
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 45
CAMPHECHLOR
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154185 7
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CAMPHECHLOR
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, properties and analytical methods
1.1.2. Uses and sources of exposure
1.1.3. Environmental concentrations and exposure
1.1.4. Kinetics and metabolism
1.1.5. Studies on experimental animals
1.1.6. Effects on man
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. PRODUCTION, USES, TRANSPORT AND DISTRIBUTION
3.1. Production and uses
3.2. Transport and distribution
3.2.1. Air
3.2.2. Water
3.2.3. Soil
3.2.4. Abiotic degradation
4 ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental levels
4.1.1. Air
4.1.2. Water
4.1.3. Food
4.1.4. Miscellaneous sources
4.1.5. Wildlife
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Human studies
5.2. Animal studies
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single exposures
6.2. Short-term exposures
6.3. Long-term exposures.
6.4. Dermal toxicity
6.5. Reproduction studies
6.6. Mutagenicity
6.7. Teratogenicity
6.8. Carcinogenicity
6.9. Other studies
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning incidents
7.2. Occupational exposure
7.3. Controlled human studies
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8.1. Aquatic organisms
8.1.1. Aquatic invertebrates
8.1.2. Fish
8.2. Terrestrial organisms
8.2.1. Insects
8.2.2. Birds
8.2.3. Wild animals
8.2.4. Plants
8.3. Microorganisms
8.4. Bioaccumulation and biomagnification
8.5. Population and community effects
8.6. Effects on the abiotic environment
9. PREVIOUS EVALUATIONS OF CAMPHECHLOR BY INTERNATIONAL BODIES
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Camphechlor toxicity
10.2. Exposure to camphechlor
10.3. Effects on the environment
10.4. Conclusions
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in
the criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of
the environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in
the criteria documents are kindly requested to make available to
the WHO Secretariat any important published information that may
have inadvertently been omitted and which may change the
evaluation of health risks from exposure to the environmental
agent under examination, so that the information may be
considered in the event of updating and re-evaluation of the
conclusions contained in the criteria documents.
* * *
A detailed data profile and a legal file can be obtained
from the International Register of Potentially Toxic
Chemicals, Palais des Nations, 1211 Geneva 10, Switzerland
(Telephone No. 988400 - 985850).
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
ORGANOCHLORINE PESTICIDES OTHER THAN DDT (CHLORDANE, HEPTACHLOR,
MIREX, CHLORDECONE, KELEVAN, CAMPHECHLOR)
Members
Dr Z. Adamis, National Institute of Occupational Health,
Budapest, Hungary
Dr D.A. Akintonwa, Department of Biochemistry, Faculty of
Medicine, University of Calabar, Calabar, Nigeriaa
Dr R. Goulding, Chairman of the Scientific Sub-committee, UK
Pesticides Safety Precautions Scheme, Ministry of
Agriculture, Fisheries & Food, London, England (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health
(Indian Council of Medical Research), Meghaninager,
Ahmedabad, India
Dr D.C. Villeneuve, Environmental Contaminants Section,
Environmental Health Centre, Tunney's Pasture, Ottawa,
Ontario, Canada (Rapporteur)
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Haddassah Medical School, Jerusalem,
Israel (Vice-Chairman)
Representatives of Other Organizations
Dr C.J. Calo, European Chemical Industry Ecology and
Toxicology Centre (ECETOC)
Mrs M.Th. van der Venne, Commission of the European
Communities, Health and Safety Directorate, Luxembourg
Dr D.M. Whitacre, International Group of National Associations
of Agrochemical Manufacturers (GIFAP)
Secretariat
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Mrs B. Goelzer, Division of Noncommunicable Diseases, Office
of Occupational Health, World Health Organization, Geneva,
Switzerland
Dr Y. Hasegawa, Division of Environmental Health,
Environmental Hazards and Food Protection, World Health
Organization, Geneva, Switzerland
------------------------------------------------------------------
a Unable to attend.
Secretariat (contd.)
Dr K.W. Jager, Division of Environmental Health,
Internationational Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr B. Labarthe, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr I.M. Lindquist, International Labour Organisation, Geneva,
Switzerland
Dr M. Vandekar, Division of Vector Biology and Control,
Pesticides Development and Safe Use Unit, World Health
Organization, Geneva, Switzerland
Mr J.D. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
ENVIRONMENTAL HEALTH CRITERIA FOR CAMPHECHLOR
Following the recommendations of the United Nations
Conference on the Human Environment held in Stockholm in 1972,
and in response to a number of World Health Assembly Resolutions
(WHA23.60, WHA24.47, WHA25.58, WHA26.68), and the recommendation
of the Governing Council of the United Nations Environment
Programme, (UNEP/GC/10, 3 July 1973), a programme on the
integrated assessment of the health effects of environmental
pollution was initiated in 1973. The programme, known as the WHO
Environmental Health Criteria Programme, has been implemented
with the support of the Environment Fund of the United Nations
Environment Programme. In 1980, the Environmental Health
Criteria Programme was incorporated into the International
Programme on Chemical Safety (IPCS). The result of the
Environmental Health Criteria Programme is a series of criteria
documents.
A WHO Task Group on Environmental Health Criteria for
Organochlorine Pesticides other than DDT met in Geneva from 28
November to 2 December 1983. Dr K.W. Jager opened the meeting on
behalf of the Director-General. The Task Group reviewed and
revised the draft criteria document and made an evaluation of the
health risks of exposure to camphechlor.
This document is a combination of drafts prepared by Dr D.C.
Villeneuve of Canada and Dr S. Dobson of the United Kingdom.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences, Research
Triangle Park, North Carolina, USA - a WHO Collaborating Centre
for Environmental Health Effects.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, properties and analytical methods
Camphechlor (toxaphene) (C10H10Cl8 approx.) is an amber,
waxy solid consisting of a complex mixture of polychlorinated
bicyclic terpenes.
Gas chromatography with electron capture detection is the
method of choice for the determination of camphechlor.
1.1.2. Use and sources of exposure
Camphechlor is a non-systemic contact and stomach insecticide
with some acaricidal action. It is often used in combination
with other pesticides.
The main source of exposure for the general population is the
residues of camphechlor in food, but these are generally very
low.
1.1.3. Environmental concentrations and exposure
Camphechlor is broken down in the environment by sunlight
(ultraviolet radiation), high temperature, and by biodegradation.
There are no details on the relative breakdown of the components
of the mixture. Camphechlor is readily lost from the soil by
evaporation, but once it penetrates the soil it is tightly bound
to soil particles and very resistent to leaching. Its half-life
in soil has been reported to vary from 70 days to 12 years,
depending on the type and condition of the soil. In some waters
it has been shown to persist for years at concentrations that
are toxic for fish.
Camphechlor is rapidly removed from crops by weathering and
evaporation.
It is toxic for aquatic species and some terrestrial species
and has been shown to bioaccumulate, mainly in aquatic species.
It may present a major hazard for aquatic organisms. It also
poses a threat to birds.
1.1.4. Kinetics and metabolism
Camphechlor is absorbed following ingestion and inhalation,
as well as through the skin. Detailed information is lacking on
its metabolism, probably because of its complex composition.
Both hydroxylation and dechlorination products have been found as
metabolites. Excretion takes place via both urine and faeces.
1.1.5. Studies on experimental animals
Camphechlor is moderately toxic, i.e., the oral LD50 values
in the rat range from 60 to 120 mg/kg body weight and can on acute
oral over-exposure give rise to salivation, vomiting, hyper-
excitability, convulsions, and death. The lethal dose for man is
estimated to be 2 - 7 g. It is an irritant for the skin.
In short-term and long-term studies on animals, hypertrophy
of the liver with increased microsomal enzyme activity and
histological changes in the liver cells occurs at high dose
levels (1000 mg/kg diet), depending on the test conditions and
the species tested. Induction of microsomal enzyme activity in
the rat has been found at levels of 5 mg or more/kg diet.
Hypertrophy of the thyroid and adrenals and degeneration of the
tubular epithelium of the kidney have also been reported. At
near lethal dosages, excitation of the CNS may occur.
Camphechlor has been shown not to have any effects on
reproduction and was not found to be teratogenic. It was mutagenic
in Salmonella typhimurium but results of a dominant lethal test
on mice were negative. It is carcinogenic for both rats and mice.
1.1.6. Effects on man
Several cases of poisoning have been described in man due to
contamination of food with camphechlor or to accidental ingestion
of camphechlor formulations. Symptomatology consists of
gastrointestinal complaints, followed by motor seizures. Some
incidents in children were lethal.
Although a survey of a population of workers in a plant
manufacturing camphechlor did not reveal any cases of ill-health
referable to their employment, some illness has been reported in
a few people coming into contact with this chemical. A group of
8 women exposed to camphechlor were reported to have a higher
incidence of chromosome abnormalities than the controls.
Available epidemiological studies are not adequate to evaluate
the carcinogenicity of camphechlor for human beings.
1.2. Recommendations
1. Careful surveillance should be maintained over the future
production of camphechlor and the nature and extent of its
use.
2. Levels in the environment should continue to be
comprehensively monitored.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1. Identity
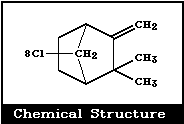 Molecular formula: C10H10Cl8 (approximately)
CAS chemical name: Toxaphene (a mixture of poly-
chlorinated bicyclic terpenes with
chlorinated camphenes predominating)
Trade names: Altox, Chem-Phene M5055, Chlor Chem
T-590, Crestoxo, Estonox, Fasco-
Terpene, Geniphene, Gy-Phene,
Hercules 3956, Huilex, Penphene,
Phenacide, Phenatox, Polychlor-
camphen, Strobane-T, Toxakil,
Toxaphene, Toxon 63
CAS registry number: 8001-35-2
Relative molecular mass: 413.8 (average)
2.2. Physical and Chemical Properties
Camphechlor is an amber, waxy solid (Canada, Department of
National Health and Welfare, 1978) with a melting range of
65 - 90 °C. Its vapour pressure is 3.3 x 10-5 mm Hg at 20 -
25 °C. Camphechlor may dechlorinate in the presence of
alkali, sunlight (UV radiation) or at temperatures above
120 °C (Canada, Department of National Health and Welfare,
1978; IARC, 1979). It is soluble in common organic solvents
but practically insoluble in water (0.4 - 3.0 mg/litre)
(Metcalf, 1976).
The exact chemical structure has not yet been elucidated
(von Rumker et al., 1974). The technical product is a complex
mixture of chlorinated bicyclic terpenes containing 67 - 69%
chlorine by weight (Canada, Department of National Health and
Welfare, 1978), which result from the chlorination of pine
resins. Camphechlor has been separated into at least 177
C10-polychloro compounds, including Cl6, Cl7, Cl8, Cl9, and
Cl10 derivatives, using absorption and gas-liquid
chromatography (Casida et al., 1974, Holmstead et al., 1974).
2.3. Analytical Methods
Much difficulty has been encountered in the determination of
camphechlor residues due to the fact that camphechlor is not a
single compound, but a mixture of over 177 compounds. In addition,
it is often used in conjunction with other pesticides, which may
cause interference in analytical procedures for camphechlor
residues.
Several analytical methods used for the determination of
camphechlor in air, water, soil, food, and animal tissues are
summarized in Table 1 (IARC, 1979).
Table 1. Methods for the determination of camphechlor
-----------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Reference
extraction/clean-up method detection
-----------------------------------------------------------------------------------
Air
workplace trap on cellulose membrane, GC/ECD 0.225 - NIOSH (1977b)
extract (petroleum ether) 1.155 mg/m3
Water
fish-tank extract (acetone-petroleum GC/ECD 10 µg/litre Stalling &
ether) in a special Huckins
syphon system, wash (1976)
resulting water-acetone layer
(petroleum ether), bulk
petroleum ether fractions, CC
Soil
moisten (water), extract GC/ECD 0.05 - Carey et al.
(hexane-isopropanol), wash 0.1 mg/kg (1976)
(water)
Food
molasses dilute (water), extract GC/ECD 0.03 mg/kg Yang et al.
(hexane-isopropanol) (1976)
fruits and extract (acetone) in blender, GC/ECD Luke et al.
vegetables filter, extract (petroleum (1975)
ether-dichloromethane), wash
aqueous phase
(dichloromethane), bulk
solvent extracts, evaporate
to small bulk, add acetone,
reduce volume and repeat, CC
Biological
tissues macerate, add anhydrous TLC 1 µg Tewari &
sodium sulfate, extract Sharma (1977)
(acetone), add water,
extract (chloroform)
wash (potassium
hydroxide solution)
-----------------------------------------------------------------------------------
CC = column chromatography;
GC = gas chromatography;
ECD = electron capture detection;
TLC = thin-layer chromatography.
3. PRODUCTION AND USES, TRANSPORT AND DISTRIBUTION
3.1. Production and Uses
Camphechlor does not occur naturally in the environment
(Canada, Department of National Health and Welfare, 1978).
Camphechlor has been in use since 1949 and, in 1975, it was the
most heavily-used insecticide in the USA (Pollock & Kilgore,
1980). In 1969, 168 uses for camphechlor were registered in the
USA.
Production in the USA has been estimated to range from 22 700
- 40 800 tonnes per year (Canada, Department of National Health
and Welfare, 1978). Von Rumker et al. (1974) gave the following
annual figures for the USA: total production 34 200 tonnes, of
which 8000 tonnes was exported and 26 500 tonnes was used in the
USA: 450 tonnes for industrial application and 25 650 tonnes for
agricultural use. Estimated consumption in the USA was 9360
tonnes in 1980 and 5400 tonnes in 1982. Since late 1982, use in
the USA has been limited to scabies control on cattle and sheep,
insect control on pineapples and bananas in the Virgin Islands
and Puerto Rico, and for emergency use against army worms and
grasshoppers on cotton, corn, and grain. These uses are
restricted to certified spray operators wearing protective
clothing (IRPTC, 1983a). Registered uses in Canada are minimal
and are decreasing.
Camphechlor is a non-systemic contact and stomach insecticide
with some acaricidal action. The primary crops on which camphechlor
is used are cotton, cereal grains, fruits, nuts, oil seeds, and
vegetables. Use on livestock is primarily for the control of ticks
and mites. The use of camphechlor on crops has not been a serious
problem for bees fertilizing the treated crops (WHO, 1975).
Camphechlor is often mixed with other pesticides and appears to act
as a solubilizer for insecticides with low solubility. The
synergistic properties of camphechlor when used with some other
insecticides have been reported (von Rumker et al., 1974).
Camphechlor is often applied together with DDT or methyl or ethyl
parathion (WHO, 1975).
3.2. Transport and Distribution
3.2.1. Air
The major route of removal of camphechlor from the soil is
through evaporation (von Rumker et al., 1974). Although there is
little information on the atmospheric transport of camphechlor,
it is likely that it may cover hundreds of miles. Camphechlor
levels in 75 out of 880 air samples from different parts of the
USA ranged between 68 and 2520 ng/m3 (Stanley et al., 1971).
Five out of 8 rainwater samples taken in Maryland, USA, contained
camphechlor (Munson, 1976).
3.2.2. Water
Accumulation of camphechlor occurs in water in areas where the
insecticide is in use and it may be quite persistent. It has been
found in water in some lakes, in toxic concentrations, for up to 5
years after fish have been killed (Canada, Department of National
Health and Welfare, 1978). Camphechlor was not found in drinking-
water supplies in Canada above a detection level of 0.01 mg/litre
(Canada, Department of National Health and Welfare, 1978). In the
USA, camphechlor concentrations were usually less than 0.001
mg/litre; nevertheless, concentrations as high as 0.065 mg/litre
were found in some areas where cotton had been sprayed several
months earlier (Bradley et al., 1972). Camphechlor concentrations
of up to about 2 g/kg were found in the sediment of a stream that
received the effluent of a camphechlor-manufacturing plant (Durant
& Reimold, 1972). Camphechlor residues in the range of 7 - 410
ng/litre were also present in all water samples taken before and
after treatment by a water treatment plant (Nicholson et al., 1964).
Although the majority of the residues are volatilized, levels
in runoff from soil surfaces or treated plants may be substantial
(von Rumker et al., 1974).
3.2.3. Soil
As camphechlor is applied topically to livestock and
agricultural crops, very little of the pesticide gets mixed into
the soil. However, that which penetrates into the soil becomes
tightly bound to soil particles and is then highly resistant to
leaching (von Rumker et al., 1974). Residues adsorbed on soil
particles may be transported via soil erosion and sediment
transport. The downward migration of camphechlor in soil is not
significant. The results of a study assessing the vertical
distribution of camphechlor indicated that, after 3 years, 85 -
90% of the remaining pesticide was in the top 23 cm of the soil
(WHO, 1975).
The half-life of camphechlor in soil has been found to vary
from 70 days (in moist, sandy soil) to 179 days (in moist clay
soil). The half-life in these same soils, if dried, became 136
and 705 days, respectively (WHO, 1975). In another study,
involving the mixing of camphechlor with soil, 45% of the
original application remained after 14 years (Nash & Woolson,
1967). Other authors have determined that the half-life of
camphechlor in soil ranges from 100 days (LaFleur et al., 1973)
up to 6 and 12 years (Alexander, 1965; Nash & Woolson, 1967;
Menzie, 1972). The disparity among these findings may be
explained by variations in soil type and climates.
No evidence has been found to suggest the existence of
conversion products of camphechlor in weathered crop residues
(WHO, 1975). However, camphechlor is rapidly removed from crops
by weathering and volatilization.
In the USA, the average level of camphechlor in cropland soil
is 0.07 mg/kg. A study on 969 soil samples from cropland soils
from 43 states and on non-cropland soils from 11 states showed
camphechlor residues in only 4.2% of the sites examined, despite
the fact that all the major camphechlor-using areas were included
in the cropland states surveyed. In the 199 non-cropland soil
samples, camphechlor occurred only once (Wiersma et al.,
1972a,b,c).
3.2.4. Abiotic degradation
Camphechlor is broken down by sunlight (ultraviolet radiation)
and high temperatures. It is also biologically degraded by soil
bacteria and fungi, the bacteria utilizing it as a source of carbon
(WHO/FAO, 1975). No definitive data have been published on the
degradation found in camphechlor (Metcalf, 1976).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Because the concentrations of camphechlor in ambient air
in unsprayed areas are in the ng/m3 range (IARC, 1979), it
seems unlikely that it would cause any health hazard.
4.1.2. Water
Only bodies of water that have been treated with camphechlor
for fish control or receive runoff from manufacturing sites or
cropland areas treated with camphechlor have been found to
contain significant amounts of camphechlor.
The lower limit of detection of camphechlor by taste is
approximately 5 µg/litre. Water treated with activated carbon (a
method used to clear water bodies of accidental camphechlor
spills) is effectively free of camphechlor (WHO, 1975). No
damage to man resulting from camphechlor residues in water has
been reported.
4.1.3. Food
Camphechlor bioaccumulates and builds up in food chains, but
not to the same extent as some other highly persistent chlorinated
hydrocarbons (von Rumker et al., 1974). For the most part,
camphechlor levels in food seem to be below the national tolerances
(WHO, 1975), which range from 2 - 7 mg/kg in Australia, Canada, and
the USA, and are as low as 0.4 mg/kg in the Federal Republic of
Germany and the Netherlands (WHO, 1975).
Camphechlor was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1968 and 1973 (FAO/WHO, 1969, 1974). In 1968,
no recommendations were made because of the many unresolved
questions. In 1973, the meeting concluded that it could not
establish an ADI for a material that varied in composition
according to the method of manufacture.
Because camphechlor is not a systemic insecticide, the
residues are more or less confined to the plant surfaces (WHO,
1975). Camphechlor residues generally occur in agricultural
crops as well as in meat from domestic livestock and fish.
Thirty-one days following an application of camphechlor to
alfalfa, 72.9% of the compound had been lost. Long-term losses
depended on the quantity applied, the formulation, and the
application route. The greatest residue was left from oily
solutions, followed by water emulsion, and by dusts (Pollock &
Kilgore, 1978). Camphechlor residues on growing leafy crops have
a half-life of 5 - 10 days. On alfalfa and clover, the half-life
is in the range of 9 - 13 days. The residue level is controlled
by time of application, dosage rate, and appropriate pre-slaughter
or pre-harvest intervals (WHO, 1975).
Fruits and vegetables have been found to contain camphechlor
residues; the highest concentrations were found in spinach
(FAO/WHO, 1969). In studies conducted during 1964-67, the average
levels of camphechlor residues found in leaf and stem vegetables
was 0.18 mg/kg and that in processed foods 0.45 mg/kg. Generally,
levels of camphechlor in all foods were low. In 1964-66,
camphechlor residues were detected in 30, 8, and 2% of the cotton-
seed, soybean, and peanut oil samples listed, respectively (Pollock
& Kilgore, 1978). Heat processing of fruits and vegetables was
found to reduce levels of camphechlor residues (WHO, 1975). Sugar
beet, sampled in the USA in the autumn of 1970, showed camphechlor
residues of 0 - 0.34 mg/kg (Yang et al., 1976). It has been
demonstrated that peeling, abrasive peeling, and washing removes
most of the camphechlor residues present on fruits and vegetables.
The addition of 0.1% synthetic detergent or 1.0% neutral soap to
the washing water increases the amount of the pesticide removed
(WHO, 1975).
Honey produced by bees exposed to 36Cl-camphechlor
contained less than 0.01 mg camphechlor/kg (WHO, 1975).
Because livestock is treated with camphechlor to remove
external insects and because some feeds are contaminated with
camphechlor, camphechlor residues are found in meat and poultry.
The residues in meat can be significantly reduced by trimming
excess fat (the primary storage site for camphechlor) and also by
cooking at temperatures sufficient to render out the fat (WHO,
1975). In animals, the level of storage of camphechlor is lower
and its elimination more rapid than with most other chlorinated
hydrocarbons (FAO/WHO, 1974), hence residues in poultry and meat
are very low (WHO, 1975). In general, the level stored in the
fat of sheep and cattle is 25 - 50% of the level in the feed.
This concentration is less in hogs, probably because of a greater
fat content (FAO/WHO, 1969).
Camphechlor has been detected in the milk from cows sprayed or
fed this insecticide (Clayborn et al., 1963; Canada, Department of
National Health and Welfare, 1978). The ratio of camphechlor
concentration in feed to that in milk is approximately 100:1.
Build-up in milk quickly fades when exposure to camphechlor is
discontinued (WHO, 1975). Residues of camphechlor in milk, due to
feed containing 5 - 20 mg camphechlor/kg, plateaued after 28 days.
Residues in milk, due to feed containing 2.5 mg camphechlor/kg,
plateaued by the 9th day. Milk contained 0.11 and 0.18 mg
camphechlor residues/litre when feed containing 10 and 20 mg/kg,
respectively. The residues in milk fell off sharply, 4 days after
camphechlor was absent from the diet and were negligible by the
14th day (Zweig et al., 1963). In the USA, camphechlor was
detected in only 5 out of 7265 meat samples and 2 out of 5504
poultry samples (Guyer et al., 1971).
In a study carried out by the Department of National Health
and Welfare, Canada, between 1969 and 1971, no detectable levels
(at the ppb level) of camphechlor were found in any of the
several hundred food items analysed (Smith, 1971; Smith et al.,
1972, 1973), while, in another study carried out by the
Department of National Health and Welfare, Canada, between 1974
and 1975, unspecified amounts of camphechlor were found in 0.6%
of the food items tested (Coffin & McLeod, 1975); thus, the
incidence was very low.
In the USA, between 1971 and 1972, among a total of 420
samples of food analysed, only one was found to contain
camphechlor (Manske & Johnson, 1975). In a study conducted in
the USA during the period of 1964-69, residues of camphechlor
were found to be the 6th most frequent in occurrence of all
pesticides in processed foods. However, levels were so low that
few, if any, exceeded the 7 mg/kg tolerance level (WHO, 1975).
4.1.4. Miscellaneous sources
Camphechlor residues have also been found in non-food items.
Camphechlor residues in chewing tobacco, smoking tobacco, snuff,
cigarettes, and cigars in the USA were found to have decreased
over the period 1971-73. The average concentration in cigarettes
decreased from 3.3 to 1.4 mg/kg (Domanski et al., 1974). In
1972, examinations showed that 6 brands of cigars purchased in 5
cities in the USA contained camphechlor residues ranging from 0.5
to 3.42 mg/kg (Domanski & Guthrie, 1974), while results of a
study conducted in 1970 (Domanski & Sheets, 1973), showed
camphechlor residues in tobacco in the USA to be as high as 12
mg/kg.
4.1.5. Wildlife
Residues of camphechlor were found in 53% of samples of fish
and invertebrates taken from waters near cotton-producing areas
along the Guatemalan Pacific Coast (Keiser et al., 1973). No
changes in camphechlor levels were observed when fish containing
5 - 10 mg/kg camphechlor were boiled or fried (Terriere &
Ingalsbe, 1953).
Residues have been found infrequently and at low levels in
non-aquatic wildlife (Pollock & Kilgore, 1978).
4.2. General Population Exposure
Camphechlor exposure of the general population can result
from residues in food.
4.3. Occupational Exposure
Results of a study conducted in camphechlor-manufacturing
plants in the USSR showed camphechlor levels 5 - 6 times the
permissible limit of 0.2 mg/m3. After a work shift, levels of
30 - 1000 mg/m2 were found on the uncovered skin of workers,
while levels on covered areas ranged up to 40 mg/m2 (Ashirova,
1971). However, no adverse effects of this occupational exposure
have been reported. Although camphechlor was used extensively
during the 1960s in agricultural and public health programmes in
Alberta, analyses of human tissue from 50 autopsies at the
University Hospital in Edmonton in 1967-68 did not reveal
camphechlor in any of the 217 tissues examined (Kadis et al.,
1970).
5. KINETICS AND METABOLISM
5.1. Human Studies
In a 9-month-old child, poisoned with a 2:1 mixture of
camphechlor and DDT, death occurred after convulsions and
respiratory failure. Ratios of camphechlor:DDT in the brain and
liver were 10:1 and, in the kidney, 3:1 (Haun & Cueto, 1967). No
significant levels of camphechlor were found in the skin and
subcutaneous tissue taken from 68 children, who died in the
perinatal period, in 13 cities in the USA (Zavon et al., 1969).
5.2. Animal Studies
The metabolism of camphechlor has been an area of little
research, because of difficulties in detecting a complex and
multicomponent substance. However, a few such studies have been
published.
About 37% of a single oral dose of technical grade
36Cl-camphechlor, administered to rats at 20 mg/kg body
weight, was eliminated in the faeces; 15% was excreted in the
urine in 9 days during the same period (Crowder & Dindal,
1974). Rats were dosed orally with solutions containing
either 36Cl-camphechlor, 36Cl-camphechlor fractions, or
14C-camphechlor. Urine and faeces samples were collected for
14 days (Ohsawa et al., 1975). The rats excreted (combined
urine and methanol extract of faeces) 76% of the 36Cl-camphechlor,
57% of the 14C-camphechlor, and 69 - 94% of the 36Cl-camphechlor
fractions. Very little of the material was excreted unmetabolized
and the camphechlor had undergone dechlorination.
A dose-related increase in the excretion of camphechlor in
the milk of cows, fed diets containing camphechlor at 2.5 - 20
mg/kg, was reported (Zweig et al., 1963). Results of in vitro
studies, using rat liver homogenates, showed that isolated
camphechlor fractions were metabolized to hydroxylated compounds,
in addition to dechlorinated products (Chandurkar & Matsumura,
1979). However, the precise chemical structures of these
metabolites were not unequivocally identified. Pollack & Kilgore
(1976) dosed rats orally with 14C-camphechlor and reported a
cumulative elimination of 58% of the dose in the urine and
faeces. Increased amounts of polar "activity" and a small
increase in non-polar fractions were found in the fat.
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single Exposures
Camphechlor is absorbed through the skin, respiratory tract,
and the intestinal tract (FAO/WHO, 1969; Gleason et al., 1969;
Gosselin et al., 1976).
The principal toxic manifestations noted in animals exposed
to a single dose of camphechlor consist of salivation, vomiting,
reflex excitability, and convulsions terminating in respiratory
failure (Taylor et al., 1979). Typical LD50 values for a variety
of animals are given in Table 2. The influence of the solvent on
camphechlor uptake can be seen from the wide range of LD50s in
this table.
