytes, glucose, blood proteins, or other chemicals in
response to treatment with 2,4-D or 2,4-D derivatives in rats,
rabbits, cattle, pigs, or sheep, or in blood from such animals to
which 2,4-D was added in vitro (Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Shevchenko, 1966;
Dzhaparov & Tsilikov, 1969; Hunt et al., 1970; Szöcs et al., 1970;
Gorshkov, 1972; Kosyan et al., 1974; Kuzminskaya & Bersan, 1975;
Chen et al., 1981). Some of the changes in transaminase (SGOT,
SGPT), blood urea nitrogen (BUN), or glucose levels appeared to be
secondary to myotoxic, nephrotoxic, or hepatotoxic effects of high
doses of 2,4-D.
7.3.1.9. Other biochemical effects observed in vivo or in vitro
Sufficiently high doses of 2,4-D may induce changes in
mitochondrial oxygen consumption and oxidative phosphorylation, in
electrolyte, ascorbic acid, glycogen, lipid, and nucleic acid
contents of organs, tissues, cells, organelles, or cell fractions
from a variety of mammals (Brody, 1952, 1973; Guseva, 1956; Baker
et al., 1960; Kuhn & Stein, 1964; Heene, 1966, 1967, 1968;
Shevchenko, 1966; Philleo & Fang, 1967; Kenigsberg, 1968; Dzhaparov
& Tsilikov, 1969; Stanosz, 1969; Buslovich & Koldobskaya, 1972;
Graff et al., 1972; Buslovich et al., 1973; Abo-khatwa &
Hollingworth, 1974; Chang et al., 1974; Nikandrov, 1974; Venkaiah &
Patwardhan, 1978; Podolak, 1979).
7.3.1.10. Pulmonary effects
No pulmonary abnormalities were reported in studies with mice,
rabbits, rats, or dogs conducted by Bucher (1946), Drill & Hiratzka
(1953), Rowe & Hymas (1954), Shillinger & Naumova (1957),
Schillinger (1960), or Björklund & Erne (1966). In contrast, Hill
& Carlisle (1947) and Palmer (1972) noted congestion of pulmonary
vessels, pulmonary petechial haemorrhages, and pulmonary edema or
emphysema in mice, rats, dogs, cattle, or sheep that died of 2,4-D
poisoning.
7.3.1.11. Hepatotoxic effects
Rabbits, rats, mice, dogs, cattle, or sheep treated for a
prolonged period with toxic doses of 2,4-D were found to develop a
subacute toxic hepatitis with congestion of hepatic blood vessels,
and cloudy swelling, fatty infiltration, local necrosis,
degeneration, or atrophy of hepatocytes, especially of the
parenchyma in the centrolobular areas (Bucher, 1946; Hill &
Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Björklund & Erne, 1966; Szöcs et al., 1970; Palmer, 1972). High
doses of 2,4-D may induce a proliferation of peroxisomes and
increased levels of mixed function oxidases in liver cells of rats
and hamsters (Buslovich et al., 1982; Vainio et al., 1982, 1983).
Changes in the levels of certain liver enzymes such as maleate
or succinic dehydrogenase, and in ascorbic acid or glycogen content
or hippuric acid production have also been reported (Shillinger &
Naumova, 1957; Baker et al., 1960; Dzhaparov & Tsilikov, 1969;
Szöcs et al., 1970; Buslovich et al., 1973; Chang et al. 1974;
Nikandrov, 1974). Some of the results concerning liver glycogen
levels in rats suggest a reverse trend at high doses, as Dzhaparov
& Tsilikov (1969) found that a dose equivalent to 1/16 LD50 per day
lowered the liver glycogen content, while Chang et al. (1974)
observed the opposite effect at doses of about 100 mg/rat per day.
7.3.1.12. Effects on the kidney
In early studies with high doses of 2,4-D, signs of impaired
kidney function, increased relative kidney weight, and of gross and
histological abnormalities (parenchymatous degeneration,
hypertrophy, and hyperplasia, cloudy swelling especially in the
cells of the proximal convoluted tubules, and glomerular lesions)
were noted in mice, rats, and dogs (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954). That the kidney
is a target organ for the structural, physiological, and chemical
effects of 2,4-D was confirmed repeatedly by later and more
detailed studies on a wider range of test species including pigs,
goats, and sheep (Schillinger, 1960; Björklund & Erne, 1966; Erne,
1966a; Stanosz, 1969; Hunt et al., 1970; Milhaud et al., 1970;
Gorshkov, 1972; Palmer, 1972; Senczuk & Pogorzelska, 1975; Koschier
et al., 1978; Orberg, 1980a). In a recent 13-week study on rats,
the no-observed-adverse-effect level for histological changes
induced with pure 2,4-D in mammalian kidney appeared to be 15 mg/kg
body weight per day (Chen et al., 1981).
7.3.1.13. Effects on endocrine organs
Swelling and congestion of the thyroid were noted by Palmer
(1972) in cattle and sheep fatally poisoned with various 2,4-D
products. Effects of 2,4-D on iodide uptake by the thyroid gland
were first decribed by Sos & Kertai (1958) and studied further by
Florsheim & Velcoff (1962), Florsheim et al. (1963), Tsilikov
(1969), and Gorshkov (1972). Their results indicate that doses of
5 - 250 mg 2,4-D sodium salt or equivalent/kg body weight per day
have a stimulatory effect on thyroid function.
Effects of 2,4-D on adrenal function and its relationship to
muscle carbohydrate metabolism and 2,4-D-induced myotonia have been
studied by Kenigsberg (1968), and by Buslovich & Koldobskaya
(1972). 2,4-D-induced changes in adrenal or thyroid function may
also be implicated in the abnormal temperature regulation in 2,4-D-
treated rats described by Sudak et al. (1966). However, it is
noteworthy that Buslovich (1963) was unable to induce a change in
the body temperature of rats by giving 15 - 20 oral doses of 2,4-D
sodium salt, amine salt, or butyl ester at a level of 1/10 or 1/5
LD50/day.
7.3.1.14. Effects on the digestive tract
Vomiting, diarrhoea, hyperaemia, bloody exudates in the gut,
necrotic changes in the mucosa, and other non-acute toxic effects
on the digestive tract have been reported after administration of
high doses of 2,4-D by either the oral or parenteral route in mice,
rats, and dogs (Bucher, 1946; Hill & Carlisle, 1947; Drill &
Hiratzka, 1953; Kosyan et al., 1974). However, Hansen et al.
(1971) did not observe any such effects in a 2-year feeding study
at dietary levels of 2,4-D corresponding to about 37.5 and 67.5 mg
2,4-D/kg body weight per day, respectively, for rats and dogs.
Their observations on dogs contrast with those of Drill & Hiratzka
(1953) who found repeated doses of 20 mg 2,4-D/kg body weight per
day to be fatal in 3 out of 4 dogs studied by them.
7.3.2. Birds
The published reports on the toxic effects of 2,4-D and 2,4-D
products in birds deal mainly with chickens (Gallus domesticus)
and game birds. The available data on cumulative oral lethal doses
for game birds have been summarized in Table 18; studies on the
reproductive, embryotoxic, and teratogenic effects of 2,4-D are
dealt with in section 7.3.6.
Table 18. Cumulative oral lethal doses of 2,4-D herbicides for game birds
------------------------------------------------------------------------------------
Product Species Dietary LC50 Cumulative LD50 References
(mg a.i./kg (mg a.i./kg bw)a
diet)a
------------------------------------------------------------------------------------
2,4-D quail (young) 5000 28 000 Dewitt et al.
dimethylamine (1962)
salt
mallard duck 5000 8250
(young) 5000 8250
(adult) > 2500 > 34 000
2,4-D quail
butoxyethanol (young) 5000 38 000
ester (adult) 5000 40 700
pheasant (young) 5000 29 500
mallard duck (adult) 5000 > 33 000
------------------------------------------------------------------------------------
a a.i. = active ingredient
The clinical signs of 2,4-D poisoning in birds appear to be
similar to those in mammals, and the kidney seems to be the most
sensitive organ. No adverse effects were reported in chickens fed
dietary levels of 1000 mg/kg for 21 days (142 mg/kg body weight)
whereas kidney enlargement occurred in chickens fed 5000 mg/kg of
diet for 21 days (Whitehead & Pettigrew, 1972).
7.3.3. Cold-blooded animals
The literature on the chronic effects of 2,4-D and its
derivatives on cold-blooded animals (poikilotherms) was not
reviewed in detail. The available reports indicate that for
aquatic vertebrates in general, 2,4-D esters are more toxic than
2,4-D amines, and that the overall no-observed-adverse-effect level
for toxic effects on fish for the esters is at or near 1 mg/litre
(King & Penfound, 1946; Zhiteneva & Chesnokova, 1973; Fabacher &
Chambers, 1974; Meehan et al., 1974). 2,4-dichlorophenol, which
may occur in water as a by-product or transformation product of
2,4-D, is similarly toxic to fish (Holcombe et al., 1980).
7.4. Fetotoxicity, Teratogenicity, and Reproductive Effects
The scientific literature contains a fairly large number of
reports on the fetotoxic, teratogenic, and reproductive effects of
2,4-D in livestock and in laboratory animals. However, most of the
observations on livestock are either too sketchy to be useful or
neglect to take into account various confounding factors. Examples
of this are studies by Björklund & Erne (1966) on a single pregnant
pig, by Sadykov et al. (1972) on sheep grazing on pastures
apparently containing a high percentage of poisonous plants, and
the report by Bodai et al., (1974), on reproductive disturbances in
cattle feeding on either 2,4-D-treated vegetation possibly
including poisonous plants, or on contaminated silage.
Some of the studies carried out with rodents under laboratory
conditions also provide little useful information, either because
it is difficult or impossible to determine the dose levels used in
the studies (Weinmann, 1957; Schuphan, 1963, 1965, 1969; Schiller,
1964; Buslovich et al., 1976); or because the information provided
is insufficient and the studies cannot be properly evaluated
(Bucher, 1946; Hansen et al., 1971; King et al., 1971). The study
by Weinmann (1957) can be considered invalid, as a high mortality
occurred in both control and experimental animals, when they were
exposed to cold stress because of construction work affecting the
animal quarters during the winter months. Moreover, Weinmann
(1957) and several other authors, including Schuphan (1963, 1965,
1969) Schiller (1964), Schillinger (1960), and Shillinger & Naumova
(1957) did not in fact examine the effects of 2,4-D, but rather the
effects of foods or food extracts prepared from crops that had been
sprayed with 2,4-D, and, in some cases, also with other plant
growth substances. As none of these authors appears to have
carried out an analysis to demonstrate the presence of 2,4-D
residues in the test material, it is questionable whether the test
animals ingested any 2,4-D or 2,4-D residues, and these studies are
therefore not reviewed in detail. Buslovich et al. (1976) tested
only a single dose level (1/2 LD50) and this reduces the usefulness
of their study. Pertinent reports are discussed below.
7.4.1. Rats
7.4.1.1. Effects on adult rats
No deleterious effects on the health or fertility of rats
receiving the maximum tolerated dose of 87.5 mg/kg body weight in
terms of 2,4-D or its molar equivalent of the isooctyl ester or the
propylene glycol butyl ether ester per day, on days 6 - 15 of
pregnancy, were reported by Schwetz et al. (1971) and Unger et al.
(1980). Even higher amounts of 2,4-D or its equivalent in 2,4-D
derivatives were used by Björklund & Erne (1966), Hansen et al.,
(1971) and Khera & McKinley (1972) with similar results.
Reduced testis and prostate size, abnormal spermatogenesis (and
also liver and kidney damage) were reported by Schillinger (1960)
in some of the male rats given 375 mg/kg body weight per day (about
1/4 LD50) of a Soviet-made 2,4-D butyl ether formulation containing
polyethylene glycol alkyl phenyl ether surfactant. These effects
were not noted at 1/10 of this dose level, i.e., at 37.5 mg/kg body
weight per day. Some of the toxic effects reported by Schillinger
(1960) may have been caused by the surfactant, but as a surfactant
control group was not included in this study, this cannot be
confirmed. Thus, the available studies suggest that the no-
observed-adverse-effect level for reproducing adult rats lies
between 37.5 and 87.5 mg 2,4-D/kg body weight per day.
7.4.1.2. Effects on offspring
Björklund & Erne (1966) gave pregnant rats a 2,4-D
concentration in drinking-water of 1000 mg/litre during pregnancy,
and for the following 10 months. No effects on reproduction were
noted.
Hansen et al. (1971) fed male and female rats dietary levels of
technical 2,4-D of 100, 500, and 1500 mg/kg (ppm), and the rats
were bred through 3 successive generations. At dietary levels of
100 and 500 mg/kg, no effects were noted. However, at a dietary
level of 1500 mg/kg, survival of pups to weaning was reduced. The
number of pups surviving ranged from 70 - 97% in the control group
and from 60 - 93% at the 100 and 500 mg/kg (ppm) dietary levels;
survival at the highest dose ranged from 20 - 62%.
Resorptions, reduced fetal weight and size, enlarged ventricles
of the brain and haemoperitoneum were found by Buslovich et al.
(1976) in the offspring of rats treated with two different 2,4-D
derivatives at a dose of one-half LD50 (value unspecified).
Schwetz et al. (1971) dosed female rats from day 6 - 15 of
pregnancy by gavage at dose levels of 0, 12.5, 25, 50, and 87.5
mg/kg body weight per day with 2,4-D or equimolar dose levels of
the propylene glycol butyl ether (PGBE) and the isooctyl esters
(IO). At doses of 50 and 87.5 mg/kg, a decrease in fetal body
weight was noted for all three compounds.
Subcutaneous oedema, delayed ossification of sternebrae,
sternebrae with split centres of ossification, wavy ribs, and
lumbar ribs increased with increasing doses, for at least one of
the agents studied. However, these anomalies were not
significantly increased at the 12.5 or 25 mg/kg dose level for the
3 compounds. A small but significant increase in the incidence of
subcutaneous oedema was observed in fetuses from dams receiving
12.5 mg/kg molar equivalent of IO. The incidence of missing
sternebrae was significantly increased at dose levels of 87.5 mg/kg
for 2,4-D and 12.5 and 87.5 mg/kg for PGBE.
Unger et al. (1980), in a later study, using the same dosing
regimens for 2,4-D IO and 2,4-D PGBE did not observe the effects
reported by Schwetz et al., except for a statistically significant
increase in rib buds at the highest dose (87.5 mg/kg) tested for
both compounds ( P < 0.05).
Khera & McKinley (1972) dosed female rats from day 6 - 15 of
pregnancy by gavage with 2,4-D, 2,4-D IO, 2,4-D butyl ester, 2,4-D
butoxyethanol ester and 2,4-D dimethylamine salt. The butyl and
isooctyl ester depressed fetal weight and decreased fetal viability
at the highest dose of 150 mg/kg body weight. Wavy ribs,
additional ribs, retarded ossification and sternal defects, fused
ribs, small-sized distorted scapula and micromelia were observed as
anomalies among the treated groups. A statistically significant
increase in malformed fetuses was noted at 2,4-D levels of 25 mg/kg
body weight ( P < 0.05) and at levels of 150 mg/kg or more for the
other compounds.
Konstantinova et al. (1975) reported hemorrhage into internal
organs in the fetuses of rats treated with a 2,4-D level of 50
mg/kg body weight.
The results of all these studies suggest that dosage levels of
less than 12.5 mg/kg body weight for the various 2,4-D derivatives
do not cause fetotoxic or teratological effects in rats, and the
results of the more recent study by Unger et al. (1980) indicate
that higher doses may be without deleterious effects on the
fetuses.
Thus, at present, a daily dose level of 10 mg 2,4-D or 2,4-D
acid equivalent/kg body weight can be considered to be without
significant fetotoxic or teratogenic effects in rats.
7.4.2. Mice
The report by Courtney (1977) indicated that in CD-1 mice,
doses of 1 mM/kg body weight of 2,4-D and its n-butyl and PGBE
esters reduced fetal body weight, and increased fetal mortality.
The compounds were also teratogenic, inducing cleft palates, at
levels of 124 mg/kg body weight per day (2,4-D or acid equivalent)
or more. However, the 2,4-D isopropyl and isooctyl esters appeared
to be less teratogenic than 2,4-D, as they did not induce birth
defects at a 2,4-D equivalent level of 124 mg/kg body weight per
day. Furthermore, the 2 esters did not induce fetal death in CD-1
mice at doses of 2,4-D equivalent up to 221 mg/kg body weight per
day.
7.4.3. Birds
In the 1960s and 1970s, a number of tests of the embryotoxic,
teratogenic, and other reproductive effects of 2,4-D were carried
out on birds' eggs and embryos. These results may not apply to
mammals, because bird embryos develop in a closed environment
different from that of mammalian embryos, and because birds differ
anatomically from mammals.
Lutz-Ostertag & Lutz (1970, 1974) reported mortality and severe
deformities in wild bird embryos exposed to 2,4-D amine salt, but
they did not provide crucial experimental details, and other
investigators (Dunachie & Fletcher, 1967, 1970; Kopischke, 1972;
Grolleau et al., 1974; Gyrd-Hansen & Dalgaard-Mikkelsen, 1974;
Somers et al., 1974a,b,c; Hilbig et al., 1976a,b; Spittler, 1976)
were unable to duplicate their results either with 2,4-D amine
salts or esters. Some of the teratogenic effects attributed to
2,4-D by Lutz-Ostertag & Lutz (1970, 1974) resemble those induced
by excessively high incubation temperatures (Nielsen, 1968), and
may thus have been experimental artifacts.
On the whole, the available studies on bird embryos indicate
that the no-observed-adverse-effect level for 2,4-D-induced
embryotoxic and teratogenic effects lies near 0.5 mg active
ingredient/egg (equivalent to about 10 mg/kg) and is thus similar
to that in mammals.
7.4.4. Cold-blooded animals
The available literature contained little information
concerning the possible reproductive, embryotoxic, or teratogenic
effects of 2,4-D or 2,4-D derivatives on cold-blooded animals.
7.4.4.1. Amphibians
Lopez (1961) noted that the motility of frog spermatozoa was
not affected by low concentrations of 2,4-D or its sodium salt, and
that the inhibition of movement, or the lysis observed under some
conditions were caused by changes in the pH of the test solution.
Aqueous solutions of less than 0.05% 2,4-D sodium salt did not
induce any macroscopic abnormalities in developing frog eggs or
embryos (Lhoste & Roth, 1946), while Cooke (1972) did not find
either toxic effects or 2,4-D residues in frog tadpoles exposed for
1 or 2 days to up to 50 mg 2,4-D/litre. According to Sanders
(1970a) a commercial 2,4-D dimethylamine salt had an LC50 of 100
mg/litre for frog tadpoles.
7.4.4.2. Fish
Only three brief reports were available on the effects of 2,4-D
on developing fish eggs and embryos. Andrusaitis (1972) found that
2,4-D reduced the oxygen consumption of 32-cell blastomeres, and
increased the oxygen consumption in 64 - 128 cell blastomeres of
Misgurnus fossilis. In a study by Mount & Stephan (1967), 2,4-D
butoxyethanol ester (BEE) at a concentration of up to 0.31
mg/litre, or 2,4-D at 0.80 mg/litre did not reduce the reproduction
rate in fathead minnows (Pimephales promelas), whereas 2,4-D BEE
at 1.5 mg/litre killed minnow eggs in 48 h. Rehwoldt et al. (1977)
similarly found that 0.1 mg 2,4-D/litre did not have any adverse
effect on reproduction in guppies.
These reports suggest that the no-observed-adverse-effect level
of 2,4-D BEE for the reproductive or teratogenic effects of 2,4-D
in fish may be about 1 mg a.i./litre water.
7.5. Mutagenicity and Related Effects
7.5.1. 2,4-D and its derivatives
Studies on the mutagenicity of 2,4-D and its derivatives have
been reviewed by Andersen et al. (1972), National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), Ramel (1978), Seiler (1978),
Vachkova-Petrova (1978), Kas'yanenko & Koroleva (1979), Murthy
(1979), Shearer (1980), US Veterans' Administration (1981), Waters
et al. (1981), and Linainmaa (1983).
A recent IARC Working Group (IARC, 1982) evaluated the activity
of 2,4-D and derivatives in short-term tests. It was reported that
2,4-D induced unscheduled DNA synthesis in cultured human
fibroblasts (Ahmed et al., 1977a), but not in rat hepatocytes
(Probst et al., 1981). 2,4-D was not mutagenic in bacterial
systems (Andersen et al., 1972; Sherasen et al., Zetterberg, 1977;
Moriya et al., 1983). 2,4-D was mutagenic in yeast, when tested at
low pH (Zetterberg et al., 1977), but was not active under other
conditions (Zetterberg, 1977) or in a host-mediated assay
(Zetterberg et al., 1977). Results of four studies on Drosophila
melanogaster were reported to be positive (Rasmuson & Persson-
Svahalin, 1978): results of three other studies were negative
Berin & Buslovich, 1971; (Vogel & Chandler, 1974; Magnusson et al.,
1977; Rasmuson & Svahlin, 1978).
2,4-D was reportedly mutagenic in cultured Chinese hamster
ovary (CHO) cells (Ahmed et al., 1977b), but did not induce a
statistically significant increase in sister chromatid exchanges
(SCEs) in CHO cells in vitro (Linainmaa, 1983). Chromosomal
effects have been reported in plants (Khalatkar & Bhargava, 1982).
Chromosomal aberrations or SCEs were found in cultured human
lymphocytes (Pilinskaya, 1974; Korte & Jatal, 1982), but
chromosomal aberrations were not found in cultured embryonic bovine
kidney cells (Bongso & Basrur, 1973).
In mice, single oral doses of 100 - 300 mg 2,4-D/kg body weight
reportedly induced chromosomal aberrations (Pilinskaja, 1974), but
micronuclei were not found in mice after ip injection of single
doses of 100 mg 2,4-D/kg body weight (Jenssen & Renberg, 1976).
2,4-D was not active in a dominant lethal test in mice (Epstein et
al., 1972), and did not induce SCEs in rats after oral
administration of 2,4-D amine salt at daily doses of 100 mg/kg body
weight for two weeks (Linnainmaa et al., 1983). Recent evidence
(Buslovich et al., 1982; Vainio et al., 1982) suggests that 2,4-D
may have an indirect effect on genetic material via the production
of active oxygen radicals derived from peroxisome proliferation,
which has been demonstrated in in vivo and in vitro studies in
liver cells of rats and hamsters (Reddy et al., 1980, 1982; Vainio
et al., 1982; Gray et al., 1983).
At present, available studies are inadequate to evaluate the
genetic effects of 2,4-D and its derivatives in short-term tests
(IARC, 1982). No data on cell transformation are available.
7.6. Carcinogenic Effects on Experimental Animals
7.6.1. 2,4-D and its derivatives
A number of studies have been carried out on mice and rats to
assess the potential carcinogenic effects of 2,4-D and its
derivatives. Two IARC Working Groups have reviewed these studies
(IARC, 1977, 1982) and concluded that it was not possible to make
an evaluation on the basis of the available data. 2,4-D and
several of its esters were tested by oral administration and by a
single sc injection in the newborn of 2 strains of mice (Innes et
al., 1969); 2,4-D or its amine salts were also tested in rats by
oral administration (Arkhipov & Kozlova, 1974; Hansen et al.,
1971).
Nearly 20 years have passed since these studies were carried
out and because of basic flaws in their design and execution, it is
unlikely that further reviews of these studies will lead to
generally accepted conclusions. The Task Group was aware that
further long-term studies in mice and rats were in progress, and
these should prove useful in the future evaluation of the potential
carcinogenicity of 2,4-D.
7.6.2. Contaminants in 2,4-D
2,7-DCDD was tested for carcinogenicity in mice and rats fed
dietary levels of 5000 and 10 000 mg/kg for 90 weeks, followed by a
short observation period of about 1 - 10 weeks, at which time all
animals were killed. Sufficient numbers of animals survived to
evaluate the development of late-appearing tumours. Although an
increased incidence of hepatocellular adenomas and carcinomas was
observed in treated male mice (20/50 in the low dose and 17/42 in
the high dose compared with 8/49 in matched controls), it was noted
that the incidence of liver tumours in historical controls ranged
from 16 - 32%. No tumorigenic effects were found in rats (US
National Cancer Institute, 1979).
8. EFFECTS ON MAN, CLINICAL AND EPIDEMIOLOGICAL STUDIES
The available clinical and epidemiological studies fall into 4
groups: (a) studies on patients treated with 2,4-D as an
anticancer drug (Apffel, 1959a) or antibiotic (Seabury, 1963), (b)
reports on acute 2,4-D poisoning due to voluntary or accidental
ingestion of herbicides (Tables 19 and 20), (c) reports on workers
(mainly men) overexposed to 2,4-D during the manufacture,
processing, or use of 2,4-D herbicides, and (d) epidemiological
studies on groups of people who were actually or potentially
exposed as a result of herbicide spray programmes, or who lived in
areas in which herbicides were used. With the exception of the
case studies of Apffel (1959a) and Seabury (1963), almost all of
the reports deal with mixed exposures to 2,4-D and other chemicals,
and therefore it is often unclear to what extent 2,4-D, its alkali
or amine salts, or its esters contributed to the effects reported
by the authors of the studies.
Much of the literature on acute poisonings and on the health
effects of occupational overexposure to 2,4-D or other
chlorophenoxy compounds or their toxic by-products has been
recently reviewed by Pocchiari et al. (1979), Bovey & Young (1980),
Huff et al. (1980), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1981),
CEC (1981), Rappe & Buser (198l), US Veterans' Administraton
(1981), Coggon & Acheson (1982), Hay (1982), IARC (1982, 1983), and
Dobrovolski (unpublished data, 1983). However, most of these
reviews concentrated on 2,4,5-T, Agent Orange, and other herbicides
used in the Vietnam war, or on industrial accidents resulting in
massive exposures to largely undefined mixtures of chlorophenols,
chlorinated dibenzodioxins, and other reaction products. In
contrast, the present review focuses mainly on 2,4-D herbicides.
In evaluating human exposure to mixtures of chemicals that
include 2,4-D and various concentrations of contaminants of 2,4-D,
it is in many instances difficult or impossible to determine
whether any of the described effects can actually be attributed to
the exposure to 2,4-D or its derivatives.
8.1. Acute Poisoning and Occupational Overexposure
Pertinent reports on acute poisoning with 2,4-D, or of the
effects of occupational overexposure to 2,4-D herbicides are
summarized or cited in Tables 19 and 20.
Signs and symptoms of acute overexposure to 2,4-D or its
derivatives occurred after ingestion or absorption of large
amounts, or where poor occupational hygiene was practised leading
to pronounced dermal absorption of the material. It is unlikely
that, with good agricultural practice, good personal protection,
and occupational hygiene, resulting in exposures to low
concentrations of 2,4-D, any of the acute symptoms and signs
reported below would be expected to occur.
Table 19. Acute toxicity of 2,4-D, fatal poisonings with herbicides containing 2,4-D
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
a) Fatal poisonings
2,4-D diethyl not stated F 50.8 kg 60-90 g 40-400 coma; death in about Curry (1962)
ester (1180-1770 mg 2 days; degeneration
(DICOTEX EXTRA) DOCOTOX/kg bw) of convoluted kidney
tubules
"2,4-D" suicide M ? ~ 125 ml ? loss of consciousness, Delarrard &
400 g (< 400 bw) coma, generalized Barbaste (1969)
a.i./litre muscular hypotonia,
loss of all reflexes,
hypotension,
hyperglycaemia,
proteinuria; death in
12 h
"pure" 2,4-D suicide M 55 kg ? 57.6-407.9 coma, myotonia, fever, Dudley &
acid in pulmonary emphysema Thapar (1972)
kerosene and edema, liver
necrosis, degeneration
of kidney tubules;
death in 6 days
"2,4-D" suicide F ? ? 20-116 loss of consciousness, Geldmacher-Van
vomiting, uterine Mallinckrodt &
bleeding, tachycardia, Lautenbach
and circulatory (1966)
failure; death in
about 30 h; edema and
congestion of brain;
fatty liver cell
changes; fatty changes
in kidney tubules;
pulmonary hyperaemia &
edema with isolated
haemorrhages
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
"2,4-D" suicide M ? "at least" stiffness in legs, Herbich &
13.5 g vomiting, loss of Machata
consciousness; death (1963)
in 14 h; hyperaemia
and edema of brain;
pulmonary edema
2,4-D suicide M 75 kg 120 ml 12.5-7700 vomiting; congestion, Nielsen et al.
dimethylamine (80 mg/kg) pulmonary emphysema; (1965)
formulation CNS congestion &
perivascular
haemorrhages, severe
degeneration of
ganglion cells; death
within hours of
ingestion
Herbicide suicide M 26 360 ml 2,4-D plasma clinical and Osterloh et al.
containing years and mecoprop 321 (1.5 h) pharmacokinetic (1983)
2,4-D, mecoprop amine salt 540.9 (21 h) study coma with
(MCPP) and (10.6%, 11.6% 480.8 (30 h) pin-point pupils
chlorpyrifos a.i.) and 360 tachycardia,
ml chlorpyrifos urine (on hypertension,
in kerosene admission): myoclonus, diarrhoea,
(6.7% a.i.) 230.3 then hypotension,
plus few cardiac arrhythmias,
granules gastric asystole, and death
Warfarin (0.025 content (on after 30 h
% a.i.) (2,4-D admission):
= 600 mg/kg bw; 108.2
mecoprop = 600
mg/kg bw) tissues
(post mortem)
brain: 186.4
blood: 389.5
liver: 293.5
heart: 301.2
kidney: 315.0
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
b) Non-fatal poisonings
"2,4-D" accidental M ? (5- ? ? drowsiness, Duric et al.
"DEHERBAN A" ingestion year unsteadiness, (1979)
herbicide old difficulties with
formulation, child) speech; dilated pupils;
400 g on fourth day, toxic
a.i./litre myocarditis with
abnormal ECG; kidney
damage indicated by
increased blood urea
levels; no liver
damage; complete
recovery within about
one month
herbicide suicide F 58 ? ? no signs of toxicity Prescott et al.
containing attempt; years on admission to (1979)
2,4-D plus ingestion of hospital with plasma
dichlorprop unspecified concentration of
(16.1% + 21.4%) amount of 335 mg 2,4-D/litre &
herbicide 400 mg mecoprop/litre;
plasma clearance
t = 143 h for
2,4-D vs 95 h for
dichlorprop; 91% of
ingested 2,4-D and
70% of ingested
dichlorprop excreted
unchanged; metabolites
not identified
----------------------------------------------------------------------------------------------------------------------- not identified
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
herbicide suicide F 30.2 kg 100 ml ? pharmacokinetic study Rivers et al.
containing attempt herbicide of 2,4-D/dicamba (1970)
salts of 2,4-D (13.6 g 2,4-D) excretion; no Young & Haley
(20.1%) and information on toxic (1977)
dicamba (1.9%) effects; excretion of
2,4-D initially slowed
by competitive
excretion of dicamba;
small amounts of 2,4-D
still excreted 3 weeks
after ingestion; only
52% of ingested 2,4-D
excreted in urine;
rest assumed to have
been excreted by
faecal route; plasma
clearance t = 59.2 h
initially, and 16.7 h
after most of the
dicamba was excreted
-----------------------------------------------------------------------------------------------------------------------
Table 20. Acute or non-acute effects attributed to occupational or bystander overexposure to 2,4-D
herbicide
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Central i) unconsciousness Radionov et al. (1967); Paggiaro et al. (1974)
nervous ii) electroencephalograph changes Kontek et al. (1973); Andreasik et al. (1979)
system iii) subjective symptoms Assouly (1951); Goldstein et al. (1959); Monarco &
Divito (1961); Foissac-Gegoux et al. (1962);
Berkley & Magee (1963); Belomyttseva (1965);
Fetisov (1966); Radionov et al. (1967); Bashirov
(1969); SARE (1972); Andreasik et al. (1979);
Kuzyk (1979)
Peripheral i) polyneuritis Foissac-Gegoux et al. (1962); Todd (1962);
nervous Belomyttseva & Karimova (1963); Bashirov (1969)
ii) partial paralysis Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Todd (1962)
iii) functional changes Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Berkley & Magee (1963); Belomyttseva
(1967); Wallis et al. (1970); Andreasik et al.
(1979); Singer et al. (1982)
iv) subjective symptoms
Skeletal i) myotonia, myokymia, fibrillation Foissac-Gegoux et al. (1962) Berkley & Magee
muscles stiffness (1963); Wallis et al. (1970); Paggiaro et al.
(1974)
ii) muscle damage or atrophy Wallis et al. (1970)
iii) subjective symptoms Assouly (1951); Foissac-Gegoux et al. (1962); Todd
(1962); Fetisov (1966); Radionov et al. (1967);
Bashirov (1969); Wallis et al. (1970)
Digestive i) vomiting, diarrhoea Goldstein et al. (1959); Monarca & Divito (1961);
system Todd (1962); Belomyttseva (1967); Radionov et al.
(1967); Paggiaro et al. (1974); Dennis (1976)
ii) various functional disorders or Assouly (1951); Monarca & Divito (1961); Bashirov
subjective symptoms (1969); Wallis et al. (1970); Kuzyk (1979)
---------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Respiratory i) irritation coughing Assouly (1951); Belomyttseva & Karimova (1963);
system Belomyttseva (1965); Wallis et al. (1970);
Andreasik et al. (1979)
ii) functional disorders Belomyttseva & Karimova (1963); Bashirov (1969);
Wallis et al. (1970)
Circulatory i) functional changes: Belomyttseva & Karimova (1963); Belomyttseva
system - cardiac involvement (1967); Winkelmann (1960); Bashirov (1969);
- vascular involvement Paggiaro et al. (1974); Andreasik et al. (1979)
ii) haematological or chemical Monarca & Divito (1961); Todd (1962); Belomyttseva
changes (1967); Radionov et al. (1967); Long et al.
(1969); Paggiaro et al. (1974); Andreasik et al.
(1979)
iii) subjective symptoms Radionov et al. (1967); Bashirov (1969); Andreasik
et al. (1979)
Liver functional abnormalities Belomyttseva & Karimova (1963); Belomyttseva
(1967); Bashirov (1969); Andreasik et al. (1979)
Kidney functional abnormalities Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Belomyttseva (1967); Bashirov (1969);
Paggiaro et al. (1974); Andreasik et al. (1979)
Skin i) irritation or allergic reactions Belomyttseva (1967); Radionov et al. (1967);
Dennis (1976); Kuzyk (1979)
ii) desquamation Foissac-Gegoux et al. (1962)
iii) chloracne Londońo (1966)
Reproductive functional abnormalities (women) Elina et al. (1975)
system decreased libido (men) Andreasik et al. (1979)
impotence Espir et al. (1970)
---------------------------------------------------------------------------------------------------------
The observations of Apffel (1959a) and Seabury (1963) on
patients treated with 2,4-D suggest that the acute no-observed-
adverse-effect level for biological effects in human beings may be
as high as 36 mg of purified 2,4-D/kg body weight, or its
equivalent in alkali or amine salts or esters. The lack of
subjective or clinical effects in studies in which volunteers
ingested low doses (5 mg/kg body weight) of purified 2,4-D also
supports the idea that a dose of a few mg/kg body weight is
unlikely to be toxic (Khanna & Kohli, 1977; Sauerhoff et al.,
1977). However, it is less certain whether this is true also of
the less pure commercial 2,4-D products. From the point of view of
occupational and bystander safety, it is reassuring that no reports
were found of fatal poisonings following dermal exposure or
inhalation, though temporary unconsciousness and other severe acute
effects have been attributed to massive combined dermal and
inhalation exposures to 2,4-D herbicides.
Most poisonings with 2,4-D herbicides involved formulations
containing more than one toxic ingredient, including solvents,
surfactants, and other additives. The symptoms and clinical signs
therefore tended to vary with different products.
8.1.1. Neurotoxic effects of 2,4-D and related compounds
Some of the case reports cited below on poisonings with, and
occupational overexposures to, herbicides indicate that 2,4-D and
other chlorophenoxy compounds may affect both the central and
peripheral nervous systems.
8.1.1.1. Effects on the central nervous system
In addition to subjective symptoms of the central nervous
system, impaired coordination, impaired responses to external
stimuli, unconsciousness, coma, and death have been observed in
human beings mainly after absorption of lethal or nearly lethal
doses of 2,4-D. The acute effects on the central nervous system
seem to resemble those produced by alcohol, sedative drugs, or
aromatic chlorinated hydrocarbons rather than those produced by
organophosphate or carbamate neurological poisons (Geldmacher-Von
Mallinckrodt & Lautenbach, 1966; Prescott et al., 1979). Acute
cerebral demyelination occurred in a suicide who ingested a
solution of 2,4-D in kerosene (Dudley & Thapar, 1972). Severe
degeneration of brain ganglion cells was observed in another
suicide who drank a herbicide product containing 2,4-D in the form
of water-soluble dimethylamine salt (Nielsen et al., 1965). It is
therefore possible that lethal doses of chlorophenoxy compounds may
cause structural as well as functional damage to the brain.
Craniocerebral and peripheral functional nerve damage was noted
by Bezuglyi et al. (1979) in a group of women occupationally
overexposed to 2,4-D herbicides and other pesticides. Electro-
encephalographic (EEG) abnormalities were observed in tractor
drivers spraying herbicides containing 2,4-D, MCPA, or mecoprop
(Kontek et al., 1973), in "individuals exposed to" chlorophenoxy
herbicides, including 2,4-D and MCPA (Bielski & Madra, 1976) and in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979). On
the other hand, a case of neuropathy with EEG abnormalities and
flaccid quadriplegia, originally attributed to occupational
overexposure to MCPA, was diagnosed as being of viral origin
(Nayrac et al., 1958), and therefore some of the neurological
damage attributed to 2,4-D, may have been caused by viruses. There
is some evidence that 2,4-D herbicides may affect the sensory
system, as Andreasik et al. (1979) and Assouly (1951) reported
intolerance to certain odours, hypersensitivity to noise, and other
sensory abnormalities in workers producing or packaging 2,4-D
herbicides, while Fetisov (1966) reported hyposmia and other
sensory deficiencies in similarly-exposed workers.
8.1.1.2. Effects on the peripheral nervous system
"Peripheral neuropathy" and reduced peripheral nerve conduction
velocities have been reported in workers producing 2,4-D and
2,4,5-T (Poland et al., 1971; Singer et al., 1982). More than one
dozen other studies of persons overexposed to chlorophenoxy
herbicides also indicated detrimental effects of 2,4-D products on
the peripheral nervous system (Goldstein et al., 1959; Goldstein &
Brown, 1960; Foissac-Gegoux et al., 1962; Todd, 1962; Berkley &
Magee, 1963; Wallis et al., 1970; Bezuglyi et al., 1979). Long-
lasting flaccid paraparesis or quadriparesis following skin contact
with 2,4-D herbicides was reported by Goldstein et al. (1959) and
Goldstein & Brown (1960). Abnormal tendon reflexes in these or
similar cases were reported by the same authors and by Andreasik et
al. (1979), Berkley & Magee (1963), and Foissac-Gegoux et al.
(1962). Cases of sensory neuropathy attributed to the ingestion
of, or dermal exposure to, 2,4-D herbicides have also been reported
by Monarca & Divito (1961), Foissac-Gegoux et al. (1962), Todd
(1962), Wallis et al. (1970), Sare (1972), and Bezuglyi et al.
(1979). However, no signs of peripheral neuropathy were reported
by Apffel (1959a), Seabury (1963), Paggiaro et al. (1974), and
Prescott et al. (1979), in similar cases of massive exposure to
2,4-D herbicide, and in patients given relatively large amounts of
purified 2,4-D or 2,4,5-T salts or esters as drugs. One
explanation for this may be great individual differences in
susceptibility to poisoning with chlorophenoxy herbicides
(Rosenberg, 1980), as attested by the case of a 58-year-old woman
who "was fully conscious with no clinical evidence of toxicity"
after ingesting enough herbicide to allegedly attain 2,4-D and
mecoprop blood plasma concentrations of 335 and 400 mg/litre
respectively (Prescott et al., 1979). By comparison, Herbich &
Machata (1963) reported that a plasma concentration of 447 mg
2,4-D/litre caused death in a 46-year-old man.
Herbicide ingredients other than 2,4-D and related compounds
might be at least partly responsible for the observed neurotoxic
effects. In particular, organic solvents, emulsifiers, and
ethylene glycol present in herbicide formulations have been
mentioned in this connection (Goldstein & Brown, 1960; Goldwater,
1960). Solvents such as alcohols and trichloroethylene, used in
the manufacture of the active herbicide ingredients, might account
for some of the abnormalities observed in workers involved in the
production of chlorophenoxy compounds (Assouly, 1951; Bashirov,
1969; Bashirov & Ter-Bagdasarova, 1970).
Some of the reported cases of central nervous system
dysfunction or peripheral neuritis may have been merely
coincidental to the herbicide exposure, as there are many known
causes of neuropathy, such as nutritional and hereditary factors,
infectious diseases, and many toxic chemicals, including alcohol
(Freemon, 1975). Thus, alcoholism may have been a contributory
factor in one case of "2,4-D polyneuropathy" reported by Brandt
(1971).
Further studies of the possible effects of 2,4-D and other
chlorophenoxy compounds or their by-products on the human nervous
system are desirable, including studies of behavioural effects
measurable by recently-developed test batteries (Baker et al.,
1983).
8.1.2. Myotoxic effects of 2,4-D
Muscle fibrillations, myotonia, myoglobinuria, muscular
weakness and other indications of a myotoxic effect of 2,4-D have
been reported in patients treated with large doses of purified
2,4-D products by Apffel (1959a) and Seabury (1963), as well as in
cases of suicidal or accidental ingestion of 2,4-D herbicides or
following occupational overexposure (Herbich & Machata, 1963;
Berwick, 1970; Dudley & Thapar, 1972; Prescott et al., 1979). In
laboratory animals, myotonia and structural or biochemical muscle
lesions can be reliably induced by doses in excess of about 100 mg
2,4-D/kg body weight. In human beings, the threshold dose for
gross myotoxic effects certainly exceeds 5 mg/kg body weight per
day, and may be above 36 mg/kg body weight per day (Seabury, 1963;
Khanna & Kohli, 1977; Sauerhoff et al., 1977).
8.1.3. Cardiopathies and cardiovascular effects
Myocardial dystrophy, myocarditis, cardiac arrhythmias or
fibrillations, a slowed heart rate, or electrocardiographic (ECG)
changes were observed in human beings who ingested herbicides
containing 2,4-D (Duric et al., 1979) or 2,4-D and mecoprop
(Prescott et al., 1979), and also after occupational overexposure
to chlorophenoxy herbicides (Belomyttseva, 1965; Khibin et al.,
1968; Zakharov et al., 1968; Paggiaro et al., 1974; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979; Prescott et al., 1979).
However, in other cases of acute poisoning with 2,4-D herbicides,
the ECG was essentially normal (Berwick, 1970; Monarca & Divito,
1961).
Thus, further studies are desirable to determine the threshold
2,4-D doses at which electrophysiological cardiac abnormalities can
be observed, and to determine whether they result from a direct
effect on the nerve conducting system of the heart, or secondarily
from a toxic action on the myocardium.
It has been suggested that allergic reactions and an increased
sensitivity may be involved in 2,4-D-related cardiac arrhythmias
(Winkelmann, 1960; Rea, 1978).
Both hypertension and hypotension have been reported following
exposure to high doses of 2,4-D (Apffel, 1959a; Kaskevich &
Sobolieva, 1978; Bezugly et al., 1979), but no conclusions could be
drawn from these studies.
8.1.4. Haematological effects
Monarca & Divito (196l), Todd (1962), Radionov et al. (1967),
Bashirov (1969), Bashirov & Ter-Bagdarasova (1970), Brandt (1971),
Andreasik et al. (1979), and Bezuglyi et al. (1979) observed
haematological changes such as mild anaemia, bone marrow
depression, mono- or lymphocytosis or eosinophilia, changes in
erythrocyte volume or size, or methaemoglobinaemia following
ingestion of, or overexposure to 2,4-D. However, these changes may
have been due to other causes, as Apffel (1959a) did not observe
either haematological changes or effects on haematopoiesis in
patients given 0.1 - 0.3 g of purified 2,4-D per day as an
anticancer drug.
8.1.5. Blood chemistry effects
Hyperglycaemia, hypercholesterolaemia, elevated levels of blood
urea, transaminase (SGOT, SGPT), and creatine phosphokinase (CPK),
or altered blood albumin, globulin, or phospholipid levels
following acute poisoning with, or occupational overexposure to
2,4-D were reported by Bashirov (1969), Bashirov & Ter-Bagdarasova
(1970), Berwick (1970), Lukoshkina et al. (1970), Brandt (1971),
Bezuglyi et al. (1979), Duric et al. (1979) and Prescott et al.
(1979). However, Bashirov (1969) reported hypoglycaemia (< 700
mg/litre) and abnormally slow return to normal values in glucose
tolerance tests in about one-third of a group of workers producing
2,4-D. Increased activity of erythrocytic glycolytic enzymes was
found in Polish workers packaging 2,4-D sodium salt (Andreasik et
al., 1979). In one case of intentional 2,4-D poisoning, there was
hyperglycaemia (De Larrard & Barbaste, 1969), while in some cases
of 2,4-D overexposure, blood glucose abnormalities were not
observed (Goldstein et al., 1959). Apffel (1959a) never observed
hyperglycaemia in his patients, on the contrary, daily doses of
1 - 1.25 g 2,4-D led to hypoglycaemia. Thus, under some
circumstances, high doses of 2,4-D apparently can affect glucose
metabolism, and produce hypo- or hyperglycaemia. Gamble (1975)
proposed that 2,4-D might inhibit certain APT-dependent enzymes and
thus affect lipid metabolism.
8.1.6. Pulmonary effects
Pulmonary emphysema, oedema, hyperaemia and haemorrhages were
found in cases of fatal poisonings due to 2,4-D herbicide ingestion
(Herbich & Machata, 1963; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972). It is not clear whether the acute
pulmonary effects were caused by the 2,4-D preparations or by the
solvents such as kerosene or fuel oil. However, it is unlikely
that the pulmonary emphysema was caused by acute exposure to 2,4-D.
Dyspnoea or respiratory tract irritation were occasionally
reported following occupational overexposure of 2,4-D production
workers or herbicide sprayers (Assouly, 1951; Belomyttseva, 1964;
Bashirov, 1969; Bezuglyi et al., 1979).
8.1.7. Hepatotoxic effects
Liver necrosis or fatty liver cell changes were observed in 2
fatal cases following 2,4-D herbicide ingestion (Geldmacher-Von
Mallindkrodt & Lautenbach, 1966; Dudley & Thapar, 1972). In
several non-fatal 2,4-D poisonings, no biochemical evidence of
liver damage was noted, and neither Apffel (1959a) nor Seabury
(1963) reported indications of liver damage in patients treated
with up to 2.5 g/day of purified 2,4-D, salts, or esters.
Hyperbilirubinaemia, elevated urobilinogen levels, or liver
enlargement were reported in workers occupationally exposed both to
2,4-D herbicides and to other chemicals (Belomyttesva, 1964, 1965;
Bashirov, 1969; Bashirov & Ter-Bagdasarova, 1970; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979).
8.1.8. Nephrotoxic effects
Degeneration of, or fatty changes in kidney tubules, or
proteinuria, increased blood urea levels, and other indications of
a nephrotoxic effects were observed in cases of fatal or nearly
fatal herbicide ingestion (Goldstein et al., 1959; Curry, 1962;
Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Brandt, 1971;
Dudley & Thapar, 1972; Duric et al., 1979). Impaired renal
function was reported in occupationally-exposed persons by Bashirov
(1969), Bashirov & Ter-Bagdasarova (1970), Paggiaro et al. (1974),
and by Andreasik et al. (1979). On the other hand, neither Apffel
(1959a) nor Seabury (1963) reported any evidence of kidney damage
in their patients, some of whom received in excess of 2 g of pure
2,4-D per day.
8.1.9. Effects on the digestive tract
Vomiting, diarrhoea, nausea, and other indications of toxic
effects on the digestive tract were observed by Apffel (1959a) in
patients injected intramuscularly with large doses (up to 2.5 g) of
purified 2,4-D products.
The same effects have also been noted after ingestion of large
doses of 2,4-D herbicides, or after combined inhalation and dermal
overexposure (Goldstein et al., 1959; Monarca & Divito, 1961;
Nielsen et al., 1965; Tsapko, 1966; Radionov et al., 1967; Paggiaro
et al., 1974; Dennis, 1976; Kuzyk, 1979; Prescott et al., 1979).
However, no gastrointestinal symptoms were reported by volunteers
who ingested a single dose of 5 mg pure 2,4-D/kg body weight
(Khanna & Kohli, 1977; Sauerhoff et al., 1977). Thus, an intake of
more than 300 mg 2,4-D per adult appears to be required to induce
acute toxic effects on the gastrointestinal tract.
8.1.10. Effects on endocrine organs
Andreasik et al. (1979) found an impaired iodine uptake by the
thyroid, and decreased thyroxine, thyroxine clearance, and
thyroxine iodine values in workers packaging 2,4-D sodium salt.
Since these workers were exposed to a variety of chemicals, these
results need confirmation.
8.1.11. Irritative and allergenic effects
Chronic tonsillitis and paranasal sinusitis were reported in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979).
Acute eye or skin irritation, as well as skin reactions of an
allergic type, including anaphylactoid purpura (allergic angiitis)
and contact eczema have been reported in agricultural and forestry
workers following occupational exposure to 2,4-D herbicides
(Winkelmann, 1960; Radionov et al., 1967; Balo-Banga et al., 1973;
Jung & Wolf, 1977; Kuzyk, 1979). Jung & Wolf (1977) found that
exposure to the vapour of a 2,4-D/2,4,5-T formulation in diesel oil
(SELEST 100) caused an acute allergic reaction in the skin of
sensitized herbicide applicators, and that the allergic reactions
were caused by the mixture of 2,4-D/2,4,5-T esters and not by the
diesel oil.
8.2. Epidemiological Studies of the Chronic Effects of 2,4-D
Much concern has been raised about the phenoxy herbicides,
including 2,4-D, especially in relation to birth defects and cancer
in human beings.
Several episodes have also been reported in which defined
populations were exposed to mixtures of 2,4-D and 2,4,5-T, in which
the 2,4,5-T was contaminated with various amounts of 2,3,7,8-
tetrachlorodibenzodioxin (2,3,7,8-TCDD) (Bleiberg et al., 1964;
Huff et al., 1980). It is now generally accepted that chloracne
and porphyria cutanea tarda observed in these studies were caused
by exposure to 2,3,7,8-TCDD and not by exposure to 2,4,5-T or 2,4-D
(Kimbrough, 1980).
The following sections concentrate on epidemiological studies
or other related studies on human beings in which actual or
potential exposures to 2,4-D products alone or to mixtures of 2,4-D
with other chlorphenoxy herbicides were demonstrated.
8.2.1. Reproductive, fetotoxic, and teratogenic effects
Although effects on reproduction have been demonstrated in
animals with 2,4,5-T, 2,4-D and 2,3,7,8-TCDD, all of the attempts
made to determine whether human beings suffer similar effects have
been frustrated by the poor design of the studies, inadequate
determination of exposure, or inadequate information about the
background incidence of spontaneous abortions and other abnormal
reproductive outcomes, by inadequate evaluation of confounding
variables, by inadequate assessment of exposure, and by mixed
exposures (Aldred et al., 1978; Lee, 1978; Field & Kerr, 1979;
Brogan et al., 1980; Hanify, 1980; Carmelli et al., 1981). For
these reasons they are not discussed in detail in this report.
Conclusive evidence of reproductive effects caused by 2,4-D in
populations that might be exposed to chlorophenoxy herbicides is
unlikely to be obtained from new epidemiological studies on
indirectly-exposed populations living in, or adjacent to, areas in
which phenoxy herbicides are used. Doses of 2,4-D absorbed by
bystanders are far below those expected to be toxic, as shown by
occupational exposure studies with 2,4-D and 2,4,5-T herbicides
(section 5). Any effects induced by such small amounts would
probably be obscured by more potent confounding factors (Janerich,
1973; Karkinen-Jääskelainen & Saxén, 1974; Saxén et al., 1974;
Elwood & Rogers, 1975; Granroth et al., 1977, 1978; James, 1977;
Holmberg, 1979; Lappe, 1979; Schacter et al., 1979).
Additional studies on female workers occupationally exposed to
significantly higher levels of 2,4-D than bystanders would be
useful to clarify some of the uncertainties raised by past studies,
if sufficiently large cohorts could be identified.
8.3. Studies on Mutagenic Effects in Workers Exposed to 2,4-D
Lymphocytes from ten workers exposed to 2,4-D esters during the
manufacture of 2,4-D herbicides, or from 15 workers packaging 2,4-D
sodium salt, did not show any chromosome abnormalities (Johnson,
1971; Andreasik et al., 1979). Chromosome or chromatid
abnormalities in lymphoctyes from some pesticide sprayers applying
a variety of agricultural chemicals, including in some cases 2,4-D,
were observed by Yoder et al. (1973) and Crossen et al. (1978).
Högstedt et al. (1980) did not observe any significant increases in
chromosome abnormalities in workers exposed to 2,4-D and other
pesticides.
The induction of SCEs among workers occupationally exposed to
the phenoxy herbicides 2,4-D and MCPA has been recently studied.
The subjects used only 2,4-D and MCPA or mixtures of the two for
spraying, and the exposure levels were estimated by determining the
urinary 2,4-D and MCPA excretion by the workers. No dose-related
differences in the frequencies of SCEs could be found either in
relation to the exposure level or to the length of the exposure
(Linnainmaa, 1983).
Although some studies suggest that occupational exposure to
2,4-D may result in chromosome abnormalities, the results are
conflicting. Moreover, the possiblity of mixed exposure and other
confounding variables cannot be excluded in the studies with
positive results.
8.4. Carcinogenic effects
8.4.1. Epidemiological studies
In two case-control studies of soft-tissue sarcoma (Hardell &
Sundström, 1979; Eriksson et al., 1981) and one of lymphoma
(Hardell et al., 1981), exposure to phenoxyacetic acids (mainly
2,4,5-T, 2,4-D, and MCPA) was associated with approximately 5-fold
increases in the risk of soft-tissue sarcomas. Exposure to 2,4-D,
either with or without MCPA exposure, also increased relative
risks. In the study of malignant lymphomas, 7 cases and 1 control
were apparently exposed to 2,4-D only (relative risk, 19.6; 95%
confidence interval, 4.3 - 89.8).
In a different case-control study with a small number of cases
and controls, no increased risk was observed (Smith et al., 1982).
A follow-up was also carried out on 348 railroad workers
exposed for less than 45 days during the period 1957 - 72 to the
herbicides 2,4-D, 2,4,5-T, atrazine, mecoprop, dichloropropionic
acid, and amitrole (Axelson & Sundell, 1974). The authors found a
significant increase in cancer mortality and morbidity among
workers exposed to amitrole.
Axelson et al. (1980) reported a further follow-up of these
workers, up to October, 1978, accumulating 5541 person-years. The
herbicide exposure of the workers was analysed in terms of exposure
to either amitrole, or phenoxy acids, or to a combination of the
two. A 10-year lapse period from the first day of exposure was
used as the induction latency. They found 15 cases of cancer
versus 6.87 expected (relative risk, 2.2). In the cohort with
combined exposure to amitrole and phenoxy acids, 6 cases were
observed versus 1.78 expected (relative risk, 3.4); in the group
exposed to amitrole alone, 3 tumours were observed versus 1.95
expected (relative risk, 1.5); and 6 cancers were observed versus
3.14 expected (relative risk, 1.9) in the phenoxy acid-exposed
group. All cancers, as well as cancers of the stomach, occurred in
statistically-significant excesses in the cohort as a whole. In
the groups exposed to amitrol plus phenoxy acids, there was a
significant excess of all cancers. In the group exposed only to
phenoxy acids, stomach cancer occurred in significant excess (2
observed, 0.33 expected; relative risk, 6.1). No soft-tissue
sarcomas were identified, but the statistical power of this study
to detect an excess of a rare cancer was limited.
