
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 50
TRICHLOROETHYLENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1985
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154190 3
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1985
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TRICHLOROETHYLENE
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Uses and sources of exposure
1.1.3. Industrial exposure
1.1.4. Environmental transport and distribution
1.1.5. Absorption, distribution, biotransformation, and
elimination
1.1.6. Effects on experimental animals
1.1.7. Effects on man
1.2. Recommendations for further research
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.2.1. Pure trichloroethylene
2.2.1.1 Chemical reactivity
2.2.1.2 Chemical degradation
2.2.1.3 Photochemical degradation
2.2.2. Commercial trichloroethylene
2.3. Analytical methods
2.3.1. Identification and purity assessment
2.3.1.1 Colorimetry tests
2.3.1.2 Infra-red spectroscopy
2.3.1.3 Gas-liquid chromatography
2.3.2. Determination in environmental media
2.3.2.1 Soil
2.3.2.2 Water
2.3.2.3 Air
2.3.2.4 Foodstuffs
2.3.3. Determination in human tissues and fluids
2.3.3.1 Trichloroethylene
2.3.3.2 Trichloroacetic acid
2.3.3.3 Trichloroethanol
2.3.3.4 Total trichloro derivatives
2.3.4. Sensitivity
3. SOURCES IN THE ENVIRONMENT, USES, AND SAFE HANDLING
3.1. Production processes, levels, and uses
3.1.1. Production processes and levels
3.1.2. Uses
3.2. Handling hazards, and precautions
3.2.1. Handling hazards
3.2.1.1 Fire, explosion, and thermal decomposition
3.2.1.2 Chemical reactivity
3.2.2. Handling precautions
3.2.2.1 Personal safeguards
3.2.2.2 Storage
3.2.3. Recovery
3.2.4. Disposal
3.2.5. Emergency measures in case of accidental spills
3.2.6. Occupational exposure
4. ENVIRONMENTAL LEVELS, TRANSPORT AND DISTRIBUTION
4.1. Environmental levels
4.1.1. Soils and sediments
4.1.2. Water
4.1.3. Air
4.1.4. Biota
4.1.5. Food
4.2. Environmental distribution and transport
4.2.1. Equilibrium distribution
4.2.2. Transformation in the environment
4.2.2.1 Air
4.2.2.2 Soils and sediments
4.2.2.3 Water
4.2.2.4 Biota
5. KINETICS AND METABOLISM
5.1. Absorption
5.1.1. Inhalation exposure
5.1.2. Oral exposure
5.1.3. Dermal exposure
5.2. Distribution and storage
5.3. Metabolic transformation
5.3.1. Animals
5.3.2. Human beings
5.3.3. Drug and other interactions
5.4. Elimination
5.4.1. Studies on animals
5.4.2. Studies on man
5.5. Biological monitoring of exposure
6. EFFECTS ON ANIMALS AND CELL SYSTEMS
6.1. Effects on animals
6.1.1. Acute toxicity
6.1.2. Short-term exposures
6.1.2.1 Oral exposures
6.1.2.2 Inhalation exposure
6.1.2.3 Parenteral exposure
6.1.3. Long-term exposure
6.1.3.1 Oral exposure
6.1.3.2 Inhalation exposure
6.1.3.3 Parenteral exposure
6.1.4. Interactions
6.1.5. Immunotoxicity
6.1.6. Effects on cell systems
6.1.7. Carcinogenicity
6.1.7.1 Conclusions
6.1.8. Mutagenicity
6.1.8.1 Gene mutation
6.1.8.2 Chromosome aberrations
6.1.8.3 DNA damage
6.1.8.4 Mammalian cells (in vitro)
6.1.8.5 Mutagenic activity of trichloroethylene
metabolites
6.1.8.6 Conclusions
6.1.9. Reproduction, embryo/fetotoxicity, and teratology
6.1.9.1 Avian embryo system
6.1.9.2 Mouse
6.1.9.3 Rat
6.1.9.4 Rabbit
7. EFFECTS ON THE ENVIRONMENT
7.1. Aquatic organisms
7.2. Uptake, distribution, storage, metabolism, and elimination
in plant and animal organisms
7.3. Effects on the stratospheric ozone layer
8. EFFECTS ON MAN
8.1. General symptoms and signs
8.1.1. Acute effects
8.1.2. Chronic effects
8.2. Effects on organs and systems
8.2.1. Effects on the nervous system
8.2.2. Effects on the cardiovascular system
8.2.3. Effects on the respiratory system
8.2.4. Effects on the urinary tract
8.2.5. Effects on the skin
8.2.6. Effects on the eye
8.2.7. Carcinogenicity
9. EVALUATION OF THE HEALTH RISKS FOR MAN
9.1. Levels of exposure
9.1.1. General population
9.1.2. Occupational exposure
9.2. Evaluation of human health risks
9.2.1. Acute effects
9.2.2. Chronic effects
9.3. Treatment of poisoning in human beings
9.3.1. Emergency measures
9.3.1.1 General points
9.3.1.2 Ingestion
9.3.1.3 Inhalation
9.3.1.4 Dermal exposure
9.3.1.5 Eye exposure
REFERENCES
APPENDIX I
REFERENCES TO APPENDIX I
TASK GROUP ON TRICHLOROETHYLENE
Members
Dr R.F. Addison, Department of Fisheries and Oceans, Bedford
Institute of Oceanography, Dartmouth, Nova Scotia, Canada
Dr O. Axelson, Department of Occupational Medicine, University
Hospital, Linköping, Sweden
Dr A. Di Domenico, High Institute of Health, Rome, Italy
Prof S.D. Gangolli, British Industries Biological Research
Association, Carshalton, Surrey, United Kingdom
Prof M. Ikeda, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan
Dr S.E. Jaggers, Central Toxicology Laboratory, ICI, Macclesfield,
Cheshire, United Kingdom
Prof N. Loprieno, Laboratory of Genetics, University of Pisa, Pisa,
Italy
Dr C. Maltoni, Oncology Institute and Tumour Centre "Felice
Addari", Bologna, Italy
Dr J.H. Mennear, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina
Dr A.C. Monster, Coronel Laboratory, University of Amsterdam,
Amsterdam, The Netherlands
Prof I.V. Sanotsky, Research Institute of Industrial Hygiene and
Occupational Diseases, USSR Academy of Medical Sciences, Moscow,
USSR
Representatives from Other Organizations
Mr J. Wilbourn, International Agency for Research on Cancer, Lyons,
France
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Observers
Prof E. Malizia, Emergency Toxicological Service, Antivenom Center,
Umberto the First Polyclinic, La Sapienza University, Rome,
Italy
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible. In the interest of
all users of the environmental health criteria documents, readers
are kindly requested to communicate any errors found to the Manager
of the International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in
the criteria documents are kindly requested to make available to
the WHO Secretariat any important published information that may
have inadvertently been omitted and which may change the evaluation
of health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conclusions contained in the
criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TRICHLOROETHYLENE
Following the recommendations of the United Nations Conference
on the Human Environment held in Stockholm in 1972, and in response
to a number of resolutions of the World Health Assembly (WHA23.60,
WHA24.47, WHA25.58, WHA26.68), and the recommendation of the
Governing Council of the United Nations Environment Programme,
(UNEP/GC/10, 3 July 1973), a programme on the integrated assessment
of the health effects of environmental pollution was initiated in
1973. The programme, known as the WHO Environmental Health
Criteria Programme, has been implemented with the support of the
Environment Fund of the United Nations Environment Programme. In
1980, the Environmental Health Criteria Programme was incorporated
into the International Programme on Chemical Safety (IPCS). The
Programme is responsible for a series of criteria documents.
A WHO Task Group on Environmental Health Criteria for
Trichloroethylene was held in Rome from 10 to 15 December, 1984.
Dr E.M. Smith opened the meeting on behalf of the Director-General.
The Task Group reviewed and revised the draft criteria document and
made an evaluation of the health risks of exposure to
trichloroethylene.
The draft criteria document was developed by the ISTITUTO
SUPERIORE DI SANITA, Rome, Director PROFESSOR F. POCCHIARI; the
principal author was DR A. DI DOMENICO.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously covered the costs of printing.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
Trichloroethylene is a colourless liquid with a characteristic,
slightly sweet odour. It is used as a solvent in a variety of
applications. There are a number of techniques suitable for
the determination of trichloroethylene including colorimetry,
infra-red spectroscopy, gas-liquid chromatography (GLC), and gas
chromatography/mass spectrometry. In GLC, the use of flame
ionization detection gives good sensitivity; however, electron
capture detection is markedly more sensitive. Methods are
available for the determination of trichloroethylene in blood, fat,
other tissues, food, water, etc.
1.1.2. Uses and sources of exposure
A major use of trichloroethylene is in metal degreasing;
other significant uses are in textile cleaning, solvent extraction
processes, and as a carrier solvent. It is no longer used as a
grain fumigant and is now only occasionally used in anaesthesia.
For practical use, trichloroethylene requires the addition of
stabilizers (up to 2%).
There may be exposure to both the vapour and the liquid in the
workplace, the highest atmospheric concentrations occurring in open
degreasing processes. Trichloroethylene may be emitted from
industrial plants in the form of a vapour and in aqueous effluent.
The major part of the annual world production of trichloro-
ethylene (estimates range from 60 to 90%) is released into the
environment.
1.1.3. Industrial exposure
Exposure in the workplace is mainly through inhalation of
trichloroethylene vapour, but skin contamination with the liquid
also occurs. The highest levels of occupational exposure occur
in metal cleaning processes. Atmospheric trichloroethylene
concentrations up to several hundred mg/m3 have been recorded.
Exposure during the actual production of trichloroethylene is
relatively low because of the nature of the process. Oral intake
is insignificant in occupational terms.
1.1.4. Environmental transport and distribution
Contamination of water has been reported but, with the
exception of contamination of water supplies through accidental
spillage, levels have been very low. Trichloroethylene is probably
widely distributed in the environment, but usually only at fairly
low levels, i.e., in the µg/kg range in sediments, in the low
µg/litre range in natural waters, in the low µg/m3 range in air,
and in the µg/kg range in aquatic biota. The limited toxicity data
available show LC50 values for aquatic biota in the mg/litre range.
Trichloroethylene is degraded in biological and abiotic systems;
in air (where most environmental trichloroethylene is expected to
occur), its lifetime is about 10 days. It seems unlikely that the
present rate of release of trichloroethylene into the environment
would contribute significantly to depletion of the stratospheric
ozone layer.
1.1.5. Absorption, distribution, biotransformation, and elimination
The most significant uptake of trichloroethylene is through
inhalation of the vapour, but uptake can also take place through
the skin or via the gastrointestinal tract. Inhalation exposure
is monitored by determining time-weighted average atmospheric
concentrations.
Following absorption, trichloroethylene is rapidly distributed
and accumulates in the adipose tissue. It easily crosses the
placental barrier. Trichloroethylene is eliminated unchanged in
exhaled air and, to a lesser extent, in faeces, sweat, and the
saliva. It is rapidly metabolized, mainly in the liver.
At least 4 mammalian metabolites of trichloroethylene have
been identified: trichoroethanol, trichloroacetic acid,
2-hydroxyacetylethanolamine, and oxalic acid; dichloroacetic acid
appears to be specific to mice. The major metabolites in human
beings, trichloroethanol and trichloroacetic acid, are excreted
in the urine. Estimations of levels of these major urinary
metabolites or total trichloro compounds in urine may be used for
the biological monitoring of exposure.
There are species differences in the rate of metabolism of
trichloroethylene to trichloroacetic acid, the rate in the mouse
being more rapid than that in the rat. Isolated hepatocytes
obtained from the mouse and the rat accurately reflect the in
vivo metabolic rates. Isolated human hepatocytes metabolize
trichloroethylene to trichloroacetic acid at a slower rate than rat
hepatocytes.
In man, the metabolism of trichloroethylene decreases when
ethanol has been ingested, and intolerance may occur.
1.1.6. Effects on experimental animals
Trichloroethylene is a moderately toxic substance. In terms of
acute toxicity, LC50 values in rodent test species range from 45 to
260 mg/m3, and oral LD50 values range from 2400 to 4920 mg/kg body
weight. The toxic effects of exposure are related to a depressant
action on the central nervous system. Central nervous system
depression can lead to coma and death. Liquid trichloroethylene
has an irritant effect on the skin and eyes; trichloroethylene
vapour is irritant to the respiratory tract. Toxic effects on the
kidneys are produced in rats by long-term oral administration.
Minimal changes in the kidneys can occur after oral administration
of 100 mg/kg body weight per day for 13 weeks and nephrotic changes
can be found following oral administration of 500 mg/kg body weight
per day, for 2 years. In mice, toxic effects on the kidney occur
after oral administration of 3000 mg/kg per day, for 13 weeks, and
mild nephrotic changes following 1000 mg/kg per day, for 2 years.
Also, in mice, oral administration of 6000 mg/kg body weight per
day for 13 weeks produced necrotic changes in the liver.
Continuous exposure of mice by inhalation to 810 mg/m3
trichloroethylene for 2 days resulted in an increased relative
liver weight, which decreased following cessation of exposure.
Some immunological changes have been observed in rodents
exposed to trichloroethylene by inhalation at concentrations
between 10 and 1000 mg/m3 for several weeks and also in those given
trichloroethylene in their drinking-water (0.1 - 5 g/litre) for a
similar period.
Trichloroethylene does not cause any biologically significant
embryotoxic or teratogenic effects.
The evidence for mutagenic effects is inconclusive.
There is clear evidence that trichloroethylene is carcinogenic
in mice with lifetime (2-year) exposures to 1620 mg/m3 by
inhalation or oral adiministration of 700 - 1200 mg/m3 body weight
per day. There is some evidence that trichloroethylene causes
tumours in rats; a low incidence of renal tumours occurred in rats
exposed for 2 years to levels of 3240 mg/m3 by inhalation or 500 -
1000 mg/kg per day by the oral route. There are species and strain
differences in carcinogenic response and the purity of the
trichloroethylene and the nature of any additives affect the
outcome.
1.1.7. Effects on man
The signs and symptoms of over-exposure in human beings are
mainly related to the central nervous system; for example,
headache, drowsiness, hyperhydrosis, tachycardia, and, in more
severe cases, stupor and coma. Trichloroethylene is analgesic and
anaesthetic; inhalation of concentrations between 27 000 mg/m3
(5000 ppm) and 108 000 mg/m3 (20 000 ppm) have been used in
anaesthetic procedures.
Fatalities have been reported through accidental or suicidal
over-exposure to trichloroethylene. In general, the lethal oral
dose for an adult is of the order of 7000 mg/kg body weight, but a
death has occurred following a single dose of 50 ml (75 g). Deaths
have been reported following inhalation of trichloroethylene,
including a number that have occurred during anaesthetic
procedures. While respiratory depression cannot be excluded,
it is more likely that cardiac arrest, related to the arrhythmic
properties of trichloroethylene, was the cause of death.
In laboratory and work-place studies, demonstrable psychomotor
impairment was found following inhalation exposure to 5400 mg/m3
(1000 ppm) for 2 h, and reaction time was increased by exposure to
a concentration of 1320 mg/m3 (245 ppm), under work-place
conditions.
Effects on the respiratory and gastrointestinal tracts
are related to the irritant properties of trichloroethylene.
Irritation of mucous membranes occurs with exposure to
trichloroethylene vapour at concentrations of 810 - 3510 mg/m3
(150 - 650 ppm). At autopsy, following fatal ingestion, lesions
of the gastrointestinal tract have been found.
Liquid trichloroethylene and its vapour at anaesthetic
concentrations (27 500 - 108 000 mg/m3) cause eye irritation and
superficial corneal damage, which normally recovers completely.
Liquid trichloroethylene is mildly irritating to the skin but, if
it is held in contact for any length of time, for example, by
clothing or footwear, it can produce marked skin irritation with
blistering. Repeated contact produces defatting of the skin and
dermatitis.
High oral doses (200 - 300 ml or more), taken suicidally or
through misuse, have produced toxic effects on the liver and
kidneys. Hepatic necrosis and nephropathy have been found at
autopsy. The use of trichloroethylene in a confined unventilated
space for 3 - 4 h has also resulted in liver and kidney damage.
Addiction to trichloroethylene ("vapour sniffing") has produced
liver and kidney damage, and deaths have occurred.
Chronic neurotoxic effects may occur, and a "psychoorganic
syndrome", with lassitude and depression, has been described but
has not been found consistently in studies on groups of
trichloroethylene workers. It is probable that many of the effects
described were due to the metabolites of trichloroethylene.
Degeneration of cranial nerves has occurred following short-
term exposures to high levels of trichloroethylene, generally in
enclosed spaces. However, it is considered that the cranial
neuropathy is probably due to breakdown products, mainly
dichloroacetylene, rather than to trichloroethylene itself.
Polyneuropathies have been reported following long-term exposure.
Data from epidemiological studies on carcinogenicity in
occupationally exposed groups are inconclusive.
1.2. Recommendations for Further Research
1. The toxic action and thresholds for toxic effects of
trichloroethylene in human beings and experimental animals, at
low levels of short- and long-term exposure, need to be defined
in more detail.
2. Studies are required for a full evaluation of the genotoxicity
of trichloroethylene. Trichloroethylene samples of high purity
(with full data on the nature and the amount of any impurities)
should be used as well as trichloroethylene samples stabilized
with non-mutagenic compounds (e.g., amines).
3. The significance for human beings of the effects seen in
rodents with long-term exposures requires further study. The
role of metabolism in carcinogenesis, both the rates and the
metabolites formed, and the production of biochemical responses
that may be the mechanisms of carcinogenic responses in target
tissues require further study and interspecies comparison.
4. In view of the equivocal evidence for mutagenicity in bacterial
and mammalian cell systems, there is an implication that
epigenetic mechanisms may be involved in the carcinogenic
effects observed in experimental animals.
It should be noted that trichloroacetic acid produced by the
metabolism of trichloroethylene has induced peroxisome
proliferation, with differences in response in isolated
hepatocytes from the mouse, rat, and human beings. Peroxisome
proliferation has been implicated in the epigenetic induction
of hepatocellular carcinoma in mice and rats.
5. The pathological significance of trichloroethylene-induced
cytomegaly and karyomegaly of renal tubular cells and the
incidence in untreated laboratory rodents of tubular renal
carcinoma should be investigated.
6. There should be further epidemiological studies to investigate
the possible carcinogenic effects of trichloroethylene exposure.
Additional cohort studies should be initiated. Registers of
TCA-monitoring data should be organized with epidemiological
studies in mind. Case-control studies, particularly of
haemolymphatic, pancreatic, and genito-urinary tract cancers,
should specifically consider exposure to trichloroethylene in
industry, dry-cleaning operations, and via food, such as
decaffeinated coffee.
7. Biological monitoring should be extended and more attention
paid to interindividual differences in toxicokinetics and to
the factors responsible for these, such as anthropometric
parameters, sex, genetic make-up, use of drugs and alcohol, and
interactions with certain chemicals in the environment.
8. Workers with moderate levels of exposure to trichloroethylene
tend to have an increased incidence of subjective symptoms.
There should be a systematic approach to the clearer
identification, analysis, and evaluation of such symptoms and
their correlation with levels of occupational exposure in
different industrial environments.
9. Although, at present, trichloroethylene does not appear to be
a major environmental problem, this assessment is based on
relatively few data describing its distribution in the
environment, and its rates and routes of degradation. More
comprehensive data should be obtained, and an assessment of
geographical or temporal changes in trichloroethylene
distribution should be made.
There should be studies on controlling the input of
trichloroethylene into the environment, and the provision of
disposal methods other than incineration should also be
studied.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Trichloroethylene is an aliphatic substance of the organic
halogen and halogen-derivative families.
Chemical structure:
H Cl
\ /
C ===== C
/ \
Cl Cl
Molecular formula: C2HCl3
IUPAC and CAS name: trichloroethene
Common synonyms: acetylene trichloride, ethinyl
trichloride, ethylene trichloride,
1-chloro-2,2-dichloroethylene,
1,1-dichloro-2-chloroethylene,
1,1,2-trichloroethylene, TCE, TRI
Common trade names: Algylen, Anamenth, Benzinol,
Blacosolv, Blancosolv, Cecolene,
Chlorilen, Chlorylen, Circosolv,
Densin-fluat, Dow-Tri, Dukeron,
Fleck-Flip, Flock-Flip, Fluate,
Gemalgene, Germ-algene, Lanadin,
Lethurin, Narcogen, Narkosoid, Nialk,
Perm-a-Chlor, Pet-zinol, Philex,
Threthylen, Threthylene, Trethylene,
Triad, Trial, Triasol, Trichloran,
Trichloren, Triclene, Trielene,
Trielin, Trielina, Triklone, Trilen,
Trilene, Triline, Trimar, Triol, Tri-
Plus, Tri Plus M, Vestrol, Vitran
CAS registry number: 79-01-6
Relative molecular mass: 131.40
Conversion factor 1 ppm trichloroethylene = 5.4 mg/m3
2.2. Physical and Chemical Properties
2.2.1. Pure trichloroethylene
In its pure state, trichloroethylene is a colourless liquid
with a characteristic, slightly sweet odour; the odour threshold
for human beings is 540 mg/m3 (100 ppm) (Torkelson & Rowe, 1982).
Some physical and chemical properties of pure trichloroethylene
are listed in Table 1.
2.2.1.1. Chemical reactivity
Trichloroethylene oxidizes to yield acids, including
hydrochloric acid (Aviado et al., 1976). Its reactivity increases
with rise in temperature and with exposure to ultraviolet radiation
(UVR). Under pressure, at 150 °C, it reacts with alkalis to
produce glycolic acid. With sulfuric acid, it reacts to produce
monochloroacetic acid (Kirk & Othmer, 1964). In the presence of
alkali, dehydrochlorination may occur in solution as well as in the
vapour phase, with the formation of dichloroacetylene, which is
highly neurotoxic and carcinogenic for animals and probably for man
(Henschler et al., 1970a).
2.2.1.2. Chemical degradation
The chemical degradation of trichloroethylene in water is very
slow. In contact with red-hot metals or a direct flame, liquid or
vapour-phase trichloroethylene decomposes to form phosgene and
hydrogen chloride (Waters et al., 1977).
2.2.1.3. Photochemical degradation
Photochemical reactions initiate the degradation of
trichloroethylene in the environment. When exposed to UVR and
humidity, the compound decomposes to form acids that have mean
half-lives ranging from 6 to 12 weeks (Correia et al., 1977). With
an OH- concentration of the order of 106 molecules/cm3 (accepted
mean value), a calculated half-life of trichloroethylene is around
5 days (De More et al., 1983). Trichloroethylene exposure to xenon
arc lamp radiation with a wavelength greater than 290 nm, at
constant temperature, produces carbon monoxide, carbon dioxide,
water, hydrogen chloride, dichloroacetyl chlorides, and phosgene;
the phosgene hydrolyses to produce carbon dioxide and hydrogen
chloride. Dichloroacetyl chlorides enter the hydrosphere as
dichloroacetate anions (McConnell et al., 1975).
Table 1. Physical and chemical properties of trichloroethylene
--------------------------------------------------------------------------
Melting point (°C) -84.8 (freeze) Windholz et al.
(1976)
-87.1 Kirk & Othmer
(1979)
-73.0 CRC (1980)
Boiling point (°C) 86.7 (760 mm Hg) Windholz et al.
(1976)
-43.8 (1 mm Hg) Windholz et al.
(1976)
--------------------------------------------------------------------------
Table 1. (contd.)
--------------------------------------------------------------------------
Specific gravity 1.46 (25/25 °C) Snell & Hilton
(1967)
1.4904 (4/4 °C) Windholz et al.
(1976)
(vapour density; air = 1) 4.53 (25 °C) Windholz et al.
(1976)
(vapour, g/litre) 4.45 (86.7 °C) Kirk & Othmer
(1979)
Vapour pressure (torr) 5.4 (-20 °C) Snell & Ettre
(1970a)
20.1 (0 °C) Snell & Ettre
(1970a)
57.8 (20 °C) Snell & Ettre
(1970a)
305.7 (60 °C) Snell & Ettre
(1970a)
Other properties:
Refraction index (nD) 1.4782 (20 °C) Kirk & Othmer
(1979)
(vapour) 1.001784 (0 °C) Kirk & Othmer
(1979)
Viscosity (cP) 0.58 (20 °C) Kirk & Othmer
(1979)
(vapour) 10 300 (60 °C) Kirk & Othmer
(1979)
Dielectric constant 3.42 (16 °C) Kirk & Othmer
(epsilon) (1979)
Coefficient of cubic 0.00119 (0 - 40 °C) Kirk & Othmer
expansion (1979)
Surface tension (dyn/cm) 26.4 (20 °C) Kirk & Othmer
(1979)
Critical temperature (°C) 271.0 Kirk & Othmer
(1979)
Critical pressure (atm) 49.7 Kirk & Othmer
(1979)
Dipole moment (debye) 0.90 Kirk & Othmer
(1979)
--------------------------------------------------------------------------
Table 1. (contd.)
--------------------------------------------------------------------------
Heat of combustion 1.751 Kirk & Othmer
(kcal/g) (1979)
Heat of formation 0.999 Kirk & Othmer
(kcal/mole) (1979)
(vapour) -7.00 Kirk & Othmer
(1979)
Latent heat of 57.4 (86.7 °C) Kirk & Othmer
vaporization (cal/g) (1979)
Flammability flash point (°C)
under various conditions Non-flammable under normal Kirk & Othmer
working conditions; Vapours (1979)
(12.5 - 90% v/v) in poorly- CRC (1967)
ventilated rooms at
temperatures between 30 and ASCHIMICI (1980)
82 °C may ignite if in
contact with high-
temperature heat sources;
Vapour ignites (t>25.5 °C) Aviado et al.
if mixed with pure oxygen (1976)
(10.3-64.5% v/v)
ignition temp. (°C) 410 ASCHIMICI (1980)
danger of explosion:
limits (% v/v in air)a 8.0 - 10.5 (25.5 °C) Kirk & Othmer
8.0 - 52.0 (100 °C) (1979)
oxidizing properties none ASCHIMICI (1980)
Solubility:
in water (g/litre) 1.07 (20 °C) Kirk & Othmer
(1979)
1.24 (60 °C) Kirk & Othmer
(1979)
in organic solvents completely Windholz et al.
miscible with (1976)
several organic
solvents
in oil miscible Windholz et al.
(1976)
--------------------------------------------------------------------------
Table 1. (contd.)
--------------------------------------------------------------------------
n-octanol/water partition Log Ko/w 2.42 Banerjee et al.
coefficient (log) (1980)
Organic carbon partition Ko/w x 0.6 Karickhoff et al.
coefficient, Koc (1979)
Bioconcentration factor, Ko/w x 0.048 Mackay (1982)
KB
--------------------------------------------------------------------------
a See section 3.2.1.1.
2.2.2. Commercial trichloroethylene
Trichloroethylene produced for chemical reagent uses has a
minimum purity of 99.85%. The commercial product can contain
impurities and stabilizers as shown in Table 2.
Table 2. Commercial trichloroethylene: examples of
impurities and commonly-used stabilizers
------------------------------------------------------
Impurities Stabilizers
------------------------------------------------------
carbon tetrachloride pentanol-2
chloroform thymol
1,2-dichloroethane triethanolamine
trans 1,2-dichloroethylene triethylamine
cis 1,2-dichloroethylene 2,2,4-trimethylpentene-1
pentachloroethane cyclohexene oxide
1,1,1,2-tetrachloroethane n-propanol
1,1,2,2-tetrachloroethane iso-butanol
1,1,1-trichloroethane n-methyl morpholine
1,1,2-trichloroethane diisopropylamine
1,1-dichloroethylene n-methyl pyrrole
bromodichloroethylene methyl ethyl ketone
perchloroethylene epichlorohydrina
bromodichloromethane
benzene
------------------------------------------------------
a Now used to a much lesser extent commercially.
Possible impurities depend on the manufacturing route, the type
and quality of feed stock used, the type of distillation equipment,
and the technical specification being met. It is uncommon for any
individual impurity to be present at a level in excess of 100 mg/kg
and for the total impurities to exceed 1000 mg/kg; not all the
impurities listed would be detected in any sample.
Stabilizers, in the form of antioxidants or acid-receptors
(such as phenolic, olephinic, pyrrolic, and/or oxiranic derivatives
and aliphatic amines), are usually added in concentrations that
normally range from 20 to 600 mg/kg. However, in some cases, for
limited quantities and special uses, concentrations as high as
5000 mg/kg are added. The stabilizers used will depend on patent
ownership and the technical specification being met.
2.3. Analytical Methods
2.3.1. Identification and purity assessment
The degree of purity of trichloroethylene can be established by
a number of methods, described by Snell & Ettre (1970a). Some
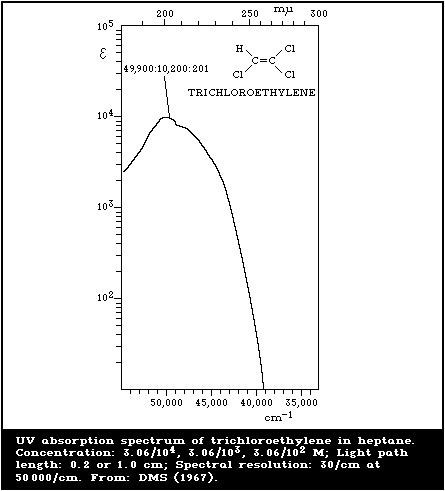
spectral features of trichloroethylene are shown in Fig. 1 - 3.
Trichloroethylene can be determined by the following analytical
methods.
 2.3.1.1. Colorimetry tests
In the Fujiwara test, trichloroethylene is treated with
pyridine in an alkaline environment. Solution absorbance is then
determined at 535 or 470 nm (absorptivity: 18 - 32 litre/g x cm)
with a sensitivity of about 1 mg/kg. This test is suitable for
other aliphatic halogenated compounds, and so is not substance-
specific. Other complementary colorimetric tests that may enable
trichloroethylene to be differentiated from other similar compounds
have been reported by Snell & Ettre (1970b).
2.3.1.1. Colorimetry tests
In the Fujiwara test, trichloroethylene is treated with
pyridine in an alkaline environment. Solution absorbance is then
determined at 535 or 470 nm (absorptivity: 18 - 32 litre/g x cm)
with a sensitivity of about 1 mg/kg. This test is suitable for
other aliphatic halogenated compounds, and so is not substance-
specific. Other complementary colorimetric tests that may enable
trichloroethylene to be differentiated from other similar compounds
have been reported by Snell & Ettre (1970b).
 2.3.1.2. Infra-red spectroscopy
In the gaseous phase, quantities are determined by measuring
the optical density of the mixture at the selected wavelength of
11.8 µm (847/cm). This corresponds to a detection sensitivity of
not less than 0.5 µg/litre (Fishbein, 1973).
In solutions of carbon disulfide, trichloroethylene can be
measured, even in the presence of similar chloro derivatives, using
the specific band at 10.8 µm (926/cm) (Snell & Ettre, 1970b;
Fishbein, 1973). Detection thresholds of some µg/litre can be
attained (Fishbein, 1973).
2.3.1.3. Gas-liquid chromatography
Generally, either packed or capillary columns (low-resolution
or high-resolution chromatography, respectively) are used; the
latter are recommended for complex mixtures containing substances
similar to trichloroethylene. A number of stationary phases can
be used, such as paraffinic hydrocarbons (squalene, hexadecane,
paraffin), Apiezon L, Carbowax (600, 4000, 20M), silicones (SE-30,
550, SF-96-350), and arylphosphates. In general, detectors such as
argon ionization or flame ionization detectors (sensitivity: ~10 ng)
are suitable for several types of analyses; the thermoconductivity
detector is little used, because it is less sensitive (sensitivity:
~250 ng). The detection threshold drops considerably (~0.02 ng in
air) with electron-capture detectors (ECD). ECD response can be
improved slightly by adding small quantities of oxygen to the
carrier gas (Miller & Grimsrud, 1979).
Gas chromatography combined with mass spectrometry (GC/MS) is
both highly selective and sensitive (Snell & Ettre, 1970b).
2.3.1.2. Infra-red spectroscopy
In the gaseous phase, quantities are determined by measuring
the optical density of the mixture at the selected wavelength of
11.8 µm (847/cm). This corresponds to a detection sensitivity of
not less than 0.5 µg/litre (Fishbein, 1973).
In solutions of carbon disulfide, trichloroethylene can be
measured, even in the presence of similar chloro derivatives, using
the specific band at 10.8 µm (926/cm) (Snell & Ettre, 1970b;
Fishbein, 1973). Detection thresholds of some µg/litre can be
attained (Fishbein, 1973).
2.3.1.3. Gas-liquid chromatography
Generally, either packed or capillary columns (low-resolution
or high-resolution chromatography, respectively) are used; the
latter are recommended for complex mixtures containing substances
similar to trichloroethylene. A number of stationary phases can
be used, such as paraffinic hydrocarbons (squalene, hexadecane,
paraffin), Apiezon L, Carbowax (600, 4000, 20M), silicones (SE-30,
550, SF-96-350), and arylphosphates. In general, detectors such as
argon ionization or flame ionization detectors (sensitivity: ~10 ng)
are suitable for several types of analyses; the thermoconductivity
detector is little used, because it is less sensitive (sensitivity:
~250 ng). The detection threshold drops considerably (~0.02 ng in
air) with electron-capture detectors (ECD). ECD response can be
improved slightly by adding small quantities of oxygen to the
carrier gas (Miller & Grimsrud, 1979).
Gas chromatography combined with mass spectrometry (GC/MS) is
both highly selective and sensitive (Snell & Ettre, 1970b).
 2.3.2. Determination in environmental media
2.3.2.1. Soil
High-resolution GC (hrGC-ECD) has been used for determining
trichloroethylene in soil (De Leon et al., 1980). The hrGC/MS
combination has been used as a confirmatory technique, with a
detection threshold of ~10 mg/kg (10 ppm).
2.3.2.2. Water
Levels of trichloroethylene in water can be determined by
hrGC/MS (Dowty et al., 1975), by GC with an electron-capture
detector or an electrolytic conductivity detector (Nicholson et
al., 1977; Dietz & Singley, 1979), and by various other techniques
such as HPLC, hrGC, GC, and GC/MS (Eklund et al., 1978; Jungclaus
et al., 1978). Where specified, detection thresholds are in the
region of 1.0 µg/litre, or lower.
2.3.2.3. Air
GC/MS can be used to measure trichloroethylene levels in the
urban atmosphere (Ioffe et al., 1977). Herbolsheimer et al.
(1972), Sawicki et al. (1975), NIOSH (1977a), Heil et al. (1979),
and Makide et al. (1979) describe sampling and sample enrichment
techniques. Detection capacity may be as low as some ng/m3.
Fujiwara's test can be used to measure trichloroethylene levels
in air (Rush, 1970).
Gas detector tubes (Kitagawa, 1961), activated carbon tubes
(NIOSH, 1977a; Shipman & Whim, 1980), and activated carbon felt
badges (Hirayama & Ikeda, 1979) are available for use in work-place
environments. Gas detector tubes are suitable for spot sampling,
while activated carbon tubes and felt are suitable for time-
weighted average concentration determinations.
2.3.2.4. Foodstuffs
High- and low-resolution GLC can be used for the determination
of trichloroethylene and other aliphatic chloro derivatives in
various foodstuffs (Entz & Hollifield, 1982). The head-space
technique is used in all cases. The sensitivity of the method
appears to be higher (< 1 µg/kg) for water-rich samples than for
fat-rich foodstuffs (10 -50 µg/kg). The coefficient of variation
can be lower than 20%.
2.3.3. Determination in human tissues and fluids
The methods described in this section are those normally
used to determine the levels of trichloroethylene or its major
metabolites (trichloroacetic acid and trichloroethanol) in
blood and urine. These methods can be used to obtain indirect
measurements of exposure. There are methods for the determination
of trichloroethylene and trichloroethanol in expired air.
2.3.3.1. Trichloroethylene
Blood or urine is distilled or aerated. Any trichloroethylene
vapour present is collected in pyridine, which is then subjected to
Fujiwara's colour test (Seto & Schultze, 1956; Tada, 1969).
Trichloroethylene detection and determination in blood and
urine samples are now generally performed by GC, which has largely
replaced colorimetric reactions. Samples are first extracted with
solvents, and trichloroethylene concentrations are then determined
in the extract. There are various modifications of this technique
(Stewart et al., 1962; Stewart & Dodd, 1964; Kylin et al., 1967;
Stewart et al., 1970; Ertle et al., 1972). Because of its
volatility, trichloroethylene can be sampled using the head-space
method (Monster & Boersma, 1975; Triebig et al., 1976; Astrand &
Ovrum, 1976). This method has also been successfully coupled with
GC/MS (Balkon & Leary, 1979).
GLC alone (Monster & Boersma, 1975; Astrand & Ovrum, 1976)
and GC/MS (Barkley et al., 1980) have been used to determine
trichloroethylene in exhaled air. The same methods, with suitable
sampling techniques, may also be used for the determination of
trichloroethylene in alveolar air.
2.3.3.2. Trichloroacetic acid
Fujiwara's colorimetry test is performed on the blood or urine
sample extract or, in the case of urine, directly on the sample
itself (Abrahamsen, 1960; Soucek & Vlachová, 1960; Bartonícek,
1962; Fawns, 1968; Tanaka & Ikeda, 1968; Tada, 1969; Weichardt &
Bardodej, 1970; Ertle et al., 1972; Kimmerle & Eben, 1973; Mantel &
Nothmann, 1977).
Trichloroacetic acid can also be determined by gas
chromatography of the extract after methylation (Ehrner-Samuel
et al., 1973; Ogata & Saeki, 1974; Nomiyama et al., 1978;
van der Hoeven et al., 1979), by direct methylation of the
specimen followed by head-space sampling and GC (Monster & Boersma,
1975; Triebig et al., 1976), or by inducing trichloroacetic acid
decarboxylation and measuring the chloroform thus formed (Müller
et al., 1972; Buchet et al., 1974). Ziglio (1979) determined
trichloroacetic acid in subjects who had absorbed trichloroethylene
in drinking-water. The extraction and methylation method, as well
as the method of inducing thermal decarboxylation and then
injecting the chloroform thus formed according to the head-space
method, were used (sensitivity of extraction and methylation
methods is better than 10 µg/litre).
2.3.3.3. Trichloroethanol
Trichloroethanol is found in the free state and as the
glucuronide (urochloralic acid) in both blood and urine. For
total trichloroethanol determination, the glucuronide is
hydrolysed. The trichloroacetic acid originally present is then
removed and the trichloroethanol is oxidized quantitatively to
trichloroacetic acid (Vlachov, 1957). The trichloroacetic acid
thus formed is then measured by one of the methods previously
described. Alternatively, trichloroethanol can be distilled in a
vapour stream and measured colorimetrically on the basis of the
condensate resulting from the reaction with pyridine and alkalis
(Bardodej, 1962). The colour test can also be carried out without
prior separation of trichloroethanol from trichloroacetic acid.
The two compounds are measured by determining absorbance at 367 or
440 nm, and at 530 nm (Cabana & Gessner, 1967; Ogata et al., 1970;
Mantel & Nothmann, 1977). Trichloroethanol can also be measured by
determining the difference between the figure obtained for the
trichloroacetic acid level and that obtained for all trichloro-
derivatives present after quantitative oxidation to trichloroacetic
acid (Seto & Schulze, 1956; Tanaka & Ikeda, 1968).
Trichloroethanol can be measured by gas chromatography after
quantitative hydrolysis of the glucuronide (Ogata et al., 1970;
Ertle et al., 1972; Kimmerle & Eben, 1973; Ogata & Saeki, 1974;
Buchet et al., 1974; Nomiyama et al., 1978). The technique of
head-space sampling has been used by Monster & Boersma (1975),
Triebig et al. (1976), and Balkon & Leary (1979).
Trichloroethanol in exhaled air can be measured directly
(Monster & Boersma, 1975).
2.3.3.4. Total trichloro derivatives
In the Imamura & Ikeda (1973) method, urine samples are
oxidized with chromium trioxide in heated nitric acid, allowed to
cool, and made alkaline; pyridine is added followed by mild heating
(Fujiwara test). Solution absorbance is then determined at 530 nm.
2.3.4. Sensitivity
Generally speaking, colorimetric test detection thresholds
range between 0.1 and 1 mg/kg. Greater sensitivity is provided by
gas chromatography, which has detection thresholds of between 10
and 100 µg/kg for trichloroethylene, trichloroacetic acid, and
trichloroethanol.
3. SOURCES IN THE ENVIRONMENT, USES, AND SAFE HANDLING
Trichloroethylene does not occur naturally.
It was first synthesized by Fisher in 1864 and became
commercially available for the first time in 1908 in Austria and in
the United Kingdom (Kirk & Othmer, 1964).
3.1. Production Processes, Levels, and Uses
3.1.1. Production processes and levels
Trichloroethylene is produced by three processes: the
dehydrochlorination of sym-tetrachloroethane, the high-temperature
oxychlorination of chlorinated products with one or two carbon
atoms, or the chlorination of ethylene.
In Western Europe, production was approximately 250 000 tonnes
in 1978. The major producing countries are the Federal Republic of
Germany, France, whose individual production capacity is of the
order of 100 000 tonnes, Italy, and the United Kingdom. Sweden
and Spain are smaller producers. In the USA the production of
trichloroethylene in 1979 was 130 000 tonnes (US ITC, 1980). In
Japan, the annual production was approximately 74 500 tonnes in
1981 and 67 500 tonnes in 1982 (Japanese Yearbook of Chemical
Industries Statistics, 1983).
3.1.2. Uses
Trichloroethylene is an industrial solvent mainly (85 - 90%)
used for the vapour degreasing and cold cleaning of fabricated
metal parts. Trichloroethylene has also been used as a carrier
solvent for the active ingredients of insecticides and fungicides;
as a solvent for waxes, fats, resins, and oils; as an anaesthetic
for medical and dental use; and as an extractant for spice
oleoresins and for caffeine from coffee. Trichloroethylene has
been used in printing inks, varnishes, adhesives, paints, lacquers,
spot removers, rug cleaners, disinfectants, and cosmetic cleansing
fluids. It may also be used as a chain terminator in polyvinyl
chloride production and as an intermediate in the production of
pentachloroethane (Defalque, 1961; Kirk & Othmer, 1963, 1979;
Wetterhahn, 1972; Valle-Riestra, 1974; US CFR, 1976; Waters et al.,
1977; IARC, 1979).
3.2. Handling Hazards, and Precautions
3.2.1. Handling hazards
3.2.1.1. Fire, explosion, and thermal decomposition
At normal handling temperatures, trichloroethylene behaves
as a non-flammable, non-burnable substance. Under normal
conditions, it is virtually impossible to induce an explosion with
trichloroethylene. In the presence of air, at temperatures above
400 °C, it produces phosgene, hydrochloric acid, and carbon
monoxide. In the vicinity of arc welding, phosgene and hydrogen
chloride can be produced from trichloroethylene. In vapour
degreasing, using combustion heaters, precautions must be taken to
prevent solvent fumes from entering the combustion air. Containers
of trichloroethylene exposed to fire should be cooled by sprinkling
with water.
3.2.1.2. Chemical reactivity
Trichloroethylene is practically non-reactive with water at
room temperature, under normal storage conditions, and the
stabilized product does not undergo any changes in the presence of
air, humidity, light, or in contact with metals. It is, however, a
wise precaution not to expose the product to temperatures exceeding
130 °C.
In the presence of strong alkalis, particularly if heated,
trichloroethylene produces dichloroacetylene (Reichert et al.,
1980a), which is highly reactive and acutely neurotoxic to both
animals and man (Reichert & Henschler, 1978). Dichloroacetylene
is also potentially carcinogenic (Reichert et al., 1980b). Under
normal circumstances in industrial use, this reaction is unlikely.
However, under occasional laboratory conditions and closed-circuit
anaesthesia, in the presence of soda lime, some dichloroacetylene
may be produced.
Dichloroacetylene may be formed from trichloroethylene by a
reaction catalysed by ionic halides in the presence of certain
epoxides, including epichlorohydrin (Dobinson & Green, 1972).
Non-stabilized trichloroethylene can react violently with
aluminium (especially in the form of dust or filings) giving off
hydrogen chloride and hexachlorobutene vapour (McNeill, 1979).
Not all stabilizers are effective in preventing the reaction with
aluminium; therefore, a suitably-stabilized product should be used
when cleaning aluminium, especially ultrasonically or where
aluminium particles are present. Suitable products are identified
in manufacturers' literature or in specifications.
3.2.2. Handling precautions
3.2.2.1. Personal safeguards
Accidental exposure to trichloroethylene under occupational
conditions is more frequently associated with the generation of
dense trichloroethylene vapour, e.g., misoperation of vapour-
degreasing apparatus (Sagawa et al., 1973) or the use of liquid
trichloroethylene for cleaning the inside of a tank.
Individual protective measures should be related to the type
and level of exposure. When significant skin contact is likely,
suitable protective clothing should be worn, bearing in mind the
limitations of such clothing and the need to maintain it properly
and replace it regularly. To control exposure through inhalation,
the use of full face masks with filters for organic vapours
(basically for short-term or emergency use), self-contained
breathing apparatus, or masks with air-line supply systems may be
necessary. Self-contained breathing apparatus should always be
available for use in emergencies.
3.2.2.2. Storage
Trichloroethylene can be safely stored in carbon steel or
stainless steel containers. It should not be kept in aluminium,
aluminium alloy, or galvanized iron containers; plastic containers
should not be used unless they are known to be suitable for the
storage of trichloroethylene. Storage areas should be cool, well-
ventilated, flame-proof, and shielded from direct sunlight, high-
temperature surfaces, or sparks. Trichloroethylene should not be
stored near food-stuffs, strong acids, alkalis, or oxidizing
agents.
3.2.3. Recovery
Used trichloroethylene can readily be recovered by
distillation. Trichloroethylene vapours in the aspiration ducts
of plants can be recovered by adsorption on activated carbon and
subsequent desorption.
3.2.4. Disposal
Where trichloroethylene is not recovered and recycled, it may
be disposed of by incineration. Incinerators must be properly
operated, at a sufficiently high temperature and for an adequate
period of time, to ensure complete combustion and prevent the
formation of other toxic chlorinated compounds. The incinerator
should incorporate a suitable scrubber to remove the acidic
breakdown products.
3.2.5. Emergency measures in case of accidental spills
The spilt liquid should be contained with earth, sand, or other
inert adsorbent material to prevent it from spreading.
If possible, remove damaged containers to an isolated and well-
ventilated area, preferably outside, or transfer contents to
another container by mechanical pumping.
Wash away small leaks with water, taking appropriate measures
to avoid creating environmental pollution problems.
When necessary, the contaminated area should be marked off
until the risk of dangerous concentrations in the air has been
eliminated.
3.2.6. Occupational exposure
Trichloroethylene is a widely-used industrial solvent and
degreasing agent. During production, exposure is relatively low
and can be controlled, but users of trichloroethylene may be
exposed to higher levels and under relatively uncontrolled
conditions depending on the type of operation involved. A WHO
Study Group has recommended a time-weighted average exposure not
exceeding 135 µg/m3 with a ceiling limit value of 1000 mg/m3 for
not more than 15 min (WHO Study Group on Recommended Health-Based
Limits in Occupational Exposure to Selected Organic Solvents,
1981). Some national occupational exposure limits are listed in
Table 3.
Table 3. Occupational exposure limits used in various
countriesa
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Australia 100 535 TWAb
Austria 50 260 TWA
Belgium 100 535 TWA
Bulgaria 2 10 TWA
Czechoslovakia 47 250 TWA
235 1250 CVc
Egypt 50 267 TWA
Finland 50 260 TWA
France 75 405 TWA
200 1080 CV
German Democratic 47 250 TWA
Republic 141 750 STd (30 min)
Germany, Federal 50 260e TWA(MAK)
Republic of
Hungary 10 50 TWA
Italy 75 400 TWA
200 1000 skin irritation
Japan 50 268 TWA
Netherlands 35 190 TWA
Poland 10 50 CV
Romania 37 200 TWA
55 300 CV
Spain 100 535 TWA
--------------------------------------------------------
Table 3. (contd.)
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Sweden 20 110 TWA
50 250 ST (15 min)
Switzerland 50 260 TWA
United Kingdom 100 535 TWA
USA
a) OSHA/NIOSH 100 536 TWA
200 1072 ST (5 min)
300 1608 CV
1000 5350 IDLHf
b) ACGIHg 100 535 TWA
150 800 ST (15 min)
USSR 2 10 CV
Yugoslavia 50 200 TWA
--------------------------------------------------------
a From: ILO (1980) and IRPTC (1984).
b TWA (time-weighted average): a mean exposure limit
averaged generally over a working day whereby, within
prescribed limits, excursions above the level
specified are permitted, provided they are compensated
for by excursions below the limit specified.
c CV (ceiling value): a maximum allowable concentration
that must not be exceeded at any time.
d ST (short-term exposure limit): a maximum
concentration allowed for a short specified duration.
e Suspected carcinogen.
f IDLH (immediately dangerous to life and health): a
maximum level from which escape is possible within
30 min without escape-impairing symptoms or any
irreversible health effects.
g Notice of intended change to TWA 270 mg/m3 (50 ppm)
and ST 805 mg/m3 (150 ppm).
Note: Occupational exposure levels and limits are derived in
different ways, possibly using different data and expressed
and applied in accordance with national practices. These
aspects should be taken into account when making
comparisons.
4. ENVIRONMENTAL LEVELS, TRANSPORT AND DISTRIBUTION
4.1. Environmental Levels
4.1.1. Soils and sediments
Trichloroethylene has been found in concentrations exceeding
100 µg/kg in soils and sediments near production sites (IARC,
1979). However, samples taken further away from production sites
show lower levels: for example, in Liverpool Bay, United Kingdom,
which is near an urban and industrialized area, concentrations in
sediments ranged from a few ng/kg to 10 µg/kg (Pearson & McConnell,
1975). An organic-rich anoxic marine sediment from the
Pettaquamscutt River in Rhode Island, USA, where there were no
obvious local sources of trichloroethylene, contained
concentrations ranging from undetectable to 70 µg/kg dry weight
(Whelan et al., 1983). Trichloroethylene was concentrated in the
upper part of the sediment core, corresponding to the period from
about 1940 to the present (determined by 210Pb dating). The
authors noted that the compound had been in use only since the mid-
1940s.
No relationship between trichloroethylene concentration and
particle size or organic matter in sediments, as noted for higher
hydrocarbons such as the DDT group (Pierce et al., 1974), has been
reported.
4.1.2. Water
Trichloroethylene is widely distributed in surface water,
rain-water, well water, and drinking-water from various sources.
Chemical industry discharges may contain concentrations up to
200 µg/litre (Eurocop-Cost, 1976); some Milan well waters contain
high concentrations (80 µg/litre) because of pollution (Cavallo &
Grassi, 1976; Ziglio et al., 1983). However, most reported levels
in water are below this, and are usually in the range of 10 -
100 µg/litre (Rook et al., 1975; Ewing et al., 1977). Rain-water
has been reported to contain concentrations in the µg/litre range
(McConnell et al., 1975) and sea water from Liverpool Bay, United
Kingdom contained a mean concentration of 0.3 µg/litre (Pearson &
McConnell, 1975). This lower range has also been reported in some
Japanese rivers (EAJ, 1983) and in some well water in the USA
(Coleman et al., 1976). Even lower concentrations (7 - 11
ng/litre) have been reported for northeast Atlantic surface water
(Murray & Riley, 1973).
While trihalomethanes are produced during the chlorination of
natural waters containing humic substances, there are no data
indicating that trichloroethylene is produced in this way (Bellar
et al., 1979; Bauer & Selenka, 1982; Otson et al., 1982). However,
treatment of sewage effluent resulted in a small increase in the
trichloroethylene level (Bellar et al., 1979). Trichloroethylene
was found in drinking-water when the original raw water source was
contaminated or when the liquid chlorine used for water treatment
contained trichloroethylene as an impurity. It is also an
intermediate in the breakdown of tetrachloroethylene in some
groundwater systems (Parsons et al., 1984).
Because data for carcinogenicity are inadequate for evaluation,
a tentative guideline value of 30 µg/litre in drinking-water has
been recommended by the World Health Organization (WHO, 1984).
4.1.3. Air
The distribution of trichloroethylene in the atmosphere has
been studied intensively, because of its possible contribution to
depletion of the ozone layer (Lovelock, 1974) (section 7.3).
Air concentrations are in the µg/m3 range (Lovelock, 1974;
Pearson & McConnell, 1975; Cronn et al., 1977; Singh et al., 1977;
Rasmusson et al., 1983). Murray & Riley (1973) reported much lower
concentrations, in the ng/m3 range, in rural areas or from sea
stations; one urban sample (Liverpool) contained 0.85 µg/m3. In
general, higher levels are found near industrialized areas (Pearson
& McConnell, 1975; Ohta et al., 1976; Correia et al., 1977; Ziglio
et al., 1983).
Data describing the partition of trichloroethylene between the
gaseous and particulate phases in the atmosphere are not available.
4.1.4. Biota
Pearson & McConnell (1975) have described trichloroethylene
concentrations in marine organisms from Liverpool Bay, United
Kingdom which is fairly close to an urban and industrialized
region. Concentrations ranged from a few ng/g to about 100 ng/g
wet weight. There was no obvious correlation between concentration
and trophic level. Typical background concentrations are probably
around 10 ng/g wet weight.
Other studies have shown the presence of trichloroethylene in
marine organisms such as invertebrates (1 µg/kg wet weight), fish
muscle (10 µg/kg), sea-bird eggs (50 µg/kg), and seal fat
(50 µg/kg) (Pearson & McConnell, 1975).
Pearson & McConnell (1975) analysed samples of marine organisms
mainly, but not exclusively, from areas near a region where major
organochlorine production plants were situated. Less than 15 µg/kg
wet weight was found in fish muscle (plaice, dab, mackerel).
Values in sea-bird eggs ranged from 2.4 µg/kg for Phalacrocorax
aristotelis (shag) to around 30 (23 - 33) µg/kg for Alca torda
(razorbill), Uria aalge (guillemot), and Rissa tridactyla
(kittiwake). Seal (Halichaerus grypus) blubber and liver from the
Faroe Islands had values ranging from 2.5 to 7.2 µg/kg.
4.1.5. Food
Trichloroethylene may be present in foodstuffs as a residue
from its use as a solvent in food processing or as the result of
environmental contamination. A study conducted by McConnell et al.
(1975) provided a table of the trichloroethylene contents of some
common foodstuffs (Table 4).
Table 4. Trichloroethylene in major foodstuffsa
------------------------------------------------
Foodstuff Concentration
(µg/kg)
------------------------------------------------
Dairy foods:
fresh milk 0.3
Cheshire cheese 3
English butter 10
eggs 0.6
Meat:
shin of beef 16
adipose tissue of beef 12
pig liver 22
Oils and fats:
margarine 6
olive oil (Spanish) 9
cod liver oil 19
vegetable oil for frying 7
Drinks:
fruit juices 5
light beer 0.7
freeze-dried coffee 4
tea in bags 60
wine (Yugoslav) 0.02
Fruit and vegetables:
potatoes 3
apples 5
pears 5
Cereals:
fresh bread 7
------------------------------------------------
a From: McConnell et al. (1975).
In some cases, upper tolerable limits for trichloroethylene
concentrations have been set; for instance, 25 mg/kg dry weight
in powdered decaffeinated coffee, 10 mg/kg dry weight in instant
coffee, and 30 mg/kg dry weight in spice oleoresins. The US FDA
has proposed the prohibition of the use of trichloroethylene in
foodstuffs. The progress of this proposal depends on the
completion of long-term toxicology and carcinogenicity studies
that are being carried out. Before 1976, the US FDA prescribed
tolerance level for trichloroethylene in decaffeinated ground
coffee was 25 mg/kg dry weight (US CFR, 1976).
Trichloroethylene has been reviewed on a number of occasions
by the Joint FAO/WHO Expert Committee on Food Additives, most
recently in 1983. An acceptable daily intake (ADI) has not been
allocated. The Joint Expert Committee recommended that the use of
trichloroethylene as an extraction solvent should be limited, in
order to ensure that its residues in food are as low as practicable
(Joint FAO/WHO Expert Committee on Food Additives, 1983).
4.2. Environmental Distribution and Transport
4.2.1. Equilibrium distribution
The distribution of trichloroethylene, which is observed in
various environmental "compartments", is similar to that which
would be expected from a consideration of its physical and chemical
properties (cf. Appendix I). The relatively high vapour pressure
at normal environmental temperatures should lead to appreciable
atmospheric concentrations; this tendency will balance the tendency
of relatively high water solubility and low Po/w to lead to high
water, biota, or sediment concentrations through either partition
or adsorption. The tendency of trichloroethylene to enter the
atmosphere is demonstrated further by its rapid evaporation from
water; its evaporation half-life is approximately 20 min at 25 °C
(Dilling, 1977).
4.2.2. Transformation in the environment
Recent studies on the degradation of trichloroethylene in
various environmental compartments are discussed below.
4.2.2.1. Air
The main removal reaction appears to be that of attack by the
tropospheric hydroxyl radical (Penkett, 1982), the steady-state
concentrations of which are around 4 x 105/cm3 (Graedel, 1978).
The decay of trichloroethylene is a function of the rate of its
(bimolecular) reaction with the hydroxyl radical (Graedel, 1978),
which is about 2.4/1012 cm3 per molecule per second at 25 °C
(Howard, 1976). This leads to a calculated reaction rate of
approximately 4/103 per h, with the calculated lifetime of
trichloroethylene in the atmosphere of around 11 days (Graedel,
1978). A half-life of the order of 5 days has been calculated by
(De More et al., 1983). Singh et al. (1977) reported a half-life
of less than 2 days in a smog chamber. Pearson & McConnell (1975),
using unrealistically high concentrations of trichloroethylene in
quartz flasks, estimated its half-life to be 11 weeks.
4.2.2.2. Soils and sediments
When methanogenic bacterial batch cultures were exposed to
low concentrations of trichloroethylene (simulating conditions in
an organic-rich sediment or in a sewage treatment system), at
35 °C, for 8 weeks, trichloroethylene concentrations were reduced
by about 40% (Bouwer & McCarty, 1983). If it is assumed that the
reaction rate is halved with every 10 °C drop in temperature, this
corresponds to an exponential decay rate (first order with respect
to trichloroethylene) of about 2/104 per h at 15 °C.
In a study on a laboratory fresh water-sediment system, it was
concluded that trichloroethylene, formed by biotransformation from
tetrachloroethylene, was itself biotransformed to chloroethane,
cis- and trans-1,2-dichloroethene, and dichloromethane (Parsons et
al., 1984).
4.2.2.3. Water
Wakeham et al. (1982) measured a trichloroethylene exponential
decay rate in a sea-water mesocosm of approximately 2.5/102 per day
at 8 - 16 °C, which is equivalent to a rate of about 1/103 per h.
This is similar to the rate described by Bouwer & McCarty (1983)
for microbial degradation. Pearson & McConnell (1975) measured a
chemical degradation rate, in sealed bottles, which led to a half-
life estimate of 2.5 years.
4.2.2.4. Biota
The only data available refer to the degradation of
trichloroethylene in a soil-plant system (Klozskowski et al.,
1981) in which the rate of trichloroethylene loss was 10% per week.
This was accounted for mainly by conversion to carbon dioxide, but
with some evaporation of organic compounds. This corresponds to an
exponential decay rate of about 6/103 per h, which is about 10
times the microbial decay rates.
5. KINETICS AND METABOLISM
5.1. Absorption
Trichloroethylene absorption in mammals can take place by the
respiratory, oral, and/or dermal routes. Intraperitoneal uptake
has been demonstrated experimentally.
5.1.1. Inhalation exposure
In all the mammalian species studied, trichloroethylene uptake
is high during the first minutes of exposure. It then decreases
until equilibrium is reached between uptake by the blood and
release from the blood to tissues, and by metabolism. After
equilibrium is reached, uptake remains constant for the remainder
of exposure (Fernandez et al., 1975; Monster et al., 1976).
In human beings, the blood/air partition coefficient ranges
from 9 to 15. Daily body uptake has been estimated to be
approximately 6 mg/kg body weight, for an exposure of 4 h at
378 mg/m3 (70 ppm), and does not seem to be greatly influenced
by the quantity of adipose tissue (Monster et al., 1976, 1979;
Monster, 1979). Trichloroethylene retention varies according to
physical activity. Under laboratory conditions, when human
volunteers at rest were exposed to concentrations of 540 or
1080 mg/m3 (100 or 200 ppm), for 30 min, 50% of the quantity
inhaled was retained. The percentage retained decreased from 50
to 25%, when activity rose from rest to a 150-watt work load, but,
because of increased ventilation, the absolute amount absorbed
still increased (Astrand & Ovrum, 1976).
5.1.2. Oral exposure
Uptake via the oral route is high because of the ease with
which trichloroethylene penetrates the gastrointestinal barrier.
In man, oral intake is a frequent cause of acute poisoning (Waters
et al., 1977).
5.1.3. Dermal exposure
In the mouse, dermal absorption increases linearly at a
constant rate with duration of exposure. For exposure periods of
between 15 min and 5 h, absorption rates ranged from 59.8 to
92.4 nmol/min per cm2 (Tsuruta, 1978).
Trichloroethylene applied to the backs of guinea-pigs (glass
depot containing at least 1.0 ml) was absorbed and produced blood
concentrations of 0.79 mg/litre after 0.5 h and decreased to
0.46 mg/litre after 6 h, in spite of continuing exposure (Jakobson
et al., 1982).
When one hand of each of 4 human male volunteers was immersed
in trichloroethylene for 30 min, Sato & Nakajima (1978) found blood
concentrations of trichloroethylene (samples taken from unexposed
arm) of 2 mg/litre, immediately after the end of immersion,
0.34 mg/litre, after 30 min, and 0.22 mg/litre, after 60 min.
The trichloroethylene concentration in the expired air was
0.28 mg/litre, 5 min after the end of immersion, 0.06 mg/litre,
30 min after, and 0.024 mg/litre 60 min after.
On the basis of these data and the results of earlier studies
by Stewart & Dodd (1964), it is thought unlikely that
trichloroethylene would be absorbed in toxic quantities through
intact skin during normal industrial use.
5.2. Distribution and Storage
After absorption, trichloroethylene is concentrated in the
cellular components but disappears rapidly (Fabre & Truhaut, 1952).
This rapid disappearance occurs because substantial amounts of
trichloroethylene are metabolized during and after exposure
(Monster, 1979). Trichloroethylene reaches the tissues via the
blood system and accumulates, particularly in adipose tissue,
because of its high liposolubility. The oil/blood partition
coefficient is approximately 750 (Droz & Fernandez, 1977; Sato &
Nakajima, 1979). Trichloroethylene crosses the placental barrier
readily and has been found in fetal blood (Laham, 1970). It
was detectable in the fetus in 2 min, and a fetal/maternal
concentration equilibrium ratio of 1:1 was reached in 6 min (La Du
et al., 1971). Data on the distribution of trichloroethylene in
the tissues of some animal species (including human beings) are
available but, because of the differences in treatment, comparative
conclusions are not possible. Trichloroethylene concentrations
found in various organs and tissues from guinea-pigs, rats, and
human beings are listed in Table 5.
5.3. Metabolic Transformation
5.3.1. Animals
Trichloroethylene is metabolized primarily in the liver and,
to a much lesser extent, in other tissues. Metabolism is by the
mixed-function oxidase system and is dependent on cytochrome P 450.
Qualitative differences between species do not seem particularly
significant with the exception of dichloroacetic acid formation,
which appears to be specific for the mouse (Hathway, 1980; Green &
Prout, in press). The major mammalian metabolites are free and
conjugated trichloroethanol and trichloroacetic acid. Other
metabolites include 2-hydroxyacetylethanolamine and oxalic acid
(Dekant & Henschler, 1982; DeKant et al., 1984). Metabolism is
illustrated in Fig. 4.
Quantitative differences in the rates of metabolism in
different species are much more significant. Mice metabolize
trichloroethylene to a much greater extent than rats (Stott et
al., 1982). It is possible to saturate the metabolism of
trichloroethylene in the rat, but not in the mouse, at doses up to
2000 mg/kg body weight (Anderson et al., 1980). Mice also produce
more reactive tissue-binding metabolites than rats in the liver and
the kidney (Stott et al., 1982).
Table 5. Tissue distribution of trichloroethylene in
(A) guinea-pigs and rats following exposure to the
compound, and (B) in human tissues obtained at autopsy
(levels of exposure not specified)
----------------------------------------------------------
(A) (B)
Organs or Guinea- Ratsb Human beingsa
tissues pigsa d e
(mg/kg) (mg/kg) (µg/kg)
----------------------------------------------------------
adrenals 22 - - -
blood 5 0.9 - -
brain 9 1.0 (1.2)c 1 -
fat 39 9.9 8.2 (32) 4.9 (11.7)
- - - 7.8 (42.2)f
kidney 14 - 2.0 -
liver 10 0.3 4.1 (5.8) 2.5
lung 7 0.7 - 2.2
muscle 2 - - 2.4 (156.6)
ovary 23 - - -
spleen 13 - - -
----------------------------------------------------------
a From: Fabre & Truhaut (1952), 6045 mg/m3 (1120 ppm)
x 5 h per day x 19 days.
b From: Savolainen et al. (1977), 1080 mg/m3 (200 ppm)
x 6 h/day x 5 days).
c Cerebellum value.
d From: McConnell et al. (1975). Mean of 8 subjects aged
48 - 52 years.
e From: Bauer (1981). Mean of 15 subjects (Figures in
parentheses are maximum values).
f Fat from kidney capsule.
2.3.2. Determination in environmental media
2.3.2.1. Soil
High-resolution GC (hrGC-ECD) has been used for determining
trichloroethylene in soil (De Leon et al., 1980). The hrGC/MS
combination has been used as a confirmatory technique, with a
detection threshold of ~10 mg/kg (10 ppm).
2.3.2.2. Water
Levels of trichloroethylene in water can be determined by
hrGC/MS (Dowty et al., 1975), by GC with an electron-capture
detector or an electrolytic conductivity detector (Nicholson et
al., 1977; Dietz & Singley, 1979), and by various other techniques
such as HPLC, hrGC, GC, and GC/MS (Eklund et al., 1978; Jungclaus
et al., 1978). Where specified, detection thresholds are in the
region of 1.0 µg/litre, or lower.
2.3.2.3. Air
GC/MS can be used to measure trichloroethylene levels in the
urban atmosphere (Ioffe et al., 1977). Herbolsheimer et al.
(1972), Sawicki et al. (1975), NIOSH (1977a), Heil et al. (1979),
and Makide et al. (1979) describe sampling and sample enrichment
techniques. Detection capacity may be as low as some ng/m3.
Fujiwara's test can be used to measure trichloroethylene levels
in air (Rush, 1970).
Gas detector tubes (Kitagawa, 1961), activated carbon tubes
(NIOSH, 1977a; Shipman & Whim, 1980), and activated carbon felt
badges (Hirayama & Ikeda, 1979) are available for use in work-place
environments. Gas detector tubes are suitable for spot sampling,
while activated carbon tubes and felt are suitable for time-
weighted average concentration determinations.
2.3.2.4. Foodstuffs
High- and low-resolution GLC can be used for the determination
of trichloroethylene and other aliphatic chloro derivatives in
various foodstuffs (Entz & Hollifield, 1982). The head-space
technique is used in all cases. The sensitivity of the method
appears to be higher (< 1 µg/kg) for water-rich samples than for
fat-rich foodstuffs (10 -50 µg/kg). The coefficient of variation
can be lower than 20%.
2.3.3. Determination in human tissues and fluids
The methods described in this section are those normally
used to determine the levels of trichloroethylene or its major
metabolites (trichloroacetic acid and trichloroethanol) in
blood and urine. These methods can be used to obtain indirect
measurements of exposure. There are methods for the determination
of trichloroethylene and trichloroethanol in expired air.
2.3.3.1. Trichloroethylene
Blood or urine is distilled or aerated. Any trichloroethylene
vapour present is collected in pyridine, which is then subjected to
Fujiwara's colour test (Seto & Schultze, 1956; Tada, 1969).
Trichloroethylene detection and determination in blood and
urine samples are now generally performed by GC, which has largely
replaced colorimetric reactions. Samples are first extracted with
solvents, and trichloroethylene concentrations are then determined
in the extract. There are various modifications of this technique
(Stewart et al., 1962; Stewart & Dodd, 1964; Kylin et al., 1967;
Stewart et al., 1970; Ertle et al., 1972). Because of its
volatility, trichloroethylene can be sampled using the head-space
method (Monster & Boersma, 1975; Triebig et al., 1976; Astrand &
Ovrum, 1976). This method has also been successfully coupled with
GC/MS (Balkon & Leary, 1979).
GLC alone (Monster & Boersma, 1975; Astrand & Ovrum, 1976)
and GC/MS (Barkley et al., 1980) have been used to determine
trichloroethylene in exhaled air. The same methods, with suitable
sampling techniques, may also be used for the determination of
trichloroethylene in alveolar air.
2.3.3.2. Trichloroacetic acid
Fujiwara's colorimetry test is performed on the blood or urine
sample extract or, in the case of urine, directly on the sample
itself (Abrahamsen, 1960; Soucek & Vlachová, 1960; Bartonícek,
1962; Fawns, 1968; Tanaka & Ikeda, 1968; Tada, 1969; Weichardt &
Bardodej, 1970; Ertle et al., 1972; Kimmerle & Eben, 1973; Mantel &
Nothmann, 1977).
Trichloroacetic acid can also be determined by gas
chromatography of the extract after methylation (Ehrner-Samuel
et al., 1973; Ogata & Saeki, 1974; Nomiyama et al., 1978;
van der Hoeven et al., 1979), by direct methylation of the
specimen followed by head-space sampling and GC (Monster & Boersma,
1975; Triebig et al., 1976), or by inducing trichloroacetic acid
decarboxylation and measuring the chloroform thus formed (Müller
et al., 1972; Buchet et al., 1974). Ziglio (1979) determined
trichloroacetic acid in subjects who had absorbed trichloroethylene
in drinking-water. The extraction and methylation method, as well
as the method of inducing thermal decarboxylation and then
injecting the chloroform thus formed according to the head-space
method, were used (sensitivity of extraction and methylation
methods is better than 10 µg/litre).
2.3.3.3. Trichloroethanol
Trichloroethanol is found in the free state and as the
glucuronide (urochloralic acid) in both blood and urine. For
total trichloroethanol determination, the glucuronide is
hydrolysed. The trichloroacetic acid originally present is then
removed and the trichloroethanol is oxidized quantitatively to
trichloroacetic acid (Vlachov, 1957). The trichloroacetic acid
thus formed is then measured by one of the methods previously
described. Alternatively, trichloroethanol can be distilled in a
vapour stream and measured colorimetrically on the basis of the
condensate resulting from the reaction with pyridine and alkalis
(Bardodej, 1962). The colour test can also be carried out without
prior separation of trichloroethanol from trichloroacetic acid.
The two compounds are measured by determining absorbance at 367 or
440 nm, and at 530 nm (Cabana & Gessner, 1967; Ogata et al., 1970;
Mantel & Nothmann, 1977). Trichloroethanol can also be measured by
determining the difference between the figure obtained for the
trichloroacetic acid level and that obtained for all trichloro-
derivatives present after quantitative oxidation to trichloroacetic
acid (Seto & Schulze, 1956; Tanaka & Ikeda, 1968).
Trichloroethanol can be measured by gas chromatography after
quantitative hydrolysis of the glucuronide (Ogata et al., 1970;
Ertle et al., 1972; Kimmerle & Eben, 1973; Ogata & Saeki, 1974;
Buchet et al., 1974; Nomiyama et al., 1978). The technique of
head-space sampling has been used by Monster & Boersma (1975),
Triebig et al. (1976), and Balkon & Leary (1979).
Trichloroethanol in exhaled air can be measured directly
(Monster & Boersma, 1975).
2.3.3.4. Total trichloro derivatives
In the Imamura & Ikeda (1973) method, urine samples are
oxidized with chromium trioxide in heated nitric acid, allowed to
cool, and made alkaline; pyridine is added followed by mild heating
(Fujiwara test). Solution absorbance is then determined at 530 nm.
2.3.4. Sensitivity
Generally speaking, colorimetric test detection thresholds
range between 0.1 and 1 mg/kg. Greater sensitivity is provided by
gas chromatography, which has detection thresholds of between 10
and 100 µg/kg for trichloroethylene, trichloroacetic acid, and
trichloroethanol.
3. SOURCES IN THE ENVIRONMENT, USES, AND SAFE HANDLING
Trichloroethylene does not occur naturally.
It was first synthesized by Fisher in 1864 and became
commercially available for the first time in 1908 in Austria and in
the United Kingdom (Kirk & Othmer, 1964).
3.1. Production Processes, Levels, and Uses
3.1.1. Production processes and levels
Trichloroethylene is produced by three processes: the
dehydrochlorination of sym-tetrachloroethane, the high-temperature
oxychlorination of chlorinated products with one or two carbon
atoms, or the chlorination of ethylene.
In Western Europe, production was approximately 250 000 tonnes
in 1978. The major producing countries are the Federal Republic of
Germany, France, whose individual production capacity is of the
order of 100 000 tonnes, Italy, and the United Kingdom. Sweden
and Spain are smaller producers. In the USA the production of
trichloroethylene in 1979 was 130 000 tonnes (US ITC, 1980). In
Japan, the annual production was approximately 74 500 tonnes in
1981 and 67 500 tonnes in 1982 (Japanese Yearbook of Chemical
Industries Statistics, 1983).
3.1.2. Uses
Trichloroethylene is an industrial solvent mainly (85 - 90%)
used for the vapour degreasing and cold cleaning of fabricated
metal parts. Trichloroethylene has also been used as a carrier
solvent for the active ingredients of insecticides and fungicides;
as a solvent for waxes, fats, resins, and oils; as an anaesthetic
for medical and dental use; and as an extractant for spice
oleoresins and for caffeine from coffee. Trichloroethylene has
been used in printing inks, varnishes, adhesives, paints, lacquers,
spot removers, rug cleaners, disinfectants, and cosmetic cleansing
fluids. It may also be used as a chain terminator in polyvinyl
chloride production and as an intermediate in the production of
pentachloroethane (Defalque, 1961; Kirk & Othmer, 1963, 1979;
Wetterhahn, 1972; Valle-Riestra, 1974; US CFR, 1976; Waters et al.,
1977; IARC, 1979).
3.2. Handling Hazards, and Precautions
3.2.1. Handling hazards
3.2.1.1. Fire, explosion, and thermal decomposition
At normal handling temperatures, trichloroethylene behaves
as a non-flammable, non-burnable substance. Under normal
conditions, it is virtually impossible to induce an explosion with
trichloroethylene. In the presence of air, at temperatures above
400 °C, it produces phosgene, hydrochloric acid, and carbon
monoxide. In the vicinity of arc welding, phosgene and hydrogen
chloride can be produced from trichloroethylene. In vapour
degreasing, using combustion heaters, precautions must be taken to
prevent solvent fumes from entering the combustion air. Containers
of trichloroethylene exposed to fire should be cooled by sprinkling
with water.
3.2.1.2. Chemical reactivity
Trichloroethylene is practically non-reactive with water at
room temperature, under normal storage conditions, and the
stabilized product does not undergo any changes in the presence of
air, humidity, light, or in contact with metals. It is, however, a
wise precaution not to expose the product to temperatures exceeding
130 °C.
In the presence of strong alkalis, particularly if heated,
trichloroethylene produces dichloroacetylene (Reichert et al.,
1980a), which is highly reactive and acutely neurotoxic to both
animals and man (Reichert & Henschler, 1978). Dichloroacetylene
is also potentially carcinogenic (Reichert et al., 1980b). Under
normal circumstances in industrial use, this reaction is unlikely.
However, under occasional laboratory conditions and closed-circuit
anaesthesia, in the presence of soda lime, some dichloroacetylene
may be produced.
Dichloroacetylene may be formed from trichloroethylene by a
reaction catalysed by ionic halides in the presence of certain
epoxides, including epichlorohydrin (Dobinson & Green, 1972).
Non-stabilized trichloroethylene can react violently with
aluminium (especially in the form of dust or filings) giving off
hydrogen chloride and hexachlorobutene vapour (McNeill, 1979).
Not all stabilizers are effective in preventing the reaction with
aluminium; therefore, a suitably-stabilized product should be used
when cleaning aluminium, especially ultrasonically or where
aluminium particles are present. Suitable products are identified
in manufacturers' literature or in specifications.
3.2.2. Handling precautions
3.2.2.1. Personal safeguards
Accidental exposure to trichloroethylene under occupational
conditions is more frequently associated with the generation of
dense trichloroethylene vapour, e.g., misoperation of vapour-
degreasing apparatus (Sagawa et al., 1973) or the use of liquid
trichloroethylene for cleaning the inside of a tank.
Individual protective measures should be related to the type
and level of exposure. When significant skin contact is likely,
suitable protective clothing should be worn, bearing in mind the
limitations of such clothing and the need to maintain it properly
and replace it regularly. To control exposure through inhalation,
the use of full face masks with filters for organic vapours
(basically for short-term or emergency use), self-contained
breathing apparatus, or masks with air-line supply systems may be
necessary. Self-contained breathing apparatus should always be
available for use in emergencies.
3.2.2.2. Storage
Trichloroethylene can be safely stored in carbon steel or
stainless steel containers. It should not be kept in aluminium,
aluminium alloy, or galvanized iron containers; plastic containers
should not be used unless they are known to be suitable for the
storage of trichloroethylene. Storage areas should be cool, well-
ventilated, flame-proof, and shielded from direct sunlight, high-
temperature surfaces, or sparks. Trichloroethylene should not be
stored near food-stuffs, strong acids, alkalis, or oxidizing
agents.
3.2.3. Recovery
Used trichloroethylene can readily be recovered by
distillation. Trichloroethylene vapours in the aspiration ducts
of plants can be recovered by adsorption on activated carbon and
subsequent desorption.
3.2.4. Disposal
Where trichloroethylene is not recovered and recycled, it may
be disposed of by incineration. Incinerators must be properly
operated, at a sufficiently high temperature and for an adequate
period of time, to ensure complete combustion and prevent the
formation of other toxic chlorinated compounds. The incinerator
should incorporate a suitable scrubber to remove the acidic
breakdown products.
3.2.5. Emergency measures in case of accidental spills
The spilt liquid should be contained with earth, sand, or other
inert adsorbent material to prevent it from spreading.
If possible, remove damaged containers to an isolated and well-
ventilated area, preferably outside, or transfer contents to
another container by mechanical pumping.
Wash away small leaks with water, taking appropriate measures
to avoid creating environmental pollution problems.
When necessary, the contaminated area should be marked off
until the risk of dangerous concentrations in the air has been
eliminated.
3.2.6. Occupational exposure
Trichloroethylene is a widely-used industrial solvent and
degreasing agent. During production, exposure is relatively low
and can be controlled, but users of trichloroethylene may be
exposed to higher levels and under relatively uncontrolled
conditions depending on the type of operation involved. A WHO
Study Group has recommended a time-weighted average exposure not
exceeding 135 µg/m3 with a ceiling limit value of 1000 mg/m3 for
not more than 15 min (WHO Study Group on Recommended Health-Based
Limits in Occupational Exposure to Selected Organic Solvents,
1981). Some national occupational exposure limits are listed in
Table 3.
Table 3. Occupational exposure limits used in various
countriesa
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Australia 100 535 TWAb
Austria 50 260 TWA
Belgium 100 535 TWA
Bulgaria 2 10 TWA
Czechoslovakia 47 250 TWA
235 1250 CVc
Egypt 50 267 TWA
Finland 50 260 TWA
France 75 405 TWA
200 1080 CV
German Democratic 47 250 TWA
Republic 141 750 STd (30 min)
Germany, Federal 50 260e TWA(MAK)
Republic of
Hungary 10 50 TWA
Italy 75 400 TWA
200 1000 skin irritation
Japan 50 268 TWA
Netherlands 35 190 TWA
Poland 10 50 CV
Romania 37 200 TWA
55 300 CV
Spain 100 535 TWA
--------------------------------------------------------
Table 3. (contd.)
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Sweden 20 110 TWA
50 250 ST (15 min)
Switzerland 50 260 TWA
United Kingdom 100 535 TWA
USA
a) OSHA/NIOSH 100 536 TWA
200 1072 ST (5 min)
300 1608 CV
1000 5350 IDLHf
b) ACGIHg 100 535 TWA
150 800 ST (15 min)
USSR 2 10 CV
Yugoslavia 50 200 TWA
--------------------------------------------------------
a From: ILO (1980) and IRPTC (1984).
b TWA (time-weighted average): a mean exposure limit
averaged generally over a working day whereby, within
prescribed limits, excursions above the level
specified are permitted, provided they are compensated
for by excursions below the limit specified.
c CV (ceiling value): a maximum allowable concentration
that must not be exceeded at any time.
d ST (short-term exposure limit): a maximum
concentration allowed for a short specified duration.
e Suspected carcinogen.
f IDLH (immediately dangerous to life and health): a
maximum level from which escape is possible within
30 min without escape-impairing symptoms or any
irreversible health effects.
g Notice of intended change to TWA 270 mg/m3 (50 ppm)
and ST 805 mg/m3 (150 ppm).
Note: Occupational exposure levels and limits are derived in
different ways, possibly using different data and expressed
and applied in accordance with national practices. These
aspects should be taken into account when making
comparisons.
4. ENVIRONMENTAL LEVELS, TRANSPORT AND DISTRIBUTION
4.1. Environmental Levels
4.1.1. Soils and sediments
Trichloroethylene has been found in concentrations exceeding
100 µg/kg in soils and sediments near production sites (IARC,
1979). However, samples taken further away from production sites
show lower levels: for example, in Liverpool Bay, United Kingdom,
which is near an urban and industrialized area, concentrations in
sediments ranged from a few ng/kg to 10 µg/kg (Pearson & McConnell,
1975). An organic-rich anoxic marine sediment from the
Pettaquamscutt River in Rhode Island, USA, where there were no
obvious local sources of trichloroethylene, contained
concentrations ranging from undetectable to 70 µg/kg dry weight
(Whelan et al., 1983). Trichloroethylene was concentrated in the
upper part of the sediment core, corresponding to the period from
about 1940 to the present (determined by 210Pb dating). The
authors noted that the compound had been in use only since the mid-
1940s.
No relationship between trichloroethylene concentration and
particle size or organic matter in sediments, as noted for higher
hydrocarbons such as the DDT group (Pierce et al., 1974), has been
reported.
4.1.2. Water
Trichloroethylene is widely distributed in surface water,
rain-water, well water, and drinking-water from various sources.
Chemical industry discharges may contain concentrations up to
200 µg/litre (Eurocop-Cost, 1976); some Milan well waters contain
high concentrations (80 µg/litre) because of pollution (Cavallo &
Grassi, 1976; Ziglio et al., 1983). However, most reported levels
in water are below this, and are usually in the range of 10 -
100 µg/litre (Rook et al., 1975; Ewing et al., 1977). Rain-water
has been reported to contain concentrations in the µg/litre range
(McConnell et al., 1975) and sea water from Liverpool Bay, United
Kingdom contained a mean concentration of 0.3 µg/litre (Pearson &
McConnell, 1975). This lower range has also been reported in some
Japanese rivers (EAJ, 1983) and in some well water in the USA
(Coleman et al., 1976). Even lower concentrations (7 - 11
ng/litre) have been reported for northeast Atlantic surface water
(Murray & Riley, 1973).
While trihalomethanes are produced during the chlorination of
natural waters containing humic substances, there are no data
indicating that trichloroethylene is produced in this way (Bellar
et al., 1979; Bauer & Selenka, 1982; Otson et al., 1982). However,
treatment of sewage effluent resulted in a small increase in the
trichloroethylene level (Bellar et al., 1979). Trichloroethylene
was found in drinking-water when the original raw water source was
contaminated or when the liquid chlorine used for water treatment
contained trichloroethylene as an impurity. It is also an
intermediate in the breakdown of tetrachloroethylene in some
groundwater systems (Parsons et al., 1984).
Because data for carcinogenicity are inadequate for evaluation,
a tentative guideline value of 30 µg/litre in drinking-water has
been recommended by the World Health Organization (WHO, 1984).
4.1.3. Air
The distribution of trichloroethylene in the atmosphere has
been studied intensively, because of its possible contribution to
depletion of the ozone layer (Lovelock, 1974) (section 7.3).
Air concentrations are in the µg/m3 range (Lovelock, 1974;
Pearson & McConnell, 1975; Cronn et al., 1977; Singh et al., 1977;
Rasmusson et al., 1983). Murray & Riley (1973) reported much lower
concentrations, in the ng/m3 range, in rural areas or from sea
stations; one urban sample (Liverpool) contained 0.85 µg/m3. In
general, higher levels are found near industrialized areas (Pearson
& McConnell, 1975; Ohta et al., 1976; Correia et al., 1977; Ziglio
et al., 1983).
Data describing the partition of trichloroethylene between the
gaseous and particulate phases in the atmosphere are not available.
4.1.4. Biota
Pearson & McConnell (1975) have described trichloroethylene
concentrations in marine organisms from Liverpool Bay, United
Kingdom which is fairly close to an urban and industrialized
region. Concentrations ranged from a few ng/g to about 100 ng/g
wet weight. There was no obvious correlation between concentration
and trophic level. Typical background concentrations are probably
around 10 ng/g wet weight.
Other studies have shown the presence of trichloroethylene in
marine organisms such as invertebrates (1 µg/kg wet weight), fish
muscle (10 µg/kg), sea-bird eggs (50 µg/kg), and seal fat
(50 µg/kg) (Pearson & McConnell, 1975).
Pearson & McConnell (1975) analysed samples of marine organisms
mainly, but not exclusively, from areas near a region where major
organochlorine production plants were situated. Less than 15 µg/kg
wet weight was found in fish muscle (plaice, dab, mackerel).
Values in sea-bird eggs ranged from 2.4 µg/kg for Phalacrocorax
aristotelis (shag) to around 30 (23 - 33) µg/kg for Alca torda
(razorbill), Uria aalge (guillemot), and Rissa tridactyla
(kittiwake). Seal (Halichaerus grypus) blubber and liver from the
Faroe Islands had values ranging from 2.5 to 7.2 µg/kg.
4.1.5. Food
Trichloroethylene may be present in foodstuffs as a residue
from its use as a solvent in food processing or as the result of
environmental contamination. A study conducted by McConnell et al.
(1975) provided a table of the trichloroethylene contents of some
common foodstuffs (Table 4).
Table 4. Trichloroethylene in major foodstuffsa
------------------------------------------------
Foodstuff Concentration
(µg/kg)
------------------------------------------------
Dairy foods:
fresh milk 0.3
Cheshire cheese 3
English butter 10
eggs 0.6
Meat:
shin of beef 16
adipose tissue of beef 12
pig liver 22
Oils and fats:
margarine 6
olive oil (Spanish) 9
cod liver oil 19
vegetable oil for frying 7
Drinks:
fruit juices 5
light beer 0.7
freeze-dried coffee 4
tea in bags 60
wine (Yugoslav) 0.02
Fruit and vegetables:
potatoes 3
apples 5
pears 5
Cereals:
fresh bread 7
------------------------------------------------
a From: McConnell et al. (1975).
In some cases, upper tolerable limits for trichloroethylene
concentrations have been set; for instance, 25 mg/kg dry weight
in powdered decaffeinated coffee, 10 mg/kg dry weight in instant
coffee, and 30 mg/kg dry weight in spice oleoresins. The US FDA
has proposed the prohibition of the use of trichloroethylene in
foodstuffs. The progress of this proposal depends on the
completion of long-term toxicology and carcinogenicity studies
that are being carried out. Before 1976, the US FDA prescribed
tolerance level for trichloroethylene in decaffeinated ground
coffee was 25 mg/kg dry weight (US CFR, 1976).
Trichloroethylene has been reviewed on a number of occasions
by the Joint FAO/WHO Expert Committee on Food Additives, most
recently in 1983. An acceptable daily intake (ADI) has not been
allocated. The Joint Expert Committee recommended that the use of
trichloroethylene as an extraction solvent should be limited, in
order to ensure that its residues in food are as low as practicable
(Joint FAO/WHO Expert Committee on Food Additives, 1983).
4.2. Environmental Distribution and Transport
4.2.1. Equilibrium distribution
The distribution of trichloroethylene, which is observed in
various environmental "compartments", is similar to that which
would be expected from a consideration of its physical and chemical
properties (cf. Appendix I). The relatively high vapour pressure
at normal environmental temperatures should lead to appreciable
atmospheric concentrations; this tendency will balance the tendency
of relatively high water solubility and low Po/w to lead to high
water, biota, or sediment concentrations through either partition
or adsorption. The tendency of trichloroethylene to enter the
atmosphere is demonstrated further by its rapid evaporation from
water; its evaporation half-life is approximately 20 min at 25 °C
(Dilling, 1977).
4.2.2. Transformation in the environment
Recent studies on the degradation of trichloroethylene in
various environmental compartments are discussed below.
4.2.2.1. Air
The main removal reaction appears to be that of attack by the
tropospheric hydroxyl radical (Penkett, 1982), the steady-state
concentrations of which are around 4 x 105/cm3 (Graedel, 1978).
The decay of trichloroethylene is a function of the rate of its
(bimolecular) reaction with the hydroxyl radical (Graedel, 1978),
which is about 2.4/1012 cm3 per molecule per second at 25 °C
(Howard, 1976). This leads to a calculated reaction rate of
approximately 4/103 per h, with the calculated lifetime of
trichloroethylene in the atmosphere of around 11 days (Graedel,
1978). A half-life of the order of 5 days has been calculated by
(De More et al., 1983). Singh et al. (1977) reported a half-life
of less than 2 days in a smog chamber. Pearson & McConnell (1975),
using unrealistically high concentrations of trichloroethylene in
quartz flasks, estimated its half-life to be 11 weeks.
4.2.2.2. Soils and sediments
When methanogenic bacterial batch cultures were exposed to
low concentrations of trichloroethylene (simulating conditions in
an organic-rich sediment or in a sewage treatment system), at
35 °C, for 8 weeks, trichloroethylene concentrations were reduced
by about 40% (Bouwer & McCarty, 1983). If it is assumed that the
reaction rate is halved with every 10 °C drop in temperature, this
corresponds to an exponential decay rate (first order with respect
to trichloroethylene) of about 2/104 per h at 15 °C.
In a study on a laboratory fresh water-sediment system, it was
concluded that trichloroethylene, formed by biotransformation from
tetrachloroethylene, was itself biotransformed to chloroethane,
cis- and trans-1,2-dichloroethene, and dichloromethane (Parsons et
al., 1984).
4.2.2.3. Water
Wakeham et al. (1982) measured a trichloroethylene exponential
decay rate in a sea-water mesocosm of approximately 2.5/102 per day
at 8 - 16 °C, which is equivalent to a rate of about 1/103 per h.
This is similar to the rate described by Bouwer & McCarty (1983)
for microbial degradation. Pearson & McConnell (1975) measured a
chemical degradation rate, in sealed bottles, which led to a half-
life estimate of 2.5 years.
4.2.2.4. Biota
The only data available refer to the degradation of
trichloroethylene in a soil-plant system (Klozskowski et al.,
1981) in which the rate of trichloroethylene loss was 10% per week.
This was accounted for mainly by conversion to carbon dioxide, but
with some evaporation of organic compounds. This corresponds to an
exponential decay rate of about 6/103 per h, which is about 10
times the microbial decay rates.
5. KINETICS AND METABOLISM
5.1. Absorption
Trichloroethylene absorption in mammals can take place by the
respiratory, oral, and/or dermal routes. Intraperitoneal uptake
has been demonstrated experimentally.
5.1.1. Inhalation exposure
In all the mammalian species studied, trichloroethylene uptake
is high during the first minutes of exposure. It then decreases
until equilibrium is reached between uptake by the blood and
release from the blood to tissues, and by metabolism. After
equilibrium is reached, uptake remains constant for the remainder
of exposure (Fernandez et al., 1975; Monster et al., 1976).
In human beings, the blood/air partition coefficient ranges
from 9 to 15. Daily body uptake has been estimated to be
approximately 6 mg/kg body weight, for an exposure of 4 h at
378 mg/m3 (70 ppm), and does not seem to be greatly influenced
by the quantity of adipose tissue (Monster et al., 1976, 1979;
Monster, 1979). Trichloroethylene retention varies according to
physical activity. Under laboratory conditions, when human
volunteers at rest were exposed to concentrations of 540 or
1080 mg/m3 (100 or 200 ppm), for 30 min, 50% of the quantity
inhaled was retained. The percentage retained decreased from 50
to 25%, when activity rose from rest to a 150-watt work load, but,
because of increased ventilation, the absolute amount absorbed
still increased (Astrand & Ovrum, 1976).
5.1.2. Oral exposure
Uptake via the oral route is high because of the ease with
which trichloroethylene penetrates the gastrointestinal barrier.
In man, oral intake is a frequent cause of acute poisoning (Waters
et al., 1977).
5.1.3. Dermal exposure
In the mouse, dermal absorption increases linearly at a
constant rate with duration of exposure. For exposure periods of
between 15 min and 5 h, absorption rates ranged from 59.8 to
92.4 nmol/min per cm2 (Tsuruta, 1978).
Trichloroethylene applied to the backs of guinea-pigs (glass
depot containing at least 1.0 ml) was absorbed and produced blood
concentrations of 0.79 mg/litre after 0.5 h and decreased to
0.46 mg/litre after 6 h, in spite of continuing exposure (Jakobson
et al., 1982).
When one hand of each of 4 human male volunteers was immersed
in trichloroethylene for 30 min, Sato & Nakajima (1978) found blood
concentrations of trichloroethylene (samples taken from unexposed
arm) of 2 mg/litre, immediately after the end of immersion,
0.34 mg/litre, after 30 min, and 0.22 mg/litre, after 60 min.
The trichloroethylene concentration in the expired air was
0.28 mg/litre, 5 min after the end of immersion, 0.06 mg/litre,
30 min after, and 0.024 mg/litre 60 min after.
On the basis of these data and the results of earlier studies
by Stewart & Dodd (1964), it is thought unlikely that
trichloroethylene would be absorbed in toxic quantities through
intact skin during normal industrial use.
5.2. Distribution and Storage
After absorption, trichloroethylene is concentrated in the
cellular components but disappears rapidly (Fabre & Truhaut, 1952).
This rapid disappearance occurs because substantial amounts of
trichloroethylene are metabolized during and after exposure
(Monster, 1979). Trichloroethylene reaches the tissues via the
blood system and accumulates, particularly in adipose tissue,
because of its high liposolubility. The oil/blood partition
coefficient is approximately 750 (Droz & Fernandez, 1977; Sato &
Nakajima, 1979). Trichloroethylene crosses the placental barrier
readily and has been found in fetal blood (Laham, 1970). It
was detectable in the fetus in 2 min, and a fetal/maternal
concentration equilibrium ratio of 1:1 was reached in 6 min (La Du
et al., 1971). Data on the distribution of trichloroethylene in
the tissues of some animal species (including human beings) are
available but, because of the differences in treatment, comparative
conclusions are not possible. Trichloroethylene concentrations
found in various organs and tissues from guinea-pigs, rats, and
human beings are listed in Table 5.
5.3. Metabolic Transformation
5.3.1. Animals
Trichloroethylene is metabolized primarily in the liver and,
to a much lesser extent, in other tissues. Metabolism is by the
mixed-function oxidase system and is dependent on cytochrome P 450.
Qualitative differences between species do not seem particularly
significant with the exception of dichloroacetic acid formation,
which appears to be specific for the mouse (Hathway, 1980; Green &
Prout, in press). The major mammalian metabolites are free and
conjugated trichloroethanol and trichloroacetic acid. Other
metabolites include 2-hydroxyacetylethanolamine and oxalic acid
(Dekant & Henschler, 1982; DeKant et al., 1984). Metabolism is
illustrated in Fig. 4.
Quantitative differences in the rates of metabolism in
different species are much more significant. Mice metabolize
trichloroethylene to a much greater extent than rats (Stott et
al., 1982). It is possible to saturate the metabolism of
trichloroethylene in the rat, but not in the mouse, at doses up to
2000 mg/kg body weight (Anderson et al., 1980). Mice also produce
more reactive tissue-binding metabolites than rats in the liver and
the kidney (Stott et al., 1982).
Table 5. Tissue distribution of trichloroethylene in
(A) guinea-pigs and rats following exposure to the
compound, and (B) in human tissues obtained at autopsy
(levels of exposure not specified)
----------------------------------------------------------
(A) (B)
Organs or Guinea- Ratsb Human beingsa
tissues pigsa d e
(mg/kg) (mg/kg) (µg/kg)
----------------------------------------------------------
adrenals 22 - - -
blood 5 0.9 - -
brain 9 1.0 (1.2)c 1 -
fat 39 9.9 8.2 (32) 4.9 (11.7)
- - - 7.8 (42.2)f
kidney 14 - 2.0 -
liver 10 0.3 4.1 (5.8) 2.5
lung 7 0.7 - 2.2
muscle 2 - - 2.4 (156.6)
ovary 23 - - -
spleen 13 - - -
----------------------------------------------------------
a From: Fabre & Truhaut (1952), 6045 mg/m3 (1120 ppm)
x 5 h per day x 19 days.
b From: Savolainen et al. (1977), 1080 mg/m3 (200 ppm)
x 6 h/day x 5 days).
c Cerebellum value.
d From: McConnell et al. (1975). Mean of 8 subjects aged
48 - 52 years.
e From: Bauer (1981). Mean of 15 subjects (Figures in
parentheses are maximum values).
f Fat from kidney capsule.
 Metabolically activated trichloroethylene binds covalently to
the hepatic microsomal proteins and DNA, in vitro. This finding
supports the formation of an epoxide intermediate (Banerjee & Van
Duuren, 1978), though this has not been demonstrated in vivo
(Parchman & Magee, 1982; Stott et al., 1982).
5.3.2. Human beings
As originally suggested by Powell (1945), the formation of the
epoxide, an intermediate reactive metabolite that binds covalently
with proteins (Bolt & Filher, 1977), has been confirmed by indirect
spectral evidence (Uehleke et al., 1977). The epoxide may undergo
intramolecular rearrangement in 2 different ways (Henschler & Hoos,
1982; DeKant & Henschler, 1983). One pathway leads to chloral
(Henschler, 1977a,b), which is further oxidized to trichloroacetic
acid (TCA), or reduced to trichloroethanol (Leibman, 1965). After
oral administration, trichloroethanol is also partly metabolized
into TCA (Müller et al., 1974). Trichloroethanol is rapidly
conjugated with glucuronic acid to form the respective glucuronide.
The other pathway leads to the formation of dichloroacetyl chloride
which, under in vitro conditions, can lead to the formation of
dichloroacetic acid. However, under normal in vivo conditions,
dichloroacetic acid is not found, except in mice following the
administration of very high doses of trichloroethylene. Under
these conditions, an "overspill" mechanism may operate (Henschler
et al., 1979; Hathway, 1980; Henschler et al., 1983). The
excretion of chloroform in expired air and monochloroacetic acid in
urine have also been proposed as minor routes of metabolism (Ogata
& Saeki, 1974; Bartonicek, 1962). Miller & Guenrich (1982)
suggested that an epoxide was not an obligatory intermediate step
and proposed an alternative model in which chlorine migration
occurs in an oxygenated trichloroethylene P 450 transition state.
Trichloroacetic acid binds well with plasma proteins and its
concentration in plasma is approximately double that in whole blood
(Müller et al., 1972).
5.3.3. Drug and other interactions
A number of commonly-used drugs might be expected to modify the
extent of metabolism of trichloroethylene during human exposure.
Although largely undocumented in man, the induction of the hepatic
microsomal mixed-function oxidase system by drugs, taken for
therapeutic reasons, or by exposure to certain environmental
chemicals (e.g., phenobarbital, toluene, PCBs) can bring about an
increased rate of trichloroethylene metabolism (Ikeda & Imamura,
1973; Ikeda, 1974).
In human beings, the simultaneous administration of ethanol and
trichloroethylene (100 mg/m3 for 6 h) causes an increase in
trichloroethylene levels in both plasma (2.4 times the normal
value) and in exhaled air (3.4 times), and a decrease in the levels
of trichloroacetic acid and trichloroethanol (Müller et al., 1975).
5.4. Elimination
5.4.1. Studies on animals
The kinetics of the distribution and elimination of
trichloroethylene, administered intravenously in Wistar rats at
dose levels of 6, 9, 12, or 15 mg/kg body weight show that the
blood concentration exhibits a first order, 2-compartment model
exponential disappearance, and it has been suggested that a dose
of 15 mg trichloroethylene/kg body weight is within the hepatic
metabolic capacity in the rat (Withey & Collins, 1980). Daniel
(1963) showed that when trichloroethylene was administered orally
to rats, the ratio of pulmonary to urinary elimination varied with
the dose and that as dose increased, pulmonary excretion increased
while urinary elimination decreased. Further evidence showing that
the metabolism of trichloroethylene is saturable in Wistar rats was
obtained by Filser & Bolt (1979) who showed that the saturation
point occurred at 350 mg/m3 (65 ppm), that the zero order Vmax was
210 µmol/h per kg body weight and that the first order clearance at
a dose of 350 mg/m3 (65 ppm) was 77 µmol/h per kg body weight.
Stott et al. (1982) found that the pulmonary elimination of
unchanged trichlorethylene in Osborn Mendel rats was only 2% of the
dose at 54 mg/m3 (10 ppm), but 21% at a dose of 3240 mg/m3
(600 ppm). In contrast to these findings of a saturable process in
the rat, The same authors showed that in the mouse, doses of
trichloroethylene up to 3240 mg/m3 (600 ppm) were completely
metabolized.
In dogs, exposed through inhalation, for 1 h, to
trichloroethylene at 3780, 8100, and 10 800 mg/m3 (700, 1500, and
2000 ppm), the excretion of trichloroethylene and trichloroacetic
acid was correlated with the trichloroethylene concentration. The
rate of trichloroacetic acid excretion was higher than that for
trichloroethanol. One hour after exposure ended, the percentage
of trichloro compounds in the urine was 0.7% of the total
trichloroethylene absorbed (Hobara et al., 1983).
5.4.2. Studies on man
It has been shown that man metabolizes trichloroethylene
extensively. Ikeda et al. (1972) showed that the capacity of
workers to metabolize trichloroethylene was nonlimiting, at least
up to a daily exposure level of 945 mg/m3 (175 ppm) for 8 h.
In human beings, trichloroethylene is eliminated unchanged
through the lungs and is eliminated in the urine in the form of
metabolites. Elimination by other routes (e.g., faeces, sweat, and
saliva) accounts for less than 10% of the total (Bartonicek, 1962).
After inhalation exposure, about 10% of the amount absorbed is
expired unchanged, about 30 - 50% is excreted as trichloroethanol
in urine, and about 10 - 30% as trichloroacetic acid in urine
(Soucek & Vlachova, 1960; Bartonicek, 1962; Monster et al., 1976,
1979).
The half-life of trichloroethylene in exhaled air and in the
blood depends on the length of exposure and on the time of sampling
after exposure. The concentration follows a multi-exponential
curve, compatible with at least 3 compartments: lungs, blood and
most other tissues, and adipose tissue. After a single exposure
to trichloroethylene, trichloroethanol reaches its maximum
concentration in blood and urine almost directly after exposure.