Table 2. The acute toxicity of camphechlor in several animal studies
-----------------------------------------------------------------------------
Species Route Vehicle LD50 (mg/kg) References
-----------------------------------------------------------------------------
Rat oral peanut oil 80 - 90 Gaines (1969)
Rat dermal xylene 780 - 1075 Gaines (1969)
Rat oral corn oil 60 US EPA (1976a)
Rat oral kerosene 120 US EPA (1976a)
Mouse oral corn oil 112 US EPA (1976a)
Dog oral corn oil 49 US EPA (1976a)
Dog oral kerosene over 250 US EPA (1976a)
Guinea-pig oral kerosene 365 US EPA (1976a)
Cat oral peanut oil 25 - 40 US EPA (1976a)
Rabbit oral peanut oil 75 - 100 US EPA (1976a)
Rabbit oral kerosene 250 - 500 US EPA (1976a)
Rabbit dermal dust over 4000 US EPA (1976a)
Rabbit dermal peanut oil over 250 US EPA (1976a)
Rabbit dermal wettable powder 1025 - 1075 Johnston &
(suspension in water) Eden (1953)
Cattle oral grain 144 US EPA (1976a)
Goat oral xylene 200 US EPA (1976a)
Sheep oral xylene 200 US EPA (1976a)
-----------------------------------------------------------------------------
The acute toxicity of camphechlor in rats is increased at least
3-fold by protein deficiency (Gleason et al., 1969). The lethal
oral dose for an adult man is estimated to be 2 -7 g (Conley, 1952).
6.2. Short-Term Exposures
Rats were fed camphechlor at levels of 0.2 - 50 mg/kg diet,
for up to 13 weeks (Kinoshita et al., 1966). Camphechlor was
observed to induce microsomal enzyme activity at levels of 5.0
mg/kg and higher.
Camphechlor did not induce any effects on the physical
appearance, gross pathology, weight gain, or liver cell histology
of albino rats fed 2.33 - 189 mg/kg diet, for up to 12 weeks
(Clapp et al., 1971).
Camphechlor, when fed to rats for 7 days at 25 mg/kg diet,
caused an increase in the metabolism of estrone and inhibited the
increase in uterine weight produced by this compound (Welch et
al., 1971).
Studies on pregnant albino rats showed that daily oral
administration of camphechlor for up to 20 days resulted in
interconnected structural and enzymic changes in the cerebral
cortex (Badaeva, 1976).
Rats were dosed once, orally, with 120 mg camphechlor/kg body
weight and monitored for up to 15 days (Peakall, 1976). Liver
weight and microsomal enzyme activity were increased after 5 and
15 days, respectively. In another study in the same report, rats
were administered camphechlor at 2.4 mg/kg body weight per day
and killed at 1, 3, and 6 months. Liver weight and microsomal
enzyme activity increased at all time intervals, but plasma
testosterone levels were not affected.
Administration to rats of a single oral dose of 120 mg/kg
body weight, as well as a daily dose of 2.4 mg/kg body weight,
for 1 or 3 months, produced a disturbance in catecholamine
metabolism (Kuzminskaya & Ivanitski, 1979).
Adult male rats were fed diets containing 0, 50, 100, 150,
and 200 mg camphechlor/kg for 14 days (Trottman & Desaiah, 1980).
No changes were observed in body weight gain, food consumption,
brain, kidney, heart, or testes weights; liver weight was
increased at 200 mg/kg and thymus weight decreased at 150 and 200
mg/kg. Increased hydroxylation of aniline was observed at all
dose levels.
Administration of 5, 50, or 500 mg camphechlor/kg diet to
quail for up to 4 months produced hypertrophy of the thyroid with
increased uptake of 131I and adrenal hypertrophy (Hurst et al.,
1974).
Feeding of camphechlor in the diet at 5, 50, or 100 mg/kg to
chickens for 31 weeks induced sternal deformation and nephrosis
(Bush et al., 1977). Occasional keel deformation, involving
cartilaginous tissue as well as an apparent increase in the
growth of cartilage, was found in birds fed 0.50 mg camphechlor/kg.
Camphechlor was administered in capsules at a daily dose of 4
mg/kg body weight to 2 dogs for 44 days and to 2 other dogs for 106
days. Occasional manifestations of acute toxicity (CNS stimulation)
occurred for a short time after administration. There were no
significant changes in body weight, blood picture, or gross
appearance of organs. Histological examination of many organs
revealed some damage to the kidney (degeneration of the tubular
epithelium) and to the liver (generalized hydropic degenerative
changes, but no destruction of the cells). Liver glycogen levels
were normal (Lackey, 1949).
6.3. Long-Term Exposures
Four groups each of 40 rats (20 males and 20 females), were
fed camphechlor in the diet at 10, 100, 1000, and 1500 mg/kg.
Effects were determined through gross observation, mortality
rates, body weight, blood tests, liver weight, liver to body-
weight ratio, gross autopsy, and histological examination of the
tissues. After 7 1/2 - 10 months of feeding, some of the rats
fed 1500 mg/kg and a few of the rats fed 1000 mg/kg suffered
occasional convulsions. The body weight gain of the rats fed the
highest feeding level (1500 mg/kg) for the first 20 weeks was
less than that in the controls, probably because of decreased
food intake through unpalatability of the diet. As the rats
became accustomed to the diet, growth rate was essentially the
same as that of the controls. There were no significant effects
on mortality rate or on the haematopoietic system. The liver
weight and liver to body weight ratio was significantly increased
only in the 1000 and 1500 mg/kg groups. Liver changes consisted
of swelling, cellular hypertrophy, and proliferation of smooth
endoplasmic reticulum with cytoplasmic margination in the
centrilobular hepatic cells. These changes occurred to a
moderate extent in the 1500 mg/kg group and to a slight extent in
the 1000 mg/kg group (Treon et al., unpublished data, 1952).
Six male and 6 female rats per group were fed 50 or 200 mg
camphechlor/kg diet for up to 9 months (Ortega et al., 1957).
There were no clinical signs of toxicity and no effects on body
weight gain, food intake, or liver weights. No histological
changes were seen in the kidney or spleen. Three out of 12 rats
fed 50 mg/kg diet for 9 months showed histological changes in the
liver consisting of centrilobular cell hypertrophy, peripheral
migration of basophilic granulation, and the presence of
liposphere inclusion bodies. Six of the 12 rats fed 200 mg/kg
showed liver changes.
Groups of rats were fed camphechlor in the diet at 25,
100, and 400 mg/kg for their lifetime (Fitzhugh & Nelson,
1951). The only organ that showed significant histological
changes was the liver and these occurred at the 100 mg/kg and 400
mg/kg levels. There was centrilobular hepatic cell enlargement
with increased oxyphilia and peripheral margination of basophilic
granules. The effects at the various feeding levels were summarized
by Lehman (1952b), as follows: 400 mg/kg, liver enlargement; 100
mg/kg, no gross effects, but tissue damage occurred; and 25 mg/kg,
no tissue damage.
Camphechlor dissolved in maize oil, in gelatin capsules, was
administered daily, for 5 days per week, to dogs. A dose of 25
mg/kg body weight was fatal. Two dogs were administered 10 mg/kg
(equivalent to 400 mg/kg diet); one dog died after 33 days, but
the other lived and was sacrificed after 3 1/2 years. Four dogs
were administered 5 mg/kg; all survived and were sacrificed after
almost 4 years. No information on the pathological findings was
reported (Lehman, 1952b).
Camphechlor was fed daily (6 days per week) for 2 years to
3 male and 5 female dogs, beginning when the animals were
approximately 4 months old. Camphechlor was added to the
diet, including their drinking liquids, at levels of 10 and
50 mg/kg. The dogs received a daily dose of 0.60 - 1.47 mg/kg
or 3.12 - 6.56 mg/kg (equivalent to approximately 40 or
200 mg/kg on a dry diet basis). Gross behaviour, body weight,
mortality, peripheral circulating blood elements, gross
pathology, organ to body weight ratios, and histopathology
were recorded (Treon et al., unpublished data, 1952). There
were no effects on behaviour, body weight, mortality, or blood
elements, but there were increases in the liver weights, liver
to body weight ratios, and moderate liver degeneration at the
higher dose level (200 mg/kg). At the lower dose level
(40 mg/kg), 1 out of 3 dogs was reported to have slight liver
enlargement and slight granularity and vacuolization of the
cytoplasm. Re-examination of the sections of these animals
failed to confirm any difference from control animals. All
other tissues were normal at both feeding levels (Brock &
Calandra, 1964).a
Groups of dogs, comprising 6 male and 6 females, were fed
camphechlor at dietary levels of 5, 10, or 20 mg/kg together with
control groups. Two male and 2 female animals were sacrificed
after 6, 12, and 24 months. None of the feeding levels produced
any changes in organ weights, gross or histological examination,
or any of the clinical or organ function tests, at any time
(Industrial Bio-Test Laboratories Inc., unpublished data, 1965)b.
---------------------------------------------------------------------
a Brock, D. & Calandra, J.C. (1964) Re-evaluation of
microscopic sections from chronic oral toxaphene study,
Industrial Bio-Test Laboratories, Inc., unpublished report.
b Industrial Bio-Test Laboratories Inc. (1965) Two-year
chronic oral toxicity of toxaphene in beagle dogs
(Unpublished report).
Two adult female monkeys were given camphechlor in their
food, 6 days per week, at a daily dose of 0.64 - 0.78 mg/kg for 2
years. A third animal served as a control. There were no signs
of intoxication and no evidence of tissue or organ damage as
evaluated by growth rate, ratios of liver to body weight, spleen
to body weight, or histological examination of the tissues (Treon
et al, unpublished data, 1952).
6.4. Dermal Toxicity
The acute LD50 for rabbits exposed dermally to camphechlor
in the dry form was > 4000 mg/kg body weight (Lehman, 1952a).
The animals showed only moderate skin irritation and systemic
effects were characterized by hyperexcitability but no deaths
occurred. The animals recovered in 5 or 6 days. The 90-day
dermal LD50 has been estimated to be approximately 40 mg/kg
body weight for rabbits (Lehman, 1952a).
A review of the effects of camphechlor on livestock
revealed that cattle, sheep, and hogs can tolerate repeated
applications of solutions containing less than 2% camphechlor
(Penumarthy et al., 1976). However, dips containing 2.5%
camphechlor emulsion caused toxicosis in cattle.
6.5. Reproduction Studies
A 3-generation, 6-litter reproduction study was conducted with
camphechlor. Groups of weanling rats were fed 25 mg/kg diet and
100 mg/kg diet for 79 days before mating. All the animals continued
on their respective dietary concentration of camphechlor during
mating, gestation, and weaning during 2 generations, or for a
period of 36 - 39 weeks. Weanlings from the second litter were
selected as parents for the second generation and continued on
their respective diets until after weaning of a second litter. A
third generation was selected in the same manner. Complete gross
and histological examination was performed on all three parental
generations after 36 weeks of camphechlor administration. The only
pathological changes found were slight alterations in the livers of
the 100 mg/kg group, similar to the changes seen in long-term
studies. Reproductive performance, fertility, and lactation were
normal. The progeny was viable and normal in size and anatomical
structure. Findings among all test animals, 3 parental generations
and 6 litters of progeny, were comparable to those in control
animals for all variables (Kennedy et al., 1973).
Groups of mice (4 males and 14 females per group) were fed
camphechlor in the diet at levels of 0 and 25 mg/kg in a 5-
generation, 2 litter-per-generation, reproduction study. No
effects were noted on any of the reproduction parameters measured
(Keplinger et al., 1970).
Camphechlor in a corn-oil carrier was injected into fertile
hen eggs. It did not cause any reduction in hatchability, even
at the highest dose used, 1.5 mg/egg (Smith et al., 1970). In
another study, white leghorn hens were fed camphechlor at 0, 10,
and 100 mg/kg diet (Arscott et al., 1976). Except for a slight
decrease in egg production, no adverse effects were observed on
fertility, hatchability, or survival of progeny.
6.6. Mutagenicity
Camphechlor did not induce dominant lethal mutations in mice
(Epstein et al., 1972). When males were injected with 36 and 180
mg/kg body weight ip and bred with untreated females during 8
weeks, the frequencies of early fetal deaths and preimplantation
losses were normal. Negative results were also obtained in animals
treated orally for 5 successive days with 40 or 80 mg/kg body
weight. In an in vitro test to determine breakage of DNA in
bacteria, camphechlor did not induce breaks at a significantly
higher rate than that occurring in controls (Griffin & Hill, 1978).
Camphechlor was mutagenic in a test using Salmonella typhimurium
without requiring activation by liver homogenate (Hooper et al.,
1979).
6.7. Teratogenicity
Camphechlor was administered to mice and rats on days 7 -
16 of gestation by oral intubation at levels of 15 - 35 mg/kg
body weight per day (Chernoff & Carver, 1976). The highest
dose produced marked maternal mortality in rats and mice and
an increase in encephaloceles among offspring of mice. Fetal
mortality was slightly increased in mice at all dose levels.
Small decreases in fetal body weight and in the number of
sternal and caudal ossification centres were observed in rats,
mostly in the 25 mg/kg dosage group (Chernoff & Carver, 1976).
6.8. Carcinogenicity
A bioassay of technical grade camphechlor was conducted in
mice and rats by administering the test chemical in the feed
(NCI, 1979b). Groups of 50 mice of each sex were given time-
weighted average doses of 99 or 198 mg/kg diet for 90 - 91 weeks.
There was a dose-related decrease in survival in male mice and a
decrease in survival in high-dose females. The incidence of
hepatocellular carcinomas was dose-related in both males and
females. The statistical significance was maintained when the
incidence of hepatocellular carcinomas was combined with that of
hyperplastic nodules of the liver. It was concluded that, under
the conditions of the assay, camphechlor was carcinogenic in male
and female B6C3F1 mice.
Fifty rats of each sex were administered camphechlor in their
diets at time-weighted average doses of 556 and 1112 mg/kg for
male rats, and 540 and 1080 mg/kg for females; the study lasted
for 108 - 110 weeks. There were no dose-related effects on
survival rates; clinical signs of toxicity in rats included
generalized body tremors at week 53 in the high-dose males and
females and later, leg paralysis, ataxia, epistaxis, haematuria,
and vaginal bleeding. Abdominal distention, diarrhoea, dyspnoea,
and rough haircoats were common to both dose groups. At the high
dose, 26% (9/35) and at the low-dose, 17% (7/41) of male rats
developed follicular-cell carcinoma or adenoma of the thyroid.
In the high-dose, female rat group, 17% (7/42) developed thyroid
tumours and in the low-dose group only 2%. On the basis of the
findings in the above study, it was concluded that the results
"suggest carcinogenicity of camphechlor for the thyroid of male
and female Osborne-Mendel rats" (NCI, 1979b).
6.9. Other Studies
Camphechlor has also been shown to increase the activity of
gluconeogenic enzymes in the rat (Desaiah et al., 1979), alter
mitochondrial electron transport in vitro (Pardino et al., 1971),
and inhibit ATPase activity in vitro (Yap et al., 1975) and in
vivo (Fattah & Crowder, 1980).
Protein deficiency was shown to increase the acute toxicity
of camphechlor, 3 to 4-fold (Boyd, 1972b).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
McGee et al. (1952) described 10 cases of poisoning with 3
deaths; the victims ranged in age from 17 months to 49 years. All
ingested camphechlor accidentally either in the form of the
insecticide, as in the case of the children, or as camphechlor-
polluted food, in the adults. Four of the cases were children
aged 17 months to 4 years, of whom three died. All the adults
survived. The onset of illness in all but one was characterized by
a major motor seizure; the latter had only gastrointestinal
complaints. The 3 deaths occurred within a matter of several hours
and those who recovered did so in 24 h.
Another poisoning incident involved a 16-month-old child who
ingested a material presumed to be camphechlor (Pollock, 1953).
Death was preceded by respiratory depression and convulsions.
Another child's death due to camphechlor poisoning was
reported in 1967 (Haun & Cueto, 1967). In this case, a 9-month-
old girl had been playing with a dust containing 13.8%
camphechlor and 7.04% DDT, which was found on her skin and in her
mouth. Convulsions, respiratory arrest, and death followed.
Cerebral oedema was found at autopsy.
7.2. Occupational Exposure
Two workers involved in harvesting cotton that had been
sprayed with camphechlor developed dyspnoea and reduced pulmonary
function and were found to have miliary opacities distributed
over their lung fields (Warraki, 1963).
Eight women working in an area that had been sprayed with
camphechlor at the rate of 2 kg/ha were reported to have a higher
incidence of chromosome aberrations (acentric fragments and
chromatid exchanges) in cultured lymphocytes (13.1%) than control
individuals (1.6%) (Samosh, 1974).
In a review of the chronic toxicity of organochlorine
pesticides in man (Deichmann, 1973), a survey of 137 workers
involved in the manufacture of camphechlor was reported. The
average length of exposure was 3.7 years, but some workers were
exposed for as long as 18 years. Physical examinations conducted
annually on these people failed to reveal any "adverse effects
that could be associated with camphechlor exposure".
An instance was reported where a rancher, who dusted his
sheep with a mixture of 5% camphechlor and 1% lindane,
demonstrated resistance to the hypothrombinemic effect of
warfarin (Jeffery et al., 1976). This effect was attributed to
the enzyme-inducing properties of camphechlor, which led to
increased metabolism of warfarin.
Two cases of acute aplastic anaemia associated with dermal
exposure to camphechlor-lindane mixtures, have been reported. One
of these cases terminated in death due to acute myelomonocytic
leukaemia (US EPA, 1976a).
In a survey of 199 employees, who worked or had worked with
camphechlor between 1949 and 1977, with exposures ranging from 6
months to 26 years (mean 5.23 years), 20 employees died, 1 with
cancer of colon; none of these deaths appeared to be related to
exposure to camphechlor (US EPA, 1978).
7.3. Controlled Human Studies
Twenty-five human volunteers were exposed to an aerosol of
camphechlor in a closed chamber, for 30 min per day, on 10
consecutive days (Shelanski, unpublished data, 1947). After 3
weeks, they received the same exposure on 3 consecutive days.
Assuming a retention of 50% of the inhaled camphechlor, each
individual absorbed 75 mg camphechlor per day or approximately 1
mg/kg body weight per day. Physical examinations and blood and
urine tests did not reveal any abnormalities.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8.1. Aquatic Organisms
8.1.1. Aquatic invertebrates
The toxicity of camphechlor for aquatic invertebrates is very
variable (Tables 3 and 4). Needham (1966) measured levels of
mortality at different concentrations of camphechlor for 14 genera
of invertebrates and determined the level that each could tolerate
for 24 h without any deaths. These are tabulated in Table 5.
Tolerance varied from 1 mg/litre for a leech to 10 µg/litre for
Haliplus sp. Values for molluscs ranged from 20 µg/litre for the
oyster to 460 mg/litre for the mactrid clam in 96-h tests, and for
crustacea from 54 ng/litre for a stage I zoeal larva of Sesarma to
290 µg/litre for a stage III zoeal larva of Rhithropanopeus, also
in 96-h tests. Resistance to camphechlor may develop. The 24-h
LD50 for fresh water shrimp taken from an area that had been
regularly treated for cotton pests was 10 times that of shrimp
taken from a wildlife reserve (Naqvi & Ferguson, 1970). Similarly,
cyclopoid copepods from a pesticide-contaminated area had a higher
tolerance for camphechlor than cyclopoids from a pesticide-free
area (Naqvi & Ferguson, 1968).
The only well-documented sublethal effects on invertebrates
are found in oysters. Yearling oysters showed a marked
inhibition in shell deposition after a 24-h exposure to 60%
camphechlor at 100 µg/litre (Butler et al., 1962). Mature
oysters showed reduced activity, as measured by shell opening
movements, during 4 weeks exposure to camphechlor at 100
µg/litre.
8.1.2. Fish
A series of studies on fathead minnows, brook trout, and
channel catfish (Mayer et al., 1975; Mehrle & Mayer, 1975a,b;
Mayer & Mehrle, 1976) document the "broken-back syndrome" in
response to camphechlor concentrations ranging from 55 to 621
µg/litre. Fish showed increased levels of calcium but decreased
levels of collagen in the backbone; phosphorus levels were
unaffected. The backbone became more brittle and sub-lethal
electric shock led to multiple fracture at all dose levels. In
addition to changes in the calcium/collagen ratio, there was a
reduction in the amino acids: alanine, valine, leucine,
isoleucine, lysine, phenylalanine, and hydroxyproline in bone
collagen. Hydroxyproline was also reduced in skin. Because of
the importance of this particular amino acid in collagen
formation, it is postulated that camphechlor may reduce the
wound-healing capacity in fish. Camphechlor, at concentrations
as low as 68 µg/litre, inhibited collagen synthesis (monitored by
hydroxyproline measurement) in brook trout fry, 7 days after
hatching. None of these effects were dose related and only at
the highest dose were any deaths observed. More recently, Mayer
& Mehrle (1976) showed that vitamin C levels in fish backbone
also decreased after camphechlor exposure, but levels in the liver
were unchanged. The authors postulated that detoxification of
camphechlor required vitamin C, which moved from storage in bone to
the liver. Vitamin C is involved in the conversion of proline to
hydroxyproline. Competition for available vitamin C was put
forward as a credible explanation for collagen synthesis effects.
Camphechlor inhibited ATPase activity in trout gill microsomes at
concentrations in incubates of 4 mg or more/litre (Davis et al.,
1972). In channel catfish, brain and gill ATPases were more
severely affected than kidney ATPase (Desaiah & Koch, 1975). Non-
mitochondrial Mg-ATPase was most severely inhibited.
Table 3. Toxicity of camphechlor for aquatic invertebratesa
-------------------------------------------------------------------------------------------------
Organism Temperature Size End point Parameter Concentration Reference
(°C) (mm) (µg/litre)
-------------------------------------------------------------------------------------------------
American oyster 27-29 shell growth 96-h EC50 16 Schimmel et al.
(Crassostrea (1977)
virginica)
crayfish from clean 11.8- no response to 48-h EC50 60.7 Albaugh (1972)
(Procambarus area 14.6 stimuli
acutus) from cotton 11.8- no response to 48-h EC50 90.2 Albaugh (1972)
field 14.6 stimuli
Daphnia pulex 15.5 immobilisation 48-h EC50 15 Sanders & Cope
first instar (1966)
pink shrimp, 96-h LC50 2.2 Courtenay &
nauplius Roberts (1973)
(Penaeus
duorarum)
pink shrimp, 24.5-26 96-h LC50 1.4 Schimmel et al.
adult (1.1-1.8) (1977)
drift line crab 25 96-h LC50 0.054 Courtenay &
(Sesarma Roberts (1973)
cinereum)
stage I zoeal
-------------------------------------------------------------------------------------------------
a A more comprehensive table listing different conditions and exposure times is available on
request from IRPTC, Geneva.
Table 4. Toxicity of camphechlor for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Stat Temp pH Size Alk End Parameter Concen- Reference
/flow (°C) (mg/ point tration
litre) (µg/litre)
---------------------------------------------------------------------------------------------------------
Goldfish stat 25 7.4 1-2 g 18 96-h LC50 5.6 Henderson et al.
(Carassius 38-64 mm (1959)
auratus)
Bluegill stat 18 7.1 0.6-1.7 g 35 96-h LC50 21 (14-30) Macek &
(Lepomis McAllister (1970)
macrochirus)
Fathead minnow stat 12.7 7.1 0.6-1.5 g 35 96-h LC50 3.2 (2.8-3.7) Macek et al. (1969)
(Pimephales stat 18 7.1 0.6-1.7 g 35 96-h LC50 14 (9-22) Macek &
promelas) McAllister (1970)
Sheepshead minnow flow 27- 21a 96-h LC50 1.1 (0.9-1.4) Schimmel et al.
(Cyprinodon 29.5 25.5 (1977)
variegatus)
Mosquito fish flow 25- 7.8 0.28 g 28a sensi- 36-h LC50 1 Burke & Ferguson
(Gambusia 26 tive (1969)
affinis) flow 25- 7.8 0.28 g 28a insen- 36-h LC50 200 Burke & Ferguson
26 sitive (1969)
Spot, 1 month flow 11- 20 mm 21b 144-h 0.5 Lowe (1964)
(Leiostomus 28 28b LC50
xanthurus)
Rainbow trout 7.2 96-h LC50 5.4 Cope (1965)
(Salmo 18.3 96-h LC50 1.8 Cope (1965)
gairdneri)
Longnose flow 28.5- 32-51 mm 10b 28-day 0.9 Schimmel et al.
killifish, juv. 31.5 20b LC50 (1977)
(Fundulus
similis)
Toad 48-h LC50 290 Guyer et al. (1971)
(Bufo woodhousi)
---------------------------------------------------------------------------------------------------------
a Hardness mg/litre.
b Salinity o/oo.
Table 5. Concentrations of camphechlor tolerated by invertebratesa
----------------------------------------------------------------
Organism Concentration for Next higherb
zero mortality concentration
(µg/litre) tested (µg/litre)
----------------------------------------------------------------
Hirudinea 1000
Gammarus sp. 100 200 (61%)
Hydracarina 1000
Callibaetis sp. 150 300 (29%)
Aeshna sp. 200 275 (16%)
Lestes sp. 450 500 (19%)
Notonecta sp. 275 300 (29%)
Signara sp. 50 75 (40%)
Limephilus sp. 500 550 (51%)
Haliplus sp. 10 40 (55%)
Hydroporus sp. 60 100 (27%)
Dytiscus sp. 15 50 (24%)
Eyrinus sp. 65 100 (22%)
Lymnara sp. 700
----------------------------------------------------------------
a From: Needham (1966).
b Value in parentheses is percentage mortality found at the next
higher concentration tested above that giving zero mortality.
The acute toxicity of camphechlor for fish species is
summarized in Table 4. Although the range of LC50 values is
not great, some species differences are clear. However, the
pattern of camphechlor toxicity is unrelated to fish families
(Macek & McAllister, 1970). Several of the studies in Table 4
illustrate the temperature dependence of camphechlor toxicity
(Hooper & Grzenda, 1955; Cope, 1965; Mahdi, 1966; Macek et al.,
1969); camphechlor being more toxic at higher temperatures. No
other test-condition variables showed such a clear relationship,
but there was a suggestion of increased toxicity with increased
alkalinity (Hooper & Grzenda, 1955; Henderson et al., 1959).
Various symptoms of camphechlor poisoning have been
described for fish (Gruber, 1959; Henderson et al., 1959;
Ludemann & Neumann, 1960; Workman & Neuhold, 1963; Schaper &
Crowder, 1976). Initial hyperactivity is followed by muscular
spasms. Later, fish show loss of equilibrium with short, jerky
movements. Mosquito fish also show respiratory distress. These
symptoms are similar to those caused by most chlorinated hydro-
carbon insecticides. The mechanism of the toxic action of
camphechlor is assumed to be mainly nervous.
There are few long-term studies on fish. Mehrle & Mayer
(1975b) showed a 46% reduction in the weight of brook trout fry
after 90 days' exposure to camphechlor at 0.039 µg/litre; which
was the lowest concentration tested. When brook trout fry,
hatched from unexposed eggs, were held in camphechlor solutions
for 90 days, the mortality rate was higher than that in unexposed
fry at 30 days and thereafter (Mayer et al., 1975). All fry
exposed to 0.288 µg/litre died before 60 days. Yearling brook
trout were less sensitive to camphechlor, showing a reduction in
weight at 0.288 µg/litre and a 40% reduction at 0.502 µg/litre
after exposure for 180 days. At these concentrations, length was
also reduced. During 150 days of exposure to camphechlor, at
concentrations ranging from 0.055 to 0.621 µg/litre, fathead
minnows showed weight reductions ranging from 10.9% to 21.1%, but
this was not dose-related (Mehrle & Mayer, 1975a).
Egg viability in female trout was significantly reduced when
they had been exposed to camphechlor at nominal concentrations of
0.075 µg or more per litre since they were yearlings (Mayer et
al., 1975). At a long-term exposure of 0.5 µg/ litre, egg
viability was reduced to zero. When eggs from unexposed females
were incubated in camphechlor solutions of between 0.039 to 0.502
µg/litre for 22 days, prior to hatching, they did not show any
reduction in hatchability (Mayer et al., 1975; Mehrle & Mayer,
1975b).
Adaptation or resistance to camphechlor may develop, since
natural populations of mosquito fish, which had either received
run-off from pesticide-treated fields or been sprayed directly,
were from 6 to 48 times more resistant to camphechlor than newly-
exposed fish (Boyd & Ferguson, 1964).
8.2. Terrestrial Organisms
8.2.1. Insects
Several studies of the effects of technical camphechlor on
honey bees gave a wide variety of results. Graves & Mackensen
(1965) determined a 24-h LD50 at 19.08 µg/bee, whereas Torchio
(1973) measured a 48-h LD50 as 0.144 µg/bee. In both these
studies, camphechlor was applied in acetone to the dorsal
thorax. Anderson & Atkins (1968) list camphechlor as
"relatively non-toxic" with an LD50 greater than 11.0 µg/bee.