Högstedt & Westerlund (1980) conducted a restrospective
mortality study on 142 forestry workers exposed to phenoxy
pesticides and 244 unexposed forestry workers, comparing their
mortality experience with national statistics. Work supervisors,
who were more highly exposed to phenoxy herbicides than the others,
had a significantly elevated tumour mortality (5 observed, 1.4
expected). No particular tumour type predominated, and no soft-
tissue sarcomas were observed, though the authors noted that the
study had limited statistical power and was inconclusive, because
of the relatively short follow-up period.
Riihimäki et al. (1982) reported on a prospective cohort study
of 2,4-D and 2,4,5-T spray personnel which was in progress.
Because of the small number of deaths and the brief follow-up
period, no conclusions can so far be drawn from this study.
Follow-up studies on cohorts of pesticide sprayers, farmers,
and agricultural workers occupationally exposed to a variety of
chemicals, in some cases including phenoxy herbicides, have been
reviewed by IARC (1983).
Follow-up studies on groups of industrial workers exposed to
chlorophenols, 2,4,5-T, or other chlorophenoxy compounds, and to
2,3,7,8-TCDD or other dioxins, during the manufacture of
chlorophenoxy herbicides, have recently been reviewed by Huff et
al. (1980) and IARC (1983).
8.4.2. Evidence on the carcinogenicity of 2,4-D
The available studies suggest that an association exists
between mixed exposure to phenoxy herbicides, chlorinated phenols,
and chlorinated dibenzodioxins, and an increased incidence of soft
tissue sarcomas and malignant lymphomas. It is not clear, at
present, whether these findings represent true associations, and
further studies are in progress (Muir & Wagner, 1981) to clarify
this point. Since many of the tumour cases had been exposed to
combinations of phenoxy herbicides and their contaminants as well
as other chemicals, it is not known whether exposure to 2,4-D is
specifically associated with the development of soft tissue
sarcomas.
8.5. Treatment of Poisoning in Human Beings
Successfully treated cases of 2,4-D poisoning indicate that
forced alkaline diuresis is helpful in reducing the level of 2,4-D
in the blood and tissues (Young & Haley, 1977; Prescott et al.,
1979). Heart and kidney damage should be anticipated and
counteracted in cases of severe poisoning (Duric et al., 1979).
In cases of acute poisoning due to 2,4-D, the nearest Poison
Control Centre should be contacted for additional information on
symptoms and recommended treatment.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO 2,4-D
9.1. General Considerations
In areas of 2,4-D herbicide production, handling, or use, the
highest exposure will be incurred by those who are directly
involved in these processes, followed by bystanders indirectly
exposed to 2,4-D vapour, dust, or droplets, or to contaminated
vegetation, soil, or water. In these two groups, exposure will
usually be via the skin. The general population in 2,4-D-use areas
would be exposed to a lesser extent, mainly through food containing
2,4-D residues and to a lesser extent through 2,4-D residues in
water. The contribution from air is negligible. As far as the
general population is concerned, 2,4-D intake from any source, is
negligible.
9.2. Estimated Intake of 2,4-D by the Population in a 2,4-D-use Area
The total contribution from air, food, and water is estimated
to be 0.03 - 2 µg/kg body weight per day (Table 13).
9.2.1. Intake by bystanders
Given the limited data available and the many uncertainties
involved, an adequate estimate of 2,4-D intake by bystanders is not
possible at this time, but it should generally be less than that
for occupationally-exposed persons.
9.2.2. Occupational intake
Workers using 2,4-D may, on average, absorb about 0.1 mg
2,4-D/kg body weight per day. However, this level may be exceeded
if good occupational hygiene is not practiced (section 5.2).
Simple precautions against excessive exposure can reduce the amount
of 2,4-D uptake.
9.3. Safety Factors
9.3.1. Definitions
For the present assessment, the safety factor is defined as the
integer obtained by dividing the overall no-observed-adverse-effect
level for a known adverse effect of 2,4-D (determined from all
available information on human beings or animals) by the daily
exposure value (absorbed dose of 2,4-D) for the various exposed
groups.
9.3.2. Determination of safety factors
9.3.2.1. Acute poisoning
Based on clinical studies in which 2,4-D was injected into
patients as a drug, the no-observed-adverse-effect level for signs
and symptoms of acute 2,4-D poisoning in children and adults
appears to be at or near 36 mg/kg body weight (section 8.1). Based
on the available studies of the amounts of 2,4-D absorbed by
occupationally-exposed person, bystanders, and populations in
2,4-D-use areas, the safety factors for acute 2,4-D poisoning are
likely to be:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) at least 360 for occupationally-exposed spraying crews.
The margin of safety for persons with excessive occupational
exposures would be smaller.
9.3.2.2. Chronic toxicity
Dose-effect relationships for the chronic toxic effects of
2,4-D or 2,4-D derivatives are available only from animal studies.
The no-observed-adverse effect levels for certain chronic toxic
effects of 2,4-D in animals have not been firmly established, and
for this reason safety factors cannot be established (section
7.2.1) for all of the chronic effects of 2,4-D.
9.3.2.3. Embryotoxic, fetotoxic, and teratogenic effects
The no-observed-adverse-effect level for embryotoxic,
fetotoxic, or teratogenic effects of 2,4-D in mammals appears to
lie at 10 mg/kg body weight per day (section 7.3.1.2). Assuming
that the same is true for human beings, then the corresponding
safety factors for the various exposed groups are:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) 100 for occupationally-exposed spraying crews using
precautions against excessive exposure.
9.3.2.4. Mutagenic effects
The available information was inadequate for an assessment of
the mutagenic potential of 2,4-D in mammals.
9.3.2.5. Carcinogenic effects
Available animal bioassays and epidemiological studies are
inadequate for an assessment of the carcinogenic potential of 2,4-D
or of its derivatives.
9.4. Evaluation of Health Risks from 2,4-D Exposure
From the data available at present, the Task Group assumes that
a possible health risk will exist, when the safety factor is less
than 100.
9.5. Recommendations on Exposure
Results of recent exposure and occupational health studies
suggest that excessive exposure to 2,4-D can be avoided by fairly
simple measures of occupational hygiene, such as those recommended
in two pertinent publications of the International Labour Office
(ILO, 1977, 1979). Laundering procedures for 2,4-D-contaminated
clothing have been published by Easley et al. (1983), and these
should be considered.
REFERENCES
ABO-KHATWA, N. & HOLLINGWORTH, R.M. (1974) Pesticidal chemicals
affecting some energy linked functions of rat mitochondria in
vitro. Bull. environ. Contam. Toxicol., 12(4): 446-454.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-induced
RNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide-induced
ouabain resistant mutants in Chinese hamster V79 cells. Chem.-biol.
Interact., 19: 369-374.
AKESSON, N.B. & YATES, W.E. (196l) Drift residues on alfalfa hay.
A progress report of investigations of pesticide drift from
aircraft applications. Calif. Agric., 15: 4-7.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous
substances. Int. Pest Control, 11(2): 29-35.
ALDRED, J.E., BELCHER, R.S., CHRISTOPHERS, A.J., CLEMENTS, A.,
DANKS, D.M., FRENCH, E.L., GALLOWAY, D.B., GARSON, O.M., PARSONS,
W., RAPER, C., ROUCH, G., & SINN, H.J. (1978) Report of the
Consultative Council on Congenital Abnormalities in the Yarram
District. Alleged relationship of congenital abnormalities to the
use of the herbicides 2,4-D and 2,4,5-T. State of Victoria,
Australia, Ministry of Health, viii + 55 pp (Document PB 338).
ALTOM, J.D. & STRITZKE, J.F. (1973) Degradation of dicamba,
picloram and familiar phenoxy herbicides in soils. Weed Sci., 21:
556-560.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20(3): 649-656.
ANDREASIK, Z., KOLON, S., & SMOLIK, R. (1979) Health status
evaluation in workers packaging the herbicide "Pielik"
(cholorophenol). Arh. Hig. Rad. Toksikol., 30 (Suppl.): 599-602.
ANDRUSHAITIS G.P. (1972) [The effect of pesticides on the
environment.] Latvi. PSR Zinatnu Akad. Vest., 4:(297) 44-49 (in
Russian, Health & Welfare Canada Translation No. 2441).
ANTONENKO, T.A. (1977) [Experimental data from a study of the
permeability of the placental barrier to the herbicide 2,4-D and
its passage with the mother's milk during feeding.] Gig. Aspekt.
Okhr. Zdor. Naseleniya (Saratov). In: Saratov, Saratov Institute
for Oral Hygiene, pp. 177-178 (in Russian, Health & Welfare Canada
Translation No. 2370).
APFFEL, C.A. (1959a) Action cytostatique de certaines auxines,
compte rendu préliminaire. Presse med., 67(6): 207-209.
ARJMAND, M., HAMILTON, R.H., & MUMMA, R.O. (1978) Separation of
amino acid conjugates of 2,4-dichlorophenoxyacetic acid by high-
pressure liquid chromatography employing ion-pair techniques.
J. agric. food Chem., 26: 971-973.
ARKHIPOV, G.N. & KOZLOVA, I.N. (1974) [A study of the
carcinogenic potential of a herbicide: 2,4-D amine salt.] Vopr.
Pit., 5: 83-84 (in Russian).
ASSOULY, M. (1951) Désherbants sélectifs et substances de
croissance aperēu technique. Effet pathologiques sur l'homme au
cours de la fabrication de l'ester du 2,4-D. Arch. Mal. prof. Hyg.
Toxicol. Ind., 12(1): 26-30.
AVERITT, W.K. (1967) An evaluation of the persistence of 2,4-D
amine in surface waters in the state of Louisiana. Proc. South.
Weed Sci. Soc., 20: 342-347.
AXELSON, O. & SUNDELL, L. (1974) Herbicide exposure, mortality
and tumor incidence. An epidemiological investigation on Swedish
railroad workers. Scand. J. Work Environ. Health, 11:21-28.
AXELSON, O., SUNDELL, L., ANDERSSON, K., EDLING, C., HOGSTEDT, C.,
& KLING, H. (1980) Herbicide exposure and tumor mortality: An
updated epidemiological investigation on Swedish railroad workers.
Scand. J. Work Environ. Health, 6: 73-79.
BACHE, C.A., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1964a)
Absence of phenoxyacid herbicide residues in the milk of dairy cows
at high feeding levels. J. dairy Sci., 47(3): 298-299.
BAKER, B.R., LEE, W.W., SKINNER, W.A., MARTINEZ, A.P., & TONG, E.
(1960) Potential anticancer agents. I. Non-classical
antimetabolites. II. Some factors in the design of exo-alkylating
enzyme inhibitors, particularly of lactic dehydrogenase. J. med.
pharm. Chem., 2(6): 633-657.
BAKER, E.L., FELDMAN, R.G., WHITE, R.F., HARLEY, J.P., DINSE, G.E.,
& BERKEY, C.S. (1983) Monitoring neurotoxins in industry:
Development of a neuro-behavioral test battery. J. occup. Med.,
25(2): 125-130.
BAKER, P.G., HOODLESS, R.A., & TYLER, J.F.C. (1981) A review of
methods for the determination of polychlorodibenzo-p-dioxins and
polychlorodibenzofurans in phenoxyalkanoic acid herbicides.
Pestic. Sci., 12: 294-304.
BALO-BANGA, M.J., ANTONI, F., ALTMANN, H., VINCZE, I., & KOCSIS, F.
(1973) Effect of 2,4-D on semiconservative and DNA repair
synthesis. (Case report: bullous dermatitis after exposure to
2,4-D). Ber. Oesterr. Studienges Atomenerg. (Forschungszentrum
Seibersdorf), 2218: 1-7.
BAMESBERGER, W.L. & ADAMS, D.F. (1966) An atmospheric survey for
aerosol and gaseous 2,4-D components. Adv. Chem. Ser., 60:
219-227.
BARTLEY, T.R. & HATTRUP, A.R. (1970) 2,4-D contamination and
persistence in irrigation water. Proc. West. Soc. Weed Sci.,
23: 10-33.
BASHIROV, A.A. (1969) [The health of workers involved in the
production of amine and butyl 2,4-D herbicides.] Vrach. Delo,
10: 92-95 (in Russian, Health & Welfare Canada Translation No.
2181).
BASHIROV, A.A. & TER-BAGDASAROVA, I.K. (1970) [The state of the
cardiovascular system in workers engaged in the production of amine
salt herbicides and 2,4-dichlorophenoxyacetic acid butyl ester.]
Azerb. Med. Zh., 47 (12): 44-48 (in Russian).
BATORA, V., VITOROUIC, S.L.J., THIER, H.P., & KLISENKO, N.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BEAVEN, F.G., RAWLS, C.K., & BECKET, G.E. (1962) Field
observations upon estuarine animals exposed to 2,4-D. Proc.
northeast. Weed Contr. Conf., 16: 449-458.
BELOMYTTSEVA, L.A. (1964) [Toxic effects of the herbicide 2,4-D
sodium on the lungs.] Sb. Nauchn. Tr. Bashk. Gos. Med. Inst.,
16(2): 135-141 (in Russian).
BELOMYTTSEVA, L.A. (1965) [In: Contributions to the 3rd All-Union
Scientific Conference on the Problems of Hygiene and Toxicology
related to the Use of Chemicals in the National Economy, Kiev,]
p. 380 (in Russian).
BELOMYTTSEVA, L.A. (1967) The toxic effect of the sodium salt of
2,4-dichlorophenoxyacetic acid. UFA 1967.
BELOMYTTSEVA, L.A. & KARIMOVA, A.K. (1963) In: [Proceedings of
the UFA Scientific Research Institute of Hygiene and Occupational
Diseases, Vol. 2.] (in Russian).
BERKLEY, M.C. & MAGEE, K.R. (1963) Neuropathy following exposure
to a dimethylamine salt of 2,4-D. Arch. internal. Med., 111:
351-352.
BERWICK, P. (1970) 2,4-dichlorophenoxyacetic acid poisoning in
man. J. Am. Med. Assoc., 214(6): 1114-1117.
BEZUGLYI, V.P., FOKINA, K.V., KOMAROVA, L.I., SIVITSKAJA, I.I.,
ILINA, V.I., & GORSKAJA, I.I. (1979) [Clinical manifestations of
long-term sequels of acute poisoning with 2,4-D.] Gig. Truda Prof.
Zabol., 3: 47-48 (in Russian).
BIELSKI, J. & MADRA, B. (1976) [Acute poisoning with pesticides.]
Pol. Tyg. Lek., 31(46): 1971-1973 (in Polish, Health & Welfare
Canada Translation No. 2245).
BJERKE, E.L., HERMAN, J.L., MILLER, P.W., & WETTERS, J.H. (1972)
Residue study of phenoxy herbicides in milk and cream. J. agric.
food Chem., 20: 963-967.
BJORKLUND, N.E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. scand., 7: 364-390.
BJORN, M.D. & NORTHEN, H.T. (1948) Effects of 2,4-dichloro-
phenoxyacetic acid on chicks. Science, 108: 479-480.
BLEIBERG, J., WALLEN, M., & BRODKIN, R. (1964) Industrially
acquired porphyria. Arch. Derm., 89: 793-797.
BODAI, J., TAMAS, L., & VEGH, A. (1974) [Reproductive
consequences of Dikonirt toxicosis in cattle.] Magy. Allatorv.
Lapja, 29(5): 319-322 (in Hungarian, Health & Welfare Canada
Translation No. 2238).
BODEM, R., PREISS, D., & KUHN, E. (1971) [On the delay of the
relaxation of the guinea pig papillary muscle by 2,4-dichloro-
phenoxy-acetic acid (2,4-D).] Klin. Wochenschr., 49(1): 227-228
(in German).
BODYAGIN, D.A., SYKIN, A.B., LUPINOSOV, Y.V., & ZAITSEVA, L.A.
(1969) [The effects of chlorophenoxyacetic acid derivatives
(herbicides) on animals and human beings. (A literature review).]
Farmakol. Toksikol., 32 (6): 747-751 (in Russian, Health & Welfare
Canada Translation No. 2031).
BOLLAG, J.M., HELLINGS, C.S., & ALEXANDER, M. (1968) 2,4-D
metabolism. Enzymatic hydroxylation of chlorinated phenols.
J. agric. food Chem., 16: 826-828.
BONGSO, T.A. & BASRUR, P.K. (1973) In vitro response of bovine
cells to 2,4-dichlorophenoxy acetic acid. In vitro, 8: 416-417.
BONTOYAN, W.R. (1977) Report on pesticide formulations. J. Assoc.
Off. Agric. Chem., 60: 324-327.
BOVAL, B. & SMITH, J.M. (1973) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. Chem. Eng. Sci., 28: 1661-1675.
BOVEY, R.W. (1980a) Residues and fate of phenoxy herbicides in
the environment. In: Bovey, R.W. & Young, A.L., ed. The science
of 2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 301-356.
BOVEY, R.W. (1980b) Human risk with phenoxy herbicides in the
environment. In: Bovey, R.W. & Young, A.L., ed. The science of
2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 425-433.
BOVEY, R.W. & YOUNG, A.L., ed. (1980) The science of 2,4,5-T and
associated phenoxy herbicides, New York, Chichester, Brisbane,
Toronto, John Willey & Sons, 462 pp.
BRADLAW, H.A., GARTHOFF, L.H., HURLEY, N.E., & FIRESTONE, D. (1980)
Comparative induction of aryl hydrocarbon hydroxylase activity
in vitro by analoques of dibenzo- p-dioxin. Food cosmet. Toxicol.,
18: 627-635.
BRANDT, M.R. (1971) [Herbatox poisoning. A short survey and a
report of a case.] Ugeskrift Laeger, 133(11): 500-503 (in Danish,
Health & Welfare Canada Translation No. 2147.
BRETAG, L.H. & CAPUTO, C. (1978) The nature of the antimyotonic
action of 2,4-D on the soleus muscle of the rat. Proc. Aust.
Physiol. Pharmacol. Soc., 9(2): 150 pp.
BRISTOL, D.W., COOK, L.W., KOTERBA, M.T., & NELSON, D.C. (1982)
Determination of free and hydrolyzable residues of 2,4-
dichlorophenoxyacetic acid and 2,4-dichlorophenol in potatoes.
J. agric. food Chem., 30: 137-144.
BRODY, I.A. (1973) Myotonia induced by monocarboxylic aromatic
acids: A possible mechanism. Arch. Neurol., 28: 243-246.
BRODY, T.M. (1952) Effect of certain plant growth substances on
oxidative phosphorylation in rat liver mitochondria. Proc. Soc.
Exp. Bio. Med., 80: 533-536.
BROGAN, W.F., BROGAN, C.E., & DADD, J.T. (1980) Herbicides and
cleft lip and palate. Lancet, 2: 597.
BUCHER, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid
on experimental animals. Proc. Soc. Exp. Biol. Med., 63: 204-205.
BURCAR, P.J., WERSHAW, R.L., GOLDBERG, M.C., & KHAN, L. (1966)
Gas chromatographic study of the behaviour of the isooctyl ester
of 2,4-D under field conditions in North Park, Colorado. Anal.
Instrum., 4: 215-224.
BURTON, J.A., GARDINER, T.H., & SCHANKER, L.S. (1974) Absorption
of herbicides from the rat lung. Arch. environ. Health, 29: 31-33.
BUSLOVICH, S.Y. (1963) [The effect of the chlorinated derivatives
of phenoxyacetic acid.] Zdrav. Beloruss., 9: 41-45 (in Russian,
Health & Welfare Canada Translation No. 2315).
BUSLOVICH, S.Y. & KOLDOBSKAYA, F.D. (1972) [Activity of
hexokinase in skeletal muscles of albino rats in experimental
myotonia.] Vopr. Med. Khim., 18(4): 403-406 (in Russian).
BUSLOVICH, S.Y & MILCHINA, M.G. (1976) [The dynamics of 2,4-D
amine decomposition in soil.] Gig. i Sanit., 5: 109-110
(in Russian).
BUSLOVICH, S.Y., VOINOVA, I.V., & MILCHINA, M.G. (1973) [The
distribution of chlorinated phenoxy acid herbicides in albino
rats.] Gig. Tr. Prof. Zabol, 17(3): 171-173 (in Russian, Health &
Welfare Canada Translation No. 2237).
BUSLOVICH, S.Y., ALEKSASHINA, Z.A., & KOLOSOVSKAYA, V.M. (1976)
[Embryotoxic effects of herbicides: Chlorinated phenoxy acids.]
Zdrav. Beloruss., 10: 83-84 (in Russian).
BUSLOVICH, S.Y., KOLDOBSKAYA, F.D., DAVYDENKO, L.I., & KRYSANOVA,
A.I. (1982) [The role of mixed-function oxidases in the
detoxication of some herbicides: Chlorinated derivatives of
phenoxyacids.] Gig. i Sanit., 10: 76-77 (in Russian).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna.
Proc. South. Weed Sci. Soc., 18: 576-580.
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON, M.H., &
MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D, DEF,
propanil, and trifluralin to the Dungeness crab, Cancer magister.
Arch. environ. contam. Toxicol., 8: 383-396.
CANADA, HEALTH & WELFARE (1980) Pesticides. In: Guidelines for
Canadian drinking water quality, 1978. Supporting documentation,
Ottawa, Supply and Services, Canada, pp. 457-488.
CARL, M. (1979) Internal laboratory quality control in the
routine determination of chlorinated pesticide residues. Adv.
pestic. Sci., 3:660-663.
CARMELLI, D., HOFHERR, L., TOMSIC, J., & MORGAN, R.W. (1981) A
case-control study of the relationship between exposure to 2,4-D
and spontaneous abortions in humans, Menlo Park, California, SRI
International (Final Report).
CARTER, E.P. (1960) Volatility of ester forms of hormone type
herbicides III. J. Assoc. Off. Anal. Chem., 43: 367-370.
CHANG, H., RIP, J.W., & CHERRY, J.H. (1974) Effects of
phenoxyacetic acids on rat liver tissues. J. agric. food Chem.,
22(1): 62-65.
CHAU, A.S.Y. & THOMSON, K. (1978) Investigation of the integrity
of seven herbicidal acids in water samples. J. Assoc. Off. Anal.
Chem., 61: 1481-1485.
CHAU, A.S.Y., AFGHAN, B.K., & ROBINSON, J.W. (1982) Analysis of
pesticides in water. Volume II. Chlorine- and phosphorus-
containing pesticides. Boca Raton (Florida), CRC Press Inc.,
pp. 238.
CHEN, W.L., GORZINSKI, S.J., KOCIBA, R.J., WADE, C.E., MORDEN,
D.C., KEYES, D.G., & SCHWETZ, B.A. (1981) 2,4-D (2,4-
dichlorophenoxy) acetic acid: results of a 13-week feeding study in
CDF Fisher 344 rats. Proc. Annu. Meet. Can. Fed. Biol. Soc.,
24: 233.
CHOI, K.L., QUE HEE, S.S., & SUTHERLAND, R.G. (1976) 2,4-D levels
in the South Saskatchewan River in 1973 as determined by a GLC
method. J. environ. Sci. Health, Part B, 11: 175-183.
CHOW, C., MONTGOMERY, M.L., & YU, T.C. (1971) Methodology and
analysis for residues of MCP and 2,4,5-T in wheat. Bull. environ.
Contam. Toxicol., 18: 361-365.
CLARK, D.E., PALMER, J.S., RADELEFF, R.D., CROOKSHANK, H.R., &
FARR, F.M. (1975) Residues of chlorophenoxy acid herbicides and
their phenolic metabolites in sheep and cattle. J. agric. food
Chem., 23(3): 573-578.
COCHRANE, W.P. (1981) Chemical derivatization in pesticide
analysis. In: Frei, R.W. & Lawrence, J.F., ed. Chemical
derivatization in analytical chemistry, I: Chromatography, New
York, Plenum Press, pp. 1-97.
COCHRANE, W.P. & RUSSELL, J.B. (1975) Residues in wheat and soil
treated with the mixed butyl esters of 2,4-D. Can. J. plant Sci.,
55: 323-325.
COCHRANE, W.P., SINGH, J., MILES, W., & WAKEFORD, B. (1981)
Determination of chlorinated dibenzo- p-dioxin contaminants in
2,4-D products by gas chromatography - mass spectrometric
techniques. J. Chromatogr., 217: 289-299.
COCHRANE, W.P., LANOUETTE, M., & SINGH, J. (1982) High pressure
liquid chromatographic determination of impurity phenols in
technical 2,4-D acid and 2,4-dichlorophenol. J. Assoc. Off. Anal.
Chem., in press.
COGGON, D. & ACHESON, E.D. (1982) Do phenoxy herbicides cause
cancer in man? Lancet, 1: 1057-1059.
COHEN, S.Z., ZWEIG, G., LAW, M., WRIGHT, D., & BONTOYAN, W.R.
(1978) Analytical determination of N-nitroso compounds in
pesticides by the United States Environmental Protection Agency.
In: Walker, E.A., Castegnaro, M., Criciute, L., & Lyle, R.E., ed.
Environmental Aspects of N-nitroso compounds, Lyons, IARC
Scientific Publications, 19: 333-342.
COLLABORATIVE INTERNATIONAL PESTICIDES ANALYTICAL COUNCIL LTD
(1970) CIPAC Handbook, Vol. I, Analysis of technical and
formulated pesticides, Harpenden, England, CIPAC, pp. 241-273.
COMMISSION OF EUROPEAN COMMUNITIES (1981) Criteria (dose/effect
relationships) for organochlorine pesticides. Oxford, New York,
Toronto, Sydney, Paris, Frankfurt, Pergamon Press, pp. 223-240.
CONNICK, W.J., Jr & SIMONEAUX, J.M. (1982) Determination of (2,4-
dichlorophenoxy) acetic acid and of 2,6-dichlorobenzo-nitrile in
water by high performance liquid chromatography. J. agric. food
Chem., 30: 258-260.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99(1): 1-12.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet
samples (V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet
samples (VI). Pestic. monit. J., 5(4): 313-330.
COURTNEY, K.D. (1977) Prenatal effects of herbicides: Evaluation
by the prenatal development index. Arch. environ. Contamin.
Toxicol., 6: 33-46.
COUTSELINIS, A., KENTARCHOV, R., & BOUKIS, D. (1977) Concentration
levels of 2,4-D and 2,4,5-T in forensic material. Forensic Sci.,
1: 203-204.
CRAFTS, A.S. (1960) Evidence for hydrolysis of esters of 2,4-D
during absorption by plants. Weeds, 8: 19-25.
CRIPPS, R.E. & ROBERTS, T. (1978) Microbial degradation of
herbicides. In: Hill, I.R. & Wright, S.J.L., ed.: Pesticide
microbiology - Microbial aspects of pesticide behaviour in the
environment, London, New York, San Francisco, Academic Press,
pp. 669-730.
CROSBY, D.G. & TUTASS, H.O. (1966) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. J. agric. Food Chem., 14: 596.
CROSBY, D.G. & WONG, A.S. (1973) Photodecomposition of 2,4,5-
trichlorophenoxyacetic acid (2,4,5-T) in water. J. agric. Food
Chem., 21(6): 1052-1054.
CROSSEN, P.E., MORGAN, W.F., HORAN, J.J., & STEWART, J. (1978)
Cytogenetic studies of pesticide and herbicide sprayers. New
Zealand med. J., 88(619): 192-195.
CRUMMET, W.P. & STEHL, R.H. (1973) Determination of chlorinated
dibenzo- p-dioxins and dibenzofurans in various materials. Environ.
Health Perspect., 5: 15-25.
CURRIE, L.A. (1968) Limits of qualitative detection and
quantitative determination. Anal. Chem., 40: 586-593.
CURRY, A.S. (1962) Twenty-one uncommon cases of poisoning. Br.
med. J., 1: 687-689.
DANON, J.M., KARPATI, G., CARPENTER, S., & WOLFE, L.S. (1976)
Experimental myotonic myopathy. Neurology, 26: 384.
DEBEER, J.O., VAN PETEGHEM, C.H., HEYNDRICKX, A.M., & VAN ZELE, W.
(1981) Mass fragmentographic determination of 2,4-D and 2,4,DP
trace levels in biosamples using intramolecular deuterated interval
standards. Med. Fac. Landbouww. Rijksuniv. Gent, 46(1): 305-316.
DELARRARD, J. & BARBASTE, M. (1969) Intoxication suicidaire
mortelle agro-chimique de l'hormone désherbante 2,4-D. Arch. Mal.
prof. Med. Trav. Secur. soc., 30(7-8): 434.
DENNIS, C.A.R. (1976) Health effects of 2,4-D. In: National
Research Council of Canada Associate Committee on Scientific
Criteria for Environmental Quality, ed. Phenoxy herbicides: their
effects on environmental quality, Ottawa, NRCC Publications
Service, p.333.
DEREUCK, J., DECOSTER, W., WILLEMS, J., & VANDER EECKEN, H. (1979)
Influence of 2,4-dichlorophenoxyacetate and of dantrolene sodium on
the target phenomenon in tenotomized rat gastrocnemius muscle.
Acta neuropathol., 46: 167-168.
DESI, I. & SOS, J. (1962a) Central nervous injury by a chemical
herbicide. Acta med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1962b) [The effect of 2,4-dichloro-
phenoxyacetic acid and triorthocresyl phosphate on the function of
the upper central nervous system (bioelectric activity and
conditioned reflexes).] Gig. i Sanit., 12: 38-46 (in Russian,
Health & Welfare Canada Translation No. 2183).
DESI, I., SOS, J., & NIKOLITS, I. (1962a) New evidence concerning
the nervous site of action of a chemical herbicide causing
professional intoxication. Pathophysiologica (Budapest), 22:
73-80.
DESI, I., SOS, J., OLASZ, J., SULE, F., & MARKUS, V. (1962b)
Nervous system effects of a chemical herbicide. Arch. environ.
Health, 4: 95-102.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B., & GEORGE, J.L. (1962)
Effects on wildlife. pp. 4-52. In: Effects of pesticides on fish
and wildlife: A review of investigations during 1960. Washington,
DC, US Dept. of the Interior (Fish and Wildlife Circ. No. 143).
DRAPER, W.M. & STREET, J.C. (1982) Applicator exposure to 2,4-D,
dicamba, and a dicamba isomer. J. environ. Sci. Health, B17(4):
321-339.
DRILL, V.A. & HIRATZKA, T. (1953) Toxicity of 2,4-D and 2,4,5-T,
a report on their acute and chronic toxicity in dogs. Arch. ind.
Hyg. occup. Med., 7: 61-67.
DUDLEY, A.W. & THAPAR, N.T. (1972) Fatal human ingestion of
2,4-D, a common herbicide. Arch. Pathol., 94: 270-275.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States (III), June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E., LIPSCOMB, G.Q., COX, E.L., HEATWOLE, R.E., & KLING,
R.C. (1971) Pesticide residue levels in foods in the United
States from July 1, 1963 to June 30, 1969. Pestic. monit. J.,
5(2): 73-80.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some herbicides
on the hatching rate of hens' eggs. Nature (Lond.), 215:
1406-1407.
DUNACHIE, J.F. & FLETCHER, W.W. (1970) The toxicity of certain
herbicides to hens' eggs assessed by the egg injection technique.
Ann. app. Biol., 66: 515-520.
DURIC, V., STOJANOVIC, V., & IGIC, B. (1979) [Presentation of
patients with pesticide poisoning.] Med. Pregl., 32(3/4): 129-133.
(In Serbo-Croatian, Health & Welfare Canada Translation No. 2382.)
DUX, E., TOTH, I., DUX, L., & JOO, F. (1978) The localization of
calcium by X-ray microanalysis in myopathic muscle fibers.
Histochemistry, 56: 239-244.
DZHAPAROV, I.R. & TSILIKOV, V.V. (1969) [The effect of the sodium
salt of 2,4-D and carbothione on the content of glycogen in the
liver and the blood sugar level in rats.] Farmakol. Tsentr.
Kholinolitikov Drugikh Neirotropnykh Sredstv., p. 180 (in
Russian).
EASLEY, C.B., LAUGHLIN, J.M., GOLD, R.E., & TUPY, D.R. (1983)
Laundering procedures for removal of 2,4-dichloro-phenoxyacetic
acid ester and amine herbicides from contaminated fabrics. Arch.
Environ. Contam. Toxicol., 12: 71-76.
EBERSTEIN, A. & GOODGOLD, J. (1979) Experimental myotonia induced
in denervated muscles by 2,4-D. Muscle Nerve, 2: 364-368.
ELO, H. & YLITALO, P. (1977) Substantial increase in the levels
of chlorophenoxyacetic acids in the CNS of rats as a result of
severe intoxication. Acta pharmacol. toxicol., 41: 280-284.
ELO, H. & YLITALO, P. (1979) Distribution of 2-methyl-4-
chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid in male
rats: evidence for the involvement of the central nervous system
in their toxicity. Toxicol. appl. Pharmacol., 51: 439-446.
ELWOOD, J.M. & ROGERS, J.R. (1975) The incidence of congenital
abnormalities in British Columbia, Alberta, Manitoba and New
Brunswick, 1966-1969. Can. J. public Health, 66: 471-475.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERICKSON, L.C., BRANNAMAN, B.L., & COGGINS, C.W. Jr. (1963)
Residues in stored lemons treated with various formulations of
2,4-D. J. agric. food Chem., 11: 437-440.
ERIKSSON, M., HARDELL, M., BERG, N.O., MOLLER, T., & AXELSON, O.
(1981) Soft-tissue sarcomas and exposure to chemical substances: a
case-referent study. Br. J. ind. Med., 38: 27-33.
ERNE, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta vet. Scand., 7: 240-256.
ERNE, K. (1966b) Studies on the animal metabolism of
phenoxyacetic herbicides. Acta vet. Scand., 7: 264-271.
ERNE, K. (1974) Weed-killers and wildlife. In: Proceedings of
the XI International Congress on Game Biology, Stockholm, 1973.
pp. 415-422 (National Swedish Environment Protection Board
Publications No. 13E).
ERNE, K. (1975) Phenoxy herbicide residues in Swedish fish and
wildlife. In: Coulston, F., Korte, F., Klein, W., & Rosenblum,
I., ed. Pesticides: Environmental quality and safety, Stuttgart,
George Thieme Publishers, Suppl. Vol. III., pp. 192-195.
ERNE, K. (1980) [Further studies of phenoxy herbicide residues in
woodland berries and mushrooms, 1973-1979.] Vr Föda, 32(5):
285-292 (in Swedish).
ERNE, K. & RUTQVIST, L. (1979) [Pesticide residues in feedstuffs
in Sweden.] Nord. Veterinaermed., 31: 263-274 (in Swedish).
ERNE, K. & SPERBER, I. (1974) Renal tubular transfer of
phenoxyacetic acids in the chicken. Acta pharmacol. toxicol., 35:
233-241.
ERNE, K. & VON HAARTMAN, U. (1973) [Phenoxy acid residues in
forest berries and mushrooms.] Vr Föda, 25: 146-153 (in Swedish).
EVANS, W.C., SMITH, B.S.W., FERNLEY, H.M., & DAVIES, J.I. (1971)
Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem. J.,
122: 543-551.
EYZAGUIRRE, C., FOLK, B.P., ZIERLER, K.L., & LILIENTHAL, J.L. Jr.
(1948) Experimental myotonia and repetitive phenomena: The
veratrinic effects of 2,4-D acetate in the rat. Am. J. Physiol.,
155: 69-77.
FABACHER, D.L. & CHAMBERS, H. (1974) Resistance to herbicides in
insecticide-resistant mosquitofish, Gambusia affinis. Environ.
Lett., 7(1): 15-20.
FAO (1981) Pesticide residues in food: 1980 evaluations. Rome,
FAO, pp. 109-110 (Plant Production and Protection Paper 26 Suppl.)
FAO/WHO (1971) 2,4-D. In: 1970 Evaluations of some pesticide
residues in food, Rome, FAO/WHO, pp. 59-86 (FAO/AGP/1970/M/ 12/1,
WHO Food Additive Series, 71, 42).
FAO/WHO (1972) 1971 evaluations of some pesticide residues in
food: 2,4-D. WHO Pesticide Residues Series, 1: 83-97.
FAO/WHO (1973) Pesticide residues in food. Report of the 1972
Joint FAO/WHO meeting, Geneva, (WHO Techn. Rep. Series No. 525).
FARWELL, S.O., BOWES, F.W., & ADAMS, D.F. (1976a) Determination
of chlorophenoxy herbicides in air by gas chromatography/mass
spectrometry: selective ion monitoring. Anal. Chem., 48: 420-426.
FARWELL, S.O., ROBINSON, E., POWELL, W.J., & ADAMS, D.F. (1976b)
Survey of airborne 2,4-D in south central Washington. J. Air
Pollut. Control Assoc., 26: 224-230.
FAUST, S.D. & ALY, O.M. (1963) Some effects of 2,4-D and 2,4-DCP
on drinking water quality. Proc. Northeast. Weed Contr. Conf., 17:
460-470.
FAUST, S.D. & SUFFET, I.H. (1966) Recovery, separation, and
identification of organic pesticides from natural and potable
waters. Residue Rev., 15: 44-116.
FEDOROVA, L.M. & BELOVA, R.S. (1974) [Inclusion of 2,4-
dichlorophenoxyacetic acid in organs of animals: Paths and
dynamics of its excretion.] Gig. i Sanit., 39(2): 105-107
(in Russian, US NTC Translation No. 76-10918-05J).
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FETISOV, M.I. (1966) Occupational hygiene in the application of
herbicides of the 2,4-D group. Hyg. Sanit., 31(7-9): 383-386.
FEUNG, C.S., HAMILTON, R.H., & WITHAM, F.H. (1971) Metabolism of
2,4-dichlorophenoxyacetic acid by soybean cotyledon callus tissue
cultures. J. agric. food Chem., 19: 474-479.
FEUNG, C.S., HAMILTON, R.H., WITHAM, F.H., & MUMMA, R.O. (1972)
The relative amounts and identification of some 2,4-
dichlorophenoxyacetic acid metabolites isolated from soybean
cotyledon callus cultures. J. agric. food Chem., 50: 80-86.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1973b) Metabolism of
2,4-dichlorophenoxyacetic acid. V. Identification of metabolites
in soybean callus tissue cultures. J. agric. food Chem., 21:
637-640.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1975) Metabolism of
2,4-dichlorophenoxyacetic acid. VII. Comparison of metabolites
from five species of plant callus tissue cultures. J. agric. food
Chem., 23: 373-376.
FEUNG, C.S., LOERCH, S.L., HAMILTON, R.H., & MUMMA, R.O. (1978)
Comparative metabolic fate of 2,4-dichlorophenoxy acetic acid in
plants and plant tissue culture. J. Agric. Food Chem., 26:
1064-1067.
FIELD, B. & KERR, C. (1979) Herbicide use and incidence of
neural tube defects. Lancet, 1: 1341-1342.
FINNISH STATE INSTITUTE OF AGRICULTURAL CHEMISTRY (Helsinki)
(1963-1969) Investigations on pesticide residues. pp. 83.
FLEEKER, J.R. & STEEN, R. (1971) Hydroxylation of 2,4-D in
several weed species. Weed Sci., 19: 507-510.
FLORSHEIM, W.H. & VELCOFF, S.M. (1962) Some effects of
2,4-dichlorophenoxyacetic acid on thyroid function in the
rat: Effects on iodine accumulation. Endocrinology, 71(1):
1-6.
FLORSHEIM, W.H., VELCOFF, S.M., & WILLIAMS, A.D. (1963) Some
effects of 2,4-D on thyroid function in the rat: Effects on
peripheral thyroxine. Endocrinology, 72: 327-333.
FOISSAC-GEGOUX, P., LELIEVRE, A., BASIN, B., & WAROT, P. (1962)
Polynevrite aprčs usage d'un desherbant: l'acide 2,4-D. Lille Med.,
7(10): 1049-1051.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry
to nine herbicides. Bull. environ. Contam. Toxicol., 15(5):
509-514.
FOLMAR, L.C. (1979) Effects of short term field applications of
acrolein and 2,4-D (DMA) on flavor of the flesh of rainbow trout,
124 pp.
FOSTER, R.K. & McKERCHER, R.B. (1973) Extraction of 14C-labelled
chlorophenoxyacetic acids from soil. Can. J. soil Sci., 53:
135-136.
FRANK, P.A. (1972) Herbicidal residues in aquatic environments.
In: Faust, S.D., ed. Fate of organic pesticides in the aquatic
environment. Washington, DC, American Chemical Society,
pp. 135-148 (Advances in Chemistry Series, No. III).
FRANK, P.A. & COMES, R.D. (1967) Herbicidal residues in pond
water and hydrosoil. Weeds, 15(3): 210-213.
FRANK, P.A., DEMINT, R.J., & COMES, R.D. (1970) Herbicides in
irrigation water following canal-bank treatment for weed control.
Weed Sci., 18(6): 687-692.
FRANK, R. & SIRONS, G.J. (1980) Chlorophenoxy and chlorobenzoic
acid herbicides; their use in eleven agricultural waterbeds and
their loss to stream waters in southern Ontario, Canada, 1975-1977.
Sci. total Environ., 15: 149-167.
FRANK, R., SIRONS, G.J., & RIPLEY, B.D. (1979) Herbicide
contamination and decontamination of well waters in Ontario,
Canada, 1969-1978. Pestic. monit. J., 13(3): 120-127.
FRANK, R., SIRONS, G.J., CAMPBELL, R.A., & MEWETT, D. (1982)
Residues of 2,4-D, dichlorprop, and picloram in wild berries from
treated rights-of-way and conifer release sites in Ontario,
1979-81. Can. J. Plant Sci., 63: 196-209.
FRANKLIN, C.A., GROVER, R., MARKHAM, J.W., SMITH, A.E., & YOSHIDA,
K. (1982) Effect of various factors on exposure of workers
involved in the aerial application of herbicides. Am. Conf. of
Govern. Ind. Hygienists, Transactions, 43: 97-117.
FREEMON, F.R. (1975) Causes of polyneuropathy. Acta neurol.
Scand., 51(Suppl. 59): 5-43.
FYODOROVA, L.M., BELOVA, R.S., KIRYUHINA, N.N., VOLCHONOK, M.G., &
KOVTUNOVA, N.Y. (1977) [Residual content of chlorophenoxyacetic
acids in the environment and human body.] Gig. i Sanit., 6: 101-103
(in Russian).
GAMBLE, W. (1975) Mechanism of action of hypolipidemic and
herbicidal aryloxy acids. J. theor. Biol., 54: 181-190.
GAVRILOVA, L.I. (1965) Experimental substantiation of the
permissible concentration of 2,4-dichlorophenoxy-gamma-butyric (DB)
acid in water bodies. Hyg. Sanit., 29(1): 11-16.
GELDMACHER- VON MALLINCKRODT, M., & LAUTENBACH, L. (1966) [Two
fatal poisonings (suicides) with chlorinated phenoxyacetic acids
(2,4-D and MCPA).] Arch. Toxicol., 21: 261-278 (in German).
GLUCK, S.J. & MELCHER, R.G. (1980) Concentration and
determination of the propylene glycol butyl ether esters of
2,4-dichlorophenoxyacetic acid (PGBE 2,4-D) in air. Am. Hyg. Ass.
J., 41(12): 932-934.
GOLDSTEIN, N.P. & BROWN, J.R. (1960) Peripheral neuropathy.
J. Am. Med. Assoc., 173: 171.
GOLDSTEIN, N.P., JONES, P.H., & BROWN, J.R. (1959) Peripheral
neuropathy after exposure to an ester of dichlorophenoxyacetic
acid. J. Am. Med. Assoc., 171: 1306-1309.
GOLDWATER, L.J. (1960) Peripheral neuropathy. J. Am. Med.
Assoc., 173(1): 171.
GORSHKOV, A.I. (1972) Hygienic evaluation of chlorocholine
chloride (Preparation CCC) and its combination with 2,4-D amine
salt. Hyg. Sanit., 36(10-12): 208-211.
GOWEN, J.A., WIERSMA, G.B., & TAI, H. (1976) Mercury and 2,4-D
levels in wheat and soils from sixteen states, 1969. Pestic. Monit.
J., 10(3): 111-113.
GRAFF, G.L.A., GUENING, C., & VANDERKELEN, B. (1972) Influence de
l'acide 2,4-dichlorophénoxyacéticque (2,4-D), un agent myotonisant,
sur le métabolisme des phosphates acidosolubles des muscle
squelettiques du rat. C. R. Soc. Biol. (Paris), 166(II):
1565-1568.
GRANROTH, G., HAKAMA, M., & SAXEN, L. (1977) Defects of the
central nervous system in Finland: I. Variations in time and
space, sex distribution, and parental age. Br. J. prev. soc.
Med., 31: 164-170.
GRANROTH, G., HAAPAKOSKI, J., & SAXEN, L. (1978) Defects of the
central nervous system in Finland: V. Multivariate analysis of
risk indicators. Int. J. Epidemiol., 7(4): 301-308.
GRANT, W. (1976) 2,4-D. In: Hart, R.W., Kraybill, H.F., &
DeSerres, F.J., ed.: A rational evaluation of pesticidal vs
mutagenic/carcinogenic action. Bethesda, Maryland, National
Institute of Health (Publication No. (NIH) 78-1306 U.S. Department
of Health, Education & Welfare, Public Health Service).
GRAY, T.J.B., LAKE, B.G., BEAMAND, J.A., FOSTER, J.R., & GANGOLLI,
S.D. (1983) Peroxisome proliferation in primary cultures of rat
hepatocytes. Toxicol. appl. Pharmacol., 67: 15-25
GREEN, S. & MORELAND, F.S. (1975) Cytogenic evaluation of
several dioxins in the rat. Toxicol. appl. Pharmacol., 33: 161.
GRIBANOV, O.I. (1968) Pollution of an open water source at
Shortandy Railroad Station by the herbicide 2,4-D butyl ester.
Hyg. Sanit., 32: 267-268.
GRIGSBY, B.H. & FARWELL, E.D. (1950) Some effects of herbicides
on pasture and on grazing livestock. Mich. Agric. Exp. St. Bull.,
32(3): 378-385.
GROLLEAU, G., DE LAVAUR, E., SIOU, G., & FROUX, Y. (1974) Effects
of 2,4-D on the reproduction of quail and partridge after the
application of the product by spraying on eggs. Ann. Zool. Ecol.
anim., 6(2): 313-331.
GROVER, R. (1976) Relative volatilities of ester and amine forms
of 2,4-D. Weed Sci., 24: 26-28.
GROVER, R. & KERR, L.A. (1981) Evaluation of polyurethane foam as
a trapping medium for herbicide vapour in air monitoring and worker
inhalation studies. J. environ. Sci. Health, B 16(1): 59-66.
GROVER, R. & SMITH, A.E. (1974) Adsorption studies with the acid
and dimethylamine forms of 2,4-D and dicamba. Can. J. Soil Sci.,
54: 179-186.
GROVER, R., MAYBANK, J., & YOSHIDA, K. (1972) Droplet and vapour
drift from butyl ester and dimethylamine salt of 2,4-D. Weed Sci.,
20: 320-324.
GROVER, R., KERR, L.A., WALLACE, K., YOSHIDA, K., & MAYBANK, J.
(1976) Residues of 2,4-D in air samples from Saskatchewan
1966-1975. J. environ. Sci. Health, B 11(4): 331-347.
GRUNOW, W. & BOHME, C. (1974) [On the metabolism of 2,4,5-T and
2,4-D in rats and mice.] Arch. Toxicol., 32: 217-225 (in German).
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1971) Determination of
some acidic herbicides by thin layer chromatography. J. agric.
food Chem., 19: 1181-1182.
GUARINO, A.M. & ARNOLD, S.T. (1979) Xenobiotic transport
mechanisms and pharmacokinetics in the dogfish shark. In: Khan,
M.A.Q., Lech, J.J., & Menn, J.J., ed. Pesticide and xenobiotic
metabolism in aquatic organisms. Washington, DC, American Chemical
Society, pp. 250-258.
GUMMER, W.D. (1980) Pesticide monitoring in the prairies of
western Canada. Environ. Sci. Res., 16: 345-372.
GUNTHER, F.A. (1962) Instrumentation in pesticide residue
determination. In: Metcalf, R.L., ed. Advances in Pest Control
Research, 5, New York, Interscience Publishers, pp. 191-319.
GUSEVA, E.N. (1956) [On the pharmacology of 2,4-dichloro-
phenoxyacetic acid.] Farmakol. Toksikol. (Mosc.), 19(4):
41-44 (in Russian).
GUTENMANN, W.H. & LISK, D.J. (1965) Conversion of 4-(2,4-DB)
herbicide to 2,4-D by bluegills. NY Fish Game J., 12(1): 108-111.
GUTENMANN, W.H., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1963)
Disappearance of 4(2,4-dichlorophenoxy-butyric) acid herbicide in
the dairy cow. J. dairy Sci., 46: 991-992.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The effects of
phenoxy herbicides on the hatchability of eggs and the viability of
the chicks. Acta pharmacol. toxicol., 35: 300-308.
HACQUE, R., DEAGEN, J., & SCHMEDDING, D. (1975) Binding of
2,4-dichloro- and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. A proton magnetic resonance study. J. agric. food Chem.,
23: 763-766.
HALLIOP, J., TOCHMAN, A., & LATALSKI, M. (1980) [Ultrastructural
investigations and myeloperoxidase determinations in rat
neutrophils in acute poisoning with 2,4-dichlorophenoxyacetic
acid.] Acta haematol. Pol., 11(4): 249-257 (in Polish).
HALTER, M. (1980) 2,4-D in the aquatic environment In: Shearer,
R., & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlobenil, diquat & endothall, Seattle
Municipality of Metropolital Seattle, January 1980, pp. 89-182.
HANIFY, J.A., METCALF, P., NOBBS, C.L., & WORSLEY, K.J. (1980)
Congenital malformations in the newborn in Northland: 1966-1977.
New Zealand med. J., 92: 245-248.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquito fish, Gambusa
affinis. Bull. environ. Contam. Toxicol., 8(1): 46-51.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973) Avoidance of
pesticides by grass shrimp (Palaeomonetes pugio). Bull. environ.
Contam. Toxicol., 9(3): 129-133.
HANSEN, W.H., QUAIFE, M.L., HABERMANN, R.T., & FITZHUGH, O.G,
(1971) Chronic toxicity of 2,4-dichlorophenoxyacetic acid in rats
and dogs. Toxicol. appl. Pharmacol., 20: 122-129.
HARDELL, L. & SANDSTRÖM, A. (1979) Case-control study: soft
tissue sarcomas and exposure to phenoxyacetic acids or
chlorophenols. Br. J. Cancer, 39: 711.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 43: 169-176.
HARRISON, J.W.E. & REES, E.W. (1946) 2,4-D toxicity - I.
Toxicity towards certain species of fish. Am. J. Pharm., 118:
422-425.
HASS, J.R., FRIESEN, M.D., & HOFFMAN, M.K. (1981) Recent mass
spectrometric techniques for the analysis of environmental
contaminants. In: McKinney, J. D., ed. Environ. Health Chem.,
Ann Arbor, Arbor Sciences, pp. 219-243.
HATCH, T.F. & GROSS, P. (1964) Pulmonary desposition and
retention of inhaled aerosols. New York, London, Academic Press,
XIV + 192 pp (American Industrial Hygiene Association Monograph
Series on Industrial Hygiene).
HAYES, W.J. (1982) 2,4-D. In: Pesticides studied in man.
Baltimore & London, Williams & Wilkins.
HEENE, R. (1966) [Histochemical proof of phosphorylase inhibition
by 2,4-D in the skeletal muscles and liver of the rat.]
Naturwissenschaften, 53: 308 (in German).
HEENE, R. (1967) [Inhibition of glycogen-forming enzymes by
2,4-D.] Histochemie, 8: 45-53 (in German).
HEENE, R. (1968) Histochemical and morphological findings in
experimental 2,4-D myopathy in warmblooded animals. Acta
neuropathol., 10: 166-169.
HEENE, R. (1975) Experimental myopathies and muscular dystrophy.
Studies in the formal pathogenesis of the myopathy of 2,4-D
acetate. Schriftenr. Neurol. Ser., 16: 1-97.
HENSHAW, B., QUE HEE, S.S., SUTHERLAND, R.F., & LEE, C.D. (1975)
Gas-liquid chromatography and gas-liquid chromatography combined
with mass spectrometry of a butyl ester formulation of 2,4-
dichlorophenoxy-acetic acid. J. Chromatogr., 106: 33-39.
HERBICH, J. & MACHATA, G. (1963). [Poisoning with
2,4-dichlorophenoxy-acetic acid (2,4-D).] Beitr. gerichtl. Med.,
22: 133-139 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies to determine
the toxic effects of derivatives of 2,4-dichloro- and 2,4,5-
trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated eggs
of pheasants, quails, and chickens. First Report.] Anz.
Schaedling-sk. D. Pflanzenschutz Umweltschutz, 49: 21-25 (in
German).
HILBIG, V., LUCAS, K., SEBEK, V., & MUNCHOW, H. (1976b) [Studies
to determine the toxic effects of derivatives of 2,4-dichloro- and
2,4,5-trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated
eggs of pheasants, quails, and chickens. Second report.] Anz.
Schaedlingsk. D. Pflanzenschutz Umweltschutz, 49: 65-68 (in
German).
HILL, E.V. & CARLISLE, H. (1947) Toxicity of 2,4-D for
experimental animals. J. ind. Hyg. Toxicol., 29(2): 85-95.
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized
fish eggs and fry. Trans. Am. Fish. Soc., 96(4): 414-416.
HOFMANN, W.W., ALSTON, W., & ROWE, G. (1966) A study of
individual neuro-muscular junctions in myotonia.
Electroencephalogr. clin. Neuro-physiol., 21: 521-537.
HOGSTEDT, C. & WESTERLUND, B. (1980) Cohort studies of cause of
death of forest workers with and without exposure to phenoxy
preparations. Läkartidninger, 77(19): 1828-1831.
HOGSTEDT, B., KOLNIG, A.M., MITELMAN, R., & SKERFVING, S. (1980)
Cytogenetic study of pesticides in agricultural work. Hered.,
92: 177-178.
HOLCOMBE, G.W., FIANDT, J.T., & PHIPPS, G.L. (1980) Effects of pH
increases and sodium chloride additions on the acute toxicity of
2,4-dichlorophenol to the fathead minnow. Water Res., 14(8):
1073-1077.
HOLMBERG, P.C. (1979) Central nervous system defects in children
born to mothers exposed to organic solvents during pregnancy.
Lancet, 2: 177-179.
HOOPER, F.N. (1958) The effect of applications of pelleted 2,4-D
upon the bottom fauna of Kemt Lake, Oakland County, Michigan. Proc.
Ann. Meet. North Centr. Weed Control Conf., 41.
HORNER, J., QUE HEE, S.S., & SUTHERLAND, R.G. (1974)
Esterification of (2,4-dichlorophenoxy) acetic acid - a
quantitative comparison of esterification techniques Anal. Chem.,
46: 110-112.
HUCKINS, J.N., STALLING, D.C., & SMITH, W.A. (1978) Foam-charcoal
chromatography for the analysis of polychlorinated dibenzodioxins
in Herbicide Orange. J. Assoc. Anal. Chem., 61: 32-38.
HUFF, J.E., MOORE, J.A., SARACCI, R., & TOMATIS, L. (1980) Long
term hazards of polychlorinated dibenzodioxins and polychlorinated
dibenzofurans. Environ. Health Persp., 36: 221-240.
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to
bluegill sunfish of phenoxy herbicides. Weeds, 11(1): 50-53.
HUNT, L.M., GILBERT, B.N., & PALMER, J.S. (1970) Effects of a
herbicide, 2-ethylhexyl ester of 2,4-D on magnesium: calcium ratios
and blood urea nitrogen levels in sheep and cattle. Bull. environ.
Contam. Toxicol., 5(1): 54-60.