Thereafter, the concentration decreases, with a half-life of
about 10 - 15 h (Müller et al., 1974; Monster et al., 1976,
1979; Vesterberg et al., 1976). After a single exposure to
trichloroethylene, the concentration of trichloroacetic acid in
both the blood and the urine increases for up to 20 - 40 h after
exposure. Thereafter, the concentration decreases with a half-life
of about 70 - 100 h (Müller et al., 1974; Monster et al., 1979).
Trichloroacetic acid, as such, has a shorter half-life of about
50 h.
In a group of workers with long-term exposure to
trichloroethylene at a concentration of 270 mg/m3 (50 ppm), median
values of trichloroethanol and trichloroacetic acid of 330 and
319 g/kg creatinine, respectively, were found at the end of a
working shift; during the work-free periods, the metabolites of
trichloroethylene were eliminated slowly (Triebig et al., 1976).
In a study on factory workers exposed to trichloroethylene,
Ikeda & Imamura (1973) observed a half-life of 41 h for the
urinary excretion of total trichloro-compounds (i.e., a combination
of trichloroacetic acid and trichloroethanol). This half-life is
somewhat longer with oral administration of trichloroethylene and
of chloral hydrate and is about 85 - 99 h after repeated exposure
to trichloroethylene, because of the delayed formation of
trichloroacetic acid from trichloroethylene and the
trichloroethanol still available from the tissues (Müller et al.,
1974). Thus, trichloroacetic acid will be found in the urine, even
when trichloroethanol is no longer detectable (Ikeda et al., 1971).
Trichloroacetic acid accumulates in the blood and urine during
daily exposures to trichlorethylene (Monster et al., 1979; Müller
et al., 1975).
5.5. Biological Monitoring of Exposure
Droz & Fernandez (1978) used a mathematical model to study the
effects of hourly and daily variations in exposure concentrations
on alveolar air trichloroethylene concentrations and on the urinary
excretion of trichloroethanol and trichloroacetic acid. The
determination of trichloroethanol in urine appeared to be more
sensitive than the determination of trichloroethylene in exhaled
air. The excretion of trichloroacetic acid can be used for the
qualitative evaluation of the preceding day's exposure. In
practice, blood analysis would be preferable to analysis of urine,
because of the smaller individual variations generally observed
with the former. In studies concerning repeated exposure to
constant concentrations, the smallest inter-individual variation
was found in the concentrations in blood (Monster et al., 1979).
The measurement of total trichloro compounds in urine was
described by Takana & Ikeda (1968). The urinary trichloroethanol
is oxidized to trichloroacetic acid and the total amount of
trichloroacetic acid is then measured with the Fujiwara reaction.
In the case of exposure to relatively steady concentrations, this
has the advantage of being able to indicate small-scale inter-
personal variations. It may be used as an index of exposure
intensity, especially when the urine samples are collected at,
or close to, the end of the workshift at the end of the work week
(Ikeda et al., 1972). However, other studies have demonstrated
poor individual correlation between trichloroethylene exposure and
the urinary elimination of the major metabolites, trichloroacetic
acid and trichloroethanol (Boudène et al., 1983). Separate
measurement of urinary metabolites provides more information on
exposure to daily fluctuating concentrations, because relatively
high concentrations of trichloroethanol indicate recent high
exposure, whereas relatively high concentrations of trichloroacetic
acid indicate long-term exposure to high concentrations (Monster et
al., 1979).
Trichloroethylene concentrations in alveolar air and in blood,
shortly after exposure, indicate recent exposure concentrations,
while the concentration several hours after exposure indicates the
average exposure over the preceding days (Stewart et al., 1974).
6. EFFECTS ON ANIMALS AND CELL SYSTEMS
6.1. Effects on Animals
Data from acute, short-term repeated dose, and long-term
toxicity studies on laboratory animals are summarized in Table 6.
6.1.1. Acute toxicity
Acute toxicity data on common laboratory animals are shown in
Tables 7 and 8.
Acute toxicity levels following inhalation exposure to
trichloroethylene are summarized in Table 7. Table 8 includes data
on acute oral, dermal, intraperitoneal (ip), subcutaneous (sc), and
intravenous (iv) LD50s for the mouse, rat, rabbit, and dog.
Von Oettingen (1955) reported that oral acutely toxic doses in
rats produced gastrointestinal irritation. Moderate increases in
aspartate aminotranferase (EC 2.6.1.1) levels were observed in rats
24, 48, and 72 h after a single 6-h exposure to trichloroethylene
vapour at concentrations of 54 mg/m3 (10 ppm) and 540 mg/m3
(100 ppm) (Deguchi, 1972); the increase at 5400 mg/m3 (1000 ppm)
was small (Deguchi, 1972), presumably due to inactivation of P-450.
According to Rigaud et al. (1977), intraperitoneal administration
resulted in a significant increase in aspartate aminotransferase
(EC 2.6.1.1), alanine aminotransferase (EC 2.6.1.2), and ornithine
carbamoyltransferase (OCT) (EC 2.1.3.3) in the rat, whereas
Wirtschafter & Cronyn (1964), administering 500 mg/kg body weight,
detected only minor hepatic effects over the 12 - 24-h period
following administration. No evidence of kidney dysfunction was
observed in mice following intraperitoneal administration of
trichloroethylene at 0.004M/kg body weight. Application of 2 ml
trichloroethylene (7800 mg/kg), under an occlusive dressing, on the
skin of 20 guinea-pigs, did not produce any deaths but, during the
35-day observation period, there were reductions in body weight at
1 week ( P < 0.001), 2 and 3 weeks ( P < 0.01), and 4 weeks
( P < 0.05) (Wahlberg & Boman, 1979).
Skin irritation
Trichloroethylene (purity 99.5%), applied (0.5 ml) to the
shaved (non-abraded) skin of rabbits, for 24 h, under an occlusive
dressing, produced severe skin irritation (Duprat et al., 1976).
In another study, trichloroethylene (1.0 ml) was applied,
occluded in a "skin depot", to the clipped skin of guinea-pigs.
Histological examinations were performed at 15 min, 1, 4, and 16 h.
Degenerative changes (pyknotic nuclei) were observed in the
epidermis after 15 min and were progressive (pyknosis, karyolysis,
junctional separation of the epidermis) up to the end of the study
at 16 h (Kronevi et al., 1981).
Table 6. Concentrations of trichloroethylene at which no effects were observed in experimental animals
exposed through inhalation
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
rat 3000 7 h mortality Adams et al. (1951)
6400 1.4 h mortality Adams et al. (1951)
12 000 0.6 h mortality Adams et al. (1951)
20 000 0.4 h mortality Adams et al. (1951)
200 7 h per day, 5 days mortality Adams et al. (1951)
per week, for 26
weeks
400 8 h per day for mortality; body weight; Battig et al. (1963)
5 days learning capacity
730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35a 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
guinea-pig 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology (1967)
6 weeks
35 90 days mortality; body weight; Prendergast et al.
haematology; histology (1967)
histology
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
guinea-pig 100 7 h per day, 5 mortality Adams et al. (1951)
(contd.) days per week for
26 weeks
monkey 730 8 h per day, 5 mortality; body weight; Prendergast et al.
(squirrel) days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
monkey 400 7 h per day, 5 mortality Adams et al. (1951)
(Rhesus) days per week for
26 weeks
rabbit 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
200 7 h per day, 5 mortality Adams et al. (1951)
days per week for
6 weeks
dog 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
dog 35 90 days mortality; body weight; Prendergast et al.
(contd.) haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
mouseb 5500 20 min anaesthesia Gehring (1968)
5500 100 min liver injury (elevated SGPT) Gehring (1968)
5500 300 min mortality Gehring (1968)
---------------------------------------------------------------------------------------------------------
a Kalashmikova et al. (1976) reported that rats exposed to 50 mg/m3 for 5 h per day, for 90 days, showed
damage to parenchyma in liver and kidney.
b Kjellstrand et al. (1983) reported that male NMRI mice
continuously exposed to 200 mg/m3 (37 ppm) for
30 days showed increased plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity; female mice did
not show any increase in BuChE activity; there was a significant increase in liver weight at 200 mg/m3
(37 ppm) in both sexes.
Table 7. Acute toxicity of trichloroethylene administered via
inhalation to laboratory animals
--------------------------------------------------------------------
Species Toxicity Exposure Reference
index ------------------------
level duration
(ppm) (mg/litre) (h)
--------------------------------------------------------------------
Rat LC100 20 000 107 0.4 Adams et al. (1951)
LC100 12 000 64.2 1.4 Adams et al. (1951)
LC100 2500 13.4 7.0 Adams et al. (1951)
LC50 26 300 140.7 1 Vernot et al. (1977)
LC50 12 500 66.9 4 Siegel et al. (1971)
LCL0a 8000 42.8 4 Smyth et al. (1969)
Mouse LC100 8000 42.7 2 Von Oettingen (1955)
LC100 5600 30.0 9.75 Gehring (1968)
LC50 8450 45.1 4 Kylin et al. (1962)
LC50 41 122 220 0.33 Aviado et al. (1976)
LC50 49 000 262 0.50 Vernot et al. (1977)
LCL0a 3000 16.0 2 Lazarev (1929)
LC50 40 4 Lazarev & Gadaskina
(1977)
Guinea- LC100 37 000 197.8 0.67 Von Oettingen (1955)
pig
Cat LCL0a 6074 32.5 2 Lehmann & Schmidt-Kehl
(1936)
Rabbit LC 5000 26.75 14.28 McCord (1932)
LC 10 000 53.5 2.5 McCord (1932)
LC 20 000 107 2 McCord (1932)
--------------------------------------------------------------------
a LCLO = lowest published lethal concentration.
Eye irritation
Instillation of 0.1 ml of trichloroethylene (purity 99.5%)
into rabbit eyes produced mild to moderate conjunctivitis with
superficial epithelial abrasion. At 7 days, there was a resolving
keratitis with complete recovery within 2 weeks (Duprat et al.,
1976).
6.1.2. Short-term exposures
6.1.2.1. Oral exposures
In a US NTP study (1983), groups of 10 male and 10 female
F344/N rats were administered trichloroethylene (in corn oil, by
gavage) at doses ranging from 125 to 2000 mg/kg body weight (males)
and 625 to 1000 mg/kg (females), 5 times per week, for 13 weeks.
All rats survived the 13-week study, but males receiving the
2000 mg/kg dose exhibited a 24% decrease in body-weight gain. At
the 1000 mg/kg dose, final body weights for males and for females
were similar to those of the controls.
Table 8. Acute oral, dermal, intraperitoneal, subcutaneous, and
intravenous LD50s for trichloroethylene in laboratory animals
--------------------------------------------------------------------------
Species Oral Dermal Intraperitoneal Subcutaneous Intravenous
(mg/kg body (ml/kg (mg/kg body (mg/kg body (mg/kg body
weight) body weight) weight) weight)
weight)
--------------------------------------------------------------------------
Rat 49201 27252
Dog 56803 2.8004 1505,a
Mouse 28506,b 32107,b 144010 3411
240012 31508,b
30009,c
1.2006,b
Rabbit 201
--------------------------------------------------------------------------
1 From: Smyth et al. (1969). a LDL0.
2 From: Rigaud et al. (1977). b In 24 h.
3 From: Christensen et al. (1974). c In 14 day.
4 From: Klaassen & Plaa (1967).
5 From: Barsoum & Saad (1934).
6 From: Aviado et al. (1976).
7 From: Klaassen & Plaa (1966).
8 From: Gehring (1968).
9 From: Gradiski et al. (1974).
10 From: Plaa et al. (1958).
11 From: NIOSH (1977b).
12 From: Tucker et al. (1982).
Histopathological examination of tissues from animals receiving
the highest doses showed minimal or mild cytomegaly and karyomegaly
of the renal tubular epithelial cells in the inner cortex in 8/9
males dosed with 2000 mg/kg per day, and the same effect, graded as
equivocal or mild, was seen in 5/10 females that had received the
1000 mg/kg per day dose.
The results of this 13-week study in F344/N rats were
essentially similar to those of an earlier 8-week study conducted
on Osborne-Mendel rats (NCI, 1976). In this study, only doses in
excess of 5000 mg/kg per day were lethal for rats. Doses of
1000 mg/kg per day had no effect on body-weight gains in males,
but depressed weight gains in females by approximately 15%.
Groups of 10 male and 10 female B6C3F1 mice received
trichloroethylene (by gavage in corn oil) at doses ranging from
375 to 6000 mg/kg body weight, 5 times per week for 13 weeks. All
males and 9/10 females receiving 6000 mg/kg, 7/10 males and 1/10
females receiving 3000 mg/kg, and 2/10 males and 1/10 females
receiving 1500 mg/kg died. Mean body weights of male mice dosed
with 750, 1500, or 3000 mg/kg were depressed by 11%, 19%, and 17%,
respectively, relative to the controls. Mean body weights of
control and treated groups of female mice were similar (US NTP,
1983).
Liver weights (both absolute and as percentage body weight)
increased in a dose-related fashion. Liver weights were increased
by more than 10% relative to the controls for males receiving
750 mg/kg body weight or more and for females receiving 1500 mg/kg
or more.
Histopathological examination showed hepatic centrilobular
necrosis (6/10 males and 1/10 females administered 6000 mg/kg).
This lesion was not seen in either males or females administered
3000 mg/kg, but 2/10 males had multifocal areas of calcification
scattered throughout their livers. Multi-focal calcification was
also seen in the liver of the single female mouse that survived the
6000 mg/kg dosage regimen. One female in the 3000 mg/kg dose group
developed a hepatocellular adenoma, an extremely rare lesion in
female mice of this age (20 weeks).
Examination of renal tissues showed the presence of mild to
moderate cytomegaly and karyomegaly of the renal tubular epithelial
cells of the inner cortex. These changes were found in only 1 of
the 23 mice (13 males and 10 females) that died after receiving
doses of 3000 or 6000 mg/kg for up to 6 weeks. However, the
changes were found in all 4 of the males that died after receiving
the 3000 mg/kg dose for 7 - 13 weeks, and in all animals that
survived the 6000 mg/kg (1/10 females) and the 3000 mg/kg doses
(3/10 males and 9/10 females). Tissues from mice receiving lower
doses of trichloroethylene were not examined.
Trichloroethylene administered orally to mice at doses in
excess of 500 mg/kg for 10 days produced proliferation of hepatic
peroxisome as demonstrated by increased cyanide-insensitive
palmitoyl CoA oxidation (PCO) and electron microscopy. In the
rat, trichloroethylene did not have any effect on peroxisome
proliferation. Trichloroacetic acid administered for 10 days
increased hepatic peroxisome proliferation in both species (Elcombe
et al., 1982). It is possible that the rapid rate of
trichloroethylene metabolism in the mouse, together with the
enterohepatic circulation of trichloroacetic acid, leads to
high steady-state blood levels of trichloracetic acid and the
concomitant proliferation of peroxisomes.
Primary cultures of isolated mouse and rat hepatocytes have
been found to metabolize trichloroethylene to trichloroacetic acid
at rates comparable to those in intact animals. Similarly, the
isolated hepatocytes respond to exposure to trichloroacetic acid
by peroxisome proliferation. Isolated human hepatocytes have been
found to produce trichloracetic acid from trichloroethylene at a
lower rate than that in the rat. Trichloroacetic acid does not
induce peroxisome proliferation in human hepatocytes (Elcombe,
1985).
6.1.2.2. Inhalation exposure
In the rat, exposure to 81 000 mg/m3 (15 000 ppm) for a period
of 2 - 4 min produced complete anaesthesia within 9 min (Schumacher
& Grandjean, 1960). In a study on mice, exposure to 36 7000 mg/m3
(6800 ppm) for 10 - 11 min or 64 800 mg/m3 (12 000 ppm) for 5 -
6 min produced complete anaesthesia (Friberg et al., 1953). In
another study on mice, exposure to 29 700 mg/m3 (5500 ppm) produced
anaesthesia in 46 min (Gehring, 1968).
Studies conducted on rats by Vissarionova et al. (1975) showed
that concentrations of 5000 mg/m3 administered for 5 h a day, for
1 week, resulted in an increase in liver and kidney weight (21.6%
and 18%, respectively), and a decrease in alkaline phosphatase and
RNA-dependent hepatic dehydrogenase. Histological changes have also
been noted in hepatic and renal parenchyma by Kalashnikova et al.
(1976).
With doses of 1080 mg/m3 (200 ppm) administered for 6 h daily
for 4 days, Savolainen et al. (1977) found that rats exhibited
greater motor activity and less cerebral RNA, 5 days after the last
exposure, and an accumulation of trichloroethylene in perirenal
fat.
Doses of 250, 500, 800, and 1200 mg/m3 administered for 15 -
90 h resulted in increases in intracellular lipids (Verne et al.,
1959).
No effects were noticed in rats administered 1080 mg/m3 (200
ppm) for 4 h a day for 4 days (Grandjean, 1960).
Continuous exposure of rats, mice, and gerbils by inhalation to
trichloroethylene at 810 mg/m3 (150 ppm), for periods ranging from
2 to 30 days, produced liver enlargement in all species; the mouse
was the most severely affected. After the end of exposure, the
liver weights of the mice decreased rapidly. An increased kidney
weight was noted in gerbils (Kjellstrand et al., 1981a).
In another study in which 7 different strains of mice (wild,
C57BL, DBA, B6CBA, A/su, NZB, and NMRI) were continuously exposed
to a trichloroethylene concentration of 810 mg/m3 (150 ppm),
Kjellstrand et al. (1983a) reported large increases in liver weight
in all strains with minimal changes in kidney and spleen weights.
Plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity
increased in males of all strains and in females of strains A/su
and NZB, but to a lesser extent than in the corresponding males.
In a further study, with continuous exposure to concentrations of
200 - 1620 mg/m3 (37 - 300 ppm), plasma BuChE increased in male
mice in a time- and concentration-dependent manner. Liver weight
increased in a time- and concentration-dependent manner in both
sexes.
Exposure of rats, guinea-pigs, rabbits, dogs, and monkeys to
3825 mg/m3, for 8 h per day, 5 days per week, for 6 weeks, resulted
in a loss of overall body weight in dogs and monkeys. There were
no changes in haematological or liver enzyme parameters
(Prendergast et al., 1967).
Groups of male Swiss Webster mice were exposed to 54 000 mg/m3
(10 000 ppm) for 1 or 4 h, daily, for 5 consecutive days. In the
4-h group, the NADPH cytochrome c reductase (EC 1.6.99.3) activity
in the lung decreased, but that in the liver increased. In the
lungs of this group, there were platelet thrombi and vacuolization
of bronchial epithelial cells. There were no changes in the liver.
It was concluded that the reduced activity of the pulmonary mixed-
function oxidase system reflected injury to the lungs (Lewis et
al., 1984).
Rabbits exposed to 15 000 mg/m3 (2790 ppm) for 4 h per day,
6 days a week for 45 days, developed severe normocytic anaemia,
leukopenia, and thrombocytopenia due to toxic effects on the bone
marrow (Mazza & Brancaccio, 1967).
In rats, urinary levels of trichloro-substituted metabolites
and the activity of drug-metabolizing enzymes (cytochrome P-450)
were related to the duration of trichloroethylene anaesthesia
(Moslen et al., 1977). Trichloroethylene hepatotoxicity in the rat
produces increased levels of serum transaminases. Hetaptotoxicity
increases the activity of drug-metabolizing enzymes (cytochrome
P-450) (correlation coefficient: 0.95) and the urinary excretion of
trichloro-metabolites (correlation coefficient: 0.88) (Moslen et
al., 1977).
Cytotoxic effects have been observed in the kidney and liver of
dogs subjected to 81 000 mg/m3 (1.5%; 15 000 ppm) trichloroethylene
anaesthesia (Kiseleva & Korolenko, 1971).
Studies on rats exposed by inhalation to trichloroethylene
concentrations of 2160, 4320, or 8640 mg/m3 (400, 800, or 1600 ppm)
for 6 h revealed no effects at the first concentration, a decrease
in swimming activity at 4320 mg/m3, and a further decrease at
8640 mg/m3 (Grandjean, 1963). Other rats exposed to 1944 -
2268 mg/m3 (360 - 420 ppm) for 8 h a day, 5 days a week for
46 weeks, exhibited no effects (Bättig & Grandjean, 1963). After
43 weeks at 2160 mg/m3 (400 ppm), rats in another set of tests by
Bättig (1964) exhibited greater maze skills. Mice exposed
intermittently to trichloroethylene showed a decrease in motor
activity at 4500 mg/m3 (900 ppm), but, at 19 440 mg/m3, it was
considerably increased (Kjellstrand et al., 1983b).
In Mongolian gerbils, continuously exposed to trichloroethylene
at 1.72 mg/m3 (320 ppm) for 9 months, there was no effect on
spatial memory, but subsequent maze performance test results were
interpreted as indicating an irreversible effect on the central
nervous system (Kjellstrand et al., 1980). In another study, 2
groups of Mongolian gerbils were continuously exposed to 810 mg
trichloroethylene/m3 (150 ppm) for 71 and 106 days, respectively.
In a series of maze tests following the end of exposure, the
treated groups performed less well than the unexposed controls
(Kjellstrand et al., 1981b).
6.1.2.3. Parenteral exposure
When male Swiss Webster mice were injected ip with 3 doses of
330 mg trichloroethylene/kg body weight (vehicle 0.2 ml of 25%
Tween 80 in saline) on alternate days, the activity of hepatic
microsomal NADPH cytochrome c reductase was increased. There
were no morphological changes in the liver (Lewis et al., 1984).
Studies conducted on rabbits showed that intramuscular
administration of 3 ml trichloroethylene, 3 times weekly for
29 days, induced neuronal damage (Bartonécek & Brun, 1970).
6.1.3. Long-term exposure
6.1.3.1. Oral exposure
The US NTP (1983) studied the effects of orally-administered
(in corn oil, by gavage) epichlorohydrin-free trichloroethylene in
male and female F344/N rats and B6C3F1 mice. Doses (500 and
1000 mg/kg body weight for rats and 1000 mg/kg for mice) were
administered 5 days per week for 103 weeks. The survival of
treated male rats and male mice was significantly reduced in
relation to that of corn-oil control animals. Mean body weights
of treated rats (both sexes) were lower than those of corn-oil
controls and the reduction in body weight gain was dose-related.
The body weights of treated female mice were similar to those of
vehicle controls.
Toxic nephrosis was found in 96/98 (98%) of the treated male
rats, in 97/97 of the treated female rats, in 45/50 (90%) of the
treated male mice, and in 48/49 (98%) of the treated female mice,
but was not found in any of the corn-oil control rats or mice.
Initially noted in rats that died early, the lesions were diagnosed
as frank enlargement of the nucleus and cytoplasm of scattered
individual tubular cells with brush borders, located near the
cortico-medullary junction. Progression of the lesions was
evident. As exposure time increased, affected tubular cells
were larger and additional tubules and tubular cells were affected.
Some tubules were enlarged or dilated to the extent that they were
difficult to identify as tubules. Eventually, there was loss of
some enlarged cells. Corresponding tubules became dilated and
portions of the basement membrane had a stripped appearance. In
the most advanced stage, the lesion had progressed to the sub-
capsular cortex, with enlarged tubular cells.
In mice, the pathological development of the renal lesion was
basically similar to that observed in rats, but it was relatively
less severe and did not develop to a stage where there was
extensive loss of cytomegalic epithelial cells and tubular
dilation.
When trichloroethylene was given in the drinking-water (0.1,
1.0, 2.5, and 5.0 g/litre) to CD-1 mice for 4 - 6 months, a
significant reduction in body weight (males and females at
5.0 g/litre), enlarged liver (males at 1.0, 2.5, and 5.0 g/litre;
females at 5.0 g/litre), and increase in kidney weight (males and
females at 5.0 g/litre) were observed. However, pathology at 4 and
6 months was unremarkable.
6.1.3.2. Inhalation exposure
Studies on rats showed that exposure to concentrations below
1080 mg/m3 (200 ppm) for 6 h a day, 5 days a week for 6 months, did
not induce any visible effects. At concentrations of 10 800 mg/m3
(2000 ppm), narcosis and loss of appetite were observed. At
16 200 mg/m3 (3000 ppm), only 33% of the test animals survived
(Taylor, 1936).
When NMRI mice were exposed to trichloroethylene at 700 mg/m3
(130 ppm) continuously for 30 days, liver weight increased by 1.5
and 1.9 times in males and females, respectively (Kanje et al.,
1981).
When rats were exposed to trichloroethylene at 1945 -
2270 mg/m3 (360 - 420 ppm), for 8 h a day, 5 days a week for
46 weeks, there were no changes in conditioned reflexes or reaction
time, but there was an increase in spontaneous climbing activity
(Bättig & Grandjean, 1963).
Rats exposed to 50 mg/m3 (0.05 mg/litre) for 5 h daily, for
3 months, showed damage to hepatic and renal parenchyma
(Kalashnikova et al., 1976).
Long-term exposure to concentrations of 100 - 200 mg/m3
(0.1 - 0.2 mg/litre) reduced the phagocytic activity of rat
leukocytes (Pelts, 1962).
In female Mongolian gerbils, exposed continuously to 270 and
810 mg/m3 (50 and 150 ppm) for 12 months, there were small changes
in the lipid composition of the cerebral cortex and hippocampus.
While protein content and lipid class distribution were virtually
unaffected, the cholesterol to phospholipid ratio in the cortex
decreased (Kyrklund et al., 1983).
6.1.3.3. Parenteral exposure
Of 6 rabbits given trichloroethylene at 200 mg/kg body weight
intramuscularly for 55 - 100 days, 2 died from renal failure
(Bartonécek & Brun, 1970). Rabbits given 2 ml (2.92 g)
intramuscularly twice a week for 41 - 247 days exhibited
neuronal damage (Bartonécek & Sovcek, 1959).
6.1.4. Interactions
Studies on rats showed that trichloroethylene was more toxic in
animals on a high-carbohydrate diet than in those on a high-protein
diet (Kalashnikova et al., 1974). In rats (Cornish & Adefuin,
1966), rabbits (Desoille et al., 1962), and human beings (Sbertoli
& Brambilla, 1962; Ferguson & Vernon, 1970; Pardys & Brotman,
1974), the presence of ethanol increased trichloroethylene
toxicity. Trichloroethylene and carbon tetrachloride acted
synergistically in producing hepatotoxicity (Deguchi, 1972;
Pessayre et al., 1982).
When trichloroethylene was administered intraperitoneally with
toluene, there was a decrease in the side-chain oxidation of the
toluene (Ikeda, 1974).
In studies by White & Carlson (1979), trichloroethylene
caused spontaneous, and potentiated epinephrine-induced, cardiac
arrhythmias in rabbits. A series of studies was conducted to
examine the effects of metabolic-inhibitors, enzyme-inducing
agents, or pre-treatment with ethanol or caffeine, on the
sensitivity of rabbits to epinephrine-induced cardiac arrythmias
(White & Carlson, 1979, 1981a, 1982). Rabbits treated with
metabolic-inhibiting agents developed more arrhythmias after
shorter exposure times in response to lower doses of epinephrine
than controls. Phenobarbitone and Aroclor 1254(R), and in a later
study benzo( a)pyrene (Carlson & Shite, 1983), were used as enzyme-
inducing agents. Phenobarbitone-treated rabbits developed
fewer cardiac arrhythmias and had lower blood levels of
trichloroethylene. Rabbits treated with benzo( a)pyrene developed
more cardiac arrhythmias and at lower doses of epinephrine when
exposed by inhalation to a trichloroethylene concentration of
43 500 mg/m3 (8100 ppm). Aroclor 1254(R) did not induce any
effects on trichloroethylene metabolism nor on the development of
epinephrine-induced cardiac arrhythmias. Rabbits pre-treated with
ethanol (1000 mg body weight, intravenously or orally) or caffeine
(10 mg/kg body weight, intraperitoneally) 30 min before exposure to
trichloroethylene at 32 400 mg/m3 (6000 ppm) by inhalation showed
an increased senstivity to epinephrine-induced cardiac arrhythmias.
Male New Zealand rabbits exposed to trichloroethylene at
10 800, 21 600, 32 400, or 43 200 mg/m3 (2000, 4000, 6000, or
8000 ppm) by inhalation for 1 h showed a concentration-related
sensitivity to epinephrine-induced cardiac arrhythmias. The blood
levels of the trichloroethylene metabolites (trichloroethanol and
trichloroacetic acid) were measured. Rabbits given chloral
hydrate (50 mg/kg body weight, intravenously), a trichloroethylene
intermediate metabolite, had blood levels of trichloroethanol and
trichloroacetic acid that were 40 - 100 times higher than those of
rabbits exposed to a trichloroethylene level of 43 200 mg/m3 (8000
ppm) but showed no increase in sensitivity to epinephrine-induced
cardiac arrhythmias (White & Carlson, 1981b). It is concluded that
trichloroethylene, rather than its metabolites, sensitizes the
rabbit myocardium.
6.1.5. Immunotoxicity
Changes in some components of antibody production (7S), cell-
mediated immunity (haemagglutination), and bone marrow stem cell
colonization have been observed in rodents (female mice were more
sensitive than males in one study; chinchillas were used in the
other) exposed to low-to-moderate levels (10 - 1000 mg/m3) by
inhalation or to 0.1 - 5 g/litre per day in the drinking-water for
periods ranging from a few weeks to 6 - 10 months (Shmuter, 1972;
Sanders et al., 1982).
6.1.6. Effects on cell systems
In vitro incubation of nerve-fibre membranes from rat spinal
cord with 10 µM trichloroethylene reduced the low relative
molecular mass protein fraction (Savolainen & Seppalainen, 1979).
A trichloroethylene concentration of 1.3/108 moles per ml was
the LD50 for HeLa (human cervix carcinoma) cells on the third day
of treatment (Gradiski et al., 1974).
Exposure of rabbit trachea cultures to trichloroethylene vapour
at 27 000 mg/m3 (5000 ppm) for 129 min and 216 000 (40 000 ppm) for
13 min, induced ciliostasis (Tomenius et al., 1979). The isolated
guinea-pig heart exposed to trichloroethylene at 530 mg/litre,
developed transitory arrhythmia. At a concentration of 1.06%,
there was evidence of a reduction in contractile force and coronary
flow, and arrhythmia (Bianchi et al., 1963; Matturro et al., 1963).
6.1.7. Carcinogenicity
Groups of 50 male and 50 female Osborne-Mendel rats, 7 weeks
old, were administered trichloroethylene (99% trichloroethylene
stabilized with 0.19% 1,2-epoxybutane and 0.09% epichlorohydrin) in
corn oil, by gavage, 5 days a week for 78 weeks. High-dose animals
received varying dose schedules of 1000 - 1500 mg/kg body weight
per day, and low-dose animals received 500 - 750 mg/kg body weight
per day. All surviving animals were killed 110 weeks after the
start of treatment. The time-weighted average doses were 549 and
1097 mg/kg body weight per day. A group of 20 male and 20 female
vehicle-treated rats served as controls. Of the males, 17/20
controls, 42/50 low-dose, and 47/50 high-dose animals died before
the end of the study; of the females, 12/20 controls, 35/48 low-
dose, and 37/50 high-dose animals died early. Median survival
times were approximately 60 weeks for high-dose males, 85 weeks
for low-dose males, and 70 weeks for high- and low-dose females.
Of the males, 5/20 controls, 7/50 low-dose, and 5/50 high-dose rats
developed tumours; of the females, 7/20 controls, 12/48 low-dose,
and 12/50 high-dose rats developed tumours. There were no liver-
cell tumours. Tumours occurred in various other organs in treated
and control animals and were mainly reticulum-cell sarcomas,
lymphosarcomas or malignant lymphomas, fibroadenomas of the
mammary gland, haemangiosarcomas at various sites, follicular
adenocarcinomas of the thyroid, chromophobe adenomas of the
pituitary, and renal hamartomas. Toxic nephropathy was observed
in rats of both sexes treated with high and low doses of
trichloroethylene (NCI, 1976).
Groups of 50 male and 50 female B6C3F1 mice, 5 weeks old,
were given trichloroethylene (99% trichloroethylene stabilized
with 0.19% 1,2-epoxybutane and 0.09% epichlorhydrin) in corn oil
by gavage, 5 days a week, for 78 weeks. High-dose males received
2000 - 2400 mg/kg body weight per day, and females 1400 - 1800
mg/kg body weight per day; low-dose males and females received
1000 - 1200 mg/kg body weight per day and 700 - 900 mg/kg body
weight per day, respectively. All surviving animals were observed
until they reached 95 weeks of age. Time-weighted average doses
were 1169 and 869 mg/kg body weight per day, respectively, in low-
dose males and females and 2339 and 1739 mg/kg body weight per day,
respectively, in high-dose males and females. Groups of 20 male
and 20 female mice served as vehicle-treated matched controls.
Survival was reduced in high-dose males and control males.
Hepatocellular carcinomas occurred in 1/20 control males and 0/20
control females; in 26/50 low-dose males and 4/50 low-dose females,
and in 31/48 high-dose males and 11/47 high-dose females.
Metastases of the liver-cell tumours to the lung were found in
7/98 treated males and in 1 control male. The first hepatocellular
carcinoma was observed in a mouse which had been treated with the
high dose of trichloroethylene and which died during week 27.
Lung tumours occurred in treated animals of both sexes: 5/50 (5
adenomas) in males and 4/50 (2 adenomas, 2 carcinomas) in females
in the low-dose group, and 2/48 (1 adenoma, 1 carcinoma) in males
and 7/47 (5 adenomas, 2 carcinomas) in females treated with the
high dose of trichloroethylene. Among controls, only one lung
adenoma was reported in a female (NCI, 1976).
In a study by Maltoni & Maioli (1977) and Maltoni et al. (in
press), groups of 30 male and 30 female, 13-week-old Sprague Dawley
rats were given trichloroethylene (highly purified and epoxide-
free, and stabilized with 20 ppm of butyl-hydroxytoluene) in olive
oil by gavage, 5 days a week, for 52 weeks. Two doses of
trichloroethylene were tested. The high-dose animals received
250 mg/kg body weight, and the low-dose animals 50 mg/kg body
weight; 30 males and 30 females received olive oil alone and served
as controls. The animals were kept until their spontaneous death
(the study lasted 140 weeks). There was no increase in specific
tumours. Abnormalities (cytokaryomegaly) in cells of the renal
tubules were observed in high-dose male rats. These changes were
not observed in females.
Groups of 30 female ICR Swiss mice and a group of 30 male
and 30 female mice of the same strain, were treated with
trichloroethylene dermally (1 mg in acetone), 3 times weekly, for
83 weeks, subcutaneously (0.5 mg in trioctanoin) once weekly for
89 weeks, and by gavage (0.5 mg in trioctanoin), once weekly, for
89 weeks. Similar-sized groups of animals served as controls. No
tumours were observed at the site of application (Van Duuren et
al., 1979). Similar negative findings were obtained following
thrice-weekly skin applications and once-weekly subcutaneous
injections of trichloroethylene oxide for life in a group of
70 female mice of the same strain (Van Duuren et al., 1983)
Groups of 30 male and 30 female Wistar rats, NMRI mice, and
Syrian hamsters were exposed by inhalation to trichloroethylene
(purified, with no detectable epoxides, and containing 15 ppm
triethanolamine as stabilizer) at 540 and 2700 mg/m3 (100 and
500 ppm). The animals were exposed for 6 h per day, 5 days per
week, for 78 weeks. The studies were terminated by killing the
surviving mice and hamsters after 130 weeks, and rats after 156
weeks. No carcinogenic effects were observed in rats, hamsters,
and male mice. A moderate increase in lymphomas was found in
treated female mice (18/28 at 2700 mg/m3; 17/30 at 540 mg/m3, and
9/29 in controls). The authors concluded that, on the basis of
these findings, there were no indications of carcinogenicity
(Henschler et al., 1980).
Groups of 49 - 50 female ICR mice, 4 weeks of age, were exposed
by inhalation to trichloroethylene (purity 99.8% with 0.128% carbon
tetrachloride, 0.019% benzene, 0.019% epichlorohydrin, and 0.01%
1,1,2-trichloroethane) at 0.270, 810, or 2430 mg/m3 (0, 50, 150,
or 450 ppm), 7 h per day for 5 days per week, for 104 weeks. The
surviving animals were killed 107 weeks after the start of the
study. Mortality was similar in control and treated groups.
Lung adenomas were found in 5, 2, 5, and 4 mice in the control,
low-dose, mid-dose, and high-dose groups, respectively. However,
it was reported that adenocarcinomas (none of which gave rise to
metastases) occurred in 1/49, 3/50, 8/50, and 7/46 mice in the
control, low-dose, mid-dose, and high-dose groups, respectively,
and that increased incidences in the mid- and high-dose groups,
were statistically significant compared with controls. In similar
studies on Sprague Dawley rats, no statistically-increased
incidence of tumours was detected; one clear-cell carcinoma of the
kidney was observed in the high-dose group (Fukuda et al., 1983).
In a large series of inhalation studies, highly purified
epoxy-free trichloroethylene (stabilized with 20 ppm butyl-
hydroxytoluene) was studied under controlled exposure conditions
in male and female Sprague Dawley rats and Swiss and B6C3F1 mice.
Treatment was for 7 h per day, 5 days per week for 8, 78, or 104
weeks. After the end of the treatment, all animals were kept under
observation until spontaneous death. The following 7 studies were
performed (Maltoni & Maioli, 1977; Maltoni et al., in press):
Study 1
Groups of 60 - 90 male and 60 - 90 female Sprague Dawley rats
were exposed to 0, 540, or 3240 mg trichloroethylene/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed. Cytomegaly and karyomegaly of tubular cells in the
kidney were not observed at either dose.
Study 2
Groups of 60 - 100 male and 60 - 100 female Swiss mice were
exposed to trichloroethylene at 0, 540, or 3240 mg/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed.
Studies 3 and 4
Groups of 90 - 95 male and 90 - 105 female Sprague Dawley rats
were exposed to trichloroethylene at 0, 540, 1620, 3240 mg/m3 (0,
100, 300, or 600 ppm) for 104 weeks. Further groups of 40 males
and 40 females were started on the same treatment 4 - 5 weeks later
(Study 4). The method and results of the 2 studies were similar
and the combined data are described. No increase in mortality was
observed in the treated animals. Cytomegaly and karyomegaly of
renal tubular cells were observed in males in the mid- and high-
dose groups and the incidence was dose-related; no such lesions
were observed in males in the low-dose group or in females at
any of the 3 dose levels, or in the controls. Five tubular
adenocarcinomas were observed in 4/130 males and 1/130 females
at the high-dose level. Leydig cell tumours of the testis were
observed in 6/135 controls, and in 16/130, 30/130, and 31/130
male rats, respectively, in the low-, mid-, and high-dose treated
groups. The average time of appearance of the tumours, from the
start of the study, was 113 weeks in controls and from 109 to
113 weeks in treated animals. A higher incidence of immunoblastic
lymphosarcomas was detected in male and female treated animals:
1/280 in controls, 9/260 at the low dose, 5/260 in the mid-dose
group, and 3/260 in the high-dose group.
Study 5
Groups of 90 male and 90 female Swiss mice were exposed to
trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0, 100, 300,
or 600 ppm) for 78 weeks. The incidence of hepatocellular
carcinomas in males was 4/90 in controls compared with 2/90 at the
low dose, 8/90 at the mid dose, and 13/90 at the high dose. One
hepatocellular carcinoma occurred in a female at the high dose.
The incidences of lung adenomas and adenocarcinomas were 25/180
in controls, 26/180 in low-dose groups, 36/180 in mid-dose, and
47/180 in the high-dose groups (males and females combined). Two
adenocarcinomas were seen in the control groups and 3 in the high-
dose group.
Studies 6 and 7
For study 6, groups of 90 male and 90 female B6C3F1 mice were
exposed to trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0,
100, 300, or 600 ppm) for 78 weeks. Because of excess mortality
due to fighting in the male groups, study 7 was started in which
trichloroethylene was tested in the same way in equal numbers of
male mice. In females, the incidence of pulmonary tumours (all
adenomas except one adenocarcinoma in the low-dose group) were 4/90
in controls compared with 6/90, 10/90, and 15/90 in the low-, mid-,
and high-dose groups, respectively. Hepatocellular carcinomas were
observed in 3/90 controls compared with 4/90, 4/90, and 9/90 in the
low-, mid-, and high-dose groups, respectively. In the males in
study 7, the incidence of pulmonary tumours was not affected by the
treatment. Hepatocellular carcinomas were observed in 14/90
controls compared with 19/90, 27/90, and 21/90 in the 3 treated
groups, respectively. Cytomegaly and karyomegaly were not observed
in either study (Maltoni et al., in press).
Groups of 50 male and 50 female rats (F344) and mice
(B6C3F1) were treated, by corn-oil gavage, with epichlorohydrin-
free trichloroethylene at 500 and 1000 mg/kg body weight (rats),
and 1000 mg/kg body weight (mice), 5 times weekly, for 103 weeks.
Similar-sized groups of male and female rats and mice treated with
corn oil alone, or without any treatment, served as controls. The
animals were killed between 103 and 107 weeks from the start of the
study. Trichloroethylene significantly reduced the survival of
male rats and mice. In male rats treated with trichloroethylene,
the incidence of tubular-cell neoplasms was increased (0/49,
untreated; 0/48, corn-oil control; 2/49, low dose; 3/49, high
dose). There was one tubular-cell tumour in a high-dose female
rat. Five low-dose male rats also had malignant peritoneal
mesotheliomas, compared with 1/50 untreated control, 1/50 vehicle
control, and 0/49 high-dose animals. Trichloroethylene also
produced nephrosis in both sexes of both species. In mice, the
administration of trichloroethylene increased the incidence of
hepatocellular carcinoma (males: 8/48, controls; 30/50, treated;
females: 2/48, controls; 13/49, treated). The incidence of
hepatocellular adenomas was also increased in male mice (3/48,
controls; 8/50, dosed) and in female mice (2/48, controls; 8/49,
dosed) (US NTP, 1983).
Groups of 50 male and 50 female Swiss mice were treated
by corn-oil gavage with daily doses of different samples of
trichloroethylene, initially at 2400 mg/kg body weight (males)
and 1800 mg/kg body weight (females), 5 days a week, for 78 weeks.
Due to toxicity, dosing and dose levels were reduced during the
study. The animals were kept under observation until
spontaneous death. The samples included: (a) highly purified
trichloroethylene stabilized with 0.0015% triethanolamine, (b)
industrial trichloroethylene (99.4% pure), (c) highly purified
trichloroethylene stabilized with 0.8% epichlorohydrin, (d) highly
purified trichloroethylene, with 0.8% 1,2-epoxybutane, and (e)
highly purified trichloroethylene with 0.25% epichlorohydrin and
0.25% epoxybutane. Similar-sized groups of each sex, treated with
corn oil alone, served as controls. Mortality was increased in
treated males and some treated female groups. No increase in
tumour incidence was observed except in groups (c) and (d) where
there was an increased incidence of forestomach cancers, attributed
to the direct alkylating properties of one of the two stabilizers
(Henschler et al., 1984).
6.1.7.1. Conclusions
Trichloroethylene (with or without epoxide stabilizers) caused
an increased incidence of hepatocellular carcinomas in 2 different
strains of mice, either when given by oral administration or by
inhalation.
Trichloroethylene also produced lung tumours in Swiss ICR mice
and in female, but not male, B6C3F1 mice.
Trichloroethylene, with added epichlorohydrin, administered by
gavage, caused an increased incidence of forestomach cancers in
WMRI mice.
Trichloroethylene (epoxide stabilizer-free) produced a low
incidence of renal tubular tumours in 2 different strains of mice
(mainly in males) following long-term oral or inhalation exposure.
These tumours occur very rarely in untreated rats of these strains.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure.
In one strain of mice and one strain of rats, epoxide
stabilizer-free trichloroethylene produced some increases in the
incidence of lymphomas. However, these data are insufficient to
draw any conclusions.
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats.
It was noted in the evaluations by IARC (1979, 1982) that
it was considered that there was limited evidence for the
carcinogenicity of trichloroethylene for experimental animals.
The significance of these findings needs to be evaluated in
the context of further studies on the mechanism of action of
trichloroethylene.
6.1.8. Mutagenicity
6.1.8.1. Gene mutation
(a) Bacteria and fungi
Pure trichloroethylene induced revertants (gene-mutations) in
the K-12 strain of Escherichia coli in the presence of fortified
mouse Arochlor(R)-induced microsomal preparations for only one gene
out of four analysed. In the positive case, the induced effect was
the doubling of spontaneous background (Greim et al., 1975).
Trichloroethylene (of unspecified purity) in vapour form was
slightly mutagenic for the TA-100 strain of Salmonella typhimurium,
in the presence of S9 mix obtained from mouse liver (control
value = 140 revert./plate; treated values: 240 revert./plate)
(Simmon et al., 1977).
Technical grade trichloroethylene (containing 1900 ppm of
1,2-epoxybutane and 900 ppm of epichlorohydrin) was mutagenic for
the TA-100 strain of S. typhimurium in a desiccator, in the absence
of a metabolic activation system (untreated 161 - 187 revert./plate;
treated: 567 - 651 revert./plate). Trichloroethylene of high
purity was not mutagenic for the TA 100 strain of S. typhimurium
in the presence of 0.5 ml of S9 mix obtained from mouse, rat, and
hamster liver; it was, however, mutagenic for this strain of
S. typhimurium in the presence of 50 - 150 µl of S9 mix from
Arochlor-treated rat liver (untreated series: 160 - 180
revert./plate; treated series: 350-379 revert./plate) (Crebelli
et al., 1982).
High-purity trichloroethylene was not mutagenic for the TA 100
strain of S. typhimurium in a plate assay, with and without
addition of S9 mixture (Henschler et al., 1977).
Trichloroethylene containing no traces of 1,2-epoxybutane or
epichlorohydrin was tested as a gas in a desiccator on the TA 1535
and TA 100 strains of S. typhimurium. Concentrations of 1 - 3%
produced a 30% increase (less than a doubling) in revertants in
strain TA 100 in the presence of a metabolic activation system from
Arochlor(R)-induced rat liver (Baden et al., 1979).
The exposure of S. typhimurium strains TA 98 and TA 100 to
0.5 - 10% vapour concentrations of trichloroethylene in sealed
vials for 48 h did not produce an increase in revertants either in
the presence or absence of rat liver homogenate (Waskell, 1978).
Trichloroethylene of high purity (99.5%) was mutagenic for the
TA-100 strain of S. typhimurium when used as a gas in a desiccator
in the presence of S9 mix (Bartsch et al., 1979). However, it
should be noted that the increase in revertants in the treated
series was less than twice that of the untreated series, but this
slight effect was consistent in repeated experiments.
Trichloroethylene of unspecified purity was reported to double
the spontaneous frequency of reverse mutations in the D-7 strain of
the yeast Saccharomyces cerevisiae, at one concentration, in the
presence of an endogenous metabolic activation system (Callen et
al., 1980).
Trichloroethylene containing impurities, which were not
specified, was mutagenic for S. cerevisiae, strain XV185-14C
(reverse mutations) in the presence of S9 mix. These results
were obtained at a level of yeast cell % survival of < 1 (1 h of
treatment) and of < 0.1 (4 h of treatment). These conditions are
unusual for the detection of mutagenic effects, since, at these
toxic values, selection of different cells (wild type and mutants)
is very efficient (Shahin & von Borstel, 1977). One of the authors
(von Borstel, personal communication, 1984) stated that, when these
studies were repeated with a pure trichloroethylene sample, it was
not mutagenic for yeast cells.
Trichloroethylene of high purity (99.8%), as well as
trichloroethylene containing stabilizers such as 1,2-epoxy-butane
(0.19%) and epichlorohydrin (0.09%), were not mutagenic for the
yeast S. pombe (forward mutations), with or without a metabolic
system obtained from rats and mice treated with phenobarbital and
beta-naphthoflavone. Using intraperitoneal or intrasanguineous,
host-mediated assay techniques with male mice inoculated with the
yeast S. pombe and treated orally with 2 g/kg body weight for
4 or 16 h, both pure trichloroethylene and the stabilized
trichloroethylene were found not to be mutagenic (Rossi et al.,
1983).
A slightly mutagenic action for cells of S. cerevisiae strain
D7 (reverse mutation at his locus) was found for trichloroethylene
of unspecified purity in the presence of a metabolic activation
system obtained from mice (untreated series: 0.79 - 0.89 his,
rev/106; treated series: 3.00 - 3.60 his rev/106 with 40 mol).
For this experiment, no survival values were reported, nor were the
actual number of revertants counted, and therefore the observed
effect cannot be definitely assigned to trichloroethylene
(Bronzetti et al., 1978). The same authors administered
trichloroethylene (unspecified purity) orally to mice (400 mg/kg
body weight) that were also inoculated with yeast cells intra-
sanguineously; a mutagenic effect was found on yeast cells (reverse
mutations at the his locus) collected from liver, lung, and kidney
but, again, the survival level was not reported nor was the number
of revertants counted (Bronzetti et al., 1978).
Trichloroethylene of high purity and containing known
contaminants (~80 ppm) was found to be active for the production
of reverse mutations (suppressor mutations) on Aspergillus
nidulans sumeth A1 strain at a dose of 27 000 mg/m3 (5000 ppm)
applied in a desiccator containing the plates inoculated with the
fungal conidia (Crebelli et al., in press).
(b) Mammalian cells (in vitro)
A pure sample of trichloroethylene, stabilized with thymol,
did not induce mutations (forward mutations) at the HPRT locus of
Chinese hamster V-79 cell line treated in vitro with or without
metabolic activation (S9 mix) (Loprieno & Abbondandolo, 1980).
(c) Mammals (in vivo)
Trichloroethylene (purity 99.5%) was evaluated for its ability
to induce somatic gene mutations in vivo in embryonic fibroblasts
of mice (spot test). Pregnant mice were injected 12 days after
copulation with a dose of 140 mg/kg body weight (50 females), or
350 mg/kg (26 females). The results observed were 2/145 (1.4%)
and 2/51 (3.9%) of progeny with "spots", respectively, whereas
the untreated series produced 0/146, 3/182 (1.6%), 6/794 (0.75%).
These results were considered by the authors to indicate a
mutagenic effect (Fahrig, 1977); however, positive data such as
those observed for trichloroethylene are among spontaneous values
for this test system.
6.1.8.2. Chromosome aberrations
(a) Mammals (in vivo)
Trichloroethylene (purity not given), repeatedly administered
intraperitoneally to ICR mice for 5 days at a dose equivalent to
half of the LD50, did not induce a significant increase in
chromosome aberration frequency in bone marrow cells from animals
killed 6, 24, and 48 h after the last treatment (Cerna & Kjpenova,
1977).
A single dose of trichloroethylene of 1000 mg/kg body weight
(stabilized with thymol), administered orally to Swiss albino
mice, did not induce a significant increase in the frequency of
chromosome aberrations in bone marrow, 24 h after treatment
(Loprieno & Abbondandolo, 1980).
The exposure of male mice (NMRI-Han/BGA) to trichloroethylene
vapour (99.5% purity) for 24 h at concentrations of 272, 1090, and
2430 mg/m3 (50, 202, and 450 ppm) did not induce dominant lethal
mutations (Slacik-Erben et al., 1980).
CDI male mice were treated orally with 3000, 2250, 1500,
1125, 750, or 375 mg/kg body weight (2 treatments in 24 h) with a
trichloroethylene sample of high purity (99.5%) and sacrificed 6 h
following the second treatment, for the analysis of the frequency
of micronucleated erythrocytes in the bone marrow cells. The
results indicated a positive dose-related effect of
trichloroethylene (Duprat & Gradiski, 1980).
Using high purity (99.8%) trichloroethylene, B6C3F1 mice (10
female and 10 male) were treated by inhalation with 3240 mg/m3
(600 ppm), for 7 h a day, 5 days a week, for a total of 52 days.
The animals were killed 6 h and 24 h after the last treatment. The
cytogenetic analysis showed the presence only of gaps without any
indication of induced chromosomal aberrations (Loprieno, personal
communication, 1984).
When trichloroethylene of high purity (99.8%) was administered
to B6C3F1 male mice, by gavage, in a single dose of 1200 mg/kg
body weight, an increased frequency (4 times) of micronucleated
erythrocytes in the bone marrow cells compared with control animals
was observed, 42 h after dosing (Sbrana et al., 1984).
6.1.8.3. DNA damage
Fungi
Trichloroethylene (unspecified purity) was positive for
the induction of mitotic gene conversion at the trp locus and
mitotic recombination in the D7 strain of S. ceverisiae, under
endogenous metabolic activation conditions (Callen et al., 1980).
Trichloroethylene (unspecified purity) was also found to be
positive for the induction of mitotic gene conversion at the trp
locus of S. cerevisiae D7 strain treated in vitro in the presence
of a metabolic activation system obtained from rat liver. Using
the host-mediated assay technique, and a single oral dose of
400 mg/kg body weight, given to mice inoculated with yeast cell
D4 strain of S. cerevisiae, a positive effect on the induction of
mitotic gene conversion at the trp 5 and ade 2 loci was observed
in the yeast cells recovered from the kidney. Repeated oral
administration of trichloroethylene to male mice, with a total
dose of 3700 mg/kg body weight, induced a positive effect (mitotic
recombination) in yeast cells recovered from the liver and kidney.
In all these studies, the actual numbers of the convertant or
recombinant colonies were not given, and the significance of the
results is questionable (Bronzetti et al., 1978).
Trichloroethylene (stabilized with thymol) was considered
inactive for the induction of mitotic recombinations in the D4
strain of S. cerevisiae following in vitro and in vivo (host-
mediated assay) studies (Loprieno & Abbondandolo, 1980).
Trichloroethylene of high purity (99.9%) induced somatic
segregants in A. nidulans strain 35 x 17, at concentrations of
40 500 and 81 000 mg/m3 (7500 and 15 000 ppm), in a desiccator
containing the plates inoculated with the fungal conidia; under
identical doses and conditions, no mitotic recombinant colonies
were induced in this strain of A. nidulans by trichloroethylene
(Crebelli et al., in press).
6.1.8.4. Mammalian cells ( in vitro)
Trichloroethylene of high purity (99.9%) induced morphological
transformations, in vitro, in rat embryo cell cultures (Price et
al., 1978).
Stabilized with thymol, trichloroethylene did not stimulate
unscheduled DNA synthesis in an HeLa human cell line grown in
vitro, in the presence or absence of a metabolic activation
system (S9 mix) (Loprieno & Abbondandolo, 1980). Trichloroethylene
(unspecified purity) elicited DNA repair synthesis in human
lymphocytes in the presence of S9 mix (Perocco & Prodi, 1981).
Exposure of Chinese hamster ovary cells (CHO) to
trichloroethylene bubbled into the growth medium for 2 min,
corresponding to a dose of 0.17% v/v for 1 h treatment time,
did not induce sister-chromatid exchange (White et al., 1979).
Trichloroethylene bound covalently to calf thymus DNA in vitro
in the presence of a rat liver metabolic activation system (Di
Renzo et al., 1982; Bergman, 1983).
When given ip to NMRI mice, twice daily for 5 days
(33.6 mg/kg), trichloroethylene bound to RNA and DNA of brain,
lung, liver, kidney, spleen, pancreas, and testis (Bergman, 1983).
6.1.8.5. Mutagenic activity of trichloroethylene metabolites
Trichloroethylene oxide was not mutagenic for the TA 1535
strain of S. typhimurium or the WP2 UVRA strain of E. coli (plate
test), but was positive in the DNA repair test on E. coli (Kline
et al., 1982).
The same compound was found to be a direct mutagen for the
yeast S. pombe (forward mutation) and for mammalian cells grown
in vitro (V-79 cell line) (forward mutation) (Loprieno &
Abbondandolo, 1980).
Syrian golden hamster embryo cells were exposed to
trichloroethylene oxide (1.1, 2.5, and 5.0 µM) at 37 °C for 30 min.
After 5 days, the number of transformed colonies was scored. A low
but dose-related increased frequency of transformed cells was
induced (Di Paolo & Doniger, 1982).
Chloral hydrate has been reported to have direct genotoxic
activity in S. typhimurium (Waskell, 1978) and in A. nidulans
(Bignami et al., 1980).
At doses of 82.7, 165.4, or 4135 mg/kg body weight, chloral
hydrate, administered intraperitoneally, induced chromosome non-
disjunction in the secondary spermatocytes of mice (Russo et al.,
1984).
6.1.8.6. Conclusions
Pure trichloroethylene acts as a weak mutagen in S. typhimurium
in the presence of a metabolic activation system. However, in all
cases, the induced effect was never more than twice the spontaneous
value. Technical grade trichloroethylene containing stabilizers
such as 1,2-epoxybutane epichlorohydrin, is mutagenic in
S. typhimurium in the absence of a metabolic activation system.
Pure trichloroethylene is not mutagenic for the yeast S. pombe,
with or without metabolic activation, in vitro or in vivo (host-
mediated assay).
Thymol-stabilized trichloroethylene is not mutagenic for V-75
hamster cells, with or without metabolic activation.
Trichloroethylene (99.5% pure) is a weak mutagen for the
induction of somatic mutations in mouse embryo.
In mice, a single oral dose of trichloroethylene (1 g/kg body
weight) or repeated exposure to 3240 mg/m3 (600 ppm) for 52 days,
7 h/day, through inhalation, did not increase chromosome
aberration frequency in bone-marrow cells. A half-LD50 dose
of trichloroethylene, administered ip to mice, did not increase
chromosomal aberration frequency in bone-marrow cells. In a
micronucleus test, a single oral treatment with 1200 mg/kg body
weight or repeated (twice) oral treatment with a series of doses
ranging from 375 to 3000 mg/kg body weight increased the frequency
of induced micronucleated erythrocytes in the bone-marrow cells of
mice. Trichloroethylene bound to DNA in vitro and in vivo to
mouse tissues.
Dominant lethal mutations were not induced in mice exposed
through inhalation to trichloroethylene vapours.
Trichloroethylene induces mitotic gene conversion in yeast
cells grown under conditions allowing an endogenous metabolic
activation system to function. The same effect reported in yeast
in the presence of an exogenous metabolic activation system
(S9 mix), or in the host-mediated assay, is questionable, because
of inadequate data and the fact that other studies produced
negative data.
A positive effect for the induction of unscheduled DNA
synthesis (UDS) in human lymphocytes, treated in vitro, was
reported by Perocco & Prodi (1981), whereas a negative response
for the same biological effect (UDS) in a human fibroblast cell
line (HeLa) grown in vitro was observed by Loprieno & Abbondandolo
(1980).
Trichloroethylene induces morphological transformation in rat
embryo cells.
The available results, shown in Table 9, are not adequate
for a complete evaluation of the genotoxic potential of
trichloroethylene. Only a few mutagenicity studies have indicated
the grade and purity of the trichloroethylene sample employed.
The confusing results could be due either to the use of
trichloroethylene samples stabilized with mutagenic compounds or to
the use of pure trichloroethylene samples which, unstablized, can
rapidly decompose to chemicals with mutagenic activity.
6.1.9. Reproduction, embryo/fetotoxicity, and teratology
The embryo/fetal toxicity and teratogenic potential of
trichloroethylene have been evaluated in the avian embryo system,
the mouse, rat, and rabbit. Reproductive function has been
examined in male rats.
6.1.9.1. Avian embryo system
Exposing avian embryos to 1% trichloroethylene (10 g/litre)
caused a significant increase in embryonic death and a slight
increase in anomalies (Fink, 1968). These observations were made
on the 3rd day of development, a time at which frank malformations
would be difficult to observe or evaluate. Elovaara et al. (1979)
reported the results of a comparative study of the teratogenic
potential of trichloroethylene and other aliphatic chlorinated
hydrocarbons in the chick embryo. Trichloroethylene (reagent
grade) in doses ranging from 5 to 1000 µmoles in olive oil (25 µl)
was injected into the air space of fertilized eggs on the 2nd and
6th days of incubation and examined on the 14th day. The LD50 for
trichloroethylene was between 50 and 100 µmoles/egg. Macroscopic
embryonic malformations occurred in 9 out of 55 surviving embryos,
5 times the incidence in the vehicle control eggs.
In another study, injection of low doses of trichloroethylene
(1, 5, 10, and 25 µmol/egg), on days 1 and 2 of embryogenesis,
resulted in embryotoxicity, growth defects, and morphological
anomalies (Bross et al., 1983).
Table 9. Summary of the mutagenicity data reported for trichloroethylene
-----------------------------------------------------------------------------------------
Organism Genotoxic effects
Gene Chromosome DNA
mutation anomalies damage
-----------------------------------------------------------------------------------------
prokaryotes weak
positive
-----------------------------------------------------------------------------------------
fungi negative ( in vitro) positive negative ( in vitro)
negative (hma) mitotic weak (hma)
positive? ( in vitro) segregation positive (yeast
positive (A. nidulans) A. nidulans metabolism)
-----------------------------------------------------------------------------------------
mammalian negative negative (SCE)
cells negative (DNA
( in vitro) repair synthesis)
positive (covalent
binding)
-----------------------------------------------------------------------------------------
mammals weak negative (1 dose) (1 g/kg body
( in vivo) weight, oral)
negative (52 doses) (3240 mg/m3,
inhalation)
negative (5 doses) (1/2 LD50, ip)
positive (micronuclei) 1200 mg/kg
positive (micronuclei) (375 -
3000 mg/kg)
negative (dominant lethals) (270,
1090, 2430 mg/m3, inhalation)
-----------------------------------------------------------------------------------------
For human cases, see Table 10.
6.1.9.2. Mouse
Swiss-Webster mice were exposed by inhalation to
trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h daily, on days
6 - 15 of gestation (Day 0 = vaginal plug). The mice were observed
daily throughout pregnancy. Maternal body weights were recorded on
days 6, 10, and 18 of gestation as an index of maternal toxicity.
On day 18, no effect was observed on the average number of
implantation sites/litter, litter size, the incidence of fetal
resorptions, fetal sex ratios, or fetal body measurements (Schwetz
et al., 1975). The incidence of gross anomalies observed by
external examination was not significantly greater than that among
the control litters. Trichloroethylene exposure did not exert any
effect on the incidence of skeletal anomalies in mice, and
microscopic examination did not reveal any abnormalities in organs,
tissues, or cells (Leong et al., 1975; Schwetz et al., 1975).
6.1.9.3. Rat
In studies similar to the preceding mouse study, exposure to
trichloroethylene at 1620 mg/m3 (300 ppm) did not produce any
evidence of maternal toxicity or teratogenicity in Sprague Dawley
rats (Leong et al., 1975; Schwetz et al., 1975) or Charles River
strain rats (Bell, 1977).
Exposing Sprague Dawley rats to trichloroethylene at 2700 mg/m3
(500 ppm) for 7 h per day, 5 days per week, during a 3-week
pregestational period, and 7 h per day each day for days 0 - 18 and
days 6 - 18 of gestation did not cause maternal or embryo/fetal
toxicity. Beliles et al. (1980) concluded that the frequency and
the character of the macro- or microscopic findings in the treated
groups did not indicate either adverse fetal effects or that
trichloroethylene, at the dose used, was teratogenic.
Dorfmueller et al. (1979) reported that female Long-Evans rats
exposed to trichloroethylene at 9720 mg/m3 (1800 ppm) before
mating, for 6 h per day, 5 days per week, for 2 weeks and/or during
pregnancy, for 6 h per day, each day, for 0 - 20 days of gestation)
did not exhibit any signs of maternal toxicity. There was no
indication of embryotoxicity, and no significant treatment effects
or interactions were found in the number of corpora lutea or
implantation sites/litter, fetal body weights, resorbed
fetuses/litter, or sex ratios. No significant treatment effects
were observed in the analysis of total soft-tissue anomalies.
However, prenatal exposure to trichloroethylene at 9720 mg/m3
(1800 ppm) caused an increase in minor anomalies of the offspring
(incomplete ossification of the sternum) indicative of development
delays in maturation, but not of major malformation.
While the study did not indicate any treatment-related effects
on general post-natal behaviour, there was a small but significant
regression in post-natal weight gain in offspring in the pre-mating
exposure group.
In groups of male rats dosed by gavage with trichloroethylene
at 10, 100, or 1000 mg/kg body weight 5 days per week, for 6 weeks,
there was no evidence of spermatotoxic effects. Trichloroethylene-
related effects of reduced weight gain and elevated liver/body
weight ratios were seen primarily in the 1000 mg/kg group.
Copulatory behaviour was initially diminished by the narcotic
properties of trichloroethylene, but was normal by the 5th week
(Zenick et al., 1984).
6.1.9.4. Rabbit
Female New Zealand white rabbits were exposed by inhalation to
trichloroethylene at 2700 mg/m3 (500 ppm), for 7 h per day, 5 days
per week, during a 3-week pregestation period, and daily, for days
0 - 21 and days 7 - 21 of gestation (Day 0 = mating day). There
was no evidence of maternal toxicity or embryotoxicity. The
occurrence of hydrocephalus in a few fetuses in one of the study
groups was reported (4 fetuses in 2 litters), but no definitive
conclusion was drawn from these findings (Beliles et al., 1980).
7. EFFECTS ON THE ENVIRONMENT
7.1. Aquatic Organisms
There is little information on the toxicity of
trichloroethylene for fish. The US Registry of Toxic Effects of
Chemical Substances (Christensen & Lugenbyhl, 1975) reports, for an
unidentified species, that exposure to a concentration range of
100 - 1000 mg/litre produced toxic effects in 96 h. Toxicity tests
carried out on salt-water flatfish, Limanda limanda (sole), 15 -
20 cm long, in a continuous water flow, established a 96-h LC50
of 16 mg/litre (Pearson & McConnell, 1975). A 96-h LC50 of
approximately 40 mg/litre (static) or 67 mg/litre (continuous flow)
has been reported for the minnow Pimephales promelas (Alexander et
al., 1978).
Verschueren (1977) established an LC100 of 600 mg/litre for
Daphnia magna. The LC50 for the balanide salt-water crustacean
nauplius (larva) (Elminius modestus) was 20 mg/litre after 46 h
(Pearson & McConnell, 1975), and the LC50 for the protozoon
Entosiphon sulcatum was established as 1200 mg/litre (Bringmann
& Kuhn, 1980).
Various LC50 values have been established for algae including
63 mg/litre for Microcystis aeruginosa, (Verschueren, 1977); a
concentration of 1000 mg/litre did not have any observable effect
on Scenedesmus quadricauda (Bringmann & Kuhn, 1980). A short-term
photosynthesis efficiency test gave an LC50 of 8 mg/litre
(Pearson & McConnell, 1975) and, finally, in tests carried out on
Thalassiosira pseudonana and Dunaliella tertiolecta, there were
observable effects at 50 and 100 µg/litre, in a mixed culture
(Biggs et al., 1979).
7.2. Uptake, Distribution, Storage, Metabolism, and Elimination in
Plant and Animal Organisms
Bioaccumulation of trichloroethylene in a marine environment
has been studied by Pearson & McConnell (1975); concentration
levels were determined for a wide variety of marine organisms,
mostly in the Bay of Liverpool.
The greatest increase in trichloroethylene concentrations in
the tissues of animals that are relatively high up in the food
chain (birds' eggs, fish liver, and seal fat) was nearly 100 times
the level in water (from 0.5/109 µg/litre in water to 50/109 µg/kg
in tissues).
These data agree with the laboratory findings of Barrows et
al. (1980) who, in a 14-day test, noted that trichloroethylene
accumulation in the sunfish species, Lepomis macrochirus was 17
times that of the aquatic medium with a halving time of less than
one day. A low bioconcentration factor (concentration in organism
divided by concentration in environment) (Uehleke et al., 1977)
has been derived using water solubility and the equation proposed
by Kenaga (1980) and Kenaga & Goring (1980).
7.3. Effects on the Stratospheric Ozone Layer
Consideration has been given to the possibility that
trichloroethylene, together with other halocarbons in the
atmosphere, may contribute to the depletion of the stratospheric
ozone layer, which would lead to atmospheric heating and increased
exposure of terrestrial biota to ultraviolet radiation (Molina &
Rowland, 1974). Atmospheric trichloroethylene concentrations seem
to be about one-fifth to one-tenth of those of other chlorocarbons
such as CH3CCl3, CH2Cl2, CCl4, or C2Cl4 or the major chlorofluoro-
carbons (Cronn et al., 1977; Penkett, 1982). The reason for this
is that while trichloroethylene emissions into the atmosphere are
of the same order as those of other halocarbons (Jesson, 1980),
trichloroethylene is efficiently scavenged by hydroxyl radicals
in the troposphere, and the reaction rate for this process is
appreciably faster for trichloroethylene than for other halocarbons
(Penkett, 1982). Thus, the predicted atmospheric lifetime for
trichloroethylene is short (about 10 - 11 days) (Derwent &
Eggleton, 1978; Graedel, 1978) compared with those for the
chlorofluorocarbons, which may be 10 years or more (Jesson, 1980).
It is not clear whether trichloroethylene is even present in the
stratosphere (Cronn et al., 1977). However, the data suggest that
trichloroethylene is unlikely to be involved in the possible
depletion of the ozone layer.
8. EFFECTS ON MAN
8.1. General Symptoms and Signs
8.1.1. Acute effects
Sorgo (1976) reported a lethal oral dose for trichloroethylene
to be 7 g/kg body weight, and Lazarev et al. (1977) reported a
fatality after the oral ingestion of less than 50 ml.
At exposure concentrations of 270 - 540 mg/m3 (50 - 100 ppm),
the most common symptoms are headache, sluggishness, sleepiness
(especially at the end of the workshift), dulling of senses,
dizziness, nausea, and vomiting (Rubino et al., 1959; Lilis et
al., 1969; Governa, 1981). At narcotic doses, vomiting may occur.
Trichloroethylene has been used as an analgesic and as an
anaesthetic on its own or in mixtures. It is now much less used
for this purpose, as safer and more effective alternatives are
available. Inhalation of 27 000 mg/m3 (5000 ppm) produces light
anaesthesia; concentrations of up to 108 000 mg/m3 (20 000 ppm)
have been used for deeper anaesthesia (Wade, 1977).
Clinical signs are not very specific. There can be enhanced
responsiveness to caloric stimulation of the labyrinth and some EEG
anomalies may be detected (Chiesura, 1980) (section 8.2). Acute
hepatic and renal toxicity has been reported (Sucui & Olinici,
1983).
A particular intolerance to ethanol may occur after ingesting
even small amounts of alcoholic beverages. This intolerance is
characterized by intense cutaneous vasodilatation, particularly in
the face, often called "degreaser's flush" (Stewart et al., 1974).
Non-specific effects on the digestive system (e.g., dyspepsia,
gastritis, and diarrhoea) have been reported in cases of suicidal
or accidental ingestion of trichloroethylene (Bozza Marrubini et
al., 1978; Governa, 1981).
8.1.2. Chronic effects
The existence of a condition of chronic trichloroethylene
poisoning is not clear (Chiesura, 1980; Boudène et al., 1983).
However, chronic effects in human beings can occur following
prolonged exposure to moderate concentrations of trichloroethylene
of about 540 mg/m3 (100 ppm). The clinical picture is mainly
related to the central nervous system (CNS), and consists of
asthenia, anorexia, headache, loss of memory, moodiness,
depression, insomnia, paraesthesia, and disturbances of the
autonomic nervous system such as hyperhydrosis, tachycardia, and
dermographism (Ahlmark & Forssman, 1951a,b; Bardodej & Viskocil,
1956; Rubino et al., 1959; Lerza et al., 1963; Castellino, 1969).
The role of trichloroethylene in inducing liver damage in
occupationally-exposed human beings is not clear (Parmeggiani,
1956; Capellini & Grisler, 1958; Rubino et al., 1959; Tolot et al.,
1964; Schuttmann, 1970; Lachnit, 1971; Thiele, 1982; Sucui &
Olinici, 1983; Svabova & Mencik, 1983).
Human subjects with high repeated, but non-occupational,
exposure may exhibit toxic effects on the liver (e.g., elevated
aspartate (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2)),
renal insufficiency, and abnormal EEG patterns (Baerg & Kimberg,
1970). Following accidental or suicidal ingestion (e.g., doses of
100 - 300 ml), hepatic necrosis and nephropathy have been found in
autopsy cases (Keinfeld & Tabershaw, 1954; Graovac-Leposavic
et al., 1964; Priest & Horn, 1965; Beisland & Wannag, 1970;
Clearfield, 1970). A decrease in sexual potency has been reported
in industrial workers exposed to trichloroethylene (Bardodej &
Vyskocil, 1956; Lazarev & Gadaskiva, 1977).
Trichloroethylene is one of the volatile solvents inhaled
("sniffed") for its euphoric effect (Hayden et al., 1976), and
abuse by oral self-administration has been reported (Wells, 1982).
Centrilobular hepatic necrosis and renal toxicity have occurred in
addicts (Baerg & Kimberg, 1970; Clearfield, 1970), and deaths have
been reported (James, 1963; Musclow & Awen, 1971). Some mixtures
sold commercially as trichloroethylene may contain other solvents,
including carbon tetrachloride, which can contribute to the toxic
manifestations (Bouyges et al., 1980; Conso et al., 1980).
Dependent patients may show withdrawal symptoms (Ikeda et al.,
1971).
8.2. Effects on Organs and Systems
8.2.1. Effects on the nervous system
Acute effects on the central nervous system (CNS) are
characterized by two sequential phases (i.e., excitation and
depression), and are usually reversible. These symptoms generally
prevail over those of other systems (Stewart et al., 1962;
Chiesura, 1980; Governa, 1981).
In the early phase of excitation, euphoria and inebriation are
present. The subsequent phase of CNS depression is characterized
by various degrees of narcosis culminating in coma (Caccuri, 1976;
Chiesura, 1980). Muscular hypotony, muscular spasms, reduced
tendon reflexes, and loss of co-ordination may occur (Huff, 1971).
Changes in EEG patterns may vary considerably. The EEG may
be normal in some cases, while substantial alterations, regressing
after a few days, may be observed in others. The most frequently
observed change is a decrease in alpha activity, which is often
irregular. Widespread rapid rhythms or low theta activity may also
be present (Chiesura, 1980).
Exposure to trichloroethylene at a concentration of 540 mg/m3
(100 ppm) produces various results on the CNS. Though there may
not be any evidence of effects on psychomotor capacities (Stewart
et al., 1970), reductions in mental performance evidenced by the
test for perception, past recall, answering speed, and response
and manual dexterity tests have been reported (Ferguson & Vernon,
1970). Visual and auditory evoked potentials were affected at
exposure levels ranging between 270 and 540 mg/m3 (50 and 100 ppm),
for 3 1/2 - 7 1/2 h (Winneke, 1982).
The behavioural effects of exposure to trichloroethylene have
been studied under laboratory and work-place conditions. Laboratory
exposure to 540 or 1080 mg/m3 (100 or 200 ppm), for 70 min, had no
effect on reaction time or short-term memory (Gamberale et al.,
1976). Volunteers exposed to 540, 1620, or 5400 mg/m3 (100, 300,
or 1000 ppm), for 2 h, showed significant impairment in a number
of psychomotor tests at 5400 mg/m3 but not at 1620 or 540 mg/m3
(Vernon & Ferguson, 1969). In a further study (Ferguson & Vernon,
1970), effects were again found at 5400 mg/m3 and marginal effects
at 1620 mg/m3. In another study, exposure of volunteers to 810
or 1620 mg/m3 (150 or 300 ppm), for 2 1/2 h, did not produce
any significant impairment in behavioural test results (Ettema
& Zielhuis, 1975). With repeated exposure under work-place
conditions, no behavioural effects were observed with mean
atmospheric levels of 270 mg/m3 (50 ppm) (Triebig et al., 1977a,b).
In a study of complex reaction time in an occupational group with a
mean exposure of 1320 mg/m3 (245 ppm), reaction time was increased
in comparison with that in a control group (Gun et al., 1978).
Motor nerve conduction velocity was not affected in a group
occupationally exposed to mean atmospheric levels of less than
270 mg/m3 (50 ppm) (Triebig et al., 1982).
A study on 122 exposed workers (Ahlmark & Forssman, 1951a)
showed that symptoms of toxicity in human beings were correlated
with urinary levels of metabolic trichloroacetic acid. With
urinary concentrations of up to 20 mg trichloroacetic acid/litre,
no particular symptoms were noted. With urinary levels ranging
between 45 and 75 mg/litre, headache, fatigue, increased need for
sleep, irritability, and alcohol intolerance were reported in 50%
of workers. When urinary levels of trichloroacetic acid exceeded
300 mg/litre, these symptoms were found in 100% of subjects.
Initial nervous-system signs of toxicity were reported to be
associated with the urinary excretion of trichloroacetic acid
at 75 mg/litre and 30 mg/litre (Lazarev & Gadaskiva, 1977).
Two types of irreversible neurotoxic effects have been reported
in man. One is the specific degeneration of the cranial nerves, in
particular the trigeminal, the olfactory, and the facial nerves
(Goldblatt, 1956; James, 1963; Kjellstrand et al., 1980; Barrett
et al., 1982). However, it is considered that this effect is
not produced by trichloroethylene itself but by its decomposition
product, dichloroacetylene (Henschler et al., 1970a). In addition,
polyneuropathies involving the whole peripheral nervous system have
been described after long-term exposure to trichloroethylene
(Szulc-Kuberska, 1972).
8.2.2. Effects on the cardiovascular system
Several cases of sudden death due to cardiac arrest have been
reported in subjects exposed to trichloroethylene, either medically
or occupationally (Ostelere, 1953; Norris & Stuart, 1957; Meyer,
1966; Wiecko, 1966; Clearfield, 1970; Tomasini, 1976). It has
been suggested that these deaths might be due to an increased
sensitivity of the myocardium to endogenous and exogenous
catecholamines. The event would entail irregular cardiac
rhythm due to the onset of ectopic foci with atrioventricular
dissociation. A range of alterations in cardiovascular function
(e.g., atrial and ventricular extrasystole, tachycardia, and
ventricular fibrillation) has been reported (Bardodej & Viskocil,
1956; Anderson, 1957; Lilis et al., 1969; Tomasini, 1976; Governa,
1981).
8.2.3. Effects on the respiratory system
Under normal conditions of exposure, trichloroethylene did not
induce any effects on the respiratory tract but, at high exposure
levels of 810 - 3510 mg/m3 (150 - 650 ppm), there may be effects
due to local irritation (Bardodej & Viskocil, 1956; Jouglard &
Vincent, 1971; Meyer, 1973; Gaultier et al., 1974; Tomasini, 1976).
Breakdown products are of more importance than trichloroethylene
with respect to the effects on the respiratory system (Castellino,
1969).
8.2.4. Effects on the urinary tract
Altered renal function has been described in both acute and
chronic trichloroethylene poisoning (Nomura, 1962; Graovac-
Leposavic et al., 1964; Clearfield, 1970). The nephropathy
observed could be due to impurities rather than to the
trichloroethylene; technical grade trichloroethylene might have
contained nephrotoxic chlorinated hydrocarbons (e.g., 1,1,2,2-
tetrachloroethane) as impurities (Chiesura, 1980).
8.2.5. Effects on the skin
When applied to the skin, trichloroethylene causes erythema
(Wahlberg, 1984). It is only mildly irritating to human skin
providing it is not held in contact (e.g., by clothing or
footwear). Chemical burning of the skin has been reported
following prolonged contact (Maloof, 1949; Tomasini, 1976). There
is a considerable difference in individual susceptibility to the
effects of repeated exposure. The reaction to repeated contact
(which will defat the skin) may take the form of an erythematous,
exudative, vesicular, eczematous, or exfoliative dermatitis (Roche
et al., 1958; Stopps & McLaughlin, 1967; Ettema et al., 1975).
Secondary infection of the skin may complicate the dermatitis.
"Degreaser's flush", a reddening of the skin in some individuals
ingesting ethanol after exposure to trichloroethylene, can occur
(section 8.1.1).
8.2.6. Effects on the eye
Liquid trichloroethylene in the eye produces pain and
discomfort and superficial damage to the cornea, but complete
recovery occurs within a few days (Grant, 1974). Exposure to high
concentrations of vapour (anaesthetic levels of 27 500 - 108 000
mg/m3) also causes eye irritation and superficial damage to the
cornea, but again with complete recovery (Grant, 1961).
8.2.7. Carcinogenicity
A number of epidemiological studies have been conducted to
examine the possible carcinogenic effects of trichloroethylene in
occupationally-exposed populations, and the major findings are
summarized in Tables 10 and 11. Some of these studies have
followed groups with specified exposure to trichloroethylene
(Axelson et al., 1978, 1984; Tola et al., 1980) (Table 10), whereas
others have involved workers in laundry and dry cleaning with more
or less mixed exposures including trichloroethylene (Blair et al.,
1979; Malek et al., 1979; Katz & Jowett, 1981) (Table 11). Other
studies are of a case-reporting or a case-referent (case-control)
type, concerning pancreatic (Lin & Kessler, 1981) and liver tumours
(Novotna et al., 1979; Paddle, 1983) or other conditions and, also,
involving exposure to other solvents (Lin & Kessler, 1981). Some
case-control studies of lymphomas, both of the Hodgkin and non-
Hodgkin type (Olsson & Brandt, 1980; Hardell et al., 1981) and of
liver cancer (Hernberg et al., 1984) have mentioned exposure to
trichloroethylene as a factor in a few cases, but the numbers are
too small for any conclusions to be drawn about trichloroethylene
as a risk factor.
Table 10. Summary of epidemiological studies on carcinogenicity with specific reference
to trichloroethylene exposure
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure intensity had been monitored since Axelson et al.
of 518 male workers 1950 in terms of urinary excretion of (1978)
(7688 person-years) trichloroacetic acid (TCA).
2. No significant excess of cancer mortality
either in the high-dose group (> 100 mg
TCA/litre urine) or the low-dose group
(< 100 mg TCA/litre urine).
3. Results of first update, see Axelson (1984)
below.
-----------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure information was based on TCA Tola et al.
of 1148 male workers determinations in the urine, permitting (1980)
and 969 female workers latency requirements of 6 - 13 years. Loss
(4269 person-years for to follow-up was 9%.
women)
2. Trichloroethylene was not carcinogenic
(given a latency period of 6 to 13 years)
among those exposed at low levels (100 mg
TCA/litre urine in 91% of the cohort).
3. Cohort to be updated at 5-year intervals.
Mortality and 1. Extension and update of the first-mentioned Axelson et al.
incidence in a study, 65% with exposure from 1970 to 1975 (1984)
cohort of 1424 men and with more than 90% having TCA levels
< 100 mg/litre.
2. Deficit found in mortality from cancers (22
versus 36.9 expected), but a significant
excess of urinary tract (11 versus 4.85) and
haematolymphatic tumours (5 versus 1.20).
3. Specifically, there were 3 urinary bladder
cancers versus 0.83 expected, 4 cancers of
the prostrate versus 2.35 expected and 2
lymphomas versus 0.27 expected, requiring
2 years or more of exposure and 10 years of
latency.
-----------------------------------------------------------------------------------------
Table 11. Summary of epidemiological studies and case reports on carcinogenicity of
mixed exposures involving trichloroethylene or trichloroethylene production
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 330 laundry 1. Significant increase in lung and cervical Blair et al.
and dry-cleaning cancer, and a slight excess of leukaemia and (1979)
workers liver cancer detected.
2. Exposed also to other chemicals such as
benzene and carbon tetrachloride.
3. To be extended to a prospective study of
2 - 5 x 103 workers.
Cohort of 37 male 1. Exposure intensity was such that TCA in Malek et al.
dry-cleaning workers urine was less than 100 mg/litre in over 60% (1979)
of the cohort.
2. 6 cases of cancer, none of which were in the
liver.
-----------------------------------------------------------------------------------------
Table 11. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 671 female 1. Death certificates were employed. Katz & Jowett
laundry/dry-cleaning (1981)
workers (controls: 2. Elevated risks of genital (unspecified) and
entire female working kidney cancers were detected, along with a
population and other smaller excess of bladder and skin cancer and
low-wage occupations) lymphoma.
109 pancreatic cancer 1. Hospital records were employed. Lin & Kessler
patients, case-control (1981)
study 2. An association was observed between
pancreatic cancer and exposure to dry-
cleaning chemicals and gasoline fumes, as
well as to drinking of decaffeinated (by
means of trichloroethylene) coffee.
3. No information was available on extent or
type of exposure.
95 liver cancers, 1. Cancer registry data on liver cancer in the Paddle (1983)
case-study area of a production plant were employed.
2. No case could be definitely tied to
trichloroethylene production.
-----------------------------------------------------------------------------------------
While some of these studies are continuing as prospective
studies to cover larger populations, they suffer, in general, from
one or more of the following factors: (a) small cohort population,
(b) young age of the cohort, (c) a rather short follow-up period,
(d) relatively few cases, (e) inaccurate or uncertain estimation
of intensity and duration of exposure to trichloroethylene, (f)
possible exposure to other chemicals, even in the more specific
studies, including potential carcinogens, and (g) inadequate
information on other general risk factors for cancer, such as
smoking, even if the influence of uncontrolled confounding is
likely to be limited in this respect.
At present, it is not possible to draw definite conclusions
from the existing epidemiological data on the carcinogenic
potential of trichloroethylene. However, the appearance of an
excess of haematolymphatic malignancies in several of the studies,
possibly associated with trichloroethylene exposure (Blair et al.,
1979; Hardell et al., 1981; Katz & Jowett, 1981; Axelson et al.,
1984) deserves attention. Furthermore, genito-urinary tumours were
over-represented in 3 studies (Blair et al., 1979; Katz & Jowett,
1981; Axelson et al., 1984), though the picture is less consistent
with regard to the specific sites involved (bladder, kidney,
prostrate, and cervix).
It was noted that IARC (1979, 1982) concluded that
epidemiological studies on human beings were inadequate to make an
evaluation.
9. EVALUATION OF THE HEALTH RISKS FOR MAN
9.1. Levels of Exposure
9.1.1. General population
The general population is exposed to very low levels of
trichloroethylene in air, water, and food (Table 12). The move
away from the use of trichloroethylene in anaesthesia, the solvent
extraction and fumigation of foodstuffs, and the dry-cleaning
of textiles has reduced exposure from these sources.
Trichloroethylene does not accumulate significantly in the food
chain. It is degraded by both biotic and abiotic processes and
its persistence in various environmental compartments is relatively
short, of the order of days or months rather than years. In ground
water, the absence of actinic radiation means that the rate of
degradation is slower, and trichloroethylene contamination may be
relatively prolonged (e.g., half-life of 2 1/2 years).
Table 12. Maximum observed concentrations of trichloroethylene
in environmental media
---------------------------------------------------------------
Media Maximum observed concentration
---------------------------------------------------------------
Open water reservoir 220 µg/litre
Industrial discharge water 200 µg/litre
Rain water ~ 1 µg/litre
Atmosphere ~ 40 µg/m3
Dairy foods 10 µg/kg
Meat 22 µg/kg
Fats and oil 19 µg/kg
---------------------------------------------------------------
Accidental or suicidal ingestion by adults occurs, but is
influenced by the limited availability of trichloroethylene for
domestic use in many countries.
9.1.2. Occupational exposure
Exposure during the actual production of trichloroethylene is
relatively low because of the nature of the process. Subsequent
uses in, for example, metal degreasing and the dry-cleaning of
textiles, can involve higher exposures. The respiratory route is
the principal route of exposure with dermal exposure as an
additional route. Oral intake is insignificant in occupational
terms.
9.2. Evaluation of Human Health Risks
9.2.1. Acute effects
The predominant effect of trichloroethylene in human beings
is on the central nervous system; liver and kidney damage can also
occur. Central nervous system depression can result in coma and
death. A lethal dose as low as 50 ml (75 g) has been reported
but, in general, the lethal dose for an adult is approximately
7000 mg/kg body weight. The threshold for demonstrable behavioural
effects, resulting from inhalation exposure, is approximately 1350
mg/m3 (250 ppm); less well-defined subjective complaints have been
reported with exposure to concentrations of 270 - 540 mg/m3 (50 -
100 ppm) for a few hours.
Cardiac arrhythmia has been reported during anaesthesia
involving trichloroethylene at inhaled concentrations of 27 000 -
54 000 mg/m3 (5000 - 10 000 ppm) (blood levels 60 - 120 mg/litre).
It should be noted that ethanol potentiates the central nervous
system effects of trichloroethylene. Exposure to trichloroethylene
at 1080 mg/m3 (200 ppm) with blood ethanol levels of 300 - 450
mg/litre produces demonstrable behavioural effects.
Irreversible neurotoxic effects on the trigeminal nerve have
been described in anaesthesia with trichloroethylene, using a
closed-circuit system with soda lime. This effect is attributed to
the generation of dichloroacetylene and not to trichloroethylene.
9.2.2. Chronic effects
The main chronic effects of trichloroethylene in human beings
are disturbances in the central nervous system, as well as effects
on the kidney and liver.
Nervous system symptoms (headache, fatigue, irritability, and
alcohol intolerance) begin to occur when levels of trichloroacetic
acid in urine are about 30 - 75 mg/litre. No symptoms have been
reported at concentrations of 20 mg/litre in urine, which
corresponds to a concentration of trichloroethylene in air of about
50 mg/m3.
For effects on other organs, there is no clear-cut quantitative
information on dose response. There are insufficient data on
possible effects on human chromosomes.
Epidemiological data on the carcinogenicity of trichloro-
ethylene are inconclusive, but a slight excess of genito-urinary
cancers and lymphomas in some studies on dry cleaners and metal
degreasers deserves further attention.
The results of long-term exposure studies on rodents have shown
that the liver and kidney are the critical organs. Cytomegaly and
karyomegaly of renal tubular cells was observed in male rats
exposed to pure trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h
per day, 5 days per week, for 104 weeks, but not at 540 mg/m3 (100
ppm).
No embryotoxic or teratogenic effects have been observed in
mice and rats with inhalation of trichloroethylene at levels of
1620 mg/m3 (300 ppm).
Data on the genetic effects of trichloroethylene are
inconclusive.
Lifetime exposure of mice to trichloroethylene at 1620 mg/m3
(300 ppm) through inhalation or to oral doses of 700 - 1200 mg/kg
body weight per day produced lung and liver tumours. A low
incidence of renal tumours was found in rats exposed to a
trichloroethylene concentration of 3240 mg/m3 (600 ppm) by
inhalation or to 500 - 1000 mg/kg body weight per day by gavage.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure to trichloroethylene
concentrations of > 540 mg/m3 (> 100 ppm).
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats. The significance of
these findings needs to be evaluated in the context of further
studies on the mechanism of action of trichloroethylene.
9.3. Treatment of Poisoning in Human Beings
9.3.1. Emergency measures
9.3.1.1. General points
In the treatment of trichloroethylene poisoning, pressor amines
should not be used because of the risk of producing arrhythmias in
the trichloroethylene-sensitized myocardium (Bozza Marrubini et
al., 1978). Hypotension may be treated by transfusion. If
necessary, anti-arrhythmic and beta-blocking agents can be
administered. Haemodialysis, haemoperfusion, or plasmapheresis
have been reported to be useful (Zaffiri et al., 1968; Roessler
& Morawiec-Borowiac, 1973; Malizia et al., 1984). Medical advice
should always be obtained in cases of over-exposure to, or frank
poisoning by, trichloroethylene.
9.3.1.2. Ingestion
Vomiting should not be induced, because of the danger of
aspiration into the larynx and lungs and consequent risks of
vagal inhibition or chemical pneumonitis. Gastric lavage can be
effective if performed within 4 h. Adsorbents such as activated
charcoal or liquid paraffin (medicinal grade) reduce intestinal
absorption; saline laxatives will speed elimination (Bothe et al.,
1973). If the patient is in a state of stupor or coma, intubation
must be performed before gastric lavage.
9.3.1.3. Inhalation
The victim should be removed from the polluted area and placed
in a semi-prone position ensuring that the airway is clear. If
the victim is in a state of stupor or coma, oxygen should be
administered. If spontaneous respiration is absent or very weak,
artifical respiration should be applied.
9.3.1.4. Dermal exposure
All contaminated clothing should be removed and the affected
parts of the body washed thoroughly with soap and plenty of water.
9.3.1.5. Eye exposure
The eyes should be thoroughly irrigated with water for at least
15 min, and ophthalmological advice obtained on the possible need
for further treatment.
REFERENCES
ABRAHAMSEN, A.M. (1960) Quantitative estimation of
trichloroacetic acid in the urine and serum in trichloroethylene
poisoning. Acta pharmacol. toxicol., 17: 288-294.
ACGIH (1982) TLVs - Threshold limit values for chemical
substances in workroom air adopted by ACGIH for 1982, Cincinnati,
Ohio, American Conference of Governmental Industrial Hygienists,
p. 32.
ADAMS, E.M., SPENCER, H.C., ROWE, V.K., MCCOLLISTER, D.D., & IRISH,
D.D. (1951) Vapor toxicity of trichloroethylene determined by
experiments on laboratory animals. Arch. ind. Hyg. occup. Med.,
4: 469-481.
AHLMARK, A. & FORSSMAN, S. (1951a) Evaluating trichloroethylene
exposures by urinalyses for trichloroacetic acid. Arch. ind. Hyg.
occup. Med., 3: 386-398.
AHLMARK, A. & FORSSMAN, S. (1951b) The effect of
trichloroethylene on the organism. Acta physiol. scand.,
22: 326-339.
ALEXANDER, H.C., MCCARTHY, W.M., & BARTLETT, E.A. (1978) Toxicity
of perchloroethylene, trichloroethylene, 1,1,1-trichloroethane,
and methylene chloride to fathead minnows. Bull. environ. Contam.
Toxicol., 20: 344-352.
ANDERSEN, M.E., GARGAS, M.L., JONES, R.A., & JENKINS, L.J., Jr
(1980) Determination of the kinetic constants for metabolism of
inhaled toxicants in vivo using gas uptake measurements. Toxicol.
appl. Pharmacol., 54(1): 100-116.
ANDERSSON, A. (1957) [Health hazards in industry on exposure to
trichloroethylene: a clinical, experimental, and technical hygienic
investigation.] Acta med. scand., 157(Suppl. 323): 1-220 (in German).
ASCHIMICI (1980) Report for Italian Working Group for
trichloroethylene.
ASTRAND, I. & OVRUM, P. (1976) Exposure to trichloroethylene.
I. Uptake and distribution in man. Scand. J. Work Environ. Health,
2: 199-211.
AVIADO, D.M., ZAKHARI, S., SIMAAN, J.A., & ULSAMER, A.G. (1976)
In: Golberg, L., ed. Methyl chloroform and trichloroethylene in
the environment, Cleveland, Ohio, CRC Press Inc., pp. 47-89.
AXELSON, O., ANDERSSON, K., HOGSTEDT, C., HOLMBERG, B., MOLINA, G.,
& DE VERDIER, A. (1978) A cohort study on trichloroethylene
exposure and cancer mortality. J. occup. Med., 20: 194-196.
AXELSON, O., ANDERSSON, K., SELDEN, A., & HOGSTEDT, C. (1984)
Cancer morbidity and exposure to trichloroethylene. In: ICOST,
International Conference on Organic Solvent Toxicity, Stockholm,
15-17 November 1984 (Abstract in: Arbete och Halsa, 29: 126).
BADEN, J.M., KELLEY, M., MAZZE, R.I., & SIMMON, V.F. (1979)
Mutagenicity of inhalation anaesthetics: trichloroethylene,
divinyl ether, nitrous oxide and cyclopropane. J. Anaesth., 51: 417.
BAERG, R.D. & KIMBERG, D.V. (1970) Centrilobular hepatic necrosis
and acute renal failure in "solvent sniffers". Ann. intern. Med.,
73: 713-720.
BALKON, J. & LEARY, J.A. (1979) An initial report on a
comprehensive, quantitative, screening procedure for volatile
compounds of forensic and environmental interest in human biofluids
by GC/MS. J. anal. Toxicol., 3: 213-215.
BANERJEE, S. & VAN DUUREN, B.L. (1978) Covalent binding of the
carcinogen trichloroethylene to hepatic microsomal proteins and to
endogenous DNA in vitro. Cancer Res., 38: 776-780.
BANERJEE, S., YALKOWSKY, S.H., & VALVANI, S.C. (1980) Water
solubility and octanol/water partition coefficients of organics.
Limitations of the solubility-partition coefficient correlation.
Environ. Sci. Technol., 14: 1227-1229.
BARDODEJ, Z. (1962) [Value and use of exposure test. VIII.
Volatile organic chloride test in urine.] Cesk. Hyg., 7: 234-239 (in
Czech).
BARDODEJ, Z. & VISKOCIL, J. (1956) The problem of
trichloroethylene metabolism and its effects on the nervous system
as a means of hygienic control. Am. Med. Assoc. Arch. ind. Health,
13: 581-592.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS (III), B.S.H., KIRKPATRICK, M.,
MICHAEL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment: a multimedia
environmental study. Biomed. mass Spectrom., 7: 139-147.
BARRETT, L., ARSAC, PH., VINCENT, M., FAURE, J., GARREL, S., &
REYMOND, F. (1982) Evoked trigeminal nerve potential in chronic
trichloroethylene intoxication. J. Toxicol. clin. Toxicol., 19:
419-423.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish (Lepomis macrochirus). In: Hague,
R., ed. Dynamics, Exposure and Hazard Assessment of Toxic
Chemicals, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BARTONICEK, V. (1962) Metabolism and excretion of
trichloroethylene after inhalation by human subjects. Br. J. ind.
Med., 19: 134-141.
BARTONICEK, V. & BRUN, A. (1970) Subacute and chronic
trichloroethylene poisoning: a neuropathological study in rabbits.
Acta pharmacol. toxicol., 28: 359-369.
BARTONICEK, V. & SOUCEK, B. (1959) Metabolism of
trichloroethylene in rabbits. Arch. Gewerbepathol. Gewerbehyg., 17:
283-293.
BARTSCH, H., MALAVEILLE, C., BARBIN, A., & PLANCHE, G. (1979)
Mutagenic and alkylating metabolites of haloethylenes,
chlorobutadienes and dichlorobutenes produced by rodent or human
liver tissues. Arch. Toxicol., 41: 249-277.
BATTIG, K. (1964) Comparison of the effects of trichloroethylene
with the effects of drugs on exploratory behaviour in the rat. In:
14th International Congress on Occupational Health, Madrid, 1963,
Vol. 2, pp. 887-889.
BATTIG, K. & GRANDJEAN, E. (1963) Chronic effects of
trichloroethylene on rat behaviour. Arch. environ. Health, 7:
694-699.
BAUER, U. (1981) [Human exposure to environmental chemicals.
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues.] Zbl. Bakt. I. Abt. Orig., B174: 200-237
(in German).
BAUER, U. & SELENKA, F. (1982) [Exposure of the population to
haloforms and chlorinated solvents in drinking water compared to
air and food.] Von Wasser, 59: 7-16 (in German).
BEISLAND, H.O. & WANNAG, S.A. (1970) [Trichloroethylene sniffing:
acute liver and kidney damage.] Tidsskr. Nor. Laegeforen., 90: 285-288
(in Norwegian).
BELILES, R.P., BRUSICK, D.J., & MECLER, F.J. (1980) Teratogenic-
mutagenic risk of workplace contaminants: trichloroethylene,
perchloroethylene, and carbon disulfide, Washington DC, US
Department of Health, Education and Welfare, pp. 234 (Report for US
DHEW Contract No. 210-77-0047) (Govt Rep. Announce. Index (US),
1982: 2728).
BELL, Z. (1977) Written communication with contractor reports of:
(a) Dominant lethal study with trichloroethylene in albino rats
exposed via inhalation (February 1977); and (b) Teratogenic study
via inhalation with trichlor 132, trichloroethylene in albino rats
(March 1977), Washington DC, US Environmental Protection Agency
(EPA-600/8-82-006).
BELLAR, T.A., BUDDE, W.L., & EICHELBERGER, J.S. (1979) The
identification and measurement of volatile organic compounds in
aqueous environmental samples. IV. In: Schuetzle, D., ed.
Monitoring toxic substances, Washington DC, American Chemical
Society (ACS Symposium Series No. 94).
BERGMAN, K. (1983) Application and results of whole-body
autoradiography in distribution studies of organic solvents. Crit.
Rev. Toxicol., 12(1): 59-118.
BIANCHI, A., DE NATALE, G., & MATTURRO, F. (1963) [Comparative
research on some pharmacological effects of fluothane and other
volatile narcotics.] G. Ital. Chir., 19: 327-349 (in Italian).
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene, and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGNAMI, M., CONTI, G., CONTI, L., CREBELLI, R., MISURACA, F.,
PUGLIA, A.M., RANDAZZO, R., SCIANDRELLO, G., & CARERE, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor, and Aspergillus nidulans.
Chem.-biol. Interact., 30: 9-23.
BLAIR, A., DECOUFLE, P., & GRAUMAN, D. (1979) Causes of death
among laundry and dry cleaning workers. Am. J. public Health, 69:
508-511.
BOLT, H.M. & FILSER, J.G. (1977) Irreversible binding of
chlorinated ethylenes to macromolecules. Environ. Health Perspect.,
21: 107-112.
BOTHE, J., BRAUN, W., & DONHARDT, A. (1973) [Effect of mineral
oil in hydrocarbon poisoning in mice.] Arch. Toxikol., 30: 243-250 (in
German).
BOUDENE, C., TURBIAUX, M., CLUET, J.-L., & TRUHAUT, R. (1983)
Contribution à la fixation d'une concentration limite tolérable de
trichloroéthylène dans l'atmosphère des locaux de travail. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 44(2): 75-91.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and 2-
carbon halogenated aliphatic organic compounds under methanogenic
conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOUYGUES, M., DANNE, O., BOUVRY, M., LUCIANI, F., & RABREAU, D.
(1980) Hépatite au trichloroéthylène. Action synergique du
tétrachlorure de carbone? Nouv. Presse méd., 9(43): 3277.
BOZZA MARRUBINI, M., GHEZZI LAURENZI, R., & UCCELLI, P. (1978)
[Acute intoxication: clinical diagnosis and treatment,] Milan,
Italy, Medico-Farmaceutica Organization Editore, p. 470 (in
Italian).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14: 231-241.
BRONZETTI, G., ZEIGER, E., & FREZZA, D. (1978) Genetic activity
of trichloroethylene in yeast. J. environ. Pathol. Toxicol., 1:
411-418.
BROSS, G., DI FRANCEISCO, D., & DESMOND, M.E. (1983) The effects
of low dosages of trichloroethylene on chick development.
Toxicology, 28: 283-294.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., DEFELD, J.M., & BAUER, H.
(1974) Le dosage par chromatographie en phase gazeuse des
métabolites urinaires du trichloréthylène: l'acide trichloro-
acétique et le trichloroéthanol. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 35(3): 395-402.
CABANA, B.E. & GESSNER, P.K. (1967) Determination of chloral
hydrate, trichloroacetic acid, trichlorethanol, and urochloralic
acid in the presence of each other and in tissue homogenates. Anal.
Chem., 39: 1449-1452.
CACCURI, S. (1976) [Labour medicine,] 3rd ed., Napoli, Italy,
Idelson, pp. 343-352 (in Italian).
CALLEN, D.F., ROLAND WOLF, C., & PHILPOT, R.M. (1980) Cytochrome
P-450 mediated genetic activity and cytotoxicity of seven
halogenated aliphatic hydrocarbons in Saccharomyces cerevisiae.
Mutat. Res., 77: 55-63.
CAPELLINI, A. & GRISLER, R. (1958) [Studies on liver function in
a group of workers chronically exposed to trichloroethylene.] Med.
Lav., 49: 167-172 (in Italian).
CARLSON, G.P. & WHITE, J.F. (1983) Cardiac arrythmogenic action
of benzo( a)pyrene in the rabbit. Toxicol. Lett., 15: 43-48.