Torchio (1973) looked at 3 bee species over different time
periods. For honey bees and alkali bees, the numbers of
deaths increased 24 - 48 h after application, but no further
deaths occurred between 48 and 72 h. All bees surviving up to
72 h also survived up to 96 h. For the leaf cutting bee,
there were more deaths between 48 and 72 h, but none between
72 and 96 h. Exposure of western yellowjacket wasps to the
topical application of 1 µliltre drops of camphechlor resulted in
mortality rates in 48 h of: 13% with exposure to 1 g/litre, 40%
with exposure to 5 g/litre, and 65% with exposure to 10 g/litre
(Johansen & Davis, 1972). Extensive research has been carried out
on target and non-target insects. This has been reviewed by the US
EPA (1979), who concluded that technical camphechlor was roughly
equally toxic for both pest and beneficial insects. Lepidopterans
were more resistant, possibly reflecting the long usage of
camphechlor to control lepidopteran pests and the consequent
development of resistance.
8.2.2. Birds
The acute toxicity of technical camphechlor for birds is
summarized in Table 6. There is some species variability; sharp-
tailed grouse, the most sensitive species, having an LD50 of 10 -
20 mg/kg body weight, and mourning dove, the least sensitive
species, having an LD50 of 200 - 250 mg/kg body weight. Dahlen &
Haugen (1954) reported a lack of motor coordination preceding death
in bobwhite quail. There was a clear age effect in the toxicity of
camphechlor for the mallard (Hudson et al., 1972), with young birds
(36 h after hatching) being the least sensitive. No satisfactory
explanation of this phenomenon is available. Two major field
studies showed that spraying with technical camphechlor could cause
death in birds. Eyer et al. (1953) applied insecticide at 2.2, 4.4,
5.5, and 6.6 kg/ha and examined domestic geese of 3 varieties. No
deaths resulted at the lowest application rate. Birds of 2 varieties
were killed within 3 h of direct contact with 4.4 kg/ha spray but
only 1 variety was killed at 5.5 kg/ha. No birds died at the
highest dose rate. The inconsistency of these results was not
explained, but it is clear that the compound killed birds. McEwen
et al. (1972) counted wild birds on control and sprayed areas of
rangeland. Spraying at 884 g/ha reduced the number of birds
relative to that in the control areas and to pre-spray observations.
Dead birds (5 larks, a cowbird, a killdeer, and a dove), found on
the sprayed area, contained between 9.6 and 0.1 mg camphechlor
residues/kg body weight. No deaths or decrease in numbers from
emigration occurred until more than a week after spraying.
Table 6. Toxicity of camphechlor for birds
-----------------------------------------------------------------------------------------------
Species Age Sex Routea Parameter Concentrationb Reference
(mg/kg)
-----------------------------------------------------------------------------------------------
Mallard 36 oral LD50 130 (80.4-210) Hudson et al. (1972)
Mallard 7 days oral LD50 30.8 (23.3-40.6) Hudson et al. (1972)
Mallard 3-5 months oral LD50 70.7 (37.6-133) Hudson et al. (1972)
Mallard 3-5 months F oral LD50 70.7 Tucker & Crabtree (1970)
Bobwhite 8 weeks oral LD50 80-100 Dahlen & Haugen (1954)
quail
Bobwhite 3 months M oral LD50 85.4 Tucker & Crabtree (1970)
quail
Mourning oral LD50 200-250 Dahlen & Haugen (1954)
dove
Sharp-tailed 1-4 years M oral LD50 10-20 Tucker & Crabtree (1970)
grouse
Fulvous 3-6 months M oral LD50 99.0 Tucker & Crabtree (1970)
tree duck
Lesser sand- F oral LD50 100-316 Tucker & Crabtree (1970)
bill crane
Pheasant 2 weeks diet 5-day LC50 500-550 Heath et al., (1970)c
Coturnix 2 weeks diet 5-day LC50 600-650 Heath et al., (1970)c
quail
Bobwhite chick diet 5-day LC50 834 Heath & Stickel (1965)
quail
Mallard duckling diet 5-day LC50 563 Heath & Stickel (1965)
-----------------------------------------------------------------------------------------------
a Oral dosing - a single dose by gelatin capsule; dietary dosing for 5 days then 3 days
of uncontaminated food.
b Concentrations - in mg/kg body weight for oral dosing; in mg/kg diet for dietary dosing.
c Unpublished data.
Long-term toxicity of camphechlor for birds is not well
documented. Keith (1966) fed sardines injected with camphechlor
to white pelicans, giving the birds a daily dose of 10 or 50 mg/kg
diet for 3 months. The mortality rate was high in all groups, but
only the higher dose resulted in definite symptoms of poisoning
prior to death. At death, birds on insecticide diets had little
subcutaneous and mesentery fat. Dietary dosing of 6-month-old
female, ring-necked pheasants for up to 3 months at 25, 100, 200,
or 300 mg/kg diet did not cause death (Genelly & Rudd, 1956a). The
initial depression in body weight observed was attributed to reduced
feeding. Autopsy of mature birds dosed for 74 days with 100 or 300
mg/kg diet showed vacuolation in the livers.
Two studies on various parameters related to reproductive
success in birds did not show any effects at doses up to 100
mg/kg diet. Bush et al. (1977) dosed female domestic chickens
from 1 day of age up to maturity with one of 4 dose levels of
camphechlor, ranging from 0.5 to 100 mg/kg diet. No significant
effects were found on egg production, hatchability, or fertility.
Genelly & Rudd (1956b) dosed female, ring-necked pheasants with
camphechlor at either 100 or 300 mg/kg diet. Significant effects
were only noticed at the higher dose rate, with reductions in egg
laying and hatchability. "Relative reproductive success rate",
calculated on the basis of several reproductive parameters, was
70% for controls, 62% for birds given camphechlor at 100 mg/kg
diet, and 46% for birds given 300 mg/kg diet. In two studies on
egg hatchability (Dunachie & Fletcher, 1969; Smith et al., 1970);
camphechlor was injected into hens' eggs with no consistent
effects. Maximum doses given were 500 mg/kg egg weight in one
study and 1.5 mg/egg in the other. Haegele & Tucker (1974)
showed that a single oral dose of technical camphechlor at 10
mg/kg body weight did not significantly alter egg shell thickness
in Japanese quail. When bobwhite quail were given technical
grade camphechlor at 5, 50, or 500 mg/kg in their feed for 4
months, thyroid growth was stimulated and adrenal hypertrophy
occurred at all dose levels (Hurst et al., 1974). However, only
the highest dose increased uptake of I131. This dose also
decreased body weight. It is difficult to attribute these
effects to a direct thyroid lesion.
8.2.3. Wild animals
Tucker & Crabtree (1970) reported a 96-h LD50 for 16 to 17-
month-old male mule deer of 139 - 240 mg/kg body weight, when
camphechlor was administered orally as a single dose in a gelatin
capsule. The effect on in vitro fermentation of dry matter
(alfalfa hay) by inocula of rumen fluid from mule deer was
investigated by Schwartz & Nagy (1974). Inhibition was found at a
dose of 1000 mg camphechlor/kg dry matter. Accidental poisoning
occurred in a female Bengal tiger that ate a llama calf which had
been dipped in a solution of camphechlor. Symptoms included
periodic convulsions and hyperreflexia to sudden auditory and
visual but not to tactile stimuli (Peavy 1975).
8.2.4. Plants
Effects of camphechlor on higher plants have only been reported
for crop varieties. In greenhouse studies on cotton, using a sandy
or clay soil, Franco et al. (1960) found some toxicity at a dose
equivalent to a field application of 72.3 kg/ha, but only with
camphechlor applied as an emulsion. When applied as a powder, the
insecticide was not phytotoxic for cotton plants at a dose equivalent
to 101.5 kg/ha. Hagley (1965) studied the growth of seedlings of 3
vegetables, including Chinese cabbage and tomatoes, treated with
camphechlor at rates of 1.57 and 15.7 kg/ha, in the greenhouse.
Both levels inhibited shoot growth in Chinese cabbage. The higher
dose rate affected average weekly growth in all 3 cultivars, with
little root inhibition. A level of 15.7 kg/ha was toxic for tomato
seedlings, with 50% dying in the second, and a further 33% in the
third week of growth. Emergence, growth, yield, and chemical
composition of soybeans were not affected by the application of
camphechlor at 44.8 kg/ha, which was considerably higher than
recommended usage level (Probst & Everly, 1957).
8.3. Microorganisms
No effects were observed on either soil bacteria or fungi
when camphechlor was applied to the soil at 22.4 kg/ha, annually,
for 5 years (Martin et al., 1959), or twice yearly for 3 years,
at 16.8 kg/ha (Eno et al., 1964). Bollen et al. (1954) showed
that camphechlor stimulated mold numbers, 10 - 20 days after field
application at 11.2 kg/ha. A higher dosage of 22.4 kg/ha, after
the same period, did not show any effects on fungi. Bacteria were
not affected by either dosage.
In greenhouse studies on the effects of camphechlor on
nodulation bacteria, doses equivalent to between 4.9 and 40.3
kg/ha did not significantly affect bacterial growth or nodulation
of legumes (Elfadl & Fahmy, 1958). Treating soil with between
12.5 and 100 mg camphechlor/kg and incubating for between 1 and
16 months in a greenhouse, did not result in any measurable
effects on numbers of soil bacteria or fungi, but did stimulate
nitrification and carbon dioxide evolution after one month; after
16 months, there were no effects (Eno & Everett, 1958).
The results of studies on marine and freshwater unicellular
algae and protozoa are summarized in Table 7. The single study
on marine algae (Ukeles, 1962) showed species variability. The
dinoflagellate, Monochrysis lutheri, is the most sensitive,
showing total inhibition of growth at camphechlor concentrations
of 0.00015 mg/litre. The study by Stadnyk et al. (1971), whilst
showing little effect on cell numbers or biomass, demonstrated
marked stimulation of carbon fixation in the presence of
camphechlor. At a concentration of 0.1 mg/litre, there was an
initial 48% increase in carbon dioxide fixation by the green alga,
Scenedesmus quadricaudata, but this became insignificant later
in the 10-day exposure period. An initial increase of 450% had
fallen to 30%, after 10 days' exposure to 1 mg camphechlor/litre.
No explanation for this stimulation seems to be available. The
complex study by O'Kelley & Deason (1976) showed that camphechlor
at 0.01 mg/litre could inhibit growth in a few strains of algae
(which had been isolated from the Warrior River) and stimulate
growth in others. More strains showed inhibited growth at 0.1
mg/litre, but 6 out of the 21 strains used were unaffected, even by
the highest dose of 1.0 mg/litre.
Table 7. Toxicity of camphechlor for unicellular algae and protozoa
-----------------------------------------------------------------------------------------------------
Organism Temp Sex Sal. Species Expo- Concen- Effect Reference
(°C) o/oo sure tration
(days) (mg/litre)
-----------------------------------------------------------------------------------------------------
Unicellular 19.5- M 22- 10 0.00015- 100% inhibition Ukeles (1962)
algae 21.5 28 0.15 of growth
Blue-green 22 F Cylindrospermum 21 2a partially toxic, Palmer &
alga licheniforme reduced growth Maloney (1955)
Green alga 22 F Scenedesmus 21 2a partially toxic, Palmer &
obliquus reduced growth Maloney (1955)
up to 14 days
no effect on
growth after
14 days
Diatom 22 F Gomphonema 21 2a partially toxic, Palmer &
parvulum reduced growth Maloney (1955)
Alga 20- F Selenastrum 0.38 EC50 inhibition US EPA (1980)
22 capricornutum of growth
Green alga F Scenedesmus up to 1.0 19% decrease in Stadnyk et al.
quadricaudata 10 cell number, (1971)
no significant
effect on biomass
Protozoa F culture dominated 1 1.3 24-h LC50 Weber et al.
by ciliates; (1982)
( Eupoletes sp.)
and microflagellates
-----------------------------------------------------------------------------------------------------
a 2 mg/litre of a 60% solution of camphechlor.
F = Freshwater.
M = Marine.
The lowest concentrations of camphechlor inducing toxic
effects in protozoa from a salt marsh, were comparable to
concentrations found in the environment (Weber et al., 1982).
When a lake was treated with camphechlor (60%) at 0.1 mg/litre,
protozoan numbers were reduced almost to zero (Hoffman & Olive,
1961). Turbidity of the lake water decreased until the bottom
(at a depth of 4.5 m) was clearly visible.
8.4. Bioaccumulation and Biomagnification
Schoettger & Olive (1961), in a study to determine the
bioaccumulation of camphechlor, exposed planktonic alga (Scenedesmus
incrassatulus) cultures to 10 mg camphechlor/litre for 384 h. The
alga did not accumulate enough camphechlor to kill fish fed on it.
However, periphytons (various algae, diatoms, and ciliates), exposed
to the same dose of camphechlor, accumulated enough to kill fish
within 24 h. Sanborn et al. (1976) reported a concentration factor
of 6902 for an alga in a model ecosystem with a water concentration
of 44.4 µg/litre. Concentration factors of 9600 for the snail and
890 for the mosquito were also reported.
After applying camphechlor at 50 µg/litre, to eradicate rough
fish, Kallman et al. (1962) studied uptake by the rooted aquatic
weed, Potamogeton. After 5 days and 9 days, mean concentrations
in plants were 4.4 mg/kg and 14.6 mg/kg, respectively, which
represents concentration factors of 4400 and 14600 over the water
concentration of 1 µg/litre. Terriere et al. (1966) also found
accumulation of camphechlor by aquatic plants. Plants absorbed up
to 15.5 mg/kg from water containing 2 µg/litre; roots contained the
highest concentrations. Salt marsh cord grass, Spartina alterniflora,
growing near the discharge point of a camphechlor manufacturing plant,
was analysed for camphechlor residues by Reimold & Durant (1972).
They found maximum levels of 72.8 mg/kg in the leaves and lower
concentrations in other parts of the plant. In contrast, Reimold
(1974) using 36Cl-labelled camphechlor, found that plant roots
and rhizomes absorbed the largest amounts. The differences between
uptake in these studies may be because of differences between sea
water and marsh soil.
Oysters exposed to 1 µg/litre for 36 weeks bioaccumulated
camphechlor throughout the pre-spawning period (Lowe et al., 1971).
Concentrations in oyster tissues at 12, 24, and 36 weeks were 20
mg/kg, 23 mg/kg, and 8 mg/kg, respectively. Johnson (1966)
measured camphechlor residues in plankton from Big Bear Lake,
California, following 2 treatments with camphechlor at rates of
0.03 and 0.1 mg/litre, applied 2 weeks apart. Camphechlor
concentrations increased to a peak of 97 mg/kg, 114 days after the
final application. Concentrations decreased with time but, even
265 days after application, were still higher (at 2.0 mg/kg) than
the original treatment level. Tubificid worms may accumulate
camphechlor since worms collected in the Mississippi River delta
contained trace amounts of camphechlor.
However, worms exposed experimentally to camphechlor did not
accumulate enough to kill the fish to which they were fed (Naqvi,
1973).
Fish are able to absorb and bioconcentrate significant amounts
of camphechlor, a large proportion being complexed with the lipid
stores. Schaper & Crowder (1976) found that exposing mosquito fish
to a fatally-toxic level of 2 mg camphechlor/litre for 8 h did not
result in bioconcentration, the average concentration in whole fish
being 0.586 mg/kg. Thus, exposure for 8 h is not sufficient for
bioaccumulation to occur in fish. When 14C-labelled camphechlor
was added to a terrestrial-aquatic model ecosystem at 44.4 µg/litre
in the water, mosquito fish concentrated camphechlor by a factor of
4247 (Sanborn et al., 1976). Hughes (1970) exposed bluegills to
17.3 µg/litre or 70.3 µg/litre, and achieved camphechlor
concentrations in the fish of 9.4 mg/kg and 7.7 mg/kg, respectively,
after 96 h. Mayer et al. (1975) carried out several studies on
brook trout. Brook trout fry, when exposed to camphechlor
concentrations of 0.041 - 0.5 µg/litre, concentrated the chemical
between 4900 and 76 000 times. Large CF values, recorded after 15
days, were partially attributed to the presence of a yolk sac,
which contains large quantities of lipid. Falling values,
occurring after 60 days, were due to the disappearance of the yolk
sac, and the rise after 90 days to the increasing lipid content of
maturing fry. Yearling brook trout bioconcentrated less camphechlor
than fry during the 161-day exposure, achieving bioconcentration
factors of between 3200 and 16000.
Residues of camphechlor found in fish from camphechlor-treated
lakes are usually similar to levels measured in the laboratory,
indicating that free-living fish can bioconcentrate camphechlor by
a factor of several thousand. Kallman et al. (1962) studied the
uptake of camphechlor by rainbow trout and bullhead in Clayton
Lake, New Mexico, following an application of the pesticide.
Seventy-two hours after treatment, the average concentration of
camphechlor in the water was 2 µg/litre, and the concentration
factors were 1700 for trout and 2100 for bullheads. After 5 days,
the average water concentration of camphechlor was 11.5 µg/litre
and, at this concentration, bullheads contained mean body residues
of 13 mg/kg and were severely affected. After the water
concentration stabilized at 1 µg/litre, trout exposed to this water
had concentration factors ranging from 800 to 2500 after 7 days,
and factors of between 1300 and 3500 after 17 days. After 108 to
170 days exposure, trout showed body residues of 0.3 mg/kg, but the
water concentration had fallen to non-detectable levels.
In a similar study, Terriere et al. (1966) monitored the
relationship between camphechlor levels in 2 Oregon lakes (Davis
Lake treated with 80 µg camphechlor/litre and Miller Lake treated
with 40 µg/litre). In Davis Lake, residues were measured in
rainbow trout and Atlantic salmon; the trout consistently
accumulated more camphechlor than the salmon. As in experimental
exposures (Mayer et al., 1975), these fish concentrated
camphechlor by a factor of several thousand. In Miller Lake,
where the water concentration of camphechlor was 0.84 (0.70 -
1.1) µg/litre, brook trout, the only species monitored in this
lake, concentrated camphechlor 14 800 times.
Hughes & Lee (1973) found that predator fish accumulated less
camphechlor than prey fish. Bluegills and suckers, both prey
fish, accumulated whole body residues of 9.4 mg/kg and 10.6
mg/kg, respectively, whereas predator fish, i.e., bass, northern
pike, and walleyes, accumulated residues of only 2.2, 3.3, and
1.2 mg/kg, respectively. This suggests that the biomagnification
of camphechlor in aquatic food chains may not be as great as
might be indicated from bioconcentration factors.
Bioconcentration factors in a freshwater model ecosystem were
shown by Metcalf (1976) to be 5 for plankton, 176 - 1152 for
carp, 200 for insects, and 200 for crayfish.
There are no data on birds and mammals from which residue
levels can be related to exposure levels.
8.5. Population and Community Effects
The effects of the application of 0.1 mg camphechlor/litre to
control rough fish on the macroscopic bottom fauna of a lake in
Colorado were studied by Cushing & Olive (1956). During the first
3 weeks following treatment, treated and control lakes were sampled
fairly frequently and, thereafter, samples were taken approximately
every other week. Tendipedes were killed immediately, and no
larvae were found in the following 8 months. Chaoborus were not
immediately affected, but numbers began to decline 3 months later.
None were found 6 months after treatment. The authors speculated
that the Chaoborus, which prey on tendipedes, starved to death.
The number of oligochaetes increased, probably due to the extra
organic matter available. In the control lake, the numbers of
tendipedid, Chaoborus, and oligochaete remained fairly constant
throughout the sampling period. Chaoborus numbers fell in late
June/August when emergence was at its peak but, unlike the
experimental lake, repopulation occurred in September.
Needham (1966) studied plankton and aquatic invertebrates in 4
North Dakota lakes. The first lake (9.7 ha, depth 2.7 m) was
treated with 35 µg camphechlor/litre. Samples were taken prior to
treatment and 1 week, 1 month, and 1 year after treatment. Plankton
were monitored as numbers of organisms per litre. Keratella
decreased from 91 pre-treatment to 15, one week after treatment,
and subsequently to very low numbers. Asplanchna increased from
73 to 106, 1 week after treatment, but none were present in samples
taken 1 month or 1 year later. Numbers of Daphnia decreased from
244 to 18, 1 week after treatment, increasing to 129 after 1 month
but dropping to 28 after 1 year. Bosmina increased from 98 to 130
following treatment, but declined to 18 after 1 month and had only
risen to 25, 1 year later. Copepoda ( Diaptomus, Cyclops, and
undetermined nauplii) declined in numbers after treatment, but the
number of nauplii had begun to increase again, 1 year after
treatment. Five invertebrate genera that lived on water plants
were found in significant numbers. Gammarus varied throughout
the study but remained abundant. Callibaetis, Caenis, and Ischnura
decreased 1 week and 1 month after treatment, but were more abundant
after 1 year. Tendipes decreased from 44 (prior to treatment) to
9, 1 week after treatment, increasing to 48, 1 month after treatment
and falling back to 25, 1 year after treatment. Gastropoda (Physa
and Gyraulus) increased from 771 before treatment to 1107, 1 week,
1366, 1 month, and 1558, 1 year after treatment. In the lake bottom
fauna, only 2 genera were abundant; Gammarus, which fluctuated
greatly during the study and tendipedes, which decreased following
treatment but, 1 year later, had increased again to original numbers
in samples. Comparison between plant-inhabiting organisms and
bottom fauna did not reveal any shifting of populations, 1 year
after treatment, apart from Physa, which increased greatly.
The second lake studied by Needham (1966) (having a surface
area of 6 ha and a maximum depth of 5.5 m) was treated twice with
camphechlor, first with 25 µg/litre and then, 53 days later, with
90 µg/litre. Samples were taken 1 day before, and 11, 33, and 371
days after the initial treatment. Plankton were monitored as
numbers of organisms per litre. A decline in population was
recorded for all genera found in significant numbers. Brachionus,
which had decreased from 114 to 108 organisms/litre, 11 days after
treatment, decreased to 15 after 33 days and to 3 after 371 days.
The numbers of the other abundant rotifer, Asplanchna increased
from 24 to 194, 11 days after treament but dropped after 33 days to
16; by 371 days, there was only one organism/litre. Daphnia,
Ceriodaphnia, and Bosmina were the most abundant zooplankton.
Both Daphnia and Ceriodaphnia showed similar patterns, an
increase in numbers, 11 days after treatment, and a subsequent
decline. Bosmina had declined from 314 to 283, 11 days after
treatment, and continued to decline reaching 50 after 33 days; none
were found in the 371-day sample. Copepods also decreased in
numbers during the study. Although the number of Cyclops had
increased between 11 and 33 days, it decreased again after 371
days.
The third lake studied by Needham (1966) (a glacial lake of
370 ha and an average depth of 2.7 m) was treated with 10 µg
camphechlor/litre followed 2 days later by 5 µg/litre, and a
fourth lake (40.5 ha, maximum depth 4.9 m) was treated with 5
µg/litre. Results from this lake were similar to those from the
second; camphechlor failed to affect any of the organisms
sampled.
When sediments containing camphechlor were disturbed by
dredging, camphechlor was released into the water leading to
higher concentrations in aquatic flora and fauna, but this did
not appear to affect the biological balance in the area (Reimold
& Durant, 1974).
8.6. Effects on the Abiotic Environment
Five annual field applications of 22.4 kg camphechlor/ha did
not have any effect on the moisture content, total infiltration,
and aggregation of the soil (Martin et al., 1959).
9. PREVIOUS EVALUATIONS OF CAMPHECHLOR BY INTERNATIONAL BODIES
Camphechlor was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1968 and 1973 (FAO/WHO, 1969, 1974). In 1968,
no recommendations were made because of the many unresolved
questions. In 1973, the meeting concluded that it could not
establish an ADI for a material that varied in composition
according to the method of manufacture.
IARC (1979) evaluated the carcinogenic hazard resulting from
exposure to camphechlor and concluded that "there is sufficient
evidence that toxaphene is carcinogenic in mice and rats. In the
absence of adequate data in humans, it is reasonable, for
practical purposes, to regard toxaphene as if it presented a
carcinogenic risk to humans".
Practical advice has been issued by WHO/FAO (1975) in the
"Data Sheets on Pesticides", No. 20, which deals with
camphechlor, and includes information on labelling, safe-
handling, transport, storage, disposal, decontamination,
selection, training and medical supervision of workers, first
aid, and medical treatment.
Over recent years, a number of official registrations for the
use of camphechlor have been drawn in several countries, for
various reasons. Further details may be obtained from IRPTC.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be
obtained from the IRPTC (International Register of Potentially
Toxic Chemicals) Legal File (IRPTC, 1983).
IPRTC (1983), in its series "Scientific reviews of Soviet
literature on toxicity and hazards of chemicals", issued a review
on camphechlor.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Camphechlor Toxicity
The acute toxicity of camphechlor in the rat is moderate,
i.e., the oral LD50 ranges from 60 to 120 mg/kg body weight.
Camphechlor is readily absorbed via all routes of entry. It is
metabolized in the body, but a certain amount of accumulation in
adipose tissue takes place on continuous exposure.
Signs and symptoms of poisoning are salivation and vomiting
and, at higher exposures, excitation of the CNS with convulsions,
respiratory failure, and death. On prolonged exposure of
animals, hypertrophy of the liver with induction of microsomal
enzymes (5 mg/kg in the diet or 0.25 mg/kg body weight per day)
are the major findings.
Camphechlor has been shown not to have an effect on reproduction
and it is not teratogenic. It is mutagenic in a bacterial test, but
the results of a dominant lethal test in mice were negative. There
is sufficient evidence for its carcinogenicity for rats and mice.
Epidemiological studies are inadequate to evaluate the carcinogenic
potential of camphechlor for human beings.
10.2. Exposure to Camphechlor
Exposure of the general population is mainly through residues
in food. However, these are normally below residue tolerances.
Accidental over-exposure has occurred as a result of contamination
of food with camphechlor.
In the past, occupational exposure to camphechlor may
occasionally have been considerable. Nevertheless, only a few
cases of adverse effects have been reported. Workplace exposures
above the permissible level have been reported.
10.3. Effects on the Environment
Movement of camphechlor through the environment is not
clearly understood, but the dominant factor in the distribution
of the chemical appears to be its high volatility. Camphechlor
is a widespread contaminant of aquatic ecosystems. Residues have
also been found in non-aquatic organisms.
Camphechlor has been used extensively as a fish poison to
clear rough fish from lakes before introducing game fish. The
toxic effects have not only been directly on adult fish but also,
via adult females, on the development of eggs and young. Non-
target aquatic organisms have also been affected. Some
invertebrates showed long-term deleterious effects of camphechlor
poisoning. The environmental levels of camphechlor in waters
where the chemical has not been deliberately applied can exceed
laboratory concentrations that have caused death or sublethal
lesions in experimental fauna. Thus, camphechlor presents a major
hazard for aquatic organisms.
Available field data suggest that birds have been adversely
affected by camphechlor.
10.4. Conclusions
1. Although no serious adverse effects on workers resulting
from occupational exposure to camphechlor have been reported,
and epidemiological studies remain inadequate, this chemical
should be considered for practical purposes as being
potentially carcinogenic for human beings.
2. For the same reason, and recognizing the limitations of
residue analysis for camphechlor, as well as the reluctance of
the JMPR to issue an ADI, reservations must remain about the
safety of this chemical in food, despite the relatively low
residues so far reported.
3. Environmentally, camphechlor is a major hazard for aquatic
and also some terrestrial species.
4. Taking into account these considerations, it is felt that
the use of this chemical should be discouraged, except where
there is no adequate alternative.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations Procambarus actus in South-Central Texas. Bull.
env. Contam. Toxicol., 8: 334-338.
ALEXANDER, M. (1965) Persistence of chlorinated hydrocarbon
insecticides in soils. Soil Sci. Soc. Am. Proc., 29: 1-7.
ANDERSON, L.D. & ATKINS, E.L. (1968) Pesticide usage in
relation to beekeeping. Annu. Rev. Entomol., 13: 213-238.
ARSCOTT, G.H., MICHENER, S., & BILLS, D.D. (1976) Effect of
toxaphene on the performance of white leghorn layers and their
progeny. Poult. Sci., 55: 1130-1131.
ASHIROVA, S.A. (1971) Work hygiene and effectiveness of
health measures in the manufacture of chlorinated terpenes.
Nauch. Tr. Leningrad Gos. Instit. Usoversh. Vrachei, 98: 26-30
(in Russian; Chem. Abstr., 79: 13949).
BADAEVA, L.N. (1976) The effect of polychlorocamphene on
some organs and their nervous apparatus in pregnant animals.
Bull. exp. Biol. Med., 82: 945-947.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect
of field treatments of insecticides on number of bacteria,
Streptomyces, and molds in soil. J. econ. Entomol., 47:
302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and
resistance of mosquito fish to several insecticides. J. econ.
Entomol., 57: 430-431.