IARC (1977) 2,4-D and esters. In: Some fumigants, the herbicides
2,4-D and 2,4,5-T, chlorinated dibenzodioxins, and miscellaneous
industrial chemicals. Lyons, International Agency for Research on
Cancer, pp. 111-138 (IARC monograph on the evaluation of the
carcinogenic risk of chemicals to man Vol. 15).
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. Lyons, International Agency for
Research on Cancer, p. 101 (IARC Monographs of 1-29, Supplement 4).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer, Vol. 30.
ILO (1977) Safe use of pesticides, Geneva, International
Labour Office (Occupational Safety and Health Series, No. 38).
ILO (1979) Guide to health and hygiene in agricultural work,
Geneva, International Labour Organisation, pp. 309.
INGELOG, T., ERNE, K., PAULSSON, A.M., JONASSON, H., & HOLMGREN, A.
(1977) [Taste of flavour changes in woodland berries after
herbicide spraying.] Vr Föda, 29(6): 1-16 (in Swedish).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, L.H.,
& GART, J.J. (1969) Bioassay of pesticides and industrial
chemicals for tumorigenicity in mice: A preliminary note. J. Natl
Cancer Inst., 42: 1101-1114.
IYER, V., WHITING, M., & FENICHEL, G. (1976) Neural influence in
experimental myotonia. Neurology, 26: 384.
JACKSON, J.W. & THOMAS, T.C. (1978) A simple and inexpensive
method for sampling 2,4-D and 2,4,5-T herbicides in air. J. Air
Pollution Control Assoc., 28(11): 1145-1147.
JAMES, M.O. (1979) Dispositon of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Palinurus argus).
Pharmacologist, 21(3): 251.
JAMES, W.H. (1977) Clomiphene, acencephaly, and spina bifida.
Lancet, 1: 603.
JANERICH, D.T. (1973) Epidemic waves in the prevalence of
anencephally and spina bifida in New York State. Teratology, 8:
253-256.
JENSEN, D.J. & GLAS, R.D. (1981) Analysis for residues of acidic
herbicides. In: Moye, H.A., ed. Analysis of pesticide residues,
New York, Chichester, Brisbane, Toronto, John Wiley & Sons, pp.
223-261.
JENSSEN, D. & RENBERG, L. (1976) Distribution and cytogenetic
test of 2,4-D and 2,4,5-T phenoxyacetic acids in mouse blood
tissues. Chem. biol. Interactions, 14: 291-299.
JOHNSON, J.E. (1971) The public health implications of widespread
use of the phenoxy herbicides and picloram. Bioscience, 27(17):
899-905.
JOHNSON, E.R., YU, T.C., & MONTGOMERY, M.L. (1977) Trapping and
analysis of atmospheric residues of 2,4-D. Bull. environ. Contam.
Toxicol., 17: 369-372.
JOHNSON, R.D. & MANSKE, D.D. (1976) Pesticide residues in total
diet samples (IX) Pestic. Monit. J., 9(4): 157-168.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples - (I) - August 1974 - July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1981a)
Pesticide, heavy metal and other chemical residues in infant and
total diet samples - (II) - August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-53.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b)
Pesticide, heavy metal, and other chemical residues in adult total
diet samples (XII) - August 1975 - July 1976. Pestic. monit. J.,
15(1): 54-69.
JUNG, H.D. & WOLF, F. (1977) [Contact eczemas due to forestry use
of the herbicide SELEST100.] Dtsch. Gesundheit., 32(31): 1464-1467
(in German).
JUZWIAK, I., JUZWIAK, J., SUCHOWIAK, J., & ORDA, E. (1973) [A
study of the extent of the pesticide threat to the health of
agricultural employees in Olesnicki Country.] Pol. Tyg. Lek.,
28(11): 419-422 (in Polish, Health & Welfare Canada Translation
No. 2157).
KAISER, H. (1973) Guiding concepts relating to trace analysis.
Pure appl. Chem., 34: 35-61.
KARKINEN-JAASKELAINEN, M. & SAXEN, L. (1974) Maternal influenza,
drug consumption, and congential defects of the central nervous
system. Am. J. Obstet. Gynecol., 118(6): 815-818.
KASKEVICH, L.M. & SOBOLOVA, L. P. (1978) [A case of acute
poisoning with herbicide 2,4-D (late after effects).] Gig. Tr.
Prof. Zabol., 22 (10): 49-50 (in Russian, US National
Translations Center, Translation No. 79-11916-06T).
KAS'JANENKO, A. G. & KOROLEVA, N. S. (1979) [An evaluation of the
genetic danger of pesticides.] Izv. Akad. Nauk SSSR Ser. Biol.,
3: 401-409 (in Russian).
KATEMAN, G. & PIJPERS, F.W. (1981) Quality Control in analytical
chemistry, New York, John Wiley & Sons, 276 pp.
KATZ, M., LEGORE, R.S., WEITKAMP, D., CUMMINS, J., ANDERSON D., &
MAY, D.R. (1972) Effects on fresh water fish. J. Water Pollut.
Control Fed., 44(6): 122-1250.
KAY, J.H., PALAZZOLO, B.S., & CALANDRA, J.C. (1965) Subacute
dermal toxicity of 2,4-D. Arch. environ. Health, 11: 648-651.
KENIGSBERG, Y.E. (1968) [Model myotonia in rats induced by
means of the diethylamine salt of 2,4-dichlorophenoxyacetic acid.]
Dok. Akad. Nauk Beloruss. SSR, 12(5): 473-475 (in Russian, Health &
Welfare Canada Translation No. 2440).
KETCHERSID, M.L., FLETCHALL, O.H., SANTELMANN, P.W., & MERKLE, M.G.
(1970) Residues in sorghum treated with the isooctyl ester of
2,4-D. Pestic. monit. J., 4(3): 111-113.
KHALATKAR, A.S. & BHARGAVA, Y.R. (1982) 2,4-dichlorophenoxy-
acetic acid. A new environmental mutagen. Mutat. Res., 103:
111-114.
KHANNA, S. & FANG, S. (1966) Metabolism of C14-labelled
2,4-dichlorophenoxyacetic acid in rats. J. agric. Food Chem.,
14: 500-503.
KHANNA, R.N. & KOHLI, J.D. (1977) Metabolism of chlorophenoxy
herbicides in man. In: Zaidi, S. H., ed. Proceedings of the
International Symposium on Industrial Toxicology, Lucknow, India,
November 1975, Lucknow, Industrial Toxicology Research Centre,
pp. 590-599.
KHERA, K.S. & McKINLEY, W.P. (1972) Pre- and postnatal studies on
2,4,5-T, 2,4-D and their derivatives in rats. Toxicol. appl.
Pharmacol., 22: 14-28.
KHERA, K.S. & RUDDICK, J.A. (1973) Polychlorodibenzo- p-dioxins.
Perinateal effects and the dominant lethal test in Wistar rats. In:
Blair, E.H., ed. Chlorodioxins - origin and fate. Washington, DC,
American Chemical Society, pp. 70-84 (Advances in Chemistry Series
No. 120).
KHIBIN, L.S., TAITSEL', L.A., & SLATOV, I.V. (1968) [On the
clinical aspects of acute poisoning with Dicotex 40.] Gig. Tr.
Prof. Zabol., 3: 52-53 (in Russian, Health & Welfare Canada
Translation No. 2240).
KIMBROUGH, R.D., ed. (1980) Halogenated biphenyls, terphenyls,
naphthalenes, debenzodioxins and related products. Amsterdam,
New York, Oxford, Elsevier, North Holland Biomedical Press.
KING, J.E. & PENFOUND, W.T. (1946) Effects of two of the new
formagenic herbicides on bream and largemouth bass. Ecology,
27(4): 327-374.
KING, C.T.G., HORIGAN, E.A., & WILK, A.L. (1971) Screening of the
herbicides 2,4,5-T and 2,4-D for cleft palate. Teratology, 4(2):
233.
KLEKOWSKI, R.Z. & ZVIRGZDS, J. (1971) [The influence of herbicide
2,4-D Na on respiration and survival of Simocephalus vetulus O.F.
Muller (Cladocera).] Pol. Arch. Hydrobiol., 18(4): 393-400 (in
Polish).
KLINGMAN, D.L., GORDON, C.H., YIP, G., & BURCHFIELD, H.P. (1966)
Residues in the forage and milk from cows grazing forage treated
with esters of 2,4-D. Weeds, 14: 164-167.
KNUTSON, J.C. & POLAND, A. (1980) Keratinization of mouse
teratoma cell line xB produced by 2,3,7,8-tetrachlorodibenzo- p-
dioxin: an in vitro model of toxicity. Cell, 22: 27-36.
KOCIBA, R.J. & SCHWETZ, B.A. (1982) A review of the toxicity of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) with a comparison to the
toxicity of other chlorinated dioxin isomers. Assoc. Food Drug
Offic. Q. Bull., 46(3): 168-188.
KOHLI, J.D., KHANNA, R.N., GUPTA, B.N., DHAR, M.M., TANDON, J.S., &
SIRCAR, K.P. (1974) Absorption and excretion of 2,4-
dichlorophenoxyacetic acid in man. Xenobiotica, 4(2): 97-100.
KOLBERG, J., HELCELAND, K., & JONSEN, J. (1973) Binding of
2,4-dichloro and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. Acta pharmacol. toxicol., 33(5-6): 470-475.
KOLMODIN-HEDMAN, B., ERNE, K., HÜKANSSON, M., & ENGQUIST, A. (1979)
[Control of occupational exposure to phenoxyacids (2,4-D and
2,4-5-T).] Arbete och Hälsa, 17: 3-26 (in Swedish).
KOLMODIN-HEDMAN, B. & ERNE, K. (1980) Estimation of occupational
exposure to phenoxy acids (2,4-D and 2,4,5-T). Arch. Toxicol.,
(Suppl. 4): 318-321.
KOLMODIN-HEDMAN, B., ERNE, K., & AKERBLOM, M. (1980) Field
application of phenoxy acid herbicides. In: Tordoir, W.F. &
Van Heemstra, E.A.H., ed. Field workers exposure during pesticide
application. New York, Elsevier Scientific Publishing Co., pp.73-77
(Studies in Environmental Science, Vol. 7).
KONSTANTINOVA, T.K. (1970) Toxicology and some problems of the
embryotropic action of the butyl ester of 2,4-D. In: Vygodchikov,
G.V., ed. Proceedings of a Conference on Problems on Hygiene and
Toxicology of Pesticides, USSR, 1967, Moscow, Meditsina, pp.
177-179.
KONSTANTINOVA, T.K., EFIMENKO, L.P., & ANTONEKO, T.A. (1975)
[Embryotropic effects of the products from the decomposition of
herbicides based on 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
11: 102-105 (in Russian, US National Translation Centre,
Translation No. 78-14230-06F.)
KONTEK, M., MARCINKOWSKA, B., & PIETRASZEK, Z. (1973) [Electro-
encephalographic investigations in farm workers exposed to
derivatives of arylakanocarboxylic acids.] Pol. Tyg. Lek.,
28(25): 937-939 (in Polish, Health & Welfare Canada
Translation No. 2215).
KOPISCHKE, E.D. (1972) The effect of 2,4-D and diesel fuel on egg
hatchability. J. Wildl. Manage., 36(4): 1353-1356.
KORTE, C. & JALAL, S.M. (1982) 2,4-D-induced clastogenicity and
elevated rates of sister chromatid exchanges in cultural human
lymphocytes. J. Hered., 73: 224-226.
KOSCHIER, F.J., GIRAD, P., & HONG, S.K. (1978) Transport of
2,4-dichlorophenoxyacetate by rat renal cortical slides. Toxicol.
appl. Pharmacol., 45: 883-894.
KOSCHIER, F.J. & PRITCHARD, J.B. (1979) Excretion of
2,4-dichlorophenoxyacetate (2,4-D) by the dogfish shark (Squalus
acanthias). Pharmacologist, 21(3):184.
KOSYAN, S.A., MARKOSYAN, V.E., SARKISYAN, L.S., & MANUKYAN, R.A.
(1974) [A toxicological evaluation of the new herbicide 3-nitro-4-
oxybenzyl ether of (2,4-dichlorophenoxy) acetic acid (NK-2).]
Zh. eksp. klin. Med., 14(3): 14-18 (in Russian, Health & Welfare
Canada Translation No. 2436).
KRAMER, D. & SCHMALAND, G. (1974) [Plant protection agent
residues in water: Problems in water management as a consequence of
biocide applications.] Wasserwirtsch. Wassertech., 24(5): 161-167
(in German).
KUHN, E. & STEIN, W. (1964) [Experimental myotonia with 2,4-D in
the rat.] Klin. Wochenschr., 42: 1215-1216 (in German).
KUHN, E. & STEIN, W. (1965) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D) in the rat. In vitro and in vivo
studies on the effects of 2,4-D on the energy metabolism of
muscle.] Klin. Wochenschr., 43: 673-677 (in German).
KUHN, E. & STEIN, W. (1966) [Model myotonia with 2,4-
dichlorophenoxyacetate (2,4-D).] Klin. Wochenschr., 44: 700-702
(in German).
KUZMINSKAJA, U.A. & BERSAN, L.V. (1975) [The effects of the
sodium salt of dichlorophenoxyacetic acid on the glycolysis,
ATPase, and transketolase activity of erythrocytes.] Farmakol.
Toksikol., 38(1): 102-104 (in Russian).
KUZYK, A. (1979) A report on a survey of the Alberta farming
community respecting the use of pesticides, Edmonton, Alberta
Department of the Environment, Pollution Control Division.
LAPPE, M. (1979) HLA homozygosity and neural-tube defects.
Lancet, 1: 1342.
LASKOWSKI, M.B. & DETTBARN, W.D. (1977) The pharmacology of
experimental myopathies. Ann. Rev. Pharmacol. Toxicol., 17:
387-409.
LAVY, T.L. ROETH, F.W., & FENSTER, C.R. (1973) Degradation of
2,4-D and atrazine at three soil depths in the field. J. Environ.
Qual., 2(1): 132-137.
LAVY, T.L., WALSTAD, J.D., FLYNN, R.R., & MATTICE, J.D. (1982)
(2,4-dichlorophenoxy) acetic acid exposure received by aerial
applications crew during forest spray operations. J. agric. food
Chem., 30: 373-381.
LEE, B. (1978) Herbicides under suspicion in Australia. New
Sci., 80(1125): 159.
LENG, M. (1972) Residues in milk and meat and safety to livestock
from the use of phenoxy herbicides in pasture and rangeland. Down
Earth, 28(1): 12-20.
LENG, M. (1977) Comparative metabolism of phenoxy herbicides in
animals. In: Ivie, G.W. & Dorough, H.W., ed. Fate of pesticides in
large animals, New York, San Francisco, London, Academic Press,
pp. 53-76.
LENG, M.L. (1979) Comparative toxicology of various chlorinated
dioxins as related to chemical structure. In: Bontoyan, W.R., ed.
Collaborative International Pesticides Analytical Council
Proceedings Symposium Series I, Cambridge, Heffers Printers Ltd.,
pp. 141-169.
LENG, M.L., LAVY, R.L., RAMSEY, J.C., & BRAUN, W.H. (1982)
Review of the studies with 2,4,5-T in humans including applicators
under field conditions, pp. 133-156. In: Plimmer, J.R., ed.:
Pesticide residues and exposure. Washington, DC, American Chemical
Society, (ACS Symposium Series 182).
LHOSTE, J. & ROTH, P. (1946) [The effect of aqueous sodium
2,4,-dichlorophenoxyacetate on the development of eggs of Rana
temporaria L.] C.R. Soc. Biol. Paris, 140: 272-274.
LIBICH, S., TO, J.C., WANG, K. Y., & WATSON, D. A. (1981) A
study to assess the occupational exposure to 2,4-D herbicides,
Toronto, Safety Services Department, Health and Safety Division.
pp. 16 (Ontario Hydro. Report SSD-81-1).
LINDQUIST, N.G. & ULLBERG, S. (1971) Distribution of the
herbicides 2,4,5-T and 2,4-D in pregnant mice. Accumulation in the
yolk sac epithelium. Experientia (Basel), 27: 1439-1441.
LINNAINMAA, K. (1983a) Non-mutagenicity of phenoxyacid herbicides
2,4-D and MCPA. In: Keith, L.H., Choudhary, G., & Rappe, C., ed.:
Chlorinated dioxins and dibenzofurans in the total environment.
Ann Arbor, Ann Arbor Sci. Publ. Inc., Vol. 1.
LINNAINMAA, K. (1983b) Induction of sister chromatid exchanges by
the peroxisome proliferators 2,4-D, MCPA, and clofibrate in vivo
and in vitro. Carcinogenesis, accepted for publication.
LIPSCOMB, G.Q. (1968) Pesticide residues in prepared baby foods
in the United States. Pestic. monit. J., 2(32):104-108.
LISK, D.J., GUTENMANN, W.H., BACHE, C.A., WARNER, R.G., & WAGNER,
D.G. (1963) Elimination of 2,4-D in the urine of steers fed 4
(2,4-DB) or 2,4-D. J. dairy Sci., 46: 1434-1435.
LIU, L.C. & CIBES-VIADE, H.R. (1973) Adsorption of fluometuron,
prometryne, Sencor and 2,4-D by soils. Univ. Puerto Rico, J.
Agric., 57: 286-293.
LOKKE, H. (1975) Analysis of free and bound chlorophenoxy-acids
in cereals. Bull. environ. Contam. Toxicol., 13: 730-736.
LOKTIONOV, V.N., BUDARKOV, V.A., KUZNETSOV, A.E., & KUZNETSOVA,
N.V. (1973) [Toxicological characteristics of 2,4-D derivatives.]
Veterinariya, 5: 107-109 (in Russian, Health & Welfare Canada
Translation No. 2173).
LONDONO, F. (1966) [Occupational acne. Five cases caused by
herbicide.] Med. cutanea, 3: 225-232 (in Spanish).
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMANN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. J. Am. Ind. Hyg.
Assoc., 20(3): 298-304.
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel
Dekker, pp. 1-49.
LOOS, M.A. (1975) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Herbicides: Chemistry, degradation and mode of
action, New York, Marcel Dekker, pp. 1-128.
LOPEZ, M.R. (1961) [Action of 2,4-dichlorophenoxyacetic acid and
its sodium salt on the spermatozoa of the frog.] Biol. R. Soc.
Esp. Hist. Nat., 59: 219-226 (in Spanish).
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960a) Metabolism of plant
growth-regulators. I. 2,4-dichlorophenoxyacetic acid in leaves of
red- and blackcurrant. Ann. appl. Biol., 48: 613-625.
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960b) Metabolism of plant
growth-regulators. II. Decarboxylation of 2,4-dichloro-
phenoxyacetic acid in leaves of apple and strawberry. Ann. appl.
Biol., 48: 626-636.
LUKOSHKINA, L.P., GUESEINOVA, R.S., & MELIK-ZADE, T.M. (1970)
Study of certain aspects of lipid-protein-carbohydrate metabolism
in workers engaged in 2,4-D production. Trud. Azerbaid. Nauchno-
Issled. Instituta Gig. prof. Zabol., No.5: 146-149.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de l'herbicide
2,4-D sur le développement embryonaire et la fécondité du gibier ą
plumes. C.R. Acad. Sci. Paris, Ser. D., 271(25): 2418-2421.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1974) Sexualité et les pesticides.
Ann. Biol. anim. (Paris), 13(3-4): 173-185.
MAAS, W. & KERSSEN, M.C. (1973) Drift studies in ULV and
conventional applications. Agric. Aviation, 15: 41-50.
MACKENTHUN, K.M. & KEUP L.E. (1972) Freshwater macroinvertebrates.
J. Water Pollut. Control Fed., 44(6): 1137-1150.
MACLEAN, G.J. & DAVIDSON, J.H. (1970) Diagnosis of disorders in
pastured livestock. Down Earth, 25(4): 12-16.
MAGNUSSON, J., RAMEL, C., & ERIKSSON, A. (1977) Mutagenic effects
of chlorinated phenoxyacetic acids in Drosophila melanogaster.
Hereditas, 85: 121-123.
MAIER-BODE, H. (1971) [Herbicides and their residues.], Stuttgart,
FRG, Verlag Eugen Ulmer, 479 pp. (in German).
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VII). Pesticide Mont. J., 8(2): 110-124.
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VIII). Pesticide Mont. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples VII. Pestic. Monit. J., 9(2): 94-105.
MARTH, P.C. & MITCHELL, J.W. (1949) Comparative volatility of
various forms of 2,4-D. Bot. Gaz., 110: 632-636.
MARTYNOV, Y.E. (1970) The effect of 2,4-d compounds on wild warm
blooded animals. Lesn. Khoz., 6: 57-59 (in Russian, Health &
Welfare Canada Translation No. 2186).
MASON, R.W. (1975) Binding of some phenoxyalkanoic acids to
bovine serum albumin in vitro. Pharmacology, 13: 177-186.
MATSUNAKA, S. (1972) Metabolism of pesticides in higher plants.
In: Matsumura, F., Boush, G.M., & Mitsato, T., ed. Environmental
toxicology of pesticides, New York, Academic Press, pp. 341-365.
MAYBANK, J. & YOSHIDA, K. (1969) Delineation of herbicide-drift
hazards on the Canadian prairies. Trans. Am. Soc. Agric. Eng.,
12(6): 759-762.
MAYBANK, J., YOSHIDA, K., & GROVER, R. (1978) Spray drift from
agricultural pesticide applications. J. Air Poll. Control Assoc.,
28: 1009-1014.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979a) Changes of metabolism
during experimentally induced myotonia of rats. I. Alterations in
lactate and malate dehydrogenase isoenzyme activities. Biochem.
Med., 22: 350-358.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979b) Changes of metabolism
during experimentally induced myotonia of rats. II. Alterations in
creatinine kinase and acid phosphatase activities: a possible
mechanism in the development of muscle damage. Biochem. Med.,
22: 359-364.
MCARDLE, F.J., MARETZKI, A.N., WILEY, R.C., & MODREY, M.G. (1961)
Influence of herbicides on flavor of processed fruits and
vegetables. J. agric. food Chem., 9: 228-230.
MCCONNELL, E.E., MOORE, J.A., HASEMAN, J.K., & HARRIS, M.W. (1978)
The comparative toxicity of chlorinated dibenzo- p-dioxins in mice
and guniea pigs. Toxicol. appl. Pharmacol., 44: 335-356.
MCLENNAN, M.W. (1974) 2,4-D toxicity in diary cattle. Aust.
vet. J., 50: 578.
MEEHAN, W.R., NORRIS, L.R., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in Southeast Alaska.
J. Fish. Res. Board Can., 31(4): 480-485.
MELNIKOV, M.N. (1971) Chemistry of pesticides. Res. Rev.,
36: 165-166.
MIERZWA, S. & WITEK, S. (1977) Gas-liquid chromatographic
method with electroncapture detection for the determination of
residues of some phenoxyacetic acid herbicides in water as their
2,2,2-trichloroethyl esters. J. Chromatogr., 136: 105-111.
MILHAUD, G., RIET ALVARIZA, F., CHARLES, E., & SABBACH, M. (1970)
Toxicologie des phytohormones. Rec. Méd. Vet., 146: 345-357.
MITCHELL, J.W., HODGSON, R.E., & GAETJENS, C.F. (1946) Tolerance
of farm animals to feed containing 2,4-dichloro-phenoxyacetic acid.
J. anim. Sci., 5(1): 226-232.
MONARCA, G. & DIVITO, G. (1961) [On acute poisoning by a herbicide
(2,4-dichlorophenoxyacetic acid).] Folia med. (Naples), 44(6):
480-485 (in Italian, Health & Welfare Canada Translation No. 2234).
MONTGOMERY, M.L., CHANG, U.L., & FREED, V.H. (1971) Comparative
metabolism of 2,4-D by bean and corn plants. J. agric. food Chem.,
19: 1219-1221.
MOREALE, A. & VAN BLADEL, R. (1980) Behaviour of 2,4-D in Belgian
soils. J. environ. Qual., 9: 627-633.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
microbial reversion assay systems. Mutat. Res., 116: 185-216.
MORRE, D.J. & ROGERS, B.J. (1960) The fate of long chain esters
of 2,4-D in plants. Weeds, 8: 436-447.
MORTON, H.L., ROBINSON, E.D., & MEYER, R.T. (1967) Persistence of
2,4-D, 2,4,5-T, and dicamba in range forage grasses. Weeds,
15: 268-271.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxy-
ethane ester of 2,4-D. Trans. Am. Fish. Soc., 96(2): 185-193.
MUIR, C.S. & WAGNER, G. (1982) Directory of on-going research in
cancer epidemiology 1982, Lyons, International Agency for Research
on Cancer, (IARC Scientific publications No. 46) pp. 86 (Abstract
No. 227), 432(1146) 460(1221).
MUKULA, J., SILTANEN, H., ROSENBERG, C., IANPAAN-HEIKKILA, J., &
LALLUKKA, R. (1978) Phenoxy herbicide residues in cowberries,
mushrooms and the twigs of forest plants. Ann. Agric. Fenn.,
17: 23-31.
MUNRO, H.E. (1972) Determination of 2,4-dichloro-phenoxyacetic
acid and 2,4,5-trichlorophenoxyacetic acid in tomato plants and
other commercial crops by microcoulometric gas chromatography.
Pestic. Sci., 3: 371-377.
MURTHY, M.S.S. (1979) Induction of gene conversion in diploid
yeast by chemicals: Correlation with mutagenic action and its
relevance in genotoxicity screening. Mutat. Res., 64: 1-17.
NASH, R.G., KEARNEY, P.C., MAITLEN, J.C., SELL, C.R., & FERTIG,
S.N. (1982) Agricultural applicators' exposure to 2,4-
dichlorophenoxyacetic acid. In: Plimmer, J.R., ed. Pesticide
residues and exposure, Washington, DC, American Chemical Society,
pp. 119-132 (ACS Symposium Series 182).
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1981)
Polychlorinated dibenzo- p-dioxins: Criteria for their effects on
man and his environment, Ottawa, NRCC Publications Service, NRCC
Publication No. 18574, 251 pp.
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1978) Phenoxy
herbicides. Their effects on environmental quality, Ottawa, NRCC
Publications Service, NRCC publication no. 16075, 437 pp.
NAYRAC, P., FONTAN, P., WAROT, P., LESCUT, J., & DELAHOUSSE, J.
(1958) Polyradiculonévrite subaciguė chez deux agriculteurs:
intoxication par des produits anti-parasitaires. Lille Med.,
3(3): 161-164.
NESBITT, H.J. & WATSON, J.R. (1980a) Degradation of the
herbicide 2,4-D in river water. I. Description of the study
area, and survey of rate determining factors. Water Res.,
14(12): 1683-1688.
NESBITT, H.J. & WATSON, J.R. (1980b) Degradation of the
herbicide 2,4-D in river water. II. The role of suspended
sediment, nutrients, and water temperature. Water Res.,
14(12): 1689-1694.
NIELSEN, N.O. (1968) Teratogenic effects of hyperthermia in
chicken embryo. Preliminary report. Teratology, 1: 102-107.
NIELSEN, K., KAEMPE, B., & JENSEN-HOLM, J. (1965) Fatal
poisoning in man by 2,4-D: Determination of the agent in
forensic materials. Acta pharmacol. toxicol., 22: 224-234.
NIKANDROV, V.N. (1974). [The activity of malate dehydrogenase in
some organs and tissues of albino rats after administration of the
amine salt of 2,4-D,] Vesti Akad. Navuk Belaruskai SSR (Ser.
Biol.) 1: 126-127 (in Belorussian).
NISHIUCHI, Y. & YOSHIDA, K. (1974) [Toxicity of new agricultural
chemicals to tadpoles.] Bull. agric. Chem. Insp. St., 14: 66-68
(in Japanese).
NORSTROM, A., RAPPE, C., LINDAHL, R., & BUSTER, H.R. (1979)
Analysis of some older Scandinavian formulations of 2,4-D and
2,4,5-T for contents of chlorinated dibenzo- p-dioxins and
dibenzofurans. Scand. J. Work Environ. Health, 5: 375-378.
OLSON, B.A., SNEATH, T.C., & JAIN, N.C. (1978) Rapid, simple
procedures for the simultaneous gas chromatographic analysis
of four chlorophenoxy herbicides in water and soil samples.
J. agric. food Chem., 26: 640-643.
ORBERG, J. (1980a) Observations on the 2,4-dichlorophenoxy-
acetic acid (2,4-D) excretion in the goat. Acta pharmacol.
toxicol., 46: 78-80.
ORBERG, J. (1980b) Effects of low protein consumption on the
renal clearance of 2,4-dichlorophenoxyacetic acid (2,4-D) in goats.
Acta pharmacol. toxicol., 46: 138-140.
OSADCHUK, M., SALAHUB, E., & ROBINSON, P. (1977) Isolation of
chlorophenoxy herbicides on columns of glass beads. J. Assoc. Off.
Anal. Chem., 60: 1327-1327.
OSTERLOH, J., LOTTI, M., & POND, S.M. (1983) Toxicologic studies
in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. anal.
Toxicol., 7: 125-129.
OU, L.T., ROTHWELL, D.F., WHEELER, W.B., & DAVIDSON, J.M. (1978)
The effect of high 2,4-D concentrations on degradation and carbon
dioxide evolution in soils. J. environmental Qual., 7(2): 241-246.
PADEROVA, V.P. (1975) [Some hygienic problems of using 2,4-D
herbicides.] Gig. i Sanit., 40(9): 96-97 (in Russian).
PAGGIARO, P.L., MARTINO, E., & MARIOTTI, S. (1974) [On a case of
poisoning by 2,4-dichlorophenoxyacetic acid.] Med. lav., 65(3-4):
128-135 (in Italian, Health & Welfare Canada Translation No. 2200).
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens. In: Production Research Report, Washington,
DC, Agricultural Research Service, pp. 1-38.
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some
organic herbicides to cattle, sheep, and chickens. In: Production
Research Report, Washington, DC, Agriculture Research Service,
pp. 1-26.
PAL'MOVA, I.V. & GALUZOVA, L.V. (1963) [Maximum permissible
concentration of sodium salt and butyl ester formulations of
2,4-D in water.] Gig. i Sanit., 28(7): 11-13 (in Russian,
Health & Welfare Canada Translation No. 2717).
PEMBERTON, J.M. (1979) Pesticide degrading plasmids: a
biological answer to environmental pollution by phenoxyherbicides.
Ambio, 8: 202-205.
PHILLEO, W.W. & FANG, S.C. (1967) Effects of 2,4-D on metabolism.
J. agric. food Chem., 15(2): 256-260.
PIERIDES, A.M., ALVAREZ-UDE, F., KERR, D.N.S., & SKILLEN, A.W.
(1975) Clofibrate-induced muscle damage in patients with chronic
renal failure. Lancet, 2: 1279-1282.
PILINSKAJA, M.A. (1974) [Cytogenetic effect of the herbicide
2,4-D on human and animal chromosomes.] Tsitol. Genet., 8(3):
202-206 (in Russian).
POCCHIARI, F., SILANO, V., & ZAMPIER, A. (1979) Human health
effects from accidental release of tetrachlorodibenzo- p-dioxin
(TCDD) at Seveso, Italy. Ann. NY Acad. Sci., 320: 311-320.
PODOLAK, M. (1979) [Effect of paraquat and 2,4-dichloro-
phenoxyacetic acid (2,4-D) on oxidative phosphorylation and
respiration of brain mitochondria in the rat.] Bromatol. Chem.
Toksykol., 12(2): 147-152 (In Polish).
POLAND, A. & GLOVER, E. (1973) 2,3,7,8-Tetrachlorodibenzo- p-
dioxin: a potent inducer of S-aminolevulinic acid synthetase.
Sci., 179: 476.
POLAND, A. & GLOVER, E. (1973) Chlorinated dibenzo- p-dioxins:
potent inducers of -aminolevulinic acid synthetase and aryl
hydrocarbon hydroxylase. Molec. Pharmacol., 9: 736-747.
POLAND, A. GLOVER, E. (1976) Sterospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo -p-dioxin by hepatic cytosol.
J. Biol. Chem., 251(16): 4936-4946.
POLAND, A. & KENDE, A. (1976) 2,3,7,8-tetra-chlorodibenzo- p-
dioxin environmental contaminant and molecular probe. Fed. Proc.,
35: 2404-2411.
POLAND, A., SMITH, D., METTER, G., & POSSICK, P. (1971) A health
survey of workers in a 2,4-D and 2,4,5-T plant. Arch. environ.
Health, 22: 316-327.
PRAVDA, O. (1973) [On the influence of herbicides on some fresh-
water animals.] Hydrobiologia, 42(1): 97-142 (in German).
PREISS, D. & ROSSNER, J.A. (1971) [Effect of 2,4-
dichlorophenoxyacetic on the transverse tubular system of the
mycardium.] Naturwissenschaften, 11: 576-577 (in German).
PRESCOTT, I.F., PARK, J., & DARRIEN, I. (1979) Treatment of
severe 2,4-D and mecoprop intoxication with alkaline diuresis.
Br. J. clin. Pharmacol., 7: 111-116.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder.
J. Pharmacol. exp. Therap., 208(2): 280-286.
PRITCHARD, J.B. & MILLER, D.S. (1980) Teleost kidney in
evaluation of xenobiotic toxicity and elimination. Fed. Proc.,
39(14): 3207-3212.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis
in primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PROVINCE OF BRITISH COLUMBIA, MINISTRY OF ENVIRONMENT (1981)
A review of the use of 2,4-D for Eurasian water milfoil control in
the Okanagan Lakes, 1976-1980. Inf. Bull., aquatic Plant Manage.
Program, 10: 1-10.
QUE HEE, S.S. & SUTHERLAND, R.G. (1974) Purity of reagent grade
p- and o-chlorophenoxyacetic acids and its biological implications.
J. agric. food Chem., 22: 726-727.
QUE HEE, S.S. & SUTHERLAND, R.G. (1975) A specific gas-liquid
chromatographic method for analysis of some amine salts of 2,4-
dichlorophenoxyacetic acid. J. agric. food hem., 23: 1007-1008.
QUE HEE, S.S. & SUTHERLAND, R.G. (1981) The phenoxyalkanoic
herbicides, Vol. I. Chemistry, analysis and environmental
pollution, Boca Raton, CRC Press, Inc., 321 pp.
RADIONOV, A.D., CHUMACHENKO, A.N., & KIRILENKO, I.I. (1967)
The toxic properties of the herbicide 2,4-D. Hyg. Sanit.,
32(4-6): 116-118.
RAMEL, C. (1978) Chlorinated phenoxy acids and their dioxins.
Mode of action, health risks, and environmental effects. Ecol.
Bull. (Stockholm), 27: 302.
RAOUL, Y. & MARNAY, O. (1948) [Effect of 3-indolacetic acid
2,4-dichlorophenoxyacetic acid on the growing rat.] C. R. Acad.
Sci. (Paris), 226: 1043-1045.
RAPPE, C. & BUSER, H.R. (1981) Occupational exposure to
polychlorinated dioxins and dibenzofurans. In: Choudhary, G.,
Chemical hazards in the workplace: Measurement and control,
Washington, DC, American Chemical Society, pp. 319-342 (ACS
Symposium Series No. 149).
RASMUSON, B. & SVAHLIN, H. (1978) Mutagenicity tests of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid in genetically stable and unstable strains of Drosophila
melanogaster. Ecol. Bull. (Stockholm), 27: 190-192.
RAWLS, C.K. (1965) Field tests of herbicide toxicity to
certain estuarine animals. Chesapeake Sci., 6(3): 150-161.
REA, W.J. (1978) Environmentally triggered cardiac disease.
Ann. Allergy, 40(4): 243-251.
REDDY, J.K., AZARNOFF, D.L., & HIGNITE, E.E. (1980)
Hypolipidaemic hepatic peroxisome proliferators form a novel
class of chemical carcinogens. Nature (Lond.), 283: 397-398.
REDDY, J.K., QURESHI, S.A., LALWANI, N.D., REDDY, M.K., & MOEHLE,
C.M. (1982a) Induction by ciprofibrate of hepatic peroxisome
proliferation in rats, pigeons, chickens, cats and rhesus monkeys.
Fed. Proc., 41: 1741.
REDDY, J.K., LALWANI, N.D., REDDY, M.K., & QURESHI, S.A. (1982b)
Excessive accumulation of autofluorescent lipofuscin in the liver
during hepatocarcinogenesis by methyl clofenapate and other
hypolipidaemic peroxisome proliferators. Cancer Res., 42: 259-266.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977) Investigations
into the acute toxicity and some chronic effects of selected
herbicides and pesticides on several fresh water fish species.
Bull. environ. Contam. Toxicol., 18(3): 361-365.
RENBERG, L. (1974) Ion exchange technique for the determination
of chlorinated phenols and phenoxy acids in organic tissue, soil
and water. Anal. Chem., 46: 459-461.
RIIHIMAKI, V., ASP, S., & HERNBERG, S. (1982) Mortality of
chlorinated phenoxyacid herbicide 2,4-D and 2,4,5-T applicators in
Finland. First report of an ongoing prospective follow-up study.
Scand. J. Work Environ. Health, 8: 37-42.
RIVERS, J.B., YAUGER, W.L., Jr., & KLEMMER, H.W. (1970)
Simultaneous gas chromatographic determination of 2,4-D and
dicamba in human blood and urine. J. Chromatogr., 50: 334-337.
ROBINSON, E. & FOX, L.L. (1978) 2,4-D herbicides in central
Washington. J. Air Poll. Control Assoc., 28: 1015-1020.
ROBSON, T.O. (1966) Some studies of the persistence of 2,4-D in
natural surface waters in Britain. Proc. Br. Weed Control Conf.,
2: 594-597.
ROSENBERG, J. (1980) 2,4-Dichlorophenoxyacetic acid (2,4-D)
- Evaluation of human health hazards, Berkeley, California,
Hazard Alert System, Epidemiological Studies Laboratory, 64 pp.
ROSS, R.D. MORRISON, J., ROUNBEHLER, D.P., FAN, S., & FINE, D.H.
(1977) N-nitroso compound impurities in herbicide formulations.
J. agric. food Chem., 25: 1416-1418.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation
of the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUDIGER, K.D., MEERBACH, W., OSSKE, G., & FISCHER, W. (1972) [On
the experimental 2,4-dichlorophenoxyacetate myopathy.] Zentralbl.
allg. Pathol., 115: 145-152 (in German).
SADYKOV, R.E., RABOCHEV, V.K., & STROKOV, Y.N. (1972) [The
effect of butyl 2,4-D treatment of pastures on the reproductive
functions of sheep.] Zhivodnovodstvo, 34(1): 73-74 (in Russian).
SANDERS, H.O. (1970a) Pesticide toxicities to tadpoles of the
western chorus frog, Pseudacris triseriata and Fowler's toad, Burfo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. (1970b) Toxicities of some herbicides to six
species of freshwater crustaceans. J. Water Pollut. Control Fed.,
42(8): 1554-1550.
SARE, W.M. (1972) The weedicide 2,4-D as a cause of headaches and
diplopia. New Z. med. J., 75(478): 173-174.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & LEBEAU, J.E. (1976)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicol. appl. Pharmacol., 37(1): 136-137.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & GEHRING, P.J. (1977)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicology, 8: 3-11.
SAXEN, L., KLEMETTI, A., & HARO, A.S. (1974) A matched-pair
register for studies of selected congenital defects. Am. J.
Epidemiol., 100(3): 297-306.
SCHACTER, B., GYVES, M., MUIR, A., & TASIN, M. (1979) HLA-A, B
compatibility in parents of offspring with neural-tube defects or
couples experiencing involuntary fetal wastage. Lancet, 1: 796-797.
SCHILLER, K. (1964) [Effects on the fertility of rats produced by
substances with special effects in potato cultivation. 1st
communication: Growth hormones and weed control agents 2,4-D and
MCPA.] Landbauforsch. Völkenrode, 14(2): 111-114 (in German).
SCHILLINGER, J.E. (1960) [Hygienic evaluation of agricultural
products cultivated with the use of herbicides.] J. Hyg.
Epidemiol. Microbiol. Immunol., 4: 243-252 (in German).
SCHNEIDER, B.A. (1979) Toxicological evaluation of pesticides by
the EPA Chemical and Biological Investigations Branch Laboratories
(1966-77). In: Toxicology handbook, mammalian and aquatic data.
Book 1: Toxicology data. Report for 1966-67, Beltsville, Md., US,
Environmental Protection Agency.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8(3): 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues at
Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7(3/4):
146-152.
SCHUPHAN, W. (1963) [Impaired health due to plant protection
agents.] Der Landarzt, 39: 1217-1222 (in German).
SCHUPHAN, W. (1965) [Plant protection problems reflected in
quality testing.] Anz. Schädlingsk. D. Pflanzenschutz.
Umweltschutz, 38(7): 97-104 (in German).
SCHUPHAN, W. (1969) [Pesticides: Usefulness and possible harm.]
Zentralbl. Bakteriol., 210: 240-258 (in German).
SCHWETZ, B.A., SPARSCHU, G.L., & GEHRING, P.J. (1971) The effect
of 2,4-D and esters of 2,4-D on rat embryonal, foetal and neonatal
growth and development. Food cosmet. Toxicol., 9: 801-817.
SCHWETZ, B.A., NORRIS, J.M., SPARSHU, G.L., ROWE, V.K., GEHRING,
P.J., EMERSON, J.L., & GERBIG, C.G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect., Exp.
Issue 5: 87-99.
SEABURY, J.H. (1963) Toxicity of 2,4-dichlorophenoxyacetic acid
for man and dog. Arch. environ. Health, 7: 202-209.
SEILER, D. (1971) The ATPase of the sarcolemma of skeletal
muscle in experimental myotonia. Experientia (Basel), 27:
1170-1171.
SEILER, J.P. (1978) The genetic toxicology of phenoxy acids
other than 2,4,5-T. Mutat. Res., 55: 197-226.
SENCZUK, W. & POGORZELSKA, H. (1975) [Urinary excretion of
2,4-dichlorophenoxyacetic acid.] Rocz. Panstw. Zakl. Hig.,
26(2): 217-222 (in Polish, Health & Welfare Canada Translation
No. 2451).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxodynamic properties of phenoxycarboxylic acid derivatives, II.
Methods of determining derivatives of phenoxyacetic and
phenoxypropionic acids in blood and urine.] Rocz. Panstw. Zakl.
Hig., 32: 339-344 (in Polish).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxicodynamic properties of derivatives of phenoxyacetic and
phenoxypropionic acid, III. The pattern of absorption into the
bloodstream and measurements of urinary excretion of phenoxyacetic
and phenoxypropionic acid.] Roczn. Panstw. Zak. Hig., 32(5-6):
419-426 (in Polish).
SENCZUK, W., POGORZELSKA, H., & DEBSKA, M. (1980) [Absorption of
2,4-dichlorophenoxyacetic acid through the skin.] Roczn. Panstw.
Zak. Hig., 31(6): 611-614 (in Polish).
SENGES, J. & RDEL, R. (1972) Experimental myotonia in mammalian
skeletal muscle: changes in contractile properties. Pflueger.
Arch., 331: 315-323.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method for
determination of low levels of exposure to 2,4-D and 2,4,5-T.
Int. J. environ. anal. Chem., 1: 23-33.
SHEARER, R.W. (1980) Public health effects of the aquatic use of
herbicides: 2,4-D dichlobenil, endothall and diquat. In: Shearer,
R.W. & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlorbenil, diquat & endothall, Municipality of
Washington, US, Metropolitan Seattle, pp. 1-76.
SHEARER, R. & HALTER, M. (1980) Literature reviews of four
selected herbicides: 2,4-D, dichlobenil, diquat, and endothall.
Municipality of Washington, US, Metropolitan Seattle.
SHILLINGER, U.Y.I. & NAUMOVA, L.P. (1957) [Hygienic evaluation of
a harvest in connection with the treatment of crops with the
herbicide 2,4-D.] Gig. i Sanit., 7: 33-38 (in Russian).
SHIM, J.C. & SELF, L.S. (1973). Toxicity of agricultural
chemicals to larvivorous fish in Korean rice fields. Trop. Med.,
15(3): 123-130.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., & RAATIKAINEN, M.
(1981). Triclopyr, glyphosate and phenoxyherbicide residues in
cowberries, bilberries and lichen. Bull. Environ. Contam Toxicol.,
27: 731-737.
SIMPSON, G.R. (1982a) Pesticide exposure - New South Wales
Services. In: Education and safe handling in pesticide application,
studies in environmental science, 18: 209-214. Amsterdam,
Oxford, New York, Elsevier Scientific Publishing Co.
SITTIG, M. ed., (1980) Pesticide manufacturing and toxic
materials control encyclopedia. Park Ridge, NJ, USA, Noyes Data
Corp., pp. 229-234.
SKELLY, N.E., STEVENS, T.S., & MAPES, D.A. (1977) Isomer-specific
assay of ester and salt formulations of 2,4-dichlorophenoxyacetic
acid by automated high pressure liquid chromatography. J. Assoc.
Off. Anal. Chem., 60: 868-872.
SMALS, A.G.H., BEEX, L.A.V.M., & KLOPPENBORG, P.W.C. (1977)
Clofibrate-induced muscle damage with myoglobinuria and
cardiomyopathy. N. Engl. J. Med., 296: 942.
SMITH, A.E. (1972) The hydrolysis of 2,4-dichlorophenoxy-acetate
esters to 2,4-dichlorophenoxyacetic acid in Saskatchewan soils.
Weed Res., 12: 364-372.
SMITH, A.E. (1976a) The hydrolysis of herbicidal phenoxy-alkanoic
esters to phenoxyalkanoic acids in Saskatchewan soils. Weed Res.,
16: 19-22.
SMITH, A.E. (1976b) Method for the determination of
2,4-dichlorophenoxyacetic acid in urine. J. Chromatogr, 171:
482-485.
SMITH, A.H., FISHER, D.O., PEARCE, N., & CHAPMAN, C.J. (1982)
Congenital defects and miscarriages among New Zealand 2,4,5-T
sprayers. Arch. environ. Health, 37(4): 197-200.
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of
large-scale applications of 2,4-D on aquatic fauna and water
quality. Pestic. monitoring J., 1(3): 16-21.
SOMERS, J., MORAN, E.T., REINHART, B.S., & STEPHENSON, G.R. (1974a)
Effect of external application of pesticides to the fertile egg on
hatching success and early chick performance. 1. Pre-incubation
spraying with DDT and commercial mixtures of 2,4-D: picloram, and
2,4-D: 2,4,5-T. Bull. environ. Contam. Toxicol., 11(1) 33-38.
SOMERS, J., MORAN, E.T., & REINHART, B.S. (1974b) Effect of
external application of pesticides to the fertile egg on
hatching success and early chick performance. 2. Commercial
herbicide mixtures of 2,4-D with pioloram or 2,4,5-T using the
pheasant. Bull. environ. Contam. Toxicol., 11(4): 339-342.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974c) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11(4): 511-516.
SOS, J. & KERTAI, P. (1958) Effect of dichlorophenoxyacetic acid
upon the I131-uptake of the thyroid. Acta physiol. Acad. Sci.
Hung., 14: 367-369.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on
the embryonic development of Phasianus colchicus (L.) and Coturni
coturnix japonica (L.).] Z. Jagdwiss., 22: 197-201 (in German).
STANLEY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANOSZ, S. (1969) [The effects of sodium salt of 2,4-
dichlorophenoxy-acetic acid on cell respiration in the parenchymal
organs of rats.] Med. Prac., 20(6): 600-605 (in Polish).
STEIN, W. & KUHN, E. (1968) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D). Isolated rat diaphragm preparation
as simple experimental object.] Klin. Wochenschr., 46: 328-330
(in German).
STEVENS, L. J., COLLIER, C. W., & WOODHAM, D. W. (1970)
Monitoring pesticides in soils from areas of regular, limited, and
no pesticide use. Pestic. Monit. J., 4(3): 145-164.
STEVENS, T.S.A., SKELLY, N.E., & GRORUD, R.B. (1978) Isomer-
specific assay of ester and salt formulations of 2,4-
dichlorophenoxyacetic acid by high pressure liquid chromatography:
Collaborative study. J. Assoc. Off. Anal. Chem., 61: 1163-1165.
STEWART, D.K.R. & GAUL, S.O. (1977) Persistence of 2,4-D,
2,4,5-T, and dicamba in a dykeland soil. Bull. environ. Contam.
Toxicol., 18(2): 210-218.
STRACH, S. & BOHOSIEWICZ, M. (1964) [Studies of the toxicity for
pigs of the weed-killer "Pielik."] Med. weter, 20(11): 662-666
(in Polish, Health & Welfare Canada Translation No. 2467).
STUPNIKOV, A.A. (1959) In: [Collected works of the Leningrad
Veterinary Sciences Research Institute.] No. 8: 289 (in Russian).
SUDAK, F.N. & CLAFF, C.L. (1960) Survival of Uca pugnax in sand,
water, and vegetation contaminated with 2,4-dichloro-phenoxyacetic
acid. Proc. Northeast. Weed Control Conf., 14: 508-510.
SUDAK, F.N., CLAFF, C.L., & CANTOR, M. (1966) Body temperature
regulation in rats treated with 2,4-dichloro-phenoxyacetic acid.
Arch. int. Pharmacodyn. Ther., 160(2): 253-264.
SUFFET, I.H. (1973a) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 2.
Selection of water: solvent ratios and number of extractions.
J. agric. food Chem., 21: 288-294.
SUFFET, I.H. (1973b) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 3.
Liquid-liquid extraction of phenoxy acid herbicides from water.
J. agric. food Chem., 21: 591-598.
SUNDSTROM, G., JENSEN, S., JANSSON, B., & ERNE, K. (1979)
Chlorinated phenoxyacetic acid derivatives and tetrachlorodibenzo- p-
dioxin in foliage after application of 2,4,5-trichlorophenoxyacetic
acid esters. Arch. environ. Contam. Toxicol., 8: 441-448.
SZOCS, J., MOLNAR, V., BALOGH, E., AJTAY, M., & FULOP, I. (1970)
[Experimental data on the toxic effects of 2,4-D (Diclordon)
herbicide.] Rev. Med. (Tirgu-Mures, Rom.), 16: 91-93 (in
Hungarian).
THIELE, E., BAUFELD, W., GRIMM, U., LEHNIGK, B., & THIELE, W.
(1981a) [Results of analysis for deriving exposure time limits in
the industrial hygiene monitoring of people working at agrochemical
centres who are exposed to pesticides.] Z. gesamte Hyg. Grenzgeb.,
27(3): 173-177 (in German).
THIELE, E., FIEBER, C., JASTROCH, S., KNOLL, W., & LANGE, B.
(1981b) [Determination of toxic concentrations at places of work
and patterns of distribution of pesticide exposure of people
working at agrochemical centres subdivided by substance groups.]
Z. gesamte Hyg. Grenzgeb., 27(3): 177-180 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & LINK, R. (1982) Mortality
study of persons exposed to dioxin in a trichlorophenol-process
accident that occurred in the BASF AG on November 17, 1953.
Am. J.ind. Med., 3: 179-189.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964a) Metabolic
fate of some chlorinated phenoxyacetic acids in the stem tissue of
Avena sativa. Nature (Lond.), 204: 286.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964b) Metabolic
fate of 2,4-dichlorophenoxyacetic acid in the stem tissue of
Phaseolus vulgaris. Nature (Lond.), 204: 884-885.
THOMSSEN, C. (1958) [Contribution to the question of possible
poisoning of domestic animals through growth hormone-based weed
control agents.] Arch. exp. Veterinaermed., 12: 216-221 (in
German).
TODD, R.L. (1962) A case of 2,4-D intoxication. J. Iowa Med.
Soc., 52: 663-664.
TSAPKO, V.P. (1966) The herbicide 2,4-D as a health hazard in
agriculture. Hyg. Sanit., 9: 449-450.
TSILIKOV, V.V. (1969) [Effect of the sodium salt of 2,4-D,
trichlorometaphos, nicotine, and arecoline on thyroid function.]
In: Denisenko, P.P., ed. Farmakol. Tsent. Kholinolitikov Drugikh
Neirootropnykh Sredstv., pp. 181-185 (in Russian, Health & Welfare
Canada Translation No. 2447).
TUINSTRA, L.G.M., ROOS, A.H., & BRONSGEEST, J.M. (1976)
Suggestions for a sensitive multi-detection high-performance
liquid chromatography method for several phenoxy-acid herbicides.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 41: 1443-1448.
TULP, M.T.M. & HUTZINGER, O. (1978) Rat metabolism of
polychlorinated dibenzo- p-dioxins. Chemosphere, 7(9): 761-768.
UNGER, T.M., KLIETHERMES, J., VAN GOEHAM, D., & SHORT, R.D.
(1980) Teratology and postnatal studies in rats on propylene
glycol butyl ether and isooctyl esters of 2,4-dichloro-
phenoxyacetic acid, Washington, US Environmental Protection
Agency (60/12-80).
US EPA (1976) National interim primary drinking water
regulations, Washington, DC, US Environmental Protection Agency.
US EPA (1980a) Environmental Protection Agency Review and
Conclusions Concerning Potential Health Effects of the Herbicide
2,4-D, April 1980, Chem. Regul. Rep., 4(5): 134-136.
US EPA (1982) Tetrachlorodibenzo- p-dioxin; re-evaluation of
disposal prohibition. Fed. Reg., 47(2): 103.
US NATIONAL CANCER INSTITUTE (1979) Bioassay of 2,7-
dichlorodibenzo-p-dioxin (DCDD) for possible carcinogenicity.
Springfield, Virginia, National Technical Information Service
Document PB 290570 (Report No. NCI-CG-TR 123).
US VETERANS' ADMINISTRATION. DEPARTMENT OF MEDICINE AND SURGERY
(1981) Review of literature on herbicide, including phenoxy
herbicides and associated dioxins. Volume I. Analysis of
literature. Volume II. Annotated bibliography, Washington, DC,
US Veterans' Administration, 397 pp.
VACHKOVA-PETROVA, R. (1978) [Mutagenic activity of pesticides.]
Hig. Zdraveopaz., 21(5): 496-506 (in Bulgarian).
VAINIO, H., NICKELS, J., & LINNAINMAA, K. (1982) Phenoxy acid
herbicides cause peroxisome proliferation in Chinese hamsters.
Scand. J. Work. Environ. Health, 8: 70-73.
VAINIO, H., LINNAINMAA, K., KAHONEN, M., NICKELS, J., HIETANEN, E.,
MARNIEMI, J., & PELTONEN, P. (1983) Hypolipidemia and peroxisome
proliferation induced by phenoxyacetic acid herbicides in rats.
Biochem. Pharmacol., (in press).
VAN DYK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air:
sampling methods. Res. Rev., 55: 91-134.
VAN PETECHEM, C.R. & HEYNDRICKX, A.M. (1975) Beta oxidation of
chlorophenoxybutyric acid herbicides in guinea pigs. Bull.
environ. Contam. Toxicol., 14(5): 632-640.
VARDIA, H.K. & DURVE, V.S. (1981) Bioassay study on some fresh
water fishes exposed to 2,4-dichlorophenoxyacetic acid. Acta
hydrochim. hydrobiol., 9(2): 219-223.
VENKAIAH, B. & PATWARDHAN, M.V. (1978) Inhibition of
mitochondrial energy transfer reactions by phenoxy acids. Indian
J. Biochem. Biophys., 15(3): 219-221.
VINOKUROVA, M.K. (1960) [On the toxicity of a herbicide: The
octyl ester of 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
12: 31-34 (in Russian, Health & Welfare Canada Translation No.
2324).
VOGEL, E. & CHANDLER, J.L.R. (1974) Mutagenicity testing of
cyclamate and some pesticides in Drosophila melanogaster.
Experientia (Basel), 30(6): 621-623.
WALLIS, W.E., VAN POZNAK, A., & PLUM, F. (1970) Generalized
muscular stiffness, fasciculations, and myokymia of peripheral
nerve origin. Arch. Neurol., 22: 430-439.