CASTELLINO, N. (1969) [Trichloroethylene: technology,
pathological manifestations and prevention,] Milano, Italy, Franco
Angeli Editore, p. 117 (in Italian).
CAVALLO, A. & GRASSI, P. (1976) Determination of chlorinated
hydrocarbons in potable waters. Boll. chim. Union Ital. Lab. prov.,
27: 337-350.
CEFIC (1982) Chlorinated solvents: trichloroethylene in metal
cleaning and other industrial applications, Brussels, Conseil
Européen des Fédérations des Industries Chimiques.
CERNA, M. & KJPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analyzed by screening system tests. Mutat. Res., 46:
214-215.
CHIESURA, P. (1980) [Halogenated aliphatic hydrocarbons.] In:
Crepet, M., ed. [Occupational medicine,] Torino, Italy, Utet (in
Italian).
CHIESURA, P. & CORSI, G. (1961) [Acute trichloroethylene
poisoning in man followed by liver disease and hyperglycaemic
glycosuria.] Folia med., 44: 121-135 (in Italian).
CHRISTENSEN, H.E., LUGINBYHL, T.T., & CARROLL, B.B., ed. (1974)
The toxic substances list 1974, Washington DC, National Institute
of Occupational Safety and Health, p. 353.
CHRISTENSEN, H.E. & LUGINBYHL, T.T. (1975) Registry of toxic
effects of chemical substances 1975, Washington DC, US Department
of Health, Education and Welfare.
CLEARFIELD, H.R. (1970) Hepatorenal toxicity from sniffing spot-
remover (trichloroethylene): Report of 2 cases. Am. J. dig. Dis.,
15: 851-856.
COLEMAN, W.E., LINGG, R.D., MELTON, R.G., & KOPFLER, F.C. (1976)
The occurrence of volatile organics in five drinking water supplies
using gas chromatography/mass spectrometry. Identification and
analysis of organisms in polluted water. In: Keith, L.H., ed.
Proceedings of the First Chemical Congress of the North American
Continent, 1975, Ann Arbor, Michigan, Ann Arbor Science,
pp. 305-327.
CONSO, F., EFTHYMIOU, M.-L., GARNIER, R., & FOURNIER; E. (1980)
Interêt de la toxicovigilance: example de certains
trichloréthylènes du commerce. Arch. Mal. prof. Méd. Trav., 41:
198-200.
CORNISH, H.H. & ADEFUIN, J. (1966) Ethanol potentiation of
halogenated aliphatic solvent toxicity. Am. Ind. Hyg. Assoc. J., 27:
57-61.
CORREIA, Y., MARTENS, G.J., VAN MENSCH, F.H., & WHIM, B.P. (1977)
The occurrence of trichloroethylene, tetrachloroethylene and 1,1,1-
trichloroethane in Western Europe in air and water. Atmos.
Environ., 11: 1113-1116.
CREBELLI, R., BIGNAMI, M., CONTI, L., & CARERE, A. (1982)
Mutagenicity of trichloroethylene in Salmonella typhimurium TA 100.
Ann. Ist. Super. Sanità, 18: 117-122.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (in press)
Mutagenicity of trichloroethylene, trichloroethanol, and chloral
hydrate in Aspergillus nidulans. Mutat. Res.
CRONN, D.R., RASMUSSEN, R.A., ROBINSON, E., & HARSCH, D.E. (1977)
Halogenated compound identification and measurement in the
troposphere and lower stratosphere. J. geophys. Res., 82: 5935-5944.
DALBEY, W. & BINGHAM, E. (1978) Metabolism of trichloroethylene
by the isolated perfused lung. Toxicol. appl. Pharmacol., 43: 267-277.
DANIEL, J.W. (1963) The metabolism of 36Cl-labelled
trichloroethylene and tetrachloroethylene in the rat. Biochem.
Pharmacol., 12: 795-802.
DEFALQUE, R.J. (1961) Pharmacology and toxicology of
trichloroethylene: A critical review of the world literature. Clin.
Pharmacol. Ther., 2: 665-688.
DEGUCHI, T. (1972) Threshold limit values for solvent mixtures in
the air. Effects of single and mixed chlorinated hydrocarbons upon
the level of serum transaminases in rats. Osaka Shiritsu Daigaku
Igaku Zasshi, 21: 187-209.
DEKANT, W. & HENSCHLER, D. (1982) Dechlorination reactions in the
course of the metabolism of trichloroethylene. Naunyn-Schmiedeb
Arch. Pharmacol., 319: R16.
DEKANT, W. & HENSCHLER, D. (1983) New pathways of
trichloroethylene metabolism. Dev. Toxicol. environ. Sci., 11:
399-402.
DEKANT, W., METZLER, M., & HENSCHLER, D. (1984) Novel metabolites
of trichloroethylene through dechlorination reactions in rats,
mice, and humans. Biochem. Pharmacol., 33(13): 2021-2027.
DE LEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, C.K., REMELE,
P.C., STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980) Rapid
gas chromatographic method for the determination of volatile and
semivolatile organochlorine compounds in soil and chemical waste
disposal site samples. J. chromatogr. Sci., 18: 85-88.
DE MORE, W.B., HAMSON, R.F., MOLINA, M.J., KURYLO, M.J., WATSON,
R.T., HOWARD, C.J., GOLDEN, D.M., & RAVISHANKARA, A.R. (1983)
Chemical kinetics and photochemical data for use in stratospheric
modeling evaluation number 6, Washington DC, National Aeronautics
and Space Administration (NASA JPL Publication No. 83-62,
15 September, 1983).
DERWENT, R.G. & EGGLETON, A.E.J. (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos. Environ., 12: 1261-1268.
DESOILLE, H., PINCHON, R.A., JANS, M., & BOUGUIGNON, A. (1962)
Intoxication expérimentale aiguë par le trichloroéthylène. Effets
aggravants de l'intoxication éthylique chronique associée. Etude
expérimentale électroencéphalographique chez le lapin. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 23: 653-664.
DIETZ, E.A., Jr & SINGLEY, K.F. (1979) Determination of
chlorinated hydrocarbons in water by headspace gas chromatography.
Anal. Chem., 51: 1809-1814.
DILLING, W.L. (1977) Interphase transfer processes. II.
Evaporation rates of chloromethanes, ethanes, ethylenes, propanes,
and propylenes from dilute aqueous solutions. Comparisons with
theoretical predictions. Environ. Sci. Technol., 11: 405.
DI PAOLO, J.A. & DONIGER, J. (1982) Neoplastic transformation of
Syrian hamster cells by putative epoxide metabolites of
commercially-utilized chloroalkenes. J. Natl Cancer Inst., 69(2):
531-534.
DI RENZO, A.B., GANDOLFI, A.J., & SPIES, I.G. (1982) Microsomal
bioactivation and covalent binding of aliphatic halides to DNA.
Toxicol. Lett., 11: 243-252.
DMS (1967) UV atlas of organic compounds, London, Dortmund,
Butterworths-Verlag Chemie, Vol. 3 (Spectrum No. A1/6).
DOBINSON, B. & GREEN, G.E. (1972) Formation of dichloroacetylene
from trichloroethylene in the presence of epoxides. Chem. Ind., 5:
214.
DORFMUELLER, M.A., HENNE, S.P., YORK, R.G., BORNSCHEIN, R.L., &
MANSON, J.M. (1979) Evaluation of teratogenicity and behavioural
toxicity with inhalation exposure of maternal rats to
trichloroethylene. Toxicology, 14: 153-166.
DOWTY, B.J., CARLISLE, D.R., & LASETER, J.L. (1975) New Orleans
drinking water sources tested by gas chromatography-mass
spectrometry. Environ. Sci. Technol., 9: 762-765.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Solubility of organic
solvents. I. Gas chromatographic determination of olive oil/gas
partition coefficient. Helv. Chim. Acta, 60: 454.
DROZ, P.O. & FERNANDEZ, J.G. (1978) Trichloroethylene exposure.
Biological monitoring by breath and urine analyses. Br. J. ind.
Med., 35: 35.
DUPRAT, P. & GRADISKI, D. (1980) Cytogenic effect of
trichloroethylene in the mouse as evaluated in the micronucleus
test. IRCS med. Sci. soc. occup. Med., 8: 182.
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir irritant
des principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur. J. Toxicol., 9(3): 171-177.
EAJ (1983) Environmental monitoring of chemicals: environmental
survey report of F.Y. 1980 and 1981, Japan, Office of Health
Studies, Department of Environmental Health, Environmental Agency
Japan, p. 144.
EHRNER-SAMUEL, H., BALMER, K., & THORSELL, W. (1973)
Determination of trichloroacetic acid in urine by a gas
chromatographic method. Am. Ind. Hyg. Assoc. J., 34: 93-96.
EKLUND, G., JOSEFFSON, B., & ROOS, C. (1978) Determination of
volatile halogenated hydrocarbons in tapwater. J. High Res. Chrom.
& Chrom. Comms., July, pp. 34-40.
ELCOMBE, C.R. (in press) Species differences in carcinogenicity
and peroxisome proliferation due to trichloroethylene: a
biochemical human hazard assessment. Arch. Toxicol.
ELCOMBE, C.R., PRATT, I.S., & GREEN, T. (1982) The rate of
trichloroacetic acid (TCA) formation determines two species
differences in hepatic peroxisome proliferation due to
trichloroethylene (TRI). Toxicologist, 2: 173.
ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979) Effects of
methylene chloride, trichloroethane, trichloroethylene,
tetrachloroethylene, and toluene on the development of chick
embryos. Toxicology, 12: 111-119.
ENTZ, R.C. & HOLLIFIELD, H.C. (1982) Headspace gas
chromatographic analysis of foods for volatile hydrocarbons.
J. agric. food Chem., 30: 84-88.
ERTLE, T., HENSCHLER, D., JULLER, G., & SPASSOWSKI, M. (1972)
Metabolism of trichloroethylene in man. Arch. Toxikol., 29: 171-188.
ETTEMA, J.J., KLEEREKOPER, L., & DUBA, W.C. (1975) Mental
stresses during short-term inhalation of trichloroethylene.
Staub-Reinhalt. Luft, 35: 409-410.
EUROCOP-COST (1976) A comprehensive list of polluting substances
which have been identified in various fresh waters, effluent
discharges, aquatic animals and plants, and bottom sediments, 2nd
ed., Luxembourg, Commission of the European Community, p. 57
(EUCO/MDU/73/76, XII/476/76).
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., & HOPKE, P.K.
(1977) Monitoring to detect previously unrecognized pollutants in
surface waters - appendix: organic analysis data, Springfield,
Virginia, US NTIS, pp. 304 (PB Report PB-273350) (Govt Rep.
Announce. Index, 1978: 199).
FABRE, R. & TRUHAUT, R. (1952) Toxicology of trichloroethylene.
II. Results of experimental animal studies. Br. J. ind. Med., 9:
39-43.
FAHRIG, R. (1977) The mammalian spot test (Fellfleckenstest) with
mice. Arch. Toxicol., 38: 87-98.
FAWNS, H.T. (1968) Trichloroacetic acid in the urine of workers
using trichloroethylene. Proc. Assoc. Clin. Biochem., 5: 110-114.
FERGUSON, R.K. & VERNON, R.J. (1970) Trichloroethylene in
combination with CNS drugs: effects on visual-motor tests. Arch.
environ. Health, 20: 462-467.
FERNANDEZ, J.G., HUMBERT, B.E., DROZ, P.O., & CAPEROS, J.R. (1975)
Exposition au trichloroéthylene. Bilan de l'absorption, de
l'excrétion, et du métabolisme sur des sujets humains. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 36(7-8): 397-407.
FERNANDEZ, J.G., DROZ, P.O., HUMBERT, B.E., & CAPEROS, J.R. (1977)
Trichloroethylene exposure: simulation of uptake, excretion, and
metabolism using a mathematical model. Br. J. ind. Med., 34: 43-55.
FILSER, J.G. & BOLT, H.M. (1979) Pharmacokinetics of halogenated
ethylenes in rats. Arch. Toxicol., 42: 123-136.
FINK, R. (1968) Toxicity of anaesthetics, Baltimore, Maryland,
Williams & Williams, pp. 270.
FISHBEIN, L. (1973) Chromatography of environmental hazards,
New York, Elsevier Scientific Publishing Company, Vol. 2,
pp. 471-490.
FOOD CHEMICAL NEWS (1978) Preliminary report on TCE study shows
no carcinogenicity in rats. Food chem. News, 20: 41-42.
FOSSA, A.A., WHITE, J.F., & CARLSON, G.P. (1982) Anti-arrhythmic
effects of disulfiram on epinephrine-induced cardiac arrthythmias
in rabbits exposed to trichloroethylene. Toxicol. appl. Pharmacol.,
66: 109-117.
FRIBERG, G.L., KYLIN, B., & NYSTROM, A. (1953) Toxicities of
trichloroethylene and tetrachloroethylene and Fujiwara's pyridine
reaction. Acta pharmacol. toxicol., 9: 303-312.
FUKUDA, K., TAKEMOTO, K., & TSURATA, H. (1983) Inhalation
carcinogenicity of trichloroethylene in mice and rats. Ind. Health,
21: 243-245.
GAMBERALE, F., ANNWALL, G., & OLSON, B.A. (1976) Exposure to
trichloroethylene. III. Psychological functions. Scand. J. Work
environ. Health, 4: 220-224.
GAULTIER, M. (1974) [Clinical toxicology,] Rome, Italy, Società
Editrice Demi (in Italian).
GEHRING, P.J. (1968) Hepatoxic potency of various chlorinated
hydrocarbon vapours relative to their narcotic and lethal potencies
in mice. Toxicol. appl. Pharmacol., 13: 287-298.
GOLDBERG, M.E., JOHNSON, H.E., POZZANI, U.C., & SMITH, H.E., Jr
(1964) Effect of repeated inhalation of vapours of industrial
solvents on animal behaviour. I. Evaluation of nine solvent vapours
on pole-climb performance in rat. Am. Ind. Hyg. Assoc. J., 25: 369-375.
GOLDBLATT, M.W. (1956) Industrial medicine and hygiene, London,
Merewether, Vol. 3.
GOVERNA, M. (1981) [Occupational diseases due to chlorine
derivatives of the aliphatic hydrocarbons.] In: Sartorelli, E., ed.
[Textbook of occupational medicine,] Padova, Italy, Vol. 1,
pp. 483-502 (in Italian).
GRADISKI, D., MAGADUR, J.-L., BAILLOT, M., DANIERE, M.C., & SCHUH,
M.B. (1974) Toxicité comparée des principaux solvants chlorés
aliphatiques. J. eur. Toxicol., 7: 247-254.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.-L., & FRANCIN,
J.M. (1978) Toxicité aiguë comparée par inhalation des principaux
solvants aliphatiques chlorés. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 39: 249-257.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
New York, Academic Press.
GRANDJEAN, E. (1960) Trichloroethylene effects on animal
behaviour. Arch. environ. Health, 1: 106-108.
GRANDJEAN, E. (1963) The effects of short exposures to
trichloroethylene on swimming performances and motor activity of
rats. Am. Ind. Hyg. Assoc. J., 24: 376-379.
GRANDJEAN, E., MUNCHINGER, R., TURRIAN, V., HAAS, P.A., KNOEPFEL,
H.K., & ROSENMUND, H. (1955) Investigations into the effects of
exposure to trichloroethylene in mechanical engineering. Br. J.
ind. Med., 12: 131-142.
GRANT, M.W. (1961) Trichoroethylene in the eye. J. Am. Med.
Assoc., 178: 359.
GRANT, M.W. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, pp. 1034-1045.
GRAOVAC-LEPOSAVIC, L., MILOSAVLJEVIC, Z., & ILIC, V. (1964)
[Liver function in workers exposed to trichloroethylene.] Arh. Hig.
Rad. Toksikol., 15: 93-97 (in Yugoslavian).
GREEN, T. & PROUT, M.S. (in press) Species differences in
response to trichloroethylene. II. Biotransformation in rats and
mice. Arch. Toxicol.
GREEN, T., PROUT, M.S., & PROVAN, W.M. (in press) Species
differences in response to trichloroethylene. I. Pharmacokinetics
in rats and mice. Arch. Toxicol.
GREIM, H., BONSE, G., RADWAN, Z., REICHERT, D., & HENSCHLER, D.
(1975) Mutagenicity in vitro and potential carcinogenicity of
chlorinated ethylenes as a function of metabolic oxirane formation.
Biochem. Pharmacol., 24: 2013-2017.
GU, Z.W., SELE, B., JALBERT, P., VINCENT, M., VINCENT, F., MARKA,
C., CHMARA, D., & FAURE, J. (1981) Induction of sister chromatid
exchanges by trichloroethylene and its metabolites. Toxicol. Eur.
Res., 3: 63-67.
GUN, R.T., GRYGORCENICZ, D., & NETTELBECK, T.J. (1978) Choice
reaction time in workers using trichloroethylene. Med. J. Aust., 1:
535-536.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 73: 169-176.
HATHWAY, D.E. (1980) Consideration of the evidence for mechanisms
of 1,1,2-trichloroethylene metabolism including new identification
of its dichloroacetic acid and trichloroacetic acid metabolites in
mice. Cancer Lett., 8: 263-269.
HAYDEN, J.W., COMSTOCK, E.G., & COMSTOCK, B.S. (1976) The
chemical toxicology of solvent abuse. Clin. Toxicol., 9: 169-184.
HEIL, E., OESER, H., HATZ, R., & KELKER, H. (1979) [Gas
chromatographic determination of traces of C- and C-fluoro- and
chlorohydrocarbons in air.] Fresenius Z. Anal. Chem., 297: 357-364
(in German).
HENSCHLER, D. (1977a) Metabolism and mutagenicity of halogenated
olefuis: a comparison of structure and activity. Environ. Health
Perspect., 21: 61-64.
HENSCHLER, D. (1977b) Metabolism of chlorinated alkenes and
alkanes as related to toxicity. J. environ. Pathol. Toxicol., 1:
125-133.
HENSCHLER, D. & HOOS, R. (1982) Metabolic activation and
deactivation mechanisms of di-, tri-, and tetrachloroethylenes.
In: Snyder, Parke, Kocsis, Jollow, Gibson, & Witmer, ed. Biological
reactive intermediates. II. Part A., New York, Plenum Publishing
Corporation, pp. 659-666.
HENSCHLER, D., BONSE, G., & GREIM, H. (1970a) [Cranial nerve
neuritis due to poisoning with chlorinated acetylenes when handling
vinylidene chloride copolymers.] Arch. Toxikol., 26: 62-75 (in
German).
HENSCHLER, D., BROSER, F., & HOPF, H.C. (1970b) Environmental
Pollution and Carcinogenic Risks, Lyons, International Agency for
Research on Cancer, pp. 171-175 (IARC Scientific Publications
No. 13).
HENSCHLER, D., EDER, E., NEUDECKER, T., & METZLER, M. (1977)
Carcinogenicity of trichloroethylene: fact or artifact? Arch.
Toxicol., 37: 233-236.
HENSCHLER, D., HOOS, W.R., FETZ, H., DALLMEIER, E., & METZLER, M.
(1979) Reactions of trichloroethylene epoxide in aqueous systems.
Biochem. Pharmacol., 28: 543-548.
HENSCHLER, D., ROMEN, W., ELSASSER, H.M., REICHERT, D., EDER, E., &
RADWAN, Z. (1980) Carcinogenicity study of trichloroethylene by
long-term inhalation in three animal species. Arch. Toxicol., 43:
237-248.
HENSCHLER, D., BONSE, G., & DEKANT, W. (1983) Mechanisms and
reactions of electroplulic intermediates of halogenated ofefins.
In: Proceedings of the 13th International Cancer Congress. Part B.
Biology of Cancer, New York, Alan R. Liss, Inc., Vol. 1,
pp. 175-183.
HENSCHLER, D., ELSASSER, H., ROMEN, W., & EDER, E. (1984)
Carcinogenicity study of trichloroethylene, with and without
epoxide stabilizers, in mice. J. Cancer Res. clin. Oncol., 107:
149-156.
HERBOLSHEIMER, R., FUNK, L., & DRASCHE, H. (1972) Use of
activated carbon as an adsorbent in the determination of
trichloroethylene in the air. Staub-Reinhalt. Luft, 32: 407-409.
HERNBERG, S., KORKATA, M.-L., ASIKAINEN, V., & RIALA, R. (1984)
Primary liver cancer and exposure to solvents. Int. Arch. occup.
environ. Health, 54: 147-153.
HIRAYAMA, T. & IKEDA, M. (1979) Applicability of carbon felt to
the dosimetry of solvent vapour mixture. Am. Ind. Hyg. Assoc. J.,
40: 1091-1096.
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., & SAKAI,
T. (1983) [Experimental studies of trichloroethylene toxicity.
II. Changes in trichloroethylene in blood serum and in urine during
and after exposure to trichloroethylene.] Nippon Eiseigaku Zasshi,
38(4): 772-779 (in Japanese with English summary).
HOWARD, C.J. (1976) Rate constants for the gas-phase reactions of
OH radicals with ethylene and halogenated ethylene compounds.
J. Chem. Phys., 65: 4771.
HUFF, J.E. (1971) New evidence on the old problems of
trichloroethylene. Ind. Med. Surg., 40: 25-33.
IARC (1979) Trichloroethylene, Lyons, International Agency for
Research on Cancer, pp. 545-572 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 247-249 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Supplement 4).
IKEDA, M. (1974) Reciprocal metabolic inhibition of toluene and
trichloroethylene in vivo and in vitro. Int. Arch. Arbeitsmed., 33:
125-130.
IKEDA, M. & IMAMURA, T. (1973) Biological half-life of
trichloroethylene and tetrachloroethylene in human subjects. Int.
Arch. Arbeitsmed., 31: 209-224.
IKEDA, M., OHTSUJI, H., KAWAI, H., & KUNIYOSHI, M. (1971)
Excretion kinetics of urinary metabolites in patient addicted to
trichloroethylene. Br. J. ind. Med., 28: 203-206.
IKEDA, M., OHTSUJI, H., IMAMAURA, T., & KOMOIKE, Y. (1972)
Urinary excretion of total trichloro-compounds, trichloroethanol,
and trichloroacetic acid as a measure of exposure to
trichloroethylene and tetrachloroethylene. Br. J. ind. Med., 29:
328-333.
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office, pp. 206-207 (Occupational Safety and Health Series No. 37).
IMAMURA, T. & IKEDA, M. (1973) A time-saving procedure for the
determination of total trichloro-compounds in human urine samples.
Int. Arch. Arbeitsmed., 31: 333-338.
IOFFE, B.V., ISIDOROV, V.A., & ZENKEVICH, I.G. (1977) Gas
chromatographic-mass spectrometric determination of volatile
organic compounds in an urban atmosphere. J. Chromatogr., 142:
787-795.
IRPTC (1984) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JAMES, W.R.L. (1963) Fatal addiction to trichloroethylene. Br. J.
ind. Med., 20: 47-49.
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G. (1982)
Uptake via the blood and elimination of 10 organic solvents
following epicutaneous exposure of anaesthetized guinea-pigs.
Toxicol. appl. Pharmacol., 63: 181-187.
JAPANESE YEARBOOK OF CHEMICAL INDUSTRIES STATISTICS (1983) Tokyo,
Ministry of International Trade and Industry.
JESSON, J.P. (1980) Release of industrial halocarbons and
tropospheric budget. In: Proceedings of the NATO Advanced Study
Institute on Atmospheric Ozone: Its Variation and Human Influences,
Washington DC, US Department of Transportation, Federal Aviation
Administration.
JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (1983)
Evaluation of certain food additives: 27th report, Geneva, World
Health Organization (Technical Report Series No. 696).
JOUGLARD, J. & VINCENT, V. (1971) Pulmonary signs of ingestions
of solvents. Marseille Med., 108: 696-697.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial waste water: a case study of their
environmental impact. Environ. Sci. Technol., 12: 88-96.
KALASHINIKOVA, V.P., VISSARIONOVA, V.Ya., BONDAREV, G.I., SKIRKO,
B.K., & KARANTIROVA, O.V. (1974) [Influence of different diets on
the course of chronic experimental trichloroethylene poisoning.]
Vopr. Pitan., No.6: 43-47 (in Russian).
KALASHINIKOVA, V.P., SKIRKO, B.K., VISSARIONOVA, V.Ya., BONDAREV,
G.I., KLEIMENOVA, N.V., POPOVA, N.S., & KARANTIROVA, O.V. (1976)
[Evaluation of the toxic effects of trichloroethylene according
to morphological and some biochemical indexes.] Gig. i Sanit., No. 2:
107-108 (in Russian).
KANJE, M., KJELLSTRAND, P., FOX, K., & WALLDORF, A. (1981)
Neurotransmitter metabolizing enzymes and plasma
butyrylcholinesterase in mice exposed to trichloroethylene. Acta
pharmacol. toxicol., 49: 205-209.
KARICKHOFF, S.W., BROWN, D.S., & SCOTT, T.A. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13: 241-248.
KATZ, R.M. & JOWETT, D. (1981) Female laundry and dry cleaning
workers in Wisconsin: a mortality analysis. Am. J. public Health,
71: 305-307.
KEINFELD, M. & TABERSHAW, I.R. (1954) Trichloroethylene toxicity:
report of 5 fatal cases. AMA Arch. ind. Hyg. occup. Med., 10: 134-141.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals.
Ecotoxicol. environ. Saf., 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (in press) Relationship between
water solubility, soil sorption, octanol-water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish,
P.R., & Hendricks, A.C., ed. Aquatic toxicology ASTM STP, 707,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
KIMMERLE, G. & EBEN, A. (1973) Metabolism, excretion, and
toxicology of trichloroethylene after inhalation. II. Experimental
human exposure. Arch. Toxicol., 30: 127-138.
KIRK, R.E. & OTHMER, D.F., ed. (1963) Anesthetics. In:
Encyclopedia of chemical technology, 2nd ed., New York, John Wiley
and Sons, Vol. 2, pp. 393-410.
KIRK, R.E. & OTHMER, D.F., ed. (1964) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 2nd
ed., New York, John Wiley and Sons, Vol. 5, pp. 183-195.
KIRK, R.E. & OTHMER, D.F., ed. (1979) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 3rd
ed., New York, John Wiley and Sons, Vol. 5, pp. 745-753.
KISELEVA, A.F. & KOROLENKO, A.M. (1971) Histoenzymic changes in
liver and kidneys during the experimental effect of anesthetic
doses of fluorothan and trylen. Eksp. Khir. Anesteziol., 16: 81-84.
KITAGAWA, T. (1961) The rapid measurement of toxic gases and
vapour. In: Proceedings of the 13th International Congress on
Occupational Health, New York, pp. 506-512.
KJELLSTRAND, P., LANKE, J., BJERKEMO, M., ZETTERQUIST, L., &
MANSSON, L. (1980) Irreversible effects of trichloroethylene
exposure on the central nervous system. Scand. J. Work environ.
Health, 6: 40-47.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M.,
MORTENSEN, I., LANKE, J., & HOLMQUIST, B. (1981a)
Trichloroethylene: effects on body and organ weights in mice,
rats, and gerbils. Toxicology, 21: 105-115.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M., MORTENSEN,
I., LANKE, J., & HOLMQUIST, B. (1981b) Effects of long-term
exposure to trichloroethylene on the behaviour of Mongolian gerbils
(Meriones unguiculatus). J. Toxicol. environ. Health, 8: 787-793.
KJELLSTRAND, P., HOLMQUIST, B., MANDAHL, N., & BJERKEMO, M.
(1983a) Effects of continuous trichloroethylene inhalation on
different strains of mice. Acta pharmacol. toxicol., 53: 369-374.
KJELLSTRAND, P., HOLMQUIST, B., ALM, P., KANJE, M., ROMARE, S.,
JONSSON, I., MANSSON, L., & BJERKEMO, M. (1983b)
Trichloroethylene: further studies of the effects on body and organ
weights and plasma butyrycholinesterase activity in mice. Acta
pharmacol. toxicol., 53: 375-384.
KLAASSEN, C.D. & PLAA, G.L. (1966) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in mice.
Toxicol. appl. Pharmacol., 9: 139-151.
KLAASSEN, C.D. & PLAA, G.L. (1967) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in dogs.
Toxicol. appl. Pharmacol., 10: 119-131.
KLINE, S.A., MCCOY, E.C., ROSENKRANZ, H.S., & VAN DUUREN, B.L.
(1982) Mutagenicity of chloroalkene epoxides in bacterial systems.
Mutat. Res., 101: 115-125.
KLOWKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981)
Laboratory screening of distribution, conversion, and
mineralisation of chemicals in the soil-plant-system and comparison
to outdoor experimental data. Chemosphere, 10: 1089-1090.
KONIETZKO, H., HABERLANDT, W., HEILBRONNER, H., REILL, G., &
WEICHARDT, H. (1978) [Chromosome studies on trichloroethylene
workers.] Arch. Toxicol., 40: 201-206 (in German).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin pathology
following epicutaneous exposure to seven organic solvents. Int. J.
Tiss. React., 111(1): 21-30.
KYLIN, B., REICHARD, H., SUMEGI, I., & YLLNER, S. (1962)
Heptatoxic effect of tri- and tetrachloroethylene on mice. Nature
(Lond.), 193: 395.
KYLIN, B., AXELL, K., EHRNER-SAMUEL, H., & LINDBORG, A. (1967)
Effect of inhaled trichloroethylene on the CNS. Arch. environ.
Health, 15: 48-52.
KYLSZEIJKO, C., LAKOMY, T., & PAPIEROWSKI, A. (1963) [The
influence of trichloroethylene during labour process in women in
comparison with the results in vitro.] Pol. Tyg. Lek., 18:
1333-1338 (in Polish).
KYRKLUND, T., ALLING, C., HAGLID, K., & KJELLSTRAND, P. (1983)
Chronic exposure to trichloroethylene: lipid and acyl group
composition in gerbil cerebral cortex and hippocampus.
Neurotoxicology, 4(4): 35-42.
LACHNIT, V. (1971) Halogenated hydrocarbons and the liver. Wein.
Klin. Wochenschr., 83: 734-737.
LA DU, B.N., MANDEL, H.G., & WAY, E.L., ed. (1971) Fundamentals
of drug metabolism and drug disposition, Baltimore, Maryland,
Williams and Wilkins.
LAHAM, S. (1970) Studies on placental transfer:
trichloroethylene. Ind. Med. Surg., 39: 46-49.
LAZAREV, N.V. (1929) [The narcotic efficacy of the vapours of the
chlorine derivatives of methane, ethane and ethylene.] Naunyn-
Schmiedeb. Arch. exp. Pathol. Pharmacol., 141: 19-24 (in German).
LAZAREV, N.V. & GADASKINA, I.D. (1977) [Harmful substances
in industry,] Leningrad, Medecine, Vol. 3 (in Russian).
LEHMANN, K.B. & SCHMIDT-KEHL, L. (1936) [Thirteen most important
chlorohydrocarbons of the paraffin series from the standpoint of
industrial hygiene.] Arch. Hyg. Bakt., 116: 131-268 (in German).
LEIBMAN, K.C. (1965) Metabolism of trichloroethylene in liver
microsomes. Mol. Pharmacol., 1: 239-246.
LEONG, B.K.J., SCHWETZ, B.A., & GEHRING, P.J. (1975) Embryo- and
fetotoxicity of inhaled trichloroethylene, perchloroethylene,
methylchloroform, and methylene chloride in mice and rats. Toxicol.
appl. Pharmacol., 33: 136.
LERZA, P., LOMBARDI, F., & VIOTTI, G. (1963) [Health and hygiene
considerations regarding work in dry-cleaning establishments using
trichloroethylene.] Lav. Um., 15: 459-478 (in Italian).
LEWIS, G.D., REYNOLDS, R.C., & JOHNSON, A.R. (1984) Some effects
of trichloroethylene on mouse lungs and livers. Gen. Pharmacol.,
15(2): 139-144.
LILIS, R., STANESCU, D., MUICA, N., & ROVENTA, A. (1969) Chronic
effects of trichloroethylene exposure. Med. Lav., 60: 595-601.
LIN, R.S. & KESSLER, I.I. (1981) A multifactoral model for
pancreatic cancer in man. J. Am. Med. Assoc., 245: 147-152.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Proceedings of the
Symposium on Short-Term Test Systmes and the Detection of
Carcinogenesis, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 333-356.
LOPRIENO, N., SBRANA, I., & LASCIALFARI, D. (in press) [Study on
the in vivo potential mutagenic effect of trichloroethylene
evaluated by cytogenetic analysis in mice treated by inhalation for
52 days.] (in Italian).
LOVELOCK, J.E. (1974) Atmospheric halocarbons and stratospheric
ozone. Nature (Lond.), 252: 292-294.
MACKAY, D. (1982) Correlations of bioconcentration factors.
Environ. Sci. Technol., 10: 274-278.
MCCONNELL, G., FERGUSON, D.M., & PEARSON, C.R. (1975) Les
hydrocarbures chlorés et l'environnement. Endeavour, 34: 13-18.
MCCORD, C.P. (1932) Toxicity of trichloroethylene. J. Am.
Med. Assoc., 99: 409.
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
MCNEILL, W.C. (1979) Chlorocarbons and chlorohydrocarbons.
Trichloroethylene. In: Kirk-Othmer's encyclopedia of chemical
technology, 3rd ed., New York, Wiley Interscience, Vol. 5,
pp. 745-753.
MAKIDE, Y., TOMINAGA, K., & ROWLAND, F.S. (1979) Gas
chromatographic analysis of halogenated hydrocarbons in air over
Japan. Chem. Lett., 1979: 355-358.
MALEK, B., KRCMAROVA, B., & RODOVA, O. (1979) [Epidemiological
study of hepatic tumour incidence in subjects working with
trichloroethylene: II. Negative result of retrospective studies in
dry cleaners.] Pracov. Lek., 31(4): 124-126 (in Czech).
MALIZIA, E., PELAIA, P., & PIROVINE, C. (1984) Experience in the
emergency treatment of acute intoxication by trichloroethylene.
Riv. Toss. Speriment Clin., 14: 437.
MALOOF, C.C. (1949) Burns of the skin produced by
trichloroethylene vapours at room temperature. J. ind. Hyg.
Toxicol., 31: 295-296.
MALTONI, I.C. & MAIOLI, P. (1977) [Long-term bioassay of
carcinogenicity of trichloroethylene. Preliminary results.]
Osp. Vita, 4: 108-110 (in Italian).
MALTONI, C., LEFEMINE, G., & COTTI, G. (in press) Experimental
research on trichloroethylene carcinogenesis. In: Maltoni, C. &
Mehlman, M.A., ed. Archives of research on industrial
carcinogenesis, Vol. 5.
MANTEL, M. & NOTHMANN, R. (1977) Rapid determination of
trichloroethanol and trichloroacetic acid in urine. Analyst, 102:
672-677.
MATTURRO, F. (1963) [Effects of some volatile narcotics on
isolated guinea-pig heart.] G. Ital. Chir., 19: 95-99 (in Italian).
MAZZA, V. & BRANCACCIO, A. (1967) [Behaviour of the formed
elements of the blood and the marrow in experimental intoxications
with Tri.] Folia med., 50: 318-324 (in Italian).
MELINO, C., MESSINEO, A., & PACELLI, E. (1979) Risk from
trichloroethylene in a factory producing electrical condensers.
Riv. Med. Lab. Ig. Ind., 3: 67-81.
MEYER, H.-J. (1966) [Trichloroethylene poisoning by the oral
route.] Arch. Toxikol., 21: 225-234 (in German).
MEYER, H.-J. (1973) Bronchopulmonary alterations induced by
trichloroethylene and other halogenated hydrocarbons. Bronches, 23:
113-124.
MILLER, D.A. & GRIMSRUD, E.P. (1979) Correlation of electron
capture response enhancements caused by oxygen with chemical
structure for chlorinated hydrocarbons. Anal. Chem., 51: 851-859.
MILLER, R.E. & GUENGERICH, F.P. (1982) Oxidation of
trichloroethylene by liver microsomal cytochrome P-450: evidence
for chlorine migration in a transition state not involving
trichloroethylene oxide. Biochemistry, 21: 1090-1097.
MOLINA, M.J. & ROWLAND, F.S. (1974) Stratospheric sink for
chlorofluoromethanes: chlorine atom-catalysed destruction of ozone.
Nature (Lond.), 294: 810-812.
MONSTER, A.C. (1979) Differences in uptake, elimination, and
metabolism in exposure to trichloroethylene, 1,1,1-trichloroethane,
and tetrachloroethylene. Int. Arch. occup. environ. Health, 42:
311-317.
MONSTER, A.C. & BOERSMA, G. (1975) Simultaneous determination of
trichloroethylene and metabolites in blood and exhaled air by gas
chromatography. Int. Arch. occup. environ. Health, 35: 155-163.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1976) Pharmaco-
kinetics of trichloroethylene in volunteers: influence of workload
and exposure concentration. Int. Arch. occup. environ. Health, 38:
87-102.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1979) Kinetics of
trichloroethylene in repeated exposure of volunteers. Int. Arch.
occup. environ. Health, 42: 283-292.
MOSLEN, M.T., REYNOLDS, E.S., & SZABO, S. (1977) Enhancement of
the metabolism and hepatoxicity of trichloroethylene and
perchloroethylene. Biochem. Pharmacol., 26: 369-375.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1972)
Trichloroethylene exposure and trichloroethylene metabolites in
urine and blood. Arch. Toxicol., 29: 335-340.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1974) Metabolism of
trichloroethylene in man. II. Pharmacokinetics of metabolites.
Arch. Toxicol., 32: 283-295.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1975) Metabolism of
trichloroethylene in man. III. Interaction of trichloroethylene and
ethanol. Arch. Toxicol., 33: 173-189.
MURRAY, A.J. & RILEY, J.P. (1973) Occurrence of some chlorinated
aliphatic hydrocarbons in the environment. Nature (Lond.), 242: 37-38.
MUSCLOW, C.E. & AWEN, C.F. (1971) Glue sniffing: report of a
fatal case. Can. Med. Assoc. J., 104: 315-319.
NCI (1976) Carcinogenesis bioassay of trichloroethylene. CAS No.
79-01-6, Washington DC, National Cancer Institute (US DHEW
Publication No. (NIH) 76-802).
NEELY, W.B. & MACKAY, D. (1982) In: Dickson, K.L., Maki, A.W., &
Cairns, J., ed. Modelling the fate of chemicals in the aquatic
environment, Ann Arbor, Michigan, Ann Arbor Science Publications.
NICHOLSON, A.A., MERESZ, O., & LEMYK, B. (1977) Determination of
free and total potential haloforms in drinking-water. Anal. Chem.,
49: 814-819.
NIOSH (1977a) Manual of analytical methods, 2nd ed., Cincinnati,
Ohio, National Institute of Occupational Safety and Health, Vol. 3,
pp. S336-1 to S336-9 (US DHEW Publication No. (NIOSH) 77-157-C).
NIOSH (1977b) National occupational hazard survey. III. Survey
analyses and supplemental tables, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, pp. 2864-2866.
NIOSH (1978) Special occupational hazard review of
trichloroethylene, Washington DC, National Institute of
Occupational Safety and Health (US DHEW Publication No. (NIOSH)
78-130).
NOMIYAMA, H., NOMIYAMA, K., & UCHIKI, H. (1978) Gas-liquid
chromatographic determination of trichloroethylene metabolites in
urine. Am. Ind. Hyg. Assoc. J., 39: 506-510.
NOMURA, S. (1962) Health hazards in workers exposed to
trichloroethylene vapour. I. Trichloroethylene poisoning in an
electroplating plant. Kumamoto med. J., 15: 29-37.
NORRIS, W. & STUART, P. (1957) Cardiac arrest during
trichloroethylene anaesthesia. Br. med. J., 1: 860-863.
NOVOTNA, E., DAVID, A., & MALEK, B. (1979) [An epidemiological
study on hepatic tumour incidence in subjects working with
trichloroethylene. I. Negative result of retrospective
investigations in subjects with primary liver carcinoma.] Pracov.
Lek., 31(4): 121-123 (in Czech).
ODUM, E.P. (1971) Fundamentals of ecology, 3rd ed.,
Philadelphia, Pennsylvania, W.B. Saunders Co.
OGATA, M. & SAEKI, T. (1974) Measurement of chloral hydrate,
trichloroethanol, trichloroacetic acid, and monochloroacetic acid
in the serum and the urine by gas chromatography. Int. Arch.
Arbeitsmed., 33: 49-58.
OGATA, M., TAKATSUKA, Y., TOMUKUNI, K., & MUROI, K. (1970) Simple
method for the quantitative analysis of urinary trichloroethanol
and trichloroacetic acid as an index of trichloroethylene exposure.
Br. J. ind. Med., 27: 378-381.
OHTA, T., MORITA, M., & MIZOGUCHI, I. (1976) Local distribution
of chlorinated hydrocarbons in the ambient air in Tokyo. Atmos.
Environ., 10: 557-560.
OLSSON, H. & BRANDT, L. (1980) Occupational exposure to organic
solvents and Hodgkin's disease in men. Scand. J. Work environ.
Health, 6: 302-305.
OSTELERE, G. (1953) Trichloroethylene anaesthesia, Edinburgh,
Scotland, Livingstone.
OTSON, R., WILLIAMS, D.T., & BIGGS, D.C. (1982) Relationships
between raw water quality treatment and the occurrence of organics
in Canadian potable water. Bull. environ. Contam. Toxicol., 28:
396-403.
PADDLE, G.M. (1983) Incidence of liver cancer and
trichloroethylene manufacture: joint study by industry and a cancer
registry. Br. med. J., 286: 876.
PARCHMAN, L.G. & MAGEE, P.N. (1982) Metabolism of
14C-trichloroethylene to 14CO2 and interaction of a metabolite
with liver DNA in rats and mice. J. Toxicol. environ. Health, 9:
797-813.
PARDYS, S. & BROTMAN, M. (1974) Trichloroethylene and alcohol: a
straight flush. J. Am. Med. Assoc., 229:: 521-522.
PARMEGGIANI, L. (1956) [A case of chronic trichloroethylene
poisoning.] Med. Lav., 47: 639-646 (in Italian).
PARSONS, F., WOOD, P.R., & DE MARCO, J. (1984) Transformation of
tetrachloroethene and trichloroethene in microcogus and ground
water. J. Am. Water Works Assoc., 76(2): 56-59.
PEARSON, C.R. & MCCONNELL, G. (1975) Chlorinated C and C
hydrocarbons in the marine environment. Proc. R. Soc. London
Ser. B, 189: 305-332.
PELTS, D.G. (1962) [Effect of trichloroethylene and
tetrachloroethylene on the phagocytic activity of blood
leukocytes.] Gig. i Sanit., 27(7): 96-99 (in Russian).
PENKETT, S.A. (1982) Non-methane organics in the remote
troposphere. In: Goldberg, E.D., ed. Atmospheric chemistry, Berlin,
Springer, pp. 329-355.
PEROCCO, P. & PRODI, G. (1981) DNA damage by haloalkanes in human
lymphocytes cultered in vitro. Cancer Lett., 13: 213-218.
PESSAYRE, D., ALLEMAND, H., WANDSCHEER, J.C., DESCATOIRE, V.,
ARTIGOU, J-Y., & BENHAMOU, J-P. (1979) Inhibition, activation,
destruction, and induction of drug-metabolizing enzymes by
trichloroethylene. Toxicol. appl. Pharmacol., 49: 355-363.
PESSAYRE, D., BARTON, C., DESCATOIRE, V., DEGOTT, C., BABANY, G.,
FUNCK-BRENTANO, C., DELAFORGE, M., & LARREY, D. (1982)
Hepatotoxicity of trichloroethylene-carbon tetrachloride mixtures
in rats. Gastroenterology, 63: 761-772.
PIERCE, R.H., OLNEY, C.E., & FELBECK, G.T. (1974) p-p'-DDT
adsorption to suspended particulate matter in sea water. Geochim.
cosmochim. Acta, 38: 1060-1073.
PLAA, G.L., EVANS, E.A., & HINE, C.H. (1958) Relative
hepatoxicity of seven halogenated hydrocarbons. J. Pharmacol. exp.
Ther., 123: 224-229.
POWELL, J.F. (1945) Trichloroethylene: absorption, elimination,
and metabolism. Br. J. ind. Med., 2: 142-145.
PRENDERGAST, J.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1967) Effects on experimental animals of long-term inhalation of
trichloroethylene, carbon tetrachloride, 1,1,1-trichloroethane,
dichlorodifluoromethane, and 1,1-dichloroethylene. Toxicol. appl.
Pharmacol., 10: 270-289.
PRICE, P.J., HASSETT, C.M., & MANSFIELD, J.I. (1978) Transforming
activities of trichloroethylene and proposed industrial
alternatives. In Vitro, 14: 290-293.
PRIEST, R.J. & HORN, R.C., Jr (1965) Trichloroethylene
intoxication: a case of acute hepatic necrosis possibly due to this
agent. Arch. environ. Health, 11: 361-365.
RASMUSSEN, R.A. & KHALIL, M.A.K. (1983) Natural and anthropogenic
trace gases in the lower troposphere of the Arctic. Chemosphere, 12:
371-375.
REICHERT, D. & HENSCHLER, D. (1978) Nephrotoxic and hepatotoxic
effects of dichloroacetylene. Food Cosmet. Toxicol., 16: 227-235.
REICHERT, D., METZLER, M., & HENSCHLER, D. (1980a) Decomposition
of the neuro- and nephrotoxic compound dichloroacetylene in the
presence of oxygen: separation and identification of novel
products. J. environ. Pathol. Toxicol., 4: 525-532.
REICHERT, D., SPENGLER, U., ROMEN, W., & HENSCHLER, D. (1980b)
Carcinogenic potential of dichloroacetylene. Dev. Toxicol. environ.
Sci., 8: 269-272.
RIGAUD, M., CHALABREYSSE, J., PROST, G., & TOLOT, F. (1977) Etude
expérimentale de la toxicité hépatique du trichloroéthylène.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 38: 263-265.
ROCHE, L., GENEVOIS, M., & MARIN, A. (1958) Trichloréthylène et
eczéma de la face. Arch. Mal. prof. Méd. Trav. Sécur. soc., 19:
615-616.
ROESSLER, R. & MORAWIEC-BOROWIAK, D. (1973) [Use of hemodialysis
in acute trichloroethylene poisoning.] Wiad. Lek., 26(13): 1271-1275 (in
Polish).
ROOK, J.J., MEIJERS, A.P., GRAS, A.A., & NOORDSIJ, A. (1975)
[Headspace analysis of volatile trace compounds in the Rhine.] Vom
Wasser, 44: 23-30 (in German).
ROSSI, A.M., MIGLIORE, L., BARALE, R., & LOPRIENO, N. (1983) In
vivo and in vitro mutagenicity studies of a possible carcinogen,
trichloroethylene, and its two stabilizers, epichlorohydrin and
1,2-epoxybutane. Teratog. Carcinog. Mutagen., 3: 75-87.
RUBINO, G.F., SCANSETTI, G., & TROMPEO, G. (1959) [Chronic
trichloroethylene poisoning. II. Absorption of trichloroethylene.]
Med. Lav., 50(12): 733-742 (in Italian).
RUSH, W.E. (1970) Quantitative analysis of gaseous pollutants,
London, Ann Arbor-Humphrey Science Publishers, Inc., pp. 229-232.
RUSSO, A., PACCHIELOTTI, F., & METALLI, P. (1984) Nondysfunction
induced in mouse spermatogenesis by chloral hydrate, a metabolite
of trichloroethylene. Environ. Mutagen., 6(5): 695-703.
SADTLER STANDARD SPECTRA (1961) 25 frequently-used spectra for
the infrared spectroscopist, London, Heyden & Son, Ltd.
SAGAWA, K., NISHITANI, Y., KAWAI, H., KUGE, Y., & IKEDA, M. (1973)
Transverse lesion of spinal cord after accidental exposure to
trichloroethylene. Int. Arch. Arbeitsmed., 31: 257-264.
SANDERS, V.M., TUCKER, A.N., WHITE, K.L., Jr, KAUFFMANN, B.M.,
HALLETT, P., CARCHMAN, R.A., BORZELLECA, J.F., & MUNSON, A.E.
(1982) Humoral and cell-mediated immune status in mice exposed to
trichloroethylene in the drinking water. Toxicol. appl. Pharmacol.,
62: 358-368.
SATO, A. & NAKAJIMA, T. (1978) Differences following skin or
inhalation exposure in the absorption and excretion kinetics of
trichloroethylene and toluene. Br. J. ind. Med., 35: 43-49.
SATO, A. & NAKAJIMA, T. (1979) A structure-activity relationship
of some chlorinated hydrocarbons. Arch. environ. Health, 34: 69.
SAVOLAINEN, H. & SEPPALAINEN, A.M. (1979) Biochemical and
physiological effects of organic solvents on rat axon membranes
isolated by a new technique. Neurotoxicology, 1: 467-477.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINIO, H. (1977)
Trichloroethylene and 1,1,1-trichloroethane: effects on brain and
liver after five days intermittent inhalation. Arch. Toxicol., 38:
229-237.
SAWICKI, E., BELSKY, T., FRIEDEL, R.A., HYDE, D.L., MONKMAN, J.L.,
RASMUSSEN, R.A., RIPPERTON, L.A., & WHITE, L.D. (1975) Organic
solvent vapours in air: analytical method. Health Lab. Sci., 12:
394-402.
SBERTOLI, C. & BRAMBILLA, G. (1962) [Three cases of
trichloroethylene poisoning presenting with intolerance to alcohol
as the only symptoms.] Med. Lav., 53: 353-358 (in Italian).
SBRANA, I., LASCIALFARI, N., & LOPRIENO, N. (1984) [Analysis of
the cytogenetic effects of trichloroethylene.] AHI AGI/ABCD/SIBBM,
21-25 October, p. 93 (in Italian).
SCHUMACHER, H. & GRANDJEAN, E. (1960) Comparative investigations
on the anaesthetic effects and acute toxicity of nine solvents.
Arch. Gewerbepathol. Gewerbehyg., 18: 109-119.
SCHUTTMANN, W. (1970) Liver damage after occupational exposure to
trichloroethylene. Dtsch. Z. Verdau Stoffwechselkr., 30: 43-45.
SCHWETZ, B.A., LEONG, B.K.J., & GEHRING, P.J. (1975) The effect
of maternally-inhaled trichloroethylene, perchloroethylene, methyl
chloroform, and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol., 32: 84-96.
SEBA, D.B. & PROSPERO, J.M. (1971) Pesticides in the lower
atmosphere of the northern equatorial Atlantic Ocean. Atmos.
Environ., 5: 1043-1050.
SELLERS, E.M., CARR, G., BERSTEIN, J.G., SELLERS, S., & KOCK-WESER,
J. (1972) Interaction of chloral hydrate and ethanol in man. II.
Hemodynamics and performance. Clin. Pharmacol. Ther., 13: 50-58.
SETO, T.A. & SCHULTZE, M.O. (1956) Determination of
trichloroethylene, trichloroacetic acid, and trichloroethanol in
urine. Anal. Chem., 28: 1625-1629.
SHAHIN, M.M. & VON BORSTEL, R.C. (1977) Mutagenic and letal
effects of alpha-benzene hexachloride, dibutyl phthalate, and
trichloroethylene in Saccharomyces cerevisiae. Mutat. Res., 48:
173-180.
SHANKS, C.A. (1964) The compatibility of octapressin with
cyclopropane, trichloroethylene, and halothane. N.Z. med. J., 63:
156-159.
SHIPMAN, A.J. & WHIM, B.P. (1980) Occupational exposure to
trichloroethylene in metal cleaning processes and to
tetrachloroethylene in the dry-cleaning industry in the U.K. Ann.
occup. Hyg., 23: 197-204.
SHMUTER, L.M. (1972) [The effect of the chronic action of low
concentrations of chlorinated hydrocarbons on the production of
various classes of immunoglobulins.] Gig. i Sanit., 37(2): 32-35 (in
Russian).
SIEGEL, J., JONES, R.A., COON, R.A., & LYON, J.P. (1971) Effects
on experimental animals of acute, repeated, and continuous
inhalation exposures to dichloroacetylene mixtures. Toxicol. appl.
Pharmacol., 18: 168-174.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.G. (1977) Mutagenic
activity of chemicals identified in drinking-water. Dev. Toxicol.
environ. Sci., 2: 249-258.
SINGH, H.B., SALAS, L.J., & CAVANAGH, L.A. (1977) Distribution,
sources, and sinks of atmospheric halogenated compounds. J. Air
Pollut. Control Assoc., 27: 332-336.
SLACIK-ERBEN, R., ROLL, R., FRANKE, G., & VEHLEKE, H. (1980)
Tichloroethylene vapours do not produce dominant lethal mutations
in mice. Arch. Toxicol., 45: 37.
SMYTH, H.F., Jr, CARPENTER, C., WEIL, C.S., POZZANI, U.C.,
STRIEGEL, J.A., & NYCUM, J.S. (1969) Range-finding toxicity data.
VII. Am. Ind. Hyg. Assoc. J., 30: 470-476.
SNELL, F.D. & ETTRE, L.S., ed. (1970a) Chlorocarbons. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 9, pp. 373-410.
SNELL, F.D. & ETTRE, L.S., ed. (1970b) Chlorocarbons. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 9, pp. 454-459.
SNELL, F.D. & HILTON, C.L., ed. (1967) Anesthetics. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 5, pp. 357, 366-367, 420-421.
SORGO, G. (1976) [Trichloroethylene, carbon tetrachloride, and
gasoline intoxication as etiological factors in the developement of
arterio- and coronary sclerosis.] Arch. Toxicol., 35: 295-318 (in German).
SOUCEK, B. & VLACHOVA, D. (1960) Excretion of trichloroethylene
metabolites in human urine. Br. J. ind. Med., 17: 60-64.
STEERE, N.V., ed. (1967) Handbook of laboratory safety,
Cleveland, Ohio, The Chemical Rubber Co., pp. 544-545.
STENHAGEN, E., ABRAHAMSSON, S., & MCLAFFERTY, F.W. (1974)
Registry of mass spectral data, New York, John Wiley and Sons,
Vol. 1, p. 246.
STEWART, R.D. & DODD, H.C. (1964) Absorption of carbon
tetrachloride, tetrachloroethylene, methylene chloride, and
1,1,1-trichloroethane through the human skin. Am. Ind. Hyg. Assoc.
J., 25: 439-446.
STEWART, R.D., GAY, H.H., ERLEY, D.S., HAKE, C.L., & PETERSON, J.E.
(1962) Observations on the concentrations of trichloroethylene in
blood and expired air following exposures of humans. Am. Ind. Hyg.
Assoc. J., 23: 167-170.
STEWART, R.D., DODD, H.C., GAY, H.H., & ERLEY, D.S. (1970)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R.D., HAKE, C.L., & PETERSON, J.E. (1974) Use of breath
analysis to monitor trichloroethylene exposures. Arch. environ.
Health, 29: 6-13.
STOPPS, G.J. & MCLAUGHLIN, M. (1967) Psychophysiological testing
of humans exposed to solvent vapors. Am. Ind. Hyg. Assoc. J., 28:
43-50.
STOTT, W.T., QUAST, J.F., & WATANABE, P.G. (1982) The
pharmacokinetics and macromolecular interactions of
trichloroethylene in mice and rats. Toxicol. appl. Pharmacol., 62:
137-151.
SUCIU, I. & OLINICI, L. (1983) Hepato-renal involvement in acute
occupational trichloroethylene intoxication. Med. Lav., 74(2): 123-128.
SVABOVA, K. & MENCIK, M. (1983) [Changes in the state of health
of workers long-term exposed to halogenated hydrocarbons.] Pracov.
Lek., 35: 105-111 (in Czech with English abstract).
SZLUC-KUBERSKA, J. (1972) Chronic industrial trichloroethylene
poisoning. Folia med. Lodz., 16: 67-90.
TADA, O. (1969) Evaluating the exposure to some chlorinated
hydrocarbons. J. Sci. Labour, Part 2, 45: 757-765.
TANAKA, S. & IKEDA, M. (1968) A method for determination of
trichloroethanol and trichloroacetic acid in urine. Br. J. ind.
Med., 25: 214-219.
TAYLOR, H. (1936) Experiments on the physiological properties of
trichloroethylene. J. ind. Hyg. Toxicol., 18: 175-193.
THIELE, D.L., EIGENBRODT, E.E., & WARE, A.J. (1982) Cirrhosis
after repeated trichloroethylene and 1,1,1-trichloroethane
exposure. Gastroenterology, 83: 926-929.
TOLA, S., VILHUNEN, R., JARVINEN, E., & KORKALA, M.-L. (1980) A
cohort study on workers exposed to trichloroethylene. J. occup.
Med., 22: 737-740.
TOLOT, F., VIALLIER, J., ROULLET, A., RIVOIRE, J., & FIGUERES, J.C.
(1964) Toxicité hepatique au trichloroéthylène. Arch. Mal. prof.
Méd. Trav., 25: 9-15.
TOMASINI, M. (1976) [Cardiac arrythmias due to intoxication by
trichloroethylene ("th").] Med. Lav., 67: 163-169 (in Italian).
TOMENIUS, L., HOLMA, B., EHRHER-SAMUEL, H., KYLIN, B., TEBROCK, O.,
& THOMASON, M. (1979) Effect of trichloroethylene on cilia
activity in rabbit trachea. Acta pharmacol. toxicol., 44: 65-70.
TORKELSON, T.R. & ROWE, V.K. (1981) Trichloroethylene. In:
Patty's industrial hygiene and toxicology, 3rd (revised) ed.,
New York, John Wiley and Sons, Vol. 2B, pp. 3553-3586.
TRIEBIG, G., GOSSLER, K., & SCHALLER, K.-H. (1976) [Simple and
reliable gas-chromatographic determination of trichloroethylene and
its metabolites in blood and urine.] Fresenius Z. Anal. Chem., 279:
115-116 (in German).
TRIEBIG, G., LEHRL, S., KINZEL, W., ERZIGHEIT, H., GALSTER, J.V., &
SCHALLER, K.H. (1977a) [Psychopathometrical results of follow-up
studies of trichloroethylene-exposed persons.] Zbl. Bakt. Hyg. I.
Abt. Orig. B., 164: 314-327 (in German with English summary).
TRIEBIG, G., SCHALLER, K.H., ERZIGHEIT, H., & VALENTIN, H. (1977b)
Biochemical investigations and psychological studies of persons
chronically exposed to trichloroethylene with regard to non-
exposure intervals. Int. Arch. occup. environ. Health, 38: 149-162.
TRIEBIG, G., TRAUTNER, P., WELTLE, D., SAURE, E., & VALENTIN, H.
(1982) Investigations on neurotoxicity of chemical substances at
the workplace. III. Determination of the motor and sensory nerve
conduction velocity in persons occupationally exposed to
trichloroethylene. Int. Arch. occup. environ. Health, 51: 25-34.
TSURUTA, H. (1978) Percutaneous absorption of trichloroethylene
in mice. Ind. Health, 16: 145-148.
TUCKER, A.N., SANDERS, V.M., BARNES, D.W., BRADSHAW, T.J., WHITE,
K.L., Jr, SAIN, L.E., BORZELLACA, J.F., & MUNSON, A.E. (1982)
Toxicology of trichloroethylene in the mouse. Toxicol. appl.
Pharmacol., 62: 351-357.
UEHLEKE, H., TABARELLI-POPLAWSKI, S., BONSE, G., & HENSCHLER, D.
(1977) Spectral evidence for 2,2,3-trichloro-oxirane formation
during microsomal trichloroethylen oxidation. Arch. Toxicol., 37:
95-105.
US CFR (1976) Code of Federal Regulations. 21 CFR, 8: 305.
US ITC (1980) Production statistics, 1979, Washington DC,
International Trade Commisssion.
US NTP (1983) Technical report on the carcinogenesis studies of
trichloroethylene (without epichlorohydrin) in F344/n rats and BCF
mice, Research Triangle Park, NC, National Toxicology Program (NIH
Publication No. 83-1979, NTP TR 243).
VALLE-RIESTRA, J.F. (1974) Food processing with chlorinated
solvents. Food Technol., 28: 25-32.
VAN DER HOEVEN, R., DROST, R.H., MAES, R.A.A., DOST, F., PLOMP,
T.A., & PLOMP, G.J.J. (1979) Improved method for the electron-
capture gas chromatographic determination of trichloroacetic acid
in human serum. J. Chromatogr., 164: 106-108.
VAN DUUREN, B.L. & BANERJEE, S. (1976) Covalent interaction of
metabolites of the carcinogen trichloroethylene in rat hepatic
microsomes. Cancer Res., 36: 2419-2422.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH, A.C.,
MELCHIONNE, S., SELDMAN, I., & ROTH, D. (1979) Carcinogenicity of
halogenated olefinic and aliphatic hydrocarbons in mice. J. Natl
Cancer Inst., 63: 1433-1439.
VAN DUUREN, B.L., KLINE, S.A., MELCHIONNE, S., & SEIDMAN, I.
(1983) Chemical structure and carcinogenicity relationships of
some chloroalkene oxides and their parent olefins. Cancer Res., 43:
159-162.
VERNE, J., CECCALDI, P.F., HEBERT, S., & ROUX, J.M. (1959)
Hepatic steatosis during intoxication by volatile organic
compounds. IV. Biochemistry and histochemistry of fatty livers
produced by trichloroethylene poisoning. Pathol. Biol. (Paris), 7:
2311-2316.
VERNON, R.J. & FERGUSON, R.K. (1969) Effects of trichloroethylene
on visual-motor performance. Arch. environ. Health, 18: 894-900.
VERNOT, E.H., MACEWEN, J.D., HAUN, C.C., & KINKEAD, E.R. (1977)
Acute toxicity and skin corrosion data for some organic and
inorganic compounds and aqueous solutions. Toxicol. appl.
Pharmacol., 42: 417-423.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Company, pp. 607-610.
VESTERBERG, O., GORCZAC, J., KRASTS, M. (1976) Exposure to
trichloroethylene. II. Metabolites in blood and urine. Scand. J.
Work environ. Health, 4: 212-219.
VISSARIONOVA, V.Ya., KALASHNIKOVA, V.P., SKIRKO, B.K., KARANTIROVA,
O.V., & LAPRUN, I.B. (1975) [Comparative evaluation of indexes of
rat liver functions in trichloroethylene poisoning.] Gig. Tr.
Prof. Zabol., No.11: 56-58 (in Russian).
VLACHOVA, D. (1957) Determination of trichloroethanol in the
urine after exposure to trichloroethylene. J. Hyg. Epidemiol.
Microbiol. Immunol., 1-2: 225-229.
VON OETTINGEN, W.F. (1955) The halogenated aliphatic, olifinic,
cyclic, aromatic, and aliphatic-aromatic hydrocarbons including the
halogenated insecticides: their toxicity and potential dangers,
Washington DC, US Government Printing Office.
WADE, A., ed. (1977) Martindale: the extra pharmacopoeia, 27th
ed., London, The Pharmaceutical Press, pp. 714-715.
WAHLBERG, J.E. (1984) Erythema-inducing effects of solvents
following epicutaneous administration to man: studied by Laser
Doppler flowmetry. Scand. J. Work environ. Health, 10: 159-162.
WAHLBERG, J.E. & BOMAN, A. (1979) Comparitive percutaneous
toxicity of ten industrial solvents in the guinea-pig. Scand. J.
Work environ. Health, 5: 345-351.
WAKEHAM, S.G., DAVIS, A.C., & GOODWIN, J.T. (1982)
Biogeochemistry of volatile organic compounds in marine
experimental ecosystems and the estuarine environment: initial
results. In: Grice, G.D. & Reeve, M.R., ed. Marine Mesocosms,
New York, Springer.
WASKELL, L. (1978) A study of the mutagenicity of anesthetics and
their metabolites. Mutat. Res., 57: 141-153.
WATERS, E.M., GERSTNER, H.B., & HUFF, J.E. (1977)
Trichloroethylene. I. An overview. J. Toxicol. environ. Health, 2:
671-707.
WEAST, R.C., ed. (1980) Handbook of chemistry and physics, 61st
ed., Boca Raton, Florida, CRC Press, Inc., p. C-317.
WEICHARDT, H. & BARDODEJ, Z. (1970) Determination of
trichloroacetic acid and trichloroethanol in the urine of
trichloroethylene workers. Zentralbl. Arbeitsmed. Arbeitsschutz.,
20: 219-221.
WELLS, J.C.D. (1982) Abuse of trichloroethylene by oral self-
administration. Anaesthesia, 37: 440-441.
WETTERHAHN, J. (1972) Eye make-up. In: Balsam, M. & Sagarin, E.,
ed. Cosmetics science and technology, New York, John Wiley and
Sons, Inc., pp. 419.
WHELAN, J.K., BLANCHETTE, M.A., & HUNT, J.M. (1983) Volatile C,-
C7 organic compounds in an anoxic sediment core from the
Pettaquamscutt River (Rhode Island, USA). Org. Geochem., 5: 29-33.
WHITE, A.E., TAKEHISA, S., EGER, E.I., WOLFF, S., & STEVENS, W.C.
(1979) Sister-chromatid exchanges induced by inhaled anaesthetics.
Anaesthesiology, 50: 426.
WHITE, J.F. & CARLSON, G.P. (1979) Influence of alterations in
drug metabolism on spontaneous and epinephrine-induced cardiac
arrhythmias in animals exposed to trichloroethylene. Toxicol. appl.
Pharmacol., 47: 515-527.
WHITE, J.F. & CARLSON, G.P. (1981a) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: potentiation
by ethanol. Toxicol. appl. Pharmacol., 60: 466-471.
WHITE, J.F. & CARLSON, G.P. (1981b) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: role of
trichloroethylene metabolites. Toxicol. appl. Pharmacol., 60: 458-465.
WHITE, J.F. & CARLSON, G.P. (1982) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: potentiation
by caffeine. Fundam. appl. Toxicol., 2: 125-129.
WHO (1984) Guidelines for drinking-water quality. I.
Recommendations, Geneva, World Health Organization, 66 pp.
WHO STUDY GROUP ON RECOMMENDED HEALTH-BASED LIMITS IN OCCUPATIONAL
EXPOSURE TO SELECTED ORGANIC SOLVENTS (1981) Recommended health-
based limits in occupational exposure to selected organic solvents,
Geneva, World Health Organization, 84 pp (Technical Report Series
No. 664).
WIECKO, W. (1966) [Oral poisoning with trichloroethylene.] Wiad.
Lek., 19: 1117-1118 (in Polish).
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & NOETHER FERTIG, M.,
ed. (1976) The Merck index, 9th ed., Rahway, New Jersey, Merck &
Co., Inc., p. 1238.
WINNEKE, G. (1982) Acute behavioural effects of exposure to some
organic solvents: psychophysiological aspects. Acta neurol. scand.,
66(suppl. 92): 117-129.
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmaocokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WIRTSCHAFTER, Z.T. & CRONYN, M.W. (1964) Relative hepato-
toxicity: pentane, trichloroethylene, benzene, carbon
tetrachloride. Arch. environ. Health, 9: 180-185.
ZAFFIRI, O., RUGGERINI, R., & FRANCESCATO, F. (1968) Anesthetics
and adrenergic receptors. Minerva Anestesiol., 34: 556-560.
ZENICK, H., BLACKBURN, K., HOPE, E., RICHDALE, N., & SMITH, M.K.
(1984) Effects of trichloroethylene exposure on male reproductive
function in rats. Toxicology, 31: 237-250.
ZIGLIO, G. (1979) [Gas-chromatographic determination of blood
trichoroacetic acid concentration in subjects not occupationally
exposed to trichloro- and tetrachloroethylene.] Ig. Mod., 72: 876-900
(in Italian).
ZIGLIO, G., FARA, G.M., BELTRAMELLI, G., & PREGLIASCO, F. (1983)
Human environmental exposure to trichloro- and tetrachloroethylene
from water and air in Milan, Italy. Arch. environ. contam.
Toxicol., 12: 57-64.
APPENDIX I
Predicting the Equilibrium Distribution of Trichloroethylene
On the basis of the physical and chemical data summarized
in Table 1 of this document, it is possible to predict the
approximate equilibrium distribution of trichloroethylene in major
environmental "compartments". The models used have been described
by Mackay & Paterson (1981). Essentially, these use physical and
chemical data and realistic compartment volumes in a "model world"
to calculate fugacity capacities and to predict the partitioning of
a chemical between various compartments. The following compartment
volumes are assumed (Neely & Mackay, 1982) (Table I.1) in an
environment, 1 km2 in area.
Table I.1 Compartment volumes for trichloroethylene
----------------------------------------------------------
Atmosphere 6 x 109 m3 1 km2 area x 6 km height
Water 7 x 106 m3 70% area x 10 m depth
Soil 4.5 x 104 m3 30% area x 15 cm depth
Sediment 2.1 x 104 m3 70% water x 3 cm depth
Suspended aquatic 35.0 m3 water volume x 5 ppm
matter
Aquatic biota 7.0 m3 water volume x 1 ppm
----------------------------------------------------------
As an example, the input of trichloroethylene is assumed to be
1000 moles/km2 per year, based on an annual production capacity of
4 x 105 tonnes per year and an approximate land area of 106 km2.
This leads to an actual production of approximately 3000 moles/km2,
but it is assumed that land represents one-third of the total area.
Physical and chemical data for trichloroethylene are given in
Table 1 (main text). Environmental temperature is assumed to be
25 °C (atmosphere) or 15 °C (land and water).
Additional compartments at appropriate volumes can be added with
appropriate characteristics, e.g., atmospheric particulates at
2 µg/m3 (Seba & Prospero, 1971) or terrestrial biota at
approximately 1 kg/m2 (Odum, 1971).
The model predicts the distributions shown in Table I.2.
These are remarkably close to the distributions observed in
most compartments and, furthermore, the ratios of concentrations
predicted to occur between compartments are remarkably similar to
those that are observed. The predicted and observed distributions
of trichloroethylene are not surprising; as noted in section 4.2.1,
its high vapour pressure would be expected to lead to high
atmospheric concentrations, and this would offset the tendency of
high water solubility and low partition coefficients (themselves
related) to lead to high water, biota, or sediment concentrations,
through either partition or adsorption.
Table I.2 Predicted and observed distribution of
trichloroethylene (TCE) in the environment using compartment
volumes and approaches described in text
--------------------------------------------------------------
Compartment Predicted Predicted TCE Observed TCE
% TCE Concentration concentration
(see text)
--------------------------------------------------------------
Air 99.7 22 µg/m3 0.01 - 10 µg/m3
Water 0.3 0.06 µg/litre 0.01 - 100 µg/litre
Sediment < 0.01 0.4 µg/kg 1 - 10 µg/kg
Aquatic < 0.01 0.7 ng/g 1 - 100 ng/g
--------------------------------------------------------------
The value of a model like this is not that it predicts the
"correct" distribution, but that it illustrates and identifies
the compartments that are important as reservoirs or as sites of
degradation of chemicals. In the present case, the size of the
atmospheric compartment and the high vapour pressure of
trichloroethylene are such that the bulk of trichloroethylene
released into the environment should be found in the atmosphere.
Therefore, atmospheric degradation processes should be important in
the eventual degradation of trichloroethylene.
This approach can be refined further by considering not only
the distribution at equilibrium (assuming no degradation), but
adding rates of degradation. For these purposes, we have assumed
the rates of degradation in the appropriate compartment, shown in
section 4.2.2. Although absolute trichloroethylene concentrations
are predicted by this model to be appreciably lower in each
compartment than those shown in Table I.2, the ratios of
concentrations between compartments do not change appreciably
(Table I.3). However, the important point is that since most of
the trichloroethylene is predicted to occur in the atmosphere, and
since atmospheric degradation rates are similar to or faster than
those in other compartments, most of the degradation should take
place in the atmosphere (Table I.3). Washout or fallout of
atmospheric particulate material is not likely to be an important
process; there seems to be little tendency for trichloroethylene to
sorb to particles, because of its low Koc, and residence times of
such particles are long compared to the the expected rate of
degradation by OH radicals. Total environmental lifetime is
controlled by the atmospheric degradation rate, and should,
therefore, be about 10 - 11 days. Degradation in sediments and/or
biota probably contribute in only a very minor way to overall
degradation.
Table I.3 Predicted distribution and compartmental
degradation rates for trichloroethylene (TCE) as %
of total environmental degradation using compartment
volumes and degradation rates in text
----------------------------------------------------
Compartment Predicted TCE Total degradation
concentration (%)
----------------------------------------------------
Air 0.6 µg/m3 99.9
Water 0.002 µg/litre 0.08
Sediment 0.01 µg/kg < 0.01
Aquatic biota 0.02 ng/g < 0.01
----------------------------------------------------
REFERENCES TO APPENDIX I
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
NEELY, W.B. & MACKAY, D. (1982) In: Dickson, K.L., Maki, A.W., &
Cairns, J., Modelling the fate of chemicals in the aquatic
environment, Ann Arbor, Michigan, Ann Arbor Science Publications.
ODUM, E.P. (1971) Fundamentals of ecology, 3rd ed., Philadelphia,
Pennsylvania, W.B. Saunders Co.
SEBA, D.B. & PROSPERO, J.M. (1971) Pesticides in the lower
atmosphere of the northern equatorial Atlantic Ocean. Atmos.
Environ., 5: 1043-1050.
Metabolically activated trichloroethylene binds covalently to
the hepatic microsomal proteins and DNA, in vitro. This finding
supports the formation of an epoxide intermediate (Banerjee & Van
Duuren, 1978), though this has not been demonstrated in vivo
(Parchman & Magee, 1982; Stott et al., 1982).
5.3.2. Human beings
As originally suggested by Powell (1945), the formation of the
epoxide, an intermediate reactive metabolite that binds covalently
with proteins (Bolt & Filher, 1977), has been confirmed by indirect
spectral evidence (Uehleke et al., 1977). The epoxide may undergo
intramolecular rearrangement in 2 different ways (Henschler & Hoos,
1982; DeKant & Henschler, 1983). One pathway leads to chloral
(Henschler, 1977a,b), which is further oxidized to trichloroacetic
acid (TCA), or reduced to trichloroethanol (Leibman, 1965). After
oral administration, trichloroethanol is also partly metabolized
into TCA (Müller et al., 1974). Trichloroethanol is rapidly
conjugated with glucuronic acid to form the respective glucuronide.
The other pathway leads to the formation of dichloroacetyl chloride
which, under in vitro conditions, can lead to the formation of
dichloroacetic acid. However, under normal in vivo conditions,
dichloroacetic acid is not found, except in mice following the
administration of very high doses of trichloroethylene. Under
these conditions, an "overspill" mechanism may operate (Henschler
et al., 1979; Hathway, 1980; Henschler et al., 1983). The
excretion of chloroform in expired air and monochloroacetic acid in
urine have also been proposed as minor routes of metabolism (Ogata
& Saeki, 1974; Bartonicek, 1962). Miller & Guenrich (1982)
suggested that an epoxide was not an obligatory intermediate step
and proposed an alternative model in which chlorine migration
occurs in an oxygenated trichloroethylene P 450 transition state.
Trichloroacetic acid binds well with plasma proteins and its
concentration in plasma is approximately double that in whole blood
(Müller et al., 1972).
5.3.3. Drug and other interactions
A number of commonly-used drugs might be expected to modify the
extent of metabolism of trichloroethylene during human exposure.
Although largely undocumented in man, the induction of the hepatic
microsomal mixed-function oxidase system by drugs, taken for
therapeutic reasons, or by exposure to certain environmental
chemicals (e.g., phenobarbital, toluene, PCBs) can bring about an
increased rate of trichloroethylene metabolism (Ikeda & Imamura,
1973; Ikeda, 1974).
In human beings, the simultaneous administration of ethanol and
trichloroethylene (100 mg/m3 for 6 h) causes an increase in
trichloroethylene levels in both plasma (2.4 times the normal
value) and in exhaled air (3.4 times), and a decrease in the levels
of trichloroacetic acid and trichloroethanol (Müller et al., 1975).
5.4. Elimination
5.4.1. Studies on animals
The kinetics of the distribution and elimination of
trichloroethylene, administered intravenously in Wistar rats at
dose levels of 6, 9, 12, or 15 mg/kg body weight show that the
blood concentration exhibits a first order, 2-compartment model
exponential disappearance, and it has been suggested that a dose
of 15 mg trichloroethylene/kg body weight is within the hepatic
metabolic capacity in the rat (Withey & Collins, 1980). Daniel
(1963) showed that when trichloroethylene was administered orally
to rats, the ratio of pulmonary to urinary elimination varied with
the dose and that as dose increased, pulmonary excretion increased
while urinary elimination decreased. Further evidence showing that
the metabolism of trichloroethylene is saturable in Wistar rats was
obtained by Filser & Bolt (1979) who showed that the saturation
point occurred at 350 mg/m3 (65 ppm), that the zero order Vmax was
210 µmol/h per kg body weight and that the first order clearance at
a dose of 350 mg/m3 (65 ppm) was 77 µmol/h per kg body weight.
Stott et al. (1982) found that the pulmonary elimination of
unchanged trichlorethylene in Osborn Mendel rats was only 2% of the
dose at 54 mg/m3 (10 ppm), but 21% at a dose of 3240 mg/m3
(600 ppm). In contrast to these findings of a saturable process in
the rat, The same authors showed that in the mouse, doses of
trichloroethylene up to 3240 mg/m3 (600 ppm) were completely
metabolized.
In dogs, exposed through inhalation, for 1 h, to
trichloroethylene at 3780, 8100, and 10 800 mg/m3 (700, 1500, and
2000 ppm), the excretion of trichloroethylene and trichloroacetic
acid was correlated with the trichloroethylene concentration. The
rate of trichloroacetic acid excretion was higher than that for
trichloroethanol. One hour after exposure ended, the percentage
of trichloro compounds in the urine was 0.7% of the total
trichloroethylene absorbed (Hobara et al., 1983).
5.4.2. Studies on man
It has been shown that man metabolizes trichloroethylene
extensively. Ikeda et al. (1972) showed that the capacity of
workers to metabolize trichloroethylene was nonlimiting, at least
up to a daily exposure level of 945 mg/m3 (175 ppm) for 8 h.
In human beings, trichloroethylene is eliminated unchanged
through the lungs and is eliminated in the urine in the form of
metabolites. Elimination by other routes (e.g., faeces, sweat, and
saliva) accounts for less than 10% of the total (Bartonicek, 1962).
After inhalation exposure, about 10% of the amount absorbed is
expired unchanged, about 30 - 50% is excreted as trichloroethanol
in urine, and about 10 - 30% as trichloroacetic acid in urine
(Soucek & Vlachova, 1960; Bartonicek, 1962; Monster et al., 1976,
1979).
The half-life of trichloroethylene in exhaled air and in the
blood depends on the length of exposure and on the time of sampling
after exposure. The concentration follows a multi-exponential
curve, compatible with at least 3 compartments: lungs, blood and
most other tissues, and adipose tissue. After a single exposure
to trichloroethylene, trichloroethanol reaches its maximum
concentration in blood and urine almost directly after exposure.
Thereafter, the concentration decreases, with a half-life of
about 10 - 15 h (Müller et al., 1974; Monster et al., 1976,
1979; Vesterberg et al., 1976). After a single exposure to
trichloroethylene, the concentration of trichloroacetic acid in
both the blood and the urine increases for up to 20 - 40 h after
exposure. Thereafter, the concentration decreases with a half-life
of about 70 - 100 h (Müller et al., 1974; Monster et al., 1979).
Trichloroacetic acid, as such, has a shorter half-life of about
50 h.
In a group of workers with long-term exposure to
trichloroethylene at a concentration of 270 mg/m3 (50 ppm), median
values of trichloroethanol and trichloroacetic acid of 330 and
319 g/kg creatinine, respectively, were found at the end of a
working shift; during the work-free periods, the metabolites of
trichloroethylene were eliminated slowly (Triebig et al., 1976).
In a study on factory workers exposed to trichloroethylene,
Ikeda & Imamura (1973) observed a half-life of 41 h for the
urinary excretion of total trichloro-compounds (i.e., a combination
of trichloroacetic acid and trichloroethanol). This half-life is
somewhat longer with oral administration of trichloroethylene and
of chloral hydrate and is about 85 - 99 h after repeated exposure
to trichloroethylene, because of the delayed formation of
trichloroacetic acid from trichloroethylene and the
trichloroethanol still available from the tissues (Müller et al.,
1974). Thus, trichloroacetic acid will be found in the urine, even
when trichloroethanol is no longer detectable (Ikeda et al., 1971).
Trichloroacetic acid accumulates in the blood and urine during
daily exposures to trichlorethylene (Monster et al., 1979; Müller
et al., 1975).
5.5. Biological Monitoring of Exposure
Droz & Fernandez (1978) used a mathematical model to study the
effects of hourly and daily variations in exposure concentrations
on alveolar air trichloroethylene concentrations and on the urinary
excretion of trichloroethanol and trichloroacetic acid. The
determination of trichloroethanol in urine appeared to be more
sensitive than the determination of trichloroethylene in exhaled
air. The excretion of trichloroacetic acid can be used for the
qualitative evaluation of the preceding day's exposure. In
practice, blood analysis would be preferable to analysis of urine,
because of the smaller individual variations generally observed
with the former. In studies concerning repeated exposure to
constant concentrations, the smallest inter-individual variation
was found in the concentrations in blood (Monster et al., 1979).
The measurement of total trichloro compounds in urine was
described by Takana & Ikeda (1968). The urinary trichloroethanol
is oxidized to trichloroacetic acid and the total amount of
trichloroacetic acid is then measured with the Fujiwara reaction.
In the case of exposure to relatively steady concentrations, this
has the advantage of being able to indicate small-scale inter-
personal variations. It may be used as an index of exposure
intensity, especially when the urine samples are collected at,
or close to, the end of the workshift at the end of the work week
(Ikeda et al., 1972). However, other studies have demonstrated
poor individual correlation between trichloroethylene exposure and
the urinary elimination of the major metabolites, trichloroacetic
acid and trichloroethanol (Boudène et al., 1983). Separate
measurement of urinary metabolites provides more information on
exposure to daily fluctuating concentrations, because relatively
high concentrations of trichloroethanol indicate recent high
exposure, whereas relatively high concentrations of trichloroacetic
acid indicate long-term exposure to high concentrations (Monster et
al., 1979).
Trichloroethylene concentrations in alveolar air and in blood,
shortly after exposure, indicate recent exposure concentrations,
while the concentration several hours after exposure indicates the
average exposure over the preceding days (Stewart et al., 1974).
6. EFFECTS ON ANIMALS AND CELL SYSTEMS
6.1. Effects on Animals
Data from acute, short-term repeated dose, and long-term
toxicity studies on laboratory animals are summarized in Table 6.
6.1.1. Acute toxicity
Acute toxicity data on common laboratory animals are shown in
Tables 7 and 8.
Acute toxicity levels following inhalation exposure to
trichloroethylene are summarized in Table 7. Table 8 includes data
on acute oral, dermal, intraperitoneal (ip), subcutaneous (sc), and
intravenous (iv) LD50s for the mouse, rat, rabbit, and dog.
Von Oettingen (1955) reported that oral acutely toxic doses in
rats produced gastrointestinal irritation. Moderate increases in
aspartate aminotranferase (EC 2.6.1.1) levels were observed in rats
24, 48, and 72 h after a single 6-h exposure to trichloroethylene
vapour at concentrations of 54 mg/m3 (10 ppm) and 540 mg/m3
(100 ppm) (Deguchi, 1972); the increase at 5400 mg/m3 (1000 ppm)
was small (Deguchi, 1972), presumably due to inactivation of P-450.
According to Rigaud et al. (1977), intraperitoneal administration
resulted in a significant increase in aspartate aminotransferase
(EC 2.6.1.1), alanine aminotransferase (EC 2.6.1.2), and ornithine
carbamoyltransferase (OCT) (EC 2.1.3.3) in the rat, whereas
Wirtschafter & Cronyn (1964), administering 500 mg/kg body weight,
detected only minor hepatic effects over the 12 - 24-h period
following administration. No evidence of kidney dysfunction was
observed in mice following intraperitoneal administration of
trichloroethylene at 0.004M/kg body weight. Application of 2 ml
trichloroethylene (7800 mg/kg), under an occlusive dressing, on the
skin of 20 guinea-pigs, did not produce any deaths but, during the
35-day observation period, there were reductions in body weight at
1 week ( P < 0.001), 2 and 3 weeks ( P < 0.01), and 4 weeks
( P < 0.05) (Wahlberg & Boman, 1979).
Skin irritation
Trichloroethylene (purity 99.5%), applied (0.5 ml) to the
shaved (non-abraded) skin of rabbits, for 24 h, under an occlusive
dressing, produced severe skin irritation (Duprat et al., 1976).
In another study, trichloroethylene (1.0 ml) was applied,
occluded in a "skin depot", to the clipped skin of guinea-pigs.
Histological examinations were performed at 15 min, 1, 4, and 16 h.
Degenerative changes (pyknotic nuclei) were observed in the
epidermis after 15 min and were progressive (pyknosis, karyolysis,
junctional separation of the epidermis) up to the end of the study
at 16 h (Kronevi et al., 1981).
Table 6. Concentrations of trichloroethylene at which no effects were observed in experimental animals
exposed through inhalation
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
rat 3000 7 h mortality Adams et al. (1951)
6400 1.4 h mortality Adams et al. (1951)
12 000 0.6 h mortality Adams et al. (1951)
20 000 0.4 h mortality Adams et al. (1951)
200 7 h per day, 5 days mortality Adams et al. (1951)
per week, for 26
weeks
400 8 h per day for mortality; body weight; Battig et al. (1963)
5 days learning capacity
730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35a 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
guinea-pig 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology (1967)
6 weeks
35 90 days mortality; body weight; Prendergast et al.
haematology; histology (1967)
histology
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
guinea-pig 100 7 h per day, 5 mortality Adams et al. (1951)
(contd.) days per week for
26 weeks
monkey 730 8 h per day, 5 mortality; body weight; Prendergast et al.
(squirrel) days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
monkey 400 7 h per day, 5 mortality Adams et al. (1951)
(Rhesus) days per week for
26 weeks
rabbit 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
200 7 h per day, 5 mortality Adams et al. (1951)
days per week for
6 weeks
dog 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
dog 35 90 days mortality; body weight; Prendergast et al.
(contd.) haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
mouseb 5500 20 min anaesthesia Gehring (1968)
5500 100 min liver injury (elevated SGPT) Gehring (1968)
5500 300 min mortality Gehring (1968)
---------------------------------------------------------------------------------------------------------
a Kalashmikova et al. (1976) reported that rats exposed to 50 mg/m3 for 5 h per day, for 90 days, showed
damage to parenchyma in liver and kidney.
b Kjellstrand et al. (1983) reported that male NMRI mice
continuously exposed to 200 mg/m3 (37 ppm) for
30 days showed increased plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity; female mice did
not show any increase in BuChE activity; there was a significant increase in liver weight at 200 mg/m3
(37 ppm) in both sexes.
Table 7. Acute toxicity of trichloroethylene administered via
inhalation to laboratory animals
--------------------------------------------------------------------
Species Toxicity Exposure Reference
index ------------------------
level duration
(ppm) (mg/litre) (h)
--------------------------------------------------------------------
Rat LC100 20 000 107 0.4 Adams et al. (1951)
LC100 12 000 64.2 1.4 Adams et al. (1951)
LC100 2500 13.4 7.0 Adams et al. (1951)
LC50 26 300 140.7 1 Vernot et al. (1977)
LC50 12 500 66.9 4 Siegel et al. (1971)
LCL0a 8000 42.8 4 Smyth et al. (1969)
Mouse LC100 8000 42.7 2 Von Oettingen (1955)
LC100 5600 30.0 9.75 Gehring (1968)
LC50 8450 45.1 4 Kylin et al. (1962)
LC50 41 122 220 0.33 Aviado et al. (1976)
LC50 49 000 262 0.50 Vernot et al. (1977)
LCL0a 3000 16.0 2 Lazarev (1929)
LC50 40 4 Lazarev & Gadaskina
(1977)
Guinea- LC100 37 000 197.8 0.67 Von Oettingen (1955)
pig
Cat LCL0a 6074 32.5 2 Lehmann & Schmidt-Kehl
(1936)
Rabbit LC 5000 26.75 14.28 McCord (1932)
LC 10 000 53.5 2.5 McCord (1932)
LC 20 000 107 2 McCord (1932)
--------------------------------------------------------------------
a LCLO = lowest published lethal concentration.
Eye irritation
Instillation of 0.1 ml of trichloroethylene (purity 99.5%)
into rabbit eyes produced mild to moderate conjunctivitis with
superficial epithelial abrasion. At 7 days, there was a resolving
keratitis with complete recovery within 2 weeks (Duprat et al.,
1976).
6.1.2. Short-term exposures
6.1.2.1. Oral exposures
In a US NTP study (1983), groups of 10 male and 10 female
F344/N rats were administered trichloroethylene (in corn oil, by
gavage) at doses ranging from 125 to 2000 mg/kg body weight (males)
and 625 to 1000 mg/kg (females), 5 times per week, for 13 weeks.
All rats survived the 13-week study, but males receiving the
2000 mg/kg dose exhibited a 24% decrease in body-weight gain. At
the 1000 mg/kg dose, final body weights for males and for females
were similar to those of the controls.
Table 8. Acute oral, dermal, intraperitoneal, subcutaneous, and
intravenous LD50s for trichloroethylene in laboratory animals
--------------------------------------------------------------------------
Species Oral Dermal Intraperitoneal Subcutaneous Intravenous
(mg/kg body (ml/kg (mg/kg body (mg/kg body (mg/kg body
weight) body weight) weight) weight)
weight)
--------------------------------------------------------------------------
Rat 49201 27252
Dog 56803 2.8004 1505,a
Mouse 28506,b 32107,b 144010 3411
240012 31508,b
30009,c
1.2006,b
Rabbit 201
--------------------------------------------------------------------------
1 From: Smyth et al. (1969). a LDL0.
2 From: Rigaud et al. (1977). b In 24 h.
3 From: Christensen et al. (1974). c In 14 day.
4 From: Klaassen & Plaa (1967).
5 From: Barsoum & Saad (1934).
6 From: Aviado et al. (1976).
7 From: Klaassen & Plaa (1966).
8 From: Gehring (1968).
9 From: Gradiski et al. (1974).
10 From: Plaa et al. (1958).
11 From: NIOSH (1977b).
12 From: Tucker et al. (1982).
Histopathological examination of tissues from animals receiving
the highest doses showed minimal or mild cytomegaly and karyomegaly
of the renal tubular epithelial cells in the inner cortex in 8/9
males dosed with 2000 mg/kg per day, and the same effect, graded as
equivocal or mild, was seen in 5/10 females that had received the
1000 mg/kg per day dose.
The results of this 13-week study in F344/N rats were
essentially similar to those of an earlier 8-week study conducted
on Osborne-Mendel rats (NCI, 1976). In this study, only doses in
excess of 5000 mg/kg per day were lethal for rats. Doses of
1000 mg/kg per day had no effect on body-weight gains in males,
but depressed weight gains in females by approximately 15%.
Groups of 10 male and 10 female B6C3F1 mice received
trichloroethylene (by gavage in corn oil) at doses ranging from
375 to 6000 mg/kg body weight, 5 times per week for 13 weeks. All
males and 9/10 females receiving 6000 mg/kg, 7/10 males and 1/10
females receiving 3000 mg/kg, and 2/10 males and 1/10 females
receiving 1500 mg/kg died. Mean body weights of male mice dosed
with 750, 1500, or 3000 mg/kg were depressed by 11%, 19%, and 17%,
respectively, relative to the controls. Mean body weights of
control and treated groups of female mice were similar (US NTP,
1983).
Liver weights (both absolute and as percentage body weight)
increased in a dose-related fashion. Liver weights were increased
by more than 10% relative to the controls for males receiving
750 mg/kg body weight or more and for females receiving 1500 mg/kg
or more.
Histopathological examination showed hepatic centrilobular
necrosis (6/10 males and 1/10 females administered 6000 mg/kg).
This lesion was not seen in either males or females administered
3000 mg/kg, but 2/10 males had multifocal areas of calcification
scattered throughout their livers. Multi-focal calcification was
also seen in the liver of the single female mouse that survived the
6000 mg/kg dosage regimen. One female in the 3000 mg/kg dose group
developed a hepatocellular adenoma, an extremely rare lesion in
female mice of this age (20 weeks).
Examination of renal tissues showed the presence of mild to
moderate cytomegaly and karyomegaly of the renal tubular epithelial
cells of the inner cortex. These changes were found in only 1 of
the 23 mice (13 males and 10 females) that died after receiving
doses of 3000 or 6000 mg/kg for up to 6 weeks. However, the
changes were found in all 4 of the males that died after receiving
the 3000 mg/kg dose for 7 - 13 weeks, and in all animals that
survived the 6000 mg/kg (1/10 females) and the 3000 mg/kg doses
(3/10 males and 9/10 females). Tissues from mice receiving lower
doses of trichloroethylene were not examined.
Trichloroethylene administered orally to mice at doses in
excess of 500 mg/kg for 10 days produced proliferation of hepatic
peroxisome as demonstrated by increased cyanide-insensitive
palmitoyl CoA oxidation (PCO) and electron microscopy. In the
rat, trichloroethylene did not have any effect on peroxisome
proliferation. Trichloroacetic acid administered for 10 days
increased hepatic peroxisome proliferation in both species (Elcombe
et al., 1982). It is possible that the rapid rate of
trichloroethylene metabolism in the mouse, together with the
enterohepatic circulation of trichloroacetic acid, leads to
high steady-state blood levels of trichloracetic acid and the
concomitant proliferation of peroxisomes.
Primary cultures of isolated mouse and rat hepatocytes have
been found to metabolize trichloroethylene to trichloroacetic acid
at rates comparable to those in intact animals. Similarly, the
isolated hepatocytes respond to exposure to trichloroacetic acid
by peroxisome proliferation. Isolated human hepatocytes have been
found to produce trichloracetic acid from trichloroethylene at a
lower rate than that in the rat. Trichloroacetic acid does not
induce peroxisome proliferation in human hepatocytes (Elcombe,
1985).
6.1.2.2. Inhalation exposure
In the rat, exposure to 81 000 mg/m3 (15 000 ppm) for a period
of 2 - 4 min produced complete anaesthesia within 9 min (Schumacher
& Grandjean, 1960). In a study on mice, exposure to 36 7000 mg/m3
(6800 ppm) for 10 - 11 min or 64 800 mg/m3 (12 000 ppm) for 5 -
6 min produced complete anaesthesia (Friberg et al., 1953). In
another study on mice, exposure to 29 700 mg/m3 (5500 ppm) produced
anaesthesia in 46 min (Gehring, 1968).
Studies conducted on rats by Vissarionova et al. (1975) showed
that concentrations of 5000 mg/m3 administered for 5 h a day, for
1 week, resulted in an increase in liver and kidney weight (21.6%
and 18%, respectively), and a decrease in alkaline phosphatase and
RNA-dependent hepatic dehydrogenase. Histological changes have also
been noted in hepatic and renal parenchyma by Kalashnikova et al.
(1976).
With doses of 1080 mg/m3 (200 ppm) administered for 6 h daily
for 4 days, Savolainen et al. (1977) found that rats exhibited
greater motor activity and less cerebral RNA, 5 days after the last
exposure, and an accumulation of trichloroethylene in perirenal
fat.
Doses of 250, 500, 800, and 1200 mg/m3 administered for 15 -
90 h resulted in increases in intracellular lipids (Verne et al.,
1959).
No effects were noticed in rats administered 1080 mg/m3 (200
ppm) for 4 h a day for 4 days (Grandjean, 1960).
Continuous exposure of rats, mice, and gerbils by inhalation to
trichloroethylene at 810 mg/m3 (150 ppm), for periods ranging from
2 to 30 days, produced liver enlargement in all species; the mouse
was the most severely affected. After the end of exposure, the
liver weights of the mice decreased rapidly. An increased kidney
weight was noted in gerbils (Kjellstrand et al., 1981a).
In another study in which 7 different strains of mice (wild,
C57BL, DBA, B6CBA, A/su, NZB, and NMRI) were continuously exposed
to a trichloroethylene concentration of 810 mg/m3 (150 ppm),
Kjellstrand et al. (1983a) reported large increases in liver weight
in all strains with minimal changes in kidney and spleen weights.
Plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity
increased in males of all strains and in females of strains A/su
and NZB, but to a lesser extent than in the corresponding males.
In a further study, with continuous exposure to concentrations of
200 - 1620 mg/m3 (37 - 300 ppm), plasma BuChE increased in male
mice in a time- and concentration-dependent manner. Liver weight
increased in a time- and concentration-dependent manner in both
sexes.
Exposure of rats, guinea-pigs, rabbits, dogs, and monkeys to
3825 mg/m3, for 8 h per day, 5 days per week, for 6 weeks, resulted
in a loss of overall body weight in dogs and monkeys. There were
no changes in haematological or liver enzyme parameters
(Prendergast et al., 1967).
Groups of male Swiss Webster mice were exposed to 54 000 mg/m3
(10 000 ppm) for 1 or 4 h, daily, for 5 consecutive days. In the
4-h group, the NADPH cytochrome c reductase (EC 1.6.99.3) activity
in the lung decreased, but that in the liver increased. In the
lungs of this group, there were platelet thrombi and vacuolization
of bronchial epithelial cells. There were no changes in the liver.
It was concluded that the reduced activity of the pulmonary mixed-
function oxidase system reflected injury to the lungs (Lewis et
al., 1984).
Rabbits exposed to 15 000 mg/m3 (2790 ppm) for 4 h per day,
6 days a week for 45 days, developed severe normocytic anaemia,
leukopenia, and thrombocytopenia due to toxic effects on the bone
marrow (Mazza & Brancaccio, 1967).
In rats, urinary levels of trichloro-substituted metabolites
and the activity of drug-metabolizing enzymes (cytochrome P-450)
were related to the duration of trichloroethylene anaesthesia
(Moslen et al., 1977). Trichloroethylene hepatotoxicity in the rat
produces increased levels of serum transaminases. Hetaptotoxicity
increases the activity of drug-metabolizing enzymes (cytochrome
P-450) (correlation coefficient: 0.95) and the urinary excretion of
trichloro-metabolites (correlation coefficient: 0.88) (Moslen et
al., 1977).
Cytotoxic effects have been observed in the kidney and liver of
dogs subjected to 81 000 mg/m3 (1.5%; 15 000 ppm) trichloroethylene
anaesthesia (Kiseleva & Korolenko, 1971).
Studies on rats exposed by inhalation to trichloroethylene
concentrations of 2160, 4320, or 8640 mg/m3 (400, 800, or 1600 ppm)
for 6 h revealed no effects at the first concentration, a decrease
in swimming activity at 4320 mg/m3, and a further decrease at
8640 mg/m3 (Grandjean, 1963). Other rats exposed to 1944 -
2268 mg/m3 (360 - 420 ppm) for 8 h a day, 5 days a week for
46 weeks, exhibited no effects (Bättig & Grandjean, 1963). After
43 weeks at 2160 mg/m3 (400 ppm), rats in another set of tests by
Bättig (1964) exhibited greater maze skills. Mice exposed
intermittently to trichloroethylene showed a decrease in motor
activity at 4500 mg/m3 (900 ppm), but, at 19 440 mg/m3, it was
considerably increased (Kjellstrand et al., 1983b).
In Mongolian gerbils, continuously exposed to trichloroethylene
at 1.72 mg/m3 (320 ppm) for 9 months, there was no effect on
spatial memory, but subsequent maze performance test results were
interpreted as indicating an irreversible effect on the central
nervous system (Kjellstrand et al., 1980). In another study, 2
groups of Mongolian gerbils were continuously exposed to 810 mg
trichloroethylene/m3 (150 ppm) for 71 and 106 days, respectively.
In a series of maze tests following the end of exposure, the
treated groups performed less well than the unexposed controls
(Kjellstrand et al., 1981b).
6.1.2.3. Parenteral exposure
When male Swiss Webster mice were injected ip with 3 doses of
330 mg trichloroethylene/kg body weight (vehicle 0.2 ml of 25%
Tween 80 in saline) on alternate days, the activity of hepatic
microsomal NADPH cytochrome c reductase was increased. There
were no morphological changes in the liver (Lewis et al., 1984).
Studies conducted on rabbits showed that intramuscular
administration of 3 ml trichloroethylene, 3 times weekly for
29 days, induced neuronal damage (Bartonécek & Brun, 1970).
6.1.3. Long-term exposure
6.1.3.1. Oral exposure
The US NTP (1983) studied the effects of orally-administered
(in corn oil, by gavage) epichlorohydrin-free trichloroethylene in
male and female F344/N rats and B6C3F1 mice. Doses (500 and
1000 mg/kg body weight for rats and 1000 mg/kg for mice) were
administered 5 days per week for 103 weeks. The survival of
treated male rats and male mice was significantly reduced in
relation to that of corn-oil control animals. Mean body weights
of treated rats (both sexes) were lower than those of corn-oil
controls and the reduction in body weight gain was dose-related.
The body weights of treated female mice were similar to those of
vehicle controls.
Toxic nephrosis was found in 96/98 (98%) of the treated male
rats, in 97/97 of the treated female rats, in 45/50 (90%) of the
treated male mice, and in 48/49 (98%) of the treated female mice,
but was not found in any of the corn-oil control rats or mice.
Initially noted in rats that died early, the lesions were diagnosed
as frank enlargement of the nucleus and cytoplasm of scattered
individual tubular cells with brush borders, located near the
cortico-medullary junction. Progression of the lesions was
evident. As exposure time increased, affected tubular cells
were larger and additional tubules and tubular cells were affected.
Some tubules were enlarged or dilated to the extent that they were
difficult to identify as tubules. Eventually, there was loss of
some enlarged cells. Corresponding tubules became dilated and
portions of the basement membrane had a stripped appearance. In
the most advanced stage, the lesion had progressed to the sub-
capsular cortex, with enlarged tubular cells.
In mice, the pathological development of the renal lesion was
basically similar to that observed in rats, but it was relatively
less severe and did not develop to a stage where there was
extensive loss of cytomegalic epithelial cells and tubular
dilation.
When trichloroethylene was given in the drinking-water (0.1,
1.0, 2.5, and 5.0 g/litre) to CD-1 mice for 4 - 6 months, a
significant reduction in body weight (males and females at
5.0 g/litre), enlarged liver (males at 1.0, 2.5, and 5.0 g/litre;
females at 5.0 g/litre), and increase in kidney weight (males and
females at 5.0 g/litre) were observed. However, pathology at 4 and
6 months was unremarkable.
6.1.3.2. Inhalation exposure
Studies on rats showed that exposure to concentrations below
1080 mg/m3 (200 ppm) for 6 h a day, 5 days a week for 6 months, did
not induce any visible effects. At concentrations of 10 800 mg/m3
(2000 ppm), narcosis and loss of appetite were observed. At
16 200 mg/m3 (3000 ppm), only 33% of the test animals survived
(Taylor, 1936).
When NMRI mice were exposed to trichloroethylene at 700 mg/m3
(130 ppm) continuously for 30 days, liver weight increased by 1.5
and 1.9 times in males and females, respectively (Kanje et al.,
1981).
When rats were exposed to trichloroethylene at 1945 -
2270 mg/m3 (360 - 420 ppm), for 8 h a day, 5 days a week for
46 weeks, there were no changes in conditioned reflexes or reaction
time, but there was an increase in spontaneous climbing activity
(Bättig & Grandjean, 1963).
Rats exposed to 50 mg/m3 (0.05 mg/litre) for 5 h daily, for
3 months, showed damage to hepatic and renal parenchyma
(Kalashnikova et al., 1976).