BOYD, E.M. (1972) Protein deficiency and pesticide toxicity,
Springfield, Illinois, Charles C. Thomas Publishing Company,
p. 231.
BRADLEY, J.R., SHEETS, T.J., & JACKSON, M.D. (1972) DDT and
toxaphene movement in surface water from cotton plots.
Environ. Qual. Saf., 1: 102-105.
BURKE, W.D. & FERGUSON, D.E. (1969) Toxicities of four
insecticides to resistant and susceptible mosquito fish in
static and flowing solutions. Mosq. News, 29: 96-101.
BUSH, P.B., KIKER, J.T., PAGE, R.K., BOOTH, N.H., & FLETCHER,
O.J. (1977) Effects of graded levels of toxaphene on poultry
residue accumulation, egg production, shell quality and
hatchability in white leghorns. J. agric. food Chem., 25:
928-932.
BUTLER, P.A. (1962) Effects on commercial fisheries,
Washington DC, US Department of the Interior, Fish and
Wildlife Service (Circular No. 143).
BUTLER, P.A. (1963) Commercial fisheries investigations,
pesticide-wildlife studies: a review of Fish and Wildlife
Service investigations during 1961 and 1962, Washington DC, US
Department of the Interior, Fish and Wildlife Service, p. 11
(Circular No. 167).
BUTLER, P.A. (1964) Pesticide-wildlife studies 1963: a
review of Fish and Wildlife Service investigations during the
calendar year, Washington DC, US Department of the Interior,
Fish and Wildlife Service, p. 5 (Circular No. 199).
BUTLER, P.A., WILSON, A.J., & RICK, A.J. (1962) Effects of
pesticides on oysters. Proc. Natl Shellfisheries Assoc., 51:
23-32.
CANADA, DEPARTMENT OF NATIONAL HEALTH AND WELFARE (1978)
Guidelines for Canadian drinking water quality, Ottawa,
Department of National Health and Welfare.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide
residues in urban soils from 14 United States cities, 1970.
Pestic. Monit. J., 10: 54-60.
CASIDA, J.E., HOLMSTEAD, R.L., KHALIFA, S., KNOX, J.R.,
OHSAWA, T., PALMER, K.J., & WONG, R.Y. (1974) Toxaphene
insecticide: A complex biodegradable mixture. Science, 183:
520-521.
CHAIYARACH, S., RATANANUN, V., & HARREL, R.C. (1975) Acute
toxicity of the insecticides toxaphene and carbaryl and the
herbicides propanil and molinate to four species of aquatic
organisms. Bull. environ. Contam. Toxicol., 14: 281-284.
CHERNOFF, N. & CARVER, B.D. (1976) Fetal toxicity of
toxaphene in rats and mice. Bull. environ. Contam. Toxicol.,
15: 660-664.
CHANDURKAR, P.S. & MATSUMURA, F. (1979) Metabolism of
toxicant B and toxicant C of toxaphene in rats. Bull. environ.
Contam. Toxicol., 21: 539-547.
CLAYBORN, H.V., MANN, H.D., IVEY, M.C., REDELEFF, R.D., &
WOODARD, G.T. (1963) Excretion of toxaphene and strobane in
the milk of dairy cows. J. agric. food Chem., 11: 286-289.
CLAPP, K.L., NELSON, D.M., BALL, J.T., & ROUSEK, E.J. (1971)
Effects of toxaphene on the hepatic cells of rats. Proc. Ann.
Meeting W. Section Am. Soc. Animal Sci., 22: 313-323.
COFFIN, D.E. & McCLEOD, H.A. (1975) Current pesticide
residue status of Canadian diets and adipose tissue. In:
Seventh Eastern Canada Symposium on Pesticide Residue Analysis
Proceedings, Toronto, May 20-23.
CONLEY, B.E. (1952) Pharmacological properties of toxaphene,
a chlorinated hydrocarbon insecticide. J. Am. Med. Assoc.,
149: 1135-1137.
COPE, O.B. (1965) Sport Fishery Investigations. In: The
effect of pesticides on fish and wildlife, Washington DC, US
Department of the Interior, Fish and Wildlife Service, pp.
51-64 (Circular No. 226).
COURTENAY, W.R. & ROBERTS, M.H. (1973) Environmental effects
on toxaphene toxicity to selected fishes and crustaceans,
Washington DC, US Environmental Protection Agency, 73 pp (US
EPA Report EPA/R3-73-035).
CROWDER, L.A. & DINDAL, E.F. (1974) Fat of 36Cl-toxaphene in
rats. Bull. environ. Contam. Toxicol., 12: 320-327.
CUSHING, C.E., & OLIVE, J.R. (1956) Effects of toxaphene and
rotenone upon macroscopic bottom fauna of two Northern
Colorado reservoirs. Trans. Am. Fish. Soc., 86: 294-301.
DAHLEN, J.H. & HAUGEN, A.O. (1954) Acute toxicity of certain
insecticides to the bobwhite quail and mourning dove. J.
Wildl., 18: 477-481.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, E.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of Na+K+-ATPase in rainbow trout
Bull. environ. Contam. Toxicol., 8: 69-72.
DEICHMANN, W.B., ed. (1973) The chronic toxicity of
organochlorine pesticides in man. In: Deichmann, W.B., ed.
Pesticides and the environment: selected papers presented at
the 8th International American Conference on Toxicology and
Occupational Medicine, University of Miami, Miami, Florida,
July, 1973, pp. 347-420.
DESAIAH, D. & KOCH, R.B. (1975) Toxaphene inhibition of
ATPase activity in catfish, Ictalurus punctatus tissues. Bull.
environ. Contam. Toxicol., 13: 238-244.
DESAIAH, D., MEHROTA, B.D., KINZEY, R., & TROTTMAN, C.H.
(1979) Effects of toxaphene, kepone and mirex on key
gluconeogenic enzymes in rat liver. Fed. Proc., 8: 538.
DOMANSKI, J.J., HAIRE, P.L., & SHEETS, T.J. (1974)
Insecticide residues on 1973 US tobacco products. Tobacco
Sci., 18: 108-109.
DOMANSKI, J.J. & GUTHRIE, F.E. (1974) Pesticide residues in
1972 cigars. Bull. environ. Contam. Toxicol., 11: 312-314.
DOMANSKI, J.J. & SHEETS, T.J. (1973) Insecticide residues on
1970 US auction market tobacco. Tobacco, 175: 29.
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of
the toxicity of insecticides to birds eggs using the egg-
injection technique. Ann. appl. Biol., 64: 409-423.
DURANT, C.J. & REIMOLD, R.J. (1972) Effects of estuarine
dredging of toxaphene-contaminated sediments in Terry Creek,
Brunswick, Georgia, 1971. Pestic. Monit. J., 6: 94-96.
ELFADL, M.A. & FAHMY, M. (1958) Effect of DDT, BHC, and
toxaphene on nodulation of legumes and soil microorganisms.
Agric. Res. Rev., 36: 339-350.
EMBRY, T.L. (1972) Search for abnormalities of heme
synthesis and sympathoadrenal activity in workers regularly
exposed to pesticides. Tex. Rep. Biol. Med., 30: 203.
ENO, C.F. & EVERETT, P.H. (1958) Effects of soil
applications of 10 chlorinated hydrocarbon insecticides on
soil microorganisms and the growth of stringless Black
Valentine beans. Proc. Soil Sci. Soc. Am., 22: 235-238.
ENO, C.F., WILSON, J.W., CHILSHOLM, R.D., & KOBLITSKY, L.
(1964) The effect of soil applications of six chlorinated
hydrocarbon insecticides on vegetable crops and soil
microorganisms. Proc. Florida State Hort. Soc., 77: 223-229.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
EYER, J.R., FALKNER, L.R., & MCCARTY, R.T. (1953) Effect of
toxaphene and DDT on geese in cotton fields. New Mexico Agric.
Exp. Sta., Bull., 1078: 1-6.
FAO/WHO (1969) Toxaphene. In: 1968 evaluations of some
pesticide residues in food, pp. 267-283.
FAO/WHO (1974) Camphechlor. In: 1973 evaluations of some
pesticide residues in food, pp. 92-118.
FATTAH, I.C.M.A. & CROWDER, L.A. (1980) Plasmamembrane
ATPC-ases from various tissues of the cockroach (Periplanita
americana) and mouse influenced by toxaphene. Bull. environ.
Contam. Toxicol., 24: 356-363.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P.
(1964) Resistance to chlorinated hydrocarbon insecticides in
three species of freshwater fish. Bioscience, 14: 43-44.
FERGUSON, D.E., CULLEY, D.D., & COTTON, W.D. (1965)
Tolerances of two populations of fresh water shrimp to five
chlorinated hydrocarbon insecticides. J. Miss. Acad. Sci., 11:
235-237.
FITZHUGH, O.G. & NELSON, A.A. (1951) Comparison of chronic
effects produced in rats by several chlorinated hydrocarbon
insecticides. Fed. Proc., 10: 295.
FRANCO, C.M., FRAGA, C.C., & NEVES, O.S. (1960) [Effects of
the addition of insecticides to the soil on the development of
cotton crops.] Bragantia, 19: 13-25 (in Portugese).
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 5-534.
GENELLY, R.E. & RUDD, R.L. (1956a) Chronic toxicity of DDT,
Toxaphene and Dieldrin to ring-necked pheasants. Calif. Fish
Game, 42: 5-14.
GENELLY, R.E. & RUDD, R.L. (1956b) Effects of DDT, Toxaphene
and Dieldrin on pheasant reproduction. AUK, 73: 529-539.
GLEASON, M.N., GOSSELIN, R.E., HODGE, H.C., & SMITH, R.P.,
ed. (1969) Clinical toxicology of commercial products -
acute poisoning, 3rd ed., Baltimore Maryland, Williams and
Wilkins Company, Section II, Ingredients Index.
GOSSELIN, R.E., HODGE, H.C., SMITH, R.P., & GLEASON, M.N.
(1976) Clinical toxicology of commercial products, Baltimore,
Maryland, Williams and Wilkins Company, p. 189.
GRAVES, J.B. & MACKENSEN, O. (1965) Topical application and
insecticide resistance studies on the honey bee. J. econ.
Entomol., 58: 990-993.
GRIFFIN, D.E. & HILL, W.E. (1978) in vitro breakage of
plasmid DNA by mutagens and pesticides. Mutat. Res., 52:
161-169.
GRUBER, R. (1959) Toxicité pour Tilapia melanopleura de deux
insecticides: l'Endrine et la Toxaphene. Bull. Agr. Congo
Belge, 50: 131-139.
GUYER, G.E., ADKISSON, P.L., DUBOIS, K., MENZIE, C.,
NICHOLSON, H.P., ZWEIG, G., & DUNN, C.L. (1971) Toxaphene
status report. Special report to Hazardous Materials Advisory
Committee, Washington DC, US Environmental Protection Agency.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutatnt on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGLEY, E.A.C. (1965) Effects of insecticides on the growth
of vegetable seedlings. J. econ. Entom., 58: 777-778.
HARRIS, R.L. & JONES, R.H. (1962) Larvicide tests with
colony-reared Culicoides variipennis. J. econ. Entomol., 55:
575-576.
HAUN, E.C. & CUETO, C. (1967) Fatal toxaphene poisoning in a
9-month-old infant. Am. J. Dis. Child, 113: 616-618.
HEATH, R.G. & STICKEL, L.F. (1965) Protocol for testing the
acute and relative toxicity of pesticides to penned birds,
Washington DC, US Department of the Interior, US Fish and
Wildlife Service, pp. 18-24 (Circular No. 226).
HEATH, R.G., et al. (1970) Unpublished data.
HENDERSON, C.F., PICKERING, Q.H., & TARZWELL, C.M. (1959)
Relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HOFFMAN, D.A. & OLIVE, J.R. (1961) The effects of rotenone
and toxaphene upon plankton of two Colorado reservoirs.
Limnol. Oceanogr., 6: 219-222.
HOLMSTEAD, R.L., KHALIFA, S., & CASIDA, J.E. (1974)
Toxaphene composition analyzed by combined gas
chromatography-chemical ionization mass spectrometry. J.
agric. food Chem., 22: 939-944.
HOOPER, F.F. & GRZENDA, A.R. (1955) The use of toxaphene as
a fish poison. Trans. Am. Fish Soc., 85: 180-190.
HOOPER, N.K., AMES, B.N., BALEK, M.A., & CASIDA, J.E. (1979)
Toxaphene, a complex mixture of polychloroterpenes and a major
insecticide, is mutagenic. Science, 205: 591-593.
HUDSON, R.H., TUCKER, R.K., & HAEGALE, M.A. (1972) Effect of
age on sensitivity. Acute oral toxicity of 14 pesticides to
mallard ducks of several ages. Toxicol. appl. Pharmacol., 22:
556-561.
HUGHES, R. (1970) Studies on the persistence of toxaphene in
treated lakes, PhD thesis, Ann Arbor, Michigan, Xerox
University Microfilms, pp 7-24, 751.
HUGHES, R.A. & LEE, G.F. (1973) Toxaphene accumulation in
fish in lakes treated for rough fish control. Environ. Sci.
Technol., 7: 934-939.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some
effects of DDT, toxaphene and polychlorinated biphenyl on
thyroid function in bobwhite quail. Poult. Sci., 53: 125-133.
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer, pp. 327-348
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IRPTC (1983a) Toxaphene. IRPTC Bull., 5: 27-28.
IRPTC (1983b) Scientific reviews of Soviet literature on
toxicity and hazards of chemicals, Toxaphene, Moscow, Centre
of International Projects (GKNT, No. 32).
IRPTC (1983c) Legal file, Vols. 1 & 2, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
JEFFERY, W.H., AHLIN, T.A., GOREN, C., & HARDY, W.R. (1976)
Loss of warfarin effect after occupational insecticide
exposure. J. Am. Med. Assoc., 236: 2881-2882.
JOHANSEN, C.A. & DAVIS, H.G. (1972) Toxicity of nine
insecticides to the western yellowjacket. J. econ. Entomol.,
65: 40-42.
JOHNSON, W.C. (1966) Toxaphene treatment of Big Bear Lake,
California. Calif. Fish Game, 52: 173-179.
JOHNSTON, B.L. & EDEN, W.G. (1953) The toxicity of aldrin,
dieldrin and toxaphene to rabbits by skin absorption. J. Econ.
Entomol., 46: 702-703.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970)
Insecticide levels in human tissues of Alberta residents. Can.
J. Public Health, 61: 413-416.
KALLMAN, B.J., COPE, O.B., & NAVARRE, R.J. (1962)
Distribution and detoxication of Toxaphene in Clayton Lake,
New Mexico. Trans. Am. Fish. Soc., 91: 14-22.
KATZ, M. (1961) Acute toxicity of some organic insecticides
to three species of salmonids and to the three-spined
stickleback. Trans. Am. Fish. Soc., 90: 264-268.
KEISER, R.K., Jr, JULIA, A.A.A., & RAFAEL, M.S. (1973)
Pesticide levels in estuarine and marine fish and
invertebrates from the Guatemalan Pacific Coast. Bull. Marine
Sci., 23: 905-924.
KEITH, J.O. (1966) Insecticide contaminations in wetland
habitats and their effects on fish-eating birds. J. appl.
Ecol. Suppl., 3: 71-85.
KENNEDY, G.L., FRAWLEY, J.P., & CALANDRA, J.C. (1973)
Multigeneration reproductive effects of three pesticides in
rats. Toxicol. appl. Pharmacol., 25: 589-596.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects
of combinations of pesticides on reproduction in mice. In:
Inter-American Conference on Toxicology and Occupational
Medicine. Pesticide Symposia, pp. 125-138.
KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P. (1966)
Quantitative measurement of induction of hepatic microsomal
enzymes by various dietary levels of DDT and toxaphene in
rats. Toxicol. appl. Pharmacol., 9: 505-513.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty
insecticides to striped bass Morone saxatilis. Calif. Fish
Game, 60: 128.
KUZMINSKAYA, U.A. & IVANITSKI, I.V.A. (1979) Study of some
biological indices of the state of the sympathoadrenaline
system under the effect of polychlorocampher. Environ. Health
Perspect., 30: 91-95.
LACKEY, R.W. (1949) Observations on the acute and chronic
toxicity of toxaphene in the dog. J. ind. Hyg. Toxicol., 3:
117-120.
LAFLEUR, K.S., WOJECK, G.A., & MCCASKILL, W.R. (1973)
Movement of toxaphene and fluoneturon through Dunbar soil to
underlying ground water. J. environ. Qual., 2: 515-518.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II. Dermal Toxicity. Assoc. Food
Drug Offic. US, Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food &
Drug Officials on current developments. Section III. Subacute
and chronic toxicity. Assoc. Food Drug Offic. US, Q. Bull.,
16: 47-53.
LOWE, J.I. (1964) Chronic exposure of spot, Leiostomus
xanthurus, to sublethal concentrations of toxaphene in sea
water. Trans. Am. Fish. Soc., 93: 396-399.
LOWE, J.I., WILSON, P.D., RICK, A.J., & WILSON, A.J. (1971)
Chronic exposure of oysters to DDT, toxaphene, and parathion.
Proc. Natl Shellfisheries Assoc., 61: 71-79.
LUEDEMANN, D. & NEUMANN, H. (1960) [Acute toxicity tests
with new contact insecticides in young carp (Cyprinus carpio
L.).] Z. Angew. Zool., 47: 11-33 (in German).
LUKE, M.A., FROBERG, J.E., & MASUMOTO, H.T. (1975)
Extraction and clean-up of organochlorine, organophosphate,
organo-nitrogen, and hydrocarbon pesticides in produce for
determination by gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem., 58: 1020-1026.
MACEK, K.J. & McALLISTER, W.A. (1970) Insecticide
susceptibility of some common fish family representatives.
Trans. Am. Fish. Soc., 99: 20-27.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) Effects of
temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MAHDI, M.A. (1966) Mortality of some species of fish to
toxaphene at three temperatures. Invest. Fish Contr., 6: 3-10.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in
total diet samples. VIII. Pestic. Monit. J., 9: 94.
MARTIN, J.P., HARDING, R.B., CANNELL, G.H., & ANDERSON, L.D.
(1959) Influence of five annual applications of organic
insecticides on soil biological and physical properties. Soil
Sci., 87: 334-338.
MAYER, F.L. & MEHRLE, P.M. (1976) Vitamin C distribution in
channel catfish as affected by toxaphene. Toxicol. appl.
Pharmacol., 37: 167-168.
MAYER, F.L., MEHRLE, P.M., & DWYER, W.P. (1975) Toxaphene
effects on reproduction, growth and mortality of brook trout,
Washington DC, US Environmental Protection Agency, Office of
Research and Development, 43 pp (US EPA 600/3-75-013).
MCEWEN, L.C., KNITTLE, C.E., & RICHMOND, M.L. (1972)
Wildlife effects from grasshopper insecticides sprayed on
short-grass range. J. Range Manage., 25: 188-194.
MCGEE, L.C., REED, H.L., & FLEMING, J.P. (1952) Accidental
poisoning by toxaphene: Review of toxicology and case reports.
J. Am. Med. Assoc., 149: 1124-1126.
MEHRLE, P.M. & MAYER, F.L. (1975a) Toxaphene effects on
growth and bone composition of fathead minnows, Pimephales
promelas. J. Fish. Res. Board Can., 32: 593-598.
MEHRLE, P.M. & MAYER, F.L. (1975b) Toxaphene effects on
growth and development of brook trout. (Salvelinus fontinalis)
J. Fish. Res. Board Can., 32: 609-613.
MENZIE, C.M. (1972) Fate of pesticides in the environment.
Ann. Rev. Entomol., 17: 199-222.
METCALF, R.L. (1976) Organochlorine insecticides: survey and
prospects. In: Metcalf, R.L. & McKelvey, J.J., ed. Future for
insecticides: needs and prospects. Advances in the
environmental science and technology, Vol. 6.
MUNSON, T.O. (1976) A note on toxaphene in environmental
samples from the Chesapeake Bay region. Bull. environ. Contam.
Toxicol., 16: 491-494.
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of
selected freshwater invertebrates. J. Miss. Acad. Sci., 14:
121-127.
NAQVI, S.M. & FERGUSON, D.E. (1970) Levels of insecticide
resistance in freshwater shrimp, Palaemontes kadiakensis.
Trans. Am. Fish. Soc., 99: 696-699.
NASH, R.C. & WOOLSON, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science, 157: 728-732.
NCI (1979b) Bioassay of toxaphene for possible
carcinogenicity, Cas Registry No. 8001-35-2, National Cancer
Institute (Technical Report Series No. 37-1979).
NEEDHAM, R.G. (1966) Effects of toxaphene on plankton and
aquatic invertebrates in North Dakota lakes, US Department of
the Interior, Fish and Wildlife Service, Bureau of Sport
Fishing and Wildlife (Resource Publication No. 8).
NICHOLSON, H.P., GRZENDA, A.R., LAUER, G.J., COX, W.S., &
TEASLEY, J.I. (1964) Water pollution by insecticides in an
agricultural river basin. I. Occurrence of insecticides in
river and treated municipal water. Limnol. Oceanogr., 9: 310.
NIOSH (1977b) NIOSH manual of analytical methods Part 1. In:
NIOSH monitoring methods, Vol. 1, 2nd ed., Method No. P Cam
225, Washington DC, US Department of Health, Education and
Welfare, pp. 225-1-256-6 (US DHEW(NIOSH), Publication No.
77-157B).
OHSAWA, T., KNOX, J.R., KHALIFA, S., & CASIDA, J.E. (1975)
Metabolic chlorination of toxaphene in rats. J. agric. food
Chem., 23: 98-106.
O'KELLEY, J.C. & DEASON, T.R. (1976) Degradation of
pesticides by algae, Athens, Georgia, US Environmental
Protection Agency, 42 pp (Report EPA-600/3-76-022).
OLSON, K.L., MATSUMURA, F., & BOUSH, G.M. (1980) Behavioral
effects on juvenile rats from perinatal exposure to low-levels
of toxaphene 6, its toxic components, toxicant-A and
toxicant-B. Arch. environ. Contam. Toxicol., 9: 247-257.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic
changes in the liver of rats after feeding low levels of
various insecticides. AMA. Arch. Pathol., 64: 614-622.
PALMER, C.M. & MALONEY, T. E. (1955) Preliminary screening
for potential algicides. Ohio J. Sci., 55: 1-8.
PARDINO, R.S., HEIDKER, J.C., & PAYNE, B. (1971) The effects
of some cyclodiene pesticides, benzenehexachloride and
toxaphene on mitochondrial electron transport. Bull. environ.
Contam. Toxicol., 6: 436-444.
PEAKALL, D.B. (1976) Effects of toxaphene on hepatic enzyme
induction and circulating steriod levels in the rat. Environ.
Health Perspect., 13: 117-120.
PEAKALL, D.B. (1979) Effect of toxaphene on pyruvic and
lactic acid levels in the rat. Environ. Health Perspect., 30:
97-98.
PEAVY, G.M. (1975) Use of diazepam and methocarbamol in the
treatment of toxaphene poisoning in a Bengal tiger. J. Am.
Vet. Med. Assoc., 167: 577-578.
PENUMARTHY, L., OEHME, F.W., SPAULDING, J.E., & RADER, W.A.
(1976) Toxaphene: livestock toxicity and tissue residues.
Vet. Tox., 18: 60-70.
POLLOCK, R.W. (1953) Toxaphene poisoning - Report of a fatal
case. Northw. Med. (Seattle), 52: 293-294.
POLLOCK, G.A. & KILGORE, W.W. (1976) The metabolism of
toxaphene components. Toxicol. appl. Pharmacol., 37: 138-139.
POLLOCK, G.A. & KILGORE, W.W. (1978) Toxaphene. Res. Rev.,
69: 87-140.
POLLOCK, G.A. & KILGORE, W.W. (1980) Toxicities and
description of some toxaphene fractions - isolation and
identification of a highly toxic component. J. Toxicol.
environ. Health, 6: 115-125.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil
insecticides on emergence, growth, yield and chemical
composition of soybeans. Agron. J., 49: 385-387.
REIMOLD, R.J. (1974) Toxaphene interactions in estuarine
ecosystems, Springfield, Virginia, National Technical
Information Service, 89 pp (COM-75-10104/8GA).
REIMOLD, R.J. & DURANT, C.J. (1972) Toxaphene levels in
Georgia survey of estuaries, Georgia, Georgia Marine Science
Center, University of Georgia, 51 pp (Technical Report No.
72-2).
REIMOLD, R.J. & DURANT, C.J. (1974) Toxaphene content of
estuarine fauna and flora before, during and after dredging
toxaphene-contaminated sediments. Pestic. Monit. J., 8: 44-49.
REUBER, M.D. (1979) Carcinogenicity of toxaphene. J.
Toxicol. environ. Health, 5: 729-748.
SAMOSH, L.V. (1974) Chromosome aberrations and character of
satellite associations after accidental exposure of the human
body to polychlorocamphene. Toxicol. Genet., 8: 24-27.
SANBORN, J.R., METCALF, R.L., BRUCE, W.N., & LU, P-Y. (1976)
The fate of chlordane and toxaphene in a terrestrial-aquatic
model ecosystem. Environ. Entomol., 5: 533-538.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several
pesticides to two species of Cladocerans. Trans. Am. Fish
Soc., 95: 165-169.
SCHAPER, R.A. & CROWDER, L.A. (1976) Uptake of
36Cl-toxaphene in mosquito fish, Gambusia affinis. Bull.
environ. Contam. Toxicol., 15: 581-587.
SCHIMMEL, S.C., PATRICK, J.N., & FORESTER, J. (1977) Uptake
and toxicity of toxaphene in several estuarine organisms.
Arch. environ. Contam. Toxicol., 5: 353-367.
SCHOETTGER, R.A. & OLIVE, J.R. (1961) Accumulation of
Toxaphene by fish-food organisms. Limnol. Oceanogr., 6:
216-219.
SCHWARTZ, C.C. & NAVY, J.G. (1974) Pesticide effects on in
vitro dry matter digestion in deer. J. Wildl. Manage., 38:
531-534.
SHELANSKI, H.A. (1947) Various untitled reports and letters
submitted to Hercules Powder Co., Smith Laboratories
(unpublished).
SMITH, D.C. (1971) Pesticide residues in the total diet in
Canada. Pestic. Sci., 2: 92-95.
SMITH, D.C., SANDI, E., & LEDUC, R. (1972) Pesticide
residues in the total diet in Canada II-1970. Pestic. Sci., 3:
207-210.
SMITH, D.C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide
residues in the total diet in Canada III-1971. Pestic. Sci.,
4: 211-214.
SMITH, S.I., WEBER, C.W., & REID, B.C. (1970) The effect of
injection of chlorinated hydrocarbon pesticides on
hatchability of eggs. Toxicol. appl. Pharmacol., 16: 179-185.
STADNYK, L., CAMPBELL, R.S., & JOHNSON, B.T. (1971)
Pesticide effect on growth and 14C assimilation in a
freshwater alga. Bull. environ. Contam. Toxicol., 6: 1-8.
STALLING, D.L. & HUCKINS, J.N. (1976) Analysis and GC-MS
characterization of toxaphene in fish and water, Washington
DC, US Environmental Protection Agency (US EPA 600/3-76-076).
STANLEY, C.W., BARNEY, J.E., II, HELTON, M.R., & YOBS, A.R.
(1971) Measurement of atmospheric levels of pesticides.
Environ. Sci. Technol., 5: 430-435.
TAYLOR, J.R., CALABRESE, V.P., & BLANKE, R.V. (1979)
Organochlorine and other insecticides. In: Vinken, P.J. &
Bruyn, G.W., ed. Handbook of clinical neurology, Vol. 36
Intoxications of the nervous system, Part. 1, Amsterdam, New
York, Elsevier/North-Holland Biomedical Press.
TERRIERE, L.C. & INGALSBE, D.W. (1953) Translocation and
residual action of soil insecticides. J. econ. Entomol., 46:
751.
TERRIERE, L.C., KIIGEMAGI, U., GERLACK, A.R., & BOROVICKA,
R.L. (1966) The persistence of Toxaphene in lake water and
its uptake by aquatic plants and animals. J. agric. food
Chem., 14: 66-69.
TEWARI, S.N. & SHARMA, I.C. (1977) Isolation and
determination of chlorinated organic pesticides by thin-layer
chromatography and the application to toxicological analysis.
J. Chromatogr., 131: 275-284.
TORCHIO, P.F. (1973) Relative toxicity of insecticides to
the honey bee, alkali bee and alfalfa leafcutting bee
(Hymenoptera, Apidae, Halictidae, Megachilidae). J. Kans.
Entomol. Soc., 46: 446-453.