WATERS, M.D., MITCHELL, A.D., JORGENSON, T.A., & VALENCIA, R.
(1981) Pesticides: Mutagenic and carcinogenic potential.
In: Bandal, S.K., Marco, G.J., Golberg, L., & Leng, M.L.,
ed., The pesticide chemist and modern toxicology. Washington,
DC, American Chemical Society (ACS Symposium Series 160).
WAY, J.M. (1969) Toxicity of hazards to man, domestic
animals and wildlife from some commonly used auxin herbicides.
Res. Rev., 26: 37-62.
WEINMANN, W. (1957) [Investigations of the influence of a
treatment of tomato plants with growth substances on the
nutritional and physiological value of the fruits. Offspring
mortality in albino rats.] Qual. Plant. Mater. Veg., 2(4):
342-349 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972) The subacute
toxicity of 2,4-dichlorophenoxyacetic acid and 2,4,5-
trichlorophenoxyacetic acid to chicks. Toxicol. appl.
Pharmacol., 21:348.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., & GANGSTAD, E.O.
(1973) The effects of a 2,4-D application on the biota and water
quality in Currituck Sound, North Carolina. Hyacinth Control J.,
11: 13-17.
WHO (1976) Pesticide residues in food. Report of the 1975
Joint FAO/WHO meeting (WHO Tech. Report Series, 592).
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residue
Series No. 1).
WHO/FAO (1976) 1975 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residues
Series No. 5).
WHO/IARC (1980) Walker, E.A., Castegnaro, M., Griciute, L.,
Börzsönyi, M., & Davis, W., ed.: N-Nitroso compounds: Analysis,
formation and occurrence. Lyons, International Agency for
Research on Cancer, 841 pp (IARC Scientific Publications No. 31).
WHO/IARC (1982) Bartsch, H., Castegnaro, M., O'Neill, I.K.,
Okada, M., & Davis, W., ed.: N-Nitroso compounds: Occurrence
and biological effects. Lyons, International Agency for Research
on Cancer, 755 pp (IARC Scientific Publications No. 41).
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972) Pesticide residue
levels in soils, F.Y. 1969 - National soils monitoring program.
Pestic. monit. J., 6(3): 194-228.
WIERZBICKA, M. (1974a) Haemolymph concentration in Cyclopoida
copepodids during active and resting stage and the effect of
2,4-D sodium salt. Pol. Arch. Hydrobiol., 21(2): 269-273.
WIERZBICKA, M. (1974b) Influence of 2,4-D sodium salt on the
survival of some Copepodia species. Pol. Arch. Hydrobiol.,
21(2): 275-282.
WINKELMANN, R.K. (1960) Diagnosis and treatment of allergic
angiitis (anaphylactoid purpura). Postgrad. Med., 27: 437-444.
WOJTALIK, T.A., HALL, T.F., & HILL, L.O. (1971). Monitoring
ecological conditions associated with wide-scale applications
of DMA-2,4-D to aquatic environmental. Pestic. monit. J.,
4(4): 184-203.
WOODHAM, D.W., MITCHELL, W.G., LOFTIS, C.D., & COLLIER, C.W.
(1971) An improved gas chromatographic method for the analysis of
2,4-D free acid in soil. J. agric. food Chem., 19: 186-188.
YIP, G. (1975) Analysis for herbicides and metabolites. J.
Chromatogr. Sci., 13: 225-230.
YODER, J., WATSON, M., & BENSON, W. (1973) Lymphocyte chromosome
analysis of agriculture workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUNG, A.L., ARNOLD, E.L., & WACHINSKI, A.M. (1974) Field
studies in soil persistence and movement of 2,4-D, 2,4,5-T and
TCDD. Weed Sci. Soc. Am., (Abstract No. 226).
YOUNG, A.L., CALCAGNI, J.A., THALKEN, C.E., & TREMBLAY, J.W.
(1978) The toxicology, environmental fate, and human risk of
Herbicide Orange and its associated dioxin. Springfield, Va.,
USA, US Air Force Occupational & Environmental Health
Laboratory (Technical Report No. TR-78-92, US National
Technical Information Service.)
YOUNG, F. & HALEY, T.J. (1977) Pharmacokinetic study of a
patient introduced with 2,4-dichlorophenoxyacetic acid and
2-methoxy-3,6-di-chlorobenzoic acid. Clin. Toxicol., 11(5)
489-500.
ZAKHAROV, G.G., SHEVCHENKO, A.M., ZUB, G.A., & PERSHAI, L.K.
(1968) [The clinical picture and prophylaxis of "Dikotex"
poisoning.] Zdrav. Beloruss., 9: 7-21 (in Russian, Health &
Welfare Canada Translation No. 2367).
ZETTERBERG, G. (1977) Experimental results of phenoxy acids
on microorganisms. In: Ramel, C., ed. Chlorinated phenoxy acids
and their dioxins: Mode of action, health risks and environmental
effects. Ecol. Bull. (Stockholm), 27.
ZETTERBERG. G., BUSK, L., ELOVSON, R., STAREC-NORDENHAMMAR, I., &
RYTTMAN, H. (1977) The influence of pH on the effects of 2,4-D on
Saccharomyces cerevisiae and Salmonella typhimurium. Mutat. Res.,
42(1): 3-18.
ZHITENEVA, L.D. & CHESNOKOVA, T.V. (1973) [Alteration of the
morphological composition of the blood in white amur larvae under
the effect of herbicides.] Eksp. vod. toksikol., 4: 56-67
(in Russian).
ZIELENSKI, W.L., Jr & FISHBEIN, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4-D and 2,4,5-T acids and
2,4-D esters in mice. J. agric food Chem., 15(5): 841-844.
ZIEM, G. & MAY, G. (1983) Tetrachlorodibenzodioxin. Br. J. ind.
Med., 40: 116.
2.1.4. Composition of technical 2,4-D materials
Technical 2,4-D may range in purity from less than 90% to 99%.
Typical levels for impurities are listed in Table 2. Trace levels
of CDDs have been found in amine and ester formulations (Table 3).
It can be seen that the amine formulations tend to be less highly
contaminated with di- and tetra-CDD than the ester products. The
structures of these impurities are shown in Fig. 2.
Table 2. Typical levels of 2,4-D and major impurities
in technical 2,4-Da
------------------------------------------------------
Component % range
------------------------------------------------------
2,4-dichlorophenoxyacetic acid 94 - 99
2,6-dichlorophenoxyacetic acid 1.5 - 0.5
2-monochlorophenoxyacetic acid 0.5 - 0.1
4-monochlorophenoxyacetic acid 0.8 - 0.2
bis(2,4-dichlorophenoxy) acetic acid 2.0 - 0.1
phenoxyacetic acid trace - 0.2
2,4-dichlorophenol 0.6 - 0.1
2,6-dichlorophenol 0.048 - 0.001
2,4,6-trichlorophenol 0.14 - 0.001
2-chlorophenol 0.04 - 0.0004
4-chlorophenol 0.005 - 0.0004
water 0.8 - 0.1
------------------------------------------------------
a From: Cochrane (1981).
Table 3. Ranges of levels of chlorinated dibenzo- p-dioxins (CDD)
in 2,4-D amine and ester formulationsa
-------------------------------------------------------------------
CDD isomers found (µg/kg)b
Type of 2,7-di- 1,3,7-tri- 1,3,6,8/ 2,3,7,8-tetra
formulation 1,3,7,9-tetra
-------------------------------------------------------------------
2,4-D amines ndc- 409 nd - 587 nd - 278 nd
2,4-D esters nd - 23815 nd - 450 nd - 8730 nd
-------------------------------------------------------------------
a From: Cochrane et al. (1981).
b Expressed in terms of 2,4-D.
c nd (not detected < 1 µg/kg).
The composition of technical 2,4-D depends on the manufacturing
process and especially on the purity of 2,4-dichlorophenol when
this is the starting material. During 2,4-D synthesis from
monochloroacetic acid and 2,4-dichlorophenol, the latter compound
as well as other ortho-chlorinated by-products can give rise to a
wide variety of chorinated by-products at a high temperature and
high pH. Self condensation of 2,4-dichlorophenol may form 2,7-
dichlorodibenzo- p-dioxin, while trichlorophenols may give rise to
a mixture of 1,3,6,8- and 1,3,7,9-tetrachlorodibenzo- p-dioxins
(but not 2,3,7,8-TCDD) by self-condensation, or to 1,3,7-
trichlorodibenzo- p-dioxin by cross-condensation with 2,4-
dichlorophenol.
A different type of toxic trace impurity, namely N-
nitrosamines, can occur in amine formulations of 2,4-D, especially
when nitrite is added as a corrosion inhibitor for containers.
Dimethyl- N-nitrosamine has been found in some 2,4-D dimethylamine
products at levels of up to 0.3 mg/litre (Ross et al., 1977; Cohen,
et al., 1978).
2.1.5. Volatility of 2,4-D derivatives
2,4-D esters with short-chain alcohols are highly volatile
(Table 1). This influences the effectiveness of their application
to target crops, their effects on neighbouring crops, and the
degree of contamination of the atmosphere. 2,4-D alkali salts or
amine salts are much less volatile than esters (Carter, 1960;
Canada, NRC, 1978; Que Hee & Sutherland, 1981, and section 4.1),
and these products are to be preferred when the use of 2,4-D esters
might lead to evaporative 2,4-D losses and to crop damage.
2.2. Determination of 2,4-D
2.2.1. General comments
General comments on criteria for acceptable analytical methods
and on other pertinent aspects of 2,4-D determination can be found
in the publications of Gunther (1962), Currie (1968), Kaiser
(1973), Carl (1979), Kateman & Pijper (1981), Que Hee & Sutherland
(1981) and Chau et al. (1982).
2.2.2. Analysis of technical and formulated 2,4-D products
In the past, the quality of 2,4-D products was assessed by an
acid-base titration or by a total chlorine determination
(Collaborative International Pesticides Analytical Council, 1970).
These non-specific and thus inaccurate methods have been superseded
by specific gas-liquid chromatography (GLC) or high pressure liquid
chromatography (HPLC), making it possible to determine various by-
products (Henshaw et al., 1975; Bontoyan, 1977; Skelly et al.,
1977; Stevens et al., l978; Cochrane et al., 1982). The isomer-
specific HPLC method is now preferred by many 2,4-D producers and
regulatory agencies. The chlorinated dibenzo- p-dioxins (CDDs) are
usually produced only in trace amounts and are difficult to
separate and identify; highly specialised equipment and skills are
necessary (Crummett & Stehl, 1973; Huckins et al., 1978; Norström
et al., 1979; Baker et al., 1981; Cochrane et al., 1981; Hass et
al., 1981, and National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1981).
2.2.3. Determination of 2,4-D residues
All exposure determinations and risk assessments ultimately
depend on accurate chemical analyses, and therefore some critical
aspects of analysis for 2,4-D residues have been included in the
present document.
Before 2,4-D residues can be measured, they have to be
quantitatively extracted and purified to remove substances that
could interfere with the final residue determination. They must
then be converted to a stable product (derivative) suitable for
determination with a given type of detector.
When comparing analytical results, it should be kept in mind
that the older methods of extraction and clean-up contained
considerable sources of errors, and that the early methods
for measuring 2,4-D residues, such as colorimetry and
spectrophotometry, were not as sensitive or specific as those
developed in recent years.
2.2.3.1. Sampling, extraction, and clean-up
Methods for the sampling, extraction, and clean-up of 2,4-D
residues in water, air, soil, and biological materials have
recently been reviewed by National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978) and by Que Hee & Sutherland (1981). Problems caused
by the conjugate formation of 2,4-D with amino acids, proteins,
sugars, or lipids, or the absorption of 2,4-D onto container
surfaces, including those of glass vessels, have been solved by
Chow et al. (1971), Renberg (1974), Osadchuk et al. (1977), Lokke
(1979), Jensen & Glas (1981), and Bristol et al. (1982). For
sampling and extracting 2,4-D residues, the following references
should also be consulted:
Air: Van Dyk & Visweswariah (1975), Farwell et al. (1976a,b),
Grover et al. (1976), Johnson et al. (1977), Gluck & Melcher
(1980), and Grover & Kerr (1981); water: Suffet (1973a,b), Renberg
(1974), Mierzwa & Witek (1977), Chau & Thomson (1978); soil:
Woodham et al. (1971); Smith (1972, 1976a), Foster & McKercher
(1973); food: Que Hee & Sutherland (1981), Bjerk et al. (1972),
Jensen & Glas (1972), Lokke (1975); biological media: Smith
(1976b), (blood, urine); Senczuk & Pogorzelska (1981).
2.2.4. Derivatization and quantification
At present, gas-liquid chromatography with electron-capture
detection (GLC-EC) is the most commonly used and generally most
sensitive method (picogram level) for measuring 2,4-D residues.
To improve the sensitivity of detection, the 2,4-D has to be
transformed (derivatized), usually to a methyl ester by reacting
with BF3-methanol, diazomethane, or with concentrated sulfuric
acid-methanol; the first method may give the best results (Munro,
1972; Horner et al., 1974; Olson et al., 1978).
For a recent review of derivatization methods and GLC columns
for various substrates see Cochrane (1981).
Thin-layer chromatography (TLC) has been used for herbicide
residue determination (Guardigli et al., 1971, Yip 1975). It has
recently been recommended by Batora et al. (1981) as a simplified
method for determining pesticide residues that requires a minimum
of costly equipment. TLC is suitable for food inspection and could
be of use in the establishment of new residue laboratories in
developing countries.
High-pressure liquid chromatography (HPLC) is less sensitive
than GLC-EC i.e., nanogram (ng) versus picogram levels, but may be
advantageous under some circumstances (Tuinstra et al., 1976;
Arjmand et al., 1978; Connick & Simoneaux, 1982). Using mass
fragmentography with deuterated internal standards it is possible
to determine nanogram amounts of 2,4-D and related compounds in
urine and plasma (De Beer et al., 1981); it is also suitable for
chemobiokinetic studies on subtoxic doses of 2,4-D in blood.
2.2.5. Confirmation
The ultimate confirmatory technique is gas chromatography
coupled with mass spectrometry and specific ion monitoring, with a
sensitivity down to the femtogram level (Farwell et al., 1976a).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use
were not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United
Kingdom. World-wide use of herbicides and annual production, which
probably exceeds 5 x 107 kg per year, are increasing, National
Research Council of Canada, Associate Committee on Scientific
Criteria for Environmental Quality, 1978; Bovey & Young, 1980).
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaf weeds in cereal crops as well as on
pastures and lawns, in parks, and on golf courses at rates of about
0.2 - 2.0 kg active ingredient (acid equivalent) per hectare.
Esters are also used at rates of up to 6 kg (acid equivalent) per
ha to suppress weeds, brush, and deciduous trees along rights-of-
way and in conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides
in or along irrigation and other canals, in ponds, and lakes at
rates ranging from 1 to 122 kg/ha (Pal'mova & Galuzova, 1963; Smith
& Isom, 1967; National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Bovey & Young, 1980).
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays
containing 20 - 40 mg 2,4-D/litre on apple trees to reduce
premature fruit drop, on potato plants to increase the proportion
of medium-size tubers or to intensify the tuber skin colour of the
red varieties (Bristol et al., 1982), and in citrus culture to
reduce pre-harvest fruit drop and to increase fruit storage life.
The highly volatile ethyl, isopropyl, and butyl esters are
being replaced by low-volatile esters or by amine salts to reduce
crop damage resulting from 2,4-D vapour drift, and to decrease
atmospheric pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public,
has been reduced in some countries, because of increasing concern
about possible toxic effects, especially in relation to CDDs.
The ecological effects of using high rates of 2,4-D and
repeated treatments have been reviewed by Bovey & Young (1980).
3.3. Disposal of wastes
3.3.1. Industrial Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localised
to the production site and to areas of waste dumping, and it is
likely to be more dispersed if disposal or leaching has occurred
into water courses. Combustion of 2,4-D and its by-products at low
temperatures could lead to the formation of CDDs. A temperature
approaching 1000 °C, however, gives almost complete destruction of
2,4-D (Sittig, ed., 1980). The spread of 2,4-D from waste dumps
may be reduced by the use of properly enclosed impermeable clay-
lined pits, away from water sources.
3.3.2. Agricultural wastes
Disposal of unused 2,4-D and washing of equipment may result in
localised land pollution and also pollution of water supplies
through direct contamination or leaching from soil.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION OF 2,4-D
2,4-D does not persist or accumulate in the environment, as it
is readily degraded by physical, chemical, and biological action.
It is susceptible to photolysis in air and water, on soil, and on
plant surfaces. Thus, the question of environmental distribution
is limited to the immediate transfer of 2,4-D between compartments
of the environment.
4.1. Drift and Volatilization in the Atmosphere
The atmosphere can be contaminated with 2,4-D during both its
manufacture and use. The production of 2,4-D may result in the
emission into the air of dichlorophenol, chloroacetic acid, and
ammonia (Sittig, ed., 1980), in addition to 2,4-D vapours (Grover
et al., 1976).
According to the formulation of 2,4-D used, environmental
transfer into the atmosphere will occur by either drift (depending
on the particle size of the droplet, the spray technique, and
climatic conditions), or by volatilization, or by a combination of
both. It is very difficult to calculate the extent to which drift
or volatisation occurs, and this is illustrated by the range of
2,4-D concentrations observed in the air after 2,4-D use (Table 4).
The factors affecting the amount of herbicide spray that lands
on a target crop and the proportion that is lost by drifting or
volatilization have been described (Grover et al., 1972; Grover,
1976; Maybank et al., 1978; Que Hee & Sutherland, 1981). Unwanted
residues may be deposited on non-target crops (Akesson & Yates,
1961; Yates & Akesson, 1973). The National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) cited reports of drift damage
caused in susceptible crops by phenoxy herbicide applications,
particularly in cotton, tobacco, tomatoes, grapes, rapeseed,
clover, and a number of horticultural species.
Widespread damage in vineyards and in other crops due to 2,4-D
drift from sprayed wheat fields was reported by Robinson & Fox
(1978) with two different damage patterns, one localized and the
other widespread. The first was characterized by severe localized
damage with a very clear gradient of decreasing severity away from
the zone, following the drift of spray droplets in the immediate
treatment area. The more widespread damage was of greater concern.
It was characterized by more or less uniform symptoms and appeared
to be attributable to the passage of a large cloud of vapour that
may have extended for several km. Both problems could have been
avoided by the use of low-volatile preparations and proper
application methods.
Table 4. Concentrations of total 2,4-D residues in ambient air
------------------------------------------------------------------------------------------------------------------
Days when 2,4-D was 2,4-D residues
Site location µg/m3air Predominant
No. of stations, height ------------------------------------------- type of 2,4-D Reference
Regional characteristics found Sample Meana Max. residue
present time
mean max. h
------------------------------------------------------------------------------------------------------------------
Saskatchewan, Canada not
(1) 150 m (aircraft) stated 1 min 1.0c 2.5 butyl ester Elias (1975)
Saskatchewan, Canada 48 33 36 24 h 0.5c 13.5 butyl ester Grover et al.
(8) 2 m above ground 24 h 0-6d isooctyl ester (1976)e,f
level, wheat area 24 h - - amine salt
California, USA 41 (1973) 24 h 0.4 0.9 high volatile Farwell et al.
(7-8) near ground 73 (1974) 24 h 0.1 0.4 low volatile (1976)
level 24 h 0.1 0.2 non volatile
Washington State, USA 105-106 81 89 24 h 0.2 2.2 isopropyl ester Adams et al.
(2) near ground 65 69 24 h 0.09 2.2 butyl ester (1964)b
level, wheat area 8 11 24 h 0.003 3.1 isooctyl ester
Washington State, 99-102 34 39 24 h 0.08 2.0 isopropyl ester droplets Bamesberger &
(2) near ground 8 15 24 h 0.08 1.3 isopropyl ester vapour Adamsb
level, wheat area 18 22 24 h 0.07 1.0 butyl ester droplets
3 5 24 h 0.03 1.3 butyl ester vapour
1 1 24 h 0.005 0.5 isooctyl ester droplets
- - 24 h - - isooctyl ester vapour
4 5 24 h 0.01 0.5 acid, salts, droplets
5 5 24 h 0.04 5.1 acid, salts, vapour
------------------------------------------------------------------------------------------------------------------
a Values measured at different sites or at different times have been averaged and reduced to a single significant
figure for simplicity.
b At the time of these studies, GLC methods were less highly developed.
c Centre of principal range observed.
d Maximum values recorded in a previous study (Que Hee et al., 1975) were shown to be equivalent to concentrations
existing directly over an open pan of formulated butyl ester; the implication was made that accidental
laboratory contamination could have occurred.
f The results of Maybank & Yoshida (1969), Maybank et al. (1978), and Stanley et al. (1971) could not be adapted
to this table.
Volatilization of 2,4-D products in the air during the spraying
operation and from the surface of plants and the soil is difficult
to distinguish from the drift of spray droplets. Evaporation
occurs to a greater extent with the highly volatile ethyl,
isopropyl, or butyl esters; very little occurs with amine salt
formulations, and it is greatly reduced when 2,4-D is dissolved in
corn oil, cottonseed oil, or diesel oil (Marth & Mitchell, 1949).
In one experiment, no significant amounts of 2,4-D amine, but 20 -
40% of the initially deposited 2,4-D butyl ester, and 10 - 15% of
the octyl ester of 2,4-D vapourized within 2 h of spraying (Grover
et al., 1972); less volatilization occurs with the higher esters of
2,4-D. For this reason, the use of the more volatile esters has
been discontinued in some countries. Studies of 2,4-D aerial drift
following ground spray operations have shown that only 3 - 8% of
the applied herbicides drift as spray droplets when low volatile
preparations are applied as large droplets. However, ultra-low-
volume (ULV) applications by aircraft, or the use of highly
volatile esters may cause as much as 25 - 30% of the 2,4-D sprayed
to drift off the target (Grover et al., 1972; Maas & Kerssen, 1973;
Maybank et al., 1978).
4.2. Movement Within and From the Soil
The movement of pesticides within and from the soil can be
divided into three categories: diffusion, leaching, and surface
movement. Diffusion is a localized process and depends on the
concentration gradient of the pesticide in the soil medium, on the
soil mineral type, and on the organic matter content, temperature,
pH, and other factors. Leaching refers to the movement of
pesticides through the soil profile with percolating water.
Surface movement refers to wind erosion of dust particles and
surface run-off in flowing water.
Examination of the behaviour of 2,4-D in soils (Liu & Cibes-
Viade, 1973; Grover & Smith, 1974; Moreale & Van Bladel, 1980) has
shown that organic matter, soil pH (surface horizons), and
exchangeable aluminium (clay sub-horizons) are the key determinants
for the percentage of 2,4-D adsorbed. As the adsorption/desorption
process is the basic mechanism influencing herbicide availability,
mobility, and degradation in soil, 2,4-D is likely to be more
strongly bound in soils with a high content of organic matter than
in those with a low content.
4.3. Contamination of Water
Residues of 2,4-D in aqueous systems can result from the
deposition of spray drifts, the "washout" of 2,4-D in the vapour or
droplet phase from the atmosphere during rainfall, the run-off from
treated fields, or following the application of 2,4-D to water for
the control of aquatic weeds. Industrial discharges, either from
accidental spills or through sewage systems, may also contribute to
the contamination of water. The National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978) has tabulated data that demonstrate
the influence of environmental factors on the clearance of 2,4-D
and its derivatives from water. The principal processes involved
are ester and amine hydrolysis, volatilization, microbial
degradation, photolysis, and sorption. There is little movement of
2,4-D into drainage water in organic soils, because it is strongly
bound to organic materials.
4.4. Environmental Transformation and Degradation Processes
4.4.1. Metabolism in plants
Plants hydrolyse 2,4-D esters to 2,4-D, which is the active
herbicide (Morton et al., 1967; Matsunaka, 1972). Further
metabolism in plants occurs through three mechanisms, namely, side-
chain degradation, hydroxylation of the aromatic ring, and
conjugation with plant constituents (Crafts, 1960; Morre & Rogers,
1960; Erickson et al., 1963).
4.4.1.1. Side-chain degradation
Degradation of the side-chain of 2,4-D has been observed in
many plants (Loos, 1969), but in only a few species or varieties
does it appear to play a major role in herbicide breakdown.
Luckwill & Lloyd Jones (1960a,b) suggested two degradation
pathways leading to the formation of 2,4-dichlorophenol.
4.4.1.2. Ring hydroxylation
Thomas et al. (1964a,b), and, more recently, Feung et al.
(1971, 1972, 1973b) identified 2,5-dichloro-4-hydroxyphenoxyacetic
acid and 2,3-dichloro-4-hydroxyphenoxyacetic acid as major and
minor phenolic acid metabolites, respectively. Evidence was found
by Fleeker & Stein (1971) indicating hydroxylation resulting in the
elimination of the 4-chloro substituent from the aromatic ring, in
addition to migration of the chlorine at the 4-position to an
adjacent carbon on the ring. A small amount of 2-chloro-4-hydroxy-
phenoxyacetic acid was produced from 2,4-D by wild buckwheat, wild
oats, leafy spurge, and yellow foxtail.
4.4.1.3. Conjugation with plant constituents
Studies indicate that resistant crops, i.e., grasses and
cereals, form water-soluble conjugates with sugars, whereas
sensitive broad-leaved crops (such as beans) form mainly water-
insoluble amino acid conjugates (Montgomery et al., 1971; Feung et
al., 1971, 1972, 1973b, 1975).
4.4.2. Degradation of 2,4-D in the soil
Deposition of 2,4-D esters on the soil is followed fairly
rapidly by hydrolysis. Burcar et al. (1966) observed that the
2,4-D isooctyl ester disappeared after 2 weeks, though free acid
could be detected up to 6 weeks after application. The breakdown
of the iso-propyl, n-butyl, and isooctyl esters of 2,4-D on three
Canadian prairie soils was studied by Smith (1972) who found that
after 24 h no iso-propyl or n-butyl esters remained, whereas
20 - 30% of the isooctyl ester was still intact. The author
concluded that an initial rapid phase of hydrolysis of the 2,4-D
esters to the anion in soil was the result of chemical and not
microbial action.
Microbial degradation of phenoxy herbicides does occur and has
been comprehensively reviewed by Loos (1975), Cripps & Roberts
(1978) and The National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, (1978).
Early studies of the persistence of 2,4-D in soil indicated that
warm moist conditions and the presence of organic matter favoured
the rapid disappearance of 2,4-D. Sterilization of the soil
inhibited breakdown, indicating that the degradation was microbial.
In addition, Pemberton (1979) reported the discovery of specific
2,4-D plasmids within some bacterial strains, transmitted from one
cell to another, and carrying with them a genetic capability
enabling the bacteria to degrade 2,4-D.
Two principal pathways have been proposed for the microbial
degradation of 2,4-D in soil. Firstly the side chain may be
removed to form 2,4-dichlorophenol, followed by orthohydroxylation
of the phenol to produce a catechol (Bollag et al., 1968). The
catechol may then be cleaved to yield a muconic acid and further
conversion products. The second possible pathway is via a
hydroxyphenoxyacetic acid intermediate (Evans et al., 1971).
4.4.3. Degradation in the aquatic ecosystem
A multitude of variables influence the partitioning and removal
of phenoxy herbicides within an aquatic ecosystem. Detectable
residues have been reported to persist for 4 weeks in some
situations and up to 4 months in others (Frank & Comes, 1967;
Wojtalik et al., 1971; Schultz & Harman, 1974). Photolysis is an
important means of degradation of 2,4-D in natural water and is
more rapid than that of 2,4,5-T (Crosby & Wong, 1973). The
partition of residues between water and sediment will have an
effect on the rate of breakdown, as will temperature and intensity
of light. Anaerobic conditions will favour microbial breakdown.
The effects of some of these factors have been tabulated by the
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (1978).
4.4.4. Photochemical degradation
Photodecomposition of 2,4-D was studied in detail by Crosby &
Tutass (1966), Boval & Smith (1973), and reviewed recently by Que
Hee & Sutherland (1981). It leads to the formation of a variety of
products but commonly involves reductive dechlorination of the
acid, esters, and salts in aqueous or in organic solutions, with
2,4-dichlorophenol acting as a catalyst for the breakdown of
2,4-D, which may involve rupture of the aromatic ring. Que Hee &
Sutherland (1987) studied the vapour and liquid phase photolysis of
the n-butyl ester of 2,4-D and observed dechlorination at the
second position with simultaneous reduction and re-arrangement to
produce a variety of photoproducts. According to Boval & Smith
(1973), carbon dioxide is the final oxidation product when aqueous
solutions of 2,4-D undergo photodecomposition.
4.5. Bioconcentration
There is no evidence that bioconcentration of 2,4-D occurs
through the food chain or in any compartment of the environment.
This has been demonstrated by large-scale monitoring for 2,4-D
residues in soils, foods, feedstuffs, wildlife, and human beings,
and from examinations of the many routes of metabolism and
degradation that exist in ecosystems (sections 5.1.3 and 5.1.4).
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1. Levels of 2,4-D Residues in the Environment
Most of the available information on 2,4-D levels in the
environment has been reviewed in detail (National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality, 1978; Ramel, 1978; Bovey & Young, 1980;
Canada, Health & Welfare, 1980; Shearer & Halter, 1980; US EPA,
1980a). In comparing early and recent results, it should be kept
in mind that the analytical procedures used before about 1965 were
often unreliable and may have resulted in under- or overestimation
of the actual levels of 2,4-D derivatives. No information is
available on the levels of 2,4-D-related dioxin by-products in the
environment.
5.1.1. In air
Some levels of 2,4-D in ambient air are shown in Table 4.
These 2,4-D residues consist mainly of esters, particularly the
highly volatile butyl esters (Bamesberger & Adams, 1966; Farwell et
al., 1976b; Grover et al., 1976). Total 2,4-D residues in the air
were found to decrease during periods of rain, suggesting a
"washout effect" (Grover et al., 1976). In the majority of cases,
the levels reported were those found shortly after spraying.
5.1.1.1. Field exposure
Concentrations of 2,4-D that occurred during and after
herbicide use in the air of the work zone of people engaged in
herbicide spray operations in various use situations, are given in
Table 5. Workers involved in these operations were exposed to
2,4-D levels of up to 0.2 mg/m3 air during the period of actual
application.
5.1.1.2. General environmental exposure
In large-scale studies in areas of intense 2,4-D use, about 40%
of all air samples were found to contain between 0.01 and 0.1 µg
2,4-D/m3 (Grover et al., 1975). In a similar study undertaken by
Que Hee et al. (1976), much higher levels were recorded in one
urban location, reaching an average of 339 µg/m3 air during 3 days.
However, Grover et al. (1976), in their subsequent work, showed
that such concentrations could only be produced under artificial
conditions that could not reflect environmental conditions. In a
general programme of air monitoring undertaken in citrus-growing
regions in the USA, only one out of 880 samples analysed was found
to contain 2,4-D, at a level of 0.004 mg/m3. The sites were not
chosen in relation to 2,4-D use (Stanley et al., 1971).
Table 5. Concentrations of total 2,4-D in air related to occupational exposure
---------------------------------------------------------------------------------------------------------
Days Mean of 2,4-D
Herbicide Circumstances Type of exposure after concentrations References
product monitoring spraying in air (mg/m3)
---------------------------------------------------------------------------------------------------------
2,4-dimethylamine agricultural spray Analyses of air 0 0.02 Thiele et al.
salt (0.9% aqueous operations with in tractor cabs (1981a,b)
solution) tractor-drawn
equipment
2,4-D butoxyethanol Exposure during Analyses of air 0 0.1-0.2 Kolmodin-Hedman
ester. 2% emulsion forest spray in breathing zone & Erne (1980),
in water operation with of workers Kolmodin-Hedman
tractor driven et al. (1979)
equipment
2,4-D isooctyl- 3-day aerial spray Analyses of air 0 0.002-01a Franklin et al.
ester in diesel oil operation with single in breathing zone (1982)
engine aircraft of pilot and
ground crew
2,4-D PGBE ester Two 1-day aerial Analyses of air 0 <0.00001b Lavy et al.
emulsion in water forest spray in breathing zone (1982)
operations by of ground crew
helicopter
---------------------------------------------------------------------------------------------------------
a Application using large spray droplets.
b One flagman was recorded as being exposed to 0.1 mg/m3.
5.1.2. In water
2,4-D, as well as chlorophenol residues resulting from the
microbial transformation of 2,4-D, may occur in raw and finished
supplies of drinking-water (Faust & Aly, 1963; US EPA, 1976, 1980a;
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality, 1978; Bovey & Young,
1980; Canada, Health & Welfare, 1980; Shearer & Halter, 1980).
Information on 2,4-D-related dioxins in water was not
available.
Drinking-water in the USA is routinely analysed by the FDA as
part of the beverage-food group in their "market basket" analysis
programme; 2,4-D has not been detected in these studies, where the
limit of detection is 0.005 mg/litre for beverages (Table 6). This
indicates that drinking-water is not a significant source of human
exposure outside directly sprayed areas.
The same conclusion can be drawn from the results of large-
scale surveys of pesticide residues, including 2,4-D in surface
waters in areas of 2,4-D use (Table 7).
Levels much higher than those found in these studies have been
observed, but only in relation to local spills or direct
contamination (Frank et al., 1979; Frank & Sirons, 1980). A very
wide fluctuation has been found in water samples following
treatment of bodies of water, shores, ditches, or stream banks with
herbicides (Averitt, 1967; Frank & Comes, 1967; Bartley & Hattrup,
1970; Frank et al., 1970; Wojtalik et al., 1970; Frank, 1972;
Whitney et al., 1973; Schultz & Harman, 1974; Schultz & Whitney,
1974; Paderova, 1975; Province of British Columbia, 1981).
Occasional high contamination levels in samples of potable water
have been reported following experimental treatments of reservoirs
with 2,4-D (Wojtalik et al., 1971). However, the mean levels
tended to remain below 2 µg/litre, even in samples of raw or
processed water from 2,4-D-treated reservoirs (Smith & Isom, 1967;
Wojtalik et al., 1971; Province of British Columbia, 1981).
Generally, 2,4-D residues were < 0.1 µg/litre in two large-scale
monitoring programmes of surface waters (Frank & Sirons, 1980;
Gummer, 1980). This is not unexpected in view of the moderately
rapid microbial degradation of 2,4-D in the environment (Robson,
1966; Averitt, 1967; Frank, 1972; Nesbitt & Watson, 1980a,b;
Province of British Columbia, 1981).
2,4-D and especially its transformation product,
dichlorophenol, at levels exceeding 20 µg/litre will impart an
objectionable odour and taste to contaminated water (Pal'mova &
Galuzova, 1963; Faust & Suffet, 1966). This organoleptic effect
may reduce the likelihood of highly contaminated water being
ingested. It is noteworthy that public water supplies containing
"traces" of 2,4-D, and wells contaminated with 2,4-D or other
herbicides have been shut down because of objectionable odours or
tastes (Gribanov, 1968; Kramer & Schmaland, 1974; Frank et al.,
1979).
Table 6. 2,4-D residues reported in market basket samples in the USA
---------------------------------------------------------------------------------------------------------
Types of samples Nature of samples % of samples Residue levels
Years analysed containing residues with residues (mg/kg) References
---------------------------------------------------------------------------------------------------------
1965-65 Total diets sugars and adjunctsa 4.2 < 0.02-0.16 Duggan & Corneliussen
1966-66 leafy vegetables (1) 3.0 < 0.02-0.03 (1972)
low fats
1967-67 leafy vegetables (2) 1.7 0.03
oil fats (1)
1968-68 dairy produce (1) 0.6 0.02-0.13
1969-69 fruits (1), sugars (2) 0.3 < 0.2
1970-70 leafy vegetables (1) 0.3 < 0.02
dairy produce (1)
1970-71 Total diets leafy vegetables (3) - 0.01-0.02 Manske & Corneliussen
(1975)
1971-72 Total diets dairy products (1) - 0.01 Manske & Johnson
(1975)
1973-80 Total diets 0 < 0.01 Manske & Johnson
(1976)
Johnson et al.
(1981a,b)
Johnson et al. (1977)
1972-73 Potatoes from raw, boiled or baked - < 0.02-0.12 Bristol et al. (1982)
fields treated
with herbicide
---------------------------------------------------------------------------------------------------------
a No. of positives not specified.
Table 7. Concentrations of 2,4-D residues in surface water samples following application of 2,4-D to
agricultural landsa
------------------------------------------------------------------------------------------------------
Site No. of samples in 2,4-D applied 2,4-D residues
Number of Stations which 2,4-D was: in watershed (µg/litre) References
Regional Characteristics ----------------- (kg/ha) ---------------
analysed found meanb max.
------------------------------------------------------------------------------------------------------
Ontario, Canada 949 66 0.8 <0.1 3.9e Frank & Sirons (1980)
11
streams
Saskatchewan, Canada 15 10 - 2 21.6 Choi et al. (1976)
5
river
Western Canada 186 10 - 0.5c 4.3d Gummer (1980)
14
diverse sites
------------------------------------------------------------------------------------------------------
a Studies in which the analytical procedures were not described or were considered unreliable have not
been included.
b Values measured at different sites or at different times have been averaged and reduced to a single
significant figure for simplicity.
c Reported data are very close to analytical detection limits.
d The maximum value, which raises the average value considerably, occurred in the effluent of an
industrial plant.
e Levels of 15.9 and 320 µg/litre were recorded at two sites but were related to spillage or actual
spraying at the sampling locality.
5.1.3. In soil
Most of the information available at present concerning 2,4-D
and other chlorophenoxy herbicide residues in soils has been
reviewed by the National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Bovey (1980a), and by Que Hee & Sutherland (1981). In highly
acidic soils, or in soils in cold or arid regions, 2,4-D
degradation is apparently slow (Lavy et al., 1973; Buslovich &
Milchina, 1976; Ou et al., 1978; Moreale & Van Bladel, 1980;
Nesbitt & Watson, 1980b). However, even at about 20 - 2000 times
the normal agricultural application rates, little or no detectable
2,4-D was left in soils under temperate climatic conditions with
prolonged winters, after intervals of 385 - 440 days (Young et al.,
1974; Stewart & Gaul, 1977; Bovey, 1980a). Furthermore, results of
a laboratory study on 2,4-D degradation in the soil showed a half-
life of 4 days (Altom & Stritzke, 1973). Several soil monitoring
studies in North America, in areas with regular 2,4-D use, have
shown residues in less than 10% of the samples, and at levels of
less than 1 mg/kg (Stevens et al., 1970; Wiersma et al., 1972;
Gowen et al., 1976).
The available data are inadequate for establishing regional and
seasonal profiles of 2,4-D soil residues and of direct population
exposure, but it is likely that direct exposure would be minor,
except during or soon after herbicide application. Indirect
exposure through the transfer of 2,4-D residues from soil to air,
or food sources is assessed separately.
5.1.4. In food sources
Although 2,4-D and its transformation products do not tend to
accumulate in plants and plant products, detectable residues of
2,4-D on food plants may be consumed by human beings or animals and
may thus contribute to the overall exposure of the human population
to this chemical.
The results of pertinent studies on 2,4-D residues on or in
foods, and in food sources for human beings and animals, are
summarized in Tables 8 - 11. Theoretically, some contribution to
the reported 2,4-D residues may have been partly derived from other
phenoxy herbicides, as 2,4-DB undergoes beta-oxidation to 2,4-D in
some plants and fish, and in cattle (Lisk et al., 1963; Gutenmann &
Lisk, 1965; Sundström et al., 1979; Bovey, 1980a).
5.1.4.1. Residues in retail food supplies
The frequency of occurrence and the levels of 2,4-D residues in
over 110 000 samples of a variety of different ready-to-eat foods,
beverages, and infant and young children's diets, have been studied
over the last 20 years in the USA (Lipscomb, 1968; Corneliussen,
1970, 1972; Duggan et al., 1971; Duggan & Corneliussen, 1972;
Johnson et al., 1979, 1981a,b). The 2,4-D residues found in such
samples are reported in Table 6. The theoretical daily intake
resulting from these residues was variously estimated to be < 1 -
5 µg/person per day (Duggan & Corneliusson, 1972).
Studies undertaken since 1970 have failed to detect residues of
2,4-D in any of the US diet samples analysed, except for a single
positive sample in the dairy product food group which was estimated
at 0.01 mg/kg (Manske & Johnson, 1975).
5.1.4.2. Residues in fish and shellfish
Fish and shellfish may be exposed to 2,4-D as a consequence of
aquatic herbicide use, or through the agricultural use of 2,4-D.
The residues in the edible portions of such fish rarely exceed 1
mg/kg wet weight (Erne, 1974, 1975 and Table 8). Residues of 2,4-D
have not been detected in retail samples of fish and shellfish
analysed as part of the US "market basket" studies (section
5.1.4.1).
There is some evidence that the organoleptic properties of the
2,4-D residues may reduce the likelihood of the consumption of fish
flesh contaminated with higher levels of 2,4-D (Gavrilova, 1965;
Folmar, 1979).
5.1.4.3. Residues in wild fruits and mushrooms
Uncultivated fruits and mushrooms taken from areas where 2,4-D
was used, or was likely to have been used, were examined for
residues of 2,4-D by Erne & von Haartman (1973), Erne, (1980),
Sietanen et al. (1981), and Frank et al. (1982). The results in
Table 9 show that residues of 2,4-D in berries in field-trial
studies have been as high as 30 mg/kg immediately after
application, but residues in berries and mushrooms taken from the
wild are generally < 1 mg/kg.
High residues of 2,4-D can produce disagreeable odours or
flavours in wild fruits and vegetables (Ingelög et al., 1977;
McArdle et al., 1961), and this may reduce the likelihood that
highly contaminated foods are ingested.
5.1.4.4. Residues in food derived from animals
Domestic meat-, milk-, and egg-producing animals, and game
animals may consume forage or feed containing 2,4-D residues, and
thus, their tissues and products may contain residues. Published
data on 2,4-D residues in feed and forage from the Northern
Hemisphere are summarized in Table 10. Immediately after
application of phenoxy herbicides, 2,4-D residues in or on grass,
generally average about 100 mg/kg for each kg of herbicide applied
per hectare. Such residues decline with a half-life of about 1 - 2
weeks, to about 20 mg/kg, within 4 weeks after an application of 1
kg/ha (Leng, 1972). Residues in 2,4-D-treated feed grains are
significantly lower than the levels reported above and no residues
would be expected in meat, milk, or eggs from such sources (Table
10).
Table 8. 2,4-D residues reported in field studies on fish and shellfish
-----------------------------------------------------------------------------------------------------
Country Year(s) 2,4-D application Types of samples 2,4-D residues in References
rate tissues (mg/kg)
-----------------------------------------------------------------------------------------------------
USA 1961 0.1 mg/litre oyster (1 species) 1.6-2.0 Butler (1965)
fish (1 species) 0.3-1.0 Cope et al. (1970)
USA 1966 44.8-112 kg/ha mussels < 0.14-1.12 Smith & Isom (1967)
clams < 0.14
fish (5 species) < 0.14
USA 1968 112 kg/ha fish (4 species) < 0.10-0.24 Whitney et al. (1973)
USA 1969 22.4-44.8 kg/ha mussels < 0.05-2.7 Wojtalik et al. (1971)
fish (8 species) < 0.10-0.34
USA 1971 2.24-8.96 kg/ha fish (3 species) < 0.005-1.075 Schultz & Harman (1974)
USA 1971 4.48 kg/ha fish (5 species) 0.000-0.162 Schultz & Whitney (1974)
-----------------------------------------------------------------------------------------------------
Table 9. 2,4-D residues in wild berries and mushrooms collected in fields or forests following application
of phenoxyalkanoic herbicides
--------------------------------------------------------------------------------------------------------------
Country Year(s) Sample 2,4-D application Days after No. samples 2,4-D residues References
rate (kg a.i./ha) treatment analysed (mg/kg)
--------------------------------------------------------------------------------------------------------------
Canadaa 1979-81 raspberries 1.1-3.9 2 124 2.6-31.0 Frank et
14-35 0.1-3.3 al. (1982)
Finlanda 1974-76 vaccinium berries 2.5 10-356 44 Mukula et
jam not known not known 1 2.2 al. (1978)
mushrooms 14-300 28 < 0.05-1.2
Finlanda 1975-76 vaccinium berries 0.25-2.25 365 not stated < 0.05 Siltanen et
al. (1981)
Swedena 1970 raspberries 1.5-2.2 2-32 9 < 0.03-0.9 Erne & Von
vaccinium berries 1.5-2.2 2-32 68 < 0.03-7.7 Haartman
blueberries 1.5-2.2 2-32 19 < 0.03-2.9 (1973)c
mushrooms 1.5-2.2 2-32 15 < 0.03
Swedena 1973-79 vaccinium berriesb 0.25-2.25 365 61 nd (< 0.05) Erne (1980)
raspberries not stated 14 not stated nd-2.5
blueberries not stated 2 not stated nd-10.0
cowberries not stated 1-28 not stated nd-6.0
mushrooms not stated 7 1 0.3
Swedena blueberries 0.4-1.5 1-35 not stated 0.2-5.3 Ingelög et
vaccinium berries 0.4-1.5 30-35 not stated 0.5-4.5 al. (1977)
raspberries 0.4-1.5 1-10 not stated 0.2-2.0
--------------------------------------------------------------------------------------------------------------
a Samples taken from areas treated with 2,4-D.
b Samples entering factory for processing.
c Data from authors' Table 1.
Table 10. 2,4-D residues reported in samples of herbicide-treated forage or feed
--------------------------------------------------------------------------------------------------------------------------
Country Year(s) Type of samples 2,4-D application Post- No. samples 2,4-D residues References
rate, (kg a.i./ha) treatment examined & (mg/kg)
interval, positive
(days)
--------------------------------------------------------------------------------------------------------------------------
Canada 1971 wheat plants 0.42 1-36 ? ? 8.35-0.011 Cochrane & Russell (1975)
Finland 1962-68 green forage 1-4 7.21 ? ? 600-3.7 Finnish State Institute of
(grass and 3.5 7-28 ? ? 13-0.4 Agriculture (1963-1969)
clover)
Finland 1974-76 aspen leaves 2.5 60-300 32 30-0.3 Mukula et al. (1978)
and twigs
birch leaves 60-300 16 31-0.1
and twigs
cowberry plants 365 8 < 26-0.05
Germany, wheat, barley, 0.375-0.735 64-101 ? ? < 0.015-0.01 Maier-Bode (1971)
Federal rye, oat grains
Republic wheat, barley, < 0.34-0.02
of rye, oat straw
Hungary 1971 silo corn 1.4-1.5 56-120 ? ? 0.8-0.075 Bodai et al. (1974)
Sweden 1972-76 barley, oats ? ? 3 2 0.7-0.4 Erne & Rutqvist (1979)
grass 7 1 0.4
lichens ? ? 2 2 0.4-0.2
USA 1949 pasture plants 4.48 1 8 8 14.6-1.65 Grigsby & Farwell (1950)
USA 1967 forage grasses 0.56-2.2 0-112 ? ? 100-1 Morton et al. (1967)
USA 1969 sorghum plants 1.4 2 ? ? 1.06 Ketchersid et al. (1970)
1.4-2.8 30-60 < 5.25-0.2
USA 1969 pasture plants 6.6-8.8 0-28 24 24 700-150 Leng (1972)
--------------------------------------------------------------------------------------------------------------------------
No residues of 2,4-D were detected (detection limit of 0.02 mg)
in the milk of dairy cows fed 2,4-D at a level of 300 mg/kg total
diet (Bjerke et al., 1972; Leng, 1972). A range of 0.06 - 0.08 mg
2,4-D/litre was found in the milk of cows fed for 3 weeks at a
level of 1000 mg 2,4-D/kg total diet.
When young beef cattle were fed 2,4-D at levels of 300, 1000,
and 2000 mg/kg total diet for 28 days, 2,4-D residue levels were
highest in the kidney and liver, but did not exceed 0.1 mg/kg in
muscle and fat, even at the highest dose level (Clark et al., 1975;
Leng, 1972, 1977). 2,4-D residues were not detected in more than
12 000 samples each of meat and dairy products analysed in the USA
between 1963 and 1969 (Duggan et al., 1971).
Results of feeding studies with hares and reindeer in
Scandinavia indicated that 2,4-D levels of 25 - 30 mg/kg forage
(equivalent to an intake of about 1 mg 2,4-D/kg body weight per
day) produce maximum 2,4-D residues of 1.1 mg/kg wet weight in
liver, and 8.9 mg/kg in kidney tissues (Erne, 1974). Residues of
2,4-D were detected in the liver and kidney of a few game animals
shot by hunters, or found dead in or near areas sprayed with
phenoxy herbicides (Table 11, Erne, 1974, 1975). The residues in
muscle tissue were not measured but would be lower than in the
liver and kidney, as indicated by the data summarized in Table 11.
On the whole, the available evidence indicates that 2,4-D is
rarely detected in commercial foods and that residues in food taken
from areas where 2,4-D has been sprayed will usually be < 1 mg/kg
food. The liver and kidney from range animals are possible
exceptions, but these contribute little to the total diet of the
general population.
5.2. Occupational Exposure to 2,4-D During the Production, Handling,
and Use of Chlorophenoxy Herbicides
During occupational exposure to 2,4-D, the chemical may be
absorbed via the inhalation, oral, and dermal routes, but more than
90% of the total amount of 2,4-D or other chlorophenoxy compounds
entering the body under these circumstances appears to be absorbed
through the skin and excreted relatively quantatively in the urine
as the phenoxy acid and readily-hydrolysed conjugates (Kolmodin-
Hedman et al., 1979, 1980; Libich et al., 1981; Draper & Street,
1982; Franklin et al., 1982; Leng et al., 1982; Nash et al., 1982)
(section 6).
Data from occupational exposure studies concerning the amounts
of 2,4-D found on the clothing or on cloth patches worn by workers
are not included in this review because the correlation between
these amounts and amounts absorbed into the body and then excreted
in urine is poor (Franklin et al., 1982; Lavy et al., 1982; Leng et
al., 1982).
Table 11. 2,4-D residues in game and domestic animals and animal products
------------------------------------------------------------------------------------------------------------------
Country Year(s) Species 2,4-D treatment Post- Type of 2,4-D residues References
rate (kg a.i./ha) treatment samples (mg/kg)
interval examined
(days)
------------------------------------------------------------------------------------------------------------------
Sweden 1968 moose (Alces game animals found ? liver and < 0.05-6 Erne (1974,
-1972 alces) dead, or shot by kidney from 1975)
deer (Capreolus hunters in herbicide- 250 animals
capreolus) treated areas found dead
hares (Lepus
lepus)
pheasants ? liver and < 0.05-4.5
grouse kidney from (2,4-D and
130 animals 2,4,5-T)
shot by
hunters
USA 1963 Jersey cow 50 ppm in diet for 4 0-2 milk < 0.1 Bache et al.
days (1964a)
USA 1974(?) adult beef 0, 9, 30 or 60 mg 0 muscle < 0.05-0.07 Clark et al.
cattle 2,4-D acid/kg bw/day 28 fat < 0.13-0.34 (1975)
for days (0, 300, liver < 0.05-0.23
1,000 2000 mg/kg feed) kidney 2.53-10.9
USA 1974(?) adult sheep 2000 mg/kg feed for 0-7 muscle < 0.05-0.06 Clark et al.
28 days fat 0.10-0.15 (1975)
liver 0.29-0.98
kidney 0.37-9.17
USA 1965(?) dairy cows animals grazing on 2 milk 0.01-0.09 Klingman et al.
pasture sprayed with 4 (1966)
herbicide at 2, 24 kg
a.i./ha
USA 1972 dairy cows 30, 300, 1000 mg/kg 0 milk < 0.05-0.16 Bjerke et al.
in feed for 2-3 weeks (1972)
1-3 < 0.05 Leng (1972)
USSR 1975 "livestock" ? ? muscle 0.04 Fyodorova et
liver 0.04 al. (1977)
kidney 0.03 (mean)
------------------------------------------------------------------------------------------------------------------
5.2.1. Industrial exposure
Several studies have been published on the levels of 2,4-D to
which workers producing or packaging 2,4-D herbicides are exposed
(Fetisov, 1966; Johnson, 1971; Juzwiak et al., 1973; Andreasik et
al., 1979). In every case the amount of 2,4-D absorbed by the
workers was uncertain and, therefore, the data are inadequate for
estimating industrial exposure to 2,4-D. Workers manufacturing
2,4-D were also exposed to other chemicals (Assouly, 1951; Bashirov
& Ter-Bagdasarova, 1970).
5.2.2. Exposure related to herbicide use
The available studies on the occupational exposure to 2,4-D of
workers during the use of 2,4-D herbicides are summarized in Table
12. Studies on the exposure of back-pack sprayers to 2,4-D have
not been published. However, comparable exposure data are
available for 2,4,5-T back-pack sprayers, and they have been
included in Table 12 for comparison. The levels of 2,4-D found in
the air of the working zone in these and other studies have already
been referred to in section 5.1.1.1 and Table 5.
In studies undertaken before 1980, only the amounts of 2,4-D in
the air, on the clothing, or on the skin were determined, except
for 2 urinary 2,4-D values reported by Shafik et al. (1971). Thus
the amounts of 2,4-D actually absorbed cannot be reliably estimated
from these early reports and are not included in Table 12.
After 1980, several detailed occupational exposure studies were
carried out to determine the amounts of 2,4-D or other chlorophenoxy
acids absorbed by various members of ground and aerial spray teams,
using a variety of equipment for dispersing aqueous or oil solutions
or emulsions (Kolmodin-Hedman et al., 1979; Kolmodin-Hedman & Erne,
1980; Libich et al., 1981; Draper & Street, 1982; Franklin et al.,
1982; Lavy et al., 1982; Leng et al., 1982; Nash et al., 1982).
The total 2,4-D urinary excretion levels reported in Table 12
reflect a wide variety of uses and show that the excretion does not
usually exceed 0.1 mg 2,4-D/kg body weight per day of exposure.
However, so far, a comprehensive comparison of the relative
exposures resulting from different methods of application and
different 2,4-D derivatives (amine salts and esters) or formulations
(aqueous, oil) cannot be carried out, because the available data are
still incomplete. The amount of 2,4-D absorbed depends on the type
of work performed, and on the degree of care taken to avoid direct
dermal contact with the herbicide concentrate, spray solution, or
spray. The most heavily-exposed workers tend to be the mixer-
loaders, who handle the herbicide concentrate, and the spray
personnel. However, if careful, they may be exposed to less 2,4-D
than, for example, a pilot of a spray plane who is not careful
(Franklin et al., 1982; Leng et al., 1982; Lavy et al., 1982; Nash
et al., 1982). The reports by Libich et al. (1981) and Leng et al.
(1982) on ground spray crews indicate that, even under unfavourable
working conditions, the amount of 2,4-D absorbed may be greatly
reduced simply by wearing clean gloves and overalls, and by making
the workers more aware of the importance of safe work habits.
Table 12. Exposure related to herbicide use
------------------------------------------------------------------------------------------------------------------------
Product No. of Type of Daily concentr- Duration of Total 2,4-D in urine References
people application ation of 2,4-D collection of excreted (mg/kg bw/
exposed in urine 24-h urine day of exposure)
(mg/litre)e samples (days)
------------------------------------------------------------------------------------------------------------------------
2,4-D and dicamba 2 boom spray 1 - 4 - - Draper & Street
dimethylene salts single use (1982)
in aqueous solution 2 repeated use 3 - 20 - -
2,4-D isooctyl 4 3 applications - 4 0.004 - 0.04 Franklin et al.
ester in diesel oil by single- (1982)
engine aircraft
2,4-D/2,4,5-T 4 tractor-drawn 1 - 14 7 - Kolmodin-Hedman
butoxyethyl sprayers, forestry (1979, 1980)
esters as 2% exposure daily,
emulsion in water for one week
2,4-D PGBE ester 26 helicopter in - 5 nd - 0.06a Lavy et al.