Long-term exposure to concentrations of 100 - 200 mg/m3
(0.1 - 0.2 mg/litre) reduced the phagocytic activity of rat
leukocytes (Pelts, 1962).
In female Mongolian gerbils, exposed continuously to 270 and
810 mg/m3 (50 and 150 ppm) for 12 months, there were small changes
in the lipid composition of the cerebral cortex and hippocampus.
While protein content and lipid class distribution were virtually
unaffected, the cholesterol to phospholipid ratio in the cortex
decreased (Kyrklund et al., 1983).
6.1.3.3. Parenteral exposure
Of 6 rabbits given trichloroethylene at 200 mg/kg body weight
intramuscularly for 55 - 100 days, 2 died from renal failure
(Bartonécek & Brun, 1970). Rabbits given 2 ml (2.92 g)
intramuscularly twice a week for 41 - 247 days exhibited
neuronal damage (Bartonécek & Sovcek, 1959).
6.1.4. Interactions
Studies on rats showed that trichloroethylene was more toxic in
animals on a high-carbohydrate diet than in those on a high-protein
diet (Kalashnikova et al., 1974). In rats (Cornish & Adefuin,
1966), rabbits (Desoille et al., 1962), and human beings (Sbertoli
& Brambilla, 1962; Ferguson & Vernon, 1970; Pardys & Brotman,
1974), the presence of ethanol increased trichloroethylene
toxicity. Trichloroethylene and carbon tetrachloride acted
synergistically in producing hepatotoxicity (Deguchi, 1972;
Pessayre et al., 1982).
When trichloroethylene was administered intraperitoneally with
toluene, there was a decrease in the side-chain oxidation of the
toluene (Ikeda, 1974).
In studies by White & Carlson (1979), trichloroethylene
caused spontaneous, and potentiated epinephrine-induced, cardiac
arrhythmias in rabbits. A series of studies was conducted to
examine the effects of metabolic-inhibitors, enzyme-inducing
agents, or pre-treatment with ethanol or caffeine, on the
sensitivity of rabbits to epinephrine-induced cardiac arrythmias
(White & Carlson, 1979, 1981a, 1982). Rabbits treated with
metabolic-inhibiting agents developed more arrhythmias after
shorter exposure times in response to lower doses of epinephrine
than controls. Phenobarbitone and Aroclor 1254(R), and in a later
study benzo( a)pyrene (Carlson & Shite, 1983), were used as enzyme-
inducing agents. Phenobarbitone-treated rabbits developed
fewer cardiac arrhythmias and had lower blood levels of
trichloroethylene. Rabbits treated with benzo( a)pyrene developed
more cardiac arrhythmias and at lower doses of epinephrine when
exposed by inhalation to a trichloroethylene concentration of
43 500 mg/m3 (8100 ppm). Aroclor 1254(R) did not induce any
effects on trichloroethylene metabolism nor on the development of
epinephrine-induced cardiac arrhythmias. Rabbits pre-treated with
ethanol (1000 mg body weight, intravenously or orally) or caffeine
(10 mg/kg body weight, intraperitoneally) 30 min before exposure to
trichloroethylene at 32 400 mg/m3 (6000 ppm) by inhalation showed
an increased senstivity to epinephrine-induced cardiac arrhythmias.
Male New Zealand rabbits exposed to trichloroethylene at
10 800, 21 600, 32 400, or 43 200 mg/m3 (2000, 4000, 6000, or
8000 ppm) by inhalation for 1 h showed a concentration-related
sensitivity to epinephrine-induced cardiac arrhythmias. The blood
levels of the trichloroethylene metabolites (trichloroethanol and
trichloroacetic acid) were measured. Rabbits given chloral
hydrate (50 mg/kg body weight, intravenously), a trichloroethylene
intermediate metabolite, had blood levels of trichloroethanol and
trichloroacetic acid that were 40 - 100 times higher than those of
rabbits exposed to a trichloroethylene level of 43 200 mg/m3 (8000
ppm) but showed no increase in sensitivity to epinephrine-induced
cardiac arrhythmias (White & Carlson, 1981b). It is concluded that
trichloroethylene, rather than its metabolites, sensitizes the
rabbit myocardium.
6.1.5. Immunotoxicity
Changes in some components of antibody production (7S), cell-
mediated immunity (haemagglutination), and bone marrow stem cell
colonization have been observed in rodents (female mice were more
sensitive than males in one study; chinchillas were used in the
other) exposed to low-to-moderate levels (10 - 1000 mg/m3) by
inhalation or to 0.1 - 5 g/litre per day in the drinking-water for
periods ranging from a few weeks to 6 - 10 months (Shmuter, 1972;
Sanders et al., 1982).
6.1.6. Effects on cell systems
In vitro incubation of nerve-fibre membranes from rat spinal
cord with 10 µM trichloroethylene reduced the low relative
molecular mass protein fraction (Savolainen & Seppalainen, 1979).
A trichloroethylene concentration of 1.3/108 moles per ml was
the LD50 for HeLa (human cervix carcinoma) cells on the third day
of treatment (Gradiski et al., 1974).
Exposure of rabbit trachea cultures to trichloroethylene vapour
at 27 000 mg/m3 (5000 ppm) for 129 min and 216 000 (40 000 ppm) for
13 min, induced ciliostasis (Tomenius et al., 1979). The isolated
guinea-pig heart exposed to trichloroethylene at 530 mg/litre,
developed transitory arrhythmia. At a concentration of 1.06%,
there was evidence of a reduction in contractile force and coronary
flow, and arrhythmia (Bianchi et al., 1963; Matturro et al., 1963).
6.1.7. Carcinogenicity
Groups of 50 male and 50 female Osborne-Mendel rats, 7 weeks
old, were administered trichloroethylene (99% trichloroethylene
stabilized with 0.19% 1,2-epoxybutane and 0.09% epichlorohydrin) in
corn oil, by gavage, 5 days a week for 78 weeks. High-dose animals
received varying dose schedules of 1000 - 1500 mg/kg body weight
per day, and low-dose animals received 500 - 750 mg/kg body weight
per day. All surviving animals were killed 110 weeks after the
start of treatment. The time-weighted average doses were 549 and
1097 mg/kg body weight per day. A group of 20 male and 20 female
vehicle-treated rats served as controls. Of the males, 17/20
controls, 42/50 low-dose, and 47/50 high-dose animals died before
the end of the study; of the females, 12/20 controls, 35/48 low-
dose, and 37/50 high-dose animals died early. Median survival
times were approximately 60 weeks for high-dose males, 85 weeks
for low-dose males, and 70 weeks for high- and low-dose females.
Of the males, 5/20 controls, 7/50 low-dose, and 5/50 high-dose rats
developed tumours; of the females, 7/20 controls, 12/48 low-dose,
and 12/50 high-dose rats developed tumours. There were no liver-
cell tumours. Tumours occurred in various other organs in treated
and control animals and were mainly reticulum-cell sarcomas,
lymphosarcomas or malignant lymphomas, fibroadenomas of the
mammary gland, haemangiosarcomas at various sites, follicular
adenocarcinomas of the thyroid, chromophobe adenomas of the
pituitary, and renal hamartomas. Toxic nephropathy was observed
in rats of both sexes treated with high and low doses of
trichloroethylene (NCI, 1976).
Groups of 50 male and 50 female B6C3F1 mice, 5 weeks old,
were given trichloroethylene (99% trichloroethylene stabilized
with 0.19% 1,2-epoxybutane and 0.09% epichlorhydrin) in corn oil
by gavage, 5 days a week, for 78 weeks. High-dose males received
2000 - 2400 mg/kg body weight per day, and females 1400 - 1800
mg/kg body weight per day; low-dose males and females received
1000 - 1200 mg/kg body weight per day and 700 - 900 mg/kg body
weight per day, respectively. All surviving animals were observed
until they reached 95 weeks of age. Time-weighted average doses
were 1169 and 869 mg/kg body weight per day, respectively, in low-
dose males and females and 2339 and 1739 mg/kg body weight per day,
respectively, in high-dose males and females. Groups of 20 male
and 20 female mice served as vehicle-treated matched controls.
Survival was reduced in high-dose males and control males.
Hepatocellular carcinomas occurred in 1/20 control males and 0/20
control females; in 26/50 low-dose males and 4/50 low-dose females,
and in 31/48 high-dose males and 11/47 high-dose females.
Metastases of the liver-cell tumours to the lung were found in
7/98 treated males and in 1 control male. The first hepatocellular
carcinoma was observed in a mouse which had been treated with the
high dose of trichloroethylene and which died during week 27.
Lung tumours occurred in treated animals of both sexes: 5/50 (5
adenomas) in males and 4/50 (2 adenomas, 2 carcinomas) in females
in the low-dose group, and 2/48 (1 adenoma, 1 carcinoma) in males
and 7/47 (5 adenomas, 2 carcinomas) in females treated with the
high dose of trichloroethylene. Among controls, only one lung
adenoma was reported in a female (NCI, 1976).
In a study by Maltoni & Maioli (1977) and Maltoni et al. (in
press), groups of 30 male and 30 female, 13-week-old Sprague Dawley
rats were given trichloroethylene (highly purified and epoxide-
free, and stabilized with 20 ppm of butyl-hydroxytoluene) in olive
oil by gavage, 5 days a week, for 52 weeks. Two doses of
trichloroethylene were tested. The high-dose animals received
250 mg/kg body weight, and the low-dose animals 50 mg/kg body
weight; 30 males and 30 females received olive oil alone and served
as controls. The animals were kept until their spontaneous death
(the study lasted 140 weeks). There was no increase in specific
tumours. Abnormalities (cytokaryomegaly) in cells of the renal
tubules were observed in high-dose male rats. These changes were
not observed in females.
Groups of 30 female ICR Swiss mice and a group of 30 male
and 30 female mice of the same strain, were treated with
trichloroethylene dermally (1 mg in acetone), 3 times weekly, for
83 weeks, subcutaneously (0.5 mg in trioctanoin) once weekly for
89 weeks, and by gavage (0.5 mg in trioctanoin), once weekly, for
89 weeks. Similar-sized groups of animals served as controls. No
tumours were observed at the site of application (Van Duuren et
al., 1979). Similar negative findings were obtained following
thrice-weekly skin applications and once-weekly subcutaneous
injections of trichloroethylene oxide for life in a group of
70 female mice of the same strain (Van Duuren et al., 1983)
Groups of 30 male and 30 female Wistar rats, NMRI mice, and
Syrian hamsters were exposed by inhalation to trichloroethylene
(purified, with no detectable epoxides, and containing 15 ppm
triethanolamine as stabilizer) at 540 and 2700 mg/m3 (100 and
500 ppm). The animals were exposed for 6 h per day, 5 days per
week, for 78 weeks. The studies were terminated by killing the
surviving mice and hamsters after 130 weeks, and rats after 156
weeks. No carcinogenic effects were observed in rats, hamsters,
and male mice. A moderate increase in lymphomas was found in
treated female mice (18/28 at 2700 mg/m3; 17/30 at 540 mg/m3, and
9/29 in controls). The authors concluded that, on the basis of
these findings, there were no indications of carcinogenicity
(Henschler et al., 1980).
Groups of 49 - 50 female ICR mice, 4 weeks of age, were exposed
by inhalation to trichloroethylene (purity 99.8% with 0.128% carbon
tetrachloride, 0.019% benzene, 0.019% epichlorohydrin, and 0.01%
1,1,2-trichloroethane) at 0.270, 810, or 2430 mg/m3 (0, 50, 150,
or 450 ppm), 7 h per day for 5 days per week, for 104 weeks. The
surviving animals were killed 107 weeks after the start of the
study. Mortality was similar in control and treated groups.
Lung adenomas were found in 5, 2, 5, and 4 mice in the control,
low-dose, mid-dose, and high-dose groups, respectively. However,
it was reported that adenocarcinomas (none of which gave rise to
metastases) occurred in 1/49, 3/50, 8/50, and 7/46 mice in the
control, low-dose, mid-dose, and high-dose groups, respectively,
and that increased incidences in the mid- and high-dose groups,
were statistically significant compared with controls. In similar
studies on Sprague Dawley rats, no statistically-increased
incidence of tumours was detected; one clear-cell carcinoma of the
kidney was observed in the high-dose group (Fukuda et al., 1983).
In a large series of inhalation studies, highly purified
epoxy-free trichloroethylene (stabilized with 20 ppm butyl-
hydroxytoluene) was studied under controlled exposure conditions
in male and female Sprague Dawley rats and Swiss and B6C3F1 mice.
Treatment was for 7 h per day, 5 days per week for 8, 78, or 104
weeks. After the end of the treatment, all animals were kept under
observation until spontaneous death. The following 7 studies were
performed (Maltoni & Maioli, 1977; Maltoni et al., in press):
Study 1
Groups of 60 - 90 male and 60 - 90 female Sprague Dawley rats
were exposed to 0, 540, or 3240 mg trichloroethylene/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed. Cytomegaly and karyomegaly of tubular cells in the
kidney were not observed at either dose.
Study 2
Groups of 60 - 100 male and 60 - 100 female Swiss mice were
exposed to trichloroethylene at 0, 540, or 3240 mg/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed.
Studies 3 and 4
Groups of 90 - 95 male and 90 - 105 female Sprague Dawley rats
were exposed to trichloroethylene at 0, 540, 1620, 3240 mg/m3 (0,
100, 300, or 600 ppm) for 104 weeks. Further groups of 40 males
and 40 females were started on the same treatment 4 - 5 weeks later
(Study 4). The method and results of the 2 studies were similar
and the combined data are described. No increase in mortality was
observed in the treated animals. Cytomegaly and karyomegaly of
renal tubular cells were observed in males in the mid- and high-
dose groups and the incidence was dose-related; no such lesions
were observed in males in the low-dose group or in females at
any of the 3 dose levels, or in the controls. Five tubular
adenocarcinomas were observed in 4/130 males and 1/130 females
at the high-dose level. Leydig cell tumours of the testis were
observed in 6/135 controls, and in 16/130, 30/130, and 31/130
male rats, respectively, in the low-, mid-, and high-dose treated
groups. The average time of appearance of the tumours, from the
start of the study, was 113 weeks in controls and from 109 to
113 weeks in treated animals. A higher incidence of immunoblastic
lymphosarcomas was detected in male and female treated animals:
1/280 in controls, 9/260 at the low dose, 5/260 in the mid-dose
group, and 3/260 in the high-dose group.
Study 5
Groups of 90 male and 90 female Swiss mice were exposed to
trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0, 100, 300,
or 600 ppm) for 78 weeks. The incidence of hepatocellular
carcinomas in males was 4/90 in controls compared with 2/90 at the
low dose, 8/90 at the mid dose, and 13/90 at the high dose. One
hepatocellular carcinoma occurred in a female at the high dose.
The incidences of lung adenomas and adenocarcinomas were 25/180
in controls, 26/180 in low-dose groups, 36/180 in mid-dose, and
47/180 in the high-dose groups (males and females combined). Two
adenocarcinomas were seen in the control groups and 3 in the high-
dose group.
Studies 6 and 7
For study 6, groups of 90 male and 90 female B6C3F1 mice were
exposed to trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0,
100, 300, or 600 ppm) for 78 weeks. Because of excess mortality
due to fighting in the male groups, study 7 was started in which
trichloroethylene was tested in the same way in equal numbers of
male mice. In females, the incidence of pulmonary tumours (all
adenomas except one adenocarcinoma in the low-dose group) were 4/90
in controls compared with 6/90, 10/90, and 15/90 in the low-, mid-,
and high-dose groups, respectively. Hepatocellular carcinomas were
observed in 3/90 controls compared with 4/90, 4/90, and 9/90 in the
low-, mid-, and high-dose groups, respectively. In the males in
study 7, the incidence of pulmonary tumours was not affected by the
treatment. Hepatocellular carcinomas were observed in 14/90
controls compared with 19/90, 27/90, and 21/90 in the 3 treated
groups, respectively. Cytomegaly and karyomegaly were not observed
in either study (Maltoni et al., in press).
Groups of 50 male and 50 female rats (F344) and mice
(B6C3F1) were treated, by corn-oil gavage, with epichlorohydrin-
free trichloroethylene at 500 and 1000 mg/kg body weight (rats),
and 1000 mg/kg body weight (mice), 5 times weekly, for 103 weeks.
Similar-sized groups of male and female rats and mice treated with
corn oil alone, or without any treatment, served as controls. The
animals were killed between 103 and 107 weeks from the start of the
study. Trichloroethylene significantly reduced the survival of
male rats and mice. In male rats treated with trichloroethylene,
the incidence of tubular-cell neoplasms was increased (0/49,
untreated; 0/48, corn-oil control; 2/49, low dose; 3/49, high
dose). There was one tubular-cell tumour in a high-dose female
rat. Five low-dose male rats also had malignant peritoneal
mesotheliomas, compared with 1/50 untreated control, 1/50 vehicle
control, and 0/49 high-dose animals. Trichloroethylene also
produced nephrosis in both sexes of both species. In mice, the
administration of trichloroethylene increased the incidence of
hepatocellular carcinoma (males: 8/48, controls; 30/50, treated;
females: 2/48, controls; 13/49, treated). The incidence of
hepatocellular adenomas was also increased in male mice (3/48,
controls; 8/50, dosed) and in female mice (2/48, controls; 8/49,
dosed) (US NTP, 1983).
Groups of 50 male and 50 female Swiss mice were treated
by corn-oil gavage with daily doses of different samples of
trichloroethylene, initially at 2400 mg/kg body weight (males)
and 1800 mg/kg body weight (females), 5 days a week, for 78 weeks.
Due to toxicity, dosing and dose levels were reduced during the
study. The animals were kept under observation until
spontaneous death. The samples included: (a) highly purified
trichloroethylene stabilized with 0.0015% triethanolamine, (b)
industrial trichloroethylene (99.4% pure), (c) highly purified
trichloroethylene stabilized with 0.8% epichlorohydrin, (d) highly
purified trichloroethylene, with 0.8% 1,2-epoxybutane, and (e)
highly purified trichloroethylene with 0.25% epichlorohydrin and
0.25% epoxybutane. Similar-sized groups of each sex, treated with
corn oil alone, served as controls. Mortality was increased in
treated males and some treated female groups. No increase in
tumour incidence was observed except in groups (c) and (d) where
there was an increased incidence of forestomach cancers, attributed
to the direct alkylating properties of one of the two stabilizers
(Henschler et al., 1984).
6.1.7.1. Conclusions
Trichloroethylene (with or without epoxide stabilizers) caused
an increased incidence of hepatocellular carcinomas in 2 different
strains of mice, either when given by oral administration or by
inhalation.
Trichloroethylene also produced lung tumours in Swiss ICR mice
and in female, but not male, B6C3F1 mice.
Trichloroethylene, with added epichlorohydrin, administered by
gavage, caused an increased incidence of forestomach cancers in
WMRI mice.
Trichloroethylene (epoxide stabilizer-free) produced a low
incidence of renal tubular tumours in 2 different strains of mice
(mainly in males) following long-term oral or inhalation exposure.
These tumours occur very rarely in untreated rats of these strains.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure.
In one strain of mice and one strain of rats, epoxide
stabilizer-free trichloroethylene produced some increases in the
incidence of lymphomas. However, these data are insufficient to
draw any conclusions.
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats.
It was noted in the evaluations by IARC (1979, 1982) that
it was considered that there was limited evidence for the
carcinogenicity of trichloroethylene for experimental animals.
The significance of these findings needs to be evaluated in
the context of further studies on the mechanism of action of
trichloroethylene.
6.1.8. Mutagenicity
6.1.8.1. Gene mutation
(a) Bacteria and fungi
Pure trichloroethylene induced revertants (gene-mutations) in
the K-12 strain of Escherichia coli in the presence of fortified
mouse Arochlor(R)-induced microsomal preparations for only one gene
out of four analysed. In the positive case, the induced effect was
the doubling of spontaneous background (Greim et al., 1975).
Trichloroethylene (of unspecified purity) in vapour form was
slightly mutagenic for the TA-100 strain of Salmonella typhimurium,
in the presence of S9 mix obtained from mouse liver (control
value = 140 revert./plate; treated values: 240 revert./plate)
(Simmon et al., 1977).
Technical grade trichloroethylene (containing 1900 ppm of
1,2-epoxybutane and 900 ppm of epichlorohydrin) was mutagenic for
the TA-100 strain of S. typhimurium in a desiccator, in the absence
of a metabolic activation system (untreated 161 - 187 revert./plate;
treated: 567 - 651 revert./plate). Trichloroethylene of high
purity was not mutagenic for the TA 100 strain of S. typhimurium
in the presence of 0.5 ml of S9 mix obtained from mouse, rat, and
hamster liver; it was, however, mutagenic for this strain of
S. typhimurium in the presence of 50 - 150 µl of S9 mix from
Arochlor-treated rat liver (untreated series: 160 - 180
revert./plate; treated series: 350-379 revert./plate) (Crebelli
et al., 1982).
High-purity trichloroethylene was not mutagenic for the TA 100
strain of S. typhimurium in a plate assay, with and without
addition of S9 mixture (Henschler et al., 1977).
Trichloroethylene containing no traces of 1,2-epoxybutane or
epichlorohydrin was tested as a gas in a desiccator on the TA 1535
and TA 100 strains of S. typhimurium. Concentrations of 1 - 3%
produced a 30% increase (less than a doubling) in revertants in
strain TA 100 in the presence of a metabolic activation system from
Arochlor(R)-induced rat liver (Baden et al., 1979).
The exposure of S. typhimurium strains TA 98 and TA 100 to
0.5 - 10% vapour concentrations of trichloroethylene in sealed
vials for 48 h did not produce an increase in revertants either in
the presence or absence of rat liver homogenate (Waskell, 1978).
Trichloroethylene of high purity (99.5%) was mutagenic for the
TA-100 strain of S. typhimurium when used as a gas in a desiccator
in the presence of S9 mix (Bartsch et al., 1979). However, it
should be noted that the increase in revertants in the treated
series was less than twice that of the untreated series, but this
slight effect was consistent in repeated experiments.
Trichloroethylene of unspecified purity was reported to double
the spontaneous frequency of reverse mutations in the D-7 strain of
the yeast Saccharomyces cerevisiae, at one concentration, in the
presence of an endogenous metabolic activation system (Callen et
al., 1980).
Trichloroethylene containing impurities, which were not
specified, was mutagenic for S. cerevisiae, strain XV185-14C
(reverse mutations) in the presence of S9 mix. These results
were obtained at a level of yeast cell % survival of < 1 (1 h of
treatment) and of < 0.1 (4 h of treatment). These conditions are
unusual for the detection of mutagenic effects, since, at these
toxic values, selection of different cells (wild type and mutants)
is very efficient (Shahin & von Borstel, 1977). One of the authors
(von Borstel, personal communication, 1984) stated that, when these
studies were repeated with a pure trichloroethylene sample, it was
not mutagenic for yeast cells.
Trichloroethylene of high purity (99.8%), as well as
trichloroethylene containing stabilizers such as 1,2-epoxy-butane
(0.19%) and epichlorohydrin (0.09%), were not mutagenic for the
yeast S. pombe (forward mutations), with or without a metabolic
system obtained from rats and mice treated with phenobarbital and
beta-naphthoflavone. Using intraperitoneal or intrasanguineous,
host-mediated assay techniques with male mice inoculated with the
yeast S. pombe and treated orally with 2 g/kg body weight for
4 or 16 h, both pure trichloroethylene and the stabilized
trichloroethylene were found not to be mutagenic (Rossi et al.,
1983).
A slightly mutagenic action for cells of S. cerevisiae strain
D7 (reverse mutation at his locus) was found for trichloroethylene
of unspecified purity in the presence of a metabolic activation
system obtained from mice (untreated series: 0.79 - 0.89 his,
rev/106; treated series: 3.00 - 3.60 his rev/106 with 40 mol).
For this experiment, no survival values were reported, nor were the
actual number of revertants counted, and therefore the observed
effect cannot be definitely assigned to trichloroethylene
(Bronzetti et al., 1978). The same authors administered
trichloroethylene (unspecified purity) orally to mice (400 mg/kg
body weight) that were also inoculated with yeast cells intra-
sanguineously; a mutagenic effect was found on yeast cells (reverse
mutations at the his locus) collected from liver, lung, and kidney
but, again, the survival level was not reported nor was the number
of revertants counted (Bronzetti et al., 1978).
Trichloroethylene of high purity and containing known
contaminants (~80 ppm) was found to be active for the production
of reverse mutations (suppressor mutations) on Aspergillus
nidulans sumeth A1 strain at a dose of 27 000 mg/m3 (5000 ppm)
applied in a desiccator containing the plates inoculated with the
fungal conidia (Crebelli et al., in press).
(b) Mammalian cells (in vitro)
A pure sample of trichloroethylene, stabilized with thymol,
did not induce mutations (forward mutations) at the HPRT locus of
Chinese hamster V-79 cell line treated in vitro with or without
metabolic activation (S9 mix) (Loprieno & Abbondandolo, 1980).
(c) Mammals (in vivo)
Trichloroethylene (purity 99.5%) was evaluated for its ability
to induce somatic gene mutations in vivo in embryonic fibroblasts
of mice (spot test). Pregnant mice were injected 12 days after
copulation with a dose of 140 mg/kg body weight (50 females), or
350 mg/kg (26 females). The results observed were 2/145 (1.4%)
and 2/51 (3.9%) of progeny with "spots", respectively, whereas
the untreated series produced 0/146, 3/182 (1.6%), 6/794 (0.75%).
These results were considered by the authors to indicate a
mutagenic effect (Fahrig, 1977); however, positive data such as
those observed for trichloroethylene are among spontaneous values
for this test system.
6.1.8.2. Chromosome aberrations
(a) Mammals (in vivo)
Trichloroethylene (purity not given), repeatedly administered
intraperitoneally to ICR mice for 5 days at a dose equivalent to
half of the LD50, did not induce a significant increase in
chromosome aberration frequency in bone marrow cells from animals
killed 6, 24, and 48 h after the last treatment (Cerna & Kjpenova,
1977).
A single dose of trichloroethylene of 1000 mg/kg body weight
(stabilized with thymol), administered orally to Swiss albino
mice, did not induce a significant increase in the frequency of
chromosome aberrations in bone marrow, 24 h after treatment
(Loprieno & Abbondandolo, 1980).
The exposure of male mice (NMRI-Han/BGA) to trichloroethylene
vapour (99.5% purity) for 24 h at concentrations of 272, 1090, and
2430 mg/m3 (50, 202, and 450 ppm) did not induce dominant lethal
mutations (Slacik-Erben et al., 1980).
CDI male mice were treated orally with 3000, 2250, 1500,
1125, 750, or 375 mg/kg body weight (2 treatments in 24 h) with a
trichloroethylene sample of high purity (99.5%) and sacrificed 6 h
following the second treatment, for the analysis of the frequency
of micronucleated erythrocytes in the bone marrow cells. The
results indicated a positive dose-related effect of
trichloroethylene (Duprat & Gradiski, 1980).
Using high purity (99.8%) trichloroethylene, B6C3F1 mice (10
female and 10 male) were treated by inhalation with 3240 mg/m3
(600 ppm), for 7 h a day, 5 days a week, for a total of 52 days.
The animals were killed 6 h and 24 h after the last treatment. The
cytogenetic analysis showed the presence only of gaps without any
indication of induced chromosomal aberrations (Loprieno, personal
communication, 1984).
When trichloroethylene of high purity (99.8%) was administered
to B6C3F1 male mice, by gavage, in a single dose of 1200 mg/kg
body weight, an increased frequency (4 times) of micronucleated
erythrocytes in the bone marrow cells compared with control animals
was observed, 42 h after dosing (Sbrana et al., 1984).
6.1.8.3. DNA damage
Fungi
Trichloroethylene (unspecified purity) was positive for
the induction of mitotic gene conversion at the trp locus and
mitotic recombination in the D7 strain of S. ceverisiae, under
endogenous metabolic activation conditions (Callen et al., 1980).
Trichloroethylene (unspecified purity) was also found to be
positive for the induction of mitotic gene conversion at the trp
locus of S. cerevisiae D7 strain treated in vitro in the presence
of a metabolic activation system obtained from rat liver. Using
the host-mediated assay technique, and a single oral dose of
400 mg/kg body weight, given to mice inoculated with yeast cell
D4 strain of S. cerevisiae, a positive effect on the induction of
mitotic gene conversion at the trp 5 and ade 2 loci was observed
in the yeast cells recovered from the kidney. Repeated oral
administration of trichloroethylene to male mice, with a total
dose of 3700 mg/kg body weight, induced a positive effect (mitotic
recombination) in yeast cells recovered from the liver and kidney.
In all these studies, the actual numbers of the convertant or
recombinant colonies were not given, and the significance of the
results is questionable (Bronzetti et al., 1978).
Trichloroethylene (stabilized with thymol) was considered
inactive for the induction of mitotic recombinations in the D4
strain of S. cerevisiae following in vitro and in vivo (host-
mediated assay) studies (Loprieno & Abbondandolo, 1980).
Trichloroethylene of high purity (99.9%) induced somatic
segregants in A. nidulans strain 35 x 17, at concentrations of
40 500 and 81 000 mg/m3 (7500 and 15 000 ppm), in a desiccator
containing the plates inoculated with the fungal conidia; under
identical doses and conditions, no mitotic recombinant colonies
were induced in this strain of A. nidulans by trichloroethylene
(Crebelli et al., in press).
6.1.8.4. Mammalian cells ( in vitro)
Trichloroethylene of high purity (99.9%) induced morphological
transformations, in vitro, in rat embryo cell cultures (Price et
al., 1978).
Stabilized with thymol, trichloroethylene did not stimulate
unscheduled DNA synthesis in an HeLa human cell line grown in
vitro, in the presence or absence of a metabolic activation
system (S9 mix) (Loprieno & Abbondandolo, 1980). Trichloroethylene
(unspecified purity) elicited DNA repair synthesis in human
lymphocytes in the presence of S9 mix (Perocco & Prodi, 1981).
Exposure of Chinese hamster ovary cells (CHO) to
trichloroethylene bubbled into the growth medium for 2 min,
corresponding to a dose of 0.17% v/v for 1 h treatment time,
did not induce sister-chromatid exchange (White et al., 1979).
Trichloroethylene bound covalently to calf thymus DNA in vitro
in the presence of a rat liver metabolic activation system (Di
Renzo et al., 1982; Bergman, 1983).
When given ip to NMRI mice, twice daily for 5 days
(33.6 mg/kg), trichloroethylene bound to RNA and DNA of brain,
lung, liver, kidney, spleen, pancreas, and testis (Bergman, 1983).
6.1.8.5. Mutagenic activity of trichloroethylene metabolites
Trichloroethylene oxide was not mutagenic for the TA 1535
strain of S. typhimurium or the WP2 UVRA strain of E. coli (plate
test), but was positive in the DNA repair test on E. coli (Kline
et al., 1982).
The same compound was found to be a direct mutagen for the
yeast S. pombe (forward mutation) and for mammalian cells grown
in vitro (V-79 cell line) (forward mutation) (Loprieno &
Abbondandolo, 1980).
Syrian golden hamster embryo cells were exposed to
trichloroethylene oxide (1.1, 2.5, and 5.0 µM) at 37 °C for 30 min.
After 5 days, the number of transformed colonies was scored. A low
but dose-related increased frequency of transformed cells was
induced (Di Paolo & Doniger, 1982).
Chloral hydrate has been reported to have direct genotoxic
activity in S. typhimurium (Waskell, 1978) and in A. nidulans
(Bignami et al., 1980).
At doses of 82.7, 165.4, or 4135 mg/kg body weight, chloral
hydrate, administered intraperitoneally, induced chromosome non-
disjunction in the secondary spermatocytes of mice (Russo et al.,
1984).
6.1.8.6. Conclusions
Pure trichloroethylene acts as a weak mutagen in S. typhimurium
in the presence of a metabolic activation system. However, in all
cases, the induced effect was never more than twice the spontaneous
value. Technical grade trichloroethylene containing stabilizers
such as 1,2-epoxybutane epichlorohydrin, is mutagenic in
S. typhimurium in the absence of a metabolic activation system.
Pure trichloroethylene is not mutagenic for the yeast S. pombe,
with or without metabolic activation, in vitro or in vivo (host-
mediated assay).
Thymol-stabilized trichloroethylene is not mutagenic for V-75
hamster cells, with or without metabolic activation.
Trichloroethylene (99.5% pure) is a weak mutagen for the
induction of somatic mutations in mouse embryo.
In mice, a single oral dose of trichloroethylene (1 g/kg body
weight) or repeated exposure to 3240 mg/m3 (600 ppm) for 52 days,
7 h/day, through inhalation, did not increase chromosome
aberration frequency in bone-marrow cells. A half-LD50 dose
of trichloroethylene, administered ip to mice, did not increase
chromosomal aberration frequency in bone-marrow cells. In a
micronucleus test, a single oral treatment with 1200 mg/kg body
weight or repeated (twice) oral treatment with a series of doses
ranging from 375 to 3000 mg/kg body weight increased the frequency
of induced micronucleated erythrocytes in the bone-marrow cells of
mice. Trichloroethylene bound to DNA in vitro and in vivo to
mouse tissues.
Dominant lethal mutations were not induced in mice exposed
through inhalation to trichloroethylene vapours.
Trichloroethylene induces mitotic gene conversion in yeast
cells grown under conditions allowing an endogenous metabolic
activation system to function. The same effect reported in yeast
in the presence of an exogenous metabolic activation system
(S9 mix), or in the host-mediated assay, is questionable, because
of inadequate data and the fact that other studies produced
negative data.
A positive effect for the induction of unscheduled DNA
synthesis (UDS) in human lymphocytes, treated in vitro, was
reported by Perocco & Prodi (1981), whereas a negative response
for the same biological effect (UDS) in a human fibroblast cell
line (HeLa) grown in vitro was observed by Loprieno & Abbondandolo
(1980).
Trichloroethylene induces morphological transformation in rat
embryo cells.
The available results, shown in Table 9, are not adequate
for a complete evaluation of the genotoxic potential of
trichloroethylene. Only a few mutagenicity studies have indicated
the grade and purity of the trichloroethylene sample employed.
The confusing results could be due either to the use of
trichloroethylene samples stabilized with mutagenic compounds or to
the use of pure trichloroethylene samples which, unstablized, can
rapidly decompose to chemicals with mutagenic activity.
6.1.9. Reproduction, embryo/fetotoxicity, and teratology
The embryo/fetal toxicity and teratogenic potential of
trichloroethylene have been evaluated in the avian embryo system,
the mouse, rat, and rabbit. Reproductive function has been
examined in male rats.
6.1.9.1. Avian embryo system
Exposing avian embryos to 1% trichloroethylene (10 g/litre)
caused a significant increase in embryonic death and a slight
increase in anomalies (Fink, 1968). These observations were made
on the 3rd day of development, a time at which frank malformations
would be difficult to observe or evaluate. Elovaara et al. (1979)
reported the results of a comparative study of the teratogenic
potential of trichloroethylene and other aliphatic chlorinated
hydrocarbons in the chick embryo. Trichloroethylene (reagent
grade) in doses ranging from 5 to 1000 µmoles in olive oil (25 µl)
was injected into the air space of fertilized eggs on the 2nd and
6th days of incubation and examined on the 14th day. The LD50 for
trichloroethylene was between 50 and 100 µmoles/egg. Macroscopic
embryonic malformations occurred in 9 out of 55 surviving embryos,
5 times the incidence in the vehicle control eggs.
In another study, injection of low doses of trichloroethylene
(1, 5, 10, and 25 µmol/egg), on days 1 and 2 of embryogenesis,
resulted in embryotoxicity, growth defects, and morphological
anomalies (Bross et al., 1983).
Table 9. Summary of the mutagenicity data reported for trichloroethylene
-----------------------------------------------------------------------------------------
Organism Genotoxic effects
Gene Chromosome DNA
mutation anomalies damage
-----------------------------------------------------------------------------------------
prokaryotes weak
positive
-----------------------------------------------------------------------------------------
fungi negative ( in vitro) positive negative ( in vitro)
negative (hma) mitotic weak (hma)
positive? ( in vitro) segregation positive (yeast
positive (A. nidulans) A. nidulans metabolism)
-----------------------------------------------------------------------------------------
mammalian negative negative (SCE)
cells negative (DNA
( in vitro) repair synthesis)
positive (covalent
binding)
-----------------------------------------------------------------------------------------
mammals weak negative (1 dose) (1 g/kg body
( in vivo) weight, oral)
negative (52 doses) (3240 mg/m3,
inhalation)
negative (5 doses) (1/2 LD50, ip)
positive (micronuclei) 1200 mg/kg
positive (micronuclei) (375 -
3000 mg/kg)
negative (dominant lethals) (270,
1090, 2430 mg/m3, inhalation)
-----------------------------------------------------------------------------------------
For human cases, see Table 10.
6.1.9.2. Mouse
Swiss-Webster mice were exposed by inhalation to
trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h daily, on days
6 - 15 of gestation (Day 0 = vaginal plug). The mice were observed
daily throughout pregnancy. Maternal body weights were recorded on
days 6, 10, and 18 of gestation as an index of maternal toxicity.
On day 18, no effect was observed on the average number of
implantation sites/litter, litter size, the incidence of fetal
resorptions, fetal sex ratios, or fetal body measurements (Schwetz
et al., 1975). The incidence of gross anomalies observed by
external examination was not significantly greater than that among
the control litters. Trichloroethylene exposure did not exert any
effect on the incidence of skeletal anomalies in mice, and
microscopic examination did not reveal any abnormalities in organs,
tissues, or cells (Leong et al., 1975; Schwetz et al., 1975).
6.1.9.3. Rat
In studies similar to the preceding mouse study, exposure to
trichloroethylene at 1620 mg/m3 (300 ppm) did not produce any
evidence of maternal toxicity or teratogenicity in Sprague Dawley
rats (Leong et al., 1975; Schwetz et al., 1975) or Charles River
strain rats (Bell, 1977).
Exposing Sprague Dawley rats to trichloroethylene at 2700 mg/m3
(500 ppm) for 7 h per day, 5 days per week, during a 3-week
pregestational period, and 7 h per day each day for days 0 - 18 and
days 6 - 18 of gestation did not cause maternal or embryo/fetal
toxicity. Beliles et al. (1980) concluded that the frequency and
the character of the macro- or microscopic findings in the treated
groups did not indicate either adverse fetal effects or that
trichloroethylene, at the dose used, was teratogenic.
Dorfmueller et al. (1979) reported that female Long-Evans rats
exposed to trichloroethylene at 9720 mg/m3 (1800 ppm) before
mating, for 6 h per day, 5 days per week, for 2 weeks and/or during
pregnancy, for 6 h per day, each day, for 0 - 20 days of gestation)
did not exhibit any signs of maternal toxicity. There was no
indication of embryotoxicity, and no significant treatment effects
or interactions were found in the number of corpora lutea or
implantation sites/litter, fetal body weights, resorbed
fetuses/litter, or sex ratios. No significant treatment effects
were observed in the analysis of total soft-tissue anomalies.
However, prenatal exposure to trichloroethylene at 9720 mg/m3
(1800 ppm) caused an increase in minor anomalies of the offspring
(incomplete ossification of the sternum) indicative of development
delays in maturation, but not of major malformation.
While the study did not indicate any treatment-related effects
on general post-natal behaviour, there was a small but significant
regression in post-natal weight gain in offspring in the pre-mating
exposure group.
In groups of male rats dosed by gavage with trichloroethylene
at 10, 100, or 1000 mg/kg body weight 5 days per week, for 6 weeks,
there was no evidence of spermatotoxic effects. Trichloroethylene-
related effects of reduced weight gain and elevated liver/body
weight ratios were seen primarily in the 1000 mg/kg group.
Copulatory behaviour was initially diminished by the narcotic
properties of trichloroethylene, but was normal by the 5th week
(Zenick et al., 1984).
6.1.9.4. Rabbit
Female New Zealand white rabbits were exposed by inhalation to
trichloroethylene at 2700 mg/m3 (500 ppm), for 7 h per day, 5 days
per week, during a 3-week pregestation period, and daily, for days
0 - 21 and days 7 - 21 of gestation (Day 0 = mating day). There
was no evidence of maternal toxicity or embryotoxicity. The
occurrence of hydrocephalus in a few fetuses in one of the study
groups was reported (4 fetuses in 2 litters), but no definitive
conclusion was drawn from these findings (Beliles et al., 1980).
7. EFFECTS ON THE ENVIRONMENT
7.1. Aquatic Organisms
There is little information on the toxicity of
trichloroethylene for fish. The US Registry of Toxic Effects of
Chemical Substances (Christensen & Lugenbyhl, 1975) reports, for an
unidentified species, that exposure to a concentration range of
100 - 1000 mg/litre produced toxic effects in 96 h. Toxicity tests
carried out on salt-water flatfish, Limanda limanda (sole), 15 -
20 cm long, in a continuous water flow, established a 96-h LC50
of 16 mg/litre (Pearson & McConnell, 1975). A 96-h LC50 of
approximately 40 mg/litre (static) or 67 mg/litre (continuous flow)
has been reported for the minnow Pimephales promelas (Alexander et
al., 1978).
Verschueren (1977) established an LC100 of 600 mg/litre for
Daphnia magna. The LC50 for the balanide salt-water crustacean
nauplius (larva) (Elminius modestus) was 20 mg/litre after 46 h
(Pearson & McConnell, 1975), and the LC50 for the protozoon
Entosiphon sulcatum was established as 1200 mg/litre (Bringmann
& Kuhn, 1980).
Various LC50 values have been established for algae including
63 mg/litre for Microcystis aeruginosa, (Verschueren, 1977); a
concentration of 1000 mg/litre did not have any observable effect
on Scenedesmus quadricauda (Bringmann & Kuhn, 1980). A short-term
photosynthesis efficiency test gave an LC50 of 8 mg/litre
(Pearson & McConnell, 1975) and, finally, in tests carried out on
Thalassiosira pseudonana and Dunaliella tertiolecta, there were
observable effects at 50 and 100 µg/litre, in a mixed culture
(Biggs et al., 1979).
7.2. Uptake, Distribution, Storage, Metabolism, and Elimination in
Plant and Animal Organisms
Bioaccumulation of trichloroethylene in a marine environment
has been studied by Pearson & McConnell (1975); concentration
levels were determined for a wide variety of marine organisms,
mostly in the Bay of Liverpool.
The greatest increase in trichloroethylene concentrations in
the tissues of animals that are relatively high up in the food
chain (birds' eggs, fish liver, and seal fat) was nearly 100 times
the level in water (from 0.5/109 µg/litre in water to 50/109 µg/kg
in tissues).
These data agree with the laboratory findings of Barrows et
al. (1980) who, in a 14-day test, noted that trichloroethylene
accumulation in the sunfish species, Lepomis macrochirus was 17
times that of the aquatic medium with a halving time of less than
one day. A low bioconcentration factor (concentration in organism
divided by concentration in environment) (Uehleke et al., 1977)
has been derived using water solubility and the equation proposed
by Kenaga (1980) and Kenaga & Goring (1980).
7.3. Effects on the Stratospheric Ozone Layer
Consideration has been given to the possibility that
trichloroethylene, together with other halocarbons in the
atmosphere, may contribute to the depletion of the stratospheric
ozone layer, which would lead to atmospheric heating and increased
exposure of terrestrial biota to ultraviolet radiation (Molina &
Rowland, 1974). Atmospheric trichloroethylene concentrations seem
to be about one-fifth to one-tenth of those of other chlorocarbons
such as CH3CCl3, CH2Cl2, CCl4, or C2Cl4 or the major chlorofluoro-
carbons (Cronn et al., 1977; Penkett, 1982). The reason for this
is that while trichloroethylene emissions into the atmosphere are
of the same order as those of other halocarbons (Jesson, 1980),
trichloroethylene is efficiently scavenged by hydroxyl radicals
in the troposphere, and the reaction rate for this process is
appreciably faster for trichloroethylene than for other halocarbons
(Penkett, 1982). Thus, the predicted atmospheric lifetime for
trichloroethylene is short (about 10 - 11 days) (Derwent &
Eggleton, 1978; Graedel, 1978) compared with those for the
chlorofluorocarbons, which may be 10 years or more (Jesson, 1980).
It is not clear whether trichloroethylene is even present in the
stratosphere (Cronn et al., 1977). However, the data suggest that
trichloroethylene is unlikely to be involved in the possible
depletion of the ozone layer.
8. EFFECTS ON MAN
8.1. General Symptoms and Signs
8.1.1. Acute effects
Sorgo (1976) reported a lethal oral dose for trichloroethylene
to be 7 g/kg body weight, and Lazarev et al. (1977) reported a
fatality after the oral ingestion of less than 50 ml.
At exposure concentrations of 270 - 540 mg/m3 (50 - 100 ppm),
the most common symptoms are headache, sluggishness, sleepiness
(especially at the end of the workshift), dulling of senses,
dizziness, nausea, and vomiting (Rubino et al., 1959; Lilis et
al., 1969; Governa, 1981). At narcotic doses, vomiting may occur.
Trichloroethylene has been used as an analgesic and as an
anaesthetic on its own or in mixtures. It is now much less used
for this purpose, as safer and more effective alternatives are
available. Inhalation of 27 000 mg/m3 (5000 ppm) produces light
anaesthesia; concentrations of up to 108 000 mg/m3 (20 000 ppm)
have been used for deeper anaesthesia (Wade, 1977).
Clinical signs are not very specific. There can be enhanced
responsiveness to caloric stimulation of the labyrinth and some EEG
anomalies may be detected (Chiesura, 1980) (section 8.2). Acute
hepatic and renal toxicity has been reported (Sucui & Olinici,
1983).
A particular intolerance to ethanol may occur after ingesting
even small amounts of alcoholic beverages. This intolerance is
characterized by intense cutaneous vasodilatation, particularly in
the face, often called "degreaser's flush" (Stewart et al., 1974).
Non-specific effects on the digestive system (e.g., dyspepsia,
gastritis, and diarrhoea) have been reported in cases of suicidal
or accidental ingestion of trichloroethylene (Bozza Marrubini et
al., 1978; Governa, 1981).
8.1.2. Chronic effects
The existence of a condition of chronic trichloroethylene
poisoning is not clear (Chiesura, 1980; Boudène et al., 1983).
However, chronic effects in human beings can occur following
prolonged exposure to moderate concentrations of trichloroethylene
of about 540 mg/m3 (100 ppm). The clinical picture is mainly
related to the central nervous system (CNS), and consists of
asthenia, anorexia, headache, loss of memory, moodiness,
depression, insomnia, paraesthesia, and disturbances of the
autonomic nervous system such as hyperhydrosis, tachycardia, and
dermographism (Ahlmark & Forssman, 1951a,b; Bardodej & Viskocil,
1956; Rubino et al., 1959; Lerza et al., 1963; Castellino, 1969).
The role of trichloroethylene in inducing liver damage in
occupationally-exposed human beings is not clear (Parmeggiani,
1956; Capellini & Grisler, 1958; Rubino et al., 1959; Tolot et al.,
1964; Schuttmann, 1970; Lachnit, 1971; Thiele, 1982; Sucui &
Olinici, 1983; Svabova & Mencik, 1983).
Human subjects with high repeated, but non-occupational,
exposure may exhibit toxic effects on the liver (e.g., elevated
aspartate (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2)),
renal insufficiency, and abnormal EEG patterns (Baerg & Kimberg,
1970). Following accidental or suicidal ingestion (e.g., doses of
100 - 300 ml), hepatic necrosis and nephropathy have been found in
autopsy cases (Keinfeld & Tabershaw, 1954; Graovac-Leposavic
et al., 1964; Priest & Horn, 1965; Beisland & Wannag, 1970;
Clearfield, 1970). A decrease in sexual potency has been reported
in industrial workers exposed to trichloroethylene (Bardodej &
Vyskocil, 1956; Lazarev & Gadaskiva, 1977).
Trichloroethylene is one of the volatile solvents inhaled
("sniffed") for its euphoric effect (Hayden et al., 1976), and
abuse by oral self-administration has been reported (Wells, 1982).
Centrilobular hepatic necrosis and renal toxicity have occurred in
addicts (Baerg & Kimberg, 1970; Clearfield, 1970), and deaths have
been reported (James, 1963; Musclow & Awen, 1971). Some mixtures
sold commercially as trichloroethylene may contain other solvents,
including carbon tetrachloride, which can contribute to the toxic
manifestations (Bouyges et al., 1980; Conso et al., 1980).
Dependent patients may show withdrawal symptoms (Ikeda et al.,
1971).
8.2. Effects on Organs and Systems
8.2.1. Effects on the nervous system
Acute effects on the central nervous system (CNS) are
characterized by two sequential phases (i.e., excitation and
depression), and are usually reversible. These symptoms generally
prevail over those of other systems (Stewart et al., 1962;
Chiesura, 1980; Governa, 1981).
In the early phase of excitation, euphoria and inebriation are
present. The subsequent phase of CNS depression is characterized
by various degrees of narcosis culminating in coma (Caccuri, 1976;
Chiesura, 1980). Muscular hypotony, muscular spasms, reduced
tendon reflexes, and loss of co-ordination may occur (Huff, 1971).
Changes in EEG patterns may vary considerably. The EEG may
be normal in some cases, while substantial alterations, regressing
after a few days, may be observed in others. The most frequently
observed change is a decrease in alpha activity, which is often
irregular. Widespread rapid rhythms or low theta activity may also
be present (Chiesura, 1980).
Exposure to trichloroethylene at a concentration of 540 mg/m3
(100 ppm) produces various results on the CNS. Though there may
not be any evidence of effects on psychomotor capacities (Stewart
et al., 1970), reductions in mental performance evidenced by the
test for perception, past recall, answering speed, and response
and manual dexterity tests have been reported (Ferguson & Vernon,
1970). Visual and auditory evoked potentials were affected at
exposure levels ranging between 270 and 540 mg/m3 (50 and 100 ppm),
for 3 1/2 - 7 1/2 h (Winneke, 1982).
The behavioural effects of exposure to trichloroethylene have
been studied under laboratory and work-place conditions. Laboratory
exposure to 540 or 1080 mg/m3 (100 or 200 ppm), for 70 min, had no
effect on reaction time or short-term memory (Gamberale et al.,
1976). Volunteers exposed to 540, 1620, or 5400 mg/m3 (100, 300,
or 1000 ppm), for 2 h, showed significant impairment in a number
of psychomotor tests at 5400 mg/m3 but not at 1620 or 540 mg/m3
(Vernon & Ferguson, 1969). In a further study (Ferguson & Vernon,
1970), effects were again found at 5400 mg/m3 and marginal effects
at 1620 mg/m3. In another study, exposure of volunteers to 810
or 1620 mg/m3 (150 or 300 ppm), for 2 1/2 h, did not produce
any significant impairment in behavioural test results (Ettema
& Zielhuis, 1975). With repeated exposure under work-place
conditions, no behavioural effects were observed with mean
atmospheric levels of 270 mg/m3 (50 ppm) (Triebig et al., 1977a,b).
In a study of complex reaction time in an occupational group with a
mean exposure of 1320 mg/m3 (245 ppm), reaction time was increased
in comparison with that in a control group (Gun et al., 1978).
Motor nerve conduction velocity was not affected in a group
occupationally exposed to mean atmospheric levels of less than
270 mg/m3 (50 ppm) (Triebig et al., 1982).
A study on 122 exposed workers (Ahlmark & Forssman, 1951a)
showed that symptoms of toxicity in human beings were correlated
with urinary levels of metabolic trichloroacetic acid. With
urinary concentrations of up to 20 mg trichloroacetic acid/litre,
no particular symptoms were noted. With urinary levels ranging
between 45 and 75 mg/litre, headache, fatigue, increased need for
sleep, irritability, and alcohol intolerance were reported in 50%
of workers. When urinary levels of trichloroacetic acid exceeded
300 mg/litre, these symptoms were found in 100% of subjects.
Initial nervous-system signs of toxicity were reported to be
associated with the urinary excretion of trichloroacetic acid
at 75 mg/litre and 30 mg/litre (Lazarev & Gadaskiva, 1977).
Two types of irreversible neurotoxic effects have been reported
in man. One is the specific degeneration of the cranial nerves, in
particular the trigeminal, the olfactory, and the facial nerves
(Goldblatt, 1956; James, 1963; Kjellstrand et al., 1980; Barrett
et al., 1982). However, it is considered that this effect is
not produced by trichloroethylene itself but by its decomposition
product, dichloroacetylene (Henschler et al., 1970a). In addition,
polyneuropathies involving the whole peripheral nervous system have
been described after long-term exposure to trichloroethylene
(Szulc-Kuberska, 1972).
8.2.2. Effects on the cardiovascular system
Several cases of sudden death due to cardiac arrest have been
reported in subjects exposed to trichloroethylene, either medically
or occupationally (Ostelere, 1953; Norris & Stuart, 1957; Meyer,
1966; Wiecko, 1966; Clearfield, 1970; Tomasini, 1976). It has
been suggested that these deaths might be due to an increased
sensitivity of the myocardium to endogenous and exogenous
catecholamines. The event would entail irregular cardiac
rhythm due to the onset of ectopic foci with atrioventricular
dissociation. A range of alterations in cardiovascular function
(e.g., atrial and ventricular extrasystole, tachycardia, and
ventricular fibrillation) has been reported (Bardodej & Viskocil,
1956; Anderson, 1957; Lilis et al., 1969; Tomasini, 1976; Governa,
1981).
8.2.3. Effects on the respiratory system
Under normal conditions of exposure, trichloroethylene did not
induce any effects on the respiratory tract but, at high exposure
levels of 810 - 3510 mg/m3 (150 - 650 ppm), there may be effects
due to local irritation (Bardodej & Viskocil, 1956; Jouglard &
Vincent, 1971; Meyer, 1973; Gaultier et al., 1974; Tomasini, 1976).
Breakdown products are of more importance than trichloroethylene
with respect to the effects on the respiratory system (Castellino,
1969).
8.2.4. Effects on the urinary tract
Altered renal function has been described in both acute and
chronic trichloroethylene poisoning (Nomura, 1962; Graovac-
Leposavic et al., 1964; Clearfield, 1970). The nephropathy
observed could be due to impurities rather than to the
trichloroethylene; technical grade trichloroethylene might have
contained nephrotoxic chlorinated hydrocarbons (e.g., 1,1,2,2-
tetrachloroethane) as impurities (Chiesura, 1980).
8.2.5. Effects on the skin
When applied to the skin, trichloroethylene causes erythema
(Wahlberg, 1984). It is only mildly irritating to human skin
providing it is not held in contact (e.g., by clothing or
footwear). Chemical burning of the skin has been reported
following prolonged contact (Maloof, 1949; Tomasini, 1976). There
is a considerable difference in individual susceptibility to the
effects of repeated exposure. The reaction to repeated contact
(which will defat the skin) may take the form of an erythematous,
exudative, vesicular, eczematous, or exfoliative dermatitis (Roche
et al., 1958; Stopps & McLaughlin, 1967; Ettema et al., 1975).
Secondary infection of the skin may complicate the dermatitis.
"Degreaser's flush", a reddening of the skin in some individuals
ingesting ethanol after exposure to trichloroethylene, can occur
(section 8.1.1).
8.2.6. Effects on the eye
Liquid trichloroethylene in the eye produces pain and
discomfort and superficial damage to the cornea, but complete
recovery occurs within a few days (Grant, 1974). Exposure to high
concentrations of vapour (anaesthetic levels of 27 500 - 108 000
mg/m3) also causes eye irritation and superficial damage to the
cornea, but again with complete recovery (Grant, 1961).
8.2.7. Carcinogenicity
A number of epidemiological studies have been conducted to
examine the possible carcinogenic effects of trichloroethylene in
occupationally-exposed populations, and the major findings are
summarized in Tables 10 and 11. Some of these studies have
followed groups with specified exposure to trichloroethylene
(Axelson et al., 1978, 1984; Tola et al., 1980) (Table 10), whereas
others have involved workers in laundry and dry cleaning with more
or less mixed exposures including trichloroethylene (Blair et al.,
1979; Malek et al., 1979; Katz & Jowett, 1981) (Table 11). Other
studies are of a case-reporting or a case-referent (case-control)
type, concerning pancreatic (Lin & Kessler, 1981) and liver tumours
(Novotna et al., 1979; Paddle, 1983) or other conditions and, also,
involving exposure to other solvents (Lin & Kessler, 1981). Some
case-control studies of lymphomas, both of the Hodgkin and non-
Hodgkin type (Olsson & Brandt, 1980; Hardell et al., 1981) and of
liver cancer (Hernberg et al., 1984) have mentioned exposure to
trichloroethylene as a factor in a few cases, but the numbers are
too small for any conclusions to be drawn about trichloroethylene
as a risk factor.
Table 10. Summary of epidemiological studies on carcinogenicity with specific reference
to trichloroethylene exposure
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure intensity had been monitored since Axelson et al.
of 518 male workers 1950 in terms of urinary excretion of (1978)
(7688 person-years) trichloroacetic acid (TCA).
2. No significant excess of cancer mortality
either in the high-dose group (> 100 mg
TCA/litre urine) or the low-dose group
(< 100 mg TCA/litre urine).
3. Results of first update, see Axelson (1984)
below.
-----------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure information was based on TCA Tola et al.
of 1148 male workers determinations in the urine, permitting (1980)
and 969 female workers latency requirements of 6 - 13 years. Loss
(4269 person-years for to follow-up was 9%.
women)
2. Trichloroethylene was not carcinogenic
(given a latency period of 6 to 13 years)
among those exposed at low levels (100 mg
TCA/litre urine in 91% of the cohort).
3. Cohort to be updated at 5-year intervals.
Mortality and 1. Extension and update of the first-mentioned Axelson et al.
incidence in a study, 65% with exposure from 1970 to 1975 (1984)
cohort of 1424 men and with more than 90% having TCA levels
< 100 mg/litre.
2. Deficit found in mortality from cancers (22
versus 36.9 expected), but a significant
excess of urinary tract (11 versus 4.85) and
haematolymphatic tumours (5 versus 1.20).
3. Specifically, there were 3 urinary bladder
cancers versus 0.83 expected, 4 cancers of
the prostrate versus 2.35 expected and 2
lymphomas versus 0.27 expected, requiring
2 years or more of exposure and 10 years of
latency.
-----------------------------------------------------------------------------------------
Table 11. Summary of epidemiological studies and case reports on carcinogenicity of
mixed exposures involving trichloroethylene or trichloroethylene production
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 330 laundry 1. Significant increase in lung and cervical Blair et al.
and dry-cleaning cancer, and a slight excess of leukaemia and (1979)
workers liver cancer detected.
2. Exposed also to other chemicals such as
benzene and carbon tetrachloride.
3. To be extended to a prospective study of
2 - 5 x 103 workers.
Cohort of 37 male 1. Exposure intensity was such that TCA in Malek et al.
dry-cleaning workers urine was less than 100 mg/litre in over 60% (1979)
of the cohort.
2. 6 cases of cancer, none of which were in the
liver.
-----------------------------------------------------------------------------------------
Table 11. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 671 female 1. Death certificates were employed. Katz & Jowett
laundry/dry-cleaning (1981)
workers (controls: 2. Elevated risks of genital (unspecified) and
entire female working kidney cancers were detected, along with a
population and other smaller excess of bladder and skin cancer and
low-wage occupations) lymphoma.
109 pancreatic cancer 1. Hospital records were employed. Lin & Kessler
patients, case-control (1981)
study 2. An association was observed between
pancreatic cancer and exposure to dry-
cleaning chemicals and gasoline fumes, as
well as to drinking of decaffeinated (by
means of trichloroethylene) coffee.
3. No information was available on extent or
type of exposure.
95 liver cancers, 1. Cancer registry data on liver cancer in the Paddle (1983)
case-study area of a production plant were employed.
2. No case could be definitely tied to
trichloroethylene production.
-----------------------------------------------------------------------------------------
While some of these studies are continuing as prospective
studies to cover larger populations, they suffer, in general, from
one or more of the following factors: (a) small cohort population,
(b) young age of the cohort, (c) a rather short follow-up period,
(d) relatively few cases, (e) inaccurate or uncertain estimation
of intensity and duration of exposure to trichloroethylene, (f)
possible exposure to other chemicals, even in the more specific
studies, including potential carcinogens, and (g) inadequate
information on other general risk factors for cancer, such as
smoking, even if the influence of uncontrolled confounding is
likely to be limited in this respect.
At present, it is not possible to draw definite conclusions
from the existing epidemiological data on the carcinogenic
potential of trichloroethylene. However, the appearance of an
excess of haematolymphatic malignancies in several of the studies,
possibly associated with trichloroethylene exposure (Blair et al.,
1979; Hardell et al., 1981; Katz & Jowett, 1981; Axelson et al.,
1984) deserves attention. Furthermore, genito-urinary tumours were
over-represented in 3 studies (Blair et al., 1979; Katz & Jowett,
1981; Axelson et al., 1984), though the picture is less consistent
with regard to the specific sites involved (bladder, kidney,
prostrate, and cervix).
It was noted that IARC (1979, 1982) concluded that
epidemiological studies on human beings were inadequate to make an
evaluation.
9. EVALUATION OF THE HEALTH RISKS FOR MAN
9.1. Levels of Exposure
9.1.1. General population
The general population is exposed to very low levels of
trichloroethylene in air, water, and food (Table 12). The move
away from the use of trichloroethylene in anaesthesia, the solvent
extraction and fumigation of foodstuffs, and the dry-cleaning
of textiles has reduced exposure from these sources.
Trichloroethylene does not accumulate significantly in the food
chain. It is degraded by both biotic and abiotic processes and
its persistence in various environmental compartments is relatively
short, of the order of days or months rather than years. In ground
water, the absence of actinic radiation means that the rate of
degradation is slower, and trichloroethylene contamination may be
relatively prolonged (e.g., half-life of 2 1/2 years).
Table 12. Maximum observed concentrations of trichloroethylene
in environmental media
---------------------------------------------------------------
Media Maximum observed concentration
---------------------------------------------------------------
Open water reservoir 220 µg/litre
Industrial discharge water 200 µg/litre
Rain water ~ 1 µg/litre
Atmosphere ~ 40 µg/m3
Dairy foods 10 µg/kg
Meat 22 µg/kg
Fats and oil 19 µg/kg
---------------------------------------------------------------
Accidental or suicidal ingestion by adults occurs, but is
influenced by the limited availability of trichloroethylene for
domestic use in many countries.
9.1.2. Occupational exposure
Exposure during the actual production of trichloroethylene is
relatively low because of the nature of the process. Subsequent
uses in, for example, metal degreasing and the dry-cleaning of
textiles, can involve higher exposures. The respiratory route is
the principal route of exposure with dermal exposure as an
additional route. Oral intake is insignificant in occupational
terms.
9.2. Evaluation of Human Health Risks
9.2.1. Acute effects
The predominant effect of trichloroethylene in human beings
is on the central nervous system; liver and kidney damage can also
occur. Central nervous system depression can result in coma and
death. A lethal dose as low as 50 ml (75 g) has been reported
but, in general, the lethal dose for an adult is approximately
7000 mg/kg body weight. The threshold for demonstrable behavioural
effects, resulting from inhalation exposure, is approximately 1350
mg/m3 (250 ppm); less well-defined subjective complaints have been
reported with exposure to concentrations of 270 - 540 mg/m3 (50 -
100 ppm) for a few hours.
Cardiac arrhythmia has been reported during anaesthesia
involving trichloroethylene at inhaled concentrations of 27 000 -
54 000 mg/m3 (5000 - 10 000 ppm) (blood levels 60 - 120 mg/litre).
It should be noted that ethanol potentiates the central nervous
system effects of trichloroethylene. Exposure to trichloroethylene
at 1080 mg/m3 (200 ppm) with blood ethanol levels of 300 - 450
mg/litre produces demonstrable behavioural effects.
Irreversible neurotoxic effects on the trigeminal nerve have
been described in anaesthesia with trichloroethylene, using a
closed-circuit system with soda lime. This effect is attributed to
the generation of dichloroacetylene and not to trichloroethylene.
9.2.2. Chronic effects
The main chronic effects of trichloroethylene in human beings
are disturbances in the central nervous system, as well as effects
on the kidney and liver.
Nervous system symptoms (headache, fatigue, irritability, and
alcohol intolerance) begin to occur when levels of trichloroacetic
acid in urine are about 30 - 75 mg/litre. No symptoms have been
reported at concentrations of 20 mg/litre in urine, which
corresponds to a concentration of trichloroethylene in air of about
50 mg/m3.
For effects on other organs, there is no clear-cut quantitative
information on dose response. There are insufficient data on
possible effects on human chromosomes.
Epidemiological data on the carcinogenicity of trichloro-
ethylene are inconclusive, but a slight excess of genito-urinary
cancers and lymphomas in some studies on dry cleaners and metal
degreasers deserves further attention.
The results of long-term exposure studies on rodents have shown
that the liver and kidney are the critical organs. Cytomegaly and
karyomegaly of renal tubular cells was observed in male rats
exposed to pure trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h
per day, 5 days per week, for 104 weeks, but not at 540 mg/m3 (100
ppm).
No embryotoxic or teratogenic effects have been observed in
mice and rats with inhalation of trichloroethylene at levels of
1620 mg/m3 (300 ppm).
Data on the genetic effects of trichloroethylene are
inconclusive.
Lifetime exposure of mice to trichloroethylene at 1620 mg/m3
(300 ppm) through inhalation or to oral doses of 700 - 1200 mg/kg
body weight per day produced lung and liver tumours. A low
incidence of renal tumours was found in rats exposed to a
trichloroethylene concentration of 3240 mg/m3 (600 ppm) by
inhalation or to 500 - 1000 mg/kg body weight per day by gavage.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure to trichloroethylene
concentrations of > 540 mg/m3 (> 100 ppm).
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats. The significance of
these findings needs to be evaluated in the context of further
studies on the mechanism of action of trichloroethylene.
9.3. Treatment of Poisoning in Human Beings
9.3.1. Emergency measures
9.3.1.1. General points
In the treatment of trichloroethylene poisoning, pressor amines
should not be used because of the risk of producing arrhythmias in
the trichloroethylene-sensitized myocardium (Bozza Marrubini et
al., 1978). Hypotension may be treated by transfusion. If
necessary, anti-arrhythmic and beta-blocking agents can be
administered. Haemodialysis, haemoperfusion, or plasmapheresis
have been reported to be useful (Zaffiri et al., 1968; Roessler
& Morawiec-Borowiac, 1973; Malizia et al., 1984). Medical advice
should always be obtained in cases of over-exposure to, or frank
poisoning by, trichloroethylene.
9.3.1.2. Ingestion
Vomiting should not be induced, because of the danger of
aspiration into the larynx and lungs and consequent risks of
vagal inhibition or chemical pneumonitis. Gastric lavage can be
effective if performed within 4 h. Adsorbents such as activated
charcoal or liquid paraffin (medicinal grade) reduce intestinal
absorption; saline laxatives will speed elimination (Bothe et al.,
1973). If the patient is in a state of stupor or coma, intubation
must be performed before gastric lavage.
9.3.1.3. Inhalation
The victim should be removed from the polluted area and placed
in a semi-prone position ensuring that the airway is clear. If
the victim is in a state of stupor or coma, oxygen should be
administered. If spontaneous respiration is absent or very weak,
artifical respiration should be applied.
9.3.1.4. Dermal exposure
All contaminated clothing should be removed and the affected
parts of the body washed thoroughly with soap and plenty of water.
9.3.1.5. Eye exposure
The eyes should be thoroughly irrigated with water for at least
15 min, and ophthalmological advice obtained on the possible need
for further treatment.
REFERENCES
ABRAHAMSEN, A.M. (1960) Quantitative estimation of
trichloroacetic acid in the urine and serum in trichloroethylene
poisoning. Acta pharmacol. toxicol., 17: 288-294.
ACGIH (1982) TLVs - Threshold limit values for chemical
substances in workroom air adopted by ACGIH for 1982, Cincinnati,
Ohio, American Conference of Governmental Industrial Hygienists,
p. 32.
ADAMS, E.M., SPENCER, H.C., ROWE, V.K., MCCOLLISTER, D.D., & IRISH,
D.D. (1951) Vapor toxicity of trichloroethylene determined by
experiments on laboratory animals. Arch. ind. Hyg. occup. Med.,
4: 469-481.
AHLMARK, A. & FORSSMAN, S. (1951a) Evaluating trichloroethylene
exposures by urinalyses for trichloroacetic acid. Arch. ind. Hyg.
occup. Med., 3: 386-398.
AHLMARK, A. & FORSSMAN, S. (1951b) The effect of
trichloroethylene on the organism. Acta physiol. scand.,
22: 326-339.
ALEXANDER, H.C., MCCARTHY, W.M., & BARTLETT, E.A. (1978) Toxicity
of perchloroethylene, trichloroethylene, 1,1,1-trichloroethane,
and methylene chloride to fathead minnows. Bull. environ. Contam.
Toxicol., 20: 344-352.
ANDERSEN, M.E., GARGAS, M.L., JONES, R.A., & JENKINS, L.J., Jr
(1980) Determination of the kinetic constants for metabolism of
inhaled toxicants in vivo using gas uptake measurements. Toxicol.
appl. Pharmacol., 54(1): 100-116.
ANDERSSON, A. (1957) [Health hazards in industry on exposure to
trichloroethylene: a clinical, experimental, and technical hygienic
investigation.] Acta med. scand., 157(Suppl. 323): 1-220 (in German).
ASCHIMICI (1980) Report for Italian Working Group for
trichloroethylene.
ASTRAND, I. & OVRUM, P. (1976) Exposure to trichloroethylene.
I. Uptake and distribution in man. Scand. J. Work Environ. Health,
2: 199-211.
AVIADO, D.M., ZAKHARI, S., SIMAAN, J.A., & ULSAMER, A.G. (1976)
In: Golberg, L., ed. Methyl chloroform and trichloroethylene in
the environment, Cleveland, Ohio, CRC Press Inc., pp. 47-89.
AXELSON, O., ANDERSSON, K., HOGSTEDT, C., HOLMBERG, B., MOLINA, G.,
& DE VERDIER, A. (1978) A cohort study on trichloroethylene
exposure and cancer mortality. J. occup. Med., 20: 194-196.
AXELSON, O., ANDERSSON, K., SELDEN, A., & HOGSTEDT, C. (1984)
Cancer morbidity and exposure to trichloroethylene. In: ICOST,
International Conference on Organic Solvent Toxicity, Stockholm,
15-17 November 1984 (Abstract in: Arbete och Halsa, 29: 126).
BADEN, J.M., KELLEY, M., MAZZE, R.I., & SIMMON, V.F. (1979)
Mutagenicity of inhalation anaesthetics: trichloroethylene,
divinyl ether, nitrous oxide and cyclopropane. J. Anaesth., 51: 417.
BAERG, R.D. & KIMBERG, D.V. (1970) Centrilobular hepatic necrosis
and acute renal failure in "solvent sniffers". Ann. intern. Med.,
73: 713-720.
BALKON, J. & LEARY, J.A. (1979) An initial report on a
comprehensive, quantitative, screening procedure for volatile
compounds of forensic and environmental interest in human biofluids
by GC/MS. J. anal. Toxicol., 3: 213-215.
BANERJEE, S. & VAN DUUREN, B.L. (1978) Covalent binding of the
carcinogen trichloroethylene to hepatic microsomal proteins and to
endogenous DNA in vitro. Cancer Res., 38: 776-780.
BANERJEE, S., YALKOWSKY, S.H., & VALVANI, S.C. (1980) Water
solubility and octanol/water partition coefficients of organics.
Limitations of the solubility-partition coefficient correlation.
Environ. Sci. Technol., 14: 1227-1229.
BARDODEJ, Z. (1962) [Value and use of exposure test. VIII.
Volatile organic chloride test in urine.] Cesk. Hyg., 7: 234-239 (in
Czech).
BARDODEJ, Z. & VISKOCIL, J. (1956) The problem of
trichloroethylene metabolism and its effects on the nervous system
as a means of hygienic control. Am. Med. Assoc. Arch. ind. Health,
13: 581-592.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS (III), B.S.H., KIRKPATRICK, M.,
MICHAEL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment: a multimedia
environmental study. Biomed. mass Spectrom., 7: 139-147.
BARRETT, L., ARSAC, PH., VINCENT, M., FAURE, J., GARREL, S., &
REYMOND, F. (1982) Evoked trigeminal nerve potential in chronic
trichloroethylene intoxication. J. Toxicol. clin. Toxicol., 19:
419-423.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish (Lepomis macrochirus). In: Hague,
R., ed. Dynamics, Exposure and Hazard Assessment of Toxic
Chemicals, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BARTONICEK, V. (1962) Metabolism and excretion of
trichloroethylene after inhalation by human subjects. Br. J. ind.
Med., 19: 134-141.
BARTONICEK, V. & BRUN, A. (1970) Subacute and chronic
trichloroethylene poisoning: a neuropathological study in rabbits.
Acta pharmacol. toxicol., 28: 359-369.
BARTONICEK, V. & SOUCEK, B. (1959) Metabolism of
trichloroethylene in rabbits. Arch. Gewerbepathol. Gewerbehyg., 17:
283-293.
BARTSCH, H., MALAVEILLE, C., BARBIN, A., & PLANCHE, G. (1979)
Mutagenic and alkylating metabolites of haloethylenes,
chlorobutadienes and dichlorobutenes produced by rodent or human
liver tissues. Arch. Toxicol., 41: 249-277.
BATTIG, K. (1964) Comparison of the effects of trichloroethylene
with the effects of drugs on exploratory behaviour in the rat. In:
14th International Congress on Occupational Health, Madrid, 1963,
Vol. 2, pp. 887-889.
BATTIG, K. & GRANDJEAN, E. (1963) Chronic effects of
trichloroethylene on rat behaviour. Arch. environ. Health, 7:
694-699.
BAUER, U. (1981) [Human exposure to environmental chemicals.
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues.] Zbl. Bakt. I. Abt. Orig., B174: 200-237
(in German).
BAUER, U. & SELENKA, F. (1982) [Exposure of the population to
haloforms and chlorinated solvents in drinking water compared to
air and food.] Von Wasser, 59: 7-16 (in German).
BEISLAND, H.O. & WANNAG, S.A. (1970) [Trichloroethylene sniffing:
acute liver and kidney damage.] Tidsskr. Nor. Laegeforen., 90: 285-288
(in Norwegian).
BELILES, R.P., BRUSICK, D.J., & MECLER, F.J. (1980) Teratogenic-
mutagenic risk of workplace contaminants: trichloroethylene,
perchloroethylene, and carbon disulfide, Washington DC, US
Department of Health, Education and Welfare, pp. 234 (Report for US
DHEW Contract No. 210-77-0047) (Govt Rep. Announce. Index (US),
1982: 2728).
BELL, Z. (1977) Written communication with contractor reports of:
(a) Dominant lethal study with trichloroethylene in albino rats
exposed via inhalation (February 1977); and (b) Teratogenic study
via inhalation with trichlor 132, trichloroethylene in albino rats
(March 1977), Washington DC, US Environmental Protection Agency
(EPA-600/8-82-006).
BELLAR, T.A., BUDDE, W.L., & EICHELBERGER, J.S. (1979) The
identification and measurement of volatile organic compounds in
aqueous environmental samples. IV. In: Schuetzle, D., ed.
Monitoring toxic substances, Washington DC, American Chemical
Society (ACS Symposium Series No. 94).
BERGMAN, K. (1983) Application and results of whole-body
autoradiography in distribution studies of organic solvents. Crit.
Rev. Toxicol., 12(1): 59-118.
BIANCHI, A., DE NATALE, G., & MATTURRO, F. (1963) [Comparative
research on some pharmacological effects of fluothane and other
volatile narcotics.] G. Ital. Chir., 19: 327-349 (in Italian).
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene, and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGNAMI, M., CONTI, G., CONTI, L., CREBELLI, R., MISURACA, F.,
PUGLIA, A.M., RANDAZZO, R., SCIANDRELLO, G., & CARERE, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor, and Aspergillus nidulans.
Chem.-biol. Interact., 30: 9-23.
BLAIR, A., DECOUFLE, P., & GRAUMAN, D. (1979) Causes of death
among laundry and dry cleaning workers. Am. J. public Health, 69:
508-511.
BOLT, H.M. & FILSER, J.G. (1977) Irreversible binding of
chlorinated ethylenes to macromolecules. Environ. Health Perspect.,
21: 107-112.
BOTHE, J., BRAUN, W., & DONHARDT, A. (1973) [Effect of mineral
oil in hydrocarbon poisoning in mice.] Arch. Toxikol., 30: 243-250 (in
German).
BOUDENE, C., TURBIAUX, M., CLUET, J.-L., & TRUHAUT, R. (1983)
Contribution à la fixation d'une concentration limite tolérable de
trichloroéthylène dans l'atmosphère des locaux de travail. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 44(2): 75-91.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and 2-
carbon halogenated aliphatic organic compounds under methanogenic
conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOUYGUES, M., DANNE, O., BOUVRY, M., LUCIANI, F., & RABREAU, D.
(1980) Hépatite au trichloroéthylène. Action synergique du
tétrachlorure de carbone? Nouv. Presse méd., 9(43): 3277.
BOZZA MARRUBINI, M., GHEZZI LAURENZI, R., & UCCELLI, P. (1978)
[Acute intoxication: clinical diagnosis and treatment,] Milan,
Italy, Medico-Farmaceutica Organization Editore, p. 470 (in
Italian).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14: 231-241.
BRONZETTI, G., ZEIGER, E., & FREZZA, D. (1978) Genetic activity
of trichloroethylene in yeast. J. environ. Pathol. Toxicol., 1:
411-418.
BROSS, G., DI FRANCEISCO, D., & DESMOND, M.E. (1983) The effects
of low dosages of trichloroethylene on chick development.
Toxicology, 28: 283-294.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., DEFELD, J.M., & BAUER, H.
(1974) Le dosage par chromatographie en phase gazeuse des
métabolites urinaires du trichloréthylène: l'acide trichloro-
acétique et le trichloroéthanol. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 35(3): 395-402.
CABANA, B.E. & GESSNER, P.K. (1967) Determination of chloral
hydrate, trichloroacetic acid, trichlorethanol, and urochloralic
acid in the presence of each other and in tissue homogenates. Anal.
Chem., 39: 1449-1452.
CACCURI, S. (1976) [Labour medicine,] 3rd ed., Napoli, Italy,
Idelson, pp. 343-352 (in Italian).
CALLEN, D.F., ROLAND WOLF, C., & PHILPOT, R.M. (1980) Cytochrome
P-450 mediated genetic activity and cytotoxicity of seven
halogenated aliphatic hydrocarbons in Saccharomyces cerevisiae.
Mutat. Res., 77: 55-63.
CAPELLINI, A. & GRISLER, R. (1958) [Studies on liver function in
a group of workers chronically exposed to trichloroethylene.] Med.
Lav., 49: 167-172 (in Italian).
CARLSON, G.P. & WHITE, J.F. (1983) Cardiac arrythmogenic action
of benzo( a)pyrene in the rabbit. Toxicol. Lett., 15: 43-48.
CASTELLINO, N. (1969) [Trichloroethylene: technology,
pathological manifestations and prevention,] Milano, Italy, Franco
Angeli Editore, p. 117 (in Italian).
CAVALLO, A. & GRASSI, P. (1976) Determination of chlorinated
hydrocarbons in potable waters. Boll. chim. Union Ital. Lab. prov.,
27: 337-350.
CEFIC (1982) Chlorinated solvents: trichloroethylene in metal
cleaning and other industrial applications, Brussels, Conseil
Européen des Fédérations des Industries Chimiques.
CERNA, M. & KJPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analyzed by screening system tests. Mutat. Res., 46:
214-215.
CHIESURA, P. (1980) [Halogenated aliphatic hydrocarbons.] In:
Crepet, M., ed. [Occupational medicine,] Torino, Italy, Utet (in
Italian).
CHIESURA, P. & CORSI, G. (1961) [Acute trichloroethylene
poisoning in man followed by liver disease and hyperglycaemic
glycosuria.] Folia med., 44: 121-135 (in Italian).
CHRISTENSEN, H.E., LUGINBYHL, T.T., & CARROLL, B.B., ed. (1974)
The toxic substances list 1974, Washington DC, National Institute
of Occupational Safety and Health, p. 353.
CHRISTENSEN, H.E. & LUGINBYHL, T.T. (1975) Registry of toxic
effects of chemical substances 1975, Washington DC, US Department
of Health, Education and Welfare.
CLEARFIELD, H.R. (1970) Hepatorenal toxicity from sniffing spot-
remover (trichloroethylene): Report of 2 cases. Am. J. dig. Dis.,
15: 851-856.
COLEMAN, W.E., LINGG, R.D., MELTON, R.G., & KOPFLER, F.C. (1976)
The occurrence of volatile organics in five drinking water supplies
using gas chromatography/mass spectrometry. Identification and
analysis of organisms in polluted water. In: Keith, L.H., ed.
Proceedings of the First Chemical Congress of the North American
Continent, 1975, Ann Arbor, Michigan, Ann Arbor Science,
pp. 305-327.
CONSO, F., EFTHYMIOU, M.-L., GARNIER, R., & FOURNIER; E. (1980)
Interêt de la toxicovigilance: example de certains
trichloréthylènes du commerce. Arch. Mal. prof. Méd. Trav., 41:
198-200.
CORNISH, H.H. & ADEFUIN, J. (1966) Ethanol potentiation of
halogenated aliphatic solvent toxicity. Am. Ind. Hyg. Assoc. J., 27:
57-61.
CORREIA, Y., MARTENS, G.J., VAN MENSCH, F.H., & WHIM, B.P. (1977)
The occurrence of trichloroethylene, tetrachloroethylene and 1,1,1-
trichloroethane in Western Europe in air and water. Atmos.
Environ., 11: 1113-1116.
CREBELLI, R., BIGNAMI, M., CONTI, L., & CARERE, A. (1982)
Mutagenicity of trichloroethylene in Salmonella typhimurium TA 100.
Ann. Ist. Super. Sanità, 18: 117-122.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (in press)
Mutagenicity of trichloroethylene, trichloroethanol, and chloral
hydrate in Aspergillus nidulans. Mutat. Res.
CRONN, D.R., RASMUSSEN, R.A., ROBINSON, E., & HARSCH, D.E. (1977)
Halogenated compound identification and measurement in the
troposphere and lower stratosphere. J. geophys. Res., 82: 5935-5944.
DALBEY, W. & BINGHAM, E. (1978) Metabolism of trichloroethylene
by the isolated perfused lung. Toxicol. appl. Pharmacol., 43: 267-277.
DANIEL, J.W. (1963) The metabolism of 36Cl-labelled
trichloroethylene and tetrachloroethylene in the rat. Biochem.
Pharmacol., 12: 795-802.
DEFALQUE, R.J. (1961) Pharmacology and toxicology of
trichloroethylene: A critical review of the world literature. Clin.
Pharmacol. Ther., 2: 665-688.
DEGUCHI, T. (1972) Threshold limit values for solvent mixtures in
the air. Effects of single and mixed chlorinated hydrocarbons upon
the level of serum transaminases in rats. Osaka Shiritsu Daigaku
Igaku Zasshi, 21: 187-209.
DEKANT, W. & HENSCHLER, D. (1982) Dechlorination reactions in the
course of the metabolism of trichloroethylene. Naunyn-Schmiedeb
Arch. Pharmacol., 319: R16.
DEKANT, W. & HENSCHLER, D. (1983) New pathways of
trichloroethylene metabolism. Dev. Toxicol. environ. Sci., 11:
399-402.
DEKANT, W., METZLER, M., & HENSCHLER, D. (1984) Novel metabolites
of trichloroethylene through dechlorination reactions in rats,
mice, and humans. Biochem. Pharmacol., 33(13): 2021-2027.
DE LEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, C.K., REMELE,
P.C., STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980) Rapid
gas chromatographic method for the determination of volatile and
semivolatile organochlorine compounds in soil and chemical waste
disposal site samples. J. chromatogr. Sci., 18: 85-88.
DE MORE, W.B., HAMSON, R.F., MOLINA, M.J., KURYLO, M.J., WATSON,
R.T., HOWARD, C.J., GOLDEN, D.M., & RAVISHANKARA, A.R. (1983)
Chemical kinetics and photochemical data for use in stratospheric
modeling evaluation number 6, Washington DC, National Aeronautics
and Space Administration (NASA JPL Publication No. 83-62,
15 September, 1983).
DERWENT, R.G. & EGGLETON, A.E.J. (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos. Environ., 12: 1261-1268.
DESOILLE, H., PINCHON, R.A., JANS, M., & BOUGUIGNON, A. (1962)
Intoxication expérimentale aiguë par le trichloroéthylène. Effets
aggravants de l'intoxication éthylique chronique associée. Etude
expérimentale électroencéphalographique chez le lapin. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 23: 653-664.
DIETZ, E.A., Jr & SINGLEY, K.F. (1979) Determination of
chlorinated hydrocarbons in water by headspace gas chromatography.
Anal. Chem., 51: 1809-1814.
DILLING, W.L. (1977) Interphase transfer processes. II.
Evaporation rates of chloromethanes, ethanes, ethylenes, propanes,
and propylenes from dilute aqueous solutions. Comparisons with
theoretical predictions. Environ. Sci. Technol., 11: 405.
DI PAOLO, J.A. & DONIGER, J. (1982) Neoplastic transformation of
Syrian hamster cells by putative epoxide metabolites of
commercially-utilized chloroalkenes. J. Natl Cancer Inst., 69(2):
531-534.
DI RENZO, A.B., GANDOLFI, A.J., & SPIES, I.G. (1982) Microsomal
bioactivation and covalent binding of aliphatic halides to DNA.
Toxicol. Lett., 11: 243-252.
DMS (1967) UV atlas of organic compounds, London, Dortmund,
Butterworths-Verlag Chemie, Vol. 3 (Spectrum No. A1/6).
DOBINSON, B. & GREEN, G.E. (1972) Formation of dichloroacetylene
from trichloroethylene in the presence of epoxides. Chem. Ind., 5:
214.
DORFMUELLER, M.A., HENNE, S.P., YORK, R.G., BORNSCHEIN, R.L., &
MANSON, J.M. (1979) Evaluation of teratogenicity and behavioural
toxicity with inhalation exposure of maternal rats to
trichloroethylene. Toxicology, 14: 153-166.
DOWTY, B.J., CARLISLE, D.R., & LASETER, J.L. (1975) New Orleans
drinking water sources tested by gas chromatography-mass
spectrometry. Environ. Sci. Technol., 9: 762-765.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Solubility of organic
solvents. I. Gas chromatographic determination of olive oil/gas
partition coefficient. Helv. Chim. Acta, 60: 454.
DROZ, P.O. & FERNANDEZ, J.G. (1978) Trichloroethylene exposure.
Biological monitoring by breath and urine analyses. Br. J. ind.
Med., 35: 35.
DUPRAT, P. & GRADISKI, D. (1980) Cytogenic effect of
trichloroethylene in the mouse as evaluated in the micronucleus
test. IRCS med. Sci. soc. occup. Med., 8: 182.
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir irritant
des principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur. J. Toxicol., 9(3): 171-177.
EAJ (1983) Environmental monitoring of chemicals: environmental
survey report of F.Y. 1980 and 1981, Japan, Office of Health
Studies, Department of Environmental Health, Environmental Agency
Japan, p. 144.
EHRNER-SAMUEL, H., BALMER, K., & THORSELL, W. (1973)
Determination of trichloroacetic acid in urine by a gas
chromatographic method. Am. Ind. Hyg. Assoc. J., 34: 93-96.
EKLUND, G., JOSEFFSON, B., & ROOS, C. (1978) Determination of
volatile halogenated hydrocarbons in tapwater. J. High Res. Chrom.
& Chrom. Comms., July, pp. 34-40.
ELCOMBE, C.R. (in press) Species differences in carcinogenicity
and peroxisome proliferation due to trichloroethylene: a
biochemical human hazard assessment. Arch. Toxicol.
ELCOMBE, C.R., PRATT, I.S., & GREEN, T. (1982) The rate of
trichloroacetic acid (TCA) formation determines two species
differences in hepatic peroxisome proliferation due to
trichloroethylene (TRI). Toxicologist, 2: 173.
ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979) Effects of
methylene chloride, trichloroethane, trichloroethylene,
tetrachloroethylene, and toluene on the development of chick
embryos. Toxicology, 12: 111-119.
ENTZ, R.C. & HOLLIFIELD, H.C. (1982) Headspace gas
chromatographic analysis of foods for volatile hydrocarbons.
J. agric. food Chem., 30: 84-88.
ERTLE, T., HENSCHLER, D., JULLER, G., & SPASSOWSKI, M. (1972)
Metabolism of trichloroethylene in man. Arch. Toxikol., 29: 171-188.
ETTEMA, J.J., KLEEREKOPER, L., & DUBA, W.C. (1975) Mental
stresses during short-term inhalation of trichloroethylene.
Staub-Reinhalt. Luft, 35: 409-410.
EUROCOP-COST (1976) A comprehensive list of polluting substances
which have been identified in various fresh waters, effluent
discharges, aquatic animals and plants, and bottom sediments, 2nd
ed., Luxembourg, Commission of the European Community, p. 57
(EUCO/MDU/73/76, XII/476/76).
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., & HOPKE, P.K.
(1977) Monitoring to detect previously unrecognized pollutants in
surface waters - appendix: organic analysis data, Springfield,
Virginia, US NTIS, pp. 304 (PB Report PB-273350) (Govt Rep.
Announce. Index, 1978: 199).
FABRE, R. & TRUHAUT, R. (1952) Toxicology of trichloroethylene.
II. Results of experimental animal studies. Br. J. ind. Med., 9:
39-43.
FAHRIG, R. (1977) The mammalian spot test (Fellfleckenstest) with
mice. Arch. Toxicol., 38: 87-98.
FAWNS, H.T. (1968) Trichloroacetic acid in the urine of workers
using trichloroethylene. Proc. Assoc. Clin. Biochem., 5: 110-114.
FERGUSON, R.K. & VERNON, R.J. (1970) Trichloroethylene in
combination with CNS drugs: effects on visual-motor tests. Arch.
environ. Health, 20: 462-467.
FERNANDEZ, J.G., HUMBERT, B.E., DROZ, P.O., & CAPEROS, J.R. (1975)
Exposition au trichloroéthylene. Bilan de l'absorption, de
l'excrétion, et du métabolisme sur des sujets humains. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 36(7-8): 397-407.
FERNANDEZ, J.G., DROZ, P.O., HUMBERT, B.E., & CAPEROS, J.R. (1977)
Trichloroethylene exposure: simulation of uptake, excretion, and
metabolism using a mathematical model. Br. J. ind. Med., 34: 43-55.
FILSER, J.G. & BOLT, H.M. (1979) Pharmacokinetics of halogenated
ethylenes in rats. Arch. Toxicol., 42: 123-136.
FINK, R. (1968) Toxicity of anaesthetics, Baltimore, Maryland,
Williams & Williams, pp. 270.
FISHBEIN, L. (1973) Chromatography of environmental hazards,
New York, Elsevier Scientific Publishing Company, Vol. 2,
pp. 471-490.
FOOD CHEMICAL NEWS (1978) Preliminary report on TCE study shows
no carcinogenicity in rats. Food chem. News, 20: 41-42.
FOSSA, A.A., WHITE, J.F., & CARLSON, G.P. (1982) Anti-arrhythmic
effects of disulfiram on epinephrine-induced cardiac arrthythmias
in rabbits exposed to trichloroethylene. Toxicol. appl. Pharmacol.,
66: 109-117.
FRIBERG, G.L., KYLIN, B., & NYSTROM, A. (1953) Toxicities of
trichloroethylene and tetrachloroethylene and Fujiwara's pyridine
reaction. Acta pharmacol. toxicol., 9: 303-312.
FUKUDA, K., TAKEMOTO, K., & TSURATA, H. (1983) Inhalation
carcinogenicity of trichloroethylene in mice and rats. Ind. Health,
21: 243-245.
GAMBERALE, F., ANNWALL, G., & OLSON, B.A. (1976) Exposure to
trichloroethylene. III. Psychological functions. Scand. J. Work
environ. Health, 4: 220-224.
GAULTIER, M. (1974) [Clinical toxicology,] Rome, Italy, Società
Editrice Demi (in Italian).
GEHRING, P.J. (1968) Hepatoxic potency of various chlorinated
hydrocarbon vapours relative to their narcotic and lethal potencies
in mice. Toxicol. appl. Pharmacol., 13: 287-298.
GOLDBERG, M.E., JOHNSON, H.E., POZZANI, U.C., & SMITH, H.E., Jr
(1964) Effect of repeated inhalation of vapours of industrial
solvents on animal behaviour. I. Evaluation of nine solvent vapours
on pole-climb performance in rat. Am. Ind. Hyg. Assoc. J., 25: 369-375.
GOLDBLATT, M.W. (1956) Industrial medicine and hygiene, London,
Merewether, Vol. 3.
GOVERNA, M. (1981) [Occupational diseases due to chlorine
derivatives of the aliphatic hydrocarbons.] In: Sartorelli, E., ed.
[Textbook of occupational medicine,] Padova, Italy, Vol. 1,
pp. 483-502 (in Italian).
GRADISKI, D., MAGADUR, J.-L., BAILLOT, M., DANIERE, M.C., & SCHUH,
M.B. (1974) Toxicité comparée des principaux solvants chlorés
aliphatiques. J. eur. Toxicol., 7: 247-254.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.-L., & FRANCIN,
J.M. (1978) Toxicité aiguë comparée par inhalation des principaux
solvants aliphatiques chlorés. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 39: 249-257.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
New York, Academic Press.
GRANDJEAN, E. (1960) Trichloroethylene effects on animal
behaviour. Arch. environ. Health, 1: 106-108.
GRANDJEAN, E. (1963) The effects of short exposures to
trichloroethylene on swimming performances and motor activity of
rats. Am. Ind. Hyg. Assoc. J., 24: 376-379.
GRANDJEAN, E., MUNCHINGER, R., TURRIAN, V., HAAS, P.A., KNOEPFEL,
H.K., & ROSENMUND, H. (1955) Investigations into the effects of
exposure to trichloroethylene in mechanical engineering. Br. J.
ind. Med., 12: 131-142.
GRANT, M.W. (1961) Trichoroethylene in the eye. J. Am. Med.
Assoc., 178: 359.
GRANT, M.W. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, pp. 1034-1045.
GRAOVAC-LEPOSAVIC, L., MILOSAVLJEVIC, Z., & ILIC, V. (1964)
[Liver function in workers exposed to trichloroethylene.] Arh. Hig.
Rad. Toksikol., 15: 93-97 (in Yugoslavian).
GREEN, T. & PROUT, M.S. (in press) Species differences in
response to trichloroethylene. II. Biotransformation in rats and
mice. Arch. Toxicol.
GREEN, T., PROUT, M.S., & PROVAN, W.M. (in press) Species
differences in response to trichloroethylene. I. Pharmacokinetics
in rats and mice. Arch. Toxicol.
GREIM, H., BONSE, G., RADWAN, Z., REICHERT, D., & HENSCHLER, D.
(1975) Mutagenicity in vitro and potential carcinogenicity of
chlorinated ethylenes as a function of metabolic oxirane formation.
Biochem. Pharmacol., 24: 2013-2017.
GU, Z.W., SELE, B., JALBERT, P., VINCENT, M., VINCENT, F., MARKA,
C., CHMARA, D., & FAURE, J. (1981) Induction of sister chromatid
exchanges by trichloroethylene and its metabolites. Toxicol. Eur.
Res., 3: 63-67.
GUN, R.T., GRYGORCENICZ, D., & NETTELBECK, T.J. (1978) Choice
reaction time in workers using trichloroethylene. Med. J. Aust., 1:
535-536.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 73: 169-176.
HATHWAY, D.E. (1980) Consideration of the evidence for mechanisms
of 1,1,2-trichloroethylene metabolism including new identification
of its dichloroacetic acid and trichloroacetic acid metabolites in
mice. Cancer Lett., 8: 263-269.
HAYDEN, J.W., COMSTOCK, E.G., & COMSTOCK, B.S. (1976) The
chemical toxicology of solvent abuse. Clin. Toxicol., 9: 169-184.
HEIL, E., OESER, H., HATZ, R., & KELKER, H. (1979) [Gas
chromatographic determination of traces of C- and C-fluoro- and
chlorohydrocarbons in air.] Fresenius Z. Anal. Chem., 297: 357-364
(in German).
HENSCHLER, D. (1977a) Metabolism and mutagenicity of halogenated
olefuis: a comparison of structure and activity. Environ. Health
Perspect., 21: 61-64.
HENSCHLER, D. (1977b) Metabolism of chlorinated alkenes and
alkanes as related to toxicity. J. environ. Pathol. Toxicol., 1:
125-133.
HENSCHLER, D. & HOOS, R. (1982) Metabolic activation and
deactivation mechanisms of di-, tri-, and tetrachloroethylenes.
In: Snyder, Parke, Kocsis, Jollow, Gibson, & Witmer, ed. Biological
reactive intermediates. II. Part A., New York, Plenum Publishing
Corporation, pp. 659-666.
HENSCHLER, D., BONSE, G., & GREIM, H. (1970a) [Cranial nerve
neuritis due to poisoning with chlorinated acetylenes when handling
vinylidene chloride copolymers.] Arch. Toxikol., 26: 62-75 (in
German).
HENSCHLER, D., BROSER, F., & HOPF, H.C. (1970b) Environmental
Pollution and Carcinogenic Risks, Lyons, International Agency for
Research on Cancer, pp. 171-175 (IARC Scientific Publications
No. 13).
HENSCHLER, D., EDER, E., NEUDECKER, T., & METZLER, M. (1977)
Carcinogenicity of trichloroethylene: fact or artifact? Arch.
Toxicol., 37: 233-236.
HENSCHLER, D., HOOS, W.R., FETZ, H., DALLMEIER, E., & METZLER, M.
(1979) Reactions of trichloroethylene epoxide in aqueous systems.
Biochem. Pharmacol., 28: 543-548.
HENSCHLER, D., ROMEN, W., ELSASSER, H.M., REICHERT, D., EDER, E., &
RADWAN, Z. (1980) Carcinogenicity study of trichloroethylene by
long-term inhalation in three animal species. Arch. Toxicol., 43:
237-248.
HENSCHLER, D., BONSE, G., & DEKANT, W. (1983) Mechanisms and
reactions of electroplulic intermediates of halogenated ofefins.
In: Proceedings of the 13th International Cancer Congress. Part B.
Biology of Cancer, New York, Alan R. Liss, Inc., Vol. 1,
pp. 175-183.
HENSCHLER, D., ELSASSER, H., ROMEN, W., & EDER, E. (1984)
Carcinogenicity study of trichloroethylene, with and without
epoxide stabilizers, in mice. J. Cancer Res. clin. Oncol., 107:
149-156.
HERBOLSHEIMER, R., FUNK, L., & DRASCHE, H. (1972) Use of
activated carbon as an adsorbent in the determination of
trichloroethylene in the air. Staub-Reinhalt. Luft, 32: 407-409.
HERNBERG, S., KORKATA, M.-L., ASIKAINEN, V., & RIALA, R. (1984)
Primary liver cancer and exposure to solvents. Int. Arch. occup.
environ. Health, 54: 147-153.
HIRAYAMA, T. & IKEDA, M. (1979) Applicability of carbon felt to
the dosimetry of solvent vapour mixture. Am. Ind. Hyg. Assoc. J.,
40: 1091-1096.
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., & SAKAI,
T. (1983) [Experimental studies of trichloroethylene toxicity.
II. Changes in trichloroethylene in blood serum and in urine during
and after exposure to trichloroethylene.] Nippon Eiseigaku Zasshi,
38(4): 772-779 (in Japanese with English summary).
HOWARD, C.J. (1976) Rate constants for the gas-phase reactions of
OH radicals with ethylene and halogenated ethylene compounds.
J. Chem. Phys., 65: 4771.
HUFF, J.E. (1971) New evidence on the old problems of
trichloroethylene. Ind. Med. Surg., 40: 25-33.
IARC (1979) Trichloroethylene, Lyons, International Agency for
Research on Cancer, pp. 545-572 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 247-249 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Supplement 4).
IKEDA, M. (1974) Reciprocal metabolic inhibition of toluene and
trichloroethylene in vivo and in vitro. Int. Arch. Arbeitsmed., 33:
125-130.
IKEDA, M. & IMAMURA, T. (1973) Biological half-life of
trichloroethylene and tetrachloroethylene in human subjects. Int.
Arch. Arbeitsmed., 31: 209-224.
IKEDA, M., OHTSUJI, H., KAWAI, H., & KUNIYOSHI, M. (1971)
Excretion kinetics of urinary metabolites in patient addicted to
trichloroethylene. Br. J. ind. Med., 28: 203-206.
IKEDA, M., OHTSUJI, H., IMAMAURA, T., & KOMOIKE, Y. (1972)
Urinary excretion of total trichloro-compounds, trichloroethanol,
and trichloroacetic acid as a measure of exposure to
trichloroethylene and tetrachloroethylene. Br. J. ind. Med., 29:
328-333.
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office, pp. 206-207 (Occupational Safety and Health Series No. 37).
IMAMURA, T. & IKEDA, M. (1973) A time-saving procedure for the
determination of total trichloro-compounds in human urine samples.
Int. Arch. Arbeitsmed., 31: 333-338.
IOFFE, B.V., ISIDOROV, V.A., & ZENKEVICH, I.G. (1977) Gas
chromatographic-mass spectrometric determination of volatile
organic compounds in an urban atmosphere. J. Chromatogr., 142:
787-795.
IRPTC (1984) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JAMES, W.R.L. (1963) Fatal addiction to trichloroethylene. Br. J.
ind. Med., 20: 47-49.
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G. (1982)
Uptake via the blood and elimination of 10 organic solvents
following epicutaneous exposure of anaesthetized guinea-pigs.
Toxicol. appl. Pharmacol., 63: 181-187.
JAPANESE YEARBOOK OF CHEMICAL INDUSTRIES STATISTICS (1983) Tokyo,
Ministry of International Trade and Industry.
JESSON, J.P. (1980) Release of industrial halocarbons and
tropospheric budget. In: Proceedings of the NATO Advanced Study
Institute on Atmospheric Ozone: Its Variation and Human Influences,
Washington DC, US Department of Transportation, Federal Aviation
Administration.
JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (1983)
Evaluation of certain food additives: 27th report, Geneva, World
Health Organization (Technical Report Series No. 696).
JOUGLARD, J. & VINCENT, V. (1971) Pulmonary signs of ingestions
of solvents. Marseille Med., 108: 696-697.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial waste water: a case study of their
environmental impact. Environ. Sci. Technol., 12: 88-96.
KALASHINIKOVA, V.P., VISSARIONOVA, V.Ya., BONDAREV, G.I., SKIRKO,
B.K., & KARANTIROVA, O.V. (1974) [Influence of different diets on
the course of chronic experimental trichloroethylene poisoning.]
Vopr. Pitan., No.6: 43-47 (in Russian).
KALASHINIKOVA, V.P., SKIRKO, B.K., VISSARIONOVA, V.Ya., BONDAREV,
G.I., KLEIMENOVA, N.V., POPOVA, N.S., & KARANTIROVA, O.V. (1976)
[Evaluation of the toxic effects of trichloroethylene according
to morphological and some biochemical indexes.] Gig. i Sanit., No. 2:
107-108 (in Russian).
KANJE, M., KJELLSTRAND, P., FOX, K., & WALLDORF, A. (1981)
Neurotransmitter metabolizing enzymes and plasma
butyrylcholinesterase in mice exposed to trichloroethylene. Acta
pharmacol. toxicol., 49: 205-209.