TREON, J.F., CLEVELAND, F.P., POYNTER, B., WAGNER, B., &
GAHEGAN, T. (1952) The physiologic effects of feeding
experimental animals on diets containing toxaphene in various
concentrations over prolonged periods, Kettering Laboratory
(unpublished report).
TROTTMAN, C.H. & DESAIAH, D. (1980) Induction of rat hepatic
microsomal enzymes by toxaphene pretreatment. J. environ. Sci.
Health (Part B: Pesticides, food Contam. agric. Wastes), 15:
121-134.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, US Department of the Interior, Fish
and Wildlife Service, Bureau for Sport Fishing and Wildlife,
pp. 116-117 (Resource Publication No. 84).
UKELES, R. (1962) Growth of pure cultures of marine
phytoplankton in the presence of toxicants. Appl. Microbiol.,
10: 532-537.
US EPA (1976a) Criteria document for toxaphene. Fed. Reg.,
440: 9-76-014.
US EPA (1976b) Economic assessment of proposed toxic
pollutant standards for manufacturers and formulators of
aldrin/dieldrin, endrin, DDT and toxaphene, Washington DC, US
Environmental Protection Agency (Prepared by Arthur D. Little
Inc. for Environmental Protection Agency) (US EPA
230/3-76-016).
US EPA (1979) Reviews of the environmental effects of
pollutants. X. Toxaphene, Washington DC, US Environmental
Protection Agency (US EPA Report 600/1-79-044).
US EPA (1980) Ambient water quality criteria for toxaphene,
Washington DC, US Environmental Protection Agency (Report US
EPA 440/5-80-076).
VON RUMKER, R., LAWLESS, L., MEINER, A.F., LAWRENCE, K.A.,
KELSO, G.L., & HORAY, F. (1974) Production, distribution,
use and environmental impact potential of selected pesticides,
Kansas City, Missouri, Midwest Research Institute, pp. 1-439
(NTIS Publication No. 236).
WARRAKI, S. (1963) Respiratory hazards of chlorinated
camphene. Arch. environ. Health, 1: 253-256.
WEBER, F.H., SHEA, T.B., & BERRY, E.S. (1982) Toxicity of
certain insecticides to protozoa. Bull. environ. Contam.
Toxicol., 28: 628-631.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, M., & CONNEY,
A.H. (1971) Effect of halogenated hydrocarbon insecticides
on the metabolism and uterotropic action of estrogens in rats
and mice. Toxicol. appl. Pharmacol., 19: 234-246.
WHO/FAO (1975) Pesticide residues in food, Vol. 97, pp. 7-37
(Technical Report Series No. 574).
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972a)
Pesticide residues in onions and soil - 1969. Pestic. Monit.
J., 5: 345-347.
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972b) Pesticide
residues in soil from eight cities - 1969. Pestic. Monit. J.,
6: 126-129.
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972c) Pesticide
residue levels in soils, FY 1969 - National Soils Monitoring
Program. Pestic. Monit. J., 6: 194-201.
WORKMAN, G.W. & NEUHOLD, J.M. (1963) Lethal concentrations
of toxaphene for goldfish, mosquito fish and rainbow trout,
with notes on detoxification. Prog. Fish Cult., 25: 23-30.
YANG, H.S.C., WIERSMA, G.B., & MITCHELL, W.G. (1976)
Organochlorine pesticide residues in sugarbeet pulps and
molasses from 16 states, 1971. Pestic. Monit. J., 10: 41-43.
YAP, H.H., DESAIAH, D., CUTKAMP, L.K., & KOCH, R.B. (1975)
In vitro inhibition of fish brain ATPase activity by
cyclodiene insecticide and related compounds. Bull. environ.
Contam. Toxicol., 14: 163-167.
ZAVON, M.R., TYE, R., & LATORRE, L. (1969) Chlorinated
hydrocarbon insecticide content of the neonate. Ann. N.Y.
Acad. Sci., 160: 196-200.
ZWEIG, G., PYE, E.L, SITLANI, R., & PEOPLES, S.A. (1963)
Residues in milk from dairy cows fed low levels of toxaphene
in their daily ration. J. agric. food Chem., 11: 70-72.
Molecular formula: C10H10Cl8 (approximately)
CAS chemical name: Toxaphene (a mixture of poly-
chlorinated bicyclic terpenes with
chlorinated camphenes predominating)
Trade names: Altox, Chem-Phene M5055, Chlor Chem
T-590, Crestoxo, Estonox, Fasco-
Terpene, Geniphene, Gy-Phene,
Hercules 3956, Huilex, Penphene,
Phenacide, Phenatox, Polychlor-
camphen, Strobane-T, Toxakil,
Toxaphene, Toxon 63
CAS registry number: 8001-35-2
Relative molecular mass: 413.8 (average)
2.2. Physical and Chemical Properties
Camphechlor is an amber, waxy solid (Canada, Department of
National Health and Welfare, 1978) with a melting range of
65 - 90 °C. Its vapour pressure is 3.3 x 10-5 mm Hg at 20 -
25 °C. Camphechlor may dechlorinate in the presence of
alkali, sunlight (UV radiation) or at temperatures above
120 °C (Canada, Department of National Health and Welfare,
1978; IARC, 1979). It is soluble in common organic solvents
but practically insoluble in water (0.4 - 3.0 mg/litre)
(Metcalf, 1976).
The exact chemical structure has not yet been elucidated
(von Rumker et al., 1974). The technical product is a complex
mixture of chlorinated bicyclic terpenes containing 67 - 69%
chlorine by weight (Canada, Department of National Health and
Welfare, 1978), which result from the chlorination of pine
resins. Camphechlor has been separated into at least 177
C10-polychloro compounds, including Cl6, Cl7, Cl8, Cl9, and
Cl10 derivatives, using absorption and gas-liquid
chromatography (Casida et al., 1974, Holmstead et al., 1974).
2.3. Analytical Methods
Much difficulty has been encountered in the determination of
camphechlor residues due to the fact that camphechlor is not a
single compound, but a mixture of over 177 compounds. In addition,
it is often used in conjunction with other pesticides, which may
cause interference in analytical procedures for camphechlor
residues.
Several analytical methods used for the determination of
camphechlor in air, water, soil, food, and animal tissues are
summarized in Table 1 (IARC, 1979).
Table 1. Methods for the determination of camphechlor
-----------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Reference
extraction/clean-up method detection
-----------------------------------------------------------------------------------
Air
workplace trap on cellulose membrane, GC/ECD 0.225 - NIOSH (1977b)
extract (petroleum ether) 1.155 mg/m3
Water
fish-tank extract (acetone-petroleum GC/ECD 10 µg/litre Stalling &
ether) in a special Huckins
syphon system, wash (1976)
resulting water-acetone layer
(petroleum ether), bulk
petroleum ether fractions, CC
Soil
moisten (water), extract GC/ECD 0.05 - Carey et al.
(hexane-isopropanol), wash 0.1 mg/kg (1976)
(water)
Food
molasses dilute (water), extract GC/ECD 0.03 mg/kg Yang et al.
(hexane-isopropanol) (1976)
fruits and extract (acetone) in blender, GC/ECD Luke et al.
vegetables filter, extract (petroleum (1975)
ether-dichloromethane), wash
aqueous phase
(dichloromethane), bulk
solvent extracts, evaporate
to small bulk, add acetone,
reduce volume and repeat, CC
Biological
tissues macerate, add anhydrous TLC 1 µg Tewari &
sodium sulfate, extract Sharma (1977)
(acetone), add water,
extract (chloroform)
wash (potassium
hydroxide solution)
-----------------------------------------------------------------------------------
CC = column chromatography;
GC = gas chromatography;
ECD = electron capture detection;
TLC = thin-layer chromatography.
3. PRODUCTION AND USES, TRANSPORT AND DISTRIBUTION
3.1. Production and Uses
Camphechlor does not occur naturally in the environment
(Canada, Department of National Health and Welfare, 1978).
Camphechlor has been in use since 1949 and, in 1975, it was the
most heavily-used insecticide in the USA (Pollock & Kilgore,
1980). In 1969, 168 uses for camphechlor were registered in the
USA.
Production in the USA has been estimated to range from 22 700
- 40 800 tonnes per year (Canada, Department of National Health
and Welfare, 1978). Von Rumker et al. (1974) gave the following
annual figures for the USA: total production 34 200 tonnes, of
which 8000 tonnes was exported and 26 500 tonnes was used in the
USA: 450 tonnes for industrial application and 25 650 tonnes for
agricultural use. Estimated consumption in the USA was 9360
tonnes in 1980 and 5400 tonnes in 1982. Since late 1982, use in
the USA has been limited to scabies control on cattle and sheep,
insect control on pineapples and bananas in the Virgin Islands
and Puerto Rico, and for emergency use against army worms and
grasshoppers on cotton, corn, and grain. These uses are
restricted to certified spray operators wearing protective
clothing (IRPTC, 1983a). Registered uses in Canada are minimal
and are decreasing.
Camphechlor is a non-systemic contact and stomach insecticide
with some acaricidal action. The primary crops on which camphechlor
is used are cotton, cereal grains, fruits, nuts, oil seeds, and
vegetables. Use on livestock is primarily for the control of ticks
and mites. The use of camphechlor on crops has not been a serious
problem for bees fertilizing the treated crops (WHO, 1975).
Camphechlor is often mixed with other pesticides and appears to act
as a solubilizer for insecticides with low solubility. The
synergistic properties of camphechlor when used with some other
insecticides have been reported (von Rumker et al., 1974).
Camphechlor is often applied together with DDT or methyl or ethyl
parathion (WHO, 1975).
3.2. Transport and Distribution
3.2.1. Air
The major route of removal of camphechlor from the soil is
through evaporation (von Rumker et al., 1974). Although there is
little information on the atmospheric transport of camphechlor,
it is likely that it may cover hundreds of miles. Camphechlor
levels in 75 out of 880 air samples from different parts of the
USA ranged between 68 and 2520 ng/m3 (Stanley et al., 1971).
Five out of 8 rainwater samples taken in Maryland, USA, contained
camphechlor (Munson, 1976).
3.2.2. Water
Accumulation of camphechlor occurs in water in areas where the
insecticide is in use and it may be quite persistent. It has been
found in water in some lakes, in toxic concentrations, for up to 5
years after fish have been killed (Canada, Department of National
Health and Welfare, 1978). Camphechlor was not found in drinking-
water supplies in Canada above a detection level of 0.01 mg/litre
(Canada, Department of National Health and Welfare, 1978). In the
USA, camphechlor concentrations were usually less than 0.001
mg/litre; nevertheless, concentrations as high as 0.065 mg/litre
were found in some areas where cotton had been sprayed several
months earlier (Bradley et al., 1972). Camphechlor concentrations
of up to about 2 g/kg were found in the sediment of a stream that
received the effluent of a camphechlor-manufacturing plant (Durant
& Reimold, 1972). Camphechlor residues in the range of 7 - 410
ng/litre were also present in all water samples taken before and
after treatment by a water treatment plant (Nicholson et al., 1964).
Although the majority of the residues are volatilized, levels
in runoff from soil surfaces or treated plants may be substantial
(von Rumker et al., 1974).
3.2.3. Soil
As camphechlor is applied topically to livestock and
agricultural crops, very little of the pesticide gets mixed into
the soil. However, that which penetrates into the soil becomes
tightly bound to soil particles and is then highly resistant to
leaching (von Rumker et al., 1974). Residues adsorbed on soil
particles may be transported via soil erosion and sediment
transport. The downward migration of camphechlor in soil is not
significant. The results of a study assessing the vertical
distribution of camphechlor indicated that, after 3 years, 85 -
90% of the remaining pesticide was in the top 23 cm of the soil
(WHO, 1975).
The half-life of camphechlor in soil has been found to vary
from 70 days (in moist, sandy soil) to 179 days (in moist clay
soil). The half-life in these same soils, if dried, became 136
and 705 days, respectively (WHO, 1975). In another study,
involving the mixing of camphechlor with soil, 45% of the
original application remained after 14 years (Nash & Woolson,
1967). Other authors have determined that the half-life of
camphechlor in soil ranges from 100 days (LaFleur et al., 1973)
up to 6 and 12 years (Alexander, 1965; Nash & Woolson, 1967;
Menzie, 1972). The disparity among these findings may be
explained by variations in soil type and climates.
No evidence has been found to suggest the existence of
conversion products of camphechlor in weathered crop residues
(WHO, 1975). However, camphechlor is rapidly removed from crops
by weathering and volatilization.
In the USA, the average level of camphechlor in cropland soil
is 0.07 mg/kg. A study on 969 soil samples from cropland soils
from 43 states and on non-cropland soils from 11 states showed
camphechlor residues in only 4.2% of the sites examined, despite
the fact that all the major camphechlor-using areas were included
in the cropland states surveyed. In the 199 non-cropland soil
samples, camphechlor occurred only once (Wiersma et al.,
1972a,b,c).
3.2.4. Abiotic degradation
Camphechlor is broken down by sunlight (ultraviolet radiation)
and high temperatures. It is also biologically degraded by soil
bacteria and fungi, the bacteria utilizing it as a source of carbon
(WHO/FAO, 1975). No definitive data have been published on the
degradation found in camphechlor (Metcalf, 1976).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Because the concentrations of camphechlor in ambient air
in unsprayed areas are in the ng/m3 range (IARC, 1979), it
seems unlikely that it would cause any health hazard.
4.1.2. Water
Only bodies of water that have been treated with camphechlor
for fish control or receive runoff from manufacturing sites or
cropland areas treated with camphechlor have been found to
contain significant amounts of camphechlor.
The lower limit of detection of camphechlor by taste is
approximately 5 µg/litre. Water treated with activated carbon (a
method used to clear water bodies of accidental camphechlor
spills) is effectively free of camphechlor (WHO, 1975). No
damage to man resulting from camphechlor residues in water has
been reported.
4.1.3. Food
Camphechlor bioaccumulates and builds up in food chains, but
not to the same extent as some other highly persistent chlorinated
hydrocarbons (von Rumker et al., 1974). For the most part,
camphechlor levels in food seem to be below the national tolerances
(WHO, 1975), which range from 2 - 7 mg/kg in Australia, Canada, and
the USA, and are as low as 0.4 mg/kg in the Federal Republic of
Germany and the Netherlands (WHO, 1975).
Camphechlor was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1968 and 1973 (FAO/WHO, 1969, 1974). In 1968,
no recommendations were made because of the many unresolved
questions. In 1973, the meeting concluded that it could not
establish an ADI for a material that varied in composition
according to the method of manufacture.
Because camphechlor is not a systemic insecticide, the
residues are more or less confined to the plant surfaces (WHO,
1975). Camphechlor residues generally occur in agricultural
crops as well as in meat from domestic livestock and fish.
Thirty-one days following an application of camphechlor to
alfalfa, 72.9% of the compound had been lost. Long-term losses
depended on the quantity applied, the formulation, and the
application route. The greatest residue was left from oily
solutions, followed by water emulsion, and by dusts (Pollock &
Kilgore, 1978). Camphechlor residues on growing leafy crops have
a half-life of 5 - 10 days. On alfalfa and clover, the half-life
is in the range of 9 - 13 days. The residue level is controlled
by time of application, dosage rate, and appropriate pre-slaughter
or pre-harvest intervals (WHO, 1975).
Fruits and vegetables have been found to contain camphechlor
residues; the highest concentrations were found in spinach
(FAO/WHO, 1969). In studies conducted during 1964-67, the average
levels of camphechlor residues found in leaf and stem vegetables
was 0.18 mg/kg and that in processed foods 0.45 mg/kg. Generally,
levels of camphechlor in all foods were low. In 1964-66,
camphechlor residues were detected in 30, 8, and 2% of the cotton-
seed, soybean, and peanut oil samples listed, respectively (Pollock
& Kilgore, 1978). Heat processing of fruits and vegetables was
found to reduce levels of camphechlor residues (WHO, 1975). Sugar
beet, sampled in the USA in the autumn of 1970, showed camphechlor
residues of 0 - 0.34 mg/kg (Yang et al., 1976). It has been
demonstrated that peeling, abrasive peeling, and washing removes
most of the camphechlor residues present on fruits and vegetables.
The addition of 0.1% synthetic detergent or 1.0% neutral soap to
the washing water increases the amount of the pesticide removed
(WHO, 1975).
Honey produced by bees exposed to 36Cl-camphechlor
contained less than 0.01 mg camphechlor/kg (WHO, 1975).
Because livestock is treated with camphechlor to remove
external insects and because some feeds are contaminated with
camphechlor, camphechlor residues are found in meat and poultry.
The residues in meat can be significantly reduced by trimming
excess fat (the primary storage site for camphechlor) and also by
cooking at temperatures sufficient to render out the fat (WHO,
1975). In animals, the level of storage of camphechlor is lower
and its elimination more rapid than with most other chlorinated
hydrocarbons (FAO/WHO, 1974), hence residues in poultry and meat
are very low (WHO, 1975). In general, the level stored in the
fat of sheep and cattle is 25 - 50% of the level in the feed.
This concentration is less in hogs, probably because of a greater
fat content (FAO/WHO, 1969).
Camphechlor has been detected in the milk from cows sprayed or
fed this insecticide (Clayborn et al., 1963; Canada, Department of
National Health and Welfare, 1978). The ratio of camphechlor
concentration in feed to that in milk is approximately 100:1.
Build-up in milk quickly fades when exposure to camphechlor is
discontinued (WHO, 1975). Residues of camphechlor in milk, due to
feed containing 5 - 20 mg camphechlor/kg, plateaued after 28 days.
Residues in milk, due to feed containing 2.5 mg camphechlor/kg,
plateaued by the 9th day. Milk contained 0.11 and 0.18 mg
camphechlor residues/litre when feed containing 10 and 20 mg/kg,
respectively. The residues in milk fell off sharply, 4 days after
camphechlor was absent from the diet and were negligible by the
14th day (Zweig et al., 1963). In the USA, camphechlor was
detected in only 5 out of 7265 meat samples and 2 out of 5504
poultry samples (Guyer et al., 1971).
In a study carried out by the Department of National Health
and Welfare, Canada, between 1969 and 1971, no detectable levels
(at the ppb level) of camphechlor were found in any of the
several hundred food items analysed (Smith, 1971; Smith et al.,
1972, 1973), while, in another study carried out by the
Department of National Health and Welfare, Canada, between 1974
and 1975, unspecified amounts of camphechlor were found in 0.6%
of the food items tested (Coffin & McLeod, 1975); thus, the
incidence was very low.
In the USA, between 1971 and 1972, among a total of 420
samples of food analysed, only one was found to contain
camphechlor (Manske & Johnson, 1975). In a study conducted in
the USA during the period of 1964-69, residues of camphechlor
were found to be the 6th most frequent in occurrence of all
pesticides in processed foods. However, levels were so low that
few, if any, exceeded the 7 mg/kg tolerance level (WHO, 1975).
4.1.4. Miscellaneous sources
Camphechlor residues have also been found in non-food items.
Camphechlor residues in chewing tobacco, smoking tobacco, snuff,
cigarettes, and cigars in the USA were found to have decreased
over the period 1971-73. The average concentration in cigarettes
decreased from 3.3 to 1.4 mg/kg (Domanski et al., 1974). In
1972, examinations showed that 6 brands of cigars purchased in 5
cities in the USA contained camphechlor residues ranging from 0.5
to 3.42 mg/kg (Domanski & Guthrie, 1974), while results of a
study conducted in 1970 (Domanski & Sheets, 1973), showed
camphechlor residues in tobacco in the USA to be as high as 12
mg/kg.
4.1.5. Wildlife
Residues of camphechlor were found in 53% of samples of fish
and invertebrates taken from waters near cotton-producing areas
along the Guatemalan Pacific Coast (Keiser et al., 1973). No
changes in camphechlor levels were observed when fish containing
5 - 10 mg/kg camphechlor were boiled or fried (Terriere &
Ingalsbe, 1953).
Residues have been found infrequently and at low levels in
non-aquatic wildlife (Pollock & Kilgore, 1978).
4.2. General Population Exposure
Camphechlor exposure of the general population can result
from residues in food.
4.3. Occupational Exposure
Results of a study conducted in camphechlor-manufacturing
plants in the USSR showed camphechlor levels 5 - 6 times the
permissible limit of 0.2 mg/m3. After a work shift, levels of
30 - 1000 mg/m2 were found on the uncovered skin of workers,
while levels on covered areas ranged up to 40 mg/m2 (Ashirova,
1971). However, no adverse effects of this occupational exposure
have been reported. Although camphechlor was used extensively
during the 1960s in agricultural and public health programmes in
Alberta, analyses of human tissue from 50 autopsies at the
University Hospital in Edmonton in 1967-68 did not reveal
camphechlor in any of the 217 tissues examined (Kadis et al.,
1970).
5. KINETICS AND METABOLISM
5.1. Human Studies
In a 9-month-old child, poisoned with a 2:1 mixture of
camphechlor and DDT, death occurred after convulsions and
respiratory failure. Ratios of camphechlor:DDT in the brain and
liver were 10:1 and, in the kidney, 3:1 (Haun & Cueto, 1967). No
significant levels of camphechlor were found in the skin and
subcutaneous tissue taken from 68 children, who died in the
perinatal period, in 13 cities in the USA (Zavon et al., 1969).
5.2. Animal Studies
The metabolism of camphechlor has been an area of little
research, because of difficulties in detecting a complex and
multicomponent substance. However, a few such studies have been
published.
About 37% of a single oral dose of technical grade
36Cl-camphechlor, administered to rats at 20 mg/kg body
weight, was eliminated in the faeces; 15% was excreted in the
urine in 9 days during the same period (Crowder & Dindal,
1974). Rats were dosed orally with solutions containing
either 36Cl-camphechlor, 36Cl-camphechlor fractions, or
14C-camphechlor. Urine and faeces samples were collected for
14 days (Ohsawa et al., 1975). The rats excreted (combined
urine and methanol extract of faeces) 76% of the 36Cl-camphechlor,
57% of the 14C-camphechlor, and 69 - 94% of the 36Cl-camphechlor
fractions. Very little of the material was excreted unmetabolized
and the camphechlor had undergone dechlorination.
A dose-related increase in the excretion of camphechlor in
the milk of cows, fed diets containing camphechlor at 2.5 - 20
mg/kg, was reported (Zweig et al., 1963). Results of in vitro
studies, using rat liver homogenates, showed that isolated
camphechlor fractions were metabolized to hydroxylated compounds,
in addition to dechlorinated products (Chandurkar & Matsumura,
1979). However, the precise chemical structures of these
metabolites were not unequivocally identified. Pollack & Kilgore
(1976) dosed rats orally with 14C-camphechlor and reported a
cumulative elimination of 58% of the dose in the urine and
faeces. Increased amounts of polar "activity" and a small
increase in non-polar fractions were found in the fat.
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single Exposures
Camphechlor is absorbed through the skin, respiratory tract,
and the intestinal tract (FAO/WHO, 1969; Gleason et al., 1969;
Gosselin et al., 1976).
The principal toxic manifestations noted in animals exposed
to a single dose of camphechlor consist of salivation, vomiting,
reflex excitability, and convulsions terminating in respiratory
failure (Taylor et al., 1979). Typical LD50 values for a variety
of animals are given in Table 2. The influence of the solvent on
camphechlor uptake can be seen from the wide range of LD50s in
this table.
Table 2. The acute toxicity of camphechlor in several animal studies
-----------------------------------------------------------------------------
Species Route Vehicle LD50 (mg/kg) References
-----------------------------------------------------------------------------
Rat oral peanut oil 80 - 90 Gaines (1969)
Rat dermal xylene 780 - 1075 Gaines (1969)
Rat oral corn oil 60 US EPA (1976a)
Rat oral kerosene 120 US EPA (1976a)
Mouse oral corn oil 112 US EPA (1976a)
Dog oral corn oil 49 US EPA (1976a)
Dog oral kerosene over 250 US EPA (1976a)
Guinea-pig oral kerosene 365 US EPA (1976a)
Cat oral peanut oil 25 - 40 US EPA (1976a)
Rabbit oral peanut oil 75 - 100 US EPA (1976a)
Rabbit oral kerosene 250 - 500 US EPA (1976a)
Rabbit dermal dust over 4000 US EPA (1976a)
Rabbit dermal peanut oil over 250 US EPA (1976a)
Rabbit dermal wettable powder 1025 - 1075 Johnston &
(suspension in water) Eden (1953)
Cattle oral grain 144 US EPA (1976a)
Goat oral xylene 200 US EPA (1976a)
Sheep oral xylene 200 US EPA (1976a)
-----------------------------------------------------------------------------
The acute toxicity of camphechlor in rats is increased at least
3-fold by protein deficiency (Gleason et al., 1969). The lethal
oral dose for an adult man is estimated to be 2 -7 g (Conley, 1952).
6.2. Short-Term Exposures
Rats were fed camphechlor at levels of 0.2 - 50 mg/kg diet,
for up to 13 weeks (Kinoshita et al., 1966). Camphechlor was
observed to induce microsomal enzyme activity at levels of 5.0
mg/kg and higher.
Camphechlor did not induce any effects on the physical
appearance, gross pathology, weight gain, or liver cell histology
of albino rats fed 2.33 - 189 mg/kg diet, for up to 12 weeks
(Clapp et al., 1971).
Camphechlor, when fed to rats for 7 days at 25 mg/kg diet,
caused an increase in the metabolism of estrone and inhibited the
increase in uterine weight produced by this compound (Welch et
al., 1971).
Studies on pregnant albino rats showed that daily oral
administration of camphechlor for up to 20 days resulted in
interconnected structural and enzymic changes in the cerebral
cortex (Badaeva, 1976).
Rats were dosed once, orally, with 120 mg camphechlor/kg body
weight and monitored for up to 15 days (Peakall, 1976). Liver
weight and microsomal enzyme activity were increased after 5 and
15 days, respectively. In another study in the same report, rats
were administered camphechlor at 2.4 mg/kg body weight per day
and killed at 1, 3, and 6 months. Liver weight and microsomal
enzyme activity increased at all time intervals, but plasma
testosterone levels were not affected.
Administration to rats of a single oral dose of 120 mg/kg
body weight, as well as a daily dose of 2.4 mg/kg body weight,
for 1 or 3 months, produced a disturbance in catecholamine
metabolism (Kuzminskaya & Ivanitski, 1979).
Adult male rats were fed diets containing 0, 50, 100, 150,
and 200 mg camphechlor/kg for 14 days (Trottman & Desaiah, 1980).
No changes were observed in body weight gain, food consumption,
brain, kidney, heart, or testes weights; liver weight was
increased at 200 mg/kg and thymus weight decreased at 150 and 200
mg/kg. Increased hydroxylation of aniline was observed at all
dose levels.
Administration of 5, 50, or 500 mg camphechlor/kg diet to
quail for up to 4 months produced hypertrophy of the thyroid with
increased uptake of 131I and adrenal hypertrophy (Hurst et al.,
1974).
Feeding of camphechlor in the diet at 5, 50, or 100 mg/kg to
chickens for 31 weeks induced sternal deformation and nephrosis
(Bush et al., 1977). Occasional keel deformation, involving
cartilaginous tissue as well as an apparent increase in the
growth of cartilage, was found in birds fed 0.50 mg camphechlor/kg.
Camphechlor was administered in capsules at a daily dose of 4
mg/kg body weight to 2 dogs for 44 days and to 2 other dogs for 106
days. Occasional manifestations of acute toxicity (CNS stimulation)
occurred for a short time after administration. There were no
significant changes in body weight, blood picture, or gross
appearance of organs. Histological examination of many organs
revealed some damage to the kidney (degeneration of the tubular
epithelium) and to the liver (generalized hydropic degenerative
changes, but no destruction of the cells). Liver glycogen levels
were normal (Lackey, 1949).
6.3. Long-Term Exposures
Four groups each of 40 rats (20 males and 20 females), were
fed camphechlor in the diet at 10, 100, 1000, and 1500 mg/kg.
Effects were determined through gross observation, mortality
rates, body weight, blood tests, liver weight, liver to body-
weight ratio, gross autopsy, and histological examination of the
tissues. After 7 1/2 - 10 months of feeding, some of the rats
fed 1500 mg/kg and a few of the rats fed 1000 mg/kg suffered
occasional convulsions. The body weight gain of the rats fed the
highest feeding level (1500 mg/kg) for the first 20 weeks was
less than that in the controls, probably because of decreased
food intake through unpalatability of the diet. As the rats
became accustomed to the diet, growth rate was essentially the
same as that of the controls. There were no significant effects
on mortality rate or on the haematopoietic system. The liver
weight and liver to body weight ratio was significantly increased
only in the 1000 and 1500 mg/kg groups. Liver changes consisted
of swelling, cellular hypertrophy, and proliferation of smooth
endoplasmic reticulum with cytoplasmic margination in the
centrilobular hepatic cells. These changes occurred to a
moderate extent in the 1500 mg/kg group and to a slight extent in
the 1000 mg/kg group (Treon et al., unpublished data, 1952).