26 forestry use - 5 nd - 0.02b (1982)
2,4,5-T PGBE ester 7 Back-pack 5 0.01 - 0.09 Leng et al.
forestry use (2,4,5-T) (1982)
single exposures,
one week apart
2,4-D/2,4-DPc and 23 roadside and < 0.01 - 8 3 - Libich et al.
2,4-D/picloramc right-of-way (one usually (1981)
ground equipment high result
incl. mist blowers of 31)
2,4-Dc 17 aircraft repeated - 7 0.006 - 0.02d Nash et al.
exposure (mean values/day) (1982)
2,4-D amine salt 26 ground equipment - 7 nd - 0.08
and ester (single exposure)
------------------------------------------------------------------------------------------------------------------------
a No special precautions taken.
b Protective clothing worn.
c Preparation used not specified.
d Mean values per day recorded for different individuals.
e It is not possible to calculate the total 2,4-D excretion in urine from these data, because of individual variations
in urine concentrations from day to day from sample to sample.
As the chemobiokinetic profiles of urinary 2,4-D output are
reported in only a few of the studies, summarized in Table 12, it
is not possible to estimate the total 2,4-D intake in all cases.
The results of the studies by Libich et al. (1981) and by
Draper & Street (1982) suggest that using single-exposure studies
to estimate the peak exposure levels reached by workers exposed
several days in succession may give an underestimation.
No information is available on the amounts of chlorinated
dibenzodioxins, or other by-products or contaminants, absorbed as a
consequence of occupational exposure to 2,4-D herbicides.
In one extensive occupational monitoring programme undertaken
in 1979 - 82, about 3000 urine samples were analysed for herbicide
residues (Simpson, 1982). The subjects included pesticide factory
staff, pest control operators, farmers, park workers, and others
potentially exposed to 2,4-D. During the first year of the study,
no 2,4-D was detected (< 0.001 mg/litre) in 735 of 973 samples.
Most of the other samples contained less than 0.1 mg/litre and only
27 contained more than 1 mg/litre. The highest value was 31
mg/litre. The study is continuing.
5.3. Exposure of Bystanders to 2,4-D
Aerial drift and other forms of pesticide transport, as well as
the contamination of surfaces during or after herbicide production,
distribution, or use, may bring 2,4-D into contact with bystanders,
i.e., persons other than those who are occupationally exposed. Few
studies of bystander exposure to 2,4-D or other chlorophenoxy
herbicides have been published. Studies available for review
included that of Lavy et al. (1982) concerning 9 supervisors and
observers present at two helicopter forest spray operations using
2,4-D propyleneglycol butylether (PGBE) ester, respectively, for
unspecified durations. These people excreted a maximum of 1.3 µg
2,4-D/kg body weight. In a forest ground spray operation with
tractor-drawn equipment, 2,4-D was not detected (< 0.05 mg/litre)
in the urine of bystanders (Kolmodin-Hedman et al., 1980).
Additional bystander exposure studies for various 2,4-D use
patterns are desirable. However, the 2,4-D intake of bystanders is
unlikely to exceed the 2,4-D intake during occupational exposure.
5.4. Estimated Exposure of the General Population in 2,4-D-Use Areas
Data useful for estimating the intake by the general population
of 2,4-D residues in the environment including those in food
sources have been generated. The present calculations of the
intake of the general population in an area of 2,4-D use are based
on these data and on a series of stated assumptions aimed at
obtaining a moderate overestimation rather than underestimation of
the actual exposure.
5.4.1. Intake of 2,4-D residues from air
On the basis of available information, it can be assumed that
the general population in areas of 2,4-D herbicide use would rarely
be exposed to 2,4-D concentrations exceeding 0.1 µg/m3 air.
Assuming an air level of 0.1 µg 2,4-D/m3, a body weight of 60
kg, an air intake of 20 m3 per day, and a 100% retention of 2,4-D,
it can be calculated that the respiratory intake would be 0.03 µg
2,4-D/kg body weight per day.
5.4.2. Intake of 2,4-D residues from potable water
The larger surveys of potable water (Table 7) show mean 2,4-D
residues in surface water to be generally < 0.1 µg/litre, but for
the present estimate, it is assumed that potable water from surface
sources or from treatment plants, during a period of about 10 days
after reservoir treatment, can contain an average 2,4-D residue
level of 2 µg/litre (Wojtalik et al., 1971 and Table 7). Assuming
a 2,4-D concentration in water of 2 µg/litre, a body weight of 60
kg, a water intake of 2 litres per day (Canada, Health & Welfare,
1980), and a 100% absorption of the ingested 2,4-D, it can be
calculated that the 2,4-D intake of the general population in a
2,4-D use area resulting from water could approach 0.07 µg/kg body
weight per day, which could occur for about 10 days.
Insufficient data are available to give a reliable estimate of
2,4-D intake from ground water sources, but it is likely to be
lower than the above value.
5.4.3. Intake of 2,4-D residues from soil
2,4-D on soil particles ingested with food or water, or carried
into the air and inhaled, is considered to be part of the exposure
due to residues in air, water, or food and is therefore assumed to
be completely covered in these exposure estimates.
5.4.4. Intake of 2,4-D residues from food
The data in Tables 8 - 11 indicate that there is unlikely to be
any exposure of the general population to 2,4-D residues in retail
food supplies. The possibility that individuals are exposed to
contaminated local sources of food has been assessed in section
5.1.4. In the case of milk or muscle meat, it can be assumed that
no individual will be exposed to levels in excess of 0.02 mg/kg of
these foods, the limit of detection of the method of analysis used.
Assuming a concentration of 0.02 mg 2,4-D/litre in milk, and a
consumption of 1.5 litre per day, the maximum intake from this
source would be 0.0005 mg/kg body weight per day for a 60 kg adult.
Individuals who consume wild berries taken from 2,4-D-treated areas
could be exposed through this food source. Assuming consumption of
100 g of berries per serving and a maximum 2,4-D concentration of 1
mg/kg, the intake from this source would be 0.002 mg/kg body weight
per serving.
5.4.5. Total exposure of the general population in a 2,4-D-use area
The above considerations suggest that the total daily 2,4-D
intake of the population in use areas will not normally exceed
about 0.002 µg/kg body weight during the application period (Table
13).
Table 13. Components of estimasted exposure to 2,4-D
-------------------------------------------------------------------
Estimated amount of
Exposed Group intake (µg 2,4-D/kg Source of 2,4-D
bw/day)
-------------------------------------------------------------------
Occupational
i. Factory workers insufficient data mainly dermal contact
ii. Applicator crews 100a
iii. Bystanders - b
General population in
areas with 2,4-D use 0.03 air
0.07 water
0.5 milk
ND retail food
2.0 wild berries, mushrooms
etc.
-------------------------------------------------------------------
a Based on total urinary output after several days of exposure.
b Unlikely to exceed occupational exposure.
5.4.6. Total exposure of persons occupationally exposed in agriculture
An accurate maximum occupational intake of 2,4-D cannot be
determined on the basis of the limited studies undertaken.
However, the available data suggest that work performed in the
preparation of, and during, agricultural application of 2,4-D
herbicide will probably result in an exposure of not more than
about 0.1 mg 2,4-D/kg body weight per day, providing that minimum
precautions are taken against excessive exposure.
5.4.7. Total exposure of the general population outside areas of 2,4-D use
Monitoring of air, water, and food outside areas of known 2,4-D
use show that intake is below present detection limits.
6. CHEMOBIOKINETICS AND METABOLISM
With the exception of recent occupational exposure studies and
studies on animals published in 1979 or later, the available
information on the uptake, distribution, transformation, and
excretion of 2,4-D by human beings and other mammals has already
been reviewed by Leng (1977), National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Young et al. (1978), Bovey (1980a,b), Shearer
(1980), and United States Veterans' Administration (1981).
6.1. Uptake via Different Routes of Exposure
6.1.1. Uptake by inhalation
6.1.1.1. Animals
Burton et al. (1974) found that small amounts of 2,4-D
instilled into the rat lung were rapidly absorbed, apparently by a
non-saturable process following first-order kinetics, with an
absorption half time of 1.4 - 1.7 min. The kinetics of the
absorption of 2,4-D vapours or aerosols in the respiratory tract of
animals have not yet been studied.
6.1.1.2. Human beings
The uptake of 2,4-D and of 2,4-D derivatives via the human
respiratory tract does not appear to have been studied under
controlled conditions. However, the observations of Kolmodin-
Hedman & Erne (1980), Libich et al. (1981), Franklin et al. (1982),
and Lavy et al. (1982) on people occupationally exposed to 2,4-D
indicated that only a small percentage of the total amount of 2,4-D
absorbed via all routes of exposure was taken in through the
respiratory tract.
6.1.2. Dermal uptake
6.1.2.1. Animals
Mice whose tails had been immersed in 2,4-D butyl or crotyl
ester solutions, 4 h daily for 3-5 days, absorbed lethal amounts of
the chemicals (Fetisov, 1966). However, the actual doses absorbed
and other details were not given. In contrast, no major ill
effects were reported in studies in which rabbits were treated
percutaneously for 2 or 3 weeks with 130 - 180 mg/kg body
weight/day of a 50% aqueous solution of 2,4-D octyl ester, or with
unspecified amounts of solutions of 2,4-D dimethylamine salt in
water, or oil solutions of 2,4-D isooctyl or butyl ester
(Vinokurova, 1960; Kay et al., 1965).
6.1.2.2. Human beings
Only 5.8% of a dilute solution of 14C-labelled 2,4-D in acetone
applied at a dose of 4 µg a.i./cm2 to the ventral forearm of adults
was excreted in the urine compared with 100% of a small intravenous
dose (Feldmann & Maibach, 1974) (Table 14). The 2,4-D excretion in
urine is delayed and more prolonged after dermal application than
after intraveneous or oral administration (Feldmann & Maibach,
1974; Sauerhoff et al., 1977), and complete elimination may take
about one week (Levy et al., 1982; Leng et al., 1982). Cases of
acute occupational 2,4-D poisoning following combined dermal and
inhalation exposures (Monarca & Divito, 1961; Tsapko, 1966;
Paggiaro et al., 1974), as well as occupational exposure studies
(Table 12), suggest a fairly efficient dermal absorption of 2,4-D.
However, the importance of solvents, surfactants, and other
ingredients of the herbicides in the uptake of 2,4-D via the dermal
route still needs to be defined.
6.1.3. Oral uptake
6.1.3.1. Animals
The uptake of 2,4-D from the gut of rats, mice, guinea-pigs,
cattle, pigs, and sheep appears to be similar in both rapidity and
extent to that observed in human beings (Mitchell et al., 1946;
Lisk et al., 1963; Bache et al., 1964a; Erne, 1966a,b; Milhaud et
al., 1970; Shafik et al., 1971; Buslovich et al., 1973; Fedorova &
Belova, 1974; Clark et al., 1975; Senczuk & Pogorzelska, 1975,
1981; Van Peteghem & Heyndrickx, 1975). In some of the ungulates,
2,4-DB acid, and 2,4-D amine salts or esters are at least partially
converted to 2,4-D in the rumen, before being absorbed (Gutenmann
et al., 1963; Lisk et al., 1963). Some of the esters may be less
well absorbed from the gut than the acid or its alkali or amine
salts (Erne, 1966a; Buslovich et al., 1973), but the uptake
mechanisms for 2,4-D and its salts or esters is not known, and thus
deserves further study.
6.1.3.2. Human beings
Information on the uptake of 2,4-D by human beings via the
oral route has been gathered in studies on two groups of 5 - 6
volunteers each, who ingested single doses of 5 mg 2,4-D/kg body
weight (Table 14), and by chemobiokinetic studies on individuals
who, with suicidal intent, swallowed lethal or non-lethal amounts
of various 2,4-D herbicides (Geldmacher-Von Mallinckrodt &
Lautenbach, 1966; Rivers et al., 1970; Kohli et al., 1974;
Sauerhoff et al., 1976, 1977; Khanna & Kohli, 1977; Young & Haley,
1977; Prescott et al., 1979) (Table 15). These results show that
single doses of 2,4-D are fairly rapidly and completely absorbed
from the human digestive tract, unless the dose is so large that
toxic effects interfere with absorption. However, in the two
studies on volunteers, considerable individual variation in the
rate and extent of absorption from the digestive tract was
observed. The absorption mechanism appears to involve first-order
kinetics (Kohli et al., 1974; Khanna & Kohli, 1977) and may fit a
single- or multi-compartment chemobiokinetic model, depending on
individual characteristics (Sauerhoff et al., 1977).
Table 14. Chemobiokinetics of 2,4-D in human beings following administration under controlled conditions
--------------------------------------------------------------------------------------------------------------------------
Product Dose and dosing Subjects Observations Toxic effects References
schedule EL NOELa
(mg/kg bw)
single dose
--------------------------------------------------------------------------------------------------------------------------
14C-2,4-D 1) Intravenous injection: 6 (sex & Scintillation counting ? ? Feldmann &
(New England Dose (7 µCi) not stated as age not 1) 100% of dose excreted in urine Maibach
Co., and 2,4-D/weight unit stated) urine in 120 h Mean t0.5 = 13 h (1974)
Amersham 2) Dermal application: 6 (sex & 2) 5.8% of applied dose excreted ? ?
Searle Co.) 1 x 4 µg 2,4-D (in acetone)/ age not in 120 h
cm2 of skin of forearm. stated)
Application site was not
washed for 24 h
2,4-D, 99% Oral administration: 6 gas chromatography of blood & ? 5 Khanna &
pure (Dow 1 x 2, 3, or 5 mg/kg bw, in (adult M) urine samples; no ill effects; no Kohli (1977);
Chemical gelatin capsule, with water, changes in clinical parameters: Kohli et al.
Co.) following breakfast blood pressure, pulse rate, Hb, WBC (1974)
counts (total & differential); mean
plasma clearance t0.5 = 33 ± 3.1 h;
peak plasma conc. at 7-24 h = 40
mg/litre; ~75% of dose excreted
in urine in 96 h
"no metabolic transformation
at up to 5 mg/kg"
2,4-D, Oral administration: 6 gas chromatography - mass ? 5 Sauerhoff
analytical 1 x 5 mg/kg bw as a slurry (adult M, spectrometry of blood & urine et al.
grade in milk, or in powder form, 70-90 kg) samples; no ill effects; (1976, 1977)
with some water, following essentially all of the dose
breakfast absorbed; peak plasma conc. = 10-30
mg/litre within 24 h; mean plasma
clearance t0.5 = 11.6 h; mean
urinary excretion t0.5 = 17.7 h;
total excreted amount ~82% of dose
administered; 4.8-27.1% of
excreted compound was conjugated
--------------------------------------------------------------------------------------------------------------------------
a NOEL = No-observed-adverse-effect level.
Table 15. Chemobiokinetics of 2,4-D by human beings following accidental or intentional ingestion of herbicides
----------------------------------------------------------------------------------------------------------------
Products Circum- Subject Observations References
stances
----------------------------------------------------------------------------------------------------------------
2,4-D suicide; F, 33 death in about 30 h; post mortem 2,4-D Geldmacher-Von
ingestion years concentration: Mallinckrodt &
of unknown mg/litre mg/kg Lautenbach (1966)
amount of blood urine brain liver lung heart
herbicide 23 164 100 116 88 63
no metabolites were identified
Herbicide suicide; F, 51 death in about 96 h; concentration of 2,4-D
containing ingestion years, plus MCPA:
2,4-D plus of unknown 66 kg mg/litre mg/kg
MCPA amount of blood urine liver kidney muscle
("U46 COMBI") herbicide 42 420 100 trace 40
(BASF 2,4-dichlorophenol not detected; several
Ludwigshafen) other metabolites or herbicide by-products
found, but not identified
Herbicide suicide M, 39 severe toxic effects; unconsciousness; Prescott et al. (1979)
containing attempt; years recovery within 11 days following treatment by
2,4-D plus ingestion alkaline diuresis; initial plasma concentration:
mecoprop of 6.7 g 2,4-D = 400 mg/litre, mecoprop = 750 mg/litre;
(10%) + (20%) 2,4-D and no pretreatment change in 2,4-D level following
7.6 g alkaline diuresis; plasma clearance t0.5 = 3.7 h
mecoprop for 2,4-D, 11-28 h for mecoprop
----------------------------------------------------------------------------------------------------------------
6.2. Distribution and Transformation in the Body
6.2.1. Animals
The absorption and distribution kinetics and metabolism of pure
2,4-D and of a variety of pure or commercial 2,4-D or 2,4-DB amine
salts and esters have been repeatedly studied both in vivo and in
vitro in a wide variety of animals including rats, mice, rabbits,
guinea-pigs, cattle, sheep, goats, pigs, chickens, fish, and spiny
lobsters (Gutenmann & Lisk, 1965; Erne & Sperber, 1974; Guarino &
Arnold, 1979; James, 1979; Koschier & Pritchard, 1979; Pritchard
& James, 1979; Pritchard & Miller 1980). The considerable
differences observed in the relative amounts of residues found in
the cells and plasma of mouse, rat, and horse blood, after dosing
animals or after in vitro addition of 2,4-D (Erne, 1966a; Jenssen
& Renberg, 1976), in different tissues of rats, mice, and sheep
(Erne, 1966a,b; Lindquist & Ullberg, 1971; Milhaud et al., 1970;
Buslovich et al., 1973; Clark et al., 1975; Elo & Ylitalo, 1979),
and in the soluble and particulate fractions of rat tissues (Khanna
& Fang, 1974) support the idea that there is more than one
physiological compartment for 2,4-D storage. The distribution
volumes appear to be equivalent to the volume occupied by about
25 - 50% of the body mass (Erne, 1966a).
2,4-D is reversibly bound to blood plasma proteins,
particularly albumins, possibly at sites for which it competes with
related compounds. The same sites are apparently also binding
sites for palmitic acid and thyroxine. The extent of 2,4-D binding
depends, in part, on pH and 2,4-D concentration (Florsheim et al.,
1963; Erne, 1966b; Kolberg et al., 1973; Hacque et al., 1975;
Mason, 1975; Orberg, 1980a), and may affect the rate and extent of
renal 2,4-D excretion (Pritchard & James, 1979; Pritchard & Miller,
1980) and thus the toxicity of 2,4-D.
In pregnant mammals, up to about 17% of a single dose of 2,4-D
may rapidly cross the placenta to reach the embryos or fetuses
(Lindquist & Ullberg, 1971; Fedorova & Belova, 1974; Antonenko,
1977).
Pigs and rats hydrolyse 2,4-D esters both in the gut and after
absorption in the body (Erne, 1966a,b). Observations from several
studies indicate that 2,4-D is not significantly metabolized in
animals, except in ruminants. No 14CO2 was produced by rats given
C1- or C2-labelled 14C-2,4-D (Khanna & Fang, 1966). No 2,4-
dichlorophenol (2,4-DCP) was detected in the tissues of mice or
rats dosed by various routes with 2,4-D (Zielinski & Fishbein,
1967; Shafik et al., 1971; Federova & Belova, 1974; Grunow & Böhme,
1974). However, residues of 2,4-DCP were detected in the milk of
dairy cows fed 100 mg 2,4-D/kg diet for 3 weeks (Bjerke et al.,
1972; Leng, 1972), and in the livers and kidneys of cattle and
sheep fed up to 2000 mg/kg diet for 4 weeks (Clark, 1975; Leng,
1972, 1977). These 2,4-DCP residues probably resulted from the
bacterial degradation of 2,4-D in the rumen of the animals.
Bacterial degradation may also account for the 2,4-DCP reported by
Antonenko (1977) in pregnant or lactating rats and rat fetuses.
Other investigators did not detect 2,4-DCP in the tissues of
mice or rats dosed with 2,4-D by various routes (Zielinski &
Fishbein, 1967; Shafik et al., 1971; Fedorova & Belova, 1974;
Grunow & Böhme, 1974).
Results of studies on experimental animals have suggested that
2,4-D conjugates are formed in the kidney tubules (Erne, 1966a,b;
Erne & Sperber, 1974; Grunow & Böhme, 1974).
Taurine and glycine conjugates, as well as various other
unidentified conjugates of 2,4-D have been found in the urine of
rats, pigs, chickens, and the dogfish shark (Squalus acanthias)
(Erne, 1966b; Erne & Sperber, 1974; Grunow & Böhme, 1974; Koschier
& Pritchard, 1979). However, in rats and pigs, only about 10-20%,
and in the chicken, less than 5% of the total amount of 2,4-D
appeared to be excreted in this form. In the dogfish shark, the
taurine conjugate may be primarily formed in the tubular cells of
the kidney (Koschier & Pritchard, 1979). The site and mechanism of
2,4-D conjugation seem to be unknown in the other species.
6.2.2. Human beings
Studies on human volunteers who ingested pure 2,4-D, and on
cases of accidental or voluntary acute poisoning with various 2,4-D
herbicides have shown that 2,4-D is very rapidly absorbed from the
gut and carried in the blood to cells and tissues throughout the
body, but that is not extensively transformed (Tables 14, 15)
(Curry, 1962; Herbich & Machata, 1963; Nielsen et al., 1965; Dudley
& Thapar, 1972; Coutselinis et al., 1977). The kinetics following
ingestion suggest a 1- or 2-compartment distribution, depending on
individual characteristics (Sauerhoff et al., 1977; Young & Haley,
1977). Following absorption of purified 2,4-D, or of herbicides
containing only 2,4-D, no transformation products, including 2,4-
dichlorophenol, were found in blood or tissues. After ingestion of
herbicides containing 2,4-D and other compounds, some 2,4-D
metabolites or manufacturing by-products were detected in tissues,
but were not identified (Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Prescott et al., 1979). Unidentified 2,4-D conjugates were
also found in urine following ingestion of pure 2,4-D. These
conjugates represented up to 27% of the 2,4-D ingested (Sauerhoff
et al., 1976, 1977). Of the 5 North American volunteers studied by
these authors, only one did not produce a conjugate; in contrast,
apparently none of the 6 Indian subjects studied by Kohli et al.
(1974) and by Khanna & Kohli (1977) produced 2,4-D metabolites.
6.3. 2,4-D Levels in Body Tissues and Fluids
6.3.1. Animals
2,4-D levels in the blood and organs of mammals have been
determined, e.g., by Erne (1966a,b), Milhaud (1970), Buslovich et
al. (1973), Khanna & Fang (1974), Clark et al. (1975), Jenssen &
Renberg (1976), and Elo & Ylitalo (1979). The highest residue
levels were usually found in liver, kidney, lungs, spleen, and
heart. In a study by Fedorova & Belova (1974), 6 - 8% of the
amount of 2,4-D administered was found in all of the tissues
examined in rats dosed orally 26 - 35 days previously with this
chemical. However, the 2,4-D residue levels quoted were close to
the limit of detection for the analytical method used.
6.3.2. Human beings
In volunteers, each of whom ingested a single dose of 5 mg
2,4-D/kg body weight, the 2,4-D levels in blood plasma reached
peaks of about 10 - 45 mg/litre within about 7 - 24 h, and then
declined (Kohli et al., 1974; Khanna & Kohli, 1977; Sauerhoff et
al., 1977). In one group of workers occupationally exposed to
2,4-D for one week while using ground equipment for spraying
(Kolmodin-Hedman et al., 1979), plasma levels ranged from the
detection limit (0.02 mg/ml) to 0.2 mg/ml, while urinary levels
ranged from 1 to 14 mg/litre. Urinary 2,4-D levels reported in
other occupational exposure studies are summarized in Table 12.
However, it should be noted that analysis of single urine specimens
is not adequate for estimating the dose absorbed by individuals,
because excretion follows a diurnal pattern and continues for
several days after dermal exposure (Leng et al., 1977; Sauerhoff et
al., 1979; Lavy et al., 1982). Thus, levels found after several
days of spraying will probably be higher than first day levels, as
reported by Libich et al. (1981) and Draper & Street (1982).
However, excretion of 2,4-D should be completed within one week
following the last exposure (Feldmann & Maibach, 1974; Lavy et al.,
1982; Leng et al., 1982).
The toxic and lethal levels of 2,4-D in human blood and tissues
are still not well defined. A woman with reportedly 335 mg
2,4-D/litre plasma did not show any signs of poisoning; in general,
the acute lethal levels of 2,4-D appear to lie between 447 and 826
mg/litre plasma (Herbich & Machata, 1963; Nielsen et al.,
1965; Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Coutselinis
et al., 1977; Prescott et al., 1979). The lowest lethal 2,4-D
levels in blood or tissues were recorded several days after the
chemical was ingested, i.e., after most of the 2,4-D had probably
been eliminated. Among the different organs examined post mortem
in cases of fatal 2,4-D poisoning, liver and kidney tended to
contain the highest concentrations of 2,4-D, while brain and other
fatty organs, and muscle including the heart, usually had lower
2,4-D levels (Table 15) (Curry, 1962; Herbich & Machata, 1963;
Nielsen et al., 1965; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972; Coutselinis et al., 1977). As in the
case of blood, the values for the different tissues may vary
according to the proportion of 2,4-D eliminated by the time death
occurs.
6.4. Elimination and Biological Half-Life
The term "biological half-life" will be used to indicate the
time required to eliminate one half of a single dose of 2,4-D, or
to reduce 2,4-D residues in the body fluids or tissues to one-half
of the peak concentration. Biological half-life, as defined here,
is a useful concept with which it is possible to make rough
comparisons of the elimination rate of 2,4-D with that of other
toxic chemicals.
6.4.1. Animals
The half-life values recorded in mammals fall into the range
observed in the rat (Erne, 1966a,b; Federova & Belova, 1974; Khanna
& Fang, 1974), with the exception of the very low value for 2,4-D
butyl ester in whole mouse carcasses (0.85 - 1.11 h) observed by
Zielinski & Fishbein (1967). Results of the investigations
conducted by these authors also suggest that prior dosing with
2,4-D ("priming") may increase the rate of elimination of 2,4-D
butyl ester in mice, presumably through stimulation of the renal
excretory mechanism.
An important correlation between diet and 2,4-D elimination
rate was observed by Orberg (1980b) in the goat. A protein-poor
diet reduced the 2,4-D plasma clearance rate by about 20 - 50%,
possibly because of decreased renal size and renal blood flow.
The many species-related factors known to affect the rate of
2,4-D elimination make interspecies comparisons difficult, and
often the published reports do not include such details as diet,
age, sex, and body weight of the test animals, type and purity of
the test compounds, and important environmental factors such as the
ambient temperature, which are necessary for valid comparisons.
6.4.2. Human beings
The studies on volunteers and on cases of accidental or
voluntary 2,4-D poisoning summarized in Tables 14 and 15 show that
human beings excrete 2,4-D mainly in the urine, and that the
blood plasma clearance times depend on the dose, individual
characteristics, and the presence or absence of compounds that may
competitively inhibit 2,4-D excretion. For single oral doses of
2,4-D, the biological half-life in blood plasma is about one day,
depending on the circumstances. However, forced alkaline diuresis
may reduce this to as little as 3.7 h (Sauerhoff et al., 1977;
Prescott et al., 1979).
In occupationally-exposed people, absorption of successive
daily doses of 2,4-D makes its biological half-life difficult to
determine, but for single occupational exposures it has been
estimated to be 35-48 h (Nash et al., 1982).
6.5. Chlorinated Dibenzo- p-Dioxins (CDDs)
The metabolism in the rat of several dibenzo- p-dioxins,
including the 2,7-CDD found in 2,4-D, was described by Tulp &
Hutzinger (1978). The primary pathway of transformation appears
to involve hydroxylation at the 2-, 3-, 7-, or 8-position in the
molecule; some sulfur-containing conversion products have also been
identified.
7. EFFECTS OF 2,4-D ON ANIMALS
7.1. General Introduction
Many studies of the toxicity of 2,4-D were carried out before
the possible toxicological importance of manufacturing by-products,
such as 2,6-dichlorophenoxyacetic acid, 2,4,6-trichlorophenoxy-
acetic acid (2,6-D and 2,4,6-T) monochlorophenoxyacetic acid, or
N-nitroso compounds, was known or appreciated.
Furthermore, 2,4-D may be contaminated with several chlorinated
dibenzo- p-dioxins (section 2.1.4). The toxicity of a number of
these contaminants has not been examined in detail, but whether or
not their presence would affect the toxicity of 2,4-D and its
derivatives would depend on the amount of CDD present in the
product, and on the inherent toxicity of the particular CDD
isomers. The most toxic CDD isomer, namely 2,3,7,8-TCDD (Schwetz
et al., 1973; McConnell et al., 1978; Leng, 1979; Kociba & Schwetz,
1982), is not normally found in 2,4-D products (see also section
2.1.4). However, there have been instances in which the same
manufacturing equipment was used to produce both 2,4,5-T and 2,4-D,
resulting in cross-contamination of 2,4-D with 2,4,5-T and 2,3,7,8-
TCDD (US EPA, 1982).
Studies of structure-activity relationships using in vitro
systems have shown that the CDDs that may be present in 2,4-D and
its derivatives have a much lower biological activity than 2,3,7,8-
TCDD (Poland & Glover, 1973; Poland & Kende, 1974; Poland et al.,
1976; Bradlaw et al., 1980; Knutson & Poland, 1980). However,
except for a study of the carcinogenic potential of 2,7-
dichlorodibenzodioxin (2,7-DCDD) (US National Cancer Institute,
1979), the chronic toxicity of the CDD detected in 2,4-D and its
derivatives has not been studied.
2,4-D has been in use as a herbicide for nearly 40 years, and
during this time a great deal of literature on the toxicology of
this chemical has accumulated. The extent to which its toxicity to
various organisms has been tested, and the types of 2,4-D products
used for such testing have varied over the years. Earlier 2,4-D
products probably contained higher concentrations of trace
contaminants than the 2,4-D in use today, and therefore it may have
been found to be more toxic in earlier than in more recent studies.
In addition, the generally accepted standards and protocols for
pesticide toxicity tests have changed, making some of the older
tests inadequate by present day standards. For these reasons,
attention has been focused on the more recent studies. The older
studies are, in many instances, only cited for completeness and
should be used with caution, especially when ascribing specific
toxic effects to unspecified 2,4-D products and when establishing
an effect level or no-observed-adverse-effect level for adverse
effects of 2,4-D. The Task Group noted that a number of additional
studies on 2,4-D are at present in progress. As the additional
information becomes available, the present document will need to be
updated.
7.2. Acute Effects
The reports of experimental studies to define the toxic and
other effects of 2,4-D or its derivatives cover a wide range of
organisms commonly referred to as "animals", including worms,
molluscs, arthropods, lower vertebrates, birds, and mammals. Much
of this information, especially on acute toxic effects, was
recently tabulated by the National Research Council of Canada,
Associate Committee on Scientific Criteria for Environmental
Quality (1978), Schneider (1979), Bovey & Young (1980), Shearer &
Halter (1980), and the Commission of the European Communities (CEC,
1981).
The usual mandatory acute and subacute safety tests for
pesticides include assays for eye and skin irritancy and for skin
sensitization, and determinations of the acute oral, percutaneous,
and parenteral lethal doses, or of the corresponding lethal
concentrations in the air, diet, or water.
7.2.1. Skin and eye irritancy
2,4-D does not appear to be an eye or skin irritant (Schneider,
l979). Adequate tests of the potential irritative properties of
2,4-D derivatives have not been reported in the literature.
7.2.2. Skin sensitization
No adequate published information is available on the dermal
sensitization potential of 2,4-D and its derivatives in mammals.
7.2.3. Lethal doses and concentrations (LD50 and L50)
The lethal potential of a chemical is usually measured as the
dose (mg/kg body weight), or as the concentration in the air, diet,
or water (mg/kg, mg/m3, mg/litre, respectively) that will kill 50%
of the test animals in a specified time interval. These amounts
are referred to as the LD50 or LC50. For 2,4-D and its derivatives,
and for 2,4-D herbicide formulations, these statistically estimated
values vary depending on the test product, the test species, and
the route and frequency of administration (Tables 16 and 17).
7.2.3.1. Acute oral LD50
7.2.3.1.1. Mammals
Published acute oral LD50 values vary for different 2,4-D
products and test species (Table 16). It appears that 2,4-D has a
moderate acute toxicity for mammals (WHO, 1976).
7.2.3.1.2. Birds
Table 17 shows published oral LD50 values for chickens.
Table 16. Acute oral toxicity of 2,4-D, esters, and salts
------------------------------------------------------------------------
Compound Species Sex LD50 Reference
(mg/kg bw)
------------------------------------------------------------------------
2,4-D mouse M 375 Hill & Carlisle (1947)
mouse M 368 Rowe & Hymas (1954)
rat M 375 Rowe & Hymas (1954)
rat 666 Hill & Carlisle (1947)
guinea-pig M & F 469 Rowe & Hymas (1954)
guinea-pig 1000 Hill & Carlisle (1947)
rabbit 800 Hill & Carlisle (1947)
dog 100 Drill & Hiratzka (1953)
butyl ester mouse 380 Konstantinova (1970)
rat 1500 Schillinger (1960)
rat 920 Konstantinova (1970)
cat 820 Konstantinova (1970)
esters of mono-, rat F 570 Rowe & Hymas (1954)
di-, and tripro-
pylene glycol
butyl ethers
isopropyl ester mouse M 541 Rowe & Hymas (1954)
rat M & F 700 Rowe & Hymas (1954)
guinea-pig M 550 Rowe & Hymas (1954)
mixed butyl mouse F 713 Rowe & Hymas (1954)
esters rat F 620 Rowe & Hymas (1954)
guinea-pig F 848 Rowe & Hymas (1954)
rabbit M & F 1420 Rowe & Hymas (1954)
sodium salt mouse 375 Rowe & Hymas (1954)
rat F 805 Rowe & Hymas (1954)
rat 2000 Schillinger (1960)
guinea-pig M 551 Rowe & Hymas (1954)
rabbit 800 Rowe & Hymas (1954)
------------------------------------------------------------------------
7.2.3.2. Acute dermal LD50
7.2.3.2.1. Mammals
Reports by Buslovich (1963) and Fetisov (1966) indicate that,
under extreme test conditions, mice and rats may absorb lethal
amounts of 2,4-D amine salts and esters through their skin, but no
acute dermal LD50 values were available for review.
7.2.3.3. Acute inhalation LC50
No accurate measurements of acute inhalation LC50 values
for 2,4-D products were available.
Table 17. Acute LD50 for various 2,4-D products in domestic chickens (Gallus domesticus)
------------------------------------------------------------------------------------------------------------------
Product Animals Procedure LD50 Acid References
(mg/kg bw) equivalent
------------------------------------------------------------------------------------------------------------------
2,4-D chick, M & F P.O., in olive oil 541 (358-817) 541 Rowe & Hymas (1954)
2,4-D Na salt adult hen P.O., in water 655 646 Loktionov et al. (1973)
2,4-D alkanolamine chick P.O., in water > 380 < 765 368 Bjorn & Northern (1948)
salts Rowe & Hymas (1954)
2,4-D amine salt 6-month chicken P.O., in water 1950 1238 Loktionov et al. (1973)
2,4-D butyl esters chick, M & F P.O., undiluted 2000 (1350-2960) 1503 Rowe & Hymas (1954)
2,4-D butoxyethyl chick, 330 g P.O., in food 900 588 Whitehead & Pettigrew
esters (1972)
2,4-D isopropyl esters chick, M & F P.O., in olive oil 1420 (1127-1789) 1145 Rowe & Hymas (1954)
2,4-D/2,4,5-T (1:1), chick, M & F P.O., undiluted 4000 (2700-5900) - Rowe & Hymas (1954)
butyl ester 3 weeks old
------------------------------------------------------------------------------------------------------------------
7.2.3.4. Parenteral LD50
The acute parenteral LD50 values reported for various
laboratory animals and 2,4-D products ranged from 220 to 666 mg
a.i./kg body weight (National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality, 1978;
Young et al., 1978; Schneider, 1979).
7.2.4. Acute toxicity in aquatic organisms
The available reports indicate great differences in acute LC50
values obtained with different test species, and with different
2,4-D derivatives and test formulations. The esters appear to be
considerably more toxic than the water-soluble salts. For fish,
the LC50 for various 2,4-D isooctyl ester products may range from 5
to 68 mg/litre for bluegill sunfish (Lepomis spp.) and from 62 to
153 mg/litre for rainbow trout (Salmo gairdneri) (Schneider,
1979). This variability may, in part, be due to differences in
test animal species and strains, in test conditions (temperature,
pH, 02 tension, mineral content of water), and, in part, to the
effects of chemicals other than 2,4-D in the herbicide
formulations, or of 2,4-dichlorophenol, which may occur in water
as a decomposition product of 2,4-D (Holcombe et al., 1980).
Discussions on this variablility can be found in numerous reviews:
Way (1969), Katz et al. (1972), National Research Council of
Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), and of Halter (1980), as well as in
the reports of studies by Harrisson & Rees (1946), King & Penfound
(1946), Lopez (1961), Hughes & Davis (1963), Butler (1965),
Hiltibran (1967), Mount & Stephan (1967), Alabaster (1969), Sanders
(1970a), Andrushaitis (1972), Cooke (1972), Shim & Self (1973),
Fabacher & Chambers (1974), Meehan et al. (1974), Nishiuchi &
Yoshida (1974), Rehwoldt et al. (1977), Vardia & Durve (1981), and
particularly in the extensive and methodical investigation of
Pravda (1973).
Similar information on aquatic invertebrates is available in
the review of Mackenthun & Keup (1972) and in the studies of Hooper
(1958), Sudak & Claff (1960), Beaven et al. (1962), Rawls (1965),
Sanders (1970b), Klekowski & Zvirgzds (1971), Wierzbicka (1974a,b),
and Caldwell et al. (1979).
The observations of Hansen et al. (1972, 1973) and Folmar
(1976) indicate that fish and aquatic invertebrates will try
to avoid water containing toxic amounts of 2,4-D products.
7.3. Subchronic and Chronic Toxicity
7.3.1. Mammals
Most of the published long-term studies with mammals have
already been reviewed by Bodyagin et al. (1969), Way (1969), IARC
(1977, 1982), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1978),
Young et al. (1978), Bovey & Young (1980), Shearer (1980), and US
Veterans' Administration (1981).
In the long-term as in the short-term studies on mammals, the
test products were mainly administered orally. The composition of
the test products was not adequately known by present standards,
but many of the tests were carried out with more or less purified
2,4-D or its alkali salts. It is difficult to decide to what
extent the available results reflect the toxicological properties
of the present 2,4-D products, in which the maximum contents of CDD
and other toxic by-products are kept at very low levels.
A long-term feeding study in the rat (Hansen, 1971) was
evaluated by FAO/WHO JMPR in 1971 (FAO/WHO, 1972). It was
concluded that a no-observed-adverse-effect level in the rat was
equivalent to 31 mg/kg body weight per day. However, new studies
on mice and rats are in progress.
7.3.1.1. Clinical signs of poisoning
Signs of toxic effects on the digestive tract, such as
diarrhoea, vomiting, dysphagia, decreased gut motility, irritation,
or necrotic changes (in dogs including necrosis of oral tissues)
are likely to appear in animals following the absorption of high
doses of 2,4-D or its derivatives by the oral, dermal, or
inhalation routes, and after parenteral injections (Bucher, 1946;
Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Thomssen, 1958; Björklund & Erne, 1966; Erne, 1966a; Palmer &
Radeleff, 1969). Blood-tinged discharges from the nose, and in
dogs also nasal and eye irritation and skin lesions may also occur
(Bucher, 1946; Hill & Carlisle, 1947; Kosyan et al., 1974). Cattle
may suffer from tympanitis, and may show signs of thirst (Björklund
& Erne, 1966). However, some of the signs of "2,4-D poisoning"
reported in herbivores pastured on 2,4-D-treated vegetation may
have been caused by the ingestion of inherently poisonous plants
(Maclean & Davidson, 1970). Pigs may refuse to eat feed containing
high amounts of 2,4-D (Strach & Bohosiewicz, 1964), but sheep have
been reported to feed avidly on 2,4-D-treated vegetation (Sadykov
et al., 1972).
Characteristic signs of severe 2,4-D poisoning in mammals
appear to be muscular weakness, stiffness, stilted gait, and muscle
spasms (myotonia), alleviated by exercise and exacerbated by rest.
There may also be muscular incoordination progressing to paralysis
especially in the hind limbs; in rodents, caudal rigidity has also
been observed. These clinical signs are the result of the myotoxic
action of 2,4-D discussed below, which, through its effects on the
heart, may also lead to hypo- or hypertension, cardiac
fibrillation, and death. Prolonged inactivity may also occur and
may lead to pulmonary congestion, emphysema, and pneumonia (Bucher,
1946; Hill & Carlisle, 1947; Drill & Hiratzka, 1953; Vinokurova,
1960; Björklund & Erne, 1966; Gorshkov, 1972; Sadykov et al., 1972;
Kosyan et al., 1974).
At high doses, 2,4-D and its derivatives may act as a central
nervous system depressant and cause lethargy, slowed respiration,
stupor, coma, and death (Bucher, 1946; Hill & Carlisle, 1947; Drill
& Hiratzka, 1953; Vinokurova, 1960; Desi et Sos, 1962a,b; Desi et
al., 1962a,b; Kosyan et al., 1974; Elo & Ylitalo, 1977, 1979).
7.3.1.2. Effects on food and water consumption, and on body weight
Relatively high concentrations of 2,4-D or its derivatives in
the diet or drinking water, or given by capsule, gavage, or
parenterally, may cause a reduction in food and water consumption,
and weight loss or reduced body weight gain in rats (Rowe & Hymas,
1954; Thomssen, 1958; Björklund & Erne, 1966; Chang et al., 1974;
Chen et al., 1981; Gorshkov, 1972), as well as in dogs (Bucher,
1946; Drill & Hiratzka, 1953), in pigs (Strach & Bohosiewicz, 1964;
Björklund & Erne, l966), in rabbits (Loktionov et al., 1973), in
cattle (Rowe & Hymas, 1954; Björklund & Erne, 1966; Palmer &
Radeleff, 1969; McLennan, 1974), and sheep (Palmer & Radeleff,
1969). However, the same authors, as well as Guseva (1956) and
Hansen et al. (1971) did not observe these effects at low doses or
low dietary concentrations of 2,4-D. In fact, in the studies by
Raoul & Marnay (1948) and Strach & Bohosiewicz (1964) on rats and
piglets fed low dietary concentrations of 2,4-D, an unimpaired
appetite and an improved body weight gain was seen in some groups
of animals. In these studies, the dosages ranged between 15 and
119 mg 2,4-D/kg body weight.
Observations of Thomssen (1958), Strach & Bohosiewicz (1964),
Martynov (1970), Gorshkov (1972), Sadykov et al. (1972), and Erne
(1974), on hares (Lepus timidus), European elk, pigs, and rats
suggest that, given a choice, animals will refuse to eat food or to
drink water containing more than a certain amount of 2,4-D, and
that this may, in part, be because of the strong characteristic
odour and taste of this compound, or of organic solvents such as
diesel fuel that are used in herbicide formulations or as diluents.
7.3.1.3. Effects on the central nervous system (CNS)
It has been suggested that the signs of central nervous system
depression in animals severely poisoned with 2,4-D are related to a
partial breakdown of the blood-brain barrier, possibly as a result
of damage to capillary vessels, and a subsequent accumulation of
2,4-D in the CNS (Desi & Sos, 1962a,b; Elo & Ylitalo, 1977, 1979).
However, further studies are needed to clarify the mechanism(s)
by which 2,4-D acts on the central nervous system.
7.3.1.4. Effects on the peripheral nervous system
No peripheral neuropathy was attributed to 2,4-D in the
available reports on short-term and long-term studies with 2,4-D in
a variety of animals (Shillinger & Naumova, 1957; Stupnikov, 1959).
The partial or complete paralysis, especially of the hind legs, of
2,4-D poisoned animals reported already by Bucher (1946) and Hill &
Carlisle (1947) may be a myotoxic rather than a neurotoxic effect
of 2,4-D. Moreover, in severe 2,4-D poisoning, a general weakness
may lead to inactivity that might be interpreted as paralysis.
No signs of neuropathy were reported by Kay et al. (1965) in
rabbits given large percutaneous doses of 2,4-D dimethylamine salt,
2,4-D butyl ester, or 2,4-D isooctyl ester. In similar studies in
which rats or rabbits were exposed by the dermal route, Buslovich
(1963) reported myotonia and death in rats given unspecified doses
of 2,4-D amine salt or 2,4-D butyl ester, but no peripheral
neuropathy, while Vinokurova (1960) found 130 - 180 mg of a 50%
aqueous emulsion of 2,4-D octyl ester/kg body weight to have "no
systemic effect" on rabbits.
The neurotoxic effects in animals of 2,4-D and of other related
compounds have not been adequately studied. Further research is
required to elucidate the mechanism of the neurotoxic and myotoxic
action of 2,4-D in animals.
7.3.1.5. Myotoxic effects
Most of the studies of the effects of 2,4-D on vertebrate
muscles were carried out because the 2,4-D-induced abnormalities
resemble a heritable muscle disorder in human beings, namely
myotonia congenita (Hofmann et al., 1966; Laskowski & Dettbarn,
1977). As a rule, high doses of 2,4-D (1/4 to 1/2 LD50) are
required to obtain severe and prolonged myotonia. The effects of
2,4-D on muscle cells are complex, and include disturbances in:
the activity of various enzymes (leading, for example, to increased
lactate production); potassium levels; membrane resistance; and in
chloride conductance. There are also shifts in calcium-binding
sites, changes in muscle and nerve cell electrical potentials, and
mitochondrial structural and ultrastructural degenerative changes
in muscle (Eyzaguirre et al., 1948; Kuhn & Stein, 1964, 1965, 1966;
Heene 1966, 1968, 1975; Hofmann et al., 1966; Kenigsberg, 1968;
Stein & Kuhn, 1968; Bodem et al., 1971; Preiss & Rossner, 1971;
Seiler, 1971; Buslovich & Koldobskaya, 1972; Rüdiger et al., 1972;
Senges & Rüdel, 1972; Brody, 1973; Danon et al., 1976; Iyer et al.,
1976; Bretag & Caputo, 1978; Dux et al. 1978; De Reuck et al.,
1979; Eberstein & Goodgold, 1979; Mazarean et al., 1979a,b).
Similar effects are produced by the chlorophenoxy drug clofibrate
(atromid) (Pierides et al., 1975; Smals et al., 1977). None of the
available studies on 2,4-D-induced myotonia were designed to
establish no-observed-adverse-effect levels for the various
myotoxic effects in intact animals, and therefore additional
studies should be carried out for this purpose.
7.3.1.6. Cardiovascular effects
As part of its myotoxic action, 2,4-D and its derivatives may
in high doses cause biochemical, physiological, and structural
damage to the myocardium in vitro and in vivo (Bodem et al., 1971;
Preiss & Rossner, 1971; Rüdiger et al., 1972; Mazarean et al.,
1979a,b).
7.3.1.7. Haematological effects
Shifts have been reported in the number or types of
erythrocytes, leukocytes, or bone marrow cells, or changes in
haemoglobin levels, in a variety of laboratory and domestic mammals
given 2,4-D or 2,4-D derivatives (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Hansen et al., 1971;
Sadykov et al., 1972; Loktionov et al., 1973; Kosyan et al., 1974;
and Halliop et al., 1980).
7.3.1.8. Effects on blood chemistry
Rowe & Hymas (1954) were apparently the first investigators to
monitor blood chemistry in 2,4-D-treated animals. They did not
observe any adverse effects at 2,4-D dietary levels as high as 300
mg 2,4-D/kg in rats treated for 113 days.
Other investigators noted changes in various serum, plasma, or
erythrocyte enzyme activity levels, and shifts in the levels of
electrolytes, glucose, blood proteins, or other chemicals in
response to treatment with 2,4-D or 2,4-D derivatives in rats,
rabbits, cattle, pigs, or sheep, or in blood from such animals to
which 2,4-D was added in vitro (Shillinger & Naumova, 1957;
Schillinger, 1960; Björklund & Erne, 1966; Shevchenko, 1966;
Dzhaparov & Tsilikov, 1969; Hunt et al., 1970; Szöcs et al., 1970;
Gorshkov, 1972; Kosyan et al., 1974; Kuzminskaya & Bersan, 1975;
Chen et al., 1981). Some of the changes in transaminase (SGOT,
SGPT), blood urea nitrogen (BUN), or glucose levels appeared to be
secondary to myotoxic, nephrotoxic, or hepatotoxic effects of high
doses of 2,4-D.
7.3.1.9. Other biochemical effects observed in vivo or in vitro
Sufficiently high doses of 2,4-D may induce changes in
mitochondrial oxygen consumption and oxidative phosphorylation, in
electrolyte, ascorbic acid, glycogen, lipid, and nucleic acid
contents of organs, tissues, cells, organelles, or cell fractions
from a variety of mammals (Brody, 1952, 1973; Guseva, 1956; Baker
et al., 1960; Kuhn & Stein, 1964; Heene, 1966, 1967, 1968;
Shevchenko, 1966; Philleo & Fang, 1967; Kenigsberg, 1968; Dzhaparov
& Tsilikov, 1969; Stanosz, 1969; Buslovich & Koldobskaya, 1972;
Graff et al., 1972; Buslovich et al., 1973; Abo-khatwa &
Hollingworth, 1974; Chang et al., 1974; Nikandrov, 1974; Venkaiah &
Patwardhan, 1978; Podolak, 1979).
7.3.1.10. Pulmonary effects
No pulmonary abnormalities were reported in studies with mice,
rabbits, rats, or dogs conducted by Bucher (1946), Drill & Hiratzka
(1953), Rowe & Hymas (1954), Shillinger & Naumova (1957),
Schillinger (1960), or Björklund & Erne (1966). In contrast, Hill
& Carlisle (1947) and Palmer (1972) noted congestion of pulmonary
vessels, pulmonary petechial haemorrhages, and pulmonary edema or
emphysema in mice, rats, dogs, cattle, or sheep that died of 2,4-D
poisoning.
7.3.1.11. Hepatotoxic effects
Rabbits, rats, mice, dogs, cattle, or sheep treated for a
prolonged period with toxic doses of 2,4-D were found to develop a
subacute toxic hepatitis with congestion of hepatic blood vessels,
and cloudy swelling, fatty infiltration, local necrosis,
degeneration, or atrophy of hepatocytes, especially of the
parenchyma in the centrolobular areas (Bucher, 1946; Hill &
Carlisle, 1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954;
Björklund & Erne, 1966; Szöcs et al., 1970; Palmer, 1972). High
doses of 2,4-D may induce a proliferation of peroxisomes and
increased levels of mixed function oxidases in liver cells of rats
and hamsters (Buslovich et al., 1982; Vainio et al., 1982, 1983).
Changes in the levels of certain liver enzymes such as maleate
or succinic dehydrogenase, and in ascorbic acid or glycogen content
or hippuric acid production have also been reported (Shillinger &
Naumova, 1957; Baker et al., 1960; Dzhaparov & Tsilikov, 1969;
Szöcs et al., 1970; Buslovich et al., 1973; Chang et al. 1974;
Nikandrov, 1974). Some of the results concerning liver glycogen
levels in rats suggest a reverse trend at high doses, as Dzhaparov
& Tsilikov (1969) found that a dose equivalent to 1/16 LD50 per day
lowered the liver glycogen content, while Chang et al. (1974)
observed the opposite effect at doses of about 100 mg/rat per day.
7.3.1.12. Effects on the kidney
In early studies with high doses of 2,4-D, signs of impaired
kidney function, increased relative kidney weight, and of gross and
histological abnormalities (parenchymatous degeneration,
hypertrophy, and hyperplasia, cloudy swelling especially in the
cells of the proximal convoluted tubules, and glomerular lesions)
were noted in mice, rats, and dogs (Bucher, 1946; Hill & Carlisle,
1947; Drill & Hiratzka, 1953; Rowe & Hymas, 1954). That the kidney
is a target organ for the structural, physiological, and chemical
effects of 2,4-D was confirmed repeatedly by later and more
detailed studies on a wider range of test species including pigs,
goats, and sheep (Schillinger, 1960; Björklund & Erne, 1966; Erne,
1966a; Stanosz, 1969; Hunt et al., 1970; Milhaud et al., 1970;
Gorshkov, 1972; Palmer, 1972; Senczuk & Pogorzelska, 1975; Koschier
et al., 1978; Orberg, 1980a). In a recent 13-week study on rats,
the no-observed-adverse-effect level for histological changes
induced with pure 2,4-D in mammalian kidney appeared to be 15 mg/kg
body weight per day (Chen et al., 1981).
7.3.1.13. Effects on endocrine organs
Swelling and congestion of the thyroid were noted by Palmer
(1972) in cattle and sheep fatally poisoned with various 2,4-D
products. Effects of 2,4-D on iodide uptake by the thyroid gland
were first decribed by Sos & Kertai (1958) and studied further by
Florsheim & Velcoff (1962), Florsheim et al. (1963), Tsilikov
(1969), and Gorshkov (1972). Their results indicate that doses of
5 - 250 mg 2,4-D sodium salt or equivalent/kg body weight per day
have a stimulatory effect on thyroid function.
Effects of 2,4-D on adrenal function and its relationship to
muscle carbohydrate metabolism and 2,4-D-induced myotonia have been
studied by Kenigsberg (1968), and by Buslovich & Koldobskaya
(1972). 2,4-D-induced changes in adrenal or thyroid function may
also be implicated in the abnormal temperature regulation in 2,4-D-
treated rats described by Sudak et al. (1966). However, it is
noteworthy that Buslovich (1963) was unable to induce a change in
the body temperature of rats by giving 15 - 20 oral doses of 2,4-D
sodium salt, amine salt, or butyl ester at a level of 1/10 or 1/5
LD50/day.
7.3.1.14. Effects on the digestive tract
Vomiting, diarrhoea, hyperaemia, bloody exudates in the gut,
necrotic changes in the mucosa, and other non-acute toxic effects
on the digestive tract have been reported after administration of
high doses of 2,4-D by either the oral or parenteral route in mice,
rats, and dogs (Bucher, 1946; Hill & Carlisle, 1947; Drill &
Hiratzka, 1953; Kosyan et al., 1974). However, Hansen et al.
(1971) did not observe any such effects in a 2-year feeding study
at dietary levels of 2,4-D corresponding to about 37.5 and 67.5 mg
2,4-D/kg body weight per day, respectively, for rats and dogs.
Their observations on dogs contrast with those of Drill & Hiratzka
(1953) who found repeated doses of 20 mg 2,4-D/kg body weight per
day to be fatal in 3 out of 4 dogs studied by them.
7.3.2. Birds
The published reports on the toxic effects of 2,4-D and 2,4-D
products in birds deal mainly with chickens (Gallus domesticus)
and game birds. The available data on cumulative oral lethal doses
for game birds have been summarized in Table 18; studies on the
reproductive, embryotoxic, and teratogenic effects of 2,4-D are
dealt with in section 7.3.6.
Table 18. Cumulative oral lethal doses of 2,4-D herbicides for game birds
------------------------------------------------------------------------------------
Product Species Dietary LC50 Cumulative LD50 References
(mg a.i./kg (mg a.i./kg bw)a
diet)a
------------------------------------------------------------------------------------
2,4-D quail (young) 5000 28 000 Dewitt et al.
dimethylamine (1962)
salt
mallard duck 5000 8250
(young) 5000 8250
(adult) > 2500 > 34 000
2,4-D quail
butoxyethanol (young) 5000 38 000
ester (adult) 5000 40 700
pheasant (young) 5000 29 500
mallard duck (adult) 5000 > 33 000
------------------------------------------------------------------------------------
a a.i. = active ingredient
The clinical signs of 2,4-D poisoning in birds appear to be
similar to those in mammals, and the kidney seems to be the most
sensitive organ. No adverse effects were reported in chickens fed
dietary levels of 1000 mg/kg for 21 days (142 mg/kg body weight)
whereas kidney enlargement occurred in chickens fed 5000 mg/kg of
diet for 21 days (Whitehead & Pettigrew, 1972).