KARICKHOFF, S.W., BROWN, D.S., & SCOTT, T.A. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13: 241-248.
KATZ, R.M. & JOWETT, D. (1981) Female laundry and dry cleaning
workers in Wisconsin: a mortality analysis. Am. J. public Health,
71: 305-307.
KEINFELD, M. & TABERSHAW, I.R. (1954) Trichloroethylene toxicity:
report of 5 fatal cases. AMA Arch. ind. Hyg. occup. Med., 10: 134-141.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals.
Ecotoxicol. environ. Saf., 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (in press) Relationship between
water solubility, soil sorption, octanol-water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish,
P.R., & Hendricks, A.C., ed. Aquatic toxicology ASTM STP, 707,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
KIMMERLE, G. & EBEN, A. (1973) Metabolism, excretion, and
toxicology of trichloroethylene after inhalation. II. Experimental
human exposure. Arch. Toxicol., 30: 127-138.
KIRK, R.E. & OTHMER, D.F., ed. (1963) Anesthetics. In:
Encyclopedia of chemical technology, 2nd ed., New York, John Wiley
and Sons, Vol. 2, pp. 393-410.
KIRK, R.E. & OTHMER, D.F., ed. (1964) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 2nd
ed., New York, John Wiley and Sons, Vol. 5, pp. 183-195.
KIRK, R.E. & OTHMER, D.F., ed. (1979) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 3rd
ed., New York, John Wiley and Sons, Vol. 5, pp. 745-753.
KISELEVA, A.F. & KOROLENKO, A.M. (1971) Histoenzymic changes in
liver and kidneys during the experimental effect of anesthetic
doses of fluorothan and trylen. Eksp. Khir. Anesteziol., 16: 81-84.
KITAGAWA, T. (1961) The rapid measurement of toxic gases and
vapour. In: Proceedings of the 13th International Congress on
Occupational Health, New York, pp. 506-512.
KJELLSTRAND, P., LANKE, J., BJERKEMO, M., ZETTERQUIST, L., &
MANSSON, L. (1980) Irreversible effects of trichloroethylene
exposure on the central nervous system. Scand. J. Work environ.
Health, 6: 40-47.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M.,
MORTENSEN, I., LANKE, J., & HOLMQUIST, B. (1981a)
Trichloroethylene: effects on body and organ weights in mice,
rats, and gerbils. Toxicology, 21: 105-115.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M., MORTENSEN,
I., LANKE, J., & HOLMQUIST, B. (1981b) Effects of long-term
exposure to trichloroethylene on the behaviour of Mongolian gerbils
(Meriones unguiculatus). J. Toxicol. environ. Health, 8: 787-793.
KJELLSTRAND, P., HOLMQUIST, B., MANDAHL, N., & BJERKEMO, M.
(1983a) Effects of continuous trichloroethylene inhalation on
different strains of mice. Acta pharmacol. toxicol., 53: 369-374.
KJELLSTRAND, P., HOLMQUIST, B., ALM, P., KANJE, M., ROMARE, S.,
JONSSON, I., MANSSON, L., & BJERKEMO, M. (1983b)
Trichloroethylene: further studies of the effects on body and organ
weights and plasma butyrycholinesterase activity in mice. Acta
pharmacol. toxicol., 53: 375-384.
KLAASSEN, C.D. & PLAA, G.L. (1966) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in mice.
Toxicol. appl. Pharmacol., 9: 139-151.
KLAASSEN, C.D. & PLAA, G.L. (1967) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in dogs.
Toxicol. appl. Pharmacol., 10: 119-131.
KLINE, S.A., MCCOY, E.C., ROSENKRANZ, H.S., & VAN DUUREN, B.L.
(1982) Mutagenicity of chloroalkene epoxides in bacterial systems.
Mutat. Res., 101: 115-125.
KLOWKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981)
Laboratory screening of distribution, conversion, and
mineralisation of chemicals in the soil-plant-system and comparison
to outdoor experimental data. Chemosphere, 10: 1089-1090.
KONIETZKO, H., HABERLANDT, W., HEILBRONNER, H., REILL, G., &
WEICHARDT, H. (1978) [Chromosome studies on trichloroethylene
workers.] Arch. Toxicol., 40: 201-206 (in German).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin pathology
following epicutaneous exposure to seven organic solvents. Int. J.
Tiss. React., 111(1): 21-30.
KYLIN, B., REICHARD, H., SUMEGI, I., & YLLNER, S. (1962)
Heptatoxic effect of tri- and tetrachloroethylene on mice. Nature
(Lond.), 193: 395.
KYLIN, B., AXELL, K., EHRNER-SAMUEL, H., & LINDBORG, A. (1967)
Effect of inhaled trichloroethylene on the CNS. Arch. environ.
Health, 15: 48-52.
KYLSZEIJKO, C., LAKOMY, T., & PAPIEROWSKI, A. (1963) [The
influence of trichloroethylene during labour process in women in
comparison with the results in vitro.] Pol. Tyg. Lek., 18:
1333-1338 (in Polish).
KYRKLUND, T., ALLING, C., HAGLID, K., & KJELLSTRAND, P. (1983)
Chronic exposure to trichloroethylene: lipid and acyl group
composition in gerbil cerebral cortex and hippocampus.
Neurotoxicology, 4(4): 35-42.
LACHNIT, V. (1971) Halogenated hydrocarbons and the liver. Wein.
Klin. Wochenschr., 83: 734-737.
LA DU, B.N., MANDEL, H.G., & WAY, E.L., ed. (1971) Fundamentals
of drug metabolism and drug disposition, Baltimore, Maryland,
Williams and Wilkins.
LAHAM, S. (1970) Studies on placental transfer:
trichloroethylene. Ind. Med. Surg., 39: 46-49.
LAZAREV, N.V. (1929) [The narcotic efficacy of the vapours of the
chlorine derivatives of methane, ethane and ethylene.] Naunyn-
Schmiedeb. Arch. exp. Pathol. Pharmacol., 141: 19-24 (in German).
LAZAREV, N.V. & GADASKINA, I.D. (1977) [Harmful substances
in industry,] Leningrad, Medecine, Vol. 3 (in Russian).
LEHMANN, K.B. & SCHMIDT-KEHL, L. (1936) [Thirteen most important
chlorohydrocarbons of the paraffin series from the standpoint of
industrial hygiene.] Arch. Hyg. Bakt., 116: 131-268 (in German).
LEIBMAN, K.C. (1965) Metabolism of trichloroethylene in liver
microsomes. Mol. Pharmacol., 1: 239-246.
LEONG, B.K.J., SCHWETZ, B.A., & GEHRING, P.J. (1975) Embryo- and
fetotoxicity of inhaled trichloroethylene, perchloroethylene,
methylchloroform, and methylene chloride in mice and rats. Toxicol.
appl. Pharmacol., 33: 136.
LERZA, P., LOMBARDI, F., & VIOTTI, G. (1963) [Health and hygiene
considerations regarding work in dry-cleaning establishments using
trichloroethylene.] Lav. Um., 15: 459-478 (in Italian).
LEWIS, G.D., REYNOLDS, R.C., & JOHNSON, A.R. (1984) Some effects
of trichloroethylene on mouse lungs and livers. Gen. Pharmacol.,
15(2): 139-144.
LILIS, R., STANESCU, D., MUICA, N., & ROVENTA, A. (1969) Chronic
effects of trichloroethylene exposure. Med. Lav., 60: 595-601.
LIN, R.S. & KESSLER, I.I. (1981) A multifactoral model for
pancreatic cancer in man. J. Am. Med. Assoc., 245: 147-152.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Proceedings of the
Symposium on Short-Term Test Systmes and the Detection of
Carcinogenesis, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 333-356.
LOPRIENO, N., SBRANA, I., & LASCIALFARI, D. (in press) [Study on
the in vivo potential mutagenic effect of trichloroethylene
evaluated by cytogenetic analysis in mice treated by inhalation for
52 days.] (in Italian).
LOVELOCK, J.E. (1974) Atmospheric halocarbons and stratospheric
ozone. Nature (Lond.), 252: 292-294.
MACKAY, D. (1982) Correlations of bioconcentration factors.
Environ. Sci. Technol., 10: 274-278.
MCCONNELL, G., FERGUSON, D.M., & PEARSON, C.R. (1975) Les
hydrocarbures chlorés et l'environnement. Endeavour, 34: 13-18.
MCCORD, C.P. (1932) Toxicity of trichloroethylene. J. Am.
Med. Assoc., 99: 409.
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
MCNEILL, W.C. (1979) Chlorocarbons and chlorohydrocarbons.
Trichloroethylene. In: Kirk-Othmer's encyclopedia of chemical
technology, 3rd ed., New York, Wiley Interscience, Vol. 5,
pp. 745-753.
MAKIDE, Y., TOMINAGA, K., & ROWLAND, F.S. (1979) Gas
chromatographic analysis of halogenated hydrocarbons in air over
Japan. Chem. Lett., 1979: 355-358.
MALEK, B., KRCMAROVA, B., & RODOVA, O. (1979) [Epidemiological
study of hepatic tumour incidence in subjects working with
trichloroethylene: II. Negative result of retrospective studies in
dry cleaners.] Pracov. Lek., 31(4): 124-126 (in Czech).
MALIZIA, E., PELAIA, P., & PIROVINE, C. (1984) Experience in the
emergency treatment of acute intoxication by trichloroethylene.
Riv. Toss. Speriment Clin., 14: 437.
MALOOF, C.C. (1949) Burns of the skin produced by
trichloroethylene vapours at room temperature. J. ind. Hyg.
Toxicol., 31: 295-296.
MALTONI, I.C. & MAIOLI, P. (1977) [Long-term bioassay of
carcinogenicity of trichloroethylene. Preliminary results.]
Osp. Vita, 4: 108-110 (in Italian).
MALTONI, C., LEFEMINE, G., & COTTI, G. (in press) Experimental
research on trichloroethylene carcinogenesis. In: Maltoni, C. &
Mehlman, M.A., ed. Archives of research on industrial
carcinogenesis, Vol. 5.
MANTEL, M. & NOTHMANN, R. (1977) Rapid determination of
trichloroethanol and trichloroacetic acid in urine. Analyst, 102:
672-677.
MATTURRO, F. (1963) [Effects of some volatile narcotics on
isolated guinea-pig heart.] G. Ital. Chir., 19: 95-99 (in Italian).
MAZZA, V. & BRANCACCIO, A. (1967) [Behaviour of the formed
elements of the blood and the marrow in experimental intoxications
with Tri.] Folia med., 50: 318-324 (in Italian).
MELINO, C., MESSINEO, A., & PACELLI, E. (1979) Risk from
trichloroethylene in a factory producing electrical condensers.
Riv. Med. Lab. Ig. Ind., 3: 67-81.
MEYER, H.-J. (1966) [Trichloroethylene poisoning by the oral
route.] Arch. Toxikol., 21: 225-234 (in German).
MEYER, H.-J. (1973) Bronchopulmonary alterations induced by
trichloroethylene and other halogenated hydrocarbons. Bronches, 23:
113-124.
MILLER, D.A. & GRIMSRUD, E.P. (1979) Correlation of electron
capture response enhancements caused by oxygen with chemical
structure for chlorinated hydrocarbons. Anal. Chem., 51: 851-859.
MILLER, R.E. & GUENGERICH, F.P. (1982) Oxidation of
trichloroethylene by liver microsomal cytochrome P-450: evidence
for chlorine migration in a transition state not involving
trichloroethylene oxide. Biochemistry, 21: 1090-1097.
MOLINA, M.J. & ROWLAND, F.S. (1974) Stratospheric sink for
chlorofluoromethanes: chlorine atom-catalysed destruction of ozone.
Nature (Lond.), 294: 810-812.
MONSTER, A.C. (1979) Differences in uptake, elimination, and
metabolism in exposure to trichloroethylene, 1,1,1-trichloroethane,
and tetrachloroethylene. Int. Arch. occup. environ. Health, 42:
311-317.
MONSTER, A.C. & BOERSMA, G. (1975) Simultaneous determination of
trichloroethylene and metabolites in blood and exhaled air by gas
chromatography. Int. Arch. occup. environ. Health, 35: 155-163.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1976) Pharmaco-
kinetics of trichloroethylene in volunteers: influence of workload
and exposure concentration. Int. Arch. occup. environ. Health, 38:
87-102.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1979) Kinetics of
trichloroethylene in repeated exposure of volunteers. Int. Arch.
occup. environ. Health, 42: 283-292.
MOSLEN, M.T., REYNOLDS, E.S., & SZABO, S. (1977) Enhancement of
the metabolism and hepatoxicity of trichloroethylene and
perchloroethylene. Biochem. Pharmacol., 26: 369-375.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1972)
Trichloroethylene exposure and trichloroethylene metabolites in
urine and blood. Arch. Toxicol., 29: 335-340.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1974) Metabolism of
trichloroethylene in man. II. Pharmacokinetics of metabolites.
Arch. Toxicol., 32: 283-295.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1975) Metabolism of
trichloroethylene in man. III. Interaction of trichloroethylene and
ethanol. Arch. Toxicol., 33: 173-189.
MURRAY, A.J. & RILEY, J.P. (1973) Occurrence of some chlorinated
aliphatic hydrocarbons in the environment. Nature (Lond.), 242: 37-38.
MUSCLOW, C.E. & AWEN, C.F. (1971) Glue sniffing: report of a
fatal case. Can. Med. Assoc. J., 104: 315-319.
NCI (1976) Carcinogenesis bioassay of trichloroethylene. CAS No.
79-01-6, Washington DC, National Cancer Institute (US DHEW
Publication No. (NIH) 76-802).
NEELY, W.B. & MACKAY, D. (1982) In: Dickson, K.L., Maki, A.W., &
Cairns, J., ed. Modelling the fate of chemicals in the aquatic
environment, Ann Arbor, Michigan, Ann Arbor Science Publications.
NICHOLSON, A.A., MERESZ, O., & LEMYK, B. (1977) Determination of
free and total potential haloforms in drinking-water. Anal. Chem.,
49: 814-819.
NIOSH (1977a) Manual of analytical methods, 2nd ed., Cincinnati,
Ohio, National Institute of Occupational Safety and Health, Vol. 3,
pp. S336-1 to S336-9 (US DHEW Publication No. (NIOSH) 77-157-C).
NIOSH (1977b) National occupational hazard survey. III. Survey
analyses and supplemental tables, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, pp. 2864-2866.
NIOSH (1978) Special occupational hazard review of
trichloroethylene, Washington DC, National Institute of
Occupational Safety and Health (US DHEW Publication No. (NIOSH)
78-130).
NOMIYAMA, H., NOMIYAMA, K., & UCHIKI, H. (1978) Gas-liquid
chromatographic determination of trichloroethylene metabolites in
urine. Am. Ind. Hyg. Assoc. J., 39: 506-510.
NOMURA, S. (1962) Health hazards in workers exposed to
trichloroethylene vapour. I. Trichloroethylene poisoning in an
electroplating plant. Kumamoto med. J., 15: 29-37.
NORRIS, W. & STUART, P. (1957) Cardiac arrest during
trichloroethylene anaesthesia. Br. med. J., 1: 860-863.
NOVOTNA, E., DAVID, A., & MALEK, B. (1979) [An epidemiological
study on hepatic tumour incidence in subjects working with
trichloroethylene. I. Negative result of retrospective
investigations in subjects with primary liver carcinoma.] Pracov.
Lek., 31(4): 121-123 (in Czech).
ODUM, E.P. (1971) Fundamentals of ecology, 3rd ed.,
Philadelphia, Pennsylvania, W.B. Saunders Co.
OGATA, M. & SAEKI, T. (1974) Measurement of chloral hydrate,
trichloroethanol, trichloroacetic acid, and monochloroacetic acid
in the serum and the urine by gas chromatography. Int. Arch.
Arbeitsmed., 33: 49-58.
OGATA, M., TAKATSUKA, Y., TOMUKUNI, K., & MUROI, K. (1970) Simple
method for the quantitative analysis of urinary trichloroethanol
and trichloroacetic acid as an index of trichloroethylene exposure.
Br. J. ind. Med., 27: 378-381.
OHTA, T., MORITA, M., & MIZOGUCHI, I. (1976) Local distribution
of chlorinated hydrocarbons in the ambient air in Tokyo. Atmos.
Environ., 10: 557-560.
OLSSON, H. & BRANDT, L. (1980) Occupational exposure to organic
solvents and Hodgkin's disease in men. Scand. J. Work environ.
Health, 6: 302-305.
OSTELERE, G. (1953) Trichloroethylene anaesthesia, Edinburgh,
Scotland, Livingstone.
OTSON, R., WILLIAMS, D.T., & BIGGS, D.C. (1982) Relationships
between raw water quality treatment and the occurrence of organics
in Canadian potable water. Bull. environ. Contam. Toxicol., 28:
396-403.
PADDLE, G.M. (1983) Incidence of liver cancer and
trichloroethylene manufacture: joint study by industry and a cancer
registry. Br. med. J., 286: 876.
PARCHMAN, L.G. & MAGEE, P.N. (1982) Metabolism of
14C-trichloroethylene to 14CO2 and interaction of a metabolite
with liver DNA in rats and mice. J. Toxicol. environ. Health, 9:
797-813.
PARDYS, S. & BROTMAN, M. (1974) Trichloroethylene and alcohol: a
straight flush. J. Am. Med. Assoc., 229:: 521-522.
PARMEGGIANI, L. (1956) [A case of chronic trichloroethylene
poisoning.] Med. Lav., 47: 639-646 (in Italian).
PARSONS, F., WOOD, P.R., & DE MARCO, J. (1984) Transformation of
tetrachloroethene and trichloroethene in microcogus and ground
water. J. Am. Water Works Assoc., 76(2): 56-59.
PEARSON, C.R. & MCCONNELL, G. (1975) Chlorinated C and C
hydrocarbons in the marine environment. Proc. R. Soc. London
Ser. B, 189: 305-332.
PELTS, D.G. (1962) [Effect of trichloroethylene and
tetrachloroethylene on the phagocytic activity of blood
leukocytes.] Gig. i Sanit., 27(7): 96-99 (in Russian).
PENKETT, S.A. (1982) Non-methane organics in the remote
troposphere. In: Goldberg, E.D., ed. Atmospheric chemistry, Berlin,
Springer, pp. 329-355.
PEROCCO, P. & PRODI, G. (1981) DNA damage by haloalkanes in human
lymphocytes cultered in vitro. Cancer Lett., 13: 213-218.
PESSAYRE, D., ALLEMAND, H., WANDSCHEER, J.C., DESCATOIRE, V.,
ARTIGOU, J-Y., & BENHAMOU, J-P. (1979) Inhibition, activation,
destruction, and induction of drug-metabolizing enzymes by
trichloroethylene. Toxicol. appl. Pharmacol., 49: 355-363.
PESSAYRE, D., BARTON, C., DESCATOIRE, V., DEGOTT, C., BABANY, G.,
FUNCK-BRENTANO, C., DELAFORGE, M., & LARREY, D. (1982)
Hepatotoxicity of trichloroethylene-carbon tetrachloride mixtures
in rats. Gastroenterology, 63: 761-772.
PIERCE, R.H., OLNEY, C.E., & FELBECK, G.T. (1974) p-p'-DDT
adsorption to suspended particulate matter in sea water. Geochim.
cosmochim. Acta, 38: 1060-1073.
PLAA, G.L., EVANS, E.A., & HINE, C.H. (1958) Relative
hepatoxicity of seven halogenated hydrocarbons. J. Pharmacol. exp.
Ther., 123: 224-229.
POWELL, J.F. (1945) Trichloroethylene: absorption, elimination,
and metabolism. Br. J. ind. Med., 2: 142-145.
PRENDERGAST, J.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1967) Effects on experimental animals of long-term inhalation of
trichloroethylene, carbon tetrachloride, 1,1,1-trichloroethane,
dichlorodifluoromethane, and 1,1-dichloroethylene. Toxicol. appl.
Pharmacol., 10: 270-289.
PRICE, P.J., HASSETT, C.M., & MANSFIELD, J.I. (1978) Transforming
activities of trichloroethylene and proposed industrial
alternatives. In Vitro, 14: 290-293.
PRIEST, R.J. & HORN, R.C., Jr (1965) Trichloroethylene
intoxication: a case of acute hepatic necrosis possibly due to this
agent. Arch. environ. Health, 11: 361-365.
RASMUSSEN, R.A. & KHALIL, M.A.K. (1983) Natural and anthropogenic
trace gases in the lower troposphere of the Arctic. Chemosphere, 12:
371-375.
REICHERT, D. & HENSCHLER, D. (1978) Nephrotoxic and hepatotoxic
effects of dichloroacetylene. Food Cosmet. Toxicol., 16: 227-235.
REICHERT, D., METZLER, M., & HENSCHLER, D. (1980a) Decomposition
of the neuro- and nephrotoxic compound dichloroacetylene in the
presence of oxygen: separation and identification of novel
products. J. environ. Pathol. Toxicol., 4: 525-532.
REICHERT, D., SPENGLER, U., ROMEN, W., & HENSCHLER, D. (1980b)
Carcinogenic potential of dichloroacetylene. Dev. Toxicol. environ.
Sci., 8: 269-272.
RIGAUD, M., CHALABREYSSE, J., PROST, G., & TOLOT, F. (1977) Etude
expérimentale de la toxicité hépatique du trichloroéthylène.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 38: 263-265.
ROCHE, L., GENEVOIS, M., & MARIN, A. (1958) Trichloréthylène et
eczéma de la face. Arch. Mal. prof. Méd. Trav. Sécur. soc., 19:
615-616.
ROESSLER, R. & MORAWIEC-BOROWIAK, D. (1973) [Use of hemodialysis
in acute trichloroethylene poisoning.] Wiad. Lek., 26(13): 1271-1275 (in
Polish).
ROOK, J.J., MEIJERS, A.P., GRAS, A.A., & NOORDSIJ, A. (1975)
[Headspace analysis of volatile trace compounds in the Rhine.] Vom
Wasser, 44: 23-30 (in German).
ROSSI, A.M., MIGLIORE, L., BARALE, R., & LOPRIENO, N. (1983) In
vivo and in vitro mutagenicity studies of a possible carcinogen,
trichloroethylene, and its two stabilizers, epichlorohydrin and
1,2-epoxybutane. Teratog. Carcinog. Mutagen., 3: 75-87.
RUBINO, G.F., SCANSETTI, G., & TROMPEO, G. (1959) [Chronic
trichloroethylene poisoning. II. Absorption of trichloroethylene.]
Med. Lav., 50(12): 733-742 (in Italian).
RUSH, W.E. (1970) Quantitative analysis of gaseous pollutants,
London, Ann Arbor-Humphrey Science Publishers, Inc., pp. 229-232.
RUSSO, A., PACCHIELOTTI, F., & METALLI, P. (1984) Nondysfunction
induced in mouse spermatogenesis by chloral hydrate, a metabolite
of trichloroethylene. Environ. Mutagen., 6(5): 695-703.
SADTLER STANDARD SPECTRA (1961) 25 frequently-used spectra for
the infrared spectroscopist, London, Heyden & Son, Ltd.
SAGAWA, K., NISHITANI, Y., KAWAI, H., KUGE, Y., & IKEDA, M. (1973)
Transverse lesion of spinal cord after accidental exposure to
trichloroethylene. Int. Arch. Arbeitsmed., 31: 257-264.
SANDERS, V.M., TUCKER, A.N., WHITE, K.L., Jr, KAUFFMANN, B.M.,
HALLETT, P., CARCHMAN, R.A., BORZELLECA, J.F., & MUNSON, A.E.
(1982) Humoral and cell-mediated immune status in mice exposed to
trichloroethylene in the drinking water. Toxicol. appl. Pharmacol.,
62: 358-368.
SATO, A. & NAKAJIMA, T. (1978) Differences following skin or
inhalation exposure in the absorption and excretion kinetics of
trichloroethylene and toluene. Br. J. ind. Med., 35: 43-49.
SATO, A. & NAKAJIMA, T. (1979) A structure-activity relationship
of some chlorinated hydrocarbons. Arch. environ. Health, 34: 69.
SAVOLAINEN, H. & SEPPALAINEN, A.M. (1979) Biochemical and
physiological effects of organic solvents on rat axon membranes
isolated by a new technique. Neurotoxicology, 1: 467-477.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINIO, H. (1977)
Trichloroethylene and 1,1,1-trichloroethane: effects on brain and
liver after five days intermittent inhalation. Arch. Toxicol., 38:
229-237.
SAWICKI, E., BELSKY, T., FRIEDEL, R.A., HYDE, D.L., MONKMAN, J.L.,
RASMUSSEN, R.A., RIPPERTON, L.A., & WHITE, L.D. (1975) Organic
solvent vapours in air: analytical method. Health Lab. Sci., 12:
394-402.
SBERTOLI, C. & BRAMBILLA, G. (1962) [Three cases of
trichloroethylene poisoning presenting with intolerance to alcohol
as the only symptoms.] Med. Lav., 53: 353-358 (in Italian).
SBRANA, I., LASCIALFARI, N., & LOPRIENO, N. (1984) [Analysis of
the cytogenetic effects of trichloroethylene.] AHI AGI/ABCD/SIBBM,
21-25 October, p. 93 (in Italian).
SCHUMACHER, H. & GRANDJEAN, E. (1960) Comparative investigations
on the anaesthetic effects and acute toxicity of nine solvents.
Arch. Gewerbepathol. Gewerbehyg., 18: 109-119.
SCHUTTMANN, W. (1970) Liver damage after occupational exposure to
trichloroethylene. Dtsch. Z. Verdau Stoffwechselkr., 30: 43-45.
SCHWETZ, B.A., LEONG, B.K.J., & GEHRING, P.J. (1975) The effect
of maternally-inhaled trichloroethylene, perchloroethylene, methyl
chloroform, and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol., 32: 84-96.
SEBA, D.B. & PROSPERO, J.M. (1971) Pesticides in the lower
atmosphere of the northern equatorial Atlantic Ocean. Atmos.
Environ., 5: 1043-1050.
SELLERS, E.M., CARR, G., BERSTEIN, J.G., SELLERS, S., & KOCK-WESER,
J. (1972) Interaction of chloral hydrate and ethanol in man. II.
Hemodynamics and performance. Clin. Pharmacol. Ther., 13: 50-58.
SETO, T.A. & SCHULTZE, M.O. (1956) Determination of
trichloroethylene, trichloroacetic acid, and trichloroethanol in
urine. Anal. Chem., 28: 1625-1629.
SHAHIN, M.M. & VON BORSTEL, R.C. (1977) Mutagenic and letal
effects of alpha-benzene hexachloride, dibutyl phthalate, and
trichloroethylene in Saccharomyces cerevisiae. Mutat. Res., 48:
173-180.
SHANKS, C.A. (1964) The compatibility of octapressin with
cyclopropane, trichloroethylene, and halothane. N.Z. med. J., 63:
156-159.
SHIPMAN, A.J. & WHIM, B.P. (1980) Occupational exposure to
trichloroethylene in metal cleaning processes and to
tetrachloroethylene in the dry-cleaning industry in the U.K. Ann.
occup. Hyg., 23: 197-204.
SHMUTER, L.M. (1972) [The effect of the chronic action of low
concentrations of chlorinated hydrocarbons on the production of
various classes of immunoglobulins.] Gig. i Sanit., 37(2): 32-35 (in
Russian).
SIEGEL, J., JONES, R.A., COON, R.A., & LYON, J.P. (1971) Effects
on experimental animals of acute, repeated, and continuous
inhalation exposures to dichloroacetylene mixtures. Toxicol. appl.
Pharmacol., 18: 168-174.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.G. (1977) Mutagenic
activity of chemicals identified in drinking-water. Dev. Toxicol.
environ. Sci., 2: 249-258.
SINGH, H.B., SALAS, L.J., & CAVANAGH, L.A. (1977) Distribution,
sources, and sinks of atmospheric halogenated compounds. J. Air
Pollut. Control Assoc., 27: 332-336.
SLACIK-ERBEN, R., ROLL, R., FRANKE, G., & VEHLEKE, H. (1980)
Tichloroethylene vapours do not produce dominant lethal mutations
in mice. Arch. Toxicol., 45: 37.
SMYTH, H.F., Jr, CARPENTER, C., WEIL, C.S., POZZANI, U.C.,
STRIEGEL, J.A., & NYCUM, J.S. (1969) Range-finding toxicity data.
VII. Am. Ind. Hyg. Assoc. J., 30: 470-476.
SNELL, F.D. & ETTRE, L.S., ed. (1970a) Chlorocarbons. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 9, pp. 373-410.
SNELL, F.D. & ETTRE, L.S., ed. (1970b) Chlorocarbons. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 9, pp. 454-459.
SNELL, F.D. & HILTON, C.L., ed. (1967) Anesthetics. In:
Encyclopedia of industrial chemical analysis, New York, John Wiley
and Sons, Vol. 5, pp. 357, 366-367, 420-421.
SORGO, G. (1976) [Trichloroethylene, carbon tetrachloride, and
gasoline intoxication as etiological factors in the developement of
arterio- and coronary sclerosis.] Arch. Toxicol., 35: 295-318 (in German).
SOUCEK, B. & VLACHOVA, D. (1960) Excretion of trichloroethylene
metabolites in human urine. Br. J. ind. Med., 17: 60-64.
STEERE, N.V., ed. (1967) Handbook of laboratory safety,
Cleveland, Ohio, The Chemical Rubber Co., pp. 544-545.
STENHAGEN, E., ABRAHAMSSON, S., & MCLAFFERTY, F.W. (1974)
Registry of mass spectral data, New York, John Wiley and Sons,
Vol. 1, p. 246.
STEWART, R.D. & DODD, H.C. (1964) Absorption of carbon
tetrachloride, tetrachloroethylene, methylene chloride, and
1,1,1-trichloroethane through the human skin. Am. Ind. Hyg. Assoc.
J., 25: 439-446.
STEWART, R.D., GAY, H.H., ERLEY, D.S., HAKE, C.L., & PETERSON, J.E.
(1962) Observations on the concentrations of trichloroethylene in
blood and expired air following exposures of humans. Am. Ind. Hyg.
Assoc. J., 23: 167-170.
STEWART, R.D., DODD, H.C., GAY, H.H., & ERLEY, D.S. (1970)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R.D., HAKE, C.L., & PETERSON, J.E. (1974) Use of breath
analysis to monitor trichloroethylene exposures. Arch. environ.
Health, 29: 6-13.
STOPPS, G.J. & MCLAUGHLIN, M. (1967) Psychophysiological testing
of humans exposed to solvent vapors. Am. Ind. Hyg. Assoc. J., 28:
43-50.
STOTT, W.T., QUAST, J.F., & WATANABE, P.G. (1982) The
pharmacokinetics and macromolecular interactions of
trichloroethylene in mice and rats. Toxicol. appl. Pharmacol., 62:
137-151.
SUCIU, I. & OLINICI, L. (1983) Hepato-renal involvement in acute
occupational trichloroethylene intoxication. Med. Lav., 74(2): 123-128.
SVABOVA, K. & MENCIK, M. (1983) [Changes in the state of health
of workers long-term exposed to halogenated hydrocarbons.] Pracov.
Lek., 35: 105-111 (in Czech with English abstract).
SZLUC-KUBERSKA, J. (1972) Chronic industrial trichloroethylene
poisoning. Folia med. Lodz., 16: 67-90.
TADA, O. (1969) Evaluating the exposure to some chlorinated
hydrocarbons. J. Sci. Labour, Part 2, 45: 757-765.
TANAKA, S. & IKEDA, M. (1968) A method for determination of
trichloroethanol and trichloroacetic acid in urine. Br. J. ind.
Med., 25: 214-219.
TAYLOR, H. (1936) Experiments on the physiological properties of
trichloroethylene. J. ind. Hyg. Toxicol., 18: 175-193.
THIELE, D.L., EIGENBRODT, E.E., & WARE, A.J. (1982) Cirrhosis
after repeated trichloroethylene and 1,1,1-trichloroethane
exposure. Gastroenterology, 83: 926-929.
TOLA, S., VILHUNEN, R., JARVINEN, E., & KORKALA, M.-L. (1980) A
cohort study on workers exposed to trichloroethylene. J. occup.
Med., 22: 737-740.
TOLOT, F., VIALLIER, J., ROULLET, A., RIVOIRE, J., & FIGUERES, J.C.
(1964) Toxicité hepatique au trichloroéthylène. Arch. Mal. prof.
Méd. Trav., 25: 9-15.
TOMASINI, M. (1976) [Cardiac arrythmias due to intoxication by
trichloroethylene ("th").] Med. Lav., 67: 163-169 (in Italian).
TOMENIUS, L., HOLMA, B., EHRHER-SAMUEL, H., KYLIN, B., TEBROCK, O.,
& THOMASON, M. (1979) Effect of trichloroethylene on cilia
activity in rabbit trachea. Acta pharmacol. toxicol., 44: 65-70.
TORKELSON, T.R. & ROWE, V.K. (1981) Trichloroethylene. In:
Patty's industrial hygiene and toxicology, 3rd (revised) ed.,
New York, John Wiley and Sons, Vol. 2B, pp. 3553-3586.
TRIEBIG, G., GOSSLER, K., & SCHALLER, K.-H. (1976) [Simple and
reliable gas-chromatographic determination of trichloroethylene and
its metabolites in blood and urine.] Fresenius Z. Anal. Chem., 279:
115-116 (in German).
TRIEBIG, G., LEHRL, S., KINZEL, W., ERZIGHEIT, H., GALSTER, J.V., &
SCHALLER, K.H. (1977a) [Psychopathometrical results of follow-up
studies of trichloroethylene-exposed persons.] Zbl. Bakt. Hyg. I.
Abt. Orig. B., 164: 314-327 (in German with English summary).
TRIEBIG, G., SCHALLER, K.H., ERZIGHEIT, H., & VALENTIN, H. (1977b)
Biochemical investigations and psychological studies of persons
chronically exposed to trichloroethylene with regard to non-
exposure intervals. Int. Arch. occup. environ. Health, 38: 149-162.
TRIEBIG, G., TRAUTNER, P., WELTLE, D., SAURE, E., & VALENTIN, H.
(1982) Investigations on neurotoxicity of chemical substances at
the workplace. III. Determination of the motor and sensory nerve
conduction velocity in persons occupationally exposed to
trichloroethylene. Int. Arch. occup. environ. Health, 51: 25-34.
TSURUTA, H. (1978) Percutaneous absorption of trichloroethylene
in mice. Ind. Health, 16: 145-148.
TUCKER, A.N., SANDERS, V.M., BARNES, D.W., BRADSHAW, T.J., WHITE,
K.L., Jr, SAIN, L.E., BORZELLACA, J.F., & MUNSON, A.E. (1982)
Toxicology of trichloroethylene in the mouse. Toxicol. appl.
Pharmacol., 62: 351-357.
UEHLEKE, H., TABARELLI-POPLAWSKI, S., BONSE, G., & HENSCHLER, D.
(1977) Spectral evidence for 2,2,3-trichloro-oxirane formation
during microsomal trichloroethylen oxidation. Arch. Toxicol., 37:
95-105.
US CFR (1976) Code of Federal Regulations. 21 CFR, 8: 305.
US ITC (1980) Production statistics, 1979, Washington DC,
International Trade Commisssion.
US NTP (1983) Technical report on the carcinogenesis studies of
trichloroethylene (without epichlorohydrin) in F344/n rats and BCF
mice, Research Triangle Park, NC, National Toxicology Program (NIH
Publication No. 83-1979, NTP TR 243).
VALLE-RIESTRA, J.F. (1974) Food processing with chlorinated
solvents. Food Technol., 28: 25-32.
VAN DER HOEVEN, R., DROST, R.H., MAES, R.A.A., DOST, F., PLOMP,
T.A., & PLOMP, G.J.J. (1979) Improved method for the electron-
capture gas chromatographic determination of trichloroacetic acid
in human serum. J. Chromatogr., 164: 106-108.
VAN DUUREN, B.L. & BANERJEE, S. (1976) Covalent interaction of
metabolites of the carcinogen trichloroethylene in rat hepatic
microsomes. Cancer Res., 36: 2419-2422.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH, A.C.,
MELCHIONNE, S., SELDMAN, I., & ROTH, D. (1979) Carcinogenicity of
halogenated olefinic and aliphatic hydrocarbons in mice. J. Natl
Cancer Inst., 63: 1433-1439.
VAN DUUREN, B.L., KLINE, S.A., MELCHIONNE, S., & SEIDMAN, I.
(1983) Chemical structure and carcinogenicity relationships of
some chloroalkene oxides and their parent olefins. Cancer Res., 43:
159-162.
VERNE, J., CECCALDI, P.F., HEBERT, S., & ROUX, J.M. (1959)
Hepatic steatosis during intoxication by volatile organic
compounds. IV. Biochemistry and histochemistry of fatty livers
produced by trichloroethylene poisoning. Pathol. Biol. (Paris), 7:
2311-2316.
VERNON, R.J. & FERGUSON, R.K. (1969) Effects of trichloroethylene
on visual-motor performance. Arch. environ. Health, 18: 894-900.
VERNOT, E.H., MACEWEN, J.D., HAUN, C.C., & KINKEAD, E.R. (1977)
Acute toxicity and skin corrosion data for some organic and
inorganic compounds and aqueous solutions. Toxicol. appl.
Pharmacol., 42: 417-423.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Company, pp. 607-610.
VESTERBERG, O., GORCZAC, J., KRASTS, M. (1976) Exposure to
trichloroethylene. II. Metabolites in blood and urine. Scand. J.
Work environ. Health, 4: 212-219.
VISSARIONOVA, V.Ya., KALASHNIKOVA, V.P., SKIRKO, B.K., KARANTIROVA,
O.V., & LAPRUN, I.B. (1975) [Comparative evaluation of indexes of
rat liver functions in trichloroethylene poisoning.] Gig. Tr.
Prof. Zabol., No.11: 56-58 (in Russian).
VLACHOVA, D. (1957) Determination of trichloroethanol in the
urine after exposure to trichloroethylene. J. Hyg. Epidemiol.
Microbiol. Immunol., 1-2: 225-229.
VON OETTINGEN, W.F. (1955) The halogenated aliphatic, olifinic,
cyclic, aromatic, and aliphatic-aromatic hydrocarbons including the
halogenated insecticides: their toxicity and potential dangers,
Washington DC, US Government Printing Office.
WADE, A., ed. (1977) Martindale: the extra pharmacopoeia, 27th
ed., London, The Pharmaceutical Press, pp. 714-715.
WAHLBERG, J.E. (1984) Erythema-inducing effects of solvents
following epicutaneous administration to man: studied by Laser
Doppler flowmetry. Scand. J. Work environ. Health, 10: 159-162.
WAHLBERG, J.E. & BOMAN, A. (1979) Comparitive percutaneous
toxicity of ten industrial solvents in the guinea-pig. Scand. J.
Work environ. Health, 5: 345-351.
WAKEHAM, S.G., DAVIS, A.C., & GOODWIN, J.T. (1982)
Biogeochemistry of volatile organic compounds in marine
experimental ecosystems and the estuarine environment: initial
results. In: Grice, G.D. & Reeve, M.R., ed. Marine Mesocosms,
New York, Springer.
WASKELL, L. (1978) A study of the mutagenicity of anesthetics and
their metabolites. Mutat. Res., 57: 141-153.
WATERS, E.M., GERSTNER, H.B., & HUFF, J.E. (1977)
Trichloroethylene. I. An overview. J. Toxicol. environ. Health, 2:
671-707.
WEAST, R.C., ed. (1980) Handbook of chemistry and physics, 61st
ed., Boca Raton, Florida, CRC Press, Inc., p. C-317.
WEICHARDT, H. & BARDODEJ, Z. (1970) Determination of
trichloroacetic acid and trichloroethanol in the urine of
trichloroethylene workers. Zentralbl. Arbeitsmed. Arbeitsschutz.,
20: 219-221.
WELLS, J.C.D. (1982) Abuse of trichloroethylene by oral self-
administration. Anaesthesia, 37: 440-441.
WETTERHAHN, J. (1972) Eye make-up. In: Balsam, M. & Sagarin, E.,
ed. Cosmetics science and technology, New York, John Wiley and
Sons, Inc., pp. 419.
WHELAN, J.K., BLANCHETTE, M.A., & HUNT, J.M. (1983) Volatile C,-
C7 organic compounds in an anoxic sediment core from the
Pettaquamscutt River (Rhode Island, USA). Org. Geochem., 5: 29-33.
WHITE, A.E., TAKEHISA, S., EGER, E.I., WOLFF, S., & STEVENS, W.C.
(1979) Sister-chromatid exchanges induced by inhaled anaesthetics.
Anaesthesiology, 50: 426.
WHITE, J.F. & CARLSON, G.P. (1979) Influence of alterations in
drug metabolism on spontaneous and epinephrine-induced cardiac
arrhythmias in animals exposed to trichloroethylene. Toxicol. appl.
Pharmacol., 47: 515-527.
WHITE, J.F. & CARLSON, G.P. (1981a) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: potentiation
by ethanol. Toxicol. appl. Pharmacol., 60: 466-471.
WHITE, J.F. & CARLSON, G.P. (1981b) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: role of
trichloroethylene metabolites. Toxicol. appl. Pharmacol., 60: 458-465.
WHITE, J.F. & CARLSON, G.P. (1982) Epinephrine-induced cardiac
arrhythmias in rabbits exposed to trichloroethylene: potentiation
by caffeine. Fundam. appl. Toxicol., 2: 125-129.
WHO (1984) Guidelines for drinking-water quality. I.
Recommendations, Geneva, World Health Organization, 66 pp.
WHO STUDY GROUP ON RECOMMENDED HEALTH-BASED LIMITS IN OCCUPATIONAL
EXPOSURE TO SELECTED ORGANIC SOLVENTS (1981) Recommended health-
based limits in occupational exposure to selected organic solvents,
Geneva, World Health Organization, 84 pp (Technical Report Series
No. 664).
WIECKO, W. (1966) [Oral poisoning with trichloroethylene.] Wiad.
Lek., 19: 1117-1118 (in Polish).
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & NOETHER FERTIG, M.,
ed. (1976) The Merck index, 9th ed., Rahway, New Jersey, Merck &
Co., Inc., p. 1238.
WINNEKE, G. (1982) Acute behavioural effects of exposure to some
organic solvents: psychophysiological aspects. Acta neurol. scand.,
66(suppl. 92): 117-129.
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmaocokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WIRTSCHAFTER, Z.T. & CRONYN, M.W. (1964) Relative hepato-
toxicity: pentane, trichloroethylene, benzene, carbon
tetrachloride. Arch. environ. Health, 9: 180-185.
ZAFFIRI, O., RUGGERINI, R., & FRANCESCATO, F. (1968) Anesthetics
and adrenergic receptors. Minerva Anestesiol., 34: 556-560.
ZENICK, H., BLACKBURN, K., HOPE, E., RICHDALE, N., & SMITH, M.K.
(1984) Effects of trichloroethylene exposure on male reproductive
function in rats. Toxicology, 31: 237-250.
ZIGLIO, G. (1979) [Gas-chromatographic determination of blood
trichoroacetic acid concentration in subjects not occupationally
exposed to trichloro- and tetrachloroethylene.] Ig. Mod., 72: 876-900
(in Italian).
ZIGLIO, G., FARA, G.M., BELTRAMELLI, G., & PREGLIASCO, F. (1983)
Human environmental exposure to trichloro- and tetrachloroethylene
from water and air in Milan, Italy. Arch. environ. contam.
Toxicol., 12: 57-64.
APPENDIX I
Predicting the Equilibrium Distribution of Trichloroethylene
On the basis of the physical and chemical data summarized
in Table 1 of this document, it is possible to predict the
approximate equilibrium distribution of trichloroethylene in major
environmental "compartments". The models used have been described
by Mackay & Paterson (1981). Essentially, these use physical and
chemical data and realistic compartment volumes in a "model world"
to calculate fugacity capacities and to predict the partitioning of
a chemical between various compartments. The following compartment
volumes are assumed (Neely & Mackay, 1982) (Table I.1) in an
environment, 1 km2 in area.
Table I.1 Compartment volumes for trichloroethylene
----------------------------------------------------------
Atmosphere 6 x 109 m3 1 km2 area x 6 km height
Water 7 x 106 m3 70% area x 10 m depth
Soil 4.5 x 104 m3 30% area x 15 cm depth
Sediment 2.1 x 104 m3 70% water x 3 cm depth
Suspended aquatic 35.0 m3 water volume x 5 ppm
matter
Aquatic biota 7.0 m3 water volume x 1 ppm
----------------------------------------------------------
As an example, the input of trichloroethylene is assumed to be
1000 moles/km2 per year, based on an annual production capacity of
4 x 105 tonnes per year and an approximate land area of 106 km2.
This leads to an actual production of approximately 3000 moles/km2,
but it is assumed that land represents one-third of the total area.
Physical and chemical data for trichloroethylene are given in
Table 1 (main text). Environmental temperature is assumed to be
25 °C (atmosphere) or 15 °C (land and water).
Additional compartments at appropriate volumes can be added with
appropriate characteristics, e.g., atmospheric particulates at
2 µg/m3 (Seba & Prospero, 1971) or terrestrial biota at
approximately 1 kg/m2 (Odum, 1971).
The model predicts the distributions shown in Table I.2.
These are remarkably close to the distributions observed in
most compartments and, furthermore, the ratios of concentrations
predicted to occur between compartments are remarkably similar to
those that are observed. The predicted and observed distributions
of trichloroethylene are not surprising; as noted in section 4.2.1,
its high vapour pressure would be expected to lead to high
atmospheric concentrations, and this would offset the tendency of
high water solubility and low partition coefficients (themselves
related) to lead to high water, biota, or sediment concentrations,
through either partition or adsorption.
Table I.2 Predicted and observed distribution of
trichloroethylene (TCE) in the environment using compartment
volumes and approaches described in text
--------------------------------------------------------------
Compartment Predicted Predicted TCE Observed TCE
% TCE Concentration concentration
(see text)
--------------------------------------------------------------
Air 99.7 22 µg/m3 0.01 - 10 µg/m3
Water 0.3 0.06 µg/litre 0.01 - 100 µg/litre
Sediment < 0.01 0.4 µg/kg 1 - 10 µg/kg
Aquatic < 0.01 0.7 ng/g 1 - 100 ng/g
--------------------------------------------------------------
The value of a model like this is not that it predicts the
"correct" distribution, but that it illustrates and identifies
the compartments that are important as reservoirs or as sites of
degradation of chemicals. In the present case, the size of the
atmospheric compartment and the high vapour pressure of
trichloroethylene are such that the bulk of trichloroethylene
released into the environment should be found in the atmosphere.
Therefore, atmospheric degradation processes should be important in
the eventual degradation of trichloroethylene.
This approach can be refined further by considering not only
the distribution at equilibrium (assuming no degradation), but
adding rates of degradation. For these purposes, we have assumed
the rates of degradation in the appropriate compartment, shown in
section 4.2.2. Although absolute trichloroethylene concentrations
are predicted by this model to be appreciably lower in each
compartment than those shown in Table I.2, the ratios of
concentrations between compartments do not change appreciably
(Table I.3). However, the important point is that since most of
the trichloroethylene is predicted to occur in the atmosphere, and
since atmospheric degradation rates are similar to or faster than
those in other compartments, most of the degradation should take
place in the atmosphere (Table I.3). Washout or fallout of
atmospheric particulate material is not likely to be an important
process; there seems to be little tendency for trichloroethylene to
sorb to particles, because of its low Koc, and residence times of
such particles are long compared to the the expected rate of
degradation by OH radicals. Total environmental lifetime is
controlled by the atmospheric degradation rate, and should,
therefore, be about 10 - 11 days. Degradation in sediments and/or
biota probably contribute in only a very minor way to overall
degradation.
Table I.3 Predicted distribution and compartmental
degradation rates for trichloroethylene (TCE) as %
of total environmental degradation using compartment
volumes and degradation rates in text
----------------------------------------------------
Compartment Predicted TCE Total degradation
concentration (%)
----------------------------------------------------
Air 0.6 µg/m3 99.9
Water 0.002 µg/litre 0.08
Sediment 0.01 µg/kg < 0.01
Aquatic biota 0.02 ng/g < 0.01
----------------------------------------------------
REFERENCES TO APPENDIX I
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
NEELY, W.B. & MACKAY, D. (1982) In: Dickson, K.L., Maki, A.W., &
Cairns, J., Modelling the fate of chemicals in the aquatic
environment, Ann Arbor, Michigan, Ann Arbor Science Publications.
ODUM, E.P. (1971) Fundamentals of ecology, 3rd ed., Philadelphia,
Pennsylvania, W.B. Saunders Co.
SEBA, D.B. & PROSPERO, J.M. (1971) Pesticides in the lower
atmosphere of the northern equatorial Atlantic Ocean. Atmos.
Environ., 5: 1043-1050.
 2.3.1.1. Colorimetry tests
In the Fujiwara test, trichloroethylene is treated with
pyridine in an alkaline environment. Solution absorbance is then
determined at 535 or 470 nm (absorptivity: 18 - 32 litre/g x cm)
with a sensitivity of about 1 mg/kg. This test is suitable for
other aliphatic halogenated compounds, and so is not substance-
specific. Other complementary colorimetric tests that may enable
trichloroethylene to be differentiated from other similar compounds
have been reported by Snell & Ettre (1970b).
2.3.1.1. Colorimetry tests
In the Fujiwara test, trichloroethylene is treated with
pyridine in an alkaline environment. Solution absorbance is then
determined at 535 or 470 nm (absorptivity: 18 - 32 litre/g x cm)
with a sensitivity of about 1 mg/kg. This test is suitable for
other aliphatic halogenated compounds, and so is not substance-
specific. Other complementary colorimetric tests that may enable
trichloroethylene to be differentiated from other similar compounds
have been reported by Snell & Ettre (1970b).
 2.3.1.2. Infra-red spectroscopy
In the gaseous phase, quantities are determined by measuring
the optical density of the mixture at the selected wavelength of
11.8 µm (847/cm). This corresponds to a detection sensitivity of
not less than 0.5 µg/litre (Fishbein, 1973).
In solutions of carbon disulfide, trichloroethylene can be
measured, even in the presence of similar chloro derivatives, using
the specific band at 10.8 µm (926/cm) (Snell & Ettre, 1970b;
Fishbein, 1973). Detection thresholds of some µg/litre can be
attained (Fishbein, 1973).
2.3.1.3. Gas-liquid chromatography
Generally, either packed or capillary columns (low-resolution
or high-resolution chromatography, respectively) are used; the
latter are recommended for complex mixtures containing substances
similar to trichloroethylene. A number of stationary phases can
be used, such as paraffinic hydrocarbons (squalene, hexadecane,
paraffin), Apiezon L, Carbowax (600, 4000, 20M), silicones (SE-30,
550, SF-96-350), and arylphosphates. In general, detectors such as
argon ionization or flame ionization detectors (sensitivity: ~10 ng)
are suitable for several types of analyses; the thermoconductivity
detector is little used, because it is less sensitive (sensitivity:
~250 ng). The detection threshold drops considerably (~0.02 ng in
air) with electron-capture detectors (ECD). ECD response can be
improved slightly by adding small quantities of oxygen to the
carrier gas (Miller & Grimsrud, 1979).
Gas chromatography combined with mass spectrometry (GC/MS) is
both highly selective and sensitive (Snell & Ettre, 1970b).
2.3.1.2. Infra-red spectroscopy
In the gaseous phase, quantities are determined by measuring
the optical density of the mixture at the selected wavelength of
11.8 µm (847/cm). This corresponds to a detection sensitivity of
not less than 0.5 µg/litre (Fishbein, 1973).
In solutions of carbon disulfide, trichloroethylene can be
measured, even in the presence of similar chloro derivatives, using
the specific band at 10.8 µm (926/cm) (Snell & Ettre, 1970b;
Fishbein, 1973). Detection thresholds of some µg/litre can be
attained (Fishbein, 1973).
2.3.1.3. Gas-liquid chromatography
Generally, either packed or capillary columns (low-resolution
or high-resolution chromatography, respectively) are used; the
latter are recommended for complex mixtures containing substances
similar to trichloroethylene. A number of stationary phases can
be used, such as paraffinic hydrocarbons (squalene, hexadecane,
paraffin), Apiezon L, Carbowax (600, 4000, 20M), silicones (SE-30,
550, SF-96-350), and arylphosphates. In general, detectors such as
argon ionization or flame ionization detectors (sensitivity: ~10 ng)
are suitable for several types of analyses; the thermoconductivity
detector is little used, because it is less sensitive (sensitivity:
~250 ng). The detection threshold drops considerably (~0.02 ng in
air) with electron-capture detectors (ECD). ECD response can be
improved slightly by adding small quantities of oxygen to the
carrier gas (Miller & Grimsrud, 1979).
Gas chromatography combined with mass spectrometry (GC/MS) is
both highly selective and sensitive (Snell & Ettre, 1970b).
 2.3.2. Determination in environmental media
2.3.2.1. Soil
High-resolution GC (hrGC-ECD) has been used for determining
trichloroethylene in soil (De Leon et al., 1980). The hrGC/MS
combination has been used as a confirmatory technique, with a
detection threshold of ~10 mg/kg (10 ppm).
2.3.2.2. Water
Levels of trichloroethylene in water can be determined by
hrGC/MS (Dowty et al., 1975), by GC with an electron-capture
detector or an electrolytic conductivity detector (Nicholson et
al., 1977; Dietz & Singley, 1979), and by various other techniques
such as HPLC, hrGC, GC, and GC/MS (Eklund et al., 1978; Jungclaus
et al., 1978). Where specified, detection thresholds are in the
region of 1.0 µg/litre, or lower.
2.3.2.3. Air
GC/MS can be used to measure trichloroethylene levels in the
urban atmosphere (Ioffe et al., 1977). Herbolsheimer et al.
(1972), Sawicki et al. (1975), NIOSH (1977a), Heil et al. (1979),
and Makide et al. (1979) describe sampling and sample enrichment
techniques. Detection capacity may be as low as some ng/m3.
Fujiwara's test can be used to measure trichloroethylene levels
in air (Rush, 1970).
Gas detector tubes (Kitagawa, 1961), activated carbon tubes
(NIOSH, 1977a; Shipman & Whim, 1980), and activated carbon felt
badges (Hirayama & Ikeda, 1979) are available for use in work-place
environments. Gas detector tubes are suitable for spot sampling,
while activated carbon tubes and felt are suitable for time-
weighted average concentration determinations.
2.3.2.4. Foodstuffs
High- and low-resolution GLC can be used for the determination
of trichloroethylene and other aliphatic chloro derivatives in
various foodstuffs (Entz & Hollifield, 1982). The head-space
technique is used in all cases. The sensitivity of the method
appears to be higher (< 1 µg/kg) for water-rich samples than for
fat-rich foodstuffs (10 -50 µg/kg). The coefficient of variation
can be lower than 20%.
2.3.3. Determination in human tissues and fluids
The methods described in this section are those normally
used to determine the levels of trichloroethylene or its major
metabolites (trichloroacetic acid and trichloroethanol) in
blood and urine. These methods can be used to obtain indirect
measurements of exposure. There are methods for the determination
of trichloroethylene and trichloroethanol in expired air.
2.3.3.1. Trichloroethylene
Blood or urine is distilled or aerated. Any trichloroethylene
vapour present is collected in pyridine, which is then subjected to
Fujiwara's colour test (Seto & Schultze, 1956; Tada, 1969).
Trichloroethylene detection and determination in blood and
urine samples are now generally performed by GC, which has largely
replaced colorimetric reactions. Samples are first extracted with
solvents, and trichloroethylene concentrations are then determined
in the extract. There are various modifications of this technique
(Stewart et al., 1962; Stewart & Dodd, 1964; Kylin et al., 1967;
Stewart et al., 1970; Ertle et al., 1972). Because of its
volatility, trichloroethylene can be sampled using the head-space
method (Monster & Boersma, 1975; Triebig et al., 1976; Astrand &
Ovrum, 1976). This method has also been successfully coupled with
GC/MS (Balkon & Leary, 1979).
GLC alone (Monster & Boersma, 1975; Astrand & Ovrum, 1976)
and GC/MS (Barkley et al., 1980) have been used to determine
trichloroethylene in exhaled air. The same methods, with suitable
sampling techniques, may also be used for the determination of
trichloroethylene in alveolar air.
2.3.3.2. Trichloroacetic acid
Fujiwara's colorimetry test is performed on the blood or urine
sample extract or, in the case of urine, directly on the sample
itself (Abrahamsen, 1960; Soucek & Vlachová, 1960; Bartonícek,
1962; Fawns, 1968; Tanaka & Ikeda, 1968; Tada, 1969; Weichardt &
Bardodej, 1970; Ertle et al., 1972; Kimmerle & Eben, 1973; Mantel &
Nothmann, 1977).
Trichloroacetic acid can also be determined by gas
chromatography of the extract after methylation (Ehrner-Samuel
et al., 1973; Ogata & Saeki, 1974; Nomiyama et al., 1978;
van der Hoeven et al., 1979), by direct methylation of the
specimen followed by head-space sampling and GC (Monster & Boersma,
1975; Triebig et al., 1976), or by inducing trichloroacetic acid
decarboxylation and measuring the chloroform thus formed (Müller
et al., 1972; Buchet et al., 1974). Ziglio (1979) determined
trichloroacetic acid in subjects who had absorbed trichloroethylene
in drinking-water. The extraction and methylation method, as well
as the method of inducing thermal decarboxylation and then
injecting the chloroform thus formed according to the head-space
method, were used (sensitivity of extraction and methylation
methods is better than 10 µg/litre).
2.3.3.3. Trichloroethanol
Trichloroethanol is found in the free state and as the
glucuronide (urochloralic acid) in both blood and urine. For
total trichloroethanol determination, the glucuronide is
hydrolysed. The trichloroacetic acid originally present is then
removed and the trichloroethanol is oxidized quantitatively to
trichloroacetic acid (Vlachov, 1957). The trichloroacetic acid
thus formed is then measured by one of the methods previously
described. Alternatively, trichloroethanol can be distilled in a
vapour stream and measured colorimetrically on the basis of the
condensate resulting from the reaction with pyridine and alkalis
(Bardodej, 1962). The colour test can also be carried out without
prior separation of trichloroethanol from trichloroacetic acid.
The two compounds are measured by determining absorbance at 367 or
440 nm, and at 530 nm (Cabana & Gessner, 1967; Ogata et al., 1970;
Mantel & Nothmann, 1977). Trichloroethanol can also be measured by
determining the difference between the figure obtained for the
trichloroacetic acid level and that obtained for all trichloro-
derivatives present after quantitative oxidation to trichloroacetic
acid (Seto & Schulze, 1956; Tanaka & Ikeda, 1968).
Trichloroethanol can be measured by gas chromatography after
quantitative hydrolysis of the glucuronide (Ogata et al., 1970;
Ertle et al., 1972; Kimmerle & Eben, 1973; Ogata & Saeki, 1974;
Buchet et al., 1974; Nomiyama et al., 1978). The technique of
head-space sampling has been used by Monster & Boersma (1975),
Triebig et al. (1976), and Balkon & Leary (1979).
Trichloroethanol in exhaled air can be measured directly
(Monster & Boersma, 1975).
2.3.3.4. Total trichloro derivatives
In the Imamura & Ikeda (1973) method, urine samples are
oxidized with chromium trioxide in heated nitric acid, allowed to
cool, and made alkaline; pyridine is added followed by mild heating
(Fujiwara test). Solution absorbance is then determined at 530 nm.
2.3.4. Sensitivity
Generally speaking, colorimetric test detection thresholds
range between 0.1 and 1 mg/kg. Greater sensitivity is provided by
gas chromatography, which has detection thresholds of between 10
and 100 µg/kg for trichloroethylene, trichloroacetic acid, and
trichloroethanol.
3. SOURCES IN THE ENVIRONMENT, USES, AND SAFE HANDLING
Trichloroethylene does not occur naturally.
It was first synthesized by Fisher in 1864 and became
commercially available for the first time in 1908 in Austria and in
the United Kingdom (Kirk & Othmer, 1964).
3.1. Production Processes, Levels, and Uses
3.1.1. Production processes and levels
Trichloroethylene is produced by three processes: the
dehydrochlorination of sym-tetrachloroethane, the high-temperature
oxychlorination of chlorinated products with one or two carbon
atoms, or the chlorination of ethylene.
In Western Europe, production was approximately 250 000 tonnes
in 1978. The major producing countries are the Federal Republic of
Germany, France, whose individual production capacity is of the
order of 100 000 tonnes, Italy, and the United Kingdom. Sweden
and Spain are smaller producers. In the USA the production of
trichloroethylene in 1979 was 130 000 tonnes (US ITC, 1980). In
Japan, the annual production was approximately 74 500 tonnes in
1981 and 67 500 tonnes in 1982 (Japanese Yearbook of Chemical
Industries Statistics, 1983).
3.1.2. Uses
Trichloroethylene is an industrial solvent mainly (85 - 90%)
used for the vapour degreasing and cold cleaning of fabricated
metal parts. Trichloroethylene has also been used as a carrier
solvent for the active ingredients of insecticides and fungicides;
as a solvent for waxes, fats, resins, and oils; as an anaesthetic
for medical and dental use; and as an extractant for spice
oleoresins and for caffeine from coffee. Trichloroethylene has
been used in printing inks, varnishes, adhesives, paints, lacquers,
spot removers, rug cleaners, disinfectants, and cosmetic cleansing
fluids. It may also be used as a chain terminator in polyvinyl
chloride production and as an intermediate in the production of
pentachloroethane (Defalque, 1961; Kirk & Othmer, 1963, 1979;
Wetterhahn, 1972; Valle-Riestra, 1974; US CFR, 1976; Waters et al.,
1977; IARC, 1979).
3.2. Handling Hazards, and Precautions
3.2.1. Handling hazards
3.2.1.1. Fire, explosion, and thermal decomposition
At normal handling temperatures, trichloroethylene behaves
as a non-flammable, non-burnable substance. Under normal
conditions, it is virtually impossible to induce an explosion with
trichloroethylene. In the presence of air, at temperatures above
400 °C, it produces phosgene, hydrochloric acid, and carbon
monoxide. In the vicinity of arc welding, phosgene and hydrogen
chloride can be produced from trichloroethylene. In vapour
degreasing, using combustion heaters, precautions must be taken to
prevent solvent fumes from entering the combustion air. Containers
of trichloroethylene exposed to fire should be cooled by sprinkling
with water.
3.2.1.2. Chemical reactivity
Trichloroethylene is practically non-reactive with water at
room temperature, under normal storage conditions, and the
stabilized product does not undergo any changes in the presence of
air, humidity, light, or in contact with metals. It is, however, a
wise precaution not to expose the product to temperatures exceeding
130 °C.
In the presence of strong alkalis, particularly if heated,
trichloroethylene produces dichloroacetylene (Reichert et al.,
1980a), which is highly reactive and acutely neurotoxic to both
animals and man (Reichert & Henschler, 1978). Dichloroacetylene
is also potentially carcinogenic (Reichert et al., 1980b). Under
normal circumstances in industrial use, this reaction is unlikely.
However, under occasional laboratory conditions and closed-circuit
anaesthesia, in the presence of soda lime, some dichloroacetylene
may be produced.
Dichloroacetylene may be formed from trichloroethylene by a
reaction catalysed by ionic halides in the presence of certain
epoxides, including epichlorohydrin (Dobinson & Green, 1972).
Non-stabilized trichloroethylene can react violently with
aluminium (especially in the form of dust or filings) giving off
hydrogen chloride and hexachlorobutene vapour (McNeill, 1979).
Not all stabilizers are effective in preventing the reaction with
aluminium; therefore, a suitably-stabilized product should be used
when cleaning aluminium, especially ultrasonically or where
aluminium particles are present. Suitable products are identified
in manufacturers' literature or in specifications.
3.2.2. Handling precautions
3.2.2.1. Personal safeguards
Accidental exposure to trichloroethylene under occupational
conditions is more frequently associated with the generation of
dense trichloroethylene vapour, e.g., misoperation of vapour-
degreasing apparatus (Sagawa et al., 1973) or the use of liquid
trichloroethylene for cleaning the inside of a tank.
Individual protective measures should be related to the type
and level of exposure. When significant skin contact is likely,
suitable protective clothing should be worn, bearing in mind the
limitations of such clothing and the need to maintain it properly
and replace it regularly. To control exposure through inhalation,
the use of full face masks with filters for organic vapours
(basically for short-term or emergency use), self-contained
breathing apparatus, or masks with air-line supply systems may be
necessary. Self-contained breathing apparatus should always be
available for use in emergencies.
3.2.2.2. Storage
Trichloroethylene can be safely stored in carbon steel or
stainless steel containers. It should not be kept in aluminium,
aluminium alloy, or galvanized iron containers; plastic containers
should not be used unless they are known to be suitable for the
storage of trichloroethylene. Storage areas should be cool, well-
ventilated, flame-proof, and shielded from direct sunlight, high-
temperature surfaces, or sparks. Trichloroethylene should not be
stored near food-stuffs, strong acids, alkalis, or oxidizing
agents.
3.2.3. Recovery
Used trichloroethylene can readily be recovered by
distillation. Trichloroethylene vapours in the aspiration ducts
of plants can be recovered by adsorption on activated carbon and
subsequent desorption.
3.2.4. Disposal
Where trichloroethylene is not recovered and recycled, it may
be disposed of by incineration. Incinerators must be properly
operated, at a sufficiently high temperature and for an adequate
period of time, to ensure complete combustion and prevent the
formation of other toxic chlorinated compounds. The incinerator
should incorporate a suitable scrubber to remove the acidic
breakdown products.
3.2.5. Emergency measures in case of accidental spills
The spilt liquid should be contained with earth, sand, or other
inert adsorbent material to prevent it from spreading.
If possible, remove damaged containers to an isolated and well-
ventilated area, preferably outside, or transfer contents to
another container by mechanical pumping.
Wash away small leaks with water, taking appropriate measures
to avoid creating environmental pollution problems.
When necessary, the contaminated area should be marked off
until the risk of dangerous concentrations in the air has been
eliminated.
3.2.6. Occupational exposure
Trichloroethylene is a widely-used industrial solvent and
degreasing agent. During production, exposure is relatively low
and can be controlled, but users of trichloroethylene may be
exposed to higher levels and under relatively uncontrolled
conditions depending on the type of operation involved. A WHO
Study Group has recommended a time-weighted average exposure not
exceeding 135 µg/m3 with a ceiling limit value of 1000 mg/m3 for
not more than 15 min (WHO Study Group on Recommended Health-Based
Limits in Occupational Exposure to Selected Organic Solvents,
1981). Some national occupational exposure limits are listed in
Table 3.
Table 3. Occupational exposure limits used in various
countriesa
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Australia 100 535 TWAb
Austria 50 260 TWA
Belgium 100 535 TWA
Bulgaria 2 10 TWA
Czechoslovakia 47 250 TWA
235 1250 CVc
Egypt 50 267 TWA
Finland 50 260 TWA
France 75 405 TWA
200 1080 CV
German Democratic 47 250 TWA
Republic 141 750 STd (30 min)
Germany, Federal 50 260e TWA(MAK)
Republic of
Hungary 10 50 TWA
Italy 75 400 TWA
200 1000 skin irritation
Japan 50 268 TWA
Netherlands 35 190 TWA
Poland 10 50 CV
Romania 37 200 TWA
55 300 CV
Spain 100 535 TWA
--------------------------------------------------------
Table 3. (contd.)
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Sweden 20 110 TWA
50 250 ST (15 min)
Switzerland 50 260 TWA
United Kingdom 100 535 TWA
USA
a) OSHA/NIOSH 100 536 TWA
200 1072 ST (5 min)
300 1608 CV
1000 5350 IDLHf
b) ACGIHg 100 535 TWA
150 800 ST (15 min)
USSR 2 10 CV
Yugoslavia 50 200 TWA
--------------------------------------------------------
a From: ILO (1980) and IRPTC (1984).
b TWA (time-weighted average): a mean exposure limit
averaged generally over a working day whereby, within
prescribed limits, excursions above the level
specified are permitted, provided they are compensated
for by excursions below the limit specified.
c CV (ceiling value): a maximum allowable concentration
that must not be exceeded at any time.
d ST (short-term exposure limit): a maximum
concentration allowed for a short specified duration.
e Suspected carcinogen.
f IDLH (immediately dangerous to life and health): a
maximum level from which escape is possible within
30 min without escape-impairing symptoms or any
irreversible health effects.
g Notice of intended change to TWA 270 mg/m3 (50 ppm)
and ST 805 mg/m3 (150 ppm).
Note: Occupational exposure levels and limits are derived in
different ways, possibly using different data and expressed
and applied in accordance with national practices. These
aspects should be taken into account when making
comparisons.
4. ENVIRONMENTAL LEVELS, TRANSPORT AND DISTRIBUTION
4.1. Environmental Levels
4.1.1. Soils and sediments
Trichloroethylene has been found in concentrations exceeding
100 µg/kg in soils and sediments near production sites (IARC,
1979). However, samples taken further away from production sites
show lower levels: for example, in Liverpool Bay, United Kingdom,
which is near an urban and industrialized area, concentrations in
sediments ranged from a few ng/kg to 10 µg/kg (Pearson & McConnell,
1975). An organic-rich anoxic marine sediment from the
Pettaquamscutt River in Rhode Island, USA, where there were no
obvious local sources of trichloroethylene, contained
concentrations ranging from undetectable to 70 µg/kg dry weight
(Whelan et al., 1983). Trichloroethylene was concentrated in the
upper part of the sediment core, corresponding to the period from
about 1940 to the present (determined by 210Pb dating). The
authors noted that the compound had been in use only since the mid-
1940s.
No relationship between trichloroethylene concentration and
particle size or organic matter in sediments, as noted for higher
hydrocarbons such as the DDT group (Pierce et al., 1974), has been
reported.
4.1.2. Water
Trichloroethylene is widely distributed in surface water,
rain-water, well water, and drinking-water from various sources.
Chemical industry discharges may contain concentrations up to
200 µg/litre (Eurocop-Cost, 1976); some Milan well waters contain
high concentrations (80 µg/litre) because of pollution (Cavallo &
Grassi, 1976; Ziglio et al., 1983). However, most reported levels
in water are below this, and are usually in the range of 10 -
100 µg/litre (Rook et al., 1975; Ewing et al., 1977). Rain-water
has been reported to contain concentrations in the µg/litre range
(McConnell et al., 1975) and sea water from Liverpool Bay, United
Kingdom contained a mean concentration of 0.3 µg/litre (Pearson &
McConnell, 1975). This lower range has also been reported in some
Japanese rivers (EAJ, 1983) and in some well water in the USA
(Coleman et al., 1976). Even lower concentrations (7 - 11
ng/litre) have been reported for northeast Atlantic surface water
(Murray & Riley, 1973).
While trihalomethanes are produced during the chlorination of
natural waters containing humic substances, there are no data
indicating that trichloroethylene is produced in this way (Bellar
et al., 1979; Bauer & Selenka, 1982; Otson et al., 1982). However,
treatment of sewage effluent resulted in a small increase in the
trichloroethylene level (Bellar et al., 1979). Trichloroethylene
was found in drinking-water when the original raw water source was
contaminated or when the liquid chlorine used for water treatment
contained trichloroethylene as an impurity. It is also an
intermediate in the breakdown of tetrachloroethylene in some
groundwater systems (Parsons et al., 1984).
Because data for carcinogenicity are inadequate for evaluation,
a tentative guideline value of 30 µg/litre in drinking-water has
been recommended by the World Health Organization (WHO, 1984).
4.1.3. Air
The distribution of trichloroethylene in the atmosphere has
been studied intensively, because of its possible contribution to
depletion of the ozone layer (Lovelock, 1974) (section 7.3).
Air concentrations are in the µg/m3 range (Lovelock, 1974;
Pearson & McConnell, 1975; Cronn et al., 1977; Singh et al., 1977;
Rasmusson et al., 1983). Murray & Riley (1973) reported much lower
concentrations, in the ng/m3 range, in rural areas or from sea
stations; one urban sample (Liverpool) contained 0.85 µg/m3. In
general, higher levels are found near industrialized areas (Pearson
& McConnell, 1975; Ohta et al., 1976; Correia et al., 1977; Ziglio
et al., 1983).
Data describing the partition of trichloroethylene between the
gaseous and particulate phases in the atmosphere are not available.
4.1.4. Biota
Pearson & McConnell (1975) have described trichloroethylene
concentrations in marine organisms from Liverpool Bay, United
Kingdom which is fairly close to an urban and industrialized
region. Concentrations ranged from a few ng/g to about 100 ng/g
wet weight. There was no obvious correlation between concentration
and trophic level. Typical background concentrations are probably
around 10 ng/g wet weight.
Other studies have shown the presence of trichloroethylene in
marine organisms such as invertebrates (1 µg/kg wet weight), fish
muscle (10 µg/kg), sea-bird eggs (50 µg/kg), and seal fat
(50 µg/kg) (Pearson & McConnell, 1975).
Pearson & McConnell (1975) analysed samples of marine organisms
mainly, but not exclusively, from areas near a region where major
organochlorine production plants were situated. Less than 15 µg/kg
wet weight was found in fish muscle (plaice, dab, mackerel).
Values in sea-bird eggs ranged from 2.4 µg/kg for Phalacrocorax
aristotelis (shag) to around 30 (23 - 33) µg/kg for Alca torda
(razorbill), Uria aalge (guillemot), and Rissa tridactyla
(kittiwake). Seal (Halichaerus grypus) blubber and liver from the
Faroe Islands had values ranging from 2.5 to 7.2 µg/kg.
4.1.5. Food
Trichloroethylene may be present in foodstuffs as a residue
from its use as a solvent in food processing or as the result of
environmental contamination. A study conducted by McConnell et al.
(1975) provided a table of the trichloroethylene contents of some
common foodstuffs (Table 4).
Table 4. Trichloroethylene in major foodstuffsa
------------------------------------------------
Foodstuff Concentration
(µg/kg)
------------------------------------------------
Dairy foods:
fresh milk 0.3
Cheshire cheese 3
English butter 10
eggs 0.6
Meat:
shin of beef 16
adipose tissue of beef 12
pig liver 22
Oils and fats:
margarine 6
olive oil (Spanish) 9
cod liver oil 19
vegetable oil for frying 7
Drinks:
fruit juices 5
light beer 0.7
freeze-dried coffee 4
tea in bags 60
wine (Yugoslav) 0.02
Fruit and vegetables:
potatoes 3
apples 5
pears 5
Cereals:
fresh bread 7
------------------------------------------------
a From: McConnell et al. (1975).
In some cases, upper tolerable limits for trichloroethylene
concentrations have been set; for instance, 25 mg/kg dry weight
in powdered decaffeinated coffee, 10 mg/kg dry weight in instant
coffee, and 30 mg/kg dry weight in spice oleoresins. The US FDA
has proposed the prohibition of the use of trichloroethylene in
foodstuffs. The progress of this proposal depends on the
completion of long-term toxicology and carcinogenicity studies
that are being carried out. Before 1976, the US FDA prescribed
tolerance level for trichloroethylene in decaffeinated ground
coffee was 25 mg/kg dry weight (US CFR, 1976).
Trichloroethylene has been reviewed on a number of occasions
by the Joint FAO/WHO Expert Committee on Food Additives, most
recently in 1983. An acceptable daily intake (ADI) has not been
allocated. The Joint Expert Committee recommended that the use of
trichloroethylene as an extraction solvent should be limited, in
order to ensure that its residues in food are as low as practicable
(Joint FAO/WHO Expert Committee on Food Additives, 1983).
4.2. Environmental Distribution and Transport
4.2.1. Equilibrium distribution
The distribution of trichloroethylene, which is observed in
various environmental "compartments", is similar to that which
would be expected from a consideration of its physical and chemical
properties (cf. Appendix I). The relatively high vapour pressure
at normal environmental temperatures should lead to appreciable
atmospheric concentrations; this tendency will balance the tendency
of relatively high water solubility and low Po/w to lead to high
water, biota, or sediment concentrations through either partition
or adsorption. The tendency of trichloroethylene to enter the
atmosphere is demonstrated further by its rapid evaporation from
water; its evaporation half-life is approximately 20 min at 25 °C
(Dilling, 1977).
4.2.2. Transformation in the environment
Recent studies on the degradation of trichloroethylene in
various environmental compartments are discussed below.
4.2.2.1. Air
The main removal reaction appears to be that of attack by the
tropospheric hydroxyl radical (Penkett, 1982), the steady-state
concentrations of which are around 4 x 105/cm3 (Graedel, 1978).
The decay of trichloroethylene is a function of the rate of its
(bimolecular) reaction with the hydroxyl radical (Graedel, 1978),
which is about 2.4/1012 cm3 per molecule per second at 25 °C
(Howard, 1976). This leads to a calculated reaction rate of
approximately 4/103 per h, with the calculated lifetime of
trichloroethylene in the atmosphere of around 11 days (Graedel,
1978). A half-life of the order of 5 days has been calculated by
(De More et al., 1983). Singh et al. (1977) reported a half-life
of less than 2 days in a smog chamber. Pearson & McConnell (1975),
using unrealistically high concentrations of trichloroethylene in
quartz flasks, estimated its half-life to be 11 weeks.
4.2.2.2. Soils and sediments
When methanogenic bacterial batch cultures were exposed to
low concentrations of trichloroethylene (simulating conditions in
an organic-rich sediment or in a sewage treatment system), at
35 °C, for 8 weeks, trichloroethylene concentrations were reduced
by about 40% (Bouwer & McCarty, 1983). If it is assumed that the
reaction rate is halved with every 10 °C drop in temperature, this
corresponds to an exponential decay rate (first order with respect
to trichloroethylene) of about 2/104 per h at 15 °C.
In a study on a laboratory fresh water-sediment system, it was
concluded that trichloroethylene, formed by biotransformation from
tetrachloroethylene, was itself biotransformed to chloroethane,
cis- and trans-1,2-dichloroethene, and dichloromethane (Parsons et
al., 1984).
4.2.2.3. Water
Wakeham et al. (1982) measured a trichloroethylene exponential
decay rate in a sea-water mesocosm of approximately 2.5/102 per day
at 8 - 16 °C, which is equivalent to a rate of about 1/103 per h.
This is similar to the rate described by Bouwer & McCarty (1983)
for microbial degradation. Pearson & McConnell (1975) measured a
chemical degradation rate, in sealed bottles, which led to a half-
life estimate of 2.5 years.
4.2.2.4. Biota
The only data available refer to the degradation of
trichloroethylene in a soil-plant system (Klozskowski et al.,
1981) in which the rate of trichloroethylene loss was 10% per week.
This was accounted for mainly by conversion to carbon dioxide, but
with some evaporation of organic compounds. This corresponds to an
exponential decay rate of about 6/103 per h, which is about 10
times the microbial decay rates.
5. KINETICS AND METABOLISM
5.1. Absorption
Trichloroethylene absorption in mammals can take place by the
respiratory, oral, and/or dermal routes. Intraperitoneal uptake
has been demonstrated experimentally.
5.1.1. Inhalation exposure
In all the mammalian species studied, trichloroethylene uptake
is high during the first minutes of exposure. It then decreases
until equilibrium is reached between uptake by the blood and
release from the blood to tissues, and by metabolism. After
equilibrium is reached, uptake remains constant for the remainder
of exposure (Fernandez et al., 1975; Monster et al., 1976).
In human beings, the blood/air partition coefficient ranges
from 9 to 15. Daily body uptake has been estimated to be
approximately 6 mg/kg body weight, for an exposure of 4 h at
378 mg/m3 (70 ppm), and does not seem to be greatly influenced
by the quantity of adipose tissue (Monster et al., 1976, 1979;
Monster, 1979). Trichloroethylene retention varies according to
physical activity. Under laboratory conditions, when human
volunteers at rest were exposed to concentrations of 540 or
1080 mg/m3 (100 or 200 ppm), for 30 min, 50% of the quantity
inhaled was retained. The percentage retained decreased from 50
to 25%, when activity rose from rest to a 150-watt work load, but,
because of increased ventilation, the absolute amount absorbed
still increased (Astrand & Ovrum, 1976).
5.1.2. Oral exposure
Uptake via the oral route is high because of the ease with
which trichloroethylene penetrates the gastrointestinal barrier.
In man, oral intake is a frequent cause of acute poisoning (Waters
et al., 1977).
5.1.3. Dermal exposure
In the mouse, dermal absorption increases linearly at a
constant rate with duration of exposure. For exposure periods of
between 15 min and 5 h, absorption rates ranged from 59.8 to
92.4 nmol/min per cm2 (Tsuruta, 1978).
Trichloroethylene applied to the backs of guinea-pigs (glass
depot containing at least 1.0 ml) was absorbed and produced blood
concentrations of 0.79 mg/litre after 0.5 h and decreased to
0.46 mg/litre after 6 h, in spite of continuing exposure (Jakobson
et al., 1982).
When one hand of each of 4 human male volunteers was immersed
in trichloroethylene for 30 min, Sato & Nakajima (1978) found blood
concentrations of trichloroethylene (samples taken from unexposed
arm) of 2 mg/litre, immediately after the end of immersion,
0.34 mg/litre, after 30 min, and 0.22 mg/litre, after 60 min.
The trichloroethylene concentration in the expired air was
0.28 mg/litre, 5 min after the end of immersion, 0.06 mg/litre,
30 min after, and 0.024 mg/litre 60 min after.
On the basis of these data and the results of earlier studies
by Stewart & Dodd (1964), it is thought unlikely that
trichloroethylene would be absorbed in toxic quantities through
intact skin during normal industrial use.
5.2. Distribution and Storage
After absorption, trichloroethylene is concentrated in the
cellular components but disappears rapidly (Fabre & Truhaut, 1952).
This rapid disappearance occurs because substantial amounts of
trichloroethylene are metabolized during and after exposure
(Monster, 1979). Trichloroethylene reaches the tissues via the
blood system and accumulates, particularly in adipose tissue,
because of its high liposolubility. The oil/blood partition
coefficient is approximately 750 (Droz & Fernandez, 1977; Sato &
Nakajima, 1979). Trichloroethylene crosses the placental barrier
readily and has been found in fetal blood (Laham, 1970). It
was detectable in the fetus in 2 min, and a fetal/maternal
concentration equilibrium ratio of 1:1 was reached in 6 min (La Du
et al., 1971). Data on the distribution of trichloroethylene in
the tissues of some animal species (including human beings) are
available but, because of the differences in treatment, comparative
conclusions are not possible. Trichloroethylene concentrations
found in various organs and tissues from guinea-pigs, rats, and
human beings are listed in Table 5.
5.3. Metabolic Transformation
5.3.1. Animals
Trichloroethylene is metabolized primarily in the liver and,
to a much lesser extent, in other tissues. Metabolism is by the
mixed-function oxidase system and is dependent on cytochrome P 450.
Qualitative differences between species do not seem particularly
significant with the exception of dichloroacetic acid formation,
which appears to be specific for the mouse (Hathway, 1980; Green &
Prout, in press). The major mammalian metabolites are free and
conjugated trichloroethanol and trichloroacetic acid. Other
metabolites include 2-hydroxyacetylethanolamine and oxalic acid
(Dekant & Henschler, 1982; DeKant et al., 1984). Metabolism is
illustrated in Fig. 4.
Quantitative differences in the rates of metabolism in
different species are much more significant. Mice metabolize
trichloroethylene to a much greater extent than rats (Stott et
al., 1982). It is possible to saturate the metabolism of
trichloroethylene in the rat, but not in the mouse, at doses up to
2000 mg/kg body weight (Anderson et al., 1980). Mice also produce
more reactive tissue-binding metabolites than rats in the liver and
the kidney (Stott et al., 1982).
Table 5. Tissue distribution of trichloroethylene in
(A) guinea-pigs and rats following exposure to the
compound, and (B) in human tissues obtained at autopsy
(levels of exposure not specified)
----------------------------------------------------------
(A) (B)
Organs or Guinea- Ratsb Human beingsa
tissues pigsa d e
(mg/kg) (mg/kg) (µg/kg)
----------------------------------------------------------
adrenals 22 - - -
blood 5 0.9 - -
brain 9 1.0 (1.2)c 1 -
fat 39 9.9 8.2 (32) 4.9 (11.7)
- - - 7.8 (42.2)f
kidney 14 - 2.0 -
liver 10 0.3 4.1 (5.8) 2.5
lung 7 0.7 - 2.2
muscle 2 - - 2.4 (156.6)
ovary 23 - - -
spleen 13 - - -
----------------------------------------------------------
a From: Fabre & Truhaut (1952), 6045 mg/m3 (1120 ppm)
x 5 h per day x 19 days.
b From: Savolainen et al. (1977), 1080 mg/m3 (200 ppm)
x 6 h/day x 5 days).
c Cerebellum value.
d From: McConnell et al. (1975). Mean of 8 subjects aged
48 - 52 years.
e From: Bauer (1981). Mean of 15 subjects (Figures in
parentheses are maximum values).
f Fat from kidney capsule.
2.3.2. Determination in environmental media
2.3.2.1. Soil
High-resolution GC (hrGC-ECD) has been used for determining
trichloroethylene in soil (De Leon et al., 1980). The hrGC/MS
combination has been used as a confirmatory technique, with a
detection threshold of ~10 mg/kg (10 ppm).
2.3.2.2. Water
Levels of trichloroethylene in water can be determined by
hrGC/MS (Dowty et al., 1975), by GC with an electron-capture
detector or an electrolytic conductivity detector (Nicholson et
al., 1977; Dietz & Singley, 1979), and by various other techniques
such as HPLC, hrGC, GC, and GC/MS (Eklund et al., 1978; Jungclaus
et al., 1978). Where specified, detection thresholds are in the
region of 1.0 µg/litre, or lower.
2.3.2.3. Air
GC/MS can be used to measure trichloroethylene levels in the
urban atmosphere (Ioffe et al., 1977). Herbolsheimer et al.
(1972), Sawicki et al. (1975), NIOSH (1977a), Heil et al. (1979),
and Makide et al. (1979) describe sampling and sample enrichment
techniques. Detection capacity may be as low as some ng/m3.
Fujiwara's test can be used to measure trichloroethylene levels
in air (Rush, 1970).
Gas detector tubes (Kitagawa, 1961), activated carbon tubes
(NIOSH, 1977a; Shipman & Whim, 1980), and activated carbon felt
badges (Hirayama & Ikeda, 1979) are available for use in work-place
environments. Gas detector tubes are suitable for spot sampling,
while activated carbon tubes and felt are suitable for time-
weighted average concentration determinations.
2.3.2.4. Foodstuffs
High- and low-resolution GLC can be used for the determination
of trichloroethylene and other aliphatic chloro derivatives in
various foodstuffs (Entz & Hollifield, 1982). The head-space
technique is used in all cases. The sensitivity of the method
appears to be higher (< 1 µg/kg) for water-rich samples than for
fat-rich foodstuffs (10 -50 µg/kg). The coefficient of variation
can be lower than 20%.
2.3.3. Determination in human tissues and fluids
The methods described in this section are those normally
used to determine the levels of trichloroethylene or its major
metabolites (trichloroacetic acid and trichloroethanol) in
blood and urine. These methods can be used to obtain indirect
measurements of exposure. There are methods for the determination
of trichloroethylene and trichloroethanol in expired air.
2.3.3.1. Trichloroethylene
Blood or urine is distilled or aerated. Any trichloroethylene
vapour present is collected in pyridine, which is then subjected to
Fujiwara's colour test (Seto & Schultze, 1956; Tada, 1969).
Trichloroethylene detection and determination in blood and
urine samples are now generally performed by GC, which has largely
replaced colorimetric reactions. Samples are first extracted with
solvents, and trichloroethylene concentrations are then determined
in the extract. There are various modifications of this technique
(Stewart et al., 1962; Stewart & Dodd, 1964; Kylin et al., 1967;
Stewart et al., 1970; Ertle et al., 1972). Because of its
volatility, trichloroethylene can be sampled using the head-space
method (Monster & Boersma, 1975; Triebig et al., 1976; Astrand &
Ovrum, 1976). This method has also been successfully coupled with
GC/MS (Balkon & Leary, 1979).
GLC alone (Monster & Boersma, 1975; Astrand & Ovrum, 1976)
and GC/MS (Barkley et al., 1980) have been used to determine
trichloroethylene in exhaled air. The same methods, with suitable
sampling techniques, may also be used for the determination of
trichloroethylene in alveolar air.
2.3.3.2. Trichloroacetic acid
Fujiwara's colorimetry test is performed on the blood or urine
sample extract or, in the case of urine, directly on the sample
itself (Abrahamsen, 1960; Soucek & Vlachová, 1960; Bartonícek,
1962; Fawns, 1968; Tanaka & Ikeda, 1968; Tada, 1969; Weichardt &
Bardodej, 1970; Ertle et al., 1972; Kimmerle & Eben, 1973; Mantel &
Nothmann, 1977).
Trichloroacetic acid can also be determined by gas
chromatography of the extract after methylation (Ehrner-Samuel
et al., 1973; Ogata & Saeki, 1974; Nomiyama et al., 1978;
van der Hoeven et al., 1979), by direct methylation of the
specimen followed by head-space sampling and GC (Monster & Boersma,
1975; Triebig et al., 1976), or by inducing trichloroacetic acid
decarboxylation and measuring the chloroform thus formed (Müller
et al., 1972; Buchet et al., 1974). Ziglio (1979) determined
trichloroacetic acid in subjects who had absorbed trichloroethylene
in drinking-water. The extraction and methylation method, as well
as the method of inducing thermal decarboxylation and then
injecting the chloroform thus formed according to the head-space
method, were used (sensitivity of extraction and methylation
methods is better than 10 µg/litre).
2.3.3.3. Trichloroethanol
Trichloroethanol is found in the free state and as the
glucuronide (urochloralic acid) in both blood and urine. For
total trichloroethanol determination, the glucuronide is
hydrolysed. The trichloroacetic acid originally present is then
removed and the trichloroethanol is oxidized quantitatively to
trichloroacetic acid (Vlachov, 1957). The trichloroacetic acid
thus formed is then measured by one of the methods previously
described. Alternatively, trichloroethanol can be distilled in a
vapour stream and measured colorimetrically on the basis of the
condensate resulting from the reaction with pyridine and alkalis
(Bardodej, 1962). The colour test can also be carried out without
prior separation of trichloroethanol from trichloroacetic acid.
The two compounds are measured by determining absorbance at 367 or
440 nm, and at 530 nm (Cabana & Gessner, 1967; Ogata et al., 1970;
Mantel & Nothmann, 1977). Trichloroethanol can also be measured by
determining the difference between the figure obtained for the
trichloroacetic acid level and that obtained for all trichloro-
derivatives present after quantitative oxidation to trichloroacetic
acid (Seto & Schulze, 1956; Tanaka & Ikeda, 1968).
Trichloroethanol can be measured by gas chromatography after
quantitative hydrolysis of the glucuronide (Ogata et al., 1970;
Ertle et al., 1972; Kimmerle & Eben, 1973; Ogata & Saeki, 1974;
Buchet et al., 1974; Nomiyama et al., 1978). The technique of
head-space sampling has been used by Monster & Boersma (1975),
Triebig et al. (1976), and Balkon & Leary (1979).
Trichloroethanol in exhaled air can be measured directly
(Monster & Boersma, 1975).
2.3.3.4. Total trichloro derivatives
In the Imamura & Ikeda (1973) method, urine samples are
oxidized with chromium trioxide in heated nitric acid, allowed to
cool, and made alkaline; pyridine is added followed by mild heating
(Fujiwara test). Solution absorbance is then determined at 530 nm.
2.3.4. Sensitivity
Generally speaking, colorimetric test detection thresholds
range between 0.1 and 1 mg/kg. Greater sensitivity is provided by
gas chromatography, which has detection thresholds of between 10
and 100 µg/kg for trichloroethylene, trichloroacetic acid, and
trichloroethanol.
3. SOURCES IN THE ENVIRONMENT, USES, AND SAFE HANDLING
Trichloroethylene does not occur naturally.
It was first synthesized by Fisher in 1864 and became
commercially available for the first time in 1908 in Austria and in
the United Kingdom (Kirk & Othmer, 1964).
3.1. Production Processes, Levels, and Uses
3.1.1. Production processes and levels
Trichloroethylene is produced by three processes: the
dehydrochlorination of sym-tetrachloroethane, the high-temperature
oxychlorination of chlorinated products with one or two carbon
atoms, or the chlorination of ethylene.
In Western Europe, production was approximately 250 000 tonnes
in 1978. The major producing countries are the Federal Republic of
Germany, France, whose individual production capacity is of the
order of 100 000 tonnes, Italy, and the United Kingdom. Sweden
and Spain are smaller producers. In the USA the production of
trichloroethylene in 1979 was 130 000 tonnes (US ITC, 1980). In
Japan, the annual production was approximately 74 500 tonnes in
1981 and 67 500 tonnes in 1982 (Japanese Yearbook of Chemical
Industries Statistics, 1983).
3.1.2. Uses
Trichloroethylene is an industrial solvent mainly (85 - 90%)
used for the vapour degreasing and cold cleaning of fabricated
metal parts. Trichloroethylene has also been used as a carrier
solvent for the active ingredients of insecticides and fungicides;
as a solvent for waxes, fats, resins, and oils; as an anaesthetic
for medical and dental use; and as an extractant for spice
oleoresins and for caffeine from coffee. Trichloroethylene has
been used in printing inks, varnishes, adhesives, paints, lacquers,
spot removers, rug cleaners, disinfectants, and cosmetic cleansing
fluids. It may also be used as a chain terminator in polyvinyl
chloride production and as an intermediate in the production of
pentachloroethane (Defalque, 1961; Kirk & Othmer, 1963, 1979;
Wetterhahn, 1972; Valle-Riestra, 1974; US CFR, 1976; Waters et al.,
1977; IARC, 1979).
3.2. Handling Hazards, and Precautions
3.2.1. Handling hazards
3.2.1.1. Fire, explosion, and thermal decomposition
At normal handling temperatures, trichloroethylene behaves
as a non-flammable, non-burnable substance. Under normal
conditions, it is virtually impossible to induce an explosion with
trichloroethylene. In the presence of air, at temperatures above
400 °C, it produces phosgene, hydrochloric acid, and carbon
monoxide. In the vicinity of arc welding, phosgene and hydrogen
chloride can be produced from trichloroethylene. In vapour
degreasing, using combustion heaters, precautions must be taken to
prevent solvent fumes from entering the combustion air. Containers
of trichloroethylene exposed to fire should be cooled by sprinkling
with water.
3.2.1.2. Chemical reactivity
Trichloroethylene is practically non-reactive with water at
room temperature, under normal storage conditions, and the
stabilized product does not undergo any changes in the presence of
air, humidity, light, or in contact with metals. It is, however, a
wise precaution not to expose the product to temperatures exceeding
130 °C.
In the presence of strong alkalis, particularly if heated,
trichloroethylene produces dichloroacetylene (Reichert et al.,
1980a), which is highly reactive and acutely neurotoxic to both
animals and man (Reichert & Henschler, 1978). Dichloroacetylene
is also potentially carcinogenic (Reichert et al., 1980b). Under
normal circumstances in industrial use, this reaction is unlikely.
However, under occasional laboratory conditions and closed-circuit
anaesthesia, in the presence of soda lime, some dichloroacetylene
may be produced.
Dichloroacetylene may be formed from trichloroethylene by a
reaction catalysed by ionic halides in the presence of certain
epoxides, including epichlorohydrin (Dobinson & Green, 1972).
Non-stabilized trichloroethylene can react violently with
aluminium (especially in the form of dust or filings) giving off
hydrogen chloride and hexachlorobutene vapour (McNeill, 1979).
Not all stabilizers are effective in preventing the reaction with
aluminium; therefore, a suitably-stabilized product should be used
when cleaning aluminium, especially ultrasonically or where
aluminium particles are present. Suitable products are identified
in manufacturers' literature or in specifications.
3.2.2. Handling precautions
3.2.2.1. Personal safeguards
Accidental exposure to trichloroethylene under occupational
conditions is more frequently associated with the generation of
dense trichloroethylene vapour, e.g., misoperation of vapour-
degreasing apparatus (Sagawa et al., 1973) or the use of liquid
trichloroethylene for cleaning the inside of a tank.
Individual protective measures should be related to the type
and level of exposure. When significant skin contact is likely,
suitable protective clothing should be worn, bearing in mind the
limitations of such clothing and the need to maintain it properly
and replace it regularly. To control exposure through inhalation,
the use of full face masks with filters for organic vapours
(basically for short-term or emergency use), self-contained
breathing apparatus, or masks with air-line supply systems may be
necessary. Self-contained breathing apparatus should always be
available for use in emergencies.
3.2.2.2. Storage
Trichloroethylene can be safely stored in carbon steel or
stainless steel containers. It should not be kept in aluminium,
aluminium alloy, or galvanized iron containers; plastic containers
should not be used unless they are known to be suitable for the
storage of trichloroethylene. Storage areas should be cool, well-
ventilated, flame-proof, and shielded from direct sunlight, high-
temperature surfaces, or sparks. Trichloroethylene should not be
stored near food-stuffs, strong acids, alkalis, or oxidizing
agents.
3.2.3. Recovery
Used trichloroethylene can readily be recovered by
distillation. Trichloroethylene vapours in the aspiration ducts
of plants can be recovered by adsorption on activated carbon and
subsequent desorption.
3.2.4. Disposal
Where trichloroethylene is not recovered and recycled, it may
be disposed of by incineration. Incinerators must be properly
operated, at a sufficiently high temperature and for an adequate
period of time, to ensure complete combustion and prevent the
formation of other toxic chlorinated compounds. The incinerator
should incorporate a suitable scrubber to remove the acidic
breakdown products.
3.2.5. Emergency measures in case of accidental spills
The spilt liquid should be contained with earth, sand, or other
inert adsorbent material to prevent it from spreading.
If possible, remove damaged containers to an isolated and well-
ventilated area, preferably outside, or transfer contents to
another container by mechanical pumping.
Wash away small leaks with water, taking appropriate measures
to avoid creating environmental pollution problems.
When necessary, the contaminated area should be marked off
until the risk of dangerous concentrations in the air has been
eliminated.
3.2.6. Occupational exposure
Trichloroethylene is a widely-used industrial solvent and
degreasing agent. During production, exposure is relatively low
and can be controlled, but users of trichloroethylene may be
exposed to higher levels and under relatively uncontrolled
conditions depending on the type of operation involved. A WHO
Study Group has recommended a time-weighted average exposure not
exceeding 135 µg/m3 with a ceiling limit value of 1000 mg/m3 for
not more than 15 min (WHO Study Group on Recommended Health-Based
Limits in Occupational Exposure to Selected Organic Solvents,
1981). Some national occupational exposure limits are listed in
Table 3.
Table 3. Occupational exposure limits used in various
countriesa
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Australia 100 535 TWAb
Austria 50 260 TWA
Belgium 100 535 TWA
Bulgaria 2 10 TWA
Czechoslovakia 47 250 TWA
235 1250 CVc
Egypt 50 267 TWA
Finland 50 260 TWA
France 75 405 TWA
200 1080 CV
German Democratic 47 250 TWA
Republic 141 750 STd (30 min)
Germany, Federal 50 260e TWA(MAK)
Republic of
Hungary 10 50 TWA
Italy 75 400 TWA
200 1000 skin irritation
Japan 50 268 TWA
Netherlands 35 190 TWA
Poland 10 50 CV
Romania 37 200 TWA
55 300 CV
Spain 100 535 TWA
--------------------------------------------------------
Table 3. (contd.)
--------------------------------------------------------
Country Exposure Limit Category
(ppm) (mg/m3) of limit
--------------------------------------------------------
Sweden 20 110 TWA
50 250 ST (15 min)
Switzerland 50 260 TWA
United Kingdom 100 535 TWA
USA
a) OSHA/NIOSH 100 536 TWA
200 1072 ST (5 min)
300 1608 CV
1000 5350 IDLHf
b) ACGIHg 100 535 TWA
150 800 ST (15 min)
USSR 2 10 CV
Yugoslavia 50 200 TWA
--------------------------------------------------------
a From: ILO (1980) and IRPTC (1984).
b TWA (time-weighted average): a mean exposure limit
averaged generally over a working day whereby, within
prescribed limits, excursions above the level
specified are permitted, provided they are compensated
for by excursions below the limit specified.
c CV (ceiling value): a maximum allowable concentration
that must not be exceeded at any time.
d ST (short-term exposure limit): a maximum
concentration allowed for a short specified duration.
e Suspected carcinogen.
f IDLH (immediately dangerous to life and health): a
maximum level from which escape is possible within
30 min without escape-impairing symptoms or any
irreversible health effects.
g Notice of intended change to TWA 270 mg/m3 (50 ppm)
and ST 805 mg/m3 (150 ppm).
Note: Occupational exposure levels and limits are derived in
different ways, possibly using different data and expressed
and applied in accordance with national practices. These
aspects should be taken into account when making
comparisons.
4. ENVIRONMENTAL LEVELS, TRANSPORT AND DISTRIBUTION
4.1. Environmental Levels
4.1.1. Soils and sediments
Trichloroethylene has been found in concentrations exceeding
100 µg/kg in soils and sediments near production sites (IARC,
1979). However, samples taken further away from production sites
show lower levels: for example, in Liverpool Bay, United Kingdom,
which is near an urban and industrialized area, concentrations in
sediments ranged from a few ng/kg to 10 µg/kg (Pearson & McConnell,
1975). An organic-rich anoxic marine sediment from the
Pettaquamscutt River in Rhode Island, USA, where there were no
obvious local sources of trichloroethylene, contained
concentrations ranging from undetectable to 70 µg/kg dry weight
(Whelan et al., 1983). Trichloroethylene was concentrated in the
upper part of the sediment core, corresponding to the period from
about 1940 to the present (determined by 210Pb dating). The
authors noted that the compound had been in use only since the mid-
1940s.
No relationship between trichloroethylene concentration and
particle size or organic matter in sediments, as noted for higher
hydrocarbons such as the DDT group (Pierce et al., 1974), has been
reported.
4.1.2. Water
Trichloroethylene is widely distributed in surface water,
rain-water, well water, and drinking-water from various sources.
Chemical industry discharges may contain concentrations up to
200 µg/litre (Eurocop-Cost, 1976); some Milan well waters contain
high concentrations (80 µg/litre) because of pollution (Cavallo &
Grassi, 1976; Ziglio et al., 1983). However, most reported levels
in water are below this, and are usually in the range of 10 -
100 µg/litre (Rook et al., 1975; Ewing et al., 1977). Rain-water
has been reported to contain concentrations in the µg/litre range
(McConnell et al., 1975) and sea water from Liverpool Bay, United
Kingdom contained a mean concentration of 0.3 µg/litre (Pearson &
McConnell, 1975). This lower range has also been reported in some
Japanese rivers (EAJ, 1983) and in some well water in the USA
(Coleman et al., 1976). Even lower concentrations (7 - 11
ng/litre) have been reported for northeast Atlantic surface water
(Murray & Riley, 1973).
While trihalomethanes are produced during the chlorination of
natural waters containing humic substances, there are no data
indicating that trichloroethylene is produced in this way (Bellar
et al., 1979; Bauer & Selenka, 1982; Otson et al., 1982). However,
treatment of sewage effluent resulted in a small increase in the
trichloroethylene level (Bellar et al., 1979). Trichloroethylene
was found in drinking-water when the original raw water source was
contaminated or when the liquid chlorine used for water treatment
contained trichloroethylene as an impurity. It is also an
intermediate in the breakdown of tetrachloroethylene in some
groundwater systems (Parsons et al., 1984).