Six male and 6 female rats per group were fed 50 or 200 mg
camphechlor/kg diet for up to 9 months (Ortega et al., 1957).
There were no clinical signs of toxicity and no effects on body
weight gain, food intake, or liver weights. No histological
changes were seen in the kidney or spleen. Three out of 12 rats
fed 50 mg/kg diet for 9 months showed histological changes in the
liver consisting of centrilobular cell hypertrophy, peripheral
migration of basophilic granulation, and the presence of
liposphere inclusion bodies. Six of the 12 rats fed 200 mg/kg
showed liver changes.
Groups of rats were fed camphechlor in the diet at 25,
100, and 400 mg/kg for their lifetime (Fitzhugh & Nelson,
1951). The only organ that showed significant histological
changes was the liver and these occurred at the 100 mg/kg and 400
mg/kg levels. There was centrilobular hepatic cell enlargement
with increased oxyphilia and peripheral margination of basophilic
granules. The effects at the various feeding levels were summarized
by Lehman (1952b), as follows: 400 mg/kg, liver enlargement; 100
mg/kg, no gross effects, but tissue damage occurred; and 25 mg/kg,
no tissue damage.
Camphechlor dissolved in maize oil, in gelatin capsules, was
administered daily, for 5 days per week, to dogs. A dose of 25
mg/kg body weight was fatal. Two dogs were administered 10 mg/kg
(equivalent to 400 mg/kg diet); one dog died after 33 days, but
the other lived and was sacrificed after 3 1/2 years. Four dogs
were administered 5 mg/kg; all survived and were sacrificed after
almost 4 years. No information on the pathological findings was
reported (Lehman, 1952b).
Camphechlor was fed daily (6 days per week) for 2 years to
3 male and 5 female dogs, beginning when the animals were
approximately 4 months old. Camphechlor was added to the
diet, including their drinking liquids, at levels of 10 and
50 mg/kg. The dogs received a daily dose of 0.60 - 1.47 mg/kg
or 3.12 - 6.56 mg/kg (equivalent to approximately 40 or
200 mg/kg on a dry diet basis). Gross behaviour, body weight,
mortality, peripheral circulating blood elements, gross
pathology, organ to body weight ratios, and histopathology
were recorded (Treon et al., unpublished data, 1952). There
were no effects on behaviour, body weight, mortality, or blood
elements, but there were increases in the liver weights, liver
to body weight ratios, and moderate liver degeneration at the
higher dose level (200 mg/kg). At the lower dose level
(40 mg/kg), 1 out of 3 dogs was reported to have slight liver
enlargement and slight granularity and vacuolization of the
cytoplasm. Re-examination of the sections of these animals
failed to confirm any difference from control animals. All
other tissues were normal at both feeding levels (Brock &
Calandra, 1964).a
Groups of dogs, comprising 6 male and 6 females, were fed
camphechlor at dietary levels of 5, 10, or 20 mg/kg together with
control groups. Two male and 2 female animals were sacrificed
after 6, 12, and 24 months. None of the feeding levels produced
any changes in organ weights, gross or histological examination,
or any of the clinical or organ function tests, at any time
(Industrial Bio-Test Laboratories Inc., unpublished data, 1965)b.
---------------------------------------------------------------------
a Brock, D. & Calandra, J.C. (1964) Re-evaluation of
microscopic sections from chronic oral toxaphene study,
Industrial Bio-Test Laboratories, Inc., unpublished report.
b Industrial Bio-Test Laboratories Inc. (1965) Two-year
chronic oral toxicity of toxaphene in beagle dogs
(Unpublished report).
Two adult female monkeys were given camphechlor in their
food, 6 days per week, at a daily dose of 0.64 - 0.78 mg/kg for 2
years. A third animal served as a control. There were no signs
of intoxication and no evidence of tissue or organ damage as
evaluated by growth rate, ratios of liver to body weight, spleen
to body weight, or histological examination of the tissues (Treon
et al, unpublished data, 1952).
6.4. Dermal Toxicity
The acute LD50 for rabbits exposed dermally to camphechlor
in the dry form was > 4000 mg/kg body weight (Lehman, 1952a).
The animals showed only moderate skin irritation and systemic
effects were characterized by hyperexcitability but no deaths
occurred. The animals recovered in 5 or 6 days. The 90-day
dermal LD50 has been estimated to be approximately 40 mg/kg
body weight for rabbits (Lehman, 1952a).
A review of the effects of camphechlor on livestock
revealed that cattle, sheep, and hogs can tolerate repeated
applications of solutions containing less than 2% camphechlor
(Penumarthy et al., 1976). However, dips containing 2.5%
camphechlor emulsion caused toxicosis in cattle.
6.5. Reproduction Studies
A 3-generation, 6-litter reproduction study was conducted with
camphechlor. Groups of weanling rats were fed 25 mg/kg diet and
100 mg/kg diet for 79 days before mating. All the animals continued
on their respective dietary concentration of camphechlor during
mating, gestation, and weaning during 2 generations, or for a
period of 36 - 39 weeks. Weanlings from the second litter were
selected as parents for the second generation and continued on
their respective diets until after weaning of a second litter. A
third generation was selected in the same manner. Complete gross
and histological examination was performed on all three parental
generations after 36 weeks of camphechlor administration. The only
pathological changes found were slight alterations in the livers of
the 100 mg/kg group, similar to the changes seen in long-term
studies. Reproductive performance, fertility, and lactation were
normal. The progeny was viable and normal in size and anatomical
structure. Findings among all test animals, 3 parental generations
and 6 litters of progeny, were comparable to those in control
animals for all variables (Kennedy et al., 1973).
Groups of mice (4 males and 14 females per group) were fed
camphechlor in the diet at levels of 0 and 25 mg/kg in a 5-
generation, 2 litter-per-generation, reproduction study. No
effects were noted on any of the reproduction parameters measured
(Keplinger et al., 1970).
Camphechlor in a corn-oil carrier was injected into fertile
hen eggs. It did not cause any reduction in hatchability, even
at the highest dose used, 1.5 mg/egg (Smith et al., 1970). In
another study, white leghorn hens were fed camphechlor at 0, 10,
and 100 mg/kg diet (Arscott et al., 1976). Except for a slight
decrease in egg production, no adverse effects were observed on
fertility, hatchability, or survival of progeny.
6.6. Mutagenicity
Camphechlor did not induce dominant lethal mutations in mice
(Epstein et al., 1972). When males were injected with 36 and 180
mg/kg body weight ip and bred with untreated females during 8
weeks, the frequencies of early fetal deaths and preimplantation
losses were normal. Negative results were also obtained in animals
treated orally for 5 successive days with 40 or 80 mg/kg body
weight. In an in vitro test to determine breakage of DNA in
bacteria, camphechlor did not induce breaks at a significantly
higher rate than that occurring in controls (Griffin & Hill, 1978).
Camphechlor was mutagenic in a test using Salmonella typhimurium
without requiring activation by liver homogenate (Hooper et al.,
1979).
6.7. Teratogenicity
Camphechlor was administered to mice and rats on days 7 -
16 of gestation by oral intubation at levels of 15 - 35 mg/kg
body weight per day (Chernoff & Carver, 1976). The highest
dose produced marked maternal mortality in rats and mice and
an increase in encephaloceles among offspring of mice. Fetal
mortality was slightly increased in mice at all dose levels.
Small decreases in fetal body weight and in the number of
sternal and caudal ossification centres were observed in rats,
mostly in the 25 mg/kg dosage group (Chernoff & Carver, 1976).
6.8. Carcinogenicity
A bioassay of technical grade camphechlor was conducted in
mice and rats by administering the test chemical in the feed
(NCI, 1979b). Groups of 50 mice of each sex were given time-
weighted average doses of 99 or 198 mg/kg diet for 90 - 91 weeks.
There was a dose-related decrease in survival in male mice and a
decrease in survival in high-dose females. The incidence of
hepatocellular carcinomas was dose-related in both males and
females. The statistical significance was maintained when the
incidence of hepatocellular carcinomas was combined with that of
hyperplastic nodules of the liver. It was concluded that, under
the conditions of the assay, camphechlor was carcinogenic in male
and female B6C3F1 mice.
Fifty rats of each sex were administered camphechlor in their
diets at time-weighted average doses of 556 and 1112 mg/kg for
male rats, and 540 and 1080 mg/kg for females; the study lasted
for 108 - 110 weeks. There were no dose-related effects on
survival rates; clinical signs of toxicity in rats included
generalized body tremors at week 53 in the high-dose males and
females and later, leg paralysis, ataxia, epistaxis, haematuria,
and vaginal bleeding. Abdominal distention, diarrhoea, dyspnoea,
and rough haircoats were common to both dose groups. At the high
dose, 26% (9/35) and at the low-dose, 17% (7/41) of male rats
developed follicular-cell carcinoma or adenoma of the thyroid.
In the high-dose, female rat group, 17% (7/42) developed thyroid
tumours and in the low-dose group only 2%. On the basis of the
findings in the above study, it was concluded that the results
"suggest carcinogenicity of camphechlor for the thyroid of male
and female Osborne-Mendel rats" (NCI, 1979b).
6.9. Other Studies
Camphechlor has also been shown to increase the activity of
gluconeogenic enzymes in the rat (Desaiah et al., 1979), alter
mitochondrial electron transport in vitro (Pardino et al., 1971),
and inhibit ATPase activity in vitro (Yap et al., 1975) and in
vivo (Fattah & Crowder, 1980).
Protein deficiency was shown to increase the acute toxicity
of camphechlor, 3 to 4-fold (Boyd, 1972b).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
McGee et al. (1952) described 10 cases of poisoning with 3
deaths; the victims ranged in age from 17 months to 49 years. All
ingested camphechlor accidentally either in the form of the
insecticide, as in the case of the children, or as camphechlor-
polluted food, in the adults. Four of the cases were children
aged 17 months to 4 years, of whom three died. All the adults
survived. The onset of illness in all but one was characterized by
a major motor seizure; the latter had only gastrointestinal
complaints. The 3 deaths occurred within a matter of several hours
and those who recovered did so in 24 h.
Another poisoning incident involved a 16-month-old child who
ingested a material presumed to be camphechlor (Pollock, 1953).
Death was preceded by respiratory depression and convulsions.
Another child's death due to camphechlor poisoning was
reported in 1967 (Haun & Cueto, 1967). In this case, a 9-month-
old girl had been playing with a dust containing 13.8%
camphechlor and 7.04% DDT, which was found on her skin and in her
mouth. Convulsions, respiratory arrest, and death followed.
Cerebral oedema was found at autopsy.
7.2. Occupational Exposure
Two workers involved in harvesting cotton that had been
sprayed with camphechlor developed dyspnoea and reduced pulmonary
function and were found to have miliary opacities distributed
over their lung fields (Warraki, 1963).
Eight women working in an area that had been sprayed with
camphechlor at the rate of 2 kg/ha were reported to have a higher
incidence of chromosome aberrations (acentric fragments and
chromatid exchanges) in cultured lymphocytes (13.1%) than control
individuals (1.6%) (Samosh, 1974).
In a review of the chronic toxicity of organochlorine
pesticides in man (Deichmann, 1973), a survey of 137 workers
involved in the manufacture of camphechlor was reported. The
average length of exposure was 3.7 years, but some workers were
exposed for as long as 18 years. Physical examinations conducted
annually on these people failed to reveal any "adverse effects
that could be associated with camphechlor exposure".
An instance was reported where a rancher, who dusted his
sheep with a mixture of 5% camphechlor and 1% lindane,
demonstrated resistance to the hypothrombinemic effect of
warfarin (Jeffery et al., 1976). This effect was attributed to
the enzyme-inducing properties of camphechlor, which led to
increased metabolism of warfarin.
Two cases of acute aplastic anaemia associated with dermal
exposure to camphechlor-lindane mixtures, have been reported. One
of these cases terminated in death due to acute myelomonocytic
leukaemia (US EPA, 1976a).
In a survey of 199 employees, who worked or had worked with
camphechlor between 1949 and 1977, with exposures ranging from 6
months to 26 years (mean 5.23 years), 20 employees died, 1 with
cancer of colon; none of these deaths appeared to be related to
exposure to camphechlor (US EPA, 1978).
7.3. Controlled Human Studies
Twenty-five human volunteers were exposed to an aerosol of
camphechlor in a closed chamber, for 30 min per day, on 10
consecutive days (Shelanski, unpublished data, 1947). After 3
weeks, they received the same exposure on 3 consecutive days.
Assuming a retention of 50% of the inhaled camphechlor, each
individual absorbed 75 mg camphechlor per day or approximately 1
mg/kg body weight per day. Physical examinations and blood and
urine tests did not reveal any abnormalities.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8.1. Aquatic Organisms
8.1.1. Aquatic invertebrates
The toxicity of camphechlor for aquatic invertebrates is very
variable (Tables 3 and 4). Needham (1966) measured levels of
mortality at different concentrations of camphechlor for 14 genera
of invertebrates and determined the level that each could tolerate
for 24 h without any deaths. These are tabulated in Table 5.
Tolerance varied from 1 mg/litre for a leech to 10 µg/litre for
Haliplus sp. Values for molluscs ranged from 20 µg/litre for the
oyster to 460 mg/litre for the mactrid clam in 96-h tests, and for
crustacea from 54 ng/litre for a stage I zoeal larva of Sesarma to
290 µg/litre for a stage III zoeal larva of Rhithropanopeus, also
in 96-h tests. Resistance to camphechlor may develop. The 24-h
LD50 for fresh water shrimp taken from an area that had been
regularly treated for cotton pests was 10 times that of shrimp
taken from a wildlife reserve (Naqvi & Ferguson, 1970). Similarly,
cyclopoid copepods from a pesticide-contaminated area had a higher
tolerance for camphechlor than cyclopoids from a pesticide-free
area (Naqvi & Ferguson, 1968).
The only well-documented sublethal effects on invertebrates
are found in oysters. Yearling oysters showed a marked
inhibition in shell deposition after a 24-h exposure to 60%
camphechlor at 100 µg/litre (Butler et al., 1962). Mature
oysters showed reduced activity, as measured by shell opening
movements, during 4 weeks exposure to camphechlor at 100
µg/litre.
8.1.2. Fish
A series of studies on fathead minnows, brook trout, and
channel catfish (Mayer et al., 1975; Mehrle & Mayer, 1975a,b;
Mayer & Mehrle, 1976) document the "broken-back syndrome" in
response to camphechlor concentrations ranging from 55 to 621
µg/litre. Fish showed increased levels of calcium but decreased
levels of collagen in the backbone; phosphorus levels were
unaffected. The backbone became more brittle and sub-lethal
electric shock led to multiple fracture at all dose levels. In
addition to changes in the calcium/collagen ratio, there was a
reduction in the amino acids: alanine, valine, leucine,
isoleucine, lysine, phenylalanine, and hydroxyproline in bone
collagen. Hydroxyproline was also reduced in skin. Because of
the importance of this particular amino acid in collagen
formation, it is postulated that camphechlor may reduce the
wound-healing capacity in fish. Camphechlor, at concentrations
as low as 68 µg/litre, inhibited collagen synthesis (monitored by
hydroxyproline measurement) in brook trout fry, 7 days after
hatching. None of these effects were dose related and only at
the highest dose were any deaths observed. More recently, Mayer
& Mehrle (1976) showed that vitamin C levels in fish backbone
also decreased after camphechlor exposure, but levels in the liver
were unchanged. The authors postulated that detoxification of
camphechlor required vitamin C, which moved from storage in bone to
the liver. Vitamin C is involved in the conversion of proline to
hydroxyproline. Competition for available vitamin C was put
forward as a credible explanation for collagen synthesis effects.
Camphechlor inhibited ATPase activity in trout gill microsomes at
concentrations in incubates of 4 mg or more/litre (Davis et al.,
1972). In channel catfish, brain and gill ATPases were more
severely affected than kidney ATPase (Desaiah & Koch, 1975). Non-
mitochondrial Mg-ATPase was most severely inhibited.
Table 3. Toxicity of camphechlor for aquatic invertebratesa
-------------------------------------------------------------------------------------------------
Organism Temperature Size End point Parameter Concentration Reference
(°C) (mm) (µg/litre)
-------------------------------------------------------------------------------------------------
American oyster 27-29 shell growth 96-h EC50 16 Schimmel et al.
(Crassostrea (1977)
virginica)
crayfish from clean 11.8- no response to 48-h EC50 60.7 Albaugh (1972)
(Procambarus area 14.6 stimuli
acutus) from cotton 11.8- no response to 48-h EC50 90.2 Albaugh (1972)
field 14.6 stimuli
Daphnia pulex 15.5 immobilisation 48-h EC50 15 Sanders & Cope
first instar (1966)
pink shrimp, 96-h LC50 2.2 Courtenay &
nauplius Roberts (1973)
(Penaeus
duorarum)
pink shrimp, 24.5-26 96-h LC50 1.4 Schimmel et al.
adult (1.1-1.8) (1977)
drift line crab 25 96-h LC50 0.054 Courtenay &
(Sesarma Roberts (1973)
cinereum)
stage I zoeal
-------------------------------------------------------------------------------------------------
a A more comprehensive table listing different conditions and exposure times is available on
request from IRPTC, Geneva.
Table 4. Toxicity of camphechlor for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Stat Temp pH Size Alk End Parameter Concen- Reference
/flow (°C) (mg/ point tration
litre) (µg/litre)
---------------------------------------------------------------------------------------------------------
Goldfish stat 25 7.4 1-2 g 18 96-h LC50 5.6 Henderson et al.
(Carassius 38-64 mm (1959)
auratus)
Bluegill stat 18 7.1 0.6-1.7 g 35 96-h LC50 21 (14-30) Macek &
(Lepomis McAllister (1970)
macrochirus)
Fathead minnow stat 12.7 7.1 0.6-1.5 g 35 96-h LC50 3.2 (2.8-3.7) Macek et al. (1969)
(Pimephales stat 18 7.1 0.6-1.7 g 35 96-h LC50 14 (9-22) Macek &
promelas) McAllister (1970)
Sheepshead minnow flow 27- 21a 96-h LC50 1.1 (0.9-1.4) Schimmel et al.
(Cyprinodon 29.5 25.5 (1977)
variegatus)
Mosquito fish flow 25- 7.8 0.28 g 28a sensi- 36-h LC50 1 Burke & Ferguson
(Gambusia 26 tive (1969)
affinis) flow 25- 7.8 0.28 g 28a insen- 36-h LC50 200 Burke & Ferguson
26 sitive (1969)
Spot, 1 month flow 11- 20 mm 21b 144-h 0.5 Lowe (1964)
(Leiostomus 28 28b LC50
xanthurus)
Rainbow trout 7.2 96-h LC50 5.4 Cope (1965)
(Salmo 18.3 96-h LC50 1.8 Cope (1965)
gairdneri)
Longnose flow 28.5- 32-51 mm 10b 28-day 0.9 Schimmel et al.
killifish, juv. 31.5 20b LC50 (1977)
(Fundulus
similis)
Toad 48-h LC50 290 Guyer et al. (1971)
(Bufo woodhousi)
---------------------------------------------------------------------------------------------------------
a Hardness mg/litre.
b Salinity o/oo.
Table 5. Concentrations of camphechlor tolerated by invertebratesa
----------------------------------------------------------------
Organism Concentration for Next higherb
zero mortality concentration
(µg/litre) tested (µg/litre)
----------------------------------------------------------------
Hirudinea 1000
Gammarus sp. 100 200 (61%)
Hydracarina 1000
Callibaetis sp. 150 300 (29%)
Aeshna sp. 200 275 (16%)
Lestes sp. 450 500 (19%)
Notonecta sp. 275 300 (29%)
Signara sp. 50 75 (40%)
Limephilus sp. 500 550 (51%)
Haliplus sp. 10 40 (55%)
Hydroporus sp. 60 100 (27%)
Dytiscus sp. 15 50 (24%)
Eyrinus sp. 65 100 (22%)
Lymnara sp. 700
----------------------------------------------------------------
a From: Needham (1966).
b Value in parentheses is percentage mortality found at the next
higher concentration tested above that giving zero mortality.
The acute toxicity of camphechlor for fish species is
summarized in Table 4. Although the range of LC50 values is
not great, some species differences are clear. However, the
pattern of camphechlor toxicity is unrelated to fish families
(Macek & McAllister, 1970). Several of the studies in Table 4
illustrate the temperature dependence of camphechlor toxicity
(Hooper & Grzenda, 1955; Cope, 1965; Mahdi, 1966; Macek et al.,
1969); camphechlor being more toxic at higher temperatures. No
other test-condition variables showed such a clear relationship,
but there was a suggestion of increased toxicity with increased
alkalinity (Hooper & Grzenda, 1955; Henderson et al., 1959).
Various symptoms of camphechlor poisoning have been
described for fish (Gruber, 1959; Henderson et al., 1959;
Ludemann & Neumann, 1960; Workman & Neuhold, 1963; Schaper &
Crowder, 1976). Initial hyperactivity is followed by muscular
spasms. Later, fish show loss of equilibrium with short, jerky
movements. Mosquito fish also show respiratory distress. These
symptoms are similar to those caused by most chlorinated hydro-
carbon insecticides. The mechanism of the toxic action of
camphechlor is assumed to be mainly nervous.
There are few long-term studies on fish. Mehrle & Mayer
(1975b) showed a 46% reduction in the weight of brook trout fry
after 90 days' exposure to camphechlor at 0.039 µg/litre; which
was the lowest concentration tested. When brook trout fry,
hatched from unexposed eggs, were held in camphechlor solutions
for 90 days, the mortality rate was higher than that in unexposed
fry at 30 days and thereafter (Mayer et al., 1975). All fry
exposed to 0.288 µg/litre died before 60 days. Yearling brook
trout were less sensitive to camphechlor, showing a reduction in
weight at 0.288 µg/litre and a 40% reduction at 0.502 µg/litre
after exposure for 180 days. At these concentrations, length was
also reduced. During 150 days of exposure to camphechlor, at
concentrations ranging from 0.055 to 0.621 µg/litre, fathead
minnows showed weight reductions ranging from 10.9% to 21.1%, but
this was not dose-related (Mehrle & Mayer, 1975a).
Egg viability in female trout was significantly reduced when
they had been exposed to camphechlor at nominal concentrations of
0.075 µg or more per litre since they were yearlings (Mayer et
al., 1975). At a long-term exposure of 0.5 µg/ litre, egg
viability was reduced to zero. When eggs from unexposed females
were incubated in camphechlor solutions of between 0.039 to 0.502
µg/litre for 22 days, prior to hatching, they did not show any
reduction in hatchability (Mayer et al., 1975; Mehrle & Mayer,
1975b).
Adaptation or resistance to camphechlor may develop, since
natural populations of mosquito fish, which had either received
run-off from pesticide-treated fields or been sprayed directly,
were from 6 to 48 times more resistant to camphechlor than newly-
exposed fish (Boyd & Ferguson, 1964).
8.2. Terrestrial Organisms
8.2.1. Insects
Several studies of the effects of technical camphechlor on
honey bees gave a wide variety of results. Graves & Mackensen
(1965) determined a 24-h LD50 at 19.08 µg/bee, whereas Torchio
(1973) measured a 48-h LD50 as 0.144 µg/bee. In both these
studies, camphechlor was applied in acetone to the dorsal
thorax. Anderson & Atkins (1968) list camphechlor as
"relatively non-toxic" with an LD50 greater than 11.0 µg/bee.
Torchio (1973) looked at 3 bee species over different time
periods. For honey bees and alkali bees, the numbers of
deaths increased 24 - 48 h after application, but no further
deaths occurred between 48 and 72 h. All bees surviving up to
72 h also survived up to 96 h. For the leaf cutting bee,
there were more deaths between 48 and 72 h, but none between
72 and 96 h. Exposure of western yellowjacket wasps to the
topical application of 1 µliltre drops of camphechlor resulted in
mortality rates in 48 h of: 13% with exposure to 1 g/litre, 40%
with exposure to 5 g/litre, and 65% with exposure to 10 g/litre
(Johansen & Davis, 1972). Extensive research has been carried out
on target and non-target insects. This has been reviewed by the US
EPA (1979), who concluded that technical camphechlor was roughly
equally toxic for both pest and beneficial insects. Lepidopterans
were more resistant, possibly reflecting the long usage of
camphechlor to control lepidopteran pests and the consequent
development of resistance.
8.2.2. Birds
The acute toxicity of technical camphechlor for birds is
summarized in Table 6. There is some species variability; sharp-
tailed grouse, the most sensitive species, having an LD50 of 10 -
20 mg/kg body weight, and mourning dove, the least sensitive
species, having an LD50 of 200 - 250 mg/kg body weight. Dahlen &
Haugen (1954) reported a lack of motor coordination preceding death
in bobwhite quail. There was a clear age effect in the toxicity of
camphechlor for the mallard (Hudson et al., 1972), with young birds
(36 h after hatching) being the least sensitive. No satisfactory
explanation of this phenomenon is available. Two major field
studies showed that spraying with technical camphechlor could cause
death in birds. Eyer et al. (1953) applied insecticide at 2.2, 4.4,
5.5, and 6.6 kg/ha and examined domestic geese of 3 varieties. No
deaths resulted at the lowest application rate. Birds of 2 varieties
were killed within 3 h of direct contact with 4.4 kg/ha spray but
only 1 variety was killed at 5.5 kg/ha. No birds died at the
highest dose rate. The inconsistency of these results was not
explained, but it is clear that the compound killed birds. McEwen
et al. (1972) counted wild birds on control and sprayed areas of
rangeland. Spraying at 884 g/ha reduced the number of birds
relative to that in the control areas and to pre-spray observations.
Dead birds (5 larks, a cowbird, a killdeer, and a dove), found on
the sprayed area, contained between 9.6 and 0.1 mg camphechlor
residues/kg body weight. No deaths or decrease in numbers from
emigration occurred until more than a week after spraying.
Table 6. Toxicity of camphechlor for birds
-----------------------------------------------------------------------------------------------
Species Age Sex Routea Parameter Concentrationb Reference
(mg/kg)
-----------------------------------------------------------------------------------------------
Mallard 36 oral LD50 130 (80.4-210) Hudson et al. (1972)
Mallard 7 days oral LD50 30.8 (23.3-40.6) Hudson et al. (1972)
Mallard 3-5 months oral LD50 70.7 (37.6-133) Hudson et al. (1972)
Mallard 3-5 months F oral LD50 70.7 Tucker & Crabtree (1970)
Bobwhite 8 weeks oral LD50 80-100 Dahlen & Haugen (1954)
quail
Bobwhite 3 months M oral LD50 85.4 Tucker & Crabtree (1970)
quail
Mourning oral LD50 200-250 Dahlen & Haugen (1954)
dove
Sharp-tailed 1-4 years M oral LD50 10-20 Tucker & Crabtree (1970)
grouse
Fulvous 3-6 months M oral LD50 99.0 Tucker & Crabtree (1970)
tree duck
Lesser sand- F oral LD50 100-316 Tucker & Crabtree (1970)
bill crane
Pheasant 2 weeks diet 5-day LC50 500-550 Heath et al., (1970)c
Coturnix 2 weeks diet 5-day LC50 600-650 Heath et al., (1970)c
quail
Bobwhite chick diet 5-day LC50 834 Heath & Stickel (1965)
quail
Mallard duckling diet 5-day LC50 563 Heath & Stickel (1965)
-----------------------------------------------------------------------------------------------
a Oral dosing - a single dose by gelatin capsule; dietary dosing for 5 days then 3 days
of uncontaminated food.
b Concentrations - in mg/kg body weight for oral dosing; in mg/kg diet for dietary dosing.
c Unpublished data.
Long-term toxicity of camphechlor for birds is not well
documented. Keith (1966) fed sardines injected with camphechlor
to white pelicans, giving the birds a daily dose of 10 or 50 mg/kg
diet for 3 months. The mortality rate was high in all groups, but
only the higher dose resulted in definite symptoms of poisoning
prior to death. At death, birds on insecticide diets had little
subcutaneous and mesentery fat. Dietary dosing of 6-month-old
female, ring-necked pheasants for up to 3 months at 25, 100, 200,
or 300 mg/kg diet did not cause death (Genelly & Rudd, 1956a). The
initial depression in body weight observed was attributed to reduced
feeding. Autopsy of mature birds dosed for 74 days with 100 or 300
mg/kg diet showed vacuolation in the livers.
Two studies on various parameters related to reproductive
success in birds did not show any effects at doses up to 100
mg/kg diet. Bush et al. (1977) dosed female domestic chickens
from 1 day of age up to maturity with one of 4 dose levels of
camphechlor, ranging from 0.5 to 100 mg/kg diet. No significant
effects were found on egg production, hatchability, or fertility.