7.3.3. Cold-blooded animals
The literature on the chronic effects of 2,4-D and its
derivatives on cold-blooded animals (poikilotherms) was not
reviewed in detail. The available reports indicate that for
aquatic vertebrates in general, 2,4-D esters are more toxic than
2,4-D amines, and that the overall no-observed-adverse-effect level
for toxic effects on fish for the esters is at or near 1 mg/litre
(King & Penfound, 1946; Zhiteneva & Chesnokova, 1973; Fabacher &
Chambers, 1974; Meehan et al., 1974). 2,4-dichlorophenol, which
may occur in water as a by-product or transformation product of
2,4-D, is similarly toxic to fish (Holcombe et al., 1980).
7.4. Fetotoxicity, Teratogenicity, and Reproductive Effects
The scientific literature contains a fairly large number of
reports on the fetotoxic, teratogenic, and reproductive effects of
2,4-D in livestock and in laboratory animals. However, most of the
observations on livestock are either too sketchy to be useful or
neglect to take into account various confounding factors. Examples
of this are studies by Björklund & Erne (1966) on a single pregnant
pig, by Sadykov et al. (1972) on sheep grazing on pastures
apparently containing a high percentage of poisonous plants, and
the report by Bodai et al., (1974), on reproductive disturbances in
cattle feeding on either 2,4-D-treated vegetation possibly
including poisonous plants, or on contaminated silage.
Some of the studies carried out with rodents under laboratory
conditions also provide little useful information, either because
it is difficult or impossible to determine the dose levels used in
the studies (Weinmann, 1957; Schuphan, 1963, 1965, 1969; Schiller,
1964; Buslovich et al., 1976); or because the information provided
is insufficient and the studies cannot be properly evaluated
(Bucher, 1946; Hansen et al., 1971; King et al., 1971). The study
by Weinmann (1957) can be considered invalid, as a high mortality
occurred in both control and experimental animals, when they were
exposed to cold stress because of construction work affecting the
animal quarters during the winter months. Moreover, Weinmann
(1957) and several other authors, including Schuphan (1963, 1965,
1969) Schiller (1964), Schillinger (1960), and Shillinger & Naumova
(1957) did not in fact examine the effects of 2,4-D, but rather the
effects of foods or food extracts prepared from crops that had been
sprayed with 2,4-D, and, in some cases, also with other plant
growth substances. As none of these authors appears to have
carried out an analysis to demonstrate the presence of 2,4-D
residues in the test material, it is questionable whether the test
animals ingested any 2,4-D or 2,4-D residues, and these studies are
therefore not reviewed in detail. Buslovich et al. (1976) tested
only a single dose level (1/2 LD50) and this reduces the usefulness
of their study. Pertinent reports are discussed below.
7.4.1. Rats
7.4.1.1. Effects on adult rats
No deleterious effects on the health or fertility of rats
receiving the maximum tolerated dose of 87.5 mg/kg body weight in
terms of 2,4-D or its molar equivalent of the isooctyl ester or the
propylene glycol butyl ether ester per day, on days 6 - 15 of
pregnancy, were reported by Schwetz et al. (1971) and Unger et al.
(1980). Even higher amounts of 2,4-D or its equivalent in 2,4-D
derivatives were used by Björklund & Erne (1966), Hansen et al.,
(1971) and Khera & McKinley (1972) with similar results.
Reduced testis and prostate size, abnormal spermatogenesis (and
also liver and kidney damage) were reported by Schillinger (1960)
in some of the male rats given 375 mg/kg body weight per day (about
1/4 LD50) of a Soviet-made 2,4-D butyl ether formulation containing
polyethylene glycol alkyl phenyl ether surfactant. These effects
were not noted at 1/10 of this dose level, i.e., at 37.5 mg/kg body
weight per day. Some of the toxic effects reported by Schillinger
(1960) may have been caused by the surfactant, but as a surfactant
control group was not included in this study, this cannot be
confirmed. Thus, the available studies suggest that the no-
observed-adverse-effect level for reproducing adult rats lies
between 37.5 and 87.5 mg 2,4-D/kg body weight per day.
7.4.1.2. Effects on offspring
Björklund & Erne (1966) gave pregnant rats a 2,4-D
concentration in drinking-water of 1000 mg/litre during pregnancy,
and for the following 10 months. No effects on reproduction were
noted.
Hansen et al. (1971) fed male and female rats dietary levels of
technical 2,4-D of 100, 500, and 1500 mg/kg (ppm), and the rats
were bred through 3 successive generations. At dietary levels of
100 and 500 mg/kg, no effects were noted. However, at a dietary
level of 1500 mg/kg, survival of pups to weaning was reduced. The
number of pups surviving ranged from 70 - 97% in the control group
and from 60 - 93% at the 100 and 500 mg/kg (ppm) dietary levels;
survival at the highest dose ranged from 20 - 62%.
Resorptions, reduced fetal weight and size, enlarged ventricles
of the brain and haemoperitoneum were found by Buslovich et al.
(1976) in the offspring of rats treated with two different 2,4-D
derivatives at a dose of one-half LD50 (value unspecified).
Schwetz et al. (1971) dosed female rats from day 6 - 15 of
pregnancy by gavage at dose levels of 0, 12.5, 25, 50, and 87.5
mg/kg body weight per day with 2,4-D or equimolar dose levels of
the propylene glycol butyl ether (PGBE) and the isooctyl esters
(IO). At doses of 50 and 87.5 mg/kg, a decrease in fetal body
weight was noted for all three compounds.
Subcutaneous oedema, delayed ossification of sternebrae,
sternebrae with split centres of ossification, wavy ribs, and
lumbar ribs increased with increasing doses, for at least one of
the agents studied. However, these anomalies were not
significantly increased at the 12.5 or 25 mg/kg dose level for the
3 compounds. A small but significant increase in the incidence of
subcutaneous oedema was observed in fetuses from dams receiving
12.5 mg/kg molar equivalent of IO. The incidence of missing
sternebrae was significantly increased at dose levels of 87.5 mg/kg
for 2,4-D and 12.5 and 87.5 mg/kg for PGBE.
Unger et al. (1980), in a later study, using the same dosing
regimens for 2,4-D IO and 2,4-D PGBE did not observe the effects
reported by Schwetz et al., except for a statistically significant
increase in rib buds at the highest dose (87.5 mg/kg) tested for
both compounds ( P < 0.05).
Khera & McKinley (1972) dosed female rats from day 6 - 15 of
pregnancy by gavage with 2,4-D, 2,4-D IO, 2,4-D butyl ester, 2,4-D
butoxyethanol ester and 2,4-D dimethylamine salt. The butyl and
isooctyl ester depressed fetal weight and decreased fetal viability
at the highest dose of 150 mg/kg body weight. Wavy ribs,
additional ribs, retarded ossification and sternal defects, fused
ribs, small-sized distorted scapula and micromelia were observed as
anomalies among the treated groups. A statistically significant
increase in malformed fetuses was noted at 2,4-D levels of 25 mg/kg
body weight ( P < 0.05) and at levels of 150 mg/kg or more for the
other compounds.
Konstantinova et al. (1975) reported hemorrhage into internal
organs in the fetuses of rats treated with a 2,4-D level of 50
mg/kg body weight.
The results of all these studies suggest that dosage levels of
less than 12.5 mg/kg body weight for the various 2,4-D derivatives
do not cause fetotoxic or teratological effects in rats, and the
results of the more recent study by Unger et al. (1980) indicate
that higher doses may be without deleterious effects on the
fetuses.
Thus, at present, a daily dose level of 10 mg 2,4-D or 2,4-D
acid equivalent/kg body weight can be considered to be without
significant fetotoxic or teratogenic effects in rats.
7.4.2. Mice
The report by Courtney (1977) indicated that in CD-1 mice,
doses of 1 mM/kg body weight of 2,4-D and its n-butyl and PGBE
esters reduced fetal body weight, and increased fetal mortality.
The compounds were also teratogenic, inducing cleft palates, at
levels of 124 mg/kg body weight per day (2,4-D or acid equivalent)
or more. However, the 2,4-D isopropyl and isooctyl esters appeared
to be less teratogenic than 2,4-D, as they did not induce birth
defects at a 2,4-D equivalent level of 124 mg/kg body weight per
day. Furthermore, the 2 esters did not induce fetal death in CD-1
mice at doses of 2,4-D equivalent up to 221 mg/kg body weight per
day.
7.4.3. Birds
In the 1960s and 1970s, a number of tests of the embryotoxic,
teratogenic, and other reproductive effects of 2,4-D were carried
out on birds' eggs and embryos. These results may not apply to
mammals, because bird embryos develop in a closed environment
different from that of mammalian embryos, and because birds differ
anatomically from mammals.
Lutz-Ostertag & Lutz (1970, 1974) reported mortality and severe
deformities in wild bird embryos exposed to 2,4-D amine salt, but
they did not provide crucial experimental details, and other
investigators (Dunachie & Fletcher, 1967, 1970; Kopischke, 1972;
Grolleau et al., 1974; Gyrd-Hansen & Dalgaard-Mikkelsen, 1974;
Somers et al., 1974a,b,c; Hilbig et al., 1976a,b; Spittler, 1976)
were unable to duplicate their results either with 2,4-D amine
salts or esters. Some of the teratogenic effects attributed to
2,4-D by Lutz-Ostertag & Lutz (1970, 1974) resemble those induced
by excessively high incubation temperatures (Nielsen, 1968), and
may thus have been experimental artifacts.
On the whole, the available studies on bird embryos indicate
that the no-observed-adverse-effect level for 2,4-D-induced
embryotoxic and teratogenic effects lies near 0.5 mg active
ingredient/egg (equivalent to about 10 mg/kg) and is thus similar
to that in mammals.
7.4.4. Cold-blooded animals
The available literature contained little information
concerning the possible reproductive, embryotoxic, or teratogenic
effects of 2,4-D or 2,4-D derivatives on cold-blooded animals.
7.4.4.1. Amphibians
Lopez (1961) noted that the motility of frog spermatozoa was
not affected by low concentrations of 2,4-D or its sodium salt, and
that the inhibition of movement, or the lysis observed under some
conditions were caused by changes in the pH of the test solution.
Aqueous solutions of less than 0.05% 2,4-D sodium salt did not
induce any macroscopic abnormalities in developing frog eggs or
embryos (Lhoste & Roth, 1946), while Cooke (1972) did not find
either toxic effects or 2,4-D residues in frog tadpoles exposed for
1 or 2 days to up to 50 mg 2,4-D/litre. According to Sanders
(1970a) a commercial 2,4-D dimethylamine salt had an LC50 of 100
mg/litre for frog tadpoles.
7.4.4.2. Fish
Only three brief reports were available on the effects of 2,4-D
on developing fish eggs and embryos. Andrusaitis (1972) found that
2,4-D reduced the oxygen consumption of 32-cell blastomeres, and
increased the oxygen consumption in 64 - 128 cell blastomeres of
Misgurnus fossilis. In a study by Mount & Stephan (1967), 2,4-D
butoxyethanol ester (BEE) at a concentration of up to 0.31
mg/litre, or 2,4-D at 0.80 mg/litre did not reduce the reproduction
rate in fathead minnows (Pimephales promelas), whereas 2,4-D BEE
at 1.5 mg/litre killed minnow eggs in 48 h. Rehwoldt et al. (1977)
similarly found that 0.1 mg 2,4-D/litre did not have any adverse
effect on reproduction in guppies.
These reports suggest that the no-observed-adverse-effect level
of 2,4-D BEE for the reproductive or teratogenic effects of 2,4-D
in fish may be about 1 mg a.i./litre water.
7.5. Mutagenicity and Related Effects
7.5.1. 2,4-D and its derivatives
Studies on the mutagenicity of 2,4-D and its derivatives have
been reviewed by Andersen et al. (1972), National Research Council
of Canada, Associate Committee on Scientific Criteria for
Environmental Quality (1978), Ramel (1978), Seiler (1978),
Vachkova-Petrova (1978), Kas'yanenko & Koroleva (1979), Murthy
(1979), Shearer (1980), US Veterans' Administration (1981), Waters
et al. (1981), and Linainmaa (1983).
A recent IARC Working Group (IARC, 1982) evaluated the activity
of 2,4-D and derivatives in short-term tests. It was reported that
2,4-D induced unscheduled DNA synthesis in cultured human
fibroblasts (Ahmed et al., 1977a), but not in rat hepatocytes
(Probst et al., 1981). 2,4-D was not mutagenic in bacterial
systems (Andersen et al., 1972; Sherasen et al., Zetterberg, 1977;
Moriya et al., 1983). 2,4-D was mutagenic in yeast, when tested at
low pH (Zetterberg et al., 1977), but was not active under other
conditions (Zetterberg, 1977) or in a host-mediated assay
(Zetterberg et al., 1977). Results of four studies on Drosophila
melanogaster were reported to be positive (Rasmuson & Persson-
Svahalin, 1978): results of three other studies were negative
Berin & Buslovich, 1971; (Vogel & Chandler, 1974; Magnusson et al.,
1977; Rasmuson & Svahlin, 1978).
2,4-D was reportedly mutagenic in cultured Chinese hamster
ovary (CHO) cells (Ahmed et al., 1977b), but did not induce a
statistically significant increase in sister chromatid exchanges
(SCEs) in CHO cells in vitro (Linainmaa, 1983). Chromosomal
effects have been reported in plants (Khalatkar & Bhargava, 1982).
Chromosomal aberrations or SCEs were found in cultured human
lymphocytes (Pilinskaya, 1974; Korte & Jatal, 1982), but
chromosomal aberrations were not found in cultured embryonic bovine
kidney cells (Bongso & Basrur, 1973).
In mice, single oral doses of 100 - 300 mg 2,4-D/kg body weight
reportedly induced chromosomal aberrations (Pilinskaja, 1974), but
micronuclei were not found in mice after ip injection of single
doses of 100 mg 2,4-D/kg body weight (Jenssen & Renberg, 1976).
2,4-D was not active in a dominant lethal test in mice (Epstein et
al., 1972), and did not induce SCEs in rats after oral
administration of 2,4-D amine salt at daily doses of 100 mg/kg body
weight for two weeks (Linnainmaa et al., 1983). Recent evidence
(Buslovich et al., 1982; Vainio et al., 1982) suggests that 2,4-D
may have an indirect effect on genetic material via the production
of active oxygen radicals derived from peroxisome proliferation,
which has been demonstrated in in vivo and in vitro studies in
liver cells of rats and hamsters (Reddy et al., 1980, 1982; Vainio
et al., 1982; Gray et al., 1983).
At present, available studies are inadequate to evaluate the
genetic effects of 2,4-D and its derivatives in short-term tests
(IARC, 1982). No data on cell transformation are available.
7.6. Carcinogenic Effects on Experimental Animals
7.6.1. 2,4-D and its derivatives
A number of studies have been carried out on mice and rats to
assess the potential carcinogenic effects of 2,4-D and its
derivatives. Two IARC Working Groups have reviewed these studies
(IARC, 1977, 1982) and concluded that it was not possible to make
an evaluation on the basis of the available data. 2,4-D and
several of its esters were tested by oral administration and by a
single sc injection in the newborn of 2 strains of mice (Innes et
al., 1969); 2,4-D or its amine salts were also tested in rats by
oral administration (Arkhipov & Kozlova, 1974; Hansen et al.,
1971).
Nearly 20 years have passed since these studies were carried
out and because of basic flaws in their design and execution, it is
unlikely that further reviews of these studies will lead to
generally accepted conclusions. The Task Group was aware that
further long-term studies in mice and rats were in progress, and
these should prove useful in the future evaluation of the potential
carcinogenicity of 2,4-D.
7.6.2. Contaminants in 2,4-D
2,7-DCDD was tested for carcinogenicity in mice and rats fed
dietary levels of 5000 and 10 000 mg/kg for 90 weeks, followed by a
short observation period of about 1 - 10 weeks, at which time all
animals were killed. Sufficient numbers of animals survived to
evaluate the development of late-appearing tumours. Although an
increased incidence of hepatocellular adenomas and carcinomas was
observed in treated male mice (20/50 in the low dose and 17/42 in
the high dose compared with 8/49 in matched controls), it was noted
that the incidence of liver tumours in historical controls ranged
from 16 - 32%. No tumorigenic effects were found in rats (US
National Cancer Institute, 1979).
8. EFFECTS ON MAN, CLINICAL AND EPIDEMIOLOGICAL STUDIES
The available clinical and epidemiological studies fall into 4
groups: (a) studies on patients treated with 2,4-D as an
anticancer drug (Apffel, 1959a) or antibiotic (Seabury, 1963), (b)
reports on acute 2,4-D poisoning due to voluntary or accidental
ingestion of herbicides (Tables 19 and 20), (c) reports on workers
(mainly men) overexposed to 2,4-D during the manufacture,
processing, or use of 2,4-D herbicides, and (d) epidemiological
studies on groups of people who were actually or potentially
exposed as a result of herbicide spray programmes, or who lived in
areas in which herbicides were used. With the exception of the
case studies of Apffel (1959a) and Seabury (1963), almost all of
the reports deal with mixed exposures to 2,4-D and other chemicals,
and therefore it is often unclear to what extent 2,4-D, its alkali
or amine salts, or its esters contributed to the effects reported
by the authors of the studies.
Much of the literature on acute poisonings and on the health
effects of occupational overexposure to 2,4-D or other
chlorophenoxy compounds or their toxic by-products has been
recently reviewed by Pocchiari et al. (1979), Bovey & Young (1980),
Huff et al. (1980), National Research Council of Canada, Associate
Committee on Scientific Criteria for Environmental Quality (1981),
CEC (1981), Rappe & Buser (198l), US Veterans' Administraton
(1981), Coggon & Acheson (1982), Hay (1982), IARC (1982, 1983), and
Dobrovolski (unpublished data, 1983). However, most of these
reviews concentrated on 2,4,5-T, Agent Orange, and other herbicides
used in the Vietnam war, or on industrial accidents resulting in
massive exposures to largely undefined mixtures of chlorophenols,
chlorinated dibenzodioxins, and other reaction products. In
contrast, the present review focuses mainly on 2,4-D herbicides.
In evaluating human exposure to mixtures of chemicals that
include 2,4-D and various concentrations of contaminants of 2,4-D,
it is in many instances difficult or impossible to determine
whether any of the described effects can actually be attributed to
the exposure to 2,4-D or its derivatives.
8.1. Acute Poisoning and Occupational Overexposure
Pertinent reports on acute poisoning with 2,4-D, or of the
effects of occupational overexposure to 2,4-D herbicides are
summarized or cited in Tables 19 and 20.
Signs and symptoms of acute overexposure to 2,4-D or its
derivatives occurred after ingestion or absorption of large
amounts, or where poor occupational hygiene was practised leading
to pronounced dermal absorption of the material. It is unlikely
that, with good agricultural practice, good personal protection,
and occupational hygiene, resulting in exposures to low
concentrations of 2,4-D, any of the acute symptoms and signs
reported below would be expected to occur.
Table 19. Acute toxicity of 2,4-D, fatal poisonings with herbicides containing 2,4-D
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
a) Fatal poisonings
2,4-D diethyl not stated F 50.8 kg 60-90 g 40-400 coma; death in about Curry (1962)
ester (1180-1770 mg 2 days; degeneration
(DICOTEX EXTRA) DOCOTOX/kg bw) of convoluted kidney
tubules
"2,4-D" suicide M ? ~ 125 ml ? loss of consciousness, Delarrard &
400 g (< 400 bw) coma, generalized Barbaste (1969)
a.i./litre muscular hypotonia,
loss of all reflexes,
hypotension,
hyperglycaemia,
proteinuria; death in
12 h
"pure" 2,4-D suicide M 55 kg ? 57.6-407.9 coma, myotonia, fever, Dudley &
acid in pulmonary emphysema Thapar (1972)
kerosene and edema, liver
necrosis, degeneration
of kidney tubules;
death in 6 days
"2,4-D" suicide F ? ? 20-116 loss of consciousness, Geldmacher-Van
vomiting, uterine Mallinckrodt &
bleeding, tachycardia, Lautenbach
and circulatory (1966)
failure; death in
about 30 h; edema and
congestion of brain;
fatty liver cell
changes; fatty changes
in kidney tubules;
pulmonary hyperaemia &
edema with isolated
haemorrhages
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
"2,4-D" suicide M ? "at least" stiffness in legs, Herbich &
13.5 g vomiting, loss of Machata
consciousness; death (1963)
in 14 h; hyperaemia
and edema of brain;
pulmonary edema
2,4-D suicide M 75 kg 120 ml 12.5-7700 vomiting; congestion, Nielsen et al.
dimethylamine (80 mg/kg) pulmonary emphysema; (1965)
formulation CNS congestion &
perivascular
haemorrhages, severe
degeneration of
ganglion cells; death
within hours of
ingestion
Herbicide suicide M 26 360 ml 2,4-D plasma clinical and Osterloh et al.
containing years and mecoprop 321 (1.5 h) pharmacokinetic (1983)
2,4-D, mecoprop amine salt 540.9 (21 h) study coma with
(MCPP) and (10.6%, 11.6% 480.8 (30 h) pin-point pupils
chlorpyrifos a.i.) and 360 tachycardia,
ml chlorpyrifos urine (on hypertension,
in kerosene admission): myoclonus, diarrhoea,
(6.7% a.i.) 230.3 then hypotension,
plus few cardiac arrhythmias,
granules gastric asystole, and death
Warfarin (0.025 content (on after 30 h
% a.i.) (2,4-D admission):
= 600 mg/kg bw; 108.2
mecoprop = 600
mg/kg bw) tissues
(post mortem)
brain: 186.4
blood: 389.5
liver: 293.5
heart: 301.2
kidney: 315.0
-----------------------------------------------------------------------------------------------------------------------
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
b) Non-fatal poisonings
"2,4-D" accidental M ? (5- ? ? drowsiness, Duric et al.
"DEHERBAN A" ingestion year unsteadiness, (1979)
herbicide old difficulties with
formulation, child) speech; dilated pupils;
400 g on fourth day, toxic
a.i./litre myocarditis with
abnormal ECG; kidney
damage indicated by
increased blood urea
levels; no liver
damage; complete
recovery within about
one month
herbicide suicide F 58 ? ? no signs of toxicity Prescott et al.
containing attempt; years on admission to (1979)
2,4-D plus ingestion of hospital with plasma
dichlorprop unspecified concentration of
(16.1% + 21.4%) amount of 335 mg 2,4-D/litre &
herbicide 400 mg mecoprop/litre;
plasma clearance
t = 143 h for
2,4-D vs 95 h for
dichlorprop; 91% of
ingested 2,4-D and
70% of ingested
dichlorprop excreted
unchanged; metabolites
not identified
----------------------------------------------------------------------------------------------------------------------- not identified
Table 19. (contd.)
-----------------------------------------------------------------------------------------------------------------------
Product(s) Circum- Sex of Body Dose ingested 2,4-D Effects and outcomes References
stances victim weight concentration
or age in tissues
(mg/kg)
-----------------------------------------------------------------------------------------------------------------------
herbicide suicide F 30.2 kg 100 ml ? pharmacokinetic study Rivers et al.
containing attempt herbicide of 2,4-D/dicamba (1970)
salts of 2,4-D (13.6 g 2,4-D) excretion; no Young & Haley
(20.1%) and information on toxic (1977)
dicamba (1.9%) effects; excretion of
2,4-D initially slowed
by competitive
excretion of dicamba;
small amounts of 2,4-D
still excreted 3 weeks
after ingestion; only
52% of ingested 2,4-D
excreted in urine;
rest assumed to have
been excreted by
faecal route; plasma
clearance t = 59.2 h
initially, and 16.7 h
after most of the
dicamba was excreted
-----------------------------------------------------------------------------------------------------------------------
Table 20. Acute or non-acute effects attributed to occupational or bystander overexposure to 2,4-D
herbicide
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Central i) unconsciousness Radionov et al. (1967); Paggiaro et al. (1974)
nervous ii) electroencephalograph changes Kontek et al. (1973); Andreasik et al. (1979)
system iii) subjective symptoms Assouly (1951); Goldstein et al. (1959); Monarco &
Divito (1961); Foissac-Gegoux et al. (1962);
Berkley & Magee (1963); Belomyttseva (1965);
Fetisov (1966); Radionov et al. (1967); Bashirov
(1969); SARE (1972); Andreasik et al. (1979);
Kuzyk (1979)
Peripheral i) polyneuritis Foissac-Gegoux et al. (1962); Todd (1962);
nervous Belomyttseva & Karimova (1963); Bashirov (1969)
ii) partial paralysis Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Todd (1962)
iii) functional changes Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Berkley & Magee (1963); Belomyttseva
(1967); Wallis et al. (1970); Andreasik et al.
(1979); Singer et al. (1982)
iv) subjective symptoms
Skeletal i) myotonia, myokymia, fibrillation Foissac-Gegoux et al. (1962) Berkley & Magee
muscles stiffness (1963); Wallis et al. (1970); Paggiaro et al.
(1974)
ii) muscle damage or atrophy Wallis et al. (1970)
iii) subjective symptoms Assouly (1951); Foissac-Gegoux et al. (1962); Todd
(1962); Fetisov (1966); Radionov et al. (1967);
Bashirov (1969); Wallis et al. (1970)
Digestive i) vomiting, diarrhoea Goldstein et al. (1959); Monarca & Divito (1961);
system Todd (1962); Belomyttseva (1967); Radionov et al.
(1967); Paggiaro et al. (1974); Dennis (1976)
ii) various functional disorders or Assouly (1951); Monarca & Divito (1961); Bashirov
subjective symptoms (1969); Wallis et al. (1970); Kuzyk (1979)
---------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------
Target organ Types of effects References
or organ
system
---------------------------------------------------------------------------------------------------------
Respiratory i) irritation coughing Assouly (1951); Belomyttseva & Karimova (1963);
system Belomyttseva (1965); Wallis et al. (1970);
Andreasik et al. (1979)
ii) functional disorders Belomyttseva & Karimova (1963); Bashirov (1969);
Wallis et al. (1970)
Circulatory i) functional changes: Belomyttseva & Karimova (1963); Belomyttseva
system - cardiac involvement (1967); Winkelmann (1960); Bashirov (1969);
- vascular involvement Paggiaro et al. (1974); Andreasik et al. (1979)
ii) haematological or chemical Monarca & Divito (1961); Todd (1962); Belomyttseva
changes (1967); Radionov et al. (1967); Long et al.
(1969); Paggiaro et al. (1974); Andreasik et al.
(1979)
iii) subjective symptoms Radionov et al. (1967); Bashirov (1969); Andreasik
et al. (1979)
Liver functional abnormalities Belomyttseva & Karimova (1963); Belomyttseva
(1967); Bashirov (1969); Andreasik et al. (1979)
Kidney functional abnormalities Monarca & Divito (1961); Foissac-Gegoux et al.
(1962); Belomyttseva (1967); Bashirov (1969);
Paggiaro et al. (1974); Andreasik et al. (1979)
Skin i) irritation or allergic reactions Belomyttseva (1967); Radionov et al. (1967);
Dennis (1976); Kuzyk (1979)
ii) desquamation Foissac-Gegoux et al. (1962)
iii) chloracne Londońo (1966)
Reproductive functional abnormalities (women) Elina et al. (1975)
system decreased libido (men) Andreasik et al. (1979)
impotence Espir et al. (1970)
---------------------------------------------------------------------------------------------------------
The observations of Apffel (1959a) and Seabury (1963) on
patients treated with 2,4-D suggest that the acute no-observed-
adverse-effect level for biological effects in human beings may be
as high as 36 mg of purified 2,4-D/kg body weight, or its
equivalent in alkali or amine salts or esters. The lack of
subjective or clinical effects in studies in which volunteers
ingested low doses (5 mg/kg body weight) of purified 2,4-D also
supports the idea that a dose of a few mg/kg body weight is
unlikely to be toxic (Khanna & Kohli, 1977; Sauerhoff et al.,
1977). However, it is less certain whether this is true also of
the less pure commercial 2,4-D products. From the point of view of
occupational and bystander safety, it is reassuring that no reports
were found of fatal poisonings following dermal exposure or
inhalation, though temporary unconsciousness and other severe acute
effects have been attributed to massive combined dermal and
inhalation exposures to 2,4-D herbicides.
Most poisonings with 2,4-D herbicides involved formulations
containing more than one toxic ingredient, including solvents,
surfactants, and other additives. The symptoms and clinical signs
therefore tended to vary with different products.
8.1.1. Neurotoxic effects of 2,4-D and related compounds
Some of the case reports cited below on poisonings with, and
occupational overexposures to, herbicides indicate that 2,4-D and
other chlorophenoxy compounds may affect both the central and
peripheral nervous systems.
8.1.1.1. Effects on the central nervous system
In addition to subjective symptoms of the central nervous
system, impaired coordination, impaired responses to external
stimuli, unconsciousness, coma, and death have been observed in
human beings mainly after absorption of lethal or nearly lethal
doses of 2,4-D. The acute effects on the central nervous system
seem to resemble those produced by alcohol, sedative drugs, or
aromatic chlorinated hydrocarbons rather than those produced by
organophosphate or carbamate neurological poisons (Geldmacher-Von
Mallinckrodt & Lautenbach, 1966; Prescott et al., 1979). Acute
cerebral demyelination occurred in a suicide who ingested a
solution of 2,4-D in kerosene (Dudley & Thapar, 1972). Severe
degeneration of brain ganglion cells was observed in another
suicide who drank a herbicide product containing 2,4-D in the form
of water-soluble dimethylamine salt (Nielsen et al., 1965). It is
therefore possible that lethal doses of chlorophenoxy compounds may
cause structural as well as functional damage to the brain.
Craniocerebral and peripheral functional nerve damage was noted
by Bezuglyi et al. (1979) in a group of women occupationally
overexposed to 2,4-D herbicides and other pesticides. Electro-
encephalographic (EEG) abnormalities were observed in tractor
drivers spraying herbicides containing 2,4-D, MCPA, or mecoprop
(Kontek et al., 1973), in "individuals exposed to" chlorophenoxy
herbicides, including 2,4-D and MCPA (Bielski & Madra, 1976) and in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979). On
the other hand, a case of neuropathy with EEG abnormalities and
flaccid quadriplegia, originally attributed to occupational
overexposure to MCPA, was diagnosed as being of viral origin
(Nayrac et al., 1958), and therefore some of the neurological
damage attributed to 2,4-D, may have been caused by viruses. There
is some evidence that 2,4-D herbicides may affect the sensory
system, as Andreasik et al. (1979) and Assouly (1951) reported
intolerance to certain odours, hypersensitivity to noise, and other
sensory abnormalities in workers producing or packaging 2,4-D
herbicides, while Fetisov (1966) reported hyposmia and other
sensory deficiencies in similarly-exposed workers.
8.1.1.2. Effects on the peripheral nervous system
"Peripheral neuropathy" and reduced peripheral nerve conduction
velocities have been reported in workers producing 2,4-D and
2,4,5-T (Poland et al., 1971; Singer et al., 1982). More than one
dozen other studies of persons overexposed to chlorophenoxy
herbicides also indicated detrimental effects of 2,4-D products on
the peripheral nervous system (Goldstein et al., 1959; Goldstein &
Brown, 1960; Foissac-Gegoux et al., 1962; Todd, 1962; Berkley &
Magee, 1963; Wallis et al., 1970; Bezuglyi et al., 1979). Long-
lasting flaccid paraparesis or quadriparesis following skin contact
with 2,4-D herbicides was reported by Goldstein et al. (1959) and
Goldstein & Brown (1960). Abnormal tendon reflexes in these or
similar cases were reported by the same authors and by Andreasik et
al. (1979), Berkley & Magee (1963), and Foissac-Gegoux et al.
(1962). Cases of sensory neuropathy attributed to the ingestion
of, or dermal exposure to, 2,4-D herbicides have also been reported
by Monarca & Divito (1961), Foissac-Gegoux et al. (1962), Todd
(1962), Wallis et al. (1970), Sare (1972), and Bezuglyi et al.
(1979). However, no signs of peripheral neuropathy were reported
by Apffel (1959a), Seabury (1963), Paggiaro et al. (1974), and
Prescott et al. (1979), in similar cases of massive exposure to
2,4-D herbicide, and in patients given relatively large amounts of
purified 2,4-D or 2,4,5-T salts or esters as drugs. One
explanation for this may be great individual differences in
susceptibility to poisoning with chlorophenoxy herbicides
(Rosenberg, 1980), as attested by the case of a 58-year-old woman
who "was fully conscious with no clinical evidence of toxicity"
after ingesting enough herbicide to allegedly attain 2,4-D and
mecoprop blood plasma concentrations of 335 and 400 mg/litre
respectively (Prescott et al., 1979). By comparison, Herbich &
Machata (1963) reported that a plasma concentration of 447 mg
2,4-D/litre caused death in a 46-year-old man.
Herbicide ingredients other than 2,4-D and related compounds
might be at least partly responsible for the observed neurotoxic
effects. In particular, organic solvents, emulsifiers, and
ethylene glycol present in herbicide formulations have been
mentioned in this connection (Goldstein & Brown, 1960; Goldwater,
1960). Solvents such as alcohols and trichloroethylene, used in
the manufacture of the active herbicide ingredients, might account
for some of the abnormalities observed in workers involved in the
production of chlorophenoxy compounds (Assouly, 1951; Bashirov,
1969; Bashirov & Ter-Bagdasarova, 1970).
Some of the reported cases of central nervous system
dysfunction or peripheral neuritis may have been merely
coincidental to the herbicide exposure, as there are many known
causes of neuropathy, such as nutritional and hereditary factors,
infectious diseases, and many toxic chemicals, including alcohol
(Freemon, 1975). Thus, alcoholism may have been a contributory
factor in one case of "2,4-D polyneuropathy" reported by Brandt
(1971).
Further studies of the possible effects of 2,4-D and other
chlorophenoxy compounds or their by-products on the human nervous
system are desirable, including studies of behavioural effects
measurable by recently-developed test batteries (Baker et al.,
1983).
8.1.2. Myotoxic effects of 2,4-D
Muscle fibrillations, myotonia, myoglobinuria, muscular
weakness and other indications of a myotoxic effect of 2,4-D have
been reported in patients treated with large doses of purified
2,4-D products by Apffel (1959a) and Seabury (1963), as well as in
cases of suicidal or accidental ingestion of 2,4-D herbicides or
following occupational overexposure (Herbich & Machata, 1963;
Berwick, 1970; Dudley & Thapar, 1972; Prescott et al., 1979). In
laboratory animals, myotonia and structural or biochemical muscle
lesions can be reliably induced by doses in excess of about 100 mg
2,4-D/kg body weight. In human beings, the threshold dose for
gross myotoxic effects certainly exceeds 5 mg/kg body weight per
day, and may be above 36 mg/kg body weight per day (Seabury, 1963;
Khanna & Kohli, 1977; Sauerhoff et al., 1977).
8.1.3. Cardiopathies and cardiovascular effects
Myocardial dystrophy, myocarditis, cardiac arrhythmias or
fibrillations, a slowed heart rate, or electrocardiographic (ECG)
changes were observed in human beings who ingested herbicides
containing 2,4-D (Duric et al., 1979) or 2,4-D and mecoprop
(Prescott et al., 1979), and also after occupational overexposure
to chlorophenoxy herbicides (Belomyttseva, 1965; Khibin et al.,
1968; Zakharov et al., 1968; Paggiaro et al., 1974; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979; Prescott et al., 1979).
However, in other cases of acute poisoning with 2,4-D herbicides,
the ECG was essentially normal (Berwick, 1970; Monarca & Divito,
1961).
Thus, further studies are desirable to determine the threshold
2,4-D doses at which electrophysiological cardiac abnormalities can
be observed, and to determine whether they result from a direct
effect on the nerve conducting system of the heart, or secondarily
from a toxic action on the myocardium.
It has been suggested that allergic reactions and an increased
sensitivity may be involved in 2,4-D-related cardiac arrhythmias
(Winkelmann, 1960; Rea, 1978).
Both hypertension and hypotension have been reported following
exposure to high doses of 2,4-D (Apffel, 1959a; Kaskevich &
Sobolieva, 1978; Bezugly et al., 1979), but no conclusions could be
drawn from these studies.
8.1.4. Haematological effects
Monarca & Divito (196l), Todd (1962), Radionov et al. (1967),
Bashirov (1969), Bashirov & Ter-Bagdarasova (1970), Brandt (1971),
Andreasik et al. (1979), and Bezuglyi et al. (1979) observed
haematological changes such as mild anaemia, bone marrow
depression, mono- or lymphocytosis or eosinophilia, changes in
erythrocyte volume or size, or methaemoglobinaemia following
ingestion of, or overexposure to 2,4-D. However, these changes may
have been due to other causes, as Apffel (1959a) did not observe
either haematological changes or effects on haematopoiesis in
patients given 0.1 - 0.3 g of purified 2,4-D per day as an
anticancer drug.
8.1.5. Blood chemistry effects
Hyperglycaemia, hypercholesterolaemia, elevated levels of blood
urea, transaminase (SGOT, SGPT), and creatine phosphokinase (CPK),
or altered blood albumin, globulin, or phospholipid levels
following acute poisoning with, or occupational overexposure to
2,4-D were reported by Bashirov (1969), Bashirov & Ter-Bagdarasova
(1970), Berwick (1970), Lukoshkina et al. (1970), Brandt (1971),
Bezuglyi et al. (1979), Duric et al. (1979) and Prescott et al.
(1979). However, Bashirov (1969) reported hypoglycaemia (< 700
mg/litre) and abnormally slow return to normal values in glucose
tolerance tests in about one-third of a group of workers producing
2,4-D. Increased activity of erythrocytic glycolytic enzymes was
found in Polish workers packaging 2,4-D sodium salt (Andreasik et
al., 1979). In one case of intentional 2,4-D poisoning, there was
hyperglycaemia (De Larrard & Barbaste, 1969), while in some cases
of 2,4-D overexposure, blood glucose abnormalities were not
observed (Goldstein et al., 1959). Apffel (1959a) never observed
hyperglycaemia in his patients, on the contrary, daily doses of
1 - 1.25 g 2,4-D led to hypoglycaemia. Thus, under some
circumstances, high doses of 2,4-D apparently can affect glucose
metabolism, and produce hypo- or hyperglycaemia. Gamble (1975)
proposed that 2,4-D might inhibit certain APT-dependent enzymes and
thus affect lipid metabolism.
8.1.6. Pulmonary effects
Pulmonary emphysema, oedema, hyperaemia and haemorrhages were
found in cases of fatal poisonings due to 2,4-D herbicide ingestion
(Herbich & Machata, 1963; Geldmacher-Von Mallinckrodt & Lautenbach,
1966; Dudley & Thapar, 1972). It is not clear whether the acute
pulmonary effects were caused by the 2,4-D preparations or by the
solvents such as kerosene or fuel oil. However, it is unlikely
that the pulmonary emphysema was caused by acute exposure to 2,4-D.
Dyspnoea or respiratory tract irritation were occasionally
reported following occupational overexposure of 2,4-D production
workers or herbicide sprayers (Assouly, 1951; Belomyttseva, 1964;
Bashirov, 1969; Bezuglyi et al., 1979).
8.1.7. Hepatotoxic effects
Liver necrosis or fatty liver cell changes were observed in 2
fatal cases following 2,4-D herbicide ingestion (Geldmacher-Von
Mallindkrodt & Lautenbach, 1966; Dudley & Thapar, 1972). In
several non-fatal 2,4-D poisonings, no biochemical evidence of
liver damage was noted, and neither Apffel (1959a) nor Seabury
(1963) reported indications of liver damage in patients treated
with up to 2.5 g/day of purified 2,4-D, salts, or esters.
Hyperbilirubinaemia, elevated urobilinogen levels, or liver
enlargement were reported in workers occupationally exposed both to
2,4-D herbicides and to other chemicals (Belomyttesva, 1964, 1965;
Bashirov, 1969; Bashirov & Ter-Bagdasarova, 1970; Kaskevich &
Sobolova, 1978; Andreasik et al., 1979).
8.1.8. Nephrotoxic effects
Degeneration of, or fatty changes in kidney tubules, or
proteinuria, increased blood urea levels, and other indications of
a nephrotoxic effects were observed in cases of fatal or nearly
fatal herbicide ingestion (Goldstein et al., 1959; Curry, 1962;
Geldmacher-Von Mallinckrodt & Lautenbach, 1966; Brandt, 1971;
Dudley & Thapar, 1972; Duric et al., 1979). Impaired renal
function was reported in occupationally-exposed persons by Bashirov
(1969), Bashirov & Ter-Bagdasarova (1970), Paggiaro et al. (1974),
and by Andreasik et al. (1979). On the other hand, neither Apffel
(1959a) nor Seabury (1963) reported any evidence of kidney damage
in their patients, some of whom received in excess of 2 g of pure
2,4-D per day.
8.1.9. Effects on the digestive tract
Vomiting, diarrhoea, nausea, and other indications of toxic
effects on the digestive tract were observed by Apffel (1959a) in
patients injected intramuscularly with large doses (up to 2.5 g) of
purified 2,4-D products.
The same effects have also been noted after ingestion of large
doses of 2,4-D herbicides, or after combined inhalation and dermal
overexposure (Goldstein et al., 1959; Monarca & Divito, 1961;
Nielsen et al., 1965; Tsapko, 1966; Radionov et al., 1967; Paggiaro
et al., 1974; Dennis, 1976; Kuzyk, 1979; Prescott et al., 1979).
However, no gastrointestinal symptoms were reported by volunteers
who ingested a single dose of 5 mg pure 2,4-D/kg body weight
(Khanna & Kohli, 1977; Sauerhoff et al., 1977). Thus, an intake of
more than 300 mg 2,4-D per adult appears to be required to induce
acute toxic effects on the gastrointestinal tract.
8.1.10. Effects on endocrine organs
Andreasik et al. (1979) found an impaired iodine uptake by the
thyroid, and decreased thyroxine, thyroxine clearance, and
thyroxine iodine values in workers packaging 2,4-D sodium salt.
Since these workers were exposed to a variety of chemicals, these
results need confirmation.
8.1.11. Irritative and allergenic effects
Chronic tonsillitis and paranasal sinusitis were reported in
workers packaging 2,4-D sodium salt (Andreasik et al., 1979).
Acute eye or skin irritation, as well as skin reactions of an
allergic type, including anaphylactoid purpura (allergic angiitis)
and contact eczema have been reported in agricultural and forestry
workers following occupational exposure to 2,4-D herbicides
(Winkelmann, 1960; Radionov et al., 1967; Balo-Banga et al., 1973;
Jung & Wolf, 1977; Kuzyk, 1979). Jung & Wolf (1977) found that
exposure to the vapour of a 2,4-D/2,4,5-T formulation in diesel oil
(SELEST 100) caused an acute allergic reaction in the skin of
sensitized herbicide applicators, and that the allergic reactions
were caused by the mixture of 2,4-D/2,4,5-T esters and not by the
diesel oil.
8.2. Epidemiological Studies of the Chronic Effects of 2,4-D
Much concern has been raised about the phenoxy herbicides,
including 2,4-D, especially in relation to birth defects and cancer
in human beings.
Several episodes have also been reported in which defined
populations were exposed to mixtures of 2,4-D and 2,4,5-T, in which
the 2,4,5-T was contaminated with various amounts of 2,3,7,8-
tetrachlorodibenzodioxin (2,3,7,8-TCDD) (Bleiberg et al., 1964;
Huff et al., 1980). It is now generally accepted that chloracne
and porphyria cutanea tarda observed in these studies were caused
by exposure to 2,3,7,8-TCDD and not by exposure to 2,4,5-T or 2,4-D
(Kimbrough, 1980).
The following sections concentrate on epidemiological studies
or other related studies on human beings in which actual or
potential exposures to 2,4-D products alone or to mixtures of 2,4-D
with other chlorphenoxy herbicides were demonstrated.
8.2.1. Reproductive, fetotoxic, and teratogenic effects
Although effects on reproduction have been demonstrated in
animals with 2,4,5-T, 2,4-D and 2,3,7,8-TCDD, all of the attempts
made to determine whether human beings suffer similar effects have
been frustrated by the poor design of the studies, inadequate
determination of exposure, or inadequate information about the
background incidence of spontaneous abortions and other abnormal
reproductive outcomes, by inadequate evaluation of confounding
variables, by inadequate assessment of exposure, and by mixed
exposures (Aldred et al., 1978; Lee, 1978; Field & Kerr, 1979;
Brogan et al., 1980; Hanify, 1980; Carmelli et al., 1981). For
these reasons they are not discussed in detail in this report.
Conclusive evidence of reproductive effects caused by 2,4-D in
populations that might be exposed to chlorophenoxy herbicides is
unlikely to be obtained from new epidemiological studies on
indirectly-exposed populations living in, or adjacent to, areas in
which phenoxy herbicides are used. Doses of 2,4-D absorbed by
bystanders are far below those expected to be toxic, as shown by
occupational exposure studies with 2,4-D and 2,4,5-T herbicides
(section 5). Any effects induced by such small amounts would
probably be obscured by more potent confounding factors (Janerich,
1973; Karkinen-Jääskelainen & Saxén, 1974; Saxén et al., 1974;
Elwood & Rogers, 1975; Granroth et al., 1977, 1978; James, 1977;
Holmberg, 1979; Lappe, 1979; Schacter et al., 1979).
Additional studies on female workers occupationally exposed to
significantly higher levels of 2,4-D than bystanders would be
useful to clarify some of the uncertainties raised by past studies,
if sufficiently large cohorts could be identified.
8.3. Studies on Mutagenic Effects in Workers Exposed to 2,4-D
Lymphocytes from ten workers exposed to 2,4-D esters during the
manufacture of 2,4-D herbicides, or from 15 workers packaging 2,4-D
sodium salt, did not show any chromosome abnormalities (Johnson,
1971; Andreasik et al., 1979). Chromosome or chromatid
abnormalities in lymphoctyes from some pesticide sprayers applying
a variety of agricultural chemicals, including in some cases 2,4-D,
were observed by Yoder et al. (1973) and Crossen et al. (1978).
Högstedt et al. (1980) did not observe any significant increases in
chromosome abnormalities in workers exposed to 2,4-D and other
pesticides.
The induction of SCEs among workers occupationally exposed to
the phenoxy herbicides 2,4-D and MCPA has been recently studied.
The subjects used only 2,4-D and MCPA or mixtures of the two for
spraying, and the exposure levels were estimated by determining the
urinary 2,4-D and MCPA excretion by the workers. No dose-related
differences in the frequencies of SCEs could be found either in
relation to the exposure level or to the length of the exposure
(Linnainmaa, 1983).
Although some studies suggest that occupational exposure to
2,4-D may result in chromosome abnormalities, the results are
conflicting. Moreover, the possiblity of mixed exposure and other
confounding variables cannot be excluded in the studies with
positive results.
8.4. Carcinogenic effects
8.4.1. Epidemiological studies
In two case-control studies of soft-tissue sarcoma (Hardell &
Sundström, 1979; Eriksson et al., 1981) and one of lymphoma
(Hardell et al., 1981), exposure to phenoxyacetic acids (mainly
2,4,5-T, 2,4-D, and MCPA) was associated with approximately 5-fold
increases in the risk of soft-tissue sarcomas. Exposure to 2,4-D,
either with or without MCPA exposure, also increased relative
risks. In the study of malignant lymphomas, 7 cases and 1 control
were apparently exposed to 2,4-D only (relative risk, 19.6; 95%
confidence interval, 4.3 - 89.8).
In a different case-control study with a small number of cases
and controls, no increased risk was observed (Smith et al., 1982).
A follow-up was also carried out on 348 railroad workers
exposed for less than 45 days during the period 1957 - 72 to the
herbicides 2,4-D, 2,4,5-T, atrazine, mecoprop, dichloropropionic
acid, and amitrole (Axelson & Sundell, 1974). The authors found a
significant increase in cancer mortality and morbidity among
workers exposed to amitrole.
Axelson et al. (1980) reported a further follow-up of these
workers, up to October, 1978, accumulating 5541 person-years. The
herbicide exposure of the workers was analysed in terms of exposure
to either amitrole, or phenoxy acids, or to a combination of the
two. A 10-year lapse period from the first day of exposure was
used as the induction latency. They found 15 cases of cancer
versus 6.87 expected (relative risk, 2.2). In the cohort with
combined exposure to amitrole and phenoxy acids, 6 cases were
observed versus 1.78 expected (relative risk, 3.4); in the group
exposed to amitrole alone, 3 tumours were observed versus 1.95
expected (relative risk, 1.5); and 6 cancers were observed versus
3.14 expected (relative risk, 1.9) in the phenoxy acid-exposed
group. All cancers, as well as cancers of the stomach, occurred in
statistically-significant excesses in the cohort as a whole. In
the groups exposed to amitrol plus phenoxy acids, there was a
significant excess of all cancers. In the group exposed only to
phenoxy acids, stomach cancer occurred in significant excess (2
observed, 0.33 expected; relative risk, 6.1). No soft-tissue
sarcomas were identified, but the statistical power of this study
to detect an excess of a rare cancer was limited.
Högstedt & Westerlund (1980) conducted a restrospective
mortality study on 142 forestry workers exposed to phenoxy
pesticides and 244 unexposed forestry workers, comparing their
mortality experience with national statistics. Work supervisors,
who were more highly exposed to phenoxy herbicides than the others,
had a significantly elevated tumour mortality (5 observed, 1.4
expected). No particular tumour type predominated, and no soft-
tissue sarcomas were observed, though the authors noted that the
study had limited statistical power and was inconclusive, because
of the relatively short follow-up period.
Riihimäki et al. (1982) reported on a prospective cohort study
of 2,4-D and 2,4,5-T spray personnel which was in progress.
Because of the small number of deaths and the brief follow-up
period, no conclusions can so far be drawn from this study.
Follow-up studies on cohorts of pesticide sprayers, farmers,
and agricultural workers occupationally exposed to a variety of
chemicals, in some cases including phenoxy herbicides, have been
reviewed by IARC (1983).
Follow-up studies on groups of industrial workers exposed to
chlorophenols, 2,4,5-T, or other chlorophenoxy compounds, and to
2,3,7,8-TCDD or other dioxins, during the manufacture of
chlorophenoxy herbicides, have recently been reviewed by Huff et
al. (1980) and IARC (1983).
8.4.2. Evidence on the carcinogenicity of 2,4-D
The available studies suggest that an association exists
between mixed exposure to phenoxy herbicides, chlorinated phenols,
and chlorinated dibenzodioxins, and an increased incidence of soft
tissue sarcomas and malignant lymphomas. It is not clear, at
present, whether these findings represent true associations, and
further studies are in progress (Muir & Wagner, 1981) to clarify
this point. Since many of the tumour cases had been exposed to
combinations of phenoxy herbicides and their contaminants as well
as other chemicals, it is not known whether exposure to 2,4-D is
specifically associated with the development of soft tissue
sarcomas.
8.5. Treatment of Poisoning in Human Beings
Successfully treated cases of 2,4-D poisoning indicate that
forced alkaline diuresis is helpful in reducing the level of 2,4-D
in the blood and tissues (Young & Haley, 1977; Prescott et al.,
1979). Heart and kidney damage should be anticipated and
counteracted in cases of severe poisoning (Duric et al., 1979).
In cases of acute poisoning due to 2,4-D, the nearest Poison
Control Centre should be contacted for additional information on
symptoms and recommended treatment.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO 2,4-D
9.1. General Considerations
In areas of 2,4-D herbicide production, handling, or use, the
highest exposure will be incurred by those who are directly
involved in these processes, followed by bystanders indirectly
exposed to 2,4-D vapour, dust, or droplets, or to contaminated
vegetation, soil, or water. In these two groups, exposure will
usually be via the skin. The general population in 2,4-D-use areas
would be exposed to a lesser extent, mainly through food containing
2,4-D residues and to a lesser extent through 2,4-D residues in
water. The contribution from air is negligible. As far as the
general population is concerned, 2,4-D intake from any source, is
negligible.
9.2. Estimated Intake of 2,4-D by the Population in a 2,4-D-use Area
The total contribution from air, food, and water is estimated
to be 0.03 - 2 µg/kg body weight per day (Table 13).
9.2.1. Intake by bystanders
Given the limited data available and the many uncertainties
involved, an adequate estimate of 2,4-D intake by bystanders is not
possible at this time, but it should generally be less than that
for occupationally-exposed persons.
9.2.2. Occupational intake
Workers using 2,4-D may, on average, absorb about 0.1 mg
2,4-D/kg body weight per day. However, this level may be exceeded
if good occupational hygiene is not practiced (section 5.2).
Simple precautions against excessive exposure can reduce the amount
of 2,4-D uptake.
9.3. Safety Factors
9.3.1. Definitions
For the present assessment, the safety factor is defined as the
integer obtained by dividing the overall no-observed-adverse-effect
level for a known adverse effect of 2,4-D (determined from all
available information on human beings or animals) by the daily
exposure value (absorbed dose of 2,4-D) for the various exposed
groups.
9.3.2. Determination of safety factors
9.3.2.1. Acute poisoning
Based on clinical studies in which 2,4-D was injected into
patients as a drug, the no-observed-adverse-effect level for signs
and symptoms of acute 2,4-D poisoning in children and adults
appears to be at or near 36 mg/kg body weight (section 8.1). Based
on the available studies of the amounts of 2,4-D absorbed by
occupationally-exposed person, bystanders, and populations in
2,4-D-use areas, the safety factors for acute 2,4-D poisoning are
likely to be:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) at least 360 for occupationally-exposed spraying crews.
The margin of safety for persons with excessive occupational
exposures would be smaller.
9.3.2.2. Chronic toxicity
Dose-effect relationships for the chronic toxic effects of
2,4-D or 2,4-D derivatives are available only from animal studies.
The no-observed-adverse effect levels for certain chronic toxic
effects of 2,4-D in animals have not been firmly established, and
for this reason safety factors cannot be established (section
7.2.1) for all of the chronic effects of 2,4-D.
9.3.2.3. Embryotoxic, fetotoxic, and teratogenic effects
The no-observed-adverse-effect level for embryotoxic,
fetotoxic, or teratogenic effects of 2,4-D in mammals appears to
lie at 10 mg/kg body weight per day (section 7.3.1.2). Assuming
that the same is true for human beings, then the corresponding
safety factors for the various exposed groups are:
(a) much greater than 1000 for the general population in 2,4-D-use
areas;
(b) 100 for occupationally-exposed spraying crews using
precautions against excessive exposure.
9.3.2.4. Mutagenic effects
The available information was inadequate for an assessment of
the mutagenic potential of 2,4-D in mammals.
9.3.2.5. Carcinogenic effects
Available animal bioassays and epidemiological studies are
inadequate for an assessment of the carcinogenic potential of 2,4-D
or of its derivatives.
9.4. Evaluation of Health Risks from 2,4-D Exposure
From the data available at present, the Task Group assumes that
a possible health risk will exist, when the safety factor is less
than 100.
9.5. Recommendations on Exposure
Results of recent exposure and occupational health studies
suggest that excessive exposure to 2,4-D can be avoided by fairly
simple measures of occupational hygiene, such as those recommended
in two pertinent publications of the International Labour Office
(ILO, 1977, 1979). Laundering procedures for 2,4-D-contaminated
clothing have been published by Easley et al. (1983), and these
should be considered.
REFERENCES
ABO-KHATWA, N. & HOLLINGWORTH, R.M. (1974) Pesticidal chemicals
affecting some energy linked functions of rat mitochondria in
vitro. Bull. environ. Contam. Toxicol., 12(4): 446-454.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide-induced
RNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide-induced
ouabain resistant mutants in Chinese hamster V79 cells. Chem.-biol.
Interact., 19: 369-374.
AKESSON, N.B. & YATES, W.E. (196l) Drift residues on alfalfa hay.
A progress report of investigations of pesticide drift from
aircraft applications. Calif. Agric., 15: 4-7.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous
substances. Int. Pest Control, 11(2): 29-35.