Because data for carcinogenicity are inadequate for evaluation,
a tentative guideline value of 30 µg/litre in drinking-water has
been recommended by the World Health Organization (WHO, 1984).
4.1.3. Air
The distribution of trichloroethylene in the atmosphere has
been studied intensively, because of its possible contribution to
depletion of the ozone layer (Lovelock, 1974) (section 7.3).
Air concentrations are in the µg/m3 range (Lovelock, 1974;
Pearson & McConnell, 1975; Cronn et al., 1977; Singh et al., 1977;
Rasmusson et al., 1983). Murray & Riley (1973) reported much lower
concentrations, in the ng/m3 range, in rural areas or from sea
stations; one urban sample (Liverpool) contained 0.85 µg/m3. In
general, higher levels are found near industrialized areas (Pearson
& McConnell, 1975; Ohta et al., 1976; Correia et al., 1977; Ziglio
et al., 1983).
Data describing the partition of trichloroethylene between the
gaseous and particulate phases in the atmosphere are not available.
4.1.4. Biota
Pearson & McConnell (1975) have described trichloroethylene
concentrations in marine organisms from Liverpool Bay, United
Kingdom which is fairly close to an urban and industrialized
region. Concentrations ranged from a few ng/g to about 100 ng/g
wet weight. There was no obvious correlation between concentration
and trophic level. Typical background concentrations are probably
around 10 ng/g wet weight.
Other studies have shown the presence of trichloroethylene in
marine organisms such as invertebrates (1 µg/kg wet weight), fish
muscle (10 µg/kg), sea-bird eggs (50 µg/kg), and seal fat
(50 µg/kg) (Pearson & McConnell, 1975).
Pearson & McConnell (1975) analysed samples of marine organisms
mainly, but not exclusively, from areas near a region where major
organochlorine production plants were situated. Less than 15 µg/kg
wet weight was found in fish muscle (plaice, dab, mackerel).
Values in sea-bird eggs ranged from 2.4 µg/kg for Phalacrocorax
aristotelis (shag) to around 30 (23 - 33) µg/kg for Alca torda
(razorbill), Uria aalge (guillemot), and Rissa tridactyla
(kittiwake). Seal (Halichaerus grypus) blubber and liver from the
Faroe Islands had values ranging from 2.5 to 7.2 µg/kg.
4.1.5. Food
Trichloroethylene may be present in foodstuffs as a residue
from its use as a solvent in food processing or as the result of
environmental contamination. A study conducted by McConnell et al.
(1975) provided a table of the trichloroethylene contents of some
common foodstuffs (Table 4).
Table 4. Trichloroethylene in major foodstuffsa
------------------------------------------------
Foodstuff Concentration
(µg/kg)
------------------------------------------------
Dairy foods:
fresh milk 0.3
Cheshire cheese 3
English butter 10
eggs 0.6
Meat:
shin of beef 16
adipose tissue of beef 12
pig liver 22
Oils and fats:
margarine 6
olive oil (Spanish) 9
cod liver oil 19
vegetable oil for frying 7
Drinks:
fruit juices 5
light beer 0.7
freeze-dried coffee 4
tea in bags 60
wine (Yugoslav) 0.02
Fruit and vegetables:
potatoes 3
apples 5
pears 5
Cereals:
fresh bread 7
------------------------------------------------
a From: McConnell et al. (1975).
In some cases, upper tolerable limits for trichloroethylene
concentrations have been set; for instance, 25 mg/kg dry weight
in powdered decaffeinated coffee, 10 mg/kg dry weight in instant
coffee, and 30 mg/kg dry weight in spice oleoresins. The US FDA
has proposed the prohibition of the use of trichloroethylene in
foodstuffs. The progress of this proposal depends on the
completion of long-term toxicology and carcinogenicity studies
that are being carried out. Before 1976, the US FDA prescribed
tolerance level for trichloroethylene in decaffeinated ground
coffee was 25 mg/kg dry weight (US CFR, 1976).
Trichloroethylene has been reviewed on a number of occasions
by the Joint FAO/WHO Expert Committee on Food Additives, most
recently in 1983. An acceptable daily intake (ADI) has not been
allocated. The Joint Expert Committee recommended that the use of
trichloroethylene as an extraction solvent should be limited, in
order to ensure that its residues in food are as low as practicable
(Joint FAO/WHO Expert Committee on Food Additives, 1983).
4.2. Environmental Distribution and Transport
4.2.1. Equilibrium distribution
The distribution of trichloroethylene, which is observed in
various environmental "compartments", is similar to that which
would be expected from a consideration of its physical and chemical
properties (cf. Appendix I). The relatively high vapour pressure
at normal environmental temperatures should lead to appreciable
atmospheric concentrations; this tendency will balance the tendency
of relatively high water solubility and low Po/w to lead to high
water, biota, or sediment concentrations through either partition
or adsorption. The tendency of trichloroethylene to enter the
atmosphere is demonstrated further by its rapid evaporation from
water; its evaporation half-life is approximately 20 min at 25 °C
(Dilling, 1977).
4.2.2. Transformation in the environment
Recent studies on the degradation of trichloroethylene in
various environmental compartments are discussed below.
4.2.2.1. Air
The main removal reaction appears to be that of attack by the
tropospheric hydroxyl radical (Penkett, 1982), the steady-state
concentrations of which are around 4 x 105/cm3 (Graedel, 1978).
The decay of trichloroethylene is a function of the rate of its
(bimolecular) reaction with the hydroxyl radical (Graedel, 1978),
which is about 2.4/1012 cm3 per molecule per second at 25 °C
(Howard, 1976). This leads to a calculated reaction rate of
approximately 4/103 per h, with the calculated lifetime of
trichloroethylene in the atmosphere of around 11 days (Graedel,
1978). A half-life of the order of 5 days has been calculated by
(De More et al., 1983). Singh et al. (1977) reported a half-life
of less than 2 days in a smog chamber. Pearson & McConnell (1975),
using unrealistically high concentrations of trichloroethylene in
quartz flasks, estimated its half-life to be 11 weeks.
4.2.2.2. Soils and sediments
When methanogenic bacterial batch cultures were exposed to
low concentrations of trichloroethylene (simulating conditions in
an organic-rich sediment or in a sewage treatment system), at
35 °C, for 8 weeks, trichloroethylene concentrations were reduced
by about 40% (Bouwer & McCarty, 1983). If it is assumed that the
reaction rate is halved with every 10 °C drop in temperature, this
corresponds to an exponential decay rate (first order with respect
to trichloroethylene) of about 2/104 per h at 15 °C.
In a study on a laboratory fresh water-sediment system, it was
concluded that trichloroethylene, formed by biotransformation from
tetrachloroethylene, was itself biotransformed to chloroethane,
cis- and trans-1,2-dichloroethene, and dichloromethane (Parsons et
al., 1984).
4.2.2.3. Water
Wakeham et al. (1982) measured a trichloroethylene exponential
decay rate in a sea-water mesocosm of approximately 2.5/102 per day
at 8 - 16 °C, which is equivalent to a rate of about 1/103 per h.
This is similar to the rate described by Bouwer & McCarty (1983)
for microbial degradation. Pearson & McConnell (1975) measured a
chemical degradation rate, in sealed bottles, which led to a half-
life estimate of 2.5 years.
4.2.2.4. Biota
The only data available refer to the degradation of
trichloroethylene in a soil-plant system (Klozskowski et al.,
1981) in which the rate of trichloroethylene loss was 10% per week.
This was accounted for mainly by conversion to carbon dioxide, but
with some evaporation of organic compounds. This corresponds to an
exponential decay rate of about 6/103 per h, which is about 10
times the microbial decay rates.
5. KINETICS AND METABOLISM
5.1. Absorption
Trichloroethylene absorption in mammals can take place by the
respiratory, oral, and/or dermal routes. Intraperitoneal uptake
has been demonstrated experimentally.
5.1.1. Inhalation exposure
In all the mammalian species studied, trichloroethylene uptake
is high during the first minutes of exposure. It then decreases
until equilibrium is reached between uptake by the blood and
release from the blood to tissues, and by metabolism. After
equilibrium is reached, uptake remains constant for the remainder
of exposure (Fernandez et al., 1975; Monster et al., 1976).
In human beings, the blood/air partition coefficient ranges
from 9 to 15. Daily body uptake has been estimated to be
approximately 6 mg/kg body weight, for an exposure of 4 h at
378 mg/m3 (70 ppm), and does not seem to be greatly influenced
by the quantity of adipose tissue (Monster et al., 1976, 1979;
Monster, 1979). Trichloroethylene retention varies according to
physical activity. Under laboratory conditions, when human
volunteers at rest were exposed to concentrations of 540 or
1080 mg/m3 (100 or 200 ppm), for 30 min, 50% of the quantity
inhaled was retained. The percentage retained decreased from 50
to 25%, when activity rose from rest to a 150-watt work load, but,
because of increased ventilation, the absolute amount absorbed
still increased (Astrand & Ovrum, 1976).
5.1.2. Oral exposure
Uptake via the oral route is high because of the ease with
which trichloroethylene penetrates the gastrointestinal barrier.
In man, oral intake is a frequent cause of acute poisoning (Waters
et al., 1977).
5.1.3. Dermal exposure
In the mouse, dermal absorption increases linearly at a
constant rate with duration of exposure. For exposure periods of
between 15 min and 5 h, absorption rates ranged from 59.8 to
92.4 nmol/min per cm2 (Tsuruta, 1978).
Trichloroethylene applied to the backs of guinea-pigs (glass
depot containing at least 1.0 ml) was absorbed and produced blood
concentrations of 0.79 mg/litre after 0.5 h and decreased to
0.46 mg/litre after 6 h, in spite of continuing exposure (Jakobson
et al., 1982).
When one hand of each of 4 human male volunteers was immersed
in trichloroethylene for 30 min, Sato & Nakajima (1978) found blood
concentrations of trichloroethylene (samples taken from unexposed
arm) of 2 mg/litre, immediately after the end of immersion,
0.34 mg/litre, after 30 min, and 0.22 mg/litre, after 60 min.
The trichloroethylene concentration in the expired air was
0.28 mg/litre, 5 min after the end of immersion, 0.06 mg/litre,
30 min after, and 0.024 mg/litre 60 min after.
On the basis of these data and the results of earlier studies
by Stewart & Dodd (1964), it is thought unlikely that
trichloroethylene would be absorbed in toxic quantities through
intact skin during normal industrial use.
5.2. Distribution and Storage
After absorption, trichloroethylene is concentrated in the
cellular components but disappears rapidly (Fabre & Truhaut, 1952).
This rapid disappearance occurs because substantial amounts of
trichloroethylene are metabolized during and after exposure
(Monster, 1979). Trichloroethylene reaches the tissues via the
blood system and accumulates, particularly in adipose tissue,
because of its high liposolubility. The oil/blood partition
coefficient is approximately 750 (Droz & Fernandez, 1977; Sato &
Nakajima, 1979). Trichloroethylene crosses the placental barrier
readily and has been found in fetal blood (Laham, 1970). It
was detectable in the fetus in 2 min, and a fetal/maternal
concentration equilibrium ratio of 1:1 was reached in 6 min (La Du
et al., 1971). Data on the distribution of trichloroethylene in
the tissues of some animal species (including human beings) are
available but, because of the differences in treatment, comparative
conclusions are not possible. Trichloroethylene concentrations
found in various organs and tissues from guinea-pigs, rats, and
human beings are listed in Table 5.
5.3. Metabolic Transformation
5.3.1. Animals
Trichloroethylene is metabolized primarily in the liver and,
to a much lesser extent, in other tissues. Metabolism is by the
mixed-function oxidase system and is dependent on cytochrome P 450.
Qualitative differences between species do not seem particularly
significant with the exception of dichloroacetic acid formation,
which appears to be specific for the mouse (Hathway, 1980; Green &
Prout, in press). The major mammalian metabolites are free and
conjugated trichloroethanol and trichloroacetic acid. Other
metabolites include 2-hydroxyacetylethanolamine and oxalic acid
(Dekant & Henschler, 1982; DeKant et al., 1984). Metabolism is
illustrated in Fig. 4.
Quantitative differences in the rates of metabolism in
different species are much more significant. Mice metabolize
trichloroethylene to a much greater extent than rats (Stott et
al., 1982). It is possible to saturate the metabolism of
trichloroethylene in the rat, but not in the mouse, at doses up to
2000 mg/kg body weight (Anderson et al., 1980). Mice also produce
more reactive tissue-binding metabolites than rats in the liver and
the kidney (Stott et al., 1982).
Table 5. Tissue distribution of trichloroethylene in
(A) guinea-pigs and rats following exposure to the
compound, and (B) in human tissues obtained at autopsy
(levels of exposure not specified)
----------------------------------------------------------
(A) (B)
Organs or Guinea- Ratsb Human beingsa
tissues pigsa d e
(mg/kg) (mg/kg) (µg/kg)
----------------------------------------------------------
adrenals 22 - - -
blood 5 0.9 - -
brain 9 1.0 (1.2)c 1 -
fat 39 9.9 8.2 (32) 4.9 (11.7)
- - - 7.8 (42.2)f
kidney 14 - 2.0 -
liver 10 0.3 4.1 (5.8) 2.5
lung 7 0.7 - 2.2
muscle 2 - - 2.4 (156.6)
ovary 23 - - -
spleen 13 - - -
----------------------------------------------------------
a From: Fabre & Truhaut (1952), 6045 mg/m3 (1120 ppm)
x 5 h per day x 19 days.
b From: Savolainen et al. (1977), 1080 mg/m3 (200 ppm)
x 6 h/day x 5 days).
c Cerebellum value.
d From: McConnell et al. (1975). Mean of 8 subjects aged
48 - 52 years.
e From: Bauer (1981). Mean of 15 subjects (Figures in
parentheses are maximum values).
f Fat from kidney capsule.
 Metabolically activated trichloroethylene binds covalently to
the hepatic microsomal proteins and DNA, in vitro. This finding
supports the formation of an epoxide intermediate (Banerjee & Van
Duuren, 1978), though this has not been demonstrated in vivo
(Parchman & Magee, 1982; Stott et al., 1982).
5.3.2. Human beings
As originally suggested by Powell (1945), the formation of the
epoxide, an intermediate reactive metabolite that binds covalently
with proteins (Bolt & Filher, 1977), has been confirmed by indirect
spectral evidence (Uehleke et al., 1977). The epoxide may undergo
intramolecular rearrangement in 2 different ways (Henschler & Hoos,
1982; DeKant & Henschler, 1983). One pathway leads to chloral
(Henschler, 1977a,b), which is further oxidized to trichloroacetic
acid (TCA), or reduced to trichloroethanol (Leibman, 1965). After
oral administration, trichloroethanol is also partly metabolized
into TCA (Müller et al., 1974). Trichloroethanol is rapidly
conjugated with glucuronic acid to form the respective glucuronide.
The other pathway leads to the formation of dichloroacetyl chloride
which, under in vitro conditions, can lead to the formation of
dichloroacetic acid. However, under normal in vivo conditions,
dichloroacetic acid is not found, except in mice following the
administration of very high doses of trichloroethylene. Under
these conditions, an "overspill" mechanism may operate (Henschler
et al., 1979; Hathway, 1980; Henschler et al., 1983). The
excretion of chloroform in expired air and monochloroacetic acid in
urine have also been proposed as minor routes of metabolism (Ogata
& Saeki, 1974; Bartonicek, 1962). Miller & Guenrich (1982)
suggested that an epoxide was not an obligatory intermediate step
and proposed an alternative model in which chlorine migration
occurs in an oxygenated trichloroethylene P 450 transition state.
Trichloroacetic acid binds well with plasma proteins and its
concentration in plasma is approximately double that in whole blood
(Müller et al., 1972).
5.3.3. Drug and other interactions
A number of commonly-used drugs might be expected to modify the
extent of metabolism of trichloroethylene during human exposure.
Although largely undocumented in man, the induction of the hepatic
microsomal mixed-function oxidase system by drugs, taken for
therapeutic reasons, or by exposure to certain environmental
chemicals (e.g., phenobarbital, toluene, PCBs) can bring about an
increased rate of trichloroethylene metabolism (Ikeda & Imamura,
1973; Ikeda, 1974).
In human beings, the simultaneous administration of ethanol and
trichloroethylene (100 mg/m3 for 6 h) causes an increase in
trichloroethylene levels in both plasma (2.4 times the normal
value) and in exhaled air (3.4 times), and a decrease in the levels
of trichloroacetic acid and trichloroethanol (Müller et al., 1975).
5.4. Elimination
5.4.1. Studies on animals
The kinetics of the distribution and elimination of
trichloroethylene, administered intravenously in Wistar rats at
dose levels of 6, 9, 12, or 15 mg/kg body weight show that the
blood concentration exhibits a first order, 2-compartment model
exponential disappearance, and it has been suggested that a dose
of 15 mg trichloroethylene/kg body weight is within the hepatic
metabolic capacity in the rat (Withey & Collins, 1980). Daniel
(1963) showed that when trichloroethylene was administered orally
to rats, the ratio of pulmonary to urinary elimination varied with
the dose and that as dose increased, pulmonary excretion increased
while urinary elimination decreased. Further evidence showing that
the metabolism of trichloroethylene is saturable in Wistar rats was
obtained by Filser & Bolt (1979) who showed that the saturation
point occurred at 350 mg/m3 (65 ppm), that the zero order Vmax was
210 µmol/h per kg body weight and that the first order clearance at
a dose of 350 mg/m3 (65 ppm) was 77 µmol/h per kg body weight.
Stott et al. (1982) found that the pulmonary elimination of
unchanged trichlorethylene in Osborn Mendel rats was only 2% of the
dose at 54 mg/m3 (10 ppm), but 21% at a dose of 3240 mg/m3
(600 ppm). In contrast to these findings of a saturable process in
the rat, The same authors showed that in the mouse, doses of
trichloroethylene up to 3240 mg/m3 (600 ppm) were completely
metabolized.
In dogs, exposed through inhalation, for 1 h, to
trichloroethylene at 3780, 8100, and 10 800 mg/m3 (700, 1500, and
2000 ppm), the excretion of trichloroethylene and trichloroacetic
acid was correlated with the trichloroethylene concentration. The
rate of trichloroacetic acid excretion was higher than that for
trichloroethanol. One hour after exposure ended, the percentage
of trichloro compounds in the urine was 0.7% of the total
trichloroethylene absorbed (Hobara et al., 1983).
5.4.2. Studies on man
It has been shown that man metabolizes trichloroethylene
extensively. Ikeda et al. (1972) showed that the capacity of
workers to metabolize trichloroethylene was nonlimiting, at least
up to a daily exposure level of 945 mg/m3 (175 ppm) for 8 h.
In human beings, trichloroethylene is eliminated unchanged
through the lungs and is eliminated in the urine in the form of
metabolites. Elimination by other routes (e.g., faeces, sweat, and
saliva) accounts for less than 10% of the total (Bartonicek, 1962).
After inhalation exposure, about 10% of the amount absorbed is
expired unchanged, about 30 - 50% is excreted as trichloroethanol
in urine, and about 10 - 30% as trichloroacetic acid in urine
(Soucek & Vlachova, 1960; Bartonicek, 1962; Monster et al., 1976,
1979).
The half-life of trichloroethylene in exhaled air and in the
blood depends on the length of exposure and on the time of sampling
after exposure. The concentration follows a multi-exponential
curve, compatible with at least 3 compartments: lungs, blood and
most other tissues, and adipose tissue. After a single exposure
to trichloroethylene, trichloroethanol reaches its maximum
concentration in blood and urine almost directly after exposure.
Thereafter, the concentration decreases, with a half-life of
about 10 - 15 h (Müller et al., 1974; Monster et al., 1976,
1979; Vesterberg et al., 1976). After a single exposure to
trichloroethylene, the concentration of trichloroacetic acid in
both the blood and the urine increases for up to 20 - 40 h after
exposure. Thereafter, the concentration decreases with a half-life
of about 70 - 100 h (Müller et al., 1974; Monster et al., 1979).
Trichloroacetic acid, as such, has a shorter half-life of about
50 h.
In a group of workers with long-term exposure to
trichloroethylene at a concentration of 270 mg/m3 (50 ppm), median
values of trichloroethanol and trichloroacetic acid of 330 and
319 g/kg creatinine, respectively, were found at the end of a
working shift; during the work-free periods, the metabolites of
trichloroethylene were eliminated slowly (Triebig et al., 1976).
In a study on factory workers exposed to trichloroethylene,
Ikeda & Imamura (1973) observed a half-life of 41 h for the
urinary excretion of total trichloro-compounds (i.e., a combination
of trichloroacetic acid and trichloroethanol). This half-life is
somewhat longer with oral administration of trichloroethylene and
of chloral hydrate and is about 85 - 99 h after repeated exposure
to trichloroethylene, because of the delayed formation of
trichloroacetic acid from trichloroethylene and the
trichloroethanol still available from the tissues (Müller et al.,
1974). Thus, trichloroacetic acid will be found in the urine, even
when trichloroethanol is no longer detectable (Ikeda et al., 1971).
Trichloroacetic acid accumulates in the blood and urine during
daily exposures to trichlorethylene (Monster et al., 1979; Müller
et al., 1975).
5.5. Biological Monitoring of Exposure
Droz & Fernandez (1978) used a mathematical model to study the
effects of hourly and daily variations in exposure concentrations
on alveolar air trichloroethylene concentrations and on the urinary
excretion of trichloroethanol and trichloroacetic acid. The
determination of trichloroethanol in urine appeared to be more
sensitive than the determination of trichloroethylene in exhaled
air. The excretion of trichloroacetic acid can be used for the
qualitative evaluation of the preceding day's exposure. In
practice, blood analysis would be preferable to analysis of urine,
because of the smaller individual variations generally observed
with the former. In studies concerning repeated exposure to
constant concentrations, the smallest inter-individual variation
was found in the concentrations in blood (Monster et al., 1979).
The measurement of total trichloro compounds in urine was
described by Takana & Ikeda (1968). The urinary trichloroethanol
is oxidized to trichloroacetic acid and the total amount of
trichloroacetic acid is then measured with the Fujiwara reaction.
In the case of exposure to relatively steady concentrations, this
has the advantage of being able to indicate small-scale inter-
personal variations. It may be used as an index of exposure
intensity, especially when the urine samples are collected at,
or close to, the end of the workshift at the end of the work week
(Ikeda et al., 1972). However, other studies have demonstrated
poor individual correlation between trichloroethylene exposure and
the urinary elimination of the major metabolites, trichloroacetic
acid and trichloroethanol (Boudène et al., 1983). Separate
measurement of urinary metabolites provides more information on
exposure to daily fluctuating concentrations, because relatively
high concentrations of trichloroethanol indicate recent high
exposure, whereas relatively high concentrations of trichloroacetic
acid indicate long-term exposure to high concentrations (Monster et
al., 1979).
Trichloroethylene concentrations in alveolar air and in blood,
shortly after exposure, indicate recent exposure concentrations,
while the concentration several hours after exposure indicates the
average exposure over the preceding days (Stewart et al., 1974).
6. EFFECTS ON ANIMALS AND CELL SYSTEMS
6.1. Effects on Animals
Data from acute, short-term repeated dose, and long-term
toxicity studies on laboratory animals are summarized in Table 6.
6.1.1. Acute toxicity
Acute toxicity data on common laboratory animals are shown in
Tables 7 and 8.
Acute toxicity levels following inhalation exposure to
trichloroethylene are summarized in Table 7. Table 8 includes data
on acute oral, dermal, intraperitoneal (ip), subcutaneous (sc), and
intravenous (iv) LD50s for the mouse, rat, rabbit, and dog.
Von Oettingen (1955) reported that oral acutely toxic doses in
rats produced gastrointestinal irritation. Moderate increases in
aspartate aminotranferase (EC 2.6.1.1) levels were observed in rats
24, 48, and 72 h after a single 6-h exposure to trichloroethylene
vapour at concentrations of 54 mg/m3 (10 ppm) and 540 mg/m3
(100 ppm) (Deguchi, 1972); the increase at 5400 mg/m3 (1000 ppm)
was small (Deguchi, 1972), presumably due to inactivation of P-450.
According to Rigaud et al. (1977), intraperitoneal administration
resulted in a significant increase in aspartate aminotransferase
(EC 2.6.1.1), alanine aminotransferase (EC 2.6.1.2), and ornithine
carbamoyltransferase (OCT) (EC 2.1.3.3) in the rat, whereas
Wirtschafter & Cronyn (1964), administering 500 mg/kg body weight,
detected only minor hepatic effects over the 12 - 24-h period
following administration. No evidence of kidney dysfunction was
observed in mice following intraperitoneal administration of
trichloroethylene at 0.004M/kg body weight. Application of 2 ml
trichloroethylene (7800 mg/kg), under an occlusive dressing, on the
skin of 20 guinea-pigs, did not produce any deaths but, during the
35-day observation period, there were reductions in body weight at
1 week ( P < 0.001), 2 and 3 weeks ( P < 0.01), and 4 weeks
( P < 0.05) (Wahlberg & Boman, 1979).
Skin irritation
Trichloroethylene (purity 99.5%), applied (0.5 ml) to the
shaved (non-abraded) skin of rabbits, for 24 h, under an occlusive
dressing, produced severe skin irritation (Duprat et al., 1976).
In another study, trichloroethylene (1.0 ml) was applied,
occluded in a "skin depot", to the clipped skin of guinea-pigs.
Histological examinations were performed at 15 min, 1, 4, and 16 h.
Degenerative changes (pyknotic nuclei) were observed in the
epidermis after 15 min and were progressive (pyknosis, karyolysis,
junctional separation of the epidermis) up to the end of the study
at 16 h (Kronevi et al., 1981).
Table 6. Concentrations of trichloroethylene at which no effects were observed in experimental animals
exposed through inhalation
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
rat 3000 7 h mortality Adams et al. (1951)
6400 1.4 h mortality Adams et al. (1951)
12 000 0.6 h mortality Adams et al. (1951)
20 000 0.4 h mortality Adams et al. (1951)
200 7 h per day, 5 days mortality Adams et al. (1951)
per week, for 26
weeks
400 8 h per day for mortality; body weight; Battig et al. (1963)
5 days learning capacity
730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35a 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
guinea-pig 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology (1967)
6 weeks
35 90 days mortality; body weight; Prendergast et al.
haematology; histology (1967)
histology
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
guinea-pig 100 7 h per day, 5 mortality Adams et al. (1951)
(contd.) days per week for
26 weeks
monkey 730 8 h per day, 5 mortality; body weight; Prendergast et al.
(squirrel) days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
monkey 400 7 h per day, 5 mortality Adams et al. (1951)
(Rhesus) days per week for
26 weeks
rabbit 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
200 7 h per day, 5 mortality Adams et al. (1951)
days per week for
6 weeks
dog 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
dog 35 90 days mortality; body weight; Prendergast et al.
(contd.) haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
mouseb 5500 20 min anaesthesia Gehring (1968)
5500 100 min liver injury (elevated SGPT) Gehring (1968)
5500 300 min mortality Gehring (1968)
---------------------------------------------------------------------------------------------------------
a Kalashmikova et al. (1976) reported that rats exposed to 50 mg/m3 for 5 h per day, for 90 days, showed
damage to parenchyma in liver and kidney.
b Kjellstrand et al. (1983) reported that male NMRI mice
continuously exposed to 200 mg/m3 (37 ppm) for
30 days showed increased plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity; female mice did
not show any increase in BuChE activity; there was a significant increase in liver weight at 200 mg/m3
(37 ppm) in both sexes.
Table 7. Acute toxicity of trichloroethylene administered via
inhalation to laboratory animals
--------------------------------------------------------------------
Species Toxicity Exposure Reference
index ------------------------
level duration
(ppm) (mg/litre) (h)
--------------------------------------------------------------------
Rat LC100 20 000 107 0.4 Adams et al. (1951)
LC100 12 000 64.2 1.4 Adams et al. (1951)
LC100 2500 13.4 7.0 Adams et al. (1951)
LC50 26 300 140.7 1 Vernot et al. (1977)
LC50 12 500 66.9 4 Siegel et al. (1971)
LCL0a 8000 42.8 4 Smyth et al. (1969)
Mouse LC100 8000 42.7 2 Von Oettingen (1955)
LC100 5600 30.0 9.75 Gehring (1968)
LC50 8450 45.1 4 Kylin et al. (1962)
LC50 41 122 220 0.33 Aviado et al. (1976)
LC50 49 000 262 0.50 Vernot et al. (1977)
LCL0a 3000 16.0 2 Lazarev (1929)
LC50 40 4 Lazarev & Gadaskina
(1977)
Guinea- LC100 37 000 197.8 0.67 Von Oettingen (1955)
pig
Cat LCL0a 6074 32.5 2 Lehmann & Schmidt-Kehl
(1936)
Rabbit LC 5000 26.75 14.28 McCord (1932)
LC 10 000 53.5 2.5 McCord (1932)
LC 20 000 107 2 McCord (1932)
--------------------------------------------------------------------
a LCLO = lowest published lethal concentration.
Eye irritation
Instillation of 0.1 ml of trichloroethylene (purity 99.5%)
into rabbit eyes produced mild to moderate conjunctivitis with
superficial epithelial abrasion. At 7 days, there was a resolving
keratitis with complete recovery within 2 weeks (Duprat et al.,
1976).
6.1.2. Short-term exposures
6.1.2.1. Oral exposures
In a US NTP study (1983), groups of 10 male and 10 female
F344/N rats were administered trichloroethylene (in corn oil, by
gavage) at doses ranging from 125 to 2000 mg/kg body weight (males)
and 625 to 1000 mg/kg (females), 5 times per week, for 13 weeks.
All rats survived the 13-week study, but males receiving the
2000 mg/kg dose exhibited a 24% decrease in body-weight gain. At
the 1000 mg/kg dose, final body weights for males and for females
were similar to those of the controls.
Table 8. Acute oral, dermal, intraperitoneal, subcutaneous, and
intravenous LD50s for trichloroethylene in laboratory animals
--------------------------------------------------------------------------
Species Oral Dermal Intraperitoneal Subcutaneous Intravenous
(mg/kg body (ml/kg (mg/kg body (mg/kg body (mg/kg body
weight) body weight) weight) weight)
weight)
--------------------------------------------------------------------------
Rat 49201 27252
Dog 56803 2.8004 1505,a
Mouse 28506,b 32107,b 144010 3411
240012 31508,b
30009,c
1.2006,b
Rabbit 201
--------------------------------------------------------------------------
1 From: Smyth et al. (1969). a LDL0.
2 From: Rigaud et al. (1977). b In 24 h.
3 From: Christensen et al. (1974). c In 14 day.
4 From: Klaassen & Plaa (1967).
5 From: Barsoum & Saad (1934).
6 From: Aviado et al. (1976).
7 From: Klaassen & Plaa (1966).
8 From: Gehring (1968).
9 From: Gradiski et al. (1974).
10 From: Plaa et al. (1958).
11 From: NIOSH (1977b).
12 From: Tucker et al. (1982).
Histopathological examination of tissues from animals receiving
the highest doses showed minimal or mild cytomegaly and karyomegaly
of the renal tubular epithelial cells in the inner cortex in 8/9
males dosed with 2000 mg/kg per day, and the same effect, graded as
equivocal or mild, was seen in 5/10 females that had received the
1000 mg/kg per day dose.
The results of this 13-week study in F344/N rats were
essentially similar to those of an earlier 8-week study conducted
on Osborne-Mendel rats (NCI, 1976). In this study, only doses in
excess of 5000 mg/kg per day were lethal for rats. Doses of
1000 mg/kg per day had no effect on body-weight gains in males,
but depressed weight gains in females by approximately 15%.
Groups of 10 male and 10 female B6C3F1 mice received
trichloroethylene (by gavage in corn oil) at doses ranging from
375 to 6000 mg/kg body weight, 5 times per week for 13 weeks. All
males and 9/10 females receiving 6000 mg/kg, 7/10 males and 1/10
females receiving 3000 mg/kg, and 2/10 males and 1/10 females
receiving 1500 mg/kg died. Mean body weights of male mice dosed
with 750, 1500, or 3000 mg/kg were depressed by 11%, 19%, and 17%,
respectively, relative to the controls. Mean body weights of
control and treated groups of female mice were similar (US NTP,
1983).
Liver weights (both absolute and as percentage body weight)
increased in a dose-related fashion. Liver weights were increased
by more than 10% relative to the controls for males receiving
750 mg/kg body weight or more and for females receiving 1500 mg/kg
or more.
Histopathological examination showed hepatic centrilobular
necrosis (6/10 males and 1/10 females administered 6000 mg/kg).
This lesion was not seen in either males or females administered
3000 mg/kg, but 2/10 males had multifocal areas of calcification
scattered throughout their livers. Multi-focal calcification was
also seen in the liver of the single female mouse that survived the
6000 mg/kg dosage regimen. One female in the 3000 mg/kg dose group
developed a hepatocellular adenoma, an extremely rare lesion in
female mice of this age (20 weeks).
Examination of renal tissues showed the presence of mild to
moderate cytomegaly and karyomegaly of the renal tubular epithelial
cells of the inner cortex. These changes were found in only 1 of
the 23 mice (13 males and 10 females) that died after receiving
doses of 3000 or 6000 mg/kg for up to 6 weeks. However, the
changes were found in all 4 of the males that died after receiving
the 3000 mg/kg dose for 7 - 13 weeks, and in all animals that
survived the 6000 mg/kg (1/10 females) and the 3000 mg/kg doses
(3/10 males and 9/10 females). Tissues from mice receiving lower
doses of trichloroethylene were not examined.
Trichloroethylene administered orally to mice at doses in
excess of 500 mg/kg for 10 days produced proliferation of hepatic
peroxisome as demonstrated by increased cyanide-insensitive
palmitoyl CoA oxidation (PCO) and electron microscopy. In the
rat, trichloroethylene did not have any effect on peroxisome
proliferation. Trichloroacetic acid administered for 10 days
increased hepatic peroxisome proliferation in both species (Elcombe
et al., 1982). It is possible that the rapid rate of
trichloroethylene metabolism in the mouse, together with the
enterohepatic circulation of trichloroacetic acid, leads to
high steady-state blood levels of trichloracetic acid and the
concomitant proliferation of peroxisomes.
Primary cultures of isolated mouse and rat hepatocytes have
been found to metabolize trichloroethylene to trichloroacetic acid
at rates comparable to those in intact animals. Similarly, the
isolated hepatocytes respond to exposure to trichloroacetic acid
by peroxisome proliferation. Isolated human hepatocytes have been
found to produce trichloracetic acid from trichloroethylene at a
lower rate than that in the rat. Trichloroacetic acid does not
induce peroxisome proliferation in human hepatocytes (Elcombe,
1985).
6.1.2.2. Inhalation exposure
In the rat, exposure to 81 000 mg/m3 (15 000 ppm) for a period
of 2 - 4 min produced complete anaesthesia within 9 min (Schumacher
& Grandjean, 1960). In a study on mice, exposure to 36 7000 mg/m3
(6800 ppm) for 10 - 11 min or 64 800 mg/m3 (12 000 ppm) for 5 -
6 min produced complete anaesthesia (Friberg et al., 1953). In
another study on mice, exposure to 29 700 mg/m3 (5500 ppm) produced
anaesthesia in 46 min (Gehring, 1968).
Studies conducted on rats by Vissarionova et al. (1975) showed
that concentrations of 5000 mg/m3 administered for 5 h a day, for
1 week, resulted in an increase in liver and kidney weight (21.6%
and 18%, respectively), and a decrease in alkaline phosphatase and
RNA-dependent hepatic dehydrogenase. Histological changes have also
been noted in hepatic and renal parenchyma by Kalashnikova et al.
(1976).
With doses of 1080 mg/m3 (200 ppm) administered for 6 h daily
for 4 days, Savolainen et al. (1977) found that rats exhibited
greater motor activity and less cerebral RNA, 5 days after the last
exposure, and an accumulation of trichloroethylene in perirenal
fat.
Doses of 250, 500, 800, and 1200 mg/m3 administered for 15 -
90 h resulted in increases in intracellular lipids (Verne et al.,
1959).
No effects were noticed in rats administered 1080 mg/m3 (200
ppm) for 4 h a day for 4 days (Grandjean, 1960).
Continuous exposure of rats, mice, and gerbils by inhalation to
trichloroethylene at 810 mg/m3 (150 ppm), for periods ranging from
2 to 30 days, produced liver enlargement in all species; the mouse
was the most severely affected. After the end of exposure, the
liver weights of the mice decreased rapidly. An increased kidney
weight was noted in gerbils (Kjellstrand et al., 1981a).
In another study in which 7 different strains of mice (wild,
C57BL, DBA, B6CBA, A/su, NZB, and NMRI) were continuously exposed
to a trichloroethylene concentration of 810 mg/m3 (150 ppm),
Kjellstrand et al. (1983a) reported large increases in liver weight
in all strains with minimal changes in kidney and spleen weights.
Plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity
increased in males of all strains and in females of strains A/su
and NZB, but to a lesser extent than in the corresponding males.
In a further study, with continuous exposure to concentrations of
200 - 1620 mg/m3 (37 - 300 ppm), plasma BuChE increased in male
mice in a time- and concentration-dependent manner. Liver weight
increased in a time- and concentration-dependent manner in both
sexes.
Exposure of rats, guinea-pigs, rabbits, dogs, and monkeys to
3825 mg/m3, for 8 h per day, 5 days per week, for 6 weeks, resulted
in a loss of overall body weight in dogs and monkeys. There were
no changes in haematological or liver enzyme parameters
(Prendergast et al., 1967).
Groups of male Swiss Webster mice were exposed to 54 000 mg/m3
(10 000 ppm) for 1 or 4 h, daily, for 5 consecutive days. In the
4-h group, the NADPH cytochrome c reductase (EC 1.6.99.3) activity
in the lung decreased, but that in the liver increased. In the
lungs of this group, there were platelet thrombi and vacuolization
of bronchial epithelial cells. There were no changes in the liver.
It was concluded that the reduced activity of the pulmonary mixed-
function oxidase system reflected injury to the lungs (Lewis et
al., 1984).
Rabbits exposed to 15 000 mg/m3 (2790 ppm) for 4 h per day,
6 days a week for 45 days, developed severe normocytic anaemia,
leukopenia, and thrombocytopenia due to toxic effects on the bone
marrow (Mazza & Brancaccio, 1967).
In rats, urinary levels of trichloro-substituted metabolites
and the activity of drug-metabolizing enzymes (cytochrome P-450)
were related to the duration of trichloroethylene anaesthesia
(Moslen et al., 1977). Trichloroethylene hepatotoxicity in the rat
produces increased levels of serum transaminases. Hetaptotoxicity
increases the activity of drug-metabolizing enzymes (cytochrome
P-450) (correlation coefficient: 0.95) and the urinary excretion of
trichloro-metabolites (correlation coefficient: 0.88) (Moslen et
al., 1977).
Cytotoxic effects have been observed in the kidney and liver of
dogs subjected to 81 000 mg/m3 (1.5%; 15 000 ppm) trichloroethylene
anaesthesia (Kiseleva & Korolenko, 1971).
Studies on rats exposed by inhalation to trichloroethylene
concentrations of 2160, 4320, or 8640 mg/m3 (400, 800, or 1600 ppm)
for 6 h revealed no effects at the first concentration, a decrease
in swimming activity at 4320 mg/m3, and a further decrease at
8640 mg/m3 (Grandjean, 1963). Other rats exposed to 1944 -
2268 mg/m3 (360 - 420 ppm) for 8 h a day, 5 days a week for
46 weeks, exhibited no effects (Bättig & Grandjean, 1963). After
43 weeks at 2160 mg/m3 (400 ppm), rats in another set of tests by
Bättig (1964) exhibited greater maze skills. Mice exposed
intermittently to trichloroethylene showed a decrease in motor
activity at 4500 mg/m3 (900 ppm), but, at 19 440 mg/m3, it was
considerably increased (Kjellstrand et al., 1983b).
In Mongolian gerbils, continuously exposed to trichloroethylene
at 1.72 mg/m3 (320 ppm) for 9 months, there was no effect on
spatial memory, but subsequent maze performance test results were
interpreted as indicating an irreversible effect on the central
nervous system (Kjellstrand et al., 1980). In another study, 2
groups of Mongolian gerbils were continuously exposed to 810 mg
trichloroethylene/m3 (150 ppm) for 71 and 106 days, respectively.
In a series of maze tests following the end of exposure, the
treated groups performed less well than the unexposed controls
(Kjellstrand et al., 1981b).
6.1.2.3. Parenteral exposure
When male Swiss Webster mice were injected ip with 3 doses of
330 mg trichloroethylene/kg body weight (vehicle 0.2 ml of 25%
Tween 80 in saline) on alternate days, the activity of hepatic
microsomal NADPH cytochrome c reductase was increased. There
were no morphological changes in the liver (Lewis et al., 1984).
Studies conducted on rabbits showed that intramuscular
administration of 3 ml trichloroethylene, 3 times weekly for
29 days, induced neuronal damage (Bartonécek & Brun, 1970).
6.1.3. Long-term exposure
6.1.3.1. Oral exposure
The US NTP (1983) studied the effects of orally-administered
(in corn oil, by gavage) epichlorohydrin-free trichloroethylene in
male and female F344/N rats and B6C3F1 mice. Doses (500 and
1000 mg/kg body weight for rats and 1000 mg/kg for mice) were
administered 5 days per week for 103 weeks. The survival of
treated male rats and male mice was significantly reduced in
relation to that of corn-oil control animals. Mean body weights
of treated rats (both sexes) were lower than those of corn-oil
controls and the reduction in body weight gain was dose-related.
The body weights of treated female mice were similar to those of
vehicle controls.
Toxic nephrosis was found in 96/98 (98%) of the treated male
rats, in 97/97 of the treated female rats, in 45/50 (90%) of the
treated male mice, and in 48/49 (98%) of the treated female mice,
but was not found in any of the corn-oil control rats or mice.
Initially noted in rats that died early, the lesions were diagnosed
as frank enlargement of the nucleus and cytoplasm of scattered
individual tubular cells with brush borders, located near the
cortico-medullary junction. Progression of the lesions was
evident. As exposure time increased, affected tubular cells
were larger and additional tubules and tubular cells were affected.
Some tubules were enlarged or dilated to the extent that they were
difficult to identify as tubules. Eventually, there was loss of
some enlarged cells. Corresponding tubules became dilated and
portions of the basement membrane had a stripped appearance. In
the most advanced stage, the lesion had progressed to the sub-
capsular cortex, with enlarged tubular cells.
In mice, the pathological development of the renal lesion was
basically similar to that observed in rats, but it was relatively
less severe and did not develop to a stage where there was
extensive loss of cytomegalic epithelial cells and tubular
dilation.
When trichloroethylene was given in the drinking-water (0.1,
1.0, 2.5, and 5.0 g/litre) to CD-1 mice for 4 - 6 months, a
significant reduction in body weight (males and females at
5.0 g/litre), enlarged liver (males at 1.0, 2.5, and 5.0 g/litre;
females at 5.0 g/litre), and increase in kidney weight (males and
females at 5.0 g/litre) were observed. However, pathology at 4 and
6 months was unremarkable.
6.1.3.2. Inhalation exposure
Studies on rats showed that exposure to concentrations below
1080 mg/m3 (200 ppm) for 6 h a day, 5 days a week for 6 months, did
not induce any visible effects. At concentrations of 10 800 mg/m3
(2000 ppm), narcosis and loss of appetite were observed. At
16 200 mg/m3 (3000 ppm), only 33% of the test animals survived
(Taylor, 1936).
When NMRI mice were exposed to trichloroethylene at 700 mg/m3
(130 ppm) continuously for 30 days, liver weight increased by 1.5
and 1.9 times in males and females, respectively (Kanje et al.,
1981).
When rats were exposed to trichloroethylene at 1945 -
2270 mg/m3 (360 - 420 ppm), for 8 h a day, 5 days a week for
46 weeks, there were no changes in conditioned reflexes or reaction
time, but there was an increase in spontaneous climbing activity
(Bättig & Grandjean, 1963).
Rats exposed to 50 mg/m3 (0.05 mg/litre) for 5 h daily, for
3 months, showed damage to hepatic and renal parenchyma
(Kalashnikova et al., 1976).
Long-term exposure to concentrations of 100 - 200 mg/m3
(0.1 - 0.2 mg/litre) reduced the phagocytic activity of rat
leukocytes (Pelts, 1962).
In female Mongolian gerbils, exposed continuously to 270 and
810 mg/m3 (50 and 150 ppm) for 12 months, there were small changes
in the lipid composition of the cerebral cortex and hippocampus.
While protein content and lipid class distribution were virtually
unaffected, the cholesterol to phospholipid ratio in the cortex
decreased (Kyrklund et al., 1983).
6.1.3.3. Parenteral exposure
Of 6 rabbits given trichloroethylene at 200 mg/kg body weight
intramuscularly for 55 - 100 days, 2 died from renal failure
(Bartonécek & Brun, 1970). Rabbits given 2 ml (2.92 g)
intramuscularly twice a week for 41 - 247 days exhibited
neuronal damage (Bartonécek & Sovcek, 1959).
6.1.4. Interactions
Studies on rats showed that trichloroethylene was more toxic in
animals on a high-carbohydrate diet than in those on a high-protein
diet (Kalashnikova et al., 1974). In rats (Cornish & Adefuin,
1966), rabbits (Desoille et al., 1962), and human beings (Sbertoli
& Brambilla, 1962; Ferguson & Vernon, 1970; Pardys & Brotman,
1974), the presence of ethanol increased trichloroethylene
toxicity. Trichloroethylene and carbon tetrachloride acted
synergistically in producing hepatotoxicity (Deguchi, 1972;
Pessayre et al., 1982).
When trichloroethylene was administered intraperitoneally with
toluene, there was a decrease in the side-chain oxidation of the
toluene (Ikeda, 1974).
In studies by White & Carlson (1979), trichloroethylene
caused spontaneous, and potentiated epinephrine-induced, cardiac
arrhythmias in rabbits. A series of studies was conducted to
examine the effects of metabolic-inhibitors, enzyme-inducing
agents, or pre-treatment with ethanol or caffeine, on the
sensitivity of rabbits to epinephrine-induced cardiac arrythmias
(White & Carlson, 1979, 1981a, 1982). Rabbits treated with
metabolic-inhibiting agents developed more arrhythmias after
shorter exposure times in response to lower doses of epinephrine
than controls. Phenobarbitone and Aroclor 1254(R), and in a later
study benzo( a)pyrene (Carlson & Shite, 1983), were used as enzyme-
inducing agents. Phenobarbitone-treated rabbits developed
fewer cardiac arrhythmias and had lower blood levels of
trichloroethylene. Rabbits treated with benzo( a)pyrene developed
more cardiac arrhythmias and at lower doses of epinephrine when
exposed by inhalation to a trichloroethylene concentration of
43 500 mg/m3 (8100 ppm). Aroclor 1254(R) did not induce any
effects on trichloroethylene metabolism nor on the development of
epinephrine-induced cardiac arrhythmias. Rabbits pre-treated with
ethanol (1000 mg body weight, intravenously or orally) or caffeine
(10 mg/kg body weight, intraperitoneally) 30 min before exposure to
trichloroethylene at 32 400 mg/m3 (6000 ppm) by inhalation showed
an increased senstivity to epinephrine-induced cardiac arrhythmias.
Male New Zealand rabbits exposed to trichloroethylene at
10 800, 21 600, 32 400, or 43 200 mg/m3 (2000, 4000, 6000, or
8000 ppm) by inhalation for 1 h showed a concentration-related
sensitivity to epinephrine-induced cardiac arrhythmias. The blood
levels of the trichloroethylene metabolites (trichloroethanol and
trichloroacetic acid) were measured. Rabbits given chloral
hydrate (50 mg/kg body weight, intravenously), a trichloroethylene
intermediate metabolite, had blood levels of trichloroethanol and
trichloroacetic acid that were 40 - 100 times higher than those of
rabbits exposed to a trichloroethylene level of 43 200 mg/m3 (8000
ppm) but showed no increase in sensitivity to epinephrine-induced
cardiac arrhythmias (White & Carlson, 1981b). It is concluded that
trichloroethylene, rather than its metabolites, sensitizes the
rabbit myocardium.
6.1.5. Immunotoxicity
Changes in some components of antibody production (7S), cell-
mediated immunity (haemagglutination), and bone marrow stem cell
colonization have been observed in rodents (female mice were more
sensitive than males in one study; chinchillas were used in the
other) exposed to low-to-moderate levels (10 - 1000 mg/m3) by
inhalation or to 0.1 - 5 g/litre per day in the drinking-water for
periods ranging from a few weeks to 6 - 10 months (Shmuter, 1972;
Sanders et al., 1982).
6.1.6. Effects on cell systems
In vitro incubation of nerve-fibre membranes from rat spinal
cord with 10 µM trichloroethylene reduced the low relative
molecular mass protein fraction (Savolainen & Seppalainen, 1979).
A trichloroethylene concentration of 1.3/108 moles per ml was
the LD50 for HeLa (human cervix carcinoma) cells on the third day
of treatment (Gradiski et al., 1974).
Exposure of rabbit trachea cultures to trichloroethylene vapour
at 27 000 mg/m3 (5000 ppm) for 129 min and 216 000 (40 000 ppm) for
13 min, induced ciliostasis (Tomenius et al., 1979). The isolated
guinea-pig heart exposed to trichloroethylene at 530 mg/litre,
developed transitory arrhythmia. At a concentration of 1.06%,
there was evidence of a reduction in contractile force and coronary
flow, and arrhythmia (Bianchi et al., 1963; Matturro et al., 1963).
6.1.7. Carcinogenicity
Groups of 50 male and 50 female Osborne-Mendel rats, 7 weeks
old, were administered trichloroethylene (99% trichloroethylene
stabilized with 0.19% 1,2-epoxybutane and 0.09% epichlorohydrin) in
corn oil, by gavage, 5 days a week for 78 weeks. High-dose animals
received varying dose schedules of 1000 - 1500 mg/kg body weight
per day, and low-dose animals received 500 - 750 mg/kg body weight
per day. All surviving animals were killed 110 weeks after the
start of treatment. The time-weighted average doses were 549 and
1097 mg/kg body weight per day. A group of 20 male and 20 female
vehicle-treated rats served as controls. Of the males, 17/20
controls, 42/50 low-dose, and 47/50 high-dose animals died before
the end of the study; of the females, 12/20 controls, 35/48 low-
dose, and 37/50 high-dose animals died early. Median survival
times were approximately 60 weeks for high-dose males, 85 weeks
for low-dose males, and 70 weeks for high- and low-dose females.
Of the males, 5/20 controls, 7/50 low-dose, and 5/50 high-dose rats
developed tumours; of the females, 7/20 controls, 12/48 low-dose,
and 12/50 high-dose rats developed tumours. There were no liver-
cell tumours. Tumours occurred in various other organs in treated
and control animals and were mainly reticulum-cell sarcomas,
lymphosarcomas or malignant lymphomas, fibroadenomas of the
mammary gland, haemangiosarcomas at various sites, follicular
adenocarcinomas of the thyroid, chromophobe adenomas of the
pituitary, and renal hamartomas. Toxic nephropathy was observed
in rats of both sexes treated with high and low doses of
trichloroethylene (NCI, 1976).
Groups of 50 male and 50 female B6C3F1 mice, 5 weeks old,
were given trichloroethylene (99% trichloroethylene stabilized
with 0.19% 1,2-epoxybutane and 0.09% epichlorhydrin) in corn oil
by gavage, 5 days a week, for 78 weeks. High-dose males received
2000 - 2400 mg/kg body weight per day, and females 1400 - 1800
mg/kg body weight per day; low-dose males and females received
1000 - 1200 mg/kg body weight per day and 700 - 900 mg/kg body
weight per day, respectively. All surviving animals were observed
until they reached 95 weeks of age. Time-weighted average doses
were 1169 and 869 mg/kg body weight per day, respectively, in low-
dose males and females and 2339 and 1739 mg/kg body weight per day,
respectively, in high-dose males and females. Groups of 20 male
and 20 female mice served as vehicle-treated matched controls.
Survival was reduced in high-dose males and control males.
Hepatocellular carcinomas occurred in 1/20 control males and 0/20
control females; in 26/50 low-dose males and 4/50 low-dose females,
and in 31/48 high-dose males and 11/47 high-dose females.
Metastases of the liver-cell tumours to the lung were found in
7/98 treated males and in 1 control male. The first hepatocellular
carcinoma was observed in a mouse which had been treated with the
high dose of trichloroethylene and which died during week 27.
Lung tumours occurred in treated animals of both sexes: 5/50 (5
adenomas) in males and 4/50 (2 adenomas, 2 carcinomas) in females
in the low-dose group, and 2/48 (1 adenoma, 1 carcinoma) in males
and 7/47 (5 adenomas, 2 carcinomas) in females treated with the
high dose of trichloroethylene. Among controls, only one lung
adenoma was reported in a female (NCI, 1976).
In a study by Maltoni & Maioli (1977) and Maltoni et al. (in
press), groups of 30 male and 30 female, 13-week-old Sprague Dawley
rats were given trichloroethylene (highly purified and epoxide-
free, and stabilized with 20 ppm of butyl-hydroxytoluene) in olive
oil by gavage, 5 days a week, for 52 weeks. Two doses of
trichloroethylene were tested. The high-dose animals received
250 mg/kg body weight, and the low-dose animals 50 mg/kg body
weight; 30 males and 30 females received olive oil alone and served
as controls. The animals were kept until their spontaneous death
(the study lasted 140 weeks). There was no increase in specific
tumours. Abnormalities (cytokaryomegaly) in cells of the renal
tubules were observed in high-dose male rats. These changes were
not observed in females.
Groups of 30 female ICR Swiss mice and a group of 30 male
and 30 female mice of the same strain, were treated with
trichloroethylene dermally (1 mg in acetone), 3 times weekly, for
83 weeks, subcutaneously (0.5 mg in trioctanoin) once weekly for
89 weeks, and by gavage (0.5 mg in trioctanoin), once weekly, for
89 weeks. Similar-sized groups of animals served as controls. No
tumours were observed at the site of application (Van Duuren et
al., 1979). Similar negative findings were obtained following
thrice-weekly skin applications and once-weekly subcutaneous
injections of trichloroethylene oxide for life in a group of
70 female mice of the same strain (Van Duuren et al., 1983)
Groups of 30 male and 30 female Wistar rats, NMRI mice, and
Syrian hamsters were exposed by inhalation to trichloroethylene
(purified, with no detectable epoxides, and containing 15 ppm
triethanolamine as stabilizer) at 540 and 2700 mg/m3 (100 and
500 ppm). The animals were exposed for 6 h per day, 5 days per
week, for 78 weeks. The studies were terminated by killing the
surviving mice and hamsters after 130 weeks, and rats after 156
weeks. No carcinogenic effects were observed in rats, hamsters,
and male mice. A moderate increase in lymphomas was found in
treated female mice (18/28 at 2700 mg/m3; 17/30 at 540 mg/m3, and
9/29 in controls). The authors concluded that, on the basis of
these findings, there were no indications of carcinogenicity
(Henschler et al., 1980).
Groups of 49 - 50 female ICR mice, 4 weeks of age, were exposed
by inhalation to trichloroethylene (purity 99.8% with 0.128% carbon
tetrachloride, 0.019% benzene, 0.019% epichlorohydrin, and 0.01%
1,1,2-trichloroethane) at 0.270, 810, or 2430 mg/m3 (0, 50, 150,
or 450 ppm), 7 h per day for 5 days per week, for 104 weeks. The
surviving animals were killed 107 weeks after the start of the
study. Mortality was similar in control and treated groups.
Lung adenomas were found in 5, 2, 5, and 4 mice in the control,
low-dose, mid-dose, and high-dose groups, respectively. However,
it was reported that adenocarcinomas (none of which gave rise to
metastases) occurred in 1/49, 3/50, 8/50, and 7/46 mice in the
control, low-dose, mid-dose, and high-dose groups, respectively,
and that increased incidences in the mid- and high-dose groups,
were statistically significant compared with controls. In similar
studies on Sprague Dawley rats, no statistically-increased
incidence of tumours was detected; one clear-cell carcinoma of the
kidney was observed in the high-dose group (Fukuda et al., 1983).
In a large series of inhalation studies, highly purified
epoxy-free trichloroethylene (stabilized with 20 ppm butyl-
hydroxytoluene) was studied under controlled exposure conditions
in male and female Sprague Dawley rats and Swiss and B6C3F1 mice.
Treatment was for 7 h per day, 5 days per week for 8, 78, or 104
weeks. After the end of the treatment, all animals were kept under
observation until spontaneous death. The following 7 studies were
performed (Maltoni & Maioli, 1977; Maltoni et al., in press):
Study 1
Groups of 60 - 90 male and 60 - 90 female Sprague Dawley rats
were exposed to 0, 540, or 3240 mg trichloroethylene/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed. Cytomegaly and karyomegaly of tubular cells in the
kidney were not observed at either dose.
Study 2
Groups of 60 - 100 male and 60 - 100 female Swiss mice were
exposed to trichloroethylene at 0, 540, or 3240 mg/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed.
Studies 3 and 4
Groups of 90 - 95 male and 90 - 105 female Sprague Dawley rats
were exposed to trichloroethylene at 0, 540, 1620, 3240 mg/m3 (0,
100, 300, or 600 ppm) for 104 weeks. Further groups of 40 males
and 40 females were started on the same treatment 4 - 5 weeks later
(Study 4). The method and results of the 2 studies were similar
and the combined data are described. No increase in mortality was
observed in the treated animals. Cytomegaly and karyomegaly of
renal tubular cells were observed in males in the mid- and high-
dose groups and the incidence was dose-related; no such lesions
were observed in males in the low-dose group or in females at
any of the 3 dose levels, or in the controls. Five tubular
adenocarcinomas were observed in 4/130 males and 1/130 females
at the high-dose level. Leydig cell tumours of the testis were
observed in 6/135 controls, and in 16/130, 30/130, and 31/130
male rats, respectively, in the low-, mid-, and high-dose treated
groups. The average time of appearance of the tumours, from the
start of the study, was 113 weeks in controls and from 109 to
113 weeks in treated animals. A higher incidence of immunoblastic
lymphosarcomas was detected in male and female treated animals:
1/280 in controls, 9/260 at the low dose, 5/260 in the mid-dose
group, and 3/260 in the high-dose group.
Study 5
Groups of 90 male and 90 female Swiss mice were exposed to
trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0, 100, 300,
or 600 ppm) for 78 weeks. The incidence of hepatocellular
carcinomas in males was 4/90 in controls compared with 2/90 at the
low dose, 8/90 at the mid dose, and 13/90 at the high dose. One
hepatocellular carcinoma occurred in a female at the high dose.
The incidences of lung adenomas and adenocarcinomas were 25/180
in controls, 26/180 in low-dose groups, 36/180 in mid-dose, and
47/180 in the high-dose groups (males and females combined). Two
adenocarcinomas were seen in the control groups and 3 in the high-
dose group.
Studies 6 and 7
For study 6, groups of 90 male and 90 female B6C3F1 mice were
exposed to trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0,
100, 300, or 600 ppm) for 78 weeks. Because of excess mortality
due to fighting in the male groups, study 7 was started in which
trichloroethylene was tested in the same way in equal numbers of
male mice. In females, the incidence of pulmonary tumours (all
adenomas except one adenocarcinoma in the low-dose group) were 4/90
in controls compared with 6/90, 10/90, and 15/90 in the low-, mid-,
and high-dose groups, respectively. Hepatocellular carcinomas were
observed in 3/90 controls compared with 4/90, 4/90, and 9/90 in the
low-, mid-, and high-dose groups, respectively. In the males in
study 7, the incidence of pulmonary tumours was not affected by the
treatment. Hepatocellular carcinomas were observed in 14/90
controls compared with 19/90, 27/90, and 21/90 in the 3 treated
groups, respectively. Cytomegaly and karyomegaly were not observed
in either study (Maltoni et al., in press).
Groups of 50 male and 50 female rats (F344) and mice
(B6C3F1) were treated, by corn-oil gavage, with epichlorohydrin-
free trichloroethylene at 500 and 1000 mg/kg body weight (rats),
and 1000 mg/kg body weight (mice), 5 times weekly, for 103 weeks.
Similar-sized groups of male and female rats and mice treated with
corn oil alone, or without any treatment, served as controls. The
animals were killed between 103 and 107 weeks from the start of the
study. Trichloroethylene significantly reduced the survival of
male rats and mice. In male rats treated with trichloroethylene,
the incidence of tubular-cell neoplasms was increased (0/49,
untreated; 0/48, corn-oil control; 2/49, low dose; 3/49, high
dose). There was one tubular-cell tumour in a high-dose female
rat. Five low-dose male rats also had malignant peritoneal
mesotheliomas, compared with 1/50 untreated control, 1/50 vehicle
control, and 0/49 high-dose animals. Trichloroethylene also
produced nephrosis in both sexes of both species. In mice, the
administration of trichloroethylene increased the incidence of
hepatocellular carcinoma (males: 8/48, controls; 30/50, treated;
females: 2/48, controls; 13/49, treated). The incidence of
hepatocellular adenomas was also increased in male mice (3/48,
controls; 8/50, dosed) and in female mice (2/48, controls; 8/49,
dosed) (US NTP, 1983).
Groups of 50 male and 50 female Swiss mice were treated
by corn-oil gavage with daily doses of different samples of
trichloroethylene, initially at 2400 mg/kg body weight (males)
and 1800 mg/kg body weight (females), 5 days a week, for 78 weeks.
Due to toxicity, dosing and dose levels were reduced during the
study. The animals were kept under observation until
spontaneous death. The samples included: (a) highly purified
trichloroethylene stabilized with 0.0015% triethanolamine, (b)
industrial trichloroethylene (99.4% pure), (c) highly purified
trichloroethylene stabilized with 0.8% epichlorohydrin, (d) highly
purified trichloroethylene, with 0.8% 1,2-epoxybutane, and (e)
highly purified trichloroethylene with 0.25% epichlorohydrin and
0.25% epoxybutane. Similar-sized groups of each sex, treated with
corn oil alone, served as controls. Mortality was increased in
treated males and some treated female groups. No increase in
tumour incidence was observed except in groups (c) and (d) where
there was an increased incidence of forestomach cancers, attributed
to the direct alkylating properties of one of the two stabilizers
(Henschler et al., 1984).
6.1.7.1. Conclusions
Trichloroethylene (with or without epoxide stabilizers) caused
an increased incidence of hepatocellular carcinomas in 2 different
strains of mice, either when given by oral administration or by
inhalation.
Trichloroethylene also produced lung tumours in Swiss ICR mice
and in female, but not male, B6C3F1 mice.
Trichloroethylene, with added epichlorohydrin, administered by
gavage, caused an increased incidence of forestomach cancers in
WMRI mice.
Trichloroethylene (epoxide stabilizer-free) produced a low
incidence of renal tubular tumours in 2 different strains of mice
(mainly in males) following long-term oral or inhalation exposure.
These tumours occur very rarely in untreated rats of these strains.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure.
In one strain of mice and one strain of rats, epoxide
stabilizer-free trichloroethylene produced some increases in the
incidence of lymphomas. However, these data are insufficient to
draw any conclusions.
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats.
It was noted in the evaluations by IARC (1979, 1982) that
it was considered that there was limited evidence for the
carcinogenicity of trichloroethylene for experimental animals.
The significance of these findings needs to be evaluated in
the context of further studies on the mechanism of action of
trichloroethylene.
6.1.8. Mutagenicity
6.1.8.1. Gene mutation
(a) Bacteria and fungi
Pure trichloroethylene induced revertants (gene-mutations) in
the K-12 strain of Escherichia coli in the presence of fortified
mouse Arochlor(R)-induced microsomal preparations for only one gene
out of four analysed. In the positive case, the induced effect was
the doubling of spontaneous background (Greim et al., 1975).
Trichloroethylene (of unspecified purity) in vapour form was
slightly mutagenic for the TA-100 strain of Salmonella typhimurium,
in the presence of S9 mix obtained from mouse liver (control
value = 140 revert./plate; treated values: 240 revert./plate)
(Simmon et al., 1977).
Technical grade trichloroethylene (containing 1900 ppm of
1,2-epoxybutane and 900 ppm of epichlorohydrin) was mutagenic for
the TA-100 strain of S. typhimurium in a desiccator, in the absence
of a metabolic activation system (untreated 161 - 187 revert./plate;
treated: 567 - 651 revert./plate). Trichloroethylene of high
purity was not mutagenic for the TA 100 strain of S. typhimurium
in the presence of 0.5 ml of S9 mix obtained from mouse, rat, and
hamster liver; it was, however, mutagenic for this strain of
S. typhimurium in the presence of 50 - 150 µl of S9 mix from
Arochlor-treated rat liver (untreated series: 160 - 180
revert./plate; treated series: 350-379 revert./plate) (Crebelli
et al., 1982).
High-purity trichloroethylene was not mutagenic for the TA 100
strain of S. typhimurium in a plate assay, with and without
addition of S9 mixture (Henschler et al., 1977).
Trichloroethylene containing no traces of 1,2-epoxybutane or
epichlorohydrin was tested as a gas in a desiccator on the TA 1535
and TA 100 strains of S. typhimurium. Concentrations of 1 - 3%
produced a 30% increase (less than a doubling) in revertants in
strain TA 100 in the presence of a metabolic activation system from
Arochlor(R)-induced rat liver (Baden et al., 1979).
The exposure of S. typhimurium strains TA 98 and TA 100 to
0.5 - 10% vapour concentrations of trichloroethylene in sealed
vials for 48 h did not produce an increase in revertants either in
the presence or absence of rat liver homogenate (Waskell, 1978).
Trichloroethylene of high purity (99.5%) was mutagenic for the
TA-100 strain of S. typhimurium when used as a gas in a desiccator
in the presence of S9 mix (Bartsch et al., 1979). However, it
should be noted that the increase in revertants in the treated
series was less than twice that of the untreated series, but this
slight effect was consistent in repeated experiments.
Trichloroethylene of unspecified purity was reported to double
the spontaneous frequency of reverse mutations in the D-7 strain of
the yeast Saccharomyces cerevisiae, at one concentration, in the
presence of an endogenous metabolic activation system (Callen et
al., 1980).
Trichloroethylene containing impurities, which were not
specified, was mutagenic for S. cerevisiae, strain XV185-14C
(reverse mutations) in the presence of S9 mix. These results
were obtained at a level of yeast cell % survival of < 1 (1 h of
treatment) and of < 0.1 (4 h of treatment). These conditions are
unusual for the detection of mutagenic effects, since, at these
toxic values, selection of different cells (wild type and mutants)
is very efficient (Shahin & von Borstel, 1977). One of the authors
(von Borstel, personal communication, 1984) stated that, when these
studies were repeated with a pure trichloroethylene sample, it was
not mutagenic for yeast cells.
Trichloroethylene of high purity (99.8%), as well as
trichloroethylene containing stabilizers such as 1,2-epoxy-butane
(0.19%) and epichlorohydrin (0.09%), were not mutagenic for the
yeast S. pombe (forward mutations), with or without a metabolic
system obtained from rats and mice treated with phenobarbital and
beta-naphthoflavone. Using intraperitoneal or intrasanguineous,
host-mediated assay techniques with male mice inoculated with the
yeast S. pombe and treated orally with 2 g/kg body weight for
4 or 16 h, both pure trichloroethylene and the stabilized
trichloroethylene were found not to be mutagenic (Rossi et al.,
1983).
A slightly mutagenic action for cells of S. cerevisiae strain
D7 (reverse mutation at his locus) was found for trichloroethylene
of unspecified purity in the presence of a metabolic activation
system obtained from mice (untreated series: 0.79 - 0.89 his,
rev/106; treated series: 3.00 - 3.60 his rev/106 with 40 mol).
For this experiment, no survival values were reported, nor were the
actual number of revertants counted, and therefore the observed
effect cannot be definitely assigned to trichloroethylene
(Bronzetti et al., 1978). The same authors administered
trichloroethylene (unspecified purity) orally to mice (400 mg/kg
body weight) that were also inoculated with yeast cells intra-
sanguineously; a mutagenic effect was found on yeast cells (reverse
mutations at the his locus) collected from liver, lung, and kidney
but, again, the survival level was not reported nor was the number
of revertants counted (Bronzetti et al., 1978).
Trichloroethylene of high purity and containing known
contaminants (~80 ppm) was found to be active for the production
of reverse mutations (suppressor mutations) on Aspergillus
nidulans sumeth A1 strain at a dose of 27 000 mg/m3 (5000 ppm)
applied in a desiccator containing the plates inoculated with the
fungal conidia (Crebelli et al., in press).
(b) Mammalian cells (in vitro)
A pure sample of trichloroethylene, stabilized with thymol,
did not induce mutations (forward mutations) at the HPRT locus of
Chinese hamster V-79 cell line treated in vitro with or without
metabolic activation (S9 mix) (Loprieno & Abbondandolo, 1980).
(c) Mammals (in vivo)
Trichloroethylene (purity 99.5%) was evaluated for its ability
to induce somatic gene mutations in vivo in embryonic fibroblasts
of mice (spot test). Pregnant mice were injected 12 days after
copulation with a dose of 140 mg/kg body weight (50 females), or
350 mg/kg (26 females). The results observed were 2/145 (1.4%)
and 2/51 (3.9%) of progeny with "spots", respectively, whereas
the untreated series produced 0/146, 3/182 (1.6%), 6/794 (0.75%).
These results were considered by the authors to indicate a
mutagenic effect (Fahrig, 1977); however, positive data such as
those observed for trichloroethylene are among spontaneous values
for this test system.
6.1.8.2. Chromosome aberrations
(a) Mammals (in vivo)
Trichloroethylene (purity not given), repeatedly administered
intraperitoneally to ICR mice for 5 days at a dose equivalent to
half of the LD50, did not induce a significant increase in
chromosome aberration frequency in bone marrow cells from animals
killed 6, 24, and 48 h after the last treatment (Cerna & Kjpenova,
1977).
A single dose of trichloroethylene of 1000 mg/kg body weight
(stabilized with thymol), administered orally to Swiss albino
mice, did not induce a significant increase in the frequency of
chromosome aberrations in bone marrow, 24 h after treatment
(Loprieno & Abbondandolo, 1980).
The exposure of male mice (NMRI-Han/BGA) to trichloroethylene
vapour (99.5% purity) for 24 h at concentrations of 272, 1090, and
2430 mg/m3 (50, 202, and 450 ppm) did not induce dominant lethal
mutations (Slacik-Erben et al., 1980).
CDI male mice were treated orally with 3000, 2250, 1500,
1125, 750, or 375 mg/kg body weight (2 treatments in 24 h) with a
trichloroethylene sample of high purity (99.5%) and sacrificed 6 h
following the second treatment, for the analysis of the frequency
of micronucleated erythrocytes in the bone marrow cells. The
results indicated a positive dose-related effect of
trichloroethylene (Duprat & Gradiski, 1980).
Using high purity (99.8%) trichloroethylene, B6C3F1 mice (10
female and 10 male) were treated by inhalation with 3240 mg/m3
(600 ppm), for 7 h a day, 5 days a week, for a total of 52 days.
The animals were killed 6 h and 24 h after the last treatment. The
cytogenetic analysis showed the presence only of gaps without any
indication of induced chromosomal aberrations (Loprieno, personal
communication, 1984).
When trichloroethylene of high purity (99.8%) was administered
to B6C3F1 male mice, by gavage, in a single dose of 1200 mg/kg
body weight, an increased frequency (4 times) of micronucleated
erythrocytes in the bone marrow cells compared with control animals
was observed, 42 h after dosing (Sbrana et al., 1984).
6.1.8.3. DNA damage
Fungi
Trichloroethylene (unspecified purity) was positive for
the induction of mitotic gene conversion at the trp locus and
mitotic recombination in the D7 strain of S. ceverisiae, under
endogenous metabolic activation conditions (Callen et al., 1980).
Trichloroethylene (unspecified purity) was also found to be
positive for the induction of mitotic gene conversion at the trp
locus of S. cerevisiae D7 strain treated in vitro in the presence
of a metabolic activation system obtained from rat liver. Using
the host-mediated assay technique, and a single oral dose of
400 mg/kg body weight, given to mice inoculated with yeast cell
D4 strain of S. cerevisiae, a positive effect on the induction of
mitotic gene conversion at the trp 5 and ade 2 loci was observed
in the yeast cells recovered from the kidney. Repeated oral
administration of trichloroethylene to male mice, with a total
dose of 3700 mg/kg body weight, induced a positive effect (mitotic
recombination) in yeast cells recovered from the liver and kidney.
In all these studies, the actual numbers of the convertant or
recombinant colonies were not given, and the significance of the
results is questionable (Bronzetti et al., 1978).
Trichloroethylene (stabilized with thymol) was considered
inactive for the induction of mitotic recombinations in the D4
strain of S. cerevisiae following in vitro and in vivo (host-
mediated assay) studies (Loprieno & Abbondandolo, 1980).
Trichloroethylene of high purity (99.9%) induced somatic
segregants in A. nidulans strain 35 x 17, at concentrations of
40 500 and 81 000 mg/m3 (7500 and 15 000 ppm), in a desiccator
containing the plates inoculated with the fungal conidia; under
identical doses and conditions, no mitotic recombinant colonies
were induced in this strain of A. nidulans by trichloroethylene
(Crebelli et al., in press).
6.1.8.4. Mammalian cells ( in vitro)
Trichloroethylene of high purity (99.9%) induced morphological
transformations, in vitro, in rat embryo cell cultures (Price et
al., 1978).
Stabilized with thymol, trichloroethylene did not stimulate
unscheduled DNA synthesis in an HeLa human cell line grown in
vitro, in the presence or absence of a metabolic activation
system (S9 mix) (Loprieno & Abbondandolo, 1980). Trichloroethylene
(unspecified purity) elicited DNA repair synthesis in human
lymphocytes in the presence of S9 mix (Perocco & Prodi, 1981).
Exposure of Chinese hamster ovary cells (CHO) to
trichloroethylene bubbled into the growth medium for 2 min,
corresponding to a dose of 0.17% v/v for 1 h treatment time,
did not induce sister-chromatid exchange (White et al., 1979).
Trichloroethylene bound covalently to calf thymus DNA in vitro
in the presence of a rat liver metabolic activation system (Di
Renzo et al., 1982; Bergman, 1983).
When given ip to NMRI mice, twice daily for 5 days
(33.6 mg/kg), trichloroethylene bound to RNA and DNA of brain,
lung, liver, kidney, spleen, pancreas, and testis (Bergman, 1983).
6.1.8.5. Mutagenic activity of trichloroethylene metabolites
Trichloroethylene oxide was not mutagenic for the TA 1535
strain of S. typhimurium or the WP2 UVRA strain of E. coli (plate
test), but was positive in the DNA repair test on E. coli (Kline
et al., 1982).
The same compound was found to be a direct mutagen for the
yeast S. pombe (forward mutation) and for mammalian cells grown
in vitro (V-79 cell line) (forward mutation) (Loprieno &
Abbondandolo, 1980).
Syrian golden hamster embryo cells were exposed to
trichloroethylene oxide (1.1, 2.5, and 5.0 µM) at 37 °C for 30 min.
After 5 days, the number of transformed colonies was scored. A low
but dose-related increased frequency of transformed cells was
induced (Di Paolo & Doniger, 1982).
Chloral hydrate has been reported to have direct genotoxic
activity in S. typhimurium (Waskell, 1978) and in A. nidulans
(Bignami et al., 1980).
At doses of 82.7, 165.4, or 4135 mg/kg body weight, chloral
hydrate, administered intraperitoneally, induced chromosome non-
disjunction in the secondary spermatocytes of mice (Russo et al.,
1984).
6.1.8.6. Conclusions
Pure trichloroethylene acts as a weak mutagen in S. typhimurium
in the presence of a metabolic activation system. However, in all
cases, the induced effect was never more than twice the spontaneous
value. Technical grade trichloroethylene containing stabilizers
such as 1,2-epoxybutane epichlorohydrin, is mutagenic in
S. typhimurium in the absence of a metabolic activation system.
Pure trichloroethylene is not mutagenic for the yeast S. pombe,
with or without metabolic activation, in vitro or in vivo (host-
mediated assay).
Thymol-stabilized trichloroethylene is not mutagenic for V-75
hamster cells, with or without metabolic activation.
Trichloroethylene (99.5% pure) is a weak mutagen for the
induction of somatic mutations in mouse embryo.
In mice, a single oral dose of trichloroethylene (1 g/kg body
weight) or repeated exposure to 3240 mg/m3 (600 ppm) for 52 days,
7 h/day, through inhalation, did not increase chromosome
aberration frequency in bone-marrow cells. A half-LD50 dose
of trichloroethylene, administered ip to mice, did not increase
chromosomal aberration frequency in bone-marrow cells. In a
micronucleus test, a single oral treatment with 1200 mg/kg body
weight or repeated (twice) oral treatment with a series of doses
ranging from 375 to 3000 mg/kg body weight increased the frequency
of induced micronucleated erythrocytes in the bone-marrow cells of
mice. Trichloroethylene bound to DNA in vitro and in vivo to
mouse tissues.
Dominant lethal mutations were not induced in mice exposed
through inhalation to trichloroethylene vapours.
Trichloroethylene induces mitotic gene conversion in yeast
cells grown under conditions allowing an endogenous metabolic
activation system to function. The same effect reported in yeast
in the presence of an exogenous metabolic activation system
(S9 mix), or in the host-mediated assay, is questionable, because
of inadequate data and the fact that other studies produced
negative data.
A positive effect for the induction of unscheduled DNA
synthesis (UDS) in human lymphocytes, treated in vitro, was
reported by Perocco & Prodi (1981), whereas a negative response
for the same biological effect (UDS) in a human fibroblast cell
line (HeLa) grown in vitro was observed by Loprieno & Abbondandolo
(1980).
Trichloroethylene induces morphological transformation in rat
embryo cells.
The available results, shown in Table 9, are not adequate
for a complete evaluation of the genotoxic potential of
trichloroethylene. Only a few mutagenicity studies have indicated
the grade and purity of the trichloroethylene sample employed.
The confusing results could be due either to the use of
trichloroethylene samples stabilized with mutagenic compounds or to
the use of pure trichloroethylene samples which, unstablized, can
rapidly decompose to chemicals with mutagenic activity.
6.1.9. Reproduction, embryo/fetotoxicity, and teratology
The embryo/fetal toxicity and teratogenic potential of
trichloroethylene have been evaluated in the avian embryo system,
the mouse, rat, and rabbit. Reproductive function has been
examined in male rats.
6.1.9.1. Avian embryo system
Exposing avian embryos to 1% trichloroethylene (10 g/litre)
caused a significant increase in embryonic death and a slight
increase in anomalies (Fink, 1968). These observations were made
on the 3rd day of development, a time at which frank malformations
would be difficult to observe or evaluate. Elovaara et al. (1979)
reported the results of a comparative study of the teratogenic
potential of trichloroethylene and other aliphatic chlorinated
hydrocarbons in the chick embryo. Trichloroethylene (reagent
grade) in doses ranging from 5 to 1000 µmoles in olive oil (25 µl)
was injected into the air space of fertilized eggs on the 2nd and
6th days of incubation and examined on the 14th day. The LD50 for
trichloroethylene was between 50 and 100 µmoles/egg. Macroscopic
embryonic malformations occurred in 9 out of 55 surviving embryos,
5 times the incidence in the vehicle control eggs.
In another study, injection of low doses of trichloroethylene
(1, 5, 10, and 25 µmol/egg), on days 1 and 2 of embryogenesis,
resulted in embryotoxicity, growth defects, and morphological
anomalies (Bross et al., 1983).
Table 9. Summary of the mutagenicity data reported for trichloroethylene
-----------------------------------------------------------------------------------------
Organism Genotoxic effects
Gene Chromosome DNA
mutation anomalies damage
-----------------------------------------------------------------------------------------
prokaryotes weak
positive
-----------------------------------------------------------------------------------------
fungi negative ( in vitro) positive negative ( in vitro)
negative (hma) mitotic weak (hma)
positive? ( in vitro) segregation positive (yeast
positive (A. nidulans) A. nidulans metabolism)
-----------------------------------------------------------------------------------------
mammalian negative negative (SCE)
cells negative (DNA
( in vitro) repair synthesis)
positive (covalent
binding)
-----------------------------------------------------------------------------------------
mammals weak negative (1 dose) (1 g/kg body
( in vivo) weight, oral)
negative (52 doses) (3240 mg/m3,
inhalation)
negative (5 doses) (1/2 LD50, ip)
positive (micronuclei) 1200 mg/kg
positive (micronuclei) (375 -
3000 mg/kg)
negative (dominant lethals) (270,
1090, 2430 mg/m3, inhalation)
-----------------------------------------------------------------------------------------
For human cases, see Table 10.
6.1.9.2. Mouse
Swiss-Webster mice were exposed by inhalation to
trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h daily, on days
6 - 15 of gestation (Day 0 = vaginal plug). The mice were observed
daily throughout pregnancy. Maternal body weights were recorded on
days 6, 10, and 18 of gestation as an index of maternal toxicity.
On day 18, no effect was observed on the average number of
implantation sites/litter, litter size, the incidence of fetal
resorptions, fetal sex ratios, or fetal body measurements (Schwetz
et al., 1975). The incidence of gross anomalies observed by
external examination was not significantly greater than that among
the control litters. Trichloroethylene exposure did not exert any
effect on the incidence of skeletal anomalies in mice, and
microscopic examination did not reveal any abnormalities in organs,
tissues, or cells (Leong et al., 1975; Schwetz et al., 1975).
6.1.9.3. Rat
In studies similar to the preceding mouse study, exposure to
trichloroethylene at 1620 mg/m3 (300 ppm) did not produce any
evidence of maternal toxicity or teratogenicity in Sprague Dawley
rats (Leong et al., 1975; Schwetz et al., 1975) or Charles River
strain rats (Bell, 1977).
Exposing Sprague Dawley rats to trichloroethylene at 2700 mg/m3
(500 ppm) for 7 h per day, 5 days per week, during a 3-week
pregestational period, and 7 h per day each day for days 0 - 18 and
days 6 - 18 of gestation did not cause maternal or embryo/fetal
toxicity. Beliles et al. (1980) concluded that the frequency and
the character of the macro- or microscopic findings in the treated
groups did not indicate either adverse fetal effects or that
trichloroethylene, at the dose used, was teratogenic.
Dorfmueller et al. (1979) reported that female Long-Evans rats
exposed to trichloroethylene at 9720 mg/m3 (1800 ppm) before
mating, for 6 h per day, 5 days per week, for 2 weeks and/or during
pregnancy, for 6 h per day, each day, for 0 - 20 days of gestation)
did not exhibit any signs of maternal toxicity. There was no
indication of embryotoxicity, and no significant treatment effects
or interactions were found in the number of corpora lutea or
implantation sites/litter, fetal body weights, resorbed
fetuses/litter, or sex ratios. No significant treatment effects
were observed in the analysis of total soft-tissue anomalies.
However, prenatal exposure to trichloroethylene at 9720 mg/m3
(1800 ppm) caused an increase in minor anomalies of the offspring
(incomplete ossification of the sternum) indicative of development
delays in maturation, but not of major malformation.
While the study did not indicate any treatment-related effects
on general post-natal behaviour, there was a small but significant
regression in post-natal weight gain in offspring in the pre-mating
exposure group.
In groups of male rats dosed by gavage with trichloroethylene
at 10, 100, or 1000 mg/kg body weight 5 days per week, for 6 weeks,
there was no evidence of spermatotoxic effects. Trichloroethylene-
related effects of reduced weight gain and elevated liver/body
weight ratios were seen primarily in the 1000 mg/kg group.
Copulatory behaviour was initially diminished by the narcotic
properties of trichloroethylene, but was normal by the 5th week
(Zenick et al., 1984).
6.1.9.4. Rabbit
Female New Zealand white rabbits were exposed by inhalation to
trichloroethylene at 2700 mg/m3 (500 ppm), for 7 h per day, 5 days
per week, during a 3-week pregestation period, and daily, for days
0 - 21 and days 7 - 21 of gestation (Day 0 = mating day). There
was no evidence of maternal toxicity or embryotoxicity. The
occurrence of hydrocephalus in a few fetuses in one of the study
groups was reported (4 fetuses in 2 litters), but no definitive
conclusion was drawn from these findings (Beliles et al., 1980).
7. EFFECTS ON THE ENVIRONMENT
7.1. Aquatic Organisms
There is little information on the toxicity of
trichloroethylene for fish. The US Registry of Toxic Effects of
Chemical Substances (Christensen & Lugenbyhl, 1975) reports, for an
unidentified species, that exposure to a concentration range of
100 - 1000 mg/litre produced toxic effects in 96 h. Toxicity tests
carried out on salt-water flatfish, Limanda limanda (sole), 15 -
20 cm long, in a continuous water flow, established a 96-h LC50
of 16 mg/litre (Pearson & McConnell, 1975). A 96-h LC50 of
approximately 40 mg/litre (static) or 67 mg/litre (continuous flow)
has been reported for the minnow Pimephales promelas (Alexander et
al., 1978).
Verschueren (1977) established an LC100 of 600 mg/litre for
Daphnia magna. The LC50 for the balanide salt-water crustacean
nauplius (larva) (Elminius modestus) was 20 mg/litre after 46 h
(Pearson & McConnell, 1975), and the LC50 for the protozoon
Entosiphon sulcatum was established as 1200 mg/litre (Bringmann
& Kuhn, 1980).
Various LC50 values have been established for algae including
63 mg/litre for Microcystis aeruginosa, (Verschueren, 1977); a
concentration of 1000 mg/litre did not have any observable effect
on Scenedesmus quadricauda (Bringmann & Kuhn, 1980). A short-term
photosynthesis efficiency test gave an LC50 of 8 mg/litre
(Pearson & McConnell, 1975) and, finally, in tests carried out on
Thalassiosira pseudonana and Dunaliella tertiolecta, there were
observable effects at 50 and 100 µg/litre, in a mixed culture
(Biggs et al., 1979).
7.2. Uptake, Distribution, Storage, Metabolism, and Elimination in
Plant and Animal Organisms
Bioaccumulation of trichloroethylene in a marine environment
has been studied by Pearson & McConnell (1975); concentration
levels were determined for a wide variety of marine organisms,
mostly in the Bay of Liverpool.
The greatest increase in trichloroethylene concentrations in
the tissues of animals that are relatively high up in the food
chain (birds' eggs, fish liver, and seal fat) was nearly 100 times
the level in water (from 0.5/109 µg/litre in water to 50/109 µg/kg
in tissues).
These data agree with the laboratory findings of Barrows et
al. (1980) who, in a 14-day test, noted that trichloroethylene
accumulation in the sunfish species, Lepomis macrochirus was 17
times that of the aquatic medium with a halving time of less than
one day. A low bioconcentration factor (concentration in organism
divided by concentration in environment) (Uehleke et al., 1977)
has been derived using water solubility and the equation proposed
by Kenaga (1980) and Kenaga & Goring (1980).
7.3. Effects on the Stratospheric Ozone Layer
Consideration has been given to the possibility that
trichloroethylene, together with other halocarbons in the
atmosphere, may contribute to the depletion of the stratospheric
ozone layer, which would lead to atmospheric heating and increased
exposure of terrestrial biota to ultraviolet radiation (Molina &
Rowland, 1974). Atmospheric trichloroethylene concentrations seem
to be about one-fifth to one-tenth of those of other chlorocarbons
such as CH3CCl3, CH2Cl2, CCl4, or C2Cl4 or the major chlorofluoro-
carbons (Cronn et al., 1977; Penkett, 1982). The reason for this
is that while trichloroethylene emissions into the atmosphere are
of the same order as those of other halocarbons (Jesson, 1980),
trichloroethylene is efficiently scavenged by hydroxyl radicals
in the troposphere, and the reaction rate for this process is
appreciably faster for trichloroethylene than for other halocarbons
(Penkett, 1982). Thus, the predicted atmospheric lifetime for
trichloroethylene is short (about 10 - 11 days) (Derwent &
Eggleton, 1978; Graedel, 1978) compared with those for the
chlorofluorocarbons, which may be 10 years or more (Jesson, 1980).
It is not clear whether trichloroethylene is even present in the
stratosphere (Cronn et al., 1977). However, the data suggest that
trichloroethylene is unlikely to be involved in the possible
depletion of the ozone layer.
8. EFFECTS ON MAN
8.1. General Symptoms and Signs
8.1.1. Acute effects
Sorgo (1976) reported a lethal oral dose for trichloroethylene
to be 7 g/kg body weight, and Lazarev et al. (1977) reported a
fatality after the oral ingestion of less than 50 ml.
At exposure concentrations of 270 - 540 mg/m3 (50 - 100 ppm),
the most common symptoms are headache, sluggishness, sleepiness
(especially at the end of the workshift), dulling of senses,
dizziness, nausea, and vomiting (Rubino et al., 1959; Lilis et
al., 1969; Governa, 1981). At narcotic doses, vomiting may occur.
Trichloroethylene has been used as an analgesic and as an
anaesthetic on its own or in mixtures. It is now much less used
for this purpose, as safer and more effective alternatives are
available. Inhalation of 27 000 mg/m3 (5000 ppm) produces light
anaesthesia; concentrations of up to 108 000 mg/m3 (20 000 ppm)
have been used for deeper anaesthesia (Wade, 1977).
Clinical signs are not very specific. There can be enhanced
responsiveness to caloric stimulation of the labyrinth and some EEG
anomalies may be detected (Chiesura, 1980) (section 8.2). Acute
hepatic and renal toxicity has been reported (Sucui & Olinici,
1983).
A particular intolerance to ethanol may occur after ingesting
even small amounts of alcoholic beverages. This intolerance is
characterized by intense cutaneous vasodilatation, particularly in
the face, often called "degreaser's flush" (Stewart et al., 1974).
Non-specific effects on the digestive system (e.g., dyspepsia,
gastritis, and diarrhoea) have been reported in cases of suicidal
or accidental ingestion of trichloroethylene (Bozza Marrubini et
al., 1978; Governa, 1981).
8.1.2. Chronic effects
The existence of a condition of chronic trichloroethylene
poisoning is not clear (Chiesura, 1980; Boudène et al., 1983).
However, chronic effects in human beings can occur following
prolonged exposure to moderate concentrations of trichloroethylene
of about 540 mg/m3 (100 ppm). The clinical picture is mainly
related to the central nervous system (CNS), and consists of
asthenia, anorexia, headache, loss of memory, moodiness,
depression, insomnia, paraesthesia, and disturbances of the
autonomic nervous system such as hyperhydrosis, tachycardia, and
dermographism (Ahlmark & Forssman, 1951a,b; Bardodej & Viskocil,
1956; Rubino et al., 1959; Lerza et al., 1963; Castellino, 1969).
The role of trichloroethylene in inducing liver damage in
occupationally-exposed human beings is not clear (Parmeggiani,
1956; Capellini & Grisler, 1958; Rubino et al., 1959; Tolot et al.,
1964; Schuttmann, 1970; Lachnit, 1971; Thiele, 1982; Sucui &
Olinici, 1983; Svabova & Mencik, 1983).
Human subjects with high repeated, but non-occupational,
exposure may exhibit toxic effects on the liver (e.g., elevated
aspartate (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2)),
renal insufficiency, and abnormal EEG patterns (Baerg & Kimberg,
1970). Following accidental or suicidal ingestion (e.g., doses of
100 - 300 ml), hepatic necrosis and nephropathy have been found in
autopsy cases (Keinfeld & Tabershaw, 1954; Graovac-Leposavic
et al., 1964; Priest & Horn, 1965; Beisland & Wannag, 1970;
Clearfield, 1970). A decrease in sexual potency has been reported
in industrial workers exposed to trichloroethylene (Bardodej &
Vyskocil, 1956; Lazarev & Gadaskiva, 1977).
Trichloroethylene is one of the volatile solvents inhaled
("sniffed") for its euphoric effect (Hayden et al., 1976), and
abuse by oral self-administration has been reported (Wells, 1982).
Centrilobular hepatic necrosis and renal toxicity have occurred in
addicts (Baerg & Kimberg, 1970; Clearfield, 1970), and deaths have
been reported (James, 1963; Musclow & Awen, 1971). Some mixtures
sold commercially as trichloroethylene may contain other solvents,
including carbon tetrachloride, which can contribute to the toxic
manifestations (Bouyges et al., 1980; Conso et al., 1980).
Dependent patients may show withdrawal symptoms (Ikeda et al.,
1971).
8.2. Effects on Organs and Systems
8.2.1. Effects on the nervous system
Acute effects on the central nervous system (CNS) are
characterized by two sequential phases (i.e., excitation and
depression), and are usually reversible. These symptoms generally
prevail over those of other systems (Stewart et al., 1962;
Chiesura, 1980; Governa, 1981).
In the early phase of excitation, euphoria and inebriation are
present. The subsequent phase of CNS depression is characterized
by various degrees of narcosis culminating in coma (Caccuri, 1976;
Chiesura, 1980). Muscular hypotony, muscular spasms, reduced
tendon reflexes, and loss of co-ordination may occur (Huff, 1971).
Changes in EEG patterns may vary considerably. The EEG may
be normal in some cases, while substantial alterations, regressing
after a few days, may be observed in others. The most frequently
observed change is a decrease in alpha activity, which is often
irregular. Widespread rapid rhythms or low theta activity may also
be present (Chiesura, 1980).
Exposure to trichloroethylene at a concentration of 540 mg/m3
(100 ppm) produces various results on the CNS. Though there may
not be any evidence of effects on psychomotor capacities (Stewart
et al., 1970), reductions in mental performance evidenced by the
test for perception, past recall, answering speed, and response
and manual dexterity tests have been reported (Ferguson & Vernon,
1970). Visual and auditory evoked potentials were affected at
exposure levels ranging between 270 and 540 mg/m3 (50 and 100 ppm),
for 3 1/2 - 7 1/2 h (Winneke, 1982).
The behavioural effects of exposure to trichloroethylene have
been studied under laboratory and work-place conditions. Laboratory
exposure to 540 or 1080 mg/m3 (100 or 200 ppm), for 70 min, had no
effect on reaction time or short-term memory (Gamberale et al.,
1976). Volunteers exposed to 540, 1620, or 5400 mg/m3 (100, 300,
or 1000 ppm), for 2 h, showed significant impairment in a number
of psychomotor tests at 5400 mg/m3 but not at 1620 or 540 mg/m3
(Vernon & Ferguson, 1969). In a further study (Ferguson & Vernon,
1970), effects were again found at 5400 mg/m3 and marginal effects
at 1620 mg/m3. In another study, exposure of volunteers to 810
or 1620 mg/m3 (150 or 300 ppm), for 2 1/2 h, did not produce
any significant impairment in behavioural test results (Ettema
& Zielhuis, 1975). With repeated exposure under work-place
conditions, no behavioural effects were observed with mean
atmospheric levels of 270 mg/m3 (50 ppm) (Triebig et al., 1977a,b).
In a study of complex reaction time in an occupational group with a
mean exposure of 1320 mg/m3 (245 ppm), reaction time was increased
in comparison with that in a control group (Gun et al., 1978).
Motor nerve conduction velocity was not affected in a group
occupationally exposed to mean atmospheric levels of less than
270 mg/m3 (50 ppm) (Triebig et al., 1982).
A study on 122 exposed workers (Ahlmark & Forssman, 1951a)
showed that symptoms of toxicity in human beings were correlated
with urinary levels of metabolic trichloroacetic acid. With
urinary concentrations of up to 20 mg trichloroacetic acid/litre,
no particular symptoms were noted. With urinary levels ranging
between 45 and 75 mg/litre, headache, fatigue, increased need for
sleep, irritability, and alcohol intolerance were reported in 50%
of workers. When urinary levels of trichloroacetic acid exceeded
300 mg/litre, these symptoms were found in 100% of subjects.
Initial nervous-system signs of toxicity were reported to be
associated with the urinary excretion of trichloroacetic acid
at 75 mg/litre and 30 mg/litre (Lazarev & Gadaskiva, 1977).
Two types of irreversible neurotoxic effects have been reported
in man. One is the specific degeneration of the cranial nerves, in
particular the trigeminal, the olfactory, and the facial nerves
(Goldblatt, 1956; James, 1963; Kjellstrand et al., 1980; Barrett
et al., 1982). However, it is considered that this effect is
not produced by trichloroethylene itself but by its decomposition
product, dichloroacetylene (Henschler et al., 1970a). In addition,
polyneuropathies involving the whole peripheral nervous system have
been described after long-term exposure to trichloroethylene
(Szulc-Kuberska, 1972).
8.2.2. Effects on the cardiovascular system
Several cases of sudden death due to cardiac arrest have been
reported in subjects exposed to trichloroethylene, either medically
or occupationally (Ostelere, 1953; Norris & Stuart, 1957; Meyer,
1966; Wiecko, 1966; Clearfield, 1970; Tomasini, 1976). It has
been suggested that these deaths might be due to an increased
sensitivity of the myocardium to endogenous and exogenous
catecholamines. The event would entail irregular cardiac
rhythm due to the onset of ectopic foci with atrioventricular
dissociation. A range of alterations in cardiovascular function
(e.g., atrial and ventricular extrasystole, tachycardia, and
ventricular fibrillation) has been reported (Bardodej & Viskocil,
1956; Anderson, 1957; Lilis et al., 1969; Tomasini, 1976; Governa,
1981).
8.2.3. Effects on the respiratory system
Under normal conditions of exposure, trichloroethylene did not
induce any effects on the respiratory tract but, at high exposure
levels of 810 - 3510 mg/m3 (150 - 650 ppm), there may be effects
due to local irritation (Bardodej & Viskocil, 1956; Jouglard &
Vincent, 1971; Meyer, 1973; Gaultier et al., 1974; Tomasini, 1976).
Breakdown products are of more importance than trichloroethylene
with respect to the effects on the respiratory system (Castellino,
1969).
8.2.4. Effects on the urinary tract
Altered renal function has been described in both acute and
chronic trichloroethylene poisoning (Nomura, 1962; Graovac-
Leposavic et al., 1964; Clearfield, 1970). The nephropathy
observed could be due to impurities rather than to the
trichloroethylene; technical grade trichloroethylene might have
contained nephrotoxic chlorinated hydrocarbons (e.g., 1,1,2,2-
tetrachloroethane) as impurities (Chiesura, 1980).
8.2.5. Effects on the skin
When applied to the skin, trichloroethylene causes erythema
(Wahlberg, 1984). It is only mildly irritating to human skin
providing it is not held in contact (e.g., by clothing or
footwear). Chemical burning of the skin has been reported
following prolonged contact (Maloof, 1949; Tomasini, 1976). There
is a considerable difference in individual susceptibility to the
effects of repeated exposure. The reaction to repeated contact
(which will defat the skin) may take the form of an erythematous,
exudative, vesicular, eczematous, or exfoliative dermatitis (Roche
et al., 1958; Stopps & McLaughlin, 1967; Ettema et al., 1975).
Secondary infection of the skin may complicate the dermatitis.
"Degreaser's flush", a reddening of the skin in some individuals
ingesting ethanol after exposure to trichloroethylene, can occur
(section 8.1.1).
8.2.6. Effects on the eye
Liquid trichloroethylene in the eye produces pain and
discomfort and superficial damage to the cornea, but complete
recovery occurs within a few days (Grant, 1974). Exposure to high
concentrations of vapour (anaesthetic levels of 27 500 - 108 000
mg/m3) also causes eye irritation and superficial damage to the
cornea, but again with complete recovery (Grant, 1961).
8.2.7. Carcinogenicity
A number of epidemiological studies have been conducted to
examine the possible carcinogenic effects of trichloroethylene in
occupationally-exposed populations, and the major findings are
summarized in Tables 10 and 11. Some of these studies have
followed groups with specified exposure to trichloroethylene
(Axelson et al., 1978, 1984; Tola et al., 1980) (Table 10), whereas
others have involved workers in laundry and dry cleaning with more
or less mixed exposures including trichloroethylene (Blair et al.,
1979; Malek et al., 1979; Katz & Jowett, 1981) (Table 11). Other
studies are of a case-reporting or a case-referent (case-control)
type, concerning pancreatic (Lin & Kessler, 1981) and liver tumours
(Novotna et al., 1979; Paddle, 1983) or other conditions and, also,
involving exposure to other solvents (Lin & Kessler, 1981). Some
case-control studies of lymphomas, both of the Hodgkin and non-
Hodgkin type (Olsson & Brandt, 1980; Hardell et al., 1981) and of
liver cancer (Hernberg et al., 1984) have mentioned exposure to
trichloroethylene as a factor in a few cases, but the numbers are
too small for any conclusions to be drawn about trichloroethylene
as a risk factor.
Table 10. Summary of epidemiological studies on carcinogenicity with specific reference
to trichloroethylene exposure
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure intensity had been monitored since Axelson et al.
of 518 male workers 1950 in terms of urinary excretion of (1978)
(7688 person-years) trichloroacetic acid (TCA).
2. No significant excess of cancer mortality
either in the high-dose group (> 100 mg
TCA/litre urine) or the low-dose group
(< 100 mg TCA/litre urine).
3. Results of first update, see Axelson (1984)
below.
-----------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure information was based on TCA Tola et al.
of 1148 male workers determinations in the urine, permitting (1980)
and 969 female workers latency requirements of 6 - 13 years. Loss
(4269 person-years for to follow-up was 9%.
women)
2. Trichloroethylene was not carcinogenic
(given a latency period of 6 to 13 years)
among those exposed at low levels (100 mg
TCA/litre urine in 91% of the cohort).
3. Cohort to be updated at 5-year intervals.
Mortality and 1. Extension and update of the first-mentioned Axelson et al.
incidence in a study, 65% with exposure from 1970 to 1975 (1984)
cohort of 1424 men and with more than 90% having TCA levels
< 100 mg/litre.
2. Deficit found in mortality from cancers (22
versus 36.9 expected), but a significant
excess of urinary tract (11 versus 4.85) and
haematolymphatic tumours (5 versus 1.20).
3. Specifically, there were 3 urinary bladder
cancers versus 0.83 expected, 4 cancers of
the prostrate versus 2.35 expected and 2
lymphomas versus 0.27 expected, requiring
2 years or more of exposure and 10 years of
latency.
-----------------------------------------------------------------------------------------
Table 11. Summary of epidemiological studies and case reports on carcinogenicity of
mixed exposures involving trichloroethylene or trichloroethylene production
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 330 laundry 1. Significant increase in lung and cervical Blair et al.
and dry-cleaning cancer, and a slight excess of leukaemia and (1979)
workers liver cancer detected.
2. Exposed also to other chemicals such as
benzene and carbon tetrachloride.
3. To be extended to a prospective study of
2 - 5 x 103 workers.
Cohort of 37 male 1. Exposure intensity was such that TCA in Malek et al.
dry-cleaning workers urine was less than 100 mg/litre in over 60% (1979)
of the cohort.
2. 6 cases of cancer, none of which were in the
liver.
-----------------------------------------------------------------------------------------
Table 11. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 671 female 1. Death certificates were employed. Katz & Jowett
laundry/dry-cleaning (1981)
workers (controls: 2. Elevated risks of genital (unspecified) and
entire female working kidney cancers were detected, along with a
population and other smaller excess of bladder and skin cancer and
low-wage occupations) lymphoma.
109 pancreatic cancer 1. Hospital records were employed. Lin & Kessler
patients, case-control (1981)
study 2. An association was observed between
pancreatic cancer and exposure to dry-
cleaning chemicals and gasoline fumes, as
well as to drinking of decaffeinated (by
means of trichloroethylene) coffee.