Genelly & Rudd (1956b) dosed female, ring-necked pheasants with
camphechlor at either 100 or 300 mg/kg diet. Significant effects
were only noticed at the higher dose rate, with reductions in egg
laying and hatchability. "Relative reproductive success rate",
calculated on the basis of several reproductive parameters, was
70% for controls, 62% for birds given camphechlor at 100 mg/kg
diet, and 46% for birds given 300 mg/kg diet. In two studies on
egg hatchability (Dunachie & Fletcher, 1969; Smith et al., 1970);
camphechlor was injected into hens' eggs with no consistent
effects. Maximum doses given were 500 mg/kg egg weight in one
study and 1.5 mg/egg in the other. Haegele & Tucker (1974)
showed that a single oral dose of technical camphechlor at 10
mg/kg body weight did not significantly alter egg shell thickness
in Japanese quail. When bobwhite quail were given technical
grade camphechlor at 5, 50, or 500 mg/kg in their feed for 4
months, thyroid growth was stimulated and adrenal hypertrophy
occurred at all dose levels (Hurst et al., 1974). However, only
the highest dose increased uptake of I131. This dose also
decreased body weight. It is difficult to attribute these
effects to a direct thyroid lesion.
8.2.3. Wild animals
Tucker & Crabtree (1970) reported a 96-h LD50 for 16 to 17-
month-old male mule deer of 139 - 240 mg/kg body weight, when
camphechlor was administered orally as a single dose in a gelatin
capsule. The effect on in vitro fermentation of dry matter
(alfalfa hay) by inocula of rumen fluid from mule deer was
investigated by Schwartz & Nagy (1974). Inhibition was found at a
dose of 1000 mg camphechlor/kg dry matter. Accidental poisoning
occurred in a female Bengal tiger that ate a llama calf which had
been dipped in a solution of camphechlor. Symptoms included
periodic convulsions and hyperreflexia to sudden auditory and
visual but not to tactile stimuli (Peavy 1975).
8.2.4. Plants
Effects of camphechlor on higher plants have only been reported
for crop varieties. In greenhouse studies on cotton, using a sandy
or clay soil, Franco et al. (1960) found some toxicity at a dose
equivalent to a field application of 72.3 kg/ha, but only with
camphechlor applied as an emulsion. When applied as a powder, the
insecticide was not phytotoxic for cotton plants at a dose equivalent
to 101.5 kg/ha. Hagley (1965) studied the growth of seedlings of 3
vegetables, including Chinese cabbage and tomatoes, treated with
camphechlor at rates of 1.57 and 15.7 kg/ha, in the greenhouse.
Both levels inhibited shoot growth in Chinese cabbage. The higher
dose rate affected average weekly growth in all 3 cultivars, with
little root inhibition. A level of 15.7 kg/ha was toxic for tomato
seedlings, with 50% dying in the second, and a further 33% in the
third week of growth. Emergence, growth, yield, and chemical
composition of soybeans were not affected by the application of
camphechlor at 44.8 kg/ha, which was considerably higher than
recommended usage level (Probst & Everly, 1957).
8.3. Microorganisms
No effects were observed on either soil bacteria or fungi
when camphechlor was applied to the soil at 22.4 kg/ha, annually,
for 5 years (Martin et al., 1959), or twice yearly for 3 years,
at 16.8 kg/ha (Eno et al., 1964). Bollen et al. (1954) showed
that camphechlor stimulated mold numbers, 10 - 20 days after field
application at 11.2 kg/ha. A higher dosage of 22.4 kg/ha, after
the same period, did not show any effects on fungi. Bacteria were
not affected by either dosage.
In greenhouse studies on the effects of camphechlor on
nodulation bacteria, doses equivalent to between 4.9 and 40.3
kg/ha did not significantly affect bacterial growth or nodulation
of legumes (Elfadl & Fahmy, 1958). Treating soil with between
12.5 and 100 mg camphechlor/kg and incubating for between 1 and
16 months in a greenhouse, did not result in any measurable
effects on numbers of soil bacteria or fungi, but did stimulate
nitrification and carbon dioxide evolution after one month; after
16 months, there were no effects (Eno & Everett, 1958).
The results of studies on marine and freshwater unicellular
algae and protozoa are summarized in Table 7. The single study
on marine algae (Ukeles, 1962) showed species variability. The
dinoflagellate, Monochrysis lutheri, is the most sensitive,
showing total inhibition of growth at camphechlor concentrations
of 0.00015 mg/litre. The study by Stadnyk et al. (1971), whilst
showing little effect on cell numbers or biomass, demonstrated
marked stimulation of carbon fixation in the presence of
camphechlor. At a concentration of 0.1 mg/litre, there was an
initial 48% increase in carbon dioxide fixation by the green alga,
Scenedesmus quadricaudata, but this became insignificant later
in the 10-day exposure period. An initial increase of 450% had
fallen to 30%, after 10 days' exposure to 1 mg camphechlor/litre.
No explanation for this stimulation seems to be available. The
complex study by O'Kelley & Deason (1976) showed that camphechlor
at 0.01 mg/litre could inhibit growth in a few strains of algae
(which had been isolated from the Warrior River) and stimulate
growth in others. More strains showed inhibited growth at 0.1
mg/litre, but 6 out of the 21 strains used were unaffected, even by
the highest dose of 1.0 mg/litre.
Table 7. Toxicity of camphechlor for unicellular algae and protozoa
-----------------------------------------------------------------------------------------------------
Organism Temp Sex Sal. Species Expo- Concen- Effect Reference
(°C) o/oo sure tration
(days) (mg/litre)
-----------------------------------------------------------------------------------------------------
Unicellular 19.5- M 22- 10 0.00015- 100% inhibition Ukeles (1962)
algae 21.5 28 0.15 of growth
Blue-green 22 F Cylindrospermum 21 2a partially toxic, Palmer &
alga licheniforme reduced growth Maloney (1955)
Green alga 22 F Scenedesmus 21 2a partially toxic, Palmer &
obliquus reduced growth Maloney (1955)
up to 14 days
no effect on
growth after
14 days
Diatom 22 F Gomphonema 21 2a partially toxic, Palmer &
parvulum reduced growth Maloney (1955)
Alga 20- F Selenastrum 0.38 EC50 inhibition US EPA (1980)
22 capricornutum of growth
Green alga F Scenedesmus up to 1.0 19% decrease in Stadnyk et al.
quadricaudata 10 cell number, (1971)
no significant
effect on biomass
Protozoa F culture dominated 1 1.3 24-h LC50 Weber et al.
by ciliates; (1982)
( Eupoletes sp.)
and microflagellates
-----------------------------------------------------------------------------------------------------
a 2 mg/litre of a 60% solution of camphechlor.
F = Freshwater.
M = Marine.
The lowest concentrations of camphechlor inducing toxic
effects in protozoa from a salt marsh, were comparable to
concentrations found in the environment (Weber et al., 1982).
When a lake was treated with camphechlor (60%) at 0.1 mg/litre,
protozoan numbers were reduced almost to zero (Hoffman & Olive,
1961). Turbidity of the lake water decreased until the bottom
(at a depth of 4.5 m) was clearly visible.
8.4. Bioaccumulation and Biomagnification
Schoettger & Olive (1961), in a study to determine the
bioaccumulation of camphechlor, exposed planktonic alga (Scenedesmus
incrassatulus) cultures to 10 mg camphechlor/litre for 384 h. The
alga did not accumulate enough camphechlor to kill fish fed on it.
However, periphytons (various algae, diatoms, and ciliates), exposed
to the same dose of camphechlor, accumulated enough to kill fish
within 24 h. Sanborn et al. (1976) reported a concentration factor
of 6902 for an alga in a model ecosystem with a water concentration
of 44.4 µg/litre. Concentration factors of 9600 for the snail and
890 for the mosquito were also reported.
After applying camphechlor at 50 µg/litre, to eradicate rough
fish, Kallman et al. (1962) studied uptake by the rooted aquatic
weed, Potamogeton. After 5 days and 9 days, mean concentrations
in plants were 4.4 mg/kg and 14.6 mg/kg, respectively, which
represents concentration factors of 4400 and 14600 over the water
concentration of 1 µg/litre. Terriere et al. (1966) also found
accumulation of camphechlor by aquatic plants. Plants absorbed up
to 15.5 mg/kg from water containing 2 µg/litre; roots contained the
highest concentrations. Salt marsh cord grass, Spartina alterniflora,
growing near the discharge point of a camphechlor manufacturing plant,
was analysed for camphechlor residues by Reimold & Durant (1972).
They found maximum levels of 72.8 mg/kg in the leaves and lower
concentrations in other parts of the plant. In contrast, Reimold
(1974) using 36Cl-labelled camphechlor, found that plant roots
and rhizomes absorbed the largest amounts. The differences between
uptake in these studies may be because of differences between sea
water and marsh soil.
Oysters exposed to 1 µg/litre for 36 weeks bioaccumulated
camphechlor throughout the pre-spawning period (Lowe et al., 1971).
Concentrations in oyster tissues at 12, 24, and 36 weeks were 20
mg/kg, 23 mg/kg, and 8 mg/kg, respectively. Johnson (1966)
measured camphechlor residues in plankton from Big Bear Lake,
California, following 2 treatments with camphechlor at rates of
0.03 and 0.1 mg/litre, applied 2 weeks apart. Camphechlor
concentrations increased to a peak of 97 mg/kg, 114 days after the
final application. Concentrations decreased with time but, even
265 days after application, were still higher (at 2.0 mg/kg) than
the original treatment level. Tubificid worms may accumulate
camphechlor since worms collected in the Mississippi River delta
contained trace amounts of camphechlor.
However, worms exposed experimentally to camphechlor did not
accumulate enough to kill the fish to which they were fed (Naqvi,
1973).
Fish are able to absorb and bioconcentrate significant amounts
of camphechlor, a large proportion being complexed with the lipid
stores. Schaper & Crowder (1976) found that exposing mosquito fish
to a fatally-toxic level of 2 mg camphechlor/litre for 8 h did not
result in bioconcentration, the average concentration in whole fish
being 0.586 mg/kg. Thus, exposure for 8 h is not sufficient for
bioaccumulation to occur in fish. When 14C-labelled camphechlor
was added to a terrestrial-aquatic model ecosystem at 44.4 µg/litre
in the water, mosquito fish concentrated camphechlor by a factor of
4247 (Sanborn et al., 1976). Hughes (1970) exposed bluegills to
17.3 µg/litre or 70.3 µg/litre, and achieved camphechlor
concentrations in the fish of 9.4 mg/kg and 7.7 mg/kg, respectively,
after 96 h. Mayer et al. (1975) carried out several studies on
brook trout. Brook trout fry, when exposed to camphechlor
concentrations of 0.041 - 0.5 µg/litre, concentrated the chemical
between 4900 and 76 000 times. Large CF values, recorded after 15
days, were partially attributed to the presence of a yolk sac,
which contains large quantities of lipid. Falling values,
occurring after 60 days, were due to the disappearance of the yolk
sac, and the rise after 90 days to the increasing lipid content of
maturing fry. Yearling brook trout bioconcentrated less camphechlor
than fry during the 161-day exposure, achieving bioconcentration
factors of between 3200 and 16000.
Residues of camphechlor found in fish from camphechlor-treated
lakes are usually similar to levels measured in the laboratory,
indicating that free-living fish can bioconcentrate camphechlor by
a factor of several thousand. Kallman et al. (1962) studied the
uptake of camphechlor by rainbow trout and bullhead in Clayton
Lake, New Mexico, following an application of the pesticide.
Seventy-two hours after treatment, the average concentration of
camphechlor in the water was 2 µg/litre, and the concentration
factors were 1700 for trout and 2100 for bullheads. After 5 days,
the average water concentration of camphechlor was 11.5 µg/litre
and, at this concentration, bullheads contained mean body residues
of 13 mg/kg and were severely affected. After the water
concentration stabilized at 1 µg/litre, trout exposed to this water
had concentration factors ranging from 800 to 2500 after 7 days,
and factors of between 1300 and 3500 after 17 days. After 108 to
170 days exposure, trout showed body residues of 0.3 mg/kg, but the
water concentration had fallen to non-detectable levels.
In a similar study, Terriere et al. (1966) monitored the
relationship between camphechlor levels in 2 Oregon lakes (Davis
Lake treated with 80 µg camphechlor/litre and Miller Lake treated
with 40 µg/litre). In Davis Lake, residues were measured in
rainbow trout and Atlantic salmon; the trout consistently
accumulated more camphechlor than the salmon. As in experimental
exposures (Mayer et al., 1975), these fish concentrated
camphechlor by a factor of several thousand. In Miller Lake,
where the water concentration of camphechlor was 0.84 (0.70 -
1.1) µg/litre, brook trout, the only species monitored in this
lake, concentrated camphechlor 14 800 times.
Hughes & Lee (1973) found that predator fish accumulated less
camphechlor than prey fish. Bluegills and suckers, both prey
fish, accumulated whole body residues of 9.4 mg/kg and 10.6
mg/kg, respectively, whereas predator fish, i.e., bass, northern
pike, and walleyes, accumulated residues of only 2.2, 3.3, and
1.2 mg/kg, respectively. This suggests that the biomagnification
of camphechlor in aquatic food chains may not be as great as
might be indicated from bioconcentration factors.
Bioconcentration factors in a freshwater model ecosystem were
shown by Metcalf (1976) to be 5 for plankton, 176 - 1152 for
carp, 200 for insects, and 200 for crayfish.
There are no data on birds and mammals from which residue
levels can be related to exposure levels.
8.5. Population and Community Effects
The effects of the application of 0.1 mg camphechlor/litre to
control rough fish on the macroscopic bottom fauna of a lake in
Colorado were studied by Cushing & Olive (1956). During the first
3 weeks following treatment, treated and control lakes were sampled
fairly frequently and, thereafter, samples were taken approximately
every other week. Tendipedes were killed immediately, and no
larvae were found in the following 8 months. Chaoborus were not
immediately affected, but numbers began to decline 3 months later.
None were found 6 months after treatment. The authors speculated
that the Chaoborus, which prey on tendipedes, starved to death.
The number of oligochaetes increased, probably due to the extra
organic matter available. In the control lake, the numbers of
tendipedid, Chaoborus, and oligochaete remained fairly constant
throughout the sampling period. Chaoborus numbers fell in late
June/August when emergence was at its peak but, unlike the
experimental lake, repopulation occurred in September.
Needham (1966) studied plankton and aquatic invertebrates in 4
North Dakota lakes. The first lake (9.7 ha, depth 2.7 m) was
treated with 35 µg camphechlor/litre. Samples were taken prior to
treatment and 1 week, 1 month, and 1 year after treatment. Plankton
were monitored as numbers of organisms per litre. Keratella
decreased from 91 pre-treatment to 15, one week after treatment,
and subsequently to very low numbers. Asplanchna increased from
73 to 106, 1 week after treatment, but none were present in samples
taken 1 month or 1 year later. Numbers of Daphnia decreased from
244 to 18, 1 week after treatment, increasing to 129 after 1 month
but dropping to 28 after 1 year. Bosmina increased from 98 to 130
following treatment, but declined to 18 after 1 month and had only
risen to 25, 1 year later. Copepoda ( Diaptomus, Cyclops, and
undetermined nauplii) declined in numbers after treatment, but the
number of nauplii had begun to increase again, 1 year after
treatment. Five invertebrate genera that lived on water plants
were found in significant numbers. Gammarus varied throughout
the study but remained abundant. Callibaetis, Caenis, and Ischnura
decreased 1 week and 1 month after treatment, but were more abundant
after 1 year. Tendipes decreased from 44 (prior to treatment) to
9, 1 week after treatment, increasing to 48, 1 month after treatment
and falling back to 25, 1 year after treatment. Gastropoda (Physa
and Gyraulus) increased from 771 before treatment to 1107, 1 week,
1366, 1 month, and 1558, 1 year after treatment. In the lake bottom
fauna, only 2 genera were abundant; Gammarus, which fluctuated
greatly during the study and tendipedes, which decreased following
treatment but, 1 year later, had increased again to original numbers
in samples. Comparison between plant-inhabiting organisms and
bottom fauna did not reveal any shifting of populations, 1 year
after treatment, apart from Physa, which increased greatly.
The second lake studied by Needham (1966) (having a surface
area of 6 ha and a maximum depth of 5.5 m) was treated twice with
camphechlor, first with 25 µg/litre and then, 53 days later, with
90 µg/litre. Samples were taken 1 day before, and 11, 33, and 371
days after the initial treatment. Plankton were monitored as
numbers of organisms per litre. A decline in population was
recorded for all genera found in significant numbers. Brachionus,
which had decreased from 114 to 108 organisms/litre, 11 days after
treatment, decreased to 15 after 33 days and to 3 after 371 days.
The numbers of the other abundant rotifer, Asplanchna increased
from 24 to 194, 11 days after treament but dropped after 33 days to
16; by 371 days, there was only one organism/litre. Daphnia,
Ceriodaphnia, and Bosmina were the most abundant zooplankton.
Both Daphnia and Ceriodaphnia showed similar patterns, an
increase in numbers, 11 days after treatment, and a subsequent
decline. Bosmina had declined from 314 to 283, 11 days after
treatment, and continued to decline reaching 50 after 33 days; none
were found in the 371-day sample. Copepods also decreased in
numbers during the study. Although the number of Cyclops had
increased between 11 and 33 days, it decreased again after 371
days.
The third lake studied by Needham (1966) (a glacial lake of
370 ha and an average depth of 2.7 m) was treated with 10 µg
camphechlor/litre followed 2 days later by 5 µg/litre, and a
fourth lake (40.5 ha, maximum depth 4.9 m) was treated with 5
µg/litre. Results from this lake were similar to those from the
second; camphechlor failed to affect any of the organisms
sampled.
When sediments containing camphechlor were disturbed by
dredging, camphechlor was released into the water leading to
higher concentrations in aquatic flora and fauna, but this did
not appear to affect the biological balance in the area (Reimold
& Durant, 1974).
8.6. Effects on the Abiotic Environment
Five annual field applications of 22.4 kg camphechlor/ha did
not have any effect on the moisture content, total infiltration,
and aggregation of the soil (Martin et al., 1959).
9. PREVIOUS EVALUATIONS OF CAMPHECHLOR BY INTERNATIONAL BODIES
Camphechlor was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1968 and 1973 (FAO/WHO, 1969, 1974). In 1968,
no recommendations were made because of the many unresolved
questions. In 1973, the meeting concluded that it could not
establish an ADI for a material that varied in composition
according to the method of manufacture.
IARC (1979) evaluated the carcinogenic hazard resulting from
exposure to camphechlor and concluded that "there is sufficient
evidence that toxaphene is carcinogenic in mice and rats. In the
absence of adequate data in humans, it is reasonable, for
practical purposes, to regard toxaphene as if it presented a
carcinogenic risk to humans".
Practical advice has been issued by WHO/FAO (1975) in the
"Data Sheets on Pesticides", No. 20, which deals with
camphechlor, and includes information on labelling, safe-
handling, transport, storage, disposal, decontamination,
selection, training and medical supervision of workers, first
aid, and medical treatment.
Over recent years, a number of official registrations for the
use of camphechlor have been drawn in several countries, for
various reasons. Further details may be obtained from IRPTC.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be
obtained from the IRPTC (International Register of Potentially
Toxic Chemicals) Legal File (IRPTC, 1983).
IPRTC (1983), in its series "Scientific reviews of Soviet
literature on toxicity and hazards of chemicals", issued a review
on camphechlor.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Camphechlor Toxicity
The acute toxicity of camphechlor in the rat is moderate,
i.e., the oral LD50 ranges from 60 to 120 mg/kg body weight.
Camphechlor is readily absorbed via all routes of entry. It is
metabolized in the body, but a certain amount of accumulation in
adipose tissue takes place on continuous exposure.
Signs and symptoms of poisoning are salivation and vomiting
and, at higher exposures, excitation of the CNS with convulsions,
respiratory failure, and death. On prolonged exposure of
animals, hypertrophy of the liver with induction of microsomal
enzymes (5 mg/kg in the diet or 0.25 mg/kg body weight per day)
are the major findings.
Camphechlor has been shown not to have an effect on reproduction
and it is not teratogenic. It is mutagenic in a bacterial test, but
the results of a dominant lethal test in mice were negative. There
is sufficient evidence for its carcinogenicity for rats and mice.
Epidemiological studies are inadequate to evaluate the carcinogenic
potential of camphechlor for human beings.
10.2. Exposure to Camphechlor
Exposure of the general population is mainly through residues
in food. However, these are normally below residue tolerances.
Accidental over-exposure has occurred as a result of contamination
of food with camphechlor.
In the past, occupational exposure to camphechlor may
occasionally have been considerable. Nevertheless, only a few
cases of adverse effects have been reported. Workplace exposures
above the permissible level have been reported.
10.3. Effects on the Environment
Movement of camphechlor through the environment is not
clearly understood, but the dominant factor in the distribution
of the chemical appears to be its high volatility. Camphechlor
is a widespread contaminant of aquatic ecosystems. Residues have
also been found in non-aquatic organisms.
Camphechlor has been used extensively as a fish poison to
clear rough fish from lakes before introducing game fish. The
toxic effects have not only been directly on adult fish but also,
via adult females, on the development of eggs and young. Non-
target aquatic organisms have also been affected. Some
invertebrates showed long-term deleterious effects of camphechlor
poisoning. The environmental levels of camphechlor in waters
where the chemical has not been deliberately applied can exceed
laboratory concentrations that have caused death or sublethal
lesions in experimental fauna. Thus, camphechlor presents a major
hazard for aquatic organisms.
Available field data suggest that birds have been adversely
affected by camphechlor.
10.4. Conclusions
1. Although no serious adverse effects on workers resulting
from occupational exposure to camphechlor have been reported,
and epidemiological studies remain inadequate, this chemical
should be considered for practical purposes as being
potentially carcinogenic for human beings.
2. For the same reason, and recognizing the limitations of
residue analysis for camphechlor, as well as the reluctance of
the JMPR to issue an ADI, reservations must remain about the
safety of this chemical in food, despite the relatively low
residues so far reported.
3. Environmentally, camphechlor is a major hazard for aquatic
and also some terrestrial species.
4. Taking into account these considerations, it is felt that
the use of this chemical should be discouraged, except where
there is no adequate alternative.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations Procambarus actus in South-Central Texas. Bull.
env. Contam. Toxicol., 8: 334-338.
ALEXANDER, M. (1965) Persistence of chlorinated hydrocarbon
insecticides in soils. Soil Sci. Soc. Am. Proc., 29: 1-7.
ANDERSON, L.D. & ATKINS, E.L. (1968) Pesticide usage in
relation to beekeeping. Annu. Rev. Entomol., 13: 213-238.
ARSCOTT, G.H., MICHENER, S., & BILLS, D.D. (1976) Effect of
toxaphene on the performance of white leghorn layers and their
progeny. Poult. Sci., 55: 1130-1131.
ASHIROVA, S.A. (1971) Work hygiene and effectiveness of
health measures in the manufacture of chlorinated terpenes.
Nauch. Tr. Leningrad Gos. Instit. Usoversh. Vrachei, 98: 26-30
(in Russian; Chem. Abstr., 79: 13949).
BADAEVA, L.N. (1976) The effect of polychlorocamphene on
some organs and their nervous apparatus in pregnant animals.
Bull. exp. Biol. Med., 82: 945-947.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect
of field treatments of insecticides on number of bacteria,
Streptomyces, and molds in soil. J. econ. Entomol., 47:
302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and
resistance of mosquito fish to several insecticides. J. econ.
Entomol., 57: 430-431.
BOYD, E.M. (1972) Protein deficiency and pesticide toxicity,
Springfield, Illinois, Charles C. Thomas Publishing Company,
p. 231.
BRADLEY, J.R., SHEETS, T.J., & JACKSON, M.D. (1972) DDT and
toxaphene movement in surface water from cotton plots.
Environ. Qual. Saf., 1: 102-105.
BURKE, W.D. & FERGUSON, D.E. (1969) Toxicities of four
insecticides to resistant and susceptible mosquito fish in
static and flowing solutions. Mosq. News, 29: 96-101.
BUSH, P.B., KIKER, J.T., PAGE, R.K., BOOTH, N.H., & FLETCHER,
O.J. (1977) Effects of graded levels of toxaphene on poultry
residue accumulation, egg production, shell quality and
hatchability in white leghorns. J. agric. food Chem., 25:
928-932.
BUTLER, P.A. (1962) Effects on commercial fisheries,
Washington DC, US Department of the Interior, Fish and
Wildlife Service (Circular No. 143).
BUTLER, P.A. (1963) Commercial fisheries investigations,
pesticide-wildlife studies: a review of Fish and Wildlife
Service investigations during 1961 and 1962, Washington DC, US
Department of the Interior, Fish and Wildlife Service, p. 11
(Circular No. 167).
BUTLER, P.A. (1964) Pesticide-wildlife studies 1963: a
review of Fish and Wildlife Service investigations during the
calendar year, Washington DC, US Department of the Interior,
Fish and Wildlife Service, p. 5 (Circular No. 199).
BUTLER, P.A., WILSON, A.J., & RICK, A.J. (1962) Effects of
pesticides on oysters. Proc. Natl Shellfisheries Assoc., 51:
23-32.
CANADA, DEPARTMENT OF NATIONAL HEALTH AND WELFARE (1978)
Guidelines for Canadian drinking water quality, Ottawa,
Department of National Health and Welfare.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide
residues in urban soils from 14 United States cities, 1970.
Pestic. Monit. J., 10: 54-60.
CASIDA, J.E., HOLMSTEAD, R.L., KHALIFA, S., KNOX, J.R.,
OHSAWA, T., PALMER, K.J., & WONG, R.Y. (1974) Toxaphene
insecticide: A complex biodegradable mixture. Science, 183:
520-521.
CHAIYARACH, S., RATANANUN, V., & HARREL, R.C. (1975) Acute
toxicity of the insecticides toxaphene and carbaryl and the
herbicides propanil and molinate to four species of aquatic
organisms. Bull. environ. Contam. Toxicol., 14: 281-284.
CHERNOFF, N. & CARVER, B.D. (1976) Fetal toxicity of
toxaphene in rats and mice. Bull. environ. Contam. Toxicol.,
15: 660-664.
CHANDURKAR, P.S. & MATSUMURA, F. (1979) Metabolism of
toxicant B and toxicant C of toxaphene in rats. Bull. environ.
Contam. Toxicol., 21: 539-547.
CLAYBORN, H.V., MANN, H.D., IVEY, M.C., REDELEFF, R.D., &
WOODARD, G.T. (1963) Excretion of toxaphene and strobane in
the milk of dairy cows. J. agric. food Chem., 11: 286-289.
CLAPP, K.L., NELSON, D.M., BALL, J.T., & ROUSEK, E.J. (1971)
Effects of toxaphene on the hepatic cells of rats. Proc. Ann.
Meeting W. Section Am. Soc. Animal Sci., 22: 313-323.
COFFIN, D.E. & McCLEOD, H.A. (1975) Current pesticide
residue status of Canadian diets and adipose tissue. In:
Seventh Eastern Canada Symposium on Pesticide Residue Analysis
Proceedings, Toronto, May 20-23.
CONLEY, B.E. (1952) Pharmacological properties of toxaphene,
a chlorinated hydrocarbon insecticide. J. Am. Med. Assoc.,
149: 1135-1137.
COPE, O.B. (1965) Sport Fishery Investigations. In: The
effect of pesticides on fish and wildlife, Washington DC, US
Department of the Interior, Fish and Wildlife Service, pp.
51-64 (Circular No. 226).
COURTENAY, W.R. & ROBERTS, M.H. (1973) Environmental effects
on toxaphene toxicity to selected fishes and crustaceans,
Washington DC, US Environmental Protection Agency, 73 pp (US
EPA Report EPA/R3-73-035).
CROWDER, L.A. & DINDAL, E.F. (1974) Fat of 36Cl-toxaphene in
rats. Bull. environ. Contam. Toxicol., 12: 320-327.
CUSHING, C.E., & OLIVE, J.R. (1956) Effects of toxaphene and
rotenone upon macroscopic bottom fauna of two Northern
Colorado reservoirs. Trans. Am. Fish. Soc., 86: 294-301.
DAHLEN, J.H. & HAUGEN, A.O. (1954) Acute toxicity of certain
insecticides to the bobwhite quail and mourning dove. J.
Wildl., 18: 477-481.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, E.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of Na+K+-ATPase in rainbow trout
Bull. environ. Contam. Toxicol., 8: 69-72.
DEICHMANN, W.B., ed. (1973) The chronic toxicity of
organochlorine pesticides in man. In: Deichmann, W.B., ed.