ALDRED, J.E., BELCHER, R.S., CHRISTOPHERS, A.J., CLEMENTS, A.,
DANKS, D.M., FRENCH, E.L., GALLOWAY, D.B., GARSON, O.M., PARSONS,
W., RAPER, C., ROUCH, G., & SINN, H.J. (1978) Report of the
Consultative Council on Congenital Abnormalities in the Yarram
District. Alleged relationship of congenital abnormalities to the
use of the herbicides 2,4-D and 2,4,5-T. State of Victoria,
Australia, Ministry of Health, viii + 55 pp (Document PB 338).
ALTOM, J.D. & STRITZKE, J.F. (1973) Degradation of dicamba,
picloram and familiar phenoxy herbicides in soils. Weed Sci., 21:
556-560.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20(3): 649-656.
ANDREASIK, Z., KOLON, S., & SMOLIK, R. (1979) Health status
evaluation in workers packaging the herbicide "Pielik"
(cholorophenol). Arh. Hig. Rad. Toksikol., 30 (Suppl.): 599-602.
ANDRUSHAITIS G.P. (1972) [The effect of pesticides on the
environment.] Latvi. PSR Zinatnu Akad. Vest., 4:(297) 44-49 (in
Russian, Health & Welfare Canada Translation No. 2441).
ANTONENKO, T.A. (1977) [Experimental data from a study of the
permeability of the placental barrier to the herbicide 2,4-D and
its passage with the mother's milk during feeding.] Gig. Aspekt.
Okhr. Zdor. Naseleniya (Saratov). In: Saratov, Saratov Institute
for Oral Hygiene, pp. 177-178 (in Russian, Health & Welfare Canada
Translation No. 2370).
APFFEL, C.A. (1959a) Action cytostatique de certaines auxines,
compte rendu préliminaire. Presse med., 67(6): 207-209.
ARJMAND, M., HAMILTON, R.H., & MUMMA, R.O. (1978) Separation of
amino acid conjugates of 2,4-dichlorophenoxyacetic acid by high-
pressure liquid chromatography employing ion-pair techniques.
J. agric. food Chem., 26: 971-973.
ARKHIPOV, G.N. & KOZLOVA, I.N. (1974) [A study of the
carcinogenic potential of a herbicide: 2,4-D amine salt.] Vopr.
Pit., 5: 83-84 (in Russian).
ASSOULY, M. (1951) Désherbants sélectifs et substances de
croissance aperēu technique. Effet pathologiques sur l'homme au
cours de la fabrication de l'ester du 2,4-D. Arch. Mal. prof. Hyg.
Toxicol. Ind., 12(1): 26-30.
AVERITT, W.K. (1967) An evaluation of the persistence of 2,4-D
amine in surface waters in the state of Louisiana. Proc. South.
Weed Sci. Soc., 20: 342-347.
AXELSON, O. & SUNDELL, L. (1974) Herbicide exposure, mortality
and tumor incidence. An epidemiological investigation on Swedish
railroad workers. Scand. J. Work Environ. Health, 11:21-28.
AXELSON, O., SUNDELL, L., ANDERSSON, K., EDLING, C., HOGSTEDT, C.,
& KLING, H. (1980) Herbicide exposure and tumor mortality: An
updated epidemiological investigation on Swedish railroad workers.
Scand. J. Work Environ. Health, 6: 73-79.
BACHE, C.A., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1964a)
Absence of phenoxyacid herbicide residues in the milk of dairy cows
at high feeding levels. J. dairy Sci., 47(3): 298-299.
BAKER, B.R., LEE, W.W., SKINNER, W.A., MARTINEZ, A.P., & TONG, E.
(1960) Potential anticancer agents. I. Non-classical
antimetabolites. II. Some factors in the design of exo-alkylating
enzyme inhibitors, particularly of lactic dehydrogenase. J. med.
pharm. Chem., 2(6): 633-657.
BAKER, E.L., FELDMAN, R.G., WHITE, R.F., HARLEY, J.P., DINSE, G.E.,
& BERKEY, C.S. (1983) Monitoring neurotoxins in industry:
Development of a neuro-behavioral test battery. J. occup. Med.,
25(2): 125-130.
BAKER, P.G., HOODLESS, R.A., & TYLER, J.F.C. (1981) A review of
methods for the determination of polychlorodibenzo-p-dioxins and
polychlorodibenzofurans in phenoxyalkanoic acid herbicides.
Pestic. Sci., 12: 294-304.
BALO-BANGA, M.J., ANTONI, F., ALTMANN, H., VINCZE, I., & KOCSIS, F.
(1973) Effect of 2,4-D on semiconservative and DNA repair
synthesis. (Case report: bullous dermatitis after exposure to
2,4-D). Ber. Oesterr. Studienges Atomenerg. (Forschungszentrum
Seibersdorf), 2218: 1-7.
BAMESBERGER, W.L. & ADAMS, D.F. (1966) An atmospheric survey for
aerosol and gaseous 2,4-D components. Adv. Chem. Ser., 60:
219-227.
BARTLEY, T.R. & HATTRUP, A.R. (1970) 2,4-D contamination and
persistence in irrigation water. Proc. West. Soc. Weed Sci.,
23: 10-33.
BASHIROV, A.A. (1969) [The health of workers involved in the
production of amine and butyl 2,4-D herbicides.] Vrach. Delo,
10: 92-95 (in Russian, Health & Welfare Canada Translation No.
2181).
BASHIROV, A.A. & TER-BAGDASAROVA, I.K. (1970) [The state of the
cardiovascular system in workers engaged in the production of amine
salt herbicides and 2,4-dichlorophenoxyacetic acid butyl ester.]
Azerb. Med. Zh., 47 (12): 44-48 (in Russian).
BATORA, V., VITOROUIC, S.L.J., THIER, H.P., & KLISENKO, N.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BEAVEN, F.G., RAWLS, C.K., & BECKET, G.E. (1962) Field
observations upon estuarine animals exposed to 2,4-D. Proc.
northeast. Weed Contr. Conf., 16: 449-458.
BELOMYTTSEVA, L.A. (1964) [Toxic effects of the herbicide 2,4-D
sodium on the lungs.] Sb. Nauchn. Tr. Bashk. Gos. Med. Inst.,
16(2): 135-141 (in Russian).
BELOMYTTSEVA, L.A. (1965) [In: Contributions to the 3rd All-Union
Scientific Conference on the Problems of Hygiene and Toxicology
related to the Use of Chemicals in the National Economy, Kiev,]
p. 380 (in Russian).
BELOMYTTSEVA, L.A. (1967) The toxic effect of the sodium salt of
2,4-dichlorophenoxyacetic acid. UFA 1967.
BELOMYTTSEVA, L.A. & KARIMOVA, A.K. (1963) In: [Proceedings of
the UFA Scientific Research Institute of Hygiene and Occupational
Diseases, Vol. 2.] (in Russian).
BERKLEY, M.C. & MAGEE, K.R. (1963) Neuropathy following exposure
to a dimethylamine salt of 2,4-D. Arch. internal. Med., 111:
351-352.
BERWICK, P. (1970) 2,4-dichlorophenoxyacetic acid poisoning in
man. J. Am. Med. Assoc., 214(6): 1114-1117.
BEZUGLYI, V.P., FOKINA, K.V., KOMAROVA, L.I., SIVITSKAJA, I.I.,
ILINA, V.I., & GORSKAJA, I.I. (1979) [Clinical manifestations of
long-term sequels of acute poisoning with 2,4-D.] Gig. Truda Prof.
Zabol., 3: 47-48 (in Russian).
BIELSKI, J. & MADRA, B. (1976) [Acute poisoning with pesticides.]
Pol. Tyg. Lek., 31(46): 1971-1973 (in Polish, Health & Welfare
Canada Translation No. 2245).
BJERKE, E.L., HERMAN, J.L., MILLER, P.W., & WETTERS, J.H. (1972)
Residue study of phenoxy herbicides in milk and cream. J. agric.
food Chem., 20: 963-967.
BJORKLUND, N.E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. scand., 7: 364-390.
BJORN, M.D. & NORTHEN, H.T. (1948) Effects of 2,4-dichloro-
phenoxyacetic acid on chicks. Science, 108: 479-480.
BLEIBERG, J., WALLEN, M., & BRODKIN, R. (1964) Industrially
acquired porphyria. Arch. Derm., 89: 793-797.
BODAI, J., TAMAS, L., & VEGH, A. (1974) [Reproductive
consequences of Dikonirt toxicosis in cattle.] Magy. Allatorv.
Lapja, 29(5): 319-322 (in Hungarian, Health & Welfare Canada
Translation No. 2238).
BODEM, R., PREISS, D., & KUHN, E. (1971) [On the delay of the
relaxation of the guinea pig papillary muscle by 2,4-dichloro-
phenoxy-acetic acid (2,4-D).] Klin. Wochenschr., 49(1): 227-228
(in German).
BODYAGIN, D.A., SYKIN, A.B., LUPINOSOV, Y.V., & ZAITSEVA, L.A.
(1969) [The effects of chlorophenoxyacetic acid derivatives
(herbicides) on animals and human beings. (A literature review).]
Farmakol. Toksikol., 32 (6): 747-751 (in Russian, Health & Welfare
Canada Translation No. 2031).
BOLLAG, J.M., HELLINGS, C.S., & ALEXANDER, M. (1968) 2,4-D
metabolism. Enzymatic hydroxylation of chlorinated phenols.
J. agric. food Chem., 16: 826-828.
BONGSO, T.A. & BASRUR, P.K. (1973) In vitro response of bovine
cells to 2,4-dichlorophenoxy acetic acid. In vitro, 8: 416-417.
BONTOYAN, W.R. (1977) Report on pesticide formulations. J. Assoc.
Off. Agric. Chem., 60: 324-327.
BOVAL, B. & SMITH, J.M. (1973) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. Chem. Eng. Sci., 28: 1661-1675.
BOVEY, R.W. (1980a) Residues and fate of phenoxy herbicides in
the environment. In: Bovey, R.W. & Young, A.L., ed. The science
of 2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 301-356.
BOVEY, R.W. (1980b) Human risk with phenoxy herbicides in the
environment. In: Bovey, R.W. & Young, A.L., ed. The science of
2,4,5-T and associated phenoxy herbicides, New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp. 425-433.
BOVEY, R.W. & YOUNG, A.L., ed. (1980) The science of 2,4,5-T and
associated phenoxy herbicides, New York, Chichester, Brisbane,
Toronto, John Willey & Sons, 462 pp.
BRADLAW, H.A., GARTHOFF, L.H., HURLEY, N.E., & FIRESTONE, D. (1980)
Comparative induction of aryl hydrocarbon hydroxylase activity
in vitro by analoques of dibenzo- p-dioxin. Food cosmet. Toxicol.,
18: 627-635.
BRANDT, M.R. (1971) [Herbatox poisoning. A short survey and a
report of a case.] Ugeskrift Laeger, 133(11): 500-503 (in Danish,
Health & Welfare Canada Translation No. 2147.
BRETAG, L.H. & CAPUTO, C. (1978) The nature of the antimyotonic
action of 2,4-D on the soleus muscle of the rat. Proc. Aust.
Physiol. Pharmacol. Soc., 9(2): 150 pp.
BRISTOL, D.W., COOK, L.W., KOTERBA, M.T., & NELSON, D.C. (1982)
Determination of free and hydrolyzable residues of 2,4-
dichlorophenoxyacetic acid and 2,4-dichlorophenol in potatoes.
J. agric. food Chem., 30: 137-144.
BRODY, I.A. (1973) Myotonia induced by monocarboxylic aromatic
acids: A possible mechanism. Arch. Neurol., 28: 243-246.
BRODY, T.M. (1952) Effect of certain plant growth substances on
oxidative phosphorylation in rat liver mitochondria. Proc. Soc.
Exp. Bio. Med., 80: 533-536.
BROGAN, W.F., BROGAN, C.E., & DADD, J.T. (1980) Herbicides and
cleft lip and palate. Lancet, 2: 597.
BUCHER, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid
on experimental animals. Proc. Soc. Exp. Biol. Med., 63: 204-205.
BURCAR, P.J., WERSHAW, R.L., GOLDBERG, M.C., & KHAN, L. (1966)
Gas chromatographic study of the behaviour of the isooctyl ester
of 2,4-D under field conditions in North Park, Colorado. Anal.
Instrum., 4: 215-224.
BURTON, J.A., GARDINER, T.H., & SCHANKER, L.S. (1974) Absorption
of herbicides from the rat lung. Arch. environ. Health, 29: 31-33.
BUSLOVICH, S.Y. (1963) [The effect of the chlorinated derivatives
of phenoxyacetic acid.] Zdrav. Beloruss., 9: 41-45 (in Russian,
Health & Welfare Canada Translation No. 2315).
BUSLOVICH, S.Y. & KOLDOBSKAYA, F.D. (1972) [Activity of
hexokinase in skeletal muscles of albino rats in experimental
myotonia.] Vopr. Med. Khim., 18(4): 403-406 (in Russian).
BUSLOVICH, S.Y & MILCHINA, M.G. (1976) [The dynamics of 2,4-D
amine decomposition in soil.] Gig. i Sanit., 5: 109-110
(in Russian).
BUSLOVICH, S.Y., VOINOVA, I.V., & MILCHINA, M.G. (1973) [The
distribution of chlorinated phenoxy acid herbicides in albino
rats.] Gig. Tr. Prof. Zabol, 17(3): 171-173 (in Russian, Health &
Welfare Canada Translation No. 2237).
BUSLOVICH, S.Y., ALEKSASHINA, Z.A., & KOLOSOVSKAYA, V.M. (1976)
[Embryotoxic effects of herbicides: Chlorinated phenoxy acids.]
Zdrav. Beloruss., 10: 83-84 (in Russian).
BUSLOVICH, S.Y., KOLDOBSKAYA, F.D., DAVYDENKO, L.I., & KRYSANOVA,
A.I. (1982) [The role of mixed-function oxidases in the
detoxication of some herbicides: Chlorinated derivatives of
phenoxyacids.] Gig. i Sanit., 10: 76-77 (in Russian).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna.
Proc. South. Weed Sci. Soc., 18: 576-580.
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON, M.H., &
MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D, DEF,
propanil, and trifluralin to the Dungeness crab, Cancer magister.
Arch. environ. contam. Toxicol., 8: 383-396.
CANADA, HEALTH & WELFARE (1980) Pesticides. In: Guidelines for
Canadian drinking water quality, 1978. Supporting documentation,
Ottawa, Supply and Services, Canada, pp. 457-488.
CARL, M. (1979) Internal laboratory quality control in the
routine determination of chlorinated pesticide residues. Adv.
pestic. Sci., 3:660-663.
CARMELLI, D., HOFHERR, L., TOMSIC, J., & MORGAN, R.W. (1981) A
case-control study of the relationship between exposure to 2,4-D
and spontaneous abortions in humans, Menlo Park, California, SRI
International (Final Report).
CARTER, E.P. (1960) Volatility of ester forms of hormone type
herbicides III. J. Assoc. Off. Anal. Chem., 43: 367-370.
CHANG, H., RIP, J.W., & CHERRY, J.H. (1974) Effects of
phenoxyacetic acids on rat liver tissues. J. agric. food Chem.,
22(1): 62-65.
CHAU, A.S.Y. & THOMSON, K. (1978) Investigation of the integrity
of seven herbicidal acids in water samples. J. Assoc. Off. Anal.
Chem., 61: 1481-1485.
CHAU, A.S.Y., AFGHAN, B.K., & ROBINSON, J.W. (1982) Analysis of
pesticides in water. Volume II. Chlorine- and phosphorus-
containing pesticides. Boca Raton (Florida), CRC Press Inc.,
pp. 238.
CHEN, W.L., GORZINSKI, S.J., KOCIBA, R.J., WADE, C.E., MORDEN,
D.C., KEYES, D.G., & SCHWETZ, B.A. (1981) 2,4-D (2,4-
dichlorophenoxy) acetic acid: results of a 13-week feeding study in
CDF Fisher 344 rats. Proc. Annu. Meet. Can. Fed. Biol. Soc.,
24: 233.
CHOI, K.L., QUE HEE, S.S., & SUTHERLAND, R.G. (1976) 2,4-D levels
in the South Saskatchewan River in 1973 as determined by a GLC
method. J. environ. Sci. Health, Part B, 11: 175-183.
CHOW, C., MONTGOMERY, M.L., & YU, T.C. (1971) Methodology and
analysis for residues of MCP and 2,4,5-T in wheat. Bull. environ.
Contam. Toxicol., 18: 361-365.
CLARK, D.E., PALMER, J.S., RADELEFF, R.D., CROOKSHANK, H.R., &
FARR, F.M. (1975) Residues of chlorophenoxy acid herbicides and
their phenolic metabolites in sheep and cattle. J. agric. food
Chem., 23(3): 573-578.
COCHRANE, W.P. (1981) Chemical derivatization in pesticide
analysis. In: Frei, R.W. & Lawrence, J.F., ed. Chemical
derivatization in analytical chemistry, I: Chromatography, New
York, Plenum Press, pp. 1-97.
COCHRANE, W.P. & RUSSELL, J.B. (1975) Residues in wheat and soil
treated with the mixed butyl esters of 2,4-D. Can. J. plant Sci.,
55: 323-325.
COCHRANE, W.P., SINGH, J., MILES, W., & WAKEFORD, B. (1981)
Determination of chlorinated dibenzo- p-dioxin contaminants in
2,4-D products by gas chromatography - mass spectrometric
techniques. J. Chromatogr., 217: 289-299.
COCHRANE, W.P., LANOUETTE, M., & SINGH, J. (1982) High pressure
liquid chromatographic determination of impurity phenols in
technical 2,4-D acid and 2,4-dichlorophenol. J. Assoc. Off. Anal.
Chem., in press.
COGGON, D. & ACHESON, E.D. (1982) Do phenoxy herbicides cause
cancer in man? Lancet, 1: 1057-1059.
COHEN, S.Z., ZWEIG, G., LAW, M., WRIGHT, D., & BONTOYAN, W.R.
(1978) Analytical determination of N-nitroso compounds in
pesticides by the United States Environmental Protection Agency.
In: Walker, E.A., Castegnaro, M., Criciute, L., & Lyle, R.E., ed.
Environmental Aspects of N-nitroso compounds, Lyons, IARC
Scientific Publications, 19: 333-342.
COLLABORATIVE INTERNATIONAL PESTICIDES ANALYTICAL COUNCIL LTD
(1970) CIPAC Handbook, Vol. I, Analysis of technical and
formulated pesticides, Harpenden, England, CIPAC, pp. 241-273.
COMMISSION OF EUROPEAN COMMUNITIES (1981) Criteria (dose/effect
relationships) for organochlorine pesticides. Oxford, New York,
Toronto, Sydney, Paris, Frankfurt, Pergamon Press, pp. 223-240.
CONNICK, W.J., Jr & SIMONEAUX, J.M. (1982) Determination of (2,4-
dichlorophenoxy) acetic acid and of 2,6-dichlorobenzo-nitrile in
water by high performance liquid chromatography. J. agric. food
Chem., 30: 258-260.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99(1): 1-12.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet
samples (V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet
samples (VI). Pestic. monit. J., 5(4): 313-330.
COURTNEY, K.D. (1977) Prenatal effects of herbicides: Evaluation
by the prenatal development index. Arch. environ. Contamin.
Toxicol., 6: 33-46.
COUTSELINIS, A., KENTARCHOV, R., & BOUKIS, D. (1977) Concentration
levels of 2,4-D and 2,4,5-T in forensic material. Forensic Sci.,
1: 203-204.
CRAFTS, A.S. (1960) Evidence for hydrolysis of esters of 2,4-D
during absorption by plants. Weeds, 8: 19-25.
CRIPPS, R.E. & ROBERTS, T. (1978) Microbial degradation of
herbicides. In: Hill, I.R. & Wright, S.J.L., ed.: Pesticide
microbiology - Microbial aspects of pesticide behaviour in the
environment, London, New York, San Francisco, Academic Press,
pp. 669-730.
CROSBY, D.G. & TUTASS, H.O. (1966) Photodecomposition of 2,4-
dichlorophenoxyacetic acid. J. agric. Food Chem., 14: 596.
CROSBY, D.G. & WONG, A.S. (1973) Photodecomposition of 2,4,5-
trichlorophenoxyacetic acid (2,4,5-T) in water. J. agric. Food
Chem., 21(6): 1052-1054.
CROSSEN, P.E., MORGAN, W.F., HORAN, J.J., & STEWART, J. (1978)
Cytogenetic studies of pesticide and herbicide sprayers. New
Zealand med. J., 88(619): 192-195.
CRUMMET, W.P. & STEHL, R.H. (1973) Determination of chlorinated
dibenzo- p-dioxins and dibenzofurans in various materials. Environ.
Health Perspect., 5: 15-25.
CURRIE, L.A. (1968) Limits of qualitative detection and
quantitative determination. Anal. Chem., 40: 586-593.
CURRY, A.S. (1962) Twenty-one uncommon cases of poisoning. Br.
med. J., 1: 687-689.
DANON, J.M., KARPATI, G., CARPENTER, S., & WOLFE, L.S. (1976)
Experimental myotonic myopathy. Neurology, 26: 384.
DEBEER, J.O., VAN PETEGHEM, C.H., HEYNDRICKX, A.M., & VAN ZELE, W.
(1981) Mass fragmentographic determination of 2,4-D and 2,4,DP
trace levels in biosamples using intramolecular deuterated interval
standards. Med. Fac. Landbouww. Rijksuniv. Gent, 46(1): 305-316.
DELARRARD, J. & BARBASTE, M. (1969) Intoxication suicidaire
mortelle agro-chimique de l'hormone désherbante 2,4-D. Arch. Mal.
prof. Med. Trav. Secur. soc., 30(7-8): 434.
DENNIS, C.A.R. (1976) Health effects of 2,4-D. In: National
Research Council of Canada Associate Committee on Scientific
Criteria for Environmental Quality, ed. Phenoxy herbicides: their
effects on environmental quality, Ottawa, NRCC Publications
Service, p.333.
DEREUCK, J., DECOSTER, W., WILLEMS, J., & VANDER EECKEN, H. (1979)
Influence of 2,4-dichlorophenoxyacetate and of dantrolene sodium on
the target phenomenon in tenotomized rat gastrocnemius muscle.
Acta neuropathol., 46: 167-168.
DESI, I. & SOS, J. (1962a) Central nervous injury by a chemical
herbicide. Acta med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1962b) [The effect of 2,4-dichloro-
phenoxyacetic acid and triorthocresyl phosphate on the function of
the upper central nervous system (bioelectric activity and
conditioned reflexes).] Gig. i Sanit., 12: 38-46 (in Russian,
Health & Welfare Canada Translation No. 2183).
DESI, I., SOS, J., & NIKOLITS, I. (1962a) New evidence concerning
the nervous site of action of a chemical herbicide causing
professional intoxication. Pathophysiologica (Budapest), 22:
73-80.
DESI, I., SOS, J., OLASZ, J., SULE, F., & MARKUS, V. (1962b)
Nervous system effects of a chemical herbicide. Arch. environ.
Health, 4: 95-102.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B., & GEORGE, J.L. (1962)
Effects on wildlife. pp. 4-52. In: Effects of pesticides on fish
and wildlife: A review of investigations during 1960. Washington,
DC, US Dept. of the Interior (Fish and Wildlife Circ. No. 143).
DRAPER, W.M. & STREET, J.C. (1982) Applicator exposure to 2,4-D,
dicamba, and a dicamba isomer. J. environ. Sci. Health, B17(4):
321-339.
DRILL, V.A. & HIRATZKA, T. (1953) Toxicity of 2,4-D and 2,4,5-T,
a report on their acute and chronic toxicity in dogs. Arch. ind.
Hyg. occup. Med., 7: 61-67.
DUDLEY, A.W. & THAPAR, N.T. (1972) Fatal human ingestion of
2,4-D, a common herbicide. Arch. Pathol., 94: 270-275.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States (III), June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E., LIPSCOMB, G.Q., COX, E.L., HEATWOLE, R.E., & KLING,
R.C. (1971) Pesticide residue levels in foods in the United
States from July 1, 1963 to June 30, 1969. Pestic. monit. J.,
5(2): 73-80.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some herbicides
on the hatching rate of hens' eggs. Nature (Lond.), 215:
1406-1407.
DUNACHIE, J.F. & FLETCHER, W.W. (1970) The toxicity of certain
herbicides to hens' eggs assessed by the egg injection technique.
Ann. app. Biol., 66: 515-520.
DURIC, V., STOJANOVIC, V., & IGIC, B. (1979) [Presentation of
patients with pesticide poisoning.] Med. Pregl., 32(3/4): 129-133.
(In Serbo-Croatian, Health & Welfare Canada Translation No. 2382.)
DUX, E., TOTH, I., DUX, L., & JOO, F. (1978) The localization of
calcium by X-ray microanalysis in myopathic muscle fibers.
Histochemistry, 56: 239-244.
DZHAPAROV, I.R. & TSILIKOV, V.V. (1969) [The effect of the sodium
salt of 2,4-D and carbothione on the content of glycogen in the
liver and the blood sugar level in rats.] Farmakol. Tsentr.
Kholinolitikov Drugikh Neirotropnykh Sredstv., p. 180 (in
Russian).
EASLEY, C.B., LAUGHLIN, J.M., GOLD, R.E., & TUPY, D.R. (1983)
Laundering procedures for removal of 2,4-dichloro-phenoxyacetic
acid ester and amine herbicides from contaminated fabrics. Arch.
Environ. Contam. Toxicol., 12: 71-76.
EBERSTEIN, A. & GOODGOLD, J. (1979) Experimental myotonia induced
in denervated muscles by 2,4-D. Muscle Nerve, 2: 364-368.
ELO, H. & YLITALO, P. (1977) Substantial increase in the levels
of chlorophenoxyacetic acids in the CNS of rats as a result of
severe intoxication. Acta pharmacol. toxicol., 41: 280-284.
ELO, H. & YLITALO, P. (1979) Distribution of 2-methyl-4-
chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid in male
rats: evidence for the involvement of the central nervous system
in their toxicity. Toxicol. appl. Pharmacol., 51: 439-446.
ELWOOD, J.M. & ROGERS, J.R. (1975) The incidence of congenital
abnormalities in British Columbia, Alberta, Manitoba and New
Brunswick, 1966-1969. Can. J. public Health, 66: 471-475.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERICKSON, L.C., BRANNAMAN, B.L., & COGGINS, C.W. Jr. (1963)
Residues in stored lemons treated with various formulations of
2,4-D. J. agric. food Chem., 11: 437-440.
ERIKSSON, M., HARDELL, M., BERG, N.O., MOLLER, T., & AXELSON, O.
(1981) Soft-tissue sarcomas and exposure to chemical substances: a
case-referent study. Br. J. ind. Med., 38: 27-33.
ERNE, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta vet. Scand., 7: 240-256.
ERNE, K. (1966b) Studies on the animal metabolism of
phenoxyacetic herbicides. Acta vet. Scand., 7: 264-271.
ERNE, K. (1974) Weed-killers and wildlife. In: Proceedings of
the XI International Congress on Game Biology, Stockholm, 1973.
pp. 415-422 (National Swedish Environment Protection Board
Publications No. 13E).
ERNE, K. (1975) Phenoxy herbicide residues in Swedish fish and
wildlife. In: Coulston, F., Korte, F., Klein, W., & Rosenblum,
I., ed. Pesticides: Environmental quality and safety, Stuttgart,
George Thieme Publishers, Suppl. Vol. III., pp. 192-195.
ERNE, K. (1980) [Further studies of phenoxy herbicide residues in
woodland berries and mushrooms, 1973-1979.] Vr Föda, 32(5):
285-292 (in Swedish).
ERNE, K. & RUTQVIST, L. (1979) [Pesticide residues in feedstuffs
in Sweden.] Nord. Veterinaermed., 31: 263-274 (in Swedish).
ERNE, K. & SPERBER, I. (1974) Renal tubular transfer of
phenoxyacetic acids in the chicken. Acta pharmacol. toxicol., 35:
233-241.
ERNE, K. & VON HAARTMAN, U. (1973) [Phenoxy acid residues in
forest berries and mushrooms.] Vr Föda, 25: 146-153 (in Swedish).
EVANS, W.C., SMITH, B.S.W., FERNLEY, H.M., & DAVIES, J.I. (1971)
Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem. J.,
122: 543-551.
EYZAGUIRRE, C., FOLK, B.P., ZIERLER, K.L., & LILIENTHAL, J.L. Jr.
(1948) Experimental myotonia and repetitive phenomena: The
veratrinic effects of 2,4-D acetate in the rat. Am. J. Physiol.,
155: 69-77.
FABACHER, D.L. & CHAMBERS, H. (1974) Resistance to herbicides in
insecticide-resistant mosquitofish, Gambusia affinis. Environ.
Lett., 7(1): 15-20.
FAO (1981) Pesticide residues in food: 1980 evaluations. Rome,
FAO, pp. 109-110 (Plant Production and Protection Paper 26 Suppl.)
FAO/WHO (1971) 2,4-D. In: 1970 Evaluations of some pesticide
residues in food, Rome, FAO/WHO, pp. 59-86 (FAO/AGP/1970/M/ 12/1,
WHO Food Additive Series, 71, 42).
FAO/WHO (1972) 1971 evaluations of some pesticide residues in
food: 2,4-D. WHO Pesticide Residues Series, 1: 83-97.
FAO/WHO (1973) Pesticide residues in food. Report of the 1972
Joint FAO/WHO meeting, Geneva, (WHO Techn. Rep. Series No. 525).
FARWELL, S.O., BOWES, F.W., & ADAMS, D.F. (1976a) Determination
of chlorophenoxy herbicides in air by gas chromatography/mass
spectrometry: selective ion monitoring. Anal. Chem., 48: 420-426.
FARWELL, S.O., ROBINSON, E., POWELL, W.J., & ADAMS, D.F. (1976b)
Survey of airborne 2,4-D in south central Washington. J. Air
Pollut. Control Assoc., 26: 224-230.
FAUST, S.D. & ALY, O.M. (1963) Some effects of 2,4-D and 2,4-DCP
on drinking water quality. Proc. Northeast. Weed Contr. Conf., 17:
460-470.
FAUST, S.D. & SUFFET, I.H. (1966) Recovery, separation, and
identification of organic pesticides from natural and potable
waters. Residue Rev., 15: 44-116.
FEDOROVA, L.M. & BELOVA, R.S. (1974) [Inclusion of 2,4-
dichlorophenoxyacetic acid in organs of animals: Paths and
dynamics of its excretion.] Gig. i Sanit., 39(2): 105-107
(in Russian, US NTC Translation No. 76-10918-05J).
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FETISOV, M.I. (1966) Occupational hygiene in the application of
herbicides of the 2,4-D group. Hyg. Sanit., 31(7-9): 383-386.
FEUNG, C.S., HAMILTON, R.H., & WITHAM, F.H. (1971) Metabolism of
2,4-dichlorophenoxyacetic acid by soybean cotyledon callus tissue
cultures. J. agric. food Chem., 19: 474-479.
FEUNG, C.S., HAMILTON, R.H., WITHAM, F.H., & MUMMA, R.O. (1972)
The relative amounts and identification of some 2,4-
dichlorophenoxyacetic acid metabolites isolated from soybean
cotyledon callus cultures. J. agric. food Chem., 50: 80-86.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1973b) Metabolism of
2,4-dichlorophenoxyacetic acid. V. Identification of metabolites
in soybean callus tissue cultures. J. agric. food Chem., 21:
637-640.
FEUNG, C.S., HAMILTON, R.H., & MUMMA, R.O. (1975) Metabolism of
2,4-dichlorophenoxyacetic acid. VII. Comparison of metabolites
from five species of plant callus tissue cultures. J. agric. food
Chem., 23: 373-376.
FEUNG, C.S., LOERCH, S.L., HAMILTON, R.H., & MUMMA, R.O. (1978)
Comparative metabolic fate of 2,4-dichlorophenoxy acetic acid in
plants and plant tissue culture. J. Agric. Food Chem., 26:
1064-1067.
FIELD, B. & KERR, C. (1979) Herbicide use and incidence of
neural tube defects. Lancet, 1: 1341-1342.
FINNISH STATE INSTITUTE OF AGRICULTURAL CHEMISTRY (Helsinki)
(1963-1969) Investigations on pesticide residues. pp. 83.
FLEEKER, J.R. & STEEN, R. (1971) Hydroxylation of 2,4-D in
several weed species. Weed Sci., 19: 507-510.
FLORSHEIM, W.H. & VELCOFF, S.M. (1962) Some effects of
2,4-dichlorophenoxyacetic acid on thyroid function in the
rat: Effects on iodine accumulation. Endocrinology, 71(1):
1-6.
FLORSHEIM, W.H., VELCOFF, S.M., & WILLIAMS, A.D. (1963) Some
effects of 2,4-D on thyroid function in the rat: Effects on
peripheral thyroxine. Endocrinology, 72: 327-333.
FOISSAC-GEGOUX, P., LELIEVRE, A., BASIN, B., & WAROT, P. (1962)
Polynevrite aprčs usage d'un desherbant: l'acide 2,4-D. Lille Med.,
7(10): 1049-1051.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry
to nine herbicides. Bull. environ. Contam. Toxicol., 15(5):
509-514.
FOLMAR, L.C. (1979) Effects of short term field applications of
acrolein and 2,4-D (DMA) on flavor of the flesh of rainbow trout,
124 pp.
FOSTER, R.K. & McKERCHER, R.B. (1973) Extraction of 14C-labelled
chlorophenoxyacetic acids from soil. Can. J. soil Sci., 53:
135-136.
FRANK, P.A. (1972) Herbicidal residues in aquatic environments.
In: Faust, S.D., ed. Fate of organic pesticides in the aquatic
environment. Washington, DC, American Chemical Society,
pp. 135-148 (Advances in Chemistry Series, No. III).
FRANK, P.A. & COMES, R.D. (1967) Herbicidal residues in pond
water and hydrosoil. Weeds, 15(3): 210-213.
FRANK, P.A., DEMINT, R.J., & COMES, R.D. (1970) Herbicides in
irrigation water following canal-bank treatment for weed control.
Weed Sci., 18(6): 687-692.
FRANK, R. & SIRONS, G.J. (1980) Chlorophenoxy and chlorobenzoic
acid herbicides; their use in eleven agricultural waterbeds and
their loss to stream waters in southern Ontario, Canada, 1975-1977.
Sci. total Environ., 15: 149-167.
FRANK, R., SIRONS, G.J., & RIPLEY, B.D. (1979) Herbicide
contamination and decontamination of well waters in Ontario,
Canada, 1969-1978. Pestic. monit. J., 13(3): 120-127.
FRANK, R., SIRONS, G.J., CAMPBELL, R.A., & MEWETT, D. (1982)
Residues of 2,4-D, dichlorprop, and picloram in wild berries from
treated rights-of-way and conifer release sites in Ontario,
1979-81. Can. J. Plant Sci., 63: 196-209.
FRANKLIN, C.A., GROVER, R., MARKHAM, J.W., SMITH, A.E., & YOSHIDA,
K. (1982) Effect of various factors on exposure of workers
involved in the aerial application of herbicides. Am. Conf. of
Govern. Ind. Hygienists, Transactions, 43: 97-117.
FREEMON, F.R. (1975) Causes of polyneuropathy. Acta neurol.
Scand., 51(Suppl. 59): 5-43.
FYODOROVA, L.M., BELOVA, R.S., KIRYUHINA, N.N., VOLCHONOK, M.G., &
KOVTUNOVA, N.Y. (1977) [Residual content of chlorophenoxyacetic
acids in the environment and human body.] Gig. i Sanit., 6: 101-103
(in Russian).
GAMBLE, W. (1975) Mechanism of action of hypolipidemic and
herbicidal aryloxy acids. J. theor. Biol., 54: 181-190.
GAVRILOVA, L.I. (1965) Experimental substantiation of the
permissible concentration of 2,4-dichlorophenoxy-gamma-butyric (DB)
acid in water bodies. Hyg. Sanit., 29(1): 11-16.
GELDMACHER- VON MALLINCKRODT, M., & LAUTENBACH, L. (1966) [Two
fatal poisonings (suicides) with chlorinated phenoxyacetic acids
(2,4-D and MCPA).] Arch. Toxicol., 21: 261-278 (in German).
GLUCK, S.J. & MELCHER, R.G. (1980) Concentration and
determination of the propylene glycol butyl ether esters of
2,4-dichlorophenoxyacetic acid (PGBE 2,4-D) in air. Am. Hyg. Ass.
J., 41(12): 932-934.
GOLDSTEIN, N.P. & BROWN, J.R. (1960) Peripheral neuropathy.
J. Am. Med. Assoc., 173: 171.
GOLDSTEIN, N.P., JONES, P.H., & BROWN, J.R. (1959) Peripheral
neuropathy after exposure to an ester of dichlorophenoxyacetic
acid. J. Am. Med. Assoc., 171: 1306-1309.
GOLDWATER, L.J. (1960) Peripheral neuropathy. J. Am. Med.
Assoc., 173(1): 171.
GORSHKOV, A.I. (1972) Hygienic evaluation of chlorocholine
chloride (Preparation CCC) and its combination with 2,4-D amine
salt. Hyg. Sanit., 36(10-12): 208-211.
GOWEN, J.A., WIERSMA, G.B., & TAI, H. (1976) Mercury and 2,4-D
levels in wheat and soils from sixteen states, 1969. Pestic. Monit.
J., 10(3): 111-113.
GRAFF, G.L.A., GUENING, C., & VANDERKELEN, B. (1972) Influence de
l'acide 2,4-dichlorophénoxyacéticque (2,4-D), un agent myotonisant,
sur le métabolisme des phosphates acidosolubles des muscle
squelettiques du rat. C. R. Soc. Biol. (Paris), 166(II):
1565-1568.
GRANROTH, G., HAKAMA, M., & SAXEN, L. (1977) Defects of the
central nervous system in Finland: I. Variations in time and
space, sex distribution, and parental age. Br. J. prev. soc.
Med., 31: 164-170.
GRANROTH, G., HAAPAKOSKI, J., & SAXEN, L. (1978) Defects of the
central nervous system in Finland: V. Multivariate analysis of
risk indicators. Int. J. Epidemiol., 7(4): 301-308.
GRANT, W. (1976) 2,4-D. In: Hart, R.W., Kraybill, H.F., &
DeSerres, F.J., ed.: A rational evaluation of pesticidal vs
mutagenic/carcinogenic action. Bethesda, Maryland, National
Institute of Health (Publication No. (NIH) 78-1306 U.S. Department
of Health, Education & Welfare, Public Health Service).
GRAY, T.J.B., LAKE, B.G., BEAMAND, J.A., FOSTER, J.R., & GANGOLLI,
S.D. (1983) Peroxisome proliferation in primary cultures of rat
hepatocytes. Toxicol. appl. Pharmacol., 67: 15-25
GREEN, S. & MORELAND, F.S. (1975) Cytogenic evaluation of
several dioxins in the rat. Toxicol. appl. Pharmacol., 33: 161.
GRIBANOV, O.I. (1968) Pollution of an open water source at
Shortandy Railroad Station by the herbicide 2,4-D butyl ester.
Hyg. Sanit., 32: 267-268.
GRIGSBY, B.H. & FARWELL, E.D. (1950) Some effects of herbicides
on pasture and on grazing livestock. Mich. Agric. Exp. St. Bull.,
32(3): 378-385.
GROLLEAU, G., DE LAVAUR, E., SIOU, G., & FROUX, Y. (1974) Effects
of 2,4-D on the reproduction of quail and partridge after the
application of the product by spraying on eggs. Ann. Zool. Ecol.
anim., 6(2): 313-331.
GROVER, R. (1976) Relative volatilities of ester and amine forms
of 2,4-D. Weed Sci., 24: 26-28.
GROVER, R. & KERR, L.A. (1981) Evaluation of polyurethane foam as
a trapping medium for herbicide vapour in air monitoring and worker
inhalation studies. J. environ. Sci. Health, B 16(1): 59-66.
GROVER, R. & SMITH, A.E. (1974) Adsorption studies with the acid
and dimethylamine forms of 2,4-D and dicamba. Can. J. Soil Sci.,
54: 179-186.
GROVER, R., MAYBANK, J., & YOSHIDA, K. (1972) Droplet and vapour
drift from butyl ester and dimethylamine salt of 2,4-D. Weed Sci.,
20: 320-324.
GROVER, R., KERR, L.A., WALLACE, K., YOSHIDA, K., & MAYBANK, J.
(1976) Residues of 2,4-D in air samples from Saskatchewan
1966-1975. J. environ. Sci. Health, B 11(4): 331-347.
GRUNOW, W. & BOHME, C. (1974) [On the metabolism of 2,4,5-T and
2,4-D in rats and mice.] Arch. Toxicol., 32: 217-225 (in German).
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1971) Determination of
some acidic herbicides by thin layer chromatography. J. agric.
food Chem., 19: 1181-1182.
GUARINO, A.M. & ARNOLD, S.T. (1979) Xenobiotic transport
mechanisms and pharmacokinetics in the dogfish shark. In: Khan,
M.A.Q., Lech, J.J., & Menn, J.J., ed. Pesticide and xenobiotic
metabolism in aquatic organisms. Washington, DC, American Chemical
Society, pp. 250-258.
GUMMER, W.D. (1980) Pesticide monitoring in the prairies of
western Canada. Environ. Sci. Res., 16: 345-372.
GUNTHER, F.A. (1962) Instrumentation in pesticide residue
determination. In: Metcalf, R.L., ed. Advances in Pest Control
Research, 5, New York, Interscience Publishers, pp. 191-319.
GUSEVA, E.N. (1956) [On the pharmacology of 2,4-dichloro-
phenoxyacetic acid.] Farmakol. Toksikol. (Mosc.), 19(4):
41-44 (in Russian).
GUTENMANN, W.H. & LISK, D.J. (1965) Conversion of 4-(2,4-DB)
herbicide to 2,4-D by bluegills. NY Fish Game J., 12(1): 108-111.
GUTENMANN, W.H., HARDEE, D.D., HOLLAND, R.F., & LISK, D.J. (1963)
Disappearance of 4(2,4-dichlorophenoxy-butyric) acid herbicide in
the dairy cow. J. dairy Sci., 46: 991-992.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The effects of
phenoxy herbicides on the hatchability of eggs and the viability of
the chicks. Acta pharmacol. toxicol., 35: 300-308.
HACQUE, R., DEAGEN, J., & SCHMEDDING, D. (1975) Binding of
2,4-dichloro- and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. A proton magnetic resonance study. J. agric. food Chem.,
23: 763-766.
HALLIOP, J., TOCHMAN, A., & LATALSKI, M. (1980) [Ultrastructural
investigations and myeloperoxidase determinations in rat
neutrophils in acute poisoning with 2,4-dichlorophenoxyacetic
acid.] Acta haematol. Pol., 11(4): 249-257 (in Polish).
HALTER, M. (1980) 2,4-D in the aquatic environment In: Shearer,
R., & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlobenil, diquat & endothall, Seattle
Municipality of Metropolital Seattle, January 1980, pp. 89-182.
HANIFY, J.A., METCALF, P., NOBBS, C.L., & WORSLEY, K.J. (1980)
Congenital malformations in the newborn in Northland: 1966-1977.
New Zealand med. J., 92: 245-248.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquito fish, Gambusa
affinis. Bull. environ. Contam. Toxicol., 8(1): 46-51.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973) Avoidance of
pesticides by grass shrimp (Palaeomonetes pugio). Bull. environ.
Contam. Toxicol., 9(3): 129-133.
HANSEN, W.H., QUAIFE, M.L., HABERMANN, R.T., & FITZHUGH, O.G,
(1971) Chronic toxicity of 2,4-dichlorophenoxyacetic acid in rats
and dogs. Toxicol. appl. Pharmacol., 20: 122-129.
HARDELL, L. & SANDSTRÖM, A. (1979) Case-control study: soft
tissue sarcomas and exposure to phenoxyacetic acids or
chlorophenols. Br. J. Cancer, 39: 711.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 43: 169-176.
HARRISON, J.W.E. & REES, E.W. (1946) 2,4-D toxicity - I.
Toxicity towards certain species of fish. Am. J. Pharm., 118:
422-425.
HASS, J.R., FRIESEN, M.D., & HOFFMAN, M.K. (1981) Recent mass
spectrometric techniques for the analysis of environmental
contaminants. In: McKinney, J. D., ed. Environ. Health Chem.,
Ann Arbor, Arbor Sciences, pp. 219-243.
HATCH, T.F. & GROSS, P. (1964) Pulmonary desposition and
retention of inhaled aerosols. New York, London, Academic Press,
XIV + 192 pp (American Industrial Hygiene Association Monograph
Series on Industrial Hygiene).
HAYES, W.J. (1982) 2,4-D. In: Pesticides studied in man.
Baltimore & London, Williams & Wilkins.
HEENE, R. (1966) [Histochemical proof of phosphorylase inhibition
by 2,4-D in the skeletal muscles and liver of the rat.]
Naturwissenschaften, 53: 308 (in German).
HEENE, R. (1967) [Inhibition of glycogen-forming enzymes by
2,4-D.] Histochemie, 8: 45-53 (in German).
HEENE, R. (1968) Histochemical and morphological findings in
experimental 2,4-D myopathy in warmblooded animals. Acta
neuropathol., 10: 166-169.
HEENE, R. (1975) Experimental myopathies and muscular dystrophy.
Studies in the formal pathogenesis of the myopathy of 2,4-D
acetate. Schriftenr. Neurol. Ser., 16: 1-97.
HENSHAW, B., QUE HEE, S.S., SUTHERLAND, R.F., & LEE, C.D. (1975)
Gas-liquid chromatography and gas-liquid chromatography combined
with mass spectrometry of a butyl ester formulation of 2,4-
dichlorophenoxy-acetic acid. J. Chromatogr., 106: 33-39.
HERBICH, J. & MACHATA, G. (1963). [Poisoning with
2,4-dichlorophenoxy-acetic acid (2,4-D).] Beitr. gerichtl. Med.,
22: 133-139 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies to determine
the toxic effects of derivatives of 2,4-dichloro- and 2,4,5-
trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated eggs
of pheasants, quails, and chickens. First Report.] Anz.
Schaedling-sk. D. Pflanzenschutz Umweltschutz, 49: 21-25 (in
German).
HILBIG, V., LUCAS, K., SEBEK, V., & MUNCHOW, H. (1976b) [Studies
to determine the toxic effects of derivatives of 2,4-dichloro- and
2,4,5-trichlorophenoxyacetic acid (2,4-D and 2,4,5-T) on incubated
eggs of pheasants, quails, and chickens. Second report.] Anz.
Schaedlingsk. D. Pflanzenschutz Umweltschutz, 49: 65-68 (in
German).
HILL, E.V. & CARLISLE, H. (1947) Toxicity of 2,4-D for
experimental animals. J. ind. Hyg. Toxicol., 29(2): 85-95.
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized
fish eggs and fry. Trans. Am. Fish. Soc., 96(4): 414-416.
HOFMANN, W.W., ALSTON, W., & ROWE, G. (1966) A study of
individual neuro-muscular junctions in myotonia.
Electroencephalogr. clin. Neuro-physiol., 21: 521-537.
HOGSTEDT, C. & WESTERLUND, B. (1980) Cohort studies of cause of
death of forest workers with and without exposure to phenoxy
preparations. Läkartidninger, 77(19): 1828-1831.
HOGSTEDT, B., KOLNIG, A.M., MITELMAN, R., & SKERFVING, S. (1980)
Cytogenetic study of pesticides in agricultural work. Hered.,
92: 177-178.
HOLCOMBE, G.W., FIANDT, J.T., & PHIPPS, G.L. (1980) Effects of pH
increases and sodium chloride additions on the acute toxicity of
2,4-dichlorophenol to the fathead minnow. Water Res., 14(8):
1073-1077.
HOLMBERG, P.C. (1979) Central nervous system defects in children
born to mothers exposed to organic solvents during pregnancy.
Lancet, 2: 177-179.
HOOPER, F.N. (1958) The effect of applications of pelleted 2,4-D
upon the bottom fauna of Kemt Lake, Oakland County, Michigan. Proc.
Ann. Meet. North Centr. Weed Control Conf., 41.
HORNER, J., QUE HEE, S.S., & SUTHERLAND, R.G. (1974)
Esterification of (2,4-dichlorophenoxy) acetic acid - a
quantitative comparison of esterification techniques Anal. Chem.,
46: 110-112.
HUCKINS, J.N., STALLING, D.C., & SMITH, W.A. (1978) Foam-charcoal
chromatography for the analysis of polychlorinated dibenzodioxins
in Herbicide Orange. J. Assoc. Anal. Chem., 61: 32-38.
HUFF, J.E., MOORE, J.A., SARACCI, R., & TOMATIS, L. (1980) Long
term hazards of polychlorinated dibenzodioxins and polychlorinated
dibenzofurans. Environ. Health Persp., 36: 221-240.
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to
bluegill sunfish of phenoxy herbicides. Weeds, 11(1): 50-53.
HUNT, L.M., GILBERT, B.N., & PALMER, J.S. (1970) Effects of a
herbicide, 2-ethylhexyl ester of 2,4-D on magnesium: calcium ratios
and blood urea nitrogen levels in sheep and cattle. Bull. environ.
Contam. Toxicol., 5(1): 54-60.
IARC (1977) 2,4-D and esters. In: Some fumigants, the herbicides
2,4-D and 2,4,5-T, chlorinated dibenzodioxins, and miscellaneous
industrial chemicals. Lyons, International Agency for Research on
Cancer, pp. 111-138 (IARC monograph on the evaluation of the
carcinogenic risk of chemicals to man Vol. 15).
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. Lyons, International Agency for
Research on Cancer, p. 101 (IARC Monographs of 1-29, Supplement 4).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer, Vol. 30.
ILO (1977) Safe use of pesticides, Geneva, International
Labour Office (Occupational Safety and Health Series, No. 38).
ILO (1979) Guide to health and hygiene in agricultural work,
Geneva, International Labour Organisation, pp. 309.
INGELOG, T., ERNE, K., PAULSSON, A.M., JONASSON, H., & HOLMGREN, A.
(1977) [Taste of flavour changes in woodland berries after
herbicide spraying.] Vr Föda, 29(6): 1-16 (in Swedish).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, L.H.,
& GART, J.J. (1969) Bioassay of pesticides and industrial
chemicals for tumorigenicity in mice: A preliminary note. J. Natl
Cancer Inst., 42: 1101-1114.
IYER, V., WHITING, M., & FENICHEL, G. (1976) Neural influence in
experimental myotonia. Neurology, 26: 384.
JACKSON, J.W. & THOMAS, T.C. (1978) A simple and inexpensive
method for sampling 2,4-D and 2,4,5-T herbicides in air. J. Air
Pollution Control Assoc., 28(11): 1145-1147.
JAMES, M.O. (1979) Dispositon of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Palinurus argus).
Pharmacologist, 21(3): 251.
JAMES, W.H. (1977) Clomiphene, acencephaly, and spina bifida.
Lancet, 1: 603.
JANERICH, D.T. (1973) Epidemic waves in the prevalence of
anencephally and spina bifida in New York State. Teratology, 8:
253-256.
JENSEN, D.J. & GLAS, R.D. (1981) Analysis for residues of acidic
herbicides. In: Moye, H.A., ed. Analysis of pesticide residues,
New York, Chichester, Brisbane, Toronto, John Wiley & Sons, pp.
223-261.
JENSSEN, D. & RENBERG, L. (1976) Distribution and cytogenetic
test of 2,4-D and 2,4,5-T phenoxyacetic acids in mouse blood
tissues. Chem. biol. Interactions, 14: 291-299.
JOHNSON, J.E. (1971) The public health implications of widespread
use of the phenoxy herbicides and picloram. Bioscience, 27(17):
899-905.
JOHNSON, E.R., YU, T.C., & MONTGOMERY, M.L. (1977) Trapping and
analysis of atmospheric residues of 2,4-D. Bull. environ. Contam.
Toxicol., 17: 369-372.
JOHNSON, R.D. & MANSKE, D.D. (1976) Pesticide residues in total
diet samples (IX) Pestic. Monit. J., 9(4): 157-168.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples - (I) - August 1974 - July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1981a)
Pesticide, heavy metal and other chemical residues in infant and
total diet samples - (II) - August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-53.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b)
Pesticide, heavy metal, and other chemical residues in adult total
diet samples (XII) - August 1975 - July 1976. Pestic. monit. J.,
15(1): 54-69.
JUNG, H.D. & WOLF, F. (1977) [Contact eczemas due to forestry use
of the herbicide SELEST100.] Dtsch. Gesundheit., 32(31): 1464-1467
(in German).
JUZWIAK, I., JUZWIAK, J., SUCHOWIAK, J., & ORDA, E. (1973) [A
study of the extent of the pesticide threat to the health of
agricultural employees in Olesnicki Country.] Pol. Tyg. Lek.,
28(11): 419-422 (in Polish, Health & Welfare Canada Translation
No. 2157).
KAISER, H. (1973) Guiding concepts relating to trace analysis.
Pure appl. Chem., 34: 35-61.
KARKINEN-JAASKELAINEN, M. & SAXEN, L. (1974) Maternal influenza,
drug consumption, and congential defects of the central nervous
system. Am. J. Obstet. Gynecol., 118(6): 815-818.
KASKEVICH, L.M. & SOBOLOVA, L. P. (1978) [A case of acute
poisoning with herbicide 2,4-D (late after effects).] Gig. Tr.
Prof. Zabol., 22 (10): 49-50 (in Russian, US National
Translations Center, Translation No. 79-11916-06T).
KAS'JANENKO, A. G. & KOROLEVA, N. S. (1979) [An evaluation of the
genetic danger of pesticides.] Izv. Akad. Nauk SSSR Ser. Biol.,
3: 401-409 (in Russian).
KATEMAN, G. & PIJPERS, F.W. (1981) Quality Control in analytical
chemistry, New York, John Wiley & Sons, 276 pp.
KATZ, M., LEGORE, R.S., WEITKAMP, D., CUMMINS, J., ANDERSON D., &
MAY, D.R. (1972) Effects on fresh water fish. J. Water Pollut.
Control Fed., 44(6): 122-1250.
KAY, J.H., PALAZZOLO, B.S., & CALANDRA, J.C. (1965) Subacute
dermal toxicity of 2,4-D. Arch. environ. Health, 11: 648-651.
KENIGSBERG, Y.E. (1968) [Model myotonia in rats induced by
means of the diethylamine salt of 2,4-dichlorophenoxyacetic acid.]
Dok. Akad. Nauk Beloruss. SSR, 12(5): 473-475 (in Russian, Health &
Welfare Canada Translation No. 2440).
KETCHERSID, M.L., FLETCHALL, O.H., SANTELMANN, P.W., & MERKLE, M.G.
(1970) Residues in sorghum treated with the isooctyl ester of
2,4-D. Pestic. monit. J., 4(3): 111-113.
KHALATKAR, A.S. & BHARGAVA, Y.R. (1982) 2,4-dichlorophenoxy-
acetic acid. A new environmental mutagen. Mutat. Res., 103:
111-114.
KHANNA, S. & FANG, S. (1966) Metabolism of C14-labelled
2,4-dichlorophenoxyacetic acid in rats. J. agric. Food Chem.,
14: 500-503.
KHANNA, R.N. & KOHLI, J.D. (1977) Metabolism of chlorophenoxy
herbicides in man. In: Zaidi, S. H., ed. Proceedings of the
International Symposium on Industrial Toxicology, Lucknow, India,
November 1975, Lucknow, Industrial Toxicology Research Centre,
pp. 590-599.
KHERA, K.S. & McKINLEY, W.P. (1972) Pre- and postnatal studies on
2,4,5-T, 2,4-D and their derivatives in rats. Toxicol. appl.
Pharmacol., 22: 14-28.
KHERA, K.S. & RUDDICK, J.A. (1973) Polychlorodibenzo- p-dioxins.
Perinateal effects and the dominant lethal test in Wistar rats. In:
Blair, E.H., ed. Chlorodioxins - origin and fate. Washington, DC,
American Chemical Society, pp. 70-84 (Advances in Chemistry Series
No. 120).