3. No information was available on extent or
type of exposure.
95 liver cancers, 1. Cancer registry data on liver cancer in the Paddle (1983)
case-study area of a production plant were employed.
2. No case could be definitely tied to
trichloroethylene production.
-----------------------------------------------------------------------------------------
While some of these studies are continuing as prospective
studies to cover larger populations, they suffer, in general, from
one or more of the following factors: (a) small cohort population,
(b) young age of the cohort, (c) a rather short follow-up period,
(d) relatively few cases, (e) inaccurate or uncertain estimation
of intensity and duration of exposure to trichloroethylene, (f)
possible exposure to other chemicals, even in the more specific
studies, including potential carcinogens, and (g) inadequate
information on other general risk factors for cancer, such as
smoking, even if the influence of uncontrolled confounding is
likely to be limited in this respect.
At present, it is not possible to draw definite conclusions
from the existing epidemiological data on the carcinogenic
potential of trichloroethylene. However, the appearance of an
excess of haematolymphatic malignancies in several of the studies,
possibly associated with trichloroethylene exposure (Blair et al.,
1979; Hardell et al., 1981; Katz & Jowett, 1981; Axelson et al.,
1984) deserves attention. Furthermore, genito-urinary tumours were
over-represented in 3 studies (Blair et al., 1979; Katz & Jowett,
1981; Axelson et al., 1984), though the picture is less consistent
with regard to the specific sites involved (bladder, kidney,
prostrate, and cervix).
It was noted that IARC (1979, 1982) concluded that
epidemiological studies on human beings were inadequate to make an
evaluation.
9. EVALUATION OF THE HEALTH RISKS FOR MAN
9.1. Levels of Exposure
9.1.1. General population
The general population is exposed to very low levels of
trichloroethylene in air, water, and food (Table 12). The move
away from the use of trichloroethylene in anaesthesia, the solvent
extraction and fumigation of foodstuffs, and the dry-cleaning
of textiles has reduced exposure from these sources.
Trichloroethylene does not accumulate significantly in the food
chain. It is degraded by both biotic and abiotic processes and
its persistence in various environmental compartments is relatively
short, of the order of days or months rather than years. In ground
water, the absence of actinic radiation means that the rate of
degradation is slower, and trichloroethylene contamination may be
relatively prolonged (e.g., half-life of 2 1/2 years).
Table 12. Maximum observed concentrations of trichloroethylene
in environmental media
---------------------------------------------------------------
Media Maximum observed concentration
---------------------------------------------------------------
Open water reservoir 220 µg/litre
Industrial discharge water 200 µg/litre
Rain water ~ 1 µg/litre
Atmosphere ~ 40 µg/m3
Dairy foods 10 µg/kg
Meat 22 µg/kg
Fats and oil 19 µg/kg
---------------------------------------------------------------
Accidental or suicidal ingestion by adults occurs, but is
influenced by the limited availability of trichloroethylene for
domestic use in many countries.
9.1.2. Occupational exposure
Exposure during the actual production of trichloroethylene is
relatively low because of the nature of the process. Subsequent
uses in, for example, metal degreasing and the dry-cleaning of
textiles, can involve higher exposures. The respiratory route is
the principal route of exposure with dermal exposure as an
additional route. Oral intake is insignificant in occupational
terms.
9.2. Evaluation of Human Health Risks
9.2.1. Acute effects
The predominant effect of trichloroethylene in human beings
is on the central nervous system; liver and kidney damage can also
occur. Central nervous system depression can result in coma and
death. A lethal dose as low as 50 ml (75 g) has been reported
but, in general, the lethal dose for an adult is approximately
7000 mg/kg body weight. The threshold for demonstrable behavioural
effects, resulting from inhalation exposure, is approximately 1350
mg/m3 (250 ppm); less well-defined subjective complaints have been
reported with exposure to concentrations of 270 - 540 mg/m3 (50 -
100 ppm) for a few hours.
Cardiac arrhythmia has been reported during anaesthesia
involving trichloroethylene at inhaled concentrations of 27 000 -
54 000 mg/m3 (5000 - 10 000 ppm) (blood levels 60 - 120 mg/litre).
It should be noted that ethanol potentiates the central nervous
system effects of trichloroethylene. Exposure to trichloroethylene
at 1080 mg/m3 (200 ppm) with blood ethanol levels of 300 - 450
mg/litre produces demonstrable behavioural effects.
Irreversible neurotoxic effects on the trigeminal nerve have
been described in anaesthesia with trichloroethylene, using a
closed-circuit system with soda lime. This effect is attributed to
the generation of dichloroacetylene and not to trichloroethylene.
9.2.2. Chronic effects
The main chronic effects of trichloroethylene in human beings
are disturbances in the central nervous system, as well as effects
on the kidney and liver.
Nervous system symptoms (headache, fatigue, irritability, and
alcohol intolerance) begin to occur when levels of trichloroacetic
acid in urine are about 30 - 75 mg/litre. No symptoms have been
reported at concentrations of 20 mg/litre in urine, which
corresponds to a concentration of trichloroethylene in air of about
50 mg/m3.
For effects on other organs, there is no clear-cut quantitative
information on dose response. There are insufficient data on
possible effects on human chromosomes.
Epidemiological data on the carcinogenicity of trichloro-
ethylene are inconclusive, but a slight excess of genito-urinary
cancers and lymphomas in some studies on dry cleaners and metal
degreasers deserves further attention.
The results of long-term exposure studies on rodents have shown
that the liver and kidney are the critical organs. Cytomegaly and
karyomegaly of renal tubular cells was observed in male rats
exposed to pure trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h
per day, 5 days per week, for 104 weeks, but not at 540 mg/m3 (100
ppm).
No embryotoxic or teratogenic effects have been observed in
mice and rats with inhalation of trichloroethylene at levels of
1620 mg/m3 (300 ppm).
Data on the genetic effects of trichloroethylene are
inconclusive.
Lifetime exposure of mice to trichloroethylene at 1620 mg/m3
(300 ppm) through inhalation or to oral doses of 700 - 1200 mg/kg
body weight per day produced lung and liver tumours. A low
incidence of renal tumours was found in rats exposed to a
trichloroethylene concentration of 3240 mg/m3 (600 ppm) by
inhalation or to 500 - 1000 mg/kg body weight per day by gavage.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure to trichloroethylene
concentrations of > 540 mg/m3 (> 100 ppm).
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats. The significance of
these findings needs to be evaluated in the context of further
studies on the mechanism of action of trichloroethylene.
9.3. Treatment of Poisoning in Human Beings
9.3.1. Emergency measures
9.3.1.1. General points
In the treatment of trichloroethylene poisoning, pressor amines
should not be used because of the risk of producing arrhythmias in
the trichloroethylene-sensitized myocardium (Bozza Marrubini et
al., 1978). Hypotension may be treated by transfusion. If
necessary, anti-arrhythmic and beta-blocking agents can be
administered. Haemodialysis, haemoperfusion, or plasmapheresis
have been reported to be useful (Zaffiri et al., 1968; Roessler
& Morawiec-Borowiac, 1973; Malizia et al., 1984). Medical advice
should always be obtained in cases of over-exposure to, or frank
poisoning by, trichloroethylene.
9.3.1.2. Ingestion
Vomiting should not be induced, because of the danger of
aspiration into the larynx and lungs and consequent risks of
vagal inhibition or chemical pneumonitis. Gastric lavage can be
effective if performed within 4 h. Adsorbents such as activated
charcoal or liquid paraffin (medicinal grade) reduce intestinal
absorption; saline laxatives will speed elimination (Bothe et al.,
1973). If the patient is in a state of stupor or coma, intubation
must be performed before gastric lavage.
9.3.1.3. Inhalation
The victim should be removed from the polluted area and placed
in a semi-prone position ensuring that the airway is clear. If
the victim is in a state of stupor or coma, oxygen should be
administered. If spontaneous respiration is absent or very weak,
artifical respiration should be applied.
9.3.1.4. Dermal exposure
All contaminated clothing should be removed and the affected
parts of the body washed thoroughly with soap and plenty of water.
9.3.1.5. Eye exposure
The eyes should be thoroughly irrigated with water for at least
15 min, and ophthalmological advice obtained on the possible need
for further treatment.
REFERENCES
ABRAHAMSEN, A.M. (1960) Quantitative estimation of
trichloroacetic acid in the urine and serum in trichloroethylene
poisoning. Acta pharmacol. toxicol., 17: 288-294.
ACGIH (1982) TLVs - Threshold limit values for chemical
substances in workroom air adopted by ACGIH for 1982, Cincinnati,
Ohio, American Conference of Governmental Industrial Hygienists,
p. 32.
ADAMS, E.M., SPENCER, H.C., ROWE, V.K., MCCOLLISTER, D.D., & IRISH,
D.D. (1951) Vapor toxicity of trichloroethylene determined by
experiments on laboratory animals. Arch. ind. Hyg. occup. Med.,
4: 469-481.
AHLMARK, A. & FORSSMAN, S. (1951a) Evaluating trichloroethylene
exposures by urinalyses for trichloroacetic acid. Arch. ind. Hyg.
occup. Med., 3: 386-398.
AHLMARK, A. & FORSSMAN, S. (1951b) The effect of
trichloroethylene on the organism. Acta physiol. scand.,
22: 326-339.
ALEXANDER, H.C., MCCARTHY, W.M., & BARTLETT, E.A. (1978) Toxicity
of perchloroethylene, trichloroethylene, 1,1,1-trichloroethane,
and methylene chloride to fathead minnows. Bull. environ. Contam.
Toxicol., 20: 344-352.
ANDERSEN, M.E., GARGAS, M.L., JONES, R.A., & JENKINS, L.J., Jr
(1980) Determination of the kinetic constants for metabolism of
inhaled toxicants in vivo using gas uptake measurements. Toxicol.
appl. Pharmacol., 54(1): 100-116.
ANDERSSON, A. (1957) [Health hazards in industry on exposure to
trichloroethylene: a clinical, experimental, and technical hygienic
investigation.] Acta med. scand., 157(Suppl. 323): 1-220 (in German).
ASCHIMICI (1980) Report for Italian Working Group for
trichloroethylene.
ASTRAND, I. & OVRUM, P. (1976) Exposure to trichloroethylene.
I. Uptake and distribution in man. Scand. J. Work Environ. Health,
2: 199-211.
AVIADO, D.M., ZAKHARI, S., SIMAAN, J.A., & ULSAMER, A.G. (1976)
In: Golberg, L., ed. Methyl chloroform and trichloroethylene in
the environment, Cleveland, Ohio, CRC Press Inc., pp. 47-89.
AXELSON, O., ANDERSSON, K., HOGSTEDT, C., HOLMBERG, B., MOLINA, G.,
& DE VERDIER, A. (1978) A cohort study on trichloroethylene
exposure and cancer mortality. J. occup. Med., 20: 194-196.
AXELSON, O., ANDERSSON, K., SELDEN, A., & HOGSTEDT, C. (1984)
Cancer morbidity and exposure to trichloroethylene. In: ICOST,
International Conference on Organic Solvent Toxicity, Stockholm,
15-17 November 1984 (Abstract in: Arbete och Halsa, 29: 126).
BADEN, J.M., KELLEY, M., MAZZE, R.I., & SIMMON, V.F. (1979)
Mutagenicity of inhalation anaesthetics: trichloroethylene,
divinyl ether, nitrous oxide and cyclopropane. J. Anaesth., 51: 417.
BAERG, R.D. & KIMBERG, D.V. (1970) Centrilobular hepatic necrosis
and acute renal failure in "solvent sniffers". Ann. intern. Med.,
73: 713-720.
BALKON, J. & LEARY, J.A. (1979) An initial report on a
comprehensive, quantitative, screening procedure for volatile
compounds of forensic and environmental interest in human biofluids
by GC/MS. J. anal. Toxicol., 3: 213-215.
BANERJEE, S. & VAN DUUREN, B.L. (1978) Covalent binding of the
carcinogen trichloroethylene to hepatic microsomal proteins and to
endogenous DNA in vitro. Cancer Res., 38: 776-780.
BANERJEE, S., YALKOWSKY, S.H., & VALVANI, S.C. (1980) Water
solubility and octanol/water partition coefficients of organics.
Limitations of the solubility-partition coefficient correlation.
Environ. Sci. Technol., 14: 1227-1229.
BARDODEJ, Z. (1962) [Value and use of exposure test. VIII.
Volatile organic chloride test in urine.] Cesk. Hyg., 7: 234-239 (in
Czech).
BARDODEJ, Z. & VISKOCIL, J. (1956) The problem of
trichloroethylene metabolism and its effects on the nervous system
as a means of hygienic control. Am. Med. Assoc. Arch. ind. Health,
13: 581-592.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS (III), B.S.H., KIRKPATRICK, M.,
MICHAEL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment: a multimedia
environmental study. Biomed. mass Spectrom., 7: 139-147.
BARRETT, L., ARSAC, PH., VINCENT, M., FAURE, J., GARREL, S., &
REYMOND, F. (1982) Evoked trigeminal nerve potential in chronic
trichloroethylene intoxication. J. Toxicol. clin. Toxicol., 19:
419-423.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish (Lepomis macrochirus). In: Hague,
R., ed. Dynamics, Exposure and Hazard Assessment of Toxic
Chemicals, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BARTONICEK, V. (1962) Metabolism and excretion of
trichloroethylene after inhalation by human subjects. Br. J. ind.
Med., 19: 134-141.
BARTONICEK, V. & BRUN, A. (1970) Subacute and chronic
trichloroethylene poisoning: a neuropathological study in rabbits.
Acta pharmacol. toxicol., 28: 359-369.
BARTONICEK, V. & SOUCEK, B. (1959) Metabolism of
trichloroethylene in rabbits. Arch. Gewerbepathol. Gewerbehyg., 17:
283-293.
BARTSCH, H., MALAVEILLE, C., BARBIN, A., & PLANCHE, G. (1979)
Mutagenic and alkylating metabolites of haloethylenes,
chlorobutadienes and dichlorobutenes produced by rodent or human
liver tissues. Arch. Toxicol., 41: 249-277.
BATTIG, K. (1964) Comparison of the effects of trichloroethylene
with the effects of drugs on exploratory behaviour in the rat. In:
14th International Congress on Occupational Health, Madrid, 1963,
Vol. 2, pp. 887-889.
BATTIG, K. & GRANDJEAN, E. (1963) Chronic effects of
trichloroethylene on rat behaviour. Arch. environ. Health, 7:
694-699.
BAUER, U. (1981) [Human exposure to environmental chemicals.
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues.] Zbl. Bakt. I. Abt. Orig., B174: 200-237
(in German).
BAUER, U. & SELENKA, F. (1982) [Exposure of the population to
haloforms and chlorinated solvents in drinking water compared to
air and food.] Von Wasser, 59: 7-16 (in German).
BEISLAND, H.O. & WANNAG, S.A. (1970) [Trichloroethylene sniffing:
acute liver and kidney damage.] Tidsskr. Nor. Laegeforen., 90: 285-288
(in Norwegian).
BELILES, R.P., BRUSICK, D.J., & MECLER, F.J. (1980) Teratogenic-
mutagenic risk of workplace contaminants: trichloroethylene,
perchloroethylene, and carbon disulfide, Washington DC, US
Department of Health, Education and Welfare, pp. 234 (Report for US
DHEW Contract No. 210-77-0047) (Govt Rep. Announce. Index (US),
1982: 2728).
BELL, Z. (1977) Written communication with contractor reports of:
(a) Dominant lethal study with trichloroethylene in albino rats
exposed via inhalation (February 1977); and (b) Teratogenic study
via inhalation with trichlor 132, trichloroethylene in albino rats
(March 1977), Washington DC, US Environmental Protection Agency
(EPA-600/8-82-006).
BELLAR, T.A., BUDDE, W.L., & EICHELBERGER, J.S. (1979) The
identification and measurement of volatile organic compounds in
aqueous environmental samples. IV. In: Schuetzle, D., ed.
Monitoring toxic substances, Washington DC, American Chemical
Society (ACS Symposium Series No. 94).
BERGMAN, K. (1983) Application and results of whole-body
autoradiography in distribution studies of organic solvents. Crit.
Rev. Toxicol., 12(1): 59-118.
BIANCHI, A., DE NATALE, G., & MATTURRO, F. (1963) [Comparative
research on some pharmacological effects of fluothane and other
volatile narcotics.] G. Ital. Chir., 19: 327-349 (in Italian).
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene, and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGNAMI, M., CONTI, G., CONTI, L., CREBELLI, R., MISURACA, F.,
PUGLIA, A.M., RANDAZZO, R., SCIANDRELLO, G., & CARERE, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor, and Aspergillus nidulans.
Chem.-biol. Interact., 30: 9-23.
BLAIR, A., DECOUFLE, P., & GRAUMAN, D. (1979) Causes of death
among laundry and dry cleaning workers. Am. J. public Health, 69:
508-511.
BOLT, H.M. & FILSER, J.G. (1977) Irreversible binding of
chlorinated ethylenes to macromolecules. Environ. Health Perspect.,
21: 107-112.
BOTHE, J., BRAUN, W., & DONHARDT, A. (1973) [Effect of mineral
oil in hydrocarbon poisoning in mice.] Arch. Toxikol., 30: 243-250 (in
German).
BOUDENE, C., TURBIAUX, M., CLUET, J.-L., & TRUHAUT, R. (1983)
Contribution à la fixation d'une concentration limite tolérable de
trichloroéthylène dans l'atmosphère des locaux de travail. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 44(2): 75-91.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and 2-
carbon halogenated aliphatic organic compounds under methanogenic
conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOUYGUES, M., DANNE, O., BOUVRY, M., LUCIANI, F., & RABREAU, D.
(1980) Hépatite au trichloroéthylène. Action synergique du
tétrachlorure de carbone? Nouv. Presse méd., 9(43): 3277.
BOZZA MARRUBINI, M., GHEZZI LAURENZI, R., & UCCELLI, P. (1978)
[Acute intoxication: clinical diagnosis and treatment,] Milan,
Italy, Medico-Farmaceutica Organization Editore, p. 470 (in
Italian).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14: 231-241.
BRONZETTI, G., ZEIGER, E., & FREZZA, D. (1978) Genetic activity
of trichloroethylene in yeast. J. environ. Pathol. Toxicol., 1:
411-418.
BROSS, G., DI FRANCEISCO, D., & DESMOND, M.E. (1983) The effects
of low dosages of trichloroethylene on chick development.
Toxicology, 28: 283-294.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., DEFELD, J.M., & BAUER, H.
(1974) Le dosage par chromatographie en phase gazeuse des
métabolites urinaires du trichloréthylène: l'acide trichloro-
acétique et le trichloroéthanol. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 35(3): 395-402.
CABANA, B.E. & GESSNER, P.K. (1967) Determination of chloral
hydrate, trichloroacetic acid, trichlorethanol, and urochloralic
acid in the presence of each other and in tissue homogenates. Anal.
Chem., 39: 1449-1452.
CACCURI, S. (1976) [Labour medicine,] 3rd ed., Napoli, Italy,
Idelson, pp. 343-352 (in Italian).
CALLEN, D.F., ROLAND WOLF, C., & PHILPOT, R.M. (1980) Cytochrome
P-450 mediated genetic activity and cytotoxicity of seven
halogenated aliphatic hydrocarbons in Saccharomyces cerevisiae.
Mutat. Res., 77: 55-63.
CAPELLINI, A. & GRISLER, R. (1958) [Studies on liver function in
a group of workers chronically exposed to trichloroethylene.] Med.
Lav., 49: 167-172 (in Italian).
CARLSON, G.P. & WHITE, J.F. (1983) Cardiac arrythmogenic action
of benzo( a)pyrene in the rabbit. Toxicol. Lett., 15: 43-48.
CASTELLINO, N. (1969) [Trichloroethylene: technology,
pathological manifestations and prevention,] Milano, Italy, Franco
Angeli Editore, p. 117 (in Italian).
CAVALLO, A. & GRASSI, P. (1976) Determination of chlorinated
hydrocarbons in potable waters. Boll. chim. Union Ital. Lab. prov.,
27: 337-350.
CEFIC (1982) Chlorinated solvents: trichloroethylene in metal
cleaning and other industrial applications, Brussels, Conseil
Européen des Fédérations des Industries Chimiques.
CERNA, M. & KJPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analyzed by screening system tests. Mutat. Res., 46:
214-215.
CHIESURA, P. (1980) [Halogenated aliphatic hydrocarbons.] In:
Crepet, M., ed. [Occupational medicine,] Torino, Italy, Utet (in
Italian).
CHIESURA, P. & CORSI, G. (1961) [Acute trichloroethylene
poisoning in man followed by liver disease and hyperglycaemic
glycosuria.] Folia med., 44: 121-135 (in Italian).
CHRISTENSEN, H.E., LUGINBYHL, T.T., & CARROLL, B.B., ed. (1974)
The toxic substances list 1974, Washington DC, National Institute
of Occupational Safety and Health, p. 353.
CHRISTENSEN, H.E. & LUGINBYHL, T.T. (1975) Registry of toxic
effects of chemical substances 1975, Washington DC, US Department
of Health, Education and Welfare.
CLEARFIELD, H.R. (1970) Hepatorenal toxicity from sniffing spot-
remover (trichloroethylene): Report of 2 cases. Am. J. dig. Dis.,
15: 851-856.
COLEMAN, W.E., LINGG, R.D., MELTON, R.G., & KOPFLER, F.C. (1976)
The occurrence of volatile organics in five drinking water supplies
using gas chromatography/mass spectrometry. Identification and
analysis of organisms in polluted water. In: Keith, L.H., ed.
Proceedings of the First Chemical Congress of the North American
Continent, 1975, Ann Arbor, Michigan, Ann Arbor Science,
pp. 305-327.
CONSO, F., EFTHYMIOU, M.-L., GARNIER, R., & FOURNIER; E. (1980)
Interêt de la toxicovigilance: example de certains
trichloréthylènes du commerce. Arch. Mal. prof. Méd. Trav., 41:
198-200.
CORNISH, H.H. & ADEFUIN, J. (1966) Ethanol potentiation of
halogenated aliphatic solvent toxicity. Am. Ind. Hyg. Assoc. J., 27:
57-61.
CORREIA, Y., MARTENS, G.J., VAN MENSCH, F.H., & WHIM, B.P. (1977)
The occurrence of trichloroethylene, tetrachloroethylene and 1,1,1-
trichloroethane in Western Europe in air and water. Atmos.
Environ., 11: 1113-1116.
CREBELLI, R., BIGNAMI, M., CONTI, L., & CARERE, A. (1982)
Mutagenicity of trichloroethylene in Salmonella typhimurium TA 100.
Ann. Ist. Super. Sanità, 18: 117-122.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (in press)
Mutagenicity of trichloroethylene, trichloroethanol, and chloral
hydrate in Aspergillus nidulans. Mutat. Res.
CRONN, D.R., RASMUSSEN, R.A., ROBINSON, E., & HARSCH, D.E. (1977)
Halogenated compound identification and measurement in the
troposphere and lower stratosphere. J. geophys. Res., 82: 5935-5944.
DALBEY, W. & BINGHAM, E. (1978) Metabolism of trichloroethylene
by the isolated perfused lung. Toxicol. appl. Pharmacol., 43: 267-277.
DANIEL, J.W. (1963) The metabolism of 36Cl-labelled
trichloroethylene and tetrachloroethylene in the rat. Biochem.
Pharmacol., 12: 795-802.
DEFALQUE, R.J. (1961) Pharmacology and toxicology of
trichloroethylene: A critical review of the world literature. Clin.
Pharmacol. Ther., 2: 665-688.
DEGUCHI, T. (1972) Threshold limit values for solvent mixtures in
the air. Effects of single and mixed chlorinated hydrocarbons upon
the level of serum transaminases in rats. Osaka Shiritsu Daigaku
Igaku Zasshi, 21: 187-209.
DEKANT, W. & HENSCHLER, D. (1982) Dechlorination reactions in the
course of the metabolism of trichloroethylene. Naunyn-Schmiedeb
Arch. Pharmacol., 319: R16.
DEKANT, W. & HENSCHLER, D. (1983) New pathways of
trichloroethylene metabolism. Dev. Toxicol. environ. Sci., 11:
399-402.
DEKANT, W., METZLER, M., & HENSCHLER, D. (1984) Novel metabolites
of trichloroethylene through dechlorination reactions in rats,
mice, and humans. Biochem. Pharmacol., 33(13): 2021-2027.
DE LEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, C.K., REMELE,
P.C., STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980) Rapid
gas chromatographic method for the determination of volatile and
semivolatile organochlorine compounds in soil and chemical waste
disposal site samples. J. chromatogr. Sci., 18: 85-88.
DE MORE, W.B., HAMSON, R.F., MOLINA, M.J., KURYLO, M.J., WATSON,
R.T., HOWARD, C.J., GOLDEN, D.M., & RAVISHANKARA, A.R. (1983)
Chemical kinetics and photochemical data for use in stratospheric
modeling evaluation number 6, Washington DC, National Aeronautics
and Space Administration (NASA JPL Publication No. 83-62,
15 September, 1983).
DERWENT, R.G. & EGGLETON, A.E.J. (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos. Environ., 12: 1261-1268.
DESOILLE, H., PINCHON, R.A., JANS, M., & BOUGUIGNON, A. (1962)
Intoxication expérimentale aiguë par le trichloroéthylène. Effets
aggravants de l'intoxication éthylique chronique associée. Etude
expérimentale électroencéphalographique chez le lapin. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 23: 653-664.
DIETZ, E.A., Jr & SINGLEY, K.F. (1979) Determination of
chlorinated hydrocarbons in water by headspace gas chromatography.
Anal. Chem., 51: 1809-1814.
DILLING, W.L. (1977) Interphase transfer processes. II.
Evaporation rates of chloromethanes, ethanes, ethylenes, propanes,
and propylenes from dilute aqueous solutions. Comparisons with
theoretical predictions. Environ. Sci. Technol., 11: 405.
DI PAOLO, J.A. & DONIGER, J. (1982) Neoplastic transformation of
Syrian hamster cells by putative epoxide metabolites of
commercially-utilized chloroalkenes. J. Natl Cancer Inst., 69(2):
531-534.
DI RENZO, A.B., GANDOLFI, A.J., & SPIES, I.G. (1982) Microsomal
bioactivation and covalent binding of aliphatic halides to DNA.
Toxicol. Lett., 11: 243-252.
DMS (1967) UV atlas of organic compounds, London, Dortmund,
Butterworths-Verlag Chemie, Vol. 3 (Spectrum No. A1/6).
DOBINSON, B. & GREEN, G.E. (1972) Formation of dichloroacetylene
from trichloroethylene in the presence of epoxides. Chem. Ind., 5:
214.
DORFMUELLER, M.A., HENNE, S.P., YORK, R.G., BORNSCHEIN, R.L., &
MANSON, J.M. (1979) Evaluation of teratogenicity and behavioural
toxicity with inhalation exposure of maternal rats to
trichloroethylene. Toxicology, 14: 153-166.
DOWTY, B.J., CARLISLE, D.R., & LASETER, J.L. (1975) New Orleans
drinking water sources tested by gas chromatography-mass
spectrometry. Environ. Sci. Technol., 9: 762-765.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Solubility of organic
solvents. I. Gas chromatographic determination of olive oil/gas
partition coefficient. Helv. Chim. Acta, 60: 454.
DROZ, P.O. & FERNANDEZ, J.G. (1978) Trichloroethylene exposure.
Biological monitoring by breath and urine analyses. Br. J. ind.
Med., 35: 35.
DUPRAT, P. & GRADISKI, D. (1980) Cytogenic effect of
trichloroethylene in the mouse as evaluated in the micronucleus
test. IRCS med. Sci. soc. occup. Med., 8: 182.
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir irritant
des principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur. J. Toxicol., 9(3): 171-177.
EAJ (1983) Environmental monitoring of chemicals: environmental
survey report of F.Y. 1980 and 1981, Japan, Office of Health
Studies, Department of Environmental Health, Environmental Agency
Japan, p. 144.
EHRNER-SAMUEL, H., BALMER, K., & THORSELL, W. (1973)
Determination of trichloroacetic acid in urine by a gas
chromatographic method. Am. Ind. Hyg. Assoc. J., 34: 93-96.
EKLUND, G., JOSEFFSON, B., & ROOS, C. (1978) Determination of
volatile halogenated hydrocarbons in tapwater. J. High Res. Chrom.
& Chrom. Comms., July, pp. 34-40.
ELCOMBE, C.R. (in press) Species differences in carcinogenicity
and peroxisome proliferation due to trichloroethylene: a
biochemical human hazard assessment. Arch. Toxicol.
ELCOMBE, C.R., PRATT, I.S., & GREEN, T. (1982) The rate of
trichloroacetic acid (TCA) formation determines two species
differences in hepatic peroxisome proliferation due to
trichloroethylene (TRI). Toxicologist, 2: 173.
ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979) Effects of
methylene chloride, trichloroethane, trichloroethylene,
tetrachloroethylene, and toluene on the development of chick
embryos. Toxicology, 12: 111-119.
ENTZ, R.C. & HOLLIFIELD, H.C. (1982) Headspace gas
chromatographic analysis of foods for volatile hydrocarbons.
J. agric. food Chem., 30: 84-88.
ERTLE, T., HENSCHLER, D., JULLER, G., & SPASSOWSKI, M. (1972)
Metabolism of trichloroethylene in man. Arch. Toxikol., 29: 171-188.
ETTEMA, J.J., KLEEREKOPER, L., & DUBA, W.C. (1975) Mental
stresses during short-term inhalation of trichloroethylene.
Staub-Reinhalt. Luft, 35: 409-410.
EUROCOP-COST (1976) A comprehensive list of polluting substances
which have been identified in various fresh waters, effluent
discharges, aquatic animals and plants, and bottom sediments, 2nd
ed., Luxembourg, Commission of the European Community, p. 57
(EUCO/MDU/73/76, XII/476/76).
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., & HOPKE, P.K.
(1977) Monitoring to detect previously unrecognized pollutants in
surface waters - appendix: organic analysis data, Springfield,
Virginia, US NTIS, pp. 304 (PB Report PB-273350) (Govt Rep.
Announce. Index, 1978: 199).
FABRE, R. & TRUHAUT, R. (1952) Toxicology of trichloroethylene.
II. Results of experimental animal studies. Br. J. ind. Med., 9:
39-43.
FAHRIG, R. (1977) The mammalian spot test (Fellfleckenstest) with
mice. Arch. Toxicol., 38: 87-98.
FAWNS, H.T. (1968) Trichloroacetic acid in the urine of workers
using trichloroethylene. Proc. Assoc. Clin. Biochem., 5: 110-114.
FERGUSON, R.K. & VERNON, R.J. (1970) Trichloroethylene in
combination with CNS drugs: effects on visual-motor tests. Arch.
environ. Health, 20: 462-467.
FERNANDEZ, J.G., HUMBERT, B.E., DROZ, P.O., & CAPEROS, J.R. (1975)
Exposition au trichloroéthylene. Bilan de l'absorption, de
l'excrétion, et du métabolisme sur des sujets humains. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 36(7-8): 397-407.
FERNANDEZ, J.G., DROZ, P.O., HUMBERT, B.E., & CAPEROS, J.R. (1977)
Trichloroethylene exposure: simulation of uptake, excretion, and
metabolism using a mathematical model. Br. J. ind. Med., 34: 43-55.
FILSER, J.G. & BOLT, H.M. (1979) Pharmacokinetics of halogenated
ethylenes in rats. Arch. Toxicol., 42: 123-136.
FINK, R. (1968) Toxicity of anaesthetics, Baltimore, Maryland,
Williams & Williams, pp. 270.
FISHBEIN, L. (1973) Chromatography of environmental hazards,
New York, Elsevier Scientific Publishing Company, Vol. 2,
pp. 471-490.
FOOD CHEMICAL NEWS (1978) Preliminary report on TCE study shows
no carcinogenicity in rats. Food chem. News, 20: 41-42.
FOSSA, A.A., WHITE, J.F., & CARLSON, G.P. (1982) Anti-arrhythmic
effects of disulfiram on epinephrine-induced cardiac arrthythmias
in rabbits exposed to trichloroethylene. Toxicol. appl. Pharmacol.,
66: 109-117.
FRIBERG, G.L., KYLIN, B., & NYSTROM, A. (1953) Toxicities of
trichloroethylene and tetrachloroethylene and Fujiwara's pyridine
reaction. Acta pharmacol. toxicol., 9: 303-312.
FUKUDA, K., TAKEMOTO, K., & TSURATA, H. (1983) Inhalation
carcinogenicity of trichloroethylene in mice and rats. Ind. Health,
21: 243-245.
GAMBERALE, F., ANNWALL, G., & OLSON, B.A. (1976) Exposure to
trichloroethylene. III. Psychological functions. Scand. J. Work
environ. Health, 4: 220-224.
GAULTIER, M. (1974) [Clinical toxicology,] Rome, Italy, Società
Editrice Demi (in Italian).
GEHRING, P.J. (1968) Hepatoxic potency of various chlorinated
hydrocarbon vapours relative to their narcotic and lethal potencies
in mice. Toxicol. appl. Pharmacol., 13: 287-298.
GOLDBERG, M.E., JOHNSON, H.E., POZZANI, U.C., & SMITH, H.E., Jr
(1964) Effect of repeated inhalation of vapours of industrial
solvents on animal behaviour. I. Evaluation of nine solvent vapours
on pole-climb performance in rat. Am. Ind. Hyg. Assoc. J., 25: 369-375.
GOLDBLATT, M.W. (1956) Industrial medicine and hygiene, London,
Merewether, Vol. 3.
GOVERNA, M. (1981) [Occupational diseases due to chlorine
derivatives of the aliphatic hydrocarbons.] In: Sartorelli, E., ed.
[Textbook of occupational medicine,] Padova, Italy, Vol. 1,
pp. 483-502 (in Italian).
GRADISKI, D., MAGADUR, J.-L., BAILLOT, M., DANIERE, M.C., & SCHUH,
M.B. (1974) Toxicité comparée des principaux solvants chlorés
aliphatiques. J. eur. Toxicol., 7: 247-254.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.-L., & FRANCIN,
J.M. (1978) Toxicité aiguë comparée par inhalation des principaux
solvants aliphatiques chlorés. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 39: 249-257.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
New York, Academic Press.
GRANDJEAN, E. (1960) Trichloroethylene effects on animal
behaviour. Arch. environ. Health, 1: 106-108.
GRANDJEAN, E. (1963) The effects of short exposures to
trichloroethylene on swimming performances and motor activity of
rats. Am. Ind. Hyg. Assoc. J., 24: 376-379.
GRANDJEAN, E., MUNCHINGER, R., TURRIAN, V., HAAS, P.A., KNOEPFEL,
H.K., & ROSENMUND, H. (1955) Investigations into the effects of
exposure to trichloroethylene in mechanical engineering. Br. J.
ind. Med., 12: 131-142.
GRANT, M.W. (1961) Trichoroethylene in the eye. J. Am. Med.
Assoc., 178: 359.
GRANT, M.W. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, pp. 1034-1045.
GRAOVAC-LEPOSAVIC, L., MILOSAVLJEVIC, Z., & ILIC, V. (1964)
[Liver function in workers exposed to trichloroethylene.] Arh. Hig.
Rad. Toksikol., 15: 93-97 (in Yugoslavian).
GREEN, T. & PROUT, M.S. (in press) Species differences in
response to trichloroethylene. II. Biotransformation in rats and
mice. Arch. Toxicol.
GREEN, T., PROUT, M.S., & PROVAN, W.M. (in press) Species
differences in response to trichloroethylene. I. Pharmacokinetics
in rats and mice. Arch. Toxicol.
GREIM, H., BONSE, G., RADWAN, Z., REICHERT, D., & HENSCHLER, D.
(1975) Mutagenicity in vitro and potential carcinogenicity of
chlorinated ethylenes as a function of metabolic oxirane formation.
Biochem. Pharmacol., 24: 2013-2017.
GU, Z.W., SELE, B., JALBERT, P., VINCENT, M., VINCENT, F., MARKA,
C., CHMARA, D., & FAURE, J. (1981) Induction of sister chromatid
exchanges by trichloroethylene and its metabolites. Toxicol. Eur.
Res., 3: 63-67.
GUN, R.T., GRYGORCENICZ, D., & NETTELBECK, T.J. (1978) Choice
reaction time in workers using trichloroethylene. Med. J. Aust., 1:
535-536.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 73: 169-176.
HATHWAY, D.E. (1980) Consideration of the evidence for mechanisms
of 1,1,2-trichloroethylene metabolism including new identification
of its dichloroacetic acid and trichloroacetic acid metabolites in
mice. Cancer Lett., 8: 263-269.
HAYDEN, J.W., COMSTOCK, E.G., & COMSTOCK, B.S. (1976) The
chemical toxicology of solvent abuse. Clin. Toxicol., 9: 169-184.
HEIL, E., OESER, H., HATZ, R., & KELKER, H. (1979) [Gas
chromatographic determination of traces of C- and C-fluoro- and
chlorohydrocarbons in air.] Fresenius Z. Anal. Chem., 297: 357-364
(in German).
HENSCHLER, D. (1977a) Metabolism and mutagenicity of halogenated
olefuis: a comparison of structure and activity. Environ. Health
Perspect., 21: 61-64.
HENSCHLER, D. (1977b) Metabolism of chlorinated alkenes and
alkanes as related to toxicity. J. environ. Pathol. Toxicol., 1:
125-133.
HENSCHLER, D. & HOOS, R. (1982) Metabolic activation and
deactivation mechanisms of di-, tri-, and tetrachloroethylenes.
In: Snyder, Parke, Kocsis, Jollow, Gibson, & Witmer, ed. Biological
reactive intermediates. II. Part A., New York, Plenum Publishing
Corporation, pp. 659-666.
HENSCHLER, D., BONSE, G., & GREIM, H. (1970a) [Cranial nerve
neuritis due to poisoning with chlorinated acetylenes when handling
vinylidene chloride copolymers.] Arch. Toxikol., 26: 62-75 (in
German).
HENSCHLER, D., BROSER, F., & HOPF, H.C. (1970b) Environmental
Pollution and Carcinogenic Risks, Lyons, International Agency for
Research on Cancer, pp. 171-175 (IARC Scientific Publications
No. 13).
HENSCHLER, D., EDER, E., NEUDECKER, T., & METZLER, M. (1977)
Carcinogenicity of trichloroethylene: fact or artifact? Arch.
Toxicol., 37: 233-236.
HENSCHLER, D., HOOS, W.R., FETZ, H., DALLMEIER, E., & METZLER, M.
(1979) Reactions of trichloroethylene epoxide in aqueous systems.
Biochem. Pharmacol., 28: 543-548.
HENSCHLER, D., ROMEN, W., ELSASSER, H.M., REICHERT, D., EDER, E., &
RADWAN, Z. (1980) Carcinogenicity study of trichloroethylene by
long-term inhalation in three animal species. Arch. Toxicol., 43:
237-248.
HENSCHLER, D., BONSE, G., & DEKANT, W. (1983) Mechanisms and
reactions of electroplulic intermediates of halogenated ofefins.
In: Proceedings of the 13th International Cancer Congress. Part B.
Biology of Cancer, New York, Alan R. Liss, Inc., Vol. 1,
pp. 175-183.
HENSCHLER, D., ELSASSER, H., ROMEN, W., & EDER, E. (1984)
Carcinogenicity study of trichloroethylene, with and without
epoxide stabilizers, in mice. J. Cancer Res. clin. Oncol., 107:
149-156.
HERBOLSHEIMER, R., FUNK, L., & DRASCHE, H. (1972) Use of
activated carbon as an adsorbent in the determination of
trichloroethylene in the air. Staub-Reinhalt. Luft, 32: 407-409.
HERNBERG, S., KORKATA, M.-L., ASIKAINEN, V., & RIALA, R. (1984)
Primary liver cancer and exposure to solvents. Int. Arch. occup.
environ. Health, 54: 147-153.
HIRAYAMA, T. & IKEDA, M. (1979) Applicability of carbon felt to
the dosimetry of solvent vapour mixture. Am. Ind. Hyg. Assoc. J.,
40: 1091-1096.
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., & SAKAI,
T. (1983) [Experimental studies of trichloroethylene toxicity.
II. Changes in trichloroethylene in blood serum and in urine during
and after exposure to trichloroethylene.] Nippon Eiseigaku Zasshi,
38(4): 772-779 (in Japanese with English summary).
HOWARD, C.J. (1976) Rate constants for the gas-phase reactions of
OH radicals with ethylene and halogenated ethylene compounds.
J. Chem. Phys., 65: 4771.
HUFF, J.E. (1971) New evidence on the old problems of
trichloroethylene. Ind. Med. Surg., 40: 25-33.
IARC (1979) Trichloroethylene, Lyons, International Agency for
Research on Cancer, pp. 545-572 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 247-249 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Supplement 4).
IKEDA, M. (1974) Reciprocal metabolic inhibition of toluene and
trichloroethylene in vivo and in vitro. Int. Arch. Arbeitsmed., 33:
125-130.
IKEDA, M. & IMAMURA, T. (1973) Biological half-life of
trichloroethylene and tetrachloroethylene in human subjects. Int.
Arch. Arbeitsmed., 31: 209-224.
IKEDA, M., OHTSUJI, H., KAWAI, H., & KUNIYOSHI, M. (1971)
Excretion kinetics of urinary metabolites in patient addicted to
trichloroethylene. Br. J. ind. Med., 28: 203-206.
IKEDA, M., OHTSUJI, H., IMAMAURA, T., & KOMOIKE, Y. (1972)
Urinary excretion of total trichloro-compounds, trichloroethanol,
and trichloroacetic acid as a measure of exposure to
trichloroethylene and tetrachloroethylene. Br. J. ind. Med., 29:
328-333.
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office, pp. 206-207 (Occupational Safety and Health Series No. 37).
IMAMURA, T. & IKEDA, M. (1973) A time-saving procedure for the
determination of total trichloro-compounds in human urine samples.
Int. Arch. Arbeitsmed., 31: 333-338.
IOFFE, B.V., ISIDOROV, V.A., & ZENKEVICH, I.G. (1977) Gas
chromatographic-mass spectrometric determination of volatile
organic compounds in an urban atmosphere. J. Chromatogr., 142:
787-795.
IRPTC (1984) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JAMES, W.R.L. (1963) Fatal addiction to trichloroethylene. Br. J.
ind. Med., 20: 47-49.
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G. (1982)
Uptake via the blood and elimination of 10 organic solvents
following epicutaneous exposure of anaesthetized guinea-pigs.
Toxicol. appl. Pharmacol., 63: 181-187.
JAPANESE YEARBOOK OF CHEMICAL INDUSTRIES STATISTICS (1983) Tokyo,
Ministry of International Trade and Industry.
JESSON, J.P. (1980) Release of industrial halocarbons and
tropospheric budget. In: Proceedings of the NATO Advanced Study
Institute on Atmospheric Ozone: Its Variation and Human Influences,
Washington DC, US Department of Transportation, Federal Aviation
Administration.
JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (1983)
Evaluation of certain food additives: 27th report, Geneva, World
Health Organization (Technical Report Series No. 696).
JOUGLARD, J. & VINCENT, V. (1971) Pulmonary signs of ingestions
of solvents. Marseille Med., 108: 696-697.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial waste water: a case study of their
environmental impact. Environ. Sci. Technol., 12: 88-96.
KALASHINIKOVA, V.P., VISSARIONOVA, V.Ya., BONDAREV, G.I., SKIRKO,
B.K., & KARANTIROVA, O.V. (1974) [Influence of different diets on
the course of chronic experimental trichloroethylene poisoning.]
Vopr. Pitan., No.6: 43-47 (in Russian).
KALASHINIKOVA, V.P., SKIRKO, B.K., VISSARIONOVA, V.Ya., BONDAREV,
G.I., KLEIMENOVA, N.V., POPOVA, N.S., & KARANTIROVA, O.V. (1976)
[Evaluation of the toxic effects of trichloroethylene according
to morphological and some biochemical indexes.] Gig. i Sanit., No. 2:
107-108 (in Russian).
KANJE, M., KJELLSTRAND, P., FOX, K., & WALLDORF, A. (1981)
Neurotransmitter metabolizing enzymes and plasma
butyrylcholinesterase in mice exposed to trichloroethylene. Acta
pharmacol. toxicol., 49: 205-209.
KARICKHOFF, S.W., BROWN, D.S., & SCOTT, T.A. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13: 241-248.
KATZ, R.M. & JOWETT, D. (1981) Female laundry and dry cleaning
workers in Wisconsin: a mortality analysis. Am. J. public Health,
71: 305-307.
KEINFELD, M. & TABERSHAW, I.R. (1954) Trichloroethylene toxicity:
report of 5 fatal cases. AMA Arch. ind. Hyg. occup. Med., 10: 134-141.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals.
Ecotoxicol. environ. Saf., 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (in press) Relationship between
water solubility, soil sorption, octanol-water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish,
P.R., & Hendricks, A.C., ed. Aquatic toxicology ASTM STP, 707,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
KIMMERLE, G. & EBEN, A. (1973) Metabolism, excretion, and
toxicology of trichloroethylene after inhalation. II. Experimental
human exposure. Arch. Toxicol., 30: 127-138.
KIRK, R.E. & OTHMER, D.F., ed. (1963) Anesthetics. In:
Encyclopedia of chemical technology, 2nd ed., New York, John Wiley
and Sons, Vol. 2, pp. 393-410.
KIRK, R.E. & OTHMER, D.F., ed. (1964) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 2nd
ed., New York, John Wiley and Sons, Vol. 5, pp. 183-195.
KIRK, R.E. & OTHMER, D.F., ed. (1979) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 3rd
ed., New York, John Wiley and Sons, Vol. 5, pp. 745-753.
KISELEVA, A.F. & KOROLENKO, A.M. (1971) Histoenzymic changes in
liver and kidneys during the experimental effect of anesthetic
doses of fluorothan and trylen. Eksp. Khir. Anesteziol., 16: 81-84.
KITAGAWA, T. (1961) The rapid measurement of toxic gases and
vapour. In: Proceedings of the 13th International Congress on
Occupational Health, New York, pp. 506-512.
KJELLSTRAND, P., LANKE, J., BJERKEMO, M., ZETTERQUIST, L., &
MANSSON, L. (1980) Irreversible effects of trichloroethylene
exposure on the central nervous system. Scand. J. Work environ.
Health, 6: 40-47.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M.,
MORTENSEN, I., LANKE, J., & HOLMQUIST, B. (1981a)
Trichloroethylene: effects on body and organ weights in mice,
rats, and gerbils. Toxicology, 21: 105-115.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M., MORTENSEN,
I., LANKE, J., & HOLMQUIST, B. (1981b) Effects of long-term
exposure to trichloroethylene on the behaviour of Mongolian gerbils
(Meriones unguiculatus). J. Toxicol. environ. Health, 8: 787-793.
KJELLSTRAND, P., HOLMQUIST, B., MANDAHL, N., & BJERKEMO, M.
(1983a) Effects of continuous trichloroethylene inhalation on
different strains of mice. Acta pharmacol. toxicol., 53: 369-374.
KJELLSTRAND, P., HOLMQUIST, B., ALM, P., KANJE, M., ROMARE, S.,
JONSSON, I., MANSSON, L., & BJERKEMO, M. (1983b)
Trichloroethylene: further studies of the effects on body and organ
weights and plasma butyrycholinesterase activity in mice. Acta
pharmacol. toxicol., 53: 375-384.
KLAASSEN, C.D. & PLAA, G.L. (1966) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in mice.
Toxicol. appl. Pharmacol., 9: 139-151.
KLAASSEN, C.D. & PLAA, G.L. (1967) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in dogs.
Toxicol. appl. Pharmacol., 10: 119-131.
KLINE, S.A., MCCOY, E.C., ROSENKRANZ, H.S., & VAN DUUREN, B.L.
(1982) Mutagenicity of chloroalkene epoxides in bacterial systems.
Mutat. Res., 101: 115-125.
KLOWKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981)
Laboratory screening of distribution, conversion, and
mineralisation of chemicals in the soil-plant-system and comparison
to outdoor experimental data. Chemosphere, 10: 1089-1090.
KONIETZKO, H., HABERLANDT, W., HEILBRONNER, H., REILL, G., &
WEICHARDT, H. (1978) [Chromosome studies on trichloroethylene
workers.] Arch. Toxicol., 40: 201-206 (in German).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin pathology
following epicutaneous exposure to seven organic solvents. Int. J.
Tiss. React., 111(1): 21-30.
KYLIN, B., REICHARD, H., SUMEGI, I., & YLLNER, S. (1962)
Heptatoxic effect of tri- and tetrachloroethylene on mice. Nature
(Lond.), 193: 395.
KYLIN, B., AXELL, K., EHRNER-SAMUEL, H., & LINDBORG, A. (1967)
Effect of inhaled trichloroethylene on the CNS. Arch. environ.
Health, 15: 48-52.
KYLSZEIJKO, C., LAKOMY, T., & PAPIEROWSKI, A. (1963) [The
influence of trichloroethylene during labour process in women in
comparison with the results in vitro.] Pol. Tyg. Lek., 18:
1333-1338 (in Polish).
KYRKLUND, T., ALLING, C., HAGLID, K., & KJELLSTRAND, P. (1983)
Chronic exposure to trichloroethylene: lipid and acyl group
composition in gerbil cerebral cortex and hippocampus.
Neurotoxicology, 4(4): 35-42.
LACHNIT, V. (1971) Halogenated hydrocarbons and the liver. Wein.
Klin. Wochenschr., 83: 734-737.
LA DU, B.N., MANDEL, H.G., & WAY, E.L., ed. (1971) Fundamentals
of drug metabolism and drug disposition, Baltimore, Maryland,
Williams and Wilkins.
LAHAM, S. (1970) Studies on placental transfer:
trichloroethylene. Ind. Med. Surg., 39: 46-49.
LAZAREV, N.V. (1929) [The narcotic efficacy of the vapours of the
chlorine derivatives of methane, ethane and ethylene.] Naunyn-
Schmiedeb. Arch. exp. Pathol. Pharmacol., 141: 19-24 (in German).
LAZAREV, N.V. & GADASKINA, I.D. (1977) [Harmful substances
in industry,] Leningrad, Medecine, Vol. 3 (in Russian).
LEHMANN, K.B. & SCHMIDT-KEHL, L. (1936) [Thirteen most important
chlorohydrocarbons of the paraffin series from the standpoint of
industrial hygiene.] Arch. Hyg. Bakt., 116: 131-268 (in German).
LEIBMAN, K.C. (1965) Metabolism of trichloroethylene in liver
microsomes. Mol. Pharmacol., 1: 239-246.
LEONG, B.K.J., SCHWETZ, B.A., & GEHRING, P.J. (1975) Embryo- and
fetotoxicity of inhaled trichloroethylene, perchloroethylene,
methylchloroform, and methylene chloride in mice and rats. Toxicol.
appl. Pharmacol., 33: 136.
LERZA, P., LOMBARDI, F., & VIOTTI, G. (1963) [Health and hygiene
considerations regarding work in dry-cleaning establishments using
trichloroethylene.] Lav. Um., 15: 459-478 (in Italian).
LEWIS, G.D., REYNOLDS, R.C., & JOHNSON, A.R. (1984) Some effects
of trichloroethylene on mouse lungs and livers. Gen. Pharmacol.,
15(2): 139-144.
LILIS, R., STANESCU, D., MUICA, N., & ROVENTA, A. (1969) Chronic
effects of trichloroethylene exposure. Med. Lav., 60: 595-601.
LIN, R.S. & KESSLER, I.I. (1981) A multifactoral model for
pancreatic cancer in man. J. Am. Med. Assoc., 245: 147-152.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Proceedings of the
Symposium on Short-Term Test Systmes and the Detection of
Carcinogenesis, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 333-356.
LOPRIENO, N., SBRANA, I., & LASCIALFARI, D. (in press) [Study on
the in vivo potential mutagenic effect of trichloroethylene
evaluated by cytogenetic analysis in mice treated by inhalation for
52 days.] (in Italian).
LOVELOCK, J.E. (1974) Atmospheric halocarbons and stratospheric
ozone. Nature (Lond.), 252: 292-294.
MACKAY, D. (1982) Correlations of bioconcentration factors.
Environ. Sci. Technol., 10: 274-278.
MCCONNELL, G., FERGUSON, D.M., & PEARSON, C.R. (1975) Les
hydrocarbures chlorés et l'environnement. Endeavour, 34: 13-18.
MCCORD, C.P. (1932) Toxicity of trichloroethylene. J. Am.
Med. Assoc., 99: 409.
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
MCNEILL, W.C. (1979) Chlorocarbons and chlorohydrocarbons.
Trichloroethylene. In: Kirk-Othmer's encyclopedia of chemical
technology, 3rd ed., New York, Wiley Interscience, Vol. 5,
pp. 745-753.
MAKIDE, Y., TOMINAGA, K., & ROWLAND, F.S. (1979) Gas
chromatographic analysis of halogenated hydrocarbons in air over
Japan. Chem. Lett., 1979: 355-358.
MALEK, B., KRCMAROVA, B., & RODOVA, O. (1979) [Epidemiological
study of hepatic tumour incidence in subjects working with
trichloroethylene: II. Negative result of retrospective studies in
dry cleaners.] Pracov. Lek., 31(4): 124-126 (in Czech).
MALIZIA, E., PELAIA, P., & PIROVINE, C. (1984) Experience in the
emergency treatment of acute intoxication by trichloroethylene.
Riv. Toss. Speriment Clin., 14: 437.
MALOOF, C.C. (1949) Burns of the skin produced by
trichloroethylene vapours at room temperature. J. ind. Hyg.
Toxicol., 31: 295-296.
MALTONI, I.C. & MAIOLI, P. (1977) [Long-term bioassay of
carcinogenicity of trichloroethylene. Preliminary results.]
Osp. Vita, 4: 108-110 (in Italian).
MALTONI, C., LEFEMINE, G., & COTTI, G. (in press) Experimental
research on trichloroethylene carcinogenesis. In: Maltoni, C. &
Mehlman, M.A., ed. Archives of research on industrial
carcinogenesis, Vol. 5.
MANTEL, M. & NOTHMANN, R. (1977) Rapid determination of
trichloroethanol and trichloroacetic acid in urine. Analyst, 102:
672-677.
MATTURRO, F. (1963) [Effects of some volatile narcotics on
isolated guinea-pig heart.] G. Ital. Chir., 19: 95-99 (in Italian).
MAZZA, V. & BRANCACCIO, A. (1967) [Behaviour of the formed
elements of the blood and the marrow in experimental intoxications
with Tri.] Folia med., 50: 318-324 (in Italian).
MELINO, C., MESSINEO, A., & PACELLI, E. (1979) Risk from
trichloroethylene in a factory producing electrical condensers.
Riv. Med. Lab. Ig. Ind., 3: 67-81.
MEYER, H.-J. (1966) [Trichloroethylene poisoning by the oral
route.] Arch. Toxikol., 21: 225-234 (in German).
MEYER, H.-J. (1973) Bronchopulmonary alterations induced by
trichloroethylene and other halogenated hydrocarbons. Bronches, 23:
113-124.
MILLER, D.A. & GRIMSRUD, E.P. (1979) Correlation of electron
capture response enhancements caused by oxygen with chemical
structure for chlorinated hydrocarbons. Anal. Chem., 51: 851-859.
MILLER, R.E. & GUENGERICH, F.P. (1982) Oxidation of
trichloroethylene by liver microsomal cytochrome P-450: evidence
for chlorine migration in a transition state not involving
trichloroethylene oxide. Biochemistry, 21: 1090-1097.
MOLINA, M.J. & ROWLAND, F.S. (1974) Stratospheric sink for
chlorofluoromethanes: chlorine atom-catalysed destruction of ozone.
Nature (Lond.), 294: 810-812.
MONSTER, A.C. (1979) Differences in uptake, elimination, and
metabolism in exposure to trichloroethylene, 1,1,1-trichloroethane,
and tetrachloroethylene. Int. Arch. occup. environ. Health, 42:
311-317.
MONSTER, A.C. & BOERSMA, G. (1975) Simultaneous determination of
trichloroethylene and metabolites in blood and exhaled air by gas
chromatography. Int. Arch. occup. environ. Health, 35: 155-163.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1976) Pharmaco-
kinetics of trichloroethylene in volunteers: influence of workload
and exposure concentration. Int. Arch. occup. environ. Health, 38:
87-102.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1979) Kinetics of
trichloroethylene in repeated exposure of volunteers. Int. Arch.
occup. environ. Health, 42: 283-292.
MOSLEN, M.T., REYNOLDS, E.S., & SZABO, S. (1977) Enhancement of
the metabolism and hepatoxicity of trichloroethylene and
perchloroethylene. Biochem. Pharmacol., 26: 369-375.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1972)
Trichloroethylene exposure and trichloroethylene metabolites in
urine and blood. Arch. Toxicol., 29: 335-340.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1974) Metabolism of
trichloroethylene in man. II. Pharmacokinetics of metabolites.
Arch. Toxicol., 32: 283-295.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1975) Metabolism of
trichloroethylene in man. III. Interaction of trichloroethylene and
ethanol. Arch. Toxicol., 33: 173-189.
MURRAY, A.J. & RILEY, J.P. (1973) Occurrence of some chlorinated
aliphatic hydrocarbons in the environment. Nature (Lond.), 242: 37-38.
MUSCLOW, C.E. & AWEN, C.F. (1971) Glue sniffing: report of a
fatal case. Can. Med. Assoc. J., 104: 315-319.
NCI (1976) Carcinogenesis bioassay of trichloroethylene. CAS No.
Metabolically activated trichloroethylene binds covalently to
the hepatic microsomal proteins and DNA, in vitro. This finding
supports the formation of an epoxide intermediate (Banerjee & Van
Duuren, 1978), though this has not been demonstrated in vivo
(Parchman & Magee, 1982; Stott et al., 1982).
5.3.2. Human beings
As originally suggested by Powell (1945), the formation of the
epoxide, an intermediate reactive metabolite that binds covalently
with proteins (Bolt & Filher, 1977), has been confirmed by indirect
spectral evidence (Uehleke et al., 1977). The epoxide may undergo
intramolecular rearrangement in 2 different ways (Henschler & Hoos,
1982; DeKant & Henschler, 1983). One pathway leads to chloral
(Henschler, 1977a,b), which is further oxidized to trichloroacetic
acid (TCA), or reduced to trichloroethanol (Leibman, 1965). After
oral administration, trichloroethanol is also partly metabolized
into TCA (Müller et al., 1974). Trichloroethanol is rapidly
conjugated with glucuronic acid to form the respective glucuronide.
The other pathway leads to the formation of dichloroacetyl chloride
which, under in vitro conditions, can lead to the formation of
dichloroacetic acid. However, under normal in vivo conditions,
dichloroacetic acid is not found, except in mice following the
administration of very high doses of trichloroethylene. Under
these conditions, an "overspill" mechanism may operate (Henschler
et al., 1979; Hathway, 1980; Henschler et al., 1983). The
excretion of chloroform in expired air and monochloroacetic acid in
urine have also been proposed as minor routes of metabolism (Ogata
& Saeki, 1974; Bartonicek, 1962). Miller & Guenrich (1982)
suggested that an epoxide was not an obligatory intermediate step
and proposed an alternative model in which chlorine migration
occurs in an oxygenated trichloroethylene P 450 transition state.
Trichloroacetic acid binds well with plasma proteins and its
concentration in plasma is approximately double that in whole blood
(Müller et al., 1972).
5.3.3. Drug and other interactions
A number of commonly-used drugs might be expected to modify the
extent of metabolism of trichloroethylene during human exposure.
Although largely undocumented in man, the induction of the hepatic
microsomal mixed-function oxidase system by drugs, taken for
therapeutic reasons, or by exposure to certain environmental
chemicals (e.g., phenobarbital, toluene, PCBs) can bring about an
increased rate of trichloroethylene metabolism (Ikeda & Imamura,
1973; Ikeda, 1974).
In human beings, the simultaneous administration of ethanol and
trichloroethylene (100 mg/m3 for 6 h) causes an increase in
trichloroethylene levels in both plasma (2.4 times the normal
value) and in exhaled air (3.4 times), and a decrease in the levels
of trichloroacetic acid and trichloroethanol (Müller et al., 1975).
5.4. Elimination
5.4.1. Studies on animals
The kinetics of the distribution and elimination of
trichloroethylene, administered intravenously in Wistar rats at
dose levels of 6, 9, 12, or 15 mg/kg body weight show that the
blood concentration exhibits a first order, 2-compartment model
exponential disappearance, and it has been suggested that a dose
of 15 mg trichloroethylene/kg body weight is within the hepatic
metabolic capacity in the rat (Withey & Collins, 1980). Daniel
(1963) showed that when trichloroethylene was administered orally
to rats, the ratio of pulmonary to urinary elimination varied with
the dose and that as dose increased, pulmonary excretion increased
while urinary elimination decreased. Further evidence showing that
the metabolism of trichloroethylene is saturable in Wistar rats was
obtained by Filser & Bolt (1979) who showed that the saturation
point occurred at 350 mg/m3 (65 ppm), that the zero order Vmax was
210 µmol/h per kg body weight and that the first order clearance at
a dose of 350 mg/m3 (65 ppm) was 77 µmol/h per kg body weight.
Stott et al. (1982) found that the pulmonary elimination of
unchanged trichlorethylene in Osborn Mendel rats was only 2% of the
dose at 54 mg/m3 (10 ppm), but 21% at a dose of 3240 mg/m3
(600 ppm). In contrast to these findings of a saturable process in
the rat, The same authors showed that in the mouse, doses of
trichloroethylene up to 3240 mg/m3 (600 ppm) were completely
metabolized.
In dogs, exposed through inhalation, for 1 h, to
trichloroethylene at 3780, 8100, and 10 800 mg/m3 (700, 1500, and
2000 ppm), the excretion of trichloroethylene and trichloroacetic
acid was correlated with the trichloroethylene concentration. The
rate of trichloroacetic acid excretion was higher than that for
trichloroethanol. One hour after exposure ended, the percentage
of trichloro compounds in the urine was 0.7% of the total
trichloroethylene absorbed (Hobara et al., 1983).
5.4.2. Studies on man
It has been shown that man metabolizes trichloroethylene
extensively. Ikeda et al. (1972) showed that the capacity of
workers to metabolize trichloroethylene was nonlimiting, at least
up to a daily exposure level of 945 mg/m3 (175 ppm) for 8 h.
In human beings, trichloroethylene is eliminated unchanged
through the lungs and is eliminated in the urine in the form of
metabolites. Elimination by other routes (e.g., faeces, sweat, and
saliva) accounts for less than 10% of the total (Bartonicek, 1962).
After inhalation exposure, about 10% of the amount absorbed is
expired unchanged, about 30 - 50% is excreted as trichloroethanol
in urine, and about 10 - 30% as trichloroacetic acid in urine
(Soucek & Vlachova, 1960; Bartonicek, 1962; Monster et al., 1976,
1979).
The half-life of trichloroethylene in exhaled air and in the
blood depends on the length of exposure and on the time of sampling
after exposure. The concentration follows a multi-exponential
curve, compatible with at least 3 compartments: lungs, blood and
most other tissues, and adipose tissue. After a single exposure
to trichloroethylene, trichloroethanol reaches its maximum
concentration in blood and urine almost directly after exposure.
Thereafter, the concentration decreases, with a half-life of
about 10 - 15 h (Müller et al., 1974; Monster et al., 1976,
1979; Vesterberg et al., 1976). After a single exposure to
trichloroethylene, the concentration of trichloroacetic acid in
both the blood and the urine increases for up to 20 - 40 h after
exposure. Thereafter, the concentration decreases with a half-life
of about 70 - 100 h (Müller et al., 1974; Monster et al., 1979).
Trichloroacetic acid, as such, has a shorter half-life of about
50 h.
In a group of workers with long-term exposure to
trichloroethylene at a concentration of 270 mg/m3 (50 ppm), median
values of trichloroethanol and trichloroacetic acid of 330 and
319 g/kg creatinine, respectively, were found at the end of a
working shift; during the work-free periods, the metabolites of
trichloroethylene were eliminated slowly (Triebig et al., 1976).
In a study on factory workers exposed to trichloroethylene,
Ikeda & Imamura (1973) observed a half-life of 41 h for the
urinary excretion of total trichloro-compounds (i.e., a combination
of trichloroacetic acid and trichloroethanol). This half-life is
somewhat longer with oral administration of trichloroethylene and
of chloral hydrate and is about 85 - 99 h after repeated exposure
to trichloroethylene, because of the delayed formation of
trichloroacetic acid from trichloroethylene and the
trichloroethanol still available from the tissues (Müller et al.,
1974). Thus, trichloroacetic acid will be found in the urine, even
when trichloroethanol is no longer detectable (Ikeda et al., 1971).
Trichloroacetic acid accumulates in the blood and urine during
daily exposures to trichlorethylene (Monster et al., 1979; Müller
et al., 1975).
5.5. Biological Monitoring of Exposure
Droz & Fernandez (1978) used a mathematical model to study the
effects of hourly and daily variations in exposure concentrations
on alveolar air trichloroethylene concentrations and on the urinary
excretion of trichloroethanol and trichloroacetic acid. The
determination of trichloroethanol in urine appeared to be more
sensitive than the determination of trichloroethylene in exhaled
air. The excretion of trichloroacetic acid can be used for the
qualitative evaluation of the preceding day's exposure. In
practice, blood analysis would be preferable to analysis of urine,
because of the smaller individual variations generally observed
with the former. In studies concerning repeated exposure to
constant concentrations, the smallest inter-individual variation
was found in the concentrations in blood (Monster et al., 1979).
The measurement of total trichloro compounds in urine was
described by Takana & Ikeda (1968). The urinary trichloroethanol
is oxidized to trichloroacetic acid and the total amount of
trichloroacetic acid is then measured with the Fujiwara reaction.
In the case of exposure to relatively steady concentrations, this
has the advantage of being able to indicate small-scale inter-
personal variations. It may be used as an index of exposure
intensity, especially when the urine samples are collected at,
or close to, the end of the workshift at the end of the work week
(Ikeda et al., 1972). However, other studies have demonstrated
poor individual correlation between trichloroethylene exposure and
the urinary elimination of the major metabolites, trichloroacetic
acid and trichloroethanol (Boudène et al., 1983). Separate
measurement of urinary metabolites provides more information on
exposure to daily fluctuating concentrations, because relatively
high concentrations of trichloroethanol indicate recent high
exposure, whereas relatively high concentrations of trichloroacetic
acid indicate long-term exposure to high concentrations (Monster et
al., 1979).
Trichloroethylene concentrations in alveolar air and in blood,
shortly after exposure, indicate recent exposure concentrations,
while the concentration several hours after exposure indicates the
average exposure over the preceding days (Stewart et al., 1974).
6. EFFECTS ON ANIMALS AND CELL SYSTEMS
6.1. Effects on Animals
Data from acute, short-term repeated dose, and long-term
toxicity studies on laboratory animals are summarized in Table 6.
6.1.1. Acute toxicity
Acute toxicity data on common laboratory animals are shown in
Tables 7 and 8.
Acute toxicity levels following inhalation exposure to
trichloroethylene are summarized in Table 7. Table 8 includes data
on acute oral, dermal, intraperitoneal (ip), subcutaneous (sc), and
intravenous (iv) LD50s for the mouse, rat, rabbit, and dog.
Von Oettingen (1955) reported that oral acutely toxic doses in
rats produced gastrointestinal irritation. Moderate increases in
aspartate aminotranferase (EC 2.6.1.1) levels were observed in rats
24, 48, and 72 h after a single 6-h exposure to trichloroethylene
vapour at concentrations of 54 mg/m3 (10 ppm) and 540 mg/m3
(100 ppm) (Deguchi, 1972); the increase at 5400 mg/m3 (1000 ppm)
was small (Deguchi, 1972), presumably due to inactivation of P-450.
According to Rigaud et al. (1977), intraperitoneal administration
resulted in a significant increase in aspartate aminotransferase
(EC 2.6.1.1), alanine aminotransferase (EC 2.6.1.2), and ornithine
carbamoyltransferase (OCT) (EC 2.1.3.3) in the rat, whereas
Wirtschafter & Cronyn (1964), administering 500 mg/kg body weight,
detected only minor hepatic effects over the 12 - 24-h period
following administration. No evidence of kidney dysfunction was
observed in mice following intraperitoneal administration of
trichloroethylene at 0.004M/kg body weight. Application of 2 ml
trichloroethylene (7800 mg/kg), under an occlusive dressing, on the
skin of 20 guinea-pigs, did not produce any deaths but, during the
35-day observation period, there were reductions in body weight at
1 week ( P < 0.001), 2 and 3 weeks ( P < 0.01), and 4 weeks
( P < 0.05) (Wahlberg & Boman, 1979).
Skin irritation
Trichloroethylene (purity 99.5%), applied (0.5 ml) to the
shaved (non-abraded) skin of rabbits, for 24 h, under an occlusive
dressing, produced severe skin irritation (Duprat et al., 1976).
In another study, trichloroethylene (1.0 ml) was applied,
occluded in a "skin depot", to the clipped skin of guinea-pigs.
Histological examinations were performed at 15 min, 1, 4, and 16 h.
Degenerative changes (pyknotic nuclei) were observed in the
epidermis after 15 min and were progressive (pyknosis, karyolysis,
junctional separation of the epidermis) up to the end of the study
at 16 h (Kronevi et al., 1981).
Table 6. Concentrations of trichloroethylene at which no effects were observed in experimental animals
exposed through inhalation
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
rat 3000 7 h mortality Adams et al. (1951)
6400 1.4 h mortality Adams et al. (1951)
12 000 0.6 h mortality Adams et al. (1951)
20 000 0.4 h mortality Adams et al. (1951)
200 7 h per day, 5 days mortality Adams et al. (1951)
per week, for 26
weeks
400 8 h per day for mortality; body weight; Battig et al. (1963)
5 days learning capacity
730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35a 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
guinea-pig 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology (1967)
6 weeks
35 90 days mortality; body weight; Prendergast et al.
haematology; histology (1967)
histology
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
guinea-pig 100 7 h per day, 5 mortality Adams et al. (1951)
(contd.) days per week for
26 weeks
monkey 730 8 h per day, 5 mortality; body weight; Prendergast et al.
(squirrel) days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
monkey 400 7 h per day, 5 mortality Adams et al. (1951)
(Rhesus) days per week for
26 weeks
rabbit 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
35 90 days mortality; body weight; Prendergast et al.
haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
200 7 h per day, 5 mortality Adams et al. (1951)
days per week for
6 weeks
dog 730 8 h per day, 5 mortality; body weight; Prendergast et al.
days per week for haematology; histology of (1967)
6 weeks heart, liver, lung, spleen,
and kidneys
--------------------------------------------------------------------------------------------------------
Table 6. (contd.)
--------------------------------------------------------------------------------------------------------
Species Concentration Duration Biological endpoints Reference
(mg/m3) at being investigated
which no effects
were observed
--------------------------------------------------------------------------------------------------------
dog 35 90 days mortality; body weight; Prendergast et al.
(contd.) haematology; histology of (1967)
heart, liver, lung, spleen,
and kidneys
mouseb 5500 20 min anaesthesia Gehring (1968)
5500 100 min liver injury (elevated SGPT) Gehring (1968)
5500 300 min mortality Gehring (1968)
---------------------------------------------------------------------------------------------------------
a Kalashmikova et al. (1976) reported that rats exposed to 50 mg/m3 for 5 h per day, for 90 days, showed
damage to parenchyma in liver and kidney.
b Kjellstrand et al. (1983) reported that male NMRI mice
continuously exposed to 200 mg/m3 (37 ppm) for
30 days showed increased plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity; female mice did
not show any increase in BuChE activity; there was a significant increase in liver weight at 200 mg/m3
(37 ppm) in both sexes.
Table 7. Acute toxicity of trichloroethylene administered via
inhalation to laboratory animals
--------------------------------------------------------------------
Species Toxicity Exposure Reference
index ------------------------
level duration
(ppm) (mg/litre) (h)
--------------------------------------------------------------------
Rat LC100 20 000 107 0.4 Adams et al. (1951)
LC100 12 000 64.2 1.4 Adams et al. (1951)
LC100 2500 13.4 7.0 Adams et al. (1951)
LC50 26 300 140.7 1 Vernot et al. (1977)
LC50 12 500 66.9 4 Siegel et al. (1971)
LCL0a 8000 42.8 4 Smyth et al. (1969)
Mouse LC100 8000 42.7 2 Von Oettingen (1955)
LC100 5600 30.0 9.75 Gehring (1968)
LC50 8450 45.1 4 Kylin et al. (1962)
LC50 41 122 220 0.33 Aviado et al. (1976)
LC50 49 000 262 0.50 Vernot et al. (1977)
LCL0a 3000 16.0 2 Lazarev (1929)
LC50 40 4 Lazarev & Gadaskina
(1977)
Guinea- LC100 37 000 197.8 0.67 Von Oettingen (1955)
pig
Cat LCL0a 6074 32.5 2 Lehmann & Schmidt-Kehl
(1936)
Rabbit LC 5000 26.75 14.28 McCord (1932)
LC 10 000 53.5 2.5 McCord (1932)
LC 20 000 107 2 McCord (1932)
--------------------------------------------------------------------
a LCLO = lowest published lethal concentration.
Eye irritation
Instillation of 0.1 ml of trichloroethylene (purity 99.5%)
into rabbit eyes produced mild to moderate conjunctivitis with
superficial epithelial abrasion. At 7 days, there was a resolving
keratitis with complete recovery within 2 weeks (Duprat et al.,
1976).
6.1.2. Short-term exposures
6.1.2.1. Oral exposures
In a US NTP study (1983), groups of 10 male and 10 female
F344/N rats were administered trichloroethylene (in corn oil, by
gavage) at doses ranging from 125 to 2000 mg/kg body weight (males)
and 625 to 1000 mg/kg (females), 5 times per week, for 13 weeks.
All rats survived the 13-week study, but males receiving the
2000 mg/kg dose exhibited a 24% decrease in body-weight gain. At
the 1000 mg/kg dose, final body weights for males and for females
were similar to those of the controls.
Table 8. Acute oral, dermal, intraperitoneal, subcutaneous, and
intravenous LD50s for trichloroethylene in laboratory animals
--------------------------------------------------------------------------
Species Oral Dermal Intraperitoneal Subcutaneous Intravenous
(mg/kg body (ml/kg (mg/kg body (mg/kg body (mg/kg body
weight) body weight) weight) weight)
weight)
--------------------------------------------------------------------------
Rat 49201 27252
Dog 56803 2.8004 1505,a
Mouse 28506,b 32107,b 144010 3411
240012 31508,b
30009,c
1.2006,b
Rabbit 201
--------------------------------------------------------------------------
1 From: Smyth et al. (1969). a LDL0.
2 From: Rigaud et al. (1977). b In 24 h.
3 From: Christensen et al. (1974). c In 14 day.
4 From: Klaassen & Plaa (1967).
5 From: Barsoum & Saad (1934).
6 From: Aviado et al. (1976).
7 From: Klaassen & Plaa (1966).
8 From: Gehring (1968).
9 From: Gradiski et al. (1974).
10 From: Plaa et al. (1958).
11 From: NIOSH (1977b).
12 From: Tucker et al. (1982).
Histopathological examination of tissues from animals receiving
the highest doses showed minimal or mild cytomegaly and karyomegaly
of the renal tubular epithelial cells in the inner cortex in 8/9
males dosed with 2000 mg/kg per day, and the same effect, graded as
equivocal or mild, was seen in 5/10 females that had received the
1000 mg/kg per day dose.
The results of this 13-week study in F344/N rats were
essentially similar to those of an earlier 8-week study conducted
on Osborne-Mendel rats (NCI, 1976). In this study, only doses in
excess of 5000 mg/kg per day were lethal for rats. Doses of
1000 mg/kg per day had no effect on body-weight gains in males,
but depressed weight gains in females by approximately 15%.
Groups of 10 male and 10 female B6C3F1 mice received
trichloroethylene (by gavage in corn oil) at doses ranging from
375 to 6000 mg/kg body weight, 5 times per week for 13 weeks. All
males and 9/10 females receiving 6000 mg/kg, 7/10 males and 1/10
females receiving 3000 mg/kg, and 2/10 males and 1/10 females
receiving 1500 mg/kg died. Mean body weights of male mice dosed
with 750, 1500, or 3000 mg/kg were depressed by 11%, 19%, and 17%,
respectively, relative to the controls. Mean body weights of
control and treated groups of female mice were similar (US NTP,
1983).
Liver weights (both absolute and as percentage body weight)
increased in a dose-related fashion. Liver weights were increased
by more than 10% relative to the controls for males receiving
750 mg/kg body weight or more and for females receiving 1500 mg/kg
or more.
Histopathological examination showed hepatic centrilobular
necrosis (6/10 males and 1/10 females administered 6000 mg/kg).
This lesion was not seen in either males or females administered
3000 mg/kg, but 2/10 males had multifocal areas of calcification
scattered throughout their livers. Multi-focal calcification was
also seen in the liver of the single female mouse that survived the
6000 mg/kg dosage regimen. One female in the 3000 mg/kg dose group
developed a hepatocellular adenoma, an extremely rare lesion in
female mice of this age (20 weeks).
Examination of renal tissues showed the presence of mild to
moderate cytomegaly and karyomegaly of the renal tubular epithelial
cells of the inner cortex. These changes were found in only 1 of
the 23 mice (13 males and 10 females) that died after receiving
doses of 3000 or 6000 mg/kg for up to 6 weeks. However, the
changes were found in all 4 of the males that died after receiving
the 3000 mg/kg dose for 7 - 13 weeks, and in all animals that
survived the 6000 mg/kg (1/10 females) and the 3000 mg/kg doses
(3/10 males and 9/10 females). Tissues from mice receiving lower
doses of trichloroethylene were not examined.
Trichloroethylene administered orally to mice at doses in
excess of 500 mg/kg for 10 days produced proliferation of hepatic
peroxisome as demonstrated by increased cyanide-insensitive
palmitoyl CoA oxidation (PCO) and electron microscopy. In the
rat, trichloroethylene did not have any effect on peroxisome
proliferation. Trichloroacetic acid administered for 10 days
increased hepatic peroxisome proliferation in both species (Elcombe
et al., 1982). It is possible that the rapid rate of
trichloroethylene metabolism in the mouse, together with the
enterohepatic circulation of trichloroacetic acid, leads to
high steady-state blood levels of trichloracetic acid and the
concomitant proliferation of peroxisomes.
Primary cultures of isolated mouse and rat hepatocytes have
been found to metabolize trichloroethylene to trichloroacetic acid
at rates comparable to those in intact animals. Similarly, the
isolated hepatocytes respond to exposure to trichloroacetic acid
by peroxisome proliferation. Isolated human hepatocytes have been
found to produce trichloracetic acid from trichloroethylene at a
lower rate than that in the rat. Trichloroacetic acid does not
induce peroxisome proliferation in human hepatocytes (Elcombe,
1985).
6.1.2.2. Inhalation exposure
In the rat, exposure to 81 000 mg/m3 (15 000 ppm) for a period
of 2 - 4 min produced complete anaesthesia within 9 min (Schumacher
& Grandjean, 1960). In a study on mice, exposure to 36 7000 mg/m3
(6800 ppm) for 10 - 11 min or 64 800 mg/m3 (12 000 ppm) for 5 -
6 min produced complete anaesthesia (Friberg et al., 1953). In
another study on mice, exposure to 29 700 mg/m3 (5500 ppm) produced
anaesthesia in 46 min (Gehring, 1968).
Studies conducted on rats by Vissarionova et al. (1975) showed
that concentrations of 5000 mg/m3 administered for 5 h a day, for
1 week, resulted in an increase in liver and kidney weight (21.6%
and 18%, respectively), and a decrease in alkaline phosphatase and
RNA-dependent hepatic dehydrogenase. Histological changes have also
been noted in hepatic and renal parenchyma by Kalashnikova et al.
(1976).
With doses of 1080 mg/m3 (200 ppm) administered for 6 h daily
for 4 days, Savolainen et al. (1977) found that rats exhibited
greater motor activity and less cerebral RNA, 5 days after the last
exposure, and an accumulation of trichloroethylene in perirenal
fat.
Doses of 250, 500, 800, and 1200 mg/m3 administered for 15 -
90 h resulted in increases in intracellular lipids (Verne et al.,
1959).
No effects were noticed in rats administered 1080 mg/m3 (200
ppm) for 4 h a day for 4 days (Grandjean, 1960).
Continuous exposure of rats, mice, and gerbils by inhalation to
trichloroethylene at 810 mg/m3 (150 ppm), for periods ranging from
2 to 30 days, produced liver enlargement in all species; the mouse
was the most severely affected. After the end of exposure, the
liver weights of the mice decreased rapidly. An increased kidney
weight was noted in gerbils (Kjellstrand et al., 1981a).
In another study in which 7 different strains of mice (wild,
C57BL, DBA, B6CBA, A/su, NZB, and NMRI) were continuously exposed
to a trichloroethylene concentration of 810 mg/m3 (150 ppm),
Kjellstrand et al. (1983a) reported large increases in liver weight
in all strains with minimal changes in kidney and spleen weights.
Plasma butyrylcholinesterase (BuChE) (EC 3.1.1.8) activity
increased in males of all strains and in females of strains A/su
and NZB, but to a lesser extent than in the corresponding males.
In a further study, with continuous exposure to concentrations of
200 - 1620 mg/m3 (37 - 300 ppm), plasma BuChE increased in male
mice in a time- and concentration-dependent manner. Liver weight
increased in a time- and concentration-dependent manner in both
sexes.
Exposure of rats, guinea-pigs, rabbits, dogs, and monkeys to
3825 mg/m3, for 8 h per day, 5 days per week, for 6 weeks, resulted
in a loss of overall body weight in dogs and monkeys. There were
no changes in haematological or liver enzyme parameters
(Prendergast et al., 1967).
Groups of male Swiss Webster mice were exposed to 54 000 mg/m3
(10 000 ppm) for 1 or 4 h, daily, for 5 consecutive days. In the
4-h group, the NADPH cytochrome c reductase (EC 1.6.99.3) activity
in the lung decreased, but that in the liver increased. In the
lungs of this group, there were platelet thrombi and vacuolization
of bronchial epithelial cells. There were no changes in the liver.
It was concluded that the reduced activity of the pulmonary mixed-
function oxidase system reflected injury to the lungs (Lewis et
al., 1984).
Rabbits exposed to 15 000 mg/m3 (2790 ppm) for 4 h per day,
6 days a week for 45 days, developed severe normocytic anaemia,
leukopenia, and thrombocytopenia due to toxic effects on the bone
marrow (Mazza & Brancaccio, 1967).
In rats, urinary levels of trichloro-substituted metabolites
and the activity of drug-metabolizing enzymes (cytochrome P-450)
were related to the duration of trichloroethylene anaesthesia
(Moslen et al., 1977). Trichloroethylene hepatotoxicity in the rat
produces increased levels of serum transaminases. Hetaptotoxicity
increases the activity of drug-metabolizing enzymes (cytochrome
P-450) (correlation coefficient: 0.95) and the urinary excretion of
trichloro-metabolites (correlation coefficient: 0.88) (Moslen et
al., 1977).
Cytotoxic effects have been observed in the kidney and liver of
dogs subjected to 81 000 mg/m3 (1.5%; 15 000 ppm) trichloroethylene
anaesthesia (Kiseleva & Korolenko, 1971).
Studies on rats exposed by inhalation to trichloroethylene
concentrations of 2160, 4320, or 8640 mg/m3 (400, 800, or 1600 ppm)
for 6 h revealed no effects at the first concentration, a decrease
in swimming activity at 4320 mg/m3, and a further decrease at
8640 mg/m3 (Grandjean, 1963). Other rats exposed to 1944 -
2268 mg/m3 (360 - 420 ppm) for 8 h a day, 5 days a week for
46 weeks, exhibited no effects (Bättig & Grandjean, 1963). After
43 weeks at 2160 mg/m3 (400 ppm), rats in another set of tests by
Bättig (1964) exhibited greater maze skills. Mice exposed
intermittently to trichloroethylene showed a decrease in motor
activity at 4500 mg/m3 (900 ppm), but, at 19 440 mg/m3, it was
considerably increased (Kjellstrand et al., 1983b).
In Mongolian gerbils, continuously exposed to trichloroethylene
at 1.72 mg/m3 (320 ppm) for 9 months, there was no effect on
spatial memory, but subsequent maze performance test results were
interpreted as indicating an irreversible effect on the central
nervous system (Kjellstrand et al., 1980). In another study, 2
groups of Mongolian gerbils were continuously exposed to 810 mg
trichloroethylene/m3 (150 ppm) for 71 and 106 days, respectively.
In a series of maze tests following the end of exposure, the
treated groups performed less well than the unexposed controls
(Kjellstrand et al., 1981b).
6.1.2.3. Parenteral exposure
When male Swiss Webster mice were injected ip with 3 doses of
330 mg trichloroethylene/kg body weight (vehicle 0.2 ml of 25%
Tween 80 in saline) on alternate days, the activity of hepatic
microsomal NADPH cytochrome c reductase was increased. There
were no morphological changes in the liver (Lewis et al., 1984).
Studies conducted on rabbits showed that intramuscular
administration of 3 ml trichloroethylene, 3 times weekly for
29 days, induced neuronal damage (Bartonécek & Brun, 1970).
6.1.3. Long-term exposure
6.1.3.1. Oral exposure
The US NTP (1983) studied the effects of orally-administered
(in corn oil, by gavage) epichlorohydrin-free trichloroethylene in
male and female F344/N rats and B6C3F1 mice. Doses (500 and
1000 mg/kg body weight for rats and 1000 mg/kg for mice) were
administered 5 days per week for 103 weeks. The survival of
treated male rats and male mice was significantly reduced in
relation to that of corn-oil control animals. Mean body weights
of treated rats (both sexes) were lower than those of corn-oil
controls and the reduction in body weight gain was dose-related.
The body weights of treated female mice were similar to those of
vehicle controls.
Toxic nephrosis was found in 96/98 (98%) of the treated male
rats, in 97/97 of the treated female rats, in 45/50 (90%) of the
treated male mice, and in 48/49 (98%) of the treated female mice,
but was not found in any of the corn-oil control rats or mice.
Initially noted in rats that died early, the lesions were diagnosed
as frank enlargement of the nucleus and cytoplasm of scattered
individual tubular cells with brush borders, located near the
cortico-medullary junction. Progression of the lesions was
evident. As exposure time increased, affected tubular cells
were larger and additional tubules and tubular cells were affected.
Some tubules were enlarged or dilated to the extent that they were
difficult to identify as tubules. Eventually, there was loss of
some enlarged cells. Corresponding tubules became dilated and
portions of the basement membrane had a stripped appearance. In
the most advanced stage, the lesion had progressed to the sub-
capsular cortex, with enlarged tubular cells.
In mice, the pathological development of the renal lesion was
basically similar to that observed in rats, but it was relatively
less severe and did not develop to a stage where there was
extensive loss of cytomegalic epithelial cells and tubular
dilation.
When trichloroethylene was given in the drinking-water (0.1,
1.0, 2.5, and 5.0 g/litre) to CD-1 mice for 4 - 6 months, a
significant reduction in body weight (males and females at
5.0 g/litre), enlarged liver (males at 1.0, 2.5, and 5.0 g/litre;
females at 5.0 g/litre), and increase in kidney weight (males and
females at 5.0 g/litre) were observed. However, pathology at 4 and
6 months was unremarkable.
6.1.3.2. Inhalation exposure
Studies on rats showed that exposure to concentrations below
1080 mg/m3 (200 ppm) for 6 h a day, 5 days a week for 6 months, did
not induce any visible effects. At concentrations of 10 800 mg/m3
(2000 ppm), narcosis and loss of appetite were observed. At
16 200 mg/m3 (3000 ppm), only 33% of the test animals survived
(Taylor, 1936).
When NMRI mice were exposed to trichloroethylene at 700 mg/m3
(130 ppm) continuously for 30 days, liver weight increased by 1.5
and 1.9 times in males and females, respectively (Kanje et al.,
1981).
When rats were exposed to trichloroethylene at 1945 -
2270 mg/m3 (360 - 420 ppm), for 8 h a day, 5 days a week for
46 weeks, there were no changes in conditioned reflexes or reaction
time, but there was an increase in spontaneous climbing activity
(Bättig & Grandjean, 1963).
Rats exposed to 50 mg/m3 (0.05 mg/litre) for 5 h daily, for
3 months, showed damage to hepatic and renal parenchyma
(Kalashnikova et al., 1976).
Long-term exposure to concentrations of 100 - 200 mg/m3
(0.1 - 0.2 mg/litre) reduced the phagocytic activity of rat
leukocytes (Pelts, 1962).
In female Mongolian gerbils, exposed continuously to 270 and
810 mg/m3 (50 and 150 ppm) for 12 months, there were small changes
in the lipid composition of the cerebral cortex and hippocampus.
While protein content and lipid class distribution were virtually
unaffected, the cholesterol to phospholipid ratio in the cortex
decreased (Kyrklund et al., 1983).
6.1.3.3. Parenteral exposure
Of 6 rabbits given trichloroethylene at 200 mg/kg body weight
intramuscularly for 55 - 100 days, 2 died from renal failure
(Bartonécek & Brun, 1970). Rabbits given 2 ml (2.92 g)
intramuscularly twice a week for 41 - 247 days exhibited
neuronal damage (Bartonécek & Sovcek, 1959).
6.1.4. Interactions
Studies on rats showed that trichloroethylene was more toxic in
animals on a high-carbohydrate diet than in those on a high-protein
diet (Kalashnikova et al., 1974). In rats (Cornish & Adefuin,
1966), rabbits (Desoille et al., 1962), and human beings (Sbertoli
& Brambilla, 1962; Ferguson & Vernon, 1970; Pardys & Brotman,
1974), the presence of ethanol increased trichloroethylene
toxicity. Trichloroethylene and carbon tetrachloride acted
synergistically in producing hepatotoxicity (Deguchi, 1972;
Pessayre et al., 1982).
When trichloroethylene was administered intraperitoneally with
toluene, there was a decrease in the side-chain oxidation of the
toluene (Ikeda, 1974).
In studies by White & Carlson (1979), trichloroethylene
caused spontaneous, and potentiated epinephrine-induced, cardiac
arrhythmias in rabbits. A series of studies was conducted to
examine the effects of metabolic-inhibitors, enzyme-inducing
agents, or pre-treatment with ethanol or caffeine, on the
sensitivity of rabbits to epinephrine-induced cardiac arrythmias
(White & Carlson, 1979, 1981a, 1982). Rabbits treated with
metabolic-inhibiting agents developed more arrhythmias after
shorter exposure times in response to lower doses of epinephrine
than controls. Phenobarbitone and Aroclor 1254(R), and in a later
study benzo( a)pyrene (Carlson & Shite, 1983), were used as enzyme-
inducing agents. Phenobarbitone-treated rabbits developed
fewer cardiac arrhythmias and had lower blood levels of
trichloroethylene. Rabbits treated with benzo( a)pyrene developed
more cardiac arrhythmias and at lower doses of epinephrine when
exposed by inhalation to a trichloroethylene concentration of
43 500 mg/m3 (8100 ppm). Aroclor 1254(R) did not induce any
effects on trichloroethylene metabolism nor on the development of
epinephrine-induced cardiac arrhythmias. Rabbits pre-treated with
ethanol (1000 mg body weight, intravenously or orally) or caffeine
(10 mg/kg body weight, intraperitoneally) 30 min before exposure to
trichloroethylene at 32 400 mg/m3 (6000 ppm) by inhalation showed
an increased senstivity to epinephrine-induced cardiac arrhythmias.
Male New Zealand rabbits exposed to trichloroethylene at
10 800, 21 600, 32 400, or 43 200 mg/m3 (2000, 4000, 6000, or
8000 ppm) by inhalation for 1 h showed a concentration-related
sensitivity to epinephrine-induced cardiac arrhythmias. The blood
levels of the trichloroethylene metabolites (trichloroethanol and
trichloroacetic acid) were measured. Rabbits given chloral
hydrate (50 mg/kg body weight, intravenously), a trichloroethylene
intermediate metabolite, had blood levels of trichloroethanol and
trichloroacetic acid that were 40 - 100 times higher than those of
rabbits exposed to a trichloroethylene level of 43 200 mg/m3 (8000
ppm) but showed no increase in sensitivity to epinephrine-induced
cardiac arrhythmias (White & Carlson, 1981b). It is concluded that
trichloroethylene, rather than its metabolites, sensitizes the
rabbit myocardium.
6.1.5. Immunotoxicity
Changes in some components of antibody production (7S), cell-
mediated immunity (haemagglutination), and bone marrow stem cell
colonization have been observed in rodents (female mice were more
sensitive than males in one study; chinchillas were used in the
other) exposed to low-to-moderate levels (10 - 1000 mg/m3) by
inhalation or to 0.1 - 5 g/litre per day in the drinking-water for
periods ranging from a few weeks to 6 - 10 months (Shmuter, 1972;
Sanders et al., 1982).
6.1.6. Effects on cell systems
In vitro incubation of nerve-fibre membranes from rat spinal
cord with 10 µM trichloroethylene reduced the low relative
molecular mass protein fraction (Savolainen & Seppalainen, 1979).
A trichloroethylene concentration of 1.3/108 moles per ml was
the LD50 for HeLa (human cervix carcinoma) cells on the third day
of treatment (Gradiski et al., 1974).
Exposure of rabbit trachea cultures to trichloroethylene vapour
at 27 000 mg/m3 (5000 ppm) for 129 min and 216 000 (40 000 ppm) for
13 min, induced ciliostasis (Tomenius et al., 1979). The isolated
guinea-pig heart exposed to trichloroethylene at 530 mg/litre,
developed transitory arrhythmia. At a concentration of 1.06%,
there was evidence of a reduction in contractile force and coronary
flow, and arrhythmia (Bianchi et al., 1963; Matturro et al., 1963).
6.1.7. Carcinogenicity
Groups of 50 male and 50 female Osborne-Mendel rats, 7 weeks
old, were administered trichloroethylene (99% trichloroethylene
stabilized with 0.19% 1,2-epoxybutane and 0.09% epichlorohydrin) in
corn oil, by gavage, 5 days a week for 78 weeks. High-dose animals
received varying dose schedules of 1000 - 1500 mg/kg body weight
per day, and low-dose animals received 500 - 750 mg/kg body weight
per day. All surviving animals were killed 110 weeks after the
start of treatment. The time-weighted average doses were 549 and
1097 mg/kg body weight per day. A group of 20 male and 20 female
vehicle-treated rats served as controls. Of the males, 17/20
controls, 42/50 low-dose, and 47/50 high-dose animals died before
the end of the study; of the females, 12/20 controls, 35/48 low-
dose, and 37/50 high-dose animals died early. Median survival
times were approximately 60 weeks for high-dose males, 85 weeks
for low-dose males, and 70 weeks for high- and low-dose females.
Of the males, 5/20 controls, 7/50 low-dose, and 5/50 high-dose rats
developed tumours; of the females, 7/20 controls, 12/48 low-dose,
and 12/50 high-dose rats developed tumours. There were no liver-
cell tumours. Tumours occurred in various other organs in treated
and control animals and were mainly reticulum-cell sarcomas,
lymphosarcomas or malignant lymphomas, fibroadenomas of the
mammary gland, haemangiosarcomas at various sites, follicular
adenocarcinomas of the thyroid, chromophobe adenomas of the
pituitary, and renal hamartomas. Toxic nephropathy was observed
in rats of both sexes treated with high and low doses of
trichloroethylene (NCI, 1976).
Groups of 50 male and 50 female B6C3F1 mice, 5 weeks old,
were given trichloroethylene (99% trichloroethylene stabilized
with 0.19% 1,2-epoxybutane and 0.09% epichlorhydrin) in corn oil
by gavage, 5 days a week, for 78 weeks. High-dose males received
2000 - 2400 mg/kg body weight per day, and females 1400 - 1800
mg/kg body weight per day; low-dose males and females received
1000 - 1200 mg/kg body weight per day and 700 - 900 mg/kg body
weight per day, respectively. All surviving animals were observed
until they reached 95 weeks of age. Time-weighted average doses
were 1169 and 869 mg/kg body weight per day, respectively, in low-
dose males and females and 2339 and 1739 mg/kg body weight per day,
respectively, in high-dose males and females. Groups of 20 male
and 20 female mice served as vehicle-treated matched controls.
Survival was reduced in high-dose males and control males.
Hepatocellular carcinomas occurred in 1/20 control males and 0/20
control females; in 26/50 low-dose males and 4/50 low-dose females,
and in 31/48 high-dose males and 11/47 high-dose females.
Metastases of the liver-cell tumours to the lung were found in
7/98 treated males and in 1 control male. The first hepatocellular
carcinoma was observed in a mouse which had been treated with the
high dose of trichloroethylene and which died during week 27.
Lung tumours occurred in treated animals of both sexes: 5/50 (5
adenomas) in males and 4/50 (2 adenomas, 2 carcinomas) in females
in the low-dose group, and 2/48 (1 adenoma, 1 carcinoma) in males
and 7/47 (5 adenomas, 2 carcinomas) in females treated with the
high dose of trichloroethylene. Among controls, only one lung
adenoma was reported in a female (NCI, 1976).
In a study by Maltoni & Maioli (1977) and Maltoni et al. (in
press), groups of 30 male and 30 female, 13-week-old Sprague Dawley
rats were given trichloroethylene (highly purified and epoxide-
free, and stabilized with 20 ppm of butyl-hydroxytoluene) in olive
oil by gavage, 5 days a week, for 52 weeks. Two doses of
trichloroethylene were tested. The high-dose animals received
250 mg/kg body weight, and the low-dose animals 50 mg/kg body
weight; 30 males and 30 females received olive oil alone and served
as controls. The animals were kept until their spontaneous death
(the study lasted 140 weeks). There was no increase in specific
tumours. Abnormalities (cytokaryomegaly) in cells of the renal
tubules were observed in high-dose male rats. These changes were
not observed in females.
Groups of 30 female ICR Swiss mice and a group of 30 male
and 30 female mice of the same strain, were treated with
trichloroethylene dermally (1 mg in acetone), 3 times weekly, for
83 weeks, subcutaneously (0.5 mg in trioctanoin) once weekly for
89 weeks, and by gavage (0.5 mg in trioctanoin), once weekly, for
89 weeks. Similar-sized groups of animals served as controls. No
tumours were observed at the site of application (Van Duuren et
al., 1979). Similar negative findings were obtained following
thrice-weekly skin applications and once-weekly subcutaneous
injections of trichloroethylene oxide for life in a group of
70 female mice of the same strain (Van Duuren et al., 1983)
Groups of 30 male and 30 female Wistar rats, NMRI mice, and
Syrian hamsters were exposed by inhalation to trichloroethylene
(purified, with no detectable epoxides, and containing 15 ppm
triethanolamine as stabilizer) at 540 and 2700 mg/m3 (100 and
500 ppm). The animals were exposed for 6 h per day, 5 days per
week, for 78 weeks. The studies were terminated by killing the
surviving mice and hamsters after 130 weeks, and rats after 156
weeks. No carcinogenic effects were observed in rats, hamsters,
and male mice. A moderate increase in lymphomas was found in
treated female mice (18/28 at 2700 mg/m3; 17/30 at 540 mg/m3, and
9/29 in controls). The authors concluded that, on the basis of
these findings, there were no indications of carcinogenicity
(Henschler et al., 1980).
Groups of 49 - 50 female ICR mice, 4 weeks of age, were exposed
by inhalation to trichloroethylene (purity 99.8% with 0.128% carbon
tetrachloride, 0.019% benzene, 0.019% epichlorohydrin, and 0.01%
1,1,2-trichloroethane) at 0.270, 810, or 2430 mg/m3 (0, 50, 150,
or 450 ppm), 7 h per day for 5 days per week, for 104 weeks. The
surviving animals were killed 107 weeks after the start of the
study. Mortality was similar in control and treated groups.
Lung adenomas were found in 5, 2, 5, and 4 mice in the control,
low-dose, mid-dose, and high-dose groups, respectively. However,
it was reported that adenocarcinomas (none of which gave rise to
metastases) occurred in 1/49, 3/50, 8/50, and 7/46 mice in the
control, low-dose, mid-dose, and high-dose groups, respectively,
and that increased incidences in the mid- and high-dose groups,
were statistically significant compared with controls. In similar
studies on Sprague Dawley rats, no statistically-increased
incidence of tumours was detected; one clear-cell carcinoma of the
kidney was observed in the high-dose group (Fukuda et al., 1983).
In a large series of inhalation studies, highly purified
epoxy-free trichloroethylene (stabilized with 20 ppm butyl-
hydroxytoluene) was studied under controlled exposure conditions
in male and female Sprague Dawley rats and Swiss and B6C3F1 mice.
Treatment was for 7 h per day, 5 days per week for 8, 78, or 104
weeks. After the end of the treatment, all animals were kept under
observation until spontaneous death. The following 7 studies were
performed (Maltoni & Maioli, 1977; Maltoni et al., in press):
Study 1
Groups of 60 - 90 male and 60 - 90 female Sprague Dawley rats
were exposed to 0, 540, or 3240 mg trichloroethylene/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed. Cytomegaly and karyomegaly of tubular cells in the
kidney were not observed at either dose.
Study 2
Groups of 60 - 100 male and 60 - 100 female Swiss mice were
exposed to trichloroethylene at 0, 540, or 3240 mg/m3 (0, 100, or
600 ppm) for 8 weeks. No increase in tumours related to treatment
was observed.
Studies 3 and 4
Groups of 90 - 95 male and 90 - 105 female Sprague Dawley rats
were exposed to trichloroethylene at 0, 540, 1620, 3240 mg/m3 (0,
100, 300, or 600 ppm) for 104 weeks. Further groups of 40 males
and 40 females were started on the same treatment 4 - 5 weeks later
(Study 4). The method and results of the 2 studies were similar
and the combined data are described. No increase in mortality was
observed in the treated animals. Cytomegaly and karyomegaly of
renal tubular cells were observed in males in the mid- and high-
dose groups and the incidence was dose-related; no such lesions
were observed in males in the low-dose group or in females at
any of the 3 dose levels, or in the controls. Five tubular
adenocarcinomas were observed in 4/130 males and 1/130 females
at the high-dose level. Leydig cell tumours of the testis were
observed in 6/135 controls, and in 16/130, 30/130, and 31/130
male rats, respectively, in the low-, mid-, and high-dose treated
groups. The average time of appearance of the tumours, from the
start of the study, was 113 weeks in controls and from 109 to
113 weeks in treated animals. A higher incidence of immunoblastic
lymphosarcomas was detected in male and female treated animals:
1/280 in controls, 9/260 at the low dose, 5/260 in the mid-dose
group, and 3/260 in the high-dose group.
Study 5
Groups of 90 male and 90 female Swiss mice were exposed to
trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0, 100, 300,
or 600 ppm) for 78 weeks. The incidence of hepatocellular
carcinomas in males was 4/90 in controls compared with 2/90 at the
low dose, 8/90 at the mid dose, and 13/90 at the high dose. One
hepatocellular carcinoma occurred in a female at the high dose.
The incidences of lung adenomas and adenocarcinomas were 25/180
in controls, 26/180 in low-dose groups, 36/180 in mid-dose, and
47/180 in the high-dose groups (males and females combined). Two
adenocarcinomas were seen in the control groups and 3 in the high-
dose group.