Pesticides and the environment: selected papers presented at
the 8th International American Conference on Toxicology and
Occupational Medicine, University of Miami, Miami, Florida,
July, 1973, pp. 347-420.
DESAIAH, D. & KOCH, R.B. (1975) Toxaphene inhibition of
ATPase activity in catfish, Ictalurus punctatus tissues. Bull.
environ. Contam. Toxicol., 13: 238-244.
DESAIAH, D., MEHROTA, B.D., KINZEY, R., & TROTTMAN, C.H.
(1979) Effects of toxaphene, kepone and mirex on key
gluconeogenic enzymes in rat liver. Fed. Proc., 8: 538.
DOMANSKI, J.J., HAIRE, P.L., & SHEETS, T.J. (1974)
Insecticide residues on 1973 US tobacco products. Tobacco
Sci., 18: 108-109.
DOMANSKI, J.J. & GUTHRIE, F.E. (1974) Pesticide residues in
1972 cigars. Bull. environ. Contam. Toxicol., 11: 312-314.
DOMANSKI, J.J. & SHEETS, T.J. (1973) Insecticide residues on
1970 US auction market tobacco. Tobacco, 175: 29.
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of
the toxicity of insecticides to birds eggs using the egg-
injection technique. Ann. appl. Biol., 64: 409-423.
DURANT, C.J. & REIMOLD, R.J. (1972) Effects of estuarine
dredging of toxaphene-contaminated sediments in Terry Creek,
Brunswick, Georgia, 1971. Pestic. Monit. J., 6: 94-96.
ELFADL, M.A. & FAHMY, M. (1958) Effect of DDT, BHC, and
toxaphene on nodulation of legumes and soil microorganisms.
Agric. Res. Rev., 36: 339-350.
EMBRY, T.L. (1972) Search for abnormalities of heme
synthesis and sympathoadrenal activity in workers regularly
exposed to pesticides. Tex. Rep. Biol. Med., 30: 203.
ENO, C.F. & EVERETT, P.H. (1958) Effects of soil
applications of 10 chlorinated hydrocarbon insecticides on
soil microorganisms and the growth of stringless Black
Valentine beans. Proc. Soil Sci. Soc. Am., 22: 235-238.
ENO, C.F., WILSON, J.W., CHILSHOLM, R.D., & KOBLITSKY, L.
(1964) The effect of soil applications of six chlorinated
hydrocarbon insecticides on vegetable crops and soil
microorganisms. Proc. Florida State Hort. Soc., 77: 223-229.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
EYER, J.R., FALKNER, L.R., & MCCARTY, R.T. (1953) Effect of
toxaphene and DDT on geese in cotton fields. New Mexico Agric.
Exp. Sta., Bull., 1078: 1-6.
FAO/WHO (1969) Toxaphene. In: 1968 evaluations of some
pesticide residues in food, pp. 267-283.
FAO/WHO (1974) Camphechlor. In: 1973 evaluations of some
pesticide residues in food, pp. 92-118.
FATTAH, I.C.M.A. & CROWDER, L.A. (1980) Plasmamembrane
ATPC-ases from various tissues of the cockroach (Periplanita
americana) and mouse influenced by toxaphene. Bull. environ.
Contam. Toxicol., 24: 356-363.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P.
(1964) Resistance to chlorinated hydrocarbon insecticides in
three species of freshwater fish. Bioscience, 14: 43-44.
FERGUSON, D.E., CULLEY, D.D., & COTTON, W.D. (1965)
Tolerances of two populations of fresh water shrimp to five
chlorinated hydrocarbon insecticides. J. Miss. Acad. Sci., 11:
235-237.
FITZHUGH, O.G. & NELSON, A.A. (1951) Comparison of chronic
effects produced in rats by several chlorinated hydrocarbon
insecticides. Fed. Proc., 10: 295.
FRANCO, C.M., FRAGA, C.C., & NEVES, O.S. (1960) [Effects of
the addition of insecticides to the soil on the development of
cotton crops.] Bragantia, 19: 13-25 (in Portugese).
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 5-534.
GENELLY, R.E. & RUDD, R.L. (1956a) Chronic toxicity of DDT,
Toxaphene and Dieldrin to ring-necked pheasants. Calif. Fish
Game, 42: 5-14.
GENELLY, R.E. & RUDD, R.L. (1956b) Effects of DDT, Toxaphene
and Dieldrin on pheasant reproduction. AUK, 73: 529-539.
GLEASON, M.N., GOSSELIN, R.E., HODGE, H.C., & SMITH, R.P.,
ed. (1969) Clinical toxicology of commercial products -
acute poisoning, 3rd ed., Baltimore Maryland, Williams and
Wilkins Company, Section II, Ingredients Index.
GOSSELIN, R.E., HODGE, H.C., SMITH, R.P., & GLEASON, M.N.
(1976) Clinical toxicology of commercial products, Baltimore,
Maryland, Williams and Wilkins Company, p. 189.
GRAVES, J.B. & MACKENSEN, O. (1965) Topical application and
insecticide resistance studies on the honey bee. J. econ.
Entomol., 58: 990-993.
GRIFFIN, D.E. & HILL, W.E. (1978) in vitro breakage of
plasmid DNA by mutagens and pesticides. Mutat. Res., 52:
161-169.
GRUBER, R. (1959) Toxicité pour Tilapia melanopleura de deux
insecticides: l'Endrine et la Toxaphene. Bull. Agr. Congo
Belge, 50: 131-139.
GUYER, G.E., ADKISSON, P.L., DUBOIS, K., MENZIE, C.,
NICHOLSON, H.P., ZWEIG, G., & DUNN, C.L. (1971) Toxaphene
status report. Special report to Hazardous Materials Advisory
Committee, Washington DC, US Environmental Protection Agency.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutatnt on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGLEY, E.A.C. (1965) Effects of insecticides on the growth
of vegetable seedlings. J. econ. Entom., 58: 777-778.
HARRIS, R.L. & JONES, R.H. (1962) Larvicide tests with
colony-reared Culicoides variipennis. J. econ. Entomol., 55:
575-576.
HAUN, E.C. & CUETO, C. (1967) Fatal toxaphene poisoning in a
9-month-old infant. Am. J. Dis. Child, 113: 616-618.
HEATH, R.G. & STICKEL, L.F. (1965) Protocol for testing the
acute and relative toxicity of pesticides to penned birds,
Washington DC, US Department of the Interior, US Fish and
Wildlife Service, pp. 18-24 (Circular No. 226).
HEATH, R.G., et al. (1970) Unpublished data.
HENDERSON, C.F., PICKERING, Q.H., & TARZWELL, C.M. (1959)
Relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HOFFMAN, D.A. & OLIVE, J.R. (1961) The effects of rotenone
and toxaphene upon plankton of two Colorado reservoirs.
Limnol. Oceanogr., 6: 219-222.
HOLMSTEAD, R.L., KHALIFA, S., & CASIDA, J.E. (1974)
Toxaphene composition analyzed by combined gas
chromatography-chemical ionization mass spectrometry. J.
agric. food Chem., 22: 939-944.
HOOPER, F.F. & GRZENDA, A.R. (1955) The use of toxaphene as
a fish poison. Trans. Am. Fish Soc., 85: 180-190.
HOOPER, N.K., AMES, B.N., BALEK, M.A., & CASIDA, J.E. (1979)
Toxaphene, a complex mixture of polychloroterpenes and a major
insecticide, is mutagenic. Science, 205: 591-593.
HUDSON, R.H., TUCKER, R.K., & HAEGALE, M.A. (1972) Effect of
age on sensitivity. Acute oral toxicity of 14 pesticides to
mallard ducks of several ages. Toxicol. appl. Pharmacol., 22:
556-561.
HUGHES, R. (1970) Studies on the persistence of toxaphene in
treated lakes, PhD thesis, Ann Arbor, Michigan, Xerox
University Microfilms, pp 7-24, 751.
HUGHES, R.A. & LEE, G.F. (1973) Toxaphene accumulation in
fish in lakes treated for rough fish control. Environ. Sci.
Technol., 7: 934-939.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some
effects of DDT, toxaphene and polychlorinated biphenyl on
thyroid function in bobwhite quail. Poult. Sci., 53: 125-133.
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer, pp. 327-348
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IRPTC (1983a) Toxaphene. IRPTC Bull., 5: 27-28.
IRPTC (1983b) Scientific reviews of Soviet literature on
toxicity and hazards of chemicals, Toxaphene, Moscow, Centre
of International Projects (GKNT, No. 32).
IRPTC (1983c) Legal file, Vols. 1 & 2, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
JEFFERY, W.H., AHLIN, T.A., GOREN, C., & HARDY, W.R. (1976)
Loss of warfarin effect after occupational insecticide
exposure. J. Am. Med. Assoc., 236: 2881-2882.
JOHANSEN, C.A. & DAVIS, H.G. (1972) Toxicity of nine
insecticides to the western yellowjacket. J. econ. Entomol.,
65: 40-42.
JOHNSON, W.C. (1966) Toxaphene treatment of Big Bear Lake,
California. Calif. Fish Game, 52: 173-179.
JOHNSTON, B.L. & EDEN, W.G. (1953) The toxicity of aldrin,
dieldrin and toxaphene to rabbits by skin absorption. J. Econ.
Entomol., 46: 702-703.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970)
Insecticide levels in human tissues of Alberta residents. Can.
J. Public Health, 61: 413-416.
KALLMAN, B.J., COPE, O.B., & NAVARRE, R.J. (1962)
Distribution and detoxication of Toxaphene in Clayton Lake,
New Mexico. Trans. Am. Fish. Soc., 91: 14-22.
KATZ, M. (1961) Acute toxicity of some organic insecticides
to three species of salmonids and to the three-spined
stickleback. Trans. Am. Fish. Soc., 90: 264-268.
KEISER, R.K., Jr, JULIA, A.A.A., & RAFAEL, M.S. (1973)
Pesticide levels in estuarine and marine fish and
invertebrates from the Guatemalan Pacific Coast. Bull. Marine
Sci., 23: 905-924.
KEITH, J.O. (1966) Insecticide contaminations in wetland
habitats and their effects on fish-eating birds. J. appl.
Ecol. Suppl., 3: 71-85.
KENNEDY, G.L., FRAWLEY, J.P., & CALANDRA, J.C. (1973)
Multigeneration reproductive effects of three pesticides in
rats. Toxicol. appl. Pharmacol., 25: 589-596.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects
of combinations of pesticides on reproduction in mice. In:
Inter-American Conference on Toxicology and Occupational
Medicine. Pesticide Symposia, pp. 125-138.
KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P. (1966)
Quantitative measurement of induction of hepatic microsomal
enzymes by various dietary levels of DDT and toxaphene in
rats. Toxicol. appl. Pharmacol., 9: 505-513.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty
insecticides to striped bass Morone saxatilis. Calif. Fish
Game, 60: 128.
KUZMINSKAYA, U.A. & IVANITSKI, I.V.A. (1979) Study of some
biological indices of the state of the sympathoadrenaline
system under the effect of polychlorocampher. Environ. Health
Perspect., 30: 91-95.
LACKEY, R.W. (1949) Observations on the acute and chronic
toxicity of toxaphene in the dog. J. ind. Hyg. Toxicol., 3:
117-120.
LAFLEUR, K.S., WOJECK, G.A., & MCCASKILL, W.R. (1973)
Movement of toxaphene and fluoneturon through Dunbar soil to
underlying ground water. J. environ. Qual., 2: 515-518.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II. Dermal Toxicity. Assoc. Food
Drug Offic. US, Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food &
Drug Officials on current developments. Section III. Subacute
and chronic toxicity. Assoc. Food Drug Offic. US, Q. Bull.,
16: 47-53.
LOWE, J.I. (1964) Chronic exposure of spot, Leiostomus
xanthurus, to sublethal concentrations of toxaphene in sea
water. Trans. Am. Fish. Soc., 93: 396-399.
LOWE, J.I., WILSON, P.D., RICK, A.J., & WILSON, A.J. (1971)
Chronic exposure of oysters to DDT, toxaphene, and parathion.
Proc. Natl Shellfisheries Assoc., 61: 71-79.
LUEDEMANN, D. & NEUMANN, H. (1960) [Acute toxicity tests
with new contact insecticides in young carp (Cyprinus carpio
L.).] Z. Angew. Zool., 47: 11-33 (in German).
LUKE, M.A., FROBERG, J.E., & MASUMOTO, H.T. (1975)
Extraction and clean-up of organochlorine, organophosphate,
organo-nitrogen, and hydrocarbon pesticides in produce for
determination by gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem., 58: 1020-1026.
MACEK, K.J. & McALLISTER, W.A. (1970) Insecticide
susceptibility of some common fish family representatives.
Trans. Am. Fish. Soc., 99: 20-27.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) Effects of
temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MAHDI, M.A. (1966) Mortality of some species of fish to
toxaphene at three temperatures. Invest. Fish Contr., 6: 3-10.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in
total diet samples. VIII. Pestic. Monit. J., 9: 94.
MARTIN, J.P., HARDING, R.B., CANNELL, G.H., & ANDERSON, L.D.
(1959) Influence of five annual applications of organic
insecticides on soil biological and physical properties. Soil
Sci., 87: 334-338.
MAYER, F.L. & MEHRLE, P.M. (1976) Vitamin C distribution in
channel catfish as affected by toxaphene. Toxicol. appl.
Pharmacol., 37: 167-168.
MAYER, F.L., MEHRLE, P.M., & DWYER, W.P. (1975) Toxaphene
effects on reproduction, growth and mortality of brook trout,
Washington DC, US Environmental Protection Agency, Office of
Research and Development, 43 pp (US EPA 600/3-75-013).
MCEWEN, L.C., KNITTLE, C.E., & RICHMOND, M.L. (1972)
Wildlife effects from grasshopper insecticides sprayed on
short-grass range. J. Range Manage., 25: 188-194.
MCGEE, L.C., REED, H.L., & FLEMING, J.P. (1952) Accidental
poisoning by toxaphene: Review of toxicology and case reports.
J. Am. Med. Assoc., 149: 1124-1126.
MEHRLE, P.M. & MAYER, F.L. (1975a) Toxaphene effects on
growth and bone composition of fathead minnows, Pimephales
promelas. J. Fish. Res. Board Can., 32: 593-598.
MEHRLE, P.M. & MAYER, F.L. (1975b) Toxaphene effects on
growth and development of brook trout. (Salvelinus fontinalis)
J. Fish. Res. Board Can., 32: 609-613.
MENZIE, C.M. (1972) Fate of pesticides in the environment.
Ann. Rev. Entomol., 17: 199-222.
METCALF, R.L. (1976) Organochlorine insecticides: survey and
prospects. In: Metcalf, R.L. & McKelvey, J.J., ed. Future for
insecticides: needs and prospects. Advances in the
environmental science and technology, Vol. 6.
MUNSON, T.O. (1976) A note on toxaphene in environmental
samples from the Chesapeake Bay region. Bull. environ. Contam.
Toxicol., 16: 491-494.
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of
selected freshwater invertebrates. J. Miss. Acad. Sci., 14:
121-127.
NAQVI, S.M. & FERGUSON, D.E. (1970) Levels of insecticide
resistance in freshwater shrimp, Palaemontes kadiakensis.
Trans. Am. Fish. Soc., 99: 696-699.
NASH, R.C. & WOOLSON, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science, 157: 728-732.
NCI (1979b) Bioassay of toxaphene for possible
carcinogenicity, Cas Registry No. 8001-35-2, National Cancer
Institute (Technical Report Series No. 37-1979).
NEEDHAM, R.G. (1966) Effects of toxaphene on plankton and
aquatic invertebrates in North Dakota lakes, US Department of
the Interior, Fish and Wildlife Service, Bureau of Sport
Fishing and Wildlife (Resource Publication No. 8).
NICHOLSON, H.P., GRZENDA, A.R., LAUER, G.J., COX, W.S., &
TEASLEY, J.I. (1964) Water pollution by insecticides in an
agricultural river basin. I. Occurrence of insecticides in
river and treated municipal water. Limnol. Oceanogr., 9: 310.
NIOSH (1977b) NIOSH manual of analytical methods Part 1. In:
NIOSH monitoring methods, Vol. 1, 2nd ed., Method No. P Cam
225, Washington DC, US Department of Health, Education and
Welfare, pp. 225-1-256-6 (US DHEW(NIOSH), Publication No.
77-157B).
OHSAWA, T., KNOX, J.R., KHALIFA, S., & CASIDA, J.E. (1975)
Metabolic chlorination of toxaphene in rats. J. agric. food
Chem., 23: 98-106.
O'KELLEY, J.C. & DEASON, T.R. (1976) Degradation of
pesticides by algae, Athens, Georgia, US Environmental
Protection Agency, 42 pp (Report EPA-600/3-76-022).
OLSON, K.L., MATSUMURA, F., & BOUSH, G.M. (1980) Behavioral
effects on juvenile rats from perinatal exposure to low-levels
of toxaphene 6, its toxic components, toxicant-A and
toxicant-B. Arch. environ. Contam. Toxicol., 9: 247-257.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic
changes in the liver of rats after feeding low levels of
various insecticides. AMA. Arch. Pathol., 64: 614-622.
PALMER, C.M. & MALONEY, T. E. (1955) Preliminary screening
for potential algicides. Ohio J. Sci., 55: 1-8.
PARDINO, R.S., HEIDKER, J.C., & PAYNE, B. (1971) The effects
of some cyclodiene pesticides, benzenehexachloride and
toxaphene on mitochondrial electron transport. Bull. environ.
Contam. Toxicol., 6: 436-444.
PEAKALL, D.B. (1976) Effects of toxaphene on hepatic enzyme
induction and circulating steriod levels in the rat. Environ.
Health Perspect., 13: 117-120.
PEAKALL, D.B. (1979) Effect of toxaphene on pyruvic and
lactic acid levels in the rat. Environ. Health Perspect., 30:
97-98.
PEAVY, G.M. (1975) Use of diazepam and methocarbamol in the
treatment of toxaphene poisoning in a Bengal tiger. J. Am.
Vet. Med. Assoc., 167: 577-578.
PENUMARTHY, L., OEHME, F.W., SPAULDING, J.E., & RADER, W.A.
(1976) Toxaphene: livestock toxicity and tissue residues.
Vet. Tox., 18: 60-70.
POLLOCK, R.W. (1953) Toxaphene poisoning - Report of a fatal
case. Northw. Med. (Seattle), 52: 293-294.
POLLOCK, G.A. & KILGORE, W.W. (1976) The metabolism of
toxaphene components. Toxicol. appl. Pharmacol., 37: 138-139.
POLLOCK, G.A. & KILGORE, W.W. (1978) Toxaphene. Res. Rev.,
69: 87-140.
POLLOCK, G.A. & KILGORE, W.W. (1980) Toxicities and
description of some toxaphene fractions - isolation and
identification of a highly toxic component. J. Toxicol.
environ. Health, 6: 115-125.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil
insecticides on emergence, growth, yield and chemical
composition of soybeans. Agron. J., 49: 385-387.
REIMOLD, R.J. (1974) Toxaphene interactions in estuarine
ecosystems, Springfield, Virginia, National Technical
Information Service, 89 pp (COM-75-10104/8GA).
REIMOLD, R.J. & DURANT, C.J. (1972) Toxaphene levels in
Georgia survey of estuaries, Georgia, Georgia Marine Science
Center, University of Georgia, 51 pp (Technical Report No.
72-2).
REIMOLD, R.J. & DURANT, C.J. (1974) Toxaphene content of
estuarine fauna and flora before, during and after dredging
toxaphene-contaminated sediments. Pestic. Monit. J., 8: 44-49.
REUBER, M.D. (1979) Carcinogenicity of toxaphene. J.
Toxicol. environ. Health, 5: 729-748.
SAMOSH, L.V. (1974) Chromosome aberrations and character of
satellite associations after accidental exposure of the human
body to polychlorocamphene. Toxicol. Genet., 8: 24-27.
SANBORN, J.R., METCALF, R.L., BRUCE, W.N., & LU, P-Y. (1976)
The fate of chlordane and toxaphene in a terrestrial-aquatic
model ecosystem. Environ. Entomol., 5: 533-538.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several
pesticides to two species of Cladocerans. Trans. Am. Fish
Soc., 95: 165-169.
SCHAPER, R.A. & CROWDER, L.A. (1976) Uptake of
36Cl-toxaphene in mosquito fish, Gambusia affinis. Bull.
environ. Contam. Toxicol., 15: 581-587.
SCHIMMEL, S.C., PATRICK, J.N., & FORESTER, J. (1977) Uptake
and toxicity of toxaphene in several estuarine organisms.
Arch. environ. Contam. Toxicol., 5: 353-367.
SCHOETTGER, R.A. & OLIVE, J.R. (1961) Accumulation of
Toxaphene by fish-food organisms. Limnol. Oceanogr., 6:
216-219.
SCHWARTZ, C.C. & NAVY, J.G. (1974) Pesticide effects on in
vitro dry matter digestion in deer. J. Wildl. Manage., 38:
531-534.
SHELANSKI, H.A. (1947) Various untitled reports and letters
submitted to Hercules Powder Co., Smith Laboratories
(unpublished).
SMITH, D.C. (1971) Pesticide residues in the total diet in
Canada. Pestic. Sci., 2: 92-95.
SMITH, D.C., SANDI, E., & LEDUC, R. (1972) Pesticide
residues in the total diet in Canada II-1970. Pestic. Sci., 3:
207-210.
SMITH, D.C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide
residues in the total diet in Canada III-1971. Pestic. Sci.,
4: 211-214.
SMITH, S.I., WEBER, C.W., & REID, B.C. (1970) The effect of
injection of chlorinated hydrocarbon pesticides on
hatchability of eggs. Toxicol. appl. Pharmacol., 16: 179-185.
STADNYK, L., CAMPBELL, R.S., & JOHNSON, B.T. (1971)
Pesticide effect on growth and 14C assimilation in a
freshwater alga. Bull. environ. Contam. Toxicol., 6: 1-8.
STALLING, D.L. & HUCKINS, J.N. (1976) Analysis and GC-MS
characterization of toxaphene in fish and water, Washington
DC, US Environmental Protection Agency (US EPA 600/3-76-076).
STANLEY, C.W., BARNEY, J.E., II, HELTON, M.R., & YOBS, A.R.
(1971) Measurement of atmospheric levels of pesticides.
Environ. Sci. Technol., 5: 430-435.
TAYLOR, J.R., CALABRESE, V.P., & BLANKE, R.V. (1979)
Organochlorine and other insecticides. In: Vinken, P.J. &
Bruyn, G.W., ed. Handbook of clinical neurology, Vol. 36
Intoxications of the nervous system, Part. 1, Amsterdam, New
York, Elsevier/North-Holland Biomedical Press.
TERRIERE, L.C. & INGALSBE, D.W. (1953) Translocation and
residual action of soil insecticides. J. econ. Entomol., 46:
751.
TERRIERE, L.C., KIIGEMAGI, U., GERLACK, A.R., & BOROVICKA,
R.L. (1966) The persistence of Toxaphene in lake water and
its uptake by aquatic plants and animals. J. agric. food
Chem., 14: 66-69.
TEWARI, S.N. & SHARMA, I.C. (1977) Isolation and
determination of chlorinated organic pesticides by thin-layer
chromatography and the application to toxicological analysis.
J. Chromatogr., 131: 275-284.
TORCHIO, P.F. (1973) Relative toxicity of insecticides to
the honey bee, alkali bee and alfalfa leafcutting bee
(Hymenoptera, Apidae, Halictidae, Megachilidae). J. Kans.
Entomol. Soc., 46: 446-453.
TREON, J.F., CLEVELAND, F.P., POYNTER, B., WAGNER, B., &
GAHEGAN, T. (1952) The physiologic effects of feeding
experimental animals on diets containing toxaphene in various
concentrations over prolonged periods, Kettering Laboratory
(unpublished report).
TROTTMAN, C.H. & DESAIAH, D. (1980) Induction of rat hepatic
microsomal enzymes by toxaphene pretreatment. J. environ. Sci.
Health (Part B: Pesticides, food Contam. agric. Wastes), 15:
121-134.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, US Department of the Interior, Fish
and Wildlife Service, Bureau for Sport Fishing and Wildlife,
pp. 116-117 (Resource Publication No. 84).
UKELES, R. (1962) Growth of pure cultures of marine
phytoplankton in the presence of toxicants. Appl. Microbiol.,
10: 532-537.
US EPA (1976a) Criteria document for toxaphene. Fed. Reg.,
440: 9-76-014.
US EPA (1976b) Economic assessment of proposed toxic
pollutant standards for manufacturers and formulators of
aldrin/dieldrin, endrin, DDT and toxaphene, Washington DC, US
Environmental Protection Agency (Prepared by Arthur D. Little
Inc. for Environmental Protection Agency) (US EPA
230/3-76-016).
US EPA (1979) Reviews of the environmental effects of
pollutants. X. Toxaphene, Washington DC, US Environmental
Protection Agency (US EPA Report 600/1-79-044).
US EPA (1980) Ambient water quality criteria for toxaphene,
Washington DC, US Environmental Protection Agency (Report US
EPA 440/5-80-076).
VON RUMKER, R., LAWLESS, L., MEINER, A.F., LAWRENCE, K.A.,
KELSO, G.L., & HORAY, F. (1974) Production, distribution,
use and environmental impact potential of selected pesticides,
Kansas City, Missouri, Midwest Research Institute, pp. 1-439
(NTIS Publication No. 236).
WARRAKI, S. (1963) Respiratory hazards of chlorinated
camphene. Arch. environ. Health, 1: 253-256.
WEBER, F.H., SHEA, T.B., & BERRY, E.S. (1982) Toxicity of
certain insecticides to protozoa. Bull. environ. Contam.
Toxicol., 28: 628-631.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, M., & CONNEY,
A.H. (1971) Effect of halogenated hydrocarbon insecticides
on the metabolism and uterotropic action of estrogens in rats
and mice. Toxicol. appl. Pharmacol., 19: 234-246.
WHO/FAO (1975) Pesticide residues in food, Vol. 97, pp. 7-37
(Technical Report Series No. 574).
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972a)
Pesticide residues in onions and soil - 1969. Pestic. Monit.
J., 5: 345-347.
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972b) Pesticide
residues in soil from eight cities - 1969. Pestic. Monit. J.,
6: 126-129.
WIERSMA, G.B., TAI, H., & SAND, P.F. (1972c) Pesticide
residue levels in soils, FY 1969 - National Soils Monitoring
Program. Pestic. Monit. J., 6: 194-201.
WORKMAN, G.W. & NEUHOLD, J.M. (1963) Lethal concentrations
of toxaphene for goldfish, mosquito fish and rainbow trout,
with notes on detoxification. Prog. Fish Cult., 25: 23-30.
YANG, H.S.C., WIERSMA, G.B., & MITCHELL, W.G. (1976)
Organochlorine pesticide residues in sugarbeet pulps and
molasses from 16 states, 1971. Pestic. Monit. J., 10: 41-43.
YAP, H.H., DESAIAH, D., CUTKAMP, L.K., & KOCH, R.B. (1975)
In vitro inhibition of fish brain ATPase activity by
cyclodiene insecticide and related compounds. Bull. environ.
Contam. Toxicol., 14: 163-167.
ZAVON, M.R., TYE, R., & LATORRE, L. (1969) Chlorinated
hydrocarbon insecticide content of the neonate. Ann. N.Y.
Acad. Sci., 160: 196-200.
ZWEIG, G., PYE, E.L, SITLANI, R., & PEOPLES, S.A. (1963)
Residues in milk from dairy cows fed low levels of toxaphene
in their daily ration. J. agric. food Chem., 11: 70-72.
 Molecular formula: C10H10Cl8 (approximately)
CAS chemical name: Toxaphene (a mixture of poly-
chlorinated bicyclic terpenes with
chlorinated camphenes predominating)
Trade names: Altox, Chem-Phene M5055, Chlor Chem
T-590, Crestoxo, Estonox, Fasco-
Terpene, Geniphene, Gy-Phene,
Hercules 3956, Huilex, Penphene,
Phenacide, Phenatox, Polychlor-
camphen, Strobane-T, Toxakil,
Toxaphene, Toxon 63
CAS registry number:
Molecular formula: C10H10Cl8 (approximately)
CAS chemical name: Toxaphene (a mixture of poly-
chlorinated bicyclic terpenes with
chlorinated camphenes predominating)
Trade names: Altox, Chem-Phene M5055, Chlor Chem
T-590, Crestoxo, Estonox, Fasco-
Terpene, Geniphene, Gy-Phene,
Hercules 3956, Huilex, Penphene,
Phenacide, Phenatox, Polychlor-
camphen, Strobane-T, Toxakil,
Toxaphene, Toxon 63
CAS registry number: 