KHIBIN, L.S., TAITSEL', L.A., & SLATOV, I.V. (1968) [On the
clinical aspects of acute poisoning with Dicotex 40.] Gig. Tr.
Prof. Zabol., 3: 52-53 (in Russian, Health & Welfare Canada
Translation No. 2240).
KIMBROUGH, R.D., ed. (1980) Halogenated biphenyls, terphenyls,
naphthalenes, debenzodioxins and related products. Amsterdam,
New York, Oxford, Elsevier, North Holland Biomedical Press.
KING, J.E. & PENFOUND, W.T. (1946) Effects of two of the new
formagenic herbicides on bream and largemouth bass. Ecology,
27(4): 327-374.
KING, C.T.G., HORIGAN, E.A., & WILK, A.L. (1971) Screening of the
herbicides 2,4,5-T and 2,4-D for cleft palate. Teratology, 4(2):
233.
KLEKOWSKI, R.Z. & ZVIRGZDS, J. (1971) [The influence of herbicide
2,4-D Na on respiration and survival of Simocephalus vetulus O.F.
Muller (Cladocera).] Pol. Arch. Hydrobiol., 18(4): 393-400 (in
Polish).
KLINGMAN, D.L., GORDON, C.H., YIP, G., & BURCHFIELD, H.P. (1966)
Residues in the forage and milk from cows grazing forage treated
with esters of 2,4-D. Weeds, 14: 164-167.
KNUTSON, J.C. & POLAND, A. (1980) Keratinization of mouse
teratoma cell line xB produced by 2,3,7,8-tetrachlorodibenzo- p-
dioxin: an in vitro model of toxicity. Cell, 22: 27-36.
KOCIBA, R.J. & SCHWETZ, B.A. (1982) A review of the toxicity of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) with a comparison to the
toxicity of other chlorinated dioxin isomers. Assoc. Food Drug
Offic. Q. Bull., 46(3): 168-188.
KOHLI, J.D., KHANNA, R.N., GUPTA, B.N., DHAR, M.M., TANDON, J.S., &
SIRCAR, K.P. (1974) Absorption and excretion of 2,4-
dichlorophenoxyacetic acid in man. Xenobiotica, 4(2): 97-100.
KOLBERG, J., HELCELAND, K., & JONSEN, J. (1973) Binding of
2,4-dichloro and 2,4,5-trichlorophenoxyacetic acid to bovine serum
albumin. Acta pharmacol. toxicol., 33(5-6): 470-475.
KOLMODIN-HEDMAN, B., ERNE, K., HÜKANSSON, M., & ENGQUIST, A. (1979)
[Control of occupational exposure to phenoxyacids (2,4-D and
2,4-5-T).] Arbete och Hälsa, 17: 3-26 (in Swedish).
KOLMODIN-HEDMAN, B. & ERNE, K. (1980) Estimation of occupational
exposure to phenoxy acids (2,4-D and 2,4,5-T). Arch. Toxicol.,
(Suppl. 4): 318-321.
KOLMODIN-HEDMAN, B., ERNE, K., & AKERBLOM, M. (1980) Field
application of phenoxy acid herbicides. In: Tordoir, W.F. &
Van Heemstra, E.A.H., ed. Field workers exposure during pesticide
application. New York, Elsevier Scientific Publishing Co., pp.73-77
(Studies in Environmental Science, Vol. 7).
KONSTANTINOVA, T.K. (1970) Toxicology and some problems of the
embryotropic action of the butyl ester of 2,4-D. In: Vygodchikov,
G.V., ed. Proceedings of a Conference on Problems on Hygiene and
Toxicology of Pesticides, USSR, 1967, Moscow, Meditsina, pp.
177-179.
KONSTANTINOVA, T.K., EFIMENKO, L.P., & ANTONEKO, T.A. (1975)
[Embryotropic effects of the products from the decomposition of
herbicides based on 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
11: 102-105 (in Russian, US National Translation Centre,
Translation No. 78-14230-06F.)
KONTEK, M., MARCINKOWSKA, B., & PIETRASZEK, Z. (1973) [Electro-
encephalographic investigations in farm workers exposed to
derivatives of arylakanocarboxylic acids.] Pol. Tyg. Lek.,
28(25): 937-939 (in Polish, Health & Welfare Canada
Translation No. 2215).
KOPISCHKE, E.D. (1972) The effect of 2,4-D and diesel fuel on egg
hatchability. J. Wildl. Manage., 36(4): 1353-1356.
KORTE, C. & JALAL, S.M. (1982) 2,4-D-induced clastogenicity and
elevated rates of sister chromatid exchanges in cultural human
lymphocytes. J. Hered., 73: 224-226.
KOSCHIER, F.J., GIRAD, P., & HONG, S.K. (1978) Transport of
2,4-dichlorophenoxyacetate by rat renal cortical slides. Toxicol.
appl. Pharmacol., 45: 883-894.
KOSCHIER, F.J. & PRITCHARD, J.B. (1979) Excretion of
2,4-dichlorophenoxyacetate (2,4-D) by the dogfish shark (Squalus
acanthias). Pharmacologist, 21(3):184.
KOSYAN, S.A., MARKOSYAN, V.E., SARKISYAN, L.S., & MANUKYAN, R.A.
(1974) [A toxicological evaluation of the new herbicide 3-nitro-4-
oxybenzyl ether of (2,4-dichlorophenoxy) acetic acid (NK-2).]
Zh. eksp. klin. Med., 14(3): 14-18 (in Russian, Health & Welfare
Canada Translation No. 2436).
KRAMER, D. & SCHMALAND, G. (1974) [Plant protection agent
residues in water: Problems in water management as a consequence of
biocide applications.] Wasserwirtsch. Wassertech., 24(5): 161-167
(in German).
KUHN, E. & STEIN, W. (1964) [Experimental myotonia with 2,4-D in
the rat.] Klin. Wochenschr., 42: 1215-1216 (in German).
KUHN, E. & STEIN, W. (1965) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D) in the rat. In vitro and in vivo
studies on the effects of 2,4-D on the energy metabolism of
muscle.] Klin. Wochenschr., 43: 673-677 (in German).
KUHN, E. & STEIN, W. (1966) [Model myotonia with 2,4-
dichlorophenoxyacetate (2,4-D).] Klin. Wochenschr., 44: 700-702
(in German).
KUZMINSKAJA, U.A. & BERSAN, L.V. (1975) [The effects of the
sodium salt of dichlorophenoxyacetic acid on the glycolysis,
ATPase, and transketolase activity of erythrocytes.] Farmakol.
Toksikol., 38(1): 102-104 (in Russian).
KUZYK, A. (1979) A report on a survey of the Alberta farming
community respecting the use of pesticides, Edmonton, Alberta
Department of the Environment, Pollution Control Division.
LAPPE, M. (1979) HLA homozygosity and neural-tube defects.
Lancet, 1: 1342.
LASKOWSKI, M.B. & DETTBARN, W.D. (1977) The pharmacology of
experimental myopathies. Ann. Rev. Pharmacol. Toxicol., 17:
387-409.
LAVY, T.L. ROETH, F.W., & FENSTER, C.R. (1973) Degradation of
2,4-D and atrazine at three soil depths in the field. J. Environ.
Qual., 2(1): 132-137.
LAVY, T.L., WALSTAD, J.D., FLYNN, R.R., & MATTICE, J.D. (1982)
(2,4-dichlorophenoxy) acetic acid exposure received by aerial
applications crew during forest spray operations. J. agric. food
Chem., 30: 373-381.
LEE, B. (1978) Herbicides under suspicion in Australia. New
Sci., 80(1125): 159.
LENG, M. (1972) Residues in milk and meat and safety to livestock
from the use of phenoxy herbicides in pasture and rangeland. Down
Earth, 28(1): 12-20.
LENG, M. (1977) Comparative metabolism of phenoxy herbicides in
animals. In: Ivie, G.W. & Dorough, H.W., ed. Fate of pesticides in
large animals, New York, San Francisco, London, Academic Press,
pp. 53-76.
LENG, M.L. (1979) Comparative toxicology of various chlorinated
dioxins as related to chemical structure. In: Bontoyan, W.R., ed.
Collaborative International Pesticides Analytical Council
Proceedings Symposium Series I, Cambridge, Heffers Printers Ltd.,
pp. 141-169.
LENG, M.L., LAVY, R.L., RAMSEY, J.C., & BRAUN, W.H. (1982)
Review of the studies with 2,4,5-T in humans including applicators
under field conditions, pp. 133-156. In: Plimmer, J.R., ed.:
Pesticide residues and exposure. Washington, DC, American Chemical
Society, (ACS Symposium Series 182).
LHOSTE, J. & ROTH, P. (1946) [The effect of aqueous sodium
2,4,-dichlorophenoxyacetate on the development of eggs of Rana
temporaria L.] C.R. Soc. Biol. Paris, 140: 272-274.
LIBICH, S., TO, J.C., WANG, K. Y., & WATSON, D. A. (1981) A
study to assess the occupational exposure to 2,4-D herbicides,
Toronto, Safety Services Department, Health and Safety Division.
pp. 16 (Ontario Hydro. Report SSD-81-1).
LINDQUIST, N.G. & ULLBERG, S. (1971) Distribution of the
herbicides 2,4,5-T and 2,4-D in pregnant mice. Accumulation in the
yolk sac epithelium. Experientia (Basel), 27: 1439-1441.
LINNAINMAA, K. (1983a) Non-mutagenicity of phenoxyacid herbicides
2,4-D and MCPA. In: Keith, L.H., Choudhary, G., & Rappe, C., ed.:
Chlorinated dioxins and dibenzofurans in the total environment.
Ann Arbor, Ann Arbor Sci. Publ. Inc., Vol. 1.
LINNAINMAA, K. (1983b) Induction of sister chromatid exchanges by
the peroxisome proliferators 2,4-D, MCPA, and clofibrate in vivo
and in vitro. Carcinogenesis, accepted for publication.
LIPSCOMB, G.Q. (1968) Pesticide residues in prepared baby foods
in the United States. Pestic. monit. J., 2(32):104-108.
LISK, D.J., GUTENMANN, W.H., BACHE, C.A., WARNER, R.G., & WAGNER,
D.G. (1963) Elimination of 2,4-D in the urine of steers fed 4
(2,4-DB) or 2,4-D. J. dairy Sci., 46: 1434-1435.
LIU, L.C. & CIBES-VIADE, H.R. (1973) Adsorption of fluometuron,
prometryne, Sencor and 2,4-D by soils. Univ. Puerto Rico, J.
Agric., 57: 286-293.
LOKKE, H. (1975) Analysis of free and bound chlorophenoxy-acids
in cereals. Bull. environ. Contam. Toxicol., 13: 730-736.
LOKTIONOV, V.N., BUDARKOV, V.A., KUZNETSOV, A.E., & KUZNETSOVA,
N.V. (1973) [Toxicological characteristics of 2,4-D derivatives.]
Veterinariya, 5: 107-109 (in Russian, Health & Welfare Canada
Translation No. 2173).
LONDONO, F. (1966) [Occupational acne. Five cases caused by
herbicide.] Med. cutanea, 3: 225-232 (in Spanish).
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMANN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. J. Am. Ind. Hyg.
Assoc., 20(3): 298-304.
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel
Dekker, pp. 1-49.
LOOS, M.A. (1975) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Herbicides: Chemistry, degradation and mode of
action, New York, Marcel Dekker, pp. 1-128.
LOPEZ, M.R. (1961) [Action of 2,4-dichlorophenoxyacetic acid and
its sodium salt on the spermatozoa of the frog.] Biol. R. Soc.
Esp. Hist. Nat., 59: 219-226 (in Spanish).
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960a) Metabolism of plant
growth-regulators. I. 2,4-dichlorophenoxyacetic acid in leaves of
red- and blackcurrant. Ann. appl. Biol., 48: 613-625.
LUCKWILL, L.C. & LLOYD-JONES, C.P. (1960b) Metabolism of plant
growth-regulators. II. Decarboxylation of 2,4-dichloro-
phenoxyacetic acid in leaves of apple and strawberry. Ann. appl.
Biol., 48: 626-636.
LUKOSHKINA, L.P., GUESEINOVA, R.S., & MELIK-ZADE, T.M. (1970)
Study of certain aspects of lipid-protein-carbohydrate metabolism
in workers engaged in 2,4-D production. Trud. Azerbaid. Nauchno-
Issled. Instituta Gig. prof. Zabol., No.5: 146-149.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de l'herbicide
2,4-D sur le développement embryonaire et la fécondité du gibier ą
plumes. C.R. Acad. Sci. Paris, Ser. D., 271(25): 2418-2421.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1974) Sexualité et les pesticides.
Ann. Biol. anim. (Paris), 13(3-4): 173-185.
MAAS, W. & KERSSEN, M.C. (1973) Drift studies in ULV and
conventional applications. Agric. Aviation, 15: 41-50.
MACKENTHUN, K.M. & KEUP L.E. (1972) Freshwater macroinvertebrates.
J. Water Pollut. Control Fed., 44(6): 1137-1150.
MACLEAN, G.J. & DAVIDSON, J.H. (1970) Diagnosis of disorders in
pastured livestock. Down Earth, 25(4): 12-16.
MAGNUSSON, J., RAMEL, C., & ERIKSSON, A. (1977) Mutagenic effects
of chlorinated phenoxyacetic acids in Drosophila melanogaster.
Hereditas, 85: 121-123.
MAIER-BODE, H. (1971) [Herbicides and their residues.], Stuttgart,
FRG, Verlag Eugen Ulmer, 479 pp. (in German).
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VII). Pesticide Mont. J., 8(2): 110-124.
MANSKE, D.D. & CORNELIUSSEN, P.E. (1974) Pesticide residues in
total diet samples (VIII). Pesticide Mont. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples VII. Pestic. Monit. J., 9(2): 94-105.
MARTH, P.C. & MITCHELL, J.W. (1949) Comparative volatility of
various forms of 2,4-D. Bot. Gaz., 110: 632-636.
MARTYNOV, Y.E. (1970) The effect of 2,4-d compounds on wild warm
blooded animals. Lesn. Khoz., 6: 57-59 (in Russian, Health &
Welfare Canada Translation No. 2186).
MASON, R.W. (1975) Binding of some phenoxyalkanoic acids to
bovine serum albumin in vitro. Pharmacology, 13: 177-186.
MATSUNAKA, S. (1972) Metabolism of pesticides in higher plants.
In: Matsumura, F., Boush, G.M., & Mitsato, T., ed. Environmental
toxicology of pesticides, New York, Academic Press, pp. 341-365.
MAYBANK, J. & YOSHIDA, K. (1969) Delineation of herbicide-drift
hazards on the Canadian prairies. Trans. Am. Soc. Agric. Eng.,
12(6): 759-762.
MAYBANK, J., YOSHIDA, K., & GROVER, R. (1978) Spray drift from
agricultural pesticide applications. J. Air Poll. Control Assoc.,
28: 1009-1014.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979a) Changes of metabolism
during experimentally induced myotonia of rats. I. Alterations in
lactate and malate dehydrogenase isoenzyme activities. Biochem.
Med., 22: 350-358.
MAZAREAN, H.H., DUX, L., & GUBA, F. (1979b) Changes of metabolism
during experimentally induced myotonia of rats. II. Alterations in
creatinine kinase and acid phosphatase activities: a possible
mechanism in the development of muscle damage. Biochem. Med.,
22: 359-364.
MCARDLE, F.J., MARETZKI, A.N., WILEY, R.C., & MODREY, M.G. (1961)
Influence of herbicides on flavor of processed fruits and
vegetables. J. agric. food Chem., 9: 228-230.
MCCONNELL, E.E., MOORE, J.A., HASEMAN, J.K., & HARRIS, M.W. (1978)
The comparative toxicity of chlorinated dibenzo- p-dioxins in mice
and guniea pigs. Toxicol. appl. Pharmacol., 44: 335-356.
MCLENNAN, M.W. (1974) 2,4-D toxicity in diary cattle. Aust.
vet. J., 50: 578.
MEEHAN, W.R., NORRIS, L.R., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in Southeast Alaska.
J. Fish. Res. Board Can., 31(4): 480-485.
MELNIKOV, M.N. (1971) Chemistry of pesticides. Res. Rev.,
36: 165-166.
MIERZWA, S. & WITEK, S. (1977) Gas-liquid chromatographic
method with electroncapture detection for the determination of
residues of some phenoxyacetic acid herbicides in water as their
2,2,2-trichloroethyl esters. J. Chromatogr., 136: 105-111.
MILHAUD, G., RIET ALVARIZA, F., CHARLES, E., & SABBACH, M. (1970)
Toxicologie des phytohormones. Rec. Méd. Vet., 146: 345-357.
MITCHELL, J.W., HODGSON, R.E., & GAETJENS, C.F. (1946) Tolerance
of farm animals to feed containing 2,4-dichloro-phenoxyacetic acid.
J. anim. Sci., 5(1): 226-232.
MONARCA, G. & DIVITO, G. (1961) [On acute poisoning by a herbicide
(2,4-dichlorophenoxyacetic acid).] Folia med. (Naples), 44(6):
480-485 (in Italian, Health & Welfare Canada Translation No. 2234).
MONTGOMERY, M.L., CHANG, U.L., & FREED, V.H. (1971) Comparative
metabolism of 2,4-D by bean and corn plants. J. agric. food Chem.,
19: 1219-1221.
MOREALE, A. & VAN BLADEL, R. (1980) Behaviour of 2,4-D in Belgian
soils. J. environ. Qual., 9: 627-633.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
microbial reversion assay systems. Mutat. Res., 116: 185-216.
MORRE, D.J. & ROGERS, B.J. (1960) The fate of long chain esters
of 2,4-D in plants. Weeds, 8: 436-447.
MORTON, H.L., ROBINSON, E.D., & MEYER, R.T. (1967) Persistence of
2,4-D, 2,4,5-T, and dicamba in range forage grasses. Weeds,
15: 268-271.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxy-
ethane ester of 2,4-D. Trans. Am. Fish. Soc., 96(2): 185-193.
MUIR, C.S. & WAGNER, G. (1982) Directory of on-going research in
cancer epidemiology 1982, Lyons, International Agency for Research
on Cancer, (IARC Scientific publications No. 46) pp. 86 (Abstract
No. 227), 432(1146) 460(1221).
MUKULA, J., SILTANEN, H., ROSENBERG, C., IANPAAN-HEIKKILA, J., &
LALLUKKA, R. (1978) Phenoxy herbicide residues in cowberries,
mushrooms and the twigs of forest plants. Ann. Agric. Fenn.,
17: 23-31.
MUNRO, H.E. (1972) Determination of 2,4-dichloro-phenoxyacetic
acid and 2,4,5-trichlorophenoxyacetic acid in tomato plants and
other commercial crops by microcoulometric gas chromatography.
Pestic. Sci., 3: 371-377.
MURTHY, M.S.S. (1979) Induction of gene conversion in diploid
yeast by chemicals: Correlation with mutagenic action and its
relevance in genotoxicity screening. Mutat. Res., 64: 1-17.
NASH, R.G., KEARNEY, P.C., MAITLEN, J.C., SELL, C.R., & FERTIG,
S.N. (1982) Agricultural applicators' exposure to 2,4-
dichlorophenoxyacetic acid. In: Plimmer, J.R., ed. Pesticide
residues and exposure, Washington, DC, American Chemical Society,
pp. 119-132 (ACS Symposium Series 182).
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1981)
Polychlorinated dibenzo- p-dioxins: Criteria for their effects on
man and his environment, Ottawa, NRCC Publications Service, NRCC
Publication No. 18574, 251 pp.
NATIONAL RESEARCH COUNCIL OF CANADA ASSOCIATE COMMITTEE ON
SCIENTIFIC CRITERIA FOR ENVIRONMENTAL QUALITY (1978) Phenoxy
herbicides. Their effects on environmental quality, Ottawa, NRCC
Publications Service, NRCC publication no. 16075, 437 pp.
NAYRAC, P., FONTAN, P., WAROT, P., LESCUT, J., & DELAHOUSSE, J.
(1958) Polyradiculonévrite subaciguė chez deux agriculteurs:
intoxication par des produits anti-parasitaires. Lille Med.,
3(3): 161-164.
NESBITT, H.J. & WATSON, J.R. (1980a) Degradation of the
herbicide 2,4-D in river water. I. Description of the study
area, and survey of rate determining factors. Water Res.,
14(12): 1683-1688.
NESBITT, H.J. & WATSON, J.R. (1980b) Degradation of the
herbicide 2,4-D in river water. II. The role of suspended
sediment, nutrients, and water temperature. Water Res.,
14(12): 1689-1694.
NIELSEN, N.O. (1968) Teratogenic effects of hyperthermia in
chicken embryo. Preliminary report. Teratology, 1: 102-107.
NIELSEN, K., KAEMPE, B., & JENSEN-HOLM, J. (1965) Fatal
poisoning in man by 2,4-D: Determination of the agent in
forensic materials. Acta pharmacol. toxicol., 22: 224-234.
NIKANDROV, V.N. (1974). [The activity of malate dehydrogenase in
some organs and tissues of albino rats after administration of the
amine salt of 2,4-D,] Vesti Akad. Navuk Belaruskai SSR (Ser.
Biol.) 1: 126-127 (in Belorussian).
NISHIUCHI, Y. & YOSHIDA, K. (1974) [Toxicity of new agricultural
chemicals to tadpoles.] Bull. agric. Chem. Insp. St., 14: 66-68
(in Japanese).
NORSTROM, A., RAPPE, C., LINDAHL, R., & BUSTER, H.R. (1979)
Analysis of some older Scandinavian formulations of 2,4-D and
2,4,5-T for contents of chlorinated dibenzo- p-dioxins and
dibenzofurans. Scand. J. Work Environ. Health, 5: 375-378.
OLSON, B.A., SNEATH, T.C., & JAIN, N.C. (1978) Rapid, simple
procedures for the simultaneous gas chromatographic analysis
of four chlorophenoxy herbicides in water and soil samples.
J. agric. food Chem., 26: 640-643.
ORBERG, J. (1980a) Observations on the 2,4-dichlorophenoxy-
acetic acid (2,4-D) excretion in the goat. Acta pharmacol.
toxicol., 46: 78-80.
ORBERG, J. (1980b) Effects of low protein consumption on the
renal clearance of 2,4-dichlorophenoxyacetic acid (2,4-D) in goats.
Acta pharmacol. toxicol., 46: 138-140.
OSADCHUK, M., SALAHUB, E., & ROBINSON, P. (1977) Isolation of
chlorophenoxy herbicides on columns of glass beads. J. Assoc. Off.
Anal. Chem., 60: 1327-1327.
OSTERLOH, J., LOTTI, M., & POND, S.M. (1983) Toxicologic studies
in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. anal.
Toxicol., 7: 125-129.
OU, L.T., ROTHWELL, D.F., WHEELER, W.B., & DAVIDSON, J.M. (1978)
The effect of high 2,4-D concentrations on degradation and carbon
dioxide evolution in soils. J. environmental Qual., 7(2): 241-246.
PADEROVA, V.P. (1975) [Some hygienic problems of using 2,4-D
herbicides.] Gig. i Sanit., 40(9): 96-97 (in Russian).
PAGGIARO, P.L., MARTINO, E., & MARIOTTI, S. (1974) [On a case of
poisoning by 2,4-dichlorophenoxyacetic acid.] Med. lav., 65(3-4):
128-135 (in Italian, Health & Welfare Canada Translation No. 2200).
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens. In: Production Research Report, Washington,
DC, Agricultural Research Service, pp. 1-38.
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some
organic herbicides to cattle, sheep, and chickens. In: Production
Research Report, Washington, DC, Agriculture Research Service,
pp. 1-26.
PAL'MOVA, I.V. & GALUZOVA, L.V. (1963) [Maximum permissible
concentration of sodium salt and butyl ester formulations of
2,4-D in water.] Gig. i Sanit., 28(7): 11-13 (in Russian,
Health & Welfare Canada Translation No. 2717).
PEMBERTON, J.M. (1979) Pesticide degrading plasmids: a
biological answer to environmental pollution by phenoxyherbicides.
Ambio, 8: 202-205.
PHILLEO, W.W. & FANG, S.C. (1967) Effects of 2,4-D on metabolism.
J. agric. food Chem., 15(2): 256-260.
PIERIDES, A.M., ALVAREZ-UDE, F., KERR, D.N.S., & SKILLEN, A.W.
(1975) Clofibrate-induced muscle damage in patients with chronic
renal failure. Lancet, 2: 1279-1282.
PILINSKAJA, M.A. (1974) [Cytogenetic effect of the herbicide
2,4-D on human and animal chromosomes.] Tsitol. Genet., 8(3):
202-206 (in Russian).
POCCHIARI, F., SILANO, V., & ZAMPIER, A. (1979) Human health
effects from accidental release of tetrachlorodibenzo- p-dioxin
(TCDD) at Seveso, Italy. Ann. NY Acad. Sci., 320: 311-320.
PODOLAK, M. (1979) [Effect of paraquat and 2,4-dichloro-
phenoxyacetic acid (2,4-D) on oxidative phosphorylation and
respiration of brain mitochondria in the rat.] Bromatol. Chem.
Toksykol., 12(2): 147-152 (In Polish).
POLAND, A. & GLOVER, E. (1973) 2,3,7,8-Tetrachlorodibenzo- p-
dioxin: a potent inducer of S-aminolevulinic acid synthetase.
Sci., 179: 476.
POLAND, A. & GLOVER, E. (1973) Chlorinated dibenzo- p-dioxins:
potent inducers of -aminolevulinic acid synthetase and aryl
hydrocarbon hydroxylase. Molec. Pharmacol., 9: 736-747.
POLAND, A. GLOVER, E. (1976) Sterospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo -p-dioxin by hepatic cytosol.
J. Biol. Chem., 251(16): 4936-4946.
POLAND, A. & KENDE, A. (1976) 2,3,7,8-tetra-chlorodibenzo- p-
dioxin environmental contaminant and molecular probe. Fed. Proc.,
35: 2404-2411.
POLAND, A., SMITH, D., METTER, G., & POSSICK, P. (1971) A health
survey of workers in a 2,4-D and 2,4,5-T plant. Arch. environ.
Health, 22: 316-327.
PRAVDA, O. (1973) [On the influence of herbicides on some fresh-
water animals.] Hydrobiologia, 42(1): 97-142 (in German).
PREISS, D. & ROSSNER, J.A. (1971) [Effect of 2,4-
dichlorophenoxyacetic on the transverse tubular system of the
mycardium.] Naturwissenschaften, 11: 576-577 (in German).
PRESCOTT, I.F., PARK, J., & DARRIEN, I. (1979) Treatment of
severe 2,4-D and mecoprop intoxication with alkaline diuresis.
Br. J. clin. Pharmacol., 7: 111-116.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder.
J. Pharmacol. exp. Therap., 208(2): 280-286.
PRITCHARD, J.B. & MILLER, D.S. (1980) Teleost kidney in
evaluation of xenobiotic toxicity and elimination. Fed. Proc.,
39(14): 3207-3212.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis
in primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PROVINCE OF BRITISH COLUMBIA, MINISTRY OF ENVIRONMENT (1981)
A review of the use of 2,4-D for Eurasian water milfoil control in
the Okanagan Lakes, 1976-1980. Inf. Bull., aquatic Plant Manage.
Program, 10: 1-10.
QUE HEE, S.S. & SUTHERLAND, R.G. (1974) Purity of reagent grade
p- and o-chlorophenoxyacetic acids and its biological implications.
J. agric. food Chem., 22: 726-727.
QUE HEE, S.S. & SUTHERLAND, R.G. (1975) A specific gas-liquid
chromatographic method for analysis of some amine salts of 2,4-
dichlorophenoxyacetic acid. J. agric. food hem., 23: 1007-1008.
QUE HEE, S.S. & SUTHERLAND, R.G. (1981) The phenoxyalkanoic
herbicides, Vol. I. Chemistry, analysis and environmental
pollution, Boca Raton, CRC Press, Inc., 321 pp.
RADIONOV, A.D., CHUMACHENKO, A.N., & KIRILENKO, I.I. (1967)
The toxic properties of the herbicide 2,4-D. Hyg. Sanit.,
32(4-6): 116-118.
RAMEL, C. (1978) Chlorinated phenoxy acids and their dioxins.
Mode of action, health risks, and environmental effects. Ecol.
Bull. (Stockholm), 27: 302.
RAOUL, Y. & MARNAY, O. (1948) [Effect of 3-indolacetic acid
2,4-dichlorophenoxyacetic acid on the growing rat.] C. R. Acad.
Sci. (Paris), 226: 1043-1045.
RAPPE, C. & BUSER, H.R. (1981) Occupational exposure to
polychlorinated dioxins and dibenzofurans. In: Choudhary, G.,
Chemical hazards in the workplace: Measurement and control,
Washington, DC, American Chemical Society, pp. 319-342 (ACS
Symposium Series No. 149).
RASMUSON, B. & SVAHLIN, H. (1978) Mutagenicity tests of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid in genetically stable and unstable strains of Drosophila
melanogaster. Ecol. Bull. (Stockholm), 27: 190-192.
RAWLS, C.K. (1965) Field tests of herbicide toxicity to
certain estuarine animals. Chesapeake Sci., 6(3): 150-161.
REA, W.J. (1978) Environmentally triggered cardiac disease.
Ann. Allergy, 40(4): 243-251.
REDDY, J.K., AZARNOFF, D.L., & HIGNITE, E.E. (1980)
Hypolipidaemic hepatic peroxisome proliferators form a novel
class of chemical carcinogens. Nature (Lond.), 283: 397-398.
REDDY, J.K., QURESHI, S.A., LALWANI, N.D., REDDY, M.K., & MOEHLE,
C.M. (1982a) Induction by ciprofibrate of hepatic peroxisome
proliferation in rats, pigeons, chickens, cats and rhesus monkeys.
Fed. Proc., 41: 1741.
REDDY, J.K., LALWANI, N.D., REDDY, M.K., & QURESHI, S.A. (1982b)
Excessive accumulation of autofluorescent lipofuscin in the liver
during hepatocarcinogenesis by methyl clofenapate and other
hypolipidaemic peroxisome proliferators. Cancer Res., 42: 259-266.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977) Investigations
into the acute toxicity and some chronic effects of selected
herbicides and pesticides on several fresh water fish species.
Bull. environ. Contam. Toxicol., 18(3): 361-365.
RENBERG, L. (1974) Ion exchange technique for the determination
of chlorinated phenols and phenoxy acids in organic tissue, soil
and water. Anal. Chem., 46: 459-461.
RIIHIMAKI, V., ASP, S., & HERNBERG, S. (1982) Mortality of
chlorinated phenoxyacid herbicide 2,4-D and 2,4,5-T applicators in
Finland. First report of an ongoing prospective follow-up study.
Scand. J. Work Environ. Health, 8: 37-42.
RIVERS, J.B., YAUGER, W.L., Jr., & KLEMMER, H.W. (1970)
Simultaneous gas chromatographic determination of 2,4-D and
dicamba in human blood and urine. J. Chromatogr., 50: 334-337.
ROBINSON, E. & FOX, L.L. (1978) 2,4-D herbicides in central
Washington. J. Air Poll. Control Assoc., 28: 1015-1020.
ROBSON, T.O. (1966) Some studies of the persistence of 2,4-D in
natural surface waters in Britain. Proc. Br. Weed Control Conf.,
2: 594-597.
ROSENBERG, J. (1980) 2,4-Dichlorophenoxyacetic acid (2,4-D)
- Evaluation of human health hazards, Berkeley, California,
Hazard Alert System, Epidemiological Studies Laboratory, 64 pp.
ROSS, R.D. MORRISON, J., ROUNBEHLER, D.P., FAN, S., & FINE, D.H.
(1977) N-nitroso compound impurities in herbicide formulations.
J. agric. food Chem., 25: 1416-1418.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation
of the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUDIGER, K.D., MEERBACH, W., OSSKE, G., & FISCHER, W. (1972) [On
the experimental 2,4-dichlorophenoxyacetate myopathy.] Zentralbl.
allg. Pathol., 115: 145-152 (in German).
SADYKOV, R.E., RABOCHEV, V.K., & STROKOV, Y.N. (1972) [The
effect of butyl 2,4-D treatment of pastures on the reproductive
functions of sheep.] Zhivodnovodstvo, 34(1): 73-74 (in Russian).
SANDERS, H.O. (1970a) Pesticide toxicities to tadpoles of the
western chorus frog, Pseudacris triseriata and Fowler's toad, Burfo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. (1970b) Toxicities of some herbicides to six
species of freshwater crustaceans. J. Water Pollut. Control Fed.,
42(8): 1554-1550.
SARE, W.M. (1972) The weedicide 2,4-D as a cause of headaches and
diplopia. New Z. med. J., 75(478): 173-174.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & LEBEAU, J.E. (1976)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicol. appl. Pharmacol., 37(1): 136-137.
SAUERHOFF, M.W., BRAUN, W.H., BLAU, G.E., & GEHRING, P.J. (1977)
The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral
administration to man. Toxicology, 8: 3-11.
SAXEN, L., KLEMETTI, A., & HARO, A.S. (1974) A matched-pair
register for studies of selected congenital defects. Am. J.
Epidemiol., 100(3): 297-306.
SCHACTER, B., GYVES, M., MUIR, A., & TASIN, M. (1979) HLA-A, B
compatibility in parents of offspring with neural-tube defects or
couples experiencing involuntary fetal wastage. Lancet, 1: 796-797.
SCHILLER, K. (1964) [Effects on the fertility of rats produced by
substances with special effects in potato cultivation. 1st
communication: Growth hormones and weed control agents 2,4-D and
MCPA.] Landbauforsch. Völkenrode, 14(2): 111-114 (in German).
SCHILLINGER, J.E. (1960) [Hygienic evaluation of agricultural
products cultivated with the use of herbicides.] J. Hyg.
Epidemiol. Microbiol. Immunol., 4: 243-252 (in German).
SCHNEIDER, B.A. (1979) Toxicological evaluation of pesticides by
the EPA Chemical and Biological Investigations Branch Laboratories
(1966-77). In: Toxicology handbook, mammalian and aquatic data.
Book 1: Toxicology data. Report for 1966-67, Beltsville, Md., US,
Environmental Protection Agency.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8(3): 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues at
Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7(3/4):
146-152.
SCHUPHAN, W. (1963) [Impaired health due to plant protection
agents.] Der Landarzt, 39: 1217-1222 (in German).
SCHUPHAN, W. (1965) [Plant protection problems reflected in
quality testing.] Anz. Schädlingsk. D. Pflanzenschutz.
Umweltschutz, 38(7): 97-104 (in German).
SCHUPHAN, W. (1969) [Pesticides: Usefulness and possible harm.]
Zentralbl. Bakteriol., 210: 240-258 (in German).
SCHWETZ, B.A., SPARSCHU, G.L., & GEHRING, P.J. (1971) The effect
of 2,4-D and esters of 2,4-D on rat embryonal, foetal and neonatal
growth and development. Food cosmet. Toxicol., 9: 801-817.
SCHWETZ, B.A., NORRIS, J.M., SPARSHU, G.L., ROWE, V.K., GEHRING,
P.J., EMERSON, J.L., & GERBIG, C.G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect., Exp.
Issue 5: 87-99.
SEABURY, J.H. (1963) Toxicity of 2,4-dichlorophenoxyacetic acid
for man and dog. Arch. environ. Health, 7: 202-209.
SEILER, D. (1971) The ATPase of the sarcolemma of skeletal
muscle in experimental myotonia. Experientia (Basel), 27:
1170-1171.
SEILER, J.P. (1978) The genetic toxicology of phenoxy acids
other than 2,4,5-T. Mutat. Res., 55: 197-226.
SENCZUK, W. & POGORZELSKA, H. (1975) [Urinary excretion of
2,4-dichlorophenoxyacetic acid.] Rocz. Panstw. Zakl. Hig.,
26(2): 217-222 (in Polish, Health & Welfare Canada Translation
No. 2451).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxodynamic properties of phenoxycarboxylic acid derivatives, II.
Methods of determining derivatives of phenoxyacetic and
phenoxypropionic acids in blood and urine.] Rocz. Panstw. Zakl.
Hig., 32: 339-344 (in Polish).
SENCZUK, W. & POGORZELSKA, H. (1981) [Chemical structure and
toxicodynamic properties of derivatives of phenoxyacetic and
phenoxypropionic acid, III. The pattern of absorption into the
bloodstream and measurements of urinary excretion of phenoxyacetic
and phenoxypropionic acid.] Roczn. Panstw. Zak. Hig., 32(5-6):
419-426 (in Polish).
SENCZUK, W., POGORZELSKA, H., & DEBSKA, M. (1980) [Absorption of
2,4-dichlorophenoxyacetic acid through the skin.] Roczn. Panstw.
Zak. Hig., 31(6): 611-614 (in Polish).
SENGES, J. & RDEL, R. (1972) Experimental myotonia in mammalian
skeletal muscle: changes in contractile properties. Pflueger.
Arch., 331: 315-323.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method for
determination of low levels of exposure to 2,4-D and 2,4,5-T.
Int. J. environ. anal. Chem., 1: 23-33.
SHEARER, R.W. (1980) Public health effects of the aquatic use of
herbicides: 2,4-D dichlobenil, endothall and diquat. In: Shearer,
R.W. & Halter, M., ed. Literature reviews of four selected
herbicides: 2,4-D dichlorbenil, diquat & endothall, Municipality of
Washington, US, Metropolitan Seattle, pp. 1-76.
SHEARER, R. & HALTER, M. (1980) Literature reviews of four
selected herbicides: 2,4-D, dichlobenil, diquat, and endothall.
Municipality of Washington, US, Metropolitan Seattle.
SHILLINGER, U.Y.I. & NAUMOVA, L.P. (1957) [Hygienic evaluation of
a harvest in connection with the treatment of crops with the
herbicide 2,4-D.] Gig. i Sanit., 7: 33-38 (in Russian).
SHIM, J.C. & SELF, L.S. (1973). Toxicity of agricultural
chemicals to larvivorous fish in Korean rice fields. Trop. Med.,
15(3): 123-130.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., & RAATIKAINEN, M.
(1981). Triclopyr, glyphosate and phenoxyherbicide residues in
cowberries, bilberries and lichen. Bull. Environ. Contam Toxicol.,
27: 731-737.
SIMPSON, G.R. (1982a) Pesticide exposure - New South Wales
Services. In: Education and safe handling in pesticide application,
studies in environmental science, 18: 209-214. Amsterdam,
Oxford, New York, Elsevier Scientific Publishing Co.
SITTIG, M. ed., (1980) Pesticide manufacturing and toxic
materials control encyclopedia. Park Ridge, NJ, USA, Noyes Data
Corp., pp. 229-234.
SKELLY, N.E., STEVENS, T.S., & MAPES, D.A. (1977) Isomer-specific
assay of ester and salt formulations of 2,4-dichlorophenoxyacetic
acid by automated high pressure liquid chromatography. J. Assoc.
Off. Anal. Chem., 60: 868-872.
SMALS, A.G.H., BEEX, L.A.V.M., & KLOPPENBORG, P.W.C. (1977)
Clofibrate-induced muscle damage with myoglobinuria and
cardiomyopathy. N. Engl. J. Med., 296: 942.
SMITH, A.E. (1972) The hydrolysis of 2,4-dichlorophenoxy-acetate
esters to 2,4-dichlorophenoxyacetic acid in Saskatchewan soils.
Weed Res., 12: 364-372.
SMITH, A.E. (1976a) The hydrolysis of herbicidal phenoxy-alkanoic
esters to phenoxyalkanoic acids in Saskatchewan soils. Weed Res.,
16: 19-22.
SMITH, A.E. (1976b) Method for the determination of
2,4-dichlorophenoxyacetic acid in urine. J. Chromatogr, 171:
482-485.
SMITH, A.H., FISHER, D.O., PEARCE, N., & CHAPMAN, C.J. (1982)
Congenital defects and miscarriages among New Zealand 2,4,5-T
sprayers. Arch. environ. Health, 37(4): 197-200.
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of
large-scale applications of 2,4-D on aquatic fauna and water
quality. Pestic. monitoring J., 1(3): 16-21.
SOMERS, J., MORAN, E.T., REINHART, B.S., & STEPHENSON, G.R. (1974a)
Effect of external application of pesticides to the fertile egg on
hatching success and early chick performance. 1. Pre-incubation
spraying with DDT and commercial mixtures of 2,4-D: picloram, and
2,4-D: 2,4,5-T. Bull. environ. Contam. Toxicol., 11(1) 33-38.
SOMERS, J., MORAN, E.T., & REINHART, B.S. (1974b) Effect of
external application of pesticides to the fertile egg on
hatching success and early chick performance. 2. Commercial
herbicide mixtures of 2,4-D with pioloram or 2,4,5-T using the
pheasant. Bull. environ. Contam. Toxicol., 11(4): 339-342.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974c) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11(4): 511-516.
SOS, J. & KERTAI, P. (1958) Effect of dichlorophenoxyacetic acid
upon the I131-uptake of the thyroid. Acta physiol. Acad. Sci.
Hung., 14: 367-369.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on
the embryonic development of Phasianus colchicus (L.) and Coturni
coturnix japonica (L.).] Z. Jagdwiss., 22: 197-201 (in German).
STANLEY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANOSZ, S. (1969) [The effects of sodium salt of 2,4-
dichlorophenoxy-acetic acid on cell respiration in the parenchymal
organs of rats.] Med. Prac., 20(6): 600-605 (in Polish).
STEIN, W. & KUHN, E. (1968) [Model myotonia after 2,4-
dichlorophenoxyacetate (2,4-D). Isolated rat diaphragm preparation
as simple experimental object.] Klin. Wochenschr., 46: 328-330
(in German).
STEVENS, L. J., COLLIER, C. W., & WOODHAM, D. W. (1970)
Monitoring pesticides in soils from areas of regular, limited, and
no pesticide use. Pestic. Monit. J., 4(3): 145-164.
STEVENS, T.S.A., SKELLY, N.E., & GRORUD, R.B. (1978) Isomer-
specific assay of ester and salt formulations of 2,4-
dichlorophenoxyacetic acid by high pressure liquid chromatography:
Collaborative study. J. Assoc. Off. Anal. Chem., 61: 1163-1165.
STEWART, D.K.R. & GAUL, S.O. (1977) Persistence of 2,4-D,
2,4,5-T, and dicamba in a dykeland soil. Bull. environ. Contam.
Toxicol., 18(2): 210-218.
STRACH, S. & BOHOSIEWICZ, M. (1964) [Studies of the toxicity for
pigs of the weed-killer "Pielik."] Med. weter, 20(11): 662-666
(in Polish, Health & Welfare Canada Translation No. 2467).
STUPNIKOV, A.A. (1959) In: [Collected works of the Leningrad
Veterinary Sciences Research Institute.] No. 8: 289 (in Russian).
SUDAK, F.N. & CLAFF, C.L. (1960) Survival of Uca pugnax in sand,
water, and vegetation contaminated with 2,4-dichloro-phenoxyacetic
acid. Proc. Northeast. Weed Control Conf., 14: 508-510.
SUDAK, F.N., CLAFF, C.L., & CANTOR, M. (1966) Body temperature
regulation in rats treated with 2,4-dichloro-phenoxyacetic acid.
Arch. int. Pharmacodyn. Ther., 160(2): 253-264.
SUFFET, I.H. (1973a) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 2.
Selection of water: solvent ratios and number of extractions.
J. agric. food Chem., 21: 288-294.
SUFFET, I.H. (1973b) The p-value approach to quantitative liquid-
liquid extraction of pesticides and herbicides from water. 3.
Liquid-liquid extraction of phenoxy acid herbicides from water.
J. agric. food Chem., 21: 591-598.
SUNDSTROM, G., JENSEN, S., JANSSON, B., & ERNE, K. (1979)
Chlorinated phenoxyacetic acid derivatives and tetrachlorodibenzo- p-
dioxin in foliage after application of 2,4,5-trichlorophenoxyacetic
acid esters. Arch. environ. Contam. Toxicol., 8: 441-448.
SZOCS, J., MOLNAR, V., BALOGH, E., AJTAY, M., & FULOP, I. (1970)
[Experimental data on the toxic effects of 2,4-D (Diclordon)
herbicide.] Rev. Med. (Tirgu-Mures, Rom.), 16: 91-93 (in
Hungarian).
THIELE, E., BAUFELD, W., GRIMM, U., LEHNIGK, B., & THIELE, W.
(1981a) [Results of analysis for deriving exposure time limits in
the industrial hygiene monitoring of people working at agrochemical
centres who are exposed to pesticides.] Z. gesamte Hyg. Grenzgeb.,
27(3): 173-177 (in German).
THIELE, E., FIEBER, C., JASTROCH, S., KNOLL, W., & LANGE, B.
(1981b) [Determination of toxic concentrations at places of work
and patterns of distribution of pesticide exposure of people
working at agrochemical centres subdivided by substance groups.]
Z. gesamte Hyg. Grenzgeb., 27(3): 177-180 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & LINK, R. (1982) Mortality
study of persons exposed to dioxin in a trichlorophenol-process
accident that occurred in the BASF AG on November 17, 1953.
Am. J.ind. Med., 3: 179-189.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964a) Metabolic
fate of some chlorinated phenoxyacetic acids in the stem tissue of
Avena sativa. Nature (Lond.), 204: 286.
THOMAS, F.W., LOUGHMAN, B.C., & POWELL, R.G. (1964b) Metabolic
fate of 2,4-dichlorophenoxyacetic acid in the stem tissue of
Phaseolus vulgaris. Nature (Lond.), 204: 884-885.
THOMSSEN, C. (1958) [Contribution to the question of possible
poisoning of domestic animals through growth hormone-based weed
control agents.] Arch. exp. Veterinaermed., 12: 216-221 (in
German).
TODD, R.L. (1962) A case of 2,4-D intoxication. J. Iowa Med.
Soc., 52: 663-664.
TSAPKO, V.P. (1966) The herbicide 2,4-D as a health hazard in
agriculture. Hyg. Sanit., 9: 449-450.
TSILIKOV, V.V. (1969) [Effect of the sodium salt of 2,4-D,
trichlorometaphos, nicotine, and arecoline on thyroid function.]
In: Denisenko, P.P., ed. Farmakol. Tsent. Kholinolitikov Drugikh
Neirootropnykh Sredstv., pp. 181-185 (in Russian, Health & Welfare
Canada Translation No. 2447).
TUINSTRA, L.G.M., ROOS, A.H., & BRONSGEEST, J.M. (1976)
Suggestions for a sensitive multi-detection high-performance
liquid chromatography method for several phenoxy-acid herbicides.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 41: 1443-1448.
TULP, M.T.M. & HUTZINGER, O. (1978) Rat metabolism of
polychlorinated dibenzo- p-dioxins. Chemosphere, 7(9): 761-768.
UNGER, T.M., KLIETHERMES, J., VAN GOEHAM, D., & SHORT, R.D.
(1980) Teratology and postnatal studies in rats on propylene
glycol butyl ether and isooctyl esters of 2,4-dichloro-
phenoxyacetic acid, Washington, US Environmental Protection
Agency (60/12-80).
US EPA (1976) National interim primary drinking water
regulations, Washington, DC, US Environmental Protection Agency.
US EPA (1980a) Environmental Protection Agency Review and
Conclusions Concerning Potential Health Effects of the Herbicide
2,4-D, April 1980, Chem. Regul. Rep., 4(5): 134-136.
US EPA (1982) Tetrachlorodibenzo- p-dioxin; re-evaluation of
disposal prohibition. Fed. Reg., 47(2): 103.
US NATIONAL CANCER INSTITUTE (1979) Bioassay of 2,7-
dichlorodibenzo-p-dioxin (DCDD) for possible carcinogenicity.
Springfield, Virginia, National Technical Information Service
Document PB 290570 (Report No. NCI-CG-TR 123).
US VETERANS' ADMINISTRATION. DEPARTMENT OF MEDICINE AND SURGERY
(1981) Review of literature on herbicide, including phenoxy
herbicides and associated dioxins. Volume I. Analysis of
literature. Volume II. Annotated bibliography, Washington, DC,
US Veterans' Administration, 397 pp.
VACHKOVA-PETROVA, R. (1978) [Mutagenic activity of pesticides.]
Hig. Zdraveopaz., 21(5): 496-506 (in Bulgarian).
VAINIO, H., NICKELS, J., & LINNAINMAA, K. (1982) Phenoxy acid
herbicides cause peroxisome proliferation in Chinese hamsters.
Scand. J. Work. Environ. Health, 8: 70-73.
VAINIO, H., LINNAINMAA, K., KAHONEN, M., NICKELS, J., HIETANEN, E.,
MARNIEMI, J., & PELTONEN, P. (1983) Hypolipidemia and peroxisome
proliferation induced by phenoxyacetic acid herbicides in rats.
Biochem. Pharmacol., (in press).
VAN DYK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air:
sampling methods. Res. Rev., 55: 91-134.
VAN PETECHEM, C.R. & HEYNDRICKX, A.M. (1975) Beta oxidation of
chlorophenoxybutyric acid herbicides in guinea pigs. Bull.
environ. Contam. Toxicol., 14(5): 632-640.
VARDIA, H.K. & DURVE, V.S. (1981) Bioassay study on some fresh
water fishes exposed to 2,4-dichlorophenoxyacetic acid. Acta
hydrochim. hydrobiol., 9(2): 219-223.
VENKAIAH, B. & PATWARDHAN, M.V. (1978) Inhibition of
mitochondrial energy transfer reactions by phenoxy acids. Indian
J. Biochem. Biophys., 15(3): 219-221.
VINOKUROVA, M.K. (1960) [On the toxicity of a herbicide: The
octyl ester of 2,4-dichlorophenoxyacetic acid.] Gig. i Sanit.,
12: 31-34 (in Russian, Health & Welfare Canada Translation No.
2324).
VOGEL, E. & CHANDLER, J.L.R. (1974) Mutagenicity testing of
cyclamate and some pesticides in Drosophila melanogaster.
Experientia (Basel), 30(6): 621-623.
WALLIS, W.E., VAN POZNAK, A., & PLUM, F. (1970) Generalized
muscular stiffness, fasciculations, and myokymia of peripheral
nerve origin. Arch. Neurol., 22: 430-439.
WATERS, M.D., MITCHELL, A.D., JORGENSON, T.A., & VALENCIA, R.
(1981) Pesticides: Mutagenic and carcinogenic potential.
In: Bandal, S.K., Marco, G.J., Golberg, L., & Leng, M.L.,
ed., The pesticide chemist and modern toxicology. Washington,
DC, American Chemical Society (ACS Symposium Series 160).
WAY, J.M. (1969) Toxicity of hazards to man, domestic
animals and wildlife from some commonly used auxin herbicides.
Res. Rev., 26: 37-62.
WEINMANN, W. (1957) [Investigations of the influence of a
treatment of tomato plants with growth substances on the
nutritional and physiological value of the fruits. Offspring
mortality in albino rats.] Qual. Plant. Mater. Veg., 2(4):
342-349 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972) The subacute
toxicity of 2,4-dichlorophenoxyacetic acid and 2,4,5-
trichlorophenoxyacetic acid to chicks. Toxicol. appl.
Pharmacol., 21:348.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., & GANGSTAD, E.O.
(1973) The effects of a 2,4-D application on the biota and water
quality in Currituck Sound, North Carolina. Hyacinth Control J.,
11: 13-17.
WHO (1976) Pesticide residues in food. Report of the 1975
Joint FAO/WHO meeting (WHO Tech. Report Series, 592).
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residue
Series No. 1).
WHO/FAO (1976) 1975 Evaluations of some pesticide residues in
food. Geneva, World Health Organisation (WHO Pesticide Residues
Series No. 5).
WHO/IARC (1980) Walker, E.A., Castegnaro, M., Griciute, L.,
Börzsönyi, M., & Davis, W., ed.: N-Nitroso compounds: Analysis,
formation and occurrence. Lyons, International Agency for
Research on Cancer, 841 pp (IARC Scientific Publications No. 31).
WHO/IARC (1982) Bartsch, H., Castegnaro, M., O'Neill, I.K.,
Okada, M., & Davis, W., ed.: N-Nitroso compounds: Occurrence
and biological effects. Lyons, International Agency for Research
on Cancer, 755 pp (IARC Scientific Publications No. 41).
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972) Pesticide residue
levels in soils, F.Y. 1969 - National soils monitoring program.
Pestic. monit. J., 6(3): 194-228.
WIERZBICKA, M. (1974a) Haemolymph concentration in Cyclopoida
copepodids during active and resting stage and the effect of
2,4-D sodium salt. Pol. Arch. Hydrobiol., 21(2): 269-273.
WIERZBICKA, M. (1974b) Influence of 2,4-D sodium salt on the
survival of some Copepodia species. Pol. Arch. Hydrobiol.,
21(2): 275-282.
WINKELMANN, R.K. (1960) Diagnosis and treatment of allergic
angiitis (anaphylactoid purpura). Postgrad. Med., 27: 437-444.
WOJTALIK, T.A., HALL, T.F., & HILL, L.O. (1971). Monitoring
ecological conditions associated with wide-scale applications
of DMA-2,4-D to aquatic environmental. Pestic. monit. J.,
4(4): 184-203.
WOODHAM, D.W., MITCHELL, W.G., LOFTIS, C.D., & COLLIER, C.W.
(1971) An improved gas chromatographic method for the analysis of
2,4-D free acid in soil. J. agric. food Chem., 19: 186-188.
YIP, G. (1975) Analysis for herbicides and metabolites. J.
Chromatogr. Sci., 13: 225-230.
YODER, J., WATSON, M., & BENSON, W. (1973) Lymphocyte chromosome
analysis of agriculture workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUNG, A.L., ARNOLD, E.L., & WACHINSKI, A.M. (1974) Field
studies in soil persistence and movement of 2,4-D, 2,4,5-T and
TCDD. Weed Sci. Soc. Am., (Abstract No. 226).
YOUNG, A.L., CALCAGNI, J.A., THALKEN, C.E., & TREMBLAY, J.W.
(1978) The toxicology, environmental fate, and human risk of
Herbicide Orange and its associated dioxin. Springfield, Va.,
USA, US Air Force Occupational & Environmental Health
Laboratory (Technical Report No. TR-78-92, US National
Technical Information Service.)
YOUNG, F. & HALEY, T.J. (1977) Pharmacokinetic study of a
patient introduced with 2,4-dichlorophenoxyacetic acid and
2-methoxy-3,6-di-chlorobenzoic acid. Clin. Toxicol., 11(5)
489-500.
ZAKHAROV, G.G., SHEVCHENKO, A.M., ZUB, G.A., & PERSHAI, L.K.
(1968) [The clinical picture and prophylaxis of "Dikotex"
poisoning.] Zdrav. Beloruss., 9: 7-21 (in Russian, Health &
Welfare Canada Translation No. 2367).
ZETTERBERG, G. (1977) Experimental results of phenoxy acids
on microorganisms. In: Ramel, C., ed. Chlorinated phenoxy acids
and their dioxins: Mode of action, health risks and environmental
effects. Ecol. Bull. (Stockholm), 27.
ZETTERBERG. G., BUSK, L., ELOVSON, R., STAREC-NORDENHAMMAR, I., &
RYTTMAN, H. (1977) The influence of pH on the effects of 2,4-D on
Saccharomyces cerevisiae and Salmonella typhimurium. Mutat. Res.,
42(1): 3-18.
ZHITENEVA, L.D. & CHESNOKOVA, T.V. (1973) [Alteration of the
morphological composition of the blood in white amur larvae under
the effect of herbicides.] Eksp. vod. toksikol., 4: 56-67
(in Russian).
ZIELENSKI, W.L., Jr & FISHBEIN, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4-D and 2,4,5-T acids and
2,4-D esters in mice. J. agric food Chem., 15(5): 841-844.
ZIEM, G. & MAY, G. (1983) Tetrachlorodibenzodioxin. Br. J. ind.
Med., 40: 116.