Studies 6 and 7
For study 6, groups of 90 male and 90 female B6C3F1 mice were
exposed to trichloroethylene at 0, 540, 1620, or 3240 mg/m3 (0,
100, 300, or 600 ppm) for 78 weeks. Because of excess mortality
due to fighting in the male groups, study 7 was started in which
trichloroethylene was tested in the same way in equal numbers of
male mice. In females, the incidence of pulmonary tumours (all
adenomas except one adenocarcinoma in the low-dose group) were 4/90
in controls compared with 6/90, 10/90, and 15/90 in the low-, mid-,
and high-dose groups, respectively. Hepatocellular carcinomas were
observed in 3/90 controls compared with 4/90, 4/90, and 9/90 in the
low-, mid-, and high-dose groups, respectively. In the males in
study 7, the incidence of pulmonary tumours was not affected by the
treatment. Hepatocellular carcinomas were observed in 14/90
controls compared with 19/90, 27/90, and 21/90 in the 3 treated
groups, respectively. Cytomegaly and karyomegaly were not observed
in either study (Maltoni et al., in press).
Groups of 50 male and 50 female rats (F344) and mice
(B6C3F1) were treated, by corn-oil gavage, with epichlorohydrin-
free trichloroethylene at 500 and 1000 mg/kg body weight (rats),
and 1000 mg/kg body weight (mice), 5 times weekly, for 103 weeks.
Similar-sized groups of male and female rats and mice treated with
corn oil alone, or without any treatment, served as controls. The
animals were killed between 103 and 107 weeks from the start of the
study. Trichloroethylene significantly reduced the survival of
male rats and mice. In male rats treated with trichloroethylene,
the incidence of tubular-cell neoplasms was increased (0/49,
untreated; 0/48, corn-oil control; 2/49, low dose; 3/49, high
dose). There was one tubular-cell tumour in a high-dose female
rat. Five low-dose male rats also had malignant peritoneal
mesotheliomas, compared with 1/50 untreated control, 1/50 vehicle
control, and 0/49 high-dose animals. Trichloroethylene also
produced nephrosis in both sexes of both species. In mice, the
administration of trichloroethylene increased the incidence of
hepatocellular carcinoma (males: 8/48, controls; 30/50, treated;
females: 2/48, controls; 13/49, treated). The incidence of
hepatocellular adenomas was also increased in male mice (3/48,
controls; 8/50, dosed) and in female mice (2/48, controls; 8/49,
dosed) (US NTP, 1983).
Groups of 50 male and 50 female Swiss mice were treated
by corn-oil gavage with daily doses of different samples of
trichloroethylene, initially at 2400 mg/kg body weight (males)
and 1800 mg/kg body weight (females), 5 days a week, for 78 weeks.
Due to toxicity, dosing and dose levels were reduced during the
study. The animals were kept under observation until
spontaneous death. The samples included: (a) highly purified
trichloroethylene stabilized with 0.0015% triethanolamine, (b)
industrial trichloroethylene (99.4% pure), (c) highly purified
trichloroethylene stabilized with 0.8% epichlorohydrin, (d) highly
purified trichloroethylene, with 0.8% 1,2-epoxybutane, and (e)
highly purified trichloroethylene with 0.25% epichlorohydrin and
0.25% epoxybutane. Similar-sized groups of each sex, treated with
corn oil alone, served as controls. Mortality was increased in
treated males and some treated female groups. No increase in
tumour incidence was observed except in groups (c) and (d) where
there was an increased incidence of forestomach cancers, attributed
to the direct alkylating properties of one of the two stabilizers
(Henschler et al., 1984).
6.1.7.1. Conclusions
Trichloroethylene (with or without epoxide stabilizers) caused
an increased incidence of hepatocellular carcinomas in 2 different
strains of mice, either when given by oral administration or by
inhalation.
Trichloroethylene also produced lung tumours in Swiss ICR mice
and in female, but not male, B6C3F1 mice.
Trichloroethylene, with added epichlorohydrin, administered by
gavage, caused an increased incidence of forestomach cancers in
WMRI mice.
Trichloroethylene (epoxide stabilizer-free) produced a low
incidence of renal tubular tumours in 2 different strains of mice
(mainly in males) following long-term oral or inhalation exposure.
These tumours occur very rarely in untreated rats of these strains.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure.
In one strain of mice and one strain of rats, epoxide
stabilizer-free trichloroethylene produced some increases in the
incidence of lymphomas. However, these data are insufficient to
draw any conclusions.
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats.
It was noted in the evaluations by IARC (1979, 1982) that
it was considered that there was limited evidence for the
carcinogenicity of trichloroethylene for experimental animals.
The significance of these findings needs to be evaluated in
the context of further studies on the mechanism of action of
trichloroethylene.
6.1.8. Mutagenicity
6.1.8.1. Gene mutation
(a) Bacteria and fungi
Pure trichloroethylene induced revertants (gene-mutations) in
the K-12 strain of Escherichia coli in the presence of fortified
mouse Arochlor(R)-induced microsomal preparations for only one gene
out of four analysed. In the positive case, the induced effect was
the doubling of spontaneous background (Greim et al., 1975).
Trichloroethylene (of unspecified purity) in vapour form was
slightly mutagenic for the TA-100 strain of Salmonella typhimurium,
in the presence of S9 mix obtained from mouse liver (control
value = 140 revert./plate; treated values: 240 revert./plate)
(Simmon et al., 1977).
Technical grade trichloroethylene (containing 1900 ppm of
1,2-epoxybutane and 900 ppm of epichlorohydrin) was mutagenic for
the TA-100 strain of S. typhimurium in a desiccator, in the absence
of a metabolic activation system (untreated 161 - 187 revert./plate;
treated: 567 - 651 revert./plate). Trichloroethylene of high
purity was not mutagenic for the TA 100 strain of S. typhimurium
in the presence of 0.5 ml of S9 mix obtained from mouse, rat, and
hamster liver; it was, however, mutagenic for this strain of
S. typhimurium in the presence of 50 - 150 µl of S9 mix from
Arochlor-treated rat liver (untreated series: 160 - 180
revert./plate; treated series: 350-379 revert./plate) (Crebelli
et al., 1982).
High-purity trichloroethylene was not mutagenic for the TA 100
strain of S. typhimurium in a plate assay, with and without
addition of S9 mixture (Henschler et al., 1977).
Trichloroethylene containing no traces of 1,2-epoxybutane or
epichlorohydrin was tested as a gas in a desiccator on the TA 1535
and TA 100 strains of S. typhimurium. Concentrations of 1 - 3%
produced a 30% increase (less than a doubling) in revertants in
strain TA 100 in the presence of a metabolic activation system from
Arochlor(R)-induced rat liver (Baden et al., 1979).
The exposure of S. typhimurium strains TA 98 and TA 100 to
0.5 - 10% vapour concentrations of trichloroethylene in sealed
vials for 48 h did not produce an increase in revertants either in
the presence or absence of rat liver homogenate (Waskell, 1978).
Trichloroethylene of high purity (99.5%) was mutagenic for the
TA-100 strain of S. typhimurium when used as a gas in a desiccator
in the presence of S9 mix (Bartsch et al., 1979). However, it
should be noted that the increase in revertants in the treated
series was less than twice that of the untreated series, but this
slight effect was consistent in repeated experiments.
Trichloroethylene of unspecified purity was reported to double
the spontaneous frequency of reverse mutations in the D-7 strain of
the yeast Saccharomyces cerevisiae, at one concentration, in the
presence of an endogenous metabolic activation system (Callen et
al., 1980).
Trichloroethylene containing impurities, which were not
specified, was mutagenic for S. cerevisiae, strain XV185-14C
(reverse mutations) in the presence of S9 mix. These results
were obtained at a level of yeast cell % survival of < 1 (1 h of
treatment) and of < 0.1 (4 h of treatment). These conditions are
unusual for the detection of mutagenic effects, since, at these
toxic values, selection of different cells (wild type and mutants)
is very efficient (Shahin & von Borstel, 1977). One of the authors
(von Borstel, personal communication, 1984) stated that, when these
studies were repeated with a pure trichloroethylene sample, it was
not mutagenic for yeast cells.
Trichloroethylene of high purity (99.8%), as well as
trichloroethylene containing stabilizers such as 1,2-epoxy-butane
(0.19%) and epichlorohydrin (0.09%), were not mutagenic for the
yeast S. pombe (forward mutations), with or without a metabolic
system obtained from rats and mice treated with phenobarbital and
beta-naphthoflavone. Using intraperitoneal or intrasanguineous,
host-mediated assay techniques with male mice inoculated with the
yeast S. pombe and treated orally with 2 g/kg body weight for
4 or 16 h, both pure trichloroethylene and the stabilized
trichloroethylene were found not to be mutagenic (Rossi et al.,
1983).
A slightly mutagenic action for cells of S. cerevisiae strain
D7 (reverse mutation at his locus) was found for trichloroethylene
of unspecified purity in the presence of a metabolic activation
system obtained from mice (untreated series: 0.79 - 0.89 his,
rev/106; treated series: 3.00 - 3.60 his rev/106 with 40 mol).
For this experiment, no survival values were reported, nor were the
actual number of revertants counted, and therefore the observed
effect cannot be definitely assigned to trichloroethylene
(Bronzetti et al., 1978). The same authors administered
trichloroethylene (unspecified purity) orally to mice (400 mg/kg
body weight) that were also inoculated with yeast cells intra-
sanguineously; a mutagenic effect was found on yeast cells (reverse
mutations at the his locus) collected from liver, lung, and kidney
but, again, the survival level was not reported nor was the number
of revertants counted (Bronzetti et al., 1978).
Trichloroethylene of high purity and containing known
contaminants (~80 ppm) was found to be active for the production
of reverse mutations (suppressor mutations) on Aspergillus
nidulans sumeth A1 strain at a dose of 27 000 mg/m3 (5000 ppm)
applied in a desiccator containing the plates inoculated with the
fungal conidia (Crebelli et al., in press).
(b) Mammalian cells (in vitro)
A pure sample of trichloroethylene, stabilized with thymol,
did not induce mutations (forward mutations) at the HPRT locus of
Chinese hamster V-79 cell line treated in vitro with or without
metabolic activation (S9 mix) (Loprieno & Abbondandolo, 1980).
(c) Mammals (in vivo)
Trichloroethylene (purity 99.5%) was evaluated for its ability
to induce somatic gene mutations in vivo in embryonic fibroblasts
of mice (spot test). Pregnant mice were injected 12 days after
copulation with a dose of 140 mg/kg body weight (50 females), or
350 mg/kg (26 females). The results observed were 2/145 (1.4%)
and 2/51 (3.9%) of progeny with "spots", respectively, whereas
the untreated series produced 0/146, 3/182 (1.6%), 6/794 (0.75%).
These results were considered by the authors to indicate a
mutagenic effect (Fahrig, 1977); however, positive data such as
those observed for trichloroethylene are among spontaneous values
for this test system.
6.1.8.2. Chromosome aberrations
(a) Mammals (in vivo)
Trichloroethylene (purity not given), repeatedly administered
intraperitoneally to ICR mice for 5 days at a dose equivalent to
half of the LD50, did not induce a significant increase in
chromosome aberration frequency in bone marrow cells from animals
killed 6, 24, and 48 h after the last treatment (Cerna & Kjpenova,
1977).
A single dose of trichloroethylene of 1000 mg/kg body weight
(stabilized with thymol), administered orally to Swiss albino
mice, did not induce a significant increase in the frequency of
chromosome aberrations in bone marrow, 24 h after treatment
(Loprieno & Abbondandolo, 1980).
The exposure of male mice (NMRI-Han/BGA) to trichloroethylene
vapour (99.5% purity) for 24 h at concentrations of 272, 1090, and
2430 mg/m3 (50, 202, and 450 ppm) did not induce dominant lethal
mutations (Slacik-Erben et al., 1980).
CDI male mice were treated orally with 3000, 2250, 1500,
1125, 750, or 375 mg/kg body weight (2 treatments in 24 h) with a
trichloroethylene sample of high purity (99.5%) and sacrificed 6 h
following the second treatment, for the analysis of the frequency
of micronucleated erythrocytes in the bone marrow cells. The
results indicated a positive dose-related effect of
trichloroethylene (Duprat & Gradiski, 1980).
Using high purity (99.8%) trichloroethylene, B6C3F1 mice (10
female and 10 male) were treated by inhalation with 3240 mg/m3
(600 ppm), for 7 h a day, 5 days a week, for a total of 52 days.
The animals were killed 6 h and 24 h after the last treatment. The
cytogenetic analysis showed the presence only of gaps without any
indication of induced chromosomal aberrations (Loprieno, personal
communication, 1984).
When trichloroethylene of high purity (99.8%) was administered
to B6C3F1 male mice, by gavage, in a single dose of 1200 mg/kg
body weight, an increased frequency (4 times) of micronucleated
erythrocytes in the bone marrow cells compared with control animals
was observed, 42 h after dosing (Sbrana et al., 1984).
6.1.8.3. DNA damage
Fungi
Trichloroethylene (unspecified purity) was positive for
the induction of mitotic gene conversion at the trp locus and
mitotic recombination in the D7 strain of S. ceverisiae, under
endogenous metabolic activation conditions (Callen et al., 1980).
Trichloroethylene (unspecified purity) was also found to be
positive for the induction of mitotic gene conversion at the trp
locus of S. cerevisiae D7 strain treated in vitro in the presence
of a metabolic activation system obtained from rat liver. Using
the host-mediated assay technique, and a single oral dose of
400 mg/kg body weight, given to mice inoculated with yeast cell
D4 strain of S. cerevisiae, a positive effect on the induction of
mitotic gene conversion at the trp 5 and ade 2 loci was observed
in the yeast cells recovered from the kidney. Repeated oral
administration of trichloroethylene to male mice, with a total
dose of 3700 mg/kg body weight, induced a positive effect (mitotic
recombination) in yeast cells recovered from the liver and kidney.
In all these studies, the actual numbers of the convertant or
recombinant colonies were not given, and the significance of the
results is questionable (Bronzetti et al., 1978).
Trichloroethylene (stabilized with thymol) was considered
inactive for the induction of mitotic recombinations in the D4
strain of S. cerevisiae following in vitro and in vivo (host-
mediated assay) studies (Loprieno & Abbondandolo, 1980).
Trichloroethylene of high purity (99.9%) induced somatic
segregants in A. nidulans strain 35 x 17, at concentrations of
40 500 and 81 000 mg/m3 (7500 and 15 000 ppm), in a desiccator
containing the plates inoculated with the fungal conidia; under
identical doses and conditions, no mitotic recombinant colonies
were induced in this strain of A. nidulans by trichloroethylene
(Crebelli et al., in press).
6.1.8.4. Mammalian cells ( in vitro)
Trichloroethylene of high purity (99.9%) induced morphological
transformations, in vitro, in rat embryo cell cultures (Price et
al., 1978).
Stabilized with thymol, trichloroethylene did not stimulate
unscheduled DNA synthesis in an HeLa human cell line grown in
vitro, in the presence or absence of a metabolic activation
system (S9 mix) (Loprieno & Abbondandolo, 1980). Trichloroethylene
(unspecified purity) elicited DNA repair synthesis in human
lymphocytes in the presence of S9 mix (Perocco & Prodi, 1981).
Exposure of Chinese hamster ovary cells (CHO) to
trichloroethylene bubbled into the growth medium for 2 min,
corresponding to a dose of 0.17% v/v for 1 h treatment time,
did not induce sister-chromatid exchange (White et al., 1979).
Trichloroethylene bound covalently to calf thymus DNA in vitro
in the presence of a rat liver metabolic activation system (Di
Renzo et al., 1982; Bergman, 1983).
When given ip to NMRI mice, twice daily for 5 days
(33.6 mg/kg), trichloroethylene bound to RNA and DNA of brain,
lung, liver, kidney, spleen, pancreas, and testis (Bergman, 1983).
6.1.8.5. Mutagenic activity of trichloroethylene metabolites
Trichloroethylene oxide was not mutagenic for the TA 1535
strain of S. typhimurium or the WP2 UVRA strain of E. coli (plate
test), but was positive in the DNA repair test on E. coli (Kline
et al., 1982).
The same compound was found to be a direct mutagen for the
yeast S. pombe (forward mutation) and for mammalian cells grown
in vitro (V-79 cell line) (forward mutation) (Loprieno &
Abbondandolo, 1980).
Syrian golden hamster embryo cells were exposed to
trichloroethylene oxide (1.1, 2.5, and 5.0 µM) at 37 °C for 30 min.
After 5 days, the number of transformed colonies was scored. A low
but dose-related increased frequency of transformed cells was
induced (Di Paolo & Doniger, 1982).
Chloral hydrate has been reported to have direct genotoxic
activity in S. typhimurium (Waskell, 1978) and in A. nidulans
(Bignami et al., 1980).
At doses of 82.7, 165.4, or 4135 mg/kg body weight, chloral
hydrate, administered intraperitoneally, induced chromosome non-
disjunction in the secondary spermatocytes of mice (Russo et al.,
1984).
6.1.8.6. Conclusions
Pure trichloroethylene acts as a weak mutagen in S. typhimurium
in the presence of a metabolic activation system. However, in all
cases, the induced effect was never more than twice the spontaneous
value. Technical grade trichloroethylene containing stabilizers
such as 1,2-epoxybutane epichlorohydrin, is mutagenic in
S. typhimurium in the absence of a metabolic activation system.
Pure trichloroethylene is not mutagenic for the yeast S. pombe,
with or without metabolic activation, in vitro or in vivo (host-
mediated assay).
Thymol-stabilized trichloroethylene is not mutagenic for V-75
hamster cells, with or without metabolic activation.
Trichloroethylene (99.5% pure) is a weak mutagen for the
induction of somatic mutations in mouse embryo.
In mice, a single oral dose of trichloroethylene (1 g/kg body
weight) or repeated exposure to 3240 mg/m3 (600 ppm) for 52 days,
7 h/day, through inhalation, did not increase chromosome
aberration frequency in bone-marrow cells. A half-LD50 dose
of trichloroethylene, administered ip to mice, did not increase
chromosomal aberration frequency in bone-marrow cells. In a
micronucleus test, a single oral treatment with 1200 mg/kg body
weight or repeated (twice) oral treatment with a series of doses
ranging from 375 to 3000 mg/kg body weight increased the frequency
of induced micronucleated erythrocytes in the bone-marrow cells of
mice. Trichloroethylene bound to DNA in vitro and in vivo to
mouse tissues.
Dominant lethal mutations were not induced in mice exposed
through inhalation to trichloroethylene vapours.
Trichloroethylene induces mitotic gene conversion in yeast
cells grown under conditions allowing an endogenous metabolic
activation system to function. The same effect reported in yeast
in the presence of an exogenous metabolic activation system
(S9 mix), or in the host-mediated assay, is questionable, because
of inadequate data and the fact that other studies produced
negative data.
A positive effect for the induction of unscheduled DNA
synthesis (UDS) in human lymphocytes, treated in vitro, was
reported by Perocco & Prodi (1981), whereas a negative response
for the same biological effect (UDS) in a human fibroblast cell
line (HeLa) grown in vitro was observed by Loprieno & Abbondandolo
(1980).
Trichloroethylene induces morphological transformation in rat
embryo cells.
The available results, shown in Table 9, are not adequate
for a complete evaluation of the genotoxic potential of
trichloroethylene. Only a few mutagenicity studies have indicated
the grade and purity of the trichloroethylene sample employed.
The confusing results could be due either to the use of
trichloroethylene samples stabilized with mutagenic compounds or to
the use of pure trichloroethylene samples which, unstablized, can
rapidly decompose to chemicals with mutagenic activity.
6.1.9. Reproduction, embryo/fetotoxicity, and teratology
The embryo/fetal toxicity and teratogenic potential of
trichloroethylene have been evaluated in the avian embryo system,
the mouse, rat, and rabbit. Reproductive function has been
examined in male rats.
6.1.9.1. Avian embryo system
Exposing avian embryos to 1% trichloroethylene (10 g/litre)
caused a significant increase in embryonic death and a slight
increase in anomalies (Fink, 1968). These observations were made
on the 3rd day of development, a time at which frank malformations
would be difficult to observe or evaluate. Elovaara et al. (1979)
reported the results of a comparative study of the teratogenic
potential of trichloroethylene and other aliphatic chlorinated
hydrocarbons in the chick embryo. Trichloroethylene (reagent
grade) in doses ranging from 5 to 1000 µmoles in olive oil (25 µl)
was injected into the air space of fertilized eggs on the 2nd and
6th days of incubation and examined on the 14th day. The LD50 for
trichloroethylene was between 50 and 100 µmoles/egg. Macroscopic
embryonic malformations occurred in 9 out of 55 surviving embryos,
5 times the incidence in the vehicle control eggs.
In another study, injection of low doses of trichloroethylene
(1, 5, 10, and 25 µmol/egg), on days 1 and 2 of embryogenesis,
resulted in embryotoxicity, growth defects, and morphological
anomalies (Bross et al., 1983).
Table 9. Summary of the mutagenicity data reported for trichloroethylene
-----------------------------------------------------------------------------------------
Organism Genotoxic effects
Gene Chromosome DNA
mutation anomalies damage
-----------------------------------------------------------------------------------------
prokaryotes weak
positive
-----------------------------------------------------------------------------------------
fungi negative ( in vitro) positive negative ( in vitro)
negative (hma) mitotic weak (hma)
positive? ( in vitro) segregation positive (yeast
positive (A. nidulans) A. nidulans metabolism)
-----------------------------------------------------------------------------------------
mammalian negative negative (SCE)
cells negative (DNA
( in vitro) repair synthesis)
positive (covalent
binding)
-----------------------------------------------------------------------------------------
mammals weak negative (1 dose) (1 g/kg body
( in vivo) weight, oral)
negative (52 doses) (3240 mg/m3,
inhalation)
negative (5 doses) (1/2 LD50, ip)
positive (micronuclei) 1200 mg/kg
positive (micronuclei) (375 -
3000 mg/kg)
negative (dominant lethals) (270,
1090, 2430 mg/m3, inhalation)
-----------------------------------------------------------------------------------------
For human cases, see Table 10.
6.1.9.2. Mouse
Swiss-Webster mice were exposed by inhalation to
trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h daily, on days
6 - 15 of gestation (Day 0 = vaginal plug). The mice were observed
daily throughout pregnancy. Maternal body weights were recorded on
days 6, 10, and 18 of gestation as an index of maternal toxicity.
On day 18, no effect was observed on the average number of
implantation sites/litter, litter size, the incidence of fetal
resorptions, fetal sex ratios, or fetal body measurements (Schwetz
et al., 1975). The incidence of gross anomalies observed by
external examination was not significantly greater than that among
the control litters. Trichloroethylene exposure did not exert any
effect on the incidence of skeletal anomalies in mice, and
microscopic examination did not reveal any abnormalities in organs,
tissues, or cells (Leong et al., 1975; Schwetz et al., 1975).
6.1.9.3. Rat
In studies similar to the preceding mouse study, exposure to
trichloroethylene at 1620 mg/m3 (300 ppm) did not produce any
evidence of maternal toxicity or teratogenicity in Sprague Dawley
rats (Leong et al., 1975; Schwetz et al., 1975) or Charles River
strain rats (Bell, 1977).
Exposing Sprague Dawley rats to trichloroethylene at 2700 mg/m3
(500 ppm) for 7 h per day, 5 days per week, during a 3-week
pregestational period, and 7 h per day each day for days 0 - 18 and
days 6 - 18 of gestation did not cause maternal or embryo/fetal
toxicity. Beliles et al. (1980) concluded that the frequency and
the character of the macro- or microscopic findings in the treated
groups did not indicate either adverse fetal effects or that
trichloroethylene, at the dose used, was teratogenic.
Dorfmueller et al. (1979) reported that female Long-Evans rats
exposed to trichloroethylene at 9720 mg/m3 (1800 ppm) before
mating, for 6 h per day, 5 days per week, for 2 weeks and/or during
pregnancy, for 6 h per day, each day, for 0 - 20 days of gestation)
did not exhibit any signs of maternal toxicity. There was no
indication of embryotoxicity, and no significant treatment effects
or interactions were found in the number of corpora lutea or
implantation sites/litter, fetal body weights, resorbed
fetuses/litter, or sex ratios. No significant treatment effects
were observed in the analysis of total soft-tissue anomalies.
However, prenatal exposure to trichloroethylene at 9720 mg/m3
(1800 ppm) caused an increase in minor anomalies of the offspring
(incomplete ossification of the sternum) indicative of development
delays in maturation, but not of major malformation.
While the study did not indicate any treatment-related effects
on general post-natal behaviour, there was a small but significant
regression in post-natal weight gain in offspring in the pre-mating
exposure group.
In groups of male rats dosed by gavage with trichloroethylene
at 10, 100, or 1000 mg/kg body weight 5 days per week, for 6 weeks,
there was no evidence of spermatotoxic effects. Trichloroethylene-
related effects of reduced weight gain and elevated liver/body
weight ratios were seen primarily in the 1000 mg/kg group.
Copulatory behaviour was initially diminished by the narcotic
properties of trichloroethylene, but was normal by the 5th week
(Zenick et al., 1984).
6.1.9.4. Rabbit
Female New Zealand white rabbits were exposed by inhalation to
trichloroethylene at 2700 mg/m3 (500 ppm), for 7 h per day, 5 days
per week, during a 3-week pregestation period, and daily, for days
0 - 21 and days 7 - 21 of gestation (Day 0 = mating day). There
was no evidence of maternal toxicity or embryotoxicity. The
occurrence of hydrocephalus in a few fetuses in one of the study
groups was reported (4 fetuses in 2 litters), but no definitive
conclusion was drawn from these findings (Beliles et al., 1980).
7. EFFECTS ON THE ENVIRONMENT
7.1. Aquatic Organisms
There is little information on the toxicity of
trichloroethylene for fish. The US Registry of Toxic Effects of
Chemical Substances (Christensen & Lugenbyhl, 1975) reports, for an
unidentified species, that exposure to a concentration range of
100 - 1000 mg/litre produced toxic effects in 96 h. Toxicity tests
carried out on salt-water flatfish, Limanda limanda (sole), 15 -
20 cm long, in a continuous water flow, established a 96-h LC50
of 16 mg/litre (Pearson & McConnell, 1975). A 96-h LC50 of
approximately 40 mg/litre (static) or 67 mg/litre (continuous flow)
has been reported for the minnow Pimephales promelas (Alexander et
al., 1978).
Verschueren (1977) established an LC100 of 600 mg/litre for
Daphnia magna. The LC50 for the balanide salt-water crustacean
nauplius (larva) (Elminius modestus) was 20 mg/litre after 46 h
(Pearson & McConnell, 1975), and the LC50 for the protozoon
Entosiphon sulcatum was established as 1200 mg/litre (Bringmann
& Kuhn, 1980).
Various LC50 values have been established for algae including
63 mg/litre for Microcystis aeruginosa, (Verschueren, 1977); a
concentration of 1000 mg/litre did not have any observable effect
on Scenedesmus quadricauda (Bringmann & Kuhn, 1980). A short-term
photosynthesis efficiency test gave an LC50 of 8 mg/litre
(Pearson & McConnell, 1975) and, finally, in tests carried out on
Thalassiosira pseudonana and Dunaliella tertiolecta, there were
observable effects at 50 and 100 µg/litre, in a mixed culture
(Biggs et al., 1979).
7.2. Uptake, Distribution, Storage, Metabolism, and Elimination in
Plant and Animal Organisms
Bioaccumulation of trichloroethylene in a marine environment
has been studied by Pearson & McConnell (1975); concentration
levels were determined for a wide variety of marine organisms,
mostly in the Bay of Liverpool.
The greatest increase in trichloroethylene concentrations in
the tissues of animals that are relatively high up in the food
chain (birds' eggs, fish liver, and seal fat) was nearly 100 times
the level in water (from 0.5/109 µg/litre in water to 50/109 µg/kg
in tissues).
These data agree with the laboratory findings of Barrows et
al. (1980) who, in a 14-day test, noted that trichloroethylene
accumulation in the sunfish species, Lepomis macrochirus was 17
times that of the aquatic medium with a halving time of less than
one day. A low bioconcentration factor (concentration in organism
divided by concentration in environment) (Uehleke et al., 1977)
has been derived using water solubility and the equation proposed
by Kenaga (1980) and Kenaga & Goring (1980).
7.3. Effects on the Stratospheric Ozone Layer
Consideration has been given to the possibility that
trichloroethylene, together with other halocarbons in the
atmosphere, may contribute to the depletion of the stratospheric
ozone layer, which would lead to atmospheric heating and increased
exposure of terrestrial biota to ultraviolet radiation (Molina &
Rowland, 1974). Atmospheric trichloroethylene concentrations seem
to be about one-fifth to one-tenth of those of other chlorocarbons
such as CH3CCl3, CH2Cl2, CCl4, or C2Cl4 or the major chlorofluoro-
carbons (Cronn et al., 1977; Penkett, 1982). The reason for this
is that while trichloroethylene emissions into the atmosphere are
of the same order as those of other halocarbons (Jesson, 1980),
trichloroethylene is efficiently scavenged by hydroxyl radicals
in the troposphere, and the reaction rate for this process is
appreciably faster for trichloroethylene than for other halocarbons
(Penkett, 1982). Thus, the predicted atmospheric lifetime for
trichloroethylene is short (about 10 - 11 days) (Derwent &
Eggleton, 1978; Graedel, 1978) compared with those for the
chlorofluorocarbons, which may be 10 years or more (Jesson, 1980).
It is not clear whether trichloroethylene is even present in the
stratosphere (Cronn et al., 1977). However, the data suggest that
trichloroethylene is unlikely to be involved in the possible
depletion of the ozone layer.
8. EFFECTS ON MAN
8.1. General Symptoms and Signs
8.1.1. Acute effects
Sorgo (1976) reported a lethal oral dose for trichloroethylene
to be 7 g/kg body weight, and Lazarev et al. (1977) reported a
fatality after the oral ingestion of less than 50 ml.
At exposure concentrations of 270 - 540 mg/m3 (50 - 100 ppm),
the most common symptoms are headache, sluggishness, sleepiness
(especially at the end of the workshift), dulling of senses,
dizziness, nausea, and vomiting (Rubino et al., 1959; Lilis et
al., 1969; Governa, 1981). At narcotic doses, vomiting may occur.
Trichloroethylene has been used as an analgesic and as an
anaesthetic on its own or in mixtures. It is now much less used
for this purpose, as safer and more effective alternatives are
available. Inhalation of 27 000 mg/m3 (5000 ppm) produces light
anaesthesia; concentrations of up to 108 000 mg/m3 (20 000 ppm)
have been used for deeper anaesthesia (Wade, 1977).
Clinical signs are not very specific. There can be enhanced
responsiveness to caloric stimulation of the labyrinth and some EEG
anomalies may be detected (Chiesura, 1980) (section 8.2). Acute
hepatic and renal toxicity has been reported (Sucui & Olinici,
1983).
A particular intolerance to ethanol may occur after ingesting
even small amounts of alcoholic beverages. This intolerance is
characterized by intense cutaneous vasodilatation, particularly in
the face, often called "degreaser's flush" (Stewart et al., 1974).
Non-specific effects on the digestive system (e.g., dyspepsia,
gastritis, and diarrhoea) have been reported in cases of suicidal
or accidental ingestion of trichloroethylene (Bozza Marrubini et
al., 1978; Governa, 1981).
8.1.2. Chronic effects
The existence of a condition of chronic trichloroethylene
poisoning is not clear (Chiesura, 1980; Boudène et al., 1983).
However, chronic effects in human beings can occur following
prolonged exposure to moderate concentrations of trichloroethylene
of about 540 mg/m3 (100 ppm). The clinical picture is mainly
related to the central nervous system (CNS), and consists of
asthenia, anorexia, headache, loss of memory, moodiness,
depression, insomnia, paraesthesia, and disturbances of the
autonomic nervous system such as hyperhydrosis, tachycardia, and
dermographism (Ahlmark & Forssman, 1951a,b; Bardodej & Viskocil,
1956; Rubino et al., 1959; Lerza et al., 1963; Castellino, 1969).
The role of trichloroethylene in inducing liver damage in
occupationally-exposed human beings is not clear (Parmeggiani,
1956; Capellini & Grisler, 1958; Rubino et al., 1959; Tolot et al.,
1964; Schuttmann, 1970; Lachnit, 1971; Thiele, 1982; Sucui &
Olinici, 1983; Svabova & Mencik, 1983).
Human subjects with high repeated, but non-occupational,
exposure may exhibit toxic effects on the liver (e.g., elevated
aspartate (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2)),
renal insufficiency, and abnormal EEG patterns (Baerg & Kimberg,
1970). Following accidental or suicidal ingestion (e.g., doses of
100 - 300 ml), hepatic necrosis and nephropathy have been found in
autopsy cases (Keinfeld & Tabershaw, 1954; Graovac-Leposavic
et al., 1964; Priest & Horn, 1965; Beisland & Wannag, 1970;
Clearfield, 1970). A decrease in sexual potency has been reported
in industrial workers exposed to trichloroethylene (Bardodej &
Vyskocil, 1956; Lazarev & Gadaskiva, 1977).
Trichloroethylene is one of the volatile solvents inhaled
("sniffed") for its euphoric effect (Hayden et al., 1976), and
abuse by oral self-administration has been reported (Wells, 1982).
Centrilobular hepatic necrosis and renal toxicity have occurred in
addicts (Baerg & Kimberg, 1970; Clearfield, 1970), and deaths have
been reported (James, 1963; Musclow & Awen, 1971). Some mixtures
sold commercially as trichloroethylene may contain other solvents,
including carbon tetrachloride, which can contribute to the toxic
manifestations (Bouyges et al., 1980; Conso et al., 1980).
Dependent patients may show withdrawal symptoms (Ikeda et al.,
1971).
8.2. Effects on Organs and Systems
8.2.1. Effects on the nervous system
Acute effects on the central nervous system (CNS) are
characterized by two sequential phases (i.e., excitation and
depression), and are usually reversible. These symptoms generally
prevail over those of other systems (Stewart et al., 1962;
Chiesura, 1980; Governa, 1981).
In the early phase of excitation, euphoria and inebriation are
present. The subsequent phase of CNS depression is characterized
by various degrees of narcosis culminating in coma (Caccuri, 1976;
Chiesura, 1980). Muscular hypotony, muscular spasms, reduced
tendon reflexes, and loss of co-ordination may occur (Huff, 1971).
Changes in EEG patterns may vary considerably. The EEG may
be normal in some cases, while substantial alterations, regressing
after a few days, may be observed in others. The most frequently
observed change is a decrease in alpha activity, which is often
irregular. Widespread rapid rhythms or low theta activity may also
be present (Chiesura, 1980).
Exposure to trichloroethylene at a concentration of 540 mg/m3
(100 ppm) produces various results on the CNS. Though there may
not be any evidence of effects on psychomotor capacities (Stewart
et al., 1970), reductions in mental performance evidenced by the
test for perception, past recall, answering speed, and response
and manual dexterity tests have been reported (Ferguson & Vernon,
1970). Visual and auditory evoked potentials were affected at
exposure levels ranging between 270 and 540 mg/m3 (50 and 100 ppm),
for 3 1/2 - 7 1/2 h (Winneke, 1982).
The behavioural effects of exposure to trichloroethylene have
been studied under laboratory and work-place conditions. Laboratory
exposure to 540 or 1080 mg/m3 (100 or 200 ppm), for 70 min, had no
effect on reaction time or short-term memory (Gamberale et al.,
1976). Volunteers exposed to 540, 1620, or 5400 mg/m3 (100, 300,
or 1000 ppm), for 2 h, showed significant impairment in a number
of psychomotor tests at 5400 mg/m3 but not at 1620 or 540 mg/m3
(Vernon & Ferguson, 1969). In a further study (Ferguson & Vernon,
1970), effects were again found at 5400 mg/m3 and marginal effects
at 1620 mg/m3. In another study, exposure of volunteers to 810
or 1620 mg/m3 (150 or 300 ppm), for 2 1/2 h, did not produce
any significant impairment in behavioural test results (Ettema
& Zielhuis, 1975). With repeated exposure under work-place
conditions, no behavioural effects were observed with mean
atmospheric levels of 270 mg/m3 (50 ppm) (Triebig et al., 1977a,b).
In a study of complex reaction time in an occupational group with a
mean exposure of 1320 mg/m3 (245 ppm), reaction time was increased
in comparison with that in a control group (Gun et al., 1978).
Motor nerve conduction velocity was not affected in a group
occupationally exposed to mean atmospheric levels of less than
270 mg/m3 (50 ppm) (Triebig et al., 1982).
A study on 122 exposed workers (Ahlmark & Forssman, 1951a)
showed that symptoms of toxicity in human beings were correlated
with urinary levels of metabolic trichloroacetic acid. With
urinary concentrations of up to 20 mg trichloroacetic acid/litre,
no particular symptoms were noted. With urinary levels ranging
between 45 and 75 mg/litre, headache, fatigue, increased need for
sleep, irritability, and alcohol intolerance were reported in 50%
of workers. When urinary levels of trichloroacetic acid exceeded
300 mg/litre, these symptoms were found in 100% of subjects.
Initial nervous-system signs of toxicity were reported to be
associated with the urinary excretion of trichloroacetic acid
at 75 mg/litre and 30 mg/litre (Lazarev & Gadaskiva, 1977).
Two types of irreversible neurotoxic effects have been reported
in man. One is the specific degeneration of the cranial nerves, in
particular the trigeminal, the olfactory, and the facial nerves
(Goldblatt, 1956; James, 1963; Kjellstrand et al., 1980; Barrett
et al., 1982). However, it is considered that this effect is
not produced by trichloroethylene itself but by its decomposition
product, dichloroacetylene (Henschler et al., 1970a). In addition,
polyneuropathies involving the whole peripheral nervous system have
been described after long-term exposure to trichloroethylene
(Szulc-Kuberska, 1972).
8.2.2. Effects on the cardiovascular system
Several cases of sudden death due to cardiac arrest have been
reported in subjects exposed to trichloroethylene, either medically
or occupationally (Ostelere, 1953; Norris & Stuart, 1957; Meyer,
1966; Wiecko, 1966; Clearfield, 1970; Tomasini, 1976). It has
been suggested that these deaths might be due to an increased
sensitivity of the myocardium to endogenous and exogenous
catecholamines. The event would entail irregular cardiac
rhythm due to the onset of ectopic foci with atrioventricular
dissociation. A range of alterations in cardiovascular function
(e.g., atrial and ventricular extrasystole, tachycardia, and
ventricular fibrillation) has been reported (Bardodej & Viskocil,
1956; Anderson, 1957; Lilis et al., 1969; Tomasini, 1976; Governa,
1981).
8.2.3. Effects on the respiratory system
Under normal conditions of exposure, trichloroethylene did not
induce any effects on the respiratory tract but, at high exposure
levels of 810 - 3510 mg/m3 (150 - 650 ppm), there may be effects
due to local irritation (Bardodej & Viskocil, 1956; Jouglard &
Vincent, 1971; Meyer, 1973; Gaultier et al., 1974; Tomasini, 1976).
Breakdown products are of more importance than trichloroethylene
with respect to the effects on the respiratory system (Castellino,
1969).
8.2.4. Effects on the urinary tract
Altered renal function has been described in both acute and
chronic trichloroethylene poisoning (Nomura, 1962; Graovac-
Leposavic et al., 1964; Clearfield, 1970). The nephropathy
observed could be due to impurities rather than to the
trichloroethylene; technical grade trichloroethylene might have
contained nephrotoxic chlorinated hydrocarbons (e.g., 1,1,2,2-
tetrachloroethane) as impurities (Chiesura, 1980).
8.2.5. Effects on the skin
When applied to the skin, trichloroethylene causes erythema
(Wahlberg, 1984). It is only mildly irritating to human skin
providing it is not held in contact (e.g., by clothing or
footwear). Chemical burning of the skin has been reported
following prolonged contact (Maloof, 1949; Tomasini, 1976). There
is a considerable difference in individual susceptibility to the
effects of repeated exposure. The reaction to repeated contact
(which will defat the skin) may take the form of an erythematous,
exudative, vesicular, eczematous, or exfoliative dermatitis (Roche
et al., 1958; Stopps & McLaughlin, 1967; Ettema et al., 1975).
Secondary infection of the skin may complicate the dermatitis.
"Degreaser's flush", a reddening of the skin in some individuals
ingesting ethanol after exposure to trichloroethylene, can occur
(section 8.1.1).
8.2.6. Effects on the eye
Liquid trichloroethylene in the eye produces pain and
discomfort and superficial damage to the cornea, but complete
recovery occurs within a few days (Grant, 1974). Exposure to high
concentrations of vapour (anaesthetic levels of 27 500 - 108 000
mg/m3) also causes eye irritation and superficial damage to the
cornea, but again with complete recovery (Grant, 1961).
8.2.7. Carcinogenicity
A number of epidemiological studies have been conducted to
examine the possible carcinogenic effects of trichloroethylene in
occupationally-exposed populations, and the major findings are
summarized in Tables 10 and 11. Some of these studies have
followed groups with specified exposure to trichloroethylene
(Axelson et al., 1978, 1984; Tola et al., 1980) (Table 10), whereas
others have involved workers in laundry and dry cleaning with more
or less mixed exposures including trichloroethylene (Blair et al.,
1979; Malek et al., 1979; Katz & Jowett, 1981) (Table 11). Other
studies are of a case-reporting or a case-referent (case-control)
type, concerning pancreatic (Lin & Kessler, 1981) and liver tumours
(Novotna et al., 1979; Paddle, 1983) or other conditions and, also,
involving exposure to other solvents (Lin & Kessler, 1981). Some
case-control studies of lymphomas, both of the Hodgkin and non-
Hodgkin type (Olsson & Brandt, 1980; Hardell et al., 1981) and of
liver cancer (Hernberg et al., 1984) have mentioned exposure to
trichloroethylene as a factor in a few cases, but the numbers are
too small for any conclusions to be drawn about trichloroethylene
as a risk factor.
Table 10. Summary of epidemiological studies on carcinogenicity with specific reference
to trichloroethylene exposure
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure intensity had been monitored since Axelson et al.
of 518 male workers 1950 in terms of urinary excretion of (1978)
(7688 person-years) trichloroacetic acid (TCA).
2. No significant excess of cancer mortality
either in the high-dose group (> 100 mg
TCA/litre urine) or the low-dose group
(< 100 mg TCA/litre urine).
3. Results of first update, see Axelson (1984)
below.
-----------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Mortality in a cohort 1. Exposure information was based on TCA Tola et al.
of 1148 male workers determinations in the urine, permitting (1980)
and 969 female workers latency requirements of 6 - 13 years. Loss
(4269 person-years for to follow-up was 9%.
women)
2. Trichloroethylene was not carcinogenic
(given a latency period of 6 to 13 years)
among those exposed at low levels (100 mg
TCA/litre urine in 91% of the cohort).
3. Cohort to be updated at 5-year intervals.
Mortality and 1. Extension and update of the first-mentioned Axelson et al.
incidence in a study, 65% with exposure from 1970 to 1975 (1984)
cohort of 1424 men and with more than 90% having TCA levels
< 100 mg/litre.
2. Deficit found in mortality from cancers (22
versus 36.9 expected), but a significant
excess of urinary tract (11 versus 4.85) and
haematolymphatic tumours (5 versus 1.20).
3. Specifically, there were 3 urinary bladder
cancers versus 0.83 expected, 4 cancers of
the prostrate versus 2.35 expected and 2
lymphomas versus 0.27 expected, requiring
2 years or more of exposure and 10 years of
latency.
-----------------------------------------------------------------------------------------
Table 11. Summary of epidemiological studies and case reports on carcinogenicity of
mixed exposures involving trichloroethylene or trichloroethylene production
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 330 laundry 1. Significant increase in lung and cervical Blair et al.
and dry-cleaning cancer, and a slight excess of leukaemia and (1979)
workers liver cancer detected.
2. Exposed also to other chemicals such as
benzene and carbon tetrachloride.
3. To be extended to a prospective study of
2 - 5 x 103 workers.
Cohort of 37 male 1. Exposure intensity was such that TCA in Malek et al.
dry-cleaning workers urine was less than 100 mg/litre in over 60% (1979)
of the cohort.
2. 6 cases of cancer, none of which were in the
liver.
-----------------------------------------------------------------------------------------
Table 11. (contd.)
-----------------------------------------------------------------------------------------
Type of study/cohort Remarks Reference
-----------------------------------------------------------------------------------------
Cohort of 671 female 1. Death certificates were employed. Katz & Jowett
laundry/dry-cleaning (1981)
workers (controls: 2. Elevated risks of genital (unspecified) and
entire female working kidney cancers were detected, along with a
population and other smaller excess of bladder and skin cancer and
low-wage occupations) lymphoma.
109 pancreatic cancer 1. Hospital records were employed. Lin & Kessler
patients, case-control (1981)
study 2. An association was observed between
pancreatic cancer and exposure to dry-
cleaning chemicals and gasoline fumes, as
well as to drinking of decaffeinated (by
means of trichloroethylene) coffee.
3. No information was available on extent or
type of exposure.
95 liver cancers, 1. Cancer registry data on liver cancer in the Paddle (1983)
case-study area of a production plant were employed.
2. No case could be definitely tied to
trichloroethylene production.
-----------------------------------------------------------------------------------------
While some of these studies are continuing as prospective
studies to cover larger populations, they suffer, in general, from
one or more of the following factors: (a) small cohort population,
(b) young age of the cohort, (c) a rather short follow-up period,
(d) relatively few cases, (e) inaccurate or uncertain estimation
of intensity and duration of exposure to trichloroethylene, (f)
possible exposure to other chemicals, even in the more specific
studies, including potential carcinogens, and (g) inadequate
information on other general risk factors for cancer, such as
smoking, even if the influence of uncontrolled confounding is
likely to be limited in this respect.
At present, it is not possible to draw definite conclusions
from the existing epidemiological data on the carcinogenic
potential of trichloroethylene. However, the appearance of an
excess of haematolymphatic malignancies in several of the studies,
possibly associated with trichloroethylene exposure (Blair et al.,
1979; Hardell et al., 1981; Katz & Jowett, 1981; Axelson et al.,
1984) deserves attention. Furthermore, genito-urinary tumours were
over-represented in 3 studies (Blair et al., 1979; Katz & Jowett,
1981; Axelson et al., 1984), though the picture is less consistent
with regard to the specific sites involved (bladder, kidney,
prostrate, and cervix).
It was noted that IARC (1979, 1982) concluded that
epidemiological studies on human beings were inadequate to make an
evaluation.
9. EVALUATION OF THE HEALTH RISKS FOR MAN
9.1. Levels of Exposure
9.1.1. General population
The general population is exposed to very low levels of
trichloroethylene in air, water, and food (Table 12). The move
away from the use of trichloroethylene in anaesthesia, the solvent
extraction and fumigation of foodstuffs, and the dry-cleaning
of textiles has reduced exposure from these sources.
Trichloroethylene does not accumulate significantly in the food
chain. It is degraded by both biotic and abiotic processes and
its persistence in various environmental compartments is relatively
short, of the order of days or months rather than years. In ground
water, the absence of actinic radiation means that the rate of
degradation is slower, and trichloroethylene contamination may be
relatively prolonged (e.g., half-life of 2 1/2 years).
Table 12. Maximum observed concentrations of trichloroethylene
in environmental media
---------------------------------------------------------------
Media Maximum observed concentration
---------------------------------------------------------------
Open water reservoir 220 µg/litre
Industrial discharge water 200 µg/litre
Rain water ~ 1 µg/litre
Atmosphere ~ 40 µg/m3
Dairy foods 10 µg/kg
Meat 22 µg/kg
Fats and oil 19 µg/kg
---------------------------------------------------------------
Accidental or suicidal ingestion by adults occurs, but is
influenced by the limited availability of trichloroethylene for
domestic use in many countries.
9.1.2. Occupational exposure
Exposure during the actual production of trichloroethylene is
relatively low because of the nature of the process. Subsequent
uses in, for example, metal degreasing and the dry-cleaning of
textiles, can involve higher exposures. The respiratory route is
the principal route of exposure with dermal exposure as an
additional route. Oral intake is insignificant in occupational
terms.
9.2. Evaluation of Human Health Risks
9.2.1. Acute effects
The predominant effect of trichloroethylene in human beings
is on the central nervous system; liver and kidney damage can also
occur. Central nervous system depression can result in coma and
death. A lethal dose as low as 50 ml (75 g) has been reported
but, in general, the lethal dose for an adult is approximately
7000 mg/kg body weight. The threshold for demonstrable behavioural
effects, resulting from inhalation exposure, is approximately 1350
mg/m3 (250 ppm); less well-defined subjective complaints have been
reported with exposure to concentrations of 270 - 540 mg/m3 (50 -
100 ppm) for a few hours.
Cardiac arrhythmia has been reported during anaesthesia
involving trichloroethylene at inhaled concentrations of 27 000 -
54 000 mg/m3 (5000 - 10 000 ppm) (blood levels 60 - 120 mg/litre).
It should be noted that ethanol potentiates the central nervous
system effects of trichloroethylene. Exposure to trichloroethylene
at 1080 mg/m3 (200 ppm) with blood ethanol levels of 300 - 450
mg/litre produces demonstrable behavioural effects.
Irreversible neurotoxic effects on the trigeminal nerve have
been described in anaesthesia with trichloroethylene, using a
closed-circuit system with soda lime. This effect is attributed to
the generation of dichloroacetylene and not to trichloroethylene.
9.2.2. Chronic effects
The main chronic effects of trichloroethylene in human beings
are disturbances in the central nervous system, as well as effects
on the kidney and liver.
Nervous system symptoms (headache, fatigue, irritability, and
alcohol intolerance) begin to occur when levels of trichloroacetic
acid in urine are about 30 - 75 mg/litre. No symptoms have been
reported at concentrations of 20 mg/litre in urine, which
corresponds to a concentration of trichloroethylene in air of about
50 mg/m3.
For effects on other organs, there is no clear-cut quantitative
information on dose response. There are insufficient data on
possible effects on human chromosomes.
Epidemiological data on the carcinogenicity of trichloro-
ethylene are inconclusive, but a slight excess of genito-urinary
cancers and lymphomas in some studies on dry cleaners and metal
degreasers deserves further attention.
The results of long-term exposure studies on rodents have shown
that the liver and kidney are the critical organs. Cytomegaly and
karyomegaly of renal tubular cells was observed in male rats
exposed to pure trichloroethylene at 1620 mg/m3 (300 ppm), for 7 h
per day, 5 days per week, for 104 weeks, but not at 540 mg/m3 (100
ppm).
No embryotoxic or teratogenic effects have been observed in
mice and rats with inhalation of trichloroethylene at levels of
1620 mg/m3 (300 ppm).
Data on the genetic effects of trichloroethylene are
inconclusive.
Lifetime exposure of mice to trichloroethylene at 1620 mg/m3
(300 ppm) through inhalation or to oral doses of 700 - 1200 mg/kg
body weight per day produced lung and liver tumours. A low
incidence of renal tumours was found in rats exposed to a
trichloroethylene concentration of 3240 mg/m3 (600 ppm) by
inhalation or to 500 - 1000 mg/kg body weight per day by gavage.
A dose-related increase in Leydig (interstitial) cell tumours
of the testis was observed in one study on male Sprague Dawley rats
following long-term inhalation exposure to trichloroethylene
concentrations of > 540 mg/m3 (> 100 ppm).
Thus, there is clear evidence that trichloroethylene is
carcinogenic in mice. There is also some evidence that
trichloroethylene causes tumours in rats. The significance of
these findings needs to be evaluated in the context of further
studies on the mechanism of action of trichloroethylene.
9.3. Treatment of Poisoning in Human Beings
9.3.1. Emergency measures
9.3.1.1. General points
In the treatment of trichloroethylene poisoning, pressor amines
should not be used because of the risk of producing arrhythmias in
the trichloroethylene-sensitized myocardium (Bozza Marrubini et
al., 1978). Hypotension may be treated by transfusion. If
necessary, anti-arrhythmic and beta-blocking agents can be
administered. Haemodialysis, haemoperfusion, or plasmapheresis
have been reported to be useful (Zaffiri et al., 1968; Roessler
& Morawiec-Borowiac, 1973; Malizia et al., 1984). Medical advice
should always be obtained in cases of over-exposure to, or frank
poisoning by, trichloroethylene.
9.3.1.2. Ingestion
Vomiting should not be induced, because of the danger of
aspiration into the larynx and lungs and consequent risks of
vagal inhibition or chemical pneumonitis. Gastric lavage can be
effective if performed within 4 h. Adsorbents such as activated
charcoal or liquid paraffin (medicinal grade) reduce intestinal
absorption; saline laxatives will speed elimination (Bothe et al.,
1973). If the patient is in a state of stupor or coma, intubation
must be performed before gastric lavage.
9.3.1.3. Inhalation
The victim should be removed from the polluted area and placed
in a semi-prone position ensuring that the airway is clear. If
the victim is in a state of stupor or coma, oxygen should be
administered. If spontaneous respiration is absent or very weak,
artifical respiration should be applied.
9.3.1.4. Dermal exposure
All contaminated clothing should be removed and the affected
parts of the body washed thoroughly with soap and plenty of water.
9.3.1.5. Eye exposure
The eyes should be thoroughly irrigated with water for at least
15 min, and ophthalmological advice obtained on the possible need
for further treatment.
REFERENCES
ABRAHAMSEN, A.M. (1960) Quantitative estimation of
trichloroacetic acid in the urine and serum in trichloroethylene
poisoning. Acta pharmacol. toxicol., 17: 288-294.
ACGIH (1982) TLVs - Threshold limit values for chemical
substances in workroom air adopted by ACGIH for 1982, Cincinnati,
Ohio, American Conference of Governmental Industrial Hygienists,
p. 32.
ADAMS, E.M., SPENCER, H.C., ROWE, V.K., MCCOLLISTER, D.D., & IRISH,
D.D. (1951) Vapor toxicity of trichloroethylene determined by
experiments on laboratory animals. Arch. ind. Hyg. occup. Med.,
4: 469-481.
AHLMARK, A. & FORSSMAN, S. (1951a) Evaluating trichloroethylene
exposures by urinalyses for trichloroacetic acid. Arch. ind. Hyg.
occup. Med., 3: 386-398.
AHLMARK, A. & FORSSMAN, S. (1951b) The effect of
trichloroethylene on the organism. Acta physiol. scand.,
22: 326-339.
ALEXANDER, H.C., MCCARTHY, W.M., & BARTLETT, E.A. (1978) Toxicity
of perchloroethylene, trichloroethylene, 1,1,1-trichloroethane,
and methylene chloride to fathead minnows. Bull. environ. Contam.
Toxicol., 20: 344-352.
ANDERSEN, M.E., GARGAS, M.L., JONES, R.A., & JENKINS, L.J., Jr
(1980) Determination of the kinetic constants for metabolism of
inhaled toxicants in vivo using gas uptake measurements. Toxicol.
appl. Pharmacol., 54(1): 100-116.
ANDERSSON, A. (1957) [Health hazards in industry on exposure to
trichloroethylene: a clinical, experimental, and technical hygienic
investigation.] Acta med. scand., 157(Suppl. 323): 1-220 (in German).
ASCHIMICI (1980) Report for Italian Working Group for
trichloroethylene.
ASTRAND, I. & OVRUM, P. (1976) Exposure to trichloroethylene.
I. Uptake and distribution in man. Scand. J. Work Environ. Health,
2: 199-211.
AVIADO, D.M., ZAKHARI, S., SIMAAN, J.A., & ULSAMER, A.G. (1976)
In: Golberg, L., ed. Methyl chloroform and trichloroethylene in
the environment, Cleveland, Ohio, CRC Press Inc., pp. 47-89.
AXELSON, O., ANDERSSON, K., HOGSTEDT, C., HOLMBERG, B., MOLINA, G.,
& DE VERDIER, A. (1978) A cohort study on trichloroethylene
exposure and cancer mortality. J. occup. Med., 20: 194-196.
AXELSON, O., ANDERSSON, K., SELDEN, A., & HOGSTEDT, C. (1984)
Cancer morbidity and exposure to trichloroethylene. In: ICOST,
International Conference on Organic Solvent Toxicity, Stockholm,
15-17 November 1984 (Abstract in: Arbete och Halsa, 29: 126).
BADEN, J.M., KELLEY, M., MAZZE, R.I., & SIMMON, V.F. (1979)
Mutagenicity of inhalation anaesthetics: trichloroethylene,
divinyl ether, nitrous oxide and cyclopropane. J. Anaesth., 51: 417.
BAERG, R.D. & KIMBERG, D.V. (1970) Centrilobular hepatic necrosis
and acute renal failure in "solvent sniffers". Ann. intern. Med.,
73: 713-720.
BALKON, J. & LEARY, J.A. (1979) An initial report on a
comprehensive, quantitative, screening procedure for volatile
compounds of forensic and environmental interest in human biofluids
by GC/MS. J. anal. Toxicol., 3: 213-215.
BANERJEE, S. & VAN DUUREN, B.L. (1978) Covalent binding of the
carcinogen trichloroethylene to hepatic microsomal proteins and to
endogenous DNA in vitro. Cancer Res., 38: 776-780.
BANERJEE, S., YALKOWSKY, S.H., & VALVANI, S.C. (1980) Water
solubility and octanol/water partition coefficients of organics.
Limitations of the solubility-partition coefficient correlation.
Environ. Sci. Technol., 14: 1227-1229.
BARDODEJ, Z. (1962) [Value and use of exposure test. VIII.
Volatile organic chloride test in urine.] Cesk. Hyg., 7: 234-239 (in
Czech).
BARDODEJ, Z. & VISKOCIL, J. (1956) The problem of
trichloroethylene metabolism and its effects on the nervous system
as a means of hygienic control. Am. Med. Assoc. Arch. ind. Health,
13: 581-592.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS (III), B.S.H., KIRKPATRICK, M.,
MICHAEL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment: a multimedia
environmental study. Biomed. mass Spectrom., 7: 139-147.
BARRETT, L., ARSAC, PH., VINCENT, M., FAURE, J., GARREL, S., &
REYMOND, F. (1982) Evoked trigeminal nerve potential in chronic
trichloroethylene intoxication. J. Toxicol. clin. Toxicol., 19:
419-423.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish (Lepomis macrochirus). In: Hague,
R., ed. Dynamics, Exposure and Hazard Assessment of Toxic
Chemicals, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BARTONICEK, V. (1962) Metabolism and excretion of
trichloroethylene after inhalation by human subjects. Br. J. ind.
Med., 19: 134-141.
BARTONICEK, V. & BRUN, A. (1970) Subacute and chronic
trichloroethylene poisoning: a neuropathological study in rabbits.
Acta pharmacol. toxicol., 28: 359-369.
BARTONICEK, V. & SOUCEK, B. (1959) Metabolism of
trichloroethylene in rabbits. Arch. Gewerbepathol. Gewerbehyg., 17:
283-293.
BARTSCH, H., MALAVEILLE, C., BARBIN, A., & PLANCHE, G. (1979)
Mutagenic and alkylating metabolites of haloethylenes,
chlorobutadienes and dichlorobutenes produced by rodent or human
liver tissues. Arch. Toxicol., 41: 249-277.
BATTIG, K. (1964) Comparison of the effects of trichloroethylene
with the effects of drugs on exploratory behaviour in the rat. In:
14th International Congress on Occupational Health, Madrid, 1963,
Vol. 2, pp. 887-889.
BATTIG, K. & GRANDJEAN, E. (1963) Chronic effects of
trichloroethylene on rat behaviour. Arch. environ. Health, 7:
694-699.
BAUER, U. (1981) [Human exposure to environmental chemicals.
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues.] Zbl. Bakt. I. Abt. Orig., B174: 200-237
(in German).
BAUER, U. & SELENKA, F. (1982) [Exposure of the population to
haloforms and chlorinated solvents in drinking water compared to
air and food.] Von Wasser, 59: 7-16 (in German).
BEISLAND, H.O. & WANNAG, S.A. (1970) [Trichloroethylene sniffing:
acute liver and kidney damage.] Tidsskr. Nor. Laegeforen., 90: 285-288
(in Norwegian).
BELILES, R.P., BRUSICK, D.J., & MECLER, F.J. (1980) Teratogenic-
mutagenic risk of workplace contaminants: trichloroethylene,
perchloroethylene, and carbon disulfide, Washington DC, US
Department of Health, Education and Welfare, pp. 234 (Report for US
DHEW Contract No. 210-77-0047) (Govt Rep. Announce. Index (US),
1982: 2728).
BELL, Z. (1977) Written communication with contractor reports of:
(a) Dominant lethal study with trichloroethylene in albino rats
exposed via inhalation (February 1977); and (b) Teratogenic study
via inhalation with trichlor 132, trichloroethylene in albino rats
(March 1977), Washington DC, US Environmental Protection Agency
(EPA-600/8-82-006).
BELLAR, T.A., BUDDE, W.L., & EICHELBERGER, J.S. (1979) The
identification and measurement of volatile organic compounds in
aqueous environmental samples. IV. In: Schuetzle, D., ed.
Monitoring toxic substances, Washington DC, American Chemical
Society (ACS Symposium Series No. 94).
BERGMAN, K. (1983) Application and results of whole-body
autoradiography in distribution studies of organic solvents. Crit.
Rev. Toxicol., 12(1): 59-118.
BIANCHI, A., DE NATALE, G., & MATTURRO, F. (1963) [Comparative
research on some pharmacological effects of fluothane and other
volatile narcotics.] G. Ital. Chir., 19: 327-349 (in Italian).
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene, and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGNAMI, M., CONTI, G., CONTI, L., CREBELLI, R., MISURACA, F.,
PUGLIA, A.M., RANDAZZO, R., SCIANDRELLO, G., & CARERE, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor, and Aspergillus nidulans.
Chem.-biol. Interact., 30: 9-23.
BLAIR, A., DECOUFLE, P., & GRAUMAN, D. (1979) Causes of death
among laundry and dry cleaning workers. Am. J. public Health, 69:
508-511.
BOLT, H.M. & FILSER, J.G. (1977) Irreversible binding of
chlorinated ethylenes to macromolecules. Environ. Health Perspect.,
21: 107-112.
BOTHE, J., BRAUN, W., & DONHARDT, A. (1973) [Effect of mineral
oil in hydrocarbon poisoning in mice.] Arch. Toxikol., 30: 243-250 (in
German).
BOUDENE, C., TURBIAUX, M., CLUET, J.-L., & TRUHAUT, R. (1983)
Contribution à la fixation d'une concentration limite tolérable de
trichloroéthylène dans l'atmosphère des locaux de travail. Arch.
Mal. prof. Méd. Trav. Sécur. soc., 44(2): 75-91.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and 2-
carbon halogenated aliphatic organic compounds under methanogenic
conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOUYGUES, M., DANNE, O., BOUVRY, M., LUCIANI, F., & RABREAU, D.
(1980) Hépatite au trichloroéthylène. Action synergique du
tétrachlorure de carbone? Nouv. Presse méd., 9(43): 3277.
BOZZA MARRUBINI, M., GHEZZI LAURENZI, R., & UCCELLI, P. (1978)
[Acute intoxication: clinical diagnosis and treatment,] Milan,
Italy, Medico-Farmaceutica Organization Editore, p. 470 (in
Italian).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14: 231-241.
BRONZETTI, G., ZEIGER, E., & FREZZA, D. (1978) Genetic activity
of trichloroethylene in yeast. J. environ. Pathol. Toxicol., 1:
411-418.
BROSS, G., DI FRANCEISCO, D., & DESMOND, M.E. (1983) The effects
of low dosages of trichloroethylene on chick development.
Toxicology, 28: 283-294.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., DEFELD, J.M., & BAUER, H.
(1974) Le dosage par chromatographie en phase gazeuse des
métabolites urinaires du trichloréthylène: l'acide trichloro-
acétique et le trichloroéthanol. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 35(3): 395-402.
CABANA, B.E. & GESSNER, P.K. (1967) Determination of chloral
hydrate, trichloroacetic acid, trichlorethanol, and urochloralic
acid in the presence of each other and in tissue homogenates. Anal.
Chem., 39: 1449-1452.
CACCURI, S. (1976) [Labour medicine,] 3rd ed., Napoli, Italy,
Idelson, pp. 343-352 (in Italian).
CALLEN, D.F., ROLAND WOLF, C., & PHILPOT, R.M. (1980) Cytochrome
P-450 mediated genetic activity and cytotoxicity of seven
halogenated aliphatic hydrocarbons in Saccharomyces cerevisiae.
Mutat. Res., 77: 55-63.
CAPELLINI, A. & GRISLER, R. (1958) [Studies on liver function in
a group of workers chronically exposed to trichloroethylene.] Med.
Lav., 49: 167-172 (in Italian).
CARLSON, G.P. & WHITE, J.F. (1983) Cardiac arrythmogenic action
of benzo( a)pyrene in the rabbit. Toxicol. Lett., 15: 43-48.
CASTELLINO, N. (1969) [Trichloroethylene: technology,
pathological manifestations and prevention,] Milano, Italy, Franco
Angeli Editore, p. 117 (in Italian).
CAVALLO, A. & GRASSI, P. (1976) Determination of chlorinated
hydrocarbons in potable waters. Boll. chim. Union Ital. Lab. prov.,
27: 337-350.
CEFIC (1982) Chlorinated solvents: trichloroethylene in metal
cleaning and other industrial applications, Brussels, Conseil
Européen des Fédérations des Industries Chimiques.
CERNA, M. & KJPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analyzed by screening system tests. Mutat. Res., 46:
214-215.
CHIESURA, P. (1980) [Halogenated aliphatic hydrocarbons.] In:
Crepet, M., ed. [Occupational medicine,] Torino, Italy, Utet (in
Italian).
CHIESURA, P. & CORSI, G. (1961) [Acute trichloroethylene
poisoning in man followed by liver disease and hyperglycaemic
glycosuria.] Folia med., 44: 121-135 (in Italian).
CHRISTENSEN, H.E., LUGINBYHL, T.T., & CARROLL, B.B., ed. (1974)
The toxic substances list 1974, Washington DC, National Institute
of Occupational Safety and Health, p. 353.
CHRISTENSEN, H.E. & LUGINBYHL, T.T. (1975) Registry of toxic
effects of chemical substances 1975, Washington DC, US Department
of Health, Education and Welfare.
CLEARFIELD, H.R. (1970) Hepatorenal toxicity from sniffing spot-
remover (trichloroethylene): Report of 2 cases. Am. J. dig. Dis.,
15: 851-856.
COLEMAN, W.E., LINGG, R.D., MELTON, R.G., & KOPFLER, F.C. (1976)
The occurrence of volatile organics in five drinking water supplies
using gas chromatography/mass spectrometry. Identification and
analysis of organisms in polluted water. In: Keith, L.H., ed.
Proceedings of the First Chemical Congress of the North American
Continent, 1975, Ann Arbor, Michigan, Ann Arbor Science,
pp. 305-327.
CONSO, F., EFTHYMIOU, M.-L., GARNIER, R., & FOURNIER; E. (1980)
Interêt de la toxicovigilance: example de certains
trichloréthylènes du commerce. Arch. Mal. prof. Méd. Trav., 41:
198-200.
CORNISH, H.H. & ADEFUIN, J. (1966) Ethanol potentiation of
halogenated aliphatic solvent toxicity. Am. Ind. Hyg. Assoc. J., 27:
57-61.
CORREIA, Y., MARTENS, G.J., VAN MENSCH, F.H., & WHIM, B.P. (1977)
The occurrence of trichloroethylene, tetrachloroethylene and 1,1,1-
trichloroethane in Western Europe in air and water. Atmos.
Environ., 11: 1113-1116.
CREBELLI, R., BIGNAMI, M., CONTI, L., & CARERE, A. (1982)
Mutagenicity of trichloroethylene in Salmonella typhimurium TA 100.
Ann. Ist. Super. Sanità, 18: 117-122.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (in press)
Mutagenicity of trichloroethylene, trichloroethanol, and chloral
hydrate in Aspergillus nidulans. Mutat. Res.
CRONN, D.R., RASMUSSEN, R.A., ROBINSON, E., & HARSCH, D.E. (1977)
Halogenated compound identification and measurement in the
troposphere and lower stratosphere. J. geophys. Res., 82: 5935-5944.
DALBEY, W. & BINGHAM, E. (1978) Metabolism of trichloroethylene
by the isolated perfused lung. Toxicol. appl. Pharmacol., 43: 267-277.
DANIEL, J.W. (1963) The metabolism of 36Cl-labelled
trichloroethylene and tetrachloroethylene in the rat. Biochem.
Pharmacol., 12: 795-802.
DEFALQUE, R.J. (1961) Pharmacology and toxicology of
trichloroethylene: A critical review of the world literature. Clin.
Pharmacol. Ther., 2: 665-688.
DEGUCHI, T. (1972) Threshold limit values for solvent mixtures in
the air. Effects of single and mixed chlorinated hydrocarbons upon
the level of serum transaminases in rats. Osaka Shiritsu Daigaku
Igaku Zasshi, 21: 187-209.
DEKANT, W. & HENSCHLER, D. (1982) Dechlorination reactions in the
course of the metabolism of trichloroethylene. Naunyn-Schmiedeb
Arch. Pharmacol., 319: R16.
DEKANT, W. & HENSCHLER, D. (1983) New pathways of
trichloroethylene metabolism. Dev. Toxicol. environ. Sci., 11:
399-402.
DEKANT, W., METZLER, M., & HENSCHLER, D. (1984) Novel metabolites
of trichloroethylene through dechlorination reactions in rats,
mice, and humans. Biochem. Pharmacol., 33(13): 2021-2027.
DE LEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, C.K., REMELE,
P.C., STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980) Rapid
gas chromatographic method for the determination of volatile and
semivolatile organochlorine compounds in soil and chemical waste
disposal site samples. J. chromatogr. Sci., 18: 85-88.
DE MORE, W.B., HAMSON, R.F., MOLINA, M.J., KURYLO, M.J., WATSON,
R.T., HOWARD, C.J., GOLDEN, D.M., & RAVISHANKARA, A.R. (1983)
Chemical kinetics and photochemical data for use in stratospheric
modeling evaluation number 6, Washington DC, National Aeronautics
and Space Administration (NASA JPL Publication No. 83-62,
15 September, 1983).
DERWENT, R.G. & EGGLETON, A.E.J. (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos. Environ., 12: 1261-1268.
DESOILLE, H., PINCHON, R.A., JANS, M., & BOUGUIGNON, A. (1962)
Intoxication expérimentale aiguë par le trichloroéthylène. Effets
aggravants de l'intoxication éthylique chronique associée. Etude
expérimentale électroencéphalographique chez le lapin. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 23: 653-664.
DIETZ, E.A., Jr & SINGLEY, K.F. (1979) Determination of
chlorinated hydrocarbons in water by headspace gas chromatography.
Anal. Chem., 51: 1809-1814.
DILLING, W.L. (1977) Interphase transfer processes. II.
Evaporation rates of chloromethanes, ethanes, ethylenes, propanes,
and propylenes from dilute aqueous solutions. Comparisons with
theoretical predictions. Environ. Sci. Technol., 11: 405.
DI PAOLO, J.A. & DONIGER, J. (1982) Neoplastic transformation of
Syrian hamster cells by putative epoxide metabolites of
commercially-utilized chloroalkenes. J. Natl Cancer Inst., 69(2):
531-534.
DI RENZO, A.B., GANDOLFI, A.J., & SPIES, I.G. (1982) Microsomal
bioactivation and covalent binding of aliphatic halides to DNA.
Toxicol. Lett., 11: 243-252.
DMS (1967) UV atlas of organic compounds, London, Dortmund,
Butterworths-Verlag Chemie, Vol. 3 (Spectrum No. A1/6).
DOBINSON, B. & GREEN, G.E. (1972) Formation of dichloroacetylene
from trichloroethylene in the presence of epoxides. Chem. Ind., 5:
214.
DORFMUELLER, M.A., HENNE, S.P., YORK, R.G., BORNSCHEIN, R.L., &
MANSON, J.M. (1979) Evaluation of teratogenicity and behavioural
toxicity with inhalation exposure of maternal rats to
trichloroethylene. Toxicology, 14: 153-166.
DOWTY, B.J., CARLISLE, D.R., & LASETER, J.L. (1975) New Orleans
drinking water sources tested by gas chromatography-mass
spectrometry. Environ. Sci. Technol., 9: 762-765.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Solubility of organic
solvents. I. Gas chromatographic determination of olive oil/gas
partition coefficient. Helv. Chim. Acta, 60: 454.
DROZ, P.O. & FERNANDEZ, J.G. (1978) Trichloroethylene exposure.
Biological monitoring by breath and urine analyses. Br. J. ind.
Med., 35: 35.
DUPRAT, P. & GRADISKI, D. (1980) Cytogenic effect of
trichloroethylene in the mouse as evaluated in the micronucleus
test. IRCS med. Sci. soc. occup. Med., 8: 182.
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir irritant
des principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur. J. Toxicol., 9(3): 171-177.
EAJ (1983) Environmental monitoring of chemicals: environmental
survey report of F.Y. 1980 and 1981, Japan, Office of Health
Studies, Department of Environmental Health, Environmental Agency
Japan, p. 144.
EHRNER-SAMUEL, H., BALMER, K., & THORSELL, W. (1973)
Determination of trichloroacetic acid in urine by a gas
chromatographic method. Am. Ind. Hyg. Assoc. J., 34: 93-96.
EKLUND, G., JOSEFFSON, B., & ROOS, C. (1978) Determination of
volatile halogenated hydrocarbons in tapwater. J. High Res. Chrom.
& Chrom. Comms., July, pp. 34-40.
ELCOMBE, C.R. (in press) Species differences in carcinogenicity
and peroxisome proliferation due to trichloroethylene: a
biochemical human hazard assessment. Arch. Toxicol.
ELCOMBE, C.R., PRATT, I.S., & GREEN, T. (1982) The rate of
trichloroacetic acid (TCA) formation determines two species
differences in hepatic peroxisome proliferation due to
trichloroethylene (TRI). Toxicologist, 2: 173.
ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979) Effects of
methylene chloride, trichloroethane, trichloroethylene,
tetrachloroethylene, and toluene on the development of chick
embryos. Toxicology, 12: 111-119.
ENTZ, R.C. & HOLLIFIELD, H.C. (1982) Headspace gas
chromatographic analysis of foods for volatile hydrocarbons.
J. agric. food Chem., 30: 84-88.
ERTLE, T., HENSCHLER, D., JULLER, G., & SPASSOWSKI, M. (1972)
Metabolism of trichloroethylene in man. Arch. Toxikol., 29: 171-188.
ETTEMA, J.J., KLEEREKOPER, L., & DUBA, W.C. (1975) Mental
stresses during short-term inhalation of trichloroethylene.
Staub-Reinhalt. Luft, 35: 409-410.
EUROCOP-COST (1976) A comprehensive list of polluting substances
which have been identified in various fresh waters, effluent
discharges, aquatic animals and plants, and bottom sediments, 2nd
ed., Luxembourg, Commission of the European Community, p. 57
(EUCO/MDU/73/76, XII/476/76).
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., & HOPKE, P.K.
(1977) Monitoring to detect previously unrecognized pollutants in
surface waters - appendix: organic analysis data, Springfield,
Virginia, US NTIS, pp. 304 (PB Report PB-273350) (Govt Rep.
Announce. Index, 1978: 199).
FABRE, R. & TRUHAUT, R. (1952) Toxicology of trichloroethylene.
II. Results of experimental animal studies. Br. J. ind. Med., 9:
39-43.
FAHRIG, R. (1977) The mammalian spot test (Fellfleckenstest) with
mice. Arch. Toxicol., 38: 87-98.
FAWNS, H.T. (1968) Trichloroacetic acid in the urine of workers
using trichloroethylene. Proc. Assoc. Clin. Biochem., 5: 110-114.
FERGUSON, R.K. & VERNON, R.J. (1970) Trichloroethylene in
combination with CNS drugs: effects on visual-motor tests. Arch.
environ. Health, 20: 462-467.
FERNANDEZ, J.G., HUMBERT, B.E., DROZ, P.O., & CAPEROS, J.R. (1975)
Exposition au trichloroéthylene. Bilan de l'absorption, de
l'excrétion, et du métabolisme sur des sujets humains. Arch. Mal.
prof. Méd. Trav. Sécur. soc., 36(7-8): 397-407.
FERNANDEZ, J.G., DROZ, P.O., HUMBERT, B.E., & CAPEROS, J.R. (1977)
Trichloroethylene exposure: simulation of uptake, excretion, and
metabolism using a mathematical model. Br. J. ind. Med., 34: 43-55.
FILSER, J.G. & BOLT, H.M. (1979) Pharmacokinetics of halogenated
ethylenes in rats. Arch. Toxicol., 42: 123-136.
FINK, R. (1968) Toxicity of anaesthetics, Baltimore, Maryland,
Williams & Williams, pp. 270.
FISHBEIN, L. (1973) Chromatography of environmental hazards,
New York, Elsevier Scientific Publishing Company, Vol. 2,
pp. 471-490.
FOOD CHEMICAL NEWS (1978) Preliminary report on TCE study shows
no carcinogenicity in rats. Food chem. News, 20: 41-42.
FOSSA, A.A., WHITE, J.F., & CARLSON, G.P. (1982) Anti-arrhythmic
effects of disulfiram on epinephrine-induced cardiac arrthythmias
in rabbits exposed to trichloroethylene. Toxicol. appl. Pharmacol.,
66: 109-117.
FRIBERG, G.L., KYLIN, B., & NYSTROM, A. (1953) Toxicities of
trichloroethylene and tetrachloroethylene and Fujiwara's pyridine
reaction. Acta pharmacol. toxicol., 9: 303-312.
FUKUDA, K., TAKEMOTO, K., & TSURATA, H. (1983) Inhalation
carcinogenicity of trichloroethylene in mice and rats. Ind. Health,
21: 243-245.
GAMBERALE, F., ANNWALL, G., & OLSON, B.A. (1976) Exposure to
trichloroethylene. III. Psychological functions. Scand. J. Work
environ. Health, 4: 220-224.
GAULTIER, M. (1974) [Clinical toxicology,] Rome, Italy, Società
Editrice Demi (in Italian).
GEHRING, P.J. (1968) Hepatoxic potency of various chlorinated
hydrocarbon vapours relative to their narcotic and lethal potencies
in mice. Toxicol. appl. Pharmacol., 13: 287-298.
GOLDBERG, M.E., JOHNSON, H.E., POZZANI, U.C., & SMITH, H.E., Jr
(1964) Effect of repeated inhalation of vapours of industrial
solvents on animal behaviour. I. Evaluation of nine solvent vapours
on pole-climb performance in rat. Am. Ind. Hyg. Assoc. J., 25: 369-375.
GOLDBLATT, M.W. (1956) Industrial medicine and hygiene, London,
Merewether, Vol. 3.
GOVERNA, M. (1981) [Occupational diseases due to chlorine
derivatives of the aliphatic hydrocarbons.] In: Sartorelli, E., ed.
[Textbook of occupational medicine,] Padova, Italy, Vol. 1,
pp. 483-502 (in Italian).
GRADISKI, D., MAGADUR, J.-L., BAILLOT, M., DANIERE, M.C., & SCHUH,
M.B. (1974) Toxicité comparée des principaux solvants chlorés
aliphatiques. J. eur. Toxicol., 7: 247-254.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.-L., & FRANCIN,
J.M. (1978) Toxicité aiguë comparée par inhalation des principaux
solvants aliphatiques chlorés. Arch. Mal. prof. Méd. Trav. Sécur.
soc., 39: 249-257.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
New York, Academic Press.
GRANDJEAN, E. (1960) Trichloroethylene effects on animal
behaviour. Arch. environ. Health, 1: 106-108.
GRANDJEAN, E. (1963) The effects of short exposures to
trichloroethylene on swimming performances and motor activity of
rats. Am. Ind. Hyg. Assoc. J., 24: 376-379.
GRANDJEAN, E., MUNCHINGER, R., TURRIAN, V., HAAS, P.A., KNOEPFEL,
H.K., & ROSENMUND, H. (1955) Investigations into the effects of
exposure to trichloroethylene in mechanical engineering. Br. J.
ind. Med., 12: 131-142.
GRANT, M.W. (1961) Trichoroethylene in the eye. J. Am. Med.
Assoc., 178: 359.
GRANT, M.W. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, pp. 1034-1045.
GRAOVAC-LEPOSAVIC, L., MILOSAVLJEVIC, Z., & ILIC, V. (1964)
[Liver function in workers exposed to trichloroethylene.] Arh. Hig.
Rad. Toksikol., 15: 93-97 (in Yugoslavian).
GREEN, T. & PROUT, M.S. (in press) Species differences in
response to trichloroethylene. II. Biotransformation in rats and
mice. Arch. Toxicol.
GREEN, T., PROUT, M.S., & PROVAN, W.M. (in press) Species
differences in response to trichloroethylene. I. Pharmacokinetics
in rats and mice. Arch. Toxicol.
GREIM, H., BONSE, G., RADWAN, Z., REICHERT, D., & HENSCHLER, D.
(1975) Mutagenicity in vitro and potential carcinogenicity of
chlorinated ethylenes as a function of metabolic oxirane formation.
Biochem. Pharmacol., 24: 2013-2017.
GU, Z.W., SELE, B., JALBERT, P., VINCENT, M., VINCENT, F., MARKA,
C., CHMARA, D., & FAURE, J. (1981) Induction of sister chromatid
exchanges by trichloroethylene and its metabolites. Toxicol. Eur.
Res., 3: 63-67.
GUN, R.T., GRYGORCENICZ, D., & NETTELBECK, T.J. (1978) Choice
reaction time in workers using trichloroethylene. Med. J. Aust., 1:
535-536.
HARDELL, L., ERIKSSON, M., LENNER, P., & LUNDGREN, E. (1981)
Malignant lymphoma and exposure to chemicals, especially organic
solvents, chlorophenols, and phenoxy acids: a case-control study.
Br. J. Cancer, 73: 169-176.
HATHWAY, D.E. (1980) Consideration of the evidence for mechanisms
of 1,1,2-trichloroethylene metabolism including new identification
of its dichloroacetic acid and trichloroacetic acid metabolites in
mice. Cancer Lett., 8: 263-269.
HAYDEN, J.W., COMSTOCK, E.G., & COMSTOCK, B.S. (1976) The
chemical toxicology of solvent abuse. Clin. Toxicol., 9: 169-184.
HEIL, E., OESER, H., HATZ, R., & KELKER, H. (1979) [Gas
chromatographic determination of traces of C- and C-fluoro- and
chlorohydrocarbons in air.] Fresenius Z. Anal. Chem., 297: 357-364
(in German).
HENSCHLER, D. (1977a) Metabolism and mutagenicity of halogenated
olefuis: a comparison of structure and activity. Environ. Health
Perspect., 21: 61-64.
HENSCHLER, D. (1977b) Metabolism of chlorinated alkenes and
alkanes as related to toxicity. J. environ. Pathol. Toxicol., 1:
125-133.
HENSCHLER, D. & HOOS, R. (1982) Metabolic activation and
deactivation mechanisms of di-, tri-, and tetrachloroethylenes.
In: Snyder, Parke, Kocsis, Jollow, Gibson, & Witmer, ed. Biological
reactive intermediates. II. Part A., New York, Plenum Publishing
Corporation, pp. 659-666.
HENSCHLER, D., BONSE, G., & GREIM, H. (1970a) [Cranial nerve
neuritis due to poisoning with chlorinated acetylenes when handling
vinylidene chloride copolymers.] Arch. Toxikol., 26: 62-75 (in
German).
HENSCHLER, D., BROSER, F., & HOPF, H.C. (1970b) Environmental
Pollution and Carcinogenic Risks, Lyons, International Agency for
Research on Cancer, pp. 171-175 (IARC Scientific Publications
No. 13).
HENSCHLER, D., EDER, E., NEUDECKER, T., & METZLER, M. (1977)
Carcinogenicity of trichloroethylene: fact or artifact? Arch.
Toxicol., 37: 233-236.
HENSCHLER, D., HOOS, W.R., FETZ, H., DALLMEIER, E., & METZLER, M.
(1979) Reactions of trichloroethylene epoxide in aqueous systems.
Biochem. Pharmacol., 28: 543-548.
HENSCHLER, D., ROMEN, W., ELSASSER, H.M., REICHERT, D., EDER, E., &
RADWAN, Z. (1980) Carcinogenicity study of trichloroethylene by
long-term inhalation in three animal species. Arch. Toxicol., 43:
237-248.
HENSCHLER, D., BONSE, G., & DEKANT, W. (1983) Mechanisms and
reactions of electroplulic intermediates of halogenated ofefins.
In: Proceedings of the 13th International Cancer Congress. Part B.
Biology of Cancer, New York, Alan R. Liss, Inc., Vol. 1,
pp. 175-183.
HENSCHLER, D., ELSASSER, H., ROMEN, W., & EDER, E. (1984)
Carcinogenicity study of trichloroethylene, with and without
epoxide stabilizers, in mice. J. Cancer Res. clin. Oncol., 107:
149-156.
HERBOLSHEIMER, R., FUNK, L., & DRASCHE, H. (1972) Use of
activated carbon as an adsorbent in the determination of
trichloroethylene in the air. Staub-Reinhalt. Luft, 32: 407-409.
HERNBERG, S., KORKATA, M.-L., ASIKAINEN, V., & RIALA, R. (1984)
Primary liver cancer and exposure to solvents. Int. Arch. occup.
environ. Health, 54: 147-153.
HIRAYAMA, T. & IKEDA, M. (1979) Applicability of carbon felt to
the dosimetry of solvent vapour mixture. Am. Ind. Hyg. Assoc. J.,
40: 1091-1096.
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., & SAKAI,
T. (1983) [Experimental studies of trichloroethylene toxicity.
II. Changes in trichloroethylene in blood serum and in urine during
and after exposure to trichloroethylene.] Nippon Eiseigaku Zasshi,
38(4): 772-779 (in Japanese with English summary).
HOWARD, C.J. (1976) Rate constants for the gas-phase reactions of
OH radicals with ethylene and halogenated ethylene compounds.
J. Chem. Phys., 65: 4771.
HUFF, J.E. (1971) New evidence on the old problems of
trichloroethylene. Ind. Med. Surg., 40: 25-33.
IARC (1979) Trichloroethylene, Lyons, International Agency for
Research on Cancer, pp. 545-572 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 247-249 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Supplement 4).
IKEDA, M. (1974) Reciprocal metabolic inhibition of toluene and
trichloroethylene in vivo and in vitro. Int. Arch. Arbeitsmed., 33:
125-130.
IKEDA, M. & IMAMURA, T. (1973) Biological half-life of
trichloroethylene and tetrachloroethylene in human subjects. Int.
Arch. Arbeitsmed., 31: 209-224.
IKEDA, M., OHTSUJI, H., KAWAI, H., & KUNIYOSHI, M. (1971)
Excretion kinetics of urinary metabolites in patient addicted to
trichloroethylene. Br. J. ind. Med., 28: 203-206.
IKEDA, M., OHTSUJI, H., IMAMAURA, T., & KOMOIKE, Y. (1972)
Urinary excretion of total trichloro-compounds, trichloroethanol,
and trichloroacetic acid as a measure of exposure to
trichloroethylene and tetrachloroethylene. Br. J. ind. Med., 29:
328-333.
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office, pp. 206-207 (Occupational Safety and Health Series No. 37).
IMAMURA, T. & IKEDA, M. (1973) A time-saving procedure for the
determination of total trichloro-compounds in human urine samples.
Int. Arch. Arbeitsmed., 31: 333-338.
IOFFE, B.V., ISIDOROV, V.A., & ZENKEVICH, I.G. (1977) Gas
chromatographic-mass spectrometric determination of volatile
organic compounds in an urban atmosphere. J. Chromatogr., 142:
787-795.
IRPTC (1984) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JAMES, W.R.L. (1963) Fatal addiction to trichloroethylene. Br. J.
ind. Med., 20: 47-49.
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G. (1982)
Uptake via the blood and elimination of 10 organic solvents
following epicutaneous exposure of anaesthetized guinea-pigs.
Toxicol. appl. Pharmacol., 63: 181-187.
JAPANESE YEARBOOK OF CHEMICAL INDUSTRIES STATISTICS (1983) Tokyo,
Ministry of International Trade and Industry.
JESSON, J.P. (1980) Release of industrial halocarbons and
tropospheric budget. In: Proceedings of the NATO Advanced Study
Institute on Atmospheric Ozone: Its Variation and Human Influences,
Washington DC, US Department of Transportation, Federal Aviation
Administration.
JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (1983)
Evaluation of certain food additives: 27th report, Geneva, World
Health Organization (Technical Report Series No. 696).
JOUGLARD, J. & VINCENT, V. (1971) Pulmonary signs of ingestions
of solvents. Marseille Med., 108: 696-697.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial waste water: a case study of their
environmental impact. Environ. Sci. Technol., 12: 88-96.
KALASHINIKOVA, V.P., VISSARIONOVA, V.Ya., BONDAREV, G.I., SKIRKO,
B.K., & KARANTIROVA, O.V. (1974) [Influence of different diets on
the course of chronic experimental trichloroethylene poisoning.]
Vopr. Pitan., No.6: 43-47 (in Russian).
KALASHINIKOVA, V.P., SKIRKO, B.K., VISSARIONOVA, V.Ya., BONDAREV,
G.I., KLEIMENOVA, N.V., POPOVA, N.S., & KARANTIROVA, O.V. (1976)
[Evaluation of the toxic effects of trichloroethylene according
to morphological and some biochemical indexes.] Gig. i Sanit., No. 2:
107-108 (in Russian).
KANJE, M., KJELLSTRAND, P., FOX, K., & WALLDORF, A. (1981)
Neurotransmitter metabolizing enzymes and plasma
butyrylcholinesterase in mice exposed to trichloroethylene. Acta
pharmacol. toxicol., 49: 205-209.
KARICKHOFF, S.W., BROWN, D.S., & SCOTT, T.A. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13: 241-248.
KATZ, R.M. & JOWETT, D. (1981) Female laundry and dry cleaning
workers in Wisconsin: a mortality analysis. Am. J. public Health,
71: 305-307.
KEINFELD, M. & TABERSHAW, I.R. (1954) Trichloroethylene toxicity:
report of 5 fatal cases. AMA Arch. ind. Hyg. occup. Med., 10: 134-141.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil
sorption coefficients of pesticides and other chemicals.
Ecotoxicol. environ. Saf., 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (in press) Relationship between
water solubility, soil sorption, octanol-water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish,
P.R., & Hendricks, A.C., ed. Aquatic toxicology ASTM STP, 707,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
KIMMERLE, G. & EBEN, A. (1973) Metabolism, excretion, and
toxicology of trichloroethylene after inhalation. II. Experimental
human exposure. Arch. Toxicol., 30: 127-138.
KIRK, R.E. & OTHMER, D.F., ed. (1963) Anesthetics. In:
Encyclopedia of chemical technology, 2nd ed., New York, John Wiley
and Sons, Vol. 2, pp. 393-410.
KIRK, R.E. & OTHMER, D.F., ed. (1964) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 2nd
ed., New York, John Wiley and Sons, Vol. 5, pp. 183-195.
KIRK, R.E. & OTHMER, D.F., ed. (1979) Chlorocarbons and
chlorohydrocarbons. In: Encyclopedia of chemical technology, 3rd
ed., New York, John Wiley and Sons, Vol. 5, pp. 745-753.
KISELEVA, A.F. & KOROLENKO, A.M. (1971) Histoenzymic changes in
liver and kidneys during the experimental effect of anesthetic
doses of fluorothan and trylen. Eksp. Khir. Anesteziol., 16: 81-84.
KITAGAWA, T. (1961) The rapid measurement of toxic gases and
vapour. In: Proceedings of the 13th International Congress on
Occupational Health, New York, pp. 506-512.
KJELLSTRAND, P., LANKE, J., BJERKEMO, M., ZETTERQUIST, L., &
MANSSON, L. (1980) Irreversible effects of trichloroethylene
exposure on the central nervous system. Scand. J. Work environ.
Health, 6: 40-47.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M.,
MORTENSEN, I., LANKE, J., & HOLMQUIST, B. (1981a)
Trichloroethylene: effects on body and organ weights in mice,
rats, and gerbils. Toxicology, 21: 105-115.
KJELLSTRAND, P., KANJE, M., MANSSON, L., BJERKEMO, M., MORTENSEN,
I., LANKE, J., & HOLMQUIST, B. (1981b) Effects of long-term
exposure to trichloroethylene on the behaviour of Mongolian gerbils
(Meriones unguiculatus). J. Toxicol. environ. Health, 8: 787-793.
KJELLSTRAND, P., HOLMQUIST, B., MANDAHL, N., & BJERKEMO, M.
(1983a) Effects of continuous trichloroethylene inhalation on
different strains of mice. Acta pharmacol. toxicol., 53: 369-374.
KJELLSTRAND, P., HOLMQUIST, B., ALM, P., KANJE, M., ROMARE, S.,
JONSSON, I., MANSSON, L., & BJERKEMO, M. (1983b)
Trichloroethylene: further studies of the effects on body and organ
weights and plasma butyrycholinesterase activity in mice. Acta
pharmacol. toxicol., 53: 375-384.
KLAASSEN, C.D. & PLAA, G.L. (1966) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in mice.
Toxicol. appl. Pharmacol., 9: 139-151.
KLAASSEN, C.D. & PLAA, G.L. (1967) Relative effects of various
chlorinated hydrocarbons on liver and kidney function in dogs.
Toxicol. appl. Pharmacol., 10: 119-131.
KLINE, S.A., MCCOY, E.C., ROSENKRANZ, H.S., & VAN DUUREN, B.L.
(1982) Mutagenicity of chloroalkene epoxides in bacterial systems.
Mutat. Res., 101: 115-125.
KLOWKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981)
Laboratory screening of distribution, conversion, and
mineralisation of chemicals in the soil-plant-system and comparison
to outdoor experimental data. Chemosphere, 10: 1089-1090.
KONIETZKO, H., HABERLANDT, W., HEILBRONNER, H., REILL, G., &
WEICHARDT, H. (1978) [Chromosome studies on trichloroethylene
workers.] Arch. Toxicol., 40: 201-206 (in German).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin pathology
following epicutaneous exposure to seven organic solvents. Int. J.
Tiss. React., 111(1): 21-30.
KYLIN, B., REICHARD, H., SUMEGI, I., & YLLNER, S. (1962)
Heptatoxic effect of tri- and tetrachloroethylene on mice. Nature
(Lond.), 193: 395.
KYLIN, B., AXELL, K., EHRNER-SAMUEL, H., & LINDBORG, A. (1967)
Effect of inhaled trichloroethylene on the CNS. Arch. environ.
Health, 15: 48-52.
KYLSZEIJKO, C., LAKOMY, T., & PAPIEROWSKI, A. (1963) [The
influence of trichloroethylene during labour process in women in
comparison with the results in vitro.] Pol. Tyg. Lek., 18:
1333-1338 (in Polish).
KYRKLUND, T., ALLING, C., HAGLID, K., & KJELLSTRAND, P. (1983)
Chronic exposure to trichloroethylene: lipid and acyl group
composition in gerbil cerebral cortex and hippocampus.
Neurotoxicology, 4(4): 35-42.
LACHNIT, V. (1971) Halogenated hydrocarbons and the liver. Wein.
Klin. Wochenschr., 83: 734-737.
LA DU, B.N., MANDEL, H.G., & WAY, E.L., ed. (1971) Fundamentals
of drug metabolism and drug disposition, Baltimore, Maryland,
Williams and Wilkins.
LAHAM, S. (1970) Studies on placental transfer:
trichloroethylene. Ind. Med. Surg., 39: 46-49.
LAZAREV, N.V. (1929) [The narcotic efficacy of the vapours of the
chlorine derivatives of methane, ethane and ethylene.] Naunyn-
Schmiedeb. Arch. exp. Pathol. Pharmacol., 141: 19-24 (in German).
LAZAREV, N.V. & GADASKINA, I.D. (1977) [Harmful substances
in industry,] Leningrad, Medecine, Vol. 3 (in Russian).
LEHMANN, K.B. & SCHMIDT-KEHL, L. (1936) [Thirteen most important
chlorohydrocarbons of the paraffin series from the standpoint of
industrial hygiene.] Arch. Hyg. Bakt., 116: 131-268 (in German).
LEIBMAN, K.C. (1965) Metabolism of trichloroethylene in liver
microsomes. Mol. Pharmacol., 1: 239-246.
LEONG, B.K.J., SCHWETZ, B.A., & GEHRING, P.J. (1975) Embryo- and
fetotoxicity of inhaled trichloroethylene, perchloroethylene,
methylchloroform, and methylene chloride in mice and rats. Toxicol.
appl. Pharmacol., 33: 136.
LERZA, P., LOMBARDI, F., & VIOTTI, G. (1963) [Health and hygiene
considerations regarding work in dry-cleaning establishments using
trichloroethylene.] Lav. Um., 15: 459-478 (in Italian).
LEWIS, G.D., REYNOLDS, R.C., & JOHNSON, A.R. (1984) Some effects
of trichloroethylene on mouse lungs and livers. Gen. Pharmacol.,
15(2): 139-144.
LILIS, R., STANESCU, D., MUICA, N., & ROVENTA, A. (1969) Chronic
effects of trichloroethylene exposure. Med. Lav., 60: 595-601.
LIN, R.S. & KESSLER, I.I. (1981) A multifactoral model for
pancreatic cancer in man. J. Am. Med. Assoc., 245: 147-152.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Proceedings of the
Symposium on Short-Term Test Systmes and the Detection of
Carcinogenesis, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 333-356.
LOPRIENO, N., SBRANA, I., & LASCIALFARI, D. (in press) [Study on
the in vivo potential mutagenic effect of trichloroethylene
evaluated by cytogenetic analysis in mice treated by inhalation for
52 days.] (in Italian).
LOVELOCK, J.E. (1974) Atmospheric halocarbons and stratospheric
ozone. Nature (Lond.), 252: 292-294.
MACKAY, D. (1982) Correlations of bioconcentration factors.
Environ. Sci. Technol., 10: 274-278.
MCCONNELL, G., FERGUSON, D.M., & PEARSON, C.R. (1975) Les
hydrocarbures chlorés et l'environnement. Endeavour, 34: 13-18.
MCCORD, C.P. (1932) Toxicity of trichloroethylene. J. Am.
Med. Assoc., 99: 409.
MACKAY, D. & PATERSON, S. (1981) Calculating fugacity. Environ.
Sci. Technol., 15: 1006-1014.
MCNEILL, W.C. (1979) Chlorocarbons and chlorohydrocarbons.
Trichloroethylene. In: Kirk-Othmer's encyclopedia of chemical
technology, 3rd ed., New York, Wiley Interscience, Vol. 5,
pp. 745-753.
MAKIDE, Y., TOMINAGA, K., & ROWLAND, F.S. (1979) Gas
chromatographic analysis of halogenated hydrocarbons in air over
Japan. Chem. Lett., 1979: 355-358.
MALEK, B., KRCMAROVA, B., & RODOVA, O. (1979) [Epidemiological
study of hepatic tumour incidence in subjects working with
trichloroethylene: II. Negative result of retrospective studies in
dry cleaners.] Pracov. Lek., 31(4): 124-126 (in Czech).
MALIZIA, E., PELAIA, P., & PIROVINE, C. (1984) Experience in the
emergency treatment of acute intoxication by trichloroethylene.
Riv. Toss. Speriment Clin., 14: 437.
MALOOF, C.C. (1949) Burns of the skin produced by
trichloroethylene vapours at room temperature. J. ind. Hyg.
Toxicol., 31: 295-296.
MALTONI, I.C. & MAIOLI, P. (1977) [Long-term bioassay of
carcinogenicity of trichloroethylene. Preliminary results.]
Osp. Vita, 4: 108-110 (in Italian).
MALTONI, C., LEFEMINE, G., & COTTI, G. (in press) Experimental
research on trichloroethylene carcinogenesis. In: Maltoni, C. &
Mehlman, M.A., ed. Archives of research on industrial
carcinogenesis, Vol. 5.
MANTEL, M. & NOTHMANN, R. (1977) Rapid determination of
trichloroethanol and trichloroacetic acid in urine. Analyst, 102:
672-677.
MATTURRO, F. (1963) [Effects of some volatile narcotics on
isolated guinea-pig heart.] G. Ital. Chir., 19: 95-99 (in Italian).
MAZZA, V. & BRANCACCIO, A. (1967) [Behaviour of the formed
elements of the blood and the marrow in experimental intoxications
with Tri.] Folia med., 50: 318-324 (in Italian).
MELINO, C., MESSINEO, A., & PACELLI, E. (1979) Risk from
trichloroethylene in a factory producing electrical condensers.
Riv. Med. Lab. Ig. Ind., 3: 67-81.
MEYER, H.-J. (1966) [Trichloroethylene poisoning by the oral
route.] Arch. Toxikol., 21: 225-234 (in German).
MEYER, H.-J. (1973) Bronchopulmonary alterations induced by
trichloroethylene and other halogenated hydrocarbons. Bronches, 23:
113-124.
MILLER, D.A. & GRIMSRUD, E.P. (1979) Correlation of electron
capture response enhancements caused by oxygen with chemical
structure for chlorinated hydrocarbons. Anal. Chem., 51: 851-859.
MILLER, R.E. & GUENGERICH, F.P. (1982) Oxidation of
trichloroethylene by liver microsomal cytochrome P-450: evidence
for chlorine migration in a transition state not involving
trichloroethylene oxide. Biochemistry, 21: 1090-1097.
MOLINA, M.J. & ROWLAND, F.S. (1974) Stratospheric sink for
chlorofluoromethanes: chlorine atom-catalysed destruction of ozone.
Nature (Lond.), 294: 810-812.
MONSTER, A.C. (1979) Differences in uptake, elimination, and
metabolism in exposure to trichloroethylene, 1,1,1-trichloroethane,
and tetrachloroethylene. Int. Arch. occup. environ. Health, 42:
311-317.
MONSTER, A.C. & BOERSMA, G. (1975) Simultaneous determination of
trichloroethylene and metabolites in blood and exhaled air by gas
chromatography. Int. Arch. occup. environ. Health, 35: 155-163.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1976) Pharmaco-
kinetics of trichloroethylene in volunteers: influence of workload
and exposure concentration. Int. Arch. occup. environ. Health, 38:
87-102.
MONSTER, A.C., BOERSMA, G., & DUBA, W.C. (1979) Kinetics of
trichloroethylene in repeated exposure of volunteers. Int. Arch.
occup. environ. Health, 42: 283-292.
MOSLEN, M.T., REYNOLDS, E.S., & SZABO, S. (1977) Enhancement of
the metabolism and hepatoxicity of trichloroethylene and
perchloroethylene. Biochem. Pharmacol., 26: 369-375.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1972)
Trichloroethylene exposure and trichloroethylene metabolites in
urine and blood. Arch. Toxicol., 29: 335-340.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1974) Metabolism of
trichloroethylene in man. II. Pharmacokinetics of metabolites.
Arch. Toxicol., 32: 283-295.
MULLER, G., SPASSOVSKI, M., & HENSCHLER, D. (1975) Metabolism of
trichloroethylene in man. III. Interaction of trichloroethylene and
ethanol. Arch. Toxicol., 33: 173-189.
MURRAY, A.J. & RILEY, J.P. (1973) Occurrence of some chlorinated
aliphatic hydrocarbons in the environment. Nature (Lond.), 242: 37-38.
MUSCLOW, C.E. & AWEN, C.F. (1971) Glue sniffing: report of a
fatal case. Can. Med. Assoc. J., 104: 315-319.
NCI (1976) Carcinogenesis bioassay of trichloroethylene. CAS No.
